Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 06/61/01. The protocol was agreed in March 2007. The assessment report began editorial review in August 2007 and was accepted for publication in November 2009. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Permissions
Copyright statement
© 2010 Queen’s Printer and Controller of HMSO. This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2010 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Diabetes
There are two main types of diabetes mellitus (‘mellitus’ is used here to distinguish it from a rarer disease called ‘diabetes insipidus’, which is not relevant to this review).
Normal blood glucose control
Glucose is the primary source of fuel for cells in the body. Carbohydrate in food is metabolised to glucose within hours of ingestion, and is absorbed from the blood into cells for use as fuel. Uptake of glucose into cells is regulated by the hormone insulin, which is released from the pancreas in response to rising blood glucose levels. Insulin also regulates the use of glucose by cells, so if there is insufficient insulin, or if cells do not respond properly to insulin (insulin insensitivity or resistance), then glucose is not used efficiently by cells, in terms of either energy or storage.
Type 1 diabetes
In type 1 diabetes mellitus (T1DM), formerly known as ‘insulin-dependent diabetes’, all or nearly all of the β-cells in the pancreas, which produce insulin, have been destroyed, usually by an autoimmune process. The cause is not known. People with T1DM have little or no ability to produce their own insulin, and would die without insulin, and so they have to inject insulin for the rest of their lives. T1DM usually starts in children or young adults, but it can have onset at any age. The incidence (number of new cases per year) has risen considerably over recent decades. Scottish data show that the rate in children has more than trebled over the last 30 years (Figure 1). The rise has been greater in the youngest age group, although absolute numbers are smaller. 1
FIGURE 1.
Scottish Study Group (SSG) incidence by age at diagnosis: 1984–2003.
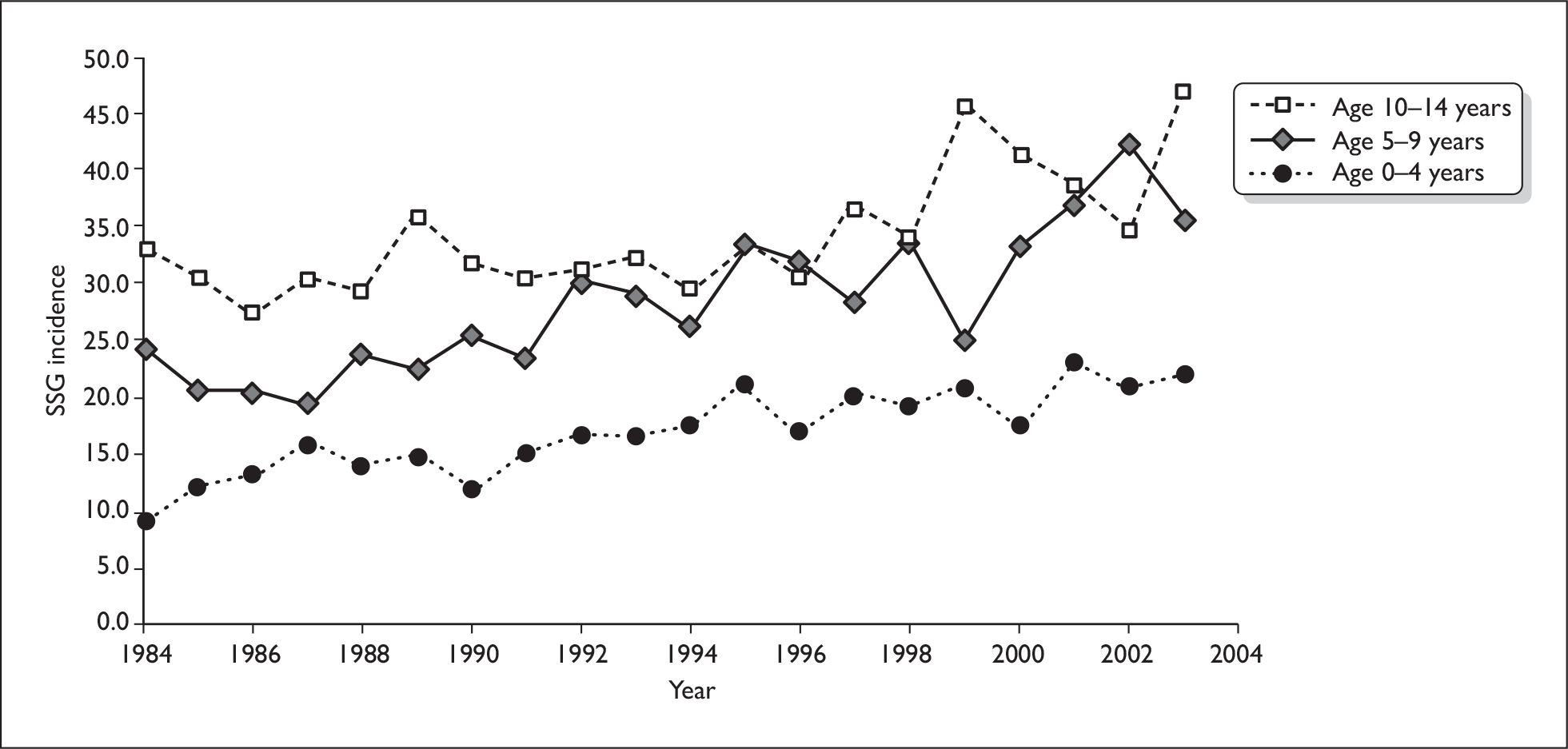
Similar rises have been reported from the Oxford region by Wilson et al. (2007). 2 The incidence of childhood diabetes (under the age of 15 years) rose from 17 per 100,000 in 1985–90 to 26.5 in 2003–4. The greatest increase was in the under-5s, in which the number who had diabetes by the age of 5 years rose fivefold.
Type 2 diabetes
Type 2 diabetes (T2DM), formerly known as ‘non-insulin-dependent diabetes’ or ‘maturity-onset diabetes’, comes on later in life than T1DM. It used to be seen almost exclusively in people over 45 years old, and was associated with overweight or obesity, but with rising prevalence of obesity it is now increasingly being seen at younger ages and even in children. Some ethnic groups, such as South Asians, have earlier onsets. The York and Humber Public Health Observatory (YHPHO) website (a very useful compendium of data on diabetes) estimates that the total prevalence of T2DM in England is 4.3%, although this includes people with undiagnosed diabetes. 3 [Some people with T2DM, perhaps 20%, have no symptoms and do not know they have it. Hence the current debate on screening, which is covered by another Health Technology Assessment (HTA) report. 4] The YHPHO estimated that the total prevalence of diabetes would rise by 15% between 2001 and 2010, with a 6% increase due to the ageing population and a 9% increase due to increasing obesity. 5
Type 2 diabetes usually starts with insulin resistance, related to overweight, with the pancreas producing more insulin than usual to overcome the resistance. Over time, the pancreas fails to produce enough, insulin production falls, blood glucose rises further, and clinical diabetes ensues. In the UK Prospective Diabetes Study (UKPDS), patients’ insulin production had fallen to about 50% of normal at the time of diagnosis. 6 Treatment starts with lifestyle measures, diet, weight loss and exercise, and, if those fail, oral drugs are added. In most patients T2DM is a progressive disease and, over time, many patients will need insulin. 7 The UKPDS showed that just over one-half of patients initially randomised to sulphonylureas (an oral drug that stimulates pancreatic insulin production) had to switch to insulin by 6 years. 8 In a population-based study in Tayside, Scotland, 6% of patients with T2DM started insulin each year. 9 Most people with T2DM starting insulin nowadays probably start with a once-daily injection of a long-acting analogue, but, over time, some will progress to multiple daily injections (MDI) in order to achieve good control.
Data from other studies have shown that many patients with T2DM are on insulin therapy. The Lothian Audit reported that 32% of people with T2DM are on insulin [J. McKnight, presentation to Royal College of Physicians of Edinburgh (RCPE) conference, 2005, formerly on RCPE website].
Control, glycated haemoglobin and insulin treatment
The term ‘control’ is a recurring one in diabetes. It refers principally to preventing blood glucose from becoming too high, but also applies to preventing it from becoming too low. High blood glucose is known as hyperglycaemia, whereas low blood glucose is called hypoglycaemia.
In the non-diabetic person, blood glucose is kept within a narrow normal range (about 4–5.6 mmol/l) through the action of insulin and other hormones. The pancreas releases a little insulin throughout the 24 hours (known as basal insulin – about 0.5–1 unit per hour in adults), but production of insulin is swiftly and markedly increased with meals, going up 5- to 10-fold in the first 30 minutes. If blood glucose falls too low, counter-regulatory hormones are released and nervous system mechanisms are activated to increase it again. A key aspect is that the brain is dependent on glucose for energy. If blood glucose falls too low, brain function is impaired, as will be described later.
Control of blood glucose is measured in three ways. First, blood glucose can be checked at any time by finger-pricking to produce a drop of blood, and testing it with a testing strip and blood glucose meter. This gives the glucose level at that time, but it may change quite rapidly after meals or either insulin or tablets. Second, longer-term control is measured by glycated haemoglobin (HbA1c) level, which reflects the average blood glucose level over 2–3 months. HbA1c has been a major advance in diabetes because by testing every 3 months, it gives an indication of how good control is. However, it provides an average and that can reflect very tight control with little fluctuation in glucose levels, or poorer control with considerable fluctuation. At the risk of considerable simplification, this can be illustrated by the averages of 4 and 8, and 2 and 10 – both equal 6. Third, new devices can now provide frequent automated testing of interstitial tissue glucose, calibrated to reflect plasma glucose, and known as ‘continuous blood glucose monitoring’. These devices are currently used more in research, but are coming into routine clinical practice in some clinics.
In T1DM, control is dependent on injected insulin, and, unfortunately, at present there is no insulin that can exactly mimic production by the normal pancreas. Even the latest rapid-acting insulins can neither achieve as rapid a rise after meals as the pancreas can, nor as rapid a fall. A key point is that natural pancreatic insulin release is regulated by the level of glucose in the blood in a way that injected insulin cannot be. A fall in blood glucose will switch off pancreatic insulin release but cannot affect injected insulin.
There are various forms of insulin, and various combinations, grouped by duration of action.
Short-acting (SA) insulin comes in three types:
-
The oldest type is called ‘regular’ or ‘soluble’; we will refer to it as short-acting (SA) soluble because some long-acting analogues are also soluble.
-
The next type is SA analogue insulin, with three varieties on the market – aspart, lispro and glulisine. SA soluble starts acting within an hour of injection, peaks at 2–4 hours, and has some effect for up to 8 hours. The SA analogues act a bit more quickly and do not last quite as long. They are therefore regarded as being closer in effect to pancreatic insulin than SA soluble insulin. However, a Cochrane review10 concluded that the advantages of SA analogues over SA soluble were minor – very little difference (0.1%) in HbA1c or total hypoglycaemic episodes, a greater (50%) but not statistically significant reduction in severe hypoglycaemic (SH) episodes (‘hypos’) in adults, but not adolescents, when used in MDI, but a greater difference (0.2%) in HbA1c in patients on continuous subcutaneous insulin infusion (CSII). The improvement in HbA1c with SA analogues rather than SA soluble in CSII, was reported to be 0.26% in a meta-analysis based on the last assessment report for the National Institute for Health and Clinical Excellence (NICE),11 and patient preference was also higher for analogues.
-
The third type is inhaled insulin, appraised by NICE in 2006 [Technology Appraisal (TA) 113] but now withdrawn from the market by the manufacturers. 12
Intermediate-acting insulins, such as neutral protamine Hagedorn (NPH) or isophane, start working in 1–2 hours, peak at about 6–10 hours, and have some effect for 16–18 hours. Unfortunately, the peaks may vary unpredictably from injection to injection, and hence from day to day.
Short- and intermediate-acting insulins can be mixed in the same syringe, and can be given as a premixed version twice daily. This is known as ‘conventional’ insulin therapy. The newer long-acting analogue insulins – glargine and detemir – are longer acting than NPH and have a long steady action, being sometimes called ‘peak-less’.
In recent years, since the Diabetes Control and Complications Trial (DCCT)13 showed that good control reduced the adverse effects of T1DM, there has been a move to more intensive insulin treatment. This consists of a combination of basal insulin using NPH (usually twice per day) or a long-acting analogue, with SA insulin at mealtimes, usually called ‘bolus’ insulin – hence the term ‘basal–bolus’ regimens. These can be given in two ways: by MDI or by CSII via insulin pumps.
History of continuous subcutaneous insulin infusion
The first studies of CSII delivered via insulin pumps came from Guy’s Hospital, London, UK in 197814 and Yale, New Haven, CT, USA in 1979. 15 CSII uses a small programmable pump with a fine tube connected to a soft plastic cannula (introduced by needle), which goes into the subcutaneous tissue under the skin, often in the abdomen. The cannula is changed every 2–4 days. The aim of CSII is to try to approximate the insulin delivery profile more closely to the pattern of output behaviour of the normal pancreas, by providing continuously infused, low-volume basal insulin for fasting periods and the delivery of increased rate boluses to cover meals. Only SA (soluble or analogue) insulin is used.
Lenhard and Reeves (2001)16 reviewed the literature in 2001 using MEDLINE only. They noted the rise in popularity of CSII after the introduction of pumps in the late 1970s and early 1980s, followed by a fall because of size, safety and efficacy concerns, followed then by a rise in usage after the publication of the DCCT study. They also noted that the newer pumps were smaller, more reliable and easier to use. They estimated that about 8% of all adults with T1DM in North America were using pumps. They concluded that there was good evidence for benefits in adults (‘comparable or slightly superior to MDI’) and some in pregnancy, but that there was little good-quality evidence in children.
Pickup and Keen,17 who were the originators of CSII, reviewed the history of, and evidence base for, CSII in 2002. They noted the considerable worldwide use of pumps (over 200,000 patients) and the disproportionately low UK use. They concluded that on CSII, blood glucose and HbA1c levels are similar or slightly lower than when using MDI, that hypoglycaemia is much less frequent, and that ketoacidosis occurs at the same rate. They concluded that the proportion of patients who would be suitable is relatively small. In a complementary paper, Pickup et al. (2002)18 carried out a meta-analysis of randomised controlled trials (RCTs) comparing CSII with MDI. They found that HbA1c level was about 0.5% better on CSII, but found that few studies reported hypoglycaemic episodes; none appeared to report effect on quality of life. The CSII group needed 14% less insulin.
The previous UK HTA report has been mentioned already and its summary is shown in Appendix 1. The Agence d’Évaluation des Technologies et des Modes d’Intervention en Santé (AETMIS),19 Quebec, Canada, published a report in June 2005, comparing MDI with CSII. It concluded that CSII might be indicated for a limited, selected group of people with T1DM, and cited various selection criteria, including:
-
inadequate glycaemic control despite a trial of intensive insulin therapy
-
recurrent, unpredictable SH episodes, nocturnal hypoglycaemia or hypoglycaemic unawareness, causing incapacitating anxiety and affecting the quality of life
-
morning hyperglycaemic episodes (morning blood glucose level of 8 mmol/l or more)
-
and for children, the above plus extreme insulin sensitivity (under 20 units of insulin per day).
At the time the report was written, glargine was not available in Quebec, Canada.
It is always interesting to know what treatments clinicians with diabetes choose for themselves. A survey of the American Association of Diabetes Educators (AADE) and the American Diabetes Association (ADA) asked members if they had diabetes, and, if so, how they were treated. 20 About 6.4% of members had diabetes, of whom 72% had T1DM. The survey found that 96% of those with T1DM used an intensive insulin regimen, and that over one-half (60% of the AADE members with diabetes and 52% of the ADA ones) used an insulin pump.
Modern pumps
Modern pumps are small and lightweight compared with the early versions. The pumps are battery operated and hold enough insulin for several days, depending on daily need. The infusion rate can be programmed for both dose and timing. Different basal rates can be preset, for example overnight could be lower than during the day, or vice versa. Bolus boosts can be given starting just before meals (if analogue insulins are used), and infusion rates can be reduced during exercise. The newer pumps are more reliable21 and may have alarms for empty cartridges, low batteries, occlusion of tubing and faulty electronics, giving rise to less fear of undetected malfunction, which was a problem with some of the older pumps.
Complications of diabetes
Diabetes causes short- and long-term problems. The short-term ones include the acute metabolic upsets shown below.
-
Diabetic ketoacidosis (DKA) Insufficiency of insulin, often at a time of incidental other illnesses when the body needs more than usual, leads to disordered metabolism, with the blood become more acidic than it should be (hence the ‘acidosis’) due to accumulation of ketones (hence the ‘keto’). DKA is a medical emergency and can be life threatening. Mortality nowadays is very low, from 0.15–0.31% in children in North America, the UK22 and India,23 but higher, at 4%, in Danish adults. 24 However, it remains a serious threat.
-
Hypoglycaemia Mild hypoglycaemia may only cause a feeling of hunger and sweating, quickly corrected by taking food or a sugary drink. However if it occurs during the night (nocturnal hypoglycaemia) it can reduce the amount and quality of sleep. More serious hypoglycaemia can mean that the diabetic person needs help in order to recover. ‘Severe hypoglycaemia’ is usually defined by the need for assistance from another person, meaning that the diabetic person cannot recover without aid. Severe hypoglycaemia can lead to behavioural disturbances, unconsciousness, convulsions (similar to an epileptic fit) or death. In very young children with frequent or SH events there may be some impairment of intellectual function (see below).
The problems mentioned above refer to physical effects, but, as has been pointed out by Cryer (2006),25 there is also psychological morbidity: ‘At the very least, an episode of hypoglycaemia is a nuisance and a distraction. It can be embarrassing and can cause social ostracism. The psychological morbidity of hypoglycaemia includes fear of hypoglycaemia, high levels of anxiety and low levels of overall happiness’.
The longer-term adverse consequences of diabetes have been traditionally known as ‘complications’, and are related to chronic hyperglycaemia. They include conditions due to damage to small blood vessels (microangiopathy) and larger ones (macrovascular disease):
-
retinopathy a disease of the eyes, which, in the past, has been the most common cause of blindness in people of working age (macular degeneration is more common in the elderly)26
-
nephropathy a disease of the kidneys, which is one of the most common causes of end-stage renal failure, leading to a need for renal dialysis or transplantation27
-
ischaemic heart disease (IHD) due to disease of the coronary arteries. People with diabetes have an increased risk of IHD28–31
-
stroke due to disease of the arteries to the brain – the risk is increased three- to fourfold in T1DM29
-
amputations due to a combination of damage to nerves (neuropathy) and to arteries in the leg (for review see Boulton et al. 2005). 32 A Welsh study reported a relative risk (RR) for amputation of 32 in people with diabetes33
-
neuropathy damage to the nervous system.
Intensified insulin therapy and better control of T1DM
Conventional insulin treatment usually means twice-daily combination of a short-acting and an intermediate-acting insulin. Intensified insulin therapy (IIT) is a combination of more frequent doses of insulin, usually one injection of a long-acting insulin per day (sometimes two) and mealtime doses of SA insulin, together with regular self-monitoring of blood glucose, self-adjustment of insulin dose, and care with diet. It requires commitment from an educated patient, and not all patients wish to move to intensified therapy. It is not just about taking insulin more often.
The DCCT in T1DM confirmed the benefits of intensified therapy, with MDI or insulin pumps, in achieving good control and thereby reducing the risk of complications. 13 It confirmed the results of smaller trials, summarised in the meta-analysis by Wang et al. (1993). 34 Since the DCCT, there has been increased emphasis on the importance of good control of blood glucose in reducing the risk of complications. The DCCT compared outcomes at an average follow-up of 6.5 years, between those randomised to intensive insulin treatment with MDI or CSII, and those randomised to conventional insulin regimens, usually two injections per day. In those who had no retinopathy (eye disease) at baseline, intensive therapy reduced the risk of retinopathy by 76% [95% confidence interval (CI) 62 to 85]: by 6 years, 7% of the intensive group and 26% of the conventional group had developed retinopathy. 13 The gap widened in later years. 35 In those who had some retinopathy at baseline, intensive therapy reduced progression by 54%, and reduced the need for laser photocoagulation therapy (a way of treating sight-threatening retinopathy) by 56%. Intensive therapy reduced the appearance of microalbuminuria, a marker for diabetic renal damage, by 39%. 13
The reduction in retinopathy was related to the improvement in HbA1c, and applied across the whole range of HbA1c. So a 10% reduction in HbA1c gave a 39% decrease in retinopathy risk, whether the reduction was from 9.0% to 8.1% or from 8.0 to 7.2%. 36 The retinopathy risk increased as the HbA1c increased, so the absolute risk reductions would be different. (For example, drops from 40% to 20% and from 20% to 10% are both 50% relative reductions, but the former is a larger absolute reduction.)
The DCCT ended after 6.5 years, and the conventional group was advised to switch to intensive therapy. Within a year, the gap in HbA1c levels had narrowed from the 1.8% seen in the trial to 0.4%, and by 5 years there was no difference. But, at 7 years, the former intensive group continued to do better, for example with progression of retinopathy at about one-third of that of the former conventional group, despite identical HbA1c level. 37 The reasons are not fully understood, but it may mean that once changes get beyond a certain point, progression is not halted by improving glucose control. This is seen in nephropathy (renal disease), which once established, progresses even if very good control of blood glucose is achieved. One finding from the DCCT was that tight control was more effective if applied early in the disease. 38
This phenomenon whereby early good control can reduce later complications, even if control worsens, has been called ‘metabolic memory’ by the DCCT/Epidemiology of Diabetes Interventions and Complications Research Group (EDIC) investigators. 39 A recent review by Ihnat et al. (2007)40 identified possible underlying biochemical mechanisms through which this could occur. If, to use Ihnat’s words, ‘hyperglycaemia can leave an early imprint in cells of the vasculature and of target organs, favouring the future development of complications’, then there are implications for diabetes care. One is that as Ihnat et al. say, ‘the existence of the metabolic memory suggests that very early aggressive treatment of hyperglycaemia is mandatory’.
Since the DCCT, there has been a move to intensified insulin regimens. A study of two cohorts of children in the USA by Svoren et al. ,41 one enrolled in 1977 and the other in 2002, found that the proportion on three or more injections per day or CSII, increased from 65% in the earlier cohort to 85% in the later one. HbA1c level dropped by 0.3% but the incidence of SH episodes and emergency room visits also dropped, by almost 50% and 25%, respectively.
Unfortunately, many patients with T1DM are poorly controlled, especially in childhood and adolescence. Two audits by the Scottish Study Group for the Care of Diabetes in the Young (SSGCDY) have examined control of hyperglycaemia as reflected by HbA1c level. The first audit, DIABAUD 2 (SSGCYD 2001),42 reported that in 1997–99, the average HbA1c level was 9.1%. Only about 10% of children were achieving the current NICE guidelines target of 7.5% or less. Nearly all children were on two injections per day; only 2% were on intensive insulin regimens of four injections per day. The second audit, DIABAUD 3 (SSGCDY 2006),43 was carried out in 2002–4. It found that mean HbA1c level had not changed (it was 9.2%) and again only 10% reached the NICE guideline target. The number of children on more than two injections per day had risen to 51% but almost all were on a three-injection regimen, splitting the evening dose. MDI was still uncommon (2.3%) and pump use was rare.
The proportion of people with diabetes who have good control, as reflected by HbA1c level, has been increasing. The National Diabetes Audit 2004–5, reported in Diabetes UK’s State of the Nations 2006 report, found that in England 62% of people with diabetes reached the target of HbA1c level ≤ 7.4%; in Wales, 61% did. 44 However, this means that 48% in England did not. For children, 84% did not achieve the target in 2004–5. Unfortunately the data, based on returns from general practices, do not provide information for T1DM separately.
Treatment of T2DM with insulin
As mentioned above, T2DM is usually a progressive disease, and about one-third of patients end up on insulin. There has been reluctance amongst both patients and clinicians to switch from oral agents to insulin in T2DM, because good control is still usually not achieved and because weight gain tends to follow insulin therapy. 45,46 Many patients with T2DM with poor control on oral agents remain on them for years before switching to insulin. 47 This may be changing for several reasons: the new general practitioner (GP) contract with incentives for reaching HbA1c targets; the evidence on the benefits of tighter control; and the greater ease of switching to insulin with once-daily long-acting analogues. Gulliford et al. (2007)48 noted that the impact of the Quality and Outcomes Framework target was seen in the proportions of patients whose HbA1c level was < 7.5%: 22% in 2000; 32% in 2001; 37% in 2002; and 57% in 2005.
For the purposes of this review, the relevant T2DM group is those who have progressed to the stage of needing intensive insulin therapy because of poor control and poor pancreatic β-cell function. Such therapy usually involves MDI, with a combination of long-acting insulin to provide a basal level of insulin throughout the 24 hours, supplemented with SA insulin at mealtimes.
Hypoglycaemia in T1DM
In the DCCT, intensification of insulin therapy was associated with a higher rate of hypoglycaemia. 13,49 Over an average follow-up of 6.5 years, 65% of patients in the intensive and 35% of those in the conventional groups had at least one SH episode. Those in the intensive group had 61.2 episodes per 100 patient-years, whereas those in the conventional group had 18.7 episodes per 100 patient-years. The average number of SH episodes a year was low, but they may have a longer effect. As one of our expert advisers, K. Tieszen (cited on p. 5 of the previous HTA report on CSII) said:50
Even though any single hypo event is short-lived in terms of its acute physiological effect, the psychological effect on many patients is not at all short-lived. It often has a profound effect so that the patient will do everything they can to avoid a recurrence. Many patients have a greater fear of hypos than of developing diabetes-related complications, and as a result will keep their blood glucose levels higher than recommended in order to avoid hypos. If they lost their fear of hypos, better glycaemic control could be achieved, resulting in a reduced risk for complications.
The NICE guidance on long-acting analogue insulins recognised that fear of hypoglycaemia was a significant factor: ‘The Committee accepted that episodes of hypoglycaemia are potentially detrimental to an individual’s quality of life. That is partly the result of an individual’s objective fear of symptomatic hypoglycaemic attacks…’. 51
A review of hypoglycaemia and diabetes noted that patients were as worried about severe hypoglycaemia as about eye disease. 52 Nordfeldt and Ludwigsson53 reported that patients (under the age of 19) who had experienced an SH episode within the previous year had a lower quality of life, and that they regarded hypoglycaemia as a bigger problem than long-term complications. Quality of life as measured by EQ-5D (European Quality of life–5 Dimensions) median was normal (1.0) in those who had not had an SH episode within the past year, but reduced (0.85) in those who had.
Fear affects not only patients, but also the patients’ families. Clarke et al. (1998)54 reported higher fear of hypoglycaemia among mothers of children with T1DM who had lost consciousness due to hypoglycaemia. They were concerned that this might cause harm in two ways: first, that the child’s blood glucose levels might be allowed to run higher than desirable in order to avoid further hypoglycaemia, and, second, that maternal reluctance to allow the child to be separated might hinder normal psychosocial development. Hypoglycaemia can be more difficult to recognise in the under-2-year-olds.
Streisand et al. (2005)55 studied what they called ‘paediatric parenting stress’ among parents of diabetic children aged 9–17 years, most on intensive insulin regimens, and noted that fear of hypoglycaemia played a significant part in raising stress levels. (Parents of the 20% of children on pumps had lower stress levels, but confounding factors must have been operating, and we cannot conclude from this study that CSII reduces stress in parents.)
Hypoglycaemia has three adverse effects:
-
hypoglycaemic episodes themselves
-
fear of recurrence
-
long-term complications, which result from allowing poorer control in order to avoid hypoglycaemia.
Hypoglycaemic unawareness
Hypoglycaemia usually causes symptoms such as hunger, sweating, tremor, palpitations or headache. Some of these are related to the activation of the autonomic nervous system, which releases the hormones adrenaline and noradrenaline into the bloodstream. These warning symptoms alert the patient to the need to take action, such as taking sugar, in order to correct the hypoglycaemia. Unfortunately, in some patients, these warning symptoms do not occur. This is called hypoglycaemic unawareness, which can be partial or complete. A review by Heller (2001)56 noted that as many as one-quarter of patients with T1DM may have partial or total unawareness. In people with diabetes who are aware of impending hypoglycaemia, the nervous system activates and causes warning symptoms at plasma glucose levels of around 3.6 mmol/l, above the level at which cognitive impairment starts (around 3.0 mmol/l). Those with hypoglycaemic unawareness have what Heller hypothesises to be a resetting of the threshold for autonomic nervous system activation, so that the cognitive impairment (drowsiness, inco-ordination, confusion) starts before the warning symptoms do, which may make it impossible for the patients to help themselves. Severe hypoglycaemia is three to six times more common in people with hypoglycaemic unawareness.
The cause is uncertain, but unawareness may be related to the frequency of previous hypoglycaemic episodes. Studies in which people with unawareness were helped to avoid hypoglycaemic episodes for several months, showed that awareness could be restored, with an apparent resetting of the threshold, so that the level at which symptoms returned rose to above the level at which cognitive impairment happens [for review see Heller (2001)56]. Nocturnal hypoglycaemia may contribute to hypoglycaemic unawareness, even when people sleep through the nocturnal hypoglycaemia.
Hypoglycaemia and cognitive impairment in children
The effects of hypoglycaemia
Under normal conditions, the brain is fuelled by glucose. It is well known that acute hypoglycaemia causes transient changes in brain function in diabetic adults, manifesting as neurobehavioural or cognitive changes, particularly in cognitive domains such as attention, information processing and both short- and long-term memory. 57 However, adult brains appear to suffer no obvious harm from moderate hypoglycaemia, and, as reported by the DCCT/EDIC group,58 even severe hypoglycaemia seems to have no long-term cognitive effects.
However, hypoglycaemia may have deleterious effects on the immature and developing brain of young diabetic children, leading to permanent effects on brain function. A review by Gold and Frier (1995)59 noted that:
-
The intelligence quotients (IQs) of diabetic children were lower.
-
Their reading skills were on average lower.
-
Cognitive impairment correlated with the frequency of SH episodes, and perhaps especially with convulsions.
-
The poorest performance was in those with onsets of diabetes under the age of 5 years.
The complexity of the issue is illustrated by research by McCarthy et al. (2003) in Iowa, USA. 60 They examined the academic performance and diabetes control in 244 children with diabetes, aged 8–18 years. Cognitive function was better if control, as reflected by HbA1c level, was better, but they note that this could mean that children with better academic skills were better at controlling their diabetes. Hospital admission, and hence time off school, could be a confounding factor. They noted that a group with good control but hospital admissions because of hypoglycaemia, had poorer academic scores, but this was a small subset (16 patients), making it difficult to draw firm conclusions.
Northam et al. (2001)61 reported that, compared with non-diabetic control subjects, and 6 years after disease onset, 90 children with T1DM onsets, aged 3–11 years performed significantly worse on measures of intelligence, attention, processing speed, long-term memory and executive skills. Some differences were more marked in those with onset of diabetes under the age of 4. Children with a history of hypoglycaemic seizures did worse.
More recently, a study by Dahlquist and Kallen (2007)62 compared the school marks of 5159 Swedish diabetic children compared with a reference population of 1,330,968 non-diabetic children. The mean of all marks obtained at the time of leaving compulsory education at the age of 16 was significantly lower for the diabetic children than for the non-diabetic children (3.15 ± 0.01 versus 3.23, p < 0.001). The maximum possible score is not clear, but may be ‘5’, in which case the difference between diabetic and non-diabetic children is only a few per cent.
The largest difference was in children with onsets under the age of 2 years, but this was not statistically significant and duration would be a confounding factor. In several subjects (mathematics, Swedish, English, sports), the chance of a diabetic child getting high or pass marks was reduced compared with non-diabetic children.
Poor cognitive performance has been suggested to be related to the age of onset of diabetes, the extent of exposure to SH episodes, number of seizures and nocturnal hypoglycaemic episodes. Desrocher and Rovet (2004)63 reviewed a number of studies of cognitive impairment in diabetic children, and noted that problems included:
-
slower motor function
-
visuospatial deficits
-
memory deficits, for example recall of words
-
reduced IQ, by 10–20 points.
However, these deficits were mostly in children diagnosed under the age of 4 or 5 years. Children who were diagnosed over the age of 5 had no IQ deficit. 63
Ferguson et al. (2005)64 reported that IQ and information-processing ability were significantly poorer (p = 0.03 and p = 0.006, respectively) in 26 children who developed diabetes before the age of 7 years compared with 45 children with later-onset diabetes. They also reported structural changes in the brain in some early-onset cases, with a reduction in volume of brain tissue.
Severe hypoglycaemia
To test the hypothesis that repeated severe hypoglycaemia, especially starting at a young age, may be detrimental to spatial memory function, Hershey et al. (2005)65 retrospectively studied a group of 103 individuals with T1DM, aged 6–18 years, who participated in three individual similar studies. Participants were categorised according to the number of SH episodes they had experienced, and according to whether they had their first SH episode before or after the age of 5. They found that, compared with non-diabetics and those with diabetes who had fewer than three episodes of severe hypoglycaemia, having more than three episodes of severe hypoglycaemia was associated with significantly reduced performance in a computerised test of spatial memory (p < 0.01), particularly in those subjects where age of onset of severe hypoglycaemia was < 5 years (p < 0.001). Long-delay (60 seconds) spatial memory, requiring long-term memory and intact medial temporal function was significantly affected, whereas no significant difference was seen in short (5 seconds) delay spatial memory. Mean HbA1c level did not correlate with spatial memory performance. It is difficult to precisely measure the occurrence of SH episode due to the possibility of under-reporting or unrecognised episodes, particularly in younger children. The authors concluded that the developing brain of very young children may be more vulnerable than the brains of older children to the effects of severe hypoglycaemia on longer-term spatial memory.
Older children seem not to be at risk of cognitive impairment after severe hypoglycaemia. Wysocki et al. (2003)66 carried out a trial of intensive versus conventional insulin treatment, in 142 6- to 15-year-old children with T1DM in the USA, achieving follow-up levels of HbA1c of 7.7% and 8.65%, respectively. They prospectively studied the frequency and severity of hypoglycaemia and found that neither the occurrence nor frequency of severe hypoglycaemia was associated with a decline in IQ or measures of cognitive function over an 18-month period. Similar findings were evident for patients who had experienced hypoglycaemic seizures or coma, two pathological situations that could independently affect cognitive function. HbA1c levels were also not associated with change in cognitive function. The authors acknowledged that sensitivity to the effects of severe hypoglycaemia may be greatest among children of 6 years or under who were not included in this study, and that the 18-month study duration may not be long enough to detect any differences that may emerge.
Conclusions
There is evidence of cognitive impairment in diabetic children with the youngest onsets. It is difficult to distinguish the components of the diabetes disease process that might account for this. Early onset of disease, episodes of severe hypoglycaemia, poor control, duration of diabetes, nocturnal hypoglycaemia and seizures are inextricably linked,66 making it difficult to draw conclusions about the relative contributions. If hypoglycaemia in the youngest children can adversely affect cognitive function, a key aim of treatment will be to minimise the incidence of hypoglycaemic episodes.
Hypoglycaemia in T2DM
Although the overall incidence of severe hypoglycaemia is much lower in people with T2DM, there is less difference from T1DM in those with T2DM who are on insulin. Leese et al. ,67 in Tayside, UK (2003), linked an area diabetes register with ambulance call-outs, accident and emergency attendances, and hospital admissions, for all hypoglycaemic episodes requiring National Health Service (NHS) assistance. The incidence of severe hypoglycaemia is shown in Table 1.
| Type of diabetes | Treatment | Incidence per 100 patient-years (95% CI) |
|---|---|---|
| Type 1 | Insulin | 11.5 (9.4 to 13.6) |
| Type 2 | Insulin | 11.8 (9.5 to 14.1) |
| Type 2 | Sulphonylurea tablets | 0.9 (0.6 to 1.3) |
| Type 2 | Metformin or diet | 0.05 (0.01 to 0.2) |
Therefore, treatment rather than type of diabetes determines the incidence of severe hypoglycaemia. The cost per episode was £375 (in 1997–8), spread as follows: ambulance service 31%; accident and emergency 14%; and hospital admissions 55%. These costs do not cover all SH episodes because some would be managed at home by family members.
A later study from Tayside, UK,68 in adults only, recruited a random sample of patients and reported that the incidence of all hypoglycaemic episodes was 0.82 episodes per week in T1DM and 0.33 episodes per week in insulin-treated T2DM. Only 10% of SH episodes in people with T1DM required medical assistance, compared with 33% of such episodes in people with T2DM.
Another, more recent UK study noted that hypoglycaemia was much less common in T2DM, but that was greater in those on insulin, and that it became more frequent over time69 (Table 2).
| Treatment | Mild hypoglycaemic episodes per person-year | Proportion having at least one mild hypoglycaemic episode per year (%) | Severe hypoglycaemic episodes per person-year | Proportion having at least one severe hypoglycaemic episode (%) |
|---|---|---|---|---|
| Tablets | 1.9 | 39 | 0.1 | 7 |
| Insulin for less than 2 years | 4.1 | 51 | 0.1 | 7 |
| Insulin for more than 5 years | 10.0 | 87 | 0.7 | 25 |
The dawn phenomenon
The ‘dawn phenomenon’ is characterised by rapidly rising blood glucose levels over the few hours before breakfast. It is usually caused by the combination of the declining effect of the previous day’s insulin and a circadian rise in growth hormone levels, which make tissues less sensitive to insulin. It can be a problem to manage. If the previous evening dose of insulin is increased, that may cause troublesome hypoglycaemia in the middle of the night. Studies in which insulin infusions have been adjusted to maintain blood glucose at a constant level in people with T1DM have shown that the amount required between 0600 and 0900 is about double the amount needed between 2400 and 0600. 70 Measures to overcome the dawn phenomenon include increasing the previous evening dose of insulin or splitting the evening dose, with SA insulin taken at evening mealtime and intermediate-acting insulin at bedtime. However, both may cause hypoglycaemia during the night, but this is less with the split dose. With CSII, different basal rates can be used, with an increase in the pre-breakfast hours, and the dawn phenomenon can be prevented. 71 In one study, CSII was used only during the night and the incidence of hypoglycaemic episodes was reduced by 32%, although this was carried out before the long-acting analogues were available. 72
Quality of life
In the last HTA report, we sought comments from a number of users of insulin pumps. One point repeatedly made was that CSII made life much more flexible, with pump users being freed from the discipline of fixed mealtimes and activities. Comments are included in Chapter 4 of the last HTA report,50 but included: ‘From my own perspective, the pump has allowed me to lead a full and active life where I control my diabetes rather than it controlling me. I have been able to travel extensively on business and for pleasure without worrying about changing time zones, strange local eating customs, and where/when the next meal might come from’; ‘Freedom, flexibility, pleasure and peace of mind on one’s daily life, almost like being a non-diabetic, compared with the uncertainty of the MDI regime’; and ‘I have experience of both injection (19 years) and insulin pump (6 years) therapy. I find pump therapy to be preferable as it gives me far more control of my insulin input and daily activities. I am now able to live a near normal lifestyle with better control of my disease.’
Interestingly, similar comments are made after DAFNE (Dose Adjustment For Normal Eating – a structured education system) courses. 73 The Diabetes Service in Aberdeen, UK, runs DAFNE courses. A book is kept for comments from participants at the end of each course, and we have seen it. Comments such as ‘I now control my diabetes rather than it controlling me’ are common.
Indications for CSII
From the above sections, we can list possible indications for CSII:
-
to improve control, as reflected in HbA1c, with a view to reducing the risk of long-term complications
-
to reduce problems with hypoglycaemia, in particular for people with hypoglycaemic unawareness, and possibly prevent cognitive impairment in young children
-
to prevent the dawn phenomenon
-
to allow for more flexible lifestyles and activities, and improve non-health-related quality of life.
The 2003 National Institute for Health and Clinical Excellence guidance
Technology Appraisal 57
The TA 57 stated that:74
1.1 CSII is recommended as an option for people with type 1 diabetes provided that:– Multiple-dose insulin (MDI) therapy (including, where appropriate the use of insulin glargine) has failed; and– Those receiving the treatment have the commitment and competence to use the therapy effectively.
1.2 People for whom MDI therapy has failed are considered to be those for whom it has been impossible to maintain a haemoglobin A1c level no greater than 7.5% (or 6.5% in the presence of microalbuminuria or adverse features of the metabolic syndrome) without disabling hypoglycaemia occurring, despite a high level of self care of their diabetes.
1.6 CSII therapy is not recommended for people with type 2 diabetes who require insulin therapy.
The evidence on which the first appraisal of CSII was based consisted of 14 trials in adults with T1DM: four in pregnancy and two in adolescents. There were no published trials in children. A few very short-term trials had been carried out in T2DM but was not considered suitable for inclusion by the Assessment Group because they were mostly of short duration.
The comparator
At the time of the first appraisal the long-acting insulin analogue, glargine, had only recently become available. The other insulin of this type, detemir, was not. The Appraisal Committee had recently considered the use of glargine, and had noted that hypoglycaemia appeared to be less of a problem with glargine than with older basal insulins, such as NPH, because it had a more prolonged action with an almost peak-less profile. 51 The Committee (para. 4.3.6) considered whether MDI therapy using glargine would reduce the need for CSII, but concluded that there would be still be some need for it. The Committee (para. 5.1) recommended that there should be a trial to compare the use of insulin glargine in MDI regimens with CSII, with particular focus on problems of hypoglycaemia and overnight control.
Searches carried out, in 2002, for the assessment report on glargine found 19 studies, but only six had been published in full. 75 Time did not permit us to do a full review to update the evidence base for the long-acting analogues, compared with older insulins, but we carried out a search (May 2007) for studies published since 2002, which compared long-acting analogues with NPH or ultralente. Brief details are given in Table 3.
| First author and year | Analogue | Comparator | Difference in HbA1c level | Difference in hypoglycaemia | Difference in nocturnal hypoglycaemia | Difference in weight |
|---|---|---|---|---|---|---|
| Ashwell (2006)76 | Glargine | NPH | 0.5% lower with glargine | 44% lower with glargine | – | |
| Chatterjee (2007)77 | Glargine | NPH | 0.19% lower | ND | ND | ND |
| De (2005)78 | Detemir | NPH | 0.04% | 32% lower | Lower with detemir | |
| Dixon (2005)79 | Glargine | NPH | ND | Fewer severe | Fewer | BMI ND |
| Fulcher (2005)80 | Glargine | NPH | 0.5% lower | Daytime similar | Fewer | – |
| Hermansen (2004)81 | Detemir | NPH | 0.22% lower | Overall 21% lower | 55% lower | 1 kg lower |
| Hershon (2004)82 | Glargine | NPH | ND | Lower | – | – |
| Home (2004)83 | Detemir | NPH | 0.18% lower | Overall lower | 53% lower | 0.7 kg lower |
| Home (2005)84 | Glargine | NPH | 0.11% lower (NS) | Lower but NS (severe 10.6% vs 15%) | Lower but NS | – |
| Kudva (2005)85 | Glargine | Ultralente | 0.02% lower | Less | ND | – |
| Murphy (2003)86 | Glargine | NPH | 0.4% lower (NS) | ND in symptomatic hypo | 43% lower | |
| Pieber (2005)87 | Detemir | NPH | ND | ND | ND | 1 kg lower |
| Porcellati (2004)88 | Glargine | NPH | 0.4% lower | All hypos halved on glargine | – | |
| Russell-Jones (2004)89 | Detemir | NPH | –0.12% (NS) | 26% lower | 0.54 kg lower | |
| Schober (2002)90 | Glargine | NPH | NSD | SH reduced by 25% (NS) | Severe hypos reduced by 30% (NS) | |
| Standl (2004)91 | Detemir | NPH | ND | RR 0.71 NS | RR 0.7 NS | 1.7 kg lower |
| Vague (2003)92 | Detemir | NPH | ND | 22% lower | 34% lower | Lower |
All of these studies were in patients with T1DM. Dixon et al. (2005)79 recruited children under 6 years of age, whereas Murphy et al. (2003)86 studied adolescents, and Schober et al. (2002)90 included children and adolescents. The other studies were in adults.
Several reviews have been undertaken since the last HTA report on glargine. Mathieu et al. (2004)93 concluded that compared with NPH MDI, detemir reduced the risk of hypoglycaemia, especially nocturnal, and gave equivalent or better levels of glycaemic control. Peterson et al. (2006)94 concluded that both detemir and glargine gave better glycaemic control, with similar or reduced hypoglycaemia. For children, the guidelines group of the International Society for Pediatric and Adolescent Diabetes concluded that the long-acting analogues had reduced day-to-day variability, and that the most marked effect was a reduction in hypoglycaemia. 95
Two analyses by the Center for Outcomes Research (CORE) group estimated that detemir-based MDI was cost-effective in the UK, at a cost of £19,285 per quality-adjusted life-year (QALY) [Palmer et al. (2004),96 sponsored by Novo Nordisk], and in the USA at a cost of US$14,974 [Valentine et al. (2006)]. 97
From the above brief review, and taking into account the NICE guidance on long-acting analogue insulins,51 we conclude that analogue-based MDI is somewhat better than NPH-based MDI in T1DM, and that it should be the comparator for CSII. Indeed, analogue-based MDI is often referred to as ‘the poor man’s pump’. However, analogue insulins provide less flexibility, either a single basal rate over almost 24 hours, or two basal rates if given twice daily, whereas insulin pumps can be set to provide a range of basal insulins at different times of day and night. These can be preset, so that a patient can go to sleep with the pump set to provide different basal rates at different periods during the night.
Analogues in T2DM
The situation may be different in T2DM. A recent Cochrane review on the long-acting analogues versus NPH, concluded that there were no benefits in terms of HbA1c level, no statistically significant reduction in SH episodes [the odds ratios (ORs) of 0.7 and 0.5 for glargine and detemir, respectively, looked promising, but had wide CIs which overlapped with the no difference line], but that both total symptomatic hypoglycaemia and nocturnal hypoglycaemia were reduced. 98
The NICE guidance (NICE 2002, TA 53) on glargine concluded (para. 4.3.9) that the cost-effectiveness of glargine in T2DM was ‘less well established’ because of the lower frequency of hypoglycaemic episodes and hence the more limited scope for improvement. However, the guidance noted that there would be some people with T2DM who could benefit, such as those who had particular problems with hypoglycaemia, and those who would otherwise need twice-daily NPH injections. 51
So in T2DM, the advantages of long-acting analogues are insufficiently proven, given their increased cost, to make them the clear comparator to CSII, and we need to include NPH-based MDI as a comparator.
The last HTA report
The summary of the last HTA report is included as Appendix 1 of this report, for convenience. 50 The Assessment Group concluded:
Control of diabetes consists of more than just control of blood glucose as reflected in glycated haemoglobin. Compared with optimised multiple injection therapy, CSII results in a modest but useful improvement in glycated haemoglobin, but its main value may be in reducing other problems such as hypoglycaemia and the dawn phenomenon, and in improving quality of life by allowing greater flexibility of lifestyle.
The Assessment Group based their primary analysis on RCTs, but noted several points. The first was that some of the RCTs were by then quite old, going back to 1982, and using older forms of insulin. The second was that some trials had used older pumps, now superseded. The third was that most trials reported less hypoglycaemia with CSII than with MDI, but the difference was less than seen in some observational studies. 99–102 The Assessment Group speculated if this was because the trials recruited unselected patients from clinics, whereas the observational studies included people having particular problems, such as hypoglycaemic episodes.
The Assessment Group also noted that most trials did not report quality of life. It obtained information from pump users with the aid of a patient-led support group (INPUT – insulin pump therapy). These pump users reported considerable gains in quality of life, some because of reduction in hypoglycaemia, some because of increased flexibility of life and greater ability to cope with activities of daily life when day-to-day variations occurred. The Assessment Group noted that many of the gains were not in health-related quality of life, but were gains in ‘social’ quality of life, which might not be picked up by the usual utility measures.
Use of CSII in the UK
Reasons for the low use of CSII in the UK were examined in the last HTA report. The low use is ironic, given that the use of insulin pumps was pioneered in the UK, by Keen and Pickup. 14,71 Likely reasons noted in the last HTA report included:
-
Fear of DKA, which had been reported in some early experiences with pumps If a pump fails for any reason, the body has no store of insulin and metabolic disturbance ensues rapidly. However, in the DCCT there was no evidence of an increased risk of DKA in pump users,13 and this has also been the experience of groups with extensive use of CSII in the UK,103 Germany104 and the USA. 105
-
Lack of funding, or competition for funding for other desirable developments at a time when the incidence of T1DM has been rising Anecdotal information following the NICE guidance suggest that some Primary Care Trusts (PCTs) are funding pumps at the lowest level suggested by NICE, of 1% of people with T1DM. NICE estimated that 1–2% of people with T1DM would use CSII (NICE 2003, Guidance 4.3.10). 74
-
Manpower shortage This applies to diabetes specialist nurses (DSNs) in particular.
-
Non-prescribability of pumps and associated consumables, such as the infusion tubing What this means is that either the hospital or the patient has to pay for the pump and the tubing. In some places patients are funding CSII themselves, whereas in other places the NHS is paying.
The submissions from both Diabetes UK and INPUT noted that there were also marked geographical differences in CSII provision in different parts of the UK.
Insulin Pumps Working Group report
The recent report106 of the Insulin Pumps Working Group, issued jointly by the Department of Health (DoH) and Diabetes UK (2007) noted that:
Collating this information, there is a consensus that several countries are now treating about 15–20% of people with type 1 diabetes by CSII (USA, Israel, Germany), and in most of the UK’s European neighbours a substantial proportion (∼10%) of people with type 1 diabetes use insulin pumps for routine management (France, Sweden and The Netherlands). In contrast, overall UK pumps usage is probably no more than 1% of people with type 1 diabetes and in some areas of the country, and in children, it is much less. Thus, the present uptake of CSII in the UK is dramatically lower than in most other countries of comparable economic standing and level of health care provision.
The Insulin Pumps Working Group report was more about how to provide a pumps service than about whether to provide it, or how much to provide – these being more within the remit of NICE. It noted that, despite the 2003 NICE guidance, there was still ‘unacceptable variation in access to CSII across the country’.
It also noted some issues for NICE to address in the review of the guidance, including:
-
lack of clarity of the term ‘failure of MDI’
-
indication in the current guidance that only 1–2% of patients with T1DM were likely to benefit from CSII was thought to be misleading
-
indications in the 2003 guidance were very limited – other possible indications suggested included:
-
– quality of life issues, including the number of injections daily required to achieve control, frequent sick days, marked glycaemic swings or dawn phenomenon, impaired exercise capacity, and difficulties with shift work or travel across time zones
-
– additional issues for children and parents, including school performance, inability to fully integrate into school life, behavioural issues, for example at meal times
-
– pregnancy, including preconception control
-
– hypoglycaemic unawareness
-
– use in people with T2DM
-
– extreme insulin sensitivity.
-
Some of these issues were raised in the ‘Patient perspectives’ chapter of the last HTA report.
Questions for this review
T1DM in adults and adolescents
The first question will be whether evidence has emerged since the last review on the use of CSII in people with T1DM. One issue will be the impact of glargine and detemir. Will hypoglycaemia be less of a problem than in the past, and if so will the need for CSII be reduced? The assessment report for the NICE appraisal of long-acting insulin analogues noted that most trials in T1DM showed no difference in HbA1c level, but that there were fewer hypoglycaemic episodes with glargine. Hence, a key question for this assessment is how CSII compares with ‘best MDI’ with long- and SA analogues in T1DM. In the clinical effectiveness analysis, we will therefore consider separately any trials of analogue-based MDI versus CSII.
New trials of CSII against NPH-based insulin regimens will be briefly reported for completeness, but we note the findings of the last review that CSII is better than NPH-based MDI, and selected meta-analyses are reproduced in Appendix 2.
An issue raised in the last HTA report, and mentioned above, was whether the RCTs might underestimate the gains in routine care. We will therefore examine the results in a number of observational studies. These are more susceptible to bias, but if the effect size is different from that seen in RCTs, then this can be used in a sensitivity analysis in the economic assessment.
Type 2 diabetes mellitus
The current guidance states that CSII is not indicated in T2DM and there is very little use of it. The Insulin Pump Clinical Database data reported only a few people with T2DM amongst well over 300 patients on CSII (R. Feltbower, University of Leeds, 2007, personal communication). The key question is therefore whether new evidence has emerged that supports the use of CSII in T2DM.
Children
Little could be said in the last appraisal on CSII in children because of a lack of evidence. However, a preliminary review of the literature shows that there are now studies of CSII in younger children.
Pregnancy
At the time of the last HTA report, there were a few trials of CSII versus MDI in pregnancy, which found little difference in results. Some trials found HbA1c levels to be lower with CSII, by 0.2% to 1.1%, but the differences were not statistically significant. The question for this review is whether any new evidence has emerged. It should also be noted that HbA1c level is not the usual measure of glycaemic control in pregnancy, because it takes too long to change, so that it cannot be used for making changes in treatment.
Chapter 2 Systematic review of clinical effectiveness
Research questions
There are five sections in this chapter:
-
CSII versus best MDI For the reasons outlined in the previous chapter, the key question was whether CSII is more effective than best MDI. For T1DM, that means MDI with short and long acting analogue insulins. However for T2DM, there is as yet no evidence that analogue-based MDI is superior to NPH-based MDI, and so we include both as comparators. 98 The first part of this chapter (CSII versus best MDI) therefore examines the RCT evidence comparing those two forms of therapy.
-
CSII versus older MDI Studies included in the previous HTA report are not re-visited, but Appendix 2 includes some of the meta-analysis summaries from the previous report. Some new studies comparing CSII with older forms of MDI have been published since the last review. These are of less relevance to our key question but are summarised, for completeness, in the section New studies of CSII against NPH-based MDI in T1DM, below (see Table 6).
-
Pregnancy A specific section on new studies of CSII in pregnancy is included (see Pregnancy and insulin pumps, below).
-
Observational studies The last review noted that some observational studies reported greater benefit than the RCTs, and we speculated that these might be a closer guide to results in routine care. We therefore include a section on recent observational studies (Observational studies reporting data before and after the initiation of CSII).
-
Other evidence This includes:
-
– two studies on the use of CSII at night only
-
– some data from pump users on use of basal insulins
-
– an unpublished meta-analysis by Pickup and Sutton (academic-in-confidence) included in the industry submission (this study has since been published107)
-
– unpublished data on pump use and results from the Insulin Pump Clinical Database (also academic-in-confidence)
-
– data on quality of life aspects of pump use, from Barnard et al. 108
-
– notes on other relevant studies and reviews published since the last appraisal by NICE. 74
-
CSII versus best MDI
The review adopted the methodological approach published by the NHS Centre for Reviews and Dissemination (CRD) (York, UK) Report No. 4. 109 Inclusion criteria are shown in Box 1.
| Intervention | CSII |
| Comparator | Best MDI: SA and long-acting (LA)analogues for T1DM, and SA and LA analogues or NPH for T2DM |
| Population | T1DM and T2DM any age |
| Study design | RCT |
| Outcomes |
Glycaemic control – HbA1c (%) Blood glucose levels and variability Quality of life Hypoglycaemia Insulin dose Weight/BMI |
Search strategy
Sensitive searches of electronic databases were performed in order to retrieve a wide range of different types of evidence and study designs. All bibliographic records retrieved were then manually screened for studies of interest. These included systematic reviews, RCTs, non-randomised trials, observational studies, and studies on economics, costs, quality of life and patient satisfaction.
The following sources were used to identify both published studies and meeting abstracts:
-
MEDLINE, 2002–June 2007; EMBASE, 2002–June 2007; Science Citation Index, 2002–June 2007 (limited to meeting abstracts only); Cochrane Library 2007 Issue 1; contact with experts; reference lists; industry submissions; website of ADA for recent meeting abstracts from the 67th Scientific Session June 22–26 2007 Chicago, IL, USA. Searches were limited to English language only.
-
Ongoing and recently completed studies were searched for using National Research Register 2007 Issue 2 and Current Controlled Trials June 2007.
-
Details of the search strategies used and a flow chart of studies identified for the clinical effectiveness sections are given in Appendix 3.
Identification of studies
Abstracts returned by the search strategy were examined independently by two researchers, and screened for inclusion and exclusion. Full texts of studies considered to be possible inclusions were obtained. Each was examined by two researchers independently.
Data extraction strategy
For each study, two reviewers extracted data regarding study design and characteristics, details of the intervention and patient characteristics and outcomes into a specially designed form. Differences in data extraction were resolved by discussion, referring back to the original papers. Interobserver differences were few. No formal calculation of interobserver agreement was carried out.
Quality assessment strategy
To assess the quality of the RCTs, the following criteria were used: (1) method and description of randomisation; (2) description of attrition/losses to follow-up; (3) specification of eligibility criteria; (4) power calculation; (5) robustness of outcome measurements; (6) similarity of group participants at baseline; and (7) data analysis. Blinding was not used as a quality criterion in this report, as it is not possible to blind patients to the wearing of an insulin pump.
Overall study quality was rated as follows: A (all quality criteria met); B (one or more of the quality criteria only partially met); or C (one or more criteria not met).
Analysis
We would have considered combining results from the trial by meta-analyses had they been sufficiently similar, but this was not considered appropriate.
CSII versus analogue MDI – quantity of research available
Four RCTs comparing CSII with analogue MDI were found in people with T1DM [two full publications, Doyle et al. (2004)110 and Thomas et al. (2007),111 and two abstracts, Maran et al. (2005)112 and Bolli et al. (2004)113].
Four RCTs in T2DM [full publications Berthe et al. (2007),114 Herman et al. (2005),115 Raskin et al. (2003)116 and Wainstein et al. (2005)117] compared CSII with MDI, one using glargine-based MDI and the others using NPH. This is a useful advance from the previous appraisal, when there were no trials of adequate duration in T2DM.
The eight trials were as follows.
Type 1 diabetes
Doyle et al. (2004)110 recruited children and adolescents with T1DM, age range 8–21 years. None had been on glargine or CSII before, and most were on conventional twice-daily insulins. Baseline HbA1c levels ranged from 6.5% to 11%.
Thomas et al. (2007)111 a pilot study, was a three-arm trial in adults with altered hypoglycaemia awareness and debilitating severe hypoglycaemia. One arm was analogue MDI, another was CSII, and the third (not further mentioned in this report) was of education and relaxation of glycaemic targets. None had been on analogues before, 15 (71%) were using human insulin MDI; and five (29%) were using twice-daily biphasic insulin mixtures. Baseline HbA1c level was 8.6%.
Maran et al. (2005)112 was a small trial in 10 adults with T1DM who had been on CSII therapy for at least 6 months. Details are sparse but the aim was presumably to find out whether the advent of glargine-based MDI means that patients on CSII could return to MDI. Mean baseline HbA1c level was 7.7%.
Bolli et al. (2004)113 recruited patients (ages not given) with T1DM naive to CSII and glargine in Italy, the UK and France. Mean baseline HbA1c level was 7.7%. Details of treatment at recruitment were not given.
Type 2 diabetes
Herman et al. (2005)115 recruited people over 60 years in the USA. Mean baseline HbA1c level was 8.25%. They were on at least one injection of insulin per day, with or without oral agents.
Wainstein et al. (2005)117 recruited obese people [body mass index (BMI) 30–45 kg/m2] with T2DM age range 30–70 years, who had not been well controlled on two or more injections per day plus metformin. All had HbA1c level over 8.5%. Insulin dosage before the trial was over 1 unit/kg per day.
Raskin et al. (2003)116 recruited adults over 35 years (mean age 56) with T2DM, on at least one injection of insulin per day, with or without an oral agent. Mean baseline HbA1c level was 8.1%.
Berthe et al. (2007)114 recruited people aged 40–65 years with a BMI of between 26 and 42 kg/m2 and an HbA1c level of ≥ 6.5% on two determinations to a randomised crossover trial. The mean HbA1c level was 9.0%.
Raskin et al. (2003)116 was funded by Novo Nordisk Pharmaceutical Industries (who do not manufacture pumps). Thomas et al. (2007)111 was supported by Sanofi-Aventis and Medtronic. Herman et al. (2005)115 was funded by the ADA. Doyle et al. (2004)110 was funded by NIH (National Institutes of Health) and Juvenile Diabetes Research Foundation (JDRF) with additional support from Medtronic. Berthe et al. (2007)114 was supported by Eli Lilly France. No details were given of funding for the Bolli et al. (2004)113, Maran et al. (2005)112 or Wainstein et al. (2005)117 trials. Table 4 gives further details of these trials.
| Study ID | Study design | Sample size | Intervention | Comparator | Concurrent treatment | Setting | Length of treatment | Any differences in educational input |
|---|---|---|---|---|---|---|---|---|
| Type 1 diabetes | ||||||||
| Doyle (2004)110 | RCT parallel (full publication) | 32 | CSII (aspart) | MDI (lispro and glargine) | None | Single centre, USA | 16 weeks |
CSII patients = 90-minute pump training session and a 45-minute follow-up 2 days later Glargine patients = 45-minute training session for use of insulin pens for pre-meal aspart insulin All other training and education equivalent |
| Thomas (2007)111 | RCT parallel | 21 (14 for MDI vs CSII) | CSII (lispro) | MDI (lispro and glargine) | None | UK | 24 weeks | Equivalent education and support to all was ensured throughout with a single additional training session for those randomised to CSII, confined to technical aspects of pump management |
| Maran (2005)112 | RCT crossover (abstract) | 10 | CSII (lispro) | MDI (lispro and glargine) | Not stated | Not stated, Italy | 4 months | None reported |
| Bolli (2004)113 | RCT parallel (abstract) | 57 | CSII (lispro) | MDI (lispro and glargine) | Not stated | Multicentre, Italy, UK, France | 6 months | None reported |
| Type 2 diabetes | ||||||||
| Herman (2005)115 | RCT parallel (full publication) | 107 | CSII (previous insulin) | MDI (lispro and glargine) | None | Multicentre (2), USA | 12 months | None reported |
| Wainstein (2005)117 | RCT crossover (full publication) analysed as parallel | 40 | CSII lispro | MDI (regular insulin or humulin R and NPH or humulin N) | Diet and metformin | Multicentre (7), Israel | 18 weeks (first treatment period of a 48-week total duration) | None reported |
| Raskin (2003)116 | RCT parallel (full publication) | 132 | CSII aspart | MDI (aspart and NPH) | None | Multicentre (14), USA | 24 weeks | None reported |
| Berthe (2007)114 | RCT crossover (full publication) | 17 | CSII lispro | MDI (lispro and NPH) | None | Multicentre (2), France | 24 weeks (12 weeks on each treatment) | Patients were hospitalised for 24–48 hours at the beginning MDI period for 5 days and at the beginning of the CSII period, in order to receive individual education sessions including pump training sessions |
The issue about differences in educational input, usually not reported in these trials, is because the amount of education is potentially a confounding factor – if the CSII group gets more education, any difference observed may be due to that rather than the CSII. The Berthe trial design gives some concern. 114
Quality of included trials
Internal validity
Sample size
Details of study power were lacking in three of the four RCTs (one full publication and two abstracts) conducted in subjects with T1DM. Thomas et al. (2007)111 stated that as they were doing a pilot study no power calculations were performed. Studies in T1DM ranged in size from 10 participants [Maran et al. (2005)]112 to 57 participants.[Bolli et al. (2004)]. 113 Of the studies conducted in subjects with T2DM, two [Wainstein et al. (2005)117 and Raskin et al. (2003)]116 were appropriately powered for the primary outcome (change in % HbA1c) under consideration. Power calculations were undertaken prior to recruitment in the Herman et al. study,115 but recruitment was terminated early due to the small effect size. Berthe et al. (2007)114 did not mention whether a power calculation was performed. Studies in T2DM ranged in size from 17 in Berthe et al. (2007)114 to 107 in Herman et al. (2005). 115
Randomisation
Doyle et al. (2004)110 was the only study in T1DM that provided details of randomisation. It used a random number table in blocks of four and stratified patients according to sex and age. Block randomisation was also used in the study of Herman et al. (2005),115 whereas Raskin et al. (2003)116 ‘randomised subjects to the lowest randomisation number with each centre to provide a treatment assignment for each centre that was as balanced as possible’; however, no criterion were used to stratify the 132 participants. Wainstein et al. (2005)117 and Berthe et al. (2007)114 provided no details of randomisation.
Similarity of groups at baseline
With the exception of the two studies presented in abstract form (which did not provide details of baseline characteristics of participants), the CSII and MDI groups mostly appear well matched at baseline. Herman et al. (2005)115 noted that there were more men in their CSII group and Berthe et al. (2007)114 (a crossover study) noted that the group 2 patients (MDI then pump) were older by a mean of 7.8 years.
Protocol violations and other problems
Protocol violations were either not described in detail [Thomas et al. (2007),111 Maran et al. (2005)112 and Bolli et al. (2004)]113 or were small in number, i.e. < 5 [Doyle et al. (2004)110 and Wainstein et al. (2005)],117 and therefore unlikely to affect results. In contrast with the other studies, Herman et al. (2005)115 described numerous technical and mechanical delivery problems in the delivery of both CSII and MDI interventions; these may have affected the results. Berthe et al. (2007)114 admitted patients for 24–48 hours at the start of MDI and for 5 days at the start of CSII, for training, which introduces a bias in favour of CSII.
Attrition bias and intention-to-treat analysis
Three studies [Doyle et al. (2004),110 Herman et al. (2005)115 and Wainstein et al. (2005)]117 conducted intention-to-treat (ITT) analysis and there were no obvious differences in dropout rates or reasons for withdrawal between CSII and MDI groups. Thomas et al. (2007)111 had no dropouts. No details of analysis were reported in the two abstracts, [Maran et al. (2005)112 and Bolli et al. (2004)]113 and Raskin et al. (2003)116 conducted only analysis based on 127/132 (96%) of subjects who received treatment.
Detection bias
For practical reasons, none of the trials were blinded. HbA1c is an objectively measured outcome but outcomes such as patient satisfaction may be more susceptible to bias.
Mean versus fluctuations in blood glucose
Glycated haemoglobin (HbA1c) was used as the primary outcome and a measurement of glycaemic control. However, a key limitation of HbA1c measurement is that it does not provide information regarding daily glucose variability. Daily glucose excursions are thought to affect the risk of complications in people with diabetes. All of the RCTs reported HbA1c levels. Additional measurements of mean daily blood glucose, mean amplitude of glucose excursions and 8-point blood glucose profiles were also reported in some studies.
External validity
Most of the trials were carried out in countries other than the UK.
See Appendix 4 for full details of study quality assessment of the trials.
Results
The following outcomes reported in the RCTs are summarised in this section:
-
mean HbA1c (%)
-
blood glucose levels
-
quality of life
-
hypoglycaemia
-
insulin dose
-
weight.
Details of all the trials are given in Appendix 4 – see Table 43 for details of the participant characteristics at baseline.
Mean glycated haemoglobin
Type 1 diabetes
All four trials in people with T1DM compared HbA1c levels at baseline compared with end of study (Table 5). Conflicting results were reported. Doyle et al. (2004)110 in the child and adolescent study, found that subjects on CSII for 16 weeks had a significantly greater reduction in HbA1c than subjects on MDI (1% versus no change: p < 0.05 between groups). In contrast, the other three studies [Maran et al. (2005),112 Bolli et al. (2004)113 and Thomas et al. (2007)]111 reported no significant difference between groups. Doyle et al. (2004)110 also reported that a greater percentage of subjects on CSII (50%) achieved the goal of having HbA1c< 7% by 16 weeks compared with 13% in the MDI group (p < 0.05 between groups).
| Study | HbA1c (%) baseline: means and SDs | HbA1c (%) end: means and SDs | Change from baseline (%) | p-value from baseline | Difference between groups at end(MDI–CSII) (%) | p-value between groups |
|---|---|---|---|---|---|---|
| Type 1 diabetes | ||||||
| Doyle (2004)110 |
CSII 8.2 ± 1.1 MDI 8.1 ± 1.2 |
CSII 7.2 ± 1.0 MDI 8.1 ± 1.2 |
CSII 1.0 MDI no change |
CSII < 0.02 MDI NS |
0.9 | < 0.05 |
| Thomas (2007)111 |
CSII 8.5 ± 1.9 MDI 8.6 ± 1 |
CSII 7.4 ± 1 MDI 7.6 ± 0.8 |
CSII 1.1 MDI 1.0 |
CSII 0.06 MDI 0.04 |
0.2 | Not stated |
| Maran (2005)112 | All 7.7 ± 0.7 |
CSII 7.2 ± 0.2 MDI 7.2 ± 0.2 |
Not clear | Not stated | No change | NS |
| Bolli (2004)113 |
CSII 7.7 ± 0.7 MDI 7.8 ± 0.6 |
CSII 7.0 ± 0.8 MDI 7.2 ± 0.7 |
CSII 0.7 MDI 0.6 |
Not stated | –0.1 (95% CI –0.5 to 0.3) | NS |
| Type 2 diabetes | ||||||
| Berthe (2007)114 |
CSII 9.0 ± 1.6 MDI 9.0 ± 1.6 |
CSII 7.7 ± 0.8 MDI 8.6 ± 1.6 |
CSII 1.3 MDI 0.4 |
Not stated | 0.9 | 0.03 |
| Herman (2005)115 |
CSII 8.4 ± 1.1 MDI 8.1 ± 1.2 |
CSII 6.6 ± 0.8 MDI 6.4 ± 0.8 |
CSII 1.7 ± 1.0 MDI 1.6 ± 1.2 |
CSII 0.0001 MDI 0.0001 |
0.1 | Not stated |
| Wainstein (2005)117 |
CSII 10.2 ± 1.4 MDI 10.3 ± 1.2 |
CSII 7.9 ± 1.0 MDI 8.4 ± 1.3 |
CSII 2.3 MDI 1.9 |
CSII 0.01 MDI 0.01 |
0.5 | NS |
| Raskin (2003)116 |
CSII 8.2 ± 1.4 MDI 8.0 ± 1.1 |
CSII 7.6 ± 1.22 MDI 7.5 ± 1.17 |
CSII 0.62 ± 1.11 MDI 0.46 ± 0.89 |
CSII 0.05 MDI 0.05 |
0.1 | NS |
Type 2 diabetes
Four trials in people with T2DM measured HbA1c levels as the primary outcome of interest. Three were parallel trials, and Berthe et al. (2007)114 was a crossover trial. All trials compared HbA1c levels at baseline compared with end of study. Herman et al. (2005)115 did not compare differences between CSII and MDI but did report that the level of HbA1c was significantly reduced from baseline to end of study in both groups (p < 0.0001). Berthe et al. (2007)114 reported that HbA1c levels decreased significantly more in patients at the end of the CSII period than at the end of MDI period. The other two studies [Wainstein et al. (2005)117 and Raskin et al. (2003)]116 reported no significant difference between groups, although both CSII and MDI reduced HbA1c levels significantly between baseline and end of study (p < 0.05).
Therefore, only two trials showed a statistically significant lower HbA1c level with CSII: Doyle et al. (2004)110 in T1DM and Berthe et al. (2007)114 in T2DM. Only two trials reported the proportions reaching targets. Doyle et al. (2004)110 noted that only three subjects met the ADA target of HbA1c < 7% at baseline; by 16 weeks, 8 out of the 16 in the CSII group and 2 out of the 16 in the MDI group had met the target.
Mean blood glucose levels
Mean daily blood glucose level was measured in all four RCTs in T1DM. Bolli et al. (2004)113 reported no significant difference between groups in daily blood glucose levels. Doyle et al. (2004)110 reported that fasting levels were the same on CSII and MDI but that mealtime levels were lower on CSII. Maran et al. (2005)112 reported lower mean glucose levels in CSII (147 mg/dl) than with MDI (189 mg/dl) (p < 0.03).
Thomas et al. (2007)111 reported median glucose levels and the results of CGMS for MDI and CSII groups at 6 months. Daytime median glucoses were similar at 7.6 and 7.8 mmol/l. Night-time median glucose was higher, with CSII at 8.4 mmol/l, but mainly because the MDI group spent, on average, 15% of time with hypoglycaemia (under 2.5 mmol/l), whereas the CSII group had very little hypoglycaemia, with 0.6% of glucose readings under 2.5 mmol/l. Neither group showed a significant change over the 24 weeks.
Glucose variability
Two studies [Bolli et al. (2004)113 and Maran et al. (2005)]112 reported measures of glucose variability. Bolli et al. (2004)113 reported no significant difference between groups in mean amplitude of glycaemic excursions (MAGE) at baseline and 6 months (CSII baseline 8 ± 2.4 to 6 months 6.4 ± 2.2 versus MDI baseline 7.6 ± 1.7 to 6 months 6.4 ± 2.1). Eight-point blood glucose profiles (coefficient of variation) measurements also revealed no significant differences between treatments at baseline and end point.
Maran et al. (2005)112 reported glucose variability as area under the curve (AUC) > 10 mmol/l, time spent at glucose level > 3.6 mmol/l and < 10 mmol/l, and time spent during night-time hours in glucose range > 3.6 mmol/l and < 10 mmol/l. There was significantly less hyperglycaemia during CSII treatment than with MDI (AUC > 10mmol/l CSII end of study 9603 ± 3941 minutes versus MDI 26445 ± 9390 minutes; p < 0.02 between groups). By the end of the study, significantly more time was spent in the glucose range > 3.6 mmol/l and < 10 mmol/l in the CSII group compared with MDI (CSII 1582 ± 212 minutes versus MDI 769 ± 158 minutes, p < 0.02 between groups). Similar results were reported for night-time glucose (CSII 298 ± 63 minutes versus MDI 194 ± 51 minutes; p < 0.02). These results do not quite fit with the lack of difference in HbA1c, unless the MDI group had much wider variation around the mean.
Berthe et al. (2007)114 used continuous blood glucose monitoring devices, and produced glucose profiles against a target of keeping glucose in the range 3.3–10 mmol/l. The target was achieved for 44% of the time on conventional insulin therapy, for 54% on MDI and for 77% on CSII (p = 0.0095 for CSII versus MDI).
Quality of life
Two studies [Doyle et al. (2004)110 and Thomas et al. (2007)]111 in people with T1DM reported quality of life outcomes. Neither study showed a significant difference in quality of life between CSII and MDI [measured using the Diabetes Quality of Life (DQoL) questionnaire]. In people with T2DM, Herman et al. (2005)115 reported no significant difference in quality of life as measured by Short Form-36 (SF-36) and the Diabetes Quality of Life Clinical Trial Questionnaire (DQoLCTQ). However, Raskin et al. (2003)116 reported a significant improvement in treatment satisfaction in CSII group compared with MDI (p < 0.001).
See Appendix 4, Table 44, for more details of quality of life and patient satisfaction outcomes in the trials.
Hypoglycaemia
All eight RCTs reported the occurrence of hypoglycaemia. 110–116 Only two RCTs in people with T1DM [Doyle et al. (2004)110 and Maran et al. (2005)]112 conducted a statistical analysis of the occurrence of hypoglycaemic episodes. Doyle et al. (2004)110 reported that significantly fewer subjects with T1DM on CSII had severe hypoglycaemia episodes by end of study compared with MDI (CSII two episodes versus MDI five episodes in four patients; p < 0.05 between groups). In contrast, Maran et al. (2005)112 (the smallest trial) reported no significant difference in hypoglycaemic reaction exposure between groups.
Bolli et al. (2004)113 commented that SH episodes were too infrequent to allow meaningful comparison. The frequency of confirmed hypoglycaemic events/patients where blood glucose fell below 4 mmol/l was similar in both groups at 6 months (CSII – 41 events in 28 people versus MDI – 35 events in 29 people). There were only two SH events so no comparison of that was possible.
Thomas et al. (2007),111 with 21 patients followed for 24 weeks, reported that non-significant trends towards reduced incidence of severe and mild symptomatic hypoglycaemia were seen in the MDI and CSII groups in comparison with the third arm, the Education Group, but no difference between MDI and CSII. This may have been due to the very small numbers involved.
None of the four RCTs in people with T2DM reported a significant difference in hypoglycaemic episodes.
See Table 45 in Appendix 4 for more details of adverse events in each of the trials.
Insulin dose
Doyle et al. (2004)110 reported an insignificant difference – 0.6 units/kg per day on CSII and 0.7 units/kg per day on MDI. Thomas et al. (2007)111 reported the daily insulin dose at zero and 24 weeks. There was a non-significant increase in the MDI group and a significant (p = 0.01) decrease in the CSII group. Both groups started on 0.7 units/kg per day. The MDI group ended at 24 weeks on 0.8 units/kg per day and the CSII on 0.4 units/kg per day. They did not test the statistical significance between MDI and CSII. The other two T1DM studies, both abstracts only, gave no results. In T2DM, no differences were found. Wainstein et al. (2005)117 noted a drop in the CSII group in the first period but it did not persist.
Weight
The only T1DM study that reported changes in weight was Thomas et al. (2007)111 where both CSII and MDI showed a non-significant change. All the T2DM studies reported that there were no significant differences in weight changes between CSII and MDI.
Summary
Type 1 diabetes
In the last guidance, NICE commented on the need for trials of CSII against analogue-based MDI in T1DM. Unfortunately, few trials have been done, most are very small, and only two have been published in full, one of which was only a pilot.
One trial [Doyle et al. (2004)]110 reports that HbA1c level is significantly lower for CSII than on analogue-based MDI in children and adolescents. The other studies in adults report no differences in HbA1c level.
Type 2 diabetes
In T2DM, there was little evidence that CSII was better than analogue-based MDI. In one study, a clinically significant difference in HbA1c level was reported, but it failed to reach statistical significance.
The Berthe et al. (2007)114 trial showed that CSII was better than NPH-based MDI.
New studies of CSII against NPH-based MDI in T1DM
The last HTA report50 included a meta-analysis (reproduced in Appendix 2) that showed that CSII gave a mean HbA1c level of about 0.6% lower than MDI in T1DM. Most of the studies used regular soluble insulin in the pumps, and a switch to a SA analogue would give a further reduction of about 0.26%. Most of the basal insulin in those trials was NPH.
Table 6 below gives brief details of trials of CSII against NPH-based MDI in T1DM, published since the last review. Some of the studies are small. Some show no differences, or differences that are not statistically significant. Those which do show significant differences favour CSII, and are thus in line with the last HTA report.
| Randomised trials – pump vs MDI with NPH in T1DM | |||
|---|---|---|---|
| First author, year, type of study | Study population, duration of study, type of insulin | Results | Summary |
| Cohen (2003),118 randomised crossover trial |
N = 16 adolescents, aged 14–18 years, with T1DM of at least 2 years. Duration: 6 months for each treatment Pump: Lispro MDI: NPH and regular insulin |
HbA1c (change from baseline): NS Pump: –0.43% MDI: +0.09% Severe hypoglycaemia (total number of events): NS Pump: 1 MDI: 4 |
HbA1c 0.52% lower with CSII and fewer hypos, but neither statistically significant, probably due to small numbers |
| DeVries (2002),119 randomised, parallel group trial |
N = 79 adults, aged 18–70 years with T1DM Duration: 16 weeks Pump: Aspart MDI: NPH and aspart |
HbA1c (change from baseline and SDs): Pump: –0.91 ± 1.28% MDI: –0.07 ± 0.70% Difference: 0.84% (–1.31 to –0.36), p = 0.002 Severe hypoglycaemia (total number of events): NS Pump: 3 MDI: 6 |
HbA1c 0.84% lower on CSII Difference in severe hypos NS |
| DiMeglio (2004),120 randomised, parallel group trial |
N = 42 children, < 5 years, with T1DM of at least 12 months Duration: 6 months Pump: Lispro MDI: maintained prestudy insulin regimens (NPH = 10, insulin IZS = 2, glargine = 1) |
HbA1c (change from baseline): NS Pump: –0.43% MDI: –0.09% Severe hypoglycaemia (total number of events): NS Pump: 1 Current therapy: 1 |
HbA1c 0.34% lower with CSII |
| Fox (2005),121 randomised, parallel group trial |
N = 26 children, aged 1–6 years with T1DM of at least 6 months Duration: 6 months Pump: NA MDI: NPH |
HbA1c (change from baseline): NS Pump: –0.19% Current therapy: –0.11% Severe hypoglycaemia (total number of events): NS Pump: 0 Current therapy: 1 |
No difference |
| Hoogma (2006),122 randomised controlled crossover trial |
N = 272 adults, aged 18–65 years with T1DM on MDI for at least 6 months Duration: 16 months Pump: Lispro MDI: NPH and lispro |
HbA1c (end of treatment): baseline from graph – CSII 7.66%, MDI 7.61% Pump: 7.45% MDI: 7.67% p < 0.001 CSII vs MDI Severe hypoglycaemia (events per patient-year): p < 0.001 CSII: 0.2 MDI: 0.5 |
Fewer hypos with CSII |
| Pozzilli (2003),123 randomised pilot study |
N = 23 patients, aged 12–35 years with T1DM, duration since beginning insulin therapy < 4 weeks Duration: 2 years Pump: Lispro MDI: NPH |
HbA1c (end of treatment, SEM): NS Pump: 6.3 ± 0.5% ISIT: 6.2 ± 0.5% Hypoglycaemic episodes: NS. Data not given |
No difference |
| Weintrob (2003)124 and (2004),125 randomised crossover trial |
N = 23 patients, aged 8–14 years, treated with insulin for at least 2 years Duration: 3.5 months for each treatment Pump: Lispro MDI: NPH and regular insulin |
HbA1c (change from baseline): NS Pump: 0.03 ± 1.0 (SD) MDI: –0.23 ± 1.0 Difference: 0.26% NS Severe hypoglycaemia (number of episodes per patient-year): NS Pump: 0.13 (0.0–0.4) MDI: 0.39 (0.0–0.84) |
No differences |
| Wilson (2005)126 |
N = 19 patients aged < 6 years with T1DM for at least 6 months Duration: 1 year Pump: six subjects (66%) used lispro MDI: At baseline, three (16%) subjects were using glargine, five (26%) were using ultralente, and 15 were using NPH (some subjects received both NPH and ultralente or glargine). Over the course of the study, the percentage of MDI subjects using glargine increased from 10% to 60% (p < 0.05) |
HbA1c (change from baseline): Pump: –0.21 ± 0.67% (SD) MDI: 0.04 ± 0.71 Difference: NS Severe hypoglycaemia Pump: one episode MDI: one episode |
No difference |
Summary
In terms of HbA1c levels, three of the new studies show no difference between MDI and CSII;121,123,126 four show differences (0.52%, 0.34%, 0.25% and 0.26%) that are not significant,118,120,124,126 and one shows a larger and statistically significant difference of 0.84%. The lack of statistical significance may sometimes be due to small numbers – the Cohen et al. (2003)118 study reported what would be seen as a clinically useful difference (0.52%), but had only 16 patients.
Pregnancy and insulin pumps
Pregnancy results in an increased metabolic demand on the body, and presents a challenge to both diabetologists and pregnant women with diabetes in maintaining glucose control to prevent poor outcomes. Inadequate control, episodes of hypoglycaemia and ketoacidosis can all have detrimental effects both on the mother and the developing fetus.
Diabetic pregnancies comprise three groups of women; those with T1DM or T2DM prepregnancy, and those with gestational diabetes, which refers to a temporary form of diabetes that comes on in pregnancy and goes away after birth. In some women, good control can be achieved with diet and exercise, but many require insulin to maintain adequate glycaemic control. Oral therapy with glibenclamide has also been used in recent years in the USA. Clinical guidelines recommend the optimal HbA1c level for pregnant women with diabetes to be between 4 and 7 mmol/l. 127,128 (Although, in practice, diabetes control in pregnancy is monitored by home-testing of blood glucose because HbA1c changes too slowly for fine tuning of insulin dose.)
Maintaining good glycaemic control is very important in pregnant women with diabetes as, compared with pregnant women in the general population, diabetic women have a higher rate of morbidity, miscarriage and stillbirth, and their babies have a higher rate of congenital malformations. 129,130 A comprehensive picture of the outcomes associated with diabetic pregnancies is discussed in the 2007 Confidential Enquiry into Maternal and Child Health report. 131
Diabetes in pregnancy requires regular maternal and fetal monitoring to ensure the best possible outcome for mother and child. Current care pathways for women with diabetes in pregnancy advocate that, ideally, all women with diabetes should be offered prepregnancy advice to achieve optimal control of diabetes (HbA1c < 7%) at least 3 months before conception. This reduces the congenital malformation rate. However, in practice many pregnancies are unplanned so achieving glycaemic control often becomes a postconception goal.
The standard treatment of diabetes in pregnancy in the UK uses the MDI regimen to deliver insulin on a frequent, self-regulated basis. The increased requirement for close monitoring of glucose levels to prevent maternal and fetal compromise has resulted in some Trusts offering pregnant women with diabetes the option of using insulin pumps.
The assessment report for the first NICE appraisal on CSII noted four RCTs of CSII versus MDI in pregnancy. 50 These showed that HbA1c was lower on CSII than MDI, but not statistically significantly so. Differences ranged from 0.2% to 1.1% lower. It concluded that there was then insufficient evidence that CSII is better than MDI in pregnancy. Since then, there seem to have been no further RCTs, but only a few observational studies, described here for completeness, although the usual caveats about observational studies should apply.
Two studies published in full [Lapolla et al. (2003)132 and Gimenez et al. (2007)]133 have retrospectively compared the outcomes of pregnancies of women with T1DM who were treated with either MDI or CSII using matched control study design.
Lapolla et al. (2003)132 reported no significant difference in HbA1c control between CSII (n = 25) and MDI (n = 68) both before and during pregnancy, although both groups progressively reduced their HbA1c levels from first to third trimester (HbA1c: CSII trimester 1, 7.4 ± 2.0; trimester 3, 6.4 ± 1.2 – p < 0.05; MDI trimester 1, 7.1 ± 1.3; trimester 3, 6.3 ± 1.0 – p < 0.05). No significant differences were reported between groups in rate of maternal (e.g. hypertension, pre-eclampsia) or fetal complications (e.g. congenital malformations). The authors noted that, compared with those who received MDI, the CSII group had diabetes for a significantly longer duration (CSII 16.0 ± 7.9 years versus MDI 11.6 ± 8.8 – p < 0.04 between groups), a significantly greater percentage had a planned pregnancy (CSII 52% versus MDI 25% – p < 0.026 between groups), and significantly more women in the CSII group were White’s class D (p < 0.02 between groups) and significantly less were White’s class B (p < 0.059 between groups). The authors concluded that CSII ‘may be used both before and during pregnancy in more complicated patients in whom conventional intensive insulin treatment fails to achieve good metabolic control’.
Similar results were reported by Gimenez et al. (2007)133 in 58 women with T1DM who received either CSII or MDI. No significant differences in glycaemic control, maternal or fetal outcomes were reported between groups.
Four additional studies (one cohort study, two case series and a matched control study) published in abstract format compared the efficacy of insulin pumps in pregnant women with T1DM. 134–137
Chen et al. (2006)134 compared 30 pregnant women with T1DM treated with CSII with 60 matched control subjects treated with MDI. No between-group differences were reported in maternal age, nulliparity rate, severity of diabetes, prepregnancy BMI and weight gain during pregnancy; however, the rate of DKA (CSII 13% versus MDI 2%, p = 0.04) and neonatal hypoglycaemia (CSII 35% versus 13%; p = 0.01) were significantly higher in the CSII group. There were no other reported differences in pregnancy outcome between groups.
Cheng et al. (2006)135 evaluated a cohort of 688 women with T1DM and compared those managed with CSII (n = 60) and MDI (n = 628). The CSII groups had significantly lower mean HbA1c levels than the MDI group (CSII 6.7% versus 7.7%; p < 0.001 between groups) and were significantly more likely to have an HbA1c level < 6% (CSII 25% versus 12.6%, adjusted OR 3.37 95% CI 1.08 to 10.5), although it is unclear at what period of the pregnancy the measurements were taken. The small number of women on CSII suggests that selection biases were operating and the results should be discounted.
Kinsley et al. (2005)136 evaluated 43 pregnant women with T1DM treated with CSII (n = 7), soluble insulin (n = 18) or analogue (n = 18). Firm conclusions regarding glucose control could not be drawn from this study as HbA1c in the CSII group was lower at baseline than in the other groups; however, the percentage of mean blood glucose readings < 2.0 mmol/l was higher in CSII than the other treatment groups at 14, 26 and 36 weeks (no statistical analysis provided). Total insulin dose was significantly lower in the CSII group than for other treatments at 14 weeks and 26 weeks (p < 0.05 between groups). No significant differences in maternal weight or birth weight were reported.
Jimenez et al. (2005)137 reported a case series of 36 pregnant women with T1DM on CSII over a 6-year period. Compared with 169 women treated with MDI who had slightly lower baseline mean BMI there were no significant differences in glycaemic control, maternal outcomes or perinatal outcomes.
In summary, most studies conclude that CSII achieves similar glycaemic control to MDI regimens in pregnant women with T1DM. Maternal and fetal outcomes are similar between treatments. One study reported more DKA with CSII and another more hypoglycaemia. As CSII gives no added benefit over MDI, but is more costly, this implies that it will not be cost-effective in diabetic pregnancies.
Observational studies reporting data before and after the initiation of CSII
Caveats
For assessing efficacy, RCTs are the gold standard. Observational studies usually provide poorer quality evidence than RCTs because there is a much greater risk of bias. For example, good results may be obtained because the recruits adhere better to therapy than most patients. Publication bias may be a problem, in that negative observational studies may be less likely to be published than positive ones. For that reason, observational studies are usually excluded from technology assessment reports (TARs) and Cochrane reviews. However, in the last TAR on CSII, we noted that the reduction in hypoglycaemia was greater in some observational studies than in the RCTs. 50 We speculated that this might be because trials were unselective in their recruitment, whereas observational studies might selectively recruit people having particular problems. If so, it is possible that the observational studies will be a better guide to results in routine care. They may also be of longer duration and hence provide useful data on discontinuation rates and side effects. It is also possible that patients will not become fully expert in pump use in short duration trials, in which case long-term follow-up might show better results. Some of the studies may give useful data on the training requirements for people starting CSII.
In this section, we give results from a group of observational studies. Some were comparisons of MDI and CSII, in matched groups or unmatched groups. Because of possible biases, and because we have evidence from RCTs, we have not used the comparative data, but have used only the CSII arms as case series. Nor have we attempted to be comprehensive.
Study characteristics
Details of 48 observational studies are given in Tables 7–9, for the different age groups. Twenty studies included adults (either adults only or mixed ages), 23 studies included children/adolescents and five studies included young children (aged ≤ 7 years).
| First author, year, country, study type | Age group, sample size | Reasons for starting on pump | Duration of follow-up | Notes – including selection criteria and training (where reported) |
|---|---|---|---|---|
| Bruttomesso (2006),141 Italy, survey | Mean age 39 years (range 4–85), n = 2702 | Main reason – poor metabolic control under intensified insulin treatment. Other reasons included desire for pregnancy, wish for more flexible lifestyle, correction of dawn phenomenon, reduction in hypo emergencies and improve quality of life; in very few cases CSII was started at patient’s request or for the presence of low insulin requirements (no percentage given) | Mean 3.9 years for adults and 2.4 years for children | Survey of 145 clinics |
| Cersosimo (2002),169 [abstract], USA, before/after study comparing CSII and MDI | Mean age 37 years, n = 35 | NR | 2 years | Multidisciplinary comprehensive education programme patients were offered to intensify glycaemic control using either pump or multiple injections |
| D’Annunzio (2005),144 [abstract], Italy, before/after study | Aged 15–29 years, n = 15 | Suggested to highly motivated patients with brittle disease (n = 8), hypoglycaemic unawareness (n = 1), pregnancy (n = 4) or need for more flexible lifestyle (n = 2) | 18 months | Training period about CSII management, both in normal conditions or during physical activity or intercurrent illnesses; a strict self-monitoring of diabetes was mandatory |
| Fahlen (2005),161 Sweden, before/after study comparing CSII and glargine using retrospective data | Mean age 40.8 (SD 12) years, n = 563 | Patients were generally selected for the therapies due to persistently high HbA1c or unstable blood glucose values, despite prolonged efforts to improve glycaemia | Median 25 months | Patients using multiple dose injections were included prior to starting on either CSII or glargine. The basic education in MDI was the same for both groups; however, the use of the pump is an additional educational tool. Initially, the visits of patients starting on CSII were more frequent, but there was no difference in the time interval between visits after 6 months |
| Garg (2004),175 USA, retrospective controlled comparison of pump vs glargine using electronic database parameters | NR | Mean 11.6 months | Training: NR | |
| Hunger-Dathe (2003),170 Germany, case series/audit | Mean 36 years (range 11–71), n = 250 | NR | Mean 1 year | Patients who participated in a structured treatment and teaching programme |
| Jankovec (2005),148 [abstract], Czech Republic, before/after study using retrospective data from national register | Mean age 38 years, all adults, n = 1051 | Poor glycaemic control (69.5%), diabetic neuropathy (22.8%) and repeated hypos (21.2%) | Mean 4.71 years | Data from national register |
| Lepore (2004),162 Italy, parallel group study | Mean age 38 years, n = 24 | Poor metabolic control (HbA1c > 8% in previous year) | 1 year | All patients had been treated with MDI (regular or lispro insulin before each meal plus NPH as basal insulin) for at least 1 year before the study. One arm of controlled clinical trial. Patients received instructions on the use of insulin infusion pumps in an outpatient basis |
| Linkeschova (2002),145 Germany, before/after study | Mean age 33 years (SD 11) (range 17–66), n = 103 | Optimisation of metabolic control and improvement of flexibility of life style in 60 patients, and prevention of severe hypoglycaemia in 43 patients | Mean 1.8 (SD 1.2) years | Prior to CSII, all patients had been on conventional intensified insulin therapy with 2 injections of NPH insulin (in the morning and at bedtime) and preprandial injections of regular human insulin |
| Nimri (2006),147 Israel, before/after study using patient file data |
Prepubertal: (median age 5.4, range 1.6–8.6 years), n = 23 Adolescent: (median age 13.7, range 9–17 years), n = 127 Young adult (median age 22.8, range 17–40 years), n = 129 Total N = 279 |
Poor glycaemic control, recurrent hypoglycaemic episodes, and patient preference | Mean 2.4 (SD 1.8) years, range 1–6 years | Only patients < 40 years included in the study. Pump therapy preceded by a training programme for the patients and their parents. Programme consisted of 3 sessions. It covered principles and mechanics of pump therapy, insertion-site care, carbohydrate counting, and insulin bolus dosing |
| Norgaard (2003),142 Denmark, survey and data collection from patient records | Mean age 48 years, n = 142 | Data for 117 patients: participation in study (24%); poor control and complications of diabetes (22%); poor control but no diabetes complications (18%); motivated to CSII (11%); prepregnancy (10%); other/no data (15%) | Mean 13 years | Survey of endocrinology departments to determine the attitudes of chief consultants to CSII. Data collection from CSII records |
| Pickup (2005),138 UK, before/after study | Mean age 39 years (SD 9.9), n = 27 | Disabling hypoglycaemia during intensive injection treatment and whose glycaemic control was unaltered by a median 5 months’ renewed MDI | Median 17 months |
After initial consultation, patients were entered into a pump assessment programme attempting to optimise glycaemic control with MDI. Those in whom glycaemic control did not improve on reoptimised multiple injections and were otherwise suitable for pump therapy were treated with CSII. Patients initially referred but considered unsuitable for pump therapy because of poor compliance, psychological problems or improvement on optimised injections (n = 3) were not offered a trial of insulin pump therapy Dietary instruction included carbohydrate counting and lifestyle advice, including advice diet, exercise and alcohol, and was essentially identical for the CSII and MDI phases of the study |
| Pickup (2006),139 UK, before/after study | Mean age 41.6 years (SD 11), n = 30 | Failed to achieve good control on MDI | 16 months | All subjects were already receiving MDI. Renewed attempts to improve control on MDI were made for a median of 5 months. Programme included frequent contact with a diabetes specialist nurse and dietitian. After switching to CSII, patients were seen at a hospital clinic for review at 2, 6, 11 and 16 months after the start of pump therapy and between times maintained regular telephone contact with the specialist nurse |
| Radermecker (20050,143 [abstract], Belgium, retrospective analysis of patient medical files | Mean age 43 years (all ages), n = 95 | Poor glycaemic control with HbA1c > 8% (n = 50), ongoing or programmed pregnancies (n = 28), recurrent hypos (n = 16), allergy to insulin (n = 1) | Mean 5.1 years; range < 5 years to > 10 years | NR |
| Reda (2007),146 New Zealand, retrospective audit | Mean age 33 years (range 6.5–66.2), n = 105 followed up out of 125 starting | All patients had two or more of the following reasons: optimisation of metabolic control (n = 25); prevention of severe hypos (n = 63); increase flexibility of lifestyle (n = 70); recurrent DKA (n = 9); poor overnight glycaemic control (n = 66) | Mean 3 years | Prior to CSII, all patients had been on MDI therapy. Pump users given intensive instructions in carbohydrate assessment, provision of correction factors and support in insulin self-adjustment |
| Rodrigues (2005),140 UK, retrospective before/after study | Mean age 33 years (range 10–62), n = 40 | Recurrent severe hypos (n = 14); own choice (n = 15); recurrent DKA (n = 5), erratic lifestyle (n = 2), gastroparesis (n = 1), pregnancy (n = 1), poor control (n = 1) | Median 20.5 months CSII (range 1–192 months) | Reviewed prior to starting CSII by liaison psychiatrist and ongoing support was available. In addition to re-education in diabetes care provided during the institution of CSII, this group was not only encouraged to use the 24-hour advice line available to them, but was contacted by the team if expected communication did not happen |
| Ronsin (2005),176 France, retrospective review of medical files | Mean age 35 years (range 15–67), n = 70 | HbA1c > 8% (n = 39) (56%); recurrent hypos (n = 1)2 (17%); planned pregnancy/during pregnancy (n = 12) (17%); planned implantation (n = 3) (4%); lipodystrophy: (n = 2) (3%); patient’s decision (n = 2) (3%) | Maximum of 2 years | At least 40% had diabetes-related medical problems. |
| Rudolph (2002),101 USA, retrospective review of medical records | Mean age 36 years (SD 10.4), n = 107 | NR | Mean 36.1 (SD 25.5 months), median 26.2 months | Review of medical records. Patients were included in the analysis if they had used an insulin pump before 31 December 1999 and had more than two follow-up visits for collection of clinical data. CSII is initiated only in patients who are willing and able to measure blood glucose levels a minimum of 4 times daily, who understand carbohydrate counting, and who comprehend how to alter insulin dose on the basis of food intake and anticipated exercise. Patients also required to attend a class on insulin pump therapy |
| Siegel-Czarkowski (2004),163 [letter], USA, retrospective before and after by chart review | Older adults (aged > 50 years), n = 34 | NR | 1 year | Patients were ‘carefully selected’. Those unwilling or unable to master the technological and other features of CSII were not included |
| Sucunza (2005),177 [abstract], Spain before/after study | Adults (mean age 35 years (range 19–73), n = 172 | NR | 2 years | Patients were consecutively started on CSII and visited individually to evaluate their pump management skills |
| First author, year, country, study type | Age group, sample size | Reasons for starting on pump | Duration of follow-up | Notes – including selection criteria and training (where reported) |
|---|---|---|---|---|
| Alemzadeh (2004),149 USA, matched before/after study |
Mean age 14.7 years (range 10.1–17.8) n = 40 |
To achieve optimal glycaemic control, to reduce hypoglycaemic events, and to provide a more flexible lifestyle by allowing variable mealtime insulin dosing | 1 year | Before initiation of CSII, patients underwent an extensive diabetes care skills and psychosocial screening to minimise non-adherence on insulin pump therapy |
| Ahern (2002),166 USA, before/after study |
Mean age 10.3 years (range 1.5–18) Preschool (1–6 years): n = 26 School age (7–11 years): n = 76 Adolescent (12–28 years): n = 59 Total n = 161 |
Offered CSII if motivated, measuring blood glucose ≥ 4 times per day or if repeated hypos | Mean 32 months (range 19–57) | Frequent telephone contacts over the first 2–3 days. Training in pump and diabetes, nurse on call 24 hours per day |
| Conrad (2002),157 USA, before/after study |
Mean age 11 years Prepubertal n = 23 Pubertal n = 42 Total N = 65 |
Patient preference, recurrent hypos, unawareness of hypos, erratic swings in blood glucose, strong dawn phenomenon, aspects of quality if life including desire for increased flexibility with meals and activity | 6 months |
Participants had to show adequate skills All children wore an insulin pump infusion set before initiation of CSII and some also wore a ‘dummy pump’, education and support |
| Garcia-Garcia (2007),167 Spain, controlled trial |
Mean age 11.6 years n = 8 |
Interest in improving control, indication for a change in therapy, HbA1c > 7.5% (> 8% in prepubertal children) or frequent episodes of hypoglycaemia | 2 years |
T1DM diagnosed before age 14 years, at least 2 years’ duration and follow-up, daily insulin requirement more than 0.75 units/kg, previous intensive treatment with more than four glycaemic analyses per day, good parental supervision and good relationship with the team (endocrinologist and nurse) Medical and nurse assistance and treatment goals were the same in CSII and MDI patients |
| Hanas (2006),150 Sweden, cross-sectional and longitudinal studies using data collected retrospectively from medical records |
Age range 7–21 years n = 27 |
High HbA1c (67%); acceptable HbA1c but too much work with multiple injections (11%), pain from insulin or needle (7%), unstable blood glucose but acceptable HbA1c (7%), high blood glucose during the night or morning (4%), wanted to try pump (4%) | 23 followed-up for 5 years on CSII | After the initial period of adjustment during the time after pump start, pump patients were not seen more often than injection patients |
| Juliusson (2006),171 Norway, before/after study |
Mean age 14.4 years (SD 1.5) (range 9.7–17.1) n = 31 |
The main indication for initiating CSII was HbA1c above the target range of 7–8% | 15 months | To initiate pump therapy, patients were admitted to the clinic on Wednesdays and discharged on Mondays. Parents were given a demonstration pump with saline. Paediatric diabetologists and specialist nurses gave practical and theoretical information |
| Kordonouri (2006),151 Germany, matched pair analysis comparing CSII vs MDI |
Mean age 6.7 years n = 59 |
Patient preference or erratic blood glucose (hypos, dawn phenomenon) | 1-year; data from 59 patients reported at 1 year |
59/85 patients on CSII were eligible (not clear why others were excluded) Training: NR |
| Liberatore (2004),172 Canada, before/after study by reviewing medical charts |
Mean age 12.9 years (range 2–17 years) n = 73 |
No restrictions on children offered CSII | At least 6 months (range 6–30 months) |
Using insulin pump for more than 6 months Intensive education about pump and management of diabetes plus close contact with children and families and more clinic visits |
| Mack-Fogg (2005),173 USA, before/after retrospective chart review |
Mean age 9.1 years (SD 2.9) Subgroups: 1. n = 9, started CSII at between 2 and 4 years old 2. n = 29, started between 5 and 9 years 3. n = 32, started between 10 and 12 years Total N = 70 |
Patients and families who were testing blood glucose at least four times daily, who were interested in achieving tighter control, and/or who had experienced several episodes of severe hypoglycaemia while attempting to maintain good glycaemic control | Mean 336 (SD 58.5) days |
Began CSII at age 12 or younger and using CSII for at least 6 months Parents and the diabetes team worked to educate the adults who were responsible for 5- to 9-year-olds while they were away from home Training in general: NR |
| McMahon (2005),152 Australia, before/after study |
Mean age 12.5 years (SD 3.8) (range 3.9–19.6 years) n = 105 |
5% were started on pump therapy because of recurrent severe hypoglycaemia, 5% because of poor control despite compliance with therapy and the remaining 90% at the request of the patient or caregiver | Mean 1.4 (SD 0.9) years (range 0.2–4.0 years) |
Patients had to be motivated and able to test blood glucose levels at least 4 times per day Insulin pump therapy started as an inpatient with a 24-hour admission. Patients and families received intensive education and glucose monitoring, and followed up by daily telephone calls for 1 week. Clinic appointments were made 2-weekly for 4 weeks, then 3-monthly |
| Mednick (2004),178 USA, survey |
Mean age 13.6 years (range 10–18 years) and parents n = 22 |
Children were transitioning to insulin pump therapy. Reasons not reported. Patients had good metabolic control prior to pump start (mean HbA1c 7.94) | Using CSII for mean 10.43 ± 5.05 months (range 3–22 months) |
Purpose of study was to describe satisfaction and subsequent QoL with the transition to insulin pump therapy among children and their parents Training: NR |
| Plotnick (2003),158 USA, before/after study |
Mean age 12 years (range 4–18 years) (29% were < 10 years old) n = 95 |
Several reasons, including better control, less blood glucose variability, fewer injections, and improvement in lifestyle flexibility | Median 15 months |
Patients were highly selected. All patients and families needed to demonstrate a desire and ability for intensive management Risks of pump use and risk prevention discussed: site infections, hyperglycaemia, ketosis and DKA. Hypoglycaemia awareness, prevention and treatment reviewed. Problem-solving strategies discussed: mechanical problems. 24- to 48-hour admission to initiate. After pump start, all patients had daily phone contact with the diabetes nurse educator for 3–7 days and then fax or phone contact 1–2 times per week for next 1–2 months |
| Raile (2002),159 Germany, prospective longitudinal non-randomised case–control study |
Mean age 13.6 years n = 12 |
Dawn phenomenon, repeated hypos especially at night, patient request for more flexibility | 1 year | Adolescents interested in CSII and fulfilling inclusion criteria were admitted to a special diabetes education programme for CSII. Prerequisites for CSII were: documented recording of blood glucose tests and adequate technical skills |
| Saha (2002),153 Finland, case series |
Mean age 8.7 years (range 0.2–16 years) n = 16 |
Marked instability in blood glucose resulting in numerous hypos, poor control and patient request since perceived as more convenient | Mean 2 years (range 0.4–4.2 years) |
Started CSII between 1992 and 1997 Training: NR |
| Schiaffini (2005),168 Italy, retrospective data collection comparing CSII with glargine |
Mean age 12.7 years (SD 1.8) n = 20 |
Suboptimal glycaemic control (HbA1c > 8.0%), wide glycaemic oscillations with fasting hyperglycaemia, frequent hypoglycaemic episodes | 1 year |
T1DM diagnosed at least 2 years; > 10 years old Support (for CSII and MDI groups) – dietary education, regular self-monitoring of blood glucose (at least 4–5 tests per day), medical and psychological care and frequent telephone consultations with the medical staff were encouraged in order to adjust the insulin dose appropriately |
| Simmons (2006), 179 [abstract], USA, matched non-randomised controlled study |
Age range 6–19 years n = 51 (aged 6–12) n = 87 (aged 13–19) Total N = 138 |
NR | Mean 1.7 years for 6–12 age group |
Subjects treated with CSII for ≥ 6 months 18% of patients 6–12 years (163/895) and 28% of patients 3–19 years (284/1025) cared for by the Barbara Davis Center were treated with CSII |
| Sulli (2003),160 Italy, prospective longitudinal trial |
Mean age 13.5 years (range 2–25 years) n = 41 |
Unstable control; high HbA1c; recurrent hypos; early onset of microangiopathic complications; dawn phenomena; difficulty matching injections to meals | 6 months | CSII suggested for 41/350 children with diabetes. All patients had undergone intensive MDI insulin therapy for at least 1 year, with 4 insulin injections per day. Patients and other family members underwent training regime. Only those cases which the patients (or parents) had shown that they had mastered the technique and had the necessary skills and knowledge for CSII therapy |
| Sullivan-Bolyai (2004),180 USA, qualitative description | 21 parents of 16 children aged < 12 years (mean age 7 years) | NR | On CSII for mean 16 ± 11 months (range 3–36 months) |
All patients < 12 years invited to participate Training: NR |
| Toni (2004),181 [letter], Italy, case series |
Mean age 14.4 years (range 9–17.8) n = 34 |
NR | 2 years |
Continued CSII for at least 1 year Training: NR |
| Ugrasbul (2006), 182 [abstract], USA, case series | Age 4–21 years n = 131 | NR | Not clear |
All patients starting CSII 2003–4 Training: NR |
| Wallach (2005),183 [abstract], USA, case series |
Mean age 12.4 years (range 2.8–21) n = 73 |
NR | Mean 2.3 years |
Consecutive patients Training: NR |
| Willi (2003),154 USA, before/after study |
Mean age 11.2 years (SD 0.3) (range 5–16) n = 51 |
Selection was not guided by any strict criteria but encompassed several features believed to be important to success. Most patients had expressed an interest, and all agreed to a trial of CSII | 1 year |
Duration of diabetes > 1.5 years or a pattern of increasing insulin requirements was present in all cases Patients and their families needed to demonstrate an ability to understand the concepts of insulin pump mechanics. The CSII training programme included individual education sessions in carbohydrate counting, insulin pump mechanics, and site insertion, culminating in a 3-day outpatient pump trial using normal saline |
| Wood (2006),155 USA, cohort study |
Mean age 14 years (range 3.7–21.7) n = 161 |
Patients and families, in collaboration with diabetes team, elected to begin pump therapy. Self-selected pump model | 3.8 years |
All youth who began pump therapy during 4 years 1998–2001 Patients and families completed clinic’s standard pump assessment and education programme |
| First author, year, country, study type | Age group, sample size | Reasons for starting on pump | Duration of follow-up | Notes – including selection criteria and training (where reported) |
|---|---|---|---|---|
| Alemzadeh (2006),184 [abstract], USA, before/after study |
Mean age 3.9 years n = 14 |
NR | 1 year | NR |
| Berhe (2006),165 USA, before/after study/retrospective chart review |
Mean age 4.6 years (range 2–6) n = 33 |
Started on CSII at discretion of attending paediatrician; no specific inclusion or exclusion criteria to start pump therapy | 1 year |
Excluded: > 6 years old at initiation of pump, still in honeymoon phase, on MDI Parents given intensive education support including initial weekly contact, additional clinic visits |
| Litton (2002),164 USA, before/after study |
Mean age 34 months (range 20–58 months) n = 9 |
On insulin for > 6 months; recurrent hypos or DKA, elevated HbA1c; erratic fluctuations in blood glucose | Mean 1 year |
After diagnosis of T1DM, families received extensive diabetes education and training, 24-hour phone access to staff Patients on CSII: it was required that they would receive constant supervision by parents or caretakers throughout the day, parental understanding of management and pump, child had to be able to tolerate equipment |
| Shehadeh (2004),174 Israel/Slovenia, before/after study |
Mean age 3.8 years (range 1.3–5.7) n = 15 |
CSII suggested for young children with diabetes ≥ 6 months | 1 year | Parental education and training, 24-hour telephone line support |
| Weinzimer (2004),156 USA, retrospective before/after study |
Mean 4.5 years (range 1.4–6.9) n = 65 |
Repeated episodes of hypoglycaemia. Initiated on pump therapy at request of parents and only with the approval of health-care team. Preinitiation requirements for pump therapy included frequent monitoring of glucose levels, adequate adult supervision, ability to comprehend and implement pump treatment | Mean 30 months |
All children initiated on pump before seventh birthday and for whom at least 3 months of prepump and 3 months of postpump data were available Families underwent education sessions. Telephone support provided |
Studies were conducted in a variety of countries most commonly in the USA (n = 20) and Europe. Three studies were conducted in the UK. 138–140 Other studies were set in Australia, New Zealand, Canada and Israel.
The observational studies incorporated a variety of study designs: surveys, audits, before/after studies with and without control group (matched and unmatched), prospective and retrospective data, primary data, patient records and national registers.
Sample size ranged from 8 to 2702. The majority of studies had a sample size of under 50. In the adult/mixed age groups, the duration of follow-up ranged from 11.5 months to 13 years, the majority being 1–2 years. In the children/adolescent groups, follow-up ranged from 6 months to 5 years, with the majority of studies being 1–2 years. Among younger children, four studies had a follow-up of 1 year and one study of a mean 30 months.
Reasons for starting CSII
Reasons for starting CSII were not reported in 13 studies. All but a few studies included poor metabolic control (including frequent hypoglycaemic episodes and the dawn phenomenon) as a reason for starting CSII.
Among adults and mixed age groups, planning for pregnancy or during pregnancy140–143 and the desire for a more flexible lifestyle140,141,144–146 were commonly cited reasons. Patient preference was included as a reason in four studies. 141,147 Other less commonly cited reasons included: quality of life,141 low insulin requirements, hypoglycaemia unawareness,144 diabetic complications,148 participation in study,142 allergy to insulin,143 gastroparesis140 and lipodystrophy. 140
Among children and adolescents, commonly cited reasons were: request of patient or parents149–156 and the desire for a more flexible lifestyle. 149,157–159 Less common reasons reported included hypoglycaemia unawareness,157 quality of life,157 early onset of diabetic complications,160 too much work with multiple injections150 and problems with injections. 150,158,160
Selection of patients for CSII
Many of the studies gave details of how patients were selected for CSII. Among adult and mixed age studies, patients were selected if already on MDI,138,139,146,161,162 and showed willingness and ability to master intensive management features of CSII. 101,163 In Pickup et al. (2005),138 patients who showed poor compliance or psychological problems were considered unsuitable for pump therapy.
Among studies including only adolescents and children, patients and families needed to demonstrate the desire and ability for intensive management. 149,152,154,157,158,160,164 Two studies reported that patients were already on MDI [Sulli and Shashaj (2003)160 and Berhe et al. (2006)]165 and two studies reported requiring prior documentation of adequate blood glucose testing. 159,166 Other inclusion criteria included good parental supervision, [Garcia-Garcia et al. (2007)167 and Litton et al. (2002)],164 minimum duration of diabetes167,168 and daily insulin requirement of more than 0.75 units/kg. 167 One study reported that patients were excluded if in the honeymoon phase. 165
Education and support for CSII
Seventeen studies did not report details of education and support. A few studies reported identical educational and support programmes for both CSII and MDI groups. [Cersosimo et al. 2002,169 Pickup et al. 138 and Garcia-Garcia et al. )]. 167,168
Several studies described intensive training/education of participants and their families. 101,140,144,146,147,152,154–162,164–166,170–174 Some training programmes included the use of a dummy saline pump. 154,157,158,171 Initiation of pump therapy involved a period of admission to clinic or hospital in a few studies. 152,158,171
Intensive ongoing support was often provided, including initial frequent visits and telephone contact, 152,156,158,161,165,166,172 and 24-hour nurse on call or telephone support. 140,164,166,174
Continuation rates
Continuation rates can be regarded as evidence of patient satisfaction. Fewer than half of the studies (22/48) reported continuation rates. Continuation rates at 1–5 years ranged from 74% to 100%. Continuation rates of 100% were reported in two studies: in adults/mixed age groups144,163 and five studies in adolescents and children. 154,156,159,164,165
A variety of reasons for discontinuing CSII were reported in 12 studies (Tables 10–12). Reasons for discontinuing included: end of pregnancy, lack of tolerance to carry the pump, perception of goals not reached, infection at insulin injection site and hypoglycaemic episodes;141 not able to cope with the technical aspects of using an insulin pump and not convinced of the advantages;145 cost and inconvenience;146 site problems, sweating, costs and other illness;140 patient’s decision (most commonly due to reluctance to wear the system), cutaneous problem and poor compliance;176 dislike of or difficulty with needle insertion, insurance difficulties, trouble keeping the infusion site clean, tape not adhering, and general dislike of the pump;101 wish to return to injections, and worsening control due to omitting bolus insulin;166 extra work involved with changing infusion sets and dislike of something being attached to body;150 psychiatric and dermatological conditions;152 inconvenience in carrying the pump;171 pump limited normal physical activity, recurrent DKA;153 and, DKA due to insulin omission, diabetes burnout, minor problems with infusion site, body image concerns and concerns about weight gain. 155
| Study | Percentage continuing on pump | Reasons for discontinuing | Duration of follow-up |
|---|---|---|---|
|
Bruttomesso (2006)141 Italy |
79 | 571 (21%) had discontinued CSII. 187 (33% of those discontinuing) discontinued at end of pregnancy; other reasons included lack of tolerance to carry pump, perception of goals not reached, infection at insulin injection site, hypos, or moving (no percentage given) | Mean 3.9 years (adults) and 2.4 years (children) |
|
D’Annunzio (2005)144 Italy |
100 | Not applicable | 18 months |
|
Linkeschova (2002)145 Germany |
97 | One patient had had a combined kidney–pancreas transplant; one did not feel able to cope with the technical aspects of using an insulin pump; one with severe hypoglycaemia on MDI stopped after 3 months of CSII as not convinced of the advantages | Mean 1.8 ± 1.2 years |
|
Reda (2007)146 New Zealand |
NR | 20 lost to follow-up for various reasons (moved to care of other physicians, moved house, discontinued CSII due to cost or inconvenience) | Mean 3 years |
|
Rodrigues (2005)140 UK |
87.5 |
15 had classic contraindications to CSII (including psychiatric disorders) 5 patients (12.5%) discontinued CSII (2 site problems, 1 sweating, 1 due to costs, 1 other illness) – all these patients were self funded |
Median 20.5 months (range 1–192 months) |
|
Ronsin (2005)176 France |
74 Including all patients who remained on some form of pump |
At least 40% had diabetes related medical problems 25 patients (36%) discontinued CSII (7 changed to implantable pump treatment; 10 patients’ decision (most commonly due to reluctance to wear system); 4 end of pregnancy; 3 cutaneous problem; 1 poor compliance) |
Maximum of 2 years |
|
Rudolph (2002)101 USA |
94.6 | Variety of reasons for discontinuing pump – usually multiple reasons; including, dislike or difficulty with needle insertion (n = 3), insurance difficulties (n = 2), trouble keeping the infusion site clean (n = 2), tape not adhering (n = 2) and general dislike of the pump (n = 2) | Mean 36.1 ± 25.5 months |
|
Siegel-Czarkowski (2004)163 USA |
100 | Not applicable | 1 year |
| Study | Percentage continuing on pump | Reasons for discontinuing | Duration of follow-up |
|---|---|---|---|
|
Ahern (2002)166 USA |
98 | 3 stopped CSII (2 discontinued due to wish to return to injections and one failed to take bolus dose and control worsened) | At least 1 year (range 19–57 months) |
|
Hanas (2006)150 Sweden |
92 |
2 discontinued (one after 3 years since no longer motivated for extra work involved with changing infusion sets, one at 2 years as she did not like something being attached to her body) Incomplete reporting of 5-year data |
5 years |
|
Juliusson (2006)171 Norway |
86 | Main reason given for withdrawing from CSII was inconvenience in carrying the pump | 15 months |
|
McMahon (2005)152 Australia |
95 | Reasons for discontinuing. 2 patients had psychiatric conditions and one had a dermatological condition. Two discontinued at the patient or parent’s request | Mean 1.4 ± 0.9 (SD) years (range 0.2–4.0 years) |
|
Raile (2002)159 Germany |
100 | Not applicable | 1 year |
|
Saha (2002)153 Finland |
75 | 4 patients discontinued (2 since pump limiting normal physical activity, 1 pump needed for another child, 1 recurrent DKA) | Mean 2 years (range 0.4–4.2 years) |
|
Toni (2004)181 [letter] Italy |
88 | 3 dropped out in year 1, one dropped out year 2 | 2 years |
|
Wallach (2005)183 [abstract] USA |
92 | 6 patients, all > 12 years, discontinued (none because of complications of therapy or weight gain) | Mean 2.3 years |
|
Willi (2003)154 USA |
100 | Not applicable | 1 year |
|
Wood (2006)155 USA |
82 | Reasons for discontinuing: major problems (n = 8, DKA, insulin omission); diabetes burnout (n = 8); minor problems (n = 6, infusion site problems), body image concerns (n = 4); concerns about weight gain (n = 3) | 3.8 years |
| Study | Percentage continuing on pump | Reasons for discontinuing | Duration of follow-up |
|---|---|---|---|
|
Berhe (2006)165 USA |
100 | Not applicable | 1 year |
|
Litton (2002)164 USA |
100 | Not applicable | Mean 1 year |
|
Shehadeh (2004)174 Israel/Slovenia |
93 | Not reported | 1 year |
|
Weinzimer (2004)156 USA |
‘None returned to MDI because of family choice or practitioner discretion’ | Not applicable | Up to 4 years |
Glycaemic control as reflected in HbA1c
Forty-six studies reported comparable before/after data on HbA1c levels (Table 13). Levels of statistical significance are reported where they were available (some papers did not report whether the change was significant or not).
| Study | Pre-CSII HbA1c level (%) (± SD%) | HbA1c level after CSII (% ± SD) (longest follow-up with HbA1c values) | Difference HbA1c% (‘before’ minus ‘after’) (+ve = decrease = improvement) | Study duration |
|---|---|---|---|---|
| Adults/mixed age group | ||||
| Cersosimo (2002)169 | 8.0 ± 1.2 | 7.1% ± 1.1 | 0.9 (p < 0.05) | 2 years |
| D’Annunzio (2005)144 | Median 9.8 | Median 8.6 | 1.2 (p = 0.014) | 18 months |
| Fahlen (2005)161 | 7.64 ± 1.5 | NR | 0.59 ± 1.19 (p < 0.001) | Median 15 months |
| Garg (2004)175 | 7.7 ± 0.1 | 7.5 ± 0.1 | 0.2 (p < 0.001) | At least 6 months |
| Hunger-Dathe (2003)170 | Relative HbA1c (absolute HbA1c/healthy mean): 1.58 ± 0.34 | Relative HbA1c (absolute HbA1c/healthy mean): 1.449 ± 0.32 | 0.45 (p < 0.0001) | 1 year |
| Jankovec (2005)148 | 9.43 ± 1.98 | 8.31 ± 1.76 | 1.1 (p < 0.001) | 1–2 years |
| Lepore (2004)162 | 9.0 ± 1.3 | 8.0 ± 1.0 (mean of 4 measures over 1 year) | 1.0 (p < 0.001) | 1 year |
| Linkeschova (2002)145 | 7.7 ± 1.1 | 7.2 ± 1.0 | 0.5 (p < 0.001) | Mean 1.8 years |
| Nimri (2006)147 |
Entire cohort: 8.4 ± 1.3 Subgroups: Prepubertal: 8.6 ± 1.2 Adolescent: 8.6 ± 1.3 Young adult: 8.1 ± 1.4 |
Entire cohort: 7.8 ± 1.3 Subgroups: Prepubertal: 8.2 ± 0.7 Adolescent: 8.3 ± 1.4 Young adult: 7.3 ± 1.0 |
Entire cohort: 0.51 (p < 0.001) Subgroups: Prepubertal: 0.48 (p <0.05) Adolescent: 0.26 (p < 0.05) Young adult: 0.76 (p <0.001) |
Mean 2.4 years |
| Norgaard (2003)142 | 8.5 ± 1.1 | 8.0 ± 1.2 | 0.5 (p < 0.05) | Mean 13 years |
| Pickup (2005)138 | 8.8 | 7.4 | 1.4 (p < 0.001) | 6 months |
| Pickup (2006)139 | 8.5 ± 1.4 | 7.3 ± 0.9 | 1.2 (p < 0.001) | 16 months |
| Radermecker (2005)143 | 8.6 ± 1.3 | 8.4 ± 1.0 | 0.2 (p < 0.001) | Mean 5.1 years |
| Reda (2007)146 |
All ages: 8.9 ± 1.3 Adults only: 8.8 ± 1.4 |
All ages: 7.9 ± 0.95 Adults only: 7.9 ± 0.79 |
All ages: 1.0 (p < 0.001) Adults only: 0.9 (p < 0.001 |
6 months |
| Rodrigues (2005)140 | 9.6 ± 2.7 | 8.3 ± 1.2 | 1.3 (p = 0.011) | Median 20 months |
| Rudolph (2002)101 | 7.6 ± 1.5 | 7.1 ± 1.1 | 0.66 (p < 0.0001) | Mean 36.1 months |
| Siegel-Czarkowski (2004)163 | 7.64 ± 0.19 | 7.01 ± 0.10 | 0.6 (p < 0.01) | 1 year |
| Sucunza (2005)177 | 8.3 ± 1.3 | 7.7 ± 1.5 | 0.6 (p < 0.005) | Mean 26 months |
| Children/adolescents | ||||
| Ahern (2002)166 |
Preschool: 7.1 ± 1.0 School age: 7.9% ± 1.0 Adolescents: 8.1% ± 1.5 |
Preschool: 6.5 ± 0.7 School age: 7.3% (SD 1.1), p < 0.02 Adolescents: 7.4% (± 1.2) p < 0.02 |
Preschool: 0.6 (p ≤ 0.02) School age: 0.6 (p ≤ 0.02) Adolescents: 0.7 (p ≤ 0.02) |
1 year |
| Alemzadeh (2004)149 | 8.4 ± 1.0 | 7.8 ± 0.8 | 0.6 (p < 0.002) | 1 year |
| Conrad (2002)157 |
All children: 8.4 ± 0.9 Prepubertal: 8.3 ± 0.7 Pubertal: 8.5 ± 0.9 |
No significant change in either prepubertal or pubertal patients; data only presented graphically | NS | 3–6 months |
| Garcia-Garcia (2007)167 | 7.62 ± 0.62 | 7.70 ± 0.64 | –0.08 | 2 years |
| Hanas (2006)150 | 8.9 ± 1.0 | Graph of 5-year data appears to show little difference between baseline and final HbAIc (no values reported at 5 years, approximately 8.7% from graph) | 0.2 (NS) | 5 years |
| Juliusson (2006)171 | 10.4 ± 1.8 | 9.6 ± 1 | 0.8 | 15 months |
| Kordonouri (2006)151 | 8.17 ± 1.03 | 8.27 ± 1.01 | –0.01 (NS) | 1 year |
| Liberatore (2004)172 | 8.3 ± 1.0 | 7.5 ± 1.1 | 0.8 (p < 0.00003) | At least 6 months |
| Mack-Fogg (2005)173 |
Overall: 7.8 ± 0.8 2- to 4-year group: 8.1 5- to 9-year group: 7.7 10- to 12-year group: 7.7 |
Overall: 7.3 ± 0.7 2- to 4-year group: 7.6 5- to 9-year group: 7.2 10- to 12-year group: 7.3 |
Overall: 0.5 (p < 0.001) 2- to 4-year group: 0.5 (NS) 5- to 9-year group: 0.5 (p < 0.05) 10- to 12-year group: 0.4 (p < 0.05) |
Mean 336 days |
| McMahon (2005)152 |
Overall: 8.3 (SEM ± 0.1) < 12 years: 8.3 ± 0.2 > 12 years: 8.4 ± 0.1 |
Overall: 7.8 (SEM ± 0.1) < 12 years: 7.5 ± 0.1 > 12 years: 7.9 ± 0.1 |
Overall: 0.5 (p < 0.0001) < 12 years: 0.8 (p < 0.001) > 12 years: 0.5 (p < 0.001) |
Mean 1.4 years |
| Mednick (2004)178 | 7.94 | 7.41 | 0.53 (p = 0.03) | 3–22 months |
| Plotnick (2003)158 | 8.1 | 7.7 | 0.4 (p < 0.001) after adjusting for duration of diabetes mellitus and age | Median 15 months |
| Schiaffini (2005)168 | 8.5 ± 1.8 | 7.6 ± 1.2 | 0.9 (p < 0.05) | 12 months |
| Simmons (2006)179 |
6–12 years: 8.3 ± 0.9 13–19 years: 8.7 ± 1.1 |
6–12 years: 7.6 ± 0.9 13–19 years: 8.4 ± 1.2 |
6–12 years: 0.7 13–19 years: 0.3 |
|
| Reda (2007)146 | 9.1 ± 1.0 | 7.9 ± 1.0 | 1.2 (p < 0.005) | 1 year |
| Raile (2002)159 | 7.4 ± 0.82 | 8.0 ± 0.7 | –0.6 | 1 year |
| Saha (2002)153 | 9.1 ± 2.4 | 8.7 ± 1.6 | 0.4 (NS) | Mean 2 years |
| Sulli (2003)160 | 9.5 ± 1.7 | 8.8 ± 1.5 | 0.7 (p < 0.05) | 6 months |
| Toni (2004)181 | 8.35 ± 1.08 | 7.81 ± 0.95 | 0.5 (p = 0.002) | 1 year |
| Ugrasbul (2006)182 | 8.5 | 8.2 | 0.3 | NR (started on CSII 2003–4) |
| Wallach (2005)183 | 8.19 ± 1.05 | 7.48 ± 0.91 | 0.7 (p = 0.126) NS | 2 years |
| Willi (2003)154 |
All groups: 8.4 ± 0.2 5–9 years: 8.4 10–12 years: 8.37 13–17 year: 8.3 (estimated from graphs) |
All groups: 7.9 ± 0.1 5–9 years: 7.72 10–12 years: 8.37 13–17 year: 7.63 |
All groups: 0.5 (p < 0.01) 5–9 years: 0.7 (p < 0.01) 10–12 years: 0 (NS) 13–17 year: 0.7 (p < 0.05) |
12 months |
| Wood (2006)155 | 8.4 ± 1.4 | 8.1 ± 1.3 | 0.3 (p < 0.01) | 12 months |
| Young children | ||||
| Alemzadeh (2006)184 | 8.0 ± 0.50 | 7.8 ± 0.40 | 0.2 (NS) | 1 year |
| Berhe (2006)165 | 8.7 ± 0.6 | 8.0 ± 0.5 | 0.7 (p < 0.001) | 1 year |
| Litton (2002)164 | 9.5 ± 0.4 | 7.9 ± 0.3 | 1.6 (p < 0.001) | 1 year |
| Shehadeh (2004)174 | 8.82 ± 0.98 | 8.18 ± 0.90 | 0.6 (p < 0.05) | 1 year |
| Weinzimer (2004)156 | 7.4 ± 1.0 | 7.1 ± 0.8 | 0.3 (p = 0.006 for all postpump values compared to prepump values) | Up to 4 years (analysed for > 162 patient-years of follow-up) |
All of the 18 studies in the adults/mixed age groups showed a significant decrease in HbA1c levels (ranging from 0.2% to 1.4%) after participants started on pumps.
Few studies reported proportions reaching targets. Targets varied. Pickup et al. (2006)139 reported that 37% of CSII subjects and 13% of MDI ones achieved HbA1c < 7%; for a target of < 8%, the proportions were 73% and 30%. Radermecker et al. (2005)143 noted that of 95 patients on CSII, only five reached HbA1c of 7% or less; most (66) were in the range 7.1% to 8.5%. In Reda et al. (2007),146 only 9 out of 105 reached the ADA target (7.0% or less) before CSII, and only 18 afterwards.
There were 23 studies in the children/adolescents age group. Three of the studies showed an increase after using pumps. In Kordonouri et al. (2006)151 (n = 59) and Garcia-Garcia et al. (2007)167 (n = 8) the increases of 0.01% and 0.08%, respectively, were neither clinically or statistically significant. The statistical significance level was not reported in Raile et al. (2002)159 (n = 12), where the increase was 0.6%. The remaining 20 studies showed an overall decrease, ranging from 0.2% to 1.2%. In 13 of these studies, the overall decrease was statistically significant. However, in Mack-Fogg et al. (2005),173 the decrease was only significant in the 10- to 12-year age groups, and not significant in the younger children. Willi et al. (2003)154 also reported the subgroups by age, and found a significance decrease in the 5- to 9-year and 13- to 17-year group, but no change in the 10- to 12-year group.
In four studies the decrease was not significant 150,153,157,183 and three studies171,179,182 did not report the significance level of the decrease. Only one study reported proportions reaching targets. Simmons et al. (2006)179 reported that 75% of 6- to 12-year-olds reached the ADA target of 8% or less (for that age group). Only 15% of adolescents (13–19) reached their age range target of < 7.5%.
There were five studies in young children and all showed decreases, which ranged from 0.2% to 1.6%. 156,164,165,174,184 Four156,164,165,174 showed a statistically significant decrease and one [Alemzadeh et al. (2006),184 n = 14] showed a non-significant decrease. Only one study reported on targets met – Berhe et al. 165 reported that 76% of patients had HbA1c levels of < 8.5% after CSII compared to 35% before.
In summary, only three out of 46 studies showed an increase in HbA1c. These studies were all in children/adolescents, and the increases were insignificant in two, and 0.6% in the third (no significance level reported). Most studies showed decreases in HbA1c varying from 0.2% to 1.6%.
Hypoglycaemic episodes
The main interest was in SH episodes. Data are included from the 26 studies reporting comparable data on the rate of SH episodes before and after CSII was initiated (Table 14). Rates were reported in different units, so rate ratios were calculated to enable comparison between studies.
| Study | Before CSII | During CSII | Difference (+ve = reduction) | Rate ratio |
|---|---|---|---|---|
| Adults/mixed age groups | ||||
| Hunger-Dathe (2003)170 | 0.46 ± 1.5 | 0.12 ± 0.51 | 0.34 (p < 0.001) | 0.26a |
| Lepore (2004)162 | 0.42 ± 0.49 | 0.17 ± 0.37 | 0.25 (p < 0.05) | 0.40a |
| Linkeschova (2002)145 | 1.23 (any external help) | 0.29 | 0.94 (p < 0.005) | 0.24a |
| 0.70 (treated with i.v. glucose or glucagon injection) | 0.06 | 0.64 (p < 0.001) | 0.09a | |
| Nimri (2006)147 | Prepubertal: 0 (per 100/patient-years) | Prepubertal: 0 | Prepubertal: 0 | |
| Adolescent: 36.5 | Adolescent: 11.1 | Adolescent: 25.4 (p = 0.002) | 0.30a | |
| Young adult: 58.1 | Young adult: 23.3 | Young adult: 34.8 (p = 0.02) | 0.40a | |
| Pickup (2005)138 | 0 | 0 | 0 | 0 |
| Pickup (2006)139 | 0 | 0 | 0 | 0 |
| Siegel-Czarkowski (2004)163 | 7/34 | 1/34 | 6/34 (p < 0.05) | 0.14a |
| Reda (2007)146 | 0.75 | 0.05 | 0.70 (p < 0.001) | 0.07a |
| Rodrigues (2005)140 | 0.92 ± 1.49 | 0.15 ± 0.38 | 0.77 (p = 0.009) | 0.16a |
| Rudolph (2002)101 | 73.2 (per 100/patient-years) | 19.1 | 54.1 (p < 0.0003) | 0.26a |
| Children/adolescents | ||||
| Ahern (2002)166 | All: 0.35 | All: 0.24 | All: 0.11 (p < 0.05) | 0.69a |
| Preschool: 0.42 | Preschool: 0.19 | Preschool: 0.23 (NS) | 0.45 | |
| School age: 0.33 | School age: 0.22 | School age: 0.11 (NS) | 0.67 | |
| Adolescents: 0.33 | Adolescents: 0.27 | Adolescents: 0.06 (NS) | 0.82 | |
| Alemzadeh (2004)149 | 20.6 (per 100 patient-years) | 8.2 | 12.4 (p < 0.05) | 0.40a |
| Garcia-Garcia (2007)167 | 0 | 0 | 0 | 0 |
| Juliusson (2006)171 | 43.8 (events per 100 patient-years) | 5.2 | 38.6 | 0.12 |
| Kordonouri (2006)151 | 19.2 (SE ± 7.3) (per 100 patients per year) | 5.8 (SE ± 3.3) | 13.4 | 0.30 |
| Mack-Fogg (2005)173 | 0.46 | 0.22 | Overall: 0.24 (NS) | 0.48 |
| 2–4 year: 0.27 (NS) | ||||
| 5–9 year: 0.16 (NS) | ||||
| 10–12 year: 0.30 (p < 0.02) | ||||
| McMahon 2005152 | Total 32.9 | Total: 11.4 | Total: 21.5 | 0.35 |
| < 12 year: 25.9 | < 12 year: 8.3 | < 12 year: 17.6 | 0.32 | |
| > 12 year: 37.5 | > 12 year: 13.5 | > 12 year: 24.0 | 0.36 | |
| (per 100 patient-years) | ||||
| Plotnick 2003158 | 14.3 (per 1,000 person-months) | 6.6 | 7.7 (NS) | 0.46 |
| Schiaffini (2005)168 | 0.25 ± 0.4 | 0.2 ± 0.3 | 0.05 (NS) | 0.80 |
| Sulli (2003)160 (hypos defined as < 3.3mmol/l) | 6.50 ± 5.50/patient/month | 3.50 ± 3.00 | 3.00 (p = 0.04) | 0.54a |
|
Wood 2006155 Subgroup of children/adolescents who remained on pump after 3 years (n = 132) |
0.23 | 0.074 | 0.16 (p = 0.001) | 0.32a |
| Young children | ||||
| Alemzadeh (2006)184 | 0.225 | 0.174 | 0.05 (NS) | 0.77 |
| Berhe (2006)165 | 0.178 | 0 | 0.178 (p < 0.001) | 0a |
| Litton (2002)164 | 0.52 ± 0.10/month | 0.09 ± 0.02/month | 0.43 (p < 0.05) | 0.17a |
| Shehadeh (2004)174 | 0.36 | 0.29 | 0.07 (NS) | 0.81 |
| Weinzimer (2004)156 | 0.78 | 0.37 | 0.41 (p = 0.02) | 0.47a |
Ten studies were in adults/mixed age groups. 101,138–140,145–147,162,163,170 Two138,139 did not report any hypoglycaemia before or after pumps use. Of the eight remaining studies, all reported a significant decrease after going on pumps. The rate ratios ranged from 0.07 to 0.4. One of these147 reported no hypoglycaemic episodes in the prepubertal group either before or after pump use.
There were 11 studies in children/adolescents. 149,151,152,155,158,160,166–168,171,173 One study167 had no SH before or after going on pumps. Of the remaining 10 studies, the overall rate ratios varied from 0.12 to 0.80.
In four studies149,155,160,166 the overall decrease was reported as statistically significant. However in Ahern et al. (2002)166 (n = 161) the decreases were not significant when broken down into three age groups (possibly due to smaller sample sizes).
Three studies151,152,171 did not report the significance level of the decrease, but showed substantial reductions (rate ratios of 0.12, 0.30 and 0.35 respectively). In the remaining three studies in children/adolescents the overall decrease was not significant. However in one of the studies173 the reduction was significant in the 10–12 year age group, but not in the 2–4 and 5–9 age groups.
There were five studies in young children,156,164,165,174,184 and the rate ratios ranged from 0 to 0.81. In three studies 156,164,165 this was significant, and the in the other two it was not significant.
In summary, of the 26 studies examined, 15 showed a statistically significant decrease in SH episodes after going on pumps, five showed a non-significant decrease, and three showed a decrease, but the significance level was not reported. The remaining three studies did not report any SH episodes before or after going on pumps.
Diabetic ketoacidosis
As reported in the last assessment report, it is likely that one of the reasons for the low use of CSII in the UK was fear of DKA. People with T1DM on CSII have no insulin store in the body, and if the pump fails, they will rapidly develop metabolic problems.
There were 15 studies that had comparable before/after data on DKA rates. 140,145–147,151,152,156,158,164–167,170,171,173 These are summarised in Table 15.
| Study | Rate per annum before CSII (unless otherwise stated) | Rate per annum after CSII (unless otherwise stated) | Difference (before/after) (+ve = reduction) |
|---|---|---|---|
| Adults/mixed age groups | |||
| Hunger-Dathe (2003)170 | 0.08 ± 0.4 (severe) | 0.05 ± 0.6 | 0.03 (p = 0.003) |
| Linkeschova (2002)145 | 0.05 | 0.01 | 0.04 |
| Nimri (2006)147 | Prepubertal: 0 | Prepubertal: 0.22 ± 0.52 | –0.22 (NS) |
| Adolescent: 0.19 ± 0.74 | Adolescent: 0.17 ± 0.46 | 0.02 (NS) | |
| Young adult: 0.12 ± 0.43 | Young adult: 0.09 ± 0.29 | 0.03 (NS) | |
| Reda (2007)146 | 0.2 | 0.05 | 0.15 |
| Rodrigues (2005)140 | 1.83 ± 4.84 | 0.27 ± 1.12 | 1.56 (p = 0.036) |
| Children/adolescents | |||
| Ahern (2002)166 | 1 episode in 161 patients over 1 year | 2 episodes in 161 patients over 1 year | –1/161 |
| Garcia-Garcia (2007)167 | 0.10 ± 0.22 | 0.20 ± 0.27 | –0.10 (NS) |
| Juliusson (2006)171 | 15.5 (/100 patient-years) | 12.9(/100 patient-years) | 2.60 (NS) |
| Kordonouri (2006)151 | 0. 9 | 0.096 (SE ± 0.041) | 0.80 (p = 0.024) |
| Mack-Fogg (2005)173 | 0 episodes | 2 episodes | –2 (NS) |
| McMahon (2005)152 | 0 | 0 | 0 |
| Plotnick (2003)158 | 0.80 (95% CI 0.11 to 5.65) (rate per 1000 person-months) | 0.55 (95% CI 0.08 to 3.91) (rate per 1000 person-months) | 0.25 (NS) |
| Young children | |||
| Berhe (2006)165 | 0 (severe) | 0 | 0 |
| Litton (2002)164 | 0.06 (SE ± 0.03) (/month) (severe) | 0.06 (SE ± 0.03) (per month) | 0 (NS) |
| Weinzimer (2004)156 | 0 (severe) | 0.04 | –0.04 |
Five studies were in adults/mixed age groups. 140,145–147,170 One study [Nimri et al. (2006)]147 showed a non-significant increase in prepubertal children and a non-significant decrease in the adolescent and young adult age groups. Two studies [Hunger-Dathe et al. (2003)170 and Rodrigues et al. (2005)]140 showed a statistically significant decrease. The patients in the study by Rodrigues et al. (2005)140 had a higher DKA rate than most other studies, but they included some having particular problems, including with DKA. Two studies [Linkeshova et al. (2002)145 and Reda et al. (2007)]146 did not report the significance level of the reduction.
There were seven studies in children/adolescents. McMahon et al. (2005)152 showed no change. Mack-Fogg et al. (2005)173 and Garcia-Garcia et al. (2007)167 showed a non-significant increase. Ahern et al. (2002)166 showed an increase (of one episode in 161 patients over 1-year) but the significance level was not reported.
Kordonouri et al. (2006)151 showed a significant decrease, and Juliusson et al. (2006)171 and Plotnick et al. (2003)158 showed a non-significant decrease.
There were three studies in young children. 156,164,165 Weinzeimer et al. (2004)156 (n = 65) showed an increase (from 0 to 0.04 per annum) but the significance level was not reported. Berhe et al. (2006)165 (n = 33) and Litton et al. (2002)164 (n = 9) showed no change, but as these studies were small they may have been underpowered to detect a statistically significant difference.
In summary, none of the 15 studies reported a statistically significant increase in DKA rates after patients going on to pumps. Three reported a significant reduction and three studies reported an increase; in one this was not significant and the other two did not report significance levels.
However, a report by Hanas et al. (2005)185 gives worrying data from Sweden, where pump use is common in children. In 1999, 7.5% of children and adolescents used pumps, and this figure rose to 11.2% in 2000. The DKA rate in CSII users was double the overall rate – 3.5 per 100 patient-years versus 1.7, but the true risk ratio will be higher, as the CSII DKA cases will presumably be included in the total for all patients. Hanas et al. note that most DKA occurred soon after CSII initiation, and that, along with the marked rise in CSII use, might perhaps suggest problems with adequate training. Full details will no doubt be published in due course.
Weight change
There were 30 studies in total reporting comparable before/after data on BMI or weight change (Table 16). 138–140,144,145,147,149–156,159,160,162–169,171–173,175,181,182 However, some of the studies that involved adolescents and young children did not take account of changes in the child’s development when considering weight change. 149,159,160,165,172,181,182
| Study | BMI/weight at baseline | BMI/weight on CSII | Difference (+ve = increase, –ve = decrease in BMI/weight) |
|---|---|---|---|
| Adults/mixed age groups | |||
| Cersosimo (2002)169 | BMI ~ 25 | Weight ~ 71 kg | NS |
| D’Annunzio (2005)144 | BMI median: 22.8 | BMI median: 23.5 | Change in BMI: +0.70 (p = 0.02) |
| Garg (2004)175 | Weight 76.2 kg | Weight 77.3 kg | +1.10 kg (p < 0.001) |
| Lepore (2004)162 | BMI 23.5 | BMI 23.9 | Change in BMI: –0.4 (NS) |
| Linkeschova (2002)145 | NR | Body weight under CSII therapy assessed by questionnaire was unchanged in 53% of the patients, increased in 22%, and decreased in 25% of the patients | |
| Nimri (2006)147 |
BMI SDS: Entire cohort: NR Prepubertal: 0.64 ± 0.8 Adolescent: 0.31 ± 0.6 Young adult: 0.35 ± 0.7 |
BMI SDS: Entire cohort: NR Prepubertal: 0.68 ± 0.81 Adolescent: 0.3 ± 0.7 Young adult: 0.28 ± 0.68 |
Change in BMI SDS: Entire cohort: –0.05 ± 0.01 (p = 0.06) NS Prepubertal: +0.04 (NS) Adolescent: –0.01 (NS) Young adult: –0.08 ± 0.37 (p = 0.016) |
| Pickup (2005)138 | Weight 71.4 ± 14.7 kg | Weight 70.0 ± 8.8 kg | –1.4 kg (NS) |
| Pickup (2006)139 | BMI 25.6 ± 3.9 | BMI 25.9 ± 4.3 | Change in BMI: –0.30 |
| Rodrigues (2005)140 | BMI 21.2 | BMI 22.1 | Change in BMI: +0.9 (NS) |
| Siegel-Czarkowski (2004)163 | BMI 23.7 | Reports no significant change at 1 year (BMI not reported) | Change in BMI: NS |
| Children/adolescents | |||
| Ahern (2002)166 | BMI z-score: | BMI z-score: | Change in BMI z -score: |
|
Preschool 1.18 ± 0.73 School age 0.94 ± 0.75 Adolescent 0.74 ± 1.41 |
Preschool 1.18 ± 0.78 School age 0.95 ± 0.84 Adolescent 0.58 ± 1.83 |
0 +0.01 –0.16 |
|
| Alemzadeh (2004)149 | BMI 21.6 ± 3.2 | BMI 23.0 ± 3.0 | Change in BMI: +1.4 (p < 0.05) |
| Garcia-Garcia (2007)167 | BMI SDS: 0.42 | BMI SDS: 0.33 | Change in BMI SDS: –0.09 |
| Hanas (2006)150 | BMI SDS: 0.65 ± 1.2 | BMI SDS: 0.81 ± 1.2 | Change in BMI SDS: +0.16 (NS) |
| Juliusson (2006)171 | BMI SDS: boys 0.43 ± 0.79; girls 1.13 ± 1.34 | BMI SDS: boys 0.68 ± 0.79; girls 1.40 ± 1.31 | Change in BMI SDS: boys: +0.25 (p = 0.14); girls: +0.27 (p = 0.01) |
| Kordonouri (2006)151 | BMI SDS: < 12 years 0.30; > 12 years 0.43 | BMI SDS: < 12 years 0.2, 8; > 12 years 0.40 | Change in BMI SDS: –0.02 (NS), –0.03 (NS) |
| Liberatore (2004)172 | BMI 22.0 | BMI 23.5 | Change in BMI: +1.5 (p = 0.00003) |
| Mack-Fogg (2005)173 | BMI z-score: NR | BMI z-score: NR | Change in BMI z-score: Overall: +0.13 (p < 0.5); 2–4 years: +0.19 (NS); 5–9 years: +0.21 (p < 0.008); 10–12 years: +0.03 (NS) |
| McMahon (2005)152 | BMI z-score: 0.81 ± 0.08 | BMI z-score: 0.75 ± 0.08 | Change in BMI z-score: –0.06 (NS) |
| Raile (2002)159 | BMI 21.3 | BMI 22.0 | Change in BMI: +0.7 (NS) |
| Saha (2002)153 | Mean relative weight: 104.1% | Mean relative weight: 107.0% | Change in mean relative weight: +2.9% (NS) |
| Schiaffini (2005)168 | BMI SDS: 1.21 ± 1.2 | BMI SDS: 1.24 ± 1.2 | Change in BMI SDS: +0.03 |
| Sulli (2003)160 | BMI 21.8 | BMI 22.32 | Change in BMI: +0.52 (NS) |
| Toni (2004)181 | BMI 20.7 ± 2.5 | BMI 21.2 ± 2.4 | Change in BMI: +0.5 (NS) |
| Ugrasbul (2006)182 | NR | NR | Change in BMI: +0.51 (p = 0.019) |
| Willi (2003)154 | Weight SDS: 0.60 SEM ± 0.13 | Weight SDS: 0.61 SEM ± 0.11 | Change in Weight SDS: +0.01 (NS) |
| Wood (2006)155 | BMI z-score: 0.79 | BMI z-score: 0.77 | Change in BMI z-score: –0.02 (NS) |
| Young children | |||
| Berhe (2006)165 | BMI 18.2 | BMI 18.4 | Change in BMI: +0.2 (NS) |
| Litton (2002)164 | Weight z-score: 0.05 | Weight z-score: 0.03 | Change in weight z-score: –0.02 (NS) |
| Weinzimer (2004)156 | BMI z-score: 0.9 | BMI z-score: 0.7 | Change in BMI z-score: –0.2 (p = 0.002) |
Seventeen studies reported a non-significant change in weight. Nimri et al. (2006)147 reported a non-significant change overall, but a significant decrease in the subgroup of young adults (but not in younger age groups).
Linkeshova et al. (2002)145 reported mixed results; i.e. unchanged in 53% of patients, an increase in 22% and decreased in 25%. Juliusson et al. (2006)171 reported a non-significant increase in boys, but a significant increase in girls.
Six studies reported a significant overall increase in BMI. 144,149,172,173,175,182 However, in Mack-Fogg et al. (2005)173 the increase was not significant in the age groups of 2–4 years and 10–12 years (but was highly significant in 5- to 9-year-olds). Also it should be noted that Alemzadeh et al. (2004)149 (mean age 14.7 years, n = 40), Liberatore et al. (2004)172 (mean age 12.9 years, n = 73) and Ugrasbul et al. (2006)182 (age range 4–21 years, n = 131) reported BMI change – not BMI z-scores – so did not take account of the child’s development.
The study by Weinzeimer et al. (2004)156 in young children showed a significant decrease in BMI z-scores. Significance levels were not reported in the four remaining studies. Pickup et al. (2006)139 and Garcia-Garcia et al. (2007)167 showed a decrease, Schiaffini et al. (2005)168 showed an increase and Ahern et al. (2002)166 showed mixed results in different subgroups, i.e. no change in preschool children, an increase in school-aged children, and a decrease in adolescents.
In summary, 17 of the 30 studies showed no significant weight change. Six showed a significant increase (but with the caveat that for three of these studies in children/adolescents the change did not measure z-scores on BMI or weight, hence did not take account of the child’s development), and one showed a significant decrease. The remaining studies either did not report significance or showed mixed results.
Insulin dose
There were 21 studies that reported comparable before/after data on insulin dose. 138–140,144,149,151,154,155,157,159–162,164–166,168,169,172,175,181 These are summarised in Table 17.
| Study | Insulin dose before CSII (units/kg per day – unless otherwise indicated) | Insulin dose on CSII (units/kg per day) | Difference (before minus after) (+ve = decrease, –ve = increase) |
|---|---|---|---|
| Adults/mixed age groups | |||
| Cersosimo (2002)169 | 0.50 | ~ 0.55 | ~ –0.05 (NS) |
| D’Annunzio (2005)144 | Median 0.92 | Median 0.90 | 0.02 (p = 0.049) |
| Fahlen (2005)161 | 0.63 ± 0.27 | 0.57 ± 0.25 | 0.06 |
| Garg (2004)175 | 43.2 units per day | 44.5 units per day | –1.3 (p < 0.001) |
| Lepore (2004)162 | 48 ± 11.7 units per day | 35.9 ± 8.5 units per day | 12.1 (p < 0.001) |
| Pickup (2005)138 | 47.1 ± 16.4 units per day | 34.1 ± 10.5 units per day. | 13.0 (p < 0.001) |
| Pickup (2006)139 | 46.1 ± 16.7 units per day | 35.7 ± 12.1 units per day | 10.4 (p < 0.001) |
| Rodrigues (2005)140 | 47.6 units per day | 37.4 units per day | 10.2 (p = 0.008) |
| Children/adolescents | |||
| Ahern (2002)166 | Preschool: 0.7 | Preschool: 0.8 | –0.1 (NS) |
| School age: 1.0 | School age: 0.9 | 0.1 (NS) | |
| Adolescent: 1.3 | Adolescent: 0.9 | 0.4 (NS) | |
| Alemzadeh (2004)149 | 0.97 ± 0.2 | 0.91 ± 0.2 | 0.06 |
| Conrad (2002)157 | Prepubertal: 0.7 ± 0.2 | Prepubertal: ~ 0.7 (estimated from graph) | Prepubertal: ~ 0 (NS) |
| Pubertal: 1.1 ± 0.3 | Pubertal: ~ 0.91 (estimated from graph) | Pubertal: ~ 0.1 (p < 0.01) | |
| Kordonouri (2006)151 | 0.96 | 0.93 | 0.03 (NS) |
| Liberatore (2004)172 | 1.10 ± 0.31 | 0.87 ± 0.17 | 0.23 (p = 0.00001) |
| Raile (2002)159 | 1.02 ± 0.27 | 0.79 ± 0.11 | 0.23 |
| Schiaffini (2005)168 | 0.93 ± 0.2 | 0.74 ± 0.15 | 0.19 (p < 0.01) |
| Sulli (2003)160 | 1.03 ± 0.30 | 0.75 ± 0.21 | 0.28 |
| Toni (2004)181 | 58.2 ± 15.3 IU | 44.4 ± 11) IU | 13.8 (p < 0.001) |
| Willi (2003)154 | 0.90 ± 0.03 | 0.61 ± 0.11 | 0.29 (NS) |
| Wood (2006)155 | 1.0 ± 0.3 | 0.8 ± 0.2 | 0.2 (p < 0.01) |
| Young children | |||
| Berhe (2006)165 | 0.74 ± 0.3 | 0.68 ± 0.25 | 0.06 (NS) |
| Litton (2002)164 | 0.61 ± 0.02 | 0.71 ± 0.07 | –0.1 (NS) |
Of the eight studies in adults,138–140,144,161,162,169,175 five showed a significant decrease138–140,144,162 and one161 showed a decrease, but the significance level was not reported. One study175 showed a significant increase and the other169 showed a non-significant increase.
There were 11 studies in children/adolescents. 149,151,154,155,157,159,160,166,168,172,181 Four155,168,172,181 showed a significant decrease, three showed a decrease but the significance level was not reported149,159,160 and two151,154 showed a non-significant decrease. Ahern et al. (2002)166 showed non-significant change in all subgroups (preschool age group increase and older age groups a decrease). Conrad et al. (2002)157 showed an almost negligible decrease in the prepubertal age group and a significant decrease in the pubertal age group.
There were two studies in young children. 164,165 One164 showed a non-significant increase and the other165 a non-significant decrease.
In summary, of the 21 studies examined, most showed a decrease in insulin dose when patient were on CSII. Five showed an increase in the insulin dose, but this increase was only significant in two studies [Garg et al. (2004),175 in adults; Conrad et al. (2002),157 in the pubertal subgroup]. The reduction in insulin dose will provide some savings to modestly offset the cost of the pump.
Quality of life
Nine studies evaluated aspects of quality of life associated with CSII use from the perspective of health-care professionals, parents or children. 140,141,145,152,153,174,178,180,184
Studies used varying methods to collect data including questionnaires, specified scales, scales developed for the study and interviews. Sample sizes were generally small; only one study evaluated more than 35 patients and this larger study assessed the views of health professionals and not patients/parents. 141
Adults/mixed age groups
Bruttomesso et al. (2006)141 sought the views of health professionals about CSII by sending a questionnaire to diabetic care centres with patients on CSII (n = 145 centres caring for 514 patients on CSII, age range 4–85 years). Patients on CSII represented about 5% of patients with diabetes. The health professionals felt that the greatest benefits of CSII were better metabolic control and greater flexibility with mealtimes and physical activity; less-important benefits included better control of dawn phenomenon and the reductions in insulin dose and hypoglycaemic episodes. Less than half of the physicians felt that CSII had improved patient comfort. The professionals felt that the main inconvenience was cost. Other inconveniences included the burden of constantly carrying an external device, the need for special education and the need of special and continuous care in wearing the pump. Only paediatricians felt that weight gain was a problem. No details were given about the questionnaire.
Rodrigues et al. (2005)140 asked patients to compare CSII with their previous treatment. All of the patients reported that they preferred CSII to previous treatment in overall terms and in terms of flexibility, convenience. All patients, including the four who discontinued CSII, would recommend CSII to others.
Linkeschova et al. (2002)145 assessed quality of life with a validated, diabetes-specific questionnaire. All quality of life parameters were significantly improved during CSII compared with insulin conventional therapy (ICT) in the 50 patients who had completed a quality of life questionnaire under ICT immediately prior to starting CSII therapy.
Children/adolescents
In the study by Mednick et al. (2004),178 parents and children (n = 22 children aged 10–18 years on CSII for between 3 and 22 months) completed the Insulin Pump Therapy Satisfaction Questionnaire (IPTSQ) that was developed specifically for this study, and children completed the Diabetes Quality of Life for Youths (DQoL-Y) questionnaire. Scores on the DQoL-Y were compared with those from children who had participated in the original DQoL-Y. In the IPTSQ, children and parents reported greatest satisfaction with flexibility in relation to meal schedules (parents 73% and children 81%), sleep schedules (parents only) and food variety (children only). About one-third (36%) of parents reported that the child was better able to manage diabetes on his/her own. Just under half of parents and children reported that the child had better control of the diabetes (parents 46% and children 43%). Rates of reporting an overall improved lifestyle were relatively low (parents 9% and children 5%). The main challenges reported were difficulties related to calculating insulin dose (parents 42% and children 41%) and difficulty inserting or changing pump cannulas (parents 38% and children 55%). Children reported that the main benefits of CSII were increased flexibility and convenience (76%) and the avoidance of painful insulin injections (33%). Just under half of the parents (45%) and about one-fifth (19%) of children would recommend the pump to others. About one-third of parents (35%) reported that the change to CSII did not go as well as anticipated. (One of our clinical experts told us that parents sometimes came back after the first 6 weeks saying that it had been harder work than they expected.) Children in the study reported lower satisfaction and less worry than the standardised sample on the DQoL-Y scale (p ≤ 0.001 for both). This may be due to small sample size, differences in mean ages of the samples or the fact that the standardisation sample included children on all types of insulin regimens. There was no significant difference between the groups for diabetes impact.
In a qualitative study involving 21 parents of 16 children (aged < 12 years), Sullivan-Bolyai et al. (2004)180 identified themes from interview audiotapes and field notes. Parents reported learning about the pump from nurses, physicians, friends and websites. They perceived that the pump would improve diabetic control. Worries included the catheter falling out or malfunctioning and the child being bullied. Parents reported that it took them between 10 days and 3 months to feel comfortable with CSII, and from 6 weeks to 9 months to feel confident. They had to alter their routines and learn to sleep through the night without checking the child’s blood glucose. They felt that older children became more involved in the management of the diabetes. On the day-to-day management of diabetes, they felt that their children had better blood glucose control and reported increased flexibility of mealtimes. Using CSII, they worried less about overall care, said that their sleep had returned to normal, that they had more free time, that children were in a better mood with increased concentration and increased participation in social life, and were more flexible about mealtimes.
Juliusson et al. (2006)171 used generic [(The Child Health Questionnaire (CHQ-CF87)] and diabetes-specific QoL instruments, and reported significant improvements in some areas of CHQ-CF87. There was a higher score on the family activity scale (p = 0.041) and change in health score (p = 0.042). However, diabetes-specific QoL was not significantly improved. The patient satisfaction data also showed a higher degree of general satisfaction, faith in disease self-management, and motivation to treatment.
In the study by McMahon et al. (2005),152 43 of the first 51 children completed the DQoL and the Self-Efficacy for Diabetes Scale (SED) questionnaires before treatment and 6 months later. The score for impact of diabetes on the patients fell indicating decreased impact (p < 0.05). Scores of individuals’ self-efficacy with diabetes increased significantly (p < 0.05). There was no significant change in worries about diabetes. Satisfaction with life did not change.
Young children
Alemzadeh et al. (USA, 2006)84 used the Preschool Children Quality of Life (TAPQOL) questionnaire to assess quality of life in young children (n = 14) and reported no significant change in TAPQoL subscales from before to after CSII (baseline to 1 year).
Saha et al. (Finland, 2002)153 stated that all parents of children under 2 years (n = 4 children) reported that CSII was easier to manage than conventional treatment.
Shehadeh et al. (Israel and Slovenia, 2004)174 compared parents’ views about the quality of life before and after 4 months of CSII use in young children (n = 15) using a modified version of the Diabetes Treatment Satisfaction Questionnaire (DTSQ, scores from 0 to a maximum of 36 for high satisfaction). Parents reported that quality of life significantly improved after CSII was started (DTSQ: 30.67 versus 19.8, p < 0.001). Both worry and impact subscales of the modified DTSQ (which measures treatment satisfaction not quality of life) were significantly improved on CSII (p < 0.001 for both). Fourteen out of the 15 families preferred CSII to the previous MDI, and refused to return to MDI.
Of the three studies that asked if patients/parents would recommend CSII to others, two studies reported that almost all would recommend CSII to others. 140,174 Just under one-half of the parents (45%) and about one-fifth (19%) of children would recommend the pump to others in the third study reporting this outcome. 178
One study, available in abstract only at present, has looked at the dermatological complications of CSII. Conwell et al. (2008),186 from Toronto, Canada, examined the skin of 50 consecutive patients and noted that most had skin lesions – scars, subcutaneous nodules, and erythema – in over half. But very few patients would consider stopping CSII because of these lesions, which suggests that the benefits outweigh the disutility of the skin lesions.
Summary of findings of observational studies
There are far more observational studies available now than there were at the last review. In general, they report:
-
Much greater improvements in HbA1c than reported in the RCTs.
-
Considerable reductions in severe hypoglycaemia. This may reflect selection for CSII of people having particular problems with hypoglycaemia, but that would make the results more applicable to the patients who would get CSII in routine care.
-
The majority of studies show no increase in DKA, and, if anything, it is decreased. The recent abstract from Sweden is concerning, but may reflect a period of very rapid expansion in CSII use.
-
Some gain in weight, but usually minor.
-
A reduction in daily insulin dose, which will provide some savings to offset the cost of CSII.
-
Gains in quality of life, with comments on items such as flexibility of meal choices and timings and other aspects of lifestyle, and diabetes being easier to manage in children. In these studies, patients prefer pumps.
Other evidence
Use of CSII at night-time only
Kanc et al. (1998)72 carried out a small trial to see if good control during the night, with avoidance of hypoglycaemia, would restore hypoglycaemic awareness in patients with T1DM who had lost it. Fourteen patients took part in a crossover study. In one arm, they continued their mealtime SA insulin and bedtime NPH. In the CSII arm, they continued their SA injections but switched to CSII at bedtime. Those who had been experiencing the dawn phenomenon used two or more basal rates during the night.
No differences in HbA1c were seen between the two arms, but hypoglycaemia was about a third less frequent (p = 0.03) and warning signs were improved. The authors believe that this was due at least partly to avoidance of nocturnal hypoglycaemia, although nocturnal testing was not frequent enough for them to be sure. Total daily insulin requirements were lower with CSII (48 units versus 56 units), with more being taken as SA at mealtimes.
Kaufman et al. (2000)187 carried out a similar study in children aged 7–10 years, with two arms in a crossover trial. In one arm, children continued with three injections of lispro and NPH. In the CSII arm the pump was used to provide basal insulin and the breakfast and dinner lispro. The duration of the trial was short, 4 weeks on each arm. During the CSII period, blood glucose control was better, as reflected in fructosamine levels and five daily measurements, including at 0300. Fear of hypoglycaemia was halved. The authors report that there was less hypoglycaemia but do not provide data. Insulin dosage was reduced from a mean of 0.9 units/kg per day to 0.7 units. Quality of life was reported to be better on CSII but no data are given.
Use of different basal rates
One of the differences between an analogue-based MDI and CSII is that, once glargine or detemir is injected, the basal rate is fixed for the day, and cannot be changed if, for example, unexpected exercise occurs. Nor can the user have different basal rates in, for example, morning and afternoon. However, pump users can programme different basal rates for different times of day, and for different days. We asked INPUT for data on how many different basal rates were used by members, and the results are shown in Table 18.
| Number of basal rates used | Percentage of members |
|---|---|
| Just 1 | 9 |
| 2 | 14 |
| 3 | 19 |
| 4 | 22 |
| 5 | 16 |
| 6 | 10 |
| 7 | 4 |
| 8 | 4 |
| 9 | 2 |
| 10 or more | 1 |
It would be interesting to look at HbA1c and hypoglycaemic episode frequency by number of basal rates used, but that is beyond the scope of this review.
The number of basal rates used raises an important issue about expertise in CSII use. Some of the trials are quite short: 16–24 weeks. Nearly all will recruit novice users [Maran et al. (2005)112 being an exception]. How long does it take a pump user to get the full benefit from a pump? Are the trials too short for users to get full benefit? Those randomised to CSII will be testing their blood glucose, and aware of hypoglycaemic episode frequency, but they will get at most one or sometimes two HbA1c results during the trial. So they will not have time to adjust their regimens, repeat the HbA1c 3 months later, and adjust again. Nor, perhaps, would they have enough time to try out different basal rates for different combinations of diet and activity.
It would be interesting to have data on HbA1c and hypoglycaemic episode frequency in pump users at 3-monthly intervals for several years.
Who benefits most from CSII?
Previous meta-analyses reported that CSII gave HbA1c levels lower, on average by 0.5% than MDI – a clinically significant, but not dramatic, improvement. The trials in these meta-analyses used mainly SA soluble insulin, rather than SA analogues. 18,50 Switching to the latter gives another 0.2% improvement. 11. A later meta-analysis of only studies using analogue insulins as the SA form, which at the time had only three trials, noted that the benefit of CSII relative to MDI was greater in those with high baseline HbA1c. 188
Pickup et al. (2005)138 explored ability to benefit further. Firstly, they studied the patients to whom NICE guidelines most applied – those who could not achieve good control without disabling hypoglycaemia. (They noted that previous trials often excluded patients who were having problems with hypoglycaemia.) In a before/after study in patients having problems with hypoglycaemia on MDI, they first tried a more intensive period of MDI for 5 months, and then, if control had not been achieved, started CSII. The improvement in HbA1c was 1.4%.
In an extension of this study with a larger group of patients, Pickup et al. (2006)139 showed that the strongest predictors of improvement in HbA1c were a high baseline level on MDI and variability of blood glucose. They noted that one of the main reasons for failing to achieve good control was hypoglycaemia, and that hypoglycaemia was associated with large swings in blood glucose, and hypothesised that subjects with wide variability in blood glucose levels would find it most difficult to achieve control on MDI because of high rates of hypoglycaemia.
More recently, Pickup and Sutton (2008)107 carried out a meta-analysis aimed specifically at identifying the impact of CSII in patients with, first, an incidence of severe hypoglycaemia that would make them fit the NICE guideline indication, and, second, an adequate duration of time on CSII (6 months or more). They did not restrict studies to RCTs; most were before/after studies.
The pooled hypoglycaemia rate on MDI was 62 events per 100 patient-years. There was a marked reduction of about 76% on CSII. HbA1c was reduced by 0.62%. The before/after studies reported a much bigger reduction in HbA1c (0.72%) than the RCTs (0.22%).
Data from the Insulin Pump Clinical Database
The following information is from unpublished data kindly supplied by R. Feltbower and the Database group, April 2007, but which is being submitted for publication.
A group of centres with considerable pumps experience, and hence with larger numbers of pump users than most clinics, have pooled their data in a project sponsored by Roche Diagnostics (Burgess Hill, UK) but run independently by the Paediatric Epidemiology Group, University of Leeds, UK. The usefulness of this data set is that it reflects, first, results in routine care outwith trials, and, second, it gives results from centres of pumps expertise.
The data currently available to us do not include details of what regimens patients were on before CSII, but we assume MDI. Current UK centres include Harrogate, Bournemouth, Leeds (paediatrics) and Middlesbrough.
The overall reduction in HbA1c was 0.9%. Table 19 shows mean changes in HbA1c by age group. These results are in some ways good, but also somewhat disappointing because the means still fell well short of targets.
| Age group (years) | Prepump HbA1c (%) | Postpump (%) | Change |
|---|---|---|---|
| 0–19 | 9.5 | 9.0 | –0.5 |
| 20–39 | 8.8 | 7.9 | –0.9 |
| 40–59 | 9.1 | 8.0 | –1.1 |
| 60–79 | 8.4 | 7.8 | –0.6 |
| All ages | 9.1 | 8.2 | –0.9 |
Short-term studies
Hirsch et al. (2005)189 carried out a crossover study comparing analogue MDI (aspart and glargine) with CSII. It was excluded from our main analysis because of the short-duration – 5 weeks on each arm, and too short to use HbA1c as a measure of glycaemic control. Fructosamine improved, and nocturnal hypoglycaemia was about 25% less frequent with CSII (p = 0.0024). Insulin dose was slightly lower on CSII.
One problem with short-term studies has been mentioned above. How long does it take users to achieve the maximum benefits of CSII, including the use of multiple basal rates when required? In their study in young children (where parents controlled the pump programming), Wilson et al. (2005)126 noted that pump users started with an average of 2.9 basal rates per day, but by the end of a year were using 4.8 different basal rates.
There are also several very short studies of CSII in insulin-resistant T2DM, used to treat insulin resistance, but we have excluded these.
Quality of life
Barnard et al. (2007)108 recently reviewed studies reporting quality of life aspects on CSII. This group has received support from Roche, one of the pump manufacturers, for pump-related research, and a conference abstract version of the review carries the Roche logo, but the review is high quality and properly critical, and was carried out for a PhD thesis (KD Barnard, University of Southampton, 2007, personal communication), not funded by Roche, and seems free of bias. Most of the 17 studies in their review are included in this review or the 2002 assessment report. 50 Barnard et al. (2007)108 pay particular attention to the quality of life instruments used, and note that some are not validated. They comment on problems with the design of the studies, such as the lack of control groups, and, in particular, the confounding role of structured education, which should be given to all before commencing CSII, but which may not be given to comparator groups if there are any. In before/after studies, it is difficult to say how much of the benefit is due to the education rather than to the CSII. They also note the small numbers in many of the studies.
They conclude that there is currently no consistent quality of life gains from CSII in the current evidence base, but accept that this may be more a problem with lack of evidence than evidence of no benefit: ‘if a minimum standard were assumed to be a randomised controlled trial, which controls for increased education and contact time, uses appropriate sensitive measures, and recruits large numbers of participants to each group, there are no current published studies which meet these criteria.’.
Barnard et al. (2007)108 recommend further research:‘… a large-scale multi-centre patient preference controlled trial is required to focus specifically on quality of life issues surrounding insulin pump therapy… It is important to be clear about what quality of life means, i.e. increased independence, greater freedom, greater flexibility, easier management of diabetes, better control, etc.’.
A key point in quality of life aspects of CSII is that some of the reported benefits are in health-related quality of life, but many are not. Some are in aspects such as social life (not having to worry abut what time meals arrive on social occasions), greater ease of travel, flexibility of lifestyle, and enhanced ability to enjoy physical activities. Once the basal insulin in MDI is in, it is there for the day and night. The pump infusion can be adjusted at any moment.
Having identified a shortage of evidence, Barnard and Skinner carried out two studies. 190 The first was a qualitative study based on interviews with 80 pump users. The study was funded by Roche Diagnostics; the patients were identified by Roche; and the interviews were carried our by Roche staff, trained by the investigators. The potential for bias seems considerable, but we think it provides reliable data, for two reasons. First, the disadvantages of CSII are dealt with as well as the benefits, and the authors report that 60% of the patients reported downsides to CSII. Second, many of the comments match those obtained from pump users in the previous assessment report for NICE, in particular those mentioning flexibility of lifestyle, the feeling of greater control over the disease, less dependence on other family members, and the reduction in hypoglycaemia.
The main disadvantages of CSII include visibility of the pump (although the latest versions may be smaller), problems with breakdowns (21% of respondents), and lack of appropriate advice from health-care professionals. Three of the 80 noted cost was a problem, presumably because they were paying that themselves: some PCTs are very restrictive in funding. But the positives outweighed the negatives, as one would expect in a group of confirmed users. The authors summarise the results thus:‘Participants overwhelmingly reported experiencing benefits and improvements in their quality of life associated with insulin pump use.’.
The second study was provided to us as part of the industry submission but has since been published. 191 It was funded by Roche Diagnostics, and two groups of patients were identified by Roche: the first from their register of pump users, the second from non-CSII users who were on the register of blood glucose monitor users (although some of the latter group were found to be pump users). The authors tried to have four non-pump users for every pump user, but response rates were very different – 85% in the pump user group versus 38% in the non-users, which must be a major source of bias.
This reflects a problem in research involving pump users. They tend to be enthusiastic and highly motivated individuals who are happy to take part in research, which may make it difficult to find a comparison group with the same characteristics.
One strength of this study was that the authors used several instruments to assess quality of life, including the World Health Organization Quality of Life Abbreviated questionnaire (WHOQOL-BREF),192 which has domains for physical health, psychological state, social relationships and ‘environment’, which includes financial matters. They added topic-specific instruments, such as questionnaires assessing aspects of insulin delivery, fear of hypoglycaemia and problem areas in diabetes. Another strength was that the authors anticipated a possible bias arising from the fact that many pump users had to be self-funding, and hence there would be social selection biases operating, and in multiple regression carried out analyses controlling for socioeconomic status.
The result suggest that pump users score better on some of the non-health related aspects of quality of life, as well as on having fewer worries about hypoglycaemia. Again, these match the comments received from a far smaller number of users for the last assessment report. 50
New systematic reviews
Weissberg-Benchell et al. 193 published a meta-analysis in 2003 but it included studies only up to 2001, and therefore does not add to the findings of the last assessment report. 50
Retnakaran et al. (2004)188 carried out a meta-analysis of trials, which used rapid-acting analogues in both MDI and CSII, and therefore included only three trials, two of which were included in the last assessment report. 50 The third is De Vries et al. (2002),119 included above. All three trials used NPH as basal insulin.
Siebenhofer et al. (2004)194 included studies comparing SA analogue and SA soluble, for both CSII and injection regimens. They included eight trials in CSII, most of which had been included in the last assessment report50 and in the journal paper by Colquitt et al. (2003). 11 Three trials were not included in the assessment report, two being definite exclusions because of short duration of follow-up. One trial in the assessment report analysis was not included by Siebenhofer.
A high-quality report from AETMIS drew heavily on the last assessment report but added trials published up to 2004. 19,50 These are included in this report.
Indications for CSII in different age groups: children and adolescents
The German working group for insulin pump treatment in paediatric patients195 has identified seven indications for CSII, in a cohort of 1567 children and adolescents:
-
dawn phenomenon (27.4%)
-
reduction of hypoglycaemia (20%)
-
flexibility (22.4%)
-
improvement of hyperglycaemia (18.1%)
-
motivation (10.4%)
-
failure of injection therapy (1.6%), by which they meant CSII was used as a ‘last resort’
-
pregnancy (0.1%).
But the proportions varied widely amongst the four age subgroups. In the under-5s, the main reason (42%) was reduction of severe hypoglycaemia, followed by flexibility (22%). In the 5- to 9-year age group, hypoglycaemia was again the top indication (32%), followed closely by the dawn phenomenon (28%). In the 10–14s, dawn phenomenon was the most common reason (32%), followed by flexibility (22%) and hypoglycaemic episode (17%) and hyperglycaemia (18%). In the 15- to 20-year range, there was an even spread – flexibility 26%, dawn phenomenon 22%, hyperglycaemia 21%, motivation 18%.
One important finding of this study was that initial reductions in HbA1c were not always sustained. Amongst those who started CSII because of hyperglycaemia, HbA1c fell from an initial mean of 8.8% to 8.5% at 12 months, but then rose back up to 8.8% at 36 months. However, this represents success, as in children HbA1c usually rises with age. 43 Those who started CSII because of hypoglycaemia had a lower starting HbA1c of 7.6%, maintained that at 12 months (7.5%), after which it rose to 7.9% at 2 years and 8.1% at 3 years.
Summary of clinical effectiveness
Since the last review, the number of observational studies has increased considerably, and there have been more trials of CSII against NPH-based MDI. We now have some trials in people with T2DM.
Unfortunately, there is a relative scarcity of trials with analogue-based MDI, and some of those are very small.
The one study of CSII versus analogue MDI in adolescents/children shows a reduction of 1% in HbA1c.
The recent RCTs of CSII versus NPH-based MDI do not add much to the previous review, which found a reduction in HbA1c of 0.6%, a similar figure to that reported by Pickup et al. in their 2002 meta-analysis. 18
The observational studies have variable results but show larger drops in HbA1c.
The Insulin Pump Clinical Database also shows a larger reduction, of 0.9% but variable amongst age groups.
Quality of life appears better on CSII, and most patients prefer it.
Most studies show a reduction in hypoglycaemia with CSII, and a reduction in insulin dose required.
Chapter 3 The industry submission
The joint submission by the pump manufacturers, under the auspices of the Association of British Healthcare Industries, started by making two points about usage:
-
there was considerable variation of provision in England, with some PCTs being more restrictive than others, and probably more restrictive than NICE intended
-
UK usage was much less than in comparable countries.
Clinical effectiveness
The main source of clinical effectiveness data was the Pickup and Sutton meta-analysis,107 described previously, which reported a 75% reduction in severe hypoglycaemia and a 0.6% improvement in HbA1c. An account of this analysis has been given previously (Chapter 2).
The submission also comments that quality of life is better on CSII and that ‘many studies fail to capture the real-life benefits, such as convenience, reduced worry, and greater freedom, reported by patients receiving insulin pump therapy’, a statement with which we agree, based on review of the literature and previous submissions by pump users or families.
The cost-effectiveness analysis in the industry submission uses three possible scenarios in terms of HbA1c benefit in T1DM, discussed below. No modelling is done in T2DM. Nor is there any modelling of hypoglycaemia-only benefit, for example in those with HbA1c under 7.5% (taking the NICE guidelines target as good control) whose HbA1c does not improve on CSII but who have less trouble with hypoglycaemia. This group was identified through the ‘Patient perspectives’ section of the previous assessment report. 50 The base-case analysis was of a cohort 38 years of age and a duration of diabetes of 10 years. Baseline HbA1c was taken to be 9.4% for MDI, with a standard deviation (SD) of 2.1%.
Immediate benefits included a reduction in SH events, as derived from the Pickup and Sutton analysis and outlined below, but it should be noted that although the CORE model used for the modelling within the industry submission permits a death rate to be associated with SH events, the modelling within the industry submission appears to have conservatively assumed that no such deaths would occur. The British Diabetic Association Cohort Study reported that of 22 out of 949 (2.3%) deaths in the cohort (patients with T1DM, diagnosed under the age of 30) were due to hypoglycaemia. 28,196 Edge et al. (1999)197 reported that in patients aged under 20 at death, and with diabetes on the death certificate, 8% of deaths (7 out of 83) related to diabetes were due to hypoglycaemia, but hypoglycaemia was also suspected in another four patients who were found ‘dead in bed’. This term refers to people found dead in an undisturbed bed, having been in apparently good health the day before, and some are known to have had problems with hypoglycaemia. 198 Tunbridge (1981)199 looking at deaths of diabetics under age 50, concluded that 3–4% were due to hypoglycaemia.
The industry submission cites a paper by Cryer,25 which estimates that 2–4% of deaths in T1DM are due to hypoglycaemia, which fits with the aforementioned studies. Other points:
-
As regards current management, the submission (p. 13) states that ‘In T1DM, therapy is mainly through intensive insulin treatment as optimised MDI or CSII’ but that may be unduly optimistic. Most children in the Scottish audit of under-15-year-olds were still on conventional insulin regimens in 2002–4. 43
-
In cost comparisons, the cost of MDI is based on glargine, but given the high proportion still on NPH-based MDI, that could be seen as possibly misleading, and as reducing the marginal cost of CSII. However, the use of MDI based on long-acting analogues is justified because current NICE guidance expects a trial of analogue MDI before CSII.
The clinical effectiveness section contains three sections:
-
the Pickup and Sutton107 meta-analysis, already described
-
quality of life data from the studies by Barnard et al. ,108,190,191 already described
-
a literature review, although it is more of an annotated bibliography than a systematic review. Most of the studies listed are in Chapter 2 of this report.
The review of cost-effectiveness studies reports the results of the Scuffham and Carr (2003)200 and Roze et al. (2005)201 papers, and the abstract of the paper by Conget et al. (2006)202 (the full paper was not translated). Our more complete review is provided in Chapter 4.
Cost-effectiveness
The cost-effectiveness analysis used the CORE model, which we consider to be a highly developed and well-tested model, and one of the foremost of its kind, although there are only a few models of T1DM. Palmer et al. (2004)203 outlined the broad structure of the CORE model for patients with T1DM and T2DM.
The CORE model can be briefly summarised as being an internet-based model, which is based upon 15 submodels that simulate the main complications of diabetes. Each submodel is a Markov model, which uses Monte Carlo simulation, incorporating the time, the state, the time in state and transition probabilities that are typically diabetes type dependent, as derived from published sources.
A common problem with standard Markov modelling is the requirement that distinct mutually exclusive memory-less disease states have to be specified. This approach would overlook the interactions between the different complications of diabetes unless a prohibitively large number of disease states were defined. CORE modelling uses tracker variables to allow interactions between the different submodels, with the progression of one or more complications influencing the transition probabilities in other submodels in which a relationship has been established. For instance, the risk of a first myocardial infarction (MI) is linked to whether gross proteinuria, microalbuminuria or end-stage renal disease has developed, an RR being specified for each of these.
The 15 submodels of CORE are: MI; angina; congestive heart failure (CHF); stroke; peripheral vascular disease; neuropathy; foot ulcer, with possible amputation; retinopathy; macular oedema; cataract; nephropathy; hypoglycaemia; ketoacidosis; lactic acidosis; and, general mortality. Note that a specific mortality is associated with the MI; CHF; stroke; foot ulcer, with possible amputation; nephropathy; hypoglycaemia; ketoacidosis and lactic acidosis submodels. For hypoglycaemia the specific mortality is specified by the user.
The population characteristics of source references for the 15 complications of diabetes submodels within the overall CORE model are briefly summarised in Appendix 5. It can be noted in passing that not all submodels are differentiated by diabetic type. MI, angina, stroke, peripheral vascular disease and foot ulcers leading to amputation are modelled as having the same inputs for patients with T1DM as for patients with T2DM. The average age within the references contributing to the modelling is also often quite high, and while some references relate their effects to age groups, the age range within these studies may still give rise to some concerns about using the CORE model among younger age groups. In particular it does not appear suitable for modelling effects of CSII started in childhood.
The baseline population characteristics within CORE can be specified in terms of age, sex, duration of diabetes, racial characteristics, glycaemic control, blood pressure, the BMI, lipid levels, smoking and baseline rates of complications. Treatments can be specified as modifying glycaemic control, hypoglycaemic event rates, SH event rates, blood pressure, the BMI and lipid levels. Typically, only glycaemic control and hypoglycaemic event rates are specified.
Palmer et al. (2004)204 undertook a validation exercise of the CORE model using published data for the incidence of the complications associated with both T1DM and T2DM. This exercise appears to show reasonably good validation for the incidence of the complications examined. However, it should be noted that for T1DM the only complications for which validation data were available were the microvascular complications of diabetes. While these showed reasonably good correspondence within the validation exercise, macrovascular complications among those with T1DM such as CHF and MI were not explored within the validation exercise.
The results of Palmer et al. (2004)204 for the validation for overall survival rates for those with T1DM used data from the US Joslin Clinic Study. 205,206 This CORE appeared to overestimate the death rate among those with T1DM, with this overestimation worsening with the time horizon used. While correspondence was reasonably good at the 10-year point, with CORE estimating 94.8% survival in contrast with 96.8% within the Joslin Clinic Study, by the 25-year point the correspondence has worsened to CORE estimating 68.8% survival as against 81.0% within the Joslin Clinic Study. The source of this is not readily apparent, but, given the validation results for the microvascular complications of diabetes in comparison with the Joslin Clinic Study, there may be a tendency for the CORE model to overestimate the incidence of macrovascular complications with an associated higher death rate. Data from the EDIC study207 suggests a link between HbA1c control and macrovascular complications, but, to the best knowledge of the authors, the predictions of CORE have not been validated against this.
Within CORE modelling any improvement in baseline HbA1c as a result of a novel treatment is typically assumed to be sustained. There is the possibility that while an improvement may be observed over a period of time, this relative improvement in HbA1c may be eroded in the medium to long term. While CORE does permit some adjustment of this assumption through the use of a long-term adjustment factor, it does not appear to permit the evolution of the gain in HbA1c to be specified in detail. Given this, the longer term adjustment to the relative improvement in HbA1c appears to be little used and the absolute gain over baseline HbA1c is typically assumed to be maintained.
In the CORE model deaths from hypoglycaemia can occur. However, given a lack of data this is typically not included, and has not been included within the industry modelling. Not including mortality is a conservative assumption, and will to a degree underestimate the QALY gain and overestimate the cost per QALY, especially as deaths from hypoglycaemia may occur in young people, hence leading to large number of life-years being lost.
Diabetes models are (if their developers submit them) tested in the Mount Hood Challenge. In the most recent of these, one test for the models was their ability to predict the outcomes of the DCCT trial. 208 The CORE model gave estimates very close to what was observed for renal disease, retinopathy and peripheral neuropathy in the intensive group, and was also close for neuropathy and renal disease in the conventional group. It did somewhat under-estimate retinopathy in the conventional group. But, overall, getting good results in a voluntary challenge reinforces our confidence that CORE is a good model, and, given the paucity of models of patients with T1DM, it is appropriate for the industry submission to have used it.
Modelling inputs
The cost-effectiveness results presented within the industry submission were reviewed in tandem with additional data supplied through the web-based CORE model implementation. As is clear from the summary of CORE above, from the summary of the population characteristics within the clinical sources used for the CORE model as outlined in Appendix 5, and from communication from the CORE modelling team, it is doubtful whether the CORE model would be applicable to the paediatric or adolescent population of patients with T1DM. The submission was been prudent in this regard, and modelled a cohort of baseline age of 38 years and an average duration of diabetes of 10 years.
The background prevalences for most of the vascular complications arising from diabetes were taken from the DCCT 1994 paper regarding the effect of intensive on the development and progression of long-term complications. 209 Baseline values for aspects such as cholesterol levels and blood pressure were drawn from two references. 68,210 As these references did not provide all of the data necessary for the CORE model, the background prevalences for angina, background diabetic retinopathy, proliferative retinopathy, macular oedema, cataract, foot ulcer and amputation were apparently set to zero. To the degree that background prevalences were underestimates within the modelling, this may have tended to slightly overstate the benefit of the anticipated improvement in HbA1c arising from adoption of CSII. But given that the baseline age of the cohort simulated it cannot be stated whether this would have necessarily been to the benefit of CSII.
The key clinical effectiveness inputs to the industry submission were drawn from the meta-analysis of Pickup and Sutton (2008),107 which analysed the effect of CSII relative to MDI within a population with T1DM with problems with severe hypoglycaemia episodes and a rate of severe episodes of more than 10 per 100 patient-years. Clinical effectiveness estimates were as shown in Table 20.
| Simulation: baseline HbA1c 9.4% (SD ± 2.1%) | Mean change from baseline | |
|---|---|---|
| CSII | MDI | |
| Trial-based analysis | ||
| HbA1c (%) |
–0.62 (SD unknown) |
0 (SD 0) |
| Severe hypoglycaemia (events/100 patient-years) |
14.8 (SD 0) |
62.0 (SD 0) |
| UK-relevant analysis | ||
| HbA1c (%) |
–1.29 (SD ± 2.98) |
0 (SD 0) |
| Severe hypoglycaemia (events/100 patient-years) |
14.8 (SD 0) |
62.0 (SD 0) |
| Conservative UK analysis | ||
| HbA1c (%) |
–0.95 (SD ± 2.98) |
0 (SD 0) |
| Severe hypoglycaemia (events/100 patient-years) |
14.8 (SD 0) |
62.0 (SD 0) |
The trial based analysis related to the meta-analysis of Pickup and Sutton, which related the average baseline HbA1c to the change that would be anticipated from the use of CSII. The average baseline HbA1c within this was 8.11% and the reduction was only –0.62%. The baseline HbA1c may seem unduly optimistic, which means that patients have less to gain in terms of complications avoided. However, those who stand to gain from CSII include patients with good or even normal HbA1c, but having problems with severe hypoglycaemia. It is therefore worth including this group.
However, the submission goes on to point out that the UK-based population within the meta-analysis had considerably worse control and an average HbA1c of 9.4%. This was mapped to given an average UK-relevant improvement from CSII of –1.29%. This might be thought to be inappropriate because the HbA1c level in all patients might be higher than in those being considered for CSII, who should already be on MDI. However, we know from unpublished data from the Insulin Pump Database (R. Feltbower, University of Leeds, 2007, personal communication) that the mean level before starting CSII was 9.1%, so the figure used in the baseline analysis is not that far away from a representative cohort.
The conservative analysis of –0.95% represents the mid-point between –0.62% and –1.29%. Note that these changes were all applied to a baseline HbA1c of 9.4%. As a consequence, MDI patients for the trial-based analysis and the conservative UK analysis will have tended to have too high a baseline HbA1c, which may have tended to bias these analyses to a degree towards CSII.
The data underlying the trial-based analysis, as summarised above (and presented within Table 20 of the economic appendix to the manufacturer’s submission), were not submitted electronically. Based upon the additional data submitted electronically, uncertainty appears to have only entered the model with respect to the baseline HbA1c level and the effect of CSII upon this.
The baseline level of HbA1c as applied to the MDI cohort, had a mean of 9.4% but this appears to have been subject to an SD of ± 2.1%. As a consequence, the range of HbA1c levels within the MDI cohort was somewhat greater than might have appeared to be the case within the submission.
Similarly, it appears that at least for the UK-relevant analysis and the conservative UK analysis, the impact of CSII upon HbA1c also involved a large range having an SD of ± 2.98%. This uncertainty as to effectiveness does not appear to have been linked to patients’ baseline levels of HbA1c, as would be implied by the logic applied within the submission to subsets within the Pickup and Sutton107 meta-analysis. This would tend to have increased the effect of CSII in patients with the worst control. Given this, some patients will have been simulated to have worse control under CSII than under MDI, but other patients using CSII will be simulated to have very tight control of HbA1c indeed.
While the submission is not explicit upon this point, the simulation inputs as uploaded by the CORE team to the CORE website implementation appear to indicate that distributions were placed upon both the baseline HbA1c and reduction in this associated with CSII. Unfortunately, the current implementation of CORE does not permit a link between baseline HbA1c and the effect of CSII upon this to be specified in the probabilistic sense: i.e. to specify a positive covariance between these variables. Given this, it may have been more appropriate to have modelled a representative patient than to have placed uncorrelated distributions upon these two variables where a clear covariance structure appears to be implied by the meta-analysis of Pickup and Sutton. 107
The direct costs of CSII and MDI treatment were drawn from industry sources and the British National Formulary (BNF), as outlined in Appendix 6 (and annualised within Table 23 of the economic appendix to the industry submission). It is not immediately clear what dose or patient weight has been assumed, but a point to note is that the industry submission anticipated a 25% reduction in the need for insulin, resulting in a cost saving from CSII of £177 per patient per year, reportedly drawing the dose assumption from the previous HTA and the cost from BNF. But the other costs of CSII more than offset this, with the annualised cost of CSII being £2770 as against £1224: an additional net cost of around £1550 from the use of CSII.
The cost of an SH event was taken to be £413, this being stated as having been drawn from the NICE inhaled insulin TAR,211 which, in turn, cites the NHS reference costs as the source. However, it should be borne in mind that this is likely to relate to a very SH event, the average length of stay within hospital for this reference cost being slightly in excess of 2 days. As outlined within the NICE glargine HTA,75 only a minority of patients are likely to be admitted to hospital following an SH event.
Given the centrality of the effect upon SH events within the submission an average cost of £413 may have been too high, and the £62 of the NICE glargine HTA may have been more appropriate or at a minimum appropriate as a sensitivity analysis. For instance, it appears that given an annual rate of 0.620 SH events under MDI compared with 0.148 under CSII, the annual cost of treating these would be around £280 for MDI compared with around £60 for CSII. This represents an annual saving of £220 from the use of CSII as against MDI. The parallel figures using the lower cost of £62 per SH event would appear to be £42 for MDI, £9 for CSII and a net annual saving of £33 per patient. Given the assumed 75% reduction in SH event from CSII and its centrality to the analysis, the assumed cost of £413 rather than £62 effectively reduces the additional annual cost of treatment with CSII by a little over 10%.
Costs of complications were mainly drawn from the Clarke et al. (2003) UKPDS65 paper,212 while utility values for were mainly drawn from the Clarke et al. (2002) UKPDS62 paper. 213 Note that UKPDS62 relates to patients with T2DM. There is no obvious reason to anticipate that the utility decrements arising from the complications associated with diabetes would be particularly different between patients with T1DM and T2DM. The appropriateness of the baseline utility value within UKPDS62 of 0.814 to patients with T1DM is a matter of conjecture, although an Australian study by Coffey et al. (2002)214 found a somewhat lower baseline value of 0.672 for those with T1DM.
Quality of life values are shown in Table 21, along with their stated sources.
| Complication | QoL | Source |
|---|---|---|
| Diabetes | 0.814 | Clarke 2002213 |
| Haemodialysis | 0.490 | Tengs 2000215 |
| Peritoneal dialysis | 0.560 | Tengs 2000215 |
| Kidney transplant | 0.762 | Tengs 2000215 |
| Background diabetic retinopathy | 0.814 | Clarke 2002213 |
| Proliferative diabetic retinopathy | 0.814 | AIHW 2003216 |
| Macular oedema | 0.794 | AIHW 2003216 |
| Severe vision loss/blindness | 0.734 | Brown 2004217 |
| Cataract | 0.794 | AIHW 2003216 |
| Neuropathy | 0.624 | AIHW 2003216 |
| Healed diabetic ulcer | 0.814 | Clarke 2002129 |
| Active ulcer | 0.600 | Carrington 1996218 |
| Amputation, year of event | –0.109 | Clarke 2002129 |
| Amputation, years 2+ after event | 0.680 | Clarke 2002213 |
Note that due to their short duration there are limited data on the quality of life impact arising from an SH event. Those studies that exist, for example Davis et al. (2005),219 Lundkvist et al. (2005),220 Tabaei et al. (2004)221 and Wikblad et al. (1996),222 are difficult to interpret due both to confounding variables and indeterminacy in terms of the duration of any quality of life impact from SH events. However, these papers do clearly show a significant effect upon quality of life from patients’ most SH events. The impact may depend on where the episode happened. An event at home may have less impact than one at work, which may lead to time lost, and loss of confidence in both subject and employer.
The submission appears to have assumed that a quality of life detriment of –0.0121 is associated with each SH event. While this appears to have been an arbitrary assumption, the value does not appear to be unreasonable. Perhaps, importantly, and as demonstrated in the discussion and modelling of the subsequent chapter, given the average rate of SH events, the results of the cost-effectiveness modelling are relatively insensitive to the quality of life detriment associated with each SH event. Results are mainly driven by the effect upon glycaemic control.
Industry submission modelling results
Given the assumptions of the modelling and the 50-year time horizon, the aggregate results of the CORE modelling can be summarised as shown in Table 22.
| CSII | MDI | Difference | |
|---|---|---|---|
| Trial-based analysis | |||
| Life expectancy | 21.29 | 20.36 | 0.93 |
| Life expectancy (discounted) | 13.97 | 13.55 | 0.42 |
| QALYs (discounted) | 9.19 | 8.69 | 0.50 |
| Treatment costs (discounted) (£) | 40,074 | 17,211 | 22,863 |
| Other costs (discounted) (£) | 36,977 | 42,682 | –5705 |
| Total costs (discounted) (£) | 77,051 | 59,893 | 17,158 |
| ICER: cost per QALY (£) | 34,330 | ||
| UK analysis | |||
| Life expectancy | 22.34 | 20.36 | 1.99 |
| Life expectancy (discounted) | 14.44 | 13.55 | 0.89 |
| QALYs (discounted) | 9.64 | 8.69 | 0.95 |
| Treatment costs (discounted) (£) | 41,329 | 17,211 | 24,118 |
| Other costs (discounted) (£) | 34,550 | 42,682 | –8132 |
| Total costs (discounted) (£) | 75,879 | 59,893 | 15,986 |
| ICER: cost per QALY (£) | 16,842 | ||
| Conservative UK analysis | |||
| Life expectancy | 21.80 | 20.36 | 1.44 |
| Life expectancy (discounted) | 14.20 | 13.55 | 0.65 |
| QALYs (discounted) | 9.41 | 8.69 | 0.72 |
| Treatment costs (discounted) (£) | 40,683 | 17,211 | 23,472 |
| Other costs (discounted) (£) | 35,613 | 42,682 | –7069 |
| Total costs (discounted) (£) | 76,296 | 59,893 | 16,403 |
| ICER: cost per QALY (£) | 22,897 | ||
While an analysis based upon the entire Pickup and Sutton (2008)107 meta-analysis results sees a cost-effectiveness of a little over £30,000 per QALY, increasing the average reduction in HbA1c from CSII to 1.29% effectively doubles the anticipated patient gain from CSII, while also slightly reducing the overall net cost given the reduced rates of complications requiring treatment.
The potential effect of the £413 cost per severe glycaemia event, as opposed to £62, may have had some impact upon the total costs as already noted, possibly being equivalent to a little over a 10% reduction in the net direct treatment costs of CSII.
Note also that within the trial base analysis the cumulative effect of CSII upon macrovascular events over the 50-year time horizon was leading to an absolute reduction in deaths from CHF of 0.7%, of deaths from MI of 0.6% and of deaths from stroke of 0.3%.
A full list of the results of the sensitivity analyses within the industry submission is presented in Appendix 6, the main results of these being summarised below.
Time horizon
As usual with diabetes, improved control now reduces complications years into the future, and so discounting has a large effect. In a relatively newly-diagnosed patient, the costs of CSII will be incurred now and every year thereafter, but the savings from, for example avoiding or postponing dialysis for end-stage renal failure, may not occur for 20–30 years. The industry submission includes various sensitivity analyses that alter the discount rates, which for the previous NICE discount rates of 1.5% for health effects and 6.0% for costs reduced the incremental cost-effectiveness ratio (ICER) for the trial-based analysis from £34,330 per QALY to £18,997 per QALY (see Table 33). The results of this sensitivity analysis were not reported for the other scenarios deemed to be more relevant to the UK setting within the industry submission.
As noted within the cost-effectiveness literature review, there may be some concerns around the possibility of CORE modelling tending to overestimate macrovascular events and in turn mortality within the population of those with T1DM. In parallel with the sensitivity analyses for discount rates, the results of sensitivity analyses on the time horizon appear only to be reported for the trials-based analyses: time horizons of 15 years, 10 years and 5 years, increasing the ICER from the base-case value of £34,330, to £42,039, £47,921 and £63,795 per QALY, respectively.
Hypoglycaemia
From the submission, it was not clear quite what the industry modelling includes in terms of the impact of reduction of hypoglycaemic episodes on quality of life, although the electronic modelling inputs uploaded to CORE website indicate a quality of life loss from each SH event of 0.0121, and also a quality of life loss from each non-SH event of 0.0052. As mentioned above, the cost of SH episodes is included at £413. The CORE model has a section for hypoglycaemic episodes, but Tables 27 and 28 of the industry submission do not mention hypoglycaemia; Table 29 includes the cost of hypoglycaemic episodes. As already noted, it appears that the industry submission has conservatively assumed that SH events have no death rate associated with them.
Fear of hypoglycaemia
The submission does not appear to include allowance for benefits such as reduction in fear of hypoglycaemias, noted in the NICE appraisal of glargine. 51 In that TA (TA 53) of long-acting insulin analogues (at that time only glargine), the NICE Appraisal Committee accepted that both hypoglycaemic episodes and the fear of such episodes recurring caused significant disutility. The relevant paragraph states:
The Committee accepted that episodes of hypoglycaemia are potentially detrimental to an individual’s quality of life. This is partly the result of an individual’s objective fear of symptomatic hypoglycaemic attacks as indicated in the economic models reviewed in the Assessment Report. In addition, as reported by the experts who attended the appraisal meeting, individuals’ quality of life is affected by increased awareness and uncertainty of their daily blood glucose status and their recognition of the need to achieve a balance between the risk of hypoglycaemia and the benefits of longer-term glycaemic control. The Committee understood that improvement in this area of concern regarding the balance between hypoglycaemia and hyperglycaemia could have a significant effect on an individual’s quality of life.
However, the guidance did not specify the amount of utility lost because of fear of hypoglycaemic episodes, and nor did the HTA report,75 because it was based on the industry submission from Aventis, which was classed as confidential. But clearly the utility gain from reducing the fear of hypoglycaemic episodes was enough to change a very large cost per QALY to an affordable one.
Other benefits not included
The submission does not factor into the ICER calculations, aspects of quality of life, reduction in depression, less cognitive impairment in children, and non-health-related quality of life gains, such as flexibility of lifestyle. Some of these omissions are understandable due to unavailability of data. In particular, it would be controversial to try to assess the impact of cognitive impairment on quality of life. If a child loses 10 points in IQ score, that does not mean that quality of life is reduced. In other cases, such as flexibility of lifestyle, the measures used most often in quality of life studies in diabetes may not capture the effect. 223
Summary
The strengths of the modelling include the use of the CORE model and the range of scenarios and sensitivity analyses.
The weaknesses are mainly due to data deficiencies (in the literature rather than the submission), which means that the effect of some benefits are not included. Modelling appears to be based only on the benefits of lowering HbA1c, mediated through the reduction in long-term complications, and on short-term costs of a reduction in severe hypoglycaemia. It is possible that the net effect is that cost-effectiveness of CSII may be underestimated, an unusual feature in industry submissions.
Chapter 4 Economics: CSII versus MDI
This chapter has four sections: the first examines the evidence on patient preference and quality of life; the second reviews the existing literature on cost-effectiveness of CSII; the third considers the costs of pumps; and the fourth provides our cost-effectiveness modelling.
Patient preference and quality of life
The previous HTA report50 identified and summarised one RCT of CSII versus MDI that also reported patient quality of life. Tsui et al. (2001)224 randomly assigned 27 T1DM patients to either CSII or MDI, and reported DQoL scores at baseline and 9-month follow-up (Table 23). Unfortunately, possibly due to the relatively small size of the trial none of the differences were significant.
| DQoL dimension | CSII score | MDI score |
|---|---|---|
| Satisfaction | 75.6 | 68.3 |
| Impact | 69.9 | 68.4 |
| Diabetic worry | 85.2 | 79.8 |
| Social worry | 89.6 | 94.0 |
| Global health | 68.2 | 67.3 |
The current review identified an additional 16 full papers that involved patient preference and quality of life for CSII (Table 24),115,116,119,120,121,140,152,171,174,178,225–230 together with an additional four papers that were available only as abstracts. Among the 16 full papers, seven were RCTs with results from an additional RCT being reported but without reference to the control arm. 115,116,119–121,225,226 All but two of the RCTs were of T1DM. 115,116 One controlled study was identified,227 with eight before/after studies being identified,140,152,171,174,178,228–230 all of which were of T1DM. 141 An additional study surveying diabetic centres was identified, the vast majority of patients covered by this having T1DM. 141
| Full papers: | ||||
|---|---|---|---|---|
| Type | Sample | Country | Results | |
| Type 1 | ||||
| Weintrob (2003)225 | Crossover RCT | 23 | Israel | DQoL-Y NS |
| Paediatric | 70% prefer CSII | |||
| Hoogma (2006)226 | RCT | 223 | 5 nations | DQoL CSII superior |
| SF-12 CSII superior mental health | ||||
| Devries (2002)119 | RCT | 79 | Netherlands | 11% randomised refuse to start CSII |
| SF-36 CSII superior general health | ||||
| SF-36 CSII superior mental health | ||||
| Dimeglio (2004)120 | RCT | 20 | USA | CSII maintained in 19/20, only one family opts to switch back to MDI |
| Paediatric | ||||
| Fox (2005)121 | RCT | 26 | USA | Parental quality of life outcomes only significantly different among fathers |
| Paediatric | ||||
| Hoogma (2004)227 | Crossover | 128 | Netherlands | DQoL NS |
| WHO well-being NS | ||||
| Garmo (2004)228 | Before/after | 27 | Sweden | DTSQ before 20 average |
| DTSQ after 32 average | ||||
| Sanfield (2002)229 | Before/after | 104 | USA | SF-36 general health, ability to perform physical activities, energy and physical pain better; CSII interfered with bathing and sexual activity |
| McMahon (2005)152 | Before/after | 100 | Australia | Impact of DQoL significant improvement |
| Paediatric | ||||
| Juliusson (2006)171 | Before/after | 31 | Norway | DQoL improved, but only significant for family activities subscale |
| Rodrigues (2005)140 | Before/after | 40 | UK | DQoL NS |
| SF-36 NS | ||||
| Shehadeh (2004)174 | Before/after | 15 | Israel | DTSQ significantly improved |
| Paediatric | DQoL significantly improved | |||
| Mednick (2004)178 | Retrospective | 22 | USA | Likert scale 1–5 values for satisfaction consistently with CSII above 3 |
| Bruttomesso (2002)230 | Retrospective | 138 | Italy | DQoL scores reported |
| Type 2 | ||||
| Raskin (2003)116 | RCT | 132 | USA | CSII reported as superior to MDI in all subscales of poorly documented TOIS questionnaire, except pain |
| Herman (2005)115 | RCT | 107 | USA | DQoL NS |
| Older patients | SF-36 NS | |||
In what follows, costs reported in foreign currencies have been converted to pounds sterling using the relevant mid-year exchange rate, or where this was not stated using the mid-year exchange rate of the date of publication.
RCT studies: T1DM
Weintrob et al. (2003)225 performed a randomised crossover trial of CSII versus MDI among 23 Israeli children with T1DM, aged 9–14 years, with a crossover period of 3.5 months after a 2-week run-in period. Quality of life aspects were measured with the DTSQ and for more general quality of life through the DQoL-Y. All children completed the two study arms. There were no significant differences in glycaemic control between the two arms. However, there was a significant difference in DTSQ scores, which averaged 21.4 at baseline, 21.9 at the end of the MDI arm and 30.6 at the end of the CSII arm. No statistically significant differences were recorded within the DQoL-Y subscales, with the end of MDI arms and the end of CSII arm displaying similar central estimates for all DQoL-Y subscales. At the end of the trial, patients were asked which regime they preferred: 70% preferred CSII on grounds of greater mealtime flexibility, avoidance of the pain of injections and better glycaemic control or profiling. Of those preferring MDI, concern as to glycaemic control, overeating and weight gain were cited, coupled with the desire to keep diabetes a secret and shame at wearing the pump were also cited, as was the required frequency of self-monitoring.
Hoogma et al. (2006)226 reported the results of a five nation randomised controlled crossover trial of CSII against MDI among 272 patients with T1DM of whom 223 completed the trial. Patient selection and patient characteristics were not presented in the paper. While not explicitly stated, it appears that patients were randomised to starting an intensified regime of either CSII or MDI, with a run-in period of 2 months. Trial duration thereafter was 6 months. HbA1c results were similar after run-in across both arms, with the mean difference at end of trial being in favour of CSII. Non-inferiority of CSII in terms of HbA1c was demonstrated, with subsequent analysis indicating statistically significant superiority. Similarly, the rate of respondent-defined SH events at 0.2 per years for CSII compared with 0.5 for MDI was also statistically significantly different.
Against this background, patients completed the DQoL and Short Form-12 (SF-12) questionnaires at baseline and at end of treatment. The overall DQoL score at end of treatment was significantly higher for CSII, the individual subscales of satisfaction, impact and diabetes-related worry also being statistically significantly better, although the social/vocational worry subscale did not reach significance. Within the SF-12 questionnaire, no significant differences in physical health were recorded, but the composite mental health subscale for CSII was statistically superior to that of MDI. The authors concluded by noting that once patients had experienced CSII they were more likely to recommend it than the MDI regimen.
Hans DeVries et al. (2002)119 reported the results of a crossover trial of 79 Dutch patients with T1DM with an average age of 37 years. Unfortunately, due to dropouts at crossover, the trial was analysed as a parallel trial using only the first half of the crossover phase. The authors noted that 11% of patients at randomisation or at crossover refused to start CSII. The trial found statistically significant differences in the general health and mental health subscales of SF-36, with CSII recording improvements of 5.9 and 5.2 on these subscales, respectively, as against falls of 1.2 and 0.6 for MDI. Scores for the other subscales were not reported. Overall treatment satisfaction was assessed using the DTSQ. No statistically significant difference was recorded, with CSII scoring an increase of 1.3 and MDI scoring an increase of 0.2.
In an RCT of CSII versus MDI in US children of under 5 years of age with T1DM, Dimeglio et al. (2004)120 reported that CSII was generally well tolerated with 19 out of the 20 families opting to continue with CSII after 6 months, rather than switch to MDI. The preferences of those in the MDI arm with regards switching of therapy at the 6-month point were not reported.
Fox et al. (2005)121 performed a 6-month RCT of CSII versus MDI among 26 US children with T1DM, average age of a little under 4 years. The children were randomly assigned to continue receiving MDI, or to switch to CSII. Given the age of participants, aspects of parental rather than patient quality of life were measured, coupled with parental perceptions of patient quality of life. While both mothers and fathers reported more psychological distress in the MDI group than in the CSII group, these differences were not statistically significant when baseline differences were controlled for. Mothers in the MDI arm reported significantly greater stress at baseline, but this difference was no longer significant at 6 months’ follow-up. However, fathers reported significantly more positive quality of life changes in the CSII group over the 6 months. The authors noted that at the end of follow-up all MDI patients started CSII therapy, while all CSII patients continued on CSII therapy.
RCT studies: T2DM
Raskin et al. (2003)116 undertook a randomised parallel trial over 24 months of CSII versus MDI among 132 US CSII-naive patients with T2DM, aged > 35 years. Patient satisfaction was measured through administering components of a poorly documented PHASE V Technologies Outcome Informations System Questionnaire. Overall satisfaction with treatment, as measured over the 10 subscales administered, was scored as an increase from 59 to 79 in the CSII group compared with an increased from 64 to 70 among the MDI group, which was described as being statistically significant. It was unclear whether the baseline for the CSII group was prior to the initiation of CSII therapy or shortly thereafter. CSII was also statistically significantly superior to MDI in all subscales other than pain, where no statistically significant difference was noted.
Herman et al. (2005)115 in a 12-month RCT of CSII versus MDI among 107 older patients with T2DM, average age 66 years, evaluated patient satisfaction and quality of life through both the DQoLCTQ and the SF-36 questionnaire. Over the period of the trial both arms reported similar increases in satisfaction with their treatment, DQoL scores for CSII increasing from 52 to 81 and for MDI increasing from 50 to 78. Likewise, changes in the composite SF-36 physical and mental health subscales were similar between both arms: the physical score for CSII rising from 40.5 to 41.1, compared with a rise from 40.6 to 41.0 for MDI, while the mental health score fell slightly for CSII from 51.0 to 50.0, compared with a slightly greater fall for MDI from 53.0 to 50.5. None of these changes was statistically significant.
Case–control study: T1DM
Hoogma et al. (2004)227 presented the results of a cross-sectional study among 128 Dutch patients with T1DM with an average age of 42 years, 49 of whom received CSII and 79 of whom received MDI. The design of the study intentionally recruited twice as many patients using MDI as patients using CSII. The selection criteria for patients using CSII patients participating in the study was not stated, although patients using MDI were reportedly randomly selected from the same outpatient clinics. Quality of life aspects were evaluated through three questionnaires: DQoL, the DTSQ and the WHO Wellbeing Questionnaire.
Self-measurement of blood glucose was once daily in 81% of CSII patients as against 63% in patients using MDI. The majority of the remaining patients using CSII, 10%, measured blood glucose one or two times a week compared with 19% for the MDI arm. Full daily blood glucose profiling showed a similar profile, with more frequent profiling being performed within the CSII arm. No differences were uncovered regarding the outcomes of DQoL, and the treatment satisfaction again showed no differences between the groups, even with regards to hypo- and hyperglycaemia. With regards to the general wellbeing questionnaire, again no statistically significant differences were noted, except for the ‘energy’ subscale, for which the MDI arm showed somewhat better results with a score of 8.7 as against 7.5 for CSII. As with much of the patient preference and quality of life literature, these results are difficult to interpret as patient characteristics and the reasons for receiving CSII were not well documented.
Before/after studies: T1DM
Garmo et al. (2004)228 report a study of 27 Swedish adults with T1DM, average age 41 years, changing from MDI at baseline to CSII with follow-up at 6 months. It appears that the change to CSII was medically driven rather than part of a research programme per se, which may make results more applicable to the sort of patients who would start CSII in routine care. Satisfaction with treatment was measured using the DTSQ, with possible values ranging from 0 to 36. Prior to the change to CSII the median DTSQ score was 20, with a range of 4–32, while at 6-month follow-up after changing to CSII the median DTSQ score was 32, with a range of 17–36. However, these results are subject to a number of criticisms, the most significant of which is the before/after non-randomised nature of the study coupled with patients being in some sense self selecting due to their previous MDI therapy being presumably unacceptable for either clinical or personal reasons. Respondents were presumably failing on MDI, this also being reflected in a self-reported fall in the frequency of unacceptable hypoglycaemic events.
Bruttomesso et al. (2002)230 retrospectively review quality of life using the DQoL questionnaire among 138 Italian patients with T1DM, who were receiving CSII, and had been on average for 7.4 years. Ninety-eight of the patients surveyed completed at least one aspect of the DQoL questionnaire, all 98 completing the satisfaction subscale, average score 72.5, and the impact of diabetes subscale, average score 71.3. 95 completed the diabetes worry subscale, average score 80.2, but only 51 completed the social worry subscale, average score 67.8. The overall average DQoL score across patients and subscales was 73.0.
Sanfield et al. (2002)229 reported the results of a before/after study of 104 US patients with T1DM prior to the initiation of CSII therapy. Patient characteristics were not reported. Prior to initiating CSII, patients underwent up to three outpatient sessions to assess suitability, a large degree of which appears to have been education around self-selection for suitability for CSII. One of the criteria within this was financial, in terms of patients having adequate financial resources to cover the initial and ongoing costs of CSII. Of the 104 patients, 35% did not proceed through all three outpatient sessions to receive CSII. The reasons for this are not outlined, but given the financial criterion the relevance of this to the UK setting is questionable.
Patients proceeding to CSII completed SF-36 and three additional trial-specific quality of life questionnaires. The great majority, 97%, of patients initiating CSII and remaining in the area remained on CSII after over 2.5 years. Few details are provided as to the quality of life measures, but the paper notes that statistically significant improvements occurred over time in the SF-36 parameters of general health, ability to perform activities, energy and physical pain. Within the trial-specific quality of life questionnaires, patients are reported as stating that eating, working, sleeping, bathing and sexual activity were the most important aspects of life. CSII was found to interfere with bathing and sexual activity, although many patients removed pumps during these activities.
McMahon et al. (2005)152 prospectively followed 100 Australian children and adolescents with T1DM who were starting CSII therapy. Those selected for CSII therapy had demonstrated some or all of severe hypoglycaemia, poor glycaemic control and erratic lifestyle with regards sport, food or routine, although commencement was typically at parental request. Quality of life was measured prior to starting CSII and after 6 months of CSII among the first 51 patients being switched to CSII through a modified DQoL questionnaire and the SED scale, with respondents being of more than 10 years of age. Within the DQoL results, the impact of diabetes score fell, on average, from 55.4 to 50.2, a difference significant at the 5% level. Worries about diabetes and satisfaction with life scores showed no significant change over the 6 months. The SED scale improved from 159 to 174, which was described as being statistically significant. But in common with the Fox et al. (2005) study,121 these results require care in their interpretation given the before/after nature of the study, coupled with patients in some sense having been failing on their previous therapy.
Juliusson et al. (2006)171 performed another before/after study of the adoption of CSII upon patient quality of life, this being among 31 children with T1DM, average age of 14 years, and who were poorly regulated on MDI with an average HbA1c of 10.4%. 171 Quality of life was measured with the DQoL questionnaire and generic CHQ-CF87 questionnaire prior to starting CSII and twice during 15 months of follow-up. Average differences in subscales of the CHQ-CF87 uniformly showed improvements, but these only reached statistical significance for the ‘family activities’ subscale. Similarly, while an improvement was recorded across the dimensions of the DQoL, none of these reached statistical significance. The authors concluded that respondents might have had moderate improvements in diabetes-specific quality of life scores, and were unlikely to have had appreciable deteriorations in them.
Rodrigues et al. (2005)140 reported the results of a before/after study of 40 UK patients with T1DM, average age 33 years, the study aiming to identify if current guidelines would correctly identify patients who would benefit from CSII. Twenty-five of the 40 patients were initiated on CSII for reasons other than SH events, while 15 patients reportedly had contraindications to CSII. The median follow-up was 20 months. Quality of life was measured although the DQoL, with the SF-36 and the Hypoglycemia Fear Survey also being administered. Only 33 questionnaires were returned, including four responses from patients who had discontinued with CSII. These four responses from those who had discontinued with CSII were excluded from the analysis. No significant differences were observed within any of the DQoL subscales. The only significant difference within the SF-36 reported was not between baseline and follow-up, but between those with and without contraindications to CSII. Those with contraindications had a significantly lower score on the mental health subscale of 47.5 than those without, who scored 69.9. Having excluded those discontinuing CSII from the analysis, all respondents, as expected, preferred CSII to their previous treatment.
Mednick et al. (2004)178 surveyed 22 US children with T1DM, average age of 14 years, and their parents, after having transferred to CSII and remaining on CSII. Data were collected between three and 22 months after CSII was initiated. Telephone interviews were conducted using the IPTSQ, which had been specifically designed for the survey by the authors, composed of 10 items ranked on a Likert scale, coupled with three open-ended questions as to life changes from pump use, the most challenging aspects of the pump and advice to prospective users to maximise their benefits from pump use. Satisfaction ratings were derived for parents and children. None was dissatisfied, and the average response on the 5-point Likert scale was consistently above ‘3’ and typically above ‘4’.
Shehadeh et al. (2004)174 briefly reported the results of a before after study of CSII among 15 Israeli children with T1DM, aged 1–6 years. Treatment satisfaction and quality of life were measured through DTSQ and DQoL, respectively, for parents at baseline and at 4 months. Both the overall DTSQ scores and the DQoL scores showed a statistically significant improvement at 4 months compared with baseline.
With all of the above before/after studies, many patients will have commenced CSII due to failure on their previous regime. As a consequence, these results may be of limited relevance to the consideration of whether those moving on to intensive insulin therapy would be best starting on CSII or starting on MDI. However, the results appear to be more useful than if the results had been representative of the total T1DM population for the situation where CSII is used in patients who are in some way failing on current therapy, as with the current NICE guidance. There is the additional difficulty that participation in the study may have affected results over the time period of the study.
Diabetic clinic survey: T1DM
Bruttomesso et al. (2006)141 reported the results of a survey of Italian diabetic centres, with 145 centres out of 179 centres responding. These covered a total of 2702 patients using CSII, of whom the average age was 39 and among whom 97% were patients with T1DM who had previously received MDI. The main reason for starting CSII was given as poor metabolic control, although quality of life, flexibility, reducing the number of hypoglycaemic events and correcting the dawn phenomenon were also cited. Reasons for not starting CSII were the inability to cope with the pump technology, lack of compliance, psychiatric problems and unwillingness to check blood glucose frequently. Notably 571 patients abandoned CSII, although quite what the relevant baseline or denominator figure for this was not clear from the paper. In total, 187 of these stopped at the end of pregnancy. Intolerance of the pump was also cited as a reason for discontinuing, coupled with disappointment as to the goals attained, infection at the infusion site and hypoglycaemia.
Meeting abstracts
Barnard and Skinner (2006)231 briefly reported the results of a qualitative telephone survey of 80 insulin pump users. The patient characteristics and method of respondent selection were not specified. Respondents were asked about the benefits of insulin pump use, and quality of life effects, any downsides to insulin pump use and whether they wished to raise any other issues. A number of positive themes emerged from the survey, with 56% reporting greater control, 41% reporting greater flexibility, 35% reporting increased freedom, 9% reporting greater convenience and 6% reporting greater independence. However, 59% also reported downsides, with 31% reporting difficulties with concealment, 21% reporting technical issues when things go wrong, 6% reporting site reactions and pain and 4% reporting cost. The authors noted that these negative factors may explain why a number of patients only remain on pump therapy for a short period of time. Given this, there may have been a degree of sample selection bias, with any results relating more to the quality of life of those finding insulin pumps of benefit in the longer term.
Reid and Lawson (2002)232 reported the results of a Canadian cross-sectional survey of 74 children with T1DM, 28 of whom had been using CSII for more than 4 months and among whom the average duration of CSII usage was 16 months. The average age of those using CSII was 14 years. Patients were matched for sex, age and duration of diabetes, with a linear regression model comparing values from the DQoL-Y questionnaire. Treatment satisfaction was significantly higher in the CSII group with a score of 33.8 as against 27.5 in the MDI group. However, despite metabolic control also being significantly better in the CSII group, with 7.7% compared with 8.9% for the MDI group, no significant differences were observed in the DQoL-Y dimensions of satisfaction, impact or worry. The authors concluded that DQoL-Y may not be the most appropriate measure of quality of life in this group.
Galatzer et al. (2002)233 compared the treatment satisfaction among 208 CSII and MDI people with T1DM of average age of 20 years, although ages ranged from 10–50 years through the use of the DQoL questionnaire. Patient selection and other characteristics were not reported. The overall treatment satisfaction score was significantly different at 30.7 among CSII patients compared with 22.7 among patients using MDI. Overall, 86% of CSII patients were reported as recommending CSII to other patients, compared with only 19% of patients using MDI.
Schweitzer et al. (2006)234 reported the results of a postal questionnaire sent to 36,450 patients using CSII, from whom a 38% response rate was achieved. The abstract restricted itself to the results of the 729 responses received from patients with T2DM, these having an average age of 56 years, an average duration of diabetes of 17 years and an average duration of CSII of 3.4 years. Most, 76%, reported selecting CSII due to poor glycaemic control on other therapies, with 44% also citing uncontrolled blood glucose fluctuations. A minority, 34% mentioned a high insulin requirement when using injections as a reason. Many (44%) patients initiated therapy as inpatients (which would be unusual in the UK), with 46% starting at a specialist diabetologist’s office, and 5% as a hospital outpatient. Only 3% initiated therapy via a GP. The authors concluded that patients were highly satisfied with CSII therapy, although the means of assessing this and associated results were not presented.
Cost-effectiveness studies
The previous HTA report did not identify any studies of the cost-effectiveness of CSII versus MDI. 50 The current HTA report has identified three full papers and eight abstracts relating to the modelling of cost-effectiveness of CSII versus MDI (Table 25). The preponderance of abstracts within the literature survey appears to be due to the recent availability of the CORE Markov model for simulating the effectiveness of CSII versus MDI among people with T1DM. This has generated a large literature, which is currently making its way into print. It appears likely that all but one of the cost-effectiveness papers and abstracts fall into this category, although not all are explicit about their use of CORE or another Markov model.
| Country | Model | Horizon | Perspective | Results | |
|---|---|---|---|---|---|
| Full papers | |||||
| Mixed | |||||
| Scuffham (2003)200 | UK | Own Markov | Lifetime | Health Service | £11,461/QALY |
| Type 1 | |||||
| Roze (2005)201 | UK | CORE | 60 years | Health Service | £26,297/QALY |
| Conget Donlo (2006)202 | Spain | CORE | Lifetime | Health Service | €29,957/QALY |
| Abstracts | |||||
| Type 1 | |||||
| Castell (2005)235 | Spain | CORE | Lifetime | Health Service | €30.453/QALY |
| Zakrzewska (2004)236 | UK | CORE | Lifetime | Health Service | £32,753/QALY |
| Zakrzewska (2005)237 | Switzerland | CORE | Lifetime | Social | CHF22,444/QALY |
| Roze (2002)238 | France | CORE | 50 years | Health Service | €1348/life-year |
| Goodall (2006)239 | Sweden | CORE(?) | Lifetime | Social | SEK58,830/QALY |
| Norway | NOK24,837/QALY | ||||
| Nicklasson (2006)240 | Sweden | CORE | Lifetime | Social | SEK227,066/QALY |
| Roze (2002)241 | USA (?) | CORE(??), paediatric | Lifetime | Health insurance | US$115,082/life-year |
Scuffham and Carr (2003)200 developed their own relatively simple Markov model to compare CSII with MDI among patients with T1DM. The perspective was as recommended in NICE guidance, the time horizon was 8 years as this is the anticipated pump longevity, and discount rates of 6.0% for costs and 1.5% for benefits were applied in the base case.
The Markov model was implemented through monthly cycles and had two principal health states: well and dead. Within this, patients who were well could experience hypoglycaemic events, which could also result in a need of inpatient treatment, and could also experience ketoacidosis. Baseline risk and death rates associated with these were taken from the literature; for hypoglycaemic events the baseline annual risk was taken to be 40% with a 0.5% death rate, whereas for ketoacidosis the baseline risk was taken to be 2.7% with a 10% death rate, which seems unusually high. Risk reductions associated with CSII upon these baseline risks were 43% for hypoglycaemic events, and apparently also a 2.5% risk reduction for ketoacidosis events. CSII also resulted in a 14% reduction in insulin use, although it is unclear whether this was restricted to basal insulin use alone.
Quality of life values were mainly drawn from the Boland et al. (1999)99 study among adolescents with T1DM. MDI was taken to result in a 5.3% utility loss, and, as a consequence, the utility of those in the ‘well’ health state receiving CSII was taken to be 1.000, whereas the utility of those ‘well but receiving MDI’ was taken to be 0.947. Similarly, both hypoglycaemic events and ketoacidosis events were taken to result in 2 days at zero utility, and so involve a disutility of 0.067. Distributions were placed upon all variables to enable probabilistic modelling.
While the annual direct costs of treatment were not stated, it appears that the annual cost of CSII appears to have been around £1380 compared with £468 for MDI. The overall cost of treatment over the 8 years was estimated as £9514 compared with £4052 for MDI: a net increase of £5462. The QALYs accrued over the 8 years were an average of 7.32 for CSII compared with 6.85 for MDI: a net increase of 0.48 QALYs, implying a cost-effectiveness of £11,461 per QALY.
A number of concerns arise with the study, not least the application of a 5.3% utility loss from MDI as against CSII and the estimates of the direct costs of treatment. The model was of relatively simple structure, and made no distinction between T1DM and T2DM or outlined any other patient characteristic that might be of interest, such as age. This is underlined by the reduction in event rates being direct, rather than modelled through the mechanism of any changes to baseline HbA1c that CSII might result in: the more common modelling approach within models of therapies for both T1DM and T2DM. Note also that although the paper was a worthwhile attempt to model the short-term impact is CSII relative to MDI, the longer-term complications of diabetes are excluded. Despite this, the cost-effectiveness estimate for CSII was considerably better than those papers that report models involving the long-term complications as reviewed below, and so it is difficult to have confidence in its results.
Most of the remaining papers modelling CSII versus MDI among people with T1DM relied upon the CORE model as reviewed in the previous chapter, although one paper, available only as an abstract, undertook an RCT of CSII against MDI, which also measured treatment costs. Most of the remaining papers list Palmer, Roze and Zakrzewska as authors, even if not as principal authors, underlining the reliance of the area upon the CORE model. This is understandable. There are few T1DM models around, and the CORE model is one of the most used, being available to use (at cost) over the internet, with many papers based on it published in peer-reviewed journals.
Roze et al. (2005)201 presented the results of a cost-effectiveness modelling exercise of CSII versus MDI implemented using the CORE model. The perspective of the analysis was as recommended in the NICE methods guidance, with a 60-year time horizon and the use of 2003 costs, but, unfortunately, the use of 3.0% discount rates for both costs and benefits.
For patients using MDI, the average HbA1c was taken to be 8.68%, coupled with an average BMI of 23.61 kg/m2. Clinical effectiveness estimates for CSII were drawn from the 2003 meta-analysis of Weissberg-Benchell et al. ,193 resulting in a relatively large improvement from baseline of 1.2% in HbA1c level but also a weight gain of 1.03 kg/m2. Roze et al. (2005)201 also assumed, given a lack of evidence to the contrary, that event rates of hypoglycaemia and ketoacidosis were the same for both treatment groups for the base case, with these rates being taken from DCCT data. These assumptions were varied in the sensitivity analyses. The rates of pre-existing complications of diabetes within the modelled cohort was not stated within the paper, although the average age at baseline was 26 years and the average duration of diabetes 12 years.
Costs of the treatment of the complications of diabetes were drawn from the literature, and were presented within the paper. Quality of life values were similarly drawn from the literature, although the values used were not presented in the paper. The direct costs of therapy were estimated to be £1482 for MDI and £2641 for CSII. The higher costs of pump and consumables of CSII of £1449 as against £149 for MDI were partially offset by a lower cost of insulin of £281 for CSII as against £422 for MDI.
Base-case results of the modelling suggested that CSII would by the end of the 60-year time horizon result in absolute reductions in the cumulative incidence of: amputation of around 1.7% (value taken from graph and reported relative percentage reduction); severe visual loss of around 4.9% (value taken from graph and reported relative percentage reduction); MI of 2.6%; and, of end-stage renal disease of 1.1%. Similar reductions were observed in the other complications of the model. These helped contribute to an estimated life expectancy of 17.44 years for CSII as against 16.73 years for MDI – an improvement of 0.71 years. Adjusting for quality of life, this increased the anticipated gain to 0.76 QALYs, i.e. the general effect of diabetes tending to reduce quality of life was more than offset by the gain in the avoidance of reduced complications.
Treatment costs were the largest cost component for the CSII arm, being £47,077 against £25,266 for MDI – an increase of £21,811. These were partially offset by lower costs of complications of £31,267 against £33,458 for the MDI arm – a net saving of £2,191 from complications. For reasons that are not apparent, Roze et al. (2005)201 report these as resulting overall average lifetime costs of £80,511 for CSII compared with £61,104 for MDI – a net additional overall cost for CSII of £19,407. Note that the direct treatment costs and costs of complications cited in the paper would appear to suggest overall costs of £78,344 for CSII and of £58,724 for MDI, to give a net costs of CSII of £19,620. Given the anticipated average gain of 0.76 QALYs this translated into a central cost-effectiveness estimate of £25,648 per QALY, and a likelihood of 74% that cost-effectiveness would lie below £30,000 per QALY. Using the NICE recommended 3.5% discount rates increased the anticipated cost-effectiveness ratio slightly to £26,297 per QALY.
Results were sensitive to the improvement in HbA1c level assumed. Application of the 0.51% improvement identified in the 2002 meta-analysis of Pickup et al. 18 increased the cost-effectiveness ratio to £61,564 per QALY. Changing the effect of CSII upon BMI from a weight gain of 1.03 kg/m2 had only a very marginal effect, improving cost-effectiveness to £25,391 per QALY. The base case assumed CSII had no effect upon the rate of hypoglycaemic events. If CSII resulted in 50% fewer hypoglycaemic events relative to MDI the cost-effectiveness improved to £20,104 per QALY, whereas a 75% reduction improved it still further to £18,047 per QALY. If CSII resulted in a doubling of ketoacidosis events, this worsened its cost-effectiveness to £28,297 per QALY.
Conget Donlo et al. (2006)202 also used the CORE model to evaluate the cost-effectiveness of CSII against MDI among people with T1DM. The perspective for cost was that of the Spanish health-care system. A lifetime horizon was applied, with all costs being at in euros at 2005 prices and a discount rate of 3.0% was applied to both costs and benefits.
Clinical effectiveness was the same as in Roze et al. (2005),201 CSII resulting in a baseline improvement of 1.2% in HbA1c but also a weight gain of 1.03 kg/m2 compared with MDI. Similarly, it was also assumed that event rates of hypoglycaemia and ketoacidosis were the same for both treatment groups. Patient characteristics were drawn from Spanish registry data relating to CSII-treated patients, the average age being 36 years. The rates of pre-existing complications of diabetes within the modelled cohort were also drawn from the Spanish registry data, with an average duration of diabetes of 15 years.
Costs of the treatment of the complications of diabetes were drawn from the literature as it related to Spain, and were presented within the paper. Aside from MI in the first year which was of somewhat higher cost than that used in Roze et al. (2005),201 the unit costs of complications were typically of similar of lower cost than those used in Roze et al. (2005). 201 Quality of life values for the complications of diabetes were mainly drawn from the Clarke et al. paper,213 which reported the results of an EQ-5D exercise conducted among UKPDS patients, and were presented within the paper.
The direct costs of therapy were estimated to be €2087 (£1410) for MDI and €3773 (£2549) for CSII. The assumed lifespan of pumps of 8 years was the same as in the study of Roze et al. , and the direct costs of treatment were consequently similar.
Conget Donlo et al. (2006)202 found that CSII would result in reductions in the cumulative incidence of; severe visual loss of 2%; MI of 1%; and, of end-stage renal disease of 7%. These reductions in the cumulative incidences of these complications are somewhat less than those reported in Roze et al. ,201 with the exception of end-stage renal disease. While the baseline average age assumed by Conget Donlo et al. was somewhat higher than that of Roze et al. , the smaller differential in rates of major complications over the period of the modelling may be mainly due to differences in the initial rates of complications that were assumed within the cohort. For instance, Conget Donlo et al. assumed that 32% had retinopathy at baseline.
Despite these possible differences at baseline, the results of Conget Donlo et al. in terms of life expectancy were surprisingly similar to those of Roze et al. , with an estimated life expectancy of 16.83 years for CSII against 15.94 years for MDI – an improvement of 0.89 years. Adjusting for quality of life, this reduced the anticipated gain to 0.85 QALYs. Again, in contrast with Roze et al. , the gain in the avoidance of complications was not sufficient to offset the general effect of diabetes tending to reduce quality of life.
The average cost per patient using CSII was estimated as €105,439 (£71,242), as opposed to €79,916 (£53,997) for MDI – an average increase of €25,523 (£17,245). Given the base-case estimate of a 0.85 QALY gain, this translated into a cost-effectiveness estimate of €29,957 (£20,234) per QALY.
Conget Donlo et al. performed similar sensitivity analyses to Roze et al. , also finding the effect upon HbA1c level to be the key variable. Adopting the 0.51% improvement identified in Pickup et al. (2002)18 increased the cost-effectiveness ratio to €103,584 (£69,989) per QALY. Assuming that CSII resulted in a 66% reduction in hypoglycaemic events improved the cost-effectiveness ratio to €25,680 (£17,351) per QALY.
The following papers in this section were available only as meeting abstracts:
-
Castell et al. (2005)235 in common with the paper of Conget Donlo et al. 202 reported above, also ran the CORE model for patients with T1DM within the Spanish setting. Note that Roze and Zakrzewska were also listed as authors. The perspective was as in the NICE guidance, with a lifetime horizon and a 3.0% discount rate for both costs and benefits. Costs were in 2004 prices. No details were supplied as to baseline patient characteristics, or the assumed efficacy of CSII versus MDI in terms of glycaemic control. The summary of their results stated that life expectancy in the CSII group was 0.859 years longer, which, when quality of life was factored in, resulted in a 0.836 QALY gain from CSII over MDI. Overall lifetime costs were €25,463 (£16,975) higher for CSII, resulting in a cost-effectiveness estimate of €30,453 (£20,302) per QALY. These results are very similar to those of the Conget Donlo paper, suggesting that Castell et al. made similar assumptions, although the slightly lower gain from CSII in terms of life expectancy and QALYs could be due to patient baseline characteristics, such as a higher baseline age and slightly greater baseline prevalence of the complications of diabetes.
-
Zakrzewska et al. (2004)236 again use the CORE model to simulate the cost-effectiveness of CSII against MDI among patients with T1DM. This was modelled from the perspective of the UK NHS, with the base year for costs being 2003 and a 3.5% discount rate being applied to both costs and benefits. Patient characteristics were an average age of 26 years, an average duration of diabetes of 12 years, and an average HbA1c of 8.68%. Unfortunately, the abstract did not itemise the assumed effectiveness of CSII relative to MDI, although life expectancy results suggest a similar effectiveness to their 2005 paper,201 published in full – a 1.2% in HbA1c but also a weight gain of 1.03 kg/m2. Patient outcomes in terms of life expectancy were marginally different compared with their paper published in full and summarised above, with an average life expectancy of 17.37 years for CSII compared with 16.66 for MDI – a gain of 0.72 years compared with 0.71 years in their fully published paper. This 0.72 life-years gain translated to a 0.59 QALY gain. Note in passing that adjusting for quality of life in their paper published in full caused the anticipated increase in life expectancy to change from 0.71 life-years to 0.76 QALYs. CSII was estimated as costing £81,115 as against £57,015 – a net increase of £19,413, which resulted in a central estimate for the cost-effectiveness of CSII of £32,753 per QALY.
-
Zakrezewska et al. (2005)237 reported cost-effectiveness results of CSII versus MDI in patients with T1DM within the Swiss setting using the CORE model. It appears that a full societal perspective was adopted for costs rather than concentrating upon health-care costs, although the abstract was not explicit about this. A lifetime horizon was adopted, with both costs and benefits being discounted at 3.0%. Costs appear to have been in 2004 prices, although again this was not explicitly stated within the abstract. Baseline patient characteristics were not stated. The clinical effectiveness of CSII against MDI in terms of glycaemic control was also not stated. Overall survival for CSII was estimated as 17.15 years as against 16.27 years for MDI: a net gain of 0.87. The use of CSII was estimated to also yield relative reductions in severe vision loss, end-stage renal disease and peripheral vascular disease were 16%, 18% and 16%, respectively, although the absolute values of these were not given. The average overall cost per patient of CSII was estimated as CHF516,745 (£224,672) as against CHF497,117 (£216,138) – a net increase of CHF19,628 (£8534). The net cost of complications were stated as being CHF10,327 (£4490) lower in the CSII group, which suggests that treatment costs were CHF29,955 (£13,024) higher for CSII than with MDI. Overall cost-effectiveness of CSII relative to MDI was estimated to be CHF22,444 (£9758) per life-year gained.
-
Roze et al. (2002)238 used the CORE model again to model the cost-effectiveness of CSII against MDI among patients with T1DM within the French setting. The cost perspective was that of the French health-care system so only including the direct health-care costs, with a 50-year time horizon and a discount rate of 5.0% being applied to costs. The base year for costs was not stated, or was the discount rate for benefits. Patient characteristics were not itemised, but were reported as being similar to the DCCT primary intervention cohort. CSII was modelled as resulting in 1% better control of HbA1c, reducing hypoglycaemic events by 50% but increasing the rate of ketoacidosis from 1.39 per 100 patient-years to 3.09 per 100 patient-years compared with MDI. The increase in life expectancy was broadly in line with that of other CORE modelling at 1.00 years, but the additional overall cost per patient was muted at only €1348 (£807). While the cost discount rate of 5.0% will have tended to reduce the cost impact over 50 years in comparison with the more usual 3.0% discount rate within the literature, the reason for the lifetime net cost of CSII being so much lower within this study are not apparent. Given the anticipated increase in survival, the cost-effectiveness was similarly estimated as €1348 (£807) per life-year gained.
-
Goodall et al. (2006)239 reported using a previously validated computer simulation model to simulate the effects of CSII versus MDI within the Norwegian and Swedish settings. It is unclear whether the model used was CORE, although the authorship would suggest so. A societal perspective was adopted for costs, although the base year was not stated, with modelling adopting a lifetime horizon and discounting costs and benefits at 3.0%. The average age of patients was 26 years, with an average duration of diabetes of 12 years and a baseline HbA1c of 8.68% as in Roze et al. (2005). 201 Baseline characteristics may have differed between the modelled populations, as the life expectancy gains from CSII differed: 0.95 years in the Norwegian setting as against 1.03 in the Swedish setting. Adjusting these figures for quality of life had relatively little effect, resulting in gains of 0.98 and 1.03 QALYs, respectively. The Norwegian modelling resulted in average lifetime costs being NOK3,505,368 (£299,604) for CSII as against NOK3,480,974 (£297,519) for MDI: a small net increase of NOK24,394 (£2085) due in part to the inclusion of indirect costs estimated through the human capital approach. This resulted in an overall cost-effectiveness estimate within the Norwegian modelling for CSII against MDI of NOK24,837 (£2123) per QALY. Within the Swedish setting, CSII was estimated as having an overall lifetime cost of SEK3,026,056 (£223,325), against SEK2,965,366 (£218,846) for MDI – again a relatively modest increase of SEK60,690 (£4479) due to the inclusion of indirect costs. This resulted in an overall cost-effectiveness estimate within the Swedish modelling for CSII against MDI of SEK 58,830 (£4432) per QALY.
-
Another study of CSII versus MDI among patients with T1DM implemented using the CORE model and based in Sweden was reported in Nicklasson et al. (2006). 240 A societal perspective was adopted, although results were also reported that included only direct treatment costs. A lifetime perspective was adopted, with the base year for costs being 2005, and a 3.0% discount rate being applied to both costs and benefits. Baseline patient characteristics were an average age of 27, a somewhat shorter duration of diabetes compared with other studies of only 6 years and an average HbA1c of 8.875%. The authors also referenced the meta-analysis of Weissberg-Benchell et al. (2003)193 and resultant improvement of 1.2% in HbA1c from the use of CSII, although no mention was made of the weight gain of 1.03 kg/m2. CSII resulted in an average life expectancy of 17.55 years compared with 16.71 for MDI – an increase of 0.84 years. Taking quality of life into account had little impact upon this, resulting in a QALY gain of 0.85 from CSII. The direct treatment costs, which appear to exclude the costs of complications, were modelled as being SEK348,582 (£25,821) higher for CSII. Including the costs of complications and anticipated productivity gains reduced this net lifetime cost of CSII relative to MDI to SEK193,078 (£14,302), and so a cost-effectiveness estimate from the societal perspective of SEK227,066 (£16,820) per QALY.
The 0.51% improvement in HbA1c from the Pickup et al. (2002) meta-analysis18 was also referenced for use in a sensitivity analysis, which resulted in a considerably lower survival gain of 0.37 years from CSII, with the quality-adjusted gain falling to 0.51 QALYs. The differential impact upon survival and quality-adjusted survival from the adoption of the 0.51% improvement in HbA1c compared with the base case 1.2% is worth noting. The reduced relative effectiveness of CSII in terms of glycaemic control should result in a more similar complications profile for CSII and MDI. It appears that the move from 1.2% impact upon HbA1c to only 0.51% causes the CORE model to reduce the impact upon complications that may be fatal, such as MI, to a greater extent than it tends to reduce the impact upon complications that are non-fatal, such as visual loss.
-
Roze and Palmer (2002)241 used a Markov model, stated as being focused upon nephropathy, in order to estimate the cost-effectiveness of CSII among newly diagnosed patients with T1DM of age 14 years. CSII appears to have been taken as improving glycaemic control to 7.5% compared with 8.3% HbA1c for MDI. A health insurance perspective was taken for costs, with results being presented for a discount rate of 3.0% for both costs and benefits. Unfortunately, the country was not specified and although the authors were based in Switzerland, the source of clinical evidence was the Adolescent Benefit from Control of Diabetes (ABC) study. 99 This study appears to have been conducted in the USA, and it seems reasonable to assume that the modelling also relates to the USA. Through the reduction of renal disease, the undiscounted life expectancy was anticipated to be 0.81 years greater with CSII. Costs were not reported separately, but the discounted cost was estimated as US$115,082 per discounted life-year of additional survival. Given the concentration upon nephropathy and the authorship, the model used may have been an early form of the CORE model. However, it may also have been an entirely separate model given the focus upon adolescents within both the modelling and the clinical effectiveness data.
Given the preponderance of CORE-based modelling, the above cost-effectiveness papers may be better viewed as a range of sensitivity analyses performed on the CORE model rather than as a range of independent cost-effectiveness studies, tending to confirm the cost-effectiveness of CSII over MDI. Given this, a number of themes clearly emerge relating to the CORE modelling and its results:
-
Patient characteristics were most commonly patients with T1DM of 26 years of age, 12 years’ diabetes duration and an HbA1c level of 8–9%.
-
The effectiveness of CSII was an improvement of 1.2% in HbA1c level in the base case.
-
The anticipated average survival was 16–17 years to give an anticipated lifespan for the modelled patients with T1DM of 42–43 years on average.
-
The anticipated survival gain from CSII was around 0.8–0.9 years.
-
The anticipated QALY gain from CSII was also around 0.8–0.9 QALYs.
-
Most modelling found CSII to be cost-effective.
-
If CSII halved hypoglycaemic events then its cost-effectiveness was much improved.
-
If CSII only resulted in a 0.5% improvement in HbA1c then its cost-effectiveness was very much worsened.
Costs of CSII versus MDI
In the light of the initial CSII HTA and NICE guidance, Feltbower et al. (2006)242 analysed the Yorkshire Register of Diabetes in Children and Young Adults. This showed an annual incidence of T1DM under 15 years of age of 19 per 100,000 person-years. The cost per patient receiving CSII was estimated as requiring an initial set-up cost of £4000 in the first year: £2000 for the pump itself, £1000 maintenance, £1000 training and other costs. Annual ongoing costs were estimated as £1800: £1200 for consumables and £600 for insulin and ongoing maintenance. No real detail was provided as to these unit cost estimates. The average annual cost for a single PCT within the then North and East Yorkshire Strategic Health Authority (SHA), and West Yorkshire SHA was estimated as being between £739 and £1322 for a take-up of 1%, rising to between £3696 and £6608 for a take-up of 5%. As a consequence, the authors concluded that the overall financial burden for a PCT of providing CSII to children with T1DM would be modest for individual PCTs.
The Canadian AETMIS review article19 identified the following average costs in 2004 prices for CSII, as shown in Table 26.
| Cost (CA$) | Cost (£) | ||
|---|---|---|---|
| Pump | 6063 | 2526 | |
| Annual consumables | |||
| Cartridge | 281 | 117 | |
| Infusion set | 2016 | 840 | |
| Batteries | 87 | 36 | |
| Total (consumables) | 2384 | 993 | |
| Initial training and set-up | |||
| Medical specialist prescription time: 1 hour | 1*96 | 96 | 40 |
| Meeting with nurse: 2 hours | 2*26 | 52 | 22 |
| Meeting with dietitian: 2 hours | 2*24 | 48 | 20 |
| Adults: 2*6-hour nurse training | 12*26 | 312 | 130 |
| Children (including parents): 20-hour nurse training | 20*26 | 520 | 217 |
| Support and follow-up | |||
| 20*30-minute care team (doctor nurse, dietitian) | 20*(48 + 13 + 12) | 1460 | 608 |
| 20*30-minute calls to nurse | 20*13 | 260 | 108 |
| Total for adult (training plus support) | 2228 | 928 | |
| Total for child (training plus support) | 2436 | 1015 | |
The review also identified some additional costs from CSII relative to MDI arising from 50% more lancets and test strips being necessary for blood glucose monitoring, these being costed at an additional CA$840 (£350), coupled with an additional CA$58 (£24) for transparent adhesive dressings. In contrast, CSII was estimated as requiring only one antiseptic swab every 4 days, in contrast with the four daily antiseptic swabs recommended for MDI (though whether necessary or used is a different matter), which increased the relative cost of MDI by CA$549 (£228). Overall, these additional costs increased the annual cost of CSII relative to MDI by CA$349 (£145).
Nuboer and Bruining (2006)243 present the results of a broad and at times qualitative review of the issues likely to affect the cost-effectiveness of CSII versus MDI. This includes a review of a German study, which evaluated the cost of CSII among 6437 children and adolescents with T1DM of up to 20 years of age (average age 12 years). This covered more than 25% of the overall T1DM German population in this age range in 2000. The overall average total annual cost of CSII was €2611 (£1740), with blood glucose self-monitoring, hospitalisation and insulin accounting for 37%, 26% and 21%, respectively, of the overall costs. Ambulatory care and injection equipment accounted for 9% and 7% of cost, respectively. Parallelling this, an American study was quoted as finding an average cost among adolescents of US$2342 (£1463) in the early 1990s, while a Finnish study in 1993 found an average cost of €2200 (£1580). Updating these prices to 2005 values the authors concluded that the average cost for CSII among children and adolescents was around €3000 (£2027). The paper also formed its own estimate of the extra costs of CSII compared to MDI among adolescents as being between €1355 (£915) and €2968 (£2005). The main source of variability in these cost estimates was the cost of the infusion sets which varied from €6 (£4) to €16 (£11).
Mbowe et al. (2004)244 estimated the costs to a Belgian university hospital of the provision of CSII to 94 patients with T1DM, six of whom were paediatric. This adopted an activity-based costing method of more than 40 microactivities, which included items such as outpatient visits, the initial provision of CSII, any initial hospitalisation coupled with any subsequent hospitalisations, and administration and maintenance. The average cost per patient to the hospital was estimated as €3045 (£2030) per year, although this appears not to include the cost of insulin. The largest cost |element at 22% was the provision of self-monitoring strips.
Ulahannan study
The industry submission was also accompanied by a 2007 study by Ulahannan et al. ,245 which reported the costs associated with CSII, and made the case that there could be short-term savings from adopting CSII. It will probably be widely quoted in support of CSII use, when pump clinics negotiate with PCTs over funding.
The study was carried out in Gloucester, UK. The aim of Ulahannan et al. (2007)245 was to collect data on NHS resource use by 34 patients starting CSII between June 2000 and June 2005. The resource use included:
-
diabetic clinical visits
-
appointments at other outpatient clinics
-
hospital admissions, whether related to diabetes or not
-
primary care contacts, at GP surgery, home visits, out of hours calls, and telephone contacts.
The aim was to collect such data for up to 5 years before and after, although for most patients it would be for much less. The exception was the subset of 17 patients for whom primary care data were collected, where the aim was to collect data for 2 years before and after. They also collected data on HbA1c levels. The average length of follow-up for hospital data was 31 months prior to CSII and 34 afterwards. The before/after HbA1c level showed an average drop of 1.2%.
The impact of CSII upon hospital resource use was reported as:
-
Consultant outpatient visits fell from 2.4 to 1.3 per year; these were costed at £88 each.
-
Nurse appointments were little changed, from 5.3 per year to 4.8 per year.
-
Hospital admissions were reported to have fallen, but few details are given. The median number of admissions per month in both periods was nil, so most patients were not admitted before or after. Means were not given, and would have been more useful. Those who were admitted before were assumed to have been so because of severe hypoglycaemia, and costed accordingly, but this could not be confirmed, because the authors received only a crude breakdown of reason for admissions into diabetes or other cause. The average length of stay for diabetes admissions in the hospital was 10.7 days. The length of stay for a hypoglycaemic episode would be much shorter. The costs were given as £757 for a hypoglycaemic episode admission, and £1932.50 for other diabetes admissions.
-
Primary care contacts were reported to have fallen by about half, from 11 to six appointments per year.
There are several problems with this study, due to there being various data deficits (i.e. some data were not available to the authors), and some inconsistencies in the paper:
-
The reduction in outpatient appointments would not release any real savings, in that the consultant would not be paid less but would do other things – an opportunity cost gain but not a financial saving. The figure used to estimate cost savings was 1.44 fewer diabetes consultant appointments per year, which does not tally with the figure of 1.1 provided from hospital records in the previous table.
-
Second, the lack of data on hospital admission costs makes any cost calculation unsafe. Table 3 of the paper suggests that 0.132 admissions per patient per year would be saved, but this is not compatible with the range of admissions per year given in the previous table, of 0–1.2.
-
Third, any reduction in admissions would not release real savings unless beds were closed and staff made redundant, which would not happen given the very small number of admissions involved. Again, although this may yield an opportunity cost gain there are unlikely to be any associated financial savings.
-
Similarly, if there is a reduction in primary care contacts, no funds would be released. The practices would be very slightly less busy.
Although the study provides some evidence for asserting that CSII may provide some gains in terms of opportunity costs and the freeing of resources to undertake other activities, the assertion that CSII will be accompanied by short-term savings in hospital and primary care cannot be supported by this study. A more detailed study is required.
Cost meeting abstracts
Bolli et al. (2004)113 undertook a 6-month multicentre RCT of CSII versus MDI among 57 patients with T1DM. Both arms of the trial showed similar improvements in glycaemic control, blood pressure and glycaemic events, with any differences in outcomes being non-significant. While treatment costs were not reported, the authors did report that CSII treatment costs were 400% those of MDI. The authors concluded that a glargine-based MDI regime was more cost-effective than CSII in an unselected T1DM population.
Cost-effectiveness modelling
Clinical effectiveness
The key clinical elements within the modelling relate to the differences between the HbA1c level and the rate of severe hypoglycaemia episodes between MDI and CSII. The base case uses the CORE model to assess the cost-effectiveness of CSII in a population of average age 40 years with T1DM within whom it seems well suited. While the CORE model is not suited to modelling adolescent and paediatric use, the effect of a younger cohort will be explored through an adoption of an average age of 30 years.
The base-case effect on the baseline HbA1c level assumes a baseline level of 8.8% HbA1c on MDI, reduced by 0.9% to 7.9% by CSII (based on the results from the Insulin Pumps Clinical Database for the 20–39 age range, which seems the most appropriate group for our purposes). Three sensitivity analyses are undertaken with regards to the effect of CSII upon glycaemic control.
-
The first uses the results from the meta-analysis by Pickup and Sutton, (2008)107 of a reduction of 0.6%.
-
The second uses the results from the study by Pickup et al. (2005)138 that show a reduction of 1.4% from a baseline of 9.0%, but no reduction in the rate of SH events
-
The third assumes that some pump users who have a high rate of SH events will gain no reduction in HbA1c levels because they start with good control hence a baseline of 7.5%, but achieve a reduction in their rate of SH events.
A general population of those with T1DM can be characterised as having a rate of SH events of 18.7 per 100 patient-years as within the NICE appraisal of the cost-effectiveness of glargine. The costing within this report has assumed the use of glargine or other long-acting analogue, which the Assessment Group for the glargine appraisal estimated could reduce the severe hypoglycaemia rate to as little as 8.8 per 100 patient-years. However, the Final Appraisal Document noted that this might be an overestimate and as a consequence a baseline rate for the general population with T1DM of 18.7 per 100 patient-years will be assumed. The effect of CSII upon this rate will be explored as a 50% reduction and a 75% reduction in SH events, with no effect upon SH events also being explored as a sensitivity analysis.
It seems likely that the main focus for NICE for the application of CSII will be on patients with more severe problems with hypoglycaemia. In common with the industry submission, this will be explored through the assumption of a rate of 62 per 100 patient-years, with reductions of 50% and 75% within this group. Simulations will also explore the possible effects of alternative cost scenarios as outlined within the costs section above. As noted within the literature review, the CORE model may have a tendency to overstate overall mortality within T1DM over longer time horizons, which may be due to a possible overestimation of macrovascular complications and their associated mortality. As a consequence, this will also be explored through 50-, 30- and 10-year time horizons coupled with a reporting of the evolution of the estimated macrovascular events and survival over this period. While the 10-year time horizon is too short for an accurate evaluation of the cost-effectiveness of CSII relative to MDI, it permits the evolution of cost-effectiveness to be explored and highlight timing of the anticipated main gains.
The group of patients who start with good control as reflected in HbA1c, but achieved at the cost of more problems with severe hypoglycaemia, will get little or no reduction in HbA1c levels. The effect of CSII upon HbA1c levels will be set to nothing for this group. However, the baseline rate of SH events will be increased to 134 per 100 patient-years as in Boland et al. (1999),99 with the effect of CSII being explored through reductions of 50% and 75%, the 50% reduction corresponding closely to the reduction to 76 per 100 patient-years from CSII as reported by Boland et al.
Treatment costs
More detail on costs is given in Appendix 7.
Capital costs
The NHS Supply Chain is currently engaged in a tendering exercise to establish a national price structure for pumps and consumables. The range of pump prices currently available within the UK is £2375 to £2750, with a usual warranty period of 4 years although the Roche Accu-Check Spirit carries a 6-year warranty.
After the warranty period, it was previously the case that an extension of the guarantee could be obtained through additional servicing. As outlined in the previous HTA report50 this could cost up to £500 in order for the guarantee to extend by an additional 2 years. This reduced the annualised cost per year of pumps under guarantee, although it was reported that this servicing was not always undertaken with pumps being disposed of when out of guarantee.
However, given the rate of change within the sector and the continuing evolution of pump types, it is reported by INPUT that extended servicing is no longer available. Pumps may be discontinued immediately after the warranty period expired with a new pump being purchased. Opinion from INPUT indicates that pump users are likely to want the most up-to-date pump and will wish to discontinue with pumps outside their warranty period. However, there is no necessary bar to using pumps outside their warranty period and a lifespan of an additional 2 years is used for sensitivity analysis.
Given this, the pumps purchase costs and their annualised values are as outlined in Table 27.
| Deltec Combo | Accu-Check | Animas IR1200 | Medtronic | |
|---|---|---|---|---|
| Purchase price (£) | 2750 | 2375 | 2600 | 2750 |
| Warranty (years) | 4 | 6 | 4 | 4 |
| Annualised (warranty) (£) | 723 | 431 | 684 | 723 |
| Annualised (warranty + 2) (£) | 499 | 334 | 471 | 499 |
With regards to the additional training that may be required for the use of CSII, this can be estimated as a one-off cost of around £240 (based on the cost of a DAFNE course in Aberdeen, UK) which would annualise to an approximate figure of £15 on the assumption that this is a one-off cost for those transferring to pumps.
In contrast, the only capital items for MDI are the two pen devices necessary, which, at a cost of around £22 each, and a possible lifespan of 3 years, would give an annualised capital cost of £15.
Consumables and total costs
The consumables for CSII of infusion sets and reservoirs as outlined within the manufacturer’s submission and by INPUT (see Appendix 8) have an annual cost of between £1090 and £1361, with an average of around £1220. However, these are pump specific. Other costs relate to the required insulin dose and the frequency of blood glucose monitoring. The meta-analysis by Pickup et al. (2002)18 noted a reduction in daily requirement for insulin of 14%, similar to the change from 0.6 IU/kg per day for CSII compared with 0.7 IU/kg per day for MDI in the study by Doyle et al. (2004)110 Thomas et al. (2007)111 also reported a baseline dosage on MDI of 0.7 IU/kg per day. In calculations, we have assumed a dose on MDI of 0.7 IU/kg per day and a 14% reduction. This may be conservative since other studies have reported large falls, such as that in Thomas et al. to 0.4 IU/kg per day on CSII.
The previous review noted that CSII had a daily requirement of four or more blood glucose tests per day compared with three or more for MDI, although concluded that on average this would not result in any real additional cost for CSII. Given this, the base case for this review will assume a common rate for both CSII and MDI.
An assumed patient weight of 80 kg translates into the CSII costs shown in Table 28.
| Deltec Combo (£) | Accu-Check (£) | Animas IR1200 (£) | Medtronic (£) | |
|---|---|---|---|---|
| Infusion sets, reservoirs | 1362 | 1214 | 1087 | 1374 |
| Insulin | 312 | 312 | 312 | 312 |
| Lancets | 36 | 36 | 36 | 36 |
| Test strips | 329 | 329 | 329 | 329 |
| Glucometer | 10 | 10 | 10 | 10 |
| Consumables | 2048 | 1900 | 1773 | 2060 |
| Total (warranty) | 2771 | 2331 | 2457 | 2783 |
| Total (warranty + 2) | 2547 | 2234 | 2245 | 2559 |
The above assumes that infusion sets are changed every 3 days as recommended. Additional data supplied by INPUT as to the frequency of infusion set changes among its members shows some variability in this, as shown in Table 29. This averages a change every 3.3 days, which, if generally applicable, would tend to reduce the total annual cost of CSII by around £100 to £130. However, the recommended change every 3 days has been retained in the base-case analysis.
| Frequency of change | Percentage |
|---|---|
| Every 2 days | 9 |
| Every 3 days | 49 |
| Twice weekly | 26 |
| Every 4 days | 10 |
| Every 5 days | 4 |
| Every 6 days | 1 |
| Weekly | 1 |
In contrast, MDI is associated with insulin costs of £466, needle costs of £32 and the same costs for lancets, test strips and glucometer of £374 to give a total annualised cost, including the £15 annualised cost of pens, of £890. Averaging the CSII costs above suggests an annualised cost of CSII of £2590, which implies a net marginal cost over MDI of £1700 for the base case.
Costs of complications
Clarke et al. (2002)213 provide costs for a number of the complications of diabetes among the patients with T2DM in the UKPDS cohort. These are broken down into the year in which the event occurred and subsequent years. Unfortunately, due to data problems the non-inpatient costs could not be determined for the individual complications but, rather, were grouped by whether the complications were macrovascular or microvascular.
Updating the costs for inflation using the Unit Costs of Health and Social Care of the Personal Social Services Research Unit (2006)246 implies the costs in 2006 prices as shown in Table 30.
| Inpatient (£) | Non-inpatient (£) | Total (£) | ||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2+ | Year 1 | Year 2+ | Year 1 | Year 2+ | |
| Fatal MI | 1474 | 0 | 403 | 0 | 1877 | 0 |
| Non-fatal MI | 5207 | 594 | 403 | 330 | 5610 | 924 |
| MI average | 3863 | 594 | 403 | 330 | 4266 | 924 |
| Fatal stroke | 4328 | 0 | 403 | 0 | 4731 | 0 |
| Non-fatal stroke | 3028 | 319 | 403 | 330 | 3431 | 649 |
| Stroke average | 3210 | 319 | 403 | 330 | 3613 | 649 |
| Angina | 2506 | 631 | 403 | 330 | 2909 | 961 |
| CHF | 2842 | 807 | 403 | 330 | 3245 | 1137 |
| Blindness in one eye | 1116 | 360 | 349 | 261 | 1465 | 621 |
| Amputation | 10,823 | 384 | 349 | 261 | 11,172 | 645 |
| Cataract extraction | 1987 | 134 | 349 | 261 | 2336 | 395 |
While these are from the same source as the industry submission, their values are typically slightly above those used in the industry submission. This difference appears to relate to the inclusions of non-inpatient costs coupled with some possible differences as to uprating for inflation, the above applying the Health and Social Care Costs index. The base case uses the above values.
In common with the industry submission, the results of Ghatnekar et al. (2001)247 are used for the costs of complications associated with uninfected ulcers, infected ulcers and gangrene, which, in 2006 prices, equate to event costs of £1643, £1684 and £2700, respectively. Again, these are slightly higher than those of the industry submission, probably due to different inflation rates having been assumed. Similarly, the costs of haemodialysis and peritoneal dialysis can be taken from UKPDS 40248 (as summarised in the industry submission), to give annual costs of £27,575 and £20,704, respectively. The other costs of treatment have been taken from the industry submission.
Cost sensitivities
As already noted, the duration of pump use is subject to some uncertainty given the changes to warranty status and extension. If pumps are used for 2 years beyond their warranty period this would suggest a reduction of around £100–200 in the cost of CSII.
Given the use of CSII in children with T1DM, and the possibility of use in overweight patients with T2DM, we need to consider the extent to which patient weight could affect costs. However, the major cost components for CSII are the consumables and capital costs that do not vary with weight or diabetes type.
For a patient weight of 80 kg, CSII results in an annual insulin cost saving of £154. Reducing the patient weight to only 30 kg reduces this cost saving to £58, so increasing the net cost of CSII by £96. In contrast, increasing the patient weight to 100 kg increases the annual insulin cost saving to £192, so reducing the net cost of CSII by £38 although this does assume the same dosing per kilogram, which may underestimate the annual saving in insulin costs.
The more pessimistic assumption of equal dosing under CSII and MDI of 0.6 IU/kg reduces, but does not eliminate, the insulin cost savings from CSII, as MDI still requires half of the insulin used to be the slightly more expensive long-acting analogues. Given this, the equalisation of insulin dosing sees CSII result in a reduced saving of £87 in the insulin cost, hence an increase in the net cost of CSII of £67.
If CSII requires one additional daily blood glucose test, the additional cost to CSII would be £120, and it should be noted that the previous HTA review50 suggested that this might be required. Similarly, the AETMIS review anticipated additional costs being associated with increased monitoring frequency from CSII compared with MDI. 19
In sensitivity testing of different costs, a high cost of CSII corresponding to equal insulin dosing and increase monitoring frequency will be explored: CSII £2710, MDI £803 resulting in a high net cost of £1907. A low net cost will also be explored, this arising from the base-case assumptions but a longer pump lifespan to give: CSII £2400, MDI £890, resulting in a low net cost of £1510.
Another element subject to a degree of uncertainty is the ongoing costs of legal blindness to the NHS/PSS (Personal Social Services). These may be somewhat higher than the cost of blindness in one eye as identified by Clarke et al. (2003). 212 Meads et al. (2003)249 estimated the average annual cost to the NHS/PSS of severe visual impairment arising in an elderly population from wet age-related macular degeneration to be £5345. Given this, the sensitivity of results to a higher ongoing cost of legal blindness of £4000 per year will be explored as an illustration, although as shown below this has minimal effect upon results.
Other model inputs and sensitivity analyses
Other baseline population characteristics, utilities and costs of complications will be as per the industry submission with the exception of the cost of an SH event, which will be taken to be £72 in the base case, reflecting the value used within the glargine appraisal, adjusted for inflation to current equivalent, rather than the £413 assumed by industry. The higher cost used by industry assumes admission, whereas most patients are not admitted to hospital after a severe hypo. Sensitivity analyses with regards to the effect of CSII upon glycaemic control, the effect upon rates of hypoglycaemic episodes, and the net treatment cost will be conducted for the base-case populations with the baseline rate of SH events of 62 per 100 patient-years.
The full list of simulations as outlined within Appendix 9 were run in CORE. However, with regards to the reduction in SH events a reduction of one SH event would be anticipated to result in an annual saving in reduced downstream costs of complications of £72 while yielding an additional annual 0.0121 QALYs in the base case.
Following from this, if there were no other benefits from CSII over MDI other than a reduction in SH events, then for a cost-effectiveness willingness to pay of £20,000 per QALY, the quality of life gain from an absolute annual reduction of one SH event could be monetised at a value of £242 (£20,000*0.0121). Given the cost saving of £72 this implies that at a threshold of £20,000 per QALY the annual willingness to pay to avoid one SH event would be £314. The parallel figure for a threshold of £30,000 per QALY would be £435. More concretely, a 50% reduction in SH events from 0.620 to 0.310 as in many of the simulations could be monetised at £95 for the £20,000 per QALY threshold (£242*0.62*50%), and at £133 for the £30,000 per QALY threshold.
Had the cost per SH event been £413 as in the industry submission, the parallel figures for a reduction of one SH event would be monetised at £655 at the £20,000 per QALY threshold, and £776 at the £30,000 per QALY threshold. As a consequence a 50% reduction in SH events from 0.620 to 0.310 would be monetised at £203 for the £20,000 per QALY threshold, and to £241 for the £30,000 per QALY threshold.
It can be seen from the above that for a cost saving per SH event of only £72, the monetised value of a reduction from 0.620 to 0310 SH events per year of between £102 and £140 arising from the adoption of CSII, while not insignificant, was not large compared with the anticipated increase in treatment costs of £1700. The base case requires there to be additional downstream gain in terms of reductions in the micro- and macrovascular complications of diabetes for CSII to be cost-effective. The adoption of the industry cost per SH event of £413 somewhat increases the monetised value of the reduction in SH events, so only requires a lesser effect upon the micro and macrovascular complications for CSII to be cost-effective. This is one of the sources of the better cost-effectiveness estimates for CSII within the industry submission compared with the current modelling.
Results
Base case for T1DM
The T1DM population has been characterised by the baseline rate of SH events being 18.7 per 100 patient-years as in the glargine appraisal. 75 Given this and the anticipated 0.9% improvement in HbA1c, the CORE model over a 50-year time horizon anticipates the results shown in Table 31.
| Type 1 | CSII | MDI | Difference |
|---|---|---|---|
| Life expectancy | 21.831 | 20.536 | 1.295 |
| Life expectancy (discounted) | 14.237 | 13.652 | 0.585 |
| QALYs (discounted) | 9.571 | 8.97 | 0.601 |
| Treatment costs (discounted) | £38,129 | £12,599 | £25,530 |
| Other costs (discounted) | £21,463 | £24,316 | –£2853 |
| Total costs (discounted) | £59,592 | £36,915 | £22,677 |
| ICER: cost per QALY | £37,712 | ||
The above results assume a 50% reduction in SH events. Other simulations for this group use a 0% reduction and a 75% reduction in SH events, the results of which are presented in Appendix 10. All these simulations show similar results given the relatively low baseline rate of SH events of 18.7 per 100 patient-years.
Higher rates of severe hypoglycaemia
For the base-case population with a baseline HbA1c of 8.8% and an SH event rate of 62 per 100 patient-years, the anticipated impact from CSII of reduction in these to 7.9% and 31 per 100 patient-years, respectively, is as outlined in Table 32.
| 50-year horizon | CSII | MDI | Difference |
|---|---|---|---|
| Life expectancy | 21.808 | 20.563 | 1.245 |
| Life expectancy (discounted) | 14.224 | 13.665 | 0.559 |
| QALYs (discounted) | 9.504 | 8.892 | 0.612 |
| Treatment costs (discounted) | £38,097 | £12,611 | £25,486 |
| Other costs (discounted) | £21,662 | £24,761 | –£3099 |
| Total costs (discounted) | £59,759 | £37,372 | £22,387 |
| ICER: cost per QALY | £36,587 | ||
Given the similarity in terms of clinical assumptions between what was labelled the conservative UK-based analysis of the industry submission, and those of the base case above, the average life expectancies are similar, at around 21.8 years for patients using CSII compared with 20.6 years for patients using MDI. The base-case analysis above anticipated a slightly smaller gain from CSII of 1.2 years compared with 1.4 years within what was labelled the conservative UK-based analysis of the industry submission. The anticipated discounted QALY gain is also a little lower in the base case, at 0.61 QALYs compared with 0.72 QALYs in the conservative UK-based analysis of the industry submission.
The small difference in cost per QALY between the two tables above, despite the difference in SH events is because the results of the CORE modelling are principally driven by the effect upon glycaemic control rather than the rates of severe hypoglycaemia. However, this assumes that the reduction in SH events does not also yield a quality of life gain from a reduction in the fear of SH events.
For the base case, while the savings from CSII within other costs provide around a 12% net cost offset to the net treatment costs, the additional total costs of £22,387 give an ICER in the base case for CSII over MDI of £36,587 per QALY.
For illustration, and to test the model, curtailing the time horizon to 10 years markedly increases the cost per QALY (Table 33).
| CSII | MDI | Difference | |
|---|---|---|---|
| 30-year horizon | |||
| QALYs (discounted) | 9.299 | 8.721 | 0.578 |
| Treatment costs (discounted) | £36,967 | £12,293 | £24,674 |
| Other costs (discounted) | £19,107 | £22,565 | –£3458 |
| Total costs (discounted) | £56,074 | £34,858 | £21,216 |
| ICER: cost per QALY | £36,710 | ||
| 10-year horizon | |||
| QALYs (discounted) | 5.603 | 5.392 | 0.211 |
| Treatment costs (discounted) | £20,637 | £7059 | £13,578 |
| Other costs (discounted) | £5062 | £6412 | –£1350 |
| Total costs (discounted) | £25,699 | £13,471 | £12,228 |
| ICER: cost per QALY | £58,013 | ||
The effect of a time horizon of 30 years is minimal, as would be anticipated given the expected average life expectancy of a little over 21 years: few live to this point and the effects of CSII are mostly contained within this horizon. However, with a time horizon of 10 years the cost-effectiveness has worsened to £58,013 per QALY. This is because there is almost no gain in anticipated average life expectancy, as almost all would live beyond the truncated time horizon whether receiving CSII or MDI. The long-term timescale for the development of complications means that the great majority of the gains from CSII occur after the 10-year point.
Curtailing the time horizon to 30 years causes relatively more of the complications of diabetes to be excluded from the CSII group than from the MDI group, as better glycaemic control under CSII tends to postpone these. This gives rise to the anticipated increase in savings among other costs, and also accounts for the relatively muted effect that curtailing the time horizon has upon the net QALY gain. Although the effect is not overly large, it can be illustrated by the cumulative incidences of three of the macrovascular complications that lead to both cost and quality of life impacts (Table 34).
| 10 years (%) | 30 years (%) | 50 years (%) | |
|---|---|---|---|
| CSII | |||
| CHF | 4.7 | 22.3 | 28.7 |
| Stroke | 1.5 | 8.1 | 11.6 |
| MI | 4.2 | 21.2 | 26.6 |
| MDI | |||
| CHF | 4.9 | 22.6 | 27.7 |
| Stroke | 1.6 | 7.9 | 10.6 |
| MI | 5.1 | 23.8 | 28.3 |
| Net | |||
| CHF | –0.2 | –0.3 | 1.0 |
| Stroke | –0.1 | 0.2 | 1.0 |
| MI | –0.9 | –2.6 | –1.7 |
Within this subset of complications, although the cumulative incidence of stroke evolves differently, its overall cumulative rate is relatively low compared with CHF and MI events. For CHF and MI events, the net effect of CSII upon these increases at the 30-year point then falls back again at the 50-year point. In addition to the explanation given above, the greater increases in the cumulative incidences of CHF and MI among the CSII group than in the MDI group will also be due, in part, to the greater proportion of CSII patients remaining alive during this period.
Younger age group
The base case and the above high SH event rates population have assumed an average age of 40 years, as seems well suited to the CORE model. Reducing this to 30 years, while retaining all of the other assumptions as within the high SH-event scenario population, leads to the data shown in Table 35.
| CSII | MDI | Difference | |
|---|---|---|---|
| Life expectancy | 25.146 | 23.498 | 1.648 |
| Life expectancy (discounted) | 15.528 | 14.854 | 0.674 |
| QALYs (discounted) | 10.357 | 9.648 | 0.709 |
| Treatment costs (discounted) | £41,352 | £13,631 | £27,721 |
| Other costs (discounted) | £23,558 | £27,055 | –£3497 |
| Total costs (discounted) | £64,910 | £40,686 | £24,224 |
| ICER: cost per QALY | £34,136 | ||
While the baseline age of the cohort has been reduced by 10 years, the average life expectancy, as modelled by CORE, increases by only a little under 5 years. As would be anticipated, the net treatment costs increase but these are offset to a slightly greater degree by increased net savings from downstream complications. Although these complications are likely to be more prevalent within the modelling for both CSII and MDI, the net effect is likely to be greater, given the greater additional life expectancy. Given these changes, the cost-effectiveness of CSII improves slightly to £34,136 per QALY. While this still does not lead to a conclusion of cost-effectiveness, it does represent an improvement of 7%.
Lesser effect upon glycaemic control
The meta-analysis of Pickup and Sutton (2008)107 suggested an overall reduction in HbA1c level with CSII of 0.6%, this also being modelled within the manufacturer’s submission as the trial-based analysis. Undertaking a similar analysis with a baseline HbA1c level of 9.0% and a 0.6% improvement, and retaining the assumption of a 50% reduction in the rate of SH events, gives the data shown in Table 36.
| CSII | MDI | Difference | |
|---|---|---|---|
| Life expectancy | 21.399 | 20.563 | 0.836 |
| Life expectancy (discounted) | 14.044 | 13.665 | 0.379 |
| QALYs (discounted) | 9.318 | 8.892 | 0.426 |
| Treatment costs (discounted) | £37,645 | £12,611 | £25,034 |
| Other costs (discounted) | £22,673 | £24,761 | –£2088 |
| Total costs (discounted) | £60,318 | £37,372 | £22,946 |
| ICER: Cost per QALY | £53,788 | ||
As would be anticipated, the net effects on life expectancy and quality of life from CSII are reduced, as are the savings. The effect upon the net discounted treatment costs is also a slight reduction but, as this arises due to the reduced impact upon net life expectancy, it is not in itself desirable. The reduced relative clinical effect has a major detrimental effect upon cost-effectiveness, increasing the anticipated ICER to £53,788 per QALY.
Note that if the effect of CSII upon SH events is a 75% reduction, the cost-effectiveness of CSII within the scenario improves slightly to £47,780 per QALY.
Greater effect upon glycaemic control
As noted within the introduction to this section, a further analysis by Pickup et al. (2005)138 indicates an improvement of 1.4% from the use of CSII on a baseline of 9.0% under MDI. However, within this analysis no effect upon SH events was recorded. This results in the following data shown in Table 37.
| CSII | MDI | Difference | |
|---|---|---|---|
| Life expectancy | 22.239 | 20.226 | 2.013 |
| Life expectancy (discounted) | 14.415 | 13.505 | 0.91 |
| QALYs (discounted) | 9.633 | 8.747 | 0.886 |
| Treatment costs (discounted) | £38,574 | £12,473 | £26,101 |
| Other costs (discounted) | £21,204 | £25,416 | –£4212 |
| Total costs (discounted) | £59,778 | £37,889 | £21,889 |
| ICER: Cost per QALY | £24,720 | ||
As would be anticipated, the anticipated life expectancy is worse for MDI, given the poorer baseline glycaemic control. Also, as CSII improves this control to 7.6%, near to good control, the anticipated lifespan under CSII is greater than in the base case and an average additional 2 years’ life expectancy under CSII are anticipated by the modelling. Net treatment costs increase slightly given this increased survival, but these are more than offset by increases net savings from reduced treatment of the complications of diabetes. The overall net cost is less than for the base case. This leads to CSII having a cost-effectiveness ratio relative to MDI of £24,720 per QALY.
Greater and lesser effects upon severe hypoglycaemic events
As has already been noted, given the baseline annual rate of severe glycaemic events of 0.620 and the implied relatively limited annual impact of these upon both quality of life and cost, the effect of CSII upon these has only a relatively limited impact upon the anticipated cost-effectiveness of CSII. If CSII has no effect upon severe hyperglycaemic events then its cost-effectiveness is anticipated to worsen from £36,587 to £41,062 per QALY, whereas a 75% reduction in SH events from CSII would improve its cost-effectiveness to £33,361 per QALY.
Utility loss from each severe hypoglycaemic event
The base case assumes a utility loss of 0.0121. Reducing this to 0.010 changes the cost per QALY to £37,172. Increasing it to 0.0142 reduces it to £35,903.
Cost per severe hypoglycaemic event
Leese et al. (2003)67 provide estimates of resource use for those seeking medical attention to cope with an SH event, also noting that the majority of SH events do not require professional medical attention. Based upon these resource use estimates but applying NHS reference costs to the costs of A&E and the costs of non-elective admissions, if 25% of SH events require professional medical attention the average cost per SH event would be around £84. Similarly, if 50% require professional medical attention the average cost would be around £168.
Assuming that CSII results in a halving of the rate of 61 SH events per 100 patient-years, as experienced under MDI, yields costs effectiveness estimates of £36,429 per QALY and £35,728 per QALY, respectively.
If we assumed that all patients having severe hypos receive health service care, at a cost of £335 [obtained by adjusting the costs of Leese et al. (2003)67 for inflation] then the cost per QALY falls to £34,335.
Deaths from severe hypoglycaemic events
It is reported that the lifetime risk of death from severe hypoglycaemia is between 2% and 4%. A survival analysis could be constructed on the basis of this, the assumed duration of diabetes and the modelled anticipated additional survival to arrive at an annual risk of dying from SH events. However, this may not apply to the groups on MDI and CSII, and what follows is only illustrative. The risk could then be applied to the assumed 61 SH events per 100 patient-years to arrive at the risk of death per SH event. However, given the uncertainties around this it was felt more sensible to undertake a threshold analysis for a willingness to pay of £30,000 per QALY. Given this, the death rate per SH event would have to approach 2% for the cost-effectiveness of CSII to fall within it. Since the death rate per SH event is far lower than this, its inclusion would improve the estimated cost-effectiveness of CSII, but is unlikely to be a driver of results.
Higher and lower treatment costs
The cost of CSII varies with both the device used and the assumptions made as to post-warranty longevity, and also with any insulin savings and monitoring differences between CSII and MDI. Given that the base-case costs result in estimates of cost-effectiveness for CSII which are outside normal cost-effectiveness limits, the impact of a high annual net treatment cost for CSII of £1907, as outlined within the section on costs above, will be explored, but only with an increased effectiveness of a 75% reduction in the baseline rate of SH episodes. The lower net cost of £1510 is explored for both the base-case situation of a 50% reduction in SH events, and a greater reduction of 75%.
Using a higher net cost of CSII increases the base case-discounted net treatment costs by £3000 – from £25,526 to £28,526. As nothing else changes, total costs show a parallel increase from £22,171 to £25,171 for the same 0.664 QALY gain, resulting in a cost-effectiveness estimate for CSII of £37,874 per QALY.
Using a lower net cost of CSII reduces the net treatment costs from £25,486 to £22,691 and results in an improvement in the cost-effectiveness estimate to £32,020 per QALY. With a larger gain of a 75% reduction in SH events, in addition to the 0.9% improvement in glycaemic control, net treatment costs are reduced from £25,526 to £22,728, with the overall cost-effectiveness ratio falling to £29,151 per QALY.
Costs of blindness
As outlined earlier, the costs of legal blindness used for the base case may be something of an underestimate. However, increasing these to £4000 per annum has only a marginal effect upon results, very slightly improving the cost-effectiveness of CSII to £36,429 per QALY.
Utility under dialysis
As drawn from the manufacturer’s submission, the utility associated with peripheral vascular disease, haemodialysis and peritoneal dialysis are 0.56, 0.49 and 0.57, respectively. These are from a different reference than the other utilities and are also somewhat lower. As an extreme sensitivity analysis the utility loss from these can be set to zero. This has minimal impact upon the estimate of cost-effectiveness, causing it to rise only slightly to £36,881 per QALY.
Fear of severe hypoglycaemic events
A reduction in the rate of SH events can give quality of life gains in two ways. First, the immediate disbenefits at the time of the hypoglycaemic episodes are avoided. Second, there is an additional gain from reduced fear of hypoglycaemic episodes – reduced worry, better mental health and improved ability to undertake usual social activities. The utility gain from this may be more likely to apply to those with more frequent severe hypoglycaemia.
For the base-case population, if there is a subgroup that derives this benefit, given an anticipated longevity of a little over 21 years an annual 0.01 quality of life increment arising from CSII for this reason would translate into approximately an additional discounted 0.15 QALY gain. Factoring this into the base-case results would be sufficient to improve the incremental cost-effectiveness ratio to approximately £29,300 per QALY. An annual increment of 0.03 would lead to an ICER of approximately £21,000 per QALY.
Davis et al. (2005)219 showed through an analysis of postal EQ-5D and SF-36 questionnaires that the severity of hypoglycaemic events is linked to a general worsening over the questionnaires’ dimensions. Unfortunately, this effect was not quantified. Tabaei et al. (2004)221 in a US study of 1522 patients with diabetes, who were roughly equally split between T1DM and T2DM, showed through the administration of Quality of Well-Being Self Administered questionnaire that there was a clear relationship between the frequency of hypoglycaemic events and quality of life. While pertinent, this appears not to have been restricted to SH events and there is no easy read across from this to quality of life values. Lundkvist et al. (2005)220 in a study of Swedish patients with diabetes administered EQ-5D and derived utilities. Although there appeared to be a negative relationship between the frequency of hypoglycaemia and utility, it was not statistically significant, and also did not confine itself to consideration of SH events.
High severe hypoglycaemia rate but good HbA1c level
As noted, some people with T1DM have good glycaemic control but when using MDI achieve that level of control at the cost of a higher baseline rate of severe hypoglycaemia, say 134 per 100 patient-years. Improvements of 50% and 75% in the rates of SH events through use of CSII can be simulated for this group, with there being no gain in glycaemic control, but these yield cost-effectiveness estimates of £273,992 per QALY and £152,058 per QALY, respectively, not allowing for any utility gain from reduced fear of hypoglycaemic episodes.
Reducing SH events with no other benefits is not cost-effective, unless we take into account that there are some patients whose fear of severe hypoglycaemia limits their ability to lead normal day-to-day lives. If CSII reduces the rate of SH events by 50%, without affecting HbA1c level, the additional annual quality of life increment from the reduced fear of severe hypoglycaemia has to be of the order of 0.05 to improve the cost-effectiveness of CSII to around £28,600 per QALY. With a 75% reduction in SH events, for CSII to move towards cost-effectiveness requires that the additional annual quality of life increment from the reduced fear of SH events to be 0.04, resulting in a cost-effectiveness of around £31,300 per QALY.
Conclusions
The possible benefits of CSII include:
-
improved glycaemic control
-
reduced frequency of hypoglycaemia
-
a reduction in the chronic fear of severe hypoglycaemia
-
a reduction in insulin dose giving modest savings
-
improved non-health related quality of life arising from greater flexibility of lifestyle.
If severe hypoglycaemia has a cost of only £65 per event, whether CSII has no effect on frequency of events, halves it or reduces it by 75% has little impact upon the estimated cost-effectiveness of CSII. This is because the main driver of cost-effectiveness in the CORE model is HbA1c level. Severe hypoglycaemia has little effect because episodes are of short duration.
This is underlined by simulation among those with high rates of severe glycaemia events but good baseline HbA1c levels, in whom CSII has no effect upon glycaemic control, but causes reductions of 50% and 75% in the rate of severe glycaemic events. Despite the larger absolute falls in severe glycaemia events within this group, the estimates of the cost-effectiveness of CSII relative to MDI rise to six figure sums per QALY. This does not take chronic fear of hypoglycaemia into account.
For the base-case estimate of a 0.9% reduction upon a baseline 8.8% HbA1c level, the central estimate of the cost-effectiveness of CSII relative to MDI is estimated as £36,587 per QALY. These figures are based upon an estimated reduction in SH events from 62 per 100 patient-years to 31 per 100 patient-years. If CSII reduces this rate to only 15.5 per 100 patient-years the cost-effectiveness of CSII for a 0.9% reduction to a baseline 8.8% HbA1c level is only slightly reduced to an estimate of £33,361 per QALY.
A lesser effect upon glycaemic control of only a 0.6% reduction upon a baseline 8.8% HbA1c level while retaining a 50% reduction in SH events leads to a cost-effectiveness estimate for CSII of £53,788. A greater effect upon glycaemic control of a 1.4% reduction upon a baseline 9.0% HbA1c level with no reduction in SH events leads to a cost-effectiveness estimate for CSII of £24,720 per QALY.
There is uncertainty as to the net treatment cost of CSII, it being plausible for this to range from a central estimate of £1700 up to a high of around £1900, and down to a low of around £1500. Despite this range, the estimate of the cost-effectiveness of CSII relative to MDI remains slightly above the usual cost-effectiveness thresholds.
However, if the net price of CSII is lower than that assumed for the base case, the impact of a 75% reduction in SH events as opposed to a 50% reduction is just sufficient to tip the cost-effectiveness of CSII from being slightly above £30,000 per QALY to being slightly below £30,000 per QALY.
One of the main uncertainties relates to the additional quality of life benefit from a reduction in the fear of severe hypoglycaemia. While this effect may apply only to a subgroup of patients, the quality of life gain from a reduced fear of severe hypoglycaemia would have to be only relatively minor to make CSII cost-effective.
An annual gain of only 0.01 in quality of life from the reduced fear of severe hypoglycaemia would be sufficient to reduce the ICER for CSII to around the £30,000 per QALY, while an annual gain of around 0.03 in quality of life would reduce it to around £20,000 per QALY. But note that this is within the base-case population: those patients with T1DM who are poorly controlled on MDI and for whom CSII results in a 0.9% improvement in glycaemic control and a halving in the rate of SH events.
Chapter 5 Patient perspectives
This chapter has three sections. First, there is an account of some of the points made in the submission to NICE from pump users. Second, insulin pump use has been increasing in very young children, in whom achieving tight control can be particularly difficult; we have therefore interviewed some parents of young children on CSII, in order to find out what the practical problems and benefits are in that age group. Third, we summarise some of the key points from the patients’ perspectives chapter of the previous assessment report.
We can think of the benefits of CSII as lying on a spectrum of different ease of measurement, with improvements in HbA1c level at the easy end to measure accurately, and ‘flexibility of lifestyle’ at the more difficult end. The published research tends to focus on the easy end. This chapter is concerned more with the other end.
The submission from INPUT
This section summarises the INPUT submission to NICE, the full version of which is available on the NICE website, in the ‘Key Documents’ section, under ‘Development History’ for the insulin pumps appraisal.
INPUT is the organisation of insulin pump users. It is a patient-led group which is independent of the manufacturers, and whose aims are: ‘to increase the awareness and understanding of insulin pump therapy, and to help, support and educate insulin pump users and their families in their use’. 250
INPUT submitted evidence to NICE, in conjunction with Insulin Pumpers UK, a web-based discussion group (INPUT ‘Joint submission from INPUT and Insulin Pumpers UK’ 2007251). Some of it provides information on aspects such as control of HbA1c and reduction in frequency of hypoglycaemia, which has been reported in Chapter 2. However, it also provides evidence on other benefits of CSII, such as quality of life.
The submission consists of two sections – a formal submission and a collection of commentaries by insulin pump users (including families). The formal section makes points including:
-
The previous NICE guidance74 has been implemented to widely varying extents in different parts of the country, ranging from full support for CSII, to capping at an arbitrary level of 1–2% of the numbers with T1DM, to no support at all (particularly for children).
-
This is partly because the guidance about who should receive CSII was open to differing interpretations.
-
In addition to tighter control of diabetes, CSII provides quality of life gains and flexibility of lifestyle – the freedom to eat only when hungry, the opportunity to sleep better without nocturnal hypoglycaemia or setting alarm clocks for blood tests, the chance to undertake sports and exercise, and greater self-confidence in education and careers. (Again, similar comments have been made after DAFNE courses, by people on MDI.)
-
That the opinion of individual diabetologists and paediatricians had a major effect on provision, and that there are ‘anti-pump’ professionals.
-
The need for a cohesive service for all people with T1DM. The submission recognises that there are resource constraints on diabetes care.
-
That if a diabetes service supports basal/bolus (MDI) insulin regimens, it should be able to support a pump service because MDI is just as demanding of specialised dietetic and nursing time as CSII.
-
That with pumps, it is easier to control the boluses, but, more importantly, the basals, particularly during the night.
-
That an algorithm should be developed to identify which patients would be suitable for CSII.
-
The need for transitional arrangements between paediatric and adult clinics.
-
Problems when patients move from an area with a pump service with funding to one without.
-
The usefulness of CSII in children who need very little insulin.
-
That in some patients, HbA1c level does not change but blood glucose is kept within a much narrower range, avoiding extreme swings.
-
That for children and families, quality of life gains include being able to eat out, to go on excursions of uncertain duration, such as school trips, to get up at different times, and to not to have to force children to eat when they do not want to.
-
That school routines are easier to manage on CSII, especially as some schools cannot cope with lunchtime injections, which prevents children moving to MDI. Children on pumps do not need to go to the medical room to inject and do not need to go to lunch first – rather they can wait and go with their peers, thus reducing social exclusion. 252
-
That examinations are easier to cope with if blood glucose is more stable.
-
That children feel more in control on CSII.
The submission is, of course, based on successful users of pumps, and INPUT accepts that not all children or adults will wish to use pumps. The individual submissions added detail to some of the above:
-
that mood often improved in children with better control
-
that, pre-pump, patients often had very wide swings in blood glucose levels – daily blood sugar ranges from 2 to 26 mmol/l. One patient reported a pre-pump range of 2–30 mmol/l, reduced to 4.8–7.5 on CSII
-
that very high levels, such as the postbreakfast spike, reduced academic ability during that period. One girl did well in most subjects but poorly in maths, which she did mid-morning. Once her blood glucose was stable on a pump and the spike was prevented, her maths improved.
-
the usefulness of being able to set different basal rates for different times of day and night, and for different activity levels (Although this can be done to some extent with twice daily detemir or NPH)
-
the ease of travelling through time zones when on business or holiday
-
going to Scout camp without mother
-
return of hypoglycaemia awareness
-
reduced insulin doses, varying amongst patients – one adult uses 30–40% less insulin on CSII
-
no support from some diabetes clinics, making travel to a pump centre a nuisance. ‘After a three-year fight to get a pump and find a suitable hospital…’
-
life more like normal youngsters… not feeling different
-
a recurring phrase – ‘being in control of the diabetes rather the other way round’ (again, echoes of comments by DAFNE graduates)
-
some very large drops in HbA1c level, such as ‘My HbA1cs were in the mid to high tens despite blood testing and injections up to 10 times per day… on pump therapy my HbA1cs dropped to around 6–7%’.
Some patients and families were self-funding.
Understanding parents’ decisions to change from injections to pumps: A qualitative study of parents’ accounts of young children with T1DM
Background
Many centres across the UK are experiencing a demand for insulin pumps from patients, as the awareness of the success of this form of therapy has entered the public domain. This has become particularly so for children and young people. 253
This section examines the accounts of parents with young children with T1DM who have changed from using injections to using pump therapy. We believe provision of CSII to young children may be one of the current growth areas and perhaps one of the most marked changes since the last assessment report. 50
Understanding patient-centred care for children and young people with T1DM, as outlined in the National Service Frameworks for both Children (DoH 2004)254 and diabetes (Scottish Executive 2002),255 requires an understanding of children’s individual autonomy as well as the executive role of parents, and the important contribution they make to the successful management of diabetes. In this section, parents’ accounts of switching to pumps from MDI are divided into themed headings. Time did not allow a larger study. This report aims to offer some background to choices of therapy made by parents with children with T1DM, and the problems they faced.
Methods and subjects
Details of methods and rationale are given in Appendix 11. The main questions posed are given below:
-
Why did parents decide to use CSII, how did they obtain information, how was CSII managed, to what extent has CSII affected diabetes outcome, and what lessons have been learned?
-
To what extent have different clinical practices affected parental use of CSII in young children, and what factors explain the variation in opinion about the use of CSII in young children, i.e. to gain a better understanding as to why the previous NICE guidance74 has been implemented to varying extents in different parts of the country?
-
What are the benefits and challenges of using CSII in young children?
The ages at onset of diabetes, at start of CSII, and at time of interview are shown in Table 38, along with funding arrangements and HbA1c changes.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Age of child (year) | 7 years | 8 years | 5 years | 5 years | 5 years | 6 years | 8 years | 7 years | 6 years | 6 years |
| Age at diagnosis (years/ months/weeks) | 5 weeks (after removal of pancreas) | 2 years | 27 months | 18 months | 14 months | 4 years | 4 years | 18 months | 2 years | 17 months |
| Age starting pump | 7 years | 7 years | 2.5 years | 4 years | 4 years | 5 years | 7 years | 6 years | 4 years | 4 years |
| Type of pumpa | S | A | S | M | A | M | A | M | M | M |
| Number of hospitals visited during seeking pump | 3 | 2 | 1 | 3 | 2 | USA | 3 | 1 | 4 | 2 |
| Paid for by PCT | ✓ (hospital and PCT) | ✓ | ✓ | ✓ | ✓ | USA (insurance) and PCT | ✓ | ✓ | ✓ | ✓ |
| Change in HbA1c (%) from injections to pump | 8.5 to 7.6 | 8 (MDI) to 7 | 10 to 5.1–5.6 | 9.5 to 6.5 | 7.6 to 6.8–6.5 | Unknown to 7.6 | 8.1 to 6.8 | 10–11 to 7.2 | 8 to 7.1–7.2 | 8.8–9 (MDI) to 7.1 |
Results
The parents’ perspective
Parents were asked to describe the history of how they found out about CSII and how they began to use it. All but two said they found out about pumps from the ‘Children with Diabetes’ website, and that they, rather than the clinical team, had raised the idea of transferring their child from insulin injections to pump therapy. One parent knew of pumps because her husband had T1DM, although he was not on CSII himself, and another said her diabetologist had recommended it. All the parents identified poor glycaemic control and the associated risks of complications as main reasons for searching for an alternative therapy to MDI:
I first heard about them through the Children with Diabetes newsgroup (internet group). At the time there were only a few people on them [pumps] so I got in touch with Medtronic and everything she said just seemed so right. His HbA1c wasn’t that bad, 8.5, but we weren’t satisfied with that. His control wasn’t horrendous but it was the feeding, you had to force feed him and then you’d have to say you can’t have anything to eat. It was just so horrible.
(Parent C)
Implicit in the accounts was the feeling that parents should not question the health professional’s authority:
It’s funny, all the literature talks about patients being experts, but I don’t think we’ve got there yet. It’s more lip service really and it’s about knowing your place and not challenging the staff’s authority, even though it’s you that’s living with your child.
(Parent J)
The consultant kept telling me 10 [blood control] was the best we could expect, but I knew it wasn’t because I’d done the reading and I’d discussed in with parents on the ‘Children with Diabetes’ web.
(Parent C)
These sentiments were echoed by another parent who said:
I read all there was and asked if I could change him from two injections a day to the pump because I had to force feed him, but the consultant was very against it.
(Parent K)
All of the parents believed the pump should be accessible to very young children including babies. In fact, a number believed they had missed out by not being able to start using the pump until their children were older:
There’s no doubt that it should be available for your child once they are diagnosed and established on insulin. It’s hell trying to feed a baby and child to fit in with the injections. If I had known how much easier the pump was I would have pushed for it earlier.
(Parent E)
The clinical team
All but two of the parents said they had had to ask their GP to refer them from their regular hospital clinic to another hospital where they could meet a consultant who was comfortable with pump therapy for their child. All had to travel significant distances to see the new diabetes team, but all felt that the journey was worthwhile, if this meant their child would receive the pump:
I went to INPUT. Then I went to my GP and asked for a referral to another hospital. We had to go about 50 miles away, it’s a trek, but she [the consultant] was fantastic; she couldn’t understand why he wasn’t on a pump. She went through all the history with him and said yes, he could have one. That was a whole year after I started trying to get one. But then I asked about the Cosmo® pump because I’d heard it was good and she recommend I go to XX [another hospital] which is even further away.
(Parent A)
He [consultant] is anti any pumps; we didn’t get the pump through him. I don’t know, it’s difficult to explain but he gave the impression that ‘I’m the consultant, I know what I’m doing and you don’t, you’re only the parent.’ And my attitude was that I wanted to do the best I could for my son and I would do whatever it took.
(Parent F)
At our clinic we were told she was too young to go on the pump. It [the pump] wasn’t good for them psychologically (5 years). Also, that she shouldn’t have her blood checked at school because it would make her stick out, which was something I couldn’t get my head around because I come from a science background and he was basically telling us that for six hours of her day we wouldn’t know what as going on. So I had to go into the school and ask if they’d go against the diabetes nurse and check her bloods to make sure she was getting enough insulin. And I have to say they did what I asked. They were very good.
(Parent H)
I’d done my homework so there wasn’t anything they [doctors] could tell me really, only confirm what I already knew and heard. Which was if you’re prepared to put the work in your child will have better control and quality of life and will be at less risk of complications. And it’s true, his last HbA1c was 6.5’ [8.5 on insulin injections].
(Parent E)
Almost all of these parents believed they needed to do ‘their homework’ because many of the consultants lacked the appropriate knowledge about diabetes, which all believed was dangerous for their child. As one parent told us:
They seem to lack the appropriate interest to find out what is best for your child, to prevent complications.
(Parent G)
We do eight blood tests a day on the pump but we did that when he was on injections. Although his consultant said we only needed to do 2. He said that ‘if you test him so may times you’ll get different reading, like 8 one time, 15 another and 10 another, so why worry yourself. So it was a case of ignorance is bliss.’ But I wanted not to have the 10s and 15s but to know about then if we did so we could do something about them. So we’ve always done a lot of blood tests anyway. It’s just a shame. When we eventually found the doctor that put X on the pump; you just want to clone them so that other parents get the benefit too.
(Parent D)
We were having a nightmare with her blood control, but I was told [by clinicians] that even though her levels were very high if I hadn’t checked it I wouldn’t have known so just leave it and they’ll sort themselves out and again, but I couldn’t get my head around it.
(Parent H)
I was doing all this reading, you know, the book by Hanas, and I was getting contrary advice from Dr A. And she [daughter] was getting headaches, her eyes were hurting and she was feeling shaky and I was told it wasn’t possible and had nothing to do with diabetes. I contacted Diabetes UK and they told me about Dr B, so I traveled to see him, just to chat about how things were progressing. It was a bit of a distance, but his approach made sense… And he [Dr B] was brilliant. The next day the diabetes nurse phoned us and said she’d written a letter for funding … and she asked if X [daughter] or I would be interested in speaking to anyone else on the pump. But I don’t think X would have cared. She was just so feed up with feeling ill on injections and her mood swings were just horrendous.’
(Parent I)
Other parents also felt the choice was ultimately their own child’s:
He’s a different child on his pump. He says he doesn’t want injections ever again.
(Parent B)
What upsets me is that when your child is first diagnosed you have total trust in the people your child sees. It’s awful how many parents lose faith so quickly and they’re being told that their child’s symptoms can be nothing to do with diabetes. And then when I manage to get transferred to in our case Dr X who is so fantastic in his level of knowledge then backs you up in everything you thought was related to the diabetes, like the headaches. There’s nothing worse than being the parent of a newly diagnosed child and being told the symptoms don’t have anything to do with diabetes and so you think OK, does that mean they have something else awful.
(Parent A)
It’s awful being told you don’t know what’s wrong with your child and that her diabetes symptoms are just because she’s upset at being diagnosed. And when I quote things out of the Hanas’s book they didn’t even know about it which to me, rings alarm bells.
(Parent I)
I think if it hadn’t been for my scientific background I probably wouldn’t have pushed so hard [for pump]. When it comes to your own child you’re at low ebb anyway, when they’ve been diagnosed with this condition that you’re trying to get your head around, you’ve no guidance from the hospital team, which you think should know. It’s very stressful. So if we hadn’t moved to Dr B then I think I would have become extremely depressed. I think I would have found it very difficult to cope with the situation of no one knowing or acknowledging your anxieties. And of course X [daughter] was beginning to stand out at school because she has to put her hand up to have biscuits. So everyone, including the hospital was beginning to label her as manipulative.
(Parent J)
In the end I had to say I’m sorry I can’t deal with this anymore and so I’ll have to transfer to Dr B if they’ll have us.
(Parent C)
Despite these parents’ beliefs in the effectiveness and safety of the pump for younger children, most did not wish to try and influence other parents. However, most believed it was important to let parents have access to the information and make the choice themselves:
I run a local support groups for parents with children with diabetes, so I hear a lot of horrendous stories. I don’t talk about insulin pumps personally, but I try to set up meetings where people can be more involved, so they are in a position to ask questions and then they can go back to their consultants and say hang on this isn’t clear and they know they can change if they need to. So now in our area, all the people from the different hospitals are talking to each other and so they know if they’re not getting the right care and that they’re not stuck and can move to someone who is knowledgeable.
(Parent A)
Another parent with a similar experience felt her doctor wasn’t sympathetic to her choice despite her child’s improved control. This was important because parents who had to be referred to another consultant for their child to go on to CSII had to return to their original outpatients clinic once pump therapy had been established:
His control was much better, but our old doctor, the one that wouldn’t put us on the pump, wasn’t very pleased. All he said was, ‘oh I see you’ve had a couple of hypos’.
So you went back to the original team?
Yes we’ve had to, but the doctor [referral to another hospital] that put him on the pump said we could ring him at anytime. He’s given us his work number, his home telephone and his mobile and his email at work and at home. And he’s just said to ring him at anytime.
Once he went on the pump [by being referred to another hospital] you then had to go back to your original clinic. Did the staff there use it [the pump] as an opportunity to learn?
No, so that’s why if there’s a problem I write an email to ‘Children with Diabetes’, but if there’s an emergency I go to A and E. I can’t use my clinic because they don’t know enough. I know more than them about pumps.
All of the parents interviewed believed that if you wanted a pump for your child you would not only need to search the literature for yourself, but also be articulate and determined. These parents believed that certain parents wanting pump therapy for their child would be at a disadvantage – those who were less confident, those that were less well read and those who were less likely to question the decision of the doctor. Most parents believed they were more determined to ‘seek out’ the best treatments for their children, whereas they might be less likely to go to such efforts for themselves.
I think it makes a difference if you’re articulate. I’ve spoken to a few parents at the hospital and they said they were interested but as soon as they mentioned it to the consultant it was ‘no, pumps are dangerous, it won’t work for your child, they’re not suitable for children at all, children on pumps go into DKA and they die.’ These views don’t seem to change even when you bring information from INPUT.
(Parent D)
I think it’s wrong that getting the pump should be such a fight. I think you have to be quite a determined person if you want your child to go on the pump, a bit evangelistic. If there’s the slightest hesitation on your part you won’t get a pump.
(Parent A)
As this parent said:
The doctor we see gets sponsored to raise funds himself so that he has personally paid for every child that wants a pump to go on one. It’s incredible.
(Parent A)
A large number of these felt that pushing for the pump labelled them as a troublesome parent by staff, rather than one wanting the best for their child:
I think if I hadn’t pushed I wouldn’t have got it, but then this makes you out to be a bit of a trouble maker rather than just wanting the best for your child. He was on multiple injections, and the school wasn’t too keen so I was determined to get him on the pump before he started, but the consultant just said he could go back on two a day. Believe it or not he said: ‘just make sure you run him high all the time so that he doesn’t have a hypo at school and there won’t be a problem.’ I was just flabbergasted. I said: ‘no there won’t be a problem because by the time the complications kick in he’ll be in the adult clinic.’ His idea was to run things between 10 and 15 every day. The school was brilliant and they said I could go in every day to give the injections. They could see how worried I was.
(Parent E)
Not all staff felt this way. However, the likelihood was that if the consultant did not support pump therapy, the other professionals in the team, such as the diabetes nurse specialists, would not have the option of going on a training course, and therefore would be unable to advise or support families wanting the pump:
The nurses can be for it, ours is about 80% certain, but they’re blocked by the consultants who are the gate keepers. So I can’t ask her for advice because she’s not trained which a shame because she can see the benefit of it. So I use the internet group if I have a problem.
(Parent E)
Education
A major concern for most parents was the lack of support they received from the school education system, and in particular the lack of knowledge most people had about diabetes:
There’s a lot of ignorance about diabetes, especially type 1. Everyone thinks it’s something you can prevent or that you bring it on yourself through lifestyle.
I since found out from the diabetes nurse and the school nurse that the head teachers was enquiring what the legal position was if they refused to take my son. They were told they couldn’t refuse him entry but that they didn’t have to do any of the treatment, but that if he was unconscious or having a fit they could call an ambulance, and that was it, there was nothing else they would do for him at four years old.
(Parent C)
Only one parent had experienced teachers who were prepared to take responsibility of her child’s diabetes.
The school he’s at is brilliant. They have three teachers who know how to do blood test.
Why is that?
Before he started I went in with the diabetes nurse and trained them. I’ve got a really good relationship with them. They check his blood in the morning before he goes out to play in the morning at lunch and in the afternoon again and any other time he needs it. The nursery nurse knew about diabetes so they were really supportive and that reassures my son and me.
Another parent had to organise a teaching assistant to help do blood tests.
Employment
All but one of the mothers, and one father, were unemployed. A large number of these gave diabetes as the reason and most of these felt it would have been impossible to work because the schools were so unhelpful when their child was on insulin therapy. MDI, even when parents were not working, were impossible in most junior and nursery schools, despite the evidence that MDI were preferable to twice-daily injections:
I did work at first, but they [the school] were struggling to keep him conscious. That’s when he was on multiple injections. It does affect you economically but it’s worth it as far as I’m concerned.
(Parent G)
Barnard et al. (2008)252 report similar findings, with some parents reporting fewer interruptions to their working day, fewer phone calls from school, and less time off work because of their child’s diabetes once the child was on CSII.
Getting used to the pump
Parents suggested that their eagerness to use pumps meant they were prepared to tackle learning the technicalities of the pump, which most felt were more complicated than the injections, but after a while reassuringly became routine.
X [child] had a saline pump for about three weeks before so I was quite confident by the time I got it. But the first night I had to check it every hour and I was thinking when will this night end and then the next night I checked it two hourly and then three hourly. For the first 3 to 4 days X’s readings were amazing, between 4 and 6 then we went from there and altered the setting. It was quite incredible the first meal I thought I don’t need to get the injections out. I kept thinking am I doing it right, what if I do it wrong.
(Parent H)
The doctor [consultant parents were referred to] set up all the basal levels and he phoned us every day in the morning and evening so we felt very supported. It was also getting used to not panicking when it read 5 on the pump, which you would on injections, but thinking this is great. So that takes a while not to panic. This is fine. Probably about two to three months to understand the trends. And because her levels are so stable you could see reactions to specific food. Whereas before it was just like white noise, now you can pinpoint certain of the variables and you can adjust for those.
(Parent I)
Pros and cons of the pump
All of the parents spoke about the major benefits of the pump. A key point for all parents was that the pump prevented blood level swings, as well as improving HbA1c:
When you go to the clinic they want to know about your HbA1c, but even it it’s good you’re still having to cope with the daily blood level swings which are a constant battle. You feel so out of control.
(Parent E)
The improvements to both their child’s and the family’s quality of life were as important as the blood glucose levels. It was important to ensure children led normal lives, even with diabetes; to prevent strain on couples’ relationships as well as between siblings. Poor control while their child was on insulin injections had, two parents believed, caused marital problems.
The pros of the pump are quality of life. He can eat what he wants, he can lie in, and he can join in any sports activity he wants. Although it’s water proof we always take it off for swimming. My daughter [9 years] knows how to look after him if they go and stay with family. She knows how to change the cannula, count carbohydrates, but she’d never do his injections.’
(Parent E)
I think the pumps are user-friendly. You can say to people now just press that and that, rather than saying ‘now here’s the needle’, so it gives him as slightly more normal identify.
(Parent F)
A few of these commented on the challenges of having a pump, although none of these would consider going back on insulin injections:
It got blocked once.
(Parent D)
Most believed that as parents they had to be committed to transfer to pump therapy:
I think if you’re not so motivated it might not be so easy because if something goes wrong you need to act on it.
(Parent B)
We were concerned that only fast acting insulin so you’re very vigilant at night. Before the pump everyone had to fit in with X’s meal times. Now we can do activities that over run and it doesn’t matter.
(Parent E)
Her blood levels were always at the high end, so we knew there would probably be complications, but the main problem for us wanting to change [to pump] was she did not feel well, she didn’t act well, she was up every night feeling shaky because her levels were high then feeling ill because her levels were plummeting, so we didn’t have a clue what was going on. So she was doing everything right, she was eating well, exercising but she still isn’t feeling well. So to me now, the insulin pump means she is a child that feels well. I means yes, I would still want her HbA1c to come down. I means since she’s been o the pump her levels are between 5, 6 and 7. But the overriding thing is that she feels well. And this is the first year ever, that she has not missed a day off school. I mean that’s not to say she doesn’t get ill, but because of the flexibility with the pump, when you first see the signs of illness you can act quickly and put up the basal levels. And you can get the illness under control quite quickly and she’ll start to feel better. Whereas, before because you didn’t have the flexibility she’d get ill for longer.
(Parent J)
It gives you the reassurance that you can sleep through the night and actually it was the first time she slept through the night. Before she needed to go to the toilet, blood levels swinging and waking her up.
(Parent J)
She’s scared of having injections, but with the pump she only has to have one injection every three days.
(Parent J)
The problem with glargine is that you give your child the dose, but if your child becomes ill you’ve not got the flexibility to change it. You’re stuck with having this amount in. And you have to make her eat when she doesn’t want to or visa versa. Also with glargine, what she needed in the day was not what she needed in the night so we had problems because after its shot into her you lose control. Whereas on the pump she can have a quiet day if she wants and I’m not having to say, no you’ve got to go on 10 minute bike ride or if she want to go absolutely mad, she can do so she can be more like a normal child.
(Parent J)
She [8 years] joined a drama club. If she’d been on injections I doubt very much I’d have allowed her to do that. So it’s given here more breadth what she can do.
(Parent H)
My only concern that is that she’s [8 years] going away on a school trip and if the cannula gets dislodged there’s no one there that knows how to put it back, so I’m very tempted to offer to go along.
(Parent H)
Body image
Most of the parents believed their children were not concerned about wearing the pump. Several felt it made them feel special, were proud of it, like the fashionable bags that came with them, felt that pumps were like other gadgets that children took for granted, such as mobile telephones. One mother, however, was concerned that her daughter might be psychologically affected by wearing the pump when she reached adolescence.
He doesn’t mind wearing pump. He just gets on with it. There’s not hiding it, but we were like that with the injections. There are other kids at school with impaired hearing so they have battery contraptions too, so it’s not so abnormal.
(Parent F)
He’d rather than the cannula in him than six injections a day. On the odd occasion when we’ve had to back to injections [pump broken] he’s got in quite state ‘I don’t need those I’ve got my pump now!
(Parent B)
Probably the worst thing [of the pump] is she gets marks left on her body. Despite the literature, they don’t fade in two to three weeks, so I worry that she’ll become more body conscious when she’s older. But despite that she says she doesn’t want to go back on the injections.
(Parent J)
Another parent was conscious that wearing the pump meant her daughter was always aware of being diabetic:
X is aware that she’s diabetic all the time because she wears it.
(Parent J)
She’s very proud of it. Apparently in the first day she wore it, I was quite surprised, she asked the teacher if she could tell the whole school about it. But now she gets fed up with people asking. And she’s going through a stage when she’s feeling vulnerable because there’s the realisation that this isn’t going to go away, so she’s not keen on the attention.
(Parent H)
Expectation of long-term use of the pump.
All parents felt that the commitment associated with CSII was worth it because it was easier to manage blood glucose control.
To minimise long term damage. I’m hoping things will develop and eventually they’ll be a cure, but we can’t hold our breath for that. But the pumps giving him a quality of life he didn’t have before.
(Parent A)
Foreseeable problems
None, nothing that I’ve heard about.
(Parent J)
I don’t know if having the pump as a constant remainder may have psychological effects in the long run.
(Parent E)
Looking at the future
I would hope that there might be like a closed loop system where it checks his blood automatically and delivers the insulin automatically. They are such ‘little things’ and they’re having sometimes up to 9 or 10 blood tests a day and sometimes he says’ Mummy I’ve just got no more blood in me anymore’.
(Parent C)
Was it worth the commitment? The options available make it valuable but I’m anxious about the future and whether wearing the pump all the time creates psychological problems.
(Parent G)
If I could look into the future I’d want doctors to understand that it’s easier for kids to deal with requirements than restrictions. For example, having insulin involves somebody to figure out what’s in the food before you eat it, you have to check your blood sugar, you have to press the button on the pump before you can do this, this and this. It’s easier than saying you can’t have a biscuit when your friends are having a biscuit because it not your set time. In a sense, the pump enables a ‘can do’ situation.
(Parent D)
Summary of perceived benefits by parents of pump therapy in children
-
To control daily blood glucose fluctuations (high and low) and HbA1c level in line with national recommendations and so prevent long-term complications.
-
To control problems of hypoglycaemia and the ‘dawn phenomenon’, which result in parents testing their children’s blood throughout the night.
-
Only having to have ‘one injection’ every 3 days rather than numerous and sometimes painful injections everyday.
-
To improve child’s flexibility of lifestyle by allowing greater flexibility in term of diet and social and physical activities.
-
In relation to the above, to improve family’s flexibility of lifestyle by allowing greater flexibility in terms of diet and social and physical activities, reduce anxiety about child’s health, especially during the night, and, as a consequence reduce tensions between family members.
-
Pumps were more acceptable in schools. Multiple injections were not an option at most schools because school staff lacked knowledge about diabetes and were not prepared to take responsibility for multiple injections, for example several parents needed to give up work so that they are available to give injections. Consequently, most relied on two daily injections before starting on the pump.
-
To control mood swings in children, particularly at school where they could be labeled ‘moody’, ‘difficult’, ‘tired’, ‘lacking concentration’ or ‘introverted’.
-
Pump represents modern technology and can be a fashionable gadget (particularly gender-specific bags), which provide kudos among peers, while at the same time allowing them to feel normal and join in everything.
-
Enhances child’s status and individuality as an expert who is mature and ‘capable’ among peers and adults (e.g. teachers).
-
Best system available to improve blood control and prevent complications until a cure is found, for example pancreatic transplant not acceptable in children.
-
Appropriate for toddlers, not only children.
Summary of perceived challenges of pump therapy by parents of young children
-
Too expensive – both the pump and the disposables.
-
Parents need to be rather ‘evangelistic’ or have contact with ‘evangelistic’ clinicians to receive a pump. Too few clinicians have expertise, and most are therefore unlikely to recommend or support parental initiatives, even when there is a chance it will improve blood control and prevent complications.
-
May have to change clinical teams (2–3 times) and travel quite long distances to receive pump. Consequently, parents rely heavily on parent network websites for advice and support.
-
Parents have to be committed in terms of regular blood tests and carbohydrate counting.
-
Easier if adults are technologically minded.
-
Worry that child may have problems with body image when wearing pump during adolescence.
-
Not enough support from patient bodies regarding changing policy (particularly education) to better accommodate T1DM and pump therapy for children.
Discussion
This qualitative study examined the beliefs and attitudes of parents of young children who have been successfully started on pump therapy in the UK in the last 5 years. While accepting that this is a biased account from parents who have had considerable difficulties in starting their children on CSII, they nonetheless reflect some important aspects of the benefits and challenges of pump therapy.
Interestingly, none of the children concerned had treated themselves for any length of time with ‘classical’ intensive insulin injection therapy – MDI. This was because of several reasons: very young age, erratic eating and exercise patterns, and a difficulty in interpreting multiple blood glucose results. However, perhaps the major factor was the reluctance of schools to take on children requiring MDI, forcing the parents to use ‘conventional’ twice daily injections of insulin. Schools frequently insisted on extra staff to supervise the intensive therapy. The parents, therefore, moved rapidly in the course of their young child’s diabetes on to pump therapy, with few difficulties. This was reflected by the schools, who also seemed more at ease with pump therapy, accepting that the parents and the children themselves were the experts and no additional supervision was necessary.
Overall for this age group the benefits of the pump outweighed significantly the challenges and difficulties. Glycaemic excursions were dramatically reduced, with improvement in overall glycaemic control, less hypoglycaemia and no episodes of DKA. The children felt better. This was with fewer injections than with MDI. The wearing of the pump did not produce significant difficulties and these young children appeared to cope well with: the practical issues; pump technology; wearing of the pump; and managing diabetes with the pump. The ‘quality of life’ for both parents and children appeared to be markedly improved.
These were all committed parents who had to seek information about the availability and the practical issues of pump therapy. A significant amount of their information came from outwith the UK. Their local diabetes teams for the majority were not supportive of pump therapy and the parents appeared to become evangelists in order to seek out pump therapy, often travelling some distance to receive sympathetic and expert advice. The majority felt this process had delayed the placing of their child on a pump and most believe that children of all ages should be considered for pump therapy from diagnosis.
The parents expressed a strong view that considerable commitment was required to master pump therapy and accepted that not all parents would be prepared and/or able to give this commitment. However, a significant factor in allowing parents to consider pump therapy would be the valuation that their clinical team places on this form of therapy.
The parents were aware of the cost issues for the NHS. However, all had made a case to their funding committees in their local PCTs within the NHS. (Eight out of the ten had their pumps paid for totally by the NHS; two parents were paying towards the cost of the consumables.) Undoubtedly, all felt that the benefits of pump therapy made it cost-effective.
Acknowledgements
We are grateful for INPUT for recruiting the families and thank the parents who freely gave us their time and intimate thoughts.
Summary of patient perspectives from last HTA report
We started the ‘Patient perspectives’ section of the last assessment report with some caveats, which bear repeating. 50 (That section of the last assessment report was written by NW, one of the authors of this report.)
Caveats
The patients’ perspectives section is based largely on written statements from pump users, and several caveats are required. First, most comments have come from members of INPUT, who have responded to a request for comments. They are likely to be a more motivated group than average and some are clearly highly organised individuals. This does not affect the validity of their comments, but may have implications for generalisability. Second, they are successful pump users and tend to be enthusiasts for the technology. That is less important because those who do not succeed will not incur the ongoing costs of pumps. Third, most have had to pay for the pumps and consumables themselves, which creates another selection bias. Fourth, because pumps are little used in the UK – it appears that most of those who have gone on to CSII have done so because they have had a lot of trouble with control of blood glucose or frequent hypoglycaemic episodes, that is, a severity bias. They may have more to gain than the average person with insulin-treated diabetes. Again, this does not affect the validity of the findings, but will be relevant to discussions about the proportion of people with diabetes who should be considered for CSII. 11
The respondents for the last assessment report mentioned the expected gains in HbA1c and hypoglycaemic episodes, but also emphasised flexibility of lifestyle and working patterns, having more energy, feeling in control, less visibility of diabetes (being able to take bolus insulin without anyone noticing), and better moods in children (an almost universal comment in submissions by parents, but not mentioned in the trials):50
Her HbA1c levels dropped from 10.6 to 8.2, and her mood and personality changed – we got our little girl back again.
(Parent 8)
One comment from several mothers was that they found it very difficult to work full-time with a young diabetic child:
I have found it hard to go back to work as I seem to be on call for him all the time. For example, I will drop him off at school at 9.30 and by 11 am they can phone me because he has gone low, and I have to go back to the school
(Parent 3)
There were many problems with schools, but the comments were consistent in saying that school life was easier on CSII and, because staff cannot take responsibility for injections, children find it easier to look after themselves, have fewer hypoglycaemic episodes, do not need to eat at special times, can miss meals if necessary, and do not need to carry insulin syringes and vials.
Reasons for difficulty in obtaining CSII
Patients and parents sometimes comment on difficulty in getting CSII, and some think that some consultants are unenthusiastic about pumps. However, as one of the (anonymous to us) peer reviewers for the HTA programme (which organises its own peer-review process in addition to ours) commented:
Within the report … considerable prominence is given to patients’ difficulties in obtaining pump treatment and accessing services trained and willing to deliver pump therapy. The clinician viewpoint is perhaps under-represented here. For example our own team has had significant difficulty in obtaining approval for pump therapy from primary care trusts both for secondary car referrals and for tertiary referrals into our service. Pump funding still requires approval on a case by case basis.
We know from contact with physicians and paediatricians that some of the reluctance to provide CSII is not due to any antipathy to pump therapy, but is because there are higher priorities for any new resources, such as shortages of diabetes specialist and dietetic time. Many diabetes services are under pressure due to rising numbers of people with diabetes. We also know that some health authorities provide very little funding for insulin pumps.
Chapter 6 Implementation
If NICE recommends increased use of CSII, and if funds are available, factors to be considered include:
-
patient selection criteria. Ideally NICE should specify these in such a way that patient selection is uniform across the country. Selection criteria are discussed below
-
training of staff
-
education of patients
-
on-going support, including initial out-of-hours telephone advice
-
the speed of roll-out, taking note of the Swedish experience185 after a time of rapid expansion.
Education of patients
Based on the previous NICE guidance, all adult patients starting CSII will presumably do so after failing to achieve satisfactory results on MDI. So they would come to CSII, being well experienced in home blood glucose testing and self-adjustment of insulin dosage. One option might be that all patients being considered for CSII should have had a trial on MDI and have attended a DAFNE or similar course. Khoo et al. (2007)256 from Nottingham reviewed the first few years of their CSII service and noted that nearly all patients had attended a DAFNE or other structured education course. If so, the additional training needed for CSII would be modest. Similarly, they will be able to cope in the event of pump failure, by reverting to MDI.
Unpublished data from Aberdeen Royal Infirmary, UK, where all patients starting pumps do the DAFNE course, show that the cost per patient of a 5-day full-time DAFNE course is about £240. The last assessment report50 estimated that staff costs involved in switching a patient from MDI to CSII was about £148 at 2002 prices. Training costs for staff were estimated to be £2715 per centre, but those were criticised as overestimating the time cost for physicians to learn about pumps; the assessment report assumed 3 days, whereas critics said that 1 day was enough.
The DAFNE programme has set-up training centres around the country and these are training staff from other centres. This may reduce the training required for clinics starting a CSII service.
However, children may come to CSII without having had a spell on MDI, due to problems with taking lunchtime insulin at school, and they and their families may require more staff support. All patients on CSII will need to have immediate access to syringes or pens, and insulin, in case of pump malfunction. If the pump infusion ceases, blood glucose levels quickly rise. Zisser (2007)257 reported that stopping CSII for 30 minutes led to a rise in blood glucose of about 0.5 mmol/l by the end of that period, and by almost 2 mmol/l 3 hours later, despite reconnection.
Barriers to implementation
These are likely to include:
-
staff time – our impression from the literature and contacts with clinicians is that people going on to CSII need more support at first, but less later
-
lack of experience with pumps amongst clinic staff
-
an apparent lack of willingness, in some centres, to move to tighter control by intensive insulin regimens
-
competing priorities for diabetes services, which are having to cope with rising numbers of patients, especially those with T2DM. This is partly due to a steep rise in age-specific prevalence rates, couple with demographic change, but is also partly due to better survival
-
financial constraints.
Professor John Pickup, in his submission on CSII to NICE for this appraisal,258 commented on reasons for the low usage of CSII in the UK:
The experiences of patients referred to our clinic are informative as to the reasons for the UK’s modest implementation of pump therapy. Our patients are registered with over 40 PCTs, about 25% referred from London, 255 from SE England (mainly Kent) and the remainder from around the UK. . . . . .This allows us a wide-ranging view of attitudes to CSII amongst health care professionals and the difficulties of access around the country. The main problems seem to be poor knowledge of the effectiveness, safety and procedures of CSII by consultants, lack of a local CSII programme and team, usually due to competition for resources, and lack of a well-defined referral procedure for areas without local pump facilities.
About half of the patients referred to Guy’s hospital come from a local network that is well informed about CSII, but Professor Pickup notes that for those from elsewhere:
The remaining 50% of patients have often received little support for a trial of CSII from their local health care professionals, sometimes have encountered considerable opposition, and have consequently researched the value of pump therapy, located a pump clinic, and asked for a referral from their GP entirely by themselves. Typical comments from patients are that their local consultant ‘does not believe in pumps’, or ‘does not know anything about pumps’ or thinks ‘pumps are dangerous’.
Professor Pickup goes on to say that considerable education of health-care professionals will be necessary, but envisages two forms of this: first, training in pump use for those who will provide it, and, second, education on CSII for those within wider medical community who will refer patients to pumps clinics.
This prompts the question of whether CSII should be provided at a limited number of centres, or whether all diabetes clinics should provide CSII? One view is that CSII is just another way of giving insulin and that all clinics should be able to provide it. However, we note from the patient and family submissions that there is clearly resistance to CSII in some centres. In some cases, this may be due not to opposition to CSII itself but to competing priorities, in that if more money is available there are higher priorities than CSII. Diabetes UK reported recently that services were being reduced in some areas because of lack of funds. A Diabetes UK news release259 stated that: ‘Four years on (from the NICE guidance), and in some areas, people with diabetes are experiencing unacceptable delays in accessing services and in some cases no services to support people using this form of therapy are available at all’.
The alternative to CSII being provided in all clinics would be, perhaps only for an interim period, that there should be a limited number of pumps services, serving populations, of say 400,000, on the assumptions that:
-
0.3% of the population have T1DM
-
5% of people with T1DM will go on to CSII (this is a guess, not a prediction)
-
about 60 people would then attend the pumps service
-
60 CSII users provides a reasonable number for a centre to develop and maintain expertise.
However, in less densely parts of the country, some services would need to cater for smaller numbers.
In the 2003 guidance, NICE expected that CSII would be initiated only by ‘a trained specialist team that comprises a physician with a specialist interest in insulin pump therapy, a diabetes specialist nurse and a dietician’ (TA 57, para. 7.5.2). 74
Selection criteria for CSII
Various sets of criteria for CSII have been produced, with inevitable overlap. The report of the Insulin Pumps Working Group106 suggested the criteria given in Table 39.
In adults, the criteria for initiating CSII are that the patient should:
|
Pickup and Keen’s (2001)21 selection criteria are given in Table 40.
|
Selection criteria for a trial of CSII Type 1 diabetic patients who have failed to achieve good glycaemic control after a 3-month trial of intensive insulin injection therapy, including re-education in injection technique, dietary advice and blood glucose self-monitoring, because of:Prerequisites for insulin pump therapy All patients should be: |
Since this list was published in 2001, an additional criterion, elevated HbA1c level and unpredictable swings in blood glucose concentrations during best MDI, has been added.
The dawn phenomenon is probably only an indication for CSII if marked and accompanied by problematic nocturnal hypoglycaemia, being less of a problem with MDI using on long-acting analogues, which can lower fasting glucose without causing hypoglycaemia as frequently as did NPH. In addition, the dawn phenomenon does not increase HbA1c level by much. 260
The Canadian review19 commented that there was a general consensus on criteria for selecting the limited number of patients for whom CSII was indicated. Their criteria are shown in Table 41 below, somewhat abbreviated.
|
The current NICE guidance is that CSII should be used in suitably committed and competent patients when MDI has failed, with failure defined as:
-
HbA1c levels of greater than 7.5%, or 6.5% in the presence of microalbuminuria or adverse features of the metabolic syndrome.
-
Achieving those targets but at the cost of disabling hypoglycaemia.
And the NICE guidance assumed that only 1–2% of people with T1DM would become pump users. However it could be argued that many people with T1DM could be fitted into the above guidance on the grounds of having higher HbA1c level. Microalbuminuria is common (about 10% of patients with T1DM), and most patients would not get down to an HbA1c level of 6.5% without hypoglycaemia. The average HbA1c level in the DCCT intensive arm was about 7%, and severe hypoglycaemia was very common with 62 episodes per 100 patient-years. 49 It should be noted that a minority of patients had frequent hypoglycaemia, but during the study, one-half of the intensive group had experienced severe hypoglycaemia. It should also be noted that in the DCCT, hypoglycaemic episodes were as common amongst those on CSII as those on MDI.
The only people on MDI who would not qualify would be those achieving the HbA1c targets without severe hypoglycaemia. Based on the DCCT, some of these patients would, if transferred to CSII, still have severe hypoglycaemia, in which case it would be logical to transfer them back to MDI.
The Insulin Pumps Working Group base their selection criteria in effect on hypoglycaemia.
The resource implications of CSII should include the ‘run-in’ period before it, when extra clinic visits will be needed, partly to select out people who are probably not suitable. Sanfield et al. (2002)229 excluded about a third by having a trial period, and thereby ensured a high continuation rate amongst those who did start. The trial period involved three visits over a 20-month period, and a trial on a saline pump for several days.
It is likely that the more rigorous the selection criteria, the lower to discontinuation rate afterwards.
The protocol for the Guy’s Hospital CSII service is shown in Figure 2.
FIGURE 2.
Guy’s Hospital CSII Protocol – strategy for treating patients by CSII. BGSM, blood glucose self-monitoring; CSII, continuous subcutaneous insulin infusion; CVD, cardiovascular disease; MDI, multiple daily injection.

The need for dietetic time is also noted.
Contracts
Some CSII centres, mindful of the extra cost to the NHS, ask patients to sign a ‘contract’, which asks them to commit to achieving good control, in return for getting a pump. The implication is that if control does not improve, the pump may be taken away (although in practice, getting it back may not be so easy, but patient could be left to pay for the consumables).
This is regarded as controversial by some clinicians, who point out that contracts are not used for other forms of care where patient compliance affects outcomes. An example of one such contract is show in Appendix 9.
One problem would be defining success. Many, perhaps most, patients on CSII do not achieve targets for HbA1c level. However, they may get a considerable improvement, or have fewer hypoglycaemic episodes, or just a feeling of greater well-being. How much benefit should the NHS expect for the extra £1700 per annum?
Chapter 7 Discussion
Statement of principal findings
The main conclusions from this review are:
-
CSII, used properly, is a safe and effective form of insulin administration.
-
In trials against traditional NPH-based MDI, CSII provides a modest but clinically useful reduction in HbA1c but a considerable reduction in hypoglycaemic episodes.
-
There are few trials against analogue MDI, which should now be the comparator in T1DM, and some are very small. Only two are published in full. One, in children and adolescents, shows a good drop in HbA1c; the other, in adults, is a pilot, which shows no advantage over MDI.
-
In T2DM, the evidence so far shows no benefit of CSII over analogue-based MDI in terms of glycaemic control, with only one trial showing no difference in HbA1c. There is now some evidence, published since the last review, that CSII may be better than NPH-based MDI in T2DM. Two trials showed clinically significant reductions in HbA1c, of 0.5% and 0.9%, but the third showed no difference (Table 5).
-
The many observational studies tend to show greater benefits than in the trials, but are more susceptible to bias. Conversely, by recruiting patients who are having particular problems with, for example, hypoglycaemia, they may be a useful guide to results in routine care.
-
The benefits of CSII include easily measurable results, such as levels of HbA1c and frequency of hypoglycaemia, but also other benefits, such as greater flexibility of lifestyle, an easier way to move to intensified insulin treatment in schoolchildren, reduction in fear of hypoglycaemia, and reduction in variability of blood glucose levels. CSII makes it easier to cope with unpredicted changes in activity or food intake. People with diabetes often report feeling more in control. Some of these benefits cannot easily be fitted into a simple cost per QALY calculation. An approach focusing more on all of the outcomes that matter to patients might have produced more evidence of benefit.
-
CSII costs about £1700 more per annum than MDI. The increased cost of CSII is only modestly offset by reduced insulin dose. The main part of the annual cost is from consumables, such as tubing.
-
Cost-effectiveness estimates vary according to assumptions used, but CSII does not appear cost-effective if based only on modest gains in reduced levels of HbA1c and frequency of hypoglycaemia. It does appear cost-effective if larger gains in HbA1c, or the disutility of chronic fear of hypoglycaemia, are factored in.
-
There is general consensus about which patients are most suitable for CSII, both in terms of clinical need, and in personal commitment and ability to use CSII.
-
CSII is far from a complete solution in T1DM, and many users still fail to achieve the NICE target of HbA1c level of 7.5% or less, although most do get improvements in HbA1c levels, or severe hypoglycaemia, or both.
Strengths and weakness in evidence
We are confident that we identified all published studies, but it is possible that there are unpublished trials, and that these are more likely to be negative than positive.
The evidence base has increased considerably since the last assessment report. 50 We now have trials and other studies in children, and in people with T2DM. We have the meta-analysis by Pickup et al. (2008),107 which focuses on patients deemed by NICE to be most suitable for CSII.
There are some new reviews, but none sufficiently up-to-date to include all of the new trials, and most had different inclusion criteria from ours (for example, including NPH-based MDI in T1DM rather than focusing on analogue-based regimens).
We have the views of successful pump users from INPUT, which reminds us of the less quantifiable benefits of CSII, and from the studies by Barnard et al. on quality of life aspects. 108,190 We have also carried out a small survey of parents of very young children, which has provided information on the benefits and costs in that group, and, in passing, on differing attitudes to CSII amongst some paediatricians.
Weaknesses in evidence include:
-
The shortage of trials against optimal MDI. We have an abundance of observational studies that have their uses, such as results in routine care, adverse effects, discontinuation rates and long-term results. But they are prone to bias, and RCTs are the gold standard for efficacy research.
-
A lack of medium-term data on NHS costs. It is likely that pump users need more support when starting, but less later.
-
The problems of fitting some benefits, especially non-health-related ones, into cost per QALY estimates.
The inclusion of observational studies is not usual in TARs for NICE, and we would probably not have done so had there been a good number of high-quality RCTs. However, in the key patient group, those with T1DM, there were only four RCTs: two available only as abstracts, and one of the full publications was only a pilot. However, the main sources of possible bias in the included studies were probably the short duration (the longest being 24 weeks in T1DM) and the sparsity of details on whether both groups had the same amount on educational input.
We used MDI based on long- and SA analogues as the key comparator, influenced by the NICE guidance on long-acting analogues. 51 We did not have time to do a full systematic review to update the evidence base on long-acting insulins. However, in the course of the review and the peer review that followed, we noted that there are reservations about the advantages of long-acting analogues over older insulins such as NPH. The Canadian Agency for Drugs and Technologies in Health reviewed the case for glargine in both 2005 and 2006, and concluded [Canadian Expert Drug Advisory Committee (CEDAC) recommendation, October 2006]261 that there was ‘no convincing evidence that insulin glargine consistently led to a reduced HbA1c’. There was some evidence of reduced problems with hypoglycaemia, but the benefits did not justify the threefold difference in cost. Doubt has also been case on the value of SA analogues over SA soluble insulin in both CSI and MDI, by Siebenhofer et al. (2004),194 whose meta-analysis found only a small benefit in HbA1c level of 0.19% in use in CSII. This is similar to the 0.26% in the meta-analysis derived from the previous assessment report for NICE,11 the difference being due to slightly different inclusions and exclusions.
We included a small qualitative study that was limited in its scope, partly because of the time and resource constraints (it is not usual to collect new data for TARs), and partly because it included only experiences of family of very young pump users. However, that is the group with most expansion in CSII use, and we felt that it would help NICE to have some understanding of the particular problems in this age group.
Research needs
The first priority is to have larger RCTs of CSII versus analogue MDI, looking at glycaemic control (HbA1c level and variability), hypoglycaemia, quality of life and costs. The costs should include not only short-term costs of starting people on CSII, but also medium-term ones, over, for example, 5 years, looking at total use of NHS resources. When appropriate, quality of life should be estimated amongst families as well as patients, particularly in parents of young children. Separate trials will be needed for children and adults, and trials in children should include measures of quality of life as assessed by the children themselves.
There is a lack of data on how long it takes to get full value out of CSII. Patients tend to increase the number of basals as they gain experience. One implication is that short-term trials, of say 12–16 weeks on CSII, may not reveal the full benefits.
As reported in this review, CSII is far from the complete answer, and many patients still do not get good control. The reasons for that should be investigated in future trials.
Our second priority would be for an RCT of MDI + DAFNE versus CSII + DAFNE. We are struck by similarities in the comments of people who have been through the DAFNE course, to those on CSII. We had access to these comments from the book kept for people who have done the DAFNE course in Aberdeen, UK. Many comments from the DAFNE graduates are about feeling empowered, such as ‘I now control the disease rather than it controlling me’ or about much improved understanding of diabetes. This raises the question as to how much of the benefit reported by those on CSII is due to the amount of education involved. No trials have compared CSII to optimised MDI with comparable structured education. Such a trial should collect data not only on biochemical and physical outcomes, but also on quality of life, ideally capturing non-health-related aspects as well.
Third, selection of patients for CSII has been mentioned, and several centres reported that they had a work-up phase involving reinforced education and MDI, or structured education such as DAFNE. It would be interesting to record how many patients heading for CSII managed without it after such intensification. There may also be a case for such interventions to be offered to patients on CSII, to see how many could revert to MDI, although anecdotal evidence suggests that once established on a pump, very few people wish to stop CSII.
Fourth, CSII is commonly used in pregnancy but in the absence of evidence of benefit. A large RCT is needed, which should start at least 6 months before conception in order to allow women to become experienced in CSII use. A recent Cochrane review by Farrar et al. (2007)262 on CSII versus MDI commented that ‘there is a dearth of robust evidence to support the use of one form of insulin administration over another for pregnant women with diabetes’.
The target group for further research on CSII in pregnancy need not include all pregnant women, but only those who meet the NICE criteria of not getting good control without severe hypoglycaemia. No RCT has yet been carried out in this group and so the effectiveness and cost-effectiveness is not known. Trials in unselected groups of pregnant women might miss benefits in higher-risk subgroups.
Fifth, several parents both in this assessment and the previous one have commented on problems at school. There is a need or a survey of school problems and policies, and consideration of solutions. One implication is that because of problems with lunchtime injections at school, children might go to CSII without trying MDI first.
The sixth issue is about quality of life measurement. We noted in Chapter 3 (see Other benefits not included) that some measures do not capture all of the benefits of CSII. The problems of quality of life measures in diabetes were reviewed by Speight and Shaw,223 who noted that a range of measures were used in the quality of life studies in CSII [for review see Barnard et al. (2007)108], and commented that very few of the measures could accurately measure the effect of CSII on quality of life. They go on to say that the measures used include health status, patient satisfaction and emotional well-being, which affect quality of life but do not measure it.
One problem, already noted, is that studies may use measures that report only on health-related quality of life. Barnard et al. (2007)108 in their review give a definition of quality of life as: ‘individual’s appraisals of the degree to which their lives contain features that they find satisfying or meaningful in terms of fulfilment or purpose, personal control, interpersonal relationships, participation in pleasant activities, personal and intellectual growth and material possessions’.
Some of these aspects will not be captured by health-related quality of life measures, for example personal control, which is often reported by patients to be one of the benefits of CSII. Future studies should include qualitative research in both intervention and control arms.
Roche Diagnostics, one of the pump manufacturers, submitted a critique that expands on the published review, and which is available on the NICE website263 with the other documents from the appraisal. It makes some suggestions for further research, including:
-
More work on patient reported outcomes.
-
Research into the suitability of the instruments used to measure quality of life in trials of CSII.
-
The development of a tool that would help clinicians select patients who are most able to benefit from CSII, based on studies of who benefits most from CSII and why.
Our last research suggestion looks to the future. Linkage of CSII to continuous glucose monitoring systems could provide automated feedback and adjustment of infusion rate. Some pumps provide continuous glucose monitoring systems. 264,265 Continuous glucose monitoring systems are moving into clinical practice, and can be used in combination with insulin pumps. However, most current use seems to be intermittent, with patients using continuous glucose monitoring systems for a few days to assess glycaemic control, rather than every day. A review by NHS Quality Improvement Scotland in 2005266 noted a paucity of evidence on the cost-effectiveness of continuous glucose monitoring systems and recommended further trials. The cost of a continuous glucose monitor to go with a pump is about £750. The problems and potential of closed-loop systems, sometimes referred to as ‘the artificial pancreas’, are reviewed by Hovorka. 267
Other types of CSII
Intraperitoneal infusion would be more physiological than subcutaneous, as insulin normally goes into the liver via the portal vein. This could be done in two ways:
-
From an external pump, as used for CSII but with the catheter into the peritoneal cavity. This would create a risk of infection, at entry site, along the tunnel through the subcutaneous tissues, and peritonitis.
-
From an implanted pump. Such pumps have been in use for over 20 years in countries such as France, the USA and the Netherlands. 268,269 The EVADIAC (Evaluation dans le Diabéte du Traitement par Implants Artifs) group, Toulouse, France, which maintains a register of patients with implanted pumps, suggests three main indications:269
-
– poor glycaemic control despite intensive CSII with good patient education and close follow-up
-
– good control achieved but with unacceptable hypoglycaemia
-
– improved quality of life.
-
Other forms of childhood diabetes
We are aware that CSII is used in children with other much less common forms of diabetes, such as cystic fibrosis-related diabetes (not common in children with cystic fibrosis, but very common in the over-15s) and associated with treatment for acute leukaemia. No research has been published for such groups. Numbers may be too small for research to be worthwhile.
Current research
The National Research Register (June 2007) shows a number of studies as being currently under way on CSII in the UK:
-
Two studies of the psychosocial impact of CSII, but with no control subjects.
-
A quality of life study in adolescents, which starts with the hypothesis that quality of life will be better on CSII. The patients are not randomly assigned to CSII, MDI or twice-daily insulins so there will be confounding variables that may make interpretation difficult.
-
A multicentre trial in East Anglia, UK of CSII versus conventional bolus insulin treatment (it is not clear if this is MDI or twice-daily mixtures) in preschool, newly diagnosed children (ISRCTN77773974).
-
Two registered studies following up patients on pumps: one of children under 16 years in Yorkshire and the other being type 1 patients over 12 years in England (presumably part of the Insulin Pump Database – see Chapter 1, Questions for this review).
The Current Controlled Trials website (accessed 29 June 2007) shows additional trials:
-
CSII versus analogue MDI in newly diagnosed adolescents, in Florida, USA (NCT00357890).
-
CSII plus continuous glucose monitoring (CGM) versus MDI (without CGM) in patients naive to pump therapy in the USA (NCT00417989).
-
CCSII versus continuous intraperitoneal insulin infusion, in patients in whom CSII was unsuccessful (defined as frequent hypoglycaemia and/or HbA1c levels above 7%) (ISRCTN68954085).
We have also heard (from one of the HTA programme referees) of a trial in children in Montreal and Quebec, Canada, due to end in 2009.
Work is emerging on the use of home blood ketone-monitoring in a large American study. 270 Only an abstract is available at present but it reports an observational study, which also notes that only 36% of pump users met HbA1c targets. Blood ketone-monitoring was used by 24% of all patients (age range 0–22 years), of whom 63% were on CSII. However, allocation to both CSII and ketone monitoring was not random, so we do not know if these patients would have done better anyway.
Conclusion
Continuous subcutaneous insulin infusion is an effective way of administering insulin, but it is little used in the UK. More people with T1DM could benefit from it than those who currently do but with present knowledge it is difficult to be precise about how many. CSII does not overcome all of the problems of exogenous insulin and is far from a complete answer. It is also more expensive than MDI, at a time when the needs for diabetic care are increasing but funds are tight. If use is expanded there will be considerable educational needs for both patients and health-care professionals. The education for patients should include structured education, such as DAFNE.
The current evidence base has various deficiencies and larger and longer trials are required.
Based on the totality of evidence, using observational studies rather than just the limited data from randomised trials against best MDI, CSII provides some advantages over MDI in T1DM. For both children and adults, these are:
-
better control of glucose levels as reflected in levels of HbA1c, with the size of improvement depending on the level before starting CSII
-
fewer problems with hypoglycaemia
-
quality of life gains, such as greater flexibility of lifestyle.
The amount of weight that we placed on the non-randomised evidence in drawing the above conclusions was questioned in the peer-review process.
Acknowledgments
We thank:
-
the Royal College of Nursing and the Royal College of Physicians of London for nominating peer reviewers
-
Billy Valentine et al. for assistance with running the CORE model
-
Katharine Barnard, John Pickup and Matt Sutton, and Richard Feltbower et al. at the Insulin Pump Clinical Database, for access to data unpublished at the time of the original review
-
John Davis and members of INPUT for answering various enquiries, and the parents of the young pump users for providing their experiences
-
our peer-review panel for useful comments on a near final draft
-
Stephanie Amiel, nominated by the Royal College of Physicians, London
-
Katharine Barnard, University of Southampton
-
Fiona Campbell, Consultant Paediatrician, Leeds
-
John Davis, on behalf of INPUT
-
Diabetes UK
-
Stephen Greene, Ninewells Hospital, Dundee
-
Jenny Hirst, on behalf of the Insulin Dependent Diabetes Trust
-
Jo Dalton and Jane Houghton, on behalf of the Royal College of Nursing
-
John Pickup, Guy’s Hospital, London
-
Elangovan Gajraj and Elisabeth George at NICE for comments on the near-final draft and for facilitating contact with the CORE team.
However, we absolve them from any faults in the report, responsibility for which rests with the Aberdeen Health Technology Assessment Group.
The Aberdeen Health Technology Assessment (HTA) Group is part of the Institute of Applied Health Sciences (IAHS), which is part of the College of Medicine and Life Sciences of the University of Aberdeen. The IAHS is made up of discrete but methodologically related research groups. The HTA Group is drawn mainly from the Health Services Research Unit, Public Health, and the Health Economics Research Unit.
The HTA Group carries out independent health technology assessments [technology appraisal reports (TARs)] for the UK HTA programme, which commissions TARs for NICE and other bodies, such as the National Screening Committee. In addition, a joint venture between the Health Services Research Unit at Aberdeen and the Medical Care Research Unit at Sheffield University informs the Review Body for Interventional Procedures Programme within NICE (ReBIP).
Contribution of authors
The review of clinical effectiveness was carried out by P Royle, A Snaith, L Robertson and L McIntyre. Literature and other searches were carried out by P Royle. The review of the industry submission, and of the cost-effectiveness literature was led by E Cummins, who carried out the economic modelling. The introduction, implementation and discussion chapters, and most of the patient perspectives one, were written by N Waugh. A Greene carried out the interviews with parents and wrote that section. Final editing was carried out by N Waugh and P Royle.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Scottish Study Group for the Care of Diabetes in the Young . The Incidence of Childhood Diabetes in Scotland 2006.
- Wilson, IV, Gale, EAM, Bingley PJ. The rising incidence of childhood Type 1 diabetes in the Oxford region 1985–2004. Diabet Med 2007;24:1-29.
- Yorkshire & Humber Public Health Observatory . PBS Diabetes Population Prevalence Model. Yorkshire &Amp; Humber Public Health Observatory 2005. www.yhpho.org.uk/viewResource.aspx?id=7 (accessed 26 December 2006).
- Waugh N, Scotland G, McNamee P, Gillet M, Brennan A, Goyder E, et al. Screening for type 2 diabetes: literature review and economic modelling. Health Technol Assess 2007;11.
- Yorkshire & Humber Public Health Observatory 2006;URL. www.yhpho.org.uk/diabetes_keyfacts.aspx (accessed 26 December 2006).
- Turner R, Cull C, Holman R. United Kingdom prospective diabetes study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control over complications in non-insulin-dependent diabetes mellitus. Ann Intern Med 1996;124:136-45.
- UK Prospective Diabetes Study . U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group 1995;44:1249-58.
- Wright A, Burden ACF, Paisey RB, Cull CA, Holman RR. Sulfonylurea inadequacy: Efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care 2002;25:330-6.
- Donnan PT, Steinke DT, Newton RW, Morris AD, Collaboration MEMO. Changes in treatment after the start of oral hypoglycaemic therapy in type 2 diabetes: a population-based study. Diabet Med 2002;19:606-10.
- Siebenhofer A, Plank J, Berghold A, Jeitler K, Horvath K, Narath M, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev 2006.
- Colquitt J, Royle P, Waugh N. Are analogue insulins better than soluble in continuous subcutaneous insulin infusion? Results of a meta-analysis. Diabet Med 2003;20:863-6.
- National Institute for Health and Clinical Excellence . Inhaled Insulin for the Treatment of Type 1 and Type 2 Diabetes: Technology Appraisal TA113. 25 January 2008 n.d. www.nice.org.uk/Guidance/TA113 (accessed 26 February 2009).
- Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86.
- Pickup JC, Keen H, Parsons JA, Alberti KG. Continuous subcutaneous insulin infusion: an approach to achieving normoglycaemia. Br Med J 1978;1:204-7.
- Tamborlane WV, Sherwin RS, Genel M, Felig P. Reduction to normal of plasma glucose in juvenile diabetes by subcutaneous administration of insulin with a portable infusion pump. N Engl J Med 1979;300:573-8.
- Lenhard MJ, Reeves GD. Continuous subcutaneous insulin infusion: a comprehensive review of insulin pump therapy. Arch Int Med 2001;161:2293-300.
- Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002;25:593-8.
- Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials. BMJ 2002;324.
- Côté B, St-Hilaire C. Comparison of the insulin pump and multiple daily insulin injections in intensive therapy for type 1 diabetes. Montreal, PQ, Canada: Agence d’évaluation des technologies et des modes d’intervention en santé (AETMIS); 2005.
- Graff MR, Rubin RR, Walker EA. How diabetes specialists treat their own diabetes: findings from a study of the AADE and ADA membership. Diabetes Educ 2000;26:460-7.
- Pickup J, Keen H. Continuous subcutaneous insulin infusion in type 1 diabetes: is beneficial in selected patients and should be more widely available. BMJ 2001;322:1262-3.
- Dunger DB, Sperling MA, Acerini CL, Bohn DJ, Daneman D, Danne TPA, et al. ESPE/LWPES consensus statement on diabetic ketoacidosis in children and adolescents. Arch Dis Child 2004;89:188-94.
- Ganesh R, Suresh N, Ramesh J. Diabetic ketoacidosis in children. Nat Med J India 2006;19:155-8.
- Henriksen OM, Roder ME, Prahl JB, Svendsen OL. Diabetic ketoacidosis in Denmark Incidence and mortality estimated from public health registries. Diabetes Res Clin Pract 2007;76:51-6.
- Cryer PE. Hypoglycemia in diabetes: pathophysiological mechanisms and diurnal variation. Prog Brain Res 2006;153:361-5.
- Ghafour IM, Allan D, Foulds WS. Common causes of blindness and visual handicap in the west of Scotland. Br J Ophthalmol 1983;67:209-13.
- NHS Scotland . The Scottish Renal Registry Report 2002–2004 2007.
- Laing SP, Swerdlow AJ, Slater SD, Botha JL, Burden AC, Waugh NR, et al. The British Diabetic Association Cohort Study, II: Cause-specific mortality in patients with insulin-treated diabetes mellitus. Diabet Med 1999;16:466-71.
- Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003;46:760-5.
- Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141.
- Stettler C, Allemann S, Juni P, Cull CA, Holman RR, Egger M, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: Meta-analysis of randomized trials. Am Heart J 2006;152:27-38.
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719-24.
- Currie CJ, Morgan CL, Peters JR. The epidemiology and cost of inpatient care for peripheral vascular disease, infection, neuropathy, and ulceration in diabetes. Diabetes Care 1998;21:42-8.
- Wang PH, Lau J, Chalmers TC. Meta-analysis of the effects of intensive glycemic control on late complications of type I diabetes mellitus. Online J Curr Clin Trials 1993;60.
- Shamoon H, Duffy H, Fleischer N, Engel S, Saenger P, Strelyzn M, et al. The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus: the diabetes control and complications trial. Arch Ophthalmol 1995;113:36-51.
- Diabetes Control and Complications Trial Research Group . The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes 1996;45:1289-98.
- Genuth S, Lipps J, Lorenzi G, Nathan DM, Davis MD, Lachin JM, et al. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287:2563-9.
- Writing Team for the Diabetes Control and Complications Trial . Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159-67.
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643-53.
- Ihnat MA, Thorpe JE, Ceriello A. Hypothesis: The ‘metabolic memory’, the new challenge of diabetes. Diabet Med 2007;24:582-6.
- Svoren BM, Volkening LK, Butler DA, Moreland EC, Anderson BJ, Laffel LM. Temporal trends in the treatment of pediatric type 1 diabetes and impact on acute outcomes. J Pediatr 2007;150:279-85.
- Scottish Study Group for the Care of the Young Diabetic . Factors influencing glycemic control in young people with type 1 diabetes in Scotland: a population-based study (DIABAUD2). Diabetes Care 2001;24:239-44.
- Scottish Study Group for the Care of the Young with Diabetes . A longitudinal observational study of insulin therapy and glycaemic control in Scottish children with type 1 diabetes: DIABAUD 3. Diabet Med 2006;23:1216-21.
- Diabetes UK. Diabetes: State of the Nations 2006. www.diabetes.org.uk/Professionals/Information_resources/Reports/Diabetes_State_of_the_Nations_2006/.
- Hayward RA, Manning WG, Kaplan SH, Wagner EH, Greenfield S. Starting insulin therapy in patients with type 2 diabetes: effectiveness, complications, and resource utilization. JAMA 1997;278:1663-9.
- Yki-Jarvinen H, Ryysy L, Kauppila M, Kujansuu E, Lahti J, Marjanen T, et al. Effect of obesity on the response to insulin therapy in non insulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1997;82:4037-43.
- Calvert MJ, McManus RJ, Freemantle N. Management of type 2 diabetes with multiple oral hypoglycaemic agents or insulin in primary care: retrospective cohort study. Br J Gen Pract 2007;57:455-60.
- Gulliford MC, Ashworth M, Robotham D, Mohiddin A. Achievement of metabolic targets for diabetes by English primary care practices under a new system of incentives. Diabet Med 2007;24:505-11.
- Diabetes Control and Complications Trial Research Group . Hypoglycemia in the diabetes control and complications trial. Diabetes 1997;46:271-86.
- Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N. Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes. Health Technol Assess 2004;8.
- National Institute for Health and Clinical Excellence . Guidance on the Use of Long-Acting Insulin Analogues for the Treatment of Diabetes: Insulin Glargine 2002. http://guidance.nice.org.uk/TA53/guidance/pdf/English (accessed 26 February 2009).
- Hepburn DA, Frier BM, Lawson DH, Toft AD. Current medicine. Edinburgh: Churchill Livingstone; 1994.
- Nordfeldt S, Ludvigsson J. Fear and other disturbances of severe hypoglycaemia in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2005;18:83-91.
- Clarke WL, Gonder-Frederick A, Snyder AL, Cox DJ. Maternal fear of hypoglycemia in their children with insulin dependent diabetes mellitus. J Pediatr Endocrinol 1998;11:189-94.
- Streisand R, Swift E, Wickmark T, Chen R, Holmes CS. Pediatric parenting stress among parents of children with type 1 diabetes: the role of self-efficacy, responsibility, and fear. J Pediatr Psychol 2005;30:513-21.
- Heller S, Gill G, Pickup J, Williams G. Difficult diabetes. Oxford: Blackwell Science; 2001.
- Warren RE, Frier BM. Hypoglycaemia and cognitive function. Diabetes Obes Metab 2005;7:493-50.
- Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, et al. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007;356:1842-52.
- Gold AE, Frier BM, Kelnar CJH. Childhood and adolescent diabetes. London: Chapman & Hall; 1995.
- McCarthy AM, Lindgren S, Mengeling MA, Tsalikian E, Engvall J. Factors associated with academic achievement in children with type 1 diabetes. Diabetes Care 2003;26:112-17.
- Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care 2001;24:1541-6.
- Dahlquist G, Kallen B. School performance in children with type 1 diabetes: a population-based register study. Diabetologia 2007;50:957-64.
- Desrocher M, Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychol 2004;10:36-52.
- Ferguson SC, Blane A, Wardlaw J, Frier BM, Perros P, McCrimmon RJ, et al. Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care 2005;28:1431-7.
- Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes. Diabetes Care 2005;28:2372-7.
- Wysocki T, Harris MA, Mauras N, Fox L, Taylor A, Jackson SC, et al. Absence of adverse effects of severe hypoglycemia on cognitive function in school-aged children with diabetes over 18 months. Diabetes Care 2003;26:1100-5.
- Leese GP, Wang J, Broomhall J, Kelly P, Marsden A, Morrison W, et al. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care 2003;26:1176-80.
- Donnelly LA, Morris AD, Frier BM, Ellis JD, Donnan PT, Durrant R, et al. Frequency and predictors of hypoglycaemia in type 1 and insulin-treated type 2 diabetes: a population-based study. Diabet Med 2005;22:749-55.
- Heller SR, Choudhary P, Davies C, Emery C, Campbell MJ, Freeman J, et al. Risk of hypoglycaemia in types 1 and 2 diabetes: Effects of treatment modalities and their duration. Diabetologia 2007;50:1140-7.
- Bolli GB, Gerich JE. The ‘dawn phenomenon’: a common occurrence in both non-insulin-dependent and insulin-dependent diabetes mellitus. N Engl J Med 1984;310:746-50.
- Pickup JC, Gill G, Pickup JC, Williams G. Difficult diabetes. Oxford: Blackwell Science; 2001.
- Kanc K, Janssen MM, Keulen ET, Jacobs MA, Popp-Snijders C, Snoek FJ, et al. Substitution of night-time continuous subcutaneous insulin infusion therapy for bedtime NPH insulin in a multiple injection regimen improves counterregulatory hormonal responses and warning symptoms of hypoglycaemia in IDDM. Diabetologia 1998;41:322-9.
- DAFNE . Dose Adjustment For Normal Eating (DAFNE) Website, 2009 n.d. www.dafne.uk.com/ (accessed 26 February 2009).
- National Institute for Clinical Excellence . Guidance on the Use of Continuous Subcutaneous Insulin Infusion: Technology Appraisal No. 57, 2003 n.d. www.nice.org.uk/guidance/TA57/ (accessed 26 February 2009).
- Warren E, Weatherley-Jones E, Chilcott J, Beverley C. Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine. Health Technol Assess 2004;8.
- Ashwell SG, Amiel SA, Bilous RW, Dashora U, Heller SR, Hepburn DA, et al. Improved glycaemic control with insulin glargine plus insulin lispro: a multicentre, randomized, cross-over trial in people with Type 1 diabetes. Diabet Med 2006;23:285-92.
- Chatterjee S, Jarvis-Kay J, Rengarajan T, Lawrence IG, McNally PG, Davies MJ. Glargine versus NPH insulin: efficacy in comparison with insulin aspart in a basal bolus regimen in type 1 diabetes: the glargine and aspart study (GLASS) a randomised cross-over study. Diabetes Res Clin Pract 2007;77:215-22.
- De L, I, Vague P, Selam JL, Skeie S, Lang H, Draeger E, et al. Insulin detemir used in basal-bolus therapy in people with type 1 diabetes is associated with a lower risk of nocturnal hypoglycaemia and less weight gain over 12 months in comparison to NPH insulin. Diabetes Obes Metab 2005;7:73-82.
- Dixon B, Peter CH, Burdick J, Fiallo-Scharer R, Walravens P, Klingensmith G, et al. Use of insulin glargine in children under age 6 with type 1 diabetes. Pediatric Diabetes 2005;6:150-4.
- Fulcher GR, Gilbert RE, Yue DK. Glargine is superior to neutral protamine Hagedorn for improving glycated haemoglobin and fasting blood glucose levels during intensive insulin therapy. Intern Med J 2005;35:536-42.
- Hermansen K, Fontaine P, Kukolja KK, Peterkova V, Leth G, Gall MA. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal-bolus therapy for patients with type 1 diabetes. Diabetologia 2004;47:622-9.
- Hershon KS, Blevins TC, Blevins TC, Blevins TC. Once-daily insulin glargine compared with twice-daily NPH insulin in patients with type 1 diabetes. Endocr Pract 2004;10:10-7.
- Home P, Bartley P, Russell-Jones D, Hanaire-Broutin H, Heeg JE, Abrams P, et al. Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes: a randomized clinical trial. Diabetes Care 2004;27:1081-7.
- Home PD, Rosskamp R, Forjanic-Klapproth J, Dressler A. European Insulin Glargine Study Group . A randomized multicentre trial of insulin glargine compared with NPH insulin in people with type 1 diabetes. Diabetes Metab Res Rev 2005;21:545-53.
- Kudva YC, Basu A, Jenkins GD, Pons GM, Quandt LL, Gebel JA, et al. Randomized controlled clinical trial of glargine versus ultralente insulin in the treatment of type 1 diabetes. Diabetes Care 2005;28:10-4.
- Murphy NP, Keane SM, Ong KK, Ford-Adams M, Edge JA, Acerini CL, et al. Randomized cross-over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with type 1 diabetes on intensive insulin regimens. Diabetes Care 2003;26:799-804.
- Pieber TR, Draeger E, Kristensen A, Grill V. Comparison of three multiple injection regimens for type 1 diabetes: morning plus dinner or bedtime administration of insulin detemir vs. morning plus bedtime NPH insulin. Diabet Med 2005;22:850-7.
- Porcellati F, Rossetti P, Pampanelli S, Fanelli CG, Torlone E, Scionti L, et al. Better long-term glycaemic control with the basal insulin glargine as compared with NPH in patients with type 1 diabetes mellitus given meal-time lispro insulin. Diabet Med 2004;21:1213-20.
- Russell-Jones D, Simpson R, Hylleberg B, Draeger E, Bolinder J. Effects of QD insulin detemir or neutral protamine Hagedorn on blood glucose control in patients with type I diabetes mellitus using a basal-bolus regimen. Clin Ther 2004;26:724-36.
- Schober E, Schoenle E, Van Dyk J, Wernicke-Panten K. Pediatric Study Group of Insulin Glargine . Comparative trial between insulin glargine and NPH insulin in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol 2002;15:369-76.
- Standl E, Lang H, Roberts A. The 12-month efficacy and safety of insulin detemir and NPH insulin in basal-bolus therapy for the treatment of type 1 diabetes. Diabetes Technol Ther 2004;6:579-88.
- Vague P, Selam JL, Skeie S, De L, I, Elte JW, Haahr H, et al. Insulin detemir is associated with more predictable glycemic control and reduced risk of hypoglycemia than NPH insulin in patients with type 1 diabetes on a basal-bolus regimen with premeal insulin aspart. Diabetes Care 2003;26:590-6.
- Mathieu C. Can we reduce hypoglycaemia with insulin detemir?. International Journal of Obesity &Amp; Related Metabolic Disorders 2004;28:S35-40.
- Peterson GE. Intermediate and long-acting insulins: a review of NPH insulin, insulin glargine and insulin detemir. Curr Med Res Opin 2006;22:2613-19.
- Bangstad HJ, Danne T, Deeb LC, Jarosz-Chobot P, Urakami T, Hanas R. Insulin treatment. Pediatr Diabetes 2007;8:88-102.
- Palmer AJ, Roze S, Valentine WJ, Smith I, Wittrup-Jensen KU. Cost-effectiveness of detemir-based basal/bolus therapy versus NPH-based basal/bolus therapy for type 1 diabetes in a UK setting: an economic analysis based on meta-analysis results of four clinical trials. Curr Med Res Opin 2004;20:1729-46.
- Valentine WJ, Palmer AJ, Erny-Albrecht KM, Ray JA, Cobden D, Foos V, et al. Cost-effectiveness of basal insulin from a US health system perspective: comparative analyses of detemir, glargine, and NPH. Adv Ther 2006;23:191-207.
- Horvath K, Jeitler K, Berghold A, Ebrahim SH, Gratzer TW, Plank J, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007.
- Boland EA, Grey M, Oesterle A, Fredrickson L, Tamborlane WV. Continuous subcutaneous insulin infusion. A new way to lower risk of severe hypoglycemia, improve metabolic control, and enhance coping in adolescents with type 1 diabetes. Diabetes Care 1999;22:1779-84.
- Mack-Fogg J, Bates S, Faro B, Sciera M, Ippolito K, Orlowski CC, et al. Safe and effective use of continuous subcutaneous insulin infusion in young children with type 1 diabetes mellitus. Pediatr Res 2002;51.
- Rudolph JW, Hirsch IB. Assessment of therapy with continuous subcutaneous insulin infusion in an academic diabetes clinic. Endocr Pract 2002;8:401-5.
- Haardt MJ, Berne C, Dorange C, Slama G, Selam J-L. Efficacy and indications of CSII revisited: The Hotel-Dieu cohort. Diabet Med 1997;14:407-8.
- Bending JJ, Pickup JC, Keen H. Frequency of diabetic ketoacidosis and hypoglycemic coma during treatment with continuous subcutaneous insulin infusion. Audit of medical care. Am J Med 1985;79:685-91.
- Chantelau E, Spraul M, Muhlhauser I, Gause R, Berger M. Long-term safety, efficacy and side-effects of continuous subcutaneous insulin infusion treatment for type 1 (insulin-dependent) diabetes mellitus: a one centre experience. Diabetologia 1989;32:421-6.
- Raskin P. Treatment of type-I diabetes with portable insulin infusion devices. Diabetes Care 1982;5:48-52.
- Department of Health . Insulin Pump Services: Report of the Insulin Pumps Working Group, 2007 n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_072777 (accessed 26 February 2009).
- Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med 2008;25:765-74.
- Barnard KD, Lloyd CE, Skinner TC. Systematic literature review: quality of life associated with insulin pump use in type 1 diabetes. Diabet Med 2007;24:607-17.
- Khan KS, ter Riet G, Glanville J, Sowden A, Kleijnen J. Undertaking Systematic Reviews of Research on Effectiveness: CRD’s Guidance for Those Carrying Out and Commissioning Reviews 2001.
- Doyle EA, Weinzimer SA, Steffen AT, Ahern JA, Vincent M, Tamborlane WV. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care 2004;27:1554-8.
- Thomas RM, Aldibbiat A, Griffin W, Cox MA, Leech NJ, Shaw JA. A randomized pilot study in type 1 diabetes complicated by severe hypoglycaemia, comparing rigorous hypoglycaemia avoidance with insulin analogue therapy, CSII or education alone. Diabet Med 2007;24:778-83.
- Maran A, Crazzolara D, Nicoletti M, Costa S, dal Pos M, Tiengo A, et al. A randomized crossover study to compare continuous subcutaneous insulin infusion (CSII) with multiple daily injection (MDI) in type 1 diabetic patients previously treated with CSII. Diabetologia 2005;48.
- Bolli GB, Capani F, Home PD, Kerr D, Thomas R, Torlone E, et al. Comparison of a multiple daily injection regimen with once-daily insulin glargine basal insulin and mealtime lispro, to continuous subcutaneous insulin infusion: a randomised, open, parallel study. Diabetes 2004;53:A107-8.
- Berthe E, Lireux B, Coffin C, Goulet-Salmon B, Houlbert D, Boutreux S, et al. Effectiveness of intensive insulin therapy by multiple daily injections and continuous subcutaneous infusion: a comparison study in type 2 diabetes with conventional insulin regimen failure. Horm Metab Res 2007;39:224-9.
- Herman WH, Ilag LL, Johnson SL, Martin CL, Sinding J, Al Harthi A, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005;28:1568-73.
- Raskin P, Bode BW, Marks JB, Hirsch IB, Weinstein RL, McGill JB, et al. Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes: a randomized, parallel-group, 24-week study. Diabetes Care 2003;26:2598-603.
- Wainstein J, Metzger M, Boaz M, Minuchin O, Cohen Y, Yaffe A, et al. Insulin pump therapy vs. multiple daily injections in obese type 2 diabetic patients. Diabet Med 2005;22:1037-46.
- Cohen D, Weintrob N, Benzaquen H, Galatzer A, Fayman G, Phillip M. Continuous subcutaneous insulin infusion versus multiple daily injections in adolescents with type I diabetes mellitus: a randomized open crossover trial. J Pediatr Endocrinol 2003;16:1047-50.
- DeVries JH, Snoek FJ, Kostense PJ, Masurel N, Heine RJ. Dutch Insulin Pump Study Group . A randomized trial of continuous subcutaneous insulin infusion and intensive injection therapy in type 1 diabetes for patients with long-standing poor glycemic control. Diabetes Care 2002;25:2074-80.
- Dimeglio LA, Pottorff TM, Boyd SR, France L, Fineberg N, Eugster EA. A randomized, controlled study of insulin pump therapy in diabetic preschoolers. J Pediatr 2004;145:380-4.
- Fox LA, Buckloh LM, Smith SD, Wysocki T, Mauras N. A randomized controlled trial of insulin pump therapy in young children with type 1 diabetes. Diabetes Care 2005;28:1277-81.
- Hoogma RP, Hoekstra JB, Michels BP, Levi M. Comparison between multiple daily insulin injection therapy (MDI) and continuous subcutaneous insulin infusion therapy (CSII), results of the five nations study. Diabetes Res Clin Pract 2006;74:S144-7.
- Pozzilli P, Crino A, Schiaffini R, Manfrini S, Fioriti E, Coppolino G, et al. A 2-year pilot trial of continuous subcutaneous insulin infusion versus intensive insulin therapy in patients with newly diagnosed type 1 diabetes (IMDIAB 8). Diabetes Technol Ther 2003;5:965-74.
- Weintrob N, Schechter A, Benzaquen H, Shalitin S, Lilos P, Galatzer A, et al. Glycemic patterns detected by continuous subcutaneous glucose sensing in children and adolescents with type 1 diabetes mellitus treated by multiple daily injections vs continuous subcutaneous insulin infusion. Arch Pediatr Adolesc Med 2004;158:677-84.
- Weintrob N, Schechter A, Bezaquen H, Shalitin S, Lilos P, Galatzer A, et al. Glycemic patterns detected by continuous subcutaneous glucose sensing in children with type 1 diabetes treated by MDI or CSII. Diabetes 2003;52.
- Wilson DM, Buckingham BA, Kunselman EL, Sullivan MM, Paguntalan HU, Gitelman SE. A two-center randomized controlled feasibility trial of insulin pump therapy in young children with diabetes. Diabetes Care 2005;28:15-9.
- Department of Health . National Service Framework for Diabetes: Standards 2001.
- Scottish Intercollegiate Guidelines Network . Management of Diabetes 2001.
- Casson IF, Clarke CA, Howard CV, McKendrick O, Pennycook S, Pharoah PO, et al. Outcomes of pregnancy in insulin dependent diabetic women: results of a five year population cohort study. BMJ 1997;315:275-8.
- Hawthorne G, Robson S, Ryall EA, Sen D, Roberts SH, Ward Platt MP. Prospective population based survey of outcome of pregnancy in diabetic women: results of the Northern Diabetic Pregnancy Audit, 1994. BMJ 1997;315:279-81.
- Confidential Enquiry into Maternal and Child Health (CEMACH) . Diabetes in Pregnancy: Are We Providing the Best Care? Findings of a National Enquiry, February 2007 n.d. www.cemach.org.uk/Final%20CEMACH%20report%20070207.pdf.
- Lapolla A, Dalfra MG, Masin M, Bruttomesso D, Piva I, Crepaldi C, et al. Analysis of outcome of pregnancy in type 1 diabetics treated with insulin pump or conventional insulin therapy. Acta Diabetologica 2003;40:143-9.
- Gimenez M, Conget I, Nicolau J, Pericot A, Levy I. Outcome of pregnancy in women with type 1 diabetes intensively treated with continuous subcutaneous insulin infusion or conventional therapy. A case-control study. Acta Diabetologica 2007;44:34-7.
- Chen R, Yogev Y, Weissman-Brenner A, Haroush AB, Hod M. Level of glycemic control and pregnancy outcome in type-1 diabetes: A comparison between multiple daily injections (MDI) and continuous subcutaneous insulin infusions (CSII). Am J Obstet Gynecol 2006;195.
- Cheng Y, Block-Kurbisch I, Inturrisi M, Ustinov A, Caughey A. Insulin pump compared to injected insulin in pregnant women with type 1 diabetes. Am J Obstet Gynecol 2006;195.
- Kinsley BT, Al-Agha R, Murray S, Firth R. Continuous subcutaneous insulin infusion (CSII) versus multiple daily injection regimes using soluble human insulin or rapid acting insulin analoque in T1DM in pregnancy (T1DMP). Diabetologia 2005;48:A314-15.
- Jimenez M, Hernaez R, Conget I, Alonso A, Yago G, Pericot A, et al. Metabolic control, maternal and perinatal outcomes in type 1 diabetic pregnancies intensively treated with conventional insulin therapy vs. continuous subcutaneous insulin infusion. Diabetologia 2005;48.
- Pickup JC, Kidd J, Burmiston S, Yemane N. Effectiveness of continuous subcutaneous insulin infusion in hypoglycaemia-prone type 1 diabetes. Pract Diabetes Int 2005;22:10-4.
- Pickup JC, Kidd J, Burmiston S, Yemane N. Determinants of glycaemic control in type 1 diabetes during intensified therapy with multiple daily insulin injections or continuous subcutaneous insulin infusion: importance of blood glucose variability. Diabetes/Metab/Res/Rev 2006;22:232-7.
- Rodrigues IA, Reid HA, Ismail K, Amiel SA. Indications and efficacy of continuous subcutaneous insulin infusion (CSII) therapy in Type 1 diabetes mellitus: a clinical audit in a specialist service. Diabet Med 2005;22:842-9.
- Bruttomesso D, Costa S, Crazzolara D, Di Bartolo P, Girelli A, Tiengo A, et al. Continuous subcutaneous insulin infusion (CSII) in Italy. Diabetes Res Clin Pract 2006;74:S130-4.
- Norgaard K. A nationwide study of continuous subcutaneous insulin infusion (CSII) in Denmark. Diabet Med 2003;20:307-11.
- Radermecker RP, Legrand DA, Scheen AJ. Continuous subcutaneous insulin therapy in type 1 diabetic patients: retrospective study of about 500 patient-years. Diabetologia 2005;48.
- D’Annunzio G, Minuto N, Serfaino M, Minicucci L, Pistorio A, Lorini R. Continuous subcutaneous insulin infusion (CSII) in young patients with type 1 diabetes mellitus (T1DM): A follow-up study. Diabetes 2005;54.
- Linkeschova R, Raoul M, Bott U, Berger M, Spraul M. Less severe hypoglycaemia, better metabolic control, and improved quality of life in Type 1 diabetes mellitus with continuous subcutaneous insulin infusion (CSII) therapy; an observational study of 100 consecutive patients followed for a mean of 2 years. Diabet Med 2002;19:746-51.
- Reda E, Von Reitzenstein A, Dunn P. Metabolic control with insulin pump therapy: the Waikato experience. New Zealand Med J 2007;120.
- Nimri R, Weintrob N, Benzaquen H, Ofan R, Fayman G, Phillip M. Insulin pump therapy in youth with type 1 diabetes: a retrospective paired study. Pediatrics 2006;117:2126-31.
- Jankovec Z, Lacigova S, Zourek M, Krcma M, Rusavy Z. Long-term results of continuous subcutaneous insulin infusion (CSII) treatment in the Czech Republic. Diabetes 2005;54.
- Alemzadeh R, Ellis JN, Holzum MK, Parton EA, Wyatt DT. Beneficial effects of continuous subcutaneous insulin infusion and flexible multiple daily insulin regimen using insulin glargine in type 1 diabetes. Pediatrics 2004;114:e91-5.
- Hanas R, Adolfsson P. Insulin pumps in pediatric routine care improve long-term metabolic control without increasing the risk of hypoglycemia. Pediatr Diabetes 2006;7:25-31.
- Kordonouri O, Hartmann R, Lauterborn R, Barnekow C, Hoeffe J, Deiss D. Age-specific advantages of continuous subcutaneous insulin infusion as compared with multiple daily injections in pediatric patients: one-year follow-up comparison by matched-pair analysis. Diabetes Care 2006;29:133-4.
- McMahon SK, Airey FL, Marangou DA, McElwee KJ, Carne CL, Clarey AJ, et al. Insulin pump therapy in children and adolescents: improvements in key parameters of diabetes management including quality of life. Diabet Med 2005;22:92-6.
- Saha M-T, Huupponen T, Knip M, Juuti M, Komulainen J. Continuous subcutaneous insulin infusion in the treatment of children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2002;15:1005-10.
- Willi SM, Planton J, Egede L, Schwarz S. Benefits of continuous subcutaneous insulin infusion in children with type 1 diabetes. J Pediatr 2003;143:796-801.
- Wood JR, Moreland EC, Volkening LK, Svoren BM, Butler DA, Laffel LM. Durability of insulin pump use in pediatric patients with type 1 diabetes. Diabetes Care 2006;29:2355-60.
- Weinzimer SA, Ahern JH, Doyle EA, Vincent MR, Dziura J, Steffen AT, et al. Persistence of benefits of continuous subcutaneous insulin infusion in very young children with type 1 diabetes: a follow-up report. Pediatrics 2004;114:1601-5.
- Conrad SC, McGrath MT, Gitelman SE. Transition from multiple daily injections to continuous subcutaneous insulin infusion in type 1 diabetes mellitus. J Pediatr 2002;140:235-40.
- Plotnick LP, Clark LM, Brancati FL, Erlinger T. Safety and effectiveness of insulin pump therapy in children and adolescents with type 1 diabetes. Diabetes Care 2003;26:1142-6.
- Raile K, Noelle V, Landgraf R, Schwarz HP. Weight in adolescents with type 1 diabetes mellitus during continuous subcutaneous insulin infusion (CSII) therapy. J Pediatr Endocrinol 2002;15:607-12.
- Sulli N, Shashaj B. Continuous subcutaneous insulin infusion in children and adolescents with diabetes mellitus: decreased HbA1c with low risk of hypoglycemia. J Pediatr Endocrinol 2003;16:393-9.
- Fahlen M, Eliasson B, Oden A. Optimization of basal insulin delivery in type 1 diabetes: a retrospective study on the use of continuous subcutaneous insulin infusion and insulin glargine. Diabet Med 2005;22:382-6.
- Lepore G, Dodesini AR, Nosari I, Trevisan R. Effect of continuous subcutaneous insulin infusion vs multiple daily insulin injection with glargine as basal insulin: an open parallel long-term study. Diabetes Nutr Metab Clin Exp 2004;17:84-9.
- Siegel-Czarkowski L, Herold KC, Goland RS. Continuous subcutaneous insulin infusion in older patients with type 1 diabetes. Diabetes Care 2004;27:3022-3.
- Litton J, Rice A, Friedman N, Oden J, Lee MM, Freemark M. Insulin pump therapy in toddlers and preschool children with type 1 diabetes mellitus. J Pediatr 2002;141:490-5.
- Berhe T, Postellon D, Wilson B, Stone R. Feasibility and safety of insulin pump therapy in children aged 2 to 7 years with type 1 diabetes: a retrospective study. Pediatrics 2006;117:2132-7.
- Ahern JA, Boland EA, Doane R, Ahern JJ, Rose P, Vincent M, et al. Insulin pump therapy in pediatrics: a therapeutic alternative to safely lower HbA1c levels across all age groups. Pediatric Diabetes 2002;3:10-5.
- Garcia-Garcia E, Galera R, Aguilera P, Cara G, Bonillo A. Long-term use of continuous subcutaneous insulin infusion compared with multiple daily injections of glargine in pediatric patients. J Pediatr Endocrinol 2007;20:37-40.
- Schiaffini R, Ciampalini P, Spera S, Cappa M, Crino A. An observational study comparing continuous subcutaneous insulin infusion (CSII) and insulin glargine in children with type 1 diabetes. Diabetes Metab Res Rev 2005;21:347-52.
- Cersosimo E, Jornsay D, Arce C, Rieff M, Feldman E, Defronzo R. Improved clinical outcomes with intensive insulin pump therapy in type 1 diabetes. Diabetes 2002;51.
- Hunger-Dathe W, Braun A, Muller UA, Schiel R, Femerling M, Risse A. Insulin pump therapy in patients with type 1 diabetes mellitus: results of the Nationwide Quality Circle in Germany (ASD) 1999–2000. Exp Clin Endocrinol Diabetes 2003;111:428-34.
- Juliusson PB, Graue M, Wentzel-Larsen T, Sovik O. The impact of continuous subcutaneous insulin infusion on health-related quality of life in children and adolescents with type 1 diabetes. Acta Paediatrica 2006;95:1481-7.
- Liberatore JR, Perlman K, Buccino J, Artiles-Sisk A, Daneman D. Continuous subcutaneous insulin infusion pump treatment in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2004;17:223-6.
- Mack-Fogg JE, Orlowski CC, Jospe N. Continuous subcutaneous insulin infusion in toddlers and children with type 1 diabetes mellitus is safe and effective. Pediatr Diabetes 2005;6:17-21.
- Shehadeh N, Battelino T, Galatzer A, Naveh T, Hadash A, de Vries L, et al. Insulin pump therapy for 1–6 year old children with type 1 diabetes. Isr Med Assoc J (IMAJ) 2004;6:284-6.
- Garg SK, Walker AJ, Hoff HK, D’Souza AO, Gottlieb PA, Chase HP. Glycemic parameters with multiple daily injections using insulin glargine versus insulin pump. Diabetes Technol Ther 2004;6:9-15.
- Ronsin O, Jannot-Lamotte MF, Vague P, Lassman-Vague V. Factors related to CSII compliance. Diabetes Metab 2005;31:90-5.
- Sucunza A, Ubeda J, Martinez C, Martinez MJ, Abraldes N, Corcoy R, et al. Unsuccessful outcome of insulin pump treatment is not predictable by clinical characteristics. Diabetes 2005;54.
- Mednick L, Cogen FR, Streisand R. Satisfaction and quality of life in children with type 1 diabetes and their parents following transition to insulin pump therapy. Child Health Care 2004;33:169-83.
- Simmons JH, McFann KK, Brown AC, Rewers A, Cruz E, Klingensmith GJ. Achieving pediatric ADA HbA1c goals: insulin pump therapy vs injection therapy. Diabetes 2006;55.
- Sullivan-Bolyai S, Knafl K, Tamborlane W, Grey M. Parents’ reflections on managing their children’s diabetes with insulin pumps. J Nurs Scholarsh 2004;36:316-23.
- Toni S, Reali MF, Fasulo A, Festini P, Medici A, Martinucci ME. The use of insulin pumps improves the metabolic control in children and adolescents with type 1 diabetes. Erratum Appears in Arch Dis Child 2004; 89: 985 – Note: ‘Festini, F’ Corrected to ‘Festini, P’ 2004;89:796-7.
- Ugrasbul F, Popovic J. BMI changes in pediatric patients with type diabetes mellitus (T1DM) treated with continuous subcutaneous insulin infusion (CSII) therapy. Diabetes 2006;55.
- Wallach EJ, Iazzetti L, Bowlby DA, Greig F, Patel A, Hyman S, et al. Sustained improvement in hemoglobin A1C during long-term treatment with continuous subcutaneous insulin infusion (CSII) in pediatric patients. Diabetes 2005;54.
- Alemzadeh R, Palma-Sisto P, Parton E, Holzum M, Kichler J. Insulin pump therapy attenuated glycemic instability without improving glycemic control in a one-year study of preschool children with type 1 diabetes. Diabetes 2006;55.
- Hanas R, Lindblad B, Lindgren F. Diabetes 2005;54.
- Conwell LS, Pope E, Artiles AM, Mohanta A, Daneman A, Daneman D. Dermatological Complications of Continuous Subcutaneous Insulin Infusion in Children and Adolescents. J Pediatr 2008;152:622-8.
- Kaufman FR, Halvorson M, Kim C, Pitukcheewanont P. Use of insulin pump therapy at nighttime only for children 7–10 years of age with type 1 diabetes. Diabetes Care 2000;23:579-82.
- Retnakaran R, Hochman J, DeVries JH, Hanaire-Broutin H, Heine RJ, Melki V, et al. Baseline A1c determines efficacy of insulin pump therapy: A pooled analysis of studies of continuous subcutaneous insulin infusion vs multiple daily injection regimens using rapid-acting insulin analogues. Diabetes 2004;53.
- Hirsch IB, Bode BW, Garg S, Lane WS, Sussman A, Hu P, et al. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005;28:533-8.
- Barnard KD, Skinner TC. Qualitative study into quality of life issues surrounding insulin pump use in type 1 diabetes. Pract Diabetes Int 2007;24:143-8.
- Barnard KD, Skinner TC. Cross-sectional study into quality of life issues surrounding insulin pump use in type 1 diabetes. Pract Diabetes Int 2008;25:194-200.
- World Health Organization . WHO Quality of Life-BREF (WHOQOL-BREF), 2004 n.d. www.who.int/substance_abuse/research_tools/whoqolbref/en/ (accessed 26 February 2009).
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003;26:1079-87.
- Siebenhofer A, Plank J, Berghold A, Horvath K, Sawicki PT, Beck P, et al. Meta-analysis of short-acting insulin analogues in adult patients with type 1 diabetes: continuous subcutaneous insulin infusion versus injection therapy. Diabetologia 2004;47.
- Kapellen TM, Heidtmann B, Bachmann J, Ziegler R, Grabert M, Holl RW. Indications for insulin pump therapy in different age groups-an analysis of 1567 children and adolescents. Diabet Med 2007;24:836-42.
- Laing SP, Swerdlow AJ, Slater SD, Botha JL, Burden AC, Waugh NR, et al. The British Diabetic Association Cohort Study, I: all-cause mortality in patients with insulin-treated diabetes mellitus. Diabet Med 1999;16:459-65.
- Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990–96. Arch Dis Child 1999;81:318-23.
- Thordarson H, Sovik O. Dead in bed syndrome in young diabetic patients in Norway. Diabet Med 1995;12:782-7.
- Tunbridge WM. Factors contributing to deaths of diabetics under fifty years of age. On behalf of the Medical Services Study Group and British Diabetic Association. Lancet 1981;2:569-72.
- Scuffham P, Carr L. The cost-effectiveness of continuous subcutaneous insulin infusion compared with multiple daily injections for the management of diabetes. Diabet Med 2003;20:586-93.
- Roze S, Valentine WJ, Zakrzewska KE, Palmer AJ. Health-economic comparison of continuous subcutaneous insulin infusion with multiple daily injection for the treatment of Type 1 diabetes in the UK. Diabet Med 2005;22:1239-45.
- Conget D, I, Serrano CD, Rodriguez Barrios JM, Levy M, I, Castell AC, Roze S. Cost-utility analysis of insulin pumps compared to multiple daily doses of insulin in patients with type 1 diabetes mellitus in Spain. Revista Espanola De Salud Publica 2006;80:679-95.
- Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. The CORE Diabetes Model: Projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20:S5-26.
- Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin 2004;20:S27-40.
- Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med 1985;78:785-94.
- National Diabetes Data Group of the National Institute of Diabetes and Digestive and Kidney Diseases . Diabetes in America 1995. http://diabetes.niddk.nih.gov/dm/pubs/america/ (accessed 26 February 2009).
- National Institute of Diabetes & Digestive & Kidney Diseases . Epidemiology of Diabetes Interventions and Complications Study (EDIC), 2008 n.d. www.niddk.nih.gov/patient/edic/edic-public.htm (accessed 26 February 2009).
- Mount Hood 4 Modeling Group . Computer modeling of diabetes and its complications: a report on the Fourth Mount Hood Challenge Meeting. Diabetes Care 2007;30:1638-46.
- Diabetes Control and Complications Trial Research Group . Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. J Pediatr 1994;125:177-88.
- Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003;26:1902-12.
- National Institute for Health and Clinical Excellence . Diabetes (type 1 and 2) – Inhaled Insulin: Technology Appraisal 113, 2006 n.d. http://guidance.nice.org.uk/TA113 (accessed 26 February 2009).
- Clarke P, Gray A, Legood R, Briggs A, Holman R. The impact of diabetes-related complications on healthcare costs: results from the United Kingdom Prospective Diabetes Study (UKPDS Study No. 65). Diabet Med 2003;20:442-50.
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340-9.
- Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, et al. Valuing health-related quality of life in diabetes. Diabetes Care 2002;25:2238-43.
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000;38:583-637.
- Australian Institute of Health and Welfare . The Burden of Disease and Injury in Australia 2003 n.d. www.aihw.gov.au/publications/hwe/bodaiia03/bodaiia03.pdf (accessed 6 August 2007).
- Brown MM, Brown GC, Sharma S. Value-based medicine and vitreoretinal diseases. Curr Opin Ophthalmol 2004;15:167-72.
- Carrington AL, Mawdsley SK, Morley M, Kincey J, Boulton AJ. Psychological status of diabetic people with or without lower limb disability. Diabetes Res Clin Pract 1996;32:19-25.
- Davis RE, Morrissey M, Peters JR, Wittrup-Jensen K, Kennedy-Martin T, Currie CJ. Impact of hypoglycaemia on quality of life and productivity in type 1 and type 2 diabetes. Curr Med Res Opin 2005;21:1477-83.
- Lundkvist J, Berne C, Bolinder B, Jonsson L. The economic and quality of life impact of hypoglycemia. Eur J Health Econ 2005;6:197-202.
- Tabaei BP, Shill-Novak J, Brandle M, Burke R, Kaplan RM, Herman WH. Glycemia and the quality of well-being in patients with diabetes. Qual Life Res 2004;13:1153-61.
- Wikblad K, Leksell J, Wibell L. Health-related quality of life in relation to metabolic control and late complications in patients with insulin dependent diabetes mellitus. Qual Life Res 1996;5:123-30.
- Speight J, Shaw JAM. Does one size really fit all? Only by considering individual preferences and priorities will the true impact of insulin pump therapy on quality of life be determined. Diabet Med 2007;24:693-5.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001;24:1722-7.
- Weintrob N, Benzaquen H, Galatzer A, Shalitin S, Lazar L, Fayman G, et al. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens in children with type 1 diabetes: a randomized open crossover trial. Pediatrics 2003;112:559-64.
- Hoogma RP, Hammond PJ, Gomis R, Kerr D, Bruttomesso D, Bouter KP, et al. Comparison of the effects of continuous subcutaneous insulin infusion (CSII) and NPH-based multiple daily insulin injections (MDI) on glycaemic control and quality of life: results of the 5-nations trial. Diabet Med 2006;23:141-7.
- Hoogma RP, Spijker AJ, Doorn-Scheele M, van Doorn TT, Michels RP, van Doorn RG, et al. Quality of life and metabolic control in patients with diabetes mellitus type 1 treated by continuous subcutaneous insulin infusion or multiple daily insulin injections. Neth J Med 2004;62:383-7.
- Garmo A, Pettersson-Frank B, Ehrenberg A. Treatment effects and satisfaction in diabetic patients changing from multiple daily insulin injections to CSII. Pract Diabetes Int 2004;21:7-12.
- Sanfield JA, Hegstad M, Hanna RS. Protocol for outpatient screening and initiation of continuous subcutaneous insulin infusion therapy: impact on cost and quality. Diabetes Educ 2002;28:599-607.
- Bruttomesso D, Pianta A, Crazzolara D, Scaldaferri E, Lora L, Guarneri G, et al. Continuous subcutaneous insulin infusion (CSII) in the Veneto region: efficacy, acceptability and quality of life. Diabet Med 2002;19:628-34.
- Barnard KD, Skinner C. Qualitative study into quality of life benefits associated with insulin pump use. Diabetes 2006;55.
- Reid SM, Lawson ML. Comparison of continuous subcutaneous insulin infusion versus conventional treatment of Type 1 diabetes with respect to metabolic control, quality of life and treatment satisfaction. Pediatric Res 2002;51:A122-3.
- Galatzer A, Weintrob N, Cohen D, Benzaquen H, Rimer A, Ofan R, et al. Treatment satisfaction of type 1 diabetic patients: continuous subcutaneous insulin infusion versus multiple daily injection. Diabetes 2002;51.
- Schweitzer MA, Pieber T, Heinemann L, Grunder S, Buhr A. Treatment satisfaction and metabolic control in type 2 patients using CSII: results from an international observational study. Diabetologia 2006;49.
- Castell C, Palmer AJ, Rodriguez JM, Roze S, Serrano-Contreras D, Zakrzewska K. Assessing the efficiency of using Continuous Subcutaneous Insulin-Infusion (CSII) versus multiple daily injections (MDI) in Spanish diabetes mellitus type-1 (DM1) patients. Cost-effectiveness analysis. Value Health 2005;8.
- Zakrzewska K, Roze S, Valentine WJ, Palmer AJ. Health economic comparison of continuous subcutaneous insulin infusion with multiple daily injection for the treatment of type 1 diabetes in the UK. Value Health 2004;7:649-50.
- Zakrzewska K, Valentine W, Roze S, Palmer A, Spinas G. Continuous subcutaneous insulin infusion reduces incidence of diabetes-related complications when compared with multiple daily injections for type 1 diabetes treatment: a health economic analysis in Switzerland. Diabetes 2005;54.
- Roze S, Palmer AJ, Foos V, Lurati FM. Cost-effectiveness of continuous subcutaneous insulin infusion versus multiple daily injections for intensive control of Type 1 diabetes: a French analysis. Diabetologia 2002;45.
- Goodall G, Nicklasson L, Zakrzewska K, Foos V, Valentine WJ, Roze S, et al. Continuous subcutaneous insulin infusion versus multiple daily injection of insulin in patients with type 1 diabetes: a long-term health economic analysis in the Norwegian and Swedish settings. Value Health 2006;9.
- Nicklasson L, Zakrzewska K, Roze S, Voss V, Attvall S, Palmer A, et al. Continuous subcutaneous insulin infusion versus multiple daily injections for type 1 diabetes: a cost-effectiveness analysis for Sweden. Diabetologia 2006;49.
- Roze S, Palmer AEJ. Long-term cost-effectiveness of the use of insulin pump compared to multiple daily injections in type 1 diabetic adolescents. Diabetes 2002;51.
- Feltbower RG, Campbell FM, Bodansky HJ, Stephenson CR, McKinney PA. Insulin pump therapy in childhood diabetes-cost implications for Primary Care Trusts. Diabet Med 2006;23:86-9.
- Nuboer R, Bruining GJ. Cost-effectiveness of continuous subcutaneous insulin infusion (CSII) in children: illusion or delusion?. Pediatr Diabetes 2006;7:39-44.
- Mbowe OT, Buysschaert M, Hermans MP. Institutional costs of continuous subcutaneous insulin infusion in type 1 diabetes: an assessment using Activity-Based Costing (ABC) method. Diabetes 2004;53.
- Ulahannan T, Myinit NN, Lonnen KF. Making the case for insulin pump therapy. Pract Diabetes Int 2007;24:252-6.
- Curtis L, Netten A. Personal Social Services Research Unit: Unit Costs of Health and Social Care 2006. www.pssru.ac.uk/pdf/uc/uc2006/uc2006.pdf.
- Ghatnekar O, Persson U, Willis M, Odegaard K. Cost effectiveness of Becaplermin in the treatment of diabetic foot ulcers in four European countries. Pharmacoeconomics 2001;19:767-78.
- UKPDS 40 . Cost effectiveness analysis of improved blood pressure control in hypertensive patients with type 2 diabetes: UKPDS 40. UK Prospective Diabetes Study Group. BMJ 1998;317:720-6.
- Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C. Clinical effectiveness and cost-utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation. Health Technol Assess 2003;7.
- INPUT . INsulin PUmp Therapy: Raising Awareness of Insulin Pump Therapy in the UK 2008. www.input.me.uk/ (accessed 26 February 2009).
- INPUT . Review of NICE Technology Appraisal No. 57 Insulin Pump Therapy Joint Submission from INPUT and Insulin Pumpers, 8 December 2007 n.d. www.nice.org.uk/guidance/index.jsp?action=download%26o=38246 (accessed 26 February 2009).
- Barnard KD, Speight J, Skinner TC. Quality of life and impact of continuous subcutaneous insulin infusion for children and their parents. Pract Diabetes Int 2008;25:278-84.
- Children with DIABETES . Children With DIABETES Online Community 2006. www.childrenwithdiabetes.com (accessed 26 February 2009).
- Department of Health . National Service Framework for Children, Young People and Maternity Services: Medicines for Children and Young People 2004.
- NHS Scotland . Scottish Diabetes Framework 2002.
- Khoo E, Miller D, Gazis A. Development and audit of a CSII service. Diabet Med 2007;24.
- Zisser H. The Impact of Insulin Pump Infusion Set Disconnects on Short-Term Glycemic Control n.d.
- Pickup J. Personal Statement on Continuous Subcutaneous Insulin Infusion 2008. www.nice.org.uk/nicemedia/pdf/JohnPickupStatement240907CF.pdf (accessed 25 February 2009).
- Diabetes UK. Postcode Lottery on Insulin Pumps 2007;URL. www.diabetes.org.uk/en/Get_involved/Campaigning/Ongoing-campaigns/Postcode-lottery-on-insulin-pumps/ (accessed 26 February 2009).
- Pickup JC. Are insulin pumps underutilized in type 1 diabetes? Yes. Diabetes Care 2006;29:1449-52.
- Canadian Agency for Drugs and Technologies in Health . CEDAC Final Recommendation and Reconsideration and Reasons for Recommendation: Insulin Glargine Resubmission, Sanofi-Aventis Canada 2006. www.cadth.ca/media/cdr/complete/cdr_complete_Lantus_Oct25–06.pdf (accessed 6 August 2007).
- Farrar D, Tuffnell D, West J. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev 2007.
- Roche Diagnostics Ltd . Additional Response: Clinical and Cost-Effectiveness of Continuous Subcutaneous Infusion for Diabetes: Updating Review, 19 November 2007 n.d. www.nice.org.uk/guidance/index.jsp?action=download%26o=38238 (accessed 26 February 2009).
- Peyrot M, Rubin RR. Patient-Reported Outcomes (PRO) for an Integrated Real-Time Continuous Glucose Monitoring Insulin Pump (RT-CGM CSII) System. Abstract No. 0461-P n.d.
- Hirsch IB, Bode BW, Abelseth J, Fischer JS, Kaufman FR, Mastrototaro J, et al. Patient-Reported Outcomes (PRO) for an Integrated Real-Time Continuous Glucose Monitoring Insulin Pump (RT-CGM CSII) System. Abstract No. 0090-OR n.d.
- NHS Quality Improvement Scotland . Continuous Glucose Monitors in Diabetes Mellitus – the Continuous Glucose Monitoring System (CGMS): Evidence Note 8, 2005 n.d. www.nhshealthquality.org/nhsqis/files/evidence_note8.pdf (accessed 6 August 2007).
- Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med 2006;23:1-12.
- Logtenberg SJJ, Van Ballegooie E, Israel-Bultman H, van Linde A, Bilo HJG. Glycaemic control, health status and treatment satisfaction in subjects with diabetes mellitus treated with continuous intraperitoneal insulin infusion (CIPII). A single centre experience. Diabetologia 2006;49:592-3.
- Renard E, Schaepelynck-Belicar P. Implantable insulin pumps. A position statement about their clinical use. Diabetes Metab 2007;33:158-66.
- Bismuth E, Svoren B, Volkening LK, Butler DA, Laffel LM. Impact of Insulin Pump Therapy and Blood [beta]-OHB Ketone Monitoring on Glycemic Outcomes in Youth with Type 1 Diabetes (T1D) n.d.
- Brinchmann-Hansen O, Dahl-Jorgensen K, Hanssen KF, Sandvik L. The response of diabetic retinopathy to 41 months of multiple insulin injections, insulin pumps, and conventional insulin therapy. Arch Ophthalmol 1988;106:1242-6.
- Chiasson JL, Ducros F, Poliquin HM, Lopez D, Lecavalier L, Hamet P. Continuous subcutaneous insulin infusion (Mill-Hill Infuser) versus multiple injections (Medi-Jector) in the treatment of insulin-dependent diabetes mellitus and the effect of metabolic control on microangiopathy. Diabetes Care 1984;7:331-7.
- Hanaire-Broutin H, Melki V, Bessieres-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. The Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000;23:1232-5.
- Home PD, Capaldo B, Burrin JM, Worth R, Alberti KG. A crossover comparison of continuous subcutaneous insulin infusion (CSII) against multiple insulin injections in insulin-dependent diabetic subjects: improved control with CSII. Diabetes Care 1982;5:466-71.
- Nathan DM, Lou PAJ. Intensive conventional and insulin pump therapy in adult type 1 diabetes. A crossover study. Ann Intern Med 1982;97:31-6.
- Saurbrey N, Arnold-Larsen S, Moller-Jensen B, Kuhl C. Comparison of continuous subcutaneous insulin infusion with multiple insulin injections using the NovoPen. Diabet Med 1988;5:150-3.
- Schiffrin A, Belmonte MM. Comparison between continuous subcutaneous insulin infusion and multiple injection of insulin. A one-year prospective study. Diabetes 1982;31:255-64.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001;24:1722-7.
- Haakens K, Hanssen KF, Dahl-Jorgensen K, Vaaler S, Aagenaes O, Mosand R. Continuous subcutaneous insulin infusion (CSII), multiple injections (MI) and conventional insulin therapy (CT) in self-selecting insulin-dependent diabetic patients. A comparison of metabolic control, acute complications and patient preferences. J Intern Med 1990;228:457-64.
- Schmitz A, Christiansen JS, Christensen CK, Hermansen K, Mogensen CE. Effect of pump versus pen treatment on glycaemic control and kidney function in long-term uncomplicated insulin-dependent diabetes mellitus (IDDM). Dan Med Bull 1989;36:176-8.
- Ziegler D, Dannehl K, Koschinsky T, Toeller M, Gries FA. Comparison of continuous subcutaneous insulin infusion and intensified conventional therapy in the treatment of type I diabetes: a two year randomized study. Diabetes Nutr Metab Clin Exp 1990;3:203-13.
- Bode BW, Steed RD, Davidson PC. Reduction in severe hypoglycemia with long-term continuous subcutaneous insulin infusion in type I diabetes. Diabetes Care 1996;19:324-7.
- Nosadini R, Velussi M, Fioretto P, Doria A, Avogaro A, Trevisan R, et al. Frequency of hypoglycaemic and hyperglycaemic–ketotic episodes during conventional and subcutaneous continuous insulin infusion therapy in IDDM. Diabetes Nutr Metab 1988;1:289-96.
- Melki V, Renard E, Lassmann-Vague V, Boivin S, Guerci B, Hanaire-Broutin H, et al. Improvement of HbA1c and blood glucose stability in IDDM patients treated with lispro insulin analog in external pumps. Diabetes Care 1998;21:977-82.
- Raskin P, Holcombe JH, Tamborlane WV, Malone JI, Strowig S, Ahern JA, et al. A comparison of insulin lispro and buffered regular human insulin administered via continuous subcutaneous insulin infusion pump. J Diabetes Complications 2001;15:295-300.
- Renner R, Pfutzner A, Trautmann M, Harzer O, Sauter K, Landgraf R. Use of insulin lispro in continuous subcutaneous insulin infusion treatment. Results of a multicenter trial. German Humalog-CSII Study Group. Diabetes Care 1999;22:784-8.
- Schmauss S, Konig A, Landgraf R. Human insulin analogue [LYS(B28), PRO(B29)]: the ideal pump insulin?. Diabet Med 1998;15:247-9.
- Zinman B, Tildesley H, Chiasson JL, Tsui E, Strack T. Insulin lispro in CSII: results of a doubleblind crossover study. Diabetes 1997;46:440-3.
- Bode B, Weinstein R, Bell D, McGill J, Nadeau D, Raskin P, et al. Comparison of insulin aspart with buffered regular insulin and insulin lispro in continuous subcutaneous insulin infusion: a randomized study in type 1 diabetes. Diabetes Care 2002;25:439-44.
- Britten N, Jones R, Murphy E, Stacy R. Qualitative research methods in general practice and primary care. Fam Pract 1995;12:104-14.
- Pope C, Mays N. Reaching the parts other methods cannot reach: an introduction to qualitative methods in health and health services research. BMJ 1995;311:42-5.
- Mason J. Qualitative researching. London: Thousand Oaks; 2002.
- Creswel JW. Qualitative inquiry and research design: choosing among five approaches. London: Sage Publications; 2003.
- van der Heijden K. Scenarios: the art of strategic conversation. Chichester: John Wiley; 2005.
- Glaser NS, Strauss A. Discovery of grounded theory. Chicago, IL: Aldine; 1967.
- Strauss A, Corbin J. Basics of qualitative research: grounded theory procedures and techniques. London: Sage; 1990.
- Mechanic D. Public trust and initiatives for new health care partnerships. Milbank Q 1998;76:281-302.
- Brownlie J, Greene A, Howson A. Researching trust and health. New York, NY: Routledge; 2008.
Appendix 1 Summary of HTA report
Reproduced with permission from Colquitt JL, Green S, Sidhu MK, Hartwell D, Waugh N. Clinical and cost-effectiveness of continuous insulin infusion for diabetes. Health Technol Assess 2004;8(43). 50
Description of the proposed service
This systematic review examines the clinical and cost-effectiveness of continuous subcutaneous insulin infusion (CSII) using insulin pumps compared with multiple daily injection (MDI) for diabetes.
Epidemiology and background
There are two main types of diabetes. Type 1 diabetes involves a process of destruction of the β-cells of the pancreas, leading to severe insulin deficiency, so that insulin treatment is required for survival. It represents about 10–15% of all diabetes in England and Wales. Type 2 diabetes is much more common, and is characterised by insulin resistance and relative insulin deficiency. Type 2 diabetes is linked to overweight and obesity, and to physical inactivity. The number of people with insulin-treated diabetes has increased due to the marked increase in incidence of type 1 diabetes and also due to a greater number of people with type 2 diabetes being treated with insulin to improve diabetic control. There has also been an increase in the prevalence of type 2 diabetes, particularly among the Asian community. Poor control of diabetes, reflected in high blood glucose levels, can, in the short term, result in diabetic ketoacidosis, a serious and potentially fatal condition, and, in the long term, increase the risk of complications, such as diabetic retinopathy and nephropathy. However, studies have shown that good diabetic control is associated with a reduced risk of these complications.
If insulin levels are too high and blood glucose falls, hypoglycaemic episodes occur. The effects of a hypoglycaemic episode depend on how low the blood glucose level falls, varying from mild and rapidly corrected by food or sugary drinks, to severe where help is required. Severe hypoglycaemia can lead to unconsciousness, convulsions or death.
There are several problems with current treatment. In the non-diabetic state, the body needs a little insulin all of the time (basal insulin), boosted by increased output after meals. This is difficult to achieve with conventional insulin injections, and, in particular, good control of blood glucose during the night is difficult. Intensive insulin regimens such as CSII aim to more closely resemble the output of a normal pancreas by providing basal insulin for fasting periods and additional short-acting supplements to cover meals.
Methods
A systematic review of the literature and an economic evaluation were undertaken.
Data sources
Electronic databases were searched, including the Cochrane Library, MEDLINE, EMBASE, PubMed, Science Citation Index, Web of Science Proceedings, DARE and HTA databases, PsycINFO, CINAHL, NHSEED, EconLit, and Health Management Information Consortium database. References of all retrieved articles were checked for relevant studies, and experts were contacted for advice and peer review, and to identify additional published and unpublished references. Manufacturers’ submissions to the National Institute of Clinical Excellence (NICE) were reviewed.
Study selection
Studies were included if they fulfilled the following criteria:
-
Interventions CSII using insulin pumps compared with optimised MDI (at least three injections per day). Analogue compared with soluble insulin in CSII.
-
Participants People with insulin-treated diabetes (type 1 or type 2). Newly diagnosed patients were excluded.
-
Outcomes Glycated haemoglobin (HbA1c), insulin dose, weight change, lipid levels, patient preference, quality of life, adverse effects.
-
Design Parallel randomised controlled trials (RCTs) and randomised and non-randomised crossover studies with a minimum duration of 10 weeks on each treatment.
Studies in non-English language or available only as abstracts were excluded from the main analysis.
For questions where no eligible studies were identified, information from selected observational studies was discussed (sections 3.2.5–3.2.7 and section 3.4).
Titles and summaries of studies being assessed for inclusion were checked by two reviewers. Full texts of selected studies were assessed for inclusion by one reviewer and checked by a second. Differences in opinion were resolved through discussion.
Data extraction and quality assessment
Data extraction and quality assessment were undertaken by one reviewer and checked by a second reviewer, with any disagreement resolved through discussion. The quality of included studies was assessed in accordance with CRD Report 4.
Data synthesis
Data on the clinical effectiveness of CSII for diabetes were synthesised through a narrative review with full tabulation of results of all eligible studies, with meta-analysis performed where appropriate. Cost-effectiveness analysis examined the marginal costs of CSII compared with MDI, and considered evidence on the marginal benefits, such as improved control, adverse events and quality of life.
Number and quality of studies
Searching identified 20 studies comparing CSII with MDI. These included eight parallel RCTs, nine randomised crossover studies and three non-random crossover studies. Fourteen studies included adults with type 1 diabetes, four studies included pregnant women, and two studies included adolescents. The quality of reporting and methodology of the studies, many of which dated from many years ago, was often poor by today’s standards, with just two studies having adequate randomisation and none reporting adequate allocation concealment.
No RCTs or crossover studies were identified in children, overnight use of CSII, in patients with poorly controlled type 2 diabetes or on discontinuation rates, therefore selected observational studies were discussed in these sections.
Six studies (one parallel RCT and five random crossover studies) were identified comparing analogue with soluble insulin in CSII. Randomisation and allocation concealment were adequate in the parallel RCT but not reported in the crossover studies.
No economic evaluations comparing CSII with optimised MDI were found.
Summary of benefits
Adults with type 1 diabetes
If all trials were included, a mean improvement in HbA1c of about 0.6% was found with CSII compared with MDI in both short-term (–0.64, 95% CI –1.28 to 0.01) and longer-term (–0.61, 95% CI –1.29 to 0.07) studies. This improvement was less if a study that used bovine ultralente in the control arm was excluded; the reduction is HbA1c is then only 0.5%. Short-term studies show a reduction in insulin dose of about 12 units (–11.90, 95% CI –18.16 to –5.63), with less difference in longer-term studies. Body weight was similar during treatment with CSII and MDI. The two studies that reported data on cholesterol levels found no significant difference between the treatments. There was no consistency between the studies in patients preferring CSII or MDI, although many of the older studies used older, bulkier and less reliable pumps, and progress has also been made with discreet ‘pen’ injectors in MDI, therefore these findings are probably not relevant to the present devices. Hypoglycaemic episodes did not differ significantly between CSII and MDI in most trials, but some found fewer episodes with CSII and one study found more hypoglycaemia and hypoglycaemic coma with CSII. In some observational studies, much greater reductions in the number of SH episodes were seen with CSII, which may be because these studies tend to select patients having particular problems.
Pregnancy
Three studies found no difference in HbA1c between CSII and MDI. Less insulin per kilogram was required by patients with CSII in one study, but two other studies found no significant difference. Patient preference and quality of life were not reported.
Adolescents
One study found no significant difference between CSII and MDI, while the second study found lower HbA1c and insulin dose with CSII. Over half of the patients chose to continue treatment with CSII in the former study.
Children
No randomised trials were identified. Case series suggest that CSII has a place in treatment of children with diabetes, but this needs to be confirmed in randomised studies.
Overnight only in children
The combination of overnight CSII and daytime MDI may help in children, by reducing nocturnal hypoglycaemic episodes and the dawn phenomenon, but no randomised trials were identified, and further research is necessary.
Short-term use in adults with poorly controlled type 2 diabetes
It has been suggested that short-term CSII may help in patients with type 2 diabetes on high doses of oral drugs and who are resistant to insulin. No good evidence was found.
Analogue versus soluble insulin
In CSII, analogue insulin was associated with lower levels of HbA1c than soluble insulin and was preferred by patients. No difference in insulin dose or weight change was observed. Some studies found fewer hypoglycaemic episodes with analogue insulin, although this varied according to the definitions used.
Costs
The extra cost of CSII compared with MDI varies according to the make of pump and the estimated life of the device, from £1075 per annum using the cheapest pump and assuming an 8-year life of the pump to £1423 per annum with the most expensive model and assuming a life of only 4 years. The largest component of cost is consumables, such as infusion sets (tubing, etc.), with the capital cost of the pump secondary. There is a need for considerable initial education.
Costs per life-year gained
There are definite benefits of CSII over MDI, including improved control of diabetes, not just as reflected in HbA1c and in a slightly reduced incidence of SH episodes, but also in flexibility of lifestyle and hence quality of life. However, evidence on quality of life is reported in only one trial, and comes mainly from testimonies of pump users.
One would expect the improvement in HbA1c to be reflected in reduced long-term complications, and for that to be accompanied by reduced costs to the NHS. However, we have not found a satisfactory method of converting the observed benefits into a cost per QALY.
The main problem with the current evidence is that it does not fully reflect the selection of patients for CSII. Most people on insulin therapy would not have much to gain from CSII, but those with particular problems, such as recurrent severe hypoglycaemia would. Their benefits would include not only fewer hypoglycaemic episodes, but also a reduction in fear of hypoglycaemic episodes. However, the utility effect of the reduction is fear of hypoglycaemic episodes has not been quantified. The cost-effectiveness of CSII is likely to be much better for certain subgroups.
Sensitivity analysis
The main costs are of consumables and pump. The price of pumps might come down with bulk purchase but this is speculative. This would not have much impact on the cost per annum.
Conclusions
Control of diabetes consists of more than just control of blood glucose as reflected in levels of HbA1c. Compared with optimised multiple injection insulin therapy, CSII results in a modest but worthwhile improvement in HbA1c, but its main value may be in reducing other problems, such as hypoglycaemia and the dawn phenomenon, and in improving quality of life by allowing greater flexibility of lifestyle. They appear to be a useful advance for patients having particular problems, rather than a dramatic breakthrough in therapy, and would probably be used by only a small percentage of patients.
Implications of approval of an increased use of CSII
Many health authorities are not funding insulin pumps, and some of those that are have restricted the number. Many patients are funding their own pumps. According to clinical consensus, it is unlikely that CSII would be used by more than a small proportion of people with type 1 diabetes, but the exact proportion is not known. We would not expect any use in true type 2 diabetes in the foreseeable future. The cost to the NHS per year would be around £3.5 million in England and Wales if 1% of people with T1DM used CSII, £10.5 million for 3%, and £17.5 million for 5%. The educational needs of patients starting CSII are significant, and it would usually be diabetes specialist nurses who would provide this. However, there are many other demands on their time.
Need for further research
The trials to date have focused on easily measurable outcomes, such as HbA1c. The main benefits may be in terms of flexibility of lifestyle and quality of life, and data on those would help with cost-effectiveness analysis. Some of the implications for patients such as the psychological impact of wearing a device for 24 hours every day have not been quantified.
Research is needed into the use of CSII in children of different ages.
Appendix 2 Selected meta-analyses from last assessment report
Reproduced with permission from Colquitt JL, Green S, Sidhu MK, Hartwell D, Waugh N. Clinical and cost-effectiveness of continuous insulin infusion for diabetes. Health Technol Assess 2004;8(43). 50
FIGURE 3.
Meta-analysis of the effect of continuous subcutaneous insulin infusion versus multiple daily injection on glycated haemoglobin in adults with type 1 diabetes. WMD, weighted mean difference. HbA1 is reported by Brinchmann-Hansen et al. (1988),271 Chiasson et al. (1984),272 Haakens et al. (1990),279 Home et al. (1982),274 Schiffrin and Belmonte (1982)277 and Ziegler et al. (1990)281 HbA1c is reported by Bode et al. (1996),282 Hanaire-Broutin et al. (2000),273 Nathan and Lou (1982),275 Nosadini et al. (1988),283 Saurbrey et al. (1998)276 Schmitz et al. (1989)280 and Tsui et al. (2001). 278 Reproduced from Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N. Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes. Health Technol Assess 2004;8(43).
FIGURE 4.
Meta-analysis of the effects of continuous subcutaneous insulin infusion versus multiple daily injection on insulin dose (units per day) in adults with type 1 diabetes. Reproduced from Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N. Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes. Health Technol Assess 2004;8(43).

FIGURE 5.
Meta-analysis of the effect of lispro versus soluble insulin on glycated haemoglobin in type 1 diabetes. Subgroup 1 ‘final values’ includes studies reporting mean glycated haemoglobin at crossover or end of study (3 months with treatment). Subgroup 2 ‘change from baseline’ includes one study reporting mean change in baseline glycated haemoglobin at end of study (4 months with treatment). Reproduced from Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N. Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes. Health Technol Assess 2004;8(43).
Appendix 3 Sources of information and search strategies used
MEDLINE and EMBASE, 2002–June 2007
1. ((insulin adj3 pump$) or csii or (continuous adj3 insulin adj3 infusion) or (subcutaneous adj3 insulin adj3 infusion) or continuous subcutaneous insulin infusion$).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
2. limit 1 to yr=‘2002 – 2007’
Cochrane Library 2007 Issue 1 – all sections
(CSII):ti,ab,kw or (continuous subcutaneous insulin infusion):ti,ab,kw or (insulin pump*):ti,ab,kw
Science Citation Index (for meeting abstracts only) 2002–June 2007
TS=(CSII or (continuous subcutaneous insulin infusion) or (insulin pump*)) AND PY=(2002–2007) DocType=Meeting Abstract; Language=All languages;
National Research Register, Current Controlled Trials and website of ADA 2007 meeting abstracts
(CSII or (continuous subcutaneous insulin infusion) or (insulin pump*))
FIGURE 6.
Flow chart of studies identified for clinical effectiveness.
Appendix 4 Characteristics of included trials of CSII versus best MDI
Type 1 diabetes
Doyle et al. (2004)110 – full publication
Description and quality of study
This RCT enrolled 32 adolescent participants with T1DM and compared CSII with MDI using parallel trial design. No power calculation was reported. Inclusion criteria were explicitly stated: T1DM, aged 8–21 years, otherwise healthy except for treated thyroid or coeliac disease, treated with insulin for at least 6 months, naive to CSII and glargine, willing to perform at least four blood glucose tests per day and screening HbA1c level between 6.5% and 11%. No specific exclusion criteria were reported. Randomisation methods were described in detail, with participants stratified according to sex and age. Treatment groups were similar at baseline and baseline analysis was reported. Analysis was ITT, using last observation-carried-forward method to account for missing values. Statistical analysis was comprehensively reported. Protocol violations were specified along with reasons for dropout; one participant in the MDI group was withdrawn after 8 weeks due to two episodes of dehydration and ketosis. The study was supported by a grant from Medtronic MiniMed.
-
Study quality = A.
Participants
Doyle et al. recruited 32 participants with T1DM. In both the CSII and MDI groups the mean age was between 12 and 13 years, 50–60% of participants were male, and the participants’ mean diabetes duration was of between 5 and 7 years. Total mean daily insulin dose before the study was between 1 and 1.5 units/kg.
Intervention
Participants were randomised either to CSII or MDI for 16 weeks, with the goal of achieving HbA1c < 7% and blood glucose levels of 70–120 mg/dl before meals and 90–150 mg/dl at bedtime. CSII intervention (Medtronic MiniMed 508 or Paradigm 511 pump with insulin aspart) consisted of an initial basal CSII dose that was 50% of the previous total daily insulin dose. MDI intervention (glargine insulin) consisted of initial dose 80% of total previous daily insulin dose, administered in the morning and at bedtime, together with aspart insulin at mealtimes (need to check whether this is correct). Both groups also participated in education sessions relevant to their treatment.
Results
Primary outcome
HbA1c
Doyle et al. assessed glycaemic control by measurement of HbA1c levels between baseline and 16 weeks. HbA1c (%) was significantly lower following CSII treatment at 16 weeks than with baseline and MDI treatment (baseline CSII 8.2% ± 1.1 versus MDI 8.1% ± 1.2; 16 weeks CSII 7.2% ± 1.0 versus MDI 8.1% ± 1.2; p < 0.05 between groups; p < 0.02 CSII versus baseline). Significantly more participants in the CSII treatment group met the HbA1c goal of ≤ 7% at 16 weeks than with the MDI group (CSII 8 versus MDI 2 participants; p < 0.05).
Secondary outcomes
Blood glucose levels
Blood glucose levels before breakfast were similar in the MDI and CSII groups (8.3 ± 5.3 versus 8.2 ± 5.2 mmol/l). However, all other mean blood glucose levels were lower in the CSII group than in the glargine group (p < 0.01).
Insulin dose requirement
The CSII group required significantly less insulin per day after 16 weeks than the MDI treatment group (CSII versus baseline, p < 0.01; CSII versus MDI at 16 weeks, p < 0.01; MDI versus baseline; p = NS).
Quality of life and treatment satisfaction
Health-related quality of life was assessed using the DQoL-Y scale, which is composed of three subscales: a Disease Impact Scale (23 items), a Disease-Related Worries Scale (11 items) and a Diabetes Life Satisfaction Scale (17 items). There was no significant difference between groups at baseline or 16 weeks. The authors noted that only half of each group successfully completed the DQoL-Y questionnaire, and that this precluded any conclusions being drawn from this study regarding impact on quality of life.
Adverse events
Participants received education on the management of hypoglycaemia and hyperglycaemia. There were no significant differences between CSII and MDI groups in the occurrence of severe hypoglycaemia. One patient on MDI was hospitalised for ketosis and dehydration and one patient on CSII had diabetic ketoacidosis.
In summary, Doyle et al. reported that ‘in contrast with those patients on MDI, CSII patients were able to significantly lower HbA1c levels and one half were able to lower HbA1c levels to ≤ 7%’. However, the authors conceded that the difference in metabolic control may be attributable to the number of dose changes and frequency of telephone contacts beyond the first 2 weeks, as these were not systematically collected. CSII patients also had longer initial education sessions.
Thomas et al. (2007)111 – full publication
Description and quality of study
This randomised open-parallel pilot trial recruited 21 participants with T1DM and compared three intervention groups over 24 weeks: CSII (lispro), MDI (lispro and glargine), and education and relaxation of glycaemic targets on existing therapy. Inclusion criteria were adults with T1DM, characterised by altered hypoglycaemia awareness and severe debilitating hypoglycaemia. Patients were naive to MDI analogue insulin therapy. As this was a pilot study, no power calculation was performed. The study was open label, so no blinding was possible. Details of statistical analysis, and withdrawals, were given, and baseline characteristics (no statistical analysis) were provided. No details of the randomisation process were provided.
-
Study quality = B.
Participants
The Thomas et al. trial recruited 21 participants with T1DM. The mean age of the participants was 43 years, mean weight was 75.6 kg, mean duration of diabetes was 25 years, and mean HbA1c level was between 8.5% and 8.6%.
Intervention
Participants were randomised into three treatment groups: CSII (lispro), MDI (lispro and glargine), and education and relaxation of glycaemic targets on existing therapy.
Results
Primary outcome
Glycaemic control – HbA1c
Thomas et al. assessed glycaemic control by measuring levels of HbA1c. Statistical analysis to assess differences within treatment groups at zero and 24 weeks was reported, but no statistical analysis on differences between groups was reported. However, levels of HbA1c declined significantly from baseline in the CSII and MDI treatment group, by 1.1% and 1.0%, respectively, but only the latter difference was reported as statistically significant (p < 0.05). There was no change in the education group.
Secondary outcomes
Mean daily blood glucose
There was no reported significant difference in mean daily blood glucose (mM) between treatment groups (CSII baseline 8.2 ± 2.5 mmol/l to 24 weeks 8.5 ± 1.5 mmol/l versus MDI baseline 9.7 ± 1.9 mmol/l to 24 weeks 9.5 ± 0.9 mmol/l). Glucose excursions below 4 mM were reduced by CSII.
Glycaemic excursions
There was no significant difference in glucose excursions between treatment groups.
Quality of life
Quality of life was assessed using DQoL and the Hypoglycaemia Fear Survey (which has a behaviour subscale of 15 items and a worry subscale of 18 items using a 0–4 Likert scale. (A high score indicates a greater degree of worry or a greater hypoglycaemia driven behavioural change.) There were no reported differences between groups.
In summary, the authors concluded that CSII reduced glucose excursions to below 4 mM and HbA1c levels declined by 1, but there was no difference from the MDI group.
Maran et al. (2005)112 – abstract
Description and quality of study
This randomised open crossover trial conducted in Italy recruited 10 participants with T1DM and compared CSII (lispro) with MDI (glargine) over 4 months. Inclusion criteria were C-peptide-negative T1DM, previously on CSII therapy for at least 6 months. No power calculation was reported, and there was no blinding, no details of randomisation, no statistical analysis, and no details of protocol violations or withdrawal. Baseline characteristics (no statistical analysis) were provided.
-
Study quality = C.
Participants
The Maran et al. trial recruited 10 participants with T1DM. Mean age was 41 ± 8 years, mean HbA1c level was 7.7 ± 0.7% and mean duration of T1DM was 19.5 ± 10 years.
Intervention
Following a 1-month run-in period, participants were randomised into two treatment groups: CSII with lispro and MDI using glargine with lispro.
Results
Primary outcome
Glycaemic control – HbA1c
Maran et al. assessed glycaemic control by measuring HbA1c. There was no significant difference between treatment groups from baseline to end point (CSII 7.2 ± 0.2 versus MDI 7.2 ± 0.2; p = NS).
Secondary outcome
Mean daily blood glucose
Mean daily blood glucose was assessed using 48 hours continuous glucose monitoring at the end of each study period. Compared with MDI, the CSII group had significantly lower mean glucose levels (CSII 8.2 ± 0.7 versus 10.5 ± 0.8 mmol/l; p < 0.03).
Adverse events
Hypoglycaemia reactions exposure (AUC < 65 mg/dl)
There was no significant difference between groups (CSII 1.88 ± 1.4 versus MDI 2.63 ± 1.88 mg/dl).
Time spent in night-time glucose range > 65 mg/dl and <180 mg/dl
The CSII participants spent significantly more time in the glucose range > 65 mg/dl and < 180mg/dl than the MDI participants (CSII 298 ± 63 versus MDI 194 ± 51 minutes; p < 0.02).
In summary, the authors of this abstract concluded that ‘CSII with insulin lispro provided lower nocturnal variability and better glycaemic control than MDI with lispro and basal glargine without increasing the risk of hypoglycaemic episodes’.
Bolli et al. (2004) – abstract113
Description and quality of study
This randomised open-parallel trial recruited 57 participants with T1DM and compared CSII (lispro) with MDI (glargine). Inclusion criteria were T1DM, HbA1c ≤ 9% and naive to SCII and glargine. Participants were randomised into two treatment groups: CSII with lispro and MDI using glargine once daily with mealtime lispro.
-
Study quality = C.
Participants
The Bolli et al. trial recruited 57 participants with T1DM.
Intervention
Participants were randomised into two treatment groups: CSII with lispro and MDI using glargine once daily with mealtime lispro.
Results
Primary outcome
Glycaemic control – HbA1c
Bolli et al. assessed glycaemic control by measuring HbA1c. There was no significant difference between treatment groups from baseline to end point (CSII 7.7 ± 0.7 to 7.0 ± 0.8 versus MDI 7.8 ± 0.6 to 7.2 ± 0.7). Baseline/centre adjusted difference –0.1 (95% CI –0.5 to 0.3; p = NS).
Secondary outcomes
Mean daily blood glucose
There was no significant difference in mean daily blood glucose (mg/dl) between treatment groups from baseline to end point (CSII baseline 9.1 ± 2.3 mmol/l end point 8.1 ± 1.8 mmol/l versus MDI baseline 8.9 ± 1.7 mmol/l end point 8 ± 1.1 mmol/l; difference 0.06 95% CI –0.77 to 0.83; p = NS).
Mean amplitude of glycaemic excursions
There was no significant difference in MAGE from baseline to end point between treatment groups (CSII baseline 144 ± 43 end point 115 ± 40 versus MDI baseline 137 ± 31 end point 115 ± 38; p = NS).
Coefficient of variation of 8-point blood glucose profiles
There was no significant difference in coefficient of variation of 8-point blood glucose profiles from baseline to end point between treatment groups (CSII baseline 53 ± 10 end point 46 ± 8 versus MDI baseline 52 ± 12 end point 47 ± 11; p = NS).
Adverse events
Confirmed hypoglycaemic events per patient
There was no significant difference in the incidence of blood glucose < 72 mg/dl between treatment groups [CSII 41 (SE ± 8) versus MDI 35 (SE ± 7); p = NS].
In summary, the authors of this abstract concluded that both CSII and once-daily glargine-based MDI regimen improved blood glucose to a similar extent, with no difference in HbA1c, mean blood glucose, blood glucose excursions and frequency of hypoglycaemia. A glargine-based MDI regimen is less expensive and therefore more cost-effective when used in an unselected population of people with T1DM.
Type 2 diabetes
Herman et al. 2005115 – full publication
Description and quality of study
This RCT enrolled 107 elderly participants with T2DM and compared CSII with MDI using parallel trial design at two centres. Power calculations estimated that 180 subjects would have the power to detect a difference in level of HbA1c of 0.5% between groups; however, recruitment was halted when an observed difference of 0.2% at interim analysis was deemed unlikely to become significant upon further recruitment. Inclusion criteria were explicitly stated: T2DM for ≥ 1 year, ≥ 60 years, taking at least one injection of insulin per day for the past month (with or without oral anti-diabetes medications), HbA1c ≥ 7%. Exclusion criteria were: BMI > 45 kg/m2; severe impairment of cardiac, hepatic or renal function; the presence of any physical, psychological or cognitive impairments that would interfere with adherence to an intensive insulin therapy programme; more than two episodes of severe hypoglycaemia in the past year or a history of hypoglycaemia unawareness. Block randomisation was used at each site. Treatment groups were similar at baseline although more men were randomised to CSII than MDI. The study was not blinded. Analysis was ITT. Statistical analysis was comprehensively reported. Protocol violations were only reported in terms of technical and mechanical problems relating to CSII and MDI delivery; however, follow-up and reasons for withdrawal were fully described. Overall, 98 (92%) participants completed the study; eight subjects withdrew (four from CSII and four from MDI), and one subject (CSII) died of cancer. The study was not supported by commercial sources.
-
Study quality = A.
Participants
Herman et al. recruited 107 participants with T2DM. In both the CSII and MDI groups the mean age was between 66 and 67 years. In the CSII group 72% of participants were male compared with 44% in the MDI group. Mean diabetes duration was between 15 and 17 years and mean HbA1c level was between 8% and 8.5%. Participants had been on insulin for a mean number of 8 to 8.3 years in both CSII and MDI groups. Authors noted that more men were randomised to CSII than MDI.
Intervention
Participants were randomised either to CSII or MDI for 12 months with the goal of achieving HbA1c < 5.6% and blood glucose levels of 80–120 mg/dl before meals and 100–150 mg/dl at bedtime without incurring unacceptable hypoglycaemia. CSII intervention (Medtronic MiniMed 508) consisted of an initial basal CSII dose 50% of previous total daily insulin dose. MDI intervention (preprandial lispro insulin and basal glargine insulin) consisted of initial basal dose of 50% of total previous daily insulin dose, and at bedtime, together with lispro insulin at mealtimes.
Results
Primary outcome
HbA1c
Herman et al. assessed glycaemic control by measurement of HbA1c between baseline and 12 months. There was no significant difference between CSII and MDI treatment groups at study end (CSII 6.6 ± 0.8% versus MDI 6.4 ± 0.8%; p = NS), although both groups had lower HbA1c than at baseline (change from baseline CSII –1.7 ± 1.0% versus MDI –1.6 ± 1.2%).
Secondary outcomes
Insulin dose requirement
There was no significant difference between CSII and MDI in mean total insulin dose requirement, mean basal insulin dose, and mean daily bolus insulin dose.
Weight
The weight of participants in both groups increased from baseline (change from baseline CSII +2.1 kg versus MDI +2.6 kg; p < 0.01 versus baseline); however, there was no significant difference between groups.
Quality of life
Health-related quality of life was assessed using the SF-36 and DQoLCTQ scale, a validated questionnaire that was used to measure treatment satisfaction, treatment flexibility, frequency and bother of symptoms, social stigma, diabetes satisfaction, diabetes impact, social worry and diabetes worry. Treatment satisfaction score, diabetes impact score and worry score all improved significantly (p < 0.05 for all three measures) from baseline in both groups; however, there was no significant difference between groups.
Adverse events
Hypoglycaemic episodes were defined as minor (≤ 65 mg/dl during week before scheduled visits every 2 months – able to treat themselves), severe (< 50 mg/dl associated with confusion, loss of consciousness or seizures that resolved with the administration of oral carbohydrates, glucagon or intravenous glucose by another person), or catastrophic (life-threatening injury to patient or another person, hospitalisation and/or death). There was no significant difference in the occurrence of episodes of minor, severe or catastrophic hypoglycaemia between groups; however, the authors noted that the rates of minor and severe hypoglycaemia were higher than those in a previous study in people with T2DM. They concluded that ‘this may be due to the older age of the study population or lower levels of HbA1c achieved in the study’.
In summary, Herman et al. reported no significant difference in reduction in mean HbA1c levels or occurrence of hypoglycaemic episodes between treatments in patients > 60 years with T2DM. The number of technical and mechanical difficulties associated with pump therapy was higher than that reported in previous studies; the authors suggests that this may have been because of ‘better ascertainment’ or (of relevance to whether pumps are suitable for certain populations) potentially because the older population in this study were ‘less technologically savvy’.
Wainstein et al. (2005)117 – full publication
Description and quality of study
This RCT enrolled 40 obese participants with T2DM and compared CSII with MDI using crossover trial design at seven centres in Israel. For this review only the first treatment period of 18 weeks was assessed. Power calculations estimated that 39 subjects would have the power to detect a HbA1c difference of 0.85% between groups. Inclusion criteria were explicitly stated: uncontrolled T2DM (HbA1c > 8.5%), obese (BMI 30–45 kg/m2), aged 30–70 years and treated for at least 3 months with diet, metformin (850 mg 2–3 times daily) and high doses of insulin (above 1 unit/kg per day), divided into two or three daily injections. Exclusion criteria were: those with new-onset diabetes (< 6 months); T1DM, or diabetes secondary to pancreatitis or other disease; history of active IHD or cerebrovascular accident (CVA) within the last 12 months; preproliferative or proliferative diabetic retinopathy; advanced nephropathy as evidenced by proteinuria or plasma creatinine > 1.5 mg/dl; liver enzymes twice above the upper limit of the normal range HbA1c > 15% at screening. No details of randomisation were reported. Baseline characteristics were reported. The study was not blinded. Analysis was ITT. Statistical analysis was comprehensively reported. Protocol violations mentioned but no details provided. Reasons for withdrawal were reported: five subjects randomised to MDI dropped out (two were non-compliant, two for protocol violations and one diagnosed with cancer) and three subjects randomised to CSII dropped out (one was unable to use pump, one had severe hypoglycaemia and one had hyperglycaemia). No competing interests were reported.
-
Study quality = B.
Participants
Baseline HbA1c levels were similar in CSII and MDI groups (CSII 10.2 ± 1.4 versus 10.3 ± 1.2). Similarly, there was no significant difference in insulin dose (CSII 99.3 ± 24.5 units per day versus MDI 113.4 ± 28.04 units per day) or weight (CSII 91.8 ± 17.4 kg versus MDI 94.01 ± 12.4 kg) between groups at baseline. It should be noted that only obese (BMI 30–45 kg/m2) participants were selected.
Intervention
Participants were randomised either to CSII (n = 20) or MDI (n = 20) for 18 weeks. Thereafter, participants crossed over to the alternative treatment (however, for this study only the first 18-week parallel period is reported). CSII intervention regimen consisted of CSII using insulin lispro. MDI regimen consisted of four injections daily using regular insulin or Humulin R and NPH or Humulin N. All participants continued with their prior treatment with diet and metformin and the goal for all treatments was to achieve HbA1c levels of < 7%.
Results
Primary outcome
HbA1c
Wainstein et al. assessed glycaemic control by measurement of HbA1c levels between baseline and 18 weeks. At the end of this period HbA1c levels had decreased significantly (CSII –2.2% versus MDI – 1.9%) in both groups (CSII from 10.2 ± 1.4 to 7.9 ± 1.0; p = 0.01 versus MDI from 10.3 ± 1.2 to 8.4 ± 1.3; p = 0.01). There was no significant difference between treatments.
Secondary outcomes
Insulin dose requirement
At the end of 18 weeks, insulin dose had decreased in the CSII group from baseline value of 99.3 ± 24.5 to 87.2 ± 25.4 units per day while dose had increased in the MDI group from 113.4 ± 28.04 to 118.7 ± 31.3 units per day. There was no significant difference between treatments.
Weight
The weight of participants in both groups remained stable throughout the study. No significant difference between treatments.
Adverse events
Hypoglycaemic episodes were defined as minor (< 3.3 mmol/l able to handle without assistance), major (< 2.8 mmol/l, symptoms remitted after intake of intravenous glucose, intramuscular glucagon or food intake and patient was unable to self treat).There was no significant difference in the occurrence of episodes of hypoglycaemia between groups.
In summary, Wainstein et al. showed that in obese insulin-treated patients with uncontrolled T2DM, CSII and MDI both significantly reduced HbA1c levels, but the decrease was not significantly different between treatments. Insulin dose, weight gain and adverse events were similar with both treatments.
Raskin et al. (2003)116 – full publication
Description and quality of study
This RCT enrolled 132 adult participants with T2DM and compared CSII with MDI using parallel trial design at 14 sites. Power calculations estimated that 102 subjects would have the power to detect a difference in HbA1c of 0.4% between groups. Inclusion criteria were explicitly stated: T2DM for ≥ 2 years, treatment for 6 months with at least one insulin dose per day (regular insulin, lispro insulin, NPH, premixed insulin, lente or ultralente, with or without an oral antidiabetic agent). Exclusion criteria were: subjects with impaired hepatic, renal or cardiac function or recurrent major hypoglycaemia; women of childbearing age if they were pregnant, breastfeeding or not practising contraception. Randomisation method was described but no stratification. Treatment groups were similar at baseline. The study was not blinded. Data were not ITT (based on the 127/132 who received treatment (five people withdrew during 2-week training period); last observation carried forward analysis). Statistical analysis was reported. Protocol violations were not reported: however, follow-up and reasons for withdrawal were fully described. Of those on CSII, six withdrew during treatment: one was non-compliant, five withdrew consent. Of those on MDI, two were non-compliant, one withdrew consent and two experienced adverse events (maculopapular rash, osteomyelitis and skin ulceration). The study was supported by Novo Nordisk Pharmaceutical Industries.
-
Study quality = B.
Participants
Raskin et al. recruited 132 participants with T2DM. In both the CSII and MDI groups the mean age was between 55 and 56 years, 36% in the CSII group were male and 43% in the MDI group, mean BMI was 32.2 kg/m2 in both groups, mean weight was between 96.4 and 96.9 kg. Mean HbA1c level at baseline was between 8.0% and 8.2%. Mean duration of diabetes was between 11.9 and 13.8 years.
Intervention
Participants were randomised either to CSII or MDI for 24 weeks with the goal of achieving fasting (prebreakfast) blood glucose levels of 4.4–6.7 mmol/l (80–120 mg/dl) without incurring unacceptable hypoglycaemia. CSII intervention (Medtronic MiniMed 507C) consisted of insulin aspart (100 units/ml) with CSII bolus doses administered just before meals. MDI intervention (preprandial insulin aspart and basal NPH). Instructions on the use of CSII and MDI were received on two separate visits, and doses of insulin were adjusted during initial 8 weeks after randomisation.
Results
Primary outcome
HbA1c
Raskin et al. assessed glycaemic control by measurement of HbA1c levels between baseline and 24 weeks. There was no significant difference between CSII and MDI treatment groups at study end (CSII 7.6 ± 1.22% versus MDI 7.5 ± 1.17%; p = NS), although both groups had lower HbA1c levels than with baseline (change from baseline CSII –0.62 ± 1.11% versus MDI –0.46 ± 0.89%; p < 0.05).
Secondary outcomes
Insulin dose requirement
There was no significant difference between CSII and MDI in mean total daily insulin dose requirement at 24 weeks (both treatment groups +0.1 units/kg; p = NS).
Weight
The weight of participants in both groups increased slightly from baseline (CSII baseline 96.4 ± 17.0 kg, 24 weeks 98.1 ± 18.1 kg; MDI baseline 96.9 ± 17.9 kg, 24 weeks 97.6 ± 19.2 kg); however, there was no significant difference between groups.
Quality of life and treatment satisfaction
Quality of life and treatment satisfaction was assessed using validated questionnaires that are components of the Phase V Technologies Outcomes Information System incorporating a diabetes treatment satisfaction components and quality of life scale. CSII had significantly greater improvement in overall treatment satisfaction than with MDI (CSII baseline 59.4 ± 2.1, 24 weeks 79.2 ± 1.8 versus MDI baseline 63.6 ± 1.9, 24 weeks 70.3 ± 2.3; p < 0.001 between groups). Of the 59/66 (89%) of CSII-treated subjects who responded to a questionnaire on CSII use, 93% preferred the pump to their previous injectable-insulin regimen.
Adverse events
Hypoglycaemia
Hypoglycaemic episodes were defined as minor (blood glucose < 2.8 mmol/l (50 mg/dl), symptoms of hypoglycaemia, i.e. palpitations, tiredness, sweating, strong hunger, dizziness, tremor, etc., and able to deal without assistance), major (blood glucose < 2.8 mmol/l (50 mg/dl) associated with severe central nervous system dysfunction that required the assistance of another person or required administration of pre-enteral glucose or glucagon). There was no significant difference between groups in the number of subjects reporting hypoglycaemic episodes (CSII 54% versus MDI 59%; p = NS) or in the mean rate of hypoglycaemic episodes per 30 days (CSII 0.8 ± 1.6 versus MDI 1.2 ± 3.1; p = NS). Nocturnal hypoglycaemic episodes were reported in 16% of CSII subjects and 22% of MDI subjects.
In summary, Raskin et al. reported no significant difference in reduction in mean HbA1c levels or occurrence of hypoglycaemic episodes between CSII and MDI in patients with T2DM. CSII subjects had significant improvements in treatment satisfaction scores compared with MDI subjects.
Berthe et al. (2007)114 – full publication
Description and quality of study
This two centre RCT set in France enrolled 17 patients with T2DM. Those eligible for inclusion were uncontrolled by two daily injections of regular plus NPH, and included those receiving insulin for > 6 months, aged 40–65 years, BMI ranging from 26 to 42 kg/m2 and willing to use an insulin pump device. The exclusion criteria were: patients with renal failure, proliferative retinopathy, high triglyceride level, use of oral glucose lowering or oral corticosteroid drugs, insulin dose requirements > 1.5 units/kg per day, and refusing pump device. The study was open label and there were no dropouts. The method of randomisation was not stated. All patients completed the study so ITT was not an issue. The trial was supported by Eli Lilly France.
-
Study quality = B.
Participants
The 17 participants were randomly assigned to either CSII or MDI for a 12-week period and thereafter switched to the other treatment for another 12-week period (hence the total study period was 24 weeks). Dietary counselling was also received at the beginning of each study period. Group 1 (n = 7) received pump then MDI and Group 2 (n = 10) received MDI then pump. The baseline characteristics of both groups were similar except that group 2 patients were older by a mean of 8 years. Patients were hospitalised for 24–48 hours at the beginning MDI period for 5 days, and at the beginning of the CSII period, in order to receive individual education sessions, including pump training sessions, MDI training sessions and instructions about hypoglycaemic and hyperglycaemic events. Patients also received dietary counselling (in accordance with ADA guidelines) at the beginning of each study period.
Intervention
The CSII used a Medtronic 508 pump delivering insulin lispro. Patients started with 70% daily dose as basal and 30% as pyramidal bolus. The MDI arm used three daily injections of premixed lispro–NPH insulin. All patients completed the study.
Results
Primary outcome
HbA1c
The HbA1c decreased from 9.0 ± 1.6% to 8.6 ± 1.6% at the end of the MDI period and to 7.7 ± 0.8% at the end of the CSII period (p < 0.03). (As this was a crossover trial it was not clear how this overall change in HbA1c was calculated for the two treatments.) A carry-over effect was tested by comparing the two groups of patients defined by the treatment order. No effect was observed.
Secondary outcome
Quality of life and treatment satisfaction
Both groups reported that they were satisfied with their insulin regimens. There was a slight but not significant preference for MDI over CSII.
Adverse event
Hypoglycaemia
There was no difference in hypoglycaemic episodes between the two groups.
In summary, Berthe et al. showed that CSII with lispro gave improved glycaemic control over MDI with three daily injections of premixed lispro–NPH insulin in patients with T2DM who had failed to respond to conventional insulin therapy. This was achieved with comparable patient satisfaction in both groups and no increase in hypoglycaemia.
| Study | Power calculation | Randomisation method | Allocation concealment | Assessors blinded | Groups similar at baseline | ITT | Protocol violations specified | Missing value treatment | Attrition | All patients accounted for |
|---|---|---|---|---|---|---|---|---|---|---|
| Type 1 diabetes | ||||||||||
| Doyle (2004)110 | No |
Stratified according to sex and age Random number table in block of four |
Open | No | Yes | Yes | Specified; two violations – would not affect results | LOCF | 31/32 (97%) completed study | Yes |
| Thomas (2007)111 | No | No details given | Open | No | Yes | Not stated | No | Not stated | Not stated | Not stated |
| Maran (2005)112 | No | No details given | Open | No | No comparison between groups | Not stated | No | Not stated | Not stated | Not stated |
| Bolli (2004)113 | No | No details given | Open | No | No baseline characteristics | Not stated | No | Not stated | Not stated | Not stated |
| Type 2 diabetes | ||||||||||
| Herman (2005)115 | Yes | Block randomisation | Open | No | Yes (although more men in CSII) | Yes | Technical and mechanical delivery violations specified – may affect results | Not stated | 98/107 (92%) completed study | Yes |
| Wainstein (2005)117 | Yes | No details given | Open | No | Yes |
Two cohorts ITT completers |
Two protocol violations resulting in dropout | LOCF in ITT analysis | 32/40 (80%) completed 18 weeks | Yes |
| Raskin (2003)116 | Yes | Inadequate method and no stratification. | Open | No | Yes | No; analysis based on 127/132 (96%) who received treatment | No | LOCF | 127/132 (96%) | Yes |
| Berthe (2007)114 | No | Crossover – no details given of method | Open | No | Yes (although group 2 patients older by 7.8 years) | Not stated | No | Not stated | Not stated | Not stated |
| Study | Number of participants | Inclusion criteria | Exclusion criteria | Mean age (years) | Mean HbA1c (%) | Mean BMI (kg/m2) [unless weight (kg) is stated] |
|---|---|---|---|---|---|---|
| Type 1 | ||||||
| Doyle (2004)110 | 32 |
T1DM Aged 8–21 years Otherwise healthy except for treated thyroid or coeliac disease Treated with insulin for at least 6 months Naive to CSII and glargine Willing to perform at least four blood glucose tests per day Screening HbA1c level between 6.5% and 11% |
Not explicitly stated |
CSII 12.5 ± 3.2 MDI 13 ± 2.8 |
CSII 8.2 ± 1.1 MDI 8.1 ± 1.2 |
Not stated |
| Thomas (2007)111 | 21 |
T1DM C-peptide negative Adults At least one episode of severe hypoglycaemia within the preceding 6 months Naive to MDI insulin analogue therapy |
Not explicitly stated |
CSII 40 ± 7 MDI 46 ± 9 |
CSII 8.5 ± 1.9 MDI 8.6 ± 1 |
CSII weight = 72.5 ± 8.6 MDI weight 78.0 ± 15.2 |
| Maran (2005)112 | 10 |
C-peptide negative T1DM Previously on CSII therapy for at least 6 months |
Not explicitly stated | All 41 ± 8 | All 7.7 ± 0.7 | Not stated |
| Bolli (2004)113 | 57 |
T1DM HbA1c < 9% Naive to CSII and glargine |
Not explicitly stated | Not stated |
CSII 7.7 ± 0.7 MDI 7.8 ± 0.6 |
Not stated |
| Type 2 | ||||||
| Herman (2005)115 | 107 |
> 60 years of age Clinical diagnosis of T2DM for at least 1 year Taking at least one injection of insulin per day for the past month (with or without oral anti-diabetes medications) HbA1c >7% |
BMI > 45 kg/m2 Severe impairment of cardiac, hepatic or renal function The presence of any physical, psychological, or cognitive impairments that would interfere with adherence to an intensive insulin therapy programme More than two episodes of severe hypoglycaemia in the past year or a history of hypoglycaemia unawareness |
CSII 66.6 ± 5.9 MDI 66.2 ± 4.5 |
CSII 8.4 ± 1.1 MDI 8.1 ± 1.2 |
CSII 32.5 ± 5.8 MDI 31.8 ± 5.8 |
| Wainstein (2005)117 | 40 |
Uncontrolled T2DM (HbA1c > 8.5%) Obese (BMI 30–45 kg/m2) Aged 30–70 years Treated for at least 3 months with diet, metformin (850 mg 2–3 times daily) and high doses of insulin (above 1 unit/kg per day), divided into two or three daily injections |
Those with new-onset diabetes (less than 6 months), T1DM, or diabetes secondary to pancreatitis or other disease, history of active IHD or CVA within the last 12 months, preproliferative or proliferative diabetic retinopathy, advanced nephropathy as evidenced by proteinuria or plasma creatinine > 1.5 mg/dl, liver enzymes twice above the upper limit of the normal range HbA1c > 15% at screening | Not stated |
CSII 10.2 ± 1.4 MDI 10.3 ± 1.2 |
Not stated |
| Raskin (2003)116 | 132 |
T2DM of 2 years’ duration ≥ 35 years Treatment for 6 months with at least one insulin dose per day (regular insulin, lispro insulin, NPH, premixed insulin, lente, or ultralente), with or without an oral antidiabetic drug |
Subjects with impaired hepatic, renal, or cardiac function or recurrent major hypoglycaemia Women of childbearing age were excluded if they were pregnant, breastfeeding, or not practising contraception |
CSII 55.1 ± 10.2 MDI 56.0 ± 8.18 |
CSII 8.2 ± 1.4 MDI 8.0 ± 1.1 |
CSII 32.2 ± 4.2 MDI 32.2 ± 5.1 |
| Berthe (2007)114 | 17 |
T2DM Receiving insulin for > 6 months Aged 40–65 years BMI ranging from 26 to 42 kg/m2 Uncontrolled by two daily injections of regular NPH (HbA1c level of ≥ 6.5% on two determinations) Willing to use an insulin pump device |
Patients with renal failure, proliferative retinopathy, high triglyceride level Use of OGLA, or oral corticosteroid drugs Insulin does requirements > 1.5 units/kg per day Refusing pump device |
Group 1 (pump then MDI) 50.6 ± 6.4 Group 2 (MDI then pump) 58.4 ± 4.6 |
CSII 9.0 ± 1.6 MDI 9.0 ± 1.6 |
Group 1 (pump then MDI) 34.6 ± 4.0 Group 2 (MDI then pump) 33.0 ± 4.9 |
| Study | Outcome measure(s) | Result |
|---|---|---|
| Type 1 diabetes | ||
| Doyle (2004)110 | DQoL-Y | Baseline to 16 weeks p = NS between CSII and MDI |
| Thomas (2007)111 | DQoL | p = NS between CSII and MDI |
| Hypoglycaemia Fear Survey | p = NS between CSII and MDI | |
| Type 2 diabetes | ||
| Herman (2005)115 | SF-36 | p = NS between CSII and MDI |
| DQoLCTQ | p = NS between CSII and MDI | |
| Raskin (2003)116 | Components of the Phase V Technologies Outcomes Information System – included a diabetes treatment satisfaction module and a quality of life summary scale | CSII vs. MDI overall treatment satisfaction p < 0.001 |
| Berthe (2007)114 | Satisfaction questionnaire (adapted from Raskin et al.116) | p = NS between CSII and MDI |
| Study | Mean blood glucose levels | Mild hypoglycaemia | Severe hypoglycaemia | Other | Hyperglycaemia | DKA |
|---|---|---|---|---|---|---|
| Type 1 diabetes | ||||||
| Doyle (2004)110 | p = NS between groups |
CSII n = 1 MDI n = 1 (hospitalised for ketosis and dehydration) |
||||
| Thomas (2007)111 | p = NS between treatment groups in mean daily blood glucose (mM) | p = NS trend toward reduced incidence in MDI and CSII groups. | p = NS trend toward reduced incidence in MDI and CSII groups | p = NS between groups in glucose excursions (< 2.4 mM/< 4 mM/< 7 mM/hour per 24 hours) | ||
| Maran (2005)112 | CSII group had significantly lower mean glucose levels (CSII 147 ± 12 vs 189 ± 14 mg/dl; p < 0.03) |
p = NS between groups in hypoglycaemia reactions exposure (AUC < 65 mg/dl) CSII spent significantly more time in glucose range > 65 mg/dl and < 180 mg/dl than MDI (CSII 298 ± 63 vs MDI 194 ± 51 minutes; p < 0.02) |
||||
| Bolli (2004)113 | CSII baseline 164 ± 41 end point 146 ± 32 vs MDI baseline 160 ± 30 end point 144 ± 20; difference 1 95% CI –14 to 15; p = NS |
p = NS between groups in incidence of blood glucose < 72 mg/dl between treatment groups (CSII 41 vs MDI 35) p = NS between groups in MAGE and 8-point blood glucose profiles |
||||
| Type 2 diabetes | ||||||
| Herman (2005)115 | p = NS between treatment groups | p = NS between groups | p = NS between groups in incidence of catastrophic hypoglycaemia | |||
| Wainstein (2005)117 | p = NS between treatment groups | p = NS between treatment groups | ||||
| Raskin (2003)116 | p = NS between groups in the number of subjects reporting hypoglycaemia episodes or mean rate of hypoglycaemic episodes |
CSII 3 (5%) reported six episodes MDI 11 (18%) reported 26 episodes |
||||
| Berthe (2007)114 | Duration (% of 24 hours) time of glucose maintained within the target (60–180 mg/dl) was significantly reduced; p = 0.0085 between groups | No difference between groups | None reported |
Rate of hyperglycaemic excursions over 24 hours; p = NS between groups Duration of hyperglycaemic excursions, % = p = 0.012 between groups |
||
Appendix 5 Structure of the CORE model
Palmer et al. (2004)203 outlines the broad structure of the CORE model for T1DM and T2DM patients, providing references for the 15 complications of diabetes submodels within the overall CORE model.
Note that where the study has analysed patients with diabetes as a specific subgroup, where the sample size is stated without qualification this refers to the size of the diabetic subgroup. Similarly, if the study was specific to diabetic patients, either entirely or as a subgroup, but without identifying or subanalysing diabetic types, this is stated as ‘yes’. Where a specific type of diabetes is analysed separately, this is stated, i.e. T1, T2 or T1 and T2.
| Submodel | |||||||
|---|---|---|---|---|---|---|---|
| MI | |||||||
| Submodel differentiated between T1 and T2: | No | ||||||
| Submodel differentiated by patient age: | Yes | ||||||
| Submodel differentiated by patient duration of diabetes: | Yes, if simulations based upon UKPDS risk engine | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA1c (%) | N | Average age |
| (a) Probability 2nd MI | 45: Herlitz 1996 | Sweden | Yes | 10 years | n/a | 96 | 72 |
| (b) MI immediate death rate | 46: Sonke 1996 | New Zealand | No | n/s | n/s | 5,106 | 55 |
| (c) MI 12-month death rate | 47: Almbrand 2000 | Sweden | Yes | n/s | n/s | 620 | n/s |
| (d) Effect of intensive insulin on (c) | 48: Malmberg 1997 | Sweden | Yes | n/s | n/s | 620 | n/s |
| Angina | |||||||
| Submodel differentiated between T1 and T2: | No | ||||||
| Submodel differentiated by patient age: | Yes | ||||||
| Submodel differentiated by patient duration of diabetes: | No | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA1c (%) | N | Average age |
| (a) Probability of developing angina | 43: DeAgostina 2000 | USA | Yes | n/s | n/s | 500 | 49 |
| (b) Cardiovascular risk multipliers | 49: Mann 2001 | Multi | Yes | n/s | n/s | 3573 | 66 |
| CHF | |||||||
| Submodel differentiated between T1 and T2: | Yes | ||||||
| Submodel differentiated by patient age: | Yes | ||||||
| Submodel differentiated by patient duration of diabetes: | No | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA1c (%) | N | Average age |
| (a) CHF risk profile | 50: Kannel 1999 | USA | Yes | n/s | n/s | 486* | 62 |
| (b) Death following CHF event | 51: Ho 1993 | USA | No | n/a | n/a | 652 | 41** |
| (c) HbA1c risk adjustment | 53: Stratton 2000 | UK | T2 | n/s | 7.1 | 3642 | 53 |
| (d) CHF risk adjustment: ACE, etc. | 54: HOPE 2000 | Multi | T2*** | 11 years | n/a | 2577 | 65 |
|
Notes: *All patients, as number of diabetic patients not stated. **At enrolment in Framingham. ***T2 constituted more than 97% study population. |
|||||||
| Stroke | |||||||
| Submodel differentiated between T1 and T2: | No | ||||||
| Submodel differentiated by patient age: | Yes | ||||||
| Submodel differentiated by patient duration of diabetes: | Yes, if simulations based upon UKPDS risk engine | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA1c (%) | N | Average age |
| (a) T2 stroke probability | 56: Kothari 2002 | UK | T2 | n/s | 6.7 | 4549 | 52** |
| (b) T2 risk adjustments | 42: Valmadrid 2000 | USA | T2 | 15 years | 9.3 | 840 | 68 |
| (c) Probability recurrent stroke | 57: Petty 1998 | USA | Yes* | n/s | n/s | 1111*** | 75 |
| (d) 12-month stroke death rate | 58: Sprafka 1994 | USA | Yes | n/s | n/s | n/s | 30–74 |
| (e) Risk adjustment: ACE, etc. | 59: ADA 2002 | USA | Yes | n/s | n/s | n/s | 30+ |
| 60: Buring 1990 | Multi | No | n/a | n/a | 17,187 | n/s | |
|
Notes: *But reference 58 appears to be used for adjusting stroke risk. **Age at diagnosis. ***All patients, not restricted to diabetic subgroup. |
|||||||
| Peripheral vascular disease | |||||||
| Submodel differentiated between T1 and T2: | No | ||||||
| Submodel differentiated by patient age: | Yes | ||||||
| Submodel differentiated by patient duration of diabetes: | Yes | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA1c (%) | N | Average age |
| (a) PVD risk profile | 61: Murabito 1997 | US | Yes | n/s | n/s | 381* | 28–62 |
| (b) T2 risk adjustment for HbA1c | 62: Stratton 2001 | UK | T2 | n/s | 7.0 | 1919 | 52 |
| *All patients – not restricted to diabetic subgroup. | |||||||
| Neuropathy | |||||||
| Submodel differentiated between T1 and T2: | Yes | ||||||
| Submodel differentiated by patient age: | No | ||||||
| Submodel differentiated by patient duration of diabetes: | Yes | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA1c (%) | N | Average age |
| (a) T1 neuropathy prevalence | 63: DCCT 1995 | USA and Canada | T1 and T2 | 1–5 years | n/s | 1441 | 26 |
| (b) T2 neuropathy prevalence | 18: Partanen 1995 | T2 | |||||
| (c) T1 transition probabilities | 63: DCCT 1995 | As above | |||||
| (d) T2 transition probabilities | 18: Partanen 1995 | As above | |||||
| (e) T1 risk adjustment for HbA1c | 63:DCCT 1995 | As above | |||||
| (f) T2 risk adjustments | 43: DeAgostina 2000 | USA | Yes | n/s | n/s | 500 | 49 |
| 53: Stratton 2000 | UK | T2 | n/s | 7.1 | 3642 | 53 | |
| 56: Kothari 2002 | UK | T2 | n/s | 6.7 | 4459 | 52 | |
| 64: Adler 2000 | UK | T2 | n/s | 7.1 | 3642 | 53 | |
| Foot ulcer and amputation | |||||||
| Submodel differentiated between T1 and T2: | No | ||||||
| Submodel differentiated by patient age: | Yes – indirectly via PVD | ||||||
| Submodel differentiated by patient duration of diabetes: | Yes – indirectly via neuropathy and PVD | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA1c | N | Average age |
| (a) Probability of developing; also linked to PVD and neuropathy | 68: Tenvall 2001 | Sweden | Yes | n/s | n/s | 1677 | 66 |
| Retinopathy | |||||||
| Submodel differentiated between T1 and T2: | Yes | ||||||
| Submodel differentiated by patient age: | No | ||||||
| Submodel differentiated by patient duration of diabetes: | Yes | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA1c (%) | N | Average age |
| (a) T1 transition probabilities | 69: DCCT 1995 | USA and Canada | Yes | 2.6 years | 8.8 | 1441 | 26 |
| (b) T1 risk adjustments | 70: DCCT 1996 | USA and Canada | Yes | 2.6 years | 8.8 | 1441 | 26 |
| 71: DCCT 1993 | USA and Canada | Yes | 2.6 years | 8.8 | 1441 | 26 | |
| 72: Malik 1998 | UK | Yes | n/s | 41 | |||
| (c) T2 transition probabilities | 73: Javitt 1994 | USA | T2 | n/s | n/s | n/s | n/s |
| 74: Klein 1989 | USA | T1* | 14 years | 12.6 | 1210 | 29 | |
| (d) Transition to SVL | 62: Stratton 2001 | UK | T2 | n/s | 7.0 | 1919 | 52 |
| (e) ACE effect on BDR and PDR | 75: Chaturvedi 1998 | Multi | T1 | 9 years | 7 | 409 | 31 |
| *Described as younger onset – prescribed insulin. | |||||||
| Macular oedema | |||||||
| Submodel differentiated between T1 and T2: | Yes | ||||||
| Submodel differentiated by patient age: | No | ||||||
| Submodel differentiated by patient duration of diabetes: | Yes | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA1c (%) | N | Average age |
| (a) T1 onset and progression SVL | 71: DCCT 1993 | USA and Canada | Yes | 2.6 years | 8.8 | 1441 | 26 |
| (b) T2 transition probabilities | 73: Javitt 1994 | USA | T2 | n/s | n/s | n/s | n/s |
| (c) T1 onset risk adjustment HbA1c | 71: DCCT 1993 | As above | |||||
| (d) T1 onset risk adjustment SBP | 64: Adler 2000 | UK | T2 | n/s | 7.1 | 3642 | 53 |
| (e) T2 onset risk adjustment HbA1c | 62: Stratton 2001 | UK | T2 | n/s | 7.0 | 1919 | 52 |
| (f) T2 onset risk adjustment SBP | 64: Adler 2000 | As above | |||||
| Cataract | |||||||
| Submodel differentiated between T1 and T2: | Yes | ||||||
| Submodel differentiated by patient age: | No | ||||||
| Submodel differentiated by patient duration of diabetes: | No | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA1c (%) | N | Average age |
| (a) T1 incidence and subsequent | 76: Janghorbani 2000 | UK | T1 and T2 | 7.6 years | ~12 | 3606 | 49 |
| (b) T2 incidence | 77: UKPDS33 1998 | UK | T2 | n/s | 7.5 | 3862 | 54 |
| (c) T2 subsequent | 76: Janghorbani 2000 | As above | |||||
| (d) T2 risk adjustment HbA1c | 53: Stratton 2000 | UK | T2 | n/s | 7.1 | 3642 | 53 |
| Nephropathy | |||||||
| Submodel differentiated between T1 and T2: | Yes | ||||||
| Submodel differentiated by patient age: | No | ||||||
| Submodel differentiated by patient duration of diabetes: | Yes | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA1c (%) | N | Average age |
| (a) T1 transition probabilities | 78: DCCT 1995 | USA and Canada | |||||
| (b) T2 transition probabilities | 79: Ritz 1996 | Multi: meta | T2 | n/s | n/s | n/s | n/s |
| 80: Wolfe 1999 | USA | Yes* | n/s | n/s | 46,164 | 40–59** | |
| 84: Ravid 1998 | Israel | T2 | n/s | ~9 | 574 | 49 | |
| 85: Ravid 1993 | Israel | T2 | 6.7 years | n/s | 108 | 44 | |
| (c) Death from ESRD | 80: Wolfe 1999 | As above | |||||
| (d) T1 risk adjustments | 71: DCCT 1993 | USA and Canada | Yes | 2.6 years | 8.8 | 1441 | 26 |
| 79: Ritz 1996 | As above | ||||||
| 80: Wolfe 1999 | As above | ||||||
| 83: Kshirsagat 2000 | Multi: meta | *** | n/a | n/a | n/a | n/a | |
| (e) T2 risk adjustment HbA1c | 87: UKPDS34 1998 | UK | T2 | 11 years | 7.7 | 753 | 53 |
| (f) T2 risk adjustment SBP | 88: UKPDS 38 1998 | UK | T2 | 2.7 years | 6.9 | 1,148 | 56 |
|
*End-stage renal patients awaiting transplantation. **Median. ***Patients with renal disease. |
|||||||
| Hypoglycaemia | |||||||
| Submodel differentiated between T1 and T2: | Yes | ||||||
| Submodel differentiated by patient age: | No | ||||||
| Submodel differentiated by patient duration of diabetes: | No | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA1c (%) | N | Average age |
| (a) T1 probability: HbA1c and age | 89: DCCT 1997 | USA and Canada | Yes | 2.6 years | 8.8 | 1,441 | 26 |
| (b) T2 probability | 90: Stepka 1993 | Poland | T1 and T2 | n/s | n/s | 20,798* | ** |
| 91: Ben Ami 1999 | Israel | T1 and T2 | n/s | n/s | 102 | 72 | |
| (c) T2 risk adjustment medication | 77: UKPDS33 1998 | UK | T2 | n/s | 7.5 | 3862 | 54 |
| (d) ACE risk adjustment | 92: Morris 1997 | UK | Yes | n/s | n/s | 500 | n/s |
| 93: Herings 1995 | Netherlands | Yes | n/s | n/s | 748 | 60–74*** | |
| (e) probability death | 70: DCCT 1993 | USA and Canada | Yes | 2.6 years | 8.8 | 1441 | 26 |
|
*Diabetic hospitalisations, of which 236 serious hypoglycaemia. **98/101 T2 patients admitted with hypoglycaemia were over 60. ***Range within which median fell. |
|||||||
| Ketoacidosis | |||||||
| Submodel differentiated between T1 and T2: | Only applies to type 1 | ||||||
| Submodel differentiated by patient age: | No | ||||||
| Submodel differentiated by patient duration of diabetes: | No | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA1c (%) | N | Average age |
| (a) Event probability | 71: DCCT 1993 | USA and Canada | Yes | 2.6 years | 8.8 | 1441 | 26 |
| (b) Probability of death | 94: MacIsaac 2002 | Australia | Yes | n/s | n/s | 312 | 33–69* |
| Review | Yes | n/a | n/a | n/a | n/a | ||
| 95: Umpierrez 1996 | *33 average for DKA, 44 average for DKA-HHS and 69 average for HHS alone. | ||||||
| Lactic acidosis | |||||||
| Submodel differentiated between T1 and T2: | Only applies to T2 (treated with metformin) | ||||||
| Submodel differentiated by patient age: | No | ||||||
| Submodel differentiated by patient duration of diabetes: | No | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA1c | N | Average age |
| (a) Event probability | 96: Campbell 1985 | Review | Yes | n/a | n/a | n/a | n/a |
| (b) Probability of death | 96: Campbell 1985 | As above | |||||
| Non-specific mortality | |||||||
| Submodel differentiated between T1 and T2: | No | ||||||
| Variable | Referencea | Country | Diabetes | Duration | HbA− | N | Average age |
| a) Non-specific mortality, USA default | 98: NVSR 2001 | USA | No | n/a | n/a | – | – |
Appendix 6 Sensitivity analyses within the industry submission
| QALY gain | Net cost | ICER (£) | |
|---|---|---|---|
| Base case: trial-based analysis | 0.500 | 17,158 | 34,330 |
| Glycaemic control | |||
| Upper 95% CI for change in HbA1c | 0.590 | 16,848 | 28,540 |
| Lower 95% CI for change in HbA1c | 0.411 | 17,283 | 42,015 |
| Probabilistic sensitivity analysis | 0.559 | 16,031 | 28,656 |
| Time horizon | |||
| 5 years | 0.085 | 5421 | 63,795 |
| 10 years | 0.189 | 9080 | 47,921 |
| 15 years | 0.275 | 11,570 | 42,039 |
| Pump price | |||
| Plus 20% | 0.500 | 18,817 | 37,649 |
| Minus 20% | 0.500 | 15,499 | 31,010 |
| Discounting | |||
| 0% costs and 0% benefits | 0.903 | 28,058 | 31,084 |
| 6% costs and 6% benefits | 0.354 | 13,090 | 36,927 |
| 6% costs and 1.5% benefits | 0.689 | 13,090 | 18,997 |
| Severe hypoglycaemic events | |||
| Upper rate | 0.526 | 16,632 | 31,636 |
| Lower rate | 0.478 | 17,761 | 37,189 |
Appendix 7 Treatment costs
Capital costs
NHS Supply Chain is currently engaging in a tendering exercise to establish a national price structure for pumps and consumables. Work to date indicates a range of pump prices from £1900 to £2600, with a usual warranty period of 4 years.
After the 4-year warranty period, servicing is required, at an average cost of around £500, in order for the pump to remain under guarantee. This subsequent guarantee lasts for between 1 and 2 years. However, NHS Supply Chain reports that as pump technology changes over time; after the initial 4-year warranty period many PCTs will simply purchase another pump, with the older newly serviced pumps possibly being retained as ‘testers’ for patients trying CSII. New pumps would be purchased for these patients if they were found to suit pump therapy.
Given this for an average pump cost of £2300 as per the industry submission, if this lasts only 4 years the annualised cost of this, given a discount rate of 3.5%, is £605. Increasing pump longevity to 6 years through servicing would reduce this annualised capital cost, including the costs of service, to £505, while a maximum lifespan of 8 years involving two services would imply an annualised cost of £455, although a lifespan of 8 years may be viewed as unlikely to occur in practise.
Similarly with regards the additional training that may be required for the use of CSII, this can be estimated as a one-off cost of around £240, which would annualise to an approximate figure of £15.
This gives an annualised capital and training costs for CSII of £620, £520 and £470 for pump lifespans of 4, 6 and 8 years, respectively. In contrast, the only capital items for MDI are the two pen devices necessary, which at a cost of around £22 each and a possible lifespan of 3 years would give an annualised capital cost of £15.
Consumable costs
Given the consumables for CSII of infusion sets and reservoirs and needles for MDI, as outlined within the manufacturer’s submission, the other consumables relate to the required insulin dose and the frequency of blood glucose monitoring.
The meta-analysis by Pickup et al. 18 noted a reduced daily requirement for insulin of 0.6 IU/kg for CSII compared to 0.7 IU/kg for MDI. These doses will be used for the base-case analysis.
In a similar vein, the previous review noted that CSII had a daily requirement of four or more blood glucose monitorings compared with three or more for MDI, although concluded that on average this would not result in any real additional cost for CSII. Given this, the base case for this review will assume a common rate for both CSII and MDI.
Total annual cost
The above assumptions coupled with an assumed patent weight of 80 kg results in the following overall annual costs for CSII and MDI.
| Costs (£) | |||
|---|---|---|---|
| CSII | MDI | Net | |
| Insulin | |||
| Humalog | 312.21 | ||
| Humalog cartridge | 200.72 | ||
| Lantus cartridge | 265.72 | ||
| Total insulin | 312.21 | 466.44 | –154.23 |
| Consumables | |||
| Infusion sets | 1058.87 | ||
| Insulin reservoir | 325.82 | ||
| Needles | 31.83 | ||
| Lancets | 35.59 | 35.59 | |
| Test strips | 328.50 | 328.50 | |
| Glucometer | 10.00 | 10.00 | |
| Total consumables | 1758.78 | 405.92 | 1352.86 |
| Capital costs | |||
| Pump: 4-year lifespan | 620.00 | 15.00 | 605.00 |
| Pump: 6-year lifespan | 520.00 | 15.00 | 505.00 |
| Pump: 8-year lifespan | 470.00 | 15.00 | 455.00 |
| Total | |||
| Pump: 4-year lifespan | 2690.99 | 887.36 | 1803.63 |
| Pump: 6-year lifespan | 2590.99 | 887.36 | 1703.63 |
| Pump: 8-year lifespan | 2540.99 | 887.36 | 1653.63 |
Given the possible role for CSII within paediatric patients with T1DM, coupled with an additional possibility of use in relatively overweight patients with T2DM, patient weight will affect relative costs. However, only insulin use and possibly dosing would vary with patient weight and type of diabetes, and, as can be seen above, the major cost components for CSII are the consumables and capital costs, which do not vary with weight or diabetes type.
As a consequence, maintaining the same dosing assumptions and assuming a pump lifespan of 6 years, a patient weight of 30 kg increases the net cost of CSII over MDI from £1703 to £1800 as the net cost of insulin drops to around a saving of £58 for CSII. In contrast, increasing the patient weight to 100 kg increases the insulin saving to around £193, thus reducing the net cost of CSII over MDI from £1703 to £1665.
The more pessimistic assumptions of equal dosing under CSII and MDI of 0.6 IU/kg, a daily requirement of four blood glucose monitorings for CSII compared with that for MDI, and both of these combined have a greater effect, resulting in the following for an 80-kg patient:
| Costs (£) | |||
|---|---|---|---|
| CSII | MDI | Net | |
| Equal insulin dose | |||
| Pump: 4-year lifespan | 2690.98 | 820.72 | 1870.26 |
| Pump: 6-year lifespan | 2590.98 | 820.72 | 1770.26 |
| Pump: 8-year lifespan | 2540.98 | 820.72 | 1720.26 |
| Higher CSII monitoring | |||
| Pump: 4-year lifespan | 2812.34 | 887.36 | 1924.98 |
| Pump: 6-year lifespan | 2712.34 | 887.36 | 1824.98 |
| Pump: 8-year lifespan | 2662.34 | 887.36 | 1774.98 |
| Equal insulin dose and higher CSII monitoring | |||
| Pump: 4-year lifespan | 2812.34 | 820.72 | 1991.62 |
| Pump: 6-year lifespan | 2712.34 | 820.72 | 1891.62 |
| Pump: 8-year lifespan | 2662.34 | 820.72 | 1841.62 |
Appendix 8 Information supplied by INPUT on the range and costs of pumps currently available within the UK
| Units | Price (£) | First-year cost (£) | |
|---|---|---|---|
| Smiths Deltec Cozmo | |||
| Pump price: models 1700 and 1701 | 1 | 2750.00 | 2750.00 |
| Cartridge, insulin, 25 | 25 | 62.50 | 312.50 |
| Comfort, single, all sizes, 10 sets | 10 | 86.00 | 1049.20 |
| Comfort, combo, five sets and five extra cannulas | 5 | 70.00 | |
| Warranty | 4 years | ||
| Roche Accu-Chek Spirit | |||
| Pump price | 1 | 2375.00 | 2375.00 |
| Accu-Chek Spirit cartridges, 25 pieces | 25 | 45.55 | 227.75 |
| Flexlink Accu-Chek Flexilink I 8/30, 10 cannulas | 10 | 80.85 | 986.37 |
| Warranty | 6 years | ||
| Animas IR1200 | |||
| Pump price | 1 | 2600.00 | 2600.00 |
| IR1200, 10 cartridges, pack of 10 | 10 | 23.50 | 282.00 |
| Infusion Set, Comfort, 17 mm 23, 10 cartridges, pack of 10 | 10 | 66.00 | 805.20 |
| Warranty | 4 years | ||
| Medtronic Paradigm | |||
| Pump price: 522/722 real-time with CGM | 1 | 3200.00 | 3200.00 |
| Pump price: 522/722 real-time without CGM | 1 | 2750.00 | 2750.00 |
| Continuous glucose monitor for real-time pump | 1 | 750.00 | 750.00 |
| Paradigm reservoir 3 ml, pack of 10 | 10 | 26.00 | 312.00 |
| Paradigm Quick set 110 cm 9 mm, pack of 10 | 10 | 87.03 | 1061.75 |
| Warranty | 4 years | ||
Worldwide use of insulin is stated as being 44 IU per day. Unlike with the previous HTA, INPUT also reports that servicing to extend the warranty period of pumps is no longer available.
Appendix 9 Cost-effectiveness simulations – assumptions used
| Simulation | HbA1c | Hypo rate | Hypo effect | Price | Horizon |
|---|---|---|---|---|---|
| Type 1 | |||||
| Sim01 | 0.9% less | 0% less | Mid | 50 years | |
| CSII | 7.9% | 0.187 | £2590 | ||
| MDI | 8.8% | 0.187 | £890 | ||
| Sim02 | 0.9% less | 50% less | Mid | 50 years | |
| CSII | 7.9% | 0.094 | £2590 | ||
| MDI | 8.8% | 0.187 | £890 | ||
| Sim03 | 0.9% less | 75% less | Mid | 50 years | |
| CSII | 7.9% | 0.047 | £2590 | ||
| MDI | 8.8% | 0.187 | £890 | ||
| Higher hypoglycaemic-event rate: time horizon | |||||
| Sim04 | 0.9% less | 50% less | Mid | 50 years | |
| CSII | 7.9% | 0.310 | £2590 | ||
| MDI | 8.8% | 0.620 | £890 | ||
| Sim05 | 0.9% less | 50% less | Mid | 30 years | |
| CSII | 7.9% | 0.310 | £2590 | ||
| MDI | 8.8% | 0.620 | £890 | ||
| Sim06 | 0.9% less | 50% less | Mid | 10 years | |
| CSII | 7.9% | 0.310 | £2590 | ||
| MDI | 8.8% | 0.620 | £890 | ||
| Higher hypoglycaemic-event rate: lesser effect upon HbA1c | |||||
| Sim07 | 0.6% less | 50% less | Mid | 50 years | |
| CSII | 8.2% | 0.310 | £2590 | ||
| MDI | 8.8% | 0.620 | £890 | ||
| Sim08 | 0.6% less | 75% less | Mid | 50 years | |
| CSII | 8.2% | 0.155 | £2590 | ||
| MDI | 8.8% | 0.620 | £890 | ||
| Higher hypoglycaemic-event rate: effect upon severe hypoglycaemia | |||||
| Sim09 | 0.9% less | 0% less | Mid | 50 years | |
| CSII | 7.9% | 0.620 | £2590 | ||
| MDI | 8.8% | 0.620 | £890 | ||
| Sim10 | 0.9% less | 75% less | Mid | 50 years | |
| CSII | 7.9% | 0.155 | £2590 | ||
| MDI | 8.8% | 0.620 | £890 | ||
| Higher hypoglycaemic-event rate: price | |||||
| Sim11 | 0.9% less | 75% less | High | 50 years | |
| CSII | 7.9% | 0.155 | £2710 | ||
| MDI | 8.8% | 0.620 | £803 | ||
| Sim12 | 0.9% less | 50% less | Low | 50 years | |
| CSII | 7.9% | 0.310 | £2400 | ||
| MDI | 8.8% | 0.620 | £890 | ||
| Sim13 | 0.9% less | 75% less | Low | 50 years | |
| CSII | 7.9% | 0.155 | £2400 | ||
| MDI | 8.8% | 0.620 | £890 | ||
| Higher hypoglycaemic-event rate: higher costs of blindness of £4000 per year | |||||
| Sim14 | 0.9% less | 50% less | Mid | 50 years | |
| CSII | 7.9% | 0.310 | £2590 | ||
| MDI | 8.8% | 0.620 | £890 | ||
| Sim15 | 0.9% less | 75% less | Mid | 50 years | |
| CSII | 7.9% | 0.155 | £2590 | ||
| MDI | 8.8% | 0.620 | £890 | ||
| High-hypoglycaemia group | |||||
| Sim16 | 0.0% less | 50% less | Mid | 50 years | |
| CSII | 7.5% | 0.670 | £2590 | ||
| MDI | 7.5% | 1.340 | £890 | ||
| Sim17 | 0.0% less | 75% less | Mid | 50 years | |
| CSII | 7.5% | 0.335 | £2590 | ||
| MDI | 7.5% | 1.340 | £890 | ||
| Higher hypoglycaemic-event rate: younger age cohort – average 30 years | |||||
| Sim18 | 0.0% less | 50% less | Mid | 50 years | |
| CSII | 7.5% | 0.670 | £2590 | ||
| MDI | 7.5% | 1.340 | £890 | ||
| Greater effect upon HbA1c | |||||
| Sim19 | 1.4% less | 0% less | Mid | 50 years | |
| CSII | 7.6% | 0.620 | £2590 | ||
| MDI | 9.0% | 0.620 | £890 | ||
Appendix 10 Results of cost-effectiveness simulations
General population
| CSII | MDI | Difference | |
|---|---|---|---|
| Sim01 General population: no hypo effect | |||
| Life expectancy | 21.848 | 20.536 | 1.312 |
| Life expectancy (discounted) | 14.244 | 13.652 | 0.592 |
| QALYs (discounted) | 9.547 | 8.97 | 0.577 |
| Treatment costs (discounted) (£) | 38,145 | 12,599 | 25,546 |
| Other costs (discounted) (£) | 21,637 | 24,316 | –2679 |
| Total costs (discounted) (£) | 59,782 | 36,915 | 22,867 |
| ICER: cost per QALY (£) | 39,586 | ||
| Sim02 General population: 50% hypo effect | |||
| Life expectancy | 21.831 | 20.536 | 1.295 |
| Life expectancy (discounted) | 14.237 | 13.652 | 0.585 |
| QALYs (discounted) | 9.571 | 8.97 | 0.601 |
| Treatment costs (discounted) (£) | 38,129 | 12,599 | 25,530 |
| Other costs (discounted) (£) | 21,463 | 24,316 | –2853 |
| Total costs (discounted) (£) | 59,592 | 36,915 | 22,677 |
| ICER: cost per QALY (£) | 37,712 | ||
| Sim03 General population: 75% hypo effect | |||
| Life expectancy | 21.855 | 20.536 | 1.319 |
| Life expectancy (discounted) | 14.246 | 13.652 | 0.594 |
| QALYs (discounted) | 9.591 | 8.97 | 0.621 |
| Treatment costs (discounted) (£) | 38,150 | 12,599 | 25,551 |
| Other costs (discounted) (£) | 21,365 | 24,316 | –2951 |
| Total costs (discounted) (£) | 59,515 | 36,915 | 22,600 |
| ICER: cost per QALY (£) | 36,373 | ||
Base case
| CSII | MDI | Difference | |
|---|---|---|---|
| Sim04 Base case: 50-year horizon | |||
| Life expectancy | 21.808 | 20.563 | 1.245 |
| Life expectancy (discounted) | 14.224 | 13.665 | 0.559 |
| QALYs (discounted) | 9.504 | 8.892 | 0.612 |
| Treatment costs (discounted) (£) | 38,097 | 12,611 | 25,486 |
| Other costs (discounted) (£) | 21,662 | 24,761 | –3099 |
| Total costs (discounted) (£) | 59,759 | 37,372 | 22,387 |
| ICER: cost per QALY (£) | 36,587 | ||
| Sim05 Base case: 30-year horizon | |||
| Life expectancy | 20.579 | 19.6 | 0.979 |
| Life expectancy (discounted) | 13.86 | 13.367 | 0.493 |
| QALYs (discounted) | 9.299 | 8.721 | 0.578 |
| Treatment costs (discounted) (£) | 36,967 | 12,293 | 24,674 |
| Other costs (discounted) (£) | 19,107 | 22,565 | –3,458 |
| Total costs (discounted) (£) | 56,074 | 34,858 | 21,216 |
| ICER: cost per QALY (£) | 36,710 | ||
| Sim06 Base case: 10-year horizon | |||
| Life expectancy | 9.416 | 9.35 | 0.066 |
| Life expectancy (discounted) | 7.863 | 7.813 | 0.05 |
| QALYs (discounted) | 5.603 | 5.392 | 0.211 |
| Treatment costs (discounted) (£) | 20,637 | 7059 | 13,578 |
| Other costs (discounted) (£) | 5062 | 6412 | –1350 |
| Total costs (discounted) (£) | 25,699 | 13,471 | 12,228 |
| ICER: cost per QALY (£) | 58,013 | ||
Lesser HbA1c effect
| CSII | MDI | Difference | |
|---|---|---|---|
| Sim07 0.6% HbA1c and 50% hypo effect | |||
| Life expectancy | 21.399 | 20.563 | 0.836 |
| Life expectancy (discounted) | 14.044 | 13.665 | 0.379 |
| QALYs (discounted) | 9.318 | 8.892 | 0.426 |
| Treatment costs (discounted) (£) | 37,645 | 12,611 | 25,034 |
| Other costs (discounted) (£) | 22,673 | 24,761 | -2,088 |
| Total costs (discounted) (£) | 60,318 | 37,372 | 22,946 |
| ICER: cost per QALY (£) | 53,788 | ||
| Sim08 0.6% HbA1c and 75% hypo effect | |||
| Life expectancy | 21.403 | 20.563 | 0.84 |
| Life expectancy (discounted) | 14.048 | 13.665 | 0.383 |
| QALYs (discounted) | 9.366 | 8.892 | 0.474 |
| Treatment costs (discounted) (£) | 37,656 | 12,611 | 25,045 |
| Other costs (discounted) (£) | 22,366 | 24,761 | –2395 |
| Total costs (discounted) (£) | 60,022 | 37,372 | 22,650 |
| ICER: cost per QALY (£) | 47,780 | ||
Effect upon SH events
| CSII | MDI | Difference | |
|---|---|---|---|
| Sim09 No hypo effect | |||
| Life expectancy | 21.831 | 20.563 | 1.268 |
| Life expectancy (discounted) | 14.235 | 13.665 | 0.57 |
| QALYs (discounted) | 9.445 | 8.892 | 0.553 |
| Treatment costs (discounted) (£) | 38,122 | 12,611 | 25,511 |
| Other costs (discounted) (£) | 21,974 | 24,761 | –2,787 |
| Total costs (discounted) (£) | 60,096 | 37,372 | 22,724 |
| ICER: cost per QALY (£) | 41,062 | ||
| Sim10 75% hypo effect | |||
| Life expectancy | 21.842 | 20.563 | 1.279 |
| Life expectancy (discounted) | 14.241 | 13.665 | 0.576 |
| QALYs (discounted) | 9.556 | 8.892 | 0.664 |
| Treatment costs (discounted) (£) | 38,137 | 12,611 | 25,526 |
| Other costs (discounted) (£) | 21,406 | 24,761 | –3355 |
| Total costs (discounted) (£) | 59,543 | 37,372 | 22,171 |
| ICER: cost per QALY (£) | 33,361 | ||
Price
| CSII | MDI | Difference | |
|---|---|---|---|
| Sim11 High price and 75% hypo effect | |||
| Life expectancy | 21.842 | 20.563 | 1.279 |
| Life expectancy (discounted) | 14.241 | 13.665 | 0.576 |
| QALYs (discounted) | 9.556 | 8.892 | 0.664 |
| Treatment costs (discounted) (£) | 39,904 | 11,378 | 28,526 |
| Other costs (discounted) (£) | 21,406 | 24,761 | –3355 |
| Total costs (discounted) (£) | 61,310 | 36,139 | 25,171 |
| ICER: cost per QALY (£) | 37,874 | ||
| Sim12 Low price and 50% hypo effect | |||
| Life expectancy | 21.808 | 20.563 | 1.245 |
| Life expectancy (discounted) | 14.224 | 13.665 | 0.559 |
| QALYs (discounted) | 9.504 | 8.892 | 0.612 |
| Treatment costs (discounted) (£) | 35,302 | 12,611 | 22,691 |
| Other costs (discounted) (£) | 21,662 | 24,761 | –3099 |
| Total costs (discounted) (£) | 56,964 | 37,372 | 19,592 |
| ICER: cost per QALY (£) | 32,020 | ||
| Sim13 Low price and 75% hypo effect | |||
| Life expectancy | 21.842 | 20.563 | 1.279 |
| Life expectancy (discounted) | 14.241 | 13.665 | 0.576 |
| QALYs (discounted) | 9.556 | 8.892 | 0.664 |
| Treatment costs (discounted) (£) | 35,339 | 12,611 | 22,728 |
| Other costs (discounted) (£) | 21,406 | 24,761 | –3355 |
| Total costs (discounted) (£) | 56,745 | 37,372 | 19,373 |
| ICER: cost per QALY (£) | 29,151 | ||
Cost of blindness
| CSII | MDI | Difference | |
|---|---|---|---|
| Sim14 Higher cost of blindness 50% hypo | |||
| Life expectancy | 21.808 | 20.563 | 1.245 |
| Life expectancy (discounted) | 14.224 | 13.665 | 0.559 |
| QALYs (discounted) | 9.504 | 8.892 | 0.612 |
| Treatment costs (discounted) (£) | 38,097 | 12,611 | 25,486 |
| Other costs (discounted) (£) | 21,993 | 25,189 | –3196 |
| Total costs (discounted) (£) | 60,090 | 37,800 | 22,290 |
| ICER: cost per QALY (£) | 36,429 | ||
| Sim15 Higher cost of blindness 75% hypo | |||
| Life expectancy | 21.842 | 20.563 | 1.279 |
| Life expectancy (discounted) | 14.241 | 13.665 | 0.576 |
| QALYs (discounted) | 9.556 | 8.892 | 0.664 |
| Treatment costs (discounted) (£) | 38,137 | 12,611 | 25,526 |
| Other costs (discounted) (£) | 21,735 | 25,189 | –3454 |
| Total costs (discounted) (£) | 59,872 | 37,800 | 22,072 |
| ICER: cost per QALY (£) | 33,213 | ||
High-glycaemia event group
| CSII | MDI | Difference | |
|---|---|---|---|
| Sim16 High-glycaemia event 50% less | |||
| Life expectancy | 22.425 | 22.394 | 0.031 |
| Life expectancy (discounted) | 14.497 | 14.481 | 0.016 |
| QALYs (discounted) | 9.702 | 9.61 | 0.092 |
| Treatment costs (discounted) (£) | 38,778 | 13,312 | 25,466 |
| Other costs (discounted) (£) | 20,908 | 21,270 | –362 |
| Total costs (discounted) (£) | 59,686 | 34,582 | 25,104 |
| ICER: Cost per QALY (£) | 273,992 | ||
| Sim17 High-glycaemia event 75% less | |||
| Life expectancy | 22.425 | 22.394 | 0.031 |
| Life expectancy (discounted) | 14.499 | 14.481 | 0.018 |
| QALYs (discounted) | 9.772 | 9.61 | 0.162 |
| Treatment costs (discounted) (£) | 38,783 | 13,312 | 25,471 |
| Other costs (discounted) (£) | 20,494 | 21,270 | –776 |
| Total costs (discounted) (£) | 59,277 | 34,582 | 24,695 |
| ICER: cost per QALY (£) | 152,058 | ||
| Sim18 Younger cohort | |||
| Life expectancy | 25.146 | 23.498 | 1.648 |
| Life expectancy (discounted) | 15.528 | 14.854 | 0.674 |
| QALYs (discounted) | 10.357 | 9.648 | 0.709 |
| Treatment costs (discounted) (£) | 41,352 | 13,631 | 27,721 |
| Other costs (discounted) (£) | 23,558 | 27,055 | –3497 |
| Total costs (discounted) (£) | 64,910 | 40,686 | 24,224 |
| ICER: cost per QALY (£) | 34,136 | ||
| Sim 19 Greater effect upon HbA1c | |||
| Life expectancy | 22.239 | 20.226 | 2.013 |
| Life expectancy (discounted) | 14.415 | 13.505 | 0.91 |
| QALYs (discounted) | 9.633 | 8.747 | 0.886 |
| Treatment costs (discounted) (£) | 38,574 | 12,473 | 26,101 |
| Other costs (discounted) (£) | 21,204 | 25,416 | –4212 |
| Total costs (discounted) (£) | 59,778 | 37,889 | 21,889 |
| ICER: cost per QALY (£) | 24,720 | ||
Appendix 11 Patient perspectives methodology
Aims
-
To examine the benefits of pump therapy (CSII) as perceived by the parents of young children (5–8 years) with T1DM. To fill in the gaps in knowledge about users’ experiences of pump therapy.
-
To assist policy-makers with the process of defining their guidance to health-care professionals in relation to developing patient-centred therapies.
Research questions
-
Why did parents decide to use CSII and how did they obtain information?
-
How was CSII managed and to what extent has CSII appeared to affect diabetes outcome?
-
What lessons have been learned in relation to pump therapy?
-
To what extent have different clinical practices affected parental use of CSII in young children?
-
What factors appear to explain the variation in opinion about the use of CSII in young children, i.e. can we gain a better understanding as to why the previous NICE guidance74 has been implemented to varying extents in different parts of the country?
-
What are the benefits and challenges of using CSII in young children from the carers’ perspective?
Subjects and design of study
The sample of volunteer parents (nine from England and one from the USA, living in England = 10) were recruited through the patient-led support group for insulin pumps – INPUT250 – and had responded to an invitation to be interviewed.
One of the authors (AG) conducted the interviews. The clinician involved in the care of the children was not involved – we did not know, and did not ask, who they were.
An invitation was posted on the INPUT website (a patient-led support group for people using insulin pumps to control their diabetes), inviting parents to participate in a telephone interview (∼1 hour), with a qualitative researcher, to describe their experiences of having children (5 years or less), with T1DM who had transferred from MDI to CSII. Inclusion criteria were: parents with children over the age of 5 years; who had not transferred from MDI to CSII. The first 10 parents meeting these criteria were selected. The sample size was chosen as it would be large enough to generate an adequate range of themes and perspectives without creating a data set too large to analyse in depth. 290,291
Recruitment through INPUT was sought as this did not involve health-care professionals and was thought, therefore, to be less likely to influence parents’ accounts or decisions to participate in the interviews.
The interviewees are likely, therefore, to be a more motivated group and some are clearly highly organised individuals. This does not affect the validity of their comments, but it may have implications for generalisability. While we accept that this study uses advocates of this treatment, our aim was to identify the reasons why they not only chose a pump for their children, but also how they secured and managed this form of therapy. It is worth considering also that they are successful pump users and they are willing to master new technology. The remaining family (originally based in the USA) paid for the pump themselves.
We have used established methods in qualitative research methodology292,293 to obtain multiple perspectives to gain an understanding of the issues that impact on the use of CSII from the perspectives of parents with young children (< 9 years) with TIDM.
The study utilised in-depth interviews that encouraged parents to display their own understandings and meanings, and permitted hypotheses to be identified and tested during the study, which might be initially anticipated. 290,291
The interview schedule was based on the concept of ‘Strategic Conversation’294 and was designed to address the key research questions and qualitative issues (summarised in Boxes 2 and 3) while maintaining sufficient flexibility to accommodate respondents’ novel ideas.
-
History and context To determine where CSII information was accessed? How parents gained access to a pump, i.e. what were perceived to have been the major barriers and facilitators to the use of CSII for children, for example attitudinal, socioeconomic, cultural and practical? Who had been the key players? What were the lines of responsibility/accountability?
-
Managing change What changes to ways of managing diabetes were anticipated by CSII? How has change been assessed? How have parents’ and children’s experiences been addressed by health professionals and policy-makers? What were the drivers and barriers to change?
-
Measuring quality and benefits What were the expected benefits of CSII for families, health professionals and policy-makers?
-
What was understood by T1DM in children, i.e. its importance and training required?
-
What were the issues relating to equity of health care and patient choice? Was it acceptable to consider selective screening for therapy, i.e. attitudinal, socioeconomic, cultural and practical? Which groups were perceived to be most at risk with different types of therapy? How should families be approached about therapy?
-
What role do the different stakeholders play in supporting education about diabetes and implementation of therapy?
-
What are the actual and potential benefits and drawbacks of diabetes management using CSII or multiple injections in children?
Interviews addressed the historical, practical and strategic issues as well as parents’ reflections on potential for change in health services for young children with T1DM.
The study was informed by grounded theory, which involved concurrent data collection and analysis, together with systematic effects to check and refine the developing categories of data. 295,296 Team members independently reviewed all data, and regular meetings were held during and after the interview phase to explore parents’ underlying reasoning, discuss deviant cases and reach agreement on recurrent themes and findings. All parents’ transcriptions were repeatedly read through and cross-compared.
Semistructured interviews were conducted by telephone and lasted around an hour, and were audiotaped, transcribed and anonymised. The interview protocols were designed to address the key research questions and qualitative issues summarised in Boxes 2 and 3, while maintaining sufficient flexibility to accommodate respondents’ novel ideas. The protocol of the study was informed by clinical expertise and policy document review. Interviews with parents addressed historical, practical and strategic issues, as well as reflections on potential for changes in practice. Their reflections also provided an indication of clinical staff perceptions of CSII and implications for practice.
Further details of qualitative approach
The time estimated for interviews took into account the breadth or specificity of issues to be explored. Anthropological field notes were taken during and immediately after each interview. These formed the main substrate of the qualitative analysis, although interviews were also recorded for transcription to enable field note validation and extraction of representative verbatim quotations. Analysis also drew on theories of trust. 297,298 Internal validation was achieved by reviewing transcripts in order to check whether all key themes were represented in the field notes. Respondent validation was sought by reflecting key aspects of the field notes back to interview participants within 1 week of the interview. External validation and inter-rater agreement was sought by asking a collaborator to independently analyse 20% of the interview summaries in order to identify key themes and then discuss areas of agreement and disagreement with the main researcher to reach consensus.
Appendix 12 Insulin pump patient contract
The contract below is used by the Diabetes Clinic of Aberdeen Royal Infirmary.
Although insulin pump therapy can be successful, this form of treatment is time-consuming and expensive. It is also important to make sure that people on pumps continue to benefit from their use in the long term. The benefits seen with a pump are:
-
less risk of severe hypoglycaemia
-
return of early warning hypoglycaemia symptoms
-
improved glucose control – lower HbA1c level
-
better quality of life.
The reduction in HbA1c level should be at least 0.5% less than your current average level.
In order to benefit from the pump, it is extremely important that you are confident in:
-
using the technical features of the pump, including temporary basal rates
-
altering the amount of insulin given depending on the carbohydrate content of meals, exercise, etc.
-
appropriate blood glucose monitoring.
We are extremely happy to provide the education necessary to make the most of insulin pump therapy and would like to formally invite you to participate. We anticipate that you will see the benefits after 3 months and these will be sustained at least for a further 6 and 12 months. We hope to measure the benefit by checking your HbA1c levels at these times. We will also check your awareness of hypoglycaemia and use a questionnaire to assess quality of life.
If, however, the pump does not prove to be successful, it would make sense to look at alternatives including the use of multiple daily insulin injections with modern insulin.
I, the undersigned, recognise that it is important that there should be demonstrable improvement in my diabetes control by continuing to use insulin pump therapy. One way of demonstrating this would be to examine my HbA1c levels in 3, 6 and 12 months’ time to make sure that they are either ≤ 8.5% or have fallen at least 0.5% below the current level. Alternative measurements of the success of insulin pump therapy may include a return of hypoglycaemia warning symptoms, less frequent severe hypoglycaemic episodes or a better quality of life.
I understand that continued funding for the pump (including consumables) is dependent on my active participation in on-going education provided by Aberdeen Diabetes Centre and by demonstrating measurable improvements in my diabetes control. I also undertake to monitor my blood sugars at least four times a day and have been informed of the dangers of omitting to do this (risk of DKA).
| Signed: | |
| Patient | Dated: |
| Name of Patient: | |
| Signed: | |
| NHS Signatory | Dated: |
| Name of NHS Signatory: | |
Glossary
- Analogue insulins
- These are newer forms of insulin, and there are two groups: (1) short-acting insulin (SA) analogues, such as aspart and lispro, that have a faster onset and shorter duration than soluble insulin, which confers theoretical advantages, as their action is closer to that of insulin produced by the pancreas; and (2) long-acting analogues, such as glargine and detemir, which last for longer than older basal insulins, such as neutral protamine Hagedorn (NPH), and, again, have advantages, such as less hypoglycaemia at night.
- Basal insulin
- Insulin given to mimic the naturally occurring level in fasting states.
- Carbohydrate
- Sugars (such as glucose, fructose, lactose, sucrose, etc.) or molecules composed of many sugar units (such as starch). Carbohydrates are important as a source of energy in living organisms. All carbohydrates are eventually broken down to the simple sugar glucose, which can then take part in energy-producing metabolic processes.
- Continuous subcutaneous insulin infusion (CSII)
- Administration of insulin under the skin by a cannula connected to an insulin pump.
- Dawn phenomenon
- The dawn phenomenon refers to rising blood glucose levels in the hours before breakfast, partly due to the effect of the previous day’s insulin wearing off, partly to rises in levels of other hormones, notably growth hormone. It can be a problem to manage because if the previous evening’s dose of insulin is increased, hypoglycaemia may occur during the night.
- Diabetes mellitus
- Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. Insulin is secreted by specialised cells in the pancreas (pancreatic β-cells) in response to a rise in blood sugar levels. A consequence of this defect is chronic hyperglycaemia (i.e. elevated levels of plasma glucose), with disturbances of carbohydrate, fat and protein metabolism. The two most common types of diabetes are type 1 diabetes mellitus (T1DM – see below) and type 2 diabetes mellitus (T2DM – see below). There are also other less common types of diabetes mellitus (see below). Individuals with any of these conditions are considered to be diabetic.
- Type 1 diabetes mellitus (formerly ‘insulin-dependent diabetes mellitus’)
- T1DM is characterised by absolute, or nearly absolute, insulin deficiency, sudden onset of symptoms, severe elevation of blood glucose levels (hyperglycaemia), rapid acidification of the blood (see Ketoacidosis), and death unless treated with insulin. The disease may occur at any age, but onset in childhood or adolescence is most common. In most cases, T1DM is caused by the immune system attacking the cells in the pancreas that produce insulin (autoimmune destruction of pancreatic β-cells). Some signs of hyperglycaemia are a great thirst, a dry mouth and a need to urinate often.
- Type 2 diabetes mellitus (formerly ‘non insulin-dependent diabetes mellitus’)
- T2DM is characterised by relative insulin deficiency. The pancreas generally retains some ability to produce insulin, but this is insufficient for the body’s needs, and the production of insulin usually falls progressively over time, so that insulin treatment is often required. Additionally, people with this type of diabetes are often resistant to the actions of insulin. Autoimmune destruction of pancreatic β-cells does not occur and ketoacidosis is rare. T2DM is usually of slow onset and the risk of developing the disease increases with age, obesity and lack of physical activity.
- Diabetic foot
- Reduced sensation, ulcers and other impairments of the foot as a complication of diabetes, resulting from the disease causing impaired nerve function (neuropathy) and predisposing to vascular disease.
- Gestational diabetes
- Diabetes that appears during pregnancy and disappears after the birth of the baby.
- Glucose
- Physiologically, one of the most important basic sugar (carbohydrate) units. For example, starch is composed of many units of glucose.
- Hyperglycaemia
- Condition characterised by too high a level of glucose (sugar) in the blood, for example in cases when diabetes is out of control. It occurs when the body does not have enough insulin to turn glucose into energy, and/or store it, or cannot use the insulin it does have.
- Hypoglycaemia
- Abnormally low concentration of glucose in the blood, which can cause muscular weakness and inco-ordination, mental confusion and sweating. If severe it may lead to hypoglycaemic coma. Hypoglycaemia most commonly occurs in diabetes mellitus as a consequence of relative insulin excess from insulin injection or insulin secretagogue therapy, associated with insufficient intake of carbohydrate, excess energy expenditure, and/or other blood glucose-lowering agents, such as alcohol. It is treated by administration of glucose or glucagon.
- INPUT
- An organisation of patients, or their parents, who use CSII.
- Insulin
- Hormone secreted by special cells of the pancreas (pancreatic β-cells) in response to blood glucose. It is involved in regulating blood glucose levels and promotes fuel storage.
- Ketoacidosis
- Complication of diabetes resulting from critical insulin deficiency with presence of elevated blood ketones. In uncontrolled T1DM, in the absence of insulin, the body starts to break down fats for fuel. Ketone bodies are a metabolic by-product of fat metabolism and can be used as fuel by muscle and brain tissue. In diabetic ketoacidosis (DKA), ketone bodies accumulate and elevated levels can be found in blood and urine, leading to a dangerous acidification of the blood.
- Multiple daily injections
- The term used to describe an intensified form of insulin regimen based on a combination of one or two injections of long-acting (basal) insulin, with injections of short-acting insulin at mealtimes. The long-acting insulin can be the older NPH human insulin or the newer synthetic insulin analogues – glargine and detemir.
- Nephropathy
- Disease of the kidney. In diabetic nephropathy, damage to the kidneys occurs as a consequence of hyperglycaemia (see above), which induces damage of blood vessels, leading to several phenomena, including impaired blood flow. Features include increased excretion of protein in the urine, increased blood pressure and declining kidney function. Severe diabetic nephropathy can lead to kidney failure and end-stage renal disease. Individuals with end-stage disease must rely on kidney dialysis, peritoneal dialysis or kidney transplantation to survive.
- Neuropathy
- Damage to nerves. High blood glucose levels in longstanding poorly controlled diabetes can damage nerves. A complication of diabetes, in some forms of which neuropathy plays a role, is the diabetic foot (see above).
- Pancreas
- Organ located behind the stomach. The exocrine pancreas secretes enzymes important in digestion. The endocrine pancreas produces two hormones vital for carbohydrate metabolism – insulin and glucagon.
- Retinopathy
- Disease of the retina (the light-sensitive layer at the back of the eye, on to which external images are projected). In diabetes, damage to blood vessels as a consequence of diabetes may lead to, for example, haemorrhages (bleeding) and retinal detachment, thereby causing impairment or loss of vision.
List of abbreviations
- AADE
- American Association of Diabetes Educators
- ADA
- American Diabetes Association
- AETMIS
- Agence d’évaluation des technologies et des modes d’intervention en Santé
- AUC
- area under the curve
- BMI
- body mass index
- BNF
- British National Formulary
- CHF
- congestive heart failure
- CHQ-CF87
- The Child Health Questionnaire
- CI
- confidence interval
- CORE
- Center for Outcomes Research
- CSII
- continuous subcutaneous insulin infusion
- CVA
- cerebrovascular accident
- DAFNE
- Dose Adjustment for Normal Eating
- DCCT
- The Diabetes Control and Complications Trial
- DKA
- diabetic ketoacidosis
- DoH
- Department of Health
- DQoL
- Diabetes Quality of Life questionnaire
- DQoLCTQ
- Diabetes Quality of Life Clinical Trial Questionnaire
- DQoL-Y
- Diabetes Quality of Life for Youths questionnaire
- DSN
- diabetes specialist nurse
- DTSQ
- Diabetes Treatment Satisfaction Questionnaire
- EDIC
- Epidemiology of Diabetes Interventions and Complications Research Group
- EQ-5D
- European Quality of life–5 Dimensions questionnaire
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HTA
- Health Technology Assessment
- IAHS
- Institute of Applied Health Sciences
- ICER
- incremental cost-effectiveness ratio
- ICT
- insulin conventional therapy
- IHD
- ischaemic heart disease
- INPUT
- Insulin Pump Therapy (patient-led support group)
- IIT
- intensive insulin therapy
- IPTSQ
- Insulin Pump Therapy Satisfaction Questionnaire
- IQ
- intelligence quotient
- ITT
- intention to treat
- JDRF
- Juvenile Diabetes Research Foundation
- MAGE
- mean amplitude of glycaemic excursions
- MDI
- multiple daily injections
- MI
- myocardial infarction
- NICE
- National Institute for Health and Clinical Excellence
- NIH
- National Institutes of Health
- NPH
- neutral protamine Hagedorn
- NS
- not significant
- OR
- odds ratio
- PCT
- Primary Care Trust
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- ReBIP
- Review Body for Interventional Procedures Programme
- RR
- relative risk
- SA
- short-acting
- SD
- standard deviation
- SDS
- standard deviation score
- SE
- standard error
- SED
- Self-Efficacy for Diabetes Scale
- SEM
- standard error of the mean
- SF-12
- Short Form-12
- SF-36
- Short Form-36
- SH
- severe hypoglycaemic
- SSGCDY
- Scottish Study Group for the Care of Diabetes in the Young
- T1DM
- type 1 diabetes mellitus
- T2DM
- type 2 diabetes mellitus
- TA
- Technology Appraisal
- TAPQOL
- Preschool children Quality of Life questionnaire
- TAR
- Technology Appraisal Report
- UKPDS
- UK Prospective Diabetes Study
- YHPHO
- York and Humber Public Health Observatory
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
‘Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of techniques.
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Reimsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D, et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
-
Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement.
By Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al.
-
Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation.
By Wang D, Cummins C, Bayliss S, Sandercock J, Burls A.
-
Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation.
By McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al.
-
Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis.
By Simpson EL, Stevenson MD, Rawdin A, Papaioannou D.
-
Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs.
By Main C, Liu Z, Welch K, Weiner G, Quentin Jones S, Stein K.
-
Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome: a systematic review and economic analysis.
By McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, Akers J, et al.
-
Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review.
By Sutcliffe P, Hummel S, Simpson E, Young T, Rees A, Wilkinson A, et al.
-
The harmful health effects of recreational ecstasy: a systematic review of observational evidence.
By Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of oesophageal Doppler monitoring in critically ill and high-risk surgical patients.
By Mowatt G, Houston G, Hernández R, de Verteuil R, Fraser C, Cuthbertson B, et al.
-
The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technology Assessment reports.
By Taylor RS, Elston J.
-
Controlling Hypertension and Hypotension Immediately Post Stroke (CHHIPS) – a randomised controlled trial.
By Potter J, Mistri A, Brodie F, Chernova J, Wilson E, Jagger C, et al.
-
Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation.
By Pilgrim H, Lloyd-Jones M, Rees A.
-
Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation.
By Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, et al.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods.
By Hobart J, Cano S.
-
Treatment of severe ankle sprain: a pragmatic randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of three types of mechanical ankle support with tubular bandage. The CAST trial.
By Cooke MW, Marsh JL, Clark M, Nakash R, Jarvis RM, Hutton JL, et al. , on behalf of the CAST trial group.
-
Non-occupational postexposure prophylaxis for HIV: a systematic review.
By Bryant J, Baxter L, Hird S.
-
Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial.
By Farmer AJ, Wade AN, French DP, Simon J, Yudkin P, Gray A, et al.
-
How far does screening women for domestic (partner) violence in different health-care settings meet criteria for a screening programme? Systematic reviews of nine UK National Screening Committee criteria.
By Feder G, Ramsay J, Dunne D, Rose M, Arsene C, Norman R, et al.
-
Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation.
By Simpson, EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J.
-
The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost-effectiveness and natural history.
By Fortnum H, O’Neill C, Taylor R, Lenthall R, Nikolopoulos T, Lightfoot G, et al.
-
Dipsticks and diagnostic algorithms in urinary tract infection: development and validation, randomised trial, economic analysis, observational cohort and qualitative study.
By Little P, Turner S, Rumsby K, Warner G, Moore M, Lowes JA, et al.
-
Systematic review of respite care in the frail elderly.
By Shaw C, McNamara R, Abrams K, Cannings-John R, Hood K, Longo M, et al.
-
Neuroleptics in the treatment of aggressive challenging behaviour for people with intellectual disabilities: a randomised controlled trial (NACHBID).
By Tyrer P, Oliver-Africano P, Romeo R, Knapp M, Dickens S, Bouras N, et al.
-
Randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of selective serotonin reuptake inhibitors plus supportive care, versus supportive care alone, for mild to moderate depression with somatic symptoms in primary care: the THREAD (THREshold for AntiDepressant response) study.
By Kendrick T, Chatwin J, Dowrick C, Tylee A, Morriss R, Peveler R, et al.
-
Diagnostic strategies using DNA testing for hereditary haemochromatosis in at-risk populations: a systematic review and economic evaluation.
By Bryant J, Cooper K, Picot J, Clegg A, Roderick P, Rosenberg W, et al.
-
Enhanced external counterpulsation for the treatment of stable angina and heart failure: a systematic review and economic analysis.
By McKenna C, McDaid C, Suekarran S, Hawkins N, Claxton K, Light K, et al.
-
Development of a decision support tool for primary care management of patients with abnormal liver function tests without clinically apparent liver disease: a record-linkage population cohort study and decision analysis (ALFIE).
By Donnan PT, McLernon D, Dillon JF, Ryder S, Roderick P, Sullivan F, et al.
-
A systematic review of presumed consent systems for deceased organ donation.
By Rithalia A, McDaid C, Suekarran S, Norman G, Myers L, Sowden A.
-
Paracetamol and ibuprofen for the treatment of fever in children: the PITCH randomised controlled trial.
By Hay AD, Redmond NM, Costelloe C, Montgomery AA, Fletcher M, Hollinghurst S, et al.
-
A randomised controlled trial to compare minimally invasive glucose monitoring devices with conventional monitoring in the management of insulin-treated diabetes mellitus (MITRE).
By Newman SP, Cooke D, Casbard A, Walker S, Meredith S, Nunn A, et al.
-
Sensitivity analysis in economic evaluation: an audit of NICE current practice and a review of its use and value in decision-making.
By Andronis L, Barton P, Bryan S.
-
Trastuzumab for the treatment of primary breast cancer in HER2-positive women: a single technology appraisal.
By Ward S, Pilgrim H, Hind D.
-
Docetaxel for the adjuvant treatment of early node-positive breast cancer: a single technology appraisal.
By Chilcott J, Lloyd Jones M, Wilkinson A.
-
The use of paclitaxel in the management of early stage breast cancer.
By Griffin S, Dunn G, Palmer S, Macfarlane K, Brent S, Dyker A, et al.
-
Rituximab for the first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma.
By Dundar Y, Bagust A, Hounsome J, McLeod C, Boland A, Davis H, et al.
-
Bortezomib for the treatment of multiple myeloma patients.
By Green C, Bryant J, Takeda A, Cooper K, Clegg A, Smith A, et al.
-
Fludarabine phosphate for the firstline treatment of chronic lymphocytic leukaemia.
By Walker S, Palmer S, Erhorn S, Brent S, Dyker A, Ferrie L, et al.
-
Erlotinib for the treatment of relapsed non-small cell lung cancer.
By McLeod C, Bagust A, Boland A, Hockenhull J, Dundar Y, Proudlove C, et al.
-
Cetuximab plus radiotherapy for the treatment of locally advanced squamous cell carcinoma of the head and neck.
By Griffin S, Walker S, Sculpher M, White S, Erhorn S, Brent S, et al.
-
Infliximab for the treatment of adults with psoriasis.
By Loveman E, Turner D, Hartwell D, Cooper K, Clegg A
-
Psychological interventions for postnatal depression: cluster randomised trial and economic evaluation. The PoNDER trial.
By Morrell CJ, Warner R, Slade P, Dixon S, Walters S, Paley G, et al.
-
The effect of different treatment durations of clopidogrel in patients with non-ST-segment elevation acute coronary syndromes: a systematic review and value of information analysis.
By Rogowski R, Burch J, Palmer S, Craigs C, Golder S, Woolacott N.
-
Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care.
By Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al.
-
A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial.
By Gray AJ, Goodacre S, Newby DE, Masson MA, Sampson F, Dixon S, et al. , on behalf of the 3CPO study investigators.
-
Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation.
By Ara R, Pandor A, Stevens J, Rees A, Rafia R.
-
Adefovir dipivoxil and pegylated interferon alpha for the treatment of chronic hepatitis B: an updated systematic review and economic evaluation.
By Jones J, Shepherd J, Baxter L, Gospodarevskaya E, Hartwell D, Harris P, et al.
-
Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis.
By Hewitt CE, Gilbody SM, Brealey S, Paulden M, Palmer S, Mann R, et al.
-
A double-blind randomised placebo-controlled trial of topical intranasal corticosteroids in 4- to 11-year-old children with persistent bilateral otitis media with effusion in primary care.
By Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, et al.
-
The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model.
By Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R.
-
Rehabilitation of older patients: day hospital compared with rehabilitation at home. A randomised controlled trial.
By Parker SG, Oliver P, Pennington M, Bond J, Jagger C, Enderby PM, et al.
-
Breastfeeding promotion for infants in neonatal units: a systematic review and economic analysis.
By Renfrew MJ, Craig D, Dyson L, McCormick F, Rice S, King SE, et al.
-
The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation.
By Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al.
-
Rapid testing for group B streptococcus during labour: a test accuracy study with evaluation of acceptability and cost-effectiveness.
By Daniels J, Gray J, Pattison H, Roberts T, Edwards E, Milner P, et al.
-
Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Honest H, Forbes CA, Durée KH, Norman G, Duffy SB, Tsourapas A, et al.
-
The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model.
By Bond M, Mealing S, Anderson R, Elston J, Weiner G, Taylor RS, et al.
-
Gemcitabine for the treatment of metastatic breast cancer.
By Jones J, Takeda A, Tan SC, Cooper K, Loveman E, Clegg A.
-
Varenicline in the management of smoking cessation: a single technology appraisal.
By Hind D, Tappenden P, Peters J, Kenjegalieva K.
-
Alteplase for the treatment of acute ischaemic stroke: a single technology appraisal.
By Lloyd Jones M, Holmes M.
-
Rituximab for the treatment of rheumatoid arthritis.
By Bagust A, Boland A, Hockenhull J, Fleeman N, Greenhalgh J, Dundar Y, et al.
-
Omalizumab for the treatment of severe persistent allergic asthma.
By Jones J, Shepherd J, Hartwell D, Harris P, Cooper K, Takeda A, et al.
-
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-Hodgkin’s lymphoma.
By Boland A, Bagust A, Hockenhull J, Davis H, Chu P, Dickson R.
-
Adalimumab for the treatment of psoriasis.
By Turner D, Picot J, Cooper K, Loveman E.
-
Dabigatran etexilate for the prevention of venous thromboembolism in patients undergoing elective hip and knee surgery: a single technology appraisal.
By Holmes M, C Carroll C, Papaioannou D.
-
Romiplostim for the treatment of chronic immune or idiopathic thrombocytopenic purpura: a single technology appraisal.
By Mowatt G, Boachie C, Crowther M, Fraser C, Hernández R, Jia X, et al.
-
Sunitinib for the treatment of gastrointestinal stromal tumours: a critique of the submission from Pfizer.
By Bond M, Hoyle M, Moxham T, Napier M, Anderson R.
-
Vitamin K to prevent fractures in older women: systematic review and economic evaluation.
By Stevenson M, Lloyd-Jones M, Papaioannou D.
-
The effects of biofeedback for the treatment of essential hypertension: a systematic review.
By Greenhalgh J, Dickson R, Dundar Y.
-
A randomised controlled trial of the use of aciclovir and/or prednisolone for the early treatment of Bell’s palsy: the BELLS study.
By Sullivan FM, Swan IRC, Donnan PT, Morrison JM, Smith BH, McKinstry B, et al.
-
Lapatinib for the treatment of HER2-overexpressing breast cancer.
By Jones J, Takeda A, Picot J, von Keyserlingk C, Clegg A.
-
Infliximab for the treatment of ulcerative colitis.
By Hyde C, Bryan S, Juarez-Garcia A, Andronis L, Fry-Smith A.
-
Rimonabant for the treatment of overweight and obese people.
By Burch J, McKenna C, Palmer S, Norman G, Glanville J, Sculpher M, et al.
-
Telbivudine for the treatment of chronic hepatitis B infection.
By Hartwell D, Jones J, Harris P, Cooper K.
-
Entecavir for the treatment of chronic hepatitis B infection.
By Shepherd J, Gospodarevskaya E, Frampton G, Cooper, K.
-
Febuxostat for the treatment of hyperuricaemia in people with gout: a single technology appraisal.
By Stevenson M, Pandor A.
-
Rivaroxaban for the prevention of venous thromboembolism: a single technology appraisal.
By Stevenson M, Scope A, Holmes M, Rees A, Kaltenthaler E.
-
Cetuximab for the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck.
By Greenhalgh J, Bagust A, Boland A, Fleeman N, McLeod C, Dundar Y, et al.
-
Mifamurtide for the treatment of osteosarcoma: a single technology appraisal.
By Pandor A, Fitzgerald P, Stevenson M, Papaioannou D.
-
Ustekinumab for the treatment of moderate to severe psoriasis.
By Gospodarevskaya E, Picot J, Cooper K, Loveman E, Takeda A.
-
Endovascular stents for abdominal aortic aneurysms: a systematic review and economic model.
By Chambers D, Epstein D, Walker S, Fayter D, Paton F, Wright K, et al.
-
Clinical and cost-effectiveness of epoprostenol, iloprost, bosentan, sitaxentan and sildenafil for pulmonary arterial hypertension within their licensed indications: a systematic review and economic evaluation.
By Chen Y-F, Jowett S, Barton P, Malottki K, Hyde C, Gibbs JSR, et al.
-
Cessation of attention deficit hyperactivity disorder drugs in the young (CADDY) – a pharmacoepidemiological and qualitative study.
By Wong ICK, Asherson P, Bilbow A, Clifford S, Coghill D, R DeSoysa R, et al.
-
ARTISTIC: a randomised trial of human papillomavirus (HPV) testing in primary cervical screening.
By Kitchener HC, Almonte M, Gilham C, Dowie R, Stoykova B, Sargent A, et al.
-
The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: a systematic review and economic evaluation.
By Black C, Clar C, Henderson R, MacEachern C, McNamee P, Quayyum Z, et al.
-
Randomised preference trial of medical versus surgical termination of pregnancy less than 14 weeks’ gestation (TOPS).
By Robson SC, Kelly T, Howel D, Deverill M, Hewison J, Lie MLS, et al.
-
Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes.
By Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, Davies S, et al.
-
VenUS II: a randomised controlled trial of larval therapy in the management of leg ulcers.
By Dumville JC, Worthy G, Soares MO, Bland JM, Cullum N, Dowson C, et al.
-
A prospective randomised controlled trial and economic modelling of antimicrobial silver dressings versus non-adherent control dressings for venous leg ulcers: the VULCAN trial
By Michaels JA, Campbell WB, King BM, MacIntyre J, Palfreyman SJ, Shackley P, et al.
-
Communication of carrier status information following universal newborn screening for sickle cell disorders and cystic fibrosis: qualitative study of experience and practice.
By Kai J, Ulph F, Cullinan T, Qureshi N.
-
Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation.
By Burch J, Paulden M, Conti S, Stock C, Corbett M, Welton NJ, et al.
-
Development of a toolkit and glossary to aid in the adaptation of health technology assessment (HTA) reports for use in different contexts.
By Chase D, Rosten C, Turner S, Hicks N, Milne R.
-
Colour vision testing for diabetic retinopathy: a systematic review of diagnostic accuracy and economic evaluation.
By Rodgers M, Hodges R, Hawkins J, Hollingworth W, Duffy S, McKibbin M, et al.
-
Systematic review of the effectiveness and cost-effectiveness of weight management schemes for the under fives: a short report.
By Bond M, Wyatt K, Lloyd J, Welch K, Taylor R.
-
Are adverse effects incorporated in economic models? An initial review of current practice.
By Craig D, McDaid C, Fonseca T, Stock C, Duffy S, Woolacott N.
-
Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE).
By Turnbull LW, Brown SR, Olivier C, Harvey I, Brown J, Drew P, et al.
-
Bevacizumab, sorafenib tosylate, sunitinib and temsirolimus for renal cell carcinoma: a systematic review and economic evaluation.
By Thompson Coon J, Hoyle M, Green C, Liu Z, Welch K, Moxham T, et al.
-
The clinical effectiveness and cost-effectiveness of testing for cytochrome P450 polymorphisms in patients with schizophrenia treated with antipsychotics: a systematic review and economic evaluation.
By Fleeman N, McLeod C, Bagust A, Beale S, Boland A, Dundar Y, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer.
By Mowatt G, Zhu S, Kilonzo M, Boachie C, Fraser C, Griffiths TRL, et al.
-
Effectiveness and cost-effectiveness of arthroscopic lavage in the treatment of osteoarthritis of the knee: a mixed methods study of the feasibility of conducting a surgical placebo-controlled trial (the KORAL study).
By Campbell MK, Skea ZC, Sutherland AG, Cuthbertson BH, Entwistle VA, McDonald AM, et al.
-
A randomised 2 × 2 trial of community versus hospital pulmonary rehabilitation, followed by telephone or conventional follow-up.
By Waterhouse JC, Walters SJ, Oluboyede Y, Lawson RA.
-
The effectiveness and cost-effectiveness of behavioural interventions for the prevention of sexually transmitted infections in young people aged 13–19: a systematic review and economic evaluation.
By Shepherd J, Kavanagh J, Picot J, Cooper K, Harden A, Barnett-Page E, et al.
-
Dissemination and publication of research findings: an updated review of related biases.
By Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al.
-
The effectiveness and cost-effectiveness of biomarkers for the prioritisation of patients awaiting coronary revascularisation: a systematic review and decision model.
By Hemingway H, Henriksson M, Chen R, Damant J, Fitzpatrick N, Abrams K, et al.
-
Comparison of case note review methods for evaluating quality and safety in health care.
By Hutchinson A, Coster JE, Cooper KL, McIntosh A, Walters SJ, Bath PA, et al.
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NETSCC, HTA
-
Dr Andrew Cook, Consultant Advisor, NETSCC, HTA
-
Dr Peter Davidson, Director of Science Support, NETSCC, HTA
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Director of NHS Support, NETSCC, HTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NETSCC, HTA
-
Dr Ruairidh Milne, Director of Strategy and Development, NETSCC
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NETSCC, HTA
HTA Commissioning Board
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, University of Sheffield
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford?
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, Univeristy of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Professor Hywel Williams, Professor of Dermato-Epidemiology, University of Nottingham
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies & Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Ms Jane Bates, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Professor Glyn Elwyn, Primary Medical Care Research Group, Swansea Clinical School, University of Wales
-
Dr Ron Gray, Consultant Clinical Epidemiologist, Department of Public Health, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, University of Sheffield
-
Dr Jennifer J Kurinczuk, Consultant Clinical Epidemiologist, National Perinatal Epidemiology Unit, Oxford
-
Dr Susanne M Ludgate, Medical Director, Medicines & Healthcare Products Regulatory Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, Joint Director, National Collaborating Centre for Mental Health, University College London
-
Mrs Una Rennard, Service User Representative
-
Dr Phil Shackley, Senior Lecturer in Health Economics, School of Population and Health Sciences, University of Newcastle upon Tyne
-
Dr W Stuart A Smellie, Consultant in Chemical Pathology, Bishop Auckland General Hospital
-
Dr Nicholas Summerton, Consultant Clinical and Public Health Advisor, NICE
-
Ms Dawn Talbot, Service User Representative
-
Dr Graham Taylor, Scientific Advisor, Regional DNA Laboratory, St James’s University Hospital, Leeds
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Pharmaceuticals Panel
-
Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor in Child Health, University of Nottingham
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Mrs Barbara Greggains, Service User Representative
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Professor Jonathan Ledermann, Professor of Medical Oncology and Director of the Cancer Research UK and University College London Cancer Trials Centre
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Dr Lesley Wise, Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Therapeutic Procedures Panel
-
Consultant Physician, North Bristol NHS Trust
-
Professor of Psychiatry, Division of Health in the Community, University of Warwick, Coventry
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School, Coventry
-
Ms Maree Barnett, Acting Branch Head of Vascular Programme, Department of Health
-
Mrs Val Carlill, Service User Representative
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Mr Mark Emberton, Senior Lecturer in Oncological Urology, Institute of Urology, University College Hospital, London
-
Professor Steve Goodacre, Professor of Emergency Medicine, University of Sheffield
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and The London School of Medicine and Dentistry
-
Mr Paul Hilton, Consultant Gynaecologist and Urogynaecologist, Royal Victoria Infirmary, Newcastle upon Tyne
-
Professor Nicholas James, Professor of Clinical Oncology, University of Birmingham, and Consultant in Clinical Oncology, Queen Elizabeth Hospital
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Kate Radford, Senior Lecturer (Research), Clinical Practice Research Unit, University of Central Lancashire, Preston
-
Mr Jim Reece Service User Representative
-
Dr Karen Roberts, Nurse Consultant, Dunston Hill Hospital Cottages
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
-
Professor Tom Walley, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist, National Commissioning Group (NCG), London
-
Director, NHS Sustainable Development Unit, Cambridge
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Professor Mike Kelly, Director, Centre for Public Health Excellence, NICE, London
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Ms Jeanett Martin, Director of Nursing, BarnDoc Limited, Lewisham Primary Care Trust
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Miss Nicky Mullany, Service User Representative
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Ken Stein, Senior Clinical Lecturer in Public Health, University of Exeter
-
Dr Kieran Sweeney, Honorary Clinical Senior Lecturer, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Professor Margaret Thorogood, Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Caroline Stone, Programme Manager, Medical Research Council
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington