Notes
Article history
This themed issue of the Health Technology Assessment journal series contains a collection of research commissioned by the NIHR as part of the Department of Health’s (DH) response to the H1N1 swine flu pandemic. The NIHR through the NIHR Evaluation Trials and Studies Coordinating Centre (NETSCC) commissioned a number of research projects looking into the treatment and management of H1N1 influenza. NETSCC managed the pandemic flu research over a very short timescale in two ways. Firstly, it responded to urgent national research priority areas identified by the Scientific Advisory Group in Emergencies (SAGE). Secondly, a call for research proposals to inform policy and patient care in the current influenza pandemic was issued in June 2009. All research proposals went through a process of academic peer review by clinicians and methodologists as well as being reviewed by a specially convened NIHR Flu Commissioning Board.
Declared competing interests of authors
AKS has an unrestricted research grant from ResMed Ltd for the investigation of an autotitrating ventilator, which is not related to the present study.
Permissions
Copyright statement
© 2010 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2010 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Respiratory viral infections, such as influenza, are spread by droplets, an aerosol of infected material or by direct or indirect contact with contaminated surfaces. The mode of transmission and the factors influencing this are important, as they have key implications for infection control in patients and staff, and therefore pandemic planning. Droplets in the respirable range (around 5 µm) may play a significant part in transmission,1 but the role of aerosols has been questioned2 and there are few studies quantifying viral load in droplets or aerosols. An observational study3 of influenza A and influenza B in exhaled breath showed viral RNA in one-third of infected patients, and 99% of particles had a diameter of < 5 µm when sampled during tidal breathing.
While some individuals recover from seasonal or H1N1 influenza, having experienced minimal symptoms, a subgroup of high-risk patients may develop complications, including respiratory failure,4,5 and, in new more pathogenic strains, such as H5N1, respiratory insufficiency may occur in more than 50% of those affected. These patients are managed with antiviral therapy and antibiotics for secondary bacterial pneumonia, but the mainstay of management is supportive respiratory care, which includes high-flow oxygen therapy (O2) for hypoxaemic patients, and ventilatory support for those with ventilatory insufficiency. 6,7 Adjunctive therapy can include nebulised bronchodilator for patients with underlying asthma or chronic obstructive pulmonary disease (COPD), and physiotherapy is used to facilitate secretion clearance for those in whom influenza has precipitated an infective exacerbation of chronic lung disease, such as COPD, bronchiectasis or cystic fibrosis.
Coughing and sneezing patients can shed relatively large particles (> 10 µm) that travel short distances and may contaminate the bedside environment. Smaller droplets or aerosols will remain airborne for longer periods and disseminate over greater distances. 1 The definition of an aerosol varies but most authorities characterise this as consisting of droplets of < 5 µm. Some medical procedures have been termed ‘aerosol generating’, as the common feature is that they are associated with high or augmented inspiratory and expiratory tidal flows, which may increase viral dissemination but this classification is based on assumptions rather than systematic evidence. The list of aerosol-generating procedures (AGPs) differs a little from country to country but in Department of Health (DH) guidance7,8 these include bronchoscopy, intubation of the airway and invasive ventilation manoeuvres, such as open suctioning, cardiopulmonary resuscitation, non-invasive ventilation (NIV) and continuous positive airway pressure (CPAP) therapy, high-frequency oscillation ventilation, and induction of sputum. Certain other procedures, such as delivery of nebulised medication therapy and high-flow O2 are considered to be possible aerosol generators, but a lesser infective risk. 8 There is an association between some of these AGPs and an increased incidence of severe acute respiratory syndrome (SARS) in health-care workers9–11 and the risk of superspreading events on wards. 12 This has implications for the safe care of patients and risk management for nurses, doctors, physiotherapists and other health-care workers, and has provoked an ethical debate on the duty of care of health-care staff in pandemics. 13,14
Much of the evidence for the link between AGPs and increased transmission of respiratory viral infection was generated during the SARS epidemic. In Toronto and Singapore, health-care workers constituted approximately 20% of critically ill cases. Infection rates were higher in doctors and nurses carrying out endotracheal intubation [relative risk (RR) 13.29, 95% confidence interval (CI) 2.99 to 59.04, p = 0.03], while nurses caring for SARS patients receiving NIV may have been at increased risk (RR 2.23), but this finding did not reach significance (95% CI 0.25 to 21.76, p = 0.5). 9 In a case–control study of dissemination of SARS from an index case to other patients on the same ward, Yu et al. 12 showed an increased risk associated with the index patient requiring O2 or bilevel NIV. Case reports15,16 have also linked transmission of infection to nebuliser use in the index patient. However, patient variables are also likely to be important, as sicker patients who may have a higher viral load are more likely to require O2 and ventilatory support, and those with underlying asthma who require nebuliser therapy may cough more due to airway hyper-reactivity. For these reasons specific infection control precautions have been introduced for unavoidable AGPs and these include use of high-efficiency FFP3 (or N95) masks, eye protection, gowns, aprons and gloves. 8 Guidelines also suggest that AGPs should only be used if necessary, and controversy has arisen over the role of NIV. 17,18 Its use is recommended with appropriate precautions in some national guidelines,7 but not in other guidelines, and NIV use is cautioned against by some authorities. 19–21
Non-invasive ventilation (NIV) and CPAP are unlikely to have a role in acute lung injury caused by influenza or in secondary bacterial pneumonia, or in patients with multisystem failure. 17 However, NIV was used successfully in some SARS cases,22,23 and as indicated in DH guidelines,24 there is potential for NIV to reduce the need for intubation in influenza pneumonia in those with chronic respiratory disease,25 to facilitate extubation, and to widen the provision of ventilatory support outside the intensive care unit. It may also be used as a ceiling of ventilatory care in patients with COPD, congestive cardiac failure and other serious comorbidities, and to palliate symptoms in those with end-stage disease in whom ICU admission is not indicated. These indications should be set against the risks of droplet dissemination during the delivery of NIV – yet at present those risks have not been quantified.
It is also important to note that there are problems in interpreting the evidence of transmission of infection during SARS. This is because transmissibility could have been increased by an inadequate use of protective personal equipment (PPE) in early cases;11,26 NIV equipment has evolved since 2003–4, and there have been subsequent experimental studies that have investigated air flows around oxygen masks and during NIV. 27–30 These studies used human simulator models or normal subjects mimicking respiratory distress. Hui et al. 28,31 have carried out a series of experimental studies analysing particle spread from NIV and oxygen masks,32 using smoke particles as a proxy of droplets in expired air. However, human simulators may not closely reflect the behaviour of sick patients, and smoke particles are considerably smaller (< 1 µm) than droplets generated by coughing and sneezing (range 5 to > 10 µm). Therefore, the behaviour of smoke particles may not accurately represent droplet dispersion. Other workers have used a Schlieren optical visualisation technique33 to demonstrate exhaled air flows in normal subjects when coughing with and without masks. These provide useful information on expiratory flow profiles but none of the investigations has been carried out using the range of common clinical interventions defined as AGPs, analysed droplet size or studied patients with respiratory infections.
This background therefore provided the rationale of this study, the aim of which was to investigate droplet dispersion during O2, NIV and nebuliser treatment in patients with coryzal symptoms, patients with an infective exacerbation of chronic lung disease and a control group of normal subjects, to inform safe use. We reasoned that patients with a chronic exacerbation of lung disease or a coryzal infection would generate droplets regardless of the aetiology of the infection, therefore we did not specify that the infection had to be due to H1N1 or any other subtype of influenza A or influenza B. We sought to:
-
determine droplet size and concentration
-
determine geographical distribution of droplets
-
compare and contrast droplets generated during different interventions
-
examine whether modifications of treatment delivery affect droplet dissemination
-
estimate droplet decay after the intervention had ceased.
Although not classified as an AGP, we added an analysis of droplet counts and dispersion during a standardised session of chest physiotherapy in the chronic respiratory patients. This was because there was a high level of concern by physiotherapists that droplet dissemination would be considerable, thus putting these health-care workers at risk. In addition, in 40 patients admitted to the respiratory wards at the Royal Brompton Hospital NHS Foundation Trust with suspected swine flu in the first and second wave of H1N1 in 2009, all had underlying respiratory disease (predominantly cystic fibrosis and asthma) or neuromuscular disease, and required chest physiotherapy as part of their clinical management.
Chapter 2 Methods
Trial design
This was an observational trial carried out in a standard single-bedside room on a respiratory ward at the Royal Brompton & Harefield NHS Foundation Trust. The study was approved by Brompton, Harefield and NHLI Research Ethics Committee (ref. no. 09/H0708/58).
Normal subjects
Normal subjects were recruited from a departmental database of normal people aged 18 years and above. Individuals with a current illness or underlying condition were excluded.
Coryzal patients
To fulfil entry criteria these patients were individuals, aged 18 years and above, who were previously well with no underlying health condition but, within the previous 24–48 hours, had developed a pyrexia or history of pyrexia and any two of the following flu-like symptoms: sore throat, muscle aches and pains, cough and/or headache.
Patients
We recruited patients with an acute infective exacerbation of chronic respiratory disease requiring admission to a respiratory ward. Inclusion criteria: aged 18 years and above, clinically confirmed infective exacerbation and with an underlying diagnosis of asthma, cystic fibrosis, COPD, bronchiectasis or chest wall disease for which O2 and/or NIV was clinically indicated. Exclusion criteria: haemodynamic instability, partial pressure of arterial oxygen (PaO2) < 7.4 kPa, partial pressure of arterial carbon dioxide (PaCO2) > 7.5 kPa, pH < 7.34 on oxygen/NIV therapy, cognitive inability such that patient was unable to understand information sheet or that the patient was unable to breathe spontaneously for more than 4 hours.
Droplet visualisation
Droplets were detected using an optical particle sizer (Aerotrak 8220, TSI Instruments Ltd, High Wycombe, UK), which counts particles in the range 0.3 to > 10 µm within ranges of 0.3–0.5, 0.5–1.0, 1.0–3.0, 3.0–5.0, 5.0–10.0 and > 10.0 µm, with a counting efficiency of 50% ± 10% at 0.3 µm and 100% ± 10% at 0.45 µm and greater. Particles or droplets are measured in size and concentration per cubic metre by detecting the light scattered from individual droplets as they are drawn through a focused laser beam. The intensity of scattered light is a composite function of the diameter, shape and refractive index of the droplet, as well as the light wavelength and the geometry of the optical detector. A photodetector within the Aerotrak measures the amount of light each droplet scatters and records a count for each size range, for example 0.3–0.5 µm, 5–10 µm, etc. The two Aerotrak devices were calibrated before and after the series of normal subject, coryzal and patient study runs, using polystyrene latex spheres made to particle standards in each size band from 0.3–10 µm. The count efficiency of the device for droplets of 0.45 µm and larger was 100 % ± 10%, and at 0.3 µm it was 50% ± 10%. The baseline zero count assurance test using a HEPA filter was passed at a count of < 1 particle per 5 minutes at a 95% confidence level in accordance with ISO (International Organisation for Standardisation) 21501–4. Sampling flow rates of both Aerotrak devices were within 5% of tolerance when calibrated before and after the study runs.
Droplet sampling was carried out over 30 seconds, at 5-minute intervals during baseline periods, and treatment interventions with an Aerotrak detector placed at two sampling points: D1, adjacent (within 20 cm) to patient/subject mouth or treatment mask/interface, and D2, 1.0 m from subject/patient at 45 degrees in the lateral plane. The position D2 was chosen to represent a typical location of a health-care worker providing assistance to the patient. Each Aerotrak counter was zeroed using a HEPA filter before each study run. To maintain accuracy and reproducibility of measurements, the Aerotrak at D1 was placed in a fixed position on a bed table and the Aerotrak at 1.0 m was mounted on a tripod, adjusted to a height of approximately 1.52 m (5 ft) from the floor, which is equivalent to the height of the nose/mouth of an average-sized health-care worker.
Equipment
Non-invasive ventilation (NIV) was provided using a VPAP ST III (ResMed UK Ltd, Abingdon, UK) bilevel positive pressure ventilator set in spontaneous timed mode. NIV was delivered (1) using a vented full-face mask that was sized to subject (ResMed vented hospital-use face mask) or (2) using a modified circuit. The modified circuit consisted of non-vented full-face mask (ResMed non-vented hospital face mask) and a viral/bacterial filter (Intersurgical filter 1944) placed between the mask and an expiratory leak so that exhalate was filtered [modified NIV (mod NIV)]. The ventilator was started once the mask was secured on the face. In the normal subjects and the coryzal group the ventilator settings were: inspiratory positive airway pressure (IPAP) 20 cmH2O, expiratory positive airway pressure (EPAP) 5 cmH2O, with back-up rate of 15 per minute. In patients the IPAP, EPAP and back-up rate were set at clinically required levels, with oxygen entrained into the NIV circuit as clinically indicated to maintain arterial oxygen saturation of > 90%. We used a standard jet nebuliser with compressor (Actineb, Clement Clark International Ltd, Harlow, UK), which was designed to generate a droplet profile of mass mean diameter 3.3 µm, with 72% droplets < 5 µm, at average flow rate of 7 l/minute. In each nebuliser intervention this delivered 4 ml of normal saline to the normal subjects, coryzal subjects and the patients.
Interventions
Normal controls and coryzal patients
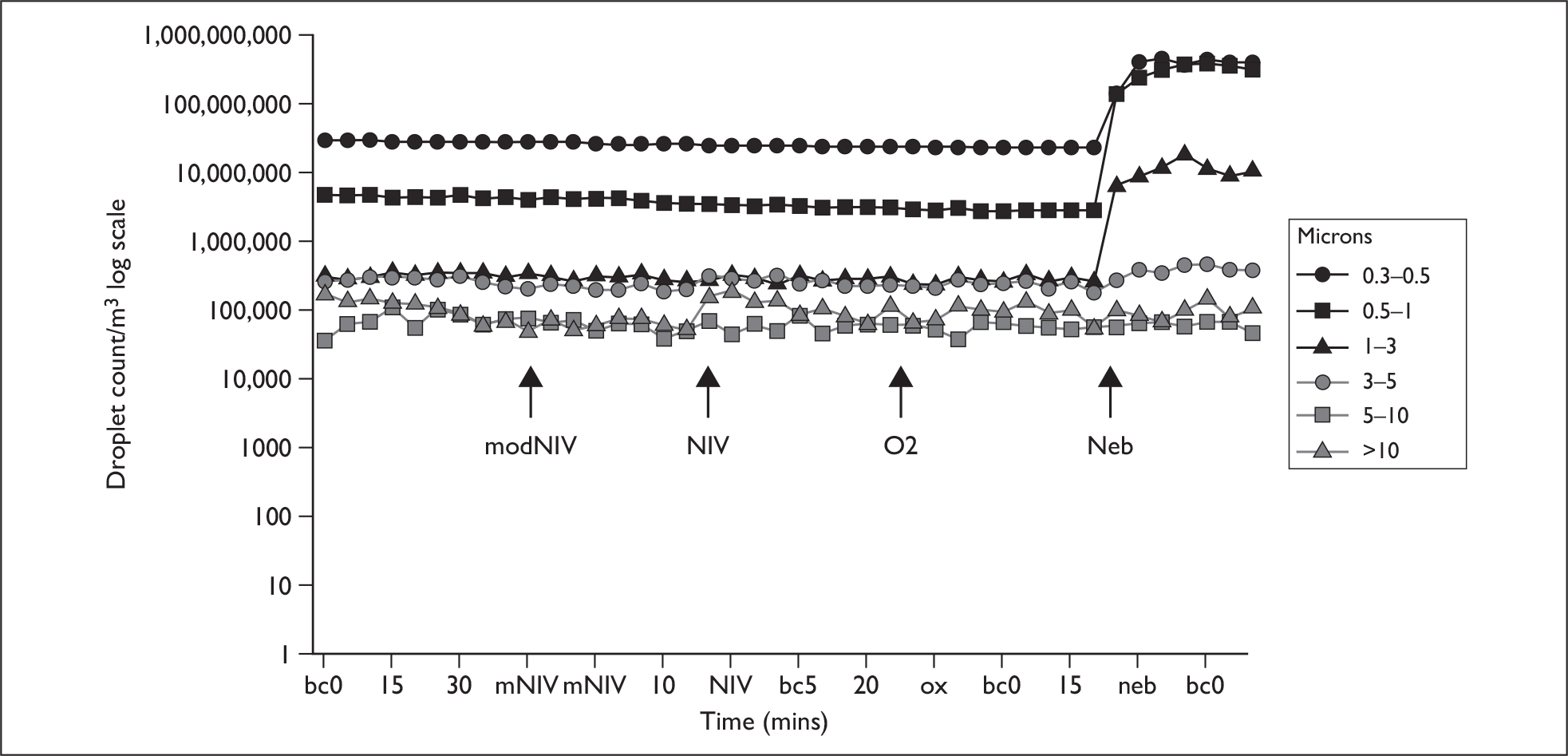
On arrival in the side room, subjects were seated in a semirecumbent position on the bed. The Aerotrak counters were aligned to the subject as described, and baseline readings of droplet counts at the two sampling positions D1 and D2 were obtained over 40 minutes, sampling at 5-minute intervals. Subjects were then asked to do a series of spontaneous coughs both without and with a surgical mask. They then received O2 via a 60% Ventimask for 20 minutes, then NIV delivered through the non-vented hospital full-face mask (ResMed) using the modified filtered circuit for 20 minutes, then NIV via standard circuit with a vented mask for 20 minutes, and, finally, 4 ml of nebulised normal saline via the mouthpiece. Between interventions there were periods of 40 minutes to allow background droplet counts to fall to baseline levels (Figure 1).
FIGURE 1.
Example of coryzal subject experimental run. bc, baseline count; mNIV, modified NIV; neb, nebuliser; ox, oxygen therapy.
Patients
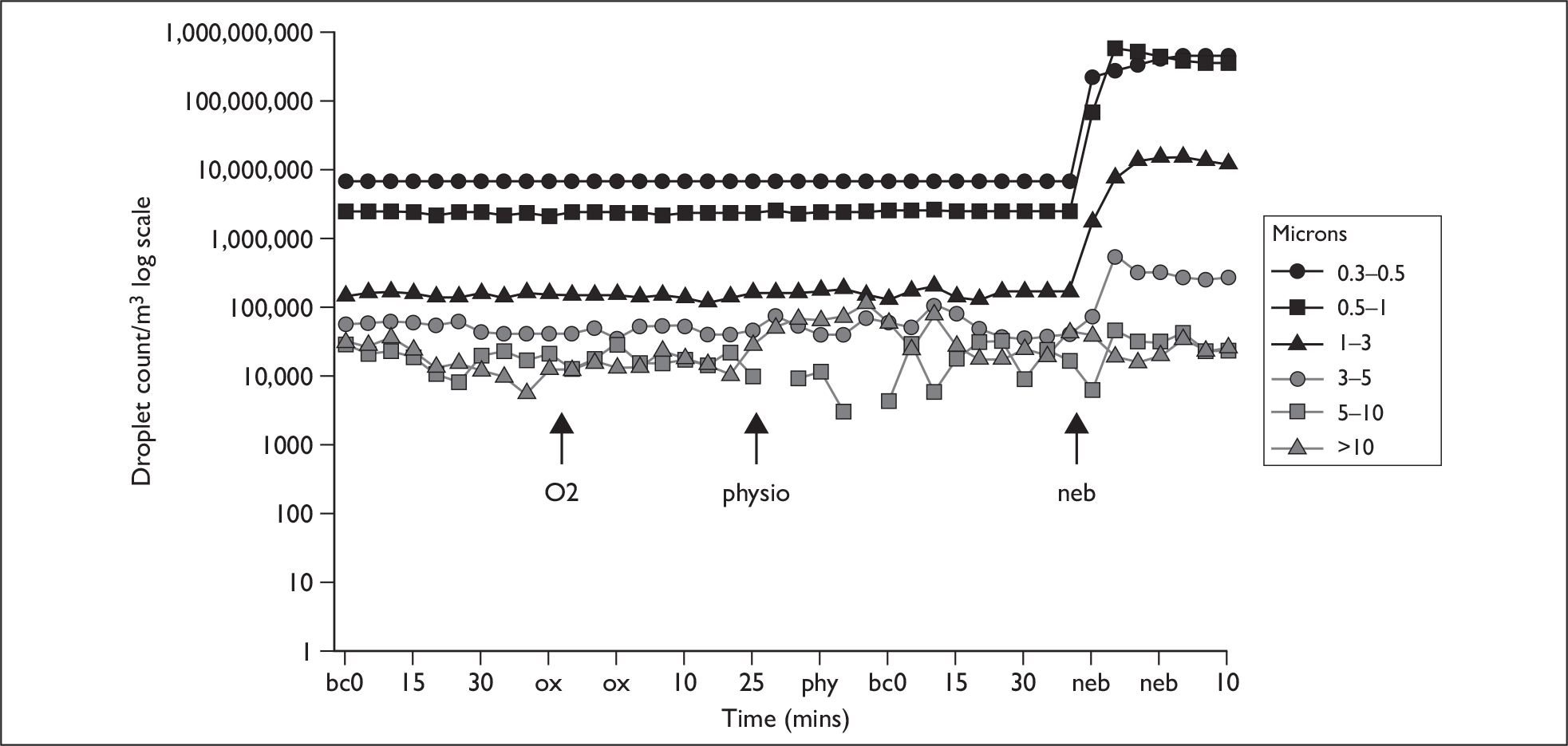
Measurements were carried out as above but with the following differences. All patients had arterial oxygen saturation (SaO2), heart rate and transcutaneous PCO2 monitored throughout the experimental interventions. After baseline measurements patients received 24% oxygen via Venturi mask for 20 minutes. Those who were using NIV received 20 minutes of ventilatory support at their current clinically indicated IPAP and EPAP settings, with oxygen entrained to maintain saturation to > 90%, first with the modified circuit and non-vented mask (2) and secondly with the vented face mask (1). The patients also underwent a standardised session of chest physiotherapy over 10 minutes. This consisted of cycles of deep breathing with percussion or shaking to loosen any secretions, followed by an assisted cough initiated manually, augmented by the physiotherapist performing inward and upwards pressure on the lower thorax to aid expectoration, after which the patient rested and cycles were repeated for 10 minutes. Throughout the study only two physiotherapists performed the physiotherapy in order to standardise the techniques as far as possible. Intervals of 40 minutes between interventions were added as in normal subjects and coryzal patients to re-establish baseline droplet levels (Figure 2).
FIGURE 2.
Example of patient experimental run. bc, baseline count; neb, nebuliser; ox, oxygen therapy.
Study area/baseline readings
A standard ward side room, of width and length 3.37 × 3.37 m and height 2.84 m, was used for all experiments. There was no external window or external ventilation system. Disturbances in the room were minimised by keeping the door shut throughout and allowing one investigator to be present. The investigator wore a surgical mask throughout, and provided the physiotherapy. The experiments were usually undertaken in two runs, in the morning and afternoon of the same day, each lasting approximately 2.5 hours. This length of time was needed in order to allow 20–40 minutes between consecutive interventions so that baseline droplet counts could be restored. Patients and subjects rested in a position of comfort in the bed throughout the interventions, and the position was not changed between the interventions in order to maintain D1 and D2 distances.
Analysis
There are few previous data on droplets generated by respiratory interventions on which to base the sample size. We reasoned that if infection is predominantly transmitted by coughing and sneezing then an increase in droplet count caused by interventions, equivalent to the increase seen from tidal breathing to spontaneous cough, would be clinically meaningful. Pilot studies suggested that a doubling in droplet size would occur in the ranges of 5–10 µm and > 10 µm, with possibly a greater increase in the aerosol range. A doubling in count of 5–10 µm or > 10 µm from a mean of 900 in 5–10 or > 10 µm range to 1800 [standard deviation (SD) 100], with a false-positive rate of 0.05 and 80% power, suggested that very small groups would be needed. We increased the patient group size to account for the possibility that counts and variability might be higher in patients and therefore aimed to study a minimum of 10 normal subjects, 10 with coryzal symptoms and 20 patients.
For each of the five interventions, droplet sampling was carried out on four occasions at 5-minute intervals before treatment, and on four occasions during the intervention, and these values were averaged to give Npre and Npost. As we were interested in the relative change due to the intervention rather than absolute values in each subject, this difference was normalised by the average of the four control samples taken before the intervention to give the normalised difference Δ or D (Npost–Npre)/Npre was calculated. The significance of this normalised difference was calculated using the two-sided Student’s t-test.
Chapter 3 Results
In total, 44 subjects and patients were studied: 12 normal controls, 11 with coryzal symptoms and 21 patients. Subject and patient characteristics are given in Tables 1–3. The patients had a range of chronic lung conditions and all had been admitted because of an acute infective exacerbation. None of the patients or coryzal individuals had an H1N1 infection. All normal subjects and 10 of the coryzal subjects completed the 60% O2, NIV, mod NIV and nebuliser therapy. One coryzal patient completed all interventions except NIV modes, as these provoked claustrophobia. All patients received physiotherapy, but normal subjects or coryzal patients did not; all patients received 24% O2 via Ventimask and nebuliser therapy. Eight patients received NIV and mod NIV, as this was indicated to manage hypercapnic respiratory failure. A total of 19 coryzal subjects and patients therefore underwent the NIV and mod NIV interventions.
| Normal subject no. | Age (years) | Trial interventions |
|---|---|---|
| 1 | 38 | O2, NIV, mod NIV, Neb |
| 2 | 35 | O2, NIV, mod NIV, Neb |
| 3 | 52 | O2, NIV, mod NIV, Neb |
| 4 | 24 | O2, NIV, mod NIV, Neb |
| 5 | 32 | O2, NIV, mod NIV, Neb |
| 6 | 52 | O2, NIV, mod NIV, Neb |
| 7 | 32 | O2, NIV, mod NIV, Neb |
| 8 | 28 | O2, NIV, mod NIV, Neb |
| 9 | 25 | O2, NIV, mod NIV, Neb |
| 10 | 24 | O2, NIV, mod NIV, Neb |
| 11 | 28 | O2, NIV, mod NIV, Neb |
| 12 | 34 | O2, NIV, mod NIV, Neb |
| Mean (SD) | 33.7 (9.6) |
| Coryzal patient no. | Age (years) | Trial interventions |
|---|---|---|
| 1 | 30 | O2, Neba |
| 2 | 24 | O2, NIV, mod NIV, Neb |
| 3 | 32 | O2, NIV, mod NIV, Neb |
| 4 | 45 | O2, NIV, mod NIV, Neb |
| 5 | 28 | O2, NIV, mod NIV, Neb |
| 6 | 37 | O2, NIV, mod NIV, Neb |
| 7 | 25 | O2, NIV, mod NIV, Neb |
| 8 | 38 | O2, NIV, mod NIV, Neb |
| 9 | 24 | O2, NIV, mod NIV, Neb |
| 10 | 28 | O2, NIV, mod NIV, Neb |
| 11 | 30 | O2, NIV, mod NIV, Neb |
| Mean (SD) | 31 (6.6) |
| Patient | Age (years) | Diagnosis | Indication for admission | Study interventions |
|---|---|---|---|---|
| 1 | 74 | Bronchiectasis | Infective exacerbation | O2, NIV, mod NIV, Neb, Physio |
| 2 | 55 | COPD | Infective exacerbation | O2, NIV, mod NIV, Neb, Physio |
| 3 | 37 | Cystic fibrosis | Infective exacerbation | O2, Physio, Neb |
| 4 | 34 | Cystic fibrosis | Infective exacerbation | O2, Physio, Neb |
| 5 | 18 | Bronchiectasis | Infective exacerbation | O2, Physio, Neb |
| 6 | 27 | Cystic fibrosis | Infective exacerbation | O2, Physio, Neb |
| 7 | 29 | Cystic fibrosis | Infective exacerbation | O2, Physio, Neb |
| 8 | 58 | Bronchiectasis | Infective exacerbation | O2, Physio, Neb |
| 9 | 62 | Bronchiectasis | Infective exacerbation | O2, Physio, Neb |
| 10 | 20 | Cystic fibrosis | Infective exacerbation | O2, Physio, Neb |
| 11 | 25 | Cystic fibrosis | Infective exacerbation | O2, Physio, Neb |
| 12 | 64 | Obesity hypoventilation syndrome | Chest infection | O2, NIV, mod NIV, Neb, Physio |
| 13 | 48 | Bronchopulmonary aspergillosis | Infective exacerbation | O2, NIV, mod NIV, Neb, Physio |
| 14 | 39 | Scoliosis | Chest infection | O2, NIV, mod NIV, Neb, Physio |
| 15 | 80 | COPD | Infective exacerbation | O2, NIV, mod NIV, Neb, Physio |
| 16 | 59 | Asthma | Infective exacerbation | O2, NIV, mod NIV, Neb, Physio |
| 17 | 58 | Bronchiectasis | Infective exacerbation | O2, NIV, mod NIV, Neb, Physio |
| 18 | 24 | Cystic fibrosis | Infective exacerbation | O2, NIV, mod NIV, Neb, Physio |
| 19 | 44 | Cystic fibrosis | Infective exacerbation | O2, Physio, Neb |
| 20 | 27 | Cystic fibrosis | Infective exacerbation | O2, Physio, Neb |
| 21 | 18 | Cystic fibrosis | Infective exacerbation | O2, Physio, Neb |
| Mean (SD) | 42.8 (19.1) |
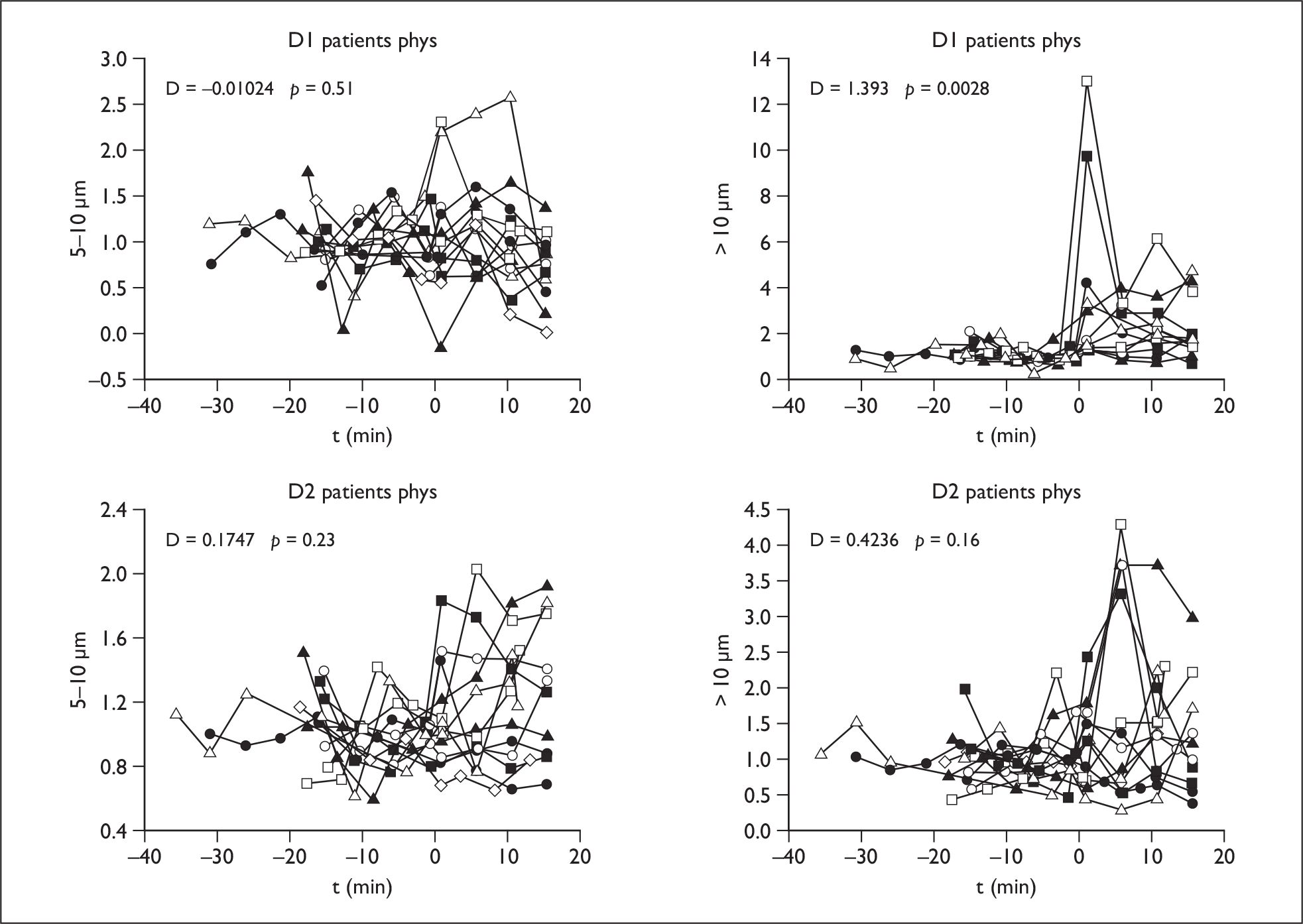
Physiotherapy
In the patients there was an increase in > 10-µm droplets at D1, but this has fallen at 1 m (D2) (p < 0.003) (Figure 3). There was no increase in the other droplet ranges.
FIGURE 3.
Physiotherapy results: droplet size > 10 µm at D1 and D2 in patients.
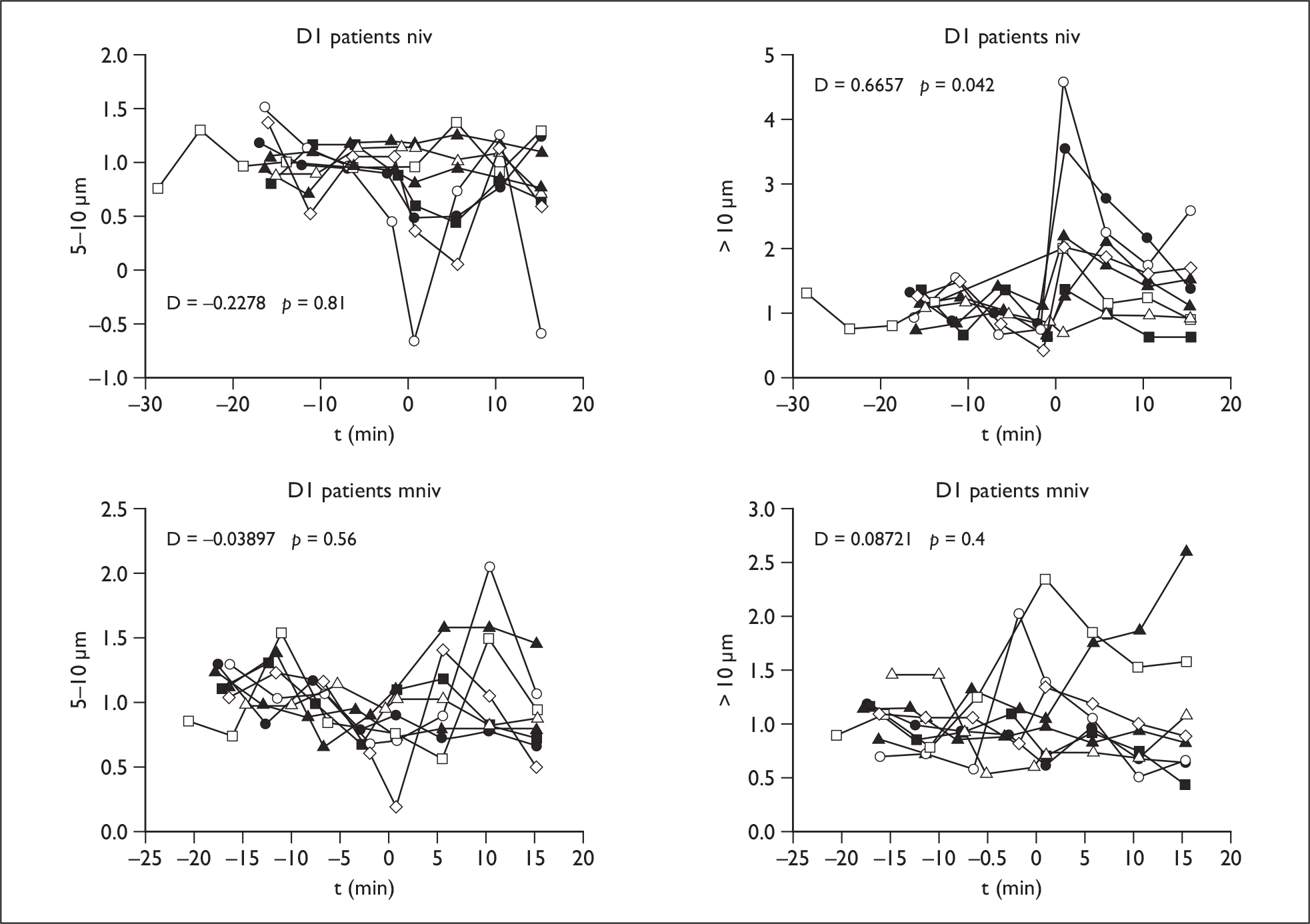
NIV using vented mask
The mean difference increased in the coryzal and patient group in the > 10-µm range at D1, but not in the normal controls, and this count was elevated at D2 in 3–5 µm, 5–10 µm and > 10-µm ranges at D2 in the coryzal subjects.
Modified NIV
Using the circuit modification the mean difference was not significantly different from baseline values on NIV in any group at D1 or D2, indicating that droplet count was significantly reduced compared with standard NIV with vented mask (Figure 4).
FIGURE 4.
Non-invasive ventilation circuit (NIV) (top row) and modified NIV results (bottom row) in patients at D1 in ranges 5–10 µm and > 10 µm.
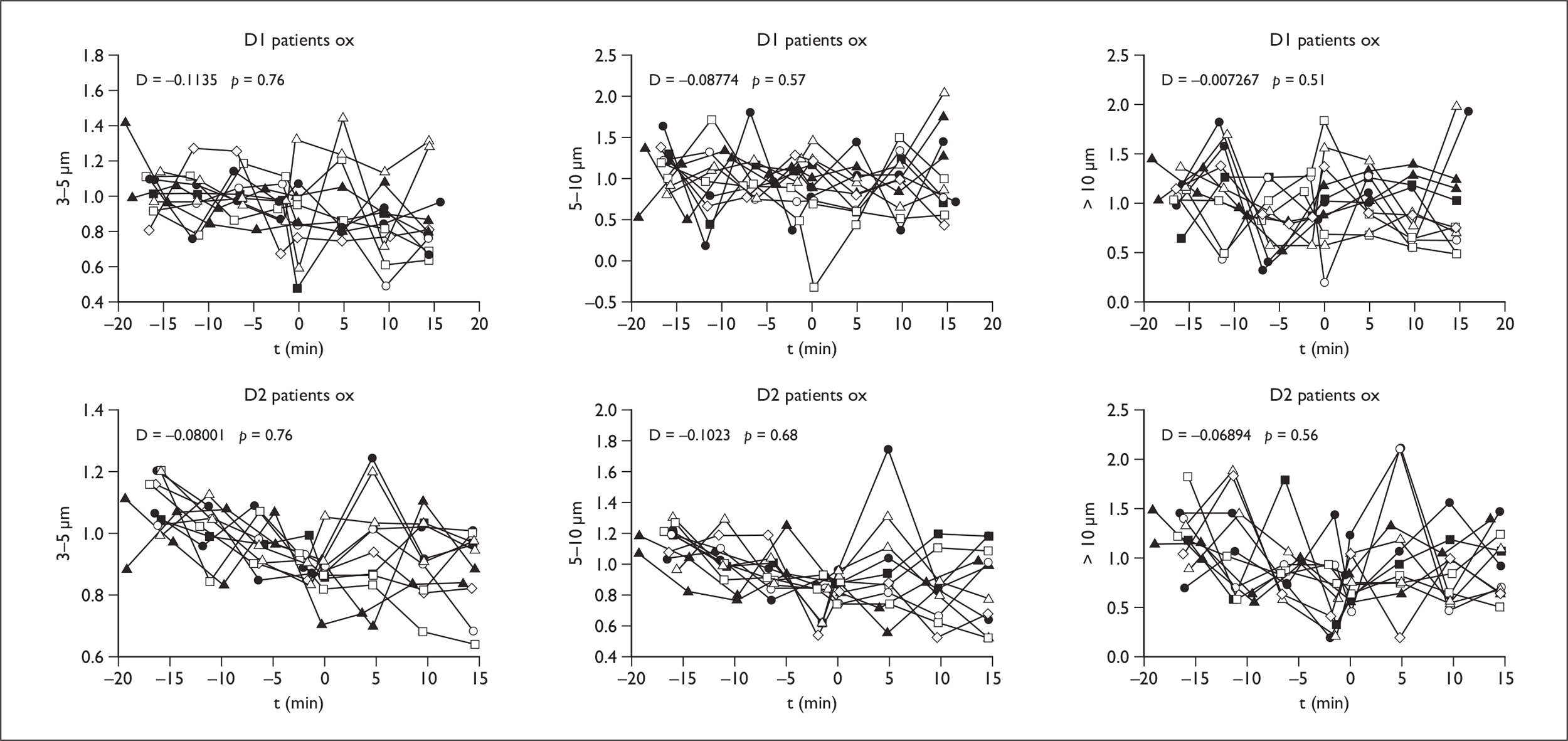
Oxygen therapy
In normal controls, the coryzal group and in patients no significant increase in droplets in aerosol or large droplet range was seen either at D1 or D2 (Figure 5).
FIGURE 5.
Oxygen results in patients at D1 and D2 in droplet ranges 3–5, 5–10 and > 10 µm.
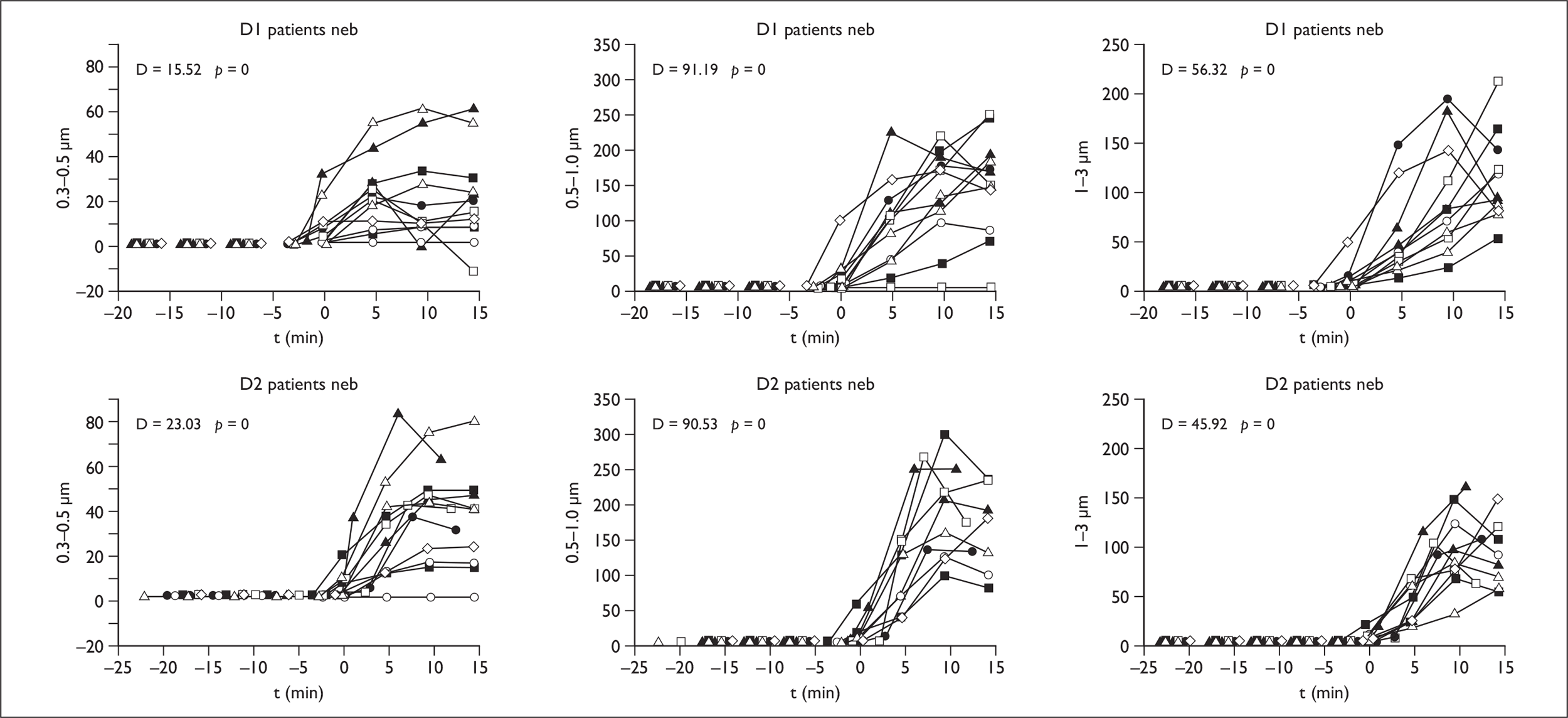
Nebuliser therapy
In all groups there was a significant increase across all droplet size ranges on therapy at D1 and D2 in normal subjects. In coryzal subjects and patients there were increases in the 0.3–0.5, 0.5–1, 1–3 and 3.5-µm aerosol ranges both at D1 and D2, but no significant mean difference in the larger droplet ranges of 5–10 and > 10 µm (Figure 6).
FIGURE 6.
Nebuliser results in patients at D1 and D2 in droplet ranges 0.3–0.5 to 1–3 µm.
Mean differences and p-values for all interventions in each group are given in Table 4.
| Microns | D1 | 0.3–0.5 | 0.5–1 | 1–3 | 3–5 | 5–10 | > 10 | D2 | 0.3–0.5 | 0.5–1 | 1–3 | 3–5 | 5–10 | > 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NIV | ||||||||||||||
| Normal | D | –0.097 | –0.103 | 0.096 | –0.073 | –0.108 | 0.148 | –0.062 | –0.065 | –0.057 | –0.013 | 0.028 | 0.100 | |
| p | 0.0991 | 0.992 | 0.863 | 0.653 | 0.636 | 0.379 | 0.968 | 0.938 | 0.873 | 0.544 | 0.449 | 0.407 | ||
| Patient | D | –0.003 | 0.004 | 0.021 | 0.143 | –0.228 | 0.666 | –0.002 | –0.002 | 0.027 | 0.067 | 0.165 | 0.184 | |
| p | 0.565 | 0.412 | 0.400 | 0.231 | 0.806 | 0.042 | 0.470 | 0.298 | 0.298 | 0.308 | 0.243 | 0.339 | ||
| Coryzal | D | –0.038 | –0.068 | –0.043 | 0.175 | –0.060 | 0.807 | –0.045 | –0.055 | 0.036 | 0.233 | 0.422 | 0.574 | |
| p | 0.894 | 0.912 | 0.639 | 0.143 | 0.552 | 0.044 | 0.908 | 0.909 | 0.238 | 0.047 | 0.018 | 0.052 | ||
| Mod NIV | ||||||||||||||
| Normal | D | –0.071 | –0.073 | –0.054 | –0.078 | –0.084 | –0.086 | –0.073 | –0.081 | –0.089 | –0.114 | –0.125 | 0.057 | |
| p | 0.971 | 0.963 | 0.703 | 0.703 | 0.690 | 0.588 | 0.975 | 0.970 | 0.963 | 0.836 | 0.734 | 0.431 | ||
| Patient | D | –0.020 | 0.005 | 0.027 | 0.013 | –0.039 | 0.087 | –0.018 | –0.017 | –0.003 | –0.014 | –0.005 | 0.236 | |
| p | 0.829 | 0.437 | 0.393 | 0.463 | 0.558 | 0.402 | 0.776 | 0.687 | 0.521 | 0.545 | 0.513 | 0.244 | ||
| Coryzal | D | –0.024 | –0.026 | –0.011 | –0.147 | –0.208 | 0.151 | –0.016 | –0.037 | –0.092 | –0.125 | –0.153 | 0.011 | |
| p | 0.776 | 0.774 | 0.539 | 0.861 | 0.770 | 0.240 | 0.671 | 0.815 | 0.857 | 0.813 | 0.745 | 0.486 | ||
| Oxygen | ||||||||||||||
| Normal | D | –0.063 | –0.083 | –0.041 | –0.003 | –0.234 | –0.013 | –0.050 | –0.057 | –0.062 | –0.049 | –0.077 | 0.081 | |
| p | 0.961 | 0.942 | 0.572 | 0.502 | 0.576 | 0.519 | 0.947 | 0.902 | 0.847 | 0.709 | 0.713 | 0.400 | ||
| Patient | D | –0.003 | –0.009 | –0.023 | –0.114 | –0.068 | –0.007 | –0.011 | –0.027 | –0.051 | –0.090 | –0.102 | –0.069 | |
| p | 0.578 | 0.636 | 0.577 | 0.748 | 0.565 | 0.507 | 0.728 | 0.789 | 0.736 | 0.764 | 0.685 | 0.554 | ||
| Coryzal | D | –0.047 | –0.067 | –0.075 | –0.108 | –0.171 | –0.012 | –0.037 | –0.045 | –0.032 | –0.001 | 0.004 | 0.058 | |
| p | 0.955 | 0.913 | 0.773 | 0.750 | 0.732 | 0.511 | 0.905 | 0.846 | 0.699 | 0.503 | 0.489 | 0.429 | ||
| Physio | ||||||||||||||
| Patient | D | –0.005 | 0.057 | 0.123 | 0.128 | –0.010 | 1.393 | –0.011 | 0.024 | 0.070 | 0.169 | 0.175 | 0.424 | |
| p | 0.610 | 0.118 | 0.164 | 0.260 | 0.511 | 0.003 | 0.702 | 0.206 | 0.151 | 0.134 | 0.228 | 0.158 | ||
| Nebuliser | ||||||||||||||
| Normal | D | 15.660 | 109.480 | 71.681 | 27.054 | 404.932 | 2.270 | 25.878 | 87.932 | 46.887 | 1.549 | 0.232 | 0.207 | |
| p | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.120 | 0.270 | ||
| Patient | D | 15.516 | 91.193 | 56.320 | 3.967 | 0.426 | 0.253 | 23.080 | 90.576 | 45.920 | 1.642 | 0.149 | 0.309 | |
| p | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.111 | 0.261 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.241 | 0.281 | ||
| Coryzal | D | 11.204 | 64.822 | 38.341 | 1.871 | 0.197 | 0.349 | 17.994 | 49.458 | 30.454 | 1.144 | 0.234 | 0.384 | |
| p | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.229 | 0.192 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.097 | 0.133 | ||
Chapter 4 Discussion
The results suggest that NIV using a vented mask in patients with an acute exacerbation of chronic lung disease disseminates large droplets locally. However, at a distance of 1 m the count has fallen significantly. There was no evidence of the generation of small droplets, i.e. an aerosol. Coryzal subjects also produced large droplets that spread for at least 1 m, which indicates that those with rhinorrhoea/upper airway inflammation also generate droplets. This group might be more representative of patients with an early progressive viral infection who are unlikely to produce large volumes of infected sputum when compared with those with cystic fibrosis or bronchiectasis. However, we did not see a difference in counts in those with markedly productive coughs compared with those with minimal sputum production (asthma, obesity hypoventilation) on the day of study. The large droplet count proximal to the mask was significantly reduced in both the patients and coryzal group in the NIV circuit with exhalation port filter, indicating that this modification minimises large droplet dissemination. These filters do not appear to increase the work of breathing if changed regularly. The finding that bilevel NIV with a vented mask disseminates large droplets is in keeping with the superspreading episodes seen in the SARS outbreak, where NIV use was found to be a risk factor on multiple logistic regression analysis. 12
Physiotherapy has not previously been included in the list of interventions in which PPE and FFP3 masks are indicated. Indeed the results show that it is not an AGP but, perhaps not surprisingly, given that the point of chest physiotherapy is to clear secretions, there was a significant increase in large droplets. As expected, these levels have dropped by D2 but the findings indicate that use of full PPE may be prudent for physiotherapists and respiratory therapists carrying out these procedures in patients with chronic respiratory disease in whom the H1N1 virus has generated an infective exacerbation or secondary bacterial pneumonia.
Oxygen therapy was not associated with an increase in droplets in any group, in any aerosol or droplet range. While it may be possible that 24% O2 might be an insufficient flow rate to shear droplets from the upper airway or disseminate those generated by spontaneous coughing or sneezing, we did not see an increase in droplet count in coryzal subjects who used 60% O2 – a flow rate more typical of that required by patients with acute lung injury due to viral pneumonia. These results should be contrasted with those from Yu et al. ,12 who showed O2 was a significant risk factor for superspreading events. However, their results were based on correlation rather than direct measurement of droplet densities and may be affected by the fact that sicker patients with higher viral loads are more likely to require O2 than those with milder disease who do not.
The association of spread of SARS with nebuliser use is controversial. Although there are case reports,15,16 in this study it is not possible to separate out droplets generated by the nebuliser itself from those generated by the patient. In addition, in clinical practice, patients being treated with nebulised bronchodilator are likely to have air flow obstruction due to asthma or COPD and are therefore more likely to be coughing and wheezing spontaneously. It is plausible that the flow from the nebuliser (either powered by a compressor or oxygen) would disseminate spontaneously generated droplets further. It is notable that the nebuliser was the only intervention that produced in droplets in the aerosol range (< 5 µm). This is entirely in line with the droplet range designed to be generated by this device, and means that this intervention also acts as a quality control confirming that the Aerotrak counters were fully able to detect particles in this range in clinical circumstances. However, in both the coryzal group and the patients we did not detect droplets in the 5- to 10-µm and > 10-µm ranges as occurred during NIV and physiotherapy. This indicates that the vast majority of droplets are likely to be nebulised saline as opposed to patient droplet secretions.
Limitations
We have used droplets as a proxy for viral dissemination, so we do not know whether an increase in droplet count confers an increased risk of infection for an exposed individual, although we believe this to be biologically plausible. This inference can be confirmed only in viral sampling studies in individuals with influenza, SARS, tuberculosis or other airborne pathogens. This further work would be valuable.
Furthermore, the patients had infective exacerbations of chronic lung disease and the pathology of this is completely different to the acute lung injury that is seen in young patients with normal lungs that are infected with H1N1 or H5N1 influenza. NIV is not indicated in patients with rapidly progressive acute lung injury, although in those with milder disease, and if used earlier in the course of the illness, it might have a role. Emerging evidence suggests that selected cases of H1N1 pneumonia worldwide were treated with NIV with variable results. 34,35 We believe, however, that the group with chronic lung disease and infectious exacerbations is the most likely to benefit from NIV, and the coryzal group used in this study may reflect airway secretion levels in viral pneumonia patients more closely. However, coryzal patients are clearly less unwell, less dyspnoeic and their lung compliance is likely to be near normal. This is relevant as decreased lung compliance enhanced the dispersion of smoke particles in the human simulator model. 36
We sampled droplets at two points – proximal to the subject’s nose/mouth/mask and at a 1-m distance. As Hui et al. 31 have shown in smoke particle experiments, flow from mask vents and leaks creates a high to low density vortex, and it is possible that we missed important sampling areas. In order to minimise this risk we used the information gained from those studies to site D1, the point of maximum density demonstrated by Hui et al. ,31 and placed D2 counter at 1 m, as DH guidelines suggest that health-care workers beyond this distance may use surgical masks as risk of transmission lower. Additionally, we placed D2 at a height of approx 1.52 m (5 ft) equivalent to the nose level of average health-care worker standing 1 m from the patient.
The experiments were carried out in a single room and we minimised disturbances, such as door opening and ventilation, to control the number of variables. Ventilation and air currents are likely to have a significant effect on small size droplets and aerosols, and, indeed, we saw a continued small fall in background count through interventions, which contributes to the mean differences seen in Table 4. However, the main impact of treatments (apart from nebuliser) was on large droplets, which, due to greater mass and terminal velocity, will be less affected by air currents.
We have carried out a series of comparisons and have expressed results as mean difference and p-values. In the discussion we have used p-values of < 0.05 to express significance. It could be argued that adjustments should be made for multiple comparisons. However, we believe the interventions to be independent, and, if comparisons are reduced by either considering one intervention at a time or pooling large versus small droplets, similar conclusions will be reached. We believe, on balance, that it is important to interpret the data erring on the side of caution with respect to risk of dissemination,37 and that these inferences are clinically plausible.
Chapter 5 Conclusions
Despite the limitations, this study indicates that NIV, O2 and physiotherapy are not AGPs. Physiotherapy and NIV generate large droplets adjacent to the patient, but these fall significantly at 1 m from the patient. A mod NIV circuit using a non-vented mask and filtered exhalate reduces the number of large droplets produced. Nebulised saline delivered by a mouthpiece produces an aerosol of droplets, but most are in the expected droplet range for the device and large droplets were not seen in patients and coryzal subjects. O2 at 60% and 24% did not appear to be an aerosol or droplet-generating procedure.
What are the implications for clinical practice and infection control? These results imply that during NIV and physiotherapy use of full PPE should be considered for health-care team members working within 1 m of the patient, as droplet count is increased. As the droplets are large and many drop out within 1 m over bedside surfaces, the crucial importance of handwashing and decontamination of near surfaces is evident, as transmission in these circumstances is likely to be direct via droplet spread or from fomites and direct contact with the patient’s local environment. As small and aerosolised particles were not demonstrated, the role other protective measures, such as negative pressure rooms, which have been advocated in some pandemic flu guidelines, may be less important.
Recommendations for further research:
-
Droplet sampling should be carried out in patients with pandemic influenza to confirm that droplets generated in this situation are comparable to those produced by patients in this study.
-
Droplet sampling sizing could be carried out in human simulator models with laser droplet imaging to corroborate results.
-
Viral carriage in different size droplets should be assessed to test whether using droplets as a proxy of infectivity risk is a realistic clinical substitute.
Acknowledgements
With thanks also to Winston Banya, Senior Statistician, Research Department, and Professor Paul Cullinan, Occupational Medicine Department, Royal Brompton Hospital, who provided statistical advice. We are grateful to the NIHR HTA panel for suggesting the addition of the coryzal group to study design.
The project was supported by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London.
Contribution of authors
AK Simonds, Consultant and Reader in Respiratory Medicine, Royal Brompton & Harefield NHS Foundation Trust, designed the study, wrote the grant application, analysed results with KP, JS and RD, and wrote the report.
A Hanak, Senior Respiratory Support Technician and Physiotherapist, Royal Brompton & Harefield NHS Foundation Trust, carried out the experiments, including performing physiotherapy, and created and maintained the database.
M Chatwin, Consultant Physiotherapist, Royal Brompton & Harefield NHS Foundation Trust, carried out the experiments, including performing physiotherapy, performed pilot studies and recruited patients.
MJ Morrell, Reader in Sleep Physiology, National Heart & Lung Institute, Imperial College, contributed to study design and critical analysis of results.
A Hall, Consultant in Microbiology & Infection Control Lead, Royal Brompton & Harefield NHS Foundation Trust, contributed to study design, provided infection control and microbiology advice, and critical analysis of results.
KH Parker, Professor Physiological Fluid Mechanics (Emeritus) Department of Bioengineering, Imperial College, provided guidance on droplet characterisation and mathematical analysis, analysed results, provided statistical advice and critical review, and contributed to writing the report and figures.
JH Siggers, Lecturer, Department Bioengineering, Imperial College, advised on study design, mathematics of droplet analysis, critical review of results and contributed to writing the report.
RJ Dickinson, Senior Lecturer, Department of Bioengineering, Imperial College, advised on study design, droplet analysis, critical review of results and contributed to writing the report.
Disclaimers
The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the Department of Health.
References
- Brankston G, Gitterman L, Hiriji Z, Lemieux C, Gardam M. Transmissin of influenza A in human beings. Lancet Infect Dis 2007;7:257-65.
- Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis 2006;12:1657-62.
- Fabian P, McDevitt JJ, DeHaan WH, Fung ROP, Cowling BJ, Chan KH, et al. Influenza virus in human exhaled breath: an observational study. PLoS ONE 2008;3.
- The ANZIC. Influenza Investigators. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 2009;361:1925-34.
- Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalised patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med 2009;361:1935-44.
- Department of Health . Pandemic Flu: A National Framework for Responding to an Influenza Pandemic 2009.
- Department of Health . Pandemic Influenza: Managing Demand and Capacity in Healthcare Organisations (surge) 2009.
- Department of Health and Health Protection Agency . Pandemic (H1N1) 2009 Influenza. A Summary of Guidance for Infection Control in Healthcare Settings 2009.
- Fowler RA, Guest CB, Lapinsky SE, Sibbald WJ, Louie M, Tang P, et al. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med 2004;169:1198-202.
- Scales DC, Green K, Chan AK, Poutanen SM, Foster D, Nowak K, et al. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerg Infect Dis 2003;9:1205-10.
- Lau JTF, Fung KS, Wong TW, Kim JH, Wong E, Chung S, et al. SARS transmission among hospital workers in Hong Kong. Emerg Infect Dis 2004;10:280-6.
- Yu IT, Xie Z, Tsoi K. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards and not others?. Clin Infect Dis 2007;44:1017-25.
- Sokol DK. Virulent epidemics and scope of healthcare workers’ duty of care. Emerg Infect Dis 2006;12:1238-41.
- Simonds AK, Sokol DK. Lives on the line? Ethics and practicalities of duty of care in pandemics and disasters. Eur Respir J 2009:1-7.
- Lee N, Hui DS, Wu A, Chan P, Cameron P, Joynt GM, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 2003;348:1986-94.
- Wong RS, Hui DS. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis 2004;10:339-41.
- Simonds AK, Simonds AK. Non-invasive respiratory support: a practical handbook. London: Hodder Arnold; 2007.
- McCracken J. Should noninvasive ventilation be considered a high-risk procedure during an epidemic?. CMAJ 2009;181:663-4.
- World Health Organization (WHO) . Clinical Management of Human Infection With Influenza A (H5N1) Virus 2007.
- Gomersall CD, Tai DYHT, Derrick JL, Goh MS, Buckley TA, Chua C, et al. Expanding ICU facilities in an epidemic: recommendations based on experience from SARS epidemic in Hong Kong and Singapore. Intensive Care Med 2006;32:1004-13.
- World Health Organization (WHO) Interim Guidelines . Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Diseases in Health Care 2007.
- Cheung TMT, Yam LYC, So LKY, Lau ACW, Poon E, Kong BMH, et al. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest 2004;126:845-50.
- Yam LYC, Chan AYF, Cheung TMT, Tsui ELH, Chan JCK, Wong VCW. Non-invasive versus invasive mechanical ventilation for respiratory failure in severe acute respiratory syndrome. Chin Med J 2005;118:1413-21.
- Department of Health . Pandemic Influenza: Surge Capacity and Prioritisation in Health Services 2008.
- Confalonieri M, Potena A, Carbone G, Della Porta R, Tolley E, Meduri GU. Acute respiratory failure in patients with severe community-acquired pneumonia. A prospective randomised evaluation of noninvasive ventilation. Am J Respir Crit Care Med 1999;160:1585-91.
- Seto WH, Tsang D, Yung RW, Ching TY, Ng TK, Ho M, et al. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet 2003;361:1519-20.
- Somogyi R, Vesely AE, Azami T, Preiss D, Fisher J, Fowler RA. Dispersal of respiratory droplets with open vs closed oxygen delivery masks: implications for transmission of severe acute respiratory syndrome. Chest 2004;125.
- Hui DS, Hall SD, Chan MTV, Chow BK, Tsou JY, Joynt GM, et al. Noninvasive positive-pressure ventilation: an experimental model to assess air and particle dispersion. Chest 2006;130:730-40.
- Ip M, Tang JW, Hui DS, Wong ALN, Chan MTV, Joynt GM, et al. Airflow and droplet spreading around oxygen masks: a simulation model for infection control research. Am J Infect Control 2007;35:684-9.
- Mardimae A, Slessarev M, Han J, Sasano H, Sasano N, Azami T, et al. Modified N95 mask delivers high inspired oxygen concentrations while effectively filtering aerosolized microparticles. Ann Emerg Med 2006;48:391-9.
- Hui DS, Chow BK, Ng SS, Chu LCY, Hall SD, Gin T, et al. Exhaled air dispersion distances during non-invasive ventilation via different Respironics facemasks. Chest 2009;136:998-1005.
- Hui DS, Hall SD, Chan MTV, Chow BK, Ng SS, Gin T, et al. Exhaled air dispersion during oxygen delivery via a simple oxygen mask. Chest 2007;132.
- Tang JW, Liebner TJ, Craven BA, Settles GS. A Schlieren optical study of the human cough with and without wearing masks for aerosol infection control. J R Soc 2009:S727-36.
- Rello J, Rodriguez A, Ibanez P, Socias L, Cebrian J, Marques A, et al. Intensive care of adult patients with severe respiratory failure caused by influenza A (H1N1) in Spain. Critical Care 2009;13.
- Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2010;302:1872-9.
- Hui DS, Chow BK, Chu LCY, Ng SS, Hall SD, Gin T, et al. Exhaled air and aerosolized droplet dispersion during application of a jet nebuliser. Chest 2009;135:648-54.
- Perneger TV. What’s wrong with Bonferroni adjustments. BMJ 1998;316:1236-8.
Appendix 1 Protocol
Evaluation of droplet dispersion during NIV, O2 and nebulised drug delivery in clinical practice
-
Project management members:
-
– Chief investigator Dr Anita K Simonds, Academic Department Sleep & Breathing, Royal Brompton Hospital, Sydney Street, London SW3 6NP, 020 73518911
-
– Co-investigators Dr R Dickinson and Dr J Siggers, Bioengineering Department, IC, Dr Michelle Chatwin & Dr Anne Hall RBH, Dr M Morrell, IC
-
– Statistician Mr Winston Banya
-
– Project manager Dr Michelle Chatwin, 020 73518911
-
– Key contact Dr Anita K Simonds (A.Simonds@rbht.nhs.uk)
-
-
Funder: NIHR HTA
-
Sponsor: Royal Brompton & Harefield NHS Foundation Trust
-
Contact: Wendy Butcher, R&D Department, Royal Brompton Hospital
Introduction
Background
Influenza viruses are spread by droplets, but aerosols may be implicated. While many patients recover without serious illness, some with H1N1 swine flu will develop pneumonia/respiratory insufficiency requiring treatment by oxygen therapy (O2), ventilatory support or nebulised drugs, and this is more likely in those with underlying respiratory or cardiac disorders, for example chronic obstructive pulmonary disease (COPD), asthma, cystic fibrosis, genetic susceptibility, pregnancy or if the virus mutates. These therapies may generate droplets or aerosol during delivery, which, in the severe acute respiratory syndrome (SARS) outbreak, were associated with an increased incidence of infection in health-care workers (Fowler et al. 2004)9 and superspreading events on hospital wards (Yu et al. 2007). 24 Non-invasive ventilation (NIV) is unlikely to be effective in patients with overwhelming acute lung injury, but in early pneumonia and in those in whom influenza has caused an exacerbation of COPD or heart failure NIV may be effective in reducing the need for intensive care unit (ICU) admission. Currently, use of NIV in pandemic flu is controversial. Department of Health Pandemic Influenza guidance recommends that NIV should be used with full infection control (aerosol-generating) precautions by experienced units employing practice guidelines that have been developed by our team (Simonds 2007),25 but there is no substantive evidence base and NIV use is not advocated in other national guidelines. Hui et al. (2006) carried out studies of NIV droplet distribution using a patient simulator and smoke particles, but there have been no systematic studies in humans or during oxygen and nebuliser therapy, or physiotherapy.
Rationale for current study
This research should provide the first analysis of droplet distribution around respiratory therapies in clinical circumstances that are relevant to H1N1 infection. Although the patients with chronic respiratory disease will not specifically have an exacerbation triggered by H1N1 influenza in this study, the results should be representative of any acute exacerbation and we will also study those with coryzal symptoms, some of whom may have H1N1 infection. The findings should enable health-care professionals to understand patterns of geographical distribution of respirable droplets when caring for patients, inform selection of circuitry and interfaces to reduce dissemination, and by modelling the profile of decay of particles after therapy we hope to guide health-care workers’ entry into rooms of unstable patients.
Impact on practice
As this is the first analysis of distribution of droplets during NIV, O2 and nebuliser therapy in representative clinical circumstances, the results obtained should influence clinical practice and policy immediately by:
-
informing the choice of interface/delivery systems
-
guiding health-care workers to safer application in pandemic flu and enable them to understand relative risks
-
reducing the risk of dissemination to other patients and staff in superspreading events
-
wider, safe use of NIV may reduce ICU bed pressures, as NIV may be performed in respiratory ward areas/high-dependency single rooms.
Study objectives
The key objective is to understand the characteristics of droplet and aerosol dispersion around delivery systems during NIV, O2 therapy, nebuliser therapy and physiotherapy procedures.
We will examine:
-
droplet size and count
-
geographical distribution of droplets
-
rise and decay of droplets over time after the therapies are initiated and discontinued
-
the impact of modifications to the delivery system to reduce droplet/aerosol dissemination in:
-
normal subjects
-
individuals with coryzal symptoms
-
patients with an acute exacerbation of chronic lung disease.
-
Primary objective
-
To evaluate the characteristics of droplets and aerosol generated using NIV, O2 therapy, nebuliser therapy and physiotherapy in clinical practice.
Secondary objectives
-
To determine whether particular delivery methods/interfaces generate more droplets.
-
To establish how can droplet characteristic information be applied to inform safe use of these therapies in patients with H1N1 swine flu, and other droplet/aerosol-borne diseases.
Study methodology
Overall design
This is an observational study with subjects and patients acting as their own control.
Setting and timescale
The study will be carried out in a single centre (Royal Brompton Hospital) over 4 months (September to December 2009).
Study outcome measures
Number of droplets in size range 0.3–10 µm, measured during conditions listed below.
Specific methods
Droplet count and sizing
We will count droplets in size range 0.3–10.0 µm within distributions of 0.3–0.5, 0.5–1.0, 1.0–3.0, 3.0–5.0, 5.0–10.0 and > 10 µm using Aerotrak Model 8220 optical particle counter with counting efficiency 50% ± 10% at 0.3 µm and 100% ± 10% at 0.45 µm and greater. We will examine dissemination of smaller droplet (aerosol) size using a P-Trak Ultrafine Condensation particle counter (particle size range 0.02–1.0 µm) at sample flow rate 100 cm3/minute. Each sampling will be carried out twice over 10 seconds, on three occasions, at sampling points: (1) adjacent (within 2 cm) to mouth or mask; (2) 0.5 m from mouth or mask; (3) 1 m from mouth or mask; and (4) 3 m from mouth or mask with sampling points (2)–(4) being carried out in radial positions – two laterally to subject/patient, one directly in front of subject/patient and one above subject. The Aerotrak and P-Trak counter devices will be mounted on tripods to maintain accuracy and reproducibility of measurements.
Mathematical modelling
We will use mathematical modelling of droplet motion and dispersion to derive the expected droplet distribution at different distances. Fitting the model with observations at a number of positions will allow interpolation and extrapolation of the measured droplet distribution as a function of size of the droplet and distance from the patient–mask interface, for a range of room conditions. In turn, this will enable us to predict the safe times and distances beyond which exposure can be considered comparable to spontaneous breathing or negligible.
Participants
Groups
We will study three groups: normal subjects, subjects with coryzal (common cold or flu-like) symptoms and adult patients with chronic lung disorders.
Inclusion criteria
Age 18 years and above. Able to speak English and understand protocol.
Age 18 years and above. Have two of any of following: raised temperature or history of raised temperature, sore throat, headache, muscle aches and pains, cough in previous 24–48 hours. Arterial oxygen saturation 95% or above on air.
A clinical diagnosis confirmed by medical consultant of COPD, asthma, cystic fibrosis, bronchiectasis, chest wall disorder or neuromuscular disease, for example Duchenne muscular dystrophy. Admitted with infective exacerbation defined by increased breathlessness, raised white cell count or temperature or CRP (C-reactive protein – raised values indicate infection or inflammation). Requiring treatment with O2 and NIV as clinically indicated.
Exclusion criteria
Current illness or underlying chronic condition. Pneumothorax in previous 3 months. Unable to understand English or trial information.
Underlying chronic condition. Arterial oxygen saturation < 95% on air. Pneumothorax in previous 3 months. Unable to understand English or trial information.
Haemodynamically unstable (systolic blood pressure < 90 mmHg, uncontrolled arrhythmia), medically unstable, arterial oxygen tension (PaO2) < 7.5 kPa on O2 or NIV, arterial carbon dioxide tension (PaCO2) > 7.5 kPa on NIV or O2, unable to breathe spontaneously for < 4 hours. Unable to understand English or trial information.
Sampling method
Will be recruited from departmental database of normal subjects who have participated in previous studies.
Will be recruited from occupational health department, and staff who develop symptoms while on duty.
Will be recruited from those already inpatients on respiratory ward, with an acute infective exacerbation of chronic lung disease. At any one time we have around 15–20 patients on the ward receiving O2/NIV. The research team are either members of the clinical team or they interact with the team on a daily basis.
Sample size
Background:
-
It should be stressed that this work is almost exclusively exploratory in nature. This is because there are very many unknowns.
-
It is not known whether the material generated by infected individuals breathing, coughing or undergoing interventions is in the form of a fine aerosol or larger droplets. 1 NIV and nebulisation have been termed ‘potential aerosol-generating procedures’ but this is based on presumption, not evidence. In the Department of Health Pandemic Flu guidelines3 it is stipulated that high-efficiency masks should be used when working within 1 m of the patient, and that beds of patients being cohort nursed should be more than 1 m apart. There is little primary evidence for either of these stipulations but in the SARS outbreak superspreading events (i.e. at least three cases arising from one index case) were associated with a distance between beds of < 1 m and index cases with the use of O2 or non-invasive ventilation. 24
-
Further, the ‘dose’ needed to infect is not clear as droplets are a proxy measure of virus presence/infectivity, and sicker patients with higher viral loads are likely to need more therapeutic interventions.
-
Moreover we do not know the rate of decay of droplets over time after interventions have been discontinued. Again, this will be partly related to size as larger droplets with greater mass will more quickly fall to the floor or onto bedding.
Droplet size and number – pilot data, variability and clinically meaningful difference:
-
We have pilot data from five normal subjects sampled at the mouth or mask and in one droplet size range (5–10 µm). This size range is known as the ‘respirable range’, representing droplets likely to be deposited in lungs; larger droplets are not inspired and very small aerosol particles do not have sufficient mass to drop out in lung and are expired as easily as they are inspired. Droplets generated by interventions (O2, NIV, etc.) should be compared with those generated by the subject/patients breathing spontaneously, as a baseline of zero droplets is not clinically realistic. We are therefore carrying out comparisons with spontaneous breathing with each subject/patient acting as their own control.
-
Our pilot data above estimated a droplet count of 900 (standard deviation = 100) with spontaneous breathing.
-
In the absence of any other published information and the uncertainties outlined above, we have chosen a doubling in this droplet count to represent a significant increase in risk of spread to health-care staff or other patients. This estimate is informed by the observation that coughing and sneezing in pilot work resulted in a count of around 1800, and that coughing and sneezing increase the risk of infection.
We used Statistical Analysis Systems (SAS), version 9.1, to estimate the required study group sizes:
-
Using our pilot estimates and a false-positive rate (α) of 0.05, calculations for a single two-group comparison with 80% power indicate that very small groups would be required.
-
We have, however, increased our group sizes to account for the possibility that variability may be higher in patients (currently unknown) and the four comparisons that are to be undertaken. Sample sizes are therefore normal subjects = 10, coryzal subjects = 10, patients = 20.
-
This model is based on droplet counts in one size range at the mouth and is suitable for our primary purpose. Again, with the lack of any information from elsewhere, we do not know whether our sample size will be sufficient for our other questions: for example, number of droplets at different distances from patient or the decay over time. Initial findings will provide further information. If variability estimates are greater or differences smaller compared to spontaneous breathing, further recruitment will be possible.
Statistical advice was provided by Mr Winston Banya, Senior Statistician, R&D Department, Royal Brompton Hospital.
Preregistration evaluations
We will check that arterial oxygen saturation level is 95% or above in normal subjects and subjects with coryzal symptoms using an oximeter ear probe. In coryzal subjects nasopharyngeal aspiration will be carried out along with throat and nasal swabs. Virology results will not be known until after study tests are done, so they will inform the analysis but are not needed for study entry as symptoms alone determine eligibility.
Patients will have an arterial oxygen saturation value of more than 88% and TcCO2 value of less than 7.5 kPa on O2 and or NIV.
Withdrawal criteria
The trial will be discontinued if the chief investigator feels it is unsafe to continue. As the therapies used are in routine clinical practice in patients, and researchers are members of the clinical team and routinely apply these therapies in patients, including those in first wave of swine flu, this risk is relatively low.
Recruitment and methodological process
Recruitment
Recruitment will take part at the Royal Brompton Hospital. Normal subjects will be recruited from departmental database and volunteers working in the hospital. Coryzal subjects will be recruited from the occupational health department and from individuals working in the hospital who develop symptoms while on duty.
Written informed consent will be obtained by the research fellow, Dr Michelle Chatwin or CI at the Royal Brompton Hospital, who have all had training in obtaining consent. Subjects and patients will be provided with information sheets. Normal subjects and patients will have 24 hours to decide whether to participate and coryzal subjects will have 1 hour to decide.
Methodological process
-
This is an observational trial that will be carried out in a single hospital side room on respiratory ward at the Royal Brompton Hospital. The aim is to measure the size and number of droplets and smaller (aerosol) particles generated during treatment with NIV, O2, nebuliser therapy and during physiotherapy.
Three groups will take part:
-
(A) normal subjects
-
(B) subjects with coryzal (common cold or flu-like) symptoms
-
(C) patients with respiratory insufficiency due to COPD, cystic fibrosis, chronic asthma, bronchiectasis, neuromuscular disease receiving NIV/O2/nebuliser therapy as indicated for an infective exacerbation. Each subject or patient will take part on one occasion, the study taking approximately 3 hours to complete.
Subjects and patients:
-
(A) Normal subjects will be recruited from our database of normals (aged 18 years and above) and above. Exclusion criteria: no current illness or underlying chronic condition.
-
(B) Individuals with common cold or flu-like (coryzal) symptoms defined by pyrexia, and two of sore throat, muscle aches and pains, headache, cough within previous 24–48 hours (age 18 years and above) will be recruited from contacts from normal patient database, occupational health department of the Royal Brompton Hospital and from staff developing symptoms while on duty. They will be studied after having nasopharyngeal swabs for viral screening, to confirm diagnosis. Exclusion criteria: no underlying chronic health conditions, medically stable.
-
(C) Patients with chronic respiratory failure will be recruited from those admitted to the ward with an infective exacerbation of chronic respiratory disease. Inclusion criteria: those with COPD, cystic fibrosis, bronchiectasis, chest wall disorder and neuromuscular disease. These groups are selected as will contain older patients with COPD and younger patients with cystic fibrosis and, for example, Duchenne muscular dystrophy, in whom NIV and O2 therapy is clinically indicated. Exclusion criteria: haemodynamically or medically unstable, PaO2 < 7.5 kPa, PaCO2 > 7.5 kPa pH < 7.34 on therapy, cognitive inability to able to understand study information sheet, able to breathe spontaneously for < 4 hours.
Technologies being assessed:
-
Non-invasive ventilation using standard bilevel pressure support device with a range of interfaces and settings, nasal continuous positive airway pressure (CPAP) therapy, O2 therapy via 60%, 35% and 24% masks
Measurements:
-
Droplets will be visualised using a Model 8220 Aerotrak Optical particle counter (TSI Inc.) with particle size detection of 0.3–10 µm, and a Model 8525 P-Trak Ultrafine Condensation particle counter (TSI Inc.) adjacent to subject/delivery system, 1 m from delivery system and 3 m from patient/subject, at six fixed radial points.
Investigation plan:
-
On arrival in the side room, subjects and patients will be assessed breathing spontaneously at rest, during simulated coughing, and then, when receiving NIV and O2, physiotherapy and nebulised saline therapy in random order.
-
Droplet distribution will be measured in the following test conditions (selected as clinically representative).
-
For (A) normal subjects and (B) subjects with coryzal symptoms:
-
Control Spontaneous breathing and simulated cough with and without surgical mask, which will take approximately 10 minutes.
-
Non-invasive ventilation A bilevel ventilator will be used: in random order delivery with non-vented full-face mask, total face mask and helmet with and without filter modification and vented full-face mask. Ventilator settings: inspiratory positive airway pressure (IPAP), expiratory airway pressure (EPAP), IPAP/EPAP 20/5 15/5 10/5 cmH2O. CPAP 5 and 10 cmH2O. This will take approximately 1 hour.
-
O2 therapy Will be delivered using 60%, 35%, 24% masks. This will take approximately 30 minutes. This will take about 20 minutes.
-
Nebulised 0.9% saline Delivered from standard nebuliser. This will take 10 minutes.
-
Standardised physiotherapy This will take 10 minutes.
-
Subjects will be able to have rest periods between the runs, as we will be sampling the room to ensure control conditions obtain and get background counts.
-
For (C) patients with respiratory insufficiency:
-
Spontaneous breathing and during simulated cough This will take approximately 10 minutes.
-
Non-invasive ventilation Using current clinically indicated NIV settings deovered in random order through non-vented full-face mask, total face mask, helmet with and without filter modification and vented mask. This will take approximately 45 minutes.
-
O2 therapy 24% Ventimask spontaneously breathing. This will take approximately 5–10 minutes.
-
Nebulised 0.9% saline Delivered by standard nebuliser. This will take approximately 10 minutes.
-
During physiotherapy using 24% O2 mask This will take about 10 minutes.
-
Patients will be monitored with arterial oxygen saturation (SaO2), transcutaneous carbon dioxide tension (TcCO2) and heart rate measurement using a non-invasive ear probe (Tosca) throughout stages (i)–(iv).
They will be able to have rest periods between the runs as we will be sampling the room to ensure control condition obtain and get background counts.
Droplet and aerosol characterisation:
-
We will count droplets in size range 0.3–10.0 µm within distributions 0.3–0.5, 0.5–1.0, 1.0–3.0, 3.0–5.0, 5.0–10.0 and > 10 µm using Aerotrak Model 8220 optical particle counter with counting efficiency 50% ± 10% at 0.3 µm and 100% ± 10% at 0.45 µm and greater. We will examine dissemination of smaller droplet (aerosol) size using a P-Trak Ultrafine Condensation particle counter (particle size range 0.02–1.0 µm) at sample flow rate 100 cm3/minute. Each sampling will be carried out twice over 10 seconds, on three occasions, at sampling points: (1) adjacent (within 2 cm) to mouth or mask; (2) 0.5 m from mouth or mask; (3) 1 m from mouth or mask; and (4) 3 m from mouth or mask, with sampling points (2)–(4) being carried out in radial positions – two laterally to subject/patient, one directly in front of subject/patient and one above subject. The Aerotrak and P-Trak counter devices will be mounted on tripods to maintain accuracy and reproducibility of measurements.
Mathematical modelling:
-
We will use mathematical modelling of droplet motion and dispersion to derive the expected droplet distribution at different distances. Fitting the model with observations at a number of positions will allow interpolation and extrapolation of the measured droplet distribution as a function of size of the droplet and distance from the patient–mask interface, for a range of room conditions. In turn, this will enable us to predict the safe times and distances beyond which exposure can be considered comparable to spontaneous breathing or negligible.
Equipment:
-
Non-invasive ventilation: we will use a Saime Elisee bilevel ventilator, which can deliver a variety of IPAP and EPAP and fixed-level CPAP through a single- limb circuit and a double-limb circuit. The pressures of IPAP/EPAP 20/5, 15/5 and 10/5 cmH2O (spontaneous triggered mode) and CPAP 5 and 10 cmH2O have been selected as clinically representative. These pressures will be used in normal subjects and those with coryzal symptoms. In the patient group we will use the IPAP/EPAP settings and back-up respiratory rate as clinically indicated.
Interfaces:
-
We will use a full-face masks (ResMed), non-vented with filtered (intersurgical) exhalation port, and vented masks (ResMed), and total masks (Respironics/Philips) in all subjects and patients, and, in five subjects, a helmet (Rusch).
Oxygen therapy:
-
Oxygen therapy 60% and 35% via high-flow reservoir mask, 24% via Venturi mask in normal subjects and those with coryzal symptoms, 24% via Venturi mask in patients.
Physiotherapy:
-
Will be standardised as cycles of deep breathing, with percussion or shaking to loosen any secretions, followed by an assisted cough initiated manually, augmented by a physiotherapist performing inwards and upwards pressure on the lower thorax to aid expectoration, after which the patient rests and the cycle repeated as required. It will be performed by one physiotherapist (MC) who has performed standardised physiotherapy manoeuvres in other randomised crossover trials.
Nebuliser:
-
Actineb nebuliser (Clement Clark) generating droplets of 3–10 µm of 0.9% saline.
There will be an interim analysis and mathematical modelling after 10 subjects and 10 patients have been studied.
Non-invasive ventilation, O2, nebuliser therapy and standardised physiotherapy will be delivered by research fellow and Dr Michelle Chatwin.
Ethical considerations
The main risk is to research staff in the dissemination of H1N1 and other coryzal viruses. Full personal protective equipment will be used – the research team members are fully familiar with this and have experience in managing H1N1 patients. Some team members have already had swine flu themselves so will be immune.
There is a very small risk of a subject or patient using NIV developing a pneumothorax. The patients already will be using NIV as part of their clinical management.
Adverse events
Potential adverse events:
-
Research team member becoming infected with swine flu. The individual would be with drawn from doing the project and treated with oseltamivir in the normal way. In practice it will be difficult to establish if the individual was infected during the study, by contact with other infected patients or from contact from within or outside the hospital
All adverse events will be reported. Depending on the nature of the event the reporting procedures below will be followed. Any questions concerning adverse event reporting will be directed to the chief investigator in the first instance.
Non-serious adverse events
All such events, whether expected or not, will be recorded.
Serious adverse events
An SAE form should be completed and faxed to the chief investigator within 24 hours. However, hospitalisations for elective treatment of a pre-existing condition will not be reported as SAEs.
All SAEs will be reported to the REC overseeing the research and the research sponsor, where, in the opinion of the chief investigator, the event was:
-
‘related’ i.e. resulted from the administration of any of the research procedures, or
-
‘unexpected’ i.e. an event that is not listed in the protocol as an expected occurrence.
Reports of related and unexpected SAEs will be submitted within 15 days of the chief investigator becoming aware of the event, using the COREC SAE form for non-IMP studies.
Investigators will report any SAEs as required by their local research ethics committee and/or research and development office.
Assessment and follow-up
We do not plan to follow up patients after the study. Virology results will be fed back to coryzal subjects and appropriate action advised.
Statistics and data analysis
Data will by analysed using ANOVA with correction for repeated measure. Statistical advice will be provided by Mr Winston Banya, R&D Department, Royal Brompton & Harefield NHS Foundation Trust.
Data and all appropriate documentation will be stored for a minimum of 5 years after the completion of the study, including the follow-up period.
Regulatory issues
Ethics approval
The chief investigator will obtain ethical approval from a research ethics committee via the IRAS system. The study will not commence until ethical approval is obtained, and will be conducted in accordance with the recommendations for physicians involved in research on human subjects adopted by the 18th World Medical Assembly, Helsinki 1964, and later revisions.
Consent
Consent to enter the study will be sought from each participant only after a full explanation has been given, an information leaflet offered and time allowed for consideration. Signed participant consent will be obtained. The right of the participant to refuse to participate without giving reasons will be respected. All participants are free to withdraw at any time from the research without giving reasons and without prejudicing further treatment. Consent will be obtained by the patient’s existing clinical consultant.
Confidentiality
The chief investigator will preserve the confidentiality of participants taking part in the study in line with the Data Protection Act 1998.
Indemnity
NHS indemnity cover.
Sponsor
Royal Brompton & Harefield NHS Foundation Trust.
Funding and costs
NIHR HTA will fund this study. Travel costs to £20 are available to normal and coryzal subjects.
Audits and inspections
Sponsor and other regulatory bodies will ensure adherence to Good Clinical Practice and the NHS Research Governance Framework for Health and Social Care (2nd edn).
Study management
The day-to-day management of the study will be co-ordinated through by Dr Michelle Chatwin (M.Chatwin@rbht.nhs.uk).
Publication policy
Results from this research will be reported and disseminated via peer-reviewed journals and via conference presentations. No personal or identifiable data will be present in any public reports of this research.
References
- Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis 2006;12:1657-62.
- Fabian P, McDevitt JJ, DeHaan WH, Fung ROP, Cowling BJ, Chan KH, et al. Influenza virus in human exhaled breath: an observational study. PLoS ONE 2008;3.
- Department of Health . Pandemic Influenza: Guidance for Infection Control in Critical Care 2008.
- Simonds AK, Sokol DK. Lives on the line? Ethics and practicalities of duty of care in pandemics and disasters. Eur Respir J 2009;34:303-9.
- Department of Health . Pandemic Flu. Managing Demand and Capacity in Health Care Organisations (surge) 2009.
- Gomersall CD, Tai DYHT, Derrick JL, Goh MS, Buckley TA, Chua C, et al. Expanding ICU facilities in an epidemic: recommendations based on experience from SARS epidemic in Hong Kong and Singapore. Intensive Care Med 2006;32:1004-13.
- Guidelines from the British Infection Society, British Thoracic Society and DH . Pandemic flu: clinical management of patients with influenza-like illness during an influenza pandemic. Thorax 2007;62:1-46.
- Levy M, Tanios MA, Nelson D, Short K, Senechia A, Vespia J, et al. Outcomes of patients with do-not-intubate orders treated with noninvasive ventilation. Crit Care Med 2004;32:2002-7.
- Fowler RA, Guest CB, Lapinsky SE, Sibbald WJ, Louie M, Tang P, et al. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med 2004;169:1198-202.
- Scales DC, Green K, Chan AK, Poutanen SM, Foster D, Nowak K, et al. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerg Infect Dis 2003;9:1205-10.
- Xiao Z, Li Y, Chen RC, Li SY, Zhong SQ, Zhong NS, et al. A retrospective study of 78 patients with severe acute respiratory syndrome. Chin Med J 2003;116:805-10.
- Varia M, Wilson S, Sarwal S, McGeer A, Gournier E, Galanis E, et al. Investigation of a nosocomial outbreak of severe acquired respiratory syndrome (SARS) in Toronto, Canada. CMAJ 2003;169:285-92.
- Cheung TMT, Yam LYC, So LKY, Lau ACW, Poon E, Kong BMH, et al. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest 2004;126:845-50.
- Yam LYC, Chan AYF, Cheung TMT, Tsui ELH, Chan JCK, Wong VCW. Non-invasive versus invasive mechanical ventilation for respiratory failure in severe acute respiratory syndrome. Chin Med J 2005;118:1413-21.
- Yu IT, Li Y, Wong TW, Tam W, Chan AT, Lee JH, et al. Evidence of airborne transmission of severe acute respiratory syndrome virus. N Engl J Med 2004;350:1731-9.
- Hugonnet S, Pittet D. Transmission of severe acute respiratory syndrome in critical care: do we need a change?. Am J Resp Crit Care Med 2004;169:1177-8.
- Seto WH, Tsang D, Yung RW, Ching TY, Ng TK, Ho M, et al. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet 2003;361:1519-20.
- Lau JTF, Fung KS, Wong TW, Kim JH, Wong E, Chung S, et al. SARS transmission among hospital workers in Hong Kong. Emerg Infect Dis 2004;10:280-6.
- Hui DS, Hall SD, Chan MTV, Chow BK, Tsou JY, Joynt GM, et al. Noninvasive positive-pressure ventilation. An experimental model to assess air and particle dispersion. Chest 2006;130:730-40.
- Somogyi R, Vesely AE, Azami T, Preiss D, Fisher J, Fowler RA. Dispersal of respiratory droplets with open vs closed oxygen delivery masks: implications for transmission of severe acure respiratory syndrome. Chest 2004;125.
- Mardimae A, Slessarev M, Han J, Sasano H, Sasano N, Azami T, et al. Modified N95 mask delivers high inspired oxygen concentrations while effectively filtering aerosolized microparticles. Ann Emerg Med 2006;48:391-9.
- Ip M, Tang JW, Hui DS, Wong ALN, Chan MTV, Joynt GM, et al. Airflow and droplet spreading around oxygen masks: a simulation model for infection control research. Am J Infect Control 2007;35:684-9.
- Keady PB. Getting data you need with particle measurements. Indoor Environ Connect 2000;2:1-5.
- Yu IT, Xie Z, Tsoi K. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards and not others?. Clin Infect Dis 2007;44:1017-25.
- Simonds AK, Simonds AK. Non-invasive respiratory support: a practical handbook. London: Hodder Arnold; 2007.
List of abbreviations
- AGP
- aerosol-generating procedure
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CPAP
- continuous positive airway pressure
- DH
- Department of Health
- EPAP
- expiratory positive airway pressure
- IPAP
- inspiratory positive airway pressure
- mod NIV
- modified NIV circuit with exhalation filter
- NIV
- non-invasive ventilation
- O2
- oxygen therapy
- PaCO2
- partial pressure of arterial carbon dioxide
- PaO2
- partial pressure of arterial oxygen
- PPE
- protective personal equipment
- RR
- relative risk
- SaO2
- arterial oxygen saturation
- SARS
- severe acute respiratory syndrome
- SD
- standard deviation
- TcCO2
- transcutaneous partial pressure of carbon dioxide
- WHO
- World Health Organization
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.