Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 06/57/01 from route sheet. The protocol was agreed in November 2006. The assessment report began editorial review in June 2007 and was accepted for publication in February 2008. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Dr Robert JO Davies, ResMed (CPAP machine manufacturer), donated US$100,000 to the Oxford Centre for Respiratory Medicine to support the running costs of a randomised trial looking at blood pressure response to CPAP treatment in sleep apnoea between 1999 and 2003. The study finished in 2003. ResMed are also supplying 400 therapeutic and subtherapeutic CPAP machines to Oxford Centre for Respiratory Medicine for use in a currently recruiting multicentre, British Heart Foundation-funded, randomised trial looking at changes in cardiovascular function in patients with mild/moderate sleep apnoea.
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NCCHTA, Alpha House, Enterprise Road, Southampton Science Park, Chilworth, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of health problem
Definition of obstructive sleep apnoea–hypopnoea syndrome
Obstructive sleep apnoea–hypopnoea is characterised by repeated, intermittent collapse and obstruction of the pharyngeal airway during sleep. Airway collapse can be complete, with total obstruction of the airway lumen and no respiratory airflow (apnoea), or partial with reduced respiratory airflow, arbitrarily often defined as at least a 50% reduction (hypopnoea). Pharyngeal patency (keeping the airway opened) depends on dilator muscles which contract during each inspiration to prevent the upper airway being closed by suction. The upper airway collapses due to falling muscle tone in the dilating muscles with sleep, leading to narrowing or total obstruction. This may result in brief awakening from sleep caused by increased respiratory effort. Recurrent arousal required to restore airway patency results in fragmentation of normal sleep architecture (structure) and a reduction in sleep quality. When obstructive sleep-disordered breathing is accompanied by clinical symptoms such as excessive daytime sleepiness, this is known as obstructive sleep apnoea–hypopnoea syndrome (OSAHS). 1–3
The most commonly reported symptoms of OSAHS are excessive daytime sleepiness, loud snoring and unrefreshing sleep. 4 Other frequent symptoms are nocturnal choking, nocturia, witnessed apopnoeas and morning headaches. Less commonly reported symptoms include reduced libido and enuresis. 4
Classification of disease severity
Diagnosis of OSHAS is usually based on recordings of multiple physiological signals during sleep polysomnography (PSG). These include the apnoea–hypopnoea index (AHI), and repetitive oxygen desaturation indices. The AHI is the frequency of apnoeas and hypopnoeas per hour of sleep; a typical cut-off for positive diagnosis is between 5 and 10 events per hour. The AHI is also used to categorise severity. Whilst definitions regarding the severity of OSAHS vary between sleep centres, recommendations for cut-offs suggest the following severity classification:5 mild OSAHS (AHI 5–15 events per hour of sleep); moderate OSAHS (AHI 15–30 events per hour of sleep); and severe OSAHS (AHI > 30 events per hour of sleep). Oxygen desaturation is calculated as the number of events causing a drop in arterial oxygen saturation per hour. Typically a diagnostic cut-off of > 4% drop is used to define an oxygen desaturation event, with thresholds approximating hypoxic dips per hour of 5–10 (mild), 10–30 (moderate) and greater than 30 (severe). The number of events can vary from night to night for individuals and these cut-off points for disease severity are considered arbitrary. 1,4 None of these measures takes into account the severity of other symptoms such as daytime sleepiness. This is considered important as the daytime consequences of OSAHS are often of more concern to the patient than are nocturnal events.
Daytime sleepiness
Several tools are available for measuring sleepiness, both subjectively and objectively. The Epworth Sleepiness Scale (ESS) is the most frequently used assessment of daytime sleepiness. This short questionnaire measures the general level of daytime sleepiness based on the subjective probability of falling asleep in a variety of situations. 6 The participant rates his or her likelihood of falling asleep in eight different daily situations, such as while sitting reading or while sitting inactive in a public place. The score range is from 0 to 24 and the higher the score the greater the sleepiness. A score of seven or less is regarded as normal sleepiness; a score of 16 or more indicates substantial daytime sleepiness. Average normal scores of 5.9 [standard deviation (SD) 2.2] with a range from 0 to 106 and 7.6 (SD 3.9)7 have been obtained in different populations without sleep disorder. The validity of the scale as a test of propensity to sleep has been established. 6 Reliability is reasonably high and the scale has high internal consistency (Cronbach’s alpha 0.88). 7 The score distribution appears to be approximately normal in OSAHS and normal populations. 6,7
The most commonly used objective measures of daytime sleepiness are the Maintenance of Wakefulness Test (MWT), which measures the capacity to stay awake, and the Multiple Sleep Latency Test (MSLT), which measures the propensity to fall asleep in favourable conditions. 8 The MWT is a 40-minute test that measures the capacity to remain awake in conditions supposedly ideal for falling sleep. If participants do not fall asleep during the test, they achieve the maximum score. The MSLT assesses the tendency to fall asleep during four or five tests at 2-hourly intervals throughout the day in conditions conducive to sleep. Both tests use a polysomnogram to establish when the participant has fallen asleep. An additional measure is the Osler test, a simplified version of the MWT, which uses a behavioural test rather than electroencephalograph recordings to define sleep onset. 9 The score derived from all these tests is the time taken to fall asleep in minutes (sleep latency). Precise normative data on time taken to fall asleep have been difficult to establish for the MWT and MSLT as many factors may affect sleep latency, such as age, prior total sleep time and variations in the testing protocol. 8 The ‘normal’ sleep latency for MSLT is around 10 minutes with an SD range of 2–19 minutes. 8 On the MWT, the mean time taken to fall asleep in a population without sleep disorder was estimated at 35.24 minutes [standard error (SE) 0.98], though this varied with age. 8 Both the MSLT and MWT are relatively poor at discriminating between sleepy and non-sleepy populations as a result of the overlap of sleep latency time in these populations. However, they are sensitive to conditions expected to increase or decrease sleepiness. 8 Performance on both tests can be affected by physiological factors such as age and circadian rhythms; psychological factors such as anxiety and depression; and test protocol factors such as the extent of activity prior to testing and the specific instructions given. The correlation between the MSLT and MWT is weak, probably because they measure different aspects of sleepiness. The MWT can have limited ability to discriminate the most alert individuals due to a ceiling effect; the MSLT can have limited ability to discriminate the most sleepy individuals due to a floor effect.
Epidemiology
The severity of sleeping upper airway collapse is a continuous variable in the community and ranges from normality, through postural and continuous snoring, postural and continuous repetitive obstructive apnoeas associated with excessive sleepiness (i.e. OSAHS) and ultimately, in the most severe cases, to daytime hypercapnic ventilatory failure, cor pulmonale and death. The major daytime symptom of the disease (excessive daytime sleepiness) also ranges from normality to very severe, disabling excessive somnolence. The severity of daytime sleepiness is moderately correlated with the objective severity of disease quantified from the number of episodes of airway obstruction per hour during sleep. 10 The treatment of obstructive sleep apnoea is targeted mainly at controlling its symptoms (particularly excessive daytime sleepiness) and consequences (such as hypertension/vascular risk), through correction of the breathing disturbance. It is therefore appropriate that disease severity should primarily be stratified using symptom severity rather than the number of episodes of airway obstruction at night.
At least 1% of men in the UK have severe obstructive sleep apnoea with both marked objective respiratory abnormality at night and substantial excessive daytime somnolence, and about 6% of men have objectively detectable disease of lesser severity. 11 The prevalence of the disease in the normal community depends on the exact definition of an episode of airway obstruction. 11 The standard definitions of an obstructive apnoea, hypopnoea or > 4% oxygen desaturation episode, used to define disease severity for this analysis, are the most frequently used disease definitions. Using these indices, it is possible to define broad disease severity subgroups, such as the mild, moderate and severe definitions used in this report. However, the variation in the absolute number of identified respiratory events produced by modest alterations in sleep study scoring definitions means that the boundaries of these groups are necessarily arbitrary and they need to be applied to clinical practice with a degree of pragmatic common sense.
The main aetiological factor for adult obstructive sleep apnoea is obesity, particularly upper body and neck obesity. Fat deposition in these areas causes airway narrowing and ultimately collapse, although the severity of obesity required to cause airway collapse depends on associated features such as facial shape and jaw structure. Therefore, the prevalence of disease varies markedly with population obesity levels11 and minimising the prevalence of OSAHS is an important potential benefit of population weight reduction strategies. Other common risk factors are enlarged tonsils and adenoids, and craniofacial abnormalities. OSAHS has also been associated with endocrine conditions such as hypothyroidism and acromegaly.
Outcomes associated with OSAHS
The major treatment goal in OSAHS is improvement in daytime sleepiness. As well as being symptomatically unpleasant, excessive sleepiness impairs function on tasks requiring vigilance such as driving, and can result in loss of employment when it causes recurrent unwanted sleep in the work environment. OSAHS is also associated with other negative consequences: deterioration in cognitive function (especially in those tasks requiring concentration, such as driving), changes in mood or personality, and impaired quality of life. Such impairments may be mediated by the severity of daytime sleepiness. 12 Other associated outcomes, with potentially major health resource implications, are hypertension, cardiovascular disease, cerebrovascular disease and stroke. A systematic review of the health effects of OSAHS concluded that OSAHS causes daytime sleepiness and possibly road traffic accidents (RTAs) but that the epidemiological evidence for a causal link with other adverse health outcomes is weak. 13 A key limitation of the evidence was the failure to sufficiently take into account the potential confounding effects of factors such as obesity and smoking and to establish a causal time sequence. 13 However, new epidemiological research has been published in the 10 years since that review, rendering it out of date, and a re-evaluation is required that incorporates the new research, although this is beyond the scope of the current review.
Cognitive function
Reported cognitive-related impairments with OSAHS include difficulties in work efficiency and performing new tasks, memory disturbance and concentration problems,12 although there is contradictory evidence regarding these effects in a population with mild to moderate disease. 14 Difficulties related to attention, memory and learning and executive performance have also been reported. 12 A systematic review of the field found that the most common aspects of executive function to be affected by OSAHS were working memory, phonological fluency, cognitive flexibility and planning (particularly non-verbal planning). 15
Accidents including road accidents
There is also evidence that symptoms of daytime sleepiness and impaired concentration arising from untreated OSAHS pose a significantly increased risk of automobile accidents and injury in the workplace. Sleepiness while driving is a recognised risk factor in road traffic and occupational accidents. 16 Studies of simulated driving tasks show that participants with OSAHS perform as poorly as alcohol-impaired participants. 17,18 A recent systematic review found an increased risk of motor vehicle collisions in drivers with OSAHS compared with those without OSAHS although the size of the estimated increased risk varied among studies. 19 The UK Driver and Vehicle Licensing Authority (DVLA) does not allow people who are prone to sleepiness that may impair vigilance while driving to hold a driving licence.
Health-related quality of life
Given the known effects of sleep apnoea on daytime sleepiness and cognitive function, an effect on measures of quality of life would be expected; a systematic review found that OSAHS significantly contributes to impairment of health-related quality of life (HRQoL). 20 It is therefore desirable to assess the impact of treatments of sleep apnoea, such as CPAP, upon quality of life. The concept of HRQoL typically refers to an individual’s perception of function in at least one of four domains: somatic sensation, physical function, emotional state and social interaction. 21 The consequences of sleep apnoea for HRQoL include the detrimental effects on physical, mental and social function, including excessive tiredness and decreased energy, decreased concentration and memory, depressive symptoms and relationship difficulties.
A number of generic instruments have been developed to measure HRQoL. These include the Medical Outcomes Study 36-item Short Form Health Survey (SF-36),22 the Nottingham Health Profile (NHP)23 and the EuroQol-5 Dimensions (EQ-5D). 24 Such instruments measure HRQoL in a standardised way that allows for comparisons across studies and conditions. However, these instruments have not been designed to specifically address the aspects of life affected by OSAHS, and as a consequence the criticism has been made that they may be less sensitive to important improvements experienced with treatment than would be a condition-specific instrument. For instance, most generic instruments do not include sleep as a specific dimension; only the NHP (Part 1) includes a sleep-specific dimension.
The two condition-specific instruments most commonly used to assess the HRQoL of people with sleep apnoea are the Functional Outcomes of Sleep Questionnaire (FOSQ)25 and the sleep apnoea quality of life index (SAQLI). 26 These are considered to have high acceptability and relevance for both patients and clinicians, and because they are disorder specific they are thought to be highly sensitive to change. The FOSQ, designed to detect the impact of disorders of excessive sleepiness on physical, mental and social functioning on everyday activities, contains 30 items grouped into five subscales: activity level, vigilance, intimacy and sexual relationships, general productivity and social outcome. Respondents are able to indicate whether lack of engagement with any of the items was a consequence of something other than sleepiness. One weakness of this instrument is that it does not measure experience of symptoms or overall well-being. In addition to a total score, the FOSQ generates a mean score for each subscale; low scores indicate poorer HRQoL. The SAQLI, designed specifically for use in clinical trials with patients experiencing sleep apnoea, contains 35 items grouped into four dimensions: daily function, social interactions, emotional functioning and symptoms. An additional domain, treatment-related symptoms, can also be added to capture the impact of treatment side effects. The SAQLI generates a total score; a low score indicates poor HRQoL. A drawback of this instrument is that it was designed to be interviewer led, although it has been used as a self-completed measure.
Cardiovascular disease
Based on three recent overviews of the evidence establishing a link between OSAHS and cardiovascular disease, the evidence seems strongest in respect of OSAHS as a risk factor for hypertension. 27–29 There is also evidence linking OSAHS with stroke and cardiac disease, although considerable uncertainties about whether it is an independent risk factor remain. 4,27,28
Current service provision
The mainstay of medical treatment of OSAHS is administration of continuous positive airway pressure (CPAP) during sleep. There are thought to be wide variations in the provision of CPAP treatment across the United Kingdom. Dental devices (also known as oral appliances) represent the main alternative group of treatments, although these are generally used only in individuals with mild to moderate OSAHS. Evidence for lifestyle modification as an efficacious treatment is weak;30 however, lifestyle management is often recommended as an adjunct to other treatments, including conservative options such as weight loss, avoidance of alcohol or sedative medication, improved sleep hygiene and use of a lateral sleeping position. In the severely obese, bariatric surgery has sometimes been used to achieve weight loss. 4 Other treatment options, such as surgery or drugs, are rarely used, and recent Cochrane reviews do not support their use for treatment of OSAHS. 31,32
Description of technology under assessment
CPAP devices
CPAP devices are small, electric pumps that deliver air to the nose or mouth via a hose and soft plastic mask during sleep. The air pressure, which can be fixed or autotitrated, opens up the airway, particularly at pharyngeal level, preventing the soft tissue from collapsing. Fixed CPAP devices deliver air at a fixed optimal pressure, usually identified by earlier observation and titration during sleep, while autotitrating CPAP devices increase pressure, as needed, to maintain airway patency, or decrease pressure if no events are detected, over a set period of time. As the minimum effective pressure delivered is automatically adjusted in autotitrating CPAP devices, the mean pressure is often lower than that from optimal fixed pressure in CPAP units. Originally developed for patients with OSAHS, CPAP is increasingly being investigated for use in populations with serious co-morbidities such as Alzheimer’s disease33 and heart failure. 34–36
It is difficult to obtain a precise estimate from the literature on rates of patient adherence to CPAP treatment. There are variations in how long-term adherence or compliance is defined, as well as in the methods used in epidemiological studies, and the influence of patient awareness that their compliance is being assessed also requires consideration. 37 There are two aspects that are of relevance when considering adherence: initial acceptance of treatment and long-term adherence (frequency of use as well as number of hours of use per night). Adherences among those accepting treatment of over 70%,38 and 80%39,40 after 1 year, have been reported, although lower rates have also been reported. Reasons for discontinuation relate primarily to physical discomfort, nasal dryness and congestion, difficulty in adapting to the pressure, dislodgement during sleep, and the social consequences of using the unit. Some patients may discontinue because they achieve an improvement in symptoms through, for example, weight loss or tonsillectomy. Serious side effects from CPAP are thought to be very rare.
A number of variations of the technology have been developed, mainly with the aim of improving adherence. The primary variations have involved the use of humidifiers, which have been shown to prevent upper airway dryness associated with CPAP use;41 and autotitrating and bi-level CPAP, which aim to vary the pressure depending on need during the night and therefore reduce both the pressure required and associated side effects. Lower treatment pressures have been reported with autotitrating than with fixed CPAP but no clinically important changes in adherence or other outcomes have been found,37,42 although one systematic review concluded that auto-CPAP may be of benefit in certain subgroups, as yet undefined. 37 Similarly, there is no evidence of increased adherence with humidified CPAP, although a need for further research has been noted. 37 Variations in the CPAP delivery interface, such as type of mask, have also been developed. A recent systematic review found a paucity of research on the impact of different masks, making it difficult to determine the best interface, but suggested that nasal pillows or the Oracle oral interface are potentially useful alternatives when patients are unable to tolerate the nasal mask. 43 For the purposes of this technology assessment report all types of CPAP device are treated as a single intervention.
Current usage in the UK
There are no routine data available on current use. Expert opinion estimates that approximately 20,000 of the probable 180,000 patients with OSAHS are using CPAP devices. Chilcott et al. 44 highlighted concerns about (1) the haphazard and sporadic provision of CPAP devices throughout the UK and (2) the potential scale of long-term costs related to provision of new devices and maintenance of an expanding pool of CPAP devices. Focusing on the Trent Region, the authors suggested that there is a great deal of variation in the pattern and range of services that are available in Trent for diagnosing and treating OSAHS. They gave the example that if 60 new CPAP devices are provided each year, as estimated in clinics in Leicester and Nottingham, the discounted cost of new investigations and maintenance of the existing pool of CPAP devices would increase exponentially. They reported the cost of a new, standard CPAP machine as £250 (no price year given) and estimated that, at that time, the annual maintenance and patient follow-up costs amount to an additional £250 per year. The cost of an initial investigation ranges from £370 to £790 per person investigated. They estimated that initial year 1 costs of about £60,000 in the Trent Region may rise to annual costs of £95,000 in year 5 and £115,000 in year 10.
Dental devices
Dental devices, also known as oral appliances, are designed to maintain the patency of the pharyngeal airway and prevent the lumen from collapsing during sleep by holding the tongue or mandible forward, thereby enlarging the posterior airspace. There are two main types – tongue repositioning devices and mandibular repositioning devices – although the latter is most commonly used for OSAHS. 45 Mandibular repositioning dental devices are either one-piece, holding the mandible in a fixed anterior position, or two-piece, allowing some movement of the mandible. 45 They can be custom-made or pre-fabricated; variations in design are available. Most side effects of treatment are reported to be minor and temporary, e.g. excessive salivation, although some are more significant, e.g. bite changes. 46 Owing to the perception that the increases in pharyngeal patency achievable with mandibular devices are modest and the lack of high-quality evidence available on their effectiveness, dental devices are currently considered appropriate for use only in mild to moderate OSAHS (where airway collapse is more easily reversed), or in patients who do not wish to use CPAP. 47
Previous systematic reviews
A number of recent systematic reviews have evaluated the effectiveness of CPAP as a treatment for OSAHS. 13,48–50 In addition, there have been systematic reviews underpinning guidelines in a number of countries, which are not discussed here. The earliest review, published ten years ago, concluded that there was a paucity of robust evidence for a clinical benefit and the cost-effectiveness of CPAP. 13 A key deficit identified was the lack of trials using a placebo that was indistinguishable from CPAP as, at that time, a pill placebo was being used. A considerable number of trials have been published subsequently. A systematic review in 2003 identified 12 trials; CPAP was compared with oral placebo, conservative therapy such as lifestyle changes and sham CPAP (a device identical to CPAP set at a non-therapeutic pressure). 49 The review investigated subjective sleepiness (ESS) and objective sleepiness (MSLT and MWT). When estimates from individual studies were pooled, there was a statistically significant improvement in the ESS score of 2.94 points (95% CI 1.61–4.26) with CPAP compared with control. There was evidence of variation in the treatment effect which remained unexplained by age, sex, body mass index (BMI), location of study or mean hours of CPAP use. Variations by study baseline disease severity and methodological quality were not investigated. The MSLT and MWT were pooled, which would seem inappropriate in view of the poor correlation between these measures. A more recent review identified a smaller number of relevant trials (n = 7) due to tighter inclusion criteria; again the comparators were oral placebo, conservative treatment and sham CPAP. 48 When estimates from individual studies were pooled, there was a statistically significant improvement on the ESS of 1.2 points (95% CI 0.5–1.9) with CPAP compared with control in patients with mild to moderate OSAHS. There was also a statistically significant improvement in sleep latency on the MWT of 2.1 minutes (95% CI 0.5–3.7) with CPAP compared with control, but no statistically significant difference on the MSLT.
The most recent and comprehensive systematic review (a Cochrane review) concluded that CPAP was effective in reducing objective and subjective symptoms of sleepiness, and improving quality of life in individuals with moderate and severe OSAHS. 50 Evidence was available from 36 trials and substantially more evidence was available from trials using sham CPAP as a comparator than had been the case in the earlier reviews. Compared with placebo (sham CPAP, oral placebo and conservative treatment), there was a statistically significant improvement in favour of CPAP of 3.83 points on the ESS (95% CI 3.09–4.57) from parallel trials and 1.92 points (95% CI 1.25–2.59) from crossover trials, although there was evidence of statistical heterogeneity (variation in the treatment effect) across the trials. There was a statistically significant benefit with CPAP compared with control in sleep latency on the MSLT (1.25 minutes, 95% CI 0.18–2.32) and on the MWT (2.36 minutes, 95% CI 0.31–4.40) from crossover trials.
Although this was a good-quality review, the current review provides an update, which includes additional studies, as well as an alternative approach to the meta-analyses; the Cochrane review analysed the data from crossover trials and parallel trials separately. While this is an appropriate approach, it does reduce the power of any subgroup analyses to investigate the influence of factors such as disease severity on treatment outcomes. 50 Such an approach also results in two treatment effects (one for parallel trials and one for crossover trials) for each outcome for use in the economic modelling. The current review uses an established method to combine the results of parallel and crossover trials for which sufficient data are available. 51,52
Systematic reviews have also been conducted on the efficacy of dental devices. The Cochrane review discussed above found that CPAP was more effective than dental devices in reducing respiratory disturbances during sleep, although no difference was shown between the treatment groups in daytime symptoms such as sleepiness. 50 A second Cochrane review, which was last updated in June 2005, compared dental devices with placebo devices that were similar devices placed in the mouth but which did not cause the mandible to protrude. 53 When parallel studies were pooled, there was a statistically significant improvement with dental devices compared with control devices on the ESS (MD –2.09, 95% CI –3.8 to –0.37), although there was high statistical heterogeneity. Crossover trials also showed a statistically significant benefit on the ESS (MD –1.81, 95% CI –2.72 to –0.90). An earlier systematic review reported a statistically significant improvement on the AHI but reported contradictory results from trials on subjective sleepiness (ESS). 45
Chapter 2 Definition of decision problem
Decision problem
Untreated OSAHS is associated with increased daytime sleepiness, impairment of cognitive function and a reduction in quality of life. Owing to increased sleepiness and impaired concentration it may have consequences for how effectively people can engage in work, home and leisure daytime activities. It has been associated with serious consequences such as increased risk of accidents and, if left untreated, it is a lifelong condition that may be a risk factor for hypertension, myocardial infarction and stroke. As a result of the association between OSAHS and obesity, the prevalence of OSAHS is expected to increase with increasing prevalence of obesity.
There is evidence from previous systematic reviews that CPAP is an effective treatment for some of the outcomes associated with OSAHS. It is the recommended first choice of treatment for moderate or severe OSAHS. Surgery and drug therapy are generally not recommended. Treatment options for mild OSAHS include conservative options such as weight loss, avoidance of alcohol or sedative medication, improved sleep hygiene and use of a lateral sleeping position. Dental devices are also considered to be a treatment option for mild to moderate disease.
However, provision of CPAP for OSAHS is variable across the UK. This is thought to be due to a combination of lack of facilities for diagnosis and treatment and a lack of recognition of the significant morbidity associated with OSAHS. An evaluation of the clinical benefit and cost-effectiveness of CPAP is required. The main focus of interest is how CPAP compares with placebo, conservative therapy and dental devices and not how different types of CPAP devices vary in effectiveness. Therefore, different CPAP devices should be treated as one technology. If the data are available, the question of whether there are subgroups of people for whom CPAP is particularly appropriate should be investigated.
Overall aims and objectives of assessment
The aim of this review was to determine the clinical effectiveness, safety, and cost-effectiveness of continuous positive airway pressure (CPAP) devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome (OSAHS) compared with best supportive care, placebo and dental devices.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing clinical effectiveness
Search strategy
The search terms used to capture the concepts of sleep apnoea and CPAP were arrived at by discussion with reviewers and experts. These search terms were then adapted for each individual database and relevant thesaurus terms used where possible. The search strategies used for each database are included in Appendix 1.
A range of databases and websites were searched to identify existing systematic reviews and guidelines on CPAP for sleep apnoea:
-
Cochrane Database of Systematic Reviews (Cochrane Library 2006 issue 3) (www.thecochranelibrary.com)
-
Database of Abstracts of Reviews of Effects [Centre for Review and Dissemination’s (CRD) administration version of the database]
-
Health Technology Assessment Database (CRD administration version of the database)
-
Scottish Intercollegiate Guidelines Network (www.sign.ac.uk)
-
National Guideline Clearinghouse (www.guideline.gov/)
-
National Research Register (2006 issue 3) (www.update-software.com/National/)
-
Health Services/Technology Assessment Text (HSTAT) (www.ncbi.nlm.nih.gov/books/bv.fcgi?rid%20=%20hstat)
-
Turning Research into Practice Database (Trip) (www.tripdatabase.com/)
-
Health Evidence Bulletins Wales (http://hebw.cf.ac.uk/index.html)
-
Clinical Evidence (www.clinicalevidence.com)
-
National Library for Health Guidelines Finder (www.library.nhs.uk/guidelinesfinder/).
Further databases were searched to identify primary studies:
-
MEDLINE (1996 to November week 3 2006) (OVID)
-
MEDLINE In-Process & Other Non-Indexed Citations (28 November 2006) (OVID)
-
EMBASE (1980 to 2006 week 47) (OVID)
-
Cochrane Central Register of Controlled Trials (Cochrane Library 2006 issue 4) (www.thecochranelibrary.com)
-
CINAHL (1982 to November week 3 2006) (OVID)
-
Science Citation Index (1900 to 25 November 2006) (Web of Knowledge)
-
ISI Proceedings Science & Technology (1990 to 25 November 2006) (Web of Knowledge)
-
Zetoc Conferences (1993 to 29 November 2006) (http://zetoc.mimas.ac.uk/)
-
SIGLE (1980 to March 2005) (SilverPlatter)
-
Index to Theses (1716 to 16 October 2006) (www.theses.com/)
-
NHS Economic Evaluation Database (NHS EED) (CRD internal administration system 13 January 2007)
-
Health Economic Evaluations Database (HEED) (1995 to January 2007) (CD-ROM)
-
Health Technology Assessment (HTA) database (CRD internal administration system 13 January 2007)
-
EconLit (1969 to October 2006) (SilverPlatter)
-
EconPapers (http://econpapers.repec.org/).
The contents pages of the following journals (selected by the review team based on included references from a previous systematic review on this topic) were also hand searched to identify reports that might not have been indexed by the electronic databases. In addition, electronic alerts were set up for each journal so that the contents page could be scanned as the latest edition was published:
-
Thorax [2005 vol 60(1) to vol 62(4)]
-
Sleep Medicine [2005 vol 6(6) to vol 7(1)]
-
European Respiratory Journal [2005 vol 26(5) to vol 29(4)]
-
Sleep [2005 vol 28(11) to vol 29(12)]
-
Respiratory Medicine [2005 vol 99(11) to vol 101(5)]
-
QJM [2005 vol 98(11) to vol 100(3)]
-
Journal of Internal Medicine [2005 vol 258(5) to vol 261(4)]
-
Journal of Sleep Research [2005 vol 14(4) to vol 16(1)]
-
European Journal of Orthodontics [2005 vol 27(6) to vol 29(1)].
The following conference proceedings were also scanned for relevant abstracts. This selection was based on recommendations from the Cochrane Airways Group:
-
American Thoracic Society international conferences 2005 and 2006 (www.thoracic.org/)
-
British Thoracic Society winter meeting 2006 (2005 winter meeting abstracts are published as part of the journal Thorax and therefore searched electronically) (www.brit-thoracic.org.uk/)
-
Thoracic Society of Australia and New Zealand annual scientific meetings 2005 and 2006 (www.thoracic.org.au/).
The industry submissions were also searched for any additional unpublished data. No additional studies were identified.
Inclusion and exclusion criteria
Titles and abstracts identified from the searches were independently screened for relevance by two reviewers and disagreements were resolved by consensus. The full papers were ordered for all potentially relevant studies. Full papers were screened independently by two reviewers based on the inclusion criteria below. Disagreements were resolved by consensus and, if necessary, a third reviewer was consulted. Studies in any language (published or unpublished or in abstract form only) were included in the review if they met the following criteria.
Population
Studies of adults (16 years or older) with a diagnosis of predominantly obstructive sleep apnoea, confirmed by use of an appropriate tool (e.g. a respiratory polysomnographic sleep study, analysed by an appropriately qualified respiratory physician, from which a standard severity criterion such as the API or arterial oxygen desaturation index was derived), were included. Populations with disease of any severity were eligible. Studies of participants with central nervous system (CNS) dysfunction (e.g. stroke or dementia such as Alzheimer’s disease) and heart failure were excluded. Both of these conditions can produce disorders of breathing control that are central in origin (i.e. breathing is interrupted by a lack of effort due to dysfunction in the part of the brain that controls breathing), in addition to OSAHS, making it difficult to determine OSAHS. Because of the complexities of differentiating obstructive from central sleep apnoea and the potential for a mixture of these disorders to complicate the interpretation of outcomes, studies conducted specifically in these patient groups were excluded. However, studies of general population groups that may have included some patients with these co-morbid conditions were included.
Intervention and comparators
Studies of fixed CPAP or autotitrating CPAP therapy were eligible for inclusion provided the treatment was of at least 1 week’s duration. For the purposes of this review, fixed and autotitrating CPAP were treated as the same intervention; studies comparing the two technologies were not eligible for inclusion. Relevant comparators were best supportive/usual care (including conservative intervention such as lifestyle advice regarding weight loss, alcohol consumption and sleep hygiene as well as sleep posture advice or treatment), placebo (including placebo pill and sham CPAP) and dental devices. For sham CPAP the subtherapeutic pressure used varies between studies. We included studies in which it was stated sham CPAP was used and did not exclude studies based on the specific subtherapeutic pressure used.
Outcomes
The following outcomes were included.
Primary outcomes
-
Subjective sleepiness as assessed by the ESS
-
Objective sleepiness as assessed by the MWT, Osler test, MSLT or equivalent measure.
Secondary outcomes
-
Blood pressure (mean day and night blood pressure were assessed separately as the mechanisms and patterns of daytime and night-time blood pressure disturbance in OSAHS vary, and the relationship between daytime blood pressure and vascular risk has been more clearly described in other studies)
-
Cardiovascular disease (e.g. myocardial infarction, stroke)
-
Accidents (e.g. driving, occupational), although it was thought unlikely that such data would be found in randomised controlled trials (RCTs)
-
Quality of life, where it was measured using a standardised scale
-
Mood, anxiety and depression, where they were measured using a standardised scale
-
Simulated driving performance
-
Neuropsychological functioning
-
AHI/desaturation rate
-
Any complications or adverse effects of treatment.
Outcomes such as changes to sleep architecture (e.g. rapid eye movement sleep, slow-wave sleep, sleep efficiency) were not considered.
Study design
RCTs using a parallel or crossover design were included. In this field there is no standard practice as to whether a washout period is used in crossover trials and, if so, how long the washout period should be. Because the effect of CPAP in relation to daytime sleepiness is thought to be short-lived, the risk of carryover was not considered to be a serious problem.
Data extraction
The authors of the recent systematic review by Giles et al. 50 provided the extracted data from their review to avoid duplication of work. This also included some unpublished data. These data had been independently extracted by two reviewers. Data from the new studies, as well as any additional data required from the studies previously extracted by Giles et al. , were extracted by one reviewer and checked by another. Discrepancies were resolved by discussion and, if necessary, a third reviewer was consulted. Where there were multiple publications from the same study, the main publication for each study was identified and data were extracted from that paper. Where additional relevant outcomes were available in a related paper these were also extracted. For some of the studies cognitive outcomes were reported for only a subset of participants from the main study. These data were extracted. Where only a conference abstract was available, authors were contacted for further data. Where necessary, authors were contacted to clarify whether published studies had any overlapping patients or to obtain missing data such as standard errors from a paired analysis in crossover trials or where data were only available in graphs.
Data were extracted into Review Manager (RevMan) and into a standard form in Microsoft Word. Data extracted included patient characteristics (age, sex, severity of OSAHS, BMI), details of the intervention (fixed or autotitrating CPAP, use of humidifier), comparator (details of placebo, conservative management or dental device), adherence (usually reported as the average number of hours the machine was running at night), length of follow-up, outcomes as identified above and study quality.
Predominantly end point data were reported in the trials, except for blood pressure, for which a mixture of change and end point data were reported. Where both end point and change data were reported, preference was given to end point data for all outcomes except blood pressure, in which case change data were used (provided the variance for the change score was reported). Where only change data were reported, the variance was imputed if necessary. Change scores may be less efficient than end point data in some situations as they have two sources of measurement error (at baseline and follow-up). 54 However, unlike end point values, the use of change scores removes a component of between-person variability. 54 Whether the between-person variation is increased or reduced by using an end point or change score depends on the size of the correlation between baseline and follow-up; therefore, it is important to specify in advance which measure will be used. 55 Use of change from baseline scores in crossover trials may increase the variation. 52 The decision was made in advance to use change data for blood pressure where they were available, as this outcome was being used in the economic model, and change in blood pressure was preferred to end point for use in this model. All outcomes were continuous data and the mean difference (MD) between CPAP and comparator was calculated for each outcome.
Paired data were extracted from crossover trials where available. If the SD or SE from a paired analysis was not reported, the SE was imputed from the t-statistic, the p-value or the CI from a paired analysis. 52 For one crossover study it was necessary to impute the SE for blood pressure:52 a within-person correlation of 0.5 was used and a within-person correlation of 0.1 and 0.9 for a sensitivity analysis. 56 It is generally recommended that when analysing a crossover trial the method of testing first for a carryover effect and then analysing only the data from the first sequence period as though it were data from a parallel trial should be avoided. 52 In the studies we included, in a few instances there was evidence of a carryover effect into the second period, but the authors reported only data from the first sequence of the crossover trial and these data were treated as data from a parallel trial. These are not ideal data but, where these were the only data available they were used in the review.
Owing to time limitations and the quantity of cognitive data from crossover trials it was not feasible to impute data for a paired analysis, where these were not reported, for all the cognitive outcomes. Where three or more studies were available for potential pooling, the SE was estimated where data were available as above. For the other cognitive outcome measures the mean end value at follow-up and the SD for the intervention and control group with the associated p-value were extracted. Where available, the SD or SE from a paired analysis was extracted.
Quality assessment
Study quality was assessed on the basis of criteria from CRD Report No. 4 and additional criteria were used to assess crossover trials (see Study quality, below). The criteria assessed were broad in anticipation that a narrative synthesis may have been necessary. Quality was assessed by one reviewer and checked by another. Discrepancies were resolved by discussion and, if necessary, a third reviewer was consulted.
Data analysis
Where sufficient data were available, they were pooled in quantitative syntheses using a random-effects model. Studies comparing CPAP with placebo or best supportive/usual care were pooled separately from studies comparing CPAP with dental devices. Where data sets included both study designs, parallel and crossover trials were pooled. 51 The generic inverse variance method in RevMan was used to pool data sets which included both parallel and crossover designs, or only crossover trials. When only parallel trials were being pooled the weighted mean difference (WMD) method in RevMan was used. To transform the parallel data for entry into the generic inverse variance facility, the SE for the MD was calculated from the 95% CI. This was calculated using the formula SE = (upper confidence limit – lower confidence limit)/3.92. This method assumes a sample size of at least 30; however, given the number of outcomes and studies included in the review it was not considered feasible in the time available to use the t-statistic.
Statistical heterogeneity between trials was assessed using the I2 statistic. 57 Five sources of potential clinical and methodological heterogeneity were identified a priori as being of priority: baseline disease severity, baseline daytime sleepiness, study design, type of placebo and study quality. We planned to investigate these for the primary outcomes using subgroup analysis, as clinically important variations in the magnitude of treatment effects are likely in different severity groups. The subgroups specified in advance were as follows:
-
population subgroups
-
– baseline disease severity, as classified using the AHI or the desaturation rate using the mean baseline score for each study: mild (AHI 5–14/hour or oxygen desaturation rate 5–10/hour), moderate (AHI 15–30/hour or oxygen desaturation rate 10–30/hour) and severe (AHI > 30/hour or oxygen desaturation rate > 30/hour)
-
– baseline symptom severity, as classified using the mean baseline ESS score for each study: mild (0–9 points), moderate (10–15 points) and severe (16–24 points)
-
-
comparator subgroups
-
– sham CPAP, oral placebo and best supportive care
-
-
study design subgroups
-
– parallel and crossover
-
– end point data and change from baseline data.
-
We planned to investigate the influence of study quality on the treatment effect by pooling studies with adequate concealment of allocation separately from those with inadequate or unclear adequacy of concealment. This analysis was limited due to the small number of studies that reported an adequate method of concealing treatment allocation.
The pooling of the primary outcomes and blood pressure were rerun using a fixed-effect model to test the impact of the model of analysis used. The robustness of the findings for these outcomes was also investigated by assessing the impact on the treatment effect of removing each study singly.
Where no new data were identified for specific outcomes subsequent to the review by Giles et al. 50 we reported the analysis based on the data sets from that review, although we report the pooling from a random-effects model, combining crossover and parallel designs, as per our protocol rather than a fixed-effect model and separate analyses by study design, as used by the earlier review.
The risk of publication bias was not formally assessed.
Results of review of clinical effectiveness
Quantity and quality of research available
The searches identified 6325 potentially relevant references (Figure 1). On the basis of screening titles and abstracts, 235 full papers were ordered for further assessment. Inclusion screening of full papers identified 48 individual relevant studies. Eighteen of these were new studies or provided additional data subsequent to the review by Giles et al. 50 Four were available at the time of the review by Giles et al. , but were classified as additional studies due to the different inclusion criteria used by the two reviews;58–61 two provided additional data as only abstracts had been available at the time of the earlier review;56,62 and 11 had become available since the earlier review had been completed. 63–73
FIGURE 1.
Study selection.
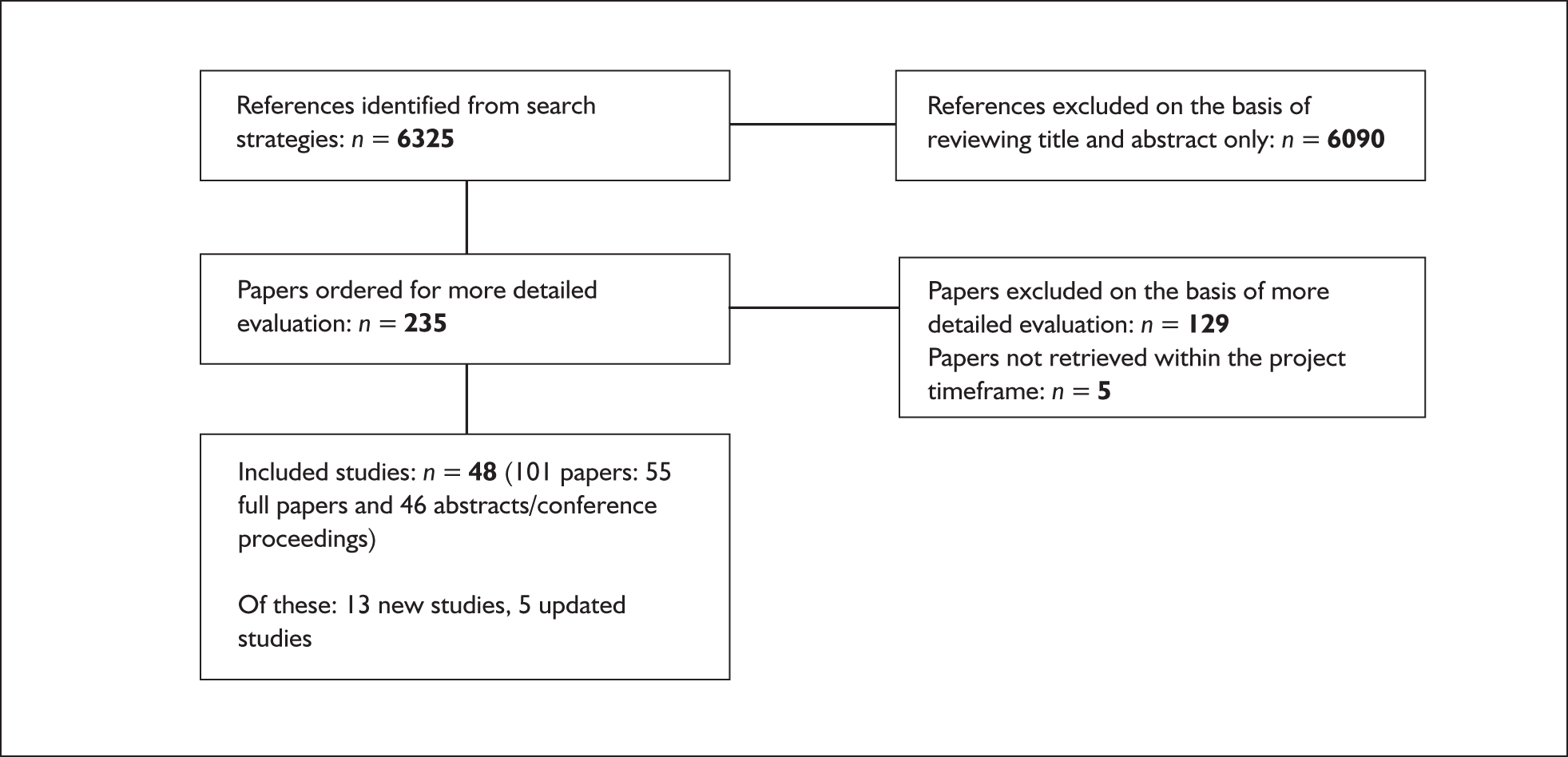
Three of the new studies were available in abstract form only and did not provide sufficient data for inclusion in the analysis. 69,71,72 Three studies that had been included in the review by Giles et al. were excluded because they focused on participants with CNS dysfunction or heart failure, and these populations were not considered in the current review. 74–76 Details of the included studies and their related papers are provided in Appendix 5. For the purpose of simplicity the main papers from individual studies are referred to in the main body of the report although data from more than one paper may have been used.
Study characteristics
The characteristics of the included studies are summarised in Table 1. This table focuses on the study characteristics that were used for the subgroup analyses: severity of daytime sleepiness at baseline (ESS), baseline disease severity (AHI), comparator and study design (parallel and crossover). Further details of study characteristics, including baseline data, are reported in Appendix 5.
| Study details | Number randomised (N) | Target population | Disease severity [AHI, mean (SD)] | Severity of sleepiness, [ESS, mean (SD)] | Treatment duration (weeks) |
|---|---|---|---|---|---|
| CPAP vs sham CPAP | |||||
| Parallel trials | |||||
| a,b Arias et al., 200663 | 23 | AHI ≥ 10 and ESS ≥ 10 | Severe, 44.1 (29.3) | NR | 12 |
| Barbé et al., 200183 | 55 | AHI ≥ 30 and no or mild daytime sleepiness | Severe, I 54 (16.2), C57 (20) | Mild, I 7 (2.2), C 7 (2) | 6 |
| Becker et al., 2003109 | 60 | AHI ≥ 5 and ESS ≥ 10 | Severe, I 62.5 (17.8), C65 (26.7) | Moderate, I 14.4 (2.5), C 14.1 (3.2) | 9 |
| aCampos-Rodriguez et al., 200665 | 72 | AHI ≥ 10 and hypertension | Severe, I 58.3 (24.6), C 59.5 (21.7) | Moderate, I 15 (3.9), C 13.6 (3.6) | 4 |
| cDimsdale et al., 200058 | 39? | RDI > 15 with or without hypertension | Severe, I RDI 53.6 (SD 23.2), C 41.7 (SD 25.6) | NR | 1 |
| dHenke et al., 200185 | 45 | AHI > 10 with daytime sleepiness or AHI > 20 with or without daytime sleepiness | Severe, I 62.1 (27.4), C 68.1 (25.2) | Severe, I 16.4 (5.6), C 16 (4.8) | 2 |
| aHui et al., 200664 | 56 | AHI ≥ 5 and daytime sleepiness or two other symptoms | Severe, I 32.9 (SE 3.2), C 29.5 (SE 3.1) | Moderate, I 10.7 (5.3), C 11.6 (5.3) | 12 |
| Jenkinson et al., 199977 | 107 | Men with > 10 episodes per hour of greater than 4% drop in SaO2 and ESS ≥ 10 | Moderate, I median 32.9 (15.5–63.4),e dips per hour > 4% SaO2, C 28.5 (10.7–68.7) | Severe, I median 16 (10.7–21.7),e C17 (10–23) | 4 |
| cNorman et al., 200673 | 46 | AHI > 15 with or without hypertension | Severe, I 66.1 (SE 29.1), C 53.9 (29.8) | Moderate, I 12 (5.5), C 12 (6.6) | 2 |
| Pepperell et al., 200287 | 118 | Men with ≥ 10 episodes per hour of greater than 4% drop in SaO2 and ESS ≥ 10 | Severe, I 38 (19.8), dips per hour > 4% SaO2, C 35.9 (19.6) | Severe, I 16.3 (3.3), C 16 (3.1) | 4 |
| aSpicuzza et al., 200666 | 25 | Moderate to severe OSAHS | Severe, I 55.3 (11.9), C 59.2 (17.3) | NR | 4 |
| aWest et al., 200667 | 42 | Men with type 2 diabetes and > 10 episodes per hour of greater than 4% drop in SaO2 and ESS ≥ 9 | Severe, I 33.1 (21.6), dips per hour > 4% SaO2, C 39.1 (24.8) | Moderate, I 14.7 (3.5), C 13.6 (3.5) | 12 |
| Crossover trials | |||||
| aArias et al., 200556 | 27 | Men with AHI ≥ 10 and ESS ≥ 10 | Severe, 44 (27.5) | NR | 12 |
| aCoughlin et al., 200762 | 35 | RDI > 15 and ESS ≥ 10 or two other symptoms | Severe, RDI 39.7 (13.8) | Moderate, 13.8 (4.9) | 6 |
| aCross et al., 2005,88 Abstract | 10 | Two major symptoms of OSAHS and > 20 episodes per hour of greater than 4% drop in SaO2 | Severe, 63 (26) | NR | 6 |
| Marshall et al., 200579 | 31 | AHI 5–30, habitual snoring or nocturnal choking and at least one symptom of daytime sleepiness or ESS ≥ 8 | Moderate, 21.6 (7.5) | Moderate, 12.5 (4.3) | 3 |
| aRobinson et al., 200668 | 35 | Patients with hypertension and > 10 episodes per hour of greater than 4% drop in SaO2 and ESS < 10 | Moderate, median 28.1 (IQR 18.0–38.0), dips per hour > 4% SaO2 | Mild, Median 5.3 (IQR 3.0–7.0) | 4 |
| CPAP vs oral placebo | |||||
| Crossover trials | |||||
| Barnes et al., 200289 | 42 | AHI 5–30 and symptoms of OSAHS | Mild, 12.9 (6.3) | Moderate, 11.2 (5) | 8 |
| Barnes et al., 200482 | 114 | AHI 5–30 | Moderate, 21.3 (13.6) | Moderate, 10.7 (6.5) | 12 |
| Engleman et al., 199490 | 35 | AHI ≥ 5 and at least two symptoms of OSAHS | Moderate, median 28 (range 7–129) | NR | 4 |
| Engleman et al., 199691 | 16 | AHI ≥ 5 and at least two symptoms of OSAHS | Severe, 49 (32.5) | NR | 3 |
| Engleman et al., 199792 | 18 | AHI 5–14.9 and at least two symptoms of OSAHS | Mild, 11 (4) | Moderate, 14 (4) | 4 |
| Engleman et al., 199893 | 23 | AHI ≥ 15 and at least two symptoms of obstructive sleep apnoea | Severe, 43 (37) | Moderate, 12 (4) | 4 |
| Engleman et al., 199978 | 37 | AHI 5–14.9 and at least two symptoms of OSAHS including daytime sleepiness (ESS ≥ 8 or reported sleepiness while driving) | Mild, 10 (3) | Moderate, 13 (3) | 4 |
| Faccenda et al., 200194 | 71 | AHI ≥ 15 and at least two symptoms of OSAHS | Severe, median 35 (range 15–129) | Moderate, median 15 (range 6–14) | 4 |
| McArdle et al., 200195 | 23 | AHI > 15 and at least two symptoms of OSAHS | Severe, median 40 (IQR 25–65) | Moderate, median 14 (IQR 10–17) | 4 |
| CPAP vs conservative/usual care | |||||
| Parallel trials | |||||
| Ballester et al., 199996 | 105 | AHI > 15 and severe clinical symptoms or AHI > 30 and mild to moderate symptoms | Severe, I 55 (22.3), C 58 (18.3) | Moderate, I 12.1 (5.0), C 11.4 (6.1) | 12 |
| Chakravorty et al., 200297 | 71 | AHI > 15 | Severe, I 55 (28.7), C35 (19.1) | Severe, I 16 (5.6), C 14 (4.2) | 12 |
| aDrager et al., 2006,69 Abstract | 16 | AHI > 30, normotensive | Severe, I 54 (8), C 65 (13) | NR | 12 |
| aLam et al., 200770 | 101 | AHI 5–40 or AHI 5–20 along with ESS > 9 | Moderate, I 23.8 (11.1), C 19.3 (10.9) | Moderate, I 12 (5.8), C 12 (5.8) | 10 |
| Lim et al., 2005,110 Abstract | 23 | Primary headache symptoms and AHI ≥ 5 | NR | NR | 4 |
| Lojander et al., 199699 | 44 | Diagnosis of OSAHS and BMI < 40 kg/m2 | Moderate | NR | 52 |
| Monasterio et al., 2001100 | 142 | AHI 10–30 and absence of severe daytime sleepiness | Moderate, I 20 (6), C 21 (6) | Moderate, I12.1 (4.9), C13.2 (4.3) | 24 |
| cRedline et al., 199859 | 111 | RDI 5–30 and absence of ‘pathological sleepiness’ | Moderate, I RDI 14.6 (9.8), C 11.8 (9.6) | Moderate, I 10.4 (4.3), C 10.6 (5.6) | 8 |
| CPAP vs posture-related device | |||||
| Crossover trials | |||||
| Jokic et al.,1999108 | 14 | AHI < 15 in the lateral position and AHI in the supine sleep position at least twice that in the lateral position | Severe, (supine) 63.8 (148.9), mild (lateral) 4.9 (SE 4.1) | Moderate, 13 (SD 1.3) | 2 |
| cSkinner et al., 200460 | 14 | AHI 10–60 and daytime symptoms of obstructive sleep apnoea | Moderate, 27 (12) | Moderate, 11.9 (4.6) | 4 |
| cSkinner et al., 200461 | 10 | AHI 10–60 and mild to moderate OSAHS | Moderate, 29.4 (13.4) | Moderate, 13.2 (SD 4.9) | 4 |
| CPAP vs dental devices | |||||
| Parallel trials | |||||
| fFleetham et al., 2002101 | 101 | AHI > 10 | Severe, I 37.6 (22.8), C38.7 (22.2) | Moderate, I 12.8 (4.1), C11.1 (4.9) | 12 |
| aHoekema et al., 2006102 | 103 | Adults with a diagnosis of OSAHS | NR | NR | 8 |
| aLam et al., 200770 | 101 | AHI 5–40 or AHI 5–20 along with ESS > 9 | Moderate, I 23.8 (11.1), C 20.9 (9.9) | Moderate, I 12 (5.8), C 12 (5.8) | 10 |
| Crossover trials | |||||
| Barnes et al., 200482 | 114 | AHI 5–30 | Moderate, 21.3 (13.6) | Moderate, 10.7 (6.5) | 12 |
| aCibele et al., 200672 Abstract | 13 | AHI ≥ 20 | Severe, 45.5 (SD 28) | Moderate, 10.6 (SD 4) | 4 |
| Ferguson et al., 199681 | 27 | AHI 15–50 | Moderate, 24.5 (8.8) | NR | 16 |
| Engleman et al., 2002103 | 51 | AHI ≥ 5 and two or more symptoms of OSAHS, including sleepiness (ESS ≥ 8 or sleepiness while driving) | Severe, 31 (26) | Moderate, 14 (4) | 8 |
| Ferguson et al., 199780 | 24 | AHI 15–55 | Moderate, 26.8 (11.9) | Moderate, I 10.3 (3.1), C 11.0 (3.8) | 16 |
| L’ Estrange et al., 1999,104 Abstract | 15 | AHI > 50 | Severe, 63.7 (10) | Moderate, 17.2 (3.8) | 8 |
| fOlson et al., 2002107 | 24 | AHI > 15 and AI > 5 or AHI > 5 and AI > 15 | NR | NR | 6 |
| Randerath et al., 2002105 | 20 | AHI 5–30 and clinical symptoms of OSAHS | Moderate, 17.5 (7.7) | NR | 6 |
| Tan et al., 2002106 | 24 | AHI < 50 | Moderate, 22.2 (9.6) | Moderate, 13.4 (4.6) | 8 |
Intervention and comparators
Forty-six of the 48 included studies that used fixed pressure CPAP. The remaining two studies used autotitrating pressure CPAP. 67,77 Three studies used humidified CPAP73,78,79 and in two studies the use of a humidifier was optional. 80,81 All CPAP interventions were treated as a single class in the analysis.
There were three three-arm trials: CPAP versus oral placebo and dental device;82 CPAP versus conservative/usual care and dental device;70 and CPAP versus sham CPAP and supplemental oxygen. 73
CPAP was compared with sham CPAP (18 studies);56,58,62–68,73,77,79,83–88 oral placebo (nine studies);78,82,89–95 conservative/usual care (eight studies);59,69,70,96–100 dental devices (12 studies);70,72,80–82,101–107 and posture-related devices (three studies). 60,61,108
Where sham CPAP was used as placebo, the subtherapeutic pressure ranged from 0 to 4 cmH2O. Where reported, the majority of studies (n = 12) used a pressure of 2 cmH2O or less; two used a pressure of between 3 and 4 cmH2O. 64,84 In the studies using oral placebo an inactive tablet was used and participants were told that the tablet was intended to improve their airway function. The information provided on usual care/conservative treatment as a comparator was limited, but generally included dietary advice, dietary advice or referral to weight loss programmes, or advice on sleep hygiene and sleep posture.
Where reported, there were two main types of dental devices used in the included studies, one-piece non-adjustable devices;70,81,103 and two-piece adjustable devices. 72,80,82,101,102,105 In four of these studies incremental mandibular advancement was used until symptoms abated or further advancement was uncomfortable. 72,80,82,102 In one study some participants used a one-piece and some used a two-piece device. 106
Studies which compared CPAP with some form of device to control sleeping position used a backpack with a soft ball inside to prevent a supine position while sleeping;108 a shoulder–head elevation pillow to maintain an upright position (60°) while sleeping;60 or a cervicomandibular collar to retain the head in a natural position and to prevent the jaw from opening during sleep. 61
Participants
The participants in the included studies were predominantly middle-aged, male and overweight or obese. The mean age in the CPAP and comparison groups at baseline ranged from 44 to 58 years. With the exception of one study,98 the majority of participants in the included studies were male; the proportion of female participants ranged from 0% to 48%. Based on the mean BMI (where reported) ten studies were of an overweight population (BMI 25–30 kg/m2) and 30 were of an obese population (BMI 30.1–40 kg/m2); the highest mean BMI at baseline was 40.1 kg/m2. 88 Two studies were of patients who were being treated for another primary disease: type 2 diabetes67 and headache symptoms. 98 Two studies specifically recruited patients with hypertension. 65,68
Table 1 provides details of baseline disease severity for the individual studies. Based on mean baseline daytime sleepiness, as reported by participants using the ESS, the majority of studies were of participants experiencing moderate sleepiness (n = 27); five of the included studies were of participants with severe daytime sleepiness and two were of participants with mild sleepiness. Symptom severity, as defined by the ESS, was not available for 14 studies. Based on disease severity at baseline, defined by AHI [or 4% oxygen desaturation or the respiratory disturbance index (RDI)], the majority of studies (n = 26) investigated a population with severe OSAHS, 15 investigated a population with moderate disease, and three investigated a population with mild disease. One study recruited patients with OSAHS that was mild in the lateral sleep position and severe in the supine position. 108 Disease severity, as defined by AHI or equivalent, was not available for three studies.
Study design
All the included studies were RCTs. There were 26 crossover trials, two partial crossover trials (only one group was crossed over in the second sequence) and 20 parallel trials. Only the data from the first sequence before crossover were used from the partial crossover trials. 85,86 For one crossover trial the outcome data appeared to be from the first sequence and these data were treated as parallel data. 63 For some individual outcomes, only the data from the first sequence of the crossover trials were reported in the papers due to detection of a carryover effect, and these were treated as parallel data in the synthesis. Studies using oral placebo as a comparator were exclusively of crossover design, as were the trials in which the comparator was postural therapy. This was also the dominant study design for trials comparing dental devices with CPAP. Parallel trials were the dominant design used in trials comparing CPAP with sham CPAP or conservative/usual treatment.
Treatment duration varied. The majority of studies were between 4 and 12 weeks’ duration. There were six studies of less than 4 weeks’ duration58,73,79,85,91,108 and four of longer than 12 weeks’ duration. 80,81,99,100 Participants were assessed at the end of treatment.
Study quality
The following checklist was used to assess the methodological quality of included studies.
Criteria
-
Was the method used to assign participants to treatment groups or the sequence of treatments really random (e.g. computer generated or random number table)?
-
Was treatment allocation concealed?
-
Were the groups similar at baseline in terms of ESS and AHI?
-
If not, were adjustments made for differences in the treatment groups?
-
Did the analysis include an intention-to-treat (ITT) analysis?
-
Were appropriate methods used to account for missing data in the ITT analysis?
-
What proportion of participants was lost to follow-up for the primary outcomes?
-
Was the study described as blind or double-blind?
-
Who was blinded?
-
Were the participants CPAP naïve?
-
Was an appropriate analysis, using paired data, conducted? (Crossover trials only.)
-
Was there a treatment by period interaction? (Crossover trials only.)
Full details of the quality assessment are presented in Appendix 3. Eighteen of 48 studies reported an adequate method of random sequence generation. The majority of studies did not report, or reported suboptimal methods of allocation concealment, with five studies reporting adequate allocation concealment, defined according to Cochrane criteria. 62,77,87,95,109 As a consequence of the comparators used, only the 18 studies using sham CPAP were double-blinded; other comparators are visibly different and cannot therefore be double-blinded. Fourteen studies reported that participants were CPAP naïve; of these, 11 studies used sham CPAP as a comparator. It was unclear in the remaining studies using sham CPAP whether participants were CPAP naïve. ITT analysis was defined as all randomised patients included in the analysis within the treatment group to which they were randomised. Although a number of studies described themselves as being ITT, only four studies61,82,87,106 used ITT analysis according to this criterion. The majority of studies reported loss to follow-up; with the exception of a few studies82,85,89,97,99,104,109 this was low (< 20%), with little difference between treatment arms. Of the 26 crossover studies included in the review, 19 reported an appropriate analysis using paired data. Fifteen studies evaluated the possibility of carryover effects, with four studies89,90,92,93 reporting carryover effects in primary or secondary outcomes.
Assessment of effectiveness
The primary outcomes of interest for clinical effectiveness were subjective daytime sleepiness as assessed by the Epworth Sleepiness Scale (ESS) and objective sleepiness as assessed by the Multiple Sleep Latency Test (MSLT) and Maintenance of Wakefulness Test (MWT) or Osler test.
Epworth Sleepiness Scale
CPAP versus placebo or conservative/usual care
Data were available for the ESS from 23 trials (1334 participants). When all the studies were pooled there was a statistically significant benefit with CPAP compared with placebo/usual care for daytime sleepiness as measured by the ESS (MD –2.7, 95% CI –3.5 to –2.0). However, heterogeneity was high (I2 = 71%) and this treatment effect is unlikely to be generalisable. The heterogeneity was investigated using subgroup analysis.
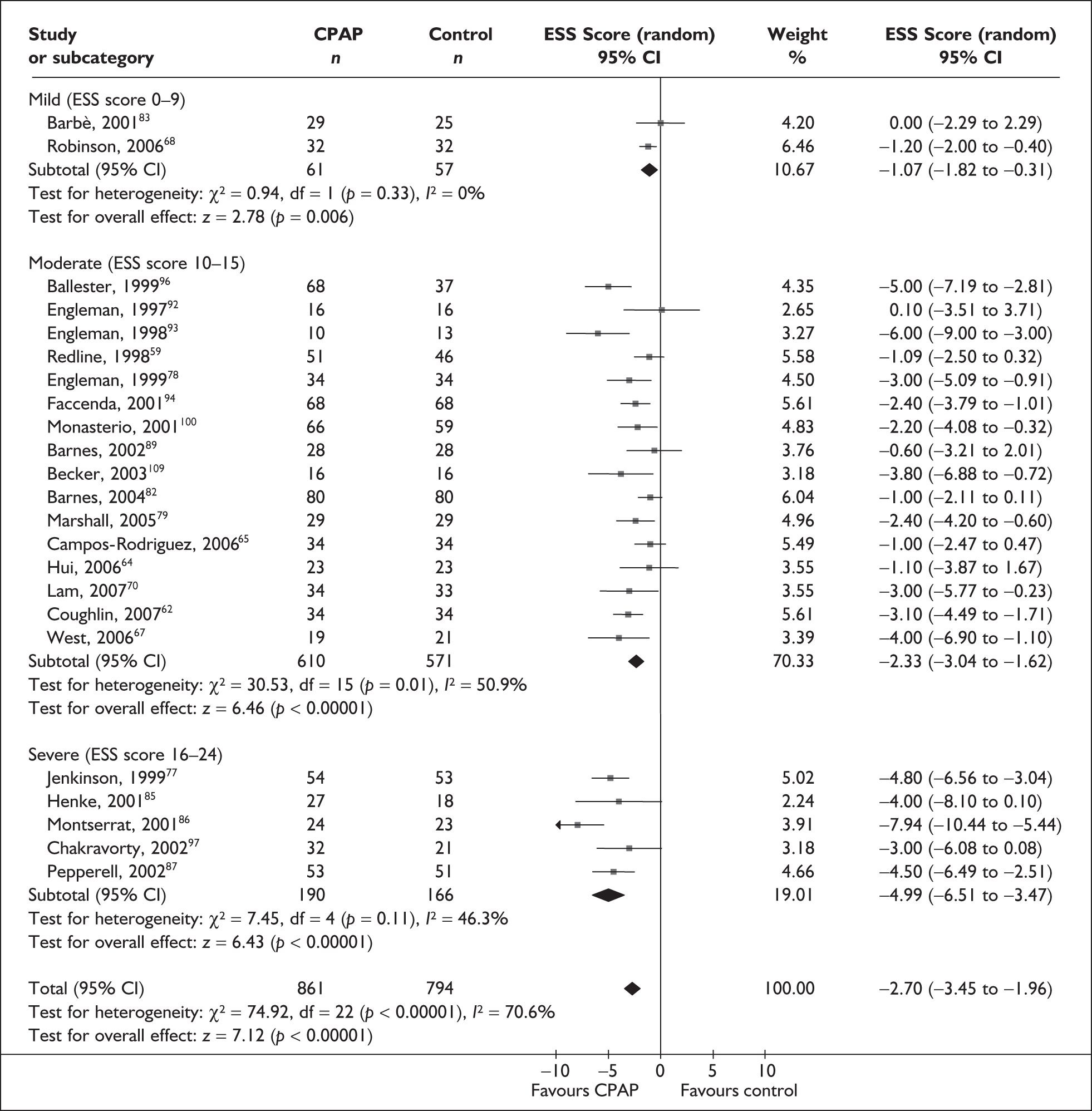
When studies were grouped by severity of daytime sleepiness at baseline (mild, moderate or severe, as defined by the ESS), heterogeneity was reduced. Although there was still evidence of moderate heterogeneity within the subgroups, with the exception of two studies, the direction of the effect was consistently in favour of CPAP (Figure 2). There was a statistically significant improvement in symptoms of daytime sleepiness with CPAP treatment compared with placebo or usual care for all levels of disease severity. The improvement was greatest in trials in which baseline sleepiness was severe (MD –5.0, 95% CI –6.5 to –3.5) and was consecutively smaller with moderate (MD –2.3, 95% CI –3.0 to –1.6) and mild severity (MD –1.1, 95% CI –1.8 to –0.3). The estimate of treatment effect for studies of mild sleepiness at baseline is based on only two studies, one which reported no difference between CPAP and placebo and one which reported a small but statistically significant improvement in favour of CPAP.
FIGURE 2.
Epworth Sleepiness Scale (CPAP versus placebo/usual care), stratified by severity of sleepiness at baseline (ESS).
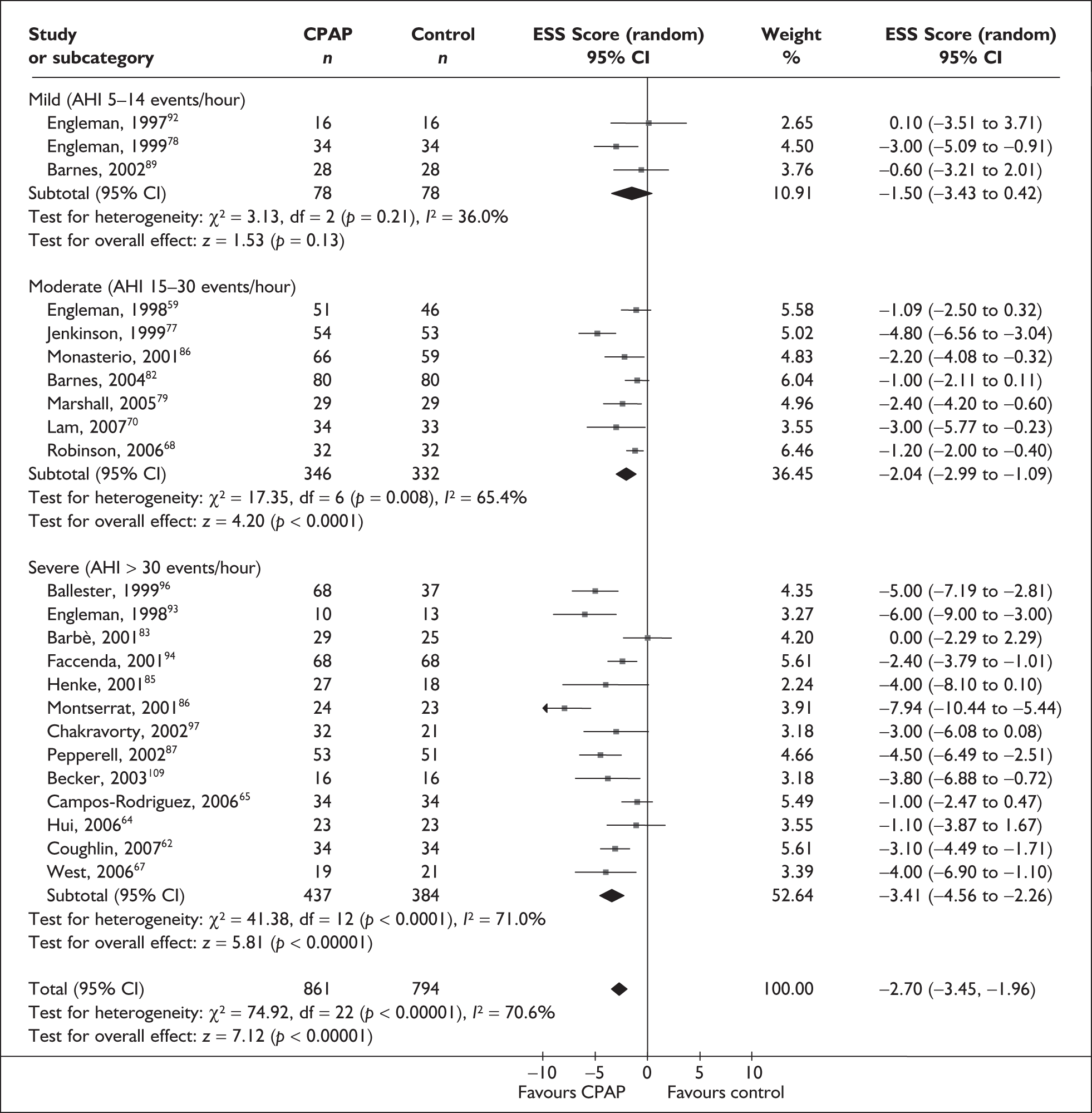
When studies were grouped by disease severity (AHI) at baseline there was a statistically significant improvement in daytime sleepiness with CPAP compared with placebo or usual care in trials of severe and moderate disease populations but not mild disease (Figure 3). As with the subgroup analysis based on ESS, the treatment effect was largest in the severe disease population and the treatment effect was consecutively smaller with moderate and mild disease. There was moderate to high statistical heterogeneity in the subgroup analyses of trials of severe (I2 = 71%) and moderate (I2 = 65%) disease. Only three trials were available for the analysis of mild disease and there was low statistical heterogeneity.
FIGURE 3.
Epworth Sleepiness Scale (CPAP versus placebo/usual care), stratified by disease severity at baseline (AHI or oxygen desaturation dip rate).
The variation in treatment effect with study design (parallel and crossover trials), type of data (end point and change scores) and comparator (sham CPAP, oral placebo and conservative/usual care) was also investigated. Each subgroup analysis was conducted for the whole data set. There was a statistically significant improvement in symptoms of daytime sleepiness (ESS) with CPAP over the comparator in each of the subgroups investigated and the treatment effects in the subgroups were consistent with each other, i.e. the 95% confidence intervals overlapped (see Appendix 4, Table 41).
Four of the five studies that reported an adequate method of concealment of allocation reported ESS as an outcome. 62,77,87,109 When these four studies were pooled, the treatment effect was consistent with the treatment effect from the overall analysis (MD –3.5, 95% CI –4.5 to –2.5). There was no statistical heterogeneity (I2 = 0%).
Further subgroup analyses were conducted on the subset of studies using sham CPAP as a comparator on a post hoc basis. Blinding of participants is particularly useful in reducing bias when subjective outcome measures such as ESS are being used. Participant blinding was possible only in the studies in which a sham CPAP was used as the comparator. Effectively, sham CPAP provides the best placebo. Therefore, further subgroup analysis was conducted on the subset of studies using sham or placebo CPAP. Studies comparing CPAP with sham CPAP were grouped by mean symptom severity at baseline (ESS) and disease severity at baseline (AHI). There was a high degree of statistical heterogeneity (I2 > 75%) in the analyses based on the mean AHI at baseline and the treatment effect is unlikely to be generalisable. When the 12 studies of CPAP versus sham CPAP were grouped based on baseline ESS the findings were similar to the subgroup analysis of symptom severity conducted on the complete data set (CPAP versus oral placebo, sham placebo and usual care) (see Appendix 4, Figure 21). The benefit of CPAP was largest in the trials in which mean baseline sleepiness was severe (MD –5.4, 95% CI –7.0 to –3.7, I2 = 46%) and was consecutively smaller in trials of moderate (MD –2.4, 95% CI –3.4 to –1.4, I2 = 31%) and mild (MD –1.1, 95% CI –1.8 to –0.3, I2 = 0%) daytime sleepiness at baseline. Statistical heterogeneity within subgroups was low to moderate.
The effect of removing individual trials from the meta-analyses in which studies were subgrouped by mean baseline severity of sleepiness was investigated. The removal of individual studies resulted in only minor variations in the size of treatment effect in the severe and moderate subjective sleepiness at baseline subgroups and the difference between CPAP and control remained statistically significant (see Appendix 4, Table 42).
Using a fixed-effect model rather than a random-effects model did not result in any substantive changes to the results (see Appendix 4, Table 41).
CPAP versus dental devices
Data were available for the ESS from six trials (n = 337). All of these trials comprised populations with moderate daytime sleepiness (ESS) at baseline. There was no statistically significant difference in the impact on daytime sleepiness (ESS) between CPAP and dental devices (MD –0.9, 95% CI –2.1 to 0.4) (Figure 4). There was evidence of moderate statistical heterogeneity (I2 = 60%) and the treatment effect ranged from MD –4.0 in favour of CPAP to a small treatment effect in favour of dental devices (MD 0.4).
FIGURE 4.
Epworth Sleepiness Scale (CPAP versus dental devices), stratified by severity of sleepiness at baseline (ESS).
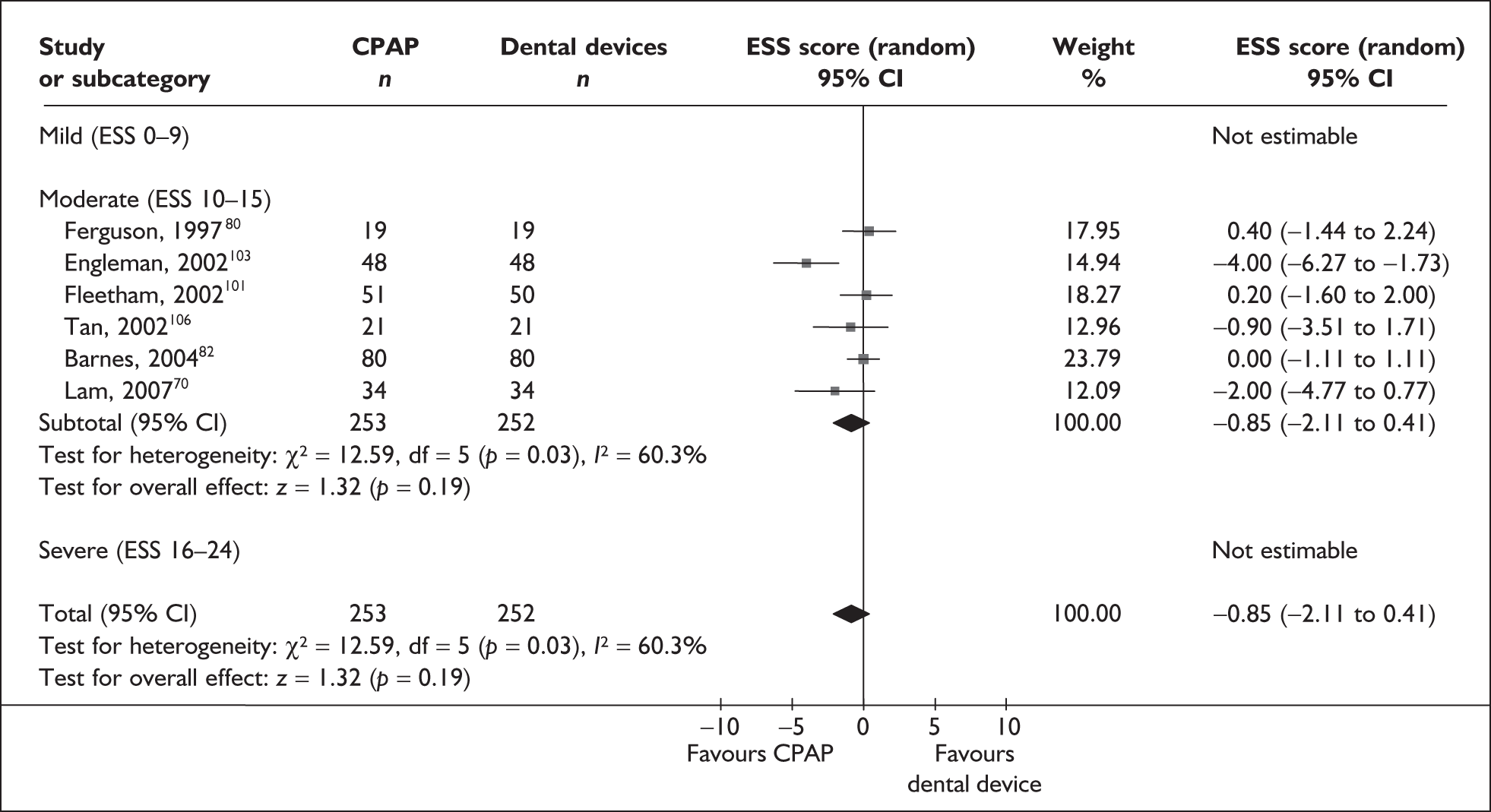
When studies were subgrouped on the basis of baseline disease severity (AHI), the findings were not substantially altered, although this analysis was limited by the small number of studies in the severe disease category and no studies in the mild group. There was no statistically significant difference between CPAP and dental devices in either the severe or moderate disease subgroup (see Appendix 4, Table 43). The treatment effects in the severe and moderate disease severity subgroups were consistent with each other, i.e. the 95% confidence intervals overlapped. The two trials of patients with severe disease were contradictory: one reported a statistically significant mean improvement of 4 points on the ESS (95% CI –6.3 to –1.7) with CPAP compared with dental devices and the other trial reported no statistically significant difference (MD 0.4, 95% CI –1.6 to 2.0).
The findings were similar within the subgroups of crossover and parallel trials, although these analyses were limited by the small number of trials. There was no statistically significant difference between CPAP and dental devices in either the crossover or parallel subgroup (see Appendix 4, Table 43).
The effect of removing individual trials from the meta-analysis of the whole data set was investigated. The removal of individual studies did not substantially alter the findings; the pooled effect size ranged from –0.1 to –1.2 and the effect remained not statistically significant (see Appendix 4, Table 44). The removal of one study that used two different dental devices dramatically reduced the statistical heterogeneity. 103 The use of a fixed-effect model did not substantially alter the findings (see Appendix 4, Table 43).
We conducted a post hoc sensitivity analysis to investigate the impact on the treatment effect of removing studies from the analysis that used a one-piece dental device,70,103,106 which is generally viewed as inferior to a two-piece adjustable device. The findings were not substantially altered, although the effect size moved in favour of dental devices (MD 0.13, 95% CI –0.71 to 0.97). When the two studies that specifically reported using incremental mandibular advancement with a two-piece device80,82 were pooled, the findings were not substantially altered (MD 0.11, 95% CI –0.84 to 1.06), although the effect size moved in favour of dental devices.
CPAP versus postural therapy
Data were available for the ESS from three small crossover trials (n = 36);60,61,108 the studies were not pooled for an overall treatment effect because of differences in the comparators used. Symptom severity was moderate in all three trial populations. No statistically significant differences were found between CPAP and postural therapy (consisting of a backpack with a soft ball inside) on the ESS in patients with positional OSAHS (i.e. AHI while sleeping on the back was two or more times the AHI during sleep in the lateral position) (MD –1.5 (95% CI –2.9 to 0.8). Similarly, there was no statistically significant difference between CPAP and a shoulder–head elevation pillow (p = 0.69 for difference in change)60 or a cervicomandibular support collar61 (p = 0.22 for difference in change) on the Scottish National Sleep Survey Questionnaire. Only overall baseline ESS scores were reported for the last two studies so change scores and the corresponding mean difference could not be calculated.
Maintenance of Wakefulness Test
CPAP versus placebo or conservative/usual care
Outcome data were available from five studies (n = 287) on the MWT. One of these studies used the Osler test. 67 There was a benefit with CPAP compared with placebo/usual care in the length of time participants could stay awake in a setting conducive to sleep (MD 3.3 minutes, 95% CI 1.3–5.3) and this was statistically significant (Figure 5). Statistical heterogeneity was low (I2 = 11%) and the treatment effect was consistently in favour of CPAP being beneficial.
FIGURE 5.
Maintenance of Wakefulness Test (CPAP versus placebo), stratified by severity of sleepiness at baseline (ESS).
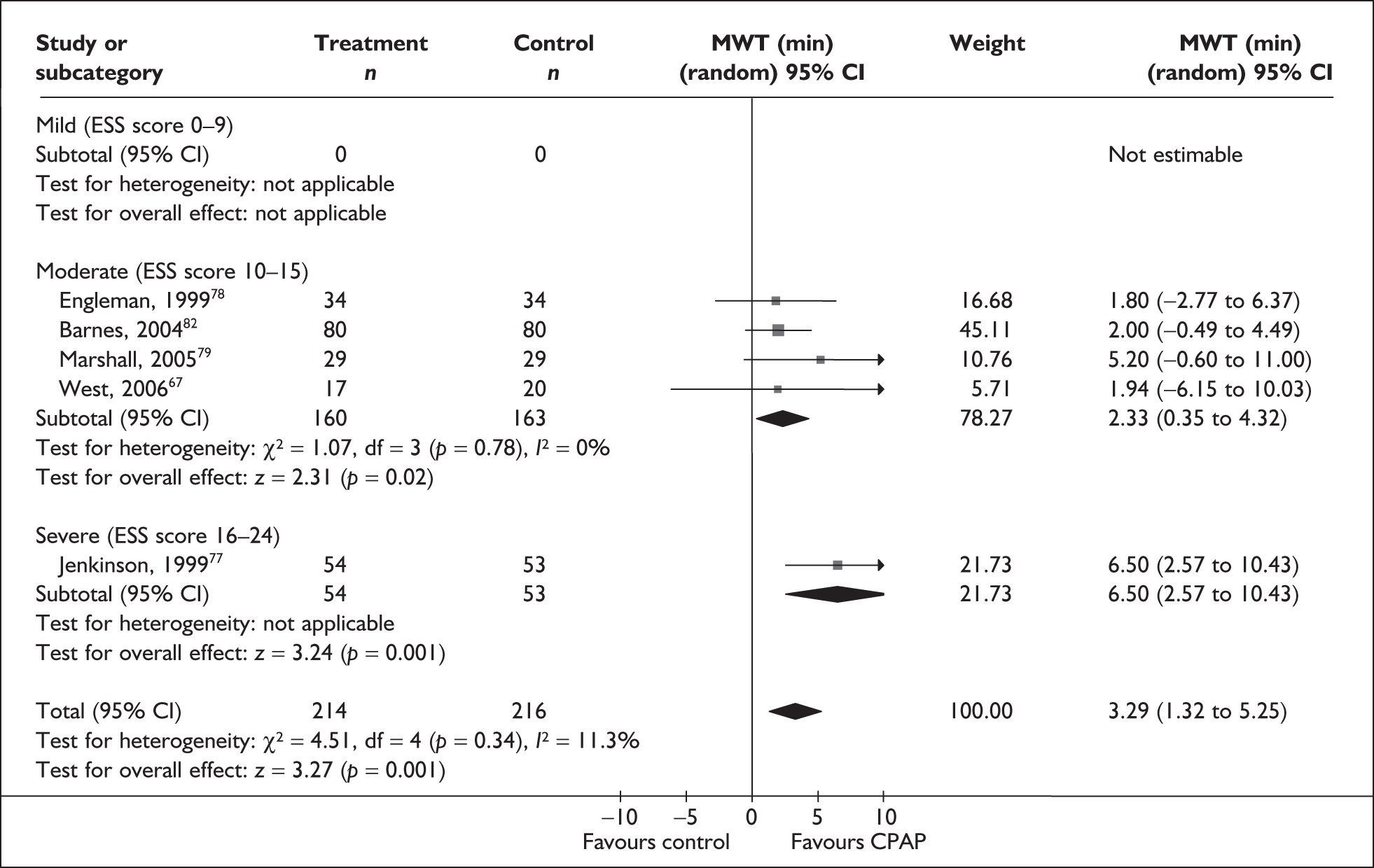
The subgroup analysis by severity of daytime sleepiness at baseline (ESS) was limited by only one study being available in the severe symptom severity group and none being available in the mild group. When studies were subgrouped there was a statistically significant improvement with CPAP compared with control in the single severe study (MD 6.5 minutes, 95% CI 2.6–10.4) and the moderate subgroup (MD 2.3 minutes, 95% CI 0.4–4.3) (see Figure 5). The benefit was greatest in the study in which symptoms were severe at baseline. The subgroup analysis by baseline disease severity (AHI) was limited by having only a single study in the mild and severe disease groups. The difference between CPAP and control was not statistically significant for the single studies of mild and severe daytime sleepiness at baseline. The treatment benefit was greatest with moderate disease and the difference between CPAP and control was statistically significant, although this analysis was limited by the small number of studies available (see Appendix 4, Table 45).
The variation in treatment effect with study design (parallel and crossover trials) was also investigated. In both subgroups there was a statistically significant benefit with CPAP compared with control. The treatment effect from the pooled crossover trials was smaller than that from parallel trials, although the 95% confidence intervals overlapped (see Appendix 4, Table 45).
The effect of removing individual trials from the meta-analysis was investigated. The statistically significant benefit of CPAP over placebo/usual care remained when individual studies were removed from the pooled analysis although the effect size ranged from 2.3 to 4.4 (see Appendix 4, Table 46). Using a fixed-effect rather than a random-effects model did not lead to any substantive changes to the results (see Appendix 4, Table 45).
CPAP versus dental devices
Data were available from two crossover trials (n = 128) on the MWT (Figure 6). In both studies baseline severity of daytime sleepiness (ESS) was classified as moderate. Neither study showed a statistically significant difference between CPAP and dental devices in the length of time participants could stay awake in a setting conducive to sleep (MD 0.7 minutes, 95% CI –1.6 to 2.9). The studies reported consistent findings.
FIGURE 6.
Maintenance of Wakefulness Test (CPAP versus dental devices).
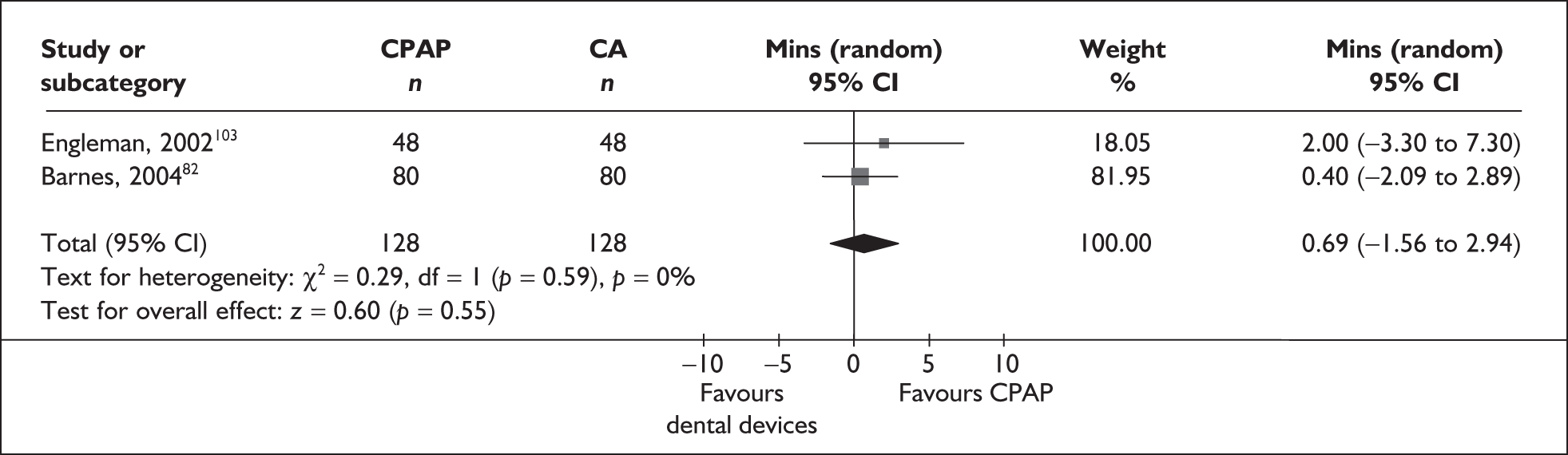
CPAP versus postural therapy
Data were available for the MWT from one small crossover trial108 (n = 13). There was no statistically significant difference between CPAP and postural therapy in the length of time participants could stay awake (MD 1.7 minutes, 95% CI –1.9 to 5.3, p = 0.32).
Multiple Sleep Latency Test
CPAP versus placebo or conservative/usual care
Outcome data were available from seven trials on the MSLT (n = 331). There was no statistically significant difference between CPAP and placebo/usual care in the length of time it took participants to fall asleep in surroundings conducive to sleep (MD 0.6 minutes, CI –0.7 to 1.9). There was evidence of moderate heterogeneity (I2 = 46%) (Figure 7).
FIGURE 7.
Multiple Sleep Latency Test (CPAP versus placebo), stratified by severity of sleepiness at baseline (ESS).
The subgroup analysis by severity of daytime sleepiness at baseline (ESS) was limited by only one study being available in the severe and mild symptom severity groups and because one study could not be classified (see Figure 7). There was no statistically significant difference between CPAP and control in the one trial of severe disease severity (MD –6.1, 95% CI –27.3 to 15.1) and the direction of the treatment effect favoured the control group. There was no statistically significant difference between CPAP and control in the moderate subgroup (MD 0.2, 95% CI –1.8 to 2.2) or in the single trial of mild symptom severity (MD 2.0, 95% CI –0.8 to 4.8). When studies were subgrouped by baseline disease severity (AHI) there was a statistically significant benefit with CPAP compared with placebo/usual care for studies of a severe disease population (MD 2.3, 95% CI 0.9–3.7, I2 = 0%) but not for those with mild- or moderate-severity disease (see Appendix 4, Table 47).
The pooled treatment effects estimated by crossover and parallel trials separately were similar; there was no statistically significant difference between CPAP and control in either subgroup (see Appendix 4, Table 47).
The effect of removing individual trials from the meta-analysis was investigated. When the Monasterio et al. trial100 was removed from the pooling, there was a statistically significant benefit in favour of CPAP (MD 1.2, 95% CI 0.0–2.4). Removing the other individual studies from the pooling did not change the overall result and the finding of no statistically significant difference between CPAP and control remained; the effect size ranged from 0 to 0.9 minutes when the individual studies were removed (see Appendix 4, Table 48). Using a fixed-effect rather than a random-effects model did not lead to any substantive changes to the results (see Appendix 4, Table 47).
CPAP versus dental devices
No data were available for the MSLT.
CPAP versus postural therapy
No data were available for the MSLT.
Summary of sleepiness outcomes
CPAP compared with control
The primary outcome of interest in the review was subjective sleepiness. Data were available on the ESS from 23 trials. Overall, CPAP reduced daytime sleepiness by a small amount compared with control; the effect probably varies among different groups of people. The average reduction on the ESS was 2.7 points, but might be anywhere between 2.0 and 3.5 points. There was considerable variation or inconsistency in the treatment effect (statistical heterogeneity); therefore some caution is needed in applying this result to all populations. Variation was reduced when studies were grouped based on baseline symptom severity and there was a trend towards a greater treatment effect with greater baseline symptom severity. It is not surprising that there would be less of a difference between CPAP and control in a population that reports only mild sleepiness at baseline.
In a severely symptomatic population the average reduction on the ESS was 5 points, but might be anywhere between 3.5 and 6.6 points; in a moderate symptom severity population the average reduction was 2.3 points, but might be anywhere between 1.6 and 3.0 points; and in mild severity the average reduction was 1.1 points, but might range anywhere between 0.3 and 1.8 points. When studies were subgrouped by disease severity at baseline, as measured by the AHI, there was a broadly similar trend. Although the definitions of disease and symptom severity used were based on current guidelines, these are arbitrary definitions and interpretation of the results for these subgroups needs to be carried out with that in mind.
The benefit with CPAP compared with control was robust across all the methodological subgroup analyses (trial design, type of data, comparator, quality) and sensitivity analyses investigating the influence of individual trials and use of a fixed-effect model.
Objective sleepiness was assessed using the MWT and MSLT. Data from the MWT were available from five trials. The length of time participants could stay awake in surroundings conducive to sleep as measured by the MWT was greater with CPAP compared with control. The average reduction in sleep latency with CPAP than with control was 3.3 minutes, but might be anywhere between 1.3 and 5.3 minutes. There was a trend towards a greater treatment effect with greater symptom severity, although this analysis was limited by the availability of only one study of a severe symptom severity population and none of a mild population. The benefit with CPAP compared with control was robust across the methodological subgroup analysis (trial design) and sensitivity analyses investigating the influence of individual trials and use of a fixed-effect model. The investigation of methodological factors was limited by the small number of trials available. There was no statistically significant difference between CPAP and control in how quickly participants could fall asleep in surroundings conducive to sleep when seven trials were pooled (MSLT).
CPAP compared with dental devices
Data were available from six trials. In a population with moderate daytime sleepiness at baseline, there was no statistically significant difference between CPAP and dental devices in the impact on daytime sleepiness. The treatment effect probably varies among different groups of people. The average effect was a reduction in sleepiness of less than one ESS point (0.9) with CPAP compared with dental devices, but might be anywhere between an increase in sleepiness of about half a point (0.4) to a decrease in sleepiness of 2.1 points. There was moderate variation in the treatment effect (statistical heterogeneity); therefore some caution needs to be taken in generalising this to all populations. The finding of no statistically significant difference between CPAP and dental devices was robust across subgroup analysis by disease severity (AHI) and trial design and sensitivity analyses investigating the influence of individual trials and use of a fixed-effect model. The investigation of methodological factors was limited by the small number of trials available.
There was no statistically significant difference between CPAP and dental devices in length of time participants could stay awake in surroundings conducive to sleep as measured by the MWT, though this is based on only two studies. No data were available for the MSLT.
Daytime blood pressure
Daytime blood pressure was the primary blood pressure outcome of interest and these data are reported below. A brief summary of the effects of treatment on night-time and 24-hour blood pressure is given below and the full data are reported in Appendix 4.
Fifteen studies reported outcome data for daytime blood pressure (Table 2); 12 used ambulatory blood pressure monitoring (ABPM) over 24 hours56,63–65,68, 82–84,87,89,91,94 and three used conventional clinic blood pressure monitoring. 62,70,100 Data were reported in graphs only for two additional studies, from which it was not possible to obtain an accurate variance estimate. 58,73 Systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP) were reported. Where daytime blood pressure was reported, four studies reported SBP, DBP and MAP,62,64,84,91 four reported MAP only,65,68,70,87 and five reported SBP and DBP but not MAP. 56,63,83,89,100 The proportion of hypertensive patients ranged from 15% to 100% and, where reported, antihypertensive medication remained unchanged throughout the studies.
| Study details | Baseline daytime BP [mean (SD)] | % (n) patients hypertensive at baseline | How BP was measured | Ambulatory blood pressure monitoring (ABPM) | Conventional clinic BP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daytime MAP | Daytime SBP | Daytime DBP | Night MAP | Night-time SBP | Night-time DBP | 24-hour MAP | 24-hour SBP | 24-hour DBP | Morning MAP | Evening MAP | ||||
| CPAP vs sham CPAP | ||||||||||||||
| Arias et al., 200556 | SBP 127 (9), DBP 79 (5) | 24-hour ABPM using oscillometric method; every 30 minutes 8 a.m.–11 p.m. and every 60 minutes 11 p.m.–8 a.m.; patients asked to be measured no later than 11 p.m. (end point data) | ✓ | ✓ | ✓ | ✓ | ||||||||
| Arias et al., 200663 | SBP 127 (9), DBP 79 (5) | 24-hour ABPM using oscillometric method (end point data) | ✓ | ✓ | ✓ | ✓ | ||||||||
| Barbé et al., 200183 | CPAP: SBP 130 (11), DBP 82 (5);Control: SBP 127 (10), DBP 80 (10) | 24-hour ABPM; at least 60 data points taken for each participant; daytime 8 a.m. to 11 p.m. (end point data) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Becker et al., 200384 | CPAP: MAP 104 (16), SBP 140 (18), DBP 86 (16); Control: MAP 104 (12), SBP 141 (14), DBP 85 (12) | CPAP: 50% (n = 8); Control: 81% (n = 13); on medication or office BP ≥ 160 and/or 90 mmHg; BP medication unchanged | ABPM measured over 20 hours using 1-minute recordings; night-time BP calculated for hours in bed and on treatment and daytime BP for remaining 12 hours (change and end point data) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Campos-Rodriguez et al., 200665 | CPAP: MAP 101 (11); Control: MAP 99 (10) | 100% (> 140/90 mmHg in three independent measurements); BP medication unchanged | 24-hour ABPM using 30-minute recordings (change and end point data) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Coughlin et al., 200762 | 79% (n = 27 (resting BP of 140/90 mmHg) | Waking BP measured between 8 a.m. and 11 a.m. in supine position after 5-minute rest; recorded as mean of three measurements taken at 1-minute intervals using automatic oscillometric digital BP monitor (end point data) | ✓ (MAP, SBP, DBP) | |||||||||||
| Hui et al., 200664 |
MAP 98 (11), SBP 128 (9), DBP 84 (9) |
50% (n = 28) (BP > 140/90 on two occasions or using antihypertensive medication; medication unchanged | 24-hour ABPM as outpatients during normal activities; BP measured every 30 minutes for 48 hours and second 24 hours of data used; patients recorded time at which they went to bed and woke up to identify sleep and wake periods (change and end point data) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Pepperell et al., 200287 | CPAP: MAP 104 (10), Control: MAP 104 (11) | 19% (n = 22) taking medication for hypertension | 24-hour ABPM measured during normal daily activities (except driving); patients kept diary and pressed event monitor to identify sleep and wake periods (change data) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Robinson et al., 200668 | CPAP: MAP 106 (14); Control: MAP 109 (13) | 100% (BP > 140/90 on 24-hour ABPM or taking hypertensive drugs); medication unchanged | 24-hour ABPM measured during normal daily activities (except driving); patients kept diary and pressed event monitor to identify sleep and wake periods (change and end point data) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| CPAP vs oral placebo | ||||||||||||||
| Barnes et al., 200289 | SBP 132 (11), DBP 84 (8) | 25% (n = 7) (24-hour SBP > 140 or DBP > 90) | 24-hour ABPM measured every 20 minutes during daytime and every 60 minutes overnight (change data) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Barnes et al., 200482 | NR | 15% (n = 14) (SBP > 140 and/or DBP > 90) | 24-hour ABPM measured every 20 minutes during daytime and every 60 minutes overnight (end point data) | ✓ | ✓ | ✓ | ||||||||
| Engleman et al., 199691 | NR | 38% (n = 5) (SBP > 134 and DBP > 84); medication unchanged | 24-hour ABPM measured at 30-minute intervals during which patients conducted normal day-to-day activities in community (end point data) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Faccenda et al., 200194 | NR | NR | 24-hour ABPM measured every 30 minutes over 48 hours during which patients went about normal daily activities, recorded in a diary; first 24 hours of data discarded (end point data) | ✓ | ✓ | ✓ | ||||||||
| CPAP vs conservative/usual care | ||||||||||||||
| Lam et al., 200770 | CPAP: SBP 128 (13), DBP 77 (11); Control: SBP 126 (20), DBP 74 (14) | 19% (n = 19); medication unchanged | Evening (8–9 p.m.) and morning (8–9 a.m; BP recorded during admission to sleep clinic; average of three readings taken at 1-minute intervals used (end point data) |
✓ (SBP, DBP) |
✓ (SBP, DBP) |
|||||||||
| Monasterio et al., 2001100 | CPAP: SBP 126 (17), DBP 81 (12); Control: SBP 132 (17), DBP 84 (11) | NR | Office daytime arterial BP recorded (end point data) | ✓ (SBP, DBP) | ||||||||||
| CPAP vs dental devices | ||||||||||||||
| Barnes et al., 200482 | NR | See above | ✓ | ✓ | ✓ | |||||||||
| Lam et al., 200770 | CPAP: See above; Dental device: SBP 127 (15), DBP 76 (12) | See above | ✓ | ✓ | ||||||||||
CPAP versus placebo/usual care
Data were available on daytime MAP for six trials (n = 309). There was an improvement in daytime MAP with CPAP compared with placebo/usual care (MD –2.1 mmHg, 95% CI –4.3 to 0.0) and this was statistically significant (Figure 8). There was moderate statistical heterogeneity (I2 = 59%).
FIGURE 8.
Daytime mean arterial pressure using ambulatory blood pressure monitoring (CPAP versus placebo/usual care), stratified by severity of sleepiness at baseline (ESS).
There was some evidence of a variation in treatment effect with severity of sleepiness at baseline (ESS), but this analysis was limited by the small number of trials (see Figure 8); only one trial each of severe and moderate baseline sleepiness was available. The single trial of severely symptomatic patients showed the largest treatment effect in favour of CPAP (MD –4.2 mmHg, 95% CI –6.4 to –2.0) and the difference between CPAP and control was statistically significant (see Figure 8). The difference between CPAP and control was not statistically significant for the moderate subgroup (MD –3.4 mmHg, 95% CI –7.9 to 1.2); the one trial of mild disease severity also reported no statistically significant difference between CPAP and control (MD 1.1 mmHg, 95% CI –2.9 to 5.1) (see Figure 8). Therefore, the overall treatment effect appears to be dominated by the one trial of severely symptomatic patients. When studies were grouped by disease severity at baseline (AHI) the treatment effect was largest with severe disease and there was a statistically significant difference in favour of CPAP; however, only one trial was available of moderate disease and none of mild disease (see Appendix 4, Table 49).
Studies were subgrouped based on whether they were crossover or parallel and whether end point or change data were used. This analysis was limited by four of the six trials being parallel trials using change data (see Appendix 4, Table 49). For the subgroup of parallel trials using change from baseline data there was a statistically significant improvement with CPAP compared with control. For the other two subgroups consisting of single studies there was no statistically significant difference between groups. The MAP treatment effect ranged from 1.1 mmHg to –3.5 mmHg (see Appendix 4, Table 49).
When studies were individually removed from the analysis the treatment effect ranged from –1.4 mmHg to –2.7 mmHg and remained statistically significant in only one instance (see Appendix 4, Table 50). There was no substantial change in the MAP results using a fixed-effect model (see Appendix 4, Table 49).
Data were available on daytime SBP and DBP from seven trials (n = 220). There was no statistically significant difference between CPAP and control for SBP although there was a small decrease in SBP in favour of CPAP (MD –1.1 mmHg, 95% CI –3.4 to 1.2) (Figure 9). There was no evidence of statistical heterogeneity. Similarly, there was no statistically significant difference between CPAP and control for DBP although there was a small decrease in favour of CPAP (MD –1.2 mmHg, 95% CI –2.9 to 0.5); heterogeneity was low (I2 = 29%) (Figure 10).
FIGURE 9.
Daytime systolic blood pressure using ambulatory blood pressure monitoring (CPAP versus placebo/usual care).
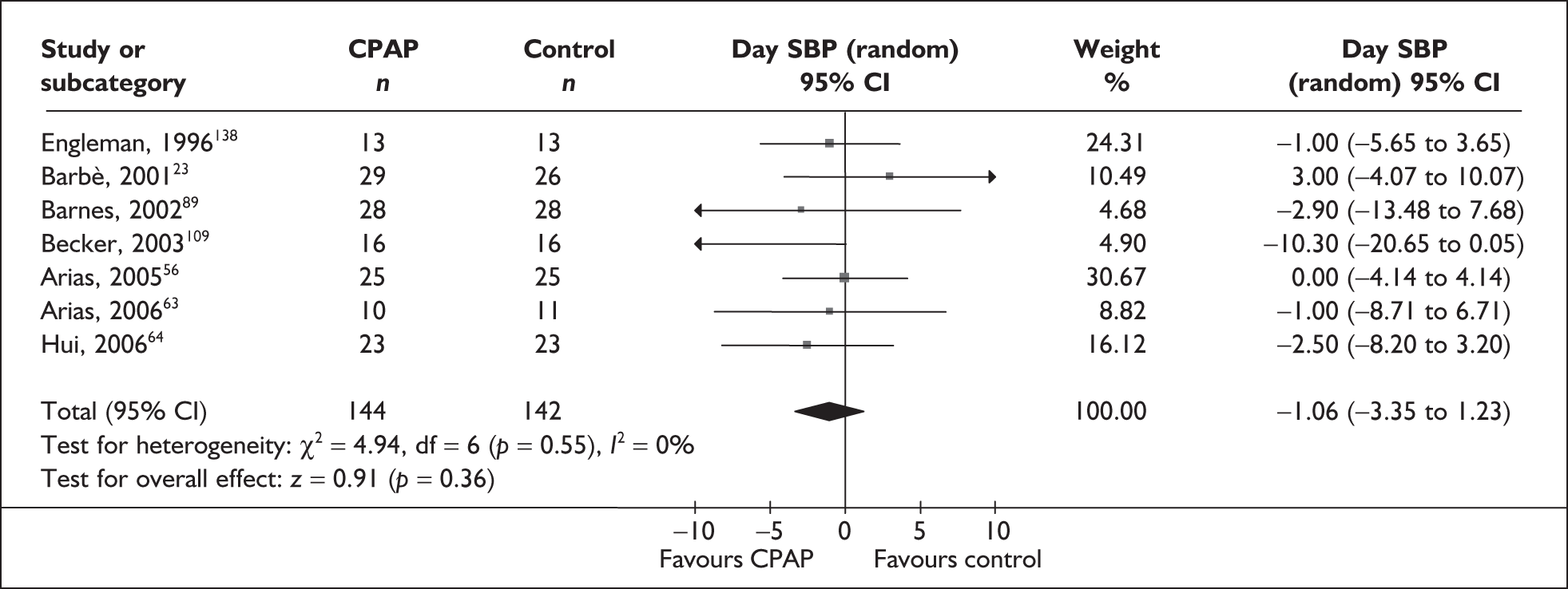
FIGURE 10.
Daytime diastolic blood pressure using ambulatory blood pressure monitoring (CPAP versus placebo/usual care).
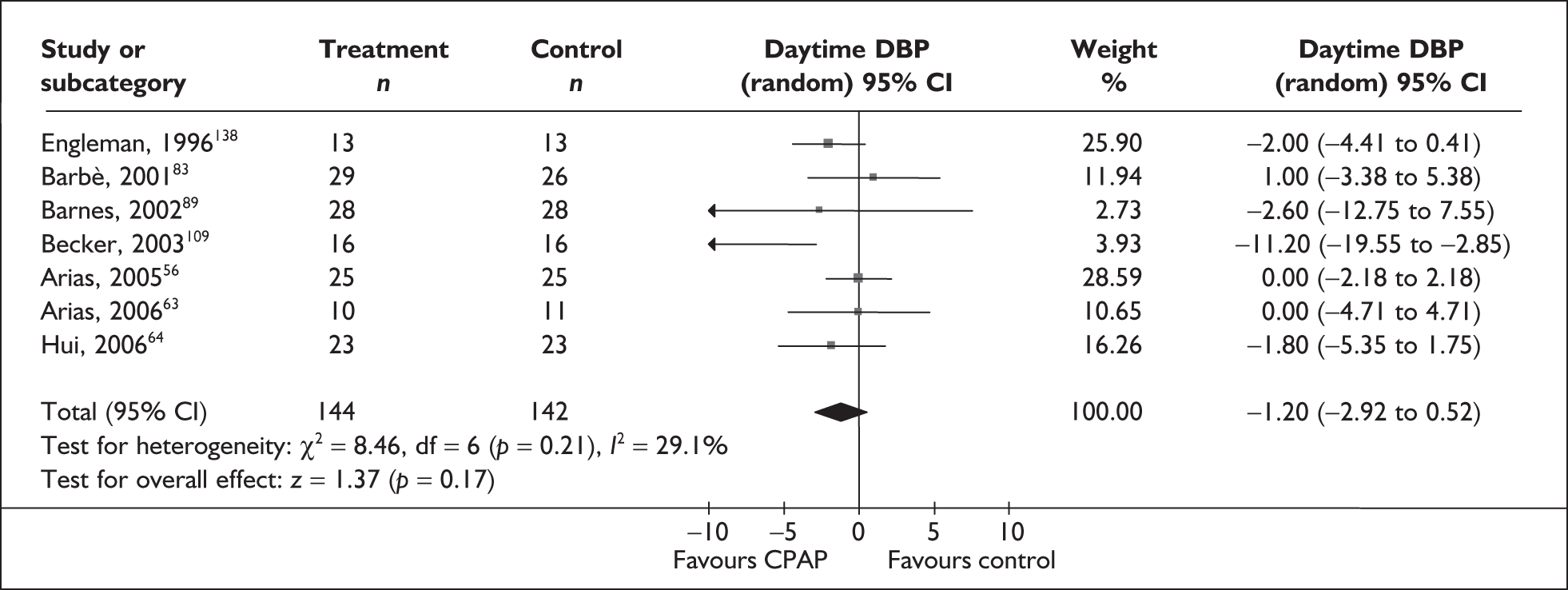
The mean baseline daytime sleepiness (ESS) was not reported for three trials56,63,91 and in the remaining trials the populations were classified as having symptoms of moderate severity at baseline. Therefore, it was not possible to explore the difference in treatment effect with different symptom severity at baseline for SBP or DBP. With the exception of one trial,89 in which the population was classified as having disease of mild severity (AHI), the study populations were all classified as having severe disease. When the single mild disease severity (AHI) study was removed from the analyses for SBP and DBP, the difference between CPAP and control remained not statistically significant (see Appendix 4, Tables 51 and 52).
The treatment effects in the crossover and parallel subgroups and the end point data and change data subgroups were consistent with each other, i.e. the 95% confidence intervals overlapped. The SBP treatment effect ranged from 1.2 for parallel trials using end point data to –5.2 for parallel trials using change from baseline data although the difference between CPAP and control was not statistically significant in any of the subgroups (see Appendix 4, Table 51). The DBP treatment effect ranged from 0.5 for parallel trials using end point data to –5.7 for parallel trials using change from baseline data although the difference between CPAP and control was not statistically significant in any of the subgroups (see Appendix 4, Table 52). These analyses are limited by the small number of studies in each of the subgroups.
The finding of no statistically significant difference between CPAP and control for SBP and DBP did not alter when a fixed-effect model was used. For DBP the treatment effect was smaller using a fixed-effect model and there was no substantial change for the SBP results using a fixed-effect model (see Appendix 4, Tables 51 and 52).
The SE for the MD in systolic and diastolic blood pressure for Arias et al. 200556 was imputed based on an estimated within-person correlation of 0.5. The meta-analyses were rerun using an SE based on an assumed within-person correlation of 0.1 and 0.9. For SBP this altered the treatment effect slightly but the finding of no statistically significant difference between CPAP and control did not change: assuming a within-patient correlation of 0.1 gave an SE of 2.75 for the study and the overall pooled treatment effect was –1.2 mmHg (95% CI –3.7 to 1.2); assuming a correlation of 0.9 gave an SE of 1.18 for the study and the overall pooled estimate was –0.6 mmHg (95% CI –2.4 to 1.1). Similarly, for DBP the treatment effect was slightly altered but the finding of no statistically significant difference between CPAP and control did not change: assuming a within-patient correlation of 0.1 gave an SE of 1.48 and the overall pooled treatment effect was –1.3 mmHg (95% CI –3.1 to 0.5); assuming a correlation of 0.9 gave an SE of 0.53 and the overall treatment effect was –1.1 mmHg (95% CI –2.7 to 0.6).
Conventional clinic blood pressure
Three studies (n = 226) used conventional or clinic daytime blood pressure: one study reported waking blood pressure recorded as the mean of three measurements taken at 1-minute intervals between 8 a.m and 11 a.m;62 one study used a similar method to record morning (8–9 a.m.) and evening (8–9 p.m.) blood pressure70 and one provided very little information. 100 The populations in all three studies were classified as having moderate daytime sleepiness at baseline. There was an improvement in daytime SBP (MD –6.62mmHg, 95% CI –9.48 to –3.76) and DBP (MD –3.47mmHg, 95% CI –6.27 to –0.68) with CPAP compared with placebo/usual care and these were both statistically significant (Figures 11 and 12). Statistical heterogeneity was low (I2 = 0% and 33% for SBP and DBP respectively).
FIGURE 11.
Daytime conventional systolic blood pressure (CPAP versus placebo/usual care).
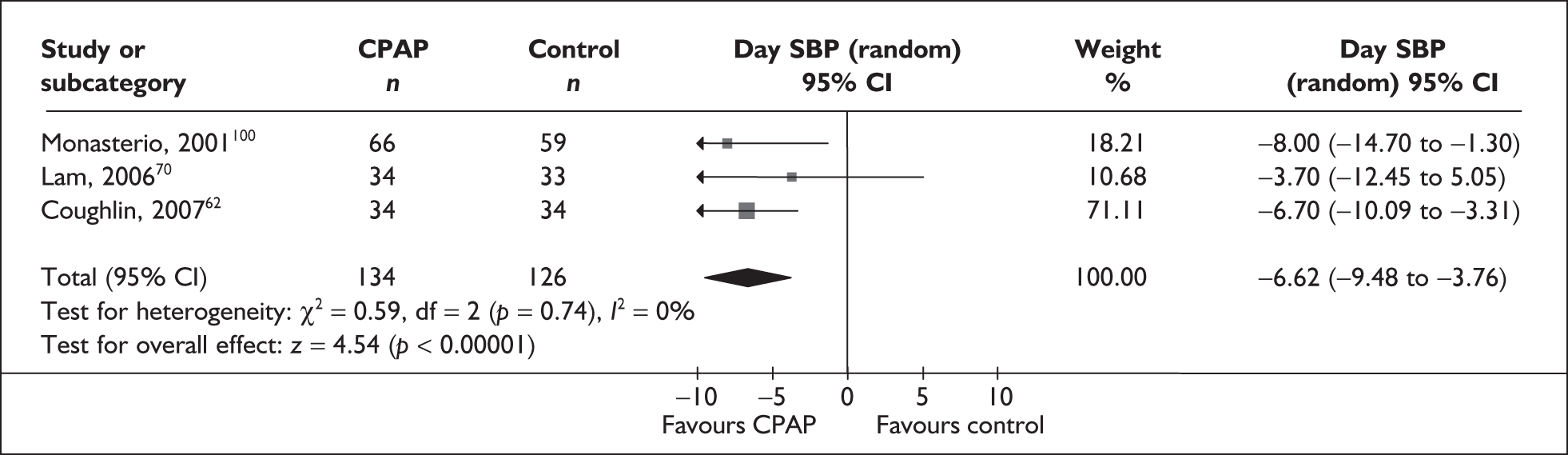
FIGURE 12.
Daytime conventional diastolic blood pressure (CPAP versus placebo/usual care).
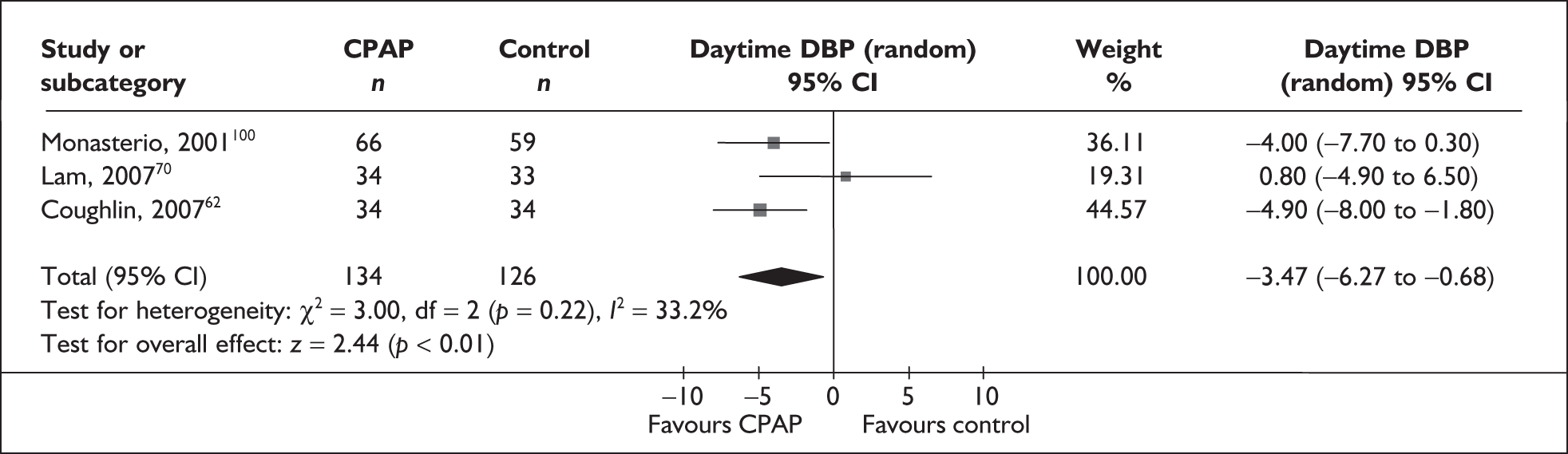
CPAP versus dental devices
No studies were found that reported daytime ABPM. One study reported morning and evening blood pressure in a population with moderate sleepiness and moderate disease severity at baseline (see Table 2). 70 This study (a parallel trial, n = 68) found no statistically significant difference between CPAP and dental devices in terms of morning SBP (MD –2.9 mmHg, 95% CI –11.0 to 5.2), evening SBP (MD –4.9 mmHg, 95% CI –14.8 to 5.0), morning DBP (MD –1.6 mmHg, 95% CI –7.4 to 4.2) and evening DBP (MD –1.9 mmHg, 95% CI –7.6 to 3.8).
Night-time and 24-hour blood pressure
CPAP versus placebo or conservative/usual care
Data were available on night-time MAP from six trials (n = 309). 64,65,68,87,91,109 Overall, there was an improvement in night-time MAP with CPAP compared with placebo/usual care (MD –3.0 mmHg, 95% CI –4.7 to –1.4) and this was statistically significant. There was no statistical heterogeneity. The pooled treatment effects estimated by crossover and parallel trials and end point and change data separately were similar (see Appendix 4, Figure 23).
Data on SBP and DBP were available from seven trials (n = 220). 56,63,64,83,89,109,138 There was no statistically significant difference between CPAP and control for night-time SBP (MD –2.9 mmHg, 95% CI –5.8 to 0.1) or DBP (MD –1.3 mmHg, 95% CI –3.2 to 0.7) (see Appendix 4, Figures 24 and 25).
Data on 24-hour blood pressure were available in one study that did not report daytime and night-time pressure separately (a crossover trial, n = 68 participants). 94 There was a statistically significant benefit with CPAP compared with oral placebo on 24-hour DBP (MD –1.5 mmHg, 95% CI –2.9 to –0.1); there was no statistically significant difference in 24-hour SBP (MD –1.3 mmHg, 95% CI –3.3 to 0.7); or 24-hour MAP (MD –1.0 mmHg, 95% CI –2.6 to 0.6).
CPAP versus dental devices
One study reported 24-hour blood pressure (crossover trial, n = 80) in a population with moderate symptom severity and moderate disease severity at baseline. 82 There was no statistically significant difference between CPAP and dental devices for 24-hour SBP (MD 0.6 mmHg, 95% CI –2.5 to 3.7) or DBP (MD 0.4 mmHg, 95% CI –1.7 to 2.5).
Summary of blood pressure outcomes
Data were available from 15 trials. Studies using 24-hour ABPM were considered separately from those using conventional clinic-based measures. Daytime and night-time blood pressure were assessed separately as the mechanisms and patterns of daytime and night-time blood pressure disturbance in OSAHS vary and the relationship between daytime blood pressure and vascular risk has been more clearly described in the literature.
CPAP versus control
Six trials reported MAP using ABPM. There was a statistically significant reduction in MAP with CPAP compared with control; the size of the effect probably varies among different groups of people. The average reduction in MAP was 2.1 mmHg, but might be anywhere between no reduction and 4.3 mmHg. There was moderate inconsistency in the treatment effect (statistical heterogeneity), but due to the small number of studies it was not possible to adequately investigate sources of this variation. Only one study was available of severely symptomatic patients and the overall treatment effect did seem to be dominated by this trial. There was no substantial change in the MAP results when a fixed-effect model was used as a sensitivity analysis. However, when individual studies were removed from the analysis the treatment effect remained statistically significant in only one instance, indicating a possible lack of statistical power due to the small number of participants.
There was no statistically significant difference between CPAP and control for SBP or DBP (measured using ABPM). The treatment effect probably varies among different groups of people. The average effect for SBP was a decrease of 1.1 mmHg with CPAP, but might be anywhere between a decrease of 3.4 mmHg and an increase of 1.2 mmHg compared with control. The average effect for DBP was a decrease of 1.2 mmHg with CPAP, but might be anywhere between a decrease of 2.9 mmHg and an increase of 0.5 mmHg compared with control. There was no inconsistency in the treatment effect (statistical heterogeneity). It was not possible to investigate whether the treatment effect varied with disease or symptom severity at baseline due to limitations in the data available. When a fixed-effect model was used, the findings were not substantially altered, except that the treatment effect for DBP was smaller. The pooling of three studies reporting conventional clinic blood pressure showed a large and statistically significant improvement in SBP and DBP with CPAP compared with control.
The results for night-time blood pressure were similar to those for daytime blood pressure. There was a statistically significant improvement in night-time MAP (using ABPM) but not SBP and DBP. The magnitude of the effects was broadly similar.
CPAP versus dental devices
Only one study was available that reported daytime blood pressure (morning and evening blood pressure using a conventional clinic method). This trial of a moderate symptom severity and moderate disease severity population found no statistically significant difference between CPAP and dental devices. Another trial that did not report daytime and night-time blood pressure separately reported no statistically significant difference in 24-hour SBP and DBP between the two interventions.
Health-related quality of life
The most commonly used quality of life measures were the FOSQ, SF-36 and NHP (Table 3). The majority of studies in which quality of life was assessed were of populations with moderate symptom severity (ESS) at baseline.
| Quality of life measure | Number of crossover trials | Number of parallel trials |
|---|---|---|
| CPAP versus placebo/usual care | ||
| EuroQol and standard gamble | 197 | |
| Functional Outcomes of Sleep Questionnaire (subscales) | 379,89,94 | 186 |
| Functional Outcomes of Sleep Questionnaire (total score) | 379,89,94 | 383,86,100 |
| Nottingham Health Profile | 478,90,92,93 | 296,100 |
| Sleep apnoea quality of life index | 267,70 | |
| SF-36 (subscales) | 378,79,89 | 370,77,86 |
| SF-36 (physical and mental component summary or total score) | 182 | 377,83,86 |
| CPAP versus dental devices | ||
| Functional Outcomes of Sleep Questionnaire | 282,103 | |
| Golombok Rust Inventory of Sexual Satisfaction | 1102a | |
| Sleep apnoea quality of life index | 370,101,107 | |
| SF-36 (subscales) | 170 | |
| SF-36 (Physical and mental component summary or total score) | 282,103 | |
CPAP versus placebo or usual care
Six studies reported the SF-36 subscales. There were three crossover trials (n = 91), all of moderate baseline symptom severity (ESS)78,79,89 and three parallel trials (n = 215), two of severe symptoms77,86 and one moderate. 70 There was no statistically significant benefit with CPAP compared with control on any of the subscales of the SF-36 although for two of the scales (vitality and physical roles) there was a trend towards improvement with CPAP (see Table 4 for the overall effect and Appendix 4, Figure 26, for the forest plots). However, the pooled estimates are likely to have limited generalisability as there was moderate to high heterogeneity in the analyses for most of the subscales and specifically for the vitality and physical role subscales. For these two subscales, the findings encompassed two studies reporting a statistically significant improvement with CPAP77,78 and the remaining studies reporting no statistically significant difference between CPAP and control (see Appendix 4, Figure 26).
| SF-36 Subscale (six trials) | Mean difference (95% CI) | Statistical heterogeneity (I2) |
|---|---|---|
| Bodily pain | 4.3 (–0.9 to 9.5) | 48% |
| Emotional role | –0.4 (–12.3 to11.5) | 72% |
| General health | 3.2 (–0.4 to 6.7) | 0% |
| Mental health | 2.2 (–2.2 to 6.7) | 52% |
| Physical function | 2.6 (–0.6 to 5.9) | 8% |
| Physical role | 6.9 (–3.8 to 17.5) | 63% |
| Social function | 1.9 (–4.4 to 8.1) | 57% |
| Vitality | 7.3 (–0.3 to 14.9) | 77% |
The treatment effects in the crossover and parallel subgroups were consistent with each other, i.e. the 95% confidence intervals overlapped (see Appendix 4, Figure 26). For bodily pain, general health and physical function there was a statistically significant benefit with CPAP compared with control for the parallel trial subgroup but not the crossover subgroup. This may be driven by two of the parallel trials being of populations with severe baseline symptoms. There was a statistically significant difference between CPAP and control for the physical component summary score but not the mental component summary scores [three trials, one of mild and two of severe symptoms (ESS)] or the total score (one trial, moderate symptom severity) (see Appendix 4, Figure 26).
Four trials reported the FOSQ subscales: three crossover trials (n = 125) of moderate symptom severity at baseline79,89,94 and one parallel trial (n = 47) of severe symptom severity. 86 There was a statistically significant benefit with CPAP compared with control for the activity level and social outcome subscales of the FOSQ (see Table 5 for the overall effect and Appendix 4, Figure 27, for the forest plots). Statistical heterogeneity was low for both of these subscales. Statistical heterogeneity was high for general productivity (I2 = 70%): there was a statistically significant benefit with CPAP compared with control for the parallel trial of severe symptom severity population but not for the subgroup of crossover, moderate disease trials. For activity level and social outcome the statistically significant benefit with CPAP did not appear to be dominated by the parallel trial of severe symptom severity (see Appendix 4, Figure 27).
| FOSQ subscale (number of trials) | Mean difference (95% CI) | Statistical heterogeneity (I2) |
|---|---|---|
| Activity level (n = 4) | 0.2 (0.0–0.3) | 34% |
| General productivity (n = 4) | 0.1 (–0.1 to 0.3) | 70% |
| Intimacy and sexual activity (n = 4) | 0.3 (–0.4 to 0.9) | 0% |
| Social outcome (n = 4) | 0.2 (0.0 to 0.4) | 0% |
| Vigilance (n = 4) | 0.2 (–0.1 to 0.5) | 76% |
| Total score (n = 6) | 51% |
Data from the NHP were reported in six studies, four of which reported NHP Part 2 (all crossover trials, n = 105), three of moderate symptom severity78,92,93 and one unclassified90 There was a statistically significant benefit with CPAP compared with placebo/usual care on the NHP Part 2 (MD –1.7, 95% CI –2.9 to –0.5) (see Appendix 4, Figure 28). There was no statistical heterogeneity. Monasterio et al. 100 did not specify which part of the NHP they used but the data presented suggest that it was probably Part 1. There was no statistically significant difference between CPAP and conservative treatment on NHP Part 1 (total score) in this parallel trial of a moderate symptom severity population (MD 0.0, 95% CI –5.8 to 5.8). Ballester et al. reported the six domains from NHP Part 1 but not the total score. 96 There was a statistically significant difference between CPAP and conservative treatment on the energy (p = 0.03) and social isolation (p < 0.005) domains but not the emotional reactions, sleep, physical mobility or pain domains.
Data were available on the SAQLI from two parallel trials, of moderate symptom severity populations. One study (n = 67) reported all the subscales70 and the overall score and one reported the overall score only (n = 41). 67 There was a statistically significant improvement with CPAP compared with conservative treatment on the daily functioning, emotional and symptoms subscales but not for the social interaction subscale (see Appendix 4, Figure 29). For the total score (A–D subscales) one study showed a significant benefit with CPAP compared with conservative treatment and one showed no significant difference between CPAP and sham CPAP (see Appendix 4, Figure 29). When change data from the latter study were used instead of end point data the difference between CPAP and sham CPAP was statistically significant and in favour of CPAP (p = 0.05). There was no baseline imbalance between groups.
One study was available of a severely symptomatic population (n = 53). 97 There was no difference between CPAP and conservative treatment in quality of life at follow-up, as measured by the EuroQol thermometer (0–100) (MD 2.0, 95% CI –8.1 to 12.1). The EuroQol-derived utility was 0.77 (SD 0.18) for CPAP versus 0.77 (SD 0.09) for conservative treatment. There was a 0.04 utility gain in the CPAP group and no change in the conservative treatment group, although the CPAP group started from a poorer baseline (EuroQol 0.73 versus 0.77 for CPAP and control respectively) and had more severe OSAHS at baseline (AHI 55 versus 35 for CPAP and control respectively).
CPAP versus dental devices
Data were available from a small number of studies comparing CPAP with dental devices. Where reported, the studies were all of moderate symptom severity populations at baseline.
Data from the FOSQ were available from two studies (crossover, n = 128) of populations with moderate symptom severity. 82,103 When both studies were pooled there was no difference between CPAP and dental devices in terms of quality of life as measured by the FOSQ (MD –0.5, 95% CI –1.4 to 0.5). These two studies had contradictory findings: one showed a statistically significant benefit with CPAP compared with dental devices and one found no difference between the two treatments (see Appendix 4, Figure 30).
One study (parallel, n = 68) reported subscale scores for the SAQLI as well as a summed score for A–D and A–E subscales. 70 Unpublished data were available from Giles et al. for two studies for a summed score,101,107 although it was unclear whether this was for subscales A–D or A–E, therefore these studies were pooled separately (see Appendix 4, Figure 31). Based on the summed score for the last two studies there was no difference between CPAP and dental devices (see Appendix 4, Figure 32). For the summed score for A–D, CPAP showed a benefit over dental devices in the third study. However, when treatment-related symptoms were included to calculate the total score for A–E for this study, CPAP no longer showed a benefit over dental devices (see Appendix 4, Figure 32).
One study reported the physical and mental component summary scores for SF-36 (crossover, n = 80), one reported the total score (crossover, n = 80) and one reported the subscale scores (parallel, n = 68). One study (see Appendix 4, Figure 33) reported a benefit with CPAP compared with dental devices on both the physical and mental component summary scores. 103 In contrast, one study reported no difference between CPAP and dental devices on the total score. 82 For one study there was a statistically significant benefit with CPAP compared with dental devices on the bodily pain subscale of SF-36 but not on any of the other subscales (see Appendix 4, Figure 34). 70
This outcome was reported in two related papers102,111 The included male participants (n = 38) who were in a heterosexual relationship and had more erectile dysfunction and sexual dissatisfaction than age-matched controls. There was no difference between CPAP and dental devices on any of the subscales at follow-up (see Appendix 4, Figure 35).
CPAP versus postural therapy
Data were available from two small crossover trials (n = 23). 60,61 The studies were not pooled for an overall treatment effect due to differences in the comparators used, i.e. a shoulder–head elevation pillow60 and a cervicomandibular support collar. 61
No statistically significant difference was found when CPAP was compared with either the shoulder–head elevation pillow (p = 0.93 for difference in change) or the cervicomandibular support collar (p = 0.85 for difference in change). Only overall baseline scores were reported so change scores and the corresponding mean difference could not be calculated.
The physical and mental component summary scores for the SF-36 were reported. There was no statistically significant difference in the impact on the summary physical component score between CPAP and the shoulder–head elevation pillow (p = 0.74 for difference in change) or the cervicomandibular support collar (p = 0.18 for difference in change). Similarly, there was no statistically significant difference in the impact on the summary mental component score between CPAP and the shoulder–head elevation pillow (p = 0.31 for difference in change) or the cervicomandibular support collar (p = 0.19 for difference in change). Only overall baseline scores were reported so change scores and the corresponding mean difference could not be calculated.
Summary of HRQoL outcomes
The majority of studies assessing quality of life were of populations with moderate symptom severity at baseline. A variety of disease-specific and generic measures were used and only those measures reported in two or more studies are summarised here. The findings for quality of life were somewhat contradictory, which may have been related to factors such as different outcome measures used, or differences in the study population or aspects of study design. Exploration of sources of heterogeneity was limited by the small number of trials.
CPAP versus control
Six studies reported the SF-36 subscales. There was no statistically significant difference between CPAP and control on any of the SF-36 subscales. There was moderate to high variation or inconsistency in the treatment effect (statistical heterogeneity) for most of the subscales, therefore some caution needs to be taken in generalising these findings to all populations receiving CPAP. Although the treatment effects from the crossover and parallel trial subgroup analysis were consistent with each other, in that their 95% confidence intervals overlapped, for bodily pain, general health and physical function there was a statistically significant benefit with CPAP compared with control for the parallel trials but not for the crossover trials. This may have been driven by two of the parallel trials being of severe symptom populations. In contrast, on the other generic scale, the NHP Part 2, there was a statistically significant benefit with CPAP compared with control based on a pooling of four studies. The treatment effect probably varies among different groups of people. The average effect was a reduction of 1.7 points with CPAP compared with control, but might fall anywhere between 0.5 and 2.9 points. There was no variation or inconsistency (statistical heterogeneity) in the treatment effect.
The findings from the disease-specific measures were also somewhat contradictory, although only a small number of studies were available. On the FOSQ (four trials), a disease-specific measure, there was a statistically significant benefit with CPAP compared with control for the activity level and social outcome subscales but not for general productivity, intimacy and sexual activity, vigilance or total score. Only two studies reported the SAQLI total score; one reported a significant benefit with CPAP compared with control and for one there was no statistically significant difference.
CPAP versus dental devices
For the majority of the quality of outcome measures only single studies were available. There was no statistically significant difference between CPAP and dental devices when two studies reporting the FOSQ and two studies reporting the SAQLI were pooled. Three studies reported the SF-36 but all used different scores and the findings were not consistent.
Psychological outcomes
There were very few new data available on psychological outcomes following the review by Giles et al. 50 One additional publication was available which reported the Profile of Mood State (POMS) from a 1-week study by the Dimsdale group58 and the Brief Symptom Inventory from a 2-week study by Norman et al. 73
CPAP versus placebo/usual care
Three studies reported on the GHQ-28 (all crossover, n = 74) and these were pooled. 90,92,93 There was no statistically significant difference between CPAP and placebo (MD –1.4, 95% CI –4.1 to1.4), although this estimate is of limited value as it was derived from only three studies, with moderate to high statistical heterogeneity (I2 = 70%) (see Appendix 4, Figure 36).
Five studies reported on the HADS and these were pooled (all crossover, n = 134). 79,90,92,93,112 There was no statistically significant difference between CPAP and placebo for the anxiety (MD –0.3, 95% CI –1.2 to 0.5) or the depression (MD –0.9, 95% CI –1.9 to 0.1) subscales. There was moderate statistical heterogeneity in both analyses (I2 = 45% and 62% respectively) (see Appendix 4, Figures 37 and 38).
One study reported the BSI global severity index and the BSI depression subscale (parallel, n = 24). 73 The standard deviation was estimated for the former but, because data were only presented as a low-scale graph, it was not possible to estimate the standard deviation for the depression subscale. There was no statistically significant difference between CPAP and sham CPAP at follow-up for the global symptom index of the BSI (See Appendix 4, Figure 39).
One study reported the POMS (parallel, n = 34). 58 There was no statistically significant difference between CPAP and placebo on any of the POMS subscales or the total score (see Appendix 4, Figure 40).
Three studies reported the energetic arousal score from the UMACL and these were pooled (all crossover, n = 73). 92,93,112 There was a statistically significant benefit in favour of CPAP compared with placebo (MD 1.7, 95% CI –0.0 to 3.3) (see Appendix 4, Figure 41). There was no statistical heterogeneity in this analysis.
CPAP versus dental devices
One study reported the HADS (crossover, n = 48). 103 There was no statistically significant difference between CPAP and dental devices for the anxiety or depression subscales (see Appendix 4, Figures 42 and 43).
Summary of psychological outcomes
CPAP versus control
Data were available for three psychological outcome measures from two or more studies. There was no statistically significant difference between CPAP and control on the GHQ-28 or the HADS. There was a statistically significant benefit in favour of CPAP compared with control on the energetic arousal score from the UMACL. There was no inconsistency (statistical heterogeneity) in the treatment effect.
CPAP versus dental devices
There was no statistically significant difference between CPAP and dental devices in one trial reporting the HADS.
Cognitive outcomes
Eighteen trials used formal testing to measure the effects of CPAP on cognitive function in adults with obstructive sleep apnoea. Ten of the studies used a crossover design,72,78,79,82,89,90,92,93,103,108 while eight used a parallel group design. 58,73,77,83,85,100,102,113 Six trials compared CPAP with sham CPAP,58,73,77,79,83,85 six trials with oral placebo,78,82,89,90,92,93 four trials with dental devices,72,82,102,103 two trials with conservative treatment100,113 and one trial with postural therapy. 108 Most of the studies included small sample sizes (range 14–125), with three studies reporting on a subgroup of the original randomised population. 77,113,114 Based on mean ESS score at baseline (where reported), the majority of trials were of reported moderate symptom severity populations, two trials were of severely symptomatic populations and one trial studied mild symptom severity. Based on mean baseline AHI, seven study populations were classified as having severe disease,58,73,83,85,93,103,108 seven as having moderate disease77,79,82,90,100,102,113 and three as having mild disease. 78,89,92
A total of 28 different cognitive tests were used, examining several areas of cognition (administered as verbal, pen-and-paper or computer-based tasks), making comparisons across trials difficult. The areas of functioning assessed were attention or vigilance, psychomotor function, construction, verbal fluency, IQ decrement, memory and learning (see Table 12). Seventeen tests were used by two trials or fewer; even when tests were used by multiple trials the scales used were not always uniform.
Testing protocols may have a confounding effect on performance; therefore assessment procedures were examined. Some variation between testing protocols existed (see Appendix 4, Table 54). Testing protocol issues include order of test presentation, which is a particular issue with test batteries containing many different types of test,115 and time of day when fluctuations in performance and levels of alertness can occur in response to circadian rhythms. In addition, performance may be improved by prior exposure to testing stimuli and procedures, which can have a significant beneficial impact on test performance when tests are administered on more than one occasion. Use of stimulants, such as nicotine and caffeine, can also modify cognitive performance, as can mood and depression. Therefore, ideally, testing protocols should employ measures to minimise risks of possible confounding, or account for potential biases in the analysis.
Nine trials administered a familiarisation session prior to baseline assessment,78,82,85,89,90,92,108,114,116–118 and four trials used alternative test forms in subsequent sessions in an attempt to minimise learning effects. 85,90,92,116 Thirteen trials reported the time of day that assessments were conducted (five in the afternoon, two in the morning, and six across the course of the day). 78,79,85,89,90,92,100,108,113,114,118 Eight trials reported administering tests in a standardised order in an attempt to control the impact of each test in relation to each other across the test session. 78,79,90,92,93,108,114,118 Four trials assessed for, or attempted to minimise, the effects of stimulants such as caffeine or nicotine, or the effects of alcohol consumption or drug intake. 83,90,108,114 No study specifically looked at the effect of mood in relation to cognitive function, although one trial82 stated that significant depression was present in 40% of the included participants. Level of baseline function, compared with normative standards, was reported in only one trial. 89 However, a number of papers79,83,100,113,117 indicated that many participants demonstrated normal values at baseline, highlighting the possibility of a ceiling effect.
All of these issues could affect the findings of the studies and should be considered when interpreting the results reported below. Owing to time limitations and the quantity of cognitive data from crossover trials it was not feasible to impute data for a paired analysis, where these were not reported, for all the cognitive outcomes. Where three or more studies were available for potential pooling the SE was estimated where appropriate data were available. A narrative synthesis was used where pooling was not feasible. Details of the individual study results are reported in Appendix 4, Table 53. Where end point data were not reported, change scores were used.
CPAP versus placebo or conservative/usual care
Seven studies used a simulated driving task. 78,83,90,92,93,100,118 Daytime sleepiness, based on ESS scores at baseline, varied between study populations; in one study daytime sleepiness was classified as severe,118 in one it was classified as mild83 and in four it was classified as moderate. 78,92,93,100
Six of the seven studies used the SteerClear simulated driving test. 78,83,90,92,93,100 SteerClear is a computerised program that attempts to mimic different components of attention involved in driving a car; the program simulates a long and monotonous highway drive that presents a number of obstacles over a period of 30 minutes.
Two studies reported performance in terms of the percentage of obstacles hit83,100 and four reported the number of obstacles hit. 78,90,92,93 These were treated separately.
There was no statistically significant difference between CPAP and oral placebo in terms of the percentage of obstacles hit (Figure 13), or in the number of obstacles hit (MD –5.74, 95% CI –14.75 to 3.27) (Figure 14). There was no statistical heterogeneity (I2 = 0%) for trials reporting the number of obstacles hit.
FIGURE 13.
SteerClear (CPAP versus placebo/usual care), percentage of obstacles hit.
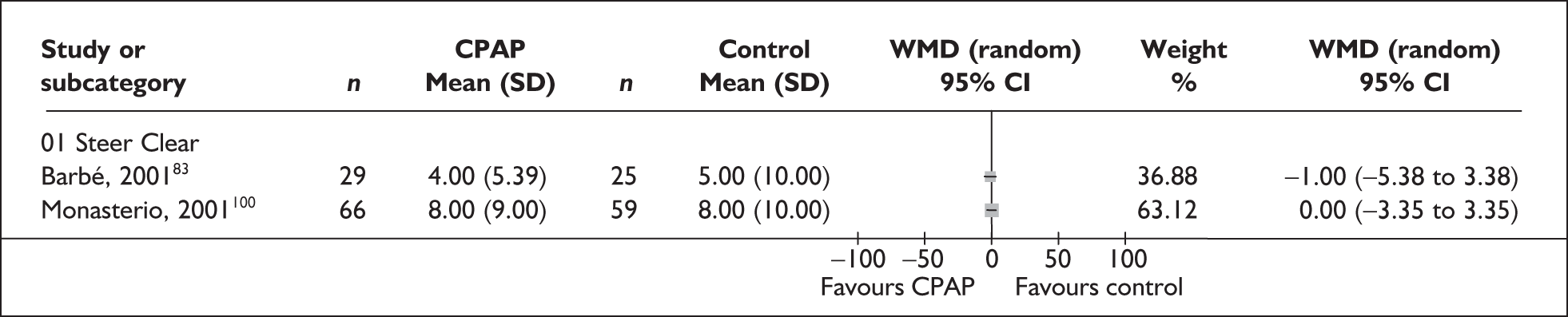
FIGURE 14.
SteerClear (CPAP versus placebo/usual care), number of obstacles hit.
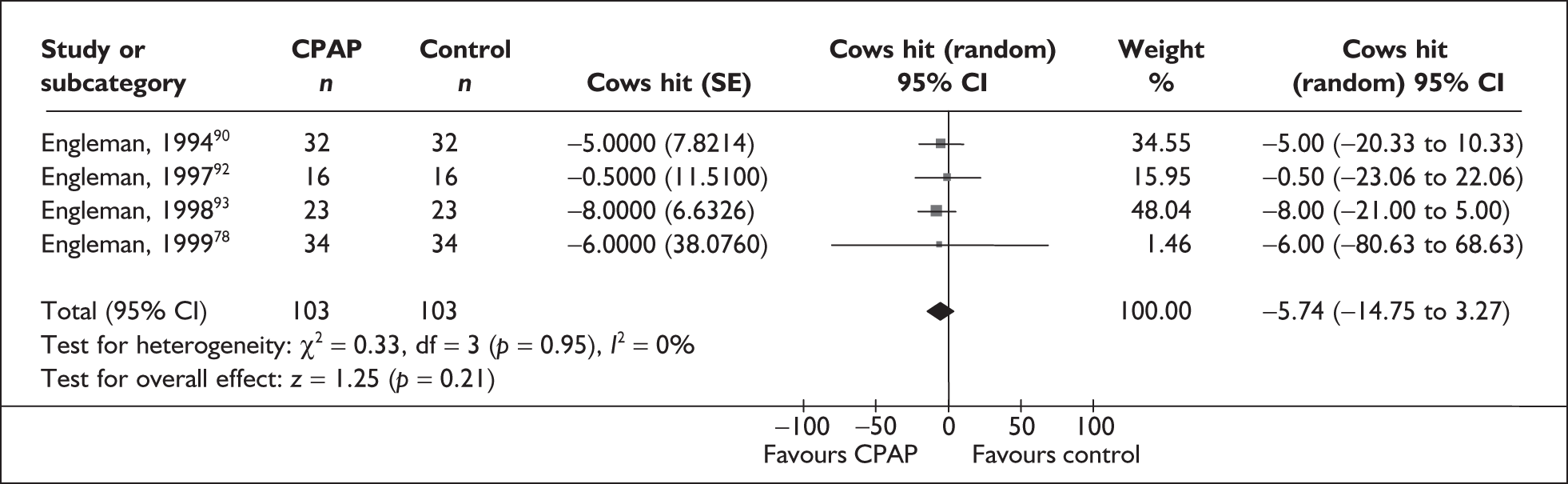
One parallel group trial118 used a different simulated driving test, based on the work of Land and Horwood. 119 This computerised program presents a white on black image (as in night driving) of the moving edges of the road with an image of the vehicle bonnet at the bottom of the screen. The primary object is to steer the centre of the vehicle as accurately as possible down the middle of the road for 30 minutes. In addition, single digits are displayed at each corner of the screen (digits change randomly at an interval of 8–10 seconds) and the participant is required to identify target digits by pressing a button on either side of the steering wheel. Baseline ESS was classified as moderate in both groups. An improvement in terms of steering performance was found with CPAP compared with sham CPAP, although not all differences were statistically significant: SD of position on road (median difference –0.1, p = 0.08), SD of deterioration (median difference –0.2, p = 0.007), and length of drive (minutes) (median difference –0.3, p = 0.08). This was based on end point data as per the protocol; when difference in change was considered, a significant difference in favour of CPAP was found for SD position on road (p = 0.03) and length of drive (p = 0.02).
The TMT is a task of complex attention given in two parts, A and B. Individuals are asked to draw lines to connect consecutively numbered circles on one work sheet (Part A), and then connect the same number of consecutively numbered and lettered circles on another work sheet, alternating between the two sequences (Part B); time taken to complete the task (seconds) and errors made are typically recorded. The test is sensitive to a range of mental processes including speed of processing and mental flexibility.
Eight studies (five parallel, three crossover, n = 260) reported on TMT Part A78,83,85,89,90,100,117,341 and 12 (six crossover, six parallel, n = 406) reported on TMT Part B. 78,82,83,85,89,90,92,93,100,113,117,341
The severity of daytime sleepiness was classified as moderate in four studies,78,89,100,341 and in one study each was classified as mild83 and severe85; two studies did not report symptom severity. 58,90 None of the studies showed a significant difference between CPAP and control in the length of time it took to complete TMT Part A (Table 6). None of the studies reported adequate allocation concealment, and it was unclear whether groups were similar at baseline in four of the included studies. 78,89,90,100
| Study | CPAP [mean (SD)] | Control [mean (SD)] | MD | p-Value |
|---|---|---|---|---|
| Crossover | ||||
| Barnes et al., 200289 | 28.1 (NR) | 27.6 (NR) | 0.5 | Not significant (precise p-value not reported) |
| Engleman et al., 199490 | NR | NR | NR | Not significant (precise p-value not reported) |
| Engleman et al., 199978 | 26 (11) | 29 (11) | –3.0 | 0.06 |
| Parallel | ||||
| Barbé et al., 200183 | 47 (NR) | 47 (NR) | 0 | > 0.20 |
| Dimsdale et al., 200058,117 | 27.4 (NR) | 27.4 (NR) | 0 | NR |
| Henke et al., 200185 | Only available in graph | Not significant (precise p-value not reported) | ||
| Monasterio et al., 2001100 | 49 (19) | 49 (20) | 0 | 0.76 |
| Norman et al., 200673,341 | 26.5 (NR) | 21.7 (NR) | 4.9 | 0.49 (relates to time × treatment interaction for three treatment groups) |
Five trials (three crossover, two parallel) did not report sufficient data to calculate a variance. 78,85,89,90,341 Therefore, only three of the eight trials reporting TMT Part A were used to generate a pooled estimate of treatment effect and are displayed on the forest plot below (Figure 15). When data from these three parallel group trials (n = 215) were pooled, no statistically significant between-group difference was found (MD 0.0, 95% CI –2.5 to 2.5). There was no statistical heterogeneity (I2 = 0%). Given that only a proportion of the available studies could be pooled, caution needs to be taken in interpreting the pooled effect.
FIGURE 15.
Trail Making Task Part A (CPAP versus placebo/usual care), stratified by type of data.
Where reported, the populations in the majority of trials were classified as having moderate baseline daytime sleepiness,78,82,89,92,93,100,341 and in one study each as having mild83 and severe85 daytime sleepiness. One trial reported a significant difference in favour of CPAP compared with oral placebo in the length of time taken to complete the task; no statistically significant between-group differences were found in the other trials (Table 7). Half of the studies did not report adequate allocation concealment, and it is unclear if the trials had sufficient power to detect a treatment effect.
| Study | CPAP [mean (SD)] | Control [mean (SD)] | MD | p-Value |
|---|---|---|---|---|
| Crossover | ||||
| Barnes et al., 200289 | 60.1 (NR) | 65.2 (NR) | –5.1 | Not significant (p-value not reported) |
| Barnes et al., 200482 | 73.3 (29.5) | 74.2 (32.2) | –0.9 | Not significant (p-value not reported) |
| Engleman et al., 199490 | 66 (28.3) | 76 (28.3) | –10 | 0.02 |
| Engleman et al., 199792 | 64.1 (22) | 77.7 (36.8) | –13.6 | 0.02 |
| Engleman et al., 199893 | 69 (32) | 68 (32) | 1.0 | Not significant (p-value not reported) |
| Engleman et al., 199978 | 63 (33) | 65 (27) | –2 | Not significant (p-value not reported) |
| Parallel | ||||
| Barbé et al., 200183 | 96 (32.3) | 110 (50) | –14.0 | 0.10 |
| Dimsdale et al., 200058,117 | 71.2 (31.8) | 87 (34.8) | –15.8 | Not significant (p-value not reported) |
| Henke et al., 200185 | Only available in graph | Not significant (p-value not reported) | ||
| Lojander et al., 1999113 | 130, median | 75, median | NR | |
| Monasterio et al., 2001100 | 106 (42) | 100 (39) | 6.0 | 0.15 (difference in change based on median values) |
Seven trials did not report sufficient data or used different scales; therefore, only data from two crossover90,93 and three parallel83,100,117 trials were pooled (n = 328) for TMT Part B (Figure 16). There was a statistically significant benefit with CPAP compared with control for time (seconds) taken to complete TMT Part B (MD –9.1, 95% CI –14.9 to –3.1). There was low statistical heterogeneity (I2 = 34%). However, as only a proportion of the studies were pooled, the treatment effect may not be generalisable.
FIGURE 16.
Trail Making Task Part B (CPAP versus placebo/usual care), stratified by type of data.
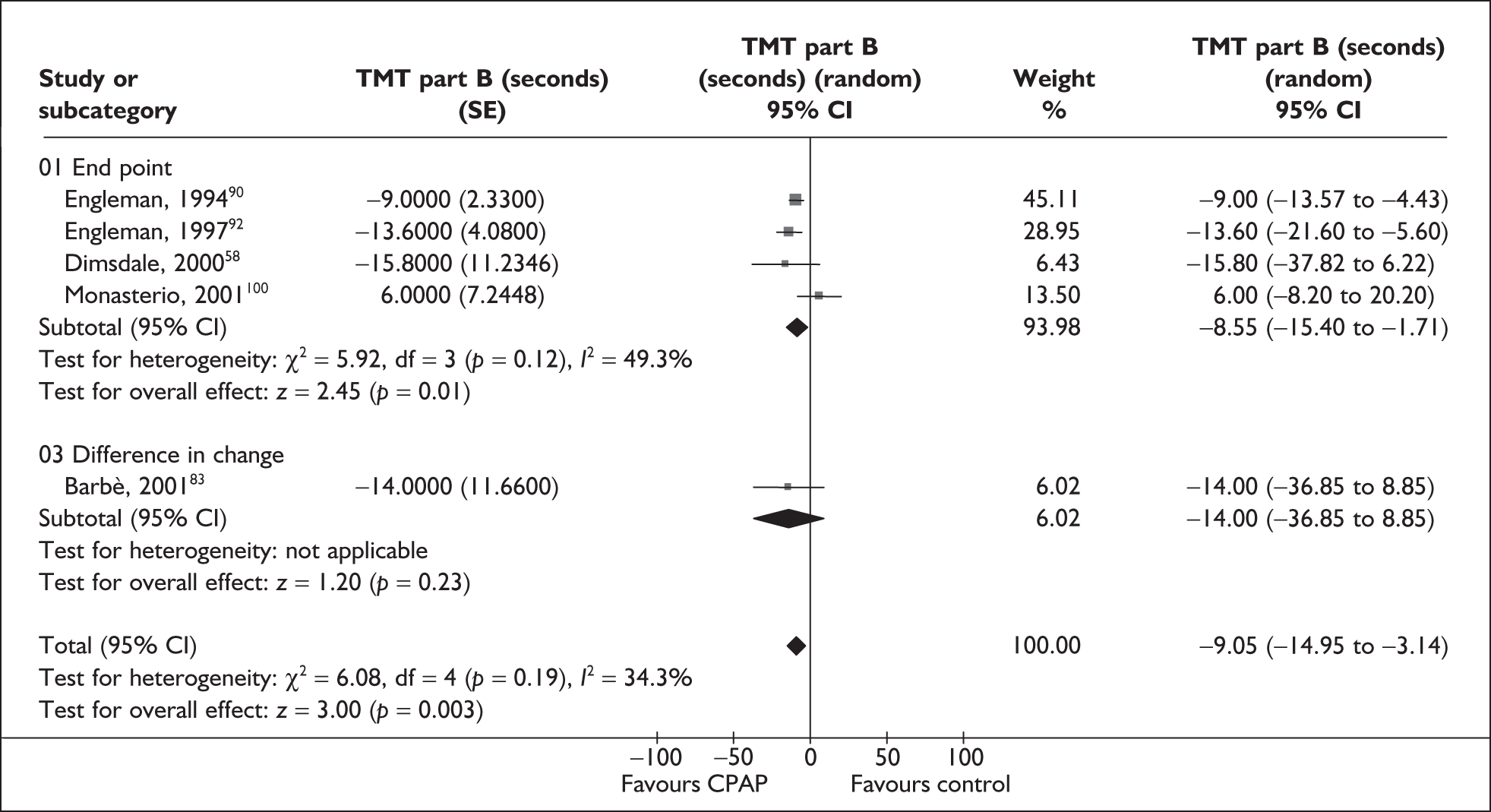
Ten studies used the DSST, a test of complex attention (five parallel and five crossover, n = 488). 73,78,82,83,85,89,90,93,100,117 In this test of attention and processing, respondents are given a code table displaying the correspondence between pairs of digits (from 1 to 9) and symbols, and then asked to fill in blank squares with the symbol that is paired to the digit displayed above the square. Six out of the eight studies reporting daytime sleepiness coud be classified as moderate78,82,89,93,100,341 and one trial each classified the study population as having mild83 and severe85 daytime sleepiness. Two trials found a significant benefit of CPAP compared with control in the number of correct responses,78,90 and one trial found a significant difference in change from baseline in favour of placebo;89 no significant between-group differences were found in the other trials (Table 8). None of the trials reported adequate allocation concealment.
| Study | CPAP [mean (SD)] | Control [mean (SD)] | MD | p-Value |
|---|---|---|---|---|
| Crossover | ||||
| Barnes et al., 200289 | 47.3 (NR) | 48 (NR) | –0.7 | 0.07 (difference in change) |
| Barnes et al., 200482 | 47.3 (3.6) | 46.8 (3.6) | 0.5 | Not significant (p-value not reported) |
| Engleman et al., 199490 | 52 (11.3) | 51 (11.3) | 1 | 0.05 |
| Engleman et al., 199893 | 52 (13) | 52 (14) | 0 | Not significant (p-value not reported) |
| Engleman et al., 199978 | 59 (12) | 57 (14) | 2 | 0.0004 |
| Parallel | ||||
| Barbé et al., 200183 | 43 (16.2) | 47 (20) | –4 | > 0.20 (difference in change) |
| Dimsdale et al., 200058,117 | 53.2 (11.2) | 53.5 (12) | –0.3 | Not significant (p-value not reported) |
| Henke et al., 200185 | Only available in graph | A binary variable of improved or not improved was assessed; not significant (p-value not reported) | ||
| Monasterio et al., 2001100 | 9 (3) scaled score | 9 (2) | 0 | 0.97 (difference in change, based on median values) |
| Norman et al., 200673,341 | 73.8 (NR) | 68.7 (NR) | 5.1 | 0.26 (based on time × treatment interaction for three-armed trial) |
Four studies did not provide sufficient data to calculate a variance and one trial used a different scale; therefore, only five trials (three crossover and two parallel, n = 170) were pooled (Figure 17);78,83,90,93,117 no statistically significant benefit with CPAP was found compared with control in terms of the number of correct responses (MD 0.2, 95% CI –0.6 to 1.0). There was no statistical heterogeneity (I2 = 0%).
FIGURE 17.
Wechsler Adult Intelligence Digit Symbol Substitution Test (CPAP versus placebo/usual care), stratified by type of data.
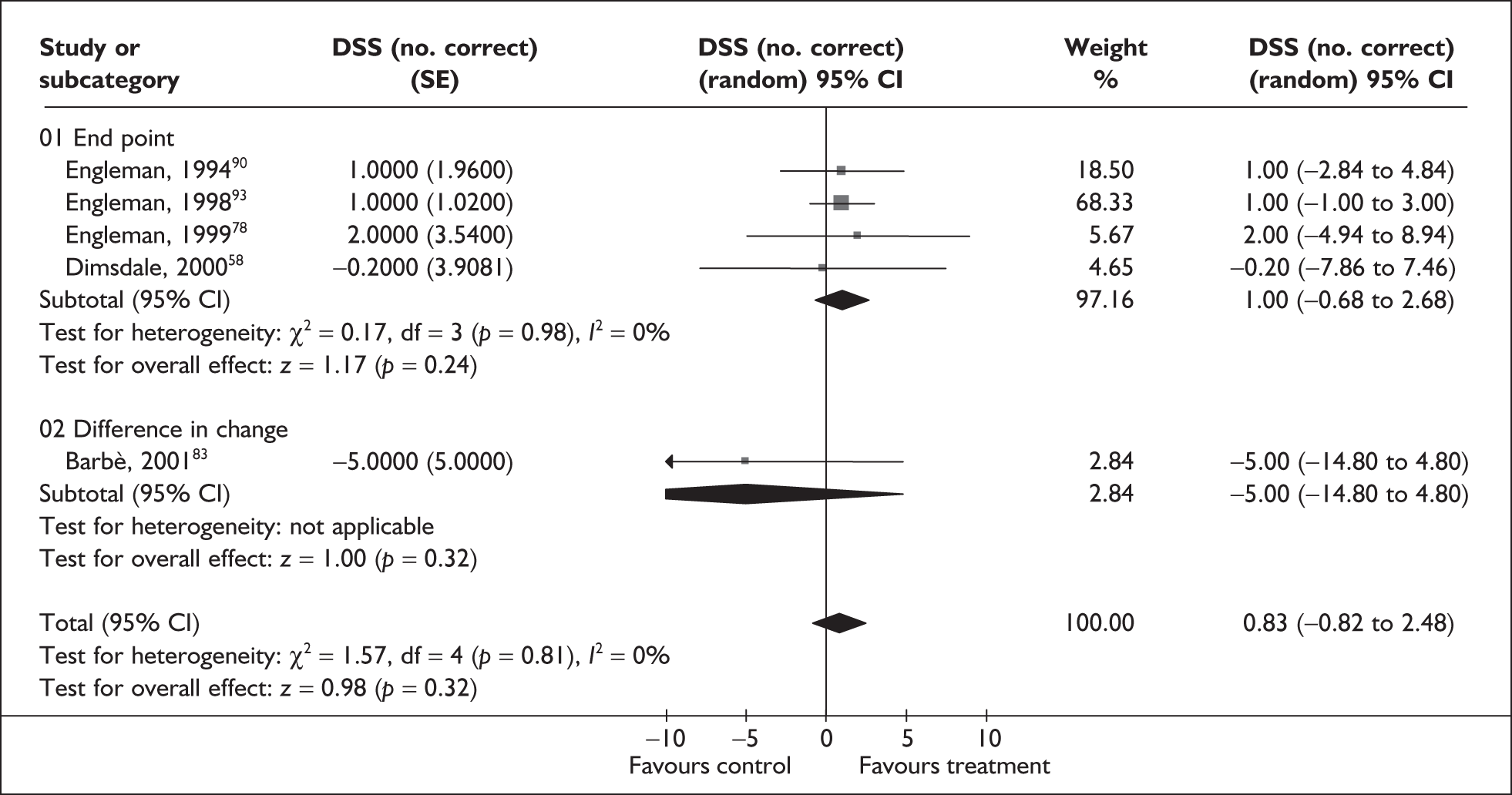
Seven studies used the PASAT test of vigilance. 78,82,83,90,92,93,100 In this computerised task, a series of digits are presented at a set rate and the respondent is asked to add the numbers in pairs, such that each number is added to the one that immediately precedes it. Presentation rates range from 1 to 4 seconds. Different formats have been developed, for example the PASAT 1.2 and the PASAT 2.4, which are thought to be more difficult than the standard PASAT 1 and PASAT 2. One study reported outcomes for the PASAT 1.2,82 six studies for the PASAT 2,78,83,90,92,93,100 one study for the PASAT 2.4,82 two studies for the PASAT 3,83,100 and two studies for the PASAT 4. 83,90
Daytime sleepiness was reported for six trial populations; five populations were classified as moderate78,90,92,93,100 and one trial as mild. 83 One crossover trial78 found a significant benefit in favour of CPAP in the number of correct responses made; no statistically significant between-group differences were found in any of the other trials (Table 9). None of the studies reported adequate allocation concealment, and one study reported a significant treatment by period interaction for the PASAT 290 indicating a potential carryover effect of treatment.
| Study | CPAP [mean (SD)] | Control [mean (SD)] | MD | p-Value |
|---|---|---|---|---|
| Crossover | ||||
| Barnes et al., 200482 | PASAT 1.2: 2.9 (0.9) | 3.4 (0.9) | –0.5 | Not significant (precise p-value not reported) |
| PASAT 2.4: 3.8 (1.8) | 3.7 (0.9) | 0.1 | Not significant (precise p-value not reported) | |
| Engleman et al., 199490 | NR | NR | NR | Not significant (precise p-value not reported) |
| Engleman et al., 199792 | PASAT 2: 37.8 (13.2) | 35.3 (11.2) | 2.5 | Not significant (precise p-value not reported) |
| Engleman et al., 199893 | PASAT 2: 37 (11) | 35 (11) | 2 | Not significant (precise p-value not reported) |
| Engleman et al., 199978 | PASAT 2: 40 (11) | 36 (14) | 4 | 0.02 |
| Parallel | ||||
| Barbé et al., 200183 | PASAT 1: 15 (5.4) | 15 (5) | 0 | > 0.20 (difference in change) |
| PASAT 2: 16 (5.4) | 15 (5) | 1 | 0.04 | |
| PASAT 3: 12 (5.4) | 12 (5) | 0 | 0.09 | |
| PASAT 4: 5 (5.4) | 5 (5) | 0 | > 0.20 | |
| Monasterio et al., 2001100 | PASAT 1: 5 (4) | 5 (3) | 0 | 0.32 (based on data for median values) |
| PASAT 2: 12(4) | 12 (4) | 0 | 0.12 | |
| PASAT 3: 15 (4) | 15 (4) | 0 | 0.20 | |
| PASAT 4: 14 (4) | 16 (4) | –2 | 0.20 | |
Data from three or more trials were available for PASAT 1 and PASAT 2. Of the three studies reporting PASAT 1, two studies82,83 did not provide sufficient data to calculate a variance for pooling, and, of the six studies reporting the PASAT 2, two studies did not provide sufficient data to calculate a variance. 90,92 Two crossover and two parallel trials were therefore pooled (n = 234) for PASAT 2 (Figure 18). No statistically significant benefit was found with CPAP compared with control for number of correct responses made (MD 2.30, 95% CI 0.24–4.37); statistical heterogeneity was low (I2 = 25%).
FIGURE 18.
Paced Auditory Serial Addition Task (PASAT) 2 (second presentation rate) (CPAP versus placebo/usual care), stratified by type of data.
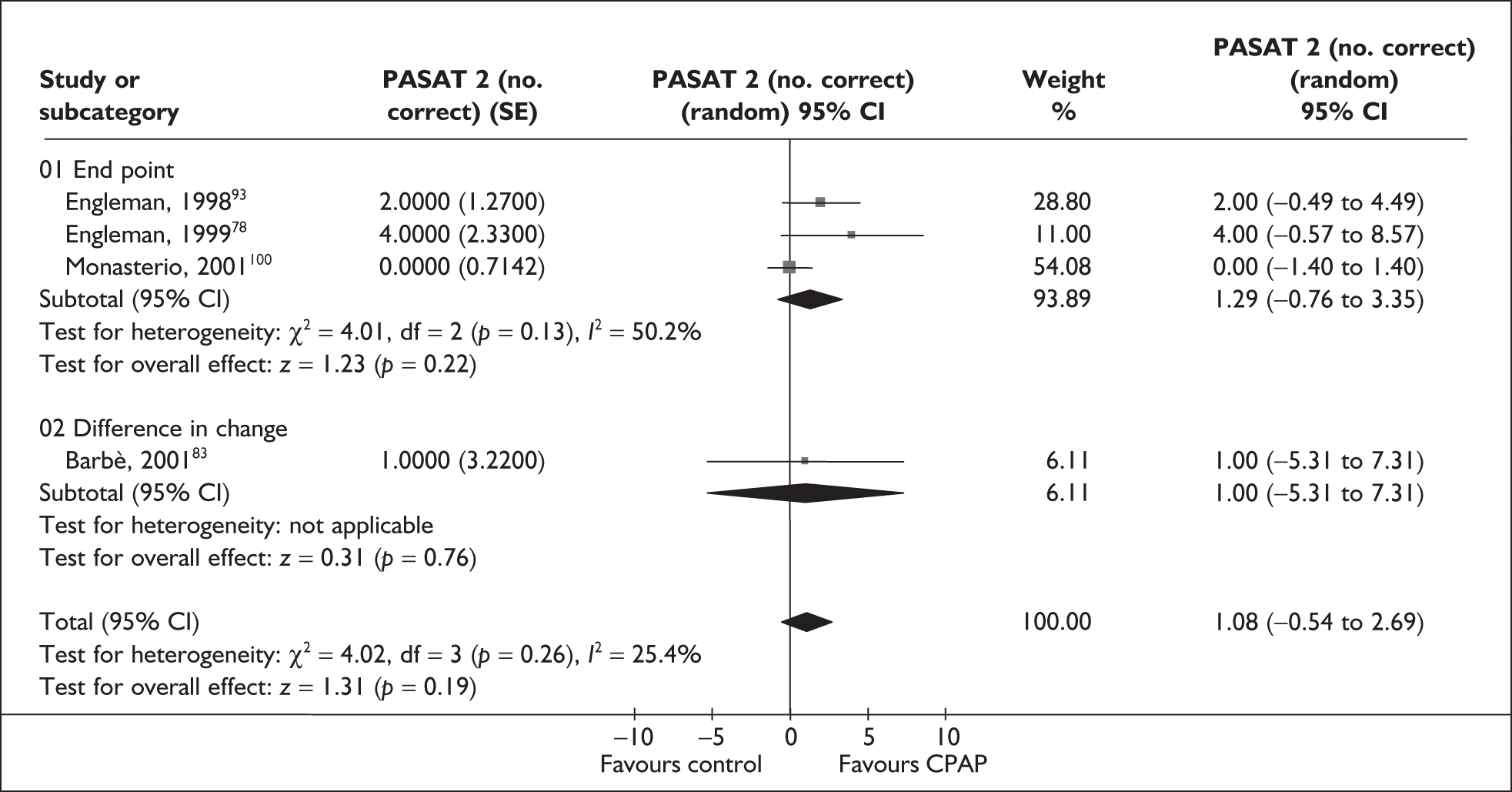
Nine trials assessed verbal fluency; there are a variety of verbal fluency tests in use and each is designed to measure the speed and flexibility of verbal thought processes. Six trials used the Controlled Oral Word Association Test (COWAT). 82,85,89,90,92,93 The remaining trials did not specify the test used. 58,73,100 Insufficient reported data, and uncertainty as to whether instruments were measuring the same thing, meant that these studies were not pooled (Table 10).
| Study | CPAP [mean (SD)] | Control [mean (SD)] | MD | p-Value |
|---|---|---|---|---|
| Crossover | ||||
| Barnes et al., 200289 |
38.7 (NR) COWAT: no. correct |
36 (NR) | 2.7 | 0.02 (difference in change) |
| Barnes et al., 200482 |
46.5 (10.7) COWAT |
46.3 (8.9) | 0.2 | NR |
| Engleman et al., 199490 |
NR COWAT |
NR | NR | Not significant (precise p-value not reported) |
| Engleman et al., 199792 |
38.5 (14.0) COWAT: no. correct |
39.2 (12.4) | –0.7 | Not significant (precise p-value not reported) |
| Engleman et al., 199893 |
41 (12) COWAT: no. correct |
42 (11) | –1 | Not significant (precise p-value not reported) |
| Parallel | ||||
| Dimsdale et al., 200058,117 |
44.5 (12.1) No. correct |
37.3 (12.8) | 7.2 | NR |
| Henke et al., 200185 |
NR COWAT |
NR | NR | Not significant (precise p-value not reported) |
| Monasterio et al., 2001100 |
69 (27) Percentile |
70 (29) | –1 | 0.53 (based on data for median values) |
| Norman et al., 200673,341 |
40.9 (NR) Total score |
45.5 (NR) | –4 | 0.15 (relates to time × treatment interaction from a three-arm trial) |
One small crossover study (n = 28)89 reported a significant improvement in the number of correct words in the CPAP group compared with the oral placebo group. However, an order effect was found; individuals receiving placebo in the first treatment period had no significant change with either treatment. No significant differences between treatment groups were found in the remaining studies.
Two parallel group trials used the DVT, which is a measure of sustained attention and psychomotor speed, using a rapid visual tracking task (Table 11). 117,341 Only one trial reported baseline ESS,341 which was classified as moderate; both trials reported severe AHI scores. Time by treatment interactions showed a significant improvement specific to CPAP for time taken to complete the task, but not errors made, in a 2-week study (n = 31) comparing CPAP with supplemental oxygen and placebo. 341 A 1-week study (n = 36) found a significant difference between CPAP and sham CPAP in the number of errors made;117 however, after controlling for pre-treatment differences, no significant difference between groups was found. A summary of the results is presented in Table 11.
| Study | CPAP [mean (SD)] | Control [mean (SD)] | MD | p-Value |
|---|---|---|---|---|
| Parallel | ||||
| Dimsdale et al., 200058,117 | Time: 6.9 (1.3 | Time: 6.6 (1.6) | 0.3 | NR |
| Errors: 10.1 (11.6) | Errors: 12.3 (12.4) | –2.2 | 0.49 | |
| Norman et al., 200673,341 | Time: 312.3 (NR) | Time: 303.1 (NR) | 9.2 | 0.02 (relates to time × treatment interaction from a three-arm trial) |
| Errors: 7.2 (NR) | Errors: 10.6 (NR) | –3.4 | 0.08 (relates to time × treatment interaction from a three-arm trial) | |
| Cognitive test | Barbé et al., 200183 | Barnes et al., 200289 | Barnes et al., 200482 | Cibele et al., 200672 | dDimsdale et al., 200058 | Engleman et al., 199490 | Engleman et al., 199792 | Engleman et al., 199893 | Engleman et al., 199978 | Engleman et al., 2002103 | Henke et al., 200185 | eHoekema et al., 2006102 | fJenkinson et al., 199977 | Jokic et al., 1999108 | Lojander et al., 1999113 | Marshall et al., 200579 | Monasterio et al., 2001100 | gNorman et al., 200673 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tests of attention | ||||||||||||||||||
| CET | ✓ | |||||||||||||||||
| DO | ✓ | |||||||||||||||||
| DVT | ✓ | ✓ | ||||||||||||||||
| PVT | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| RT (8-C) | ✓ | ✓ | ||||||||||||||||
| RVIP | ✓ | ✓ | ||||||||||||||||
| Stroop | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| TMT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| PASAT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| BW | ✓ | |||||||||||||||||
| SteerClear | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓c | ✓ | ||||||||||
| Other driving tests | ✓ | ✓ | ||||||||||||||||
| Tests of verbal fluency | ||||||||||||||||||
| COWAT | ✓ | ✓ | ✓a | ✓a | ✓a | ✓ | ||||||||||||
| VFT | ✓ | ✓ | ✓ | |||||||||||||||
| Tests of memory | ||||||||||||||||||
| BVM | ✓ | |||||||||||||||||
| BVRT | ✓ | ✓ | ✓ | |||||||||||||||
| CT | ✓ | |||||||||||||||||
| HVL | ✓ | |||||||||||||||||
| WMS | ✓ | ✓(s) | ✓ | ✓ | ✓(s) | |||||||||||||
| WPMR | ✓ | |||||||||||||||||
| MDT | ✓ | |||||||||||||||||
| Tests of motor performance | ||||||||||||||||||
| PP | ✓ | |||||||||||||||||
| FTT | ✓ | |||||||||||||||||
| Tests of construction | ||||||||||||||||||
| CFD | ✓ | |||||||||||||||||
| Copying | ✓ | |||||||||||||||||
| CFT | ✓b,c | |||||||||||||||||
| Neurocognitive test batteries | ||||||||||||||||||
| WAIS | ✓(s) | ✓(s) | ✓(s) | ✓(s) | ✓(s) | ✓(s) | ✓(s) | ✓(s) | ✓(s) | ✓(s)c | ✓(s) | ✓(s) | ✓(s) | |||||
| IQ decrement | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
A number of additional cognitive tests were also used by individual studies, including Stroop colour and word test, psychomotor vigilance, brief visuospatial memory and a concentration endurance test; however, no statistically significant between-group differences were found. It was unclear whether most of these studies were appropriately powered to detect an effect. In addition, few trials reported adequate allocation concealment, baseline comparisons were not always reported and intention-to-treat analysis was seldom conducted. Results for these studies are presented in Appendix 4, Table 53.
CPAP versus dental devices
Cognitive outcomes were reported in four studies (two crossover and two parallel group trials, n = 160). 72,82,103,114 Where reported, symptom severity was classified as moderate, and in all but one trial103 disease severity was also reported as moderate. There were no statistically significant differences between treatment groups on any of the cognitive tests assessed (see Appendix 4, Table 53). None of these trials reported appropriate allocation concealment, and only one trial82 reported adequate randomisation methods and used intention-to-treat analysis.
CPAP versus postural therapy
Data were available for one small crossover study (n = 14). 108 No statistically significant between-group differences were found on any of the cognitive tests administered (see Appendix 4, Table 53). It is unclear whether performance differences at baseline existed, or whether the study was appropriately powered to detect an effect.
Apnoea–hypopnoea index
CPAP versus placebo/usual care
Nine studies reported the AHI at follow-up. 66,70,73,82,85,87,97,100,109 There was high statistical heterogeneity (I2 = 97%) and any pooled effect is likely to be meaningless. All the studies reported a statistically significant reduction in the AHI with CPAP compared with placebo/usual care and the effect size ranged from –9.2 (95% CI –18.3 to –0.1) to –60.0 (95% CI –72.1 to –47.5) (see Appendix 4, Figure 44).
CPAP versus dental devices
Nine studies reported the AHI at follow-up. 70,80–82,103,105–107,119 There was a statistically significant reduction in AHI in favour of CPAP compared with dental devices (MD –8.4, 95% CI –10.5 to –6.3) (see Appendix 4, Figure 45). Statistical heterogeneity was low to moderate (I2 = 40%).
CPAP versus postural therapy
Data were available for the AHI from three small crossover trials (n = 36). 60,61,108 There was a statistically significant benefit with CPAP on the AHI compared with postural therapy (shoulder–head elevation pillow, MD 15.5, p = 0.008; cervicomandibular support collar, MD in change 16.8, p = 0.001; backpack with soft ball inside, MD 6.1, 95% CI 2.0–10.2, p = 0.007).
Adverse effects
Reporting of adverse effects was patchy across studies. Reported adverse effects with CPAP were related mainly to discomfort with the equipment (e.g. machine noise, a feeling of pressure and mask discomfort), dry mouth and stuffy or runny nose (Table 13). Reported adverse effects with use of dental devices were related mainly to excess salivation, tooth discomfort and temporomandibular joint discomfort.
| Effect | Study | CPAP (n/n) | Control (n/n) | Dental device (n/n) |
|---|---|---|---|---|
| Mask discomfort or other problems with mask/headgear | Engleman et al., 199978 | 8/34 | 0/34 | – |
| Machine noise | Lojander et al., 199699 | 2/13 | 0/20 | – |
| Lam et al., 200770 | 8/34 | – | 0/34 | |
| Sleep disturbance | Engleman et al., 199978 | 8/34 | 0/34 | – |
| Engleman et al., 2002103 | 16/48 | – | 12/48 | |
| Lojander et al., 199699 | 1/13 | 0/20 | – | |
| Difficulty falling asleep with prescribed pressure | Engleman et al., 199978 | 1/34 | 0/34 | – |
| Feeling of pressure | Lam et al., 200770 | 11/34 | – | 0/34 |
| Pressure (on face) | Randerath et al., 2002105 | 8/19 | – | 2/19 |
| Pressure (in mouth) | Randerath et al., 2002105 | 0/19 | – | 2/19 |
| Early awakening | Engleman et al., 199978 | 1/34 | 0/34 | – |
| Residual sleepiness | Engleman et al., 199978 | 0/34 | 3/34 | – |
| Dry throat/nose/mouth | Engleman et al., 199978 | 4/34 | 0/34 | – |
| Engleman et al., 2002103 | 5/48 | – | 0/48 | |
| Lojander et al., 199699 | 2/13 | 0/20 | – | |
| Lam et al., 200770 | 16/34 | – | 11/34 | |
| Rhinorrhoea | Lojander et al., 199699 | 7/13 | 0/20 | – |
| Skin irritation or abrasion | Redline et al., 199859 | 2/51 | 0/46 | – |
| Lam et al., 200770 | 7/34 | – | 0/34 | |
| Minor nosebleeds (related to nasal spray) | Redline et al., 199859 | 1/51 | 2/46 | – |
| Use of antibiotics during intervention period | Redline et al., 199859 | 7/51 | 2/46 | – |
| Excess salivation | Engleman et al., 2002103 | 0/48 | – | 9/48 |
| Lam et al., 200770 | 0/34 | – | 19/34 | |
| Tooth discomfort | Lam et al., 200770 | 0/34 | – | 11/34 |
| Tooth damage | Engleman et al., 2002103 | 0/48 | – | 3/48 |
| Temporomandibular joint discomfort | Engleman et al., 2002103 | 0/48 | – | 33/48 |
| Lam et al., 200770 | 0/34 | – | 13/34 | |
| Randerath et al., 2002105 | 0/19 | – | 8/19 | |
| Removal of appliance during sleep | Engleman et al., 2002103 | 7/48 | – | 19/48 |
| Leakage | Engleman et al., 2002103 | 11/48 | – | 0/48 |
| Stuffy nose | Engleman et al., 2002103 | 8/48 | – | 0/48 |
| Inconvenience | Engleman et al., 2002103 | 6/48 | – | 0/48 |
| Side-effect severity | ||||
| None | Ferguson et al., 199681 | 11/25 | – | 10/21 |
| Ferguson et al., 199780 | 10/20 | – | 7/20 | |
| Mild | Ferguson et al., 199681 | 1/25 | – | 9/21 |
| Ferguson et al., 199780 | 4/20 | – | 9/20 | |
| Moderate | Ferguson et al., 199681 | 5/25 | – | 5/21 |
| Ferguson et al., 199780 | 3/20 | – | 4/20 | |
| Severe | Ferguson et al., 199681 | 4/25 | – | 1/21 |
| Ferguson et al., 199780 | 3/20 | – | 1/20 |
CPAP versus postural therapy
Data were available from two trials (n = 23). 60,61 No statistically significant difference in the overall number of self-reported adverse events was found when CPAP was compared with a shoulder–head elevation pillow (MD –0.8, p = 0.16). However, there were significantly fewer self-reported adverse events with a cervicomandibular support collar than with CPAP (MD in change 4.2, p = 0.01). Type of adverse event and indication of perceived severity were not reported in either study.
Patient preference
New data were not found regarding patient preference for CPAP compared with dental devices. Giles et al. highlighted the difficulties in interpreting the data they found: preferences can be measured only when participants receive both treatment options (i.e. in crossover trials), leading to possible order effects. 50 It was not considered appropriate to pool the data. 50 Based on two studies (n = 15) of patients who had a successful outcome with both treatments there was a preference for CPAP. When all patients were considered (four studies, n = 164), in one study participants showed a statistically significant preference for dental devices over CPAP, but there was no statistically significant difference in the other studies and they did not show a consistent direction for preference. 50 Participants were more likely to withdraw while using dental devices than with CPAP [odds ratio (OR) 0.46, 95% CI 0.25–0.84] (see Appendix 4, Figure 46).
Chapter 4 Assessment of cost-effectiveness evidence
The examination of the cost-effectiveness of continuous positive airway pressure (CPAP) for the treatment of obstructive sleep apnoea–hypopnoea syndrome (OSAHS) comprises:
-
A systematic review of existing evidence on the cost-effectiveness of CPAP, against relevant comparators, including dental devices and conservative management. The review includes the manufacturer ResMed’s submission120 to the National Institute for Health and Clinical Excellence (NICE).
-
Employment of this systematic review to inform the development of an economic model to evaluate the cost-effectiveness of CPAP for the treatment of OSAHS.
Systematic review of existing cost-effectiveness evidence
Cost-effectiveness review methods
A systematic review of cost-effectiveness studies was undertaken to compare CPAP with other interventions routinely used for the treatment of OSAHS in the National Health Service (NHS). The review comprised manufacturer submissions to NICE and relevant, published cost-effectiveness analyses. To obtain the latter, papers obtained from the clinical effectiveness review (see Chapter 3) were scanned to check whether they included cost-effectiveness data. In addition, several economic databases were searched for cost-effectiveness studies as listed below (for full details see Appendix 1, Cost-effectiveness searches).
-
MEDLINE and in process MEDLINE and other non-indexed citations (1950–Jan 10 2007) (OVID)
-
EMBASE (1980–2007 week 1) (OVID)
-
Cochrane Central Register of Controlled Trials (Cochrane Library 2006, issue 4) (www.thecochranelibrary.com)
-
NHS Economic Evaluation Database (NHS EED) (CRD internal administration system 13/1/07)
-
Health Economic Evaluations Database (HEED) (1995–Jan 2007) (CD-ROM)
-
HTA database (CRD internal administration system 13/1/07)
-
EconLit (1969–2006/10) (SilverPlatter)
-
EconPapers (http://econpapers.repec.org/).
A broad range of studies was considered in the assessment of cost-effectiveness, including economic evaluations conducted alongside trials, modelling studies and analyses of administrative databases. Studies were included in the cost-effectiveness review if they considered the costs and outcomes associated with two or more interventions in the treatment of OSAHS. Therefore, studies based on cost–consequence analysis, cost–utility analysis, cost-effectiveness analysis, cost minimisation analysis and cost–benefit analysis were eligible for inclusion.
Data were extracted using a data extraction form that was developed for use in previous technology assessment reviews. The quality of the cost-effectiveness studies was assessed based on a checklist developed by Drummond et al. (2005)121 and which reflects the criteria for economic evaluation detailed in the methodological guidance developed by NICE (www.nice.org.uk/) (see Appendix 6 for economic evaluation data extraction table and Table 40 for economic evaluation quality assessment table).
Cost-effectiveness review results
The above searches identified four full economic evaluations for inclusion in the cost-effectiveness review of published studies. 44,122,123,124 One manufacturer (ResMed) submitted a full cost-effectiveness study to NICE. 120 Two manufacturers, Fisher Paykel Ltd125 and Respironics (UK) Ltd,126 submitted a partial economic evaluation. Full economic evaluations, including ResMed’s submission and the four published economic evaluations (i.e. Ayas et al. 122, Mar et al. 123, the Trent Report by Chilcott et al. 44 and Tousignant et al. 124), are reviewed next and reported in Table 40. These are followed by an overall summary of the cost-effectiveness evidence base.
Review of manufacturers’ submissions
ResMed model120
ResMed performed a cost–utility analysis comparing CPAP using fixed pressure and CPAP using autotitrated pressure with a ‘do nothing’ alternative for the treatment of patients with severe OSAHS. 120 The hypothetical patient population depicted by the model consisted of 55-year-old patients with severe OSAHS as defined by an AHI > 30 and daytime sleepiness represented by a score of 12 on the ESS. The analysis was undertaken from the NHS and PSS perspective.
ResMed produced a cohort-based Markov model with a 14-year time horizon and each Markov cycle lasted a year. 120 Patients enter the model following an initial outpatient visit or a diagnostic sleep study test. Treatment begins 8.4 months after whichever visit takes place first. For each year in the model patients can remain event free in the severe OSAHS state, or can have a non-fatal or fatal stroke, a cardiovascular event (CVE), e.g. myocardial infarction or a road traffic accident (RTA), as illustrated in Figure 19. In each subsequent year patients who have had a non-fatal CVE or an RTA can have a stroke, CVE or RTA. However, patients who have a stroke can no longer drive and, therefore, are not at subsequent risk of an RTA.
FIGURE 19.
Structure of ResMed Model (adaptation of Figure 6.1, p. 9, v in ResMed submission).
The primary measure of cost-effectiveness was incremental cost per quality-adjusted life-year (QALY) gained, and the secondary measure was cost per life-year gained. The QALY estimate incorporated the impact on health-related quality of life (HRQoL) of stroke, CVE and RTA. Effectiveness estimates and utilities were drawn from the HRQoL published literature and government statistics, and based on the authors’ assumptions. Data on patient management and resource use were obtained from 19 clinicians throughout the UK who had relevant clinical experience. Unit cost data on CPAP treatment, and resource use associated with CVEs and RTAs, were obtained from list prices, the published literature and government statistics. The authors undertook several univariate sensitivity analyses and probabilistic sensitivity analysis to test the robustness of findings.
Summary of effectiveness data
For the base-case analysis, utility values for treated and untreated OSAHS were obtained from Mar et al. 123 In this study, a survey of 51 OSAHS patients who attended a sleep clinic in Spain was undertaken before the initiation of CPAP and 3 months after initiation of CPAP in order to generate ‘do nothing’ and nasal CPAP (nCPAP) utility values, respectively. The EQ-5D instrument (EuroQoL Group127) was used to describe patient health states and completed data for 46 patients were obtained. These were then elicited using the time trade-off technique and valued based on UK societal preferences (Dolan et al. 128). No information was obtained on the HRQoL of OSAHS patients with stroke and coronary heart disease (CHD). To estimate these utilities, the authors assumed quality adjustment factors of 0.8 and 0.9 in relation to standard OSAS patient utilities (Table 14, based on Torrance et al. 129) To estimate utility associated with a non-fatal RTA, ResMed took the average utility for OSAHS and a non-fatal CVE in treated and untreated patients.
| Health state | Utility values | |
|---|---|---|
| Untreated OSAS patients | nCPAP OSAS patients | |
| OSAS | 0.738 | 0.811 |
| Non-fatal stroke | 0.590 | 0.649 |
| Non-fatal CHD | 0.664 | 0.730 |
| Non-fatal RTA | 0.701 | 0.771 |
The annual incidence rates of fatal and non-fatal cardiovascular and cerebrovascular events in patients with severe OSAHS (AHI > 30) were calculated for CPAP-treated and untreated patients using the results of a long-term observational study by Marin et al. 130 The untreated patients comprised those who had refused CPAP treatment on initial referral to the sleep clinic. The baseline characteristics of these patients may differ compared with the treated patients for reasons other than chance, thus undermining the internal validity of the study results. Results were extrapolated from years 12–14. No method of extrapolation was reported. The authors justified the use of a 14-year time horizon because 14 is divisible by seven: the estimated NHS shelf-life of CPAP according to the authors. ResMed used the Mar et al. 123 study to estimate the ratio of CHD and stroke in patients with untreated severe OSAHS as 1.185 and 1.353 respectively compared with treated OSAHS. Therefore, they estimated the ratio of developing CHD to stroke as 1 : 1.13. Based on the same data, ResMed estimated the ratio of CHD to stroke in treated patients as 1 : 1. Thus, treatment with CPAP was assumed to reduce the incidence of CHD and stroke, and to reduce the proportion of total CHD and stroke events. Using these estimates, ResMed calculated the annual risk of CVE and stroke.
To estimate the risk of an RTA, ResMed took the average risk increase of an RTA in patients with OSAHS based on two studies. One study assessed RTAs in patients with OSAHS before and after treatment with CPAP (George et al. ). 131 Patients were followed-up for at least 3 years. The other study (Mazza et al. 132) measured driving ability in OSAHS patients before and after CPAP treatment using a ‘road safety platform’ (i.e. a stretch of road to test driving ability). The risk of an RTA was estimated as 2.6 times greater than the risk among controls, whereas the risk among treated patients was assumed to be equivalent to that among controls. Using data from the Department of Transport133 and assuming that all OSAHS patients were drivers of a licensed motor vehicle, ResMed estimated that the risk of an RTA among the control group and treated OSAHS patients was 0.009 per year and 0.023 per year for untreated OSAHS patients.
ResMed reviewed the published literature to obtain data on compliance among OSAHS patients with fixed CPAP. Compliance was defined as the percentage of patients with OSAHS of all severity levels who have not discontinued using their CPAP device. ResMed estimated that 79% of patients would continue to use CPAP after the first year of treatment, based on the results of four studies that followed patient compliance for at least 1 year. 39,40,134,135 It took the average compliance across the studies, the follow-up time for which varied between 2 and 7 years. For patients who continued their use of the device for at least 1 year, it was assumed that there would be no further loss to compliance, based on expert clinical opinion. For autotitrating CPAP it was estimated that compliance would be 84%. The increase in compliance with autotitrating CPAP compared with fixed CPAP was based on expert opinion.
Summary of resource utilisation and cost data
The opinion of 19 clinical experts was sought in order to estimate the health-care resource use associated with the management of OSAHS in the UK. Resource use and unit costs were reported separately and these are detailed in Tables 15 and 16 respectively. Unit costs were reported in 2005 prices and were based on list prices, ResMed estimates, the published literature and government statistics. Costs were calculated by multiplying the resource use by the relevant unit costs (Table 17). Confidence intervals were calculated based on the resource use estimates provided by clinical experts.
| Resource use |
Probability (95% CI) |
|---|---|
| Probability of having an initial outpatient visit before a diagnostic sleep study | 0.31 (0.11–0.51) |
| Probability of one outpatient visit after a diagnostic sleep study | 0.69 (0.49–0.89) |
| Probability of having a home sleep study | 0.75 (0.59–0.90) |
| Probability of having a home titration study | 0.99 (0.97–1.00) |
| Probability of having a titration study in hospital | 0.04 (0.00–0.05) |
| Probability of using CPAP (fixed) for titration | 0.19 (0.01–0.36) |
| Probability of using CPAP (auto) for titration | 0.81 (0.64–0.99) |
| Probability of seeing a consultant during the titration phase | 0.40 (0.05–0.52) |
| Probability of seeing a specialist nurse during the titration phase | 1.00 (0.53–1.00) |
| Probability of seeing a technician during the titration phase | 0.48 (0.10–0.93) |
| Probability of having a humidifier | 0.38 (0.22–0.50) |
| Probability of switching from fixed to autotitrating CPAP in the second year | 0.06 (0.04–0.07) |
| Probability of switching from fixed to autotitrating CPAP in subsequent years | 0.01 (0.00–0.02) |
| Probability of a non-compliant patient returning his or her machine | 0.75 (0.50–1.00) |
| Probability of having a follow-up visit within 3 months of starting CPAP | 0.75 (0.50–1.00) |
| Probability of having a follow-up visit within 4–6 months of starting CPAP | 0.75 (0.75–1.00) |
| Probability of annual follow-up visits after starting CPAP with a consultant | 0.13 (0.00–0.27) |
| Probability of annual follow-up visits after starting CPAP with a specialist nurse | 0.61 (0.33–0.79) |
| Probability of annual follow-up visits after starting CPAP with a technician | 0.26 (0.09–0.54) |
| Probability of a dead patient’s machine being returned | 0.90 (0.75–1.00) |
| Resource | Cost | Source |
|---|---|---|
| Myocardial infarction episode | £1694.51 | Department of Health, 2005137 |
| Home-based cardiac rehabilitation for the first year following a myocardial infarction | £3702.49 | Taylor et al., 2006138 |
| Stroke episode | £1667.23 | Department of Health, 2005137 |
| Annual cost of stroke rehabilitation | £10,140 | Department of Health, 2005139 |
| Initial outpatient visit with specialist | £115.00 | Department of Health, 2005137 |
| Follow-up outpatient visit with specialist | £108.00 | Department of Health, 2005137 |
| Initial sleep study | £115.35 | Department of Health, 2005137 |
| Follow-up sleep study | £107.87 | Department of Health, 2005137 |
| Specialist nurse visit (30-minute appointment) | £34.00 | Department of Health, 2005137 |
| Technician visit (30-minute appointment) | £9.50 | Department of Health, 2005137 |
| Fatal car accident | £5688.23 | Department of Transport, 2004133 |
| Serious/slight car accident | £12,019.89 | Department of Transport, 2004133 |
| CPAP S8 Escape | £280.00 | ResMed121 |
| APAP S8 AutoSet Spirit | £410.00 | ResMed121 |
| HumidAire H3i (Humidifier) | £150.00 | ResMed121 |
| Ultra Mirage II Nasal (Mask) | £80.00 | ResMed121 |
| Miscellaneous spare parts for CPAP | £100.00 | Estimate |
| Cost of using CPAP (auto) for dose titration | £2.51 | Estimate |
| Cost of using CPAP (fixed) for dose titration | £1.71 | Estimate |
| Nightly cost of using Embletta X10 for a diagnostic sleep study | £6.69 | Estimate |
Examination of the electronic model submitted by ResMed revealed a number of modelling errors that may have affected the ability of the model to provide an accurate estimate of mean costs and QALYs. The beta distributions used to characterise the uncertainty around transition probabilities and utilities were mis-specified. The alpha and beta parameters were correctly estimated from the mean and SD, but the scale parameter was set equal to the mean, effectively truncating the distributions at the mean. This meant that for the probabilistic analysis the mean and SD of every transition probability was lower than indicated by the source data. In addition, a number of other distributions were truncated; for example, the relative risk of an RTA was modelled as a normal distribution with mean = 2.6, SD = 0.26, and truncated at a lower limit of 2.4 and an upper limit of 2.9. The reason for the truncation is unclear, and it effectively reduces the uncertainty around the model input parameters. The uncertainties around resource use and cost data were characterised using normal distributions, which allow negative values to be drawn for simulations in the probabilistic analysis.
The numbers of CHD and stroke events were calculated as a proportion of the number of patients alive in the first year of the model for every cycle rather than as a proportion of those patients at risk at a given time point. This led to an overestimate of the number of events, as patients who die as the model progresses are not removed from the pool of those at risk.
The proportion of males in the hypothetical patient population was modelled with uncertainty. Risks that differed according to gender, such as mortality risks, were averaged according to the proportion of males and females at the start of the model. Averaging over heterogeneous subgroups in this manner can produce misleading results. For example, as the risk of death is greater among men, the proportion of men in the hypothetical patient population would be expected to fall over time. By not reflecting this in the model the number of deaths will be overestimated.
A number of other minor errors were also found. The lack of internal validity indicates that the model results should be interpreted with caution.
Summary of cost-effectiveness
The expected outcomes associated with severe OSAHS at 14 years from commencement of treatment with CPAP compared with no treatment are provided in Table 18. Based on the Markov model, differences in health gain between patients receiving CPAP and patients who are untreated becomes apparent after 2–3 years of treatment.
The primary cost driver in patients with severe OSAHS was managing stroke [i.e. 68% of the total cost for the no treatment group, 48% for the CPAP (fixed) and 45% for CPAP (auto) groups]. The secondary cost driver in untreated patients was the cost associated with managing RTAs (i.e. 23% of the total cost for the no treatment group), whereas in CPAP-treated patients it was the cost associated with the device itself [i.e. 22% for the CPAP (fixed) and 26% for CPAP (auto) groups].
| CPAP (fixed) | CPAP (auto) | |
|---|---|---|
| Year 1 | ||
| Initial outpatient visit | £35.65 | £35.65 |
| Diagnostic sleep study at home | £5.02 | £5.02 |
| Diagnostic sleep study at hospital | £115.35 | £115.35 |
| Follow-up outpatient visit after sleep study | £74.52 | £74.52 |
| Dose titration study (home) | £2.34 | – |
| Dose titration study (inpatient) | £4.31 | – |
| Consultant visit during titration phase | £43.20 | – |
| Specialist nurse visit during titration phase | £38.08 | – |
| Technician visit during titration phase | £90.25 | – |
| CPAP machine | £280.00 | £410.00 |
| Mask | £80.00 | £80.00 |
| Humidifier | £57.00 | £57.00 |
| Sundries | £100.00 | £100.00 |
| Follow-up visit within 3 months of starting CPAP | £81.00 | £81.00 |
| Follow-up visit within 4–6 months of starting CPAP | £81.00 | £81.00 |
| Year1 total | £1087.72 | £1039.54 |
| Years 2–7 and 9–14 | ||
| Follow-up outpatient visit | £37.25 | £37.25 |
| Replacement mask | £80.00 | £80.00 |
| Sundries | £100.00 | £100.00 |
| Total for each year (2–7 and 9–14) | £217.25 | £217.25 |
| Year 8 | ||
| Follow-up outpatient visit | £37.25 | £37.25 |
| Replacement CPAP machine | £280.00 | £410.00 |
| Replacement mask | £80.00 | £80.00 |
| Replacement humidifier | £57.00 | £57.00 |
| Sundries | £100.00 | £100.00 |
| Year 8 total | £554.25 | £684.25 |
| Outcome | No treatment | CPAP (fixed) | CPAP (auto) |
|---|---|---|---|
| CVE | 0.57 (0.55–0.66) | 0.74 (0.69–0.80) | 0.78 (0.73–0.81) |
| Stroke | 0.35 (0.20–0.53) | 0.16 (0.08–0.26) | 0.14 (0.07–0.25) |
| RTA | 0.39 (0.23–0.60) | 0.17 (0.08–0.29) | 0.15 (0.07–0.28) |
| Event-free survival | 0.30 (0.15–0.47) | 0.63 (0.52–0.73) | 0.68 (0.56–0.74) |
| QALYs | 7.22 (6.85–7.62) | 8.19 (7.79–8.69) | 8.32 (7.97–8.81) |
Key results of the cost-effectiveness model are shown in Table 19. The incremental cost-effectiveness ratio (ICER) was based on deterministic analysis and over 14 years it was estimated that CPAP dominates no treatment (i.e. CPAP was associated with lower costs and higher effects than no treatment). However, at 1 year, the cost per QALY for CPAP is expected to exceed £20,000. After 2 years the expected cost per QALY gained is £10,000 or less, and after 11 years CPAP is the dominant treatment. CPAP (fixed) was compared with no treatment and CPAP (auto) was compared with no treatment. Based on this indirect analysis, the authors found that CPAP (auto) dominated CPAP (fixed).
| Model result | CPAP (fixed) | CPAP (auto) | No treatment |
|---|---|---|---|
| Base case | |||
| Cost per QALY gained compared with no treatment |
–£1620 95% CI –£4123 to £259 |
–£1845 95% CI –£3936 to £37 |
– |
| Cost per life-year gained compared with no treatment |
–£9215 95% CI –£19,824–£22,224 |
–£9845 95% CI –£18,530 to £218 |
– |
| Cost per event-free life-year gained |
–£4813 95% CI –£10,195 to £1158 |
–£5441 95% CI –£10,005 to £135 |
– |
| Secondary analysis | |||
| Cost per QALY gained compared with no treatment when clinical outcomes relating to cardiovascular and cerebrovascular events are excluded |
£2311 95% CI £483–£3254 |
£2173 95% CI £460–£2912 |
– |
| Cost per QALY gained compared with no treatment when clinical outcomes relating to cardiovascular and cerebrovascular events and RTAs are excluded |
£4551 95% CI £2259–£6597 |
£4219 95% C £2124–£5799 |
– |
| Expected alive | 74% | 78% | 57% |
| Increased probability of survival compared with no treatment | 26% | 32% | – |
| Expected event-free survival | 63% | 68% | 30% |
| Increased probability of event-free survival compared with no treatment | 100% | 113% | – |
| Reduction in relative risk of having a CVE compared with no treatment | 55% | 60% | – |
| Reduction in relative risk of having a stroke compared with no treatment | 57% | 63% | – |
| Reduction in relative risk of having an RTA compared with no treatment | 36% | 41% | – |
| Expected cost (NHS perspective) per patient |
£9086 95% CI £6851–£11,117 |
£8622 95% CI £6712–£10,947 |
£10,645 95% CI £7912–£14,177 |
| Reduction in NHS management costs compared with no treatment | 15% (£1559) | 19% (£2,023) | – |
Several univariate sensitivity analyses were undertaken and demonstrated that the cost-effectiveness of CPAP was robust to changes in some input values. However, the model was sensitive to the following parameters: time to start of treatment, compliance rate with CPAP, risk of having a cardiovascular event or a cerebrovascular event, risk of having an RTA, utility for treated and untreated OSAHS, cost of managing a non-fatal RTA and cost of managing stroke rehabilitation.
The results of the univariate sensitivity analyses are reported in Table 20. No rationale was provided for the ranges over which input values were varied.
| Scenario | Base-case value | Effect |
|---|---|---|
| Model results that are sensitive to input values | ||
| Time to start of treatment: range 4.8 months to 12.0 months | 8.400 | Relative cost–utility changes marginally but CPAP (fixed) and CPAP (auto) remain dominant vs no treatment |
| Compliance rate with CPAP (fixed): range 0.5–1.0 | 0.790 | Relative cost–utility of CPAP (fixed): range £703 to –£2543; at a compliance rate < 0.6 CPAP (fixed) is no longer dominant treatment; relative cost–utility of CPAP (auto) remains unchanged |
| Compliance rate with CPAP (auto): range 0.7–1.0 | 0.790 | Relative cost–utility of CPAP (auto): range –£1,574 to –£2564; relative cost–utility of CPAP (fixed) remains unchanged |
| Risk of CVEs among treated and untreated patients: range 50% below to 75% above the base-case value | 1.000 | As risk increases, relative cost–utility of CPAP (fixed) ranges from £24 to –£2843, CPAP (auto) ranges from –£218 to –£2999; if risk falls to 60% below the base-case value, CPAP (fixed) ceases to be dominant |
| Ratio of CVEs to stroke among untreated OSAHS patients: range 1 : 4 to 1 : 0 | 0.47 : 0.53 | As the ratio increases, the relative cost–utility of CPAP (fixed) ranges from –£404 to –£3534 and of CPAP (auto) ranges from –£870 to £3374 |
| Risk of untreated OSAHS patients having an RTA: range 1.0–5.0 above background rate | 2.600 | As risk increases, relative cost–utility of CPAP (fixed) ranges from –£498 to –£3107 and of CPAP (auto) ranges from –£812 to £3244 |
| Utility for untreated OSAHS: range 0.5–0.9 | 0.738 | If utility falls below 0.68 CPAP ceases to be most cost-effective alternative, assuming treated OSAHS unchanged |
| Utility for treated OSAHS: range 0.5–0.9 | 0.811 | If utility rises above 0.89 CPAP ceases to be most cost-effective alternative, assuming untreated OSAHS unchanged |
| Utility of non-fatal stroke in untreated OSAHS patients: range 0.5–0.9 | 0.590 | As utility increases, relative cost–utility of CPAP (fixed) ranges from –£1532 to –£2020 and of CPAP (auto) ranges from –£1751 to –£2262 |
| Utility of non-fatal stroke in treated OSAHS patients: range 0.5–0.9 | 0.649 | As utility increases, relative cost–utility of CPAP (fixed) ranges from –£1694 to –£1510 and of CPAP (auto) ranges from –£1914 to £1739 |
| Utility of non-fatal CVE in untreated OSAHS patients: range 0.5–0.9 | 0.664 | As utility increases, relative cost–utility of CPAP (fixed) ranges from –£1565 to –£1708 and of CPAP (auto) ranges from –£1787 to £1936 |
| Utility of non-fatal cardiovascular event in treated OSAHS patients: range 0.5–0.9 | 0.730 | As utility increases, relative cost–utility of CPAP (fixed) ranges from –£1646 to –£1602 and of CPAP (auto) ranges from –£1858 to £1835 |
| Utility of non-fatal RTA in untreated OSAHS patients: range 0.5–0.9 | 0.701 | As utility increases, relative cost–utility of CPAP (fixed) ranges from –£1573 to –£1901 and of CPAP (auto) ranges from –£1792 to £1901 |
| Utility of non-fatal RTA in treated OSAHS patients: range 0.5–0.9 | 0.771 | As utility increases, relative cost–utility of CPAP (fixed) ranges from –£1649 to –£1607 and of CPAP (auto) ranges from –£1876 to £1831 |
| NHS cost of cardiac rehabilitation in the first year following a non-fatal CVE: range £1851–£7404 | £3702 | As cost increases, relative cost–utility of CPAP (fixed) ranges from –£1445 to –£1970 and of CPAP (auto) ranges from –£1677 to –£2180 |
| Annual NHS cost of stroke rehabilitation following non-fatal stroke: range £5070–£20,280 | £10,140 | As cost increases, relative cost–utility of CPAP (fixed) ranges from –£287 to –£4286 and of CPAP (auto) ranges from –£456 to £4623 |
| NHS cost of managing a non-fatal RTA: range £6000–£22,000 | £12,020 | As cost increases, relative cost–utility of CPAP (fixed) ranges from –£1113 to –£2461 and of CPAP (auto) ranges from –£1384 to –£2609 |
| Model results that are not sensitive to input values | ||
| Per cent of OSAHS patients who are male: range 0.5–1.0 | 0.700 | Relative effect unchanged |
| Probability of having a home diagnostic sleep study: range 0.5–1.0 | 0.750 | Relative effect unchanged |
| Probability of having a home titration study: range 0.5–1.0 | 0.990 | Relative effect unchanged |
| Probability of switching (fixed) to (auto) CPAP in the 2nd year: range 0.0–0.1 | 0.060 | Relative effect unchanged |
| Probability of a clinician visit within 3 months of starting treatment: range 0.5–1.0 | 0.750 | Relative effect unchanged |
| Probability of an annual visit with a consultant: range 0.0–0.5 | 0.130 | Relative effect unchanged; relative cost–utility of CPAP (fixed) and CPAP (auto) changes marginally |
| Probability of an annual visit with a specialist nurse: range 0.4–1.0 | 0.610 | Relative effect unchanged |
| Initial NHS cost of managing a CVE: range £1000–£2500 | £1695 | Relative effect unchanged; relative cost–utility of CPAP (fixed) and CPAP (auto) changes marginally |
| Initial NHS cost of managing an episode of stroke: range £1000–£2500 | £1667 | Relative effect unchanged; relative cost–utility of CPAP (fixed) and CPAP (auto) changes marginally |
| NHS cost of managing a fatal RTA: range £2000–£8000 | £5688 | Relative effect unchanged |
| Annual discount: rate 0–6% | 3.5% | Relative effect unchanged; relative cost–utility of CPAP (fixed) and CPAP (auto) changes marginally |
Cost-effectiveness acceptability curves (CEACs) were presented for CPAP (auto) and CPAP (fixed). Each was assessed in a pair-wise comparison against a ‘do nothing’ alternative. The CEAC showed that CPAP (auto) has a marginally higher probability of being cost-effective compared with a ‘do nothing’ option than CPAP (fixed) compared with a ‘do nothing’ option at a willingness to pay threshold of less than £5000 per QALY in all simulations.
Comments on methodology
ResMed used the results of a before and after study (Mar et al. , 2003)123 to examine the impact of no treatment compared with CPAP on HRQoL associated with sleepiness. There are numerous weaknesses associated with before and after data which might undermine results. A number of factors may bias and confound the results, for example a placebo effect, a Hawthorne effect, regression to the mean and/or co-intervention. As Chapter 3 demonstrates, a considerable RCT-based literature exists which examines the efficacy and effectiveness of CPAP compared with other therapies in the treatment of OSAHS. The study by Mar et al. 123 did not report the change in ESS or any other measure in the utility study that would have allowed comparison with the results of the systematic review of trial evidence in Chapter 3.
ResMed did not include the full range of comparators that are discussed in Chapter 3. For patients with diagnosed severe OSAHS it is not clear that a ‘do nothing’ option represents routine clinical practice. Incremental cost-effectiveness analysis examines the relative cost-effectiveness of treatment options. It is possible that by comparing CPAP with a ‘do nothing’ alternative, the comparative benefit of CPAP is increased, compared, for example, with dental devices. ResMed briefly describes the recent systematic review by Giles et al. 50 This review suggests that symptoms post treatment did not show a significant difference between CPAP and dental devices. However, Giles et al. also suggest that additional data are required. 50
ResMed modelled cost-effectiveness results over a 14-year time horizon. However, OSAHS is a chronic condition; therefore, given the NICE guidelines, it is appropriate to model the results for a lifetime horizon. ResMed used a 14-year time horizon for analytical convenience. It was assumed that the device life of CPAP was 7 years and a 14-year time horizon is a multiple of seven. The device life of CPAP was not tested in the univariate sensitivity analysis. However, it was found that results were sensitive to the second most important cost driver in CPAP-treated patients: the cost associated with the device itself [i.e. 22% for CPAP (fixed) and 26% for CPAP (auto)]. Ayas et al. 122 and Mar et al. 123 assumed the device life of CPAP to be 5 years. The shorter the device life, the greater the cost associated with the relevant treatment.
There were shortcomings in the internal validity of the electronic model that may have led to inaccurate estimates of costs and QALYs.
Review of published economic evaluations
Review of Ayas et al.122
Overview
Ayas et al. 122 performed a cost–utility analysis comparing CPAP with a ‘do nothing’ alternative for the treatment of patients with moderate to severe OSAHS. The base-case analysis was patients aged between 25 and 54 years who were newly diagnosed with moderate to severe OSAHS, classified as having an AHI ≥ 15 events per hour. The analysis was undertaken from the US third-party payer perspective and the societal perspective.
The authors developed a Markov model with a 5-year time horizon. Each Markov cycle lasted 1 year. The primary outcome measure used in the analysis was incremental cost per QALY. The QALY estimate for CPAP incorporated the expected gains in HRQoL due to a reduction in RTAs. Effectiveness, utility and resource use estimates were obtained from the published literature and administrative databases. A number of univariate sensitivity analyses were undertaken. In addition, the authors undertook a probabilistic sensitivity analysis.
Summary of effectiveness data
For the base-case analysis, QALY estimates were obtained using utilities valued based on the standard gamble, in patients with OSAHS, pre and post CPAP therapy (Chakravorty et al. 97) Therefore, patient preferences rather than societal preferences were used for the valuations. Overall, a weighted average of the findings was obtained for patients by age group and sex. These were adjusted for the impact of CPAP on RTAs and the impact of death due to natural causes, as explained below.
Estimates of effect were calculated based on the proportion of patients in one of six patient groups (i.e. ages 25–34, 35–44, 45–54 by sex), reflecting the distribution found in a sample of 99 patients with OSAHS: a distribution comparable to that in the United States. The probabilities of RTAs were stratified by the relevant patient groups. An RTA could either result in a fatality or property damage only or be injury related. The injury-related RTAs were stratified into one of five levels on the Maximum Abbreviated Injury Scale (MAIS), with scores ranging from one (minimal injury) to five (most severely injured). Of the patients having an RTA, the severity of injury was estimated as 85.6%, 10.5%, 3.3%, 0.4% and 0.2% for severity levels 1–5. RTA survivors in level 5 MAIS state were assumed to be unable to drive again and therefore were confined to a tunnel state in the model.
The effect of CPAP on RTAs was based on a random-effects meta-analysis of eight observational studies in which actual RTAs were observed in patients pre and post initiation of CPAP (Table 21). 131,136–142 It excluded driving simulator studies. A pooled reduction in RTA risk and an improvement in HRQoL due to CPAP were calculated by a random-effects meta-analysis using the inverse variance of the logarithm of the odds ratio. It was assumed that the RTA crash rate in OSAHS patients who received CPAP equalled that in the general population. The RTA rates in OSAHS patients who were not receiving CPAP were obtained taking the general population RTA rates and dividing these by the proportionate reduction in RTAs associated with CPAP therapy.
| Source | Country | Number of patients | Mean AHI | Mean age | Definition of crash | Rates of RTA | |
|---|---|---|---|---|---|---|---|
| CPAP | No CPAP | ||||||
| George, 2001131 | Canada | 210 | 54 | 52 | From provincial insurance database | CPAP | No CPAP |
| Findley et al., 1988137 | US | 50 | 37 | 56 | State DMV (injury or property damage > $500) | 0.06 per year | 0.18 per year |
| Krieger et al., 1997136 | France | 547 | 59.8 | 56.6 | Self-report | 0 per year | 0.07 per year |
| Engleman et al., 1996138 | Scotland | 215 | 47 | 53 | Self-report (major incidents) | 0.0256 per year | 0.084 per year |
| Horstmann et al., 2000139 | Switzerland | 85 | NA | NA | Self-report | 0.001 per 16,000 km driven | 0.005 per 16,000 km driven |
| Suratt and Findley, 1992140 | US | 22 | NA | NA | NS | 2.7 per 1,000,000 km driven | 10.6 per 1,000,000 km driven |
| Cassel et al., 1996141 | Germany | 59 | 38.9 | 49 | Self-report | 0.023 per year | 0.30 per year |
| Yamamoto et al., 2000142 | Japan | 39 | 55.7 | 48 | Self-report | 0.14 per 100,000 km driven | 0.8 per 100,000 km driven |
Based on one study (Chakravorty et al. 97) it was assumed that the average utility in patients receiving CPAP was 0.55, an increase in utility of 0.23 compared with baseline (no CPAP). The utilities were valued using the standard gamble. Quality weights for the five MAIS injury levels were obtained using the Functional Capacity Index (FCI), which used rating scale preferences from patients who had functional limitations exceeding 1 year. The FCI weights were applied using similar methods to those of Graham et al. 143
The transition probability of death was estimated by applying the yearly, sex-specific probability of an RTA death to the rate of death due to natural causes, based on US life tables.
Summary of resource utilisation and cost data
The base-case analysis included third-party payer, direct medical costs only. For the first year, the total cost of CPAP was calculated using the US Medicare fee schedule. For year 2 and year 5, ongoing annual CPAP cost components were included. The costs included those of the device and the time of medical and emergency specialists. For the first 15 months, rental fees were applied based on Medicare guidelines. Following this, patients incurred a rental fee. The CPAP machine was assumed to have a device life of 5 years.
For the analysis undertaken from a societal perspective, productivity losses associated with RTAs were also included, e.g. losses in household and market productivity, and associated workplace costs were calculated using the human capital approach. Non-medical costs including legal costs and insurance administration costs were also included. Societal costs of RTAs were stratified by level of severity using the MAIS scale and were based on national data. Lifetime costs of RTA outcomes and costs were based on public sources. In addition, it was assumed that all RTA costs were uniformly distributed over a future of 40 years for all patient groups. Unit costs were reported separately from resource use. Costs were reported for the year 2003. A discount rate of 3% was applied to the costs and effects for the base-case analysis.
Patient compliance with CPAP was included in the analysis. A compliance rate of 70% was assumed, based on findings in one article (McArdle et al. 39) It was assumed that non-compliant patients incurred rental costs for the device and the cost of a single visit to their doctor over a 3-month period but did not benefit from the device over the period.
Summary of cost-effectiveness
From a third-party payer perspective, CPAP was more costly and more effective than no CPAP and the ICER was $3354 per QALY (95% CI $1062 per QALY to $9715 per QALY). From the societal perspective CPAP was also found to be more costly and more effective and had an ICER of $314 (95% CI cost saving to $6114). Based on the probabilistic sensitivity analysis, if the value of society’s willingness to pay for a QALY is $50,000, 100% of the 1000 Monte Carlo simulations favoured the cost-effectiveness of CPAP from the third-party payer and the societal perspective. From the societal perspective, 42% of the ICERs from the simulations indicated that CPAP was dominant, i.e. it was associated with lower costs and greater effects than was no treatment.
Based on the univariate sensitivity analyses, ICER estimates were shown to be robust to many assumptions associated with the parameter estimates chosen. The analytical perspective had a substantial influence on the ICER, resulting in a more than 10-fold higher ICER when comparing the third-party payer perspective with the societal perspective. The type of utility estimate used also had considerable influence on the ICER. When EQ-5D utility estimates were used in place of standard gamble estimates, the ICER estimate increased more than fivefold.
Comments on Ayas et al.122
A single study (Chakravorty et al. 97) was used to measure the treatment effect of CPAP on sleepiness and hence HRQoL. This treatment effect was obtained from one arm of an RCT, with the pre-CPAP utility estimate used for the no treatment arm and the 3-month post-CPAP utility estimate used for the CPAP arm. As revealed by the systematic review (see Chapter 3), a considerable number of other data are available on the treatment effect of CPAP. Since only one arm of an RCT was used, in effect the data were subject to the same limitations associated with the before and after study design as mentioned in the discussion of the ResMed submission. It is worth noting that the mean change in ESS in the study arm used to inform the utility estimates was –8, which is considerably greater than the reduction with CPAP indicated by the weight of evidence incorporated in the systematic review in Chapter 3.
The impact of CPAP on RTAs was based on eight before and after studies, pre and post CPAP. Another influence that might undermine the results derived from this study design is referral bias. Patients may have been referred for suspected sleep-disordered breathing because they were involved in an RTA, thereby falsely inflating rates prior to using CPAP. The authors undertook a sensitivity analysis, using the odds ratio of the lower end of the confidence interval, which gave an ICER of around $3530 per QALY. The utility values were adjusted for various MAIS injury levels using the FCI. FCI weights represent rating scale preferences, whereas the NICE guidance focuses on choice-based measures of valuation.
For the base-case analysis, patient preferences were used to estimate utilities, based on the standard gamble technique. NICE methods guidance focuses on the use of societal preferences to inform health-care decision-making. However, it is worth noting that the authors applied EQ-5D estimates based on societal preferences in a sensitivity analysis. 123,144 The resultant CPAP ICER was within ranges typically considered to be cost-effective.
CPAP is a chronic condition; therefore, it would have been appropriate to extrapolate results over the life course.
Review of Mar et al.123
Overview
Mar et al. 123 performed a cost–utility analysis comparing nCPAP with a ‘do nothing’ alternative for the treatment of patients with OSAHS. The base case was defined as a 50-year-old male patient with moderate to severe OSAHS, classified as having an AHI ≥ 30 per hour and an ESS > 10. The analysis was undertaken from the Spanish health-care system perspective.
The authors developed a semi-Markov (time-varying) model with a time horizon of 5 years and over the lifetime. Each Markov cycle lasted 1 year. The primary outcome measure used in the analysis was incremental cost per QALY. The QALY estimate for nCPAP incorporated the expected gains in HRQoL due to a reduction in stroke and CHD as well as the impact of these events on mortality and RTAs. At the end of each cycle patients could be in an OSAS state, have a non-fatal stroke or non-fatal CHD or die. During the cycle, patients could experience a temporary event: a stroke, CHD or an RTA. Cost estimates were also adjusted for the reduced risk of these three events. Effectiveness, utility and resource use estimates were obtained from the published literature and administrative databases. A number of univariate sensitivity analyses were undertaken. In addition, the authors undertook a probabilistic sensitivity analysis.
Summary of effectiveness data
The effect of CPAP on stroke, CHD, RTAs and HRQoL was incorporated in a series of steps. To model the reduced risk of stroke and CHD due to nCPAP compared with no CPAP, it was assumed that use of nCPAP returned blood pressure to its pre-OSAHS levels, as a consequence of the reduction in AHI by nCPAP. Based on an assessment of CVE risk, it was assumed that OSAHS patients with an AHI of greater than 30 had an increase in diastolic arterial pressure of 3.6 mmHg. It was estimated that increases in blood pressure were linearly correlated with the natural logarithm of the risk of stroke and CHD, based on MacMahon et al. 145 Therefore, applying this to the 3.6 mmHg increase in diastolic pressure, the natural logarithms of the risk could be obtained, thus estimating the relative risks of stroke and CHD. It was assumed that relative risk of these events remained constant at a given blood pressure and that absolute risk was best estimated by applying this relative risk to the baseline absolute risk for patient and age and sex using rates tables.
Mortality rates for stroke, CHD, RTAs and all causes by age and sex were based on an administrative database from the Basque country, Spain. The transition probabilities from OSAHS to death were based on the mortality rates of all causes, excluding stroke, CHD and RTAs. Age- and sex-specific mortality rates for the general population were multiplied by the corresponding relative risks to calculate the corrected rate for nCPAP and no CPAP groups (Table 22).
| Probabilities (relative risks) | Untreated OSAS patients | nCPAP OSAS patients |
|---|---|---|
| CHD | 1.185 | 1.0 |
| Stroke | 1.353 | 1.0 |
| Car accident | 8.1 | 1.0 |
| Death after stroke | 1.1 | 1.1 |
| Death after CHD | 1.1 | 1.1 |
Utility values were obtained as described in Summary of effectiveness review for the ResMed submission (see pp. 54–5).
Summary of resource utilisation and cost data
The cost analysis was undertaken from the health-care perspective. The following direct costs were considered: costs of investigation, diagnosis and treatment of OSAHS and costs attributable to CVE morbidity, costs associated with in-home technical maintenance and medical follow-up. The nCPAP was assumed to have a device life of 5 years. Data relating to the last 5000 patients who had suspected OSAHS and who attended a sleep clinic (in the Basque country) were used to estimate the cost of diagnosis and the proportion of patients abandoning treatment during the first year. It was assumed that no benefits of treatment accrued to these patients.
Costs were reported for the year 2000 and were converted from Spanish pesetas to euros. A discount rate of 3% was applied to the costs and effects for the base-case analysis.
Summary of cost-effectiveness
The base-case analysis found that CPAP was more costly and more effective than no CPAP. The ICER for CPAP was €7861 per QALY over a 5-year time horizon and €4938 per QALY for the lifetime horizon.
Based on the univariate and multivariate sensitivity analyses, ICER estimates were robust to many assumptions associated with the parameter estimates chosen and remained in the region of €5000–€10,000. The only case where the ICER was found to exceed €20,000 per QALY was for a worst-case scenario analysis in which the authors used the lower limit of the CI obtained from the patient preference utility survey and a 5- year time horizon. As anticipated, the authors found that the cost-effectiveness ratio increases as the cost of nCPAP increases. For example, the authors assessed the impact of an increase in diagnostic costs on cost-effectiveness and the impact of two different types of diagnostic protocol on the ICER. As well as this, they presented disaggregated incremental effectiveness data (for CVE risk, RTAs and utility effect) and disaggregated incremental cost information for a 50-year-old male using nCPAP for both a 5-year and a lifespan time horizon. They found that the results were very sensitive to the time horizon specified. Over the lifespan of the patient, improvements in quality of life accounted for 84% of the incremental effectiveness of nCPAP. The purchase and maintenance costs of nCPAP accounted for 86% of the overall incremental costs. When the time horizon was reduced to 5 years, these costs amounted to 98% and 61% of the overall incremental costs respectively.
Overall, therefore, the authors suggested that nCPAP is cost-effective in patients with an AHI ≥ 30 and who also exhibit symptoms of daytime sleepiness. The authors suggested that the improvement in HRQoL associated with nCPAP is the main force behind its clinical effectiveness, as measured in QALYs, being seven times greater than that of reduced CVE mortality, which in turn is seven times greater than that of decreased numbers of RTAs. The authors suggested that the remaining uncertainties about the impact of nCPAP on long-term mortality have relatively little impact on the clinical and economic efficiency of treatment.
Comments on Mar et al.123
Data from a single survey, conducted as part of the study, were used to measure the treatment effect (in the form of change in sleepiness in terms of utilities) of CPAP versus no CPAP. As mentioned in the comments on the Ayas et al. 122 study, a large number of other data were available but were not used to inform this estimate, and the data were based on a before and after design, which represents a weak source of evidence of effectiveness.
No account in the model was taken of the impact of an RTA on morbidity/utility or costs. It is not clear that the mortality rates for RTAs (or stroke or CHD) were related to OSAHS. Probabilistic sensitivity analysis was not undertaken.
Review of the Chilcott et al. Trent Report44
Overview
The Trent Report, written by Chilcott et al. ,44 provided the foundation for the work undertaken by Mar et al. 123 A cost–utility analysis was undertaken which compared nCPAP with a ‘do nothing’ alternative for the treatment of patients with OSAS. Summary results were reported comparing nCPAP and dental devices. The authors undertook the analysis based on a review of the literature and using clinical opinion. The analysis was undertaken from the UK health-care system perspective. The primary outcome measure used in the analysis was incremental cost per QALY. Cost estimates were also adjusted for the reduced risk of these three events. Effectiveness, utility, resource use estimates and costs were obtained from the published literature, administrative databases and clinical expert opinion. Several univariate sensitivity analyses were undertaken.
Summary of effectiveness data
No relevant utility data were available; therefore, an indirect approach was undertaken to estimate utilities. No data were reported on any change in ESS associated with treatment. The authors used SF-36 data generated by the Sleep Disorders Unit at the Royal Hallamshire Hospital in Sheffield. This involved a cohort study in which patients who were referred to the Unit for suspected OSAHS completed the SF-36 questionnaire before and after initiation of treatment, as reported in a conference abstract (Waterhouse et al. 146,147). Given that the data were not randomised, the authors attempted to validate them by comparing the results with data presented in Jenkinson et al. 77 They found that the before and after results were broadly similar for all SF-36 dimensions in the two studies. However, the population from the Waterhouse et al. 147 study appeared to have a lower initial baseline health status. The 1998 Brazier algorithm (Brazier et al. 148) (and another algorithm: no further details) was applied to the Waterhouse results to derive a preference-based single index measure of health. The QALY results at 1 year are reported in Table 23.
| Mean | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|
| All study participants | 0.10 | 0.07 | 0.12 |
| Participants offered long-term nCPAP treatment | 0.12 | 0.09 | 0.16 |
Summary of resource utilisation and cost data
The cost analysis was undertaken from the health-care perspective. As for the Mar et al. 123 analysis, the Trent Report considered the costs of investigation, diagnosis and treatment of OSAHS and maintenance and medical follow-up costs. In contrast to Mar et al. ,123 any costs attributable to CVE morbidity were not included in the analysis. The report did not mention the financial year of the cost data. The device life of nCPAP was estimated to be 5 years. A discount rate of 6% was applied to the costs and a discount rate of 1.5% was applied to effects for the base-case analysis.
Summary of cost-effectiveness
The results of the base-case analysis are reported in Table 24. The results were extrapolated on a time horizon of up to 5 years, assuming that the benefits accrued in the trial period were maintained.
| Time horizon | Cost per QALY gained |
|---|---|
| 1 month | £99,000 |
| 1 year | £8300 |
| 2 years | £5200 |
| 5 years | £3200 |
Several univariate sensitivity analyses were undertaken, comprising impact of the analytical time horizon; costs of investigation for nCPAP; long-term costs of maintenance, follow-up and other health-care resource usage; long-term impact of gross annual health-care costs; potential impact of improved mortality from use of nCPAP treatment; impact of uncertainty in morbidity benefits from nCPAP therapy; and discount rate. All estimates of cost-effectiveness over 1 year were < £16,000 per QALY gained.
The authors suggested that the cost-effectiveness of dental devices compared with no treatment was likely to be similar or worse than the cost-effectiveness of nCPAP therapy compared with no treatment. The costs of nCPAP and dental devices were similar. Based on two studies,80,149 they found small differences in clinical effectiveness and costs when comparing nCPAP with dental devices. The implied differences in costs and outcomes, and the considerable level of uncertainty associated with both, suggested to the authors that the incremental cost-effectiveness of nCPAP over dental advancement devices was likely to be highly uncertain.
Comments on the Trent Report
Data used to estimate effectiveness were short term and based on observational data (Waterhouse et al. , 2 weeks’ duration;146,147 Jenkinson et al. , 4 weeks’ duration77). No account was taken of the potential impact of nCPAP on the risk of CVE or RTAs in terms of costs or effects. The analysis was deterministic.
Review of Tousignant et al.124
Overview
Tousignant et al. 124 performed a cost–utility analysis retrospectively comparing the impact on HRQoL of pre-treatment with treatment using nCPAP. In this way nCPAP was compared with a ‘do nothing’ option in 19 patients with moderate to severe OSAHS. The study took place at a sleep clinic in Montreal, Canada.
Summary of effectiveness data
Patients attending a hospital sleep clinic (mean age 57 years, SD 10) and who had been receiving nCPAP treatment for an average of 9 months completed a standard gamble exercise. The health states valued were receiving treatment with nCPAP, pre-treatment, full health and immediate death. To assess the reliability of the results, patients completed the exercise on two occasions 2–3 weeks apart. The mean utility score for the pre-treatment health state was 0.63 (± 0.29) and the mean utility score for the nCPAP treatment health state was 0.87 (± 0.17). The intraclass correlation coefficients for the retest data were above 0.7 for both the treatment health state and pre-treatment health states. Patient life expectancy was estimated using Canadian life tables. The difference in utility pre and post treatment was multiplied by the life-years to calculate QALYs.
Summary of resource utilisation and cost data
The perspective of the cost analysis was not stated but appears to be the health-care system perspective. Costs included the cost of supplies and the rental and maintenance costs of the nCPAP device and associated devices (e.g. tubing and masks). It was estimated that the yearly cost of treating a patient in Quebec was CAN$2348 (price year not given). Costs also included the cost of one overnight sleep study at the outset of treatment at CAN$500, which included physician fees, technician salaries, supplies and amortisation of capital costs over 7 years. Alternatively, an ongoing cost of treatment per patient per year was estimated at CAN$800.
Summary of cost-effectiveness
Based on the use of the different cost estimates (above) a high estimate of CAN$9792 per QALY gained by nCPAP was calculated as well as a low estimate of CAN$3397 per QALY gained. Three patients had particularly large treatment effects. The authors explored the impact of excluding the three patients on the cost–utility ratio. Without the three patients, based on the high cost estimate, the cost–utility ratio increased to CAN$18,637 per QALY.
Comments on Tousignant et al.124
As all the patients were currently receiving nCPAP therapy, their valuation of the pre-treatment health state was done retrospectively. Given this, it is difficult to ascertain the extent to which the difference in pre-treatment and treatment utility scores is a real difference reflecting the impact of nCPAP treatment and the extent to which it reflects some sort of measurement error due to bias in recall. In addition, the results may be unreliable due to the weaknesses associated with observational data. It appears that costs (and effects) were not discounted to present values. For the most part, resource use estimates were reported separately from costs. Only the impact of treatment effects over the short term was considered. The analysis was deterministic.
Discussion of manufacturers’ submissions and published cost-effectiveness studies
Of the studies reviewed, none compared all therapies identified in the NICE scope, i.e. none compared CPAP with dental devices and conservative management. The NICE Reference Case states that costs included in the economic evaluation should be based on the NHS and PSS perspective. Only two studies (ResMed120 and Chilcott et al. 44) examined the treatment of OSAHS in the UK NHS context, the others focusing on the US,122 Spanish123 and Canadian124 health-care systems.
The existing cost-effectiveness studies had several limitations that need to be addressed in order to assess the value for money of these technologies. The key limitations were:
-
The cost-effectiveness studies did not use the full range of clinical trial evidence for estimating the impact of treatment on daytime sleepiness, blood pressure, HRQoL and other relevant outcomes.
-
There was a lack of trial-based evidence to compare the utility associated with different treatments for OSAHS.
-
There were limited data (in terms of quantity and quality) on the long-term impact of OSAHS on HRQoL, CVE, RTAs and other outcomes.
-
None of the evaluations examined all the comparators relevant to this review.
In an attempt to make full use of all of the available evidence on therapies for the treatment of OSAHS and in order to overcome some of the limitations noted above, a new cost-effectiveness model was developed.
York economic model
The objective of the York economic assessment was to assess the cost-effectiveness of CPAP by developing a clinically and economically appropriate decision model structured to characterise OSAHS and the impact of the different therapies. Several sources of evidence were used to inform the analysis. The model was developed using the methodological guidance for the NICE Reference Case (www.nice.org) as reported in Table 25. The development of the model was informed by research in the published literature including the clinical effectiveness systematic review reported in Chapter 3, published cost-effectiveness analyses, previously performed economic models and the advice of clinical experts participating in this technology assessment review. The methods used for decision modelling are based on those described in Briggs et al. 150
| Element of health technology assessment | Reference case |
|---|---|
| Defining the decision problem | Scope developed by the Institute |
| Comparator | Alternative therapies routinely used in the NHS |
| Perspective on costs | NHS and PSS |
| Perspective on outcomes | All health effects on individuals |
| Types of economic evaluation | Cost-effectiveness analysis |
| Synthesis of evidence on outcomes | Based on a systematic review |
| Measure of health benefits | QALYs |
| Description of health states for calculation of QALYs | Health states described using a standardised and validated generic instrument |
| Methods of preference elicitation for health state valuation | Choice-based method, e.g. time trade-off, standard gamble (not rating scale) |
| Source of preference data | Representative sample of the public |
| Discount rate | Annual rate of 3.5% on both costs and health effects |
| Equity provision | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit |
The new economic evaluation, undertaken by a team in York (and termed the York economic model from now on), is described in two parts. First, the methods used to perform the economic analysis are described; these comprise the structure of the model, the parameter estimates including a brief summary of the literature searches undertaken to inform the model, and the assumptions underlying the base-case analysis. Second, the results of the base-case analysis are presented and the role of parameter uncertainty is investigated by means of a probabilistic sensitivity analysis.
Methods of York economic model
Overview
A cost–utility analysis was undertaken that compared CPAP with use of dental devices and conservative management, using comparators relevant to the NHS. The scope developed by NICE indicated that CPAP devices should be treated as a single class of intervention, and this was reflected in the modelling. However, in the secondary analysis, various adjuncts to CPAP, e.g. the use of a humidifier, were examined to assess their impact on the costs associated with CPAP. The costs and QALYs associated with CPAP, dental devices and conservative management were compared over a lifetime time horizon as OSAHS is a chronic condition. The cost of the resource use associated with each intervention was estimated from the NHS and PSS perspective for England and Wales. Costs relating to the financial year 2005 were reported.
The health effects of OSAHS, and the impacts of alternative treatments, were expressed in terms of QALYs. Given the dearth of HRQoL data expressed in terms of utility in the randomised trials (see Chapter 3), it was necessary to estimate the relationships between clinical end points and QALYs using other data. Three clinical end points were related to QALYs. The first was difference in ESS between treatments; ESS was taken as the main measure of sleepiness given that it was reported in most trials, and in many it was the primary end point (see Chapter 3). The second clinical end point was differential treatment effect on blood pressure, which was reported in trials, and was related to CVE risks and hence to QALYs in the model. The third end point was differences in the risk of RTAs, which was based on non-randomised evidence and was related to QALYs in the model.
HRQoL in terms of utilities was expressed on the basis of generic HRQoL instruments, the EQ-5D and the SF-6D (a preference-based single-index measure for health, based on SF-36 and SF-12, allowing QALYs to be obtained for use in cost–utility analysis), and valued using the public preferences associated with those instruments. An annual discount rate of 3.5% was applied to costs and benefits to discount them to present values. The assumed target patient population is adults (16 years or older) with a diagnosis of OSAHS confirmed by use of an appropriate tool (e.g. the AHI or arterial oxygen desaturation index and the ESS). The model was run separately by age and sex, given the availability of age- and sex-specific mortality data and CVE risks. The base-case analysis is based on a male aged 50, as the average age of patients in the RCTs was around 50 [at baseline the mean age range was 44–58 years (see Chapter 3)] and the majority of participants in the included RCT studies were male.
The following analyses were undertaken to explore the robustness of the findings in the York economic model.
Base-case analysis
-
The base-case analysis compared the costs and QALYs of CPAP versus dental devices versus conservative management in a male aged 50 years.
-
Subgroup analyses were undertaken by gender, OSAHS severity (as measured by ESS) and other relevant baseline patient characteristics.
Secondary analysis
-
Scenario analyses undertaken to explore the impact on cost-effectiveness of:
-
[dash]excluding the impact of treatment on CVE
-
excluding the impact of treatment on RTAs
-
excluding the impact of treatment on both CVE and RTAs
-
change in ESS linked to SF-6D utility score rather than EQ-5D
-
relative risk reduction for CVE based on DBP and MacMahon et al. 145
-
autotitrating positive airway pressure (APAP) machine with 5-year life span and humidifier
-
treatment effects from bivariate random-effects meta-analysis (BRMA) (APBM only)
-
treatment effects from BRMA (APBM and office measurements).
-
-
Subgroup analyses
-
– for a cohort aged (35 and 65).
-
-
– Other relevant modelling assumptions.
-
– Uncertainty and value of information analysis.
The York economic model is fully probabilistic and the results from the model are presented probabilistically to reflect the implications of parameter uncertainty on decision uncertainty.
To inform research priorities, the expected value of perfect information (EVPI) was calculated for the decision problem. 150 This represents the value of obtaining perfect information on all the model parameters to eliminate the decision uncertainty (given acceptance of the model structure and evidence base). The EVPI can be compared with the potential costs of additional research to indicate whether there is value in further research to reduce the decision uncertainty.
Structure of the York economic model
A Markov state transition cohort model was developed in Microsoft® Excel 2002 and the Bayesian evidence synthesis was undertaken using WinBUGS 1.4 (the WinBUGS code is reported in Appendix 8). The structure of the model is shown in Figure 20. The model characterises the patient’s prognosis over his or her lifetime in terms of four health states: (1) OSAHS; (2) OSAHS post coronary heart disease (CHD); (3) OSAHS post stroke; and (4) death. Yearly cycles were chosen for the current model. The model records the ESS score of the hypothetical patient cohort and any change in ESS associated with treatment. As described in Chapter 3, the evidence suggests that interventions for OSAHS might have a beneficial effect on sleepiness, which may in turn affect the risk of RTAs. The trial data also describe the effect of treatment on blood pressure, which may in turn affect the incidence of CHD and stroke. Therefore, these events are included in the model. Based on the model structure, patients can remain in the initial OSAHS state until death. Alternatively, they could experience CHD, and those that survive move to an OSAHS post-CHD state that incorporates the increased mortality and morbidity associated with having had a first CHD event. They could then remain in this state until death, experience an RTA or have a stroke that may render them disabled.
FIGURE 20.
York economic model structure
Alternatively, they could experience a stroke from the initial OSAHS health state. Patients who survive a stroke enter an OSAHS post-stroke health state which incorporates the increased mortality and morbidity associated with having had a first stroke. Patients who are not disabled remain at risk of an RTA. In contrast, if they become disabled post stroke, it is assumed that they are no longer able to drive and therefore incur no further risk of a driving accident. Once in the OSAHS post-stroke health state it is assumed that they will remain in this health state until death, which could happen directly or following an RTA. The model does not record CHD events separately following a stroke.
Patients in the initial OSAHS state might at some point have an RTA which could be fatal or non-fatal. In the latter case they would return to the OSAHS state.
Parameter estimates for inclusion in the York economic model
This section reports the methods used to estimate parameters for the base-case analysis and secondary analyses. It describes the approach used to estimate the utility, resource use and costs associated with CPAP, dental devices and conservative management in the treatment of OSAHS.
The evidence used to populate the parameters of the economic model comprises the RCT data reviewed in Chapter 3 as well as relevant evidence from non-randomised trials, modelling studies, analyses of administrative databases and expert clinical opinion. In addition, individual patient data from three trials87,144,151 were obtained from the clinical experts on this technology assessment (RJOD/JS).
Several searches were undertaken to populate specific parameters of the economic model. Searches in MEDLINE were conducted to identify data to inform three elements of the model: (1) HRQoL studies, utilities and QALYs; (2) literature linking CVE, particularly stroke and CHD, to OSAHS; and (3) literature linking RTAs to OSAHS. The search strategies are presented in Appendix 1, Cost-effectiveness searches.
Utility estimation for inclusion in the York economic model
As reviewed in Chapter 3, the evidence base on the effectiveness of CPAP in OSAHS consists of a number of randomised trials of varying designs (crossover and parallel), which display heterogeneity in their inclusion criteria and which report a considerable range of outcome measures covering sleepiness, HRQoL and blood pressure. Analysis was required to link the short-term outcome measures of clinical effectiveness to a preference-based measure of HRQoL in terms of utilities. The randomised trial data provided evidence on what can be viewed as intermediary outcomes in terms of sleepiness and blood pressure, but did not measure either treatment effects in terms of a reduction in the number of CVEs or a reduction in the risk of RTAs. Therefore, a model was required to link the available clinical data to long-term outcomes, HRQoL and costs in order to estimate the long-term cost-effectiveness of treatment with CPAP.
Valuing clinical effectiveness in terms of utility
The NICE Reference Case indicates that the measure of health outcome used in the cost-effectiveness analysis should be QALYs calculated with utility values derived from a validated generic, preference-based measure of HRQoL. The systematic review reported in Chapter 3 highlighted the measures used to describe the efficacy of treatments for OSAHS in RCTs, among which the ESS was the most frequently reported (n = 27 trials). Utility values and quality-adjusted survival were infrequently reported (n = 1 trial comparing CPAP to placebo) (Chakravorty et al. 97). Therefore, an additional literature search was undertaken, and this identified four other papers that contained potentially relevant HRQoL/utility data for inclusion in the model.
Table 26 reports key information on the four studies (see Appendix 7 for more details). Jenkinson et al. 152 and Chakrovarty et al. 97 also reported pre- and post-ESS scores. The former reported a pre-treatment ESS of 14 (SD 5) and a post-treatment score of 8.2 (SD 4.8). In a CPAP treatment arm, Chakrovarty et al. 97 reported a pre-treatment ESS of 16 (SD 6) and a post-treatment score of 8 (SD 6). In the lifestyle arm, Chakrovarty et al. 97 reported a pre-treatment ESS of 14 (SD 4) and a post-treatment score of 11 (SD 5). Note that none of the studies reporting utility data assessed the use of dental devices.
| Authors | Method | Study design | Utility values [mean (SD)] | Source of values |
|---|---|---|---|---|
| Tousignant et al., 1994124 | SG | Retrospective before and after study. Patients did SG exercise twice (2–3 weeks apart) to assess reliability. Health states valued were: receiving nCPAP treatment and pre-treatment | Pre-treatment health state = 0.63 (0.29); nCPAP treatment health state = 0.87 (0.17) | Patients attending hospital sleep clinic who had been receiving nCPAP (for around 9 months) (n = 19) |
| Jenkinson et al., 1997152 | EQ-5D | Before and after study. Patients completed EQ-5D before commencing treatment with nCPAP and 5 weeks later | Baseline EQ-5D index = 0.79 (0.21); post-treatment EQ-5D index = 0.84 (0.25) | Patients attending sleep clinic for nCPAP therapy (n = 108) |
| Chakravorty et al., 200297 | EQ-5D, SG | RCT comparing 3 months’ treatment with CPAP with lifestyle management. EQ-5D and SG were completed before randomisation and at 3 months. In SG patients were asked whether they would choose their current state of health or treatment with two potential outcomes: complete cure or failure leading to a worst health state/death |
CPAP group: SG pre-treatment = 0.32 (0.17); SG post-treatment = 0.55 (0.26); EQ-5D index pre-treatment = 0.73 (0.18); EQ-5D index post-treatment = 0.77 (0.18) Lifestyle group: SG pre-treatment = 0.31 (0.13); SG post-treatment = 0.35 (0.12); EQ-5D index pre-treatment = 0.77 (0.12); EQ-5D index post-treatment = 0.77 (0.09) |
Patients referred to hospital sleep clinic (n = 71) |
| Mar et al., 2003123 | EQ-5D | Before and after study. Patients completed EQ-5D pre-treatment and after using nCPAP for 3 months | Baseline EQ-5D index = 0.74; post-treatment EQ-5D index = 0.81 | Patients referred to sleep unit (n = 46) |
In order to use the trial data and to allow a comparison between CPAP and dental devices, there was a need to link the data on clinical efficacy, in the form of the disease-specific ESS, to utility. Data on mean difference in ESS were available for 23 studies comparing CPAP with placebo and six studies comparing CPAP with dental devices. To achieve the link between change in ESS and change in utility, three sets of individual patient data were obtained, two that measured ESS and SF-36 profile in the same patients87,151 and one that measured ESS, SF-36 profile and EQ-5D in the same patients. 144 The SF-36 data were used to calculate utility values based on the SF-6D, using an algorithm developed by Brazier et al. 153 based on UK public preferences.The EQ-5D data were used to calculate utility based on general UK population tariff values. 128 The three data sets were then used to develop prediction models to estimate the relationship between ESS and (1) utility values based on SF-6D and (2) utility scores based on EQ-5D.
A simple linear regression model was fitted to predict absolute utility scores from absolute ESS, controlling for baseline utility and baseline ESS. A larger number of observations were available with the SF-6D in comparison with the EQ-5D and, where multiple observations were available on the same patient, analyses were adjusted to reflect the dependence between repeated observations on the same individual. All variables were treated as continuous data, and baseline scores were centred prior to estimation. The use of ordinary least squares (OLS) regression relies on the assumption that the error terms are normally distributed. Assessment of the residuals estimated in both regressions revealed that this assumption appeared reasonable for utility scores based on the SF-6D (Figure 21). However, typically, EQ-5D scores do not follow a normal distribution, and as expected the EQ-5D scores in the data set formed a distribution skewed to the left, with a large number of observations clustered at a score of 1. Figure 21 shows that the residuals from the regression of EQ-5D scores on ESS deviate somewhat from a normal distribution. Although OLS regression methods have been found to perform well when predicting EQ-5D scores,154 a generalised linear model was also fitted to the data to ascertain whether an alternative error distribution such as a gamma might produce a better fit. However, this model did not improve the fit on the basis of the Akaike Information Criterion and so the results from the OLS model were used for both the EQ-5D and the SF-6D.
FIGURE 21.
Standardised normal plot of residuals for use in York economic model.
The results of the regression analyses are shown in Tables 27 and 28. The models indicate that an increase of 1 point in ESS is associated with a fall in utility of 0.01 and this is true for both the SF-6D and EQ-5D instruments, the results of which were remarkably similar. A test was performed to see if there was evidence for a change in relationship between different levels of baseline ESS (i.e. a change in the slope of the regression line for particular cut-off values of ESS) but there was no evidence to support such a subgroup effect.
| Utility | Coefficient | SE | p-Value | 95% CI | |
|---|---|---|---|---|---|
| OLS model for utility based on SF-6D (n = 294) | |||||
| ESS | –0.0095213 | 0.0013849 | 0.000 | –0.0122512 | –0.0067915 |
| Baseline ESS | 0.0050331 | 0.0011942 | 0.000 | 0.0026791 | 0.0073871 |
| Baseline utility | 0.5588972 | 0.0534972 | 0.000 | 0.4534455 | 0.6643489 |
| Constant | 0.8067555 | 0.0115013 | 0.000 | 0.7840845 | 0.8294265 |
| OLS model for utility based on EQ-5D (n = 94) | |||||
| ESS | –0.0096984 | 0.003947 | 0.016 | –0.0175364 | –0.0018604 |
| Baseline ESS | 0.0029526 | 0.0033693 | 0.383 | –0.0037382 | 0.0096435 |
| Baseline utility | 0.6287684 | 0.1346153 | 0.000 | 0.3614492 | 0.8960877 |
| Constant | 0.8925207 | 0.0286109 | 0.000 | 0.8357052 | 0.9493363 |
| Utility | Mean | SD | Source |
|---|---|---|---|
| OSAHS untreated (baseline) | Baseline ESS × (–0.01 + 0.89)a | Estimated from prediction equation (see Table 27) | |
| OSAHS treated with CPAP (change from baseline) | MD_ESSCPAP_CM × –0.01 | Estimated from prediction equation (see Table 27) | |
| OSAHS treated with dental devices (change from baseline) | MD_ESSDD_CM × –0.01 | Estimated from prediction equation (see Table 27) | |
| Stroke (absolute decrement) | –0.0524 | 0.0002 | Sullivan and Ghushchyan, 2006155 |
| CHD (absolute decrement) | –0.0635 | 0.0001 | Sullivan and Ghushchyan, 2006155 |
| RTA (absolute utility) | 0.62 | 0.27 | Currie et al., 2005156 |
| Age decrement (per year) | –0.0007 | 0 | Sullivan and Ghushchyan, 2006155 |
The Cholesky decomposition of the covariance matrix from the regressions was employed to characterise the uncertainty around the estimated coefficients and to reflect the correlation between coefficients in the probabilistic sensitivity analysis. 150 The baseline utility for the hypothetical patient population was predicted from the specified baseline ESS score. Changes in ESS associated with treatment were converted to changes in utility (utility increments) using the predicted relationship between ESS and utility.
The utility decrements associated with stroke, CHD and age were based on the regression analysis reported by Sullivan et al. 155 and are reported in Table 28. Utility decrements and increments can be applied to the baseline utility of the hypothetical cohort to reflect the utility associated with being in any health state in the model. The EQ-5D scores used in the analysis by Sullivan et al. were calculated using US community preferences. However, equivalent decrements were not available based on UK community preferences. The uncertainty around the utility decrements was characterised using a normal distribution, as the utility decrements are described by the coefficients from a regression analysis. The standard errors are small enough that there is no risk of unsuitable values being selected in the probabilistic analysis.
The utility associated with experiencing an RTA was based on EQ-5D measures from the Health Outcomes Data Repository (HODaR). 156 HODaR recorded EQ-5D data for individuals 6 weeks after their inpatient episode (at Cardiff Hospital, UK) for injuries sustained from an RTA. Data were extracted for all patients who had a traffic accident as a motorcycle rider, an occupant of a three-wheeled motor vehicle, a car occupant or an occupant of a pick-up truck or a van [V20 to V59, International Classification of Diseases-10 (ICD-10) codes]. Results were found for 56 patients. A gamma distribution was used to characterise the uncertainty around the utility decrements associated with clinical events.
For the base-case analysis the effect of treatment on ESS was derived by pooling all of the available trial data to obtain an overall effect. However, in Chapter 3 it was noted that there was a high level of heterogeneity in this overall analysis that was reduced when trials were grouped by baseline severity of OSAHS. The treatment effects estimated by pooling trials grouped according to average baseline ESS (mild, moderate or severe) were applied in three separate analyses (see Chapter 3, Figure 2). These analyses cannot be interpreted as subgroup analyses reflecting differential treatment effects according to OSAHS severity as they are based on a study-level covariate in the form of average baseline ESS. In order to conduct a true subgroup analysis, trial data would have to be available that estimated the relationship at the patient level between baseline ESS and change in ESS with treatment.
Linking reduction in blood pressure to cardiovascular events
As noted above, the randomised trials provided information on the effect of CPAP on blood pressure but, for the economic model, the implications of this treatment effect for clinical events need to be estimated. The Framingham risk equations provide a link between risk factors such as blood pressure and the incidence of fatal and non-fatal CVEs. Published risk equations predict the risk of CHD and stroke157 over a range of 4–12 years as a function of either SBP or DBP. Anderson et al. 157 state that, of the two alternative measures, SBP was the best predictor of stroke, and therefore this was selected as the measure of blood pressure to be included in the economic model. We did not identify corresponding risk equations that incorporated MAP. The risk equations were estimated separately for men and women using the baseline characteristics of the hypothetical patient population shown in Table 29, which were determined from the RCT data where possible and based on plausible assumptions otherwise. It was also possible to incorporate the increased baseline risk associated with high BMI using the relative risk published by Mora et al. 158 However, as none of the relevant comparators demonstrated efficacy in terms of weight loss and reducing BMI, this was not included in the analysis.
| Age | 50 |
| SBP | 130 |
| Smoking (0 = no; 1 = yes) | 1 |
| Total cholesterol (mg/dl) | 224 |
| HDL-cholesterol (mg/dl) | 43 |
| Diabetes (0 = no; 1 = yes) | 1 |
| ECG-LVH (0 = no; 1 = yes) | 0 |
| Ten-year probability of stroke event | 3.4% (male); 3.7% (female) |
| Ten-year probability of death from CVD | 3.8% (male); 3.6% (female) |
| Ten-year probability of CHD | 19.7% (male); 19.2% (female) |
| Ten-year probability of death from CHD | 3.9% (male); 3.7% (female) |
It was assumed that the only risk factor affected by use of CPAP was blood pressure. The Framingham risk equations are based on Weibull models, and so the predicted risk is non-linear with respect to each risk factor. To determine whether the use of the mean change in blood pressure would bias the results, a check was performed on a set of individual patient data that reported change in blood pressure. The risk of CHD and stroke was predicted for each patient individually, and the mean of the individual predicted risks was compared with the risk based on the mean change in blood pressure for the whole group. The risks were found to be identical to two decimal places, and so it was felt that, although the equations are non-linear, the use of the mean change would not bias the model results.
In order to estimate the probability of CHD and stroke events per model cycle, a piece-wise exponential was assumed. The equations were used to predict the 4-year probability of an event every 4 years given the current age of the hypothetical patient cohort and starting from year 0. For the intervening years it was assumed that survival followed an exponential distribution, and so each 4-year probability was converted into a constant yearly probability to be applied over the relevant 4-year interval. When multiple equations from the Framingham set are used they should be applied in random order to take into account competing risks. However, in a cohort model structure the risk equations must be applied in the same order across the entire cohort for any given model cycle. This is relevant to patients in the initial state of the model who may experience either CHD or a stroke event. Rather than specify an order in which to apply the equations, the probability of any event was calculated by summing the hazards and then the proportion of events that were CHD or stroke events was calculated.
The relative risk reduction for CVE implied by the difference in SBP with CPAP compared with usual care is estimated to be relatively low using the Framingham risk equations (RR ≈ 0.98 for mean reduction in SBP of 1.06 mmHg). It has been posited that the Framingham risk equations may be subject to regression dilution bias when describing the relationship between a change in blood pressure and the change in risk of cardiovascular disease (CVD) events. Random fluctuations in blood pressure may cause the relationship between blood pressure and incidence of CVEs to be underestimated if the analysis is conducted on the basis of single baseline assessment of blood pressure. The Framingham risk equations specified in Anderson et al. 157 are based on the average of two office measurements of blood pressure (systolic or diastolic). MacMahon et al. 145 conducted an analysis to estimate the change in risk of stroke and CHD as a function of DBP in which they correct for regression dilution bias by incorporating data on usual DBP (average DBP over several years) as well as baseline DBP. Their results indicate that the percentage reduction in risk of stroke or CHD is approximately linearly related to DBP. They estimate that a 7.5 mmHg fall in DBP is associated with a 46% (SD 2%) reduction in risk of stroke and a 29% (SD 1%) reduction in risk of CHD. MacMahon et al. did not estimate the absolute risk of stroke or CHD events associated with particular levels of DBP. Therefore, a scenario analysis was conducted in which the baseline risks of stroke and CHD events were determined by the Framingham risk equations, but the change in risk associated with treatment was modelled using the relationship provided by MacMahon et al. The relative risk reduction for CVE implied by the difference in DBP with CPAP compared with usual care is estimated to be higher based on the MacMahon et al. analysis in comparison with the Framingham risk equations (RR = 0.96 for CHD and RR = 0.94 for stroke given a mean reduction in DBP of 1.20 mmHg).
Evidence synthesis on change in ESS and SBP
The model incorporates data on the effectiveness of treatments for OSAHS in terms of change in ESS and change in SBP. The use of a BRMA allows the incorporation of the between- and within-study correlation in the treatment effect in these two end points. 159,160 The between-study correlation is estimated in the meta-analysis on the basis of those studies that report both outcomes. However, none of the studies provided an estimate of the within-study correlation between the mean change in ESS and the mean change in SBP. As it was felt that these treatment effects might be correlated, a set of patient-level data were obtained from which an informative prior distribution for the within-study correlation could be estimated. 87,144,151 Note that the assumption in the BRMA is that treatment effects on different outcomes may be correlated, and not that measures of ESS and blood pressure might themselves be correlated. The meta-analysis was performed in WinBUGS, and the code and data appear in Appendix 8.
The results of the BRMA are shown in Table 30. The estimate for the mean change in ESS is similar to that reported in Chapter 3. This is not surprising given the relatively small number of data points that inform this estimate. However, the estimate for the mean change in blood pressure differs somewhat from that reported in Chapter 3. This is because the BRMA in essence imputes the missing SBP for the 19 studies that did not report that end point on the basis of the observed between-study correlation. Only four trials reported both ESS and daytime SBP based on ABPM. 64,83,89,109 This provides limited data to inform the estimation of the parameters of the BRMA relating to between-study correlation. For this reason the mean changes estimated in separate univariate meta-analyses in Chapter 3 were applied in the base-case analysis, and these were used regardless of whether the differences were statistically significant. The estimates from the BRMA were applied in a sensitivity analysis. Three additional trials reported both ESS and daytime SBP based on office measurements. 62,70,100 While the absolute SBP recorded by ABPM may be expected to differ from that recorded by an office-based measure, it could be argued that the changes in SBP may be comparable. If this assumption is acceptable, then a BRMA could be estimated based on seven trials that report both outcome measures, as shown in Table 30.
| Measure | ESS [MD (SD)] | SBP [MD (SD)] |
|---|---|---|
| BRMA incorporating trials that report SBP based on ABPM | –2.65 (0.43) | –1.64 (1.72) |
| BRMA incorporating trials that report SBP based on ABPM or office measures | –2.62 (0.43) | –3.69 (1.55) |
No trials reported change in daytime SBP based on ABPM for the comparison of CPAP with dental devices. For the base-case analysis, it was assumed that the ratio of the treatment effects on daytime SBP for CPAP and dental devices compared with placebo would be equal to the ratio of the observed treatment effects on ESS. The mean differences in ESS for CPAP versus conservative management (MD_ESSCPAP_CM) and for CPAP versus dental devices (MD_ESSCPAP_DD) were reported in Chapter 3. The mean difference in ESS for dental devices versus conservative management was calculated from this information using standard methods for an indirect comparison (MD_ESSDD_CM = MD_ESSCPAP_CM – MD_ESSCPAP_DD) (Bucher et al. 161). The mean difference in SBP for dental devices compared with conservative management was therefore calculated as MD_SBPDD_CM = MD_SBPCPAP_CM × (MD_ESSDD_CM/MD_ESSCPAP_CM).
Where parameters were estimated in WinBUGS, the output from 10,000 Monte Carlo iterations was used directly to characterise the uncertainty around the estimated treatment effects and to incorporate the correlation between outcomes. The uncertainty around treatment effects estimated in the meta-analysis reported in Chapter 3 was characterised using a normal distribution.
Estimating the treatment effect of interventions on RTAs
To estimate the impact of CPAP on RTAs, the meta-analysis of before and after studies undertaken by Ayas et al. 122 was updated (see Table 21). Only one additional study was found (Barbé et al. 162). Because this study reported a relative risk rather than an odds ratio (as in the Ayas et al. paper122), the data reported on events and non-events were used to (re)calculate an odds ratio. The log odds ratios were then pooled by means of inverse variance weighting. The separate and pooled odds ratios are reported in Table 31. Note that, although the relative risk reduction of experiencing an RTA with CPAP treatment is high, the absolute baseline risk is very low.
| Source | Odds ratio | Variance |
|---|---|---|
| Ayas et al., 2006122 (based on eight studies) | 0.15 | 0.00094 |
| Barbé et al., 2007162 (single study) | 0.33 | 0.02075 |
| Pooled data Ayas et al., 2006122 and Barbé et al., 2007162 | 0.17 | 0.00098 |
The literature search did not identify any studies that assessed the impact of treatment with dental devices on RTAs. For the base-case analysis an adjusted odds ratio for dental devices compared with conservative management was estimated by applying the ratio of the treatment effects on ESS for CPAP and dental devices versus conservative management to the odds ratio for RTAs for CPAP versus conservative management.
It was assumed that patients left disabled following a first stroke event would no longer be at risk of an RTA. For the base-case analysis the proportion of first strokes that were disabling was estimated to be 30.9% based on the Second European Stroke Prevention Study (ESPS-2). 163 Note that the base-case analysis applies to patients who hold a driving licence. For OSAHS sufferers who do not drive the appropriate analysis is one in which the risk of RTA is excluded.
In summary, Table 32 shows the treatment effects used to populate the York economic model.
| CPAP vs CM [mean (SD)] | CPAP vs DD [mean (SD)] | DD vs CM [mean (SD)] | |
|---|---|---|---|
| ESS (mean difference) | |||
| Overalla | –2.7 (0.38) | –0.85 (0.64) | –1.85b |
| Mild baseline severity (ESS) | –1.07 (0.39) | NA | NA |
| Moderate | –2.33 (0.36) | –0.85 (0.64) | –1.48b |
| Severe | –4.99 (0.76) | NA | NA |
| Blood pressure (mean difference) | |||
| SBPa | –1.06 (1.17) | NA | –0.73b |
| DBP | –1.20 (0.88) | NA | –0.82b |
| RTA (odds ratio)a | 0.17 (0.001) | NA | 0.25b |
Compliance
The long-term compliance with CPAP will have implications for the estimated effectiveness in the target population. The majority of the trial data were based on less than 12 weeks’ follow-up. Given this, long-term compliance with CPAP was estimated on the basis of observational data provided by McArdle et al. 39 This study reported compliance over 6 years in a cohort of Scottish patients with a median age of 50 and an average ESS score at baseline of 12. The results indicated that 84% of patients continued to use CPAP 1 year after initiation of treatment, and that compliance was steady after a period of 4 years, with 68% of patients continuing treatment. The percentage of patients compliant at 2 and 3 years after treatment initiation was read from the survival curve (74% and 73% respectively) and these data were then used to model the rate of discontinuation from years 1 to 4 in the model. Patients discontinuing treatment were assumed to return immediately to the levels of ESS, SBP and utility associated with no treatment. In the base-case analysis it was assumed that 90% of patients who discontinued treatment with CPAP would return their machine. Equivalent data were not available for dental devices, and so in the base-case analysis it was assumed that compliance with dental devices was equivalent to that for CPAP.
Mortality rates
Table 33 reports the parameters associated with CHD, stroke and RTAs.
| Parameter | Mean | 95% CI | Source |
|---|---|---|---|
| CHD | |||
| Relative risk of death following CHD | 3.2 | 2.67–3.83 | Rosengren et al., 1998165 |
| Stroke | |||
| Relative risk of death following stroke | 2.3 | 2.0–2.7 | Dennis et al., 1993164 |
| RTAs | |||
| Rate of non-fatal RTAs for male licence holders | 0.0089 | b | Department of Transport, 2004133 |
| Rate of non-fatal RTA for female licence holders | 0.0082 | b | Department of Transport, 2004133 |
| Rate of fatal RTA for male licence holders | 0.00014 | b | Department of Transport, 2004133 |
| Rate of fatal RTA for female licence holders | 0.000060 | b | Department of Transport, 2004133 |
The mortality rate for individuals who have not experienced CHD or stroke (by age and sex) was taken from the UK life tables of the Government Actuary Department (www.gad.gov.uk). For each age band, the all-cause hazard was reduced by the proportion of people dying of cardiovascular disease (CVD) or ischaemic heart disease (IHD) causes to get the hazard of death for non-CVD or non-IHD causes using methods developed by Chiang. 168 For patients who experienced CHD or stroke, an elevated mortality rate was used based on relative risks from the literature. For patients who experienced CHD and stroke, relative risks of death of 3.2165 and 2.3,164 respectively, were employed. These relative risks were applied to the non-cardiovasular/ischaemic heart disease mortality rates in the UK population (by age and sex).
Resource use and cost estimation for the York economic model
The costs of the three interventions for OSAHS included the initial costs of the interventions as well as the ongoing costs of care associated with the interventions. The costs included the cost of the devices, staff time and overheads associated with providing the interventions and the cost of other NHS health care and PSS related to OSAHS. Costs were reported in prices relating to 2005 and any costs that related to previous years were uprated using the Hospital and Community Health Services (HCHS) pay and prices index (2006). 166
The review of the published cost-effectiveness studies identified limited information on resource use associated with CPAP treatment. Only one study related to the UK setting. 44 This study included the costs of investigation and diagnosis for OSAHS and nCPAP. In contrast, the York model includes adults who have already been diagnosed with OSAHS and therefore does not incorporate this cost. The current study attempts to take into account the impact of treatment in terms of the utilisation of other health-care resources comprising any health-care use due to stroke, CHD and RTAs. None of the existing published cost-effectiveness studies included the full range of relevant costs and none compared dental devices with CPAP for OSAHS in the UK.
The costs of CPAP, dental devices and conservative management used in the York economic model are shown in Table 34. The majority of CPAP costs and resource use were obtained from the ResMed submission, which was informed by a survey of 19 clinical experts. Other relevant data were obtained from published studies and from correspondence with clinical experts.
| Parameters | Costs (2006) | Probability [mean (SD)] | Number | Source |
|---|---|---|---|---|
| CPAP and APAP initial costs | ||||
| Outpatient visit | ||||
| Unit cost of outpatient (consultant) visit | £107.87 | NHS reference costs 2006 | ||
| Probability of having a follow-up outpatient visit | 0.69 (0.3) | ResMed survey of clinicians | ||
| Total cost of follow-up outpatient visit | £74.52 | |||
| Home titration | ||||
| Probability of home titration | 0.99 (0.01) | ResMed survey of clinicians | ||
| Probability of using APAP | 0.81 (0.19) | ResMed survey of clinicians | ||
| APAP machine | £410 | ResMed | ||
| Probability of using CPAP | 0.19 | ResMed survey of clinicians | ||
| CPAP machine | £280 | ResMed | ||
| Number of times CPAP/APAP used for dose titration | 163 | |||
| Total cost of home titration | £2.34 | |||
| Inpatient titration | ||||
| Probability of inpatient titration | 0.01 | Assume if not home titration must be inpatient titration (1–0.99) | ||
| Unit cost of sleep study follow up | £107.87 | NHS reference costs 2006 | ||
| Total cost of inpatient titration | £1.08 | |||
| Titration by specialist nurse | ||||
| Probability of seeing a specialist nurse for titration | 1 | Assumption | ||
| Unit cost of 30-minute appointment with specialist nurse | £34 | NHS reference costs 2006 | ||
| Total cost of titration by specialist nurse | £34 | |||
| Titration by consultant | ||||
| Probability of seeing a consultant for titration | 0.4 | ResMed survey of clinicians | ||
| Total cost of titration by consultant | £43.10 | |||
| Total cost of 30-minute appointment with technician | £9.50 | |||
| Total initial costs (first year) | ||||
| CPAP | £164.64 | |||
| APAPa | £108 | |||
| CPAP ongoing costs | ||||
| Interest rate | 3.5% | NICE methods guidance | ||
| Estimated device life of CPAP | 7 years | |||
| Annual equivalent cost of CPAP device | £44.24 | 280/annuity factor | ||
| Annual equivalent cost of CPAP mask | £80 | ResMed | ||
| Annual sundries | £15 | Clinical opinion | ||
| Annual follow-up | £79 | Clinical expert referring to NHS tariff | ||
| Total CPAP ongoing costs (yearly) | £218.24 | £44.24 + £80 + £15 + £79 | ||
| APAP ongoing costs | ||||
| APAP machine | £410 | ResMed | ||
| Humidifier | £150 | ResMed | ||
| Estimated device life of APAP and humidifier | 5 years | ResMed | ||
| Annual equivalent cost of APAP with humidifier | £100 | 560/annuity factor | ||
| Annual sundries | £100 | ResMed | ||
| Annual equivalent cost of CPAP mask | £80 | ResMed | ||
| Annual follow-up | £79 | Clinical expert referring to NHS tariff | ||
| Total APAP ongoing costs (yearly)a | £359 | £100 + £100 + £80 + £79 | ||
| Dental device costs | ||||
| Estimated device life of dental device | 2 years | |||
| NHS cost of dental device and its provision | £250.92 | 12 units of dental activity × 20.91 | ||
| Total dental device costs (yearly) | £128.82 | |||
| Dental device ongoing costs (yearly) | ||||
| Maintenance of dental device | £19.47 | Edwards et al., 1999,168 cost of a consultant appointment | ||
| Conservative management cost | ||||
| One-off consultation with a GP | £21 | Curtis and Netten, 2006166 | ||
It was assumed that the CPAP machine has a device life of 7 years (which was the device life used by ResMed and confirmed by a clinical expert) and that a dental device lasts for 2 years (based on clinical opinion). The costs of the devices were expressed as equivalent annual costs121 using the public sector discount rate of 3.5%.
No published NHS cost of dental devices for the treatment of OSAHS was found; therefore, to fulfil the scope of the review, the cost was estimated based on clinical opinion. It was assumed that the dentist provided a Thornton Adjustable Positioner (TAP), a device that is commonly used for the treatment of OSAHS in the UK. Under the new NHS Dental Contract a course of treatment is classified into a treatment band. It is appropriate to classify TAP provision as Band 3 as such treatment requires laboratory work (www.ic.nhs.uk/). Twelve units of dental activity (UDAs) are applied to Band 3. 167 The national average reimbursement rate for a UDA is not known. The value of UDAs varies because of a number of factors, including the contract values negotiated locally by primary care trusts (PCTs), differences in the treatment patterns, treatment needs in different areas and the degree to which PCTs may have set broader service objectives for contractors that cannot be measured by units of dental activity (Department of Health, personal communication). Therefore, the value of a UDA was obtained from published material (Bath & North East Somerset Primary Care Trust: www.banes-pct.nhs.uk/about/BoardPapers/2007/May/Agenda%20Item%2010%20Annex%201.pdf. The average reimbursement per UDA was estimated to be £20.91; therefore, multiplied by 12 UDAs, this gives an estimate of approximately £251 for the total cost of a dental device. Based on clinical expert opinion, it was assumed that the patient would have a yearly check-up appointment.
The cost of non-compliance was determined by the proportion of CPAP machines not returned (10%) and the cost of dental devices no longer used. The data were entered probabilistically based on estimates of the service and resource use data obtained from ResMed’s survey of clinicians. The uncertainty around the probability distributions was characterised by beta distributions.
On the basis of clinical advice, conservative management was estimated as the cost of a one-off GP consultation during which the patient may receive advice on posture, dietary habits and lifestyle. A unit cost of a GP appointment was obtained from the Personal Social Services Research Unit (PSSRU) costs of health and social care report. 166
The unit costs associated with stroke, CHD and RTAs are reported in Table 35. Published references were used to estimate these parameters. The uncertainty around the costs was characterised by gamma distributions, where mean costs and SD were presented, and by normal distributions, where the costs were based on coefficients from a regression analysis. The Department of Transport information regarding the costs of RTAs was presented as point estimates. In order to characterise the uncertainty around these estimates it was assumed that the SD would be equal to that reported by HODaR (Currie et al. 156) for the hospital cost associated with non-fatal RTAs (mean £2437, SD £1643).
| Parameter | Mean cost | SD | Source |
|---|---|---|---|
| CHD and stroke | |||
| Cost of fatal CVE | £3021 | 367 | Briggs et al., 2007168 |
| Acute cost of CHD | £9997 | 428 | Briggs et al., 2007168 |
| Ongoing cost of CHD | £751 | 117 | Briggs et al., 2007168 |
| Acute cost of stroke | £9067 | 294 | Bravo et al., 2007169 |
| Ongoing cost of stroke | £2392 | 282 | Bravo et al., 2007169 |
| RTA | |||
| Cost of RTA (all injuries) | £2700 | 1643 | Department of Transport, 2004133 |
| Cost of fatal RTA | £5450 | 1643 | Department of Transport, 2004133 |
Results of York economic model
Base-case analysis
The base-case analysis is based on a hypothetical cohort of men aged 50 with the baseline cardiovascular risk factors described in Table 36. In this cohort CPAP was associated with both higher costs and higher QALYs in comparison with treatment with dental devices or conservative management. The incremental cost-effectiveness of CPAP compared with dental devices is estimated to be £4000 per QALY. CPAP might therefore be considered cost-effective at a cost-effectiveness threshold per QALY of £20,000.
| Conservative management | Dental device | CPAP | |
|---|---|---|---|
| Treatment costs | £21 | £1726 | £2465 |
| RTA costs | £2201 | £1138 | £904 |
| CVE costs | £5918 | £5932 | £5931 |
| Total costs | £8140 | £8797 | £9301 |
| Total QALYs | 11.93 | 12.26 | 12.39 |
| ICER | £2000 | £3899 | |
| Probability of being cost-effective for threshold | |||
| £10,000 per QALY | 0.01 | 0.32 | 0.66 |
| £20,000 per QALY | 0.00 | 0.20 | 0.80 |
| £30,000 per QALY | 0.00 | 0.17 | 0.83 |
Similar results were obtained for a hypothetical cohort of women aged 50, as shown in Table 37.
| Conservative management | Dental device | CPAP | |
|---|---|---|---|
| Treatment costs | £21 | £1824 | £2608 |
| RTA costs | £2139 | £1108 | £878 |
| CVE costs | £5840 | £5829 | £5820 |
| Total costs | £7999 | £8762 | £9306 |
| Total QALYs | 12.71 | 13.02 | 13.15 |
| ICER | £2432 | £4335 | |
| Probability of being cost-effective for threshold | |||
| £10,000 per QALY | 0.02 | 0.33 | 0.64 |
| £20,000 per QALY | 0.01 | 0.21 | 0.78 |
| £30,000 per QALY | 0.00 | 0.17 | 0.83 |
For the base-case analysis the effect of CPAP on ESS was derived by pooling all of the available trial data to obtain an overall effect. The treatment effects estimated by pooling trials, grouped according to average baseline ESS (mild, moderate or severe), were applied in three separate analyses and the results are shown in Table 38. Note that the trials comparing CPAP with dental devices all had a mean baseline ESS that would classify them as moderate OSAHS. Because it was not possible to estimate the differential effect of baseline severity of OSAHS on CVD and RTA risks, these risks have not been included in the results in Table 38 (i.e. these cost-effectiveness results by severity include only treatment effects on ESS). It can be seen that cost-effectiveness varies according to severity, with CPAP most cost-effective (lower ICER) in severely affected patients. However, CPAP has an ICER below a cost-effectiveness threshold of £20,000 for moderate and severe levels using baseline ESS score. The ICER in a subgroup with mild disease was estimated to be £20,585.
| Conservative management | Dental devicea | CPAP | |
|---|---|---|---|
| Mild OSAHS, male aged 50 (mean baseline ESS = 7) | |||
| Total cost | £21 | NA | £2726 |
| Total QALYs | 14.56 | NA | 14.69 |
| ICER | £20,585 | ||
| Probability of being cost-effective for threshold | |||
| £10,000 per QALY | 0.95 | NA | 0.05 |
| £20,000 per QALY | 0.57 | NA | 0.43 |
| £30,000 per QALY | 0.32 | NA | 0.68 |
| Moderate OSAHS, male aged 50 (mean baseline ESS = 13) | |||
| Total cost | £21 | £1906 | £2726 |
| Total QALYs | 13.51 | 13.70 | 13.80 |
| ICER | ED | £9391 | |
| Probability of being cost-effective for threshold | |||
| £10,000 per QALY | 0.40 | 0.24 | 0.36 |
| £20,000 per QALY | 0.09 | 0.21 | 0.70 |
| £30,000 per QALY | 0.04 | 0.18 | 0.78 |
| Severe OSAHS, male aged 5 (mean baseline ESS = 16) | |||
| Total cost | £21 | NA | £2726 |
| Total QALYs | 13.01 | NA | 13.62 |
| ICER | £4413 | ||
| Probability of being cost-effective for threshold | |||
| £10,000 per QALY | 0.05 | NA | 0.95 |
| £20,000 per QALY | 0.02 | NA | 0.98 |
| £30,000 per QALY | 0.01 | NA | 0.99 |
Secondary analysis
There are a number of uncertainties over several of the modelling assumptions, and results are shown as a set of subgroup and scenario analyses in Table 39. In each case the variable or assumption altered from the base-case analysis is indicated in the title of the scenario analysis, and all other variables and assumptions were left unchanged. It can be seen that, although ICERs for CPAP vary according to the different assumptions, they consistently fall below a threshold of £20,000 per QALY. The greatest effect on the CPAP ICER comes from applying the highest feasible acquisition cost for the treatment by including the costs of an APAP machine and a humidifier.
| Male | Female | |||||
|---|---|---|---|---|---|---|
| CM | DD | CPAP | CM | DD | CPAP | |
| Subgroup analysis | ||||||
| Cohort aged 35 | ||||||
| Cost | £8521 | £9356 | £10,034 | £8177 | £9155 | £9868 |
| QALY | 15.55 | 15.99 | 16.15 | 16.21 | 16.60 | 16.76 |
| ICER | £1894 | £4143 | £2477 | £4454 | ||
| Cohort aged 65 | ||||||
| Cost | £5969 | £6398 | £6728 | £5159 | £5709 | £6078 |
| QALY | 7.95 | 8.17 | 8.26 | 8.89 | 9.12 | 9.21 |
| ICER | £1866 | £2960 | £2426 | £3944 | ||
| Scenario analysis | ||||||
| Change in ESS linked to SF-6D utility score rather than EQ-5D | ||||||
| Cost | £8129 | £8781 | £9295 | £8003 | £8761 | £9307 |
| QALY | 10.66 | 10.95 | 11.06 | 11.35 | 11.62 | 11.74 |
| ICER | £2258 | £4451 | £2748 | £4669 | ||
| Relative risk reduction for CVE based on DBP and MacMahon et al., 1990145 | ||||||
| Cost | £8133 | £8734 | £9207 | £7949 | £8656 | £9189 |
| QALY | 11.92 | 12.28 | 12.42 | 12.70 | 13.04 | 13.19 |
| ICER | £1678 | £3330 | £2040 | £3756 | ||
| Exclude CVE events from model | ||||||
| Cost | £2488 | £3171 | £3736 | £2389 | £3252 | £3894 |
| QALY | 13.41 | 13.77 | 13.90 | 14.48 | 14.82 | 14.96 |
| ICER | £1896 | £4184 | £2557 | £4732 | ||
| Exclude CVE and RTA events from model | ||||||
| Cost | £21 | £1906 | £2726 | £21 | £2038 | £2920 |
| QALY | 13.69 | 13.92 | 14.02 | 14.69 | 14.93 | 15.04 |
| ICER | ED | £8098 | ED | £8113 | ||
| APAP machine with 5-year life span and humidifier | ||||||
| Cost | £8150 | £8816 | £10,939 | £7979 | £8741 | £11,036 |
| QALY | 11.92 | 12.25 | 12.38 | 12.70 | 13.01 | 13.14 |
| ICER | £2017 | £16,362 | £2408 | £18,356 | ||
| Treatment effects from BRMA (APBM only) | ||||||
| Cost | £8132 | £8799 | £9283 | £7973 | £8737 | £9275 |
| QALY | 11.93 | 12.26 | 12.40 | 12.69 | 13.01 | 13.14 |
| ICER | £2003 | £3678 | £2412 | £4093 | ||
| Treatment effects from BRMA (APBM and office measurements) | ||||||
| Cost | £8139 | £8771 | £9237 | £7989 | £8728 | £9222 |
| QALY | 11.92 | 12.27 | 12.42 | 12.70 | 13.04 | 13.19 |
| ICER | £1787 | £3097 | £2170 | £3249 | ||
| Cost-effectiveness | Ayas et al., 2006122 | Mar et al., 2003123 | ResMed, 2007120 | Chilcott et al., 200044 |
|---|---|---|---|---|
| Study question | ||||
| Were costs and effects examined? | ✓ | ✓ | ✓ | ✓ |
| Were alternatives compared? | ✓ | ✓ | ✓ | ✓ |
| Are viewpoint/s clearly stated? | ✓ | ✓ | ✓ | ✓ |
| Selection of alternatives | ||||
| All relevant alternatives were compared | ✓ | ✓ | ✓ | ✓ |
| For the alternatives compared are all clearly described? | ✓ | ✓ | ✓ | ✓ |
| The rationale for choosing the alternative programmes compared is stated | ✓ | ✓ | ✓ | ✓ |
| Form of evaluation | ||||
| The choice of form of economic evaluation is justified in relation to questions addressed | ✓ | ✓ | ✓ | ✓ |
| If a cost minimisation analysis is chosen, have equivalent outcomes been adequately demonstrated? | NA | NA | NA | NA |
| Effectiveness data | ||||
| The sources of effectiveness estimates used are stated | ✓ | ✓ | ✓ | ✓ |
| Effectiveness data are from RCTs or review of RCTs | X | X | X | X |
| Potential biases are identified | ✓ | ✓ | X | ✓ |
| Details of method of synthesis or meta-analysis of estimates are given | ✓ | ✓ | ✓ | ✓ |
| Costs | ||||
| All the important and relevant resource use is included | ✓ | ✓ | ✓ | ✓ |
| All the important and relevant resource use is measured accurately | ✓ | ✓ | ✓ | ✓ |
| Appropriate unit costs are estimated | ✓ | ✓ | ✓ | ✓ |
| Unit costs are reported separately from resource use data | ✓ | ✓ | ✓ | ✓ |
| If productivity costs are included, are they treated separately from other costs? | ✓ | NA | X | X |
| The year and country to which unit costs apply are stated with appropriate adjustments for inflation and/or currency conversion | ✓ | ✓ | ✓ | X |
| Benefit measurement and valuation | ||||
| The primary outcome measure for the economic evaluation is clearly stated | ✓ | ✓ | ✓ | ✓ |
| Methods to value health states and other benefits are stated | ✓ | ✓ | ✓ | ✓ |
| Details of the individuals from whom valuations were obtained are given | ✓ | ✓ | ✓ | ✓ |
| Decision modelling | ||||
| Details of any model used are given | ✓ | ✓ | ✓ | NU |
| The choice of model used and the key input parameters on which it is based are adequately detailed and justified | ✓ | ✓ | ✓ | NU |
| All model outputs are described adequately | ✓ | ✓ | ✓ | NU |
| Discounting | ||||
| Discount rates are used for both costs and benefits | ✓ | ✓ | ✓ | ✓ |
| Do discount rates accord with current NHS guidance? | X | X | ✓ | X |
| Allowance for uncertainty: stochastic analysis of patient-level data | ||||
| Details of statistical tests and confidence intervals are given for stochastic data | NA | NA | NA | NA |
| Uncertainty around cost-effectiveness estimates are expressed | NA | NA | NA | NA |
| Sensitivity analysis is used to assess uncertainty in non-stochastic variables and analytic methods | NA | NA | NA | NA |
| Allowance for uncertainty: stochastic analysis of decision models | ||||
| Are all appropriate input parameters included with uncertainty? | ✓ | ✓ | ✓ | NU |
| Is second-order uncertainty (uncertainty in means) included rather than first-order uncertainty (uncertainty between patients)? | ✓ | X | ✓ | NU |
| Are the probability distributions adequately detailed and appropriate? | ✓ | X | X | NU |
| Sensitivity analysis is used to assess uncertainty in non-stochastic variables (e.g. unit costs) and analytic decisions (e.g. methods to handle missing data) | ✓ | ✓ | ✓ | NU |
| Deterministic analysis | ||||
| The approach to sensitivity analysis is given | ✓ | ✓ | ✓ | ✓ |
| The choice of variables for sensitivity analysis is justified | ✓ | ✓ | ✓ | ✓ |
| The ranges over which the variables are varied are stated | ✓ | ✓ | ✓ | ✓ |
| Presentation of results | ||||
| Incremental analysis is reported using appropriate decision rules | ✓ | ✓ | ✓ | ✓ |
| Major outcomes are presented in a disaggregated as well as an aggregated form | ✓ | ✓ | ✓ | ✓ |
| They are applicable to the UK setting | X | X | ✓ | ✓ |
Value of information analysis
The base case per episode EVPI was estimated to be £183 (male) and £202 (female) for a cost-effectiveness threshold of £20,000 per QALY. Assuming a lifetime for the technology of 5 years and incidence of OSAHS of 0.1% in the UK population aged between 16 and 65 (39 million) gives an effective population of 0.18 million (http://www.statistics.gov.uk/cci/nugget.asp?ID=949). This corresponds to a population EVPI of £33 million (male). When CVE and RTA events were excluded from the model, the population EVPI rises to approximately £51 million (based on per episode EVPI of £277 in men).
Chapter 5 Assessment of factors relevant to the NHS and other parties
It is unlikely that the implementation of CPAP as a treatment for OSAHS would have training requirements for clinicians that have major resource implications for the NHS. Consultant-level respiratory physicians are currently required to have completed a basic sleep apnoea training programme. Appropriate diagnosis is important and may have additional cost implications. The trials included in this technology assessment mainly used thorough diagnostic assessment (encompassing recordings of multiple physiological signals during sleep) to establish a diagnosis of OSAHS, and the findings of this review are applicable to a population where there has been an adequate diagnostic assessment. Trained staff and structured induction programmes are another feature of specialist units. The detailed consideration of what would constitute an appropriate diagnostic assessment and the associated cost implications were outside the remit of this technology appraisal.
In practice, dental devices are unlikely to be provided under NHS dental care, and in regions where they may be available under the NHS waiting times for treatment may need consideration. The cost-effectiveness model in this appraisal (the York model) considered NHS costs and PSS costs; incorporating private costs of dental care would reduce the cost-effectiveness of dental devices.
Chapter 6 Discussion
Statement of principal findings
Clinical effectiveness
The clinical effectiveness and safety of CPAP compared with best supportive care, placebo and dental devices for the treatment of OSAHS was investigated using systematic review and meta-analyses. The majority of studies in the review included participants with moderate daytime symptom severity (ESS) at baseline, who were male and overweight or obese. Several studies excluded patients who reported sleepiness while driving; this may indirectly have led to most studies having a mean baseline symptom severity that was classified as moderate. When disease at baseline was classified based on the AHI, most included studies were classified as being of severe disease populations.
The mean age of participants in the included studies ranged from 44 to 58 years and the duration of follow-up in most studies was between 4 and 12 weeks. We excluded studies that were restricted to patients with serious co-morbid conditions such as heart failure or Alzheimer’s disease; therefore, the findings may not be generalisable to those groups. Although 48 relevant studies were identified, the outcomes investigated varied and data for some outcomes were available from only a small number of studies. In general, there was inconsistency (statistical heterogeneity) in the treatment effect within groups of studies with the same comparators. Heterogeneity for the primary outcome of subjective sleepiness (as measured by the ESS) was reduced when studies were subgrouped based on mean severity of daytime symptoms at baseline, but not when subgrouping was based on the mean number of episodes of airway obstruction at night (AHI). This was possibly because ESS and AHI are not strongly correlated. It was considered appropriate to focus on the stratification of studies by symptom severity rather than the number of episodes of airway obstruction at night as the treatment of OSAHS is targeted mainly at controlling its symptoms and consequences, e.g. hypertension, rather than correcting the breathing disturbance itself. Any variation in the treatment effect discussed below is in relation to disease severity based on severity of daytime symptoms at baseline, as measured by the ESS.
There was clear evidence of a benefit with CPAP compared with placebo, conservative treatment/best supportive care, on two of the three primary outcomes, one assessing subjective symptoms of daytime sleepiness (ESS) and one objective measure of sleepiness (MWT). The benefit with CPAP on daytime sleepiness was robust across all the methodological subgroup analyses and sensitivity analyses. There was consistent evidence that the treatment effect increased with symptom severity at baseline. The evidence for any benefit with CPAP was less clear on the secondary outcome measures, although there was some evidence of a beneficial impact on quality of life and daytime MAP. The identified studies comparing CPAP with dental devices were in populations with symptoms of moderate severity. There was no statistically significant difference between CPAP and dental devices on any of the measures investigated. However, only a small number of studies was available and there was some inconsistency in the findings, making it difficult to draw firm conclusions. Despite obesity being an important aetiological factor for adult OSAHS, there were no studies directly comparing CPAP with weight loss interventions; where a weight loss programme was used, both the intervention and comparator group received this.
There was a lack of evidence on long-term outcomes such as stroke and cardiac events (with changes in event rates in the economic model being inferred from blood pressure changes rather than being measured directly), and a lack of direct evidence on RTAs and accidents in the workplace.
Daytime sleepiness
The primary outcome of interest for the clinical treatment of OSAHS is the control of excessive daytime sleepiness, for its symptomatic benefits and the consequences for tasks that require vigilance and the resistance of sleep onset, e.g. driving and employment performance. In this review, sleepiness was quantified as subjective daytime sleepiness as measured by the ESS and objective sleepiness as measured by the MWT and MSLT. These objective and subjective measures of sleepiness were used as primary outcomes, as the daytime consequences of OSAHS are the primary concern for patients. There was evidence that CPAP was more effective than placebo or conservative treatment/usual care in reducing symptoms of daytime sleepiness as measured by the ESS and MWT but not the MSLT. There was no statistically significant difference between CPAP and dental devices or CPAP and postural therapy on any of the three primary outcomes in a population classified as having moderate symptom severity at baseline.
The random-effects model that we used for the statistical pooling assumes that the effect of treatment differs in different populations, but that these effects cluster around a mean. The estimated MD for daytime sleepiness in the overall pooling was 2.7 points on the ESS, but might be anywhere between 2.0 and 3.5. However, this probably has limited generalisability, due to high statistical heterogeneity or inconsistency in the treatment effect. Heterogeneity was reduced when estimates were generated for studies subgrouped by mean baseline symptom severity. The benefit with CPAP was greatest in the group of trials of severe symptoms (MD –5.0 points, 95% CI –6.5 to –3.5), and was smaller with moderate (MD –2.3 points, 95% CI –3.0 to –1.6) and mild symptoms (MD –1.1 points, 95% CI –1.8 to –0.3). These were all statistically significant. It is not surprising to find a smaller benefit in the studies of populations that only had mild sleepiness prior to treatment. The estimate for mild disease was based on only two trials; therefore, this finding may not be robust. Although there was still moderate statistical heterogeneity in the subgroups, the direction of the treatment effect was consistently in favour of CPAP, with the exception of two studies.
There was a statistically significant benefit with CPAP compared with placebo/usual care on the ability to stay awake in a setting conducive to sleep (MWT). It is not possible to make a firm conclusion about whether the benefit with CPAP varied by disease severity as there were no trials of mild symptom severity and only one of severe. There was a benefit with CPAP in the single trial of a severe symptom severity population, which was apparently greater than that in the group of trials of moderate disease symptoms. There was no statistically significant difference between CPAP and control in the length of time it took participants to fall asleep in a setting conducive to sleep (MSLT), for any level of disease severity. This finding may have limited generalisability due to the evidence of inconsistency in the treatment effect which could not be adequately investigated. Again, only single trials were available of mild and severe symptomatic populations, making it impossible to draw firm conclusions about whether there may be a variation in the treatment effect in populations with different disease severity.
It is not surprising that the findings from the MSLT and MWT do not correspond, as, although the two tests appear to measure the same thing, time to onset of sleep, low correlations have been found between the two tests, implying that there is not a simple single dimension of sleepiness. 170 It has been suggested that the MSLT measures underlying arousal as well as propensity to sleep. American Academy of Sleep Medicine practice parameters state that use of MSLT is not routinely indicated for the diagnosis of OSAHS or for assessment of response to treatment. 171 The ESS and MWT may be more clinically meaningful in that they measure the ability to resist sleep. This has potentially more applicability to real-life situations, in which the ability to resist sleep while driving or carrying out a daily activity is important, than does the length of time it takes a person to fall asleep, when instructed to do so, as measured by the MSLT.
There was no statistically significant difference between CPAP and dental devices amongst a population with moderate symptom severity at baseline (where reported). The treatment effect is likely to differ in different groups of people, based on the random-effects model used. The average effect was a reduction in sleepiness of less than 1 point (0.9) in favour of CPAP compared with dental devices, but might be anywhere between an increase in sleepiness of 0.4 points with CPAP and a decrease in sleepiness of 2.1 points. However, it is unclear how generalisable this is within a moderate disease population as there was evidence of moderate inconsistency (heterogeneity) in the treatment effect. The effectiveness of CPAP compared with dental devices in severe and mild severity populations could not be estimated due to a lack of studies investigating these populations. Overall it is difficult to draw firm, clinically useful conclusions from the studies comparing CPAP with dental devices and postural therapy. Assessment of the comparative clinical effectiveness of CPAP and dental devices or postural therapy is limited by the number and consistency of available data and by the spectrum of patients studied. The studies of postural therapy, in particular, were very small trials and there was only one trial on each type of postural therapy.
Blood pressure
The studies assessing blood pressure had diverse populations; the proportion of hypertensive patients ranged from 15% to 100%. Day and night blood pressure were considered separately as the mechanisms and patterns of daytime and night-time blood pressure disturbance in OSAHS vary. Priority was given to daytime measures as the relationship between daytime blood pressure and vascular risk has been more clearly established and was more useful for the economic model. Based on studies using ABPM, there was a statistically significant benefit with CPAP compared with placebo/usual care in daytime MAP. Based on the random-effects pooling used, the size of the effect probably varies among different groups of people: the average reduction in daytime MAP was 2.1 mmHg, but might be anywhere between no reduction and 4.3 mmHg. When SBP and DBP were considered separately, the differences between CPAP and control were not statistically significant although there was a small decrease in both measures in favour of CPAP (MD –1.1 mmHg, 95% CI –3.4 to 1.2, and MD –1.2 mmHg, 95% CI –2.9 to 0.5, respectively). It should be noted that not all the trials in the analysis of MAP were the same as those in the SBP and DBP analyses. Therefore, the lack of a statistically significant effect for SBP and DBP may be due to differences in the study populations or methods.
The overall treatment effect for MAP did not appear to be robust. When individual studies were removed from the pooling the treatment effect remained statistically significant in only one instance. The analyses for all three blood pressure measures were based on a small number of trials and participants, and blood pressure was not always the primary outcome in the studies. Therefore, the risk of the analyses being underpowered to detect an effect is an important consideration. The subgroup analysis exploring variation in treatment effect with symptom severity was limited by the small number of trials available. The pooling of a small group of studies using conventional clinic blood pressure measurement showed a large and statistically significant improvement in SBP and DBP with CPAP compared with control. Given the evidence that a person’s actual blood pressure is more accurately reflected by the repeated measurements of ABPM than by conventional clinic measures,172 the results of the studies using ABPM probably provide a more generalisable estimate of the effect of treatment on blood pressure. However, there is always the possibility that there are important differences between these studies other than the method of blood pressure measurement.
Health-related quality of life (HRQoL)
There was evidence of a beneficial impact on HRQoL although the findings were somewhat inconsistent. This may have been related to a number of factors including the different types of quality of life outcome measures used, the small number of trials available or aspects of study design. It was not possible to explore these factors due to the small number of trials available. In general, the available data sets were too small to allow meaningful investigation of potential sources of heterogeneity. The included studies reporting these outcomes were of moderate and severely symptomatic populations; it is unclear whether similar benefits would be experienced by a mild disease population.
The most commonly reported quality of life measures were the FOSQ, the NHP and the SF-36, although the number of trials available for any single quality of life measure was small. Only one trial was identified that used a utility-based measure to inform the cost-effectiveness model. The majority of the trials were of moderate symptom severity populations (based on ESS). There was a statistically significant benefit with CPAP compared with placebo/usual care on the activity level and social outcome dimensions of the FOSQ (a condition-specific measure) when three trials of moderate disease and one of severe disease were pooled; and on the NHP (Part 2) in a moderate disease severity population. There was no statistically significant difference between CPAP and control on the SF-36 subscales. However, there was high inconsistency (statistical heterogeneity) for the emotional role and vitality subscales, limiting the reliability of these findings. It is therefore not appropriate to draw general conclusions from these analyses. When parallel and crossover trials were pooled separately, there was a benefit with CPAP compared with control on the SF-36 bodily pain, general health and physical function subscales in the parallel trial subgroup; this may have been driven by two trials of severely symptomatic populations.
Quality of life data regarding CPAP compared with dental devices (all moderate symptom severity) were inconsistent: on the FOSQ one study showed a statistically significant benefit with CPAP compared with dental devices and one found no difference; there was no statistically significant benefit on the SAQLI with CPAP; and on the SF-36 one study reported a statistically significant benefit with CPAP compared with dental devices on the physical and mental component subscales, one reported a statistically significant benefit on the bodily pain subscale with CPAP and there was no statistically significant difference on the total score in one study. There was no statistically significant difference between CPAP and postural therapy in any quality of life measure studied.
Psychological and cognitive outcomes
Assessment of the effects of CPAP on psychological outcomes was limited by the small number of trials investigating these outcomes. Subgroup analysis by baseline symptom severity was not feasible. The most commonly used scales were the General Health Questionnaire-28 (GHQ-28), Hospital Anxiety and Depression Scale (HADS) and University of Wales Mood Adjective Checklist (UMACL). There was no statistically significant benefit with CPAP compared with placebo/usual care on the GHQ-28 or HADS. However, there was evidence of inconsistency in the treatment effect (statistical heterogeneity) which could not be explored, making any firm conclusions difficult. On the UMACL there was a statistically significant benefit with CPAP compared with placebo. There was no statistically significant difference between CPAP and dental devices in a single trial using HADS.
Despite the substantial number of trials investigating cognitive outcomes, interpretation was difficult due to the wide range of tests used, non-uniform use of the same scales, variation in testing protocols and the difficulty in assessing the risk of a ceiling effect due to lack of information on how baseline performance compared with normative performance. The findings were contradictory from trials for individual cognitive tests, with some showing a benefit with CPAP and others not.
Cost-effectiveness
Published evidence and company submissions
Only one manufacturer submitted a full economic evaluation of CPAP – ResMed. This analysis used decision modelling and evidence drawn from a range of sources to estimate the costs and QALYs associated with CPAP versus a ‘do nothing’ option. The company’s estimated cost-effectiveness over 14 years was that CPAP dominated no treatment (i.e. CPAP was associated with higher QALYs and lower costs), although this varied over shorter time horizons. Comparing CPAP (auto) and CPAP (fixed), the analysis suggested that the former dominated the latter. There are a number of methodological weaknesses associated with the ResMed analysis including the following:
-
The results of a before and after study123 were used to examine the impact of no treatment compared with CPAP on HRQoL (in terms of utilities) associated with sleepiness. There are numerous limitations to this type of study in estimating treatment effects. Furthermore the approach effectively ignores the considerable RCT-based literature examining the efficacy and effectiveness of CPAP compared with other therapies.
-
ResMed did not include the full range of comparators and, at least for patients diagnosed with moderate/severe OSAHS, it is not clear that a ‘do nothing’ option represents typical clinical practice.
-
ResMed modelled cost-effectiveness results over a 14-year time horizon. However, OSAHS is a chronic condition; therefore, it is appropriate to model the results for a lifetime horizon.
-
There were shortcomings in the internal validity of the electronic model that may have led to inaccurate estimates of costs and QALYs.
Four published full economic evaluations of CPAP were identified and reviewed. 44,122,123124 Although they varied in terms of their detailed methods, there was moderate consistency in the estimates of cost-effectiveness with CPAP; estimates of the incremental cost per QALY gained with CPAP against no therapy ranged from about £1500 to £3000. These studies had several limitations including:
-
the failure to use a full range of clinical trial evidence for estimating the impact of treatment on daytime sleepiness, blood pressure, HRQoL and other relevant outcomes
-
a lack of evidence to compare the utility associated with different treatments for OSAHS
-
limited evidence (in terms of quantity and quality) on the long-term impact of OSAHS on HRQoL, CVEs and RTAs
-
the fact that none of the evaluations examined all the comparators relevant to this review.
York economic model
As a result of the limitations of existing cost-effectiveness studies of CPAP, a new model was developed. Its key features (compared with earlier models) were that it compared CPAP with relevant alternative treatment options (taken as conservative management and dental devices); it based the main estimate of effectiveness on the RCT evidence on sleepiness symptoms (based on the ESS), which were ‘mapped’ to utilities using individual patient data from a subset of studies; and it used trial evidence on changes in blood pressure following intervention to estimate differences in the rates of CVEs over time.
The York model found that, on average, CPAP was associated with higher costs and benefits compared with dental devices or conservative management. The incremental cost per QALY gained with CPAP, compared with dental devices, using base-case assumptions and an assumed age of 50 years, was £3899 for men and £4335 for women; the probability of CPAP being more cost-effective than dental devices and conservative management at a threshold of £20,000 per QALY was 0.78 and 0.80 for men and women respectively.
The York model is the first to compare CPAP with dental devices. It was noted earlier that differences between dental devices and CPAP in the effect on the ESS were not statistically significant. However, those differences were incorporated into the cost-effectiveness analysis and the uncertainty in these, as well as all other, parameters are reflected in the expressed decision uncertainty. The systematic review detailed in Chapter 3 included only trials in which CPAP was a comparator. As a consequence, trials comparing dental devices with placebo will not have been identified. Hence, the comparison between dental devices and conservative management is not based on the full range of available data. However, a recent systematic review of dental devices by Hoekema et al. identified few additional dental device versus placebo studies. 173
Clinically, the treatment effect on the ESS from CPAP, relative to conservative therapy, was greater in patients with greater baseline severity of OSAHS. When this was reflected in the cost-effectiveness analysis by looking at the cost-effectiveness of CPAP in separate severity groups, the ICER (probability of being cost-effective at a threshold of £20,000 per QALY) varied between £20,585 (0.43) and £4413 (0.98) in patients with mild and severe disease respectively. In mild and severe disease, it was only possible to compare the cost-effectiveness of CPAP with conservative management given the absence of trials of dental devices in those patients. Furthermore, given the lack of evidence on the relative treatment effects of the alternative therapies on blood pressure and RTAs by baseline OSAHS severity, these estimates do not factor in differential effects on CVEs or RTAs, so are likely to be an underestimate.
A series of other subgroup and scenario analyses found that the ICER of CPAP was consistently below £20,000 per QALY gained (when there is no distinction between baseline severity of disease). Typically the cost-effectiveness of interventions was lower (i.e. the ICER was higher) in women than in men; this may be due to the fact that women have a lower baseline risk of CVD and RTAs, giving less potential for QALY gains. They also typically have a longer life expectancy, resulting in higher treatment costs compared with no treatment. CPAP remained cost-effective when the age of the hypothetical cohort was increased or decreased by 15 years. Although the target population for this appraisal is patients aged 16 or older, the clinical trials typically included older patients and so the results may not be generalisable to a younger cohort. As mentioned previously, the generalisability to cohorts older than the patient population included in the trials may be compromised by the presence of additional co-morbidity in older people.
The greatest contribution to QALY gain was found to be the gain in utility associated with a reduction in ESS score with CPAP. The next most important factor in differentiating between the alternative treatments in terms of QALY gain was the rate of RTAs. The inclusion of CVEs reduced quality-adjusted survival by similar amounts for all three alternatives. A similar pattern was observed for costs, with the greatest difference between alternatives contributed by the cost of the device and associated care, followed by RTA costs and finally CVE costs.
The cost of the CPAP device is higher than the cost of dental devices or conservative management. However, the NHS and PSS costs of RTAs were lower with CPAP than with those relating to dental devices and conservative management as fewer events occur. The costs of CVD differed little between the alternative treatment strategies. A consequence of the reduced risk of fatal RTAs or fatal CVEs with CPAP was that more patients remained alive and at risk of a non-fatal CVE, partially offsetting any savings from a reduced risk of events overall. Omitting the impact of CVD had little effect on the study results. Even when incorporating the larger relative risk reduction for CVEs implied by the MacMahon et al. 145 study, the reduction in blood pressure associated with CPAP contributed little to the estimation of its cost-effectiveness. When the impact of CVD and RTAs was omitted, the incremental cost of CPAP compared with usual care increased, but the ICER remained low at £8098. Note that the results of this analysis are relevant for patients who do not drive.
The per episode EVPI was high, indicating that the cost of decision uncertainty may be high. Reliable data relating to incidence of OSAHS were not found; therefore, the mortality rate of men aged 35 was used as an approximation (0.1%) in order to calculate the population EVPI, assuming a lifetime for the technology of 5 years. This indicated that the upper boundary for the value of further research was between £33 million and £50 million. Further investigation may be warranted to identify those parameters that contribute most to the decision uncertainty. Including the prevalent patient population in those able to benefit from additional research would increase the EVPI considerably. The indication is that the value of information gained from further research may well exceed the costs of undertaking that research.
Based on the regression analysis to predict utility scores, the EQ-5D and SF-6D utility models indicated that an increase of 1 point in ESS was associated with a decline in utility of 0.01. A crude comparison of the ESS and utility data from Chakravorty et al. 97 indicates that, for a 1-point drop in ESS, an increase in utility of 0.005 was found based on the EQ-5D valuations and 0.03 based on the standard gamble valuations. The 0.23 improvement in utility post treatment, on the basis of a before and after analysis of standard gamble valuations, has been used in a previous economic evaluation by Ayas et al. 122 without an attempt to link in the available clinical evidence. The crude comparison of ESS and utility data from Jenkinson et al. 152 indicated that, for a 1-point drop in ESS, an increase in utility was found of 0.009 based on the EQ-5D valuations. Therefore, our analysis seems to be in line with previously published estimates.
Hypothetically, the sensitivity of EQ-5D scores to changes in sleepiness could be limited as the instrument does not contain a question specifically directed at sleepiness or energy and wakefulness. However, the EQ-5D instrument could still capture the health effects of sleepiness, e.g. in terms of its effects on usual activities or anxiety and depression. This analysis suggests that this concern may be unfounded, as employing the utility scores calculated from SF-6D, which does include a question about energy and vitality, produced strikingly similar results in the set of individual patient-level data available.
The York model considered NHS and PSS costs and, therefore, omitted any private costs of health care. In practice, however, according to our clinical experts, dental devices are infrequently provided under NHS dental care. Private costs of dental devices were estimated at an initial cost of over £600 which, if included in the analysis, would reduce the cost-effectiveness of dental devices.
Strengths and limitations of the assessment
Clinical effectiveness
While there is clear and robust evidence of a benefit with CPAP compared with placebo/usual care in relation to daytime sleepiness, the finding of a variation in the treatment effect with disease severity needs to be interpreted with some caution. The factors of interest investigated (except for one post hoc analysis) were specified in advance and the number of factors investigated was kept as small as possible. In addition, the findings from the subgroup analyses make clinical sense. However, the subgroup analyses are based on summary data and the comparisons are therefore observational and are not based on randomised comparisons as in a trial or an individual patient data analysis. Therefore, the trend of a treatment effect by disease severity should not be considered definitive. In addition, although the cut-off points used to define disease (AHI) and symptom severity (ESS) are based on those used clinically, these are arbitrary cut-off points. The subgroup analyses for other outcomes were limited by the small number of studies available. However, because disease and symptom severity are thought to be clinically important factors in the response to treatment we have tried to make clear the clinical populations to which the findings refer.
The subgroup analyses also do not account for any potential confounding between the factors investigated; for example, studies using a sham CPAP comparator were less likely to be crossover trials. Where the treatment effect varied between crossover and parallel trials, this may not have been due to the study design but may have been related to the comparator used. There were other factors that may have had an influence on the treatment effect that it was not feasible to investigate, due to the limitations of the available data, such as study duration and the subtherapeutic pressure used for sham CPAP.
The findings for the primary outcome of subjective sleepiness were robust when only studies with adequate concealment of allocation were considered. However, the investigation of the impact of study quality (as defined by adequacy of concealment of allocation) on the findings was limited by the fact that only five studies reported that an adequate method had been used. It was also possible to investigate whether the findings where robust, for the ESS as an outcome measure, when only the studies using sham CPAP as a comparator were considered. Participant blinding was possible only in the studies in which CPAP was used as the comparator; effectively it provides the best placebo in this field. When only studies using a sham CPAP comparator were subgrouped by baseline symptom severity (ESS) the findings were similar to those using the complete data set subgrouped by baseline symptom severity (ESS), i.e. there was a statistically significant benefit with CPAP compared with sham CPAP for each of the three subgroups, and the treatment effect was largest for the severe symptom subgroup and was consecutively smaller for the moderate and mild groups. Only a small subset of studies included all the randomised patients in the analysis; therefore, as a group of studies there is a risk that the size of the treatment effect may have been slightly overestimated. It was not possible to investigate the impact of this; however, loss to follow-up was reasonably low in the majority of studies.
The benefit with CPAP compared with control on the ESS and MWT is consistent with previous systematic reviews. 48,50 A previous subgroup analysis reported a more pronounced effect of CPAP in participants with moderate and severe symptoms at baseline, although this was apparent in parallel trials only. 50 A systematic review published just as the current review was completed reported 24-hour mean blood pressure, using ABPM, as a primary outcome. 174 A small but statistically significant decrease in 24-hour MAP of 1.7 mmHg was reported (MD –1.7 mmHg, 95% CI –2.7 to –0.7). There was a statistically significant decrease in daytime MAP of a similar magnitude to the current review (–1.8 mmHg, 95% CI –3.3 to –0.2) compared with 2.1 mmHg (MD –2.1, 95% CI –4.3 to 0.0) in the current review. Unlike the current review, there was a significant improvement with CPAP compared with placebo for SBP [–2.3 mmHg, (95% CI –4.3 to –0.2) compared with –1.1 mmHg (95% CI –3.4 to 1.2)] in the current review and DBP [–2.9mm Hg, (95% CI –4.4 to –0.4) compared with –1.2 mmHg (95% CI –2.9 to 0.5)]. The recently published review used estimates for MAP from SBP and DBP where MAP was not reported and included data from two studies from which we could not get accurate estimates from the graphs and were not able to obtain the data from the authors. The additional power in the analysis may have been important in deriving a statistically significant benefit on SBP and DBP.
Cost-effectiveness
The York model is the first cost-effectiveness study to seek to reflect the implications for long-term costs and QALYs of a broad range of trial evidence on sleepiness and to compare all relevant treatment options in the NHS. It explores a range of scenarios and quantifies decision uncertainty and the EVPI. The analysis suggests that CPAP is cost-effective compared with dental devices and conservative management, assuming a cost-effectiveness threshold of £20,000, with one exception, i.e. the ICER in a subgroup with mild disease in terms of baseline ESS score was estimated to be £20,585.
This is consistent with previously published economic evaluations. However, the York model additionally provided an estimate of the value of further research, which indicated that the cost of the uncertainty associated with the model parameters was high. The EVPI was calculated based only on the incident patient population and does not incorporate uncertainty in model structure, modelling assumptions and data quality. Given this, it may underestimate the cost of the decision uncertainty. When interpreting the results of the York model some caveats must be borne in mind:
-
The translation of health benefits in terms of ESS to utility scores was based on simple regression models derived from just three sets of patient-level data.
-
The patient-level data on which the regression models were based came from predominantly patients receiving CPAP. To address this problem, future trials would ideally incorporate generic instruments to provide a direct measure of preference-based HRQoL.
-
The effect of CPAP treatment on reducing RTAs was derived from observational studies. While it would not seem feasible to conduct an RCT to measure such a rare effect, it would be preferable to have been able to link this information in some way to the information obtained in the systematic review.
-
While some trials report the impact of CPAP on blood pressure, this outcome is infrequently reported, and the trials are too short in length to directly measure impact on CVEs, and so estimated changes in CVE rates are inferred from other published risk equations.
Uncertainties
-
The effectiveness (and hence cost-effectiveness) of using CPAP to treat mild disease remains uncertain due to a paucity of research; the treatment effect for daytime sleepiness in the current review is based on only two studies.
-
The relative treatment benefits with CPAP according to symptom severity are based on summary data and cannot be viewed as definitive.
-
The patients studied in most trials tend to be middle-aged and predominantly male. It is unclear whether therapeutic benefits are similar in other groups, in particular the elderly, in whom cognitive impairment and cerebrovascular disease are more prevalent and the OSAHS may be complicated.
-
There is evidence of a benefit with CPAP on MAP although this finding was not robust, possibly due to an underpowered analysis. In addition, it remains unclear in what patient groups this benefit might be expected to be found in terms of disease severity and blood pressure status at baseline.
-
The evidence of a fall in MAP implies a reduction in cardiovascular risk, but this has not been directly studied and the magnitude of the risk for end-organ cardiovascular damage is therefore uncertain.
-
Dental devices may be a treatment option in moderate disease. However, there was inconsistency in the treatment effect comparing CPAP and dental devices, possibly due to the variety of dental devices investigated. It remains unclear precisely what type of devices may be effective and in which populations with OSAHS. The effectiveness of dental devices compared with CPAP in mild and severe disease populations is unclear.
-
Only two outcome measures from the clinical trial data (effect of treatment on ESS and SBP) were incorporated in the economic model. Potentially some of the other measures reported in the trials could impact on HRQoL independently of ESS and this is not reflected in the current model. In particular, the model does not differentiate between conservative management, dental devices and CPAP in terms of the disutility associated with undesirable side effects from treatments themselves, which may be expected to differ between the technologies.
-
The estimates of cost-effectiveness of CPAP by baseline severity in OSAHS should be considered with caution. Although there was clear heterogeneity in ESS treatment effects in the overall meta-analysis in Chapter 3, it was only possible to group trials by severity using average study-level data. Furthermore, because it was not possible to estimate treatment effects on blood pressure or RTAs by baseline OSAHS severity, these effects have been removed entirely from this analysis.
Other relevant factors
The trials included in this technology assessment mainly used thorough diagnostic assessment to establish a diagnosis of OSAHS and the findings of this review are applicable to a population in which there has been an adequate diagnostic assessment. In view of the diagnostic complexity of sleep apnoea, adequate diagnostic assessment should include a multichannel sleep study reported by an appropriately trained physician (such as a consultant respiratory physician). The detailed consideration of what would constitute an appropriate multichannel sleep study and the associated cost implications were outside the remit of this technology appraisal.
CPAP does not deal with one of the key underlying causes of the disease, which is obesity. It was not possible to assess the relative benefits of CPAP over weight loss interventions due to a lack of appropriate data. The main focus of the current review was CPAP for the treatment of OSAHS, but a recent systematic review comparing lifestyle interventions with placebo found no relevant studies and identified a need for research addressing obesity. 30
Chapter 7 Conclusions
Implications for service provision
-
CPAP is an effective treatment for OSAHS compared with best supportive care and placebo in populations with moderate to severe symptoms and there may be benefits where the disease is mild. In populations with moderate to severe symptoms there is robust evidence of improvement in symptoms of daytime sleepiness.
-
There is evidence of benefit in blood pressure and quality of life with CPAP, although some uncertainty remains about these outcomes.
-
Dental devices may be a treatment option in moderate disease but some uncertainty remains.
-
On average, CPAP was associated with higher costs and higher benefits than were dental devices or conservative management. The probability of CPAP being more cost-effective than dental devices or conservative management was high for a cost-effectiveness threshold of £20,000 per QALY gained.
Suggested research priorities
-
An EVPI analysis suggested that because the cost of any decision uncertainty may be high, the value of further research to investigate the parameters contributing most to the decision uncertainty may exceed the costs of that research.
-
There is uncertainty about the effectiveness of CPAP for populations with OSAHS with mild daytime sleepiness and further investigation of the effectiveness of CPAP for this population is required.
-
Although dental devices are not as cost-effective as CPAP, they do provide an alternative treatment option for patients who cannot tolerate CPAP. The trial evidence comparing dental devices with CPAP was not extensive and had some limitations; therefore, further trials may be useful. Given the heterogeneity of devices in use, the research should identify the most effective type and address whether there are specific patient groups that do or do not benefit.
-
Further investigation of the effect of CPAP on hypertension and which populations might be expected to benefit in terms of OSAHS disease severity and normotensive and hypertensive patients would be beneficial.
-
Currently changes in cardiovascular events have to be inferred from changes in blood pressure; therefore, clinical trials that are adequately powered to identify changes in CVEs would be beneficial.
-
The populations studied in current trials are mostly male and middle-aged. Clinical trials to define treatment effects at the extremes of age (particularly in the elderly, in whom cardiovascular co-morbidity complicates assessment) and in women would be beneficial.
-
More comprehensive data are required on side effects, and this information should be systematically obtained and reported in any future research.
Acknowledgements
Thanks to Professor John Stradling for providing clinical expertise and interpretation of evidence used in the assessment and to Dr Mark Ter-Berg and Adrian Zacher for information on resource use associated with dental devices.
Thanks to the following authors of clinical effectiveness trials who responded to queries or provided data: Aarnoud Hoekema, John Wilding, Melanie Cross, Joel Dimsdale, David Hui, Francisco García Río and Sophie West. Thanks also to TJ Lasserson and colleagues for providing data from their Cochrane review.
Contribution of authors
Catriona McDaid (Research Fellow) was involved in the clinical effectiveness part of the review, writing the protocol, study selection, data extraction, quality assessment, data analysis and report writing. Susan Griffin (Research Fellow) was involved in the cost-effectiveness section, study selection, development of the economic model and report writing. Helen Weatherly (Research Fellow) was involved in the cost-effectiveness section, study selection, development of the economic model and report writing. Kate Durée (Research Fellow) was involved in the clinical effectiveness section including writing the protocol, study selection, data extraction, quality assessment, data analysis and report writing. Mariëlle van der Burgt (Visiting Researcher) was involved in the cost-effectiveness section, study selection, development of the economic model and report writing. Sue van Hout (Research Fellow) contributed to the quality of life section for the background and summarised the studies reporting utility data which were used to inform the economics section. Jo Akers (Information Officer) devised the search strategy, carried out the literature searches and wrote the search methodology sections of the report. Robert Davies (Reader in Respiratory Medicine) provided technical and clinical advice and commented on drafts of the report. Mark Sculpher provided input at all stages of the review, commented on drafts of the report and took overall responsibility for the economic section. Marie Westwood (Reviews Manager) provided input at all stages of the review, commented on drafts of the report and took overall responsibility for the review.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA Programme or the Department of Health.
References
- Stradling JR, Davies RJ. Sleep 1: Obstructive sleep apnoea/hypopnoea syndrome: definitions, epidemiology, and natural history. Thorax 2004;59:73-8.
- Douglas NJ, Polo O. Pathogenesis of obstructive sleep apnoea/hypopnoea syndrome. Lancet 1994;344:653-5.
- Gibson GJ, Douglas NJ, Stradling JR, London DR, Semple SJ. Sleep apnoea: clinical importance and facilities for investigation and treatment in the UK: addendum to the 1993 Royal College of Physicians sleep apnoea report. J R Coll Physicians Lond 1998;32:540-4.
- Gibson GJ. Obstructive sleep apnoea syndrome: underestimated and undertreated. Br Med Bull 2004;72:49-64.
- Sleep related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research . The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667-89.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5.
- Johns MW. Reliability and factor analysis of the Epworth sleepiness scale. Sleep 1992;15:376-81.
- Arand D, Bonnet M, Hurwitz T, Mitler M, Rosa R, Sangal RB. The clinical use of the MSLT and MWT. Sleep 2005;28:123-44.
- Krieger AC, Ayappa I, Norman RG, Rapoport DM, Walsleben J. Comparison of the maintenance of wakefulness test (MWT) to a modified behavioral test (OSLER) in the evaluation of daytime sleepiness. J Sleep Res 2004;13:407-11.
- Bennett LS, Langford BA, Stradling JR, Davies RJ. Sleep fragmentation indices as predictors of daytime sleepiness and nCPAP response in obstructive sleep apnea. Am J Respir Crit Care Med 1998;158:778-86.
- Davies RJ, Stradling JR. The epidemiology of sleep apnoea. Thorax 1996;51:S65-70.
- Engleman HM, Douglas NJ. Sleep. 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax 2004;59:618-22.
- Wright J, Johns R, Watt I, Melville A, Sheldon T. Health effects of obstructive sleep apnoea and the effectiveness of continuous positive airways pressure: a systematic review of the research evidence. BMJ 1997;314:851-60.
- Boland LL, Shahar E, Iber C, Knopman DS, Kuo TF, Nieto FJ, et al. Measures of cognitive function in persons with varying degrees of sleep-disordered breathing: the Sleep Heart Health Study. J Sleep Res 2002;11:265-72.
- Saunamaki T, Jehkonen M. A review of executive functions in obstructive sleep apnea syndrome. Acta Neurol Scand 2007;115:1-11.
- Engleman HM, Douglas NJ. Sleep, driving and the workplace. Clin Med 2005;5:113-17.
- Hack MA, Choi SJ, Vijayapalan P, Davies RJ, Stradling JR. Comparison of the effects of sleep deprivation, alcohol and obstructive sleep apnoea (OSA) on simulated steering performance. Respir Med 2001;95:594-601.
- George CF, Boudreau AC, Smiley A. Simulated driving performance in patients with obstructive sleep apnea. Am J Respir Crit Care Med 1996;154:175-81.
- Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep 2004;27:453-8.
- Moyer CA, Sonnad SS, Garetz SL, Helman JI, Chervin RD. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med 2001;2:477-91.
- Shipper H, Clinch J, Powell V, Spilker B. Quality of life assessments in clinical trials. New York, NY: Raven Press; 1990.
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 health survey: manual and interpretation guide. Lincoln, RI: QualityMetric Inc; 2000.
- Hunt SM, McEwen J, McKenna SP. Measuring health status: a new tool for clinicians and epidemiologists. J R Coll Gen Pract 1985;35:185-8.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208.
- Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 1997;20:835-43.
- Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med 1998;158:494-503.
- McNicholas WT, Bonsignore MR. the Management Committee of EU Cost Action B26. Sleep apnoea as an independent risk factor for cardiovascular disease. Current evidence, basic mechanisms and research priorities. Eur Respir J 2007;29:156-78.
- Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 2003;290:1906-14.
- Robinson GV, Stradling JR, Davies RJ. Sleep 6: Obstructive sleep apnoea/hypopnoea syndrome and hypertension. Thorax 2004;59:1089-94.
- Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2001;1. https://doi.org/10.1002/14651858.CD002875.
- Smith I, Lasserson TJ, Wright J. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database of Systematic Reviews 2006;2. 10.1002/14651858.CD003002.pub2.
- Sundaram S, Bridgman SA, Lim J, Lasserson TJ. Surgery for obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2005;4. 10.1002/14651858.CD001004.pub2.
- Chong MS, Ayalon L, Marler M, Loredo JS, Corey-Bloom J, Palmer BW, et al. Continuous positive airway pressure reduces subjective daytime sleepiness in patients with mild to moderate Alzheimer’s disease with sleep disordered breathing. J Am Geriatr Soc 2006;54:777-81.
- Xie A, Skatrud JB, Puleo DS, Dempsey JA. Influence of arterial O2 on the susceptibility to posthyperventilation apnea during sleep. J Appl Physiol 2006;100:171-7.
- Nadar S, Prasad N, Taylor RS, Lip GY. Positive pressure ventilation in the management of acute and chronic cardiac failure: a systematic review and meta-analysis. Int J Cardiol 2005;99:171-85.
- Ryan CM, Usui K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax 2005;60:781-5.
- Haniffa M, Lasserson TJ, Smith I. Interventions to improve compliance with continuous positive airway pressure for obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2004;4. 10.1002/14651858.CD003531.pub2.
- Popescu G, Latham M, Allgar V, Elliott MW. Continuous positive airway pressure for sleep apnoea/hypopnoea syndrome: usefulness of a 2 week trial to identify factors associated with long term use. Thorax 2001;56:727-33.
- McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999;159:1108-14.
- Krieger J, Kurtz D, Petiau C, Sforza E, Trautmann D. Long-term compliance with CPAP therapy in obstructive sleep apnea patients and in snorers. Sleep 1996;19:S136-43.
- Martins de Araujo MT, Vieira SB, Vasquez EC, Fleury B. Heated humidification or face mask to prevent upper airway dryness during continuous positive airway pressure therapy. Chest 2000;117:142-7.
- Hailey D, Jacobs P, Mayers I, Mensinkai S. The current status of autotitrating continuous positive airway pressure systems in the management of obstructive sleep apnea. Can Respir J 2005;12:271-6.
- Chai CL, Pathinathan A, Smith B. Continuous positive airway pressure delivery interfaces for obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2006;4. 10.1002/14651858.CD005308.pub2.
- Chilcott J, Clayton E, Chada N, Hanning CD, Kinnear W, Waterhouse JC. Nasal continuous positive airways pressure in the management of sleep apnoea. Leicester: Trent Institute for Health Services Research; 2000.
- Hoekema A, Stegenga B, De Bont LG. Efficacy and co-morbidity of oral appliances in the treatment of obstructive sleep apnea–hypopnea: a systematic review. Crit Rev Oral Biol Med 2004;15:137-55.
- Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep 2006;29:244-62.
- Kushida CA, Morgenthaler TI, Littner MR, Alessi CA, Bailey D, Coleman J, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep 2006;29:240-3.
- Marshall NS, Barnes M, Travier N, Campbell AJ, Pierce RJ, McEvoy RD, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax 2006;61:430-4.
- Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003;163:565-71.
- Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database of Systematic Reviews 2006;3. 10.1002/14651858.CD001106.pub3.
- Curtin F, Altman DG, Elbourne D. Meta-analysis combining parallel and cross-over clinical trials. I: continuous outcomes. Stat Med 2002;21:2131-44.
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 2002;31:140-9.
- Lim J, Lasserson TJ, Fleetham J, Wright J. Oral appliances for obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2006;1. 10.1002/14651858.CD004435.pub3.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 2006. www.cochrane.dk/cochrane/handbook/handbook.htm.
- Vickers AJ, Altman DG. BMJ 2001;323:1123-4.
- Arias MA, Garcia-Rio F, Alonso-Fernandez A, Mediano O, Martinez I, Villamor J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation 2005;112:375-83.
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60.
- Dimsdale JE, Loredo JS, Profant J. Hypertension 2000;35:144-7.
- Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med 1998;157:858-65.
- Skinner MA, Kingshott RN, Jones DR, Homan SD, Taylor DR. Elevated posture for the management of obstructive sleep apnea. Sleep Breath 2004;8:193-200.
- Skinner MA, Kingshott RN, Jones DR, Taylor DR. Lack of efficacy for a cervicomandibular support collar in the management of obstructive sleep apnea. Chest 2004;125:118-26.
- Coughlin SR, Mawdsley L, Mugarza JA, Wilding JPH, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese men with OSA [epub ahead of print]. Eur Respir J 2007. 10.1183/09031936.0043306.
- Arias MA, Garcia-Rio F, Alonso-Fernandez A, Martinez I, Villamor J. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure. A randomized, controlled cross-over study. Eur Heart J 2006;27:1106-13.
- Hui DS, To KW, Ko FW, Fok JP, Chan MC, Ngai JC, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness. Thorax 2006;61:1083-90.
- Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, Merino-Sanchez M, Gonzalez-Benitez MA, Beltran-Robles M, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest 2006;129:1459-67.
- Spicuzza L, Bernardi L, Balsamo R, Ciancio N, Polosa R, Di Maria G. Effect of treatment with nasal continuous positive airway pressure on ventilatory response to hypoxia and hypercapnia in patients with sleep apnea syndrome. Chest 2006;130:774-9.
- West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. The effect of CPAP on insulin resistance and HBA1C in people with obstructive sleep apnoea and type 2 diabetes: a randomised controlled trial. Thorax 2006;61.
- Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J 2006;27:1229-35.
- Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Continuous positive airway pressure therapy reverses the impaired arterial stiffness in normotensive patients with obstructive sleep apnea. Sleep Med 2006;7.
- Lam B, Sam K, Mok W, Cheung MT, Fong DY, Lam JC, et al. A randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax 2007;62:354-9.
- Hoekema A. Efficacy and comorbidity of oral appliances in the treatment of obstructive sleep apnea–hypopnea: a systematic review and preliminary results of a randomized trial. Sleep Breath 2006;10:102-3.
- Cibele D-F, Garbuio SA, D’Almeida V, Santos RF, Bittencourt LR, Tufik S. Efficacy of an oral appliance (OA) compared with nCPAP over oxidative stress parameters in obstructive sleep apnea (OSA) patients: preliminary results. Sleep Med 2006;7.
- Norman D, Loredo JS, Nelesen RA, Ancoli-Israel S, Mills PJ, Ziegler MG, et al. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure. Hypertension 2006;47:840-5.
- Egea C, Pinto J, Ayuela J, Ballester E, Zamarron C, Sojo A, et al. Efficacy of the treatment with CPAP in patients with chronic heart failure and sleep apnoea. Eur Respir J 2004;24.
- Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003;348:1233-41.
- Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 2004;169:361-6.
- Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet 1999;353:2100-5.
- Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999;159:461-7.
- Marshall NS, Neill AM, Campbell AJ, Sheppard DS. Randomised controlled crossover trial of humidified continuous positive airway pressure in mild obstructive sleep apnoea. Thorax 2005;60:427-32.
- Ferguson KA, Ono T, Lowe AA, al Majed S, Love LL, Fleetham JA. A short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. Thorax 1997;52:362-8.
- Ferguson KA, Ono T, Lowe AA, Keenan SP, Fleetham JA. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild–moderate obstructive sleep apnea. Chest 1996;109:1269-75.
- Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med 2004;170:656-64.
- Barbé F, Mayoralas LR, Duran J, Masa JF, Maimo A, Montserrat JM, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med 2001;134:1015-23.
- Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Peter JH. Effect of therapeutic and subtherapeutic nasal continuous positive airway pressure (nCPAP) treatment on blood pressure in obstructive sleep apnea (OSA). Sleep Med 2003;4.
- Henke KG, Grady JJ, Kuna ST. Effect of nasal continuous positive airway pressure on neuropsychological function in sleep apnea–hypopnea syndrome. A randomized, placebo-controlled trial. Am J Respir Crit Care Med 2001;163:911-17.
- Montserrat JM, Ferrer M, Hernandez L, Farre R, Vilagut G, Navajas D, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med 2001;164:608-13.
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling JR, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002;359:204-10.
- Cross MD, Al-Abri M, Newby DE, Riha R, Vennelle M, Douglas NJ. RCT evidence that CPAP improves endothelial function in obstructive sleep apnea–hypopnea syndrome (OSAHS) 2005. www.abstracts2view.com/ats05/.
- Barnes M, Houston D, Worsnop CJ, Neill AM, Mykytyn IJ, Kay A, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med 2002;165:773-80.
- Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet 1994;343:572-5.
- Engleman HM, Gough K, Martin SE, Kingshott RN, Padfield PL, Douglas NJ. Ambulatory blood pressure on and off continuous positive airway pressure therapy for the sleep apnea/hypopnea syndrome: effects in “non-dippers”. Sleep 1996;19:378-81.
- Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of CPAP therapy on daytime function in patients with mild sleep apnoea/hypopnoea syndrome. Thorax 1997;52:114-9.
- Engleman HM, Martin SE, Kingshott RN, Mackay TW, Deary IJ, Douglas NJ. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax 1998;53:341-5.
- Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea–hypopnea syndrome. Am J Respir Crit Care Med 2001;163:344-8.
- McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea–hypopnea syndrome: a randomized controlled trial. Am J Respir Crit Care Med 2001;164:1459-63.
- Ballester E, Badia JR, Hernandez L, Carrasco E, de Pablo J, Fornas C, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999;159:495-501.
- Chakravorty I, Cayton RM, Szczepura A. Health utilities in evaluating intervention in the sleep apnoea/hypopnoea syndrome. Eur Respir J 2002;20:1233-8.
- Lim LL. Effect of CPAP therapy on pain, disability and quality of life in chronic daily headache and sleep apnea: a randomized controlled trial. Neurology 2005;64.
- Lojander J, Maasilta P, Partinen M, Brander PE, Salmi T, Lehtonen H. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest 1996;110:114-9.
- Monasterio C, Vidal S, Duran J, Ferrer M, Carmona C, Barbé F, et al. Effectiveness of continuous positive airway pressure in mild sleep apnea–hypopnea syndrome. Am J Respir Crit Care Med 2001;164:939-43.
- Fleetham JA, Lowe A, Vazquez JC, Ferguson K, Flemons W, Remmers JA. A long term randomized parallel multicentre study of an oral appliance vs nCPAP in the treatment of obstructive sleep apnea. Am J Respir Crit Care Med 2002.
- Hoekema A, Stegenga B, Wijkstra H, van der Hoeven JH, Meinesz AF, de Bont LG. Effectiveness of obstructive sleep apnea therapy: a randomized parallel clinical trial of oral-appliance versus continuous positive airway pressure therapy. Sleep Med 2006;7.
- Engleman HM, McDonald JP, Graham D, Lello GE, Kingshott RN, Coleman EL, et al. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med 2002;166:855-9.
- L’Estrange PR, Smith C, Grant HR, Makker HK, Spiro SG. Pilot randomised trial of nasal continuous positive airway pressure (CPAP) ventilation versus a mandibular advancement splint (MAS) in severe obstructive sleep apnoea (OSA): early difficulties 1999.
- Randerath WJ, Heise M, Hinz R, Ruehle KH. An individually adjustable oral appliance vs continuous positive airway pressure in mild-to-moderate obstructive sleep apnea syndrome. Chest 2002;122:569-75.
- Tan YK, L’Estrange PR, Luo YM, Smith C, Grant HR, Simonds AK, et al. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: a randomized cross-over trial. Eur J Orthod 2002;24:239-49.
- Olson LG, Ambrogetti A, Trevillian Z. A randomised crossover comparison of nasal CPAP and a mandibular advancement splint in mild obstructive sleep apnea 2002.
- Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest 1999;115:771-81.
- Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003;107:68-73.
- Lim LL. Effect of CPAP therapy on pain, disability and quality of life in chronic daily headache and sleep apnea: a randomized controlled trial. Neurology 2005;64:A422-3.
- Hoekema A, Stel A-L, Stegenga B, van der Hoeven JH, Wijkstra PJ, van Driel F, et al. Sexual function and obstructive sleep apnea–hypopnea: a randomized clinical trial evaluating the effects of oral-appliance and continuous positive airway pressure therapy. J Sex Med 2006. 10.1111/j.743–6109.2006.00341.x.
- Engleman HM, Carruthers ER, Kingshott RN, McArdle N, Faccenda JF, Douglas NJ. Randomized trial of MRS vs CPAP therapy for the sleep apnea/hypopnea syndrome (SAHS). Am J Respir Crit Care Med 1999;159.
- Lojander J, Kajaste S, Maasilta P, Partinen M. Cognitive function and treatment of obstructive sleep apnea syndrome. J Sleep Res 1999;8:71-6.
- Hoekema A, Stegenga B, Bakker M, Brouwer WH, de Bont LG, Wijkstra H, et al. Simulated driving in obstructive sleep apnoea–hypopnoea; effects of oral appliances and continuous positive airway pressure. Sleep Breath 2007. 10.1007/s11325-006-0093-7.
- Lezak MD. Neuropsychological assessment. New York: Oxford University Press; 1995.
- Lim W, Bardwell WA, Loredo JS, Kim E, Ancoli-Israel S, Dimsdale JE. Effects of two-week continuous positive airway pressure treatment and supplemental oxygen on neuropsychological functioning in patients with obstructive sleep apnea: a randomized placebo-controlled study. Sleep 2005;28.
- Bardwell WA, Ancoli-Israel S, Berry CC, Dimsdale JE. Neuropsychological effects of one-week continuous positive airway pressure treatment in patients with obstructive sleep apnea: a placebo-controlled study. Psychosom Med 2001;63:579-84.
- Hack M, Davies RJ, Mullins R, Choi SJ, Ramdassingh-Dow S, Jenkinson C, et al. Randomised prospective parallel trial of therapeutic versus subtherapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnoea. Thorax 2000;55:224-31.
- Land M, Horwood J. Which parts of the road guide steering?. Nature 1995;377:339-40.
- Fleetham JA, Lowe A, Vazquez JC, Ferguson K, Flemons W, Remmers JA. A long term randomized parallel multicentre study of an oral appliance vs nCPAP in the treatment of obstructive sleep apnoea. Am J Respir Crit Care Med 1998.
- ResMed . Cost-Effectiveness and cost–utility of Using Continuous Positive Airways Pressure in the Treatment of Severe Obstructive Sleep Apnoea Hypopnoea Syndrome in the UK. ResMed Submission for CPAP Technology Appraisal 2007.
- Drummond M, Sculpher M, Torrance G, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Ayas NT, FitzGerald JM, Fleetham JA, White DP, Schulzer M, Ryan CF, et al. Cost-effectiveness of continuous positive airway pressure therapy for moderate to severe obstructive sleep apnea/hypopnea. Arch Intern Med 2006;166:977-84.
- Mar J, Rueda JR, Duran-Cantolla J, Schechter C, Chilcott J. The cost-effectiveness of nCPAP treatment in patients with moderate-to-severe obstructive sleep apnoea. Eur Respir J 2003;21:515-22.
- Tousignant P, Cosio MG, Levy RD, Groome PA. Quality adjusted life years added by treatment of obstructive sleep apnea. Sleep 1994;17:52-60.
- Fisher & Paykel Healthcare . Continuous Positive Airways Pressure (CPAP) for the Treatment of Obstructive Sleep Apnoea Hypopnoea Syndrome (OSAHS). MTA Submission to the National Institute for Health and Clinical Excellence 2007.
- Respironics (UK) Ltd . Continuous Positive Airway Pressure Treatment for Obstructive Sleep Apnoea Hypopnoea Syndrome (OSAHS). Submission to the National Institute for Health and Clinical Excellence 2007.
- EuroQoL Group . EuroQoL – A new facility for measurement of health-related quality of life. Health Policy 1990;16:199-208.
- Dolan P, Gudex C, Kind P, Williams A. The time trade-off method: results from a general population study. Health Econ 1996;5:141-54.
- Torrance GW, Feeny D. Utilities and quality-adjusted life years. Int J Technol Assess Health Care 1989;5:559-75.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53.
- George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001;56:508-12.
- Mazza S, Pepin JL, Naegele B, Rauch E, Deschaux C, Ficheux P, et al. Driving ability in sleep apnoea patients before and after CPAP treatment: evaluation on a road safety platform. Eur Respir J 2006;28:1020-8.
- Department of Transport . Highways Economic Note No.1 2004. www.dft.gov.uk/pgr/roadsafety/ea/highwayseconomicnoteno12004.
- Findley L, Smith C, Hooper J, Dineen M, Suratt PM. Treatment with nasal CPAP decreases automobile accidents in patients with sleep apnea. Am J Respir Crit Care Med 2000;161:857-9.
- Marquez-Baez C, Paniagua-Soto J, Castilla-Garrido JM. Treatment of sleep apnea syndrome with CPAP: compliance with treatment, its efficacy and secondary effects. Rev Neurol 1998;26:375-80.
- Department of Health . NHS Reference Costs 2005–06 2006. www.dh.gov.uk/PublicationsAndStatistics/Publications/PublicationsPolicyAndGuidance/PublicationsPolicyAndGuidanceArticle/fs/en?CONTENT_ID=4141135%26chk=7wrsFg.
- Taylor RS, Watt A, Dalda HM, . Home-based cardiac rehabilitation versus hospital-based rehabilitation: a cost-effectiveness analysis. Int J Cardiol 2006.
- Department of Health . Reducing Brain Damage: Faster Access to Better Stroke Care 2005.
- Krieger J, Meslier N, Lebrun T, Levy P, Phillip-Joet F, Sailly JC, et al. Accidents in obstructive sleep apnea patients treated with nasal continuous positive airway pressure: a prospective study. Chest 1997;112:1561-6.
- Findley LJ, Unverzagt ME, Suratt PM. Automobile accidents involving patients with obstructive sleep apnea. Am Rev Respir Dis 1988;138:337-40.
- Engleman H, Asgari-Jihandeh N, McLeod L, Ramsay CF, Deary IJ, Douglas NJ. Self-reported use of CPAP and benefits of CPAP therapy: a patient survey. Chest 1996;109:1470-6.
- Horstmann S, Hess CW, Bassetti C, Gugger M, Mathis J. Sleepiness-related accidents in sleep apnea patients. Sleep 2000;23:383-9.
- Suratt P, Findley L. Effect of nasal CPAP treatment on automobile driving simulator performance and on self-reported automobile accidents in subjects with sleep apnea. Am Rev Respir Dis 1992;145.
- Cassel W, Ploch T, Becker C, Dugnus D, Peter JH, Von Wichert P. Risk of traffic accidents in patients with sleep-disordered breathing: reduction with nasal CPAP. Eur Respir J 1996;9:2606-11.
- Yamamoto H, Akashiba T, Kosaka N, Ito D, Horie T. Long-term effects of nasal continuous positive airway pressure on daytime sleepiness, mood and traffic accidents in patients with obstructive sleep apnoea. Respir Med 2000;94:87-90.
- Graham JD, Thompson KM, Goldie SJ, Segui-Gomez M, Weinstein MC. The cost-effectiveness of air bags by seating position. JAMA 1997;278:1418-25.
- Jenkinson C, Davies RJ, Mullins R, Stradling JR. Long-term benefits in self-reported health status of nasal continuous positive airway pressure therapy for obstructive sleep apnoea. QJM 2001;94:95-9.
- MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990;335:765-74.
- Waterhouse JC, Brazier JE, Billings CG, . Can a two week trial of CPAP treatment return patients’ perception of vitality to that of the local population?. Eur Respir J 2000;16.
- Waterhouse JC, Brazier JE, Billings CG, . Can a health status questionnaire demonstrate change after a two week trial of CPAP treatment?. Eur Respir J 2000;16.
- Brazier J, Usherwood T, Harper R, Thomas K. Deriving a preference-based single index from the UK SF-36 Health Survey. J Clin Epidemiol 1998;51:1115-28.
- Clark GT, Blumenfeld I, Yoffe N, Peled E, Lavie P. A crossover study comparing the efficacy of continuous positive airway pressure with anterior mandibular positioning devices on patients with obstructive sleep apnea. Chest 1996;109:1477-83.
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. The effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes [personal communication] n.d.
- Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res 1997;6:199-204.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J. Health Econ. 2002;21:271-92.
- Chung L-H, Kind P. Exchanging SF-12 to EQ-5D: caveat emptor [submitted to journal]. Med Decis Making 2007.
- Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006;26:410-20.
- Currie C, McEwan P, Peters J, Patel TC, Dixon S. The routine collation of health outcomes data from hospital treated subjects in the Health Outcomes Data Repository (HODaR): Descriptive analysis from the first 20,000 subjects. Value Health 2005;8:581-90.
- Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J 1990;121:293-8.
- Mora S, Yanek L, Moy T, Fallin D, Becker L, Becker D. Interaction of Body Mass Index and Framingham Risk Score in predicting incident coronary disease in families. Circulation 2005;111:1871-6.
- Riley R, Abrams K, Lambert P, Sutton AJ, Thompson JR. An evaluation of bivariate random-effects meta-analysis for the joint synthesis of two correlated outcomes. Stat Med 2006;26:78-97.
- Riley RD, Abrams KR, Sutton AJ, Lambert PC, Thompson JR. Bivariate random effects meta-analysis and the estimation of between-study correlation. BMC Med Res Methodol 2007;7. 10.1186/471-2288-7-3.
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomised controlled trials. J Clin Epidemiol 1997;50:683-91.
- Barbé F, Sunyer J, de la Pena A, Pericas J, Mayoralas LR, Anto JM, et al. Effect of continuous positive airway pressure on the risk of road accidents in sleep apnea patients. Respiration 2007;74:44-9.
- Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al. Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation. Health Technol Assess 2004;8.
- Chiang CL. Introduction to stochastic processes in biostatistics. New York: John Wiley; 1968.
- Dennis MS, Burn JP, Sandercock PA, Bamford JM, Wade DT, Warlow CP. Long-term survival after first-ever stroke: the Oxfordshire Community Stroke. Stroke 1993;24:796-800.
- Rosengren A, Wilhelmsen L, Hagman M, Wedel H. Natural history of myocardial infarction and angina pectoris in a general population sample of middle-aged men: a 16-year follow-up of the Primary Prevention Study, Göteborg, Sweden. J Intern Med 1998;244:495-50.
- Curtis L, Netten A. Unit costs of health and social care 2006. Personal Social Services Research Unit (PSSRU); 2006.
- The Information Centre . NHS Dental Statistics for England. Quarter 1: 30 June 2006 2006. www.ic.nhs.uk/pubs/nhsdentq2.
- Edwards MJ, Brickley MR, Goodey RD, Shephard JP. The cost, effectiveness and cost-effectiveness of removal and retention of asymptomatic, disease-free third molars. Br Dent J 1999;187:380-4.
- Briggs A, Mihaylova B, Sculpher M, Hall A, Wolstenholme J, Simoons M, et al. The cost-effectiveness of perindopril in reducing cardiovascular events in patients with stable coronary artery disease using data from the EUROPA study [online publication]. Heart 2006. 10.1136/hrt.2005.086728.
- Bravo-Vergel Y, Palmer S, Asseburg C, Fenwick E, de Belder M, Abrams K, et al. Is primary angioplasty cost-effective in the UK? Results of a comprehensive decision analysis [in press]. Heart 2007.
- Bonnet MH. ACNS clinical controversy: MSLT and MWT have limited clinical utility. J Clin Neurophysiol 2006;23:50-8.
- Littner MR, Kushida CA, Wise MS, Davila D, Morgenthaler TI, Lee-Chiong T, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep 2005;28:113-21.
- O’Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens 2003;21:821-48.
- Hoekema A, Stegenga B, De Bont LG. Efficacy and co-morbidity of oral appliances in the treatment of obstructive sleep apnea–hypopnea: a systematic review. Crit Rev Oral Biol Med 2004;15:137-55.
- Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome. Arch Intern Med 2007;167:757-65.
- Evaluation of the Application of Nocturnal Nasal Continuous Positive Airway Pressure in the Treatment of Obstructive Sleep Apnoea 1992.
- Resta O, Guido P, Picca V, Scarpelli F, Foschino MP, Legari G, et al. Bilevel positive pressure (BiPAP) in sleep apnea (SA) patients. Chest 1997;112.
- Shashikumar RH, Fulambarker A, Crisostomo I, Cohen M. Long-term effectiveness of nasal continuous positive airway pressure in mild sleep apnea compared with conservative measures. Chest 2002;122.
- Martinez-Garcia MA, Galiano-Blancart R, Roman-Sanchez P, Soler-Cataluna JJ, Cabero-Salt L, Salcedo-Maiques E. Continuous positive airway pressure treatment in sleep apnea prevents new vascular events after ischemic stroke. Chest 2005;128:2123-9.
- Adlakha A, Gross PT. Comparison of sleep arousals before and after CPAP titration in patients with obstructive sleep apnea. Sleep 2006;29.
- Ahmed T, Zein JG, Younis WG, Ali F, Tawk MM, Kinasewitz GT. Long-term effect of CPAP therapy on blood pressure control in patients with obstructive sleep apnea (OSA). Chest 2005;128.
- Akashiba T, Minemura H, Horie T. The influence of nasal continuous positive airways pressure (CPAP) on nocturnal hypertension in obstructive sleep apnea (OSA) patients. Sleep 1993;16:S35-6.
- Almirall J, Masdeu MJ, Ferrer A, Martinez-Ocana JC, Lopez T, Prats T, et al. Prevalence of obstructive sleep apnea among patients with resistant arterial hypertension. Effects of CPAP on blood pressure. J Hypertens 2005;23.
- Anonymous . Nasal CPAP reduces day and night blood pressure in obstructive sleep apnea. Cardiology Review 2003;20.
- Antic N, Clark R, Hensley M, Naughton M, Rowland S, Windler S, et al. Assessing neurocognitive function in moderate to severe obstructive sleep apnea before and after CPAP therapy. Sleep 2006;29.
- Ayas NT, Mancini GBJ, Fleetham J. Does CPAP delay the development of cardiovascular disease in patients with obstructive sleep apnoea hypopnoea?. Thorax 2006;61:459-60.
- Babar SI, Quan SF. Continuous positive airway pressure: Placebo power, or does it really work?. Arch Intern Med 2003;163:519-20.
- Badia JR, Hernandez L, Leon C, Alarcon A, Ballester E, Rodriguez-Roisin R, et al. Efficacy of CPAP treatment in moderate to severe sleep apnea hypopnea syndrome (SAHS). Eur Respir J 1997;10.
- Bakshi MS, Hoskere G, Mehta JB, Chidambaram B, Ponder M, Girish MR. Circulatory response in sleep apnea patients during sleep before and after CPAP treatment. Sleep 2005;28.
- Barry H. Is nasal continuous positive airway pressure effective in reducing symptoms of obstructive sleep apnea?. Evid Based Pract 1999;2:9-10.
- Becker H, Kohler U, Peter JH, von Wichert P, Horne JA. Sleep ‘88. Stuttgart: Hustav Fischer Verlag; 1989.
- Becker H, Grote L, Ploch T, Schneider H, Stammnitz A, Peter JH, et al. Intrathoracic pressure changes and cardiovascular effects induced by nCPAP and nBiPAP in sleep apnoea patients. J Sleep Res 1995;4:125-9.
- Beecroft JM, Zanon S, Lukic D, Hanly PJ. Oral CPAP for sleep apnea: Effectiveness, patient preference and compliance. Sleep 2003;26:A209-10.
- Berka C, Westbrook PR, Lumicao MN, Olmstead R, Levendowski DJ, Davis G, et al. Influence of sleep-disordered breathing on neurocognitive functions pre- and posttreatment with CPAP. Sleep 2006;29:A208-9.
- Bloch MJ, Basile J. Short-term treatment of sleep apnea with nocturnal continuous positive airway pressure does not improve blood pressure in patients with well controlled hypertension. J Clin Hypertens 2006;8:673-5.
- Bradley TD, Takasaki Y, Orr D, Popkin J, Liu P, Rutherford R, et al. Sleep and respiration: proceedings of the first International Symposium on Sleep and Respiration held in Banff, Alberta, April 1–4, 1989. New York, NY: Wiley-Liss; 1990.
- Braghiroli A, Sacco C, Carli S, Rossi S, Donner CF. Autocontinuous positive airway pressure in the diagnosis and treatment of obstructive sleep apnoea. Monaldi Arch Chest Dis 1998;53:621-4.
- Buechner NJ, Zidek W, Esser M, Haske M, Sanner BM. Obstructive sleep apnea syndrome. Effects of therapy on dyslipidemia. Somnologie 2001;5:97-102.
- Buttner A, Ruhle KH. Quality of life before and during nCPAP. Comparison of different questionnaires – SWLS, MLDL and SF-36. Pneumologie 2004;58:651-9.
- Castronovo VE, Baietto C, Castaldi P, Di Gioia MR, Zucconi M, Ferini-Strambi L. Obstructive sleep apnea syndrome and cognitive functions: the effect of CPAP treatment. Sleep 2003;26:A252-3.
- Chakravorty I, Sapiano S, Ruprai M, Cayton R. Quality of life in patients on long term continuous positive airway pressure for obstructive sleep apnoea. Thorax 1998;53.
- Chasens ER, Weaver TE, Maislin G, Dinges D, Pack A. Sleepiness and adherence to CPAP treatment in adults with OSA. Sleep 2003;26.
- Chazan R, Bielicki P, Byskiniewicz K, Rubinsztajn R, Kumor M. The influence of 8 months of therapy with nasal continuous positive airway pressure (NCPAP) on leptin serum concentration and sympathetic activity in patients with obstructive sleep apnea syndrome (OSAS). Chest 2004;126.
- Chrysostomakis SI, Simantirakis EN, Marketou ME, Schiza SE, Mavrakis HE, Klapsinos NC, et al. Effects of continuous positive airway pressure therapy on autonomic nervous system activity in patients with obstructive sleep apnoea hypopnoea syndrome. Eur Heart J 2004;25.
- Ciftci TU, Ciftci B, Guven SF, Kokturk O, Turktas H. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respir Med 2005;99:529-34.
- Deegan PC, McNicholas WT. Effects of (nCPAP) on cardiac function awake and asleep. J Sleep Res 1995;4:59-63.
- Dhillon S, Chung SA, Fargher TJ, Shapiro CM. CPAP decreases blood pressure in sleep apnea patients with hypertension. Sleep 2003;26.
- Donadio V, Montagna P, Vetrugno R, Contin M, Falzone F, Baruzzi A, et al. CPAP treatment lowers sympathetic nerve activity and blood pressure in hypertensive obstructive sleep apnoea patients. J Neurol 2006;253.
- Dorkova Z, Tkacova R. Initiating treatment with auto-CPAP in patients with severe obstructive sleep apnea and arterial hypertension: effects on systemic blood pressure and heart rate. J Sleep Res 2006;15.
- Douglas NJ, Engleman HM. CPAP therapy: outcomes and patient use. Thorax 1998;53:S47-8.
- Douglas NJ. Treatment of the obstructive sleep apnea/hypopnea syndrome: the effect on blood pressure. Sleep 2004;27:842-3.
- Drummond FE, Doelken P, Frye MD. Empiric treatment of clinically diagnosed obstructive sleep apnea using auto-titrating continuous positive airway pressure. Chest 2005;128.
- Engleman HM, Cheshire KE, Deary IJ, Douglas NJ. Daytime sleepiness, cognitive performance and mood after continuous positive airway pressure for the sleep apnoea/hypopnoea syndrome. Thorax 1993;48:911-14.
- Engleman HM. When does ‘mild’ obstructive sleep apnea/hypopnea syndrome merit continuous positive airway pressure treatment?. Am J Respir Crit Care Med 2002;165:743-5.
- Fairbairn SEA, Richards J, Gregory M, Hack M. The effect of continuous positive airway pressure and mandibular advancement splint therapy on steering simulation in obstructive sleep apnoea syndrome (OSAS). American Thoracic Society; 2006.
- Ficker JH, Fischer C, Wiest GM, Lehnert G, Hahn EG. Efficacy of auto-CPAP in the treatment of obstructive sleep apnoea syndrome (OSA). Eur Respir J 1997;10.
- Ficker JH, Clarenbach CF, Neukirchner C, Fuchs FS, Wiest GH, Schahin SP, et al. Efficacy and compliance of autoadjusting CPAP therapy based on the forced oscillation technique. Am J Respir Crit Care Med 2002;165.
- Fitzpatrick MF, Driver HS, Willing S, San PM. Does continuous positive airway pressure (CPAP) after nasal resistance [Abstract online]. American Thoracic Society; 2005.
- Flemons W, Lowe A, Vazquez JC, Ferguson K, Remmers J, Fleetham J. Quality of life in patients with obstructive sleep apnea treated either with an oral appliance or CPAP. Am J Respir Crit Care Med 1998;157.
- Fletcher EC. Cardiovascular effects of continuous positive airway pressure in obstructive sleep apnea. Sleep 2000;23:S154-7.
- Gagnadoux F. The mandibular advancement splint: a true therapeutic alternative. Rev Mal Respir 2006;23:7S51-4.
- Golish JA. Therapeutic NCPAP was more effective than subtherapeutic NCPAP in obstructive sleep apnea. Commentary on Jenkinson C, Davies RJ, Mullins R et al. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet 1999 Jun 19;353: 2100– 5. ACP J Club 2000;132.
- Goncalves MA, Guilleminault C, Ramos E, Palha A, Paiva T. Erectile dysfunction, obstructive sleep apnea syndrome and nasal CPAP treatment. Sleep Med 2005;6:333-9.
- Gotsopoulos H, Chen C, Qian L, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med 2002;166:743-8.
- Grimm W, Koehler U, Fus E, Hoffmann J, Menz V, Funck R, et al. Outcome of patients with sleep apnea-associated severe bradyarrhythmias after continuous positive airway pressure therapy. Am J Cardiol 2000;86:688-92.
- Hahn PY, Hauri PJ, Sahai A, Staats BA. Insomnia and obstructive sleep apnea (OSA): evaluation and CPAP therapy. Sleep 2003;26:A300-1.
- Hermida RC, Zamarron C, Ayala DE, Fontao MJ, Ricoy J, Calvo C. Continuous positive airway pressure therapy has no effect on nocturnal blood pressure in patients with obstructive sleep apnea. J Hypertens 2003;21.
- Hermida RC, Zamarron C, Ayala DE, Fontao MJ, Ricoy J, Calvo C. Effect of continuous positive airway pressure therapy on ambulatory blood pressure in patients with obstructive sleep apnea. Am J Hypertens 2003;16.
- Hermida RC, Zamarron C, Ayala DE, Calvo C. Effect of continuous positive airway pressure on ambulatory blood pressure in patients with obstructive sleep apnoea. Blood Press Monit 2004;9:193-202.
- Hernandez L, Farre R, Ballester E, Navajas D, Montserrat JM. Compliance of sham-cpap compared with optimal cpap in sleep apnea syndrome. Eur Respir J 1999;14.
- Hetzel C, Weess HG, Schroeder A, Steinberg R. Parameters of subjective well-being of obstructive sleep apnea patients before and after nCPAP-therapy. Wien Med Wochenschr 1994;145:510-11.
- Hira HS. Obstructive sleep apnea syndrome: evaluation of subcritical continuous positive airway pressure. J Assoc Physicians India 1998;46:796-7.
- Hirshkowitz M, Sharafkhaneh A. Positive airway pressure therapy of OSA. Semin Respir Crit Care Med 2005;26:68-79.
- Hla KM, Skatrud JB, Finn L, Palta M, Young T. The effect of correction of sleep-disordered breathing on BP in untreated hypertension. Chest 2002;122:1125-32.
- Hoster M, Schlenker E, Ruhle KH, Schlafke ME. Leistung und Schlaf: Beitrage zum 3 Kongre- der Deutschen Gesellschaft fur Schlafforschung und Schlafmedizin (DGSM) vom 19 bis 21 Oktober 1995 an der Ruhr-Universitat Bochum. Germany. Wien: Bochum; 1995.
- Huang R, Huang XZ, Xiao Y. Evaluation of an auto-CPAP device for treatment of obstructive sleep apnea. Beijing Med J 2001;23:350-2.
- Iellamo F, Montana N. Continuous positive airway pressure treatment: Good for obstructive sleep apnea syndrome, maybe not for hypertension?. Chest 2006;129:1403-5.
- Itzhaki S, Pillar G, Lavie P, Lavie L. Endothelial function of patients with obstructive sleep apnea improves following 3 months on CPAP. J Sleep Res 2006;253.
- Juhasz J, Cassel W, Gutheil T, Becker H, Peter JH. Proportional positive airway pressure for the therapy of obstructive sleep apnea (OSA). Eur Respir J 1997;10.
- Kajaste S, Brander PE, Telakivi T, Partinen M, Mustajoki P. A cognitive-behavioral weight reduction program in the treatment of obstructive sleep apnea syndrome with or without initial nasal CPAP: a randomized study. Sleep Med 2004;5:125-31.
- Kaleth AS, Chittenden TW, Hawkins BJ, Gregg JM, Zedalis D, Pierson LM, et al. The effects of nCPAP therapy and aerobic exercise on daytime heart rate variability in patients with obstructive sleep apnea. Sleep 2003;26.
- Kaleth A, Chittenden TW, Hawkins BJ, Hargens TA, Guill S, Zedalis D, et al. Nasal CPAP therapy and aerobic exercise training: effects on the heart rate response to graded exercise in obstructive sleep apnea patients. Sleep 2005;28:A206-7.
- Karacan I, Karatas M. Annual Meeting of the American Psychiatric Association. Miami, FL: American Psychiatric Association; 1995.
- Kiely JL, Murphy M, McNicholas WT. Subjective efficacy of nasal CPAP therapy in obstructive sleep apnoea syndrome: a prospective controlled study. Eur Respir J 1999;13:1086-90.
- Konermann M, Sanner B, Klewer J, Kreuzer I, Laschewski F, Burmann-Urbanek M, et al. Leistung und Schlaf: Beitrage zum 3 Kongre- der Deutschen Gesellschaft fur Schlafforschung und Schlafmedizin (DGSM) vom 19 bis 21 Oktober 1995 an der Ruhr-Universitat Bochum. Bochum; Germany: Wien; 1995.
- Krieger J, Racineux JL, Huber P, Sautegeau A, Redondo J, Castaing Y, et al. A multicenter trial of a device for treating obstructive sleep apnea by continuous positive pressure. Bull Eur Physiopathol Respir 1986;22:393-7.
- Lafond C, Series F, Lemiere C. Impact of continuous positive airway pressure (CPAP) on airway responsiveness and asthma quality of life in subjects with asthma and sleep apnea syndrome. Chest 2005;128:S165-6.
- Lafond C, Series F, Lemiere C. Eur Respir J 2007;29:307-11.
- Lewis KE, Watkins AJ, Seale L, Bartle IE, Ebden P. Continuous positive airways pressure (CPAP) treatment can reduce anxiety and depression in obstructive sleep apnoea. Thorax 2002;57.
- Li F, Feng Q, Zhang X, Liu Q. Treatment for erectile dysfunction patients with obstructive sleep apnea syndrome by nasal continual positive airway pressure. Zhong Hua Nan Ke Xue 2004;10:355-7.
- Litvin A, Pevzner AV, Golitsyn PV, Galiavi RA, Mazygula EP, Nesterenko L, et al. New approaches to treatment of bradyarrhythmia in patients with obstructive sleep apnea syndrome. Ter Arkh 2006;78:41-7.
- Logan AG, Tkacova R, Perlikowski SM, Leung RS, Tisler A, Floras JS, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J 2003;21:241-7.
- Mador MJ, Krauza M, Pervez A, Pierce D, Braun M. Effect of heated humidification on nasal CPAP compliance and quality of life in patients with sleep apnea 2005. www.abstracts2view.com/ats05/.
- Malow B. Effects of treating obstructive sleep apnea in epilepsy [online project record]. National Library of Medicine; 2005.
- Marrone O, Milone F, Ferrara G, Romano L, Bellia V. Transmural pulmonary artery pressure during CPAP in obstructive sleep apnoea syndrome. Eur Respir J 1990;11:S544-5.
- Marshall MJ, Coughlin SR. Does positive airway pressure therapy (PAP) provide a symptomatic benefit at low levels of compliance in obstructive sleep apnoea patients?. Thorax 2004;59.
- Mayer J, Weichler U, Cassel W, Ploch T, Peter JH, von Wichert P. Does placebo treatment lower nocturnal blood pressure in patients with sleep-related breathing disorders and arterial hypertension?. Cardiology 1993;82:69-78.
- McArdle N, Kingshott R, Engleman HM, Mackay TW, Douglas NJ. Partners of patients with sleep apnoea/hypopnoea syndrome: effect of CPAP treatment on sleep quality and quality of life. Thorax 2001;56:513-18.
- McEvoy RD, Thornton AT. Treatment of obstructive sleep apnea syndrome with nasal continuous positive airway pressure. Sleep 1984;7:313-25.
- McFadyen TA, Espie CA, McArdle N, Douglas NJ, Engleman HM. Controlled, prospective trial of psychosocial function before and after continuous positive airway pressure therapy. Eur Respir J 2001;18:996-1002.
- McNab BD, Dang B, Cotton D, Skomro R, Gjevre J, Reid J. Patient satisfaction with empiric CPAP therapy for suspected obstructive sleep apnea. Sleep 2006;29.
- Morrish R, Shneerson JM, Smith IE. Comparison of mortality risk between men and women using CPAP for obstructive sleep apnea. Sleep 2004;27.
- Mulgrew AT, Fox N, Ayas NT, Ryan CF. Diagnosis and initial management of obstructive sleep apnea without polysomnography. Ann Intern Med 2007;146:157-66.
- Nagasaka Y, Nambu Y, Fujita E, Hazu R, Yoshida M, Ikeshita K. Comparison of the effect of nasal CPAP and mandibular advancement splint (MAS) in patients with obstructive sleep apnea (OSA). Eur Respir J 1997;10.
- Newsom-Davis IC, Lyall RA, Leigh PN, Moxham J, Goldstein LH. J Neurol Neurosurg Psychiatry 2001;71:482-7.
- Nooman YM, Hamid S, Woolford M, Sutton A. Experience with mandibular repositioning in the treatment of snoring and the obstructive apnoea–hypopnoea syndrome with and without nasal continuous positive airway pressure (N-CPAP). Thorax 1998;53.
- Pepin J, Baguet J, Lebrun M, Levy P, Pierre H, Peeters M, et al. Obstructive sleep apnea syndrome and left ventricular diastolic function: effects of nasal continuous positive airway pressure (CPAP). J Sleep Res 2006;15.
- Phillips BA, Schmitt FA, Berry DT, Lamb DG, Amin M, Cook YR. Treatment of obstructive sleep apnea. A preliminary report comparing nasal CPAP to nasal oxygen in patients with mild OSA. Chest 1990;98:325-30.
- Poluektov MG, Eligulashvili TS. Long-term positive air pressure in the treatment of sleep apnea syndrome. Ter Arkh 1994;66:85-7.
- Risk CG, Winzer JA. Impact of continuous positive airway pressure treatment on attention deficit in obstructive sleep apnea. Chest 2004;126.
- Rosenthal LD, Meixner RM, Schmidt-Nowara WW, Becker PM, Jamieson AO. The functional status outcome of OSA patients after two weeks of CPAP therapy. Sleep 2003;26.
- Rosenthal L, Dolan DC, Taylor DJ. Response to CPAP therapy in sleepy and non-sleepy OSA patients. Sleep 2006;29.
- Sakakibara H. Treatment of sleep apnea syndrome with oral appliances. Kokyu To Junkan 2005;53:301-8.
- Sanders MH, Strollo PJ. Positive airway pressure in the treatment of sleep-related breathing disorders. Semin Respir Crit Care Med 1998;19:147-56.
- Sanders MH. Article reviewed: daytime sleepiness and EEG spectral analysis in apneic patients before and after treatment with continuous positive airway pressure. Sleep Med 2001;2:263-5.
- Sanner B, Fluerenbrock N, Kleiber-Imbeck A, Mueller J, Zidek W. Effect of CPAP therapy on infectious complications in patients with OSAS. Eur Respir J 2000;16.
- Sanner B, Markmann A, Zidek W, Tepel M. Continuous positive airway pressure therapy reduces blood pressure in hypertensive patients with obstructive sleep apnea – a prospective study. J Hypertens 2003;21.
- Schutte-Rodin S, Spencer G, Stache S, Colleen B, Staley B, Katzka D, et al. Defining and scoring gastroesophageal reflux events in obstructive sleep apnea patients during polysomnography with and without CPAP. Sleep 2006;29.
- Seiler SC, Keller M, Hurlimann U, Klinger K, Brandli O. Effect of CPAP-therapy on neuropsychological functioning in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 1998;157.
- Shadan F, Jalowayski AA, Kline LE, Dawson A. Nasal inflammation and carriage of Staphylococcus aureus in patients with obstructive sleep apnea treated with nasal CPAP. Sleep 2006;29:A193-4.
- Shimizu M, Tachibana N, Nagasaka Y, Goto M. Obstructive sleep apnea (OSA) in RA patients and effect of CPAP on RA activity. Arthritis Rheum 2003;48.
- Skomro RP, King L, Mink J. Efficacy and safety of empiric continuous positive airway pressure therapy in subjects with obstructive sleep apnea. Chest 2003;124.
- Smith LJ, Skrekas JE, Arnedt J, Millman RP, Stanchina M, Aloia MS. CPAP use predicts change in depressive symptoms 3 months post-treatment in obstructive sleep apnea (OSA). Sleep 2005;28.
- Stammnitz A, Becker H, Schneider H, Peter JH, Wichert PV. Risks and complications during positive airway pressure treatment of obstructive sleep apnea. Pneumologie 1993;49:190-4.
- Stoohs RA, Facchini FS, Philip P, Valenciaflores M, Guilleminault C. Selected cardiovascular risk-factors in patients with obstructive sleep-apnea – effect of nasal continuous positive airway pressure (N-Cpap). Sleep 1993;16:S141-2.
- Suhner AG, Darko DD, Erman MK, Riel KF, Mitler MM. Depressive symptoms in patients with OSA and the impact of nasal CPAP treatment. Sleep 2003;26.
- Veale D, Cornette A, Joet FP, Pepin JL, Escourrou P, Meurice JC. Evaluation of CPAP efficacy in long term home treatment for obstructive sleep apnea syndrome. American Thoracic Society; 2003.
- Verbraecken JA, Willemen M, Van de Heyning P, De Backer WA. Daytime lung inflation after bilevel positive airway pressure therapy (bipap) in obstructive sleep apnea with and without overlapsyndrome. Eur Respir J 2002;20.
- Voronin IM, Belov AM, Chuchalin AG. Placebo-controlled study of a short-term hypotensive effect of intranasal ventilation with continuous positive airway pressure therapy in patients with arterial hypertension stage I. Ter Arkh 2002;74:25-30.
- Weaver T. Impact of CPAP on functional outcomes in milder obstructive sleep apnea (CATNAP) [project record online]. National Library of Medicine; 2004.
- Weisfogel GM, Ash CE, Walters AS. Nocturnal angina caused by OSA with symptoms relieved by positive airway pressure. Sleep 2003;26.
- Weissenberg A, Zulley J, Bauer M. The influence of CPAP-treatment on stress coping and well-being sleep apnea patients. Wien Med Wochenschr 1994;145:523-4.
- Westbrook PR. Treatment of sleep disordered breathing: nasal continuous positive airway pressure (CPAP). Prog Clin Biol Res 1990;345:387-93.
- Westbrook PR, Berka C, Levendowski DJ, Lumicao M, Davis G, Olmstead R, et al. Quantification of alertness, memory and neurophysiological changes in sleep apnea patients following treatment with nCPAP. Sleep 2004;27.
- Wiest GH, Ficker JH, Lehnert G, Hahn EG. Prospective, randomised, placebo-controlled study on the effectiveness of a heated humidifier in the therapy of dry upper respiratory tracts under nCPAP by OSAS. Pneumologie 1998;52.
- Woodson BT, Steward DL, Weaver EM. Outcomes of non-attended home autosetting CPAP titration. Sleep Med 2003;4.
- Woodson BT, Steward DL, Weaver EM, Javaheri S. A randomized trial of temperature-controlled radiofrequency, continuous positive airway pressure, and placebo for obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 2003;128:848-61.
- Worsnop C, Naughton M, Barter C, Morgan T, Anderson A, Pierce R. Blood pressure and humoral effects of nasal continuous positive airway pressure (NCPAP) in hypertensives with obstructive sleep apnoea (OSA). Aust N Z J Med 1994;24.
- Yen FC, Behbehani K, Burk JR, Lucas EA, Axe J. Long term performance evaluation of an automatic airway positive pressure device. Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society 1997;18:2113-14.
- Zimmerman ME, Arnedt J, Skrekas J, Harris S, Smith L, Aloia MS. The relationship between time-on-CPAP and vigilance in sleep apnea at baseline, 3-months, and 6-months. Sleep 2005;28.
- Doff MH, Hoekema A, Stegenga B, Bakker M, Brouwer WH, de Bont LG, et al. Effectiveness of oral appliances and continuous positive airway pressure on simulated driving performance in obstructive sleep apnea–hypopnea syndrome: a randomised trial. Sleep Med 2006;7.
- Alonso-Fernandez A, Arias MA, Garcia-Rio F, Ramirez M, Fernandez-Lahera J. Impaired left ventricular performance during exercise in patients with obstructive sleep apnea–hypopnea syndrome improves with continuous positive airway pressure 2005. www.abstracts2view.com/ats05/.
- Bardwell WA, Loredo JS, Lim W, Ancoli-Israel S, Dimsdale JE. Effects of two-weeks nocturnal oxygen supplementation and continuous positive airway pressure treatment on psychological symptoms in patients with obstructive sleep apnea: a randomized placebo-controlled study. Sleep 2005;28.
- Arias MA, Alonso-Fernandez A, Garcia-Rio F, Santiago A, Lores V. Obstructive sleep apnea is independently associated with daytime pulmonary arterial hypertension that improves with continuous positive airway pressure treatment [Poster abstract 526] 2005. www.abstracts2view.com/ats05/.
- Arias MA, Alonso-Fernandez A, Garcia-Rio F, Mediano O, Rojo B. Obstructive sleep apnea syndrome affects left ventricular diastolic function effects on nasal continuous positive airway pressure [Poster abstract 525] n.d. www.abstracts2view.com/ats05/view.php?nu%20=%20ATS5L_1594.
- Becker H, Jerrentrup A, Ploch T, Grote L, Penzel T, Peter JH. Prospective randomized study on the effect of therapeutic and subtherapeutic nasal continuous positive airway pressure (NCPAP) treatment on blood pressure in obstructive sleep apnea (OSA). Am J Respir Crit Care Med 2001;163.
- Chakravorty I, Cayton RM, Szczepura A. Cost effectiveness analysis of nasal CPAP and lifestyle intervention in obstructive sleep apnoea. Thorax 2001;56:27-8.
- Coughlin SR, Mugarza JA, Mawdsley L, Wilding JP, Calverley PM. Continuous positive airways pressure treatment reduces the cardiovascular risk factors associated with obstructive sleep apnoea [Poster abstract 111] n.d. www.abstracts2view.com/ats/.
- Cross MD, Al-Abri M, Newby DE, Riha R, Vennelle M, Douglas NJ. Randomised controlled trial evidence that continuous positive airway pressure improves vascular function in obstructive sleep apnoea hypopnoea syndrome. Thorax 2005;60.
- Profant J, Ancoli-Israel S, Dimsdale JE. A randomized, controlled trial of 1 week of continuous positive airway pressure treatment on quality of life. Heart Lung 2003;32:52-8.
- Bao X, Nelesen RA, Loredo JS, Dimsdale JE, Ziegler MG. Blood pressure variability in obstructive sleep apnea: role of sympathetic nervous activity and effect of continuous positive airway pressure. Blood Press Monit 2002;7:301-7.
- Ziegler MG, Mills PJ, Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest 2001;120:887-93.
- Nelesen RA, Yu H, Ziegler MG, Mills PJ, Clausen JL, Dimsdale JE. Continuous positive airway pressure normalizes cardiac autonomic and hemodynamic responses to a laboratory stressor in apneic patients. Chest 2001;119:1092-101.
- Yu BH, Ancoli-Israel S, Dimsdale JE. Effect of CPAP treatment on mood states in patients with sleep apnea. J Psychiatr Res 1999;33:427-32.
- Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure vs placebo continuous positive airway pressure on sleep quality in obstructive sleep apnea. Chest 1999;116:1545-9.
- Engleman H, McDonald J, Graham D, Lello G, Douglas N. Randomized controlled crossover trial of CPAP vs MRS therapy for the sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 2001;163.
- Faccenda J, Boon N, Mackay T, Douglas N. Quality of life on and off CPAP in patients with sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 2000;161.
- Faccenda JF, Boon NA, Douglas NJ. Randomised trial of CPAP on blood pressure in SAHS. Am J Respir Crit Care Med 1999;159.
- Hui DS, To KW, Ko FW, Fok JP, Chan MC, Ngai JC, et al. A randomized subtherapeutic CPAP controlled study of the effects of nasal CPAP on 24-hour systemic blood pressure in obstructive sleep apnoea syndrome n.d. www.abstracts2view.com/ats06/.
- Hack M, Choi R, Mullins R, Dow S, Davies RJ, Stradling JR. Performance of patients with obstructive sleep apnoea (OSA) on a steering simulation after six months treatment with nasal continuous positive airway pressure (NCPAP). Thorax 1999;54.
- Hack MA, Davies RJ, Stradling JR. Randomised, sham placebo, parallel study of the effect of nasal continuous positive airway pressure (NCPAP) on steering performance in patients with obstructive sleep apnoea (OSA) – interim analysis. Thorax 1998;53.
- Stradling JR, Jenkinson C, Davies RJ, Mulllins B. Randomised, sham-placebo controlled, parallel study of nasal continuous positive airway pressure (NCPAP) for the treatment of obstructive sleep apnea [abstract]. Am J Respir Crit Care Med 1998;157.
- Stradling JR, Jenkinson C, Davies RJ, Mullins B. Randomised, sham-placebo, parallel study of nasal continuous positive airway pressure (NCPAP) for the treatment of obstuctive sleep apnoea (OSA). Thorax 1998;53.
- Hack MA, Davies RJO, Mullins R, Stradling JR. Randomised, sham placebo controlled study of nasal continuous positive airway pressure (NCPAP) on simulated steering in obstructive sleep apnoea (OSA). Am J Respir Crit Care Med 1999;159.
- Stradling JR, Jenkinson C, Davies RJ, Mullins B. Randomised, sham placebo controlled, parallel study of nCPAP on quality of life (SF36) in obstructive sleep apnoea. Am J Respir Crit Care Med 1999;159.
- Lam B, Sam K, Orth M, Mok W, Lam J, Yam L, et al. A randomized study of three non-surgical treatment options in mild to moderate obstructive sleep apnea 2005. www.abstracts2view.com/ats05/.
- Lam B, Sam K, Cheung MT, Mok W, Lam J, Tsang KWT, et al. A randomized controlled study for the treatment of mild/moderate onstructive sleep apnea (OSA). Sleep Med 2003;4.
- Campbell AJ, Marshall NS, Sheppard DS, Neil AM. Randomised placebo controlled trial of humidified CPAP in mild obstructive sleep apnoea (OSA). Respirology 2004;9.
- Marshall N, Sheppard DS, Campbell AJ, Neill AM. Randomised controlled crossover trial of continuous positive airway pressure in the treatment of mild–moderate obstructive sleep apnoea using a mechanical placebo. Intern Med J 2004;34.
- Vidal S, Navarro A, Duran J, Bariera R, Carmona C, Botebol G, et al. Effectiveness of CPAP in mild sleep apnea/hypopnea syndrome (SAHS) compared with conservative treatment. Eur Respir J 2000;16.
- Loredo JS, Ancoli-Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP-controlled study. Sleep 2006;29:564-71.
- Mills PJ, Kennedy BP, Loredo JS, Dimsdale JE, Ziegler MG. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol 2006;100:343-8.
- von Kanel R, Loredo JS, Ancoli-Israel S, Dimsdale JE. Association between sleep apnea severity and blood coagulability: treatment effects of nasal continuous positive airway pressure. Sleep Breath 2006;10:139-46.
- Loredo JS, Ancoli-Israel S, Kim E, Lim W, Dimsdale JE. Effect of continuous positive airway positive airway pressure and supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP controlled study. Sleep 2005;28.
- Bardwell WA, Norman D, Ancoli-Israel S, Loredo JS, Lowery A, Lim W, et al. Effects of 2-week nocturnal oxygen supplementation and continuous positive airway pressure treatment on psychological symptoms in patients with obstructive sleep apnea: a randomized placebo-controlled study. Behav Sleep Med 2007;5:21-38.
- Lim W, Loredo JS, Kim E, Ancoli-Israel S, Morgan EE, Heaton RK, et al. Neuropsychological effects of two week continuous positive airway pressure treatment and supplemental oxygen in patients with obstructive sleep apnea: a randomized controlled study. J Clin Sleep Med 2007;3:380-6.
- Pepperell JC, Mullins B, Dow SR, Crosthwaite N, Stradling JR, Davies RJ. Blood pressure change after treatment for obstructive sleep apnoea (OSA) with continuous positive airway pressure (CPAP). Thorax 2000;55.
- Pepperell JC. Obstructive sleep apnoea and cardiovascular risk: the effects of nasal continuous positive airway pressure therapy. Cambridge: Cambridge University; 2005.
- Randerath W, Heise M, Galetke W, Hinz R, Ruhle KH. Prospective randomised comparison of a three-dimensional variable protrusion splint with CPAP in mild obstructive sleep apnoea syndrom (OSAS). Pneumologie 2001;55.
- Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. CPAP does not reduce 24 hour blood pressure in hypertensive obstructive sleep apnoea patients without daytime sleepiness. Thorax 2004;59.
- Tan YK, L’Estrange PR, Grant HR, Smith C, Simonds AK, Spiro SG. A randomised crossover study of continuous positive airway pressure (CPAP) vs mandibular advancement splint (MAS) in mild and moderate obstructive sleep apnoea (OSA). Thorax 1998;53.
- Tan YK, L’Estrange PL, Grant HR, Smith C, Simonds AK, Spiro SG. A randomised crossover study of continuous positive airway pressure (CPAP) vs mandibular advancement splint (MAS) in mild and moderate obstructive sleep apnoea (OSA). Eur Respir J 1998;12.
- Tan YK, L’Estrange PR, Grant HR, Smith C, Simonds AK, Spiro SG. Subjective assessment of continuous positive airway pressure (CPAP) and a mandibular advancement splint (MAS) in a randomised crossover study of patients with mild or moderate obstructive sleep apnoea (OSA). Thorax 1998;53.
- Jenkinson C, Stradling J, Petersen S. How should we evaluate health status? A comparison of three methods in patients presenting with obstructive sleep apnoea. Qual Life Res 1998;7:95-100.
Appendix 1 Literature search strategies
Searches for systematic reviews and guidelines
Cochrane Database of Systematic Reviews (Cochrane Library 2006, issue 3) (www.thecochranelibrary.com)
Searched 17 October 2006. The 53 records retrieved were scanned to remove references to infants and children and 11 records were downloaded.
-
MeSH descriwptor Sleep Apnea Syndromes explode all trees
-
apnea or apnoea
-
hypopnea or hypopnoea
-
hypoapnea or hypoapnoea
-
“sahs” or “shs” or “osas” or “osa”
-
(#1 OR #2 OR #3 OR #4 OR #5)
-
MeSH descriptor Positive-Pressure Respiration explode all trees
-
cpap or apap or ncpap or autocpap or auto-cpap
-
positive near3 airway near3 pressure
-
(#7 OR #8 OR #9)
-
(#6 AND #10)
Database of Abstracts of Reviews of Effects (CRD administration database)
Searched 17 October 2006. Sixty-six records were retrieved.
-
s apnea or apnoea or hypopnea or hypopnoea or hypoapnea or hypoapnoea
-
s sahs or shs or osas or osa
-
s s1 or s2
-
s cpap or apap or ncpap or autocpap
-
s positive(3w)airway(3w)pressure
-
s s4 or s5
-
s s3 and s6
Health Technology Assessment Database (CRD administration database)
Searched 17 October 2006. Eight records were retrieved.
-
s apnea or apnoea or hypopnea or hypopnoea or hypoapnea or hypoapnoea
-
s sahs or shs or osas or osa
-
s s1 or s2
-
s cpap or apap or ncpap or autocpap
-
s positive(3w)airway(3w)pressure
-
s s4 or s5
-
s s3 and s6
National Research Register (2006, issue 3)(www.update-software.com/National/)
Searched 17 October 2006. Seventy-seven records were retrieved.
-
SLEEP APNEA SYNDROMES explode all trees (MeSH)
-
(apnea or apnoea or hypopnea or hypopnoea or hypoapnea or hypoapnoea)
-
(sahs or shs or osas or osa)
-
(#1 or #2 or #3)
-
POSITIVE-PRESSURE RESPIRATION explode all trees (MeSH)
-
(cpap or apap or ncpap or autocpap)
-
(positive near airway near pressure)
-
(#5 or #6 or #7)
-
(#4 and #8)
Scottish Intercollegiate Guidelines Network (www.sign.ac.uk)
Searched 17 October 2006.The websitwe was scanned and one record was retrieved.
National Guideline Clearinghouse (www.guideline.gov/)
Searched 17 October 2006. The following search terms were used. The results were scanned and seven records were retrieved.
-
apnea
-
apnoea
-
hypopnea
-
hypopnoea
-
hypoapnea
-
hypoapnoea
-
sahs
-
shs
-
osas
-
osa
Health Services/Technology Assessment Text (HSTAT) (www.ncbi.nlm.nih.gov/books/bv.fcgi?rid%20=%20hstat)
Searched 17 October 2006. The following search terms were used. The results were scanned and one record was retrieved.
-
apnea
-
apnoea
-
hypopnea
-
hypopnoea
-
hypoapnea
-
hypoapnoea
Turning Research into Practice Database (Trip)(www.tripdatabase.com/)
Searched 17 October 2006. The following search terms were used. The results were scanned and two records were retrieved.
-
cpap or apap or ncpap or autocpap
-
apnea or apnoea or hypopnea or hypopnoea or hypoapnea or hypoapnoea
Health Evidence Bulletins Wales (http://hebw.cf.ac.uk/index.html)
Searched 17 October 2006. The website was scanned and no records were retrieved.
Clinical Evidence (www.clinicalevidence.com)
Searched 17 October 2006. Chapters were scanned online and eight records were retrieved.
National Library for Health Guidelines Finder (www.library.nhs.uk/guidelinesfinder/)
Searched 20 October 2006. One record was retrieved.
-
apnea or apnoea
-
hypopnoea or hypopnea
-
hypoapnea or hypoapnoea
Searches for trials
MEDLINE (1966 to November week 3 2006) (OVID)
Searched 29 November 2006. 2346 records were retrieved.
-
exp sleep apnea syndromes/
-
(apnea or apnoea).ti,ab.
-
(hypopnea or hypopnoea).ti,ab.
-
(hypoapnea or hypoapnoea).ti,ab.
-
sleep disordered breathing.ti,ab.
-
(sleep adj2 respirat$disorder$).ti,ab.
-
sahs.ti,ab.
-
shs.ti,ab.
-
osa.ti,ab.
-
osas.ti,ab.
-
osahs.ti,ab.
-
or/1–11
-
exp positive-pressure respiration/
-
(positive adj3 airway adj3 pressure).ti,ab.
-
(cpap or ncpap or apap or bipap).ti,ab.
-
(c pap or bi pap or nc pap).ti,ab.
-
autocpap.ti,ab.
-
or/13–16
-
12 and 18
MEDLINE In-Process & Other Non-Indexed Citations (28 November 2006) (OVID)
Searched 29 November 2006. 113 records were retrieved.
-
(apnea or apnoea).ti,ab.
-
(hypopnea or hypopnoea).ti,ab.
-
(hypoapnea or hypoapnoea).ti,ab.
-
sleep disordered breathing.ti,ab.
-
(sleep adj2 respirat$disorder$).ti,ab.
-
sahs.ti,ab.
-
shs.ti,ab.
-
osa.ti,ab.
-
osas.ti,ab.
-
osahs.ti,ab.
-
or/1–10
-
(positive adj3 airway adj3 pressure).ti,ab.
-
(cpap or ncpap or apap or bipap).ti,ab.
-
(c pap or bi pap or nc pap).ti,ab.
-
autocpap.ti,ab.
-
or/12–15
-
11 and 16
EMBASE (1980 to 2006 week 47) (OVID)
Searched 29 November 2006. 2744 records were retrieved.
-
Sleep Apnea Syndrome/
-
(apnea or apnoea).ti,ab.
-
(hypopnoea or hypopnea).ti,ab.
-
(hypoapnea or hypoapnoea).ti,ab.
-
Sleep Disordered Breathing/
-
sleep disordered breathing.ti,ab.
-
(sleep adj2 respirat$disorder$).ti,ab.
-
sahs.ti,ab.
-
shs.ti,ab.
-
osa.ti,ab.
-
osas.ti,ab.
-
osahs.ti,ab.
-
or/1–12
-
positive end expiratory pressure/
-
(positive adj3 airway adj3 pressure).ti,ab.
-
(cpap or ncpap or apap or bipap).ti,ab.
-
(c pap or bi pap or nc pap).ti,ab.
-
autocpap.ti,ab.
-
or/14–18
-
0. 13 and 19
Cochrane Central Register of Controlled Trials (Cochrane Library 2006, issue 4) (www.thecochranelibrary.com)
Searched 29 November 2006. 461 records were retrieved.
-
MeSH descriptor Sleep Apnea Syndromes explode all trees
-
apnea or apnoea
-
hypopnea or hypopnoea
-
hypoapnea or hypoapnoea
-
“sleep disordered breathing”
-
sleep near2 respirat* disorder*
-
sahs or shs or osa or osas or osahs
-
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
-
MeSH descriptor Positive-Pressure Respiration explode all trees
-
positive near3 airway near3 pressure
-
cpap or ncpap or apap or bipap
-
c-pap or bi-pap or nc-pap
-
autocpap
-
(#9 OR #10 OR #11 OR #12 OR #13)
-
(#8 AND #14)
CINAHL (1982 to November week 3 2006) (OVID)
Searched 29 November 2006. 419 records were retrieved.
-
exp Sleep Apnea Syndromes/
-
(apnea or apnoea).ti,ab.
-
(hypopnea or hypopnoea).ti,ab.
-
(hypoapnea or hypoapnoea).ti,ab.
-
sleep disordered breathing.ti,ab.
-
(sleep adj2 respirat$disorder$).ti,ab.
-
sahs.ti,ab.
-
shs.ti,ab.
-
osa.ti,ab.
-
osas.ti,ab.
-
osahs.ti,ab.
-
or/1–11
-
exp Positive Pressure Ventilation/
-
(positive adj3 airway adj3 pressure).ti,ab.
-
(cpap or ncpap or apap or bipap).ti,ab.
-
(c pap or bi pap or nc pap).ti,ab.
-
autocpap.ti,ab.
-
or/13–17
-
12 and 18
Science Citation Index (1900 to 25 November 2006) (Web of Knowledge)
Searched 29 November 2006. 2745 records were retrieved.
-
TS = (apnea or apnoea)
-
TS = (hypopnea or hypopnoea)
-
TS = (hypoapnea or hypoapnoea)
-
TS = “sleep disordered breathing”
-
TS = (sahs or shs or osa or osas or osahs)
-
#1 or #2 or #3 or #4 or #5
-
TS = (positive same airway same pressure)
-
TS = (cpap or ncpap or apap or bipap)
-
TS = (“c pap” or “nc pap” or “bi pap”)
-
TS = autocpap
-
#7 or #8 or #9 or #10
-
#6 and #11
ISI Proceedings Science & Technology (1990 to 25 November 2006) (Web of Knowledge)
Searched 29 November 2006. 407 records were retrieved.
-
TS = (apnea or apnoea)
-
TS = (hypopnea or hypopnoea)
-
TS = (hypoapnea or hypoapnoea)
-
TS = “sleep disordered breathing”
-
TS = (sahs or shs or osa or osas or osahs)
-
#1 or #2 or #3 or #4 or #5
-
TS = (positive same airway same pressure)
-
TS = (cpap or ncpap or apap or bipap)
-
TS = (“c pap” or “nc pap” or “bi pap”)
-
TS = autocpap
-
#7 or #8 or #9 or #10
-
#6 and #11
Zetoc Conferences (1993 to 29 November 2006)(http://zetoc.mimas.ac.uk/)
Searched 29 November 2006. 113 records were retrieved.
-
conference: autocpap
-
conference: bi pap
-
conference: c pap
-
conference: nc pap
-
conference: bipap
-
conference: apap
-
conference: ncpap
-
conference: cpap
-
conference: positive airway pressure
The records retrieved from this search in Zetoc were loaded into an endnote library and duplicates were removed. The following search was then carried out within that endnote library:
-
apnea or apnoea or hypopnea or hypopnoea or hypoapnea or hypoapnoea
-
sleep or sahs or shs or osa or osas or osahs
SIGLE (1980 to March 2005) (SilverPlatter)
Searched 29 November 2006. Three records were retrieved.
-
(apnea or apnoea) in ti,ab
-
(hypopnea or hypopnoea) in ti,ab
-
(hypoapnea or hypoapnoea) in ti,ab
-
sleep disordered breathing in ti,ab
-
(sleep near2 respirat* disorder*) in ti,ab
-
(sahs or shs or osa or osas or osahs) in ti,ab
-
#1 or #2 or #3 or #4 or #5 or #6
-
(positive near3 airway near3 pressure) in ti,ab
-
(cpap or ncpap or apap or bipap) in ti,ab
-
(c pap or bi pap or nc pap) in ti,ab
-
autocpap in ti,ab
-
#8 or #9 or #10 or #11
-
#7 and #12
Index to Theses (1716 to 16 October 2006) (www.theses.com/)
Searched 29 November 2006. Fourteen records were retrieved.
-
(apnea or apnoea or hypopnea or hypopnoea or hypoapnea or hypoapnoea or sleep) and (cpap or ncpap or apap or bipap or c pap or bi pap or nc pap or autocpap)
-
(apnea or apnoea or hypopnea or hypopnoea or hypoapnea or hypoapnoea or sleep)and (positive airway pressure)
-
(sahs or shs or osa or osas or osahs) and (cpap or ncpap or apap or bipap or c pap or bi pap or nc pap or autocpap)
-
(sahs or shs or osa or osas or osahs) and (positive airway pressure)
NHS Economic Evaluation Database (NHS EED) (CRD internal administration system)
Searched 1 December 2006. Twenty-four records were retrieved.
-
s apnea or apnoea
-
s hypopnea or hypopnoea
-
s hypoapnea or hypoapnoea
-
s sleep(w)disordered(w)breathing
-
s sleep(2w)respirat$(w)disorder$
-
s sahs or shs or osa or osas or osahs
-
s s1 or s2 or s3 or s4 or s5 or s6
-
s positive(3w)airway(3w)pressure
-
s cpap or ncpap or apap or bipap
-
s c(w)pap or bi(w)pap or nc(w)pap
-
s autocpap
-
s s8 or s9 or s10 or s11
-
s s7 and s12
Health Economic Evaluations Database (HEED) (1995 to November 2006) (CD-ROM)
Searched 1 December 2006. Seventeen records were retrieved.
-
ax = apnea or apnoea or hypopnea or hypopnoea or hypoapnea or hypoapnoea
-
ax = sleep and disorder*
-
ax = sahs or shs or osa or osas or osahs
-
cs = 1 or 2 or 3
-
ax = positive and airway and pressure
-
ax = cpap or ncpap or apap or bipap or pap or autocpap
-
cs = 5 or 6
-
cs = 4 and 7
EconLit (1969 to October 2006) (SilverPlatter)
Searched 1 December 2006. No records were retrieved.
-
(apnea or apnoea) in ti,ab
-
(hypopnea or hypopnoea) in ti,ab
-
(hypoapnea or hypoapnoea) in ti,ab
-
sleep disordered breathing in ti,ab
-
(sleep near2 respirat* disorder*) in ti,ab
-
(sahs or shs or osa or osas or osahs) in ti,ab
-
#1 or #2 or #3 or #4 or #5 or #6
-
(positive near3 airway near3 pressure) in ti,ab
-
(cpap or ncpap or apap or bipap) in ti,ab
-
(c pap or bi pap or nc pap) in ti,ab
-
autocpap in ti,ab
-
#8 or #9 or #10 or #11
-
#7 and #12
EconPapers (http://econpapers.repec.org/)
Searched 1 December 2006. The search results were scanned and no records were retrieved.
-
apnea
-
apnoea
-
hypopnea
-
hypopnoea
-
hypoapnea
-
hypoapnoea
-
sleep and disorder*
Cost-effectiveness searches
The following databases were searched for economic evaluations of sleep apnoea.
NHS Economic Evaluation Database (NHS EED) (CRD internal administration system)
Searched 13 January 2007. Forty-two records were retrieved.
-
S sleep(w)apn$
-
S apn$
-
S hypoapn$
-
S sleep(w)disordered(w)breathing
-
S sleep(2w)respirat$(2w)disorder$
-
S sahs or shs or osa or osas or soahs
-
S s1 or s2 or s3 or s4 or s5 or s6
Health Technology Assessment Database (CRD administration database)
Searched 13 January 2007. Eight records were retrieved.
-
S sleep(w)apn$
-
S apn$
-
S hypoapn$
-
S sleep(w)disordered(w)breathing
-
S sleep(2w)respirat$(2w)disorder$
-
S sahs or shs or osa or osas or soahs
-
S s1 or s2 or s3 or s4 or s5 or s6
-
S econ$or cost$
-
S s7 and s8
Health Economic Evaluations Database (HEED) (1995 to January 2007) (CD-ROM)
Searched 13 January 2007. Seventy records were retrieved.
-
Apn* or hypoapn*
-
‘Sleep disordered’ within 3
-
sahs or shs or osa or osas or soahs
IDEAS (http:\\ideas.repec.org)
Searched 13 January 2007. No records were retrieved.
-
Apnoea or apnea or hypoapnoea or hypoapnea
MEDLINE (1950 to 10 January 2007) (OVID)
Searched 13 January 2007. 494 records were retrieved.
-
exp sleep apnea syndromes/(12843)
-
(apnea or apnoea).ti,ab. (17402)
-
(hypopnea or hypopnoea or hypoapnea or hypoapnoea).ti,ab. (2515)
-
sleep disordered breathing.ti,ab. (1603)
-
(sleep adj2 respirat$disorder$).ti,ab. (154)
-
(sahs or shs or osa or osas or osahs).ti,ab. (4375)
-
or/1–6 (21688)
-
economics/(24617)
-
exp “costs and cost analysis”/(125794)
-
economic value of life/(4779)
-
economics, medical/(6672)
-
economics,nursing/(3725)
-
(econom$or cost or costs or costly or costing or prices or pricing or pharmacoeconomic$).ti,ab. (239906)
-
(expenditure$not energy).ti,ab. (10278)
-
(value adj3 money).ti,ab. (472)
-
budget$.ti,ab. (10745)
-
or/8–16 (337377)
-
7 and 17 (521)
-
(letter or editorial or historical-article).pt. (1006834)18 not 19 (504)
-
animals/not (humans/and animals/) (3010245)
-
20 not 21 (494)
EMBASE (1980 to 2007 week 1) (OVID)
Searched 13 January 2007. 569 records were retrieved.
-
sleep apnea syndrome/(11977)
-
(apnea or apnoea).ti,ab. (15034)
-
(hypopnoea or hypopnea or hypoapnea or hypoapnoea).ti,ab. (2219)
-
sleep disordered breathing/(484)
-
sleep disordered breathing.ti,ab. (1555)
-
(sleep adj2 respirat$disorder$).ti,ab. (100)
-
(sahs or shs or osa or osas or osahs).ti,ab. (3866)
-
or/1–7 (19042)
-
health economics/(8941)
-
exp economic evaluation/(84200)
-
exp health care cost/(85556)
-
exp pharmacoeconomics/(44276)
-
(econom$or cost or costs or costly or costing or prices or pricing or pharmacoeconomic$).ti,ab. (189706)
-
(expenditure$not energy).ti,ab. (8248)
-
(value adj3 money).ti,ab. (376)
-
budget$.ti,ab. (7647)
-
or/9–16 (280800)
-
8 and 17 (655)
-
(letter or editorial or note).pt. (712099)
-
8 not 19 (586)
-
(energy or oxygen) adj3 (cost or expenditure$)).ti,ab. (10286)
-
(metabolic adj3 cost$).ti,ab. (502)
-
exp animal/or exp animal experiment/(1206737)
-
(rat or rats or mouse or mice or hamster$ or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (1857239)
-
or/21–24 (2100839)
-
20 not 25 (569)
Searches to inform the model
All searches were conducted in Ovid MEDLINE.
Traffic accidents and cardiovascular events
The following strategy was used to identify literature linking traffic accidents and cardiovascular events (particularly stroke and coronary heart disease) to sleep apnoea.
MEDLINE (In-Process & Other Non-Indexed Citations and MEDLINE 1950 to Present)
Searched 15 January 2007. The results of set 13 were scanned for epidemiological records and sets of selected records were downloaded.
-
Accidents, Traffic/
-
road accidents.ti,ab.
-
traffic accidents.ti,ab.
-
(stroke or strokes).ti,ab.
-
(chd or cardiovascular disease).ti,ab.
-
exp heart diseases/or exp vascular diseases/
-
exp Cerebrovascular Accident/
-
or/1–7
-
exp sleep apnea syndromes/
-
8 and 9
-
ep.fs.
-
10 and 11
-
limit 12 to yr = “1990 – 2007”
Quality of life studies
The following strategy was used to identify quality of life studies/utilities studies in MEDLINE.
MEDLINE (In-Process & Other Non-Indexed Citations and MEDLINE 1950 to Present)
Searched 8 July 2007. 491 records were downloaded (set 43) and assessed for relevance.
-
exp sleep apnea syndromes/
-
(apnea or apnoea).ti,ab.
-
(hypopnea or hypopnoea).ti,ab.
-
(hypoapnea or hypoapnoea).ti,ab.
-
sleep disordered breathing.ti,ab.
-
(sleep adj2 respirat$disorder$).ti,ab.
-
(sahs or shs or osa or osas or osahs).ti,ab.
-
or/1–7
-
quality of life/
-
(quality adj2 life).ti,ab.
-
utility.ti,ab.
-
utilities.ti,ab.
-
standard gamble.ti,ab.
-
tto.ti,ab.
-
(time tradeoff or time trade off).ti,ab.
-
(eq or euroqol).ti,ab.
-
osa 18.ti,ab.
-
sf 36.ti,ab.
-
sgrq.ti,ab.
-
respiratory questionnaire.ti,ab.
-
practical sleep scale.ti,ab.
-
sleep scale.ti,ab.
-
scopa.ti,ab.
-
objective daytime sleepiness.ti,ab.
-
oxford sleep resistance.ti,ab.
-
osler test.ti,ab.
-
stai.ti,ab.
-
emotional control scale.ti,ab.
-
cecs.ti,ab.
-
life orientation test.ti,ab.
-
satisfaction with life scale.ti,ab.
-
swls.ti,ab.
-
calgary sleep apnea quality.ti,ab.
-
(functional outcomes adj2 sleep).ti,ab.
-
osa patient oriented severity.ti,ab.
-
osa18.ti,ab.
-
cohen$pediatric osa.ti,ab.
-
(comment or letter or editorial).pt.
-
or/9–37
-
8 and 39
-
40 not 38
-
limit 41 to yr = “2000 – 2007”
-
limit 42 to english language (491)
Rates of road accidents
Searches for recent studies on rates of road accidents were undertaken using the strategy described by Ayas and colleagues in their meta-analysis of all studies that examined RTA rates in patients with OSAHS before and after CPAP. 122 Ayas et al. reported their search as follows: ‘A comprehensive search of MEDLINE (1966 to March 2005) using Ovid was conducted using the following exploded MESH terms: sleep apnea syndromes AND positive pressure respiration OR continuous positive airway pressure AND automobile driving OR accident.’
This search was rerun in Ovid MEDLINE (1950 to March week 1 2007) and included searches of in process citations. The search results were limited to those added to MEDLINE after February 2005 to ensure that no records were missed. The correct medical subject heading (MeSH) term ACCIDENTS was used rather than the term ACCIDENT noted by Ayas et al. , on the assumption that this was a transcription error in their paper.
Eight new records were identified.
-
exp sleep apnea syndromes/
-
exp positive pressure respiration/
-
exp continuous positive airway pressure/
-
1 and (2 or 3)
-
exp automobile driving/
-
exp accidents/
-
5 or 6
-
4 and 7
-
200503$.ep.
-
(200504$or 200505$or 200506$or 200507$or 200508$or 200509$or 20051$).ep.
-
(2006$or 2007$).ep.
-
(200503$or 200504$or 200505$or 200506$or 200507$or 200508$or 200509$or 20051$).ed.
-
(2006$or 2007$).ed.
-
or/9–13
-
8 and 14 (8)
Search for data on life expectancy for individuals who have suffered a stroke
Ovid MEDLINE (Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1950 to Present>) was searched on 27 April 2007 for a known paper on post-stroke life expectancy by Dennis and Burn. 164 Once identified, the ‘Find similar’ option was selected and also the citing papers option to identify similar records. After assessing those references for relevance, a series of searches was undertaken and the results in sets 5, 11, 12, 15, 17, 22 and 24 were assessed to identify further relevant studies.
-
dennis $.au. and stroke.ti. (130)
-
burn $.au. (1073)
-
1 and 2 (6)
-
find similar to Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. (76)
-
from 5 keep 2–3, 5–6 (4)
-
*cerebrovascular disorders/(27223)
-
*survival rate/(335)
-
7 and 8 (0)
-
stroke$1.ti. (30599)
-
8 and 10 (2)
-
(7 or 10) and survival rate/(634)
-
from 12 keep 4,8,14,17,33,44,81,109,132–134,148,159 (13)
-
*cerebrovascular accident/(16575)
-
8 and 14 (2)
-
14 and survival rate/(386)
-
16 not 13 (375)
-
cerebrovascular accident/(22109)
-
survival rate/(77068)
-
survival analysis/(59922)
-
exp great britain/(220221)
-
8 and (19 or 20) and 21 (48)
-
stroke register.ti,ab. (162)
-
23 and 21 (33)
Google was searched (27 April 2007) using the following search terms:
-
life expectancy stroke
-
stroke life expectancy
-
life tables stroke.
Appendix 2 Excluded studies
| Study | Reasons(s) for exclusion | ||||
|---|---|---|---|---|---|
| Appropriate interventiona | Relevant comparatorb | Appropriate study designc | Appropriate participantsd | Appropriate outcome measurese | |
| Abe et al., 2005178 | No | No | No | Yes | No |
| Adlakha and Gross, 2006179 | No | No | No | Yes | No |
| Ahmed et al., 2005180 | Yes | No | No | Yes | Yes |
| Akashiba et al., 1993181 | No | No | No | Yes | Yes |
| Almirall et al., 2005182 | Yes | No | No | Yes | Yes |
| ANDEM, 1992175 | No | No | No | No | No |
| Anonymous, 2003183 | No | No | No | No | No |
| Antic et al., 2006184 | Yes | No | No | Yes | Yes |
| Ayas et al., 2006185 | No | No | No | No | No |
| Babar and Quan, 2003186 | No | No | No | No | No |
| Badia et al., 1997187 | Yes | Yes | Unclear | Yes | Yes |
| Bakshi et al., 2005188 | No | No | No | Yes | No |
| Barry, 1999189 | No | No | No | No | No |
| Becker et al., 1989190 | No | No | No | Yes | No |
| Becker et al., 1995191 | No | No | No | Yes | No |
| Beecroft et al., 2003192 | Yes | No | No | Yes | Yes |
| Berka et al., 2006193 | No | No | No | Yes | Yes |
| Bloch and Basile, 2006194 | No | No | No | No | No |
| Bradley et al., 1990195 | No | No | No | No | No |
| Braghiroli et al., 1998196 | No | No | No | No | No |
| Buechner et al., 2001197 | No | No | No | Yes | Yes |
| Buttner and Ruhle, 2004198 | Yes | No | No | Yes | Yes |
| Castronovo et al., 2003199 | Yes | No | No | Yes | Yes |
| Chakravorty et al., 1998200 | Yes | No | No | Yes | Yes |
| Chasens et al., 2003201 | Yes | No | No | Yes | Yes |
| Chazan et al., 2004202 | Yes | No | No | Yes | No |
| Chrysostomakis et al., 2004203 | No | No | No | Yes | No |
| Ciftci et al., 2005204 | Yes | No | No | No | Yes |
| Clark et al., 1996149 | Yes | Yes | No | Yes | Yes |
| Deegan and McNicholas, 1995205 | No | No | No | No | No |
| Dhillon et al., 2003206 | Yes | No | No | Yes | Yes |
| Donadio et al., 2006207 | Yes | Yes | No | Yes | Yes |
| Dorkova and Tkacova, 2006208 | No | No | No | Yes | Yes |
| Douglas and Engleman, 1998209 | No | No | No | No | No |
| Douglas, 2004210 | No | No | No | No | No |
| Drummond et al., 2005211 | Yes | Yes | Yes | No | Yes |
| Engleman et al., 1993212 | Yes | Yes | No | Yes | Yes |
| Engleman, 2002213 | No | No | No | No | No |
| Fairbairn et al., 2006214 | Yes | Yes | No | Yes | Yes |
| Ficker et al., 1997215 | No | No | Yes | Yes | Yes |
| Ficker et al., 2002216 | Yes | No | Yes | Yes | Yes |
| Fitzpatrick et al., 2005217 | No | Yes | Yes | No | No |
| Flemons et al., 1998218 | Yes | Yes | Yes | Yes | Yes |
| Fletcher, 2000219 | No | No | No | No | No |
| Gagnadoux, 2006220 | No | No | No | No | No |
| Golish, 2000221 | No | No | No | No | No |
| Goncalves et al., 2005222 | Yes | No | No | Yes | Yes |
| Gotsopoulos 2002223 | No | No | Yes | Yes | Yes |
| Grimm et al., 2000224 | Yes | No | No | No | Yes |
| Hahn et al., 2003225 | No | No | No | Yes | Yes |
| Hermida et al., 2003226 | Yes | Yes | No | Yes | Yes |
| Hermida et al., 2003227 | Yes | Yes | No | Yes | Yes |
| Hermida et al., 2004228 | Yes | Yes | No | Yes | Yes |
| Hernandez et al., 1999229 | Yes | Yes | Yes | Yes | No |
| Hetzel et al., 1994230 | Yes | No | No | Yes | Yes |
| Hira, et al., 1998231 | Yes | Yes | No | Yes | Yes |
| Hirshkowitz and Sharafkhaneh, 2005232 | No | No | No | No | No |
| Hla et al., 2002233 | Yes | No | No | No | Yes |
| Hoster et al., 1995234 | No | No | Yes | Yes | No |
| Huang et al., 2001235 | No | No | Unclear | No | Yes |
| Iellamo and Montana, 2006236 | No | No | No | No | No |
| Itzhaki et al., 2006237 | Yes | No | No | Yes | Yes |
| Jenkinson et al., 2001144 | Yes | Yes | Yes | No | Yes |
| Juhasz et al., 1997238 | No | No | Yes | Yes | No |
| Kajaste et al., 2004239 | Yes | Yes | Yes | Yes | Yes |
| Kaleth et al., 2003240 | Yes | No | No | Yes | No |
| Kaleth et al., 2005241 | Yes | No | Yes | Yes | No |
| Karacan and Karatas, 1995242 | No | No | No | Yes | Yes |
| Kiely et al., 1999243 | Yes | No | No | Yes | Yes |
| Konermann et al., 1995244 | Yes | No | No | Yes | Yes |
| Krieger et al., 1986245 | No | No | No | No | Yes |
| Lafond et al., 2005246 | Yes | No | No | Yes | Yes |
| Lafond et al., 2007247 | No | No | Yes | Yes | Yes |
| Lewis et al., 2002248 | Yes | No | No | Yes | Yes |
| Li et al., 2004249 | Yes | Unclear | Yes | No | Yes |
| Litvin et al., 2006250 | No | No | No | Yes | No |
| Logan et al., 2003251 | Yes | No | No | Yes | Yes |
| Mador et al., 2005252 | Yes | No | Yes | Yes | Yes |
| Malow, 2005253 | Yes | Yes | Yes | Yes | No |
| Marrone et al., 1990254 | No | No | No | Yes | No |
| Marshall and Coughlin, 2004255 | Yes | No | No | Yes | Yes |
| Mayer et al., 1993256 | No | No | Yes | Yes | Yes |
| McArdle et al., 2001257 | Yes | Yes | Yes | Yes | No |
| McEvoy and Thornton, 1984258 | Yes | No | No | Yes | Yes |
| McFadyen et al., 2001259 | Yes | Yes | No | Yes | Yes |
| McNab et al., 2006260 | No | No | No | Yes | Yes |
| Morrish et al., 2004261 | No | No | No | Yes | No |
| Mulgrew et al., 2007262 | Yes | No | Yes | Yes | Yes |
| Nagasaka et al., 1997263 | No | Yes | No | Yes | No |
| Newsom-Davis et al., 2001264 | No | No | No | No | Yes |
| Nooman et al., 1998265 | No | No | No | Yes | Yes |
| Pepin et al., 2006266 | Yes | Yes | Unclear | Yes | No |
| Phillips et al., 1990267 | Yes | No | Unclear | Yes | Yes |
| Poluektov and Eligulashvili, 1994268 | No | No | No | No | No |
| Resta et al., 1997176 | No | No | No | Yes | No |
| Risk and Winzner, 2004269 | No | No | No | Yes | Yes |
| Rosenthal et al., 2003270 | Yes | No | No | Yes | Yes |
| Rosenthal et al., 2006271 | No | No | No | Yes | Yes |
| Sakakibara, 2005272 | No | No | No | No | No |
| Sanders and Strollo, 1998273 | No | No | No | No | No |
| Sanders, 2001274 | No | No | No | No | No |
| Sanner et al., 2000275 | Yes | Yes | No | Yes | Yes |
| Sanner et al., 2003276 | Yes | No | No | Yes | Yes |
| Schutte-Rodin et al., 2006277 | No | No | No | No | No |
| Seiler et al., 1998278 | Yes | No | Yes | Yes | Yes |
| Shadan et al., 2006279 | Yes | No | No | Yes | No |
| Shashikumar et al., 2002177 | No | Yes | No | Yes | Yes |
| Shimizu et al., 2003280 | Yes | No | No | Yes | No |
| Skomro et al., 2003281 | Yes | No | No | No | Yes |
| Smith et al., 2005282 | Yes | No | No | Yes | Yes |
| Stammnitz et al., 1993283 | No | No | No | Yes | Yes |
| Stoohs et al., 1993284 | Yes | No | No | Yes | No |
| Suhner et al., 2003285 | Yes | No | No | Yes | Yes |
| Veale et al., 2003286 | Yes | No | No | Yes | Yes |
| Verbraecken et al., 2002287 | Yes | Unclear | No | No | No |
| Voronin et al., 2002288 | No | Yes | No | No | Yes |
| Weaver, 2004289 | Yes | Yes | Yes | Yes | No |
| Weisfogel et al., 2003290 | Yes | No | No | No | Yes |
| Weissenberg et al., 1994291 | No | No | No | Yes | Yes |
| Westbrook, 1990292 | No | No | No | No | No |
| Westbrook et al., 2004293 | Yes | No | No | Yes | Yes |
| Wiest et al., 1998294 | No | No | No | No | No |
| Woodson et al., 2003295 | Yes | No | Yes | Yes | Yes |
| Woodson et al., 2003296 | Yes | No | Yes | Yes | Yes |
| Worsnop et al., 1994297 | Yes | Yes | Yes | No | Yes |
| Yen et al., 1997298 | Yes | No | No | Yes | No |
| Zimmerman et al., 2005299 | Yes | No | No | Yes | Yes |
Appendix 3 Quality assessment
| Source | Was the method used to assign participants to treatment groups or the sequence of treatments really random? | Was treatment allocation concealed? | Were the groups similar at baseline in terms of ESS and AHI? | If not, were adjustments made for differences in baseline characteristics of the treatment groups? | Did the analysis include an intention-to-treat analysis? | Were appropriate methods used to account for missing data in the intention-to-treat analysis? | What proportion of participants was lost to follow-up for the primary outcomes? | Was the study described as blind or double-blind? | Who was blinded? | Studies using sham CPAP as comparator | Was the study parallel design? (sham CPAP) | Were the participants CPAP naïve? (sham CPAP) | Crossover trials | Was an appropriate analysis using paired data performed? (crossover) | Was there a treatment by period interaction? (crossover) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arias et al., 200556 | ? | ? | ? | X | X | NA | 7.4% | ✓ | Participants | ✓ | X | ✓ | ✓ | ✓ | X |
| Arias et al., 200663 | ✓ | ? | ? | NA | X | NA | 8.7% | ✓ | Participants | ✓ | X | ✓ | ✓ | X | ? |
| Ballester et al., 199996 | ? | ? | ✓ | NA | ? | NA | NR | X | NA | NA | ✓ | ? | X | NA | NA |
| Barbé et al., 200183 | ✓ | ? | ✓ | NA | X (1 not included) | NA | 1.8% | ✓ | Participants, psychologist administering tests | ✓ | ✓ | ? | X | NA | NA |
| Barnes et al., 200289 | ✓ | X | ? | NA | ✓ | ✓ | 23% | X |
Staff administering psychometric tests |
NA | X | ? | ✓ | ✓ | X |
| Barnes et al., 200482 | ✓ | X | ? | NA | X (14 not included) | NA | 33.3% | X | Staff administering psychometric tests | NA | X | ? | ✓ | ✓ | ✓ (ESS) |
| Becker et al., 2003109 | ? | ✓ | ✓ | NA | X (28 not included) | NA | 46.6% | X | NA | ✓ | ✓ | ? | X | NA | NA |
| Campos-Rodriguezet al., 200665 | ? | X | ✓ | NA | X (4 not included) | NA | 5.6% | ✓ | Participants, outcome assessor and nurse following CPAP monitors | ✓ | ✓ | ✓ | X | NA | NA |
| Chakravorty et al., 200297 | ? | ? | ?e | NA | X (18 not included) | NA | 25.4% | X | NA | NA | ✓ | ? | X | NA | NA |
| Cibele et al., 200672 | ? | ? | ? | NA | ? | NA | ? | ✓ | ? | NA | X | ? | ✓ | ? | ? |
| Coughlinet al., 200762 | ✓ | ✓ | ? | NA | X (1 not included) | NA | 2.3% | ✓ | Participants and investigators | ✓ | X | ✓ | ✓ | ✓ | X |
| Cross et al., 200588 | ? | ? | ? | NA | ? | NA | NR | ✓ | Participants and outcome assessors | ✓ | X | ? | ✓ | ? | ? |
| Dimsdale et al., 200058 | ✓ | Xb | ✓ (RDI) | NA | X | NA | ? | ✓ | Participants and investigators (not involved with CPAP titration) | ✓ | ✓ | ✓ | X | NA | NA |
| Drager et al., 200669 | ? | ? | ? | ? | ? | ? | ? | ✓ | Outcome assessors | NA | ✓ | ? | X | NA | NA |
| Engleman et al., 2002103 | ? | ? | ? | NA | X (3 not included) | NA | 5.8% | ✓ | Staff scoring sleep data | NA | X | ? | ✓ | ✓ | ? |
| Engleman et al., 199978 | ? | ? | ? | NA | X (3 not included and 2 additional for PASAT) | NA | 8.1% | X | NA | NA | X | ? | ✓ | ✓ | X |
| Engleman et al., 199893 | ? | ? | ? | NA | X (1 not included) | NA | 4.3%c | ✓ | NA | NA | X | ? | ✓ | ✓ | ✓ (ESS) d |
| Engleman et al., 199792 | ? | ? | ? | NA | X (2 not included) | NA | 11.1% | X | NA | NA | X | ? | ✓ | ✓ | ✓ (MSLT) |
| Engleman et al., 199691 | ? | ? | ? | NA | X (3 not included) | NA | 18.8% | X | NA | NA | X | ? | ✓ | ✓ | ? |
| Engleman et al., 199490 | ? | ? | ? | NA | X (3 not included) | NA | 8.6% | X | NA | NA | X | ? | ✓ | ✓ | ✓ (PASAT 2)d |
| Faccenda et al., 200194 | ✓ | ? | ? | NA | X (3 not included) | NA | 4.2% | X | NA | NA | X | ? | ✓ | ✓ | |
| Ferguson et al., 199681 | ? | ? | ✓ (AHI) | NA | X (2 not included) | NA | 7.4% | X | NA | NA | X | ? | ✓ | ✓ | X |
| Ferguson et al., 199780 | ? | ? | ✓ | NA | X (4 not included) | NA | 16.7% | X | NA | NA | X | ? | ✓ | ✓ | X |
| Fleetham et al., 2002101 | ? | ? | ✓ | NA | ? | ? | ? | X | NA | NA | ✓ | ? | NA | NA | NA |
| Henke et al., 200185 | ? | ? | ✓ | NA | X (10 not included) | NA | 22.2%e | ✓ | Participants and staff in contact with them | ✓ | Partial crossover | ? | X | NA | NA |
| Hoekema et al., 2006102 | ✓ | ? | ✓ | NA | ✓ | ? | None reported | NA | NA | NA | ✓ | NA | X | NA | NA |
| Hui et al., 200664 | ✓ | ? | ✓ | NA | X (10 not included) | NA | 17.9% | ✓ | Participants and outcome assessors | ✓ | ✓ | ✓ | X | NA | NA |
| Jenkinson et al., 199977 | ✓ | ✓ | ✓ | NA | ? | NA | 5.6% | ✓ | Patients and staff scoring and administering | ✓ | ✓ | ✓ | X | NA | NA |
| Jokic et al., 1999108 | ? | ? | ? | NA | X (1 not included) | NA | 0% | ✓ | Staff scoring sleep data and administering psychometric tests | NA | X | ? | ✓ | Yes | ? |
| Lam et al., 200770 | ✓ | ? | ✓ | NA | X (10 not included) | NA | 9.9% | X | NA | NA | ✓ | ? | X | NA | NA |
| L’Estrange et al., 1999104 | ? | ? | NA | ? | ? | NA | 40% | X | NA | NA | X | ? | ✓ | ? | ? |
| Lim, 2005110 | ? | ? | ✓ | NA | ? | NA | ? | X | NA | NA | ✓ | ? | X | NA | NA |
| Lojander et al., 199699 | ? | ? | ✓ | NA | X (5 not included, 4 not analysed as per protocol) | NA | 33.3% | X | NA | NA | ✓ | ? | X | NA | NA |
| McArdle and Douglas, 200195 | ✓ | ✓ | ? | NA | ? | NA | 4.3% | X | NA | NA | X | ? | ✓ | ✓ | X |
| Marshall et al., 200579 | ✓ | X | ? | NA | X (2 not included) | NA | 6.4% | ✓ | Participants, investigator collecting daytime data | ✓ | X | ✓ | ✓ | ✓ | X |
| Monasterio et al., 2001100 | ✓ | ? | ✓ | NA | X (17 not included) | NA | 12% | ✓ | Staff who did data entry and analysis | NA | ✓ | ? | X | NA | NA |
| Montserrat et al., 200186 | ✓ | ? | ✓ (AHI) | NA | X (2 not included) | NA | 4.3% | ✓ | Patients and interviewers | ✓ | Partial crossover | ? | X | NA | NA |
| Norman et al., 200673 | ✓ | Xb | ✓ CPAP had higher baseline SBP than control | ✓ | X | NA | ? | ✓ | Participants and investigators (not involved with CPAP titration) | ✓ | ✓ | ✓ | X | NA | NA |
| Olson et al., 2002107 | ? | ? | ? | ? | ? | ? | ? | X | NA | X | Partial crossover | ✓ | ✓ | ? | ? |
| Pepperell et al., 200287 | ? | ✓ | ✓ | NA | ✓ | ✓ | 11.9% | ✓ | Patients and investigators | ✓ | ✓ | ? | X | NA | NA |
| Randerath et al., 2002105 | ? | ? | ✓ (AHI) | NA | ? | NA | NR | X | NA | NA | X | ? | ✓ | X | ? |
| Redline et al., 199859 | ✓ | ? | ✓ (ESS) | NA | X (14 not included) | NA | 12.6% | NA | NA | NA | ✓ | ? | X | NA | NA |
| Robinson et al., 200668 | ? | Xb | ? | ? | ? | NA | 8.6% | ✓ | Participants and outcome assessors | ✓ | X | ? | ✓ | ✓ | X |
| Skinner et al., 200460 | ? | ? | ? | NA | X (1 not included) | NA | 7.1% | X | NA | NA | X | ✓ | ✓ | ✓ | ? |
| Skinner et al., 200461 | ? | Un-clear | Unclear | NA | ✓ | NA | 0% | X | NA | NA | X | ✓ | ✓ | ✓ | X |
| Spicuzza et al., 200666 | ? | ? | ✓ (AHI not assessed) | NA | ? | ? | NR | ? | Participants and clinical staff in contact with them | ✓ | ✓ | ✓ | X | NA | NA |
| Tan et al., 2002106 | ? | ? | ? | NA | ✓ (3 not included) | ? | 12.5% | X | NA | ✓ | X | ? | ✓ | ✓ | X |
| West et al., 200667 | ✓ | ? | ✓ | NA | X (1 participant received a defective machine delivering minimal pressure; data were included in sham CPAP group) | NA | 4.8% | ✓ | Participants and investigators | ✓ | ✓ | ✓ | X | NA | NA |
Appendix 4 Clinical effectiveness
| Number of trials | Random effects [MD (95% CI)] | Fixed effect [MD (95% CI)] | Statistical heterogeneity (I2) | |
|---|---|---|---|---|
| Baseline ESS | ||||
| Mild (0–9) | 2 | –1.1 (–1.8 to –0.3) | 0% | |
| Moderate (10–15) | 16 | –2.3 (–3.0 to –1.6) | –2.1 (–2.6 to –1.7) | 51% |
| Severe (16–24) | 5 | –5.0 (–6.5 to –3.5) | –5.0 (–6.1 to –4.0) | 46% |
| Baseline AHI | ||||
| Mild (AHI 5–14) | 3 | –1.5 (–3.4 to 0.4) | 36% | |
| Moderate (AHI 15–30) | 7 | –2.0 (–3.0 to –1.1) | 65% | |
| Severe (AHI > 30) | 13 | –3.4 (–4.6 to –2.3) | 71% | |
| Study design | ||||
| Crossover | 7 | –2.0 (–4.5 to –1.7) | 36% | |
| Parallel | 13 | –3.5 (–4.8 to –2.3) | 73% | |
| Change data | 3 (two parallel and one crossover) | –1.5 (–2.6 to –0.4) | ||
| Comparator | ||||
| Sham CPAP | 12 | –3.0 (–4.2 to –1.9) | 36% | |
| Oral placebo | 6 | –2.1 (–3.5 to –0.8) | 63% | |
| Conservative/usual care | 5 | –2.7 (–4.1 to –1.3) | 56% | |
| Baseline symptom severity | Overall treatment effect [MD (95% CI)] | Statistical heterogeneity (I2) |
|---|---|---|
| Mild (ESS 0–9) | ||
| Barbé et al., 200183 | 0.0 (–2.3, 2.3) | |
| Robinson et al., 200668 | –1.2 (–2.0, –0.4) | |
| Moderate (ESS 10–15) | ||
| Ballester et al., 199996 | –2.1 (–2.8, –1.5) | 41% |
| Barnes et al., 200289 | –2.4 (–3.2, –1.7) | 52% |
| Barnes et al., 200482 | –2.5 (–3.2, –1.8) | 46% |
| Becker et al., 2003109 | –2.3 (–3.0, –1.6) | 52% |
| Campos-Rodriguez et al., 200665 | –2.5 (–3.2, –1.7) | 50% |
| Coughlin et al., 200762 | –2.3 (–3.0, –1.5) | 51% |
| Engleman et al., 199792 | –2.4 (–3.1, –1.7) | 52% |
| Engleman et al., 199893 | –2.2 (–2.8, –1.5) | 42% |
| Engleman et al., 199978 | –2.3 (–3.0, –1.6) | 53% |
| Faccenda et al., 200194 | –2.3 (–3.1, –1.6) | 54% |
| Hui et al., 200664 | –2.4 (–3.1, –1.7) | 53% |
| Lam et al., 200770 | –2.3 (–3.0, –1.6) | 54% |
| Marshall et al., 200579 | –2.3 (–3.1, –1.6) | 54% |
| Monasterio et al., 2001100 | –2.4 (–3.1, –1.6) | 54% |
| Redline et al., 199859 | –2.5 (–3.2, –1.7) | 50% |
| West et al., 200667 | –2.3 (–3.0, –1.5) | 52% |
| Severe (ESS 14–24) | ||
| Chakravorty et al., 200297 | –5.4 (–7.0, –3.7) | 46% |
| Henke et al., 200185 | –5.1 (–6.9, –3.4) | 58% |
| Jenkinson et al., 199977 | –5.0 (–7.2, –2.8) | 59% |
| Montserrat et al., 200186 | –4.4 (–5.5, –3.2) | 0% |
| Pepperell et al., 200287 | –5.1 (–7.2, –3.0) | 58% |
FIGURE 22.
Epworth Sleepiness Scale (CPAP versus sham CPAP) stratified by severity of sleepiness at baseline (ESS).
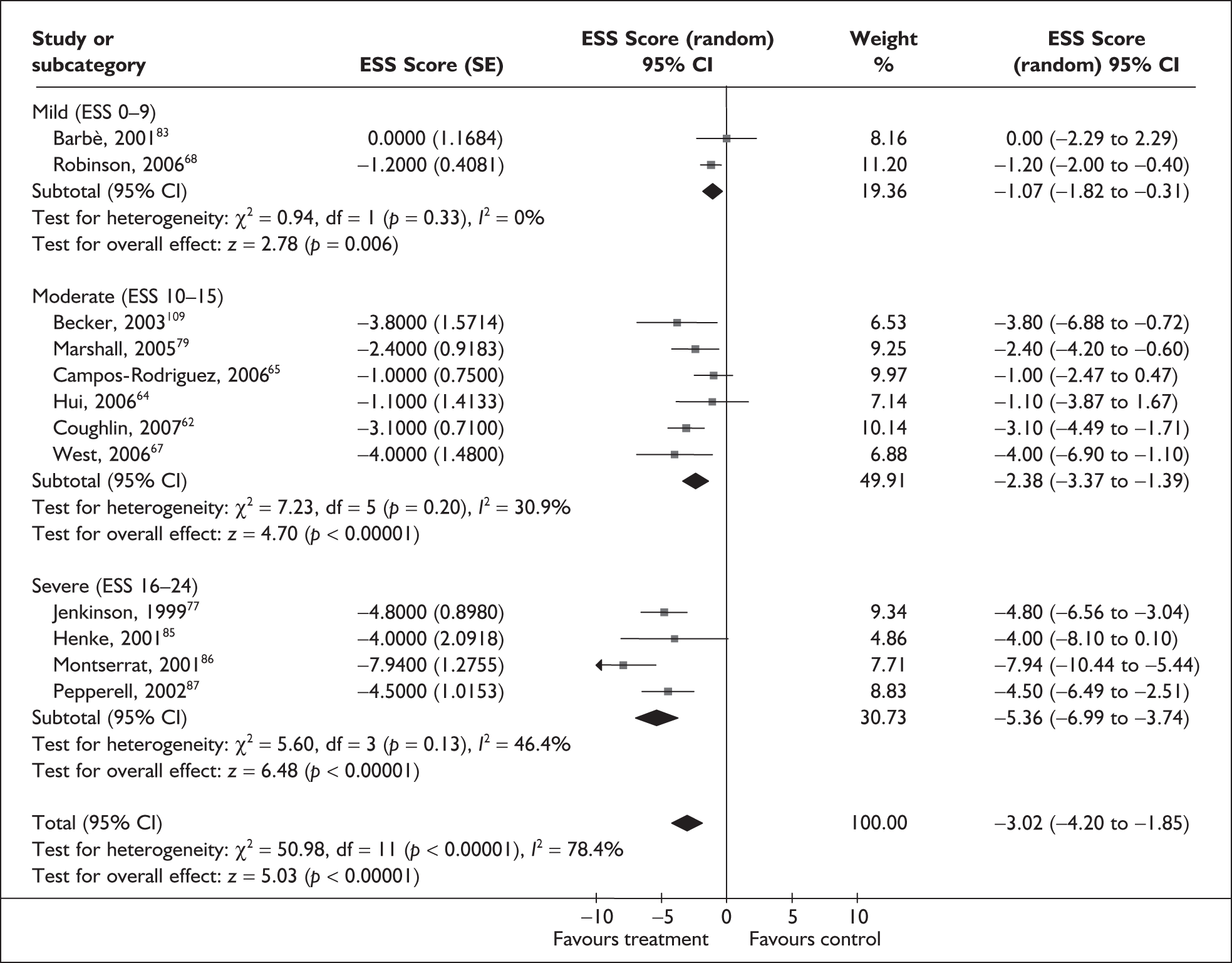
| Number of trials | Random effects (MD (95% CI)] | Fixed effect [MD (95% CI)] | Statistical heterogeneity (I2) | |
|---|---|---|---|---|
| Baseline ESS | ||||
| Mild (0–9) | 0 | |||
| Moderate (10–15) | 6 | –0.9 (–2.1 to 0.4) | –0.5 (–1.3 to 0.2) | 0% |
| Severe (16–24) | 0 | |||
| Baseline AHI | ||||
| Mild (AHI 5–14) | 0 | |||
| Moderate (AHI 15–30) | 4 | –0.2 (–1.1 to 0.7) | 0% | |
| Severe (AHI > 30) | 2 | –1.8 (–6.0 to 2.3) | 88% | |
| Study design | ||||
| Crossover | 4 | –1.0 (–1.1 to 0.7) | 72% | |
| Parallel | 2 | –0.6 (–2.7 to 1.5) | 41% | |
| Baseline symptom severity | Overall treatment effect [MD (95% CI)] | Statistical heterogeneity (I2) |
|---|---|---|
| Moderate (ESS 10–15) | ||
| Barnes et al., 200482 | –1.2 (–2.8 to 0.5) | 64% |
| Engleman et al., 2002103 | –0.1 (–0.9 to 0.6) | 0% |
| Ferguson et al., 199780 | –1.2 (–2.7 to 0.4) | 65% |
| Fleetham et al., 2002101 | –1.1 (–2.7 to 0.4) | 66% |
| Lam et al., 200770 | –0.7 (–2.1 to 0.7) | 65% |
| Tan et al., 2007106 | –0.9 (–2.3 to 0.6) | 68% |
| Number of trials | Random effects [MD (95% CI)] | Fixed effect [MD (95% CI)] | Statistical heterogeneity (I2) | |
|---|---|---|---|---|
| Baseline ESS | ||||
| Mild (0–9) | 0 | |||
| Moderate 10–15) | 4 | 2.3 (0.4–4.3) | 2.3 (0.4–4.3) | 0% |
| Severe (16–24) | 1 | 6.5 (2.6–10.4) | 6.5 (2.6–10.4) | |
| Overall effect | 3.3 (1.3, 5.3) | 3.2 (1.4–5.0) | 11.3% | |
| Baseline AHI | ||||
| Mild (AHI 5–14) | 1 | 1.8 (–2.8 to 6.4) | ||
| Moderate (AHI 15–30) | 3 | 4.1 (1.0–7.3) | 50% | |
| Severe (AHI > 30) | 1 | 1.9 (–6.2 to 10.0) | ||
| Study design | ||||
| Crossover | 3 | 2.4 (0.3–4.4) | 0% | |
| Parallel | 2 | 5.6 (2.1–9.2) | 0% | |
| Study | Overall treatment effect [MD (95% CI)] | Statistical heterogeneity (I2) |
|---|---|---|
| Barnes et al., 200482 | 4.4 (1.9–6.9) | 0% |
| Engleman et al., 199978 | 3.8 (1.2–6.3) | 27% |
| Jenkinson et al., 199977 | 2.3 (0.4–4.3) | 0% |
| Marshall et al., 200579 | 3.1 (0.8–5.5) | 25% |
| West et al., 200667 | 3.5 (1.1–5.9) | 32% |
| Number of trials | Random effects [MD (95% CI)] | Fixed effect [MD (95% CI)] | Statistical heterogeneity (I2) | |
|---|---|---|---|---|
| Baseline ESS | ||||
| Mild (0–9) | 1 | 2.0 (–0.8 to 4.8) | ||
| Moderate 10–15) | 4 | 0.2 (–1.8 to 2.2) | 69% | |
| Severe (16–24) | 1 | –6.1 (–27.3 to 15.1) | ||
| Not reported | 1 | 1.1 (–0.8 to 3.0) | ||
| Overall effect | 0.6 (–0.7 to 1.9) | 0.8 (–0.1 to 1.6) | 46% | |
| Baseline AHI | ||||
| Mild (AHI 5–14) | 2 | –0.7 (–2.9 to 1.4) | 0% | |
| Moderate (AHI 15–30) | 2 | 0.0 (–2.1 to 2.1) | 60% | |
| Severe (AHI > 30) | 3 | 2.3 (0.9–3.7) | 0% | |
| Study design | ||||
| Crossover | 4 | 1.0 (–0.6 to 2.5) | 45% | |
| Parallel | 3 | 0.2 (–2.4 to 2.7) | 43% | |
| Study | Overall treatment effect [MD (95% CI)] | Statistical heterogeneity (I2) |
|---|---|---|
| Barbé et al., 200183 | 0.4 (–1.1 to 1.9) | 52% |
| Barnes et al., 200289 | 0.9 (–0.4 to 2.3) | 45% |
| Chakravorty et al., 200297 | 0.7 (–0.7 to 2.0) | 54% |
| Engleman et al., 199490 | 0.5 (–1.2 to 2.2) | 55% |
| Engleman et al., 199792 | 0.7 (–0.8 to 2.1) | 55% |
| Engleman et al., 199898 | 0.0 (–1.0 to 1.2) | 9% |
| Monasterio et al., 2001100 | 1.2 (0.0–2.4) | 19% |
| Number of trials | Random effects [MD (95% CI)] | Fixed effect [MD (95% CI)] | Statistical heterogeneity (I2) | |
|---|---|---|---|---|
| Baseline ESS | ||||
| Mild (0–9) | 1 | 1.1 (–2.9 to 5.1) | ||
| Moderate 10–15) | 3 | –3.4 (–7.9 to 1.2) | 58% | |
| Severe (16–24) | 1 | –4.2 (–6.4 to –2.0) | ||
| Not reported | 1 | –1.0 (–2.7 to 0.7) | ||
| Overall effect | –2.1 (–4.3 to 0.0) | –2.00 (–3.16 to –0.83) | 59% | |
| Baseline AHI | ||||
| Mild (AHI 5–14) | ||||
| Moderate (AHI 15–30) | 1 | 1.1 (–2.9 to 5.1) | ||
| Severe (AHI > 30) | 5 | –2.7 (–4.9 to –0.4) | 59% | |
| Study design | ||||
| Crossover (end point) | 1 | –1.0 (–2.7 to 0.7) | ||
| Parallel (end point) | 0 | |||
| Crossover (change) | 1 | 1.1 (–2.9 to 5.1) | ||
| Parallel (change) | 4 | –3.5 (–6.2 to –0.7) | 48% | |
| Study | Overall treatment effect [MD (95% CI)] | Statistical heterogeneity (I2) |
|---|---|---|
| Becker et al., 2003109 | –1.7 (–3.5 to 0.1) | 48% |
| Campos-Rodriguezet al., 200665 | –2.4 (–4.9 to 0.1) | 66% |
| Engleman et al., 199691 | –2.6 (–5.4 to 0.3) | 60% |
| Hui et al., 200664 | –2.2 (–4.7 to 0.4) | 67% |
| Pepperell et al., 200287 | –1.4 (–3.6 to 0.8) | 42% |
| Robinson et al., 200668 | –2.7 (–4.9 to –0.4) | 59% |
| Number of trials | Random effects [MD (95% CI)] | Fixed effect [MD (95% CI)] | Statistical heterogeneity (I2) | |
|---|---|---|---|---|
| Baseline AHI | ||||
| Mild (AHI 5–14) | 1 | –2.9 (–13.5 to 7.7) | ||
| Moderate (AHI 15–30) | 0 | |||
| Severe (AHI > 30) | 6 | –1.0 (–3.3 to 1.4) | 0% | |
| Overall effect | 7 | –1.1 (–3.4 to 1.2) | –1.1 (–3.4 to 1.2) | 0% |
| Study design | ||||
| Crossover (end point) | 2 | –0.4 (–3.5 to 2.7) | 0% | |
| Parallel (end point) | 2 | 1.2 (–4.0 to 6.4) | 0% | |
| Crossover (change) | 1 | –2.9 (–13.5 to 7.7) | ||
| Parallel (change) | 2 | –5.2 (–12.4 to 2.1) | ||
| Number of trials | Random effects [MD (95% CI)] | Fixed effect [MD (95% CI)] | Statistical heterogeneity (I2) | |
|---|---|---|---|---|
| Baseline AHI | ||||
| Mild (AHI 5–14) | 1 | –2.6 (–12.8 to 7.6) | ||
| Moderate (AHI 15–30) | 0 | |||
| Severe (AHI > 30) | 6 | –1.2 (–3.1 to 0.7) | 40% | |
| Overall effect | 7 | –1.2 (–2.9 to 0.5) | –0.06 (–2.37 to 0.25) | 29% |
| Study design | ||||
| Crossover (end point) | 2 | –0.9 (–2.9 to 1.0) | 31% | |
| Parallel (end point) | 2 | 0.5 (–2.7 to 3.7) | 0% | |
| Crossover (change) | 1 | –2.6 (–12.8 to 7.6) | ||
| Parallel (change) | 2 | –5.7 (–14.8 to 3.4) | 76% | |
FIGURE 23.
Night-time mean blood pressure (CPAP versus placebo/usual care).
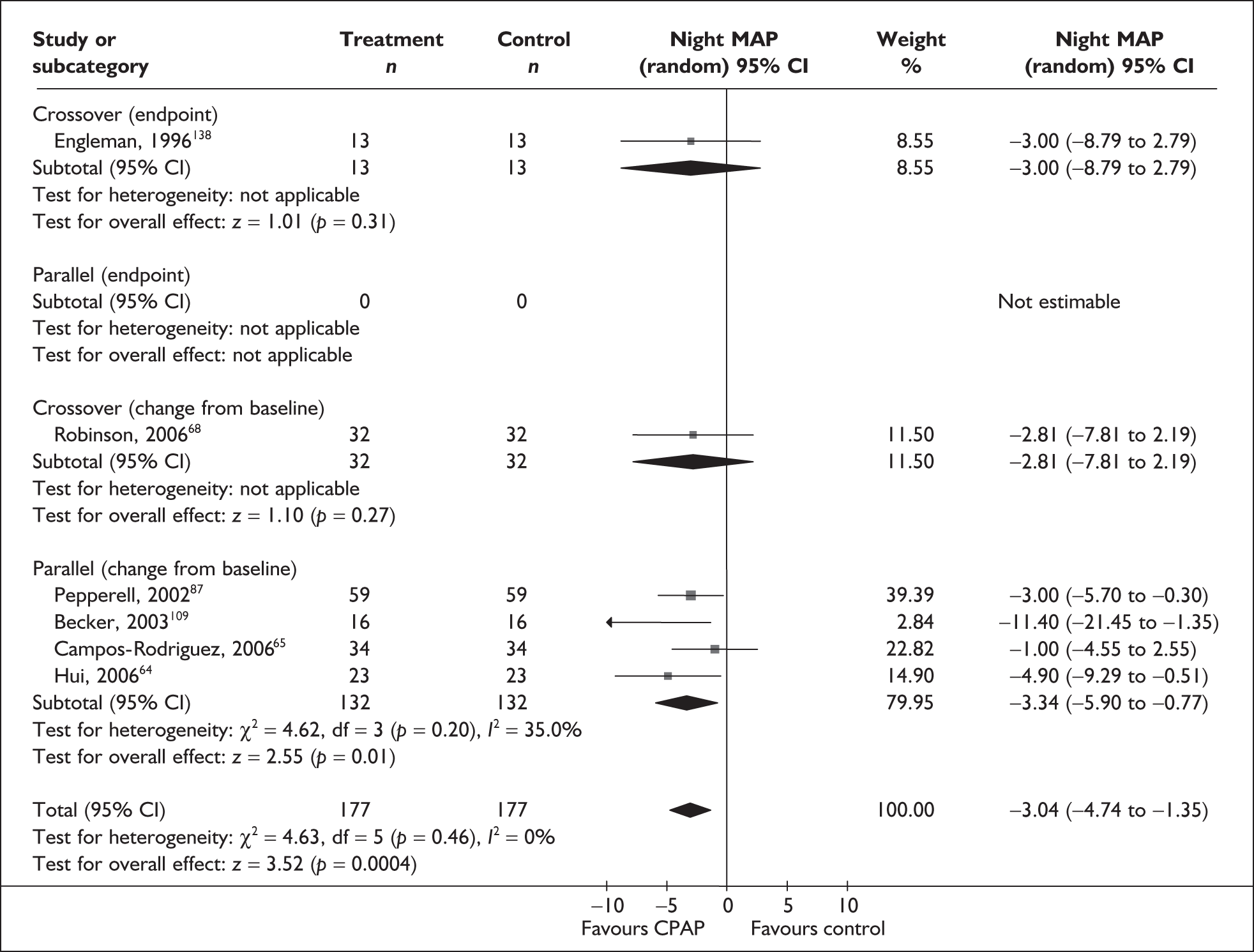
FIGURE 24.
Night-time systolic blood pressure (CPAP versus placebo/usual care).
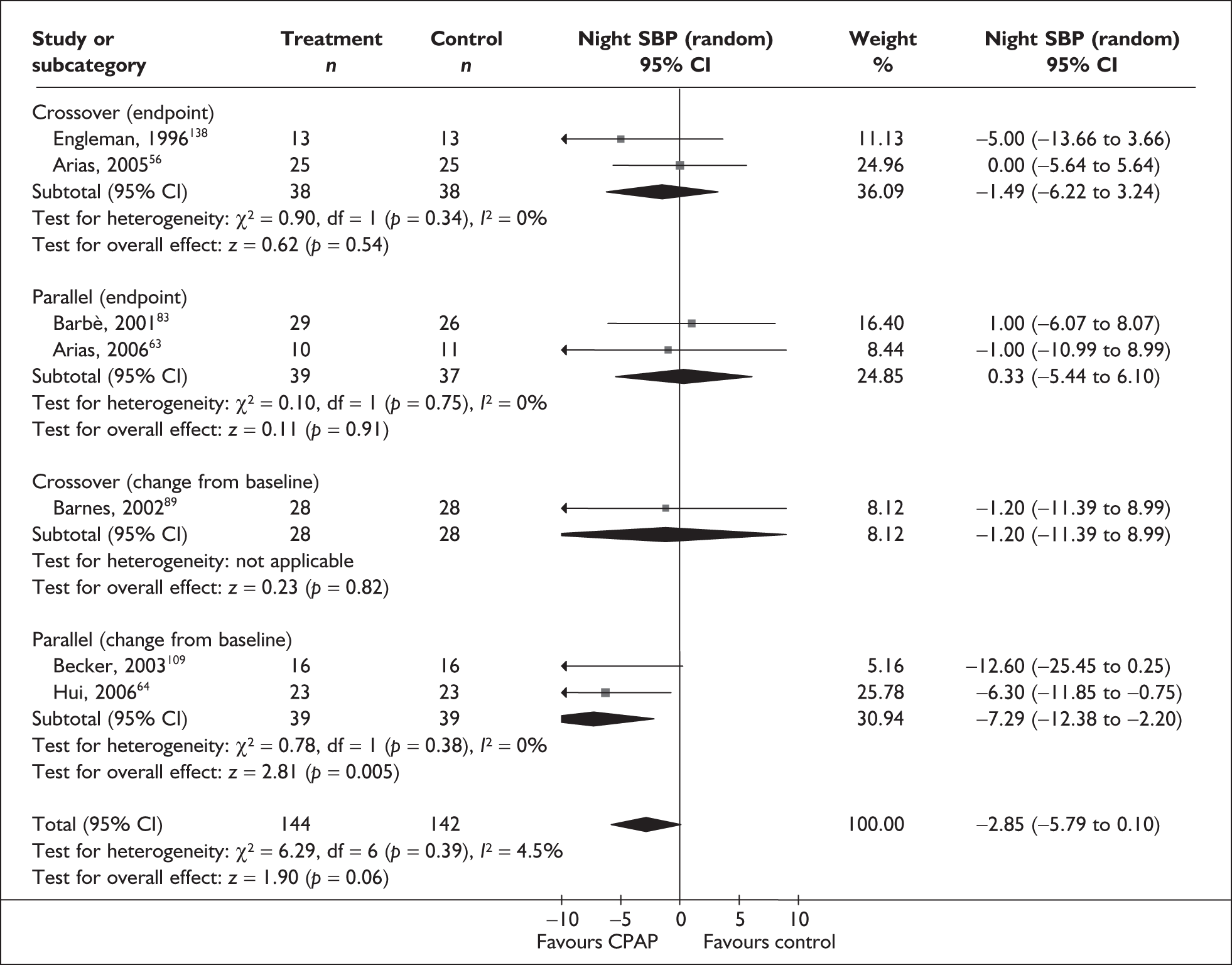
FIGURE 25.
Night-time diastolic blood pressure (CPAP versus placebo/usual care).
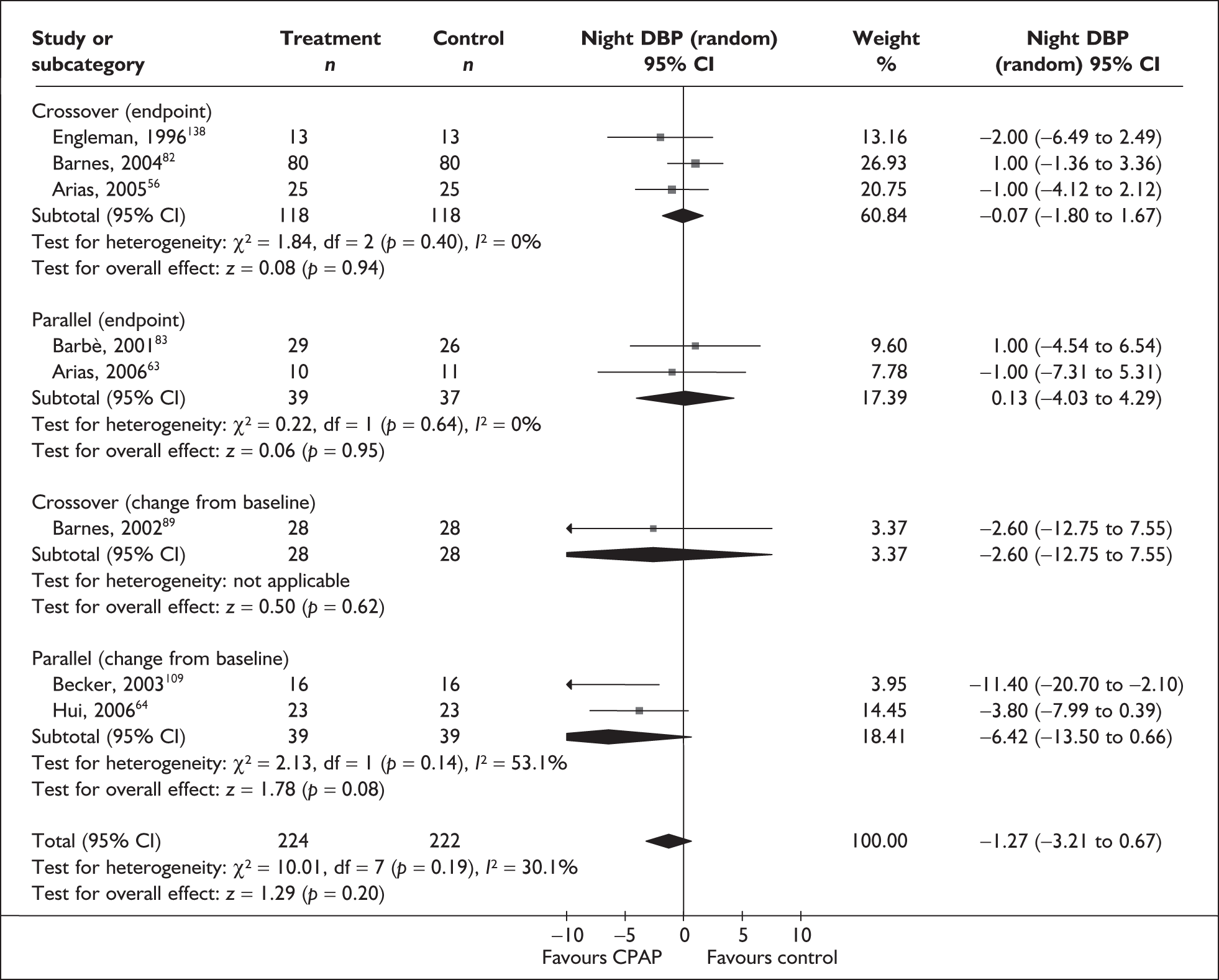
FIGURE 26a.
SF-36 subscales (CPAP versus placebo/usual care): bodily pain.

FIGURE 26b.
SF-36 subscales (CPAP versus placebo/usual care): emotional role.
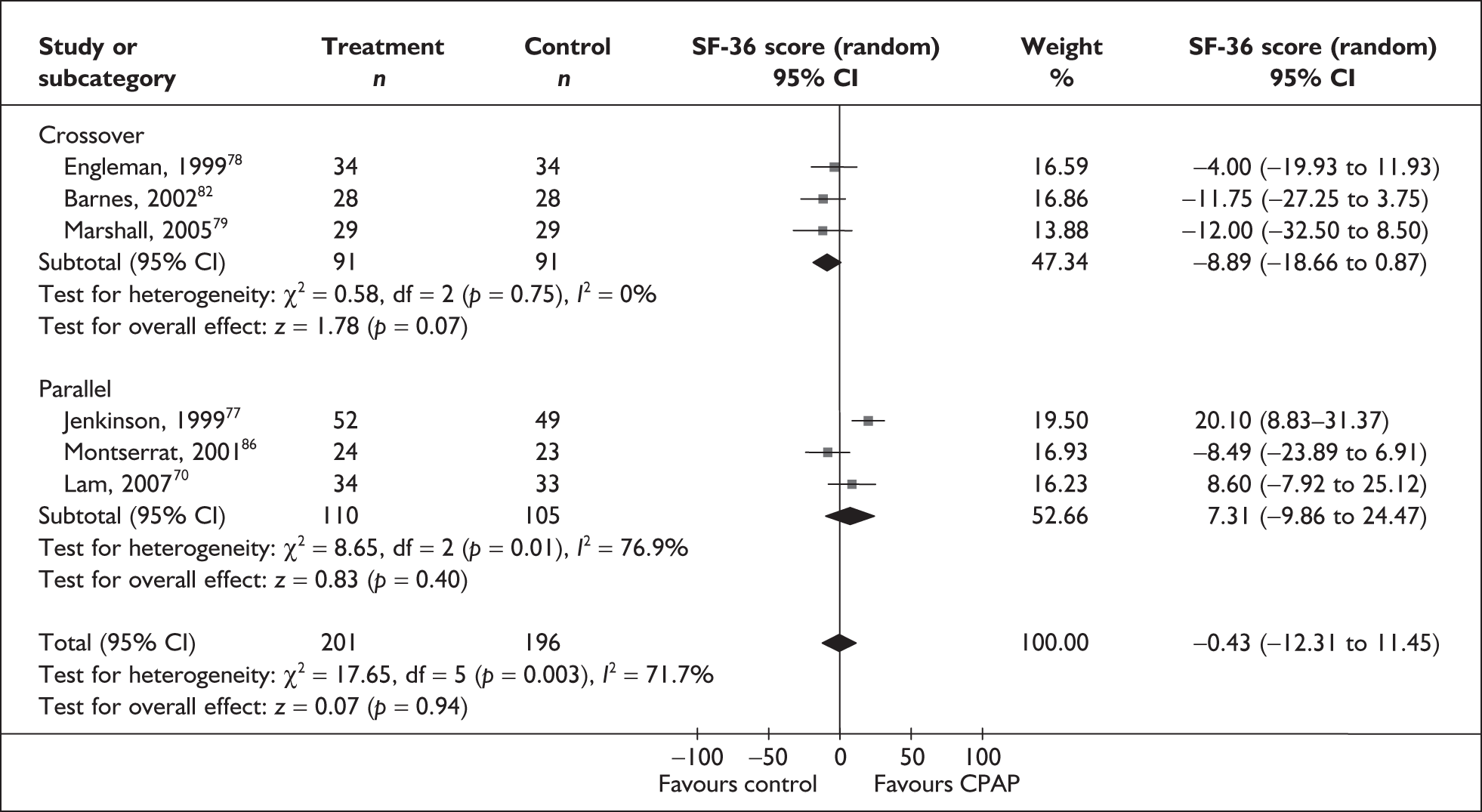
FIGURE 26c.
SF-36 subscales (CPAP versus placebo/usual care): general health.
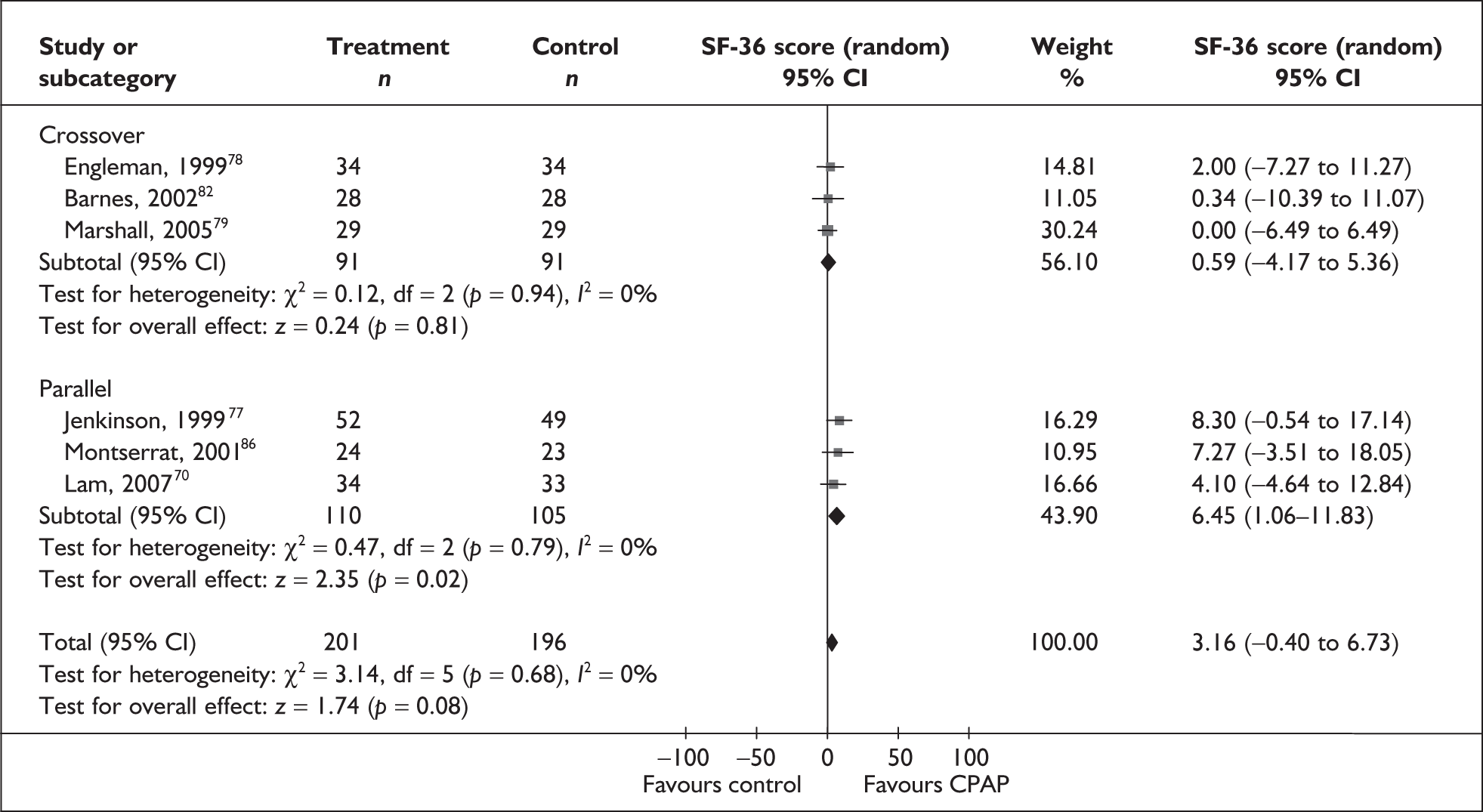
FIGURE 26d.
SF-36 subscales (CPAP versus placebo/usual care): mental health.
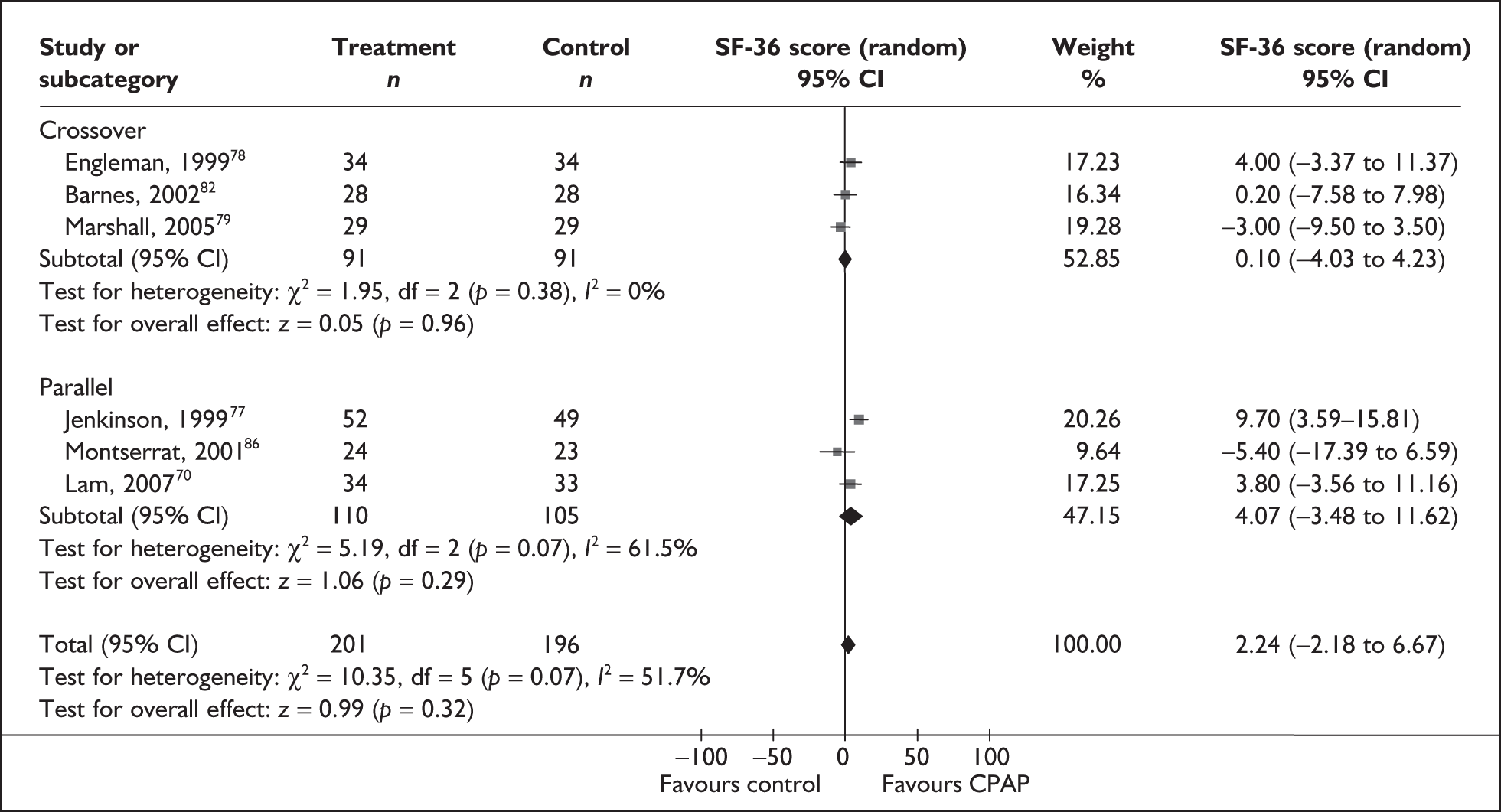
FIGURE 26e.
SF-36 subscales (CPAP versus placebo/usual care): physical function.
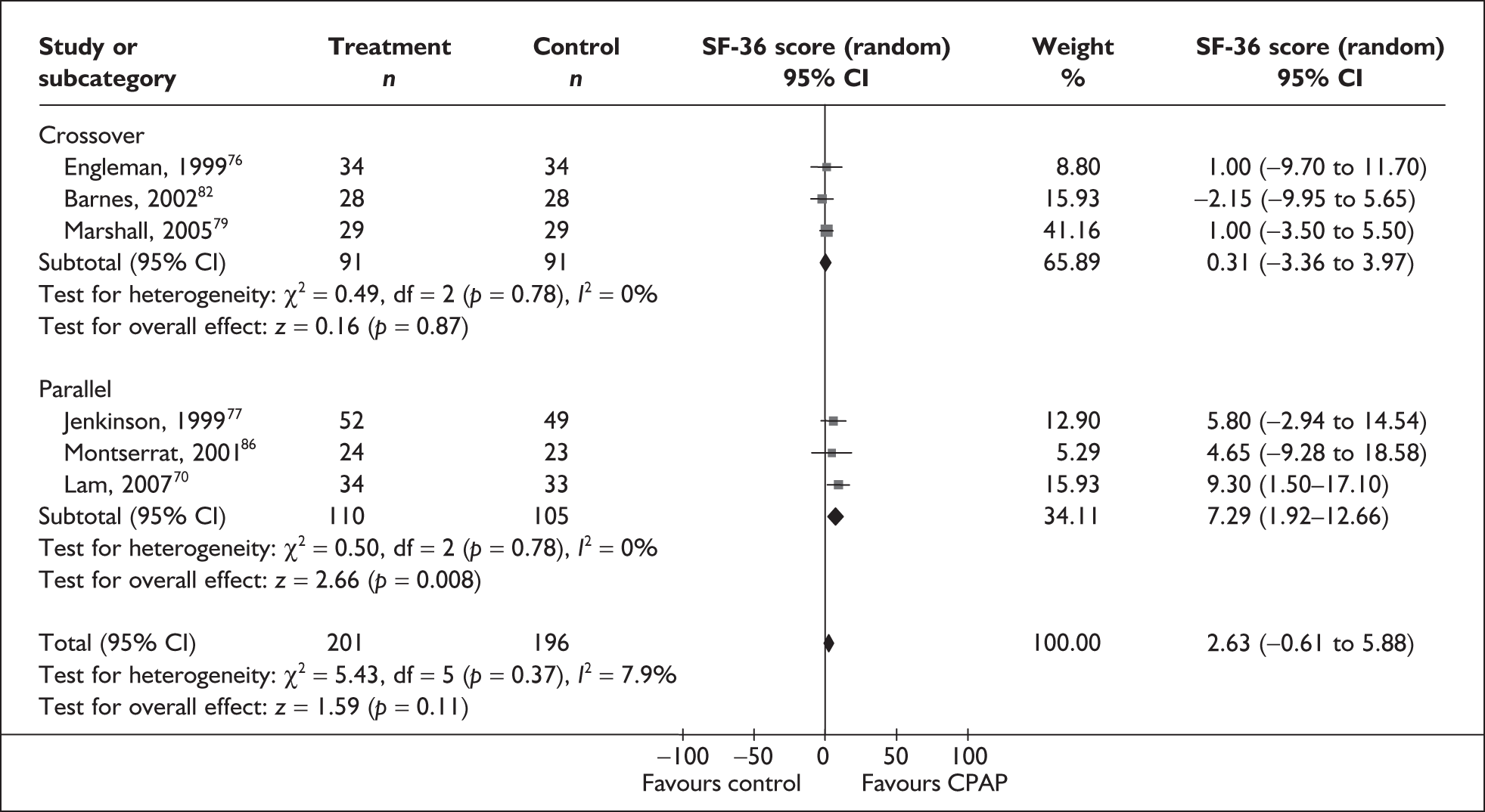
FIGURE 26f.
SF-36 subscales (CPAP versus placebo/usual care): physical role.
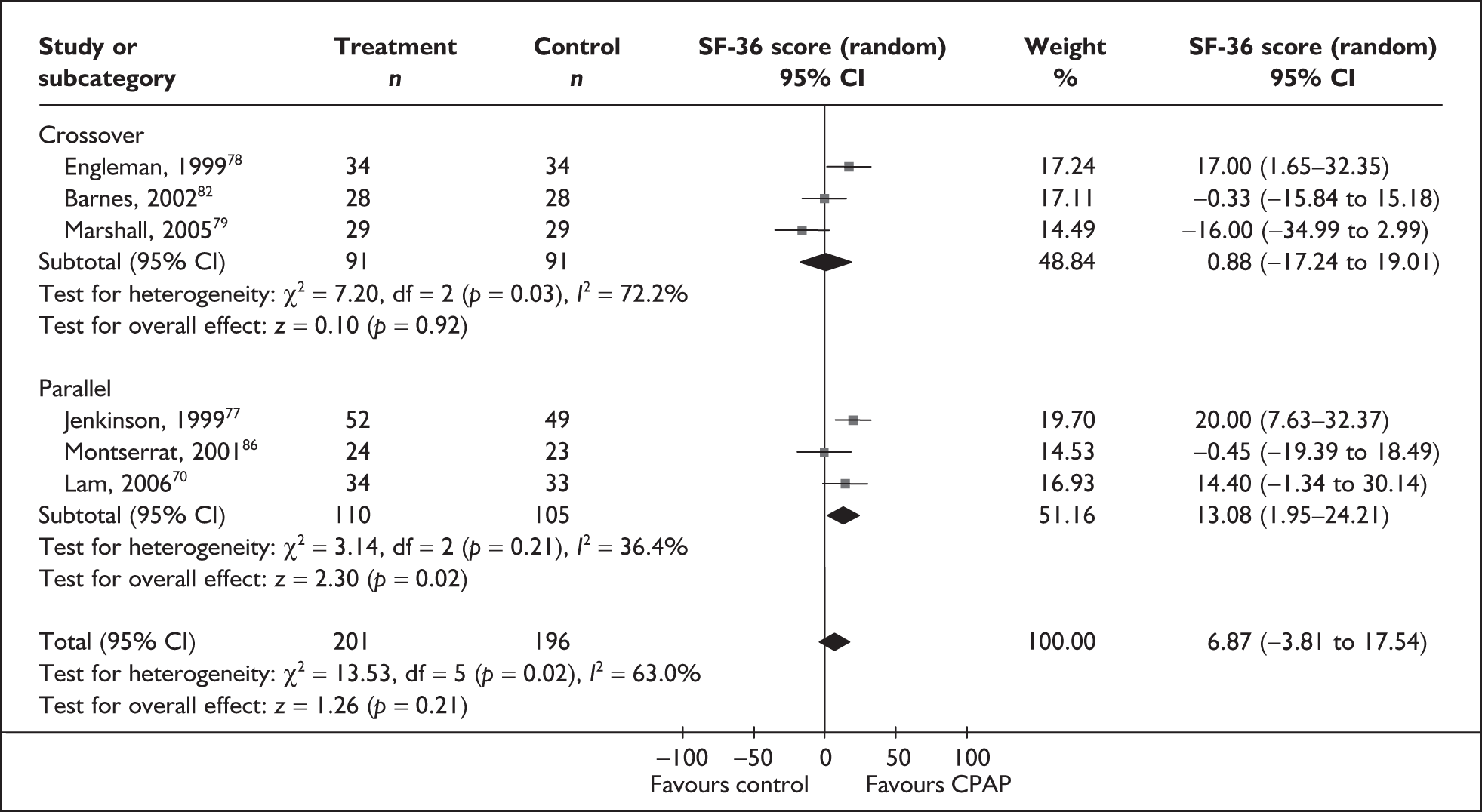
FIGURE 26g.
SF-36 subscales (CPAP versus placebo/usual care): social function.
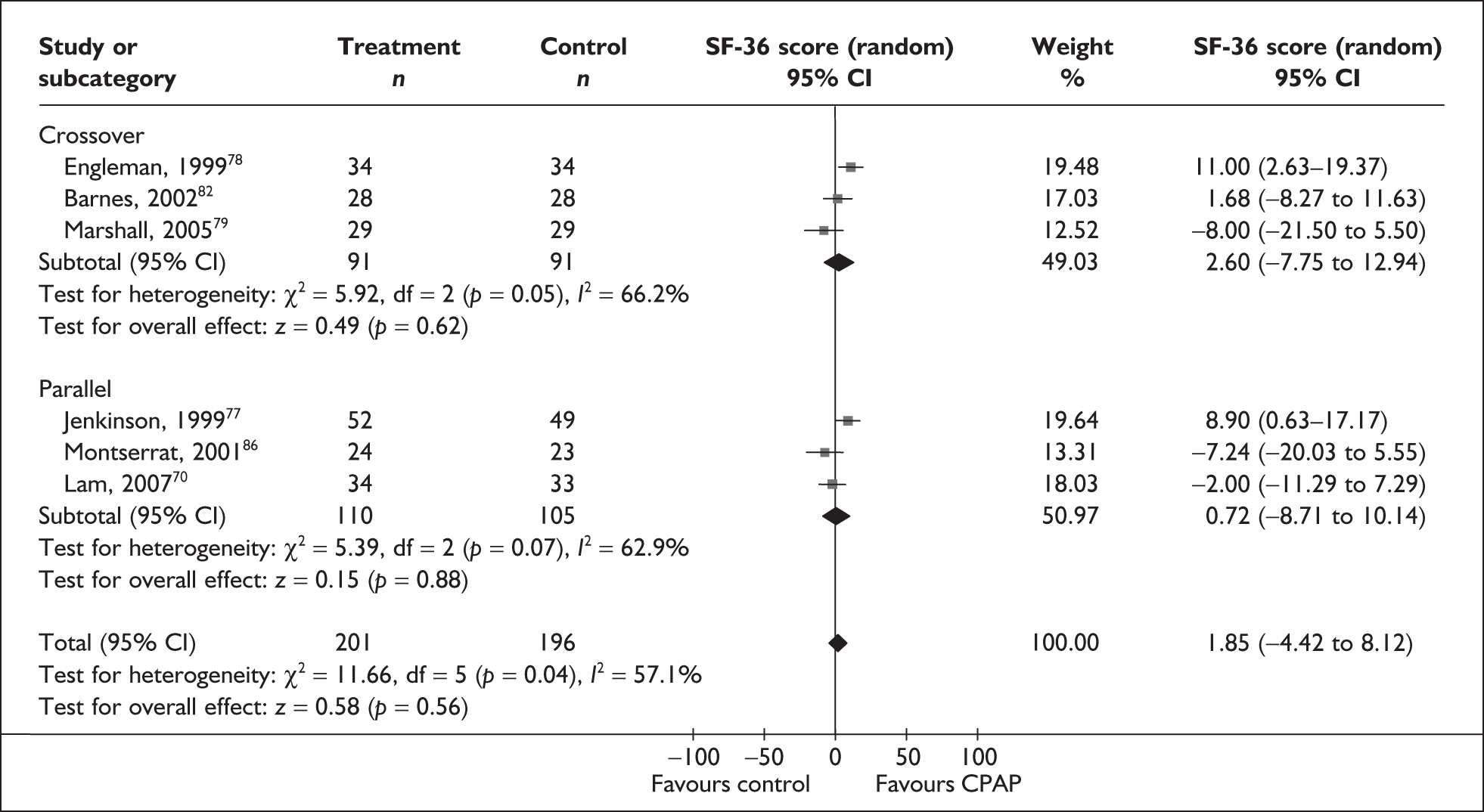
FIGURE 26h.
SF-36 subscales (CPAP versus placebo/usual care): vitality.
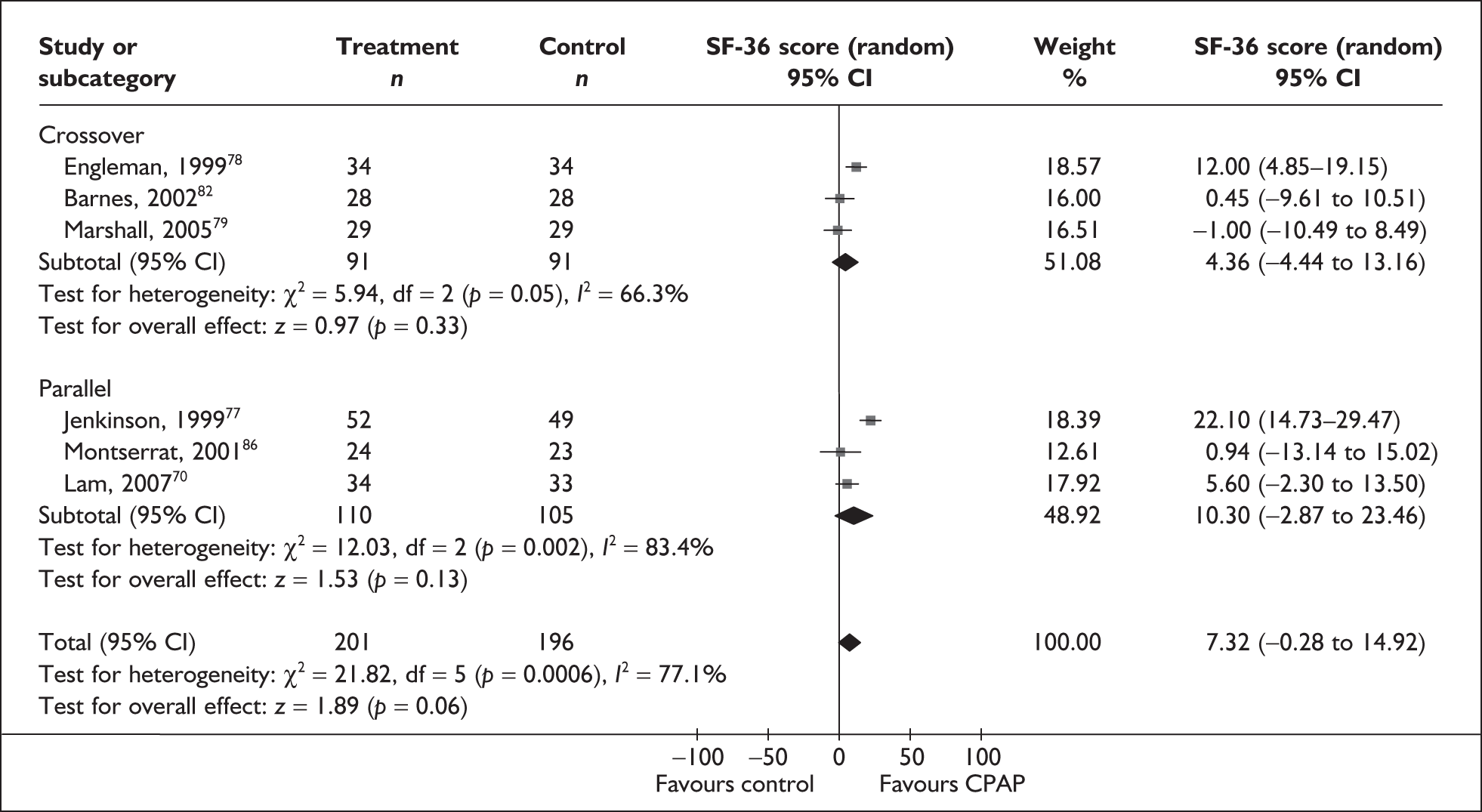
FIGURE 26j.
SF-36 subscales (CPAP versus placebo/usual care): SF-36 component score.
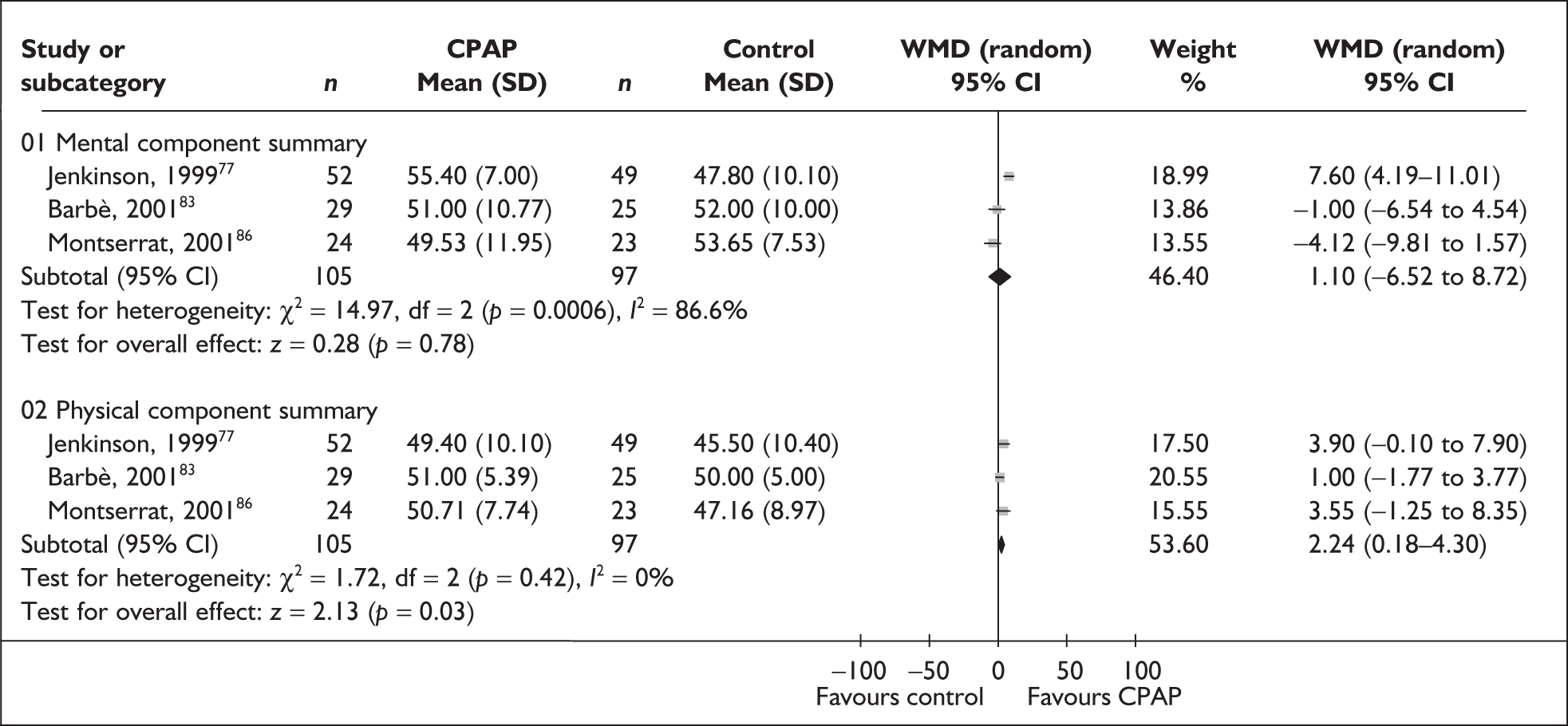
FIGURE 26k.
SF-36 subscales (CPAP versus placebo/usual care): SF-36 total score.
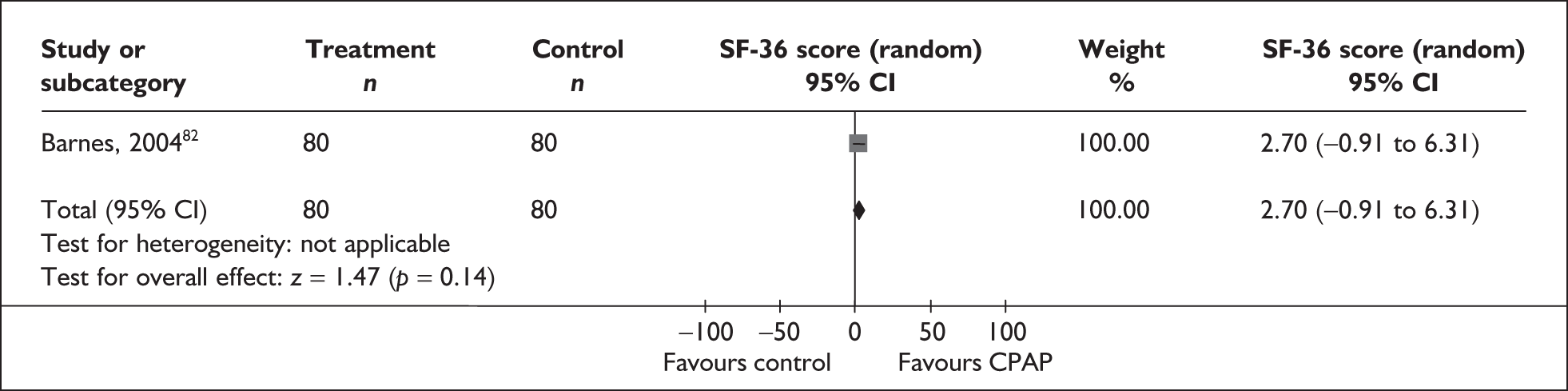
FIGURE 27a.
Functional Outcomes of Sleep Questionnaire subscales (CPAP versus control):FOSQ activity level.
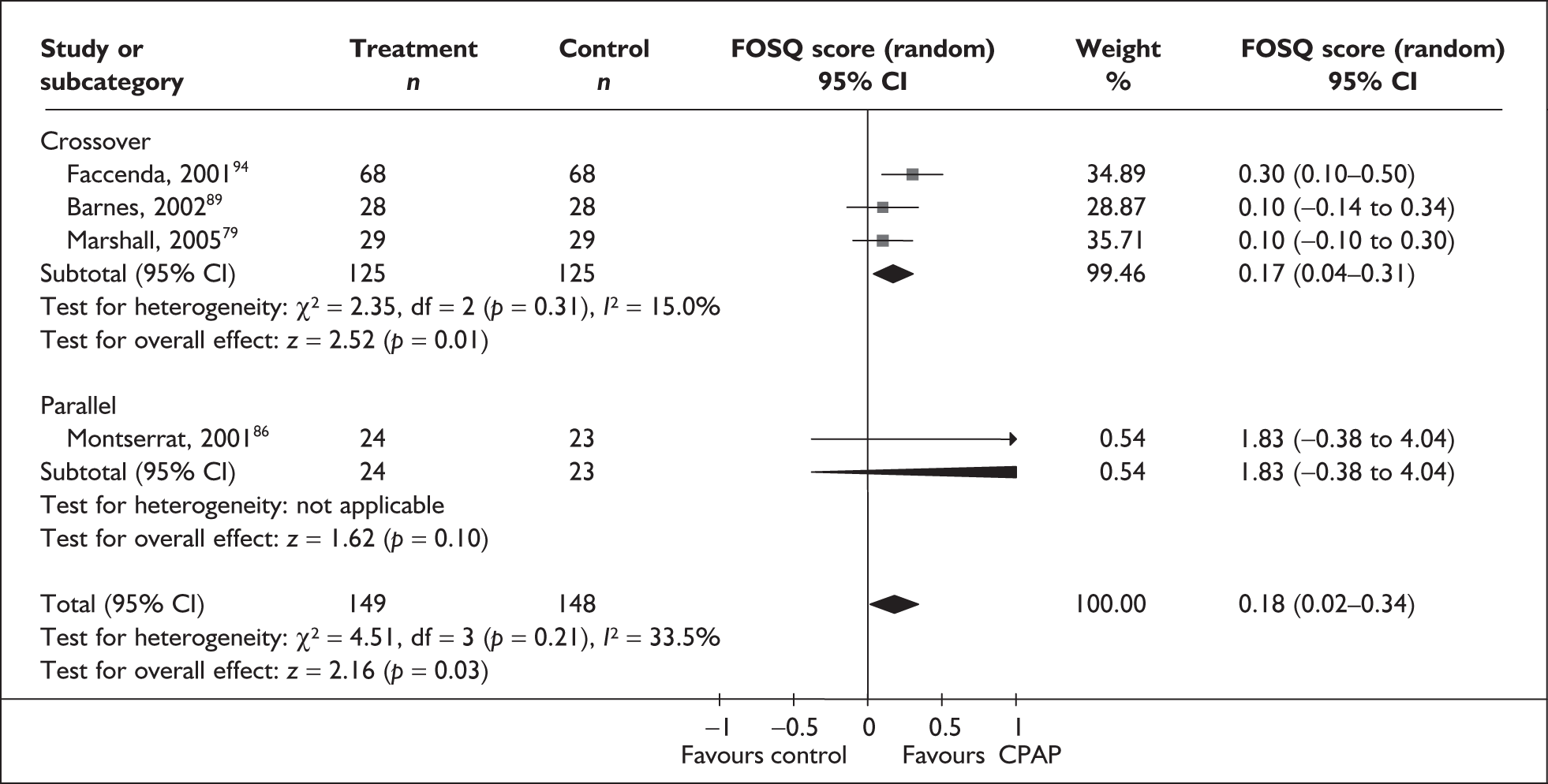
FIGURE 27b.
Functional Outcomes of Sleep Questionnaire subscales (CPAP versus control): FOSQ general productivity.
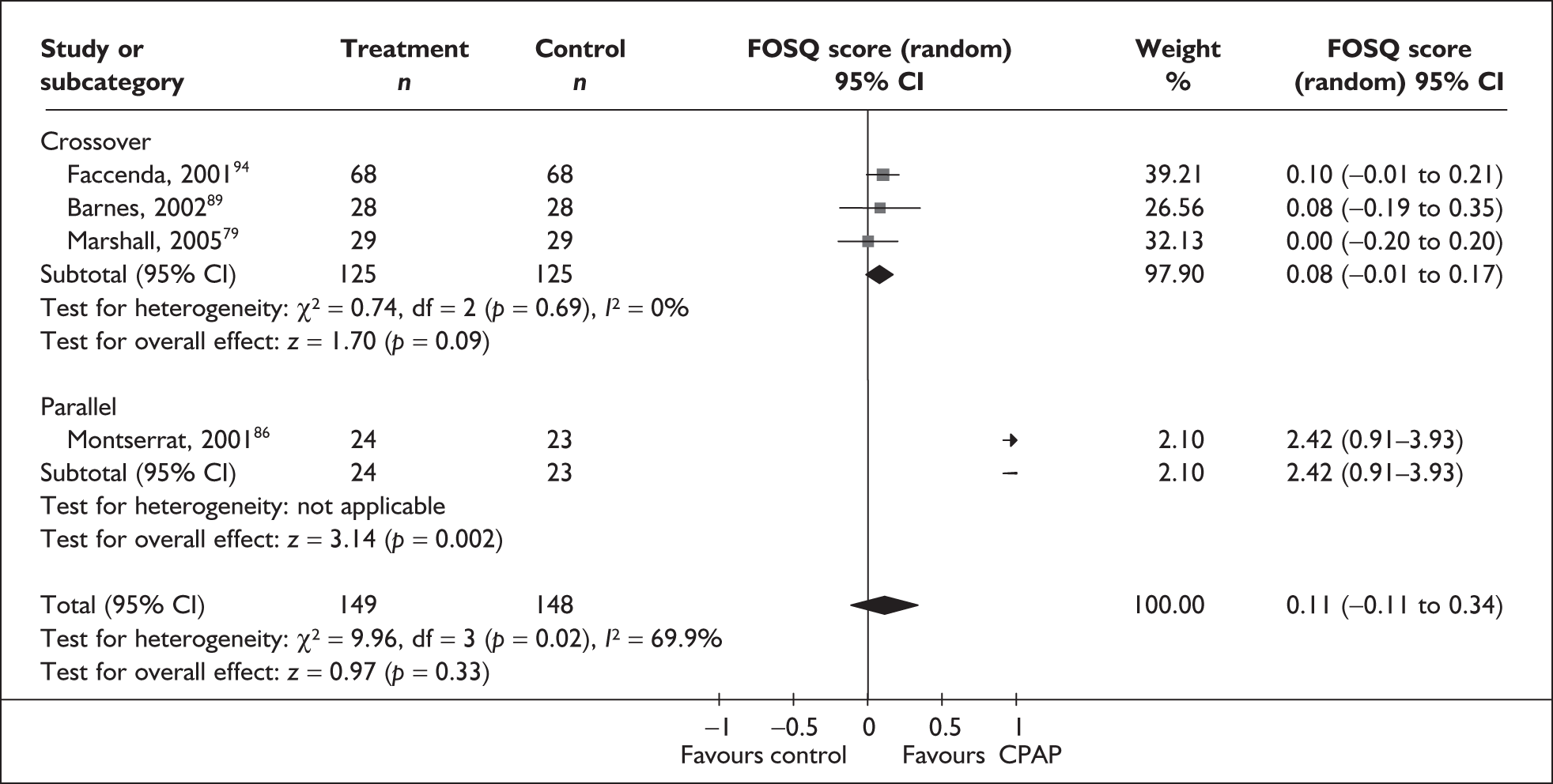
FIGURE 27c.
Functional Outcomes of Sleep Questionnaire subscales (CPAP versus control): FOSQ intimacy and sexual activity.
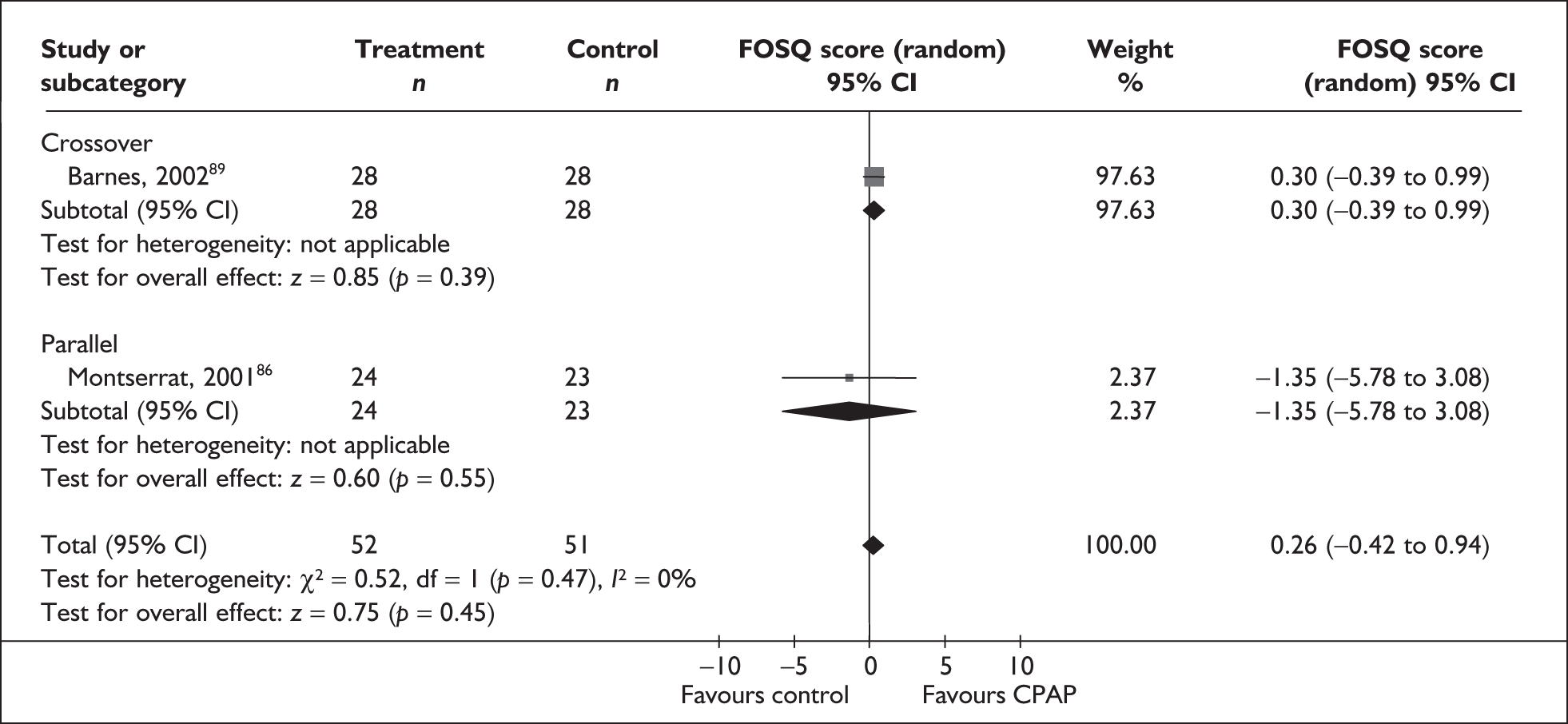
FIGURE 27d.
Functional Outcomes of Sleep Questionnaire subscales (CPAP versus control): FOSQ social outcome.
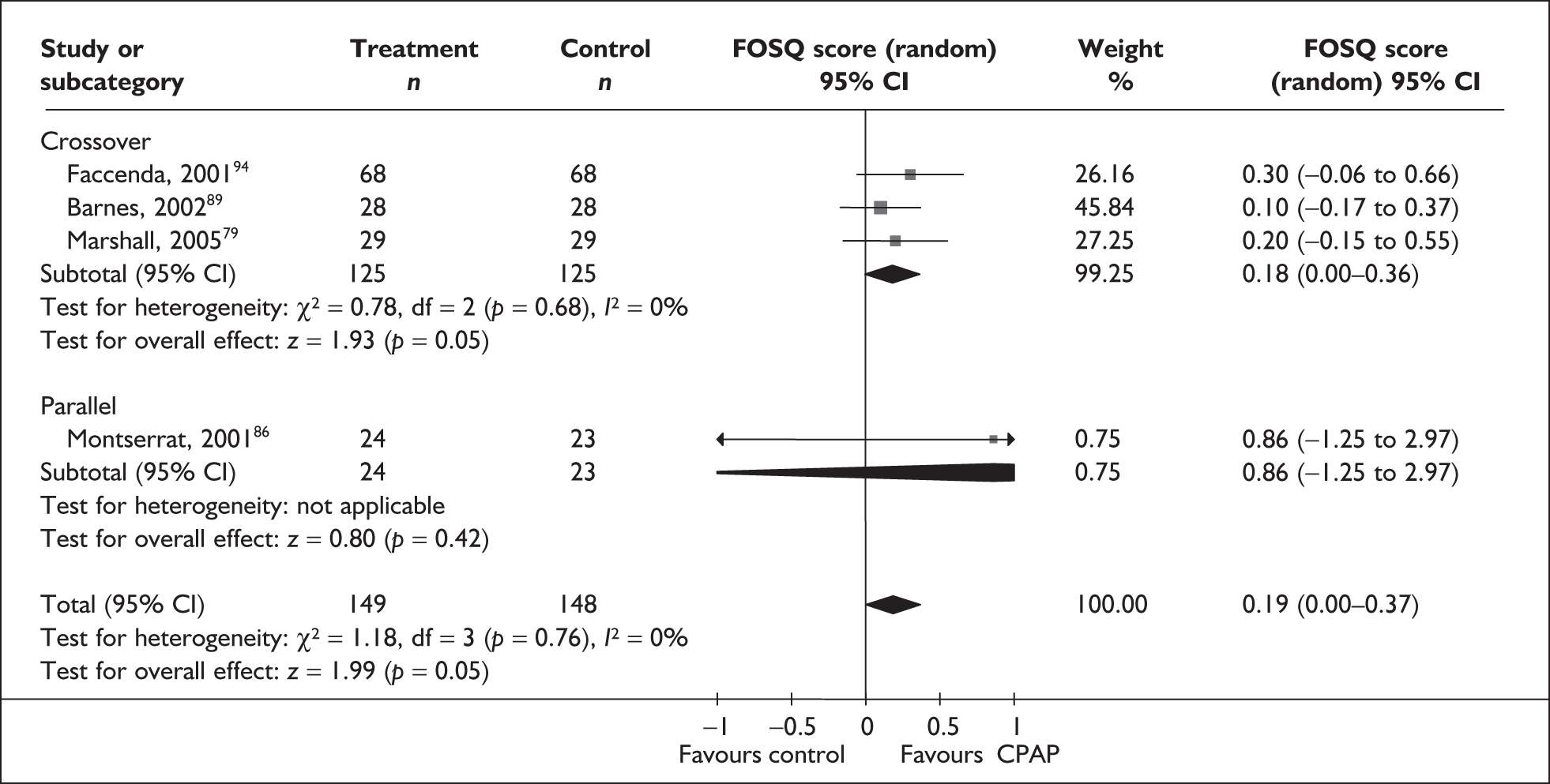
FIGURE 27e.
Functional Outcomes of Sleep Questionnaire subscales (CPAP versus control): FOSQ vigilance.
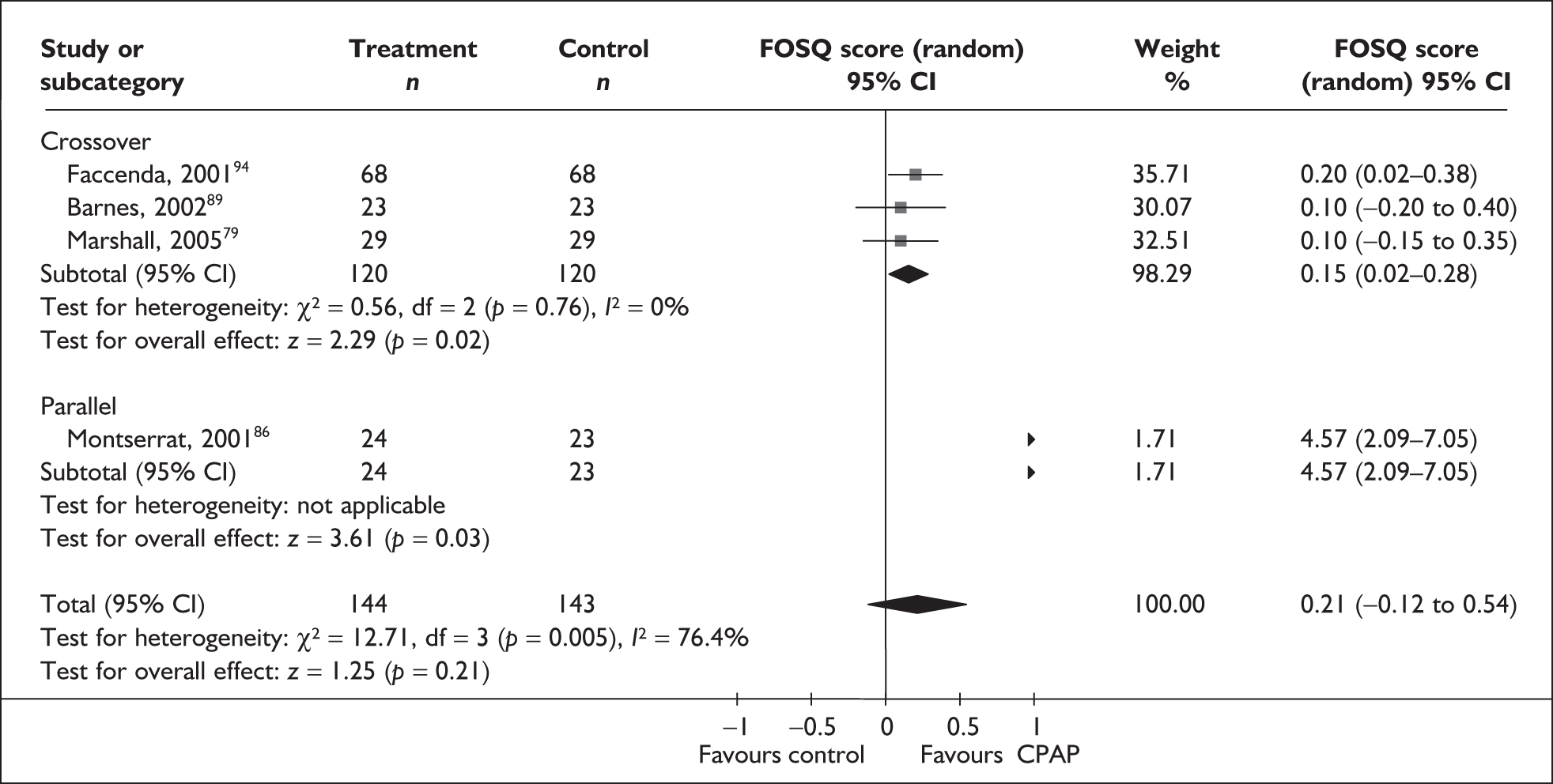
FIGURE 27f.
Functional Outcomes of Sleep Questionnaire subscales (CPAP versus control): FOSQ total score.
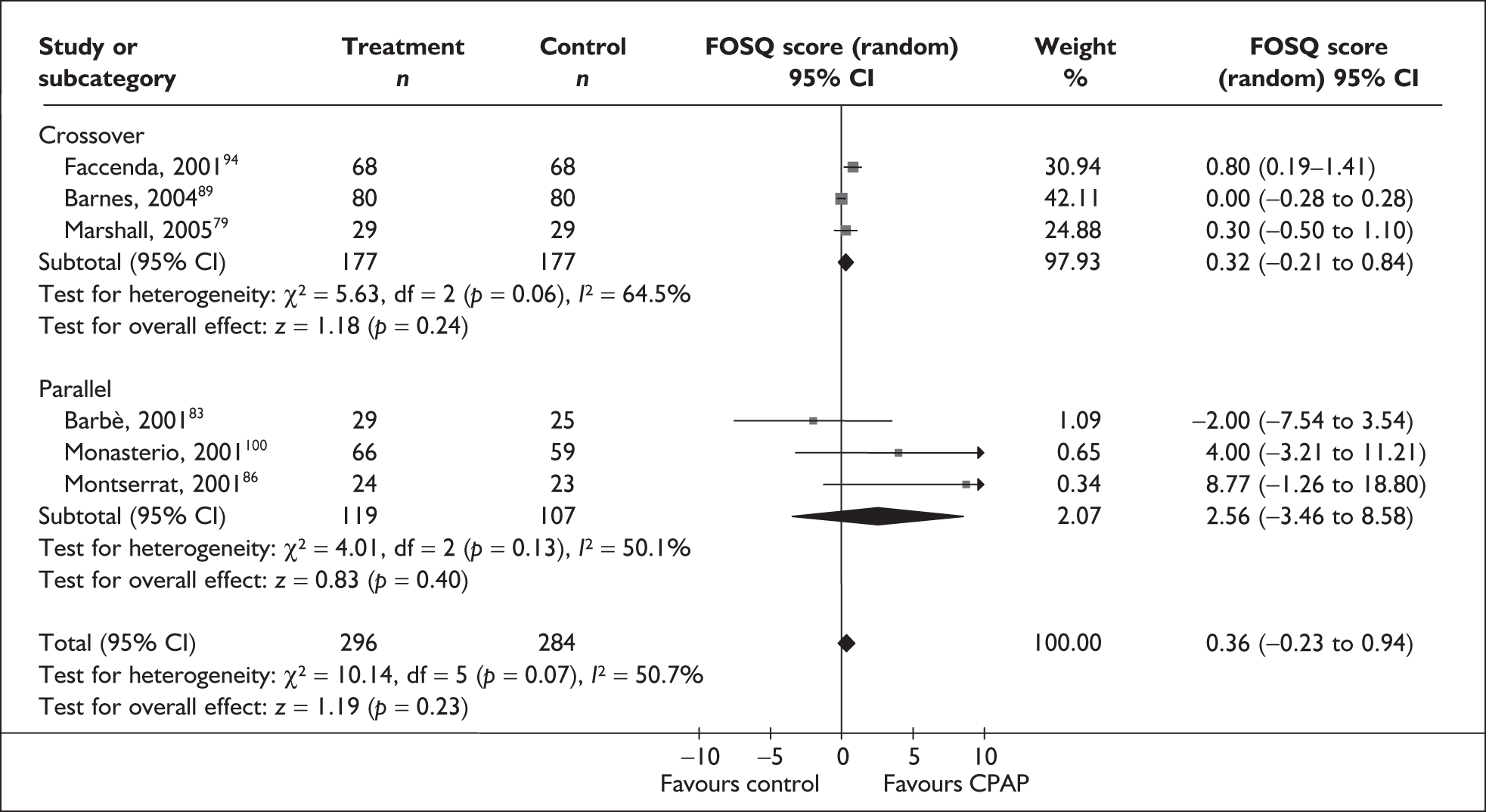
FIGURE 28.
Nottingham Health Profile (Part 2) (CPAP versus placebo/usual care).
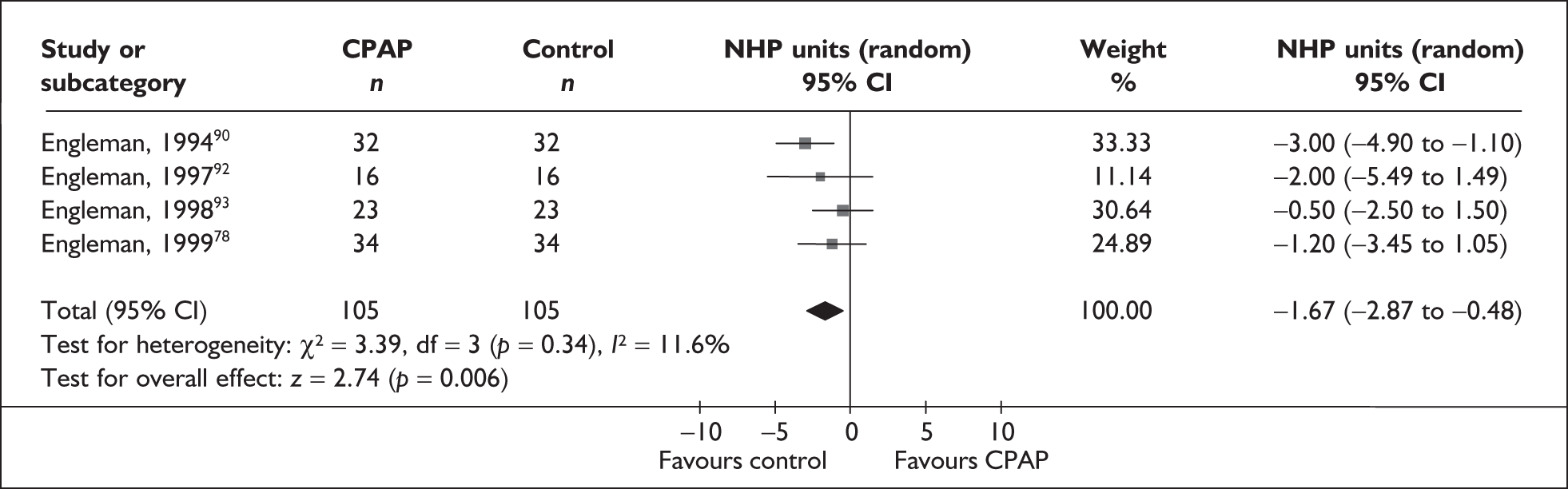
FIGURE 29.
Sleep apnoea quality of life index (CPAP versus placebo/usual care).
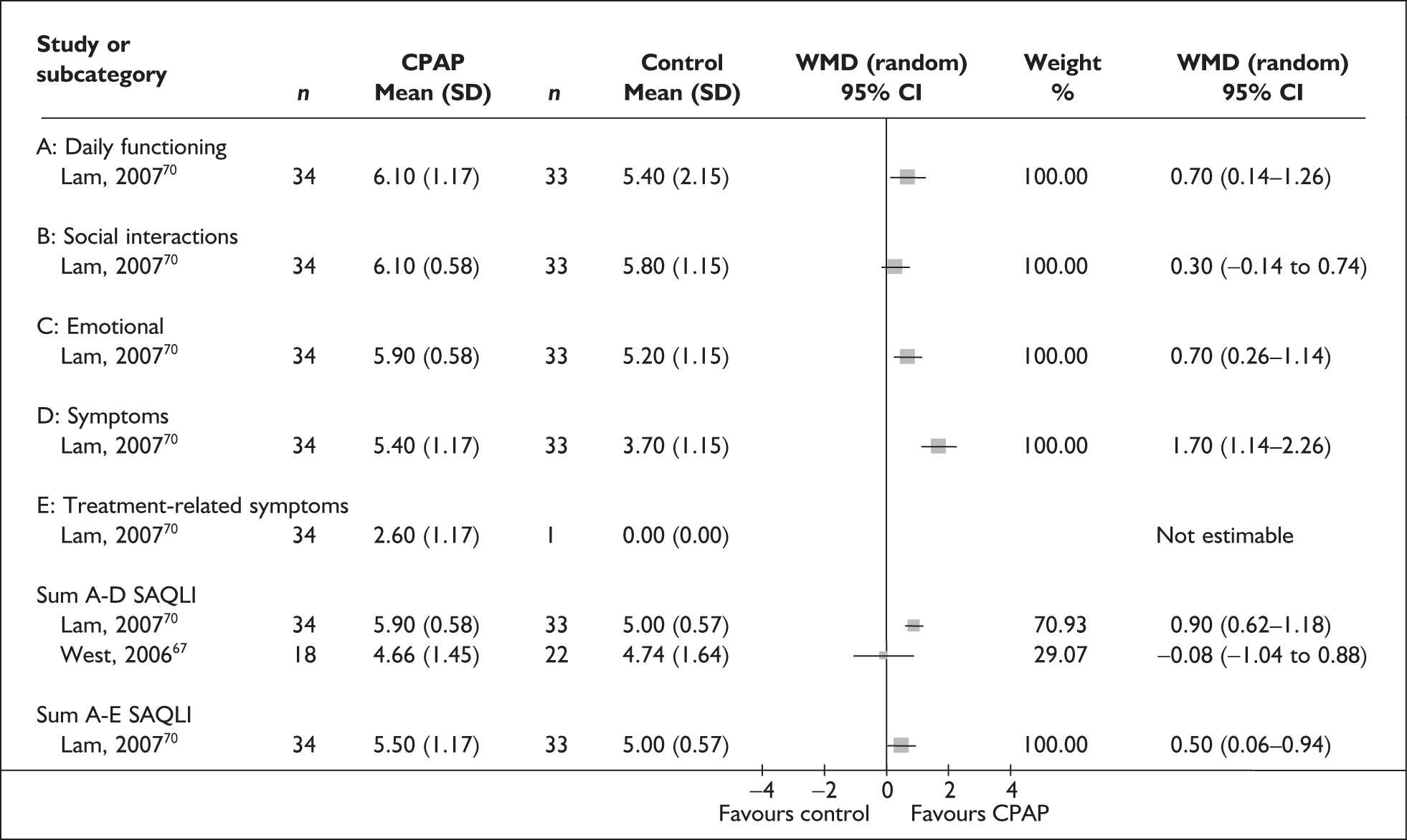
FIGURE 30.
Functional Outcomes of Sleep Questionnaire (CPAP versus dental devices).
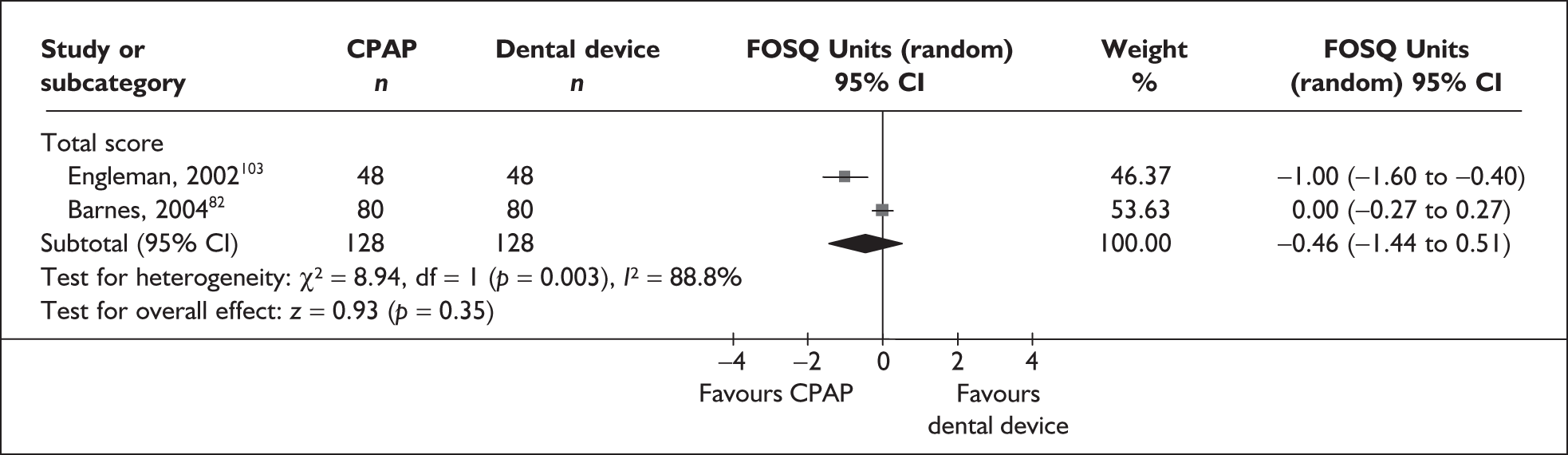
FIGURE 31.
Sleep apnoea quality of life index – summed score (CPAP versus dental devices).
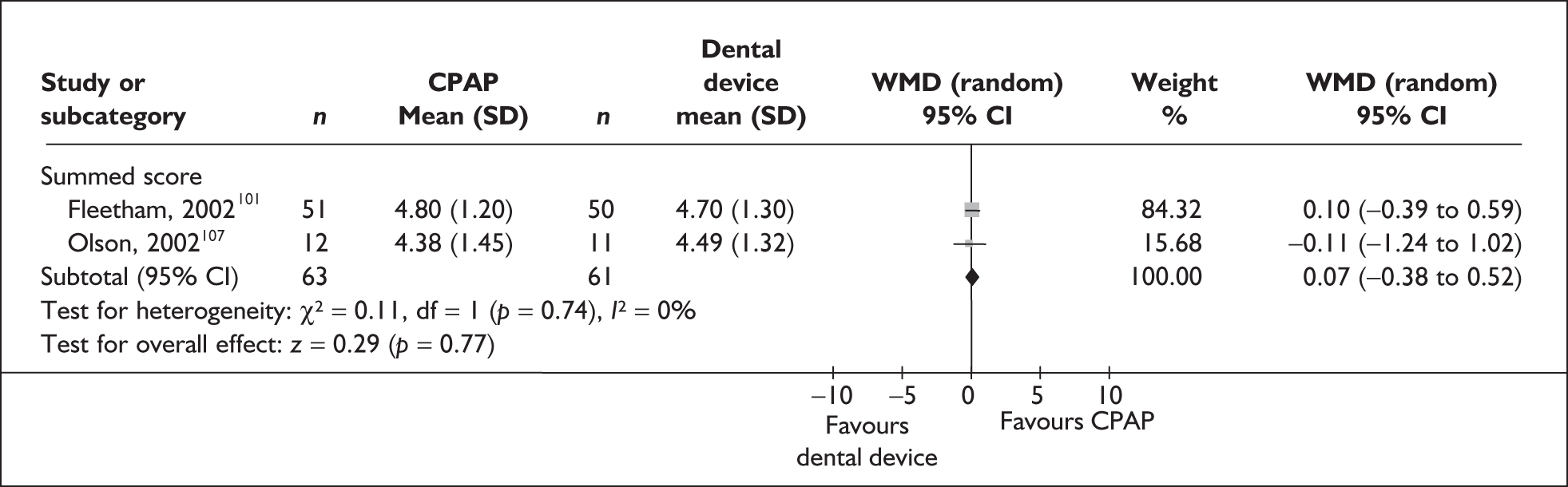
FIGURE 32.
Sleep apnoea quality of life index – subscales and summed scores A–D and A–E (CPAP versus dental devices).
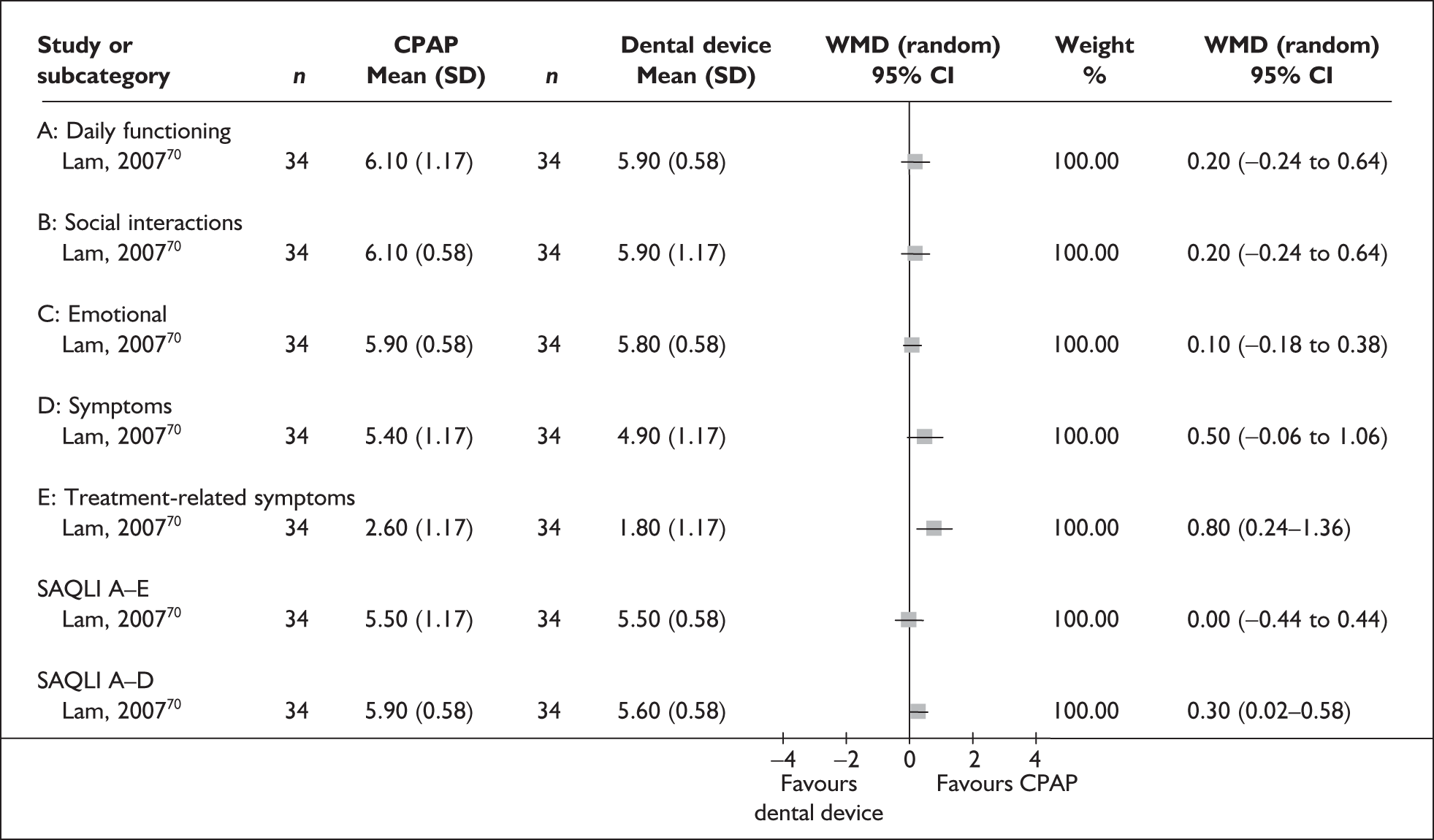
FIGURE 33.
SF-36 summary and total scores (CPAP versus dental devices).
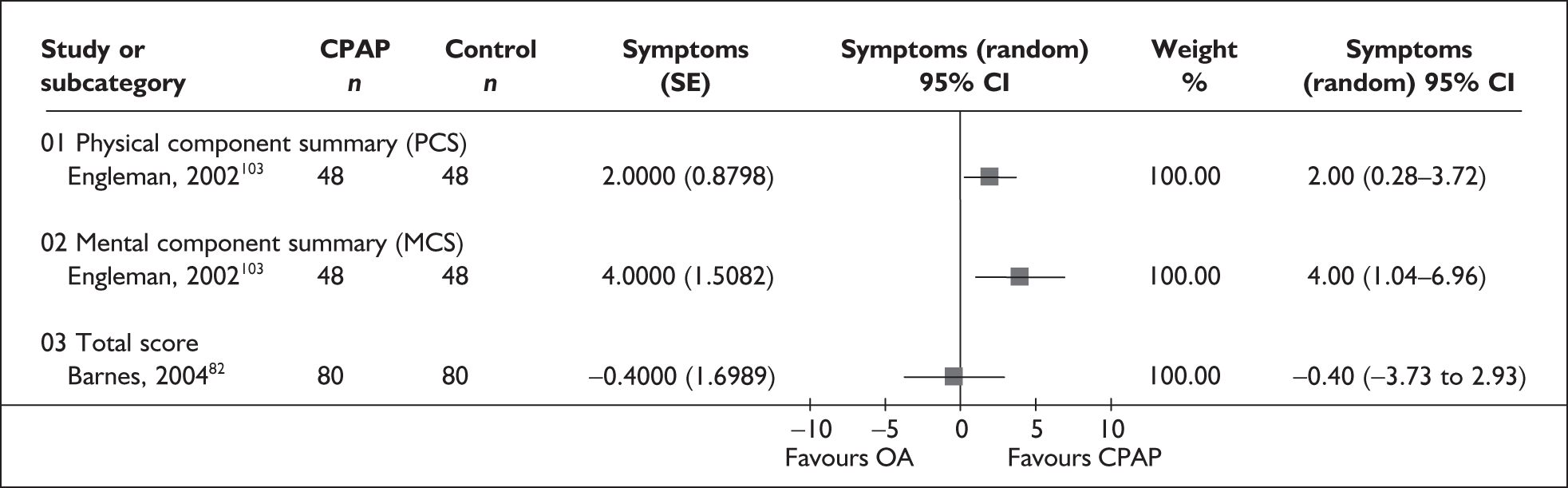
FIGURE 34.
SF-36 subscales (CPAP versus dental devices).
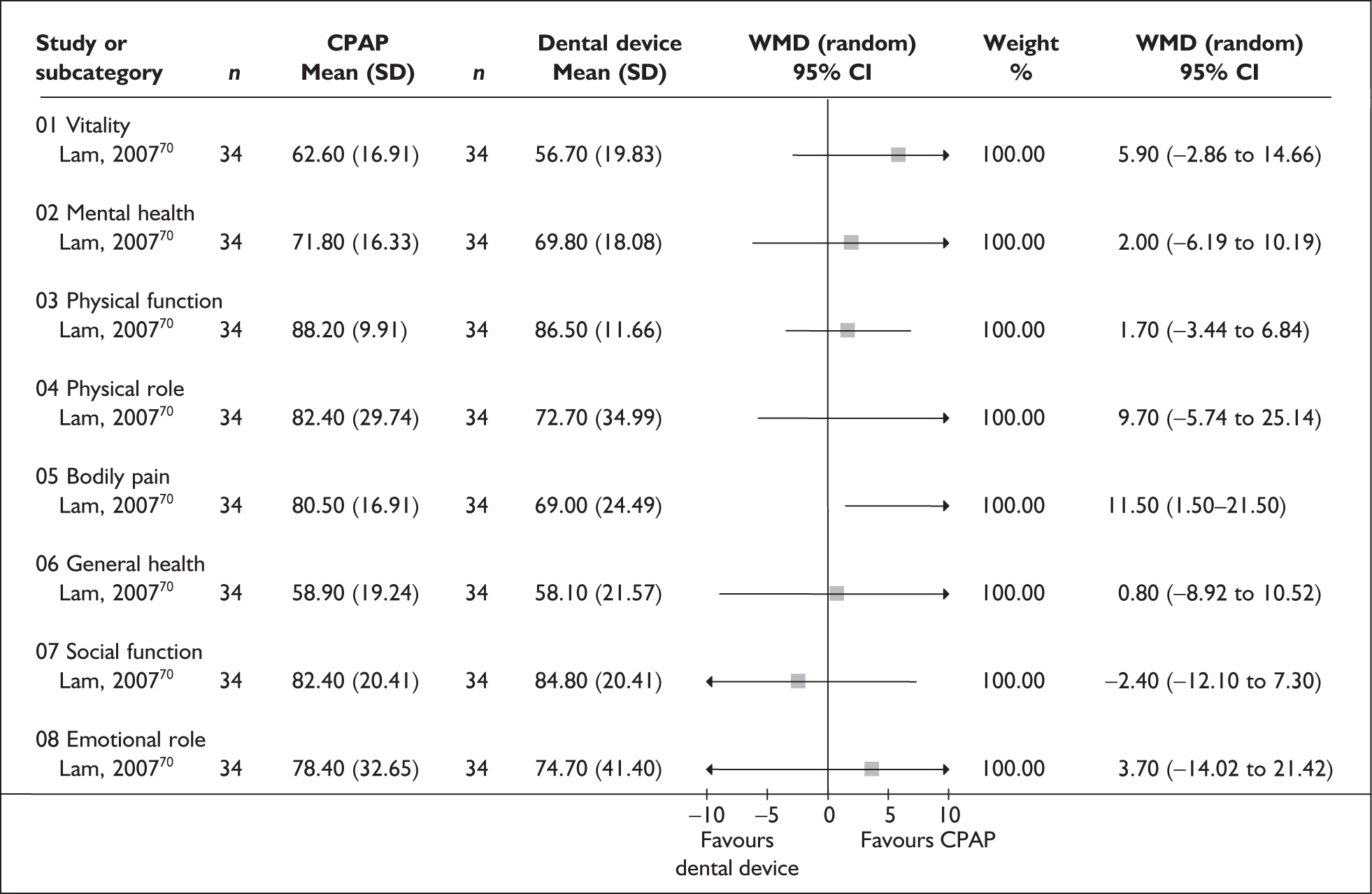
FIGURE 35.
Golombok Rust Inventory of Sexual Satisfaction (CPAP versus dental devices).
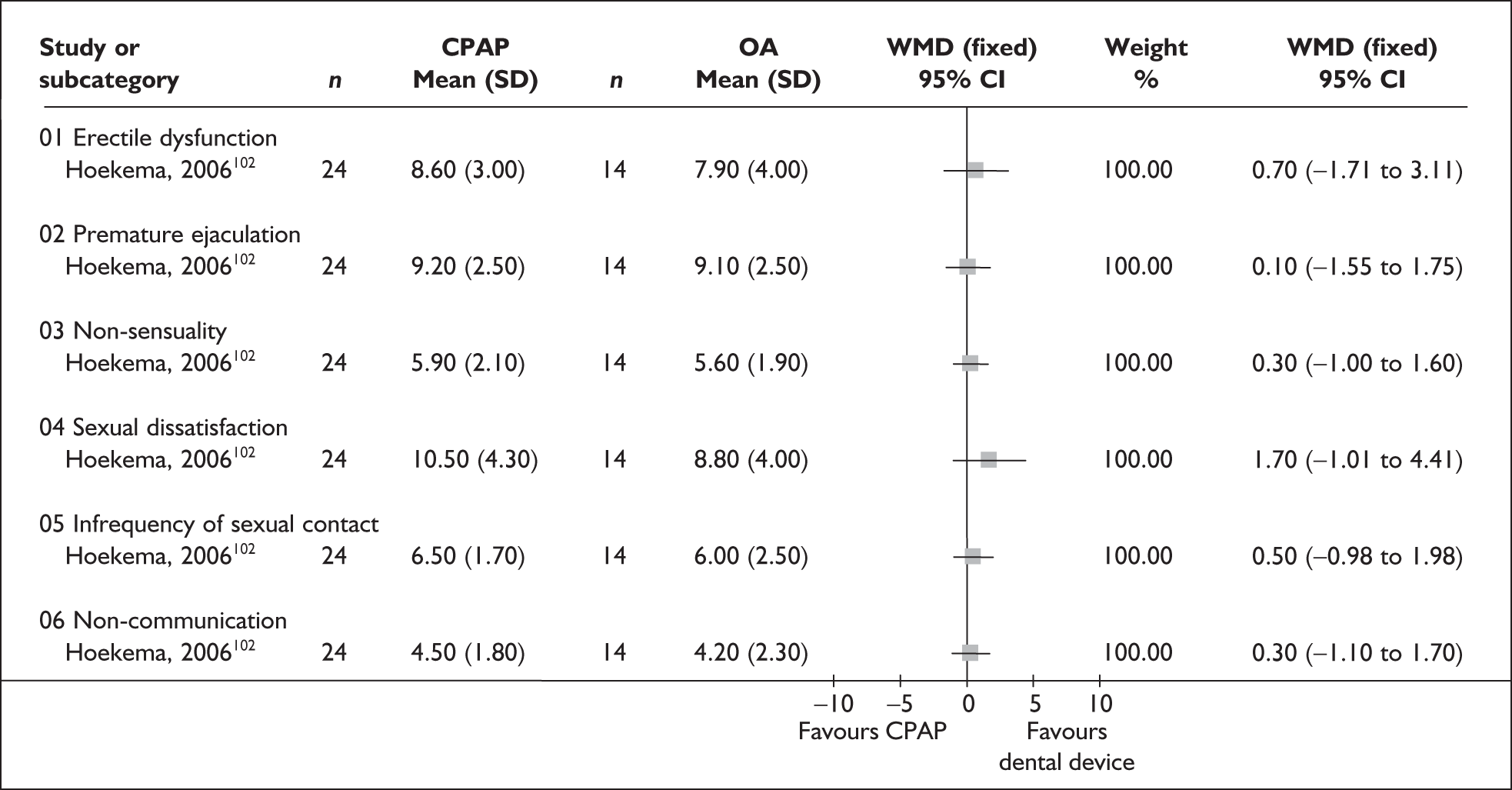
FIGURE 36.
General Health Questionnaire-28 (CPAP versus placebo).
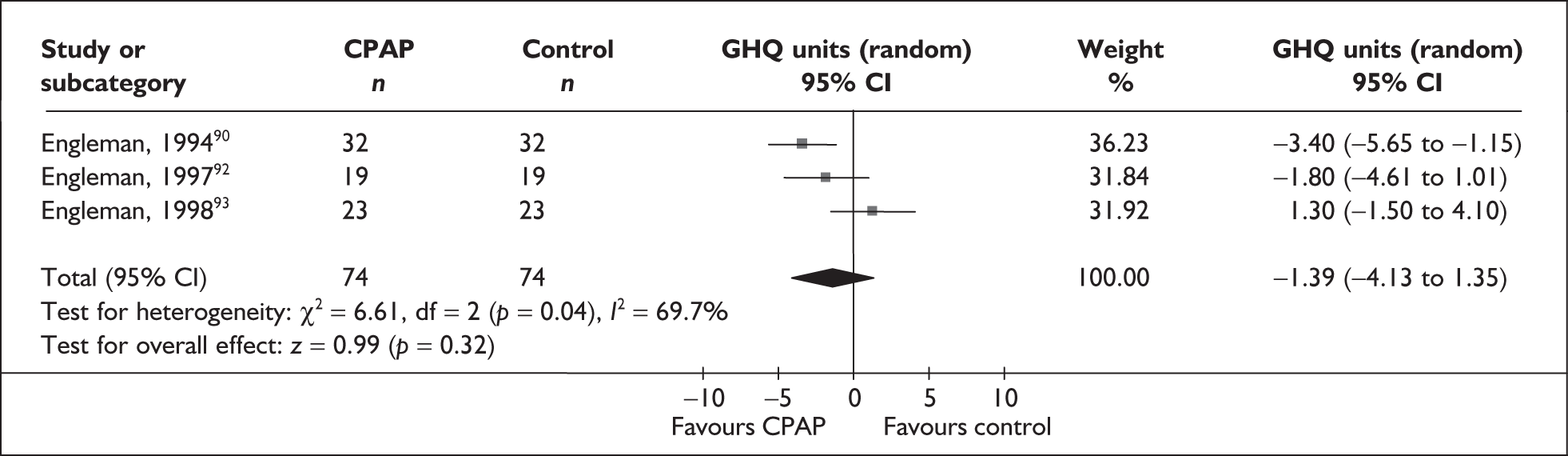
FIGURE 37.
Hospital Anxiety and Depression Scale – anxiety (CPAP versus placebo).
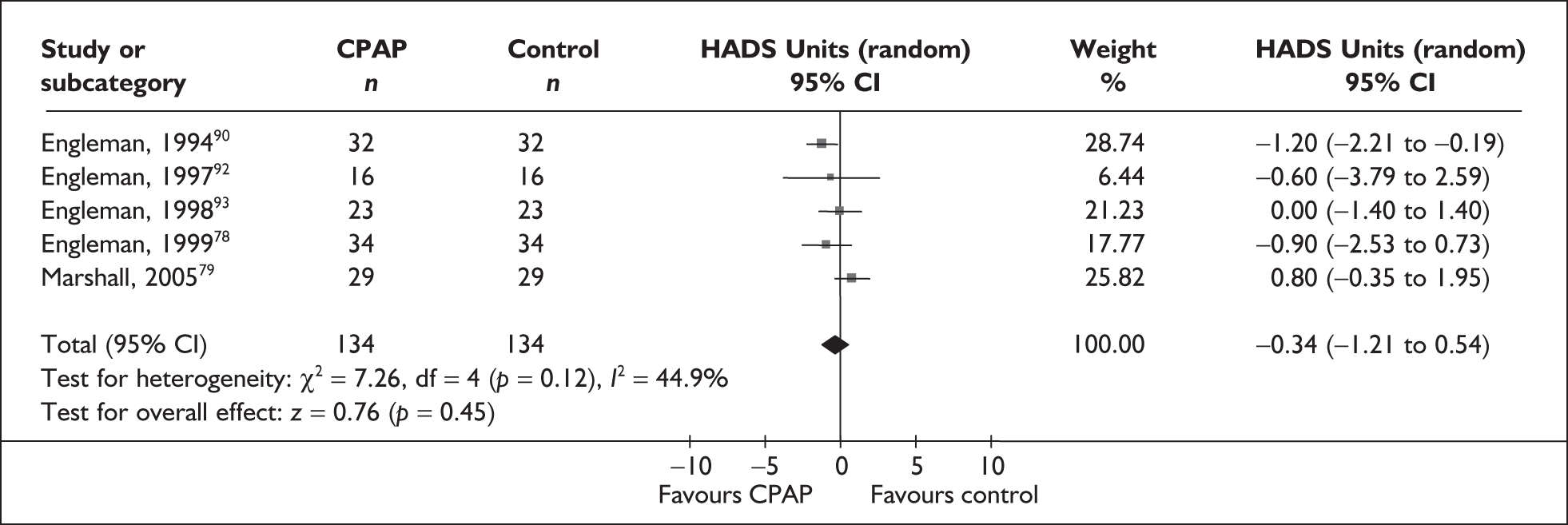
FIGURE 38.
Hospital Anxiety and Depression Scale – depression (CPAP versus placebo).
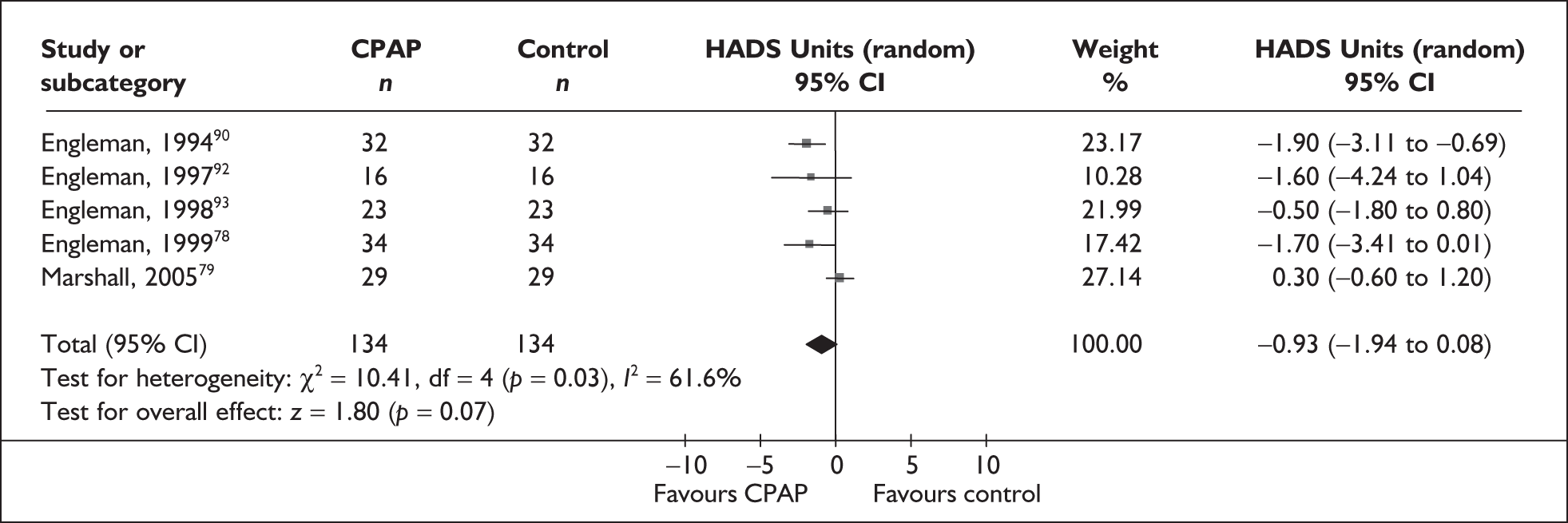
FIGURE 39.
Brief Symptom Inventory (CPAP versus placebo).
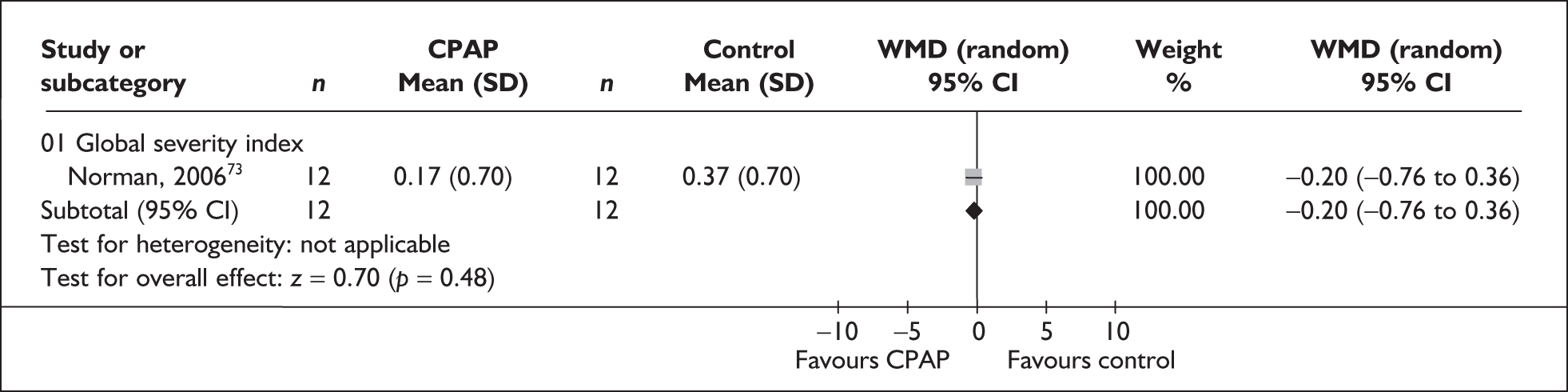
FIGURE 40.
Profile of Mood State (CPAP versus placebo).
FIGURE 41.
UWIST Mood Adjective Checklist – energetic arousal score (CPAP versus control).
FIGURE 42.
Hospital Anxiety and Depression Scale – anxiety (CPAP versus dental devices).
FIGURE 43.
Hospital Anxiety and Depression Scale – depression (CPAP versus dental devices).
| Baseline | Follow-up 1 | Follow-up 2 | |
|---|---|---|---|
| Benton Visual Retention Test (direction of improvement +): This is a visuospatial memory test composed of 10 geometric designs; each design is exposed for 10 seconds, after which time the subject is required to reproduce the design from memory | |||
| Crossover trials | |||
| Engleman et al., 199490 | CPAP: NR; OP: NR; both: NR | Data not reported because there was no statistically significant difference between CPAP and oral placebo | |
| Engleman et al., 199792 | CPAP: NR; OP: NR; both: NR | CPAP: 7.3 (SE 0.6); OP: 7.3 (SE 0.6); MD (SD): 0, p-value: ns | |
| Engleman et al., 199893 | CPAP: NR; OP: NR; both: 7.3 (SD 2.3), n = 23 | CPAP: 7.7 (SD 1.5); OP: 7.7 (SD 1.7); MD (SD): 0, p-value: ns | |
| Parallel trials | |||
| aLojander et al., 1999113 | Correct | Correct | Correct |
| CPAP: 8.5 (6–10), n = 10; CM: 7 (3–10), n = 17 | CPAP: 8.5, n = 10; CM: 7, n = 16; p-value: ns | CPAP: 9.5, n = 9; CM: 5, n = 12; p-value: ns | |
| Delayed | Errors | Errors | |
| CPAP: 4 (4–4), n = 10; CM: 4 (1–4), n = 17 | CPAP: 3, n = 10; CM: 5, n = 17; p-value: ns | CPAP: 1, n = 10; CM: 5, n = 17; p-value: ns | |
| Errors | Delayed | Delayed | |
| CPAP: 2 (0–9), n = 10; CM: 5 (0–9), n = 17 | CPAP: 4, n = 10; CM: 4, n = 17; p-value: ns | CPAP: 4, n = 10; CM: 4, n = 17; p-value: ns | |
| Brief Visuospatial Memory (direction of improvement +): The respondent is briefly shown a number of geometric figures presented on a single page and is asked to reproduce as many figures as possible in their correct location. After a delay, which includes primarily verbal activities, the task is repeated. The respondent is then asked to identify, from 12 figures, which were included in the six geometric figures originally presented. An optional copy trial may be administered. | |||
| Parallel trials | |||
| Norman et al., 199673 (related paper341) | BVM-TR | BVM-TR | |
| CPAP: 26.4, n = 17; sham CPAP: 26.1, n = 14 | CPAP: 29.7, n = 17; sham CPAP: 25.7 (SE 0.6); MD (SD): 4, p-value: 0.143 | ||
| BVM-DL | BVM-DL | ||
| CPAP: 10.8, n = 17; sham CPAP: 10.5, n = 14 | CPAP: 10.7, n = 17; sham CPAP: 9.4, n = 14; MD (SD): 1.3, p-value: 0.138 | ||
| NB p-value based on treatment × time interaction (three treatment arms) | |||
| Bourdon-Wiersma Test (direction of improvement +, except ‘errors’): After scanning a series of rows containing groups of black dots, respondents are asked to strike out all groups of four dots. | |||
| Parallel trials | |||
| aLojander et al., 1999113 | Marked | Marked | Marked |
| CPAP: 128 (56–160), n = 10; CM: 120 (62–400), n = 17 | CPAP: 118, n = 10; CM: 111, n = 16; p-value: ns | CPAP: 103, n = 9; CM: 116, n = 12; p-value: ns | |
| Errors | Errors | Errors | |
| CPAP: 11 (1–18), n = 10; CM: 4 (0–44), n = 17 | CPAP: 12, n = 10; CM: 4, n = 16; p-value: ns | CPAP: 12, n = 9; CM: 4, n = 12; p-value: ns | |
| Concentration endurance test (direction of improvement +, except errors): This test of sustained attention and visual scanning consists of 14 lines with 47 characters in each line (each character consists of a letter, ‘d’ or ‘p’, marked with one, two, three or four small dashes). The respondent is required to scan the lines and cross out all occurrences of the letter ‘d’ with two dashes while ignoring all other characters. | |||
| Crossover trials | |||
| Jokic et al., 1999108 | Total score | Total score | |
| Both: 482.3 (SD 38.6), n = 13 | CPAP: 532.3 (SD 73.5); CM: 530 (SD 55.6); MD (SD): 2.3, p-value: 2.36 | ||
| % Errors | % Errors | ||
| Both: 7.4 (SD 3.1), n = 13 | CPAP: 4.4 (SD 5.4); CM: 4.5 (SD 6.4); MD (SD): –0.1, p-value: 2.82 | ||
| Clock face drawing (direction of improvement –): Respondents are asked to draw the face of a clock and then add in a specified time. | |||
| Parallel trials | |||
| aLojander et al., 1999113 | CPAP: 1 (1–3), n = 10; CM: 1 (1–3), n = 17 | CPAP: 1, n = 10; CM: 1, n = 16; p-value: ns (change) | CPAP: 1, n = 9; CM: 2, n = 12; p-value: ns (change) |
| Copying (direction of improvement –): Respondents are asked to reproduce a set of designs | |||
| Parallel trials | |||
| aLojander et al., 1999113 | CPAP: 1 (1–3), n = 10; CM: 2 (1–4) | CPAP: 1, CM: 2; p-value: ns | CPAP: 1, CM: 2; p-value: ns |
| Complex figure test (direction of improvement +): The subject is required to make a pen/paper copy of a complex figure. | |||
| Parallel trials | |||
| Henke et al., 200185 | CPAP: NR; sham CPAP: NR | Data presented in graph only; no statistically significant difference between groups at follow-up | |
| Consonant trigram (direction of improvement +): In this distractor task respondents are asked to count backwards from a given number on hearing/seeing the stimulus item. When signalled to stop counting respondents are asked to report or identify the stimulus item | |||
| Crossover trials | |||
| Jokic et al., 1999108 | 3-second delay | 3-second delay | |
| Both: 12.3 (SD 4.4), n = 13 | CPAP: 13.2 (SD 1.5); CM other: 13.4 (SD 1.7); MD (SD): –0.2, p-value: 1.86 | ||
| 9-second delay | 9-second delay | ||
| Both: 9.8 (SD 2.5), n = 13 | CPAP: 10.5 (SD 1.5); CM other: 10.2 (SD 2.6); MD (SD): 0.3, p-value: 1.94 | ||
| 18-second delay | 18-second delay | ||
| Both: 6.6 (SD 0.6), n = 13 | CPAP: 8.0 (SD 3.6); CM other: 8.2 (SD 1.0); MD (SD): –0.2, p-value: 2.34 | ||
| Total correct position | Total correct position | ||
| Both: 34.3 (SD 7), n = 13 | CPAP: 40.2 (SD 10.6); CM other: 41.5 (SD 11.6); MD (SD): –1.3, p-value: 2.88 | ||
| Total correct sequence | Total correct sequence | ||
| Both: 38.9 (SD 9), n = 13 | CPAP: 37.1 (SD 8.0); CM other: 38.4 (SD 11.1); MD (SD): –1.3, p-value: 0.77 | ||
| Controlled Oral Word Association Test (direction of improvement +): In these three word-naming trials respondents are asked to name words that begin with a specified set of letters in 60 seconds. | |||
| Crossover trials | |||
| Barnes et al., 200289 | CPAP: NR; OP: NR; both: 35.3 (SD 10.7), n = 28 | No. of words correct | |
| CPAP: 38.7; OP: 36.0; MD (SD): 2.7, p-value: 0.02 (difference in change) | |||
| NB After treatment × period interaction accounted for p = ns | |||
| Barnes et al., 200482 | CPAP: NR; comparator: NR; both: 43.2 (SE 1.1), n = 80 | CPAP: 46.5 (SE 1.2); OA: 46.3 (SE 1.1); OP: 46.3 (SE 1.0); CPAP/OA MD (SD): 0.2; CPAP/OP MD (SD): 0.2; p-value: ns | |
| Engleman et al., 199490 | CPAP: NR; OP: NR | Data not reported because there was no statistically significant difference between CPAP and oral placebo | |
| Engleman et al., 199792 | CPAP: NR; OP: NR; n = 16 | No. of words correct | |
| CPAP: 38.5 (SE 3.5); OP: 39.2 (SE 3.1); MD (SD): –0.7, p-value: ns | |||
| Engleman et al., 199893 | CPAP: NR; OP: NR; both: 39 (SD 12), n = 23 | No. of words correct | |
| CPAP: 41 (SD 12); OP: 42 (SD 11); MD (SD): –1, p-value: ns | |||
| Parallel trials | |||
| Henke et al., 200185 | CPAP: NR; sham CPAP: NR | Data not reported; no statistically significant difference between groups at follow-up | |
| Digit ordering (direction of improvement +): Respondents are asked to recall digit sequences in ascending order. | |||
| Parallel trials | |||
| Dimsdale et al., 200058 (related paper117) | No. correct | No. correct | |
| CPAP: 87.8 (SE 1.9), n = 20; sham CPAP: 85.7 (SE 2.2), n = 16 | CPAP: 90.6 (SE 1.7), n = 20; sham CPAP: 86.1 (SE 2.0), n = 16; MD (SD): 4.5, p-value: ns | ||
| No. of items | No. of items | ||
| CPAP: 4.3 (SE 0.7), n = 20; sham CPAP: 2.6 (SE 0.8), n = 16 | CPAP: 4.9 (SE 0.7), n = 20; sham CPAP: 2.7 (SE 0.8), n = 16; MD (SD): 2.2, p-value: ns | ||
| Driving simulator test (direction of improvement –): Respondents are asked to steer an image of a car bonnet down the centre of a winding road as accurately as possible using a standard computer game steering wheel. | |||
| Crossover trials | |||
| Engleman et al., 199490 (SteerClear) | Obstacles hit | Obstacles hit | |
| CPAP: NR; OP: NR; n = 32 | CPAP: 76 (SE 5); OP: 81 (SE 6); MD (SD): –5, p-value: 0.01 | ||
| Engleman et al., 199792 (SteerClear) | Obstacles hit | Obstacles hit | |
| CPAP: NR; OP: NR; n = 16 | CPAP: 74.8 (SE 7.3); OP: 75.3 (SE 8.9); MD (SD): –0.5, p-value: ns | ||
| Engleman et al., 199893 (SteerClear) | Obstacles hit | Obstacles hit | |
| CPAP: NR; OP: NR; both: 100 (SD 63), n = 22 | CPAP: 63 (SD 27); OP: 71 (SD 40); MD (SD): –8, p-value: ns | ||
| Engleman et al., 199978 (SteerClear) | Obstacles hit | Obstacles hit | |
| CPAP: NR; OP: NR; both: 295 (SD 183), n = 34 | CPAP: 189 (SD 156); OP: 195 (SD 158); MD (SD): –6, p-value: ns | ||
| Engleman et al., 2002103 (SteerClear) | Obstacles hit | Obstacles hit | |
| CPAP: NR; OA: NR; n = 48 | CPAP: 49 (SD 60); OA: 50 (SD 44); MD (SD): 1, p-value: 0.266 | ||
| Parallel trials | |||
| Barbé et al., 200183 (SteerClear) | Obstacles hit (%) | Obstacles hit (%) | |
| CPAP: 5 (SE 1), n = 29; sham CPAP: 6 (SE 2), n = 25 | CPAP: 4 (SE 1), n = 29; sham CPAP: 5 (SE 2), n = 25; MD (SD): –1, p-value: > 0.20 (difference in change) | ||
| bHoekema et al., 2006102 (related paper117) | Lapses of attention (total) | Lapses of attention (total) | |
| CPAP: 10.0 (1–16.8), n = 10; OA: 5.0 (2–14), n = 9 | CPAP: 0.5 (0–5.3), n = 10; OA: 0.0 (0–2), n = 9; p-value: ns | ||
| Lapses of attention (0–5 minutes) | Lapses of attention (0–5 minutes) | ||
| CPAP: 0.0 (0–0), n = 10; OA: 0.0 (0–1), n = 9 | CPAP: 0.0 (0–0), n = 10; OA: 0.0 (0–0.5), n = 9; p-value: ns | ||
| Lapses of attention (6–10 minutes) | Lapses of attention (6–10 minutes) | ||
| CPAP: 0.0 (0–1), n = 10; OA: 0.0 (0–1), n = 9 | CPAP: 0.0 (0–0.3), n = 10; OA: 0.0 (0–0), n = 9; p-value: ns | ||
| Lapses of attention (11–15 minutes) | Lapses of attention (11–15 minutes) | ||
| CPAP: 1.0 (0–2.5), n = 10; OA: 0.0 (0–5), n = 9 | CPAP: 0.0 (0–1), n = 10; OA: 0.0 (0–0), n = 9; p-value: ns | ||
| Lapses of attention (16–20 minutes) | Lapses of attention (16–20 minutes) | ||
| CPAP: 3.0 (0.8–7.8), n = 10; OA: 2.0 (0–5.5), n = 9 | CPAP: 0.0 (0–0.5), n = 10; OA: 0.0 (0–0.5), n = 9; p-value: ns | ||
| Lapses of attention (21–25 minutes) | Lapses of attention (21–25 minutes | ||
| CPAP: 4.0 (0.0–8.5), n = 10; OA: 2.0 (0–4), n = 9 | CPAP: 0.0 (0.0–2.5), n = 10; OA: 0.0 (0–1), n = 9; p-value: ns | ||
| cJenkinson et al., 199977 (other DST) (related paper118) | SD position on road | SD position on road | |
| CPAP: 0.36 (0.15–1.12), n = 26; sham CPAP: 0.35 (0.15–1.17), n = 33 | CPAP: 0.21 (0.14–0.63), n = 26; sham CPAP: 0.30 (0.14–1.19), n = 33; MD (SD): –0.07, p-value: 0.08 | ||
| SD deterioration (SD/hour) | SD deterioration (SD/hour) | ||
| CPAP: 0.18 (–1.14 to 30.3), n = 26; sham CPAP: 0.18 (–0.02 to 2.67), n = 33 | CPAP: 0.06 (–1.02 to 0.40), n = 26; sham CPAP: 0.24 (–0.04 to 2.64), n = 33; MD (SD): –0.18, p-value: 0.007 | ||
| Off-road events (no./hour) | Off-road events (no./hour | ||
| CPAP: 17.8 (0.4–149), n = 26; sham CPAP: 34.8 (0.9–149), n = 33 | CPAP: 9 (0–76), n = 26; sham CPAP: 23 (0–150), n = 33; MD (SD): –14, p-value: 0.07 | ||
| Length of drive (minutes) | Length of drive (minutes) | ||
| CPAP: 24.8 (7.6–30), n = 26; sham CPAP: 27.6 (10.9–30), n = 33 | CPAP: 30 (17.6–30), n = 26; sham CPAP: 26.9 (9.1–30), n = 33; MD (SD): 3.1, p-value: 0.08 | ||
| Reaction time (seconds) | Reaction time (seconds) | ||
| CPAP: 2.8 (1.8–4.9), n = 26; sham CPAP: 2.8 (1.7–5.5), n = 33 | CPAP: 2.3 (1.5–3.5), n = 26; sham CPAP: 2.7 (1.6–4.0), n = 33; MD (SD): –0.4, p-value: 0.04 | ||
| Monasterio et al., 2001100 (SteerClear) | Obstacles hit (%) | Obstacles hit (%) | |
| CPAP: 10 (SD 8), n = 66; CM: 10 (SD 3), n = 59 | CPAP: 8 (SD 9), n = 66; CM: 8 (SD 10), n = 59; MD (SD): 0, p-value: 0.88 | ||
| Digit Vigilance Test (direction of improvement –): Respondents are asked to find and cross out either 6s (standard administration) or 9s (alternate administration), which appear randomly within 59 rows of single digits. These 59 rows of digits are printed in red on the first stimulus page and in blue on the second. | |||
| Parallel trials: | |||
| Dimsdale et al., 200058 (related paper117) | Time (minutes) | Time (minutes) | |
| CPAP: 7.5 (SE 0.4), n = 20; sham CPAP: 6.4 (SE 0.4), n = 16 | CPAP: 6.9 (SE 0.3), n = 20; sham CPAP: 6.6 (SE 0.4), n = 16; MD (SD): –0.3, p-value: ns | ||
| Errors | Errors | ||
| CPAP: 7.1 (SE 3.1), n = 20; sham CPAP: 18.3 (SE 3.7), n = 16 | CPAP: 10.1 (SE 2.6), n = 20; sham CPAP: 12.3 (SE 3.1), n = 16; MD (SD): –2.2, p-value: ns | ||
| Norman et al., 200673 (related paper341) | Time (seconds) | Time (seconds) | |
| CPAP: 350.9, n = 17; sham CPAP: 326.4, n = 14 | CPAP: 312.3, n = 17; sham CPAP: 303.1, n = 14; MD (SD): 9.2, p-value: 0.02 | ||
| Errors | Errors | ||
| CPAP: 5.6, n = 17; sham CPAP: 14.1, n = 14 | CPAP: 7.2, n = 20; sham CPAP: 10.6, n = 16; MD (SD): –2.4, p-value: 0.08 | ||
| NB p-value based on treatment × time interaction (three treatment arms) | |||
| Finger tapping task (direction of improvement +): Speed of finger tapping is measured for the index finger of the right and left hands separately; five times for a period of 10 seconds each. | |||
| Parallel | |||
| aLojander et al., 1999113 | CPAP: 48 (42–55), n = 10; CM other: 44 (34–55), n = 17 | CPAP: 48, n = 10; CM other: 44, n = 16; p-value: ns | CPAP: 45, n = 9; CM other: 43, n = 12; p-value: ns |
| IQ decrement (direction of improvement +): NART score minus WAIS-R subtests. | |||
| Crossover | |||
| Engleman et al., 199490 | CPAP: NR; OP: NR; n = 32 | CPAP: 4.0 (SE 2.1); OP: 7.2 (SE 2.0); MD (SD): –3.2, p-value: 0.04 | |
| Engleman et al., 199792 | CPAP: NR; OP: NR; n = 16 | CPAP: 7.0 (SE 3.1); OP: 5.3 (SE 3.5); MD (SD): 1.7, p-value: ns | |
| Engleman et al., 199893 | CPAP: NR; OP: NR; both: 6 (SD 12); n = 23 | CPAP: 3.0 (SD 11); OP: 4.0 (SD 11); MD (SD): –1, p-value: ns | |
| Engleman et al., 2002103 | CPAP: NR; OA: NR; n = 34 | CPAP: –1.0 (SD 14); OA: –2.0 (SD 14); MD (SD): 1, p-value: 0.549 | |
| Hopkins Verbal Learning Task (direction of improvement +): Respondents are asked to verbally repeat a list of words (immediately and after a delay) and to identify the words from the list from a verbal presentation (including both the target words and the distractors). | |||
| Parallel trials | |||
| Norman et al., 200673 (related paper341) | Immediate recall | Immediate recall | |
| CPAP: 24.7, n = 17; OA: 26.3, n = 14 | CPAP: 24.2, n = 17; OA: 25.5, n = 14; MD (SD): –1.3, p-value: 0.486, p-value: 0.679 (difference in change) | ||
| Delayed recall | Delayed recall | ||
| CPAP: 8.9, n = 17; OA: 9, n = 14 | CPAP: 8.8, n = 17; OA: 8.3, n = 14; MD (SD): 0.5, p-value: 0.641, p-value: 0.347 (difference in change) | ||
| NB p-value based on treatment × time interaction (three treatment arms) | |||
| Memory distractor task (direction of improvement –): This is a test of verbal and visual spatial memory using the Brown–Peterson technique. | |||
| Parallel trials | |||
| aLojander et al., 1999113 | CPAP: 3 (1–3), n = 10; CM: 3 (1–5), n = 17 | CPAP: 3; CM: 3; p-value: ns | CPAP: 3; CM: 3; p-value: ns |
| Paced Auditory Serial Addition Task (direction of improvement +): A series of single digits is presented at a set rate; the respondents are asked to add the numbers in pairs, such that each number is added to the one that immediately precedes it. | |||
| Crossover trials | |||
| Barnes et al., 200482 | PASAT 1.2 | PASAT 1.2 | |
| CPAP: NR; comparator: NR; both: 3.4 (SE 0.2), n = 80 | CPAP: 2.9 (SE 0.1); OA: 2.6 (SE 0.03);OP: 3.4 (0.1); CPAP/OA MD (SD): 0.3; CPAP/OP MD (SD): –0.5, p-value: ns | ||
| PASAT 2.4 | PASAT 2.4 | ||
| CPAP: NR; comparator: NR; both: 4.2 (SE 0.2), n = 80 | CPAP: 3.8 (SE 0.2); OA: 3.7 (SE 0.1); OP: 3.7 (SE 0.1); CPAP/OA MD (SD): 0.1; CPAP/OP MD (SD): 0.1, p-value: ns | ||
| Engleman et al., 199490 | PASAT 2 | There was an improvement with CPAP but also an order effect. Data were analysed from first assessment only and no statistically significant difference between CPAP and placebo was found. Data were not reported because there was no statistically significant difference between CPAP and oral placebo | |
| CPAP: NR; OP: NR | |||
| PASAT 4 | |||
| CPAP: NR; OP: NR | |||
| Engleman et al., 199792 | PASAT 2 | PASAT 2 | |
| CPAP: NR; OP: NR; n = 16 | CPAP: 37.8 (SE 3.3); OP: 35.3 (SE 2.8); MD (SD): 2.5, p-value: ns | ||
| Engleman et al., 199893 | PASAT 2 | PASAT 2 | |
| CPAP: NR; OP: NR; both: 31 (SD 8), n = 23 | CPAP: 37 (SD 11); OP: 35 (SD 11); MD (SD): 2, p-value: ns | ||
| Engleman et al., 199978 | PASAT 2 | PASAT 2 | |
| CPAP: NR; OP: NR; both: 31 (SD 12), n = 32 | CPAP: 40 (SD 11); OP: 36 (SD 14); MD (SD): 4, p-value: 0.02 | ||
| Engleman et al., 2002103 | PASAT 2 | PASAT 2 | |
| CPAP: NR; OA: NR; both: NR, n = 48 | CPAP: 40 (SD 11); OA: 39 (SD 10); MD (SD): 1, p-value: 0.064 | ||
| Parallel trials | |||
| Barbé et al., 200183 | PASAT 1 | PASAT 1 | |
| CPAP: 15 (SE 1), n = 29; sham CPAP: 14 (SE 1), n = 25 | CPAP: 15 (SE 1); sham CPAP: 15 (SE 1); MD (SD): 0, p-value: > 0.20 (difference in change) | ||
| PASAT 2 | PASAT 2 | ||
| CPAP: 14 (SE 1), n = 29; sham CPAP: 15 (SE 1), n = 25 | CPAP: 16 (SE 1); sham CPAP: 15 (SE 1); MD (SD): 1, p-value: 0.04 (difference in change) | ||
| PASAT 3 | PASAT 3 | ||
| CPAP: 10 (SE 1), n = 29; sham CPAP: 11 (SE 1), n = 25 | CPAP: 12 (SE 1); sham CPAP: 12 (SE 1); MD (SD): 0, p-value: 0.09 (difference in change) | ||
| PASAT 4 | PASAT 4 | ||
| CPAP: 5 (SE 1), n = 29; sham CPAP: 4 (SE 1), n = 25 | CPAP: 5 (SE 1); sham CPAP: 5 (SE 1); MD (SD): 0, p-value: > 0.20 (difference in change) | ||
| Monasterio et al., 2001100 | PASAT 1 | PASAT 1 | |
| CPAP: 4 (SD 3), n = 66; CM: 4 (SD 3), n = 59 | CPAP: 5 (SD 4); CM: 5 (SD 3); MD (SD): 0, p-value: 0.32 | ||
| PASAT 2 | PASAT 2 | ||
| CPAP: 10 (SD 4), n = 66; CM: 10 (SD 5), n = 59 | CPAP: 12 (SD 4); CM: 12 (SD 4); MD (SD): 0, p-value: 0.12 | ||
| PASAT 3 | PASAT 3 | ||
| CPAP: 14 (SD 5), n = 66; CM: 13 (SD 5), n = 59 | CPAP: 15 (SD 4); CM: 15 (SD 4); MD (SD): 0, p-value: 0.20 | ||
| PASAT 4 | PASAT 4 | ||
| CPAP: 13 (SD 5), n = 66; CM: 13 (SD 4), n = 59 | CPAP: 14 (SD 4); CM: 16 (SD 4); MD (SD): –2, p-value: 0.20 | ||
| NB Reduced version with 20 items for each condition. It is unclear whether the score is for number correct or percentage correct. | |||
| Purdue Pegboard (direction of improvement +): This is a test of manual dexterity. Following standard instructions the subject places pegs onto a pegboard. | |||
| Crossover trials | |||
| Jokic et al., 1999108 | Dominant hand (no. correct) | Dominant hand (no. correct) | |
| CPAP: NR; CM other: NR; both: 13 (SD 3.6), n = 13 | CPAP: 15.3 (SD 1.7); CM other: 14.3 (SD 1.7); MD (SD): 1, p-value: 0.25 | ||
| Non-dominant hand (no. correct) | Non-dominant hand (no. correct) | ||
| CPAP: NR; CM other: NR; both: 13 (SD 1.5), n = 13 | CPAP: 14.1(SD 2.3); CM other: 12.8 (SD 2.3); MD (SD): 1.3, p-value: 0.16 | ||
| Both hands (no. correct) | Both hands (no. correct) | ||
| CPAP: NR; CM other: NR; both: 10.8 (SD 1.5), n = 13 | CPAP: 11.1(SD 1.0); CM other: 11.5 (SD 1.0); MD (SD): –0.4, p-value:1.41 | ||
| Right/left/both hands (no. correct) | Right/left/both hands (no. correct) | ||
| CPAP: NR; CM other: NR; both: 36.8 (SD 6.7), n = 13 | CPAP: 39.6 (SD 10.7); CM other: 38.6 (SD 4.9); MD (SD): 1, p-value: 1.47 | ||
| Assembly | Assembly | ||
| CPAP: NR; CM other: NR; both: 34.6 (SD 5.7), n = 13 | CPAP: 35.1(SD 7.6); CM other: 35.4 (SD 8.1); MD (SD): –0.3, p-value: 2.05 | ||
| Psychomotor Vigilance Test (direction of improvement –): This is a test of sustained attention. Typically it requires the subject to respond as quickly as possible to a specific stimulus while maintaining accuracy (e.g. pressing a button on seeing a dot/light on the computer screen) | |||
| Crossover trials | |||
| Barnes et al., 200289 | Inverse of mean of slowest 10% | Inverse of mean of slowest 10% | |
| CPAP: NR; OP: NR; both: 2.7 (SE 0.5), n = 28 | CPAP: 2.6; OP: 2.6; MD (SD): 0, p-value: ns (difference in change) | ||
| Barnes et al., 200482 | Inverse of mean of slowest 10% | Inverse of mean of slowest 10% | |
| CPAP: NR; comparator: NR; both: 2.7 (SE 0.1), n = 80 | CPAP: 2.7 (SE 0.1); OA: 2.7 (SE 0.1); OP: 2.6 (SE 0.1); CPAP/OA MD (SD): 0; CPAP/OP MD (SD): 0.1, p-value: ns | ||
| Lapses (> 500 milliseconds RT) | Lapses (> 500 milliseconds RT) | ||
|
CPAP: NR, comparator: NR Both: 2.5 (SE 0.3), n = 80 |
CPAP: 2.1 (SE 0.2); OA: 2.2 (SE 0.2); OP: 2.7 (SE 0.3); CPAP/OA MD (SD): –0.1; CPAP/OP MD (SD): –0.6, p-value: < 0.05 | ||
| Errors | Errors | ||
|
CPAP: NR, comparator: NR Both: 7.4 (SE 0.8), n = 80 |
CPAP: 7.4 (SE 0.7); OA: 7.5 (SE 0.8); OP: 7.8 (SE 0.8); CPAP/OA MD (SD): –0.1; CPAP/OP MD (SD): –0.4, p-value: ns | ||
| Cibele et al., 200672 | NR | NR | |
| Marshall et al., 200579 | Mean RT (milliseconds) | Mean RT (milliseconds) | |
| CPAP: NR; sham CPAP: NR; both: 264 (SE 5), n = 29 | CPAP: 266 (SE 5); sham CPAP: 259 (5); MD (SD): 7, p-value: NR | ||
| Lapses (> 500 milliseconds RT) | Lapses (> 500 milliseconds RT) | ||
| CPAP: NR; sham CPAP: NR; both: 1.3 (SE 0.3), n = 29 | CPAP: 1.3 (SE 0.4); sham CPAP: 1.0 (SE 0.4); MD (SD): 0.3, p-value: NR | ||
| Errors | Errors | ||
| CPAP: NR; sham CPAP: NR; both: 2.8 (SE 0.5), n = 29 | CPAP: 3.2 (SE 0.7); sham CPAP: 3.3 (SE 0.7); MD (SD): –.01, p-value: NR | ||
| Reaction time, milliseconds (direction of improvement –): This is the elapsed time between the presentation of a sensory stimulus and the subsequent behavioural response. | |||
| Crossover trials | |||
| Engleman et al., 199490 | CPAP: NR; OP: NR | Data not reported because there was no statistically significant difference between CPAP and oral placebo | |
| Engleman et al., 199792 | CPAP: NR; OP: NR; n = 16 | CPAP: 365 (SE 16); OP: 356 (SE 14); MD (SD): 9, p-value: ns | |
| Engleman et al., 199893 | CPAP: NR; OP: NR; both: 346 (SD 57), n = 23 | CPAP: 327 (SD 46); OP: 325 (SD 38); MD (SD): 2, p-value: ns | |
| Rapid Visual Information Processing Task (direction of improvement +): Single digits are presented in quick succession on a computer screen, and respondents are asked to identify (button press) target sequences of numbers. | |||
| Crossover trials | |||
| Engleman et al., 199490 | CPAP: NR; OP: NR | Data not reported because there was no statistically significant difference between CPAP and oral placebo | |
| Engleman et al., 199792 | CPAP: NR; OP: NR; n = 16 | No. correct | |
| CPAP: 36.9 (SE 3.2); OP: 34.8 (SE 3.2); MD (SD): 2.1, p-value: ns | |||
| Engleman et al., 199893 | CPAP: NR; OP: NR; both: 28 (SD 10), n = 23 | No. correct | |
| CPAP: 34 (SD 15); OP: 35 (SE 13); MD (SD): –1, p-value: ns | |||
| STROOP colour and word test (direction of improvement +): Respondents are asked to name colour-words printed in different colours, name the printed colours, or read a set of colour-words while naming colours of another set of colour-words. | |||
| Crossover trials | |||
| Barnes et al., 200482 | CPAP: NR; comparator: NR; both: 4.8 (SE 0.8), n = 80 | CPAP: 9.3 (SE 0.9); OA: 10.3 (SE 0.9); OP: 9.2 (SE 0.9); CPAP/OA MD (SD): –1; CPAP/OP MD (SD): 0.1, p-value: ns | |
| Parallel trials | |||
| Dimsdale et al., 200058 (related paper117) | Naming (no. correct) | Naming (no. correct) | |
| CPAP: 73.6 (SE 2.5), n = 20; sham CPAP: 77.7 (SE 2.9), n = 16 | CPAP: 80.3 (SE 2.7), n = 20; sham CPAP: 82.2 (SE 3.2), n = 16; MD (SD): 1.9, p-value: ns | ||
| Naming (errors) | Naming (errors) | ||
| CPAP: 0.3 (SE 0.2), n = 20; sham CPAP: 0.2 (SE 0.2), n = 16 | CPAP: 0.1 (SE 0.1), n = 20; sham CPAP: 0.3 (SE 0.1), n = 16; MD (SD): –0.2, p-value: ns | ||
| Reading (no. correct) | Reading (no. correct) | ||
| CPAP: 93.1 (SE 1.9), n = 20; sham CPAP: 92.4 (SE 2.2), n = 16 | CPAP: 95.6 (SE 2.0), n = 20; sham CPAP: 92.8 (SE 2.3), n = 16; MD (SD): 2.8, p-value: ns | ||
| Reading (errors) | Reading (errors) | ||
| CPAP: 0.2 (SE 0.2), n = 20; sham CPAP: 0.2 (SE 0.2), n = 16 | CPAP: 0.1 (SE 0.1), n = 20; sham CPAP: 0.01 (SE 0.1), n = 16; MD (SD): 0.19, p-value: ns | ||
| Inference (no. correct) | Inference (no. correct) | ||
| CPAP: 40 (SE 1.6), n = 20; sham CPAP: 38.5 (SE 1.9), n = 16 | CPAP: 44.2 (SE 1.8), n = 20; sham CPAP: 41 (SE 2.1), n = 16; MD (SD): 3.2, p-value: ns | ||
| Inference (errors) | Inference (errors) | ||
| CPAP: 0.5 (SE 0.3), n = 20; sham CPAP: 0.4 (SE 0.3), n = 16 | CPAP: 0.3 (SE 0.3), n = 20; sham CPAP: 0.8 (SE 0.3), n = 16; MD (SD): –0.5, p-value: ns | ||
| Norman et al., 200673 (related paper341) | Colour | Colour | |
| CPAP: 67.7, n = 17; sham CPAP: 73.7, n = 14 | CPAP: 72.3, n = 17; sham CPAP: 77.9, n = 14; MD (SD): –5.6, p-value: 0.532 | ||
| Colour-word ratio | Colour-word ratio | ||
| CPAP: 37.7, n = 17; sham CPAP: 37.9, n = 14 | CPAP: 37.3, n = 17; sham CPAP: 41.9, n = 14; MD (SD): –4.6, p-value: 0.061 | ||
| NB p-value based on treatment × time interaction (three treatment arms) | |||
| Trail Making Task (direction of improvement –): This is a complex attention task in which respondents are asked to draw lines to connect consecutively numbered circles on one sheet (Part A), and then connect the same number of consecutively numbered and lettered circles on another sheet by alternating between the two sequences (Part B). | |||
| Crossover trials | |||
| Engleman et al., 199490 | Part A/Part B | Part A | |
| CPAP: NR; OP: NR; n = 32 | Data not reported because there was no statistically significant difference between CPAP and oral placebo | ||
| Part B | |||
| CPAP: 66 (SE 5); OP: 76 (SE 5); MD (SD): –9, p-value: 0.02 | |||
| Engleman et al., 199792 | Part B | Part B | |
| CPAP: NR; OP: NR; n = 16 | CPAP: 64.1 (SE 5.5); OP: 77.7 (SE 9.2); MD (SD): –13.6, p-value: 0.02 | ||
| Engleman et al., 199893 | Part B | Part B | |
| CPAP: NR; OP: NR; both: 84 (SD 41), n = 23 | CPAP: 69 (SD 32); OP: 68 (SD 32); MD (SD): 1, p-value: ns | ||
| Engleman et al., 199978 | Part A | Part A | |
| CPAP: NR; OP: NR; both: 34 (SD 12), n = 34 | CPAP: 26 (SD 11); OP: 29 (SD 11); MD (SD): –3, p-value: 0.06 | ||
| Part B | Part B | ||
| CPAP: NR; OP: NR; both: 76 (SD 36), n = 34 | CPAP: 63 (SD 33); OP: 65 (SD 27); MD (SD): –2, p-value: ns | ||
| Engleman et al., 2002103 | Part B | Part B | |
| CPAP: NR; OA: NR; n = 34 | CPAP: 59 (SD 21); OA: 64 (SD 28); MD (SD): –5, p-value: 0.106 | ||
| Barnes et al., 200289 | Part A | Part A | |
| CPAP: NR; OP: NR; both: 28.2 (SD 9), n = 28 | CPAP: 28.1; OP: 27.6; MD (SD): 0.5, p-value: ns | ||
| Part B | Part B | ||
| CPAP: NR; OP: NR; both: 65.3 (SD 30.2), n = 28 | CPAP: 60.1; OP: 65.2; MD (SD): –5.1, p-value: ns | ||
| Barnes et al., 200482 | Part B | Part B | |
| CPAP: NR; OA:NR; OP: NR; all: 85.9 (SE 4.4), n = 80 | CPAP: 73.3 (SE 3.3); OA: 76.0 (SE 3.7); OP: 74.2 (SE 3.6); CPAP/OA MD (SD): –2.7; CPAP/OP MD (SD): –0.9, p-value: ns | ||
| Jokic et al., 1999108 | Part A | Part A | |
| CPAP: NR; CM other: NR; both: 28.1 (SD 2.1), n = 13 | CPAP: 20.5 (SD 3.2); CM other: 21.9 (SD 7.9); MD (SD): –1.4, p-value: 1.17 | ||
| Part B | Part B | ||
| CPAP: NR; CM other: NR; both: 73.8 (SE 34.7), n = 13 | CPAP: 56.5 (SD 7); CM other: 57.7 (SD 6.6); MD (SD): –1.2, p-value: 2.18 | ||
| Parallel trials | |||
| Barbé et al., 200183 | Part A (seconds) | Part A (seconds) | |
| CPAP: 49 (SE 4), n = 29; sham CPAP: 49 (SE 4), n = 25 | CPAP: 47 (SE 3), n = 29; sham CPAP: 47 (SE 3), n = 25; MD (SD): 0, p-value: > 0.2 (difference in change) | ||
| Part B (seconds) | Part B (seconds) | ||
| CPAP: 122 (SE 16), n = 29; sham CPAP: 108 (SE 11), n = 25 | CPAP: 96 (SE 6), n = 29; sham CPAP: 110 (SE 10), n = 25; MD (SD): –14, p-value: 0.1 (difference in change) | ||
| Dimsdale et al., 200058 (related paper117) | Part A | Part A | |
| CPAP: 33.3 (SE 2.2), n = 20; sham CPAP: 32.4 (SE 2.7), n = 16 | CPAP: 27.4 (SE 1.6), n = 20; sham CPAP: 27.4 (SE 2), n = 16; MD (SD): 0, p-value: ns | ||
| Part A (errors) | Part A (errors) | ||
| CPAP: 0.2 (SE 0.1), n = 20; sham CPAP: 0.1 (SE 0.1), n = 16 | CPAP: 0.03 (SE 0.1), n = 20; sham CPAP: 0.2 (SE 0.1), n = 16; MD (SD): –0.17, p-value: ns | ||
| Part B | Part B | ||
| CPAP: 81.1 (SE 8), n = 20; sham CPAP: 88.3 (SE 9.8), n = 16 | CPAP: 71.2 (SE 7.1), n = 20; sham CPAP: 87 (SE 8.7), n = 16; MD (SD): –14.5, p-value: ns | ||
| Part B (errors) | Part B (errors) | ||
| CPAP: 1.2 (SE 0.3), n = 20; sham CPAP: 0.8 (SE 0.4), n = 16 | CPAP: 0.5 (SE 0.2), n = 20; sham CPAP: 1.1 (SE 0.3), n = 16; MD (SD): –0.6, p-value: ns | ||
| Henke et al., 200185 | Part A/Part B | ||
| Data presented as graph. No statistically significant difference between groups at follow-up | |||
| aLojander et al., 1999113 | Part B | Part B | Part B |
| CPAP: 111 (65 to 180), n = 10; CM: 83 (50 to 290), n = 17 | CPAP: 128; CM: 82; p-value: ns | CPAP: 130; CM: 75; p-value: ns | |
| Monasterio et al., 2001100 | Part A | Part A (seconds) | |
| CPAP: 56 (SD 26), n = 66; CM: 54 (SD 18), n = 59 | CPAP: 49 (SD 19), n = 66; CM: 49 (SD 20), n = 59; MD (SD): 0, p-value: 0.76 | ||
| Part B | Part B (seconds) | ||
| CPAP: 121 (SD 44), n = 66; CM other: 125 (SD 47), n = 59 | CPAP: 106 (SD 42), n = 66; CM: 100 (SD 39), n = 59; MD (SD): 6, p-value: 0.15 | ||
| Norman et al., 200673 (related paper341) | Part A | Part A | |
| CPAP: 32.4, n = 17; sham CPAP: 25.5, n = 14 | CPAP: 26.5, n = 17; sham CPAP: 21.7, n = 14; MD (SD): 4.8, p-value: 0.494 | ||
| Part B | Part B | ||
| CPAP: 70.8, n = 17; sham CPAP: 70.3, n = 14 | CPAP: 63.4, n = 17; sham CPAP: 59.6, n = 14; MD (SD): 3.8, p-value: 0.823 | ||
| NB p-value based on treatment × time interaction (three treatment arms) | |||
| Verbal fluency test (direction of improvement +): This is a word-naming task. | |||
| Parallel trials | |||
| Dimsdale et al., 200058 (related paper117) | No. correct | No. correct | |
| CPAP: 40.6 (SE 2.9), n = 20; sham CPAP: 35.9 (SE 3.4), n = 16 | CPAP: 44.5 (SE 2.7), n = 20; sham CPAP: 37.3 (SE 3.2), n = 16; MD (SD): 7.2, p-value: ns | ||
| Perseveration | Perseveration | ||
| CPAP: 1.1 (SE 0.4), n = 20; sham CPAP: 0.9 (SE 0.5), n = 16 | CPAP: 0.8 (SE 0.3), n = 20; sham CPAP: 1.2 (SE 0.3), n = 16; MD (SD): –0.4, p-value: ns | ||
| Intrusions | Intrusions | ||
| CPAP: 0.2 (SE 0.1), n = 20; sham CPAP: 0.5 (SE 0.1), n = 16 | CPAP: 0.1 (SE 0.1), n = 20; sham CPAP: 0.4 (SE 0.2), n = 16; MD (SD): –0.3, p-value: ns | ||
| Variations | Variations | ||
| CPAP: 0.5 (SE 0.3), n = 20; sham CPAP: 0.7 (SE 0.3), n = 16 | CPAP: 0.6 (SE 0.2), n = 20; sham CPAP: 0.8 (SE 0.3), n = 16; MD (SD): –0.2, p-value: ns | ||
| Monasterio et al., 2001100 | Score: percentile | Score: percentile | |
| CPAP: 69 (SD 29), n = 66; CM: 66 (SD 28), n = 59 | CPAP: 69 (SD 27), n = 66; CM: 70 (SD 29), n = 59; MD (SD): –1, p-value: 0.53 | ||
| Norman et al., 200673 (related paper341) | Total score | Total score | |
| CPAP: 38.4, n = 17; sham CPAP: 42.3, n = 14 | CPAP: 40.9, n = 17; sham CPAP: 45.5, n = 14; MD (SD): –4.6, p-value: 0.149 | ||
| NB p-value based on treatment × time interaction (three treatment arms) | |||
| Wechsler Adult Intelligence Scale (direction of improvement +): This is a neuropsychological test battery. The full-scale IQ test is broken down into 14 subtests comprising the verbal (seven subtests) and performance (seven subtests) scales. | |||
| Crossover trials | |||
| Barnes et al., 200289 | DSST | DSST | |
| CPAP: NR; OP: NR; both: 46.5 (SD 4.0), n = 28 | CPAP: 47.3; OP: 48.0; MD (SD): –0.7, p-value: 0.07 (difference in change) | ||
| Barnes et al., 200482 | DSST | DSST | |
| CPAP: NR; OA: NR; OP: NR; all: 46.4 (SE 0.4), n = 80 | CPAP: 47.3 (SE 0.4); OA: 47.5 (SE 0.4); OP: 46.8 (SE 0.4); CPAP/OA MD (SD): –0.2; CPAP/OP MD (SD): 0.5; p-value: ns | ||
| Digit backwards | Digit backwards | ||
| CPAP: NR; OA: NR; OP: NR; all: 4.4 (SE 0.1), n = 80 | CPAP: 4.6 (SE 0.1); OA: 4.6 (SE 0.1); OP: 4.8 (SE 0.1); CPAP/OA MD (SD): 0; CPAP/OP MD (SD): –0.2; p-value: ns | ||
| Engleman et al., 199490 | DSST | DSST | |
| CPAP: NR; OP: NR; n = 32 | CPAP: 52.0 (SE 2.0); OP: 51.0 (SE 2.0); MD (SD): 1, p-value: 0.05 | ||
| Block design | Block design | ||
| CPAP: NR; OP: NR; n = 32 | Data not reported because there was no statistically significant difference between CPAP and oral placebo | ||
| Engleman et al., 199893 | DSST | DSST | |
| CPAP: NR; OP: NR; both: 48.0 (SD 12.0), n = 23 | CPAP: 52.0 (SD 13.0); OP: 52.0 (SD 14.0); MD (SD): 0, p-value: ns | ||
| Block design | Block design | ||
| CPAP: NR; OP: NR; both: 29.0 (SD 11.0), n = 23 | CPAP: 33.0 (SD 9.0); OP: 31.0 (SD 8.0); MD (SD): 2, p-value: ns | ||
| Engleman et al., 199978 | DSST | DSST | |
| CPAP: NR; OP: NR; both: 54.0 (SD 12.0), n = 34 | CPAP: 59.0 (SD 12.0); OP: 57.0 (SD 14.0); MD (SD): 2, p-value: 0.0004 | ||
| Block design | Block design | ||
| CPAP: NR; OP: NR; both: 29.0 (SD 10.0), n = 34 | CPAP: 31.0 (SD 12.0); OP: 32.0 (SD 10.0); MD (SD): –1, p-value: ns | ||
| Parallel trials | |||
| Barbé et al., 200183 | Digit symbols | Digit symbols | |
| CPAP: 42.0 (SE 2.0), n = 29; OP: 44.0 (SE 4.0), n = 25 | CPAP: 43.0 (SE 3.0), n = 29; OP: 47 (SE 4.0), n = 25; MD (SD): –4, p-value: > 0.20 (difference in change) | ||
| Block design | Block design | ||
| CPAP: 33.0 (SE 1.0), n = 29; OP: 32.0 (SE 2.0), n = 25 | CPAP: 34.0 (SE 1.0), n = 29; OP: 33.0 (SE 2.0), n = 25; MD (SD): 1, p-value: > 0.20 (difference in change | ||
| Digit forwards | Digit forwards | ||
| CPAP: 9.0 (SE 0.3), n = 29; OP: 10.0 (SE 0.4), n = 25 | CPAP: 9.0 (SE 0.3), n = 29; OP: 10.0 (SE 1.0), n = 25; MD (SD): –1, p-value: > 0.20 (difference in change) | ||
| Dimsdale et al., 200073 (related paper117) | Digit symbols | Digit symbols | |
| CPAP: 51.3 (SE 2.3), n = 20; sham CPAP: 52.9 (SE 2.8), n = 16 | CPAP: 53.2 (SE 2.5), n = 20; sham CPAP: 53.5 (SE 3.0), n = 16; MD (SD): –0.3, p-value: ns | ||
| Digit forwards | Digit forwards | ||
| CPAP: 8.1 (SE 0.7), n = 20; sham CPAP: 8.6 (SE 0.8), n = 16 | CPAP: 8.6 (SE 0.6), n = 20; sham CPAP: 8.7 (SED 0.7), n = 16; MD (SD): –0.1, p-value: ns | ||
| Digit forwards and backwards | Digit forwards and backwards | ||
| CPAP: 14.7 (SE 1.1), n = 20; sham CPAP: 14.7 (SE 1.2), n = 16 | CPAP: 16.2 (SE 1.1), n = 20; sham CPAP: 15.1 (SE 1.3), n = 16; MD (SD): 1.1, p-value: ns | ||
| Henke et al., 200185 | Digit symbols and digit backwards | Data presented as graphs. Rather than assess change scores a binary variable was created of improved or not improved and the two groups were compared based on this. No significant difference between groups | |
| aLojander et al., 1999113 | Verbal intelligence quotient | Verbal intelligence quotient | Verbal intelligence quotient |
| CPAP: 121 (110–148), n = 10; CM: 111 (91–140), n = 17 | CPAP: 115, n = 10; OP: 104, n = 16; p-value: ns | CPAP: 114, n = 9; OP: 105, n = 12; p-value: ns | |
| Performance quotient | Performance quotient | Performance quotient | |
| CPAP: 115 (110–143), n = 10; CM: 114 (84–147), n = 17 | CPAP: 123, n = 10; OP: 109, n = 16; p-value: ns | CPAP: 106, n = 9; OP: 109, n = 12; p-value: ns | |
| Monasterio et al., 2001100 | Digit symbols | Digit symbols (scaled score) | |
| CPAP: 9 (SD 3), n = 66; CM: 9 (SD 3), n = 59 | CPAP: 9.0 (SD 3.0), n = 66; CM: 9.0 (SD 2.0), n = 59; MD (SD): 0, p-value: 0.97 (difference in change) | ||
| Block design | Block design (scaled score) | ||
| CPAP: 10 (SD 3), n = 66; CM: 10 (SD 3), n = 59 | CPAP: 11.0 (SD 3.0), n = 66; CM: 11.0 (SD 3.0), n = 59; MD (SD): 0, p-value: 0.82 (difference in change) | ||
| Digit forwards and backwards | Digit forwards and backwards (scaled score: mean 10, SD 3) | ||
| CPAP: 10 (SD 2), n = 66; CM: 10 (SD 3), n = 59 | CPAP: 11.0 (SD 3.0), n = 66; CM: 11.0 (SD 2.0), n = 59; MD (SD): 0, p-value: 0.56 (difference in change) | ||
| Norman et al., 200673( related paper341) | Digit symbols | Digit symbols (no. correct) | |
| CPAP: 65.8, n = 17; sham CPAP: 67.6, n = 14 | CPAP: 73.8, n = 17; CM: 68.7, n = 14; MD (SD): 5.1, p-value: 0.256 | ||
| Digit forwards | Digit forwards (total score) | ||
| CPAP: 18.6, n = 17; sham CPAP: 21.2, n = 14 | CPAP: 26.4, n = 17; CM: 22.5, n = 14; MD (SD): 3.9, p-value: 0.378 | ||
| Letter sequencing | Letter sequencing | ||
| CPAP: 11.0, n = 17; sham CPAP: 11.7, n = 14 | CPAP: 11.9, n = 17; CM: 12.9, n = 14; MD (SD): –1, p-value: 0.827 | ||
| Symbol search | Symbol search (no. correct) | ||
| CPAP: 31.8, n = 17; sham CPAP: 32.1, n = 14 | CPAP: 33.8, n = 17; CM: 37.7, n = 14; MD (SD): –3.9, p-value: 0.614 | ||
| NB p-value based on treatment × time interaction (three treatment arms) | |||
| Wechsler Memory Scale (direction of improvement +): This is a battery of memory tests. | |||
| Crossover trials | |||
| Barnes et al., 200289 | Visual reproduction | Visual reproduction | |
| CPAP: NR; OP: NR; both: 33.5 (SD 5.4), n = 28 | CPAP: 34.7; OP: 35.1; MD (SD): –0.4, p-value: ns | ||
| Jokic et al., 1999108 | No baseline data; n = 13 | Visual reproduction | |
| CPAP: 10.6 (SD 4.4); CM other: 10.3 (SD 2); MD (SD): 0.3, p-value: 0.72 | |||
| Orientation | |||
| CPAP: 5.0 (SD 0); CM other: 5.0 (SD 0); MD (SD): 0, p-value: 1 | |||
| Information | |||
| CPAP: 5.5 (SD 1.2); CM other: 5.9 (SD 0); MD (SD): –0.4, p-value: 0.14 | |||
| Mental control | |||
| CPAP: 7.3 (SD 2.1); CM other: 7.4 (SD 1.2); MD (SD): –0.1, p-value: 0.9 | |||
| Logical memory | |||
| CPAP: 11.4 (SD 3.3); CM other: 12.0 (SD 3.8); MD (SD): –0.6, p-value: 0.42 | |||
| Associate learning | |||
| CPAP: 14. 8 (SD 1.0); CM other: 16.6 (SD 4.2); MD (SD): –1.8, p-value: 0.06 | |||
| Digit forwards | |||
| CPAP: 6.5 (SD 2.1); CM other: 6.5 (SD 1.5); MD (SD): 0, p-value: 1 | |||
| Digit backwards | |||
| CPAP: 4.9 (SD 2.1); CM other: 5.0 (SD 1.7); MD (SD): –0.1, p-value: 0.86 | |||
| Recall (logical memory) | |||
| CPAP: 9.0 (SD 5.0); CM other: 9.5 (SD 3.5); MD (SD): –0.5, p-value: 0.52 | |||
| Recall (visual reproduction) | |||
| CPAP: 9.2 (SD 4.5); CM other: 9.9 (SD 2.1); MD (SD): –0.7, p-value: 0.15 | |||
| Recall (associate learning) | |||
| CPAP: 6.1 (SD 3.1); CM other: 6.3 (SD 3.3); MD (SD): –0.2, p-value: 0.54 | |||
| Memory quotient | |||
| CPAP: 122.2 (SD 24.7); CM other: 122.8 (SD 20.2); MD (SD): –0.6, p-value: 0.83 | |||
| Parallel trials | |||
| Barbé et al., 200183 | Mental control | Mental control | |
| CPAP: 6.0 (SE 0.4), n = 29; sham CPAP: 6.0 (SE 1.0), n = 25 | CPAP: 6.0 (SE 0.4), n = 29; sham CPAP: 7.0 (SE 0.4); MD (SD): –1, p-value: > 0.20 (difference in change) | ||
| Verbal paired associate | Verbal paired associate | ||
| CPAP: 14 (SE 1.0), n = 29; sham CPAP: 15 (SE 1.0), n = 25 | CPAP: 15.0 (SE 1.0), n = 29; sham CPAP: 15.0 (SE 1.0), n = 25; MD (SD): 0, p-value: > 0.20 (difference in change) | ||
| aLojander et al., 1999113 | Memory quotient | Memory quotient | Memory quotient |
| CPAP: 128 (105 to 151), n = 10; CM: 118 (99 to 150), n = 17 | CPAP: 129 n = 10; CM: 115, n = 16; p-value: ns | CPAP: 121 n = 9; CM: 120, n = 12; p-value: ns | |
| Monasterio et al., 2001100 | No baseline data reported | Mental control (percentile) | |
| CPAP: 51.0 (SD 274), n = 66; CM: 53.0 (SD 27.0), n = 59; MD (SD): –2, p-value: 0.08 (difference in change) | |||
| Verbal paired associate (percentile) | |||
| CPAP: 41.0 (SD 30.0), n = 66; CM: 43.0 (SD 32.0), n = 59; MD (SD): –2, p-value: 0.63 (difference in change) | |||
| Verbal paired associate (percentile) | |||
| CPAP: 61.0 (SD 24.0), n = 66; CM: 63.0 (SD 25.0), n = 59; MD (SD): –2, p-value: 0.06 (difference in change) | |||
| Word Paired Memory Recall (direction of improvement +): Respondents were asked to learn a series of word-pair associates; this was followed by a retention period, after which respondents were asked to recall target words. | |||
| Crossover trials | |||
| Barnes et al., 200289 | CPAP: NR; OP: NR; both: 1.5 (SD 1.2), n = 28 | CPAP: 1.9; OP: 1.55; MD (SD): 0.35, p-value: ns | |
| Study | Time of day | Caffeine/medication | Test order | Environment | Special exclusion criteria | Test–retest |
|---|---|---|---|---|---|---|
| Barbé et al., 200183 | NR | Drug intake and alcohol consumption were assessed at each test period | NR | NR | Cognitive deterioration of any cause, illicit drugs or excessive alcohol use | NR |
| Barnes et al., 200289 | The NAB was administered three times during the day of the MSLT and a mean score was computed to account for time of day effects. Other NC tests were only administered during the first session | NR | NR | NR | Fluency of English language, no history of cerebrovascular disease, closed head injury associated with loss of consciousness > 15 minutes, psychiatric illness, or drug or alcohol abuse | Participants attended a familiarisation session 1 week prior to baseline assessment and performed an abbreviated version of the neurocognitive tests |
| Barnes et al., 200482 | NR | Clinically significant depression was present in 40% of the OSAHS participants (as measured by the BDI) | NR | NR | Fluency of English language, no history of cerebrovascular disease, closed head injury associated with loss of consciousness > 15 minutes, psychiatric illness, or drug or alcohol abuse | Participants attended a familiarisation session 1 week prior to baseline assessment and performed an abbreviated version of the neurocognitive tests |
| Cibele et al., 200672 | NR | NR | NR | NR | NR | NR |
| Bardwell et al., 2000117 | Tests were administered early afternoon | NR | NR | NR | No major illness other than OSA, hypertension medication tapered before participation | Alternate forms were used for the word fluency test (considered to be the most likely test to show a learning effect) |
| Engleman et al., 199490 | Cognitive function was assessed in between MSLT naps across the course of a day | Participants were asked to avoid caffeine-containing drinks before attending assessments and were offered only decaffeinated drinks during the assessment day | Tests were conducted on the last day of each treatment period and were administered in a standardised order at the same time of day | NR | NR | Participants attended a familiarisation session with the psychometric battery prior to baseline assessment. Alternate forms of two neurocognitive tests were used at follow-up assessment |
| Engleman et al., 199792 | Assessments were conducted throughout the day | NR | Tests were conducted on the last day of each treatment period and were administered in a standardised order | NR | Individuals with co-existing neurological disorders were excluded | Participants attended a familiarisation session with the psychometric battery prior to baseline assessment. Alternate forms of two neurocognitive tests were used at follow-up assessment |
| Engleman et al., 199893 | NR | NR | Tests were administered in a standardised order | NR | Individuals with co-existing neurological disorders were excluded | NR |
| Engleman et al., 199978 | Afternoon, during ‘post-lunch dip’ in performance | NR | Tests were administered in a standardised order | NR | NR | Participants attended a familiarisation session with the psychometric battery prior to baseline assessment |
| Engleman et al., 2002103 | NR | NR | NR | NR | NR | NR |
| Henke et al., 200185 | Tests were administered between 2 p.m. and 6 p.m. | NR | NR | NR | NR | Participants attended a familiarisation session with the psychometric battery prior to baseline assessment. Four parallel test packets were administered on a randomised counterbalanced schedule |
| Hoekema et al., 2006102,114 | Between noon and 2 p.m | Participants were instructed to avoid stimulating products, such as caffeine, up to 3 hours before testing and not to smoke in the 30 minutes before testing | The test was administered at the same time of day in subsequent sessions | Room conditions: lights were dimmed, noise was excluded, and room temperature was kept at 22°C. Participants were alone during testing | Individuals without a driving licence, or who were involved in shift work or night-time work, were excluded | A practice session immediately prior to baseline assessment was provided |
| Jenkinson et al., 199977,118 | Three 30-minute drives (11 a.m, 12 p.m. and 2 p.m) | NR | The tests were performed at the same time of day on each occasion | NR | Individuals considered too mentally impaired to provide reliable informed consent were excluded | An initial training drive was administered prior to the first drive on each day |
| Jokic et al., 1999108 | Cognitive tests were administered after the third MSLT test, which was conducted 2 hours after waking and given at 2-hourly intervals | Participants were asked to avoid caffeine-containing drinks before attending assessments and were offered only decaffeinated drinks during the assessment day. A urine toxicology screen was performed on day 15 of each study limb for use of any drugs that might affect sleep quality or daytime function | Each test was administered at the same time of day on each study limb. The order was balanced throughout the study | NR | NR | Participants attended a familiarisation session with the psychometric tests prior to baseline assessment |
| Lojander et al., 1999113 | Tests were performed in the morning | NR | NR | NR | Individuals with other diseases and daytime hypoxaemia were excluded | NR |
| Marshall et al., 200579 | PVT was administered twice a day with a 1-minute practice session before each 10-minute data collection session at 2.30 p.m. and 4.30 p.m. on each of the four study days (beginning and end of each treatment period) | NR | Testing procedures were time of day fixed | NR | Individuals who performed shift work, had extreme somnolence requiring immediate treatment, had a chronic sleep restriction, were taking sedatives, had an alcohol intake > 3 standard units/24 hours or had a caffeine dependency were excluded | NR |
| Monasterio et al., 2001100 | Tests were administered at 9 a.m. | NR | Testing procedures were administered at the same time each session | NR | Individuals with hazardous jobs (drivers or those handling dangerous machinery), with conditions that might affect cognition or quality of life (severe neurological disease, psychiatric disease, severe chronic disease or illiteracy) were excluded | NR |
| Norman et al., 200673,341 | Tests were administered at 1 p.m. | NR | NR | NR | Individuals were excluded if they had a history of heart, liver or renal disease, diabetes, psychosis, narcolepsy, current drug or alcohol abuse, severe asthma or cerebrovascular disease, were pregnant, or were taking prescription medications except hypertensive medication. Those taking hypertensive medication underwent a 3-week washout period before study entry | Employed alternate forms for tests |
FIGURE 44.
Apnoea–hypopnoea index (CPAP versus placebo/usual care).
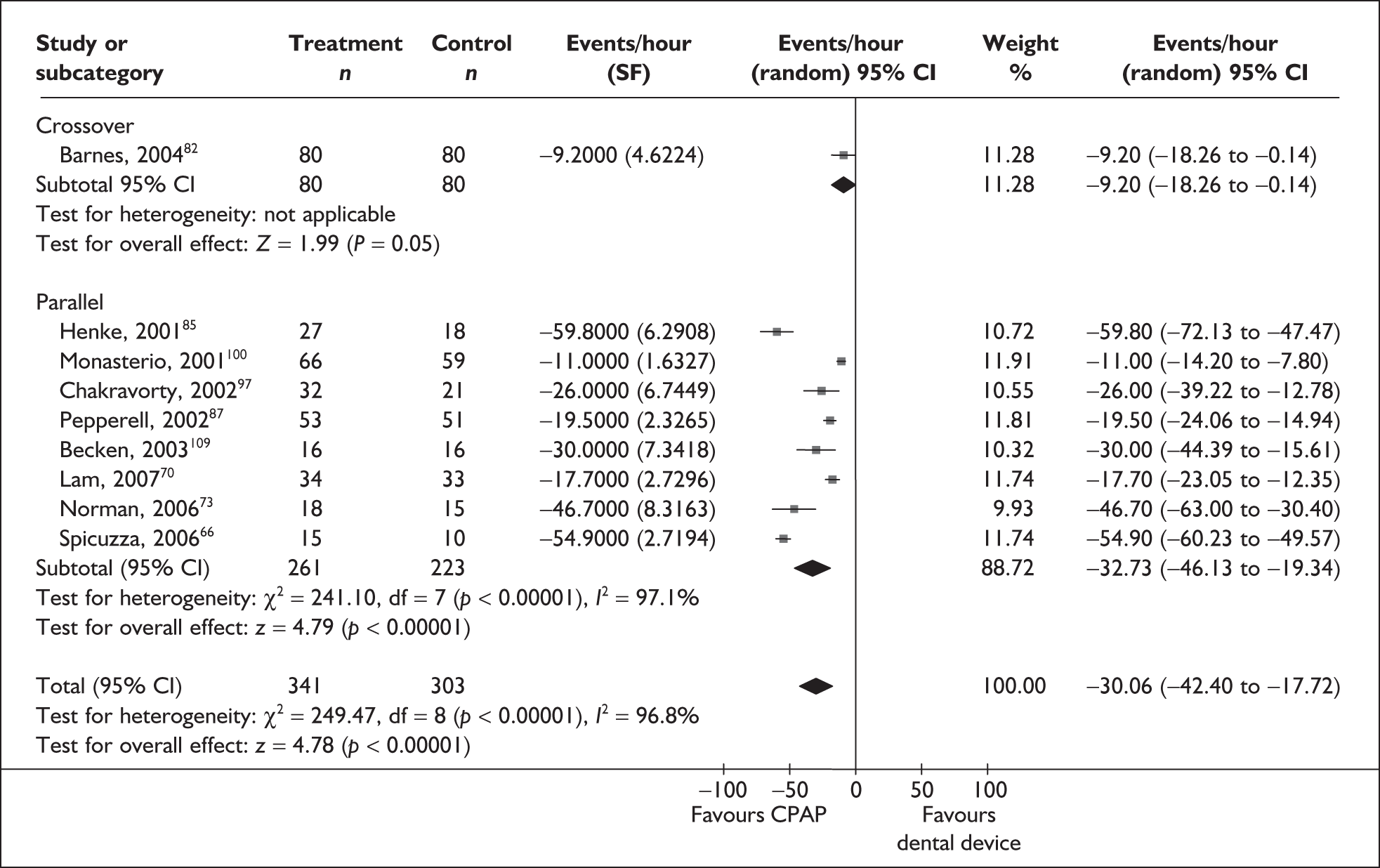
FIGURE 45.
Apnoea–hypopnoea index (CPAP versus dental devices).
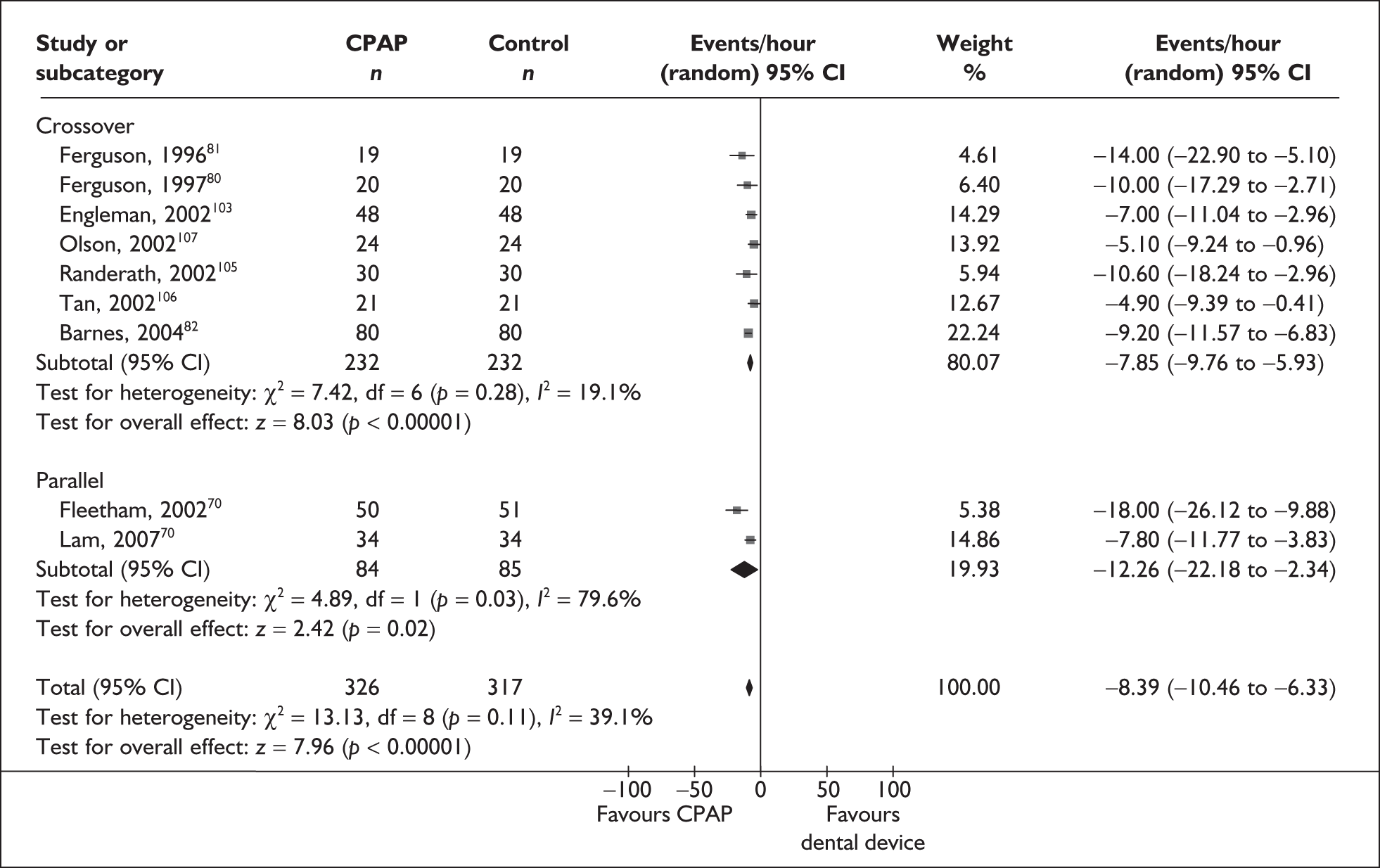
FIGURE 46.
Withdrawals (CPAP versus dental devices).
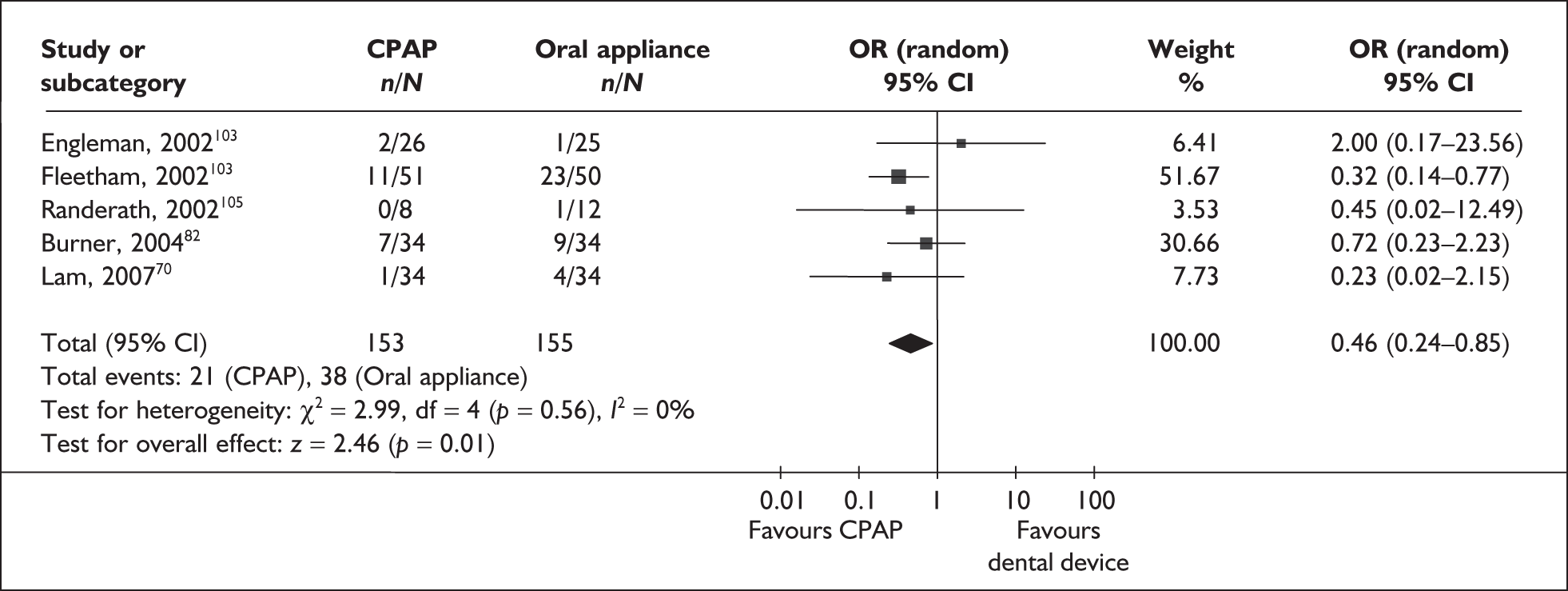
Appendix 5 Data extraction for clinical effectiveness trials
| Study details | Intervention | Participants | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Arias et al. , 200556 (related papers301–304) Setting. Spain Design. Crossover study. Duration. Two · 12 weeks (no washout period) Inclusion criteria. Men with OSAHS. Exclusion criteria. Obstructive or restrictive lung disease demonstrated on pulmonary function testing, current use of cardioactive medication, cardiac rhythm disturbances, hypertension or 24-hour mean BP of 135 and/or ≥ 85 mmHg, left ventricular ejection fraction < 50%, ischaemic or valvular heart disease, hypertrophic restrictive or infiltrative cardiomyopathy, pericardial disease or stroke, diabetes mellitus, morbid obesity, daytime hypoxaemia or hypercapnia |
CPAP (following full-night titration using an automated pressure setting device to set the pressure) Comparator. Sham nCPAP |
Number randomised. Total: 27 [baseline data for BP are presented for completers (n = 25)] Number of withdrawals. Total: 2 (CPAP: 2, comparator: 0) Reasons for withdrawals. Participants were not included in analysis because of insufficient use of CPAP Baseline characteristics TotalCPAPComparatorAge (years)52 (SD 13)SexMale (n = 27)AHI29.0 (SD 26.9)ESSNot assessedBMI (kg/m2)30.5 (SD 4.0)BP (mmHg) Daytime SBP127 (SD 9) Daytime DBP79 (SD 5) Night-time SBP118 (SD 11) Night-time DBP71 (SD 7) Additional information. 24-hour BP, urinary catecholamines, heart rate and echocardiographic parameters. Outcomes were assessed at study entry, after first treatment period and after second treatment period. BP was measured every 30 minutes from 8 a.m. to 11 p.m. and every 60 minutes from 11 p.m. to 8 a.m. using a cuff. |
Total | CPAP | Comparator | Age (years) | 52 (SD 13) | Sex | Male (n = 27) | AHI | 29.0 (SD 26.9) | ESS | Not assessed | BMI (kg/m2) | 30.5 (SD 4.0) | BP (mmHg) | Daytime SBP | 127 (SD 9) | Daytime DBP | 79 (SD 5) | Night-time SBP | 118 (SD 11) | Night-time DBP | 71 (SD 7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (years) | 52 (SD 13) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 27) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 29.0 (SD 26.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2) | 30.5 (SD 4.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP (mmHg) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Daytime SBP | 127 (SD 9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Daytime DBP | 79 (SD 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Night-time SBP | 118 (SD 11) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Night-time DBP | 71 (SD 7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Arias et al. , 2006 63 Setting. Spain Design. Crossover trial (outcome data appeared to be from the first sequence and data were treated as parallel data) Duration. Two · 12 weeks (no washout period) Inclusion criteria. Individuals with OSA (AHI > 9 and ESS > 9), and in whom pulmonary artery systolic pressure could be estimated Exclusion criteria. Obstructive or restrictive lung disease demonstrated on pulmonary function test, connective tissue or chronic thromboembolic diseases, current cardioactive medication, cardiac rhythm disturbances, known hypertension or 24-hour mean BP of 135 and/or ≥ 85 mmHg, left ventricular ejection fraction < 50%, ischaemic or valvular heart disease, cardiomyopathy, pericardial disease or stroke, diabetes, morbid obesity, day-time hypoxaemia or hypercapnia, history of cocaine or antidepressant drug use |
CPAP (following full-night titration using an automated pressure setting device to set the pressure). Adherence: 6.2 hours/night (SD 1.1) Comparator. Sham CPAP. Adherence: 5.8 hours/night (SD 1.4) |
Number randomised. Total: 23 (CPAP: NR, comparator: NR) Number of withdrawals. Total: 2 (CPAP: NR, comparator: NR) Reasons for withdrawals. Two participants not included in the analyses because of insufficient (< 3.5 hour/night) CPAP use Baseline characteristics TotalCPAPComparatorAge51 (SD 13)SexMale (n = 22), female (n = 1)AHI44.1 (SD 29.3)ESSNot assessedBMI30.9 (SD 4)BP Daytime SBP127 (SD 10) Daytime DBP79 (SD 5) Night-time SBP118 (SD 12) Night-time DBP71 (SD 7) Additional information. 24-hour BP, heart rate, echocardiographic parameters, urinary catecholamines and levels of pulmonary artery pressure were assessed at study entry and after each 12-week treatment period. Ambulatory BP monitoring was performed using an oscillometric method. |
Total | CPAP | Comparator | Age | 51 (SD 13) | Sex | Male (n = 22), female (n = 1) | AHI | 44.1 (SD 29.3) | ESS | Not assessed | BMI | 30.9 (SD 4) | BP | Daytime SBP | 127 (SD 10) | Daytime DBP | 79 (SD 5) | Night-time SBP | 118 (SD 12) | Night-time DBP | 71 (SD 7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 51 (SD 13) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 22), female (n = 1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 44.1 (SD 29.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 30.9 (SD 4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Daytime SBP | 127 (SD 10) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Daytime DBP | 79 (SD 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Night-time SBP | 118 (SD 12) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Night-time DBP | 71 (SD 7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Ballester et al. , 1999 96 Setting. Spain Design. Parallel Duration. 12 weeks Notes. Two patients randomised to CPAP group for every one randomised to control group; nine patients excluded Inclusion criteria. Men and women with severe symptoms and AHI > 15 or mild to moderate symptoms and AHI > 10 |
CPAP (following full-night titration using an automated pressure setting device) and conservative treatment. Adherence: 5.2 hours/night (± 2). Adequate compliance (defined as > 4.5 hours/night) was achieved in 73% of participants Comparator. Conservative treatment (postural advice, avoid sedatives and alcohol, lose weight) |
Number randomised. Total: n = 105 (CPAP: n = 68, comparator: n = 37) Number of withdrawals. Total: unclear (CPAP: n = 0, comparator: NR) Reasons for withdrawals. No record of dropouts recorded for control group Baseline characteristics TotalCPAPComparatorAge53 (SD 10)53 (SE 1.3)54 (SE 1.5)SexMale (n = 92), female (n = 13)Male, n = 60; female, n = 8Male, n = 32; female,n = 5AHI56 (SD 20)55 (SE 2.7)58 (SE 3)ESS12 (SD5)12.1 (SE 0.6)11.4 (SE 1)BMI32 (SD 6)32 (SE 0.6)34 (SE 1.2)BPNot assessed––Sleep questionnaire23.2 (SE 0.8)21.0 (SE 1.2)Associated symptoms43.4 (SE 0.8)41.8 (SE 1.1)Daytime function33.9 (SE 1.3)32.3 (SE 1.7)NHP Emotional reactions28.4 (SE 3.3)29.4 (SE 5.0) Sleep30.1 (SE 3.3)23.1 (SE 3.8) Physical24.2 (SE 2.6)25.0 (SE 3.6) Social isolation14.2 (SE 2.3)13.2 (SE 3.0) Pain20.5 (SE 3.3)20.6 (SE 4.0) Energy34.3 (SE 4.7)23.2 (SE 4.6) |
Total | CPAP | Comparator | Age | 53 (SD 10) | 53 (SE 1.3) | 54 (SE 1.5) | Sex | Male (n = 92), female (n = 13) | Male, n = 60; female, n = 8 | Male, n = 32; female,n = 5 | AHI | 56 (SD 20) | 55 (SE 2.7) | 58 (SE 3) | ESS | 12 (SD5) | 12.1 (SE 0.6) | 11.4 (SE 1) | BMI | 32 (SD 6) | 32 (SE 0.6) | 34 (SE 1.2) | BP | Not assessed | – | – | Sleep questionnaire | 23.2 (SE 0.8) | 21.0 (SE 1.2) | Associated symptoms | 43.4 (SE 0.8) | 41.8 (SE 1.1) | Daytime function | 33.9 (SE 1.3) | 32.3 (SE 1.7) | NHP | Emotional reactions | 28.4 (SE 3.3) | 29.4 (SE 5.0) | Sleep | 30.1 (SE 3.3) | 23.1 (SE 3.8) | Physical | 24.2 (SE 2.6) | 25.0 (SE 3.6) | Social isolation | 14.2 (SE 2.3) | 13.2 (SE 3.0) | Pain | 20.5 (SE 3.3) | 20.6 (SE 4.0) | Energy | 34.3 (SE 4.7) | 23.2 (SE 4.6) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 53 (SD 10) | 53 (SE 1.3) | 54 (SE 1.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 92), female (n = 13) | Male, n = 60; female, n = 8 | Male, n = 32; female,n = 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 56 (SD 20) | 55 (SE 2.7) | 58 (SE 3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 12 (SD5) | 12.1 (SE 0.6) | 11.4 (SE 1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 32 (SD 6) | 32 (SE 0.6) | 34 (SE 1.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sleep questionnaire | 23.2 (SE 0.8) | 21.0 (SE 1.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Associated symptoms | 43.4 (SE 0.8) | 41.8 (SE 1.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Daytime function | 33.9 (SE 1.3) | 32.3 (SE 1.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NHP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Emotional reactions | 28.4 (SE 3.3) | 29.4 (SE 5.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sleep | 30.1 (SE 3.3) | 23.1 (SE 3.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical | 24.2 (SE 2.6) | 25.0 (SE 3.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social isolation | 14.2 (SE 2.3) | 13.2 (SE 3.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pain | 20.5 (SE 3.3) | 20.6 (SE 4.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Energy | 34.3 (SE 4.7) | 23.2 (SE 4.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Barbé et al. , 2001 83 Setting. Spain Design. Parallel Duration. 6 weeks Inclusion criteria. Men and women with AHI > 30, ESS ≤ 10 and with no or mild symptoms of daytime sleepiness. Exclusion criteria. Severe cardiac disease |
CPAP (following full-night titration). Adherence: 5 hours/night Comparator. Sham CPAP (no pressure added) following mock titration Adherence was not reported for the control group. However, adherence was reported to be similar in both groups |
Number randomised. Total: 55 (CPAP: 29, comparator: 26) Number of withdrawals. Total: 1 (CPAP: 0, comparator: 1) Reasons for withdrawals. Loss to follow-up due to change in residence Baseline characteristics (baseline data are presented for completers, n = 54) TotalCPAPComparatorAge54 (SD 2)52 (SD 2)SexMale (n = 49), female (n = 5)Male (n = 26), female (n = 3)Male (n = 23), female (n = 2)AHI54 (SD 3)57 (SD 4)ESS7 (SE 0.4)7 (SE 0.4)BMI29 (SD 1)29 (SD 0.4)BP 24-Hour SBP126 (SE 2)123 (SE 2) Diurnal SBP130 (SE 2)127 (SE 2) Nocturnal SBP118 (SE 2)118 (SE 3) 24-Hour DBP79 (SE 1)77 (SE 2) Diurnal DBP82 (SE 1)80 (SE 2) Nocturnal DBP73 (SE 1)73 (SE 2)SF-36 PCS score49 (SE 1)48 (SE 1) MCS score51 (SE 2)50 (SE 2)FOSQ102 (SE 3)107 (SE 3)MSLT (minutes)12 (SE 1)10 (SE 1) Additional information. Outcomes: ESS, FOSQ, BP, AHI, MSLT, SF-36, and cognitive function (see Table 53). |
Total | CPAP | Comparator | Age | 54 (SD 2) | 52 (SD 2) | Sex | Male (n = 49), female (n = 5) | Male (n = 26), female (n = 3) | Male (n = 23), female (n = 2) | AHI | 54 (SD 3) | 57 (SD 4) | ESS | 7 (SE 0.4) | 7 (SE 0.4) | BMI | 29 (SD 1) | 29 (SD 0.4) | BP | 24-Hour SBP | 126 (SE 2) | 123 (SE 2) | Diurnal SBP | 130 (SE 2) | 127 (SE 2) | Nocturnal SBP | 118 (SE 2) | 118 (SE 3) | 24-Hour DBP | 79 (SE 1) | 77 (SE 2) | Diurnal DBP | 82 (SE 1) | 80 (SE 2) | Nocturnal DBP | 73 (SE 1) | 73 (SE 2) | SF-36 | PCS score | 49 (SE 1) | 48 (SE 1) | MCS score | 51 (SE 2) | 50 (SE 2) | FOSQ | 102 (SE 3) | 107 (SE 3) | MSLT (minutes) | 12 (SE 1) | 10 (SE 1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 54 (SD 2) | 52 (SD 2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 49), female (n = 5) | Male (n = 26), female (n = 3) | Male (n = 23), female (n = 2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 54 (SD 3) | 57 (SD 4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 7 (SE 0.4) | 7 (SE 0.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 29 (SD 1) | 29 (SD 0.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-Hour SBP | 126 (SE 2) | 123 (SE 2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diurnal SBP | 130 (SE 2) | 127 (SE 2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nocturnal SBP | 118 (SE 2) | 118 (SE 3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-Hour DBP | 79 (SE 1) | 77 (SE 2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diurnal DBP | 82 (SE 1) | 80 (SE 2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nocturnal DBP | 73 (SE 1) | 73 (SE 2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PCS score | 49 (SE 1) | 48 (SE 1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MCS score | 51 (SE 2) | 50 (SE 2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FOSQ | 102 (SE 3) | 107 (SE 3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSLT (minutes) | 12 (SE 1) | 10 (SE 1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Barnes et al. , 2002 89 Setting. Australia Design. Crossover Duration. Two periods of 8 weeks (no washout period) Inclusion criteria. Men and women with AHI 5–30 with symptoms of sleep-disordered breathing Exclusion criteria. Excessive daytime sleepiness requiring urgent treatment |
CPAP. Adherence: 3.53 hours/night (n = 23) Comparator. Oral placebo (lactose tablet) |
Number randomised. Total: n = 42 Number of withdrawals. Total: n = 14 (CPAP: NR, comparator: NR) Reasons for withdrawals. Work commitments (n = 6), intolerance of CPAP (n = 5), unrelated surgery (n = 1), subsequent diagnosis of periodic limb movement syndrome (n = 1) and loss of interest (n = 1)Baseline characteristics TotalCPAPComparatorAge45.5 (SD 10.7)SexMale (n = 35), female (n = 7)AHI12.9 (SD 4.24)ESS11.2 (SD 5)BMI30.2 (SD 4.8)BPNot assessedMSLT (minutes)12.5 (SD 4.8)Symptom questionnaire Sleepiness26.3 (SD 9.4) Sleep fragmentation21.9 (SD 9.5) Personality change8.6 (SD 6.1) Morning confusion6.4 (SD 5.3) Total score63.2 (SD 21.7)FOSQ General productivity3.3 (SD 0.6) Social outcome3.2 (SD 1.0) Activity level3.1 (SD 0.6) Vigilance3.0 (SD 0.7) Intimate relationships3.0 (SD 1.3) Total score0.8 (SD 0.1) Additional information. Seven participants were hypertensive at study entry (defined as BP > 140 mmHg, or a mean 24-hour DBP > 90 mmHg). Outcomes: ESS, FOSQ, AHI, MSLT, symptoms, and cognitive function (see Table 53). |
Total | CPAP | Comparator | Age | 45.5 (SD 10.7) | Sex | Male (n = 35), female (n = 7) | AHI | 12.9 (SD 4.24) | ESS | 11.2 (SD 5) | BMI | 30.2 (SD 4.8) | BP | Not assessed | MSLT (minutes) | 12.5 (SD 4.8) | Symptom questionnaire | Sleepiness | 26.3 (SD 9.4) | Sleep fragmentation | 21.9 (SD 9.5) | Personality change | 8.6 (SD 6.1) | Morning confusion | 6.4 (SD 5.3) | Total score | 63.2 (SD 21.7) | FOSQ | General productivity | 3.3 (SD 0.6) | Social outcome | 3.2 (SD 1.0) | Activity level | 3.1 (SD 0.6) | Vigilance | 3.0 (SD 0.7) | Intimate relationships | 3.0 (SD 1.3) | Total score | 0.8 (SD 0.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 45.5 (SD 10.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 35), female (n = 7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 12.9 (SD 4.24) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 11.2 (SD 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 30.2 (SD 4.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSLT (minutes) | 12.5 (SD 4.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptom questionnaire | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sleepiness | 26.3 (SD 9.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sleep fragmentation | 21.9 (SD 9.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Personality change | 8.6 (SD 6.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Morning confusion | 6.4 (SD 5.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total score | 63.2 (SD 21.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FOSQ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General productivity | 3.3 (SD 0.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social outcome | 3.2 (SD 1.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Activity level | 3.1 (SD 0.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vigilance | 3.0 (SD 0.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Intimate relationships | 3.0 (SD 1.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total score | 0.8 (SD 0.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Barnes et al. , 2004 82 Setting. Australia Design. Three-way crossover trial Duration. Three × 12 weeks (2-week washout period between each treatment). Notes. Authors report that 80 participants completed all three treatment arms Inclusion criteria. Patients with mild to moderate OSA |
CPAP. Adherence (machine usage): 3.6 hours/night (± 0.3) and 4.2 nights/per week (± 0.3) Comparator. Mandibular advancement splint. Mean mandibular advancement was 10.3 mm (range 1–13 mm). MAS was advanced weekly during a wash-in period as tolerated by the participant until the maximum comfortable protrusion was reached. The screw was sealed. Adherence (self-reported): 5.5 hours/night (± 0.3) and 5.3 nights/per week (± 0.3) Placebo (dummy pill). Adherence (pill count): tablets taken for 94.3% (± 1.2) of treatment nights |
Number randomised. Total: unclear (at least 99 randomised). Number of withdrawals. Total: 30 (CPAP: 8, MAS: 14, oral: 8) Reasons for withdrawals. CPAP: time commitments (n = 5), relocated (n = 1), intolerant to CPAP (n = 1), illness (n = 1). MAS: teeth unsuitable for MAS (n = 5), time commitments (n = 2), did not tolerate MAS (n = 2), unrelated illness (n = 1), lost weight and felt better (n = 1), lost to follow-up (n = 1). Placebo: time commitments (n = 6), wanted CPAP treatment (n = 1), illness (n = 1) Baseline characteristics TotalCPAPComparatorAge47 (SE 0.9)SexMale (79.8%), female (n = 16)AHI21.3 (SE 1.3)ESS10.7 (SE 0.4)BMI 31.1 (SE 0.5)BP 24-Hour SBP126.5 (SE 1.0) 24-Hour DBP76.3 (SE 0.8) Night-time DBP69.4 (SE 1.3)FOSQ, mean score3.1 (SE 0.1)SASQ64.7 (SE 1.7)POMS, total mood disorder15.5 (SE 2.0)BDI9.2 (SE 0.5)SF-3669.4 (SE 1.3) Additional information. 16 participants were hypertensive (defined as: SBP > 140 and/or DBP > 90 mmHg). Outcomes were measured at baseline and at the end of each intervention period: ESS, FOSQ, ODI 4%, AHI, MWT, SF-36 and cognitive function (see Table 53). |
Total | CPAP | Comparator | Age | 47 (SE 0.9) | Sex | Male (79.8%), female (n = 16) | AHI | 21.3 (SE 1.3) | ESS | 10.7 (SE 0.4) | BMI | 31.1 (SE 0.5) | BP | 24-Hour SBP | 126.5 (SE 1.0) | 24-Hour DBP | 76.3 (SE 0.8) | Night-time DBP | 69.4 (SE 1.3) | FOSQ, mean score | 3.1 (SE 0.1) | SASQ | 64.7 (SE 1.7) | POMS, total mood disorder | 15.5 (SE 2.0) | BDI | 9.2 (SE 0.5) | SF-36 | 69.4 (SE 1.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 47 (SE 0.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (79.8%), female (n = 16) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 21.3 (SE 1.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 10.7 (SE 0.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 31.1 (SE 0.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-Hour SBP | 126.5 (SE 1.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-Hour DBP | 76.3 (SE 0.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Night-time DBP | 69.4 (SE 1.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FOSQ, mean score | 3.1 (SE 0.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SASQ | 64.7 (SE 1.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| POMS, total mood disorder | 15.5 (SE 2.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BDI | 9.2 (SE 0.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | 69.4 (SE 1.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Becker et al. , 2003109 (related papers84,305) Setting. Germany Design. Parallel placebo controlled trial Duration. 9 weeks Notes. A maximum of four patients per week could be included in the study. If more than one patient was eligible on 1 day, the patient with the most pronounced sleep apnoea was invited to participate first Inclusion criteria. Men and women with OSA (AHI ≥ 5 and excessive daytime sleepiness) Exclusion criteria. Predominantly central sleep apnoea, respiratory failure, heart failure, myocardial infarction 3 months before the study, relevant cardiac arrhythmia and professional driver |
CPAP (following overnight manual titration). Adherence: 5.5 hours/night (SD 2.0) Comparator. Sham CPAP (pressure 3 or 4 cmH2O). Adherence: 5.4 hours/night (SD 2.2) |
Number randomised. Total: 60 (CPAP: 30, comparator: 30) Number of withdrawals. Total: 28 (CPAP: 14, comparator: 14) Reasons for withdrawals. CPAP: refused to continue (n = 3), technical fault with BP device (n = 6), antihypertensive medication change (n = 3), other (n = 2). Comparator: refused to continue (n = 2), technical fault with BP device (n = 5), antihypertensive medication change (n = 4), other (n = 3) Baseline characteristics (presented for completers only) TotalCPAPComparatorAge54.4 (SD 8.9)52.3 (SD 8.4)SexMale (n = 29), female (n = 3)Male (n = 15), female (n = 1)Male (n = 14), female (n = 2)AHI62.5 (SD 17.8)65.0 (SD 26.7)ESS14.4 (SD 2.5)14.1 (SD 3.2)BMI 33.3 (SD 5.1) 33.5 (SD 6.0) BP 24-Hour BP100.4 (SD 15.9)99.5 (SD 12.3) 24-Hour SBP135.9 (SD 17.5)136.2 (SD 13.1) 24-Hour DBP83.4 (SD 15.9)81.1 (SD 12.3) Daytime BP103.6 (SD 16.1)103.5 (SD 12.1) Daytime SBP140.1 (SD 17.6)141.0 (SD 13.8) Daytime DBP86.4 (SD 16.1)85.4 (SD 12.3) Night-time BP96.2 (SD 17.6)93.6 (SD 15.2) Night-time SBP129.9 (SD 20.0)129.2 (SD 16.9) Night-time DBP79.1 (SD 17.0)75.4 (SD 15.2) Additional information. 8/16 in CPAP group were hypertensive; 13/16 in control group were hypertensive. Outcomes were measured at baseline and at 9 weeks: daytime MAP, night-time MAP, ESS, AHI, REM (%), minimum SaO2. BP was measured using a cuff (recording time was limited to 20 hours). During the diagnostic period participants spent 7.2 hours in bed; night-time BP was calculated for this time period. Daytime BP was recorded for the remaining 12 hours. |
Total | CPAP | Comparator | Age | 54.4 (SD 8.9) | 52.3 (SD 8.4) | Sex | Male (n = 29), female (n = 3) | Male (n = 15), female (n = 1) | Male (n = 14), female (n = 2) | AHI | 62.5 (SD 17.8) | 65.0 (SD 26.7) | ESS | 14.4 (SD 2.5) | 14.1 (SD 3.2) | BMI | 33.3 (SD 5.1) | 33.5 (SD 6.0) | BP | 24-Hour BP | 100.4 (SD 15.9) | 99.5 (SD 12.3) | 24-Hour SBP | 135.9 (SD 17.5) | 136.2 (SD 13.1) | 24-Hour DBP | 83.4 (SD 15.9) | 81.1 (SD 12.3) | Daytime BP | 103.6 (SD 16.1) | 103.5 (SD 12.1) | Daytime SBP | 140.1 (SD 17.6) | 141.0 (SD 13.8) | Daytime DBP | 86.4 (SD 16.1) | 85.4 (SD 12.3) | Night-time BP | 96.2 (SD 17.6) | 93.6 (SD 15.2) | Night-time SBP | 129.9 (SD 20.0) | 129.2 (SD 16.9) | Night-time DBP | 79.1 (SD 17.0) | 75.4 (SD 15.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 54.4 (SD 8.9) | 52.3 (SD 8.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 29), female (n = 3) | Male (n = 15), female (n = 1) | Male (n = 14), female (n = 2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 62.5 (SD 17.8) | 65.0 (SD 26.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 14.4 (SD 2.5) | 14.1 (SD 3.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 33.3 (SD 5.1) | 33.5 (SD 6.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-Hour BP | 100.4 (SD 15.9) | 99.5 (SD 12.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-Hour SBP | 135.9 (SD 17.5) | 136.2 (SD 13.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-Hour DBP | 83.4 (SD 15.9) | 81.1 (SD 12.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Daytime BP | 103.6 (SD 16.1) | 103.5 (SD 12.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Daytime SBP | 140.1 (SD 17.6) | 141.0 (SD 13.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Daytime DBP | 86.4 (SD 16.1) | 85.4 (SD 12.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Night-time BP | 96.2 (SD 17.6) | 93.6 (SD 15.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Night-time SBP | 129.9 (SD 20.0) | 129.2 (SD 16.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Night-time DBP | 79.1 (SD 17.0) | 75.4 (SD 15.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Campos-Rodriguez et al. , 2006 65 Setting. Spain. Design. Parallel group trial Duration. 4 weeks. Inclusion criteria. Adults with OSAHS and hypertension (defined as > 140/90 mmHg in three independent measurements Exclusion criteria. Greater than 30% central sleep apnoea, respiratory failure, heart failure, ischaemic heart disease, cardiac arrhythmia, neoplastic or systemic diseases, patients with secondary hypertension, or professional driver. Participants were also excluded if their antihypertensive medication was changed during the course of the trial |
CPAP (following a full-night titration). Adherence not reported Comparator. Sham CPAP (pressure < 2 cmH2O) (following a mock titration night) |
Number randomised. Total: 72 (CPAP: 36, comparator: 36) Number of withdrawals. Total: 4 (CPAP: 2, comparator: 2) Reasons for withdrawals. CPAP: one participant changed treatment, and one did not attend follow-up. Comparator: one did not tolerate placebo and one changed antihypertensive treatment Baseline characteristics. n = 68 TotalCPAPComparatorAge55.3 (SD 9.6)58.0 (SD 7.0)SexMale (n = 41), female (n = 27)NRNRAHI58.3 (SD 24.6)59.5 (SD 21.7)ESS15.0 (SD 3.9)13.6 (SD 3.6)BMI35.7 (SD 5.6)33.8 (SD 6.3)BP 24-Hour BP97.7 (SD 10.7)96.2 (SD 10.1) 24-Hour SBP131.9 (SD 13.5)130.4 (SD 15.9) 24-Hour DBP78.4 (SD 10.3)77.6 (SD 8.7) Daytime BP100.8 (SD 10.7)98.9 (SD 10.0) Night-time BP94.6 (SD 11.1)93.5 (SD11.4) Additional information. ESS, AHI and 24-hour BP. Participants were assessed at trial entry and after 4 weeks of treatment. BP was measured using cuff inflation every 30 minutes for 24 hours. All participants were naïve to CPAP treatment. |
Total | CPAP | Comparator | Age | 55.3 (SD 9.6) | 58.0 (SD 7.0) | Sex | Male (n = 41), female (n = 27) | NR | NR | AHI | 58.3 (SD 24.6) | 59.5 (SD 21.7) | ESS | 15.0 (SD 3.9) | 13.6 (SD 3.6) | BMI | 35.7 (SD 5.6) | 33.8 (SD 6.3) | BP | 24-Hour BP | 97.7 (SD 10.7) | 96.2 (SD 10.1) | 24-Hour SBP | 131.9 (SD 13.5) | 130.4 (SD 15.9) | 24-Hour DBP | 78.4 (SD 10.3) | 77.6 (SD 8.7) | Daytime BP | 100.8 (SD 10.7) | 98.9 (SD 10.0) | Night-time BP | 94.6 (SD 11.1) | 93.5 (SD11.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 55.3 (SD 9.6) | 58.0 (SD 7.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 41), female (n = 27) | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 58.3 (SD 24.6) | 59.5 (SD 21.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 15.0 (SD 3.9) | 13.6 (SD 3.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 35.7 (SD 5.6) | 33.8 (SD 6.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-Hour BP | 97.7 (SD 10.7) | 96.2 (SD 10.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-Hour SBP | 131.9 (SD 13.5) | 130.4 (SD 15.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-Hour DBP | 78.4 (SD 10.3) | 77.6 (SD 8.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Daytime BP | 100.8 (SD 10.7) | 98.9 (SD 10.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Night-time BP | 94.6 (SD 11.1) | 93.5 (SD11.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Chakravorty et al. , 200297 (related paper306) Setting. UK Design. Parallel group trial Duration. 12 weeks Inclusion criteria. Participants with AHI ≥ 15 |
CPAP (following home-based titration using an automated pressure setting device). Adherence not reported Comparator. Conservative management (verbal advice, leaflet listing strategies for sleep hygiene, quitting smoking, reducing alcohol consumption, and controlling stress, verbal and written advice on ideal body weight, weight reduction and exercise) |
Number randomised. Total: 71 (CPAP: 37, comparator: 34) Number of withdrawals. Total: 18 (CPAP: 5, comparator: 13) Reasons for withdrawals. CPAP: failed to tolerate CPAP (n = 4), subsequently underwent surgery (n = 1). Comparator: excluded due to health reasons (n = 5), preferred surgery (n = 2), other (n = 6) Baseline characteristics (based on completers only) TotalCPAPComparatorAge50 (SD 11)49 (SD 11)52 (SD 9.6)SexNRNRNRAHI49 (SD 28)55 (SD 28.7)35 (SD 19.1)ESS14 (SD 5)16 (SD 5.6)14 (SD 4.2)BMI37 (SD 12)40 (SD 14.5)32.3 (SD 5.5)BPNot assessed––EuroQol (0–100)59 (SD 19.8)68 (SD 16.8)Ueqa0.73 (SD 0.18)0.77 (SD 0.12)Usgb0.32 (SD 0.17)0.31 (SD 0.13)abAdditional information. Outcomes include ESS, AHI, quality of life and utility. |
Total | CPAP | Comparator | Age | 50 (SD 11) | 49 (SD 11) | 52 (SD 9.6) | Sex | NR | NR | NR | AHI | 49 (SD 28) | 55 (SD 28.7) | 35 (SD 19.1) | ESS | 14 (SD 5) | 16 (SD 5.6) | 14 (SD 4.2) | BMI | 37 (SD 12) | 40 (SD 14.5) | 32.3 (SD 5.5) | BP | Not assessed | – | – | EuroQol (0–100) | 59 (SD 19.8) | 68 (SD 16.8) | Ueqa | 0.73 (SD 0.18) | 0.77 (SD 0.12) | Usgb | 0.32 (SD 0.17) | 0.31 (SD 0.13) | a | b | Additional information. Outcomes include ESS, AHI, quality of life and utility. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 50 (SD 11) | 49 (SD 11) | 52 (SD 9.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | NR | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 49 (SD 28) | 55 (SD 28.7) | 35 (SD 19.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 14 (SD 5) | 16 (SD 5.6) | 14 (SD 4.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 37 (SD 12) | 40 (SD 14.5) | 32.3 (SD 5.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EuroQol (0–100) | 59 (SD 19.8) | 68 (SD 16.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ueqa | 0.73 (SD 0.18) | 0.77 (SD 0.12) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Usgb | 0.32 (SD 0.17) | 0.31 (SD 0.13) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| a | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| b | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional information. Outcomes include ESS, AHI, quality of life and utility. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Cibele et al. , 2006 72 Setting. Brazil Design. Crossover trial Duration. 1 month OA or OA placebo and then 1 month nCPAP (1-week washout period between OA devices and CPAP treatment) Notes. Conference abstract – insufficient outcome data available Inclusion criteria. Adults with moderate to severe OSA (AHI ≥ 20) |
CPAP. Adherence not reported Comparator. OA (repositioning mandibular appliance with progressive adjustment) or placebo OA (splint at lower arch) |
Number randomised. Total: 13 Number of withdrawals. The authors do not report any losses to follow-up Reasons for withdrawals. NA Baseline characteristics TotalCPAPComparatorAge44 (SD 8)SexMale (n = 12), female (n = 1)AHI45.5 (SD 28)ESS10.6 (SD 4)BMI27.4 (SD 5)BPNot assessed Additional information. Outcomes include: ESS, SF-36, PVT (see Table 53), and oxidative stress. Outcomes were assessed before treatment and at each treatment phase. |
Total | CPAP | Comparator | Age | 44 (SD 8) | Sex | Male (n = 12), female (n = 1) | AHI | 45.5 (SD 28) | ESS | 10.6 (SD 4) | BMI | 27.4 (SD 5) | BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 44 (SD 8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 12), female (n = 1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 45.5 (SD 28) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 10.6 (SD 4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 27.4 (SD 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Coughlin et al. , 200762 (related paper307) Setting. UK Design. Crossover trial Duration. Two × 6 weeks (no washout period) Notes. Participants spent significantly more each night on the therapeutic CPAP machine than on the sham CPAP machine (p < 0.01). No evidence of a carryover effect for any outcome variable Inclusion criteria. Untreated male patients with OSAHS Exclusion criteria. Any abnormality identified on baseline ECG, or if there was evidence of diabetes, renal liver or cardiac disease, or if participants had symptoms of peripheral neuropathy or waking blood pressure ≥ 180/110 mmHg, or a level of blood pressure requiring treatment |
CPAP (following titration in sleep laboratory using automated pressure setting device). Adherence (machine running time): 3.9 hours/night (range 0–7.4) Comparator. Sham CPAP (pressure < 1 cmH2O). Adherence (machine running time): 2.6 hours/night (range 0–7.5) |
Number randomised. Total: 35 Number of withdrawals. Total: 1 (CPAP: 1, comparator: 0) Reasons for withdrawals. One participant withdrew during the first treatment period (CPAP limb) for personal reasons Baseline characteristics (based on 34 participants) TotalCPAPComparatorAge49.0 (SD 8.3)SexMale (n = 34)RDI39.7 (SD 13.8)ESS13.8 (SD 4.9)BMI36.1 (SD 7.6)BPNR Additional information. 27 participants (79%) were hypertensive (resting blood pressure of 140/90 mmHg). Outcomes included ESS, waking BP, fasting glucose, baroreceptor sensitivity, fasting insulin, cholesterol and incidence of metabolic syndrome. Outcomes were assessed at baseline and after each intervention period. Waking BP was measured from 8 a.m. to 11 a.m. in a supine position after a 5-minute rest. It was recorded as the mean of three measurements taken at 1-minute intervals. |
Total | CPAP | Comparator | Age | 49.0 (SD 8.3) | Sex | Male (n = 34) | RDI | 39.7 (SD 13.8) | ESS | 13.8 (SD 4.9) | BMI | 36.1 (SD 7.6) | BP | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 49.0 (SD 8.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 34) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RDI | 39.7 (SD 13.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 13.8 (SD 4.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 36.1 (SD 7.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Cross et al. , 2005308 (related paper88) Setting. UK Design. Crossover trial Duration. Two × 6 weeks (washout period not reported) Notes. Conference abstract Inclusion criteria. Adults with severe OSA (two major symptoms of OSAHS, > 20 of 4% nocturnal desaturation/hour) |
CPAP. Adherence: 5.5 hours/night (1.2) Comparator. Sham CPAP. Adherence: 3.3 hours/night (2.2) |
Number randomised. Total: 31 Number of withdrawals. Total: NR (CPAP: NR, comparator: NR) Reasons for withdrawals. NA Baseline characteristics TotalCPAPComparatorAge51 (SD 5)SexMale (n = 30), female (n = 1)AHI63 (SD 26)ESSNot assessedBMI40.1 (SD 8.4)BP NR Additional information. Outcomes include bilateral forearm blood flow (measured using venous occlusion plethysomography with unilateral intrabrachial infusions). |
Total | CPAP | Comparator | Age | 51 (SD 5) | Sex | Male (n = 30), female (n = 1) | AHI | 63 (SD 26) | ESS | Not assessed | BMI | 40.1 (SD 8.4) | BP | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 51 (SD 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 30), female (n = 1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 63 (SD 26) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 40.1 (SD 8.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Dimsdale et al. , 200058 (related papers117,309–314) Setting. USA Design. Parallel group trial Duration. 1 week Notes. Patients were treatment naïve Inclusion criteria. Adults (aged 30–65 years), within 100–170% of ideal weight and RDI > 15 Exclusion criteria. Major ongoing illness other than sleep apnoea and hypertension (> 140/90 mmHg but < 180/110 mmHg) |
CPAP (following manual overnight titration). Compliance was reported to be > 5 hours/night Comparator. Sham CPAP (pressure: 2 cmH2O) (following mock titration). Adherence was reported to be > 5 hours/night |
Number randomised. Unclear: 39 participants completed the study, but related papers indicate that a greater number of participants may have originally been randomised (n = 38–48) [CPAP (n = 21), comparator (n = 18)] Number of withdrawals. The authors do not report any withdrawals for BP or QoL outcomes. The papers report that POMS had 34 participants [CPAP (n = 20)] Reasons for withdrawals. NR Baseline characteristics TotalCPAPComparatorAge47.7 (SD 8.1)48.9 (SD 9.9)SexMale (n = 15), female (n = 6)Male (n = 16), female (n = 2)AHINot assessed––ESSNot assessed––BMI32.7 (SD 4.9)28.5 (SD 5.0)BP Mean screening SBP128 (SD 15)123 (SD 12) Mean screening DBP82 (SD 8)78 (SD 9)RDI53.6 (SD 23.2)41.7 (SD 25.6)POMS Tension10.9 (SD 7.1)9.9 (SD 5.5) Depression12.5 (SD 15.1)12.7 (SD 10.1) Fatigue13.3 (SD 8.2)11.6 (SD 6.6) Confusion7.6 (SD 5.0)8.3 (SD 3.5) Vigour15.3 (SD 7.7)15.3 (4.7) Anger14.3 (SD 11.9)9.2 (SD 6.5) Total mood disturbance43.2 (SD 48.5)36.5 (SD 28.4) Additional information. Ten participants were hypertensive (CPAP 6, sham CPAP 4). Participants receiving antihypertensive medication had their medication tapered and their BP status confirmed after a 3-week washout. Outcomes included BP and RDI. Participants wore an ambulatory BP monitor for a 24-hour period on three occasions: before randomisation, after 1 day of treatment and after 1 week of treatment. BP was taken every 15 minutes 6 a.m. to 10 p.m. and every 30 minutes 10 p.m. to 6 a.m. Related papers report QoL (MOS) (Profant309), mood (POMS; Yu313), and cognitive outcomes (Bardwell117) (see Table 53); assessed before treatment and after 1 week of treatment. |
Total | CPAP | Comparator | Age | 47.7 (SD 8.1) | 48.9 (SD 9.9) | Sex | Male (n = 15), female (n = 6) | Male (n = 16), female (n = 2) | AHI | Not assessed | – | – | ESS | Not assessed | – | – | BMI | 32.7 (SD 4.9) | 28.5 (SD 5.0) | BP | Mean screening SBP | 128 (SD 15) | 123 (SD 12) | Mean screening DBP | 82 (SD 8) | 78 (SD 9) | RDI | 53.6 (SD 23.2) | 41.7 (SD 25.6) | POMS | Tension | 10.9 (SD 7.1) | 9.9 (SD 5.5) | Depression | 12.5 (SD 15.1) | 12.7 (SD 10.1) | Fatigue | 13.3 (SD 8.2) | 11.6 (SD 6.6) | Confusion | 7.6 (SD 5.0) | 8.3 (SD 3.5) | Vigour | 15.3 (SD 7.7) | 15.3 (4.7) | Anger | 14.3 (SD 11.9) | 9.2 (SD 6.5) | Total mood disturbance | 43.2 (SD 48.5) | 36.5 (SD 28.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 47.7 (SD 8.1) | 48.9 (SD 9.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 15), female (n = 6) | Male (n = 16), female (n = 2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 32.7 (SD 4.9) | 28.5 (SD 5.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean screening SBP | 128 (SD 15) | 123 (SD 12) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean screening DBP | 82 (SD 8) | 78 (SD 9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RDI | 53.6 (SD 23.2) | 41.7 (SD 25.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| POMS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tension | 10.9 (SD 7.1) | 9.9 (SD 5.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Depression | 12.5 (SD 15.1) | 12.7 (SD 10.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fatigue | 13.3 (SD 8.2) | 11.6 (SD 6.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Confusion | 7.6 (SD 5.0) | 8.3 (SD 3.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vigour | 15.3 (SD 7.7) | 15.3 (4.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Anger | 14.3 (SD 11.9) | 9.2 (SD 6.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total mood disturbance | 43.2 (SD 48.5) | 36.5 (SD 28.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Drager et al. , 2006 69 Setting. Brazil Design. Parallel group trial Duration. 12 weeks Notes. Conference abstract – insufficient outcome data available Inclusion criteria. Normotensive participants with OSA |
CPAP. Adherence not reported Comparator. Usual care |
Number randomised. Total: 16 (CPAP: NR, comparator: NR) Number of withdrawals. Total: no reported dropouts Reasons for withdrawals. NA Baseline characteristics TotalCPAPComparatorAge45 (SE 3)47 (SE 4)SexNRNRNRAHI54 (SE 8)65 (SE 13)ESSNot assessed––BMI31 (SE 1)30 (SE 1)BP Mean SBP118 (SE 4)125 (SE 5) Additional information. Outcomes included BP, carotid–femoral pulse wave velocity, cholesterol level and heart rate. Participants were assessed at baseline and after 3 months. |
Total | CPAP | Comparator | Age | 45 (SE 3) | 47 (SE 4) | Sex | NR | NR | NR | AHI | 54 (SE 8) | 65 (SE 13) | ESS | Not assessed | – | – | BMI | 31 (SE 1) | 30 (SE 1) | BP | Mean SBP | 118 (SE 4) | 125 (SE 5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 45 (SE 3) | 47 (SE 4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | NR | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 54 (SE 8) | 65 (SE 13) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 31 (SE 1) | 30 (SE 1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean SBP | 118 (SE 4) | 125 (SE 5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Engleman et al. , 1994 90 Setting. UK Design. Crossover trial Duration. Two × 2 weeks (no washout period) Notes. Significant learning effect on some outcome measures, especially cognitive tests Inclusion criteria. Men and women with AHI ≥ 5 and at least two symptoms of obstructive sleep apnoea. Included consecutive patients referred for investigation of OSA Exclusion criteria. No co-existing disorder causing excessive sleepiness and lives within 50 miles of the laboratory. |
CPAP (following overnight titration). Adherence: 3.7 hours/night Comparator. Oral placebo (inactive ranitidine) |
Number randomised. Total: 35 Number of withdrawals. Total: 3 (CPAP: NR, comparator: NR) Reasons for withdrawals. Pressure at work (n = 1), relocation (n = 1), reluctance to use CPAP (n = 1) Baseline characteristics (based on completers only) TotalCPAPComparatorAge49 (SE 1.5)SexMale (n = 26), female (n = 6)AHI (median)28ESSNot assessedBMI33 (1.6)BPNot assessed Additional information. These outcomes were measured on the last day of each treatment period: MSLT, cognitive function (NART, WAIS, TMT A and B, SteerClear, RVIPT, PASAT, Borkowski Test, BVRT-R) (see Table 53), in-house symptom score, HADS, GHQ, NHP, energetic arousal score compliance, patient preference. |
Total | CPAP | Comparator | Age | 49 (SE 1.5) | Sex | Male (n = 26), female (n = 6) | AHI (median) | 28 | ESS | Not assessed | BMI | 33 (1.6) | BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 49 (SE 1.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 26), female (n = 6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI (median) | 28 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 33 (1.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Engleman et al. , 1996 91 Setting. UK Design. Crossover trial Duration. Two × 4 weeks (no washout period) Inclusion criteria. Men and women with AHI ≥ 5 and at least two symptoms of obstructive sleep apnoea |
CPAP. Adherence: 4.3 hours/night (SE 0.6) Comparator. Oral placebo (inactive ranitidine) |
Number randomised. Total: 16 Number of withdrawals. Total: 3 (CPAP: NR, comparator: NR) Reasons for withdrawals. Equipment unavailability (n = 1), poor ambulatory BP (n = 1) and non-attendance (n = 1) Baseline characteristics (completers only, n = 13) TotalCPAPComparatorAge51 (SE 3)SexMale (n = 11), female (n = 2)AHI49 (SE 9)ESSNot assessedBMI36 (SE 2.6)BPNR Additional information. Five participants were classified as hypertensive (24-hour BP > 134 and DBP > 84 mmHg), and four participants were taking antihypertensive medication; medication did not change throughout the trial period. Outcome was 24-hour ambulatory BP. BP was recorded every 30 minutes for a 24-hour period with a cuff. |
Total | CPAP | Comparator | Age | 51 (SE 3) | Sex | Male (n = 11), female (n = 2) | AHI | 49 (SE 9) | ESS | Not assessed | BMI | 36 (SE 2.6) | BP | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 51 (SE 3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 11), female (n = 2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 49 (SE 9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 36 (SE 2.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Engleman et al. , 1997 92 Setting. UK Design. Crossover trial Duration. Two × 4 weeks (no washout period) Notes. No tests reported for differential carryover for period or washout effect. Ten patients refused to participate in the study Inclusion criteria. Patients with mild sleep apnoea (AHI 5–14.9) and at least two symptoms of obstructive sleep apnoea |
CPAP (following titration study). Adherence (machine usage): 3.2 hours/night (SE 0.7) Comparator. Oral placebo (inactive ranitidine) |
Number randomised. Total: 18 Number of withdrawals. Total: 2 (CPAP: 2, comparator: 0) Reasons for withdrawals. Relocated (n = 1), intolerant of noise from CPAP unit and declined to complete treatment limb (n = 1) Baseline characteristics (based on completers) TotalCPAPComparatorAge52 (SE 2)SexMale (n = 12), female (n = 4)AHI11 (SE 1)ESS14 (SE 1)BMI29.8 (SE 1.8)BPNot assessed Additional information. Outcomes were measured on the last day of each treatment: MSLT, cognitive function (NART, WAIS, TMT A and B, SteerClear, RVIPT, PASAT, Borkowski Test, BRVT-R) (see Table 53), in-house symptom score, HADS, GHQ, NHP, energetic arousal score, compliance, patient preference. |
Total | CPAP | Comparator | Age | 52 (SE 2) | Sex | Male (n = 12), female (n = 4) | AHI | 11 (SE 1) | ESS | 14 (SE 1) | BMI | 29.8 (SE 1.8) | BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 52 (SE 2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 12), female (n = 4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 11 (SE 1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 14 (SE 1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 29.8 (SE 1.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Engleman et al. , 1998 93 Setting. UK Design. Crossover trial Duration. Two × 4 weeks (no washout period) Inclusion criteria. Patients with AHI of at least 15 and two or more symptoms of sleep-disordered breathing Exclusion criteria. Lung disease, neurological disorders, co-existing sleep disorder and individuals who lived more than 50 miles from the Scottish National Sleep Centre |
CPAP (following overnight titration). Adherence: 3.2 hours/night Comparator. Oral placebo |
Number randomised. Total: 23 Number of withdrawals. Total: 1 (CPAP: 1, comparator: 0) Reasons for withdrawals. Myocardial infarction during CPAP limb. Participant position refilled by next available recruit Baseline characteristics TotalCPAPComparatorAge47 (SD 12)SexMale (n = 21), female (n = 2)AHI43 (SD 37)ESS12.0 (SD 4)BMIBMI 30 (SD 7)BPNot assessedUMACL, energetic arousal score21 (SD 5)Symptom score, total 5.1 (SD 1.5)HADS Anxiety8.3 (SD 4.4) Depression5.7 (SD 4.4)GHQ-286.6 (SD 6.5)NHP part 28.0 (SD 5.0) Additional information. Outcomes were measured on the last day of each treatment: ESS, AHI, NHP, MSLT, HADS, GHQ-28, UMACL, cognitive function (see Table 53), preference. |
Total | CPAP | Comparator | Age | 47 (SD 12) | Sex | Male (n = 21), female (n = 2) | AHI | 43 (SD 37) | ESS | 12.0 (SD 4) | BMI | BMI 30 (SD 7) | BP | Not assessed | UMACL, energetic arousal score | 21 (SD 5) | Symptom score, total | 5.1 (SD 1.5) | HADS | Anxiety | 8.3 (SD 4.4) | Depression | 5.7 (SD 4.4) | GHQ-28 | 6.6 (SD 6.5) | NHP part 2 | 8.0 (SD 5.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 47 (SD 12) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 21), female (n = 2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 43 (SD 37) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 12.0 (SD 4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | BMI 30 (SD 7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UMACL, energetic arousal score | 21 (SD 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptom score, total | 5.1 (SD 1.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HADS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Anxiety | 8.3 (SD 4.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Depression | 5.7 (SD 4.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GHQ-28 | 6.6 (SD 6.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NHP part 2 | 8.0 (SD 5.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Engleman et al. , 1999 78 Setting. UK Design. Crossover trial Duration. Two × 4 weeks (no washout period) Inclusion criteria. New outpatient attendees with at least two symptoms of OSA including sleepiness (ESS ≥ 8) and mild sleep apnoea (AHI 5–14.9/hour) |
CPAP (following overnight titration). Heated humidified nCPAP. Adherence (machine usage): 3.2 hours/night (SD 2.4) Comparator. Oral placebo |
Number randomised. Total: 37 Number of withdrawals. Total: 3 (CPAP: NR, comparator: NR) Reasons for withdrawals. Unwilling to persist with CPAP treatment (n = 1), failed to attend final assessment (n = 1), travel to centre too demanding (final CPAP limb) (n = 1) Baseline characteristics (based on completers only) TotalCPAPComparatorAge44 (SD 8) SexMale (n = 21), female (n = 13)AHI10 (SD 3)ESS13 (SD 3)BMI 30 (SD 5) BPNot assessedSymptoms (total score)22 (SD 6)UMACL, energetic arousal score18 (SD 5)HADS Anxiety9.0 (SD 4.2) Depression7.4 (SD 4.1)NHP part 210.5 (SD 4.8)SF-36 Health transition3.1 (SD 0.6) Physical function75 (SD 27) Role – physical58 (SD 36) Role – emotional62 (SD 38) Bodily pain68 (SD 31) Mental health64 (SD 19) Social function60 (SD 27) General health68 (SD 21) Vitality33 (SD 19) Additional information. Outcomes included MWT, ESS, UMACL, symptom questionnaire, HADS, NHP part 2, SF-36, WAIS-R, cognitive function (see Table 53). |
Total | CPAP | Comparator | Age | 44 (SD 8) | Sex | Male (n = 21), female (n = 13) | AHI | 10 (SD 3) | ESS | 13 (SD 3) | BMI | 30 (SD 5) | BP | Not assessed | Symptoms (total score) | 22 (SD 6) | UMACL, energetic arousal score | 18 (SD 5) | HADS | Anxiety | 9.0 (SD 4.2) | Depression | 7.4 (SD 4.1) | NHP part 2 | 10.5 (SD 4.8) | SF-36 | Health transition | 3.1 (SD 0.6) | Physical function | 75 (SD 27) | Role – physical | 58 (SD 36) | Role – emotional | 62 (SD 38) | Bodily pain | 68 (SD 31) | Mental health | 64 (SD 19) | Social function | 60 (SD 27) | General health | 68 (SD 21) | Vitality | 33 (SD 19) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 44 (SD 8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 21), female (n = 13) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 10 (SD 3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 13 (SD 3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 30 (SD 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms (total score) | 22 (SD 6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UMACL, energetic arousal score | 18 (SD 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HADS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Anxiety | 9.0 (SD 4.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Depression | 7.4 (SD 4.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NHP part 2 | 10.5 (SD 4.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health transition | 3.1 (SD 0.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical function | 75 (SD 27) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role – physical | 58 (SD 36) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role – emotional | 62 (SD 38) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bodily pain | 68 (SD 31) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental health | 64 (SD 19) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social function | 60 (SD 27) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General health | 68 (SD 21) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitality | 33 (SD 19) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Engleman et al. , 2002103 (related papers112,315) Setting. UK Design. Crossover trial Duration. Two × 8 weeks (no washout period) Inclusion criteria. Patients aged 18–70 years with AHI ≥ 5 and two symptoms of OSAHS Exclusion criteria. Individuals with fewer than four teeth remaining in either arch, co-existing narcolepsy, periodic limb movement of more than 10/hour, major medical illness, shift work, or living more than 50 miles away from Edinburgh |
CPAP (following all-night titration). Adherence: 6.1 hours/night (± 1.9) Comparator. Oral appliance. Participants were randomised to receive one of two oral devices: (1) two mouth guards providing complete occlusal coverage constructed by an ethylenemethylacrylate/polystyrene material and the two units sealed in protrusion; (2) device manufactured from less flexible 1MEDL™ dual laminate material, without occlusal coverage. Both were individually fitted to produce 80% of maximal comfortable mandibular protrusion, with 2–4 mm of interdental clearance. Adherence: 5.6 hours/night (± 2.0) |
Number randomised. Total: 51 Number of withdrawals. Total: 3 (CPAP: 2, comparator: 1) Reasons for withdrawals. Uncontactable (first CPAP limb) (n = 1), unable to spare time due to starting a new job (during first CPAP and first OA limb) Baseline characteristics (n = 48) TotalCPAPComparatorAge46 (SD 9)SexMale (n = 36), female (n = 12)AHI31 (SD 26)ESS14 (SD 4)BMINRBPNot assessed Additional information. Outcomes were measured on the last day of each treatment: SF-36, ESS, MWT, HADS, AHI, FOSQ, PASAT, cognitive function (see Table 53), preference. |
Total | CPAP | Comparator | Age | 46 (SD 9) | Sex | Male (n = 36), female (n = 12) | AHI | 31 (SD 26) | ESS | 14 (SD 4) | BMI | NR | BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 46 (SD 9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 36), female (n = 12) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 31 (SD 26) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 14 (SD 4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Faccenda et al. , 200194 (related papers316, 317) Setting. UK Design. Crossover trial. Duration. Two × 4 weeks (no washout period) Inclusion criteria. Patients that exhibit two symptoms of sleep apnoea/hypopnoea syndrome and an AHI ≥ 15 Exclusion criteria. Individuals taking hypotensive medication |
CPAP (following full-night titration using an automated pressure setting device). Adherence: 3.3 hours/night (range 0–8.1) Comparator. Oral placebo. Adherence (capsule counting): a median of 0 tablets (95th percentile, 1.4 tablets) were missed over the 1-month period |
Number randomised. Total: 71 Number of withdrawals. Total: 3 (CPAP: 2, comparator: 1) Reasons for withdrawals. NR Baseline characteristics (n = 68) TotalCPAPComparatorAge50 (29–72)SexMale (n = 55), female (n = 13)AHI35 (15–129)ESS15 (6–24)BMI30 (21–53)BPNR Data presented as median (range) unless otherwise stated Additional information. Outcomes were measured on the last day of each 1-month period treatment: ESS; AHI; BP. BP was measured via arm cuff, programmed to record every 30 minutes for a 48-hour period. Data for second 24-hour period used (6 p.m.–6 p.m.). |
Total | CPAP | Comparator | Age | 50 (29–72) | Sex | Male (n = 55), female (n = 13) | AHI | 35 (15–129) | ESS | 15 (6–24) | BMI | 30 (21–53) | BP | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 50 (29–72) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 55), female (n = 13) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 35 (15–129) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 15 (6–24) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 30 (21–53) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Ferguson et al. , 199681 Setting. Canada Design. Crossover trial Duration. Two × 16 weeks (2-week washout period) Notes. Patients randomised after 2-week wash-in period. Unclear about patient preference – only provided results for seven patients who were treatment successes with both treatments. No evidence of carryover effect between treatment periods Inclusion criteria. Patients with mild to moderate sleep apnoea and at least 10 teeth in each of the mandibular and maxillary arches. |
CPAP (following overnight titration). The use of a humidifier was optional, but encouraged. Intranasal corticosteroids and/or anticholinergic medications to relieve nasal symptoms caused by CPAP use were used. Adherence (% of the night treatment used): 10 patients used CPAP 100%, four patients used CPAP > 75%, three patients used CPAP 25–75%, four patients used CPAP < 25% Adherence (% of the night treatment used): nine patients used CPAP 100%, five patients used CPAP > 75%, three patients used CPAP 25–75%, four patients used CPAP < 25% Comparator. Oral appliance. OA was constructed to position the mandible 3 mm posterior to the position of maximal acceptable advance and with a 7 mm opening between the upper and lower incisors. Material was added to increase the vertical dimension of the appliance in a few participants. Oral device was constructed of an acrylic polymer Adherence (% nights treatment used): 15 patients used OA 100%, nine patients used OA > 75%, one patient used OA 25–75% Adherence (% nights treatment used): 12 patients used OA 100%, eight patients used OA > 75%, four patients used OA 25–75%, one patient used OA < 25% |
Number randomised. Total: 26 Number of withdrawals. Total: 1 (CPAP: 0, comparator: 1) Reasons for withdrawals. Relocated (n = 1) Baseline characteristics (n = 27, based on number recruited for washout period before randomisation) TotalCPAPComparatorAge46.2 (SD 10.9)SexMale (n = 24, female n = 3)AHI24.5 (SD 8.8)ESSNot assessedBMI30.4 (SD 4.8)BPNot assessed Additional information. Outcomes were measured at end of each 4-month period: sleep variables, symptom questionnaire, patient preference, side effects. |
Total | CPAP | Comparator | Age | 46.2 (SD 10.9) | Sex | Male (n = 24, female n = 3) | AHI | 24.5 (SD 8.8) | ESS | Not assessed | BMI | 30.4 (SD 4.8) | BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 46.2 (SD 10.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 24, female n = 3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 24.5 (SD 8.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 30.4 (SD 4.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Ferguson et al. , 1997 80 Setting. Canada Design. Crossover trial Duration. Two × 16 weeks (2-week washout period) Notes. Patients randomised after 2-week wash-in period. No evidence of carryover effect Inclusion criteria. Patients with symptomatic mild to moderate sleep apnoea (AHI 15–55), and at least 10 teeth in each of the mandibular and maxillary arches |
CPAP. Participants used a variety of airway access devices based on their own preference. The use of a cold flow-by humidifier was optional but encouraged. Intranasal corticosteroids and/or anti-cholinergic medications were used to relieve nasal symptoms Comparator. Oral appliance. The amount of mandibular advancement was initially set at 70% of maximal mandibular advancement. The amount of mandibular advancement was increased over the treatment period by a mean of 1.8 mm (SD 1.2) until snoring stopped and symptoms improved, or until participants could not tolerate further advancement |
Number randomised. Total: 24 [baseline data for ESS are presented for completers (n = 20)] Number of withdrawals. Total: 4 (CPAP: NR, comparator: NR) Reasons for withdrawals. Refused follow-up assessments (n = 1), refused to cross over from OA to CPAP (n = 3) Baseline characteristics TotalCPAPComparatorAge44 (SD 11)SexMale (n = 19), female (n = 5)AHI27 (SD 12)ESSNR11 (SD 3.8)10.3 (SD 3.1)BMI32 (SD 8)BPNot assessed Additional information. Outcomes were measured at the end of each treatment period: sleep variables, symptom questionnaire, patient preference, side effects. |
Total | CPAP | Comparator | Age | 44 (SD 11) | Sex | Male (n = 19), female (n = 5) | AHI | 27 (SD 12) | ESS | NR | 11 (SD 3.8) | 10.3 (SD 3.1) | BMI | 32 (SD 8) | BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 44 (SD 11) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 19), female (n = 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 27 (SD 12) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | NR | 11 (SD 3.8) | 10.3 (SD 3.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 32 (SD 8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fleetham et al. , 200250 (unpublished data from Gileset al. , 2006) (related papers101,119,218) Setting. Canada Design. Parallel group trial Duration. 12 weeks Inclusion criteria. AHI > 10 |
CPAP. Adherence: NR Comparator. Adjustable oral appliance |
Number randomised. Total: 101 (CPAP: NR, comparator: NR) Number of withdrawals. Total: NR Reasons for withdrawals. NA Baseline characteristics TotalCPAPComparatorAge49.0 (± 9.4)6.2 (± 11.3)SexMale (n = 96), female (n = 5)AHI37.6 (± 22.8)38.7 (± 22.2)ESS12.8 (± 4.1)11.1 (± 4.9)BMI32.0 (± 5.5)1.4 (± 5.7)BPNot assessed––SAQLI4.2 (± 1.1)4.2 (± 1.0)Minimum SaO275.8 (± 12.7)73.6 (± 1.8) Additional information. Outcomes included: AHI, ESS, minimum SaO2, quality of life index. |
Total | CPAP | Comparator | Age | 49.0 (± 9.4) | 6.2 (± 11.3) | Sex | Male (n = 96), female (n = 5) | AHI | 37.6 (± 22.8) | 38.7 (± 22.2) | ESS | 12.8 (± 4.1) | 11.1 (± 4.9) | BMI | 32.0 (± 5.5) | 1.4 (± 5.7) | BP | Not assessed | – | – | SAQLI | 4.2 (± 1.1) | 4.2 (± 1.0) | Minimum SaO2 | 75.8 (± 12.7) | 73.6 (± 1.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 49.0 (± 9.4) | 6.2 (± 11.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 96), female (n = 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 37.6 (± 22.8) | 38.7 (± 22.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 12.8 (± 4.1) | 11.1 (± 4.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 32.0 (± 5.5) | 1.4 (± 5.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAQLI | 4.2 (± 1.1) | 4.2 (± 1.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum SaO2 | 75.8 (± 12.7) | 73.6 (± 1.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Henke et al. , 2001 85 Setting. USA Design. Partial crossover trial Duration. Six weeks (no washout period). Sham-CPAP group received treatment for 15 days then crossed over and received CPAP for rest of treatment period. CPAP received treatment for entire period Notes. Only data from the first sequence were used, thus data were treated as parallel data Inclusion criteria. Males and females with diagnosed symptoms of sleep apnoea/hypopnoea syndrome with AHI > 10 plus daytime sleepiness or AHI > 20 without daytime sleepiness Exclusion criteria. Oxygen saturation < 85% for > 50% of sleep time, clinical signs of right-sided congestive heart failure, claustrophobia or nasal obstruction preventing use of nasal CPAP |
CPAP (following laboratory titration). Adherence (hours/night): first limb 5.9 (SD 1.8); second limb 5.8 (SD 2.0) Comparator. Sham CPAP. Adherence (hours/night): first limb 5.2 (SD 2.2); second limb 4.9 (SD 2.4) |
Number randomised. Total: 45 (CPAP: 27, comparator: 18) Number of withdrawals. Total: 4 (T3 parallel analysis) (CPAP: NR, comparator: NR) Reasons for withdrawals. Baseline polysomnography was mistakenly performed on effective CPAP rather than ineffective CPAP for four participants from placebo group; data were not included in the analysis Baseline characteristics TotalCPAPComparatorAgeNR50.2 (SD 10.4)50.6 (SD 9.7)SexMale (n = 25), female (n = 20)NRNRAHINR62.1 (SD 27.4)68.1 (SD 25.2)ESS1616.4 (SD 5.6)16.0 (SD 4.8)BMINR42.7 (SD 10.5)42.2 (SD 11.9)BPNot assessed–– Additional information. Outcomes were measured on the last day of each treatment: ESS, AHI, ODI, minimum SaO2, SteerClear (see Table 53). |
Total | CPAP | Comparator | Age | NR | 50.2 (SD 10.4) | 50.6 (SD 9.7) | Sex | Male (n = 25), female (n = 20) | NR | NR | AHI | NR | 62.1 (SD 27.4) | 68.1 (SD 25.2) | ESS | 16 | 16.4 (SD 5.6) | 16.0 (SD 4.8) | BMI | NR | 42.7 (SD 10.5) | 42.2 (SD 11.9) | BP | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | NR | 50.2 (SD 10.4) | 50.6 (SD 9.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 25), female (n = 20) | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | NR | 62.1 (SD 27.4) | 68.1 (SD 25.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 16 | 16.4 (SD 5.6) | 16.0 (SD 4.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | NR | 42.7 (SD 10.5) | 42.2 (SD 11.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Hoekema et al. , 2006102 (related papers71,111,114,300) Setting. Netherlands Design. Parallel group trial Duration. 8 weeks Notes. Abstract only for main efficacy data. Data for driving simulator test come from related paper (Hoekema et al., 2006114). Data for sexual dysfunction (GRISS) taken from related paper (Hoekema et al., 2006111). Duration (for the two related papers). 8–12 weeks. After 8 weeks of treatment participants were assessed with a second polysomnographic study; for participants with AHI ≥ 5, treatment was adjusted if possible to improve effectiveness, and in these participants the follow-up period was extended for another 4 weeks. Adjustment sequence was continued until AHI < 5 or until adjustments become uncomfortable Inclusion criteria. Adults with OSAHS (AHI > 5) |
CPAP. Adherence: NR Comparator. Oral appliance Related papers. The oral appliance was a two-part adjustable appliance set at 5 degrees of patients’ maximum advancement to begin with; patients could adjust the amount of mandibular advancement in increments of 0.2 degrees and were instructed to adjust by 0.2–0.4 degrees in weeks 2–8 until symptoms abated or any further advancement was uncomfortable |
Number randomised. Total: 103 (CPAP: 52, comparator: 51) Number of withdrawals. NR Reasons for withdrawals. NR Baseline characteristics TotalCPAPComparatorAgeNRSexNRAHI (median)NRESSNot assessedBMINot assessedBPNot assessedDriving simulator test (n = 19) Lapses of attention, median10 (IQR 1.0–16.8)5.0 (IQR 2.0–14.0)GRISS (n = 47) Erectile dysfunction9.5 (SD 4.2)7.9 (SD 3.5) Premature ejaculation9.0 (SD 2.7)9.5 (SD 3.5) Non-sensuality5.8 (SD 2.1)5.4 (SD 2.0) Avoidance4.0 (IQR 4.0–5.5)4.0 (IQR 4.0–5.0) Sexual dissatisfaction10.2 (SD 4.3)8.5 (SD 3.9) Infrequency of sexual contact7.0 (SD 1.7)5.9 (SD 2.0) Non-communication4.6 (SD 1.8)4.0 (SD 1.9) Additional information. Primary outcome for main paper: AHI. Primary outcomes for related papers: driving simulator test and sexual dysfunction. Participants undertaking the driving simulator test were assessed between noon and 2 p.m. Room conditions: lights were dimmed, noise was excluded and room temperature was kept at 22˚C. The driving test was completed alone. Participants were instructed to refrain from stimulating products such as caffeine in the 3 hours before testing and not to smoke for 30 minutes before testing. Outcomes were assessed at baseline and after 8 weeks of treatment. In some participants final assessment occurred at a later stage [median final review was 81 days (IQR 72–93) in the OA group and 79 days (IQR 63–102) in the CPAP group]. |
Total | CPAP | Comparator | Age | NR | Sex | NR | AHI (median) | NR | ESS | Not assessed | BMI | Not assessed | BP | Not assessed | Driving simulator test (n = 19) | Lapses of attention, median | 10 (IQR 1.0–16.8) | 5.0 (IQR 2.0–14.0) | GRISS (n = 47) | Erectile dysfunction | 9.5 (SD 4.2) | 7.9 (SD 3.5) | Premature ejaculation | 9.0 (SD 2.7) | 9.5 (SD 3.5) | Non-sensuality | 5.8 (SD 2.1) | 5.4 (SD 2.0) | Avoidance | 4.0 (IQR 4.0–5.5) | 4.0 (IQR 4.0–5.0) | Sexual dissatisfaction | 10.2 (SD 4.3) | 8.5 (SD 3.9) | Infrequency of sexual contact | 7.0 (SD 1.7) | 5.9 (SD 2.0) | Non-communication | 4.6 (SD 1.8) | 4.0 (SD 1.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI (median) | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Driving simulator test (n = 19) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lapses of attention, median | 10 (IQR 1.0–16.8) | 5.0 (IQR 2.0–14.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRISS (n = 47) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Erectile dysfunction | 9.5 (SD 4.2) | 7.9 (SD 3.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Premature ejaculation | 9.0 (SD 2.7) | 9.5 (SD 3.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-sensuality | 5.8 (SD 2.1) | 5.4 (SD 2.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Avoidance | 4.0 (IQR 4.0–5.5) | 4.0 (IQR 4.0–5.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sexual dissatisfaction | 10.2 (SD 4.3) | 8.5 (SD 3.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Infrequency of sexual contact | 7.0 (SD 1.7) | 5.9 (SD 2.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-communication | 4.6 (SD 1.8) | 4.0 (SD 1.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Hui et al. , 200664 (related paper318) Setting. Hong Kong Design. Parallel group trial Duration. 3 months Inclusion criteria. CPAP-naïve adults with OSA. Participants with hypertension were eligible as long as there was no change in antihypertensive medication Exclusion criteria. Individuals with problems staying awake during driving, professional drivers, shift workers, recent myocardial infarction, unstable angina or underlying malignancy |
CPAP (following overnight titration using an automated pressure setting device). Adherence (machine running time): 5.1 hours/night (SE 0.4) Comparator. Sham CPAP (pressure: 4 cmH2O). Adherence (machine running time): 2.6 hours/night (SE 0.4) |
Number randomised. Total: 56 (CPAP: 28, comparator: 28) Number of withdrawals. Total: 10 (CPAP: 5, comparator: 5) Reasons for withdrawals. CPAP: defaulted ambulatory BP measurement (n = 3), ambulatory BP recording failed to record (n = 2); Comparator: discomfort with treatment (n = 5) Baseline characteristics TotalCPAPComparatorAge50.8 (SE 1.7)50.3 (SE 1.6)51.2 (SE 1.8)SexMale (n = 43), female (n = 13)Male (n = 22), female (n = 6)Male (n = 21), female (n = 7)AHI31.2 (SE 2.2)32.9 (SE 3.2)29.5 (SE 3.1)ESS11.1 (SE 0.7)10.7 (SE 1.0)11.6 (1.0)BMI27.2 (SE 0.5)27.5 (SE 0.6)26.9 (SE 0.7)BP 24-hour SBP123.7 (SE 1.8)125.4 (SE 2.6)122.0 (SE 2.7) 24-hour DBP 80.9 (SE 1.2)81.8 (SE 1.9)80.0 (SE 1.7) 24-hour MAP95.2 (SE 1.3)96.2 (SE 1.8)94.1 (SE 2.0) Wake time SBP127.8 (SE 1.8)128.6 (SE 2.6)127.2 (SE 2.7) Wake time DBP83.6 (SE 1.2)83.7 (SE 1.9)83.7 (SE 1.7) Wake time MAP98.1 (SE 1.5)98.3 (SE 1.8)97.9 (SE 1.9) Sleep time SBP115.7 (SE 2.0)117.7 (SE 2.9)113.9 (SE 3.0) Sleep time DBP74.8 (SE 1.4)75.8 (SE 2.1)74.1 (SE 2.0) Sleep time MAP89.1 (SE 1.5)90.6 (SE 2.1)87.8 (SE 2.3) Additional information. 28 participants had hypertension (previously documented BP > 140/90 mmHg on at least two occasions or receiving antihypertensive medication). There were no changes in antihypertensive medication during the study. Outcomes included ESS, change in 24-hour arterial BP, change in SBP and DBP, change in mean BP awake and asleep. Outcomes were assessed at baseline and 48 hours before end of treatment (BP) or 3 months after treatment (ESS). BP was measured every 30 minutes for 48 hours using cuff inflation; the second 24 hours of data were used. |
Total | CPAP | Comparator | Age | 50.8 (SE 1.7) | 50.3 (SE 1.6) | 51.2 (SE 1.8) | Sex | Male (n = 43), female (n = 13) | Male (n = 22), female (n = 6) | Male (n = 21), female (n = 7) | AHI | 31.2 (SE 2.2) | 32.9 (SE 3.2) | 29.5 (SE 3.1) | ESS | 11.1 (SE 0.7) | 10.7 (SE 1.0) | 11.6 (1.0) | BMI | 27.2 (SE 0.5) | 27.5 (SE 0.6) | 26.9 (SE 0.7) | BP | 24-hour SBP | 123.7 (SE 1.8) | 125.4 (SE 2.6) | 122.0 (SE 2.7) | 24-hour DBP | 80.9 (SE 1.2) | 81.8 (SE 1.9) | 80.0 (SE 1.7) | 24-hour MAP | 95.2 (SE 1.3) | 96.2 (SE 1.8) | 94.1 (SE 2.0) | Wake time SBP | 127.8 (SE 1.8) | 128.6 (SE 2.6) | 127.2 (SE 2.7) | Wake time DBP | 83.6 (SE 1.2) | 83.7 (SE 1.9) | 83.7 (SE 1.7) | Wake time MAP | 98.1 (SE 1.5) | 98.3 (SE 1.8) | 97.9 (SE 1.9) | Sleep time SBP | 115.7 (SE 2.0) | 117.7 (SE 2.9) | 113.9 (SE 3.0) | Sleep time DBP | 74.8 (SE 1.4) | 75.8 (SE 2.1) | 74.1 (SE 2.0) | Sleep time MAP | 89.1 (SE 1.5) | 90.6 (SE 2.1) | 87.8 (SE 2.3) | ||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 50.8 (SE 1.7) | 50.3 (SE 1.6) | 51.2 (SE 1.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 43), female (n = 13) | Male (n = 22), female (n = 6) | Male (n = 21), female (n = 7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 31.2 (SE 2.2) | 32.9 (SE 3.2) | 29.5 (SE 3.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 11.1 (SE 0.7) | 10.7 (SE 1.0) | 11.6 (1.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 27.2 (SE 0.5) | 27.5 (SE 0.6) | 26.9 (SE 0.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-hour SBP | 123.7 (SE 1.8) | 125.4 (SE 2.6) | 122.0 (SE 2.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-hour DBP | 80.9 (SE 1.2) | 81.8 (SE 1.9) | 80.0 (SE 1.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-hour MAP | 95.2 (SE 1.3) | 96.2 (SE 1.8) | 94.1 (SE 2.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wake time SBP | 127.8 (SE 1.8) | 128.6 (SE 2.6) | 127.2 (SE 2.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wake time DBP | 83.6 (SE 1.2) | 83.7 (SE 1.9) | 83.7 (SE 1.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wake time MAP | 98.1 (SE 1.5) | 98.3 (SE 1.8) | 97.9 (SE 1.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sleep time SBP | 115.7 (SE 2.0) | 117.7 (SE 2.9) | 113.9 (SE 3.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sleep time DBP | 74.8 (SE 1.4) | 75.8 (SE 2.1) | 74.1 (SE 2.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sleep time MAP | 89.1 (SE 1.5) | 90.6 (SE 2.1) | 87.8 (SE 2.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Jenkinson et al. , 199977 (related papers118,319–324) Setting. UK Design. Parallel group trial Duration. 4 weeks Inclusion criteria. Men aged 30–75 years with ESS ≥ 10 and ODI (SaO2) > 4% |
CPAP (autotitrating). Adherence: 5.4 (2.2–7.4) hours/night Comparator. Sham CPAP (pressure: approx. 1 cmH2O). Adherence: 4.6 (0.7–8.5) hours/night |
Number randomised. Total: 107 (CPAP: 54, comparator: 53) Number of withdrawals. Total: 6 (CPAP: 2, comparator: 4) Reasons for withdrawals. CPAP: did not use nCPAP and failed to reattend (n = 2); Comparator: did not use nCPAP and failed to reattend (n = 3), unexplained collapse (n = 1) Baseline characteristics TotalCPAPComparatorAge49 (34–70) 50 (33–71)48 (36–68)Sex Male (n = 107)Male (n = 54)Male (n = 53)AHINot assessed––ESSNR16.0 (10.7–21.7)17 (10.0–23.0)BMI35 (26–50)35.1 (25.8–44.3)35.0 (26.9–51.4)BP 4% SaO2 (dips per hour)31 (13–65) 32.9 (15.5–63.4)28.5 (10.7–68.7)MWT22.5 (7.6–40.0)20.0 (3.5–40.0)SF-36* Mental component44.8 (SD 10.4)43.5 (SD 10.7) Physical component43.7 (SD 11.6)42.6 (SD 10.1) General health perception59.2 (SD 18.4)59.5 (SD 20.4) Physical functioning80.9 (SD 22.7)78.6 (SD 22.1) Social functioning73.5 (SD 26.1)73.0 (SD 26.1) Physical role62.0 (SD 37.2)58.7 (SD 37.0) Mental role73.7 (SD 33.2)68.7 (SD 36.3) Bodily pain82.1 (SD 23.8)76.2 (SD 25.5) Mental health73.2 (SD 16.8)68.7 (SD 18.2) Energy and vitality35.4 (SD 22.4)33.9 (SD 17.5) *With exception of mean (SD) data are median (5th–95th centiles) Additional information. Outcomes include ESS, MWT, daytime saturation, SF-36. Related paper reports cognitive outcomes (Hack et al. , 2000118) (see Table 53). |
Total | CPAP | Comparator | Age | 49 (34–70) | 50 (33–71) | 48 (36–68) | Sex | Male (n = 107) | Male (n = 54) | Male (n = 53) | AHI | Not assessed | – | – | ESS | NR | 16.0 (10.7–21.7) | 17 (10.0–23.0) | BMI | 35 (26–50) | 35.1 (25.8–44.3) | 35.0 (26.9–51.4) | BP | 4% SaO2 (dips per hour) | 31 (13–65) | 32.9 (15.5–63.4) | 28.5 (10.7–68.7) | MWT | 22.5 (7.6–40.0) | 20.0 (3.5–40.0) | SF-36* | Mental component | 44.8 (SD 10.4) | 43.5 (SD 10.7) | Physical component | 43.7 (SD 11.6) | 42.6 (SD 10.1) | General health perception | 59.2 (SD 18.4) | 59.5 (SD 20.4) | Physical functioning | 80.9 (SD 22.7) | 78.6 (SD 22.1) | Social functioning | 73.5 (SD 26.1) | 73.0 (SD 26.1) | Physical role | 62.0 (SD 37.2) | 58.7 (SD 37.0) | Mental role | 73.7 (SD 33.2) | 68.7 (SD 36.3) | Bodily pain | 82.1 (SD 23.8) | 76.2 (SD 25.5) | Mental health | 73.2 (SD 16.8) | 68.7 (SD 18.2) | Energy and vitality | 35.4 (SD 22.4) | 33.9 (SD 17.5) | ||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 49 (34–70) | 50 (33–71) | 48 (36–68) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 107) | Male (n = 54) | Male (n = 53) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | NR | 16.0 (10.7–21.7) | 17 (10.0–23.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 35 (26–50) | 35.1 (25.8–44.3) | 35.0 (26.9–51.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4% SaO2 (dips per hour) | 31 (13–65) | 32.9 (15.5–63.4) | 28.5 (10.7–68.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MWT | 22.5 (7.6–40.0) | 20.0 (3.5–40.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36* | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental component | 44.8 (SD 10.4) | 43.5 (SD 10.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical component | 43.7 (SD 11.6) | 42.6 (SD 10.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General health perception | 59.2 (SD 18.4) | 59.5 (SD 20.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical functioning | 80.9 (SD 22.7) | 78.6 (SD 22.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social functioning | 73.5 (SD 26.1) | 73.0 (SD 26.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical role | 62.0 (SD 37.2) | 58.7 (SD 37.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental role | 73.7 (SD 33.2) | 68.7 (SD 36.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bodily pain | 82.1 (SD 23.8) | 76.2 (SD 25.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental health | 73.2 (SD 16.8) | 68.7 (SD 18.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Energy and vitality | 35.4 (SD 22.4) | 33.9 (SD 17.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Jokic et al. , 1999 108 Setting. Canada Design. Crossover trial Duration. Two × 2 weeks (no washout period) Notes. Unclear how many originally eligible Inclusion criteria. Outpatients referred with daytime sleepiness. Postural OSA (AHI during supine sleep that was two or more times AHI during sleep in the lateral position; AHI < 15 in lateral position; daytime sleepiness) |
CPAP (following manual titration) Comparator. Postural therapy with backpack with soft ball inside to prevent subjects from sleeping supine |
Number randomised. Total: 14 participants completed study Number of withdrawals. Total: 1 (exclusion) (CPAP: NR, comparator: NR) Reasons for withdrawals. NR Baseline characteristics. n = 13 TotalCPAPComparatorAge51 (SD 9)SexMale (n = 12), female (n = 1)AHI17 (SD 8)ESS13 (SD 1.3)BMI30 (SD 4)BPNot assessed Additional information. Outcomes include MWT, ESS, HADS, UMACL, NHP, TMT A and B, GHQ, SDMT, WMS I and II, and cognitive outcomes (see Table 53). |
Total | CPAP | Comparator | Age | 51 (SD 9) | Sex | Male (n = 12), female (n = 1) | AHI | 17 (SD 8) | ESS | 13 (SD 1.3) | BMI | 30 (SD 4) | BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 51 (SD 9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 12), female (n = 1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 17 (SD 8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 13 (SD 1.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 30 (SD 4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
L’Estrange et al. , 1999 104 Setting. UK Design. Crossover trial Duration. Two × 2 months (washout period not reported) Notes. Conference abstract – insufficient outcome information Inclusion criteria. Patients with severe OSAHS (AHI > 50) |
CPAP. Adherence: NR Comparator. Oral appliance (mandibular advancement splint) |
Number randomised. Total: 15 Number of withdrawals. Total: unclear, appears that six participants were not included in the analysis Reasons for withdrawals. Two participants failed to tolerate CPAP and four participants failed to complete OA treatment period Baseline characteristics TotalCPAPComparatorAge52.9 (± 6.3)SexNRAHI63.7 (± 10.0)ESS17.2 (± 3.8)BMI34.2 (± 7.2)BPNot assessed Additional information. Outcomes include ESS and AHI. |
Total | CPAP | Comparator | Age | 52.9 (± 6.3) | Sex | NR | AHI | 63.7 (± 10.0) | ESS | 17.2 (± 3.8) | BMI | 34.2 (± 7.2) | BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 52.9 (± 6.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 63.7 (± 10.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 17.2 (± 3.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 34.2 (± 7.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Lam et al. , 200770 (related papers325,326) Setting. Hong Kong Design. Parallel group trial Duration. 10 weeks Inclusion criteria. Patients with mild to moderate OSA Exclusion criteria. Sleepiness which may present a risk to self or others, unstable medical disease, co-existence of sleep disorders other than OSA, previous surgery to upper airway (except for nasal problems) and pregnant women |
CPAP (at a pre-titrated pressure) and conservative management (advice on sleep hygiene and attendance at a weight control programme if overweight) plus CPAP Adherence to active treatment: 4.2 (SEM 0.1) hours/night and 4.4 (SEM 0.1) nights/week Comparator. CM (advice on sleep hygiene and attendance at a weight control programme if overweight using Asian criteria of BMI ≥ 23 kg/m²); CM plus OA: tailor-made non-adjustable device made of dental acrylic from a functional activator (Harvold type) Adherence to active treatment (self-reported): 6.4 (SEM 0.2) hours/night and 5.2 (SEM 0.3) nights/week |
Number randomised. Total: 101 (CPAP: 34, OA: 34, CM: 33) Number of withdrawals. Total: 10 (CPAP: 1, OA: 4, CM: 5) Reasons for withdrawals. CPAP: intolerance of device (n = 1), OA: gum problems (n = 4), CM: refused final PSG (n = 5) Baseline characteristics CPAPOACMAge45 (SE 1)45 (SE 2)47 (SE 2)SexMale (n = 27), female (n = 7)Male (n = 26), female (n = 8)Male (n = 26), female (n = 7)AHI23.8 (SE 1.9)20.9 (SE 1.7) 19.3 (SE 1.9)ESS12 (SE 1)12 (SE 1)12 (SE 1)BMI27.6 (SE 0.6)27.3 (SE 0.6)27.3 (SE 0.6)BP Morning SBP127.9 (SE 2.3)127.1 (SE 2.6)125.5 (SE 3.5) Morning DBP77.0 (SE 1.8)76.2 (SE 2.1)74.2 (SE 2.4) Evening SBP130.9 (SE 2.4)131.9 (SE 3.1)127.2 (SE 3.2) Evening DBP78.0 (SE 1.9)77.8 (SE 2.2)73.5 (SE 1.9) Additional information. 19 participants were hypertensive and on treatment [CPAP (n = 7), OA (n = 4), CM (n = 8)]. There was no change in antihypertensive medications during the study period. Outcomes included AHI, ESS, QoL (SF-36, SAQLI), morning BP (8–9 a.m.), evening BP (8–9 p.m.), treatment adherence (assessed at 4 weeks and 10 weeks) and adverse events. BP was the average of three readings taken at 1-minute intervals. |
CPAP | OA | CM | Age | 45 (SE 1) | 45 (SE 2) | 47 (SE 2) | Sex | Male (n = 27), female (n = 7) | Male (n = 26), female (n = 8) | Male (n = 26), female (n = 7) | AHI | 23.8 (SE 1.9) | 20.9 (SE 1.7) | 19.3 (SE 1.9) | ESS | 12 (SE 1) | 12 (SE 1) | 12 (SE 1) | BMI | 27.6 (SE 0.6) | 27.3 (SE 0.6) | 27.3 (SE 0.6) | BP | Morning SBP | 127.9 (SE 2.3) | 127.1 (SE 2.6) | 125.5 (SE 3.5) | Morning DBP | 77.0 (SE 1.8) | 76.2 (SE 2.1) | 74.2 (SE 2.4) | Evening SBP | 130.9 (SE 2.4) | 131.9 (SE 3.1) | 127.2 (SE 3.2) | Evening DBP | 78.0 (SE 1.9) | 77.8 (SE 2.2) | 73.5 (SE 1.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CPAP | OA | CM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 45 (SE 1) | 45 (SE 2) | 47 (SE 2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 27), female (n = 7) | Male (n = 26), female (n = 8) | Male (n = 26), female (n = 7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 23.8 (SE 1.9) | 20.9 (SE 1.7) | 19.3 (SE 1.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 12 (SE 1) | 12 (SE 1) | 12 (SE 1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 27.6 (SE 0.6) | 27.3 (SE 0.6) | 27.3 (SE 0.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Morning SBP | 127.9 (SE 2.3) | 127.1 (SE 2.6) | 125.5 (SE 3.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Morning DBP | 77.0 (SE 1.8) | 76.2 (SE 2.1) | 74.2 (SE 2.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Evening SBP | 130.9 (SE 2.4) | 131.9 (SE 3.1) | 127.2 (SE 3.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Evening DBP | 78.0 (SE 1.9) | 77.8 (SE 2.2) | 73.5 (SE 1.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Lim 2005 110 Setting. USA Design. Parallel group trial Duration. 4 weeks Notes. Abstract – insufficient outcome data Inclusion criteria. Adults with chronic daily headache and AHI ≥ 5 |
CPAP. Adherence :NR Comparator. Conservative management |
Number randomised. Total: 23 (CPAP: 12, comparator: 11) Number of withdrawals. No dropouts were reported Reasons for withdrawals. NA Baseline characteristics TotalCPAPComparatorAgeNRSexMale (n = 5), female (n = 18)AHINRESSNot assessedBMINot assessedBPNot assessed Additional information. There were no changes in medication throughout the study period. |
Total | CPAP | Comparator | Age | NR | Sex | Male (n = 5), female (n = 18) | AHI | NR | ESS | Not assessed | BMI | Not assessed | BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 5), female (n = 18) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Lojander et al. , 199699 (related paper113) Setting. Finland Design. Parallel group trial Duration. 52 weeks Notes. Patients were reviewed by a panel of experts and allocated to two groups: candidates for UPP surgery (n = 23) and candidates for CPAP (n = 27). The latter group was randomly allocated to CPAP or conservative follow-up Inclusion criteria. Patients with OSA and BMI of < 40 kg/m2 |
CPAP. Nasal CPAP (initiated in the hospital). Adherence: 9 out of 10 participants complied with CPAP use (a minimum of 4 hours/night for at least five nights/week) Comparator. Conservative management (avoidance of alcohol at bedtime and weight reduction) |
Number randomised. Total: 27 (CPAP: 10, comparator: 17) Number of withdrawals. Total: 9 (CPAP: unclear, comparator: unclear) Reasons for withdrawals. Refused to attend follow-up visits due to relocation or other personal reason (n = 5). Four participants randomised to CM were put on CPAP (n = 3) or operated on (n = 1) due to a worsening of symptoms Baseline characteristics TotalCPAPComparatorAge50 (41–60)51 (43–65)SexNRNRNRAHINot assessed––ESSNot assessed––BMI 31 (25–38)33 (26–41)BPNot assessed––ODI 4% 31 (10–67)26 (11–96)Daytime somnolence (VAS)31 (21–80)58 (8–90) Data are median (range) Additional information. Follow-up at 3 and 12 months: ODI 4%, ODI 10%, VAS for sleepiness, frequency and loudness of snoring questionnaire. Cognitive outcomes were also reported (see Table 53). |
Total | CPAP | Comparator | Age | 50 (41–60) | 51 (43–65) | Sex | NR | NR | NR | AHI | Not assessed | – | – | ESS | Not assessed | – | – | BMI | 31 (25–38) | 33 (26–41) | BP | Not assessed | – | – | ODI 4% | 31 (10–67) | 26 (11–96) | Daytime somnolence (VAS) | 31 (21–80) | 58 (8–90) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 50 (41–60) | 51 (43–65) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | NR | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 31 (25–38) | 33 (26–41) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ODI 4% | 31 (10–67) | 26 (11–96) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Daytime somnolence (VAS) | 31 (21–80) | 58 (8–90) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Marshall et al. , 200579 (related papers327,328) Setting. New Zealand Design. Crossover trial Duration. Two × 3 weeks (2-week washout period) Inclusion criteria. AHI 5–30, age ≥18 years; daytime symptoms of sleepiness/ESS ≥ 8, CPAP naïve, English-speaking |
CPAP. Humidified CPAP (following manual titration overnight). Adherence (machine running time): 4.9 hours/night (range 0–8.4) Comparator. Humidified sham CPAP (pressure: < 1 cmH2O). Adherence (machine running time): 4.9 hours/night (range 0–8.32) |
Number randomised. Total: 31 Number of withdrawals. Total: 2 (CPAP: 1, comparator: 1) Reasons for withdrawals. Non-fatal MI during sham treatment (n = 1), and CPAP intolerant (n = 1) Baseline characteristics (completers only, n = 29) TotalCPAPComparatorAge range26–67 SexMale (n = 22), female (n = 7)AHI22 (14.5)ESS12.5 (SE 0.8)BMINot assessedBPNot assessedMWT20.9 (SE 2.5)SF-36 Mental health75 (SE 3) Bodily pain73 (SE 4) Social functioning79 (SE 4) Vitality44 (SE 3) Role – emotional78 (SE 7) Role – physical63 (SE 8) Physical functioning82 (SE 3) General health74 (SE 3)HADS Anxiety6.8 (SE 0.7) Depression4.2 (SE 0.5)FOSQ Total12.6 (SE 0.3) Activity3.0 (SE 0.1) Social outcomes3.2 (SE 0.1) Vigilance3.0 (SE 0.1) General product3.3 (SE 0.1) Additional information. Outcomes: ESS, FOSQ, SF-36, HADS (anxiety), HADS (depression), MWT and cognitive function (see Table 53). |
Total | CPAP | Comparator | Age range | 26–67 | Sex | Male (n = 22), female (n = 7) | AHI | 22 (14.5) | ESS | 12.5 (SE 0.8) | BMI | Not assessed | BP | Not assessed | MWT | 20.9 (SE 2.5) | SF-36 | Mental health | 75 (SE 3) | Bodily pain | 73 (SE 4) | Social functioning | 79 (SE 4) | Vitality | 44 (SE 3) | Role – emotional | 78 (SE 7) | Role – physical | 63 (SE 8) | Physical functioning | 82 (SE 3) | General health | 74 (SE 3) | HADS | Anxiety | 6.8 (SE 0.7) | Depression | 4.2 (SE 0.5) | FOSQ | Total | 12.6 (SE 0.3) | Activity | 3.0 (SE 0.1) | Social outcomes | 3.2 (SE 0.1) | Vigilance | 3.0 (SE 0.1) | General product | 3.3 (SE 0.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age range | 26–67 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 22), female (n = 7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 22 (14.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 12.5 (SE 0.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MWT | 20.9 (SE 2.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental health | 75 (SE 3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bodily pain | 73 (SE 4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social functioning | 79 (SE 4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitality | 44 (SE 3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role – emotional | 78 (SE 7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role – physical | 63 (SE 8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical functioning | 82 (SE 3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General health | 74 (SE 3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HADS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Anxiety | 6.8 (SE 0.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Depression | 4.2 (SE 0.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FOSQ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 12.6 (SE 0.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Activity | 3.0 (SE 0.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social outcomes | 3.2 (SE 0.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vigilance | 3.0 (SE 0.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General product | 3.3 (SE 0.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
McArdle and Douglas, 2001 95 Setting. UK Design. Crossover trial Duration. Two × 4 weeks. No washout phase described Inclusion criteria. Men and women with AHI> 15 and at least two symptoms of obstructive sleep apnoea Exclusion criteria. Shift workers, those with driving problems due to sleepiness, consumption of > 21 g alcohol/week, medication/comorbidity likely to disturb sleep quality |
CPAP. Adherence: NR Comparator. Oral placebo (lactose capsule) |
Number randomised. Total: 23 Number of withdrawals. Total: 1 (CPAP: 1, comparator: 0) Reasons for withdrawals. Refused to continue with CPAP treatment Baseline characteristics TotalCPAPComparatorAge53 (SD 11)SexMale (n = 20), female (n = 3)AHI (median)40 (IQR 25–65)ESS (median)14 (IQR 10–17)BMI31 (SD 5)BPNot assessed Additional information. Outcomes: ESS; sleep efficiency. |
Total | CPAP | Comparator | Age | 53 (SD 11) | Sex | Male (n = 20), female (n = 3) | AHI (median) | 40 (IQR 25–65) | ESS (median) | 14 (IQR 10–17) | BMI | 31 (SD 5) | BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 53 (SD 11) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 20), female (n = 3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI (median) | 40 (IQR 25–65) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS (median) | 14 (IQR 10–17) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 31 (SD 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Monasterio et al. , 2001100 (related paper329) Setting. Spain. Design. Parallel group trial Duration. 24 weeks Inclusion criteria. Men and women with AHI 10–30 and absence of severe daytime sleepiness Exclusion criteria. Apnoea index > 20, hazardous jobs, notable cardiovascular disease and conditions that may affect cognitive or quality of life evaluation (severe neurological or psychiatric disorder, severe chronic disease or illiteracy) |
CPAP. CPAP plus CM. Adherence: 4.8 hours/night (SD 2.2) at 6 months; at 6 months 64% of participants used CPAP for > 4 hours/night Comparator. CM: weight loss programme following home diet if BMI > 27, avoidance of sedatives and alcohol consumption, supine position during sleep and adequate hours of sleep every night |
Number randomised. Total: 142 (CPAP: 77, comparator: 65) Number of withdrawals. Total: 17 (CPAP: 11, comparator: 7) Reasons for withdrawals. NR Baseline characteristics TotalCPAPComparatorAge54 (SD 9)53 (SD 9)54 (SD 9)Sex (%)Male (86%)Male (81%)Male (91%)AHI20 (SD 6)20 (SD 6)21 (SD 6)ESS12.6 (SD 4.6)12.1 (SD 4.9)13.2 (SD 4.3)BMI29 (SD 4)29.4 (SD 3.7)29.5 (SD 3.3)BP SBP126 (SD 17)132 (SD 17) DBP81 (SD 12)84 (SD 11)FOSQ101 (SD 18)100 (SD 15)NHP21 (SD 20)20 (SD 16)MSLT (minutes)10 (SD 5)11 (SD 5)SAHS-related symptoms21 (SD 4)21 (SD 3) Additional information. Outcomes were measured at study entry and after 3 months and 6 months of treatment. Outcomes included symptom measures (SAHS symptom questionnaire), sleepiness (ESS, MSLT), quality of life (NHP, FOSQ) and cognitive function (see Table 53). |
Total | CPAP | Comparator | Age | 54 (SD 9) | 53 (SD 9) | 54 (SD 9) | Sex (%) | Male (86%) | Male (81%) | Male (91%) | AHI | 20 (SD 6) | 20 (SD 6) | 21 (SD 6) | ESS | 12.6 (SD 4.6) | 12.1 (SD 4.9) | 13.2 (SD 4.3) | BMI | 29 (SD 4) | 29.4 (SD 3.7) | 29.5 (SD 3.3) | BP | SBP | 126 (SD 17) | 132 (SD 17) | DBP | 81 (SD 12) | 84 (SD 11) | FOSQ | 101 (SD 18) | 100 (SD 15) | NHP | 21 (SD 20) | 20 (SD 16) | MSLT (minutes) | 10 (SD 5) | 11 (SD 5) | SAHS-related symptoms | 21 (SD 4) | 21 (SD 3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 54 (SD 9) | 53 (SD 9) | 54 (SD 9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex (%) | Male (86%) | Male (81%) | Male (91%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 20 (SD 6) | 20 (SD 6) | 21 (SD 6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 12.6 (SD 4.6) | 12.1 (SD 4.9) | 13.2 (SD 4.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 29 (SD 4) | 29.4 (SD 3.7) | 29.5 (SD 3.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SBP | 126 (SD 17) | 132 (SD 17) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DBP | 81 (SD 12) | 84 (SD 11) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FOSQ | 101 (SD 18) | 100 (SD 15) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NHP | 21 (SD 20) | 20 (SD 16) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSLT (minutes) | 10 (SD 5) | 11 (SD 5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAHS-related symptoms | 21 (SD 4) | 21 (SD 3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Montserrat et al. , 2001 86 Setting. Spain Design. Partial crossover trial Duration. 6 weeks Notes. CPAP group had 6 weeks study period using the intervention; sham CPAP group trialled 6 weeks on each intervention. 10-day washout period Inclusion criteria. Patients previously diagnosed with AHI > 10 and excessive daytime somnolence Exclusion criteria. Severe or unstable cardiovascular disease or hazardous job (professional driver or handling dangerous machinery) |
CPAP (following overnight manual titration). Adherence: 4.25 hours/night Comparator. Sham CPAP All participants were encouraged to follow a diet and sleep regimen regardless of treatment group assigned |
Number randomised. Total: 48 (CPAP: 24, comparator: 24) [baseline data for age, AHI and BMI (n = 45), other outcomes (n = 46)] Number of withdrawals. Total: 2 (first treatment period), 1 (crossover) [CPAP: 2 (n = 1 first treatment period, n = 1 crossover), comparator: 1) Reasons for withdrawals. NR Baseline characteristics TotalCPAPComparatorAge55.65 (SD 9.41)52.59 (SD 10.93)SexNRNRNRAHI50.52 (SD 19.83)57.14 (SD 21.14)ESS16.13 (SD 1.03)16.86 (SD 1.20)BMI30.31 (SD 4.49)33.73 (SD 6.62)BPNot assessed––SF-36 PCS46.53 (SD 1.92)45.54 (SD 2.17) MCS48.21 (SD 2.06)48.73 (SD 2.49) Physical functioning76.96 (SD 5.66)78.18 (SD 4.80) Role – physical71.74 (SD 8.79)78.41 (SD 8.11) Bodily pain75.26 (SD 5.26)58.32 (SD 7.27) General health60.48 (SD 3.05)61.55 (SD 5.61) Vitality56.52 (SD 5.93)58.03 (SD 6.23) Social functioning82.61 (SD 3.91)82.39 (SD 5.37) Role – emotional84.06 (SD 7.52)75.76 (SD 8.55) Mental health71.83 (SD 3.93)77.45 (SD 3.67)FOSQ General productivity19.18 (SD 1.06)20.25 (SD 0.89) Social18.96 (SD 1.26)18.00 (SD 1.66) Activity level16.71 (SD 0.81)17.21 (SD 1.23) Vigilance13.66 (SD 1.16)14.17 (SD 1.48) Intimacy16.40 (SD 1.57)17.43 (SD 1.63) Total score84.45 (SD 4.63)86.16 (SD 5.96)SAHS-related symptoms39.70 (SD 1.14)38.86 (SD 1.14) Additional information. Outcomes were measured on the last day of each treatment: ESS, SF-36, FOSQ. |
Total | CPAP | Comparator | Age | 55.65 (SD 9.41) | 52.59 (SD 10.93) | Sex | NR | NR | NR | AHI | 50.52 (SD 19.83) | 57.14 (SD 21.14) | ESS | 16.13 (SD 1.03) | 16.86 (SD 1.20) | BMI | 30.31 (SD 4.49) | 33.73 (SD 6.62) | BP | Not assessed | – | – | SF-36 | PCS | 46.53 (SD 1.92) | 45.54 (SD 2.17) | MCS | 48.21 (SD 2.06) | 48.73 (SD 2.49) | Physical functioning | 76.96 (SD 5.66) | 78.18 (SD 4.80) | Role – physical | 71.74 (SD 8.79) | 78.41 (SD 8.11) | Bodily pain | 75.26 (SD 5.26) | 58.32 (SD 7.27) | General health | 60.48 (SD 3.05) | 61.55 (SD 5.61) | Vitality | 56.52 (SD 5.93) | 58.03 (SD 6.23) | Social functioning | 82.61 (SD 3.91) | 82.39 (SD 5.37) | Role – emotional | 84.06 (SD 7.52) | 75.76 (SD 8.55) | Mental health | 71.83 (SD 3.93) | 77.45 (SD 3.67) | FOSQ | General productivity | 19.18 (SD 1.06) | 20.25 (SD 0.89) | Social | 18.96 (SD 1.26) | 18.00 (SD 1.66) | Activity level | 16.71 (SD 0.81) | 17.21 (SD 1.23) | Vigilance | 13.66 (SD 1.16) | 14.17 (SD 1.48) | Intimacy | 16.40 (SD 1.57) | 17.43 (SD 1.63) | Total score | 84.45 (SD 4.63) | 86.16 (SD 5.96) | SAHS-related symptoms | 39.70 (SD 1.14) | 38.86 (SD 1.14) | ||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 55.65 (SD 9.41) | 52.59 (SD 10.93) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | NR | NR | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 50.52 (SD 19.83) | 57.14 (SD 21.14) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 16.13 (SD 1.03) | 16.86 (SD 1.20) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 30.31 (SD 4.49) | 33.73 (SD 6.62) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PCS | 46.53 (SD 1.92) | 45.54 (SD 2.17) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MCS | 48.21 (SD 2.06) | 48.73 (SD 2.49) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical functioning | 76.96 (SD 5.66) | 78.18 (SD 4.80) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role – physical | 71.74 (SD 8.79) | 78.41 (SD 8.11) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bodily pain | 75.26 (SD 5.26) | 58.32 (SD 7.27) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General health | 60.48 (SD 3.05) | 61.55 (SD 5.61) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitality | 56.52 (SD 5.93) | 58.03 (SD 6.23) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social functioning | 82.61 (SD 3.91) | 82.39 (SD 5.37) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role – emotional | 84.06 (SD 7.52) | 75.76 (SD 8.55) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental health | 71.83 (SD 3.93) | 77.45 (SD 3.67) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FOSQ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General productivity | 19.18 (SD 1.06) | 20.25 (SD 0.89) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social | 18.96 (SD 1.26) | 18.00 (SD 1.66) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Activity level | 16.71 (SD 0.81) | 17.21 (SD 1.23) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vigilance | 13.66 (SD 1.16) | 14.17 (SD 1.48) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Intimacy | 16.40 (SD 1.57) | 17.43 (SD 1.63) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total score | 84.45 (SD 4.63) | 86.16 (SD 5.96) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAHS-related symptoms | 39.70 (SD 1.14) | 38.86 (SD 1.14) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Norman et al. , 200673 (related papers 116,302,330–335) Setting. USA Design. Parallel group trial Duration. 2 weeks Notes. At time of initial screening, CPAP group had a higher baseline SBP than sham CPAP group (p = 0.042). Participants whose BP was > 170/105 mmHg were excluded and re-started on BP medication Inclusion criteria. Men and women between 25 and 65 years within 100–170% of their ideal body weight, with AHI > 15 Exclusion criteria. Major ongoing illness other than sleep apnoea and hypertension. Individuals who had had previous treatment with CPAP or undergone pharyngeal surgery for OSA were also excluded |
CPAP. Humidified CPAP (following manual titration) plus an oxygen concentrator that provided room air. Adherence: 6.7 hours/night (SE 1.2) Comparator. Sham CPAP (0–0.5 cmH2O) following mock titration plus an oxygen concentrator that provided room air. Adherence: 6.0 hours/night (SE 2.4) Supplemented oxygen (3 l/m oxygen concentrator) plus sham CPAP. Adherence: 6.7 hours/night (SE 1.2) |
Number randomised. Total: 46 (CPAP: 18, sham CPAP: 15, oxygen: 13) Number of withdrawals. No withdrawals reported Reasons for withdrawals. NA Baseline characteristics TotalCPAPSham CPAPAge49.7 (SE 2.5)49.3 (SE 2.7)SexMale (n = 15), female (n = 3)Male (n = 13), female (n = 2)AHI66.1 (SD 29.1)53.9 (SD 29.8)ESS12.0 (SE 1.3)12.0 (SE 1.7)BMI31.5 (SE 1.4)29.9 (SE 1.3)BP SBP135.1 (SE 3.8)122.5 (SE 3.3) DBP79.6 (SE 1.7)75.6 (SE 2.5) MAP98.1 (SE 2.5)91.2 (SE 2.5) Additional information. Outcomes: BP (mean 24-hour ambulatory BP, daytime SBP and DBP, night-time SBP and DBP), AHI and various polysomnographic parameters. BP was taken every 15 minutes between 6 a.m. and 10 p.m. (daytime) and every 30 minutes between 10 p.m. and 6 a.m. (night-time) using a cuff. Related papers report psychological symptoms (Bardwell et al., 2007334) and cognitive outcomes (Lim et al., 2005116) (see Table 53). |
Total | CPAP | Sham CPAP | Age | 49.7 (SE 2.5) | 49.3 (SE 2.7) | Sex | Male (n = 15), female (n = 3) | Male (n = 13), female (n = 2) | AHI | 66.1 (SD 29.1) | 53.9 (SD 29.8) | ESS | 12.0 (SE 1.3) | 12.0 (SE 1.7) | BMI | 31.5 (SE 1.4) | 29.9 (SE 1.3) | BP | SBP | 135.1 (SE 3.8) | 122.5 (SE 3.3) | DBP | 79.6 (SE 1.7) | 75.6 (SE 2.5) | MAP | 98.1 (SE 2.5) | 91.2 (SE 2.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Sham CPAP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 49.7 (SE 2.5) | 49.3 (SE 2.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 15), female (n = 3) | Male (n = 13), female (n = 2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 66.1 (SD 29.1) | 53.9 (SD 29.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 12.0 (SE 1.3) | 12.0 (SE 1.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 31.5 (SE 1.4) | 29.9 (SE 1.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SBP | 135.1 (SE 3.8) | 122.5 (SE 3.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DBP | 79.6 (SE 1.7) | 75.6 (SE 2.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MAP | 98.1 (SE 2.5) | 91.2 (SE 2.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Olson et al. , 2002107 (unpublished data from Gileset al. , 2006) Setting. NR Design. Crossover trial Duration. Two × 6 weeks (2-week washout period) Inclusion criteria. AHI > 15, or apnoea index > 5, or AHI > 5 and arousal index > 15 Exclusion criteria. Poor dentition, temporomandibular joint pain, or previous treatment with oral appliances or CPAP |
CPAP. Adherence: NR Comparator. Oral appliance |
Number randomised. Total: unclear, 24 participants included Number of withdrawals. NR Reasons for withdrawals. NA Baseline characteristics TotalCPAPComparatorAgeNRSexNRAHI (range)8.1–36.9ESSNRBMINRBPNR Additional information. Outcomes: total sleep time, sleep efficiency, % REM sleep, AHI, arousal index, SAQLI. |
Total | CPAP | Comparator | Age | NR | Sex | NR | AHI (range) | 8.1–36.9 | ESS | NR | BMI | NR | BP | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI (range) | 8.1–36.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | NR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Pepperell et al. , 200287 (related papers336,337) Setting. UK Design. Parallel group trial Duration. 4 weeks Notes. A specialist nurse helped all participants with advice by telephone, and masks were further adjusted if necessary Inclusion criteria. Men with > 10 episodes/hour of > 4% drop in SaO2 and ESS ≥ 10 Exclusion criteria. Required urgent CPAP therapy, were about to lose their job as a result of sleepiness, declined to participate, preferred alternative treatment, unable to give informed consent |
CPAP (following overnight titration using an automated pressure setting device). Adherence: 4.5 hours/night (SD 2.4) Comparator. Subtherapeutic CPAP (pressure < 1 cmH2O). Adherence: 4.9 hours/night (SD 204) |
Number randomised. Total: 118 (CPAP: 59, comparator: 59) Number of withdrawals. Total: 14 (CPAP: 6, comparator: 8) Reasons for withdrawals. CPAP: discontinued CPAP (n = 2), did not attend post-treatment BP (n = 4); Sham CPAP: discontinued CPAP (n = 2), did not attend post-treatment BP (n = 6). In addition, post-BP recordings were inadequate in 10 participants (n = 5 per treatment arm) Baseline characteristics TotalCPAPComparatorAge50.1 (SD 10.4)51.0 (SD 9.8)SexMale (n = 118)AHINot assessedESS16.3 (SD 3.3)16.0 (SD 3.1)BMI34.6 (SD 8.5)35.3 (SD 6.0)BP SBP132.5 (SD 15.3)134.9 (SD 18.7) DBP85.1 (SD 8.7)85.1 (SD 8.9) 24-Hour mean BP101.0 (SD 9.8)101.7 (SD 10.8) Sleep period BP93.7 (SE 1.6)96.2 (SE 1.6) Wake period BP104.3 (SE 1.3)104.2 (SE 1.4) Additional information. 22 participants (11 in each group) were taking medication for hypertension. Outcomes: BP, withdrawal, ESS, AHI. BP was recorded every 30 minutes for a 24-hour period, and measured with a cuff. |
Total | CPAP | Comparator | Age | 50.1 (SD 10.4) | 51.0 (SD 9.8) | Sex | Male (n = 118) | AHI | Not assessed | ESS | 16.3 (SD 3.3) | 16.0 (SD 3.1) | BMI | 34.6 (SD 8.5) | 35.3 (SD 6.0) | BP | SBP | 132.5 (SD 15.3) | 134.9 (SD 18.7) | DBP | 85.1 (SD 8.7) | 85.1 (SD 8.9) | 24-Hour mean BP | 101.0 (SD 9.8) | 101.7 (SD 10.8) | Sleep period BP | 93.7 (SE 1.6) | 96.2 (SE 1.6) | Wake period BP | 104.3 (SE 1.3) | 104.2 (SE 1.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 50.1 (SD 10.4) | 51.0 (SD 9.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 118) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 16.3 (SD 3.3) | 16.0 (SD 3.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 34.6 (SD 8.5) | 35.3 (SD 6.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SBP | 132.5 (SD 15.3) | 134.9 (SD 18.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DBP | 85.1 (SD 8.7) | 85.1 (SD 8.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-Hour mean BP | 101.0 (SD 9.8) | 101.7 (SD 10.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sleep period BP | 93.7 (SE 1.6) | 96.2 (SE 1.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wake period BP | 104.3 (SE 1.3) | 104.2 (SE 1.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Randerath et al. , 2002105 (related paper338) Setting. Germany Design. Crossover trial Duration. Two × 6 weeks Inclusion criteria. Patients with AHI 5–30 and clinical symptoms of OSAS Exclusion criteria. AHI > 30, temporomandibular joint disorders, bruxism, participants with gaps in their teeth preventing fitting of device |
CPAP. Adherence (self-reported): > 8 hours/night, 9%; 6–7 hours/night, 27%; 4–5 hours/night, 64%; 2–3 hours/night, 0%. All participants used CPAP on at least five nights/week Comparator. Oral appliance (two thin thermoplastic plates, worn on the upper and lower jaws connected by two adjustable telescopic guide rods). The maximum forward protrusion of the mandible was measured and this amount reduced to about two-thirds before mounting the casts. Adherence (self-reported): > 8 hours/night, 33%; 6–7 hours/night, 53%; 4–5 hours/night, 7%; 2–3 hours/night, 7%. All participants used OA on at least five nights/ week |
Number randomised. Total: 20 Number of withdrawals. No dropouts reported Reasons for withdrawals. NA Baseline characteristics TotalCPAPComparatorAge56.5 (SD 10.2)SexMale (n = 16), female (n = 4)AHI17.5 (SD 7.7)ESSNot assessedBMI31.2 (SD 6.4)BPNot assessed Additional information. Outcomes: AHI; snoring (epochs/hour); SaO2 (%); TST (minutes); wake after sleep onset; sleep stages 1, 2, 3, 4; REM sleep; arousals/hour; respiration-induced arousals per hour of TST. |
Total | CPAP | Comparator | Age | 56.5 (SD 10.2) | Sex | Male (n = 16), female (n = 4) | AHI | 17.5 (SD 7.7) | ESS | Not assessed | BMI | 31.2 (SD 6.4) | BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 56.5 (SD 10.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 16), female (n = 4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 17.5 (SD 7.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 31.2 (SD 6.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Redline et al. , 1998 59 Setting. USA Design. Parallel group trial Duration. 8–16 weeks Inclusion criteria. Adults, aged 25–65 years, with mild to moderate sleep disordered breathing (RDI 5–30) without subjective pathological sleepiness Exclusion criteria. Any severe or unstable medical disease documented in previous 3 months, neurological disease, alcohol or drug abuse, regular use of medications that impair sensorium and < 8 years of education |
CPAP. CPAP plus CM (advice on sleep, posture and sleep hygiene. Weight reduction and counselling was provided to patients with a BMI > 29 kg/m2 and nasal steroid spray for those with nasal congestion). Adherence (machine usage): 44% (SD 34) of the time subjects were estimated to be asleep (3.1 hours) Comparator. Conservative treatment. Control patients were also given a supply of nasal dilators. Use of mechanical nasal dilators: 82% (SD 28) of intervention nights |
Number randomised. Total: 111 (CPAP: 59, comparator: 52) Number of withdrawals. Total: 14 (CPAP: 8, comparator: 6) Reasons for withdrawals. CPAP: three withdrew due to problems in using CPAP and five due to an inability to schedule all-day testing battery; Comparator: all withdrew due to an inability to schedule all-day testing battery Baseline characteristics (presented for completers only, n = 97) TotalCPAPComparatorAge48.1 (SD 9.2)49.2 (SD 10.5). SexMale (n = 30), female (n = 21)Male (n = 20), female (n = 26)AHINot assessed––ESS10.4 (SD 4.3)10.6 (SD 5.6)BMI33.4 (SD 6.9)32.0 (SD 8.5) BPNot assessed––RDI14.6 (SD 9.8)11.8 (SD 9.6)MSLT (minutes)9.9 (SD 4.8)10.3 (SD 5.0)POMS Fatigue score44.2 (SD 8.2)41.8 (SD 7.6)SF-36 Fatigue/energy51.7 (SD 19.8)58.3 (SD 19.0) General health perception66.4 (SD 18.2)69.8 (SD 19.5) Social role functioning88.4 (SD 18.3)91.4 (SD 14.0) Role limitations – physical70.6 (SD 34.5)88.1 (SD 22.5) Role limitation – emotional85.6 (SD 26.9)82.8 (SD 31.1)PANAS Positive affect32.9 (SD 7.3)32.3 (SD 6.8) Negative affect16.3 (SD 5.4)16.0 (SD 6.0) Additional information. Polysomnographic parameters and daytime test battery: mood (POMS, PANAS, well-being and functional status (SF-36) and objective measure of sleepiness (MSLT). Participants were evaluated at baseline and at 8–16 weeks, and after at least 2 weeks following any intercurrent illness. Change on these measures was used to calculate an overall treatment response score. ESS was also assessed. Three participants were re-tested after the 16-week period (two at 17 weeks and 1 at 19 weeks). |
Total | CPAP | Comparator | Age | 48.1 (SD 9.2) | 49.2 (SD 10.5). | Sex | Male (n = 30), female (n = 21) | Male (n = 20), female (n = 26) | AHI | Not assessed | – | – | ESS | 10.4 (SD 4.3) | 10.6 (SD 5.6) | BMI | 33.4 (SD 6.9) | 32.0 (SD 8.5) | BP | Not assessed | – | – | RDI | 14.6 (SD 9.8) | 11.8 (SD 9.6) | MSLT (minutes) | 9.9 (SD 4.8) | 10.3 (SD 5.0) | POMS | Fatigue score | 44.2 (SD 8.2) | 41.8 (SD 7.6) | SF-36 | Fatigue/energy | 51.7 (SD 19.8) | 58.3 (SD 19.0) | General health perception | 66.4 (SD 18.2) | 69.8 (SD 19.5) | Social role functioning | 88.4 (SD 18.3) | 91.4 (SD 14.0) | Role limitations – physical | 70.6 (SD 34.5) | 88.1 (SD 22.5) | Role limitation – emotional | 85.6 (SD 26.9) | 82.8 (SD 31.1) | PANAS | Positive affect | 32.9 (SD 7.3) | 32.3 (SD 6.8) | Negative affect | 16.3 (SD 5.4) | 16.0 (SD 6.0) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 48.1 (SD 9.2) | 49.2 (SD 10.5). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 30), female (n = 21) | Male (n = 20), female (n = 26) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 10.4 (SD 4.3) | 10.6 (SD 5.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 33.4 (SD 6.9) | 32.0 (SD 8.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RDI | 14.6 (SD 9.8) | 11.8 (SD 9.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSLT (minutes) | 9.9 (SD 4.8) | 10.3 (SD 5.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| POMS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fatigue score | 44.2 (SD 8.2) | 41.8 (SD 7.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fatigue/energy | 51.7 (SD 19.8) | 58.3 (SD 19.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General health perception | 66.4 (SD 18.2) | 69.8 (SD 19.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Social role functioning | 88.4 (SD 18.3) | 91.4 (SD 14.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role limitations – physical | 70.6 (SD 34.5) | 88.1 (SD 22.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Role limitation – emotional | 85.6 (SD 26.9) | 82.8 (SD 31.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PANAS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Positive affect | 32.9 (SD 7.3) | 32.3 (SD 6.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Negative affect | 16.3 (SD 5.4) | 16.0 (SD 6.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Robinson et al. , 200668 (related paper339) Setting. UK Design. Crossover trial Duration. Two × 4 weeks (2-week washout period) Inclusion criteria. Adults with moderate to severe OSA (without hypersomnolence) and hypertension. Hypertension was defined as BP > 140/90mmHg, or currently using hypertensive medication Exclusion criteria. Respiratory failure |
CPAP (following titration using an automated pressure device). Adherence (mean machine usage): 5.2 hours/night (SD 2.1) Comparator. Sham CPAP (pressure < 1 cmH2O). Adherence (mean machine usage): 4.3 hours/night (SD 2.4) |
Number randomised. Total: 35 Number of withdrawals. Total: 3 (CPAP: 3, comparator: 0) Reasons for withdrawals. CPAP arm: two before completing first month’s treatment period (one due to intolerance of BP cuff and one due to inadequate BP data), and one during the second treatment period due to intolerance of BP cuff Baseline characteristics TotalCPAPComparatorAge54 (SD 8)SexMale (n = 31), female (n = 4)AHINot assessedESS (median)5.3 (IQR 3.0–7.0)BMI33.2 (SD 5.3)BP 24-hour BP (mmHg)NR103.4 (SD 11.6)105.1 (SD 12.1) 24-hour SBP140.3 (SD 16.1)143.0 (SD 17.33) 24-hour DBP85.3 (SD 11.2)86.7 (SD 11.1) Wake BP106.1 (SD 13.6)108.8 (SD 13.0) Sleep BP96.0 (SD 11.5)98.0 (SD 14.8)Osler test [minutes (median)]40 (IQR 40–40)Dips in SaO2 of > 4%/hour sleep (median)28.1(IQR 18.0–38.0) Additional information. 77% (27) of participants were receiving medication for hypertension; these participants were instructed not to change their antihypertensive medication during the study period. Outcomes: oxygen saturation, ESS and 24-hour BP. Outcomes were assessed before and after each treatment period. BP was measured every 30 minutes over 24 hours using an arm cuff. |
Total | CPAP | Comparator | Age | 54 (SD 8) | Sex | Male (n = 31), female (n = 4) | AHI | Not assessed | ESS (median) | 5.3 (IQR 3.0–7.0) | BMI | 33.2 (SD 5.3) | BP | 24-hour BP (mmHg) | NR | 103.4 (SD 11.6) | 105.1 (SD 12.1) | 24-hour SBP | 140.3 (SD 16.1) | 143.0 (SD 17.33) | 24-hour DBP | 85.3 (SD 11.2) | 86.7 (SD 11.1) | Wake BP | 106.1 (SD 13.6) | 108.8 (SD 13.0) | Sleep BP | 96.0 (SD 11.5) | 98.0 (SD 14.8) | Osler test [minutes (median)] | 40 (IQR 40–40) | Dips in SaO2 of > 4%/hour sleep (median) | 28.1(IQR 18.0–38.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 54 (SD 8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 31), female (n = 4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS (median) | 5.3 (IQR 3.0–7.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 33.2 (SD 5.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-hour BP (mmHg) | NR | 103.4 (SD 11.6) | 105.1 (SD 12.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-hour SBP | 140.3 (SD 16.1) | 143.0 (SD 17.33) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24-hour DBP | 85.3 (SD 11.2) | 86.7 (SD 11.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wake BP | 106.1 (SD 13.6) | 108.8 (SD 13.0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sleep BP | 96.0 (SD 11.5) | 98.0 (SD 14.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Osler test [minutes (median)] | 40 (IQR 40–40) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dips in SaO2 of > 4%/hour sleep (median) | 28.1(IQR 18.0–38.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Skinner et al. , 2004 60 Setting. New Zealand Design. Crossover trial Duration. Two × 4 weeks (1-week washout period) Inclusion criteria. Men and women with OSA (AHI 10–60 and symptoms of daytime somnolence) Exclusion criteria. History of cardiovascular, neurological and/or psychological disorders affecting sleep; known cervical, shoulder or thoracic wall abnormalities and/or chronic pain; or previous treatment for OSA |
CPAP (the pressure was set following 3–5 nights on an automated titrating device). Adherence (machine recorded): 4.7 hours/night (SD 2.6) Comparator. Shoulder–head elevation pillow (SHEP): designed to keep the patient in an upright posture (60˚) during sleep. Adherence (self-reported): 6.3 hours/night (SD 1.6) |
Number randomised. Total: 14 Number of withdrawals. Total: 1 (CPAP: 1, comparator: 0) Reasons for withdrawals. Declined to use portable sleep monitor at end of CPAP treatment phase Baseline characteristics TotalCPAPComparatorAge54 [SD 10 (range 39–69)]SexMale (n = 12), female (n = 2)AHI27 (SD 12)ESS11.9 (SD 4.6)BMI34 (SD 7)BPNot assessedSHS (%)53.6 (SD 13.7)SF-36 Physical score42.6 (SD 11.3) Mental score47.9 (SD 12.3)FOSQ12.1 (SD 1.9) Additional information. Outcomes: ESS, AHI, FOSQ, SF-36 and Scottish SHS were assessed at study entry and at the end of each treatment period. In addition, adverse events were assessed using a questionnaire at the end of each treatment period; a questionnaire comprising 19 adverse events most likely to be associated with SHEP was administered and a similar questionnaire was completed after CPAP. |
Total | CPAP | Comparator | Age | 54 [SD 10 (range 39–69)] | Sex | Male (n = 12), female (n = 2) | AHI | 27 (SD 12) | ESS | 11.9 (SD 4.6) | BMI | 34 (SD 7) | BP | Not assessed | SHS (%) | 53.6 (SD 13.7) | SF-36 | Physical score | 42.6 (SD 11.3) | Mental score | 47.9 (SD 12.3) | FOSQ | 12.1 (SD 1.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 54 [SD 10 (range 39–69)] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 12), female (n = 2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 27 (SD 12) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 11.9 (SD 4.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 34 (SD 7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHS (%) | 53.6 (SD 13.7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical score | 42.6 (SD 11.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental score | 47.9 (SD 12.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FOSQ | 12.1 (SD 1.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Skinner et al. , 2004 61 Setting. New Zealand Design. Crossover trial Duration. Two × 4 weeks (no report of washout period) Inclusion criteria. Men and women with AHI 10–60 and mild to moderate obstructive sleep apnoea Exclusion criteria. History of cardiovascular, neurological or psychological disorders affecting sleep, known cervical or temporomandibular joint dysfunction and/or pain |
CPAP (the pressure was set following 3–5 nights on an automated titrating device). Adherence: 68% (SD 24%) of available study nights, mean nightly duration 4.4 hours (SD1.2) Comparator. Cervicomandibular collar (designed to retain head in natural head position and prevent jaw opening during sleep). Adherence: 89% (SD 23%) of total available study nights, mean nightly duration 5.2 hours (SD 1.2) |
Number randomised. Total: 10 Number of withdrawals. None Reasons for withdrawals. NA Baseline characteristics TotalCPAPComparatorAge48.6 (SD 14.8). SexMale (n = 8), female (n = 2)AHI29.4 (SD 13.4)ESS13.2 (SD 4.9)BMI34.1 (SD 5.6)BPNot assessedSF-36 Physical score45.3 (SD 10.4) Mental score43.8 (SD 13.1)FOSQ12.2 (SD 3.1)SHS59.7 (SD 11.9) Additional information. Outcomes: sleep parameters, ESS, SF-36, FOSQ and SHS were assessed before treatment and after each treatment period. Adverse effects of treatment were also assessed at the end of each treatment period (self-reported diary and questionnaire). Mean scores were annotated from responses to 19 self-reported questions (score 0–3). Overall benefit of each treatment was assessed at the end of the study. |
Total | CPAP | Comparator | Age | 48.6 (SD 14.8). | Sex | Male (n = 8), female (n = 2) | AHI | 29.4 (SD 13.4) | ESS | 13.2 (SD 4.9) | BMI | 34.1 (SD 5.6) | BP | Not assessed | SF-36 | Physical score | 45.3 (SD 10.4) | Mental score | 43.8 (SD 13.1) | FOSQ | 12.2 (SD 3.1) | SHS | 59.7 (SD 11.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 48.6 (SD 14.8). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 8), female (n = 2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 29.4 (SD 13.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 13.2 (SD 4.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 34.1 (SD 5.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SF-36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical score | 45.3 (SD 10.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mental score | 43.8 (SD 13.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FOSQ | 12.2 (SD 3.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SHS | 59.7 (SD 11.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Spicuzza et al. , 2006 66 Setting. Italy Design. Parallel group trial Duration. 4 weeks Inclusion criteria. Men and women with moderate to severe obstructive sleep apnoea Exclusion criteria. Presence of hypertension and/or other cardiovascular diseases, diabetes, thyroid disorders, chronic obstructive/restrictive lung diseases or respiratory failure, and smokers |
CPAP (following overnight titration). Adherence (machine usage): 6.0 hours (SD 1.1) Comparator. Sham CPAP (pressure 1–2 cmH2O). Adherence (machine usage): 6.5 hours (SD 2.4) |
Number randomised. Total: 25 (CPAP: 15, comparator: 10) Number of withdrawals. The authors do not report withdrawals Reasons for withdrawals. NA Baseline characteristics TotalCPAPComparatorAge55.9 (SD 9.4)55.1 (SD 9.3)SexMale (n = 20), female (n = 5)AHI55.3 (SD 11.9)59.2 (SD 17.3)ESSNot assessedBMI31.1 (SD 4.2)33.5 (SD 5.5)BP SBP145.4 (SD 4.7)149.5 (SD 7.2) DBP87.9 (SD 4.6)85.0 (SD 3.8) Additional information. Outcomes: AHI and ventilatory control measures. Outcomes were assessed at baseline and at the end of the study. |
Total | CPAP | Comparator | Age | 55.9 (SD 9.4) | 55.1 (SD 9.3) | Sex | Male (n = 20), female (n = 5) | AHI | 55.3 (SD 11.9) | 59.2 (SD 17.3) | ESS | Not assessed | BMI | 31.1 (SD 4.2) | 33.5 (SD 5.5) | BP | SBP | 145.4 (SD 4.7) | 149.5 (SD 7.2) | DBP | 87.9 (SD 4.6) | 85.0 (SD 3.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 55.9 (SD 9.4) | 55.1 (SD 9.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 20), female (n = 5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 55.3 (SD 11.9) | 59.2 (SD 17.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 31.1 (SD 4.2) | 33.5 (SD 5.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SBP | 145.4 (SD 4.7) | 149.5 (SD 7.2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DBP | 87.9 (SD 4.6) | 85.0 (SD 3.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Tan et al. , 2002106 (related papers340–342) Setting. UK Design. Crossover trial Duration. Two × 8 weeks (2-week washout) Notes. Baseline: O2 desaturation: 7.1 ± 2.7. Arousals/hour: 19.3 ± 9.6 Inclusion criteria. Men and women with AHI < 50, with adequate dentition and periodontal status for support and retention of oral appliance Exclusion criteria. Temporomandibular joint dysfunction, medical contraindications, significant heart disease, co-existent chronic obstructive pulmonary disease, regular hypnotic use, epilepsy, arterial oxygen saturation < 60% during initial sleep study, ability to give informed consent |
CPAP. Two different models of nCPAP compressor were used. Nasal corticosteroid sprays were prescribed to relieve nasal congestion where necessary. Adherence: NR Comparator. Oral appliance. A soft one-piece MAS was used for the first 10 participants. A two part semi-rigid MAS was used for the remainder of the study; this appliance permitted some mandibular opening during sleep. Adherence: NR |
Number randomised. Total: 24 Number of withdrawals. Total: 3 (CPAP: 2, comparator: 1) Reasons for withdrawals. Two participants did not tolerate nCPAP, one participant did not tolerate MAS Baseline characteristics TotalCPAPComparatorAge50.9 (SD 10.1)SexMale (n = 20), female (n = 4)AHI22.2 (SD 9.6)ESS13.4 (4.6)BMI31.9 (SD 6.8)BPNot assessed Additional information. Outcomes were measured on the last day of each treatment: ESS, AHI, ODI, REM %, sleep efficacy, preference. |
Total | CPAP | Comparator | Age | 50.9 (SD 10.1) | Sex | Male (n = 20), female (n = 4) | AHI | 22.2 (SD 9.6) | ESS | 13.4 (4.6) | BMI | 31.9 (SD 6.8) | BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 50.9 (SD 10.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 20), female (n = 4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | 22.2 (SD 9.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 13.4 (4.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 31.9 (SD 6.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
West et al. , personal communication151 (unpublished data supplied by the author) (related papers67) Setting. UK Design. Parallel group trial Duration. 12 weeks Notes. One participant in the active treatment group received a defective machine which delivered minimal pressure and was analysed with sham CPAP group Inclusion criteria. Men with OSA (> 10 SaO2 dips of greater than 4% per hour) and type 2 diabetes Exclusion criteria. Urgent CPAP required or unstable diabetes requiring an escalation in treatment. Primary care physicians were requested not to change participants’ medications, unless essential |
CPAP. CPAP (autotitrating). Adherence (machine usage): 3.6 hours/night (SD 2.8) Comparator. Sham CPAP (pressure < 1 cmH2O and > 0 cmH2O). Adherence (machine usage): 3.3 hours/night (SD 3.0) |
Number randomised. Total: 42 (CPAP: 20, comparator: 22) Number of withdrawals. Total: 2 (CPAP: 2, comparator: 0) Reasons for withdrawals. Unrelated surgery (n = 1), unwilling to continue using CPAP (n = 1) Baseline characteristics TotalCPAPComparatorAge57.8 (SD 10.4)54.5 (SD 9.4)SexMale (n = 21)Male (n = 21)AHINot assessed––ESS14.7 (SD 3.5)13.5 (SD 3.5)BMI36.6 (SD 4.9)36.8 (SD 4.6)BPNot assessed––> 4% SaO2 dips/hour33.1 (SD 21.6)39.1 (SD 24.8)MWT (minutes)21.9 (SD 12.8)32 (SD 10.8)SAQLI4.3 (SD 1.1)4.4 (SD 0.9) Additional information. Outcomes: ESS, MWT (Osler), SAQLI, change in HbA1c, insulin sensitivity and adverse events. Outcomes were assessed at baseline and at end of treatment. |
Total | CPAP | Comparator | Age | 57.8 (SD 10.4) | 54.5 (SD 9.4) | Sex | Male (n = 21) | Male (n = 21) | AHI | Not assessed | – | – | ESS | 14.7 (SD 3.5) | 13.5 (SD 3.5) | BMI | 36.6 (SD 4.9) | 36.8 (SD 4.6) | BP | Not assessed | – | – | > 4% SaO2 dips/hour | 33.1 (SD 21.6) | 39.1 (SD 24.8) | MWT (minutes) | 21.9 (SD 12.8) | 32 (SD 10.8) | SAQLI | 4.3 (SD 1.1) | 4.4 (SD 0.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | CPAP | Comparator | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age | 57.8 (SD 10.4) | 54.5 (SD 9.4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | Male (n = 21) | Male (n = 21) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AHI | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESS | 14.7 (SD 3.5) | 13.5 (SD 3.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI | 36.6 (SD 4.9) | 36.8 (SD 4.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BP | Not assessed | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| > 4% SaO2 dips/hour | 33.1 (SD 21.6) | 39.1 (SD 24.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MWT (minutes) | 21.9 (SD 12.8) | 32 (SD 10.8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAQLI | 4.3 (SD 1.1) | 4.4 (SD 0.9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Appendix 6 Economic evaluation data extraction
| Ayas et al., 2006122 | Mar et al., 2003123 | ResMed, 2007120 | Chilcott et al., 200044 | |
|---|---|---|---|---|
| Type of economic evaluation | Cost–utility analysis | Cost–utility analysis | Cost–utility analysis and cost-effectiveness analysis | Cost–utility analysis |
| Currency used, year | US$, 2003 | Euros (converted from Spanish pesetas), 2000 | £ sterling, 2005 | £ sterling, no report of financial year of costs |
| Study design | Markov model using effectiveness estimates from one study and adjusting these for the impact of RTAs, based on a random-effects meta-analysis of eight before and after studies | Semi-Markov model using effectiveness estimates from a before and after study | Markov model using effectiveness estimates from a before and after study | Review synthesis. Effectiveness estimates based on results from two studies. Estimates based on before and after data |
| Perspective | Third-party payer. Societal perspective | Health-care perspective | Health-care perspective | Health-care perspective |
| Participants | Data from 99 patients with moderate to severe OSAHS were used to establish the proportion of patients in each sex/age group | Based on a cohort of 5000 patients with moderate to severe OSAS to establish the proportion of patients in each sex/age group | Based on a simulated cohort of 2000 patients with moderate to severe OSAHS | Patients referred to a sleep clinic. Typically middle-age group (45+ years old) |
| Setting, country of study | US | Spain | UK | UK |
| Intervention group | CPAP | nCPAP | CPAP (fixed) | nCPAP |
| Control group | No CPAP | No CPAP | CPAP (auto); no CPAP | Dental devices; no CPAP |
| Resources used | Health care; costs of diagnosis and treatment of OSAHS, costs attributable to motor vehicle accidents and maintenance costs of the devices and costs of medical follow-up | Health care; costs of investigation, diagnosis and treatment of OSAS, costs attributable to CVE morbidity and maintenance costs of the devices and costs of medical follow-up | Health care; costs of investigation, titration, diagnosis and treatment of OSAHS, costs attributable to CVE and RTA morbidity and maintenance costs of the devices and costs of medical follow-up | Health care; costs of investigation, diagnosis and treatment of OSAHS and maintenance costs of the devices and costs of medical follow-up |
| Source of effectiveness data | Base case used single study (Chakravorty et al., 200297). For patients who had an RTA, utility estimates were adjusted using the FCI | Survey of OSAS patients before the initiation of CPAP and 3 months post CPAP | Base case used Mar et al., 2003123 results | Three studies (Waterhouse et al., 2000150,151 and Jenkinson et al., 199977) using before and after (up to 4 weeks) initiation of CPAP Jenkinson, 199977 |
| Length of follow-up | 5 years | Results were extrapolated to 5 years and over the lifespan of the patient | 14 years. Used results of Marin et al., 2005130 and Mar et al., 2003123 to calculate annual incidence of fatal and non-fatal CVE and cerebrovascular events in CPAP-treated and untreated patients with severe OSAHS (AHI ≥ 30) to 12 years and extrapolated these results over another 2 years. Used the Mar et al., 2003123 results to estimate the ratio of CHD and stroke in patients with untreated severe OSAHS as 1.185 and 1.353 respectively. Estimated the ratio of developing CHD to stroke as 1 : 1.13. Estimated ratio of CHD to stroke in treated patients as 1 : 1. Using these estimates, ResMed calculated the annual risk of CVE and stroke | 5 years |
| Source of resource use data | A primary referral centre and national data. Not all sources of resource use data were reported | Single sleep centre located in a hospital | 19 clinicians | Published literature, administrative database and clinical opinion |
| Source of unit cost data | The cost of CPAP was obtained from Medicare fee schedules. Costs of RTAs were obtained from the National Highway Traffic Safety Administration | The device cost was the price of the CPAP S VI Plus model. Regional hospital costs were used for the health-care costs | List prices, published literature, government statistics | Published literature, administrative database and clinical opinion |
| Link between cost and effectiveness data | Cost and effectiveness data were not linked directly | Cost and effectiveness data were not linked directly | Cost and effectiveness data were not linked directly | Cost and effectiveness data were not linked directly |
| Clinical outcomes measured and methods of valuation used | The base case used a single study (Chakravorty et al., 200297) to obtain the relative treatment effect of CPAP vs do nothing. The utilities were elicited using patient preferences and were valued using the standard gamble. For the secondary analysis, EQ-5D estimates were used based on societal preferences (Jenkinson et al., 1998349 and Mar et al., 2003123). For patients who had an RTA, utility estimates were adjusted using rating scale preference weights obtained from the FCI (Graham et al., 1997143) | Utility values were obtained using the EQ-5D. The health states for 46 patients were elicited and societal preferences were applied using the time trade-off technique | Utility values were obtained from the EQ-5D using the Mar et al., 2003123 results. The health states for 46 patients were elicited and societal preferences were applied using the time trade-off technique | Utility values generated via SF-36 survey, using the Brazier et al., 1998148 algorithm. Societal preferences were applied using the TTO and/or standard gamble |
| No data were available on quality of life in OSAS patients with stroke and CHD; therefore this was modelled by assigning quality adjustment factors of 0.8 and 0.9 respectively to these health states, based on an estimate in the published literature | No data were available on quality of life in OSAHS patients with stroke and CHD; therefore this was modelled by assigning quality adjustment factors of 0.8 and 0.9 respectively to these health states, based on an estimate in the published literature. To estimate utility associated with a non-fatal RTA, ResMed took the average utility for OSAHS and a non-fatal CVE in treated and untreated patients | |||
| Outcome results/adverse events | CPAP utility = 0.55, no CPAP utility = 0.32. Treatment with CPAP reduced the rate of RTAs sevenfold (OR of RTA with CPAP vs no CPAP = 0.15, 95% CI 0.10–0.22. No consideration was given to the effect of adverse events due to CPAP | Among the patients who were diagnosed with moderate to severe OSAS, there was a 10% dropout rate from treatment in the first year. In the analysis this impacted on costs but was not included in terms of outcomes | The utility associated with untreated OSAHS is 0.738 and with treated OSAHS is 0.811. The utility of non-fatal stroke in untreated OSAHS patients was 0.590 and in treated patients was 0.649. The utility of non-fatal CVE in untreated OSAHS patients was 0.664 and in treated patients was 0.730. The utility of non-fatal RTA in untreated OSAHS patients was 0.701 and was 0.771 in treated patients | The gain in HRQoL as measured by the SF-36 single index was 0.12 QALYs (95% CI 0.09–0.16) over 1 year |
| The incremental QALY for CPAP was 0.75 QALYs, i.e. 2.22 QALYs (95% CI 0.86–3.89) vs 1.47 QALYs (95% CI 0.28–3.08) | The mean EQ-5D score was 0.738 (0.646–0.829) before beginning nCPAP. The mean gain 3 months later was 0.073 (0.015–0.131) | At 14 years the estimated QALY gains were 7.22 (6.85–7.62) for no treatment, 8.19 (7.79–8.69) for CPAP (fixed) and 8.32 (7.97–8.81) for CPAP (auto) | ||
| QALYs were 3.73 at 5 years and 14.38 over the lifespan in the nCPAP arm, and 3.39 at 5 years and 12.90 over the lifespan in the no CPAP arm | It was estimated that 79% of patients would continue to use CPAP (fixed) and 84% CPAP (auto) after the first year of treatment and that following this there would be no further loss to compliance | |||
| Cost data handled appropriately | Some unit costs and resource use were reported separately | Unit costs and resource use were reported separately | Unit costs and resource use were reported separately | Unit costs and resource use were reported separately |
| Cost results |
From the third-party payer perspective, the incremental cost for CPAP was $2519, i.e. $4177 (95% CI $2804–$6057) vs $1659 (95% CI $283–$3936) |
Costs were €2719 at 5 years and €7902 over the lifespan in the nCPAP arm, and €55 at 5 years and €591 over the lifespan in the no CPAP arm | Costs were estimated over 14 years. From the NHS perspective the cost of no treatment was £10,645 (95% CI £7912–£14,177), and £9086 (95% CI £6851–£11,117) for CPAP (fixed) and £8622 (95% CI £6712–£10,947) for CPAP (auto) | Total recurring annual cost of £250 per patient on long-term CPAP therapy |
| From the societal perspective, CPAP was more costly, i.e. $7123 (95% CI $4324–$11,906) vs $6887 (95% CI $3113–$14,843) | ||||
| Subgroup analysis | Not undertaken | Not undertaken | Not undertaken | Not undertaken |
| Modelling summary | Third-party payer perspective: CPAP more costly and more effective than no CPAP. ICER = $3354 per QALY (95% CI $1062–$9715) | nCPAP was estimated to be more costly and more effective than no CPAP. The ICER for CPAP was €7861 per QALY over a 5-year time horizon and €4938 per QALY for the lifetime horizon | CPAP was estimated to be more costly and more effective than no CPAP | Authors undertook a review of the evidence. nCPAP was estimated to be more costly and more effective than no CPAP. The ICER for CPAP was £8300 at 1 year and £3200 at year 5. Small differences in clinical effectiveness and cost were found when comparing nCPAP with dental devices but these were not explicitly quantified |
| Societal perspective: CPAP more costly and more effective. ICER = $314 (95% CI cost saving to $6114) | ||||
| From the third-party payer perspective and the societal perspective, 100% of simulations favoured the cost-effectiveness of CPAP, assuming the threshold value is $50,000 per QALY | ||||
| Direction of result with appropriate quadrant location | North-east quadrant | North-east quadrant | South-east quadrant | North-east quadrant |
| Statistical analysis for patient-level stochastic data | Not undertaken | Not undertaken | Not undertaken | Not undertaken |
| Appropriateness of statistical analysis | Not undertaken | Not undertaken | Yes | Not undertaken |
| Uncertainty around cost-effectiveness expressed and appropriateness of method of dealing with uncertainty around this | Yes | No | Yes | No |
| Sensitivity analysis and appropriateness | Sensitivity analysis was undertaken on utility values, discount rate, compliance rate, time horizon, scaling factor for converting lifetime costs to the 5-year model time frame and reduction in rates of RTAs according to the 95% confidence limits determined in the meta-analysis. Probabilistic sensitivity analysis was also undertaken. The analyses were appropriate | A series of univariate and multivariate sensitivity analyses were conducted by age, sex, relative risk of stroke (untreated), different utility estimates, benefit of nCPAP on blood pressure, dropout rate, cost of nCPAP and discount rate/s | A series of univariate and multivariate sensitivity analyses were conducted as reported in Table 20 | A series of univariate sensitivity analyses were conducted on impact of analytical time horizon, costs of investigation for nCPAP, long-term costs of maintenance, follow-up and other health-care resource usage, long-term impact of gross annual health-care costs, potential impact of improved mortality from use of nCPAP treatment, impact of uncertainty in morbidity benefits from nCPAP therapy and discount rate |
| It was found that the estimation of the ICER was sensitive to the time horizon | ||||
| Modelling inputs and appropriate techniques | Markov model using second-order Monte Carlo simulations to generate 1000 incremental cost and effectiveness pairs was appropriate | Yes | Yes | Not undertaken |
| Authors’ conclusions | Treatment for OSAHS with CPAP has a cost-effectiveness in line with that of other commonly funded treatments such as antihypertensive drugs | Treatment for OSAS with nCPAP has a cost-effectiveness in line with that of other commonly funded treatments such antihypertensive drugs. The key clinical benefit of nCPAP is improvement in the quality of life of patients with OSAS | CPAP (fixed) dominates no treatment and CPAP (auto) dominates no treatment after a minimum of 2 years’ treatment. Based on current evidence, use of CPAP (auto) is associated with marginally better outcomes and no additional cost compared with CPAP (fixed) | Treatment for OSAHS with CPAP has a cost-effectiveness in line with that of other commonly funded treatments. The incremental cost-effectiveness of nCPAP over dental devices was likely to be highly uncertain |
Appendix 7 Review of utility data
The approach recommended by the National Institute for Health and Clinical Excellence (NICE) and other bodies, when undertaking cost-effectiveness analyses, is to measure the incremental cost per quality-adjusted life-year (QALY) gained of the intervention under study versus an appropriate comparator. QALYs are calculated by multiplying the amount of time spent in a health outcome by the preference value or utility attached to that outcome. This latter quality adjustment is based on a set of values or weights for each possible health state that reflect the relative desirability of that health state as judged by individuals or society. In the case of cost-effectiveness studies of CPAP, utility values are required that quantify the impact on HRQoL of experiencing sleep apnoea and also the influence of receiving CPAP therapy on HRQoL. This report reviews the clinical and cost-effectiveness literature on CPAP and sleep apnoea in order to identify possible utility values for the economic analysis.
A search was undertaken of the MEDLINE database in order to identify relevant literature. The search strategy identified 160 abstracts. Abstracts were screened by one reviewer (SvH) and copies of those that were considered relevant were obtained. Four papers were identified as containing potentially relevant HRQoL/utility data. These are appraised below with respect to their utility data.
Tousignant et al., 1994124
This study assessed the impact of nCPAP therapy on the quality of life of 19 patients with sleep apnoea.
Patients attending a hospital sleep clinic (mean age 57 years, SD 10), who had been receiving nCPAP treatment for an average of 9 months, completed a standard gamble exercise. The health states valued were receiving treatment for nCPAP, pre-treatment, full health and immediate death. To assess the reliability of the results patients completed the exercise on two occasions 2–3 weeks apart. The mean utility score for the pre-treatment health state was 0.63 (0.29) and the mean utility score for the nCPAP treatment health state was 0.87 (0.17). The intraclass correlation coefficients for the retest data were above 0.7 for both the treatment health state and the pre-treatment health state.
Comments
As all the patients were currently receiving nCPAP therapy, the valuation of their pre-treatment health state was done retrospectively. Given this, it is difficult to ascertain the extent to which the difference in pre-treatment and treatment utility scores is a real difference, reflecting the impact of nCPAP treatment and the extent to which it demonstrates some sort of measurement error due to bias in recall.
Jenkinson et al., 1997152
This study compared the performance of three commonly used quality of life measures [SF-36, EQ-5D and the Functional Limitations Profile (FLP)] in patients with sleep apnoea before and after nCPAP therapy and compared the data with those for a normal population.
One hundred and eight male patients (mean age 50 years, SD 10) with a mean baseline ESS of 14 (SD 5) attending a sleep clinic for a therapeutic assessment of nCPAP therapy completed the three HRQoL measures before and 5 weeks after commencing treatment.
At baseline the mean EQ-5D index score was 0.79 (0.21) and after treatment the score had increased to 0.84 (0.25), which was not statistically significant and indicates only minor benefits from nCPAP therapy. In contrast, both SF-36 and the FLP showed statistically significant improvements in scores on the majority of their dimensions. The authors suggest that the failure of EQ-5D to show a similar magnitude of change to the other measures may be because it does not contain questions that specifically address areas of health thought to be affected by sleep apnoea such as sleep, tiredness, energy and social functioning. With this in mind, they caution the use of measures such as EQ-5D when evaluating therapy in this area.
Comments
Unlike the SF-36 and FLP, EQ-5D does not contain dimensions relating to sleep and/or vitality, which undoubtedly are of importance in this area; however, its usual activities dimension does arguably measure aspects of social functioning. If the intention were to measure change in sleepiness or tiredness then EQ-5D would not be the first choice. However, if the intention is to measure change in overall HRQoL then EQ-5D is a well-validated, globally used measure that, unlike the other two measures, generates a score that is weighted by the values of the general population. The EQ-5D index score is the main output of interest to those who require a score for use in decisions relating to resource allocation. However, within the context of this paper, it is not clear why the authors restrict their assessment of the performance of EQ-5D to the performance of the EQ-5D index score alone and appear to disregard the EQ-5D visual analogue scale score or, indeed, the score on the five dimension questions separately.
Chakravorty et al., 200297
This study compared the effectiveness of CPAP therapy with a conservative lifestyle management as assessed using two different methods for eliciting health utilities (EQ-5D and a standard gamble task).
Seventy-one patients referred to a hospital sleep clinic with a history of snoring and excessive daytime sleepiness were recruited to the study and randomised to receive either CPAP therapy or lifestyle management. Each treatment phase lasted for 3 months. Prior to randomisation and at the end of treatment patients completed EQ-5D and a standard gamble task in which they were asked whether they would choose to stay in their current state of health or receive treatment leading to one of two outcomes: either complete cure or failure leading to a worst health state/death.
Standard gamble scores showed a mean gain of 0.23 (from 0.32 to 0.55) for the CPAP group compared with a gain of 0.04 (from 0.31 to 0.35) for the lifestyle group. In comparison, EQ-5D index scores showed a much smaller mean gain of 0.04 (from 0.73 to 0.77) for the CPAP group and no change for the lifestyle group. Both groups showed significant improvements in their ESS score, and the CPAP group but not the lifestyle group also showed significant improvement in their AHI score.
The authors suggest that the results indicate that EQ-5D may not be an appropriate instrument to use among patients with sleep apnoea because it only showed a mild change in patients with an effective positive treatment response to CPAP, and failed to record the small improvement seen in the lifestyle group (as measured using standard gamble). However, it should be noted that the lifestyle group did not show significant improvement in terms of the objective polysomnographic measures reported in the study.
Comments
EQ-5D scores were relatively high at baseline and were similar to those reported in other studies for this patient group.
It is not surprising that the utility values obtained using the two methods are different as they were elicited by means of completely different questions. Given this, the authors’ conclusions about EQ-5D appear to be somewhat unwarranted.
Comparing utility values elicited using two different methods is problematic; the authors appear to treat the utilities elicited using the standard gamble approach as the ‘gold standard’ but their justifications for doing so in this context, other than describing the standard gamble approach as the ‘classical approach to calculating utilities’, are unclear.
Mar et al., 2003123
This paper aimed to analyse the long-term cost-effectiveness of nCPAP treatment in patients with moderate to severe obstructive sleep apnoea in comparison with conventional treatment.
The authors undertook a survey to obtain EQ-5D utility values for patients with sleep apnoea. Forty-six patients referred to a hospital sleep unit were recruited and interviewed twice, once before commencing treatment and again after using nCPAP for 3 months. The mean age of the sample was 53 years (SD 12), 87% were male and their mean ESS was 13.8 (SD 5.8). Before starting nCPAP the mean EQ-5D index score for the sample was 0.738 (0.646–0.829); 3 months later the mean gain was 0.073 (0.015–0.131). Utilities for non-fatal stroke and non-fatal CHD also included in the model were obtained from the literature but were not specific to patients with sleep apnoea.
Comments
EQ-5D utility values were chosen as the study was designed to consider CPAP within the context of resource allocation; in addition EQ-5D has been validated in a Spanish population. 124 Improvement was similar to that reported in the study by Jenkinson et al. , 1997152 (see above) which used EQ-5D in a similar population.However, the authors point out that the lack of a control group is a limitation of most studies that measure utility values in patients with sleep apnoea, and warn that the improvement in HRQoL observed may be an overestimation due to the placebo effect.
Appendix 8 Bivariate random-effects meta-analysis in WinBUGS
The randomised controlled trials identified in the systematic review reported a range of outcomes that were potentially relevant to the appraisal of CPAP for the treatment of OSAHS. In Chapter 3 a separate univariate meta-analysis was performed for each outcome of interest (where there were sufficient data from comparable studies). An alternative approach would be to perform a multivariate meta-analysis to jointly calculate pooled estimates for each outcome. By performing a multivariate meta-analysis the correlation between outcomes can be estimated and incorporated. Under a univariate approach, outcomes that are not reported are assumed to be missing completely at random (MCAR) and the between-study correlation between treatment effects on different outcomes is assumed to be zero. Using the multivariate approach, outcomes that are not reported are assumed to be missing at random (MAR), with the mechanism for missingness informed by the between-study correlation and the treatment effects for those outcomes that are reported.
Two outcomes identified in the systematic review were selected to inform the York economic model: mean difference in ESS score and mean difference in SBP. A random-effects analysis was performed in both the univariate and bivariate meta-analysis approaches, where each study’s summary statistics [essi (essVari), bpi (bpVari)] were assumed to represent an estimate of different underlying true values (essMui, bpMui), and these underlying true values were assumed to be drawn from a distribution with a particular mean (essReMu, bpReMu) and variance (essReVar, bpReVar). The framework for the bivariate approach is as follows:
where ϕiessVari is the within-study covariance and ΦessReVar is the between-study covariance. None of the trials reported within-study covariance between mean difference in ESS and mean difference in SBP, and so a set of patient-level data containing both outcomes was obtained from which an informative prior could be specified. In addition, owing to the small number of studies reporting both outcomes when incorporating only SBP measured by ABPM, an informative prior was specified for the variance (essVari, bpVari) to be used where studies did not report one of the outcomes of interest. This prior was specified by multiplying the variance by the sample size, with a crude adjustment for the study design (i.e. doubling the sample size for crossover studies).
A product-normal approach was used to specify the model to estimate the effect of CPAP compared with conservative management on ESS score and SBP. The WinBUGS code for the model and the data sets used in the analyses are presented below. The output from 10,000 iterations was used to inform the York economic model after discarding the first 50,000 iterations.
WinBUGS code for bivariate random-effects meta-analysis
model{ bpReMu ∼ dnorm(0,1.0E–6) essReMu ∼ dnorm(0,1.0E–6) essReTau <- 1/pow(essReSe,2) zReTau <- 1/(bpReVar – pow(bBeta,2)*essReVar) bpReVar <- pow(bpReSe,2) essReVar <- pow(essReSe,2) bpReSe ∼ dunif(0,10) essReSe ∼ dunif(0,10) bPsi ∼ dunif(–0.99,0.99) bBeta <- (pow(bpReVar,0.5)/pow(essReVar,0.5))*bPsi essVarPA ∼ dgamma(0.001,0.001) essVarPB∼ dgamma(0.001,0.001) bpVarPA ∼ dgamma(0.001,0.001) bpVarPB ∼ dgamma(0.001,0.001) for (i in 1:nStudies) { essMu[i] ∼ dnorm(essReMu,essReTau) bpMu[i] ∼ dnorm(bpReMuD[i],zReTau) bpReMuD[i] <- bpReMu –bBeta*(essReMu-essMu[i]) ess[i] ∼ dnorm(essMu[i],essTau[i]) essTau[i] <- 1/essVar[i] essVar[i] <- essPopVar[i]/(n[i]*typ[i]) essPopVar[i] ∼ dgamma(essVarPA,essVarPB) bpVar[i] <- bpPopVar[i]/(n[i]*typ[i]) bpPopVar[i] ∼ dgamma(bpVarPA,bpVarPB) bp[i] ∼ dnorm(bpMuD[i],zTau[i]) bpMuD[i] <- bpMu[i] – wBeta[i]*(essMu[i]-ess[i]) zTau[i] <- 1/(bpVar[i] –pow(wBeta[i],2)*essVar[i]) wBeta[i] <- pow(bpVar[i],0.5)/pow(essVar[i],0.5)*wPsiD[i] wPsiD[i] ∼ dnorm(wPsi,t)I(–1,1)} wPsi <-0.2852149 t <- 1/pow(.0739224,2)}
| Data typ | ess | essVar | bp | bpVar | n |
|---|---|---|---|---|---|
| 1 | 0 | 1.36515856 | 3 | 13.01117041 | 54 |
| 2 | –1.2 | 0.16654561 | NA | NA | 35 |
| 1 | –5 | 1.24835929 | NA | NA | 105 |
| 2 | 0.1 | 1.401856 | NA | NA | 18 |
| 2 | –6 | 2.3409 | NA | NA | 23 |
| 1 | –1.09 | 0.5184 | NA | NA | 111 |
| 2 | –3 | 1.138489 | NA | NA | 37 |
| 2 | –2.4 | 0.5041 | NA | NA | 71 |
| 1 | –2.2 | 0.92006464 | NA | NA | 142 |
| 2 | –0.6 | 1.7689 | –2.9 | 29.16 | 42 |
| 1 | –3.8 | 2.46929796 | –10.3 | 27.88473636 | 60 |
| 2 | –1 | 0.32069569 | NA | NA | 114 |
| 2 | –2.4 | 0.84327489 | NA | NA | 31 |
| 1 | –1 | 0.5625 | NA | NA | 72 |
| 1 | –1.1 | 1.99741689 | –2.5 | 8.4681 | 56 |
| 1 | –3 | 1.99741689 | NA | NA | 101 |
| 2 | –3.1 | 0.5041 | NA | NA | 35 |
| 1 | –4 | 2.1904 | NA | NA | 42 |
| 1 | –4.8 | 0.806404 | NA | NA | 107 |
| 1 | –4 | 4.37562724 | NA | NA | 45 |
| 1 | –7.94 | 1.62690025 | NA | NA | 48 |
| 1 | –3 | 2.46929796 | NA | NA | 71 |
| 1 | –4.5 | 1.03083409 | NA | NA | 118 |
| 2 | NA | NA | –1 | 5.6169 | 13 |
| 2 | NA | NA | 0 | 4.4521 | 25 |
| 1 | NA | NA | –1 | 15.47399569 | 21 |
| typ | ess | essVar | bp | bpVar | n |
|---|---|---|---|---|---|
| 1 | 0 | 1.36515856 | 3 | 13.01117041 | 54 |
| 2 | –1.2 | 0.16654561 | NA | NA | 35 |
| 1 | –5 | 1.24835929 | NA | NA | 105 |
| 2 | 0.1 | 1.401856 | NA | NA | 18 |
| 2 | –6 | 2.3409 | NA | NA | 23 |
| 1 | –1.09 | 0.5184 | NA | NA | 111 |
| 2 | –3 | 1.138489 | NA | NA | 37 |
| 2 | –2.4 | 0.5041 | NA | NA | 71 |
| 1 | –2.2 | 0.92006464 | NA | NA | 142 |
| 2 | –0.6 | 1.7689 | –2.9 | 29.16 | 42 |
| 1 | –3.8 | 2.46929796 | –10.3 | 27.88473636 | 60 |
| 2 | –1 | 0.32069569 | NA | NA | 114 |
| 2 | –2.4 | 0.84327489 | NA | NA | 31 |
| 1 | –1 | 0.5625 | NA | NA | 72 |
| 1 | –1.1 | 1.99741689 | –2.5 | 8.4681 | 56 |
| 1 | –3 | 1.99741689 | NA | NA | 101 |
| 2 | –3.1 | 0.5041 | NA | NA | 35 |
| 1 | –4 | 2.1904 | NA | NA | 42 |
| 1 | –4.8 | 0.806404 | NA | NA | 107 |
| 1 | –4 | 4.37562724 | NA | NA | 45 |
| 1 | –7.94 | 1.62690025 | NA | NA | 48 |
| 1 | –3 | 2.46929796 | NA | NA | 71 |
| 1 | –4.5 | 1.03083409 | NA | NA | 118 |
| 2 | NA | NA | –1 | 5.6169 | 13 |
| 2 | NA | NA | 0 | 4.4521 | 25 |
| 1 | NA | NA | –1 | 15.47399569 | 21 |
Glossary
- Apnoea
- The cessation of airflow during sleep as the result of an obstruction, preventing air from entering the lungs. Arbitrarily defined in adults as a 10-second breathing pause.
- Auto-positive airway pressure
- A type of continuous positive airway pressure (CPAP) machine that monitors changes in breathing and compensates automatically by making appropriate adjustments in pressure.
- Continuous positive airway pressure (CPAP)
- Device used to treat sleep apnoea that delivers a stream of compressed air at a prescribed pressure via a nose or full-face mask and hose, splinting the airway (keeping it open under air pressure) so that unobstructed breathing becomes possible.
- Cost–benefit analysis
- An attempt to give a monetary value to the consequences of the alternative interventions. In this way, the consequences can be more easily weighed against the costs of the intervention. This involves measuring individuals’ ‘willingness to pay’ for given outcomes and can present difficulties.
- Cost–consequence analysis
- Costs and health effects are reported separately.
- Cost-effectiveness acceptability curve (CEAC)
- A graphical representation of the probability of an intervention being cost-effective over a range of monetary values against society’s willingness to pay for an additional unit of health gain.
- Cost-effectiveness analysis
- The consequences of the alternatives are measured in natural units, such as years of life gained. These consequences are not given a monetary value.
- Cost-minimisation
- When two alternatives are found to have equal efficacy or outcomes (consequences), i.e. the only difference between the two is cost. This is sometimes considered to be a subtype of cost-effectiveness analysis.
- Cost–utility analysis
- The consequences of alternatives are measured in ‘health state preferences’, which are given a weighting score. In this type of analysis, different consequences are valued in comparison with each other, and the outcomes (e.g. life-years gained) are adjusted by the weighting assigned. In this way, an attempt is made to value the quality of life associated with the outcome so that life-years gained become quality-adjusted life-years gained.
- Disutility
- The reduction in utility in comparison with a healthy population.
- Hypopnoea
- Reduction of airflow during sleep. Arbitrarily defined in adults as a 10-second breathing event where there is continuous breathing but ventilation is reduced by at least 50%.
- Mandibular advancement device
- Dental device that holds the lower jaw and tongue forward to allow more space to breathe and to prevent snoring.
- Markov chain Monte Carlo (MCMC)
- A mathematical model containing a finite number of mutually exclusive and exhaustive health states, with uniform time periods, in which the probability of movement from one state to another depends on the current state and remains constant over time.
- Mixed treatment comparison
- A form of meta-analysis used to strengthen inference concerning the relative efficacy of two treatments. It uses data based on direct comparisons (A versus B and B versus C trials) and indirect comparisons (A versus C trials), and also facilitates simultaneous inference regarding all treatments in order to select those most appropriate.
- Odds ratio
- A way of comparing whether the probability of a certain event is the same for two groups; refers to the ratio of the number of people having an event to the number not having an event.
- Oxygen desaturation
- Less than the normal amount of oxygen carried by haemoglobin in the blood. Values below 90% are considered abnormal.
- Polysomnography
- Procedure involved in the evaluation of sleep disorders, often conducted overnight, that consists of a simultaneous recording of multiple physiological parameters related to sleep and wakefulness.
- Quality of life (health-related quality of life)
- A concept incorporating all the factors that might impact on an individual’s life, including factors such as the absence of disease or infirmity as well as other factors that might affect their physical, mental and social well-being.
- Quality-adjusted life-year (QALY)
- An index of health gain whereby survival duration is weighted or adjusted by the patient’s quality of life during the survival period. QALYs have the advantage of incorporating changes in both quantity (mortality) and quality (morbidity) of life.
- Random-effects model
- A statistical model sometimes used in meta-analysis in which both within-study sampling error (variance) and between-study variation are included in the assessment of the uncertainty (confidence interval) of the results of a meta-analysis.
- Sensitivity analysis
- A mathematical method that examines uncertainty associated with parameters estimated into the analysis to test the robustness of the analysis findings. In one-way sensitivity analysis each parameter is varied individually, and in multi-way analysis two or more parameters are varied at the same time. Threshold analysis identifies the critical values above or below which the results of a study vary and analysis of extremes is used to examine the most pessimistic and the most optimistic scenarios. Finally, probabilistic sensitivity analysis attributes distributions of probabilities to uncertain variables that are incorporated within a model.
- Standard gamble
- Measuring a health-state utility by comparing life in a particular given health state to a gamble with a probability that perfect health is the outcome or that immediate death is the outcome. The probability is varied until a point of indifference between the two choices (i.e. until the preference for the given health state is equal to the preference for the gamble).
- Time trade-off
- Measuring a health state by trading off life-years in a state of less than perfect health for a shorter lifespan in a state of perfect health.
- Utility
- A measure of the strength of an individual’s preference for a given health state or outcome. Utilities assign numerical values on a scale from 0 (death) to 1 (optimal or ‘perfect’ health), and provide a single number that summarises health-related quality of life.
- Weighted mean difference (in meta-analysis)
- A method of meta-analysis used to combine measures on a continuous scale, where the mean, standard deviation and sample size in each group are known. The weight given to each study is determined by the precision of its estimate of effect and is equal to the inverse of the variance. This method assumes that all the trials have measured the outcome on the same scale.
List of abbreviations
- ABPM
- ambulatory blood pressure monitoring
- AHI
- apnoea–hypopnoea index
- APAP
- autotitrating positive airway pressure
- BMI
- body mass index
- BRMA
- bivariate random-effects meta-analysis
- BSJ
- Brief Symptom Inventory
- CEAC
- cost-effectiveness acceptability curve
- CHD
- coronary heart disease
- CI
- confidence interval
- CNS
- central nervous system
- COWAT
- Controlled Oral Word Association Test
- CPAP
- continuous positive airway pressure
- CRD
- Centre for Reviews and Dissemination
- CVD
- cardiovascular disease
- CVE
- cardiovascular event
- DBP
- diastolic blood pressure
- DSST
- Digit Symbol Substitution Test
- DVT
- Digit Vigilance Test
- EQ-5D
- EuroQoL-5 Dimensions
- ESS
- Epworth Sleepiness Scale
- EVPI
- expected value of perfect information
- FCI
- Functional Capacity Index
- FLP
- Functional Limitations Profile
- FOSQ
- Functional Outcomes of Sleep Questionnaire
- GHQ
- General Health Questionnaire
- GRISS
- Golombok Rust Inventory of Sexual Satisfaction
- HADS
- Hospital Anxiety and Depression Scale
- HCHS
- Hospital and Community Health Services
- HODaR
- Health Outcomes Data Repository
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IHD
- ischaemic heart disease
- IQR
- interquartile range
- ITT
- intention-to-treat
- MAIS
- Maximum Abbreviated Injury Scale
- MAP
- mean arterial pressure
- MCAR
- missing completely at random
- MD
- mean difference
- MeSH
- medical subject heading
- MSLT
- Multiple Sleep Latency Test
- MWT
- Maintenance of Wakefulness Test
- NA
- not applicable
- nCPAP
- nasal continuous positive airway pressure
- NHP
- Nottingham Health Profile
- NICE
- National Institute for Health and Clinical Excellence
- NR
- not reported
- OA
- oral appliance
- OLS
- ordinary least squares
- OP
- oral placebo
- OR
- odds ratio
- OSAHS
- obstructive sleep apnoea–hypopnoea syndrome
- OSAS
- obstructive sleep apnoea syndrome
- PASAT
- Paced Auditory Serial Addition Task
- POMS
- Profile of Mood State
- PSG
- polysomnography
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RDI
- respiratory disturbance index
- RTA
- road traffic accident
- SAQLI
- sleep apnoea quality of life index
- SBP
- systolic blood pressure
- SD
- standard deviation
- SE
- standard error
- SF-36
- Medical Outcomes Study 36-item Short Form Health Survey
- TAP
- Thornton Adjustable Positioner
- TMT
- Trail Making Task
- UDA
- unit of dental activity
- UMACL
- University of Wales Institute of Science and Technology Mood Adjective Checklist
- WAIS
- Wechsler Adult Intelligence Scale
- WMD
- weighted mean difference
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
-
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
'Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
-
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
-
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of
techniques.
-
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Reimsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
-
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D,
-
et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Düdar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
-
Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement.
By Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al.
-
Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation.
By Wang D, Cummins C, Bayliss S, Sandercock J, Burls A.
-
Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation.
By McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al.
-
Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis.
By Simpson EL, Stevenson MD, Rawdin A, Papaioannou D.
-
Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs.
By Main C, Liu Z, Welch K, Weiner G, Jones SQ, Stein K.
Health Technology Assessment Programme
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Deputy Director, Professor Jon Nicholl,
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NCCHTA
-
Dr Andrew Cook, Consultant Advisor, NCCHTA
-
Dr Peter Davidson, Director of Science Support, NCCHTA
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Director of NHS Support, NCCHTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NCCHTA
-
Dr Ruairidh Milne, Director of Strategy and Development, NETSCC
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NCCHTA
HTA Commissioning Board
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology,
-
University of Sheffield Professor Allan House,
-
Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, Univeristy of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Professor Hywel Williams, Professor of Dermato-Epidemiology, University of Nottingham
-
Ms Kay Pattison, Section Head, NHS R&D Programmes, Research and Development Directorate, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies & Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Ms Jane Bates, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Professor Glyn Elwyn, Primary Medical Care Research Group, Swansea Clinical School, University of Wales
-
Dr Ron Gray, Consultant Clinical Epidemiologist, Department of Public Health, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, University of Sheffield
-
Dr Jennifer J Kurinczuk, Consultant Clinical Epidemiologist, National Perinatal Epidemiology Unit, Oxford
-
Dr Susanne M Ludgate, Medical Director, Medicines & Healthcare Products Regulatory Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, Joint Director, National Collaborating Centre for Mental Health, University College London
-
Mrs Una Rennard, Service User Representative
-
Dr Phil Shackley, Senior Lecturer in Health Economics, School of Population and Health Sciences, University of Newcastle upon Tyne
-
Dr W Stuart A Smellie, Consultant in Chemical Pathology, Bishop Auckland General Hospital
-
Dr Nicholas Summerton, Consultant Clinical and Public Health Advisor, NICE
-
Ms Dawn Talbot, Service User Representative
-
Dr Graham Taylor, Scientific Advisor, Regional DNA Laboratory, St James’s University Hospital, Leeds
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Pharmaceuticals Panel
-
Chair, Professor Robin Ferner,
Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor in Child Health, University of Nottingham
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health,
-
Bury Primary Care Trust Dr Ben Goldacre,
-
Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Mrs Barbara Greggains, Service User Representative
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Professor Jonathan Ledermann, Professor of Medical Oncology and Director of the Cancer Research UK and University College London Cancer Trials Centre
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Dr Lesley Wise, Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Therapeutic Procedures Panel
-
Consultant Physician, North Bristol NHS Trust
-
Professor of Psychiatry, Division of Health in the Community, University of Warwick, Coventry
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School, Coventry
-
Ms Maree Barnett, Acting Branch Head of Vascular Programme, Department of Health
-
Mrs Val Carlill, Service User Representative
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Mr Mark Emberton, Senior Lecturer in Oncological Urology, Institute of Urology, University College Hospital, London
-
Professor Steve Goodacre, Professor of Emergency Medicine, University of Sheffield
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and The London School of Medicine and Dentistry
-
Mr Paul Hilton, Consultant Gynaecologist and Urogynaecologist, Royal Victoria Infirmary, Newcastle upon Tyne
-
Professor Nicholas James, Professor of Clinical Oncology, University of Birmingham, and Consultant in Clinical Oncology, Queen Elizabeth Hospital
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Kate Radford, Senior Lecturer (Research), Clinical Practice Research Unit, University of Central Lancashire, Preston
-
Mr Jim Reece Service User Representative
-
Dr Karen Roberts, Nurse Consultant, Dunston Hill Hospital Cottages
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
-
Professor Tom Walley, Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist, National Commissioning Group (NCG), London
-
Director, NHS Sustainable Development Unit, Cambridge
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Professor Mike Kelly, Director, Centre for Public Health Excellence, NICE, London
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Ms Jeanett Martin, Director of Nursing, BarnDoc Limited, Lewisham Primary Care Trust
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Miss Nicky Mullany, Service User Representative
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Ken Stein, Senior Clinical Lecturer in Public Health, University of Exeter
-
Dr Kieran Sweeney, Honorary Clinical Senior Lecturer, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Professor Margaret Thorogood, Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Caroline Stone, Programme Manager, Medical Research Council
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington