Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 08/11/01. The contractual start date was in November 2008. The draft report began editorial review in August 2009 and was accepted for publication in May 2010. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
None
Permissions
Copyright statement
© 2010 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2010 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Accurate and efficient diagnostic procedures are paramount in optimising patient management and use of health-care resources. If a correct diagnosis is not made patients may receive inaccurate information regarding their prognosis, may undergo inappropriate medical treatment, or the correct treatment may be withheld. This can result in less than optimal outcomes, both in terms of the clinical management of patients and the use of health-care resources. Furthermore, following the use of different tests to establish a diagnosis, further tests are often required to monitor disease progression, or to screen for the presence of other risk factors or concomitant disease.
To reach a diagnosis or manage a patient, a clinician may choose to order one or more medical tests. In this sense, ‘diagnostic test’ refers to any procedure that tries to confirm or identify the presence or absence of a patients’ symptoms or signs or alteration in a patient’s condition. This includes laboratory measurements, e.g. biochemistry, haematology, bacteriology, imaging, and invasive procedures. There are a number of factors which may influence a clinician’s decision to order a test including:
-
– a patient’s medical history, signs and symptoms
-
– therapeutic and prognostic factors, such as deciding on an appropriate course of treatment
-
– patient-related factors such as demographics or patient preference
-
– factors related to both the individual clinician and health-care organisation. 1
A recent systematic review of reasons and context for test ordering by clinicians highlighted that the majority of factors associated with test ordering were clinician related, including level of clinical experience, confidence in their clinical judgement, speciality, and working patterns. Availability of tests, type of health-care organisation (salaried health-care professionals vs fee for service approach), and size of the primary care practice were also found to influence test requesting patterns. 1 This review therefore highlights the fact that clinician test ordering behaviour is influenced by a multitude of interactive factors, and therefore may be difficult to standardise as it will depend not only on the nature of clinical consultation, but also on the individual clinician working within a specific organisational environment. Many potential benefits of order communication systems (OCS) (termed Computerised Physician Order Entry or CPOE systems in the USA) in hospitals have been identified. These include improvements in clinician ordering patterns, optimisation of clinical time, and aiding communication processes between clinicians and different departments. 2–5 These systems have the potential to automate the clinical test ordering process and to improve the quality and safety of patient care. 6–9 Many OCS now include computerised decision support systems (CDSS). These incorporate features such as decision support mechanisms, including alerts of critical values, reminders of overdue preventative health tasks, (including laboratory or radiology imaging tests), built-in alerts, rule-based prompts, advice for drug prescribing, critiques of existing health-care orders, and suggestions for various care issues. However, as a number of reviews have highlighted CDSS do not always improve clinical practice. In a recent review of computer-based systems, (including but not restricted to just CDSS) of 100 randomised controlled trials (RCTs) assessing a wide range of indications for OCS and CDSS use (diagnosis, reminder systems, disease management systems, and drug-dosing or prescribing systems), most [62/97 (64%)] significantly improved practice in some way, but 36% did not. 10 Furthermore, there is relatively little sound scientific evidence available to explain why some systems succeed and some systems fail.
Computerised decision support systems in health care are information systems designed to improve clinical decision making, and by and large are intended to support health-care workers in the normal course of their duties, assisting in tasks that rely on the manipulation of data and knowledge. Although there is no consensus on the definition of a CDSS, the definition used in three systematic reviews conducted at McMaster University, Hamilton, ON, Canada9–11 is an:
active knowledge systems which use two or more items of patient data to generate case-specific advice. 9–11
Computerised clinical decision support systems match characteristics of an individual patient to a computerised knowledge base, with software algorithms used to generate patient-specific recommendations. Clinicians, health-care staff or patients can manually enter patient characteristics into the computer system, or alternatively electronic medical records can be queried for retrieval of patient characteristics. Computer-generated recommendations are then delivered through the electronic medical record, by pager, e-mail, or through printouts placed in a patient’s paper chart. Additionally, CDSS can be used to check the potential duplication of services and highlight test orders that should be considered when one order is placed (‘corollary’ orders).
A large proportion of orders processed through order communication systems are for pathology and imaging services. The use of laboratory services for diagnostic testing has increased in many health-care jurisdictions around the world. 12–14 The Healthcare Commission report ‘Getting results: Pathology services in acute and specialist trusts’, highlights the fact that in the UK pathology is the largest diagnostic service in the number of requests it meets annually (175 million), in expenditure (£1.8B in 2005–6 and 5.1% of the total budget of NHS Trusts) and in the proportion of clinical decisions that it affects (reputedly over 70%). 15 Moreover the number of requests for biochemistry, haematology and microbiology tests continues to increase, and there is also an increase in the number of tests requested per sample. The report also highlights that in 2005 while tests were generally completed more quickly that in 2003, there was still considerable variation between laboratories in test turn around times. Additionally, many non-urgent tests were being completed more quickly than in 2003, raising the question of whether improved turnaround results in clinical benefits that may justify additional marginal costs.
In the test ordering process there are two distinct aspects to order communication systems:
-
Test requesting – the process of making a request to a diagnostic service.
-
Results reporting – the process of electronic reporting of results to the clinician.
Figure 1 outlines the flow of information in the test requesting and reporting process and the stages in which CDSS and OCS can have an impact.
FIGURE 1.
Information flow in order communication.
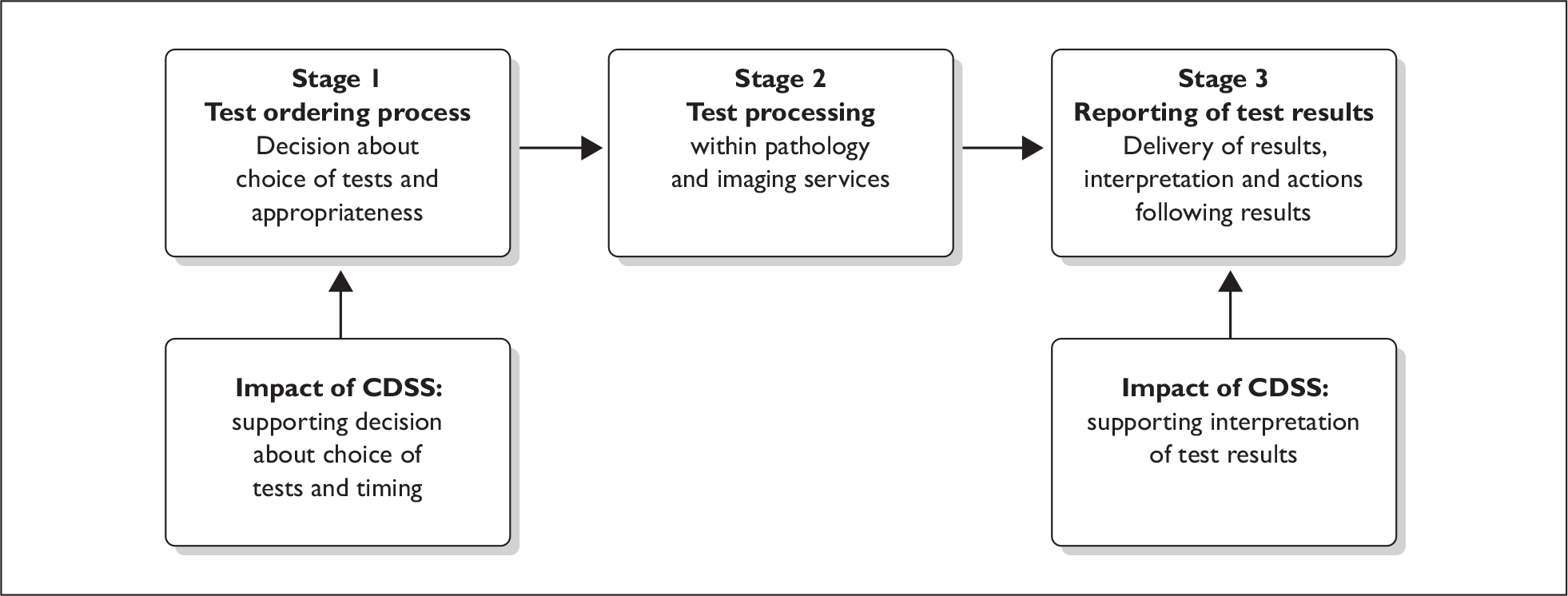
In the test ordering process the use of CDSS in OCS has the potential to: reduce the number of redundant tests that are ordered; ensure necessary tests are performed at the correct intervals by prompting clinicians; ensure tests appropriate to the specific clinical circumstances are ordered; and correct sampling procedures for the tests that are ordered.
In the results reporting process the potential impact of CDSS in OCS with intelligent feedback lies in the provision of context-specific interpretative comments to help the clinician with the interpretation of test results (either alone or in addition to those provided by pathology or imaging services), and provide advice on the best course of action given a specific result, e.g. to undertake further investigations and the timing of such tests.
Types of order communication and CDSS
Order communication systems vary in their level of sophistication with a distinction between systems which provide only knowledge support, those which provide audit feedback on aggregated data, and those which provide real-time decision support embedded in the clinical process. OCS can vary in at least four different ways, which have the potential to impact on any benefits and costs associated with the system:
-
Functions of the system. There are at least four important dimensions along which systems can differ:
-
only order tests versus order and display past/current results
-
ordering/result display alone versus system with knowledge support (e.g. an electronic laboratory handbook for browsing) versus system with decision support
-
with or without a regular audit report on the number and type of tests ordered by the user
-
location of the access points: fixed access points versus mobile computers
-
patient identification aids: none versus bar coding versus other identification such as radio frequency identification tags.
-
-
Scope of the orders covered:
-
orders for tests from one single laboratory versus
-
for all laboratories versus
-
for laboratories plus imaging and electrocardiograms, etc.
-
versus for all tests and therapies and drugs versus
-
all orders integrated into full electronic patient record.
-
-
Purpose of the tests ordered:
-
test ordering for preventive care or screening versus
-
diagnostic purposes versus
-
monitoring of long-term conditions and drug dosing (e.g. insulin, warfarin).
-
-
Aim of the advice offered by system:
-
to increase appropriate use of tests versus
-
to decrease over use of tests.
-
Additionally, as the systematic review including a meta-analysis and meta-regression by Kawamoto and colleagues16 highlights, other specific system features may be related to the success or failure of the CDSS in significantly improving clinical practice. In their review, which included 70 studies comparing 71 relevant comparisons, 15 decision support features whose importance had been repeatedly suggested in the literature as having an impact on the effects of CDSS were assessed using univariate analyses for each selected feature to determine whether or not it had a statistically or clinically significant impact on clinical practice. The authors did not report how a ‘significant impact on clinical practice’ was defined within the review. Nor did it appear that the included studies reported a significant improvement in practice using the same definition. The presence or absence of each of the 15 features within a system were then used as predictors of system success or failure in terms of having a significant impact using multiple regression models. The 15 features assessed in the review are listed in Table 1. Further explanatory variables to account for decision support subject matter (acute vs non-acute care) and two indicators for the study setting (academic vs non academic, and outpatient vs inpatient care) were also entered into the regression models. The authors found that four system features were independent predictors of improved clinical practice: automatic provision of decision support as part of clinician workflow, provision of recommendations rather than just assessments, provision of decision support at the time and location of decision making, and computer-based decision support. 16 However, the odds ratios for the two most important predictors identified by the authors, namely automatic provision of decision support as part of clinician workflow, and provision of recommendations rather than just assessments, were implausibly high with associated very wide confidence intervals (CIs). As the authors acknowledge these two features that were included in the multivariate model may have had a significant effect in the regression model due to model over-fitting. 16
| Features and sourcesa |
|---|
| General system features |
| Integration with charting or order entry system to support workflow integration.17–21 |
| ± Use of computer to generate the decision support.b,22–31 |
| Clinical–system interaction features |
| ± Automatic provision of decision support as part of clinician workflow.18,19,32–41 |
| No need for additional clinician data entry.17,32,33,36,42–6 |
| Request documentation of the reason for not following CDSS recommendations.42,45–8 |
| ± Provision of decision support at time and location of decision making.5,20,22,23,25,31,35,37–41,44,49–51 |
| Recommendations executed by noting agreement.24,46,47,52 |
| Communication content features |
| ± Provision of a recommendation, not just an assessment.45,49,53 |
| Promotion of action rather than inaction.36,39,54 |
| Justification of decision support via provision of reasoning.17,47,54,55 |
| Justification of decision support via provision of research evidence.29,34,54,55 |
| Auxiliary features |
| Local user involvement in development process.5,18,45,54–62 |
| Provision of decision support results to patients as well as providers.25,63–7 |
| CDSS accompanied by periodic performance feedback.34,40,50,54,60,68 |
| CDSS accompanied by conventional education.28,50,69–71 |
Additionally, as well as CDSS systems varying in their degree of sophistication, location of access points and timeliness and mode of feedback, and the information system in which they are located (Laboratory/Radiology Information Systems; Hospital Information Systems, or GP Practice Systems), CDSS also vary in the reasoning methods used to generate advice and the source of the information from which advice is generated. A typology of six types of reasoning methods for decision tools was described by Liu and colleagues. 72 This categorised the reasoning methods as either (1) Bayesian methods, (2) logistic regression extensions of Bayes’ theorem, (3) based on discrimination rules, (4) clinical algorithms, (5) expert systems or (6) machine learning methods.
Bayesian methods
Bayes’ theorem describes how the probability that an individual has a disease (known as the pre-test or prior probability) changes when the result of a diagnostic test is obtained (post-test probability), dependent on the performance characteristics of the test. Bayes’ theorem can be extended to combine multiple pieces of diagnostic information, with the post-test probability obtained from the first test acting as the prior probability for the next test. However, this approach, known as naive Bayes, has been shown to give over-optimistic predictions when individual test results are not independent due to the double counting of diagnostic information.
Logistic regression extensions of Bayes’ theorem
The problem of double counting of diagnostic information is removed by using logistic regression models that account for correlations between the different pieces of diagnostic information. In this method the links between Bayes’ theorem and logistic regression models can be fitted by re-expressing the theorem using ‘weights of evidence’ or log likelihood ratios to account for the correlations. 73 Adjustments for correlations between diagnostic items are made by estimating a beta parameter for each test, which either increases or decreases the likelihood ratio for the test. Bivariate and multivariate model fitting approaches can then be used to remove redundant symptoms and select those to keep in the final model.
Discrimination rules
Discrimination rules use standard statistical methods to produce a rule that can be used to discriminate between individuals on the basis of symptoms or test results, and to allocate them to the group to which they are most likely to belong, for example, diseased versus non-diseased. These methods rely on producing predictions from either logistic regression models or discriminant functional analyses to predict membership of two or more groups. The application of logistical regression in this instance differs in two important ways from that applied to Bayes’ theorem. Firstly, there is no direct way of altering predictions to allow for differences in pre-test probabilities, and secondly, there is no quantification of the uncertainty around group allocation.
Clinical algorithms
An algorithm is a process for carrying out a complex task which is broken down into a series of simple decision and action steps. 74 Clinical algorithms can either be represented as paper-based flowcharts or as computer programs. These algorithms have a number of limitations including the need to have all the data specified in the algorithm available, space restrictions if the algorithm is paper based, and the practical difficulties of breaking down many clinical problems into a set of discrete decisions that can then be represented in computer language. Optimal clinical algorithms can be developed using statistical methods known as classification and regression trees.
Expert systems
An expert system is a computer program that simulates human thought processes ‘to provide the kind of problem analysis and advice that the expert might provide’. 74
Machine learning
Machine learning can either be supervised or unsupervised. In supervised learning the system is provided with a sample of input data and the content designated on how to identify and classify patterns within the data. In unsupervised learning the system is provided with data, but is left to identify patterns without external assistance using a form of cluster analysis. There are a number of different types of machine learning methods, including decision trees, artificial neural networks and genetic algorithms. For all methods, internal weights within the system are adjusted during training until a pre-specified performance level is attained. Limitations of these systems include the fact that although experts can evaluate a decision tree generated by a machine system, the system cannot often provide understandable reasons for the advice it generates. Furthermore experts can rarely evaluate the reasoning behind the classifiers generated by neural networks and genetic algorithms as these systems are ‘black boxes’.
Evaluation of CDSS
Wyatt and Spiegelhalter describe a systematic approach to laboratory and field testing of CDSS, suggesting that the final stages should include evaluation of effects on health-care processes and patient outcomes. 75 However, if a CDSS is to have an ultimate impact on health-care processes or patient outcomes then acceptance of the system and usage rates must be high. User acceptance and satisfaction with a CDSS is therefore highly important; if users are satisfied they are likely to modify their behaviour to use the system to their advantage, but if they are not then they will either not use the system or will use it in a suboptimal manner. 76
A literature review by Ohmann and colleagues, which focused on user satisfaction with computer-based systems, highlights the fact that satisfaction is a complex interplay between both system-dependent and system-independent factors. 77 System-dependent factors include ‘satisfaction with the content of the CDSS’ and ‘satisfaction with the interface of the system’, whereas system-independent factors include personal factors, such as ‘computer anxiety’ and ‘attitudes towards computers’ as well as organisational factors, including the environment in which the system is used.
In terms of system-dependent factors acceptance of CDSS depends on a number of factors including:
-
time taken to get access to the CDSS
-
time taken to use the CDSS
-
conceptual complexity of the CDSS (which affects ease of understanding and usage)
-
number of data items to collect (if the data are not already available in electronic patient records)
-
ease of data entry
-
ease of interpreting the results (numbers, probabilities, graphs, advice, etc.)
-
perceived applicability of the CDSS knowledge base to the clinician’s own patients.
Although it is recognised that system-independent factors are additionally likely to impact on user acceptance of CDSS, it is necessary to assess what features of CDSS are likely to make the system more or less acceptable to clinicians or patients when developing prototypes and final versions of the system, as ultimately acceptance of the system will impact on usage rates, and may influence both process and patient outcomes. Thus during the development stage as highlighted in the review by Kawamoto and colleagues16 it may be important to involve local users in the development process, as ultimately they will be the system users, and may be able to provide useful feedback on the different functionalities of the system as the development phase progresses.
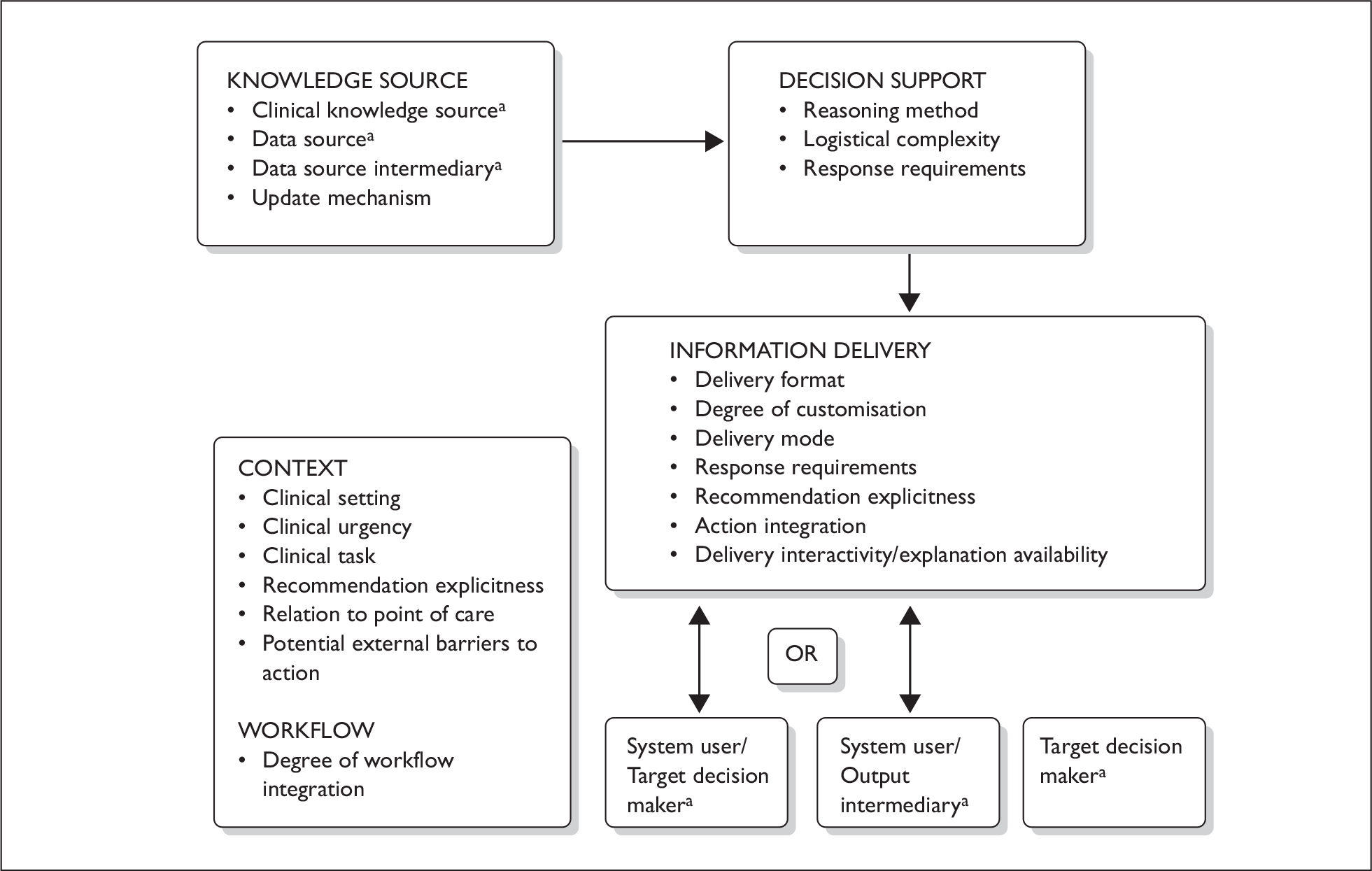
Figure 2 adapted from Sim and colleagues, highlights the complex interplay between the knowledge source that underpins the CDSS, the CDSS characteristics, the information delivery and the clinical work context. 55
FIGURE 2.
Overview of CDSS and the interplay with the context in which it is used. a denotes human and possible human roles.
Current service provision
The National Programme for Information Technology (NpfiT) is a 10-year programme that will procure, develop and implement modern, integrated information technology (IT) infrastructure and systems for all NHS organisations, and was originally described as one of the world’s biggest IT projects projected to cost £ 6.2B. However due to the complexity and delays in completing the project, in 2006 a report from the National Audit Office suggested that spending on the NpfiT would actually reach £ 12.4B by the year 2014. 78
The key elements of the programme are:
-
The NHS Care Records Service, with a record for each individual patient, which can be accessed securely by both the patient and health-care providers.
-
Choose and Book, an electronic booking service aiming to give patients a greater choice of hospital or clinic and more convenience in the date and time of their appointment.
-
A system for Electronic Transmission of Prescriptions, to make GP prescribing and dispensing safer and easier.
-
A national network for the NHS (N3), providing IT infrastructure and broadband connectivity to meet all NHS computing needs.
-
Picture Archiving and Communications System (PACS) to capture, store and distribute static and moving medical images.
-
The Quality and Management and Analysis System giving GP practices and primary care trusts objective evidence and feedback on the question of care delivered to patients.
-
NHS e-mail, including a directory service for the NHS.
Originally the agency responsible for delivering the NpfIT was NHS Connecting for Health (CFH), with a number of core contractors employed to deliver specific aspects of the programme at either a national or regional level. Table 2 below shows the original core contractors and the services which they were responsible for delivering.
| Contract | Area | Company | Duration |
|---|---|---|---|
| NHS Care Records Service – NASP | National | BT | 10 years |
| NHS Care Records Service – LSP | North East | CSC | 10 years |
| NHS Care Records Service – LSP | Eastern | CSC | 10 years |
| NHS Care Records Service – LSP | London | Capital Care Alliance (BT) | 10 years |
| NHS Care Records Service – LSP | North West and West Midlands | CSC | 10 years |
| NHS Care Records Service – LSP | Southern | The Fujitsu Alliance | 10 years |
| N3 | National | BT | 7 years |
| Choose and book | National | Atos Origin | 5 years |
| NHS Mail | National | Cable and Wireless | 10 years |
However, accountability for the delivery of the programme was transferred to Strategic Health Authorities (SHAs) in April 2007, as part of the NpfiT Local Ownership Programme. Currently programme activity is split into three programmes for IT, each of which is hosted by the SHAs and has a Local Service Provider. These are comprised of:
-
London Programme for IT (LpfiT) for which the local service provider is BT.
-
North Midlands and East (NME) Programme for IT (NMEPfIT) for which the local service provider is Computer Sciences Corporation. This covers the six SHAs: East Midlands SHA, East of England SHA, North East SHA, North West SHA, West Midlands SHA and Yorkshire and Humberside SHA.
-
Southern Programme for IT which covers three SHAs: South Central SHA, South East Coast SHA and South West SHA.
Furthermore, an Additional Supply and Capacity service Contract (ASCC) was established for specific technical aspects of the project in recognition of the fact that the original NpfIT contract was likely to require additional capability and capacity over and above that of the original contractors. Additional Supply and Capacity Service contractors were established to provide specialist knowledge, skills and services not currently or readily available from the existing NpfIT suppliers at either the local, regional, pan-SHA or national level. In relation to the provision of decision support (Service Category 2.20), 24 additional suppliers were contracted, 12 at the national level and 12 at the level of small to medium-sized enterprises (SMEs). These additional 24 decision support suppliers along with the core contractors formed the basis of the sampling frame used to identify which CDSS in OCS are currently either in use or being implemented in the UK. A list of these suppliers is given in Table 3.
| National |
|---|
| AGFA Healthcare (UK) Ltd |
| Atos Origin |
| British Telecommunications plc |
| Cerner Ltd |
| CSE Servelec Ltd |
| FileTek UK Ltd |
| Fujitsu Services Ltd |
| ISoft plc |
| Perot Systems Europe Ltd |
| Siemens plc |
| Steria Ltd [formerly known as Xansa (UK) Ltd] |
| TATA Consultancy Services Ltd |
| Specialist SME |
| Adastra Software Ltd |
| ALERT Life Sciences Computing, SA |
| Oasis Medical Solutions Ltd (formerly known as Capula Healthcare Ltd) |
| CAS Services Ltd (formerly known as Clinical Solutions Ltd) |
| CSW Group Ltd |
| Egton Medical Information Systems Ltd |
| Infermed Ltd |
| Map of Medicine (formerly known as Informa UK Ltd) |
| Plain Healthcare |
| Sowerby Centre for Health Informatics at Newcastle Ltd |
| Stalis Ltd |
Up-to-date information (current as of 1 July 2009) regarding the deployment of the different key elements of the NpfIT project by the three programmes for IT and the service providers is available at www.connectingforhealth.nhs.uk/newsroom/statistics/deployment/commsrep.pdf. However it should be noted that this does not include data on deployment of CDSS, although does provide minimal information on the deployment of OCS.
Chapter 2 Scope of the technology assessment
Aims and objectives
The purpose of this report was to assess:
-
Which CDSS in OCS for test ordering are currently in use within the UK, and what are their main characteristics and their intended/actual scope of use?
-
What is the impact of CDSS in OCS for diagnostic, screening or monitoring test ordering compared to OCS without CDSS on process outcomes, patient outcomes and adverse events/safety?
-
What features of CDSS are associated with clinician or patient acceptance of CDSS in OCS?
-
What is known about the cost-effectiveness of CDSS in diagnostic, screening or monitoring test OCS compared to OCS without CDSS?
-
In order to address the questions specified, the assessment was comprised of:
-
A survey of the 24 manufacturers/suppliers currently contracted by NpfIT to provide either national or specialist SME decision support systems, NHS CFH, eHealth Strategy Board, ‘Informing Health Care’, the Healthcare Commission,15 NHS Purchasing Suppliers, and the NHS Supply Chain.
-
Two linked systematic reviews to assess, firstly, the impact of CDSS in OCS for diagnostic, screening or monitoring test ordering compared to OCS without CDSS on process and patient outcomes, and secondly, to examine what specific features of CDSS may be associated with clinician or patient acceptance of the system.
-
A systematic review of economic evaluations and cost-comparison studies of CDSS in diagnostic, screening or monitoring test OCS compared to OCS CDSS.
-
Interventions
The report assesses CDSS in OCS for diagnostic, screening or monitoring test ordering compared to OCS without CDSS evaluated in a clinical setting. For the purpose of this assessment a CDSS is defined as:
an active knowledge system which uses two or more items of patient data to generate patient-specific assessments or recommendations that are then presented to clinicians for consideration. 9–11
Studies in which a CDSS has not been evaluated in a clinical setting are not included. Additionally, studies in which the OCS: (1) only provides summaries of patient information (i.e. no specific test ordering or test interpretation advice); (2) provides feedback on groups of patients without individual assessment; (3) only provides computer-aided instruction (i.e. provides generic rather than patient specific advice); or (4) is used in image analysis are not included.
Population
Studies which include health-care workers (e.g. physicians, nurses, dentists, psychiatrists, physiotherapists) in practice or training, or patients undergoing testing for diagnostic, screening or monitoring purposes in a primary or secondary care setting are included.
Relevant comparators
CDSS in OCS are compared with OCS without CDSS.
Outcomes
Study question two:
Studies which report an objective measure of process of care, e.g. test volumes, compliance with guidelines implemented via the CDSS, appropriateness of the test(s) ordered, patient outcomes, or adverse events, are included. Studies which only report the diagnostic accuracy of the CDSS compared to a gold standard (such as a diagnosis reached by the clinician without use of the CDSS) (i.e. sensitivity and specificity) are excluded. These studies are excluded as the outcome of interest is the impact of CDSS in conjunction with OCS for test ordering, rather than the accuracy of CDSS compared with a clinician in providing a correct clinical diagnosis.
Study question three:
Outcomes that were included were clinician or patient self-reported acceptability of the CDSS. Acceptability was defined according to the definitions used in the primary studies.
Study question 4:
Studies which reported the cost-effectiveness of CDSS are included.
Study designs
For study questions two and three randomised, cluster randomised, and non-randomised trials with a contemporaneous control group, interrupted time series (ITS), and controlled and uncontrolled pre–post studies (CPPs and UPPs) are included. In addition for review question three cross sectional and longitudinal surveys and qualitative studies are also included. For review question four, the systematic review of economic evaluations, cost–comparison studies, full cost-effectiveness analyses (CEA), cost–utility analyses (CUA) and cost–consequence analyses (CCA) are included.
Publication language and status
A full English language text copy of the study has to be available for it to be included. Studies which are reported in abstract form only and where no further information is available were excluded. Foreign language papers were also excluded.
Overall aims and objectives of assessment
This assessment aimed to establish which CDSS in OCS for test ordering were currently in use or being implemented in the UK, and the main characteristics and intended/actual scope of their use. The assessment also reviews the evidence on the impact of CDSS in OCS, the specific CDSS features which may be associated with clinician or patient acceptance of the system, and the cost-effectiveness of CDSS in OCS compared to OCS alone through three linked systematic reviews. Additionally, through drawing together the evidence on the impact of CDSS on clinical processes and patient outcomes, and the likely cost-effectiveness of CDSS, systems for which future primary research would be of benefit will be identified.
Chapter 3 Methods to address the questions
Study question 1: identification of CDSS in OCS for diagnostic, screening and monitoring test ordering currently in use within the UK
Computerised decision support systems in diagnostic, screening and monitoring test OCS currently in use or being implemented within the UK were identified through contact with the 24 manufacturers/suppliers currently contracted by NpfIT (service category 2.20) to provide either national or specialist SME decision support. Manufacturers were contacted by e-mail and asked to stipulate whether their specific system was currently being piloted, in use or being implemented in the UK. They were additionally asked to state the number and at which sites their CDSS was installed. Where they considered this data to be commercial-in-confidence (CIC) they were asked to state this, but at least respond as to whether the CDSS was currently deployed in the UK. Non-responders to the survey were followed-up twice, at two weekly intervals.
In addition, NHS CFH, the Healthcare Commission,15 NHS Purchasing Suppliers, and the NHS Supply Chain were contacted. These contacts did not yield any additional information.
Generic methods for the conduct of reviews to assess the impact, acceptability and cost-effectiveness of CDSS systems in test order communication systems
Standard systematic review methods following the guidance on the conduct of systematic reviews published by the Centre for Reviews and Dissemination (CRD)79 was used to undertake the reviews of the impact, acceptability, and cost-effectiveness of CDSS. The generic methods for the conduct of the reviews are outlined below with the specific inclusion criteria for each of the reviews, data extracted, and methods of synthesis for each outlined for the specific review questions in turn.
Identification of relevant studies
A generic search to identify potentially relevant studies for inclusion in the three reviews was conducted. This was used to identify relevant clinical, cost-effectiveness and cost–comparison studies indexed on the following medical and social science databases between 1974 (the year of publication of the first article to evaluate the effect of a CDSS on clinician performance by De Dombal and colleagues)80 and 2008: The searches were then updated in April 2009.
-
MEDLINE
-
EMBASE
-
Cochrane Controlled Trials Register (CCTR)
-
CINAHL (Cumulative Index to Nursing and Allied Health Literature)
-
DARE (Database of Abstracts of Reviews of Effects)
-
Health Technology Assessment (HTA) database
-
IEEE (Institute of Electrical and Electronic Engineers) Xplore
-
NHS Economic Evaluation Database (NHS EED)
-
EconLit.
No study design filters were applied to the search strategy.
The literature searches retrieved 22,109 unique references after de-duplication. All references were managed using reference manager, software version 11. Full details of the search strategies are presented in Appendix 1. In addition, bibliographies of all included studies were checked to identify further relevant studies.
Selection of primary studies for the reviews
Relevant studies were identified in two stages. One reviewer screened titles and abstracts returned by the database searches, and a random 20% of these were checked for agreement by a second reviewer. Cohen’s unweighted κ-statistic for disagreements at the title/abstract screening stage between reviewers was 0.89, indicating a good level of agreement. The full texts of any references that were considered relevant by either reviewer were obtained where available. The relevance of each paper was assessed according to the criteria set out below for each review question. Any discrepancies between the reviewers were resolved by recourse to the papers, and if necessary a third reviewer was consulted. All duplicate papers were double-checked and excluded. The extent of disagreements between reviewers for study inclusion in the reviews was again quantified using Cohen’s unweighted κ-statistic with an agreement level of 0.91 between reviewers. 81 Further bibliographic details of excluded studies, along with reasons for their exclusion are detailed in Appendix 2.
Data extraction and quality assessment processes
Data were extracted from the included studies using a standardised data extraction form developed for each of the reviews. The quality of the individual studies was assessed according to study design by one reviewer and checked for accuracy by a second. RCTs, cluster randomised controlled trials (CRCTs), controlled clinical trials (CCTs), and CPPs and UPPs were assessed according to methodological criteria listed in the up-dated CRD Report 4;79 ITS studies were assessed according to criteria specified by the Cochrane Effective Practice and Organisation of Care (EPOC) Group;82 economic evaluations were assessed using the Consensus on Health Economic Criteria list questions developed by Evers and colleagues. 83
The main criteria assessed according to study design are outlined below.
RCTs, CRCTs and CCTs
The assessment of internal validity was examined: the methods of randomisation (RCTs and CRCTs), the handling of potential confounders (baseline imbalance, cointervention), blinding of assessors and data analysts, the rate of attrition and the appropriateness of data analyses. In addition, for CRCTs whether the analysis took clustering into account was also examined.
ITS
In line with the quality assessment criteria suggested by the EPOC Group for ITS studies,82 quality assessment criteria focused on whether the intervention was independent of other changes over time, whether sufficient data points were presented to enable reliable statistical inference and whether a formal test for trend was presented. Additionally, the reliability of the primary outcome measure, whether the intervention was likely to affect the methods of data collection, blinding of outcome assessors, and rates of attrition were assessed.
Controlled and uncontrolled pre–post studies
Assessment of validity for both controlled and uncontrolled pre–post studies was undertaken by assessing the adequacy of baseline details, rates of attrition and the appropriateness of data analyses (i.e. whether analyses were conducted on the basis of the ‘intention to provide or communicate information’).
Study question 2: What is the impact of CDSS in OCS for diagnostic, screening or monitoring test ordering compared to OCS without CDSS on process and patient outcomes?
Inclusion and exclusion criteria
The inclusion and exclusion criteria to select studies for the review on the impact of CDSS in OCS on process and patient outcomes were the same as those outlined in Chapter 2, for the participants, interventions, relevant comparators, outcomes and study designs.
Data extraction strategy
Data were extracted on the study setting, clinician and patient characteristics (where reported), study design and methods, intervention and comparator systems, area of impact, CDSS characteristics, including the presence or absence of the 14 relevant features of CDSS identified as being potentially related to system success by Kawamoto and colleagues,16 and outcomes. Outcomes were summarised using descriptive summary statistics, including proportions (with 95% CIs) for categorical variables and mean [standard deviation (SD)] for continuous variables.
Data synthesis
Due to considerable heterogeneity between the studies, in terms of the type of CDSS evaluated, the output format of the CDSS, study designs and setting, and outcomes assessed, results were first broadly grouped according to the intervention (i.e. the type of information provided by the CDSS, for example, the display of test costs, corollary orders, or advice/recommendations). Studies within each broad intervention group were then further grouped according to study design. Due to the heterogeneity between studies results were therefore combined using a narrative synthesis84,85 with key demographic data for each study and relevant quantitative results tabulated. All data extraction tables are presented in Appendix 3.
Study question 3: What features of CDSS are associated with clinician or patient acceptance of CDSS in OCS?
Inclusion and exclusion criteria
Inclusion criteria for eligible studies were the same as those specified in Chapter 2, for study participants. Inclusion criteria for the interventions were also the same as those listed in Chapter 2, apart from a comparator system, i.e. an OCS without CDSS was not required for studies to be eligible for inclusion. The study outcomes that were included were clinician or patient self-reported acceptability of the CDSS. Acceptability was defined according to the definitions used in the primary studies. Study designs that were included were the same as those used to address review question 2 (i.e. RCTs, CRCTs, CCTs, ITS, CPPs and UPPs) but in addition cross sectional and longitudinal surveys, and qualitative studies were also eligible for inclusion.
Data extraction strategy
The data extracted included the study setting; clinician and patient characteristics; study methods; intervention and comparator systems (where applicable); self-reported rates/scores of clinician or patient acceptability of the CDSS; and CDSS characteristics including where reported, time taken to obtain the CDSS, time taken to use the CDSS, methods of system reasoning, CDSS knowledge base, number of data items to collect (if the data are not already available in electronic patient records), ease of data entry, ease of interpreting results and perceived applicability of the CDSS knowledge base to the clinician’s own patients. All data on clinician or patient acceptance of the CDSS were summarised using appropriate descriptive summary measures including proportions (with 95% CIs) for categorical variables.
Data synthesis
Due to considerable heterogeneity between the studies, in terms of the type of CDSS evaluated, the output format of the CDSS, study designs and setting, and outcomes assessed, results were first broadly grouped according to the intervention (i.e. the type of information provided by the CDSS, for example, the display of test costs, recommendations, or restricted lists). Where studies incorporated a second subsidiary CDSS intervention the study was grouped according to the primary outcome and aim of evaluating the CDSS, with the secondary outcome also reported within this category. Studies within each broad intervention group were then further subgrouped according to study design. Due to the heterogeneity between studies results were combined using a narrative synthesis84,85 with key demographic data for each study and relevant quantitative results tabulated.
Studies were grouped according to the type of CDSS system with key data on acceptability and specific system features presented in tables. Results were then combined using a narrative synthesis. 84,85 Differences in rates/scores of acceptability between studies were explored narratively by recourse to differences in the study setting, and CDSS characteristics.
Study question 4: What is the cost-effectiveness of CDSS in diagnostic, screening or monitoring test OCS compared to OCS without CDSS?
Inclusion and exclusion criteria
The inclusion and exclusion criteria for the systematic review of economic evaluations and cost–comparison studies were identical to those specified in Chapter 2, apart from the study design criteria. For the review, full CEA, CUA, cost–benefit analyses (CBA), CCA, and cost–comparison studies were included. Economic evaluations that only reported average cost-effectiveness ratios were only eligible for inclusion if incremental ratios could be calculated from the available published data.
Data extraction strategy
Data on study setting, clinician and patient characteristics (where reported), intervention, comparators, and outcomes were tabulated. In addition data were extracted on the study design (CEA, CUA or cost-analysis), model type or trial based study, research question, perspective, time horizon, discounting, main costs included, and sensitivity analyses.
Methods of data synthesis
Studies results were presented narratively and where possible key results presented in tables. Differences in the cost-effectiveness of CDSS in comparison with OCS alone were explored narratively by considering differences in the setting, type of tests ordered, type of CDSS and OCS, perspective, time horizon and methods of discounting.
Chapter 4 Survey results
The results from the survey of manufacturers and suppliers under the ASCC was extremely low at only 17%, with only four manufacturers providing any type of feedback. All of this was classified as being CIC and therefore does little to provide any information on the current deployment or implementation of CDSS within the NHS at the present time. For this reason and due to the lack of information provided the results of the survey are not presented within the main text of the report, but are presented in Appendix 4.
Chapter 5 Systematic reviews of the impact and acceptability of CDSS
Aims
-
1. To summarise existing published research evidence on the impact of CDSS in OCS for diagnostic, screening or monitoring test ordering compared with OCS alone on process, patient outcomes, and adverse events.
-
2. To examine what can be gleaned from the existing research evidence on the specific features of CDSS that are likely to be associated with clinician or patient acceptance of systems.
This chapter therefore firstly presents the results from the literature searches outlined in Chapter 3, Identification of relevant studies, undertaken to identify potentially relevant studies for the three reviews, followed by:
-
3. the results from the systematic review to assess the impact of CDSS with OCS versus OCS alone on process, patient outcomes and adverse events and
-
4. the results from the systematic review to examine what specific features of CDSS are likely to be associated with acceptance of the system.
Quantity and quality of research available for reviews
A total of 22,109 titles and abstracts were screened for inclusion in the three reviews to assess: (1) the impact of CDSS in OCS for diagnostic, screening, or monitoring test ordering; (2) the features of CDSS associated with clinician or patient acceptance of CDSS in OCS; and (3) the cost-effectiveness of CDSS compared to OCS without CDSS. Of the titles and abstracts screened 130 were ordered as full papers and assessed in detail. Two papers were unavailable at the time of the assessment. Of the full papers screened 95 related to the impact of CDSS on processes of care, patient outcomes or adverse events; 31 papers related to the acceptability of CDSS features to clinicians and patients; and four papers related to the cost-effectiveness of different CDSS. The overall process of study selection is shown in Figure 3.
FIGURE 3.
Process of study selection for the three reviews.
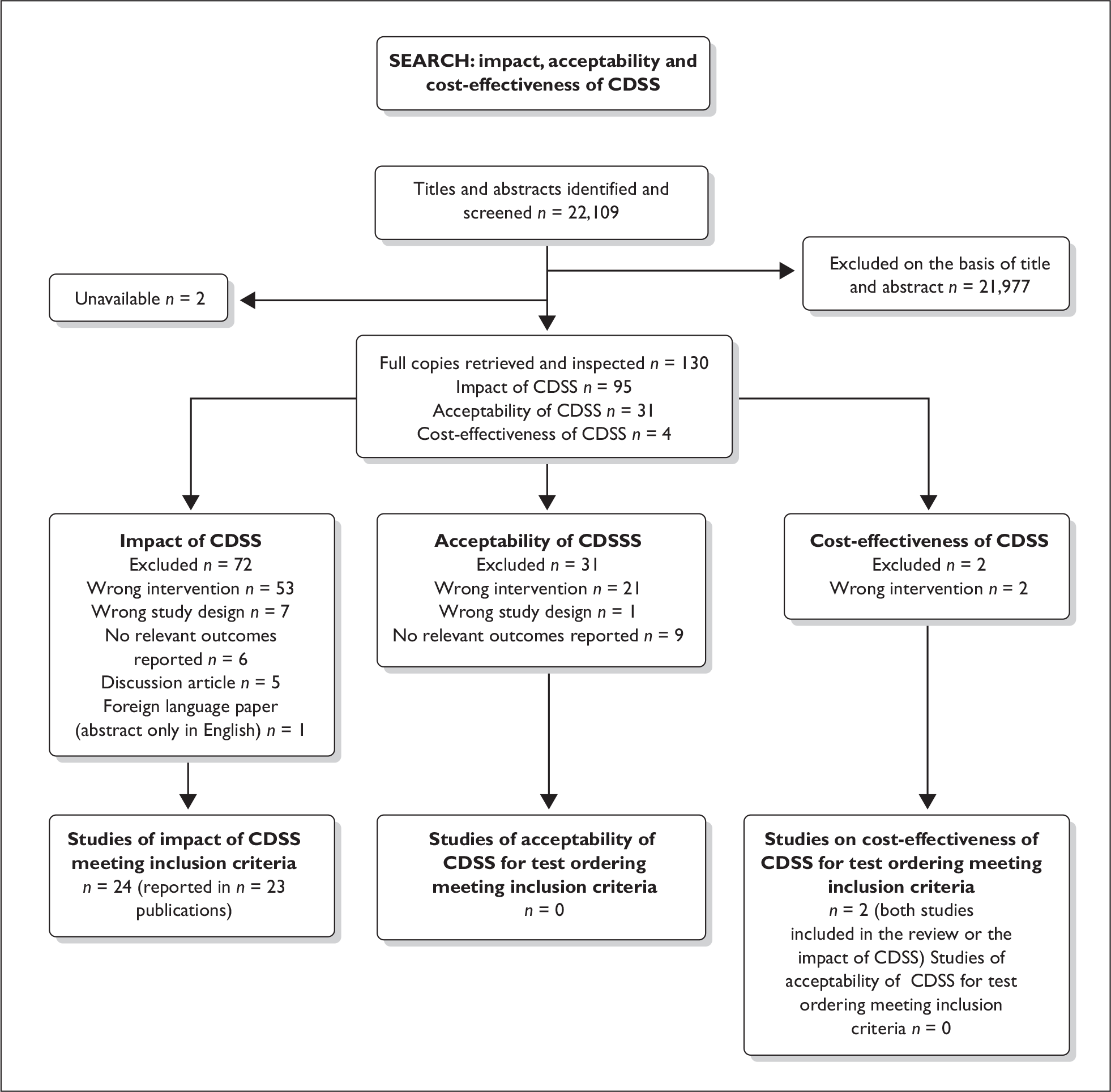
In total, therefore, 24 studies reported in 23 publications met the inclusion criteria for the review of the impact of CDSS in OCS versus OCS alone on process, patient outcomes, and adverse events. 29,32,48,50,57,86–100 Two of these studies also reported limited cost–comparison data between CDSS in conjunction with OCS versus OCS alone and were included in the systematic review of economic evaluations. 96,98 No studies were identified that met the inclusion criteria to address study question 3, on the specific features of CDSS that may be associated with physician or patient acceptance of the system.
Study question 2: What is the impact of CDSS in OCS for diagnostic, screening or monitoring test ordering compared to OCS without CDSS on process and patient outcomes?
Due to the considerable heterogeneity between the studies, in terms of the type of CDSS evaluated, the output format of the CDSS, study designs and settings, and outcomes assessed, the review of the impact of CDSS in OCS is presented as follows:
-
Overview of the quantity and quality of the included studies and CDSS characteristics.
-
Review of the evidence for studies assessing the impact of the CDSS presenting:
-
– test charges (i.e. the costs the patient would pay on that day for the specific tests ordered)
-
– previous test results (i.e. as physicians wrote an order for a specific test the patients previous test results for that test or set of previous tests results were displayed)
-
– reminders (i.e. reminders to undertake preventative patient care measures, order appropriate laboratory tests for medication monitoring, reminders regarding tests that may be redundant within that specific time interval, and reminders for appropriate guidelines for laboratory or radiological test ordering)
-
– restricted lists (i.e. restriction on the ordering of multiple laboratory tests simultaneously with or without limits on forward ordering)
-
– recommendations [i.e. guideline based recommendations for appropriate screening or monitoring intervals, referral for an MRI (magnetic resonance imaging) or CT (computerised tomography) scan, or the number of tests conducted per patient/day based on the CDSS recommendations].
-
For each intervention, text and summary tables are presented on:
-
the quantity and quality of the studies
-
the study characteristics (summary table)
-
CDSS characteristics (text and summary table)
-
the study results
-
an overview of the impact of the CDSS intervention(s).
Overview of the quantity and quality of the research available
A total of 24 studies reported in 23 publications met the inclusion criteria. 29,32,48,50,57,86–101 The results from two RCTs, one assessing the impact of the display of test charges on clinical laboratory test orders and the other the impact on radiological test volumes conducted at the same institution, were reported together in one paper by Bates and colleagues. 101 Two further studies included in the review of the impact of CDSS in OCS also reported limited cost–comparison data and were therefore also included in the review of the cost-effectiveness of CDSS in OCS compared to OCS alone. 96,98
As previously stated there was considerable heterogeneity between the identified studies in terms of the type of CDSS assessed, the settings in which the studies were conducted, the patient populations, whether the studies focused on the impact of the CDSS on a single type of laboratory or imaging test order or on multiple tests and study designs. The nomenclature used to attempt to group the studies is therefore highly simplistic, as a limited number of studies assessed the impact of multiple interventions. However, in grouping the studies the predominant CDSS intervention, for example the display of restricted test order lists, was chosen for the grouping. This means that the results of these studies are more likely to be confounded from concomitant interventions than is readily apparent from rudimentary methods used to devise the study groupings.
Overall, in total, the 24 studies could broadly be grouped into those that assessed the impact of the display of test charges (n = 3),86,101 those that displayed patients previous test results (n = 2),88,89 those that provided reminders (n = 10),24,29,48,87,91–94,102,103 studies that displayed restricted lists of test orders (n = 2),95,96 and those in which the CDSS provided a recommendation (n = 7). 29,32,48,50,57,86–101 A summary of the type of CDSS intervention by the number of studies and study design is displayed in Table 4.
| Study design | CRCT | RCT | CCT | X-over | ITS | CPP | UPP | Total |
|---|---|---|---|---|---|---|---|---|
| Intervention group | ||||||||
| Display of test charges | 1 | 2 | – | – | – | – | – | 3 |
| Previous test results | – | – | 1 | – | – | – | 1 | 2 |
| Display of reminders | 4 | 1 | 1 | 1 | 1 | – | 2 | 10 |
| Restricted test lists | – | – | – | – | 1 | 1 | – | 2 |
| Recommendations | 2 | 1 | – | – | – | – | 4 | 7 |
| Total | 7 | 4 | 2 | 1 | 2 | 1 | 7 | 24 |
Across the 24 studies, seven CRCTs (29%),24,32,48,50,86,90,91 four RCTs (17%),92,97,101 two non-randomised controlled trials (8%),57,88 one randomised crossover trial (4%),29 two ITS studies (one with a AB-AB-AB design) (8%),93,95 one CPP study (4%),96 and seven UPP studies (29%)87,89,94,98–100,104 were identified. Duration of follow-up varied widely with a median of 7 months (range: 2–72). Sixty-five per cent of studies described funding from the public sector,24,29,48,86,89–92,94–97,101 13% stated funding was from the private sector,50,57,98 while 22% did not report the funding source. 32,87,93,99,100 Developers of the CDSS software were also outcome evaluators in 62.5% of the studies,24,29,48,86,88,89,91–93,95,96,99,101,104 were evaluators in part (collaboration) in one study (8%),90 and were not involved in the system evaluation in 29% of the studies. 50,57,87,94,97,98,105
In terms of the study settings, of the 24 studies the majority (17, 71%) were conducted in the USA,23,28,47,86–91,94–96,98,103 followed by two (8%) each conducted in the UK32,99 and Spain. 7,98 The remaining three studies were conducted in France (4%),93 the Netherlands (4%),96 and Belgium (4%) respectively. 100 Of the studies conducted in the USA, 12 of the 17 studies had been undertaken at one of three specific sites: four studies were conducted at the Wishard Memorial Hospital, Indianapolis, IN, or at one or their outpatient centres;24,29,48,86 five had been conducted at the Brigham and Women’s Hospital, Boston, MA;88,91,92,101 and three had been conducted at the Vanderbilt University Medical Center. 89,95,104 Across the 24 studies, eight were conducted in a primary care setting,32,50,57,86,90,94,96,98 one was conducted in both a primary and secondary outpatient care setting,91 two were conducted in secondary care outpatients,29,97 11 were conducted in secondary care inpatients,24,48,87,88,92,95,99–101,104 one was conducted in a secondary care intensive care unit (ICU),89 and one in an accident and emergency department. 93
In relation to the patients indication(s) for undergoing either laboratory or radiological test imaging, the majority of the studies (n = 10) included patients with a mixture of diagnoses that were unspecified. 24,29,48,86,87,90–92,94,97 Two studies included only medical or surgical patients,101 and one included patients undergoing testing for suspected rheumatic disease. 88 The focus of a further three studies were test specific, and so included patients undergoing arterial blood gas (ABG) laboratory testing,89 serum magnesium level testing,95 or a range of blood tests96 respectively. Of the remaining studies, one included patients with a diagnosis of diabetes mellitus,57 three included patients with hyperlipidemia,32,50,98 two included patients undergoing assessment or liver transplantation,101,102 and two included patients in which radiological imaging was indicated. 93,104
The outcomes reported reflected the intended impact of the CDSS and included test volumes,32,50,86,88,89,95,96,98,99,101,104,105 test costs,86,97,99,101, compliance with reminders,24,29,48,90,91,94 compliance with recommendations,97,104 guideline compliance,87,93 and order appropriateness in terms of test frequency. 57,92 Only four studies reported potential adverse effects of test cancellations. 86,92,101 Additionally, all the studies focused on the decision to order a laboratory or imaging test, rather than on the impact of CDSS on the interpretation of test results. Of the 24 included studies, six were focused more broadly on patient management, and included the ordering or appropriateness of pharmacological prescriptions, vaccinations or health-care advice. Therefore only limited outcomes in terms of the impact of CDSS on laboratory test rates or appropriateness were reported in these studies. 24,29,48,50,97,98
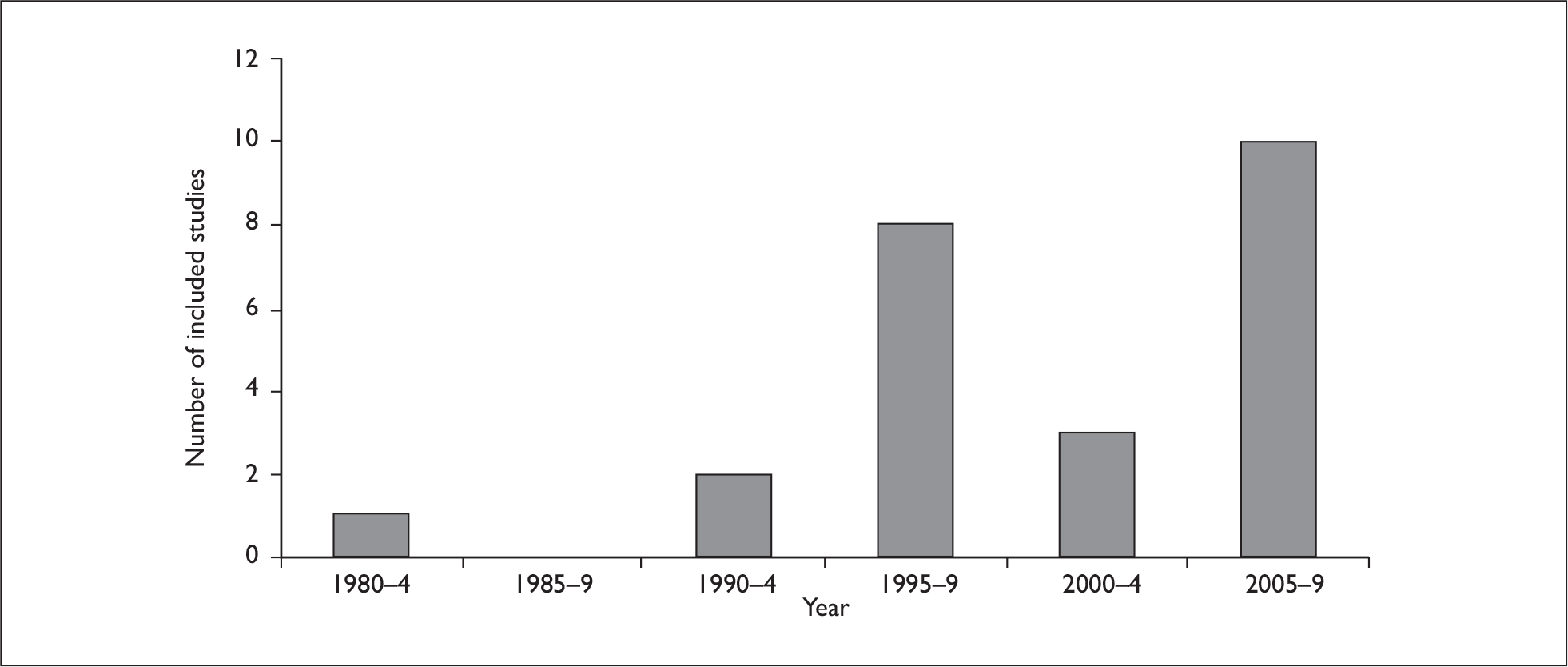
As well as the studies being heterogeneous in terms of the type of CDSS assessed, the settings, patient groups and study designs, the year in which they were undertaken and published also varied considerably. Of the 24 eligible studies, one was published in the period 1980–4, two in 1990–4, eight in 1995–9, three in 2000–4 and 10 in 2004–9.
While the year of study publication can only act as a proxy for the year(s) in which the research was conducted, it can be postulated, particularly in terms of the older CDSS, that these systems may now be obsolete, or will have been upgraded and changed considerably with further technological development in clinical settings. Many of the older studies may therefore now not be of direct relevance to CDSS that are currently available, and greater weight should be given to the more recently published studies that have assessed technologies that are still available or may have undergone limited changes. A summary of the number of studies identified by year is displayed in Figure 4 and a summary of study characteristics of the 24 identified studies in Table 5.
FIGURE 4.
Number of included studies by year.
| Study ID | Intervention type | Specific CDSS | Study design | Country in which study was conducted | CDSS primary users | N primary results based on | Health-care setting | Patient condition(s) | Test indication |
|---|---|---|---|---|---|---|---|---|---|
| Tierney (1990)86 | Display of test charges | Home-grown (Wishard Memorial Hospital) | CRCT | USA | Physicians | 125 physicians | Primary care | Mixed | D |
| Bates (1997)101 | Home-grown (Brigham and Women’s Hospital) | 2 RCTs | USA | Physicians |
Laboratory trial n = 7090 patients Radiology trial n = 17,381 patients |
Secondary care (inpatients) | Medical and surgical | NR | |
| Solomon (1999)88 | Display of previous test results | Home-grown (Brigham and Women’s Hospital) | CCT | USA | Physicians | 225 physicians | Secondary care (inpatients) | Rheumatic disease | D |
| Bansal (2001)89 | Home-grown (Vanderbilt University Medical Centre) | UPP | USA | Physicians | 6 ICUs | Secondary care (ICU) | Mixed (ABG test indicated | D and M | |
| Overhage (1996)48 | Reminders | Home-grown (Wishard Memorial Hospital) | CRCT | USA | Physicians | 78 physicians | Secondary care (inpatients) | Mixed | S |
| Overhage (1997)24 | Home-grown (Wishard Memorial Hospital) | CRCT | USA | Physicians | 86 physicians | Secondary care (inpatients) | Mixed (general medicine) | M | |
| Palen (2006)90 | Clinical Information System (IBM, CO) | CRCT | USA | Physicians | 207 physicians | Primary care | Mixed | M | |
| Matheny (2008)91 | Home-grown (Brigham and Women’s Hospital) | CRCT | USA | Physicians | 1922 patients | Primary care and secondary care (outpatients) | Mixed | M | |
| Bates (1999)92 | Home-grown (Brigham and Women’s Hospital) | RCT | USA | Physicians | 11,586 patients | Secondary care (inpatients) | Mixed | NR | |
| O’Connor (2005)57 | Epic Systems | CCT | USA | Physicians | 122 patients | Primary care | Diabetes | M | |
| McDonald (1980)29 | Home-grown (Wishard Memorial Hospital) | Randomised crossover trial | USA | Residents, interns, nurses, clinicians | 31 physicians | Secondary care (outpatients) | Mixed | NR | |
| Carton (2002)93 | NR | ITS (AB-AB-AB design | France | Physicians | 6434 imaging tests | Secondary care (A & E) | Mixed (radiological imaging indicated) | D and S | |
| Abboud (2006)87 | INVISION®, (Siemens Medical Solutions, PA) | UPP | USA | Physicians | 275 patients | Secondary care (paediatric inpatients) | Mixed (paediatric inpatients) | M | |
| Steele (2005)94 | NR | UPP | USA | Physicians | 19,076 patients | Primary care | Mixed | M | |
| Rosenbloom (2005)95 | Restricted lists | WizOrder (Vanderbilt University Medical Centre) | ITS | USA | Physicians, nurses, practitioners, medical students | 194,192 patients | Secondary care (inpatients) | Mixed (serum magnesium test indicated) | M |
| Poley (2007)96 | NR (Home-grown) | CPP | Netherlands | Physicians | 134 practices | Primary care | Mixed (blood test indicated) | NR | |
| Hobbs (1996)32 | Recommendations | Primed (Wolfson Research Laboratories, University of Birmingham, UK) | CRCT | UK | Physicians | 25 practices | Primary care | Hyperlipidemia | S |
| Cobos (2005)50 | NR | CRCT | Spain | Physicians | 2221 patients | Primary care | Hyperlipidemia | S and M | |
| Apkon (2005)97 | DSIT tool Problem-Knowledge Couplers (PKC Corp, VT) | RCT | USA | Physicians | 1902 patients | Secondary care (outpatients) | Mixed | S and M | |
| Bassa (2005)98 | NR | UPP | Spain | Physicians | 500 patients | Primary care | Hyperlipidemia | S and M | |
| Sanders (2001)104 | WizOrder (Vanderbilt University Medical Centre) | UPP | USA | Physicians, nurses, receptionists, medical students | 1446 physicians | Secondary care (inpatients) | Head or brain CT or MRI indicated | D, S and M | |
| Nightingale (1994)99 | Home-grown (Wolfson Computer Laboratory, Queen Elizabeth Hospital, Birmingham, UK) | UPP | UK | Physicians | 1487 patients | Secondary care (inpatients) | Patients undergoing assessment or liver transplantation | D, S and M | |
| Boon-Falleur (1995)100 | Computer Laboratory, Queen Elizabeth Hospital, Birmingham, UK) | UPP | Belgium | Physicians | 217 patients | Secondary care (inpatients) | Patients undergoing assessment or liver transplantation | D, S and M |
Study quality
As can be expected from the heterogeneity of the identified studies, the level of reporting and study quality was highly variable. Across the studies the median length of follow-up was 7 months, but this ranged dramatically, from 2 months to 72 months. An assessment of study quality is provided in each specific section of the report according to CDSS intervention type.
Overview of the CDSS characteristics according the 15 features of CDSS suggested by Kawamoto and colleagues16 as having a potential impact on CDSS effectiveness
An overview of the CDSS characteristics from the 24 studies postulated by Kawamoto and colleagues16 as having a potential impact on the effectiveness of the CDSS is displayed in Figure 5. The figure depicts 14 of the characteristics, but omits the use of a computer to generate the decision support, as this formed part of the inclusion criteria by which studies were selected for inclusion in the review, and therefore is not relevant to the current review scope.
FIGURE 5.
Overview of the CDSS characteristics of the included studies. NA, not applicable; NR, not reported.
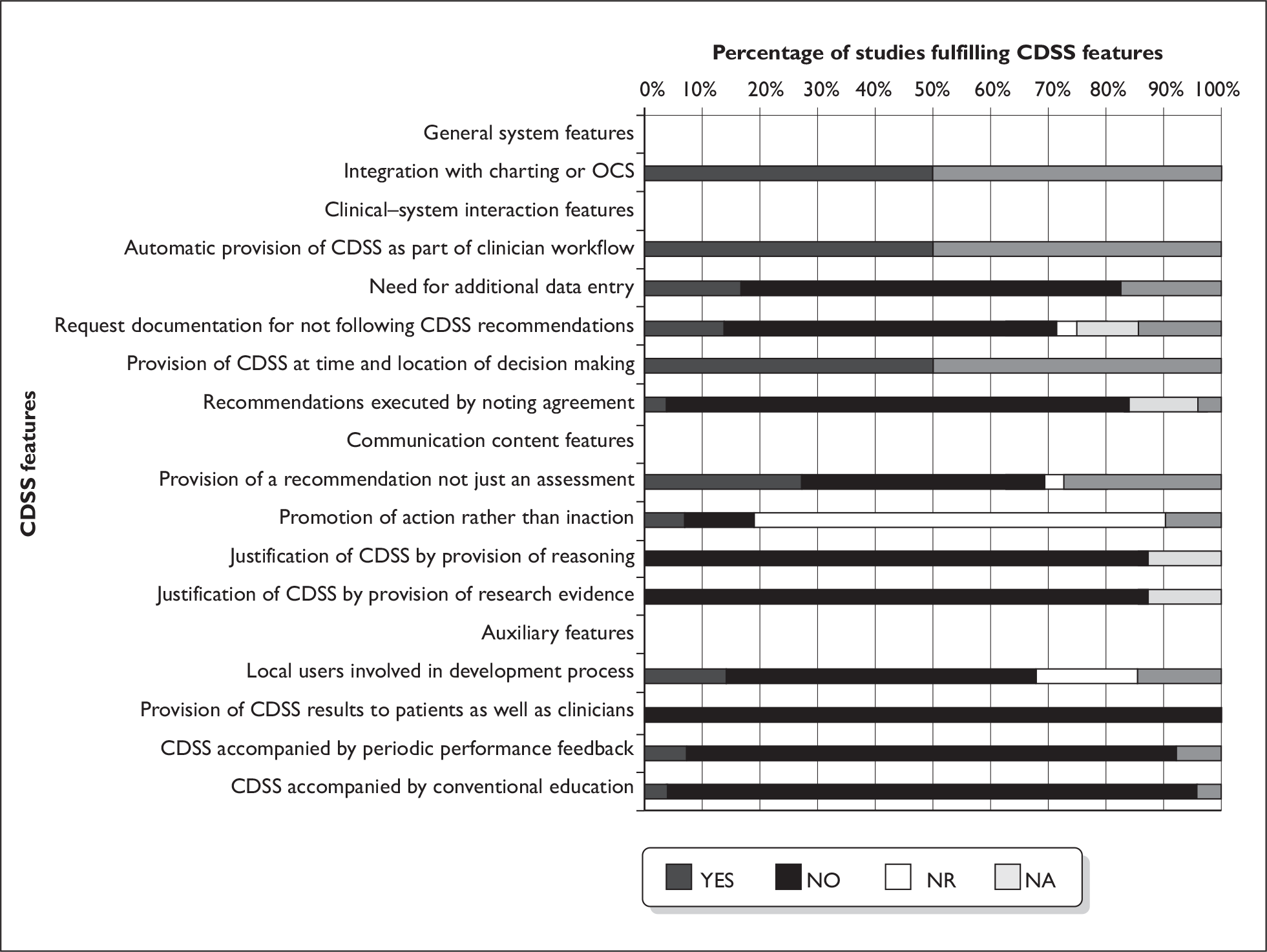
As can be seen from Figure 5 all the studies were integrated with charting or OCS to support workflow integration, provided automatic decision support as part of the clinician workflow, and provided support at the time and location of decision-making. In the majority of studies (79%, 19/24) there was no need for additional data entry by the clinician. Again in the majority of studies (67%, 16/24) the CDSS did not request documentation for not following the recommendations (where these were made), and in 83% (20/24) of studies there was no need for the clinician to execute any recommendations by noting agreement.
Only 38% (9/24) of studies provided a recommendation with the rest displaying either test charges, previous test results, reminders, or displaying restricted test ordering lists. In the majority of studies (79%, 19/24) it was not reported whether the CDSS output would be likely to promote action by the clinician rather than inaction (for example, suggesting a different course of action or test if appropriate rather than suggesting that the test order was cancelled). Additionally in 88% (21/24) of studies no justification of the CDSS output was provided either by recourse to the provision of CDSS reasoning or the research evidence on which this was based.
Local users of the CDSS were involved in the development process in only 17% (4/24) of the studies, with the majority of studies (63%, 15/24) not involving the health-care professionals who would ultimately use the system either in the development or piloting of the system. None of the studies provided decision support results to patients as well as clinicians, and only 8% (2/24) of the studies provided periodic performance feedback to clinicians. Additionally, only 4% (1/24) of the studies provided concomitant conventional education alongside use of the CDSS.
Studies assessing the impact of the display of test charges
Quantity and quality of the studies
The impact of the display of test charges on the number of laboratory and radiology test orders was assessed in one CRCT and two RCTs reported in two publications. 86,101 In all three trials, the objective was to reduce the number of tests that were ordered. The CRCT, by Tierney and colleagues86 was conducted in the outpatient General Medicine Practice of the Regenstrief Health Centre, Indianapolis, IN, USA (the primary outpatient facility for the Wizard Memorial Hospital). In the CRCT the physician was the unit of randomisation, with 121 physicians with a total of 8392 patient visits during the intervention period included. 86 The trial consisted of a 14-week pre-intervention period, a 26-week intervention, and a 26-week post-intervention period conducted simultaneously from January 1988. The trial included all patients and visits to physicians, whether scheduled or unscheduled. It also included all outpatient diagnostic tests (i.e. all laboratory and radiological imaging studies) but excluded 24-hour electrocardiographic monitoring, treadmill exercise testing, and endoscopic procedures. 86 The specific CDSS evaluated was not reported, but as the primary care practice was associated with the Wishard Memorial Hospital, it can be postulated to be part of the ‘home-grown/in-house’ OCS and CDSS developed specifically for use at this site. Physicians in both the intervention and control groups entered all their orders for tests through a computer workstation. The CDSS intervention in the trial consisted of the display of the charge the patient (or the insurer) would pay for the current test when ordered and the total charges for all tests ordered for that patient on that day. Additional fees (e.g. for the interpretation of test results) were not included.
The two simultaneously conducted RCTs by Bates and colleagues101 were undertaken on all medical and surgical inpatients at Brigham and Women’s Hospital, Boston, MA, USA. The trials consisted of an assessment of the impact of the display of test charges on laboratory and radiological imaging test orders (length of trial follow-up 4 and 7 months respectively). The patient was the unit of randomisation in the trials, with 7090 patients included in the laboratory trial, and 17,381 in the radiology trial. Therefore physicians could treat both intervention and control group patients, therefore contamination was a risk thereby potentially reducing the effect size of any potential benefits of the CDSS. The trials were conducted between February and October 1994. The trials included all laboratory test orders and the 35 most frequently ordered imaging tests. Charges for the remainder of the radiological tests were not displayed. Physicians could enter test orders for patients though the OCS, with individual and total test charges for each patient order displayed to physicians in the intervention group. Alternatively during the trial periods, specimens could be obtained and sent directly to the laboratory. Therefore the number of tests ordered during the trials was less than the number performed. For the laboratory and radiological trials respectively only 53% and 74% of tests performed had a corresponding computer order. Again, the specific CDSS assessed was not reported, but would appear to be part of the home-grown/in-house OCS and CDSS developed by Brigham and Women’s Hospital. A summary of the study characteristics from the trials is displayed in Table 6.
| Study ID | Setting/country | Study design | Number of sites | Intervention (I)/Comparison (C) | Study description | Area of impact | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| Process outcomes | Adverse effects of test cancellation | |||||||
| Tierney (1990)86 | Outpatient physicians, Wishard Memorial Hospital, USA | CRCT | 1 |
(I) OCS + CDSS: test charges displayed (C) OCS alone |
Physicians (n = 121) were randomly allocated into intervention and control groups. Trial conducted over a 26-week period with 8392 patients with n = 4254 intervention group and n = 4138 control group | Test volume and costs |
Number of tests ordered per visit Charges for tests ordered per visit (US$) Number of tests (scheduled visit) Number of tests (unscheduled visit) Charges for tests ordered (scheduled visit) (US$) Charges for tests ordered (unscheduled visit) (US$) Number of tests ordered per visit (post-intervention period) Charges for tests ordered per visit (post-intervention period) (US$) |
Number of hospitalisations Number of A&E visits Number of outpatient visits |
| Bates (1997)101 | Medical and surgical inpatients, Brigham and Women’s Hospital, USA | 2 RCTs | 1 |
(I) OCS + CDSS: laboratory and radiology test charges displayed (C) OCS alone |
Two RCTs including laboratory and radiological imaging tests. Patients n = 7090 laboratory tests (n = 3536 intervention group and n = 3553 control group) and n = 17,381 imaging tests (n = 8728 intervention group and n = 8653 control group) randomised to intervention and control groups. Trials conducted over 4 and 7 months | Test volume and costs |
Number of tests ordered per admission Number of tests performed per admission Charges for tests ordered per admission (US$) Charges for tests performed per admission (US$) Total hospital charges (US$) |
Length of patient stay (days) |
Outcomes
Process outcomes
The areas of impact in the test ordering process assessed in all three trials were test volume and costs. 86,101 These included the number of tests ordered per visit/admission,86,101 charges for tests per visit/admission,86,101 and total hospital charges per admission. 101
Adverse effects of test cancellation
All three trials assessed and reported the impact of the display of test charges on the subsequent decision by the physician on the basis of the display of test costs to order or cancel the indicated test(s) and the potential associated adverse effects. 86,101 Tierney and colleagues86 assessed patients’ use of other resources and their health outcomes through assessing the number of hospitalisations, visits to the accident and emergency department, and outpatient visits during both the trial intervention period, and a 26-week post-intervention follow-up. Bates and colleagues101 assessed the length of patients hospital stay in both the laboratory and radiology trials.
Study quality
The methods of randomisation and whether allocation concealment was attained was not reported in any of the three trials. 86,101 Trial eligibility criteria were adequately specified and sufficient details of physician/patient baseline characteristics were reported. In all three trials baseline characteristics were balanced between treatment groups, indicating that the method of randomisation, while not specified, was probably appropriate. Blinding of physicians as in all trials of CDSS plus OCS to treatment allocation was not possible, and it was unclear whether outcome assessors were blinded. Moreover, as the unit of randomisation was the patient in the two trials by Bates and colleagues101 contamination bias may be present. This could potentially lead to an underestimation of the impact of the CDSS. Data analyses to account for clustering by physician in the trial by Tierney and colleagues86 was adequate and appropriate. Data analyses were only conducted on an ‘intention to provide or communicate information’ basis in the two trials by Bates and colleagues. 101 However, the rate of attrition (3.2%) was low in the trial by Tierney and colleagues86 and therefore failure to conduct the analysis on an ‘intention to provide or communicate information’ basis is unlikely to impact significantly on the results attained.
CDSS characteristics
A summary of the key characteristics, including the 14 features of CDSS proposed by Kawamoto and colleagues16 as predictors of system success or failure are displayed in Table 7. The specific CDSS, as stated in Quantity and quality of the studies was not reported in any of the trials, but in the trial by Tierney and colleagues86 would appear to be the home-grown/in-house OCS and CDSS developed by the Wishard Memorial Hospital, Indianapolis, IN, USA. In the two trials by Bates and colleagues101 the CDSS again appears to be a home-grown/in-house system, this time developed specifically by Brigham and Women’s Hospital, Boston, MA, USA for use at this site.
| Study ID | Tierney (1990)86 | Bates (1997)101 |
|---|---|---|
| CDSS characteristics | ||
| 1. Name of CDSS (if any) | NR | NR |
| 2. CDSS reasoning methods | NR | NR |
| 3. CDSS knowledge base | Test costs | Test costs |
| 4. Information used in CDSS | Costs of biochemical and radiology tests | Costs of biochemical and radiology tests |
| 5. Time to complete CDSS (minutes) | 11.5 seconds compared with 10 seconds in control group | NR |
| 6. CDSS output format | Display of test costs | Display of test costs |
| 7. Is a description of pilot testing with users prior to implementation provided? | Yes | No |
| 8. Is user instructional training at the time of implementation described? | No | No |
| General system features | ||
| 9. Is the CDSS integrated with charting or OCS to support workflow integration?a | Yes | Yes |
| Clinician–system interaction features | ||
| 10. Is automatic provision of CDSS output provided as part of clinician workflow?a | Yes | Yes |
| 11. Is there a need for additional data entry by the clinician other then the specification of which test orders?a | No | No |
| 12. Does the CDSS request documentation of the reason for not following CDSS recommendations?a | NA | NA |
| 13. Does CDSS provide output at the time and location of decision making?a | Yes | Yes |
| 14. Are the CDSS recommendations executed by the clinician noting agreement?a | NA | NA |
| Communication content features | ||
| 15. Does the CDSS provide a recommendation rather than just an assessment?a | No | No |
| 16. Does the CDSS promote action rather than inaction?a | NR | NR |
| 17. Does the CDSS justify the output of provision of reasoning?a | NA | NA |
| 18. Does the CDSS justify the output by provision of research evidence?a | NA | NA |
| Auxiliary features | ||
| 19. Were the local users involved in the CDSS development process?a | No | NR |
| 20. Is the CDSS output provided to patients as well as clinicians?a | No | No |
| 21. Does the CDSS provide periodic summaries of performance feedback?a | No | No |
| 22. Is the CDSS used in conjunction with conventional education?a | No | No |
The CDSS reasoning methods were not reported in any of the three trials. 86,101 Both systems used laboratory and radiological test costs as the system information source, and the display of test costs as the output format. 86,101 The time to complete the CDSS was only reported in the trial by Tierney and colleagues86 with physicians in the intervention group taking an average of 11.5 seconds for test ordering, compared with 10 seconds taken by physicians in the control group. None of the trials describe any form of user training prior to implementation of the CDSS. 86,101
In relation to the 14 features of CDSS proposed by Kawamoto and colleagues16 the CDSS in all three trials was integrated as part of the OCS, did not require additional data entry by the physician, and provided output automatically as part of the consultation workflow. 86,101 No reasons were required to be documented by the system for not following CDSS recommendations, as only test and total test charges were displayed, and therefore no specific recommendations to order or cancel specific tests were provided by the system. Again, as only test charges were displayed rather than specific recommendations, there was no need for these to be justified by recourse to either the system reasoning methods or the provision of research evidence supporting the recommendations.
It was unclear whether local system users were directly involved in the CDSS development. The data provided by the CDSS was used by the physician alone, and did not appear to provide period summaries of performance feedback. In all three trials the CDSS output information appeared to be used alone, and was not combined in conjunction with educational information regarding the charges for specific tests, and the indications for test ordering. 86,101
Results
Process outcomes
In the CRCT by Tierney and colleagues86 during the 14-week pre-intervention period there were no significant differences between the intervention and control groups in the number of tests ordered per visit [intervention group: 1.8 (SD: 0.9); control group: 1.7 (SD: 0.8)], or the total charges for tests per visit [intervention group: US$54.7 (SD: 28); control group: US$54.8 (SD: 22)]. During the 26-week trial intervention period, significantly fewer tests (–14%) were ordered per patient visit by intervention group physicians compared to those in the control group [intervention group: 1.6 (SD: 0.7) tests per patient; control group: 1.8 (SD: 0.9); % difference: –14; p < 0.005]. Correspondingly patient test charges were –13% (US$6.7), significantly lower in the intervention group relative to those in the control group [intervention group: US$45.1 (SD: 22.0); control group: US$51.8 (SD: 22.0); % difference: –13; p < 0.05].
Analyses of data by physician status [residents (n = 99); faculty (n = 22)] indicated that residents in the intervention group ordered 15.0% fewer tests than the residents in the control group [intervention group: 1.6 (SD: 0.7); control group: 1.9 (SD: 0.9); % difference: –15.0; p < 0.005], resulting in a 13.0% (US$7.1 per visit) reduction in test charges [intervention group: US$45.9 (SD: 22.0); control group: US$53.0 (SD: 22.2); % difference: –13.0; p < 0.05]. However, while faculty members in the intervention group ordered 7.9% fewer tests than those in the control group [intervention group: 1.4 (SD: 0.60); control group: 1.50 (SD: 0.70); % difference: –8.0; p > 0.05] this was not significantly different between the groups. Corresponding test charges per visit, while lower (–11.0%) in the intervention group [$41.8 (SD: 23.0)] were also not significantly different from those in the control group [US$47.1 (SD: 21.3); p > 0.05]. Differences in the size of the reduction in the number of tests ordered between resident and faculty physicians may reflect differences between the two physician groups in baseline test ordering rates, where it was observed in the pre-intervention period that facility physicians ordered 17% less tests [intervention group: 1.5 (SD: 0.6); control group: 1.5 (SD: 0.7)] than residents [intervention group: 1.9 (SD: 1.0); control group: 1.8 (SD: 0.80)]. Therefore it can be posited that the incremental effect of displaying test charges on test ordering rates may be smaller when test ordering rates are lower, or that the display of test charges lowers residents test ordering rates bringing closer to those observed by faculty members.
Further separate analyses of scheduled visits (return appointments and appointments for new patients) which constituted 80% of appointments during the intervention phase, versus unscheduled visits, indicated that physicians in the intervention group ordered 16.8% fewer tests than those in the control group during scheduled visits [intervention group: 1.6 (SD: 0.8); control group: 1.9 (SD: 0.9); % difference: –17; p < 0.01]. This equated to a significant 15.3% reduction (US$8.2) in test charges per visit [intervention group: US$45.3 (SD: 22.8); control group: US$53.4 (SD: 23.0); % difference: –15.3; p < 0.01]. Likewise, for unscheduled visits, physicians in the intervention group ordered significantly (–11.4%) fewer tests [1.2 (SD: 0.7)] compared to the control group, [1.3 (SD: 0.9)] which resulted in a significant [–9.7% (US$3.8)] reduction in charges per visit [intervention group: US$35.6 (SD: 21.3); control group: US$39.9 (SD: 25.1); % difference: –9.7; p < 0.05]. Differences in test ordering rates between scheduled and unscheduled visits may reflect the fact that for scheduled visits physicians’ habits and practice patterns may be the main factors in decision to order tests, whereas for unscheduled visits, patients’ symptoms and clinical condition may dominate decisions about testing.
Post-intervention follow-up
Further data on 74 physicians (n = 39 intervention group; n = 35 control group) who remained in the practice during the 19-week post-intervention period were collected in order to assess any lasting effects of the intervention. During the pre-intervention period, there were no statistically significant differences in test ordering rates between the physicians in the intervention group who remained in the practice and control [1.8 (SD: 0.9) vs 1.8 (SD: 0.9)] tests per visit respectively. However, during the intervention period, physicians in the intervention group ordered 17.6% fewer tests than the control group [intervention group: 1.6 (SD: 0.7) vs control group: 1.9 (SD: 1.0); % change: –17.6%; p < 0.05]; resulting in a significantly [14.7% (US$7.9)] lower charges per visit [intervention group: US$45.8 (SD: 22.1) vs control group: US$53.7 (SD: 24.8); p = 0.08]. During the post-intervention period, physicians in the intervention group ordered only 7.7% fewer tests [1.7 (SD: 0.8) vs 1.8 (SD: 0.9); p > 0.05] per visit. The lasting effect on charges was even weaker. Charges for tests ordered by physicians in the intervention group was only 3.5% (US$1.8 per visit) lower than test charges ordered by control group physicians [US$48.3 (SD: 22.6) vs US$50.0 (SD: 21.7) respectively; p > 0.05]. This indicates that there was little evidence of any learning effect from the intervention of the display of test charges during the intervention period.
Adverse effects of test cancellation
To assess whether any potential cost savings resulting from the intervention were potentially offset by increases in patients’ use of other resources or worse outcomes, the number of hospitalisations, emergency room visits, and visits to the General Medicine Practice and all other outpatient clinics were recorded for all patients who attended the practice during the intervention phase, and follow-up throughout the 26-week post-intervention period. There were no significant differences in the number of hospitalisations between patients in the intervention and control groups [0.2 (SD: 0.6) vs 0.2 (SD: 0.6)], emergency room visits [1.0 (SD: 1.7) vs 1.00 (SD: 1.7)] or outpatient visits [4.3 (SD: 3.4) vs 4.3 (SD: 3.4)] respectively.
In both the laboratory and radiological imaging test order trials conducted by Bates and colleagues101 patients groups were well balanced with respect to age, gender, race, insurer, hospital service at admission and diagnostic-related group (DRG) at baseline. In the laboratory test order trial, during the 4-month intervention period, there were no significant differences between intervention and control groups for the mean number of tests ordered per admission [intervention group: 25.6 (SD: 38.0); control group: 26.8 (SD: 43.4); % differences: –4.5; p = 0.074]. Correspondingly there were also no significant differences in charges for tests ordered per admission between the two groups [intervention group: US$739 (SD: 1129); control group: US$771 (SD: 1310); % difference: –4.2; p = 0.97]. The mean values for the number of tests performed as opposed to ordered and their associated costs per admission, while higher in both groups than the number of tests performed, were not significantly different between groups, with the mean number of tests performed being –5.4% lower in the intervention group [intervention: 46.9 (SD: 79.2); control group: 49.6 (SD: 94.4); % change: –5.4%; p = 0.87]; and the costs being –4.9% lower in this group [intervention group: US$1423 (SD: 2730); control group: US$1496 (SD: 3147); % change: –4.9%; p = 0.89]. This translated into a US$73 reduction in the costs for tests performed per admission between the intervention and control groups. Multiple linear regression analyses that adjusted for age, gender, race, admission service, and DRG weight also showed no significant differences for the number of laboratory tests that were ordered and performed per admission [intervention group: 25.7 (SD: 0.6); control group: 26.6 (SD: 0.6); % change: –3.4%; and intervention group: 47.4 (SD:1.1); control group: 49.1 (SD: 1.1); % change: –3.3%, for the number of tests ordered and performed respectively]. Charges for tests ordered and performed in the analyses were correspondingly not significantly different between groups [intervention group: US$743 (SD: 17); control group: US$766 (SD: 17); % difference: –3.0 and intervention group: US$1440 (SD: 37); control group: 1478 (SD: 38); % change: –2.6% respectively].
For the radiological imaging test trial, during the 7-month intervention period the differences between the intervention and control groups were smaller than for the clinical laboratory test trial. The number of tests ordered and performed per admission were nearly identical between groups {[intervention group: 1.8 (SD: 4.4); control group: 1.8 (SD: 4.7); % change: 0; p = 0.13] and [intervention group: 1.5 (SD: 3.6); control group: 1.5 (SD: 4.1); % change: 0; p = 0.10 respectively]}. Correspondingly, there were only minor insignificant changes in both the costs for tests ordered and those performed. The costs for tests ordered per admission only decreased by –0.4% [intervention group: US$275 (SD: 688); control group: US$276 (SD: 737); % change: –0.4; p = 0.10], whilst those for tests performed increased by 2.3% [intervention group: US$220 (SD: 473); control group: US$215 (SD: 515); % change: +2.3%; p = 0.03]. Multiple linear regression analyses again adjusting for age, gender, race, admission service, and DRG weight also showed no significant differences for the number of tests ordered [intervention group: 1.9 (SD: 0.04); control group: 1.9 (SD: 0.04); % change: +0.5] and tests performed [intervention group: 1.6 (SD: 0.03); control group: 1.6 (SD: 0.03): % change: +0.6]. Again, due to insignificant differences between the groups in the number of tests ordered and performed, there were no significant differences between groups for the costs of tests ordered [intervention group: US$296 (SD: 7.0); control group: US$296 (SD: 7.1); % change: 0] or performed [intervention group: US$234 (SD: 4.7); control group: US$228 (SD: 4.8);% change: +2.6].
Adverse effects of test cancellation
Comparison of the total length of hospital stay between groups in both the laboratory and radiological imaging test trials showed no significant differences between groups in either of the trials. The median length of stay in both groups in the laboratory trial was 4 days (range: 2–7), and in the radiology trial was 3 days in both groups (range: 2–7 and 2–6) for the intervention and control groups respectively.
Summary of studies assessing the impact of the display of test charges
Process outcomes
Three trials, including a total of 32,863 patients assessed the impact of the display of test charges on the number of tests ordered. In both studies the aim was to reduce the number of tests ordered and their associated charges. Both studies were conducted in the USA, with one conducted in outpatients at a general medicine practice,86 and the other two conducted on medical and surgical inpatients. 101 The duration of follow-up across the trials ranged from 4 to 14 months (including the post-intervention follow-up undertaken in the CRCT by Tierney and colleagues). 86 Two of the trials focused predominantly on the effects of the display of test charges on laboratory test orders,86,101 while the other focused on radiological imaging test orders. 101 Results across the three trials were equivocal. The trial by Tierney and colleagues86 conducted in outpatients found a significant decrease (–14.3%) in the number of tests ordered per patient visit in the intervention group [1.6 (SD: 0.7)] compared to the control group [1.8 (SD: 0.9)]. Corresponding patient test charges were also significantly lower in the intervention group [US$45.1 (SD: 22.0)] compared to the control group [(US$51.8 (SD: 22.0)]. However, the reduction in the number of tests ordered and the subsequent charges to patients, appeared to be driven more by the reduction in the number of tests ordered by residents physicians as opposed to faculty physicians, for whom it was observed that baseline test ordering rates were lower. It would therefore appear that the display of test costs may be differentially effective in reducing the number of test orders and their corresponding costs according to baseline ordering rates. Furthermore, additional post-intervention follow-up indicated that the effect of displaying test charges may be relatively transient, as there were no significant differences between intervention and control physicians in the number of tests ordered in this period.
Contrary to the results of the CRCT by Tierney and colleagues,86 Bates and colleagues101 found no significant impact of the display of test costs on either the number of laboratory or radiological imaging tests ordered between intervention and control groups per patient admission. Correspondingly there were no significant differences in either of the trials in test costs between the intervention and control groups. A summary of the results of the process outcomes for the three trials is displayed in Table 8.
| Study ID | Tierney (1990)86 | Bates (1997) (laboratory test trial)101 | Bates (1997) (radiological imaging trial)101 | |||
|---|---|---|---|---|---|---|
| Study design | CRCT | RCT | RCT | |||
| Intervention | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS |
| N | 59 | 62 | 3554 | 3536 | 8653 | 8728 |
| Process outcomes | ||||||
| Number of tests ordered per visit/admission | 1.8 ± 0.9 | 1.6 ± 0.7 | 26.8 ± 43.4 | 25.6 ± 37.9 | 1.76 ± 4.68 | 1.76 ± 4.43 |
| % difference between groups | –14 | –4.5 | 0 | |||
| p-value for difference between groups | p < 0.005 | p = 0.074 | p = 0.13 | |||
| Charges for tests ordered per visit/admission (US$) | 51.8 ± 22.0 | 45.1 ± 22.0 | 771 ± 1310 | 739 ± 1129 | 276 ± 737 | 275 ± 688 |
| % difference between groups | –13 | –4.2 | –0.4 | |||
| p-value for difference between groups | p < 0.05 | p = 0.97 | p = 0.1 | |||
| Number of tests (scheduled visit) | 1.9 ± 0.9 | 1.6 ± 0.8 | ||||
| % difference between groups | –16.8 | |||||
| p-value for difference between groups | p < 0.01 | |||||
| Charges for test ordered (scheduled visit) (US$) | 53.4 ± 23 | 45.3 ± 22.8 | ||||
| % difference between groups | –15.3 | |||||
| p-value for difference between groups | p < 0.01 | |||||
| Number of tests (unscheduled visit) | 1.3 ± 0.9 | 1.2 ± 0.7 | ||||
| % difference between groups | –11.4 | |||||
| p-value for difference between groups | p < 0.05 | |||||
| Charges for test ordered (unscheduled visit) (US$) | 39.4 ± 25.1 | 35.6 ± 21.3 | ||||
| % difference between groups | –9.7 | |||||
| p-value for difference between groups | p < 0.05 | |||||
| N | 35 | 39 | ||||
| Number of tests ordered per visit (post-intervention period) | 1.8 ± 0.9 | 1.7 ± 0.8 | ||||
| % difference between groups | –7.7 | |||||
| p-value for difference between groups | p > 0.05 | |||||
| Charges for tests ordered per visit (post-intervention period) (US$) | 50.0 ± 21.7 | 48.3 ± 22.6 | ||||
| % difference between groups | –3.5 | |||||
| p-value for difference between groups | p > 0.05 | |||||
| Number of tests performed per admission | 49.6 ± 94.4 | 46.9 ± 79.2 | 1.53 ± 4.1 | 1.5 ± 3.6 | ||
| % difference between groups | –5.4 | 0 | ||||
| p-value for difference between groups | p = 0.87 | p = 0.10 | ||||
| Charges for test ordered per admission (US$) | 1496 ± 3147 | 1423± 2730 | 215 ± 515 | 220 ± 473 | ||
| % difference between groups | –4.9 | +2.3 | ||||
| p-value for difference between groups | p = 0.89 | p = 0.03 | ||||
Adverse effects of test cancellation
In all three trials there were no significant differences in the patient outcomes of number of hospitalisations, emergency room visits, visits to the General Medicine Practice or other outpatient clinics,85 or length of hospital stay. 101 Therefore it would appear that any potential cost savings achieved in the trials resulting from a reduction in the number of tests performed were not offset by increases in patient’s use or other resources of worse outcomes. A summary of the results of patient outcomes for the trials is displayed in Table 9.
| Study ID | Tierney (1990)86 | Bates (1997) (laboratory test trial)101 | Bates (1997) (radiological imaging trial)101 | |||
|---|---|---|---|---|---|---|
| Intervention | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS |
| N | 5962 | 62 | 3554 | 3536 | 8653 | 8728 |
| Patient outcomesa | ||||||
| Number of hospitalisations/patient | 0.2 ± 0.6 | 0.2 ± 0.6 | ||||
| p-value for difference between groups | p > 0.05 | |||||
| Number of emergency room visits/patient | 1.0 ± 1.7 | 1.0 ± 1.7 | ||||
| p-value for difference between groups | p > 0.05 | |||||
| Number of outpatient visits/patient | 4.3 ± 3.4 | 4.3 ± 3.4 | ||||
| p-value for difference between groups | p > 0.05 | |||||
| Length of hospital stay (days) b | 4 (2.7) | 4 (2.7) | 3 (2–6) | 3 (2–7) | ||
| p-value for difference between groups | p > 0.05 | p > 0.05 | ||||
Studies assessing the impact of display of previous test results
Quantity and quality of the studies
Two studies, one CCT and one UPP study assessed the impact of the display of previous test results on subsequent test order volumes. 88,89
The CCT conducted by Solomon and colleagues88 was carried out at the Brigham and Women’s Hospital, Boston, MA, USA, and was focused on reducing unnecessary serological testing in the diagnosis of suspected systemic rheumatic disease. 88 The trial was conducted over a 10-month period, with the intervention and control groups formed according to the indication for test(s) ordered. All physicians ordering a rheumatoid factor (RF) or antinuclear antibody (ANA) test for the suspected indications of rheumatoid arthritis, systemic lupus erythematosus, primary systemic sclerosis, mixed connective tissue disease, or Sjögren’s syndrome were assigned to the intervention group. All test orders for RF or ANA for the suspected indications of systemic vasculitis and cryoglobulinemia, or a complement test for any condition during the study period were assigned to the control group. Therefore, during the trial physicians were exposed to both the intervention and control conditions. Tests were selected as target tests for the trial on the basis that estimates of the test’s sensitivity and specificity for each of the suspected indications existed in the literature. The CDSS intervention required physicians to state their estimate of the pre-test probability of disease in the patient. The CDSS then calculated the post-test positive and negative predictive values, based on the physician’s estimated pre-test probability. These calculations were based on sensitivity and specificity values abstracted from relevant literature. 106–112 During the 10-month trial 71 physicians wrote test orders for 99 patients in the intervention group, while 154 physicians wrote orders for 236 patients in the control group. The two groups were well balanced in terms of both physician (age, gender, postgraduate year, department) and patient (age, gender, length of hospital stay, total hospital charges) baseline characteristics.
The UPP study conducted by Bansal and colleagues89 aimed to assess the impact of a computer-based intervention on ABG usage in an ICU setting. The study was conducted at Vanderbilt University Medical Centre, Nashville, TN, USA, and included six conditions managed in ICU (trauma, general surgery, medical, cardiac, burn and neurology). The study was conducted over a 12-week period, consisting of a 5-week pre-intervention and a 7-week intervention period. There were no restrictions on the ordering of ABG tests during the pre-intervention period (OCS alone).
During the intervention the CDSS provided the user with a graphical display of the patient’s previous ABG values; pO2 pCO2, HCO3, O2 saturation, pH, and FiO2. All values, except O2 saturations, reflected previous ABGs performed during the patient’s current hospitalisation. In addition cointervention educational text was provided alongside the graphical display of results and test ordering was limited to within 24 hours so no multi-day orders were allowed. The default (pre-populated) response was to cancel the order. However, the final decision to test or not was left to the user’s discretion. Table 10 displays a summary of the key characteristics of both studies.
| Study ID | Setting/country | Study design | Number of sites | Intervention (I)/Comparison (C) | Study description | Area of impact | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| Process outcomes | Adverse effects of test cancellation | |||||||
| Solomon (1999)88 | Inpatients, Brigham and Women’s Hospital, Boston, MA, USA | CCT | 1 |
(I) OCS + CDSS: CDSS required physicians to enter their estimate of the pre-test probability of disease and the sensitivity and specificity of the test ordered. CDSS then calculated +ve and –ve predictive values based on the values for test sensitivity and specificity derived from a literature review. (C) OCS alone (no +ve and –ve predicative values displayed) |
Trial conducted over a 10-month period. Study intervention and control groups were formed according to the type of test ordered. Intervention tests included all RF or ANA tests for the suspected indications of rheumatoid arthritis, systemic lupus erythematosus, primary systemic sclerosis, mixed connective tissue disease, or Sjögren’s syndrome. Control tests included RF or ANA for systemic vasculitis and cryoglobulinemia, or a complements level for any condition. Over the 10-month trial 71 physicians wrote test orders for 99 patients in the intervention group and 154 physicians wrote orders for 236 patients in the control group | Test volume |
Number of tests cancelled Number of positive tests for known rheumatic disease |
NR |
| Bansal (2001)89 | Inpatients, Vanderbilt University Hospital, Nashville, TN, USA | UPP | 1 (6 ICUs) |
(I) OCS + CDSS: Baseline: OCS alone (no previous test results or limits on forward ordering) (I) OCS + CDSS: graphical display of patients previous ABG results; educational text and tests ordering limited to within 24-hours |
Study consisted of 12 weeks; 5 weeks pre-intervention and 7 weeks intervention period. Intervention implemented on 6 ICUs. Number of ABG test requested per week compared in pre- and intervention periods | Test volume | Number of ABG test orders pre- and post-intervention | NR |
Outcomes
Process outcomes
The area of impact in the test ordering process assessed in both studies was test volumes, although only limited outcomes were reported in both studies. 88,89 Solomon and colleagues88 assessed the number of cancelled test orders, and the number of positive tests for known rheumatic disease, while Bansal and colleagues89 examined the number of ABG test orders both pre- and post-intervention.
Study quality
In both studies adequate eligibility criteria were specified, and detailed baseline characteristics were provided in the study by Solomon and colleagues88 that indicated the two study groups were well balanced at baseline in terms of both physician and patient characteristics. However, only details on the CDSS users were reported by Bansal and colleagues89 with no details on patient characteristics provided. It is therefore unclear whether differences in patient characteristics between the pre- and post-intervention periods may have potentially confounded study results.
In the study by Solomon and colleagues88 physicians were exposed to both the intervention and control conditions. This has the potential to impact on the study results through contamination, with learning through exposure to the intervention condition impacting on test ordering behaviour in the control condition. The impact of this, if any, would potentially be to underestimate the impact of the CDSS intervention.
In both studies the tests used to conduct the statistical analyses were appropriate, and greater than 80% of physicians/patients were included in the follow-up assessment. However, it is unclear in the study by Bansal and colleagues89 whether analyses were conducted on an ‘intention to provide or communicate information’ basis. In both studies there were therefore a number of factors that may have biased the results and the results should be interpreted in light of these.
CDSS characteristics
The specific CDSS evaluated were the home-grown/in-house OCS and CDSS developed by Brigham and Women’s Hospital, Boston, MA, USA, and Vanderbilt University Hospital, Nashville, TN, USA, in the studies by Solomon and colleagues88 and Bansal and colleagues89 respectively. The CDSS in the study by Solomon and colleagues88 used naive Bayesian methods of reasoning, with the physician’s pre-test probability of disease as the system input information. The CDSS output format were post-test positive and negative predictive values based on values for each specific indication for test sensitivity and specificity derived from a review of the literature. The CDSS reasoning methods were not reported by Bansal and colleagues,89 but the system input information included six previous test ABG input parameters (ABG values; pO2 pCO2, HCO3, O2 saturation, pH, and FiO2); the results of these were presented to the system user as a graphical display.
Neither of the studies reported the time needed to use the CDSS, or pilot testing with users prior to implementation, but instructional training was provided to users in the study by Solomon and colleagues. 88 In both of the studies the CDSS was integrated with OCS, and CDSS output was provided as part of the physician workflow, providing output at the time and location of decision making. The study by Solomon and colleagues88 required the input of additional information by the physician in the form of a pre-test probability of disease. Neither of the studies required a reason to be documented for not following the CDSS output, as only previous test results were displayed, and therefore no specific recommendations to order or cancel specific tests were provided by the system. It was unclear in both studies whether the display of previous test results were likely to promote action rather than inaction on the part of the physician. 88,89 Additionally, it would appear that local system users were not directly involved in the CDSS development process of either system. The data provided by the CDSS was used by the physician alone, and did not appear to provide period summaries of performance feedback.
In the study by Solomon and colleagues88 the previous test results appeared to be used alone, while in the study by Bansal and colleagues89 they were combined with educational text regarding the interpretation of previous ABG results, and limitations on further test ordering within the consecutive 24-hour period. A summary of the key CDSS characteristics for each study is displayed in Table 11.
| Study ID | Solomon (1999)88 | Bansal (2001)89 |
|---|---|---|
| CDSS characteristics | ||
| 1. Name of CDSS (if any) | NRb | NRc |
| 2. CDSS reasoning methods | NBM | NR |
| 3. CDSS knowledge base | Sensitivity and specificity values abstracted from the literature | NR |
| 4. Information used in CDSS | Pre-test probability estimates | 6 previous ABG results (pO2, pCO2, HCO3, pH, FiO2,O2 saturations |
| 5. Time to complete CDSS (minutes) | NR | NR |
| 6. CDSS output format | Post test +ve and -ve predictive values | Graphical display of previous test results |
| 7. Is a description of pilot testing with users prior to implementation provided? | No | No |
| 8. Is user instructional training at the time of implementation described? | Yes | No |
| General system features | ||
| 9. Is the CDSS integrated with charting or OCS to support workflow integration?a | Yes | Yes |
| Clinician–system interaction features | ||
| 10. Is automatic provision of CDSS output provided as part of clinician workflow?a | Yes | Yes |
| 11. Is there a need for additional data entry by the clinician other then the specification of which test orders?a | Yes | No |
| 12. Does the CDSS request documentation of the reason for not following CDSS recommendations?a | No | No |
| 13. Does CDSS provide output at the time and location of decision making?a | Yes | Yes |
| 14. Are the CDSS recommendations executed by the clinician noting agreement?a | No | Yes |
| Communication content features | ||
| 15. Does the CDSS provide a recommendation rather than just an assessment?a | No | No |
| 16. Does the CDSS promote action rather than inaction?a | No | No |
| 17. Does the CDSS justify the output of provision of reasoning?a | No | No |
| 18. Does the CDSS justify the output by provision of research evidence?a | No | No |
| Auxiliary features | ||
| 19. Were the local users involved in the CDSS development process?a | NR | Yes |
| 20. Is the CDSS output provided to patients as well as clinicians?a | No | No |
| 21. Does the CDSS provide periodic summaries of performance feedback?a | No | No |
| 22. Is the CDSS used in conjunction with conventional education?a | No | No |
Results
Process outcomes
In the CCT by Solomon and colleagues88 during the 10-month trial period significantly more test orders [11 out of 99 tests (11%)] were cancelled compared to those in the control group [1 out of 236 (0.4%); p = 0.001]. There were no associations between the physicians’ pre-test probability estimates and whether the test was cancelled (p = 0.59). Additionally, only 43 of the 335 test orders (13%) yielded positive results, but from these only four patients (1%) were given new diagnoses of rheumatic disease.
Results from the UPP study by Bansal and colleagues89 showed no significant differences in the number of ABG test orders placed pre- and post-intervention (376 and 387 respectively; p = 0.09).
Summary of studies assessing the impact of the display of previous test results
Only two studies, one CCT and one UPP study, assessed the impact of the display of previous test results on subsequent test ordering. 88,89 Both studies reported only very limited results, and were focused upon specific test types, namely RF and ANA,88 and ABG. 89 It is therefore difficult to know the extent to which the results from these studies could be extrapolated to a wider context in which a broader spectrum of tests were being ordered.
Results between the two studies were contradictory, with the CCT by Solomon and colleagues88 showing a significant increase (11.1%) in the number of tests cancelled in the intervention group compared to the control group (0.42%) despite the fact that the same physicians ordered tests for both intervention and control patients. The UPP study by Bansal and colleagues, in contrast, found no significant differences in the number of ABG test orders between the pre- and post-intervention periods. A summary of the results from both studies is displayed in Table 12.
| Study ID | Solomon (1999)88 | Bansal (2001)89 | ||
|---|---|---|---|---|
| Study design | CCT | UPP | ||
| Intervention | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS |
| Pre- | Post- | |||
| N | 154 | 71 | NR | NR |
| Process outcomes | ||||
| Number of cancelled tests (n %) | 11 (11)) | 1 (0.4) | ||
| p-valuea | p = 0.001 | |||
| Number of ABG test orders (n) | 376 | 387 | ||
| p-valueb | p = 0.09 | |||
Studies assessing the impact of the display of reminders
Quantity and quality of the studies
The impact of the display of reminders was assessed in 10 studies:24,29,48,57,87,90–94
-
four CRCTs, two published by Overhage and colleagues in 1996 and 1997,24,48 one by Palen and colleagues,90 one by Matheny and colleagues91
-
one RCT undertaken by Bates and colleagues92
-
one CCT by O’Connor and colleagues57
-
one randomised crossover trial by McDonald and colleagues29
-
one ITS study with an AB-AB-AB design by Carton and colleagues93 and
-
two UPP studies by Steele and colleagues and Abboud and colleagues. 87,94
Across the studies, nine of the 10 were conducted in the USA,24,29,48,57,87,90–92,94 while the remaining study was conducted in France. 93 Of those undertaken in the USA, three studies were undertaken at the Wishard Memorial Hospital, Indianapolis, IN,24,29,48 two of which were undertaken in an inpatient setting24,48 and one in an outpatient setting. 29 Two studies were conducted at the Brigham and Women’s Hospital, and associated community and outpatient clinics,91,92 one of which included inpatients and the other outpatients. Of the remaining four studies undertaken in the USA, three were undertaken in an outpatient setting (group-model managed care organisation, Kaiser Permanente; Health Partners Medical Group, MN; and Sam Sandos Family Health Clinic, Denver Health respectively)57,90,94 and one was undertaken in a paediatric inpatient population at Cincinnati Children’s Hospital Medical Centre, OH. 87 Of these nine studies, one assessed compliance with reminders to undertake preventative care measures,48 seven assessed compliance with reminders to undertake appropriate laboratory testing,24,29,57,87,90,91,94 and one examined compliance with reminders about redundant tests. 92 The focus in three of the studies, the two CRCTs by Overhage and colleagues2,48 and the randomised crossover trial by McDonald and colleagues29 was on both reminders to order medication, screening, and other health-care procedures. In these three studies therefore only limited results were presented for laboratory test ordering alone.
The one study conducted in France, was undertaken in two accident and emergency departments, and assessed the impact of reminders to undertake the most appropriate radiological tests across all the physicians involved in the study. 93 This study therefore assessed the impact of reminders on the number of tests that complied with guideline recommendations. 93 A summary of study characteristics from the eight studies that assessed the impact of reminders is displayed in Table 13.
| Study ID | Setting/Country | Study design | Number of sites | Intervention (I)/Comparison (C) | Study description | Area of impact | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| Process outcomes | Adverse effects of test cancellation | |||||||
| Overhage (1996)48,a | Inpatient general medicine ward, Wishard Memorial Hospital, Indianapolis, IN, USA | CRCT | 1 (6 independent medical services within the ward) |
(I) OCS + CDSS: reminders to physicians to undertake preventative care measures (11 measures included laboratory tests) (C) OCS alone (reminders suppressed) |
Services were randomly allocated to intervention and control groups (n = 78 physicians; n not reported by group). The trial was conducted over a 6-month period including 1622 patients (n = 821 intervention group; n = 801 control group) | Reminder compliance | Compliance with reminders for preventative care measures | NR |
| Overhage (1997)24,b | Inpatient general medicine ward, Wishard Memorial Hospital, Indianapolis, IN, USA | CRCT | 1 (6 independent medical services within the ward) |
(I) OCS + CDSS: reminders about recommended corollary test orders (C) OCS alone (reminders suppressed) |
Services were randomly allocated to intervention (n = 45 physicians) or control (n = 41 physicians) groups. The trial was 6-month duration including 1686 patients (n = 814 intervention; n = 872 control) | Reminder compliance | Rate of 24-hour compliance for the 18 most common laboratory test corollary orders | NR |
| Palen (2006)90 | Outpatients, group-model managed care organisation (Kaiser Permanente), USA | CRCT | 16 |
(I) OCS + CDSS: reminders to physicians ordering medication for concomitant laboratory test orders (C) OCS alone (reminders suppressed) |
Physicians were randomly allocated into intervention (n = 104) or control groups (n = 103). The trial was conducted over a 12-month period and included 26,586 patients (intervention n = 14,376; control n = 12,210) prescribed one of the 34,242 prescriptions for one or more of the 25 target prescriptions | Reminder compliance |
Compliance with recommended laboratory monitoring tests Compliance with recommended tests by gender and medication type |
NR |
| Matheny (2008)91 | Partners Healthcare system (including Brigham and Women’s Hospital, Massachusetts General Hospital, and a number of community hospitals and outpatient clinics) | CRCT | 20 |
(I) OCS + CDSS: reminders to physicians to order a test (C) OCS alone (reminders suppressed) |
Physicians were randomly allocated into intervention (n = 145) or control groups (n = 158). The trial was conducted over a 6-month period and included 1922 patients (intervention n = 924; control n = 998) on at least one of the 15 target study medications for whom no laboratory monitoring test had been conducted in the previous year | Reminder compliance | Number of appropriate laboratory tests ordered when overdue within 14 days | NR |
| Bates (1999)92 | All inpatients Brigham and Women’s Hospital, USA | RCT | 1 |
(I) OCS + CDSS: reminders to physicians about redundant tests (C) OCS alone (reminders suppressed) |
Patients were randomly allocated into intervention (n = 5700) and control groups (n = 5886). The trial was conducted over a 15-week period and included 5059 instances of the target tests (n = 2478 intervention group; n = 2581 control group) |
Order appropriateness Hospital costs (estimated total annual saving) |
Proportion of tests cancelled Proportion of test performed after reminder Proportion of tests performed earlier than test-specific intervals Proportion of justified overrides of reminders Charge saving associated with reminders for redundant tests |
Adverse effects of test cancellation (new abnormal results for the same test performed within 3 days of cancellation) |
| McDonald (1980)29 | Outpatients, Wishard Memorial Hospital, Indianapolis, IN, USA | Randomised crossover trial | 1 |
(I) OCS + CDSS: reminders to physicians to order a test (S1) (either with or without bibliographic citations supporting the reminders (S2) (C) OCS alone (reminders suppressed) |
Study consisted of 15 weeks; 5 weeks control period; 5 weeks of S1 and 5 weeks of S2. 31 care providers were randomly allocated to each condition and assessed in the three treatment cross-over l | Reminder compliance | Compliance with reminders to order a test(s) | NR |
| O’Connor (2005)57 | Outpatients with an established diagnosis of diabetes, HealthPartners Medical Group, MN, USA | CCT | 2 |
(I) OCS + CDSS: prompts and reminders. Prompts were included when a patient had no HbA1C test within 6 months, no urine microalbuminiuria test within 1 year, had blood pressures of ≥ 130/85 mm Hg, LDL levels of ≥ 130 mg/dL, HbA1C levels of ≥ 8% or no aspirin use if aged 40 years or older (C) OCS alone |
Frequency of tests for glycated haemoglobin and low-density lipoprotein were compared at baseline and three time points over 5-year follow-up between the intervention (n = 57 patients) and control clinic (n = 65 patients) which used a common diabetes care guideline | Order appropriateness (appropriate test frequency) |
Number of HbA1C tests Number of LDL cholesterol tests Percentage of patients having at least two HbA1C tests, one LDL test, or two HbA1C and one LDL test |
NR |
| Carton (2002)93 | Accident and emergency department physicians treating patients with an indication for radiological imaging tests, Boulogne and Rennes, France | ITS (AB-AB-AB design) | 2 |
(I) OCS + CDSS: reminders displaying the appropriate guideline recommendations for radiological imaging tests (Baseline) OCS alone (reminders suppressed) |
Study consisted of 6 months; three control periods and three intervention periods run alternately with no delay between periods. A total of 15,086 patients were seen during the study, with 6869 radiological examinations undertaken | Guideline compliance |
Number of requests complying with guideline recommendations Type of requests not complying with guideline recommendations Most frequent requests not complying with guideline recommendations |
NR |
| Steele (2005)96 | Outpatient physicians, Sam Sandos Family Health Clinic, Denver Health, CO, USA | UPP | 1 |
(I) OCS + CDSS: reminders to physicians for drug–laboratory interactions for potential drug induced hypokalemia; hyperkalemia; nephrotoxicity; thrombocytopenia or hepatotoxicity. Physicians were also alerted to rule-associated laboratory test orders |
Baseline (17 weeks): No reminders were displayed to physicians. Intervention period (21 weeks): Reminders were displayed for drug-laboratory test interactions and associated laboratory test orders. A total of 19,076 patients were seen during the intervention period, with 9274 prescriptions triggering an alert. Differences between the number of laboratory test orders pre- and during the intervention period were compared |
Compliance with reminders for drug–laboratory interactions |
Number of rule associated laboratory tests ordered: no alert displayed Number of rule associated laboratory tests ordered: alert displayed Number of rule associated laboratory tests ordered: ‘abnormal labs’ message displayed Number of rule associated laboratory tests ordered: ‘no labs’ message displayed |
NR |
| Abboud (2006)87 | Paediatric inpatients, Cincinnati Children’s Hospital Medical Centre, OH, USA | UPP | 1 |
(I) OCS + CDSS: reminders about corollary order blood tests for patients receiving aminoglycosides (Baseline) OCS alone (reminders suppressed) |
Study consisted of 6 months; 3 months pre-intervention and 3 months post-intervention. The number of orders for aminoglycosides during study period was 336 (159 pre- and 177 post-intervention) | Guideline compliance |
Number of courses of aminoglycosides with appropriate laboratory test monitoring. Frequency of therapeutic, toxic, subtherapeutic, or toxic and subtherapeutic laboratory values during courses of therapy |
NR |
The two CRCTs conducted by Overhage and colleagues24,48 were both conducted on six inpatient medical wards (three intervention and three control) at the Wishard Memorial Hospital, Indianapolis, IN, USA. Both of the trials therefore used the site-specific home-grown Regenstrief Medical Record System which interfaced with two CDSS that appear to have been developed specifically for the trials. 24,48 The first trial published in 1996 included 78 physicians treating 1622 patients (821 intervention group and 801 control group) on one of the six wards.
During the 6-month trial period, wards were randomised to either receive or not receive preventative care measure recommendations provided by the CDSS. Any patient who had at least one preventative care recommendation was eligible for inclusion in the intervention group. The preventative care recommendation reminders were based on 22 preventative care measures from the US Preventive Services Task Force recommendations, and included vaccination measures, prescription of prophylactic medication, as well as laboratory screening test reminders. 113 Only 10 of the 22 preventive care recommendations were related to the use of laboratory testing and therefore of relevance to the present review. Data on these 10 were therefore extracted and presented, and included: (1) cervical cytology, (2) mammography, (3) thyrotropin screen, (4) hepatitis B screen, (5) screening urinalysis, (6) cholesterol test, (7) human immunodeficiency virus (HIV) screen, (8) 24-hour urine protein test, (9) sickle cell screen, and (10) sexually transmitted disease (STD) screen.
While the trial groups were well balanced in terms of age, gender, ethnic origin, and primary discharge diagnosis, data were presented only for the overall trial groups, not just patients who received laboratory test preventative care recommendations. It should therefore be noted that there may be potential baseline imbalances within the intervention and control groups, which could bias the results for these specific outcomes from this trial.
In the second trial, published in 1997, 86 physicians were randomised (45 intervention group and 41 control group) to receive or not receive reminders to order suggested corollary tests or medications needed to detect or ameliorate adverse effects to any one of the selected 87 trial tests or treatments. Standard reference texts114 and drug package inserts were used to identify 87 trial target orders (76 drugs and 11 tests) that were paired with one or more corollary orders. For example, aminoglycoside prescribing was paired with peak and trough aminoglycoside levels, and warfarin with prothrombin time. The CDSS then issued a reminder during the 6-month trial period to intervention physicians when any of the 87 target orders were placed, to consider ordering the linked corollary tests or medications. When suggesting corollary orders, the CDSS took into account the status of the order (a new order or a revision of an old order); the time elapsed since the last time the order being suggested was written; and whether any orders for a new equivalent item had already been written.
Physicians were free to accept or reject the suggested corollary orders. All inpatients who had at least one order written that triggered a suggested corollary order were eligible for trial inclusion, with 814 patients included in the intervention group and 872 in the control group. Again, groups were well balanced at baseline between the two groups in terms of age, gender, ethnic group, and primary diagnostic group. However, as the trial considered compliance with reminders to order both corollary medications and laboratory tests, again only the data for compliance with the ordering of laboratory tests was extracted and is presented. Therefore, as patient sociodemographic data were presented for the overall trial groups (not by type of order) then it is again possible that there were baseline differences between the intervention and control groups that may potentially confound the results for the laboratory outcomes of interest.
The CRCT by Palen and colleagues90 like the trial by Overhage and colleagues24 also focused upon the impact of reminders when ordering medications on compliance with guidelines for laboratory test monitoring. The trial, conducted at 16 primary care sites within the group model managed care organisation Kaiser Permanente, included 207 physicians (104 intervention group and 103 control group) who ordered 34,242 prescriptions for study medication for 26,586 different patients during the 12-month trial period. Physicians were randomised to receive or not receive non-intrusive drug–laboratory reminders for 25 selected medications within the OCS. This information was specific for the individual medication and presented guidelines for appropriate baseline monitoring. The 25 medications chosen were selected based on the presence of US Food and Drug Administration (FDA) black box warnings, published clinical guidelines, and the potential for adverse clinical consequences related to lack of monitoring.
The guideline recommendations for laboratory test monitoring for each medication were developed from information presented in national and internal clinical guidelines, and through discussion with physicians, pharmacists, and clinician leaders within the health-plan group. These guidelines then formed the basis for the reminders that were presented via the CDSS, which was implemented within the existing proprietary system (Clinical Information System) that was developed in collaboration with IBM (Boulder, CO).
For each of the 25 target medications, prescribing data from the 12-month trial period were analysed to assess whether appropriate laboratory tests had been completed within either 2 weeks after the medication order or had been ‘recently performed’. ‘Recently performed’ tests were defined as those completed within 180 days before medication dispensing or 2 weeks after dispensing. Physicians were defined as having followed the laboratory monitoring guideline if results of completed laboratory tests were available for review within these time frames (i.e. 180 days pre-dispensing and 2 weeks post-dispensing).
Matheny and colleagues91 in their CRCT also assessed the impact of reminders on appropriate laboratory monitoring of maintenance therapy with a focus on the monitoring of potassium, creatinine, liver function, thyroid function, and therapeutic drug levels for appropriate medications. The specific medications assessed were selected for inclusion in the reminder system based on (1) prevalence of their use, and (2) potential morbidity associated with failure to perform appropriate laboratory monitoring. These were based on evidence-based guidelines that were reviewed for routine medication monitoring. 115–118 The specific 15 target drugs included in the system were:
-
non-steroidal anti-inflammatory drugs (NSAIDs)
-
angiotensin receptor blockers (ARBs)
-
metformin
-
potassium supplements
-
potassium sparing diuretic
-
thiazide diurectic
-
angiotensin-converting enzyme inhibitors
-
HMG (3-hydroxy-3-methyl-glutaryl)-CoA reductase inhibitors (statins)
-
thyroxine
-
carbamazapine
-
ciclosporin
-
phenobarbital
-
phenytoin
-
proc-NAPA
-
valproate.
The interval chosen for appropriate monitoring was annual, and therefore the trial included all outpatients (total n = 1922; 924 intervention group and 998 control group) seen during the 6-month study period on one or more of the 15 target study drugs for at least 365 days for which no relevant laboratory monitoring tests were conducted in the preceding year. The two groups were reasonably well matched in terms of age, gender, race and insurance status, and these factors were included in the analytic model to assess appropriate laboratory testing rates. Physicians from the Partners Healthcare system, which included two academic teaching hospitals (Brigham and Women’s Hospital and Massachusetts General Hospital), and a number of community hospitals and outpatient clinics were randomised from a total of 20 sites, either to receive reminders (n = 145 physicians) or for reminders to be suppressed (n = 158). The two physician trial groups were well balanced at baseline in terms of both age and gender.
The RCT conducted by Bates and colleagues,92 ‘a randomised trial of a computer-based intervention to reduce utilization of redundant laboratory tests’, was undertaken at Brigham and Women’s Hospital, Boston, MA, USA and included 5,700 inpatients in the intervention group and 5,886 in the control group admitted during the study period (between 28 June and 30 October 1994). Thirteen specific tests were chosen as appropriate candidates for redundant reminders either because they were commonly ordered or because the marginal cost of performing the test was high. The specific candidate tests were chemistry-20 profiles; urinalyses; urine, sputum and stools cultures; serum digoxin, tobramycin, aminophylline, vancomycin, and gentamicin levels; Clostridium difficile cultures;119 and fibrin split products.
Test-specific intervals within which a second test was considered redundant, were based on a review of the literature, and evaluated retrospectively by applying them to a random sample of patients. 120 ‘Target tests’ were defined as tests repeated earlier than the test-specific interval, which for all tests except fibrin split products were 20 hours. The test specific interval for fibrin split products was 8 hours. For digoxin and aminophylline levels, reminders were sent only if the first test result was within the reference range. A patient admission formed the unit of randomisation with physicians entering orders for both intervention and control patients using the home-grown site-specific Brigham and Women’s Hospital OCS.
In the intervention group, if a test had previously been ordered within its test-specific interval, the physician received a reminder that the test had been performed recently or was pending, and the result given if available. For the control group, redundancy was determined in the same way, but the reminder was suppressed. In the intervention group, when a reminder was delivered, the default response was to cancel the test. Physicians could override a reminder, but were required to choose from a menu of reasons for the override. For technical reasons, checking for redundancy was performed only for tests ordered from the main OCS screens and not for tests ordered using order sets or templates. Consequently, of the redundant laboratory tests performed, only 44% had an associated computer order. In total the trial included 5059 admissions (2478 in the intervention group and 2581 in the control group) who had at least one of the specified target tests within the 15-week trial period, with 13,425 study tests ordered in the intervention group versus 13,847 in the control group. Both groups were well matched at baseline in terms of age and gender. No details on sociodemographic data for the physicians were reported, or the number involved in the trial.
The randomised crossover trial by McDonald and colleagues,29 like the two CRCTs conducted by Overhage and colleagues,24,48 was undertaken at the Wishard Memorial Hospital, Indianapolis, IN, USA. However, the trial was undertaken in an outpatient population, and the CDSS system used, although part of the site-specific hospital system, is unlikely to be comparable to that assessed in the other trials due to considerable differences in the years in which the studies were conducted. It should also be noted that as the trial was conducted in 1980 the CDSS is likely now to be obsolete, and not of direct relevance to service providers needing to implement CDSS within the NHS at the present time.
The aim of this trial was to assess whether the CDSS that was designed to detect and remind physicians about clinical events that might need corrective action significantly increased response rates to events. The trial, which included 31 care providers (interns, residents and practice nurses) lasted 15 weeks: 5 weeks control period (no reminders sent); 5 weeks study period 1 (S1) and 5 weeks study period 2 (S2). During S1 care providers were sent reminders about the detected conditions alone, and during S2 were sent reminders plus bibliographic citations supporting the reminders. The correct action to the reminder was predetermined and compliance assessed. The three study periods, were presented in six possible temporal sequences, with care providers randomly assigned to a sequence. No information on patient sociodemographic status or presenting conditions was reported.
O’Connor and colleagues57 undertook a 5-year longitudinal study (CCT) to assess whether implementation of an electronic medical record with CDSS or the medical record system alone improved the process or outcomes of care in patients with an established diagnosis of diabetes mellitus. The trial was conducted in two clinics at the HealthPartners Medical Group, MN, USA and included all patients with a diagnosis of diabetes at study baseline (1996) in both clinics. The clinic that the patient attended was then identified in 1998 and 2000 based on administrative data. Patients were only eligible for inclusion in the analyses if they attended their original study clinic in all 3 years, with a total of 57 patients included in the intervention group and 65 in the control group. Patient baseline characteristics were well balanced between the two clinics in terms of age, gender, and Charlson comorbidity score. 121 As modified by Deyo and colleagues,122 and Rush and colleagues. 123 No sociodemographic data on the physicians participating in the trial were presented, but physicians in both clinics participated in the same diabetes-related care improvement activities within the medical group over the trial period. Similarly, both clinics had access to diabetes specific registries, in-clinic diabetes teaching nurses for patient education, and a common diabetes clinical guideline developed by the Institute for Clinical Systems Improvement (www.icsi.org/).
The CDSS used was a commercial system developed by Epic Systems (Madison, WI) that provided reminders to physicians when a patient had no HbA1C test within 6 months, no urine microalbuminiuria test within 1 year, had blood pressures of ≥ 130/85 mmHg, low-density lipid (LDL) levels of ≥130 mg/dL, HbA1C levels of ≥ 8%, or no aspirin use if aged 40 years or older. The reminders were displayed on screen, but a response to them was not obligatory. In the control group the reminders were suppressed. The electronic medical record system used a Windows interface and a Visual Basic (Microsoft Corp, Redmond, Washington, DC, USA) operating system linked to laboratory test results and pharmacy databases.
The process of care measures included the number of HbA1C tests and LDL cholesterol tests conducted for patients in each clinic in 1996, 1998, and 2000. Additional process measures assessed whether patients met minimum thresholds for HbA1C and LDL testing. Specifically, three threshold measures assessed whether the patient had at least two HbA1C tests per annum; at least one LDL test per year; or at least two HbA1C and one LDL test. Intermediate outcome measures included glycemic and lipid control, as assessed by HbA1C and LDL test values in each of the three study years.
The only study conducted outside the USA was undertaken by Carton and colleagues,93 and aimed to assess the impact of guidelines on medial imaging referral practice in two hospital accident and emergency departments, the Hospital Ambroise Pare, Boulonge-Billancourt and Hospital de Pontchaillous, Rennes, France. The study was an ITS with an AB-AB-AB design, with the intervention implemented with three control periods and three intervention periods running alternatively over 6 months, with no delay between periods, and each period 1 month in length. The study included all physicians working in either emergency department who ordered a radiological examination within the study period. The number of physicians included in the study and details of their sociodemographic status were not reported. During intervention periods the CDSS displayed the appropriate guideline recommendation on screen for the patient’s indication; if the request did not conform to the guidelines, confirmation was requested before submitting the request. The request could still be fulfilled even if it was not in agreement with the guidelines. During the control periods, radiological requests were recorded, but no reminder displayed.
The CDSS knowledge base was written by the Collège des Enseignants de Radiologie de France (French Society of Radiologists) based on the results of a review of the literature, existing guidelines and the expertise of all the societies of radiologists and clinicians. During the study period a total of 15,086 patients were seen in the two departments, with 6,434 radiological requests recorded. Of these, 743 (11%) were discarded from the analysis because the radiological examination and/or clinical context had been profoundly changed by hand. The primary outcome measure was the number of requests complying with the guideline reminders, and secondary outcomes being the type of requests not in compliance.
The primary focus of the pre–post study by Steele and colleagues94 was on medication ordering, and drug–laboratory interactions. The primary outcome measure was therefore the number of medication orders cancelled after a reminder for a drug–laboratory interaction was displayed. The secondary outcome measures were the number of times the indicated laboratory test(s) were ordered once a reminder had been displayed, the number of associated test(s) ordered when an ‘abnormal labs’ message was displayed, and the number of appropriate test(s) ordered when a ‘no labs’ reminder was given. The study was conducted at the Sam Sandos Family Health Clinic, Denver Health outpatient primary care clinics, Denver, CO, USA. The study consisted of a 17-week pre-intervention period, and a 21-week intervention period, with a total of 19,076 patients seen during this 9-month period. This provided a total of 54,206 patient visits with medications ordered on 17,444 (32%) of visits. The rule processed on 49% of all medication orders during the entire study period. During the post-intervention period, a reminder was displayed to the care provider for 11.8% (1,093 out of 9,274) of the times the rule processed. Among these reminders, 5.6% were for only ‘missing laboratory values’, 6.0% were for only ‘abnormal laboratory values’, and 0.2% were for both types of reminders. This means that there were 14,297 medication orders across the study period where the rule was triggered, but it did not meet the criteria to display a reminder. All provider staff were allowed to enter medication orders, including physicians, allied health-care providers (nurse practitioners, physician assistants), and residents. No further information was reported on health-care provider demographics. All registered patients were eligible for inclusion in the study.
The CDSS was developed in collaboration with Thomson Micromedex and Siemens Medical Solutions, and used commercially available rules developed in Ardex Syntax language as Medical Logic Modules, which were then modified to meet local needs. The study focused upon rules that were determined as being the most appropriate to addressing patient safety in an outpatient setting. The rules covered the following five areas:
-
potential drug-induced hyperkalemia (covering interactions with captopril, lisinopril, spironolactone, and enalapril)
-
hypokalemia (covering interactions with chlorthalidone, furosemide, hydrochlorothiazide, metolazone and ethacrynic acid)
-
thrombocytopenia (covering interactions with ranitidine)
-
elevated creatinine levels (covering interactions with amiloride, chlorthalidone, enalapril, furosemide, hydrochlorothiazide, lisinopril, metolazone and spironolactone)
-
elevated transaminase (covering interactions with atorvastatin, citalopram, fluoxetine, paroxetine, rosiglitazone and sertaline).
For each drug–laboratory interaction, rules were written identifying medications, routes of administration, and abnormal laboratory threshold levels for inclusion in the rule. The laboratory cut-off values for triggering a reminder were the same as for the Denver Health abnormal laboratory reference ranges. In addition, a determination was made for each medication as to whether a reminder should be provided for an abnormal laboratory value only, an abnormal or missing laboratory value, or whether despite an association with a laboratory abnormality, no reminder would be displayed. In response to reminders, CDSS users could decide to order, revise or delete the medication order. They could also order any rule-associated laboratory tests. Users did not need to respond to the reminder, but needed to select ‘Continue’ to proceed with the drug ordering session.
In the 17-week pre-intervention period, the rules were turned on in the background, but no reminders were displayed to users. Baseline ordering behaviour was then compared in a pre-post design with ordering behaviour during the 21-week intervention period.
The UPP study conducted by Abboud and colleagues87 was undertaken in paediatric inpatients at the Cincinnati Children’s Hospital Medical Centre, Cincinnati, OH, USA. The study consisted of a 3-month pre-intervention period followed by a 3-month intervention period, with a total study duration of 6 months. The study was specifically aimed at assessing the impact of a CDSS corollary order for aminoglycoside laboratory blood monitoring levels in all patients who received aminoglycosides for 4 or more days duration during the study period, as this was identified as being suboptimal at study baseline. This interval was chosen by investigator consensus as the minimum duration of therapy that would require monitoring of aminoglycoside levels. The CDSS was developed and implemented in the existing hospital information system (invision®, Siemens Medical Solutions, Malven, PA, USA).
During the intervention phase, immediately after placement of an order for aminoglycosides, the physician was reminded to check peak, trough, peak and trough, or random blood levels for the drug prescribed. This could be undertaken during the same session as the prescribing session. During the pre-intervention phase no reminders were presented on screen.
The study included 159 courses of antibiotic therapy of 4 or more days duration (n = 125 patients) in the pre-intervention phase, and 177 (n = 150 patients) in the intervention phase. No specific patient demographics were reported, but groups were well matched in the pre- and post-intervention phases in terms of the number of courses of aminoglycosides prescribed, total number of laboratory results obtained, and the number of laboratory results per patient. For the analyses, all antibiotic levels ordered were utilised in assessing the response to the reminder, but only true peak and trough levels were used to assess toxicity and subtherapeutic values. These were defined according the hospital laboratory guidelines, with peak and trough levels further divided into toxic or subtherapeutic. In the study a peak level was predefined as a level obtained 50–120 minutes after drug administration, and a trough defined as being obtained 0–120 minutes prior to drug administration.
Outcomes
Process outcomes
The area of impact in the test ordering process assessed in six of the studies was reminder compliance,24,29,48,90,91,94 while two studies assessed compliance with guideline recommendations,87,93 and two assessed order appropriateness. 57,92 The outcomes assessed in the six studies which assessed reminder compliance included rates of compliance with reminders to undertake preventative care measures,48 and appropriate laboratory tests for drug monitoring. 24,29,90,91,94 In the two studies that examined compliance with guideline recommendations, the outcomes included the number of requests that complied with recommendations,93 and the number of courses of drug therapy with appropriate laboratory test monitoring. 87 Outcomes in the two studies that assessed order appropriateness included the proportion of tests that were cancelled or performed after a reminder, the proportion of tests performed earlier than specified test-specific intervals,92 and the number of HbA1C, and of LDL cholesterol tests performed. 56
Adverse effects of test cancellation
Only the RCT by Bates and colleagues92 assessed potential adverse effects associated with test cancellation as advocated by the reminders. These were defined as new abnormal results for the same test performed within 3 days of the original test cancellation.
Study quality
Controlled studies
Study quality was generally reasonable apart from in the randomised crossover trial by McDonald and colleagues29 in which the methods were very poorly reported. The method of randomisation was adequate in the four CRCTs,24,48,90,91 and the RCT conducted by Bates and colleagues,92 but unclear in the randomised crossover trial by McDonald and colleagues. 29 It was therefore unclear whether true randomisation was carried out. Adequate study eligibility criteria were reported in the four CRCTs,24,48,90,91 the RCT,92 and the CCT,57 but again were lacking in the trial by McDonald and colleagues. 29
Only partial baseline details on the physicians and patients included in the two trials conducted by Overhage24,48 and the RCT by Bates and colleagues92 were reported, and again this information was lacking in the trial by McDonald and colleagues. 29 However, where this information were reported it does appear that intervention and control groups were reasonably well balanced in terms of baseline prognostic factors. 24,48,57,90,92 The exception to this was the CRCT conducted by Matheny and colleagues,91 in which baseline imbalances in terms of patients’ gender, age and insurance status were adjusted for in the analyses. Only the CCT by O’Connor and colleagues57 administered any cointerventions, and these were similar between the two treatment groups. Care provider blinding to treatment allocation, as expected with this type of intervention was not attained in any of the trials. Data analyses were appropriate in three of the CRCTs,24,48,91 and the RCT,92 but clustering was not taken into account in the analyses in the CRCT by Palen and colleagues. 90 Again due to lack of adequate reporting it was unclear whether the analyses undertaken by McDonald and colleagues29 were appropriate. Apart from the CRCT by Overhage and colleagues,48 analyses in all of the trials was undertaken on an ‘intention to provide or communicate information’ basis. All the trials attained a > 80% follow-up of patients.
Uncontrolled studies
The uncontrolled studies consisted of an ITS study by Carton and colleagues93 and two UPP studies by Steele and colleagues94 and Abboud and colleagues. 87 The studies were of variable quality. In the ITS it was unclear whether the intervention was independent of other secular changes over time which may have confounded the results. Moreover, the AB-AB-AB employed is likely to have confounded results due to intervention carry over effects into the control periods. The effect of this if any, would be to underestimate the effects of the CDSS intervention. Additionally, although there were an adequate number of data points collected to allow for reliable statistical analysis, no formal tests for trends were undertaken. On a more positive note, the method of data collection was reliable and unlikely to be affected by the intervention, and outcome assessment was blinded. Furthermore, there was a low rate of attrition with > 80% of episodes of care included in the follow-up assessment. 93 In the pre–post study by Steele and colleagues94 adequate, although limited study eligibility criteria were reported for both health-care providers and patients. However, no further sociodemographic information were reported on the health-care providers in the study. Detailed sociodemographic information on all patients seen during the study period were reported, but this was not reported separately for pre- and intervention periods. It is therefore unclear whether any differences in patient socio-demographics between the two study periods could have potentially biased the results obtained. All statistical analyses undertaken were appropriate, with analysis conducted on an ‘intention to provide or communicate information’ basis. Overall, the study was reasonably well designed and conducted, with the results likely to be robust. The study by Abboud and colleagues87 was of a somewhat poor methodological quality. Although study eligibility criteria were adequately specified, no baseline sociodemographic data on either the patients or physicians involved in the study were presented. This means that differences in patient baseline characteristics between the pre- and post-intervention periods could potentially confound the results. Data analyses were appropriate and conducted on an ‘intention to provide or communicate information’ basis, with > 80% of episodes of care included in the analyses.
CDSS characteristics
A summary of the key characteristics of the CDSS used in the 10 studies are displayed in Table 14. The specific CDSS used in the studies were only reported in four of the studies,57,87,90,94 but in the two CRCTs by Overhage and colleagues24,48 and the study by McDonald and colleagues29 would appear to be the home-grown/inhouse OCS and CDSS developed by Wishard Memorial Hospital, Indianapolis, IN, USA. Likewise, in the CRCT by Matheny and colleagues91 and the RCT by Bates and colleagues92 the system would again appear to one of the site specific ones, this time belonging to Brigham and Women’s Hospital, Boston, MA, USA. In the four studies that reported the systems used, the CRCT undertaken by Palen and colleagues90 used a study-specific CDSS (Clinical Information System) developed in collaboration with IBM (Boulder, CO, USA) that was implemented within the existing Kaiser Permanente proprietary system; the CCT by O’Connor and colleagues57 used what appears to be a commercially available diabetes mellitus-specific CDSS developed by Epic Systems (Madison, WI, USA); and the two pre–post studies by Steele and colleagues94 and Abboud and colleagues87 used study-specific CDSS that were developed in collaboration with Thomson Micromedex and Siemens Medical Solutions using commercially available Medical Logical Modules modified to meet the needs of the Denver Health laboratory,94 and a CDSS developed by invision®, Siemens Medical Solutions, Malven, PA, USA that was then implemented within the existing hospital information system. 87 The CDSS used in the ITS by Carton and colleagues93 was not reported.
| Study ID | Overhage (1996)48 | Overhage (1997)24 | Palen (2006)90 | Matheny (2008)91 | Bates (1999)92 | McDonald (1980)29 | O’Connor (2005)57 | Carton (2002)93 | Steele (2005)94 | Abboud (2006)87 |
|---|---|---|---|---|---|---|---|---|---|---|
| CDSS characteristics | ||||||||||
| 1. Name of CDSS (if any) | NR | NR | Clinical Information Systema | NR (Brigham and Women’s Hospital) | NR | NRb | Epic Systems | NR | NRc | invision® |
| 2. CDSS reasoning methods | DR | DR | NR | DR | NR | NR | NR | NR | DR | NR |
| 3. CDSS knowledge base | Guidelines (US Preventive Services Task Force Recommendations) | Standard reference books, drug package inserts and local practice guidelines | Published and internal guidelines, and expert opinion | Guidelines | Test-specific intervals based on a review of the literature | NR | NR | Guidelines based on a review of the literature and clinician expertise | Commercially available Medical Logic Modules developed and adapted to meet local needs | NR |
| 4. Information used in CDSS | NR | NR | Medication related alerts for baseline laboratory monitoring tests | Time interval from previously ordered test | Published and internal guidelines and expert opinion | NR | NR | NR | Biochemical tests | Test orders |
| 5. Time to complete CDSS (minutes) | NR | NR | NR | NR | NR | NR | NR | 1 min | NR | NR |
| 6. CDSS output format | R | R | R | R | R | R | R | R | R | R |
| 7. Is a description of pilot testing with users prior to implementation provided? | No | No | No | No | No | No | No | No | No | No |
| 8. Is user instructional training at the time of implementation described? | No | No | Yes | No | Yes | No | Yes | No | No | Yes |
| General system features | ||||||||||
| 9. Is the CDSS integrated with charting or OCS to support workflow integration?d | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Clinician–system interaction features | ||||||||||
| 10. Is automatic provision of CDSS output provided as part of clinician workflow?d | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 11. Is there a need for additional data entry by the clinician other than the specification of which test orders?d | No | No | No | No | No | No | No | Yes | No | No |
| 12. Does the CDSS request documentation of the reason for not following CDSS recommendations?d | No | No | No | No | No | No | No | Yes | No | No |
| 13. Does CDSS provide output at the time and location of decision making?d | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 14. Are the CDSS recommendations executed by the clinician noting agreement?d | No | No | No | No | No | No | No | No | No | No |
| Communication content features | ||||||||||
| 15. Does the CDSS provide a recommendation rather than just an assessment?d | NR | No | No | Yes | No | No | Yes | No | No | No |
| 16. Does the CDSS promote action rather than inaction?d | No | NR | NR | Yes | NR | NR | Yes | NR | No | NR |
| 17. Does the CDSS justify the output of provision of reasoning?d | No | No | No | No | No | No | No | No | NR | No |
| 18. Does the CDSS justify the output by provision of research evidence?d | No | No | No | No | No | No | No | No | No | No |
| Auxiliary features | ||||||||||
| 19. Were the local users involved in the CDSS development process?d | No | No | No | No | No | No | No | No | Yes | No |
| 20. Is the CDSS output provided to patients as well as clinicians?d | No | No | No | No | No | No | No | No | No | No |
| 21. Does the CDSS provide periodic summaries of performance feedback?d | No | No | No | No | No | No | No | No | No | No |
| 22. Is the CDSS used in conjunction with conventional education?d | No | No | No | No | No | No | Yes | No | No | Yes |
In the four studies that reported the CDSS reasoning methods, these were all based on discrimination rules. 24,48,91,94 Where reported the CDSS knowledge base was based upon either reviews of the literature,92,93 guidelines,48 standard reference books, and drug packet inserts,24 or existing database information. 94 The CDSS output in all studies was the display of reminders.
In relation to the 14 features of CDSS proposed by Kawamoto and colleagues16 the CDSS was not reported as being piloted with users prior to implementation in any of the 10 studies, but user instructional training at the time of implementation was reported in four. 57,90,92,100 The CDSS in all 10 studies was integrated as part of the OCS, and provided output automatically as part of the consultation workflow. Only in the UPP study by Carton and colleagues was there a need for additional data entry by the physician,93 and only in this study and the CRCT by Overhage and colleagues48 was a reason requested for not following the CDSS reminders. None of the studies required the user to note agreement with the reminder before implementation. The study by O’Connor and colleagues57 was the only one in which a recommendation rather than just a reminder was issued. In none of the studies was the reminder justified by recourse to the CDSS reasoning methods or evidence upon which these were based. Additionally, it would appear that local system users were only involved in the CDSS development process in the study by Steele and colleagues. 94 The data provided by the CDSS was used by the physician alone, and in all studies did not appear to provide periodic summaries of performance feedback. Two studies, those by O’Connor and colleagues57 and Abboud and colleagues87 both combined CDSS implementation with concomitant coeducation of users. None of the other studies deployed any concomitant cointerventions.
Results
Process outcomes
In the first CRCT by Overhage and colleagues48 there were no significant differences between intervention and control physicians in compliance with suggested preventative laboratory testing guidelines for cervical cytology screening, mammography, thyrotropin screen, hepatitis B screen, urinalysis, cholesterol testing, human immunodeficiency virus (HIV) screening, 24-hour urine protein testing, sickle cell screening or screening for STDs. Of note in this trial, no further significant differences were observed in compliance with reminders for preventive care measures between intervention and control physicians for either recommendations to undertake vaccinations or for the prescription of prophylactic medication. 48 A summary of the specific results from the trial by laboratory test type is displayed in Table 15.
| Intervention | OCS alone | OCS + CDSS | |||
|---|---|---|---|---|---|
| Preventive laboratory test | n | Compliance (%) | n | Compliance (%) | p-value |
| Cervical cytology study | 329 | 2.8 | 323 | 2.8 | 0.41 |
| Mammography | 131 | 1.5 | 125 | 5.6 | 0.08 |
| Thyrotropin screen | 118 | 9.3 | 112 | 16.1 | 0.12 |
| Hepatitis B screen | 92 | 2.2 | 88 | 8.0 | 0.08 |
| Screening urinalysis | 75 | 34.7 | 68 | 32.4 | 0.77 |
| Cholesterol test | 58 | 13.8 | 70 | 14.3 | 0.94 |
| HIV Screen | 43 | 9.3 | 44 | 4.6 | 0.38 |
| 24-hour urine protein test | 23 | 4.4 | 24 | 25.0 | 0.05 |
| Sickle cell screen | 14 | 0.0 | 22 | 9.0 | 0.25 |
| STD screen | 6 | 16.7 | 2 | 50.0 | 0.35 |
In the second CRCT, also conducted by Overhage and colleagues24 only relevant data on 24-hour compliance rates for the 18 most common laboratory test corollary orders were presented separately and were of relevance to the current assessment. The overall trial results indicate that the display of reminders had a significant effect on the number of corollary orders placed, with compliance rates of 46.3% in the intervention group compared with 21.9% in the control group for immediate compliance with test orders (p < 0.0001). The data on compliance with the ordering of common laboratory tests supports there being a significant effect of reminders on corollary test ordering, with compliance rates being higher in the intervention group than the control for every type of test order. The increase in compliance with suggested corollary orders ranged from 7.1% to 72.6% according to the type of laboratory test suggested, in the intervention group compared to the control. A specific breakdown of compliance rates by trial group and suggested laboratory test order is given in Table 16.
| Intervention | Total (n) | OCS alone | OCS + CDSS | Compliance increase (%) |
|---|---|---|---|---|
| % | % | |||
| Suggested test order (compliance) (%) | ||||
| Serum creatinine | 1209 | 41.2 | 48.3 | 7.1 |
| Serum electrolytes | 1034 | 70.9 | 87.0 | 16.2 |
| Glycosylated HbA1C | 821 | 7.4 | 23.7 | 16.3 |
| Activated partial thromboplastin time | 615 | 59.6 | 89.2 | 29.7 |
| SGPT (ALT) | 569 | 1.9 | 12.6 | 10.8 |
| SGOT (AST) | 467 | 0 | 7.1 | 7.1 |
| Capillary glucose | 446 | 4.4 | 30.8 | 26.7 |
| Blood cell profile | 382 | 51.4 | 80.5 | 29.0 |
| Stool occult blood test | 374 | 12.1 | 60.9 | 48.9 |
| Prothrombin time | 320 | 45.5 | 64.6 | 19.1 |
| Theophylline level | 270 | 46.5 | 75.9 | 29.4 |
| Platelet count | 236 | 15.1 | 70.0 | 54.9 |
| Reticulocyte count | 205 | 11.4 | 19.7 | 8.3 |
| Fe-TIBC | 149 | 0 | 12.6 | 12.6 |
| Vancomycin | 143 | 65.2 | 90.7 | 25.6 |
| Phenytoin level | 140 | 38.4 | 73.1 | 34.8 |
| A-V blood gas | 123 | 0 | 72.6 | 72.6 |
| Gentamicin level | 118 | 75.9 | 90.0 | 14.1 |
In contrast to the results of the CRCT by Overhage and colleagues,24 Palen and colleagues90 found no significant differences between intervention and control group physicians in the overall rate of compliance with ordering the recommended laboratory monitoring tests for patients prescribed one or more of the 25 target study medications. Laboratory monitoring was performed within the recommended guidelines 56.6% of the time (10,494 of 18,556 index dispensings) in the intervention group compared with 57.1% of the time (8957 of 15,686 index dispensings) in the control group (p = 0.31). Analysis of guideline compliance rates by patient gender also showed no significant differences between the intervention and control groups.
Male patients who had medication orders placed by intervention group physicians had a laboratory monitoring rate of 57.5% compared with 58.5% for control physicians (p = 0.18). The comparative percentages for female patients were 55.7% and 55.9% respectively (p = 0.82). Compliance rates with guidelines for laboratory monitoring for individual medications varied from 0.0% to 93.7%. A summary of compliance rates for each of the 25 individual medications is shown in Table 17. Although the overall results showed no significant difference between the two groups, this was not true across all individual medications. In the four drugs in which a statistically, or borderline statistically, significant difference was observed, the improvement in monitoring rates favoured the patients of physicians in the intervention group. The laboratory monitoring rates among patients prescribed medications by the intervention group compared with control group was 52.8% versus 46.0% for colchicine (p = 0.05), 71.2% versus 62.3% for gemfibrozil (p = 0.003), 42.9% versus 0.0% for methotrexate (p = 0.03), and 75.7% versus 73.9% for statins (p = 0.05).
| Medication | OCS alonea | OCS + CDSSa | p-valueb |
|---|---|---|---|
| ACE inhibitors | 2729 (47.5) | 3099 (47.0) | 0.681 |
| Allopurinol | 355 (61.1) | 429 (57.6) | 0.31 |
| Carbamazepine | 119 (35.3) | 153 (34.6) | 0.91 |
| Colchicine | 400 (46.0) | 411 (52.8) | 0.05 |
| Digoxin | 178 (48.9) | 242 (55.0) | 0.22 |
| Diuretic | 4270 (45.6) | 5384 (44.0) | 0.11 |
| Gemfibrozil | 454 (62.3) | 569 (71.2) | 0.003 |
| Isoniazid | 36 (19.4) | 33 (15.2) | 0.64 |
| Losartan potassium | 433 (52.7) | 506 (52.0) | 0.84 |
| Metformin hydrochloride | 940 (7.6) | 1098 (67.7) | 0.14 |
| Methotrexate | 9 (0.0) | 7 (42.9) | 0.03 |
| Niacin | 36 (47.2) | 34 (67.7) | 0.084 |
| Phenytoin sodium | 52 (25.0) | 83 (32.5) | 0.35 |
| Pioglitazone hydrochloride | 63 (93.7) | 76 (92.1) | 0.73 |
| Potassium chloride | 1291 (57.8) | 1623 (54.3) | 0.06 |
| Rifampincin | 6 (50.0) | 7 (14.3) | 0.20 |
| Statins | 4245 (73.9) | 4717 (75.7) | 0.05 |
| Valproic acid | 70 (38.6) | 85 (36.5) | 0.79 |
| Total | 15,686 | 18,556 | 0.79 |
Likewise, Matheny and colleagues91 also found no significant differences between intervention and control group physicians in the overall rate of compliance with ordering the recommended laboratory monitoring tests for one or more of the 15 study target drugs in patients who had not received a laboratory monitoring test in the previous year. Rates of appropriate laboratory monitoring within 14 days of an office visit ranged from 12.5% for therapeutic drug levels to 64% for potassium levels. A summary of compliance rates for each of the 15 individual medications is shown in Table 18. The authors postulated that the impact of reminders in the intervention group was small due to the already high rates of appropriate laboratory monitoring of patients. In the study medication-laboratory monitoring non-compliance ranged from 1.6% (21/1330) for potassium supplementation to 6.3% (1287/20,376) for statin use.
| Medication-laboratory reminder | Visits (n) | Visits with laboratory overdue (n; %) | Laboratory ordered when overdue (n; %) | Odds Ratio (adjusted) (95% CI) | p-value |
|---|---|---|---|---|---|
| NSAID-Cr | |||||
| OCS + CDSS | 8487 | 442 (5.2%) | 150 (33.9%) | 1.24 (0.71 to 2.15) | 0.457 |
| OCS alone | 9307 | 428 (4.6%) | 136 (31.8%) | ||
| ARB-Cr | |||||
| OCS + CDSS | 751 | 31 (4.1%) | 17 (54.8%) | 0.24 (0.04 to 1.34) | 0.104 |
| OCS alone | 832 | 27 (3.2%) | 17 (63.0%) | ||
| Metformin-Cr | |||||
| OCS + CDSS | 857 | 20 (2.3%) | 7 (35.0%) | 0.53 (0.05 to 5.34) | 0.594 |
| OCS alone | 781 | 16 (2.1%) | 6 (37.5%) | ||
| K Supplement-K | |||||
| OCS + CDSS | 579 | 12 (2.1%) | 7 (58.3%) | 0.91 (0.03 to 24.44) | 0.956 |
| OCS alone | 751 | 9 (1.2%) | 5 (55.5%) | ||
| K Sparing Diuretic-K | |||||
| OCS + CDSS | 761 | 19 (2.5%) | 13 (68.4%) | 0.82 (0.12 to 5.60) | 0.836 |
| OCS alone | 875 | 28 (3.2%) | 17 (60.7%) | ||
| Thiazide Diuretic-K | |||||
| OCS + CDSS | 1997 | 62 (3.1%) | 40 (64.5%) | 1.30 (0.63 to 2.67) | 0.473 |
| OCS alone | 2508 | 89 (3.5%) | 46 (51.7%) | ||
| ACE Inhibitor-K | |||||
| OCS + CDSS | 2279 | 119 (5.2%) | 57 (47.9%) | 1.00 (0.43 to 2.30) | 0.993 |
| OCS alone | 2790 | 80 (2.9%) | 40 (50.0%) | ||
| Statin-ALT | |||||
| OCS + CDSS | 9441 | 613 (6.5%) | 291 (47.5%) | 0.89 (0.43 to 1.81) | 0.740 |
| OCS alone | 10935 | 674 (6.2%) | 358 (53.1%) | ||
| Thyroxine-TSH | |||||
| OCS + CDSS | 897 | 38 (4.2%) | 22 (57.9%) | 1/19 (0.40 to 3.53) | 0.747 |
| OCS alone | 1233 | 44 (3.6%) | 25 (56.8%) | ||
| Therapeutic levela | |||||
| OCS + CDSS | 514 | 16 (3.1%) | 2 (12.5%) | 0.55 (0.03 to 8.94) | 0.677 |
| OCS alone | 755 | 26 (3.4%) | 4 (15.4%) | ||
Bates and colleagues92 reported 939 apparently redundant laboratory tests ordered over the 4-month trial period, including 437 (47%) in the intervention group. In this group, suggestions to cancel the test were accepted 69% (300 out of 437 tests) of the time, and therefore 31% of reminders were overridden. The rate of actual performance of the redundant test orders was significantly reduced in the intervention group, being 27% (117) compared to the 51% (257) observed in the control group, and therefore there was an absolute difference in the proportion of redundant tests performed of 24% (p < 0.001). Of note, the rate of test performance for tests that would have received a reminder was reasonably low at 51% in the control group. A pre–post comparison of the number of target tests performed earlier than the test specific interval comparing the 4 months immediately preceding the trial and results from the 15-week study period, indicated that during the preceding period, 20.5% of target tests were performed earlier than the specific intervals. During the study period this was significantly lower in the intervention group at 18.5% (p = 0.004) but not in the control group (19.6%; p = 0.19). Additionally, in the 4-month period preceding the intervention, there were 4.84 target tests per admission, compared with 4.24 during the study period in the intervention group and 4.28 in the control group (p < 0.0001).
Adverse effects of test cancellation
To determine whether the cancellation of a test had potentially adverse effects new abnormal results for the same test performed with 3 days of cancellation was conducted. Chemistry-20 profiles were excluded from the analysis due to high probability of an abnormal result on at least one of the tests in the panel. Of the remaining 225 accepted reminders, 119 (53%) were followed by another test of the same type within 72 hours; 55 (24%) of these were abnormal. Only 10 (4%) of these had not been preceded by a similar abnormal result within 24 hours before the cancelled test, and two were duplicate orders for the same patient. Therefore only 8 (4%) of the tests provided new information.
Process outcomes
McDonald and colleagues29 reported limited results from their randomised crossover trial for test ordering alone. These indicated the presentation of reminders (either with or without supporting bibliographic citations) significantly increased compliance with test ordering in both residents and interns. These increased from 20% and 9% during the control phase in which reminders were suppressed to 49% and 38% during their presentation for each of the groups respectively. The presence of reminders had no significant effect on the test ordering behaviour of nurse clinicians with compliance rates of 15% and 24% in each trial condition.
Results from the CCT by O’Connor and colleagues57 in outpatients with diabetes showed that the number of HbA1C tests performed per patient per year in the intervention clinic increased significantly relative to the number of HbA1C tests in the comparison clinic at both 2-year (p < 0.04) and 4-year follow-up (p < 0.001) after the CDSS was introduced. In the first year of the introduction of the system (1996) 1.67 HbA1C tests were performed per patient in the 12-month period in the intervention clinic, with this increasing to 2.2 in 1998 and 2.46 in 2000. The comparative figures for the control clinical were 1.75, 1.83 and 1.63 for the years 1996, 1998, and 2000 respectively. Therefore while an increase in the number of tests performed was observed in the intervention clinic, a comparable rise was not observed in the control clinic. There were no significant effects of any other covariates in the model (patient age, gender and baseline Charlson comorbidity score) observed. For the number of LDL cholesterol tests performed, the number increased in both clinics from 1996 (0.54 in the intervention clinic vs 0.49 in the control clinic), through 1998 (0.87 in the intervention clinic vs 0.59 in the control clinic) to 2000 (1.45 in the intervention clinic vs 0.92 in the control clinic). However there were no significant differences in LDL cholesterol test rates between the clinics (p = 0.33 and p = 0.19) for the years 1998 and 2000 respectively. Again there were no significant interactions in the model for any of the covariates.
Results of the third model which tested whether the proportion of patients who met both the HbA1C and LDL testing thresholds increased with time and by clinic showed the proportion increased significantly from 1996 (29.8 for the intervention clinic vs 30.8 in the control clinic) to 1998 (57.9 for the intervention clinic vs 46.2 in the control clinic (between time points; < 0.05) and showed an even greater increase between 1996 and 1998 in the intervention clinic (70.2), but remained stable in the control clinic (46.2) (between time points; p < 0.01). There was no significant difference between the intervention and control clinics for the time period of 1996 to 1998 (p = 0.27), but due to the increase in the proportion of patients meeting the criteria in the intervention clinic between 1998 and 2000 was significantly different (p = 0.03).
There were no significant differences between clinics for the outcome of HbA1C values either for the time period of 1996 (7.80 for the intervention clinic vs 7.35 for the control clinic) to 1998 (7.90 for the intervention clinic vs 7.26 for the control clinic) (p = 0.10) nor the period 1998 to 2000 (7.71 for the intervention clinic vs 7.11 for the control clinic) (p = 0.27). The only significant effect involving covariates showed that older patients had lower HbA1C values. There were too few patients with LDL cholesterol level measurements throughout the two follow-up periods for the data to produce reliable statistical estimates. Therefore these data were not analysed.
Carton and colleagues93 in their ITS study to assess the effects of CDSS on radiology referral practice compared with a set of guidelines in two French accident and emergency departments, found a small but significant decrease in the proportion of requests that did not conform to the guideline from 33% when the guidelines were inactive to 27% when recommendations were active (p < 0.0001). However, there were considerable differences between the two hospitals in the number of requests that did not conform to the guidelines, with 353 (18%) of requests not conforming in hospital A compared to 1693 (35%) in hospital B (p < 0.0001). The three most common examinations (abdominal plain radiographs, chest radiographs and CT of the brain) represented more than 90% of all examinations not in agreement with the guidelines. When considering each of these examinations separately, approximately 76.5% of abdominal plain radiographs, 24.9% of chest radiographs and 15.8% of CT examinations did not conform to guidelines. Overall, the requests from junior practitioners more frequently disagreed with recommendations than those from senior practitioners (30.8% vs 24.0% respectively; p < 0.0001). Additionally, analyses of the number of requests not conforming with the guidelines by temporal period (i.e. change from intervention period one to control period one) showed an increase on each of the three successive occasions when the recommendations were switched off: from 27.5% to 29.8% (relative increase of 8.4%), from 27.0% to 37.8% (relative increase of 40%), and from 26.0% to 26.9% (relative increase of 3.5%) indicating that there did not appear to be a learning effect regarding guideline appropriate test ordering that carried through into the control periods.
Results from the pre–post study by Steele and colleagues94 showed that comparison of the pre- and post-intervention periods for medication orders for which no reminder was displayed showed no significant differences in the percentage of time the provider ordered the drug rule associated laboratory test (17.0% during pre-intervention period vs 16.2% during the post-intervention period; p = 0.38). This indicates that there was no trend to increased laboratory test ordering during the post-intervention period.
In contrast there was a significant increase during the intervention period that the rule associated laboratory test was ordered when an alert was displayed, from 347 (38.5%) during the pre-intervention period to 559 (51.1%) in the intervention period (% change: +32.73; p-value <0.0001).
In the UPP study conducted by Abboud and colleagues87 there were 336 courses of aminoglycoside therapy prescribed for 4 or more days duration in 275 patients (1.2 courses per patient). In total there were 548 laboratory results obtained (2.0 per patient). However of these, 114 results (20.8%) did not meet the predefined criteria for a peak or trough level, resulting in 434 analysed laboratory results. In the pre- and post-intervention periods there were no significant differences between the number of aminoglycoside prescriptions with appropriate laboratory test monitoring: 128 (80.5%) vs 146 (82.5%) for each time period respectively (p > 0.05). Additionally, there were no significant differences observed in the frequency in which patients had all therapeutic levels [n = 94 (84.7%) in the pre-intervention phase vs n = 100 (80.0%) in the post-intervention phase]; any toxic level [n = 9 (8.1%) in the pre-intervention phase vs n = 15 (12.0%) in the post-intervention phase]; or any subtherapeutic levels [n = 8 (7.2%) in the pre-intervention phase vs n = 7 (5.6%) in the post-intervention phase].
Summary of studies assessing the impact of the display of reminders
Process outcomes
Ten studies, including four CRCTs,24,48,90,91 one RCT,92 one CCT,57 one randomised crossover trial,29 one ITS with an AB-AB-AB design,93 and two UPP studies87,94 assessed the impact of the display of reminders. Across the studies nine of the 10 were conducted in the USA,24,29,48,57,87,90–92,94 and assessed the impact of the reminders on compliance to undertake preventative care measures48, laboratory test ordering for medication monitoring,24,29,57,87,90,91,94 or the ordering of redundant tests. 92 Two of the studies were either undertaken on specific patient groups, namely those with diabetes,57 or assessed one specific drug (aminoglycosides). 87 All the rest of the studies assessed the impact on the monitoring of a number of different pre-specified drugs or test orders.
The final study, which was conducted in France, assessed the impact of reminders on compliance with guidelines for radiology imaging referral. Results from the one CRCT that assessed compliance with reminders to undertake preventative care measures suggests that contrary to findings in an outpatient setting, these had no impact on increasing compliance with guidelines for undertaking preventative laboratory screening in patients in an inpatient setting, as there were no significant differences between the intervention and control groups observed. 93
In relation to compliance with guidelines for undertaking suggested laboratory monitoring for pre-specified drugs, the results across the studies were mixed and equivocal. Of the six studies reviewed, the CRCT by Overhage and colleagues,24 the pre–post study by Steele and colleagues,94 and the randomised crossover trial by McDonald and colleagues29 all reported a significant impact of the display of reminders on compliance with undertaking the necessary laboratory tests. However, in the trial by McDonald and colleagues29 this impact was limited only to physicians and did not extend to nurse practitioners. In the other three studies, the two CRCTs by Palen and colleagues90 and Matheney and colleagues91 and the pre–post study by Abboud and colleagues,87 reminders did not significantly improve compliance with suggested laboratory test monitoring across a range of different pharmacological therapeutic interventions in both primary and secondary care settings.
Again, the impact of the display of reminders on compliance with guidelines for laboratory test monitoring in patients with diabetes was mixed in the CCT by O’Connor and colleagues. 57 Reminders had a significant impact on the number of patients undergoing HbA1C tests, but no significant impact on the number of LDL cholesterol tests undertaken. Likewise, there were mixed effects on the proportion of patients meeting both the HbA1C and LDL testing thresholds, with no significant differences between intervention and control groups observed at 2-year follow-up, but a significant impact in favour of the intervention group observed at 4-year follow-up. In terms of actual HbA1C values there were no significant differences observed at either time point between patients in the intervention and control groups. Thus suggesting that while reminders may have some impact (dependent on the test type) on the number of tests undertaken, this does not translate into actual clinical differences that may impact on the patient’s disease process and management.
Results from the one RCT by Bates and colleagues92 assessing the impact of reminders about redundant tests, suggested that these have a significantly positive impact on the number of redundant test orders placed, with a 27% absolute reduction in rates observed between the intervention and control groups. This did not appear to have adverse effects in terms of there being new abnormal results that would have provided new information from the cancelled redundant test, and therefore potentially impacted upon patient care outcomes.
Likewise, the ITS by Carton and colleagues93 found the display of reminders had a small but significant impact on the number of radiology imaging referrals that conformed to guidelines, with an increase from 66.8% compliance without reminders to 73.1% with reminders. A summary of the results of the eight studies which assessed the impact of the display of reminders on compliance with preventative care measures or laboratory medication monitoring is displayed in Table 19, while the results for the impact of reminders on redundant laboratory tests and compliance with radiology referral guidelines is displayed in Table 20.
| Study ID | Overhage (1996)48 | Overhage (1997)24 | Palen (2006)90 | Matheny (2008)91 | McDonald (1980)29 | O’Connor (2005)57 | Steele (2005)94 | Abboud (2006)87 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design | CRCT | CRCT | CRCT | CRCT | Randomised x-over trial | CRCT | UPP | UPP | ||||||||
| OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | |
| Pre- | Post- | Pre- | Post- | |||||||||||||
| n | 821 | 801 | 814 | 872 | 14,376 | 12,210 | 924 | 998 | 31 | 57 | 65 | 19.076a | 159 | 177 | ||
| Compliance with suggested preventative screening tests | NS | |||||||||||||||
| 24-hour compliance with suggested laboratory corollary orders (%) | 46.3 | 46.3 | 21.9 | |||||||||||||
| p-valueb | p < 0.0001 | |||||||||||||||
| Compliance with suggested laboratory monitoring tests (%) | 56.6 | 57.1 | NR overall by group | NR overall by group |
Resident: 20% Interns: 9% Nurses: 15% |
49% 38% 24% |
38.5 | 51.1 | 80.5 | 82.5 | ||||||
| p-valueb | p = 0.31 | p > 0.05 | p < 0.0001 | p > 0.05 | ||||||||||||
|
Number of HbA1c tests performed per patient year Baseline: 2-year follow-up 4-year follow-up |
1.7 22.2 2.5 |
1.8 1.8 1.6 |
||||||||||||||
|
p-value 2-year follow-up 4-year follow-up |
p < 0.04 p < 0.001 |
|||||||||||||||
|
Number of LDL cholesterol tests performed per patient year Baseline: 2-year follow-up 4-year follow-up |
0.54 0.87 1.45 |
0.49 0.59 0.92 |
||||||||||||||
|
p-value 2-year follow-up 4-year follow-up |
p = 0.33 p = 0.19 |
p = 0.33 p = 0.19 |
||||||||||||||
|
Proportion of patients meeting both HbA1C and cholesterol tests thresholds Baseline: 2-year follow-up 4-year follow-up |
29.8 57.9 70.2 |
30.8 46.2 46.2 |
||||||||||||||
|
p-value 2-year follow-up 4-year follow-up |
p < 0.05 p < 0.01 |
p < 0.05 p < 0.01 |
||||||||||||||
| Study ID | Bates (1999)92 | Carton (2002)93 | ||
|---|---|---|---|---|
| Study design | RCT | ITS | ||
| Intervention | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS |
| n | 5700 | 5886 | 6869 | |
| Number of redundant test orders (n; %) | 437 (47) | 502 (53) | ||
| Difference between groups in proportion of redundant tests performed (%) | 24 | |||
| p-valuea | p < 0.001 | |||
| % requests not complying with radiology referral guidelines | 26.9 | 33.2 | ||
| p-valuea | p < 0.0001 | |||
Studies assessing the impact of the display of restricted lists
Quantity and quality of the studies
Assessment of the display of restricted lists was examined in one ITS study by Rosenbloom and colleagues95 and one CPP study by Poley and colleagues. 96 The study by Poley and colleagues96 also included a cost-comparison analyses, the results of which are reported in Chapter 6, Systematic review of economic evaluations. Both studies targeted specific tests, with the ordering of serum magnesium level tests being the subject of the ITS by Rosenbloom and colleagues95 and blood test ordering targeted by Poley and colleagues. 96 The area of impact in the test ordering process targeted in both studies was therefore test volumes. 95,96 The ITS by Rosenbloom and colleagues95 was conducted on 30 of the 33 inpatient wards at the Vanderbilt University Hospital, Nashville, TN, USA over a 6-year period (1 January 1998 to 31 December 2003). The specific CDSS evaluated was the WizOrder Care Provider Order Entry System, a home-grown system developed by the medical centre. The system assessed in the study was a non-commercialised form of the software code used at the hospital. All patients admitted to any ward where the CDSS was implemented over the study period were eligible for inclusion; with a total of 194,192 patients admitted. The CDSS users were all physicians, nurse practitioners or medical students working within the specific wards. No further data on CDSS user sociodemographic variables were presented.
The study consisted of three different CDSS intervention protocols that were implemented sequentially over the study period. The Vanderbilt University Medical Centre Resource Utilization Committee firstly identified two ‘normal’ ranges for serum magnesium test results that were considered appropriate. One was based on the institutional laboratory’s statistically normal range, 1.5–2.5 mg/dL, and other represented a ‘physiologically appropriate’ range (i.e. serum magnesium was unlikely to directly cause clinical sequelae if within this range) of 1.0–3.9 mg/dL. These were then implemented within the CDSS, with changes to the three different interventions made based on the results of the previous intervention.
At study baseline (1 January 1998 to 4 December 1999), no protocols were in place restricting the frequency, context or volume of serum magnesium test ordering. The first CDSS intervention (implemented from 5 December 1999 to 21 March 2000), targeted all open-ended laboratory and radiology orders, with tests scheduled more than 72 hours into the future flagged, and users prompted to consider discontinuing the order. The second intervention, which was implemented from 20 June 2000 to 30 November 2001, was also a broad-based CDSS intervention, that globally addressed the ordering of multiple laboratory tests simultaneously. The specific tests addressed were magnesium, calcium, and phosphorus.
The intervention included a graphical display of patients’ recent serum magnesium, calcium, and phosphorus test results, educational material outlining indications for magnesium testing, and test interpretation. This intervention also limited orders to one test per order (i.e. no recurrent testing was possible). CDSS users could bypass the intervention only by ordering magnesium testing from disease-specific order sets or by specifically ordering a single magnesium test (rather than recurrent testing) from the standard OCS. The third intervention, implemented from 1 December 2001 to 31 December 2003, focused only on the ordering of magnesium tests. This intervention included a graphical display of patients’ most recent serum magnesium result, and a graphical display of a calculated corrected magnesium value. Test volumes were also limited to one test per order. In addition users had to enter a reason for testing after reviewing a list of indications. CDSS users could bypass the intervention by ordering magnesium tests from a disease-specific order set. Both of the second and third interventions implemented therefore included a concomitant CDSS intervention, such as the display of educational material, and were not limited to just the use of test restrictions. Follow-up within the study was conducted at 11 months, 27 months; 47 months and 72 months. 95
The CPP study by Poley and colleagues96 was conducted in 134 primary care practices in the Netherlands, with the study consisting of a 6-month pre-intervention period and a 6-month post-intervention period. The total length of study follow-up was therefore 12 months. The study included 234 primary care physicians (159 in the intervention group and 75 in the control group), with study inclusion criteria including submission of greater than 80% of blood test orders to one of the 27 laboratories participating in the study, and use of one of three information systems (Elias, MicroHIS, or Promedico) for which the CDSS was developed. There were no statistically significant differences in baseline sociodemographic variables either between physicians in the intervention and control groups, or between physicians in the intervention group and national figures on physicians from the Netherlands Institute for Health Services Research. The CDSS was comprised of an optimal but restricted list of blood tests based on recommendations for blood test ordering for the patient’s indication, selected by the physician based on guidelines from the Dutch College of General Practitioners (http://nhg.artsennet.nl/). The GP could adhere to the proposed list or add or remove tests from the list as appropriate. Additionally, the GPs were not obliged to use the CDSS. The specific CDSS developed was a study specific system, that is available (in Dutch only) from the authors, and is integrated with the three OCS specified above as in the study. 96 A summary of the study characteristics from the two studies is displayed in Table 21.
| Study ID | Setting/country | Study design | Number of sites | Intervention (I)/Comparison (C) | Study description | Area of impact | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| Process outcomes | Adverse effects of test cancellation | |||||||
| Rosenbloom (2005)95 | Inpatients, Vanderbilt University Hospital, Nashville, TN, USA | ITS | 1 (30 out of 33 inpatient wards) |
Three different interventions were implemented sequentially. Baseline: no restrictions in place on frequency, context or volume of magnesium test ordering. (I) OCS + CDSS (time 1): targeted all open-ended laboratory and radiology orders; tests scheduled >72 hours into the future flagged, and user prompted to consider discontinuing order (time 2): targeted magnesium, calcium and phosphorus tests; display of previous test results and educational material displayed; test orders limited to one test per order (no recurrent testing possible) (time 3): targeted magnesium tests; display of previous test results and corrected magnesium values; test orders limited to one test per order; user had to enter a reason for test ordering. |
Study conducted over a 6-year period January 1998 to December 2003 with 194,192 patients admitted during this period. All patients admitted to any hospital unit where the CDSS was implemented who required one of the indicated tests were eligible for inclusion. Follow-up was conducted at 23 months, 27 months, 47 months and 72 months | Test volumes |
Weekly rates of serum magnesium test orders Number of magnesium test orders per patient Reported magnesium test results Number of calcium tests ordered concurrently with serum magnesium tests Number of phosphorus tests ordered concurrently serum magnesium tests |
NR |
| Poley (2007)96 | Outpatient practices throughout the Netherlands | CPP | 134 |
(I) OCS + CDSS: optimal, but restricted list of blood (C): OCS alone |
Study consisted of 12-months; 6-months pre-intervention period where physicians ordered blood tests from an unrestricted list. 6-month intervention period where optimal but restricted list of blood tests were displayed for the patients suspected indication in the intervention group (n = 87 physicians). No restriction were placed on lists in control group (n = 47 physicians) | Test volumes |
Number of laboratory request forms submitted Number of tests per order form |
NR |
Outcomes
Process outcomes
The area of impact in the test ordering process assessed in both studies were test volumes. 95,96 As both studies assessed the impact of CDSS on specific test types, these included test volumes pertaining to either serum magnesium test orders or the volume of blood test orders placed. The specific outcomes reported were weekly instances of serum magnesium test orders,95 number of magnesium test orders per patient admission, reported magnesium test results,95 the proportion of either calcium or phosphorus tests ordered concurrently with magnesium test orders,95 the number of laboratory request forms submitted,96 and the number of tests per order form. 96 Additionally, as previously stated, Poley and colleagues96 also reported a cost-comparison for the development and implementation of the CDSS, compared to OCS alone, the results of which are reported in Chapter 6, Systematic review of economic evaluations.
Study quality
In both studies, study eligibility criteria were adequately reported,95,96 and groups were balanced in terms of sociodemographic variables at baseline in the pre–post study by Poley and colleagues. 96 In both studies the intervention appeared to be implemented independently of other changes in the study period, which is of particular import in the ITS study conducted by Rosenbloom and colleagues,95 and in the study by Poley and colleagues was controlled for by use of a separate group that were not exposed to the CDSS intervention. 96 In both studies therefore, steps were taken to limit bias from exposure to other concomitant interventions or independent secular changes over time.
The intervention could not influence the methods of data collection in either of the studies, and the primary outcome measure of the number of test orders was reliable. 95,96 In the ITS study by Rosenbloom and colleagues95 sufficient data points over time were reported for reliable statistical inference, and formal tests for trends were conducted. 95 In both studies statistical analyses were appropriate, and in the controlled pre–post study by Poley and colleagues comparisons of pre- and post-intervention periods, and between group comparisons were conducted. 96 However, data were not analysed on an ‘intention to provide or communicate information’ basis in this study, and the rate of attrition in the intervention group (23%) was reasonably high. This has the potential to bias the results, and no sensitivity analyses were conducted to explore differences between study completers, and those who dropped out. 96 In the ITS study by Rosenbloom and colleagues95 analyses were conducted for all instances of test ordering, and results are therefore unlikely to be biased.
CDSS characteristics
A summary of the CDSS characteristics in both studies is displayed in Table 22. In the ITS by Rosenbloom and colleagues95 the specific CDSS evaluated was the WizOrder Care Provider Order Entry System, a home-grown/inhouse system from Vanderbilt University Medical Centre. In this particular study a non-commercialised form of the software code was implemented. The CDSS used in the controlled pre–post study by Poley and colleagues was not reported, but a Dutch language version of the CDSS is available on request from the authors. Neither of the studies reported the CDSS reasoning methods, but the information used in the CDSS appeared to the normal reference ranges for serum magnesium test results in the study by Rosenbloom and colleagues95 and information from the relevant guideline in the study by Poley and colleagues. 96
| Study ID | Rosenbloom (2005)95 | Poley (2007)96 |
|---|---|---|
| CDSS characteristics | ||
| 1. Name of CDSS (if any) | WizOrder Care Provider Order Entry Systema | NR |
| 2. CDSS reasoning methods | NR | NR |
| 3. CDSS knowledge base | Serum magnesium test results cut-offs | Guidelines |
| 4. Information used in CDSS | NR | NR |
| 5. Time to complete CDSS (minutes) | NR | NR |
| 6. CDSS output format | Restricted lists | Restricted lists |
| 7. Is a description of pilot testing with users prior to implementation provided? | No | No |
| 8. Is user instructional training at the time of implementation described? | No | No |
| General system features | ||
| 9. Is the CDSS integrated with charting or OCS to support workflow integration?b | Yes | Yes |
| Clinician–system interaction features | ||
| 10. Is automatic provision of CDSS output provided as part of clinician workflow?b | Yes | Yes |
| 11. Is there a need for additional data entry by the clinician?b | No | No |
| 12. Does the CDSS request documentation of the reason for not following CDSS recommendations?b | No | No |
| 13. Does CDSS provide output at the time and location of decision making?b | Yes | Yes |
| 14. Are the CDSS recommendations executed by the clinician noting agreement?b | No | No |
| Communication content features | ||
| 15. Does the CDSS provide a recommendation rather than just an assessment?b | No | No |
| 16. Does the CDSS promote action rather than inaction?b | NR | NR |
| 17. Does the CDSS justify the output of provision of reasoning?b | No | No |
| 18. Does the CDSS justify the output by provision of research evidence?b | No | No |
| Auxiliary features | ||
| 19. Were the local users involved in the CDSS development process?b | NR | No |
| 20. Is the CDSS output provided to patients as well as clinicians?b | No | No |
| 21. Does the CDSS provide periodic summaries of performance feedback?b | No | No |
| 22. Is the CDSS used in conjunction with conventional education?b | No | No |
In both studies the output format was primarily restricted lists, but also included restrictions on forward ordering, a graphical display of previous test results, and education material as concomitant interventions at different phases of the ITS by Rosenbloom. 95 Neither of the studies reported the time to complete the CDSS, or whether pilot testing and user training were provided prior to implementation. Both the CDSS were integrated with OCS and provided output at the time and location of decision making. Neither of the systems required the additional input of information. Additionally as output was limited to the display of restricted lists only and no specific recommendations were provided, the user was not required to document a reason for not following the CDSS recommendations. It was unclear in both studies whether the display of restricted lists was likely to promote action rather than inaction on the part of the physician. The data provided by the CDSS was used by the physician alone, and did not appear to provide periodic summaries of feedback performance.
Results
Process outcomes
In the ITS study by Rosenbloom and colleagues95 at baseline when no intervention was in place the weekly rate of new serum magnesium test requests was 539. This decreased significantly to 380 per week after implementation of the first CDSS intervention (p = 0.001) (which targeted all open-ended laboratory and radiology orders; flagged tests scheduled >72 hours into the future and prompted the user to discontinue the order), increased significantly to 491 per week after the second intervention (p < 0.001) (which targeted magnesium, calcium and phosphorus tests; provided the display of previous test results and educational material, and limited tests to one per order, i.e. no recurrent testing was possible) and then decreased significantly to 276 per week after the third intervention (p < 0.001) (which targeted magnesium tests alone; displayed previous test results and corrected test values, limited test orders to one test per order; and prompted the user for a reason for the test ordering). Predicated upon this, the net serum magnesium test orders per patient followed a similar trend. CDSS users ordered a baseline mean of 0.87 test instances per admitted patient on all study wards. This decreased significantly to 0.59 net instances per patient with the first intervention (p < 0.001), increased significantly to 0.87 per patient after the second intervention (p = 0.001), and decreased significantly to 0.39 per patient after the third intervention (p = 0.003). There were no significant trend changes in net requests per patient with any of the interventions. At the end of the study period, the expected rate of magnesium testing had dropped to 0.41 requests per patient. Overall, magnesium serum testing was ordered on 21% of all admitted patients at baseline, and had dropped to 14% at the end of the study period. During all periods of the study, 14% of serum magnesium results fell outside the laboratory normal range, and 0.4% of results fell outside the physiologically acceptable range; there was no change in these rates with any of the interventions.
During the baseline period, 33% of orders for calcium testing were entered simultaneously with orders for serum magnesium testing. This did not change immediately upon implementation of the first intervention, but had dropped significantly over time to 23% prior to implementation of the second intervention (p < 0.001). Concurrent magnesium and calcium test ordering then increased significantly to 37% after implementation of the second intervention (p < 0.001), and then dropped significantly to 25% following implementation of the third (p < 0.001). During the baseline period, 48% of phosphorous test orders were entered simultaneously with orders for magnesium serum testing. This increased significantly to 80% with the implementation of the second CDSS intervention (p < 0.001), and then decreased significantly to 47% with implementation of the third (p < 0.001).
In the controlled pre–post study by Poley and colleagues96 there was a significant decrease in the number of tests requested per order form between groups in the post-intervention period (p < 0.001). In the CDSS plus OCS group 5.9 (SD 1.5) tests were requested per form in the pre-intervention period compared with 5.5 (SD 1.4) requested with implementation of the CDSS [change within group: –0.4 (SD 0.7)]. This compared with 5.8 (SD 1.3) ordered in the pre-intervention period in the control group, and 5.8 (SD 1.3) in the post-intervention period [mean change within group: –0.01 (SD 0.4)] to give a significant difference between groups of –0.38 (95% CI –0.61 to –0.16) in the number of requests ordered per form.
However, there was no significant effect upon the number of laboratory request forms submitted with implementation of the CDSS. The mean number of forms submitted in the OCS plus CDSS group in the pre-intervention period was 358 (SD 174) compared with 356 (SD 177) with implementation of the system. The change of –2 (SD 53) forms between the pre- and post-intervention periods was not significant. Likewise, there were no significant differences between the number of forms submitted in either period between the intervention and control groups, with a mean change between groups of –7 forms (95% CI –26 to 11). In the control group 376 (SD 194) forms were submitted in the pre-intervention period, and 382 (SD 208) in the post-intervention period [change within group: +6 (SD 52)].
Summary of studies assessing the impact of the display of restricted lists
Two studies, one ITS study conducted on inpatients in the USA, and one CPP study conducted in general practice in the Netherlands assessed the impact of the display of restricted lists. The aim in both of the studies was to limit unnecessary test orders, and therefore the outcomes of interest were test volumes. Both of the studies focused on specific test types with Rosenblooom and colleagues95 focusing primarily on serum magnesium test orders, and as secondary outcomes calcium and phosphorus test instances. Poley and colleagues96 targeted a range of blood tests.
Results from the ITS by Rosenbloom and colleagues,95 generally showed a significant reduction from baseline in mean weekly requests for serum test orders, from 539 at baseline, to 380 after the implementation of the first intervention, and 277 after the third. Paradoxically, there was a significant increase in test order rates after implementation of the second intervention to 491. Net requests of magnesium test orders per patient also followed this trend. Concurrent calcium and phosphorus test ordering with serum magnesium tests also showed a similar pattern, both decreasing significantly from baseline after implementation of the first intervention, increasing significantly after introduction of the second, and then decreasing significantly after the third. Overall, therefore, it would appear in this study that while the CDSS had the potential to regulate and limit unnecessary serum magnesium test ordering, other concomitant CDSS interventions may interact with this aim, and have the potential to paradoxically increase test ordering rates.
Limited results from the CPP study by Poley and colleagues96 indicated that implementation of an optimal but restricted list of blood tests within the CDSS did not significantly decrease the mean number of laboratory request forms over and above the use of OCS alone. However, there was a small (–0.38) but significant decrease noted in the number of test requests per form in favour of the intervention group. No results were reported for the overall number of blood tests conducted. A summary of the results of the two studies is displayed in Table 23.
| Study ID | Rosenbloom (2005)95 | Poley (2007)96 | ||||||
|---|---|---|---|---|---|---|---|---|
| Study design | ITS | Controlled pre–post | ||||||
| Intervention | Baseline | Int 1 | Int 2 | Int 3 | OCS alone | OCS + CDSS | ||
| Pre- | Post- | Pre- | Post- | |||||
| N (practices in analyses) | NR | NR | NR | NR | 47 | 47 | 87 | 87 |
| Process outcomes | ||||||||
| Mean instances of weekly serum magnesium test orders | 539 | 380 | 491 | 277 | ||||
| p-value between periods | – | 0.001 | < 0.001 | < 0.001 | ||||
| Net instances of magnesium test orders per patient | 0.9 | 0.6 | 0.9 | 0.4 | ||||
| p-value between periods | – | < 0.001 | 0.001 | 0.003 | ||||
| Proportion of calcium tests ordered concurrently with serum magnesium tests (%) | 33 | 23 | 37 | 25 | ||||
| p-value between periods | – | < 0.001 | < 0.001 | < 0.001 | ||||
| Proportion of phosphorus tests ordered concurrently serum magnesium tests (%) | 48 | NR | 80 | 47 | ||||
| p-value between periods | – | NR | < 0.001 | < 0.001 | ||||
| Mean number of laboratory request forms (n; SD) | 376 ± 194 | 382 ± 208 | 358 ± 174 | 356 ± 177 | ||||
| Change within group (n; SD) | +6 ± 52 | –2 ± 53 | ||||||
| Mean difference in change between groups (n; 95% CI) | –7 (–26 to 11) | |||||||
| Number of test requests per order form (n; SD) | 5.8 ± 1.3 | 5.8 ± 1.35 | 5.9 ± 1.5 | 5.5 ± 1.4 | ||||
| Change within group (n; SD) | –0.01 ± 0.4 | –0.39 ± 0.7 | ||||||
| Mean difference in change between groups (n; 95% CI) | –0.38 (–0.61 to –0.16) | |||||||
Studies assessing the impact of the display of recommendations
Quantity and quality of the studies
The effect of the CDSS providing a recommendation was assessed in seven studies;32,50,97–100,104 two CRCTs published by Hobbs and colleagues32 and Cobos and colleagues50 respectively; one RCT conducted by Apkon and colleagues;97 and four pre–post studies undertaken by Bassa and colleagues,98 Sanders and colleagues,104 Nightingale and colleagues99 and Boon-Falleur and colleagues. 100
Across the studies, two each were conducted in the UK,32,99 Spain,50,98 and the USA,97,104 with the remaining study being undertaken in Belgium. 100 Three of the studies which all focused on the management of hypercholesterolaemia were undertaken in primary care settings,32,50,98 (one from the UK,32 and two from Spain)50,98 while of the remaining four studies conducted in a secondary care setting, one assessed the impact of CDSS recommendations on the number of health-care opportunities fulfilled in terms of the number of laboratory and radiology screening tests undertaken,97 one examined the impact on test ordering patterns of neuroradiology head imaging studies,104 while the further two studies assessed the impact on the number of laboratory test requests in patients either being assessed for or having undergone a liver transplantation. 99,100 The RCT which assessed the impact of recommendations on the number of laboratory and radiology screening test opportunities undertaken was conducted in the USA at two military treatment facilities97 and reported the number of health-care opportunities fulfilled for a wide range of health problems including vaccinations, pharmacological treatments, smoking cessation, and diet and exercise counselling. As only the number of opportunities fulfilled, in terms of laboratory test screening and back pain imaging were of relevance to the current review, only data on these outcomes were extracted and are reported. A summary of study characteristics from the seven studies that assessed the impact of recommendations is displayed in Table 24.
| Study ID | Setting/country | Study design | Number of sites | Intervention (I)/Comparison (C) | Study description | Area of impact | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| Process outcomes | Adverse effects of test cancellation | |||||||
| Hobbs (1996)32,a | Outpatients in 25 practices, Birmingham, UK | CRCT | 25 |
Baseline: OCS alone in all 25 practices (I) OCS + CDSS: recommendations on patient management (C) OCS alone: (no recommendations) |
Practices were randomly allocated to intervention (n = 21) and control (n = 4) groups. Study comprised a 3-month baseline period in both groups where OCS alone was used, followed by a 6-month intervention period | Test volumes |
Lipid test rates Number of patients receiving a full lipid profile (cholesterol, fasting triglyceride and HDL) |
NR |
| Cobos (2005)50 | Outpatients with hypercholesterolemia in 44 practices in Spain (mainly the Catalonia region) | CRCT | 44 |
(I) OCS + CDSS: recommendations on therapy, frequency of follow-up visits, and laboratory tests. (C) OCS alone (no recommendations) |
General practices were randomly allocated to intervention (n = 22 physicians; n = 1046 patients) or control (n = 22 physicians; n = 1145 patients) groups. Trial conducted over a 12- month period | Test volumes |
Number of lipid assessments per visit Number of AST/ALT tests per visit Number of CK tests per visit |
NR |
| Apkon (2005)97,b | Outpatients at two military treatment hospitals, Ireland Army Community Hospital and Clinic, Fort Knox, KT, and Mayport Branch Health Clinic, Mayport, FL, USA | RCT | 2 |
(I) OCS + CDSS: patients entered medical histories into appropriate Coupler. This information was used by CDSS to provide recommendation for 24 health-care measures (screening/prevention and acute/chronic disease management) (C) OCS alone (no Coupler or recommendation provided) |
Patients were randomly allocated to intervention (Coupler) (n = 936) or control group (n = 966). Patients follow-up at 60 days to assess the ‘quality of care’ as defined by the number of opportunities for prevention/screening or acute/chronic disease management fulfilled |
Compliance with recommendations Resource consumption |
Number of health-care opportunities fulfilled within 60 days of index visit Laboratory test resource consumption within 60 days of index visit Diagnostic imaging test resource consumption within 60 days of index visit |
NR |
| Bassa (2005)98,c | Outpatients with hypercholesterolemia, Vila Olimpica Primary Health Care Centre, Barcelona, Spain | UPP | 1 |
Baseline: OCS alone (no recommendations (I) OCS + CDSS: recommendations on therapy, frequency of follow-up visits, and laboratory tests |
Study consisted of 12-month pre-intervention period (OCS alone) and 12-month intervention period, with 500 patients included over both periods | Test volumes | Number of lipid profile tests carried out pre- and post intervention | NR |
| Sanders (2001)104 | Inpatients;, Vanderbilt University Hospital, Nashville, TN, USA | UPP | 1 |
Baseline: OCS alone (I) OCS + CDSS: recommendations to physicians for ordering an MRI of the brain or a CT of the head. |
Study consisted of 17 weeks: 9-week pre-intervention and 8-week intervention. 742 orders were made pre-intervention and 704 during the intervention period | Test volumes |
Number of tests ordered pre-and post- intervention Number of orders complying with the recommendation |
NR |
| Nightingale (1994)99 | Inpatients, Supraregional Liver Unit, The Queen Elizabeth Hospital, Birmingham, UK | UPP | 1 |
Baseline: OCS alone (no management protocol in place) (I) OCS + CDSS: recommendations for tests to be performed the following day based on latest test results and patient clinical categories |
Study consisted of 2-year period; 1-year pre-intervention and 1-year intervention. Total patients (N = 1487; n = 654 pre-intervention and n = 833 intervention period) | Test volumes |
Total number of tests requested per patient day Number of out of hours tests requested per patient day Direct laboratory costs per patient day Number of plasma urea and electrolyte tests, liver function tests, bone profile, calcium and other tests requested per patient day |
NR |
| Boon-Falleur (1995)100,d | Inpatients, Paediatric Liver Transplantation Unit, Cliniques Universitaires St. Luc, Universite Catholique de Louvain, Brussels, Belgium | UPP | 1 |
Baseline: OCS alone (no management protocol in place) (I) OCS + CDSS: recommendations for tests to be performed the following day based on latest test results and patient clinical categories |
Study consisted of 12 months: 6-month pre-intervention and 6-month intervention period. Total patients (N = 2041; n = 1,153 pre-intervention and n = 888 intervention period) | Test volumes |
Number of tests per patient (assessment protocols) Number of tests per patient (transplant protocols) Mean number of urgently requested tests per patient |
NR |
The first CRCT which assessed the impact of the provision of recommendations was undertaken in 25 primary care practices (21 intervention and 4 control) in Birmingham, UK, by Hobbs and colleagues. 32 The aim of the 9-month trial, which included a 3-month historical baseline control period and a 6-month intervention period, was to examine the effect of CDSS on the management of hyperlipidaemia in patients not previously diagnosed with the problem. The primary outcome of interest in relation to this review was lipid test rates between intervention and control groups, although changes in prescribing and referral practices were also reported. The CDSS used was the Primed system designed for use in general practice by Wolfson Research Laboratories, University of Birmingham, UK. The software was a rule-based system, which provided an initial screening prompt for capture of patient sociodemographic and cardiovascular risk factor data. This also included the input of the patients’ current cholesterol level. The patients’ coronary risk score was then displayed on screen, with a score greater than 10 being in the top quintile for risk of a coronary event within the next 5 years.
Based on the risk score, the CDSS provided advice on patient management, with the rules underpinning the recommendations derived from a protocol developed by a lipid specialist. Overall, the trial was subject to high rates of attrition with eight clusters (seven intervention and one control) withdrawing (the total number of physicians within the trial was not reported). Furthermore, only limited results were reported, and all comparisons were analysed as a change from the 3-month baseline period, rather than as between group comparisons, thus limiting their utility in assessing the effects of the CDSS in conjunction with OCS compared to OCS alone.
The second non-inferiority CRCT conducted by Cobos and colleagues50 in general practices in Spain (mainly drawn from the Catalonia region) aimed to assess the cost-effectiveness of CDSS for adapted recommendations based on guidelines from the European Society of Cardiology and other societies for Hypercholesterolemia Management. 124 The 12-month pragmatic trial, included 44 practices (22 in the intervention group and 22 in the usual care group) with a total of 2191 patients (1046 in the intervention group and 1145 in the usual care group) with a diagnosis of hypercholesterolemia. This was defined as a pre-treatment total cholesterol concentration > 200 mg/dL, and < 400 mg/dL. Patients who lacked a baseline lipid profile were excluded from all analyses. Patient groups were well matched at baseline in terms of age, gender, mean BMI, and other concomitant risk factors for hypercholesterolemia. No sociodemographic data on the physicians participating in the trial was reported.
The CDSS was based on adapted algorithms that were implemented in the intervention group and withheld in the usual care group that issued recommendations on therapy, frequency of follow-up visits, and laboratory tests according to the patient’s cardiovascular risk factors LDL-C goals. Physicians were free to either adopt or ignore the CDSS recommendations. Adherence to the guideline was monitored by the CDSS and a reason requested in case of any discrepancy between the treatment recommended and prescribed. A concomitant intervention, of the provision of items such as table cloths and fridge magnets with relevant promotional messages was also undertaken in intervention group practices, but withheld in the usual care practices. Only data on the number of laboratory test analyses were extracted and are therefore reported in this review.
Like the trial conducted by Hobbs and colleagues32 this study was subject to a high rate of missing post-baseline data with 26% of the intervention group and 28% of the control group having no post-baseline lipid profile. Additionally, only 59% of the intervention group and 47% of the usual care group had a follow-up of 9 months or more. However, appropriate sensitivity analyses to missing data (no post-baseline assessment and < 9 months follow-up) were conducted. Among patients lost to follow-up after the first (baseline) visit no change in lipids or coronary vascular risk (CVR) was assumed. This assumption was then tested in two different analyses, one including patients with at least one post-baseline assessment (per protocol analysis) and patients with a length of follow-up of at least 9 months.
The RCT conducted at the Ireland Army Community Hospital and Clinic, Fort Knox, KT, USA, and the Mayport Branch Health Clinic, Mayport, FL, USA, within two military treatment facilities by Apkon and colleagues97 aimed to assess the impact of the use of CDSS on quality of patient care. This was defined as the total percentage of any of 24 health-care quality process measures (opportunities to provide evidence-based care) that were fulfilled within 60 days of a patients’ index visit. The trial measured and reported a wide range of health-care opportunities, but as only the results of those that have an impact on laboratory testing or radiology imaging rates are relevant to the present review, only data for these outcomes were extracted and reported.
A total of seven outcomes were deemed relevant to this review:
-
cervical cancer screening
-
screening for Chlamydia
-
colorectal cancer screen
-
lipid level testing
-
back pain imaging
-
screening for diabetes using glycosylated haemoglobin levels
-
screening for lipid abnormalities.
In addition laboratory test resource and diagnostic test imaging consumption were reported.
These were calculated from the Centers for Medicare and Medicaid Services 2003 fee schedule using the relative value unit conversion rate of US$36.8. The 60-day trial included 4639 health-care opportunities (2374 in the intervention group and 2265 in the control group) among study patients at their index visit. Patient characteristics were reasonably well balanced at baseline in terms of age, gender, and type of visit (acute, established, routine, wellness or other). However, as data were reported by trial group rather than by patient indication, it is possible that there were baseline imbalances between the two groups which could potentially bias results. No sociodemographic data or the number of physicians involved in the trial were stated.
The CDSS intervention which used the DSIT Problem-Knowledge Couplers (PKC Corp, Burlington, VT, USA) involved the use of a number of ‘Couplers’ which were available for a wide range of preventive and acute/chronic disease management needs. Patients entered data into the Coupler appropriate for their complaint, or when no condition-specific Coupler was appropriate, a generic medical history and screening Coupler, prior to seeing the physician. This took approximately 20 minutes. Physicians treating intervention group patients could enter additional information before reviewing Coupler outputs outlining investigation or treatment options. Patients in the control group had no exposure to Couplers. In total there were 667 health-care opportunities (325 in the intervention group and 342 in the control group) from the seven of relevance to this review.
Of the four pre–post studies, the first conducted by Bassa and colleagues98 was undertaken at the Vila Olimpica Primary Health Care Centre, Barcelona, Spain and included 500 patients randomly selected from the Primary Health Care centre database with hypercholesterolemia. These patients had a median age of 67 years, 65.8% (329/500) were female, 18% had a concomitant diagnosis of diabetes mellitus (90/500), 16.6% (83/500) were current smokers, and 52.4% (262/500) had a sedentary lifestyle. In terms of the number of cardiovascular risk factors per patient at baseline in the study, 4.4% (22/500) had none, 34.8% (174/500) had one, 53.4% (267/500) had two, and 7.4% (37/500) had more than two. The number of physicians involved in the study or sociodemographic data on them were not reported.
The aim of the study was to assess the impact in terms of effectiveness and costs of CDSS and to implement a practice guideline for patient management. The study was carried out over a 2-year period, with 1-year pre-implementation of the CDSS and 1-year post-implementation. The CDSS recommendations were based on the Sociedad Española de Medicina de Familia y Comunitania’s clinical guidelines for dyslipidemia management and cost-effectiveness data published in a meta-analysis. 125,126 Based on the patient’s data (personal history of cardiovascular disease, cardiovascular risk factors, and lipid profile), the CDSS established the therapeutic objectives in terms of LDL cholesterol levels and issued testing, therapeutic and follow-up recommendations for the patient. Physicians were free to accept or decline the recommendations, but were prompted for a reason when they declined. The recommendations included dietary treatment, lipid-lowering drugs, monitoring of hepatic and muscular enzymes and a recommended date for the follow-up visit. As only data on the number of lipid-profile tests conducted and their costs are of relevance to the present review only these data were abstracted and are reported. Data associated with the mean cost of undertaking laboratory assessment tests and the data sources and method used to calculate these are reported in Chapter 6, Systematic review of economic evaluations.
The second pre–post study undertaken by Sanders and colleagues104 was conducted at the Vanderbilt University Medical Centre, Nashville, TN, USA, using a module specifically designed for use in the University-specific WizOrder entry system. The study consisted of a 9-week control (pre-intervention) period and an 8-week intervention phase. The aim of the study was to assess the effects of guidelines implemented within the CDSS for the ordering of neurological head imaging studies on test ordering patterns and guideline compliance.
All physicians, nurses, medical students and receptionists who entered an order via the system for one or more head CT or brain MRI scans during the study period were eligible for inclusion in the study, with a total of 1446 orders included in the analyses (742 pre-implementation and 704 post-implementation). To develop the CDSS a list of common indications for ordering an imaging examination of the head or brain was created based on prior free text indications at the time of order entry, historical International Classification of Diseases, Ninth Edition (ICD-9) coding data and local clinical guidelines for the tests. These were then mapped to ICD-9 codes, and for each indication, the most appropriate imaging test determined.
The CDSS required input of the patients’ acuity and indication by the user and provided a recommended test (head CT without contrast; head CT with contrast; head CT with and without contrast; brain MRI without contrast; and brain MRI with and without contrast). If a suggestion was given, this choice was defaulted. The user was able to override the recommendation and select any of the listed studies, but had to type the reason for doing so. If no indication for requesting the test was given by the user or ‘other’ was chosen, no CDSS recommendation was provided. Analyses were then conducted between study periods to evaluate changes in the distribution of test ordering patterns.
The last two pre–post studies were both conducted in secondary care settings involving patients either being assessed for, or undergoing, liver transplantation. 99,100 The first of the two studies, by Nightingale and colleagues99 was undertaken in adult patients at the Supraregional Liver Unit, The Queen Elizabeth Hospital, Birmingham, UK. The study aimed to assess the effects of a CDSS protocol management system on the number of tests, costs, and the appropriateness of laboratory investigations requested. The study consisted of a 1-year pre-implementation phase during which 654 patients were assessed, and a 1-year post CDSS implementation phase during which 833 patients were assessed. Patient categories in terms of the numbers undergoing initial assessment, reassessment, transplant, post-transplantation or being an emergency were fairly well matched between the two study periods. No socio-demographic data on the physicians involved in the study or their numbers were reported. The study specific CDSS was developed by Wolfson Computer Laboratories at the hospital, and aimed to implement the unit’s existing investigation protocols developed by senior clinicians. These were based on information regarding the category of the patient’s clinical state (e.g. assessment, transplant) and the use of the latest test results to propose the laboratory investigations to be performed on the following day. The system was based upon a combination of static and dynamic rules. Static rules were those that applied to all patients with a certain classification for a certain number of days, and dynamic rules were those which used the results of previous laboratory results to determine which investigations to propose. Once the physician had viewed the proposed tests they were free to accept or modify them as required.
The same CDSS as used in the study by Nightingale and colleagues99 was adapted to a multilingual version and exported for use at the Paediatric Liver Transplantation Unit, Cliniques Universitaires Saint-Luc, Université catholique de Louvai, Brussels, Belgium by Boon-Falleur and colleagues. 100 This study, like that by Nightingale and colleagues,99 aimed to assess the impact of the system on the number of laboratory tests performed pre-implementation and post-implementation. However, this study only included paediatric inpatients who were undergoing either a pre-transplant assessment protocol (n = 183; 32 pre-intervention and 151 post-intervention) or transplant protocol (n = 34; 10 pre-implementation and 24 post-implementation). No sociodemographic data on the patients in either study period were reported, and so it is not possible to comment on whether there were systematic differences in the patient populations in the pre- and post-intervention periods that may potentially confound the results. Likewise, no socio-demographic data on the physicians or the number involved in the study were reported.
The pre-implementation phase consisted of a 6-month period, but the length of post-intervention assessment was not reported. Additionally, the results were reported according to the number of tests per patient, rather than the number of tests per patient per day, which potentially confounds length of stay with number of tests, and is not an appropriate outcome measure.
Outcomes
Process outcomes
The area of impact in the test ordering process assessed in all seven studies were test volumes. 32,50,97–100,104 Additionally, the RCT by Apkon and colleagues97 and the UPP study by Sanders and colleagues104 also assessed compliance with guidelines for laboratory or imaging test protocols. As three of the studies, by Hobbs and colleagues,32 Cobos and colleagues50 and Bassa and colleagues,98 assessed the impact of CDSS on hypercholesterolemia testing rates (either in patients diagnosed with the conditions or as a screening procedure in previously undiagnosed patients), the specific outcomes in these studies included lipid profile test rates,32,50,98 number of patients receiving full lipid profile tests, (cholesterol, fasting triglyceride and HDL),32 and the number of aspartate aminotransferase/alanine aminotransferase (AST/ALT) and creatine kinase (CK) tests per visit. 50 The RCT by Apkon and colleagues97 assessed the number of health-care opportunities fulfilled within 60 days of the patients’ index visit, so not only did this study report compliance with recommended laboratory/imaging recommendations, but also the number of tests completed between the intervention and control groups. The study also reported outcomes on the resources used in terms of both laboratory and imaging tests. 97 The pre–post study by Sanders and colleagues104 being the only study to assess the impact of guidelines implemented within CDSS for CT/MRI head imaging protocols reported both the number of imaging tests ordered both pre- and post-implementation of the CDSS, but also the number of test orders that complied with guideline recommendations. The two pre–post studies that were undertaken in adult and paediatric liver transplantation patients by Nightingale and colleagues99 and Boon-Falleur and colleagues100 both reported changes in test volumes,99,100 as well as additionally the number of out of hours requests,99 number of specific tests requested for plasma urea and electrolyte, liver function, bone profile, and calcium levels,99 number of urgently requested tests,100 and laboratory costs per patient day. 99
Study quality
Controlled studies
In the CRCT by Hobbs and colleagues32 and the RCT by Apkon and colleagues97 the methods of randomisation were not stated, and it was also unclear whether allocation concealment were attained in these trials. Additionally, in the RCT by Apkon and colleagues97 contamination may have occurred, as physicians treated both intervention and control group patients, and therefore any ‘learning effect’ from use of the CDSS Coupler may have passed onto the control group. The effect of this if any would be to underestimate the treatment effect in the Coupler intervention group. Additionally the results of this RCT are likely to be confounded by the completion of a 20 minute patient-completed computer-based questionnaire. In contrast, the CRTC by Cobos and colleagues50 was properly randomised and took clustering into account in both the design and analysis. Eligibility criteria, baseline details and baseline similarity between groups were attained in both the CRCT by Cobos and colleagues50 and RCT by Apkon and colleagues. 97 However, only partial details were presented on trial eligibility by Hobbs and colleagues,32 and no sociodemographic data on participants were reported. It was therefore unclear whether groups were balanced at baseline. As can be expected in CDSS trials, physicians were not blinded to treatment allocation, and it was unclear whether outcome assessors were blinded. Data analysis were appropriate in the trials by Cobos and colleagues50 and Apkon and colleagues,97 and greater than 80% follow-up was attained in both trials. However, only Cobos and colleagues50 undertook their analyses on an ‘intention to provide or communicate information’ basis. In the trial by Hobbs and colleagues32 little detail regarding the analyses were reported, but no between group comparisons or specific point estimates were given. The few results reported, were reliant on differences between pre- and post-intervention rates, and were therefore not appropriate.
Uncontrolled studies
Study eligibility criteria were adequately reported by Bassa and colleagues,98 Sanders and colleagues,104 and Nightingale and colleagues,99 but were only partially reported in the study by Boon-Faulleur and colleagues. 100 Baseline details were fully reported in only one of the four studies, that by Nightingale,99 were partially reported in a further two by Bassa and colleagues98 and Sanders and colleagues,104 but no details were given in the paper by Boon-Faulleur and colleagues. 100 In relation to this it was impossible to tell whether there were potential differences in patient baseline characteristics between the pre- and post-intervention phases that may potentially confound the study results. Data analyses were appropriate in three of the studies,98,99,104 but only conducted on an ‘intention to provide or communicate information’ basis in the studies by Sanders and colleagues104 and Nightingale and colleagues. 99 Both of these studies additionally achieved a greater than 80% follow-up rate. In the study by Boon-Falleur and colleagues100 the analyses were unclear, and the presentation of the results by the number of tests per patient rather than the number of tests per patient day confounds the length of hospital stay with the number of tests. The results of this study should therefore be interpreted with caution.
CDSS characteristics
A summary of the key characteristics of the CDSS used in the seven studies is displayed in Table 25. The specific CDSS used was only reported in three of the studies,32,97,104 these included the DSIT tool Problem-Knowledge Couplers (PKC Corp, Burlington, VT, USA) implemented within the existing Military Health System’s electronic medical system (Composite Healthcare System),97 the Primed system developed specifically for use in patients with hyperlipidemia in a primary care setting by Wolfson Research Laboratories, University of Birmingham, UK,32 and the WizOrder entry system, the home-grown/in-house system from Vanderbilt University Medical Center, Nashville, TN, USA. 104 In the other studies, three of the other four CDSS appeared to have been developed and implemented specifically for the sites and specialities in which it was used (in two studies screening for hyperlipidemia50,98 and in the third for the assessment and management of patients undergoing liver transplantation). 99 This CDSS was developed again by Wolfson Research Laboratories, University of Birmingham, UK, and adapted to a multilingual version for use in the study by Boon-Falleur and colleagues,100 again for the management of liver transplantation patients.
| Study ID | Apkon (2005)97 | Hobbs (1996)32 | Cobos (2005)50 | Bassa (2005)98 | Sanders (2001)104 | Nightingale (1994)99 | Boon-Falleur (1995)100 |
|---|---|---|---|---|---|---|---|
| CDSS characteristics | |||||||
| 1. Name of CDSS (if any) | DSIT tool Problem-Knowledge Couplersa | Primed systemb | NR | NR | WizOrderc | NRb | NRb |
| 2. CDSS reasoning methods | NR | DR | CA | CA | NR | DR | DR |
| 3. CDSS knowledge base | NR | Expert opinion (protocol developed by lipid specialist) | Recommendations of the European Society of Cardiology and other societies for Hypercholesterolemia Management | SEMFYC (Sociedad Española de Medicina de Familia y Comunitania’s) clinical guidelines for dyslipidemia management and cost-effectiveness data published in a meta-analysis | Prior free indications at the time of order entry, historical ICD-9 coding data and local published guidelines | Expert opinion | Expert opinion |
| 4. Information used in CDSS | Patients entered their medical histories into the appropriate Coupler tool for their complaint | Sociodemographic details; CV risk factors; cholesterol level |
History Biochemical laboratory test results (number of items NR) |
History of CV disease; CV risk factors and lipid profile | Acuity and indication | NR | NR (signs; symptoms; history, biochemical test results) |
| 5. Time to complete CDSS (minutes) | 30 | NR | NR | NR | NR | NR | NR |
| 6. CDSS output format | R | R | R | R | R | R | R |
| 7. Is a description of pilot testing with users prior to implementation provided? | No | No | No | No | No | No | No |
| 8. Is user instructional training at the time of implementation described? | No | Yes | No | No | No | Yes | No |
| General system features | |||||||
| 9. Is the CDSS integrated with charting or OCS to support workflow integration?a | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Clinician–system interaction features | |||||||
| 10. Is automatic provision of CDSS output provided as part of clinician workflow?a | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 11. Is there a need for additional data entry by the clinician other then the specification of which test orders?a | No | Yes | No | No | No | Yes | Yes |
| 12. Does the CDSS request documentation of the reason for not following CDSS recommendations?a | ? | NR | Yes | Yes | Yes | No | No |
| 13. Does CDSS provide output at the time and location of decision making?a | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 14. Are the CDSS recommendations executed by the clinician noting agreement?a | No | No | No | No | No | No | No |
| Communication content features | |||||||
| 15. Does the CDSS provide a recommendation rather than just an assessment?a | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 16. Does the CDSS promote action rather than inaction?a | ? | NR | NR | NR | NR | NR | NR |
| 17. Does the CDSS justify the output of provision of reasoning?a | No | No | No | No | No | No | No |
| 18. Does the CDSS justify the output by provision of research evidence?a | No | No | No | No | No | No | No |
| Auxiliary features | |||||||
| 19. Were the local users involved in the CDSS development process?a | No | No | No | NR | Yes | Yes | No |
| 20. Is the CDSS output provided to patients as well as clinicians?a | No | No | No | No | No | No | No |
| 21. Does the CDSS provide periodic summaries of performance feedback?a | No | No | No | No | Yes | Yes | No |
| 22. Is the CDSS used in conjunction with conventional education?a | No | No | No | No | No | No | No |
In three of the seven studies the CDSS reasoning methods were discrimination rules,32,99,100 and in a further two clinical algorithms. 50,98 Reasoning methods were not clear in the remaining two studies. 97,104 The CDSS knowledge base was either expert opinion,32,99,100 clinical guidelines,98,104 or a combination of prior indications at the time of order entry, historical ICD-9 coding data and guidelines. 104 The information used in the CDSS was the patient’s medical history in one RCT which patients entered themselves into the appropriate Coupler,97 CV risk factors coupled with either cholesterol level or lipid profile in three studies,32,50,98 acuity and indication in the pre–post study assessing the impact of CDSS implemented guidelines for radiological imaging in one study,104 and not reported in the remaining two studies. 99,100 In these two studies it would, however, appear to have been based on the patients condition, history, and results of any previous laboratory tests. 99,100 The CDSS outputs in all the studies was the display of recommendations. Only the RCT by Apkon and colleagues97 reported the length of time to complete the CDSS Coupler which was 20 minutes and patient completed. In relation to the 14 features of CDSS proposed by Kawamoto and colleagues16 as being important to the success or failure of a CDSS, the CDSS was not reported as being piloted with users prior to implementation in any of the seven studies, and user instructional training was provided in only one. 32 In all seven studies the CDSS was integrated as part of the OCS, and provided output automatically as part of the consultation workflow. In three of the studies there was a need for additional data entry by the physician,32,99,100 while in a further study this was optional. 97 Additionally, in three studies there was a need for documentation for not following the CDSS recommendations,50,98,104 however, none of the studies required the physician to note agreement with the recommendations in order for these to be implemented. In none of the studies was a recommendation justified by recourse to the CDSS reasoning methods or evidence upon which these were based. Additionally it would appear that local users were directly involved in the CDSS development process in only two of the studies. 100,104 The data provided by the CDSS was used by the physician alone, and only in two studies was periodic performance feedback provided. 99,104 None of the studies deployed any concomitant educational cointerventions.
Results
In the CRCT by Hobbs and colleagues32 which assessed the impact of CDSS on screening patients with previously undiagnosed hyperlipedmia no point estimates for pre- and post-implementation rates of lipid test rates were reported. Likewise, no between group comparisons were conducted. The authors stated that in the 9-month post-implementation period the mean rate of testing was 4.4 tests/1000 population/month. This rate was not significantly different from the pre-intervention phase. However, the authors stated that there was a significant increase in the number of patients receiving a full lipid profile in the intervention phase, and a decrease in those having only a partial investigation compared with the baseline period (p < 0.05). Again the exact figures were not reported.
Similarly, in the 1-year CRCT conducted by Cobos and colleagues50 there were no significant differences between the treatment and usual care groups (n = 1046 and 1145 respectively) in the number of lipid assessments conducted per patient visit. This was 1.8 in the intervention group compared with 1.8 in the control group (p = 0.298). However, there was a significant increase in the number of patients receiving AST/ALT tests per visit in the intervention group, 1.4 compared to the control group, 1.3 (p = 0.033). No significant differences were observed in the number of CK tests performed per patient visit, with 0.54 and 0.24 conducted in the intervention and control groups respectively (p = 0.053).
The 60-day trial undertaken by Apkon and colleagues97 to assess the impact of CDSS Coupler recommendations on the number of health-care opportunities fulfilled 60-days after the patient’s initial index visit, showed no statistically significant differences between the treatment groups in terms of any of the seven opportunities related to laboratory or imaging tests screening. A break down of the number of opportunities fulfilled in each group by opportunity type is displayed in Table 26.
| Opportunity type | OCS + Coupler (n = 325) | OCS alone (n = 342) | Difference between groups |
|---|---|---|---|
| Cervical cancer | 26/95 (27.4%) | 22/98 (22.4%) | p = 0.47 |
| Chlamydia | 22/73 (30.1%) | 19/64 (29.7%) | p = 0.90 |
| Colorectal cancer | 4/32 (12.5%) | 2/58 (3.4%) | p = 0.15 |
| Lipids | 13/49 (26.5%) | 18/48 (37.5%) | p = 0.32 |
| Back pain imaging | 4/4 (100%) | 2/2 (100%) | NA |
| Diabetes – glycosylated haemoglobin | 3/6 (50%) | 1/3 (33.3%) | p = 0.48 |
| Lipid abnormalities | 12/66 (18.2%) | 11/69 (15.9%) | p = 0.81 |
However, the authors reported a statistically significant increase in median laboratory test resource consumption in the intervention group of US$43 (range: 0–144) compared to the usual care group, US$31 (range: 0–139) (p = 0.04). No differences were observed between the two groups in terms of diagnostic test imaging consumption, with these being US$31 (range: 0–148) and US$29 (range: 0–127) in the intervention and control groups respectively (p = 0.26).
Bassa and colleagues98 from their 2-year pre–post study on the effects of the implementation of guidelines for the treatment of patients with hypercholesterolmia implemented via CDSS, reported no significant differences in the number of lipid tests carried out in the pre-and post-implementation phases of the study. At baseline a total of 773 tests were conducted per annum compared with 763 post-intervention (p = 0.59).
In the 17-week pre–post study by Sanders and colleagues104 examining the effects of implementation of a guideline for ordering head or brain imaging studies, there was a small significant decrease in the number of imaging tests ordered after implementation of the CDSS. This fell from 742 tests ordered in the pre-implementation phase to 704 tests post-intervention (p = 0.048). Compliance with ordering the CDSS recommended tests however, was still fairly low at only 60%.
The two pre–post studies by Nightingale and colleagues99 and Boon-Falleur and colleagues100 assessing the impact of CDSS recommendations for adult and paediatric inpatients pre- and post-liver transplantation protocols showed slightly disparate results. In the 2-year study by Nightingale and colleagues99 there was a significant (17%) overall reduction in the number of tests requested per patient day from 8.5 (SD 3.6) pre-intervention to 7.0 (SD 3.5) with the implementation of the protocols (p < 0.001). This reduction was consistent across all patient categories apart from patients undergoing routine reassessment in which an insignificant reduction from 4.8 (SD 2.7) to 3.7 (SD 2.7) tests per patient day was observed, and those undergoing an annual post-transplant review in which an insignificant 12% reduction in the number of tests ordered from 7.4 (SD 4.1) to 6.6 (SD 2.9) per patient day was observed. Additionally, there was an increase in the number of tests ordered for patients with emergency acute hepatic failure of 17%, from 6.7 (SD 3.8) at baseline to 7.8 (SD 4.0) per patient day post-implementation. A summary of the changes in the number of tests ordered per patient per day by patient category is displayed in Table 27.
| Patient category | Pre-a | Post-a | % change | Student’s t-statistic |
|---|---|---|---|---|
| Initial assessment | 7.1 (2.9) | 5.4 (3.0) | –25 | 5.23b |
| Reassessment (routine) | 4.8 (2.7) | 3.7 (2.7) | –22 | 1.92 |
| Reassessment (problem) | 7.7 (2.1) | 6.3 (2.3) | –19 | 2.67c |
| Transplant | 11.0 (2.8) | 9.6 (3.3) | –13 | 3.37b |
| Post-transplant (problem) | 7.8 (2.5) | 6.9 (2.3) | –11 | 2.62c |
| Post-transplant (t tube removal) | 6.6 (4.0) | 5.0 (2.1) | –25 | 2.41d |
| Post-transplant (annual review) | 7.4 (4.1) | 6.6 (2.9) | –12 | 1.73 |
| Emergency (acute hepatic failure) | 6.7 (3.8) | 7.8 (4.0) | +17 | 1.37 |
| Emergency (acute problem – chronic disease) | 11.1 (4.2) | 8.0 (4.1) | –28 | 2.57d |
| Other | 6.2 (4.3) | 5.5 (4.1) | –11 | 0.73 |
| Total | 8.5 (3.6) | 7.0 (3.5) | –17 | 8.10 b |
The implementation of the CDSS protocols resulted in a significant 48% decrease in the number of out of hours tests requested per patient day, from a pre–post baseline of 0.31 to a post-intervention number of 0.16 (p < 0.001). Likewise, the median number of plasma urea and electrolyte tests (p < 0.05), bone profile tests (p < 0.001), and calcium tests (p < 0.005) were all significantly reduced. However, there was no significant reduction in the number of liver function tests conducted, and a minor non-significant increase in the number of others tests undertaken. The overall reduction in the number of tests conducted was reflected in direct laboratory costs per patient days with a significant 28% reduction observed (p < 0.001).
Results of the study by Boon-Falleur and colleagues100 in contrast to those of Nightingale and colleagues99 showed a 13% increase in the number of tests ordered per patient stay for patients undergoing pre-treatment assessment protocols. The authors did not state whether this was statistically significantly different from the number of tests ordered prior to implementation of the system. Interestingly, the largest increase in test orders (46%) was observed in the ‘other test’ category which consisted of special chemistry, serology, nuclear medicine and bacteriology tests, suggesting that more specialised diagnostic tests were requested more frequently after introduction of the CDSS. A summary of the number and type of tests ordered for patients undergoing assessment protocols pre- and post-implementation of the CDSS is displayed in Table 28.
| Test category | |||
|---|---|---|---|
| Number of tests per patient stay: pre-treatment assessment protocols | Pre- (n = 32) | Post- (n = 151) | Δ% |
| General chemistry | 46 | 53 | +15 |
| Virology | 22 | 18 | –18 |
| Haematology and coagulation | 23 | 30 | +30 |
| Others | 13 | 19 | +46 |
| Total | 106 | 120 | +13 |
| Number of tests per patient stay: transplant protocols | Pre- (n = 10) | Post- (n = 24) | Δ% |
| General chemistry | 368 | 273 | –26 |
| Virology | 70 | 49 | –30 |
| Haematology and coagulation | 345 | 268 | –22 |
| Others | 264 | 178 | –33 |
| Total | 1047 | 768 | –27 |
In contrast to patients undergoing an assessment protocol, there was a 27% decrease observed in the number of tests requested per patient stay for those undergoing a transplant protocol, from 1047 pre-implementation of the system, to 768 post-implementation. This was most marked for the ‘other test’ category in which a 33% decrease in the number of tests ordered was observed. Again the authors did not state whether this decrease from baseline levels was statically significant. There was also a 44% decrease in the number of urgently requested tests per patient from 65 per patient prior to CDSS implementation to 36 post-implementation.
Summary of studies assessing the impact of the display of recommendations
The effect of the CDSS providing a recommendation was assessed in seven studies;32,50,97–100,104 two CRCTs,32,50 one RCT,97 and four pre–post studies. 98–100,104 Study quality was variable and only limited results were reported in one of the CRCTs by Hobbs and colleagues32 and in the RCT by Apkon and colleagues. 97
In the three studies that focused on the impact of the CDSS providing recommendations for the management of patients with hyperlipidemia, there was no significant effect in terms of increasing lipid test rates. 32,50,98 However, there was some limited impact in two, of increasing either the number of patients receiving a full lipid profile,32 or receiving an AST/ALT test. 50 However, to what degree these marginal increases would translate into improved management of patients with hyperlipidemia is unclear.
Likewise, the one RCT that assessed the impact of recommendations provided by a CDSS Coupler on the number of patient health-care opportunities fulfilled showed no significant benefit in terms of the number of either laboratory or diagnostic screening imaging tests undertaken compared with usual care. 97 In fact, there was a significant increase in laboratory test resource consumption compared with the usual care group.
In the one UPP study that assessed the impact of CDSS guideline recommendations conducted by Sanders and colleagues104 a small but significant reduction in the number of head or brain imaging studies was observed. However, despite this reduction compliance with the tests indicated by the guideline recommendations remained relatively low at only 60%. 104
Overall the results of the studies by Nightingale and colleagues99 and Boon-Falleur and colleagues100 conducted in specialist liver transplant centres showed that the implementation of guideline protocols for patient management may have differential effects according to the patient group. Overall, there tended to be a significant decrease in the number of laboratory tests ordered, the number of out of hours tests requested, and a reduction in laboratory costs. However, as appropriate levels of testing are driven by the patients’ condition and disease stage there were some increases observed in test requesting in certain patient groups. This was most pronounced in the study by Boon-Falleur and colleagues100 in which a 13% increase in the number of laboratory tests ordered in patients undergoing an initial assessment protocol was observed. A summary of the results from the studies assessing the impact of the display of recommendations is given in Table 29.
| Study ID | Hobbs (1996)32,a | Cobos (2005)50,a | Apkon (2005)97,b | Bassa (2005)98 | Sanders (2001)104 | Nightingale (1994)99 | Boon-Falleur (1999)100 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design | CRCT | CRCT | RCT | UPP | UPP | UPP | UPP | |||||||
| OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | OCS alone | OCS + CDSS | |
| Process outcomes | Pre- | Post- | Pre- | Post- | Pre- | Post- | Pre- | Post- | ||||||
| N | 21 | 4 | 22 | 22 | 325 | 342 | 500 | 404 | NR | NR | 654 | 833 | 42 | 175 |
| Lipid test ratesc | 4.4 | 4.4 | ||||||||||||
| p-valued | p > 0.05 | |||||||||||||
| Number of patients receiving a full lipid profile | NR | NR | ||||||||||||
| p-valued | p < 0.05 | |||||||||||||
| Number of lipid assessments/patient visit | 1.8 | 1.89 | ||||||||||||
| p-valuee | p = 0.298 | |||||||||||||
| Number of AST/ALT tests/patient visit | 1.4 | 1.3 | ||||||||||||
| p-valuee | p = 0.033 | |||||||||||||
| Number of CK tests/patient visit | 0.5 | 0.2 | ||||||||||||
| p-valuee | p = 0.053 | |||||||||||||
| Number of health-care opportunities fulfilled | NR | NR | ||||||||||||
| p-valuee | p > 0.05 | |||||||||||||
| Laboratory test resource consumption (US$) | 43 (range: 0–144) | 31 (range: 0–139) | ||||||||||||
| p-valuee | p = 0.04 | |||||||||||||
| diagnostic test imaging consumption (US$) | 31 (range: 0–148) | 29 (range: 0–127) | ||||||||||||
| p-valuee | p = 0.26 | |||||||||||||
| Lipid test rates (annum) | 773 | 763 | ||||||||||||
| p-valued | p = 0.59 | |||||||||||||
| Number of imaging tests orders | 742 | 704 | ||||||||||||
| p-valued | p = 0.048 | |||||||||||||
|
Number of laboratory tests/ patient day |
8.5 ± 3.6 | 7.0 ± 3.5 | ||||||||||||
| p-valued | p < 0.001 | |||||||||||||
| Number of laboratory out of hours requests/patient day | 0.32 | 0.16 | ||||||||||||
| p-valued | p < 0.001 | |||||||||||||
| Process outcomes | Pre- | Post- | ||||||||||||
| Number of laboratory tests/patient stay (assessment protocol) | 106 | 120 | ||||||||||||
| p-valued | NR | |||||||||||||
| Number of laboratory tests/patient stay (transplant protocol) | 1047 | 768 | ||||||||||||
| p-valued | NR | |||||||||||||
Study question 3. What features of CDSS are associated with clinician or patient acceptance of CDSS in order communication systems?
A total of 31 papers were screened for relevance to address the above question, however, none of these finally met the inclusion criteria. For the majority of these this was due to the fact that studies had assessed the acceptability of the overall CDSS for both pharmaceutical ordering as well as laboratory and imaging test ordering. Therefore it was not possible to discern the acceptability of the system to physicians for test ordering alone from these studies. A list of the 31 excluded studies and their reasons for exclusions are displayed in Appendix 2.
Chapter 6 Systematic review of economic evaluations
Aim
To summarise existing published research evidence on both the costs and cost-effectiveness of CDSS in conjunction with OCS for diagnostic, screening or monitoring test ordering compared with OCS alone, with particular emphasis on the potential generalisability of previous studies to the current NHS policy and clinical context.
Methods
Search strategy
The generic search strategy used to identify relevant studies for inclusion in the three reviews is described in the section Identification of relevant studies, Chapter 3, and the search strategy documented in Appendix 1.
Study selection criteria
Apart from the study design criteria, the inclusion and exclusion criteria as outlined in Chapter 3 were identical to those for question two, the review of studies to assess the impact of CDSS in OCS versus OCS alone for diagnostic, monitoring or screening test ordering. For this review full CEA, CUA, CBA, CCA, and cost–comparison studies were eligible for inclusion. Economic evaluations that only reported the average cost-effectiveness ratios were also eligible for inclusion provided the incremental ratios could be calculated from the available published data. Based on the above inclusion/exclusion criteria, initial study selection was made on the basis of titles and abstracts from the search results by one reviewer, and a random 20% of these checked, unblinded by a second reviewer.
Data extraction strategy
Data were extracted by one reviewer and checked for accuracy by a second. Data extraction was limited by data availability in many studies. Relevant results were tabulated alongside the data for the main review to address the impact of CDSS in conjunction with OCS versus OCS alone (study question 2) and are presented in Appendix 3.
Quality assessment strategy
Both of the included identified studies primarily assessed the impact of CDSS plus OCS versus OCS alone, and were reported alongside evaluations of the impact of CDSS on process and patient outcomes. Therefore the reported cost comparisons were reported as secondary outcomes. Due to this the methodological quality of the studies was assessed according to the criteria for each study design outlined for question two, rather than by specific criteria for studies of economic evaluations. The methodological quality of both of the identified studies is previously discussed under the section including the review by Poley and colleagues96 (Chapter 5, Studies assessing the impact of the display of restricted lists) and the review by Bassa and colleagues98 (Chapter 5, Studies assessing the impact of the display of recommendations).
Results
As previously stated only two studies met the inclusion criteria, both of which were cost analyses. 96,98 A full description of both of the studies is reported in Chapter 5 in the sections Studies assessing the impact of the display of restricted lists and Study question 3. What features of CDSS are associated with clinician or patient acceptance of CDSS in order communication systems? on the impact of CDSS plus OCS versus OCS alone. One of the studies by Poley and colleagues96 was conducted in the Netherlands, while the second study by Bassa and colleagues98 was conducted in Spain. The perspective taken in both studies was the societal level. 96,98 The first study was a CPP study by Poley and colleagues96 conducted in 134 primary care practices including 234 primary care physicians (159 in the intervention group and 75 in the control group) which aimed to evaluate the cost analyses of a computer-based CDSS for ordering blood tests in a primary care setting compared with OCS alone. 96 The study consisted of a 6-month pre-intervention period and a 6-month post-intervention period. The CDSS comprised an optimal but restricted list of blood tests based on recommendations for blood test ordering for the patient’s indication, selected by the physician based on guidelines from the Dutch College of General Practitioners (http://nhg.artsennet.nl/). Minimum, maximum and base-case intervention costs (comprising the costs of both developing and installing the CDSS) were calculated. Development costs comprised: (1) personnel costs for reviewing 83 different guidelines for possible recommendations on blood tests, (2) writing the content, programming the software, testing prototypes, writing an explanatory leaflet about the CDSS, and writing instructions for installation and use. A summary of the minimum, maximum and base-case interventions costs is displayed in Table 30.
| Minimum estimate | Base-case estimate | Maximum estimate | ||||
|---|---|---|---|---|---|---|
| Hours | Costs (€) | Hours | Costs (€) | Hours | Costs (€) | |
| Developing the CDSS | ||||||
| Writing CDSS content | 108 | 3000 | 138 | 4000 | 168 | 5000 |
| Expert meeting | 228 | 9000 | 228 | 9000 | 228 | 9000 |
| Software programming | 480 | 15,000 | 560 | 17,000 | 640 | 19,000 |
| Testing prototypes | 64 | 2000 | 64 | 2000 | 64 | 2000 |
| Writing instructions | 400 | 12,000 | 400 | 12,000 | 400 | 12,000 |
| Subtotal | 1280 | 41,000 | 1390 | 44,000 | 1500 | 48,000 |
| Costs per practice (n = 118) | 349 | 377 | 405 | |||
| Installing CDSS; performed by: | ||||||
| Our team (n = 90 practices) | 463 | 15,000 | 1048 | 30,000 | 1634 | 46,000 |
| Physician (n = 8) | 26 | 1000 | 33 | 1000 | 41 | 1000 |
| Colleague physician (n = 20) | 54a | 2000 | 74 a | 3000 | 94 a | 4000 |
| Subtotal | 542 | 18,000 | 1155 | 35,000 | 1769 | 51,000 |
| Costs per practice (n = 118) | 153 | 293 | 434 | |||
| Total | 1821 | 59,000 | 2545 | 79,000 | 3268 | 99,000 |
| Total costs per practice ( n = 118) | 502 | 670 | 839 | |||
The minimum, base-case and maximum costs for installing the CDSS per practice were therefore €502, €670 and €839. As the CDSS was ultimately installed in 118 practices the total minimum and maximum estimate of the costs of developing and installing the CDSS was €41,000 and €48,000 respectively, with a base-case estimate of €44,000.
As the cost of laboratory requests depended on (1) the number of blood samples obtained and (2) the number and type of laboratory tests performed, data on the number of blood samples and blood tests performed in the pre-intervention and post-intervention phases were obtained from the laboratories. Costs were calculated by multiplying the number of blood samples and the number of tests by their unit costs. All unit costs were obtained from the national list of charges established by the Dutch Board for Health Care Tariffs (year 2003). The cost for obtaining a blood sample was set at €11.50, with the cost per test varying from €1.47 to €33.19 depending on the type of test. In addition to these costs (which included the cost of materials, laboratory personnel, and housing) the salary costs of a clinical chemist or medical microbiologist were included.
As previously stated in the review of studies on the impact of CDSS in OCS for diagnostic, screening or monitoring test ordering on process or patient outcomes, there was a significant decrease in the intervention group in the number of tests per order form (–6%) compared to the control group (+0%) (p = 0.001). This in combination with the type of laboratory blood test ordered and performed resulted in an insignificant mean cost decrease of 3% (€639) in the intervention group in the post-intervention phase, compared with a 2% (€208) increase in the control group (p = 0.09). Thus the CDSS yielded a mean cost saving of €847 per practice per 6 months (i.e. €639 plus €208).
The break-even point at which savings on laboratory costs exceeded the intervention costs was therefore reached after 5 months. Sensitivity analysis using the best-case scenario (upper limit of the 95% CI of the difference in laboratory cost requests; i.e. yearly savings of €3,669 per practice) and the minimum estimate of the intervention costs (€502 per practice) indicated intervention costs would be offset by savings as early as 2-months post-intervention implementation. Sensitivity analysis using the worst-case scenario (lower limit of the 95% CI of the difference in laboratory cost requests; i.e. increase of €282 per practice and the maximum estimate of the intervention costs (€838 per practice per year) indicated intervention costs would not be outweighed by savings on laboratory costs.
The second UPP study by Bassa and colleagues98 which assessed the impact on the effectiveness and costs of a practice guideline implemented through CDSS for the management of patients with hypercholesterolemia in a primary care setting, reported cost data pre- and post-implementation of the CDSS for pharmacological treatment, and laboratory tests. Only very minimal data were reported. The specific tests assessed were lipid profile and safety analyses (transaminases and muscular enzymes). These were costed using the Soikos database of health-care costs,127 with €0.46 for total cholesterol, €2 for LDL, €3 for HDL, €4 for triglycerides, €15 of CK, €1 for serum glutamic oxaloacetic transaminase, and €1 for serum glutamic pyruvic transaminase respectively. Despite the fact that there were no significant differences in the number of lipid profile tests conducted in the pre- and post-intervention periods (773 pre-intervention and 763 post-intervention, there was a significant increase in laboratory costs per patient from €41.8 per annum in the pre-implementation phase to €47.2 post-intervention [difference: +5.4 (95%: 2.0; 8.7) p = 0.0017]. The authors reported that this was due to a significantly higher number of safety analyses conducted in the post-intervention phase compared to the pre-intervention phase (803 compared with 734 respectively). However, overall patient treatment costs were reduced by a total of €78.4 per patient in the 1-year post-intervention phase mainly due to a decrease in the number of patients treated pharmacologically.
Chapter 7 Discussion and conclusions
Statement of principal findings
Study question 1: Which CDSS in OCS for test ordering are currently in use in the UK?
As stated in Chapter 4, the response rate from the survey of the 24 manufacturers and suppliers commissioned under the ASCC to provide CDSS support and functionality within the systems currently being deployed in the NHS was extremely disappointing, at 17%. The results have therefore been included in an Appendix rather than reported in the main body of the report as we do not consider them to be informative. Where any responses were received these were generally classified as being commercially sensitive data, and did little to elucidate which CDSS are currently either being trialled, deployed or implemented within the NHS.
Further contact with NHS CFH, the Healthcare Commission,15 NHS Purchasing Suppliers, and the NHS Supply Chain was made. However, as the contractual level is now managed at the individual SHA level, through the ASCC, no further useful information was gained as NHS Purchasing Suppliers and the NHS Supply Chain are not involved in this deployment.
Due to the time constraints of the assessment it was not possible to make contact with individual SHAs and PCTs to ascertain whether they are currently implementing CDSS and OCS as part of the NPfIT, and which systems if any they are implementing. It was therefore not possible within this assessment to ascertain which CDSS are currently being used within the NHS.
Study question 2: What is the impact of CDSS in OCS for diagnostic, screening or monitoring test ordering compared to OCS without CDSS on process and adverse events?
This section discusses the principal finding from the 24 studies that assessed the impact of CDSS in conjunction with OCS compared to OCS alone for test ordering. In the main body of the report the results from the studies have been discussed under the nominal categories according to predominant type of CDSS intervention(s). This section therefore follows the same format with the principal findings for (1) studies assessing the impact of the display of test costs (n = 3), (2) those assessing the impact of the display of previous test results (n = 2), (3) studies assessing the impact of reminders (n = 10), (4) studies assessing the effects of restricted test lists(n = 2), and (5) those assessing the impact of recommendations (n = 7) presented.
Impact of the display of test charges
Evidence from one CRCT by Tierney and colleagues86 and two RCTs by Bates and colleagues101 both conducted in the USA, to support there being an impact of the display of laboratory or radiological imaging test charges on test volumes and costs is equivocal.
Two of the trials focused predominantly on the effects of the display of test charges on laboratory test orders,86,101 while the other focused on radiological imaging test orders. 101 The CRCT by Tierney and colleagues86 showed a statistically significant decrease in the number of tests ordered per patient visit in the intervention group compared to the control group. Corresponding patient test charges were significantly lower in the intervention group relative to the control group. 86 However, post-intervention follow-up indicated the effect of the display of test charges may be transient, and there may be little learning effect from their previous display as no significant differences in test ordering rates were observed between the groups in this period. In contrast to the results of the CRCT,86 in the two RCTs by Bates and colleagues there were no significant differences between treatment groups in either the laboratory or radiological imaging RCTs on either test volumes or costs per patient admission. 101
Impact of the display of previous test results
The impact of the display of previous test results was assessed in one CCT by Solomon and colleagues88 and one UPP study by Bansal and colleagues. 89 Both studies were conducted in the USA. The CCT focused on the reduction of reducing unnecessary serological testing in the diagnosis of suspected systemic rheumatic disease, and also included the interpretation of future test results,88 while the focus of the pre–post study was on reducing ABG usage in an ICU setting. This study also included the provision of educational text and limitations on 24-hour multiple test ordering. 89
Again, results between the two studies were contradictory. In the CCT by Solomon and colleagues88 significantly more test orders (11%) were cancelled in the intervention group compared to the control group (0.42%). Whereas in the UPP study by Bansal and colleagues89 there were no significant differences between the number of ABG test orders placed pre- and post- intervention (376 and 387 respectively).
Impact of the display of reminders
The impact of the display of reminders was assessed in 10 studies:24,29,48,57,87,90–94 four CRCTs; two by Overhage and colleagues in 1996 and 1997,24,48 one by Palen and colleagues,90 and one by Matheny and colleagues;91 one RCT undertaken by Bates and colleagues,92 one CCT by O’Connor and colleagues,57 one randomised crossover trial by McDonald and colleagues,29 one ITS study with an AB-AB-AB design by Carton and colleagues,93 and two UPP studies by Steele and colleagues and Abboud and colleagues. 87,94 Nine of the 10 studies were conducted in the USA,24,29,48,57,87,90–92,94 while the remaining study was conducted in France. 93
Nine studies assessed the impact of reminders; one for compliance to undertake preventative care measures,48 seven for reminders to undertake appropriate laboratory test ordering for medication monitoring,24,29,57,87,90,91,94 and one for reminders regarding the ordering of redundant laboratory tests. 92 Two of the studies were undertaken on specific patient groups, namely those with diabetes57 or assessed specific drug monitoring of aminoglycosides. 87 The remaining five studies all assessed the impact of reminders on the monitoring of pre-specified study specific target medications. 24,29,90,91,94 The remaining study assessed the impact of reminders based on guidelines for radiology imaging referral practice. 93
Results across the studies were mixed and equivocal. The one CRCT by Overhage and colleagues24 assessing compliance with reminders to undertake laboratory or imaging preventative care measures in an inpatient setting showed no significant differences between treatment groups. 48 The results from the seven studies that assessed reminders to undertake appropriate laboratory test ordering for medication monitoring were also mixed, both between and within studies. 24,29,57,87,90,91,94 Among the seven studies, one CRCT by Overhage and colleagues and one pre–post study by Steele and colleagues,24,94 showed a statistically significant benefit with the display of reminders in terms of compliance to undertake appropriate laboratory tests for medication ordering. In the randomised cross-over trial by McDonald and colleagues conducted in a mixed outpatient population,29 and the CCT by O’Connor and colleagues57 conducted in patients with diabetes, results were inconsistent. McDonald and colleagues29 found a significant impact on compliance rates for medication laboratory test monitoring in physicians but not in nurse practitioners. In the CCT by O’Connor and colleagues reminders had a beneficial impact on the number of diabetic patients undergoing HbA1C tests, but no significant impact on the number of LDL cholesterol tests undertaken. 57 In terms of HbA1C values observed in the study, there were no significant differences between intervention and control groups at either 2- or 4-year follow-up, suggesting that while reminders may have some impact (dependent on the test type) on the number of tests undertaken, this does not necessarily translate into actual clinical differences that may impact on the patient’s disease process and management. None of the other three studies, (two CRCTs and a pre–post study)87,90,91 showed there to be any significant benefit with the display of reminders for compliance with recommended medication laboratory test monitoring.
In contrast, the RCT by Bates and colleagues92 which assessed the impact of reminders for redundant laboratory test orders showed a statistically significant reduction in test ordering between the intervention and control groups (27% versus 51% respectively); and therefore an absolute difference in the proportion of redundant tests performed between the groups of 24% in favour of the intervention group. Likewise, the ITS by Carton and colleagues93 found the display of reminders had a small but statistically significant impact on the number of radiology imaging referrals that conformed to guidelines, with an increase from 66.8% compliance without reminders to 73.1% with reminders.
Impact of the display of restricted lists
One ITS by Rosenbloom and colleagues95 and one CPP study by Poley and colleagues96 assessed the impact of the display of restricted lists. 95,96 The ITS was conducted in the USA and the pre–post study in the Netherlands. The former study focused primarily on the restriction of the ordering of serum magnesium tests, with calcium and phosphorus test instances as secondary outcomes, whilst the latter study targeted a range of blood tests. 96 Both studies showed that in general the use of restricted lists significantly reduced test volumes, although the ITS highlighted the complexity of the implementation of CDSS and how unexpected effects due to part(s) of the CDSS intervention may occur.
In this study, two of the three CDSS interventions reduced test ordering rates, but an increase in test volumes was noted after implementation of the second of the three interventions. The CDSS was then amended, and a significant reduction in the volume of test orders was observed. Results from the controlled pre–post study showed a small (–0.38) but statistically significant decrease in the number of test requests per form with implementation of the CDSS, but no differences in the number of laboratory request forms submitted compared to OCS alone. 96
Impact of the display of recommendations
The effects of CDSS recommendations were assessed in seven studies: two CRCTs by Hobbs and colleagues,32 and Cobos and colleagues;50 one RCT by Apkon and colleagues;97 and four pre–post studies by Bassa and colleagues,98 Sanders and colleagues,104 Nightingale and colleagues,99 and Boon-Falleur and colleagues. 100 The CRCT by Hobbs and colleagues32 and the pre–post study by Nightingale and colleagues99 were both conducted in the UK; the two studies by Apkon and colleagues97 and Sanders and colleagues104 were undertaken in the USA; the studies by Cobos and colleagues50 and Bassa and colleagues98 were conducted in Spain, while the study by Boon-Falleur and colleagues100 was undertaken in Belgium. Overall, the results both within and between studies were mixed and equivocal. All three studies focusing on the provision of recommendations for the management of patients with hyperlipidemia (Hobbs and colleagues,32 Cobos and colleagues50 and Bassa and colleagues98) showed no significant beneficial impact in terms of increasing lipid test rates. However, there was some limited impact in two of the studies on increasing either the number of patients receiving a full lipid profile,32 or receiving an AST/ALT test. 50 Likewise, the RCT by Apkon and colleagues that assessed the impact of recommendations provided by a CDSS Coupler on the number of patient health-care opportunities fulfilled showed no significant benefit in terms of the number of either laboratory or diagnostic screening imaging tests undertaken compared with usual care. 97 However, there was a significant increase in laboratory test resource consumption compared with the usual care group.
In one UPP study by Sanders and colleagues assessing the impact of CDSS guideline recommendation for undertaking head or brain imaging scans,104 a small but statistically significant reduction in the number of imaging studies was observed, with a decrease from 742 scans undertaken pre-implementation of the CDSS to 704 after implementation. However, despite this reduction compliance with the tests indicated by the guideline recommendations remained relatively low at only 60%. 104
Overall the results of the two pre–post studies by Nightingale and Boon-Falleur implementing guideline protocols for the management of liver transplant patients,99,100 were somewhat mixed, but tended to show a significant decrease in the number of laboratory tests ordered,99,100 the number of out of hours tests requested,99 and a reduction in laboratory costs. 99 However, as appropriate levels of testing are driven by the patients’ condition and disease stage there were some increases observed in test requesting in certain patient groups in both studies. This was most pronounced in the study by Boon-Falleur and colleagues100 in which a 13% increase in the number of laboratory tests ordered in patients undergoing an initial assessment protocol was observed, but a 27% reduction in test orders for those undergoing transplant protocols was shown.
Study question 3. What features of CDSS are associated with clinician or patient acceptance of CDSS in OCS?
No studies were identified which met the inclusion criteria on the acceptability of CDSS to physicians or patients and therefore it was not possible to address this question within the context of this review.
Study question 4: What is the cost-effectiveness of CDSS in diagnostic, screening or monitoring test OCS compared to OCS without CDSS?
Only two studies met the inclusion criteria, both of which were cost-comparison analyses,96,98 although some limited cost data were also reported in the three trials that assessed the impact of the display of test charges by Tierney and collegues86 and the two RCTs by Bates and colleagues101 which are included in the review of the impact of CDSS plus OCS versus OCS alone for test ordering. None of these three trials met the inclusion criteria for the systematic review of economic evaluations of CDSS and were therefore not included in this section. Of the two included cost-comparison analyses the one by Poley and colleagues was conducted alongside a CPP study,96 and the one by Bassa and colleagues alongside a UPP study. 98 A full description of both of the studies is reported in Chapter 5, Studies assessing the impact of the display of recommendations and Study question 3. What features of CDSS are associated with clinician or patient acceptance of CDSS in order communications systems – on the impact of CDSS plus OCS versus OCS alone. Both studies found that the impact of CDSS plus OCS versus OCS alone had no significant impact on test costs.
Analyses conducted alongside the CPP study by Poley and colleagues found a mean cost decrease of 3% for blood tests orders (€639) in each of the intervention clinics compared with a 2% (€208) increase in the control clinics in test costs. However, this difference failed to reach conventional levels of statistical significance. 96 Likewise, the analysis conducted alongside the UPP study by Bassa and colleagues found a significant increase in the cost of laboratory tests (triglycerides, CK, serum glutamic oxaloacetic transaminase, and serum glutamic pyruvic transaminase) from €41.8 per patient per annum to €47.2 post implementation of the intervention. However, overall, patient treatment costs were reduced by a total of €78.4 per patient in the 1-year post-intervention phase, mainly due to a decrease in the number of patients treated pharmacologically.
Discussion
There is a growing body of research evidence which has examined the impact of CDSS in OCS versus OCS alone for diagnostic, screening or monitoring tests purposes on process outcomes and adverse events, although this lags far behind the volume of literature on the use of CDSS for medication ordering. This review identified 24 empirical studies which have examined the impact of CDSS plus OCS on test rates or compliance with guidelines for laboratory or radiological test imaging published between 1980 and 2009, with 10 of these published in the last 4 years.
However, there are a number of limitations to the evidence-base reviewed. All the 24 identified studies focused upon the decision to order a test, its appropriateness and timing. No studies were identified that addressed the results reporting process with the provision of context specific interpretative comments to help clinicians with the interpretation of the test results, and provide advice on the best course of action given a specific test result, for example to undertake further investigations and the timing of such tests. Moreover, only two of the 24 included studies, those by Bansal and colleagues89 and Rosenbloom and colleagues,95 assessed CDSS with a number of different functions, such as limitations on forward ordering of tests, the display of educational text, graphical display of recent test results and calculated correct test results. The remainder of the included studies tended to focus on one specific CDSS function, e.g. display of test charges, reminders, the display of restricted lists or recommendations. While it is useful to evaluate these single CDSS functions alone, in order to assess their impact prior to potentially incorporating them in a system with multiple functions, it limits the external validity of the results of these studies, as the majority of CDSS within OCS are likely to be multi-functionality systems, which may address issues such as corollary order sets, recommendations, the display of previous test results, and interpretative comments for results reporting, as well as the facility for medication ordering. Therefore, the results of these studies may do little to elucidate how multi-functional CDSS may actually be used by health-care professionals and perform in practice once implemented in a clinical setting.
Furthermore, of the 24 studies identified, 17 were conducted within the USA,23,28,47,86–91,94–96,98,103 and of these 17, 12 were undertaken in three large academic centres which are well renowned for being ‘leaders’ at the forefront of CDSS and OCS development and implementation. Four studies had been conducted at the Wishard Memorial Hospital, Indianapolis, IN, or at one of their outpatient centres,24,29,48,86 five at the Brigham and Women’s Hospital, Boston, MA,88,91,92,101 and three at the Vanderbilt University Medical Center, Nashville, TN. 89,95,104 The systems used within these centres are all home-grown, and sharply focused on specific wards or units, and/or display a technical novelty side to their investigation. Additionally, in all 12 studies the system developers were also the evaluators. The results from these studies are potentially likely to be more biased in finding a significant benefit in favour of CDSS plus OCS use versus OCS use alone, as the systematic review by Garg and colleagues10 highlights that studies in which the authors also developed and evaluated the CDSS were approximately three times more likely to show a beneficial effect in terms of CDSS use than when the authors were not the developers (74% success vs 28% success respectively). Additionally, it highlights the complexity of the development, implementation and use of CDSS which can be highly context specific and as Ash and colleagues103 highlight can be highly dependent upon the organisational context, support for the system by both management and staff, and people with the specialist knowledge and skills who are able to develop systems that are likely to have a beneficial effect upon clinical practice. This means that systems that developers need to have a strong insight into the clinical process which they are attempting to address, and highlights the fact that systems that may have a beneficial impact on practice in some wards, units or hospitals may not do so when transferred into a different environmental context.
Only two studies were conducted within the UK. 32,99 Both of these were focused on specific groups, namely people being screened for hyperlipidemia,32 and those being assessed for or undergoing liver transplantation. 99 Additionally, both of the studies, and therefore the systems, are relatively old with the studies having been published in 1994 and 1996 respectively. 32,99
The implications of the evidence-base reviewed are threefold; first, it is very difficult to extrapolate the findings from studies conducted in the USA to the UK, as the health-care system is generally insurance based, and ‘baseline’ rates of laboratory testing or radiological imaging tend to be more than twice that observed within the NHS. Second, reliance on results from an evidence-base where 50% of the studies have been conducted within three known leading academic institutions is problematic, and may be more biased in terms of finding a statistically significant effect for CDSS use than would be observed in everyday general clinical practice. Furthermore in today’s NHS these home-grown systems may be of little value, as ‘off the shelf’ systems have greater potential for wider deployment application. 103
Third, of the 24 studies identified,24,29,32,48,50,57,86–101,104 only 10 were published within the period of 2004–9,50,57,87,90,91,94–98 with the remaining 13 studies being published either within or prior to 1999. It can therefore be postulated that many of these systems, particularly in terms of the older CDSS may now be obsolete or have been totally upgraded or updated with the further technological developments that are occurring rapidly within the growth and development of CDSS. Greater weight should therefore be given to the results of the studies that have been published more recently in which the technologies are still potentially available and may have undergone limited changes.
In terms of the findings of this review, as previously stated the findings were mixed and equivocal, often both within and between studies, which is not surprising given the heterogeneity between the study settings, patient indications, outcome assessed and the different types of CDSS evaluated. Overall, if the findings of both primary and secondary outcomes are taken into account then CDSS significantly improved practitioner performance in 15 out of 24 studies (62.5%),24,29,32,50,57,86,88,92–96,99,100,104 including one of three studies (33.3%) assessing the impact of the display of costs,86,101 one of the two studies (50%) assessing the impact of the display of previous test results,99,89 six of the 10 studies (60%) examining the use of reminders,24,29,48,57,87,90–94 one of the two studies (50%) that displayed previous test results,95,96 and five of the seven studies (71.4%) that assessed the impact of the display of recommendations. 32,50,97–100,104
Four studies also assessed the impact of test cancellation or delay on potential adverse events. 86,92,101 There were no significant differences between treatment groups in any of the four trials in terms of extra health-care utilisation by patients or adverse events. Therefore the impact of cancelling either costly or redundant tests on adverse outcomes currently appears to be negligible. Overall therefore it would appear that the implementation of CDSS in conjunction with OCS versus OCS alone for test ordering shows no evidence of harm even in studies in which the aim was primarily to reduce the rate of test ordering.
Due to the heterogeneity between studies it is very difficult to conclude why some CDSS are successful in terms of either decreasing laboratory or imaging test rates, or increasing test rates to the appropriate specified standards. In terms of the three system features that were found to be independent predictor of improved clinical practice by Kawamoto and colleagues16 (automatic provision of CDSS output provided as part of clinician workflow; output at the time and location of decision making; and provision of a recommendation rather than just an assessment), there were few differences between the 24 studies, apart from in the provision of recommendations. All 24 studies provided automatic CDSS output as part of the clinician workflow and at the time and location of decision making. However, only seven of the studies provided a recommendation, of which five of seven found that the CDSS recommendations had a significant positive impact on either a primary or secondary outcome measure. 32,50,99,100,104
With respect to the CDSS developers also being the outcome evaluators compared to independent evaluators assessing system impact, a less pronounced effect was observed in this review than that shown in the systematic review by Garg and colleagues. 10 In this review when system developers were also the evaluators a statistically significant positive impact of the CDSS on either a primary or secondary outcome measure was observed in 67% of the studies,24,29,86,88,92,93,95,96,99,104 compared with 43% when the evaluator was not the developer. 57,94,105 These figures show a slightly different distribution to those found by Garg and colleagues who found a 74% success rate when the authors were also the system evaluator versus a 28% success rate when the evaluation was undertaken independently. 10
It is often posited in the literature that studies of a less rigorous methodological design, such as CCTs, controlled and uncontrolled pre–post studies are more likely to be biased in terms of finding a significant effect. When an interaction between study design and a significant or non-significant effect in terms of the impact of the CDSS was assessed no such interaction was observed. Nor was there any interaction between year of study publication and a significant or non-significant impact of CDSS on process outcomes or practitioner performance.
Strengths and limitations of the assessment
The strengths of this assessment lie in the review of the impact of CDSS on process and patient outcomes. Extensive searches were undertaken, although restricted to English language articles, and studies included that reported any relevant outcome on the impact of CDSS on laboratory or radiological imaging included, even if this was not the primary aim of the study. The systematic review searches were updated in April 2009, and should therefore provide a comprehensive up-to-date review of the evidence available.
However, there are a number of limitations in the assessment. Firstly, although manufacturers and suppliers under the ASCC were contacted regarding the deployment or implementation of their CDSS within the NHS the response rate despite of follow-up was extremely low at 17%. Most of the information supplied by them was also classified as being CIC and therefore is not useful. We were therefore unable to address this question as set out in the original report protocol.
Secondly, in terms of the systematic review of the impact of CDSS plus OCS versus OCS alone on process and patient outcomes, the identified studies were so heterogeneous in terms of the settings, patient indications and CDSS assessed that any pooling of studies was not possible. This has meant that, while studies have been broadly grouped according to the type of CDSS intervention, the review has been presented on a study-by-study basis, rather than as a complete synthesis of the results for each type of intervention. This makes the interpretation of the results of the studies somewhat more complex, challenging and ultimately of less value to decision makers in the NHS. Furthermore, due to heterogeneity between the studies, particularly in terms of study design, we were unable to use formal meta-regression techniques to investigate the impact of the presence or absence of different CDSS features on the study results obtained. Additionally it should be noted that many of the studies identified were published a number of years ago, with only 10 of the 24 studies included in the review published post 2004. Therefore many of the CDSS evaluated within the review may now be obsolete and no longer used in practice or have been upgraded or changed in response to the rapid changes taking place within pathology practice and the implementation of CDSS and OCS.
No studies were identified which have addressed the question of what features of CDSS are associated with clinician or patient acceptance of CDSS in OCS. As previously stated, this was due to the fact that while 31 studies were identified that had examined the acceptability of CDSS to physicians, the focus of these was generally on the ordering of medications, and results for laboratory or radiological test ordering were either not reported at all or not reported separately. These studies were therefore outside the scope of the current assessment.
The systematic review of economic evaluations is severely limited, and includes only two studies neither of which were conducted within the UK. Both of these were cost comparisons that compared the use of CDSS plus OCS versus OCS alone, and were focused on specific indications, namely blood test ordering and the management of patients with hypercholesterolemia. One of the studies reported only very limited results. It was therefore difficult to extrapolate the results of these studies to the wider context in which test ordering generally occurs, and on the basis of such limited evidence makes it impossible to comment on the cost-effectiveness of CDSS as they would be implemented and used in a wider clinical setting within OCS in the NHS.
Conclusions
Review question 1: Although a survey of manufacturers and suppliers under the ASCC was undertaken to establish the present deployment or implementation of CDSS within the NHS, the survey response rate was extremely low at only 17%. Most of the very limited data provided by contractors was designated as being CIC and therefore it was not possible to address the question of which CDSS are currently being used within the NHS in this assessment.
Review question 2: The findings from the review on the impact of CDSS plus OCS versus OCS alone are mixed and equivocal. Overall, if the findings of both primary and secondary outcomes are taken into account then CDSS showed a significant benefit on either process or practitioner performance outcomes in 15 out of 24 studies (62.5%). Additionally in four studies that assessed adverse effects of either test cancellation or delay, no significant detrimental effects in terms of patients extra utilisation of health-care resources or adverse events were observed. However, none of the studies assessed patient outcomes such as complications, disease progression or quality of life, and therefore it is unclear whether the use of CDSS either for curtailing unnecessary or redundant tests, or increasing the appropriateness of tests and their timing has any potential impact on health-care outcomes that are relevant to patients. Furthermore, although CDSS appears to have a potentially small positive impact on diagnostic, screening or monitoring test ordering, the majority of the included studies come from a limited number of institutions in the USA with home-grown systems, and it is unclear how well these results would extrapolate to the current NHS situation in which ‘off the shelf’ systems are being installed
Review question 3: No studies were identified which assessed the features of CDSS that are associated with clinician or patient acceptance of CDSS in OCS. This question was therefore not addressed within the context of this review.
Review question 4: Given the very limited data available on the cost-effectiveness of CDSS plus OCS compared with OCS alone, and the highly specific indications in which both of the identified studies were undertaken, it is not possible to extrapolate the findings of these studies to the wider context in which diagnostic, screening or monitoring test ordering occurs within the NHS. It is therefore not possible to comment on the likely cost-effectiveness of CDSS within OCS as they would be implemented and used within a wider NHS clinical setting.
Research recommendations
There is a need to establish which CDSS in OCS are currently being piloted, implemented or already deployed within the NHS and the type of systems, e.g. hospital or laboratory information systems, with which they interface. A comprehensive survey of individual SHAs, user sites, primary care trusts, CFH via their IT investment survey, pathology services, the Royal Colleges of Pathologists, and Radiologists is therefore warranted to establish which systems are in place or likely to be implemented within the context of the NpfIT. The results of such a survey would hopefully inform system commissioners as to the best manner in which to conduct a rigorous evaluation of the CDSS within OCS that are already being implemented or currently ‘rolled out’.
Currently there is very little evidence from the UK on the impact of CDSS in OCS compared to OCS alone, and no evidence on the impact of ‘off the shelf’ CDSS which are of relevance to the NpfIT and the NHS. There is therefore a need to establish whether there is any ‘grey’ literature available from NHS Trusts that have already implemented OCS as this would be potentially of use in informing how to design and implement evaluation studies of CDSS within OCS within the NHS.
We believe the key current need is for a well designed and comprehensive survey, and on the basis of the results of this potentially for evaluation studies in the form of CRCTs or RCTs which incorporate process, and patient outcomes, as well as full economic evaluations alongside the trials to assess the impact of CDSS in conjunction with OCS versus OCS alone for diagnostic, screening or monitoring test ordering in the NHS. The economic evaluation should incorporate the full costs of potentially developing, testing, and installing the system, including staff training costs.
Acknowledgements
We particularly acknowledge the help of Mrs Mary Bond, Research Fellow, PenTAG for screening titles, abstracts and full papers for inclusion in the reviews and Ms Zulian Liu, Research Assistant, PenTAG for checking data extraction. We also thank Ms Kate Boddy for undertaking the update searches.
The views expressed in this report are those of the authors and not necessarily those of the NHS R&D HTA Programme. Any errors are the responsibility of the authors.
About PenTAG
The Peninsula Technology Assessment Group (PenTAG) is part of the Institute of Health Service Research at the Peninsula Medical School. PenTAG was established in 2000 and carries out independent health technology assessments for the UK HTA Programme, systematic reviews and economic analyses for the NICE Centre for Public Health Excellence, and systematic reviews as part of the Cochrane Collaboration Heart Group, as well as for other local and national decision-makers. The group is multi-disciplinary and draws on individuals’ backgrounds in public health, health services research, computing and decision analysis, systematic reviewing, statistics and health economics. The Peninsula Medical School is a school within the Universities of Plymouth and Exeter. The Institute of Health Research is made up of discrete, but methodologically related research groups, across which health technology assessment is a strong and recurring theme.
Expert Advisory Group
| Dr Susan Clamp | Yorkshire Centre for Health Informatics, Leeds Institute of Health Sciences, University of Leeds, Leeds, UK |
| Dr Ian Bailey | Informatics Advisor, Royal College of Pathologists, 2 Carlton House Terrace, London, UK |
| Dr Ed Keedwell | Lecturer in Computer Science, University of Exeter, Exeter, UK |
Contributions of authors
Ms Caroline Main was responsible for project coordination, drafting the protocol, conducting the survey, undertaking study selection, data extraction and quality assessment, data synthesis, and drafting the final report.
Ms Tiffany Moxham was responsible for devising the search strategy, conducting the literature searches, and commenting on the final report.
Professor Jeremy C Wyatt was responsible for providing informatics and clinical input at all project stages, commenting on the draft protocol and draft report.
Professor Jonathan Kay was responsible for providing informatics and clinical input at all project stages, commenting on the draft protocol and draft report.
Dr Rob Anderson was responsible for providing input at all project stages, editing the systematic review of economic evaluations, and commenting on the draft protocol and draft report.
Professor Ken Stein was responsible for project direction, input to the protocol and draft report and has overall responsibility for report content.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Whiting P, Toerien M, de Salis I, Sterne JAC, Dieppe P, Egger M, et al. A review identifies and classifies reasons for ordering diagnostic tests. J Clinl Epidemiol 2007;60:981-9.
- Berger R, Kichak B. Computerized physician order entry: helpful or harmful?. J Am Med Inform Assoc 2004;11:100-3.
- Hwang JI, Park HA, Bakken S. Impact of a physician’s order entry (POE) system on physicians’ ordering patterns and patient length of stay. Int J Med Inform 2002;65:213-23.
- Sittig D, Stead W. Computer-based physician order entry: state of the art. J Am Med Inform Assoc 1994;1:108-23.
- Tierney WM. Improving clinical decisions and outcomes with information: a review. Int J Med Inform 2001;62:1-9.
- Kuperman GJ, Gibson RF. Computer physician order entry: benefits, costs and issues. Ann Intern Med 2003;139:3-9.
- Doolan DF, Bates DW, James BC. The use of computers for clinical care: a case series of advanced U.S. sites. J Am Med Inform Assoc 2003;10:94-107.
- Tierney WM, Overhage JM, Takesue L, Harris M, Murray D, Vargo C. Computerizing guidelines to improve care and patients outcomes: example of heart failure. J Am Med Inform Assoc 1995;2:316-22.
- Hunt DL, Haynes RB, Hanna SE, Smith K. Effects of computer-based clinical decision support systems on physician performance and patient outcomes: a systematic review. JAMA 1998;280:1339-46.
- Garg AX, Adhikari NKJ, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: A systematic review. JAMA 2005;293:1223-38.
- Johnston ME, Langton KB, Haynes RB, Mathieu A. Effects of computer-based clinical decision support systems on clinician performance and patient outcome. A critical appraisal of research. Ann Intern Med 1994;120:135-42.
- Danzon PM, Manning WG, Marquis MS. Factors affecting laboratory test use and prices. Health Care Financ Rev 1984;5:23-32.
- Fawkes FG, Catford JC, Logan RF. Containing the use of laboratory tests. BMJ 1985;290:488-90.
- Shawstack JA, Schroeder SA, Matsumoto MF. Changes in the use of medical technologies, 1972–1977: a study of 10 inpatient diagnoses. N Engl J Med 1982;306:706-12.
- Commission for Healthcare Audit and Inspection . Getting Results: Pathology Services in Acute and Specialist Trusts 2007.
- Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005;330.
- Wendt T, Knaup-Gregori P, Winter A. Decision support in medicine: a survey of problems of user acceptance. Stud Health Technol Inform 2000;77:852-6.
- Wetter T. Lessons learnt from bringing knowledge-based decision support into routine use. Artif Intell Med 2002;24:195-203.
- Aronsky D, Chan KJ, Haug PJ. Evaluation of a computerized diagnostic decision support system for patients with pneumonia: study design considerations. J Am Med Inform Assoc 2001;8:473-85.
- Ramnarayan P, Britto J. Paediatric clinical decision support systems. Arch Dis Child 2002;87:361-2.
- Boekeloo BO, Becker DM, Levine DM, Belitsos PC, Pearson TA. Strategies for increasing house staff management of cholesterol with inpatients. Am J Prev Med 1990;6:5-9.
- Ryff-de-Lèche A, Engler H, Nutzi E, Berger M, Berger W. Clinical applications of two computerized diabetes managment systems: comparison with the log-book method. Diabetes Res 1992;19:97-105.
- Rossi RA, Every NA. A computerized intervention to decrease the use of calcium channel blockers in hypertension. J Gen Intern Med 1997;12:672-8.
- Overhage JM, Tierney WM, Zhou XH, McDonald CJ. A randomized trial of ‘corollary orders’ to prevent errors of omission. J Am Med Inform Assoc 1997;4:364-75.
- Frame PS, Zimmer JG, Werth PL, Hall WJ, Eberly SW. Computer-based versus manual health maintenance tracking. A controlled trial. Arch Fam Med 1994;3:581-8.
- Barnett GO, Winickoff RN, Morgan MM, Zielstorff RD. A computer-based monitoring system for follow-up of elevated blood pressure. Med Care 1983;21:400-9.
- McPhee SJ, Bird JA, Fordham D, Rodnick JE, Osborn EH. Promoting cancer prevention activities by primary care physicians. Results of a randomized, controlled trial. JAMA 1991;266:538-44.
- Tierney WM, McDonald CJ, Hui SL, Martin DK. Computer predictions of abnormal test results. Effects on outpatient testing. JAMA 1988;259:1194-8.
- McDonald CJ, Wilson GA, McCabe GP. Physician response to computer reminders. JAMA 1980;244:1579-81.
- Dexter PR, Wolinsky FD, Gramelspacher GP. Effectiveness of computer-generated reminders for increasing discussions about advance directives and completion of advance directive forms. A randomized, controlled trial. Ann Intern Med 1998;128:102-10.
- Chambers CV, Balaban DJ, Carlson BL, Grasberger DM. The effect of microcomputer-generated reminders on influenza vaccination rates in a university-based family practice center. J Am Board Fam Pract 1991;4:19-26.
- Hobbs FD, Delaney BC, Carson A, Kenkre JE. A prospective controlled trial of computerized decision support for lipid management in primary care. Fam Pract 1996;13:133-7.
- Payne TH. Computer decision support systems. Chest 2000;118:47-52.
- Shiffman RN, Brand CA, Liaw Y, Corb GJ. A design model for computer-based guideline implementation based on information management services. J Am Med Inform Assoc 1999;6:99-103.
- Trivedi MH, Kern JK, Marcee A, Grannemann B, Kleiber B, Bettinger T. Development and implementation of computerized clinical guidelines: barriers and solutions. Methods Inf Med 2002;41:435-42.
- Bates DW, Kuperman GJ, Wang S, Gandhi T, Kittler A, Volk L, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc 2003;10:523-30.
- Hersh WR. Medical informatics: improving health care through information. JAMA 2002;288:1955-8.
- Bodenheimer T, Grumbach K. Electronic technology: a spark to revitalize primary care?. JAMA 2003;290:259-64.
- Christakis DA, Zimmerman FJ, Wright JA, Garrison MM, Rivara FP, Daivs RL. A randomized controlled trial of point-of-care evidence to improve the antibiotic prescribing practices for otitis media in children. Pediatrics 2001;107.
- Lobach DF. Electronically distributed, computer-generated, individualized feedback enhances the use of a computerized practice guideline 1996:493-7.
- Eccles M, McColl E, Steen N. Effect of computerised evidence based guidelines on management of asthma and angina in adults in primary care: cluster randomised controlled trial. BMJ 2002;325.
- Bennett JW, Glasziou PP. Computerised reminders and feedback in medication management: a systematic review of randomised controlled trials. Med J Aust 2003;178:217-22.
- Lowensteyn I, Joseph L, Levinton C, Abrahamowicz M, Steinert Y, Grover S. Can computerized risk profiles help patients improve their coronary risk? The results of the coronary health assessment study (CHAS). Prev Med 1998;27:730-7.
- Miller RA. Medical diagnostic decision support systems-past, present, and future: a threaded bibliography and brief commentary. J Am Med Inform Assoc 1994;1:8-27.
- Morris AH. Academia and clinic. Developing and implementing computerized protocols for standardization of clinical decisions. Ann Intern Med 2001;132:373-83.
- Lobach DF, Hammond WE. Computerized decision support based on a clinical practice guideline improves compliance with care standards. Am J Med 1997;102:89-98.
- Litzelman DK, Dittus RS, Miller ME, Tierney WM. Requiring physicians to respond to computerized reminders improves their compliance with preventive care protocols. J Gen Intern Med 1993;8:311-17.
- Overhage JM, Tierney WM, McDonald CJ. Computer reminders to implement preventive care guidelines for hospitalized patients. Arch Intern Med 1996;156:1551-6.
- Meyer TJ, Van Kooten D, Marsh S, Prochazka AV. Reduction of polypharmacy by feedback to clinicians. J Gen Intern Med 1991;6:133-6.
- Cobos A, Vilaseca J, Asenjo C, Pedro-Botet J, Sanchez E, Val A, et al. Cost effectiveness of a clinical decision support system based on the recommendations of the European Society of Cardiology and other societies for the management of hypercholesterolemia: Report of a cluster-randomized trial. Dis Manag Health Outcomes 2005;13:421-32.
- Nicholls GM, Dissanayake AM, Hazell W. Notification of random hyperglycaemia to general practitioners by an emergency medicine team: impact of a simple intervention plan. Diabet Med 2008;25:751-4.
- Kuperman GJ, Teich JM, Tanasijevic MJ, Ma’Luf N, Rittenberg E, Jha A, et al. Improving response to critical laboratory results with automation: results of a randomized controlled trial. J Am Med Inform Assoc 1999;6:512-22.
- Stock JL, Waud CE, Coderre JA, Overdoft JH, Janikas JS, Heiniluoma KM. Clinical reporting to primary care physicians leads to increased use and understanding of bone densitometry and affects the management of oesteoporosis. A randomized trial. Ann Intern Med 1998;128:996-9.
- Harpole LH, Khorasani R, Fiskio J, Kuperman GJ, Bates DW. Automated evidence-based critiquing of orders for abdominal radiographs: impact on utilization and appropriateness. J Am Med Inform Assoc 1997;4:511-21.
- Sim I, Gorman P, Greenes RA, Haynes RB, Kaplan B, Lehmann H. Clinical decision support systems for the practice of evidence-based medicine. J Am Med Inform Assoc 2001;8:527-34.
- Ash JS, Stavri PZ, Kuperman GJ. A consensus statement on considerations for a successful CPOE implementation. J Am Med Inform Assoc 2003;10:229-34.
- O’Connor PJ, Crain AL, Rush WA, Sperl-Hillen JM, Gutenkauf JJ, Duncan JE. Impact of an electronic medical record on diabetes quality of care. Ann Fam Med 2005;3:300-6.
- Heathfield HA, Wyatt J. Philosophies for the design and development of clinical decision-support systems. Methods Inf Med 1989;32:1-8.
- Frances CD, Alperin P, Adler JS, Grady D. Does a fixed physician reminder system improve the care of patients with coronary artery disease? A randomized controlled trial. West J Med 2001;175:165-6.
- Belcher DW, Berg AO, Inui TS. Practical approaches to providing better preventive care: are physicians the problem or a solution?. Am J Prev Med 1988;4.
- Tierney WM, Hui SL, McDonald CJ. Delayed feedback of physician performance versus immediate reminders to perform preventive care. Med Care 1986;24:659-66.
- White P, Atherton A, Hewett G, Howells K. Using information from asthma patients: a trial of information feedback in primary care. BMJ 1995;311:1065-9.
- Balas EA, Weingarten S, Garb CT, Blumenthal D, Boren SA, Brown GD. Improving preventive care by prompting physicians. Arch Intern Med 2000;160:301-8.
- McPhee SJ, Detmer WM. Office-based interventions to improve delivery of cancer prevention services by primary care physicians. Cancer 1993;72:1100-12.
- McPhee SJ, Bird JA, Jenkins CN, Fordham D. Promoting cancer screening. A randomized, controlled trial of three interventions. Arch Intern Med 1989;149:1866-72.
- Becker DM, Gomez EB, Kaiser DL, Yoshihasi A, Hodge RH. Improving preventive care at a medical clinic: how can the patient help?. Am J Prev Med 1989;5:353-9.
- Fordham D, McPhee SJ, Bird JA, Rodnick JE, Detmer WM. The Cancer Prevention Reminder System. MD Computing 1990;7:289-95.
- Thompson W, Dodek PM, Norena M, Dodek J. Computerized physician order entry of diagnostic tests in an intensive care unit is associated with improved timeliness of service. Crit Care Med 2004;32:1306-9.
- Strecher VJ, O’Malley MS, Villagra VG, Campbell EE, Gonzalez JJ, Irons TG. Can residents be trained to counsel patients about quitting smoking? Results from a randomized trial. J Gen Intern Med 1991;6:9-17.
- McAlister NH, Covvey HD, Tong C, Lee A, Wigle ED. Randomised controlled trial of computer assisted management of hypertension in primary care. Br Med J 1986;293:670-4.
- Buchsbaum DG, Buchanan RG, Lawton MJ, Elswick RK, Schnoll SH. A program of screening and prompting improves short-term physician counseling of dependent and nondependent harmful drinkers. Arch Intern Med 1993;153:1573-7.
- Liu JLY, Wyatt J, Deeks JJ, Clamp S, Keen J, Verde P, et al. Systematic reviews of clinical decision tools for acute abdominal pain. Health Technol Assess 2006;10.
- Spiegelhalter D, Knill-Jones R. Statistical and knowledge-based approaches to clinical decision support systems, with an application to gastroenterology. J R Stat Soc 1984;147:35-77.
- Wyatt J. Decision support systems. J R Soc Med 2000;93:629-33.
- Wyatt J, Spiegelhalter D. Evaluating medical expert systems: what to test and how?. Med Inform 1990;15:205-17.
- Balas EA, Boren SA, Berner ES. Clinical decision support systems: theory and practice. New York, NY: Springer; 1999.
- Ohmann C, Boy O, Yang Q. A systematic approach to the assessment of user satisfaction with health care systems: constructs, models and instruments. Stud Health Technol Inform 1997;43:781-5.
- Ranger S. NHS IT Spending Will Reach 12.4 Bn 2009. www.silicon.com/publicsector/0.3800010403.39159681n (accessed 6 June 2009).
- Khan K, Ter Riet G, Glanville J, Sowden A, Kleijnen J. Undertaking systematic reviews of research on effectiveness: CRD’s guidance for those carrying out or commissioning reviews. York, UK: Centre for Reviews and Dissemination; University of York; 2001.
- De Dombal FT, Leaper DJ, Horrocks JC, Staniland JR, McCann AP. Human and computer-aided diagnosis of abdominal pain: further report with emphasis on performance of clinicians. BMJ 1974;1:376-80.
- Cohen JA. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37-46.
- Cochrane Effective Practice and Organisation of Care Group . EPOC Methods Paper: Including Interrupted Time Series (ITS) Designs in a EPOC Review 2009.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Popay J, Roberts HT, Sowden AP, Britten N, Arai L. Developing guidance on the conduct of narrative synthesis in systematic reviews. J Epidemiol Community Health 2005;59.
- Popay J, Roberts HT, Sowden AP, Arai L, Rodgers M. Guidance on the conduct of narrative synthesis in systematic reviews. 2006. ESRC Research Methods Programme n.d.
- Tierney WM, Miller ME, McDonald CJ. The effect on test ordering of informing physicians of the charges for outpatient diagnostic tests. N Engl J Med 1990;322:1499-504.
- Abboud PA, Ancheta R, McKibben M, Jacobs BR, Group CIOR. Impact of workflow-integrated corollary orders on aminoglycoside monitoring in children. Health Inform J 2006;12:187-98.
- Solomon DH, Shmerling RH, Schur PH, Lew R, Fiskio J, Bates DW. A computer based intervention to reduce unnecessary serologic testing. J Rheumatol 1999;26:2578-84.
- Bansal P, Aronsky D, Aronsky D, Talbert D, Miller RA. A computer based intervention on the appropriate use of arterial blood gas 2001.
- Palen TE, Raebel M, Lyons E, Magid DM. Evaluation of laboratory monitoring alerts within a computerized physician order entry system for medication orders. Am J Manag Care 2006;12:389-95.
- Matheny ME, Sequist TD, Seger AC, Fiskio JM, Sperling M, Bugbee D, et al. A randomized trial of electronic clinical reminders to improve medication laboratory monitoring. J Am Med Inform Assoc 2008;15:424-9.
- Bates DW, Kuperman GJ, Rittenberg E, Teich JM, Fiskio J, Ma’Luf N, et al. A randomized trial of a computer-based intervention to reduce utilization of redundant laboratory tests. Am J Med 1999;106:144-50.
- Carton M, Auvert B, Guerini H, Boulard JC, Heautot JF, Landre MF, et al. Assessment of radiological referral practice and effect of computer-based guidelines on radiological requests in two emergency departments. Clin Radiol 2002;57:123-8.
- Steele AW, Eisert S, Witter J, Lyons P, Jones MA, Gabow P, et al. The effect of automated alerts on provider ordering behavior in an outpatient setting. PLoS Med 2005;2.
- Rosenbloom ST, Chiu KW, Byrne DW, Talbert DA, Neilson EG, Miller RA. Interventions to regulate ordering of serum magnesium levels: report of an unintended consequence of decision support. J Am Med Inform Assoc 2005;12:546-53.
- Poley MJ, Edelenbos KI, Mosseveld M, van Wijk MA, de Bakker DH, van der LJ, et al. Cost consequences of implementing an electronic decision support system for ordering laboratory tests in primary care: evidence from a controlled prospective study in the Netherlands. Clin Chem 2007;53:213-19.
- Apkon M, Mattera JA, Lin Z, Herrin J, Bradley EH, Carbone M, et al. A randomized outpatient trial of a decision-support information technology tool. Arch Intern Med 2005;165:2388-94.
- Bassa A, Del VM, Cobos A, Torremade E, Bergonon S, Crespo C, et al. Impact of a clinical decision support system on the management of patients with hypercholesterolemia in the primary healthcare setting. Dis Manag Health Outcomes 2005;13:65-72.
- Nightingale PG, Peters M, Mutimer D, Neuberger JM. Effects of a computerised protocol management system on ordering of clinical tests. Qual Health Care 1994;3:23-8.
- Boon-Falleur L, Sokal E, Peters M, Ketelslegers JM. A rule-based decision support application for laboratory investigations management 1995:314-18.
- Bates DW, Kuperman GJ, Jha A, Teich JM, Orav EJ, Ma’Luf N, et al. Does the computerized display of charges affect inpatient ancillary test utilization?. Arch Intern Med 1997;157:2501-8.
- Subramanian U, Fihn SD, Weinberger M, Plue L, Smith FE, Udris EM, et al. A controlled trial of including symptom data in computer-based care suggestions for managing patients with chronic heart failure. Am J Med 2004;116:375-84.
- Ash JS, Stavri PZ, Dykstra R, Fournier L. Implementing computerized physician order entry: the importance of special people. Int J Medl Inform 2003;69:235-50.
- Sanders DL, Miller RA. The effects on clinician ordering patterns of a computerized decision support system for neuroradiology imaging studies 2001.
- Sittig DF, Krall MA, Dykstra RH, Russell A, Chin HL. A survey of factors affecting clinician acceptance of clinical decision support. BMC Med Inform Decis Mak 2006;6.
- Suleebe G, Morcka K. Diagnostic and prognostic significance of different antinuclear antibodies in more than 1000 consecutive Albanian patients with rheumatic diseases. Clin Exp Rheumatol 1992;10:255-61.
- Hochberg MC, Boyd RE, Ahearn JM. Systemic lupus erythematosus: a review of clinico-laboratory features and innunogenetic markers in 150 patients with emphasis on demographic subsets. Medicine 1985;64:285-95.
- Reichlin M, Harley JB, Wallace DJ, Hahn BH. Dubois’ lumus erythematosus. Baltimore, MD: Williams and Wilkins; 1997.
- Wolfe F, Cathey MA, Roberts FK. The latex test revisited: rheumatoid factor testing in 8287 rheumatic disease patients. Arthritis Rheum 1991;34:951-60.
- Nimelstein SH, Brody S, McShane D, Holman HR. Mixed connective tissue disease: a subsequent evaluation of the original 25 patients. Medicine 1980;59:239-48.
- Bloch KJ, Buchanan WW, Wohl MJ, Bunim JJ. Sjogren’s syndrome: a clinical, pathological, and serological study of sixty-two cases. Medicine 1965;44:187-225.
- Association . Subcommittee for scleroderma criteria for the American Rheumatism. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 1980;23:581-90.
- US Preventitive Services Task Force . Guide to Clinical Preventive Services: An Assessment of the Effectiveness of 169 Interventions 1989.
- Knoben J. P. A. Handbook of Clinical Drug Data. Hamilton, NY: Drug Intelligence Publications; 1988.
- American Diabetes Association . Standards of Medical Care in Diabetes. Diabetes Care 2005;28:4-36.
- Henrich WL, Agoda LE, Barrett B, Bennett WM, Blantz RC, Buckalew VM. Analgesics and the kidney: summary and recommendations to the Scientific Advisory Board of the National Kidney Foundation from an Ad Hoc Committee of the Natinoal Kidney Foundation. Am J Kidney Dis 1996;27:162-5.
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560-72.
- Expert consensus guideline series . Optimizing pharmacologic treatment of psychotic disorders. J Clin Psych 2003;64:2-97.
- Renshaw AA, Stelling JM, MH D. The lack of value of repeated Clostiridium difficile cytotoxity assays. Arch Pathol Lab Med 1996;120:49-52.
- Bates DW, Kuperman GJ, Boyle DL, Rittenberg E, Menkin V, Tanasijevic M. Clinical laboratory tests: what proportion are redundant and potentially eliminable using simple computerized rules?. Am J Med 1998;104:261-38.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: develoment and validation. J Chroni Dis 1987;40:373-83.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-19.
- Rush WA, O’Connor PJ, Goodman MJ. Validation of a Modified Charlson Score for Using Health Plan Claims Data n.d.
- Second Joint Task Force of European and Other Societies on Coronary Prevention . Prevention of coronary heart disease in clinical practice. Eur Heart 1998;19:1434-503.
- Lago F, bad Perez JJ, Alverez A. Recomendaciones semFYC-Dislipemias. Barcelona, SemFYC; 1997.
- Cobos A, Jovell AJ, Garcoa A. Which statin is most efficient for the treatment of hypocholesterolemia? A cost-effectivneness analysis. Clin Therapy 1999:1924-36.
- Oblikue Consulting . Base de Datos de Costes Sanitarios. SOIKOS 2009. www.oblikue.com/bddcostes.
- Ali NA, Mekhjian HS, Kuehn PL, Bentley TD, Kumar R, Ferketich AK, et al. Specificity of computerized physician order entry has a significant effect on the efficiency of workflow for critically ill patients. Crit Care Med 2005;33:110-14.
- Beyond guidelines: tool arms physicians with critical knowledge at the point of care . Dis Manag Advis 2001;8:9-12.
- Computerized system alerts docs to costs . Ed Management 1999;11:100-2.
- Anton C, Nightingale PG, Adu D, Lipkin G, Ferner RE. Improving prescribing using a rule based prescribing system. Qual Saf Health Care 2004;13:186-90.
- Augstein P, Vogt L, Kohnert KD, Freyse EJ, Heinke P, Salzsieder E. Outpatient assessment of Karlsburg Diabetes Management System-based decision support. Diabetes Care 2007;30:1704-8.
- Ayanian JZ, Sequist TD, Zaslavsky AM, Johannes RS. Physician reminders to promote surveillance colonoscopy for colorectal adenomas: A randomized controlled trial. J Gen Intern Med 2008;23:762-7.
- Bernstein RM, Hollingworth GR, Wood WE. Prompting physicians for cost-effective test ordering in the low prevalence conditions of family medicine 1994:824-8.
- Berry DL, Wolpin SE, Lober WB, Ellis WJ, Russell KJ, Davison BJ. Actual use and perceived usefulness of a web-based, decision support program for men with prostate cancer. Stud Health Technol Inform 2006;122:781-2.
- Bindels R, Hasman A, van Wersch JW, Talmon J, Winkens RA. Evaluation of an automated test ordering and feedback system for general practitioners in daily practice. Intern J Med Inform 2004;73:705-12.
- Blaser R, Schnabel M, Biber C, Baumlein M, Heger O, Beyer M, et al. Improving pathway compliance and clinician performance by using information technology. Intern J Med Inform 2007;76:151-6.
- Braham RL, Ruchlin HS. Physician practice profiles: a case study of the use of audit and feedback in an ambulatory care group practice. Health Care Manage Rev 1987;12:11-6.
- Cannon-Wagner M, Soule DS, Walker DR, Vance RP. An independent practice association-supported disease management program: Impact on patients with coronary artery disease. Dis Manag Health Outcomes 2002;10:571-7.
- Chambers CV, Balaban DJ, Carlson BL, Ungemack JA, Grasberger DM. Microcomputer-generated reminders. Improving the compliance of primary care physicians with mammography screening guidelines. J Fam Prac 1989;29:273-80.
- Christensen-Szalanski JJ, Diehr PH, Wood RW, Tompkins RK. Phased trial of a proven algorithm at a new primary care clinic. Am J Pub Health 1982;72:16-21.
- Chu S. Non-intrusive guideline-based electronic disease management programme: Principles and evaluation of a pilot. J Inform Technol Healthcare 2004;2:263-80.
- Chu S. Computerised clinical pathway management systems and the implications. Collegian: 2001;8:19-24.
- Colombet I, Bura-Riviere A, Chatila R, Chatellier G, Durieux P, . Personalized versus non-personalized computerized decision support system to increase therapeutic quality control of oral anticoagulant therapy: an alternating time series analysis. BMC Health Serv Res 2004;4.
- Colombet I, Dart T, Leneveut L, Zunino S, Menard J, Chatellier G. Combining risks estimations and clinical practice guidelines in a computer decision aid: a pilot study of the EsPeR system. Stud Health Technol Inform 2003;95:525-30.
- Connelly DP, Sielaff BH, Willard KE. The clinical workstation as a means of improving laboratory use. Clinica Chimica Acta 1996;248:51-64.
- Connelly DP, Willard KE, Hallgren JH, Sielaff BH. Closing the clinical laboratory testing loop with information technology. Am J Clin Pathol 1996;105.
- Connelly DP, Sielaff BH, Willard KE. A clinician’s workstation for improving laboratory use. Integrated display of laboratory results. Am J Clin Pathol 1995;104:243-52.
- Cordero L, Kuehn L, Kumar RR, Mekhjian HS. Impact of computerized physician order entry on clinical practice in a newborn intensive care unit. J Perinatol 2004;24:88-93.
- De Wilde EJL, Pop P, Hasman A, Blom JA. OpenLabs services for ordering laboratory investigations. Comput Methods Programs Biomed 1996;50:135-41.
- Demakis JG, Beauchamp C, Cull WL, Denwood R, Eisen SA, Lofgren R, et al. Improving residents’ compliance with standards of ambulatory care: results from the VA Cooperative Study on Computerized Reminders. JAMA 2000;284:1411-16.
- Emerson JF, Emerson SS. The impact of requisition design on laboratory utilization. Am J Clin Pathol 2001;116:879-84.
- Fihn SD, McDonell MB, Vermes D, Henikoff JG, Martin DC, Callahan CM, et al. A computerized intervention to improve timing of outpatient follow-up: a multicenter randomized trial in patients treated with warfarin. National Consortium of Anticoagulation Clinics. J Gen Internl Med 1994;9:131-9.
- Fitzmaurice DA, Hobbs FD, Murray ET, Holder RL, Allan TF, Rose PE. Oral anticoagulation management in primary care with the use of computerized decision support and near-patient testing: a randomized, controlled trial. Arch Intern Med 2000;160:2343-8.
- Fransen J, Twisk JW, Creemers MC, van Riel PL. Design and analysis of a randomized controlled trial testing the effects of clinical decision support on the management of rheumatoid arthritis. Arthritis Rheum 2004;51:124-7.
- Georgiou A, Williamson M, Westbrook JI, Ray S. The impact of computerised physician order entry systems on pathology services: a systematic review. Intern J Med Inform 2007;76:514-29.
- Glasgow RE, Nutting PA, King DK, Nelson CC, Cutter G, Gaglio B, et al. Randomized effectiveness trial of a computer-assisted intervention to improve diabetes care. Diabetes Care 2005;28:33-9.
- Groopman DS, Powers RD. Effect of ‘standard order’ deletion on emergency department coagulation profile use. Ann Emerg Med 1992;21:524-7.
- Guss DA, Chan TC, Killeen JP. The impact of a pneumatic tube and computerized physician order management on laboratory turnaround time. Ann Emerg Med 2008;51:181-5.
- Harris RP, O’Malley MS, Fletcher SW, Knight BP. Prompting physicians for preventive procedures: a five-year study of manual and computer reminders. Am Journal Prev Med 1990;6:145-52.
- Hasman A, Pop P, Winkens RA, Blom JL. To test or not to test, that is the question. Clinica Chimica Acta 1993;222:49-56.
- Hetlevik I, Holmen J, Kruger O. Implementing clinical guidelines in the treatment of hypertension in general practice. Evaluation of patient outcome related to implementation of a computer-based clinical decision support system. Scand J Prim Health Care 1999;17:35-40.
- Hetlevik I, Holmen J, Kruger O, Kristensen P, Iversen H. Implementing clinical guidelines in the treatment of hypertension in general practice. Blood Press 1998;7:270-6.
- Holleman DR, Simel DL. Effectiveness of automatic diagnostic test result feedback on outpatient laboratory and radiology testing in veterans. A controlled trial. Med Care 1996;34:857-61.
- Kern LM, Barron Y, Blair AJ, Salkowe J, Chambers D, Callahan MA, et al. Electronic result viewing and quality of care in small group practices. J Genl Intern Med 2008;23:405-10.
- Kinney WC. Web-based clinical decision support system for triage of vestibular patients. Otolaryngol Head Neck Surg 2003;128:48-53.
- Koide D, Ohe K, Kitamura K, Kitagawa M, Yoshihara H, Nagase T, et al. System for warning on excessive laboratory tests. Japan J Med Inform 1995;15:217-27.
- Maass MC, Asikainen P, Maenpaa T, Wanne O, Suominen T. Usefulness of a Regional Health Care Information System in primary care: a case study. Comput Methods Programs Biomed 2008;91:175-81.
- Mantha S, Roizen MF, Madduri J, Rajender Y, Shanti NK, Gayatri K. Usefulness of routine preoperative testing: a prospective single-observer study. J Clin Anesth 2005;17:51-7.
- Martens JD, van der AA, Panis B, van der WT, Winkens RA, Severens JL. Design and evaluation of a computer reminder system to improve prescribing behaviour of GPs. Studies Health Technol Inform 2006;124:617-23.
- Modai I, Sigler M. A computerized laboratory alerting system in a psychogeriatric unit. MD Computing 1998;15:95-9.
- Mutimer D, McCauley B, Nightingale P, Ryan M, Peters M, Neuberger J. Computerised protocols for laboratory investigation and their effect on use of medical time and resources. J Clin Pathol 1992;45:572-4.
- Nam HS, Han SW, Ahn SH, Lee JY, Choi HY, Park IC, et al. Improved time intervals by implementation of computerized physician order entry-based stroke team approach. Cerebrovasc Dis 2007;23:289-93.
- Neilson EG, Johnson KB, Rosenbloom ST, Dupont WD, Talbert D, Giuse DA, et al. The impact of peer management on test-ordering behavior. Ann Intern Med 2004;141:196-204.
- Nilasena DS, Lincoln MJ. A Computer-Generated Reminder System Improves Physician Compliance With Diabetes Preventive Care Guidelines 1995:640-5.
- Ornstein SM, Garr DR, Jenkins RG, Musham C, Hamadeh G, Lancaster C. Implementation and evaluation of a computer-based preventive services system. Fam Med 1995;27:260-6.
- Patkar V, Hurt C, Steele R, Love S, Purushotham A, Williams M, et al. Evidence-based guidelines and decision support services: A discussion and evaluation in triple assessment of suspected breast cancer. Br J Cancer 2006;95:1490-6.
- Payne TH, Hoey PJ, Nichol P, Lovis C. Preparation and use of preconstructed orders, order sets, and order menus in a computerized provider order entry system. J Am Med Inform Assoc 2003;10:322-9.
- Perkins J, Ambroson R, Kinsey K. Bring ‘order’ to diabetes management. Nurs Manage 2008;39:24-30.
- Pham DQ, Pham AQ, Ullah E, McFarlane SI, Payne R. Evaluating the appropriateness of thromboprophylaxis in an acute care setting using a computerised reminder, through order-entry system. Int J Clin Pract 2008;62:134-7.
- Piva E, Sciacovelli L, Zaninotto M, Laposata M, Plebani M. Evaluation of effectiveness of a computerized notification system for reporting critical values. Am J Clin Pathol 2009;131:432-41.
- Rosenthal DI, Weilburg JB, Schultz T, Miller JC, Nixon V, Dreyer KJ, et al. Radiology order entry with decision support: initial clinical experience. J Am Coll Radiol 2006;3:799-806.
- Stair TO. Reduction of redundant laboratory orders by access to computerized patient records. J Emerg Med 1998;16:895-7.
- Studnicki J, Bradham DD, Marshburn J, Foulis PR, Straumfjord JV. A feedback system for reducing excessive laboratory tests. Arch Pathol Lab Med 1993;117:35-9.
- Tierney WM, Miller ME, Overhage JM, McDonald CJ. Physician inpatient order writing on microcomputer workstations. Effects on resource utilization. JAMA 1993;269:379-83.
- Valenstein PN, Howanitz PJ. Ordering accuracy: A College of American Pathologists Q-probes study of 577 institutions. Arch Pathol Lab Med 1995;119:117-22.
- van Wijk MA, van der LJ, Mosseveld M, Bohnen AM, van Bemmel JH. Assessment of decision support for blood test ordering in primary care. A randomized trial. Ann Intern Med 2001;134:274-81.
- Vashitz G, Meyer J, Gilutz H. General practitioners’ adherence with clinical reminders for secondary prevention of dyslipidemia. Proceedings of the AMIA Annual Symposium 2007:766-70.
- Aarts J, Berg M. A tale of two hospitals: a sociotechnical appraisal of the introduction of computerized physician order entry in two Dutch hospitals. Medinfo 2004;11:2-1002.
- Ahearn MD, Kerr SJ. General practitioners’ perceptions of the pharmaceutical decision-support tools in their prescribing software. Med J Aust 2003;179:34-7.
- Alberdi E, Gilhooly K, Hunter J, Logie R, Lyon A, McIntosh N, et al. Computerisation and decision making in neonatal intensive care: A cognitive engineering investigation. J Clin Monit Comput 2000;16:85-94.
- Asaro PV, Sheldahl AL, Char DM. Physician perspective on computerized order-sets with embedded guideline information in a commercial emergency department information system 2005;10.
- Ash JS, Sittig DF, Poon EG, Guappone K, Campbell E, Dykstra RH. The extent and importance of unintended consequences related to computerized provider order entry. J Am Med Inform Assoc 2007;14:415-23.
- Ash JS, Gorman PN, Lavelle M, Stavri PZ, Lyman J, Fournier L, et al. Perceptions of physician order entry: results of a cross-site qualitative study. Methods Inf Med 2003;42:313-23.
- Aydin CE, Anderson JG, Rosen PN, Felitti VJ, Weng HC. Computers in the consulting room: a case study of clinician and patient perspectives. Health Care Manag Sci 1998;1:61-74.
- Banet GA, Jeffe DB, Williams JA, Asaro PV. Effects of implementing computerized practitioner order entry and nursing documentation on nursing workflow in an emergency department. J Healthc Inf Manag 2006;20:45-54.
- Bindels R, Hasman A, Derickx M, van Wersch JW, Winkens RA. User satisfaction with a real-time automated feedback system for general practitioners: a quantitative and qualitative study. Int J Qual Health Care 2003;15:501-8.
- Bonnevie L, Thomsen T, Jorgensen T. The use of computerized decision support systems in preventive cardiology-principal results from the national PRECARD survey in Denmark. Eur J Cardiovasc Prev Rehabil 2005;12:52-5.
- Bossen C. Evaluation of a computerized problem-oriented medical record in a hospital department: does it support daily clinical practice?. Int J Med Inform 2007;76:592-600.
- Briggs JS, Fitch CJ. The ISABEL user survey. Med Inform Internet Med 2005;30:167-72.
- Callen JL, Braithwaite J, Westbrook JI. Cultures in hospitals and their influence on attitudes to, and satisfaction with, the use of clinical information systems. Soc Sci Med 2007;65:635-9.
- Campbell EM, Sittig DF, Ash JS, Guappone KP, Dykstra RH. Types of unintended consequences related to computerized provider order entry. J Am Med Inform Assoc 2006;13:547-56.
- Fung CH, Tsai JS, Lulejian A, Glassman P, Patterson E, Doebbeling BN, et al. An evaluation of the Veterans Health Administration’s clinical reminders system: a national survey of generalists. J Gen Intern Med 2008;23:392-8.
- Gandhi TK, Poon EG, Sequist TD, Johnson R, Pizziferri L, Karson AS, et al. Primary care clinician attitudes towards ambulatory computerized physician order entry 2005.
- Grundmeier R, Johnson K. Housestaff attitudes toward computer-based clinical decision support 1999;70.
- Jaspers MWM, Peute LWP, Lauteslager A, Bakker PJM. Pre-post evaluation of physicians’ satisfaction with a redesigned electronic medical record system. Studies Health Technol Inform 2008;136:303-8.
- Kailajarvi M, Takala T, Gronroos P, Tryding N, Viikari J, Irjala K, et al. Reminders of drug effects on laboratory test results. Clin Chem 2000;46:1395-400.
- Krall MA, Sittig DF. Clinician’s assessments of outpatient electronic medical record alert and reminder usability and usefulness requirements 2002;4.
- Krall MA, Sittig DF. Subjective assessment of usefulness and appropriate presentation mode of alerts and reminders in the outpatient setting 2001;8.
- Lee F, Teich JM, Spurr CD, Bates DW. Implementation of physician order entry: user satisfaction and self-reported usage patterns. J Am Med Inform Assoc 1996;3:42-55.
- Murff HJ, Kannry J. Physician satisfaction with two order entry systems. J Am Med Inform Assoc 2001;8:499-50.
- Rapley T, May C, Heaven B, Murtagh M, Graham R, Kaner EF, et al. Doctor–patient interaction in a randomised controlled trial of decision-support tools. Soc Sci Med 2006;62:2267-78.
- Ridderikhoff J, van HB. Who is afraid of the system? Doctors’ attitude towards diagnostic systems. Int J Med Inform 1999;53:91-100.
- Rosenbloom ST, Talbert D, Aronsky D. Clinicians’ perceptions of clinical decision support integrated into computerized provider order entry. Int J Med Inform 2004;73:433-41.
- Short D, Frischer M, Bashford J. Barriers to the adoption of computerised decision support systems in general practice consultations: a qualitative study of GPs’ perspectives. Int J Med Inform 2004;73:357-62.
- Barnett PG, Rodgers JH. Use of the Decision Support System for VA cost-effectiveness research. Med Care 1999;37.
- Ohsfeldt RL, Ward MM, Schneider JE, Jaana M, Miller TR, Lei Y, et al. Implementation of hospital computerized physician order entry systems in a rural state: feasibility and financial impact. J Am Med Inform Assoc 2005;12:20-7.
- Cobos A, Jovell AJ, Garcia-Altes A, Garcia-Closas R, Serra-Majem L. Which statin is most efficient for the treatment of hypocholesterolemia? A cost-effectiveness analysis. Clin Ther 1999;21:1924-36.
- Broughton PMG, Hogan TL. A new approach to the costing of clinical laboratory tests. Ann Clin Biochem 1981;18:330-42.
Appendix 1 Search strategy
CDSS Search strategies
Ovid MEDLINE
Current: Ovid MEDLINE 1950 to July Week 1 2008. Search Date: 9 July 2008. Update 2 April 2009.
| Set number | Search term | Number of hits returned |
|---|---|---|
| 1 | (computer* or microcomputer* or electronic* or automat* or web).tw. | 305,363 |
| 2 | computers/or microcomputers/ | 57,081 |
| 3 | (remind* or alert* or notif*).ti,ab. | 32,570 |
| 4 | (screen* or monitor* or feedback).ti,ab. | 641,672 |
| 5 | ((diagnos$or screen$or monitor$) and (order$or test$or laborator$or endoscop$or imag$)).tw. | 507,094 |
| 6 | (order$and test$).tw. | 80,981 |
| 7 | 1 or 2 | 331,025 |
| 8 | 3 or 4 or 5 or 6 | 1,038,072 |
| 9 | 7 and 8 | 57,217 |
| 10 | 3 or 5 or 6 | 600,044 |
| 11 | exp Unnecessary Procedures/ | 1622 |
| 12 | exp Reminder Systems/ | 1216 |
| 13 | exp Decision Support Techniques/ | 55,089 |
| 14 | decision making, computer-assisted/or diagnosis, computer-assisted/or therapy, computer-assisted/or drug therapy, computer-assisted/ | 20,401 |
| 15 | exp Physician’s Practice Patterns/ | 26,391 |
| 16 | “Laboratory Techniques and Procedures”/sn, ut [Statistics & Numerical Data, Utilization] | 695 |
| 17 | exp Medical Records Systems, Computerized/or Medical Records/ | 43,823 |
| 18 | exp Diagnostic Tests, Routine/ | 4736 |
| 19 | Point-of-Care Systems/ | 3448 |
| 20 | “Diagnostic Techniques and Procedures”/ | 1230 |
| 21 | (decision adj2 support).tw. | 4112 |
| 22 | or/11–21 | 154,807 |
| 23 | 9 and 22 | 6962 |
| 24 | Clinical Laboratory Information Systems/ | 1530 |
| 25 | Decision Support Systems, Clinical/ | 2482 |
| 26 | Medical Order Entry Systems/ | 476 |
| 27 | order$communicat$system$.tw. | 8 |
| 28 | (decision adj2 support adj2 system$).tw. | 1683 |
| 29 | 24 or 25 or 26 or 27 or 28 | 5480 |
| 30 | artificial intelligence/ | 10,687 |
| 31 | “neural networks (computer)”/ | 10,704 |
| 32 | 30 or 31 | 20,047 |
| 33 | 10 and 32 | 2141 |
| 34 | (expert system$or neural network$or artificial intellig$or bayes$).tw. | 23,684 |
| 35 | 10 and 34 | 3075 |
| 36 | (CDSS or OCS or CPOE or (order adj entry)).tw. | 3339 |
| 37 | (diagnos$or screen$or monitor$).tw. | 1,639,950 |
| 38 | 36 and 37 | 506 |
| 39 | (case reports or comment or congresses or editorial or historical article or interview or letter or news).pt. | 2,596,968 |
| 40 | (animals not humans).sh. | 3,235,272 |
| 41 | 39 or 40 | 5,763,459 |
| 42 | 23 or 29 or 33 or 35 or 38 | 15,318 |
| 43 | 42 not 41 | 14,264 |
| 44 | 43 | 14,264 |
| 45 | limit 44 to (english language and yr=“1974 – 2008”) | 12,926 |
| 46 | 35 not 45 | 500 |
EMBASE
17 July 2008 Current: EMBASE 1980 to 2008 Week 28.
-
(computer* or microcomputer* or electronic* or automat* or web).tw.
-
computer/or computer system/or microcomputer/
-
(remind* or alert* or notif*).ti,ab.
-
(screen* or monitor* or feedback).ti,ab.
-
((diagnos$or screen$or monitor$) and (order$or test$or laborator$or endoscop$or imag$)).tw.
-
(order$and test$).tw.
-
1 or 2
-
3 or 4 or 5 or 6
-
7 and 8
-
unnecessary procedure/
-
reminder system/
-
computer assisted diagnosis/
-
computer assisted drug therapy/
-
clinical practice/
-
medical record/
-
laboratory/
-
medical information system/or medical record/
-
hospital information system/
-
electronic medical record/
-
information system/
-
”point of care testing”/
-
diagnostic approach route/
-
diagnostic procedure/
-
computer assisted therapy/
-
medical informatics/
-
medical order/
-
decision making/
-
(decision adj2 support).tw.
-
Feedback System/
-
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
-
9 and 30
-
order$communicat$system$.tw.
-
(decision adj2 support adj2 system$).tw.
-
decision support system/
-
computerized physician order entry/
-
32 or 33 or 34 or 35
-
artificial intelligence/
-
Artificial Neural Network/
-
(expert system$or neural network$or artificial intellig$or bayes$).tw.
-
Expert System/
-
37 or 38 or 39 or 40
-
9 and 41
-
(CDSS or OCS or CPOE or (order adj entry)).tw.
-
(diagnos$or screen$or monitor$).tw.
-
43 and 44
-
31 or 36 or 42 or 45
-
(book or editorial or letter or press or release).pt.
-
((animal or nonhuman) not human).sh.
-
47 or 48
-
46 not 49
-
50
-
limit 51 to (english language and yr=“1974 – 2008”)
Total pr-dedupluication = 8928
CINAHL
20 July 2008 CINAHL – 1982 to date (NAHL)
DIALOG DATASTAR web version
| Set number | Search term | Number of hits returned | ||
|---|---|---|---|---|
| 1 | CINAHL – 1982 to date | (computer$4 OR microcomputer$4 OR electronic$4 OR automat$4 OR web).TI,AB. | unrestricted | 39,999 |
| 2 | CINAHL – 1982 to date | Computers-and-Computerization.DE. | unrestricted | 4232 |
| 3 | CINAHL – 1982 to date | Microcomputers.W..DE. | unrestricted | 944 |
| 4 | CINAHL – 1982 to date | (remind$4 OR alert$4 OR notif$4).TI,AB. | unrestricted | 6097 |
| 5 | CINAHL – 1982 to date | (screen$4 OR monitor$4 OR feedback).TI,AB. | unrestricted | 57,895 |
| 6 | CINAHL – 1982 to date | (diagnos$4 OR screen$4 OR monitor$4).TW. AND (order$4 OR test$4 OR laborator$4 OR endoscop$4 OR imag$4).TW. | unrestricted | 161,729 |
| 9 | CINAHL – 1982 to date | order$4.TI,AB. AND test$4.TI,AB. | unrestricted | 4842 |
| 10 | CINAHL – 1982 to date | 1 OR 2 OR 3 | unrestricted | 42,332 |
| 11 | CINAHL – 1982 to date | 4 OR 5 OR 6 OR 9 | unrestricted | 199,115 |
| 12 | CINAHL – 1982 to date | 10 AND 11 | unrestricted | 8748 |
| 13 | CINAHL – 1982 to date | Reminder-Systems.DE. | unrestricted | 622 |
| 14 | CINAHL – 1982 to date | Decision-Making-Computer-Assisted.DE. OR Diagnosis-Computer-Assisted.DE. OR Therapy-Computer-Assisted.DE. OR Drug-Therapy-Computer-Assisted.DE. | unrestricted | 2687 |
| 15 | CINAHL – 1982 to date | Computers-and-Computerization.DE. | unrestricted | 4232 |
| 17 | CINAHL – 1982 to date | 15 | various | 3557 |
| 19 | CINAHL – 1982 to date | 15 NOT 17 | various | 675 |
| 20 | CINAHL – 1982 to date | Management-Information-Systems#.DE. | unrestricted | 1257 |
| 21 | CINAHL – 1982 to date | Clinical-Information-Systems#.DE. | unrestricted | 9766 |
| 22 | CINAHL – 1982 to date | Diagnostic-Tests-Routine.DE. | unrestricted | 528 |
| 24 | CINAHL – 1982 to date | (decision$2 ADJ support$2).TI,AB. | unrestricted | 885 |
| 25 | CINAHL – 1982 to date | Patient-Record-Systems#.DE. | unrestricted | 5325 |
| 26 | CINAHL – 1982 to date | Artificial-Intelligence#.DE. | unrestricted | 1771 |
| 27 | CINAHL – 1982 to date | Neural-Networks-Computer#.DE. | unrestricted | 210 |
| 29 | CINAHL – 1982 to date | (expert ADJ system$2 OR neural ADJ network$2 OR artificial ADJ intellig$5 OR bayes$4).TI,AB. | unrestricted | 815 |
| 30 | CINAHL – 1982 to date | 13 OR 14 OR 19 OR 20 OR 21 OR 22 OR 24 OR 25 OR 26 OR 27 OR 29 | various | 17,394 |
| 31 | CINAHL – 1982 to date | 12 AND 30 | various | 1571 |
| 32 | CINAHL – 1982 to date | Electronic-Order-Entry.DE. | unrestricted | 637 |
| 33 | CINAHL – 1982 to date | Decision-Support-Systems-Clinical.DE. | unrestricted | 707 |
| 34 | CINAHL – 1982 to date | (order$2 ADJ communicat$5 ADJ system$2).TI,AB. | unrestricted | 2 |
| 35 | CINAHL – 1982 to date | (decision$2 ADJ support$2 ADJ system$2).TI,AB. | unrestricted | 306 |
| 36 | CINAHL – 1982 to date | 32 OR 33 OR 34 OR 35 | unrestricted | 1434 |
| 37 | CINAHL – 1982 to date | (CDSS OR OCS OR CPOE OR order ADJ entry).TI,AB. | unrestricted | 732 |
| 38 | CINAHL – 1982 to date | (diagnos$4 OR screen$4 OR monitor$4).TI,AB. | unrestricted | 125,575 |
| 39 | CINAHL – 1982 to date | 37 AND 38 | unrestricted | 77 |
| 40 | CINAHL – 1982 to date | 31 OR 39 | various | 1620 |
| 41 | CINAHL – 1982 to date | PT=CASE-STUDY OR PT=CEU OR PT=COMMENTARY OR PT=EDITORIAL OR PT=LETTER | unrestricted | 323,950 |
| 42 | CINAHL – 1982 to date | 40 NOT 41 | various | 1471 |
| 1. | SEARCH: | (computer$4 OR microcomputer$4 OR electronic$4 OR automat$4 OR web).TI,AB. | ||
| 2. | SEARCH: | Computers-and-Computerization.DE. | ||
| 3. | SEARCH: | Microcomputers.W..DE. | ||
| 4. | SEARCH: | (remind$4 OR alert$4 OR notif$4).TI,AB. | ||
| 5. | SEARCH: | (screen$4 OR monitor$4 OR feedback).TI,AB. | ||
| 6. | SEARCH: | (diagnos$4 OR screen$4 OR monitor$4).TW. AND (order$4 OR test$4 OR laborator$4 OR endoscop$4 OR imag$4).TW. | ||
| 7. | SEARCH: | order$4.TI,AB. AND test$4.TI,AB. | ||
| 8. | SEARCH: | 1 OR 2 OR 3 | ||
| 9. | SEARCH: | 4 OR 5 OR 6 OR 7 | ||
| 10. | SEARCH: | 8 AND 9 | ||
| 11. | SEARCH: | Reminder-Systems.DE. | ||
| 12. | SEARCH: | Decision-Making-Computer-Assisted.DE. OR Diagnosis-Computer-Assisted.DE. OR Therapy-Computer-Assisted.DE. OR Drug-Therapy-Computer-Assisted.DE. | ||
| 13. | SEARCH: | Computers-and-Computerization.DE. | ||
| 14. | SEARCH: | 13 (restricted to 1987-current) | ||
| 15. | SEARCH: | 13 NOT 14 | ||
| 16. | SEARCH: | Management-Information-Systems#.DE. | ||
| 17. | SEARCH: | Clinical-Information-Systems#.DE. | ||
| 18. | SEARCH: | Diagnostic-Tests-Routine.DE. | ||
| 19. | SEARCH: | (decision$2 ADJ support$2).TI,AB. | ||
| 20. | SEARCH: | Patient-Record-Systems#.DE. | ||
| 21. | SEARCH: | Artificial-Intelligence#.DE. | ||
| 22. | SEARCH: | Neural-Networks-Computer#.DE. | ||
| 23. | SEARCH: | (expert ADJ system$2 OR neural ADJ network$2 OR artificial ADJ intellig$5 OR bayes$4).TI,AB. | ||
| 24. | SEARCH: | 11 OR 12 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 | ||
| 25. | SEARCH: | 10 AND 24 | ||
| 26. | SEARCH: | Electronic-Order-Entry.DE. | ||
| 27. | SEARCH: | Decision-Support-Systems-Clinical.DE. | ||
| 28. | SEARCH: | (order$2 ADJ communicat$5 ADJ system$2).TI,AB. | ||
| 29. | SEARCH: | (decision$2 ADJ support$2 ADJ system$2).TI,AB. | ||
| 30. | SEARCH: | 26 OR 27 OR 28 OR 29 | ||
| 31. | SEARCH: | (CDSS OR OCS OR CPOE OR order ADJ entry).TI,AB. | ||
| 32. | SEARCH: | (diagnos$4 OR screen$4 OR monitor$4).TI,AB. | ||
| 33. | SEARCH: | 31 AND 32 | ||
| 34. | SEARCH: | 25 OR 33 | ||
| 35. | SEARCH: | PT=CASE-STUDY OR PT=CEU OR PT=COMMENTARY OR PT=EDITORIAL OR PT=LETTER | ||
| 36. | SEARCH: |
34 NOT 35 TOTAL CINAHL pre de-dup: 1471 |
||
Cochrane Library
Cochrane Library Edition 2008:3 – online
Date searched: 20 July 2008
NOTE: search excluded EMBASE and Pubmed references as searched separately
Total: SRs: 420
CENTRAL: 540
HTA: 150
NHS EED: 103
DARE: 22
| Set number | Search term | Number of hits returned |
|---|---|---|
| #1 | (computer* or microcomputer* or electronic* or automat* or web):ti,ab | 12,133 |
| #2 | MeSH descriptor Computers explode all trees | 717 |
| #3 | (remind* or alert* or notif*):ti,ab | 2559 |
| #4 | (screen* or monitor* or feedback):ti,ab | 33,706 |
| #5 | ((diagnos* or screen* or monitor*) and (order* or test* or laborator* or endoscop* or imag*)):ti,ab | 19,057 |
| #6 | (order* and test*):ti,ab | 6492 |
| #7 | (#1 OR #2) | 12,413 |
| #8 | (#3 OR #4 OR #5 OR #6) | 49,173 |
| #9 | (#7 AND #8) | 3291 |
| #10 | (#3 OR #5 OR #6) | 26,784 |
| #11 | MeSH descriptor Unnecessary Procedures explode all trees | 78 |
| #12 | MeSH descriptor Reminder Systems explode all trees | 360 |
| #13 | MeSH descriptor Decision Support Techniques explode all trees | 2981 |
| #14 | MeSH descriptor Decision Making, Computer-Assisted explode all trees | 2021 |
| #15 | MeSH descriptor Drug Therapy, Computer-Assisted explode all trees | 101 |
| #16 | MeSH descriptor Physician’s Practice Patterns explode all trees | 996 |
| #17 | MeSH descriptor Laboratory Techniques and Procedures explode all trees with qualifier: SN | 175 |
| #18 | MeSH descriptor Medical Records Systems, Computerized explode all trees | 190 |
| #19 | MeSH descriptor Medical Records explode all trees | 1490 |
| #20 | MeSH descriptor Diagnostic Tests, Routine explode all trees | 231 |
| #21 | MeSH descriptor Point-of-Care Systems explode all trees | 192 |
| #22 | MeSH descriptor Diagnostic Techniques and Procedures, this term only | 78 |
| #23 | (decision support):ti,ab | 728 |
| #24 | (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23) | 8756 |
| #25 | (#9 AND #24) | 476 |
| #26 | (order* communicat* system*):ti,ab | 40 |
| #27 | (expert system* or neural network* or artificial intellig* or bayes*):ti,ab | 1255 |
| #28 | (CDSS or OCS or CPOE or (order entry)):ti,ab | 457 |
| #29 | (#9 OR #23 OR #25 OR #26 OR #27 OR #28) | 5528 |
| #30 | “accession number” NEAR pubmed | 316,701 |
| #31 | “accession number” near2 embase | 51,982 |
| #32 | (#30 OR #31) | 368,683 |
| #33 | (#29 AND NOT #32) |
EconLit (EconLit)
EconLit (EconLit)
Search Date: 23 July 2008
Via: First Search, 1969 to present
Monthly; Last update: 18 July 2008
| Set number | Search term | Number of hits returned |
|---|---|---|
| #1 | de: computers or de: computer and ln= “english” | 7809 |
| #2 | kw: decision and kw: support and kw: systems and ln= “english” | 384 |
| #3 | su= “Introductory Material” | 2089 |
| #4 | ti: remind* or su= “alert* or notif*” or ab: remind* or su= “alert* or notif*” | 193 |
| #5 | ti: remind* or su= “alert* or notif*” or ab: remind* or su= “alert* or notif*” | 193 |
| #6 | ti: screen* or su= “monitor* or feedback” and ab: screen* or su= “monitor* or feedback” | 225 |
| #7 | (ti: diagnos* or ti: screen* or ti: monitor*) and (su= “order* or test* or laborator* or endoscop* or imag*”) and (ab: diagnos* or ab: screen* or ab: monitor*) and (su= “order* or test* or laborator* or endoscop* or imag*”) | 0 |
| #8 | (ti: diagnos* or ti: screen* or ti: monitor*) and (ti: order* or ti: test* or ti: laborator* or ti: endoscop* or ti: imag*) and (ab: diagnos* or ab: screen* or ab: monitor*) and (ab: order* or ab: test* or ab: laborator* or ab: endoscop* or ab: imag*) | 38 |
| #9 | (ti: order* and ti: test*) or (ab: order* and ab: test*) | 2554 |
| #10 | (ti: remind* or su= “alert* or notif*” or ab: remind* or su= “alert* or notif*”) or (ti: remind* or su= “alert* or notif*” or ab: remind* or su= “alert* or notif*”) or (ti: screen* or su= “monitor* or feedback” and ab: screen* or su= “monitor* or feedback”) or ((ti: diagnos* or ti: screen* or ti: monitor*) and (ti: order* or ti: test* or ti: laborator* or ti: endoscop* or ti: imag*) and (ab: diagnos* or ab: screen* or ab: monitor*) and (ab: order* or ab: test* or ab: laborator* or ab: endoscop* or ab: imag*)) or ((ti: order* and ti: test*) or (ab: order* and ab: test*)) | 2991 |
| #11 | (de: computers or de: computer and ln= “english”) and ((ti: remind* or su= “alert* or notif*” or ab: remind* or su= “alert* or notif*”) or (ti: remind* or su= “alert* or notif*” or ab: remind* or su= “alert* or notif*”) or (ti: screen* or su= “monitor* or feedback” and ab: screen* or su= “monitor* or feedback”) or ((ti: diagnos* or ti: screen* or ti: monitor*) and (ti: order* or ti: test* or ti: laborator* or ti: endoscop* or ti: imag*) and (ab: diagnos* or ab: screen* or ab: monitor*) and (ab: order* or ab: test* or ab: laborator* or ab: endoscop* or ab: imag*)) or ((ti: order* and ti: test*) or (ab: order* and ab: test*))) | 17 |
| #12 | ti: comput* or ab: comput* | 13,200 |
| #13 | (ti: remind* or su= “alert* or notif*” or ab: remind* or su= “alert* or notif*”) or (ti: remind* or su= “alert* or notif*” or ab: remind* or su= “alert* or notif*”) or (ti: screen* or su= “monitor* or feedback” and ab: screen* or su= “monitor* or feedback”) or ((ti: diagnos* or ti: screen* or ti: monitor*) and (ti: order* or ti: test* or ti: laborator* or ti: endoscop* or ti: imag*) and (ab: diagnos* or ab: screen* or ab: monitor*) and (ab: order* or ab: test* or ab: laborator* or ab: endoscop* or ab: imag*)) or ((ti: order* and ti: test*) or (ab: order* and ab: test*)) and (ti: comput* or ab: comput*) | 158 |
| #14 | (de: computers or de: computer and ln= “english”) and ((ti: remind* or su= “alert* or notif*” or ab: remind* or su= “alert* or notif*”) or (ti: remind* or su= “alert* or notif*” or ab: remind* or su= “alert* or notif*”) or (ti: screen* or su= “monitor* or feedback” and ab: screen* or su= “monitor* or feedback”) or ((ti: diagnos* or ti: screen* or ti: monitor*) and (ti: order* or ti: test* or ti: laborator* or ti: endoscop* or ti: imag*) and (ab: diagnos* or ab: screen* or ab: monitor*) and (ab: order* or ab: test* or ab: laborator* or ab: endoscop* or ab: imag*)) or ((ti: order* and ti: test*) or (ab: order* and ab: test*))) or ((ti: remind* or su= “alert* or notif*” or ab: remind* or su= “alert* or notif*”) or (ti: remind* or su= “alert* or notif*” or ab: remind* or su= “alert* or notif*”) or (ti: screen* or su= “monitor* or feedback” and ab: screen* or su= “monitor* or feedback”) or ((ti: diagnos* or ti: screen* or ti: monitor*) and (ti: order* or ti: test* or ti: laborator* or ti: endoscop* or ti: imag*) and (ab: diagnos* or ab: screen* or ab: monitor*) and (ab: order* or ab: test* or ab: laborator* or ab: endoscop* or ab: imag*)) or ((ti: order* and ti: test*) or (ab: order* and ab: test*)) and (ti: comput* or ab: comput*)) | 170 |
| #15 | ti: decsion* n3 support* or ab: decsion* n3 support* or kw: decsion* n3 support* | 0 |
| #16 | ti: decision* n3 support* or ab: decision* n3 support* or kw: decision* n3 support* | 936 |
| #17 | (ti: decision* n3 support* or ab: decision* n3 support* or kw: decision* n3 support*) AND (ti: medical* or ab: medical* or kw: medical*) | 11 |
| #18 | (ti: decision* n3 support* or ab: decision* n3 support* or kw: decision* n3 support*) AND su= “hospitals” | 0 |
| #19 | (ti: decision* n3 support* or ab: decision* n3 support* or kw: decision* n3 support*) AND kw: hospital | 15 |
| #20 | (ti: decision* n3 support* or ab: decision* n3 support* or kw: decision* n3 support*) AND (ti: medical* or ab: medical* or kw: medical*) or ((ti: decision* n3 support* or ab: decision* n3 support* or kw: decision* n3 support*) AND kw: hospital) | 21 |
| #21 | (ab: CDSS or ab: OCS or ab: CPOE or ab: order w entry) or (ti: CDSS or ti: OCS or ti: CPOE or ti: order w entry) | 59 |
| #22 | ab: CDSS | 12 |
| #23 | (ab: point and ab: care) and de: hospital | 11 |
Appendix 2 Excluded studies
Study question 2: What is the impact of CDSS in OCS for diagnostic, screening or monitoring test ordering compared to order communication systems without CDSS on process and patient outcomes?
| Study ID | Reason for exclusion |
|---|---|
| Ali (2005)128 | No test order outcomes |
| Anonymous (2002)129 | No OCS alone at baseline |
| Anonymous (1999)130 | No OCS alone at baseline |
| Anton (2009)131 | Overview of system; no outcome data reported |
| Augstein (2007)132 | No OCS alone (continuous glucose monitoring system versus monitoring system plus CDSS); no test outcomes |
| Ayanian (2008)133 | No CDSS or OCS |
| Bernstein (1994)134 | No CDSS; no evaluation; discussion of development of system |
| Berry (2006)135 | No CDSS; cross sectional survey |
| Bindels (2004)136 | OCS + CDSS in both groups; randomised to provide recommendations for different test ordering between the groups |
| Blaser (2006)137 | Discussion article (no evaluation) |
| Braham (1987)138 | No CDSS; audit and feedback of test costs to physicians for tests ordered; aggregate data only |
| Cannon-Wagner (2002)139 | No CDSS; no test ordering outcomes |
| Chambers (1989)140 | No CDSS |
| Christensen-Szalanski (1982)141 | Comparator not OCS alone |
| Chu (2004)142 | No CDSS; no OCS alone at baseline; system not used for test ordering |
| Chu (2001)143 | Discussion article on CDSS and OCS (no evaluation) |
| Colombet (2004)144 | Discussion article on development of system and study design for evaluation (no evaluation reported) |
| Colombet (2003)145 | Focus group conducted on CDSS using scenarios in a non-clinical setting |
| Connelly (1996)146 | No OCS alone comparator; audit |
| Connelly (1996)147 | Discussion article; no evaluation |
| Connelly (1995)148 | OCS + CDSS compared with no OCS at baseline |
| Cordero (2004)149 | Evaluates implementation of OCS + CDSS versus no OCS |
| de Wilde (1996)150 | No OCS at baseline; all orders entered on paper |
| Demakis (2000)151 | Comparator not OCS alone; both groups CDSS |
| Emerson (2001)152 | No CDSS |
| Fihn (1994)153 | No CDSS; no test outcomes (scheduling of return visits) |
| Fitzmaurice (2000)154 | Comparator not OCS alone |
| Fordham (1990)67 | CDSS + OCS not compared to CDSS alone; system is a manual audit for producing reminders |
| Fransen (2004)155 | No evaluation |
| Georgiou (2007)156 | Whole system analysis; not just CDSS in OCS versus OCS alone |
| Glasgow (2005)157 | No CDSS |
| Groopman (1992)158 | No CDSS (OCS alone) |
| Guss (2008)159 | No CDSS |
| Harpole (1997)54 | CDSS plus CS (standard) versus CDSS plus OCS (amended); no OCS alone comparator |
| Harris (1990)160 | No CDSS |
| Hasman (1993)161 | No evaluation of CDSS |
| Hetlevik (1999)162 | Not test ordering |
| Hetlevik (1998)163 | CDSS not compared with OCS alone |
| Holleman (1996)164 | No CDSS; health summaries and test results created manually pre-patient visit |
| Hwang (2002)3 | No CDSS just OCS |
| Kern (2007)165 | No CDSS; not compared to OCS alone; cross-sectional study |
| Kinney (2003)166 | Diagnostic accuracy study |
| Koide (1995)167 | Japanese language article (abstract only in English) |
| Kuperman (1999)52 | Comparator not OCS alone |
| Litzelman (1993)47 | CDSS in OCS requiring response to alert from clinicians versus CDSS in OCS not requiring response (CDSS versus CDSS) |
| Lobach (1996)40 | Effect of an audit programme for monitoring physician guideline adherence rates. Data feedback on aggregate patient group not individual patients |
| Maass (2008)168 | CDSS plus OCS versus OCS alone not compared |
| Mantha (2005)169 | Cross sectional study (no pre, just post) |
| Martens (2006)170 | No CDSS; no test outcomes (prescribing behaviour) |
| McPhee (1989)65 | No CDSS all patient records searched and audit |
| McPhee (1991)27 | CDSS with OCS not compared to OCS alone |
| Modai (1998)171 | CDSS implemented at the same time as OCS; no pre-implementation data reported |
| Mutimer (1992)172 | No OCS in pre-implementation phase |
| Nam (2007)173 | OCS alone no CDSS |
| Neilson (2004)174 | No CDSS |
| Nicholls (2008)51 | No CDSS |
| Nilasena (1995)175 | Comparator not OCS alone; outcomes for laboratory test/referral rates are pooled between the two groups and analysed as a post intervention change from baseline |
| Ornstein (1995)176 | No CDSS; audit of preventive care services (including screening) appropriate to age and gender, only aggregate data reported |
| Patkar (2006)177 | CDSS used on hypothetical cases |
| Payne (2003)178 | No CDSS (OCS alone) |
| Perkins (2008)179 | Discussion article |
| Pham (2008)180 | No CDSS: not test ordering |
| Piva (2009)181 | No CDSS: not test ordering |
| Rosenthal (2006)182 | Only frequency of system use reported pre- and post-addition of CDSS |
| Stair (1998)183 | No pre-and post-assessment |
| Studnicki (1993)184 | No CDSS |
| Subramanian (2004)102 | CDSS versus CDSS; no OCS comparator |
| Thompson (2004)68 | No CDSS just OCS alone |
| Tierney (1993)185 | CDSS + OCS compared to no OCS |
| Valenstein (1995)186 | Survey on accuracy of tests orders transmitted to the laboratory |
| van Wijk (2001)187 | Compares two different CDSS + OCS with each other |
| Vashitz (2007)188 | Not CDSS with OCS; reminders sent to physicians via a hard copy every 4 months |
Study question 3: What features of CDSS are associated with clinician or patient acceptance of CDSS in order communication systems?
| Study ID | Reason for exclusion |
|---|---|
| Aarts (2004)189 | Not test ordering (prescribing); no acceptability outcome measure |
| Ahearn (2003)190 | No CDSS; no acceptability outcome measure |
| Alberdi (2000)191 | Not test ordering; no CDSS; no acceptability outcome measures |
| Asaro (2005)192 | No CDSS; no acceptability outcome measure |
| Ash (2007)193 | Not test ordering |
| Ash (2003)194 | Not test ordering |
| Aydin (1998)195 | OCS not CDSS |
| Banet (2006)196 | OCS + CDSS in both groups; randomised to provide recommendations for different test ordering between the groups therefore no evaluation of OCS alone at baseline |
| Bindels (2003)197 | CDSS not used for test ordering; no acceptability outcomes; no evaluation |
| Bonnevie (2005)198 | No acceptability outcome measure |
| Bossen (2007)199 | Not CDSS within OCS (stand alone internet CDSS – ISABEL); no acceptability outcomes |
| Briggs (2005)200 | OCS not CDSS |
| Callen (2007)201 | No acceptability outcomes; not evaluation of implementation of CDSS |
| Campbell (2006)202 | No acceptability rates reported |
| Doolan (2003)7 | No acceptability rates reported |
| Fung (2008)203 | OCS without CDSS; orders for laboratory tests completed using paper forms |
| Gandhi (2005)204 | No acceptability rates reported |
| Grundmeier (1999)205 | Attitudes towards future addition of CDSS to OCS |
| Jaspers (2008)206 | CDSS in OCS not used for test ordering; no acceptability outcomes |
| Kailajarvi (2000)207 | No CDSS; focus groups used to discuss general usefulness of alerts/reminders (not test ordering specific) |
| Krall (2002)208 | No CDSS; not used for test ordering; no acceptability outcomes |
| Krall (2001)209 | OCS not CDSS |
| Lee (1996)210 | Not test ordering |
| Martens (2006)170 | Not test ordering |
| Murff (2001)211 | No test ordering results, evaluation on number of preventive care recommendations flagged. |
| Nilasena (1995)175 | No test ordering results, evaluation on number of preventive care recommendations |
| Rapley (2005)212 | Not conducted in a clinical setting; diagnostic accuracy outcomes |
| Ridderikhoff (1999)213 | No acceptability outcomes |
| Rosenbloom (2004)214 | Not test ordering; no acceptability outcome measure |
| Short (2004)215 | CDSS in OCS not used for test ordering; no acceptability outcomes |
| Sittig (2006)105 | CDSS in OCS not used for test ordering; no acceptability outcomes |
Study question 4: What is known about the cost-effectiveness of CDSS in diagnostic, screening or monitoring test order communication systems compared to order communication systems without CDSS?
Appendix 3 Data extraction tables
Studies assessing the display of test charges (n = 3)
| Study demographics | ||||
|---|---|---|---|---|
| Author; year; study ID: Tierney (1990)86 | ||||
| Title: The effect on test ordering of informing physicians of the charges for outpatient diagnostic tests | ||||
| Country: USA | ||||
| Specific setting: General Medicine Practice of the Regenstrief Health Center, Indianapolis, IN, USA (The centre is the primary outpatient facility for Wishard Memorial Hospital) and provides primary care to more than 12,000 patients | ||||
| Study objectives: To assess the effects of informing physicians of the charges for outpatient diagnostic tests on test ordering practices | ||||
| Health-care setting: Primary care | ||||
| If secondary care, academic status of the hospital: Academic Primary Care Centre | ||||
| Health-care system: USA (Medicare; Medicaid; commercial insurance; Hospital’s programme for indigent patients; no health insurance coverage) | ||||
| Study design: RCT (14-week pre-intervention period; 26-week intervention period; 19-week post-intervention period) | ||||
| Number of sites: 1 | ||||
| Funding source: Public sector | ||||
| Was evaluator of tool also its developer? Unclear | ||||
| System users | ||||
| Intervention group | Control group | |||
| CDSS user(s) | Physicians | Physicians | ||
| Pre-intervention period (14 weeks) | ||||
| Physicians (n) | 62 | 59 | ||
| Residents (n) | 51 | 48 | ||
| Faculty (n) | 11 | 11 | ||
| Intervention period (26 weeks) | ||||
| Physicians (n) | 62 | 59 | ||
| Residents (n) | 51 | 48 | ||
| Faculty (n) | 11 | 11 | ||
| Post-intervention period (19 weeks) | ||||
| Physicians (n) | 39 | 35 | ||
| Residents (n) | 32 | 26 | ||
| Faculty (n) | 7 | 9 | ||
| Practitioners (n) in analysis: | ||||
| Pre-intervention period | 121 | |||
| Intervention period | 74 | |||
| Post-intervention period | 74 | |||
| Inclusion criteria: Physicians using OCS with or without CDSS for laboratory or radiological test ordering for all outpatient visits within the study period | ||||
| Exclusion criteria: NR | ||||
| Patient baseline demographicsa | ||||
| Inclusion criteria: All outpatients attending for either scheduled or unscheduled visits at the practice within the study period | ||||
| Exclusion criteria: NR | ||||
| Intervention group | Control group | |||
| Pre-intervention period (14 weeks) | ||||
| N | 3511 | 3362 | ||
| Visits (n) | 5229 | 5040 | ||
| Intervention period (26 weeks) | ||||
| N | 4254 | 4138 | ||
| Visits (n) | 7800 | 7457 | ||
| Post-intervention period (19 weeks) | ||||
| N | 2784 | 2806 | ||
| Visits (n) | 4461 | 4259 | ||
| a No further baseline details were reported on study participants. | ||||
| Interventions | ||||
|
Intervention: OCS plus CDSS (display of test charges). The computer displayed the charge the patient (or insurer) would pay for the current test and total charges for all tests ordered for the patient on that day. Physicians were then given the option of cancelling the test(s) or continuing with the order. The charges displayed were the hospital’s current charges to the patients or their insurance carriers; additional fees (e.g. for interpretation of the roentrogenograms) were not included. Tests included all laboratory and imaging studies; 24-hour electrocardiographic monitoring tests, treadmill exercise testing, and endoscopic procedures were excluded During the pre-intervention period and post-intervention period no messages about test charges appeared when tests were ordered Comparator: OCS alone (no display of test charges) Concomitant interventions: NA |
||||
| CDSS tool | ||||
| Name of CDDS (if any) | NR | |||
| CDSS reasoning method | NR | |||
| CDSS knowledge base | Hospital test costs | |||
| Did study use training set and test set data? |
Training set: NR Test set: NR |
|||
| For training set data complete the following: | ||||
| Design | NR | |||
| Target decision | NR | |||
| Sample characteristics | Intervention | Control | ||
| Sample size (n) | NR | NR | ||
| Age (mean; range) | NR | NR | ||
| Gender (n male; n female) | NR | NR | ||
| Disease type | NR | NR | ||
| For test set data complete the following: | ||||
| Properties of test set data | NR | |||
| Test centre | NR | |||
| Target decision | NR | |||
| Other CDSS information | ||||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | NR; costs of biochemical and radiology tests | |||
| 2. Time to complete the CDSS (minutes) | NR | |||
| 3. CDSS output format: (score; probability graph; advice, etc). | Display of test costs | |||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | |||
| 5. Is user instructional training at the time of implementation described? | No | |||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | |||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | |||
| 8. Is there a need for additional data entry by the clinician? | No | |||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | |||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | |||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | |||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | No | |||
| 13. Does the CDSS promote action rather than inaction? | NR | |||
| 14. Does the CDSS justify the output by provision of reasoning? | No | |||
| 15. Does the CDSS justify the output by provision of research evidence? | No | |||
| 16. Were local users involved in the CDSS development process? | No | |||
| 17. Is the CDSS output provided to patients as well as clinician? | No | |||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | |||
| 19. Is the CDSS used in conjunction with conventional education? | No | |||
| Outcome measures | ||||
| Outcome 1: Mean number of tests ordered per visit | ||||
| Outcome 2: Mean charges for tests ordered per visit | ||||
| Outcome 3: Mean number of tests ordered per scheduled visit | ||||
| Outcome 4: Mean number of tests ordered per unscheduled visit | ||||
| Outcome 5: Mean charges for tests ordered per scheduled visit | ||||
| Outcome 6: Mean charges for tests ordered per unscheduled visit | ||||
| Outcome 7: Mean number of tests ordered per visit in post-intervention period | ||||
| Outcome 8: Mean charges for tests ordered per visit in post-intervention period | ||||
| Outcome 9: Number of hospitalisations per patient in the intervention and post-intervention period | ||||
| Outcome 10: Number of emergency room visits per patient in the intervention and post-intervention period | ||||
| Outcome 11: Number of outpatient visits per patient in the intervention and post-intervention period | ||||
| Total length of follow-up: 40 weeks; 26 weeks for the intervention period (14 weeks pre-intervention period and 26 weeks post-intervention period) | ||||
| Follow-up assessment times: Baseline (end of pre-intervention period); 26 weeks (post-intervention); and 52 weeks (end of post-intervention period) | ||||
| Rate of attrition at each follow-up time: Baseline (end of pre-intervention period): 4/125 (n = 121; 3.2% attrition); end of intervention period: 51/125 (n = 74; 40.8% attrition); end of post-intervention period: 51/125 (n = 74; 40.8% attrition) | ||||
| Methods of statistical analysis: For each physician the mean number of tests ordered and the mean charge for tests per patient visit was calculated separately for each study period (pre-intervention, intervention, and post-intervention). The analysis was weighted by the reciprocal of the sum of the variance within and between physicians. Weighted analysis of covariance was used to compare differences between groups in the intervention period, with each physician’s pre-intervention mean entered as a covariate. All two-tailed p-values < 0.05 were considered statistically significant. Rates of hospitalisations, emergency room visits and outpatient visits were compared using the Wilcoxon rank-sum test | ||||
| Results: mean ± SD | ||||
| Outcome 1: Mean number of tests ordered per visit | ||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | % difference | p-value |
| All physicians | 1.56 ± 0.72 | 1.82 ± 0.90 | –14.3% | p < 0.005 |
| Residents | 1.60 ± 0.73 | 1.89 ± 0.93 | –15.3% | p < 0.005 |
| Faculty | 1.39 ± 0.64 | 1.51 ± 0.71 | –7.9% | p > 0.05 |
| Outcome 2: Mean charges for tests ordered per visit | ||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | % difference | p-value |
| All physicians | 45.13 ± 21.98 | 51.81 ± 21.99 | –12.9% | p < 0.05 |
| Residents | 45.90 ± 21.9 | 52.99 ± 22.21 | –13.4% | p < 0.05 |
| Faculty | 41.84 ± 23.01 | 47.12 ± 21.28 | –11.2% | p > 0.05 |
| Outcome 3: Mean number of tests ordered per scheduled visit | ||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | % difference | p-value |
| All physicians | 1.59 ± 0.75 | 1.91 ± 0.94 | –16.8% | p < 0.01 |
| Outcome 4: Mean number of tests ordered per unscheduled visit | ||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | % difference | p-value |
| All physicians | 1.17 ± 0.67 | 1.32 ± 0.94 | –11.4% | p < 0.05 |
| Outcome 5: Mean charges for tests ordered per scheduled visit | ||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | % difference | p-value |
| All physicians | 45.26 ± 22.79 | 53.43 ± 22.97 | –15.3% | p < 0.01 |
| Outcome 6: Mean charges for tests ordered per unscheduled visit | ||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | % difference | p-value |
| All physicians | 35.55 ± 21.32 | 39.38 ± 25.10 | –9.7% | p < 0.05 |
| Outcome 7: Mean number of tests ordered per visit in post-intervention period | ||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | % difference | p-value |
| All physicians | 1.67 ± 0.77 | 1.81 ± 0.9 | –7.7% | p > 0.05 |
| Outcome 8: Mean charges for tests ordered per visit in post-intervention period | ||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | % difference | p-value |
| All physicians | 48.28 ± 22.55 | 50.04 ± 21.73 | –3.5% | p > 0.05 |
| Outcome 9: Number of hospitalisations per patient in the intervention and post-intervention period | ||||
| Intervention | OCS + CDSS | OCS alone | p-value | |
| Number of hospitalisations/patient | 0.19 ± 0.62 | 0.17 ± 0.55 | p > 0.05 | |
| Outcome 10: Number of emergency room visits per patient in the intervention and post-intervention period | ||||
| Intervention | OCS + CDSS (costs – US$s) | OCS alone | p-value | |
| Number of emergency room visits/patient | 1.03 ± 1.73 | 1.00 ± 1.74 | p > 0.05 | |
| Outcome 11: Number of outpatient visits per patient in the intervention and post-intervention period | ||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | p-value | |
| Outpatient visits/patient | 4.30 ± 3.39 | 4.30 ± 3.44 | p > 0.05 | |
| Authors’ conclusions: In the 14 weeks before the trial, the number of tests orders and the average charges for test per patient visit were similar for the intervention and control groups. During the trial displaying the charges for diagnostic tests significantly reduced the number and cost of tests ordered, especially for patients with scheduled visits. The effect of this intervention did not persist after it was discontinued | ||||
| Methodological assessment criteria (Tierney) (1990)86 | ||||
| 1. Is the study properly randomised? | Unclear | |||
| 2. Is allocation of treatment concealed? | Unclear | |||
| 3. Were the study eligibility criteria specified? | Yes | |||
| 4. Are adequate baseline details presented? | Yes | |||
| 5. Are groups similar at baseline? | Yes | |||
| 6. Are any baseline imbalances adequately adjusted for in the analysis? | NA | |||
| 7. Are similar cointerventions administered? | NA | |||
| 8. Are physician’s blinded to treatment allocation? | No | |||
| 9. Are outcome assessors blinded? | Unclear | |||
| 10. Are data analyses appropriate? | Yes | |||
| 11. Is analysis conducted on an ‘intention to provide or communicate’ information basis? | No | |||
| 12. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | |||
| 13. Are the conclusions supported by the results? | Yes | |||
| Comments: None | ||||
| Study demographics | |||||
|---|---|---|---|---|---|
| Author; year; study ID: Bates (1997)101 | |||||
| Title: Does the computerised display of charges affect inpatient ancillary test utilisation? | |||||
| Country: USA | |||||
| Specific setting: Brigham and Women’s Hospital, Boston, MA, USA (720-bed tertiary care hospital) | |||||
| Study objectives: To assess whether the computerised display of charges for clinical laboratory or radiological tests affects physician-ordering behaviour | |||||
| Health-care setting: Secondary care (medical and surgical l inpatients) | |||||
| If secondary care, academic status of the hospital: Teaching hospital | |||||
| Health-care system: Medicare; HMO; private insurance; uninsured | |||||
| Study design: 2 RCTs | |||||
| Number of sites: 1 (720-bed hospital) | |||||
| Funding source: Public sector | |||||
| Was evaluator of tool also its developer? Yes | |||||
| System users (both RCTs) | |||||
| Intervention group | Control group | ||||
| CDSS user(s) | Physician | Physician | |||
| Practitioners (n) | NR | NR | |||
| Practitioners (n) in analysis | NR | NR | |||
| a No further baseline details were reported on study physicians. | |||||
| Inclusion criteria: All physicians using OCS with or without CDSS for laboratory or radiological test ordering on the medical or surgical wards during the study period. The clinical laboratory study period was 4 months (February to May 1994) and Radiology study period was 7 months (April to October 1994) | |||||
| Exclusion criteria: NR | |||||
| Patient baseline demographics | |||||
| Inclusion criteria: All inpatients who were admitted to the medical or surgical wards during and discharged before the last day of the study periods | |||||
| Exclusion criteria: NR | |||||
| Clinical laboratory trial | |||||
| Intervention group | Control group | ||||
| N | 3536 | 3554 | |||
| Age (years) (mean; SD) | 55.3 ± 17.8 | 54.7 ± 17.7 | |||
| Gender (Male, n; %) | 1847 (49.4) | 1732 (48.7) | |||
| Service (n; %) | |||||
| Medical | 2081 (55.7) | 1952 (55.9) | |||
| Surgical | 1658 (44.3) | 1602 (45.1) | |||
| DRG weighta,b | 1.09 (0.76–1.97) | 1.07 (0.76–1.97) | |||
| White (n; %) | 2690 (71.9) | 2574 (72.4) | |||
| Medicare, HMO, or private insurance (n; %) | 3242 (86.7) | 3088 (68.9) | |||
| Length of stay (days)b | 4 (2–7) | 4 (2–7) | |||
| Radiology trial | |||||
| N | 8728 | 8653 | |||
| Age (mean; SD) | 54.0 ± 17.9 | 53.2 ± 17.9 | |||
| Gender (Male, n; %) | 3894 (44.6) | 3877 (44.8) | |||
| Service (n; %) | |||||
| Medical | 4194 (52.0) | 4099 (51.9) | |||
| Surgical | 3874 (48.0) | 3799 (48.1) | |||
| DRG weighta,b | 1.02 (0.76–1.96) | 1.02 (0.75–1.89) | |||
| White (n; %) | 6388 (73.2) | 6329 (73.1) | |||
| Medicare, HMO, or private insurance (n; %) | 7611 (87.2) | 7545 (87.2) | |||
| Length of stay (days)b | 3 (2–7) | 3 (2–6) | |||
| a DRG indicates diagnosis related group. | |||||
| b Medians; ranges (in parentheses) are 25% and 75% quartiles. | |||||
| Interventions | |||||
| Intervention: OCS plus CDSS (display of test charges). For the laboratory test trial the costs for 19 commonly ordered laboratory tests were displayed. Other less frequently ordered laboratory tests were entered from other checkbox screens or via textual input. This was used to parse a coded test name. The coded test name and test charge were then displayed. For the radiological procedures, charges for 35 of the most frequently ordered tests were displayed at the time of ordering. Charges for the remainder of the radiological tests were not displayed. In both trials, in addition to the display of charges, a ‘cash register’ window that showed physicians the sum of the total charges for the tests during the ordering session was displayed. Charges that were displayed were the hospital’s charges to the patients or their insurers for the tests only; other charges (e.g. for interpretation of radiographs) were not displayed. | |||||
| Comparator: OCS alone | |||||
| Note: During the study periods tests could still be ordered directly from the laboratory thus by-passing the use of the OCS. Additionally, radiological studies that were performed in the operating room (6.3% of all radiological studies) or in the emergency department (10% of all studies) were not ordered via OCS. Therefore in the study periods only 53% of the laboratory tests performed and 74% of the radiology tests performed had a corresponding computer order. | |||||
| Concomitant interventions: NA | |||||
| CDSS tool | |||||
| Name of CDDS (if any) | NR | ||||
| CDSS reasoning method | NR | ||||
| CDSS knowledge base | Hospital test costs | ||||
| Did study use training set and test set data? |
Training set: NR Test set: NR |
||||
| For training set data complete the following: | |||||
| Design | NR | ||||
| Target decision | NR | ||||
| Sample characteristics: | Intervention | Control | |||
| Sample size (n) | NR | NR | |||
| Age (mean; range) | NR | NR | |||
| Gender (n male; n female) | NR | NR | |||
| Disease type | NR | NR | |||
| For test set data complete the following: | |||||
| Properties of test set data | NR | ||||
| Test centre | NR | ||||
| Target decision | NR | ||||
| Other CDSS information | |||||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | NR; biochemical and radiology test orders | ||||
| 2. Time to complete the CDSS (minutes) | NR | ||||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Display of test costs | ||||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||||
| 5. Is user instructional training at the time of implementation described? | No | ||||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||||
| 8. Is there a need for additional data entry by the clinician? | No | ||||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | ||||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | No | ||||
| 13. Does the CDSS promote action rather than inaction? | NR | ||||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||||
| 16. Were local users involved in the CDSS development process? | Unclear | ||||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | ||||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||||
| Outcome measures | |||||
| Outcome 1: Mean number of tests ordered per admission | |||||
| Outcome 2: Mean number of tests performed per admission | |||||
| Outcome 3: Mean charges for tests ordered per admission | |||||
| Outcome 4: Mean charges for tests performed per admission | |||||
| Outcome 5: Mean length of patient stay (days) | |||||
| Outcome 6: Mean total hospital charges | |||||
| Total length of follow-up: Clinical laboratory trial – 4 months; radiology trial – 7 months | |||||
| Follow-up assessment times: Clinical laboratory trial – 4 months; radiology trial – 7 months | |||||
| Rate of attrition at each follow-up time: NA | |||||
| Methods of statistical analysis: Results for the number of tests and charges were reported as medians (with 25% and 75% quartiles) and as means (SDs). Univariate comparisons between intervention and comparator groups were made with Wilcoxon rank-sum statistic or t-tests. Multiple linear regression analysis were performed adjusting for age, gender, race, primary insurer, and DRG weight | |||||
| Results | |||||
| Outcome 1: Mean number of tests ordered per admission | |||||
| Clinical laboratory triala | |||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | Δ (%) | p-valueb | |
| N in analysis | 3739 | 3554 | – | – | |
| 25.6 ± 37.9 | 26.8 ± 43.4 | –4.5 | 0.74; 0.21 | ||
| 15 (6–31) | 15 (6–31) | ||||
| Radiology triala | |||||
| N in analysis | 8728 | 8653 | – | – | |
| 1.76 ± 4.43 | 1.76 ± 4.68 | 0 | 0.13; 0.95 | ||
| 0 (0–2) | 0 (0–2) | ||||
| a Results reported as mean ± SD with medians with 25th and 75th percentiles in parentheses. | |||||
| b First p-value by Wilcoxon rank-sum test; second p-value by t-test. | |||||
| Outcome 2: Mean number of tests performed per admission | |||||
| Clinical laboratory triala | |||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | Δ (%) | p-valueb | |
| N in analysis | 3739 | 3554 | – | – | |
| 46.9 ± 79.2 | 49.6 ± 94.4 | –5.4 | 0.87; 0.18 | ||
| 25 (13–50) | 24 (13–52) | ||||
| Radiology triala | |||||
| N in analysis | 8728 | 8653 | – | – | |
| 1.53 ±3.58 | 1.53 ± 4.06 | 0 | 0.06; 0.99 | ||
| 0 (0–1) | 0 (0–1) | ||||
| a Results reported as mean ± SD with medians with 25th and 75th percentiles in parentheses. | |||||
| b First p-value by Wilcoxon rank-sum test; second p-value by t-test. | |||||
| Outcome 3: Mean charges for tests ordered per admission ($) | |||||
| Clinical laboratory triala | |||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | Δ (%) | p-valueb | |
| N in analysis | 3739 | 3554 | – | – | |
| 739 ± 1129 | 771 ± 1310 | –4.2 | 0.97; 0.25 | ||
| 392 (151–907) | 399 (149–883) | ||||
| Radiology triala | |||||
| N in analysis | 8728 | 8653 | – | – | |
| 275 ± 688 | 276 ± 737 | -0.4 | 0.10; 0.88 | ||
| 0 (0–266) | 0 (0–209) | ||||
| a Results reported as mean ± SD with medians with 25th and 75th percentiles in parentheses. | |||||
| b First p-value by Wilcoxon rank-sum test; second p-value by t-test. | |||||
| Outcome 4: Mean charges for tests performed per admission | |||||
| Clinical laboratory triala | |||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | Δ (%) | p-valueb | |
| N in analysis | 3739 | 3554 | – | – | |
| 1423 ± 2730 | 1496 ± 3147 | –4.9 | 0.89; 0.29 | ||
| 649 (309–1409) | 565 (305–1463) | ||||
| Radiology triala | |||||
| N in analysis | 8728 | 8653 | – | – | |
| 220 ± 473 | 215 ± 515 | + 2.3 | 0.03; 0.50 | ||
| 0 (0–266) | 0 (0–200) | ||||
| a Results reported as mean ± SD with medians with 25th and 75th percentiles in parentheses. | |||||
| b First p-value by Wilcoxon rank-sum test; second p-value by t-test. | |||||
| Outcome 5: Mean length of patient stay (days) | |||||
| Clinical laboratory triala | |||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | Δ (%) | p-value | |
| N in analysis | 3536 | 3363 | – | – | |
| 6.25 ± 0.11 | 6.50 ± 0.11 | –3.8 | NS | ||
| Radiology triala | |||||
| N in analysis | 8728 | 8653 | – | – | |
| 5.97 ± 0.07 | 5.86 ± 0.07 | + 1.9 | NS | ||
| a Adjusted by age, diagnosis related group weight, race, service, and insurance status | |||||
| Outcome 6: Mean total hospital charges ($) | |||||
| Clinical laboratory triala | |||||
| Intervention | OCS + CDSS (costs – US$) | OCS alone | Δ (%) | p-value | |
| N in analysis | 3536 | 3363 | – | – | |
| 16,298 ± 318 | 16,734 ± 326 | –2.6 | NS | ||
| Radiology triala | |||||
| N in analysis | 8728 | 8653 | – | – | |
| 16,842 ± 264 | 16,417 ± 267 | + 2.6 | NS | ||
| a Adjusted by age, diagnosis related group weight, race, service, and insurance status. | |||||
| Authors’ conclusions: The computerised display of charges had no statistically significant effect on the number of clinical laboratory tests or radiological procedures ordered or performed, although small trends were present for clinical laboratory tests. More intensive interventions may be needed to affect physician test utilisation | |||||
| Methodological assessment criteria (Bates) (1997)101 | |||||
| 1. Is the study properly randomised? | Unclear | ||||
| 2. Is allocation of treatment concealed? | Unclear | ||||
| 3. Were the study eligibility criteria specified? | Yes | ||||
| 4. Are adequate baseline details presented? | Yes | ||||
| 5. Are groups similar at baseline? | Yes | ||||
| 6. Are any baseline imbalances adequately adjusted for in the analysis? | NA | ||||
| 7. Are similar cointerventions administered? | NA | ||||
| 8. Are physician’s blinded to treatment allocation? | No | ||||
| 9. Are outcome assessors blinded? | Unclear | ||||
| 10. Are data analyses appropriate? | Yes | ||||
| 11. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Yes | ||||
| 12. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | ||||
| 13. Are the conclusions supported by the results? | Yes | ||||
| Comments: contamination likely as patients are the unit of randomisation and physicians treated patients in both groups (with and without test costs displayed). Trials may lack power to detect any differences between the two groups as only 53% of the laboratory tests performed and 74% of the radiology tests performed had a corresponding computer order. | |||||
Studies assessing the impact of display of previous test results (n = 2)
| Study demographics | ||||
|---|---|---|---|---|
| Author; year; study ID: Solomon (1999)88 | ||||
| Title: A computer-based intervention to reduce unnecessary serologic testing | ||||
| Country: USA | ||||
| Specific setting: Brigham and Women’s Hospital, Boston, MA, USA | ||||
| Study objectives: To assess whether physicians would reduce their use of unnecessary serologic tests if provided with information on the likelihood that a antinuclear antibody (ANA), rheumatoid factor (RF), or complement level test would change their estimate of disease | ||||
| Health-care setting: Secondary care (inpatients) | ||||
| If secondary care, academic status of the hospital: NR | ||||
| Health-care system: NR | ||||
| Study design: CCT | ||||
| Number of sites: 1 (720-bed hospital) | ||||
| Funding source: Public sector | ||||
| Was evaluator of tool also its developer? Yes | ||||
| System users | ||||
| Intervention group | Control group | p-value | ||
| CDSS user(s) | Physician | Physician | ||
| Practitioners (n) | 71 | 154 | ||
| Age (year) (mean; SD) | 30 ± 4 | 30 ± 4 | 0.5 | |
| Gender (Female, %) | 28 | 36 | 0.2 | |
| Postgraduate year (%)a | ||||
| First | 65 | 52 | ||
| Second | 13 | 27 | ||
| Third | 16 | 14 | ||
| Fourth | 5 | 7 | 0.08 | |
| Department (%)a | ||||
| Medicine | 86 | 82 | ||
| Surgery | 2 | 7 | ||
| Neurology | 7 | 8 | ||
| Obstetrics-gynaecology | 5 | 3 | 0.2 | |
| a Percentages may not sum due to rounding (reported by authors). | ||||
| b Calculated from chi-squared tests for categorical data and Wilcoxon rank sum tests for ordinal data. | ||||
| Practitioners (n) in analysis | NA | NA | ||
| Inclusion criteria: All physicians ordering an RF or ANA test for the suspected indications of rheumatoid arthritis, systemic lupus erythematosus, primary systemic sclerosis, mixed connective tissue disease, or Sjögren’s syndrome during the 10-month study period (September 1996 to June 1997) were assigned to the intervention group. All physicians ordering an RF or ANA for the suspected indications of systemic vasculitis and cryoglobulinemia, or a complement test for any condition during the study period were assigned to the control group | ||||
| Exclusion criteria: When multiple orders for the same test for the same patient during one calendar day were made, only the last order was included | ||||
| Patient baseline demographics | ||||
| Inclusion criteria: All inpatients with an order written for an ANA, RF or complement level test during the 10-month study period | ||||
| Exclusion criteria: NR | ||||
| Patient characteristics | Intervention group | Control group | p-valuea | |
| N | 99 | 236 | ||
| Age (year) (mean; SD) | 55 ± 19 | 54 ± 17 | 0.7 | |
| Gender (female) (%) | 66 | 66 | 1.0 | |
| Length of stay (days)b | 6 (3,12) | 6 (3,11) | 0.9 | |
| Total charges (US$)b | 13,415 (7636; 27,951) | 13,217 (7337; 25,634) | 0.6 | |
| Died in hospital (n) | 4 | 6 | 0.4 | |
| a Calculated from chi-squared tests for categorical data and Wilcoxon rank-sum tests for ordinal data. | ||||
| b Median (25%; 75%). | ||||
| Interventions | ||||
| Intervention: OCS plus CDSS. The CDSS required the physician to enter into the computer their estimate of the pre-test probability of disease. The CDSS then calculated the positive and negative post-test predictive values based on estimates of the sensitivity and specificity of each test derived from the literature | ||||
| Comparator: OCS alone | ||||
| Concomitant interventions: NA | ||||
| CDSS tool | ||||
| Name of CDDS (if any) | NR (home-grown system Brigham and Women’s Hospital) | |||
| CDSS reasoning method | Naive Bayesian methods | |||
| CDSS knowledge base | Sensitivity and Specificity values abstracted from the literature | |||
| Did study use training set and test set data? |
Training set: NR Test set: NR |
|||
| For training set data complete the following: | ||||
| Design | NR | |||
| Target decision | NR | |||
| Sample characteristics: | Intervetion | Control | ||
| Sample size (n) | NR | NR | ||
| Age (mean; range) | NR | NR | ||
| Gender (n male; n female) | NR | NR | ||
| Disease type | NR | NR | ||
| For test set data complete the following: | ||||
| Properties of test set data | ||||
| Test centre | NR | |||
| Target decision | NR | |||
| Other CDSS information | ||||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | 1; pre-test probability estimate | |||
| 2. Time to complete the CDSS (minutes) | NR | |||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Positive and negative predictive values (post-test) | |||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | |||
| 5. Is user instructional training at the time of implementation described? | Yes | |||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | |||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | |||
| 8. Is there a need for additional data entry by the clinician? | Yes | |||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | |||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | |||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | |||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | No | |||
| 13. Does the CDSS promote action rather than inaction? | No | |||
| 14. Does the CDSS justify the output by provision of reasoning? | No | |||
| 15. Does the CDSS justify the output by provision of research evidence? | No | |||
| 16. Were local users involved in the CDSS development process? | NR | |||
| 17. Is the CDSS output provided to patients as well as clinician? | No | |||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | |||
| 19. Is the CDSS used in conjunction with conventional education? | No | |||
| Outcome measures | ||||
| Outcome 1: Number of tests cancelled | ||||
| Outcome 2: Yield of positive tests for known or new rheumatic disease | ||||
| Total length of follow-up: 10 months (September 1996 to June 1997) | ||||
| Follow-up assessment times: 10 months | ||||
| Rate of attrition at each follow-up time: 348 patients had a ANA, RF or complement test ordered, of these 13 duplicate orders were excluded. N = 335 tests (attrition rate = 3.7%) | ||||
| Methods of statistical analysis: Chi-squared tests and Wilcoxon rank-sum tests for univariate analysis | ||||
| Results | ||||
| Outcome 1: Number of tests cancelled | ||||
| OCS + CDSS | OCS alone | p-value | ||
| N in analysis | 99 | 236 | – | |
| Number of tests cancelled n (%) | 11 (11.1%) | 1 (0.42%) | p = 0.001 | |
|
Outcome 2: Yield of positive tests for known or new rheumatic disease The charts of 43 patients with positive tests were reviewed to determine whether the positive test yielded a new diagnosis of a rheumatic condition. 26/43 of the positive tests were in patients with known rheumatic disease. Only 4/43 new diagnosis of rheumatic disease were made, which account for 1.2% of all tests ordered |
||||
| Authors’ conclusions: The computer-based intervention resulted in a small but statistically significant decrease in orders for AAN and RF levels by 10%. Further reductions without clinical harm are probably possible, since the yield of testing for new rheumatic diseases was low | ||||
| Methodological assessment criteria Solomon (1999)88 | ||||
| 1. Were the study eligibility criteria specified? | Yes | |||
| 2. Are adequate baseline details presented? | Yes | |||
| 3. Are groups similar at baseline? | Yes | |||
| 4. Are any baseline imbalances adequately adjusted for in the analysis? | NA | |||
| 5. Are similar cointerventions administered? | NA | |||
| 6. Are physician’s blinded to treatment allocation? | No | |||
| 7. Are outcome assessors blinded? | No | |||
| 8. Are data analyses appropriate? | Yes | |||
| 9. Is analysis conducted on an ‘intention to provide or communicate’ information basis? | Yes | |||
| 10. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | |||
| 11. Are the conclusions supported by the results? | Yes | |||
| Comments: None | ||||
| Study demographics | ||
|---|---|---|
| Author; year; study ID: Bansal (2001)89 | ||
| Title: A computer-based intervention on the appropriate use of ABG | ||
| Country: USA | ||
| Specific setting: Vanderbilt University Medical Center, Nashville, TN, USA (630-bed hospital with 31,000 admissions per year) | ||
| Study objectives: To evaluate the impact of a computer-based intervention on ABG usage in an intensive care setting | ||
| Health-care setting: Secondary care (ICUs) | ||
| If secondary care, academic status of the hospital: University | ||
| Health-care system: NR | ||
| Study design: Pre–post | ||
| Number of sites: 1 (six ICUs; trauma; general surgery; medical; cardiac; burn; neurology) | ||
| Funding source: Public sector | ||
| Was evaluator of tool also its developer? Yes | ||
| System users | ||
| CDSS user(s)a | Physicians’; respiratory therapists; nurses; medical receptionists | |
| Practitioners (n) | NR | |
| Orders entered by user type at baseline | ||
| User typea | n | % |
| Ancillary staff | 24 | 1.8% |
| Physicians | 366 | 28.0% |
| Other users | 8 | 0.6% |
| Nurses | 813 | 62.0% |
| Respiratory therapists | 80 | 6.1% |
| a Staff who were not MD had the ability to enter verbal or written orders from physicians. | ||
| Inclusion criteria: All users with the authority to enter orders via the OCS during the study period | ||
| Exclusion criteria: NR | ||
| Patient baseline demographics | ||
| Inclusion criteria: All patients on the six ICUs; (trauma; general surgery; medical; cardiac; burn; neurology) who had a ABG test ordered during the study perioda | ||
| Exclusion criteria: NR | ||
| a No further data on patient baseline demographics was reported. | ||
| Interventions | ||
| Intervention: OCS plus CDSS. CDSS provided the ordering clinician with educational text alongside a graphical display of the patient’s previous ABG values (pO2, pCO2, HCO3, pH, FiO2) and O2 saturations. Advanced ordering of ABG tests was also limited to within 24 hours so no multiday orders were allowed. The default response was to cancel the order, but the final decision regarding the test order was left to the user’s discretion | ||
| Comparator: OCS alone (pre-intervention) | ||
| Concomitant interventions: NA | ||
| CDSS tool | ||
| Name of CDDS (if any) | NR (home-grown system Vanderbilt University Medical Center) | |
| CDSS reasoning method | NR | |
| CDSS knowledge base | NR | |
| Did study use training set and test set data? |
Training set: No Test set: No |
|
| For training set data complete the following: | ||
| Design | NR | |
| Target decision | NR | |
| Sample characteristics: | ||
| Sample size (n) | NR | |
| Age (mean; range) | NR | |
| Gender (n male; n female) | NR | |
| Disease type | NR | |
| For test set data complete the following: | ||
| Properties of test set data | NR | |
| Test centre | NR | |
| Target decision | NR | |
| Other CDSS information | ||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | Six previous ABG results (pO2, pCO2, HCO3, pH, FiO2,O2 saturations | |
| 2. Time to complete the CDSS (minutes) | NR | |
| 3. CDSS output format: (score; probability graph; advice; etc.) | Graphs | |
| 4. Is a description of pilot testing with users prior to implementation provided? | No | |
| 5. Is user instructional training at the time of implementation described? | No | |
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | |
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | |
| 8. Is there a need for additional data entry by the clinician? | No | |
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | |
| 10. Does CDSS provide output at the time and location of decision making? | Yes | |
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | Yes | |
| 12. Does the CDSS provide a recommendation rather than just an assessment? | No | |
| 13. Does the CDSS promote action rather than inaction? | No | |
| 14. Does the CDSS justify the output by provision of reasoning? | No | |
| 15. Does the CDSS justify the output by provision of research evidence? | No | |
| 16. Were local users involved in the CDSS development process? | Yes | |
| 17. Is the CDSS output provided to patients as well as clinician? | No | |
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | |
| 19. Is the CDSS used in conjunction with conventional education? | No | |
| Outcome measures | ||
| Outcome 1: Number of ABG test orders placed pre- and post-intervention | ||
| Total length of follow-up: 12 weeks (pre-intervention 5 weeks; post-intervention 7 weeks). Study period: 1 November 2000 to 23 January 2001 | ||
| Follow-up assessment times: 12 weeks | ||
| Rate of attrition at each follow-up time: NA | ||
| Methods of statistical analysis: ANOVA and linear regression analysis | ||
| Results | ||
| Outcome 1: Number of ABG test orders placed pre- and post-intervention | ||
| Pre-intervention (n) | Post-intervention (n) | p-value |
| 376 | 387 | p = 0.09 |
| Authors’ conclusions: Study did not demonstrate significant change. Longer study periods are therefore needed. The impact could be improved in the future by targeting physician users and tailoring the intervention to specific workflow patterns of high utilisation units | ||
| Methodological assessment criteria (Bansal) (2001)89 | ||
| 1. Were the study eligibility criteria specified? | Yes | |
| 2. Are adequate baseline details presented? | Partial | |
| 3. Are similar cointerventions administered in both study periods? | No | |
| 4. Are data analyses appropriate? | Yes | |
| 5. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Unclear | |
| 6. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | |
| 7. Are the conclusions supported by the results? | Yes | |
| Comments: The numbers presented in the abstract for change in the number of test orders placed pre- and post-intervention do not tally with those presented in the text on page 34. It is unclear whether the post-intervention results reported in both the abstract and text (p. 34) pertain to just the implemented units or all units together. Numbers reported for outcome 1 are taken from the abstract | ||
Studies assessing the impact of the display of reminders (n = 10)
| Study demographics | |||||
|---|---|---|---|---|---|
| Author; year; study ID: Overhage (1996)48 | |||||
| Title: Computer reminders to implement preventive care guidelines for hospitalised patients | |||||
| Country: USA | |||||
| Specific setting: Wishard Memorial Hospital, Indianapolis, IN, USA | |||||
| Study objectives: To determine if computer reminders increase the provision on inpatient preventive care | |||||
| Health-care setting: Inpatient general medicine ward | |||||
| If secondary care, academic status of the hospital: Teaching hospital | |||||
| Health-care system: NR | |||||
| Study design: CRCT | |||||
| Number of sites: 1; 6 independent medical services within the ward (3 intervention; 3 control) | |||||
| Funding source: Public sector | |||||
| Was evaluator of tool also its developer? Yes | |||||
| System users | |||||
| Intervention group | Control group | ||||
| CDSS user(s) | Physician | Physician | |||
| Practitioners (n) | 78 (24 unique teams of physicians and medical students rotated through the 6 medical services) | ||||
| If the CDSS user is a doctor, complete n for the following: | NR | NR | |||
| Consultant (attending) | |||||
| Registrar (chief resident) | |||||
| SHO (resident) | |||||
| HO (intern) | |||||
| Practitioners (n) in analysis | 74 | ||||
| Inclusion criteria: NR | |||||
| Exclusion criteria: Physicians who rotated through the service more than once and could not be assigned to the same study status (i.e. intervention or control were excluded in the analysis) | |||||
| Patient baseline demographics | |||||
| Inclusion criteria: All patients admitted to the inpatient general medicine ward during the study period for whom the computer generated at least one preventive care recommendation | |||||
| Exclusion criteria: Data from patients who were (1) admitted before the study began, (2) discharged after the study period, (3) had already been hospitalised during the study period or (4) remained in the hospital less than 18 hours were excluded from the analysis | |||||
| Intervention group | Control group | ||||
| N | 821 | 801 | |||
| Age (years) (mean; SD) | 51 ± 18 | 51 ± 18 | |||
| Gender (% male) | 50% | 50% | |||
| White ethnic group (%) | 50.1% | 48.4% | |||
| Primary discharge diagnosis (%) | |||||
| Chest pain, angina, MI | 7.6% | 8.1% | |||
| Pneumonia | 5.7% | 6.7% | |||
| Congestive heart failure | 4.8% | 4.9% | |||
| Pancreatitis | 3.0% | 3.6% | |||
| Asthma | 3.6% | 3.6% | |||
| Gastrointestinal bleeding | 3.3% | 3.6% | |||
| Diabetic ketoaciodosis | 3.0% | 3.1% | |||
| Abdominal pain | 1.0% | 3.1% | |||
| Diabetes mellitus | 2.2% | 2.8% | |||
| Interventions | |||||
|
Intervention: OCS plus reminders for preventive care measures. Twenty-two preventive care measures from the US Preventive Services Task Force recommendations were developed and presented for each patient to physicians as reminders on printed daily reports for each patient and also in the OCS used for all order writing. These were the only reminders that were active during the study period. The preventive care actions included: (1) cervical cytology study; (2) pneumococcal vaccination; (3) aspirin; (4) oestrogen treatment; (5) calcium treatment; (6) ophthalmologic referral; (7) mammography; (8) thyrotropin screen; (9) hepatitis B screen; (10) rubella screen; (11) screening urinalysis; (12) cholesterol test; (13) pregnancy test; (14) HIV screen; (15) ACE inhibitor; (16) heparin prophylaxis; (17) 24-hour urine protein test; (18) sickle cell screen; (19) cholesterol treatment; (20) screening electrocardiogram; (21) beta-blocker; (22) STD screen Note: only data on (1) cervical cytology study; (2) mammography; (3) thyrotropin screen; (4) hepatitis B screen; (5) screening urinalysis; (6) cholesterol test; (7) HIV screen; (8) 24-hour urine protein test; (9) Sickle cell screen; (10) screening electrocardiogram; (11) STD screen impact on test volumes/rates and are therefore extracted |
|||||
| Comparator: OCS alone without reminders | |||||
| Concomitant interventions: NA | |||||
| CDSS tool | |||||
| Name of CDDS (if any) | NR | ||||
| CDSS reasoning method | Discrimination rules | ||||
| CDSS knowledge base | US Preventive Services Task Force Recommendations (Guidelines) | ||||
| Did study use training set and test set data? |
Training set: Yes Test set: NR |
||||
| For training set data complete the following: | |||||
| Design | Retrospective test against data from 1000 medical inpatients | ||||
| Target decision | NR | ||||
| Sample characteristics: | Intervention | Control | |||
| Sample size (n) | NR | NR | |||
| Age (mean; range) | NR | NR | |||
| Gender (n male; n female) | NR | NR | |||
| Disease type | NR | NR | |||
| For test set data complete the following: | |||||
| Properties of test set data | NR | ||||
| Test centre | Same as for training set | ||||
| Target decision | NR | ||||
| Other CDSS information | |||||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | NR | ||||
| 2. Time to complete the CDSS (minutes). | NR | ||||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Reminders | ||||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||||
| 5. Is user instructional training at the time of implementation described? | No | ||||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||||
| 8. Is there a need for additional data entry by the clinician? | No | ||||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | ||||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | No | ||||
| 13. Does the CDSS promote action rather than inaction? | NR | ||||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||||
| 16. Were local users involved in the CDSS development process? | No | ||||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | ||||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||||
| Outcome measures | |||||
| Outcome 1: Compliance with reminders for preventive care measures | |||||
| Total length of follow-up: 6 months (beginning 26 October 1992) | |||||
| Follow-up assessment times: 6 months | |||||
| Rate of attrition at each follow-up time: During the study period 1929 patients received care during 2595 hospitalisations. Data were eliminated from 973 hospitalisations (38%) because (1) the patient was admitted before the study began (n = 76), (2) the patient was discharged after the study ended (n = 95), (3) the patient had already been hospitalised once during the study (n = 412), (4) the patient remained in hospital less than 18 hours (n = 226), (5) other (n = 164) (unspecified). After exclusions data from 1622 hospitalisations remained | |||||
| Methods of statistical analysis: Results for individual preventive care measures and all measures combined were analysed using the Kleinman β-binomial model. A 2-tailed p-value ≤ 0.05 was considered significant | |||||
| Results | |||||
| Outcome 1: Compliance with reminders for preventative care measures | |||||
| Preventive Care Action | OCS + CDSS (alerts) | OCS alone | p-value | ||
| N | Compliance (%) | N | Compliance (%) | ||
| Cervical cytology study | 323 | 2.8 | 329 | 2.8 | p = 0.41 |
| Mammography | 125 | 5.6 | 131 | 1.5 | p = 0.08 |
| Thyrotropin screen | 112 | 16.1 | 118 | 9.3 | p = 0.12 |
| Hepatitis B screen | 88 | 8.0 | 92 | 2.2 | p = 0.08 |
| Screening urinalysis | 68 | 32.4 | 75 | 34.7 | p = 0.77 |
| Cholesterol test | 70 | 14.3 | 58 | 13.8 | p = 0.94 |
| HIV screen | 44 | 4.6 | 43 | 9.3 | p = 0.38 |
| 24-hour urine protein test | 24 | 25.0 | 23 | 4.4 | p = 0.05 |
| Sickle cell screen | 22 | 9.0 | 14 | 0.0 | p = 0.25 |
| Screening electrocardiogram | 13 | 0.0 | 14 | 21.4 | p = 0.08 |
| STD screen | 2 | 50.0 | 6 | 16.7 | p = 0.35 |
| HIV, human immunodeficiency virus; STD, sexually transmitted disease. | |||||
| Authors’ conclusions: Using a moderately intensive intervention we were unable to increase the provision of preventive care during hospitalisations. The physicians providing care during the hospitalisations were not the patients’ primary care physicians which proved to be an important barrier | |||||
| Methodological assessment criteria (Overhage) (1996)48 | |||||
| 1. Is the study properly randomised? | Yes | ||||
| 2. Did the analysis take clustering into account? | Yes | ||||
| 3. Were the study eligibility criteria specified? | Yes | ||||
| 4. Are adequate baseline details presented? | Partial | ||||
| 5. Are groups similar at baseline? | Yes | ||||
| 6. Are any baseline imbalances adequately adjusted for in the analysis? | NA | ||||
| 7. Are similar cointerventions administered? | NA | ||||
| 8. Are physician’s blinded to treatment allocation? | No | ||||
| 9. Are outcome assessors blinded? | Unclear | ||||
| 10. Are data analyses appropriate? | Yes | ||||
| 11. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | No | ||||
| 12. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | ||||
| 13. Are the conclusions supported by the results? | Yes | ||||
| Comments: None. | |||||
| Study demographics | |||||
|---|---|---|---|---|---|
| Author; year; study ID: Overhage (1997)24 | |||||
| Title: A randomised trial of ‘corollary’ to prevent errors of omission | |||||
| Country: USA | |||||
| Specific setting: Wishard Memorial Hospital, Indianapolis, IN, USA | |||||
| Study objectives: To determine if automated, guideline-based reminders to physicians provided as they wrote orders could reduce errors of omission to order tests or treatments needed to monitor/ameliorate the effects of other tests or treatments | |||||
| Health-care setting: Inpatient general medicine ward | |||||
| If secondary care, academic status of the hospital: Teaching hospital | |||||
| Health-care system: NR | |||||
| Study design: CRCT | |||||
| Number of sites: 1; 6 independent medical services within the ward (3 intervention; 3 control) | |||||
| Funding source: Public sector | |||||
| Was evaluator of tool also its developer? Yes | |||||
| System users | |||||
| Intervention group | Control group | ||||
| CDSS user(s) | Housestaff physician | Housestaff physician | |||
| Practitioners (n) | 45 | 41 | |||
| If the CDSS user is a doctor, complete n for the following: | NR | NR | |||
| Consultant (attending) | |||||
| Registrar (chief resident) | |||||
| SHO (resident) | |||||
| HO (intern) | |||||
| Practitioners (n) in analysis | 45 | 41 | |||
|
Inclusion criteria: All housestaff physicians who received five or more suggestions for corollary orders during the study period (October 1992 to April 1993) Exclusion criteria: Housestaff physicians who received fewer than five suggestions about corollary orders were excluded from the analysis. Additionally, data from physicians who rotated through the service more than once and could not be assigned to the same study status (i.e. intervention or control) were excluded from all rotations after the physician’s original study status changed. Data on suggested orders that occurred when physicians’ and patients’ study status differed were also excluded |
|||||
| Patient baseline demographics | |||||
| Inclusion criteria: All inpatients who had at least one order written that triggered a suggestion for a corollary order during the study period | |||||
| Exclusion criteria: NR | |||||
| Intervention group | Control group | ||||
| N | 814 | 872 | |||
| Age (years) (mean; SD) | 54 ± 18 | 53 ± 18 | |||
| Gender (Male; %) | 45 | 51 | |||
| White ethnic group (%) | 50 | 49 | |||
| Problem list (%) | |||||
| Hypertension | 5.2 | 5.6 | |||
| Heart failure | 3.4 | 3.2 | |||
| Diabetes mellitus | 3.0 | 3.0 | |||
| Chest pain | 3.5 | 2.9 | |||
| Pneumonia | 2.5 | 2.7 | |||
| Urinary tract infection | 2.4 | 2.2 | |||
| Anaemia | 2.4 | 2.2 | |||
| Gastrointestinal tract infection | 1.9 | 1.7 | |||
| Diabetic ketoacidosis | 0.6 | 0.5 | |||
| Interventions | |||||
|
Intervention: OCS plus CDSS (suggested corollary orders linked to trigger orders). Eighty-seven target orders (76 drugs and 11 tests) were identified that could be paired with one or more corollary orders. Examples of the target orders identified were (1) heparin infusion; (2) IV fluids; (3) insulin (all kinds); (4) oral hypoglycaemic agents; (5) narcotics (class II); (6) nonsteroidals; (7) aminoglycosides; (8) vancomycin intravenously; (9) warfarin; (10) amphotericin B; (11) angiotensin-converting enzyme inhibitions; (12) choloramphenicol; (13) air contrast barium enema; (14) isoniazid; (15) potassium supplements; (16) pulmonary artery catheter; (17) ventilator orders; and (18) vasopressin drip Each time one of the target orders was placed, the CDSS suggested that the other linked corollary orders be considered. When suggesting orders the CDSS took into account, the status of the order (a new order or a revision of an old order); the time elapsed since the last time the order being suggested was written; and whether any orders for a new equivalent item had already been written. Physicians were free to accept or reject the suggested corollary orders Note: only data on 24-hour suggested laboratory test corollary orders is extracted, as the main reported results are a combination of all orders (i.e. both pharmaceutical and laboratory orders) and therefore do not report laboratory/radiology orders separately (extracted data taken from Table 16,page 54) |
|||||
| Comparator: OCS alone (no suggested corollary orders) | |||||
| Concomitant interventions: NA | |||||
| CDSS tool | |||||
| Name of CDDS (if any) | NR | ||||
| CDSS reasoning method | Discrimination rules | ||||
| CDSS knowledge base | Standard reference text books, drug package inserts and local practice guidelines | ||||
| Did study use training set and test set data? |
Training set: No Test set: No |
||||
| For training set data complete the following: | |||||
| Design | NR | ||||
| Target decision | NR | ||||
| Sample characteristics: | Intervention | Control | |||
| Sample size (n) | NR | NR | |||
| Age (mean; range) | NR | NR | |||
| Gender (n male; n female) | NR | NR | |||
| Disease type | NR | NR | |||
| For test set data complete the following: | |||||
| Properties of test set data | NR | ||||
| Test centre | NR | ||||
| Target decision | NR | ||||
| Other CDSS information | |||||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | NR | ||||
| 2. Time to complete the CDSS (minutes) | NR | ||||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Suggested corollary orders | ||||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||||
| 5. Is user instructional training at the time of implementation described? | No | ||||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||||
| 8. Is there a need for additional data entry by the clinician? | No | ||||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | ||||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | Yes | ||||
| 13. Does the CDSS promote action rather than inaction? | NR | ||||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||||
| 16. Were local users involved in the CDSS development process? | No | ||||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | ||||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||||
| Outcome measures | |||||
| Outcome 1: 24-hour compliance rates for the 25 most common corollary orders (only data on laboratory test orders extracted) | |||||
| Outcome 2: Length of hospital stay | |||||
| Outcome 3: Total inpatient charges | |||||
| Total length of follow-up: 6 months (beginning October 1992) | |||||
| Follow-up assessment times: 6 months | |||||
| Rate of attrition at each follow-up time: NA | |||||
| Methods of statistical analysis: General estimating equation models and t-tests were used to analyse the immediate 24-hour and hospital stay compliance with suggested collory orders. T-tests were used to assess differences between the intervention group and comparator group for length of hospital stay and costs, and t- and chi-squared tests were used to assess baseline differences between groups | |||||
| Results | |||||
| Outcome 1: 24-hour compliance rates for the 25 most common corollary orders (only data on laboratory test orders extracted) | |||||
| Suggested order | Total orders | OCS + CDSS compliance (%) | OCS alone compliance (%) | Compliance increase (%) | |
| Serum creatinine | 1209 | 48.28% | 41.18% | 7.10% | |
| Serum electrolytes | 1034 | 87.03% | 70.86% | 16.18% | |
| Glycosylated HbA1C | 821 | 23.71% | 7.39% | 16.32% | |
| Activated partial thromboplastin time | 615 | 89.21% | 59.56% | 29.65% | |
| Serum glutamic pyruvic transaminase (alanine aminotransferase) | 569 | 12.63% | 1.87% | 10.76% | |
| Serum glutamic oxaloacetic transaminase (aspartate aminotransferase) | 467 | 7.14% | 0.00% | 7.14% | |
| Capillary glucose | 446 | 30.77% | 4.41% | 26.36% | |
| Blood cell profile | 382 | 80.46% | 51.44% | 29.02% | |
| Stool occult blood test | 374 | 60.94% | 12.09% | 48.85% | |
| Prothrombin time | 320 | 64.57% | 45.52% | 19.05% | |
| Theophylline level | 270 | 75.89% | 46.51% | 29.38% | |
| Platelet count | 236 | 70.00% | 15.09% | 54.91% | |
| Reticulocyte count | 205 | 19.66% | 11.36% | 8.29% | |
| Fe-TIBC | 149 | 12.64% | 0.00% | 12.64% | |
| Vancomycin | 143 | 90.74% | 65.17% | 25.57% | |
| Phenytoin level | 140 | 73.13% | 38.36% | 34.78% | |
| A-V blood gas | 123 | 72.60% | 0.00% | 72.60% | |
| Gentamicin level | 118 | 90.00% | 75.86% | 14.14% | |
| Authors’ conclusions: This study demonstrates that physician workstations, linked to a comprehensive electronic medical record, can be an efficient means for decreasing errors of omissions and improving adherence to practice guidelines | |||||
| Methodological assessment criteria (Overhage) (1997)24 | |||||
| 1. Is the study properly randomised? | Yes | ||||
| 2. Did the analysis take clustering into account? | Yes | ||||
| 3. Were the study eligibility criteria specified? | Yes | ||||
| 4. Are adequate baseline details presented? | Partial | ||||
| 5. Are groups similar at baseline? | Yes | ||||
| 6. Are any baseline imbalances adequately adjusted for in the analysis? | NA | ||||
| 7. Are similar cointerventions administered? | NA | ||||
| 8. Are physician’s blinded to treatment allocation? | No | ||||
| 9. Are outcome assessors blinded? | Unclear | ||||
| 10. Are data analyses appropriate? | Yes | ||||
| 11. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Yes | ||||
| 12. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | ||||
| 13. Are the conclusions supported by the results? | Yes | ||||
| Comments: None | |||||
| Study demographics | |||||||
|---|---|---|---|---|---|---|---|
| Author; year; study ID: Palen (2006)90 | |||||||
| Title: Evaluation of laboratory monitoring alerts within a computerized physician order entry system for medication orders | |||||||
| Country: USA | |||||||
| Specific setting: Kaiser Permanente group-model managed care organisation with more than 350,000 members | |||||||
| Study objectives: To evaluate if computerised non-intrusive reminders presented during OCS for medications would increase physicians’ compliance with guidelines for laboratory monitoring at initiation of therapy | |||||||
| Health-care setting: Primary care | |||||||
| If secondary care, academic status of the hospital: NA | |||||||
| Health-care system: Group-model managed care organisation (Kaiser Permanente) | |||||||
| Study design: CRCT | |||||||
| Number of sites: 16 | |||||||
| Funding source: Public sector | |||||||
| Was evaluator of tool also its developer? In part | |||||||
| System users | |||||||
| Intervention group | Control group | ||||||
| CDSS user(s) | Physician | Physician | |||||
| Practitioners (n) | 104 | 103 | |||||
| If the CDSS user is a doctor, complete n for the following: | Primary-care physicians | Primary-care physicians | |||||
| Consultant (attending) | |||||||
| Registrar (chief resident) | |||||||
| SHO (resident) | |||||||
| HO (intern) | |||||||
| Practitioners (n) in analysis | 104 | 103 | |||||
| Inclusion criteria: NR | |||||||
| Exclusion criteria: NR | |||||||
| Patient baseline demographics | |||||||
| Inclusion criteria: All patients must have (1) been a health plan member for at least 180 days before and 14 days after drug dispensing, (2) received the drug dispensing between 1 November 2002, and 31 October 2003, (3) not received a dispensing of that drug within the previous 180 days (i.e. the drug dispensing was the index dispensing), and (4) had the drug order by a physician in the control or intervention study group | |||||||
| Exclusion criteria: NR | |||||||
| Intervention group | Control group | p-value | |||||
| N Index Dispensings | 14,084 | 12,502 | – | ||||
| Age (median; 5th and 95th percentile) | 64 (40; 85) | 64 (40; 84) | 0.90 | ||||
| Age at dispensing (mean; SD; range) | 63.20 ± 13.98 (range: 18; 89) | ||||||
| Gender (female: n; %) | 7523 (53.4%) | –6853 (54.8%) | 0.22 | ||||
| Prescribed 1 medication (n; %) | 20,433 (76.9%) | – | |||||
| Prescribed 2 medications (n; %) | 4903 (18.4%) | – | |||||
| Prescribed 3–6 medications (n; %) | 1250 (4.7%) | – | |||||
| Interventions | |||||||
| Intervention: OCS plus CDSS. All physicians had a ‘custom formulary’ of medications loaded into their OCS. In addition to this list, an additional field contained information specific to recommended laboratory monitoring for the selected study medications. This information was specific for the individual medication and presented guidelines for appropriate baseline and on-going monitoring. The selected study medications (25) were: angiotensin-converting enzyme inhibitors (captopil, lisinopril); angiotensin-II receptor blocker (losartan potassium); antiarrhythmic (digoxin); antiinfective agents (isoniazid, rifampin); antigout (allopurinol, colchicine); cholesterol-lowering (atorvastatin calcium, gemfibrozil, lovastatin, niacin, simvastatin); diurectics (bumetanide, furosemide, hydrochlorothiazide, spironolactone, triamterene/hydrochlorothiazide); hyperglycemics (methotrexate, pioglitazone); and neurologica (carbamazepine, phenytoin sodium, valproic acid). At the end of the study period prescribing data for study medications was analysed to assess if appropriate laboratory tests had been completed within either 2 weeks after the medication order or had been ‘recently performed’. ‘Recently performed’ tests were defined as those completed within 180 days before medication dispensing and 2 weeks after dispensing. Physicians were defined as having followed the laboratory monitoring guideline if results of completed laboratory tests were available for review in EMR within these time frames (i.e. 180 days pre-dispensing and 2 weeks post-dispensing) | |||||||
| Comparator: OCS alone. Physicians received the standard list of medications loaded into their custom formularies | |||||||
| Concomitant interventions: NA | |||||||
| CDSS tool | |||||||
| Name of CDDS (if any) | Clinical Information System; IBM; Boulder, CO, USA | ||||||
| CDSS reasoning method | NR | ||||||
| CDSS knowledge base | Published guidelines and internal clinical guidelines; expert physician and clinical pharmacist opinion | ||||||
| Did study use training set and test set data? |
Training set: NR Test set: NR |
||||||
| For training set data complete the following: | |||||||
| Design | NR | ||||||
| Target decision | NR | ||||||
| Sample characteristics: | Intervention | Control | |||||
| Sample size (n) | NR | NR | |||||
| Age (mean; range) | NR | NR | |||||
| Gender (n male; n female) | NR | NR | |||||
| Disease type | NR | NR | |||||
| For test set data complete the following: | |||||||
| Properties of test set data | NR | ||||||
| Test centre | NR | ||||||
| Target decision | NR | ||||||
| Other CDSS information | |||||||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | Medication related alerts for baseline laboratory monitoring tests | ||||||
| 2. Time to complete the CDSS (minutes) | NR | ||||||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Alert | ||||||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||||||
| 5. Is user instructional training at the time of implementation described? | Yes | ||||||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||||||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||||||
| 8. Is there a need for additional data entry by the clinician? | No | ||||||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | ||||||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||||||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||||||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | No | ||||||
| 13. Does the CDSS promote action rather than inaction? | NR | ||||||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||||||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||||||
| 16. Were local users involved in the CDSS development process? | No | ||||||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||||||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | ||||||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||||||
| Outcome measures | |||||||
| Outcome 1: Compliance with recommended laboratory monitoring tests | |||||||
| Outcome 2:.Compliance with recommended laboratory monitoring tests by patient gender | |||||||
| Outcome 3:.Compliance with recommended laboratory monitoring tests by medication | |||||||
| Total length of follow-up: 12 months (1 November 2002 to 31 October 2003) | |||||||
| Follow-up assessment times: 12 months | |||||||
| Rate of attrition at each follow-up time: NA | |||||||
| Methods of statistical analysis: Chi-squared test was used to compare differences in laboratory monitoring rates between groups, by drug or drug class and by gender | |||||||
| Results | |||||||
| Outcome 1: Compliance with recommended laboratory monitoring tests | |||||||
| Intervention | OCS + CDSS (alerts) | OCS alone | Difference between groupsb | ||||
| N in analysis | 18,556 | 15,686 | – | ||||
| Compliance rates (n: %) | 10,494 (56.6%) | 8957 (57.1%) | p = 0.31 | ||||
| Outcome 2: Compliance with recommended laboratory monitoring tests by patient gender | |||||||
| Intervention | OCS + CDSS (alerts) | OCS alone | Difference between groupss | ||||
| Compliance rates in males (%) | 57.5% 58.5% | 58.5% | p = 0.18 | ||||
| Compliance rates in females (%) | 55.7% | 55.9% | p = 0.82 | ||||
| Outcome 3: Compliance with recommended laboratory monitoring tests by medicationa | |||||||
| Medication | All dispensings | Intervention | Control | p-valueb | |||
| ACE inhibitors | 5828 (47.2%) | 3099 (47.0%) | 2729 (47.5%) | 0.681 | |||
| Allopurinol | 784 (59.2%) | 429 (57.6%) | 355 (61.1%) | 0.31 | |||
| Carbamazepine | 272 (34.9%) | 153 (34.6%) | 119 (35.3%) | 0.91 | |||
| Colchicine | 811 (49.6%) | 411 (52.8%) | 400 (46.0%) | 0.05 | |||
| Dogoxin | 420 (52.4%) | 242 (55.0%) | 178 (48.9%0 | 0.22 | |||
| Diuretic | 9654 (44.7%) | 5384 (44.0%) | 4270 (45.6%) | 0.11 | |||
| Gemfibrozil | 1023 (67.3%) | 569 (71.2%) | 454 (62.3%) | 0.003 | |||
| Isoniazid | 69 (17.4%) | 33 (15.2%) | 36 (19.4%) | 0.64 | |||
| Losartan pottassium | 939 (52.3%) | 506 (52.0%) | 433 (52.7%) | 0.84 | |||
| Metformin hydrochloride | 2038 (69.0%) | 1098 (67.7%) | 940 (7.6%) | 0.14 | |||
| Methotrexate | 16 (18.7%) | 7 (42.9%) | 9 (0.0%) | 0.03 | |||
| Niacin | 70 (57.1%) | 34 (67.7%) | 36 (47.2%) | 0.084 | |||
| Phenytoin sodium | 135 (29.6%) | 83 (32.5%) | 52 (25.0%) | 0.35 | |||
| Pioglitaone hydrochloride | 139 (92.8%) | 76 (92.1%) | 63 (93.7%) | 0.73 | |||
| Potassium chloride | 2914 (55.8%) | 1623 (54.3%) | 1291 (57.8%) | 0.06 | |||
| Rifampin | 13 (30.8%) | 7 (14.3%) | 6 (50.0%) | 0.20 | |||
| Statins | 8962 (74.9%) | 4717 (75.7%) | 4245 (73.9%) | 0.05 | |||
| Valproic acid | 155 (37.4%) | 85 (36.5%) | 70 (38.6%) | 0.79 | |||
| Total | 34,242 | 18,556 | 15,686 | 0.79 | |||
| a Data are given as number (% monitored) unless otherwise indicated. | |||||||
| b Chi-squared test. | |||||||
| Authors’ conclusions: As OCS becomes more prevalent, additional research is needed to determine effective decision support tools. These findings then should be communicated to the developers and users of computerised medical record systems | |||||||
| Methodological assessment criteria (Palen) (2006)90 | |||||||
| 1. Is the study properly randomised? | Yes | ||||||
| 2. Did the analysis take clustering into account? | No | ||||||
| 3. Were the study eligibility criteria specified? | Partial | ||||||
| 4. Are adequate baseline details presented? | Yes | ||||||
| 5. Are groups similar at baseline? | Yes | ||||||
| 6. Are any baseline imbalances adequately adjusted for in the analysis? | NA | ||||||
| 7. Are similar cointerventions administered? | NA | ||||||
| 8. Are physician’s blinded to treatment allocation? | No | ||||||
| 9. Are outcome assessors blinded? | Unclear | ||||||
| 10. Are data analyses appropriate? | Partial | ||||||
| 11. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Yes | ||||||
| 12. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | ||||||
| 13. Are the conclusions supported by the results? | Yes | ||||||
| Comments: No ICC reported in design and clustering by physician was not taken into account in the analysis (unit of analysis was patient–drug therapy combination) | |||||||
| Study demographics | |||||
|---|---|---|---|---|---|
|
Author; year; study ID: Matheny (2008)91 Title: A randomised trial of electronic clinical reminders to improve medication laboratory monitoring Country: USA Specific setting: Partners Healthcare system (including Brigham and Women’s Hospital, Massachusetts General Hospital, and a number of community hospitals and outpatient clinics) Study objectives: To assess the impact of electronic reminders delivered to primary care physicians on rates of appropriate routine medication laboratory monitoring Health-care setting: Outpatient clinics (4 community health centres, 9 hospital-based clinics, 7 off-site practices) If secondary care, academic status of the hospital: Academic teaching Health-care system: NR Study design: CRCT Number of sites: 20 (10 intervention, 10 control) Funding source: Public sector Was evaluator of tool also its developer? Yes |
|||||
| System users | |||||
| Intervention group | Control group | ||||
| CDSS user(s) | Physician | Physician | |||
| Practitioners (n) | 145 | 158 | |||
|
If the CDSS user is a doctor, complete n for the following: Consultant (attending) Registrar (chief resident) SHO (resident) HO (intern) |
NR | NR | |||
| Age (mean; SD) | 40.5 (11.1) | 40.6 (11.2) | |||
| Female (%) | 90 (62.1) | 93 (58.8) | |||
|
Inclusion criteria: All physicians using OCS with a patient on one of the 15 target study medications for at least 365 days in which no relevant laboratory monitoring test had been undertaken. The study target medications were selected for inclusion due to (1) the prevalence of their use; (2) potential morbidity associated with failure to perform appropriate laboratory monitoring, and were based on a review of evidence based guidelines for routine medication laboratory monitoring. The 15 target medications included: (1) non-steroidal anti-inflammatory drugs (NSAIDs); (2) angiotensin receptor blockers (ARB); (3) metformin; (4) potassium supplements; (5) potassium sparing diuretic; (6) thiazide diurectic; (7) angiotensin-converting enzyme inhibitors; (8) HMG-CoA reductase inhibitors (statins); (9) thyroxine; (10) carbamazapine; (11) ciclosporin; (12) phenobarbital; (13) phenytoin; (14) proc-NAPA; and (15) valproate. Laboratory monitoring focused on tests for potassium, creatinine, liver function, thyroid function, and therapeutic levels as appropriate for the other medications Exclusion criteria: NR |
|||||
| Patient baseline demographics | |||||
|
Inclusion criteria: All outpatients seen during the 6-month study period (1 Janary 2004 to 30 June 2004) on one or more of the 15 target study for at least 365 days for which no relevant laboratory monitoring tests were conducted in the preceding year Exclusion criteria: NR |
|||||
| Patient characteristics | Intervention group | Control group | p -value | ||
| N | 924 | 998 | – | ||
| Age (mean, yr, SD) | 60.2 (14.3) | 60.2 (14.6) | 0.996 | ||
| Female (%) | 530 (57.4) | 605 (60.6) | 0.150 | ||
| Race (%) | |||||
| White | 509 (55.1) | 596 (59.7) | 0.042 | ||
| African American | 107 (11.6) | 82 (8.2) | 0.041 | ||
| Hispanic | 153 (16.6) | 66 (6.6) | <0.001 | ||
| Other | 30 (3.2) | 43 (4.3) | 0.234 | ||
| Unknown | 125 (13.5) | 211 (21.1) | <0.001 | ||
| Insurance (%) | |||||
| Medicare | 326 (35.3) | 328 (32.9) | 0.268 | ||
| Medicaid | 126 (13.6) | 74 (7.4) | <0.001 | ||
| Private | 447 (48.4) | 579 (58.0) | <0.001 | ||
| Self-pay | 25 (2.7) | 17 (1.7) | 0.160 | ||
| Interventions | |||||
|
Intervention: OCS with reminder generated if patient was on one or more of the study target medications for at least 365 days and there was no relevant laboratory test for that medication within that timeframe Comparator: OCS with reminder suppressed Concomitant interventions: NA |
|||||
| CDSS tool | |||||
| Name of CDDS (if any) | NR (Brigham and Women’s Hospital) | ||||
| CDSS reasoning method | DR | ||||
| CDSS knowledge base | Test-specific monitoring intervals of 1 year were based on a review of the literature. | ||||
| Did study use training set and test set data? |
Training set: NR Test set: NR. |
||||
| For training set data complete the following: | |||||
| Design | NR | ||||
| Target decision | NR | ||||
|
Sample characteristics: Sample size (n) Age (mean; range) Gender (n male; n female) Disease type |
Intervention NR NR NR NR |
Control NR NR NR NR |
|||
| For test set data complete the following: | |||||
| Properties of test set data | NR | ||||
| Test centre | NR | ||||
| Target decision | NR | ||||
| Other CDSS information | |||||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | 1; time interval from previously ordered test | ||||
| 2. Time to complete the CDSS (minutes) | NR | ||||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Reminder | ||||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||||
| 5. Is user instructional training at the time of implementation described? | No | ||||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||||
| 8. Is there a need for additional data entry by the clinician? | No | ||||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | ||||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | Yes | ||||
| 13. Does the CDSS promote action rather than inaction? | Yes | ||||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||||
| 16. Were local users involved in the CDSS development process? | Unclear | ||||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | ||||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||||
| Outcome measures | |||||
|
Outcome 1: Number of appropriate laboratory tests ordered when overdue within 14 days Total length of follow-up: 6 months (1 January 2004 to 30 June 2004) Follow-up assessment times: 6 months Rate of attrition at each follow-up time: NA Methods of statistical analysis: All reminders for therapeutic drug level monitoring were aggregated into one category for analyses. Multivariable logistic regression models were used to assess the impact of the reminder system on rates of appropriate laboratory monitoring with adjustment for patient age, gender, race and insurance status as well as provider age and gender. GENMOD procedure within sas was used to account for clustering of patients by surgery site |
|||||
| Results | |||||
| Outcome 1: Number of appropriate laboratory tests ordered when overdue within 14 days | |||||
| Medication-laboratory reminder | Visits (n) | Visits with laboratory overdue (n; %) | Laboratory ordered when overdue (n; %) | Odds Ratio (adjusted) (95% CI) | p-value |
| NSAID-Cr | |||||
| OCS + CDSS | 8487 | 442 (5.2%) | 150 (33.9%) | 1.24 (0.71 to 2.15) | 0.457 |
| OCS alone | 9307 | 428 (4.6%) | 136 (31.8%) | ||
| ARB-Cr | |||||
| OCS + CDSS | 751 | 31 (4.1%) | 17 (54.8%) | 0.24 (0.04 to 1.34) | 0.104 |
| OCS alone | 832 | 27 (3.2%) | 17 (63.0%) | ||
| Metformin-Cr | |||||
| OCS + CDSS | 857 | 20 (2.3%) | 7 (35.0%) | 0.53 (0.05 to 5.34) | 0.594 |
| OCS alone | 781 | 16 (2.1%) | 6 (37.5%) | ||
| K Supplement-K | |||||
| OCS + CDSS | 579 | 12 (2.1%) | 7 (58.3%) | 0.91 (0.03 to 24.44) | 0.956 |
| OCS alone | 751 | 9 (1.2%) | 5 (55.5%) | ||
| K Sparing Diuretic-K | |||||
| OCS + CDSS | 761 | 19 (2.5%) | 13 (68.4%) | 0.82 (0.12 to 5.60) | 0.836 |
| OCS alone | 875 | 28 (3.2%) | 17 (60.7%) | ||
| Thiazide Diuretic-K | |||||
| OCS + CDSS | 1997 | 62 (3.1%) | 40 (64.5%) | 1.30 (0.63 to 2.67) | 0.473 |
| OCS alone | 2508 | 89 (3.5%) | 46 (51.7%) | ||
| ACE Inhibitor-K | |||||
| OCS + CDSS | 2279 | 119 (5.2%) | 57 (47.9%) | 1.00 (0.43 to 2.30) | 0.993 |
| OCS alone | 2790 | 80 (2.9%) | 40 (50.0%) | ||
| Statin-ALT | |||||
| OCS + CDSS | 9441 | 613 (6.5%) | 291 (47.5%) | 0.89 (0.43 to 1.81) | 0.740 |
| OCS alone | 10935 | 674 (6.2%) | 358 (53.1%) | ||
| Thyroxine-TSH | |||||
| OCS + CDSS | 897 | 38 (4.2%) | 22 (57.9%) | 1/19 (0.40 to 3.53) | 0.747 |
| OCS alone | 1233 | 44 (3.6%) | 25 (56.8%) | ||
| Therapeutic level a | |||||
| OCS + CDSS | 514 | 16 (3.1%) | 2 (12.5%) | 0.55 (0.03 to 8.94) | 0.677 |
| OCS alone | 755 | 26 (3.4%) | 4 (15.4%) | ||
| a Represents the aggregated reminders for therapeutic monitoring for carbamazapine, ciclosporin, phenobarbital, phenytoin, proc-NAPA and valproate. | |||||
| Authors’ conclusions: High rates of appropriate baseline laboratory monitoring were identified, and electronic reminders did not significantly improve these monitoring rates. Future studies should focus on settings with lower baseline adherence rates and alternate drug–laboratory combinations | |||||
| Methodological assessment criteria Matheny (2008)91 | |||||
| 1. Is the study properly randomised? | Yes | ||||
| 2. Did the analysis take clustering into account? | Yes | ||||
| 3. Were the study eligibility criteria specified? | Yes | ||||
| 4. Are adequate baseline details presented? | Yes | ||||
| 5. Are groups similar at baseline? | No | ||||
| 6. Are any baseline imbalances adequately adjusted for in the analysis? | Yes | ||||
| 7. Are similar cointerventions administered? | NA | ||||
| 8. Are physician’s blinded to treatment allocation? | No | ||||
| 9. Are outcome assessors blinded? | Unclear | ||||
| 10. Are data analyses appropriate? | Yes | ||||
| 11. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Yes | ||||
| 12. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | ||||
| 13. Are the conclusions supported by the results? | Yes | ||||
| Comments: None | |||||
| Study demographics | |||
|---|---|---|---|
|
Author; year; study ID: Bates (1999)92 Title: A randomised trial of a computer-based intervention to reduce utilization of redundant laboratory tests Country: USA Specific setting: Brigham and Women’s Hospital, Boston, MA, USA Study objectives: To determine the degree to which reminders for apparently redundant laboratory tests affects (1) the number of tests ordered, (2) the number of test performed, (3) to evaluate what proportion of overrides of reminders are justified, (4) whether the cancellation of tests resulted in adverse effects for patients, and (5) to assess the charge savings and potential for additional savings associated with giving reminders for apparently redundant tests Health-care setting: Secondary care (all inpatients) If secondary care, academic status of the hospital: NR Health-care system: NR Study design: RCT Number of sites: 1 (720-bed hospital) Funding source: Public sector Was evaluator of tool also its developer? Yes |
|||
| System users | |||
| Intervention group | Control group | ||
| CDSS user(s) | Physician | Physician | |
| Practitioners (n) | NR | NR | |
|
If the CDSS user is a doctor, complete n for the following: Consultant (attending) Registrar (chief resident) SHO (resident) HO (intern) |
NR | NR | |
| Practitioners (n) in analysis | NR | NR | |
|
Inclusion criteria: All physicians using OCS during the study period to order any one of the following 13 tests: chemistry-20 profiles; aminophyilline level; digoxin level; vancomycin level; gentamicin level; tobramycin level; amikacin level; urinalysis; urine culture; stool culture; sputum culture; Clostridium difficile toxin assay or fibrin split products. Test were chosen as candidates for redundant reminders as they were either commonly ordered or because the marginal cost of performing the test was high. Additionally, there needed to be published literature or clinical consensus about how often the tests should be performed. The interval in which a test was considered redundant was 20 hours in most instances. During the study period physicians could order tests through the OCS or by sending specimens directly to the laboratory in a labelled envelope, without using the OCS Exclusion criteria: NR * Note: 56% of redundant test performed did not have a computer order and 50% of tests with a computer order were not screened for redundancy because they were ordered as part of an order set |
|||
| Patient baseline demographics | |||
|
Inclusion criteria: All inpatients during the 4-month period between 28 June and 30 October, 1994, excluding 3 weeks from 27 July 1994 to 16 August 1994 Exclusion criteria: NR |
|||
| Intervention group | Control group | ||
| All admissions in study period (n) | 5700 | 5886 | |
| Age (mean; SD) | 38.7 ± 24.6 | 39.2 ± 24.8 | |
| Gender (female %) | 65 | 66 | |
| Medical condition(s) | NR | NR | |
| Total number of tests | 131,563 | 132,068 | |
| Number of study tests | 13,425 | 13,847 | |
| Admissions with ≥ 1 study test (n)a | 2478 | 2581 | |
| Age (mean; range) | 50.0 ± 22.0 | 50.9 ± 2.0 | |
| Gender (female %) | 59 | 59 | |
| Medical condition(s) | NR | NR | |
| a Compared to the entire sample patients with ≥ 1 study test were older and more frequently male. | |||
| Interventions | |||
|
Intervention: OCS with alert if test had previously been ordered within its test-specific interval. Previous test results were displayed where available. Checking for redundancy was only performed for tests ordered from the main order entry screens and not for tests ordered using order sets or templates (tools that allow many orders to be entered at once). When a reminder was delivered the default response was to cancel the test Comparator: OCS with reminder suppressed Concomitant interventions: NA |
|||
| CDSS tool | |||
| Name of CDDS (if any) | NR | ||
| CDSS reasoning method | NR | ||
| CDSS knowledge base | Test-specific intervals within which a second test was considered redundant were based on a review of the literature. They were evaluated retrospectively by applying them to a random sample of patients from an earlier study. This information and clinical evaluation was used to select the intervals used in the study | ||
| Did study use training set and test set data? |
Training set: NR Test set: NR |
||
| For training set data complete the following: | |||
| Design | NR | ||
| Target decision | NR | ||
|
Sample characteristics: Sample size (n) Age (mean; range) Gender (n male; n female) Disease type |
Intervention NR NR NR NR |
Control NR NR NR NR |
|
| For test set data complete the following: | |||
| Properties of test set data | NR | ||
| Test centre | NR | ||
| Target decision | NR | ||
| Other CDSS information | |||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | 1; time interval from previously ordered test | ||
| 2. Time to complete the CDSS (minutes) | NR | ||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Reminder | ||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||
| 5. Is user instructional training at the time of implementation described? | No | ||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||
| 8. Is there a need for additional data entry by the clinician? | No | ||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | Yes | ||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | No | ||
| 13. Does the CDSS promote action rather than inaction? | No | ||
| 14. Does the CDSS justify the output by provision of reasoning? | Yes | ||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||
| 16. Were local users involved in the CDSS development process? | Unclear | ||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||
| 18. Does the CDSS provide periodic summaries of performance feedback? (yes; no; unclear) | No | ||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||
| Outcome measures | |||
|
Outcome 1: Proportion of reminders accepted Outcome 2: Proportion of test performed after reminder Outcome 3: Proportion of tests performed earlier than test-specific intervals Outcome 4: Proportion of justified overrides of reminders by specific test Outcome 5: Adverse effects of test cancellation (new abnormal results for the same test performed within 3 days of cancellation) Outcome 6: Charge savings associated with reminders for redundant tests Total length of follow-up: 4 months from 28 June to 30 October 1994. Data were also collected from the preceding 4-month period to assess any changes in the overall frequency of test ordering Follow-up assessment times: 4 months Rate of attrition at each follow-up time: NA Methods of statistical analysis: Comparisons between intervention and control group and between different time periods made using Student’s t-test for continuous variables and chi-square test for categorical variables. Annual charge savings estimated by multiplying the 1994 charges for each test by the number of test cancelled, and annualised to 1 year. Statistical significance set at p < 0.05 (two sided) |
|||
| Results | |||
| Outcome 1: Proportion of reminders accepted | |||
| Intervention | OCS + CDSS (reminders) | OCS alone | Difference between groups |
| N in analysis | 437 | 502a | – |
| 300 (69%) | NAb | ||
|
a Tests that would have received reminders. b Not applicable. |
|||
| Outcome 2: Proportion of test performed after reminder | |||
| Intervention | OCS + CDSS (reminders) | OCS alone | Difference between groups |
| N in analysis | 437 | 502a | |
| 117 (27%) | 257 (51%) | p < 0.0001 | |
| a Tests that would have received reminders. | |||
| Outcome 3: Proportion of tests performed earlier than test-specific intervals (change from baseline) | |||
| Baseline (both groups) | OCS + CDSS (reminders) | OCS alone | Difference from baseline |
| 20.5% | 18.5% | 19.6% |
p = 0.004a p = 0.19b |
|
a Change from baseline in OCS + CDSS group. b Change from baseline in OCS alone group. |
|||
| Outcome 4: Proportion of justified overrides of reminders by specific test | |||
| Test | Number ordered | Number of overrides | Override justified |
| Urinalysis | 136 | 46 (34%) | 26 (57%) |
| Chemistry-20 profile | 113 | 38 (34%) | 24 (63%) |
| Urine culture | 110 | 25 (23%) | 3 (12%) |
| Sputum culture | 39 | 10 (26%) | 2 (20%) |
| Stool culture | 15 | 7 (47%) | 0 (0%) |
| Other | 24 | 11 (46%) | 1 (9%) |
| Total | 437 | 137 (31%) | 56 (41%) |
| Outcome 5: Adverse effects of test cancellation (new abnormal results for the same test performed within 3 days of cancellation): Chemsitry-20 profiles were excluded from the analysis due to high probability of an abnormal result on at least one of the tests in the panel. Of the remaining 225 accepted reminders, 119 (53%) were followed by another test of the same type within 72 hours; 55 (24%) of these were abnormal. Only 10 (4%) of these had not been preceded by a similar abnormal result within 24 hours before the cancelled test, and two were duplicate orders for the same patient. Therefore only 8(4%) of tests provided new information | |||
| Outcome 6: Charge savings associated with reminders for redundant tests (1994) rates: US$35,000 | |||
| Authors’ conclusions: Reminders about orders for apparently redundant laboratory tests are effective when delivered. However, the overall effect was limited because many tests were performed without corresponding computer orders, and many orders were not screened for redundancy | |||
| Methodological assessment criteria (Bates) (1999)92 | |||
| 1. Is the study properly randomised? | Yes | ||
| 2. Is allocation of treatment concealed? | Yes | ||
| 3. Were the study eligibility criteria specified? | Yes | ||
| 4. Are adequate baseline details presented? | Partial | ||
| 5. Are groups similar at baseline? | Yes | ||
| 6. Are any baseline imbalances adequately adjusted for in the analysis? | NA | ||
| 7. Are similar cointerventions administered? | NA | ||
| 8. Are physician’s blinded to treatment allocation? | No | ||
| 9. Are outcome assessors blinded? | Yes | ||
| 10. Are data analyses appropriate? | Yes | ||
| 11. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Yes | ||
| 12. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | ||
| 13. Are the conclusions supported by the results? | Yes | ||
| Comments: contamination likely as patients are the unit of randomisation and physicians both received reminders/had reminders suppressed at the same time | |||
| Study demographics | |||||
|---|---|---|---|---|---|
|
Author; year; study ID: O’Connor (2005)57 Title: Impact of an electronic medical record on diabetes quality of care Country: USA Specific setting: HealthPartners Medical Group; Minnesota (Multi-specialty medical group that provided care to 175,000 adults in 18 clinics in 1996) Study objectives: To assess whether implementation of an Electronic Medical Record (EMR) in a primary care clinic significantly improves process of care [appropriate frequency of testing for HbA1C and low-density lipoprotein (LDL)] or intermediate outcomes of care (change in HbA1C and LDL levels) for adults with diabetes mellitus Health-care setting: Primary care If secondary care, academic status of the hospital: NA Health-care system: NR Study design: CCT Number of sites: 2 Funding source: Private Sector (HealthPartners Medical Group) Was evaluator of tool also its developer? No |
|||||
| System users | |||||
| Intervention group | Control group | ||||
| CDSS user(s) | Physician | Physician | |||
| Practitioners (n) | 4 or 5 | NR | |||
|
If the CDSS user is a doctor, complete n for the following: Consultant (attending) Registrar (chief resident) SHO (resident) HO (intern) |
NR | NR | |||
| Practitioners (n) in analysis | NR | NR | |||
|
Inclusion criteria: NR Exclusion criteria: NR |
|||||
| Patient baseline demographics | |||||
|
Inclusion criteria: All adult patients (> 18 years) with an established diagnosis of diabetes at study baseline (1996) in both clinics. Patients were classified as having diabetes if in calendar year 1994 they had either (1) 1 or more inpatient or 2 or more outpatient ICD-9 codes for diabetes or (2) filled a prescription for a diabetes-specific drug (insulins, sulfonylureas, metformin, thiazolidinediones, alpha-glucosidase inhibitors, medlitinides). The clinic that each patient attended was identified in 1996, 1998 and 2000 based on number of visits and administrative data. Patients were included in the analysis only if they attended their original study clinical in all 3 study years and were still alive and enrolled in HealthPartners Medical Group on 31 December 2000 Exclusion criteria: NR |
|||||
| Patient characteristics | Intervention group | Control group | p-value | ||
| N | 57 | 65 | |||
| Age (years) (mean; SE) | 60.6 ± 1.62 | 59.4 ± 1.72 | p = 0.34 | ||
| Gender (male, %) | 54.4 | 58.5 | p = 0.65 | ||
| Charlson < 2 (%)a | 73.7 | 75.4 | p = 0.97 | ||
| Charlson = 2 (%)a | 15.8 | 15.4 | – | ||
| Charlson > 2 (%)a | 10.5 | 9.2 | – | ||
| a Charlson comorbidy score was based on the method of Charlson and colleagues121 and modified by Deyo and colleagues122 and Rush and colleagues.123 | |||||
| Interventions | |||||
|
Intervention: OCS plus CDSS (prompts and reminders). Prompts were included when a patient had no HbA1C test within 6 months, no urine micoalbuminiuria test within 1 year, had blood pressures of ≥130/85 mmHg, LDL levels of ≥130 mg/dL, HbA1C levels of ≥8% or no aspirin use if aged 40 years or older Comparator: OCS alone Concomitant interventions: Other diabetes-related care improvement activities (both clinics) |
|||||
| CDSS tool | |||||
| Name of CDDS (if any) | Epic Systems | ||||
| CDSS reasoning method | NR | ||||
| CDSS knowledge base | NR | ||||
| Did study use training set and test set data? |
Training set: NR Test set: NR |
||||
| For training set data complete the following: | |||||
| Design | NR | ||||
| Target decision | NR | ||||
|
Sample characteristics: Sample size (n) Age (mean; range) Gender (n male; n female) Disease type |
Intervention NR NR NR NR |
Control NR NR NR NR |
|||
| For test set data complete the following: | |||||
| Properties of test set data | |||||
| Test centre | NR | ||||
| Target decision | NR | ||||
| Other CDSS information | |||||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | NR | ||||
| 2. Time to complete the CDSS (minutes) | NR | ||||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Prompts and Reminder | ||||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||||
| 5. Is user instructional training at the time of implementation described? | Yes | ||||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||||
| 8. Is there a need for additional data entry by the clinician? | No | ||||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | ||||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | Yes | ||||
| 13. Does the CDSS promote action rather than inaction? | Yes | ||||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||||
| 16. Were local users involved in the CDSS development process? | No | ||||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | ||||
| 19. Is the CDSS used in conjunction with conventional education? | Yes | ||||
| Outcome measures | |||||
|
Outcome 1: Number of HbA1C tests in each study clinic in years 1996, 1998, and 2000 Outcome 2: Number of LDL cholesterol tests in each study clinic in years 1996, 1998, and 2000 Outcome 3: Percentage of patients having at least two HbA1C tests, one LDL test, or two HbA1C and one LDL test in each study clinic in 1996, 1998 and 2000 Outcome 4: Mean HbA1C test values in each study clinic in years 1996, 1998, and 2000 Total length of follow-up: 4 years Follow-up assessment times: Baseline (1996), 2 years (1998) and 4 years (2000) Rate of attrition at each follow-up time: For the outcome of mean HbA1C test values in each study clinic in years 1996, 1998 and 2000, 11 in the intervention group and 15 in the comparison group Methods of statistical analysis: Generalised linear models were used to assess whether the independent variables of electronic medical record (EMR) use and study year (1996, 1998 and 2000) were predictors of the number of tests performed, the proportion of patients with the recommended number of tests in a given year, and changes in test values. Patient age, gender and Charlson comorbidity score were entered as covariates into the model. In all models the unit of analysis was the patient, and the covariance structure among the repeated measures unspecified |
|||||
| Results | |||||
| Outcome 1: Number of HbA1C tests in each study clinic in years 1996, 1998, and 2000a | |||||
| Year | OCS + CDSS | OCS alone | Time by EMR p-value | ||
| N in analysis | 57 | 65 | – | ||
| 1996 | 1.67 | 1.75 | – | ||
| 1998 | 2.20 | 1.83 | 0.04 | ||
| 2000 | 2.46 | 1.63 | 0.001 | ||
| a Results adjusted for age, gender and Charlson comorbidity score. | |||||
| Outcome 2: Number of LDL cholesterol tests in each study clinic in years 1996, 1998, and 2000a | |||||
| Year | OCS + CDSS | OCS alone | Time by EMP p-value | ||
| N in analysis | 57 | 65 | – | ||
| 1996 | 0.54 | 0.49 | – | ||
| 1998 | 0.87 | 0.59 | 0.33 | ||
| 2000 | 1.45 | 0.92 | 0.19 | ||
| a Results adjusted for age, gender and Charlson comorbidity score. | |||||
| Outcome 3: Percentage of patients having at least two HbA1C tests, one LDL test, or two HbA1C and one LDL test in each study clinic in 1996, 1998 and 2000a | |||||
| OCS + CDSS | OCS alone | Time by EMP p-value | |||
| N in analysis | 57 | 65 | – | ||
| Test | |||||
| ≥2 HbA 1C tests | |||||
| 1996 | 47.4 | 55.4 | – | ||
| 1998 | 73.7 | 63.1 | 0.09 | ||
| 2000 | 78.9 | 53.9 | 0.002 | ||
| ≥1 LDL test | |||||
| 1996 | 42.1 | 46.2 | – | ||
| 1998 | 68.4 | 55.4 | 0.12 | ||
| 2000 | 84.2 | 72.3 | 0.12 | ||
| ≥2 HbA 1C and 1 LDL test | |||||
| 1996 | 29.8 | 30.8 | – | ||
| 1998 | 57.9 | 46.2 | 0.27 | ||
| 2000 | 70.2 | 46.2 | 0.03 | ||
| a Results adjusted for age, gender and Charlson comorbidity score. | |||||
| Outcome 4: Mean HbA1C test values in each study clinic in years 1996, 1998, and 2000a | |||||
| OCS + CDSS | OCS alone | Time by EMP p-value | |||
| N in analysis | 46 | 50 | – | ||
| 1996 | 7.80 | 7.35 | – | ||
| 1998 | 7.90 | 7.26 | 0.10 | ||
| 2000 | 7.71 | 7.11 | 0.27 | ||
| a Predicted least squares mean adjusted for age, gender and Charlson comorbidity score. | |||||
| Authors’ conclusions: EMR (with CDSS) led to an increased number of HbA1C and LDL tests but not to better metabolic control as evidence by patients HbA1C test values. If EMRs are to fulfil their promise as care improvement tools, improved implementation strategies and more sophisticated clinical decision support may be needed | |||||
| Methodological assessment criteria (O’Connor) (2005)57 | |||||
| 1. Were the study eligibility criteria specified? | Yes | ||||
| 2. Are adequate baseline details presented? | Yes | ||||
| 3. Are groups similar at baseline? | Yes | ||||
| 4. Are any baseline imbalances adequately adjusted for in the analysis? | NA | ||||
| 5. Are similar cointerventions administered? | Yes | ||||
| 6. Are physician’s blinded to treatment allocation? | No | ||||
| 7. Are outcome assessors blinded? | Unclear | ||||
| 8. Are data analyses appropriate? | Yes | ||||
| 9. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Yes | ||||
| 10. Are greater than 80% of physicians/patients included in the follow-up assessment? | Partial (outcome measure dependent) | ||||
| 11. Are the conclusions supported by the results? | Yes | ||||
| Comments: Small number of patients (n = 122) | |||||
| Study demographics | |||
|---|---|---|---|
|
Author; year; study ID: McDonald (1980)29 Title: Physicians response to computer reminders Country: USA Specific setting: Wishard Memorial Hospital, Indianapolis, IN, USA Study objectives: To assess whether a medical record system designed to detect and remind physicians about clinical events that might need corrective action significantly increases response rates to such events Health-care setting: Secondary care (all outpatients) If secondary care, academic status of the hospital: NR Health-care system: NR Study design: Randomised crossover trial Number of sites: 1 Funding source: Public Sector Was evaluator of tool also its developer? NR |
|||
| System users | |||
| Intervention group 1 | Intervention group 2 | Control group | |
| N | 31 | 31 | 31 |
| CDSS user(s) | Interns (n = 9) | Interns (n = 9) | Interns (n = 9) |
| Residents (n = 17) | Residents (n = 17) | Residents (n = 17) | |
| Nurse practitioners (n = 5) | Nurse practitioners (n = 5) | ||
| N in analysis | 31 | 31 | 31 |
|
Inclusion criteria: Interns, Residents, and Practice Nurses of the General Medicine Clinic involved in the diagnosis, and management of patients in the 15-week study period Exclusion criteria: NR |
|||
| Patient baseline demographics | |||
|
Inclusion criteria: NR Exclusion criteria: NR |
|||
| Patient characteristics | Intervention group | Control group | p-value |
| N | NR | NR | – |
| Age (mean; SD) | NR | NR | NR |
| Gender (female, %) | NR | NR | NR |
| Interventions | |||
|
Intervention: OCS plus CDSS (reminders). Study consisted of 15 weeks; 5 weeks of control period (C); 5 weeks of study period 1 (S1) and five weeks of study period 2 (S2). During S1 care providers were sent reminders about the detected conditions alone, and during S2 were sent reminders plus bibliographic citations supporting the reminders. The 3 intervention periods, C, S1 and S2 could be presented in six possible temporal orders Comparator: Control conditions with no reminders sent to care providers Concomitant interventions: NA |
|||
| CDSS tool | |||
| Name of CDDS (if any) | NR (home-grown system Wishard Memorial Hospital) | ||
| CDSS reasoning method | NR | ||
| CDSS knowledge base | NR | ||
| Did study use training set and test set data? |
Training set: NR Test set: NR |
||
| For training set data complete the following: | |||
| Design | NR | ||
| Target decision | NR | ||
| Sample characteristics: | Intervention 1 | Intervention 2 | Control |
| Sample size (n) | NR | NR | NR |
| Age (mean; range) | NR | NR | NR |
| Gender (n male; n female) | NR | NR | NR |
| Disease type | NR | NR | NR |
| For test set data complete the following: | |||
| Properties of test set data | |||
| Test centre | NR | ||
| Target decision | NR | ||
| Other CDSS information | |||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | NR | ||
| 2. Time to complete the CDSS (minutes) | NR | ||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Reminders | ||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||
| 5. Is user instructional training at the time of implementation described? | No | ||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||
| 8. Is there a need for additional data entry by the clinician? | No | ||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | ||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | No | ||
| 13. Does the CDSS promote action rather than inaction? | NR | ||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||
| 16. Were local users involved in the CDSS development process? | No | ||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | ||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||
| Outcome measures | |||
|
Outcome 1: Compliance with reminders to order a test Total length of follow-up: 15 weeks Follow-up assessment times: 15 weeks Rate of attrition at each follow-up time: NR Methods of statistical analysis: A variance-stabilising transformation and F-root transformation. Data from S1 and S2 periods were analysed together, as differences in compliance with reminder rates were not different between the two periods (S1 + S2/2) |
|||
| Results | |||
| Outcome 1: Compliance with reminders to order a test | |||
| OCS + CDSS | OCS alone | p-value | |
| Resident | |||
| Events detected (n) | 725 | 374 | – |
| Compliance with reminder (%) | 49.0% | 20.0% | p < 0.001 |
| Intern | |||
| Events detected (n) | 226 | 108 | – |
| Compliance with reminder (%) | 38.0% | 9.0% | p < 0.017 |
| Nurse clinician | |||
| Events detected (n) | 289 | 89 | – |
| Compliance with reminder (%) | 24.0% | 15.0% | Not statistically significant |
| Authors’ conclusions: Reminders significantly increased care providers response rate (in terms of test orders and treatment changes) to clinical events that might need corrective action | |||
| Methodological assessment criteria (McDonald) (1980)29 | |||
| 1. Is the study properly randomised? | No | ||
| 2. Were the study eligibility criteria specified? | No | ||
| 3.. Are adequate baseline details presented? | No | ||
| 4. Are groups similar at baseline? | Unclear | ||
| 5. Are any baseline imbalances adequately adjusted for in the analysis? | Unclear | ||
| 6. Is the ‘wash out’ period adequate? | No | ||
| 7. Are physician’s blinded to treatment allocation? | No | ||
| 8. Are outcome assessors blinded? | No | ||
| 9. Are data analyses appropriate? | Unclear | ||
| 10. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Yes | ||
| 11. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | ||
| 12. Are the conclusions supported by the results? | Partial | ||
| Comments: Very small number of participants (n = 31) in a sequential crossover trial. Potential for temporal effects due to learning through completing periods S1 and S2 before control condition | |||
| Study demographics | |||
|---|---|---|---|
|
Author; year; study ID: Carton (2002)93 Title: Assessment of radiological referral practice and effect of computer-based guidelines on radiological requests in two emergency departments Country: France Specific setting: Hopital Ambroise Pare, Boulogne-Billancourt and Hopital de Pontchaillou, Rennes, France Study objectives: To assess medical emergency radiology referral practice compared with a set of French guidelines and to measure the efficiency of computer-based guidelines on unnecessary medical imaging Health-care setting: Secondary care (A&E) If secondary care, academic status of the hospital: Teaching Health-care system: NR Study design: ITS (AB-AB-AB design) Number of sites: 2 Funding source: NR Was evaluator of tool also its developer? Yes |
|||
| System users | |||
| CDSS user(s) | Physician | ||
| Practitioners (n) | NR | ||
|
If the CDSS user is a doctor, complete n for the following: Consultant (attending) Registrar (chief resident) SHO (resident) HO (intern) |
NR | ||
| Practitioners (n) in analysis | NR | ||
|
Inclusion criteria: All physicians working in the emergency departments of either two hospitals who ordered a radiological examination within the study period. Study was conducted over 6 months between June and November 1998. Three control periods and three intervention periods were run alternately with no delay between periods; each period was about 1-month long Exclusion criteria: NR |
|||
| Patient baseline demographics | |||
|
Inclusion criteria: All patients for who a radiological examination was ordered during the study period in either emergency department Exclusion criteria: NR |
|||
| Number of patients seen in emergency departments (n) | 15,086 | ||
| Number of documented radiological requests (n) | 6434 | ||
| Number of requests excluded (n;%)a | 743 (11) | ||
| a 743 (11%) requests were excluded from analysis because the radiological examination and/or clinical context had been profoundly altered by hand. | |||
| Interventions | |||
|
Intervention: OCS with CDSS (reminders displaying the appropriate guideline recommendation). The guidelines were written by the Collège des Enseignants de Radiologie de France (French Society of Radiologists) based on the results of a review of the literature, existing guidelines and the expertise of all the societies of radiologists and clinicians. During the control periods, radiological requests were recorded but no action taken. During intervention periods, reminders displayed on screen the appropriate recommendations for the given clinical context Comparator: NA Concomitant interventions: NA |
|||
| CDSS tool | |||
| Name of CDDS (if any) | NR | ||
| CDSS reasoning method | NR | ||
| CDSS knowledge base | Guidelines based on a review of the literature, existing guidelines and the clinician expertise | ||
| Did study use training set and test set data? |
Training set: NR Test set: NR |
||
| For training set data complete the following: | |||
| Design | NR | ||
| Target decision | NR | ||
| Sample characteristics: | |||
| Sample size (n) | NR | ||
| Age (mean; range) | NR | ||
| Gender (n male; n female) | NR | ||
| Disease type | NR | ||
| For test set data complete the following: | |||
| Properties of test set data | NR | ||
| Test centre | NR | ||
| Target decision | NR | ||
| Other CDSS information | |||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | NR | ||
| 2. Time to complete the CDSS (minutes) | 1 minute | ||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Reminder | ||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||
| 5. Is user instructional training at the time of implementation described? | No | ||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Partial | ||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||
| 8. Is there a need for additional data entry by the clinician? | Yes | ||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | Yes | ||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | Yes | ||
| 13. Does the CDSS promote action rather than inaction? | NR | ||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||
| 16. Were local users involved in the CDSS development process? | No | ||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | ||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||
| Outcome measures | |||
|
Outcome 1: Number of radiography requests complying with guideline recommendations Outcome 2:.Type of radiography requests not complying with guideline recommendations Outcome 3: Most frequent radiography requests not complying with guideline recommendations Outcome 4: Number of radiography requests not complying with guideline recommendations by control and intervention periods Total length of follow-up: 6 months between June and November 1998 Follow-up assessment times: 6 months Rate of attrition at each follow-up time: 743/6434 (11%) requests were excluded from analysis because the radiological examination and/or clinical context had been profoundly altered by hand Methods of statistical analysis: comparison of the distribution of categorical variables was made using Pearson chi-squared test. All p-values were two-tailed, with values lower than 5% considered significant |
|||
| Results | |||
| Outcome 1: Number of radiography requests complying with guideline recommendations | |||
| Centre A (n; %) | Centre B (n; %) | Total (n; %) | |
| In agreement | 1657 (82.4) | 3166 (65.2) | 4823 (70.2) |
| Not in agreement | 353 (17.6) | 1693 (34.8) | 2046 (29.8) |
| Outcome 2: Type of radiography requests not complying with guideline recommendations | |||
| Radiography | Total (n) | (%)a | (%)b |
| Abdominal plain radiographs | 861 | 42.1 | 76.5 |
| Chest radiographs | 891 | 43.5 | 24.9 |
| CT of the brain | 162 | 7.9 | 15.8 |
| Others | 132 | 6.5 | 11.6 |
| Total | 2046 | 100 | 29.8 |
|
a Percentage of total examinations that were not in agreement with guideline. b Percentage of examinations that were not in agreement with guideline for a given radiography. |
|||
| Outcome 3: Most frequent radiography requests not complying with guideline recommendations | |||
| Clinical context | n (%) | ||
| Chest radiographs for systematic entry check-up | 611 (29.9) | ||
| Abdominal plain radiographs or abdominal ultrasonography for abdominal pain | 398 (19.4) | ||
| Chest radiographs for acute bronchitis | 145 (7.1) | ||
| CT of the brain for a first epileptic fit, without fever or neurological symptoms | 83 (4.1) | ||
| Abdominal plain radiographs for diarrhoea | 82 (4.0) | ||
| Abdominal plain radiographs for constipation | 68 (3.3) | ||
| Radiograph of the ribs for minor thoracic trauma | 44 (2.2) | ||
| Othersa | 615 (30.1) | ||
| a Represents 54 different requests that were not in agreement with guidelines, each with a frequency of under 2%. | |||
| Outcome 4: Number of radiography requests not complying with guideline recommendations by control and intervention periods | |||
| The proportion of requests not conforming to guidelines increased on each of three success occasions when the recommendations displayed on screen were switched off: from 27.5% to 29.8% (relative increase of 8.4%), from 27.0% to 37.8% (relative increase of 40%), and from 26.0% to 26.9% (relative increase of 3.5%) | |||
| Authors’ conclusions: While the computer provided advice that was tailored to the needs of individual patients concurrent with care, the effect of the intervention was weak. However, our study identified the few situations that were responsible for the majority of unnecessary radiological requests | |||
| Methodological assessment criteria (Carton) (2002)93 | |||
| 1. Is the intervention independent of other changes over time? | Unclear | ||
| 2. Are there sufficient data points to enable reliable statistical inference? | Yes | ||
| 3. Does the analysis include a formal test for trend? | No | ||
| 4. Is the intervention unlikely to affect data collection? | Yes | ||
| 5. Is the assessment of primary outcome blinded? | Yes | ||
| 6. Are greater than 80% of physicians/patients/episodes of care included in the follow-up assessment? | Yes | ||
| 7. Is the primary outcome measure reliable? | Yes | ||
| Comments: AB-AB-AB design and therefore it is possible that intervention effects were carried over into the control periods. No tests for trends were conducted | |||
| Study demographics | |||||
|---|---|---|---|---|---|
|
Author; year; study ID: Steele (2005)94 Title: The effect of automated alerts on provider ordering behaviour in an outpatient setting Country: USA Specific setting: Sam Sandos Family Health Clinic, Denver Health outpatient primary-care clinics, Denver, CO, USA Study objectives: To assess the effects of implementation of OCS with CDSS providing automated alerts on medication errors related to drug–laboratory interactions in an outpatient primary care setting Health-care setting: Primary care If secondary care, academic status of the hospital: NA Health-care system: Mixed (Medicaid; Medicare; private/commercial; uninsured) Study design: Pre–post Number of sites: 1 Funding source: Public sector Was evaluator of tool also its developer? No |
|||||
| System users | |||||
| CDSS user(s)a | Physicians, allied health providers (nurse practitioners, physician assistants), residents | ||||
| Practitioners (n) | NR | ||||
| a No further baseline details were reported on study physicians. | |||||
|
Inclusion criteria: All users who entered medication orders during the study period Exclusion criteria: NR |
|||||
| Patient baseline demographics | |||||
|
Inclusion criteria: All registered patients were eligible for inclusion Exclusion criteria: NR |
|||||
| N | % | ||||
| N | 19,076 | 100.0% | |||
| Age (mean) | 25.3 | – | |||
| Gender (female) | 12,241 | 64.2% | |||
| Ethnic group | |||||
| African American | 514 | 2.7% | |||
| Caucasian | 2081 | 10.9% | |||
| Hispanic | 15,708 | 82.3% | |||
| Other | 773 | 4.1% | |||
| Insurance | |||||
| Medicare | 8049 | 42.2% | |||
| Medicaid | 1249 | 6.5% | |||
| Private/commercial | 1174 | 6.2% | |||
| Uninsured | 7832 | 41.1% | |||
| Other | 772 | 4.0% | |||
| Interventions | |||||
|
Intervention: OCS plus CDSS. The CDSS used commercially available rules developed by Thomson Micromedex as Medical Logic Modules (MLM) and modified them to meet local needs. The rules chosen focused upon those appropriate to addressing patient safety in an outpatient setting and covered the following five areas: (1) medication use that can lead to hyperkalemia, (2) hypokalemia, (3) nephrotoxicity, (4) thrombocytopenia, and (5) heptic inflammation. In addition a determination was made for each medication as to whether an alert should be provided for (1) an abnormal laboratory value only; (2) either an abnormal laboratory value or a missing laboratory value, or (3) no alert displayed. The laboratory cut-off values for triggering an alert were the same as the Denver Health abnormal laboratory reference ranges In response to the alerts, providers could decide to keep, revise or delete the medication order. They could also order any rule associated laboratory tests Comparator: OCS alone (pre-intervention) Concomitant interventions: NA |
|||||
| CDSS tool | |||||
| Name of CDDS (if any) | NR (developed in collaboration with Thomson Micromedex and Siemens Medical Solutions) | ||||
| CDSS reasoning method | Discrimination rules | ||||
| CDSS knowledge base | Commercially available Medical Logic Modules developed by Thomson Micromedex and then adapted to meet local needs | ||||
| Did study use training set and test set data? |
Training set: No Test set: No |
||||
| For training set data complete the following: | |||||
| Design | NR | ||||
| Target decision | NR | ||||
| Sample characteristics: | |||||
| Sample size (n) | NR | ||||
| Age (mean; range) | NR | ||||
| Gender (n male; n female) | NR | ||||
| Disease type | NR | ||||
| For test set data complete the following: | |||||
| Properties of test set data | NR | ||||
| Test centre | NR | ||||
| Target decision | NR | ||||
| Other CDSS information | |||||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | Biochemical tests | ||||
| 2. Time to complete the CDSS (minutes) | NR | ||||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Alert | ||||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||||
| 5. Is user instructional training at the time of implementation described? | No | ||||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||||
| 8. Is there a need for additional data entry by the clinician? | No | ||||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | ||||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | Yes | ||||
| 13. Does the CDSS promote action rather than inaction? | NR | ||||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||||
| 16. Were local users involved in the CDSS development process? | Yes | ||||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | ||||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||||
| Outcome measures | |||||
|
Outcome 1: Percentage of time rule associated laboratory test ordered: no alert displayed Outcome 2: Percentage of time rule associated laboratory test ordered: alert displayed Outcome 3: Percentage of time rule associated laboratory test ordered: ‘abnormal labs’ message displayed Outcome 4: Percentage of time rule associated laboratory test ordered: ‘no labs’ message displayed Total length of follow-up: 9 months (pre-intervention 17 weeks; post-intervention 21 weeks). Study period: 1 August 2002 to 30 April 2003 Follow-up assessment times: 9 months Rate of attrition at each follow-up time: NA Methods of statistical analysis: Fisher’s exact test or generalised estimating equations |
|||||
| Results | |||||
| Outcome 1: Percentage of time rule associated laboratory test ordered: no alert displayeda | |||||
| Pre-intervention (n; %) | Post-intervention (n; %) | % change | p-value | ||
| 1042 (17) | 1322 (16.20) | –4.71 | 0.38 | ||
| a There was no significant change in the % time provider ordered the rule associated laboratory test pre- and post-intervention indicating there was no trend to increased laboratory test ordering during the study period. | |||||
| Outcome 2: Percentage of time rule associated laboratory test ordered: alert displayed | |||||
| Pre-intervention (n;%) | Post-intervention (n;%) | % change | p-value | ||
| 347 (38.50) | 559 (51.10) | 32.73 | <0.0001 | ||
| Outcome 3: Percentage of time rule associated laboratory test ordered: ‘abnormal labs’ message displayed | |||||
| Pre-intervention (n;%) | Post-intervention (n;%) | % change | p-value | ||
| 152 (33.80) | 240 (41.70) | 23.37 | 0.0771 | ||
| Outcome 4: Percentage of time rule associated laboratory test ordered: ‘no labs’ message displayed | |||||
| Pre-intervention (n;%) | Post-intervention (n;%) | % change | p-value | ||
| 198 (43.0) | 331 (62.0) | 44.19 | <0.001 | ||
| Authors’ conclusions: Providers will adhere to alerts and will use this information to improve patient care. Specifically, in response to drug–laboratory interaction alerts, providers will significantly increase the ordering of laboratory tests | |||||
| Methodological assessment criteria (Steele) (2005)94 | |||||
| 1. Were the study eligibility criteria specified? | Yes | ||||
| 2. Are adequate baseline details presented? | Partial | ||||
| 3. Are similar cointerventions administered in both study periods? | NA | ||||
| 4. Are data analyses appropriate? | Yes | ||||
| 5. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Yes | ||||
| 6. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | ||||
| 7. Are the conclusions supported by the results? | Yes | ||||
| Comments: None | |||||
| Study demographics | |||
|---|---|---|---|
|
Author; year; study ID: Abboud (2006)87 Title: Impact of workflow-integrated corollary orders on aminoglycoside monitoring in children Country: USA Specific setting: Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA (423-bed tertiary care children’s hospital with over 700,000 total patient visits per annum) Study objectives: To assess the impact of an electronic workflow-integrated aminoglycoside corollary order in paediatric patients Health-care setting: Secondary care (paediatric inpatients) If secondary care, academic status of the hospital: NR Health-care system: NR Study design: Pre–post Number of sites: 1 (423 bed hospital) Funding source: NR Was evaluator of tool also its developer? No |
|||
| System users | |||
| CDSS user(s)a | Physicians | ||
| Practitioners (n) | NR | ||
| a No further baseline details were reported on study physicians. | |||
|
Inclusion criteria: All users who entered aminoglycosides medication orders for 4 or more days duration during the study period (October 2003 to March 2004) Exclusion criteria: NR |
|||
| Patient baseline demographics | |||
|
Inclusion criteria: All patients who received aminoglycosides for 4 or more days duration during the study period Exclusion criteria: NR |
|||
| Baseline period (September 2003 to November 2003) | Intervention period (January 2004 to March 2004) | p-value | |
| Courses of antibiotic therapy ≥ 4 days | 159 | 177 | n.s |
| Total number of patients: | 125 | 150 | n.s |
| 1 course of therapy | 101 | 128 | n.s |
| 2 courses of therapy | 19 | 19 | n.s |
| 3 courses of therapy | 4 | 2 | n.s |
| ≥4 courses of therapy | 1 | 1 | n.s |
| Courses of antibiotic therapy/patient | 1.3 | 1.2 | n.s |
| Total number of laboratory results obtained | 262 | 286 | n.s |
| Laboratory results analysed based on predefined criteria (% of total)a | 215 (82.1%) | 219 (76.6%) | n.s |
| Laboratory results per antibiotic course (range) | 1.6 (0–9) | 1.6 (0–8) | n.s |
| Laboratory results per patient (range) | 2.1 (0–11) | 1.9 (0–10) | n.s |
|
n.s, not statistically significant. a Laboratory values were analysed only if obtained at predefined peak (obtained 50–120 minutes after drug administration) or trough (obtained 0–120 minutes prior to drug administration) times. |
|||
| Interventions | |||
|
Intervention: OCS plus CDSS (corollary order). For all aminoglycoside orders of 4 or more days duration, CDSS prompted the physician in whether they were interested in checking peak, trough, peak and trough or random blood level for the drug being prescribed as well as the date and time when they wanted the blood sample obtained Comparator: OCS alone (pre-intervention) Concomitant interventions: NA |
|||
| CDSS tool | |||
| Name of CDDS (if any) | invision®, Siemens Medical Solutions, Malvern, PA, USA | ||
| CDSS reasoning method | NR | ||
| CDSS knowledge base | NR | ||
| Did study use training set and test set data? |
Training set: No Test set: No |
||
| For training set data complete the following: | |||
| Design | NR | ||
| Target decision | NR | ||
| Sample characteristics: | |||
| Sample size (n) | NR | ||
| Age (mean; range) | NR | ||
| Gender (n male; n female) | NR | ||
| Disease type | NR | ||
| For test set data complete the following: | |||
| Properties of test set data | NR | ||
| Test centre | NR | ||
| Target decision | NR | ||
| Other CDSS information | |||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | Test order | ||
| 2. Time to complete the CDSS (minutes) | NR | ||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Prompt | ||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||
| 5. Is user instructional training at the time of implementation described? | Yes | ||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||
| 8. Is there a need for additional data entry by the clinician? | No | ||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | ||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | No | ||
| 13. Does the CDSS promote action rather than inaction? | NR | ||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||
| 16. Were local users involved in the CDSS development process? | No | ||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | ||
| 19. Is the CDSS used in conjunction with conventional education? | Yes | ||
| Outcome measures | |||
|
Outcome 1: Number of courses of aminoglycosides with appropriate laboratory test monitoring Outcome 2: Frequency of therapeutic, toxic, subtherapeutic, or toxic and subtherapeutic laboratory values during courses of therapy Total length of follow-up: 6 months (3 months pre- and 3 months post-intervention). Study period: October 2003 to March 2004 Follow-up assessment times: 6 months Rate of attrition at each follow-up time: NA Methods of statistical analysis: Categorical data were analysed using descriptive statistics and chi-square tests. Continuous parametric data were analysed by t-tests and non-parametric data by Mann-Whitney rank-sum test. p-Values <0.05 were considered statistically significant |
|||
| Results | |||
| Outcome 1: Number of courses of aminoglycosides with appropriate laboratory test monitoring | |||
| Pre-intervention (n; %) | Post-intervention (n; %) | p-value | |
| N in analysis | 159 | 177 | – |
| 128 (80.5%) | 146 (82.5%) | n.s | |
| n.s, not statistically significant at the p < 0.05 level. | |||
| Outcome 2: Frequency of therapeutic, toxic, subtherapeutic, or toxic and subtherapeutic laboratory values during courses of therapy | |||
| Pre-intervention (n; %) | Post-intervention (n; %) | p-value | |
| N in analysis | 111 | 125 | – |
| All therapeutic levels | 94 (84.7%) | 100 (80.0%) | p = 0.44 |
| Any toxic levels | 9 (8.1%) | 15 (12%) | p = 0.44 |
| Any subtherapeutic levels | 8 (7.2%) | 7 (5.6%) | p = 0.81 |
| Both toxic and sub-therapeutic levels | 0 | 3 (2.4%) | p = 0.29 |
| Authors’ conclusions: The introduction of a computerised corollary order for aminoglycoside blood level monitoring tests did not significantly improve laboratory monitoring rates, nor did it result in a reduction in the rate of either toxic or subtherapeutic levels. However, aminoglycoside corollary orders may have an important role in institutions where pharmacists are not actively involved in monitoring therapy | |||
| Methodological assessment criteria (Abboud) (2006)87 | |||
| 1. Were the study eligibility criteria specified? | Yes | ||
| 2. Are adequate baseline details presented? | No | ||
| 3. Are similar cointerventions administered in both study periods? | NA | ||
| 4. Are data analyses appropriate? | Yes | ||
| 5. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Yes | ||
| 6. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | ||
| 7. Are the conclusions supported by the results? | Yes | ||
| Comments: None | |||
Studies assessing the impact of the display of restricted lists (n = 2)
| Study demographics | ||||
|---|---|---|---|---|
|
Author; year; study ID: Solomon (1999)88 Title: A computer-based intervention to reduce unnecessary serologic testing Country: USA Specific setting: Brigham and Women’s Hospital, Boston, MA, USA Study objectives: To assess whether physicians would reduce their use of unnecessary serologic tests if provided with information on the likelihood that an antinuclear antibody (ANA), rheumatoid factor (RF), or complement level test would change their estimate of disease Health-care setting: Secondary care (inpatients) If secondary care, academic status of the hospital: NR Health-care system: NR Study design: CCT Number of sites: 1 (720-bed hospital) Funding source: public sector Was evaluator of tool also its developer? Yes |
||||
| System users | ||||
| Intervention group | Control group | p-valueb | ||
| CDSS user(s) | Physician | Physician | ||
| Practitioners (n) | 71 | 154 | ||
| Age (year) (mean; SD) | 30 ± 4 | 30 ± 4 | 0.5 | |
| Gender (female, %) | 28 | 36 | 0.2 | |
| Postgraduate yeara (%) | ||||
| First | 65 | 52 | ||
| Second | 13 | 27 | ||
| Third | 16 | 14 | ||
| Fourth | 5 | 7 | 0.08 | |
| Department (%)a | ||||
| Medicine | 86 | 82 | ||
| Surgery | 2 | 7 | ||
| Neurology | 7 | 8 | ||
| Obstetrics-gynaecology | 5 | 3 | 0.2 | |
|
a Percentages may not sum due to rounding (reported by authors). b Calculated from chi-squared tests for categorical data and Wilcoxon rank-sum tests for ordinal data. |
||||
| Practitioners (n) in analysis | NA | NA | ||
|
Inclusion criteria: All physicians ordering an RF or ANA test for the suspected indications of rheumatoid arthritis, systemic lupus erythematosus, primary systemic sclerosis, mixed connective tissue disease, or Sjögren’s syndrome during the 10-month study period (September 1996 to June 1997) were assigned to the intervention group. All physicians ordering an RF or ANA for the suspected indications of systemic vasculitis and cryoglobulinemia, or a complement test for any condition during the study period were assigned to the control group Exclusion criteria: When multiple orders for the same test for the same patient during one calendar day were made, only the last order was included |
||||
| Patient baseline demographics | ||||
|
Inclusion criteria: All inpatients with an order written for an ANA, RF or complement level test during the 10-month study period Exclusion criteria: NR |
||||
| Patient characteristics | Intervention group | Control group | p-valuea | |
| N | 99 | 236 | ||
| Age (year) (mean; SD) | 55 ± 19 | 54 ± 17 | 0.7 | |
| Gender (female) (%) | 66 | 66 | 1.0 | |
| Length of stay (days)b | 6 (3,12) | 6 (3,11) | 0.9 | |
| Total charges (US$)b | 13,415 (7636; 27,951) | 13,217 (7337; 25,634) | 0.6 | |
| Died in hospital (n) | 4 | 6 | 0.4 | |
|
a Calculated from chi-squared tests for categorical data and Wilcoxon rank-sum tests for ordinal data. b Median (25%; 75%). |
||||
| Interventions | ||||
|
Intervention: OCS plus CDSS. The CDSS required the physician to enter into the computer their estimate of the pre-test probability of disease. The CDSS then calculated the positive and negative post-test predictive values based on estimates of the sensitivity and specificity of each test derived from the literature Comparator: OCS alone Concomitant interventions: NA |
||||
| CDSS tool | ||||
| Name of CDDS (if any) | NR (home-grown system Brigham and Women’s Hospital) | |||
| CDSS reasoning method | Naive Bayesian methods | |||
| CDSS knowledge base | Sensitivity and Specificity values abstracted from the literature | |||
| Did study use training set and test set data? |
Training set: NR Test set: NR |
|||
| For training set data complete the following: | ||||
| Design | NR | |||
| Target decision | NR | |||
| Sample characteristics: | Intervention | Control | ||
| Sample size (n) | NR | NR | ||
| Age (mean; range) | NR | NR | ||
| Gender (n male; n female) | NR | NR | ||
| Disease type | NR | NR | ||
| For test set data complete the following: | ||||
| Properties of test set data | ||||
| Test centre | NR | |||
| Target decision | NR | |||
| Other CDSS information | ||||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | 1; pre-test probability estimate | |||
| 2. Time to complete the CDSS (minutes) | NR | |||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Positive and negative predictive values (post-test) | |||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | |||
| 5. Is user instructional training at the time of implementation described? | Yes | |||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | |||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | |||
| 8. Is there a need for additional data entry by the clinician? | Yes | |||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | |||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | |||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | |||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | No | |||
| 13. Does the CDSS promote action rather than inaction? | No | |||
| 14. Does the CDSS justify the output by provision of reasoning? | No | |||
| 15. Does the CDSS justify the output by provision of research evidence? | No | |||
| 16. Were local users involved in the CDSS development process? | NR | |||
| 17. Is the CDSS output provided to patients as well as clinician? | No | |||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | |||
| 19. Is the CDSS used in conjunction with conventional education? | No | |||
| Outcome measures | ||||
|
Outcome 1: Number of tests cancelled Outcome 2: Yield of positive tests for known or new rheumatic disease Total length of follow-up: 10 months (September 1996 to June 1997) Follow-up assessment times: 10 months Rate of attrition at each follow-up time: 348 patients had a ANA, RF or complement test ordered, of these 13 duplicate orders were excluded. N = 335 tests (attrition rate = 3.7%) Methods of statistical analysis: Chi-squared tests and Wilcoxon rank-sum tests for univariate analysis |
||||
| Results | ||||
| Outcome 1: Number of tests cancelled | ||||
| OCS + CDSS | OCS alone | p-value | ||
| N in analysis | 99 | 236 | – | |
| Number of tests cancelled n (%) | 11 (11.1%) | 1 (0.42%) | p = 0.001 | |
|
Outcome 2: Yield of positive tests for known or new rheumatic disease The charts of 43 patients with positive tests were reviewed to determine whether the positive test yielded a new diagnosis of a rheumatic condition. 26/43 of the positive tests were in patients with known rheumatic disease. Only 4/43 new diagnosis of rheumatic disease were made, which account for 1.2% of all tests ordered |
||||
| Authors’ conclusions: The computer-based intervention resulted in a small but statistically significant decrease in orders for AAN and RF levels by 10%. Further reductions without clinical harm are probably possible, since the yield of testing for new rheumatic diseases was low | ||||
| Methodological assessment criteria Solomon (1999)88 | ||||
| 1. Were the study eligibility criteria specified? | Yes | |||
| 2. Are adequate baseline details presented? | Yes | |||
| 3. Are groups similar at baseline? | Yes | |||
| 4. Are any baseline imbalances adequately adjusted for in the analysis? | NA | |||
| 5. Are similar cointerventions administered? | NA | |||
| 6. Are physician’s blinded to treatment allocation? | No | |||
| 7. Are outcome assessors blinded? | No | |||
| 8. Are data analyses appropriate? | Yes | |||
| 9. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Yes | |||
| 10. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | |||
| 11. Are the conclusions supported by the results? | Yes | |||
| Comments: None | ||||
| Study demographics | |||
|---|---|---|---|
|
Author; year; study ID: Bansal (2001)89 Title: A computer-based intervention on the appropriate use of arterial blood gas Country: USA Specific setting: Vanderbilt University Medical Center, Nashville, TN, USA (630-bed hospital with 31,000 admissions per year) Study objectives: To evaluate the impact of a computer-based intervention on ABG usage in an intensive care setting Health-care setting: Secondary care (ICUs) If secondary care, academic status of the hospital: University Health-care system: NR Study design: Pre–post Number of sites: 1 (6 ICUs; trauma; general surgery; medical; cardiac; burn; neurology) Funding source: Public sector Was evaluator of tool also its developer? Yes |
|||
| System users | |||
| CDSS user(s)a | Physicians; respiratory therapists; nurses; medical receptionists | ||
| Practitioners (n) | NR | ||
| Orders entered by user type at baseline | |||
| User typea | n | % | |
| Ancillary staff | 24 | 1.8% | |
| Physicians | 366 | 28.0% | |
| Other users | 8 | 0.6% | |
| Nurses | 813 | 62.0% | |
| Respiratory therapists | 80 | 6.1% | |
| a Staff who were not medical doctors had the ability to enter verbal or written orders from physicians. | |||
|
Inclusion criteria: All users with the authority to enter orders via the OCS during the study period Exclusion criteria: NR |
|||
| Patient baseline demographics | |||
|
Inclusion criteria: All patients on the six ICUs (trauma; general surgery; medical; cardiac; burn; neurology) who had a ABG test ordered during the study perioda Exclusion criteria: NR |
|||
| a No further data on patient baseline demographics was reported. | |||
| Interventions | |||
|
Intervention: OCS plus CDSS. CDSS provided the ordering clinician with educational text alongside a graphical display of the patient’s previous ABG values (pO2, pCO2, HCO3, pH, FiO2) and O2 saturations. Advanced ordering of ABG tests was also limited to within 24 hours so no multiday orders were allowed. The default response was to cancel the order, but the final decision regarding the test order was left to the user’s discretion Comparator: OCS alone (pre-intervention) Concomitant interventions: NA |
|||
| CDSS tool | |||
| Name of CDDS (if any) | NR (home-grown system Vanderbilt University Medical Center) | ||
| CDSS reasoning method | NR | ||
| CDSS knowledge base | NR | ||
| Did study use training set and test set data? |
Training set: No Test set: No |
||
| For training set data complete the following: | |||
| Design | NR | ||
| Target decision | NR | ||
| Sample characteristics: | |||
| Sample size (n) | NR | ||
| Age (mean; range) | NR | ||
| Gender (n male; n female) | NR | ||
| Disease type | NR | ||
| For test set data complete the following: | |||
| Properties of test set data | NR | ||
| Test centre | NR | ||
| Target decision | NR | ||
| Other CDSS information | |||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | Six previous ABG results (pO2, pCO2, HCO3, pH, FiO2,O2 saturations | ||
| 2. Time to complete the CDSS (minutes) | NR | ||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Graphs | ||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||
| 5. Is user instructional training at the time of implementation described? | No | ||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||
| 8. Is there a need for additional data entry by the clinician? | No | ||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | ||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | Yes | ||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | No | ||
| 13. Does the CDSS promote action rather than inaction? | No | ||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||
| 16. Were local users involved in the CDSS development process? | Yes | ||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | ||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||
| Outcome measures | |||
|
Outcome 1: Number of ABG test orders placed pre- and post-intervention Total length of follow-up: 12 weeks (pre-intervention 5 weeks; post-intervention 7 weeks). Study period: 1 November 2000 to 23 January 2001 Follow-up assessment times: 12 weeks Rate of attrition at each follow-up time: NA Methods of statistical analysis: ANOVA (analysis of variance) and linear regression analysis |
|||
| Results | |||
| Outcome 1: Number of ABG test orders placed pre- and post-intervention | |||
| Pre-intervention (n) | Post-intervention (n) | p-value | |
| 376 | 387 | p = 0.09 | |
| Authors’ conclusions: Study did not demonstrate significant change. Longer study periods are therefore needed. The impact could be improved in the future by targeting physician users and tailoring the intervention to specific workflow patterns of high utilisation units | |||
| Methodological assessment criteria (Bansal) (2001)89 | |||
| 1. Were the study eligibility criteria specified? | Yes | ||
| 2. Are adequate baseline details presented? | Partial | ||
| 3. Are similar cointerventions administered in both study periods? | No | ||
| 4. Are data analyses appropriate? | Yes | ||
| 5. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Unclear | ||
| 6. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | ||
| 7. Are the conclusions supported by the results? | Yes | ||
| Comments: The numbers presented in the abstract for change in the number of test orders place pre- and post-intervention do not tally with those presented in the text on page 34. It is unclear whether the post-intervention results reported in both the abstract and text (p 34) pertain to just the implemented units or all units together. Numbers reported for outcome 1 are taken from the abstract | |||
Studies assessing the impact of recommendations (n = 7)
| Study demographics | ||
|---|---|---|
|
Author; year; study ID: Hobbs (1996)32 Title: A prospective controlled trial of computerized decision support for lipid management in primary care Country: UK Specific setting: Primary care; 25 practices covering a population of 150,000 in Birmingham, UK Study objectives: To explore the uptake and effect in primary care of a computerised decision support system for the management of hyperlipidaemia Health-care setting: Primary care If secondary care, academic status of the hospital: NA Health-care system: NHS Study design: CRCT Number of sites: 25 practices (21 intervention; 4 control) Funding source: NR Was evaluator of tool also its developer? No |
||
| System users | ||
| Intervention group | Control group | |
| CDSS user(s) | Physician | Physician |
| Practitioners (n) | NR | NR |
|
If the CDSS user is a doctor, complete n for the following: Consultant (attending) Registrar (chief resident) SHO (resident) HO (intern) |
NR | NR |
| Practitioners (n) in analysis | NR | NR |
|
Inclusion criteria: NR Exclusion criteria: Practices with previous experience of CDSS were excluded |
||
| Patient baseline demographics | ||
|
Inclusion criteria: NR Exclusion criteria: NR |
||
| Intervention group | Control group | |
| Age (mean; SD) | NR | NR |
| Gender (female %) | NR | NR |
| Interventions | ||
|
Intervention: OCS with CDSS (Primed system) using the hyperlipidaemia decision support module Comparator: OCS without CDSS Concomitant interventions: NA |
||
| CDSS tool | ||
| Name of CDDS (if any) | Primed system (Wolfson Research Laboratories, University of Birmingham) | |
| CDSS reasoning method | Discrimination rules | |
| CDSS knowledge base | Physician opinion (protocol developed by a lipid specialist) | |
| Did study use training set and test set data? |
Training set: NR Test set: NR |
|
| For training set data complete the following: | ||
| Design | [Consecutive; random; retrospective; unclear; other: (specify)] | |
| Target decision | State | |
| Sample characteristics: | Intervention | Control |
| Sample size (n) | NR | NR |
| Age (mean; range) | NR | NR |
| Gender (n male; n female)Disease type | NR | NR |
| Disease type | NR | NR |
| For test set data complete the following: | ||
| Properties of test set data | NR | |
| Test centre | NR | |
| Target decision | NR | |
| Other CDSS information | ||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | Sociodemographic details; cardiovascular risk factors; cholesterol level | |
| 2. Time to complete the CDSS (minutes) | NR | |
| 3. CDSS output format: (score; probability graph; advice; etc.) | Advice | |
| 4. Is a description of pilot testing with users prior to implementation provided? | No | |
| 5. Is user instructional training at the time of implementation described? | Yes | |
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | No | |
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | |
| 8. Is there a need for additional data entry by the clinician? | Yes | |
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | NR | |
| 10. Does CDSS provide output at the time and location of decision-making? | Yes | |
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | |
| 12. Does the CDSS provide a recommendation rather than just an assessment? | Yes | |
| 13. Does the CDSS promote action rather than inaction? | NR | |
| 14. Does the CDSS justify the output by provision of reasoning? | No | |
| 15. Does the CDSS justify the output by provision of research evidence? | No | |
| 16. Were local users involved in the CDSS development process? | No | |
| 17. Is the CDSS output provided to patients as well as clinician? | No | |
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | |
| 19. Is the CDSS used in conjunction with conventional education? | No | |
| Outcome measures | ||
|
Outcome 1: Lipid test rates Outcome 2: Number of patients receiving a full lipid profile (cholesterol, fasting triglyceride and HDL) Total length of follow-up: 9 months (3 months historical control period and 6 months intervention period Follow-up assessment times: 9 months Rate of attrition at each follow-up time: eight practices (three practices failed to record any data; three practices lost all collected data; one practice dropped out; data from one practice was lost in the post) Methods of statistical analysis: Comparisons between pre- and intervention time for each practice was compared using paired t-tests and Wilcoxon tests. No between group comparisons were conducted with all analysis conducted as a change from baseline period |
||
| Results | ||
|
Outcome 1: Lipid test rates The mean rate of testing was 4.4 tests/1000 population/month. The authors report that rates of testing did not show any significant differences between the pre- and intervention period (actual figures not reported) |
||
|
Outcome 2: Number of patients receiving a full lipid profile (cholesterol, fasting triglyceride and HDL) The authors report there was a significant increase in the number of patients receiving a full lip profile in the study period and a decrease in those having only partial investigations (χ2 = 49.5; df = 3; p < 0.05) (actual figures not reported) |
||
| Authors’ conclusions: Greater integration of CDSS software and practice based data handling systems is needed. The mode of data capture, and hence both the content and form of knowledge representation, in CDSS must take greater account of the primary care consultation process if such systems are to be of use to practitioners | ||
| Methodological assessment criteria (Hobbs) (1996)32 | ||
| 1. Is the study properly randomised? | Unclear | |
| 2. Did the analysis take clustering into account? | No | |
| 3. Were the study eligibility criteria specified? | Partial | |
| 4. Are adequate baseline details presented? | No | |
| 5. Are groups similar at baseline? | Unclear | |
| 6. Are any baseline imbalances adequately adjusted for in the analysis? | No | |
| 7. Are similar cointerventions administered? | NA | |
| 8. Are physician’s blinded to treatment allocation? | No | |
| 9. Are outcome assessors blinded? | Unclear | |
| 10. Are data analyses appropriate? | No | |
| 11. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | No | |
| 12. Are greater than 80% of physicians/patients included in the follow-up assessment? | No | |
| 13. Are the conclusions supported by the results? | Yes | |
| Comments: attrition 8/25 clusters; no intraclass correlation reported in design and clustering does not appear to have been taken into account in analysis; no between group differences reported, analysis conducted as a change in pre- and post- intervention rates | ||
| Study demographics | |||||
|---|---|---|---|---|---|
|
Author; year; study ID: Apkon (2005)97 Title: A randomised outpatient trial of a decision-support information technology tool Country: USA Specific setting: Two military treatment facilities (Ireland Army Community Hospital and Clinic, Fort Knox, KY, USA and Mayport Branch Health Clinic, Mayport, FL, USA) Study objectives: To conduct a RCT to evaluate the impact of the DSIT tool Problem-Knowledge Couplers within a computerised medical record on quality of care, and resource consumption Health-care setting: Secondary care (all outpatients) If secondary care, academic status of the hospital: NR Health-care system: USA military treatment facilities Study design: RCT Number of sites: 2 Funding source: Public sector Was evaluator of tool also its developer? No |
|||||
| System users | |||||
| Intervention group | Control group | ||||
| CDSS user(s) | Physician | Physician | |||
| Practitioners (n) | NR | NR | |||
|
If the CDSS user is a doctor, complete n for the following: Consultant (attending) Registrar (chief resident) SHO (resident) HO (intern) |
NR | NR | |||
| Practitioners (n) in analysis | NR | NR | |||
|
Inclusion criteria: All physicians treating patients at either the Ireland Army Community Hospital and Clinic, Fort Knox, KY, USA, or Mayport Branch Health Clinic, Mayport, FL, USA. No further inclusion criteria were reported Exclusion criteria: NR |
|||||
| Patient baseline demographics | |||||
|
Inclusion criteria: Patients ≥18 years with scheduled appointments, who could speak and read English, were not scheduled for obstetric care, and who had no emergency medical condition and who had not completed a previous Coupler session Exclusion criteria: NR |
|||||
| Intervention group | Control group | ||||
| Age (mean; SD) | 34.4 ± 10.4 | 35.3 ± 11.0 | |||
| Gender (female n; %) | 593 (63.4) | 587 (60.8) | |||
| Visit type (n: %) | |||||
| Acute | 383 (40.9) | 416 (43.1) | |||
| Established | 47 (5.0) | 27 (2.8) | |||
| Routine | 365 (39.0) | 375 (38.8) | |||
| Wellness | 126 (13.5) | 139 (14.4) | |||
| Other | 15 (1.6) | 9 (0.9) | |||
| Interventions | |||||
|
Intervention: OCS plus Coupler. Couplers were available for a wide range of preventive health-care needs and conditions or management of acute conditions. Patients completed the Coupler appropriate for their complaint, or when no condition-specific Coupler was appropriate, a generic history and screening Coupler. Physicians treating Coupler patients could enter additional information before reviewing Coupler outputs. Data only extracted on the seven outcomes using either laboratory tests or imaging Comparator: OCS alone (usual care) Concomitant interventions: NA |
|||||
| CDSS tool | |||||
| Name of CDDS (if any) | DSIT tool Problem-Knowledge Couplers (PKC Corp, Burlington, VT, USA) | ||||
| CDSS reasoning method | NR | ||||
| CDSS knowledge base | NR | ||||
| Did study use training set and test set data? |
Training set: NR Test set: NR |
||||
| For training set data complete the following: | |||||
| Design | NR | ||||
| Target decision | NR | ||||
| Sample characteristics: | Intervention | Control | |||
| Sample size (n) | NR | NR | |||
| Age (mean; range) | NR | NR | |||
| Gender (n male; n female) | NR | NR | |||
| Disease type | NR | NR | |||
| For test set data complete the following: | |||||
| Properties of test set data | NR | ||||
| Test centre | NR | ||||
| Target decision | NR | ||||
| Other CDSS information | |||||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | Patients entered their medical histories into the appropriate Coupler tool for their complaint | ||||
| 2. Time to complete the CDSS (minutes) | 30 | ||||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Advice | ||||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||||
| 5. Is user instructional training at the time of implementation described? | No | ||||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||||
| 8. Is there a need for additional data entry by the clinician? | No, but physician could enter additional information if necessary | ||||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | Unclear | ||||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | Unclear | ||||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | Unclear | ||||
| 13. Does the CDSS promote action rather than inaction? | Unclear | ||||
| 14. Does the CDSS justify the output by provision of reasoning? | Unclear | ||||
| 15. Does the CDSS justify the output by provision of research evidence? | Unclear | ||||
| 16. Were local users involved in the CDSS development process? | No | ||||
| 17. Is the CDSS output provided to patients as well as clinician? | Unclear | ||||
| 18. Does the CDSS provide periodic summaries of performance feedback? (yes; no; unclear) | Unclear | ||||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||||
| Outcome measures | |||||
|
Outcome 1: Number of health-care opportunities fulfilled within 60 days of index visit (data extracted for laboratory and radiology test screening only) Outcome 2: Laboratory test resource consumption within 60 days of index visit Outcome 3: Diagnostic imaging test resource consumption within 60 days of index visit Total length of follow-up: 60 days Follow-up assessment times: 60 days Rate of attrition at each follow-up time: 74.9% (77% intervention group; 72.9% control group). In intervention group 15.3% had missing/incomplete medical records at index visit, 6% had no health-care opportunity at index visit and 1.7% had missing/incomplete medical records for 60-day follow-up. In control group 16.8% had missing/incomplete medical records at index visit, 7.9% had no health-care opportunity at index visit and 2.5% had missing/incomplete medical records for 60-day follow-up Methods of statistical analysis: Likelihood of health-care opportunities being fulfilled was compared using a Mantel-Haenszel chi-squared test of homogeneity, stratified by physician and adjusted for clustering by patient. Dollar values for laboratory and diagnostic imaging test resource consumption were taken from the Centers for Medicare and Medicaid Services 2003 fee schedule using a relative value unit conversion rate of US$36.7856. Where not coded in the Composite Healthcare System, Current Procedural Terminology codes were assigned for the midlevel service for each visit type. Differences in median resource use between groups was compared using Wilcoxon rank-sum of equality of distribution |
|||||
| Results | |||||
| Outcome 1: Number of health care opportunities fulfilled within 60 days of index visit (data extracted for laboratory and radiology test screening only) | |||||
| Opportunity type | OCS + Coupler | OCS alone | Difference between groups | ||
| Cervical cancer | 26/95 (27.4%) | 22/98 (22.4%) | p = 0.47 | ||
| Chlamydia | 22/73 (30.1%) | 19/64 (29.7%) | p = 0.90 | ||
| Colorectal cancer | 4/32 (12.5%) | 2/58 (3.4%) | p = 0.15 | ||
| Lipids | 13/49 (26.5%) | 18/48 (37.5%) | p = 0.32 | ||
| Back pain imaging | 4/4 (100%) | 2/2 (100%) | NA | ||
| Diabetes – glycosylated haemoglobin | 3/6 (50%) | 1/3 (33.3%) | p = 0.48 | ||
| Lipid abnormalities | 12/66 (18.2%) | 11/69 (15.9%) | p = 0.81 | ||
| NA, not available. | |||||
| Outcome 2: Laboratory test resource consumption within 60 days of index visit | |||||
| Intervention | OCS + Coupler | OCS alone | Difference between groups | ||
| Median (interquartile range US$) | 43 (0–144) | 31 (0–139) | p = 0.04 | ||
| Outcome 3: Diagnostic imaging test resource consumption within 60 days of index visit | |||||
| Intervention | OCS + Coupler | OCS alone | Difference between groups | ||
| Median (interquartile range US$) | 31 (0–148) | 29 (0–127) | p = 0.26 | ||
| Authors’ conclusions: The results provide no strong evidence to support the utility of this decision-support tool, but the study demonstrates the value of rigorous evaluation of decision-support information technology | |||||
| Methodological assessment criteria (Apkon) (2005)97 | |||||
| 1. Is the study properly randomised? | Unclear | ||||
| 2. Is allocation of treatment concealed? | Unclear | ||||
| 3. Were the study eligibility criteria specified? | Yes | ||||
| 4. Are adequate baseline details presented? | Yes | ||||
| 5. Are groups similar at baseline? | Yes | ||||
| 6. Are any baseline imbalances adequately adjusted for in the analysis? | NA | ||||
| 7. Are similar cointerventions administered? | NA | ||||
| 8. Are physician’s blinded to treatment allocation? | No | ||||
| 9. Are outcome assessors blinded? | Unclear | ||||
| 10. Are data analyses appropriate? | Yes | ||||
| 11. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | No | ||||
| 12. Are greater than 80% of physicians/patients included in the follow-up assessment? | No | ||||
| 13. Are the conclusions supported by the results? | Yes | ||||
| Comments: contamination likely as patients are the unit of randomisation and physicians at both sites treatment Coupler and usual care groups | |||||
| Study demographics | |||||
|---|---|---|---|---|---|
|
Author; year; study ID: Bassa (2005)98 Title: Impact of a clinical decision support system on the management of patients with hypercholesterolemia in the primary health care setting Country: Spain Specific setting: Vila Olimpica Primary Health Care Centre, Barcelona, Spain Study objectives: To assess the impact on the effectiveness and costs of a practice guideline implemented through a clinical decision support system for the management of patients with hypercholesterolemia in the primary health-care setting Health-care setting: Primary care If secondary care, academic status of the hospital: NA Health-care system: Private model of management Study design: Pre–post Number of sites: One Funding source: Private sector (Novartis Pharmaceuticals) Was evaluator of tool also its developer? No |
|||||
| System users | |||||
| CDSS user(s)a | Physicians | ||||
| Practitioners (n) | NR | ||||
| a No further baseline details were reported on study physicians. | |||||
|
Inclusion criteria: Physicians treating study eligible participants during the post-intervention study phase Exclusion criteria: NR |
|||||
| Patient baseline demographics | |||||
|
Inclusion criteria: Patients with hypercholesterolemia randomly selected from the practice database Exclusion criteria: NR |
|||||
| N | 500 | ||||
| Age; median (IQR) | 67 (14) | ||||
| Gender (female, %) | 329 (65.8%) | ||||
| Diabetes mellitus (n; %) | 90 (18%) | ||||
| Current smoker (n; %) | 83 (16.6%) | ||||
| Sedentary lifestyle (n; %) | 262 (52.4%) | ||||
| Number of CVRFs | |||||
| None (n; %) | 22 (4.4%) | ||||
| One (n; %) | 174 (34.8%) | ||||
| Two (n: %) | 267 (53.4%) | ||||
| More than two (n; %) | 37 (7.4%) | ||||
| IQR, interquartile range; CVRF, cardiovascular risk factors. | |||||
| Interventions | |||||
|
Intervention: OCS plus CDSS (recommendations). The CDSS recommendations were based on the SEMFYC clinical guidelines for dyslipidemia management and cost-effectiveness data published in a meta-analysis [Cobos and colleagues (1999)218]. Based on the patient’s data (personal history of cardiovascular disease, cardiovascular risk factors, and lipid profile), the CDSS established the therapeutic objectives in terms of LDL and issued therapeutic and follow-up recommendations for the patient. The therapeutic recommendations included dietary treatment and lipid-lowering drugs. The follow-up recommendations included the monitoring of hepatic and muscular enzymes and a recommended date for the subsequent control visit Physicians were free to accept or decline the recommendations issued by the CDSS, but were prompted for a reason when they declined To estimate the costs in the pre- and post-intervention periods, the consumption of the following resources was considered: (1) the number of physician visits related to the management of hypercholesterolemia, (2) the number of laboratory assessments (lipid profile, transaminases, and muscular enzymes), (3) lipid-lowering drugs prescribed. The unit cost used for cost estimation were obtained from the SOIKOS database of health-care costs and were set to €12 for visits, €0.46 for total cholesterol, €2 for LDL, €3 for HDL, €4 for triglycerieds, €15 for creatine kinase, €1 for serum glutamic oxaloacetic transaminase, and €1 for serum glutamic pyruvic transaminase. Costs were estimated from the societal perspective (year of costing 2002) Comparator: OCS alone (pre-intervention) Concomitant interventions: NA |
|||||
| CDSS tool | |||||
| Name of CDDS (if any) | NR | ||||
| CDSS reasoning method | Clinical algorithm | ||||
| CDSS knowledge base | SEMFYC clinical guidelines for dyslipidemia management and cost-effectiveness data published in a meta-analysis | ||||
| Did study use training set and test set data? |
Training set: NR Test set: NR |
||||
| SEMFYC, Sociedad Española de Medicina de Familia y Comunitania’s. | |||||
| For training set data complete the following: | |||||
| Design | NR | ||||
| Target decision | NR | ||||
| Sample characteristics: | |||||
| Sample size (n) | NR | ||||
| Age (mean; range) | NR | ||||
| Gender (n male; n female) | NR | ||||
| Disease type | NR | ||||
| For test set data complete the following: | |||||
| Properties of test set data | NR | ||||
| Test centre | NR | ||||
| Target decision | NR | ||||
| Other CDSS information | |||||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | History of cardiovascular (CV) disease; CV risk factors and lipid profile | ||||
| 2. Time to complete the CDSS (minutes) | NR | ||||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Recommendation | ||||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||||
| 5. Is user instructional training at the time of implementation described? | No | ||||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||||
| 8. Is there a need for additional data entry by the clinician? | No | ||||
| 9. Does the CDSS request documentation of the reason for not following CDSS 9? | Yes | ||||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | Yes | ||||
| 13. Does the CDSS promote action rather than inaction? | NR | ||||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||||
| 16. Were local users involved in the CDSS development process? | NR | ||||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | ||||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||||
| Outcome measures | |||||
|
Outcome 1: Number of lipid-profile tests carried out in pre- and post-intervention periods Outcome 2: Mean costs of lipid profile and safety analyses (transaminases and muscular enzymes) tests carried out pre- and post-intervention periods Total length of follow-up: 2 years (pre-intervention 1 year; post- intervention 1 year). Study period: October 1998 to October 2000 Follow-up assessment times: 2 years Rate of attrition at each follow-up time: 19.2% (96/500 patients). None of the 96 patients had an LDL assessment after the beginning of the intervention. For 62 of these patients, the CDSS had recommended to carry out the following control after 1–5 years because they were low-risk. The remaining 36 patients (7.2% from initial sample) were lost to follow-up Methods of statistical analysis: Difference in the pre- and post-intervention periods were compared using the McNemar test for dichotomous variables and the Wilcoxon test for continuous variables. Paired t-test was used to compare pre- and post-intervention cost data without any transformation |
|||||
| Results | |||||
| Outcome 1: Number of lipid-profile tests carried out in pre- and post-intervention periods | |||||
| Pre-intervention (n) | Post-intervention (n) | p-value | |||
| 773 | 763 | p = 0.59 | |||
| Outcome 2: Mean costs of lipid profile and safety analyses (transaminases and muscular enzymes) tests carried out pre- and post-intervention periods (euros) | |||||
| Pre-intervention | Post-intervention | Difference (95% CI) | p-valuea | ||
| 41.8 | 47.2 | 5.4 (2.0 to 8.7) | p = 0.0017 | ||
| a Paired t-test. | |||||
| Authors’ conclusions: The results of the present study suggest that it is possible to optimise the efficiency of the management of hypercholesterolemia in standard practice by the implementation of a CDSS | |||||
| Methodological assessment criteria (Bassa) (2005)98 | |||||
| 1. Were the study eligibility criteria specified? | Yes | ||||
| 2. Are adequate baseline details presented? | Partial | ||||
| 3. Are similar cointerventions administered in both study periods? | NA | ||||
| 4. Are data analyses appropriate? | Yes | ||||
| 5. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | No | ||||
| 6. Are greater than 80% of physicians/patients included in the follow-up assessment? | 80% of patients included in follow-up | ||||
| 7. Are the conclusions supported by the results? | Yes | ||||
| Comments: Very few relevant outcomes that could be extracted with only minimal data | |||||
| Study demographics | |||||
|---|---|---|---|---|---|
|
Author; year; study ID: Sanders (2001)104 Title: The effects on clinician ordering patterns of a computerized decision support system for neuroradiology imaging studies Country: USA Specific setting: Vanderbilt University Medical Center (630 bed academic hospital with approximately 31,000 admissions per year) Study objectives: To evaluate the impact of computerised ordering guidelines on clinical (clinician) ordering patterns for neuroradiology imaging studies of the head Health-care setting: Secondary care (inpatient) If secondary care, academic status of the hospital: University Health-care system: NR Study design: Pre–post Number of sites: One Funding source: Public sector Was evaluator of tool also its developer? Yes |
|||||
| System users | |||||
| CDSS user(s) | Physicians; nurses; medical students; receptionists; other (unspecified) | ||||
| Practitioners (n) | NR | ||||
| Orders entered by user type at baseline | |||||
| User typea | n | % | |||
| Medical doctor | 617 | 83% | |||
| Nurse | 102 | 14% | |||
| Receptionist | 14 | 2% | |||
| Medical student | 3 | <1% | |||
| Other | 6 | 1% | |||
| Total | 742 | 100% | |||
| a Staff who were not medical doctors had the ability to enter verbal or written orders from physicians. | |||||
|
Inclusion criteria: All users who entered an order via WizOrder for one or more head CT or brain MRI imaging examinations on inpatients during the study period Exclusion criteria: NR |
|||||
| Patient baseline demographics | |||||
|
Inclusion criteria: All inpatients who had a head CT or brain MRI imaging examination ordered via WizOrder in the study perioda Exclusion criteria: NR |
|||||
| a No further data on patient baseline demographics was reported. | |||||
| Interventions | |||||
|
Intervention: OCS plus CDSS. A list of common indications for ordering an imaging examination of the head or brain was created and mapped to ICD-9 codes. For each indication, the most appropriate imaging test was determined. The CDSS required input of the patients’ acuity and indication by the user and provided a recommended test (head CT without contrast; head CT with contrast; head CT with and without contrast; brain MRI without contrast; and brain MRI with and without contrast). If a suggestion was given, this choice was defaulted. The user was able to override the recommendation and select any of the listed studies but had to type the reason for doing so. If no indication for requesting the test was given by the user or ‘other’ was chosen, no CDSS recommendation was provided Comparator: OCS alone (pre-intervention) Concomitant interventions: NA |
|||||
| CDSS tool | |||||
| Name of CDDS (if any) | WizOrder (home-grown system Vanderbilt University Medical Center) | ||||
| CDSS reasoning method | NR | ||||
| CDSS knowledge base | Prior free indications at the time of order entry, historical ICD-9 coding data and Local Medical Review Policy published guidelines | ||||
| Did study use training set and test set data? |
Training set: NR Test set: NR |
||||
| For training set data complete the following: | |||||
| Design | NR | ||||
| Target decision | NR | ||||
| Sample characteristics: | |||||
| Sample size (n) | NR | ||||
| Age (mean; range) | NR | ||||
| Gender (n male; n female) | NR | ||||
| Disease type | NR | ||||
| For test set data complete the following: | |||||
| Properties of test set data | NR | ||||
| Test centre | NR | ||||
| Target decision | NR | ||||
| Other CDSS information | |||||
| 1. Information used in CDSS [number of items: signs; symptoms; history; biochemical tests (list)] | 4 | ||||
| 2. Time to complete the CDSS (minutes) | NR | ||||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Recommendation | ||||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||||
| 5. Is user instructional training at the time of implementation described? | No | ||||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||||
| 8. Is there a need for additional data entry by the clinician? | No | ||||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | Yes | ||||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | Yes | ||||
| 13. Does the CDSS promote action rather than inaction? | NR | ||||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||||
| 16. Were local users involved in the CDSS development process? | Unclear | ||||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | ||||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||||
| Outcome measures | |||||
|
Outcome 1: Number of tests ordered pre- and post-intervention Outcome 2: Number of orders complying with the recommendation Total length of follow-up: 17 weeks (9 weeks pre-intervention, 8 weeks post-intervention) (study conducted between 30 September 2000 and 30 January 2001) Follow-up assessment times: 17 weeks Rate of attrition at each follow-up time: NA Methods of statistical analysis: Chi-squared tests were performed to evaluated changes in the distribution of ordering patterns |
|||||
| Results | |||||
| Outcome 1: Number of tests ordered pre-and post- intervention (by user type) | |||||
| User typea | Pre- (n; %) | Post- (n; %) | p-value | ||
| MD | 617 (83%) | 596 (85%) | NR | ||
| Nurse | 102 (14% | 84 (12%) | NR | ||
| Receptionist | 14 (2%) | 18 (3%) | NR | ||
| Medical student | 3 (<1%) | 3 (<1%) | NR | ||
| Other | 6 (1%) | 3 (<1%) | NR | ||
| Total | 742 (100%) | 704 (100%) | 0.048 | ||
| a Staff who were not MD had the ability to enter verbal or written orders from physicians. | |||||
| Outcome 2: Number of orders complying with the recommendation | |||||
| N in analysis | 551 | ||||
| Orders complying with recommendation (n; %) | 328 (60%) | ||||
| Orders not complying with recommendation (n; %) | 223 (40%) | ||||
| Authors’ conclusions: This study was successful in showing that a computerised implementation of guidelines for head and brain imaging studies influenced ordering patterns | |||||
| Methodological assessment criteria (Sanders) (2001)104 | |||||
| 1. Were the study eligibility criteria specified? | Yes | ||||
| 2. Are adequate baseline details presented? | Partial | ||||
| 3. Are similar cointerventions administered in both study periods? | NA | ||||
| 4. Are data analyses appropriate? | Yes | ||||
| 5. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Yes | ||||
| 6. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | ||||
| 7. Are the conclusions supported by the results? | Yes | ||||
| Comments: None | |||||
| Study demographics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Author; year; study ID: Nightingale (1994)99 Title: Effects of a computerised protocol management system on ordering of clinical tests Country: UK Specific setting: Supraregional Liver Unit, The Queen Elizabeth Hosptial, Birmingham, UK Study objectives: To assess the effects of a computerised protocol management system on the number, cost, and appropriateness of laboratory investigations requested Health-care setting: Secondary and tertiary (inpatients) If secondary care, academic status of the hospital: Teaching Health-care system: NHS Study design: Pre–post Number of sites: 1 Funding source: NR Was evaluator of tool also its developer? Yes |
|||||||||
| System users | |||||||||
| CDSS user(s)a | Physicians | ||||||||
| Practitioners (n) | NR | ||||||||
| a No further baseline details were reported on study physicians. | |||||||||
|
Inclusion criteria: All physicians on the unit within the study period Exclusion criteria: NR |
|||||||||
| Patient baseline demographics | |||||||||
|
Inclusion criteria: All patients admitted to the unit during the study period Exclusion criteria: NR |
|||||||||
| N (%) of patients | |||||||||
| Patient category | Pre-intervention | Post-intervention | |||||||
| Initial assessment | 177 (27%) | 153 (18%) | |||||||
| Reassessment (routine) | 33 (5%) | 67 (8%) | |||||||
| Reassessment (problem) | 30 (5%) | 45 (5.5%) | |||||||
| Transplant | 106 (16%) | 112 (13.5%) | |||||||
| Post-transplant (problem) | 84 (13%) | 112 (13.5%) | |||||||
| Post-transplant (t tube removal) | 41 (6%) | 48 (6%) | |||||||
| Post-transplant (annual review) | 86 (13%) | 146 (18%) | |||||||
| Emergency (acute hepatic failure) | 39 (6%) | 62 (7.5%) | |||||||
| Emergency (acute problem – chronic disease) | 32 (5%) | 19 (2%) | |||||||
| Other | 26 (4%) | 69 (8%) | |||||||
| Total | 654 (100%) | 833 (100%) | |||||||
| Interventions | |||||||||
|
Intervention: OCS plus CDSS. The CDSS used the latest available test results along with the clinical categories applicable to the patient to propose the test investigations to be performed on the following day. The system was based upon a combination of static and dynamic rules. Static rules were those that applied to all patients with a certain classification for a certain number of days, and dynamic rules were those which used the results of previous laboratory results to determine which investigations to propose Once the physician had viewed the proposed tests they were free to accept or modify them as required Comparator: OCS alone (pre-intervention) Concomitant interventions: NA |
|||||||||
| CDSS tool | |||||||||
| Name of CDDS (if any) | NR (home-grown system developed by the Wolfson Computer Laboratory, Queen Elizabeth Hospital, Birmingham, UK) | ||||||||
| CDSS reasoning method | Discrimination rules | ||||||||
| CDSS knowledge base | Protocol developed by Senior Clinicians | ||||||||
| Did study use training set and test set data? |
Training set: No Test set: No |
||||||||
| For training set data complete the following: | |||||||||
| Design | NR | ||||||||
| Target decision | NR | ||||||||
| Sample characteristics: | |||||||||
| Sample size (n) | NR | ||||||||
| Age (mean; range) | NR | ||||||||
| Gender (n male; n female) | NR | ||||||||
| Disease type | NR | ||||||||
| For test set data complete the following: | |||||||||
| Properties of test set data | NR | ||||||||
| Test centre | NR | ||||||||
| Target decision | NR | ||||||||
| Other CDSS information | |||||||||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | NR (signs; symptoms; history, biochemical tests) | ||||||||
| 2. Time to complete the CDSS (minutes) | NR | ||||||||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Advice | ||||||||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||||||||
| 5. Is user instructional training at the time of implementation described? | Yes | ||||||||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||||||||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||||||||
| 8. Is there a need for additional data entry by the clinician? | Yes | ||||||||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | ||||||||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||||||||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||||||||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | Yes | ||||||||
| 13. Does the CDSS promote action rather than inaction? | NR | ||||||||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||||||||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||||||||
| 16. Were local users involved in the CDSS development process? | Yes | ||||||||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||||||||
| 18. Does the CDSS provide periodic summaries of performance feedback? | Yes | ||||||||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||||||||
| Outcome measures | |||||||||
|
Outcome 1: Total number of tests requested per patient day (pre- and post-intervention) Outcome 2: Number of out of hours tests requested per patient day (pre- and post-intervention) Outcome 3: Direct laboratory costs per patient day (pre- and post-intervention) Outcome 4: Number of plasma urea and electrolyte tests, liver function tests, bone profile, calcium and other tests requested per patient day (pre- and post-intervention) Total length of follow-up: 2 years (pre-intervention 1 year; post-intervention 1 year). Study period: January 1990 to December 1991 Follow-up assessment times: 2 years Rate of attrition at each follow-up time: NA Methods of statistical analysis: Comparison between pre- and post-intervention periods were made using 2-sample Student’s t-tests or Mann-Whitney U-tests. Direct costs of laboratory tests were calculated by the methods of Broughton and Hogan219 |
|||||||||
| Results | |||||||||
| Outcome 1: Total number of tests requested per patient day (pre- and post-intervention) | |||||||||
| Patient category | Pre-a | Post-a | % change | Student’s t-statistic | |||||
| Initial assessment | 7.1 (2.9) | 5.4 (3.0) | –25 | 5.23d | |||||
| Reassessment (routine) | 4.8 (2.7) | 3.7 (2.7) | –22 | 1.92 | |||||
| Reassessment (problem) | 7.7 (2.1) | 6.3 (2.3) | –19 | 2.67c | |||||
| Transplant | 11.0 (2.8) | 9.6 (3.3) | –13 | 3.37d | |||||
| Post-transplant (problem) | 7.8 (2.5) | 6.9 (2.3) | –11 | 2.62c | |||||
| Post transplant (t tube removal) | 6.6 (4.0) | 5.0 (2.1) | –25 | 2.41b | |||||
| Post-transplant (annual review) | 7.4 (4.1) | 6.6 (2.9) | –12 | 1.73 | |||||
| Emergency (acute hepatic failure) | 6.7 (3.8) | 7.8 (4.0) | +17 | 1.37 | |||||
| Emergency (acute problem – chronic disease) | 11.1 (4.2) | 8.0 (4.1) | –28 | 2.57b | |||||
| Other | 6.2 (4.3) | 5.5 (4.1) | –11 | 0.73 | |||||
| Overall | 8.5 (3.6) | 7.0 (3.5) | –17 | 8.10d | |||||
|
a Mean (standard deviation) values. b p < 0.05. c p < 0.01. d p < 0.001. |
|||||||||
| Outcome 2: Number of out of hours tests requested per patient day (pre- and post-intervention) | |||||||||
| Pre-intervention (n;%) | Post-intervention (n;%) | % change | p-value | ||||||
| 0.31 | 0.16 | –48 | p < 0.001 | ||||||
| Outcome 3: Direct laboratory costs per patient day (pre- and post-intervention) | |||||||||
| Patient category | Pre-a | Post-a | % change | Mann-Whitney statistic | |||||
| Initial assessment | 2.54 (1.37–4.61) | 2.46 (1.54–3.83) | –3 | 0.21 | |||||
| Reassessment (routine) | 0.35 (0.21–0.94) | 0.76 (0.21–1.94) | +117 | 1.38 | |||||
| Reassessment (problem) | 1.67 (1.21–2.25) | 0.83 (0.38–1.23) | –50 | 3.24c | |||||
| Transplant | 2.84 (1.94–4.18) | 2.51 (1.86–3.42) | –12 | 1.46 | |||||
| Post-transplant (problem) | 1.65 (1.03–2.37) | 1.43 (0.78–1.90) | –13 | 2.44b | |||||
| Post transplant (t tube removal) | 1.16 (0.91–1.52) | 0.72 (0.60–1.34) | –38 | 3.09c | |||||
| Post-transplant (annual review) | 1.29 (0.91–1.43) | 0.86 (0.74–1.29) | –33 | 5.50d | |||||
| Emergency (acute hepatic failure) | 2.34 (0.91–3.77) | 1.68 (0.60–2.56) | –28 | 1.74 | |||||
| Emergency (acute problem – chronic disease) | 2.92 (1.93–5.59) | 2.44 (0.92–3.49) | –16 | 1.20 | |||||
| Other | 0.00 (0.00–1.16) | 0.12 (0.00–1.19) | NR | 0.10 | |||||
| Overall | 1.79 (0.94–2.96) | 1.29 (0.71–2.37) | –28 | 6.86d | |||||
|
a Median (interquartile range) values. b p < 0.05. c p < 0.01. d p < 0.001. |
|||||||||
| Outcome 4: Number of plasma urea and electrolyte tests, liver function tests, bone profile, calcium and other tests requested per patient day (pre- and post-intervention) | |||||||||
| Requests | Pre-a | Post-a | % change | Mann-Whitney statistic | |||||
| Plasma urea and electrolytes | 0.67 (0.42–0.95) | 0.56 (0.33–0.83) | –16 | 2.32b | |||||
| Liver function tests | 0.60 (0.39–0.88) | 0.50 (0.33–0.80) | –17 | 1.92 | |||||
| Bone profile | 0.40 (0.17–0.67) | 0.00 (0.00–0.10) | –100 | 18.4c | |||||
| Calcium | 0.50 (0.33–0.72) | 0.07 (0.00–0.25) | –86 | 16.3c | |||||
| Others | 1.27 (0.50–2.67) | 1.38 90.33–3.14) | +9 | 0.32 | |||||
|
a Median (interquartile range) values. b p < 0.05. c p < 0.001. |
|||||||||
| Authors’ conclusions: Use of the computerised protocol management system resulted in closer compliance with the protocols and a significant reduction in the overall level of requesting | |||||||||
| Methodological assessment criteria (Nightingale) (1994)99 | |||||||||
| 1. Were the study eligibility criteria specified? | Yes | ||||||||
| 2. Are adequate baseline details presented? | Yes | ||||||||
| 3. Are similar cointerventions administered in both study periods? | NA | ||||||||
| 4. Are data analyses appropriate? | Yes | ||||||||
| 5. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Yes | ||||||||
| 6. Are greater than 80% of physicians/patients included in the follow-up assessment? | Yes | ||||||||
| 7. Are the conclusions supported by the results? | Yes | ||||||||
| Comments: None | |||||||||
| Study demographics | |||||
|---|---|---|---|---|---|
|
Author; year; study ID: Boon-Falleur (1995)100 Title: A rule-based decision support application for laboratory investigations management Country: Belgium Specific setting: Paediatric Liver Transplantation Unit, Cliniques Universitaires Saint-Luc, Université catholique de Louvain, Brussels, Belgium Study objectives: To assess the impact of a rule-based expert system for clinical laboratory investigations management in a paediatric liver transplantation unit If secondary care, academic status of the hospital: University Health-care system: NR Study design: Pre–post Number of sites: One Funding source: NR Was evaluator of tool also its developer? No |
|||||
| System users | |||||
| CDSS user(s)a | Physicians | ||||
| Practitioners (n) | NR | ||||
| a No further baseline details were reported on study physicians. | |||||
|
Inclusion criteria: All physicians on the unit within the study period treating patients with either an assessment protocol or immediate post-transplant monitoring protocol Exclusion criteria: NR Patient baseline demographicsa Inclusion criteria: All patients admitted to the unit during the study period who were classified as managed by either an assessment protocol or immediate post-transplant monitoring protocol Exclusion criteria: NR |
|||||
| a No further baseline details were reported on study patients. | |||||
| Interventions | |||||
|
Intervention: OCS plus CDSS. The CDSS used the latest available test results along with the clinical categories applicable to the patient to propose the test investigations to be performed on the following day. The system was based upon a combination of static and dynamic rules. Static rules were those that applied to all patients with a certain classification for a certain number of days, and dynamic rules were those which used the results of previous laboratory results to determine which investigations to propose. The parameters evaluated by the rules were current patient status, current and previous test(s) results including trend analysis, and previous proposals. All patients classified according to the clinical categories as being eligible to be managed by either the assessment protocol or immediate post-transplant monitoring protocol were included in the study Once the physician had viewed the proposed tests they were free to amend them by adding or removing requests from the proposed schedule Comparator: OCS alone (pre-intervention) Concomitant interventions: NA |
|||||
| CDSS tool | |||||
| Name of CDDS (if any) | NR (home-grown system developed by the Wolfson Computer Laboratory, Queen Elizabeth Hospital, Birmingham, UK) | ||||
| CDSS reasoning method | Discrimination rules | ||||
| CDSS knowledge base | Protocol developed by senior clinicians | ||||
| Did study use training set and test set data? |
Training set: No Test set: No |
||||
| For training set data complete the following: | |||||
| Design | NR | ||||
| Target decision | NR | ||||
| Sample characteristics: | |||||
| Sample size (n) | NR | ||||
| Age (mean; range) | NR | ||||
| Gender (n male; n female) | NR | ||||
| Disease type | NR | ||||
| For test set data complete the following: | |||||
| Properties of test set data | NR | ||||
| Test centre | NR | ||||
| Target decision | NR | ||||
| Other CDSS information | |||||
| 1. Information used in CDSS [number of items; signs; symptoms; history; biochemical tests (list)] | NR (signs; symptoms; history, biochemical tests) | ||||
| 2. Time to complete the CDSS (minutes) | NR | ||||
| 3. CDSS output format: (score; probability graph; advice; etc.) | Advice | ||||
| 4. Is a description of pilot testing with users prior to implementation provided? | No | ||||
| 5. Is user instructional training at the time of implementation described? | No | ||||
| 6. Is the CDSS integrated with charting or OCS to support workflow integration? | Yes | ||||
| 7. Is automatic provision of CDSS output provided as part of clinician workflow? | Yes | ||||
| 8. Is there a need for additional data entry by the clinician? | Yes | ||||
| 9. Does the CDSS request documentation of the reason for not following CDSS recommendations? | No | ||||
| 10. Does CDSS provide output at the time and location of decision making? | Yes | ||||
| 11. Are the CDSS recommendations executed by the clinician noting agreement? | No | ||||
| 12. Does the CDSS provide a recommendation rather than just an assessment? | Yes | ||||
| 13. Does the CDSS promote action rather than inaction? | NR | ||||
| 14. Does the CDSS justify the output by provision of reasoning? | No | ||||
| 15. Does the CDSS justify the output by provision of research evidence? | No | ||||
| 16. Were local users involved in the CDSS development process? | No | ||||
| 17. Is the CDSS output provided to patients as well as clinician? | No | ||||
| 18. Does the CDSS provide periodic summaries of performance feedback? | No | ||||
| 19. Is the CDSS used in conjunction with conventional education? | No | ||||
| Outcome measures | |||||
|
Outcome 1: Number of tests per patient – assessment protocols (pre- and post-intervention) Outcome 2: Number of tests per patient – transplant protocols (pre- and post-intervention) Outcome 3: Number of urgently requested tests per patient (pre- and post-intervention) Total length of follow-up: Unclear (6 month pre-intervention baseline conducted between June and December 1993. Post-intervention from March 1994; length not reported) Follow-up assessment times: Unclear Rate of attrition at each follow-up time: NA Methods of statistical analysis: NR |
|||||
| Results | |||||
| Outcome 1: Number of tests per patient – assessment protocols | |||||
| Test category | Pre- (n = 32) | Post- (n = 151) | Δ% | ||
| General chemistry | 46 | 53 | 15% | ||
| Virology | 22 | 18 | –18% | ||
| Haematology and coagulation | 23 | 30 | 30% | ||
| Others | 13 | 19 | 46% | ||
| Total | 106 | 120 | 13% | ||
| Outcome 2: Number of tests per patient – transplant protocols | |||||
| Test category | Pre- (n = 10) | Post- (n = 24) | Δ% | ||
| General chemistry | 368 | 273 | –26% | ||
| Virology | 70 | 49 | –30% | ||
| Haematology and coagulation | 345 | 268 | –22% | ||
| Others | 264 | 178 | –33% | ||
| Total | 1047 | 768 | –27% | ||
| Outcome 3: Number of urgently requested tests per patient (mean) | |||||
| Pre- | Post- | Δ% | |||
| 65.0 | 36 | –44% | |||
| Authors’ conclusions: The system was perceived by the clinicians as increasing the overall benefits in use of clinical resources, improving the laboratory data management, and saving time for the execution of laboratory ancillary tasks | |||||
| Methodological assessment criteria (Boon-Falleur) (1995)100 | |||||
| 1. Were the study eligibility criteria specified? | Partial | ||||
| 2. Are adequate baseline details presented? | No | ||||
| 3. Are similar cointerventions administered in both study periods? | NA | ||||
| 4. Are data analyses appropriate? | Unclear | ||||
| 5. Is analysis conducted on an ‘intention to provide or communicate information’ basis? | Unclear | ||||
| 6. Are greater than 80% of physicians/patients included in the follow-up assessment? | Unclear | ||||
| 7. Are the conclusions supported by the results? | Yes | ||||
| Comments: Study quality and level of reporting is poor. The presentation of results by the number of tests per patient, rather than the number of tests per patient per day, confounds length of stay with number of tests. Length of stay is not reported and therefore it is not possible to assess whether this was similar between the groups of patients in the pre- and post-intervention periods. Results should be interpreted with caution | |||||
Appendix 4 Responses to survey by CDSS manufacturers/suppliers
| Manufacturer/Supplier | Specific CDSS | Deployed in UK | Currently being implemented in UK | Number of sites | Sites where deployed |
|---|---|---|---|---|---|
| National | |||||
| AGFA Healthcare (UK) Ltd | NR | NR | NR | NR | NR |
| Atos Origin | NR | NR | NR | NR | NR |
| British Telecommunications plc | NR | NR | NR | NR | NR |
| Cerner Ltd | NR | NR | NR | NR | NR |
| CSE Servelec Ltd | RiO Care Records System | Yes | Yes | 46 NHS organisations and 2 commercial companies including primary and secondary care, and mental health and learning disabilities | Across London and the south-east |
| FileTek UK Ltd | NR | NR | NR | NR | NR |
| Epic Systems Ltd | NR | No | No | NR | NR |
| Fujitsu Services Ltd | NR | NR | NR | NR | NR |
| ISoft plc | NR | NR | NR | NR | NR |
| Perot Systems Europe Ltd | NR | NR | NR | NR | NR |
| Siemens plc | NR | NR | NR | NR | NR |
| Steria Ltd [formerly known as Xansa (UK) Ltd] | NR | NR | NR | NR | NR |
| TATA Consultancy Services Ltd | NR | NR | NR | NR | NR |
| Specialist SME | |||||
| Adastra Software Ltd | NR | NR | NR | NR | NR |
| ALERT Life Sciences Computing, SA | NR | No | Will be implemented in one private hospital run by Circle Health by end 2009 and expects to interface with PAS, Pathology, PACS, and pharmacy stock control systems | 1 | NR |
| Oasis Medical Solutions Ltd (formerly known as Capula Healthcare Ltd) | NR | NR | NR | NR | NR |
| CAS Services Ltd (formerly known as Clinical Solutions Ltd) | NR | NR | NR | NR | NR |
| CSW Group Ltd | NR | NR | NR | NR | NR |
| Egton Medical Information Systems Ltd | NR | NR | NR | NR | NR |
| Infermed Ltd | NR | NR | NR | NR | NR |
| Map of Medicine (formerly known as Informa UK Ltd) | NR | NR | NR | NR | |
| Plain Healthcare | Odyssey FacetoFace | Yes | Unclear | NR | NR |
| Sowerby Centre for Health Informatics at Newcastle Ltd | NR | ||||
| Stalis Ltd | NR | NR | NR | NR | NR |
Glossary
- Algorithm
- A process for carrying out a complex task broken down into simple decision and action steps. Often assists the requirements analysis process carried out before programming.
- Applicability
- The extent to which the results of a study or review can be applied to the target population in practice.
- Appraisal of evidence
- Formal assessment of the quality of research evidence and its relevance to the clinical question according to predetermined criteria.
- Bias
- Systematic errors in the design and execution of a study which may lead to an over- or underestimation of the ‘true’ effect of a treatment or intervention.
- Blinding
- The practice of keeping the investigators or patients in a study ignorant of the group to which a participant has been assigned or of the population from which the participant has come from. The purpose of ‘blinding’ is to protect against bias.
- Computerised decision support systems (CDSS)
- An active knowledge system, which uses two or more items of patient data to generate case-specific advice.
- Clinical effectiveness
- How well a drug, procedure, device or package of care works to produce good outcomes for patients.
- Clinical trial
- Research study conducted with patients, usually to evaluate a new drug, device or procedure. Each trial is designed to answer scientific questions and to find better ways to treat individuals with a specific disease. See also randomised controlled trial.
- Cochrane Library
- The Cochrane Library consists of a regularly updated collection of evidence-based medicine databases including the Cochrane Database of Systematic Reviews. The Cochrane Library is available on CD-ROM and on the Internet.
- Computerised tomography
- A technique whereby X-rays are used to map the inside of the body.
- Confidence interval
- This helps us assess the likely effect of an intervention by describing the range of possible effects that are consistent with the results of a study (or a combination of studies). A wide confidence interval indicates a lack of certainty or precision about the true size of the clinical effect and is seen in studies with too few patients. Where confidence intervals are narrow they indicate more precise estimates of effects and a larger sample of patients studied. We usually interpret a 95% confidence interval as the range of effects within which we are 95% confident that the true effect lies.
- Confounding factor
- Something that introduces uncertainty and bias into an observed outcome, complicating interpretation of the result.
- Control group
- A group of patients recruited into a study that receives no treatment, a treatment of known effect, or a placebo in order to provide a comparison for a group receiving an experimental treatment, such as a new procedure.
- Controlled clinical trial
- A study that includes some form of a control group that is not randomised.
- Diagnostic work-up
- The process of making a diagnosis through tests, clinical history and clinical judgement.
- Effectiveness
- The extent to which a specific procedure or device, when used under usual or everyday conditions, does what it is intended to do.
- Electronic patient record
- A computer-based clinical data system designed to replace paper patient records.
- Extrapolation
- The application of research evidence based on studies of a specific population to another population with similar characteristics.
- Heterogeneity
- The term is used in meta-analysis and systematic reviews when the results or estimates of effects from separate studies seem to have different magnitude or even different sign or direction. Differences in the interventions, patient populations, outcome measures, definition of variables and duration of follow-up of the studies included in the analysis create problems of non-compatibility. See also homogeneity.
- Homogeneity
- This means that the results of studies included in a systematic review are similar and there is no evidence of heterogeneity. Results are usually regarded as homogeneous when differences between studies could reasonably be expected to occur by chance.
- Inclusion criteria
- See selection criteria.
- Intention to provide or communicate information
- Analyses of a clinical trial where patients are analysed according to the group to which they were initially randomly allocated, regardless of whether or not they dropped out, fully complied with the treatment, or cross over and received the alternative treatment.
- Knowledge base
- A store of knowledge represented explicitly so that a computer can search and reason with it automatically; often uses a clinical coding system to label the concepts.
- Knowledge-based system (expert system)
- A computer decision support system with an explicit knowledge base and separate reasoning program that uses this to give advice or interpret patient data.
- Methodological quality
- The extent to which a study has conformed to recognised good practice in the design and execution of its research methods.
- Non-experimental study
- A study based on participants selected on the basis of their availability, with no attempt having been made to avoid problems of bias.
- Objective measure
- A measurement that follows a standardised procedure which is less open to subjective interpretation by potentially biased observers or study participants.
- Pre–post study
- A study design which measures outcomes in one group of people, first before, and then after, an intervention is given or initiated.
- Probability
- How likely an event is to occur, for example how likely a treatment or intervention will alleviate the symptom.
- Prognostic factor
- Patient or disease characteristics which influence the course of a particular condition. In a randomised trial to compare two treatments, chance imbalances in prognostic factors that influence patient outcomes are possible, especially if the size of the study is fairly small. In terms of analysis these prognostic factors become confounding factors.
- p-value
- If a study is done to compare two treatments, then the p-value is the probability of obtaining the results, or something more extreme, if there really was no difference between treatments. By convention, where the value of p is below 0.05 (i.e. < 5%) the result is seen as statistically significant.
- Randomised controlled trial
- A trial in which people are randomly assigned to two (or more) groups: one (the experimental group) receiving the treatment that is being tested, and the other (comparison or control group) receiving an alternative treatment, a placebo, or no treatment. The two groups are followed-up to compare differences in outcomes between the two groups.
- Reliability
- Reliability refers to a method of measurement that consistently gives the same results.
- Sample
- A part of the study’s target population from which the participants of the study will be recruited. If participants are drawn in an unbiased way from a particular population, the results can be generalised from the sample to the population as a whole.
- Selection criteria
- Explicit criteria used in systematic reviews to decide which studies should be included and excluded from consideration as potential sources of evidence.
- Standard deviation
- A measure of the spread, scatter or variability of a set of measurements.
- Validity
- Assessment of how well a tool or instrument measures what it is intended to measure.
List of abbreviations
- ABG
- arterial blood gas
- ALT
- alanine aminotransferase
- ANA
- antinuclear antibody
- ARB
- angiotensin receptor blocker
- ASCC
- Additional Supply and Capacity service Contract
- AST
- aspartate aminotransferase
- CBA
- cost–benefit analyses
- CCA
- cost–consequence analyses
- CCT
- controlled clinical trial
- CDSS
- computerised decision support system
- CEA
- cost-effectiveness analyses
- CFU
- Connecting for Health
- CI
- Confidence interval
- CIC
- commercial-in-confidence
- CK
- creatine kinase
- CPOE
- computerised physician order entry
- CPP
- controlled pre–post study
- CRCT
- cluster randomised controlled trial
- CRD
- Centre for Reviews and Dissemination
- CT
- computerised tomography
- CUA
- cost–utility analyses
- CVR
- coronary vascular risk
- DRG
- diagnostic-related group
- HIV
- human immunodeficiency virus
- ICD-9
- International Classification of Diseases, Ninth Edition
- ICU
- intensive care unit
- IT
- information technology
- ITS
- interrupted time series
- LDL
- low-density lipid
- MRI
- magnetic resonance imaging
- NSAID
- non-steroidal anti-inflammatory drug
- NPfIT
- National Project for Information Technology
- OCS
- order communication system
- PACS
- picture archiving and communication system
- RCT
- randomised controlled trial
- RF
- rheumatoid factor
- SD
- standard deviation
- SHA
- Strategic Health Authority
- SME
- small to medium-sized enterprise
- STD
- sexually transmitted disease
- UPP
- uncontrolled pre–post study
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
‘Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of techniques.
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Riemsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA, Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D, et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant LD, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
-
Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement.
By Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al.
-
Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation.
By Wang D, Cummins C, Bayliss S, Sandercock J, Burls A.
-
Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation.
By McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al.
-
Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis.
By Simpson EL, Stevenson MD, Rawdin A, Papaioannou D.
-
Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs.
By Main C, Liu Z, Welch K, Weiner G, Quentin Jones S, Stein K.
-
Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome: a systematic review and economic analysis.
By McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, Akers J, et al.
-
Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review.
By Sutcliffe P, Hummel S, Simpson E, Young T, Rees A, Wilkinson A, et al.
-
The harmful health effects of recreational ecstasy: a systematic review of observational evidence.
By Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of oesophageal Doppler monitoring in critically ill and high-risk surgical patients.
By Mowatt G, Houston G, Hernández R, de Verteuil R, Fraser C, Cuthbertson B, et al.
-
The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technology Assessment reports.
By Taylor RS, Elston J.
-
Controlling Hypertension and Hypotension Immediately Post Stroke (CHHIPS) – a randomised controlled trial.
By Potter J, Mistri A, Brodie F, Chernova J, Wilson E, Jagger C, et al.
-
Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation.
By Pilgrim H, Lloyd-Jones M, Rees A.
-
Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation.
By Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, et al.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods.
By Hobart J, Cano S.
-
Treatment of severe ankle sprain: a pragmatic randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of three types of mechanical ankle support with tubular bandage. The CAST trial.
By Cooke MW, Marsh JL, Clark M, Nakash R, Jarvis RM, Hutton JL, et al. , on behalf of the CAST trial group.
-
Non-occupational postexposure prophylaxis for HIV: a systematic review.
By Bryant J, Baxter L, Hird S.
-
Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial.
By Farmer AJ, Wade AN, French DP, Simon J, Yudkin P, Gray A, et al.
-
How far does screening women for domestic (partner) violence in different health-care settings meet criteria for a screening programme? Systematic reviews of nine UK National Screening Committee criteria.
By Feder G, Ramsay J, Dunne D, Rose M, Arsene C, Norman R, et al.
-
Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation.
By Simpson EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J.
-
The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost-effectiveness and natural history.
By Fortnum H, O’Neill C, Taylor R, Lenthall R, Nikolopoulos T, Lightfoot G, et al.
-
Dipsticks and diagnostic algorithms in urinary tract infection: development and validation, randomised trial, economic analysis, observational cohort and qualitative study.
By Little P, Turner S, Rumsby K, Warner G, Moore M, Lowes JA, et al.
-
Systematic review of respite care in the frail elderly.
By Shaw C, McNamara R, Abrams K, Cannings-John R, Hood K, Longo M, et al.
-
Neuroleptics in the treatment of aggressive challenging behaviour for people with intellectual disabilities: a randomised controlled trial (NACHBID).
By Tyrer P, Oliver-Africano P, Romeo R, Knapp M, Dickens S, Bouras N, et al.
-
Randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of selective serotonin reuptake inhibitors plus supportive care, versus supportive care alone, for mild to moderate depression with somatic symptoms in primary care: the THREAD (THREshold for AntiDepressant response) study.
By Kendrick T, Chatwin J, Dowrick C, Tylee A, Morriss R, Peveler R, et al.
-
Diagnostic strategies using DNA testing for hereditary haemochromatosis in at-risk populations: a systematic review and economic evaluation.
By Bryant J, Cooper K, Picot J, Clegg A, Roderick P, Rosenberg W, et al.
-
Enhanced external counterpulsation for the treatment of stable angina and heart failure: a systematic review and economic analysis.
By McKenna C, McDaid C, Suekarran S, Hawkins N, Claxton K, Light K, et al.
-
Development of a decision support tool for primary care management of patients with abnormal liver function tests without clinically apparent liver disease: a record-linkage population cohort study and decision analysis (ALFIE).
By Donnan PT, McLernon D, Dillon JF, Ryder S, Roderick P, Sullivan F, et al.
-
A systematic review of presumed consent systems for deceased organ donation.
By Rithalia A, McDaid C, Suekarran S, Norman G, Myers L, Sowden A.
-
Paracetamol and ibuprofen for the treatment of fever in children: the PITCH randomised controlled trial.
By Hay AD, Redmond NM, Costelloe C, Montgomery AA, Fletcher M, Hollinghurst S, et al.
-
A randomised controlled trial to compare minimally invasive glucose monitoring devices with conventional monitoring in the management of insulin-treated diabetes mellitus (MITRE).
By Newman SP, Cooke D, Casbard A, Walker S, Meredith S, Nunn A, et al.
-
Sensitivity analysis in economic evaluation: an audit of NICE current practice and a review of its use and value in decision-making.
By Andronis L, Barton P, Bryan S.
-
Trastuzumab for the treatment of primary breast cancer in HER2-positive women: a single technology appraisal.
By Ward S, Pilgrim H, Hind D.
-
Docetaxel for the adjuvant treatment of early node-positive breast cancer: a single technology appraisal.
By Chilcott J, Lloyd Jones M, Wilkinson A.
-
The use of paclitaxel in the management of early stage breast cancer.
By Griffin S, Dunn G, Palmer S, Macfarlane K, Brent S, Dyker A, et al.
-
Rituximab for the first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma.
By Dundar Y, Bagust A, Hounsome J, McLeod C, Boland A, Davis H, et al.
-
Bortezomib for the treatment of multiple myeloma patients.
By Green C, Bryant J, Takeda A, Cooper K, Clegg A, Smith A, et al.
-
Fludarabine phosphate for the firstline treatment of chronic lymphocytic leukaemia.
By Walker S, Palmer S, Erhorn S, Brent S, Dyker A, Ferrie L, et al.
-
Erlotinib for the treatment of relapsed non-small cell lung cancer.
By McLeod C, Bagust A, Boland A, Hockenhull J, Dundar Y, Proudlove C, et al.
-
Cetuximab plus radiotherapy for the treatment of locally advanced squamous cell carcinoma of the head and neck.
By Griffin S, Walker S, Sculpher M, White S, Erhorn S, Brent S, et al.
-
Infliximab for the treatment of adults with psoriasis.
By Loveman E, Turner D, Hartwell D, Cooper K, Clegg A
-
Psychological interventions for postnatal depression: cluster randomised trial and economic evaluation. The PoNDER trial.
By Morrell CJ, Warner R, Slade P, Dixon S, Walters S, Paley G, et al.
-
The effect of different treatment durations of clopidogrel in patients with non-ST-segment elevation acute coronary syndromes: a systematic review and value of information analysis.
By Rogowski R, Burch J, Palmer S, Craigs C, Golder S, Woolacott N.
-
Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care.
By Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al.
-
A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial.
By Gray AJ, Goodacre S, Newby DE, Masson MA, Sampson F, Dixon S, et al. , on behalf of the 3CPO study investigators.
-
Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation.
By Ara R, Pandor A, Stevens J, Rees A, Rafia R.
-
Adefovir dipivoxil and pegylated interferon alpha for the treatment of chronic hepatitis B: an updated systematic review and economic evaluation.
By Jones J, Shepherd J, Baxter L, Gospodarevskaya E, Hartwell D, Harris P, et al.
-
Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis.
By Hewitt CE, Gilbody SM, Brealey S, Paulden M, Palmer S, Mann R, et al.
-
A double-blind randomised placebo-controlled trial of topical intranasal corticosteroids in 4- to 11-year-old children with persistent bilateral otitis media with effusion in primary care.
By Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, et al.
-
The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model.
By Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R.
-
Rehabilitation of older patients: day hospital compared with rehabilitation at home. A randomised controlled trial.
By Parker SG, Oliver P, Pennington M, Bond J, Jagger C, Enderby PM, et al.
-
Breastfeeding promotion for infants in neonatal units: a systematic review and economic analysis.
By Renfrew MJ, Craig D, Dyson L, McCormick F, Rice S, King SE, et al.
-
The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation.
By Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al.
-
Rapid testing for group B streptococcus during labour: a test accuracy study with evaluation of acceptability and cost-effectiveness.
By Daniels J, Gray J, Pattison H, Roberts T, Edwards E, Milner P, et al.
-
Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Honest H, Forbes CA, Durée KH, Norman G, Duffy SB, Tsourapas A, et al.
-
The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model.
By Bond M, Mealing S, Anderson R, Elston J, Weiner G, Taylor RS, et al.
-
Gemcitabine for the treatment of metastatic breast cancer.
By Jones J, Takeda A, Tan SC, Cooper K, Loveman E, Clegg A.
-
Varenicline in the management of smoking cessation: a single technology appraisal.
By Hind D, Tappenden P, Peters J, Kenjegalieva K.
-
Alteplase for the treatment of acute ischaemic stroke: a single technology appraisal.
By Lloyd Jones M, Holmes M.
-
Rituximab for the treatment of rheumatoid arthritis.
By Bagust A, Boland A, Hockenhull J, Fleeman N, Greenhalgh J, Dundar Y, et al.
-
Omalizumab for the treatment of severe persistent allergic asthma.
By Jones J, Shepherd J, Hartwell D, Harris P, Cooper K, Takeda A, et al.
-
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-Hodgkin’s lymphoma.
By Boland A, Bagust A, Hockenhull J, Davis H, Chu P, Dickson R.
-
Adalimumab for the treatment of psoriasis.
By Turner D, Picot J, Cooper K, Loveman E.
-
Dabigatran etexilate for the prevention of venous thromboembolism in patients undergoing elective hip and knee surgery: a single technology appraisal.
By Holmes M, C Carroll C, Papaioannou D.
-
Romiplostim for the treatment of chronic immune or idiopathic thrombocytopenic purpura: a single technology appraisal.
By Mowatt G, Boachie C, Crowther M, Fraser C, Hernández R, Jia X, et al.
-
Sunitinib for the treatment of gastrointestinal stromal tumours: a critique of the submission from Pfizer.
By Bond M, Hoyle M, Moxham T, Napier M, Anderson R.
-
Vitamin K to prevent fractures in older women: systematic review and economic evaluation.
By Stevenson M, Lloyd-Jones M, Papaioannou D.
-
The effects of biofeedback for the treatment of essential hypertension: a systematic review.
By Greenhalgh J, Dickson R, Dundar Y.
-
A randomised controlled trial of the use of aciclovir and/or prednisolone for the early treatment of Bell’s palsy: the BELLS study.
By Sullivan FM, Swan IRC, Donnan PT, Morrison JM, Smith BH, McKinstry B, et al.
-
Lapatinib for the treatment of HER2-overexpressing breast cancer.
By Jones J, Takeda A, Picot J, von Keyserlingk C, Clegg A.
-
Infliximab for the treatment of ulcerative colitis.
By Hyde C, Bryan S, Juarez-Garcia A, Andronis L, Fry-Smith A.
-
Rimonabant for the treatment of overweight and obese people.
By Burch J, McKenna C, Palmer S, Norman G, Glanville J, Sculpher M, et al.
-
Telbivudine for the treatment of chronic hepatitis B infection.
By Hartwell D, Jones J, Harris P, Cooper K.
-
Entecavir for the treatment of chronic hepatitis B infection.
By Shepherd J, Gospodarevskaya E, Frampton G, Cooper K.
-
Febuxostat for the treatment of hyperuricaemia in people with gout: a single technology appraisal.
By Stevenson M, Pandor A.
-
Rivaroxaban for the prevention of venous thromboembolism: a single technology appraisal.
By Stevenson M, Scope A, Holmes M, Rees A, Kaltenthaler E.
-
Cetuximab for the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck.
By Greenhalgh J, Bagust A, Boland A, Fleeman N, McLeod C, Dundar Y, et al.
-
Mifamurtide for the treatment of osteosarcoma: a single technology appraisal.
By Pandor A, Fitzgerald P, Stevenson M, Papaioannou D.
-
Ustekinumab for the treatment of moderate to severe psoriasis.
By Gospodarevskaya E, Picot J, Cooper K, Loveman E, Takeda A.
-
Endovascular stents for abdominal aortic aneurysms: a systematic review and economic model.
By Chambers D, Epstein D, Walker S, Fayter D, Paton F, Wright K, et al.
-
Clinical and cost-effectiveness of epoprostenol, iloprost, bosentan, sitaxentan and sildenafil for pulmonary arterial hypertension within their licensed indications: a systematic review and economic evaluation.
By Chen Y-F, Jowett S, Barton P, Malottki K, Hyde C, Gibbs JSR, et al.
-
Cessation of attention deficit hyperactivity disorder drugs in the young (CADDY) – a pharmacoepidemiological and qualitative study.
By Wong ICK, Asherson P, Bilbow A, Clifford S, Coghill D, R DeSoysa R, et al.
-
ARTISTIC: a randomised trial of human papillomavirus (HPV) testing in primary cervical screening.
By Kitchener HC, Almonte M, Gilham C, Dowie R, Stoykova B, Sargent A, et al.
-
The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: a systematic review and economic evaluation.
By Black C, Clar C, Henderson R, MacEachern C, McNamee P, Quayyum Z, et al.
-
Randomised preference trial of medical versus surgical termination of pregnancy less than 14 weeks’ gestation (TOPS).
By Robson SC, Kelly T, Howel D, Deverill M, Hewison J, Lie MLS, et al.
-
Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes.
By Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, Davies S, et al.
-
VenUS II: a randomised controlled trial of larval therapy in the management of leg ulcers.
By Dumville JC, Worthy G, Soares MO, Bland JM, Cullum N, Dowson C, et al.
-
A prospective randomised controlled trial and economic modelling of antimicrobial silver dressings versus non-adherent control dressings for venous leg ulcers: the VULCAN trial.
By Michaels JA, Campbell WB, King BM, MacIntyre J, Palfreyman SJ, Shackley P, et al.
-
Communication of carrier status information following universal newborn screening for sickle cell disorders and cystic fibrosis: qualitative study of experience and practice.
By Kai J, Ulph F, Cullinan T, Qureshi N.
-
Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation.
By Burch J, Paulden M, Conti S, Stock C, Corbett M, Welton NJ, et al.
-
Development of a toolkit and glossary to aid in the adaptation of health technology assessment (HTA) reports for use in different contexts.
By Chase D, Rosten C, Turner S, Hicks N, Milne R.
-
Colour vision testing for diabetic retinopathy: a systematic review of diagnostic accuracy and economic evaluation.
By Rodgers M, Hodges R, Hawkins J, Hollingworth W, Duffy S, McKibbin M, et al.
-
Systematic review of the effectiveness and cost-effectiveness of weight management schemes for the under fives: a short report.
By Bond M, Wyatt K, Lloyd J, Welch K, Taylor R.
-
Are adverse effects incorporated in economic models? An initial review of current practice.
By Craig D, McDaid C, Fonseca T, Stock C, Duffy S, Woolacott N.
-
Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE).
By Turnbull LW, Brown SR, Olivier C, Harvey I, Brown J, Drew P, et al.
-
Bevacizumab, sorafenib tosylate, sunitinib and temsirolimus for renal cell carcinoma: a systematic review and economic evaluation.
By Thompson Coon J, Hoyle M, Green C, Liu Z, Welch K, Moxham T, et al.
-
The clinical effectiveness and cost-effectiveness of testing for cytochrome P450 polymorphisms in patients with schizophrenia treated with antipsychotics: a systematic review and economic evaluation.
By Fleeman N, McLeod C, Bagust A, Beale S, Boland A, Dundar Y, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer.
By Mowatt G, Zhu S, Kilonzo M, Boachie C, Fraser C, Griffiths TRL, et al.
-
Effectiveness and cost-effectiveness of arthroscopic lavage in the treatment of osteoarthritis of the knee: a mixed methods study of the feasibility of conducting a surgical placebo-controlled trial (the KORAL study).
By Campbell MK, Skea ZC, Sutherland AG, Cuthbertson BH, Entwistle VA, McDonald AM, et al.
-
A randomised 2 × 2 trial of community versus hospital pulmonary rehabilitation for chronic obstructive pulmonary disease followed by telephone or conventional follow-up.
By Waterhouse JC, Walters SJ, Oluboyede Y, Lawson RA.
-
The effectiveness and cost-effectiveness of behavioural interventions for the prevention of sexually transmitted infections in young people aged 13–19: a systematic review and economic evaluation.
By Shepherd J, Kavanagh J, Picot J, Cooper K, Harden A, Barnett-Page E, et al.
-
Dissemination and publication of research findings: an updated review of related biases.
By Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al.
-
The effectiveness and cost-effectiveness of biomarkers for the prioritisation of patients awaiting coronary revascularisation: a systematic review and decision model.
By Hemingway H, Henriksson M, Chen R, Damant J, Fitzpatrick N, Abrams K, et al.
-
Comparison of case note review methods for evaluating quality and safety in health care.
By Hutchinson A, Coster JE, Cooper KL, McIntosh A, Walters SJ, Bath PA, et al.
-
Clinical effectiveness and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes: systematic review and economic evaluation.
By Cummins E, Royle P, Snaith A, Greene A, Robertson L, McIntyre L, et al.
-
Self-monitoring of blood glucose in type 2 diabetes: systematic review.
By Clar C, Barnard K, Cummins E, Royle P, Waugh N.
-
North of England and Scotland Study of Tonsillectomy and Adeno-tonsillectomy in Children (NESSTAC): a pragmatic randomised controlled trial with a parallel non-randomised preference study.
By Lock C, Wilson J, Steen N, Eccles M, Mason H, Carrie S, et al.
-
Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial.
By Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I, et al.
-
A randomised controlled multicentre trial of treatments for adolescent anorexia nervosa including assessment of cost-effectiveness and patient acceptability – the TOuCAN trial.
By Gowers SG, Clark AF, Roberts C, Byford S, Barrett B, Griffiths A, et al.
-
Randomised controlled trials for policy interventions: a review of reviews and meta-regression.
By Oliver S, Bagnall AM, Thomas J, Shepherd J, Sowden A, White I, et al.
-
Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery: a systematic review.
By McDaid C, Maund E, Rice S, Wright K, Jenkins B, Woolacott N.
-
A systematic review of outcome measures used in forensic mental health research with consensus panel opinion.
By Fitzpatrick R, Chambers J, Burns T, Doll H, Fazel S, Jenkinson C, et al.
-
The clinical effectiveness and cost-effectiveness of topotecan for small cell lung cancer: a systematic review and economic evaluation.
By Loveman E, Jones J, Hartwell D, Bird A, Harris P, Welch K, et al.
-
Antenatal screening for haemoglobinopathies in primary care: a cohort study and cluster randomised trial to inform a simulation model. The Screening for Haemoglobinopathies in First Trimester (SHIFT) trial.
By Dormandy E, Bryan S, Gulliford MC, Roberts T, Ades T, Calnan M, et al.
-
Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis.
By Black C, Sharma P, Scotland G, McCullough K, McGurn D, Robertson L, et al.
-
A randomised controlled trial of cognitive behaviour therapy and motivational interviewing for people with Type 1 diabetes mellitus with persistent sub-optimal glycaemic control: A Diabetes and Psychological Therapies (ADaPT) study.
By Ismail K, Maissi E, Thomas S, Chalder T, Schmidt U, Bartlett J, et al.
-
A randomised controlled equivalence trial to determine the effectiveness and cost–utility of manual chest physiotherapy techniques in the management of exacerbations of chronic obstructive pulmonary disease (MATREX).
By Cross J, Elender F, Barton G, Clark A, Shepstone L, Blyth A, et al.
-
A systematic review and economic evaluation of the clinical effectiveness and cost-effectiveness of aldosterone antagonists for postmyocardial infarction heart failure.
By McKenna C, Burch J, Suekarran S, Walker S, Bakhai A, Witte K, et al.
-
Avoiding and identifying errors in health technology assessment models: qualitative study and methodological review.
By Chilcott JB, Tappenden P, Rawdin A, Johnson M, Kaltenthaler E, Paisley S, et al.
-
BoTULS: a multicentre randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A.
By Shaw L, Rodgers H, Price C, van Wijck F, Shackley P, Steen N, et al. , on behalf of the BoTULS investigators.
-
Weighting and valuing quality-adjusted life-years using stated preference methods: preliminary results from the Social Value of a QALY Project.
By Baker R, Bateman I, Donaldson C, Jones-Lee M, Lancsar E, Loomes G, et al.
-
Cetuximab for the first-line treatment of metastatic colorectal cancer.
By Meads C, Round J, Tubeuf S, Moore D, Pennant M, Bayliss S.
-
Infliximab for the treatment of acute exacerbations of ulcerative colitis.
By Bryan S, Andronis L, Hyde C, Connock M, Fry-Smith A, Wang D.
-
Sorafenib for the treatment of advanced hepatocellular carcinoma.
By Connock M, Round J, Bayliss S, Tubeuf S, Greenheld W, Moore D.
-
Tenofovir disoproxil fumarate for the treatment of chronic hepatitis B infection.
By Jones J, Colquitt J, Shepherd J, Harris P, Cooper K.
-
Prasugrel for the treatment of acute coronary artery syndromes with percutaneous coronary intervention.
By Greenhalgh J, Bagust A, Boland A, Saborido CM, Fleeman N, McLeod C, et al.
-
Alitretinoin for the treatment of severe chronic hand eczema.
By Paulden M, Rodgers M, Griffin S, Slack R, Duffy S, Ingram JR, et al.
-
Pemetrexed for the first-line treatment of locally advanced or metastatic non-small cell lung cancer.
By Fleeman N, Bagust A, McLeod C, Greenhalgh J, Boland A, Dundar Y, et al.
-
Topotecan for the treatment of recurrent and stage IVB carcinoma of the cervix.
By Paton F, Paulden M, Saramago P, Manca A, Misso K, Palmer S, et al.
-
Trabectedin for the treatment of advanced metastatic soft tissue sarcoma.
By Simpson EL, Rafia R, Stevenson MD, Papaioannou D.
-
Azacitidine for the treatment of myelodysplastic syndrome, chronic myelomonocytic leukaemia and acute myeloid leukaemia.
By Edlin R, Connock M, Tubeuf S, Round J, Fry-Smith A, Hyde C, et al.
-
The safety and effectiveness of different methods of earwax removal: a systematic review and economic evaluation.
By Clegg AJ, Loveman E, Gospodarevskaya E, Harris P, Bird A, Bryant J, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of rapid point-of-care tests for the detection of genital chlamydia infection in women and men.
By Hislop J, Quayyum Z, Flett G, Boachie C, Fraser C, Mowatt G.
-
School-linked sexual health services for young people (SSHYP): a survey and systematic review concerning current models, effectiveness, cost-effectiveness and research opportunities.
By Owen J, Carroll C, Cooke J, Formby E, Hayter M, Hirst J, et al.
-
Systematic review and cost-effectiveness evaluation of ‘pill-in-the-pocket’ strategy for paroxysmal atrial fibrillation compared to episodic in-hospital treatment or continuous antiarrhythmic drug therapy.
By Martin Saborido C, Hockenhull J, Bagust A, Boland A, Dickson R, Todd D.
-
Chemoprevention of colorectal cancer: systematic review and economic evaluation.
By Cooper K, Squires H, Carroll C, Papaioannou D, Booth A, Logan RF, et al.
-
Cross-trimester repeated measures testing for Down’s syndrome screening: an assessment.
By Wright D, Bradbury I, Malone F, D’Alton M, Summers A, Huang T, et al.
-
Exploring the needs, concerns and behaviours of people with existing respiratory conditions in relation to the H1N1 ‘swine influenza’ pandemic: a multicentre survey and qualitative study.
By Caress A-L, Duxbury P, Woodcock A, Luker KA, Ward D, Campbell M, et al.
-
Influenza A/H1N1v in pregnancy: an investigation of the characteristics and management of affected women and the relationship to pregnancy outcomes for mother and infant.
By Yates L, Pierce M, Stephens S, Mill AC, Spark P, Kurinczuk JJ, et al.
-
The impact of communications about swine flu (influenza A H1N1v) on public responses to the outbreak: results from 36 national telephone surveys in the UK.
By Rubin GJ, Potts HWW, Michie S.
-
The impact of illness and the impact of school closure on social contact patterns.
By Eames KTD, Tilston NL, White PJ, Adams E, Edmunds WJ.
-
Vaccine effectiveness in pandemic influenza – primary care reporting (VIPER): an observational study to assess the effectiveness of the pandemic influenza A (H1N1)v vaccine.
By Simpson CR, Ritchie LD, Robertson C, Sheikh A, McMenamin J.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses: a Cochrane review.
By Jefferson T, Del Mar C, Dooley L, Ferroni E, Al-Ansary LA, Bawazeer GA, et al.
-
Randomised controlled trial and parallel economic evaluation of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR).
By Peek GJ, Elbourne D, Mugford M, Tiruvoipati R, Wilson A, Allen E, et al.
-
Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation.
By Waugh N, Cummins E, Royle P, Clar C, Marien M, Richter B, et al.
-
Barrett’s oesophagus and cancers of the biliary tract, brain, head and neck, lung, oesophagus and skin.
By Fayter D, Corbett M, Heirs M, Fox D, Eastwood A.
-
Towards single embryo transfer? Modelling clinical outcomes of potential treatment choices using multiple data sources: predictive models and patient perspectives.
By Roberts SA, McGowan L, Hirst WM, Brison DR, Vail A, Lieberman BA.
-
Sugammadex for the reversal of muscle relaxation in general anaesthesia: a systematic review and economic assessment.
By Chambers D, Paulden M, Paton F, Heirs M, Duffy S, Craig D, et al.
-
Systematic review and economic modelling of the effectiveness and cost-effectiveness of non-surgical treatments for women with stress urinary incontinence.
By Imamura M, Abrams P, Bain C, Buckley B, Cardozo L, Cody J, et al.
-
A multicentred randomised controlled trial of a primary care-based cognitive behavioural programme for low back pain. The Back Skills Training (BeST) trial.
By Lamb SE, Lall R, Hansen Z, Castelnuovo E, Withers EJ, Nichols V, et al.
-
Recombinant human growth hormone for the treatment of growth disorders in children: a systematic review and economic evaluation.
By Takeda A, Cooper K, Bird A, Baxter L, Frampton GK, Gospodarevskaya E, et al.
-
A pragmatic randomised controlled trial to compare antidepressants with a community-based psychosocial intervention for the treatment of women with postnatal depression: the RESPOND trial.
By Sharp DJ, Chew-Graham C, Tylee A, Lewis G, Howard L, Anderson I, et al.
-
Group cognitive behavioural therapy for postnatal depression: a systematic review of clinical effectiveness, cost-effectiveness and value of information analyses.
By Stevenson MD, Scope A, Sutcliffe PA, Booth A, Slade P, Parry G, et al.
-
Screening for hyperglycaemia in pregnancy: a rapid update for the National Screening Committee.
By Waugh N, Royle P, Clar C, Henderson R, Cummins E, Hadden D, et al.
-
Open-label, randomised, parallel-group, multicentre study to evaluate the safety, tolerability and immunogenicity of an AS03B/oil-in-water emulsion-adjuvanted (AS03B) split-virion versus non-adjuvanted wholevirion H1N1 influenza vaccine in UK children 6 months to 12 years of age.
By Waddington CS, Andrews N, Hoschler K, Walker WT, Oeser C, Reiner A, et al.
-
Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections.
By Simonds AK, Hanak A, Chatwin M, Morrell MJ, Hall A, Parker KH, et al.
-
Evaluation of triage methods used to select patients with suspected pandemic influenza for hospital admission: cohort study.
By Goodacre S, Challen, K, Wilson R, Campbell M.
-
Virus shedding and environmental deposition of novel A (H1N1) pandemic influenza virus: interim findings.
By Killingley B, Greatorex J, Cauchemez S, Enstone JE, Curran M, Read R, et al.
-
Neuraminidase inhibitors for preventing and treating influenza in healthy adults: a Cochrane review.
By Jefferson T, Jones M, Doshi P, Del Mar C, Dooley L, Foxlee R.
-
Intensity-modulated radiotherapy for the treatment of prostate cancer: a systematic review and economic evaluation.
By Hummel S, Simpson EL, Hemingway P, Stevenson MD, Rees A.
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NETSCC, HTA
-
Dr Andrew Cook, Consultant Advisor, NETSCC, HTA
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Consultant Adviser, NETSCC, HTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NETSCC, HTA
-
Professor Ruairidh Milne, Director of NETSCC External Relations
-
Ms Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NETSCC, HTA
HTA Commissioning Board
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Warwick Clinical Trials Unit
-
Director, Nottingham Clinical Trials Unit
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, University of Sheffield
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford?
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Ms Kay Pattison, NHS R&D Programme/DH, Leeds
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Mr A S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital
-
Dr Dianne Baralle, Consultant & Senior Lecturer in Clinical Genetics, Human Genetics Division & Wessex Clinical Genetics Service, Southampton, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Service User Representative
-
Professor Anthony Robert Kendrick, Professor of Primary Medical Care, University of Southampton
-
Dr Susanne M Ludgate, Director, Medical Devices Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr David Mathew Service User Representative
-
Dr Michael Millar, Lead Consultant in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, University College London
-
Mrs Una Rennard, Service User Representative
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr W Stuart A Smellie, Consultant, Bishop Auckland General Hospital
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Alan J Williams, Consultant in General Medicine, Department of Thoracic Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist Commissioning Advisory Group (NSCAG), Department of Health
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook Clinical Programmes Director, Bazian Ltd, London
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr Colin Greaves Senior Research Fellow, Peninsular Medical School (Primary Care)
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Miss Nicky Mullany, Service User Representative
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Service User Representative
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Ms Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Dr Caroline Stone, Programme Manager, Medical Research Council
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust, Bristol
-
Reader in Wound Healing and Director of Research, University of Leeds, Leeds
-
Professor Bipin Bhakta Charterhouse Professor in Rehabilitation Medicine, University of Leeds, Leeds
-
Mrs Penny Calder Service User Representative
-
Professor Paul Carding, Professor of Voice Pathology, Newcastle Hospital NHS Trust, Newcastle
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry, London
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol, Bristol
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and the London School of Medicine and Dentistry, London
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester, Manchester
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham, Nottingham
-
Dr Kate Radford, Division of Rehabilitation and Ageing, School of Community Health Sciences. University of Nottingham, Nottingham
-
Mr Jim Reece, Service User Representative
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton, Southampton
-
Dr Pippa Tyrrell, Stroke Medicine, Senior Lecturer/Consultant Stroke Physician, Salford Royal Foundation Hospitals’ Trust, Salford
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford, Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University, Cardiff
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health , London
-
Ms Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, MRC, London
-
Dr Ursula Wells PRP, DH, London
Interventional Procedures Panel
-
Consultant Surgeon & Honorary Clinical Lecturer, University of Sheffield
-
Mr David P Britt, Service User Representative, Cheshire
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Mr Michael Thomas, Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Mrs Isabel Boyer, Service User Representative, London
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Professor Robin Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Ms Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Service User Representative, Gloucestershire
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician & Cardiologist, County Durham & Darlington Foundation Trust
-
Mr John Needham, Service User, Buckingmashire
-
Ms Mary Nettle, Mental Health User Consultant, Gloucestershire
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Howard Ring, Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry & Warwickshire Partnership Trust
-
Dr Alastair Sutcliffe, Senior Lecturer, University College London
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Ms Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, MRC, London
-
Professor Tom Walley, HTA Programme Director, Liverpool
-
Dr Ursula Wells, Policy Research Programme, DH, London
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington