Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 06/59/01. The protocol was agreed in November 2006. The assessment report began editorial review in March 2008 and was accepted for publication in December 2008. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
None
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the problem
Hearing loss
Loss of hearing is a common problem, generally associated with increasing age. 1 In the UK about 40% of those over 50 years of age have some degree of deafness. 2 A person who can detect tones at an average level below 20 decibels hearing level (dB HL) is considered to have normal hearing. Those with a severe loss of hearing cannot detect tones at an average level below 70–94 dB HL in their better-hearing ear. Those with a profound loss of hearing cannot detect tones at an average level below 95 dB HL in their better-hearing ear.
Traditional acoustic hearing aids may improve hearing function but are diminishingly ineffective for many people with severe to profound sensorineural loss of hearing. 3 For some of this group the advent of cochlear implants has provided an alternative treatment. 4
Epidemiology
Incidence and/or prevalence
Children (0–16 years)
An estimated 371 [95% confidence interval (CI) 327–421] children in England and 21 (95% CI 18–24) children in Wales are born annually with permanent severe to profound deafness. 5 The prevalence for severe to profound deafness is about 59 cases per 100,000 children. 5 About 1 in 1000 children is severely or profoundly deaf at 3 years old. This rises to 2 in 1000 children aged 9–16 years. 6
Adults (over 16 years)
Significant hearing loss affects one-third of those over 60 years and half of those over 75 years. 4 In the UK around 3% of those over 50 years and 8% of those over 70 years have severe to profound hearing loss. 2 People with severe to profound hearing loss make up around 8% of the adult deaf population. 2 This number is likely to rise with the increasingly elderly population. In those over 60 years the prevalence of hearing impairment is higher in men than in women (55% and 45%, respectively, for all degrees of deafness). 1
Aetiology
Children
A 15-year study by Fortnum and colleagues7 that examined birth cohorts of those born in the UK between 1980 and 1995 found nearly 3600 (21%) cases of children with permanent severe hearing loss (71–95 dB) and 4262 (25%) cases with permanent profound hearing loss (> 95 dB). The aetiology of severe hearing loss was 22% more likely than other levels of deafness to have perinatal causes (p < 0.001). Those with profound deafness were more likely to have a genetic (42%; p < 0.001), postnatal (20%; p < 0.001) or prenatal (12%; p < 0.001) aetiology. Fortnum and colleagues also looked at the subset of children with cochlear implants. Here, significantly more of these children had a postnatal aetiology (47.7%; p < 0.001) than those profoundly deaf children not implanted.
Adults
The most common cause of eventual severe to profound deafness in the elderly is presbycusis. 1 This is progressive hearing loss due to the failure of hair-cell receptors in the inner ear, in which the highest frequencies are affected first. Hearing loss may also be due to noise exposure, ototoxic drugs, metabolic disorders, infections or genetic causes. 8 Communication problems from deafness may lead to social isolation and depression. 9–11
Pathology
Hearing impairment can be classified as conductive or sensorineural. Conductive deafness is caused by disease of the external, or more commonly middle, ear, which prevents the conduction of sound waves to the cochlea where they are sensed. Cochlear implantation is not a treatment for conductive deafness, which will not be considered further.
Sensorineural hearing loss occurs when there is damage to the inner ear (cochlea) or to the nerve pathways from the inner ear (retro cochlea) to the brain. Sensorineural hearing loss is permanent and not only involves a reduction in the ability to hear faint sounds but also affects speech understanding and discrimination.
Sensorineural hearing loss can be caused by disease, birth injury, drugs that are toxic to the auditory system, and genetic syndromes. It may also occur as a result of noise exposure, viruses, head trauma, ageing and tumours. It can be much more severe than conductive hearing loss, causing insensitivity to even the loudest sounds (total deafness).
Co-morbidities
Hearing loss is often associated with other health problems; Fortnum and colleagues7 found that 27% of children who were deaf had additional disabilities. In total, 7581 disabilities were reported in 4709 children; however, this may be an underestimate as ‘no disability’ and ‘missing data’ responses were not distinguished. Abutan and colleagues1 found that 11% of adults over 60 years with hearing loss also had tinnitus. About 23,000 (0.3%) of the population of deaf people are also blind and 250,000 (2.7%) of hearing-impaired people have some degree of additional sensory disability. 2 Additionally, 45% of severely or profoundly deaf people under 60 years have other disabilities, usually physical; this rises to 77% of those over 60 years. 2
Measurement of hearing sensitivity
The degree of sound intensity that can be heard is measured in decibels (dB); this is a relative not an absolute measure. Hearing loss is characterised as the additional intensity that pure tones must possess to be detected by an individual relative to the intensity that can be detected by young adults free from auditory pathology. The additional intensity is measured in units of decibels hearing level (dB HL) and is usually averaged across frequencies from 500 to 4000 Hz.
Communication with hearing loss
People with hearing loss communicate face to face in two different ways:
-
oral communication – this includes auditory–oral skills, which can range from emphasising auditory information without lip-reading to cued speech in which hand cues supplement lip-reading
-
total communication – this emphasises both signed and spoken communication with considerable variation from one setting to another in the emphasis placed on each modality.
Of those with severe or profound deafness, about 450,000 cannot hear well enough to use a voice telephone. 2
It is estimated that about 50,000 people, mainly those who were born deaf or who lost their hearing early in life, use British sign language as their first language. 2 It is difficult to accurately estimate the number of lip-readers as this skill is used in varying degrees by most deaf people. 2
Impact of deafness
Children
In children, hearing loss may have significant consequences for linguistic, cognitive, emotional and social development. 12 Many deaf children live in relative isolation in their early years and their first contact with other deaf children may be when they attend school. 13
Early life may be dominated by trying to adapt to their impairment. This may involve learning to lip-read and/or using cued speech or sign language, either at mainstream or special schools. 14 The inability to communicate wants and needs may alienate children from family members. 12
At school, deaf children may also exhibit more behavioural problems than their hearing peers. Greater problems are evident in those with bilateral severe to profound deafness. 13,15 Congenitally deaf children fare poorly academically. 13,15 In the longer term children with uncorrected hearing loss are at an increased risk of becoming underemployed. 16,17
Measurement of quality of life in young children (i.e. < 5 years) is often by proxy through parents (or teachers). 18,19 In total, 90% of deaf children have two hearing parents and 95% have at least one. 13,20 There are no standardised measures to assess quality of life specific to deaf children, deaf adolescents or their parents. 15 However, two generic profile measures have been used to assess quality of life in deaf children, the Child Health Questionnaire (CHQ)15,21 and the Munich Quality of Life Questionnaire for Children (KINDLr). 19,22 Both have been used to assess quality of life in children with severe to profound deafness, including those who are prelingually deafened, either using an acoustic hearing aid or with a cochlear implant. Prelingual deafness refers to deafness occurring before a child has developed speech, with an age of 3 years often taken as a proxy for this. Postlingual deafness refers to deafness occurring after this time.
An Australian cross-sectional study15 used the 28-item short version of the parent proxy report CHQ to compare quality of life in children aged 7–8 with significant congenital hearing loss (with mild to profound hearing impairment, including cochlear implant users) with their hearing peers. The CHQ has 12 subscales – physical functioning, role (social–physical), role (social–emotional), bodily pain, behaviour, mental health, self-esteem, general health, parent impact (emotional), parental impact (time) and family activities – and produces two summary scores (physical and psychosocial). The CHQ has only been provisionally validated. 23 Children with congenital hearing loss scored significantly worse in six domains [role (social–physical), behaviour, mental health, parent impact (emotional), parental impact (time) and family activities]. The psychosocial summary score (out of 100) was also significantly lower in children with congenital hearing loss (49.2, SD 9.6) than in children with normal hearing (53.1, SD 8.2). Ceiling scores of 100 were reported on four subscales in both groups. 15 The study did not control for differences in parental level of tertiary education or co-morbidity.
An Austrian study22 used the KINDLr to assess the quality of life of children (aged 8–16 years) with cochlear implants. It has six domains (physical health, general health, family functioning, self-esteem, social functioning and school functioning). Total self-reported scores for boys (67.5, SD 9.6) and girls (63.1, SD 8.6) were significantly below those of their hearing peers (76.8, SD 8.6 and 76.7, SD 8.7 respectively).
Adults
Studies indicate that deafness may adversely affect the quality of life of adults24–29 and that of their family members. 31,32 Mulrow and colleagues33 reported that 82% of the elderly deaf stated that deafness had an adverse effect on their quality of life and 24% felt depressed.
Commonly reported difficulties identified by postlingually deaf adults include feelings of isolation, loss of confidence and tinnitus. 10 In social settings, in particular those with background noise, communicating with others can be very challenging. 17 In a study of 47 severely to profoundly postlingually deafened adults in Wales,34 nearly two-thirds identified feelings of isolation, loss of confidence and loss of social life as causing them difficulties. Such difficulties may influence the viability of personal networks and, therefore, the sense of self. 16 These effects can lead to reduced feelings of well-being. 17 The difficulties caused by hearing loss may result in withdrawal from social activities, reducing intellectual and cultural stimulation and cognitive functioning. 12,17
In an Italian study of 1191 non-institutionalised elderly,35 those with hearing impairment had twice the risk [odds ratio (OR) 2.1, 95% CI 1.36–3.25] of poor functioning in daily living activities compared with non-impaired elderly, with over 20% of the elderly deaf having a level of functioning classified as poor by the Instrumental Activities of Daily Living (IADL) scale. A similar relationship between hearing loss and self-sufficiency was seen among middle-aged adults (51–61 years) living in the community. 36
A US cross-sectional study31 of 178 adults (17–84 years) with profound postlingual deafness showed that 13% showed clinically elevated levels of depressed mood (T-score ≥ 70) and 16% had feelings of significant social isolation on the Minnesota Multiphasic Personality Inventory (MMPI). Their levels of anxiety in social contexts, measured using the Social Avoidance and Distress (SAD) scale, were also greater than those of people diagnosed with simple phobias. 31 A follow-on study involving 95 of these participants also showed that they had lower levels of social participation than 44 age-matched hearing control subjects. 31 Candidates experienced lower levels of pleasant social events (16%) and non-social events (19%) than control subjects (23% and 27% respectively; p < 0.05). 31
Dalton and colleagues27 used the Short-Form 36 (SF-36) to measure the impact of hearing impairment on quality of life in 2688 adults. The SF-36 measures physical functioning, role limitation because of health problems, social functioning, role–emotional, general health, bodily pain, mental health and vitality on a scale from 0 to 100. They found that severity of hearing loss was significantly associated with worse quality of life. Those with moderate to severe hearing loss (> 40 dB) had the lowest scores. Scores were 1.9–5.9 points lower than in those without hearing loss across six of the eight domains. The greatest differences were in the domains of role (physical) (5.9), physical functioning (5.2), vitality (4.2) and role (emotional) (3.9). There was no association with general health (2.1) or bodily pain (1.9), although scores did decline with hearing loss. 27 There was also a statistically significant difference in the two adjusted summary component scores in physical and mental health between people with no hearing loss (40.3, SE 1.87 and 50.2, SE 1.59 respectively) and those with moderate to severe hearing loss (38.8, SE 1.89 and 49.0, SE 16.1 respectively). 27 The impact of increasing deafness on quality of life has been shown in other studies. 37 It is not clear to what extent these relationships are causally related to the hearing impairment rather than to other disabilities or diseases associated with ageing or the aetiology of deafness (e.g. premature birth).
In a Dutch study38 of 46 people waiting for cochlear implants, SF-36 scores in those with profound postlingual deafness, mean age 51 years (SD 16), were between 60.2 (SD 41.5) [role (physical) domain] and 79.2 (SD 24.8) (physical functioning domain). A Norwegian study39 of 27 postlingually deaf, cochlear implant candidates compared pre- and postimplantation scores. They found that postimplantation participants had similar physical functioning scores (80.8) as preimplantation participants but higher role (physical) scores (71.0, SD 40.0). The vitality domain had the lowest scores (58.8, SD 21.8).
Tinnitus
Tinnitus is often associated with sensorineural hearing loss. 28,40 One in five people reported tinnitus as severely annoying,28,40 affecting speech discrimination, concentration and sleeping patterns. 29,41 The Norwegian study40 found that 67% of people with subjective hearing loss had tinnitus. Similarly, the prevalence of tinnitus was 70% in those with severe to profound deafness. 40
In an American study29 25% of adults with tinnitus attending an audiology clinic had moderate to severe depression, impacting on their quality of life. Self-assessment using the Quality of Well-being Scale (QWBS) was 0.53 (SD, 0.15), with a score of 0 equating to death and 1 to complete functioning. Thus, combined with hearing loss, tinnitus may exacerbate problems with maintaining a social life. 29
Quality of life in families of people with hearing loss
As the majority of parents and relatives of deaf people have no previous experience of deafness13 they may need to spend time and effort managing communication problems or assisting their deaf relative when engaging in social activities. 42,43 Over time this additional load may result in reduced physical health and elevated levels of emotional and psychological distress,15,42 the magnitude of which may be moderated by personal and external resources or the severity of the impairment. 32 However, the evidence for effects on health in families with a hearing-impaired child is inconclusive. 32
Whose quality of life? – The deaf world perspective
The deaf world community do not consider that deafness is an impairment. 44 From their perspective, deafness is a variation of normality. 45,46 Therefore, people who use sign language do not require hearing to be functional, productive and happy. 44 Growing up or living in a deaf community provides social and emotional support against the difficulties commonly associated with deafness,13,47 as well as a cultural identity. 47 As such, the hearing world may undervalue the quality of life experienced by deaf people. A Dutch study44 has shown that degree of deafness is not associated with a respondent’s happiness or perceived quality of life. Wald and Knutson47 have shown that deaf people who have a deaf identity have higher self-esteem than with those who do not. Some deaf activists argue that providing cochlear implants for prelingually deaf children will result in a declining deaf community. 46 They believe that the provision of cochlear implants poses a long-term indirect threat to the survival of the deaf world.
However, in assessing arguments about the ethics of providing cochlear implantation to deaf children, it is necessary to dissociate the needs of a community for recruits to ensure its survival from issues of what is right and best for children. Indeed, Arlinger17 has shown that deaf people are not always aware of all of the consequences of their condition and therefore may underestimate the impact of deafness on the quality of their own lives.
Current service provision
Relevant national guidelines
-
National action plan for audiology – Improving Access to Audiology Services in England. 48 This framework document sets out how health reform levers can be brought to bear to improve quality, efficiency and access to audiology services. It also describes national work intended to support this for adults and children.
-
MHAS – Modernisation of Hearing Aid Services (Adults)
-
MCHAS – Modernisation of Hearing Aid Services (Children) (2001)
-
NHS Newborn Hearing Screening Programme – seeks to identify deafness within 26 days of birth.
Significance to the NHS
Although deafness per se is not an illness, it does impact on NHS resources through the need for procedures of diagnosis and assessment and the possible provision of acoustic hearing aids and cochlear implants with the associated follow-up and support required.
Cochlear implants have been available in England and Wales since the late 1980s. Currently there are 14 tertiary implant centres in England and three in Wales. Treatment is provided by multidisciplinary teams of clinical scientists in audiology, audiologists, surgeons, speech and language therapists, hearing therapists and administrators. Within paediatric services, teams also include teachers of the deaf. Some units use or have access to clinical and/or educational psychology, link nurses and paediatricians. 49
The recently published best practice guidance Improving Access to Audiology Services in England48 seeks to improve the responsiveness of audiological services to cut waiting times to a maximum of 18 weeks.
Management of hearing loss
NHS Newborn Hearing Screening Programme
Nationally all newborns are screened for hearing problems within 26 days of birth with positive cases referred to NHS audiology departments. If confirmed deaf the baby should be provided with a hearing aid within 2 months. Referrals to other services are usually coordinated by the audiology department. These include:
-
paediatric services, to assess for possible co-morbidities
-
ear, nose and throat services, to consider surgery, including possible referral to a tertiary centre for cochlear implant assessment
-
educational services
-
social services.
Variation in services for hearing loss
There is geographical variation in the way that hearing impairment is managed in different parts of the UK. In general, models of adult services tend to follow the MHAS. Differences occur in the types of digital hearing aids fitted, the diagnostic facilities available and the access to hearing therapists. The professionals providing services may also vary. The larger departments and teaching hospitals tend to employ clinical scientists (audiology) whereas local district general hospitals are more likely to employ audiologists.
Paediatric services also vary; there are different models of newborn hearing screening, with some being maternity-unit based and others community based. In some areas second-tier paediatric services are delivered in the community, usually by community paediatricians with support from audiologists. In other areas these services have been integrated into the main audiology department and are clinical scientist/audiologist led. Hearing aid services for paediatrics will usually follow the MCHAS model.
The referral process for cochlear implants may also vary, but referrals will go to the major centres (14 in England and three in Wales). Similar protocols are used for adults and children.
There may be slight variation nationally in the initial screening and diagnostic services described above. Although follow-up care may vary more, the following description may be considered reasonably typical (expert advisory group, 2006, personal communication).
For children, following diagnosis and fitting with an initial hearing aid 2 months later, services generally conform to the following pattern:
-
visit to the audiology department every 2 weeks for new ear moulds for 6 months
-
visit from educational services every week
-
formal diagnosis of level of hearing loss at 3 months
-
potential cochlear implant use considered very early on, i.e. usually within the first year
-
audiological assessment at 6 months, then every 6 months until 2 years old or until hearing aid use is stable and consistent
-
once stable audiological checks every 6 months until 5 years old, then annually until adult services take over at 18 when there are 4-yearly reassessments.
Adults make up the vast majority of people seen in the NHS for hearing problems. The NHS provides over 2,600,000 adult hearing aid services per year; 600,000 of these are assessments of hearing, 500,000 are hearing aid fittings, 500,000 are follow-up appointments and more than 1,000,000 are for ‘repairs’ of devices. 50 Services are coordinated by audiology departments. Adults normally have a 4-yearly review, although this varies across the UK (Dr Jonathan Parsons, Mid, East Devon and Exeter Area Primary Care Trust, 2006, personal communication).
Description of technology under assessment
Summary of intervention
Cochlear implants first became available on the NHS in the 1980s. These were single channel devices that used simple coding strategies to interpret speech into intelligible sounds. These early devices gave 15–35% word or sentence understanding. 51 Cochlear implants and their coding strategies have been continually developed since then, with step changes in the quality of performance coming from the arrival of multichannel devices and whole speech coding strategies in the mid-1990s, giving up to 90% understanding of words or sentences. 51 It is these later multichannel whole-speech processing devices that this technology assessment will consider.
Aim of cochlear implants
The aim of cochlear implants is to improve quality of life by enabling people with hearing loss to hear and interpret sounds, thus improving their ability to understand others, communicate effectively and move safely in their environment.
Description of cochlear implants
Cochlear implant systems consist of the following components (Figure 1). A microphone, worn behind the ear, is connected by a wire to a sound processor. The sound processor is connected by a wire to a transmitter coil, worn on the side of the head. The transmitter coil transmits electrical power (by induction) and data (as a radio-frequency signal) to a receiver coil. The receiver coil is part of a receiver/stimulator package that is placed in a depression fashioned surgically in the mastoid bone behind the ear. The transmitter coil is held in place, and is aligned with the receiver coil, because the coils surround magnets of opposing polarity. The stimulator is a microprocessor that receives electrical power and digital data from the receiver coil. The microprocessor translates the data into biphasic charge-balanced electrical pulses, which are delivered to an array of electrodes that are placed surgically within the cochlea. The primary neural targets of the electrodes are the spiral ganglion cells, which innervate fibres of the auditory nerve.
FIGURE 1.
Ear with cochlear implant.
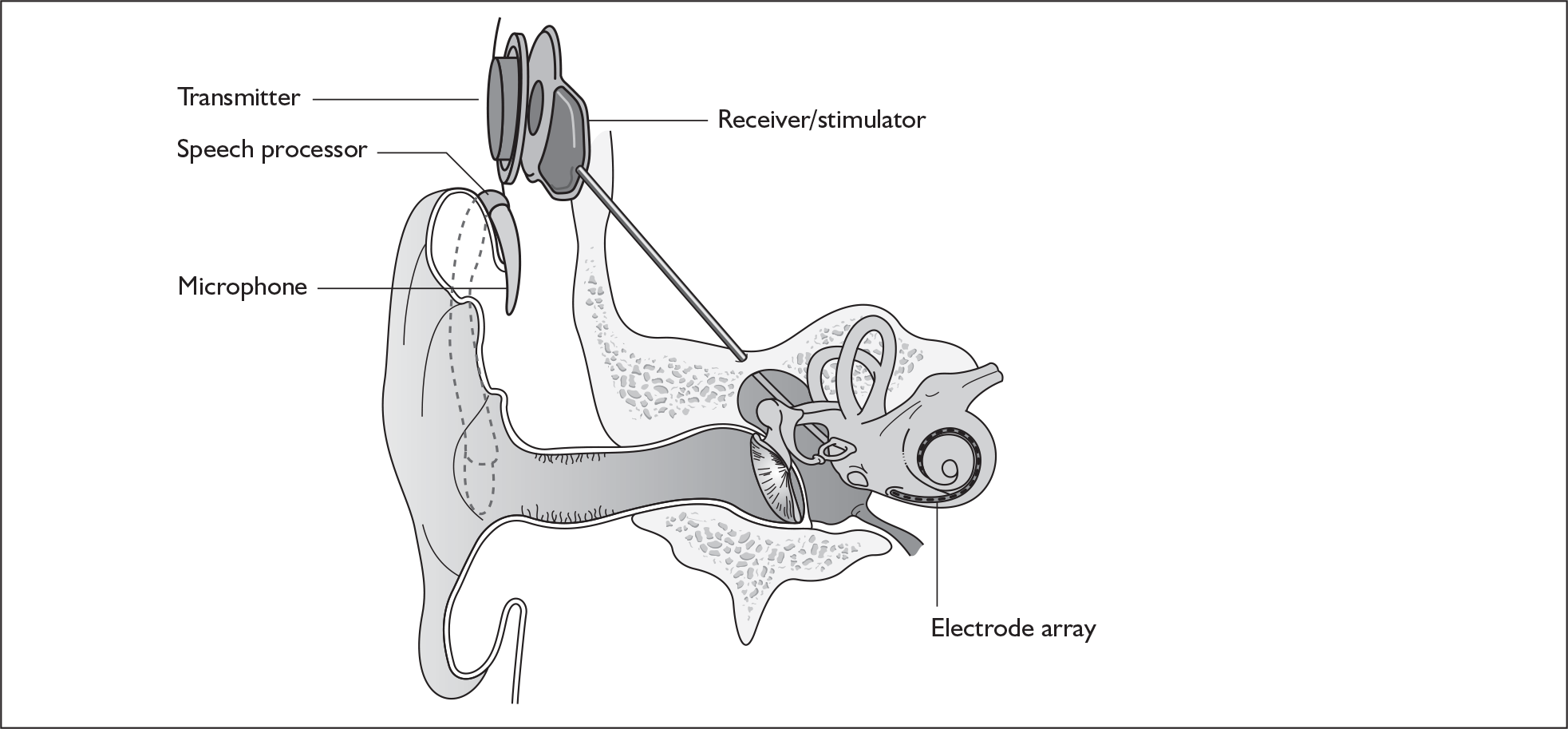
When the electrodes are activated by a signal they send a current along the auditory nerve, which produces a sensation of hearing. This is not a restoration of hearing. A normal ear can resolve patterns of sound energy in about 60 distinct bands of frequency in the range from 100 Hz to 20,000 Hz. The best that users of implants achieve is 6–8 bands, regardless of whether they have 24, 16 or 12 electrodes (Professor Quentin Summerfield, University of York, May 2007, personal communication).
One of the limitations of implants arises because electrical stimulation spreads widely within the cochlea. This means that a single electrode excites spiral ganglion cells that would normally respond to a wide range of frequencies. In comparison, the tuning of the basilar membrane in the normal cochlea restricts the spread of excitation to a narrow range of spiral ganglion cells.
Initially, sound processors were about the size of a packet of cigarettes and were worn clipped to clothing or, in the case of a young child, held in a harness. More recently, miniaturisation of electronic circuitry and the increased capacity of small batteries have allowed the processor to be combined with the microphone in an assembly worn behind the ear, like an acoustic hearing aid. Body-worn processors are still used by infants because the processor can be held securely in place in a harness. Behind-the-ear assemblies are used by older children and adults.
Insertion procedure
The procedure for cochlear implant surgery takes between 2 and 3 hours under general anaesthetic. It involves the insertion of the electrode array into the cochlea through a tunnel that has been drilled above the external ear canal, bypassing the mastoid cavity. 52
Criteria for candidacy for cochlear implantation
Currently there are no nationally agreed criteria for candidacy for cochlear implantation, although the British Cochlear Implant Group (BCIG) is due to produce a position statement in 2007. A summary of its recent audit of UK practice can be found in Appendix 4.
The joint submission to NICE of the British Academy of Audiology (BAA), BCIG and the British Association of Otorhinolaryngologists (ENT UK) in March 200749 states that, in broad terms, criteria for candidacy in the UK are based on:
-
failure to achieve adequate benefit from conventional acoustic amplification in cases of severe to profound sensorineural deafness
-
organisation of the cochlea together with the presence of viable spiral ganglion cells and auditory nerve capable of stimulation
-
the ability to gain surgical access to the cochlea
-
the ability of the patient to utilise the auditory input from the cochlear implant.
A number of other issues should be considered in relation to candidacy:
-
In the UK there are no upper or lower age limits for consideration for cochlear implantation; however, hearing evaluation tests mean that implantation is unlikely before 9 months.
-
Profound deafness of greater than 30 years has been linked to poorer outcomes;53 however, positive outcomes are possible and therefore skilled candidate selection is essential.
-
Progressive and fluctuating loss can give rise to a greater degree of difficulty experienced by the patient than the audiogram may suggest at any particular time. Patients within these groups require careful multidisciplinary monitoring and intervention in a timely fashion. 54
-
Patients with a profound unilateral loss, or an asymmetric profound/severe loss, can also experience high levels of difficulty in adapting to a cochlear implant.
It might be assumed that the inability to detect tones at severe and profoundly deaf levels correlates directly with speech perception; however, while there is some correlation this is not total (Professor Quentin Summerfield, University of York, May 2007, personal communication). This raises a problem because in clinical situations older children and adults are assessed for implant suitability on the basis of functional outcomes, i.e. ability to understand prerecorded sentences without lip-reading; however, the inclusion criteria of most research studies are based on the average ability to detect tones in the better-hearing ear. Thus, people who are classified as profoundly deaf do not form a homogeneous group, so that a person may meet the candidacy criteria on functional outcomes but not on audiological ones. This causes a potential mismatch between clinical assessment for candidacy and the research on which effectiveness for particular levels of deafness is based.
Assessment for cochlear implantation
Assessment for cochlear implantation is undertaken by a multidisciplinary team whose aim is to select people who are medically, audiologically and psychologically suitable. Assessment comprises a number of evaluations:
-
Audiological – This involves a pure-tone audiogram to give an indication of the degree of hearing deficit. If this results in a likely indication for cochlear implantation then patients undertake a 3-month trial with acoustic hearing aids to confirm that these do not provide sufficient support.
-
Functional hearing – This is tested using optimally fitted acoustic hearing aids to find out if cochlear implants are likely to improve hearing outcomes.
-
Speech, language and communication – This is difficult in prelingual children and requires a specialist speech and language therapist to assess abilities in relation to normal development and contribute to judgements about the level of functional hearing. Most adult candidates are postlingually deafened and so their ability to communicate and comprehend in social situations is assessed.
-
Medical – This involves an assessment of fitness for surgery, the aetiology of hearing loss and whether there are other disabilities or medical conditions present that may affect the success of implantation.
-
Radiological – This involves an examination of the anatomical structure of the cochlea and the auditory nerve for anomalies that might contraindicate surgery or require a modified implantation. This is carried out using computerised tomography (CT) and magnetic resonance imaging scanning, under general anaesthetic in young children.
-
Psychological assessment – This may be carried out to ensure that realistic expectations of the benefits and the demands of training are understood. Children may also be evaluated by teachers of the deaf.
Setting and equipment required
Specialist surgical equipment is needed to perform the operation, in particular, specialist drills for shaping the mastoid bone and monitoring equipment to check the integrity of the facial nerve. Intraoperative CT scanning may be used to check the position of the electrode array.
Follow-up required for children
Cochlear implantation requires a commitment from the child’s carers to long-term involvement in rehabilitation. Children receive individualised programmes of audiological training once they have shown that they are able to detect sound after implantation (the device is switched on approximately 1 month after insertion). Intensive training over several months is undertaken by a speech and language therapist and a teacher of the deaf. Tuition addresses sound discrimination, recognition with associated meaning and the appropriate response to verbal cues (comprehension). The development of speech is encouraged by imitation and concurrent articulation, progressing to sentence production. Complete training may take many years; however, initial benefit occurs within 6–18 months. Typically, a child’s progress is assessed at approximately 3, 6 and 12 months post implant and then annually. These evaluations involve a variety of measures to test understanding of others’ speech and the intelligibility of their own to others.
Identification of important subgroups
As far as the included data permit we look at the issues of pre- and postlingual implantation in children and differences in outcomes between adults who were born deaf and those who later became deaf.
Bilateral implantation
Bilateral implantation has the potential to provide a number of benefits above those of unilateral implantation:
-
Localisation of sounds. The ability to detect the direction that a sound comes from can be measured either by the minimum audible angle in the frontal horizontal plane, which is a measure of the least separation that two sources of sound need to have to be able to tell which direction the sound comes from, or by the accuracy with which someone can localise the sources of sound to more than two locations.
-
Measures of the ability to use both ears to improve the accuracy with which speech is understood in noise:
-
– binaural summation is shown when both speech and noise come from the same place, and ability with both ears is significantly better than ability with the better-hearing single ear
-
– the head shadow effect is shown when speech and noise come from separate locations, and ability is better when listening with both implants than with a single implant for the ear closer to the noise
-
– binaural squelch is shown when speech and noise come from separate locations, and ability is better when listening with both implants than with a single implant for the ear closer to the speech.
-
-
The assurance that the better of the two ears receives an implant.
-
That two ears are often better than one even when there is no difference between the sound reaching the two ears.
These potential benefits of bilateral implantation are outcomes that are measured in the systematic review in Chapters 4 and 5.
Current usage in the NHS
By the year ending March 2007 there were 374 adults and 221 children implanted with cochlear implants in England and eight adults and 22 children in Wales. A further 451 adults and 446 children are under assessment. A summary of the results of an audit by the BCIG of cochlear implant services for the year ending March 2007 is shown in Table 1. Ages ranged from babies of less than 12 months to adults of over 80 years. 49
| Total registered | Implanted, current year | Under assessment | Waiting time first OPD1 (mean months) | |||||
|---|---|---|---|---|---|---|---|---|
| Adults | Children | Adults | Children | Adults | Children | Adults | Children | |
| England | 2599 | 2474 | 374 | 221 | 416 | 434 | 5.4 | 5.6 |
| Wales | 72 | 45 | 8 | 22 | 35 | 12 | 4 | 5 |
Bilateral implantation in the UK
Throughout the UK there had been 115 bilateral implantations by the year ending April 2006; of these, 33 were simultaneous implantations (both ears implanted in the same operation) and 82 were sequential implantations (ears implanted in different operations). In the year ending March 2007 there were an additional 32 child and 11 adult bilateral implantations. There had also been 34 bilateral reimplantations to this date either because of contraindication of more surgery to the first ear or because residual function of the first device was considered likely to contribute to the benefit from a second implant. 49
Estimated future demand
The BAA, BCIG and ENT UK joint submission to NICE in March 200749 estimated (based on the assumption that 25% of severely deaf people with > 85 dB HL may benefit from an implant) that in 2005 there were potentially 625 children and 1620 adults per year who could benefit from implantation.
Anticipated costs associated with cochlear implantation
The costs of cochlear implantation to the NHS mainly comprise the resources involved in assessing deaf people for possible implantation, the purchase costs of the devices (implanted components and speech processors), the costs of surgery and postsurgical care, the costs of tuning (setting the implant to individual requirements) and training to use the devices, and any costs over the lifetime of the implant recipient associated with hardware failures, other complications or routine external device replacements or upgrades.
Cochlear implant devices are currently purchased by the NHS under a long-term procurement contract (framework agreement) between the four main manufacturers and the NHS Supply Chain (formerly part of the NHS Purchasing and Supply Agency). This contract (contract reference number CM/RSG/05/3419) was established in November 2005 and applies until 31 October 2008, with an option to extend for a further 24 months (www.pasa.nhs.uk/PASAweb).
The suppliers and different products included in this agreement are listed in Table 2, together with the price for each product (the ‘applicable national price band’ for buying a full implant system for an NHS trust). The full agreement involves adjustment of these price bandings according to actual sales volumes (price adjustments not shown here). The price of single systems varies from £12,250 to £15,600. One of the suppliers, Neurelec, provides a two-system pack of Digisonic SP cochlear implant devices. The same supplier also offers a 24-channel ‘binaural’ device, which comprises one device and two electrode arrays. Although this is part of the NHS Supply Chain contract it is not shown here because it is not a bilateral cochlear implant.
| Supplier | Product | Cost (£)a | Cost (£) if low sales | Cost (£) if high sales |
|---|---|---|---|---|
| Advanced Bionics | CLARION ICS HiRes 90K Bionics Ear (HF IJ CI-1400–01) | 14,900 | 16,550 | 12,900 |
| HiRes CI 24R with HiFocus Helix Electrode (CI-1400–02H) | 14,900 | 16,500 | 12,900 | |
| Cochlear UK | Nucleus CI 24R (ST) ‘K’ with a Sprint or ESPrit 3G Speech Processor | 14,350 | 14,350b | 14,350b |
| Nucleus CI 24R (CA) Advanced with a Sprint or ESPrit 3G Speech Processor | 14,350 | 14,350b | 14,350b | |
| Nucleus CI11 + 11 + 2 Double Array with a Sprint or ESPrit 3G Speech Processor | 14,350 | 14,350b | 14,350b | |
| Nucleus Freedom with either BTE or BWP optionc | 15,250 | 15,250b | 15,250b | |
| Nucleus Freedom with both BTE and BWP optionc | 15,550 | 15,550b | 15,550b | |
| MED-EL | Pulsar CI-100 (implant and patient kit) | 15,600 | 17,375 | 13,500 |
| Pulsar CI-100 (implant alone) | 13,500 | 13,500 | 13,500 | |
| Neurelec | DIGISONIC SP with Digi SP or Digi SP*K (model no. DX10/SP/K) | 12,250 | 12,250 | 10,200 |
| DIGISONIC SP for bilateral implantation – two full systems (model no. DX10/SP-BILAT) | 18,375 | 18,375 | 15,300 |
Bilateral implantation essentially involves the use of two systems in the same person. However, a range of price discounts are offered by manufacturers to reduce the per system price (usually by offering a percentage discount on the second implant system). These price discounts are discussed more fully in the assessment of cost-effectiveness (Chapter 6).
The other main costs associated with cochlear implantation have been estimated in two relatively recent UK-based studies. 52,54 They are summarised in Table 3 and discussed in more detail in the assessment of cost-effectiveness (Chapter 6). Note that the costs of tuning and maintenance in Table 3 include some costs for repairs and replacements, which under current warranty arrangements would be covered by the manufacturer.
| Cost type/stage of use | Children (£) | Adults (£) |
|---|---|---|
| Assessment | 2843 | 4011 |
| Implantation (excluding hardware costs) | 3480 | 2814 |
| Tuning (first year post implantation) | 9148 | 5262 |
| First year of maintenance | 4716 | 1060 |
| Second year of maintenance | 3640 | 1018 |
| Each subsequent year | 1897 | 861 |
These NHS costs reflect the current organisation of NHS service provision for cochlear implantation, which is via 20 regional tertiary cochlear implant centres in the UK (14 in England, three in Wales, two in Scotland and one in Northern Ireland).
Chapter 2 Definition of the decision problem
Decision problem
The purpose of this report is to assess the clinical effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults.
Because cochlear implants may be placed in either one or both ears, and because having one cochlear implant may be an intermediate step between having none and having two, there are in fact two decision problems in the severely and profoundly deaf population: (1) should people without a cochlear implant have one implanted and (2) should people who already have one (unilateral) cochlear implant receive a second one in the other ear (i.e. bilateral cochlear implantation).
More fully, therefore, the policy questions to be answered are:
-
For severely or profoundly deaf people (who may be either using or not using a hearing aid), is it effective and cost-effective to implant a first (i.e. unilateral) cochlear implant?
-
For severely or profoundly deaf people with a single cochlear implant (either unilateral or unilateral with a hearing aid), is it effective and cost-effective to implant a second (i.e. bilateral) cochlear implant?
In the clinical effectiveness systematic review these questions are answered by looking at eight independent comparisons. These are:
-
In children:
-
– unilateral cochlear implants versus non-technological support (no devices of any kind)
-
– unilateral cochlear implants versus acoustic hearing aids
-
– unilateral cochlear implants versus bilateral cochlear implants
-
– bilateral cochlear implants versus unilateral cochlear implants and acoustic hearing aids.
-
-
In adults:
-
– unilateral cochlear implants versus non-technological support (no devices of any kind)
-
– unilateral cochlear implants versus acoustic hearing aids
-
– unilateral cochlear implants versus bilateral cochlear implants
-
– bilateral cochlear implants versus unilateral cochlear implants and acoustic hearing aids.
-
Although the two policy questions above set out the two main logical comparisons (going from using no cochlear implant to one cochlear implant, and going from using one to two cochlear implants), there is also the clinical reality – and different decision problem – of going straight from having no cochlear implant to bilateral implantation. This is why, in the absence of reliable outcome (especially utility) data to answer the second policy question, the cost-effectiveness of simultaneous and sequential bilateral cochlear implantation is assessed in this report, that is, in deaf adults and children who are not currently cochlear implant users.
Interventions
This assessment considers multichannel cochlear implants using whole-speech processing coding strategies, for example advanced combination encoder (ACE), spectral peak (SPEAK), continuous interleaved sampling (CIS) and speech and motion sensor (SMP) (i.e. devices that are the same as, or comparable with, those currently available on the NHS).
Population including subgroups
The population is children and adults with severe to profound deafness. People with a severe loss of hearing cannot detect tones at an average level below 70–94 dB HL in their better-hearing ear. People with a profound loss of hearing cannot detect tones at an average level below 95 dB HL in their better-hearing ear.
The assessment considered the following groups of people depending on the availability and quality of the data:
-
children who were born deaf or who became deaf before the age of 3 years (prelingually deaf)
-
children who were post lingual (3 years or older) when they became deaf
-
adults who became deaf after learning spoken language compared with adults who were born deaf or who became deaf before acquiring spoken language
-
adults who were born deaf.
The comparison between having no cochlear implant and having one cochlear implant was analysed separately for those already using hearing aids and those only using non-auditory methods to aid communication. (However, we acknowledge that many people who only use non-auditory methods may either be clinically ineligible to receive a cochlear implant or would choose not to have one for the same reasons that they may choose not to use hearing aids.)
The comparison between having one and two cochlear implants was analysed separately for those with a contralateral hearing aid and those with a cochlear implant but no hearing aid in their other ear. This is because the use of a hearing aid, either with or without a cochlear implant, indirectly reflects both the severity and the cause of deafness; they are thus more appropriately defined as subgroups rather than comparators in this assessment.
The extent to which the degree of residual hearing (e.g. severe deafness, profound deafness) and the presence of other additional needs (e.g. dual sensory impairments, learning disabilities) may influence costs and outcomes could be considered but was constrained by lack of data; no utilities were found for severe deafness or co-disabilities. Additionally, a sensitivity analysis, including the wider costs and benefits of educational placement, which are not reflected in health-related quality of life measures, was conducted.
Outcomes
The outcome measures found in the studies included in the systematic reviews were:
-
sensitivity to sound
-
speech perception
-
speech production
-
adverse effects of treatment
-
health-related quality of life
-
educational outcomes.
Overall aims and objectives of assessment
This project will review the evidence for the effectiveness and cost-effectiveness of cochlear implants for children and adults who have severe to profound or profound deafness. The assessment will look at multichannel devices used in one or both ears and will draw together the relevant evidence about unilateral and bilateral cochlear implants and try to determine what, if any, is the incremental cost-effective benefit of the population using one implant rather than acoustic hearing aids or non-auditory support and if there is an additional benefit from using two cochlear implants.
Chapter 3 Clinical effectiveness systematic review methods and search results
Methods for reviewing effectiveness
The clinical effectiveness of cochlear implantation was assessed by a systematic review of published research evidence. The review was undertaken following the general principles published by the NHS Centre for Reviews and Dissemination. 57
Identification of studies
Electronic databases were searched for published systematic reviews and/or meta-analyses, randomised controlled trials (RCT) and ongoing research in October 2006 and this search was updated in July 2007. The updated search revealed one new cross-sectional study. Appendix 1 shows the databases searched and the strategies in full. Bibliographies of articles were also searched for further relevant studies, and the US Food and Drug Administration (FDA) and European Regulatory Agency Medical Device Safety Service websites were searched for relevant material. The search was limited to English language papers only.
Relevant studies were identified in two stages. Abstracts returned by the search strategy were examined independently by two researchers (MB and JE) and screened for inclusion. Disagreements were resolved by discussion. Full texts of the identified studies were obtained. Two researchers (MB and JE) examined these independently for inclusion or exclusion and disagreements were again resolved by discussion. The process is illustrated by the flow chart in Appendix 2.
Inclusion and exclusion criteria
Intervention
This assessment considers one or two multichannel cochlear implants using whole-speech processing coding strategies that attempt to transmit as much sound signal information as possible, for example ACE, SPEAK and CIS, rather than earlier feature extraction strategies. In cases in which the coding strategy was not disclosed in the research paper, attempts were made to contact authors for this information. When there was no response it was assumed that studies which collected data after 1995 used whole-speech processing and that those before did not.
This distinction between coding strategies was made following expert advice that whole-speech processing strategies are considered more effective and that older coding strategies are no longer being implanted by the NHS (Professor Quentin Summerfield, University of York, January 2007, personal communication). The devices currently supplied to the NHS and those in the included studies are shown in Table 4. There are currently 11 cochlear implant devices sold on contract to the NHS. Only two of these were used in the studies included in this report. Fourteen others were used in the studies but are no longer supplied under contract to the NHS.
| Supplier | Brand and model no.a | Year of introduction |
|---|---|---|
| Advanced Bionics | HiRes 90K with HiFocus Helix Electrode CI-1400-02H | 2005 |
| CLARION ICS HiRes 90K Bionic Ear HF I J CI-400-01 | 2003 | |
| CLARION CII HiFocus | 2001 | |
| BI CLARION Platinum Aura | ||
| CLARION multistrategy implant with CIS | 1994 | |
| CLARION 1.2 | ||
| Cochlear UK | Nucleus Freedom with either the BTE or BWP option | 2006 |
| Nucleus CI 24R (ST) ‘K’ with a Sprint or ESPrit 3G speech processor | ||
| Nucleus CI 24R (CA) Advanced with a Sprint or ESPrit 3G speech processor | 2003 | |
| Nucleus CI11+11+2 double array with a Sprint or ESPrit 3G speech processor | 2000 | |
| Nucleus 24 contour | 1997 | |
| Nucleus 24 | 1997 | |
| Nucleus 22 with SPEAK | 1994 | |
| Nucleus multichannel | ||
| MED-EL | Pulsar CI-100 (implant and patient kit) | 2004 |
| Pulsar CI-100 (implant alone) | 2004 | |
| COMBI 40+ | 1996 | |
| COMBI 40 | 1996 | |
| Neurelec | DIGISONIC SP with Digi SP or Digi SP*K, model no. DX10/SP-K | |
| DIGISONIC SP binaural 24 channel, model no. DX10/SP-BIN | ||
| DIGISONIC SP for bilateral implantation, two full systems, model no. DX10/SP-BILAT | ||
| Manufacturers not reported | Tempo+ | |
| Spectra | ||
| CIS Pro+ | ||
| SPRINT |
Comparator
One cochlear implant was compared with non-auditory support, acoustic hearing aids and two cochlear implants. Two cochlear implants were compared with one cochlear implant plus a contralateral acoustic hearing aid.
Population
The population was children aged from 12 months to 18 years and adults.
Outcomes
These included:
-
sensitivity to sound
-
speech perception
-
speech production
-
psychological outcomes
-
educational outcomes
-
adverse events
-
health-related quality of life.
Relevance to the UK NHS of the technology
Studies were included if they were in health-care settings that were considered to be sufficiently similar to the UK to be relevant to this assessment (e.g. Europe, North America and Australasia).
Overview of the policy questions
This technology assessment report seeks to respond to the following NHS policy questions:
-
For severely or profoundly sensorineurally deaf people (who may be either using or not using acoustic hearing aids), is it effective and cost-effective to implant a first (i.e. unilateral) cochlear implant? This first question is addressed by the following comparisons:
-
unilateral cochlear implant versus no other hearing aid (non-technological support)
-
unilateral cochlear implant versus an acoustic hearing aid.
-
-
For severely or profoundly sensorineurally deaf people with a single cochlear implant (either unilateral or unilateral with a hearing aid), is it effective and cost-effective to implant a second (i.e. bilateral) cochlear implant? This second question is addressed by the following comparisons:
-
bilateral cochlear implants versus unilateral cochlear implant
-
bilateral cochlear implants versus unilateral cochlear implant and acoustic hearing aid.
-
Study design hierarchy
Systematic reviews and randomised controlled trials
All systematic reviews and RCTs were included, including those with waiting list controls. Systematic reviews ideally only consider well-conducted RCTs; however, in this instance the evidence base is methodologically highly variable across the policy questions of interest. The inclusion criteria for studies of clinical effectiveness were as follows.
Controlled studies
Other types of controlled studies (i.e. non-RCTs, cross-sectional studies and pre/post studies with people acting as their own controls) were included. These designs, including within-subject designs, were considered acceptable because levels of sensitivity to sound outcome at preimplantation were near or at zero and because hearing loss was unlikely to improve over time. Thus, benefits seen over time can be attributed to the intervention. However, with speech outcomes for children it could be expected that there would be a natural improvement over time. Prospective cohort designs, in which other people acted as control subjects, were included when baseline levels of hearing loss between the two groups were similar.
The inclusion of prospective cohort studies in a systematic review requires caution. The absence of randomisation introduces the possibility of bias in the selection of participants so that the group receiving the intervention may have different characteristics from the control group. These dissimilarities may cause confounding. Further bias may occur in measurement, for example ceiling effects from the benefit of a unilateral cochlear implant may obscure the benefit of an additional implant.
A number of the included studies were prospective case series; although these had the advantage of allowing participants to be their own controls, the validity of the results obtained is uncertain as familiarity with test materials, and therefore procedural learning, may affect results. 58 In observational studies confounding is a greater issue than lack of statistical power. A review59 evaluating non-randomised intervention studies has concluded that:
Results from non-randomised studies sometimes, but not always, differ from results of randomised studies of the same intervention. Non-randomised studies may still give seriously misleading results when treated and control groups appear similar in key prognostic factors.
Data abstraction strategy
Data were independently abstracted by one of five researchers (MB, SM, JE, ZL and CM). Each data extraction form was checked by another researcher. Disagreements were resolved by discussion.
Critical appraisal strategy
Assessments of study quality were performed using the indicators shown in the following sections. Results were tabulated and these aspects described in Chapter 4 and Chapter 5.
Internal validity
Consideration of internal validity addressed the selection of appropriate study groups, the identification of sources of possible confounders and their effects on analyses, whether the study was prospective, the blinding of assessors and data analysts, the validity and reliability of outcome measures, the reporting of attrition and the appropriateness of data analysis.
External validity
External validity was judged according to the ability of a reader to consider the applicability of findings to a patient group in practice. Study findings can only be generalisable if they (1) describe a cohort that is representative of the affected population at large or (2) present sufficient detail in their outcome data to allow the reader to extrapolate findings to a patient group with different characteristics. Studies that appeared representative of the UK population with regard to these factors were judged to be externally valid.
Data synthesis
The high degree of clinical heterogeneity of the studies combined with generally poor reporting of methods, plus a preponderance of non-randomised studies, meant that quantitative pooling of the data has not been possible. Instead, narrative syntheses of studies with tabulated quantitative results have been given.
Clinical effectiveness search results
Structure of the clinical effectiveness results section
The assessment of clinical effectiveness will be presented as follows:
-
a brief summary of the history of cochlear implant research
-
an overview of the quantity and quality of included studies
-
a description of the outcome measures used in the included studies.
Then, separately for children and adults we present:
-
a critical review of the evidence for cochlear implantation with each comparison reviewed in turn, including the type and quality of studies; a summary table of key quality indicators; study results, presented as a narrative description and as tables giving a visual overview of study results; and a summary of the comparison results
-
at the end of the child and adult comparisons a review of studies reporting quality of life outcomes outside the population intervention comparator outcome setting (PICOS) criteria
-
at the end of the children’s section a review of studies reporting educational outcomes outside the PICOS criteria
-
at the end of the child and adult sections a summary of all of the clinical effectiveness studies
-
a review of the safety and reliability of cochlear implants.
Summary of cochlear implant research history
In the late 1970s and early 1980s the earliest research prototype cochlear implants provided totally deaf people with a sensation of sound. This enabled them to identify environmental sounds and possibly a few words. The research issues at that time were those of safety and efficacy and understanding the differences in outcome that people experienced.
In 1993 an RCT50 compared single channel and multichannel devices and showed that multichannel implants had significant advantages. This study led to the end of single channel implantation. Also, in the early 1990s the Iowa research group60 compared the leading makes of multichannel implants by allocating recipients alternately to either device. As well as showing no differences between devices, this group demonstrated that a large number of people would be needed to show significant differences between devices. Thus, the research agenda shifted to studies of small numbers of carefully selected people to test different processing strategies, and large-scale RCTs were not undertaken.
Quantity and quality of studies found
The systematic search of electronic databases for clinical effectiveness studies produced 1581 abstracts/titles.
From the search results 1436 items were excluded; reasons for these exclusions included that items were narrative reviews, preclinical or technical papers, uncontrolled studies, conference abstracts, not relevant to the UK setting, animal studies or outside the PICOS criteria for this assessment. The movement of papers can be seen in the QUOROM flow chart in Appendix 2. One meta-analysis and 144 other primary research papers were obtained for further examination. This led to the exclusion of 97 papers, leaving 47.
Further papers (n = 27) were obtained from the reference lists of the included papers; when these had been assessed four papers were added to the review giving a total of 51 primary research papers in the review of clinical effectiveness.
Because of the large number of eligible studies (n = 51), some of which included a very small sample size (range n = 3 to n = 311), and constraints on resources, we evaluated sufficient studies for each of the eight comparisons (see Chapter 2, Decision problem) to have at least an arbitrary 75% of the total eligible study population for that comparison, starting with the largest studies. We would have preferred to make these further exclusions on grounds of quality; however, on examination it was found that the heterogeneity amongst the studies was such that there was no logical way to pursue this. The 75% population cut-off left a total of 33 studies (13 adult studies and 20 child studies). All of the excluded studies used non-randomised designs.
The main theoretical implication of not including all eligible studies is that the excluded studies may contain evidence that contradicts that presented. In reality this is unlikely to be the case as, although there is a large amount of heterogeneity between the included studies in terms of design, numbers, outcome measures, etc., the results all go in the same direction. It is therefore unlikely that the excluded smaller studies would contradict this finding. Another potential problem could occur if data were pooled, as the results of the excluded studies could change the central estimate; however, in this review, because of heterogeneity, there is no pooling of data. Furthermore, the excluded studies may contain particular information that is not available in the other studies.
The meta-analysis by Cheng and colleagues61 was a comparison of published and unpublished literature on child cochlear implantation. However, all of the included studies were of old technologies excluded from this review. Table 5 provides a summary of the types and numbers of studies included. The relaxing of criteria to include non-RCTs permits the introduction of many sources of bias and limits the possible statistical analyses.
| Comparison | Design | Total studies | n in each group | % of potential participants included | |||
|---|---|---|---|---|---|---|---|
| Waiting list RCTs | Pre/post studies | Cross-sectional studies | Prospective cohort studies | ||||
| Adult groups | |||||||
| One CI vs NT | 0 | 4 | 0 | 0 | 4 | 984 | 89 |
| One CI vs AHA | 0 | 2 | 1 | 1 | 4 | 248 | 91 |
| Two CI vs 1CI | 2 | 2 | 1 | 0 | 5 | 147 | 77 |
| Two CI vs 1CI and AHA | 0 | 0 | 0 | 0 | 0 | 0 | – |
| Total adults | 2 | 8 | 2 | 1 | 13 | 1379 | 88 |
| Child groups | |||||||
| One CI vs NT | 0 | 8 | 0 | 0 | 8 | 848 | 97 |
| One CI vs AHA | 0 | 2 | 1 | 3 | 6 | 535 | 87 |
| Two CI vs 1CI | 0 | 0 | 3 | 0 | 3 | 61 | 84 |
| Two CI vs 1CI and AHA | 0 | 1 | 2 | 0 | 3 | 69 | 100 |
| Total children | 0 | 11 | 6 | 3 | 20 | 1513 | 93 |
| Total both groups | 2 | 19 | 8 | 4 | 33 | 2892 | 90 |
A summary of ongoing trials can be found in Appendix 14.
Definitions of study design used in this report
-
Waiting list RCTs – These are RCTs in which participants are randomly allocated to have the intervention immediately or to go onto a waiting list and have the intervention in the future. Outcomes from both groups are then compared at baseline and at the same time points from baseline. The weakness of this design is that confounding variables may affect the control group in the time before they receive the intervention.
-
Pre/post studies – This design consists of measuring and comparing outcomes before and after the intervention, with participants usually acting as their own controls. The main weaknesses of this design are its inability to account for maturation effects and selection bias.
-
Cross-sectional studies – These measure differences in outcomes between intervention and control groups at one point in time. Usually the intervention and control groups are two different groups of people. However, in the case of cochlear implants they may be the same, as the external component of an implant can be removed and outcomes measured without the device. The main weaknesses of this design are that it cannot report changes over time and if different groups are measured then selection bias may occur.
-
Prospective cohort studies – In this design the intervention group is compared with control subjects who have been selected to have similar characteristics. The weakness of this design is the lack of randomisation, which would control for selection bias and potential confounders.
Summary tables for each comparison are shown at the beginning of the relevant section.
Only seven studies reported both sensitivity to sound and functional measures of severity of deafness. Moreover, there were insufficient studies (with the same comparators) to reveal any apparent relationships between the preimplantation sensitivity to sound hearing level and size of functional outcome.
Of the studies reporting both types of measures of deafness (sound sensitivity and functional ability) only one62 used health utility outcomes; this study classified implant recipients according to preimplantation speech perception using standard sentence tests and when using optimally fitted hearing aids. This can be viewed as a classification according to level of ‘functional hearing’, and was predictive of levels of utility gain with implantation. Given that, in the current UK NHS, ability to benefit from cochlear implantation is primarily judged on the basis of level of functional hearing ability, it is unfortunate that the vast majority of the evaluative research on this technology only reports the audiologically measured severity of deafness of implantation candidates.
Number and type of studies excluded
Studies of single channel implants or those that used feature extraction or compressed analogue coding strategies were excluded as they are not comparable with current NHS practice. In total, 132 studies were excluded from the clinical systematic review. This was for a variety of reasons, for example the outcome measures or comparisons were outside our inclusion criteria, they included technologies that are no longer in current use, none of the data published was usable, they described technical details of the technologies, they were literature reviews or conference proceedings or they had very small sample sizes.
Quality of life and educational outcome studies
The study selection process found only three studies that included measures of quality of life, and no studies with educational outcomes. Therefore, the searches were screened again for studies with these outcomes using broader inclusion criteria that allowed normal-hearing control subjects and no control subjects; further searches were carried out; and references from included studies were checked. Seven studies were identified that included educational outcomes for children with cochlear implantation. For the quality of life of cochlear implant users four studies in children and six studies in adults were found. Quality of life and educational outcomes are therefore reported separately for children and adults in Chapters 4 and 5 respectively.
Outcome measures
This section reports an overview of outcome measures used in the included studies. The outcome measures selected by the authors of the included studies reflect the hypothesised benefits that may come from cochlear implantation. These are enhanced auditory receptive skills with evidence of emergence of aural/oral communication modes, followed by useful levels in ability in spoken language; improved performance at school in terms of academic achievement and reduced levels of specialist educational support, leading to enhanced social skills; a successful transition to secondary education; and better educational outcomes with better further educational and employment prospects, which may lead to greater independence and quality of life.
The outcome measures can be categorised as sensitivity to sound, speech perception, speech production, quality of life and educational. Because of the large numbers of measures (n = 62) reported in the included studies they are described in more detail in Appendix 5. Here we present a brief description of the different types of outcome measure followed by a list of outcomes by type and the number of studies that used each one. In Tables 6–10, measures shaded in dark grey were used with adults, those shaded with light grey were used with adults and children and those unshaded were used with children.
| Measure | No. of studies using this measure |
|---|---|
| Basal auditory ability64 | 1 |
| CAP – Categories of Auditory Performance65 | 1 |
| MAA – Minimal audible angle | 3 |
| MAIS (IT-MAIS) – Meaningful Auditory Integration Scale62 | 2 |
| PTA – Pure-tone audiometry | 2 |
| SSQ – Speech Hearing, Spatial Hearing and Qualities of Hearing questionnaires63 | 1 |
| Measure | No. of studies using this measure |
|---|---|
| One-syllable test70 | 1 |
| Two-syllable test70 | 1 |
| AB monosyllables – Arthur Boothroyd monosyllabic word test71 | 1 |
| AVGN – A normalised index of audiovisual gain | 1 |
| BKB – Bamford–Kowal–Bench sentences67 | 5 |
| CAP – Categories of Auditory Performance72 | 1 |
| CDT – Connected discourse tracking73 | 1 |
| CID sentences – Central Institute for the Deaf sentences74 | 1 |
| CNC – Consonant Nucleus Consonant monosyllabic word test75 | 4 |
| Common Phrases Test76 | 3 |
| CUNY – City University of New York77 | 5 |
| ESP – Early Speech Perception battery68 | 5 |
| FMWT – Freiburger monosyllabic word test78 | 1 |
| GASP – Glendonald Auditory Screening Procedure69 | 7 |
| Gottinger speech lists79 | 1 |
| HINT – Hearing in Noise Test80 | 3 |
| HINT-C – Hearing in Noise Test for Children80 | 3 |
| HSM sentences – Hochmaier, Schultz and Moser sentence test81 | 2 |
| IMST – Iowa Matrix Sentence Test82 | 1 |
| LNT – Lexical Neighbourhood Test83 | 2 |
| MAC – Minimal Auditory Capabilities84 | 1 |
| Minimal Pairs Test76 | 1 |
| MLNT – Multisyllabic Lexical Neighbourhood Test85 | 2 |
| Mr Potato Head86 | 3 |
| NU-6 – Northwestern University Auditory Test number 687 | 1 |
| OLSA – Oldenburg sentence test88 | 1 |
| PB-K – Phonetically Balanced Kindergarten Word List89 | 5 |
| RITLS – Rhode Island Test of Language Structure90 | 1 |
| SECSHIC – Scales of Early Communication Skills for Hearing Impaired Children91 | 1 |
| TAC – Test for Auditory Comprehension of Language92 | 1 |
| TAPS – Test for Auditory Perception and Speech93 | 1 |
| TROG – Test for the Reception of Grammar94 | 1 |
| Measure | No. of studies using this measure |
|---|---|
| CRISP – Children’s Realistic Intelligibility and Speech Perception test95 | 2 |
| IPSyn – Index of Productive Syntax96 | 1 |
| SIR – Speech Intelligibility Rating97 | 1 |
| Measure | No. of studies using this measure |
|---|---|
| APHAB – Abbreviated Profile of Hearing Aid Benefit98 | 1 |
| AQoL – Assessment of Quality of Life99 | 2 |
| Everyday Life Questionnaire100 | 1 |
| EQ-5D – EuroQol 5 dimensions101,102 | 1 |
| GBI – Glasgow Benefit Inventory103 | 2 |
| GHSI – Glasgow Health Status Inventory104 | 2 |
| HHIA – Hearing Handicap Inventory for Adults105 | 1 |
| HPS – Hearing Participation Scale106 | 1 |
| HUI-3 – Health Utilities Index 3107 | 2 |
| IRQF – Index Relative Questionnaire Form108 | 1 |
| KINDLr – Munich Quality of Life Questionnaire for Children109 | 1 |
| NCIQ – Nijmegen Cochlear Implant Questionnaire110 | 1 |
| PQLF – Patient Quality of Life Form108 | 1 |
| Quality of life questionnaire111 | 2 |
| SF-36 – Short-Form 36112 | 1 |
| Symptom Checklist 90-R113 | 1 |
| Tinnitus Questionnaire114 | 1 |
| ULS – Usher Lifestyle Questionnaire115 | 1 |
| VAS quality of life scale – Visual analogue scale101,102 | 1 |
| Measure | No. of studies using this measure |
|---|---|
| AMP – Assessment of Mainstream Performance116 | 1 |
| SIFTER – Screening Instrument for Targeting Educational Risk117 | 1 |
Sensitivity to sound measures
Six different sensitivity to sound measures were used in 10 studies, nine of which were studies of children (Table 6).
Some of these measures used everyday sounds, for example the basal auditory ability test, which determines whether a child can correctly associate a common sound with its source, such as a door bell. Real-life listening behaviours of children were measured by proxy from carer questionnaires with the Meaningful Auditory Integration Scale (MAIS). 63 Other instruments were laboratory-based, measuring the ability to detect the direction of sound (Speech, Spatial and Quality of Hearing questionnaires64) or the smallest change in position that could be discriminated (minimal audible angle, MAA).
Speech perception measures
Most studies reported speech perception measures. In total, 32 measures were used; 11 measures were used for adults, one measure was used for adults and children (Bamford–Kowal–Bench sentences67) and 20 were used only on children (Table 7). The tests consisted of lists of phonemes, words or sentences that had to be correctly identified. Some tests included word recognition tasks in which a word is spoken and the correct picture has to be pointed to [e.g. the Early Speech Perception (ESP) battery68]. Other tests [e.g. the Glendonald Auditory Screening Procedure (GASP)69] required a verbal response and so could also be used to measure speech production. These outcome measures place varying cognitive demands on people to complete the tasks, i.e. perception, discrimination, recognition and understanding at different levels (word, sentence, phoneme). This means that the tests and results are not all comparable and cannot be considered as equally difficult.
Speech production measures
Speech production measures were less frequently used, including three measures in four studies, all of which were in children (Table 8). Measures evaluated the intelligibility of whole speech by a range of listeners (Speech Intelligibility Rating) and parts of speech such as noun phrases (Index of Productive Syntax).
Quality of life measures
Quality of life with cochlear implants was measured in 23 studies using 19 different instruments (children, five; adults, 13; both, one) (Table 9). A range of experience was covered by the measures, which included ad hoc, condition-specific questionnaires (Everyday Life Questionnaire) to generic, validated measures of utility (Health Utilities Index 3). Other instruments measured particular aspects of quality of life and psychological, social, emotional and physical states (Glasgow Health Status Inventory) or focused on specific diseases or symptoms (Usher Lifestyle Questionnaire and the Tinnitus Questionnaire).
Educational measures
Only two questionnaire measures of educational outcomes were used (Table 10). These measured the skills that deaf children need to succeed in mainstream education [Assessment of Mainstream Performance (AMP)] and school performance [Screening Instrument for Targeting Educational Risk (SIFTER)].
Other considerations about measures and their implementation
There is some evidence that the choice of speech recognition test can affect outcomes; sentences that have more syllables per minute are harder to recognise. 118 It has also been shown that a known voice is easier to understand than an unknown one. 119
Chapter 4 Results of the clinical effectiveness evidence for children
The majority of studies reported results in figures (usually bar charts) rather than in the text or in tables. To maximise accurate data extraction the figures had to be enlarged (×400%) to enable reading of the study results. Thus, values may deviate from values measured by the original authors. Summary tables of study characteristics and results can be found in Appendix 3.
Unilateral cochlear implants versus non-technological support – children
This section considers studies in which the comparisons did not include devices of any kind.
Type and quality of studies
Eight studies were included in the review of evidence for one cochlear implant versus non-technological support (i.e. the absence of acoustic or tactile aids but permitting sign language and lip-reading). All used pre/post designs, with participants acting as their own controls. Two of the studies were based in the UK,120,121 two in other European countries,70,122 two in Canada123,124 and two in the USA. 125,126 There were 848 participants in total, with sample sizes ranging from 49 to 182. Participants’ ages were between 1 year 3 months and 17 years 11 months. Follow-up times ranged from 6 months to 12 years. Two studies were excluded on the grounds of population size (n = 10 and n = 19); however, 97% of the total population was included.
Surprisingly two studies did not report the degree of deafness of participants; four of the studies had a profoundly deaf population; and the other two studies’ populations were severe to profoundly deaf. The outcome measures used varied widely between studies and covered measures of sensitivity to sound, speech perception and speech production. See Appendix 3 for summary tables of study characteristics and results.
As can be seen from Table 11, overall the studies were of moderate to poor quality, with inadequate descriptions of methods and lack of reporting of important quality markers. None of the studies was an RCT, yet possible confounding factors were scarcely reported, and in only one case120 were they accounted for in the analysis. Furthermore, not all participants were accounted for, and neither was the treatment of missing data reported.
| Quality criteria | Harrison 2005122 | Nikolopoulos 2004119 | Manrique 2004121 | Staller 2002123 | MED-EL 2001124 | Nikolopoulos 1999120 | Illg 199969 | Kessler 1997125 |
|---|---|---|---|---|---|---|---|---|
| Was the study prospective? | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Selection bias | ||||||||
| Eligibility criteria stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Appropriate? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | – |
| Were the participants representative of the population? | Yes | Partlya | Partlya | Yes | Yes | Yes | Yes | Yes |
| Were potential confounders reported? | Yes | Yes | Yes | No | No | No | Some | No |
| Were they accounted for in the design or analysis? | No | Yes | NR | NR | NR | NR | NR | NR |
| Assessment bias | ||||||||
| Were the outcome measures relevant to the research question? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Independent blind assessment? | NR | NR | NR | NR | NR | NR | NR | NR |
| Objective? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Attrition bias | ||||||||
| Was attrition reported? | No | Yes | No | No | Yes | Yes | Yes | Yes |
| Were all participants accounted for? | No | No | No | No | Yes | Yes | Yes | No |
| How were missing data accounted for? | NR | NR | NR | NR | NR | NR | NR | NR |
| Protocol violations specified? | NR | NR | NR | NR | NR | NR | NR | NR |
| Power and analysis | ||||||||
| Data analysis | ANOVA | DS | t-test | DS | DS | DS | DS | DS |
| Was the analysis appropriate? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was there a power calculation? | No | No | No | No | No | No | No | No |
| Other | ||||||||
| Was ethical approval given? | NR | NR | NR | NR | NR | NR | NR | NR |
| Generalisability? | Yes | Somewhat | Somewhat | Yes | Yes | Yes | Yes | Yes |
| Intercentre variability? | NA | NA | NA | NR | NR | NA | NA | NA |
Study results for unilateral cochlear implants versus non-technological support
Despite the large variety of outcome measures used the overall results for all outcomes from all studies were in favour of cochlear implants. The outcomes used in the studies can be classified as sensitivity to sound, speech perception or speech production. For further clarification of the measures see Tables 6–10, Chapter 3 (Clinical effectiveness search results) and Appendix 5 (Tables 110–114). Table 12a–c provides a visual summary of the results by type of outcome measure. A summary of the characteristics and results of the included studies can be found in Appendix 3 (Tables 92 and 93 respectively).
| Study design (follow-up, months) | Study | n | Audiological outcomes |
|---|---|---|---|
| PTA | |||
| PP (P) (144) | Manrique 2004;122 2004127 | 182 |
| Study design (follow-up, months) | Study | n | Speech perception outcomes | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAIS | IT-MAIS | CAP | GASP (words/sentences) | PKB (words/phonemes) | TAC | TROG | ESP | LNT | HINT-C | MLNT | BKB sentences | Communicative skills checklist | IMST | CDT | TAPS | Mr Potato Head | Monosyllable word test | Pattern perception | One-syllable test | Two-syllable test | MPT | |||||
| PP (R) (96) | Harrison 2005122 | 82 | ||||||||||||||||||||||||
| PP (P) (60) | Nikolopoulos 2004120 | 82 | ||||||||||||||||||||||||
| PP (P) (6) | Staller 2002124 | 78 | ||||||||||||||||||||||||
| PP (P) (6) | MED-EL 2001126 | 82 | ||||||||||||||||||||||||
| PP (P) (72) | Nikolopoulos 1999121 | 126 | ||||||||||||||||||||||||
| PP (P) (36) | Illg 199970 | 167 | ||||||||||||||||||||||||
| PP (P) (6) | Kessler 1997126 | 49 | ||||||||||||||||||||||||
| Study design (follow-up, months) | Study | n | Speech production outcomes |
|---|---|---|---|
| SIR | |||
| PP (P) (72) | Nikolopoulos 1999121 | 126 |
Sensitivity to sound
Only one study, conducted by Manrique and colleagues (n = 182),122,127 measured sensitivity to sound in this comparison. They found a significant improvement in pure-tone audiometry (PTA) scores (p < 0.05) at 12 months post activation compared with preimplantation (preimplantation = 115.8, SD 3.25; 12 months post implantation = 34.3, SD 8.25). This indicates that a fundamental change in the children’s ability to detect sound had occurred.
Speech perception
In total, 666 children were measured for speech perception ability across seven studies using 22 different instruments.
Staller and colleagues (n = 78)124 used four measures of speech perception [ESP battery, GASP, Lexical Neighbourhood Test (LNT) and the Hearing In Noise Test for Children (HINT-C)]. The results showed a range of improvements for children (age 3–17 years) over 6 months, from a 35% difference with the LNT (word recognition) to a 50% difference with the HINT-C (sentence recognition).
Further evidence for the benefits of cochlear implants came from the MED-EL report (for the FDA) (n = 82). 125 This measured speech perception 6 months post activation with six instruments [ESP battery, GASP, Communicative Skills Checklist for all ages (18 months to < 18 years) and the Multisyllabic Lexical Neighbourhood Test (MLNT), LNT and Bamford–Kowal–Bench (BKB) test for older children (≥ 5 to < 18 years)]. The scores for younger children ranged from a 50% difference on the ESP spondee identification test (two long syllables) to a 70% difference on the ESP battery (pattern perception test). Older children’s scores ranged from a 53% difference with the BKB test (simple sentences) to a 79% difference with the ESP spondee identification test and the GASP. However, not all children were entered for all tests.
An earlier study by Illg and colleagues (n = 167)70 reported on seven measures in children from 12 months to 15 years over a 2-year follow-up period [Test for Auditory Perception and Speech (TAPS), GASP (word and sentence recognition), Mr Potato Head (following instructions to assemble the toy), pattern perception, one- and two-syllable tests and a minimal pairs test]. All results showed a trend favouring cochlear implants, with scores for younger children (< 7 years) ranging from an 8% (SD 8.5) difference with the GASP to a 59% difference with a pattern perception test. Older children’s (7–15 years) scores ranged from a 15% (SD 14.5) difference with the Mr Potato Head assembly task and the minimal pairs test (words that differ by one feature) to a 39% difference with a pattern perception test.
Kessler and colleagues’ much smaller study (n = 49)126 measured speech perception with the Phonetically Balanced Kindergarten Word List (PB-K), ESP, GASP and Mr Potato Head over 6 months for children aged 7 years or over. They found that all outcome measures showed a benefit from cochlear implants, with a range of improvement in scores from a 32% difference with the PB-K to a 54% difference with the ESP.
Additionally, the MED-EL, Staller and Kessler studies reported parental ratings of listening behaviours (e.g. responding to a door bell) using the MAIS as the mean percentage point improvement over 6 months. Although significance was not reported, all found increased scores (MED-EL = 38%, Staller = 16%, Kessler = 20%).
All of these four studies70,124–126 found a positive association between early age at implantation and improvements in speech understanding. However, only one study reported significance levels.
Harrison and colleagues (n = 82)123 used four speech perception tests [Test for Auditory Comprehension (TAC), GASP, PB-K word test and PB-K phoneme test] with children aged from 2 to 13 years. They found a positive trend associated with earlier implantation with mean differences from preimplantation ranging from 6.36% with the TAC to 84.25% with the GASP. A similar association between age at implantation and positive outcome was found by Nikolopoulos and colleagues (n = 82)120 who examined participants’ understanding of English grammar and found a link between earlier age at implantation and greater understanding of the construction of grammar. The proportion of those with understanding comparable to normal-hearing peers rose from 2% at preimplantation to a remarkable 67% after 5 years when measured with the Test for the Reception of Grammar (TROG).
In an earlier study Nikolopoulos and colleagues121 found significant negative correlations with age at implantation at 3 (–0.38) and 4 (–0.58) years from baseline on the connected discourse tracking (CDT) measure of auditory performance (p < 0.01), and at 4 years (–0.44) with the Iowa Matrix Sentence Test (IMST), a closed-set sentence test (p < 0.05), thus further indicating that increased benefit from cochlear implants was associated with earlier implantation. Interestingly, Nikolopoulos and colleagues also showed significantly greater improvements for younger-implanted children than older-implanted children in Categories of Auditory Performance (CAP; a measure of performance on a range of auditory tasks performed in a quiet situation); scores were recorded at between 24 and 48 months of implant use (correlation coefficients with age at implantation: 24 months = –0.32, p = 0.006; 36 months = –0.48, p = 0.0007; 48 months = –0.58, p = 0.002). This indicates that in quiet situations there was again benefit from earlier implantation.
Speech production
Results for speech production were similarly positive. Only one study, that by Nikolopoulos and colleagues (n = 126),121 examined the effects of age at implantation on speech production. Using the Speech Intelligibility Rating (SIR) scale they found that at 4 years post activation there was a significant correlation (–0.49) between earlier implantation and better speech production (p < 0.01).
Summary: effectiveness of unilateral cochlear implants versus non-technological support – children
There is considerable heterogeneity in the studies of unilateral cochlear implants versus non-technological support. The variety of outcome measures used, the range of methods of data analysis and the limited reporting mean that pooling of data was not possible and drawing firm conclusions is difficult. However, weight should be given to the large total number of participants (n = 848) and the prospective design of most of the studies. All studies reported gains on all reported outcome measures, some demonstrating greater gain from earlier implantation.
Measures of sensitivity to sound provide the strongest evidence to support the use of cochlear implants. Clear gains were made from 6 months post activation, with PTA thresholds ranging from 32 to 44 dB HL post implantation. 122
The results of speech perception and production outcomes have almost certainly been biased by confounding from maturation: as children grow older their ability to understand and produce language may have improved independently. However, the degree of improvement in the ability to understand the speech of others and to produce intelligible speech is likely to be greater than that due to ageing alone, for example a 50% improvement in understanding speech in noise124 and a correlation coefficient between the ability of other people to understand their speech after 4 years and age at implantation of –0.49. 121
Overall conclusions
Unilateral cochlear implants improve the hearing, speech perception and speech production of severely to profoundly and profoundly sensorineurally deaf children, and additional benefit may be gained by early implantation.
Unilateral cochlear implants versus acoustic hearing aids – children
Type and quality of studies
Six studies were included in the review of evidence for unilateral cochlear implants versus acoustic hearing aids. Two of the studies were prospective cohorts, two were prospective pre/post studies with repeated measures and participants acting as their own controls, one was a cross-sectional study and one was a retrospective cohort design. Four studies were from the USA and two were from Europe. There were 535 participants in total, with population sizes ranging from 30 to 297. Ages of participants ranged from 9 months to 17 years, and all children were profoundly deaf. Three studies were excluded on the grounds of the small size of the study population (total n = 70; range 20–26), leaving 87% of the total population included. Again, outcome measures varied widely between studies. A summary of the characteristics and results of the included studies can be found in Appendix 3 (Tables 94 and 95 respectively).
Table 13 gives a summary of the key quality indicators for the included studies. Overall the studies were of moderate to poor quality, with inadequate descriptions of methods and lack of reporting of important quality markers. The lack of randomisation potentially introduces bias to all of these studies. No information was given about how participants were selected; frequently the results were only presented in figures, which were read off with a degree of inaccuracy. Two of the studies used different participants as control subjects; however, the groups were poorly matched. Generally not enough information was given to assess fully how studies were conducted.
| Quality criteria | Mildner 2006128 | Tomblin 1999129 | Osberger 1999130 | Svirsky 1999131 | Osberger 1998132 | van den Borne 199865 |
|---|---|---|---|---|---|---|
| Was the study prospective? | No, cross-sectional | Yes | Yes | No | Yes | Yes |
| Selection bias | ||||||
| Eligibility criteria stated? | No | Yes | Yes | Yes | Yes | Yes |
| Appropriate? | – | Yes | Yes | Yes | Yes | Yes |
| Were the participants representative of the population? | Yes | Somewhata | Somewhatb | Yes | Somewhatb | Yes |
| Were potential confounders reported? | No | Yes | Yes | Yes | Yes | No |
| Were they accounted for in the design or analysis? | – | Yes | Yes | Yes | Yes | – |
| Assessment bias | ||||||
| Were the outcome measures relevant to the research question? | Yes | Yes | Yes | Yes | Yes | Yes |
| Independent blind assessment? | NR | Yes | NR | NR | NR | NR |
| Objective? | Yes | Yes | Yes | Yes | Yes | Yes |
| Attrition bias | ||||||
| Was attrition reported? | No | Yes | No | Yes | No | Yes |
| Were all participants accounted for? | Yes | Yes | Unclear | No | Unclear | No |
| How were missing data accounted for? | NR | NR | NR | NR | NR | NR |
| Protocol violations specified? | NR | NR | NR | NR | NR | NR |
| Power and analysis | ||||||
| Data analysis | DS, chi-squared test | t-test, ANOVA | DS, ANOVA | DS, linear regression, ANOVA | ANOVA | DS |
| Was the analysis appropriate? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was there a power calculation? | No | No | No | No | No | No |
| Other | ||||||
| Was ethical approval given? | NR | NR | NR | NR | NR | NR |
| Generalisability? | Yes | Somewhat | Somewhat | Yes | Somewhat | Somewhat |
Study results for unilateral cochlear implants versus acoustic hearing aids
The studies covered sensitivity to sound, speech perception or speech production outcomes, with the overall results being in favour of cochlear implants. However, there were some equivocal results from the study by van den Borne and colleagues65 for speech perception, possibly because of lower baseline scores for the cochlear implant group.
Sensitivity to sound
In the study by van den Borne and colleagues65 a total of 43 children had auditory outcomes measured. The ability to detect everyday sounds was measured on a scale from 1 to 4; both groups were measured before implant and at 6-month intervals, up to 24 months post implant. Both groups were measured with acoustic hearing aids before implant after which the cochlear implant group were measured with implants alone. The score in the cochlear implant group improved by 3.5 points and that in the acoustic hearing aid group by 1.9 points during this time.
Speech perception
Across all studies a total of 209 children had their ability to understand speech measured; two studies reported significance levels.
Mildner and colleagues (n = 49)128 used a cross-sectional study design to compare children with cochlear implants or acoustic hearing aids. They found a mean percentage gain in understanding visually and orally presented words for the cochlear implant group, with an overall difference in word scores of 22.4%, (p < 0.01) (cochlear implant group = 82.8%, acoustic hearing aid group = 60.4%).
An earlier pre/post implantation study by Osberger and colleagues (n = 58)130 measured speech perception using five tests (ESP, GASP, Mr Potato Head, common phrases test, PB-K phonemes and words). Improvements were seen on all measures over 18 months, ranging from a mean score difference between times of 19.9 on the common phrases test to 56.5 on the ESP. All measures showed a significant difference in favour of cochlear implants (p < 0.0001).
A much larger study by Svirsky and colleagues (n = 297)131 compared the difference between actual PB-K words scores for implanted children and predicted PB-K scores for children using acoustic hearing aids. However, they reported insufficient information to calculate the difference in scores for the acoustic hearing aid group. The cochlear implant group mean scores improved by 6.3% over 18 months for those aged less than 6 years and by 6.5% over 12 months for those aged between 6 and 12 years.
A small study by Osberger and colleagues (n = 30)131 measured speech perception using three instruments (ESP, GASP words and sentences, PB-K phonemes and words). Measures were taken before implantation with acoustic hearing aids and 6 months post implantation with cochlear implants. The results showed improvements on all measures over 6 months for the cochlear implant group. The difference in scores between the groups ranged from a mean percentage score difference of 33.3% on PB-K phonemes to 49.6% on PB-K words; however, statistical significance was not reported. These participants may be a subset of those of Osberger and colleagues. 130
van den Borne and colleagues (n = 43)65 also reported on speech perception, this time using the Scales of Early Communication Skills for Hearing Impaired Children (SECSHIC), in a prospective cohort study. Their results showed a small relative improvement in verbal receptive skills over baseline for cochlear implant users compared with those using acoustic hearing aids of 0.1 over 24 months. However, the actual scores at 24 months were better for acoustic hearing aid users (cochlear implant group = 50, acoustic hearing aid group = 54), although it should be noted that the baseline scores were lower for the cochlear implant group (cochlear implant group = 43, acoustic hearing aid group = 47.5). As both groups made gains from their baseline scores (cochlear implant group = +7.0, acoustic hearing aid group = +6.9) it would appear that maturation effects contributed to improvements in receptive language.
Speech production
Only Tomblin and colleagues (n = 58)129 reported speech production measures, using the Index of Productive Syntax (IPSyn) to analyse transcripts of children retelling stories in a prospective cohort study. Their results showed a mean difference in 5-year total scores of 19.6 in favour of cochlear implants. However, these results may be susceptible to bias as the cochlear implant group had the advantage of repeated exposure to the test whereas the acoustic hearing aid group had only one exposure. Regression analysis showed that, when age was included, length of use of cochlear implants was the main factor in IPSyn scores.
The visual summary of results in Table 14a–c shows the overall positive impact of cochlear implants compared with hearing aids for the profoundly deaf children who participated in these studies.
| Study design (follow-up, months) | Study | n | Auditory outcomes |
|---|---|---|---|
| Basal sound identification | |||
| PC (36) | van den Bourne 199864 | 43 |
| Study design (follow-up, months) | Study | n | Speech perception outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall word scores | Response to vowels | Response to consonants | ESP | GASP (words/sentences) | Mr Potato Head | Common phrases | PKB (words/phonemes) | SECSHIC | ||||||
| XS | Mildner 2006128 | 49 | ||||||||||||
| PP (P) (18) | Osberger 1999130 | 58 | ||||||||||||
| PC (18) | Svirsky 1999131 | 29 | ||||||||||||
| PP (P) (6) | Osberger 1998132 | 30 | ||||||||||||
| PC (36) | van den Bourne 199865 | 43 | ||||||||||||
| Study design (follow-up, months) | Study | n | Speech production outcomes |
|---|---|---|---|
| IPSyn | |||
| PC (60) | Tomblin 1999128 | 58 |
Summary of studies: unilateral cochlear implants versus acoustic hearing aids
Again, heterogeneity and limited reporting precluded meta-analysis. However, the results on a variety of outcomes for 535 profoundly sensorineurally deaf children (range > 98 to ≥ 110 dB HL) indicate that for this group greater gains in sensitivity to sound, speech perception and speech production can be made with cochlear implants compared with acoustic hearing aids.
Only one study reported sensitivity to sound, showing that children with cochlear implants had mean scores 1.6 points above those of children with acoustic hearing aids on a 4-point scale measuring the ability to identify everyday sounds.
In addition to poor reporting, some studies excluded children with other physical or learning disabilities, and this, together with the diverse outcomes, makes comparison between studies difficult. However, all of the speech perception outcomes measured were in favour of cochlear implants. They ranged from a difference from baseline of 0.1 on the SECSHIC to 56.5 on the ESP.
The results for speech production are weakened by the bias introduced from the greater test exposure given to the cochlear implant group.
Overall conclusions
The evidence suggests that cochlear implants facilitate improved sensitivity to sound and speech outcomes for profoundly sensorineurally deaf children when compared with acoustic hearing aids. However, methodological variation and other study limitations affect the certainty of this conclusion.
Unilateral cochlear implants versus bilateral cochlear implants – children
Type and quality of studies
Three studies have compared unilateral cochlear implantation with bilateral cochlear implantation in children. These were cross-sectional studies with participants acting as their own controls, that is, all children had been bilaterally implanted; tests were taken with either one or both external components in place. The study by Peters and colleagues133 was a larger study with a pre/post repeated measures design of bilateral implants versus a unilateral implant and acoustic hearing aid and so this study is also reported in the next comparison. Two of the studies were from the USA and one from Europe. A total of 61 children participated, with sample sizes ranging from 13 to 30. Ages of participants ranged from 2 years 11 months to 13 years. All of the studies were funded by manufacturers of the devices. One study was excluded on the grounds of sample size (n = 10); 86% of the possible total population was included. All of the participants in these studies were severe to profoundly deaf. One study measured sensitivity to sound; the other two looked at different speech perception outcomes. A summary of the characteristics and results of the included studies can be found in Appendix 3 (Tables 96 and 97 respectively).
Table 15 gives a summary of the key quality indicators. The quality of the studies varied from moderate to poor. In only one study134 were potential confounding factors identified and accounted for in the design or analysis. In another study79 the eligibility criteria were not stated and so it is not possible to say whether the results are generalisable. It is assumed that because the children were bilaterally implanted they were severe to profoundly sensorineurally deaf.
| Quality criteria | Peters 2007133 | Litovsky 2006134 | Kuhn-Inacker 200479 |
|---|---|---|---|
| Was the study prospective? | Yes | Yes | Yes |
| Selection bias | |||
| Eligibility criteria stated? | Yes | Yes | No |
| Appropriate? | Yes | Yes | Unknown |
| Were the participants representative of the population? | Somewhata | Yes | Unknown |
| Were potential confounders reported? | Yes | Yes | No |
| Were they accounted for in the design or analysis? | No | Yes | Yes |
| Assessment bias | |||
| Were the outcome measures relevant to the research question? | Yes | Yes | Yes |
| Independent blind assessment? | NR | NR | NR |
| Objective? | Yes | Yes | Yes |
| Attrition bias | |||
| Was attrition reported? | No | NA | NA |
| Were all participants accounted for? | No | Yes | No |
| How were missing data accounted for? | NR | NR | NR |
| Protocol violations specified? | No | No | No |
| Power and analysis | |||
| Data analysis | DS | DS | DS |
| Was the analysis appropriate? | Yes | Yes | Yes |
| Was there a power calculation? | NR | NR | NR |
| Other | |||
| Was ethical approval given? | NR | Yes | NR |
| Generalisability? | Somewhat | Yes | Unknown |
Study results for unilateral implants versus bilateral implants
The outcomes measured in these studies were either sensitivity to sound or speech perception. The studies all showed a direction of change in favour of bilateral implantation.
Sensitivity to sound
Only one small study, that by Litovsky and colleagues (n = 13),134 reported sensitivity to sound, using the MAA, which assess the narrowest angle at which a person can detect a change in sound direction. This is used to determine whether there is an advantage of bilateral implantation in improving the ability to tell the direction that a sound has come from. However, of the 13 participants recruited, only the nine who found the task easiest were measured, somewhat undermining these results. Litovsky and colleagues found that these children were able to discriminate sound location better with two implants than with one (mean score first side 27.7%, second side 29.7%, bilateral 16.2%; p < 0.001).
Speech perception
Two studies with a total of 48 participants measured speech perception using six tests.
The cross-sectional study by Peters and colleagues (n = 30)133 measured speech perception in quiet conditions with the MLNT, LNT and HINT-C and in noise with the Children’s Realistic Intelligibility and Speech Perception (CRISP) test. The participants were grouped by age (group 1: 3–5 years, group 2: 5.1–8 years, group 3: 8.1–13 years). Children recruited were not a representative sample of candidates for cochlear implantation, as only those who already had open-set speech perception abilities with their first implant were eligible.
None of the results in quiet conditions reached significance. However, all of the results showed a trend in favour of the use of bilateral implants. The difference in scores ranged from 5% with the HINT-C in the oldest group to 13% with the LNT for the youngest group. They found that children who received their second implant when they were younger than 8 years old did better at word recognition than those who received their second implant when they were older (10.6% mean improvement < 8 years, 5.5% mean improvement 8–13 years). The CRISP test directs sound from the front and at both ears individually to test the ability to identify picture and sound combinations. All sound directions showed a significant bilateral advantage with the greatest advantage when noise was directed at the ear that was implanted first (mean improvement of 13.2%; p < 0.0001).
Similar results were found in an earlier small cross-sectional study by Kuhn-Inacker and colleagues (n = 18). 79 They measured speech perception with the Gottinger test in quiet conditions and with a discrimination in noise test. Both tests showed a trend in favour of bilateral implantation. Mean scores in quiet conditions were 70% and 71% for each ear independently and 87% bilaterally. When tested in noise the unilateral mean score was 60% and the bilateral mean score was 80%. However, this study was very poorly reported with no description of the selection criteria and other key quality indicators.
Table 16a and b provides a visual summary of the results.
| Study design (follow-up, months) | Study | n | Auditory outcomes |
|---|---|---|---|
| MAA degrees azimuth | |||
| XS (OC) | Litovsky 2006134 | 13 |
| Study design (follow-up, months) | Study | n | Speech perception outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MLNT words | LNT words | HINT-C | CRISP (noise)a | Gottinger test (quiet/noise) | |||||
| XS (OC) | Peters 200770 | 30 | |||||||
| XS (OC) | Kuhn-Inacker 20048 | 18 | |||||||
Summary of studies for unilateral cochlear implants versus bilateral cochlear implants – children
The heterogeneity between the studies, small numbers of participants, weaknesses in design, poor reporting of methods and lack of controlling for confounding factors mean that it is difficult to come to firm conclusions regarding the benefits of bilateral versus unilateral implantation for children.
In laboratory conditions Litovsky and colleagues134 found that the most able children in their study had an improved ability to detect sound direction of 12.5° azimuth. However, it is hard to generalise from this small sample (n = 13) in artificial conditions; larger numbers tested in noise are needed to accurately gauge differences between these two modalities in real-life conditions.
The strongest evidence for an advantage from bilateral implantation comes from speech perception outcomes measured in noise. Peters and colleagues133 found a mean improvement after bilateral implantation compared with unilateral implantation of +13.2% (p < 0.0001).
Peters and colleagues133 also found that age at second implant affected the speed and final level of improvement. In addition, Kuhn-Inacker and colleagues79 found a greater degree of improvement in noise than in quiet (20% in noise and 16.5% in quiet).
Overall conclusions
The evidence from these studies, albeit with important limitations, suggests that there may be an advantage of bilateral implantation over unilateral implantation in children. However, in our opinion, larger, better-quality studies are needed to establish this with certainty.
Bilateral cochlear implants versus unilateral cochlear implant and an acoustic hearing aid – children
Type and quality of studies
One pre/post repeated measures study and two cross-sectional studies were included in the comparison of two cochlear implants versus one cochlear implant with an acoustic hearing aid. Two of the studies133,134 were included in the previous comparison but different outcomes are reported here. All of the studies were from the USA. There were 69 participants in total, with sample sizes ranging from 19 to 30. The pre/post study by Peters and colleagues133 followed up participants at 12 months. Litovsky and colleagues134 did not report the degree of deafness of participants; participants in the other two studies were severe to profoundly deaf. No studies were excluded from this comparison on the grounds of sample size. A summary of the characteristics and results of the included studies can be found in Appendix 3 (Tables 98 and 99 respectively).
Peters and colleagues133 measured the ability of children to understand speech in noise and quiet. Participants were only selected if they had shown an ability to perceive speech when using one implant. The children were assessed in three age groups (3–5 years, 5.1–8 years, 8.1–13 years).
Litovsky and colleagues134 measured the ability to detect the direction of sound. Participants attended between one and three sessions at 3–15 months following the second implant (mean 7 months). Results from participants who attended more than one session were reported for the latest measurement. Implantation was sequential, with a range of 1–11.6 years between implants (mean 3.9 years).
Litovsky and colleagues135 measured speech intelligibility and the ability to detect the direction of sound. Outcomes were measured at 3–26 months post implant (mean 13.5 months). Implantation was sequential with 0.8–6.4 years between implants (mean 3.2 years).
The studies were of moderate to poor quality with little description of how participants were selected. Litovsky and colleagues135 did not state the level of the bilateral participants’ previous hearing loss. An inadequate description of participants’ characteristics was given so that it was not possible to tell if the two groups considered were well matched; however, this was the only study to report age at implantation and whether the children were pre- or postlingually deaf.
No mention was made in any study of approaches to blinding assessors or whether data analyses were conducted blind to study group. However, the studies were prospective and used validated outcome measures and appropriate statistical analyses; for a summary of study quality indicators see Table 17.
| Quality criteria | Peters 2007133 | Litovsky 2006134 | Litovsky 2006135 |
|---|---|---|---|
| Was the study prospective? | Yes | Yes | Yes |
| Selection bias | |||
| Eligibility criteria stated? | Yes | Yes | Yes |
| Appropriate? | Yes | Yes | Yes |
| Were the participants representative of the population? | Somewhata | Yes | Unknown |
| Were potential confounders reported? | Yes | Yes | Yes |
| Were they accounted for in the design or analysis? | No | Yes | Yes |
| Assessment bias | |||
| Were the outcome measures relevant to the research question? | Yes | Yes | Yes |
| Independent blind assessment? | NR | NR | NR |
| Objective? | Yes | Yes | Yes |
| Attrition bias | |||
| Was attrition reported? | No | NA | NA |
| Were all participants accounted for? | No | Yes | No |
| How were missing data accounted for? | NR | NR | NR |
| Protocol violations specified? | No | No | No |
| Power and analysis | |||
| Data analysis | DS | DS | ANOVA |
| Was the analysis appropriate? | Yes | Yes | Yes |
| Was there a power calculation? | NR | NR | NR |
| Other | |||
| Was ethical approval given? | NR | Yes | NR |
| Generalisability? | Somewhat | Yes | unknown |
Study results for bilateral cochlear implants versus unilateral cochlear implant and acoustic hearing aid – children
The outcomes for these studies were either sensitivity to sound or speech perception. The results all showed a greater benefit from bilateral implantation than from a unilateral cochlear implant and acoustic hearing aid.
Sensitivity to sound
A total of 39 children had sensitivity to sound measured by determining their ability to detect the direction that sounds came from using the MAA.
Litovsky and colleagues (n = 19)134 found that bilaterally implanted children were significantly better than children with one implant plus a hearing aid at detecting sound direction measured using the MAA (which indicates the smallest change in position of a sound that can be detected, with lower scores being better) (bilateral = 28.0°, unilateral + acoustic hearing aid = 44.4°; p < 0.05). In another study (n = 20)135 with the same outcome measure a similar result was obtained (bilateral = 20.0°, unilateral + acoustic hearing aid = 27.0°; p < 0.05).
Speech perception
Peters and colleagues133 measured the ability of 50 children to understand speech using three different instruments [MLNT words (age 3–5 years), LNT words (age 5.1–13 years) and HINT-C sentences (age 8.1–13years)]. All results showed a trend in favour of bilateral implantation; for some groups this reached significance (MLNT, 3–5 years: bilateral = 92.3, unilateral + acoustic hearing aid = 67.3, p = 0.003; LNT, 5.1–13 years, bilateral = 86.0, unilateral + acoustic hearing aid = 69.4, p = 0.004).
Speech production
Litovsky and colleagues (n = 20)135 measured speech production using the CRISP test in quiet and in noise. Both conditions showed a significant benefit for bilateral implantation (quiet: bilateral = 20.00, unilateral + acoustic hearing aid = 24.00, p < 0.0001; noise: bilateral = 11.00, unilateral + acoustic hearing aid = 17.50, p < 0.005).
Table 18a and b provides a visual summary of the overall benefit from bilateral implantation reported by these studies.
| Study design (follow-up, months) | Study | n | Auditory outcomes |
|---|---|---|---|
| MAA degrees azimuth | |||
| XS (NRC) | Litovsky 2006134 | 19 | |
| XS (NRC) | Litovsky 2006135 | 20 |
| Study design (follow-up, months) | Study | n | Speech perception outcomes | |||||
|---|---|---|---|---|---|---|---|---|
| MLNT words | LNT wordsa | HINT-C sentences | CRISP (quiet/noise) | |||||
| PP (P) (12) | Peters 2007133 | 30 | ||||||
| XS (NRC) | Litovsky 2006134 | 20 | ||||||
Summary: bilateral cochlear implants versus unilateral cochlear implant and acoustic hearing aid – children
Again, small sample sizes, poor reporting and design, and a lack of consideration of confounding factors mean that evidence for a definitive benefit for bilateral implants compared with one implant plus an acoustic hearing aid is somewhat unclear.
The psychoacoustics results give the most consistent evidence as, with a small number of participants, significant improvement was shown in the ability to detect the direction of sound (bilateral = 28.0°, unilateral + acoustic hearing aid = 44.4°, p < 0.05).
Speech perception, measured by an ability to understand words and sentences, was better for children with bilateral implants. The degree of benefit ranged from a mean difference of 4.0 for the CRISP test in quiet conditions to 25.0 for the MLNT words in quiet conditions.
Overall conclusions
From the limited number of studies it seems that there may be an additional benefit for children from having two cochlear implants compared with one plus an acoustic hearing aid, although the methodological quality of these studies was limited.
Quality of life – children
As stated in Chapter 3 (see Quality of life and educational outcome studies) the results of the systematic review identified no studies of quality of life in children. The review of the original searches with expanded inclusion criteria (admitting uncontrolled studies and surveys) and further searches found four studies that did not meet the original systematic review inclusion criteria. Three of the studies were cross-sectional surveys and one was a retrospective controlled study. See Appendix 3 for summary tables of these studies and their results (Tables 100 and 101). This second review was restricted to non-preference-based studies (see Chapter 6, Utilities, for a review of these studies).
The quality of these studies varied from moderate to poor with some papers inadequately describing participants, procedures and results. The degree of deafness was only reported by two studies; it is assumed that all other participants were severely or profoundly sensorineurally deaf. Table 19 gives a summary of the key quality indicators.
| Quality criteria | Damen 2006136 | Huber 200522 | Spahn 200442 | Chmiel 2000137 |
|---|---|---|---|---|
| Was the study prospective? | No | NA | NA | NA |
| Selection bias | ||||
| Eligibility criteria stated? | Minimal | Yes | Yes | Minimal |
| Appropriate? | Yes | Yes | Yes | Yes |
| Were the participants representative of the population? | No, Usher syndrome | Somewhata | Yes | Yes |
| Were potential confounders reported? | No | No | No | No |
| Were they accounted for in the design or analysis? | No | No | No | No |
| Assessment bias | ||||
| Were the outcome measures relevant to the research question? | Yes | Yes | Yes | Yes |
| Independent blind assessment? | NR | NR | NR | NR |
| Objective? | No | No | No | No |
| Attrition bias | ||||
| Was attrition reported? | No | Yes | No | No |
| Were all participants accounted for? | Yes | Yes | No | Yes |
| How were missing data accounted for? | NR | NR | NR | NR |
| Protocol violations specified? | No | No | No | No |
| Power and analysis | ||||
| Data analysis | DS | Mean scores | t-tests | NR |
| Was the analysis appropriate? | Yes | Yes | Yes | |
| Was there a power calculation? | NR | NR | NR | NR |
| Other | ||||
| Was ethical approval given? | NR | NR | Yes | NR |
| Generalisability? | No | Somewhat | Yes | Yes |
Study results – quality of life in children and their carers
Huber22 investigated health-related quality of life using the KINDLr. In total, 37 children and seven of their parents completed this cross-sectional survey; results were compared with normal hearers. The total score for the cochlear implant children aged 8–12 years was below that of normal hearers (cochlear implant = 64.6, normal = 76.8, p < 0.001) and less than parent ratings (80.8, p < 0.0001). The total score for older children (13–16 years) was within the norm (72.1) with no significant difference between children and parents.
Chmiel and colleagues137 examined quality of life using an ad hoc questionnaire within a cross-sectional survey of parents (n = 11) and children with cochlear implants (n = 11) from the same families. They found that parents and children rated the benefits of cochlear implants similarly, with both groups indicating that they found the implant to be ‘a lot of help’. The ability to hear environmental sounds was held to be the greatest benefit by both groups. All of the children reported that the implant helped them to ‘feel happier’.
Damen and colleagues136 retrospectively evaluated the health-related quality of life of children with Usher syndrome type 1 who used cochlear implants. They used proxy measures from parents by comparing the responses on two quality of life measures from parents of children with (n = 7) and without (n = 2) cochlear implants. They found an increased quality of life reported by parents of children with cochlear implants measured on the Nijmegen Cochlear Implant Questionnaire (NCIQ) (mean scores, with cochlear implant = 66, without cochlear implant = 41). However, the results of the Usher Lifestyle Questionnaire (ULS) were similar between groups and more difficult to interpret because of the disparity in group numbers.
The study by Spahn and colleagues42 (n = 74)investigated the quality of life of parents of children with cochlear implants. It used a cross-sectional design, using the Symptom Checklist 90-R to measure psychological distress, and the Everyday Life Questionnaire to measure quality of life. They used a postal questionnaire, comparing parents with population norms. Results of the distress scale showed that parents of children with cochlear implants had heightened psychological distress (cochlear implant = 79%, norm = 21%). The results of the quality of life measure were compared with those of various disease groups and students; parents of cochlear implant children had a better quality of life than cardiac patients but a worse quality of life than students (cochlear implant = 168, cardiac patients = 151, students = 172). However, there was no comparison group of parents with similarly deaf children who had not received cochlear implants and so it is not possible to say whether these findings are due to cochlear implantation or deafness.
Summary of quality of life studies – children
The quality of life studies for children with cochlear implants all used different measures. Two studies directly measured children’s ratings of quality of life, three used parents’ proxy ratings and one measured only the quality of life of parents.
The results showed that, in comparison to preimplantation, cochlear implants improved children’s quality of life and that deaf children with cochlear implants had a higher parent-rated quality of life than those without. However, this remained below that of normal hearers. Parents rated their children’s quality of life at least as highly as their children did. When parents were asked about psychological distress and their own quality of life they rated their levels of distress much higher than those of general population norms and their quality of life as better than that of cardiac patients but worse than that of students. The difficulties in measuring quality of life, particularly in children, together with the quality of these studies mean that these results are uncertain.
Chapter 6 (see Utilities) summarises a more specific review of studies that reported utility values for paediatric cochlear implantation.
Conclusions
Cochlear implants may improve the quality of life of child users.
Educational outcomes
The clinical evidence from this systematic review suggests that cochlear implants improve speech perception and production in children, and that the degree of improvement is linked to the age at implantation and duration of deafness. Improvements may be substantial, for example a 57% mean score increase in ESP understanding of speech pattern scores post implantation. 130 It follows that there may be consequent effects on educational outcomes.
However, the results of the following review should be read with caution for a number of reasons: first, because of the potential for bias to have confounded the results because of lack of randomisation; second, because of changes in government policy over the years, with increasing emphasis being put on the integration of children with disabilities within mainstream schools; and, third, because the effects of differing socioeconomic status, social support structures and the presence of other disabilities may not have been taken into account in the analyses.
As stated in Chapter 3 (see Quality of life and educational outcome studies), none of the studies originally included in the systematic review measured educational outcomes. Therefore, the searches were reviewed with the inclusion criteria relaxed. Seven studies were found that compared cochlear implant users with either normal-hearing peers or non-implanted hearing-impaired peers. Three of the studies measured academic outcomes and five investigated educational placement.
The quality of the studies was generally good; Table 20 gives a summary of the key quality indicators.
| Quality criteria | Barton 2006138 | Stacey 200621 | Damen 2006139 | Thoutenhoofd 2006140 | Archbold 2002141 | Fortnum 2002142 | Archbold 1998143 |
|---|---|---|---|---|---|---|---|
| Was the study prospective? | NA | NA | NA | Yes | NA | No | Yes |
| Selection bias | |||||||
| Eligibility criteria stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Appropriate? | Yes | Yes | Yesa | Yes | Yes | Yes | Yes |
| Were the participants representative of the population? | Yes | Yes | Somewhatb | Yes | Yes | Yes | Yes |
| Were potential confounders reported? | No | No | No | No | No | No | No |
| Were they accounted for in the design or analysis? | No | No | No | No | No | No | No |
| Assessment bias | |||||||
| Were the outcome measures relevant to the research question? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Independent blind assessment? | NR | NR | NR | NR | NR | NR | NR |
| Objective? | No | No | No | Yes | Yes | Yes | Yes |
| Attrition bias | |||||||
| Was attrition reported? | NA | NA | NA | Yes | NA | NA | NA |
| Were all participants accounted for? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| How were missing data accounted for? | NR | Identified by logistic regression | NR | NR | NR | NR | NR |
| Protocol violations specified? | No | No | No | No | No | No | No |
| Power and analysis | |||||||
| Data analysis | Linear regression | Linear and logistic regression | Mann–Whitney test | DS | Chi-squared test | Chi-squared test and logistic regression | Chi-squared test, ANOVA and Mann–Whitney test |
| Was the analysis appropriate? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was there a power calculation? | No | No | No | No | No | No | No |
| Other | |||||||
| Was ethical approval given? | NR | NR | NR | NR | NR | NR | NR |
| Generalisability? | Yes | Yes | Somewhat | Yes | Yes | Yes | Yes |
Review of educational studies
Barton and colleagues138 conducted a large cross-sectional survey of teachers, asking them to state the educational placement of a representative sample of deaf children amongst other outcomes (costs are reported in Chapter 6). A total of 383 teachers of children with cochlear implants returned questionnaires between May 1999 and October 2001. They found that 76% of children with cochlear implants compared with 49% of those profoundly sensorineurally deaf at an average hearing level (AHL) > 105 dB, 70% of those profoundly sensorineurally deaf at AHL 96–105 dB and 76% of those severely deaf (AHL 71–95 dB) were in primary or secondary school. The proportion of implanted children in schools for deaf children was less than that of the most profoundly sensorineurally deaf unimplanted children (AHL > 105 dB) (17% and 34% respectively). However, less of those profoundly sensorineurally deaf at AHL 96–105 were in a school for the deaf (15%) (Table 21). These results are from the same research project as that of Stacey and colleagues. 21
| School placement | Severe (AHL 71–95 dB) | Profound (AHL 96–105 dB) | Profound (AHL > 105 dB) | Implanted | Total |
|---|---|---|---|---|---|
| Nursery | 5 (1%) | 2 (1%) | 2 (0%) | 15 (4%) | 24 |
| Primary | 282 (42%) | 132 (35%) | 104 (25%) | 239 (62%) | 757 |
| Secondary | 228 (34%) | 132 (35%) | 100 (24%) | 54 (14%) | 514 |
| School for the deaf | 50 (7%) | 57 (15%) | 141 (34%) | 64 (17%) | 312 |
| Special school | 58 (9%) | 18 (5%) | 29 (7%) | 5 (1%) | 110 |
| Further education | 41 (6%) | 29 (8%) | 37 (9%) | 3 (1%) | 110 |
| Left school | 4 (1%) | 6 (2%) | 4 (1%) | 0 (0%) | 14 |
| Other | 3 (0%) | 1 (0%) | 2 (0%) | 3 (1%) | 9 |
| Total | 671 | 377 | 419 | 383 | 1850 |
Stacey and colleagues21 used a cross-sectional design to look at variables affecting different outcomes, including education, in children with cochlear implants. (Other aspects of the same study covered auditory performance, academic achievements, health-related quality of life and costs of special education.) They invited the parents of 993 children with cochlear implants, 3288 profoundly sensorineurally deaf children and 3580 severely deaf children in the UK to take part in a questionnaire survey. Teachers of participating children were invited to judge academic abilities. In total, 468 parents and 383 teachers of children with cochlear implants returned the questionnaires. Children were stratified by age at implantation and duration of use of implants.
Educational data were analysed by multiple regression to control for associations between outcomes and potentially confounding variables, for example age, age of onset of hearing loss, degree of hearing impairment, socioeconomic status and number of disabilities. The results showed an inconsistent association between implantation and enhanced educational outcomes, and also few of the possible associations reached significant levels. The authors hypothesised that this pattern would arise if the measures used were unresponsive to change in outcome.
Stacey and colleagues found that reading (assessed by teachers) was positively significantly associated with implantation before the age of 5 years and with between 2 and 4 years’ experience of implantation (1.721, p < 0.01). However, for this same group there was a negative significant association with academic ability (assessed by parents) (–0.234, p < 0.01). The authors suggest that this anomaly may be explained by the greater amount of missing data from parents compared with teachers on educational outcomes, and the possibly higher educational expectations of these parents compared with those of non-implanted children.
Two significant associations were found for children implanted before the age of 5 years and with at least 4 years’ cochlear implant experience (assessed by teachers); these were academic ability (0.185, p < 0.05) and participation and engagement (0.224, p < 0.05).
Damen and colleagues139 compared prelingually deaf children with cochlear implants (n = 32) in mainstream education with normal-hearing peers (n = 35) in a cross-sectional study in the Netherlands. The implanted children had a mean age of 9.0 years (range 4.5–13.0), had been deaf for a mean of 3.4 years (range 0.4–9.7), had been implanted at a mean age of 3.7 years (range 1.0–9.7) and had used their implants for a mean of 5.0 years (range 1.0–9.1). The normal-hearing control subjects were quasi-randomly selected from their classmates. The children were assessed by their teachers using two questionnaires: the AMP, a measure of the skills needed to succeed in mainstream schools, and the SIFTER, which measures school performance.
The results were analysed with non-parametric statistics (Mann–Whitney) and a general linear model to look for correlations between AMP scores, SIFTER scores or class ranking and different variables.
For the AMP, Damen and colleagues found that, for kindergarten-aged children (3–5 years), the cochlear-implanted and normal-hearing children spent a similar amount of time performing to their ability in class [mean (SD) AMP scores 4.6 (0.94) and 5.3 (0.25) respectively]. The older (elementary age) deaf children (6–13 years) showed significantly less regular participation and appropriate communicative behaviour compared with their normal-hearing peers [cochlear implant = 4.1 (0.68), normal = 5.0 (0.59), p < 0.001]. When teachers were asked to estimate the children’s class level compared with their peers all of the cochlear-implanted children scored ‘above average’ and all of the normal hearers scored ‘good’; these differences were not significant (kindergarten cochlear implant = 3.33 (0.82), normal = 3.58 (0.67); elementary cochlear implant = 3.07 (1.00), normal = 3.55 (0.83), p = 0.08).
For elementary age children negative correlations were found between the AMP scores and age at implantation and duration of deafness (–0.06, p < 0.001 and –0.66, p < 0.001 respectively), indicating that earlier implantation and shorter time between deafness and implantation had educational benefits, with a greater effect for duration of deafness.
The SIFTER is divided into five subscales (academic, attention, communication, class participation and school behaviour). No significant differences were found between the kindergarten-aged cochlear-implanted and normal-hearing children. However, the normal-hearing elementary school-aged children did significantly better than the cochlear-implanted elementary school-aged children on the attention [cochlear implant = 8.52 (2.79), normal = 10.96 (2.32), p < 0.001], communication [cochlear implant = 7.32 (2.53), normal = 11.43 (2.01), p < 0.001] and class participation [cochlear implant = 9.17 (2.63), normal = 12.33 (2.25), p < 0.001] subscales.
The duration of deafness in implanted elementary school-aged children was correlated negatively with academics (–0.53, p = 0.01), attention (–0.46, p = 0.02), communication (–0.52, p < 0.001) and class participation (–0.048, p = 0.02), showing that the shorter the period of deafness and the longer the period of cochlear implant use the better the outcomes. Similarly, kindergarten-aged cochlear-implanted children had negative correlations between duration of deafness and communication (–0.88, p = 0.02). Duration of implant use was positively correlated with attention (0.81, p = 0.05) and social behaviour (0.84, p = 0.04).
Thoutenhoofd140 studied a cohort of cochlear-implanted children in Scotland from 2000 to 2004. There were 105 primary school-aged children with a mean age of 8.06 (SD 2.1) years, a mean age at implantation of 3.02 (SD 1.6) years and a mean of 4.01 (SD 1.9) years of cochlear implant experience. A total of 47 secondary school-aged children were included with a mean age of 14.07 (SD 1.9) years, a mean age at implantation of 7.07 (SD 4.1) years and a mean of 5.03 (SD 3.0) years of cochlear implant experience.
A total of 139 of these children were in full-time educational placements: 56 (40.2%) were in mainstream schools, 14 (10.1%) were in a designated integrated placement, 48 (34.5%) were in a special unit placement and 21 (15.1%) were in schools for deaf pupils.
National test scores for reading, writing and mathematics were taken by normal-hearing children (n = 478,931), bilaterally profoundly deafened (≥ 95 dB HL) children without cochlear implant (n = 78) and cochlear implant students (n = 89) in the years 2000–4. It is not reported whether the profoundly sensorineurally deaf children were matched for level of hearing loss with the cochlear implant group. The results showed that the deaf students did not attain the same level as normal hearers and that as demands rose the deaf students fell further behind. The results of the students with cochlear implants were closer to those of normal hearers than the profoundly sensorineurally deaf pupils without implants.
Table 22 shows the differences in mean scores between profoundly deaf non-cochlear-implanted and cochlear-implanted children and normal hearers. The results indicate educational gains in all three categories from cochlear implants, most apparent in mathematics (grade F: cochlear implant difference from normal hearers = 1.4%, profoundly deaf without implants difference from normal hearers = 9.5%, i.e. those with implants had scores that were closer to those of their normal-hearing peers). Additionally, although the difference in scores between normal hearers and the profoundly deaf increases as the tasks get harder, the increase in the difference scores is less marked for profoundly deaf cochlear implant users than for those who do not use implants. However, most deaf children, including cochlear implant users, fell below the national average.
| Category | Group | Average population size | Lowest grade in national tests (A+), % | Highest grade in national tests (F+), % |
|---|---|---|---|---|
| Reading | National data normal hearers | 478,931 | 90.4 | 17.5 |
| Profoundly deaf without CI | 78 | 28.8a | 16.1a | |
| CI users | 89 | 22.2a | 8.0a | |
| Writing | National data normal hearers | 478,931 | 88.5 | 49.8 |
| Profoundly deaf without CI | 78 | 39.2a | 48.3a | |
| CI users | 89 | 24.1a | 45.0a | |
| Maths | National data normal hearers | 478,931 | 98.4 | 11.0 |
| Profoundly deaf without CI | 78 | 27.7a | 9.5a | |
| CI users | 89 | 20.1a | 1.4a |
Archbold and colleagues141 compared the educational settings in the UK, 3 years after implantation, of profoundly deaf children using cochlear implants (n = 42) and aged-matched peers using acoustic hearing aids (severely deaf n = 635, profoundly deaf n = 511). Participants had received their implants before 5 years of age. The severely deaf comparison group had pure-tone hearing threshold levels between 71 and 95 dB and the profoundly deaf group had a pure-tone hearing threshold level > 95 dB.
They found that, after 3 years of implantation, the cochlear implant group had 38% of its members in mainstream education, 57% in a unit or special class in a mainstream school and 5% in a school for the deaf. This contrasts favourably with profoundly sensorineurally deaf hearing aid users, of whom 12% were in mainstream school, 55% were in a unit or class in a mainstream school and 33% were in schools for the deaf. The results for the severely deaf children were closer to those of the cochlear implant group: 38% were in mainstream schools, 51% were in a unit of a mainstream school and 11% were in a special school. A comparison between the placement of cochlear-implanted children and the placement of profoundly sensorineurally deaf children was significant at p < 0.00001. There was no significant difference between the placement of severely deaf children and the placement of those with cochlear implants.
Thus, profoundly sensorineurally deaf children with cochlear implants (> 95 dB HL) who are implanted for less than 5 years may have similar educational placement expectations to severely deaf non-implanted children.
Fortnum and Marshall142 studied a cohort of deaf children born between 1980 and 1997 (n = 12,255). They reported on population data collected in 1998 (n = 2938, profoundly sensorineurally deaf with cochlear implants = 608, profoundly sensorineurally deaf without cochlear implants = 2330). Analyses showed that a number of variables, including cochlear implantation, were independently associated with educational settings that had lower levels of support. The results for profoundly sensorineurally deaf children with cochlear implants showed that 18% were in mainstream schools, 58% were in units within mainstream schools, 21% were in schools for the deaf and 3% were placed elsewhere. In comparison, of profoundly sensorineurally deaf children without a cochlear implant, 11% were in mainstream education, 36% were in a unit within a mainstream school, 46% were in a school for the deaf and 7% were placed elsewhere.
Archbold and colleagues143 looked at the educational placement of 121 profoundly deaf children before and 2 years after cochlear implantation. In particular, they looked at the effect of whether children were implanted before (n = 47) or after (n = 74) they had started school. They found that 53% of preschool-implanted children were in mainstream education 2 years later, compared with 6% of children who were already in school when implanted. Similarly, 13% of preschool-implanted children were in schools for the deaf compared with 33% of children implanted after starting school, and 33% of preschool-implanted children were in special units compared with 61% who were in education before implantation. The difference in educational setting was significant (p = 0.004).
Archbold and colleagues also looked at the effects of age at implantation and duration of deafness. They found that the mean age at implantation for those in a school for the deaf or in a unit was 72 months, and for those in mainstream education it was 49 months. This was significantly younger than in the other settings (p < 0.01). For duration of deafness the mean length of deafness before implantation was 58 months for those in special schools, 54 months for those in units and 25 months for those in mainstream education. These differences were significant (p = 0.004) and indicate that children who are given implants before they enter education may be more likely to go into mainstream education than those who are implanted after they have begun school. Once a child is in a particular education setting they may be less likely to change that setting than when they are at the preschool stage and choosing the most appropriate educational placement. However, the results from this retrospective review may be affected by biases that have not been controlled for; the early implanted group may have had different characteristics to those implanted later which meant that they were selected for implantation at an earlier age.
Summary of education
Educational placement
The data in Table 23 indicate that, taken together:
-
children with cochlear implants are more likely to be in mainstream education, including a special unit within the school (75–95%), than in a school for the deaf (5–21%).
-
children with cochlear implants are less likely to be in schools for the deaf (5–21%) than profoundly deaf children without cochlear implants (29–46%).
| School placement | Barton 2006138 | Archbold 2002141 | Fortnum 2002142 | Thoutenhoofd 2006140 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Severe (AHL 71–95 dB), n (%) | Profound (AHL 96–105 dB) , n (%) | Profound (AHL > 105 dB) , n (%) | Implanted, n (%) | Severe (AHL 71–95 dB) , n (%) | Profound (AHL > 95 dB) , n (%) | Implanted, n (%) | Profound (AHL > 95 dB) , n (%) | Implanted, n (%) | Implanted, n (%) | |
| Mainstream including unit | 510 (76) | 164 (70) | 204 (49) | 293 (76) | 565 (89) | 342 (67) | 40 (95) | 1088 (47) | 459 (76) | 104 (75) |
| School for the deaf | 50 (7) | 57 (15) | 141 (34) | 64 (17) | 70 (11) | 167 (33) | 2 (5) | 1073 (46) | 128 (21) | 21 (15) |
| Nursery | 5 (1) | 2 (1) | 2 (0) | 15 (4) | ||||||
| Special school | 58 (9) | 18 (5) | 29 (7) | 5 (1) | ||||||
| Further education | 41 (6) | 29 (8) | 37 (9) | 3 (1) | ||||||
| Left school | 4 (1) | 6 (2) | 4 (1) | 0 (0) | ||||||
| Other | 3 (0) | 1 (0) | 2 (0) | 3 (1) | 169 (7) | 20 (3) | 14 (10) | |||
Effect of implanting before or after starting school on educational placement
Archbold and colleagues143 looked at the effect of implantation before and after children had started school on educational placement. They found that 53% of children who were implanted before starting school were in mainstream schools compared with 6% of those who were implanted after (Table 24 and Figure 2).
| Educational setting | Implanted before starting school (n = 47) | Implanted after starting school (n = 74) |
|---|---|---|
| Mainstream school | 53.0% | 6.0% |
| Special unit within mainstream school | 33.0% | 61.0% |
| School for the deaf | 13.0% | 33.0% |
FIGURE 2.
The effect of implanting before and after starting school on educational placement.
Educational attainment
Academic outcomes
Damen and colleagues (n = 32)139 found that before the age of 5 years cochlear implantation was associated with improved scores on a measure of skills needed for mainstream education (AMP) (age = – 0.06, p < 0.001; duration of deafness = –0.66, p < 0.001).
Stacey and colleagues (n = 7861)21 compared cochlear-implanted children with non-implanted severely and profoundly sensorineurally deaf children. They found that a lower duration of deafness was associated with improved academic attainment, reading level (assessed by teachers: 1.721, p < 0.01), academic ability (assessed by teachers: 0.185, p < 0.05; assessed by parents: –0.234, p < 0.01), participation and engagement (assessed by teachers: 0.224, p < 0.05).
Thoutenhoofd (n = 152)140 compared profoundly sensorineurally deaf children with cochlear implants with age-matched normal-hearing children and profoundly sensorineurally deaf children without cochlear implants. The difference in reading scores for profoundly sensorineurally deaf children with cochlear implants compared with normal-hearing peers was less than the difference in reading scores for similar children without cochlear implants compared with normal-hearing peers (cochlear implant difference = 8%, no cochlear implant difference = 16.1%).
Similarly, the differences in writing scores and mathematics scores for profoundly sensorineurally deaf children with cochlear implants compared with normal-hearing peers were less than those for similar children without cochlear implants compared with normal-hearing peers (writing : cochlear implant difference = 45%, no cochlear implant difference = 48.3%; mathematics: cochlear implant difference = 1.4%, no cochlear implant difference = 9.5%).
Conclusions
Cochlear implantation may have educational benefits in terms of academic outcomes. Children who are implanted before they attend school may be more likely to achieve better academic results and be in mainstream education than those who are implanted after they reach school age. Profoundly sensorineurally deaf children with cochlear implants performed at similar levels to moderately or severely deaf children without implants.
Overall summary of effectiveness in children
Table 25 gives an overview of the outcomes from the children’s studies included in the clinical effectiveness systematic review. It shows that all outcomes were either positively significant (n = 19) or showed a positive trend (n = 41) (significance not reported or results not significant).
| Comparison | Total outcomes, n (no. reporting significance) | Positive significant outcomes (p < 0.05), n (%) | Positive trend (not significant, not reported) outcomes, n (%) | Negative trend (not significant, not reported) outcomes, n (%) | Negative significant outcomes (p < 0.05), n (%) |
|---|---|---|---|---|---|
| Cochlear implant vs non-auditory support | |||||
| Audiological outcomes | 1 (1) | 1 (100) | |||
| Speech perception | 31 (3) | 3 (10) | 28 (90) | ||
| Speech production | 1 (1) | 1 (100) | |||
| Cochlear implant vs acoustic hearing aid | |||||
| Audiological outcomes | 1 (1) | 1 (100) | |||
| Speech perception | 12.5 (7) | 7 (56) | 5.5 (44) | ||
| Speech production | 1 (0) | 1 (100) | |||
| One cochlear implant vs two cochlear implants | |||||
| Audiological outcomes | 1 (1) | 1 (100) | |||
| Speech perception | 5 (5) | 1 (20) | 4 (80) | ||
| Two cochlear implants vs one cochlear implant and acoustic hearing aid | |||||
| Audiological outcomes | 2 (2) | 2 (100) | |||
| Speech perception | 4 (4) | 2.5 (63) | 1.5 (37) | ||
Summary of clinical effectiveness studies – children
The 20 studies of children in this systematic review had a total population of 1513. The heterogeneity between the studies and the large number of outcome measures (n = 38) meant that pooling of data was not possible.
Clinical summary
-
All of the studies were in favour of one cochlear implant over acoustic hearing aids or non-technological support and of bilateral over unilateral implants with or without a contralateral acoustic hearing aid.
-
A small number of studies showed that cochlear implants improved quality of life compared with preimplantation and with profoundly deaf non-implanted children.
-
Educationally, cochlear implants may benefit profoundly sensorineurally deaf children in terms of their academic achievement.
-
Profoundly deaf children with cochlear implants may be more likely to attend mainstream school.
-
Positive outcomes may be associated with earlier age at implantation and a shorter duration between deafness and implantation.
-
No adverse events were reported by the included studies. Adverse events for children are considered alongside those of adults in Chapter 5 (see Safety and reliability of cochlear implants – children and adults).
Methodological summary
-
Overall the studies were of moderate to poor quality with some weaknesses in design and internal validity. In particular, outcomes were sometimes measured at different times for different groups.
-
Our assessment of confounding factors showed that very few studies reported or allowed exploration of how outcomes varied with different age at implantation, different duration of deafness or different levels of audiologically measured hearing impairment. No effectiveness studies separately reported outcomes for subgroups of deaf children with different levels of ‘functional hearing’, or for children with other sensory impairments or complex needs defined in other ways.
-
The participants were not always clearly a representative sample and this potentially limits generalisability; in some cases those with other disabilities or who performed less well on screening tests were excluded. There was a lack of power calculation in all cases.
-
Many of the studies were poorly reported. Results were not reported in the text but had to be interpreted from figures; the methods of participant selection were not well documented; attrition and accounting for all participants did not always occur; and it is not known whether those who assessed or analysed the outcomes were blinded to the condition of the participants.
Chapter 5 Results of the clinical effectiveness evidence for adults
Unilateral cochlear implants versus non-technological support – adults
Type and quality of studies
Four studies are included in this comparison. Two have prospective pre/post designs, one is a prospective cohort study and one is a retrospective review of data from one UK implant centre. In all studies participants were their own controls. Two studies were based in the UK and two in the USA. There were 984 participants in total, with sample sizes ranging from 214 to 311. Follow-up times ranged from 3 months to 24 months and the age of participants ranged from 16 years to 82 years. Six studies were excluded on the grounds of size of population, with a total n = 127 (range 4–41); 89% of the total possible population was included. Two of the studies included populations that were profoundly deaf, with the other two having populations that were severe to profoundly deaf. A wide range of speech perception outcomes were measured by these studies. A summary of the characteristics and results of the included studies can be found in Appendix 3 (Tables 102 and 103 respectively).
The quality of the studies ranged from good to poor, with some not reporting or accounting for confounding factors, follow-up of all participants, missing data, power calculations and whether ethical approval was given. A summary of the key quality indicators is given in Table 26.
| Quality criteria | UKCISG 200462,144 | Mawman 2004145 | Parkinson 2002146 | Kessler 1997126 |
|---|---|---|---|---|
| Was the study prospective? | Yes | No | Yes | Yes |
| Selection bias | ||||
| Eligibility criteria stated? | Yes | No | Yes | Yes |
| Appropriate? | Yes | – | Yes | Yes |
| Were the participants representative of the population? | Yes | Yes | Yes | Yes |
| Were potential confounders reported? | Yes | Yes | No | No |
| Were they accounted for in the design or analysis? | Yes | Yes | No | No |
| Assessment bias | ||||
| Were the outcome measures relevant to the research question? | Yes | Yes | Yes | Yes |
| Independent blind assessment? | NR | NA | NR | NR |
| Objective? | Yes | Yes | Yes | Yes |
| Attrition bias | ||||
| Was attrition reported? | Yes | Yes | No | No |
| Were all participants accounted for? | Yes | Yes | No | No |
| Were missing data accounted for? | Yes | NR | NR | NR |
| Protocol violations specified? | No | No | No | No |
| Power and analysis | ||||
| Data analysis | DS | DS | DS | DS |
| Was the analysis appropriate? | Yes | Yes | Yes | Yes |
| Was there a power calculation? | NR | NR | NR | NR |
| Other | ||||
| Was ethical approval given? | NR | NR | NR | NR |
| Generalisability? | Yes | Yes | Yes | Yes |
Study results
The studies measured either speech perception or quality of life. All outcomes showed a significant benefit or a non-significant trend towards benefit from unilateral cochlear implants.
Speech perception
The total number of participants in this comparison was 984, with the four studies using nine instruments; however, there is some overlap between those taking part in the UK Cochlear Implant Study Group (UKCISG)62,144 study and those in the Mawman and colleagues study145 (number unknown); therefore the actual total number will be less.
The UKCISG study (n = 316)62,144 measured speech perception with the BKB and a normalised index of audiovisual gain (AVGN) preimplantation and 9 months later. Participants were divided into ‘traditional candidates’ [TC: mean hearing level = 117.1 dB (95% CI 115.7–118.5)] or ‘marginal hearing aid users’ [MHU: mean hearing level = 108.7 dB (95% CI 106.8–110.5)] on the basis of their score on speech intelligibility tests taken before implantation. Both groups were profoundly sensorineurally deaf. The MHU results are also reported in the next comparison (one cochlear implant versus acoustic hearing aid) as their preimplantation measures were with acoustic hearing aids. They are recorded here to show the comparative effects of level of hearing loss.
The mean scores for both outcome measures improved at 9 months compared with preimplantation, with the TC group showing significantly more improvement than the MHU group [BKB: TC = 53.0 (95% CI 48–58), MHU = 44.0 (95% CI 37–51), p < 0.05; AVGN: TC = 68.0 (95% CI 63–71), MHU = 31.0 (95% CI 26–37), p < 0.001].
Further evidence for the benefits of cochlear implants came in the same year from Mawman and colleagues (n = 214),145 who looked retrospectively at patient records from one UK cochlear implant centre. Speech perception results were measured with BKB sentences and Arthur Boothroyd (AB) monosyllable words preimplantation and 18 months later. They found non-significant trends in favour of cochlear implants for both measures [BKB mean difference = 64.0 (SD 24.0); AB mean difference = 50.0 (SD 17.3)].
An earlier study by Parkinson and colleagues (n = 216)146 used a pre-/postimplantation design and evaluated speech perception in quiet conditions using City University New York (CUNY) sentences and words and HINT sentences, and in noise using CUNY sentences. They found significant positive benefits for cochlear implants at 3 months post implant (p < 0.001 for all measures). Mean change (SD) scores from pre- to post implantation ranged from 34.5 (22.6) for CUNY words in quiet to 67.0 (31.5) for CUNY sentences in quiet.
Kessler and colleagues (n = 238)126 found similar benefits from cochlear implants when they measured outcomes from preimplantation to 12 months post implantation on a range of instruments [Minimal Auditory Capabilities (MAC) vowels and consonants, CUNY sentences, Central Institute for the Deaf (CID) sentences, Northwestern University Auditory Test number six (NU-6), words and everyday sentences listened to over the telephone]. Positive trends were found on all measures; these ranged from a median 36% improvement in score with NU-6 words to a 73% improvement with everyday telephone sentences.
Quality of life
Only the UKCISG study62 measured quality of life, using three instruments [Health Utilities Index 3 (HUI-3), Glasgow Health Status Inventory (GHSI) and the Glasgow Benefit Inventory (GBI)]. This study compared the preimplant scores of 64 people with their 9-month post implant scores. All measures showed a trend towards improvement in quality of life. In particular, the HUI-3 showed that traditional candidates had a significantly better health-related quality of life than marginal hearing aid users after 9 months [mean changes: TC = 0.22 (95% CI 0.19–0.24); MHU = 0.15 (95% CI 0.11–0.19), p < 0.01].
Association with age at implantation
The UKCISG study62 also considered the association between speech perception and quality of life and age at implantation. Participants were divided into six age groups (years): < 30, 30–39, 40–49, 50–59, 60–69, ≥ 70.
There were no strong links between speech perception and quality of life and age at implantation. Pearson correlations revealed that there was a non-significant decline in speech intelligibility as age at implantation increased when measured with the BKB. However, there was a significant increase in benefit with age in audiovisual gain at 9 months post operation [r = 0.164 shown by the AVGN (p < 0.01)]. The HUI-3 and GHSI quality of life measures declined with age at implantation, significantly with the latter measure (r = –0.114, p < 0.05). The GBI did not vary significantly with age.
Association with duration of deafness
The UKCISG study62 showed a stronger effect on speech perception and quality of life with duration of deafness, with greater effectiveness being associated with implantation in the ear with a shorter duration of profound deafness. On all measures effectiveness declined with duration of deafness (r = –0.203, p < 0.01), with a significant difference being found between the group with the shortest duration of deafness and those with more than 30 years of deafness.
Table 27a and b provides a visual summary of these outcomes showing the pattern of results.
| Study design (follow-up, months) | Study | n | Speech perception outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BKB | AVGN | AB monosyllabic words | CUNY words | CUNY sentences | CUNY sentences in noise | HINT sentences | MAC vowels | MAC consonants | CID sentences | NU-6 monosyllabic word test | Everyday telephone sentences | |||
| C (P) (9) | UK Cochlear Implant Study Group 200462,144 | 316 | ||||||||||||
| PP (R) (> 18) | Mawman 2004145 | 214 | ||||||||||||
| PP (P) (3) | Parkinson 2002146 | 216 | ||||||||||||
| PP (P) (24) | Kessler 1997126 | 238 | ||||||||||||
| Study design (follow-up, months) | Study | n | Quality of life outcomes | ||
|---|---|---|---|---|---|
| HUI-3 | GHSI | GBI | |||
| PP (P) (9) | UK Cochlear Implant Study Group 200462,146 | 316 | |||
Summary: unilateral cochlear implants versus non-technological support – adults
Again, heterogeneity between studies precluded pooling. There was a large variation in the quality of studies, with the UKCISG providing the most comprehensive reporting of methods, quality indicators and outcomes.
All studies measured speech perception and all found a benefit from cochlear implants. Measures were taken before implantation and post implantation at various time intervals with participants acting as their own controls. Mean change (SD) scores from pre- to post implantation ranged from 34.5 (SD 22.56) with CUNY words in quiet to 67.0 (SD 31.5) for CUNY sentences in quiet.
The results also indicate an improvement in quality of life from cochlear implant use with a HUI-3 gain for traditional candidates of 0.22 (95% CI 0.19–0.24) and for marginal hearing aid users of 0.15 (95% CI 0.11–0.19).
There were no strong links between speech perception and quality of life and age at implantation. A greater effect is seen in the correlation between duration of deafness and effectiveness (r = –0.203, p < 0.01), with people who had been profoundly deaf for more than 30 years before implantation not showing a significant benefit.
Overall conclusions
This evidence, which ranges from good to poor quality, suggests that cochlear implants improve the ability of severe to profound or profoundly sensorineurally deaf adults to understand speech as well as improving their quality of life. There is a weak correlation with age at implantation and a slightly stronger correlation with duration of deafness before implantation.
Unilateral cochlear implants versus acoustic hearing aids – adults
Type and quality of studies
Four studies are included in the review of evidence for one cochlear implant versus acoustic hearing aids in adults. Two were prospective cohort studies (one with the same participants in both groups), one was a prospective pre/post study and one had a cross-sectional design with participants acting as their own controls. One study was from the UK, one from Europe, one from the USA and one from Australia. There were 248 participants in total, with study sizes ranging from 21 to 106. Mean ages ranged from 37 to 62 years. Three of the studies were of people with severe to profound deafness; the other study’s population was profoundly deaf people. This comparison had a wider range of outcome measures than the previous one, including sensitivity to sound, quality of life, speech production and speech perception. Three studies were excluded on the grounds of the size of population, with a total n = 25 (range 3–12); 91% of the total possible population was included. Appendix 3 provides a summary of the characteristics and results of the included studies (Tables 104 and 105 respectively).
The quality of the included studies ranged from good to poor. One study had a separate control group;147 however, their mean level of deafness was only severe compared with a mean (SD) level of profound sensorineural deafness in the intervention group [cochlear implant = 105 (5) dB HL, acoustic hearing aid = 85 (10) dB HL]. The reporting of inclusion and exclusion criteria ranged from good to inadequate with no information given about exclusions in two cases and minimal reporting of inclusion criteria in one case, making judgements about the generalisability of some of the results difficult. Three of the studies acknowledged confounding factors and accounted for them in analyses. Two studies reported attrition, but none reported whether they had estimated power requirements or obtained ethical approval. Table 28 gives a summary of the key quality indicators for these studies.
| Quality criteria | UKCISG 200462 | Ching 2004148 | MED-EL 2001125 | Hamzavi 2001147 |
|---|---|---|---|---|
| Was the study prospective? | Yes | NA | Yes | Yes |
| Selection bias | ||||
| Eligibility criteria stated? | Yes | Minimal | Yes | Yes |
| Appropriate? | Yes | Yes | Yes | Yes |
| Were the participants representative of the population? | Yes | Yes | Yes | Yes |
| Were potential confounders reported? | Yes | Some | No | Yes |
| Were they accounted for in the design or analysis? | Yes | Yes | No | Yes |
| Assessment bias | ||||
| Were the outcome measures relevant to the research question? | Yes | Yes | Yes | Yes |
| Independent blind assessment? | NR | NR | NR | NR |
| Objective? | Yes | Yes + subjective | Yes | Yes |
| Attrition bias | ||||
| Was attrition reported? | Yes | NA | Yes | No |
| Were all participants accounted for? | Yes | NA | Yes | NR |
| Were missing data accounted for? | Yes | NR | NR | NR |
| Protocol violations specified? | No | No | No | No |
| Power and analysis | ||||
| Data analysis | DS | ANOVA | DS | Mann–Whitney |
| Was the analysis appropriate? | Yes | Yes | Yes | Yes |
| Was there a power calculation? | NR | NR | NR | NR |
| Other | ||||
| Was ethical approval given? | NR | NR | NR | NR |
| Generalisability? | Yes | Yes | Yes | Yes |
Study results: unilateral cochlear implants versus acoustic hearing aids – adults
The results of these studies showed a greater benefit for unilateral cochlear implants for this population than for acoustic hearing aids. The outcomes of these studies included sensitivity to sound, speech perception, speech production, functional performance, quality of life and adverse events.
Sensitivity to sound
Ching and colleagues (n = 21)148 conducted the only study that measured sensitivity to sound in people with severe to profound deafness. They used a cross-sectional design and measured the ability of participants to detect the direction of sound in quiet laboratory conditions. They found a minimal benefit for cochlear implants by measuring the average root mean squared errors [cochlear implant = 4.5 (95% CI 4.1–4.9), acoustic hearing aid = 4.6 (95% CI 4.3–4.9)].
Speech perception
All studies measured speech perception, using six instruments; the total number of participants was 121.
The UKCISG study (n = 84)62 used a prospective cohort design to measure speech perception with the BKB and AVGN before implantation and 9 months later in people who were profoundly deaf. Marginal hearing aid users were classified on the basis of their score on speech intelligibility tests taken before implantation [mean hearing level = 108.7 dB (95% CI 106.8–110.5)].
Results showed an improvement in scores on both outcome measures at 9 months [BKB: MHU = 44.0 (95% CI 37–51); AVGN: MHU = 31.0 (95% CI 26–37)]. This is the same study as reported in the previous comparison.
Ching and colleagues (n = 21)148 used a cross-sectional design in a small study to measure the same people with cochlear implants or acoustic hearing aids. Before each condition was measured participants used only that type of device in the preceding week. Ching and colleagues used BKB sentences in noise to measure speech perception and found a significant benefit for cochlear implant users (mean scores, cochlear implant = 39, acoustic hearing aid = 2, p < 0.001).
A few years earlier the MED-EL study (n = 63)125 measured speech perception in quiet conditions with HINT and CUNY sentences and in noise with HINT sentences and Consonant Nucleus Consonant (CNC) words. They compared participants’ scores preimplantation with hearing aids to scores with cochlear implants 6 months later. They conducted two sets of subgroup analyses: (1) pre- and postlingually deaf and (2) duration of deafness in postlingually deaf people (more or less than 25 years old). The mean difference (pre/post) for postlingually deaf people in quiet was 62%, with people with 25 years or less of hearing loss showing greater benefit from cochlear implants than those with more than 25 years of hearing loss (≤ 25 years = 71%, > 25 years = 53%). Prelingually deaf participants had a mean benefit in quiet of 20%. In noisy conditions postlingually deaf people with 25 years or less of deafness again did better than those with more than 25 years of deafness (mean scores: ≤ 25 years = 40% and > 25 years = 29% with CNC words).
Hamzavi and colleagues (n = 37)147 used number and monosyllable tests to measure speech perception preimplantation and 12 months later in participants who were severe to profoundly deaf, with cochlear implants or acoustic hearing aids, in a small prospective cohort study. They also measured changes between 12 and 36 months post implant in quiet and noise with the Hochmaier, Schultz and Moser (HSM) sentence test. They found that people with cochlear implants had a mean improvement in pre/post implant scores of 90% whereas over the same time acoustic hearing aid users’ mean scores improved by 37%. The monosyllable word test showed a mean improvement of 43% for cochlear implant users and 19% for acoustic hearing aid users. Over 2 years the HSM scores in quiet improved by 16% for cochlear implant users and 0% for acoustic hearing aid users. In noise, acoustic hearing aid users again showed no improvement over a range of decibels; however, cochlear implant users showed improvement over all levels, ranging from 3.5% at a signal to noise ratio (SNR) of 0 dB to 19.5% at a SNR of 10 dB.
Speech production
The MED-EL study (n = 63)125 measured speech production with CID sentences via a telephone. As with the speech perception results, the MED-EL study found an advantage for those who had been deaf for less than 25 years. Cochlear implant recipients were more able to correctly repeat back uncommon sentences (≤ 25 years = 68% and > 25 years = 42%).
Functional performance
Ching and colleagues (n = 21)148 measured functional performance in real-life situations by giving participants an ad hoc questionnaire after they had used each condition unaided by the other for a week. The questions considered the use of the devices, their performance in quiet and noisy conditions and awareness of environmental sounds. Participants with cochlear implants had significantly higher overall scores than those with acoustic hearing aids [cochlear implant = 59% (95% CI 52–65), acoustic hearing aid = 40% (95% CI 36–44), p < 0.001], indicating greater satisfaction with the functional performance of cochlear implants.
Quality of life
The UKCISG study (n = 84)62 measured quality of life with the HUI-3, GHSI and GBI. This study compared preimplant scores with scores 9 months post implant. All measures showed a trend towards improvement in quality of life [mean scores (95% CI): HUI-3 = 0.15 (0.11–0.19); GHSI = 0.19 (0.16–0.22); GBI = 42.0 (37–47)].
Participants in the MED-EL study (n = 63)125 were given an ad hoc quality of life questionnaire after 6 months with a cochlear implant. Overall, 84% of postlingually and 83% of prelingually deaf people were quite or very positive about the impact of cochlear implants on their quality of life.
Adverse events
Adverse events were measured by the UKCISG53 and the MED-EL study. 125 The UKCISG found that, out of 311 participants, there were 37 adverse events in 27 (9%) participants. Twelve of these events required readmission but did not lead to revision surgery and 25 events did lead to revision surgery. Eleven people had wound infections treatable by antibiotics. Six people had wound revisions, one of which went onto permanent explantation; one person had the device explanted and the other ear implanted; six people needed the device electrodes replacing; two needed the electrodes repositioning; and one needed wound revision. Three became non-users (1%); one was explanted because of complications; one had vertigo; and one had poor non-specified outcomes.
The MED-EL results were taken from all 106 adults implanted in the USA with a COMBI 40+. A total of 22 adverse events occurring in 20 (19%) people were reported. Seven of these were medical and 15 were device related. Only one of these required revision surgery (0.9%).
A visual summary of the results for this comparison is shown in Table 29a–d.
| Study design (follow-up, months) | Study | n | Auditory outcome |
|---|---|---|---|
| Sound direction | |||
| XSOC (NA) | Ching 2004148 | 21 |
| Study design (follow-up, months) | Study | n | Speech perception outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BKB sentences (noise) | AVGN | CNC words | CUNY sentences (quiet) | HINT sentences (quiet) | HINT sentences (noise) | HSM in quiet | HSM in noise | CNC words | |||
| C (P) | UKCISG62 | 84 | |||||||||
| XSOC (NA) | Ching 2004148 | 21 | |||||||||
| PP (P) (6) | MED-EL 2001125 | 106 | |||||||||
| C (P) (36) | Hamzavi 2001147 | 37 | |||||||||
| Study design (follow-up, months) | Study | n | Speech production outcome |
|---|---|---|---|
| CID sentences (telephone) | |||
| PP (P) (6) | MED-EL 2001125 | 106 |
| Study design (follow-up, months) | Study | n | Quality of life outcomes | ||||
|---|---|---|---|---|---|---|---|
| HUI-3 | GHSI | GBI | Functional performance in real life | Quality of life questionnaire | |||
| C (P) | UKCISG | 84 | |||||
| XSOC (NA) | Ching 2004148 | 21 | |||||
| PP (P) (6) | MED-EL 2001125 | 106 | |||||
Summary of studies: unilateral cochlear implants versus acoustic hearing aids – adults
Although the studies ranged from good to poor, some inadequate reporting and weak design again made it difficult to come to firm conclusions about the validity of these results.
Audiologically the results are inconclusive; the measure of the average root mean squared errors [cochlear implant = 4.5 (95% CI 4.1–4.9); acoustic hearing aid = 4.6, (95% CI 4.3–4.9)] from Ching and colleagues148 is ambiguous, possibly due to levels of residual hearing in participants, as the mean (SD) level of deafness was severe rather than profound [83.3 dB HL (18.9)].
Speech perception was measured in a variety of ways, all showing benefits from cochlear implants. The clearest benefit was indicated by Ching and colleagues148 who showed a mean score advantage of 37 points for cochlear implants over acoustic hearing aids in noise with BKB sentences (p < 0.001), showing that implanted adults were able to correctly repeat back significantly more sentences than when they used hearing aids alone. However, prelingually deaf people had less benefit, gaining mean change scores in quiet of 20% compared with 62% for the postlingually deaf. It is difficult to comment on the results from Hamzavi and colleagues147 as the cochlear implant and acoustic hearing aid groups had different degrees of deafness [mean (SD): cochlear implant = 105 dB (5), acoustic hearing aid = 85 dB (10)].
The degree of benefit for speech perception and production was linked to the duration of deafness before implantation, with those postlingually deaf who had been deaf for 25 years or less faring better in quiet and noise than those who had been deaf for longer on all measures. For example, with the CID sentence test of uncommon sentences, given over the telephone, those who had been deaf for less than 25 years were able to correctly repeat a greater proportion than those who had been deaf for at least 25 years (≤ 25 years = 68% and > 25 years = 42%). 125
Participants were asked to rate the functional performance of cochlear implants and their effects on quality of life in the studies by Ching and colleagues148 and MED-EL125 respectively. Ching and colleagues148 found that cochlear implants were given a higher functional performance rating (cochlear implant = 59%, acoustic hearing aid = 40%). The MED-EL125 study found commensurate gains in quality of life with 84% of participants quite or very positive about the impact of cochlear implants on their lives.
The rate of major surgical complications requiring revision surgery found by the UKCISG study53 was fairly low (8%) but not as low as that of the MED-EL study (0.9%). 125
Overall conclusions
These studies indicate that there may be additional benefits from having cochlear implants over acoustic hearing aids. These benefits become clearer in noisy conditions with greater gain being experienced by adults who are postlingually rather than prelingually deaf. People with cochlear implants may find that their functional hearing and quality of life improve.
Bilateral cochlear implants versus unilateral cochlear implants – adults
Type and quality of studies
Five studies are included in the comparison of unilateral versus bilateral cochlear implantation. Two studies were RCTs with waiting list controls and two studies were pre/post repeated measure designs with their own controls and one was a cross-sectional study. Three of the studies were based in the UK, one in Europe and one in the USA. There were 147 participants in total. Follow-up ranged from 6 to 9 months post implantation and mean ages ranged from 46 to 59 years. All studies used the Nucleus CI 24 device.
It should be noted that there is an overlap of participants between the studies of Summerfield and colleagues,149 Ramsden and colleagues150 and Vershurr and colleagues. 151 Ramsden and colleagues recruited all of the participants from Summerfield and colleagues plus a further six people, and Vershurr and colleagues’ participants were a mixture of those randomised by Summerfield and colleagues and others. Surprisingly two of the studies did not report the degree of deafness of their participants; two of the remaining studies used severely to profoundly deaf people and the other one only the profoundly deaf. Three studies were excluded on the grounds of the size of the population, with a total n = 43 (range 5–20); 77% of the total possible population was included.
Summary tables of the characteristics and results of the included studies can be found in Appendix 3 (Tables 106 and 107 respectively).
The studies were of good to moderate quality. All studies were prospective and had eligibility criteria appropriate to the research question and representative populations. The reporting of the degree of participants’ deafness and attrition over the period of the trials and the addressing of potential confounding factors varied. The blinding of assessors or data analysts, methods for accounting for missing data and power calculations were not reported by any study. Table 30 provides a summary of the key study quality indicators.
| Quality criteria | Summerfield 2006149 | Litovsky 2006152 | UK Bi trial 2005 | Laszig 2004153 | Verschuur 2005151 |
|---|---|---|---|---|---|
| Was the study prospective? | Yes | Yes | Yes | Yes | Yes |
| Selection bias | |||||
| Eligibility criteria stated? | Yes | Yes | Yes | Yes | Yes |
| Appropriate? | Yes | Yes | Yes | Yes | Yes |
| Were the participants representative of the population? | Yes | Yes | Yes | Yes | Yes |
| Were potential confounders reported? | No | No | Yes | No | Yes |
| Were they accounted for in the design or analysis? | No | No | Yes | No | Yes |
| Assessment bias | |||||
| Were the outcome measures relevant to the research question? | Yes | Yes | Yes | Yes | Yes |
| Independent blind assessment? | NR | NR | NR | NR | NR |
| Objective? | No | No | Yes | Yes | No |
| Attrition bias | |||||
| Was attrition reported? | Yes | Yes | Yes | No | NA |
| Were all participants accounted for? | Yes | No | Yes | No | Yes |
| Were missing data accounted for? | NR | NR | NR | NR | NR |
| Protocol violations specified? | No | No | No | Partial | No |
| Power and analysis | |||||
| Data analysis | ANOVA | ANOVA | ANOVA + t-test | NR | ANOVA |
| Was the analysis appropriate? | Yes | Yes | Yes | – | Yes |
| Was there a power calculation? | NR | NR | NR | NR | NR |
| Other | |||||
| Was ethical approval given? | NR | NR | Yes | Yes | NR |
| Intercentre variability reported? | NR | NR | NR | NR | NR |
| Generalisability: | Yes | Yes | Yes | Yes | Yes |
Study results: unilateral cochlear implant versus bilateral cochlear implants – adults
The sensitivity to sound and speech perception results showed a binaural advantage; however, the quality of life results varied with some positive and a few negative trends for bilateral implantation. The outcomes from these studies were either sensitivity to sound, speech perception or quality of life.
Sensitivity to sound
A total of 44 people in two studies had sensitivity to sound measured in laboratory conditions.
The RCT of Summerfield and colleagues (n = 24)149 measured self-reported spatial hearing, qualities of hearing and hearing for speech (Speech Hearing, Spatial Hearing and Qualities of Hearing questionnaires, SSQ) in adults who either had sequentially received a second cochlear implant or were waiting for one. The scores are an average score for the domain in question with a range of 0–10. They found that there was a significant benefit for spatial hearing at 3 and 9 months post implantation compared with preimplantation [mean difference (SD) scores: 3 months = 1.46 (0.83–2.09), p < 0.01; 9 months = 0.71 (0.08–1.33), p < 0.01]. When the groups’ bilateral results were pooled a stronger effect was seen [3 months = 1.56 (0.95–2.17), p < 0.001; 9 months = 2.00 (1.47–2.53), p < 0.001]. Pooling of the group results showed significant binaural gains for quality of hearing and hearing for speech [quality of hearing: 3 months = 0.9 (0.5–1.3), p < 0.05; 9 months = 0.7 (0.2–1.2), p < 0.05; hearing for speech: 3 months = 6.00 (0.00–12.00), p < 0.01; 9 months = 9.00 (3.00–15.00), p < 0.01].
Verschuur and colleagues (n = 20)151 investigated the ability to detect the direction of sound with either unilateral or sequential bilateral implants. They found that bilaterally aided participants made significantly fewer errors in sound direction detection, however speakers were positioned (mean absolute angular error scores: unilateral = 67°, bilateral = 24°, p < 0.001).
Speech perception
Three studies measured speech perception in a total of 103 participants using seven outcome measures.
Litovsky and colleagues (n = 37)152 used three outcome measures (CNC words and HINT sentences in quiet conditions and BKB sentences in noise) to measure speech perception in simultaneously implanted adults. They found significant binaural gains on all instruments (CNC: left ear 40%, right ear 36%, bilaterally 54%, p < 0.0001; HINT: left ear 66%, right ear 67%, bilaterally 76%, p < 0.0001).
In particular, bilaterally implanted participants were able to use the head shadow effect when in noise. This occurs when speech and noise come from different directions producing a difference in the SNR because of the presence of the head. The mean (SD) head shadow effects were 4.95 dB (3.6) for noise right and 6.34 dB (3.8) for noise left, i.e. a slightly greater effect for noise left. When speech reception thresholds were compared for bilateral implants and either ear unilaterally there was a significant gain for bilateral versus unilateral implants (data not reported, p < 0.0001).
An earlier study similarly evidenced the benefits of bilateral implantation in noise. The RCT of Ramsden and colleagues (n = 29)150 measured speech perception with the CNC and CUNY in quiet and noise in sequentially implanted adults. They found a significant binaural benefit over the first ear alone for speech and noise from the front (12.6 ± 5.4%, p < 0.001) and when noise was ipsilateral to the first ear (21 ± 6%, p < 0.001). No bilateral advantage over the first ear was found in quiet.
Improved speech perception through accessing the head shadow effect was found by Laszig and colleagues (n = 37). 153 They used three tests in this pre/post study with its own controls [the Freiburger monosyllabic word test (FMWT) words and HSM sentences in quiet, and HSM and Oldenburg sentence test (OLSA) sentences in noise]. They found a significant binaural benefit in quiet conditions compared with the poorer unilateral ear alone (mean score: unilateral = 49%, bilateral = 58%, p = 0.00009). In noisy conditions they found a significant head shadow effect, with bilateral advantage greater when the better ear was closest to the speech source than when the poorer ear was closest (poorer ear closest to noise –10 dB and better ear closest to noise –11.4 dB, p < 0.00001).
Quality of life
Quality of life was measured for 54 participants in two studies with five different instruments.
The RCT of Summerfield and colleagues (n = 24)149 measured quality of life with five instruments [GHSI, HUI-3, overall quality of life visual analogue scale (VAS), EuroQol 5 dimensions (EQ-5D) and a tinnitus questionnaire]. At 9 months post implantation they found that scores on the GHSI showed a positively significant result in favour of bilateral implantation [GHSI = 4.00 (95% CI 1.00–0.08), p < 0.05]. Other measures showed neutral or negative mean differences between unilateral and bilateral conditions at 9 months [HUI-3: –0.01 (95% CI –0.1 to 0.08), not significant; VAS: –0.06 (95% CI 0.12–0.00), not significant; EQ-5D: –4.5 (95% CI –12.0 to 3.0), p < 0.05]. These results were coincidental with worsening tinnitus that followed the second implantation (seven out of 16 people who reported tinnitus before the second implant said that tinnitus worsened after the second implant). The reduction in quality of life because of tinnitus reached significance at 3 months (mean score on the tinnitus questionnaire: 12 (95% CI 1.0–23), p < 0.05). Summerfield and colleagues examined these outcomes with multivariate analyses, which showed that the positive gains that came from improved hearing were offset by worsening tinnitus.
Litovsky and colleagues (n = 37)152 used the Abbreviated Profile of Hearing Aid Benefit (APHAB) to measure quality of life. On four of the subscales they found significant gains for bilateral implantation; these ranged from mean scores of 4.4% (p < 0.0001) for reverberant conditions and background noise to 5.7% (p < 0.0001) for communication. Table 31a–c provides a visual summary of these results.
| Study design (follow-up, months) | Study | n | Bilateral cochlear implant condition, auditory outcomes | |||
|---|---|---|---|---|---|---|
| SSQ | Quality of hearing | Hearing for speech | Mean absolute angular error | |||
| RCT (WLC) (9) | Summerfield 2006149 | 24 | ||||
| XSOC | Verschuur 2005151 | 20 | ||||
| Study design (follow-up, months) | Study | n | Bilateral cochlear implant condition, speech perception outcomes | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BKB sentences (noise left) | BKB sentences (noise right) | BKB sentences (noise front) | BKB binaural redundancy | CNC words (quiet) | CUNY sentences (quiet) | CUNY sentences (S&NF) | CUNY sentences (SFN1) | CUNY sentences (SFN2) | HINT sentences (quiet) | FMW words | HSM sentences | HSM sentences (noise) | OLSA (front) | |||||||
| PP (P) (6) | Litovsky 2006152 | 37 | ||||||||||||||||||
| RCT (WLC) (9) | Ramsden 2005150 | 29 | NS | |||||||||||||||||
| PP (P) (6) | Laszig 2004153 | 37 | ||||||||||||||||||
| Study design (follow-up, months) | Study | n | Bilateral cochlear implant condition, quality of life outcomes | |||||
|---|---|---|---|---|---|---|---|---|
| EQ-5D | GHSI | HUI-3 | Tinnitus questionnaire | VAS overall quality of life | APHAB | |||
| RCT (WLC) (9) | Summerfield 2006149 | 24 | ||||||
| PP (P) (6) | Litovsky 2006152 | 37 | ||||||
Summary: bilateral cochlear implants versus unilateral cochlear implants – adults
This comparison included two well-reported RCTs and two less well-reported prospective pre/post studies. Again, heterogeneity meant that pooling of data was not possible.
The sensitivity to sound results are fairly robust (internally valid), although the number of participants is low (n = 44). Both studies that measured this outcome found significant binaural advantages. The RCT found a mean difference for spatial hearing of 0.71 (95% CI 0.08–1.33, p < 0.01), a mean difference for quality of hearing of 0.7 (95% CI 0.2–1.2, p < 0.05) and a mean difference for hearing for speech of 9.00 (95% CI 3.00–15.00, p < 0.01) with self-reported tests with scores between 0 and 10; the result for detection of sound direction was 24° (p < 0.001).
Binaural benefits for speech perception were found to be significant in noisy conditions on all measures. These ranged from 12.6 for CUNY sentences (p < 0.001) to 76% for HINT sentences (p < 0.0001). In particular, advantages were shown for the head shadow effect (–3.5, p < 0.0001). Not all measures in quiet conditions showed significant gains.
Quality of life was measured with generic and disease-specific instruments. Two measures (GHSI and APHAB) found significant quality of life benefits from bilateral implantation [GHSI: 2.00 (95% CI 1.00–7.00), p < 0.05; APHAB communication: 5.7 (SE 0.2), p < 0.0001]. However, neutral and negative results came from the HUI-3 [–0.01 (95% CI –0.1 to 0.08), not significant], VAS [–0.06 (95% CI 0.12–0.00), not significant] and EQ-5D [–4.5 (95% CI –12.0 to 3.0), p < 0.05]. Multiple regression indicated that the negative results for quality of life after bilateral implantation in one study might have been due to worsening tinnitus following the second implant in that study. The non-disease-specific measures showed no benefit.
Overall conclusions
Bilateral implantation increases the ability to hear clearly, detect the direction of sound in noisy conditions and understand speech and may improve quality of life in the absence of worsening tinnitus.
Bilateral cochlear implants versus unilateral cochlear implant and an acoustic hearing aid – adults
This systematic review did not find any studies of two cochlear implants versus one cochlear implant with an acoustic hearing aid.
Additional studies on quality of life – adults
Three studies met the systematic review inclusion criteria and measured quality of life; these have been discussed previously in this chapter in the comparisons of unilateral cochlear implants and non-technological support, unilateral cochlear implants and acoustic hearing aids and bilateral and unilateral cochlear implants. Improvement in quality of life may be considered the primary benefit from cochlear implants; therefore, to gain a better picture of the effects of cochlear implants on the quality of life of adults the original searches and papers obtained were reviewed for further studies outside the systematic review inclusion criteria.
A meta-analysis of cost–utility data was found; this contained seven studies, six of which were excluded as their publication dates were 1995 or earlier, the cut-off date for this systematic review because of technological advances since then (see Chapter 3, Inclusion and exclusion criteria). One study from the meta-analysis was included with a further five studies from the other searches. Four studies were prospective designs, three with their own controls and one with cochlear implant candidate controls, one study was cross-sectional and one was a qualitative interview study. See Appendix 3 for a summary of the characteristics and results of these studies (Tables 108 and 109 respectively).
The quality of these studies varied from moderately good to poor. Descriptions of participants were given rather than specific inclusion criteria, there was a failure to acknowledge or account for any potential confounding factors and the numbers of participants recruited were not always accounted for. Table 32 gives a summary of the key quality indicators.
| Quality criteria | Mo 200539 | Vermeire 2005154 | Hallberg 200418 | Hawthorne 2004155 | Hogan 200143 | Palmer 1999156 |
|---|---|---|---|---|---|---|
| Was the study prospective? | Yes | NA | NA | Yes | NA | Yes |
| Selection bias | ||||||
| Eligibility criteria stated? | Minimal | Minimal | Minimal | Minimal | Yes | Yes |
| Appropriate? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the participants representative of the population? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were potential confounders reported? | No | No | NA | No | No | No |
| Were they accounted for in the design or analysis? | No | No | NA | No | No | No |
| Assessment bias | ||||||
| Were the outcome measures relevant to the research question? | Yes | Yes | Yes | Yes | Yes | Yes |
| Independent blind assessment? | NR | NR | NA | NR | NR | NR |
| Objective? | Yes | Yes | No | Yes | Yes | Yes |
| Attrition bias | ||||||
| Was attrition reported? | No | No | NA | Yes | NA | Yes |
| Were all participants accounted for? | Yes | No | NA | Yes | NA | Yes |
| How were missing data accounted for? | NR | NR | NA | Within dimension computations or regression | NR | NR |
| Protocol violations specified? | NR | NR | NA | No | No | |
| Power and analysis | ||||||
| Data analysis | t-test, Wilcoxon, linear regression | t-test, ANOVA | Grounded theory | ANOVA | Multiple regression, ANCOVA | ANOVA |
| Was the analysis appropriate? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was there a power calculation? | NR | NR | NA | NR | NR | NR |
| Other | ||||||
| Was ethical approval given? | Yes | NR | NR | NR | Yes | NR |
| Generalisability | Yes | Yes | No | Yes | Yes | Yes |
Study results – additional studies of quality of life in adults
The six studies evaluated the health-related quality of life of 431 participants. Three studies were carried out in Europe, two in Australia and New Zealand and one in the USA.
Mo and colleagues (n = 27)39 prospectively measured the quality of life of postlingually deaf adult cochlear implant recipients. They used three measures [Patient Quality of Life Form (PQLF), Index Relative Questionnaire Form (IRQF) and SF-36]. Over the 15-month follow-up period they found significant differences in the total mean (SD) scores of the PQLF [0.62 (0.47), p < 0.01] and IRQF [0.37 (0.39), p < 0.01]. However, the SF-36 showed a significant improvement only on the general health subscale [7.2 (14.5), p < 0.05]. The greatest mean (SD) improvements were in PQLF communication [0.93 (0.64), p < 0.01], feeling being a burden [0.87 (0.90), p < 0.01], isolation and relationships with friends [0.60 (0.64), p < 0.01] and relations to close individuals [0.29 (0.44), p < 0.01].
Vermeire and colleagues (n = 89)154 used the Hearing Handicap Inventory for Adults (HHIA) and the GBI to prospectively measure quality of life in 89 postlingually deafened adults. They found that HHIA postoperative mean (SD) scores were significantly better than preoperative mean (SD) scores [pre = 69 (0.69), post = 48 (25.28), p < 0.001]. GBI scores, which range from 0 (low) to 100 (high), were taken post implant and gave a mean total score of 35.16 (SD 19.61).
Hawthorne and colleagues (n = 34)155 prospectively measured quality of life with the Assessment of Quality of Life (AQL) and the Hearing Participation Scale (HPS). They found that after 6 months with a cochlear implant quality of life had improved significantly for the profoundly deaf participants [mean (SD) scores: AQL, difference 0.28 (0.36), p < 0.01; HPS, difference 0.20 (0.23), p < 0.01].
Hogan and colleagues (n = 202)43 used a cross-sectional design to measure quality of life with the AQL. Of the six subscales they found significant differences between the intervention and control groups’ mean (SD) scores on physical senses [intervention = 0.78 (0.19), control = 0.58 (0.19), p < 0.01] and utilities [intervention = 0.57 (0.27), control = 0.38 (0.22), p < 0.01].
Palmer and colleagues (n = 62)156 used a repeated measures pre/post design with non-randomised control subjects and 12 months’ follow-up to measure quality of life with the HUI-3. They found a mean (SD) utility gain of 0.20 (0.24) for the implanted group.
Hallberg and Ringdahl (n = 17)16 conducted a grounded theory157 analysis of interviews with 17 adult, profoundly sensorineurally deaf, cochlear implantees. Participants had used their implant for a mean of 4.1 years (range 1–12). They found that the overarching core category was ‘coming back to life’, which reflected perceived harmony in life and becoming part of the living world. This was related to four subcategories: preventing disappointment, waiting in silence, retraining the brain and strengthening of self-worth. These told a story of the process of decision-making to undergo implantation, balancing a feeling of having nothing to lose with low expectations of the result. Postoperatively participants had to ‘wait in silence’ with uncertainty about the outcome. This was followed by the ‘significant revelation’ following switching the device on and was the emotional starting point of their coming back to life. This was followed by the lengthy training process of learning to hear and listen with the implant. Finally, self-worth was strengthened by being less dependent and having increased social participation. In all, cochlear implants were represented as making a substantial improvement in their recipients’ quality of life.
Table 33 provides a visual summary of these quality of life results.
| Study design (follow-up, months) | Study | n | Cochlear implant condition, quality of life outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PQLF | IRQF | SF-36 | HHIA | GBI | AQL | HPS | HUI-3 | |||||
| PP (P) (15) | Mo 200539 | 27 | ||||||||||
| PP (P) (??) | Vermeire 2005154 | 89 | ||||||||||
| PP (P) (6) | Hawthorne 2004155 | 34 | ||||||||||
| XS (NRC) | Hogan 200143 | 202 | ||||||||||
| PP (P) (12) | Palmer 1999156 | 62 | ||||||||||
Summary of quality of life studies – adults
There are five quantitative and one qualitative study in this extended review of adult quality of life. The eight measures used by the studies showed either significant gains or trends towards gains from using cochlear implants. The studies that used pre/post measures (within subjects) were more likely to find significant results than those that used other control subjects (between subjects). The degree of improvement ranged from a mean (SD) gain of 7.2 (14.5) on the SF-36 to 21 (25.29) on the HHIA. The qualitative study found that all 17 interviewees thought that cochlear implants had substantially improved their quality of life.
A section in Chapter 7 summarises a more specific review of studies that reported utility values for cochlear implantation in adults (see Utilities).
Conclusions
Cochlear implants improve quality of life in suitable candidates.
Overall summary of effectiveness in adults
Table 34 provides an overall summary of the effectiveness of cochlear implants in adults. This table gives an overview of the outcomes from the adult studies included in the clinical effectiveness systematic review. It shows that the outcomes were positively significant (n = 26), showed a positive trend (n = 27) (because of significance not being reported or the results not being significant), showed a negative trend (n = 2) or were negatively significant (n = 1). The negative results were related to the effects of tinnitus on quality of life.
| Comparison | Total outcomes, n(no. reporting significance) | Positively significant outcomes, n (%)(p < 0.05) | Positive trend outcomes (NS/NR), n (%) | Negative trend outcomes (NS/NR), n (%) | Negatively significant outcomes, n (%) (p < 0.05) |
|---|---|---|---|---|---|
| Cochlear implants vs non-auditory support | |||||
| Sensitivity to sound | – | – | – | – | – |
| Speech perception | 14 (6) | 6 (43) | 8 (57) | – | – |
| Speech production | – | – | – | – | – |
| Health-related quality of life | 3 (1) | 1 (33) | 2 (66) | – | – |
| Cochlear implants vs acoustic hearing aids | |||||
| Sensitivity to sound | 1 (1) | 0 | 1 (100) | – | – |
| Speech perception | 10 (3) | 3 (30) | 7 (70) | – | – |
| Speech production | 1 (0) | 0 | 1 (100) | – | – |
| Health-related quality of life | 2 (2) | 2 (100) | – | – | – |
| Unilateral cochlear implants vs bilateral cochlear implants | |||||
| Sensitivity to sound | 4 (4) | 4 (100) | – | – | – |
| Speech perception | 18 (9.5) | 10 (56) | 6 (33) | 2 (11) | – |
| Speech production | – | – | – | – | – |
| Health-related quality of life | 6 (6) | 2 (33) | 1 (17) | 2 (33) | 1 (17) |
| Bilateral cochlear implants vs unilateral cochlear implants and acoustic hearing aids | |||||
| Sensitivity to sound | – | – | – | – | – |
| Speech perception | – | – | – | – | – |
| Speech production | – | – | – | – | – |
| Health-related quality of life | – | – | – | – | – |
Summary of adult studies of clinical effectiveness
In total, 13 studies were included in the systematic review. There were 1379 adults (92%) who were severely to profoundly or profoundly sensorineurally deaf, with ages ranging from 16 to 87 years.
Clinical summary
-
When cochlear implants are compared with non-technological support the evidence indicates that cochlear implants lead to improvements in the ability to understand speech and quality of life. This is moderately associated with age at implantation and more strongly associated with duration of deafness before implantation.
-
There may be additional benefits from having cochlear implants compared with acoustic hearing aids for adults with less severe hearing loss. These gains may be greater in noisy conditions, especially amongst people who are postlingually deaf, although greater gains in noise may be due to ceiling effects of the tests used to measure performance in quiet conditions. Furthermore, functional hearing and quality of life may be improved.
-
Bilateral cochlear implantation increases the ability to hear clearly, detect the direction of sound in noisy conditions and understand speech, and may improve quality of life when compared with unilateral cochlear implantation.
-
Widening the scope of the review of quality of life confirms the finding that cochlear implants may improve quality of life for severely to profoundly or profoundly sensorineurally deaf adults.
Methodological summary
-
Two of the studies were RCTs with waiting list controls, seven were pre-/post implant studies, one was a prospective cohort study and one used a cross-sectional design. Nine papers used participants as their own controls and one used a comparator group of non-implanted severely deaf people. Heterogeneity meant that pooling of data was not possible.
-
The quality of the studies was variable, ranging from good to poor. Generally there was inadequate reporting of methods in the non-randomised studies, thus threatening their internal validity.
Safety and reliability of cochlear implants – children and adults
Adverse events were only reported by two studies in the clinical systematic review; therefore, the original clinical searches were reviewed for adverse event studies. Further evidence came from the economic systematic review, which is presented here as adverse events are also a factor in clinical effectiveness. The numbers of adverse events reported were small and similar in children and adults, thus these groups will be considered together. The reliability of cochlear implants is also reviewed.
Adverse events
Abandoned initial procedure
Abandoned procedures represent the irreversible failure of an implant operation.
The study by Ray and colleagues158 reported on complications experienced in 844 consecutive implants in a mixture of adults and children, the majority of which were performed after 1994. Only one (0.12%) operation was ‘abandoned’. Similarly, Bhatia and colleagues159 report that in 300 consecutive paediatric implantations no major postsurgical complications occurred perioperatively and only one (0.33%) operation was abandoned.
In an Australian model-based analysis of the cost-effectiveness of cochlear implants for both adults and children, published in 1999, Carter and Hailey160 made provision for 5% of operations to result in some form of complication and assumed a 99% clear-up rate.
Major complications
A major complication is defined as one that leads to revision surgery under general anaesthetic. These may include flap breakdown, cholesteatoma, ear drum perforation, facial nerve damage, persistent infection, meningitis, extrusion of the electrode array or device failure. Revision surgery may also be required to reposition a suboptimally placed electrode array. The surgery to implant the device may mean the loss of residual hearing and it is not possible to predict which patients may suffer such loss. 4
In considering the safety and effectiveness of the COMBI 40+ implant system MED-EL125 collected adverse event data on 106 adults and 82 children. The cumulative implant experiences were 713 months and 533 months respectively. In adults there was one major complication requiring revision surgery. In children there were three major postsurgical complications, two involving resuturing and one explantation. The corresponding complication rates are therefore 1.7 events per 100 patient-years for adults and 6.8 events per 100 patient-years for children.
Two other papers allow the approximation of cumulative implant experience. Proops and colleagues161 reported on complications occurring in 100 adults who received devices as part of the UK-based implant programme. Implants were fitted between December 1990 and May 1996 and the number of operations per year reported. Assuming that all operations occurred in the middle of each year gives an approximate follow-up period of 2638 patient-months. Over that time there were four events reported. The crude rate is therefore 1.8 events per 100 patient-years.
Dutt and colleagues162 report the same type of data for a different group of 100 adults from the same implant programme. The study period was 1999–2001, over which period 122 operations were carried out. This follow-up period is relatively short and assuming that the same number of operations was carried out in each month gives an approximate follow-up period of 2257 patient-months. Three events classified as major postsurgical complications were reported, giving a crude rate of 1.6 events per 100 patient-years.
Fayad and colleagues163 studied the clinical outcomes of children following revision surgery. In total, 28 of the 496 children required some form of revision surgery, leading to an ‘overall revision rate’ of 5.6%. However, without knowing how many children had implants in each year it is impossible to calculate the cumulative implant experience.
Minor complications
Minor complications may resolve with conservative treatment and may include wound infections, flap oedema, haematoma, facial nerve stimulation, tinnitus and temporary vertigo.
In evaluating the safety and effectiveness of the COMBI 40+ implant system125 MED-EL noted that there were 21 ‘minor’ events in adults and 16 such events in children. These correspond to event rates of 35.3 minor complications per 100 patient-years for adults and 34.7 events per 100 patient-years for children.
Meningitis
Before 2003 an increased risk of meningitis associated with cochlear implantation was reported. 164 Summerfield and colleagues165 ascertained that, of 1851 children implanted in the UK before October 2002, none had contracted meningitis and there were no significant differences compared with the general population. Of 1779 adults, five had contracted meningitis, of whom three died. 165 This incidence was significantly higher than that in the general population. For the total UK cochlear implant cohort the incidence rate per 100,000 population was 29 cases (95% CI 9–68), compared with 1.31 per 100,000 population in the general population. 165
Since 2002 the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK has advised that patients should be routinely vaccinated against pneumococcal meningitis before surgery for cochlear implantation. 166 An international consensus on meningitis and cochlear implants167 reported that since these and other measures have been in place the incidence of meningitis has fallen to ‘its previously low, acceptable level, and may even have fallen below it’. Nevertheless, the risk of meningitis is discussed with prospective implantees or their carers and is therefore included in sensitivity analyses in the PenTAG cost-effectiveness model but not in the base case.
The increased incidence of meningitis among patients with cochlear implants in 2002 was shown to be significantly associated with a particular type of electrode array, which was subsequently withdrawn from use. It may be that the withdrawal of this array, plus careful attention to preoperative vaccination and postoperative intervention with antibiotics in case of middle ear infection, has reduced the incidence of postoperative meningitis worldwide.
A similar investigation to that of Summerfield and colleagues165 was undertaken in the USA by Reefhuis and colleagues168 of paediatric cochlear implant users. The person-years of exposure in this cohort was much larger than that analysed by Summerfield and colleagues (9652 person-years compared with 2478). Reefhuis and colleagues noted 10 cases of meningitis in this cohort, giving an incidence rate of 104 episodes per 100,000 patient-years.
Non-use of devices
Non-use of devices refers to the choice of recipients of cochlear implants to no longer use them for a variety of reasons.
Summerfield and Marshall169 published a paper reporting the incidence of elective non-use among the first cohort of adult patients to receive implants in the UK (n = 313); they found that cumulative elective non-use was stable at 6.3% (95% CI 3.6–9.1%) between 4 and 7 years post implantation but rose to 11.0% (95% CI 1.75–20.3%) at 7.5 years post implantation. Risk factors for non-use were low auditory performance (odds ratio = 8.2, 95% CI 2.1–31.9), low self-reported benefit (odds ratio = 19.6, 95% CI 4.6–84.4) and experiencing a major complication (odds ratio = 3.2, 95% CI 1.0–10.6).
More recently, Bhatt and colleagues170 conducted a retrospective case review of 214 adults who received implants between June 1988 and June 2002. They found that 29 (13.6%) had at some time not used their device for more than 4 consecutive weeks. The cumulative follow-up period was 1126 patient-years. Over that period two people (0.93%) elected for non-use, three (1.40%) became non-users because of co-morbid illnesses and one (0.47%) became a non-user because of audiological complications. Two people (1%) became non-users because of the deterioration of hearing.
Archbold and colleagues171 looked at long-term cochlear implant use in children (n = 138). They found that over 7 years 83% of children wore their implants full-time, 12% most of the time, 2% some of the time and 3% not at all. When the children were classified according to age at implantation they found a significant effect. Those who were full-time users had a median age at implantation of 4.4 years, whereas those who were not full-time users had a median age at implantation of 5.5 years (p = 0.0009). All of the children who were total implant non-users had been implanted over the age of 5 years.
These results are similar to those of Ray and colleagues172 who retrospectively looked at 172 children and 251 adults implanted in the Birmingham programme between 1990 and 2000. They found that five (2.9%) of the children (mean age 11 years) and three (1.2%) of the adults (mean age 42 years) chose not to use their cochlear implants. For children the main reason for non-use was peer pressure and for adults reasons included depression, tinnitus, concomitant neurological problems and non-auditory stimulation.
Non-reimplantation of a cochlear implant during a revision procedure
A cochlear implant may need to be removed for a variety of reasons, for example infection or device failure. Normally, once the problem has been dealt with the ear is reimplanted; however, this is not always the case. Available information concerning reported instances of cochlear implants being permanently explanted is summarised in Table 35.
| Source | Population group | No. explanted | No. not reimplanted | Comments |
|---|---|---|---|---|
| Dutt 2005162 | Adults | 5 | 1 | Follow-up period 1999–2001 |
| Ray 2004158 | Adults | 15 | 1 | Follow-up period 1990–2002 |
| Bhatia 2004159 | Children | 8 | 2 | Follow-up period not reported; paper reports eight reoperations |
| Balkany 1999173 | Mixture of adults and children | 16 | 0 | Follow-up period 1990–7 |
| Stratigouleas 2006174 | Mixture of adults and children | 6 | 1 | Follow-up period not reported; paper reports seven major postsurgical complications but only six revision operations |
| Lassig 2005175 | Mixture of adults and children | 60 | 2 | Follow-up period 1985–2003; overall number |
Reliability of cochlear implants
The reliability of cochlear implants refers to the length of time implants work for before they need replacing.
Failure and replacement of cochlear implant internal components
Maurer and colleagues176 conducted a review of studies looking at the reliability of cochlear implants. However, only two (Von Wallenberg and Brinch177 and Ajayi and colleagues178) of the nine studies reviewed reported the cumulative reliability of devices (Table 36).
| Study | Time period covered in years | Number of devices | Cumulative reliability |
|---|---|---|---|
| Maurer 2005176 | 11 | 192 | 91.7% |
| Conboy 2004179 | 13 | 363 | 90.0% |
| Lehnhardt 2000180 | 12 | 16,427 | 94.9% |
| Ajayi 1997178 | 2 | 118 | 99.1% |
| Von Wallenberg 1995177 | 5 | 8804 | 92.2% |
Maurer and colleagues176 then looked at the reliability of 192 devices implanted over 11 years (1990–2001) and found an overall cumulative survival of device rate of 91.7% over 11 years.
Conboy and colleagues179 followed 363 devices for 13 years (1989–2002) and found a similar cumulative survival (90.0%) to that of Maurer and colleagues. 176 Data on the number of implants for each year as well as cumulative device survival are reported. Overall, 94.3% of devices survived to 7 years and 90.0% to 13 years. Again, neither confidence intervals nor standard errors were presented for each time point. This makes it difficult to assess whether the differences between devices reported in the two studies are significantly different. This finding is less favourable than that from Lehnhardt and colleagues,180 who reported a cumulative reliability for 16,427 devices over 12 years of 94.9%.
In a review of cochlear implant failures and revisions in 2005, Lassig and colleagues175 found that, in 900 cochlear implant patients from one centre, 27 (3%) underwent revision surgery because of the failure of the internal device.
The Cochlear Europe submission to NICE presented information about the numbers of devices given to children and the cumulative survival for several different devices. The information was reportedly correct as of 30 June 2006, with each graph representing a type of Nucleus® device based on the receiver/stimulator portion. These data are reproduced in Figure 3 alongside the annual data presented in Conboy and colleagues. 179
FIGURE 3.
Paediatric cumulative survival plots for a range of cochlear implants as reported by Cochlear Europe and Conboy and Gibbin. 179
The Cochlear Europe submission also contains cumulative survival curves for various devices as used in adults. These are reproduced in Figure 4.
FIGURE 4.
Cumulative survival plots for a range of cochlear implants given to adults as reported by Cochlear Europe.
Summary of safety and reliability
-
Cochlear implants are safe and reliable. The rate of abandoned operations is low (0.12%).
-
The incidence of major complications is 6.8 per 100 patient-years in children and 1.4–1.7 per 100 patient-years in adults.
-
The incidence of minor complications is 35.3 per 100 patient-years in adults and 34.7 per 100 patient-years in children.
-
Cochlear implants are reliable with 92% of devices lasting 11 years.
Chapter 6 Assessment of cost-effectiveness
Systematic review of economic evaluations
Aim
To summarise existing published research evidence on both the costs and cost-effectiveness of unilateral cochlear implantation (compared with living without a cochlear implant) and bilateral implantation (compared with either unilateral implantation or no implant), with particular emphasis on the potential generalisability of previous studies to the current NHS policy and clinical context.
Methods
Search strategy
Appendix 1 describes the range of sources searched and the search strategy. The search was limited to English language papers only. Databases were searched from their inception to the most recent date available.
Study selection criteria
The inclusion and exclusion criteria for the systematic review of economic evaluations were identical to those for the systematic review of clinical effectiveness, except that:
-
decision model-based analyses or analyses of patient-level cost and effectiveness data alongside observational studies were included
-
only full cost-effectiveness analyses, cost–utility analyses, cost–benefit analyses and cost–consequence analyses were included (economic evaluations that report only average cost-effectiveness ratios were included only if the incremental ratios could easily be calculated from the published data)
-
stand-alone cost analyses based in the UK NHS were also sought.
Using these inclusion/exclusion criteria, initial study selection was made on the basis of titles and abstracts from the search results by one reviewer (ZL), with unblinded checking by a second reviewer (RA).
Data extraction strategy
Data were extracted by one researcher (ZL) and checked by another (RA) into two summary tables, one to describe elements of the study design of each economic evaluation and the other to describe the main results (see Appendix 6).
For each study the following information was recorded in the study design table: author and year, whether model or trial based, type of model (when relevant), design type (e.g. cost-effectiveness analysis, cost–utility analysis or cost analysis), service setting/country, study population, comparators, research question(s), perspective, time horizon and discounting, main costs included, main outcomes included and sensitivity analyses conducted (see Appendix 7).
In the main results table, incremental costs and benefits as well as the incremental cost-effectiveness ratio (ICER) were recorded for each reported pairwise comparison. Occurrences of either dominance or extended dominance were also noted.
Study quality assessment
The methodological quality of any full UK-based economic evaluations was assessed using the international consensus-developed criteria reported by Evers and colleagues. 181 This formed the basis of a fuller narrative appraisal of these studies. Because of the relatively large number of full economic evaluations discovered we did not conduct a full assessment of the quality of studies from outside the UK.
Results
In total, 24 studies were identified that reported cost-effectiveness or cost–benefit ratios and, of these, 20 were classified as full economic evaluations. Of the four excluded studies, two (Lea and Hailey182 for adults and children, and Evans and colleagues197) were not considered to be full economic evaluations [as they reported only the ratio of cost per quality-adjusted life-year (QALY) without providing further separate information on costs and benefits], and another by Sach and colleagues184 was primarily a willingness to pay analysis. The other study, by Cheng and Niparko,185 was a meta-analysis, which, unusually, pooled utility estimates and cost–utility ratios from seven other studies (conducted in a variety of countries and in different years) to produce overall estimates.
Of the 20 full economic evaluations, three included an assessment of cochlear implantation in both adults and children, six were only in children and 11 were only in adults (see Appendix 7). Eight were analysed primarily from a UK NHS perspective and were usually based on patient-level clinical and resource use data specifically collected from UK cochlear implant centres. All but one of the economic evaluations in children also included educational cost savings in either their main or a subsidiary analysis. A further two studies183,186 were based on data collected by UK-based cochlear implantation programmes but did not clearly state the perspective used in the analysis. Four were analysed from a US perspective and based mainly on cochlear implant programmes conducted in the USA. Another four studies were analysed from a variety of other national perspectives (Australian: n = 2,160,182 Norwegian: n = 1,187 German: n = 1188). In the remainder, neither the perspective from which, nor the context in which, the analysis was conducted was reported.
Because of the wide variation in health system settings and study perspectives in the identified studies from outside the UK, these studies were deemed irrelevant to the current decision problem facing the UK NHS. A detailed appraisal of these studies was therefore not carried out (but they are summarised in table form in Appendix 11). Some review papers and other studies that, although not included in the systematic review, were thought to be relevant are also summarised later in this chapter (see Summary of reviews and other studies).
UK-based full economic evaluation studies
Four of the eight full economic evaluations analysed from a UK perspective involved postlingually deafened adults,53,189–191 one involved children with prelingual deafness190 and the remaining three either failed to report whether the children were deafened pre- or post lingually or contained a mixture of pre- and postlingually profoundly deafened children. 192–194 All of the UK-based full economic evaluations were published between 1995 and 2006. Four of the nine studies are at least a decade old.
No studies were identified in which prelingually deafened adults were analysed from a UK perspective. The clinical and service settings, comparators and basic designs of the eight studies are summarised in Tables 37 and 38.
| Study | Study type | Analysis type | Participants, country/setting | Comparators/comparisons | Perspective |
|---|---|---|---|---|---|
| Summerfield 2002189 | Empirical utility elicitation study and decision model | CUA | Adults undergoing unilateral implantation who did not benefit from hearing aids or benefited marginally, in 14 hospitals in the UK NHS (using HUI-2), and staff and volunteers in one Medical Research Council research unit (for TTO exercise) |
Unilateral implantation vs no implant Unilateral implantation vs hearing aids Simultaneous bilateral implantation vs unilateral implantation Sequential bilateral implantation vs unilateral implantation |
UK NHS |
| Summerfield 1997190 | Decision model | CUA | Postlingually deafened adults receiving a cochlear implant in the UK | Unilateral implant vs no implant | UK NHS |
| Summerfield and Marshall 1995191 | Empirical utility elicitation study and decision model | CUA | Profoundly postlingually deafened adults who received a cochlear implant under the UK National Cochlear Implantation Programme (1990–4) at hospitals in England, Scotland and Northern Ireland | 22-channel implanta vs no implant | UK NHS |
| UK Cochlear Implant Study Group 200453 | Prospective cohort study and decision model | CUA | Profoundly hearing-impaired postlingually deafened adults received multichannel cochlear implants, in 13 hospitals in the UK NHS | Unilateral implant vs no implant | UK NHS |
| Study | Study type | Analysis type | Participants, country/setting | Comparators/comparisons | Perspective |
|---|---|---|---|---|---|
| Summerfield 1997190 | Decision model | CUA | UK, prelingually (?) deafened children at the Nottingham Paediatric Programme with three educational settings: school for deaf children, special unit attached to mainstream school and mainstream school with support | Unilateral cochlear implant vs no cochlear implant | UK NHS + education costs |
| Barton 2006192 | Cross-sectional survey | CUA | Profoundly deaf children (implanted both prelingually and at an older age) in the UK with permanent bilateral hearing impairment | Cochlear implanta vs no cochlear implant | UK NHS, UK societal |
| Hutton 1995193 | Decision model/basic calculation | CUA | UK (pre- or postlingually deaf unspecified) | Cochlear implanta vs no cochlear implant | UK societal (NHS + education + home support) |
| O’Neill 2001,194 O’Neill 2000186 | Basic calculation | CUA | UK, profoundly hearing-impaired children (pre- or postlingually deaf unspecified) at the Nottingham Paediatric Cochlear Implant Programme | Cochlear implanta vs no cochlear implant | UK NHS + education costs |
All of these studies were cost–utility analyses. Five were based on clinical effectiveness results from UK-based cochlear implant programmes. 53,189–191,194 Although the settings in the other three studies190,192,193 were not explicitly reported in the papers it is apparent from related papers that they were also based on NHS treatment settings.
All of the UK-based full economic evaluations used average remaining life expectancy as the time horizon. All of the studies applied discounting to both costs and benefits. None of the included studies was funded by manufacturers of cochlear implants.
UK-based economic evaluations of cochlear implantation in adults
All four studies in adults presented cost–utility analyses and used a decision model to produce cost and utility estimates. All four were based on cochlear implant programmes conducted in the UK NHS (see Table 37). Summary information on the results is shown in Table 39.
| Study | Analysis year | Setting | Source of effectiveness data | Comparator | ICERa |
|---|---|---|---|---|---|
| Summerfield 2002189 | 2000 | UK, 14 hospitals in the UK NHS and one Medical Research Council research unit |
Subset of patients subsequently reported by the UK Cochlear Implant Study Group Volunteers with normal hearing recruited specifically for the study for their knowledge of the impacts of deafness and cochlear implantation |
Unilateral implantation vs no intervention | £16,774 |
| Unilateral implantation vs hearing aids | £27,401 | ||||
| Simultaneous bilateral implantation vs unilateral implantation | £61,734 | ||||
| ‘Additional bilateral implantation’ vs unilateral implantation | £68,916 | ||||
| Summerfield 1997190 | 1996 | UK | Study by Summerfield and Marshall 1995191 | Unilateral implantation vs no cochlear implant | £13,300 |
| Summerfield and Marshall 1995191 | 1991/2 | UK, the Adult Cochlear Implant Programmes at hospitals in England, Scotland and Northern Ireland |
Theoretical mappings of data from the programme onto various health-state classification systems (e.g. HUI and EuroQol) Empirical: visual analogue scales used in the programme |
22-channel implantb vs no treatment | £11,440 |
| UK Cochlear Implant Study Group 200453 | Resources 1998/9, costs 2001/2 | 13 hospitals in the UK NHS | The study cohort | Unilateral implantation vs no cochlear implant | €27,142 (= £17,625)c |
All of the four studies examined costs and effects of cochlear implantation as a treatment and reported the cost–utility of cochlear implants relative to either non-implanted or preimplanted adults. None of the studies, however, reported both costs and effects of the comparator.
The earliest UK-based analysis of multichannel cochlear implantation in adults was that conducted by the MRC Institute for Hearing Research, assessing the cost-effectiveness of the technology as used from 1990 to 1994. 191 Using a decision model, over their remaining lifetimes of 26 years, the base-case cost–utility of cochlear implantation was estimated as £11,440 per QALY (with costs and benefits discounted at 6% per year). Although this was higher than cost-effectiveness estimates from US-based studies, this partly reflects the high discount rate used. However, they also speculated that the cost-effectiveness of the technology would improve over time because of longer duration of implant use (with people implanted sooner after a diagnosis of profound deafness, and people also living longer); expected further increases in utility gain because of improvements in electrode and speech processor technology; and efficiency gains in the organisation and provision of services. This was also the first study to publish comprehensive sensitivity analyses.
The UK-based cost-effectiveness analyses published before 2003 serve to highlight the widespread use of health-related quality of life as the only sensible outcome measure for use in cost-effectiveness analyses of the technology; the lack of large well-designed studies of the quality of life impact (and utility gains) associated with cochlear implantation; the critical importance of study perspective (and particularly the potential inclusion of educational cost savings in assessment of paediatric cochlear implantation); the importance of including both device (‘hardware’) costs and postimplantation tuning, rehabilitation and maintenance costs in determining cost-effectiveness; and the paucity of economic studies evaluating bilateral implantation.
Many of these parameters were not adequately empirically assessed until the series of linked studies by the UK Cochlear Implant Study Group (in adults) and Barton and colleagues (in children) published from 2003 to 2006. 22,53,55,62,138,192,195,196 These used unit cost data from the majority of the UK cochlear implant centres, combined with resource use and outcome data from much larger numbers of implant recipients than any of the earlier studies. A fuller description of the costing methods used in these studies is provided later in this chapter (see Resource use estimation, Costs for cochlear implant model).
The only two currently published economic studies of bilateral cochlear implantation were both based in the UK and assessed the technology in adults from an NHS perspective. 149,189 The more recent (2006) study,149 based on a small RCT, was not classed as a full economic evaluation as the exploratory cost–utility analysis forms just a small part of the discussion. The earlier of the two studies189 elicited health-state values from 70 normal-hearing volunteers who were clinical professionals from cochlear implant centres or academics with experience of profoundly deaf people and cochlear implantation. The other study149 obtained HUI-3 scores alongside an RCT of 24 adults, 12 of whom were randomised to receive a second implant immediately, the other 12 subjects having their second implant after a 12-month wait. The unadjusted between-trial arm results of this trial for the HUI-3 outcome at 9 months after the second implant are only reported in diagram form. They appear to show a modest but non-significant increase in utility [approximately +0.11, 95% CI –0.11 to +0.29, whereas the whole group result (i.e. before versus after difference) is approximately –0.01, 95% CI –0.1 to +0.08]. On the VAS and EQ-5D there are small negative differences in quality of life at 9 months after the second implant (with the whole group and between-trial arm analyses).
However, this is a small trial and because these negative impacts on quality of life were largely explained by a few trial participants who experienced worsening tinnitus after their second implant (and because research on unilateral implantation suggests that there is usually an overall positive impact on the prevalence and intensity of tinnitus) the authors decided to adjust for the impact of tinnitus using a regression model. Using this model (Table 1 in the paper), and therefore assuming an overall neutral impact of tinnitus, gives an estimated utility gain from the second cochlear implant of +0.03 (95% CI –0.045 to +0.104). It is this figure that is used in the discussion section to generate an estimate of cost-effectiveness, and this is also the initial estimate used in our model-based analysis of bilateral implantation.
This HUI-3 measured utility gain from the RCT assumes a neutral impact of change in tinnitus, which was not the case in the raw results of this small study (the gain of 0.03 is based on a regression analysis, i.e. after adjusting for the impacts of tinnitus). Also, as second implant recipients in this trial had been unilateral implant users for between 1 and 6 years, these results may not reflect the actual gains of simultaneous bilateral implantation nor those of second implant recipients who have been unilateral implant users for more than 6 years. The cost-effectiveness estimates of bilateral compared with unilateral cochlear implantation from these two studies are £68,916 and £66,600 per QALY, assuming an overall neutral impact of tinnitus on quality of life. However, the authors are duly cautious about these estimates given the weaknesses in the data on utility gains.
The most recent study was by the UKCISG53 and examined both the expected lifetime costs incurred by the UK NHS of providing and maintaining a cochlear implant and the gains in health benefits (measured using HUI-3) associated with implant use. A total of 311 profoundly deaf adults were classified as belonging to one of four subgroups. These subgroups were chosen to represent a progressive relaxation of implant candidacy criteria relating to severity of deafness. Preimplantation HUI-3 utility values were elicited for each of the groups.
The economic evaluation published in 2002 by Summerfield and colleagues189 reported estimates of incremental costs, benefits and cost–utility ratios for both unilateral implantation compared with either non-technological support or hearing aids and bilateral implantation (simultaneous and sequential) compared with unilateral implantation. Regardless of comparator, health-related quality of life was measured using the HUI-2 tool.
UK-based economic evaluations of cochlear implants in children
The remaining five included studies were in groups of children. Although the primary perspective was that of the NHS all also performed some analyses which included education cost savings. One included the cost of support services at home196 and another study costs to the family. 195
Three of the five studies involved either a mixture of pre- and postlingually deafened children or failed to specify their age of onset of deafness, with the other study reporting that only prelingually deafened children were included. 190
The results of the five paediatric evaluations are shown in Table 40.
| Study | Analysis year | Setting | Effectiveness data source | Comparisona | ICERs (per QALY) |
|---|---|---|---|---|---|
| Barton 2006192 (from an NHS perspective) | 2001/2 | UK | The survey | Unilateral implantation vs no cochlear implantation | |
| Barton 2006192 (from a societal perspective) | 2001/2 | UK | The survey | Unilateral implantation vs no cochlear implantation | |
| O’Neill 2000186 | 1997/8 | UK, the Nottingham Paediatric Cochlear Implant Programme | Study by Summerfield and Marshall 1995197 | Unilateral implantation vs no cochlear implantation | £2532 |
| O’Neill 2001194 | 1997/8 | UK, the Nottingham Paediatric Cochlear Implant Programme | Studies by O’Neill 2000186 and Summerfield and Marshall 1995197 | Unilateral implantation vs no cochlear implantation | Results stratified by education authority:f county: £8310; London: £12,282; Metropolitan: £11,177; unitary: £10,360 |
| Summerfield 1997190 | 1996 | The Nottingham Paediatric Programme with three educational settings: school for deaf children, special unit attached to mainstream school, and main stream school with support | Cost data derived from Summerfield and Marshall 1995191 | Unilateral implantation vs no cochlear implantation |
£15,600g £12,100,g taking into account saved costs in education £10,000,g taking into account cost savings of special equipment for daily living in adulthood |
| Hutton 1995193 | 1994 | UK | Assumption | Unilateral implantation vs no cochlear implantation | £16,214 |
The earliest UK-based study in children was by Hutton and colleagues193 and was explicitly only a preliminary analysis, primarily of health system costs and potential cost savings when education costs are included. Their cost-effectiveness estimates were only tentative (e.g. using the speculative assumption that cochlear implantation would increase the utility of deaf people from 0.6 to 0.7, and assuming zero costs for the no implantation alternative). Two later studies of paediatric cochlear implantation, by O’Neill and colleagues,186,194 were both based on the Nottingham Paediatric Cochlear Implant Programme; they included the cost savings associated with different postimplantation schooling and educational support needs (the second paper mainly highlighted how regional variations in these costs can critically alter the cost-effectiveness of the technology). With the inclusion of educational savings, and relying on a mean utility gain of 0.23 (extrapolated from adult studies), they estimated that unilateral paediatric cochlear implantation achieved an ICER of £2532 per QALY gained (in 1998 UK pounds). Another early study190 of unilateral cochlear implantation similarly used cost data from the Nottingham Programme and also relied on the assumed average utility gain of 0.23 (from adults); their projected lifetime cost-effectiveness estimates were £15,600 per QALY without educational cost savings, and from £10,000 to £12,100 per QALY when educational cost savings and/or special equipment for daily living were included (all in 1996 UK pounds).
The most recent study, published in 2006 by Barton and colleagues,192 used regression analysis of a large sample of individual patient data – including 403 implant recipients – to examine the gain in health utility associated with the implant. They used a version of the HUI-3 instrument with slightly adapted and simplified wording for the UK context. Utility was modelled as a linear function of preoperative average hearing level, age at implantation, the time period over which gains are accumulated and level of deafness (profound or severe). They combined these estimates of utility gain with comprehensive NHS costs (from Barton and colleagues55) and other cost estimates to produce a range of incremental cost–utility estimates for children at two different ages (3 and 6 years old), for three different levels of preimplantation hearing loss and according to three analytical perspectives (NHS, NHS plus education sector, and ‘societal perspective’).
Finally, two other minor sources of data on paediatric cochlear implantation were identified. One was a brief study by Summerfield and colleagues190 in which paediatric cochlear implantation was discussed alongside a main study on implantation in adults, and the other was a section in the study by Summerfield and Marshall191 in which the costs of paediatric implantation were discussed.
Summary of reviews and other studies
One systematic review of economic evaluations198 and one meta-analysis of a number of cost–utility and cost–benefit analyses185 were also included. Quite unusually, this latter study involved the pooling of cost–utility ratios across a number of studies.
There was also a fairly recent and very comprehensive costing study by Barton and colleagues55 that reported an audit and survey of resource use in all 12 UK cochlear implant centres in 1998/9. This study provided the per patient costs for their economic evaluation and also for our cost–utility analysis later in this assessment report. Another related study195 showed that much of the variation in costs between implant centres could be explained by differences in the volume of implantation activity in that centre. Finally, a study by Sach and colleagues,199 using time-adjusted individual patient outcome and cost data from the Nottingham Paediatric Cochlear Implant Programme from 1989 to 1996, showed that the per patient cost of the programme was reducing over time during this period. This was thought to be partly explained by learning effects and economies of scale.
Assessment of industry submissions to NICE
There were three industry submissions made to NICE. These are critiqued in Appendix 8.
PenTAG cost–utility analysis
Decision problem
To reflect both current policy and clinical practice and possible changes to UK NHS practice we aimed to assess, based on available data, the following two policy questions:
-
For profoundly sensorineurally deaf people (who may be either using or not using acoustic hearing aids), is it cost-effective to implant a first (i.e. unilateral) cochlear implant?
-
For profoundly sensorineurally deaf people (who may be either using or not using acoustic hearing aids), is it cost-effective to simultaneously implant two cochlear implants or to implant two cochlear implants sequentially in relatively close succession?
Note that the population in this second policy question – i.e. deaf people currently not using a cochlear implant – differs from that set out in the original decision problem (see Chapter 2, Decision problem) and project protocol. This is primarily because of a lack of utility data that would inform an assessment of the cost-effectiveness of providing a second cochlear implant to someone who had been a cochlear implant user for some years. The second question (see Chapter 1, Description of the problem) was therefore reframed after examination of the available data.
Throughout this report ‘simultaneous bilateral implantation’ refers to two devices being fitted during the same operation and ‘sequential bilateral implantation’ refers to two devices being fitted in two operations, with these operations being 3 years apart.
The focus of these model-based analyses is therefore population-level policy decisions rather than clinical decisions as such, and it is important that the complete range of relevant policy comparators is included. In Table 41 we show the main policy comparators included in our analysis, together with the relevant populations.
| Population (starting cohort in model) | Treatment strategies (policies) to be compared | Assumptions about possible pathways following treatment strategy |
|---|---|---|
| People with profound deafness and who have no cochlear implants and use acoustic hearing aid(s) as necessary | Continue life without a cochlear implant | Continue using acoustic hearing aids as required for most of remaining life (except when age-related worsening of hearing causes their gradual non-use) |
| Add a cochlear implant in one ear; (other ear remains as before or with bilateral hearing aid if it improves hearing in combination with the implant) | No possibility of upgrade to bilateral cochlear implant | |
| Add two cochlear implants in close succession (e.g. within 1 year) for those who might benefit (sequential bilateral implantation), or one in those for whom two implants is not indicated |
No possibility of upgrade to bilateral cochlear implant (for those previously judged clinically ineligible) Possible failure and explantation of second implant |
|
| Add two cochlear implants during the same operation (simultaneous bilateral implantation) |
No possibility of upgrade to bilateral cochlear implant (for those previously judged clinically ineligible) Possible failure and explantation of second implant |
|
| People with profound deafness and who have one cochlear implant (a hearing aid in the other ear if required) |
Continue life with one cochlear implant and a hearing aid in the other ear if necessary Add a cochlear implant in second ear |
No possibility of upgrade to bilateral cochlear implant in the future Possible failure and explantation of second implant |
Methods overview
We developed a state-transition (Markov) model to represent the main care pathways that deaf people might follow (with or without cochlear implantation)200 and, for those using a cochlear implant, the main complications and device failures associated with significant health or cost impacts. The main care pathways concern whether people have surgery for cochlear implantation (or not, if assessed as unlikely to benefit) and also whether the implant has to be permanently removed at any point or is not used voluntarily.
The model does not attempt to simulate the possible progression of deafness or associated impacts, nor is it stratified according to the severity of deafness. This lack of an underlying model of the natural history of deafness is justified because, although there are degrees of deafness, it is not necessarily a progressive condition. Also, although some cost-effectiveness studies have estimated QALY gains and cost–utility for people of different deafness severity, as these groups would be mutually exclusive, their cost-effectiveness can be evaluated using an unstratified model. The populations that we mainly investigate are profoundly deaf and have mostly therefore already reached the extreme end of the scale.
The cohorts that start in the model are characterised by their level of audiologically measured deafness, age when deafened (pre- or postlingual) and age at referral for implantation. All cohorts are modelled until death regardless of these factors.
Costs included in the analysis are those associated with assessment for implantation, device hardware, the surgical procedure and hospital stay, tuning and rehabilitation, regular maintenance and monitoring, and dealing with device failures and complications. When necessary, annual costs were converted to 6-month (per cycle) costs by dividing by two. When relevant, the cost of digital acoustic hearing aids is factored into the costs for people without acoustic hearing aids and who use cochlear implants in conjunction with an (‘contralateral’) acoustic hearing aid. Although the primary outcome of the analysis is QALYs, the model also calculates intermediate outcomes such as lifetime complications or device failure rates. Costs and benefits are both discounted using an annual rate of 3.5%. 201
The model was developed in Microsoft Excel® (Microsoft Corporation, Redmond, WA) with structure informed by expert clinical opinion on the management of people using either cochlear implants or conventional acoustic hearing aids. The costs and benefits associated with conventional best practice (non-auditory support in combination with an acoustic hearing aid if deemed necessary) were also estimated using a version of the same model. The costs and benefits of giving users already familiar with the technology an additional device were also estimated using a variation on the original model.
Model structure
The model has a two-level hierarchical structure with the higher level (as depicted in Figure 5) primarily reflecting the pathways by which people come to have either one, two or no cochlear implants. The lower level contains the various clinical and device-related events that might occur for those people in the model who are cochlear implant users, such as internal device (electrode) failures, external device (coil and speech processor) failures and major postsurgical complications (primarily wound infections and revisions).
FIGURE 5.
Main care pathways in the model for (a) unilateral implantation and (b) bilateral implantation.
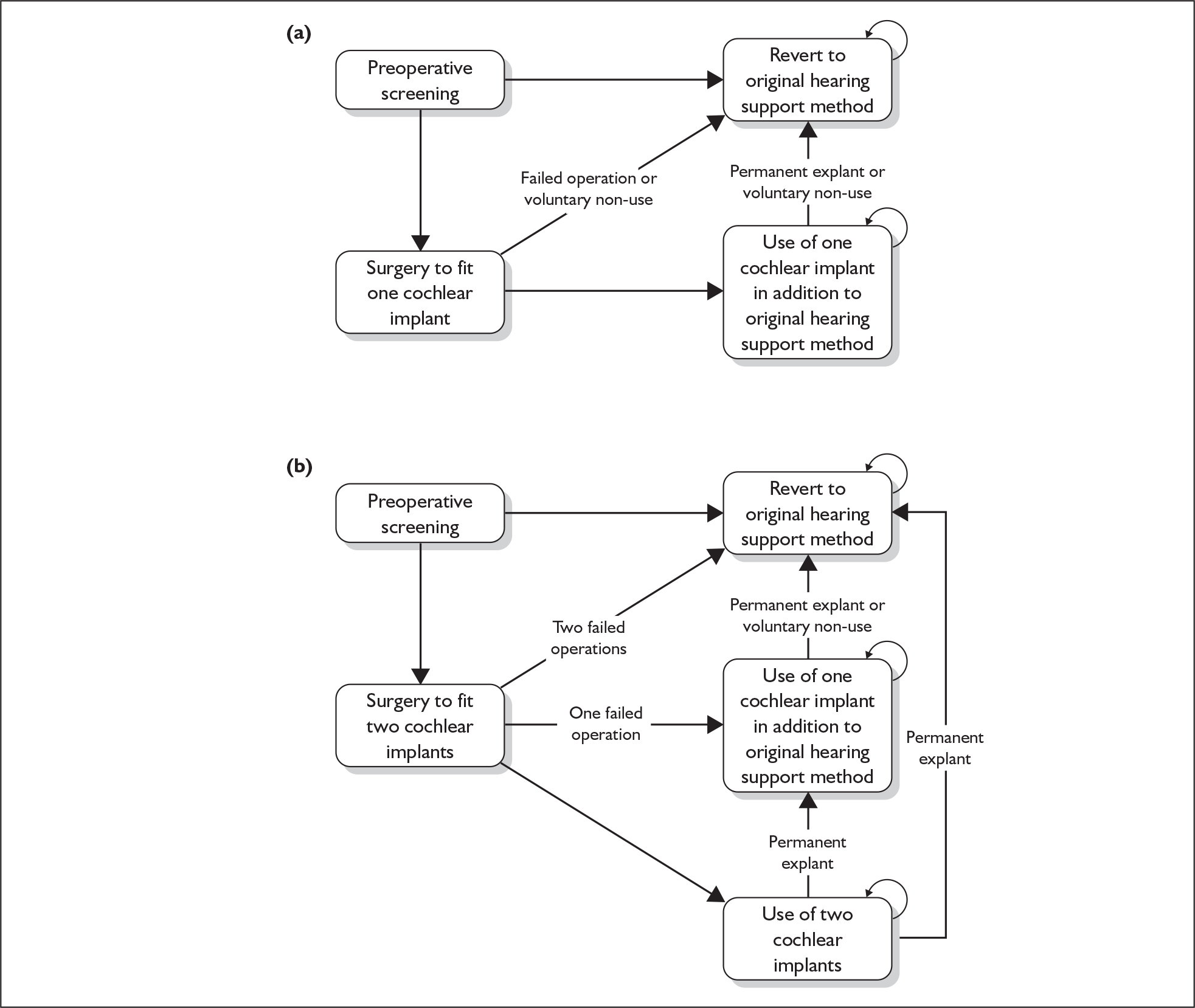
Figure 6 shows the main Markov states for users of cochlear implants and Table 42 shows all of the Markov states used in the PenTAG model.
FIGURE 6.
States and allowable state transitions corresponding to use of a single cochlear implant (CI). Note: In addition to the transitions shown, all those with a cochlear implant may become implant non-users voluntarily (modelled as a one-off risk after several years’ use).
| Markov state(s) | Description |
|---|---|
| No cochlear implant | Individuals not using any cochlear implants (i.e. using acoustic hearing aids, lip-reading, sign language, etc.) |
| Preoperative assessment | Implant candidate undergoes a period of preoperative screening to assess whether they are (1) suitable for and (2) ready for implantation |
| Implantation surgery (unilateral) | Individuals undergo procedure to have a cochlear implant fitted |
| Implantation surgery to have first of two scheduled implants | Individuals scheduled to receive two cochlear implants undergo procedure to have the initial device implanted |
| Surgery to have second of two scheduled implants | Individuals scheduled to receive two cochlear implants undergo procedure to have second device implanted. Initial operation successful |
| Surgery to have second of two scheduled implants (operation for first ear failed) | Individuals initially scheduled to receive two cochlear implants undergo procedure to have second device implanted. Initial operation unsuccessful |
| Surgery to have two implants implanted simultaneously | Individuals have two cochlear implants fitted during a single procedure |
| Failed initial operation (if scheduled for two) | Failed initial operation in individuals scheduled to receive two cochlear implants |
| Cochlear implant working | All fitted cochlear implant(s) and acoustic hearing aids working. No adverse events (other than minor wound problems, which are successfully treated with antibiotics) |
| Major complication | Individuals experience a major complication as a result of having a cochlear implant, which requires some form of reoperation. Such reoperations may include wound revision, reimplantation following wound-related problems or repositioning of electrode |
| Internal failure | Individuals experience a problem with the internal component of a cochlear implant device requiring some form of reoperation |
| External failure | Individuals experience a problem with the external component of a cochlear implant, which needs to be replaced |
| Death | Death |
A two-state Markov model (individuals are either alive or not) is used to simulate living without a cochlear implant, with the cost of the only key event for this group – replacement of acoustic hearing aid – being estimated.
Relevant population(s)
The population used in all base-case analyses is people who are profoundly deaf. Adults (18 years old) and children (< 18 years old) are modelled separately. Children are assumed to have been implanted before the onset of speech development (i.e. prelingually). Profoundly deaf children who were implanted at a later stage of childhood do not form part of the base-case analyses and are assessed separately using a scenario analysis. The gender and clinical characteristics of each of the cohorts reflect those of the general population.
As discussed in Chapter 1 (see Criteria for candidacy for cochlear implantation), candidacy is measured in clinical settings using functional rather than purely audiological measures. However, the model population is based on profound deafness. This is because, with the exception of the study by Summerfield and colleagues,62 the studies used as the sources for model utilities recruited participants based on their audiological rather than functional hearing ability. We acknowledge that this does not mean that the profoundly deaf are a homogeneous group, but we are constrained by the available data from using functional ability, which would more accurately reflect clinical practice.
Age at implantation
A mean implant age of 50 years was used for all postlingually deafened adult cohorts. Prelingually deafened children are assumed to be implanted at 1 year of age. A non-reference case analysis of children implanted at age 8 is also conducted (although separate utility gain estimates for postlingually deafened children are not available).
Simulation
For each comparator, single birth cohorts of either adults or children were modelled independently and results used to produce a deterministic ICER (i.e. using best point estimates for each input parameter). A cycle length of 6 months was used to suitably capture the complexity of the process and to maintain flexibility in the model. The impact of running the model using different time horizons was assessed in sensitivity analysis.
Policy comparisons
The primary research questions investigated in this report are listed in Table 43.
| Description of question | Patient groups in reference case analyses |
|---|---|
| Compared with no cochlear implantation, is unilateral cochlear implantation cost-effective in people currently using only conventional best practice (non-acoustic support in combination with an acoustic hearing aid as required)? | Prelingually deafened children and postlingually deafened adults |
| Compared with unilateral cochlear implantation, is simultaneous bilateral cochlear implantation cost-effective in people currently using only conventional best practice? | Prelingually deafened children and postlingually deafened adults |
| Compared with unilateral cochlear implantation, is sequential bilateral cochlear implantation cost-effective in people currently using only conventional best practice? | Prelingually deafened children and postlingually deafened adults |
Even though, strictly, ‘no cochlear implantation’ should be compared with three main policy comparators – unilateral cochlear implantation and both types of bilateral implantation (simultaneous and sequential) – we have chosen to break down the decision problem into simpler pairwise comparisons. This is for two main reasons. First, in terms of both costs and effectiveness, unilateral cochlear implantation is inherently intermediate between having no cochlear implant and having two. Second, the current dominant de facto clinical practice in the NHS for the patient groups in our reference case analyses is unilateral implantation. It therefore makes sense to examine the cost and QALY implications of changing policy from this current standard clinical practice.
In addition, we have chosen not to present a head-to-head formal comparison of simultaneous versus sequential bilateral implantation. This is primarily because the difference in QALY gains between these two strategies is entirely due to the difference in age at implantation (and hence life expectancy) when the second implant is put in, rather than to any known difference in the effectiveness between the two strategies of bilateral cochlear implantation. To be consistent with NICE’s principles for the use of social value judgements about age in the development of NICE guidance (Principle 6202) – and in the absence of reliable clinical evidence that outcomes such as speech perception and quality of life are different between children implanted by each method – then any modelled difference in QALY gain between sequential and simultaneous bilateral implantation should be ignored as purely resulting from the difference in age (and life expectancy) at implantation.
Furthermore, although there is a difference in the surgical procedure costs of simultaneous versus sequential bilateral implantation, these are small compared with the initial device hardware and other maintenance costs involved.
Transition probabilities
Transitions between individual states used in the Markov model are driven by a sequence of probabilities. In the PenTAG model there are occasions when there are multiple pathways to leave a particular health state and arrive in another. All of these possible pathways must be incorporated into the transition probability used to capture such a move. This is achieved using probability trees. 200 A selection of the probability trees used to generate the PenTAG model are shown in Appendix 9.
Replacement of acoustic hearing aids
Although the approximate proportion of hearing aid users in the overall underlying population of profoundly deaf individuals is known, information on subgroups of profoundly deaf individuals who either do or do not gain benefit from acoustic hearing aids was not identified. The PenTAG model, therefore, does not subdivide non-cochlear implant users on the basis of acoustic hearing aid use.
In relation to the chosen cycle length (6 months), the ease and low cost of replacing an acoustic hearing aid means that the period of time for which an individual is without any acoustic support is minimal following the failure of an acoustic hearing aid. The impact on health states in terms of a reduction in health-related quality of life is therefore also minimal. Consequently a separate health state is not needed to represent acoustic hearing aid replacement and so it can be modelled purely as a cost. During each cycle the number of individuals incurring this cost is based on the underlying proportion of each cohort who are hearing aid users and the probability of device failure.
Model assumptions
A number of assumptions underpin the base case of the model; these are simplifications of real life so that the model is not overly complex but contains sufficient detail to capture key events in the decision process. In the absence of citable sources of research evidence they are based mainly on expert input from our expert advisory group (Table 44).
| Assumption | Comments |
|---|---|
| All reimplants occur in the same ear from which the device was temporarily explanted | Loss of all residual hearing is not automatic post implantation |
| Any complication that occurs during any particular cycle is assumed to affect only one ear. Equally, only one ear can experience a major complication during any particular cycle | Adverse events are rare and therefore the chances of problems in both ears within one cycle are minimal |
| There is no significant difference in aggregate lifetimes of the internal components of the cochlear implants between manufacturers | Long-term safety data not collected for more recent devices. When aggregated over all devices offered no one company appears to have a significantly better range than any other |
| Use of either a cochlear implant or an acoustic hearing aid does not alter life expectancy | Initial implant operation is safe, risk of meningitis is not significantly different to that in the general population and device-related side effects do not significantly impact on mortality |
| All initial operations to fit a cochlear implant are successful | Rates of abandonment discussed in Chapter 5 (see Non-use of devices) are very low |
| Death from any state involving surgery is the same as for states not involving surgery | Death rate attributable to general anaesthesia extremely low |
| Meningitis not included in the patient pathway | Risk of meningitis in the general population extremely low. Changes to general preoperative practice mean rates in cochlear implant users also very lowa |
| Individuals who gain benefit from acoustic hearing aids receive them | As is the case in general clinical practice |
| Failure rates for cochlear implants (both external and internal) do not vary significantly between manufacturers | Cochlear implants as a health technology is being modelled. No distinction between products made |
| Of those people who are lifelong acoustic hearing aid users, in the absence of cochlear implants, 50% will use two acoustic aids and the remaining 50% one acoustic aid | Broadly reflects clinical practice |
| Individuals that enter the preoperative screening stage but do not go on to receive an implant only incur 25% of the costs of those who do go on to receive an implant | Broadly reflects clinical practice |
| Major complications occur sooner rather than later. Rate for the first year is ten times higher than the rate used in the rest of the model | Broadly reflects clinical practice |
Time horizon
The model uses a lifetime time horizon; cohorts are followed until death (defined as less than one person alive). The effects of imposing fixed time horizons on the base case ICER are explored in sensitivity analyses.
Discount rates (costs and benefits)
In accordance with Treasury advice, costs and benefits were discounted at an annual rate of 3.5%. 201
Model parameters
Cohort characteristics
Gender distribution
Hospital Event Statistics (HES) have reported cochlear implantation as a distinct category [Healthcare Resource Group (HRG) C60 ‘Cochlear implants’] since 2003/4.
Table 45 shows the number of finished consultant episodes for males and females during the period 2003–6 as well as the average over this period. The data corresponds to HRG v3.5 category C60 and represents the totals for all English strategic health authorities.
| Age category (years) | 2003/4 | 2004/5 | 2005/6 | Average |
|---|---|---|---|---|
| Males | ||||
| 0–14 | 120 | 143 | 141 | 134.67 |
| 15–59 | 60 | 62 | 42 | 54.67 |
| 60–74 | 28 | 33 | 28 | 26.67 |
| 75+ | 11 | 2 | 6 | 6.33 |
| All ages | 219 | 240 | 217 | 225.33 |
| Ages 15+ | 99 | 97 | 76 | 90.66 |
| Females | ||||
| 0–14 | 120 | 111 | 133 | 121.33 |
| 15–59 | 86 | 91 | 102 | 93.00 |
| 60–74 | 27 | 20 | 33 | 26.67 |
| 75+ | 6 | 8 | 14 | 9.33 |
| All ages | 239 | 230 | 282 | 250.33 |
| Ages 15+ | 119 | 119 | 149 | 129 |
Using the 0–14 years category for children, on the basis of the 3-year averages, the male–female ratio is approximately 52:48. A similar calculation can be performed using the age 15+ category as a proxy for adults. The resulting male–female ratio is approximately 41:59.
Starting ages
Children
ICERs for two distinct profoundly deaf paediatric subgroups are produced: a base case for those who enter the model at age 1 year; and an older group of children, mean age 8 years (90% of whom are prelingually deafened21,192), whose results are explored using scenario analysis. These ages were chosen to reflect the earliest age currently implanted by the NHS and the mean age of a subgroup of older children implanted after the age of 4 years in the study by Barton and colleagues192 and further investigated by Stacey and colleagues. 21
Adults
In a UK-based cost-effectiveness analysis the UKCISG53 analysed data from 311 individuals implanted between 1997 and 2000. The mean implant age was 50.8 years (range 16–82 years). This value is similar to that in a study by Summerfield and colleagues165 of all UK implantees who received their devices before 2002. The median age of implantation amongst the 1779 adults was 51.5 years (interquartile range 39.4–63.6 years). HES also reports the mean age corresponding to each category in Table 45. Taking the 3-year average, and using the gender proportions calculated above as weights, the average age for a finished consultant episode for adults (aged 15 or over) is 49.7 years.
On the basis of this we have used a starting age of 50 years for all adult cohorts. The effect on all pairwise comparisons of changing this starting age is explored in sensitivity analyses.
Severity of baseline hearing impairment
In all base-case analyses individuals are assumed to be profoundly deaf (average hearing level in better ear of > 95 dB). The cost-effectiveness of cochlear implantation in severely deaf individuals is not presented as there are no published utility gain values for this subgroup of cochlear implant recipients, and no studies that would allow them to be reliably estimated.
Benefit from acoustic hearing aids
It was not possible to generate separate parameter values for implant users who would otherwise have been users of either acoustic hearing aids or non-acoustic support because of the lack of research reporting whether hearing aids were used.
We have therefore chosen to match the underlying baseline populations used in each of the cohorts to the real-world situation faced by audiologists. Therefore, we have assumed that, in the absence of cochlear implants, 50% of profoundly deaf individuals will gain benefit from hearing aids (2007, personal communication with expert advisory group).
Event probabilities
Unsuccessful candidacy
No published information on the current UK situation was found; however, clinical opinion suggests that around 20% of paediatric and 30% of adult referrals do not go on to receive an implant (Professor Quentin Summerfield, University of York, 2007, personal communication). These values have been used in all model cohorts.
Background mortality
The most up-to-date life tables (2003–5) produced by the UK government actuarial department203 were consulted for gender-specific annual values for inhabitants of England and Wales. Equivalent cycle values were generated and combined using the proportions above to derive the required parameter values.
Rare adverse events
By definition mathematical models are simplifications of reality and, as such, decisions about what not to incorporate into the model have to be made. In representing the patient pathway experienced by cochlear implant users the following events were deemed ‘rare’ and were therefore not included in the base-case analysis: abandoned initial implant procedures, surgical death and the likelihood of contracting meningitis.
For people scheduled to have sequential bilateral implantation, a proportion of candidates may forego the second operation and remain as users of either one cochlear implant or, if the first operation failed, no implants. This event has also been classified as rare and not included in the base-case analysis.
The effect of introducing these variables is explored in sensitivity analyses.
Number of acoustic hearing aids used
All potential implantees (adults or children) are normally provided with two acoustic hearing aids before implantation as part of the assessment process. We have assumed that, in the absence of cochlear implants, of individuals who showed signs of benefiting from these devices, 50% will remain using two acoustic hearing aids and the remaining 50% will use only one acoustic hearing aid.
Furthermore, the assumption has been made that 70% of adults and 80% of children who undergo unilateral implantation use a contralateral acoustic hearing aid (2007, personal communication with members of the expert advisory group]. Clearly, no one who receives bilateral implants continues to use an acoustic hearing aid.
Expected lifetime of an acoustic hearing aid
In an assessment of best practice standards for adult audiology published in 2002204 the Royal National Institute for the Deaf (RNID) stated that the ‘full patient journey’ (assessment, fitting and follow-up) should reoccur every 3 years with an upgrade in technology. Currently, independent sector contracts use a patient journey of 5 years (Jonathan Parsons, Consultant Clinical Scientist, Clinical Director of Audiology, East, Mid Devon and Exeter Area, 2007, personal communication). The assumption has been made that these contracts rather than the guidelines produced by the RNID reflect current practice within the NHS. Therefore, the lifetime of a conventional acoustic hearing aid has been assumed to be 5 years. All individuals in the model who are still alive and users of acoustic hearing aids are given new devices every 5 years.
Failure and replacement of cochlear implant external components
In the absence of any published data, information from the Advanced Bionics submission was used to generate a parameter estimate. This submission reported that around 12% of Auria processors required replacing over a 1-year period. This value was also used in their economic evaluation. Assuming a constant hazard, the expected lifetime of the external components is approximately 7.8 years. The values used in the model are summarised in Table 46.
| Parameter | Submodel | Annual value | Source |
|---|---|---|---|
| Annual probability of failure and replacement of external component of a cochlear implant | All adult submodels; all child submodels | 0.12 | Advanced Bionics submission (based on company data for failure rates of external and internal components) |
Failure and replacement of cochlear implant internal components
Analysis of internal device reliability is usually presented in the form of cumulative survival graphs. 176,180,205 Such graphs show the proportions of devices fitted that survive to a particular time point.
Conboy and Gibbin179 present results relating to the reliability of 377 paediatric implantations carried out as part of a UK programme between 1989 and 2002. Data on the number of implants for each year as well as cumulative device survival are reported. Overall, 94.3% of devices survived to 7 years and 90.0% to 13 years.
The Cochlear Europe submission presented information about the number of devices given to children and the cumulative survival for several different devices. The information was correct as of 30 June 2006 and each graph in the submission represents a different type of Nucleus® device based on the receiver/stimulator.
Information was pooled from both of these sources to generate a survival curve that is representative of all cochlear implant devices currently in use. The resulting curve is shown in Figure 7. The time-dependent probability for internal device failure was calculated from the cumulative survival values using standard formulae. 206 The construction of this curve is explained in detail in Appendix 10.
FIGURE 7.
Cumulative survival for internal components in cochlear implants worn by children.
The same process can be undertaken to generate a combined survival curve for internal failure for cochlear implants worn by adults. Figure 8 shows the resulting survival curve.
FIGURE 8.
Cumulative survival for internal components in cochlear implants worn by adults.
Major complications
In the PenTAG model a major complication refers to any adverse event that is not related to device failure and which results in some form of reoperation. As discussed in Chapter 2 only four studies were identified that contained enough information to derive estimates of the likelihood of an event during a model cycle. The relevant information is summarised in Table 47.
| Study | n | Cumulative follow-up (patient-years) | No. events |
|---|---|---|---|
| MED-EL (for FDA) 2001125 | 106 adults | 59.4 | 1 |
| 82 children | 44.4 | 3 | |
| Proops 1999161 | 116 implants in adults | 117a | 1 |
| Dutt 2005162 | 122 implants in adults | 32.5a | 1 |
| UKCISG 200453 | 311 adults | 233a | 14b |
On the basis of information presented in the study by the UKCISG (Figure 3, p. 317) the consequences of our definition mean that the vast majority of major complications are wound related. These require wound revision, electrode replacement and, more rarely, device explantation followed by implantation of the other ear. On the basis of this we have assumed that it was inappropriate to use the same event probability during each cycle and instead have used one probability for major complications during the first year post surgery and another for all years thereafter.
Of the studies in Table 47 two53,125 have an average per individual follow-up period of less than 1 year and the remainder report the number of events that occur within the first year. Therefore, the weighted average of the complication rates for these studies can be used to derive a cycle probability for the first year post implantation. Although Dutt and colleagues162 and Proops and colleagues161 both follow groups of patients for longer than 1 year, not enough information is presented to calculate the probability of major complications after the first year. Therefore, we have simply assumed that the long-term probability is a tenth of the year 1 probability. The values used in the model are summarised in Table 48.
| Parameter | Submodel | Annual probability | Source |
|---|---|---|---|
| Probability of a major complication when using unilateral implants | All adult submodels; all child submodels | Year 1: 0.041 | Derived from pooled average of rates for adults and children reported in FDA report for COMBO 40 + system,124 Dutt 2005164 and Proops 1999163 |
| Year 2 +: 0.004 | Assumed to be 1/10 of the value used in year 1 | ||
| Probability of a major complication when bilateral implants are implanted simultaneously | All adult submodels; all child submodels | Year 1: 0.082 | Assumed to be twice the value for one device |
| Year 2 +: 0.008 | |||
| Probability of a major complication when bilateral implants are implanted sequentially | All adult submodels; all child submodels | Year 1: 0.041 | Combination of values for unilateral and bilateral implant use applied to this particular cohort |
| Year 2–3: 0.004 | |||
| Year 4: 0.045 | |||
| Year 5 +: 0.008 |
Permanent elective non-use
To incorporate information on elective non-use (reported in Chapter 2, Non-use of devices) we have assumed a trial period during which individuals use their devices before deciding whether to continue or not. We have therefore made the assumption that all non-use occurs after 2 years (i.e. at the start of the third year). Thereafter, all remaining users are assumed to use their implants fully (Table 49).
| Parameter | Submodel | Cycle | Value | Source |
|---|---|---|---|---|
| Probability of voluntary non-use of a functioning cochlear implant | All adult submodels; all child submodels | 1–4 | 0.00 | Modeller assumption |
| All adult submodels; all child submodels | 5 | 0.0236 | Weighted average of values presented in Ray 2006171 for adults and children | |
| All adult submodels; all child submodels | 6 + | 0.00 | Modeller assumption |
Device explantation without reimplantation
Because of the small number of studies that report these results for adults and children, the small numbers of events and the wide range of values, the assumption that the probability of permanent removal is the same for adults and children has been made. The value derived from data presented in Table 35 has therefore been applied throughout the model.
Sequential bilateral implantation
Conditional on successful assessment, individuals in the cohort used to model sequential bilateral implantation have two operations scheduled. In a UK-based multicentre study by Verschuur and colleagues151 of 20 individuals who underwent sequential implantation, the mean delay between the two operations was 35 months. The interval used in the model is therefore 3 years.
A summary of the PenTAG model parameters, values and sources is presented in Table 50.
| Parameter (short description) | Base-case value | Source | Justification |
|---|---|---|---|
| Time horizon | Lifetime | NICE requirement | All cohorts modelled until death regardless of starting age |
| Annual discount rate | 3.5% | UK Treasury recommendations201 | Value applied to costs and utilities |
| Starting age (adults) | 50 years | Hospital Event Statistics (HES) database (2003–6) | 3-year weighted average for Healthcare Resource Group (HRG) C60 finished consultant episodes |
| Starting age (children) | Prelingually deafened: 1 year; older profoundly deaf: 8 years | Barton 2006;192 Stacey 200621 | Prelingual value chosen to reflect current clinical practice |
| Gender distribution (children) | Male: 52%; female: 48% | HES database (2003–6) | 3-year weighted average for HRG C60 finished consultant episodes |
| Gender distribution (adults) | Male: 41%; female: 59% | HES database (2003–6) | 3-year weighted average for HRG C60 finished consultant episodes |
| Proportion of candidates for cochlear implantation who gain benefit from acoustic hearing aids | 50% | None | Personal communication (2007)with expert advisory group; assumed to be the same for adults and children |
| Proportion of unilateral cochlear implant users using contralateral acoustic hearing aids | Adult: 70%; children: 80% | None | Personal communication (2007) with expert advisory group |
| General mortality | Age dependant | UK government actuarial department life tables203 | Age-specific values for men and women pooled using relevant gender distribution |
| Proportion of initial referrals not undergoing an operation to fit a cochlear implant | Adults: 30%; children: 20% | None | Personal communication (Professor Quentin Summerfield, 2007) |
| Probability of surgical death | 0 | NA | Classified as a rare event and therefore not included in base-case analysis |
| Proportion of initial procedures abandoned during implant operation | 0% | NA | Classified as a rare event and therefore not included in base-case analysis |
| Mean lifetime of an acoustic hearing aid | 5 years | None | Based on personal communication (Jonathon Parsons). Value used for length of patient journey in independent sector contracts. Assumed same for NHS |
| 6-month probability of cochlear implant external component failure (unilateral) | 0.062 (adults and children) | Value for annual replacement taken from industry submission | Assumed same for adults and children |
| 6-month probability of cochlear implant external component failure (bilateral) | 0.124 (adults and children) | None | Assumed to be twice the value derived for unilateral use |
| 6-month probability of cochlear implant internal component failure (unilateral) | Time dependant (different values for adults and children) | Kaplan–Meier curves reported in industry submission for a variety of devices | Reported curves approximated using functions. Weighed average of functions used to generate failure probability |
| 6-month probability of cochlear implant internal component failure (bilateral) | Time dependant (different values for adults and children) | None | Assumed to be twice the value derived for unilateral use |
| 6-month probability of major complication (unilateral) | Year 1: 0.02 (adults and children) | Combo 40 + FDA submission 2001;125 Proops 1999;161 Dutt 2005162 | Weighted average of adult and children values applied to all models |
| Year 2 +: 0.002 (adults and children) | None | Assumed to be 1/10 of the year 1 value | |
| 6-month probability of major complication (simultaneous bilateral) | Year 1: 0.041 (adults and children); year 2 +: 0.0041 (adults and children) | None | Assumed to be twice the value derived for unilateral use |
| 6-month probability of major complication (sequential bilateral) | Year 1: 0.041; years 2–3: 0.004; year 4: 0.045; year 5 +: 0.008 | None | Combination of values for unilateral and bilateral implant use applied to this particular cohort |
| Additional risk of meningitis in cochlear implant users compared with the general population | 0% (adults and children) | NA | Risk in cochlear implant users assumed to be not significantly greater than the risk in the general population |
| Additional risk of death from meningitis compared with background death probability in cochlear implant users | 0% (adults and children) | NA | Probability of death from meningitis assumed to be the same as the age-specific background death value for cochlear implant users |
| Probability of voluntary (permanent) non-use of implants | 2.36% of cochlear implant users stopping at the end of 2 years; full compliance before and after assumed | Weighted average of values presented in Ray 2006172 for adults and children | Insufficient evidence to justify a more complex pattern of non-use |
| Probability of non-reimplantation of cochlear implant internal component during any surgical procedure | 0.115 (adults and children) | Dutt 2005;162 Ray 2004;158 Bhatia 2004;159 Balkany 1999;173 Stratigouleas 2006;174 Lassig 2005175 | Pooled value applied to all models |
| Interoperative period between sequential bilateral operations | 3 years (adults and children) | None | Modeller assumption + expert advisory group |
| Proportion of bilateral candidates choosing not to have second operation | 0% | None | Best case scenario assumed (100% uptake) |
Resource use estimation
Costs for cochlear implant model
The costs for the model can be broadly divided into those applicable to Markov states in the model (or stages in the clinical pathway) or to events that occur within states or when moving between states.
We have made particular use of the two recent and large UK-based studies that have evaluated the resource use and costs associated with paediatric and adult cochlear implantation: Barton and colleagues55 for paediatric costs and UKCISG53 for adult costs.
In the UKCISG study53 in adults, costs were assigned to 316 severely to profoundly hearing-impaired postlingually deafened adults who had received multichannel cochlear implants in 13 hospitals in the NHS between June 1997 and May 2000. The costing method is described more fully in the paper but, briefly, in relation to assessment and rehabilitation, costs included ‘costs incurred in providing acoustic hearing aids when assessing the suitability of a subject for cochlear implantation’. These resources were ‘identified and valued in consultation with audiologists’. The analysis also included the ‘core cost of providing an implant’. This involved identifying, measuring and valuing the resources used in each of five NHS hospitals (using data on salaries of staff; salary overheads; accommodation of cochlear implantation programme; incidental running costs of the cochlear implantation programme; costs of capital equipment, radiology and surgery; cost of a 72-hour inpatient stay; cost of implant hardware). First, the costs due to salaries, salary overheads, accommodation, running costs and capital equipment were based on retrospective records of the five NHS hospital programmes since their inception up until March 1999. Second, for each of these programmes a profile of patient care was identified, that is, the pattern of appointments with different clinical professionals and the duration of those appointments during different phases of assessment and postimplantation care. Third, information from steps one and two were combined to arrive at an average cost per contact hour, projected (because they declined during the 1990s) to a 2001/2 value. Monthly contact time costs were then aggregated by treatment phase (e.g. assessment, tuning). Finally, specific procedure or test costs (e.g. preoperative imaging, surgical session, postoperative radiography, implant system, spares and repairs) were estimated in consultation with clinicians and hospital accountants. The cost of the hospital stay for implantation was also included in this last costing step.
For the costs of paediatric implantation Barton and colleagues55 summarise how various categories of resource use were measured and valued in their study to estimate costs incurred in the 1998/9 financial year in all 16 UK hospitals that provided cochlear implants to children at that time. Resource use categories included were staff, accommodation, equipment, incidentals (e.g. office supplies, travel and conferences), inpatient care, implant device and adverse events. Data were obtained from the clinical coordinator of each programme by questionnaire, telephone calls, e-mail and a face-to-face interview. This included developing a description of the profile of care (pattern and length of clinical appointments) for paediatric implant recipients. The clinical case notes of the first 909 children implanted in the UK also fed into this costing exercise, as well as the annual survey of UK cochlear implantation programmes (conducted since 1991).
Tables 51 and 52 show the main data that we have used from these two studies and how we have calculated the relevant parameter values.
| Type of costs | 2001/2 (€) | 2001/2 (£) | 2005/6 (£) | 2005/6 (£ less repairs) |
|---|---|---|---|---|
| First year of care: assessment | 5286 | 3432 | 4011 | 4011 |
| Implantation: excluding hardware | 3709 | 2408 | 2814 | 2814 |
| Second year of care: tuning | 6935 | 4503 | 5262 | 5000 |
| Third year of care: maintenance | 1397 | 907 | 1060 | 798 |
| Fourth year of care: maintenance | 1341 | 871 | 1018 | 756 |
| Future years: maintenance | 1135 | 737 | 861 | 599 |
| Cost type/stage of use | 2001/2 (€) | 2001/2 (£) | 2005/6 (£) | 2005/6 (£ less repairs) |
|---|---|---|---|---|
| Assessment | 3743 | 2433 | 2843 | 2843 |
| Implantation: excluding hardware | 4582 | 2978 | 3480 | 3480 |
| ‘Tuning’ (first year post implantation) | 12,044 | 7829 | 9148 | 9148 |
| First year of maintenance | 6209 | 4036 | 4716 | 4184 |
| Second year of maintenance | 4792 | 3115 | 3640 | 3107 |
| Each subsequent year | 2497 | 1623 | 1897 | 1364 |
In addition, data presented in Figure 3 of the UKCISG study53 was used to estimate the cost of major postsurgical complications (which are mostly wound related, see below).
We have sought input from the current membership of the BCIG and they have assured us that in nearly all respects the pattern of care in UK implant centres, both before and after cochlear implantation, is still very similar to that when these costing studies were carried out.
States in the model
Candidacy/assessment for implantation
Candidacy or assessment costs are all NHS costs incurred between referral to a cochlear implant centre and the day of the implant operation. We used the converted and inflated costs reported in the published studies, as in Tables 3 and 51.
First implantation (unilateral cochlear implant)
The mean NHS cost for ‘implantation of intracochlear prosthesis’ or ‘implantation of extracochlear prosthesis’ has the HRG code of C60. The National Schedule of Reference Costs (NSRC) 2005/6 cost of this inpatient episode is £18,005. However, the NHS Supply Chain agency has also provided us with detailed data on the prices currently paid by the NHS under an NHS purchasing contract for cochlear implants. Depending on the exact cochlear implant model and manufacturer, these prices (for ‘applicable national price bands’) vary from £12,250 to £15,550 for single implant systems. Within this contract (for one manufacturer’s products) there is also a single price for two full implant systems for bilateral implants of £18,375.
There is therefore a choice between using the NSRC cost for the HRG code for cochlear implants and using separate estimates for the costs of the devices and the costs of preoperative, operative and perioperative procedures and care. To retain more flexibility we have decided to use current device costs as provided by the NHS Supply Chain and the converted and inflated costs from the two UK costing studies described above.
All but one of the cochlear implant systems are for use in either children or adults. One of the DIGISONIC products from Neurelec is intended only for use in children under the age of 3 years.
In children
In children the price of a cochlear implant system used in the model is the mean cost of the nine devices in the NHS Supply Chain purchasing contract (£14,611), plus the cost of the implantation procedure and hospital stay (£3480) derived from the Barton and colleagues study. 55
In adults
In adults the price of a cochlear implant system used in the model is the mean cost of the nine devices in the NHS Supply Chain purchasing contract (£14,611), plus the cost of the implantation procedure and hospital stay (£2814) derived from the UKCISG study. 53
Bilateral cochlear implantation
In the reference case analysis we assume that bilateral implantation requires two complete (unilateral) cochlear implant systems and therefore the device costs are twice the device costs of a unilateral implant. However, it is current practice for all four of the manufacturers that sell cochlear implant systems to the UK NHS to offer price discounts when two systems are being implanted in the same person (information supplied by manufacturers and also suggested in the joint submission to NICE from BAA/BCIG/ENT UK). Nevertheless, the continued presence and size of these discounts in the future is impossible to guarantee and so we have decided initially to assess the technology on the basis of those prices that are contractually agreed with the NHS (via the NHS Supply Chain).
Therefore, for both simultaneous and sequential bilateral implantation, the device costs are twice those for a single implant system (£14,611 × 2 = £29,222). The cost of the implantation procedure and hospital stay is assumed to be incurred twice for sequential cochlear implantation (£5628 in adults, £6960 in children, derived from the two previous UK costing studies53,55). However, although for simultaneous bilateral implantation only one surgical procedure and hospital stay is required, we assume that these costs are 50% higher than for unilateral implantation (£4221 in adults, £5220 in children), mainly because of the additional time in surgery.
With regard to preimplantation assessment costs we assume that for either simultaneous or sequential bilateral implantation these costs are incurred only once and at the same level as for unilateral implantation. However, the cost of tuning and rehabilitation (in the first year after implantation) is assumed to be incurred after each implantation operation and is therefore incurred twice for sequential implantees. The long-term costs of routine maintenance (4+ years post implantation) are assumed to be the same whether people have one or two cochlear implants, although the risks of device failures and major complications are doubled in those using two implants (see below).
Device tuning and other early postimplantation costs
In the first year after a successful operation to implant a cochlear implant the recipient requires various specialist appointments during which the devices themselves are adjusted and the person is further assessed and ‘trained’ to maximise their capacity to benefit from the implants, for example in terms of speech perception and other goals.
We have used the costs of tuning and other care in the first year post implantation from the two recent UK-based studies,53,192 as cited at the beginning of this section. After inflation and conversion to 2005/6 UK pounds these NHS care costs in the first year after implantation are £9148 in children and £5000 in adults.
Routine maintenance costs
The routine costs of device maintenance used are those derived from the two previous UK costing studies53,55 (from year 4 onwards post implantation: £1364 per year for children and £599 per year for adults). The only exception is that, in generating cost–utility ratios for all paediatric subgroups, the model assumes that children will at some point incur the lower annual costs of device maintenance and hearing support which adults experience. In our model, from the age of 16 years, children incur the annual adult cost (£599) for the remainder of their lives as cochlear implant users rather than the estimated annual cost for children (£1364).
Device failure – internal
In the model internal device failures were attributed the mean NHS cost of a replacement implant device (electrode) (£14,498) plus the operation costs to implant it (£2814 in adults, £3480 in children).
The internal component of a cochlear implant is under warranty for free repairs and/or replacements (information supplied to NICE by manufacturers) and therefore separate costs need to be used for the periods of time inside and outside the warranty.
During the first 10 years after initial implantation all devices are assumed to be within warranty and therefore upon failure individuals only incur the costs associated with implantation. Thereafter, during each model cycle a proportion of internal failures are assumed to be in warranty and the remainder not (and hence incurring the full cost of replacement). The proportions used were derived using the relevant event probabilities in adults and children.
Device failure – external
Similarly, the external component of a cochlear implant is also under warranty for free repairs and/or replacements, with the warranty period being 3 rather than 10 years (information supplied to NICE by manufacturers).
During the initial warranty period we have assumed that all replacements incur no cost; thereafter, a proportion incur the full NHS cost of a replacement speech processor (£4114) and the remainder do not. These proportions were again calculated on the basis of the relevant event probabilities for adults and children.
Major complications
Major complications are defined for our modelling purposes as those requiring a reoperation at the implantation site but not associated with a device failure. Most complications are wound related; more rarely they result in operations to reposition the electrode or receiver/stimulator. We estimated the cost of these on the basis of data on wound-related complications in adults from the UKCISG study53 (specifically, data presented in Figure 3 of that paper).
For 21 out of the 311 patients in this study a profile is provided of complications that required treatment (e.g. a course of antibiotics); these included wound revision, electrode repositioning, electrode replacement (functioning electrode but wound-affected) and in some circumstances cochlear implant removal and a new cochlear implant in the other ear. We calculated a weighted average of these reported costs (inflated to 2005/6 prices and converted from euros to UK pounds), except using current reimplantation and device costs (as described in the previous section).
The resultant costs of treating major postsurgical complications were £7777 in adults and £7935 in children for unilateral implantees and £6117 in adults and £6212 in children for bilateral implantees (for bilateral implantees complications are, on average, slightly cheaper to treat because implantation in the other ear is not an option).
Speech processor upgrades
These are assumed to take place every 10 years and attract the same cost as a replacement external processor due to device failure (£4114).
Digital hearing aids
In the model, digital hearing aids may be used either in conjunction with cochlear implants or by deaf people in the absence of cochlear implantation. The cost (2007 prices) to the NHS of a moderate-power digital hearing aid varies from £68 to £118, and the cost of a high-power digital hearing aid from £105 to £152. As there are a vast number of products, many with different prices, we have made the reference case assumption that on average they cost £100 each and are replaced every 5 years. (We have not taken into account the cost of hearing aid batteries supplied by the NHS because they are relatively inexpensive.)
Summary of cost parameters
Table 53 lists the cost parameters included in the model, together with their base-case value and source. It should be noted that although the NHS Purchasing and Supply Agency (PASA; now NHS Supply Chain) prices are cited for the 2005/6 contract period the same contract (and prices) have been extended to September 2008 (NHS Supply Chain, audiology, 2007, personal communication).
| Parameter name (short description) | Value (2006 £) | Source |
|---|---|---|
| Presurgical candidacy costs (adults) | 4011 | Table 2 in UKCISG 200453 converted from euros to pounds and inflated to 2005/6 prices |
| Presurgical candidacy costs (children) | 2843 | Table 3 in Barton 200655 converted from euros to pounds and inflated to 2005/6 prices |
| Unilateral implantation costs (excluding system cost, adults) | 2814 | Table 2 in UKCISG 200453 converted from euros to pounds and inflated to 2005/6 prices |
| Unilateral implantation costs (excluding device cost, children) | 3480 | Table 3 in Barton 200655 converted from euros to pounds and inflated to 2005/6 prices |
| Mean cost of unilateral cochlear implant system (adults) | 14,611 | NHS PASA purchasing contract for November 2005–October 2006; ‘applicable national price bands for NHS Trusts’; mean cost of nine devices |
| Mean cost of unilateral cochlear implant system (children) | 14,611 | NHS PASA purchasing contract for November 2005–October 2006; ‘applicable national price bands for NHS Trusts’; mean cost of nine devices |
| Bilateral implantation costs (excluding system cost, adults) | 4221 | Table 2 in UKCISG 200453 converted from euros to pounds and inflated to 2005/6 prices; unilateral costs multiplied by 1.5 to reflect additional surgery costs for bilateral operative procedure |
| Bilateral implantation costs (excluding device cost, children) | 5220 | Table 3 in Barton 200655 converted from euros to pounds and inflated to 2005/6 prices; unilateral implantation costs multiplied by 1.5 to reflect additional surgery costs for bilateral operative procedure |
| Cost of bilateral cochlear implant system (adults) | 29,222 | NHS PASA purchasing contract for November 2005–October 2006; ‘applicable national price bands for NHS Trusts’; mean cost of nine devices |
| Cost of bilateral cochlear implant system (children) | 29,222 | NHS PASA purchasing contract for November 2005–October 2006; ‘applicable national price bands for NHS Trusts’; mean cost of nine devices |
| Mean replacement cost of a digital hearing aid (adults) | 100 | NHS Supply Chain (2007 audiology brochure) |
| Mean replacement cost of a digital hearing aid (children) | 100 | NHS Supply Chain (2007 audiology brochure) |
| Postimplantation costs | ||
| Tuning and maintenance costs in year 1 (adults) | 5000 | Table 2 in UKCISG 200453 converted from euros to pounds and inflated to 2005/6 prices |
| Tuning costs in year 1 (children) | 9148 | Table 3 in Barton 200655 converted from euros to pounds and inflated to 2005/6 prices |
| Maintenance costs in year 1 (children) | 4184 | Table 3 in Barton 200655 converted from euros to pounds and inflated to 2005/6 prices |
| Maintenance costs in year 2 (adults) | 798 | Table 2 in UKCISG 200453 converted from euros to pounds and inflated to 2005/6 prices |
| Maintenance costs in year 2 (children) | 3107 | Table 3 in Barton 200655 converted from euros to pounds and inflated to 2005/6 prices |
| Maintenance costs in year 3 (adults) | 756 | Table 2 in UKCISG 200453 converted from euros to pounds and inflated to 2005/6 prices |
| Maintenance costs in year 3 (children) | 1364 | Table 3 in Barton 200655 converted from euros to pounds and inflated to 2005/6 prices |
| Maintenance costs in years 4+ (adults) | 596 | Table 2 in UKCISG 200453 converted from euros to pounds and inflated to 2005/6 prices |
| Maintenance costs in years 4–15 (children) | 1364 | Table 3 in Barton 200655 converted from euros to pounds and inflated to 2005/6 prices |
| Maintenance costs in years 16+ (children) | Table 2 in UKCISG 200453 converted from euros to pounds and inflated to 2005/6 prices | |
| Processor upgrade every 10 years (adults and children) | 4114 | NHS PASA purchasing contract for November 2005–October 2006; mean cost of 10 systems |
| Cost of major complications (unilateral) | Adult: 7777; child: 7935 | Source for mix of mostly wound-related complications: Figure 3 in UKCISG 200453 study of adults (excluding six who had electrode replacements unrelated to wound problems) |
| Cost of major complications (bilateral) | Adult: 6117; child: 6212 | Source for mix of mostly wound-related complications: Figure 3 in UKCISG 200453 study of adults (excluding six who had electrode replacements unrelated to wound problems and excluding any costs for implanting other ear) |
| Cost of internal component failure (during warranty period) | Adult: 2814; child: 3480 | Unilateral implantation costs (excluding device cost) (sources as per initial implantation above) |
| Cost of internal component failure (in years after warranty period) | Adult: 17,425; child: 18,091 | Unilateral implantation costs (including device cost) (sources as per initial implantation above) |
| Proportion of internal component failures occurring during warranty period | Adult: 0.7%; children: 0.9% | Values derived using time-dependant event probabilites for internal component failure in adults and children |
| Cost of external component failure (during warranty period) | Adult: 0; child: 0 | Authors’ assumption |
| Cost of external component failure (in years after warranty period) | Adult: 4114; child: 4114 | NHS PASA purchasing contract for November 2005–October 2006; mean cost of 10 systems |
| Proportion of external component failures occurring during warranty period | Adult: 31.8%; children: 31.8% | Values derived using event probabilities for external component failure in adults and children |
| Annual NHS or social services cost of non-acoustic support | 0 | Not included |
Reduced costs of education as a result of cochlear implantation
The review of clinical effectiveness studies has summarised evidence on the impact of cochlear implantation in children on both their educational attainment and the levels of special educational support required at school (i.e. the destination of deaf pupils in terms of mainstream schools, special schools or dedicated schools for the deaf). Although the research evidence is not extensive, the broad conclusion is that for many deaf children cochlear implantation leads to improved academic performance and a greater chance of placement in a mainstream school.
Four studies138,186,188,207 have concluded that cochlear implantation is associated with reduced costs of education. The most recent of these is a UK-based study, published in 2006 by Barton and colleagues,138 that includes data on 2241 hearing-impaired children, of whom 383 were cochlear implant users. The data were obtained from May 1999 to October 2001 using a questionnaire survey of teachers of the sampled hearing-impaired children. Of the implanted children whose educational costs were estimated, most (62%) were in a mainstream primary school, 17% were in a school for the deaf and 14% were in a mainstream secondary school. The remainder were in nursery (n = 15; 4%), at special schools (n = 3; 1%) or in further education (n = 3; 1%).
This study directly elicited resource use (e.g. staff contact time, size of teaching groups) and educational support information about specific deaf children in particular educational settings and also adjusted for a range of other factors that would influence educational costs (using regression analysis). We have therefore used the results of this study to inform a supplementary cost–utility analysis that includes educational cost savings resulting from cochlear implantation (i.e. in addition to those ‘reference case’ costs that fall on the NHS). Table 54 shows the mean estimated annual educational cost savings due to cochlear implantation at three preoperative average hearing levels (and after adjustment for other factors). We have assumed that £2359 per year is saved in educational costs from age 5 to 16 years inclusive (which assumes that the mean average hearing level of children currently implanted is the same as that when this study was conducted).
| AHL 105 dB | AHL 115 dB | AHL 125 dB | |
|---|---|---|---|
| 2001/2 euros saved during 12 years at schoola | 17,826 | 37,265 | 48,376 |
| Annual savings in 2001/2 euros | 1486 | 3105 | 4031 |
| Annual savings converted to 2001/2 poundsb | 966 | 2019 | 2620 |
| Annual savings inflated to 2005/6 poundsc | 1128 | 2359 | 3062 |
| 2005/6 pounds saved during 12 years at school | 13,540 | 28,304 | 36,744 |
| If discounted at 3.5% per year and incurred from age 5 to 16 yearsd | 9834 | 20,558 | 26,687 |
Utilities
Utilities were derived wherever possible from the published research literature, following a systematic search for all studies that reported utility values for:
-
being severely or profoundly deaf (with or without acoustic hearing aids)
-
living with one or two cochlear implants.
The search strategy involved a wide range of search terms spanning various synonyms for quality of life, quality-adjusted life-year and utility, as well as specific acronyms for the main quality of life instruments (e.g. EQ-5D, SF-36 and HUI-2/HUI-3), which can be used to derive utility estimates. Any cost–utility analyses in the systematic review of economic evaluations were also examined for their sources of utility estimates. The complete list of papers reviewed for obtaining utility values is shown in Appendix 11.
Studies were included if they involved the empirical elicitation of utility values relating to being deaf with or without a cochlear implant. We included but gave much less weight to those studies that simply used the utility decrement associated with the levels of hearing impairment as specified in the HUI instrument (i.e. as based on the original Canadian exercise for deriving utility weights).
In accordance with NICE methodological guidance201 we tried to obtain utility values from studies of severe or profoundly deaf people who had reported their health-related quality of life using a standardised and validated generic quality of life instrument, and for which the value of changes in health states have been based on public preferences elicited using choice-based methods. In practice, for capturing the quality of life impacts of cochlear implants on deaf people this means finding studies that have used the HUI. This is because, in contrast to alternative generic health-related quality of life measures, such as the SF-36 or EQ-5D, the HUI is the only standard instrument that includes statement items relating to functional limitations because of impaired hearing or speech. The HUI-3 has therefore become the standard outcome instrument used by the UKCISG for quality of life and cost–utility studies. 53,62 This is despite the fact that the utility (social preference) weights available for the HUI-3 instrument are only from Canadian and US populations.
Also, there are few studies that have used this instrument with the same cohort of deaf people both before and after cochlear implantation, and the medium- to longer-term impacts on health-related quality of life are still largely undocumented.
As the research literature is dominated by non-randomised studies it should be noted that people’s reported quality of life immediately before being considered for a cochlear implant will not necessarily be the same as their actual future health-related quality of life had they not received an implant. For example, in postlingually deafened adults their deafness may be progressively worsening and consequently also lowering their future quality of life compared with same-aged people with normal-hearing ability. On the other hand, in prelingually deafened children it might be assumed that their ability to communicate by other means (and hence their quality of life) may gradually improve during childhood.
Another consequence of the requirement to use a standardised and validated generic instrument for estimating the quality of life impacts of deafness and cochlear implantation is that utility estimates for deafness in children will generally have to be obtained from proxy adults, usually their parents.
Utility of being severely or profoundly deaf
Adults
The best study that estimates the utility associated with being a severely or profoundly deaf adult is that by the UKCISG,53 which elicited values from 311 postlingually deafened adults who completed the HUI-3 instrument both before and after cochlear implantation (Table 55). Alternative possible sources that were less suitable were studies by Summerfield and colleagues189 [smaller sample (n = 202), and HUI-2 instrument], Francis and colleagues208 [smaller sample (n = 47) and older sample, retrospective assessments, algorithm for utility derivation not stated], Wyatt and colleagues209 [smaller sample (n = 32), US sample], Lee and colleagues210 [small sample (n = 11), Korean implantees, retrospective assessments, HUI version not stated] and Krabbe and colleagues211 [smaller sample (n = 45), Netherlands sample, retrospective assessments, HUI-2 used].
| Type of cochlear implant candidatea | n | Mean preimplantation utility (HUI-3) | 95% confidence interval |
|---|---|---|---|
| All | 311 | 0.433 | 0.411–0.455 |
| All traditional candidates | 227 | 0.410 | 0.386–0.435 |
| Non-benefiting traditional candidates | 134 | 0.365 | 0.332–0.398 |
| Benefiting traditional candidates | 93 | 0.475 | 0.443–0.508 |
| All marginal hearing aid users | 84 | 0.494 | 0.447–0.540 |
| Non-scoring marginal hearing aid users | 53 | 0.495 | 0.432–0.557 |
| Scoring marginal hearing aid users | 31 | 0.492 | 0.422–0.562 |
In the UKCISG study53 the age at time of implant ranged from 18 to 82 years in the whole group. Although their AHL in the better-hearing ear ranged from 85 dB HL to 140 dB HL, nearly all were profoundly deaf; the mean preoperative AHL in their better ear ranged from 119 dB HL (95% CI 117.7–121.3 dB HL) in ‘non-benefitting traditional candidates’ to 107.4 dB HL (95% CI 104.3–110.6 dB HL) in ‘scoring marginal hearing aid users’ (Table 4, p319, in UKCISG 2004 REF CEA I paper61).
Children
The best study that estimates the utility associated with being a deaf child is that by Barton and colleagues,192 which elicited proxy values from the parents of a representative sample of hearing-impaired British children using an adapted version of the HUI-3 instrument (Table 56). We could not find any studies that had tried to directly elicit utility values from deaf children. Alternative possible sources were less suitable, either because they used estimated values for the hypothetical pure state of ‘being deaf’ (on the HUI instrument) or because they were based on much smaller samples of children in the USA.
| Severity of deafness | n | Mean utility (HUI-3) | 95% CI |
|---|---|---|---|
| Severe (AHL 71–95 dB HL) | 464 | 0.616 | 0.598–0.634 |
| ‘Group profound’ (AHL 96–105 dB HL) | 259 | 0.497 | 0.469–0.535 |
| Profound (AHL > 105 dB HL) | 290 | 0.353 | 0.327–0.379 |
Utility following unilateral cochlear implantation
Adults
The best study that estimates the utility associated with unilateral cochlear implantation in deaf adults is that by the UKCISG,53 which elicited values from 311 postlingually deafened adults who completed the HUI-3 instrument both before and after cochlear implantation. Alternative possible sources that were less suitable were rejected for the same reasons as already listed in the previous section.
Table 57 shows the mean utility at 9 months post implantation and the resultant mean change in utility compared with preimplantation. All improvements in utility were statistically significant at the 95% confidence level. On average, traditional candidates were older (mean age 52.5 years) than the marginal hearing aid users (mean age 46.3 years) at the time of implantation.
| Type of cochlear implant candidatea | n | Mean postimplantation utility (at 9 months) | Mean change in utility |
|---|---|---|---|
| All | 311 | 0.630 | 0.197 |
| All traditional candidates | 227 | 0.624 | 0.214 |
| Non-benefiting traditional candidates | 134 | 0.597 | 0.232 |
| Benefiting traditional candidates | 93 | 0.666 | 0.188 |
| All marginal hearing aid users | 84 | 0.645 | 0.151 |
| Non-scoring marginal hearing aid users | 53 | 0.627 | 0.132 |
| Scoring marginal hearing aid users | 31 | 0.676 | 0.184 |
A recently published study by Damen and colleagues212 is the first to have evaluated long-term changes in quality of life following cochlear implantation. In a group of 37 implant recipients followed for 6 years, and using a number of different quality of life measures, including the SF-36 and HUI-3, they showed that the health-related quality of life of adult implant recipients appears to decrease slightly over time, although this may reflect ageing rather than any supposed diminishing benefits of cochlear implant use.
Children
The study by Barton and colleagues192 of the cost–utility of paediatric cochlear implantation in the UK provides the most relevant utility estimates for this analysis. The parents of 403 profoundly deaf children with unilateral cochlear implants completed a modified HUI-3 instrument according to their perception of their children’s health-related quality of life, together with the parents of 549 profoundly deaf children and 464 severely deaf children without cochlear implants. The responses in relation to the implanted children yielded a mean post implant utility of 0.575 (95% CI 0.553–0.598). This utility weight was intermediate between the raw utility weights of 0.616 for children with severe deafness (AHL 71–95 dB HL) and 0.497 for those with an AHL between 96 and 105 dB HL. The mean preoperative AHL of children with implants was 115 dB and approximately 93% had an AHL of between 100 dB and 130 dB (i.e. they were nearly all profoundly deaf before implantation).
However, linear regression analysis of these data including child-specific data on age, age at onset of hearing impairment, severity of preoperative hearing impairment and years since implantation reveals considerable variations in the estimated utility gain from implantation. The main results of this regression analysis are shown in Table 58. The study shows that the estimated utility change due to cochlear implantation, even in profoundly deaf children (AHL ≥ 105 dB HL), varies considerably from a non-significant (p > 0.05) increase of 0.066 to a significant increase of 0.232, depending on preoperative AHL, number of years of use and age at implantation. However, amongst profoundly deaf children who have been implant users for more than 4 years the estimated utility gain is at least 0.183.
| Age at implantation | |||
|---|---|---|---|
| < 5 years | ≥ 5 years | ||
| Duration of use of implant | < 2 years | 0.066 (–0.013 to 0.144) | 0.130 (0.053–0.206) |
| ≥ 2 and < 4 years | 0.212 (0.161–0.263) | 0.172 (0.103–0.240) | |
| ≥ 4 years | 0.232 (0.184–0.280) | 0.183 (0.126–0.239) | |
No regression analysis was conducted involving cochlear implant variables stratified by age at onset of hearing impairment and so the possible different utility gains for postlingually deafened children were not specifically estimated. The results shown should be regarded as relating to children who became deaf prelingually [of those implanted at < 5 years of age less than 2% became deaf while aged 4 years and only 10% of those implanted at age 5 years or over became deaf after the age of 3 years (using data from Table 4 in Stacey and colleagues21)].
Utility and utility changes following bilateral implantation
Adults
Only two published studies have assessed the utility of bilateral cochlear implantation. Summerfield and colleagues189 used the time trade-off method to elicit values from 70 normal-hearing volunteers, all of whom had familiarity with deaf adults and cochlear implantation in a professional capacity (either clinicians working in the UK adult cochlear implant programme or staff at the MRC Institute of Hearing Research). A more recent study, also by Summerfield and colleagues,149 randomised 24 adult unilateral cochlear implant users to receive a second cochlear implant either immediately (12 users) or 12 months later (12 patients). At 9 months after bilateral implantation there was only a small and non-significant difference in HUI-3 estimated utility between the bilateral and unilateral groups, of +0.030 (95% CI –0.045 to +0.104). This very modest utility increment, although based on a small sample of actual implant users, is remarkably similar to the utility increment estimated from the time trade-off exercise with normal-hearing volunteers in the earlier study (+0.031). However, it should be noted that the (HUI-3) utility gain of +0.03 estimated from the RCT assumes a neutral impact from changes in tinnitus; we believe this is justified because a larger body of evidence about the impact of unilateral implantation on tinnitus experience implies reductions in tinnitus are more likely. Also, changes in utility at 3 months and 9 months on the EQ-5D and VAS were neutral or negative.
Although the population in the RCT had been unilateral implant users for between 1 and 6 years, we have assumed that the utility gain estimate from this study more closely applies to simultaneous bilateral implanted adults and those adults sequentially implanted in relatively close succession (3 years in our base case). Unfortunately, neither of the two studies that report utility estimates for bilateral implantation in adults report empirical data from deaf adults who received their second implant more than 5 years after the first implant and so there are no utility gain estimates for this group of potential bilateral implant recipients.
Children
We could not find any published studies evaluating the impact of bilateral cochlear implantation on the quality of life of deaf children; therefore, we assume the same value as for adults, +0.03.
Summary of utility values used in PenTAG base-case analyses
Given the limited availability of high-quality data on utility improvements following unilateral cochlear implantation we have decided to restrict our analyses for these comparisons to two reference cases: one for profoundly deaf adults and one for profoundly deaf children. We have also used information from the single source for the incremental benefit associated with bilateral implantation and applied the result to both adults and children. These are defined in Table 59, together with the relevant best estimate of short-term utility gain.
| Group implanted | Utility without cochlear implant | Years since implant | Estimated utility gain, unilateral (95% CI) | Estimated utility gain, bilateral (95% CI) | Source |
|---|---|---|---|---|---|
| Profoundly deaf prelingually deafened children | 0.421 | NA | Weighted mean of data relating to profound and ‘group profound’ in Barton 2006193 | ||
| < 2 years | 0.066 (–0.013 to 0.144) | Data relating to those implanted at < 5 years of age in Barton 2006192 | |||
| ≥ 2 and < 4 years | 0.212 (0.161–0.263) | ||||
| ≥ 4 years | 0.232 (0.184–0.280) | ||||
| NA | 0.03 (–0.045 to 0.104) (versus unilateral) | Authors’ assumption | |||
| Profoundly deaf postlingually deafened adults | 0.433 | NA | Data relating to all 311 implanted adults in UKCISG 200453 | ||
| 0.197 (0.176–0.218) | Data relating to all 311 implanted adults in UKCISG 200453 | ||||
| NA | 0.03 (–0.045 to 0.104) (versus unilateral) | Summerfield 2006149 |
We were unable to find any reliable published estimates of the utility gain from cochlear implantation for the following specific subgroups of deaf people:
-
severely deaf adults or children
-
postlingually deafened children
-
prelingually deafened adults
-
established unilateral implant recipients (e.g. for > 5 years) receiving a second implant
-
unilateral implant recipients with (or without) a contralateral hearing aid.
Declining utility gain for scenario analysis
In the base-case analysis for adults the incremental benefit associated with unilateral implantation was modelled as a single value (+0.197). This gain was assumed to hold for the remainder of an individual’s expected lifetime. However, published evidence shows that the utility of a normal-hearing person decreases with age. 213 A potential weakness of using a single, age-independent value for utility gain is that a profoundly deaf cochlear implant recipient could end up having a better estimated quality of life than their normal-hearing peers.
Cost-effectiveness results were therefore generated using a gradually diminishing (i.e. age-dependent) rather than fixed incremental utility. The baseline values used in this analysis were set to the original deterministic values (age = 50 years, utility gain = +0.197). For each age band a scaling factor was calculated using the formula:
This scaling factor is then multiplied by the baseline utility gain to obtain the values used in the model. These are summarised in Table 60.
| Age band (years) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 50–54 | 55–59 | 60–64 | 65–69 | 70–74 | 75–79 | 80–84 | 85+ | |
| Scaling factor | 100% | 98.0% | 98.0% | 96.0% | 91.0% | 84.0% | 72.0% | 50.0% |
| Utility gain (one cochlear implant) | 0.197 | 0.193 | 0.193 | 0.189 | 0.179 | 0.165 | 0.142 | 0.099 |
| Utility gain (two cochlear implants) | 0.227 | 0.222 | 0.222 | 0.218 | 0.207 | 0.191 | 0.163 | 0.114 |
Cost-effectiveness of adding a second cochlear implant for existing unilateral cochlear implant users
In the protocol for this technology assessment we stated that we would assess the cost-effectiveness of implanting a second cochlear implant for severely or profoundly deaf people already using a single cochlear implant. That is, what is the cost-effectiveness of implanting a second cochlear implant when someone has been a unilateral cochlear implant user for a number of years. [This should be distinguished from the decision problem in which people with no cochlear implants might receive either one implant or two implants simultaneously (or in relatively close succession) and for whom the decision concerning suitability for bilateral implantation is initially made before the patient has received a cochlear implant.]
We have decided not to present any cost-effectiveness analyses to assess this decision problem, mainly because of a lack of clearly relevant effectiveness evidence. In particular, there was only one study that could provide an estimate of the utility gain associated with unilateral cochlear implant users having a second cochlear implant. This was a small RCT149 of those who had been using a single cochlear implant for between 1 and 6 years (mean not stated) and therefore is of uncertain relevance for people implanted with the second implant more than 6 years after their first implant. (Note also that we have already assumed that the utility estimates from this study are more generalisable to the comparisons involving simultaneous and sequential bilateral implantation, and we use them as the sole source for our analyses of these strategies.)
Furthermore, of published bilateral implantation studies (included in the review of clinical effectiveness, and in either children or adults) there are no studies using comparable outcomes and in comparable populations of deaf people that would allow investigation of the relationship between the effectiveness of bilateral implantation and the number of years between the first and second implant. However, given the well-documented negative relationship between duration of deafness and a person’s ability to benefit from cochlear implants62,125,139,143 it can be reasonably assumed that bilateral cochlear implantation following a number of years as a unilateral implant user will probably be less cost-effective than simultaneous bilateral implantation (in people of equivalent age, hearing impairment and age at onset of deafness).
Chapter 7 Results of cost-effectiveness assessment
PenTAG cost and QALY outputs by age
Figures 9–11 summarise the main simulated outputs of the PenTAG cost–utility model for the main comparators and in profoundly postlingually deaf adults and profoundly prelingually deaf children. (All data shown for bilateral implantation are for simultaneous bilateral implantation.)
FIGURE 9.
Breakdown of costs for each main comparator in the PenTAG analyses, undiscounted and discounted. CI, cochlear implant; HA, hearing aid.
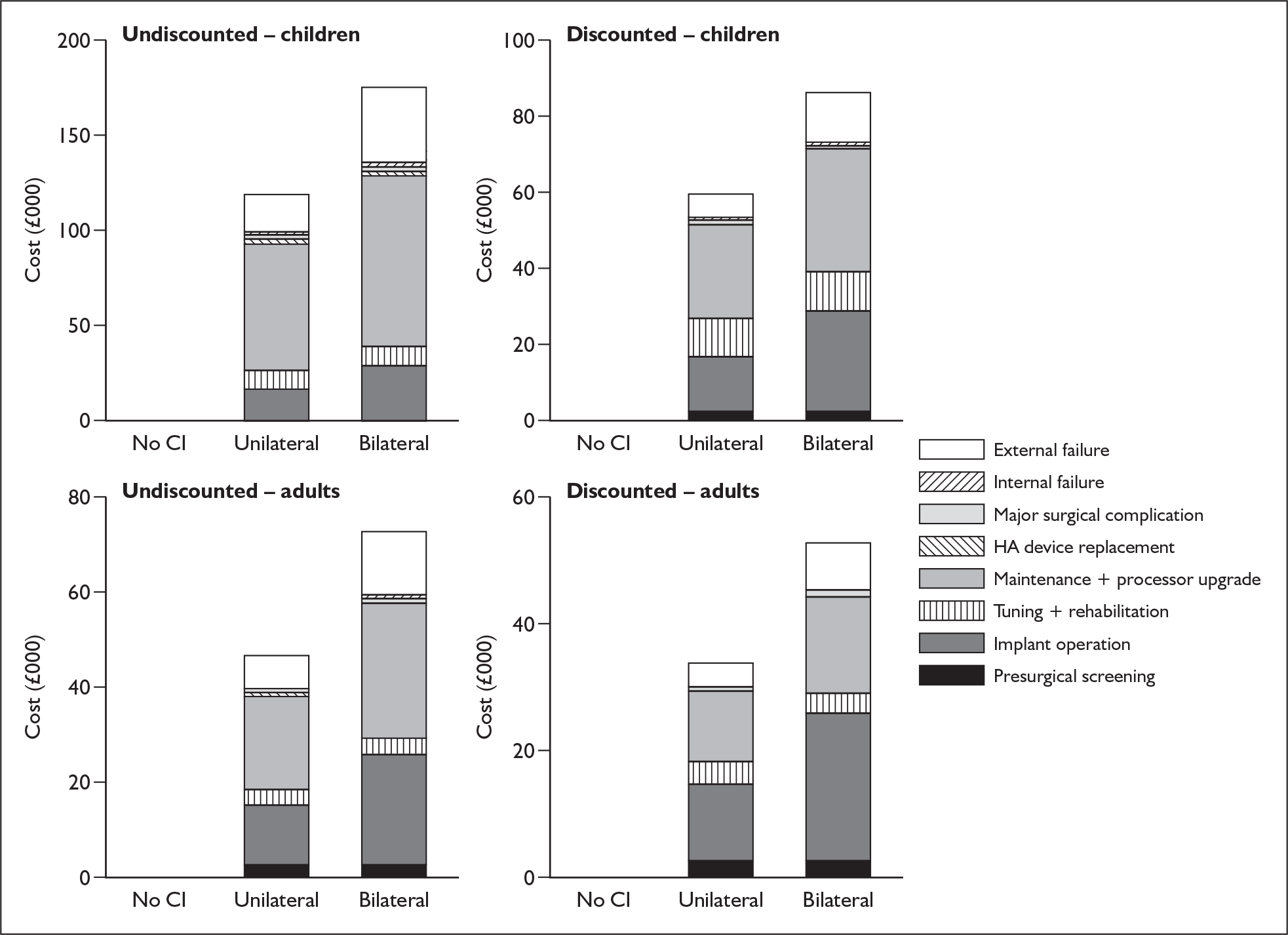
FIGURE 10.
Undiscounted costs of unilateral and bilateral implantation by age in children (implanted at age 1 year) and adults (implanted at age 50 years).
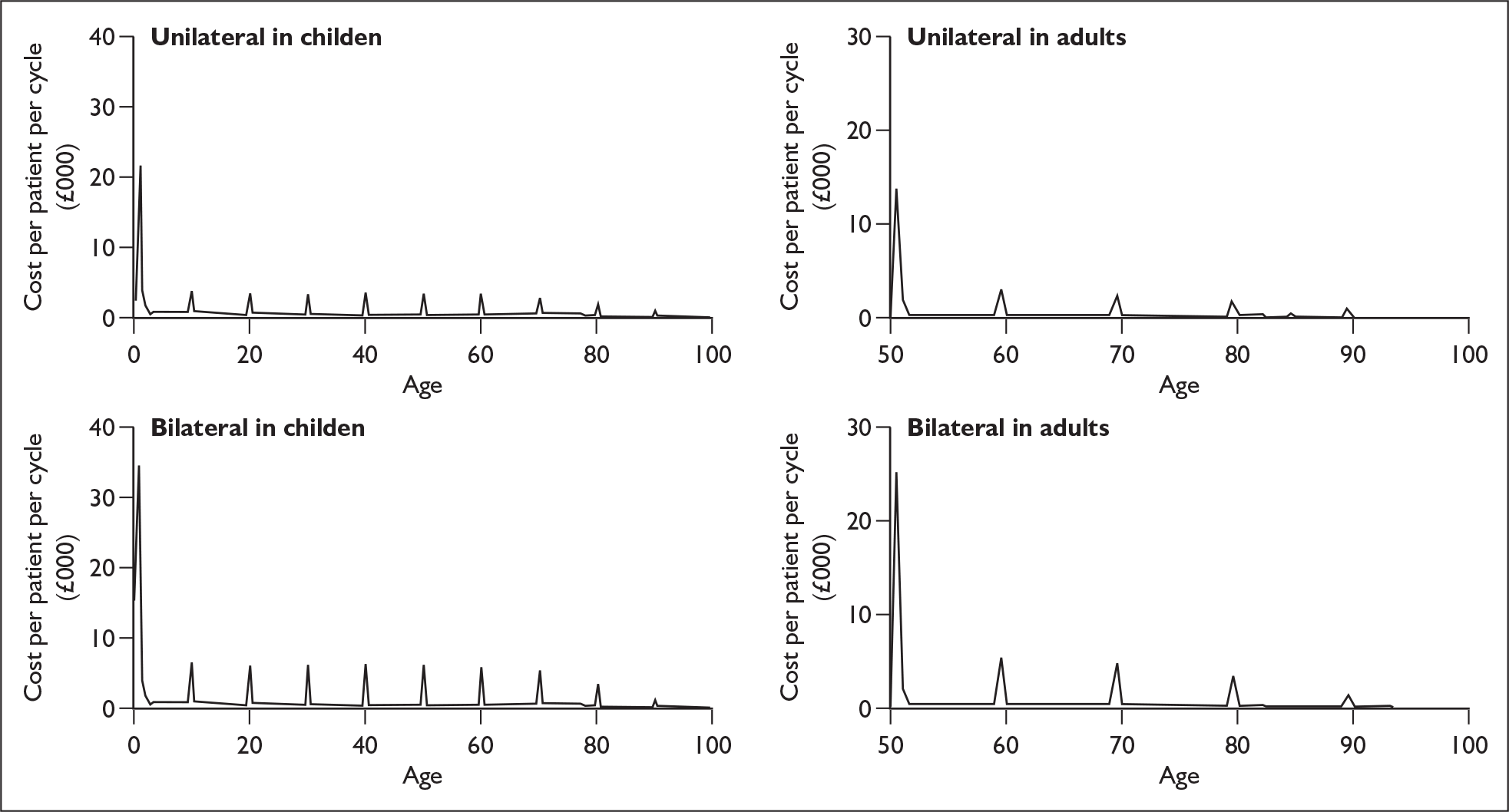
FIGURE 11.
Discounted and undiscounted mean quality-adjusted life-years (QALYs) per person for unilateral and bilateral cochlear implantation and no provision of cochlear implantation by age, in adults and children. CI, cochlear implant.
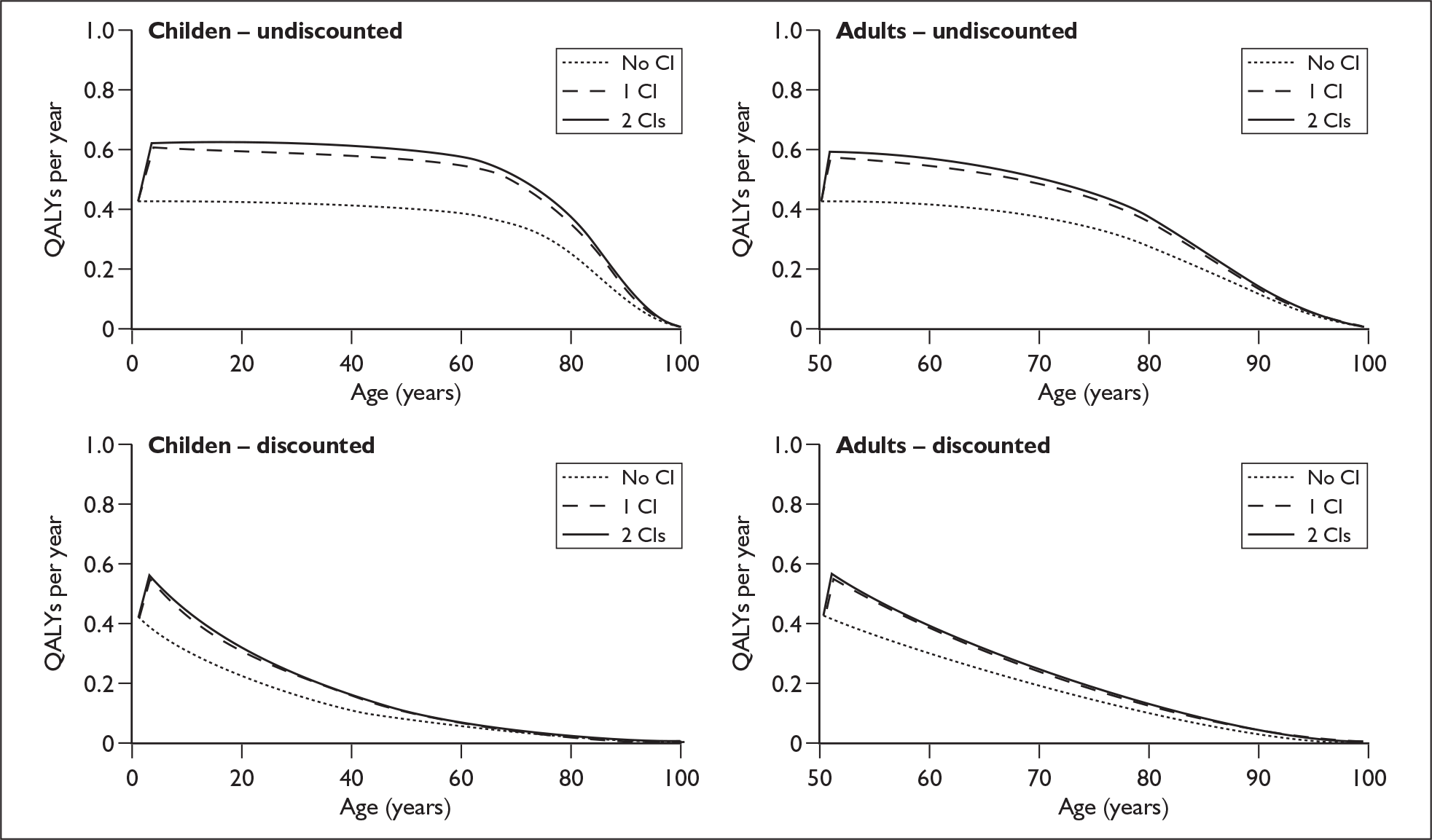
Figure 9 shows the origin of the costs that make up the total lifetime cost of each of the main comparators (undiscounted and discounted). Figure 10 shows the estimated lifetime pattern of undiscounted costs produced by the model. Similarly, for the benefits, Figure 11 shows the estimated lifetime pattern of utility associated with unilateral or bilateral cochlear implantation in children and in adults.
Results of cost-effectiveness in prelingually implanted profoundly deaf children
Unilateral implantation compared with best standard care without cochlear implantation
Base-case results produced by the decision model for a cohort of profoundly deaf children entering the candidacy process at age 1 year are shown in Table 61. In comparison to no cochlear implantation, the provision of unilateral cochlear implantation provides an extra 4.48 QALYs. This improvement would cost the NHS £60,070 per patient to achieve.
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| No cochlear implant use | 371 | 11.36 | – | – | – |
| Unilateral cochlear implant use | 60,441 | 15.84 | 60,070 | 4.48 | 13,413 |
Model outputs
Expected lifetime of cohort
Simulated children survive to a mean age of 80 years, similar to mortality in government actuarial life tables. 203 This is because we assume no mortality impact of deafness or the evaluated technologies and use the same life tables to determine mortality in the model. The expected lifetime over which events occur is therefore 79 years.
Event counts
During each cycle of the model a proportion of the cohort used to model unilateral implantation either transfers from one health state to another or remains within their current state. Such transfers can be considered as events. For example, moving from ‘device working’ to ‘cochlear implant external failure’ is an indication of the event of receiving a new speech processor and/or transmitter. These events can be aggregated to provide useful comparative outputs as well as a validation tool against published data and clinical experience.
The model outputs for the whole unilateral cohort as well as the subset of successful cochlear implant recipients are shown in Table 62. With the exception of voluntary non-use, all model outputs represent the number of events that an individual can expect to experience over their lifetime. When relevant, results are also reported at the rate per 100 patient-years.
Cohorts of individuals without a cochlear implant are modelled separately and the only event that they can experience is the replacement of an acoustic hearing aid. These individuals can expect to receive 11.4 new acoustic hearing aids over the course of their lifetimes.
| Whole cohort (including non-recipients) | Unilateral cochlear implant recipients | |||
|---|---|---|---|---|
| Lifetime | Event rate/100 patient-years | Lifetime | Event rate/100 patient-years | |
| New cochlear implant internal components | 0.07 | 0.09 | 0.09 | 0.12 |
| New cochlear implant external components | 12.94 | 16.37 | 16.17 | 20.5 |
| Major complications | 0.26 | 0.32 | 0.32 | 0.4 |
| Initial implant operations | 0.8 | NA | 1.0 | NA |
| New acoustic hearing aids | 12.02 | 15.21 | 12.17 | 15.4 |
| Permanent explants | 0.04 | 0.05 | 0.05 | 0.06 |
| Voluntary non-compliance | 0.19 | 0.02 | 0.02 | 0.03 |
Model validation
The validation of model outputs against data reported in empirical studies is not straightforward. First, data have been extrapolated a long way into the future. Second, cochlear implantation is a rapidly evolving technology and therefore any data from studies with a long follow-up period may well be obsolete.
Analysis of uncertainty
The ICER is the ratio of the incremental cost of treatment and the incremental benefits of treatment (i.e. difference in costs/difference in QALYs) between two interventions. Although this is useful in many situations, the fact that the ICER is a ratio measure makes the metric unstable. As the difference in health benefits between the two health technologies approaches zero the ICER is often difficult to interpret in one-way sensitivity analysis in which effects may be non-linear.
Net benefit214,215 is calculated by first assigning a willingness to pay value to a benefit unit. The incremental benefit of the treatment arm of the model can then be rescaled in terms of cost using this valuation. The net benefit of the treatment can then be calculated by offsetting the incremental cost against the incremental benefits of treatment.
The advantage of reporting net benefit is that it behaves in a more linear way than the ICER and incorporates a notional willingness to pay threshold which makes it easier to interpret. The disadvantage of using net benefit is that it relies on a specific level of valuation for each unit of benefit. In our analysis we have assumed a willingness to pay of £30,000 per QALY unless otherwise stated.
Deterministic sensitivity analysis
Extensive one-way sensitivity analyses were undertaken to explore which of the input parameters, when varied alone, had the greatest impact on the cost-effectiveness of unilateral cochlear implantation of prelingually deafened children in comparison to no cochlear implant use. One-way sensitivity analyses also allow the impact of the uncertainty in each parameter to be assessed.
These analyses examined the impact of:
-
structural assumptions –including changes in the time horizon and discount rates for costs and QALYs
-
event probabilities – including the probability of experiencing both internal and external cochlear implant failure as well as major postsurgical complications
-
survival curve fitting – this included looking at the impact of using just one curve for modelling internal failure instead of the pooled value
-
utility values – these include baseline values as well as time-dependant gains
-
costs – including the costs of initial implantation, maintenance and tuning as well as all replacements and reoperations.
The results of these analyses have been expressed graphically showing the ICER associated with each new parameter value. Results have been presented as separate graphs for structural parameters (Figure 12), utilities (Figure 13), event-related probabilities and survival curves (Figure 14) and costs (Figure 15).
FIGURE 12.
One-way sensitivity analysis for structural inputs. Incremental cost-effectiveness ratios of paediatric unilateral cochlear implantation compared with no cochlear implantation use. BC, base-case value; QALY, quality-adjusted life-year.
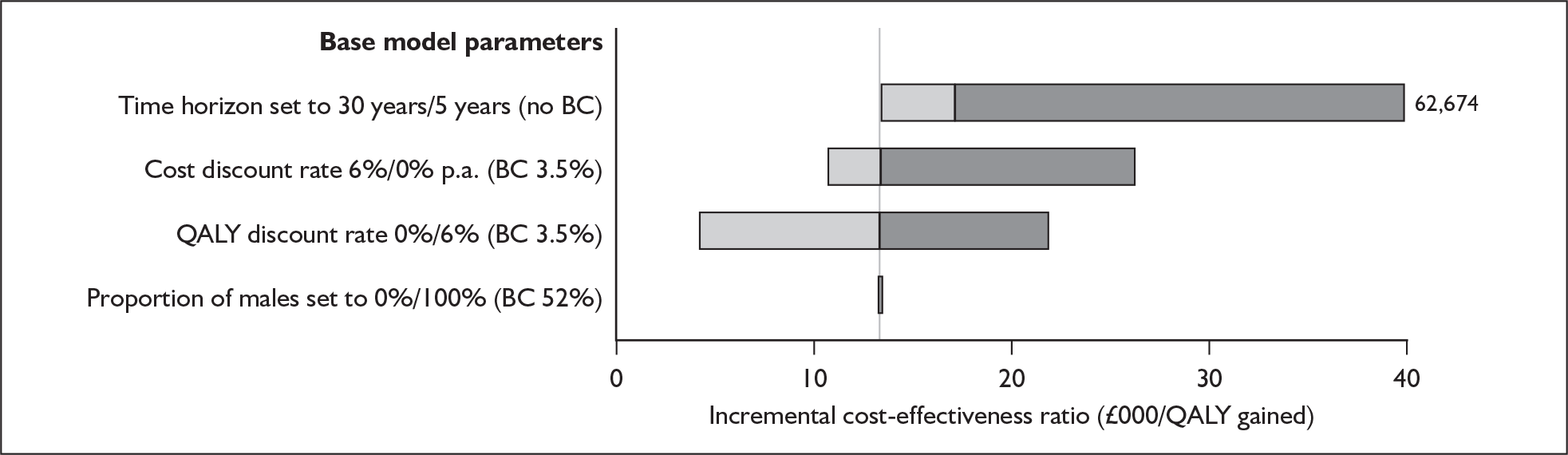
FIGURE 13.
One-way sensitivity analysis for utility gain. Incremental cost-effectiveness ratios of paediatric unilateral cochlear implantation compared with no cochlear implantation. BC, base-case value; QALY, quality-adjusted life-year.
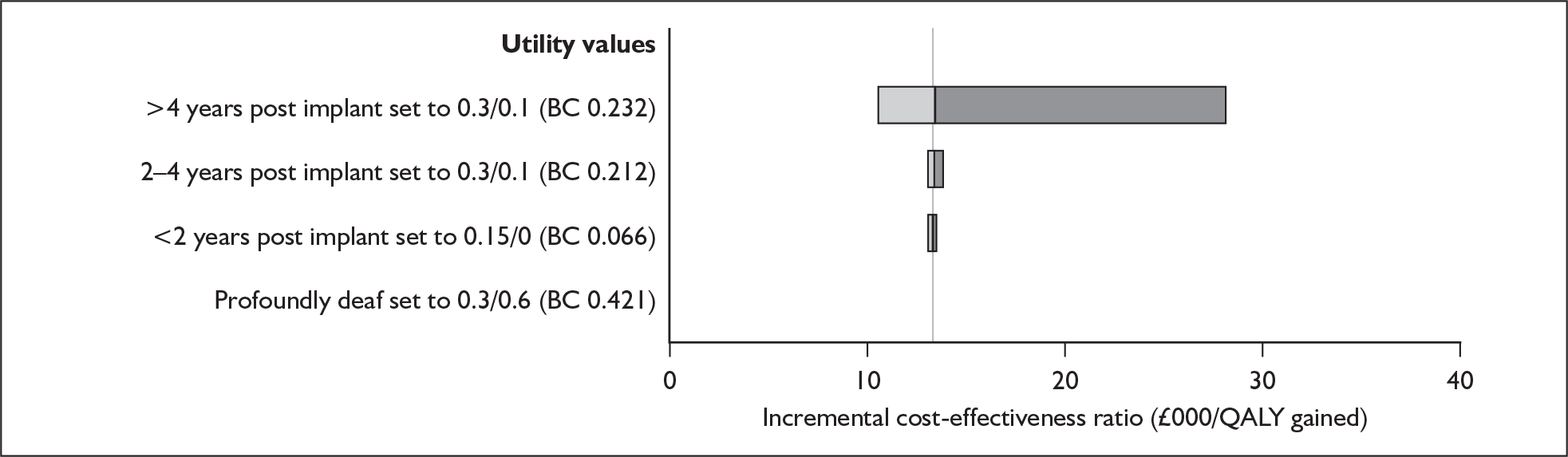
FIGURE 14.
One-way sensitivity analysis for event probabilities. Incremental cost-effectiveness ratios of paediatric unilateral cochlear implantation compared with no cochlear implantation use. BC, base-case value; CI, cochlear implant; HA, hearing aid; QALY, quality-adjusted life-year.
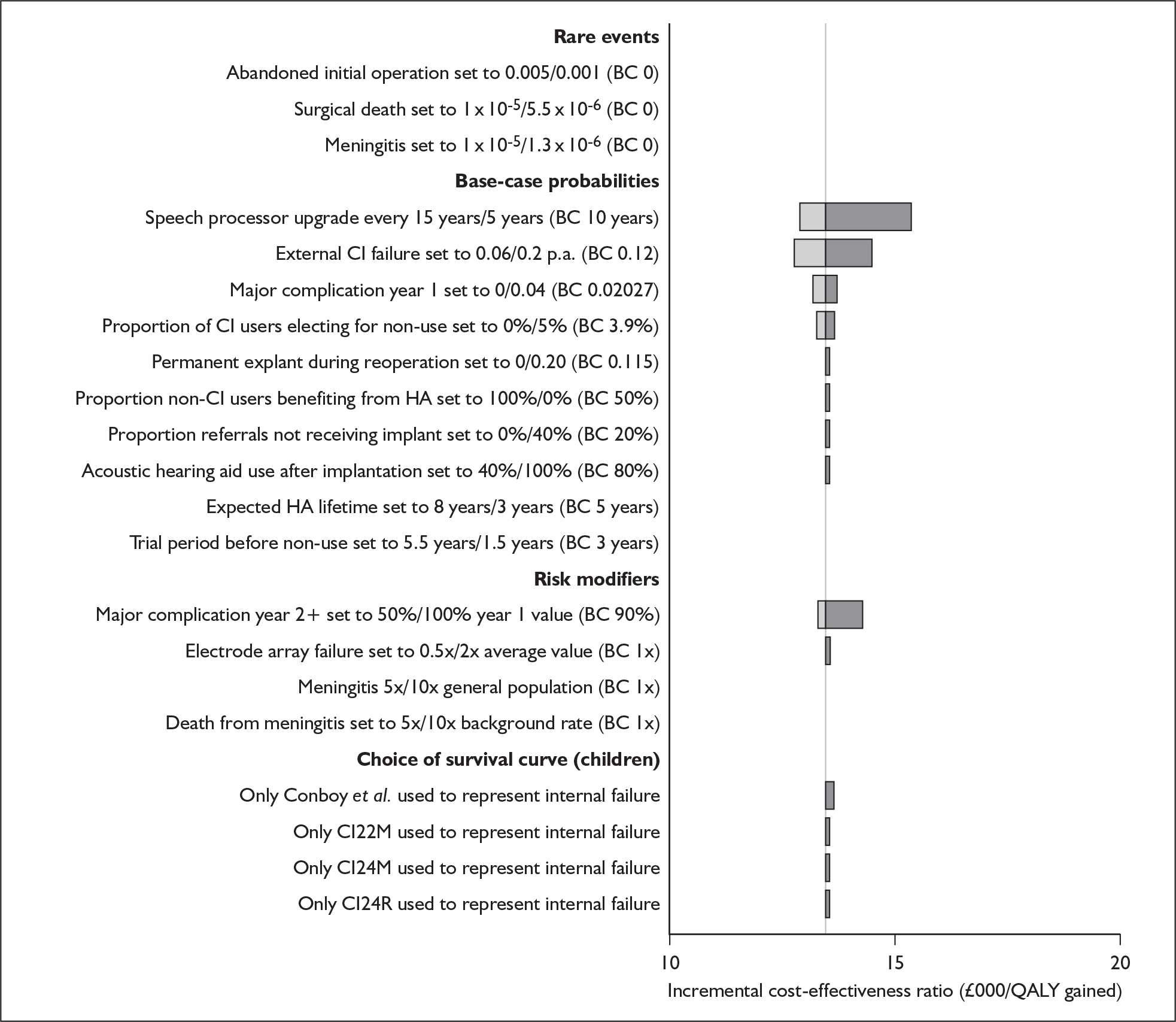
FIGURE 15.
One-way sensitivity analysis for costs. Incremental cost-effectiveness ratios of paediatric unilateral cochlear implantation compared with no cochlear implantation use. BC, base-case value; CI, cochlear implant; QALY, quality-adjusted life-year.
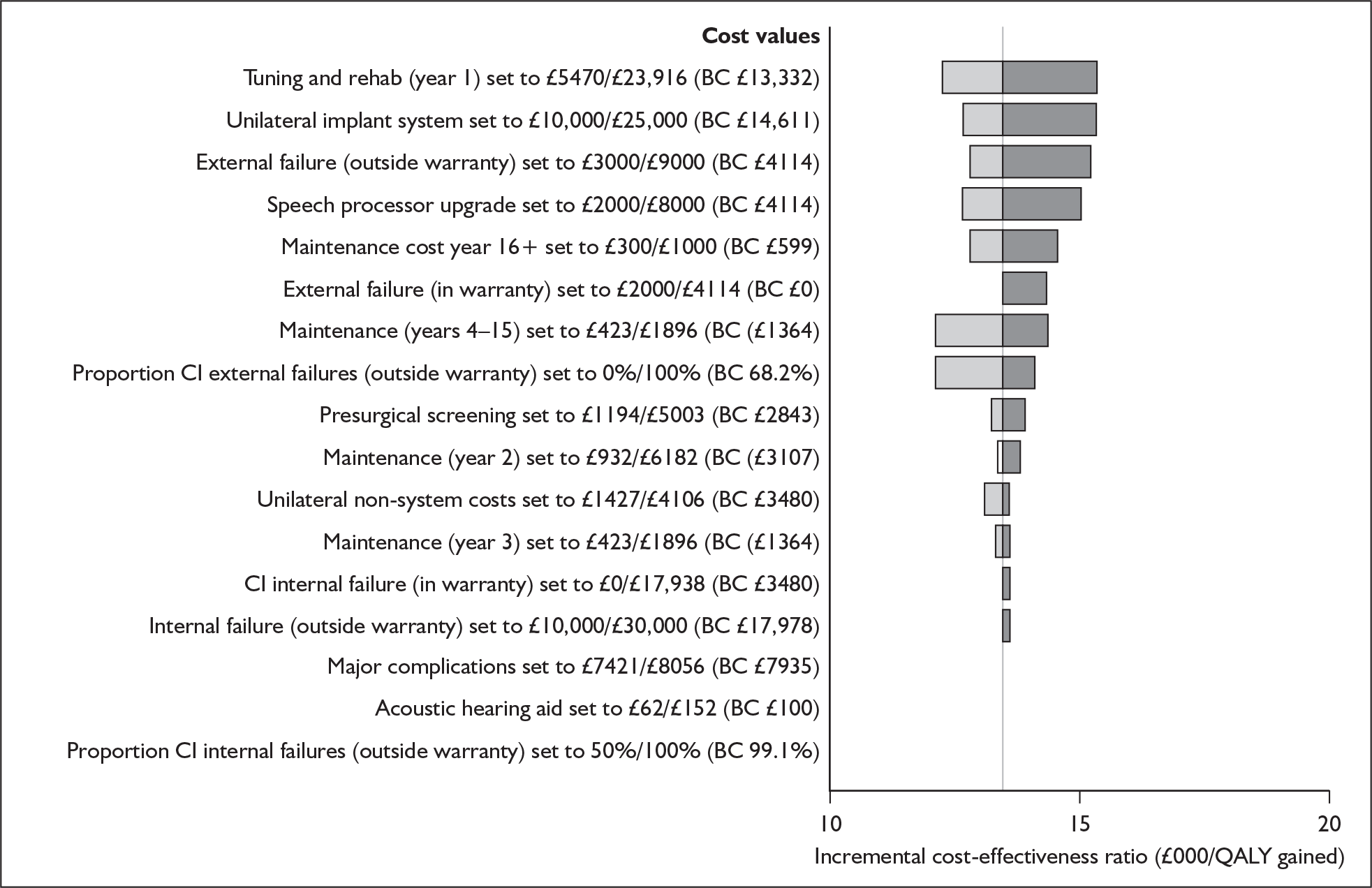
In this analysis of the effect of changes in individual parameters on the cost-effectiveness of paediatric unilateral cochlear implantation the base-case ICER appears particularly sensitive to changes in the following parameters:
-
the time horizon of the model
-
the discount rates applied to both costs and health benefits
-
the incremental utility gain associated with unilateral use as opposed to non-device use (> 4 years post implant operation)
-
maintenance costs from year 4 onwards.
Threshold analysis
The deterministic analyses presented in the previous section identified the inputs to which the model is most sensitive. By systematically varying each parameter within plausible ranges it is possible to identify the value at which the incremental net benefit changes from positive to negative. This point represents the parameter value at which unilateral implantation goes from being cost-effective to being cost-ineffective. The graphical output is expressed in terms of the incremental net benefit at an assumed willingness to pay threshold of £30,000 per QALY rather than as ICERs. Cost-effectiveness is represented as a positive net benefit. We considered only the utility gain associated with unilateral implant use of more than 4 years post fitting and the time horizon because cost-effectiveness is particularly sensitive to these parameters.
Utility gain associated with unilateral implant use of more than 4 years post fitting
Figure 16 shows that at a willingness to pay threshold of £30,000 per QALY unilateral implantation only becomes cost-ineffective below a value of approximately 0.09. At a willingness to pay threshold of £20,000 per QALY unilateral implantation becomes cost-ineffective with a utility gain below approximately 0.15.
FIGURE 16.
Threshold analysis for utility gain associated with unilateral cochlear implantation of profoundly deaf children at more than 4 years after initial fitting. QALY, quality-adjusted life-year.
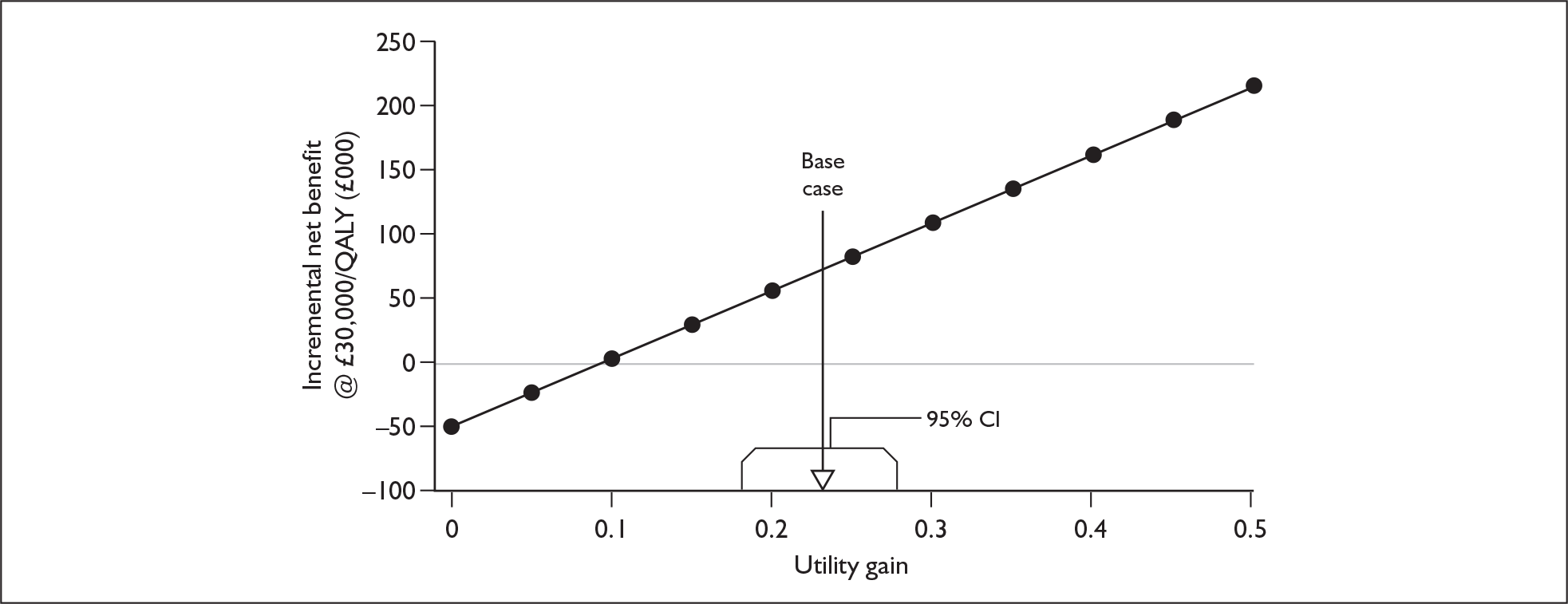
Time horizon used in analysis
The cost-effectiveness of unilateral cochlear implantation of prelingually deafened children at various time points is shown in Figure 17. At a willingness to pay threshold of £30,000 per QALY the procedure becomes cost-effective after approximately 11 years. At a willingness to pay threshold of £20,000 per QALY the procedure becomes cost-effective after approximately 26 years.
FIGURE 17.
Threshold analysis for model time horizon associated with paediatric cochlear implantation. QALY, quality-adjusted life-year.
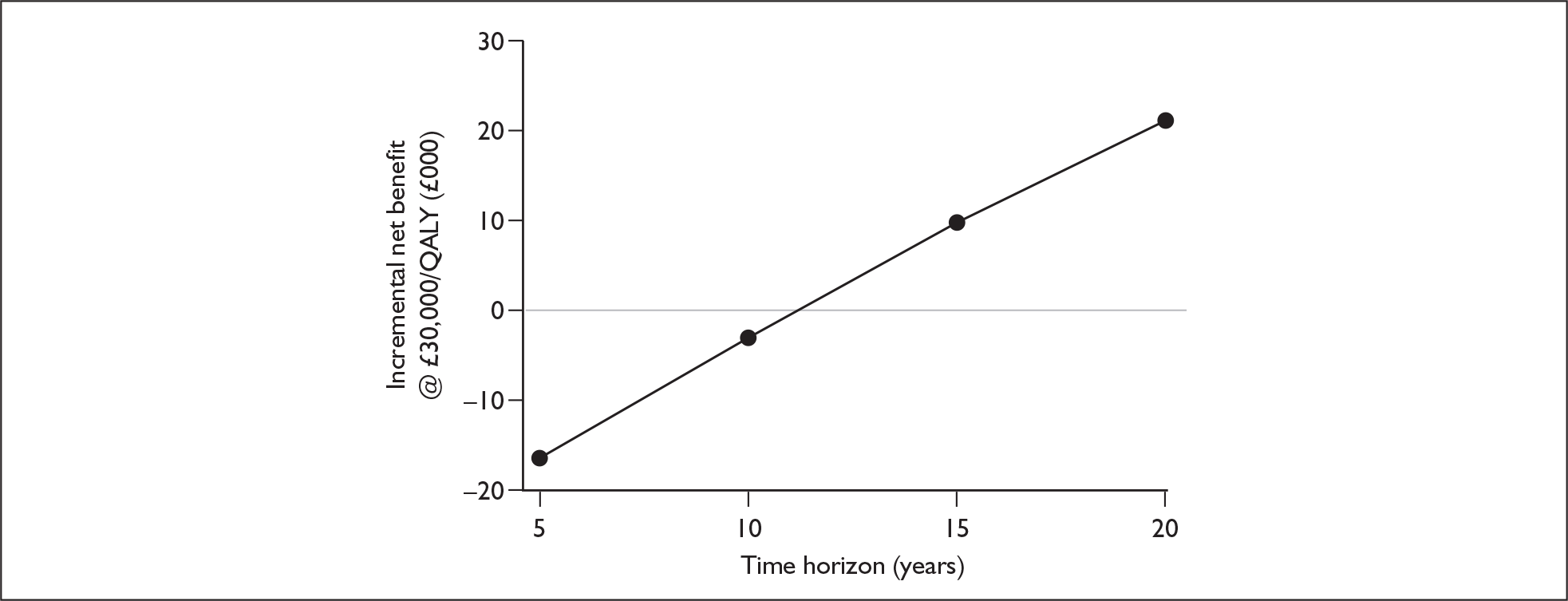
Probabilistic sensitivity analysis
A Monte Carlo simulation was used to explore the impact of underlying parameter uncertainty on cost-effectiveness. In these simulations, ranges and distributions used were sampled from the events, utility values and costs shown in Appendix 12.
The simulation output (based on 1000 runs of the model) shows that at a willingness to pay threshold of £20,000 per QALY unilateral implantation of children is cost-effective in 99.9% of simulations. At a threshold of £30,000 per QALY unilateral implantation of children is cost-effective in 100% of simulations and was dominated in 0% of simulations (creating higher costs compared with non-use of cochlear implants but lower QALYs). The probabilistic mean incremental net benefit is £76,081 (95% Cr I £75,214–76,948) and the probabilistic median incremental net benefit is £75,684.
Outputs from the Monte Carlo simulation are shown graphically in Figure 18. The two lines represent the willingness to pay thresholds used by NICE in the decision-making process. The cost-effectiveness acceptability curves (CEACs) for unilateral implantation are shown in Figure 19. The CEACs show that unilateral implantation would be considered cost-effective only if the willingness to pay threshold was increased beyond approximately £13,500 per QALY.
FIGURE 18.
Simulation output (cohort based, 1000 trials) for the cost-effectiveness of paediatric unilateral implantation in comparison to non-use of cochlear implants. QALY, quality-adjusted life-year.
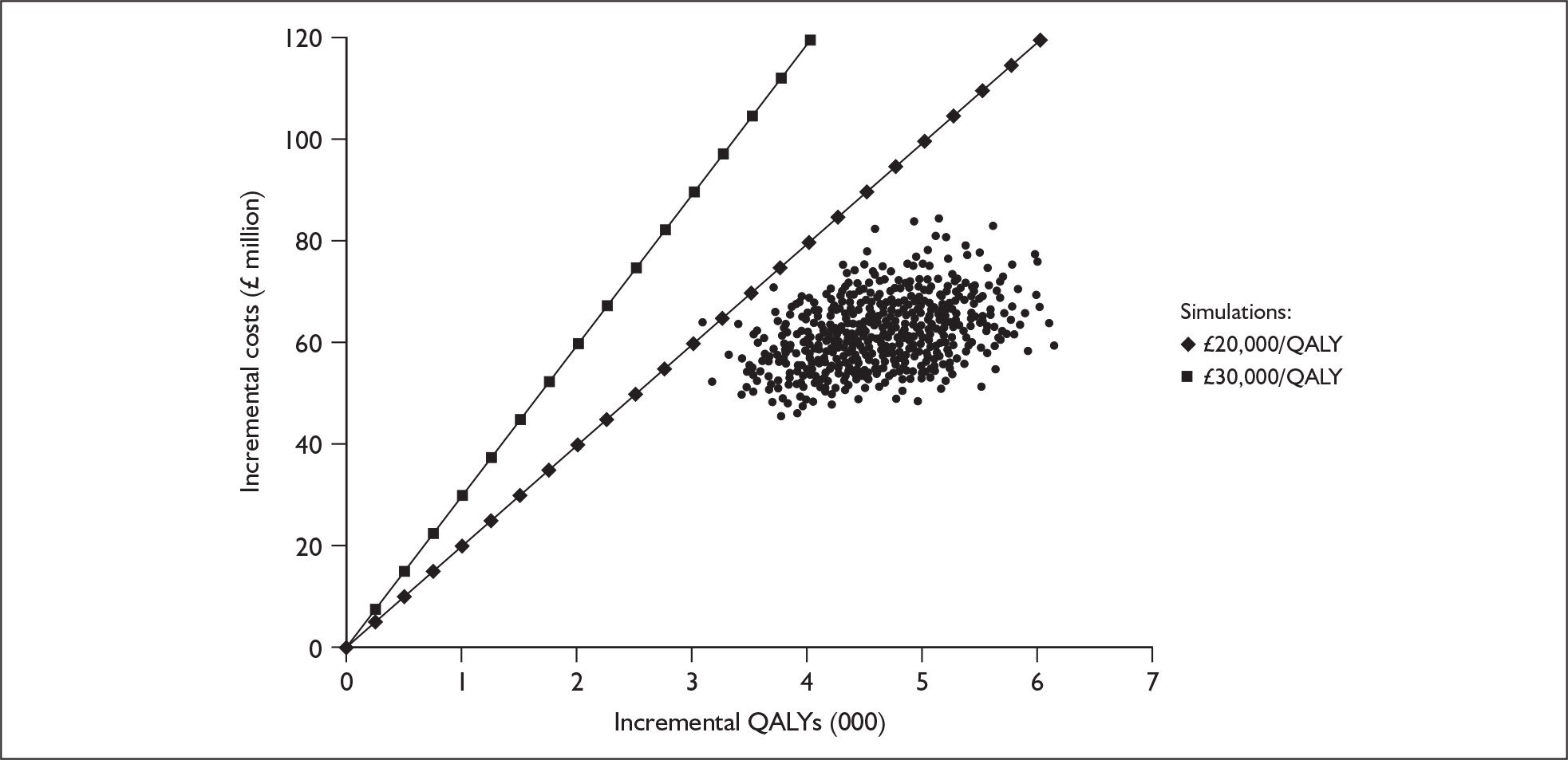
FIGURE 19.
Cost-effectiveness acceptability curves for unilateral cochlear implantation of children in comparison to non-use of cochlear implants. QALY, quality-adjusted life-year.
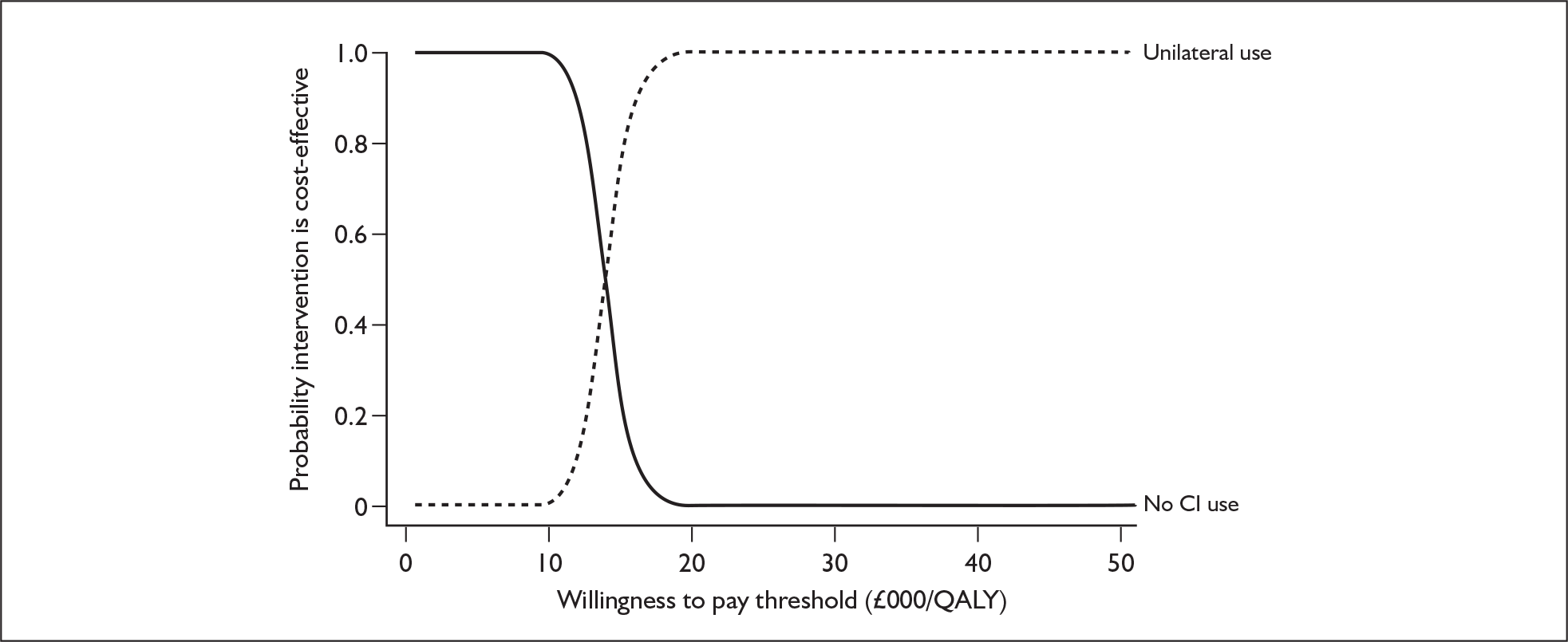
Bilateral implantation compared with unilateral implantation in prelingually implanted children
Base-case results for a cohort of children entering the precandidacy screening process at age 1 year are shown on a per-patient basis for simultaneous bilateral implantation in Table 63 and for sequential bilateral implantation in Table 64.
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| Unilateral implantation | 60,441 | 15.84 | – | – | – |
| Simultaneous bilateral implantation | 87,546 | 16.51 | 27,105 | 0.67 | 40,410 |
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| Unilateral implantation | 60,441 | 15.84 | – | – | – |
| Sequential bilateral implantation | 93,098 | 16.45 | 32,657 | 0.60 | 54,098 |
In comparison to unilateral cochlear implantation, simultaneous bilateral cochlear implantation produces an extra 0.67 QALYs. This health gain would cost the NHS £27,105 per patient to achieve.
In contrast, when compared with unilateral cochlear implantation, sequential bilateral implantation confers an additional 0.6 QALYs at an additional cost of £32,657 per person.
Because of space constraints some model outputs and uncertainty analyses will only be presented for simultaneous implantation. The overall pattern of results – that sequential bilateral implantation will generate slightly fewer QALYs and cost around £5000 more than simultaneous bilateral implantation –should be fairly stable.
Model outputs
Expected lifetime of cohort
Bilateral implantation has no significant impact on background mortality and therefore the expected lifetime of the bilateral implant cohort is exactly the same as for the unilateral cohort (79 years).
Device use
Table 65 shows the number of devices used over the course of an individual’s expected lifetime. Results for both the whole bilateral implant cohort as well as for the subset of bilateral recipients are reported.
| Whole cohort (including non-recipients) | Bilateral cochlear recipients | |
|---|---|---|
| Proportion of lifetime using two devices | 73% | 91% |
| Proportion of lifetime using one device | 4% | 5% |
| Proportion of lifetime using no devices | 23% | 4% |
The results of this analysis show that if an individual successfully receives two devices there is a 91% chance that they will remain using two devices for the whole of their lives.
Event counts
The event counts for the whole paediatric cohort as well as the subset of successful cochlear implant recipients are shown in Table 66. With the exception of voluntary non-use all model outputs represent the number of events that an individual can expect to experience over their lifetime. When relevant, results are also reported at the rate per 100 patient-years.
| Whole cohort (including non-recipients) | Bilateral cochlear implant recipients | |||
|---|---|---|---|---|
| Lifetime | Event rate/100 patient-years | Lifetime | Event rate/100 patient-years | |
| New cochlear implant internal components | 0.15 | 0.18 | 0.18 | 0.23 |
| New cochlear implant external components | 25.9 | 32.7 | 32.3 | 40.9 |
| Major complications | 0.51 | 0.64 | 0.64 | 0.81 |
| Initial implant operations | 0.8 | NA | 1.0 | NA |
| New acoustic hearing aids | 3.04 | 3.84 | 0.93 | 1.18 |
| Permanent explants | 0.07 | 0.09 | 0.09 | 0.12 |
| Voluntary non-compliance | 0.019 | 0.02 | 0.024 | 0.03 |
The corresponding per-person event counts for unilateral implantation of the same patient group are reported earlier in this chapter.
Analysis of uncertainty
Deterministic sensitivity analysis
Separate graphs are again presented for structural parameters (Figure 20), utilities (Figure 21), event-related probabilities and survival curves (Figure 22) and costs (Figure 23).
FIGURE 20 One-way sensitivity analysis for structural inputs.
Incremental cost-effectiveness ratios of paediatric simultaneous bilateral cochlear implantation compared with unilateral implantation. BC, base-case value; QALY, quality-adjusted life-year.
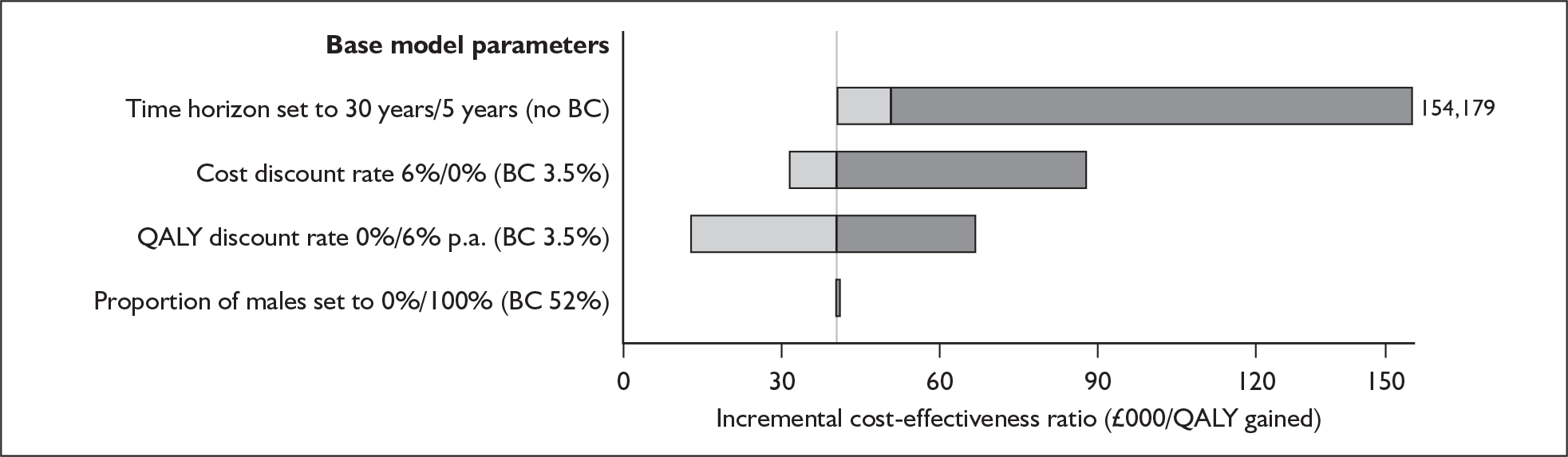
FIGURE 21 One-way sensitivity analysis for utilities.
Incremental cost-effectiveness ratios of paediatric simultaneous bilateral cochlear implantation compared with unilateral implant use. BC, base-case value; CI, cochlear implant; QALY, quality-adjusted life-year.
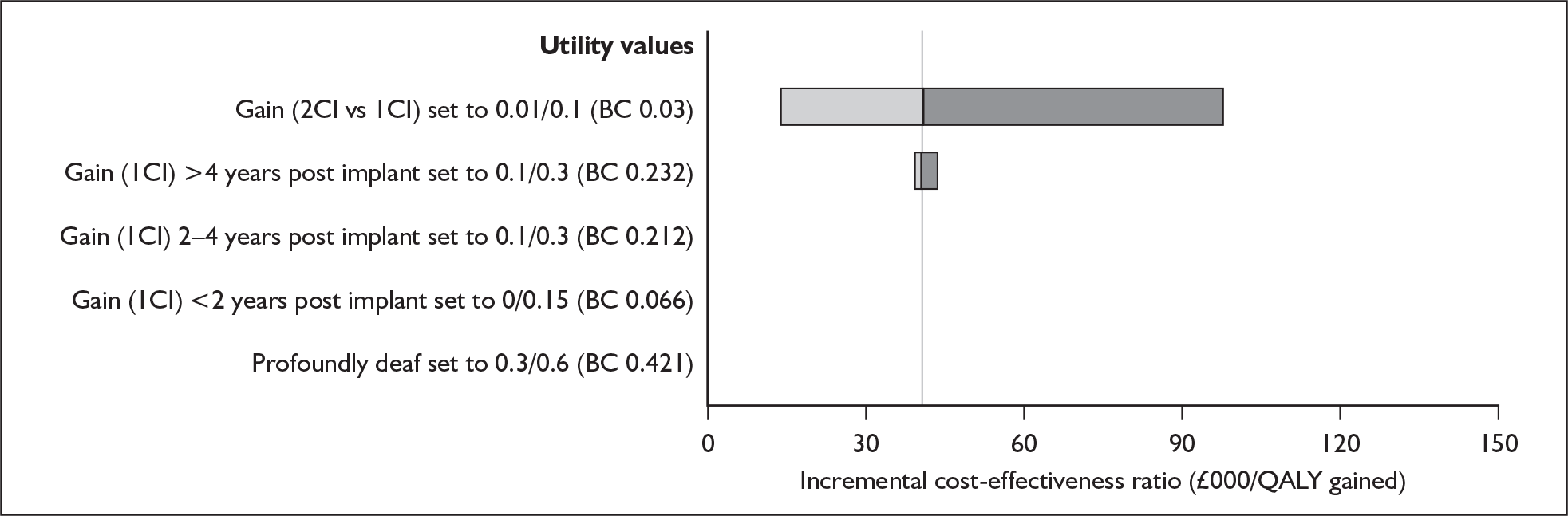
FIGURE 22 One-way sensitivity analysis for event probabilities.
Incremental cost-effectiveness ratios of simultaneous bilateral cochlear implantation compared with unilateral use. BC, base-case value; CI, cochlear implant; HA, hearing aid; QALY, quality-adjusted life-year.
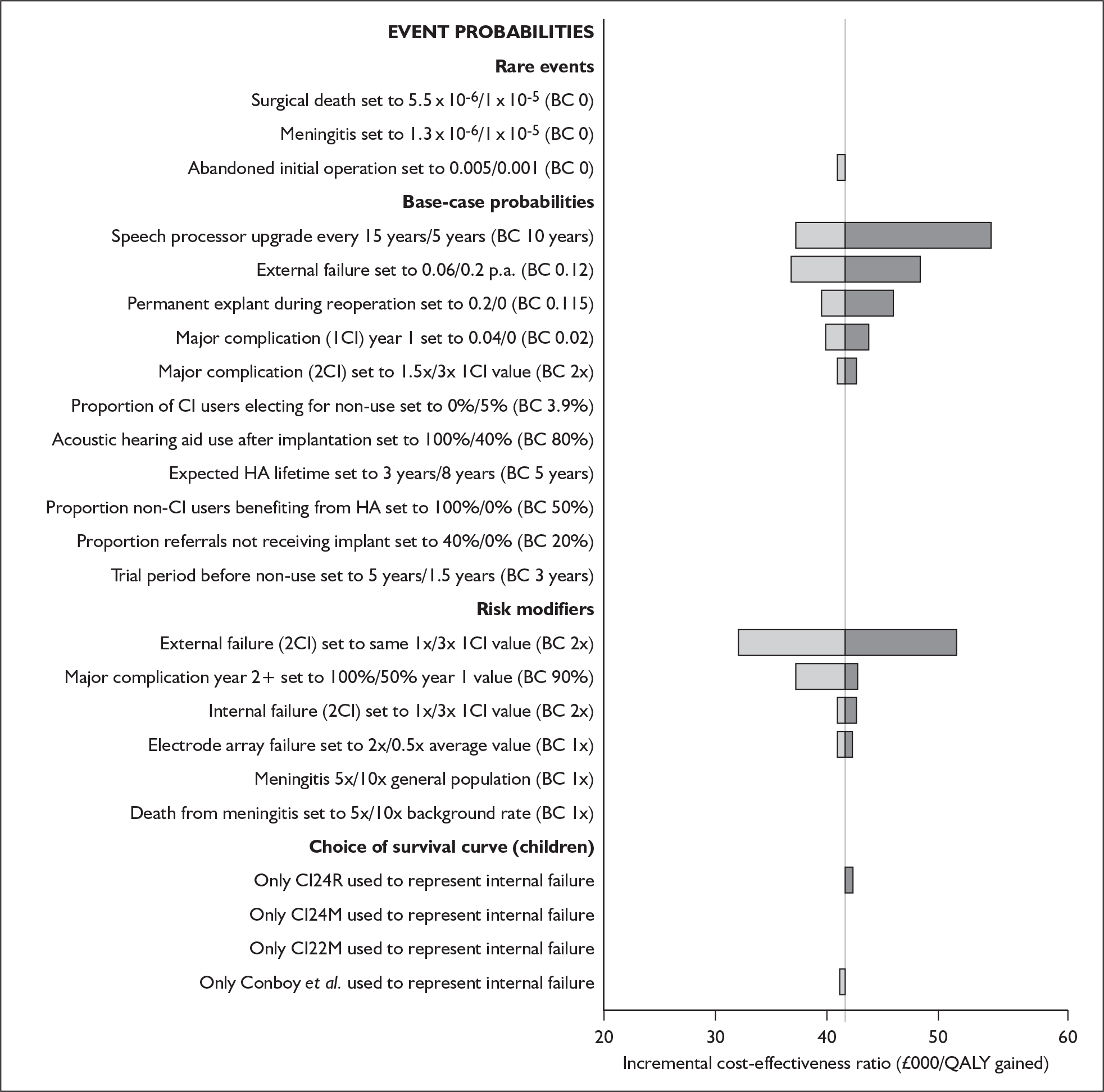
FIGURE 23 One-way sensitivity analysis for costs.
Incremental cost-effectiveness ratios of paediatric simultaneous bilateral cochlear implantation compared with unilateral use. BC, base-case value; CI, cochlear implant.
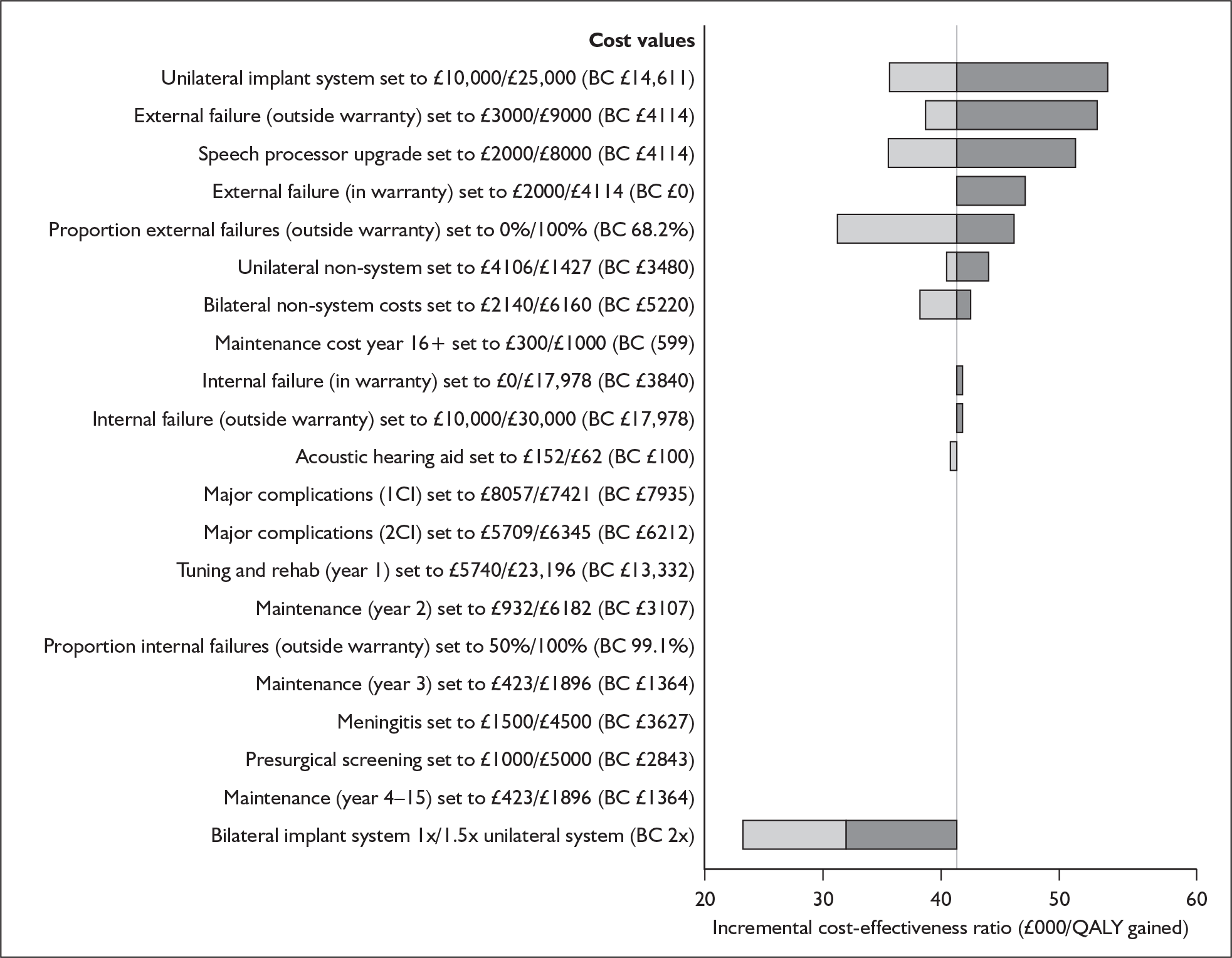
The base-case ICER appears particularly sensitive to changes in:
-
the time horizon used in the model
-
the discount rates applied to both costs and health benefits
-
the incremental utility associated with bilateral use compared with unilateral use
-
the proportion of external failures that occur outside of the 3-year warranty period
-
the price discount applied to the cost of the second implant system.
Threshold analysis
Utility gain associated with bilateral compared with unilateral device use
Analysis of the incremental utility associated with bilateral cochlear implant use compared with unilateral cochlear implant use shows that at a willingness to pay threshold of £30,000 per QALY simultaneous bilateral implantation becomes cost-effective when the utility gain associated with bilateral implantation rises above a value of approximately 0.04 (Figure 24). At a willingness to pay threshold of £20,000 per QALY bilateral implantation becomes cost-effective when the parameter value is above approximately 0.07. Both of these values are very close to the value assumed in the base case (0.03).
FIGURE 24.
Threshold analysis for paediatric utility gain associated with bilateral as opposed to unilateral cochlear implant use. QALY, quality-adjusted life-year.
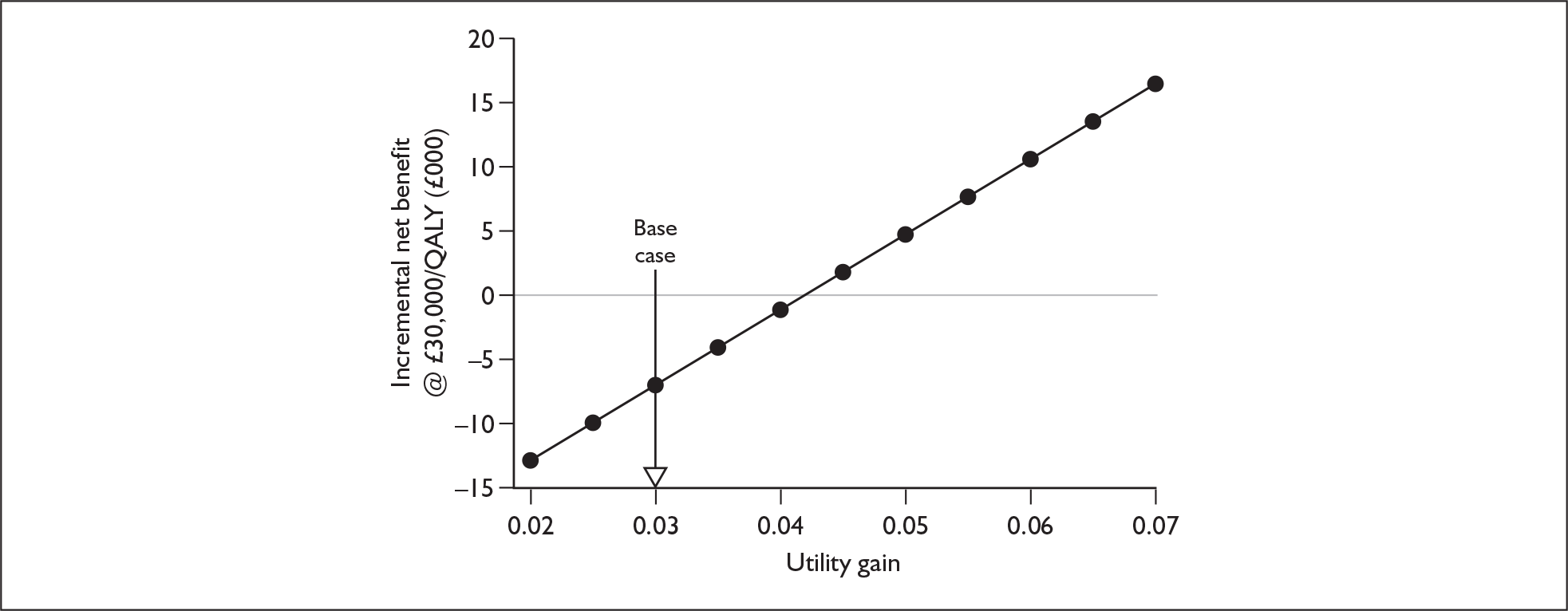
As stated in Chapter 6 (see Utility and utility changes following bilateral implantation) the 95% confidence interval for this parameter is –0.045 to +0.104. Therefore, regardless of which of the threshold values are used the model is extremely sensitive to changes in this parameter.
Although this interval may be statistically meaningful, individuals who receive two cochlear implants will only have a worse quality of life than those with only one implant if the negative impacts on utility, because of, for example, surgical complications or changes in tinnitus, are greater than the other documented benefits of binaural hearing. On current evidence, in particular the typically ameliorating impacts on tinnitus of cochlear implantation (see Utility and utility changes following bilateral implantation), it seems more reasonable to assume that health-related quality of life may increase rather than decrease with a second device. Table 67 shows the range of ICERs corresponding to positive parameter values within the confidence interval.
| Utility gain | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| –0.01 | 0 | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 | 0.06 | 0.07 | 0.08 | 0.09 | 0.10 | |
| ICER (£) | Dominated | NA | 97,340 | 57,111 | 40,410 | 31,267 | 25,498 | 21,526 | 18,625 | 16,413 | 14,670 | 13,262 |
Cost of bilateral implant system
Analysis of the cost of a bilateral implant system shows that at a willingness to pay threshold of £30,000 per QALY simultaneous bilateral implantation becomes cost-effective when a discount of approximately 60% is offered on the cost of the second implant system (Figure 25). In the base-case analysis we have assumed that no such discount exists.
FIGURE 25.
Threshold analysis of discount offered on second paediatric implant system used in simultaneous bilateral implantation. QALY, quality-adjusted life-year.
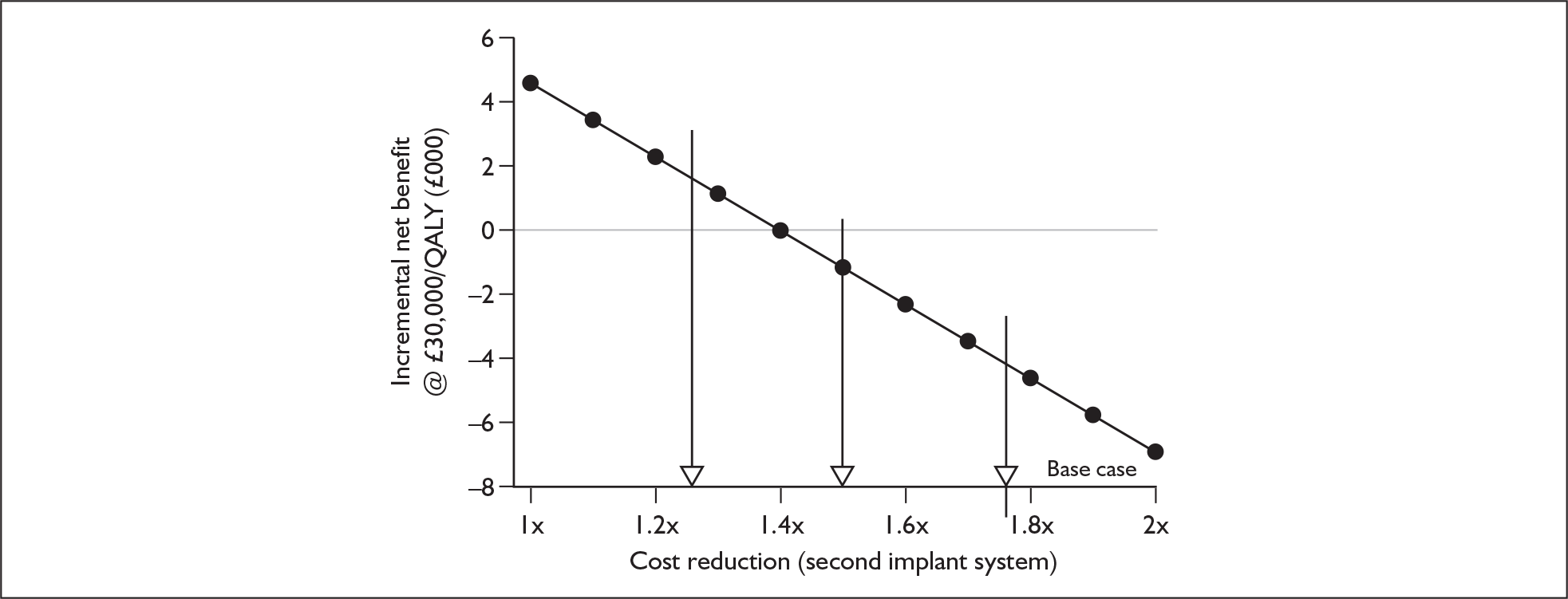
Table 68 shows the range of ICERs generated when a range of discounts are applied to the cost of an implant system.
| Discount offered on cost of second implant systema | |||||
|---|---|---|---|---|---|
| 0% | 25% | 50% | 75% | 100% | |
| Cost of bilateral implant systemb | £29,222 | £25,569 | £21,916 | £18,263 | £14,611 |
| ICER | £40,410 | £36,139 | £31,867 | £27,595 | £23,325 |
At a willingness to pay threshold of £20,000 per QALY no feasible value for system costs makes bilateral implantation appear cost-effective.
Cost of speech processor
Analysis of the cost of a speech processor shows that at a willingness to pay threshold of £30,000 per QALY bilateral implantation becomes a cost-effective alternative to unilateral implantation when the cost of a speech processor falls below approximately £2000 (Figure 26). No realistic parameter value can make bilateral cochlear implantation appear cost-effective at a willingness to pay threshold of £20,000 per QALY.
FIGURE 26.
Threshold analysis for speech processor costs in children. QALY, quality-adjusted life-year.
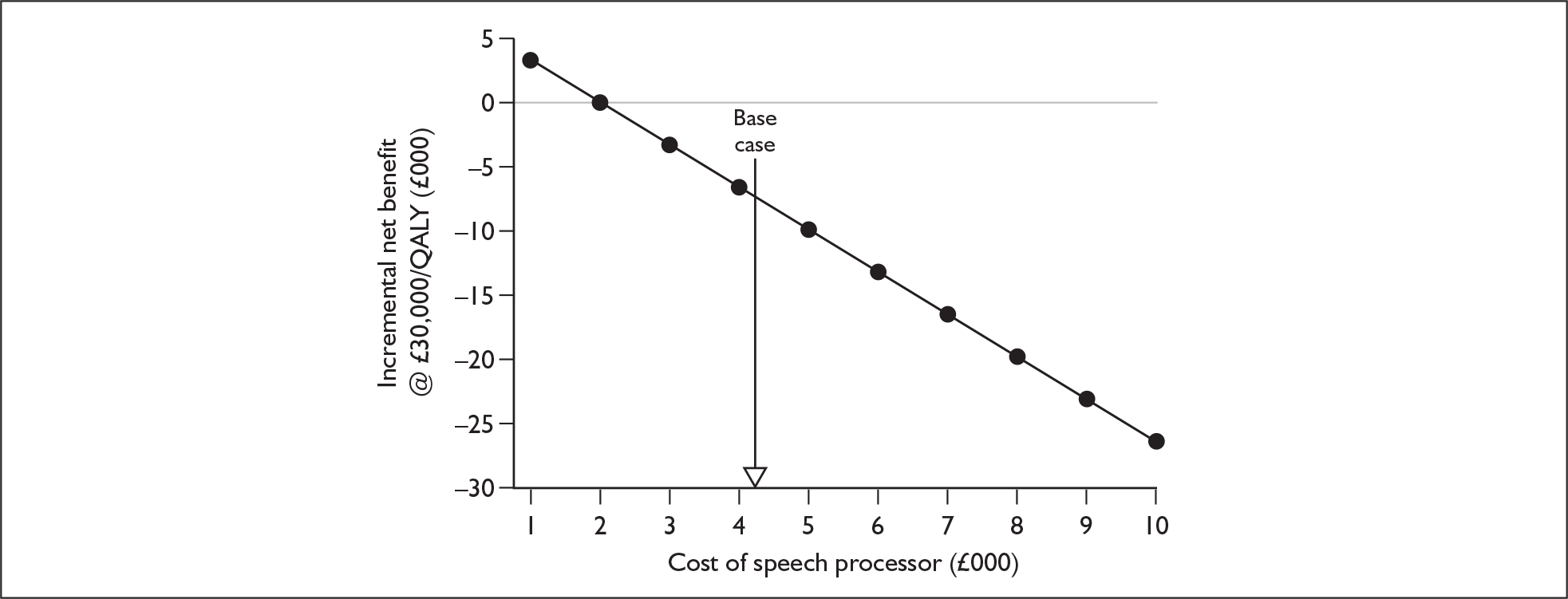
Cost of specific implant systems
Figure 27 shows the range of ICERs for simultaneous bilateral implantation of children aged 1 year generated by varying the cost of a unilateral implant system. No price discount on the cost of a second system has been applied (i.e. bilateral implant system cost is twice the cost of unilateral implant system). No specific devices appeared cost-effective at £30,000 per QALY; however, the cheapest implant/processor combination reduced the ICER from around £40,500 to approximately £37,500.
FIGURE 27.
Device-specific incremental cost-effectiveness ratios (ICERs) in paediatric bilateral implantation (see Table 2 for actual prices in current NHS Supply Chain contract). A: Advanced Bionics CLARION® ICS HiRes 90K; B: Advanced Bionics CLARION® HiRes 90K with HiFocus Helix; C: Cochlear Europe Nucleus® CI24R (ST) ‘K’ with a Sprint or ESPrit 3G Processor; D: Cochlear Europe Nucleus® CI24R (CA) Advanced with a Sprint or ESPrit 3G Processor; E: Cochlear Europe Nucleus® CI11 + 11 + 2 double array with a Sprint or ESPrit 3G Processor; F: Cochlear Europe Nucleus® Freedom with either BTE or BWP option; G: Cochlear Europe Nucleus® Freedom with both BTE and BWP option; H: MED-EL UK Pulsar CI-100; I: Neurelec DIGISONIC SP with Digi SP or Digi SP*K.
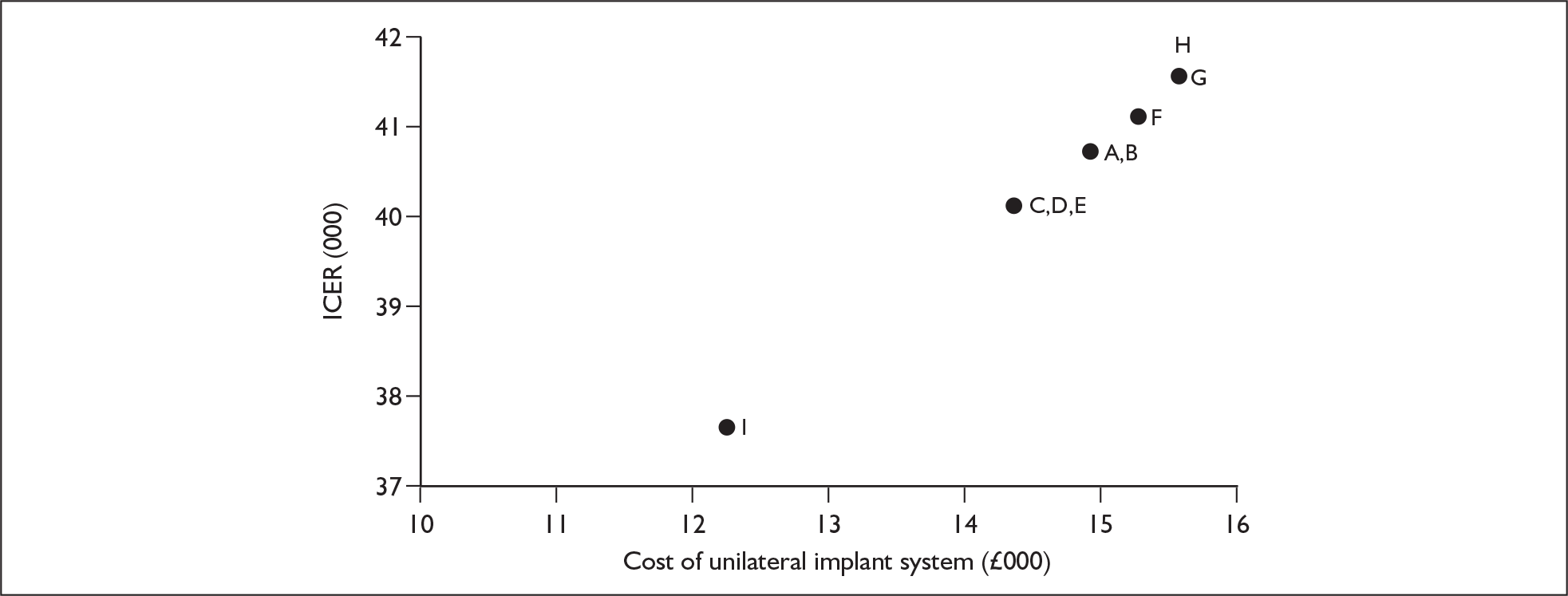
Probabilistic sensitivity analysis
Simultaneous bilateral implantation versus unilateral implantation
The simulation output (based on 1000 runs of the model) shows that, for profoundly deaf, non-cochlear implanted children, at a willingness to pay threshold of £20,000 per QALY simultaneous provision of two cochlear implants is cost-effective in 16.6% of simulations; at £30,000 per QALY it is cost-effective in 34.9% of simulations.
At a willingness to pay threshold of £30,000 per QALY simultaneous bilateral implantation was dominated in 16.9% of simulations (creating higher costs compared with unilateral implantation but lower QALYs). The probabilistic mean incremental net benefit is –£7990 (95% Cr I –£9375 to –£6605) and the probabilistic median incremental net benefit is –£7400.
Outputs from the Monte Carlo simulation are shown graphically in Figure 28, and the CEACs for simultaneous bilateral cochlear implantation are shown in Figure 29. The CEACs show that simultaneous bilateral implantation would be considered cost-effective only if the willingness to pay threshold was increased beyond approximately £41,000 per QALY.
FIGURE 28.
Simulation output (cohort based, 1000 trials) for the cost-effectiveness of simultaneous bilateral implantation in comparison to unilateral implantation in profoundly deaf children not using cochlear implants. QALY, quality-adjusted life-year.
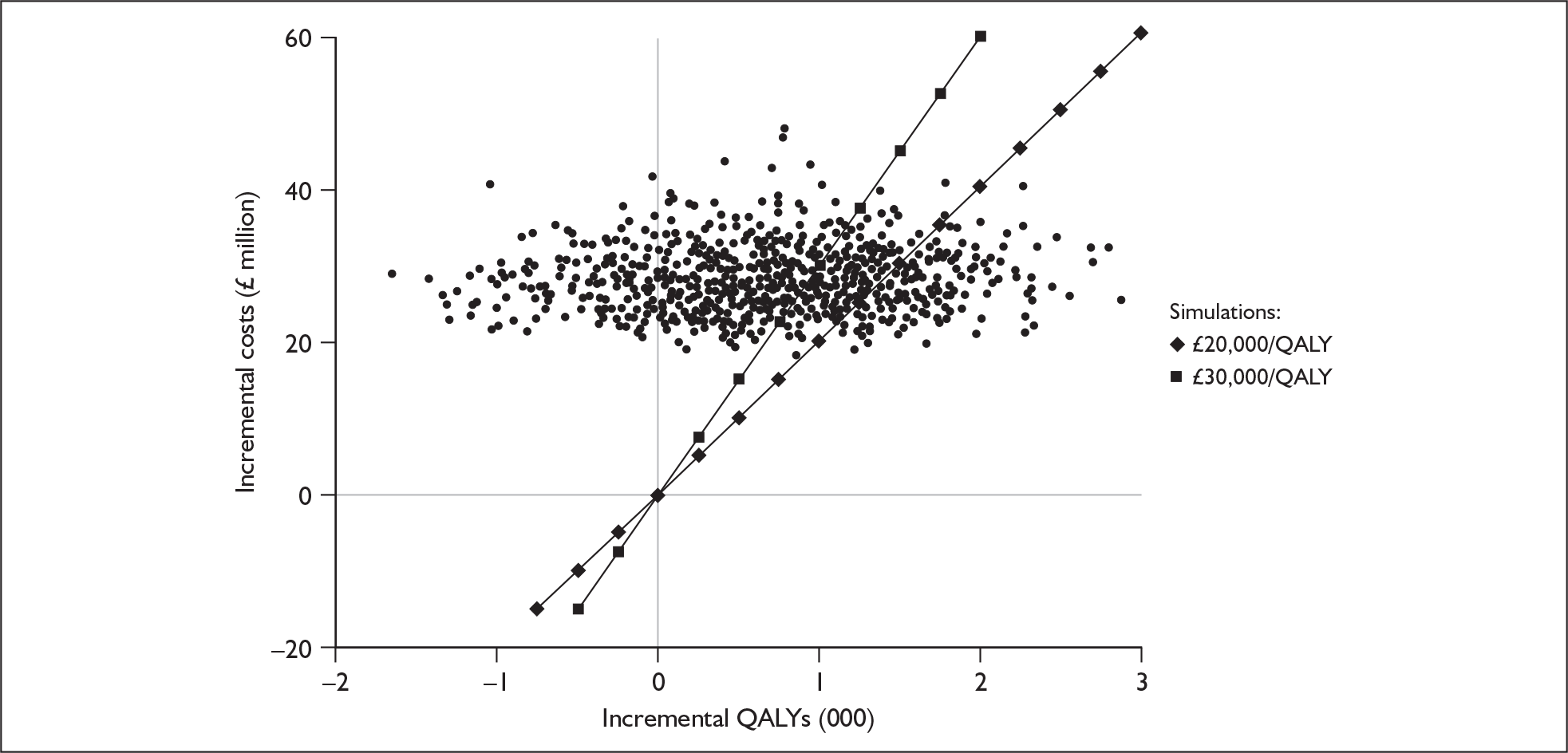
FIGURE 29.
Cost-effectiveness acceptability curves for simultaneous bilateral implantation vs unilateral cochlear implantation in profoundly deaf children not using cochlear implants. QALY, quality-adjusted life-year.
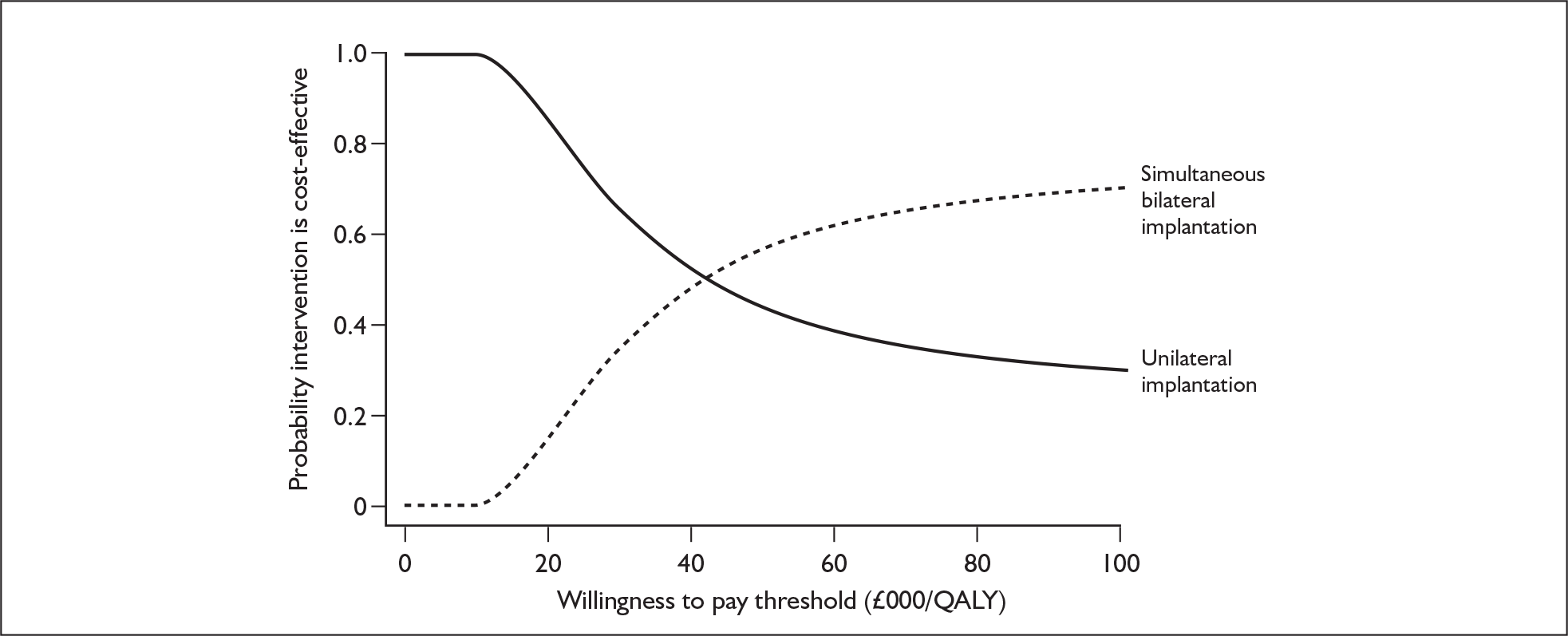
Sequential bilateral implantation versus unilateral implantation
The simulation output (based on 1000 runs of the model) shows that, for profoundly deaf, non-cochlear implanted children, at a willingness to pay threshold of £20,000 per QALY sequential provision of two cochlear implants is cost-effective in 5.5% of simulations; at £30,000 per QALY it is cost-effective in 21.3% of simulations.
At a willingness to pay threshold of £30,000 per QALY sequential bilateral implantation was dominated in 16.2% of simulations (creating higher costs compared with unilateral implantation but lower QALYs). The probabilistic mean incremental net benefit is –£15,548 (95% Cr I –£16,793 to –£14,303) and the probabilistic median incremental net benefit is –£14,739.
Outputs from the Monte Carlo simulation are shown graphically in Figure 30, and the CEACs for sequential bilateral cochlear implantation are shown in Figure 31. The CEACs show that sequential bilateral implantation would be considered cost-effective only if the willingness to pay threshold was increased beyond approximately £55,000 per QALY.
FIGURE 30.
Simulation output (cohort based, 1000 trials) for the cost-effectiveness of sequential bilateral implantation in comparison to unilateral implantation in profoundly deaf children. QALY, quality-adjusted life-year.
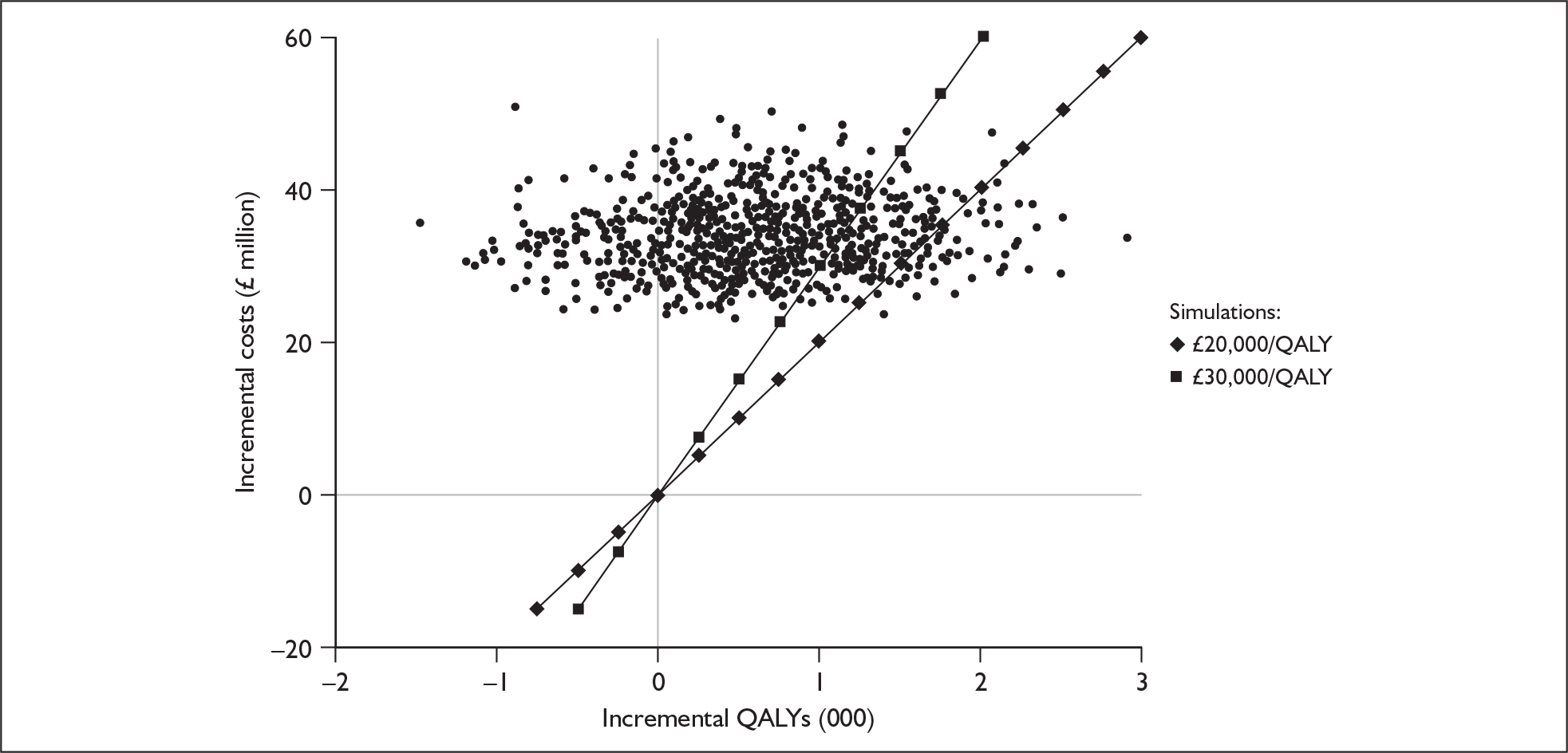
FIGURE 31.
Cost-effectiveness acceptability curves for sequential bilateral implantation vs unilateral cochlear implantation in profoundly deaf children. QALY, quality-adjusted life-year.
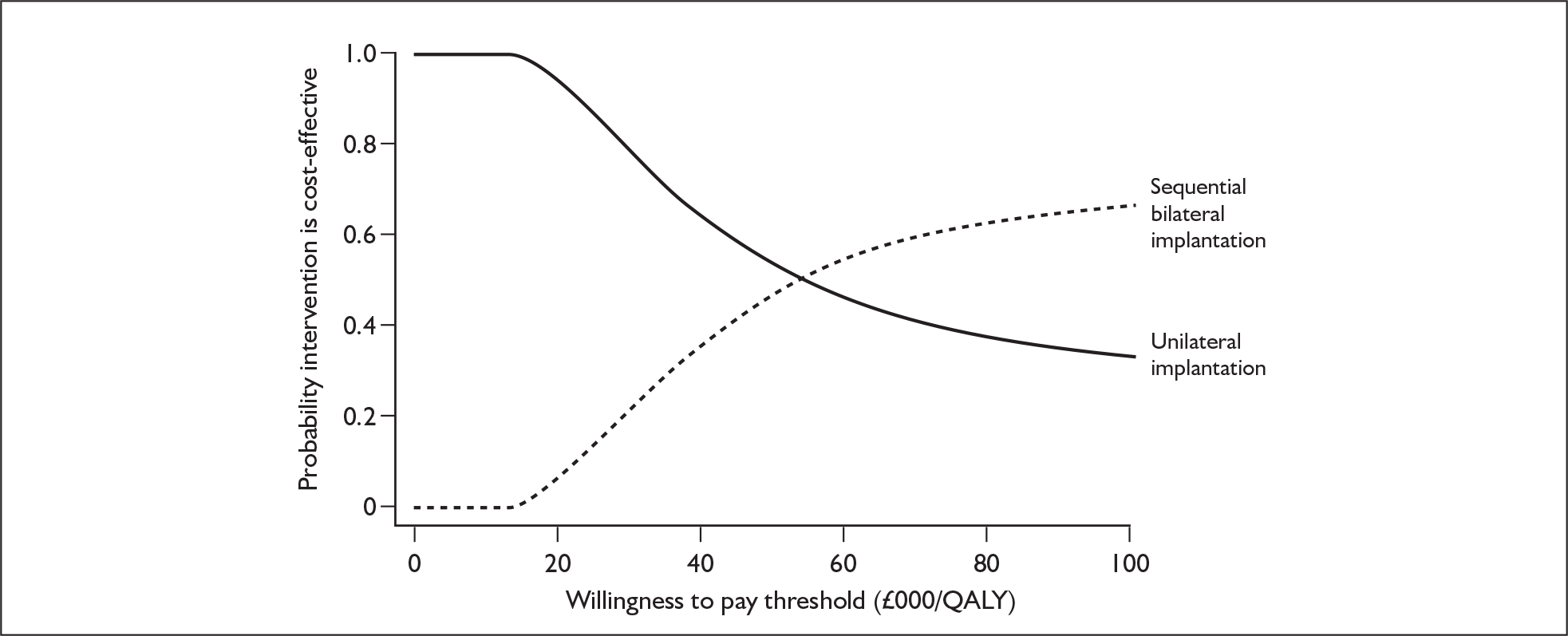
Scenario analyses
Cost-effectiveness of paediatric cochlear implantation assuming no product warranties
We examined the impact on cost-effectiveness of the scenario in which product warranties (i.e. free repairs and replacements for a number of years) are no longer offered. The results are shown in Tables 69 and 70. Without warranties the ICER increases by approximately 7% for unilateral implantation in comparison to no cochlear implantation and by approximately 15% for bilateral implantation compared with unilateral implantation. However, all of the previous uncertainties surrounding discounts and incremental utility remain.
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| No cochlear implant use | 371 | 11.36 | – | – | – |
| Unilateral implantation | 64,491 | 15.84 | 64,120 | 4.48 | 14,317 |
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| Unilateral implantation | 64,491 | 15.84 | – | – | – |
| Simultaneous bilateral implantation | 95,647 | 16.51 | 31,156 | 0.67 | 46,449 |
Early unilateral implantation of children (including educational costs)
As discussed in Chapter 4 (see Educational outcomes, Review of educational studies), early implantation of children leads to a greater number attending normal schools as opposed to schools for the deaf. From a societal perspective this leads to savings in educational costs. Although not a reference case analysis, an estimate of the cost-effectiveness of unilateral implantation when these cost savings are introduced can be made.
For the comparison of unilateral implantation with non-cochlear implant use the results for a cohort of non-cochlear implant users entering the precandidacy screening process at age 1 year are shown in Table 71. As with the reference case analysis, in comparison to no cochlear implant use, unilateral implantation confers an extra 4.48 QALYs. However, the costs incurred over an individual’s lifetime fall from £60,070 to £44,403. This leads to the ICER falling from £13,413 per QALY to £9,915 per QALY.
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| No cochlear implant use | 371 | 11.36 | – | – | – |
| Unilateral implantation | 44,774 | 15.84 | 44,403 | 4.48 | 9915 |
No information was found in which the impact of bilateral implantation on schooling was reported. However, it seems reasonable to assume that the impact on schooling when two devices are used is at least as large as the impact with one device. Therefore, assuming that the same cost savings apply to this patient group, the ICER falls from £40,410 per QALY to £40,185 per QALY (Table 72).
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| Unilateral implantation | 44,774 | 15.84 | – | – | – |
| Simultaneous bilateral Implantation | 71,728 | 16.51 | 26,954 | 0.67 | 40,185 |
Differential results for paediatric subgroups
Profoundly deaf children implanted later in childhood
Base-case results for a cohort of non-cochlear implant users entering the precandidacy screening process at age 8 years are shown for unilateral implantation in Table 73 and for simultaneous bilateral implantation in Table 74.
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| No cochlear implant use | 364 | 11.18 | – | – | – |
| Unilateral implantation | 57,197 | 15.06 | 56,832 | 3.88 | 14,665 |
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| Unilateral implantation | 57,197 | 15.06 | – | – | – |
| Simultaneous bilateral implantation | 83,917 | 15.70 | 26,721 | 0.64 | 41,501 |
In comparison to no cochlear implant use, unilateral implantation confers an extra 3.88 QALYs. This improvement would cost the NHS £56,832 per patient to achieve. In contrast, when compared with unilateral cochlear implantation, simultaneous bilateral implantation confers an additional 0.64 QALYs at an additional cost of £26,721 per person.
Results of cost-effectiveness in adults
Unilateral implantation compared with best standard care
Base-case results produced by the decision model for a cohort of postlingually deafened adults entering the candidacy screening process at age 50 years are shown in Table 75. In comparison to no cochlear implantation the provision of unilateral cochlear implantation provides an extra 2.4 QALYs. This improvement would cost the NHS an extra £33,959 per patient to achieve.
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| No cochlear implant use | 248 | 8.20 | – | – | – |
| Unilateral cochlear implant use | 34,207 | 10.60 | 33,959 | 2.40 | 14,163 |
The ICER suggests that unilateral cochlear implantation may be slightly more cost-effective in adults than in children. The reasons for this appear to be that in the first few years post implantation children incur higher tuning and maintenance costs than adults, and that adults have a larger, fixed gain in health-related quality of life. In contrast, in children this gain is time dependant and lower in the first few years than the fixed value used for adults.
Model outputs
Expected lifetime of cohort
Simulated adults survive to a mean age of 82 years, similar to mortality in government actuarial life tables. 203 This value is not the same as the one used in the analyses of prelingually deafened children for reasons of differences in gender mix and also the fact that individuals who have survived to the age of 50 years have an older expected age of death than those who have survived to the age of 1 year. The assumption is again made that neither deafness nor the evaluated technologies carry with them an increased mortality risk. The expected lifetime over which events occur is therefore 32 years.
Event counts
The model outputs for the whole adult cohort as well as the subset of successful cochlear implant recipients are shown in Table 76. With the exception of voluntary non-use, all model outputs represent the number of events that an individual can expect to experience over their lifetime. When relevant, results are also reported as the rate per 100 patient-years.
| Whole cohort (including non-recipients) | Unilateral cochlear implant recipients | |||
|---|---|---|---|---|
| Lifetime | Event rate/100 patient-years | Lifetime | Event rate/100 patient-years | |
| New cochlear implant internal components | 0.02 | 0.05 | 0.02 | 0.08 |
| New cochlear implant external components | 4.45 | 13.77 | 6.36 | 19.67 |
| Major complications | 0.10 | 0.3 | 0.14 | 0.44 |
| Initial implant operations | 0.7 | NA | 1.0 | NA |
| New acoustic hearing aids | 4.24 | 13.11 | 4.15 | 12.85 |
| Permanent explants | 0.01 | 0.04 | 0.02 | 0.06 |
| Voluntary non-compliance | 0.016 | 0.05 | 0.023 | 0.07 |
A separate cohort is used to generate results for adults not using any form of cochlear implant. The only event such individuals can experience is the replacement of an acoustic hearing aid. An individual can expect to receive 4.4 new acoustic hearing aids over the course of their lifetime.
Analysis of uncertainty
Deterministic sensitivity analysis
Results are again presented separately for structural parameters (Figure 32), utilities (Figure 33), event-related probabilities and survival curves (Figure 34) and costs (Figure 35).
FIGURE 32.
One-way sensitivity analysis for structural inputs. Incremental cost-effectiveness ratios of adult unilateral cochlear implantation compared with no cochlear implant use. BC, base-case value; QALY, quality-adjusted life-year.
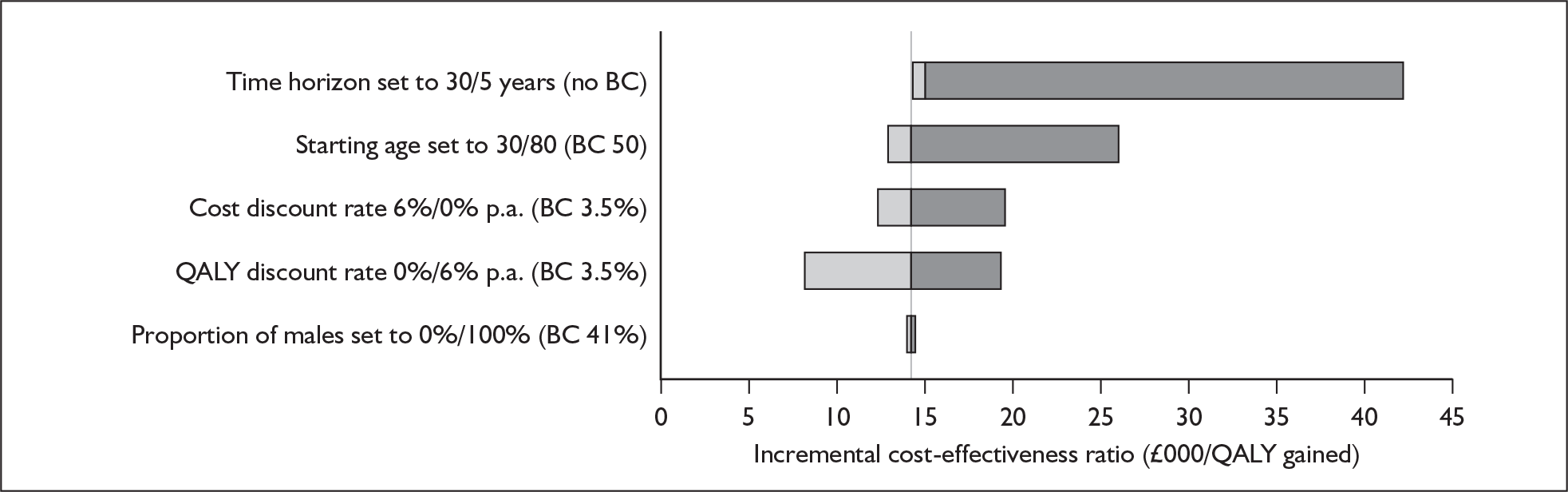
FIGURE 33.
One-way sensitivity analysis for utilities. Incremental cost-effectiveness ratios of adult unilateral cochlear implantation compared with no cochlear implant use. BC, base-case value; CI, cochlear implant; QALY, quality-adjusted life-year.
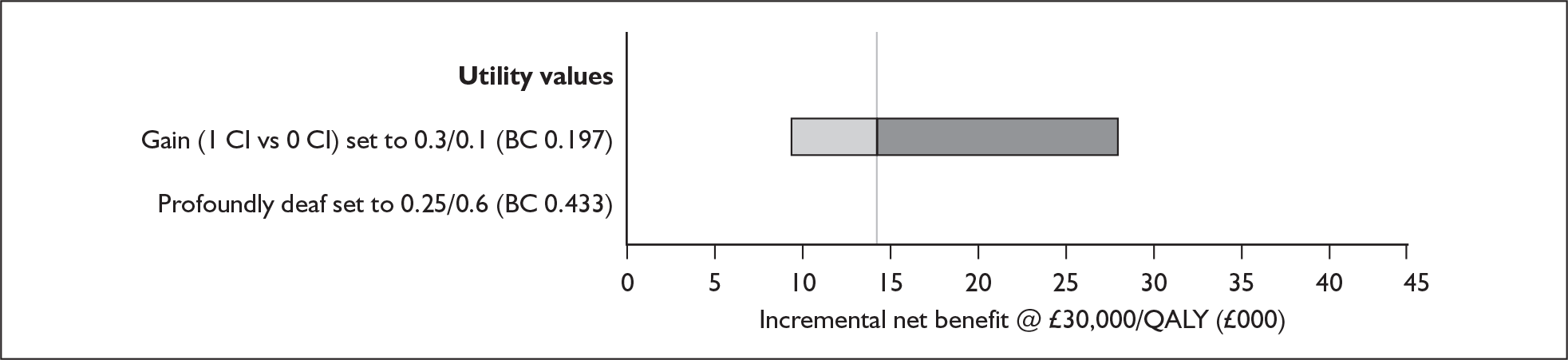
FIGURE 34.
One-way sensitivity analysis for event probabilities. Incremental cost-effectiveness ratios of adult unilateral cochlear implantation compared with no cochlear implant use. BC, base-case value; CI, cochlear implant; HA, hearing aid; QALY, quality-adjusted life-year.
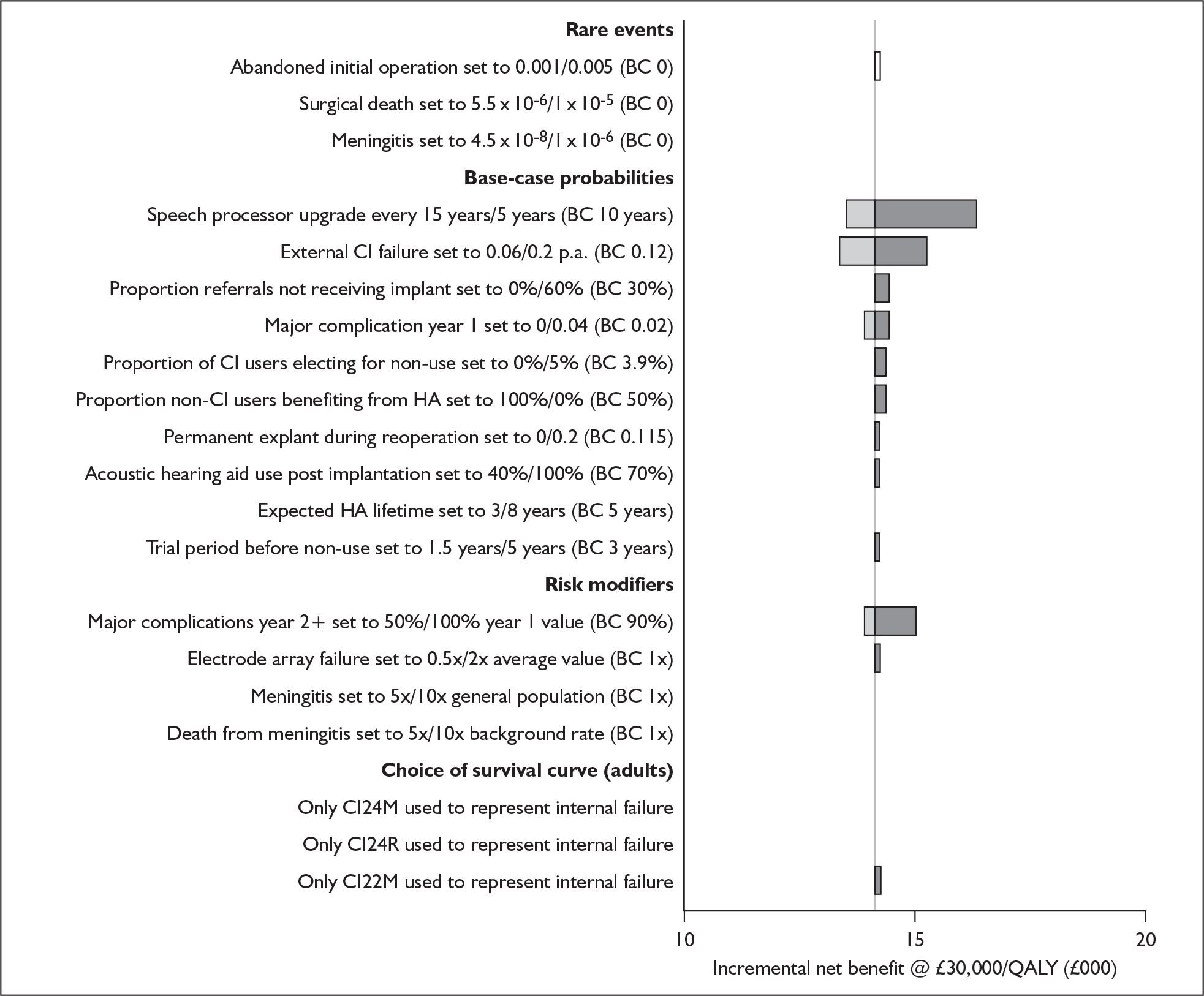
FIGURE 35.
One-way sensitivity analysis for costs. Incremental cost-effectiveness ratios of adult unilateral cochlear implantation compared with no cochlear implant use. BC, base-case value; CI, cochlear implant; QALY, quality-adjusted life-year.
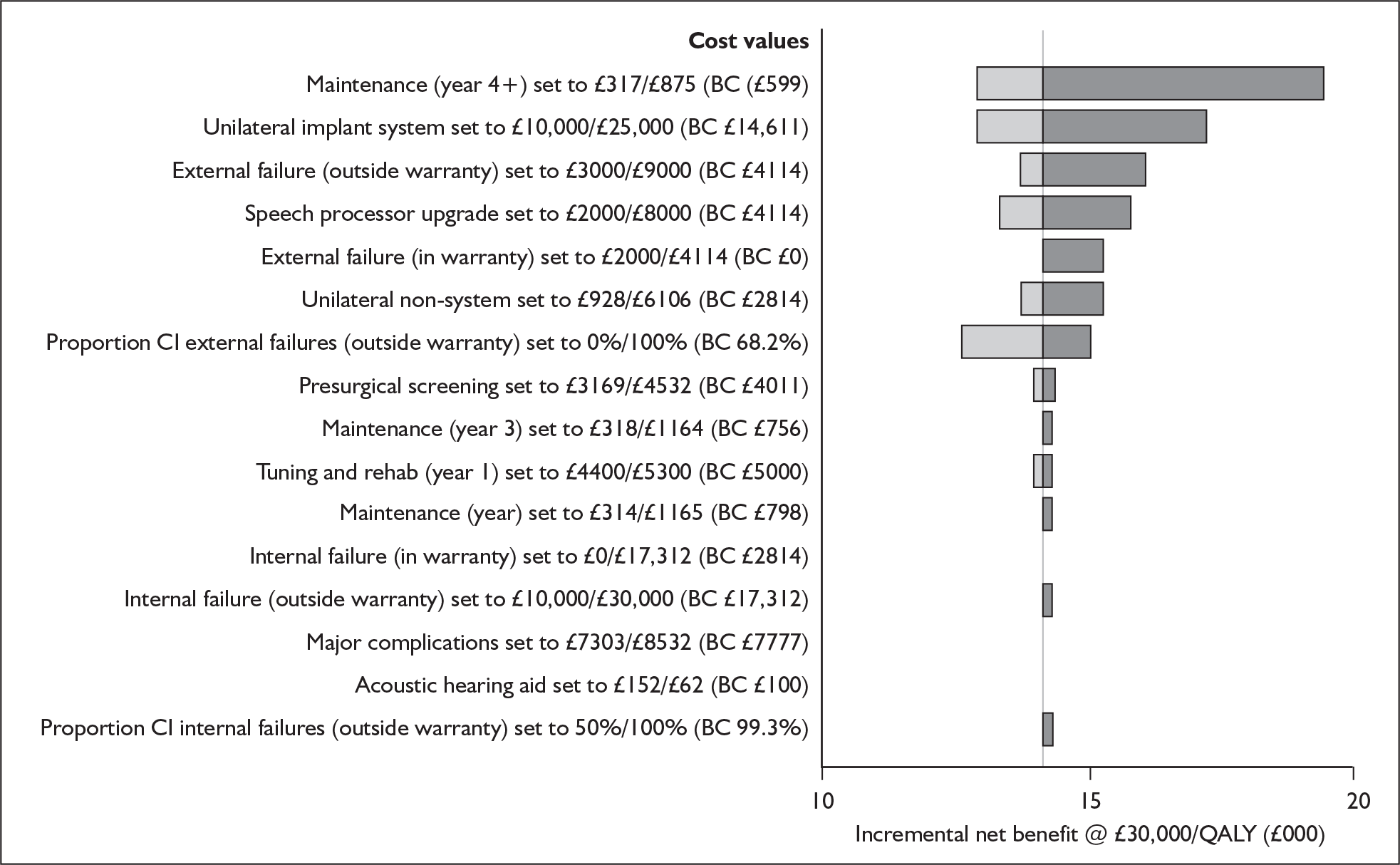
In this analysis of the effect of changes in individual parameters on the cost-effectiveness of unilateral cochlear implantation in adults compared with no cochlear implant use the base-case ICER appears particularly sensitive to changes in the following parameters:
-
time horizon used in the model
-
annual discount rate applied to health benefits
-
starting age of the cohort
-
incremental utility associated with unilateral use compared with implant non-use.
Threshold analyses
We considered the imposition of a fixed time horizon, the starting age of the cohort and the incremental utility gain because the model is particularly sensitive to these parameters.
Cohort starting age
Threshold analysis of the starting age of the adult cohort shows that at a willingness to pay threshold of £30,000 per QALY unilateral implantation represents a cost-effective treatment option for all realistic input values (Figure 36). At a willingness to pay threshold of £20,000 per QALY unilateral implantation ceases to appear cost-ineffective when the cohort starting age increases above approximately 70 years.
FIGURE 36.
Threshold analysis for starting age of adult cohort (unilateral implantation). QALY, quality-adjusted life-year.
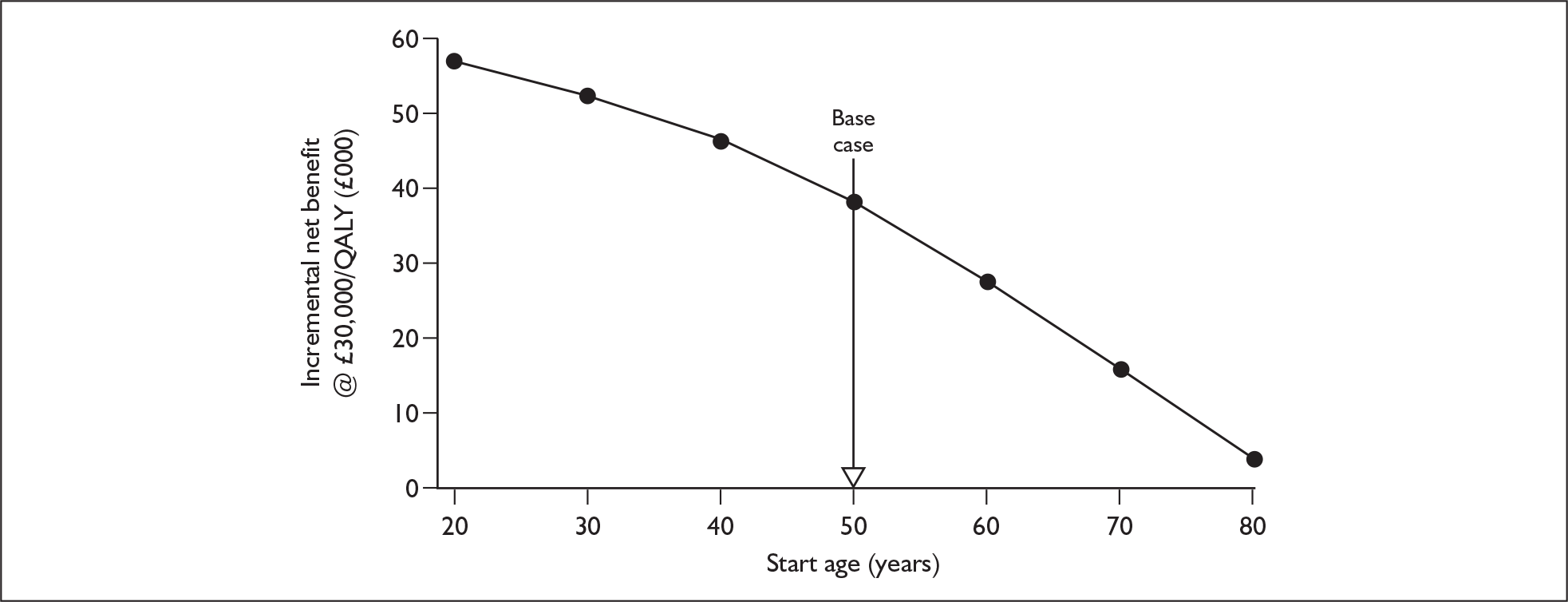
Utility gain associated with unilateral cochlear implant use compared with no implant use
Figure 37 shows that at a willingness to pay threshold of £30,000 per QALY unilateral implantation becomes cost-ineffective only when the utility gain associated with unilateral cochlear implantation as opposed to no implant use falls below a value of approximately 0.1. At a willingness to pay threshold of £20,000 per QALY unilateral implantation becomes cost-ineffective below a value of approximately 0.15.
FIGURE 37.
Threshold analysis for utility gain associated with unilateral cochlear implant use compared with no device use in adults.
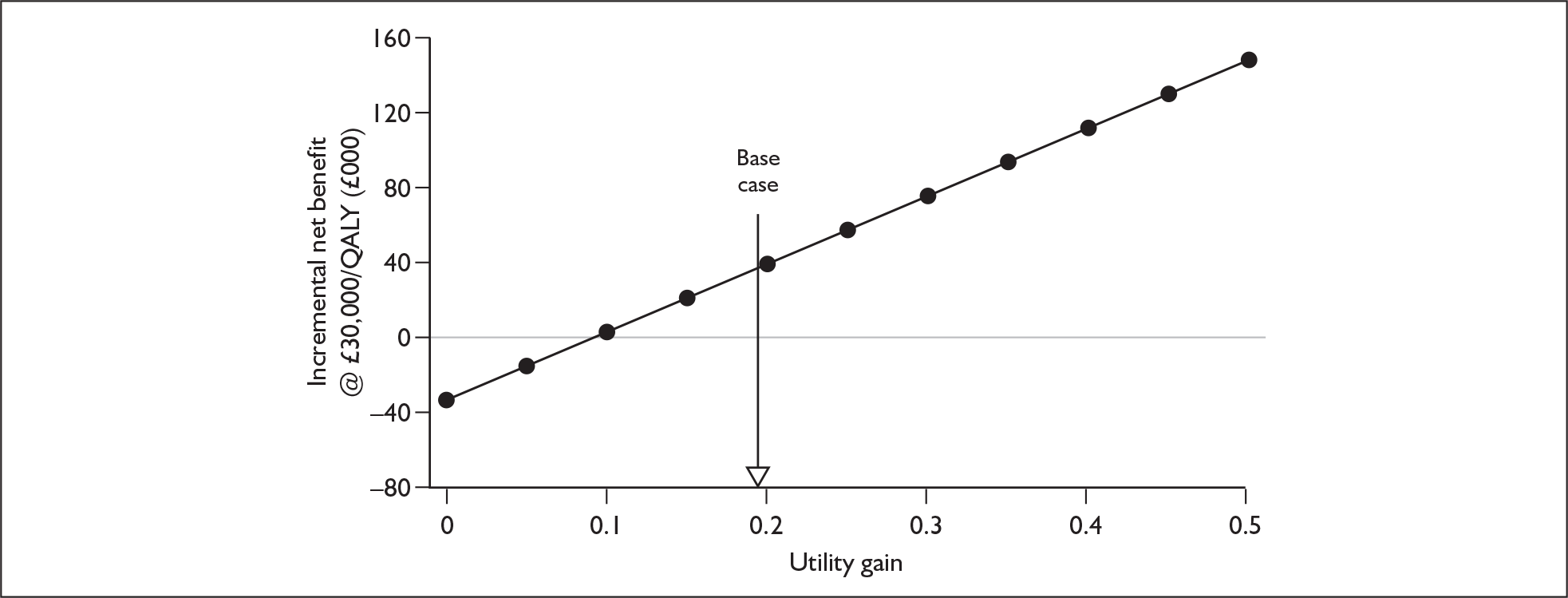
Model time horizon
The cost-effectiveness of unilateral cochlear implantation of adults at various time points is shown in Figure 38. At a willingness to pay threshold of £30,000 per QALY the procedure becomes cost-effective after approximately 8 years. At a willingness to pay threshold of £20,000 per QALY the procedure becomes cost-effective after approximately 14 years.
FIGURE 38.
Threshold analysis for model time horizon associated with unilateral cochlear implantation of adults at age 50 years. QALY, quality-adjusted life-year.
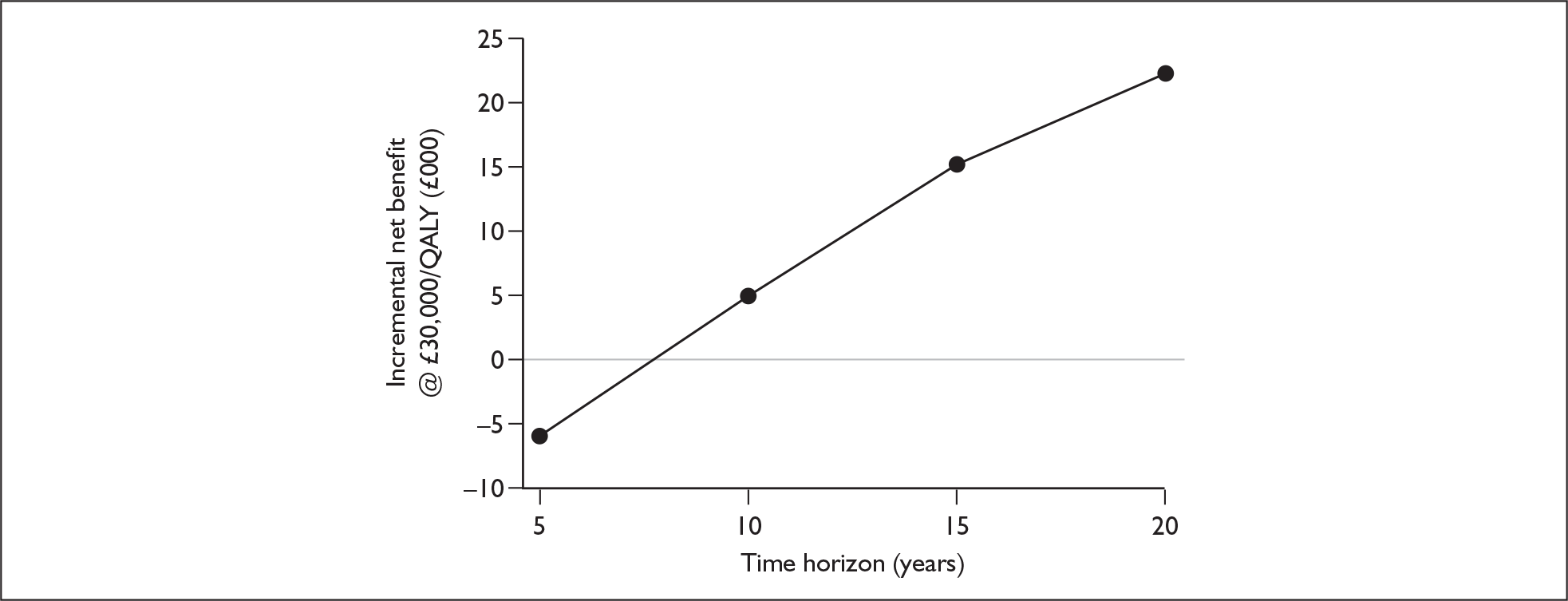
Probabilistic sensitivity analysis
The simulation output (based on 1000 runs of the model) shows that at both £20,000 per QALY and £30,000 per QALY unilateral implantation of profoundly deaf adults is cost-effective in 100% of simulations.
At a willingness to pay threshold of £30,000 per QALY unilateral implantation was dominated in 0% of simulations (creating higher costs compared with no cochlear implant use but lower QALYs). The probabilistic mean incremental net benefit is £37,390 (95% Cr I £36,999–37,781) and the probabilistic median incremental net benefit is £37,131.
Outputs from the Monte Carlo simulation are shown graphically in Figure 39, and the CEACs are shown in Figure 40. The CEACs show that unilateral implantation would be considered cost-effective only if the willingness to pay threshold was increased beyond approximately £14,500 per QALY.
FIGURE 39.
Simulation output (cohort based, 1000 trials) for the cost-effectiveness of unilateral cochlear implantation of adults in comparison to no cochlear implant use. QALY, quality-adjusted life-year.
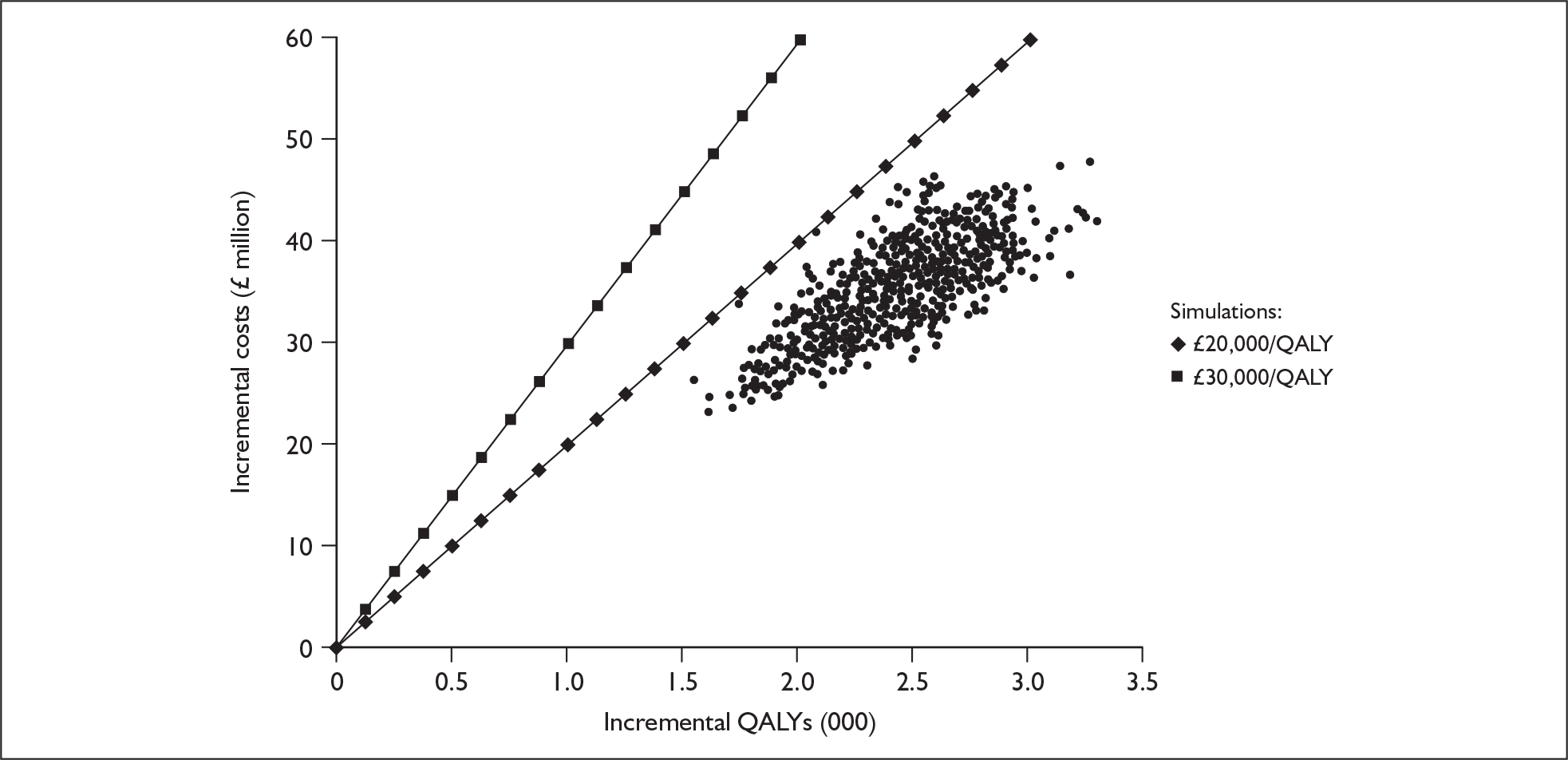
FIGURE 40.
Cost-effectiveness acceptability curves for unilateral cochlear implantation of adults vs non-cochlear implant use. QALY, quality-adjusted life-year.
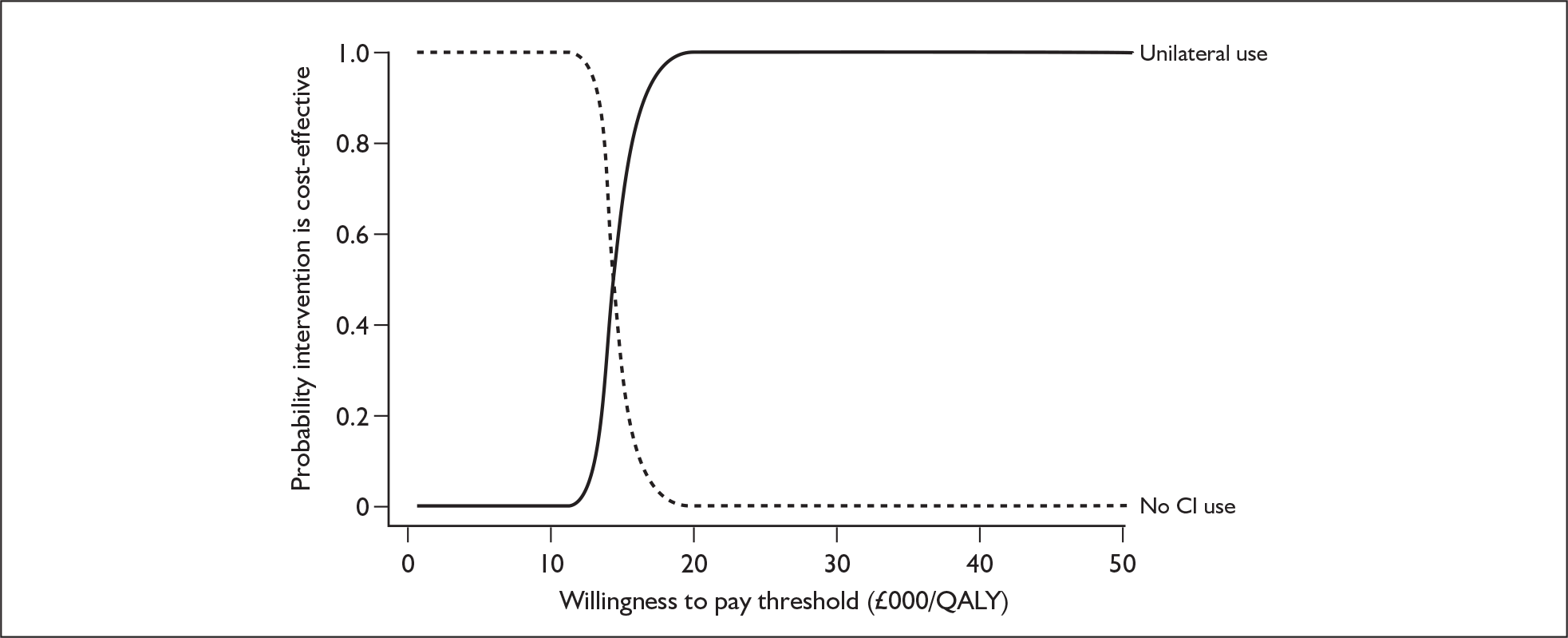
Scenario analysis
Cost-effectiveness of unilateral implantation compared with non-use of cochlear implants (age-dependant utility gain)
The results for this scenario are summarised in Table 77. Overall, the ICER is 7.5% higher than that generated in the base-case scenario.
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| No cochlear implant use | 248 | 7.66 | – | – | – |
| Unilateral implantation | 34,207 | 9.89 | 33,959 | 2.23 | 15,226 |
Bilateral implantation compared with unilateral implantation
Base-case results produced by the decision model for a cohort of postlingually deafened adults entering the candidacy screening process at age 50 years are shown for simultaneous bilateral implantation in Table 78 and for sequential bilateral implantation in Table 79.
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| Unilateral implantation | 34,207 | 10.60 | – | – | – |
| Simultaneous bilateral implantation | 53,255 | 10.99 | 19,048 | 0.38 | 49,559 |
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| Unilateral implantation | 34,207 | 10.60 | – | – | – |
| Sequential bilateral implantation | 53,886 | 10.93 | 19,678 | 0.33 | 60,301 |
In comparison to unilateral cochlear implantation, simultaneous bilateral cochlear implantation provides an extra 0.38 QALYs. This improvement would cost the NHS an additional £19,048 per patient to achieve.
In contrast, when also compared with unilateral cochlear implantation, sequential bilateral implantation confers an additional 0.33 QALYs at an additional cost of £19,678 per person.
As with the analysis of paediatric implantation, all of the following results refer to simultaneous bilateral implantation unless otherwise stated.
Model outputs
Expected lifetime of cohort
Bilateral implantation has no significant impact on background mortality and therefore the expected lifetime following implantation of the bilateral implant cohort is exactly the same as for the unilateral cohort (32 years).
Device use
Table 80 shows the number of devices used over the course of an individual’s expected lifetime. Results for the whole bilateral cohort as well as the subset of bilateral recipients are reported.
| Whole cohort (including non-recipients) | Bilateral cochlear recipients | |
|---|---|---|
| Proportion of lifetime using two devices | 65% | 93% |
| Proportion of lifetime using one device | 2% | 2% |
| Proportion of lifetime using no devices | 33% | 5% |
If an individual successfully receives two devices there is an 93% chance that they will remain using two devices for the remainder of their life.
Event counts
The event counts for the whole bilateral cohort as well as the subset of simultaneous bilateral recipients are shown in Table 81. With the exception of voluntary non-use, all model outputs represent the number of events that an individual can expect to experience over their remaining lifetime.
| Whole cohort (including non-recipients) | Only bilateral cochlear implant recipients | |||
|---|---|---|---|---|
| Lifetime | Event rate/100 patient-years | Lifetime | Event rate/100 patient-years | |
| New cochlear implant internal components | 0.03 | 0.11 | 0.05 | 0.15 |
| New cochlear implant external components | 8.90 | 27.53 | 12.71 | 39.34 |
| Major complications | 0.20 | 0.61 | 0.28 | 0.87 |
| Initial implant operations | 0.70 | NA | 1.00 | NA |
| New acoustic hearing aids | 1.47 | 4.56 | 0.20 | 0.63 |
| Permanent explants | 0.03 | 0.08 | 0.04 | 0.12 |
| Voluntary non-compliance | 0.016 | 0.05 | 0.024 | 0.07 |
Corresponding per-person event counts for unilateral implantation of the same patient group are reported earlier in this chapter.
Analysis of uncertainty
Deterministic sensitivity analysis
Results have again been presented separately for structural parameters (Figure 41), utilities (Figure 42), event-related probabilities and survival curves (Figure 43) and costs (Figure 44).
FIGURE 41.
One-way sensitivity analysis for structural inputs. Incremental cost-effectiveness ratios of adult simultaneous bilateral cochlear implantation compared with unilateral use. BC, base-case value; QALY, quality-adjusted life-year.
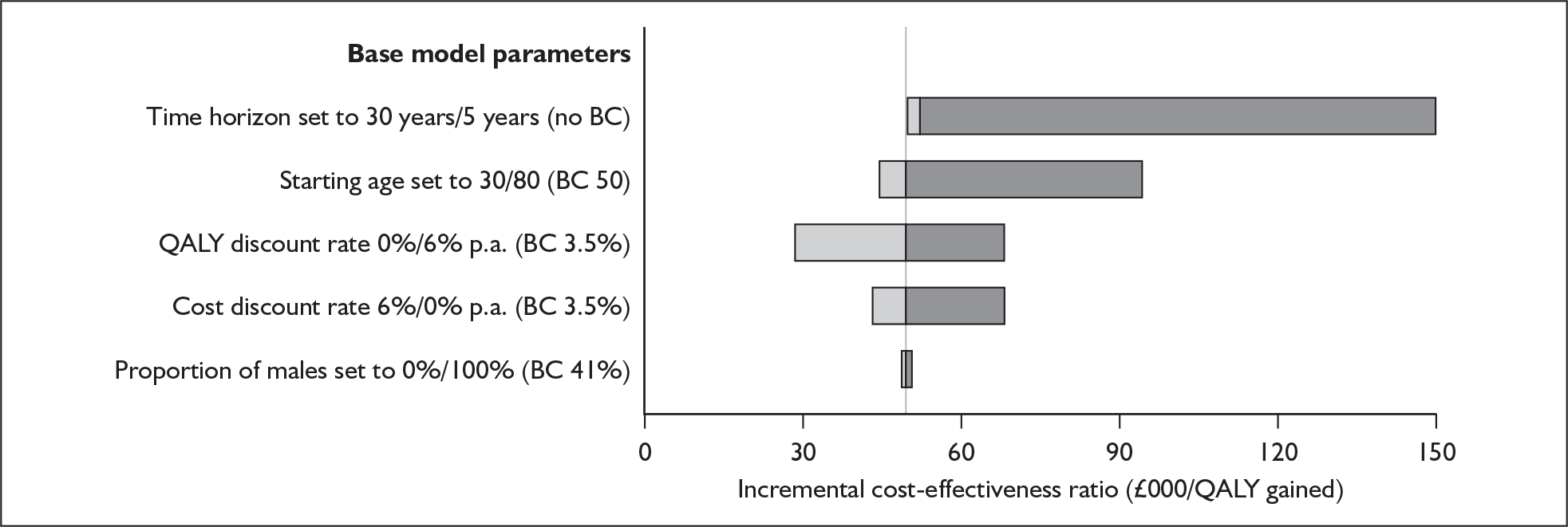
FIGURE 42.
One-way sensitivity analysis for utilities. Incremental cost-effectiveness ratios of adult simultaneous bilateral implantation compared with unilateral use. BC, base-case value; CI, cochlear implant; QALY, quality-adjusted life-year.
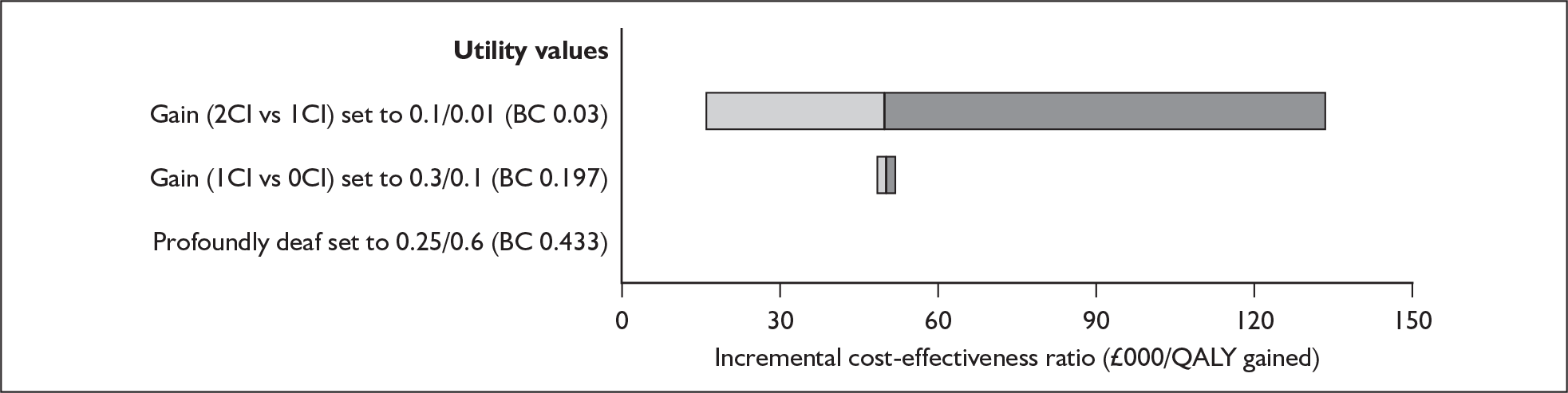
FIGURE 43.
One-way sensitivity analysis for event probabilities. Incremental cost-effectiveness ratios of adult simultaneous bilateral implantation compared with unilateral use. BC, base-case value; CI, cochlear implant; HA, hearing aid; QALY, quality-adjusted life-year.
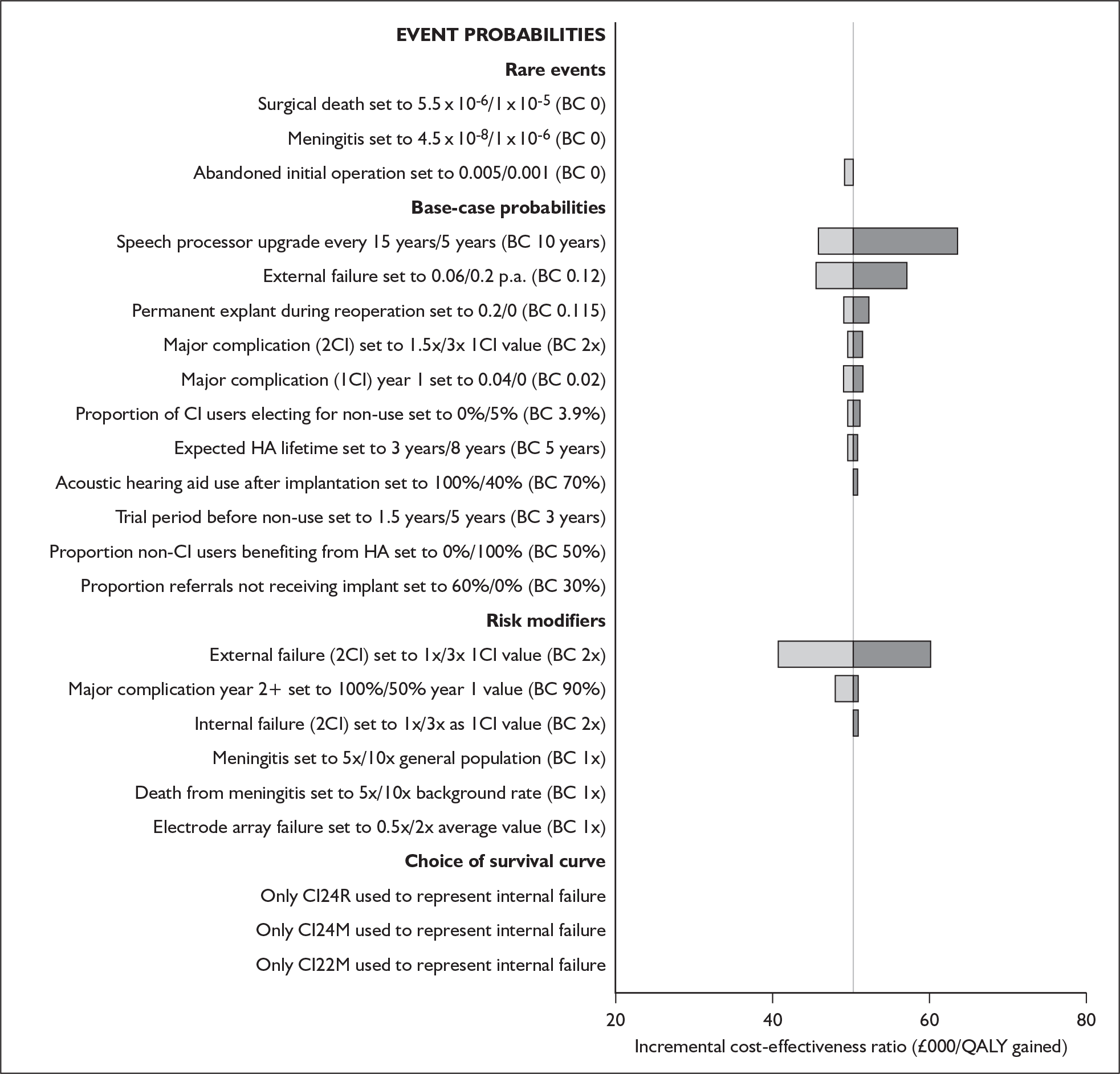
FIGURE 44.
One-way sensitivity analysis for costs. Incremental net benefit of adult simultaneous bilateral implantation compared with unilateral implantation at a willingness to pay threshold of £30,000 per QALY. BC, base-case value; CI, cochlear implant; QALY, quality-adjusted life-year.
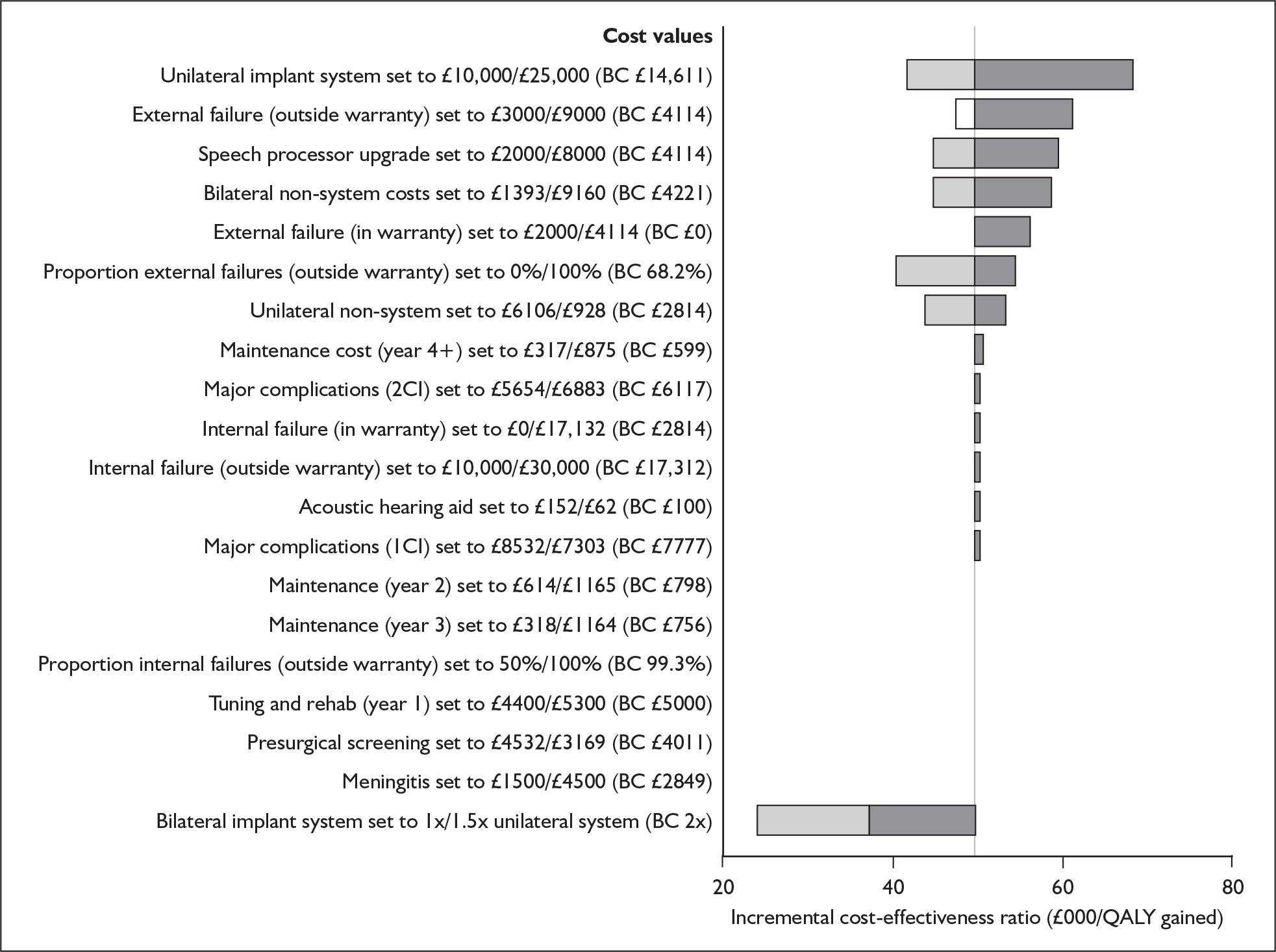
The baseline ICER corresponding to the comparison of simultaneous bilateral and unilateral cochlear implantation of adults aged 50 years appears particularly sensitive to changes in the following parameters:
-
the time horizon used in the model
-
the annual discount rate applied to health benefits
-
the incremental value associated with bilateral implant use in comparison to unilateral implant use
-
the cost of bilateral implant hardware as a proportion of the cost of unilateral implant hardware.
Threshold analyses
Cohort starting age
Threshold analysis of the starting age of the adult cohort shows that at a willingness to pay threshold of £30,000 per QALY bilateral implantation never represents a cost-effective treatment option for any feasible input values (Figure 45).
FIGURE 45.
Threshold analysis for starting age of adult cohort (bilateral implantation). QALY, quality-adjusted life-year.
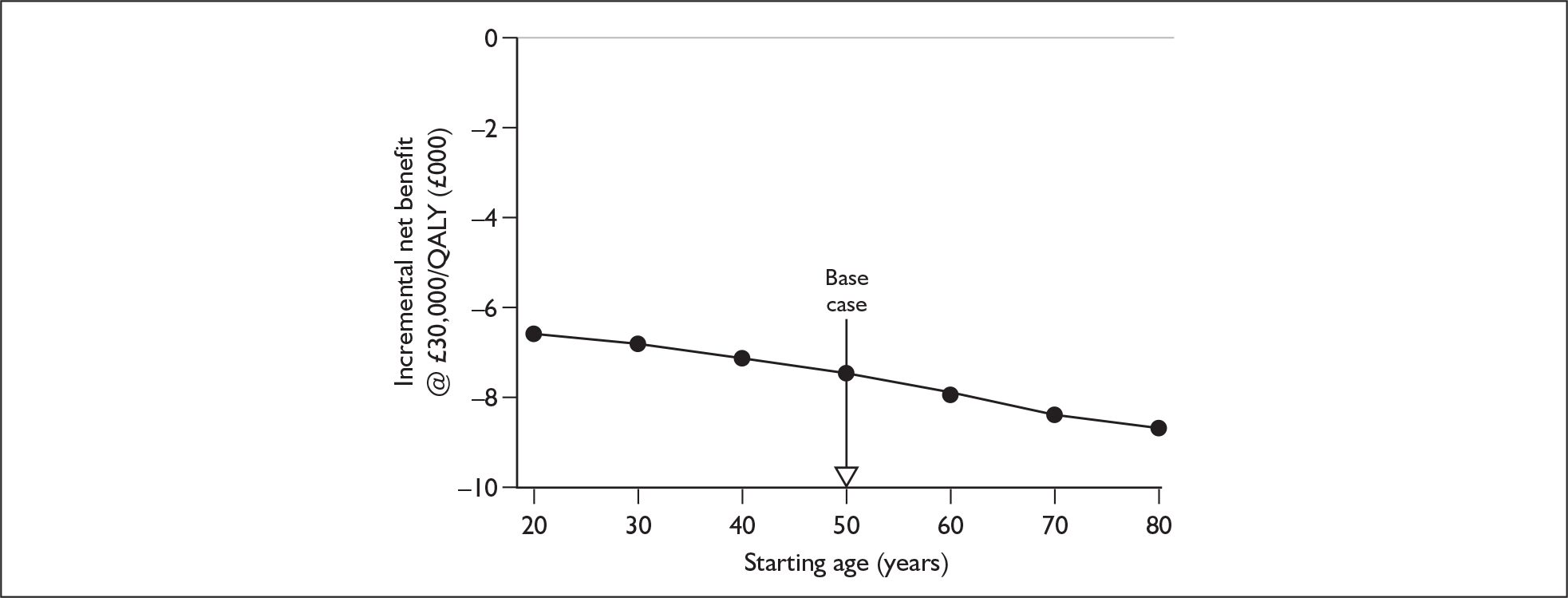
Utility gain associated with bilateral compared with unilateral device use
Analysis of the incremental utility associated with bilateral cochlear implant use compared with unilateral cochlear implant use shows that at a willingness to pay threshold of £30,000 per QALY simultaneous bilateral implantation becomes cost-effective above a value of approximately 0.05 (Figure 46). At a willingness to pay threshold of £20,000 per QALY bilateral implantation becomes cost-effective when the parameter value is above approximately 0.08. Both of these are close to the value assumed in the base case (0.03). However, because the adult ICER is higher than the corresponding value for children, additional benefit is needed to make the technology appear cost-effective.
FIGURE 46.
Threshold analysis for utility gain in adults associated with bilateral as opposed to unilateral cochlear implant use. QALY, quality-adjusted life-year.
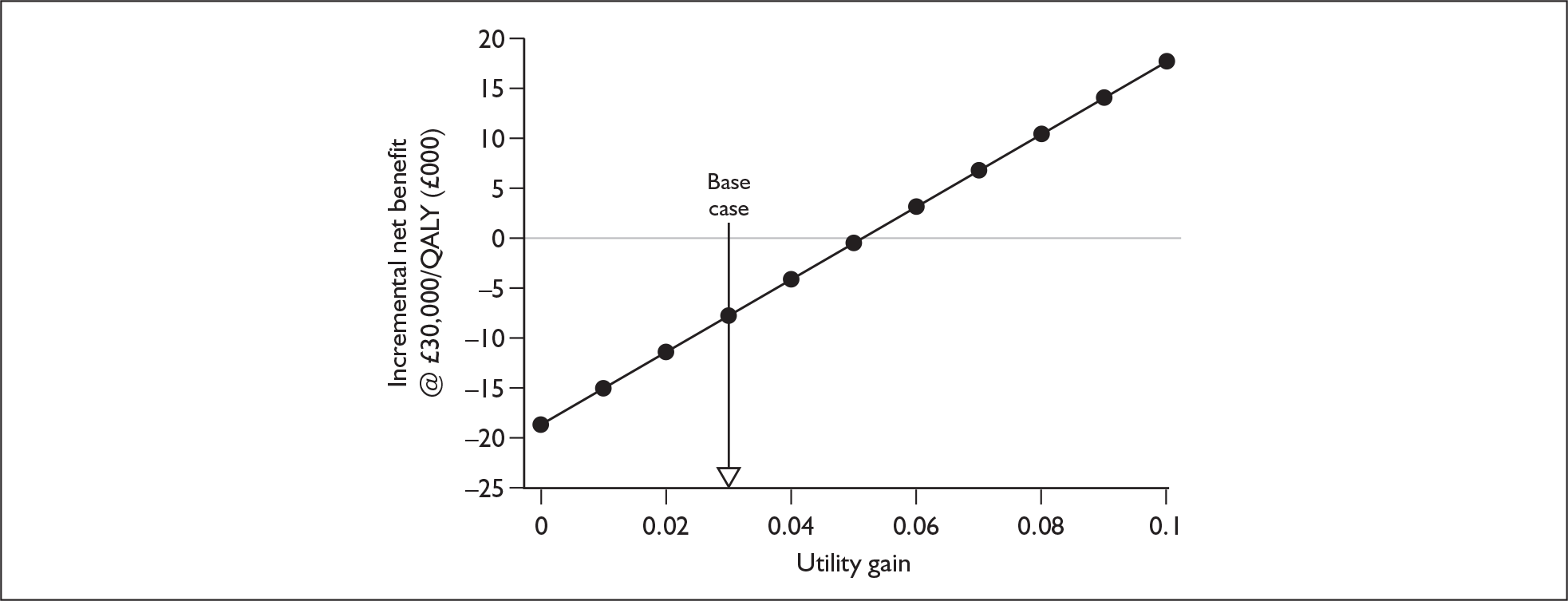
As stated in Chapter 6 (see Utility and utility changes following bilateral implantation) the 95% confidence interval for this regression model-derived parameter is –0.045 to +0.104. Regardless of which of the threshold values are used the model is extremely sensitive to changes in this parameter.
Although this interval may be statistically meaningful, individuals who receive two cochlear implants will only have a worse quality of life than with only one implant if the negative impacts on utility, because of, for example, surgical complications or changes in tinnitus, are greater than the other documented benefits of binaural hearing. On current evidence, in particular the typically ameliorating impacts on tinnitus of cochlear implantation (see Utility and utility changes following bilateral implantation), it seems more reasonable to assume that health-related quality of life may increase rather than decrease with a second device. Table 82 shows the range of ICERs corresponding to positive parameter values within the confidence interval.
| Utility gain | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| –0.01 | 0 | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 | 0.06 | 0.07 | 0.08 | 0.09 | 0.10 | |
| ICER value | Dominated | NA | £132,986 | £72,208 | £49,559 | £37,725 | £30,454 | £25,532 | £21,980 | £19,296 | £17,196 | £15,508 |
Cost of bilateral implant system
Figure 47 shows that at a willingness to pay threshold of £30,000 per QALY for simultaneous bilateral implantation to become cost-effective a discount of approximately 75% on the cost of the second implant system is required. In the base-case analysis no discount has been applied. The discount is greater than the corresponding value for prelingually deafened children because of the base-case ICER being higher.
FIGURE 47.
Threshold analysis of discount offered on second adult implant system used in simultaneous bilateral implantation. QALY, quality-adjusted life-year.
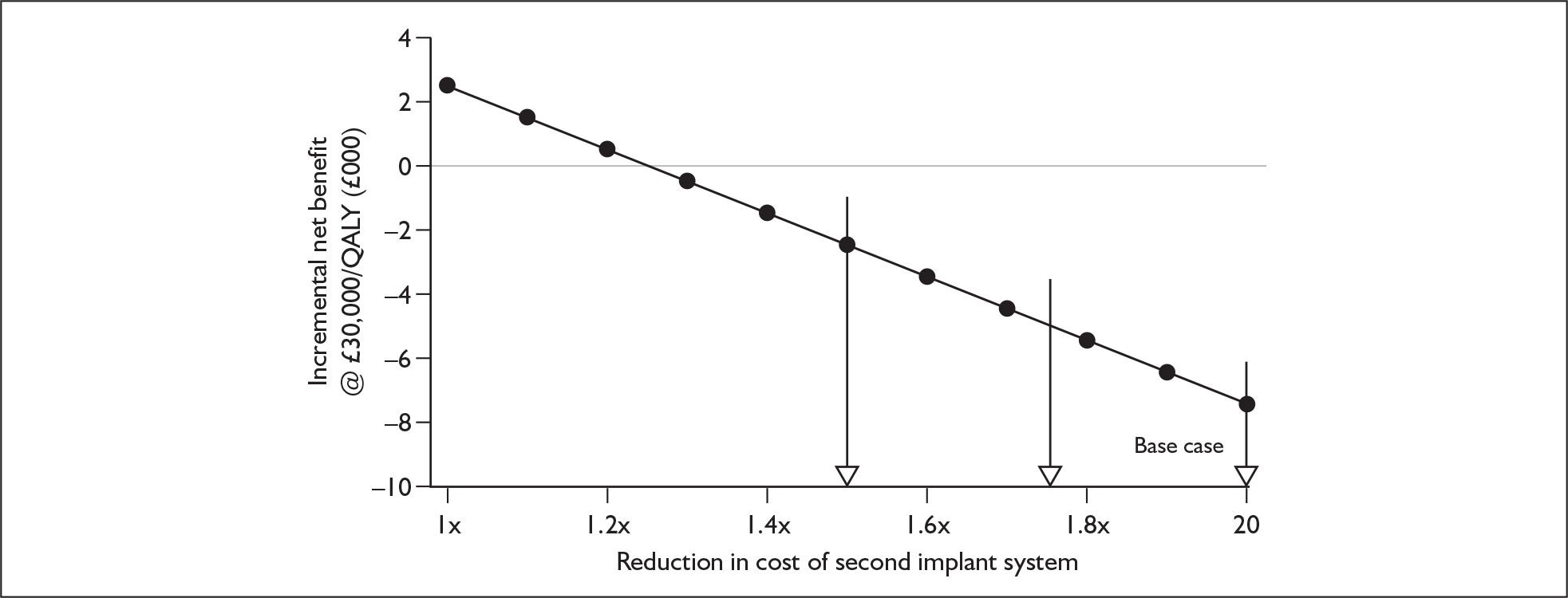
Table 83 shows the range of ICERs generated when a range of discounts are applied to the cost of a unilateral implant system.
| Discount offered on cost of second implant systema | |||||
|---|---|---|---|---|---|
| 0% | 25% | 50% | 75% | 100% | |
| Cost of bilateral implant systemb | £29,222 | £25,569 | £21,916 | £18,263 | £14,611 |
| ICER | £49,559 | £43,028 | £36,497 | £29,966 | £23,438 |
At a willingness to pay threshold of £20,000 per QALY, no feasible value for system costs makes bilateral implantation appear cost-effective.
Cost of unilateral implant system
Figure 48 shows the range of ICERs for simultaneous bilateral implantation of adults aged 50 years generated by varying the cost of a unilateral implant system. No discount on the cost of the second system has been applied (i.e. the cost of a bilateral implant system is twice the cost of a unilateral implant system). No devices appeared cost-effective at £30,000 per QALY. However, the cheapest implant/processor combination reduced the ICER from around £50,000 to approximately £45,000.
FIGURE 48.
Device-specific incremental cost-effectiveness ratios (ICERs) in adult bilateral implantation. A: Advanced Bionics CLARION® ICS HiRes 90K; B: Advanced Bionics CLARION® HiRes 90K with HiFocus Helix; C: Cochlear Europe Nucleus® CI24R (ST) ‘K’ with a Sprint or ESPrit 3G Processor; D: Cochlear Europe Nucleus® CI24R (CA) Advanced with a Sprint or ESPrit 3G Processor; E: Cochlear Europe Nucleus® CI11+11+2 double array with a Sprint or ESPrit 3G Processor; F: Cochlear Europe Nucleus® Freedom with either BTE or BWP option; G: Cochlear Europe Nucleus® Freedom with both BTE and BWP option; H: MED-EL UK Pulsar CI-100; I: Neurelec DIGISONIC SP with Digi SP or Digi SP*K.
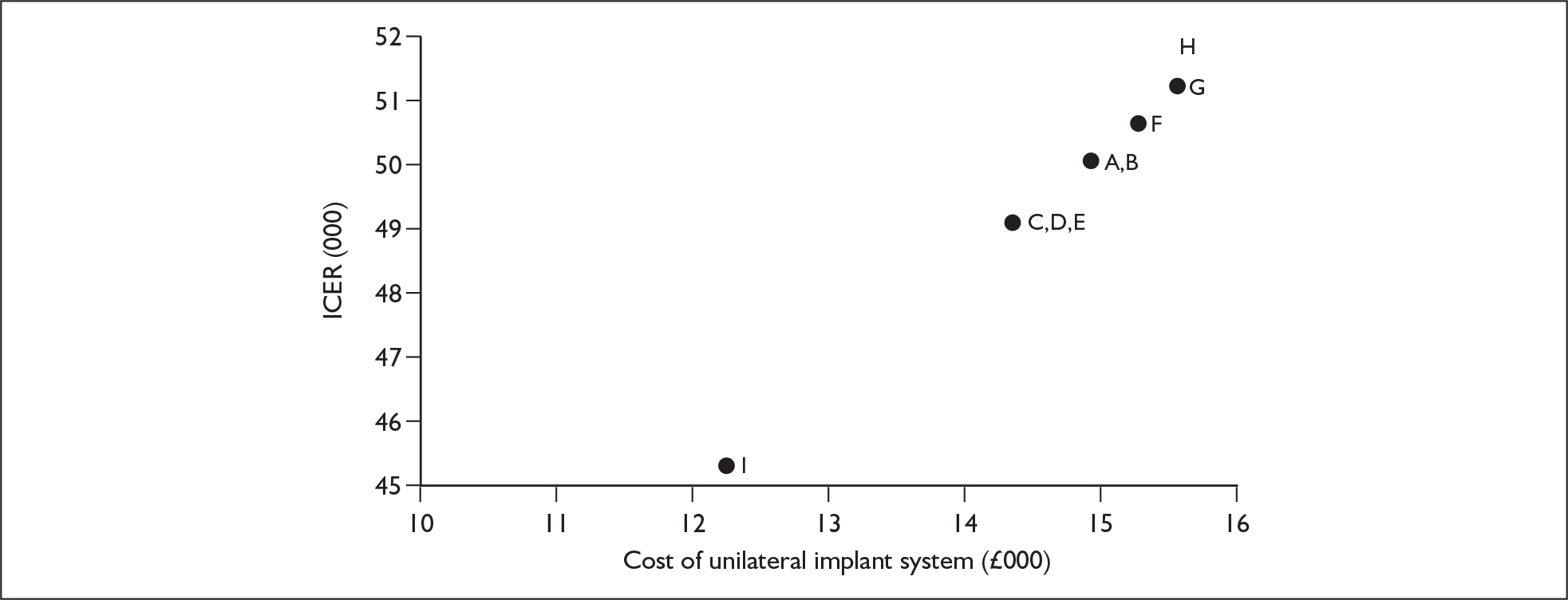
Probabilistic sensitivity analysis
Simultaneous bilateral implantation versus unilateral implantation
The simulation output (based on 1000 runs of the model) shows that at £20,000 per QALY, in profoundly deaf adults who are initially not cochlear implant users, simultaneous bilateral implantation is cost-effective in 3% of simulations; at £30,000 per QALY it is cost-effective in 20.7% of simulations.
At a willingness to pay threshold of £30,000 per QALY, simultaneous bilateral implantation was dominated by unilateral implantation in 13.2% of simulations (creating higher costs but lower QALYs). The probabilistic mean incremental net benefit is –£8868 (95% Cr I –£9525 to –£8212) and the probabilistic median incremental net benefit is –£8256.
Outputs from the Monte Carlo simulation are shown graphically in Figure 49, and the CEACs are shown in Figure 50. The CEACs show that simultaneous bilateral implantation would be considered cost-effective only if the willingness to pay threshold was increased beyond approximately £50,000 per QALY.
FIGURE 49.
Simulation output (cohort based, 1000 trials) for the cost-effectiveness of simultaneous bilateral implantation of profoundly deaf, non-cochlear implant using adults in comparison to unilateral implantation. QALY, quality-adjusted life-year.
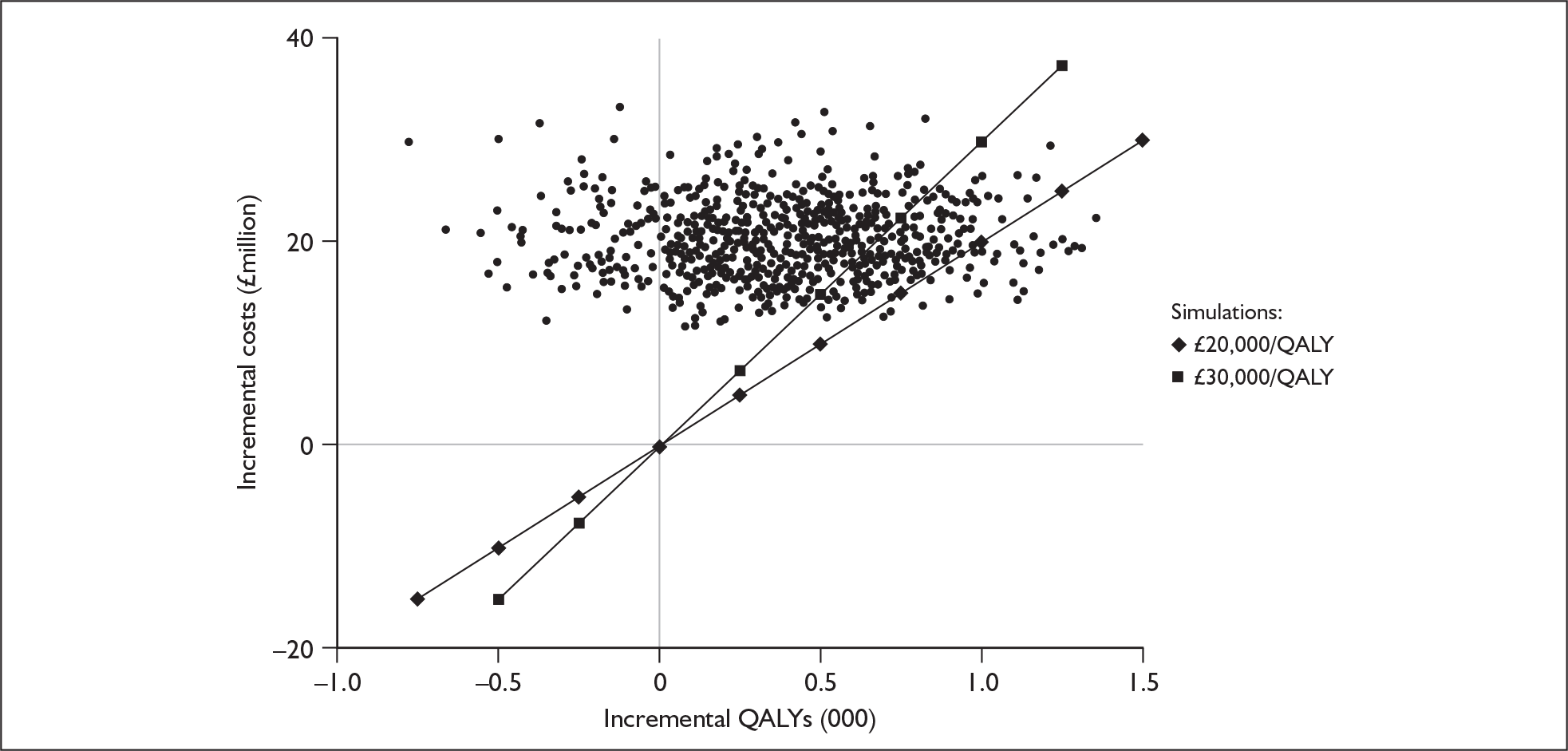
FIGURE 50.
Cost-effectiveness acceptability curves for simultaneous bilateral implantation of profoundly deaf, non-cochlear implant using adults vs unilateral implantation. QALY, quality-adjusted life-year.
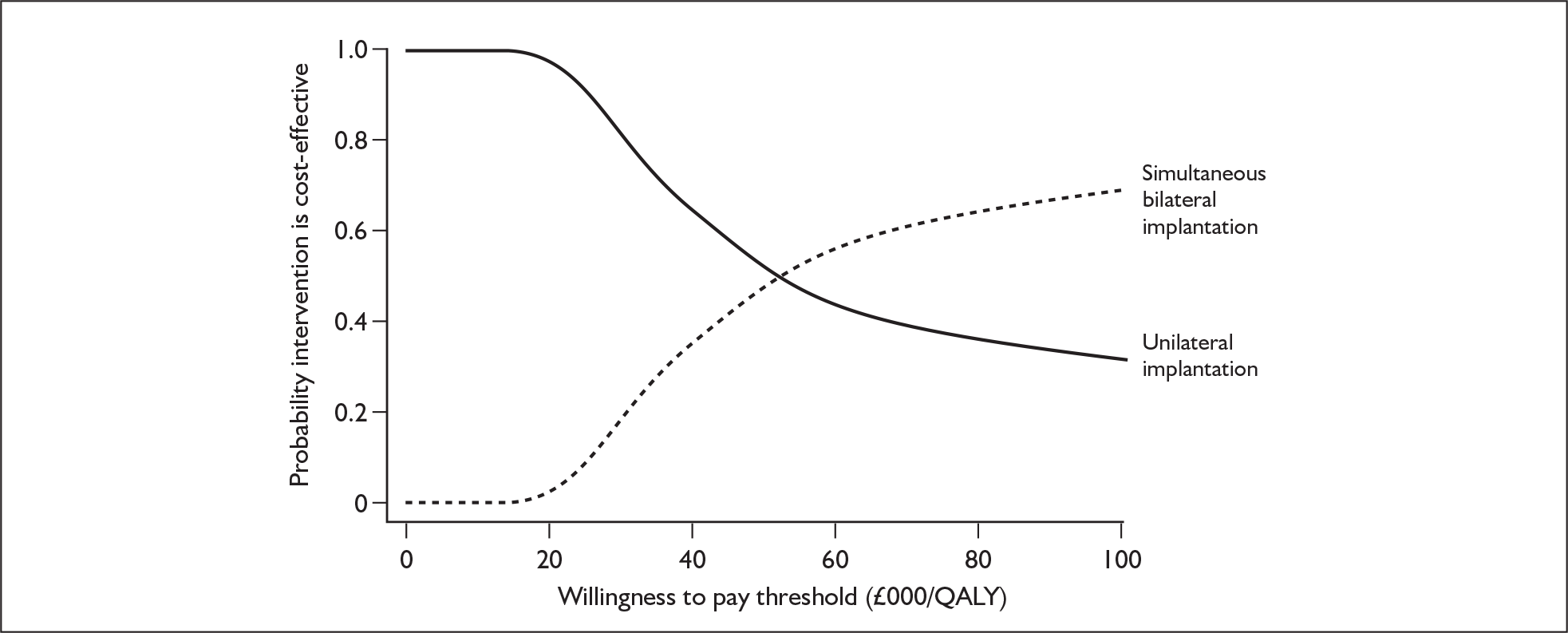
Sequential bilateral implantation versus unilateral implantation
The simulation output (based on 1000 runs of the model) shows that at £20,000 per QALY, in profoundly deaf adults who are initially not cochlear implant users, sequential bilateral implantation is cost-effective in 0.7% of simulations; at £30,000 per QALY it is cost-effective in 8.9% of simulations.
At a willingness to pay threshold of £30,000 per QALY, sequential bilateral implantation was dominated by unilateral implantation in 12.8% of simulations (creating higher costs but lower QALYs). The probabilistic mean incremental net benefit is –£11,311 (95% Cr I –£11,869 to –£10,572) and the probabilistic median incremental net benefit is –£10,394. Outputs from the Monte Carlo simulation are shown graphically in Figure 51, and the CEACs are shown in Figure 52. The CEACs show that sequential bilateral implantation would be considered cost-effective only if the willingness to pay threshold was increased beyond approximately £61,000 per QALY.
FIGURE 51.
Simulation output (cohort based, 1000 trials) for the cost-effectiveness of sequential bilateral implantation of profoundly deaf, non-cochlear implant using adults in comparison to unilateral implantation. QALY, quality-adjusted life-year.
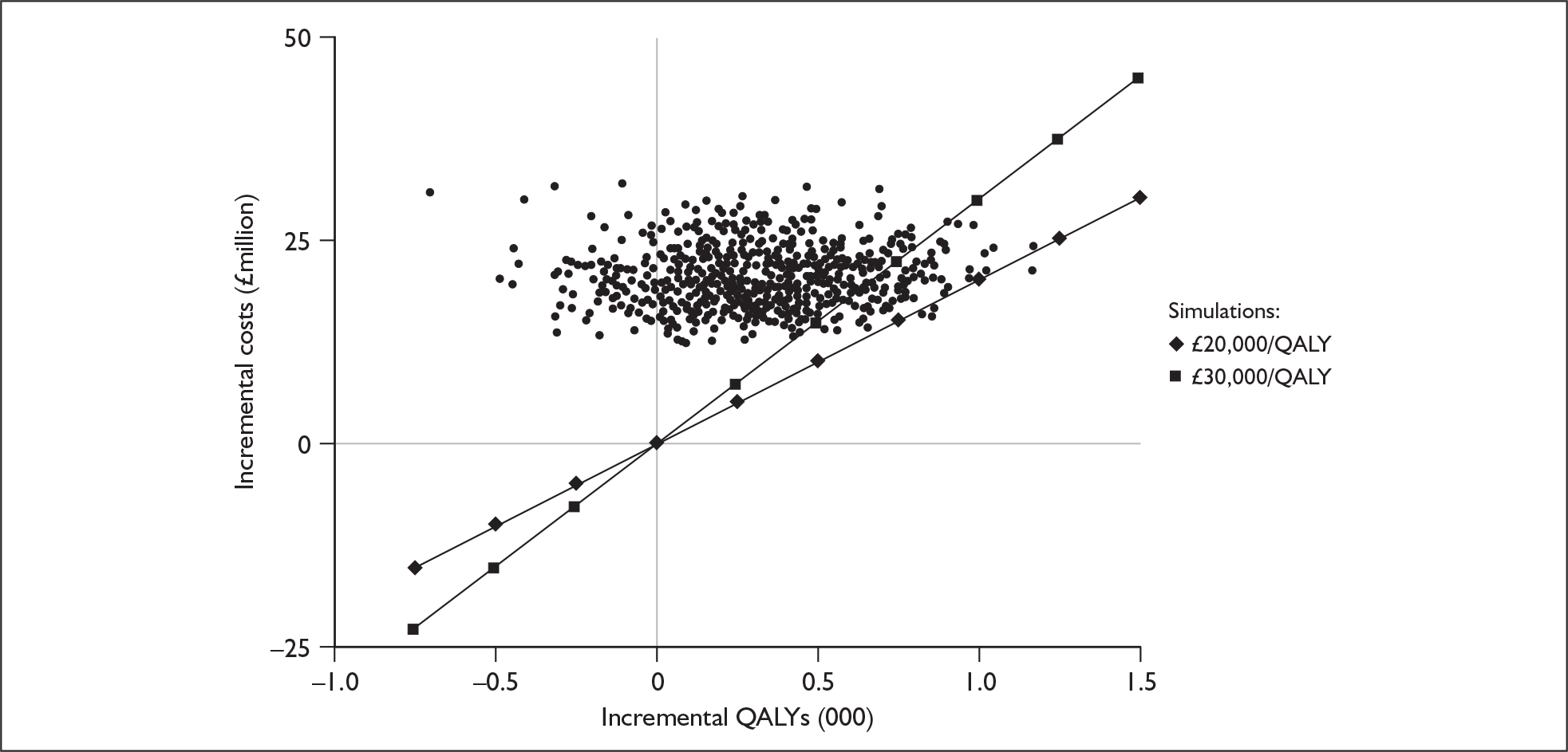
FIGURE 52.
Cost-effectiveness acceptability curves for sequential bilateral implantation of profoundly deaf, non-cochlear implant using adults vs unilateral implantation. QALY, quality-adjusted life-year.
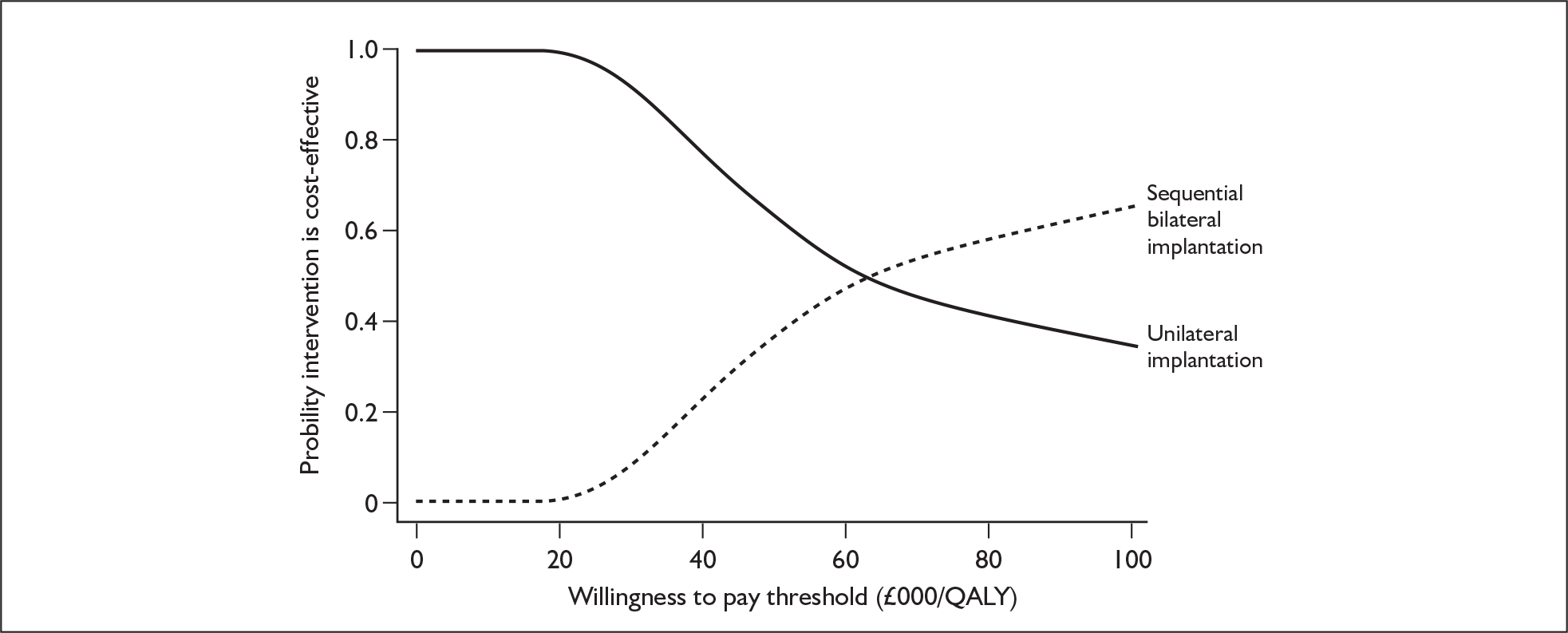
Scenario analyses
Cost-effectiveness of bilateral implantation compared to unilateral implantation (alternative utility scenario)
In the base-case analysis the incremental utilities associated with both unilateral and bilateral cochlear implant use are assumed to be fixed. In this scenario these incremental utilities are assumed to decline with age.
The cost-effectiveness results for this scenario are summarised in Tables 84 and 85. Overall, the cost-effectiveness ratios for simultaneous and sequential implantation are approximately 8% and 9% higher, respectively, than those generated in the base-case scenarios.
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| Unilateral implantation | 34,207 | 9.89 | – | – | – |
| Simultaneous bilateral implantation | 53,255 | 10.24 | 19,048 | 0.36 | 53,441 |
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| Unilateral implantation | 34,207 | 9.89 | – | – | – |
| Sequential bilateral implantation | 53,886 | 10.18 | 19,678 | 0.30 | 65,933 |
Cost-effectiveness of adult cochlear implantation assuming no product warranties
The results for this scenario are shown in Tables 86 and 87. Without warranties the ICER increases by approximately 7% for unilateral implantation in comparison to no cochlear implantation and by approximately 13% for bilateral implantation compared with unilateral implantation. However, all of the previous uncertainties surrounding discounts and incremental utility remain.
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| No cochlear implant use | 248 | 8.2 | – | – | – |
| Unilateral implantation | 36,701 | 10.6 | 36,453 | 2.40 | 15,203 |
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£/QALY) | |
|---|---|---|---|---|---|
| Unilateral implantation | 36,701 | 10.6 | – | – | – |
| Simultaneous bilateral implantation | 58,242 | 10.99 | 21,541 | 0.38 | 56,046 |
Comparison of industry-submitted analyses with PenTAG cost–utility analyses
Tables 88–91 compare the key inputs and key results from the PenTAG cost–utility analyses with the analyses submitted to NICE by Cochlear Europe and Advanced Bionics Europe. (The other two suppliers of cochlear implants to the NHS did not submit any original economic analyses.) Cochlear Europe was the only manufacturer that provided a cost–utility analysis of bilateral cochlear implantation.
| PenTAG analysis | Cochlear Europe | Advanced Bionics | Difference: Cochlear Europe | Difference: Advanced Bionics | |
|---|---|---|---|---|---|
| Key input values | |||||
| Degree of deafness | Profound | Severe to profound | Profound | ||
| Age at implantation (years) | 1.5 | 3 | 3 | –1.5 | –1.5 |
| Mean survival (age, years) | 80 | Not stated | Not stated | ||
| Resources for cochlear implantation: assessment; cochlear implant system; surgery; tuning/rehabilitation; maintenance; major complications; device failures (internal or external); routine replacements | All | All | All | ||
| Cost of assessment | £2843 | £4925 | £3017 | –£2082 | –£174 |
| Cost of implant system | £14,611 | £15,250 | £16,000 | –£639 | –£1,389 |
| Cost of implantation surgery | £3480 | £5087 | £3693 | –£1607 | –£213 |
| Cost of tuning/rehabilitation (year 1) | £9148 | £9487 | £9708 | –£339 | –£560 |
| Cost of maintenance (years 4–15) | £1364 | £1972 | £1447 | –£608 | –£83 |
| Cost of maintenance (years 16+) | £599 | £1972 | £1447 | –£1373 | –£848 |
| Cost of processor repair | £0 (years 1–3); £4114 | £300pa | £0 (years 1–3); £312pa | ||
| Cost of processor upgrade | £4114 | £3500 | NR | +£614 | |
| Types of device failures included | Implant or external | Implant or processor | |||
| Utility gain for cochlear implant users | 0.232 | 0.224 | 0.256 | + 0.008 | –0.024 |
| Utility of being profoundly deaf | 0.421 | Not used | 0.39 | + 0.031 | |
| Other factors or events included | Voluntary non-use of device | Declining HRQoL with age | Voluntary non-use; duration of deafness | ||
| Key results | |||||
| Lifetime discounted cost with unilateral cochlear implants | £60,070 | £82,888 | £84,820 | –£22,447 | –£24,379 |
| Lifetime discounted cost without cochlear implants | £371 | £11,706 | £1732 | –£11,706 | –£1361 |
| Incremental cost (discounted) | £60,070 | £71,182 | £83,088 | –£11,112 | –£23,018 |
| Lifetime discounted QALYs with unilateral cochlear implants | 15.84 | 23.15 | 16.53 | –7.31 | –0.69 |
| Lifetime discounted QALYs without cochlear implants | 11.36 | 16.40 | 10.30 | –5.04 | + 1.06 |
| Incremental QALYs (discounted) | 4.48 | 6.75 | 6.23 | –2.27 | –1.75 |
| Incremental cost per QALY | £13,413 | £10,542 | £13,337 | +£2871 | +£76 |
| Cost per QALY 95% confidence interval (from PSA) | £8804–£12,655 | £1945–dominated | |||
| % PSA ICERs < £30,000 per QALY | 100% | 98%a | 87.8% | + 2.0% | + 12.2% |
| PenTAG analysis | Cochlear Europe | Difference: Cochlear Europe | |
|---|---|---|---|
| Key input values | |||
| Age at implantation (years) | 1.5 | 3 | –1.5 |
| Mean survival (age, years) | 80 | Not stated | Not stated |
| Resources for cochlear implantation: assessment; cochlear implant system; surgery; tuning/rehabilitation; maintenance; major complications; device failures (internal or external); routine replacements | All | All | |
| Cost of assessment | £2843 | £4925 | –£2082 |
| Cost of two implant systems | £29,222 | £35,439 | –£6217 |
| Cost of implantation surgery | £5220 | £7258 | –£2038 |
| Cost of tuning/rehabilitation (year 1) | £9148 | £11,384 | –£2236 |
| Cost of maintenance (years 4–15) | £1364 | £1872 | –£508 |
| Cost of maintenance (years 16+) | £599 | £1872 | –£1273 |
| Cost of processor repair | £0 (years 1–3); £4114 | £600pa | |
| Cost of processor upgrade | £4114 | £3500 | £614 |
| Types of device failures included | Implant or external | Apparently internal or external | |
| Utility gain for bilateral implant users | 0.030 | + 15% = 0.0336 | –0.004 |
| Utility of having a unilateral implant | 0.653 | ||
| Other factors or events included | Voluntary non-use | Declining HRQoL with age | |
| Key results | |||
| Lifetime discounted cost with bilateral cochlear implants | £87,546 | £122,436 | –£34,890 |
| Lifetime discounted cost with unilateral cochlear implants | £60,441 | £82,888 | –£22,447 |
| Incremental cost (discounted) | £27,104 | £39,549 | –£12,445 |
| Lifetime discounted QALYs with bilateral cochlear implants | 16.51 | 24.17 | –7.66 |
| Lifetime discounted QALYs with unilateral cochlear implants | 15.84 | 23.15 | –7.31 |
| Incremental QALYs (discounted) | 0.67 | 1.01 | –0.34 |
| Incremental cost per QALY | £40,410 | £39,049 | +£1361 |
| Cost per QALY 95% confidence interval (from PSA) | £31,426–49,798 | ||
| % PSA ICERs < £30,000 per QALY | 34.9% | 24%a | + 10.9% |
| PenTAG analysis | Cochlear Europe | Advanced Bionics | Difference: Cochlear Europe | Difference: Advanced Bionics | |
|---|---|---|---|---|---|
| Key input values | |||||
| Age at implantation (years) | 50 | 62 | 50 | –12 | 0 |
| Mean survival (age, years) | 82 | Not stated | Not stated | ||
| Resources for cochlear implantation: assessment; cochlear implant system; surgery; tuning/rehabilitation; maintenance; major complications; device failures (internal or external); routine replacements | All | All | All | ||
| Cost of assessment | £4011 | £4193 | £3017 | –£182 | +£994 |
| Cost of implant system | £14,611 | £15,250 | £16,000 | –£639 | –£1389 |
| Cost of implantation surgery | £4221 | £3349 | £3693 | +£872 | +£528 |
| Cost of tuning/rehabilitation (year1) | £5000 | £5226 | £9708 | –£226 | –£4708 |
| Cost of maintenance (years 4+) | £599 | £625 | £1447 | –£26 | –£848 |
| Cost of processor repair | £0 (years 1–3); £4114 | £300pa | £0 (years 1–3); £312 | ||
| Cost of processor upgrade | £4114 | £3500 | £0 | £614 | £4114 |
| Types of device failures included | Implant or external | Implant or external | Implant or processor | ||
| Utility gain for cochlear implant users | 0.197 | 0.394–0.360 | 0.214 | 0.197 | 0.017 |
| Utility of being profoundly deaf | 0.433 | 0.365–0.333 | 0.41 | 0.068 | 0.023 |
| Other factors or events included | Voluntary non-use of device | Declining HRQoL with age | Voluntary non-use, duration of deafness | ||
| Key results | |||||
| Lifetime discounted cost with unilateral cochlear implants | £34,207 | £43,524 | £59,510 | –£9317 | –£25,303 |
| Lifetime discounted cost without cochlear implants | £248 | £7400 | £1031 | –£7152 | –£783 |
| Incremental cost (discounted) | £33,959 | £36,124 | £58,479 | –£2165 | –£24,520 |
| Lifetime discounted QALYs with unilateral cochlear implants | 10.60 | 10.13 | 9.56 | 0.45 | 1.04 |
| Lifetime discounted QALYs without cochlear implants | 8.20 | 5.07 | 6.64 | 3.13 | 1.56 |
| Incremental QALYs (discounted) | 2.40 | 5.06 | 2.92 | –2.66 | –0.52 |
| Incremental cost per QALY | £14,163 | £7145 | £20,027 | +£7018 | –£5864 |
| Cost per QALY 95% confidence interval (from PSA) | £5907–7794 | £2396–dominated | |||
| % PSA ICERs < £30,000 per QALY | 100% | 100%a | 68.7% | 0% | 31.3% |
| PenTAG analysis | Cochlear Europe | Difference: Cochlear Europe | |
|---|---|---|---|
| Key input values | |||
| Age at implantation (years) | 50 | 62 | –12 |
| Mean survival (age, years) | 82 | Not stated | |
| Resources for cochlear implantation: assessment; cochlear implant system; surgery; tuning/rehabilitation; maintenance; major complications; device failures (internal or external); routine replacements | All | All | |
| Cost of assessment | £4011 | £4193 | –£182 |
| Cost of implant system | £29,222 | £30,500 | –£1278 |
| Cost of implantation surgery | £4221 | £4476 | –£255 |
| Cost of tuning/rehabilitation (year 1) | £5000 | £6271 | –£1271 |
| Cost of maintenance (years 4+) | £599 | £626 | –£27 |
| Cost of processor repair | £0 (years 1–3); £4114 | ||
| Cost of processor upgrade | £4114 | £3500 | +£614 |
| Types of device failures included | Implant or external | Implant or external | |
| Utility gain for bilateral implant users | 0.03 | 0.114 | –0.084 |
| Utility of having a unilateral implant | 0.63 | 0.759–0.693 | |
| Other factors or events included | Voluntary non-use of device | Declining HRQoL with age | |
| Key results | |||
| Lifetime discounted cost with bilateral cochlear implants | £53,255 | £68,481 | –£152,26 |
| Lifetime discounted cost with unilateral cochlear implants | £34,207 | £43,524 | –£9317 |
| Incremental cost (discounted) | £19,048 | £24,956 | –£5908 |
| Lifetime discounted QALYs with bilateral cochlear implants | 10.99 | 10.89 | 0.10 |
| Lifetime discounted QALYs with unilateral cochlear implants | 10.60 | 10.13 | 0.47 |
| Incremental QALYs (discounted) | 0.38 | 0.76 | –0.38 |
| Incremental cost per QALY | £49,559 | £32,909 | +£17,050 |
| Cost per QALY 95% confidence interval (from PSA) | £24,051–44,582 | ||
| % PSA ICERs < £30,000 per QALY | 20.7% | 32%a | 0.8% |
In terms of differences in key input parameters, in general the PenTAG analyses used slightly lower device costs; slightly lower assessment, tuning/rehabilitation and ongoing maintenance costs; and more conservative but still similar estimates of utility gain. The generally lower lifetime estimates of QALY gain from the PenTAG model may be explained by the fact that these analyses are based on the whole cohort originally referred for assessment for implantation, of whom some (20% of children, 30% of adults) do not go on to receive an implant (and then accrue the cost and QALY profiles of non-implanted profoundly deaf people). Although it is not entirely clear, in the industry-submitted analyses, the unilateral and bilateral cochlear implantation comparators involve all simulated individuals initially receiving one or two implants.
It can be seen from Tables 88 and 90 that, in general, the incremental cost-effectiveness estimates for unilateral cochlear implantation (compared with no cochlear implant provision) were similar between analyses. In profoundly prelingually deaf children the three estimates ranged from £10,542 to £13,413 per QALY, whereas in profoundly postlingually deaf adults they ranged from £7145 to £20,027 per QALY (with the PenTAG analysis providing the intermediate estimate of £14,163 per QALY). Whereas the PenTAG and Cochlear Europe ICERs for unilateral implantation were slightly lower in adults than in children, the Advanced Bionics Europe ICER for adults was over 50% higher than that in children; this is largely explained by the substantially higher costs used in this analysis (for adults costs were taken from the study by Barton and colleagues55 of paediatric cochlear implantation). The lowest ICERs for unilateral cochlear implantation, in both adults and children, were those estimated by Cochlear Europe, largely because of the significantly higher estimates of the lifetime QALY gain (which, in adults, was related to the high utility increment assumed to be associated with unilateral implantation).
However, in all three analyses of unilateral cochlear implantation in young children, the probabilistic ICERs were less than £30,000 per QALY in over 87% of simulations. In adults, although both the PenTAG and Cochlear Europe probabilistic analyses resulted in 100% of simulations generating ICERs less than this threshold, in the Advanced Bionics Europe analysis 68.7% of simulations were below this threshold.
For bilateral cochlear implantation in profoundly deaf adults (Table 91), the deterministic analysis from PenTAG generated a significantly higher ICER than that estimated by Cochlear Europe (£49,500 per QALY versus £32,900 per QALY). This was mainly explained by a smaller estimated difference in the incremental QALY gain (0.38 in the PenTAG analysis versus 0.76 in the Cochlear Europe analysis). This, in turn, is mainly a result of an assumed gain in utility in the Cochlear Europe analysis for bilateral versus unilateral implantation of about 0.11 (versus 0.03 in the PenTAG analysis). Although both of these utility gain estimates are from the same source study, Cochlear Europe chose to treat it as a relative utility increment, that is, as a proportion (15%) of the utility value for unilateral implantation (for which they already employ a comparatively high value of 0.759).
In children, despite generating quite similar ICERs (Table 89), the estimates should be treated with considerable caution, given that the utility gains from bilateral implantation in children have not yet been the subject of any empirical study. Again, the similarity in the ICERs conceals a quite different estimate of the incremental cost of bilateral implantation (£29,000 in the PenTAG analysis versus £39,500 in the Cochlear Europe analysis), and also a proportionally quite different – although in absolute terms very small – lifetime QALY gain (0.67 versus 1.01).
Summary of key results
Profoundly deaf children
-
A mixed sex cohort of 1000 children aged 1 year who were not already users of cochlear implants was modelled until death.
-
No studies were identified that contained values for the incremental utility associated with bilateral cochlear implant use as opposed to unilateral implant use.
-
The base-case analyses showed that:
-
in comparison to no cochlear implant use, unilateral implantation conferred an additional 4.48 QALYs for an additional £60,070 per person, giving an ICER of £13,413 per QALY
-
assuming that the mean incremental utility gain associated with bilateral cochlear implant use is the same in children as in adults, the following speculative results are obtained: (a) simultaneous bilateral implantation versus unilateral implantation confers an additional 0.67 QALYs for an additional £27,105 per person, giving an ICER of £40,410 per QALY; (b) sequential bilateral implantation versus unilateral implantation confers an additional 0.60 QALYs for an additional £32,657 per person, giving an ICER of £54,098 per QALY.
-
-
One-way sensitivity analyses showed that these results were sensitive to changes in discount rates, the time horizon used in the analysis, the discount offered on the cost of a second implant system and the long-term utility gain associated with unilateral implant use (versus no cochlear implant).
-
One-way sensitivity analyses showed that the results for bilateral implantation were extremely sensitive to the incremental utility associated with bilateral cochlear implant use (versus unilateral implant use).
-
Probabilistic sensitivity analysis based on 1000 simulated trials showed that at £30,000 per QALY (and at £20,000 per QALY):
-
unilateral implantation versus no cochlear implant use: unilateral implantation conferred the greatest net benefit in 100% (99.9%) of simulations and was dominated (fewer QALYs for greater cost) in 0% of simulations
-
again, assuming that the mean incremental utility gain associated with bilateral cochlear implant use is the same in children as in adults, the following speculative results are obtained: (a) simultaneous bilateral implantation versus unilateral implantation: simultaneous bilateral implantation conferred the greatest net benefit in 34.9% (16.6%) of simulations and was dominated in 16.9% of simulations; (b) sequential bilateral implantation versus unilateral implantation: sequential bilateral implantation conferred the greatest net benefit in 21.3% (5.5%) of simulations and was dominated in 16.2% of simulations.
-
Profoundly deaf adults
-
A mixed sex cohort of 1000 adult non-cochlear implant users aged 50 years was modelled until death.
-
The base case showed that:
-
in comparison to no cochlear implant use, unilateral implantation conferred an additional 2.40 QALYs for an additional £33,959 per person, giving an ICER of £14,163 per QALY
-
simultaneous bilateral implantation versus unilateral implantation conferred an additional 0.38 QALYs for an additional £19,048 per person, giving an ICER of £49,559 per QALY
-
sequential bilateral implantation versus unilateral implantation conferred an additional 0.33 QALYs for an additional £19,678 per person, giving an ICER of £60,301 per QALY.
-
-
One-way sensitivity analyses showed that these results were sensitive to changes in discount rates, the time horizon used in the analysis, the discount offered on the cost of a second implant system and the long-term utility gain associated with unilateral implant use (versus no cochlear implant).
-
One-way sensitivity analyses showed that the results for bilateral implantation were extremely sensitive to the incremental utility associated with bilateral cochlear implant use (versus unilateral implant use).
-
Probabilistic sensitivity analysis based on 1000 simulated trials showed that at £30,000 per QALY (and at £20,000 per QALY):
-
unilateral implantation versus no cochlear implant use: unilateral implantation conferred the greatest net benefit in 100% (100%) of simulations and was dominated (fewer QALYs for greater cost) in 0% of simulations
-
simultaneous bilateral implantation versus unilateral implantation: simultaneous bilateral implantation conferred the greatest net benefit in 20.7% (3%) of simulations and was dominated in 13.2% of simulations
-
sequential bilateral implantation versus unilateral implantation: sequential bilateral implantation conferred the greatest net benefit in 8.9% (0.7%) of simulations and was dominated in 12.8% of simulations.
-
-
Only one study was identified containing a value for the incremental utility associated with bilateral implant use as opposed to unilateral implant use. This study was very small (24 participants) and the values generated assumed that tinnitus was not a problem.
Chapter 8 Assessment of factors relevant to the NHS and other parties
The effects of cochlear implantation on employment
Cochlear implants improve the ability of deaf people to communicate, and in children may improve their educational attainment (see Chapter 4, Educational attainment). It might therefore be expected that this would have an impact on the type and level of employment attained or retained.
Kos and colleagues216 conducted a survey of the effects of cochlear implantation on professional occupation in 60 adults with a mean age at implantation of 50 years (range 18–77 years); however, without a matched control group their results are inconclusive. The employment prospects of people with cochlear implants are an area that would benefit from further comparative research (e.g. using age- and sex-matched profoundly deaf control subjects).
Implications for service provision
The numbers of adults and children implanted in the UK have risen each year since 1989 with 57% of cochlear implant centres reporting unmet demand, 10% unable to assess and 33% being content with their level of supply and demand. These figures come from the British Academy of Audiology, BCIG and ENT UK who have voiced concern about the recruitment, training and retention of staff to meet increasing demand (from the BAA/BCIG/ENT UK joint submission to NICE49).
A recent email survey of English and Welsh cochlear implant centres (n = 9) conducted by a member of our expert advisory group showed that waiting times varied between centres. The mean paediatric time from referral to operation was 7 months (range 3–17 months), with urgent cases usually seen within 6 weeks (range 1–8 months). The mean time that adults wait from referral to operation was less than 13 months (range 3–26 months), with urgent cases generally seen within 6 weeks (range 1–6 weeks). The waiting times include the time it takes to confirm funding, any treatment for co-morbidities and patient choice.
The BCIG service audit examined the staff mix involved in providing cochlear implant services (Figure 53). Note that this does not capture other support services for the cochlear implants provided by, for example, local education authorities or primary care trusts.
FIGURE 53.
BCIG NHS UK service audit 2007: staff employed in cochlear implant programmes. Audiology, audiological scientists; HT, hearing therapists; Other, audiological physicians, medical physics, family liaison officers, clinical psychologists, paediatricians, deaf advocate; SLT, speech and language therapists; ToD, teachers of the deaf. From Appendix 2 in the BAA/BCIG/ENT UK joint submission to NICE. 49
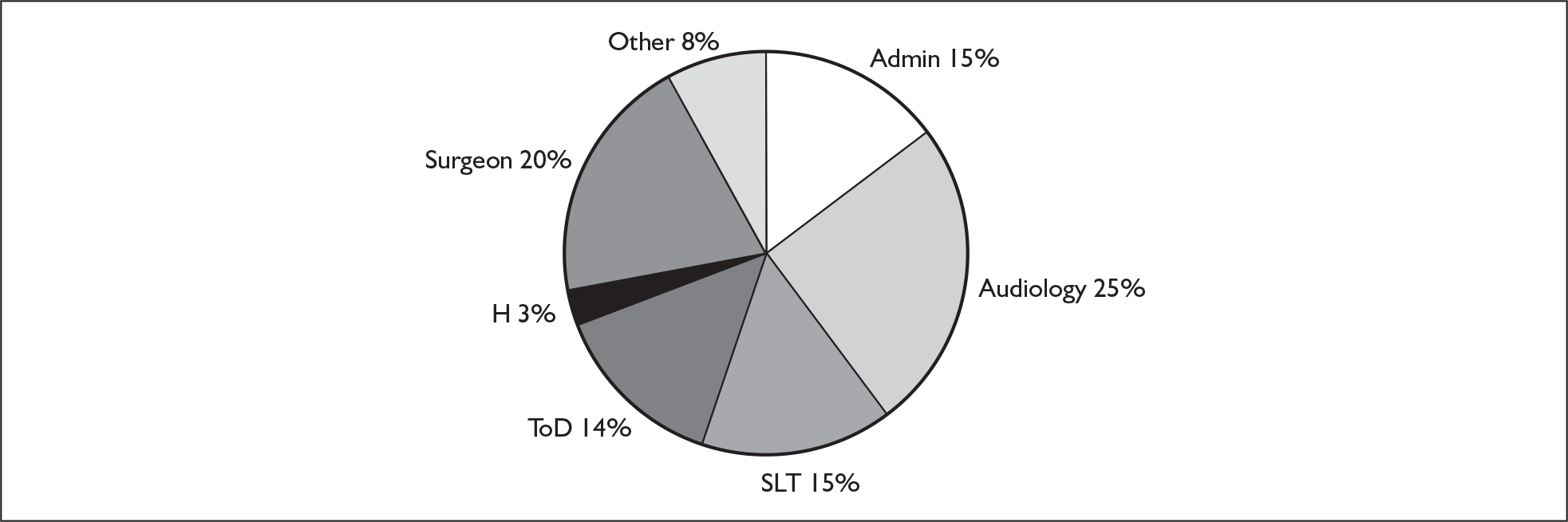
Although there are a number of part-time staff, the work force equates (2007) to nearly 260 whole time equivalent (WTE) staff who are involved with specialist service delivery for paediatric and adult care within the UK. Recruitment, training and retention are concerns expressed by most centres, especially in audiology.
Given increasing demand for new cochlear implants, and the growing population of deaf people, the current system of specialist regional tertiary centres may not be a sustainable model of service provision in the longer term. Any changes towards more service provision from a larger number of more generalist audiological departments in NHS trusts will alter the NHS cost profiles used in the analyses presented in this report and affect the travel costs to patients (which may be substantial).
Out-of-pocket costs and time costs for families
As well as being a relatively expensive technology for the NHS, families of children with cochlear implants also bear some of the cost of using the technology. A relatively recent interview study217 of 216 parents of children who had received cochlear implants via the Nottingham Cochlear Implant Programme (over a period of 13 years) estimated that the time and out-of-pocket costs were £3090 per year during the first 2 years post implant, £2159 per year in years 3–5 and approximately £1815 per year thereafter. Time costs (e.g. lost parental wages or non-employment productivity) and travel costs accounted for most of these costs.
We are not aware of any comparable studies that have estimated out-of-pocket costs or the time costs incurred by adults receiving cochlear implants.
Support services for optimising the benefits of cochlear implantation
A comprehensive and long-term programme of speech and language therapy is considered necessary for successful use of a cochlear implant, particularly in children. Some of these services may be provided by the cochlear implant centre (requiring outpatient visits and home visits for some). Others are provided by teachers of the deaf and audiological sociologists. They start in conjunction with the device ‘switch on’ process and mapping/tuning and continue as part of the rehabilitation process for a number of years.
In addition, some families of children with implants may receive visits from a community paediatrician or receive psychological support when needed.
It should be stressed that most of these support services, but programmes of speech and language therapy in particular (such as auditory verbal therapy), are considered by clinicians to critically rely on the time and effort of parents and others to achieve the best improvements from cochlear implantation.
Equity and current access to bilateral implantation under the NHS
At present, bilateral implantation is not routinely provided on the NHS to all deaf children or adults who might benefit. UK cochlear implant teams are offering bilateral implants to certain groups of deaf children and adults, either on the basis of particular clinical needs or as part of research studies (from the BAA/BCIG/ENT UK joint submission to NICE, March 200749). However, whether bilateral implantation is ultimately carried out will also depend on whether a person’s local primary care trust is willing to fund it.
Severely and profoundly deaf with special needs and multiple sensory handicaps
A relatively high proportion of people (27% of deaf children7 and 45% of severely or profoundly deaf people over the age of 60 years2) have other special needs or other sensory handicaps (such as blindness) (see Chapter 1, Pathology).
Because of significant heterogeneity amongst deaf children and adults who have other needs or handicaps, the effectiveness and cost-effectiveness of cochlear implantation in these subgroups has rarely been studied. In the studies included in the review of clinical effectiveness none reported on the effects of cochlear implants for those with multiple disabilities or focused on those whose cause of deafness involved wider impairments or needs. One study in the additional quality of life review for children looked at the educational impact of cochlear implants for those with Usher type 1 syndrome. 136 Three studies excluded those with other disabilities. None of the studies that did not exclude for other disabilities separately reported outcomes for this group.
Chapter 9 Discussion
The purpose of this report is to assess the effectiveness and cost-effectiveness of cochlear implants for children and adults with severe to profound deafness.
Statement of principal findings
The results for unilateral implantation will be summarised first, followed by those for bilateral implantation.
Unilateral implantation
Clinical effectiveness
The review of clinical effectiveness studies for children indicates that unilateral implantation in severe to profoundly deaf children consistently produces better outcomes than acoustic hearing aids or non-technological support for:
-
sensitivity to sound outcomes (e.g. mean difference of 1.6 points favouring cochlear implants over acoustic hearing aids on a 4-point scale)
-
speech perception (e.g. mean differences ranging from 19.9 on the common phrases test to 56.6 on the ESP battery, both measures favouring cochlear implants over acoustic hearing aids, p < 0.0001)
-
speech production measures (a Pearson correlation of –0.49 between age at implantation and better speech production).
These results may be associated with age at implantation for unilateral and bilateral implantation, children implanted at a younger age obtaining greater benefit than older implantees [e.g. correlation coefficient –0.44 (p < 0.05) for speech perception score].
Similar benefits were found in the adult population. Compared with non-technological support, cochlear implant users had improved understanding of speech ranging from mean (SD) differences of 34.5% (22.56) for CUNY words in quiet to 67.0% (31.5) for CUNY sentences in quiet, as well as quality of life gains with a HUI-3 mean change score for traditional candidates of 0.22 (95% CI 0.19–0.24) and for marginal hearing aid users of 0.15 (95% CI 0.11–0.19) (traditional candidates are profoundly deaf, mean hearing level 117.1 dB; marginal hearing aid users are profoundly deaf, mean hearing level 108.7 dB). These were associated with duration of deafness before implantation and age at implantation. Additional benefit was found compared with acoustic hearing aids, with greater gains in noisy conditions, especially amongst the postlingually deaf (mean score advantage of 37 points, p < 0.001).
Summary of PenTAG’s cost–utility analysis – unilateral
The PenTAG model used a lifetime time horizon. Parameters were obtained from a variety of sources including published clinical and cost-effectiveness studies, national statistical databases, the national NHS purchasing agency, expert opinion and the industry submissions to NICE.
The deterministic results showed that, compared with no provision of cochlear implants, profoundly prelingually deaf children, implanted at age 1 year, benefited from unilateral implantation. The devices conferred an additional 4.48 QALYs for an additional £60,070 per person, giving an estimated ICER of £13,413 per QALY gained.
A similar benefit from unilateral implantation was found for profoundly and postlingually deaf adults implanted at age 50 years compared with non-use of cochlear implants. Here unilateral implantation conferred an additional 2.40 QALYs for an additional £33,959 per person, giving an estimated ICER of £14,163 per QALY gained.
Sensitivity analysis
Deterministic one-way sensitivity analyses showed that the model was extremely sensitive to utility gain, model time horizon, discount rate, major postsurgical complications and maintenance costs. Additionally, the model was also sensitive to changes in discount rates, the time horizon used in the analysis, the discount offered on the cost of a second implant system and the long-term utility gain associated with unilateral implant use as opposed to non-use of cochlear implants.
Probabilistic sensitivity analysis
All results cited below are based on a willingness to pay threshold of £30,000 per QALY and were generated using 1000 Monte Carlo simulations.
In comparison to no provision of cochlear implants, for children, unilateral implantation had the highest net benefit in 100% of simulations and was dominated in 0% of simulations (creating higher costs compared with non-use of cochlear implants but lower QALYs).
Four studies were identified in which the impact of cochlear implant use on the costs of schooling were assessed. On the basis of the most recent study138 the estimate of mean annual savings in educational costs for children between the ages of 5 and 16 years inclusive was £2359. When this value was introduced into all arms of the model in which individuals may benefit from cochlear implants, the baseline ICER for unilateral implantation of children at age 1 year fell from £13,413 per QALY to £9915 per QALY.
In comparison to no provision of cochlear implants, unilateral implantation of adults aged 50 years generated the greatest net benefit in 100% of the Monte Carlo simulations and was dominated in 0% of simulations. The probabilistic mean incremental net benefit was £37,362 (95% Cr I £36,987–37,738) and the probabilistic median incremental net benefit was £37,181.
Bilateral implantation
Clinical effectiveness
Bilateral implantation shows greater benefits than unilateral implantation for children, whether or not the unilateral aid is used with a contralateral acoustic hearing aid. The additional gain is mainly in ‘real life’ noisy situations in which the child is more able to detect the direction that a sound is coming from and pick out a voice from background noise (e.g. mean improvement with bilateral implants of 13.2% over unilateral implants for speech perception in noise).
Adults also benefited from bilateral implantation. Our results showed that they were able to hear more clearly [0.71 (95% CI 0.08–1.33), p < 0.01] (measured on the SSQ scale), better detect the direction of sound in noisy conditions (24°, p < 0.001) and understand speech better [9.00 (95% CI 3.00–15.00), p < 0.01] and that they may have an improved quality of life when compared with quality of life with unilateral implantation. However, the results for improved quality of life for bilateral implantation were ambiguous with positive scores for APHAB communication [5.7 (SE 0.2), p < 0.0001] and non-significant negative results with the HUI-3 [–0.01 (95% CI –0.1 to 0.08), not significant], although the negative results were mainly due to the effects of worsening tinnitus that a few people experienced after their second implant.
Summary of PenTAG’s cost–utility analysis – bilateral
It should be noted that bilateral ICERs for children are speculative as no utility values were found for children.
The speculative results for children when simultaneous bilateral implantation is compared with unilateral implantation (using an assumed utility gain of +0.03) indicate that bilateral implants confer an additional 0.67 QALYs for an additional £27,105 per person, giving an estimated ICER of £40,410 per QALY. With the same assumed utility gain sequential bilateral implantation provides an additional 0.60 QALYs for an additional £32,657 giving an estimated ICER of £54,098 per QALY.
In adults, when simultaneous bilateral implantation is compared with unilateral implantation, bilateral implants confer an additional 0.38 QALYs for an additional £19,048 per person, giving an ICER of £49,559 per QALY. Sequential bilateral implantation provides an additional 0.33 QALYs for an additional £19,678, giving an estimated ICER of £60,301 per QALY.
Similarly there is a high degree of uncertainty surrounding the bilateral ICERs for adults as the utility values are based on one small (n = 24) study.
Sensitivity analysis
For bilateral implantation the deterministic one-way sensitivity analyses showed that the model was extremely sensitive to the incremental utility associated with bilateral cochlear implant use.
In comparison to unilateral implantation, and assuming the same utility gain and associated uncertainty as used in the analysis for adults (i.e. 0.03, which in turn assumes an overall neutral impact of tinnitus), simultaneous (within the same operation) bilateral implantation in children had the greatest net benefit in 34.9% of simulations and was dominated in 16.9% of simulations. The probabilistic mean incremental net benefit is –£7989 (95% Cr I –£9375 to –£6605) and the median incremental net benefit is –£7400. In contrast, sequential (3 years after the first implant) bilateral implantation in children generated the greatest net benefit in 21.3% of simulations and was dominated in 16.2% of simulations. No studies were identified that reported the impact of educational cost savings and which contained values for the incremental utility associated with bilateral cochlear implant use as opposed to unilateral implant use. Assuming the cost savings for simultaneous bilateral use are the same as for unilateral use, the ICER for the same patient group falls from £40,410 per QALY to £40,185 per QALY.
In comparison to unilateral implantation, simultaneous bilateral implantation of adults aged 50 years generated the greatest net benefit in 20.7% of the Monte Carlo simulations and was dominated by unilateral implantation in 13.2% of simulations. In contrast, sequential bilateral implantation of adults aged 50 years generated the greatest net benefit in 8.9% of simulations and was dominated by unilateral implantation in 12.8% of simulations.
However, these results are based on only one study that contained a value for the additional utility associated with bilateral implants versus unilateral implants. This study was very small (24 participants) and the values used here assume that tinnitus had an overall neutral impact on quality of life.
Adverse events
The number of adverse events associated with cochlear implant use is small and similar for adults and children, and the rate of abandoned operations is also low (0.12%). The rate of major complications ranges from 1.4 to 1.7 per 100 patient-years in adults and is 6.8 per 100 patient-years in children (in the first year or two post implantation). The rate of minor complications is 35.3 per 100 patient-years in adults and 34.7 per 100 patient-years in children. Cochlear implants are reliable with 92% of devices lasting 11 years.
Summary of previously published economic evaluations
All systematic reviews of economic evaluations are limited in terms of the extent to which they can produce generalisable conclusions about the cost-effectiveness of interventions in any particular jurisdiction. 218,219 This is a consequence of the typically wide variation in care settings and countries, year of analysis, treatment comparators and specific methods of analysis used in different studies. We therefore concentrated on appraising high-quality recent economic evaluations conducted in the UK. The broad conclusions possible from the review are:
-
In the UK, unilateral implantation has generally been assessed to be cost-effective in either profoundly deaf adults or profoundly deaf children who have been clinically selected for implantation at UK cochlear implant centres.
-
A comprehensive assessment of the resource implications of cochlear implantation should include all care costs from the time of referral for assessment for possible implantation, through surgery and postimplantation treatment of complications, tuning and rehabilitation, to the lifelong costs for device maintenance, repairs and routine replacements. The assessment costs before implantation, and the costs of medical care and other support following implantation, account for a high proportion of the overall health-care costs of providing the technology.
-
There is a paucity of economic studies that have used utility estimates which have been derived from large well-controlled studies of the quality of life of deaf people living with and without cochlear implants.
-
The inclusion of educational cost savings in analyses of cochlear implantation in children can have a significant impact on the resulting cost–utility ratios.
-
Two particular studies on unilateral cochlear implantation stand out as being the most recent, well-conducted and reported studies, as well as being relevant to current NHS provision. 53,192
-
Although the only economic evaluation of bilateral implantation (in adults) was based on an RCT and conducted from a UK NHS perspective, it has some serious limitations (notably a sample size of only 24, and recruitment of people who had been unilateral implant users for between 1 and 6 years).
Candidacy
The criteria for candidacy for cochlear implants are central to the current clinical debate and also to estimates of effectiveness and cost-effectiveness. Unfortunately, largely because of shortcomings in most of the published research literature, we have not been able to address the full range of patient factors that appear to determine the effectiveness of cochlear implantation. However, we would like to note its importance and have suggested some areas for research priority.
In particular, the issue of cost-effectiveness and candidacy centres on at what point the level of residual hearing reaches before it becomes cost-ineffective to provide a cochlear implant rather than an acoustic hearing aid. Profound deafness covers a wide range of loss. Those unable to hear less than about 110 dB are unlikely to use acoustic hearing aids and will score zero on preoperative tests of speech perception. Those unable to hear between 95 and 110 dB probably use acoustic hearing aids and may score above zero on tests of speech identification without lip-reading. Going further into the severe category there are people who will score higher with acoustic hearing aids than those who are most successful with their cochlear implants. It is important to know where on this continuum the boundary of candidature for implantation should be drawn as well as how formal assessments of functional hearing ability should alter candidacy judgements made on the basis of audiologically measured sensitivity to sound.
Although most trials mainly base their inclusion criteria largely on sensitivity to sound (severe > 70 dB HL, profound > 95 dB HL), clinical judgements are more likely to refer to the functional ability of being able to understand prerecorded sentences without lip-reading. These two types of measure may not completely correlate, i.e. two people with the same pure-tone hearing level may have different abilities at understanding speech. Thus, profoundly or severely deaf people do not form a homogeneous group.
Impact of tinnitus
Tinnitus is associated with being deaf and is also positively associated with the severity of deafness. 40,220 A person’s experience of tinnitus may be altered by receiving a cochlear implant, and most evidence points to cochlear implants suppressing tinnitus. For example, in a study by Ruckenstein and colleagues,221 35 of 38 cochlear implant recipients reported a reduction in tinnitus intensity, and in a study by Mo and colleagues40 32 of 59 recipients reported that their tinnitus was better (and a further 21 reported that there was no change in their tinnitus experience). Demajumdar and colleagues222 similarly reported ‘marked suppression’ of tinnitus in a study of 99 implantees, which was often experienced in both the implanted and contralateral ear, and in many of these suppression was also seen when the implant was switched off.
However, in these and other studies a minority of cochlear implant patients report experiencing worsening tinnitus (e.g. 3 out of 22,223 5 out of 59,40 and 4 out of 60224 implant recipients). For these patients the tinnitus may clearly contribute to lower estimates of quality of life and may also be a factor in the non-use of devices by implantees (assuming that their tinnitus is reduced by not having the device switched on). Although for unilateral implant recipients such adverse effects may be relatively small (e.g. compared with the perceived quality of life benefits of enhanced speech perception and production), in bilateral implantation there is some evidence that the experience of worsening tinnitus in a minority may be significant enough to offset any smaller utility gains. 149
Strengths and limitations of the assessment
Strengths of the systematic review of clinical effectiveness
The strengths of this systematic review are that it is systematic, up-to-date and conducted by an independent research team, to address an explicit policy decision problem.
Limitations of the systematic review of clinical effectiveness
There are a number of limitations of the clinical effectiveness systematic review:
-
The systematic review of clinical effectiveness is limited as the number of studies reviewed represents a proportion (at least 75%) of the possible total population in the studies for each comparison, starting with the largest studies. This restriction was made because of limited resources and the large number of eligible studies (n = 51). All of the studies excluded had non-randomised designs and individual sample sizes ranging from three to 41. It is theoretically possible that the results of the excluded studies may have been contrary to those of the included studies. However, we believe that this is unlikely, because of both the large amount of heterogeneity in the included studies and the consistency of the direction of their results.
-
Most of the reviewed studies were of moderate to poor quality; this reflects the standard of reporting more than the choice of design. The absence of key information for quality appraisal and a preference for reporting results graphically rather than in text made it difficult at times to determine exactly how participants had been selected, what the results were and what factors may have confounded the results.
-
The included studies generally measured degree of hearing loss with pure-tone thresholds rather than the functional ability of being able to understand sentences, which is how candidacy is assessed in clinical practice. This may affect the generalisability of the results.
-
The large number of outcomes measured (n = 62) together with the heterogeneity of the studies and lack of RCTs meant that pooling of data was not possible.
-
We were unable to find any studies of adults that compared two cochlear implants with one cochlear implant plus a contralateral acoustic hearing aid.
Strengths of the independent cost–utility analysis
We believe that our analysis represents a valid and reliable attempt to address questions concerning the long-term effectiveness and cost-effectiveness of cochlear implantation, given currently available published evidence and other knowledge about the current provision of the technology in the NHS. In particular:
-
We have made best use of two relatively recent studies of the costs and effectiveness (including HUI-3-measured utility) of paediatric and adult cochlear implantation in UK NHS cochlear implant centres.
-
Our model captures the cost implications of a wide range of events related to preimplantation assessment, implantation surgery and postsurgical care, tuning/rehabilitation and lifelong maintenance. It also included the cost impacts of any major postsurgical complications (usually wound-related) and internal or external device failures. Furthermore (and in contrast to the analyses submitted by manufacturers), it included the assessment costs of those referred deaf people who were ultimately not given a cochlear implant.
-
Our utility estimates were chosen on the basis of a systematic review of all empirical studies reporting the health-related quality of life impacts, or elicited utility values of being severely or profoundly deaf or of receiving a cochlear implant.
-
Both deterministic and fully probabilistic results are produced.
-
No artificial time horizon is imposed on the cohorts; instead they are followed until death.
-
Our model allows for the components of the device to change (because of either device failure or routine replacement).
-
Internal device failure is modelled using techniques from survival analysis rather than assuming a constant failure rate.
-
When possible, costs represent those paid by NHS purchasing units.
-
Subgroups of infant and older child implantees have been investigated.
-
The impact of educational costs have been included in sensitivity analyses.
-
The model also allows people to have failed operations and revert back to a non-implanted status over the course of their remaining lives, reflecting real-world clinical practice.
Limitations of the independent cost–utility analysis
There are two major general limitations to our cost–utility analysis, which we believe any cost–utility analysis in this clinical area would also currently face.
The first is the paucity of high-quality long-term studies that have measured the health-related quality of life associated with having different levels of severe to profound or profound deafness with or without cochlear implantation, in both adults and children, and also that have used a generic instrument that can be responsive to changes in sensory impairments such as deafness. Few large studies have measured quality of life gains for longer than a year after implantation. Also, in the absence of RCT evidence, estimates of utility gain in decision models such as ours inevitably have to assume that the difference between preimplantation- and postimplantation-measured utility is a reasonable proxy for the actual utility gain (i.e. had the person remained without a cochlear implant). There were no studies that estimated the utility gain from bilateral implantation in children, and the only study in adults was a very small (n = 24) RCT.
The second major limitation is that there are a considerable number of other interrelated individual-level factors that are known to impact on the effectiveness (and hence cost-effectiveness) of cochlear implantation relative to alternative acoustic hearing aids, and empirical studies have not always clearly reported these factors or been large enough to explore or statistically control for them (such as audiologically measured severity of deafness, duration of deafness, age at implantation, whether deafened pre- or postlingually).
As discussed elsewhere (under candidacy), although there is a definite positive relationship between increasing severity/profoundness of deafness and measured benefit from cochlear implants this relationship is not perfect; the clinical community increasingly uses an assessment of a deaf person’s functional hearing to predict the likely benefit from a cochlear implant, using audiologically measured deafness in conjunction with other assessments (e.g. in adults, performance on speech perception tests without lip-reading and whilst using optimally fitted hearing aids).
As functional hearing or the ability to benefit materially from acoustic hearing aids currently has no standard single measure and cannot be assessed in the same way for adults and children (and there are other factors that are believed to impact on the likely improvement in performance with a cochlear implant), the effectiveness and cost-effectiveness of cochlear implantation is critically dependent on who is defined as a suitable candidate.
Consequently, our cost–utility results relating to profoundly deaf adults or children should be interpreted as relating to those who both are profoundly deaf (AHL > 95 dB) and have a low level of functional hearing when optimally acoustically aided. Moreover, even within those in this group we have been unable to identify subgroups who had different levels of functional hearing at preimplantation. Any reliable definition of the subgroup of severe to profoundly deaf individuals in whom cochlear implantation is cost-effective would require empirical evidence from studies that have followed a large number of cochlear implant recipients for a number of years post implantation (especially in children), used a valid and appropriate generic measure of health-related quality of life (e.g. HUI-3), and collected preimplantation data on a range of known confounders such as audiologically measured hearing level, standard test scores for assessing functional hearing, duration of severe/profound deafness, age at implantation and age at onset of deafness.
Subgroups and co-factors not assessed
Primarily because of the lack of valid and reliable utility estimates we were unable to assess the cost-effectiveness of unilateral implantation in several potentially important subgroups of deaf people:
-
postlingually deafened children
-
severely deaf adults or children
-
people who have been unilateral cochlear implant users for several years (bilateral implantation)
-
postmeningitic deaf people
-
children and adults with multiple disabilities.
Although the economic evaluation submitted to NICE by Advanced Bionics Europe purported to present estimates of the incremental cost–utility of unilateral cochlear implantation both in ‘severely deaf adults’ and in ‘profoundly postlingually deaf children’, the actual patients from whom the utility estimates were obtained were, respectively, (less) profoundly deaf adults and older, but dominantly still prelingually, deafened children. There has not yet been a study that measures the utility gain from unilateral cochlear implantation specifically in severely deaf adults or children or in postlingually deafened children.
We were also unable to assess the impact on the cost-effectiveness of cochlear implantation of their use with and without a contralateral hearing aid. However, except in the very profoundly deaf it has become common clinical practice to encourage most unilateral cochlear implant users to try out their new device with a contralateral hearing aid (and so this potential subgroup may be irrelevant in assessments of unilateral implantation).
There have now been two large, relatively recent and UK-based empirical studies into the effectiveness and cost–utility of unilateral cochlear implantation, and these have allowed some regression modelling to be undertaken to explore the factors that appear to determine greater short-term utility gains. However, the impact of factors such as functional hearing ability and the presence of complex or additional needs are still quite under-researched, despite both factors being important in current decisions about whether a child or adult is chosen as an appropriate candidate for an implant.
In relation to postmeningitic patients, in whom rapid ossification of the cochleas would usually prevent a second implant at a later date, some people advance arguments that there is a stronger case for simultaneous bilateral implantation. With unilateral implantation in postmeningitic patients, if the implant fails and needs to be explanted, the chance of successful reimplantation in either ear is minimal and so bilateral implantation in these patients – aside from its other potential benefits –serves as a form of ‘insurance policy’ against this eventuality.
Warranties and price discounts
We have chosen to include the cost reductions resulting from device warranties (10 years for internal devices such as electrodes or receiver/stimulators, 3 years for speech processors and other external components) in the base-case analysis. This was justified on the basis that these warranties are standard across the current manufacturers and arguably, therefore, less likely to be withdrawn given their role in assuring device reliability for the clinical community of users. Although this choice is not strictly in line with NICE reference case requirements (which is to use the nationally available list price, without discounts) we felt that it would have been a more inaccurate assessment of the true cost to the NHS of this technology to ignore the warranties. Having said that, any internal device or external device replacements needed within the warranty periods would still incur some operative and other repair/assessment costs to the NHS, which we have not included.
In contrast, price discounts on cochlear implant systems used for bilateral implantation were not included in our base-case analysis. In contrast to device warranties, price discounts for bilateral implantation were different between the manufacturers. Nevertheless we explored this in a sensitivity analysis.
Other potential limitations
-
We have modelled only the profoundly deaf (AHL > 95 dB). Currently, most effectiveness data for cochlear implantation in the profoundly deaf relate to children or adults with higher levels of profound deafness (i.e. AHL > 110 dB).
-
Although the HUI-3 (used in this analysis) has become a commonly used generic instrument for assessing health-related quality of life changes in deaf people, and has some advantages over instruments such as the EQ-5D or SF-6D (SF-36), it still has limitations. For example, it has quite complex wording, it could be criticised for being ‘semigeneric’ (being focused on disability rather than explicitly on health-related quality of life) and it also imposes an artificial ceiling on the health-related quality of life of respondents who depend on devices that assist hearing. Also, the social preference weights (or utilities) currently available for the HUI-3 are not from the UK general public (the main published utility weights are from the public in Ontario or in Canada as a whole). It is therefore possible that valuations of improved hearing and communication by members of the UK public, relative to changes in other aspects of quality of life, may be different from the Canadian values (and might have yielded different estimates of utility gain in this assessment).
-
There is a paucity of high-quality long-term outcome data, particularly in relation to utility estimates but also for key parameters such as complication rates, device failure rates (for recent models), the need for device replacements and upgrades, and voluntary non-use of devices.
-
Ears have not been modelled separately although hearing loss between ears may vary and this may alter the ability to benefit from unilateral cochlear implantation (especially with a contralateral hearing aid) or bilateral cochlear implantation.
-
We have assumed that the initial operation is always successful.
-
Minor complications have not been modelled.
-
Finally, there is also, inevitably, some structural uncertainty in the PenTAG model’s underlying main assumptions. The impact of this on cost–utility estimates is not captured with techniques such as probabilistic sensitivity analysis and other methods for assessing parameter uncertainty. For example, by not having an underlying dynamic model of deafness, we have effectively assumed that being severely or profoundly deaf is a non-progressive condition. For adults this may not be true, but for children it may lead to overestimates of the quality of life impact of being deaf (if relying on differences between preimplantation and postimplantation assessments of utility). Time constraints meant that the impact of structural uncertainty was not explored as much as it could be.
Suggested future research questions and priorities
-
Candidacy:
-
– How much residual hearing can remain before it becomes cost-ineffective to provide an implant rather than an acoustic hearing aid?
-
– What is the earliest age at which the implantation of a congenitally deaf child is safe and effective?
-
– In what ways, if any, should the functionality of a child’s family inform the decision whether or not to offer an implant?
-
-
Utilities:
-
– What is the utility gain for children from bilateral implantation compared with unilateral implantation?
-
– Studies are needed in children and adults that enable mapping (i.e. reliable prediction) from measures of speech perception and production and hearing to validated generic utility assessment instruments.
-
-
Employment:
-
– What are the effects of using cochlear implants on employment prospects, in adults or children compared with profoundly/severely deaf people?
-
-
Long-term follow-up:
-
– Larger studies are needed that follow up implant recipients for longer, use standard measures for outcomes and quality of life impact, and record full information on known covariates of postimplantation speech and quality of life outcomes. There may be a strong case for a national research registry of all cochlear implantees in the UK. Large sample sizes would enable better exploration of implant candidacy, including the relationship between hearing ability, timing of and age at implantation and the presence of additional/complex needs, and key outcomes; this would enable multicriteria models to be developed to help predict the likely benefit profiles of individual candidates (see also the following point).
-
-
Other:
-
– Given that in the UK it now seems to be a central concept in determining which deaf people should be offered a cochlear implant, there may be a case for developing a standard classification system for defining levels of functional hearing (or classes of deaf people with different combinations of performance on standard sound sensitivity tests and standard speech perception tests).
-
– More comparative empirical research is needed into the relative effectiveness of, and patient and clinician preferences for, simultaneous versus sequential bilateral implantation.
-
– Further research is needed on the clinical effectiveness and cost-effectiveness of cochlear implants for children and adults with multiple disabilities and the effects of implants on quality of life.
-
Chapter 10 Conclusion
Unilateral implantation
Despite reservations about the quality of some of the studies included in the clinical effectiveness review we conclude that unilateral cochlear implantation is safe and effective for adults and children; it improves the ability to understand and produce speech and improves quality of life compared with acoustic hearing aids or non-technological support. For children it seems likely that unilateral implantation increases the likelihood of mainstream education. Greater benefits are found with earlier implantation and shorter duration of deafness before implantation.
For profoundly deaf adults and profoundly and prelingually deaf children, unilateral cochlear implants present a cost-effective response. Probabilistic threshold analyses estimate that, when measured on a lifetime horizon and compared with non-technological support or acoustic hearing aids, cochlear implants are highly likely to be considered cost-effective for adults and children at willingness to pay thresholds of £20,000 and £30,000 per QALY.
When potential savings in educational costs (£2359 per annum) for children are introduced into the model, the baseline ICER for unilateral implantation of children at age 1 year falls from £13,413 per QALY to £9915 per QALY.
Bilateral implantation
The clinical effectiveness evidence for bilateral implantation suggests that there is additional gain from having two devices; these may enable people to hold conversations in social situations by being able to filter out voices from background noise and tell the direction that sounds are coming from.
Any conclusion about the cost-effectiveness of bilateral cochlear implants should take into account the high degree of uncertainty within the PenTAG model and its input parameters, most particularly surrounding the utility gain when comparing bilateral with unilateral implantation. This is especially the case for children for whom there were no empirical utility data. However, overall, in both adults and children, our model and the highly uncertain utility gain estimates contained within it suggest that both simultaneous and sequential bilateral implantation would be unlikely to be judged as cost-effective as unilateral implantation (given currently accepted levels of willingness to pay for a QALY in the UK NHS).
There is further uncertainty surrounding any discount offered on the second implant system. Our main estimates have assumed that there are no price discounts and so the ICERs for both adults and children are clearly higher than would be the case with such discounts factored in. The combination of these two areas of uncertainty will have a major impact on any decisions about the adoption of bilateral implantation in the NHS.
Acknowledgements
We would like to acknowledge the help of Sue Whiffin and Jo Perry for their administrative support and Caroline Main for data abstraction.
Expert advisory group
We would particularly like to thank the expert advisory group for their help throughout the project: Professor Q Summerfield, Anniversary Professor of Psychology, University of York; Mr G Barton, Lecturer in Health Economics, University of East Anglia; Dr J Parsons, Consultant Clinical Scientist, Mid, East Devon and Exeter Areas, Devon Primary Care Trust; Ms J Martin, Specialist Advisory Teacher of the Deaf, YCIS Service Coordinator, Bradford Royal Infirmary; Professor GM O’Donoghue, Professor of Otology and Neurotology, Queen’s Medical Centre, Nottingham; Miss T Twomey, Head of Nottingham Cochlear Implant Programme, Nottingham University Hospitals, Chair British Cochlear Implant Group; Dr J Niparko, Director, Division of Otology, Neurotology and Skull Base Surgery, Department of Otolaryngology – Head and Neck Surgery, Johns Hopkins University, USA.
Competing interests of expert advisory group
Professor GM O’Donoghue has given professional advice and received hospitality from all cochlear implant manufacturers. Ms Jane Martin works as service coordinator on a cochlear implant team and would offer professional advice. Professor Q Summerfield from time to time has given unpaid advice to manufacturers of cochlear implants. He has presented scientific data at meetings organised by manufacturers of cochlear implants and has accepted their hospitality. Likewise, from time to time he has given unpaid advice to clinicians in the NHS and to charities with which they are involved. He has presented scientific data at meetings organised by clinicians in the NHS and by charities with which they are involved, and has accepted their hospitality. Dr John Niparko has provided consultations to the US FDA and Centers for Medicine/Medicaid Services and to the Cochlear Corporation, Advanced Bionics Corporation and the Medtronics Corporation on clinical results and cost–utility outcomes with cochlear implantation. He has received travel support only and no personal remuneration for these consultations. These arrangements have been, and continue to be, reviewed by the Johns Hopkins Conflict of Interest Committee. No conflicts have been identified at any time.
Contribution of authors
Rob Anderson oversaw the cost-effectiveness aspects of the analysis and report and obtained costs for the model, contributed to writing the report, led the critique of the economic evaluations provided by the manufacturers and contributed to the design and development of the model and editing of the report. Mary Bond provided overall project management, wrote the protocol, assessed abstracts for inclusion and exclusion, contributed to writing and editing of the report and contributed to the design of the model. Julian Elston assessed abstracts for inclusion and exclusion, contributed to writing and editing of the report and contributed to the design of the model. Martin Hoyle verified and contributed to the model and reviewed and edited the economic section of the report. Zulian Liu assessed abstracts for inclusion and exclusion, reviewed published economic evaluations and contributed to writing and editing of the report. Stuart Mealing led the design, development and execution of the economic model and contributed to writing of the report (economics section). Alison Price undertook literature searches for the systematic reviews. Ken Stein contributed to the design of the assessment, the design and development of the model and the preparation and editing of the report. Rod Taylor contributed to the design of the model, advised on analysis of the clinical effectiveness data and contributed to the editing of the report. Graeme Weiner provided clinical input into the design of the model, advised on clinical matters and contributed to the editing of the report.
About PenTAG
The Peninsula Technology Assessment Group (PenTAG) is part of the Institute of Health Service Research at the Peninsula Medical School. PenTAG was established in 2000 and carries out independent health technology assessments for the UK HTA Programme and other local and national decision-makers. The group is multidisciplinary and draws on individuals’ backgrounds in public health, health services research, computing and decision analysis, systematic reviewing, statistics and health economics. The Peninsula Medical School is a school within the Universities of Plymouth and Exeter. The Institute of Health Research is made up of discrete but methodologically related research groups, among which health technology assessment is a strong and recurring theme. Projects to date include:
Screening for hepatitis C among injecting drug users and in genitourinary medicine (GUM) clinics: systematic reviews of effectiveness, modelling study and national survey of current practice. Health Technol Assess 2000;6(31).
Systematic review of endoscopic sinus surgery for nasal polyps. Health Technol Assess 2003;7(17).
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: systematic review and economic modelling. Health Technol Assess 2004;8(3).
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis. Health Technol Assess 2002;8(28).
Do the findings of case series studies vary significantly according to methodological characteristics? Health Technol Assess 2005;9(2).
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation. Health Technol Assess 2005;9(29).
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation. Health Technol Assess 2005;9(43).
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling. Health Technol Assess 2006;10(8).
The cost-effectiveness of testing for hepatitis C (HCV) in former injecting drug users. Health Technol Assess 2006;10(32).
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation. Health Technol Assess 2007;11(18).
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly-diagnosed high-grade glioma: a systematic review and economic evaluation. Health Technol Assess 2007;11(45).
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model. Health Technol Assess 2007;11(47).
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Abutan BB, Hoes AW, Van Dalsen CL, Verschuure J, Prins A. Prevalence of hearing impairment and hearing complaints in older adults: a study in general practice. Fam Pract 1993;10:391-5.
- Royal National Institute for the Deaf . Information and Resources for Deaf and Hard of Hearing People, Their Families, Friends and Employers, and Professionals 2006. www.rnid.org.uk/information_resources/.
- Owens D, Espeso A, Hayes J, Williams RG. Cochlear implants: referral, selection and rehabilitation. Curr Paediatr 2006;16:360-5.
- Copeland BJ, Pillsbury HC. Cochlear implantation for the treatment of deafness. Annu Rev Med 2004;55:157-67.
- Davis A, Bamford J, Ramkalawan T, Forshaw M, Wright S. A critical review of the role of neonatal screening in the detection of hearing impairment. Health Technol Assess 1997;1.
- Fortnum HM, Summerfield AQ, Marshall DH, Davis AC, Bamford JM. Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire-based ascertainment study [see comment]. Br Med J 2001;323:536-40.
- Fortnum HM, Marshall DH, Summerfield AQ. Epidemiology of the UK population of hearing-impaired children, including characteristics of those with and without cochlear implants – audiology, aetiology, comorbidity and affluence. Int J Audiol 2002;41:170-9.
- Weinstein BE. Geriatric hearing loss: myths, realities, resources for physicians. Geriatrics 1989;44:42-8.
- Sataloff J. Hearing loss. Philadelphia and Toronto: JB Lippincott; 1966.
- Sangster JF, Gerace M, Seewald RC. Hearing loss in elderly patients in a family practice. Can Med Assoc J 1991;2144:981-4.
- Gilhome Herbst KR, Humphrey C. Hearing impairment and mental state in the elderly living at home. Br Med J 1980;281:903-5.
- Eisenberg LS. Use of the cochlear implant by the prelingually deaf. Ann Otol Rhinol Laryngol Suppl 1982;91:62-6.
- Fellinger J, Holzinger D, Dobner U, Gerich J, Lehner R, Lenz G, et al. Mental distress and quality of life in a deaf population. Soc Psychiatr Psychiatr Epidemiol 2005;40:737-42.
- Beadle EA, McKinley DJ, Nikolopoulos TP, Brough J, O’Donoghue GM, Archbold SM. Long-term functional outcomes and academic–occupational status in implanted children after 10 to 14 years of cochlear implant use. Otol Neurotol 2005;26:1152-60.
- Wake M, Hughes EK, Collins CM, Poulakis Z. Parent-reported health-related quality of life in children with congenital hearing loss: a population study. Ambul Pediatr 2004;4:411-17.
- Hallberg LRM, Ringdahl A. Living with cochlear implants: experiences of 17 adult patients in Sweden. Int J Audiol 2004;43:115-21.
- Arlinger S. Negative consequences of uncorrected hearing loss – a review. Int J Audiol 2003;42:2S17-20.
- De Civita M, Regier D, Alamgir AH, Anis AH, FitzGerald MJ, Marra CA. Evaluating health-related quality-of-life studies in paediatric populations: some conceptual, methodological and developmental considerations and recent applications. Pharmacoeconomics 2005;23:659-85.
- Lin FR, Niparko JK. Measuring health-related quality of life after pediatric cochlear implantation: a systematic review. Int J Pediatr Otorhinolaryngol 2006;70:1695-706.
- Balkany T, Hodges AV, Goodman KW. Ethics of cochlear implantation in young children. Otolaryngol Head Neck Surg 1996;114:748-55.
- Stacey PC, Fortnum HM, Barton GR, Summerfield AQ. Hearing-impaired children in the United Kingdom. I: Auditory performance, communication skills, educational achievements, quality of life, and cochlear implantation. Ear Hear 2006;27:161-86.
- Huber M. Health-related quality of life of Austrian children and adolescents with cochlear implants. Int J Pediatr Otorhinolaryngol 2005;69:1089-101.
- Hallman JL. The development of a scoring system for the cochlear implant questionnaire for parents for assessing the quality of life of pediatric cochlear implant recipients. Dissertation Abstracts International 2003;63.
- Joore MA, Potjewijd J, Timmerman AA, Anteunis LJ. Response shift in the measurement of quality of life in hearing impaired adults after hearing aid fitting. Qual Life Res 2002;11:299-307.
- Karinen PJ, Sorri MJ, Valimaa TT, Huttunen KH, Lopponen HJ. Cochlear implant patients and quality of life. Scand Audiol Suppl 2001:48-50.
- Barton GR, Bankart J, Davis AC. A comparison of the quality of life of hearing-impaired people as estimated by three different utility measures. Int J Audiol 2005;44:157-63.
- Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist 2003;43:661-8.
- Robinson SK, McQuaid JR, Viirre ES, Betzig LL, Miller DL, Bailey KA, et al. Relationship of tinnitus questionnaires to depressive symptoms, quality of well-being, and internal focus. Int Tinnitus J 2003;9:97-103.
- Erlandsson SI, Hallberg LR. Prediction of quality of life in patients with tinnitus. Br J Audiol 2000;34:11-20.
- Knutson JF, Johnson A, Murray KT. Social and emotional characteristics of adults seeking a cochlear implant and their spouses. Br J Health Psychol 2006;11:279-92.
- Knutson JF, Murray KT, Husarck S, Westerhouse K, Woodworth G, Gantz B, et al. change over 54 months of cochlear implant use. Ear Hear 1998;19:191-20.
- Burger T, Spahn C, Richter B, Eissele S, Lohle E, Bengel J. Psychic stress and quality of life in parents during decisive phases in the therapy of their hearing-impaired children. Ear Hear 2006;27:313-20.
- Mulrow CD, Aguilar C, Endicott JE, Tuley MR, Velez R, Charlip WS, et al. Quality-of-life changes and hearing impairment. A randomized trial. Ann Intern Med 1990;113:188-94.
- Bai Z, Stephens D. Subjective outcome measures after cochlear implantation: overall measures. Audiol Med 2005;3:212-19.
- Carabellese C, Appollonio I, Rozzini R, Bianchetti A, Frisoni GB, Frattola L, et al. Sensory impairment and quality of life in a community elderly population. J Am Geriatr Soc 1993;41:401-7.
- Lee PP, Smith JP, Kington RS. The associations between self-rated vision and hearing and functional status in middle age. Ophthalmology 1999;106:401-5.
- Pugh KC, Crandell CC. Hearing loss, hearing handicap, and functional health status between African American and Caucasian American seniors. J Am Acad Audiol 2002;13:493-502.
- Cruickshanks KJ, Tweed TS, Wiley TL, Klein BE, Klein R, Chappell R, et al. The 5-year incidence and progression of hearing loss: the Epidemiology of Hearing Loss Study. Arch Otolaryngol Head Neck Surg 2003;129:1041-6.
- Mo B, Lindbaek M, Harris S. Cochlear implants and quality of life: a prospective study. Ear Hear 2005;26:186-94.
- Mo B, Harris S, Lindboek M. Tinnitus in cochlear implant patients – a comparison with other hearing-impaired patients. Int J Audiol 2002;41:527-34.
- Yardley L, Dibb B, Osborne G. Factors associated with quality of life in Meniere’s disease. Clin Otolaryngol Allied Sci 2003;28:436-41.
- Spahn C, Burger T, Loschmann C, Richter B. Quality of life and psychological distress in parents of children with a cochlear implant. Cochlear Implants Int 2004;5:13-27.
- Hogan A, Hawthorne G, Kethel L, Giles E, White K, Stewart M, et al. Health-related quality-of-life outcomes from adult cochlear implantation: a cross-sectional survey. Cochlear Implants Int 2001;2:115-28.
- Gaines RA. The value of deaf culture: should states have the right to mandate placement of cochlear implants?. Curr Surg 2003;60:600-1.
- Nunes R. Ethical dimension of paediatric cochlear implantation. Theor Med Bioeth 2001;22:337-49.
- Knights BR. A review of the educational, cost-effectiveness, and cultural considerations of cochlear implantation. Aust J Otolaryngol 2000;3:523-7.
- Wald RL, Knutson JF. Deaf cultural identity of adolescents with and without cochlear implants. Ann Otol Rhinol Laryngol Suppl 2000;185:87-9.
- Department of Health . Improving Access to Audiology Services in England 2007.
- Twomey T, Craddock L, Raine C. Cochlear implants for deafness in children and adults. BAA/BCIG/ENT UK; 2007.
- Cohen NL, Waltzman SB, Fisher SG. A prospective, randomized study of cochlear implants. The Department of Veterans Affairs Cochlear Implant Study Group. N Engl J Med 1993;328:233-7.
- Rubinstein JT, Parkinson WS, Tyler RS, Gantz BJ. Residual speech recognition and cochlear implant performance: effects of implantation criteria. Am J Otol 1999;20:445-52.
- Arnoldner C, Baumgartner WD, Gstoettner W, Hamzavi J. Surgical considerations in cochlear implantation in children and adults: a review of 342 cases in Vienna. Acta Otolaryngol 2005;125:228-34.
- UK Cochlear Implant Study Group . Criteria of candidacy for unilateral cochlear implantation in postlingually deafened adults. II: Cost-effectiveness analysis. Ear Hear 2004;25:336-60.
- Gray RF, Jones SE, Court I. Cochlear implantation for progressive hearing loss. Arch Dis Child 2003;88:708-11.
- Barton GR, Bloor KE, Marshall DH, Summerfield AQ. Health-service costs of pediatric cochlear implantation: multi-center analysis. Int J Pediatr Otorhinolaryngol 2003;67:141-9.
- Curtis L, Netten A. Unit costs of health and social care 2006. Canterbury, PSSRU: University of Kent; 2006.
- NHS Centre for Reviews and Dissemination . Undertaking Systematic Reviews of Research on Effectiveness 2001.
- Drummond MF, Drummond MF, Maynard A, Wells N. Purchasing and providing cost-effective health care. Edinburgh: Churchill Livingstone; 1993.
- Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7.
- Gantz BJ, Woodworth GG, Knutson JF, Abbas PJ, Tyler RS. Multivariate predictors of audiological success with multichannel cochlear implants. Ann Otol Rhinol Laryngol 1993;102:909-16.
- Cheng AK, Grant GD, Niparko JK. Meta-analysis of pediatric cochlear implant literature. Ann Otol Rhinol Laryngol Suppl 1999;177:124-8.
- UK Cochlear Implant Study Group . Criteria of candidacy for unilateral cochlear implantation in postlingually deafened adults. I: Theory and measures of effectiveness. Ear Hear 2004;25:310-35.
- Robbins AM, Renshaw J, Berry SW. Evaluating meaningful auditory integration in profoundly hearing-impaired children. Am J Otol 1991;12:144-50.
- Gatehouse S, Noble W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Audiology 2004;43:85-99.
- van den Borne S, Snik AF, Hoekstra CC, Vermeulen AM, van den Broek P, Brokx JP. Assessment of basal sound identification skills and communication abilities in profoundly deaf children fitted with hearing aids or a cochlear implant. Clin Otolaryngol Allied Sci 1998;23:455-61.
- Archbold S, Robinson K. A European perspective on pediatric cochlear implantation, rehabilitation services, and their educational implications. Am J Otol 1997;18:S75-8.
- Bench J, Bamford J. Speech-hearing tests and the spoken language of hearing-impaired children. London: Academic Press; 1979.
- Moog JS, Geers AE. Early speech perception test. St Louis, MO: Central Institute for the Deaf; 1990.
- Erber NP. Auditory training. Washington, DC: Alexander Graham Bell Association for the Deaf; 1982.
- Illg A, von der Haar-Heise S, Goldring JE, Lesinski-Schiedat A, Battmer RD, Lenarz T. Speech perception results for children implanted with the CLARION cochlear implant at the Medical University of Hannover. Ann Otol Rhinol Laryngol Suppl 1999;177:93-8.
- Boothroyd A. Developments in speech audiometry. Br J Audiol 1968;2:3-10.
- Archbold S, Lutman ME, Marshall DH. Categories of auditory performance. Ann Otol Rhinol Laryngol 1995;104:312-14.
- De Filippo CL, Scott B. A method for training and evaluating the reception of ongoing speech. J Acoust Soc Am 1978;63:1186-92.
- Hirsh IJ, Davis H, Silverman SR, Reynolds EG, Eldert E, Bensen RW. Development of materials for speech auditometry. J Speech Hear Disord 1952;17:321-37.
- Peterson FE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord 1962;27:62-70.
- Robbins AM, Osberger M. Common phrases test. Indianapolis, IN: Indiana University School of Medicine; 1988.
- Boothroyd A, Hannin L, Hnath T. A Sentence Test of Speech Perception: Reliability Set Equivalence and Short Term Learning (Internal Report RCI 10) 1985.
- Bosman AJ. Moderne Verfahren der Sprachaudiometrie. Heidelberg: Medion-Verlag von Killisch-Horn; 1992.
- Kuhn-Inacker H, Shehata-Dieler W, Muller J, Helms J. Bilateral cochlear implants: a way to optimize auditory perception abilities in deaf children?. Int J Pediatr Otorhinolaryngol 2004;68:1257-66.
- Nilsson MJ, Soli S, Sullivan J. Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc Am 1994;95:1085-99.
- Hochmair-Desoyer I, Schulz E, Moser L, Schmidt M. The HSM sentence test as a tool for evaluating the speech understanding in noise of cochlear implant users. Am J Otol 1997;18.
- Tyler R, Holstad B. A closed set speech perception test for hearing-impaired children. Iowa City, IA: University of Iowa; 1987.
- Kirk KI, Pisoni D, Osberger M. Lexical effects on spoken word recognition by pediatric cochlear implant users. Ear Hear 1995;16:470-81.
- Owens E, Kessler DK, Raggio M, Schubert ED. Analysis and revision of the Minimal Auditory Capabilities (MAC) battery. Ear Hear 1985;6:285-7.
- Kirk KI, Pisoni DB, Sommers MS, Young M, Evanson C. New directions for assessing speech perception in persons with sensory aids. Ann Otol Rhinol Laryngol 1995;104:300-3.
- Robbins AM. Mr Potato Head task. Indianapolis, IN: Indiana University School of Medicine; 1993.
- Tillman TW, Carhart R. An expanded test for speech recognition utilizing CNC monosyllabic words. San Antonio, TX: Brooks Air Force Base; 1966.
- Wagener K, Kuhnel K, Kollmeier B. Development and evaluation of a German sentence test. I. Design of the Oldenburger sentence test. Audiology 1999;38:4-15.
- Haskins HA. A phonetically balanced test of speech discrimination for children. Evanston, IL: Northwestern University; 1949.
- Engen E, Engen T. Rhode Island test of language structure. Baltimore, MD: University Park Press; 1983.
- Moog JS, Geers AE. Scales of early communication skills for hearing impaired children. St Louis, MO: Central Institute for the Deaf; 1975.
- Office of the Los Angeles County Superintendent of Schools . Test of Auditory Comprehension 1976.
- Demenko G, Rychter L, Pruszewicz A, Szyfter W, Woznica B. Tests of auditory perception of speech TAPS for children with cochlear implants. Otolaryngol Pol 1996;50:628-32.
- Bishop DVM. Test for the reception of grammar. Manchester: University of Manchester Age and Cognitive Performance Research Centre; 1989.
- Litovsky RY. Method and system for rapid and reliable testing of speech intelligibility in children. Acoust Soc Am 2004;115.
- Scarborough HS. Index of productive syntax. Appl Psycholinguist 1990;11:1-22.
- Dyar D, McCormick B, Archbold S, Sheppard S. Cochlear implants for young children. London: Whurr Publishers; 1994.
- Cox RM, Alexander GC. The abbreviated profile of hearing aid benefit. Ear Hear 1995;16:176-83.
- Hawthorne G, Richardson J, Osborne R. The Assessment of Quality of Life (AQoL) instrument: a psychometric measure of health-related quality of life. Qual Life Res 1999;8:209-24.
- Kelsay DM, Tyler RS. Advantages and disadvantages expected and realized by pediatric cochlear implant recipients as reported by their parents. Am J Otol 1996;17:866-73.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72.
- Dolan P, Gudex C, Kind P, Williams A. A social tariff for the EuroQol: results from a UK general population survey. York: University of York, Centre for Health Economics; 1995.
- Robinson K, Gatehouse S, Browning GG. Measuring patient benefit from otorhinolaryngological surgery and therapy. Ann Otol Rhinol Laryngol 1996;105:415-22.
- MRC Institute of Hearing Research . The Glasgow Health Status Questionnaires Manual 1998.
- Newman CW, Weinstein BE, Jacobson GP, Hug GA. The hearing handicap inventory for adults: psychometric adequacy and audiometric correlates. Ear Hear 1990;11:430-3.
- Hawthorne G, Hogan A. Measuring disability-specific patient benefit in cochlear implant programs: developing a short form of the Glasgow Health Status Inventory, the Hearing Participation Scale. Int J Audiol 2002;41:535-44.
- Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ 1986;5:1-30.
- Crary WG, Wexler M, Berliner KI, Miller LW. Psychometric studies and clinical interviews with cochlear implant patients. Ann Otol Rhinol Laryngol Suppl 1982;912:55-8.
- Ravens-Sieberer U, Bullinger M. Fragenbogen zur Erfassung der gesundheitsbezogenen Lebensqualitat bei Kindern und Jugendlichen. Hamburg: University of Hamburg; 2000.
- Hinderink JB, Krabbe PF, Van Den Broek P. Development and application of a health-related quality-of-life instrument for adults with cochlear implants: the Nijmegen cochlear implant questionnaire. Otolaryngol Head Neck Surg 2000;123:756-65.
- Bullinger M, Kirchberger I, von Steinbuchel N. The Everyday Life Questionnaire – EDLQ – an instrument for the assessment of health related quality of life. Z Med Psychol 1993;3:121-31.
- Ware JE, Snow KK, Kosinski M. Health survey: manual and interpretation guide. Lincoln, RI: Quality Metric; 2000.
- Frank G. The Symptom Checklist of Derogatis 1995.
- Jakes SC, Hallam RS, Chambers C, Hinchcliffe R. A factor analytic study of tinnitus complaint behaviour. Audiology 1985;24:195-206.
- Damen GW, Krabbe PF, Kilsby M, Mylanus E. The Usher lifestyle survey; a multicentre study. Int J Rehabil Res 2005;28:309-20.
- Chute PM. Assessing Mainstream Performance in Children With Cochlear Implants n.d.
- Anderson KL. SIFTER Screening Identification for Targeting Educational Risk in Children Identified by Hearing Screening or Who Have Minimal Hearing Loss Users Manual. Danville, IL: The Interstate Printers and Publisher; 1989.
- Kiefer J, Muller J, Pfennigdorff T, Schon F, Helms J, von Ilberg C, et al. Speech understanding in quiet and in noise with the CIS speech coding strategy (MED-EL Combi-40) compared to the multipeak and spectral peak strategies (nucleus). J Otorhinolaryngology Rel Spec 1996;58:127-35.
- Loizou PC, Dorman M, Powell V. The recognition of vowels produced by men, women, boys and girls by cochlear implant patients using a six-channel CIS processor. J Acoust Soc Am 1998;103:1141-9.
- Nikolopoulos TP, Dyar D, Archbold S, O’Donoghue GM. Development of spoken language grammar following cochlear implantation in prelingually deaf children. Arch Otolaryngol Head Neck Surg 2004;130:629-33.
- Nikolopoulos TP, O’Donoghue GM, Archbold S. Age at implantation: its importance in pediatric cochlear implantation. Laryngoscope 1999;109:595-9.
- Manrique M, Cervera-Paz FJ, Huarte A, Molina M. Prospective long-term auditory results of cochlear implantation in prelinguistically deafened children: the importance of early implantation. Acta Otolaryngol 2004:55-63.
- Harrison RV, Gordon KA, Mount RJ. Is there a critical period for cochlear implantation in congenitally deaf children? Analyses of hearing and speech perception performance after implantation. Dev Psychobiol 2005;46:252-61.
- Staller S, Parkinson A, Arcaroli J, Arndt P. Pediatric outcomes with the nucleus 24 contour: North American clinical trial. Ann Otol Rhinol Laryngol Suppl 2002;189:56-61.
- MED-EL . Summary of Safety and Effectiveness Data 2001. http://www.fda.gov/cdrh/pdf/P000025b.pdf (accessed February 2007).
- Kessler DK, Osberger MJ, Boyle P. CLARION patient performance: an update on the adult and children’s clinical trials. Scand Audiol Suppl 1997;47:45-9.
- Manrique M, Cervera-Paz FJ, Huarte A, Molina M. Advantages of cochlear implantation in prelingual deaf children before 2 years of age when compared with later implantation. Laryngoscope 2004;114:1462-9.
- Mildner V, Sindija B, Zrinski KV. Speech perception of children with cochlear implants and children with traditional hearing aids. Clin Linguist Phon 2006;20:219-29.
- Tomblin JB, Spencer L, Flock S, Tyler R, Gantz B. A comparison of language achievement in children with cochlear implants and children using hearing aids. J Speech Lang Hear Res 1999;42:497-511.
- Osberger MJ, Zimmerman-Phillips S, Barker M, Geier L. Clinical trial of the CLARION cochlear implant in children. Ann Otol Rhinol Laryngol Suppl 1999;177:88-92.
- Svirsky MA, Meyer TA. Comparison of speech perception in pediatric CLARION cochlear implant and hearing aid users. Ann Otol Rhinol Laryngol Suppl 1999;177:104-9.
- Osberger MJ, Fisher L, Zimmerman-Phillips S, Geier L, Barker MJ. Speech recognition performance of older children with cochlear implants. Am J Otol 1998;19:152-7.
- Peters BR, Litovsky R, Parkinson A, Lake J. Importance of age and postimplantation experience on speech perception measures in children with sequential bilateral cochlear implants. Otol Neurotol 2007;29:649-57.
- Litovsky RY, Johnstone PM, Godar S, Agrawal S, Parkinson A, Peters R, et al. Bilateral cochlear implants in children: localization acuity measured with minimum audible angle. Ear Hear 2006;27:43-59.
- Litovsky RY, Johnstone PM, Godar SP. Benefits of bilateral cochlear implants and/or hearing aids in children. Int J Audiol 2006;45:S78-91.
- Damen GW, Pennings RJ, Snik AF, Mylanus EA. Quality of life and cochlear implantation in Usher syndrome type I. Laryngoscope 2006;116:723-8.
- Chmiel R, Sutton L, Jenkins H. Quality of life in children with cochlear implants. Ann Otol Rhinol Laryngol Suppl 2000;185:103-5.
- Barton GR, Stacey PC, Fortnum HM, Summerfield AQ. Hearing-impaired children in the United Kingdom. II: Cochlear implantation and the cost of compulsory education. Ear Hear 2006;27:187-20.
- Damen GWJ, van den Oever-Goltstein MH, Langereis MC, Chute P, Mylanus EAM. Classroom performance of children with cochlear implants in mainstream education. Ann Otol Rhinolaryngol 2006;115:542-52.
- Thoutenhoofd E. Cochlear implanted pupils in Scottish Schools: 4-year school attainment data (2000–2004). J Deaf Stud Deaf Educ 2006;11:171-88.
- Archbold SM, Nikolopoulos TP, Lutman ME, O’Donoghue GM. The educational settings of profoundly deaf children with cochlear implants compared with age-matched peers with hearing aids: implications for management. Int J Audiol 2002;41:157-61.
- Fortnum HM, Marshall DH. Hearing-impaired children in the UK: education setting and communication approach. Deafness Educ Int 2002;4:123-41.
- Archbold S, Nikolopoulos TP, O’Donoghue GM, Lutman ME. Educational placement of deaf children following cochlear implantation. Br J Audiol 1998;32:295-300.
- UK Cochlear Implant Study Group . Criteria of candidacy for unilateral cochlear implantation in postlingually deafened adults. III: Prospective evaluation of an actuarial approach to defining a criterion. Ear Hear 2004;25:361-74.
- Mawman DJ, Bhatt YM, Green KMJ, O’Driscoll MP, Saeed SR, Ramsden RT. Trends and outcomes in the Manchester adult cochlear implant series. Clin Otolaryngol 2004;29:331-9.
- Parkinson AJ, Arcaroli J, Staller SJ, Arndt PL, Cosgriff A, Ebinger K. The nucleus 24 contour cochlear implant system: adult clinical trial results. Ear Hear 2002;23:41S-48S.
- Hamzavi J, Franz P, Baumgartner WD, Gstoettner W. Hearing performance in noise of cochlear implant patients versus severely-profoundly hearing-impaired patients with hearing aids. Audiology 2001;40:26-31.
- Ching TY, Incerti P, Hill M. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear Hear 2004;25:9-21.
- Summerfield AQ, Barton GR, Toner J, McAnallen C, Proops D, Harries C, et al. Self-reported benefits from successive bilateral cochlear implantation in post-lingually deafened adults: randomised controlled trial. Int J Audiol 2006;45:S99-107.
- Ramsden R, Greenham P, O’Driscoll M, Mawman D, Proops D, Craddock L, et al. Evaluation of bilaterally implanted adult subjects with the Nucleus 24 cochlear implant system. Otol Neurotol 2005;26:988-98.
- Verschuur CA, Lutman ME, Ramsden R, Greenham P, O’Driscoll M. Auditory localization abilities in bilateral cochlear implant recipients. Otol Neurotol 2005;26:965-71.
- Litovsky R, Parkinson A, Arcaroli J, Sammeth C. Simultaneous bilateral cochlear implantation in adults: a multicenter clinical study. Ear Hear 2006;27:714-31.
- Laszig R, Aschendorff A, Stecker M, Muller-Deile J, Maune S, Dillier N, et al. Benefits of bilateral electrical stimulation with the nucleus cochlear implant in adults: 6-month postoperative results. Otol Neurotol 2004;25:958-68.
- Vermeire K, Brokx JPL, Wuyts FL, Cochet E, Hofkens A, Van de Heyning PH. Quality-of-life benefit from cochlear implantation in the elderly. Otol Neurotol 2005;26:188-95.
- Hawthorne G, Hogan A, Giles E, Stewart M, Kethel L, White K, et al. Evaluating the health-related quality of life effects of cochlear implants: a prospective study of an adult cochlear implant program. Int J Audiol 2004;43:183-92.
- Palmer CS, Niparko JK, Wyatt JR, Rothman M, de Lissovoy G. A prospective study of the cost-utility of the multichannel cochlear implant. Arch Otolaryngol Head Neck Surg 1999;125:1221-8.
- Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. New Brunswick: Aldine Transaction; 1967.
- Ray J, Gibson WPR, Sanli H. Surgical complications of 844 consecutive cochlear implantations and observations on large versus small incisions. Cochlear Implants Int 2004;5:87-95.
- Bhatia K, Gibbin KP, Nikolopoulos TP, O’Donoghue GM. Surgical complications and their management in a series of 300 consecutive pediatric cochlear implantations. Otol Neurotol 2004;25:730-9.
- Carter R, Hailey D. Economic evaluation of the cochlear implant. Int J Technol Assess Health Care 1999;15:520-30.
- Proops DW, Stoddart RL, Donaldson I. Medical, surgical and audiological complications of the first 100 adult cochlear implant patients in Birmingham. J Laryngol Otol Suppl 1999;24:14-7.
- Dutt SN, Ray J, Hadjihannas E, Cooper H, Donaldson I, Proops DW. Medical and surgical complications of the second 100 adult cochlear implant patients in Birmingham. J Laryngol Otol 2005;119:759-64.
- Fayad JN, Eisenberg LS, Gillinger M, Winter M, Martinez AS, Luxford WM. Clinical performance of children following revision surgery for a cochlear implant. Otolaryngol Head Neck Surg 2006;134:379-84.
- Josefson D. Cochlear implants carry risk of meningitis, agencies warn. Br Med J 2002;325.
- Summerfield AQ, Cirstea SE, Roberts KL, Barton GR, Graham JM, O’Donoghue GM. Incidence of meningitis and of death from all causes among users of cochlear implants in the United Kingdom. J Public Health 2004;27:55-61.
- MHRA . Cochlear Implants 2002.
- Cohen N, Ramos A, Ramsden R, Baumgarten W, Lesisnski A, O’Donoghue G, et al. International consensus on meningitis and cochlear implants. Acta Otolaryngol 2005;125:916-17.
- Reefhuis J, Honein MA, Whitney CG, Chamany S, Mann EA, Biernath KR, et al. Risk of bacterial meningitis in children with cochlear implants [see comment]. N Engl J Med 2003;349:435-45.
- Summerfield A Q, Marshall D. Non-use of cochlear implants by post-lingually deafened adults. Cochlear Implants Int 2000;1:18-39.
- Bhatt YM, Green KMJ, Mawman DJ, Aplin Y, O’Driscoll MP, Saeed SR, et al. Device nonuse among adult cochlear implant recipients. Otol Neurotol 2005;26:183-7.
- Archbold SM, Nikolopoulos TP, Lloyd H. Long-term use of cochlear implant systems in paediatric recipients and factors contributing to non-use 2006:1-30.
- Ray J, Wright T, Fielden C, Cooper H, Donaldson I, Proops DW. Non-users and limited users of cochlear implants. Cochlear Implants Int 2006;7:49-58.
- Balkany TJ, Hodges AV, Gomez-Marin O, Bird PA, Dolan-Ash S, Butts S, et al. Cochlear reimplantation. Laryngoscope 1999;109:351-5.
- Stratigouleas ED, Perry BP, King SM, Syms CA. Complication rate of minimally invasive cochlear implantation. Otolaryngol Head Neck Surg 2006;135:383-6.
- Lassig AA, Zwolan TA, Telian SA. Cochlear implant failures and revision. Otol Neurotol 2005;26:624-34.
- Maurer J, Marangos N, Ziegler E. Reliability of cochlear implants. Otolaryngol Head Neck Surg 2005;132:746-50.
- Von Wallenberg EL, Brinch JM. Cochlear implant reliability. Ann Otol Rhinolaryngol 1995;104:441-3.
- Ajayi F, Garnham C, O’Donoghue G. Pediatric experience of the reliability of the Nucleus Mini 22-channel cochlear implant. Am J Otol 1997;18:44-5.
- Conboy PJ, Gibbin KP. Paediatric cochlear implant durability: the Nottingham experience. Cochlear Implants Int 2004;5:131-7.
- Lehnhardt M, Von Wallenberg EL, Brinch JM. Reliability of the nucleus CI22 and CI24M cochlear implants. Ann Otol Rhinol Laryngol Suppl 2000;185:14-6.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Lea AR, Hailey DM. The cochlear implant. A technology for the profoundly deaf. Med Prog Technol 1995;21:47-52.
- Summerfield AQ, Marshall DH, Davis AC. Cochlear implantation: demand, costs, and utility. Ann Otol Rhinol Laryngol 1995;104:S245-8.
- Sach TH, Whynes DK, O’Neill C, O’Donoghue GM. Willingness-to-pay for pediatric cochlear implantation. Int J Pediatr Otorhinolaryngol 2004;68:91-9.
- Cheng AK, Niparko JK. Cost-utility of the cochlear implant in adults: a meta-analysis. Arch Otolaryngol Head Neck Surg 1999;125:1214-18.
- O’Neill C, O’Donoghue GM, Archbold SM, Normand C. A cost-utility analysis of pediatric cochlear implantation [see comment]. Laryngoscope 2000;110:156-60.
- Neilson AR. Cost-effectiveness of cochlear implantation in adults. Oslo: Norwegian Knowledge Centre for the Health Services; 2006.
- Schulze-Gattermann H, Illg A, Schoenermark M, Lenarz T, Lesinski-Schiedat A. Cost-benefit analysis of pediatric cochlear implantation: German experience. Otol Neurotol 2002;23:674-81.
- Summerfield AQ, Marshall DH, Barton GR, Bloor KE. A cost-utility scenario analysis of bilateral cochlear implantation. Arch Otolaryngol Head Neck Surg 2002;128:1255-62.
- Summerfield AQ, Marshall DH, Archbold S. Cost-effectiveness considerations in pediatric cochlear implantation. Am J Otol 1997;18:S166-8.
- Summerfield AQ, Marshall DH. Cochlear Implantation in the UK 1990–1994 1995.
- Barton GR, Stacey PC, Fortnum HM, Summerfield AQ. Hearing-impaired children in the United Kingdom. IV: Cost-effectiveness of pediatric cochlear implantation. Ear Hear 2006;27:575-88.
- Hutton J, Politi C, Seeger T. Cost-effectiveness of cochlear implantation of children. A preliminary model for the UK. Adv Otorhinolaryngol 1995;50:201-6.
- O’Neill C, Archbold SM, O’Donoghue GM, McAlister DA, Nikolopoulos TP. Indirect costs, cost-utility variations and the funding of paediatric cochlear implantation. Int J Pediatr Otorhinolaryngol 2001;58:53-7.
- Barton GR, Bloor KE, Marshall DH, Summerfield AQ. Health service costs of paediatric cochlear implantation: influence of the scale and scope of activity. Int J Audiol 2004;43:369-76.
- Barton GR, Fortnum HM, Stacey PC, Summerfield AQ. Hearing-impaired children in the United Kingdom. III: Cochlear implantation and the economic costs incurred by families. Ear Hear 2006;27:563-74.
- Evans AR, Seeger T, Lehnhardt M. Cost-utility analysis of cochlear implants. Ann Otol Rhinol Laryngol 1995;104:S239-40.
- Sach T, O’Neill C. Cochlear implantation: cost creating or cost saving?. Oxford: Hughes Associates; 2002.
- Sach T, O’Neill C, Whynes DK, Archbold SM, O’Donoghue GM. Evidence of improving cost-effectiveness of pediatric cochlear implantation. Int J Technol Assess Health Care 2003;19:421-31.
- Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993;13:322-38.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal 2004.
- NICE . Social Value Judgements: Principles for the Development of NICE Guidance 2005.
- Government Actuarial Department n.d. www.gad.gov.uk/Life_Tables/Interim_Life_Tables.htm (accessed 20 April 2007).
- Royal National Institute for the Deaf . Best Practice Standards for Adult Audiology n.d. www.rnid.org.uk/information_resources/factsheets/hearing_aids/factsheets_leaflets/best_practice_standards_for_adult_audiology.htm (accessed 26 March 2007).
- Boor S, Maurer J, Mann W, Stoeter P. Virtual endoscopy of the inner ear and the auditory canal. Neuroradiology 2000;42:543-7.
- Briggs A, Sculpher M, Claxton K. Decision modelling in health economic evaluation. Oxford: Oxford University Press; 2006.
- Francis HW, Koch ME, Wyatt JR, Niparko JK. Trends in educational placement and cost-benefit considerations in children with cochlear implants. Arch Otolaryngol Head Neck Surg 1999;125:499-505.
- Francis HW, Chee N, Yeagle J, Cheng A, Niparko JK. Impact of cochlear implants on the functional health status of older adults. Laryngoscope 2002;112:1482-8.
- Wyatt JR, Niparko JK, Rothman M, de Lissovoy G. Cost utility of the multichannel cochlear implant in 258 profoundly deaf individuals. Laryngoscope 1996;106:816-21.
- Lee HY, Park EC, Joong KH, Choi JY, Kim HN. Cost-utility analysis of cochlear implants in Korea using different measures of utility. Acta Otolaryngol 2006;126:817-23.
- Krabbe PF, Hinderink JB, Van Den Broek P. The effect of cochlear implant use in postlingually deaf adults. Int J Technol Assess Health Care 2000;16:864-73.
- Damen GW, Beynon AJ, Krabbe PF, Mulder JJ, Mylanus EA. Cochlear implantation and quality of life in postlingually deaf adults: long-term follow-up. Otolaryngol Head Neck Surg 2007;136:597-604.
- Kind P, Hardman G, Macran S. UK population norms for EQ-5D. York: University Of York, Centre for Health Economics; 2002.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:S68-80.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87.
- Kos MI, Degive C, Boex C, Guyot JP. Professional occupation after cochlear implantation. J Laryngol Otol 2007;121:215-18.
- Sach TH, Whynes DK, Archbold SM, O’Donoghue GM. Estimating time and out-of-pocket costs incurred by families attending a pediatric cochlear implant programme. Int J Pediatr Otorhinolaryngol 2005;69:929-36.
- Mugford M, Donaldson C, Mugford M, Vale L. Evidence-based health economics: from effectiveness to efficiency in systematic review. London: BMJ Books; 2002.
- Pignone M, Saha S, Hoerger T, Lohr K, Teutsch S, Mandelblatt J. Challenges in systematic reviews of economic analyses. Ann Intern Med 2005;142:1073-9.
- Sanchez L. The epidemiology of tinnitus. Audiol Med 2004;2:8-17.
- Ruckenstein MJ, Hedgepeth C, Rafter KO, Montes ML, Bigelow DC. Tinnitus suppression in patients with cochlear implants. Otol Neurotol 2001;22:200-4.
- Demajumdar R, Stoddart R, Donaldson I, Proops DW. Tinnitus, cochlear implants and how they affect patients. J Laryngol Otol Suppl 1999;24:24-6.
- Tyler RS. Tinnitus in the profoundly hearing-impaired and the effects of cochlear implants. Ann Otol Rhinol Laryngol Suppl 1995;165:25-30.
- Ito J, Sakakihara J. Suppression of tinnitus by cochlear implantation. Am J Otolaryngol Head Neck Med Surg 1994;15:145-8.
Appendix 1 Literature search strategies
A wide range of databases and other information resources were searched to locate details of both published and unpublished studies and other information on the clinical effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness.
All resources were searched from their inception to the most recent date available. There was no restriction on study by publication date. The search was limited to English language papers only. The bibliographies of retrieved references were checked for additional publications. The results of the searches were imported into Reference Manager 11 bibliographic management software and deduplicated. All initial searches were carried out in October 2006 and the update searches were rerun in July 2007.
The following databases were searched: MEDLINE (Ovid), EMBASE (Ovid), Ovid MEDLINE® In-Process & Other Non-Indexed Citations, ISI Science Citation Index, Cochrane Database of Systematic Reviews, CENTRAL, NHS EED, DARE, HTA (NHS-CRD), EconLit, Biosis Previews, ISI Proceedings, Current Controlled Trials, National Research Register and ClinicalTrials.gov.
Relevant internet sites were searched for information including the following regulatory sites: Medical Health and Regulatory Agency (MHRA), US Food and Drug Administration (FDA) and the European Regulatory Agency – Medical Device Safety Service (MDSS).
Full search strategies are listed in the following sections.
Search strategy: cochlear implants for severe to profound deafness in children and adults
Clinical searches: searched by Alison Price 12–19 October 2006, updated July 2007
Cochrane Library – CDSR – Issue 3/2006
-
#1. MeSH descriptor Hearing Loss explode all trees
-
#2. MeSH descriptor Deafness explode all trees
-
#3. MeSH descriptor Hearing Disorders explode all trees
-
#4. severe to profound deafness
-
#5. (severe NEAR/5 deaf*)
-
#6. (profound* NEAR/5 deaf*)
-
#7. deaf*
-
#8. (hear* NEAR/5 loss)
-
#9. (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8)
-
#10. MeSH descriptor Cochlear Implants explode all trees
-
#11. MeSH descriptor Cochlear Implantation explode all trees
-
#12. (cochlea* NEAR/10 (implant* or device* or prosthe*))
-
#13. (#10 OR #11 OR #12)
-
#14. (#9 AND #13)
Cochrane Library – CENTRAL – Issue 3/2006
As above
Ovid MEDLINE® – 1966 to October Week 1 2006
Searched 12 October 2006, saved as med-cochlear-clini-effect-final-all
-
exp hearing loss/(37541)
-
exp hearing loss, sensorineural/(14163)
-
“Hearing Loss, Bilateral”/(1190)
-
exp deafness/(17839)
-
severe to profound deafness.mp. (15)
-
(severe adj4 deaf$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (327)
-
(profound adj4 deaf$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (542)
-
Hearing Loss, Unilateral/(34)
-
exp Hearing Disorders/(49630)
-
deaf$.ti,ab. (19840)
-
(hear$adj5 loss).ti,ab. (19425)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (61873)
-
exp ear, middle/or exp ear, inner/(47537)
-
12 or 13 (98248)
-
Cochlear Implants/(4019)
-
Cochlear Diseases/(557)
-
Cochlear Implantation/(1476)
-
(cochlear adj10 (implant$or device$)).ti,ab. (4660)
-
15 or 16 or 17 or 18 (6089)
-
14 and 19 (4964)
-
limit 20 to (humans and english language) (3781)
-
randomized controlled trial.pt. (238589)
-
controlled clinical trial.pt. (77157)
-
randomized controlled trials/(49082)
-
random allocation/(59319)
-
double-blind method/(91964)
-
single-blind method/(10803)
-
exp evaluation studies/(602476)
-
exp clinical trials/(196879)
-
clinical trial.pt. (463567)
-
(clin$adj5 trial$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (599913)
-
22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 (1058607)
-
((singl$or doubl$or tripl$or trebl$) adj5 (blind$or mask$)).tw. (89036)
-
exp placebos/(26048)
-
placebo$.tw. (102143)
-
random$.tw. (373635)
-
exp research design/(219553)
-
33 or 34 or 35 or 36 or 37 (553879)
-
32 or 38 (1269102)
-
limit 39 to human (1064650)
-
21 and 40 (463)
-
(review or review-tutorial or review-academic).pt. (1271244)
-
(Medline or medlars or embase).ti,ab,sh. (22444)
-
(scisearch or psychinfo or psycinfo).ti,ab,sh. (1115)
-
(Psychlit or psyclit).ti,ab,sh. (704)
-
cinahl.ti,ab,sh. (1949)
-
((hand adj59 search$) or (manual$adj9 search$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (3717)
-
(electronic database$or bibliographic database$or computeri#ed database$or online database$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (3852)
-
(pooling or pooled or mantel haenszel).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (25514)
-
(peto or dersimonian or der simonian or fixed effect).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (1060)
-
43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 (50925)
-
42 and 51 (18566)
-
meta-analysis.pt. (14472)
-
meta-analysis.sh. (7449)
-
(meta-analys$or meta analys$or metaanalys$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (26392)
-
(systematic$adj9 review$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (12274)
-
(systematic$adj9 overview$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (425)
-
(quantitativ$adj9 review$).mp. (1686)
-
(quantitativ$adj9 overview$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (151)
-
(quantitativ$adj9 synthesis$).mp. (1331)
-
(methodologic$adj9 review$).mp. (2451)
-
(methodologic$adj9 overview$).mp. (145)
-
(integrative research review$or research integration).mp. (49)
-
or/53–63 (39296)
-
52 or 64 (51255)
-
21 and 65 (10)
-
41 not 66 (461)
-
Waiting Lists/(4600)
-
(wait$adj10 (surgery or operat$or implant$or list$or control$)).ti,ab. (5725)
-
exp case-control studies/or exp cohort studies/(889029)
-
68 or 69 or 70 (895766)
-
71 and 21 (726)
-
72 not (66 or 67) (620)
EMBASE – 1980 to 2006 Week 41
Searched 16 October 2006
-
exp Hearing Impairment/(35069)
-
exp Congenital Deafness/(2186)
-
Perception Deafness/(6680)
-
hearing loss/or mixed hearing loss/or unilateral hearing loss/(10373)
-
exp ear disease/(47491)
-
severe to profound deafness.mp. (18)
-
(severe adj4 deaf$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (272)
-
(profound adj4 deaf$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (457)
-
deaf$.ti,ab. (13992)
-
(hear$adj5 loss).ti,ab. (16635)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 (76335)
-
cochlea prosthesis/(4375)
-
Implantation/(13919)
-
(cochlea$adj10 (implant$or device$or prosthe$)).ti,ab. (4396)
-
12 or 13 or 14 (18264)
-
11 and 15 (3683)
-
limit 16 to (humans and english language) (2934)
-
randomization/(20626)
-
controlled study/(2270014)
-
single blind procedure/(6140)
-
placebo/(90992)
-
double blind procedure/(61537)
-
clinical trial/(398197)
-
crossover procedure/(17930)
-
placebo$.tw. (94492)
-
blind$fashion.tw. (3499)
-
random$.tw. (317764)
-
clinical trial?.tw. (93827)
-
or/18–28 (2655359)
-
limit 29 to human (1686676)
-
17 and 30 (801)
-
exp meta analysis/(27478)
-
meta#analy$.ab,sh,ti. (27479)
-
methodologic$review$.ab,sh,ti. (124)
-
methodologic$overview$.ab,sh,ti. (29)
-
(integrative research adj5 review$).mp. or research integration.ab,ti. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (24)
-
quantitat$synthesis.ab,sh,ti. (87)
-
quantitat$review$.ab,sh,ti. (245)
-
quantitat$overview$.ab,sh,ti. (58)
-
systematic$review$.ab,sh,ti. (19122)
-
systematic$overview$.ab,sh,ti. (276)
-
32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 (39054)
-
17 and 42 (7)
-
43 not 31 (4)
-
cohort analysis/(37235)
-
(wait$adj10 (surgery or operat$or implant$or list$or control$)).ti,ab. (4919)
-
45 or 46 (42058)
-
17 and 47 (25)
-
48 not (44 or 31) (11)
Ovid MEDLINE® In-Process & Other Non-Indexed Citations – 13 October 2006
Searched 16 October 2006
-
severe to profound deafness.mp. (1)
-
(severe adj4 deaf$).mp. (10)
-
(profound adj4 deaf$).mp.
-
deaf$.ti,ab. (357)
-
(hear$adj5 loss).ti,ab. (613)
-
1 or 2 or 3 or 4 or 5 (880)
-
(cochlear adj10 (implant$or device$)).ti,ab. (173)
-
6 and 7 (82)
-
limit 8 to english language (69)
-
randomized controlled trial.pt. (282)
-
controlled clinical trial.pt. (20)
-
clinical trial.pt. (309)
-
(clin$adj5 trial$).mp. (3970)
-
10 or 11 or 12 or 13 (4254)
-
((singl$or doubl$or tripl$or trebl$) adj5 (blind$or mask$)).tw. (1668)
-
placebo$.tw. (2272)
-
random$.tw. (16771)
-
15 or 16 or 17 (17799)
-
14 or 18 (20448)
-
19 and 9 (3)
-
(review or review-tutorial or review-academic).pt. (496)
-
meta-analysis.pt. (2)
-
(meta-analys$or meta analys$or metaanalys$).mp. [mp=title, original title, abstract, name of substance word] (912)
-
(systematic$adj9 review$).mp. [mp=title, original title, abstract, name of substance word] (990)
-
(systematic$adj9 overview$).mp. [mp=title, original title, abstract, name of substance word] (18)
-
(quantitativ$adj9 review$).mp. (76)
-
(quantitativ$adj9 overview$).mp. [mp=title, original title, abstract, name of substance word] (6)
-
(quantitativ$adj9 synthesis$).mp. (41)
-
(methodologic$adj9 review$).mp. (107)
-
(methodologic$adj9 overview$).mp. (9)
-
(integrative research review$or research integration).mp. (3)
-
or/21–31 (1891)
-
32 and 9 (1)
-
(wait$adj10 (surgery or operat$or implant$or list$or control$)).ti,ab.
-
34 and 9 (1)
Web of Knowledge (SCI-EXPANDED) – 1970 to present
| #14 | 82 |
#13 AND #12 DocType = All document types; Language = English; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #13 | >100,000 |
TS = (trial* or random*) DocType = All document types; Language = English; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #12 | 1699 |
#11 AND #6 DocType = All document types; Language = English; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #11 | 4048 |
#10 OR #9 OR #8 OR #7 DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #10 | 262 |
TS = (cochlea* SAME prosthe*) DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #9 | 300 |
TS = (cochlea* SAME device*) DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #8 | 3786 |
TS = (cochlea* SAME implant*) DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #7 | 3945 |
TS = (Cochlea* Implant*) DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #6 | 30,982 |
#5 OR #4 OR #3 OR #2 OR #1 DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #5 | 6563 |
TS = (hear* SAME/5 loss) DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #4 | 15,103 |
TS = (deaf*) DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #3 | 219 |
TS = (profound* SAME/5 deaf*) DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #2 | 235 |
TS = (severe SAME/5 deaf*) DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #1 | 20,949 | TS = (Hearing Loss or Deafness or Hearing Disorders) |
Web of Science Proceedings – 1990 to present
| #7 | 8 |
#6 AND #5 DocType = All document types; Language = English; Database = STP; Timespan = 2003–2006 |
| #6 | 55,983 |
TS = (trial* or random*) DocType = All document types; Language = English; Database = STP; Timespan = 2003–2006 |
| #5 | 126 |
#4 AND #3 DocType = All document types; Language = English; Database = STP; Timespan = 2003–2006 |
| #4 | 302 |
TI = (cochlea* SAME (implant* or device* or prosthe*)) DocType = All document types; Language = English; Database = STP; Timespan = 2003–2006 |
| #3 | 1,129 |
#2 OR #1 DocType = All document types; Language = All languages; Database = STP; Timespan = 2003–2006 |
| #2 | 574 |
TS = (deaf*) DocType = All document types; Language = All languages; Database = STP; Timespan = 2003–2006 |
| #1 | 889 | TS = (Hearing Loss or Deafness or Hearing Disorders) |
Web of Knowledge BIOSIS Previews – 1990–2006
| #9 | 4 |
#9 DocType = All document types; LitType = Meeting Abstract; Language = English; Taxa Notes = Humans; Database = BIOSIS Previews; Timespan = 2003–2006 |
| #8 | 20 |
#8 DocType = All document types; LitType = Meeting Abstract; Language = English; Taxa Notes = Humans; Database = BIOSIS Previews; Timespan = 1990–2006 |
| #7 | 63 |
#6 AND #5 DocType = All document types; LitType = All literature types; Language = English; Taxa Notes = All Taxa Notes; Database = BIOSIS Previews; Timespan = 1990–2006 |
| #6 | >100,000 |
TS = (trial* or random*) DocType = All document types; LitType = All literature types; Language = English; Taxa Notes = All Taxa Notes; Database = BIOSIS Previews; Timespan = 1990–2006 |
| #5 | 2,007 |
#4 AND #1 DocType = All document types; LitType = All literature types; Language = English; Taxa Notes = All Taxa Notes; Database = BIOSIS Previews; Timespan = 1990–2006 |
| #4 | 35903 |
#3 OR #2 DocType = All document types; LitType = All literature types; Language = English; Taxa Notes = All Taxa Notes; Database = BIOSIS Previews; Timespan = 1990–2006 |
| #3 | 6966 |
TS = (hear* SAME/5 loss) DocType = All document types; LitType = All literature types; Language = English; Taxa Notes = All Taxa Notes; Database = BIOSIS Previews; Timespan = 1990–2006 |
| #2 | 29,809 |
TS = (deaf*) DocType = All document types; LitType = All literature types; Language = English; Taxa Notes = All Taxa Notes; Database = BIOSIS Previews; Timespan = 1990–2006 |
| #1 | 2916 |
TS = (cochlea* SAME (implant* or device* or prosthe*)) DocType = All document types; LitType = All literature types; Language = English; Taxa Notes = All Taxa Notes; Database = BIOSIS Previews; Timespan = 1990–2006 |
DARE
(cochlea* AND (implant* or device* or prosthe*))
HTA database (on CRD databases)
(cochlea* SAME (implant* or device* or prosthe*))
NRR (National Research Register)
| #1 | (cochlea* near implant*) | 177 |
| #2 | (cochlea* near device*) | 8 |
| #3 | (cochlea* near prosthe*) | 2 |
| #4 | (hearing near loss) | 302 |
| #5 | deaf* | 426 |
| #6 | (#1 or #2 or #3) | 177 |
| #7 | (#4 or #5) | 664 |
| #8 | (#7 and #6) | 73 |
Current Controlled Trials including MRC Trials dB (http://controlled-trials.com/)
“Cochlear implants”
ClinicalTrials.gov (http://clinicaltrials.gov/)
Cochlear implant*
National Guidelines Clearinghouse
Cochlear implant*
FDA Center for Devices and Radiological Health (www.fda.gov)
Cochlear implant*
Medical Healthcare and Regulatory Authority
Cochlear implants
PsycINFO including PsycARTICLES – 1985 to present
-
severe to profound deafness.mp. (2)
-
(severe adj4 deaf$).mp. [mp=title, abstract, heading word, table of contents, key concepts] (52)
-
(profound adj4 deaf$).mp. [mp=title, abstract, heading word, table of contents, key concepts] (64)
-
exp Hearing Disorders/(6023)
-
deaf$.ti,ab. (4905)
-
(hear$adj5 loss).ti,ab. (1927)
-
1 or 2 or 3 or 4 or 5 or 6 (7807)
-
Cochlear Implants/(506)
-
(cochlear adj10 (implant$or device$)).ti,ab. (641)
-
8 or 9 (666)
-
7 and 10 (458)
-
(random$or trial$).mp. [mp=title, abstract, heading word, table of contents, key concepts] (80452)
-
11 and 12 (17)
-
limit 13 to (human and english language) (16)
Economics searches
Cochrane Library – CENTRAL – Issue 3/2006
-
#1. MeSH descriptor Hearing Loss explode all trees
-
#2. MeSH descriptor Deafness explode all trees
-
#3. MeSH descriptor Hearing Disorders explode all trees
-
#4. severe to profound deafness
-
#5. (severe NEAR/5 deaf*)
-
#6. (profound* NEAR/5 deaf*)
-
#7. deaf*
-
#8. (hear* NEAR/5 loss)
-
#9. (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8)
-
#10. #10 MeSH descriptor Cochlear Implants explode all trees
-
#11. MeSH descriptor Cochlear Implantation explode all trees
-
#12. (cochlea* NEAR/10 (implant* or device* or prosthe*))
-
#13. (#10 OR #11 OR #12)
-
#14. (#9 AND #13)
-
#15. MeSH descriptor Costs and Cost Analysis explode all trees
-
#16. MeSH descriptor Models, Economic explode all trees
-
#17. MeSH descriptor Cost-Benefit Analysis explode all trees
-
#18. (cost* NEAR (benefit* or utilit* or minim* or effective))
-
#19. (#15 OR #16 OR #17 OR #18)
-
#20. (#19 AND #14)
Ovid MEDLINE® – 1966 to October Week 1 2006
-
exp hearing loss/(37560)
-
exp hearing loss, sensorineural/(14170)
-
“Hearing Loss, Bilateral”/(1190)
-
exp deafness/(17844)
-
severe to profound deafness.mp. (15)
-
(severe adj4 deaf$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (327)
-
(profound adj4 deaf$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (542)
-
Hearing Loss, Unilateral/(34)
-
exp Hearing Disorders/(49653)
-
deaf$.ti,ab. (19850)
-
(hear$adj5 loss).ti,ab. (19438)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (61902)
-
exp ear, middle/or exp ear, inner/(47551)
-
12 or 13 (98289)
-
Cochlear Implants/(4022)
-
Cochlear Diseases/(557)
-
Cochlear Implantation/(1477)
-
(cochlear adj10 (implant$or device$)).ti,ab. (4664)
-
cochleostomy.ti,ab. (105)
-
15 or 16 or 17 or 18 or 19 (6114)
-
14 and 20 (4988)
-
limit 21 to (humans and english language) (3791)
-
exp ECONOMICS/(363519)
-
exp ECONOMICS, HOSPITAL/(14374)
-
exp ECONOMICS, PHARMACEUTICAL/(1692)
-
exp ECONOMICS, NURSING/(3702)
-
exp ECONOMICS, DENTAL/(3386)
-
exp ECONOMICS, MEDICAL/(10144)
-
exp “Costs and Cost Analysis”/(128319)
-
Cost-Benefit Analysis/(39954)
-
VALUE OF LIFE/(4935)
-
exp MODELS, ECONOMIC/(4995)
-
exp FEES/and CHARGES/(7025)
-
exp BUDGETS/(9323)
-
(economic$or price$or pricing or financ$or fee$or pharmacoeconomic$or pharma economic$).tw. (315970)
-
(cost$or costly or costing$or costed).tw. (187673)
-
(cost$adj2 (benefit$or utilit$or minim$or effective$)).tw. (48482)
-
(expenditure$not energy).tw. (10092)
-
(value adj2 (money or monetary)).tw. (565)
-
budget$.tw. (10347)
-
(economic adj2 burden).tw. (1322)
-
“resource use”.ti,ab. (2043)
-
or/23–41 (733772)
-
news.pt. (105429)
-
letter.pt. (582201)
-
editorial.pt. (199280)
-
comment.pt. (318112)
-
or/44–47 (924522)
-
43 not 48 (677588)
-
22 and 49 (148)
-
exp Hearing Disorders/ec [Economics] (132)
-
23 or 29 or 30 or 31 or 32 or 33 or 34 (367556)
-
51 and 52 (103)
-
limit 53 to english language (86)
-
54 not 48 (75)
-
55 or 50 (206)
EMBASE – 1980 to 2006 Week 41
-
exp Hearing Impairment/(35069)
-
exp Congenital Deafness/(2186)
-
Perception Deafness/(6680)
-
hearing loss/or mixed hearing loss/or unilateral hearing loss/(10373)
-
exp ear disease/(47491)
-
severe to profound deafness.mp. (18)
-
(severe adj4 deaf$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (272)
-
(profound adj4 deaf$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (457)
-
deaf$.ti,ab. (13992)
-
(hear$adj5 loss).ti,ab. (16635)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 (76335)
-
cochlea prosthesis/(4375)
-
Implantation/(13919)
-
(cochlea$adj10 (implant$or device$or prosthe$)).ti,ab. (4396)
-
12 or 13 or 14 (18264)
-
11 and 15 (3683)
-
limit 16 to (humans and english language) (2934)
-
(cost$adj2 effective$).ti,ab. (35195)
-
(cost$adj2 benefit$).ti,ab. (7330)
-
cost effectiveness analysis/(45567)
-
cost benefit analysis/(24596)
-
budget$.ti,ab. (7519)
-
cost$.ti. (33258)
-
(cost$adj2 (effective$or utilit$or benefit$or minimi$)).ab. (38347)
-
(economic$or pharmacoeconomic$or pharmaco economic$).ti. (12947)
-
(price$or pricing$).ti,ab. (9586)
-
(financial or finance or finances or financed).ti,ab. (19752)
-
(fee or fees).ti,ab. (4563)
-
cost/(18617)
-
cost minimization analysis/(1039)
-
cost of illness/(3417)
-
cost utility analysis/(1754)
-
drug cost/(27654)
-
health care cost/(49256)
-
health economics/(8752)
-
economic evaluation/(3338)
-
economics/(4999)
-
pharmacoeconomics/(875)
-
budget/(6673)
-
economic burden.ti,ab. (1284)
-
“resource use”.ti,ab. (1791)
-
or/18–41 (203782)
-
(editorial or letter).pt. (495203)
-
42 not 43 (181874)
-
17 and 44 (95)
-
*”cost benefit analysis”/or *”cost effectiveness analysis”/or *”cost minimization analysis”/or *”cost of illness”/or *”cost utility analysis”/(9260)
-
11 and 46 (65)
-
45 or 47 (152)
-
limit 48 to (human and english language) (141)
Ovid MEDLINE® In-Process & Other Non-Indexed Citations – 18 October 2006
-
severe to profound deafness.mp. (1)
-
(severe adj4 deaf$).mp. [mp=title, original title, abstract, name of substance word] (10)
-
(profound adj4 deaf$).mp. [mp=title, original title, abstract, name of substance word] (10)
-
deaf$.ti,ab. (360)
-
(hear$adj5 loss).ti,ab. (624)
-
(cochlear adj10 (implant$or device$)).ti,ab. (174)
-
1 or 2 or 3 or 4 or 5 (894)
-
6 and 7 (83)
-
limit 8 to english language (69)
-
(economic$or price$or pricing or pharmacoeconomic$or pharma economic$).tw. (3454)
-
(cost$or budget$).tw. (7154)
-
(cost$adj2 (benefit$or utilit$or minim$)).tw. (399)
-
(value adj2 (money or monetary)).tw. (23)
-
10 or 11 or 12 or 13 (9666)
-
9 and 14 (4)
-
7 and 14 (22)
-
15 or 16 (22)
SCI-EXPANDED – 1970–2006
| #12 | 57 |
#11 AND #6 DocType = All document types; Language = English; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #11 | 1720 |
#10 AND #9 DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #10 | 3904 |
TS = (cochlea* SAME (implant* or device* or prosthes*)) DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #9 | 26,686 |
#8 OR #7 DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #8 | 15,113 |
TS = (deaf*) DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #7 | 14,551 |
TS = (hearing SAME (loss or disorders)) DocType = All document types; Language = All languages; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #6 | >100,000 |
#5 OR #4 OR #3 OR #2 OR #1 DocType = All document types; Language = English; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #5 | 51,944 |
TS = (cost* SAME effective*) DocType = All document types; Language = English; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #4 | 818 |
TS = (value SAME (money or monetary)) DocType = All document types; Language = English; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #3 | 36,218 |
TS = (cost* SAME (benefit* or utilit* or minim*)) DocType = All document types; Language = English; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #2 | >100,000 |
TS = (cost* or costly or costing* or costed) DocType = All document types; Language = English; Database = SCI-EXPANDED; Timespan = 1970–2006 |
| #1 | >100,000 |
TS = (economic* or price* or pricing or pharmacoeconomic* or pharma economic*) DocType = All document types; Language = English; Database = SCI-EXPANDED; Timespan = 1970–2006 |
NHS EED (CRD database)
(cochlea* SAME (implant* or device* or prosthe*))
EconLit
hearing disorder or deaf*
Epidemiology searches
Ovid MEDLINE® – 1966 to September Week 3 2006
-
1. exp hearing loss/(10251)
-
2. exp hearing loss, sensorineural/(7929)
-
3. “Hearing Loss, Bilateral”/(10251)
-
4. exp deafness/(34934)
-
5. Deafness/ep, et [Epidemiology, Etiology] (2073)
-
6. severe to profound deafness.mp. (18)
-
7. (severe adj4 deaf$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (272)
-
8. (profound adj4 deaf$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (456)
-
9. 7 or 8 (672)
-
10. or/1–9 (35041)
-
11. exp Incidence/(98905)
-
12. exp Prevalence/(111736)
-
13. incidence.ti. (28941)
-
14. prevalence.ti. (32779)
-
1. Risk Factors/(180965)
-
2. epidemiol$.ti. (38186)
-
3. etiolog$.ti. (10334)
-
4. aetiolog$.ti. (3549)
-
5. or/11–18 (411077)
-
6. *Epidemiology/(9142)
-
7. *Incidence/(1536)
-
8. *Prevalence/(2300)
-
9. incidence.ti. (28941)
-
10. prevalence.ti. (32779)
-
11. epidemiol$.ti. (38186)
-
12. etiolog$.ti. (10334)
-
13. aetiolog$.ti. (3549)
-
14. or/20–27 (116821)
-
15. (uk or united kingdom or england or scotland or britain or wales or great britain).in. (842631)
-
16. 10 and 19 and 29 (262)
-
17. united kingdom.cp. or united kingdom.sh. (2004975)
-
18. (uk or united kingdom or england or scotland or britain or wales or great britain).in,cp. (2306024)
-
19. 10 and 28 and 32 (161)
-
20. limit 33 to (human and english language) (155)
-
21. from 34 keep 3 (1)
-
22. exp hearing disorder/or exp hearing impairment/or hearing loss/or exp congenital deafness/or exp perception deafness/(43021)
-
23. hearing loss.ti,ab. (15512)
-
24. deafness.ti,ab. (7544)
-
25. (hearing adj5 disability).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (410)
-
26. 1 or 6 or 7 or 8 or 36 or 37 or 38 or 39 (46690)
-
27. 28 and 32 and 40 (188)
-
28. limit 41 to (human and english language) (180)
EMBASE – 1980 to 2006 Week 39
-
exp hearing loss/(10251)
-
exp hearing loss, sensorineural/(7929)
-
“Hearing Loss, Bilateral”/(10251)
-
exp deafness/(34934)
-
Deafness/ep, et [Epidemiology, Etiology] (2073)
-
severe to profound deafness.mp. (18)
-
(severe adj4 deaf$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (272)
-
(profound adj4 deaf$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (456)
-
7 or 8 (672)
-
or/1–9 (35041)
-
exp Incidence/(98905)
-
exp Prevalence/(111736)
-
incidence.ti. (28941)
-
prevalence.ti. (32779)
-
Risk Factors/(180965)
-
epidemiol$.ti. (38186)
-
etiolog$.ti. (10334)
-
aetiolog$.ti. (3549)
-
or/11–18 (411077)
-
*Epidemiology/(9142)
-
*Incidence/(1536)
-
*Prevalence/(2300)
-
incidence.ti. (28941)
-
prevalence.ti. (32779)
-
epidemiol$.ti. (38186)
-
etiolog$.ti. (10334)
-
aetiolog$.ti. (3549)
-
or/20–27 (116821)
-
(uk or united kingdom or england or scotland or britain or wales or great britain).in. (842631)
-
10 and 19 and 29 (262)
-
united kingdom.cp. or united kingdom.sh. (2004975)
-
(uk or united kingdom or england or scotland or britain or wales or great britain).in,cp. (2306024)
-
10 and 28 and 32 (161)
-
limit 33 to (human and english language) (155)
-
from 34 keep 3 (1)
-
exp hearing disorder/or exp hearing impairment/or hearing loss/or exp congenital deafness/or exp perception deafness/(43021)
-
hearing loss.ti,ab. (15512)
-
deafness.ti,ab. (7544)
-
(hearing adj5 disability).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (410)
-
1 or 6 or 7 or 8 or 36 or 37 or 38 or 39 (46690)
-
28 and 32 and 40 (188)
-
limit 41 to (human and english language) (180)
Quality of life searches
Cochrane Library – CENTRAL – Issue 3/2006
Ovid MEDLINE® – 1966 to October Week 1 2006
-
exp hearing loss/(37541)
-
exp hearing loss, sensorineural/(14163)
-
“Hearing Loss, Bilateral”/(1190)
-
exp deafness/(17839)
-
severe to profound deafness.mp. (15)
-
(severe adj4 deaf$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (327)
-
(profound adj4 deaf$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (542)
-
Hearing Loss, Unilateral/(34)
-
exp Hearing Disorders/(49630)
-
deaf$.ti,ab. (19840)
-
(hear$adj5 loss).ti,ab. (19425)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (61873)
-
exp ear, middle/or exp ear, inner/(47537)
-
12 or 13 (98248)
-
Cochlear Implants/(4019)
-
Cochlear Diseases/(557)
-
Cochlear Implantation/(1476)
-
(cochlear adj10 (implant$or device$)).ti,ab. (4660)
-
cochleostomy.ti,ab. (105)
-
15 or 16 or 17 or 18 or 19 (6110)
-
14 and 20 (4985)
-
limit 21 to (humans and english language) (3788)
-
exp ECONOMICS/(363215)
-
exp ECONOMICS, HOSPITAL/(14367)
-
exp ECONOMICS, PHARMACEUTICAL/(1688)
-
exp ECONOMICS, NURSING/(3702)
-
exp ECONOMICS, DENTAL/(3386)
-
exp ECONOMICS, MEDICAL/(10140)
-
exp “Costs and Cost Analysis”/(128174)
-
Cost-Benefit Analysis/(39904)
-
VALUE OF LIFE/(4933)
-
exp MODELS, ECONOMIC/(4990)
-
exp FEES/and CHARGES/(7018)
-
exp BUDGETS/(9317)
-
(economic$or price$or pricing or financ$or fee$or pharmacoeconomic$or pharma economic$).tw. (315608)
-
(cost$or costly or costing$or costed).tw. (187439)
-
(cost$adj2 (benefit$or utilit$or minim$or effective$)).tw. (48416)
-
(expenditure$not energy).tw. (10082)
-
(value adj2 (money or monetary)).tw. (565)
-
budget$.tw. (10335)
-
(economic adj2 burden).tw. (1320)
-
“resource use”.ti,ab. (2035)
-
or/23–41 (733038)
-
news.pt. (105269)
-
letter.pt. (581686)
-
editorial.pt. (199009)
-
comment.pt. (317617)
-
or/44–47 (923485)
-
43 not 48 (676925)
-
22 and 49 (148)
-
49 and 12 (1988)
-
limit 51 to (humans and english language) (1453)
-
23 or 24 or 25 or 26 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 37 or 39 or 41 or 42 (392242)
-
12 and 53 (714)
-
limit 54 to (humans and english language) (563)
-
50 or 55 (644)
-
54 not 50 (647)
-
55 not 50 (496)
-
*”Hearing Loss”/(3032)
-
from 58 keep 12,14,17,21,27,47,50,53,61,71,73,87–88,90–91,93 (16)
-
exp Hearing Disorders/ec [Economics] (132)
-
(61 not 60) or 50 (257)
-
61 not (60 or 50) (109)
-
limit 63 to (humans and english language) (86)
-
from 64 keep 16,18,20,26–27,31,34–37,41–42,51,57,59,64–65,77 (18)
-
exp Hearing Loss, Sensorineural/ec [Economics] (38)
-
limit 66 to (humans and english language) (30)
-
67 not (64 or 60 or 50) (0)
-
50 or 60 or 65 (182)
-
from 69 keep 1–182 (182)
-
value of life/(4933)
-
quality adjusted life year/(2787)
-
quality adjusted life.ti,ab. (2002)
-
(qaly$or qald$or qale$or qtime$).ti,ab. (1580)
-
disability adjusted life.ti,ab. (336)
-
daly$.ti,ab. (410)
-
health status indicators/(10691)
-
(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirstysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab. (5977)
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab. (669)
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab. (717)
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab. (14)
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab. (268)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab. (888)
-
(hql or hqol or h qol or hrqol or hr qol).ti,ab. (2089)
-
(hye or hyes).ti,ab. (51)
-
health$year$equivalent$.ti,ab. (31)
-
health utilit$.ab. (371)
-
(hui or hui1 or hui2 or hui3).ti,ab. (392)
-
disutil$.ti,ab. (76)
-
rosser.ti,ab. (59)
-
quality of well being.ti,ab. (204)
-
quality of wellbeing.ti,ab. (1)
-
qwb.ti,ab. (111)
-
willingness to pay.ti,ab. (740)
-
standard gamble$.ti,ab. (414)
-
time trade off.ti,ab. (353)
-
time tradeoff.ti,ab. (122)
-
tto.ti,ab. (235)
-
(index adj2 well being).mp. (257)
-
(quality adj2 well being).mp. (455)
-
(health adj3 utilit$ind$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (277)
-
((multiattribute$or multi attribute$) adj3 (health ind$or theor$or health state$or utilit$or analys$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (148)
-
quality adjusted life year$.mp. (3647)
-
(15D or 15 dimension$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (556)
-
(12D or 12 dimension$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (184)
-
rating scale$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (49351)
-
linear scal$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (293)
-
linear analog$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (695)
-
visual analog$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (14155)
-
(categor$adj2 scal$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (695)
-
or/71–110 (92434)
-
(letter or editorial or comment).pt. (825243)
-
111 not 112 (89676)
-
(Vertigo Symptom Scale or Hearing Disability Handicap Scale or Tinnitus Severity Questionnaire or Sense of Coherence Scale).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (78)
-
113 or 114 (89735)
-
22 and 115 (50)
-
12 and 115 (340)
-
limit 117 to (humans and english language) (297)
-
116 or 118 (297)
-
*”Quality of Life”/(24727)
-
from 119 keep 5 (1)
-
from 116 keep 1–50 (50)
-
12 and 120 (143)
-
limit 123 to (humans and english language) (127)
-
124 not 116 (116)
-
from 125 keep 1,3–4,6–7,10–15,17–18,20,23–26,28,30–31,33,37–43,45–49,53–55,58–59,61–63,65–71,73–76,78–80,82–84,86–88,91–93,95,97,100–104,109–110,112,115–116 (77)
EMBASE – 1980 to 2006 Week 42
-
exp Hearing Impairment/(35130)
-
exp Congenital Deafness/(2189)
-
Perception Deafness/(6698)
-
hearing loss/or mixed hearing loss/or unilateral hearing loss/(10402)
-
exp ear disease/(47570)
-
severe to profound deafness.mp. (18)
-
(severe adj4 deaf$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (272)
-
(profound adj4 deaf$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (457)
-
deaf$.ti,ab. (14005)
-
(hear$adj5 loss).ti,ab. (16667)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 (76451)
-
cochlea prosthesis/(4382)
-
Implantation/(13947)
-
(cochlea$adj10 (implant$or device$or prosthe$)).ti,ab. (4402)
-
12 or 13 or 14 (18298)
-
11 and 15 (3685)
-
limit 16 to (humans and english language) (2935)
-
exp “quality of life”/(71508)
-
quality adjusted life year/(2694)
-
quality adjusted life.ti,ab. (1882)
-
(qaly$or qald$or qale$or qtime$).ti,ab. (1467)
-
disability adjusted life.ti,ab. (316)
-
daly$.ti,ab. (356)
-
(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirstysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab. (5643)
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab. (758)
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab. (655)
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab. (11)
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab. (177)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab. (869)
-
(hql or hqol or h qol or hrqol or hr qol).ti,ab. (1955)
-
(hye or hyes).ti,ab. (25)
-
health$year$equivalent$.ti,ab. (23)
-
((health or cost) adj5 utilit$).ab,ti. (2116)
-
(hui or hui1 or hui2 or hui3).ti,ab. (298)
-
disutil$.ti,ab. (71)
-
rosser.ti,ab. (48)
-
quality of well being.ti,ab. (175)
-
quality of wellbeing.ti,ab. (5)
-
qwb.ti,ab. (96)
-
willingness to pay.ti,ab. (724)
-
standard gamble$.ti,ab. (373)
-
time trade off.ti,ab. (334)
-
time tradeoff.ti,ab. (115)
-
tto.ti,ab. (248)
-
(index adj2 well being).mp. (235)
-
(quality adj2 well being).mp. (417)
-
(health adj3 utilit$ind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (255)
-
((multiattribute$or multi attribute$) adj3 (health ind$or theor$or health state$or utilit$or analys$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (134)49 quality adjusted life year$.mp. (3325)
-
(15D or 15 dimension$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (550)
-
(12D or 12 dimension$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (177)
-
rating scale$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (50552)
-
linear scal$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (245)
-
linear analog$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (641)
-
visual analog$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (15842)
-
(categor$adj2 scal$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (595)
-
or/18–56 (137020)
-
(letter or editorial or comment).pt. (496255)
-
57 not 58 (128805)
-
59 and 17 (141)
Ovid MEDLINE® In-Process & Other Non-Indexed Citations – 20 October 2006
-
severe to profound deafness.mp. (1)
-
(severe adj4 deaf$).mp. [mp=title, original title, abstract, name of substance word] (10)
-
(profound adj4 deaf$).mp. [mp=title, original title, abstract, name of substance word] (10)
-
deaf$.ti,ab. (367)
-
(hear$adj5 loss).ti,ab. (645)
-
(cochlear adj10 (implant$or device$)).ti,ab. (177)
-
1 or 2 or 3 or 4 or 5 (919)
-
6 and 7 (86)
-
limit 8 to english language (71)
-
(economic$or price$or pricing or pharmacoeconomic$or pharma economic$).tw. (3526)
-
(cost$or budget$).tw. (7307)
-
(cost$adj2 (benefit$or utilit$or minim$)).tw. (407)
-
(value adj2 (money or monetary)).tw. (23)
-
10 or 11 or 12 or 13 (9869)
-
9 and 14 (4)
-
7 and 14 (22)
-
15 or 16 (22)
-
quality adjusted life.ti,ab. (114)
-
(qaly$or qald$or qale$or qtime$).ti,ab. (120)
-
disability adjusted life.ti,ab. (23)
-
daly$.ti,ab. (25)
-
(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirstysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab. (358)
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab. (77)
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab. (49)
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab. (0)
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab. (0)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab. (74)
-
(hql or hqol or h qol or hrqol or hr qol).ti,ab. (126)
-
(hye or hyes).ti,ab. (0)
-
health$year$equivalent$.ti,ab. (0)
-
health utilit$.ab. (13)
-
(hui or hui1 or hui2 or hui3).ti,ab. (27)
-
disutil$.ti,ab. (1)
-
rosser.ti,ab. (0)
-
quality of well being.ti,ab. (4)
-
quality of wellbeing.ti,ab. (1)
-
qwb.ti,ab. (2)
-
willingness to pay.ti,ab. (60)
-
standard gamble$.ti,ab. (14)
-
time trade off.ti,ab. (10)
-
time tradeoff.ti,ab. (3)
-
tto.ti,ab. (14)
-
(index adj2 well being).mp. (8)
-
(quality adj2 well being).mp. (17)
-
(health adj3 utilit$ind$).mp. [mp=title, original title, abstract, name of substance word] (11)
-
((multiattribute$or multi attribute$) adj3 (health ind$or theor$or health state$or utilit$or analys$)).mp. [mp=title, original title, abstract, name of substance word] (5)
-
quality adjusted life year$.mp. (113)
-
(15D or 15 dimension$).mp. [mp=title, original title, abstract, name of substance word] (40)
-
(12D or 12 dimension$).mp. [mp=title, original title, abstract, name of substance word] (18)
-
rating scale$.mp. [mp=title, original title, abstract, name of substance word] (621)
-
linear scal$.mp. [mp=title, original title, abstract, name of substance word] (97)
-
linear analog$.mp. [mp=title, original title, abstract, name of substance word] (23)
-
visual analog$.mp. [mp=title, original title, abstract, name of substance word] (592)
-
(categor$adj2 scal$).mp. [mp=title, original title, abstract, name of substance word] (20)
-
or/18–54 (2167)
-
(letter or editorial or comment).pt. (21716)
-
55 not 56 (2150)
-
9 and 57 (3)
-
7 and 57 (10)
-
59 or 58 (10)
PsycINFO including PsycARTICLES – 1985 to present
-
severe to profound deafness.mp. (2)
-
(severe adj4 deaf$).mp. [mp=title, abstract, heading word, table of contents, key concepts] (52)
-
(profound adj4 deaf$).mp. [mp=title, abstract, heading word, table of contents, key concepts] (64)
-
exp Hearing Disorders/(6023)
-
deaf$.ti,ab. (4905)
-
(hear$adj5 loss).ti,ab. (1927)
-
1 or 2 or 3 or 4 or 5 or 6 (7807)
-
Cochlear Implants/(506)
-
(cochlear adj10 (implant$or device$)).ti,ab. (641)
-
8 or 9 (666)
-
7 and 10 (458)
-
(random$or trial$).mp. [mp=title, abstract, heading word, table of contents, key concepts] (80452)
-
11 and 12 (17)
-
limit 13 to (human and english language) (16)
-
from 14 keep 1–16 (16)
-
exp “quality of life”/(11199)
-
11 and 16 (7)
Appendix 2 Quality assessment.
QUOROM statement flow diagram for the quality of studies in this technology assessment review
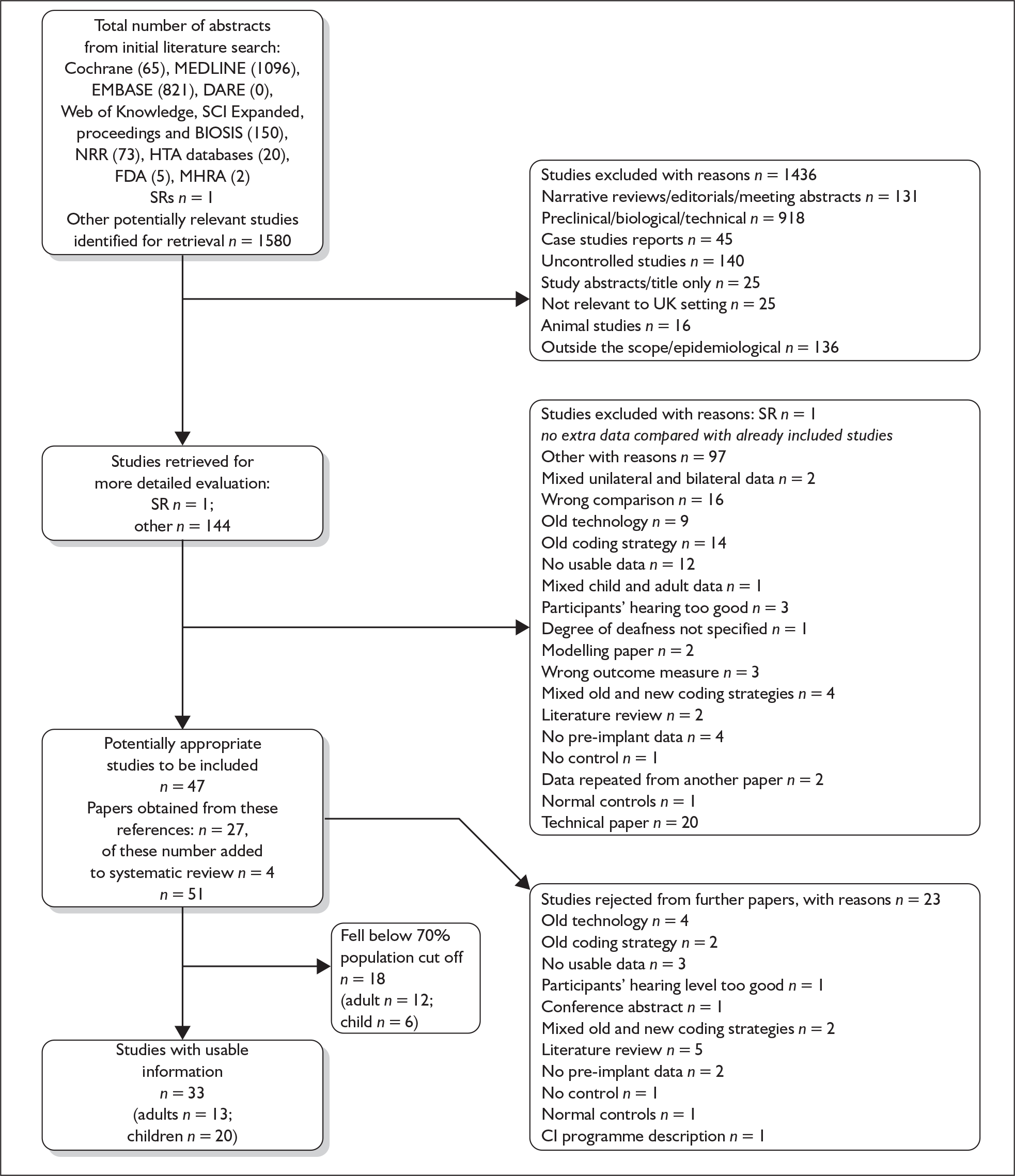
Appendix 3 Summary of study characteristics and results tables
For definitions of outcome measures see Tables 6–10.
Children
| Study | Design | Participants | Intervention and control | Outcomes – auditory | Outcomes – speech |
|---|---|---|---|---|---|
|
Harrison 20051 Canada Length of follow-up: 8 years |
Pre/post retrospective longitudinal analysis Own control |
n = 82 Age: 2–13 years Mean age at implant: 5.4 years Degree of deafness: severe to profound Prelingually deaf Mean time between deafness and implantation: 5.4 years |
Nucleus multichannel cochlear implant Coding strategy: NR |
Speech perception: closed set: TAC; open set: GASP, PB-K | |
|
Nikolopoulos 20042 UK Length of follow-up: 5 years |
Pre/post prospective Repeated measures Own control |
n = 82 Age: < 7 years at implantation Mean age at implant: NR Degree of deafness: profound Prelingually deaf Mean time between deafness and implantation: NR |
Nucleus multichannel cochlear implant Coding strategy: NR |
Speech perception: TROG | |
|
Manrique 20043 Spain Length of follow-up: 12 years |
Pre/post prospective Repeated measures Own control |
n = 182 Age: 0–14 years Mean age at implant: 4.8 years Degree of deafness: profound Prelingually deaf Mean time between deafness and implantation: NR |
Multichannel cochlear implants: Nucleus 22, n = 86; Nucleus 24, n = 96 Coding: SPEAK |
Auditory: PTA | |
|
Staller 20024 Canada Length of follow-up: 6 months Funded by Cochlear Corporation |
Pre/post prospective Repeated measures Own control |
n = 78 Age: 1–17 years Mean age at implant: ≥ 2 years = 1.5 years; 2–4 years = 3.4 years; > 5 years = 9.7 years Degree of deafness: profound Pre- and postlingually deaf Mean time between deafness and implantation: NR |
Nucleus 24 cochlear implant Coding strategy: ACE, SPEAK, CIS |
Auditory: 1–2 years: IT-MAIS ≥ 5 years: MAIS |
Speech perception: 1–2 years: ESP, GASP, MLNT ≥ 5 years: ESP, GASP, LNT, HINT-C |
|
MED-EL 20015 USA Length of follow-up: 6 months Funded by MED-EL for the FDA |
Pre/post prospective Repeated measures Own control |
n = 82 Age: 18 months–17 years Mean age at implant: 8.8 years Degree of deafness: profound Pre- and postlingually deaf Mean time between deafness and implantation: NR |
COMBI 40+ cochlear implant Coding strategy: CIS PRO+ |
Auditory: < 5 years: IT-MAIS, auditory skills checklist, open set word recognition ≥ 5 years: IT-MAIS, auditory skills checklist, all communication skills checklist |
Speech perception: < 5 years: ESP, GASP ≥ 5 years: ESP, MLNT, LNT, GASP, BKB |
|
Nikolopoulos 19996 UK Length of follow-up: 6 years |
Pre/post prospective Repeated measures Own control |
n = 126 Age: > 7 years at implantation Mean age at implant: 4.2 years Degree of deafness: NR Pre- and postlingually deaf Mean time between deafness and implantation: NR |
Nucleus multichannel cochlear implant Coding strategy: NR |
Speech perception: closed set: IMST; open set: CDT, CAP Speech production: SIR |
|
|
Illg 19997 Germany Length of follow-up: 3 years |
Pre/post prospective Repeated measures Own control |
n = 167 Age: 1.25–15 years Mean age at implant: NR Degree of deafness: severe to profound Pre- and postlingually deaf Mean time between deafness and implantation: NR |
Cochlear implants CLARION 1.2 Coding: CIS |
Speech perception: closed set: pattern perception, two-syllable word test, monosyllable word test, Minimal Pairs Test; open set: TAPS, monosyllable word test, GASP sentences, common phrases, Mr Potato Head | |
|
Kessler 19978 USA Length of follow-up: 6 months Funded by Advanced Bionics |
Pre/post CLARION clinical trial, prospective Repeated measures Own control |
n = 49 Age: ≥ 7 years Mean age at implant: NR Degree of deafness: not reported (assumed same as adult trial – profound) Pre- and postlingually deaf Mean time between deafness and implantation: NR |
CLARION multistrategy cochlear implant system Coding strategy: CIS 93%; compressed analogue 7% |
Auditory: MAIS | Speech perception: PB-K, ESP, GASP, common phrases, Mr Potato Head |
| Study | Audiological | Speech perception | Speech production | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Mean (%) (SD) | p-value | Direction of change | Outcome | Mean (%) | p-value | Direction of change | Outcome | Mean (%) (SD) | p-value | Direction of change | p-value | Direction of change | |
|
Harrison 20051 n = 82 |
% mean difference from baseline at 5 years | |||||||||||||
| TAC(n = 71) | 6.36 | – | + early implanta | |||||||||||
| GASP (n = 71) | 84.25 | – | + early implant | |||||||||||
| PB-K words (n = 77) | 48.28 | – | + early implant | |||||||||||
| PB-K phonemes (n = 77) | 65.85 | – | + early implant | |||||||||||
|
Nikolopoulos 20042 n = 82 |
TROG | % mean difference from normal percentiles | ||||||||||||
| Preimplant: | ||||||||||||||
| < 1% | 98 | – | + early implant | |||||||||||
| 1–25% | 2 | – | + early implant | |||||||||||
| 25–75% | 0 | – | + early implant | |||||||||||
| 75–100% | 0 | – | + early implant | |||||||||||
| Post 3 years: | ||||||||||||||
| < 1% | 60 | – | + early implant | |||||||||||
| 1–25% | 33 | – | + early implant | |||||||||||
| 25–75% | 3.5 | – | + early implant | |||||||||||
| 75–100% | 3.5 | – | + early implant | |||||||||||
| Post 5 years: | ||||||||||||||
| < 1% | 33 | – | + early implant | |||||||||||
| 1–25% | 47 | – | + early implant | |||||||||||
| 25–75% | 17 | – | + early implant | |||||||||||
| 75–100% | 3 | – | + early implant | |||||||||||
|
Manrique 20043 n = 182 |
PTA 1 year | Mean difference from baseline: 81.53 (5.00) | < 0.05 | + | ||||||||||
|
Staller 20024 n = 78 |
12 months–2 years: IT-MAIS | No usable data reported | Total mean % difference between baseline and 3 months’ follow-up | |||||||||||
| 3–17 years: MAIS (n = 20) | Total mean % difference between baseline and 6 months post activation: + 16 | – | + in favour of cochlear implants | 3–17 years: | ||||||||||
| ESP: | ||||||||||||||
| Pattern (n = 25) | + 37.0 | – | + | |||||||||||
| Spondee (n = 18) | + 44.2 | – | + | |||||||||||
| Monosyllable (n = 17) | + 35.4 | – | + | |||||||||||
| 6 months’ follow-up | ||||||||||||||
| GASP (n = 25) | + 37.1 | – | + | |||||||||||
| ≥ 5–17 years: | ||||||||||||||
| LNT (n = 43) | + 34.5 | |||||||||||||
| HINT-C (n = 21) | + 49.5 | |||||||||||||
|
MED-EL 20015 n = 82; < 5 years = 35, ≥ 5 years = 47 |
Auditory skills checklist | Results not reported | < 5 years: | Total mean % difference between baseline and 6 months post activation | ||||||||||
| Total mean % difference between baseline and 6 months post activation | ESP: | |||||||||||||
| Pattern perception | 70.0 | – | + | |||||||||||
| Spondee ID | 50.0 | – | + | |||||||||||
| Monosyllable word ID | 48.0 | – | + | |||||||||||
| GASP | 59.0 | – | + | |||||||||||
| IT-MAIS | 53.7 | – | + | ≥ 5 years: | ||||||||||
| MAIS | 38.2 | – | + | ESP: | ||||||||||
| Pattern perception | 68.0 | – | + | |||||||||||
| Spondee ID | 79.0 | – | + | |||||||||||
| Monosyllable word ID | 68.0 | – | + | |||||||||||
| GASP | 79.0 | – | + | |||||||||||
| MLNT | 73.5 | – | + | |||||||||||
| LNT | 76.0 | – | + | |||||||||||
| BKB sentences | 53.0 | – | + | |||||||||||
| All ages: | ||||||||||||||
| Communicative skills checklist | 39.5 | – | + | |||||||||||
|
Nikolopoulos 19996 n = 126 |
Correlation with age at implantation | Correlation with age at implantation | Correlation with age at implantation | |||||||||||
| n = 74 | Post 2 years: | Post 2 years: | Post 2 years: | |||||||||||
| CAP | –0.32 | 0.006 | + early implant | IOWA | + 0.06 | NS | SIR | –0.06 | NS | NS | ||||
| CDT | + 0.05 | NS | ||||||||||||
| n = 50 | Post 3 years: | Post 3 years: | Post 3 years: | |||||||||||
| CAP | –0.48 | 0.0007 | + early implant | IOWA | –0.24 | NS | SIR | –0.49 | NS | NS | ||||
| CDT | –0.38 | 0.007 | + early implant | |||||||||||
| n = 29 | Post 4 years: | Post 4 years: | Post 4 years: | |||||||||||
| CAP | –0.58 | 0.002 | + early implant | IOWA | –0.44 | 0.02 | + early implant | SIR | –0.49 | 0.01 | + early implant | 0.01 | + early implant | |
| CDT | –0.58 | 0.0008 | + early implant | |||||||||||
|
Illg 19997 n = 167 |
> 7 years: | 2-year differences from baseline, mean % (SD) | ||||||||||||
| TAPS | 56 (70.93) | – | + | |||||||||||
| Monosyllable word test | 54 (73.31) | – | + | |||||||||||
| GASP sentences | 8 (36.12) | – | + | |||||||||||
| Mr Potato Head | 22 (78.73) | – | + | |||||||||||
| Pattern perception | 59 | – | + | |||||||||||
| Two-syllable test | 54 | – | + | |||||||||||
| One-syllable test | 51 | – | + | |||||||||||
| Minimal Pairs Test | 10 | – | + | |||||||||||
| 7–15 years: | ||||||||||||||
| TAPS | 37 (67.96) | – | + | |||||||||||
| Monosyllable word test | 36 (62.23) | – | + | |||||||||||
| GASP sentences | 23 (50.93) | – | + | |||||||||||
| Common phrases | 15 (43.09) | – | + | |||||||||||
| Pattern perception | 39 | – | + | |||||||||||
| Two-syllable test | 25 | – | + | |||||||||||
| One-syllable test | 35 | – | + | |||||||||||
| Minimal Pairs Test | 15 | – | + | |||||||||||
|
Kessler 19978 n = 49 |
Total mean % difference between baseline and 6 months post activation | Total mean % difference between baseline and 6 months post activation | ||||||||||||
| MAIS (n = 14) | + 20 | – | + in favour of cochlear implantation | PB-K (n = 49) | + 31.5 | – | + | |||||||
| ESP (n = 49) | + 54.0 | – | + | |||||||||||
| GASP (n = 49) | + 33.0 | – | + | |||||||||||
| Mr Potato Head (n = 49) | + 45.0 | – | + | |||||||||||
| Study | Design | Intervention group | Control group | Intervention and control | Outcomes – speech |
|---|---|---|---|---|---|
|
Mildner 20069 Croatia n = 49 Length of follow-up: NA |
Cross-sectional |
n = 29 Age: mean 11.6 (7–15) years Degree of deafness: profound > 98 dB HL Mean age at implant: 8.2 (2–12) years Mean time between deafness and implantation: NR |
n = 20 Age: mean 12.9 (7–15) years Degree of deafness: profound > 98 dB HL |
Intervention: ESPrit 3G 45%, Tempo+ 24%, Spectra 21%, CIS PRO+ 7%, SPRINT 3% Coding strategy: ACE 48%, CIS 31%, SPEAK 21% Control: acoustic hearing aids |
Speech perception: 52 one- and two-syllable words, words presented visually and orally, 34 word pairs, five nonsense words |
|
Tomblin 199910 USA n = 58 Length of follow-up: 5 years |
Non-randomised controlled prospective trial with cross-overs allowed |
n = 29 Age, mean (SD): 10 (2.9) years Degree of deafness: profound Prelingually deaf Mean (SD, range) age at implant: 4.76 (1.57, 2–13) years Mean time between deafness and implantation: NR |
n = 29 Age, mean (SD): 9 (3.65) years Degree of deafness: profound Prelingually deaf |
Intervention: Nucleus 22 Coding strategy: NR Control: acoustic hearing aids |
Speech perception: RITLS Speech production: expressive sentence usage, IPSyn |
|
Osberger 199911 USA n = 58 Length of follow-up: 1.5 years |
Pre/post prospective Repeated measures Own control |
Participantsn = 58 Age, mean: 5.4 years Degree of deafness: profound, mean = 110 dB HL Prelingually deaf Mean age at implant: 5.4 years Mean time between deafness and implantation: NR |
Intervention: CLARION multistrategy implant Coding strategy: CIS Control: acoustic hearing aids |
Speech perception: closed set: ESP; open set: GASP, PB-K Recognition of key words: Mr Potato Head, common phrases test |
|
|
Svirsky 199912 USA n = 297 Length of follow-up: 1.5 years |
Non-randomised controlled retrospective trial |
n = 222 Age, mean: 4.2 years Degree of deafness: profound Prelingually deaf Mean age at implant: 4.2 years Duration of implant use: NR Mean time between deafness and implantation: NR |
n = 75 Age, mean: 8.4 years Degree of deafness: profound Prelingually deaf |
Intervention: CLARION multistrategy implant Coding strategy: CIS Control: acoustic hearing aids |
Speech perception: PB-K |
|
Osberger 199813 USA n = 30 Length of follow-up: 0.5 years |
Pre/post prospective Repeated measures Own control |
n = 30 Age, mean: 9.0 years Degree of deafness: profound, mean 110 dB HL Prelingually deaf Mean age at implant: 9.0 years Duration of implant use: NR Mean time between deafness and implantation: 8.2 years |
Intervention: CLARION multistrategy implant Coding strategy: CIS Control: acoustic hearing aids |
Speech perception: closed set: ESP; open set: GASP, PB-K | |
|
van den Borne 199814 Netherlands n = 43 Length of follow-up: 3 years |
Non-randomised controlled prospective trial |
n = 20 Age, mean (range): 5.9 (3.3–9.3) years Degree of deafness: profound, ≥ 110 dB HL Prelingually deaf Mean age at implant: NR Mean time implant use: 3.4 (1.7–6.0) years Mean time (range) between deafness and implantation: 5.9 (1.5–8.6) years |
n = 23 Age, mean: 3.6 years Degree of deafness: profound, ≥ 110 dB HL Prelingually deaf |
Intervention: Nucleus multichannel Coding strategy: NR Control: acoustic hearing aids |
Speech production: SECSHIC Auditory outcomes: basal auditory ability, average unaided pure-tone thresholds |
| Study | Outcome | Cochlear implants | Acoustic hearing aids | Difference | p-value | Direction of change | ||
|---|---|---|---|---|---|---|---|---|
|
Mildner 20069 n = 49 |
Speech perception (one time point): | n | Mean (%) | n | Mean (%) | |||
| Overall word scores | 29 | 82.8 | 20 | 60.4 | 22.4 | 0.000 | + | |
| Response to vowels: | + | |||||||
| Nonsense words | 29 | 57.5 | 20 | 31.6 | 25.9 | + | ||
| Minimal pairs | 29 | 66.6 | 20 | 46.5 | 20.1 | 0.000 | + | |
| Individual vowels | 29 | 76.8 | 20 | 55.0 | 21.8 | ≤ 0001 | + | |
| Individual vowel pairs | 29 | 76.0 | 20 | 54.0 | 22.0 | + | ||
| Response to consonants: | ||||||||
| According to features | 29 | 61.0 | 20 | 41.4 | 19.6 | + | ||
| According to consonant category | 29 | 61.0 | 20 | 39.3 | 21.7 | < 0.01 | + | |
|
Tomblin 199910 n = 58 |
Speech perception: | n | Mean (SD) | n | Mean (SD) | |||
| RITLS at latest visit | 28 | 42.2 (15.7) | – | |||||
| Speech production: | ||||||||
| Difference in scores between baseline and 5-year follow-up | – | + | ||||||
| IPSyn: | ||||||||
| Total final score | 29 | 60.3 (15.2) | 29 | 40.7 (17.4) | 19.6 (23.10) | + | ||
| Subscale scores: | ||||||||
| Noun phrase | 29 | 17.8 | 29 | 13.8 | 4.1 | + | ||
| Verb phrase | 29 | 18.1 | 29 | 11.0 | 7.1 | + | ||
| Negative question | 29 | 1.9 | 29 | 1.0 | 0.9 | + | ||
| Sentence structure | 29 | 21.5 | 29 | 15.4 | 6.1 | + | ||
| Correlation of age in regression analysis: | ||||||||
| IPSyn score and age | 29 | 0.4 | – | < 0.03 | + | |||
| IPSyn score and months of cochlear implant use | 29 | 0.6 | – | < 0.0001 | + | |||
| IPSyn score and months of cochlear implant use with age partialled out | 29 | 0.6 | – | < 0.001 | + | |||
|
Osberger 199911 n = 58 |
Speech perception: | n | Mean | n | Mean | |||
| Difference between preoperative scores with acoustic hearing aids and 18-month postoperative scores with cochlear implants: | ||||||||
| ESP | 58 | 83.2 | 58 | 26.7 | 56.5 | < 0.0001 | + | |
| GASP | 58 | 55.0 | 58 | 9.7 | 45.3 | < 0.0001 | + | |
| Mr Potato Head | 58 | 31.4 | 58 | 2.1 | 29.3 | < 0.0001 | + | |
| Common phrases | 58 | 24.5 | 58 | 4.6 | 19.9 | < 0.0001 | + | |
| PK-B phonemes | 58 | 47.1 | 58 | 4.3 | 42.8 | < 0.0001 | + | |
| PK-B words | 58 | 27.6 | 58 | 0 | 27.6 | < 0.0001 | + | |
|
Svirsky 199912 n = 297 |
Speech perception: | n | Mean (95% CI) | n | Mean (95% CI) | |||
| Difference between preoperative and final scores: cochlear implant scores compared with predicted hearing aid scores: | Measured cochlear implants | Predicted acoustic hearing aid | ||||||
| PB-K words: | Insufficient data given to calculate the mean difference in scores | |||||||
| < 6 years at 18 months | 222 | 6.3 | – | – | + | |||
| 6–12 years at 12 months | 222 | 6.5 | – | – | + | |||
|
Osberger 199813 n = 30 |
Speech perception: | n | Mean % score at 6 months | n | Mean % score at baseline | |||
| Difference between preoperative scores with acoustic hearing aids and 6-month scores postoperatively with cochlear implants: | ||||||||
| ESP monosyllable words | 30 | 62.7 | 30 | 26.8 | 35.9 | – | + | |
| GASP words | 30 | 60.1 | 30 | 15.7 | 44.4 | – | + | |
| GASP sentences | 30 | 43.7 | 30 | 8.6 | 35.1 | – | + | |
| PB-K phonemes | 30 | 39.6 | 30 | 6.3 | 33.3 | – | + | |
| PB-K words | 30 | 49.6 | 30 | 0 | 49.6 | – | + | |
|
van den Borne 199814 n = 43 |
Auditory: | n | Mean | n | Mean | |||
| Difference between preoperative scores and 24-month scores: | ||||||||
| Basal sound identification | 20 | + 3.5 | 23 | + 1.9 | 1.6 | – | + | |
| Speech perception: | ||||||||
| SECSHIC | 20 | + 7.0 | 23 | + 6.9 | 0.1 | – | +/– | |
| Study | Design | Participants 1 | Participants 2 | Intervention | Outcomes – speech |
|---|---|---|---|---|---|
|
Peters 200715 USA Length of follow-up: NA Funded by Cochlear America |
Cross-sectional Own control Sequential implantsa |
n = 30 Age range: 3–13 years; group 1: 3–5 years, group 2: 5.1–8 years, group 3: 8.1–13 years Mean age at implant: NR |
Degree of deafness: severe to profound Prelingually deaf: NR Mean time between deafness and implantation: NR Time between implants: ≥ 5 years |
First ear: Nucleus 22, 24 or 24 contour Coding strategy: NR Second ear: Nucleus 24 contour or Nucleus 24 contour advance |
Speech perception: Group 1 MLNT words; group 2 LNT words; group 3 LNT words, HINT-C sentences, CRISP |
|
Litovsky 200616 USA Length of follow-up: NA Funded by Cochlear America |
Cross-sectional Own control Sequential implants |
n = 13 Age range: 3–6 years Mean age at implant: NR Time between implants, mean (range): 4.6 (1.6–13.0) years |
Degree of deafness: severe to profound Prelingually deaf: 12; postlingually deaf: 1 Time between deafness and implantation, mean (range): 3.1 (1.5–6.0) years |
Nucleus 22 = 1; Nucleus 24 = 22; Nucleus 24 contour = 1; BI CLARION (platinum/auria) = 2 Coding strategy: NR |
Auditory: MAA |
|
Kuhn-Inacker 200417 Germany Length of follow-up: NA Funded by MED-EL |
Cross-sectional Own control Timing of second implant: simultaneousb = 1; sequential = 17 |
n = 18 Age range: 2.11–9.1 years Age range at first implant: 2.0–6.11 years; age range at second implant: 2.1–8.0 years Time between implants: range 0 years–4 years 5 months |
Degree of deafness: severe to profound Prelingually deaf: 14; postlingually deaf: 4 Mean time between deafness and implantation: NR |
MED-EL COMBI 40, MED-EL COMBI 40+ Coding strategy: NR |
Speech perception: discrimination in noise; discrimination in quiet: Gottinger test |
| Study | Outcome | First side only | Second side only | Bilateral | p-value | Direction of change | |||
|---|---|---|---|---|---|---|---|---|---|
|
Peters 200715 n = 30 |
Speech perception: | n | Mean % (range) | n | Mean % (range) | ||||
| In quiet, differences between unilateral implant and bilateral implants at 12 months after the second implant: | |||||||||
| MLNT words (group 1) | 7 | 84.5 (71–96) | 7 | 92.3 (71–100) | NS | + | |||
| LNT words (group 2) | 8 | 68.0 | 8 | 81.0 | NS | + | |||
| LNT words (group 3) | 13 | 81.0 | 13 | 86.5 | NS | + | |||
| HINT-C sentences (group 3) | 12 | 89 | 12 | 94.0 | NS | + | |||
| In noise, differences between unilateral implant and bilateral implants at 9 months after the second implant: | Difference | ||||||||
| n | Mean % (range) | ||||||||
| CRISP all participants: | |||||||||
| Noise directed at the front | 19 | 6.8 (62.1–68.9) | 0.018 | + | |||||
| Noise directed at first implant | 15 | 13.2 (68.5–55.3) | 0.0001 | + | |||||
| Noise directed at second implant | 18 | – (72.2–79.0) | 0.018 | + | |||||
|
Litovsky 200616 n = 13 |
Auditory: | n | Mean % score | n | Mean % score | n | mean % score | ||
| MAA in degrees azimuth (data are reported on the nine children who found the task easiest) | 9 | 27.7 | 9 | 29.7 | 9 | 16.2 | < 0.001 | + | |
| Outcome | Left ear only | Right ear only | Bilateral | p-value | Direction of change | ||||
|
Kuhn-Inacker 200417 n = 18 |
Speech perception: | n | Mean % | n | Mean % | n | |||
| Discrimination in quiet – Gottinger test | 18 | 70 | 18 | 71 | 18 | + | |||
| Unilateral | Bilateral | p-value | Direction of change | ||||||
| Discrimination in noise | 18 | 60 | 18 | + | |||||
| Study | Design | Participants 1 | Participants 2 | Intervention | Outcomes |
|---|---|---|---|---|---|
|
Peters 200715 USA Length of follow-up: 1 year n = 30 Funded by Cochlear America |
Pre/post repeated measures Own control Sequential implants |
n = 30 Age range: 3–13 years; group 1: 3–5 years, group 2: 5.1–8 years, group 3: 8.1–13 years Mean age at implant: NR Degree of deafness: severe to profound Prelingually deaf: NR Mean time between deafness and implantation: NR Time between implants: ≥ 5 years |
First ear: Nucleus 22, 24 or 24 contour Coding strategy: NR Second ear: Nucleus 24 contour or Nucleus 24 contour advance Coding strategy: NR |
Speech perception: group 1 MLNT words; group 2 LNT words; group 3 LNT words, HINT-C sentences | |
|
Litovsky 200616 USA n = 19 Length of follow-up: NA Participants had measures taken between one and three times. The latest time recorded was between 3 and 15 months after the second implant (mean 7 months) Funded by Cochlear America |
Cross-sectional Sequential implants ≥ 1.6 years apart |
Bilateral cochlear implant: n = 13 Age range: 3–6 years Mean age (range) at implant: 4.6 (1.6–13.0) years Time between implants, mean (range): 3.9 (1–11.6) years Degree of deafness: severe to profound Prelingually deaf: 12; postlingually deaf: 1 Time between deafness and implantation, mean (range): 3.1 (1.5–6.0) years |
Cochlear implant + acoustic hearing aid: n = 6 Age range: 4–14 years Mean age (range) at implant: 5.6 (3.6–8.6) years Degree of deafness: severe to profound Prelingually deaf: NR; postlingually deaf: NR Time between deafness and implantation, mean (range): 5.3 (3.6–8.6) years |
Bilateral cochlear implant group: Nucleus 22 = 1; Nucleus 24 = 22; Nucleus 24 contour = 1; Bi CLARION (platinum/auria) = 2 Coding strategy: NR Cochlear implant + acoustic hearing aid group: Nucleus 24 = 2; Nucleus 24 contour = 2; CLARION II HiFocus = 1; MED-EL C40+ = 1 Acoustic hearing aids Coding strategy: NR |
Auditory: MAA |
|
Litovsky 200618 USA n = 20 Length of follow-up: NA Data collection varied between 3 and 26 months post second implant (mean 13.5 months) |
Cross-sectional |
Bilateral cochlear implants: n = 10 Age range: 3–14 years Mean age at implant: NR Time between implants: NR Degree of deafness: NR Prelingually deaf: NR; postlingually deaf: NR Time between deafness and implantation: NR |
Cochlear implants and acoustic hearing aids: n = 10 Age range: 6–14 years Mean age at implant: NR Degree of deafness: NR Prelingually deaf: NR Postlingually deaf: NR Time between deafness and implantation: NR |
Bilateral cochlear implant group: Nucleus 22 = 3; Nucleus 24 = 12; Nucleus 24 contour = 3; BI CLARION = 2 Coding strategy: NR Cochlear implant + acoustic hearing aid group: Nucleus 22 = 1; Nucleus 24 = 6; Nucleus Freedom = 1; MED-EL C40+ = 2 Acoustic hearing aids Coding strategy: NR |
Auditory: MAA Speech production: CRISP |
| Study | Outcome | One cochlear implant + acoustic hearing aid | Bilateral | p-value | Direction of change | ||
|---|---|---|---|---|---|---|---|
|
Peters 200715 n = 30 |
Speech perception: | n | Mean % (range) | n | Mean % (range) | ||
| In quiet, differences between pre second implant and 1-year follow-up post second implant: | |||||||
| MLNT words (group 1) | 7 | 67.3 (38–100) | 7 | 92.3 (71–100) | 0.003 | + | |
| LNT words (group 2) | 8 | 71.0 | 8 | 81.0 | NS | + | |
| LNT words (group 3) | 13 | 69.4 | 13 | 86.0 | 0.004 | + | |
| HINT-C sentences (group 3) | 12 | 88.0 | 13 | 94.0 | NS | + | |
|
Litovsky 200616 n = 19 |
Auditory: | n | Mean degrees azimuth | n | Mean degrees azimuth | ||
| Differences in the ability to detect the direction of sound, MAA degrees azimuth | 5 | 44.4 | 12 | 28.0 | < 0.05 | + | |
|
Litovsky 200618 n = 20 |
Auditory: | n | Mean degrees azimuth (SD) | n | Mean degrees azimuth (SD) | ||
| Differences in the ability to detect the direction of sound, MAA degrees azimuth | 8 | 27.0 (± 23) | 6 | 20.0 (± 10) | < 0.05 | + | |
| Speech production: | |||||||
| CRISP test: | n | Mean % (range) | n | Mean % (range) | |||
| In quiet | 10 | –24.0 (–18 to –35) | 10 | –20.0 (–12 to –28) | < 0.0001 | + | |
| In noise | 10 | –17.5 (–13 to –18) | 10 | –11.0 (–5 to –17) | < 0.005 | + | |
| Study | Design | Participants – intervention | Participants – control | Outcomes |
|---|---|---|---|---|
|
Damen 200619 Netherlands n = 9 |
Retrospective controlled study |
Parents of children or children with Usher syndrome type 1: n = 7 Age, mean (SD): 12.4 (2.9) years Used cochlear implants |
Parents of children or children with Usher syndrome type 1: n = 2 Age, mean: 15.4 years Without cochlear implants |
NCIQ, ULS |
|
Huber 200520 Austria n = 44 |
Cross-sectional survey |
Children 8- to 16-years-old (n = 37) and their parents (n = 7) Used cochlear implants |
KINDLr | |
|
Spahn 200421 Germany n = 94 |
Cross-sectional survey | Parents of children with cochlear implants: mothers: n = 52; fathers: n = 42 | Symptom checklist 90-R, Everyday Life Questionnaire | |
|
Chmiel 200022 USA n = 22 |
Cross-sectional survey |
Parents of children with cochlear implants: n = 11; age range: 3–20 years; length of cochlear implant use, mean years: 4.0 Children (same families) with cochlear implants: n = 11; age range: 6–20 years; length of cochlear implant use, mean years: 4.78 |
Quality of life questionnaire |
| Study | Quality of life outcomes | p-value | Direction of change | |||
|---|---|---|---|---|---|---|
|
Damen 200619 n = 9 |
Intervention (n = 7), mean (range) | Control (n = 2), mean (range) | ||||
| NCIQ – parents: | ||||||
| Sound perception – basic | 75.4 (52.5–85.0) | 21.3 (0–42.5) | + | |||
| Sound perception – advanced | 67.9 (32.5–95.0) | 21.3 (10.0–32.5) | + | |||
| Speech production | 42.5 (22.5–57.5) | 47.0 (20.0–75.0) | – | |||
| Self-esteem | 65.4 (40.0–90.0) | 50.0 (47.5–52.5) | + | |||
| Activity limitations | 74.2 (63.9–88.9) | 53.6 (47.2–60.0) | + | |||
| Social interactions | 70.9 (60.0–77.5) | 53.8 (47.5–60.0) | + | |||
| Intervention (n = 7), median (range) | Control (n = 2), median (range) | |||||
| ULS: | ||||||
| Access to information | 2.3 (1–4) | 2.5 (2–4) | ||||
| Communication | 1.75 (0–5) | 1.75 (0–2) | ||||
| Mobility | 0.5 (0–1) | 1 (0–1) | ||||
|
Huber 200520 n = 44 (children = 37; parents = 7 |
Cochlear implant children, mean (SD; 95% CI) | Hearing children, mean (SD) | ||||
| KINDLr: | ||||||
| Age range 8–12 years (n = 18) | 64.6 (8.9; 60.4–68.7); parents 80.8 (5.4; 78.4–83.3) | 76.8 (8.6) | < 0.001 | – | ||
| Age range 13–16 years (n = 19) | 72.1 (10.3; 66–78.1); parents 76.3 (10.2; 70.3–82.3) | |||||
|
Spahn 200421 n = 94 |
SCL 90-R – distress | Parents (n = 94): global severity index: T = 78.7% | Norms: global severity index: T = 21.3% | |||
| EDLQ – quality of life | Cochlear implant parents, mean score = 168 | Cardiac patients, mean score = 151 | Students, mean score = 172 | |||
|
Chmiel 200022 n = 22 |
Parents (n = 11), mean score | Children (n = 11), mean score | ||||
| Quality of life questionnaire mean benefit ratings, scale of 1–5 (5 is better): | ||||||
| Hearing environmental sounds | 4.55 | 4.40 | ||||
| Imitate or produce speech | 4.30 | 3.64 | ||||
| Speech-reading and understanding | 4.00 | 3.89 | ||||
| Child’s attitude or behaviour | 3.64 | 4.00 | ||||
| Larger variety of activities | 3.55 | 4.18 | ||||
| Make new friends | 3.45 | 3.72 | ||||
| Child’s level of frustration | 3.45 | 3.55 | ||||
| Telephone use | 3.18 | 3.73 | ||||
| Mean benefit rating | 3.76 | 3.89 | ||||
| Mean problem ratings: | ||||||
| Extra care needed | 2.36 | 2.64 | ||||
| Loud sounds bothersome | 2.09 | 3.16 | ||||
| Cumbersome equipment | 2.09 | 2.91 | ||||
| Acceptance by peers | 2.00 | 2.20 | ||||
| Embarrassment from device | 1.82 | 1.82 | ||||
| Child’s resistance to wearing | 1.55 | 1.70 | ||||
| Mean problem rating | 1.99 | 2.40 | ||||
Adults
| Study | Design | Participants 1 | Participants 2 | Intervention | Outcomes |
|---|---|---|---|---|---|
|
UK Cochlear Implant Study Group 200423,24 UK Length of follow-up: 9 months |
Prospective cohort Own control |
n = 311: TC = 227; MHU = 84a Participants were defined by their preoperative BKB scores, MHUs aided acoustically and without lip-reading Age (range): 50.6 (16–82) years |
Degree of deafness: profound Mean (range) dB HL: 115 (85–140); TC = 117.1 (116–119); MHU = 108.7 (107–111) Postlingually deaf mean (range) duration of deafness: TC = 14.2 (12.2–16.2) years; MHU = 10.6 (8.1–13.0) years |
Nucleus CI 24 = 215; CLARION = 63; COMBI 40+ = 38 Coding strategy: CIS, SPEAK, compressed analogue |
Speech perception: BKB sentences, AVGN, CUNY sentences Quality of life: HUI-3, GHSI, GBI |
|
Mawman 200425 2004 UK Length of follow-up: > 18 months |
Retrospective pre/post analysis Own control |
n = 214 Age at implantation, mean (SD): 50.4 (12.8) years |
Degree of deafness: severe to profound Postlingually deaf Mean time (range) between deafness and implantation: 16.3 years (2 months–53 years) |
Nucleus 22M = 88; Nucleus 24M = 56; Nucleus 24K Contour = 23; Nucleus 24K = 7; Nucleus 24 double array = 1; CLARION High Focus II = 1; MED-EL C40+ = 41; MED-EL C40 = 17; MED-EL S = 3; Ineraid = 2 Coding strategy: Nucleus: SPEAK or ACE; MED-EL: CIS; CLARION: MPS; Ineraid: CIS |
Speech perception: BKB sentences, AB monosyllables |
|
Parkinson 200226 USA Length of follow-up: 3 months |
Pre/post repeated measures prospective Own control |
n = 216 Age at implantation, mean (SD): 50.4 (12.8) years |
Degree of deafness: severe to profound Postlingually deaf Mean time (SD) between deafness and implantation: 10.9 (11.8) years |
Nucleus 24 contour Coding strategy: ACE, SPEAK, CIS |
Speech perception: HINT sentences, CUNY, CNC |
|
Kessler 19978 USA Length of follow-up: 24 months |
Pre/post repeated measures prospective Own control |
n = 238 Age at implantation, mean (range): 51 (18–81) years |
Degree of deafness: profound Postlingually deaf Mean time (range) between deafness and implantation: 11 (0–73) years |
CLARION multistrategy Coding strategy: CIS, 93%; CA, 7% |
Speech perception: MAC vowels, MAC consonants, CUNY lip-reading, CUNY implant, NU-6 mono words, telephone sentences |
| Study | Speech perception | Quality of life | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Mean (95% CI) | p-value | Direction of change | Outcome | Mean (95% CI) | p-value | Direction of change | |||
|
UK Cochlear Implant Study Group 200423 n = 316 |
Mean difference between preimplant and 9-month postoperative scores: | TC | MHU | Mean difference between preimplant and 9-month postoperative scores: | TC | MHU | ||||
| BKB | 53.0 (48–58) | 44.0 (37–51) | < 0.05 | + | HUI-3 | 0.22(0.19, 0.24) | 0.15 (0.11–0.19) | < 0.01 | + | |
| AVGN | 68.0 (63–71) | 31.0 (26–37) | < 0.001 | + | GHSI | 0.17 (0.15–0.18) | 0.19 (0.16–0.22) | + | ||
| GBI | 45.0 (43–48) | 42.0 (37–47) | + | |||||||
| Association with age at implantation at 9 months (n = 311): | All | Association with age at implantation at 9 months (n = 311): | All | |||||||
| < 30 years: BKB 39 | 58.0 (48–58) | + | < 30 years: HUI-3 39 | 0.24 (0.18–0.3) | + | |||||
| AVGN | 50.0 (40–60) | + | GHSI | 20.0 (16–24) | + | |||||
| < 30–40 years: BKB 35 | 49. 0 (35–62) | + | GBI | 45 (38–52) | + | |||||
| AVGN | 57.0 (45–69) | + | < 30–40 years: HUI-3 35 | 0.23 (0.16–0.29) | + | |||||
| < 40–50 years: BKB 59 | 49.0 (40–58) | + | GHSI | 16.0 (12–20) | + | |||||
| AVGN | 55.0 (45–64) | + | GBI | 43 (36 to 49) | + | |||||
| < 50–60 years: BKB 75 | 53.0 (44–62) | + | < 40–50 years: HUI-3 59 | 0.21 (0.16–0.26) | + | |||||
| AVGN | 51.5 (44–59) | + | GHSI | 17.5 (13–22) | + | |||||
| < 60–70 years: BKB 70 | 47.5 (40–55) | + | GBI | 42 (36–47) | + | |||||
| AVGN | 64.5 (57–72) | + | < 50–60 years: HUI-3 75 | 0.16 (0.11–0.20) | + | |||||
| ≥ 70 years: BKB 33 | 46.0 (36–56) | + | GHSI | 21.0 (16–23) | + | |||||
| AVGN | 69.0 (59–79) | + | GBI | 45 (39–50) | + | |||||
| < 60–70 years: HUI-3 70 | 0.20 (0.15–0.24) | + | ||||||||
| GHSI | 18.0 (13–20) | + | ||||||||
| GBI | 47 (42–52) | + | ||||||||
| ≥ 70 years: HUI-3 33 | 0.17 (0.09–0.25) | + | ||||||||
| GHSI | 12.0 (8–16) | + | ||||||||
| GBI | 40 (34–45) | + | ||||||||
| Association with age, 9-month and preimplant scores: | Pearson correlation coefficients | Association with age, 9-month and preimplant scores: | Pearson correlation coefficients | |||||||
| BKB | r = – 0.062 | NS | + | HUI-3 | r = – 0.108 | NS | + | |||
| AVGN | r = 0.164 | < 0.01 | + | GHSI | r = –0.114 | < 0.05 | + | |||
| GBI | r = 0.003 | NS | + | |||||||
| Association with duration of deafness in years, change at 9 months from baseline (n = 311) | All | Association with duration of deafness in years, change at 9 months from baseline (n = 311) | All | |||||||
| 0 to < 10 years: BKB 168 | 58 (53–63) | + | 0 to < 10 years: HUI-3 168 | 0.23 (0.2–0.25) | + | |||||
| AVGN | 63 (58–67) | + | GHSI | 20 (18–22) | + | |||||
| 10 to < 20 years: BKB 64 | 49 (51–67) | + | GBI | 47 (44–50) | + | |||||
| AVGN | 56 (47–64) | + | 10 to < 20 years: HUI-3 64 | 0.18 (0.14–0.21) | + | |||||
| 20 to < 30 years: BKB 29 | 43 (30–56) | + | GHSI | 18 (15–20) | + | |||||
| AVGN | 60 (50–70) | + | GBI | 45 (40–50) | + | |||||
| 30–40 years: BKB 24 | 26 (14–38) | + | 20 to < 30 years: HUI-3 29 | 0.23 (0.15–0.30) | + | |||||
| AVGN | 44 (27–60) | + | GHSI | 16 (11–20) | + | |||||
| 40–50 years: BKB 15 | 9 (–1 to 18) | + | GBI | 42 (35–48) | + | |||||
| AVGN | 32 (17–46) | + | 30–40 years: HUI-3 24 | 0.13 (0.05–0.21) | + | |||||
| ≥ 50 years: BKB 11 | 14 (–2 to 30) | + | GHSI | 9 (4–14) | + | |||||
| AVGN | 37 (13–60) | + | GBI | 36 (28–43) | + | |||||
| Both measures declined significantly as duration of deafness increased | r < –0.203 | < 0.01 | + | 40–50 years: HUI-3 15 | 0.90 (–0.03 to 0.21) | + | ||||
| GHSI | 8 (0–15) | + | ||||||||
| GBI | 31 (15–47) | + | ||||||||
| ≥ 50 years: HUI-3 11 | 0.80 (–0.01 to 0.25) | + | ||||||||
| GHSI | 12 (3–21) | + | ||||||||
| GBI | 27 (11–38) | + | ||||||||
| Quality of life measures declined significantly as duration of deafness increased | r < –0.203 | < 0.01 | + | |||||||
| Outcome | n, mean (SD) | p-value | Direction of change | |||||||
|
Mawman 200425 n = 214 |
Difference in scores between preimplant and 18 months post implant: | |||||||||
| BKB sentences | n = 44, 63.97 (42.96) | + | ||||||||
| AB monosyllable words | n = 43, 49.91 (30.87) | + | ||||||||
|
Parkinson 200226 n = 216 |
Difference in scores between preimplant and 3 months post implant: | |||||||||
| Open set in quiet: | ||||||||||
| CUNY sentences | n = 56, 67.0 (33.69) | < 0.001 | + | |||||||
| CUNY words | n = 56, 34.5 (23.12) | < 0.001 | + | |||||||
| HINT sentences | n = 56, 57.0 (31.79) | < 0.001 | + | |||||||
| Open set in noise: | ||||||||||
| CUNY sentences in noise (+10 dB SNR) | n = 56, 55.2 (35.60) | < 0.001 | + | |||||||
| Outcome | n, mean % | p-value | Direction of change | |||||||
|
Kessler 19978 n = 238 |
Difference in scores between preimplant and 12 months post implant: | |||||||||
| MAC vowels | n = 120, 39 | + | ||||||||
| MAC consonants | n = 120, 39 | + | ||||||||
| CUNY sentences | n = 119, 42 | + | ||||||||
| Difference in scores between preimplant and 24 months post implant: | n, median % | |||||||||
| CID sentences | n = 61, 89 | + | ||||||||
| NU-6 monosyllabic word test | n = 61, 36 | + | ||||||||
| Everyday telephone sentences | n = 61, 73 | + | ||||||||
| Study | Design | Participants 1 | Participants 2 | Intervention and control | Outcomes |
|---|---|---|---|---|---|
|
UK Cochlear Implant Study Group 200423a UK n = 84 Length of follow-up: 9 months |
Prospective cohort Own control |
MHU = 84b Participants were defined by their preoperative BKB scores, aided acoustically and without lip-reading Age, mean (range): 50.6 (16–82) years |
Degree of deafness: profound Mean (range) dB HL: 108.7 (107–111) Postlingually deaf mean duration of deafness: 10.6 (8.1–13.0) years |
Nucleus CI 24 = 215; CLARION = 63; COMBI 40+ = 38 Coding strategy: CIS, SPEAK, compressed analogue |
Speech perception: BKB sentences, AVGN Quality of life: HUI-3, GHSI, GBI |
|
Ching 200427 Australia n = 21 Length of follow-up: NA |
Cross-sectional Own control |
Age, mean (range): 62 (25–84) years Degree of deafness: severe or profound: mean (SD) 83.3 (18.9) dB HL Postlingually deaf |
Time between implantation and recruitment: > 6 months Duration of deafness, mean (range): 29 (8–62) years Duration of cochlear implant use, mean (range): 4 (1.0–8.8) years |
Intervention: Nucleus CI 24 = 18; Nucleus CI 22 = 3 Coding strategy: ACE, SPEAK Control: Acoustic hearing aids |
Auditory: direction of sound Speech perception: BKB/A sentences, functional performance, structured interview questionnaire |
|
MED-EL 20015 USA n = 106 (fitted); efficacy n = 63, safety n = 50 Length of follow-up: 6 months Funded by MED-EL |
Pre/post prospective repeated measures Own control |
Degree of deafness: severe or profound: ≥ 70 dB HL Postlingually deaf: n = 45 Age at implantation, mean: 53 years Duration of deafness, mean: 28 years |
Prelingually deaf: n = 18 Age at implantation: 37.4 years Duration of deafness, mean: 36.5 years |
Intervention: COMBI 40+ Coding strategy: CIS PRO+ Control: Preoperative acoustic hearing aids |
Speech perception: in quiet: HINT sentences, CUNY; in noise: HINT sentences, CNC words Speech production: telephone sentences, CID sentences Quality of life: questionnaire, adverse events |
|
Hamzavi 200128 Austria n = 37 Length of follow-up: 36 months |
Prospective cohort |
Intervention group: n = 22 Age, mean (range): 53 (31–76) years Degree of deafness: severe/profound: mean (SD): 105 (5) dB HL Postlingually deaf Time between implantation and baseline measures: 36 months |
Control group: n = 15 Age, mean (range): 53 (23–71) years Degree of deafness: severe/profound: mean (SD): 85 (10) dB HL |
Intervention: COMBI 40/40+ Coding strategy: NR Control: Acoustic hearing aids |
Speech perception: in quiet and noise: HSM sentences, open set |
| Study | Outcome | Cochlear implant | p-value | Direction of change | |
|---|---|---|---|---|---|
|
UK Cochlear Implant Study Group 200423 n = 84 |
Speech perception: | ||||
| Mean difference between preimplant scores with acoustic hearing aids and 9-month postoperative cochlear implant scores: | |||||
| BKB | 44.0 (37–51) | + | |||
| AVGN | 31.0 (26–37) | + | |||
| Quality of life: | |||||
| Mean difference between preimplant scores with acoustic hearing aids and 9-month postoperative cochlear implant scores: | |||||
| HUI-3 | 0.15 (0.11–0.19) | + | |||
| GHSI | 0.19 (0.16–0.22) | + | |||
| GBI | 42.0 (37.0–47.0) | + | |||
| Outcome | Cochlear implant | Acoustic hearing aid | p-value | Direction of change | |
| Auditory: | n, mean (95% CI) | n, mean (95% CI) | |||
|
Ching 200427 n = 21 |
Sound direction (averaged root mean squared errors, horizontal localization) | n = 18, 4.5 (4.1–4.9) | n = 18, 4.6 (4.3–4.9) | NS | +/– |
| Speech perception: | |||||
| BKB sentences in noise | n = 21, 39 | n = 21, 2 | < 0.001 | ||
| Functional performance in real life, questionnaire scores: | |||||
| Use | n = 20, 87 (83–92) | n = 20, 84 (78–90) | < 0.001 | + | |
| Quiet | n = 20, 51 (40–62) | n = 20, 22 (12–31) | < 0.001 | + | |
| Noise | n = 20, 45 (43–56) | n = 20, 18 (12–25) | < 0.001 | + | |
| Environmental alertness | n = 20, 46 (40–52) | n = 20, 28 (22–34) | < 0.001 | + | |
| Overall score | n = 20, 59 (52–65) | n = 20, 40 (36–44) | < 0.001 | + | |
| Outcome | ≤ 25 years hearing loss | ≥ 25 years hearing loss | p-value | Direction of change | |
|
MED-EL 20015 n = 63 |
Speech perception: | Mean % | Mean % | ||
| Changes between baseline with hearing aid and 6 months follow-up with cochlear implants: | |||||
| Postlingually deaf (n = 45): | |||||
| In quiet: | |||||
| HINT sentences | + 70 | + 50 | + | ||
| CUNY sentences | + 72 | + 56 | + | ||
| In noise: | |||||
| HINT sentences | + 61 | + 41 | |||
| CNC words | + 40 | + 29 | |||
| Prelingually deaf (n = 18): | |||||
| In quiet: | |||||
| HINT sentences | + 19 | + | |||
| CUNY sentences | + 21 | + | |||
| In noise: | |||||
| HINT sentences | + 12 | + | |||
| CNC words | + 10 | + | |||
| CID sentences via telephone | |||||
| Speech production: | + | ||||
| CID sentences via telephone, mean increase in ability between baseline and 6-month follow-up: | |||||
| Postlingually deaf | + 68 | + 42 | + | ||
| Prelingually deaf | + 20 | + | |||
| Outcome | All | p-value | Direction of change | ||
| Quality of life: | |||||
| Impact of the implant on lifestyle (n = 45): | % gaining benefit | ||||
| Quite positive | 40 | + | |||
| Very positive | 44 | + | |||
| Quality of life questionnaire: | |||||
| Postlingually deaf: | |||||
| Impact of the implant on lifestyle (n = 45): | % gaining benefit | ||||
| Quite positive | 40 | + | |||
| Very positive | 44 | + | |||
| Significant improvement shown on 18 items in the questionnaire | < 0.05 | + | |||
| Prelingually deaf (n = 18): | % gaining benefit | ||||
| Quite positive | 33 | + | |||
| Very positive | 50 | + | |||
| Adverse events (n = 106) | |||||
| Total adverse events (people/events) | 20/22 | + | |||
| Medical events | 7 | + | |||
| Device related | 15 | ||||
| Outcome | Cochlear implant | Acoustic hearing aid | p-value | Direction of change | |
|
Hamzavi 200128 n = 37 |
Speech perception: | n , mean (SD) | n , mean (SD) | ||
| Changes between preimplantation and 12 months post implantation: | |||||
| HSM: | |||||
| Numbers | n = 22, 90 (10) | n = 15, 35 (11.40) | + | ||
| Monosyllables | n = 22, 43 (20) | n = 15, 18 (12.17) | + | ||
| Changes between 12 months and 36 months post implantation: | |||||
| HSM sentences: | |||||
| In quiet: | n = 22, 16.2 (34.61) | n = 15, 0 | < 0.001 | + | |
| In noise: | |||||
| SNR: 15 dB | n = 22, 14.4 (38.09) | n = 15, 0 | < 0.007 | + | |
| SNR: 10 dB | n = 22, 19.5 (30.69) | n = 15, 0 | < 0.003 | + | |
| SNR: 5 dB | n = 22, 13.0 (9.62) | n = 15, 0 | < 0.006 | + | |
| SNR: 0 dB | n = 22, 3.5 (4.68) | n = 15, 0 | 0.1 | + | |
| Study | Design | Participants 1 | Participants 2 | Intervention | Outcomes – speech |
|---|---|---|---|---|---|
|
Summerfield 200629 UK n = 24 Length of follow-up: 9 months |
RCT Waiting list control subjects Sequential implants |
Age, median (range): 56 (29–82) years Already use one implant |
Postlingually deaf Degree of deafness: NR Length of time using one implant, median (IQR): 2.7 (1.7) years |
Intervention: Nucleus CI 24 Coding strategy: SPEAK Control: Waiting list control subjects |
Auditory: SSQ Quality of life, GHSI, HUI-3, VAS quality of life, EQ-5D, tinnitus questionnaire |
|
Litovsky 200630 USA n = 37 Length of follow-up: 6 months Funded by Cochlear America |
Pre/post prospective Repeated measures Own control Simultaneous implants |
Age, median (range): 53.6 (26.6–86.6) years |
Postlingually deaf Degree of deafness: severe to profound: > 70 dB HL Duration of deafness, mean (range): 5.6 (1 month–15 years) years |
Nucleus CI 24 contour Coding strategy: SPEAK, ACE, CIS |
Speech perception: in quiet: CNC, HINT; in noise: BKB Quality of life: APHAB |
|
Ramsden 2005;31 UK multicentre trial of bilateral cochlear implants UK n = 29 Length of follow-up: 9 months |
RCT Waiting list control subjects Sequential implants |
Age, mean (range): 57 (29–87) years Time between implants, mean (range): 36 (12–84) months |
Degree of deafness: severe to profound: mean (range): 102.79 (67 to > 130) dB HL Duration of deafness, mean (range): 6.14 (1–15) years |
Nucleus CI 24 (M or RST) Coding strategy: SPEAK, ACE |
Speech perception: in quiet: CUNY, CNC; in noise: CUNY noise front, CUNY noise left, CUNY noise right Adverse events |
|
Verschuur 200532 UK n = 20 Length of follow-up: NA Trial funded by Cochlear Ltd |
Cross-section (from larger RCT) Own control Sequential implants |
Age at first implant, mean (SD): 58.9 (12.67) years Time between implants, mean (SD): 37.0 (14.40) months |
Degree of deafness: NR Postlingually deaf Duration of deafness (years): first ear, mean (SD): 7.9 (4.34); second ear, mean (SD): 10.0 (3.73) |
Nucleus CI 24M, Nucleus CI 24K Coding strategy: SPEAK, ACE |
Auditory: detection of sound direction |
|
Laszig 200433 Germany and Switzerland n = 37 Length of follow-up: 6 months |
Pre/post prospective Repeated measures Own control Simultaneous = 22; sequential = 15 |
Age: ≥18 years Age at bilateral implantation, mean (SD): 46 (11) years Time between sequential implants, mean (SD): 2.2 (1.4) years |
Degree of deafness: profound Time between deafness and implantation, mean: 10 years |
Nucleus CI 24 Coding strategy: SPEAK |
Speech perception: in quiet and noise: HSM, OLSA; in quiet: FMWT |
| Outcome | Two cochlear implants | One cochlear implant | Direction of change | Pooled within groups pre/post | Direction of change | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Differences between measuring sessions, 9 months from 3 months and 3 months from 1 month preimplant | |||||||||||
| Auditory: | n | Mean (95% CI) | p-value | n | Mean (95% CI) | p-value | n | Mean (95% CI) | p-value | ||
| SSQ: spatial hearing: | |||||||||||
| 3 months | 12 | 1.46 (0.83–2.09) | < 0.01 | 12 | – | NS | 24 | + | 1.56 (0.95–2.17) | < 0.001 | + |
| 9 months | 12 | 0.71 (0.08–1.33) | < 0.01 | 12 | – | NS | 24 | + | 2.00 (1.47–2.53) | < 0.001 | + |
| Qualities of hearing: | |||||||||||
| 3 months | 12 | 0.5 (–0.3 to 1.30) | NS | 12 | – | NS | 24 | + | 0.9 (0.5–1.3) | < 0.05 | + |
| 9 months | 12 | 1.76 (–0.6 to 2.8) | < 0.01 | 12 | – | NS | 24 | + | 0.7 (0.2–1.2) | < 0.05 | + |
| Hearing for speech: | |||||||||||
| 3 months | 12 | 1.1 (–1.00 to 2.3) | NS | 12 | – | NS | 24 | + | 6.00 (0.00–12.00) | < 0.01 | + |
| 9 months | 12 | 1.1 (–0.3 to 2.5) | NS | 12 | – | NS | 24 | + | 9.00 (3.00–15.00) | < 0.01 | + |
| Quality of life: | |||||||||||
| GHSI: | |||||||||||
| 3 months | 12 | 0.00 (–8.00 to 8.00) | NS | 12 | – | NS | 24 | + | 3.00 (–1.00 to 6.00) | NS | + |
| 9 months | 12 | 6.00 (–7.00 to 13.00) | NS | 12 | – | Ns | 24 | + | 4.00 (1.00 to 7.00) | < 0.05 | + |
| HUI-3: | |||||||||||
| 3 months | 12 | 0.1 (–0.1 to 0.3) | NS | 12 | – | NS | 24 | + | –0.03 (–0.11 to 0.08) | NS | – |
| 9 months | 12 | 0.11 (–0.08 to 0.29) | NS | 12 | – | NS | 24 | + | –0.01 (–0.1 to 0.08) | NS | – |
| VAS overall quality of life: | |||||||||||
| 3 months | 12 | –1.5 (–9.5 to 6.5) | NS | 12 | – | NS | 24 | + | –0.06 (–0.15 to 0.03) | NS | – |
| 9 months | 12 | –3.0 (-9.0 to 3.0) | NS | 12 | – | NS | 24 | + | –0.06 (0.12–00) | NS | – |
| EQ-5D: | |||||||||||
| 3 months | 12 | –0.04 (–0.13 to 0.05) | NS | 12 | – | NS | 24 | + | –3.5 (–0.11 to 4.00) | NS | – |
| 9 months | 12 | –0.01 (–0.09 to 0.07) | NS | 12 | – | NS | 24 | + | –4.5 (–12.0 to 3.0) | < 0.05 | – |
| Tinnitus questionnaire: | |||||||||||
| 3 months | 12 | 10 (–5.0 to 25.0) | NS | 12 | – | ns | 24 | + | 12 (1.0 to 23) | < 0.05 | + |
| 9 months | 12 | 14 (–1.0 to 29) | NS | 12 | – | NS | 24 | + | 8 (–4.0 to 20) | NS | + |
| Outcome | n | Left ear alone | Right ear alone | Bilateral | p-value | Direction of change |
|---|---|---|---|---|---|---|
| Speech perception: | ||||||
| Difference between preimplant and 6 months post activation: | Mean % | Mean % | Mean % | |||
| In quiet: | ||||||
| CNC | 33 | 40.1 | 35.6 | 54 | 0.0001 | + |
| HINT | 33 | 66.0 | 66.9 | 76 | 0.0001 | + |
| Difference between 3 and 6 months post activation: | ||||||
| In noise: | ||||||
| BKB: | ||||||
| Noise left | 29 | –1.8 | –2.7 | –3.5 | 0.0001 | + |
| Noise right | 29 | –0.8 | –1.1 | –1.1 | 0.0001 | + |
| Noise front | 29 | –0.5 | –1.3 | 0.02 | 0.0001 | – |
| Speech in noise: | ||||||
| Binaural redundancy effect | 34 | 17.0 (3) | 14.0 (3) | 9.5 (3) | + | |
| Noise right, mean (SD) | Noise left, mean (SD) | |||||
| Head shadow effect | 32 | 4.95 (3.6) | 6.34 (3.8) | Binaural advantage p < 0.0001 | ||
| Unilateral mean % (+1 SE) | Bilateral mean % (+1 SE) | |||||
| Quality of life: | ||||||
| APHAB: | ||||||
| Communication | 30 | 4.4 (0.2) | 5.7 (0.2) | < 0.0001 | + | |
| Reverberant conditions | 30 | 3.0 (0.2) | 4.4 (0.2) | < 0.0001 | + | |
| Background noise | 30 | 3.1 (0.2) | 4.4 (0.2) | < 0.0001 | + | |
| Aversion to sounds | 30 | 3.2 (0.3) | 3.0 (0.2) | > 0.05 | + | |
| Outcome | n | First ear | p-value | n | Second ear | p-value | n | Bilateral | p-value | Direction of change |
|---|---|---|---|---|---|---|---|---|---|---|
| Speech perception – 3 months compared with pre second implant: | ||||||||||
| In quiet: | ||||||||||
| CNC words | 24 | NS | 25 | NS | 26 | NS | ||||
| CUNY sentences | 25 | 6.5 ± 3.2 | 0.004 | 26 | NS | 26 | NS | + | ||
| In noise: | ||||||||||
| CUNY front | 25 | NS | 25 | NS | 25 | NS | ||||
| CUNY left | 25 | NS | 25 | NS | 25 | NS | ||||
| CUNY right | 25 | NS | 25 | NS | 25 | NS | ||||
| Outcome | n | p-value | n | p-value | n | p-value | Direction of change | |||
| Speech perception: | Bilateral compared with best monaural 9 months | Bilateral compared with first ear at 9 months | Bilateral compared with second ear at 9 months | |||||||
| Speech and noise from front speaker: | ||||||||||
| CUNY sentences | 7.7 ± 5.3% | < 0.002 | 12.6 ± 5.4% | < 0.001 | – | + | ||||
| Speech from front, noise into first ear: | ||||||||||
| CUNY sentences | 21 ± 6% | < 0.001 | 11.7 ± 6% | < 0.001 | + | |||||
| Head shadow effect | – | NS | – | NS | – | NS | ||||
| Speech from front, noise into second ear: | ||||||||||
| CUNY sentences | – | NS | – | NS | 49.8 ± 5.8% | < 0.001 | + | |||
| First ear score compared with second ear score 9 months | ||||||||||
| Head shadow effect | 45.2 ± 5.8% | < 0.001 | ||||||||
| Binaural squelch effect | – | NS | ||||||||
| Outcome | n | Right implant only | n | Left implant only | n | Bilateral | p-value for condition | p-value for bilateral | Direction of change |
|---|---|---|---|---|---|---|---|---|---|
| Auditory: | 20 | 20 | 20 | ||||||
| Mean (SD) absolute angular error | 67° (10) unilateral | 67° (9) | 24° (5) | < 0.001 | |||||
| Speaker to the front, mean (SD) angular error | 73° | 23° | < 0.001 | < 0.001 | + | ||||
| Speaker to the side, mean (SD) angular error | 54° | 26° | < 0.001 | < 0.05 | + | ||||
| Device opposite speaker | Device same side | ||||||||
| Mean error from left speaker as a function of listening condition | 95° | 33° | 24° | < 0.001 | < 0.05 | + | |||
| Mean error from right speaker as a function of listening condition | 96° | 36° | 24° | < 0.001 | < 0.001 | + | |||
| Outcome | n | Poorer ear – unilateral [mean % correct (SD)] | p-value | n | Better ear – unilateral [mean % correct (SD)] | p-value | n | Bilateral [mean % correct (SD)] | p-value | Direction of change |
|---|---|---|---|---|---|---|---|---|---|---|
| Speech perception: | ||||||||||
| In quiet: | ||||||||||
| FMW words: | ||||||||||
| 6-month score | 30 | 49 | 30 | 58 | 30 | 58 | 0.00009a | +~ | ||
| Difference between 6 and 3 months post second implant | 30 | 17 | 30 | 11 | 30 | 48 | + | |||
| HSM sentences: | ||||||||||
| 6-month score | 14 | 78 | 14 | 80 | 14 | 81 | +~ | |||
| Difference between 6 and 3 months post second implant | 14 | 10 (41.34) | 14 | 0 (30.02) | 14 | –2 (35.38) | Poorer ear = 0.01; better ear = NS | +~ | ||
| OLSA sentences: | ||||||||||
| 6-month score | 19 | 72 | 19 | 74 | 19 | 78 | + | |||
| Difference between 6 and 3 months post second implant | 19 | 10 | 19 | 6 | 19 | 7 | + | |||
| In noise: | ||||||||||
| HSM sentences: | ||||||||||
| 6-month score: | ||||||||||
| Sound front | 23 | 29 (21) | 23 | 50 (31) | 23 | 54 (31) | + | |||
| SBNP | 23 | 8 (14) | 23 | 63 (32) | 23 | 70 (32) | + | |||
| SPNB | 23 | 13 (15) | 23 | 55 (38) | 23 | 59 (36) | + | |||
| OLSA sentences:b | ||||||||||
| 6-month score: | ||||||||||
| Sound front | 20 | +0.46 (7.59) | 20 | –1.15 (4.14) | 0.01 | 20 | –2.76 (5.29) | 0.04 | + | |
| SBNP | 20 | +7.36 (13.80) | 20 | –4.14 (8.97) | 20 | –5.06 (–11.04) | + | |||
| SPNB | 20 | +4.14 (11.73) | 20 | +5.98 (12.42) | 20 | –2.76 (9.43) | – | |||
| Bilateral head shadow effect: | ||||||||||
| Better ear closer to noise | 20 | –11.4 dB (6 dB) | < 0.00001 | + | ||||||
| Poorer ear closer to noise | 20 | –10 dB (8 dB) | < 0.00001 | + | ||||||
| Study | Design | Participants 1 | Participants 2 | Outcomes |
|---|---|---|---|---|
|
Mo 200534 Norway n = 27 Length of follow-up: 15 months |
Prospective Pre/post repeated measures Own control |
Mean (SD) age adults: 57.6 (14.5) years Duration of deafness prior to surgery (SD): 8.5 (10.3) years |
Postlingually deaf | PQLF, IRQF, SF-36 |
|
Vermeire 200535 Netherlands n = 89 |
Prospective Pre/post repeated measures Own control |
Mean (SD) age adults: 58 (15) years Postlingually deaf Degree of deafness: profound |
HHIA, GBI | |
|
Hallberg 200436 Sweden n = 17 |
Grounded theory Interviews |
Mean (range) age adults: 56.5 (29–78) years Cochlear implant usage, mean (range): 4.1 (1–12) years |
Emergent categories | |
|
Hawthorne 200437 Australia and New Zealand n = 34 Length of follow-up: 6 months Partly funded by Cochlear Ltd |
Prospective Pre/post repeated measures Own control |
Mean (SD) age adults: 49 (13) years Degree of deafness: profound |
Years since hearing loss: from birth = 8; ≤ 6 years = 10; ≥ 5 years = 14 | AQoL, HPS |
|
Hogan 200138 Australia and New Zealand n = 202 |
Cross-sectional Non-randomised control |
Intervention: n = 148 Cochlear implant user Time since implantation, mean (range): 4.9 (0–16) years |
Control: n = 54 Cochlear implant candidate |
AQoL |
|
Palmer 199939 USA n = 62 Length of follow-up: 12 months Partly funded by Cochlear Corporation |
Prospective Pre/post repeated measures |
n = 46 Age at study, mean (SD): 49.0 (14.5) years Postlingually deaf Degree of deafness: severe or profound |
n = 16 Age at study, mean (SD): 56.0 (15.4) years Postlingually deaf Degree of deafness: severe or profound |
HUI-3 |
| Study | Outcome | Intervention | Control | p-value | Direction of change |
|---|---|---|---|---|---|
|
Mo 200534 n = 27 |
PQLF total score: | Mean (SD) | |||
| Preimplant | 2.94 (0.54) | ||||
| Post implant | 3.56 (0.44) | ||||
| Difference | 0.62 (0.47) | < 0.01 | + | ||
| IRQF total score: | |||||
| Preimplant | 3.35 (0.41) | ||||
| Post implant | 3.72 (0.44) | ||||
| Difference | 0.37 (0.39) | < 0.01 | + | ||
| SF-12 – general health: | |||||
| Preimplant | 72.6 (21.6) | ||||
| Post implant | 79.8 (21.4) | ||||
| Difference | 7.2 (14.5) | 0.02 | + | ||
| Other SF-12 subscales were not significantly different | |||||
|
Vermeire 200535 n = 89 |
HHIA: | Mean (SD) | |||
| Preoperative total score | 69 (0.69) | ||||
| Postoperative total score | 48 (25.28) | < 0.001 | + | ||
| GBI: | |||||
| Postoperative total score | 35.16 (19.61) | ||||
|
Hawthorne 200437 n = 34 |
AQoL: | n, mean (SD) | |||
| Preimplant | n = 31, 0.36 (0.23) | ||||
| 6 months post implant | n = 31, 0.64 (0.28) | ||||
| Difference | n = 31, 0.28 (0.36) | < 0.01 | + | ||
| HPS: | |||||
| Preimplant | n = 34, 0.48 (0.15) | ||||
| 6 months post implant | n = 34, 0.68 (0.18) | ||||
| Difference | n = 34, 0.20 (0.23) | < 0.01 | + | ||
|
Hogan 200138 n = 202 |
n = 46 | n = 16 | |||
| AQoL: | Mean (SD) | Mean (SD) | |||
| Illness | 0.64 (0.35) | 0.69 (0.32) | NS | - | |
| Independent living | 0.89 (0.16) | 0.87 (0.16) | NS | + | |
| Social relationships | 0.84 (0.22) | 0.82 (0.21) | NS | + | |
| Physical senses | 0.78 (0.19) | 0.58 (0.19) | < 0.01 | + | |
| Psychological well-being | 0.88 (0.17) | 0.83 (0.19) | NS | + | |
| Utilities | 0.57 (0.27) | 0.38 (0.22) | < 0.01 | + | |
|
Palmer 199939 n = 62 |
n = 62 | n = 16 | |||
| HUI-3 | Mean (SD) | Mean (SD) | |||
| Preimplant | 0.58 (0.17) | 0.58 (0.20) | |||
| 12 months post implant | 0.78 (0.17) | 0.58 (0.23) | + | ||
| Utility gain | 0.20 (0.24) | + | |||
|
Hallberg 200436 n = 17 |
Reported above | ||||
Appendix 4 Summary of audit of clinical practice in the UK by the British Cochlear Implant Group – criteria for candidacy
Referral recommendations (children and adults)
All programmes recommend referral of patients with severe to profound hearing loss for assessment for cochlear implantation. Referrals for lower levels of loss are accepted if functional hearing is poor.
Hearing aid trial (children and adults)
All programmes require a valid hearing aid trial to be undertaken, the majority recommending a 3-month trial unless the patient is post meningitic.
Unaided hearing level (children)
The most common current practice in the UK incorporates a guideline for unaided hearing levels of profound bilateral loss in the high frequencies (> 90 dB HL at 2 and 4 kHz) with the proviso that all programmes require the flexibility to implant at lower levels for individual patients with poor functional hearing as appropriate. A reliability (test–retest) margin of +/– 10 dB is applicable in paediatric testing. This is used in conjunction with a multidisciplinary functional hearing assessment.
Unaided hearing level (adults)
The most common current practice in the UK incorporates a guideline for unaided hearing levels of severe to profound bilateral loss across the frequency range, typically > 90 dB HL at 2 and 4 kHz, with the proviso that all programmes require the flexibility to implant at lower levels for individual patients with poor functional hearing as appropriate. This is used in conjunction with a functional hearing measure, most commonly a score of < 50% correct on BKB sentence testing in the best aided condition.
Aided hearing levels (children and adults)
No programme uses aided hearing thresholds as a criterion or guideline for cochlear implantation.
Functional hearing (children)
All programmes concur that they use functional hearing as the primary factor/indicator for implantation.
Factors include failure to develop, progress or maintain speech, language and listening skills appropriate to age, development and cognitive ability, measured by a multidisciplinary range of age-appropriate assessments and questionnaires. The child should also be likely to benefit from increased access to audition, with increased potential for improvement in linguistic and communication skills, monitoring own environment, and psychosocial factors.
The weight of influence of particular components of the assessment of functional hearing on whether to implant or not must be considered on an individual patient basis.
Functional hearing (adults)
All programmes use speech perception testing in conjunction with other assessments, most commonly a score of ≤ 50% correct on BKB sentence testing in the best aided condition, with the proviso that all programmes require the flexibility to implant at lower levels for individual patients with poor functional hearing as appropriate.
Other factors include consideration of the probability of benefit from increased access to audition in terms of likelihood of improvement in linguistic skills, communication skills, monitoring own environment, and psychosocial factors.
Additional needs (children and adults)
All programmes state that patients with additional or complex needs are considered on an individual basis, for both children and adults.
Age at implantation (children and adults)
No programme has a lower age limit for cochlear implantation in children, nor an upper age limit for adults. Some programmes will impose an upper limit to duration of profound deafness for both children and adults.
Guidelines/criteria (children and adults)
All programmes indicated that their guidelines are continuously evolving in line with developments in clinical experience, technology and peer-reviewed published evidence-based outcomes.
Funding issues (children and adults)
The majority of programmes experience funding problems for patients recommended for cochlear implantation.
Appendix 5 Outcome measures in reviewed studies
The outcome measures used in the included studies can be categorised as audiological, speech perception, speech production, quality of life and educational. Because of the large numbers of measures reported in the included studies (n = 62) they will be described in a series of tables. Measures shaded dark grey were used with adults, those shaded light grey were used with adults and children and those unshaded were used with children. For details of references for included measures see Tables 6–10.
| Measure | Description | No. of studies using each measure |
|---|---|---|
| Basal auditory ability (SRPx) | First detection of sounds is observed, then the ability to derive meaning from the sounds presented is assessed by finding out if the child can associate the sounds with their sources. These are everyday sounds presented randomly; the child is watched to see if they respond at all to the sound | 1 |
| MAA – Minimal audible angle (P) | This is the smallest change in the position of a sound source that can be reliably discriminated | 3 |
| PTA – Pure-tone audiometry (P) | Measures hearing sensitivity in laboratory conditions. This measure involves the peripheral and central auditory systems. Pure-tone thresholds indicate the softest sound audible to an individual at least 50% of the time. The average unaided threshold is taken | 2 |
| SSQ – Speech Hearing, Spatial Hearing and Qualities of Hearing questionnaires (SR) | This measure has three sections: spatial hearing, which is concerned with the location and tracking of sounds (17 questions); quality of hearing, which is about the clarity and naturalness of sounds (19 questions); hearing for speech, which assesses the capacity to focus attention on a single source and to divide attention between sources (14 items) | 1 |
| Measure | Description | No. of studies using each measure |
|---|---|---|
| One-syllable test | Closed-set ad hoc test. The child has to recognise monosyllabic words | 1 |
| Two-syllable test | Closed-set ad hoc test. The child has to recognise familiar two-syllable words | 1 |
| AVGN – A normalised index | Using CUNY scores a normalised index (AVGN) can be calculated: AVGN = 100 × (CUNYAVpre – CUNYVpre)/(100–CUNYVpre). This is used to assess the arithmetically possible improvement in performance over lip-reading alone. Using AVGN scores before and after the intervention allows the change in performance to be evaluated (AVGNpre – AVGNpost) | 1 |
| AB monosyllables – Arthur Boothroyd monosyllabic word test | Listener has to identify phonemes from a list of recorded words. Each list consists of 10 words and each word is constructed as consonant–vowel–consonant (CVC isophonemes). Score is based on the phonemes correct out of 30 | 1 |
| BKB – Bamford–Kowal–Bench sentences | These are 21 lists of 16 sentences of simple syntactical structure presented in auditory alone conditions in quiet and noise of 10 dB signal to noise ratio. The listener repeats back what they have heard. Performance is scored as the number of key words reported correctly, using the loose key word scoring method | 5 |
| CAP – Categories of Auditory Performance | This measures real-life auditory receptive abilities and is a non-linear, hierarchical scale of auditory receptive abilities, from no awareness of environmental sounds to the ability to use a telephone with a known speaker | 1 |
| CDT – Connected discourse tracking | Measures open-set speech perception. Stories are read, phrase by phrase, to a child who repeats them back. This is done without lip-reading. The number of correct words per minute is calculated. Generic measure | 1 |
| CID sentences – Central Institute for the Deaf sentences | Subjects listen to a list of 20 sentences, varying in length and structure and spoken with minimal inflection. Sentences are drawn from 10 sets of 10 sentences, with 50 ‘target’ words in each set. These are played at 70 dB (A) under different contextual conditions (i.e. with or without lip-reading). Subjects repeat the sentences or undertake a written task. Sentences are uncommon and not likely to be heard on a regular basis. Examples of typical sentences are ‘The vacuum is in the back of the closet’ or ‘The book is on the top shelf next to the pencil’ | 1 |
| CNC – Consonant Nucleus Consonant monosyllabic word test | This open-set word recognition test consists of 10 lists with 50 monosyllabic words in each list. Patients are scored for words correct and phonemes correct | 4 |
| Common Phrases Test | Open-set test. Assesses understanding of familiar phrases spoken in everyday situations. Ten simple phrases are repeated by the child and one mark is given for each correct answer | 3 |
| CUNY – City University of New York | Measure of the benefit of lip-reading from hearing, obtained by presenting high-quality audiovisual recordings of 12 sentences from 25 possible lists. Listeners have to report as many spoken words as possible in different conditions (background noise) and locations using lip-reading only (CUNYVpre), audiovisual with the right ear aided (CUNYAVRpre) and audiovisual with the left ear aided (CUNYAVLpre). Performance is scored by the percentage of all words reported correctly. All of the tests are of a man’s voice and are played at 70 dB | 5 |
| ESP – Early Speech Perception battery | Examines children’s speech perception abilities according to four categories: no pattern perception, pattern perception, some word identification and consistent word identification. Pictures are identified by pointing after hearing spoken language | 5 |
| FMWT – Freiburger monosyllabic word test | This test comprises two lists of 20 German words for each listening condition (quiet and background noise), for which a percentage total word score is obtained. Words are presented at 70 dB | 1 |
| GASP – Glendonald Auditory Screening Procedure | Words: 12 words of different syllable numbers and/or stress patterns. The child repeats the word presented by the examiner. Sentences: 10 questions are asked; responses can be to repeat or answer the question by verbal/signed response | 7 |
| Gottinger speech lists | These are used in speech audiometry; they consist of two lists of bisyllabic words (30 words each) administered in an open-set format. Toddlers have a closed set (8 word list) | 1 |
| HINT – Hearing in Noise Test | This test measures sentence speech reception thresholds in quiet and noise. It consists of 13 lists of 10 sentences with all correctly identified words tabulated to derive a percentage score. A child version is also available | 3 |
| HINT-C – Hearing in Noise Test for Children | Measures sentence speech reception thresholds in quiet and noise. It consists of 13 lists of 10 sentences with all correctly identified words tabulated to derive a percentage score | 3 |
| HSM sentences – Hochmaier, Schultz and Moser sentence test | This test is specifically designed for cochlear implant users and is composed of 600 everyday sentences arranged in 30 lists of 20 sentences, each varying in structure and length and played at 80 dB. Lists are prepared with five different levels of noise in the background at various signal to noise ratios of between 15 dB and 0 dB | 2 |
| IMST – Iowa Matrix Sentence Test | Assesses closed-set speech perception. Controls for learning effects and consists of four 2 × 3 picture matrices. These word sentences are presented by voice but without lip-reading. The child responds by pointing to pictures or retelling the sentence | 1 |
| LNT – Lexical Neighbourhood Test | Open-set test of word recognition, based on word frequency and neighbourhood density, it uses words found in the vocabulary of children aged 3–5 years. The child repeats a spoken word. Can also be used for speech perception | 2 |
| MAC – Minimal Auditory Capabilities | A battery of tests graded in difficulty specifically targeted at cochlear implant patients. There are 14 subtests (13 audio and one video), which include gross sound identification, inflection detection, contrast detection, accent discrimination and word identification. Although designed for postlingual hearing loss it can be used to evaluate the hearing abilities of persons for whom traditional speech materials are too difficult. The second edition has been standardised | 1 |
| MAIS (IT-MAIS) – Meaningful Auditory Integration Scale (SRPx) | A parental rating scale of listening behaviours. There is also an infant/toddler version (IT-MAIS). Evaluates meaningful use of sound in everyday situations. It provides information about consistency of device use and response to sound in everyday listening using 10 questions, e.g. ‘Do they respond to the doorbell?’ | |
| Minimal Pairs Test | Assesses auditory discrimination skills for minimal pair words. Closed-set single word pairs differing by one feature (e.g. place, manner, articulation). The child has to find the picture corresponding to one or two similar words. Results are scored by feature errors | 1 |
| MLNT – Multisyllabic Lexical Neighbourhood Test | Similar to the LNT. Can also be used for speech production. Open-set test of word recognition, based on word frequency and neighbourhood density, it uses words found in the vocabulary of children aged 3–5 years. The child repeats a spoken word | 2 |
| Mr Potato Head | This is a modified open-set speech perception task. Children are asked to carry out 10 commands presented aurally to assemble the toy. A sentence score is given for the number of commands correctly carried out and a word score for the number of key words correctly identified, e.g. choosing the right colour shoes | 3 |
| NU-6 – Northwestern University Auditory Test number 6 | Listeners repeat back a series of prerecorded phonetically balanced CNC monosyllabic words from four lists of 50 words, each recorded in four randomisations | 1 |
| OLSA – Oldenburg sentence test | This test comprises two lists of 10 German sentences each consisting of 50 words. A percentage correct score is obtained for each listening condition (quiet and background noise) | 1 |
| PB-K – Phonetically Balanced Kindergarten Word List | Word identification test, with two levels, used to evaluate open-set word recognition. Includes 50 phonetically balanced words that are within the vocabularies of normal-hearing 5-year-olds. Scores indicate the numbers of words and phonemes correctly identified | 5 |
| RITLS – Rhode Island Test of Language Structure | This is a test of sentence comprehension. A sentence is presented in signed English or voice. The child chooses a picture from a set of three that best represents the sentence. The final score is the total number of sentences chosen in error | 1 |
| SECSHIC – Scales of Early Communication Skills for Hearing Impaired Children | Evaluates speech and language development in deaf children. There are four subscales: verbal receptive language, verbal expressive language, non-verbal receptive skills and non-verbal expressive skills (verbal receptive skills only used) | 1 |
| TAC – Test for Auditory Comprehension of Language | Measures the auditory comprehension of language with a battery of increasingly difficult auditory/speech discrimination tasks. Closed-set test | 1 |
| TAPS – Test for Auditory Perception and Speech | Designed for children with cochlear implants. It consists of six subtests: sound detection, synthetic syllables, syllable/word identification, phrases of different length and stress and sentences of similar length and stress. Open set | 1 |
| TROG – Test for the Reception of Grammar | An individually administered, multiple-choice test of understanding of English grammar for children aged 4–13 years. Includes 20 blocks of four items, each assessing a specific type of grammatical contrast. The child selects the correct picture from an array that corresponds to a word order or grammatical construction | 1 |
| Measure | Description | No. of studies using each measure |
|---|---|---|
| CRISP – Children’s Realistic Intelligibility and Speech Perception test | Used to evaluate bilateral enhancement in noise. It uses known words to identify picture and sound combinations. Can be used to measure speech perception | 2 |
| IPSyn – Index of Productive Syntax | A system for scoring transcriptions of expressive language recorded on videotape. There are four grammatical domains: noun phrases, verb phrases, questions and negotiations, and simple and complex sentence forms. These are combined to give a total score | 1 |
| SIR – Speech Intelligibility RATING | This is a 5-point scale describing degrees of speech intelligibility, ranging from unintelligible speech to that understandable by all listeners (1 = least intelligible and 5 = most intelligible) | 1 |
| Measure | Description | No. of studies using each measure |
|---|---|---|
| APHAB – Abbreviated Profile of Hearing Aid Benefit | This is a condition-specific 24-item self-assessment quality of life inventory in which patients report the amount of trouble that they are having with communication or noises in various everyday situations. Benefit is calculated by comparing the patient’s reported difficulty in the unaided condition with their amount of difficulty when using amplification. It produces scores for four subscales: ease of communication (EC), reverberation (RV), background noise (BN) and aversiveness (AV) | 1 |
| AQoL – Assessment of Quality of Life | A multiattribute utility instrument designed for economic evaluation. It contains 15 items across five domains (illness, independent living, social relationships, physical senses and psychological well-being). The last four domains are combined using time trade-off weights derived from the Australian population to provide a utility score ranging from –0.04 (health state worse than death) to 1 (best health state) | 2 |
| Everyday Life Questionnaire | Ad hoc cochlear implant-specific questionnaire with most questions derived from Kelsay and Tyler (reference 99 in main reference list). Parent questionnaire has 54 questions assessing problems and benefits. A modified version has fewer items for children. Items are scored on a Likert scale, with higher scores indicating a better quality of life | 1 |
| EQ-5D – EuroQol 5 dimensions | This is a generic measure of health-related quality of life that contains five questions about the extent of problems in mobility, self-care, daily activities, pain and anxiety/depression. Each question has three possible responses: no problem, mild problem or severe problem. Each possible combination of responses to the five questions constitutes a ‘health state’. The last section of the questionnaire also contains a visual analogue scale (VAS) (see below) on general health. States have been valued by a cross-section of the British public to allow a societal value to be assigned to a patient’s health state on a scale in which 0 corresponds to death and 1 to full health | 1 |
| GBI – Glasgow Benefit Inventory | The GBI provides a direct self-reported measure of change in health status in relation to a specific event (e.g. an operation). It contains 18 questions covering three domains: psychological (optimism and self-confidence), social and emotional. Responses are made on a 5-point Likert scale and scored on a scale of –2 to +2. Scores are summed across questions and the total multiplied by 2.778 to give an aggregate score | 2 |
| GHSI – Glasgow Health Status Inventory | This (quasi)-generic profile measure assesses the effect of a health problem on a person’s quality of life across five domains: overall life, general, physical, health and social support | 2 |
| HHIA – Hearing Handicap Inventory for Adults | This is a 25-item hearing-specific quality of life scale used to assess hearing handicap in adults. It has two subscales that measure the emotional and situational impact of hearing loss. The scale is scored from 0 to 100, with 100 representing the maximum handicap possible | 1 |
| HPS – Hearing Participation Scale | This is a shortened form of the GHSI with 11 items. It is scored from 0.00 to 1.00. A low score indicates that there are profound effects on quality of life due to deafness | 1 |
| HUI-3 – Health Utilities Index 3 | This is a population-based instrument measuring quality of life on 9 domains: hearing, speech, vision, emotion, pain, ambulation, dexterity, cognition and self-care. Respondents are mapped onto a health state depending on functional capacity. Based on a 15-item questionnaire with specific questions related to hearing.40 These assume that patients have no co-morbidity. Utilities are calculated for each domain and an overall utility value derived using an algorithm to weight each domain41 | 2 |
| IRQF – Index Relative Questionnaire Form | This is a condition-specific health-related quality of life measure developed for cochlear implantation by the House Ear Institute, Los Angeles. It contains 31 questions relating to a relative’s experience with the patient as hearing impaired, the effect of the handicap on their daily activities and the patient’s adaptation to the implant. Answers are scaled from 1 to 5, where a low score may represent either a positive or a negative response | 1 |
| KINDLr – Munich Quality of Life Questionnaire for Children | This is a 24-item Likert-scaled generic questionnaire completed by children or adults, with different age versions. There are six dimensions: physical well-being, emotional well-being, self-esteem, family, friends and everyday functioning. These can be combined to produce a total score | 1 |
| NCIQ – Nijmegen Cochlear Implant Questionnaire | This is a condition-specific instrument with questions in three domains, physical, psychological and social functioning, each containing 10 items. Scores range from 0 to 100 (optimal) | 1 |
| PQLF – Patient Quality of Life Form | This is a condition-specific health-related quality of life instrument for cochlear implantation developed by the House Ear Institute, Los Angeles. It contains 43 questions concerning patients’ coping with their hearing loss, adaptation to the implant and emotional alteration since implantation. Answers are scaled from 1 to 5, where a low score may represent either a positive or a negative response | 1 |
| Quality of life questionnaire | This is a generic behaviour-oriented instrument. It has 42 items on a Likert scale, with subscales for the physical, psychological, social, medical treatment, well-being and functional components of quality of life. The total range of scores is from 42 to 210 with a high score showing a better quality of life | 2 |
| SF-36 – Short-Form 36 | This is a widely used generic health-related quality of life instrument, which contains 36 questions across eight domains. Each domain focuses on different aspects of quality of life (physical functioning, role limitation because of health problems, bodily pain, social function, role–emotional, mental health, vitality), except for one domain, which provides an overall evaluation (general health). Responses to questions in each domain are summed to provide eight scores between 0 and 100, where a high score means a better result | 1 |
| Symptom Checklist 90-R | This measures psychological symptoms in the last 7 days. It contains 90 items on a Likert scale combining nine scales and a severity index to provide a measure of psychological distress | 1 |
| Tinnitus Questionnaire | This questionnaire provides an index of tinnitus-related distress and its degree of severity. It is a 52-item self-rating scale covering emotional and cognitive distress, intrusiveness, auditory perceptual difficulties, sleep disturbances and somatic complaints | 1 |
| ULS – Usher Lifestyle Questionnaire | This is a descriptive questionnaire consisting of nine main questions divided into subgroups. It covers domains of independence affected by deaf–blindness, communication, access to information and mobility | 1 |
| VAS quality of life scale – Visual analogue scale | This provides a summary of overall quality of life by asking subjects to indicate their quality of life on a 100-point visual analogue scale, ranging from 0 (the worst imaginable quality of life) to 100 (the best imaginable quality of life) | 1 |
| Measure | Description | No. of studies using each measure |
|---|---|---|
| AMP – Assessment of Mainstream Performance | This is a deaf-specific measure which determines the skills that children need to possess to be successful in mainstream school. There is a 3- to 5-year-old version with 16 items and an older children’s version with 22 items. It measures the child’s ability to participate in a range of classroom activities and age-appropriate behaviours. Answers are coded as the percentage of time that a child spends doing an activity or behaviour | 1 |
| SIFTER – Screening Instrument for Targeting Educational Risk | This deaf-specific measure has had content and score reliability shown. It rates the child in comparison to others in the classroom on 15 items in five areas: academics, attention, communication, classroom participation and school behaviour. The scores are summed for each area to give a profile of failure, marginal or sufficient | 1 |
Appendix 6 Data extraction tables for the systematic review of economic evaluations
| Study | Analysis type, year | Country, setting | Population | Comparators | Perspective | Time horizon, discounting | Costs | Effects for ICER | Sensitivity analyses |
|---|---|---|---|---|---|---|---|---|---|
| Barton, 200642 | Cross-sectional survey-based CUA; in 2001/2 UK pounds | UK, specialist cochlear implant centres | Children in the UK with permanent bilateral hearing impairment > 40 dB HL in the better-hearing ear. n = 8876, including 993 with cochlear implants (modal age at implantation 3 years, mean age at implantation 8 years), 3580 profoundly impaired and 1015 moderately impaired |
Cochlear implant (presumed unilateral) No cochlear implant |
UK NHS and societal | 15 years and lifetime, discounted at 3% per annum for both cost and utility |
Cost to the NHS of providing implant/acoustic hearing aids Annual educational cost Cost to family of child’s impaired hearing: out-of pocket expenditure, time away from usual activities by parents for accompanying child to clinical and hospital appointments |
QALYs (adapted HUI-3, completed by parents; effect of cochlear implant estimated via regression analysis) | One way |
| Carter 199943 | Community survey and expert opinion exercise with decision model (project pathway)-based CUA; in 1994 Australian dollars | Australian implantation programmes | Deaf children (severity not reported)a |
Cochlear implant (presumed unilateral) No cochlear implant |
Australian government/service provider | 10 years, 15 years and 20 years; discounting rate at 5% for both costs and life-years |
Ongoing costs for consecutive cohorts of patients (costs for recipients and their families are included only insofar as they are related to the funder/service provider perspective): selection of recipients, surgery/implantation, rehabilitation/implant maintenance over useful life of the implant Offsetting savings achieved through being able to attend ordinary schools rather than requiring special educationb |
QALYs (using the Sintonen HRQoL-15D instrument) | One way |
| Cheng 200044 | Retrospective before-and-after study-based CUA; in 1999 US dollars | Hearing clinic at a US academic medical centre | Profoundly deaf children (pre- or postlingually deaf not reported), average age 7.5 years, who received cochlear implant |
Cochlear implant (unknown bilateral or unilateral) No cochlear implant (pre- vs post implantation) |
US societal [= health care (Medicare) + patient + education + productivity] | 73 remaining years of life; discounting rate at 3% for both cost and utility |
Direct costs: b Preoperative costs Operative costs: implant device, hospital and surgery, medical complications if any Postoperative costs: audiology follow-up, rehabilitation, device failure if any, loss or damage insurance, extended warranty, external, special batteries, processor upgrade Indirect costs:b Time off from work Travel and parking Change in educational costs and future earnings Special equipment |
QALY (using TTO, VAS and HUI – version not stated)b | One way |
| O’Neill 200145 | Simple calculation (no model)-based CUA; in 1997/8 UK pounds | UK, the Nottingham Paediatric Cochlear Implant Programme | Profoundly hearing-impaired children |
Cochlear implant (unknown bilateral or unilateral) No cochlear implant |
UK NHS | Lifetime; discounting rate at 6% for both costs and utility |
Assessment Maintenance Rehabilitation Education cost savingsb |
QALY (assumed from HUI-2 generic health state of being deaf)b | Not reported |
| Summerfield 199746c | Decision model-based CUA; in 1996 UK pounds | UK, Nottingham Paediatric Programme with three educational settings: school for deaf children, special unit attached to mainstream school, and mainstream school with support | Deaf children in the UK |
Unilateral cochlear implant No cochlear implant (pre- vs post implantation) |
British health-care system | Lifetime; discounting at 6% for both costs and benefits | Direct costs of medical and rehabilitative management: assessing referrals who prove unwilling or unsuitable to proceed to treatment, remedying medical/surgical/technical complications, managing patients who subsequently become non-users, maintenance and upgrades for patients who continue to use their device | QALY (using HUI Index) | One way |
| O’Neill 200047 | Simple calculation (no model)-based CUA; in 1997/8 UK pounds | A major paediatric implant centre, alternative educational setting in the UK | Profoundly hearing-impaired children (hearing level > 95 dB) in the UK |
Cochlear implant (unknown bilateral or unilateral) No cochlear implant |
UK NHS (health authority purchaser + educational savings) | Compulsory school years (between age 4 and 16 years), and 70 years lifetime; discounting rate for costs at 6% (for benefits not reported) |
Direct costs to purchasers of a paediatric cochlear implant over first 4 years Education costs: educating children with differing degrees of hearing impairment, communication needs, the number of extra teaching staff as opposed to non-teaching staff hours provided for the pupil, any single or recurrent cash allocations involved (e.g. for special equipment) |
QALY (assumed from HUI-2 generic health state of being deaf)b | Not reported |
| Schulze-Gattermann 200248 | Cost-offset analysis based on a retrospective analysis; in 1999 euros | At the centre for the hearing impaired at the Medical University of Hanover in Hildesheim in Germany; educational settings were used to measure for benefits | Congenitally deaf and prelingually deafened children aged 1–6.9 years, with a severe hearing impairment (click-evoked hearing level of approximately 95 dB) |
Cochlear implant (unknown bilateral or unilateral) Hearing aid |
Germany payers’ perspective | Up to 16 years (end of education); discounting rate at 6% for costs and savings | Costs of medical and educational services that were related to the hearing impairment on an individual level: direct (medical) costs, indirect costs (e.g. travelling costs), educational expenses covered by public authorities | No ICER – educational costs (savings) offset against health-care costs | One way |
| Hutton 199549 | Simple calculation, CUA; 1992 | UK, setting not stated | Children |
Postlingually implanted Non-implanted |
NHS and education sector | Lifetime, discounting at 6% |
Implantation Rehabilitation Education cost/saving Special living equipment |
QALY (assumed utility gain of 0.1) | None |
| Wong 200050 | CUA; in Hong Kong dollars, US dollars and UK pounds, year not stated | Hong Kong | Prelingually deafened children,d n = 22; mean age at implantation 6.42 years |
Cochlear implant (unknown bilateral or unilateral) No cochlear implant |
Service providers | Life expectancy of 75 years; discount rate at 6% for both costs and effects |
Selection of implantees Provision of cochlear implants to the implantees Surgery including preoperative evaluations and postoperative medical care: rehabilitative measures; social, audiological and postoperation speech training and maintenance |
QALY (using HRQoL 15-D instrument) | Not reported |
| Study | From treatment | To treatment | Incremental cost | Incremental effects | ICER (discounted) | ICER (not discounted) |
|---|---|---|---|---|---|---|
| Barton, 200642 | No cochlear implant | Cochlear implant |
Societal perspective: Lifetime period: implanted at age 3 years: €119,591,a €102,373b and €92,525c implanted at age 6 years: €119,565,a €109,388b and €102,572c Health sector: Lifetime period: |
Lifetime period: implanted at age 3 years: 4.894 QALYs,a 7.363 QALYsb and 8.569 QALYsc implanted at age 6 years: 4.073 QALYs,a 5.668 QALYsb and 6.447 QALYsc |
Societal perspective: Lifetime period, cost per QALY: implanted at age 3 years: €24,436,a €13,904b and €10,798c implanted at age 6 years: €29,355,a €19,299b and €15,910c Health sector: Lifetime period, cost per QALY: implanted at age 3 years: €26,982,a €17,933b and €15,410c implanted at age 6 years: €32,235,a €23,164b and €20,366c (Cost per QALY from the perspective of health and education sectors combined was also reported) |
|
| Carter 199943 | No cochlear implant | Cochlear implant | Not reported |
Improvement in HRQoL-15D score: Low value:d 17% Middle value:e 26% High value:f 38% |
Cost per QALY: 10-year period: A$13,020,d A$8440e and A$5940f |
|
| Cheng 200044 | No cochlear implant | Cochlear implant |
Total direct costs: US$60,228 Total indirect costs: – US$113,426 Total costs: – US$53,198 |
TTO: 6.54 QALYs VAS: 8.03 QALYs HUI: 11.59 QALYs |
Using direct medical costs: TTO: US$9209 per QALY VAS: US$7500 per QALY HUI: US$5197 per QALY Savings to society (using total costs): TTO: –$53,198 |
|
| O’Neill 200047 | No cochlear implant | Cochlear implant |
Discounted value of savings attributable to cochlear implant: Cost over compulsory school years: $48,756.58 Education savings: $26,781.36 Net cost over compulsory school years: $21,975.23 Costs over 70 years of life: $68,130.90 Net cost over 70 years of life: $41,349.55 |
2.99 QALYs (discounted) 5.98 QALYs (undiscounted) |
£2532 ($4051) per QALY | |
| O’Neill 200145 | No treatment | Cochlear implant |
Mean net discounted implantation costs by education authority type:g County: £33,241 London: £49,130 Metropolitan: £44,709 Unitary: £41,442 |
4 QALYs (assuming a child is implanted at age 4 years and lives for 75 years) |
Net cost per QALY by education authority type:g County: £8310 London: £12,282 Metropolitan: £11,177 Unitary: £10,360 |
|
| Summerfield 199746 | No cochlear implant | Unilateral cochlear implant | Cumulative cost of medical and rehabilitative care: £60,000 | Cumulative 3.8 QALYs |
£15,600 per QALY £12,100 per QALY (taking into account saved costs in education) £10,000 per QALY (taking into account savings in costs for special equipment for daily living in adulthood) |
|
| Schulze-Gattermann 200248 | Hearing aid | Cochlear implant | NA (cost-offset analysis) | NA (cost-offset analysis) | NA (cost-offset analysis) | |
| Hutton 199549 | Non-implanted | Unilateral cochlear implant |
£59,343 (health-care cost) £15,906 (net saving after educational costs) |
3.66 QALYs | £16,213 | |
| Wong 200050 | Non-cochlear implantation | Postcochlear implantation | HK$250,628 | Not reported | HK$183,100 or US$23,474 or £14,084 per QALYh |
| Study | Analysis type, year | Country, setting | Population | Comparators | Perspective | Time horizon, discounting | Costs | Effects for ICER | Sensitivity analyses |
|---|---|---|---|---|---|---|---|---|---|
| Bichey 200251 | Retrospective cohort study-based CUA; year not stated | Indiana University Medical Center in the USA | Patients (both children and adults) with large vestibular aqueduct syndrome, at the medical centre, who were postlingually deafened and severely deaf: 10 with cochlear implants vs 10 with hearing aids |
Cochlear implant (unknown bilateral or unilateral) Hearing aid |
Not reported | Age 76 years; QALYs were discounted at 5% |
Preoperative assessment costs Surgical fees Cost of anaesthesia Cost of hospitalisation Cost of implant Cost of postoperative audiological and communication assessments Cost of postoperative surgerya |
QALYs (based on the Ontario HUI-3)a | One way |
| Carter 199943 | Community survey and expert opinion exercise with decision model (project pathway)-based CUA; in 1994 Australian dollars | Australian implantation programmes | Postlingually and profoundly deaf adultsb |
Cochlear implant (unknown bilateral or unilateral) No cochlear implant (pre- vs post implantation) |
Australian government/service provider | 15 years; discounting rate at 5% for both costs and life-years | Ongoing costs for consecutive cohorts of patients (costs for recipients and their families are included only insofar as they are related to the funder/service provider perspective): selection of recipients, surgery/implantation, rehabilitation/implant maintenance over useful life of the implanta | QALYs (based on the Sintonen HRQoL-15D instrument)a | One way |
| Lee 200652 | Small cohort study-based CUA, in 2002 $ (US?) | A hospital in Seoul in Korea |
11 postlingually deaf adults who had received cochlear implants in that hospital between 1990 and 2002 and who had used the device for at least 1 year and were available for a direct interview Mean age at analysis 49.6 years Mean age at onset of deafness 33.4 years Mean time that the cochlear implant device was used 5.6 years |
Cochlear implant (unknown bilateral or unilateral) No cochlear implant (pre- vs post implantation) |
Health sector | Expected lifetime (years not reported); discounting rate at both 0% and 5% for costs and annual discounting rate at 3% for QALYs |
Preoperative: outpatient hospital fees, audiological and radiological evaluation Operative: costs of surgery, hospital accommodation, cochlear implant device, treatments for medical complications Postoperative: rehabilitation fees, audiological follow-up examinations during the first year following surgery, batteries for the device, processor upgrades, device failure from the second year following surgery until death Costs estimated based on the references; hospital data and other reports were also useda |
QALY, using VAS (visual analogue scale), TTO (time trade-off), QWB (quality of well-being) index, EQ-5D (EuroQol) indexa | One way |
| Palmer 199939c | Comparative cohort study and calculation-based CUA; 1996 US$ | One Canadian and 16 US implantation centres, i.e. hospital-based and patient resource clinics, from October 1994 to February 1996 | Severely to profoundly hearing-impaired adult recipients of a cochlear implant (n = 66) and adults eligible for the device who had not yet received it (n = 24), aged ≥ 18 years |
Nucleus 22-channel cochlear implant (unilateral) No cochlear implant |
Canadian and US health sector | 22 years; discounting rate at 3% for both costs and benefits |
Assessments prior to surgery Facility charge (inpatient or day surgery) Cochlear implant device Surgery professional fee Anaesthesiology professional fee 1-year follow-up carea |
QALY (using HUI scores) | One way |
| Summerfield 200253 | Empirical utility elicitation study and decision model-based CUA; in 2000 UK pounds | 14 hospitals in the UK NHS and one Medical Research Council research unit | Normal-hearing adult volunteers (n = 70) and adults undergoing unilateral implantation who either did not benefit from acoustic hearing aids preoperatively (n = 87) or benefited marginally (n = 115) |
Unilateral implant vs no intervention Unilateral implant vs hearing aids Unilateral implant vs simultaneous bilateral implants Provision of additional implant vs no additional intervention |
Health care | 30 years; discounting rate at 6% |
Staff Incidentals Accommodation and equipment CT and/or MRI Surgical session Inpatient stay Radiographic examination Implant system Spares and repairsa |
QALY (using HUI-2) | One-way |
| Summerfield 199746d | Decision model-based CUA; in 1996 UK pounds | UK | Profoundly deaf adults |
Unilateral cochlear implant No cochlear implant (pre- vs post implantation) |
British health-care and education system | Remaining lifetime of 26 years; discounting at 6% for both costs and benefits | Direct costs of medical and rehabilitative management: assessing referrals who prove unwilling or unsuitable to proceed to treatment, remedying medical/surgical/technical complications, managing patients who subsequently become non-users, maintenance and upgrades for patients who continue to use their devicea | QALY (using HUI scores)a | One way |
| Summerfield 199554 | CUA based on a UK national cochlear implantation programme (1990–4) and a decision model; costs are expressed in UK pounds at 1991–2 price levels | UK, the adult programmes at hospitals in England, Scotland and Northern Ireland | Profoundly postlingually deafened adults who received Nucleus 22-channel implant system under the programme |
22-channel implant (unknown bilateral or unilateral) No treatment |
UK’s purchaser/provider of health care | 26 years (remaining of lifetime); discounting rate at 6% per annum for the cost and utility |
Salaries Salary overheads Accommodation Incidentals Capital equipment Radiology Surgery Hotel charges Implant hardware Maintenance and upgradesa |
QALY (various derivation methods: VAS, Rosser Index, Ontario HUI) | One way |
| UK Cochlear Implant Study Group 200440 | Before-and-after cohort study and decision tree-based CUA;e costs inflated to 2001/2 financial year levels and converted into euros (£1 = €1.54) | 13 hospitals in the UK NHS | 316 profoundly hearing-impaired postlingually deafened adults who received multichannel cochlear implants in 13 hospitals in the NHS between 1 June 1997 and 31 May 2000 |
Unilateral cochlear implantation No cochlear implantation |
UK NHS | Lifetime; discounting rate at 6% for both costs and benefits |
Cost of providing acoustic hearing aids when assigning the suitability of a subject for cochlear implantation Costs averted if acoustic hearing aids would not be provided to the subject after cochlear implantation Core cost of providing and maintaining implant Costs of managing medical/surgical complications Cost of replacing electrode arrays that faila |
QALY (based on HUI-3 scores) | One-way |
| Wong 200050f | Cohort study-based CUA in Hong Kong dollars, US dollars and UK pounds; year not stated | Hong Kong | Postlingually deafened adults, n = 13; mean age at implantation 41.2 years, average life of implantation 33.8 years |
Cochlear implant (unknown bilateral or unilateral) No cochlear implant (pre- vs post implantation) |
US service providers | Mean 33.8 years of remaining life expectancy (to age 75 years); discount rate at 6% for both costs and effects |
Selection of implantees Provision of cochlear implants to the implantees Surgery including preoperative evaluations and postoperative medical care Rehabilitative measures Social, audiological and postoperation speech training and maintenancea |
QALY (using HRQoL 15-D instrument)a | Not reported |
| Francis 200255 | Cohort study-based CUA; study year for CUA not stated | Johns Hopkins Hospital in the USA | Pre- (n = 6) and postlingually (n = 41) deaf adults aged ≥ 50 years who have multiple channel cochlear implants received at the hospital between June 1989 and February 2000 |
Cochlear implant (unknown bilateral or unilateral) No cochlear implant (pre- vs post implantation) |
21 years (remaining of 85 years life expectancy); discounting rate at 3% for both cost and utility | Costs associated with cochlear implantation: presurgical evaluation, cost of device, surgeon’s and anaesthesiologist’s fees, hospital costs, postoperative services including programming, insurance, extended warranty and miscellaneous hardware costsa | QALY (based on HUI-3 score)a | Not reported | |
| Neilson 200656 | CUA based on a systemic review and a simple patient care pathway decision model; in 2005/6 Norwegian kroner | Norway | Severe to profoundly deaf adults |
Unilateral cochlear implant No intervention (pre- vs post implantation) |
Norwegian health-care system perspective, and perspective of patients | 25 years of device/assumed remaining life expectancy; discounting rate at 4% for both cost and utility |
Preimplant outpatient assessment and testing CT and/or MRI scan Hospitalisation, surgery and implanted device Postimplant outpatient follow-up and rehabilitation Cost of managing (major) complications with readmissions Ongoing periodic follow-up of patient’s progress and equipment maintenancea |
QALY | One way |
| Summerfield 199557 (short report) | CUA based on a UK national cochlear implantation programme (1990–4); costs are expressed in UK pounds at 1992–3 price levels | UK, the adult programmes at hospitals in England, Scotland and Northern Ireland | Profoundly postlingually deafened adults who received Nucleus 22-channel implant system under the programme |
22-channel implant (unknown bilateral or unilateral) No treatment (pre- vs post implantation) |
Not reported | 12 years; discounting rate at 6% per annum for the cost and utility |
Salaries Salary overheads Accommodation Incidentals Capital equipment Radiology Surgery Hotel charges Implant hardware Maintenance and upgradesa |
QALY (VAS and ad hoc mapping to an unstated HRQoL measure) | Not reported |
| Wyatt 199658 | Comparative survey (for utilities) and decision-analytic model-based CUA; in 1993 US dollars | Johns Hopkins Hospital in USA | Adults with profound hearing loss (acquired or born deaf not reported) underwent cochlear implantation between July 1993 and June 1994 at the hospital |
Nucleus 22-channel implant (unknown bilateral or unilateral) No cochlear implant (pre- vs post implantation or with cochlear implant vs awaiting cochlear implant?) |
American third-party payors?? | 23 years of remaining life expectancy; discounting rate at 5% for both cost and utility | Direct medical costs: preoperative evaluation, surgery (hospital, surgeon’s fee, anaesthesia), initial device, rehabilitation, follow-up, audiological testing and device maintenance, expected cost of a minor and a major complicationa | QALY (using Ontario HUI score)a | One way |
| Wyatt 199559 | Decision-analytic model-based CUA; 1992 US dollars | USA | Profoundly deaf adults (acquired or born deaf not reported) who underwent cochlear implantation at the Johns Hopkins Hospital (USA) between February 1990 and May 1993. Mean age 45 years |
Nucleus 22-channel implant (unknown bilateral or unilateral) No cochlear implant |
American third-party payors | 33 years of remaining life expectancy; discounting rate at 5% for both cost and utility | Direct medical costs: preoperative evaluation, surgery (hospital, surgeon’s fee, anaesthesia), initial device, rehabilitation, follow-up, audiological testing and device maintenance, expected cost of a minor and a major complicationa | QALY(using the Ontario HUI-3)a | One way |
| Study | From treatment | To treatment | Incremental cost | Incremental effects | ICER (discounted) | ICER (not discounted) |
|---|---|---|---|---|---|---|
| Bichey 200251 | Hearing aid | Cochlear implant | US$37,320 (mean charges for cochlear implant in the medical centre) | Average HUI score: 0.2 (SD 0.13, 95% CI 0.12–0.28) | US$12,774 per QALYa | US$6426 per QALYa |
| Carter 199943 | No cochlear implant | Cochlear implant | Not reported | Not reported |
Cost per QALY (in A$): 10-year period: $45,630,b $22,045c and $14,115d |
|
| Lee 200652 | No cochlear implant | Cochlear implant | Average lifetime discounted = $22,320 (base-case cost reported in Table VIII is different to this) |
Gain in QALYs: VAS: 1.16 HUI: 1.28 EQ-5D: 0.91 QWB: 0.55 Mean = 0.98 (95% CI 0.66–1.2) |
$ per QALY: VAS: 19,223 HUI: 17,387 EQ-5D: 24,604 QWB: 40,474 Mean = 25,424 |
|
| Palmer 199939 | No cochlear implant | Cochlear implant | $37,405; $34,460 after imputation of missing data | Gain in QALYs = 3.26(calculated by the reviewers) | US$14,670 per QALY (95% CI US$8241–30,347) | |
| Summerfield 200253 | No intervention | Unilateral implant | £41,136 | 2.45 QALYs | £16,774 | |
| Hearing aids | Unilateral implant | £39,029 | 1.42 QALYs | £27,401 | ||
| Unilateral implant | Simultaneous bilateral implant | £27,001 | 0.44 QALYs | £61,734 | ||
| No additional intervention | Additional implant | £30,142 | 0.44 QALYs | £68,916 | ||
| No additional intervention | Simultaneous bilateral implant | £68,137 | 2.89 QALYs | £23,578 | ||
| Hearing aids | Simultaneous bilateral implant | £65,165 | 1.86 QALYs | £35,002 | ||
| Summerfield 199554 | No treatment | 22-channel implant | £34,215 |
2.99 QALYs (discounted) 5.98 QALYs (undiscounted) |
£11,440 per QALY | £5722 per QALY |
| Summerfield 199746 | No cochlear implant | Cochlear implant (unilateral) | £40,000 accumulated costs of medical management | 3 QALYs | £13,300 per QALY | |
| UK Cochlear Implant Study Group 200440 | No cochlear implant | Cochlear implant (unilateral) |
All groups: €67,017 All TC: €67,076 TC-I: €66,808 TC-II: €67,439 All MHU: €66,854 MHU-I: €67,266 MHU-II: €66,206 |
QALYs gained: All groups: 2.46 All TC: 2.64 TC-I: 2.75 TC-II: 2.49 All MHU: 1.99 MHU-I: 1.73 MHU-II: 2.44 |
€ per QALY, calculated using ‘net benefit approach’: All groups: 27,142 All TC: 25,336 TC-I: 24,032 TC-II: 27,062 All MHU: 33,512 MHU-I: 39,009 MHU-II: 27,092 |
|
| Wong 200050 | No cochlear implant | Cochlear implant | HK$250,628 | 1.8 QALYs (calculated by the reviewers) | UK£10,237 or HK$133,087 or US$17,195 per QALYe | |
| Francis 200255 | No cochlear implant | Cochlear implant | US$36,025 | 3.78 QALYs | US$9530 per QALY | |
| Neilson 200656 | Unilateral cochlear implant | No intervention | Kr537,175 | 3.12 QALYs | Kr17,200 per QALY | |
| Summerfield 199557 | No cochlear implant | 22-channel implant | £28,318 | 3.9 or 1.3 QALYs (0.3 or 0.1 utility gain for 26 years, discounted at 6%) | £8624 or £25,871 | |
| Wyatt 199658 | No cochlear implant | Cochlear implant | US$53,838 | 2.83 QALYs (calculated by the reviewers) | US$15,928 per QALY | |
| Wyatt 199559 | No alternatives | Nucleus 22-channel cochlear implant | US$53,058 | 4.41 QALYs (calculated by the reviewers) | US$15,593 per QALY | |
| Wyatt 199560 | No cochlear implant | Nucleus 22-channel cochlear implant | Not reported | Mean difference in health status rating: 30.4% (95% CI 29.98–33.84) | US$9325 per QALY (unknown if this is discounted or undiscounted) |
Appendix 7 Quality assessment tables of UK-based economic evaluations and industry-submitted cost–utility analyses
| Criteria | Summerfield 199554 | Summerfield 199746a | O’Neil 200047 | O’Neil 200145 | UKCISG 200440 | Barton 200642 | Summerfield 200253 |
|---|---|---|---|---|---|---|---|
| Unilateral in adults | Unilateral in children | Unilateral in children | Unilateral in children | Unilateral in adults | Unilateral in children | Bilateral in adults | |
| Is the study population clearly described? | Yes | Partly | Yes | Yes | Yes | Yes | Yes |
| Are competing alternatives clearly described? | Yes/nob | No | Yes | Yes | Yes | Yes | Yes |
| Is a well-defined research question posed in answerable form? | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Is the economic study design appropriate to the stated objective? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Is the chosen time horizon appropriate to include relevant costs and consequences? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Is the actual perspective chosen appropriate? | Yes | Yesc | Yesd | Yesd | Yes | Yes | Yes |
| Are all important and relevant costs for each alternative identified? | Yes/nob | Yes/no | Yes | Yes | Yes | Yes | Yes |
| Are all resources measured appropriately in physical units? | Yes | Yes | Noe | Noe | Yes | Yes | Yes |
| Are resources valued appropriately? | Yes | Yes | NA | NA | Yes | Yes | Yes |
| Are all important and relevant outcomes for each alternative identified? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Are all outcomes measured appropriately in physical units? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Are outcomes valued appropriately? | Nof | Nof | Nof | Nof | Yes (HUI-3) | Yes (HUI-3) | Yes (HUI-2) |
| Is an incremental analysis of costs and outcomes performed? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Are all future costs and outcomes discounted appropriately? | Yes (6%) | Yes (6%)g | Yes (6%) | Yes (6%) | Yes (6%) | Yes (6%) | Yes (6%) |
| Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | Yes (one way) | Yes | Yes | Yes | Yes | Yes | Mostly |
| Do the conclusions follow from the data reported? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Does the study discuss the generalisability of the results to other settings and patient/client groups? | Yes | No | No | No | Yes | Yes | Yes |
| Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | No | No | No | No | No | No | No |
| Are ethical and distributional issues discussed appropriately? | Noh | No | No | Yes | No | No | No |
| Criteria | Cochlear Europe | Assessment |
|---|---|---|
| Is the study population clearly described? | Yes | Yes |
| Are competing alternatives clearly described? | Yes | Yes |
| Is a well-defined research question posed in answerable form? | Yes | Not explicitly |
| Is the economic study design appropriate to the stated objective? | Yes | Yes |
| Is the chosen time horizon appropriate to include relevant costs and consequences? | Yes | Yes |
| Is the actual perspective chosen appropriate? | Yes | Yes |
| Are all important and relevant costs for each alternative identified? | Mostly, except not clear whether treatment of postsurgical complications was included. Omitted patient assessment costs of those ultimately not implanted. Also, assumed that 0% of device failures received a subsequent implant | Mostly, except not clear whether treatment of postsurgical complications was included, and omitted preimplantation assessment costs of those ultimately not implanted |
| Are all resources measured appropriately in physical units? | Yes | Yes |
| Are resources valued appropriately? | Yes | Yes |
| Are all important and relevant outcomes for each alternative identified? | Yes | Yes |
| Are all outcomes measured appropriately in physical units? | Yes (life-years) | Yes (life-years) |
| Are outcomes valued appropriately? | Yes (QALY weights from HUI-3) | Yes (QALY weights from HUI-3) |
| Is an incremental analysis of costs and outcomes performed? | Yes | Yes |
| Are all future costs and outcomes discounted appropriately? | Yes (at 3.5% pa) | Yes (at 3.5% pa) |
| Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | Yes (one-way and PSA) | Yes (one-way and PSA) |
| Do the conclusions follow from the data reported? | Yes | Yes |
| Does the study discuss the generalisability of the results to other settings and patient/client groups? | Yes | Yes |
| Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | NA | NA |
| Are ethical and distributional issues discussed appropriately? | Yes/no | Yes/no |
Appendix 8 Assessment of industry submissions to NICE
Industry-submitted economic evaluations
There were three industry submissions made to NICE as part of the appraisal process of cochlear implantation. These were from Cochlear Europe, Advanced Bionics Europe and MED-EL UK. Only those submissions from Cochlear Europe and Advanced Bionics Europe contained new economic analyses and we mainly focus on these below. The submission from MED-EL UK mainly summarised the results of the 2004 UKCISG paper (on adults)40 and the 2006 paper by Barton and colleagues42 on the cost-effectiveness of paediatric cochlear implantation.
Cost-effectiveness analyses from Cochlear Europe
This submission took a Markov model-based approach to evaluating the cost-effectiveness of unilateral and bilateral cochlear implantation compared with ‘standard of care’, from an NHS and Personal Social Services (PSS) perspective. They assessed the NICE decision problem in relation to their Nucleus® and Nucleus® Freedom™ implants, using costs and failure rates specific to these systems.
Costs included were those associated with assessment (imaging); surgery and inpatient stay; outpatient resources (years 1–20); the implant device; processor upgrade; and spares and repairs. However, their analyses omitted the assessment costs of those referred deaf people who ultimately did not receive a cochlear implant.
Outcomes were estimated as QALYs gained. The estimated utility gain resulting from unilateral cochlear implantation was 0.394 in adults and 0.224 in children.
In adults they reduced the baseline utility of being deaf and the utility gain due to implantation, as people in the model aged in proportion to the age-related decline in utility of the general population (using UK population age-specific EQ-5D utility weights). In this way both the utility associated with being deaf and that associated with implantation would be a fixed proportion of the population norm utility for that age. Without this adjustment – that is, with the same absolute utility gain at all ages – it is possible that implanted individuals might end up with greater utility than their normal-hearing peers (although this effect would occur only when people are very old).
In summary, with two notable exceptions, both the model structure and parameter estimates for effectiveness, resource use, costs and other assumptions were generally reasonable and evidence-based, and in most other respects the analyses met the reference case criteria stipulated in NICE methodological guidance (Table 121).
| Reference case requirement | Reviewer comment | |
|---|---|---|
| Decision problem: as per NICE project scope | Yes | Simultaneous bilateral implantation considered (not sequential or additional) |
| Comparator(s): alternative therapies routinely used in the UK | Yes | ‘Standard of care’ with or without a hearing aid |
| Perspective on costs: NHS and PSS | Yes | But education costs included as sensitivity analysis |
| Perspective on outcomes: all health effects on individuals | Yes | |
| Type of economic evaluation: cost-effectiveness analysis | Yes | Cost per QALY |
| Adequate time horizon | Yes | |
| Synthesis of evidence on outcomes: based on a systematic review | Yes/no | No synthesis, but evidence of a review of some relevant studies before choice of utility estimate |
| Measure of health benefits: QALYs | Yes | |
| Description of health states for QALY calculations: use of a standardised and validated generic instrument | Yes/no | HUI-3 utility values mapped from speech recognition scores (not using an validated algorithm) |
| Method of preference elicitation for health state values: choice-based method (e.g. TTO, SG, not rating scale) | Yes | HUI-3 preference weights derived using standard gamble |
| Source of preference data: representative sample of the UK public | No | From Ontario general population |
| Evidence on costs: prices relevant to NHS and PSS | Yes | Recent NHS-based studies. Plausible assumptions used to estimate costs of bilateral implantation |
| Discount rate: 3.5% per annum for costs and health effects | Yes |
They produced two base-case ICERs (£ per QALY) for deaf adults and deaf children. These are reproduced in Table 122.
| Comparison | ICER | 95% CI |
|---|---|---|
| In adults | ||
| Unilateral implantation vs standard care | £7145 | £5907–7794 |
| Bilateral vs unilateral implantation | £32,909 | £24,051–44,582 |
| In children | ||
| Unilateral implantation vs standard care | £10,542 | £8804–12,655 |
| Bilateral vs unilateral implantation | £39,049 | £31,426–49,798 |
There are two aspects of the model’s assumptions that are worth closer scrutiny, both of which favour the cost-effectiveness of cochlear implantation.
Estimation of utility gains
First, in preference to using directly derived utility gain values – which are available for unilateral implantation (in children and adults) and bilateral implantation (only in adults) – they chose to indirectly map word perception scores to predict utility gain as a percentage of age-adjusted normal utility. For adults the word perception score gains were from Balkany and colleagues63 and cochlear ‘data on file’, and for children they were from Staller and colleagues. 4 Use of these particular studies was justified only on the basis of them including the ‘most recently implanted patients’.
However, the logic used to map changes in sentence recognition scores to utility gains is the step in the process that appears most questionable. From correlation data on only 28 patients (about half of the patients studied in Francis and colleagues55) they assume that a 100% improvement in monosyllabic word scores is associated with a gain in utility of 0.73. This is on the basis of a weak correlation (r = 0.55), on a different word scoring system (monosyllabic sentences), and inspection of best fit lines. Furthermore, our inspection of the best fit lines in the publication suggests that the estimated HUI utility gain associated with a 100% increase in monosyllabic sentence scores is 0.47 rather than 0.73.
Cost of treatment failures
Second, in the base-case analyses, the proportion of treatment failures that receive a subsequent implant was 0%. This seems highly implausible and notably contrasts with a statement in the Advanced Bionics Europe submission that ‘reimplantation following [implant] failure is near universal’ (p. 28), and information in the 2004 UKCISG study in which 11 of the 27 adults who experienced adverse events had either their electrode replaced or the other ear implanted.
Cost-effectiveness analyses from Advanced Bionics Europe
Methods
This submission also took a Markov model-based approach to evaluating the cost-effectiveness of unilateral cochlear implantation compared with no cochlear implant, from an NHS and PSS perspective. They produced reference case analyses for unilateral cochlear implantation versus no implantation in:
-
prelingually deafened profoundly deaf children
-
postlingually deafened profoundly deaf children
-
postlingually deafened profoundly deaf adults (profoundly deaf for 10 years)
-
postlingually deafened severely deaf adults.
They chose not to present any cost-effectiveness analysis of bilateral implantation in adults or children on the basis of a lack of sufficient current research evidence. For the same reason no analysis in severely deaf children was presented.
Costs included were those associated with preimplantation assessment; implant surgery; the implant device; programming and initial rehabilitation; ongoing support/maintenance; processor upgrade; and spares and repairs. The main data source for non-device costs was the 2003 study by Barton and colleagues64 of the cost of paediatric implantation in 12 UK implantation programmes from the early 1990s to 1998/9. However, their analyses omitted the assessment costs of those referred deaf people who ultimately did not receive a cochlear implant.
They use these costs in their analyses of cochlear implantation in both children and adults and acknowledge that they consequently may have overestimated the cost of cochlear implants in adults. The hardware costs are from their own company records, notably including a zero cost for implant replacements (in the first 10 years) and a zero cost for processor replacement repair in the first 3 years (as per their current sales policy).
Outcomes were estimated as QALYs gained. Based directly on the related study by Barton and colleagues42 the estimated long-term utility gains (4 years after implantation and onwards) due to unilateral cochlear implantation in children were 0.256 if implanted at age 3 years and 0.196 if implanted at age 6 years. The utility value used for assessment of the benefits of unilateral implantation for adults was 0.214, based on the large 2004 study by the UKCISG. 40 In adults, to reflect a documented inverse relationship between duration of deafness before implantation and utility gain from implantation, a utility decrement (of 0.002) was subtracted from the utility gain for each year of deafness.
In summary, both the model structure and parameter estimates that determine effectiveness (QALYs), resource use, costs and other assumptions appeared reasonable and evidence-based where possible, and in most other respects the analyses met the reference case criteria stipulated in NICE methodological guidance (Table 123).
| Reference case requirement | Reviewer comment | |
|---|---|---|
| Decision problem: as per NICE project scope | Yes | Except no estimation of cost-effectiveness of bilateral implantation. Analysis of technology in severely deaf actually uses data for profoundly deaf ‘marginal hearing aid users’ (as defined by UKCISG 2004) |
| Comparator(s): alternative therapies routinely used in the UK | Yes | |
| Perspective on costs: NHS and PSS | Yes | Education costs included in a sensitivity analysis |
| Perspective on outcomes: all health effects on individuals | Yes | |
| Type of economic evaluation: cost-effectiveness analysis | Yes | Cost per QALY |
| Adequate time horizon | Yes | |
| Synthesis of evidence on outcomes: based on a systematic review | Yes/no | No synthesis, but evidence of a review of some relevant studies before choice of utility estimate |
| Measure of health benefits: QALYs | Yes | |
| Description of health states for QALY calculations: use of a standardised and validated generic instrument | Yes | HUI-3 completed |
| Method of preference elicitation for health state values: choice-based method (e.g. TTO, SG, not rating scale) | Yes | HUI-3 preference weights derived using standard gamble |
| Source of preference data: representative sample of the UK public | No | From Ontario general population |
| Evidence on costs: prices relevant to NHS and PSS | Yes | A recent NHS-based study in children used as source of model costs for both children and adults |
| Discount rate: 3.5% per annum for costs and health effects | Yes |
Main results
The main probabilistic results for lifetime analyses are summarised in Table 124.
| Unilateral cochlear implantation compared with no implantation in: | ICER (£/QALY) | 95% CI (£/QALY) | % of samples with ICER < £30,000/QALY |
|---|---|---|---|
| Prelingually deafened profoundly deaf children implanted at age 3 years | 13,337 | 1945 – Dominated | 87.8 |
| Postlingually deafened profoundly deaf children implanted at age 6 years | 17,210 | 2137 – Dominated | 79.2 |
| Postlingually deafened profoundly deaf adults implanted at age 50 years | 20,027 | 2396 – Dominated | 68.7 |
| Postlingually deafened severely deaf adults implanted at age 50 years | 37,012 | 2660 – Dominated | 37.4 |
In addition they presented an analysis for prelingually deafened profoundly deaf children, implanted at age 3 years, which included estimated educational savings. This reduced the previous (health-care cost only) ICER by about £4500 to £8875 per QALY gained.
Cost-effectiveness analyses from MED-EL UK
The submission by Med-EL UK to NICE did not include an economic model and primarily takes the form of a narrative summary of a selection of the published literature on the efficacy, quality of life impacts and cost-effectiveness of cochlear implantation. Search strategies to identify the studies or any inclusion/exclusion criteria used to choose the final studies reviewed were not presented.
In relation to cost-effectiveness the submission described evidence relating to four different uses of cochlear implantation: unilateral implantation in children; unilateral implantation in adults; bilateral implantation in adults; and ‘bimodal stimulation’ in adults (unilateral cochlear implant with a contralateral hearing aid).
For unilateral implantation in adults separate results are presented for ‘traditional implant candidates’ (those who scored 0% without lip-reading on speech perception tests and who did not significantly improve when aided) and ‘marginal hearing aid users’ (those whose scores did significantly improve).
Cost-effectiveness results
The results presented in this submission that are most relevant to the UK NHS context are summarised in Table 125. The source papers from which cost-effectiveness results are quoted are those by UKCISG,40 Barton and colleagues,42 Summerfield and colleagues53 and Summerfield and colleagues. 29 As these studies have been summarised in more detail elsewhere in this report we do not elaborate on their methods and other results here.
| Comparison | ICER (€/QALY) | ICER (£/QALY) | Source paper |
|---|---|---|---|
| Unilateral implantation in children | €25,629 | Barton 200642 | |
| Unilateral implantation in adults (all) | €27,142 | £20,595 | UKCISG 200440 |
| Unilateral implantation in adults (traditional candidates) | €25,336 | £19,224 | UKCISG 200440 |
| Unilateral implantation in adults (marginal hearing aid users) | €33,512 | £25,428 | UKCISG 200440 |
Major limitations
The major limitations of the economic evidence presented in this submission are that:
-
the questions being answered are not well-defined and have to be inferred from the section headings; in most cases the comparator interventions are stated in the source paper but not in the submission
-
the information presented is not based on a systematic review or on a rigorously informed decision model; any process of selecting studies cited and how their quality was judged are not described.
Summary of industry-submitted economic evaluations
Two of the manufacturers submitted original economic analyses that met most NICE reference case criteria, whereas a third manufacturer provided a narrative review of published economic evaluations.
The base-case cost-effectiveness ratios from submissions are summarised in Table 126. However, these results should be viewed with some caution. The cost–utility estimates from Cochlear Europe were based on an estimate of the utility gain from unilateral cochlear implantation in adults of 0.394, which is almost twice the typical estimate from other studies. This high estimate was derived from weak correlations between sentence recognition scores and utility scores in one small study. They also further assumed that no device failures would involve reimplantation of a new cochlear implant. The analyses by Advanced Bionics Europe were clear and comprehensive in terms of the range of costs and events captured in the model and the range of analyses conducted. However, they applied the same costs of assessment, surgery, tuning/rehabilitation and maintenance to both adults and children despite reliable evidence from the UK that most of these costs are lower for adults. Advanced Bionics Europe did not present an analysis of bilateral cochlear implantation, but suggested that this use of cochlear implantation be appraised after better evidence becomes available. Finally, neither the analysis submitted by Cochlear Europe nor that submitted by Advanced Bionics Europe included any costs for those referred and assessed for implantation but who ultimately did not receive a cochlear implant. Table 126 summarises the main results of these two submissions.
| Policy comparison assessed | Cochlear Europe | Advanced Bionics Europe | ||
|---|---|---|---|---|
| Base-case ICER | PSA % ICERs < £30,000/QALY | Base-case ICER | PSA % ICERs < £30,000/QALY | |
| In children | ||||
| Degree of deafness | Severe to profound | Profound | ||
| Unilateral cochlear implantation vs none (profoundly deaf, aged 3 years) | £10,542 | 98%a | £13,337 | 87.8% |
| Unilateral cochlear implantation vs none (profoundly deaf, aged 6 years) | £17,210 | 79.2% | ||
| Bilateral vs unilateral implantation (profoundly deaf, aged 3 years)b | £39,049 | 24%a | NC | NC |
| In adults | ||||
| Degree of deafness | Severe to profound | Profound | ||
| Unilateral cochlear implantation vs none (profoundly deaf, aged 50 years) | £7145 | 100%a | £20,027 | 68.7% |
| Unilateral cochlear implantation vs none (severely deaf, aged 50 years) | £37,012 | 37.4% | ||
| Bilateral vs unilateral implantation (profoundly deaf, aged 50 years)b | £32,909 | 32%a | NC | NC |
Comparison of PenTAG’s analysis with the industry-submitted analyses
For unilateral cochlear implantation there were relatively minor differences in the estimated ICERs; all base-case ICERs were less than £15,000 per QALY except for the Advanced Bionics Europe estimate for unilateral implantation in adults (mostly because they used relatively high paediatric costs in their analysis). The major difference between the industry-submitted and PenTAG economic analyses was for bilateral implantation in adults, for which Cochlear Europe’s estimated ICER (of £32,900 per QALY) was some £17,000 lower than PenTAG’s estimate (£49,500 per QALY). This difference is primarily due to the manufacturer using a substantially higher assumed utility gain from cochlear implantation (0.114) than that used in PenTAG’s analysis, which was based on an estimated utility gain from bilateral implantation of 0.03.
Appendix 9 Probability trees used in the PenTAG model
The full probability trees used in the construction of the model are shown in Figures 55–61. In the base-case analyses a number of event probabilities were set to zero, meaning that several of the branches are redundant. Several of the trees are used to generate transition probabilities for multiple health states. Table 127 summarises which trees correspond to which health states. The probability trees correspond to the full model and therefore include rare events.
FIGURE 55.
Probability tree associated with the procedure to fit one cochlear implant. CI, cochlear implant.
FIGURE 56.
Probability tree associated with the procedure to fit two cochlear implants simultaneously. CI, cochlear implant.
FIGURE 57.
Probability tree associated with the procedure to fit a second cochlear implant in individuals already using one. CI, cochlear implant.
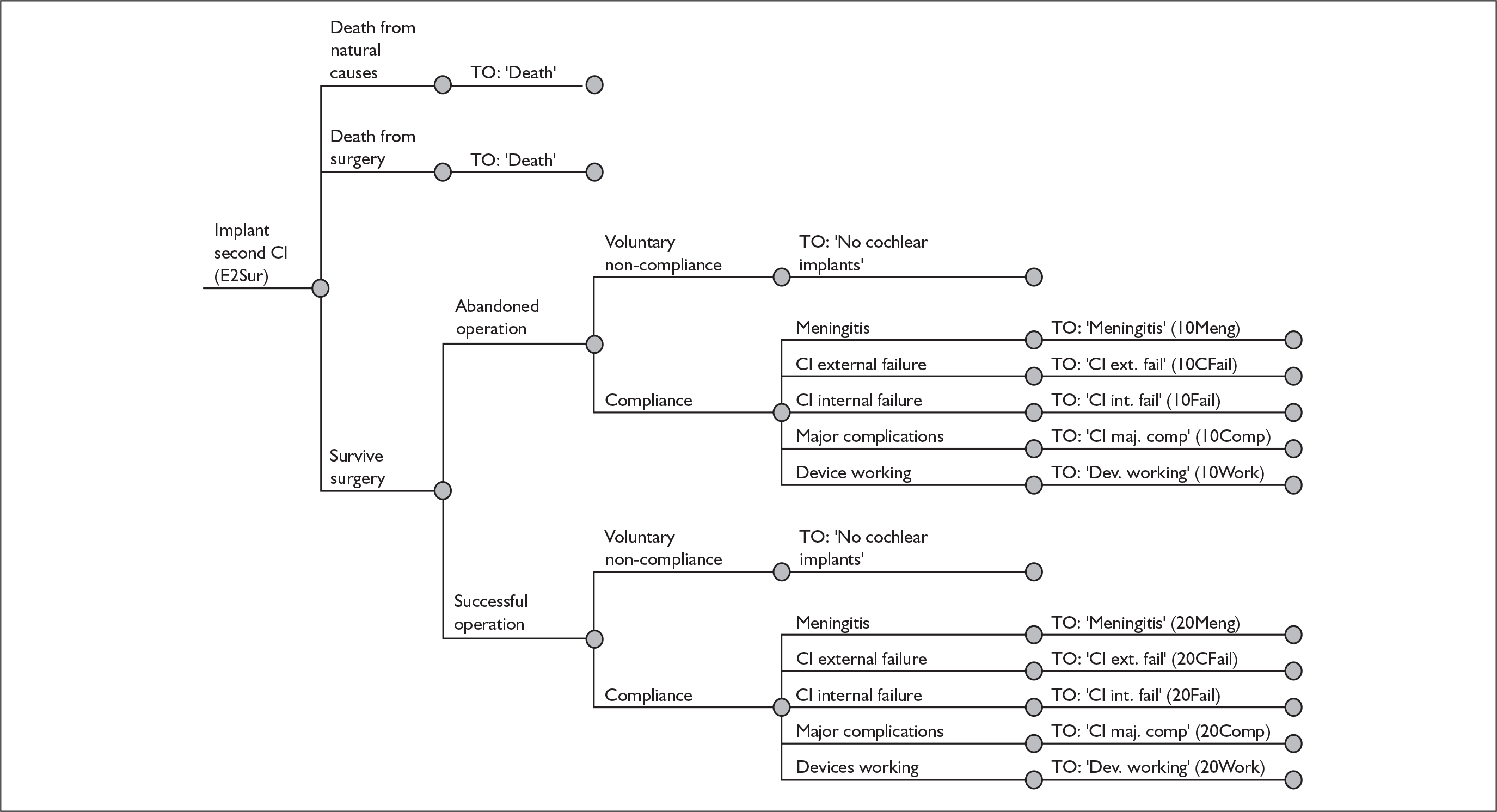
FIGURE 58.
Probability tree associated with health state ‘20Work’. CI, cochlear implant.
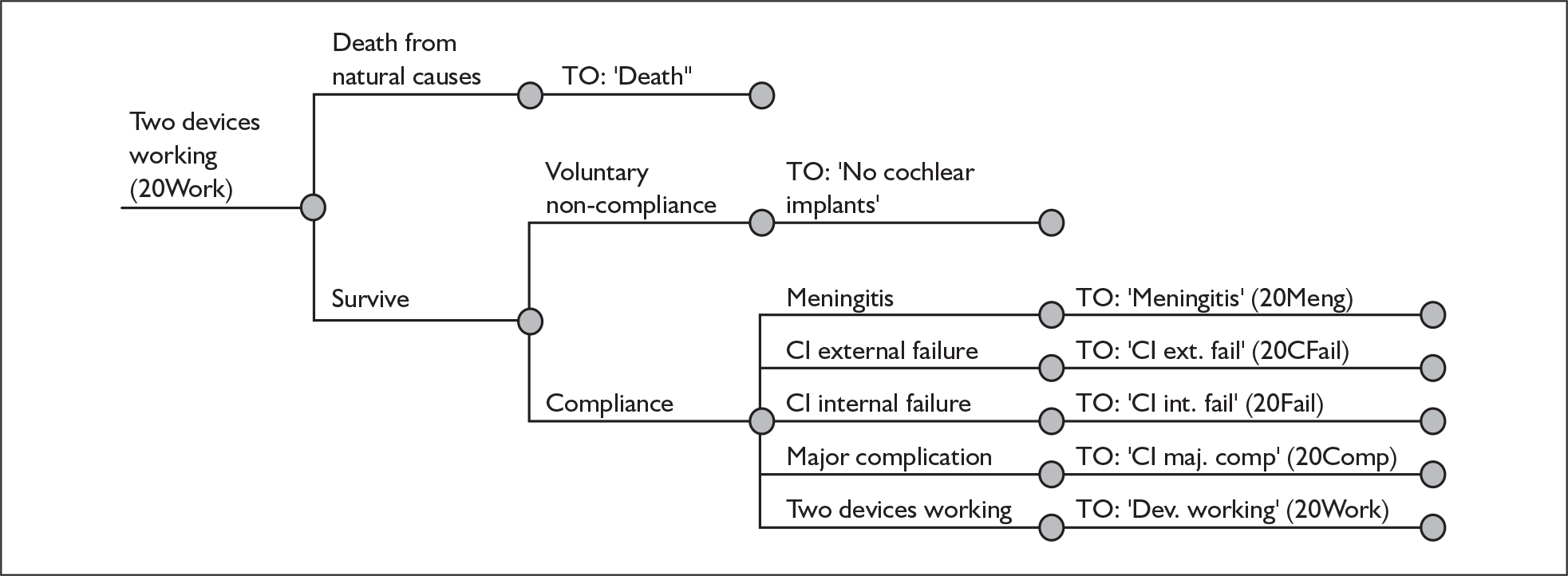
FIGURE 59.
Probability tree associated with health state ‘20Fail’. CI, cochlear implant.
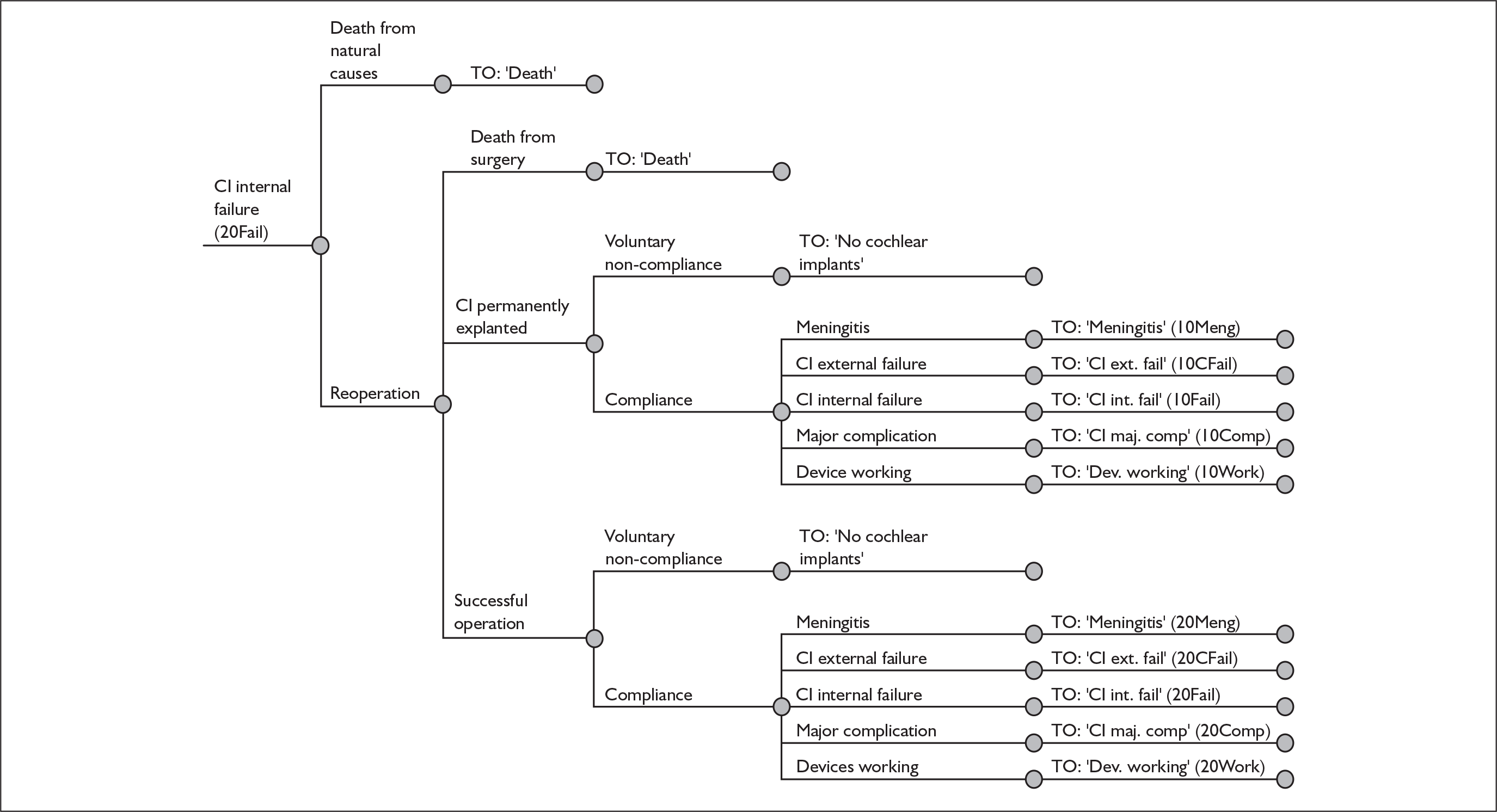
FIGURE 60.
Probability tree associated with health state ‘10Work’. CI, cochlear implant.
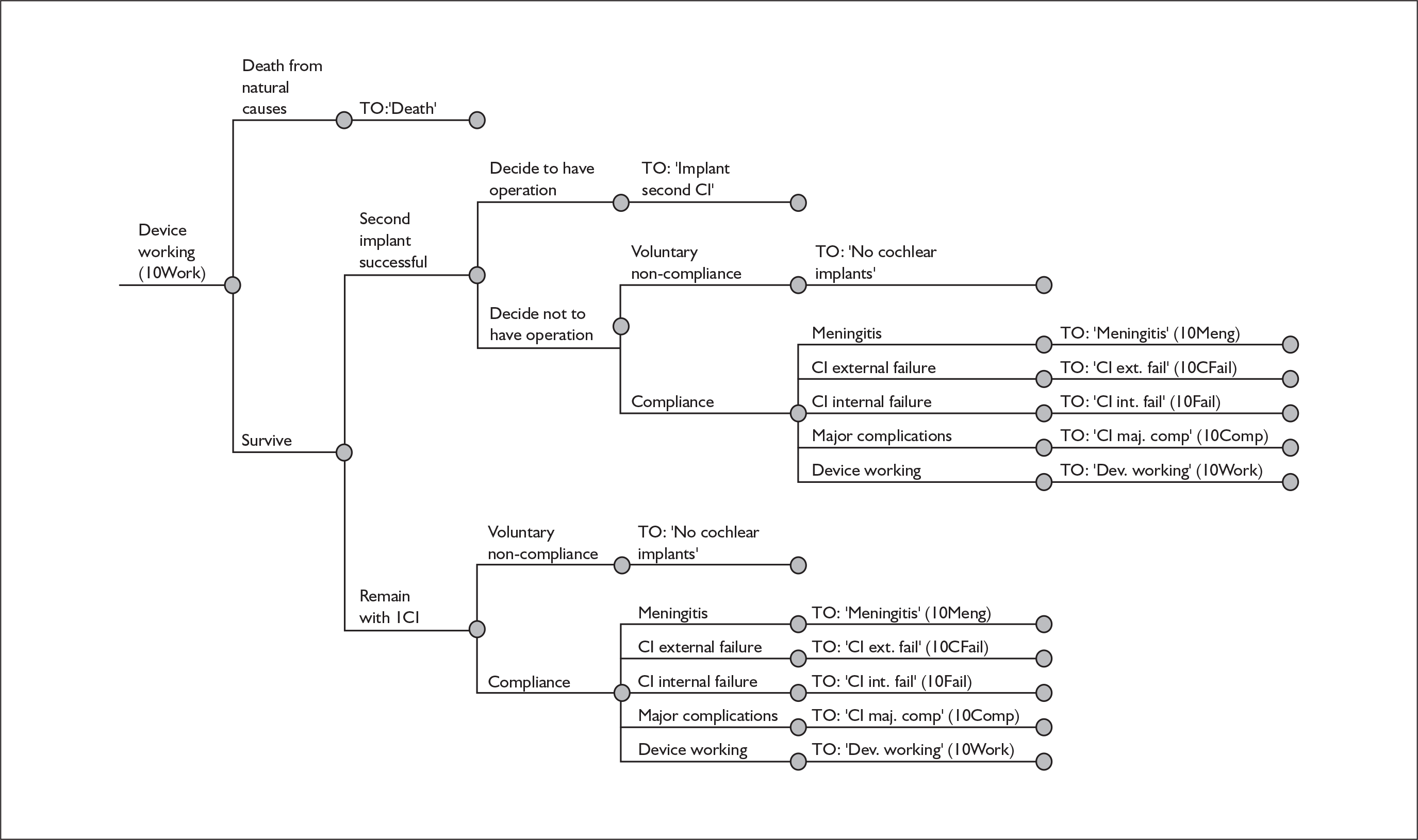
FIGURE 61.
Probability tree associated with health state ‘10Fail’. CI, cochlear implant.
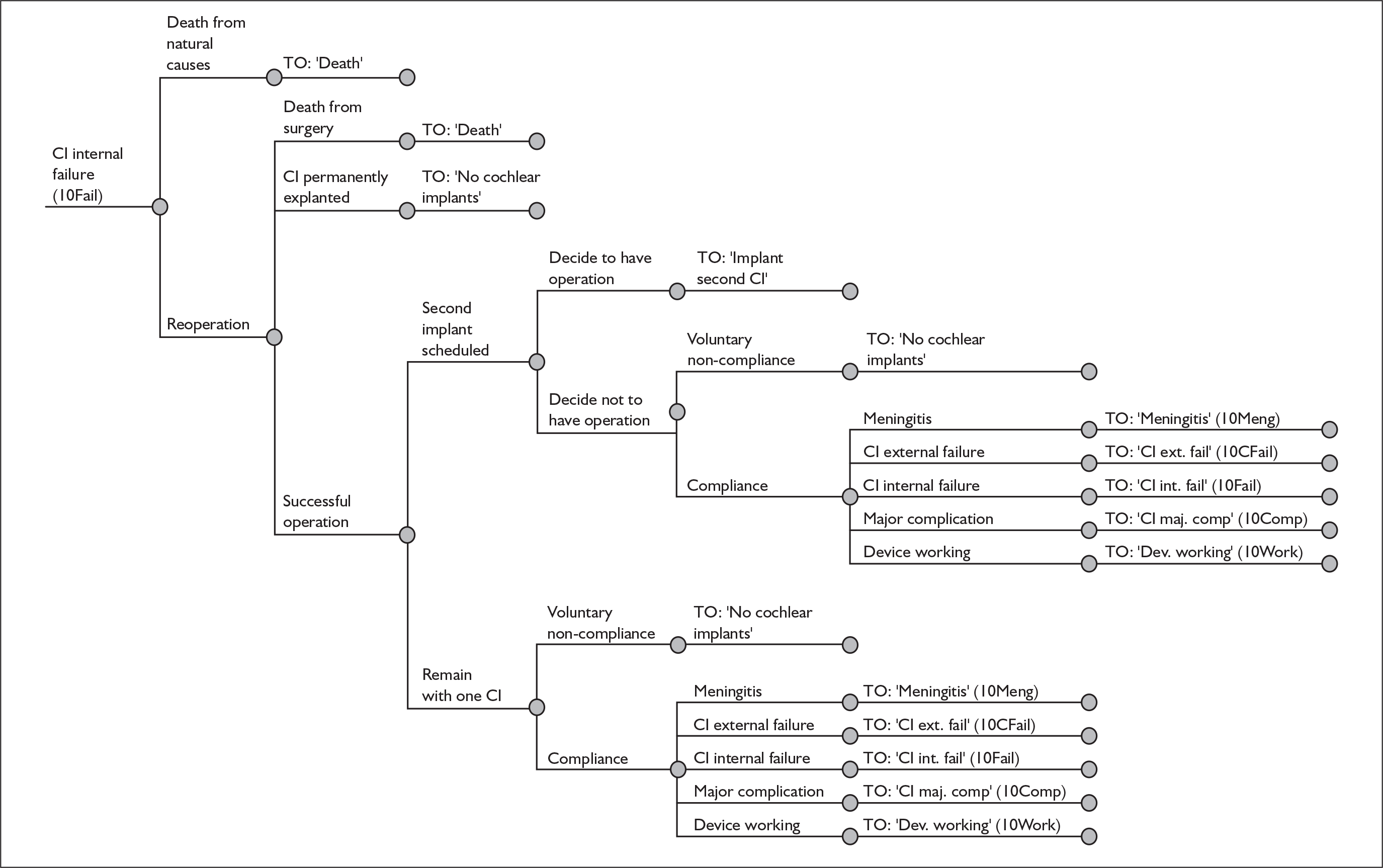
Appendix 10 Fitting parametric curves to survival data.
General strategy
The initial stage of generating the survival curves used to model internal device failure was to find functional approximations to each of the individual curves reported in both the Cochlear Europe submission and Conboy and Gibbin. 65 For each published curve several different choices were explored (exponential, Weibull, Gompertz and linear), with goodness of fit assessed using the mean absolute percentage error (MAPE). 66
An additional analysis of the numbers of each type of device implanted was also performed to generate a set of weights. Finally, these weights are used in combination with the values generated by the individual curves to produce a combined survival curve for the relevant patient group.
Calculation of MAPE
The method used is derived from that outlined in Makridakis and colleagues. 66 For each time point reported in one of the studies, the estimated value (Ŝ(t)) and observed value (S(t)) were used to generate the percentage error using the formula:
From these values the MAPE is derived using the formula:
where n is the number of reported values for a particular survival curve.
Linear approximation
The general form of the survival function is:
To generate estimates for the survival function, ordinary least squares (OLS) regression was performed with time as the independent variable to generate estimates for alpha and beta.
Exponential distribution
The exponential distribution is uniquely defined by a single parameter (lambda). In general:
where S(t) represents the cumulative survival function. 67 To fit curves to published data an initial estimate of lambda (λstart) is made. This estimate is used to generate estimates of the associated survivor function (Ŝ(t)). Microsoft Solver® was then used to find the lambda value that minimises the MAPE.
Weibull distribution
The Weibull distribution is uniquely defined by two parameters (lambda and gamma). The general survivor function67 can be written as:
Rearranging this gives:
OLS regression analysis using transformed values for each time point and the corresponding cumulative regression is then performed to derive estimates for lambda and gamma.
Gompertz distribution
The Gompertz distribution is uniquely defined by two parameters (lambda and theta) and the general survivor function67 can be written as:
Unfortunately there is no obvious transformation that can be performed to generate estimates for lambda and theta. Therefore, a solution was found in Microsoft Excel® using the Solver® function. Combinations of lambda and theta were analysed and the pair that minimised the MAPE was sought.
Survival analysis in children
The cumulative survival plots reported in the Cochlear Europe submission are reproduced in Figure 62 alongside the data presented in Conboy and Gibbin. 65
FIGURE 62.
Paediatric cumulative survival plots for a range of cochlear implants as reported by Cochlear Europe and Conboy and Gibbin. 65
Table 128 summarises the curve-fitting process for paediatric cochlear implants.
| Receiver/stimulator or study | Functional approximation | Label | MAPE |
|---|---|---|---|
| Conboy 200465 | Exponential | F(t) | 0.64% |
| CI22M | Weibull | G(t) | 0.19% |
| CI24RE | NA | NA | |
| CI24R | Weibull | H(t) | 0.04% |
| CI24M (all) | Exponential | J(t) | 0.48% |
| CI24M (post) | NA | NA |
Table 129 summarises the information concerning the numbers of paediatric implants.
| Receiver/stimulator or study | Number implanted | Proportion of total implants |
|---|---|---|
| Conboy 200465 | 377 | 0.95% |
| CI22M | 8225 | 20.82% |
| CI24R | 19,942 | 50.47% |
| CI24M (all) | 10,968 | 27.76% |
| Total | 39,512 | 100% |
The combined survival function used to model the cumulative survival of cochlear implants in children is therefore:
Survival analysis in adults
The cumulative survival values for several different devices are reproduced in Figure 63 and the fitting process summarised in Table 130. Published data for the CI24RE and CI24M post modification are again not used for the same reason as in the child approximation. The values used to generate the weights are summarised in Table 131.
FIGURE 63.
Cumulative survival plots for a range of cochlear implants given to adults.
| Receiver/stimulator | Functional approximation | Label | MAPE |
|---|---|---|---|
| CI22M | Linear | A(t) | 0.09% |
| CI24R | Weibull | B(t) | 0.02% |
| CI24M (all) | Weibull | C(t) | 0.03% |
| Receiver/stimulator | Number implanted | Proportion of total implants |
|---|---|---|
| CI22M | 9940 | 30.16% |
| CI24R | 15,743 | 47.77% |
| CI24M (all) | 7272 | 22.07% |
| Total | 32,955 | 100% |
The function used to generate the combined cumulative survival curve for adult cochlear implant users is therefore:
Appendix 11 Studies reviewed to identify utility values for model
The following is a complete list of the studies retrieved and examined to identify the most valid and reliable utility and utility gain estimates for use in the model:
-
Barton GR, Stacey PC, Fortnum HM, Summerfield AQ. Hearing-impaired children in the United Kingdom. IV: Cost-effectiveness of pediatric cochlear implantation. Ear Hear 2006;27:575–88.
-
Cheng AK, Rubin HR, Powe NR, Mellon NK, Francis HW, Niparko JK. Cost-utility analysis of the cochlear implant in children. JAMA 2000;284:850–6.
-
Francis HW, Chee N, Yeagle J, Cheng A, Niparko JK. Impact of cochlear implants on the functional health status of older adults. Laryngoscope 2002;112:1482–8.
-
Krabbe PF, Hinderink JB, Van Den Broek P. The effect of cochlear implant use in postlingually deaf adults. Int J Technol Assess Health Care 2000;16:864–73.
-
Lee HY, Park EC, Joong KH, Choi JY, Kim HN. Cost-utility analysis of cochlear implants in Korea using different measures of utility. Acta Otolaryngol 2006;126:817–23.
-
O’Neill C, O’Donoghue GM, Archbold SM, Normand C. A cost-utility analysis of pediatric cochlear implantation. Laryngoscope 2000;110:156–60.
-
O’Neill C, Archbold SM, O’Donoghue GM, McAlister DA, Nikolopoulos TP. Indirect costs, cost-utility variations and the funding of paediatric cochlear implantation. Int J Pediatr Otorhinolaryngol 2001;58:53–7.
-
Palmer CS, Niparko JK, Wyatt JR, Rothman M, de Lissovoy G. A prospective study of the cost-utility of the multichannel cochlear implant. Arch Otolaryngol Head Neck Surg 1999;125:1221–8.
-
Summerfield A, Stacey PC, Roberts KL, Fortnum H, Barton GR. Economic analysis and cochlear implantation. Int Congr Ser 2003;1254:313–19.
-
Summerfield AQ, Marshall DH, Archbold S. Cost-effectiveness considerations in pediatric cochlear implantation. Am J Otol 1997;18(6 Suppl.):S166–8.
-
Summerfield AQ, Marshall DH, Barton GR, Bloor KE. A cost-utility scenario analysis of bilateral cochlear implantation. Arch Otolaryngol Head Neck Surg 2002;128:1255–62.
-
Summerfield AQ, Barton GR, Toner J, McAnallen C, Proops D, Harries C, et al. Self-reported benefits from successive bilateral cochlear implantation in post-lingually deafened adults: randomised controlled trial. Int J Audiol 2006;45(Suppl. 1):S99–107.
-
UK Cochlear Implant Study Group. Criteria of candidacy for unilateral cochlear implantation is postlingually deafened adults. II: Cost-effectiveness analysis. Ear Hear 2004;25:336–60.
-
Wyatt JR, Niparko JK, Rothman ML, de Lissovoy G. Cost effectiveness of the multichannel cochlear implant. Am J Otol 1995;16:52–62.
-
Wyatt JR, Niparko JK, Rothman M, de Lissovoy G. Cost utility of the multichannel cochlear implant in 258 profoundly deaf individuals. Laryngoscope 1996;106:816–21.
Appendix 12 Ranges and distributions used in the probabilistic sensitivity analysis
| Parameter | Subgroup | Available range | Source | Data type | Distribution |
|---|---|---|---|---|---|
| Utilities | |||||
| Profound deafness | Adults | (0.411–0.455) | UKCISG 200440 | 95% CI | Beta |
| Children | (0.393–0.452) | Barton 200642 | 95% CI | Beta | |
| Utility gain (one cochlear implant vs no cochlear implants) | Adults | (0.176–0.218) | UKCISG 200440 | 95% CI | Beta |
| Children, < 2 years post implant | (–0.013 to 0.144) | Barton 200642 | 95% CI | Beta | |
| Children, 2–4 years post implant | (0.161–0.263) | Barton 200642 | 95% CI | Modified beta | |
| Children, > 4 years post implant | (0.184–0.280) | Barton 200642 | 95% CI | Modified beta | |
| Utility gain (two cochlear implants vs one cochlear implant) | Adults | (–0.045 to 0.104) | Summerfield 200253 | 95% CI | Initially 1-U transformation applied. Lognormal distribution used on transformed variable |
| Children | None | Assumption that effect in children is identical to effect in adults made | Assumption | Initially 1-U transformation applied. Lognormal distribution used on transformed variable | |
| Costs | |||||
| Presurgical candidacy | Adults |
(£3169–4532) (–21% to + 13%) |
UKCISG 200440 | Min./max. values. Assumed to represent 99.9% CI | Lognormal |
| Children |
(£1194–5003) (–58% to + 76%) |
Barton 200642 | Min./max. values assumed to represent 99.9% CI | Lognormal | |
| Unilateral implant (non-system) | Adults |
(£928–6106) (–67% to + 117%) |
UKCISG 200440 | Min./max. values assumed to represent 99.9% CI | Lognormal |
| Children |
(£1427–4106) (–59% to + 18%) |
Barton 200642 | Min./max. values assumed to represent 99.9% CI | Lognormal | |
| Bilateral implant (non-system) | Adults |
(£1393–9160) (–67% to + 117%) |
UKCISG 200440 | Min./max. values assumed to represent 99.9% CI | Lognormal |
| Children |
(£2140–6160) (–59% to + 18%) |
Barton 200642 | Min./max. values assumed to represent 99.9% CI | Lognormal | |
| Tuning and maintenance (year 1) | Adults |
(£4400–5300) (–12% to + 6%) |
UKCISG 200440 | Min./max. values assumed to represent 99.9% CI | Lognormal |
| Tuning (year 1) | Children |
(£4025–17,473) (–56% to + 91%) |
Barton 200642 | Min./max. values assumed to represent 99.9% CI | Lognormal |
| Maintenance (year 1) | Children |
(£1715–6443) (–59% to + 54%) |
Barton 200642 | Min./max. values assumed to represent 99.9% CI | Lognormal |
| Maintenance (year 2) | Adults |
(£614–1165) (–23% to + 46%) |
UKCISG 200440 | Min./max. values assumed to represent 99.9% CI | Lognormal |
| Children |
(£932–6182) (–70% to + 99%) |
Barton 200642 | Min./max. values assumed to represent 99.9% CI | Lognormal | |
| Maintenance (year 3) | Adults |
(£318–1164) (–58% to + 54%) |
UKCISG 200440 | Min./max. values assumed to represent 99.9% CI | Log Normal |
| Children |
(£423–1896) (–69% to 39%) |
Barton 200642 | Min./max. values assumed to represent 99.9% CI | Lognormal | |
| Maintenance (year 4+) | Adults |
(£317–875) (–47% to 46%) |
UKCISG 200440 | Min./max. values assumed to represent 99.9% CI | Lognormal |
| Children |
(£423–1896) (–69% to + 39%) |
Barton 200642 | Min./max. values assumed to represent 99.9% CI | Lognormal | |
| Digital hearing aid | Adults | (£62–152) | NHS Supply Chain | Min./max. values assumed to represent 99.9% CI | Lognormal |
| Children | |||||
| Cochlear implant internal failure (outside warranty) | Adults | NA | NA | Assumed to be the same as sampled value for non-implant system cost | NA |
| Children | |||||
| Cochlear implant external failure (in warranty) | Adults | (£0–100) | Values represent median cost of outpatient audiological appointment | Assumption. Assumed to represent 99.9% CI | Lognormal |
| Children | |||||
| Major complication (unilateral cochlear implant use) | Adults | (£7303–8532) | NA | Values a consequence of uncertainty in other parameters. Assumed to represent 99.9% CI | Lognormal |
| Children | (£7421–8057) | NA | Values a consequence of uncertainty in other parameters. Assumed to represent 99.9% CI | Lognormal | |
| Major complication (bilateral cochlear implant use) | Adults | (£5654–6883) | NA | Values a consequence of uncertainty in other parameters. Assumed to represent 99.9% CI | Lognormal |
| Children | (£5709–6345) | NA | Values a consequence of uncertainty in other parameters. Assumed to represent 99.9% CI | Lognormal | |
| Proportion internal failures outside warranty | Adults | (50–75%) | None | Assumption | Beta |
| Children | (50–75%) | None | Assumption | Beta | |
| Proportion external failures outside warranty | Adults | (99–99.9%) | None | Assumption | Beta |
| Children | (99–99.9%) | None | Assumption | Beta | |
| Event probabilities – internal device failure (adults) | |||||
| CI22M | Alpha: (0.9928–0.9945); beta: (–0.0009 to –0.0008) | Values derived by OLS regression using Cochlear Corporation reliability data | 95% CI | Bivariate normal | |
| CI24M (all) | Lambda: (0.0005–0.0014); gamma: (0.402–0.858) | Values derived by OLS regression using Cochlear Corporation reliability data | 95% CI | Bivariate normal | |
| CI24R | Lambda: (0.001–0.002); gamma: (0.391–0.712) | Values derived by OLS regression using Cochlear Corporation reliability data | 95% CI | Bivariate normal | |
| Proportion of above devices used to generate pooled survival curve | Baseline values derived from overall device use (Cochlear Corporation reliability data) | NA | Dirichlet | ||
| Event probabilities – internal device failure (children) | |||||
| Conboy 200465 | Lambda: (0.002–0.006) | Values derived by OLS regression using information derived from original source | 95% CI | Truncated normal distribution | |
| CI22M | Lambda: (0.005–0.0064); gamma: (0.735–0.822) | Values derived by OLS regression using Cochlear Corporation reliability data | 95% CI | Bivariate normal | |
| CI24R | Lambda: (0.002–0.004); gamma: (0.507 0.821) | Values derived by OLS regression using Cochlear Corporation reliability data | 95% CI | Bivariate normal | |
| CI24M | Lambda: (0.002–0.003) | Values derived by OLS regression using Cochlear Corporation reliability data | 95% CI | Truncated normal distribution | |
| Proportion of above devices used to generate pooled survival curve | Baseline values derived from overall device use (Cochlear Corporation reliability data) | NA | Dirichlet | ||
| Event probabilities – other | |||||
| Referrals not receiving an implant | Adults | None | SE assumed to be ¼ central estimate | Assumption | Beta |
| Children | None | SE assumed to be ¼ central estimate | Assumption | Beta | |
| Major complication (unilateral use) | Adults | None | SE assumed to be ¼ central estimate | Assumption | Beta |
| Children | None | SE assumed to be ¼ central estimate | Assumption | Beta | |
| Permanent device explantation during reoperation | Adults | (0.023–0.203) | Derived from meta-analysis of data presented in Dutt 2005,68 Ray 2004,69 Bhatia 2004,70 Balkany 1999,71 Stratigouleas 2006,72 and Lassig 200573 | 95% CI | Beta |
| Children | (0.023–0.203) | Assumed to be same as for adults | Assumption | Beta | |
| External cochlear implant failure (annual probability) | Adults | None | SE assumed to be ¼ central estimate | Assumption | Beta |
| Children | None | SE assumed to be ¼ central estimate | Assumption | Beta | |
| Elective (permanent) non-use | Adults | None | SE assumed to be ¼ central estimate | Assumption | Beta |
| Children | |||||
| Risk modifiers | |||||
| Major complication (bilateral use compared with unilateral use) | Adults | None | SE assumed to be ¼ log mean value | Assumption | Constrained lognormal |
| Children | None | SE assumed to be ¼ log mean value | Assumption | Constrained lognormal | |
| Cochlear implant internal failure (bilateral use compared with unilateral use) | Adults | None | SE assumed to be ¼ log mean value | Assumption | Constrained lognormal |
| Children | None | SE assumed to be ¼ log mean value | Assumption | Constrained lognormal | |
| Cochlear implant external failure (bilateral use compared with unilateral use) | Adults | None | SE assumed to be ¼ log mean value | Assumption | Constrained lognormal |
| Children | None | SE assumed to be ¼ log mean value | Assumption | Constrained lognormal | |
| Device lifetimes | |||||
| Acoustic hearing aid | Adults | None | Variance assumed to be same as mean lifetime | Assumption. Need to generate an integer value | Poisson |
| Children | None | Variance assumed to be same as mean lifetime | Assumption. Need to generate an integer value | Poisson | |
| Cochlear implant speech processor | Adults | None | Variance assumed to be same as mean warranty | Assumption. Need to generate an integer value | Poisson |
| Children | None | Variance assumed to be same as mean warranty | Assumption. Need to generate an integer value | Poisson | |
| Other model parameters | |||||
| Delay between operations (sequential implantation) | Adults | None | Variance assumed to be same as mean | Assumption. Need to generate an integer value | Poisson |
| Children | None | Variance assumed to be same as mean | Assumption. Need to generate an integer value | Poisson | |
| Proportion of non-cochlear implant users gaining benefit from an acoustic hearing aid | Adults | None | SE assumed to be ¼ central estimate | Assumption | Beta |
| Children | None | SE assumed to be ¼ central estimate | Assumption | Beta | |
| Proportion of unilateral implantees using a contralateral acoustic hearing aid | Adults | None | SE assumed to be ¼ central estimate | Assumption | Beta |
| Children | None | SE assumed to be ¼ central estimate | Assumption | Beta | |
| Length of trial period before elective non-use | Adults | None | Variance assumed to be same as mean | Assumption. Need to generate an integer value | Poisson |
| Children | None | Variance assumed to be same as mean | Assumption. Need to generate an integer value | Poisson | |
| Proportion of initial costs incurred by unsuccessful referrals | Adults | None | SE assumed to be ¼ central estimate | Assumption | Beta |
| Children | None | SE assumed to be ¼ central estimate | Assumption | Beta | |
| Proportion of non-cochlear implant users who gain benefit from acoustic hearing aids only using one rather than two | Adults | None | SE assumed to be ¼ central estimate | Assumption | Beta |
| Children | None | SE assumed to be ¼ central estimate | Assumption | Beta | |
Appendix 13 Speculative cost-effectiveness acceptability curves for a range of bilateral utility gain values
Children
As stated previously (see Chapter 5, Safety and reliability of cochlear implants – children and adults) our systematic review of utility values discovered no studies in children in which the incremental gain associated with using two cochlear implants versus one was measured or elicited. We therefore made the provisional modelling assumption that the utility gain in children would be the same as in adults.
This assumption is incorporated into the PSA outputs generated for the comparison of bilateral and unilateral cochlear implant use (see Chapter 7, Results of cost-effectiveness in prelingually implanted profoundly deaf children). We felt that because of the extreme uncertainty in this key parameter we would also generate a set of speculative, hypothetical CEACs corresponding to a range of possible estimates for this parameter.
In the absence of any data to inform this analysis we have assumed that the standard error is one-half of the central estimate and used these values to parameterise a range of beta distributions. The results for both early (implant age 1.5 years) simultaneous and early sequential bilateral implantation compared with unilateral implantation are presented graphically in Figures 64 and 65 and tabulated in Table 133.
FIGURE 64.
Indicative cost-effectiveness acceptability curves for a range of utility gain values associated with simultaneous paediatric bilateral implantation.
FIGURE 65.
Indicative cost-effectiveness acceptability curves for a range of utility gain values associated with sequential paediatric bilateral implantation.
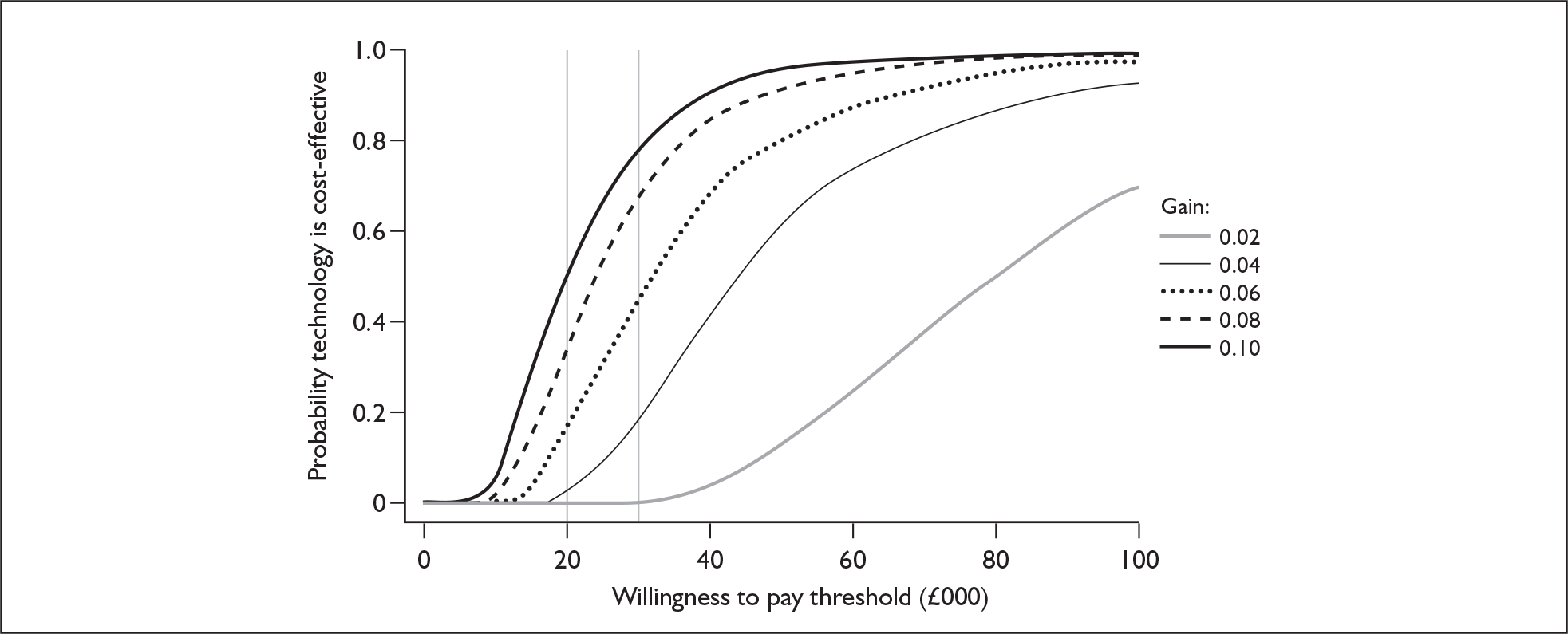
| Mean utility gain | |||||
|---|---|---|---|---|---|
| 0.02 | 0.04 | 0.06 | 0.08 | 0.1 | |
| Range used | 0.0004–0.04 | 0.001–0.079 | 0.0012–0.112 | 0.002–0.158 | 0.002–0.198 |
| Likelihood simultaneous implantation cost-effective at £20,000/QALY | 0.2% | 11.9% | 35.2% | 58.8% | 72% |
| Likelihood simultaneous implantation cost-effective at £30,000/QALY | 3.0% | 40.1% | 67.5% | 83.9% | 90.2% |
| Likelihood sequential implantation cost-effective at £20,000/QALY | 0% | 3.3% | 15.4% | 35.1% | 49.0% |
| Likelihood sequential implantation cost-effective at £30,000/QALY | 0.3% | 19.8% | 44.4% | 66.5% | 78.6% |
Adults
The systematic review of studies that produced utility estimates highlighted one study in which the incremental benefit associated with bilateral compared with unilateral implantation was elicited. This study was, however, very small (n = 24) and had a short-term follow-up period (9 months). The resultant parameter estimate is, therefore, subject to a high degree of uncertainty.
We therefore produced CEACs for a range of other possible parameter estimates for adults in the same way as for children. The results are summarised in Table 134 numerically and in Figures 66 and 67 graphically.
| Mean utility gain | |||||
|---|---|---|---|---|---|
| 0.02 | 0.04 | 0.06 | 0.08 | 0.1 | |
| Range used | 0.0004–0.034 | 0.001–0.079 | 0.0012–0.112 | 0.002–0.158 | 0.002–0.198 |
| Likelihood simultaneous implantation cost-effective at £20,000/QALY | 0% | 5.0% | 24.1% | 42.5% | 61.0% |
| Likelihood simultaneous implantation cost-effective at £30,000/QALY | 1.0% | 25.9% | 55.5% | 71.3% | 82.8% |
| Likelihood sequential implantation cost-effective at £20,000/QALY | 0% | 1.2% | 12.0% | 27.5% | 44.4% |
| Likelihood sequential implantation cost-effective at £30,000/QALY | 0.2% | 13.4% | 38.7% | 57.0% | 73.5% |
FIGURE 66.
Indicative cost-effectiveness acceptability curves for a range of utility gain values associated with simultaneous adult bilateral implantation.
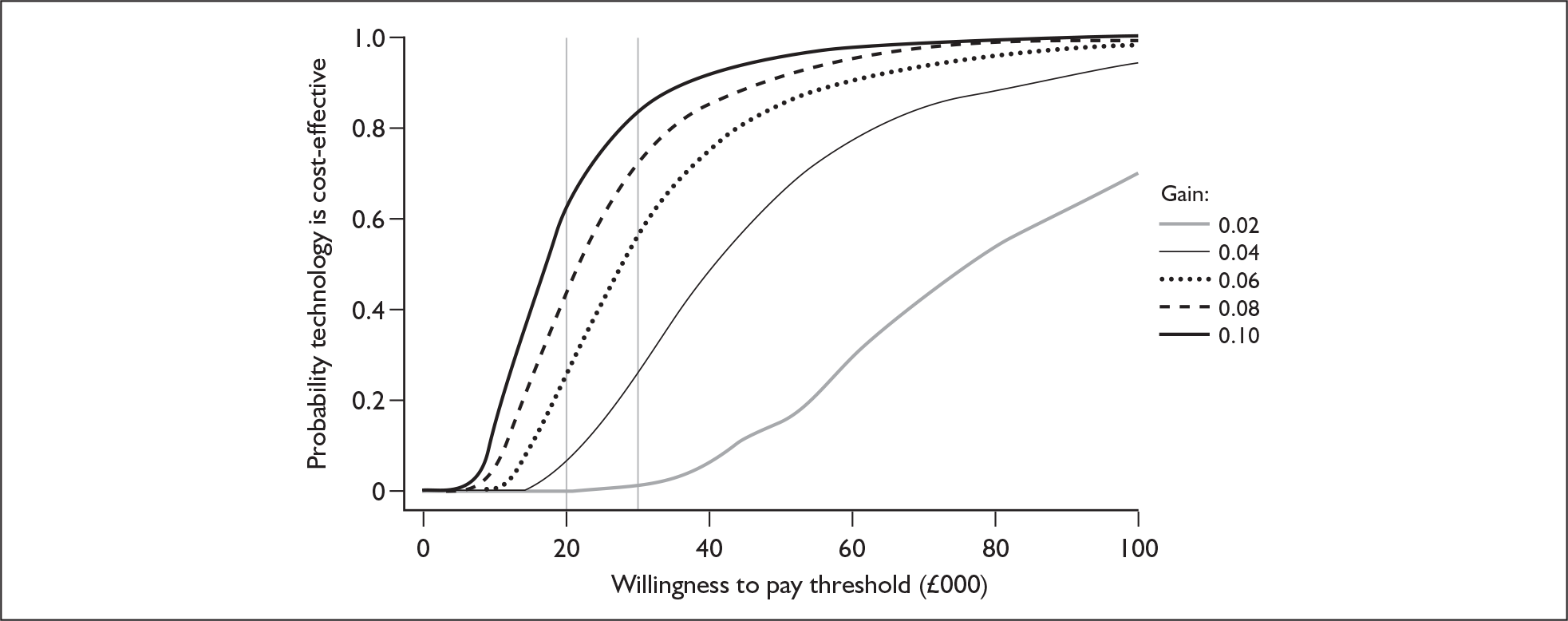
FIGURE 67.
Indicative cost-effectiveness acceptability curves for a range of utility gain values associated with sequential adult bilateral implantation.
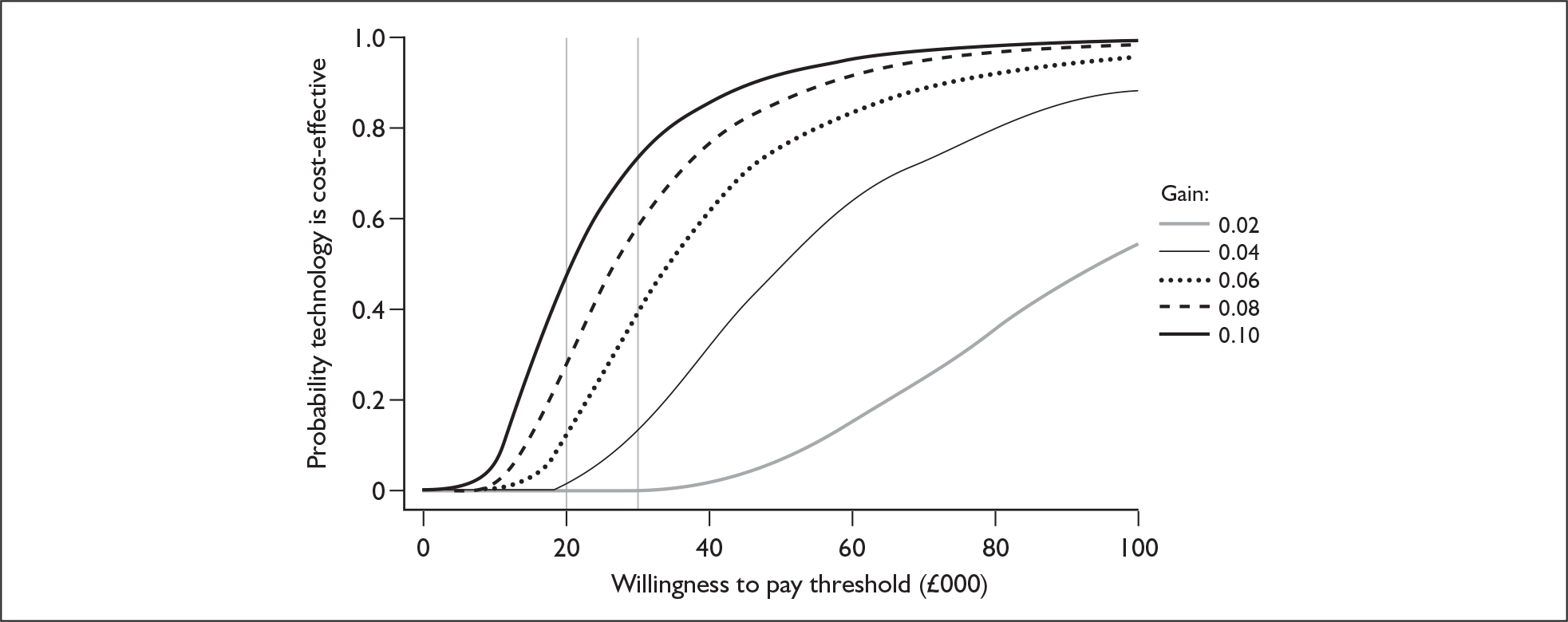
Appendix 14 Ongoing trials
Ongoing cochlear implant trials on the National Research Register, 18 October 2007
The NRR shows the following eight ongoing trials (see following section for details):
-
Candidacy of cochlear implants and hearing amplification devices in the Cambridgeshire area.
-
Cortical activity in cochlear implant users – a positron emission tomography study.
-
Maximising outcomes for adult cochlear implant users by auditory training (Bradford and Nottingham studies).
-
Quality of life of patients with binaural hearing aids and bilateral cochlear implants.
-
Health-related quality of life in children and adolescents with cochlear implants.
-
Improving outcomes for users of cochlear implants and bilateral cochlear implants by measuring primary psychophysical modifying speech processors and fitting schemes.
-
The effect of cochlear implantation on middle ear mechanics.
NRR multicentre ongoing trials
Record #1 of 1
TI:Candidacy of cochlear implants and hearing amplification devices in the Cambridgeshire area
PI:N0544194751
PR:Cambridge Consortium – Addenbrookes
RE:Eastern Regional Office
MR:We wish to calculate the prevalence of patient suitability (on audiological criteria) for cochlear implants and hearing amplification devices among patients who have undergone audiological investigations in hospitals in England.
MT:Aim: assessing the prevalence of candidacy for cochlear implants and hearing amplification devices among the patients previously assessed at audiology departments of selected hospitals
1− All patients in audiology database searched for audiological criteria for cochlear implants and hearing amplification devices.
2− Notes of patients fitting audiological criteria in certain/borderline cases are assessed for medical and otological exclusion criteria.
3− Outcome: suitability of patients grouped as candidates or not. This does not entail the device being offered because of resource restriction.
OU:Prevalence for candidacy for cochlear implants and hearing amplification devices in patients who have been investigated in selected hospitals’ audiology departments.
MC:This record is from the lead centre of a multicentre study.
EC:06/MRE05/11
PC:Addenbrooke’s Hospital
SD:3/8/2005
ED:3/8/2008
ST:Ongoing
AU:Mr Elias Koury
AD:ENT
F1:Own account
PK:MeSH terms not yet assigned
NRR participant centre ongoing trials
Record #1 of 4
TI:Cortical activity in cochlear implant users – a positron emission tomography study
PI:N0063116034
PR:Christie Hospital NHS Trust
RE:North West Regional Office
MR:Which areas of the auditory cortex are involved in patient response to cochlear implants?
MT:Neuroactivation study with FDG PET
OU:Patient response
MC:This record refers to a multicentre study led by another centre
LC:Manchester Royal Infirmary Hospital
SD:1/1/2002
ED:31/12/2008
ST:Ongoing
AU:Dr D Hastings
AD:North West Medical Physics, Christie Hospital NHS Trust, Wilmslow Road, Withington, Manchester, M20 4BX
PH:0161 446 3546
PN:RBV
PT:Greater Manchester Cancer Programme
F1:Royal College of Surgeons of Edinburgh
F3:140000
F4:NHS R&D Support Funding
F5:2007/08
PK:MeSH terms not yet assigned
Record #2 of 4
TI:Maximising outcomes for adult cochlear implant users by auditory training
PI:N0050182727
PR:Bradford Teaching Hospitals NHS Foundation Trust
RE:Northern/Yorkshire Regional Office
MR:Cochlear implantation is a surgical and therapeutic intervention that restores useful auditory sensations to people who are profoundly deaf and who do not benefit materially from acoustic hearing aids. The aim of this research is to measure the effectiveness of a computer-based, self-administered auditory training package designed to improve the speech perception abilities of adults who perform poorly with their cochlear implants. The training package will be implemented on laptop computers and used by patients in their own homes. If successful the training package will reduce the need for costly one-to-one speech and language therapy.
MT:The research planned with cochlear implant users follows on from work that has been carried out with normally hearing listeners who listen to speech through simulation of a cochlear implant system. The study has developed auditory training packages based around discriminating between quasi-minimal pairs of words and around discriminating words in sentences.
The study intends to evaluate the effectiveness of a training package that includes both word- and sentence-based auditory training. Participants will be eight cochlear implant users who have limited speech recognition abilities. An initial meeting between the investigators and each participant will take place at the local cochlear implant programme. Thereafter, all training and testing will be carried out in participants’ own homes.
Participants will carry out extensive auditory training via laptop computers. Participants will be asked to complete an hour of training a day, 5 days a week, for a period of 3 weeks. They will be asked to complete half an hour of word training and half an hour of sentence training each day. Tests of speech perception will be administered on four occasions to allow the study to evaluate the effectiveness of auditory training.
SA:The lead surgeons at Nottingham and Yorkshire have agreed to identify suitable participants for this study. Suitable participants will be approached by clinicians at the cochlear implant programmes. The study intends to include participants who have limited speech perception abilities as they are seeking to develop a training package that will improve the speech perception skills of people who perform poorly with their implants. Participants will be native speakers of British English, as all of the tests of speech perception are administered in English. The study intends to recruit eight participants to this study; four will be recruited from the Bradford Teaching Hospitals NHS Trust.
OU:Improvement on a test of sentence recognition, which will be administered at baseline and after 1, 2 and 3 weeks of training.
AI:This research study is being undertaken as part of a PhD degree at the University of York. Educational supervisor is Professor Quentin Summerfield, Department of Psychology. Tel: 01904 432913. Email: aqs1@york.ac.uk.
MC:This record refers to a multicentre study led by another centre.
LC:University of York
EC:05/Q1205/258
SD:25/1/2006
ED:31/3/2008
ST:Ongoing
AU:Dr Paula Stacey
AD:Division of Psychology, Nottingham Trent University, Burton Street, Nottingham, NG1 4BU, UK
PH:07977 448415
EM:p.stacey@psychology.york.ac.uk
F1:Deafness Research UK (the Hearing Research Trust)
PK:MeSH terms not yet assigned
Record #3 of 4
TI:Maximising outcomes for adult cochlear implant users by auditory training
PI:N0192182458
PR:Nottingham University Hospitals NHS Trust
RE:Trent Regional Office
MR:Cochlear implantation is a surgical and therapeutic intervention that restores useful auditory sensations to people who are profoundly deaf and who do not benefit materially from acoustic hearing aids. The aim of this research is to measure the effectiveness of a computer-based, self-administered auditory training package designed to improve the speech perception abilities of adults who perform poorly with their cochlear implants. The training package will be implemented on laptop computers and used by patients in their own homes. If successful the training package will reduce the need for costly one-to-one speech and language therapy. Secondary research objectives: n/a.
MT:Case–control and questionnaire.
SA:Participants will be native speakers of British English who have limited speech perception abilities.
OU:Improvement on a test of sentence recognition, which will be administered at baseline and after 1, 2 and 3 weeks of training. Secondary outcome measures: improvement on tests of consonant and vowel recognition, which will be administered at baseline and after 1, 2 and 3 weeks of training.
MC:This record refers to a multicentre study led by another centre.
LC:University of York
SD:15/5/2006
ED:1/10/2007
ST:Ongoing
AU:Professor G O’Donoghue
AD:Queens Medical Centre, University Hospital Nottingham NHS Trust, Derby Road, Nottingham, NG7 2UH, UK
PH:0115 9249924
FA:0115 9249924
EM:g.o’donoghue@nottingham.ac.uk/
PN:RFKRA
PT:Optimising care for the management of common ENT conditions
F1:Deafness Research UK
F2:406:YOR:PS
F4:NHS R&D Support Funding
F5:2006/07
PK:MeSH terms not yet assigned
Record #4 of 4
TI:Quality of life of patients with binaural hearing aids and bilateral cochlear implants
PI:N0265147240
PR:University Hospital Birmingham NHS Foundation Trust
RE:West Midlands Regional Office
MR:To investigate the quality of life of patients with hinatmrai hearing aids and bilateral cochlear implants. To design and validate questionnaires that measure the quality of life of these patients.
MT:The study sample for this study will consist of patients from the UK National Health Service (NEd) who have received two hearing aids or cochlear implants. The clinicians at their own audiology centres will ask them if they would like to participate in the study and inform them that it will only involve filling in a questionnaire. It will also be emphasised that their participation or lack of it will not affect their treatment and that the only person who will know their responses will be the chief investigator as the questionnaires will be sent directly to the University of Southampton.
Patients agreeing to participate in the study will be given an envelope containing an information sheet (with the contact details of the chief investigator too), a consent form, an open-ended questionnaire and a prepaid envelope. The contents of the envelope are attached to the form. Patients will be able to fill in the questionnaires in their own homes. Questions will aim to prompt responses about the patients’ views of how the second hearing aid or cochlear implant has changed their lifestyle, comparing their present quality of life to when they had only one aid or implant.
The responses from the open-ended questionnaire will then be used to develop a closed-ended questionnaire for the same purposes. These questionnaires will eventually be validated with the help of more patients from the NI-IS during the next stage of the PhD. It is envisaged that the same cochlear implantees will be used for the validation process of the implant questionnaire (as the number of bilateral implantees in the country is limited) but different patients will be asked to participate in the validation process of the hearing aid questionnaire. The validation of the closed-ended questionnaires will involve separate MREC approval.
SA:Hearing aid patients – some of these patients might be taking part in the Modernising Hearing Aid Services programme, which is happening across the UK. This involves filling in questionnaires, which do not affect the present study or vice versa. Cochlear implant patients – these patients are taking part in a multicentric study on the benefits of bilateral implantation. It involves studies on speech recognition in noise and the ability to localise sounds in an echo chamber. Once again these studies will not interfere with this study or vice versa.
OU:Unknown
MC:This record refers to a multicentre study led by another centre
EC:03/11/112
SD:7/8/2004
ED:7/8/2008
ST:Ongoing
AU:Mr H Cooper
AD:Audiology, Selly Oak Hospital, Birmingham, B29 6JD, UK
PH:0121 627 1627
PN:RRK
PT:Neurosciences and ageing
F1:Unfunded
F4:NHS R&D Support Funding
F5:2007/08
F6:12500.24
PK:MeSH terms not yet assigned
NRR single centre ongoing trials
Record #1 of 3
TI:Health-related quality of life in children and adolescents with cochlear implants
PI:N0013192684
PR:Guy’s and St. Thomas’ NHS Foundation Trust
RE:London Regional Office
MR:To evaluate the health-related quality of life of established paediatric cochlear implant users from St Thomas’ Hospital Paediatric Cochlear Implant Programme, aged 4–16 years, via a generic, health-related quality of life questionnaire – KINDLr (Ravens-Sieberer U, Bullinger M. Fragenbogen zur Erfassung der gesundheitsbezogenen Lebensqualitat bei Kindern und Jugendlichen. Hamburg: University of Hamburg; 2000.)
MT:A qualitative cross-sectional study to investigate self-reported health-related quality of life questionnaire (HRQoL) ratings obtained from paediatric cochlear implant users and their parents using a standardised generic HRQoL questionnaire, and a qualitative component in which the key HRQoL issues for paediatric cochlear implant users will be explored through small focus groups containing children and adolescents with cochlear implants.
SA:71
OU:The primary outcome measure is the total score from the HRQoL questionnaire, the KINDLr. The raw total score will be transformed to a standardised score out of 100 so that total scores from the different versions of the KINDLr questionnaires can be compared. Domain scores will also be obtained for each domain within the questionnaire.
MC:This record refers to a single-centre study.
SD:4/12/2006
ED:4/11/2007
ST:Ongoing
AU:Miss Emma Stark
AD:St Thomas’, Lambeth Palace Road, London, SE1 7EH
PH:020 7188 2197
EM:emma.stark@gstt.nhs.uk
PN:RJ1
PT:Improving children’s health and quality of life
F1:Own account
F4:NHS R&D Support Funding
F5:2007/08
F6:7351
PK:MeSH terms not yet assigned
Record #2 of 3
TI:Improving outcomes for users of cochlear implants and bilateral cochlear implants by measuring primary psychophysical modifying speech processors and fitting schemes
PI:N0265147271
PR:University Hospital Birmingham NHS Foundation Trust
RE:West Midlands Regional Office
MR:Many users of unilateral cochlear implants can achieve a high degree of spoken word recognition when the speech is presented in quiet. However, even the most successful users experience difficulty in the presence of competing sounds and are poor at identifying where sounds come from. Our research aims to improve speech reception in noise. In one part of this we are investigating a new method for fitting bilateral implants, so that the same frequency band of speech results in stimulation of matched regions of the two cochleae. We have demonstrated that this matching is a necessary first step to allow patients to make full use of between-ear differences, in order to localise sounds and to extract speech from noisy backgrounds. We have also argued that the majority of existing cochlear implant speech processors, which preserve only the slowly varying ‘envelope’ information, are unlikely to permit full use of these binaural timing cues even when the electrodes are matched. To test this we have proposed a series of experiments to examine the conditions under which patients better hear speech in noisy situations and to localise sounds. The results should guide the selection of speech processing strategies used in monolateral and bilateral implants. We have funding from the Royal National Institute for Deaf People (RNID) for this project.
MT:Our overall strategy for the psychophysical experiments is to use stimuli that are relevant for the perception of speech whilst being sufficiently tightly controlled to allow generalisable conclusions to be drawn from the results. Because the number of bilaterally implanted listeners, although growing, is still quite small, we focus on detailed measurements with about four implant users per experiment, backed up by parallel studies with normally hearing users. This dual approach has been very successful in unilateral studies that we have published, yielding similar results with acoustic and electric stimulation. This permits us to fine-tune experimental design using normal listeners, allowing the most efficient use of implant users’ time. When appropriate, additional tests with monolaterally implanted users will be performed.
The psychophysical experiments with implant users will generally use 2 μs/phase biphasic pulse trains presented in monopolar (‘MPI + 2’) mode, presented at a comfortable and loudness-matched level in each ear. There are 10 bilaterally implanted adult users of the Cochlear Limited C124 device seen at Birmingham Selly Oak Hospital. There are about 300 monolaterally implanted adult users of the Cochlear Limited C124 device seen there. Our psychophysical experiments use the SPEAR3 processor, which allows bilateral stimulation together with precise control of the timing between the two devices. This is controlled via specialised software.
The parallel psychoacoustic experiments will be performed at the Cambridge MRC-CBJJ and are covered by separate ethical approval.
SA:Clinicians in audiology at Selly Oak Hospital will identify prospective candidates who have a bilateral cochlear implant or a monolateral cochlear implant and a willingness to take part and the agreement of their audiologist and surgeon.
OU:Unknown
MC:This record refers to a single-centre study
SD:17/9/2004
ED:17/9/2008
ST:Ongoing
AU:Mr H Cooper
AD:Audiology, Selly Oak Hospital, Birmingham, B29 6JD
PH:0121 627 1627
PN:RRK
PT:Neurosciences and ageing
F1:Medical Research Council
F4:NHS R&D Support Funding
F5:2007/08
F6:12500.24
PK:MeSH terms not yet assigned
Record #3 of 3
TI:The effect of cochlear implantation on middle ear mechanics
PI:N0013164881
PR:Guy’s and St. Thomas’ NHS Foundation Trust
RE:London Regional Office
MR:Does a cochlear implant alter the mechanics of the middle ear?
MT:During implant operation the middle ear mechanics will be assessed by laser Doppler vibrometry.
SA:10 patients undergoing cochlear implants will be recruited into the study.
OU:Whether the mechanics of the middle ear are affected by the implant.
MC:This record refers to a single-centre study
SD:1/10/2006
ED:1/10/2009
ST:Ongoing
AU:Mr Alec Fitzgerald O’Connor
AD:Ear Nose & Throat, 2nd Floor, Lambeth Wing, St. Thomas’ Hospital, Lambeth Palace Road, London, SE1 7EH, UK
PH:020 7188 2190
EM:Alec.FitzgeraldOConnor@gstt.nhs.uk
PN:RJ1
PT:Improving children’s health and quality of life
F1:RNID
F3:64491
F4:NHS R&D Support Funding
F5:2007/08
F6:45963
PK:COCHLEAR IMPLANTS [adverse-effects]; EAR MIDDLE [injuries]
SK:HUMANS
Appendix 15 References for appendices
References
- Harrison RV, Gordon KA, Mount RJ. Is there a critical period for cochlear implantation in congenitally deaf children? Analyses of hearing and speech perception performance after implantation. Dev Psychobiol 2005;46:252-61.
- Nikolopoulos TP, Dyar D, Archbold S, O’Donoghue GM. Development of spoken language grammar following cochlear implantation in prelingually deaf children. Arch Otolaryngol Head Neck Surg 2004;130:629-33.
- Manrique M, Cervera-Paz FJ, Huarte A, Molina M. Prospective long-term auditory results of cochlear implantation in prelinguistically deafened children: the importance of early implantation. Acta Otolaryngol Suppl 2004;552:55-63.
- Staller S, Parkinson A, Arcaroli J, Arndt P. Pediatric outcomes with the Nucleus 24 contour: North American clinical trial. Ann Otol Rhinol Laryngol Suppl 2002;189:56-61.
- MED-EL . Summary of Safety and Effectiveness Data 2001. URL: www.docstoc.com/docs/575634/Part-Summary-of-Safety-and-Effectiveness-P000025.
- Nikolopoulos TP, O’Donoghue GM, Archbold S. Age at implantation: its importance in pediatric cochlear implantation. Laryngoscope 1999;109:595-9.
- Illg A, von der Haar-Heise S, Goldring JE, Lesinski-Schiedat A, Battmer RD, Lenarz T. Speech perception results for children implanted with the CLARION cochlear implant at the Medical University of Hannover. Ann Otol Rhinol Laryngol Suppl 1999;177:93-8.
- Kessler DK, Osberger MJ, Boyle P. CLARION patient performance: an update on the adult and children’s clinical trials. Scand Audiol Suppl 1997;47:45-9.
- Mildner V, Sindija B, Zrinski KV. Speech perception of children with cochlear implants and children with traditional hearing aids. Clin Linguist Phon 2006;20:219-29.
- Tomblin JB, Spencer L, Flock S, Tyler R, Gantz B. A comparison of language achievement in children with cochlear implants and children using hearing aids. J Speech Lang Hear Res 1999;42:497-511.
- Osberger MJ, Zimmerman-Phillips S, Barker M, Geier L. Clinical trial of the CLARION cochlear implant in children. Ann Otol Rhinol Laryngol Suppl 1999;177:88-92.
- Svirsky MA, Meyer TA. Comparison of speech perception in pediatric CLARION cochlear implant and hearing aid users. Ann Otol Rhinol Laryngol Suppl 1999;177:104-9.
- Osberger MJ, Fisher L, Zimmerman-Phillips S, Geier L, Barker MJ. Speech recognition performance of older children with cochlear implants. Am J Otol 1998;19:152-7.
- van den Borne S, Snik AF, Hoekstra CC, Vermeulen AM, van den Broek P, Brokx JP. Assessment of basal sound identification skills and communication abilities in profoundly deaf children fitted with hearing aids or a cochlear implant. Clin Otolaryngol Allied Sci 1998;23:455-61.
- Peters BR, Litovsky R, Parkinson A, Lake J. Importance of age and postimplantation experience on speech perception measures in children with sequential bilateral cochlear implants. Otol Neurotol 2007;29:649-57.
- Litovsky RY, Johnstone PM, Godar S, Agrawal S, Parkinson A, Peters R, et al. Bilateral cochlear implants in children: localization acuity measured with minimum audible angle. Ear Hear 2006;27:43-59.
- Kuhn-Inacker H, Shehata-Dieler W, Muller J, Helms J. Bilateral cochlear implants: a way to optimize auditory perception abilities in deaf children?. Int J Pediatr Otorhinolaryngol 2004;68:1257-66.
- Litovsky RY, Johnstone PM, Godar SP. Benefits of bilateral cochlear implants and/or hearing aids in children. Int J Audiol 2006;45:S78-91.
- Damen GW, Pennings RJ, Snik AF, Mylanus EA. Quality of life and cochlear implantation in Usher syndrome type I. Laryngoscope 2006;116:723-8.
- Huber M. Health-related quality of life of Austrian children and adolescents with cochlear implants. Int J Pediatr Otorhinolaryngol 2005;69:1089-101.
- Spahn C, Burger T, Loschmann C, Richter B. Quality of life and psychological distress in parents of children with a cochlear implant. Cochlear Implants Int 2004;5:13-27.
- Chmiel R, Sutton L, Jenkins H. Quality of life in children with cochlear implants. Ann Otol Rhinol Laryngol Suppl 2000;185:103-5.
- UK Cochlear Implant Study Group . Criteria of candidacy for unilateral cochlear implantation in postlingually deafened adults. I: Theory and measures of effectiveness. Ear Hear 2004;25:310-35.
- UK Cochlear Implant Study Group . Criteria of candidacy for unilateral cochlear implantation in postlingually deafened adults. III: Prospective evaluation of an actuarial approach to defining a criterion. Ear Hear 2004;25:361-74.
- Mawman DJ, Bhatt YM, Green KMJ, O’Driscoll MP, Saeed SR, Ramsden RT. Trends and outcomes in the Manchester adult cochlear implant series. Clin Otolaryngol 2004;29:331-9.
- Parkinson AJ, Arcaroli J, Staller SJ, Arndt PL, Cosgriff A, Ebinger K. The Nucleus 24 contour cochlear implant system: adult clinical trial results. Ear Hear 2002;23:41S-48S.
- Ching TY, Incerti P, Hill M. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear Hear 2004;25:9-21.
- Hamzavi J, Franz P, Baumgartner WD, Gstoettner W. Hearing performance in noise of cochlear implant patients versus severely-profoundly hearing-impaired patients with hearing aids. Audiology 2001;40:26-31.
- Summerfield AQ, Barton GR, Toner J, McAnallen C, Proops D, Harries C, et al. Self-reported benefits from successive bilateral cochlear implantation in post-lingually deafened adults: randomised controlled trial. Int J Audiol 2006;45:S99-S107.
- Litovsky R, Parkinson A, Arcaroli J, Sammeth C. Simultaneous bilateral cochlear implantation in adults: a multicenter clinical study. Ear Hear 2006;27:714-31.
- Ramsden R, Greenham P, O’Driscoll M, Mawman D, Proops D, Craddock L, et al. Evaluation of bilaterally implanted adult subjects with the Nucleus 24 cochlear implant system. Otol Neurotol 2005;26:988-98.
- Verschuur CA, Lutman ME, Ramsden R, Greenham P, O’Driscoll M. Auditory localization abilities in bilateral cochlear implant recipients. Otol Neurotol 2005;26:965-71.
- Laszig R, Aschendorff A, Stecker M, Muller-Deile J, Maune S, Dillier N, et al. Benefits of bilateral electrical stimulation with the nucleus cochlear implant in adults: 6-month postoperative results. Otol Neurotol 2004;25:958-68.
- Mo B, Lindbaek M, Harris S. Cochlear implants and quality of life: a prospective study. Ear Hear 2005;26:186-94.
- Vermeire K, Brokx JPL, Wuyts FL, Cochet E, Hofkens A, Van de Heyning PH. Quality-of-life benefit from cochlear implantation in the elderly. Otol Neurotol 2005;26:188-95.
- Hallberg LRM, Ringdahl A. Living with cochlear implants: experiences of 17 adult patients in Sweden. Int J Audiol 2004;43:115-21.
- Hawthorne G, Hogan A, Giles E, Stewart M, Kethel L, White K, et al. Evaluating the health-related quality of life effects of cochlear implants: a prospective study of an adult cochlear implant program. Int J Audiol 2004;43:183-92.
- Hogan A, Hawthorne G, Kethel L, Giles E, White K, Stewart M, et al. Health-related quality-of-life outcomes from adult cochlear implantation: a cross-sectional survey. Cochlear Implants Int 2001;2:115-28.
- Palmer CS, Niparko JK, Wyatt JR, Rothman M, de Lissovoy G. A prospective study of the cost-utility of the multichannel cochlear implant. Arch Otolaryngol Head Neck Surg 1999;125:1221-8.
- UK Cochlear Implant Study Group . Criteria of candidacy for unilateral cochlear implantation in postlingually deafened adults. II: Cost-effectiveness analysis. Ear Hear 2004;25:336-60.
- Torrance GW, Furlong W, Feeny D, Boyle M. Multi-attribute preference functions. Health Utilities Index. Pharmacoeconomics 1995;7:503-20.
- Barton GR, Stacey PC, Fortnum HM, Summerfield AQ. Hearing-impaired children in the United Kingdom. IV: Cost-effectiveness of pediatric cochlear implantation. Ear Hear 2006;27:575-88.
- Carter R, Hailey D. Economic evaluation of the cochlear implant. Int J Technol Assess Health Care 1999;15:520-30.
- Cheng AK, Rubin HR, Powe NR, Mellon NK, Francis HW, Niparko JK. Cost-utility analysis of the cochlear implant in children. JAMA 2000;284:850-6.
- O’Neill C, Archbold SM, O’Donoghue GM, McAlister DA, Nikolopoulos TP. Indirect costs, cost-utility variations and the funding of paediatric cochlear implantation. Int J Pediatr Otorhinolaryngol 2001;58:53-7.
- Summerfield AQ, Marshall DH, Archbold S. Cost-effectiveness considerations in pediatric cochlear implantation. Am J Otol 1997;18:S166-S168.
- O’Neill C, O’Donoghue GM, Archbold SM, Normand C. A cost-utility analysis of pediatric cochlear implantation [see comment]. Laryngoscope 2000;110:156-60.
- Schulze-Gattermann H, Illg A, Schoenermark M, Lenarz T, Lesinski-Schiedat A. Cost-benefit analysis of pediatric cochlear implantation: German experience. Otol Neurotol 2002;23:674-81.
- Hutton J, Politi C, Seeger T. Cost-effectiveness of cochlear implantation of children. A preliminary model for the UK. Adv Otorhinolaryngol 1995;50:201-6.
- Wong BY, Hui Y, Au D, Wei W. Economic evaluation of cochlear implantation. Adv Otorhinolaryngol 2000;57:377-81.
- Bichey BG, Hoversland JM, Wynne MK, Miyamoto RT. Changes in quality of life and the cost-utility associated with cochlear implantation in patients with large vestibular aqueduct syndrome. Otol Neurotol 2002;23:323-7.
- Lee HY, Park EC, Joong KH, Choi JY, Kim HN. Cost-utility analysis of cochlear implants in Korea using different measures of utility. Acta Otolaryngol 2006;126:817-23.
- Summerfield AQ, Marshall DH, Barton GR, Bloor KE. A cost-utility scenario analysis of bilateral cochlear implantation. Arch Otolaryngol Head Neck Surg 2002;128:1255-62.
- Summerfield AQ, Marshall DH. Cochlear implantation in the UK 1990–1994. London: HMSO; 1995.
- Francis HW, Chee N, Yeagle J, Cheng A, Niparko JK. Impact of cochlear implants on the functional health status of older adults. Laryngoscope 2002;112:1482-8.
- Neilson AR. Cost-effectiveness of cochlear implantation in adults. Oslo: Norwegian Knowledge Centre for the Health Services; 2006.
- Summerfield AQ, Marshall DH, Davis AC. Cochlear implantation: demand, costs, and utility. Ann Otol Rhinol Laryngol 1995;104:S245-8.
- Wyatt JR, Niparko JK, Rothman M, de Lissovoy G. Cost utility of the multichannel cochlear implant in 258 profoundly deaf individuals. Laryngoscope 1996;106:816-21.
- Wyatt JR, Niparko JK, Rothman ML, DeLissovoy G. Cost effectiveness of the multichannel cochlear implant. Am J Otol 1995;16:52-6.
- Wyatt JR, Niparko JK, Rothman ML, Delissovoy GV. Cost-effectiveness of the multichannel cochlear implant. Ann Otol Rhinol Larygol 1995;166:248-50.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Drummond M, Sculpher M, Torrance GW, O’Brien B, Stoddart GL. Methods for the economic evaluation of health care programmes. New York: Oxford University Press; 2005.
- Balkany T, Hodges A, Menapace C, Hazard L, Driscoll C, Gantz B, et al. Nucleus Freedom North American clinical trial. Otolaryngol Head Neck Surg 2007;136:757-62.
- Barton GR, Bloor KE, Marshall DH, Summerfield AQ. Health-service costs of pediatric cochlear implantation: multi-center analysis. Int J Pediatr Otorhinolaryngol 2003;67:141-9.
- Conboy PJ, Gibbin KP. Paediatric cochlear implant durability: the Nottingham experience. Cochlear Implants Int 2004;5:131-7.
- Makridakis S, Wheelwright SC, Hyndman RJ. Forecasting: methods and applications. New York: Wiley; 1998.
- Collett D. Modelling survival data in medical research. New York: Chapman and Hall; 2003.
- Dutt SN, Ray J, Hadjihannas E, Cooper H, Donaldson I, Proops DW. Medical and surgical complications of the second 100 adult cochlear implant patients in Birmingham. J Laryngol Otol 2005;119:759-64.
- Ray J, Gibson WPR, Sanli H. Surgical complications of 844 consecutive cochlear implantations and observations on large versus small incisions. Cochlear Implants Int 2004;5:87-95.
- Bhatia K, Gibbin KP, Nikolopoulos TP, O’Donoghue GM. Surgical complications and their management in a series of 300 consecutive pediatric cochlear implantations. Otol Neurotol 2004;25:730-9.
- Balkany TJ, Hodges AV, Gomez-Marin O, Bird PA, Dolan-Ash S, Butts S, et al. Cochlear reimplantation. Laryngoscope 1999;109:351-5.
- Stratigouleas ED, Perry BP, King SM, Syms CA. Complication rate of minimally invasive cochlear implantation. Otolaryngol Head Neck Surg 2006;135:383-6.
- Lassig AA, Zwolan TA, Telian SA. Cochlear implant failures and revision. Otol Neurotol 2005;26:624-34.
List of abbreviations
- ACE
- advanced combination encoder
- AHA
- acoustic hearing aids
- AQL
- Assessment of Quality of Life
- BCIG
- British Cochlear Implant Group
- BKB
- Bamford–Kowal–Bench test
- CAP
- Categories of Auditory Performance
- CDT
- connected discourse tracking
- CEAC
- cost-effectiveness acceptability curve
- CHQ
- Child Health Questionnaire
- CI
- confidence interval
- CIS
- continuous interleaved sampling
- Cr I
- credibility interval
- CRISP
- Children’s Realistic Intelligibility and Speech Perception
- CT
- computerised tomography
- dB
- decibels
- dB HL
- decibels hearing level
- ESP
- Early Speech Perception
- FDA
- Food and Drug Administration
- GASP
- Glendonald Auditory Screening Procedure
- GBI
- Glasgow Benefit Inventory
- GHSI
- Glasgow Health Status Inventory
- HES
- Hospital Event Statistics
- HHIA
- Hearing Handicap Inventory for Adults
- HINT-C
- Hearing in Noise Test for Children
- HPS
- Hearing Participation Scale
- HRG
- Healthcare Resource Group
- HSM
- Hochmaier, Schultz and Moser sentence test
- HUI-3
- Health Utilities Index 3
- IADL
- Instrumental Activities of Daily Living scale
- ICER
- incremental cost-effectiveness ratio
- IOWA
- Iowa Matrix Sentence Test
- IRQF
- Index Relative Questionnaire Form
- KINDLr
- Munich Quality of Life Questionnaire for Children
- LNT
- Lexical Neighbourhood Test
- MAA
- minimal audible angle
- MAIS
- Meaningful Auditory Integration Scale (IT-MAIS, infant/toddler version)
- MAPE
- mean absolute percentage error
- MHAS
- Modernisation of Hearing Aid Services
- MHRA
- Medicines and Healthcare Products Regulatory Agency
- MHU
- marginal hearing aid users
- MLNT
- Multisyllabic Lexical Neighbourhood Test
- MMPI
- Minnesota Multiphasic Personality Inventory
- NCIQ
- Nijmegen Cochlear Implant Questionnaire
- OC
- oral communication
- OLS
- ordinary least squares
- OR
- odds ratio
- PQLF
- Patient Quality of Life Form
- PTA
- pure-tone audiometry
- PTT
- pure-tone thresholds
- QALY
- quality-adjusted life-year
- QWBS
- Quality of Well-being Scale
- RCT
- randomised controlled trial
- RNID
- Royal National Institute for the Deaf
- SAD
- Social Avoidance and Distress scale
- SF-36
- Short-Form 36
- SIFTER
- Screening Instrument for Targeting Educational Risk
- SIR
- Speech Intelligibility Rating scale
- SNR
- signal to noise ratio
- SPEAK
- spectral peak
- TAPS
- Test for Auditory Perception and Speech
- TC
- traditional candidates
- UKCISG
- UK Cochlear Implant Study Group
- ULS
- Usher Lifestyle Survey
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
‘Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of techniques.
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Reimsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D, et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
-
Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement.
By Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al.
-
Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation.
By Wang D, Cummins C, Bayliss S, Sandercock J, Burls A.
-
Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation.
By McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al.
-
Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis.
By Simpson EL, Stevenson MD, Rawdin A, Papaioannou D.
-
Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs.
By Main C, Liu Z, Welch K, Weiner G, Quentin Jones S, Stein K.
-
Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome: a systematic review and economic analysis.
By McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, Akers J, et al.
-
Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review.
By Sutcliffe P, Hummel S, Simpson E, Young T, Rees A, Wilkinson A, et al.
-
The harmful health effects of recreational ecstasy: a systematic review of observational evidence.
By Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of oesophageal Doppler monitoring in critically ill and high-risk surgical patients.
By Mowatt G, Houston G, Hernández R, de Verteuil R, Fraser C, Cuthbertson B, et al.
-
The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technology Assessment reports.
By Taylor RS, Elston J.
-
Controlling Hypertension and Hypotension Immediately Post Stroke (CHHIPS) – a randomised controlled trial.
By Potter J, Mistri A, Brodie F, Chernova J, Wilson E, Jagger C, et al.
-
Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation.
By Pilgrim H, Lloyd-Jones M, Rees A.
-
Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation.
By Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, et al.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods.
By Hobart J, Cano S.
-
Treatment of severe ankle sprain: a pragmatic randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of three types of mechanical ankle support with tubular bandage. The CAST trial.
By Cooke MW, Marsh JL, Clark M, Nakash R, Jarvis RM, Hutton JL, et al. , on behalf of the CAST trial group.
-
Non-occupational postexposure prophylaxis for HIV: a systematic review.
By Bryant J, Baxter L, Hird S.
-
Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial.
By Farmer AJ, Wade AN, French DP, Simon J, Yudkin P, Gray A, et al.
-
How far does screening women for domestic (partner) violence in different health-care settings meet criteria for a screening programme? Systematic reviews of nine UK National Screening Committee criteria.
By Feder G, Ramsay J, Dunne D, Rose M, Arsene C, Norman R, et al.
-
Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation.
By Simpson, EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J.
-
The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and costeffectiveness and natural history.
By Fortnum H, O’Neill C, Taylor R, Lenthall R, Nikolopoulos T, Lightfoot G, et al.
-
Dipsticks and diagnostic algorithms in urinary tract infection: development and validation, randomised trial, economic analysis, observational cohort and qualitative study.
By Little P, Turner S, Rumsby K, Warner G, Moore M, Lowes JA, et al.
-
Systematic review of respite care in the frail elderly.
By Shaw C, McNamara R, Abrams K, Cannings-John R, Hood K, Longo M, et al.
-
Neuroleptics in the treatment of aggressive challenging behaviour for people with intellectual disabilities: a randomised controlled trial (NACHBID).
By Tyrer P, Oliver-Africano P, Romeo R, Knapp M, Dickens S, Bouras N, et al.
-
Randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of selective serotonin reuptake inhibitors plus supportive care, versus supportive care alone, for mild to moderate depression with somatic symptoms in primary care: the THREAD (THREshold for AntiDepressant response) study.
By Kendrick T, Chatwin J, Dowrick C, Tylee A, Morriss R, Peveler R, et al.
-
Diagnostic strategies using DNA testing for hereditary haemochromatosis in at-risk populations: a systematic review and economic evaluation.
By Bryant J, Cooper K, Picot J, Clegg A, Roderick P, Rosenberg W, et al.
-
Enhanced external counterpulsation for the treatment of stable angina and heart failure: a systematic review and economic analysis.
By McKenna C, McDaid C, Suekarran S, Hawkins N, Claxton K, Light K, et al.
-
Development of a decision support tool for primary care management of patients with abnormal liver function tests without clinically apparent liver disease: a record-linkage population cohort study and decision analysis (ALFIE).
By Donnan PT, McLernon D, Dillon JF, Ryder S, Roderick P, Sullivan F, et al.
-
A systematic review of presumed consent systems for deceased organ donation.
By Rithalia A, McDaid C, Suekarran S, Norman G, Myers L, Sowden A.
-
Paracetamol and ibuprofen for the treatment of fever in children: the PITCH randomised controlled trial.
By Hay AD, Redmond NM, Costelloe C, Montgomery AA, Fletcher M, Hollinghurst S, et al.
-
A randomised controlled trial to compare minimally invasive glucose monitoring devices with conventional monitoring in the management of insulin-treated diabetes mellitus (MITRE).
By Newman SP, Cooke D, Casbard A, Walker S, Meredith S, Nunn A, et al.
-
Sensitivity analysis in economic evaluation: an audit of NICE current practice and a review of its use and value in decision-making.
By Andronis L, Barton P, Bryan S.
-
Trastuzumab for the treatment of primary breast cancer in HER2-positive women: a single technology appraisal.
By Ward S, Pilgrim H, Hind D.
-
Docetaxel for the adjuvant treatment of early node-positive breast cancer: a single technology appraisal.
By Chilcott J, Lloyd Jones M, Wilkinson A.
-
The use of paclitaxel in the management of early stage breast cancer.
By Griffin S, Dunn G, Palmer S, Macfarlane K, Brent S, Dyker A, et al.
-
Rituximab for the first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma.
By Dundar Y, Bagust A, Hounsome J, McLeod C, Boland A, Davis H, et al.
-
Bortezomib for the treatment of multiple myeloma patients.
By Green C, Bryant J, Takeda A, Cooper K, Clegg A, Smith A, et al.
-
Fludarabine phosphate for the firstline treatment of chronic lymphocytic leukaemia.
By Walker S, Palmer S, Erhorn S, Brent S, Dyker A, Ferrie L, et al.
-
Erlotinib for the treatment of relapsed non-small cell lung cancer.
By McLeod C, Bagust A, Boland A, Hockenhull J, Dundar Y, Proudlove C, et al.
-
Cetuximab plus radiotherapy for the treatment of locally advanced squamous cell carcinoma of the head and neck.
By Griffin S, Walker S, Sculpher M, White S, Erhorn S, Brent S, et al.
-
Infliximab for the treatment of adults with psoriasis.
By Loveman E, Turner D, Hartwell D, Cooper K, Clegg A
-
Psychological interventions for postnatal depression: cluster randomised trial and economic evaluation. The PoNDER trial.
By Morrell CJ, Warner R, Slade P, Dixon S, Walters S, Paley G, et al.
-
The effect of different treatment durations of clopidogrel in patients with non-ST-segment elevation acute coronary syndromes: a systematic review and value of information analysis.
By Rogowski R, Burch J, Palmer S, Craigs C, Golder S, Woolacott N.
-
Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care.
By Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al.
-
A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial.
By Gray AJ, Goodacre S, Newby DE, Masson MA, Sampson F, Dixon S, et al. , on behalf of the 3CPO study investigators.
-
Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation.
By Ara R, Pandor A, Stevens J, Rees A, Rafia R.
-
Adefovir dipivoxil and pegylated interferon alpha for the treatment of chronic hepatitis B: an updated systematic review and economic evaluation.
By Jones J, Shepherd J, Baxter L, Gospodarevskaya E, Hartwell D, Harris P, et al.
-
Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis.
By Hewitt CE, Gilbody SM, Brealey S, Paulden M, Palmer S, Mann R, et al.
-
A double-blind randomised placebocontrolled trial of topical intranasal corticosteroids in 4- to 11-year-old children with persistent bilateral otitis media with effusion in primary care.
By Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, et al.
-
The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model.
By Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R.
-
Rehabilitation of older patients: day hospital compared with rehabilitation at home. A randomised controlled trial.
By Parker SG, Oliver P, Pennington M, Bond J, Jagger C, Enderby PM, et al.
-
Breastfeeding promotion for infants in neonatal units: a systematic review and economic analysis.
By Renfrew MJ, Craig D, Dyson L, McCormick F, Rice S, King SE, et al.
-
The clinical effectiveness and costeffectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation.
By Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al.
-
Rapid testing for group B streptococcus during labour: a test accuracy study with evaluation of acceptability and cost-effectiveness.
By Daniels J, Gray J, Pattison H, Roberts T, Edwards E, Milner P, et al.
-
Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Honest H, Forbes CA, Durée KH, Norman G, Duffy SB, Tsourapas A, et al.
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NETSCC, HTA
-
Dr Andrew Cook, Consultant Advisor, NETSCC, HTA
-
Dr Peter Davidson, Director of Science Support, NETSCC, HTA
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Director of NHS Support, NETSCC, HTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NETSCC, HTA
-
Dr Ruairidh Milne, Director of Strategy and Development, NETSCC
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NETSCC, HTA
HTA Commissioning Board
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, University of Sheffield
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford?
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, Univeristy of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Professor Hywel Williams, Professor of Dermato-Epidemiology, University of Nottingham
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies & Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Ms Jane Bates, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Professor Glyn Elwyn, Primary Medical Care Research Group, Swansea Clinical School, University of Wales
-
Dr Ron Gray, Consultant Clinical Epidemiologist, Department of Public Health, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, University of Sheffield
-
Dr Jennifer J Kurinczuk, Consultant Clinical Epidemiologist, National Perinatal Epidemiology Unit, Oxford
-
Dr Susanne M Ludgate, Medical Director, Medicines & Healthcare Products Regulatory Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, Joint Director, National Collaborating Centre for Mental Health, University College London
-
Mrs Una Rennard, Service User Representative
-
Dr Phil Shackley, Senior Lecturer in Health Economics, School of Population and Health Sciences, University of Newcastle upon Tyne
-
Dr W Stuart A Smellie, Consultant in Chemical Pathology, Bishop Auckland General Hospital
-
Dr Nicholas Summerton, Consultant Clinical and Public Health Advisor, NICE
-
Ms Dawn Talbot, Service User Representative
-
Dr Graham Taylor, Scientific Advisor, Regional DNA Laboratory, St James’s University Hospital, Leeds
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Pharmaceuticals Panel
-
Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor in Child Health, University of Nottingham
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Mrs Barbara Greggains, Service User Representative
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Professor Jonathan Ledermann, Professor of Medical Oncology and Director of the Cancer Research UK and University College London Cancer Trials Centre
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Dr Lesley Wise, Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Therapeutic Procedures Panel
-
Consultant Physician, North Bristol NHS Trust
-
Professor of Psychiatry, Division of Health in the Community, University of Warwick, Coventry
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School, Coventry
-
Ms Maree Barnett, Acting Branch Head of Vascular Programme, Department of Health
-
Mrs Val Carlill, Service User Representative
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Mr Mark Emberton, Senior Lecturer in Oncological Urology, Institute of Urology, University College Hospital, London
-
Professor Steve Goodacre, Professor of Emergency Medicine, University of Sheffield
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and The London School of Medicine and Dentistry
-
Mr Paul Hilton, Consultant Gynaecologist and Urogynaecologist, Royal Victoria Infirmary, Newcastle upon Tyne
-
Professor Nicholas James, Professor of Clinical Oncology, University of Birmingham, and Consultant in Clinical Oncology, Queen Elizabeth Hospital
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Kate Radford, Senior Lecturer (Research), Clinical Practice Research Unit, University of Central Lancashire, Preston
-
Mr Jim Reece Service User Representative
-
Dr Karen Roberts, Nurse Consultant, Dunston Hill Hospital Cottages
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
-
Professor Tom Walley, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist, National Commissioning Group (NCG), London
-
Director, NHS Sustainable Development Unit, Cambridge
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Professor Mike Kelly, Director, Centre for Public Health Excellence, NICE, London
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Ms Jeanett Martin, Director of Nursing, BarnDoc Limited, Lewisham Primary Care Trust
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Miss Nicky Mullany, Service User Representative
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Ken Stein, Senior Clinical Lecturer in Public Health, University of Exeter
-
Dr Kieran Sweeney, Honorary Clinical Senior Lecturer, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Professor Margaret Thorogood, Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Caroline Stone, Programme Manager, Medical Research Council
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington