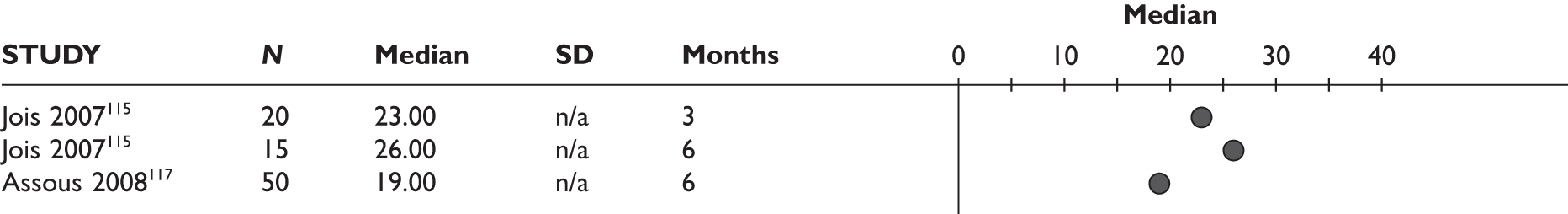Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 04/26/02. The protocol was agreed in July 2009. The assessment report began editorial review in November 2009 and was accepted for publication in June 2010. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Anne Fry-Smith was one of the authors of the technology assessment reports compiled to inform the following technology appraisals: TA130 adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis; and TA36 etanercept and infliximab for the treatment of rheumatoid arthritis. She also formed part of the Evidence Review Group for the single technology appraisal of tocilizumab for rheumatoid arthritis in 2009.
Dr David Moore and Kinga Malottki were part of the Evidence Review Group for the single technology appraisal of certolizumab pegol for rheumatoid arthritis.
Dr Pelham Barton constructed the Birmingham Rheumatoid Arthritis Model, which has been used in several National Institute for Health and Clinical Excellence (NICE) technology assessments/appraisals related to rheumatoid arthritis.
Angelos Tsourapas was part of the Evidence Review Group for the single technology appraisal of tocilizumab for rheumatoid arthritis in 2009.
Dr Paresh Jobanputra has participated in several technology assessments/appraisals related to rheumatoid arthritis as the clinical expert of the assessment group. He works as consultant rheumatologist in a large department of rheumatology. His department has taken part in clinical trials of etanercept, adalimumab, rituximab and tocilizumab. The department receives funding for nurses from Schering-Plough Ltd to administer infliximab to patients currently being treated with this drug. Currently he is the chief investigator of a clinical trial comparing adalimumab versus etanercept (ISRCTN95861172). He has, in the past, received support for educational purposes from Wyeth Pharmaceuticals (etanercept), Abbott (adalimumab) and Roche (rituximab). He has also been involved in an advisory board for Roche in relation to tocilizumab and rituximab, and has accepted support from UCB Pharma for the purposes of study leave to attend an American College for Rheumatology conference in 2009. He has not been involved in any pharmaceutical company submissions to the NICE and has no stocks or shares in any of these companies. Dr Jobanputra was also part of the Evidence Review Group for the single technology appraisal of tocilizumab for rheumatoid arthritis in 2009.
Dr Martin Connock was part of the Evidence Review Group for the single technology appraisal of tocilizumab and certolizumab pegol for rheumatoid arthritis.
Dr Yen-Fu Chen was one of the authors of the technology assessment report that was compiled to inform technology appraisal No. 130 entitled adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis.
Permissions
Copyright statement
© 2011 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of underlying health problem
Clinical features of rheumatoid arthritis
Rheumatoid arthritis (RA) typically begins in middle age and more affects more women than men. Pathologically the disease is characterised by an inflammatory reaction and increased cellularity of the lining layer of synovial joints. Joints such as the proximal interphalangeal joints, metacarpophalangeal joints, wrists, elbows, cervical spinal joints, knees, ankle and foot joints are commonly affected. Affected joints become stiff after periods of inactivity, for example in the morning, become swollen and are variably painful. Other organ systems may also be affected. Patients commonly experience fatigue and blood abnormalities such as anaemia and a raised platelet count. Weight loss, lymph node enlargement, lung diseases (such as pleurisy, pleural fluid and alveolitis), pericarditis, vascular inflammation (vasculitis), skin nodules and eye diseases (reduced tear production or inflammation) may also occur.
The severity of disease, its clinical course and individual responses to treatment vary greatly. Symptoms of RA may develop within days or evolve over many weeks and months. 1 Several distinct patterns of joint disease are recognised, including predominantly small or medium joint disease; predominantly large joint disease; flitting or transient attacks of joint pain (palindromic rheumatism); pain and stiffness of the shoulder and pelvic girdles (polymyalgic disease); disease associated with weight loss and fever (systemic onset); or any combination of these. Pain and disability in early RA is linked to disease severity and to measures of psychological distress. 2 Disease progression can be relentless or punctuated by partial or complete remissions of variable and unpredictable intervals.
Diagnosis of rheumatoid arthritis
Rheumatoid arthritis is diagnosed from a constellation of clinical, laboratory and radiographic abnormalities. Diagnosis may be obvious or may need specialist assessment or a period of clinical observation. Internationally agreed classification criteria for RA are used widely in contemporary research studies,3 but it is widely acknowledged that current criteria need to be revised. Current criteria include morning stiffness in joints exceeding 1 hour, physician-observed arthritis of three or more areas, arthritis involving hand joints, symmetrical arthritis, rheumatoid skin nodules, a positive blood test for rheumatoid factor (RF) and radiographic changes typical of rheumatoid disease. Such criteria have limited utility in routine practice and most clinicians diagnose RA without reference to them, as many patients do not meet formal disease classification criteria early in their disease. 4
Epidemiology
Rheumatoid arthritis affects around 0.5%–1% of the population, three times as many women as men and at age of onset peaks between the ages of 40 years and 70 years. Prevalence of disease at 65 years of age is six times that at 25 years of age. Recent estimates from England and Wales show an annual incidence of 31 per 100,000 women and 13 per 100,000 men, suggesting a decline in recent decades, and a prevalence of 1.2% in women and 0.4% in men. 5 The National Audit Office (NAO) estimates that around 580,000 people have RA in England and that 26,000 patients are diagnosed each year. 6
Aetiology
A specific cause for RA has not been identified. There appear to be many contributing factors including genetic and environmental influences. Genetic influence is estimated at 50%–60%. 7 The risk of RA in both members of a pair of monozygotic twins is 12%–15% and a family history of RA gives an individual a risk ratio of 1.6 compared with the expected population rate. 8 Many of the genes associated with susceptibility to RA are concerned with immune regulation. For example, the human leucocyte antigen HLA-DRB1, which contributes the greatest risk, and PTPN22, which makes the second most important genetic contribution in Caucasian populations, are both involved in T-lymphocyte activation and signalling. 9,10
Infectious agents have been suspected but no consistent relationship with an infective agent has been shown. Sex hormones have also been suspected because of the higher prevalence of RA in women and a tendency for disease to improve in pregnancy. However, a precise relationship has not been identified. A causal link with lifestyle factors such as diet, occupation or smoking has not been shown.
Pathology
Synovial joints occur where the ends of two bones, covered with hyaline cartilage, meet in a region where free movement is desirable. This joint space is encapsulated by a fibrous capsule lined on the inside by a synovial membrane, which functions to secrete fluid to lubricate and nourish hyaline cartilage. In RA the synovial layer of affected joints becomes enlarged as a result of increased cellularity or hyperplasia, infiltration by white blood cells and formation of new blood vessels. This is accompanied by increased fluid in the joint cavity, which contains white blood cells and a high level of protein (an exudate), contributing to the joint swelling. Bony erosions of cartilage and bone occur where synovial tissue meets cartilage and bone. This occurs through the combined actions of synovial tissue (pannus) and resident cartilage and bone cells. Erosions and loss of cartilage are rarely reversible. Such damage, therefore, compromises the structure and function of a normal joint.
Pathogenesis and biological targets in rheumatoid arthritis
A detailed discussion of the pathogenesis of RA is beyond the scope of this report. This subject is reviewed comprehensively elsewhere. 11–13 The synovial membrane in RA contains activated immune cells such as B and T lymphocytes and macrophages. These cells accumulate in synovial tissue. Cells resident in normal joints including synovial fibroblasts, cartilage cells (chondrocytes) and bone cells (osteoclasts) are also activated. Different cytokines, or small proteins, are produced by particular resident and infiltrating cells and aid intercellular communication and influence cellular and tissue behaviour.
A number of cytokines involved in this inflammatory cascade are seen as potential targets for intervention in RA. Drugs that target cytokines and which are licensed or are at a late stage of development currently include anakinra (directed against interleukin-1), tocilizumab [(TOC, RoActemra®, Roche) targeting interleukin-6] and tumour necrosis factor (TNF) inhibitors [including adalimumab (ADA, Humira®, Abbott Laboratories), certolizumab (Cimzia®, UCB), etanercept (ETN, Enbrel®, Wyeth Pharmaceuticals), golimumab (Simponi®, Schering-Plough Ltd) and infliximab (IFX, Remicade®, Schering-Plough Ltd)]. Other agents include abatacept [(ABT, Orencia®, Bristol-Myers Squibb Ltd) also known as CTLA4Ig], which interferes with T-cell activation, and rituximab (RTX, Mabthera®, Roche), which depletes B lymphocytes. Many other potential targets have been identified and a number of novel agents are in clinical trials. 14
Management of rheumatoid arthritis
The current management of RA is described in detail in a recent National Institute for Health and Clinical Excellence (NICE) guideline. 15 An exhaustive review of management is not provided here. We focus on aspects of disease management that are relevant to the decision problem in this appraisal.
Non-steroidal anti-inflammatory drugs (NSAIDs) and analgesics are commonly used for symptom relief in RA. These drugs do not modify the disease process and key recommendations in NICE guidance centre on minimising the use of NSAIDs because of the potential toxicity of these agents. Corticosteroids are used widely and in a variety of ways. High doses given orally or parenterally (by a variety of routes) are used for the short-term control of disease while waiting for the effects of disease-modifying antirheumatic drugs (DMARDs). Low-dose glucocorticoids are also commonly used either as sole therapy or in combination with DMARDs. Low-dose glucocorticoids have important disease-modifying effects in RA. 16
Disease-modifying antirheumatic drugs may be divided into conventional DMARDs, which include azathioprine (AZA), ciclosporin A (CyA), gold [GST (given by intra-muscular injection)], hydroxychloroquine (HCQ), leflunomide (LEF), methotrexate (MTX) and sulfasalazine,17–19 and newer targeted biological agents, described below. Conventional DMARDs such as penicillamine are now used rarely. 18 Conventional DMARDs may be used in combination, especially where there is a poor response to a single DMARD. For example, in early disease MTX is commonly combined with sulfasalazine and HCQ. There are few direct comparisons of individual DMARDs in early disease, but MTX is regarded as the standard against which other drugs should be compared. Most conventional DMARDs have specific dosing and monitoring schedules that require regular visits to a health-care facility and blood tests. How this is managed varies greatly in the UK; for example, in some centres all patients are seen in hospital clinics for drug monitoring whereas in others this occurs largely in the community.
The key objective in early RA management is to achieve remission. Many patients with early inflammatory arthritis (which often does not meet international classification criteria for RA) are able to achieve remission and treatment may be withdrawn in a proportion without relapse. 20 This occurs in randomised trials or therapeutic studies with conventional DMARDs21–24 used as monotherapy or in combination, conventional DMARDs combined with TNF inhibitors and also in observational studies. While these reports focus on the excellent outcomes achieved, it is important to recall that 57% of patients with early RA treated with a protocol designed to minimise disease do not achieve remission, around one-third do not achieve their treatment goal and between 31% and 54% of patients have progressive joint damage depending on the treatment strategy after 4 years of treatment. 25
The NICE RA guidance recommends the use of MTX combined with another DMARD and corticosteroids (used short term) for disease control in early, severe RA. Practice varies; however, and evidence for combining DMARDs is limited and controversial. 26–28 Not all rheumatologists accept the need for DMARD combinations. Some prefer to step up therapy by adding another DMARD to MTX if the disease is inadequately controlled and others choose to replace the first DMARD with a second drug. 29 A necessity for long-term use of multiple medications plainly requires an open dialogue and shared decision making between patients and health professions,30 especially where expert opinion differs.
In England and Wales patients who have failed to respond to (or tolerate) at least two DMARDs, including MTX at optimal doses, are eligible for TNF inhibitors subject to NICE guidance. Patients who do not respond to TNF inhibitors may be treated with RTX, a monoclonal antibody that depletes B lymphocytes. Other biological therapies such as anakinra, ABT and TOC are not currently approved for use by NICE. The relevant NICE guidance concerned with biologic therapies is described briefly below (see Current service provision).
Controlling symptoms of joint pain and stiffness, minimising loss of function, improving quality of life (QoL) and reducing the risk of disability associated with joint damage and deformity are central objectives in the management of RA at all stages. These objectives are not met with drug therapy alone: patients often need advice and support from a multidisciplinary team including specialist nurses, podiatrists, physiotherapists and occupational therapists. Since RA is a heterogeneous disease, which may vary over time, a long-term plan with regular clinical evaluation to assess disease status, disease complications, comorbidity, patient preferences and psychosocial factors is essential and is aided by well-informed and satisfied patients and carers. 31,32 Indeed a key element of a Scottish trial reporting excellent outcomes was frequent specialist review with a focus on tight disease control. 21
With advanced joint damage surgical intervention such as joint replacement arthroplasty, joint fusion or osteotomy may be necessary. Long-term observations show that around a quarter of patients with RA undergo a total joint arthroplasty. 33 It cannot, of course, be assumed that all such surgery is directly attributable to RA, especially as osteoarthritis is the most prevalent form of arthritis. Other surgical interventions such as removal of synovial tissues and rheumatoid nodules, peripheral nerve decompression (such as in carpal tunnel syndrome) or soft tissue procedures such as tendon release or repair may be necessary at any stage of disease.
Assessment of response to disease-modifying antirheumatic drugs and biologic therapies
ACR response criteria
Modern clinical trials rely on composite end points such as the American College of Rheumatology (ACR) definition of improvement and the Disease Activity Score (DAS). The ACR response requires an improvement in the counts of the number of tender and swollen joints (using designated joints) and at least three items from the following: observer evaluation of overall disease activity; patient evaluation of overall disease activity; patient evaluation of pain; a score of physical disability [such as the Health Assessment Questionnaire (HAQ); see below]; and improvements in blood acute phase responses [e.g. erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP)].
Response is defined as ACR20, ACR50 or ACR70, where figures refer to the percentage improvement of these clinical measures. This creates a dichotomous outcome of responders and non-responders. Achieving an ACR20 response has been regarded as a low hurdle, but in clinical practice patients who achieve this hurdle often gain a worthwhile clinical response, especially in early RA. ACR response criteria are described in more detail in Appendix 1.
DAS response criteria
The DAS score is calculated using a formula that includes counts for tender and swollen joints, an evaluation by the patient of general health (on a scale of 0 to 100) and blood acute phase (usually a log of the ESR, but more recently using CRP). DAS response criteria are described in more detail in Appendix 1. Originally DAS was based on an assessment of 53 joints for tenderness and 44 joints for swelling. Disease Activity Score 28 (DAS28), based on an evaluation of 28 joints, is used widely in routine clinical practice, partly as a result of NICE guidance on use of TNF inhibitors. DAS28, like DAS, is a continuous scale with a theoretical range from 0 to 10. Thresholds have been suggested for the scale such that a score greater than 5.1 is regarded as indicating high disease activity, a score of less than 3.2 low disease activity and a score of less than 2.6 remission. 34,35 Achieving a DAS28 score of less than or equal to 3.2 and improving the score by greater than 1.2 is regarded to be a good response while achieving a score of less than or equal to 3.2 and improving by greater than 0.6 but less than 1.2 is regarded as a moderate response. Current NICE guidance for TNF inhibitors demands that patients should improve DAS28 by 1.2 in order to justify continuing treatment. It has been suggested that NICE guidance should be altered to allow patients who have attained a moderate response to continue treatment with a TNF inhibitor. 36
While DAS28 scores are a very valuable tool for assessing treatment responses in groups of patients, scores have important limitations when used for individual patient decisions. For example, DAS28 does not incorporate ankle and foot disease. Thus, a patient with disease localised here may not attain a sufficiently high score to be eligible for a TNF inhibitor. DAS28 also shows poor concordance with clinical judgement (based on a wide range of parameters). 37 In addition, the degree of measurement error in a test–retest reliability study indicates that the faith placed in DAS28 as the sole decision-making tool is misplaced. 38 For example, the smallest detectable difference which should be exceeded if a clinician is to be 95% confident that a change exceeds measurement variability was 1.32 for DAS28.
The Health Assessment Questionnaire
The HAQ is a family of questionnaires designed to assess functional capacity of patients. 39 The most widely used version of HAQ is the modified HAQ (MHAQ) score which comprises eight items such as an ability to dress, get in and out of bed, lift a cup, walk outdoors and wash. MHAQ is reported as an average score across the eight categories such that 0 indicates an ability to achieve tasks without difficulty and 3 reflects an inability to achieve tasks. Scores therefore range between 0 and 3 with an interval of 0.125. Low scores indicate better function. Care is needed in the interpretation of HAQ scores in published studies because there are several modifications to HAQ. The HAQ score is described in more detail in Appendix 1.
Radiographic measures
Radiographic outcomes are believed by many to be the most important outcome measure in RA. However, variation in joint inflammation has a more profound and immediate impact on disability compared with the slow and cumulative effect of radiographic damage on disability. 40 The most commonly used tools for assessing joint damage are the Sharp and Larsen methods and their modifications (see Appendix 1), which rely on evaluations of plain radiographs of hands and feet. Plain radiographs are rather insensitive to change but are cheap and widely available. A majority of patients show only mild or no progression on plain radiographs over periods of 1–2 years, highlighting one of their limitations in modern clinical trials. 41
Prognosis
The impact of RA on an individual can be viewed from a variety of perspectives including employment status, economic costs to the individual or society, QoL, physical disability, life expectancy and medical complications such as extra-articular disease and joint deformity, radiographic damage or the need for surgery. In general, persistent disease activity is associated with poorer outcomes, although in the first 5 years of disease physical function is especially labile. Greater physical disability at presentation is associated with greater disability later in disease. Other factors linked with poorer function include older age at presentation, the presence of rheumatoid nodules, female sex, psychological distress and degree of joint tenderness. 42 Continued employment is related to the type of work and other aspects of the workplace such as pace of work, physical environment, physical function, education and psychological status; work disability is not necessarily linked to measures of disease activity. 43,44 Radiographic damage in RA joints is also influenced by RF status, age, disease duration and extent of disease and perhaps genetic factors.
Life expectancy in RA is reduced and is related to age, disability, disease severity, comorbidity and RF status, in particular. 45–48 For example, a 50-year-old woman with RA is expected to live for 4 years less than a 50-year-old woman without RA. 49 Patients with RA have a significantly increased risk of ischaemic heart disease. Heart disease is the principal reason for an approximately 60% increased mortality risk in RA. 50 However, other factors such as infection associated with aspects such as comorbidity, including lung disease, extra-articular manifestations of disease, reduced white cell count and corticosteroid use, also contribute. 51,52
Burden of illness
Early in disease indirect costs exceed costs due to health-care utilisation and medication (direct costs) by twofold. 53 It is also clear that informal caregivers shoulder a considerable burden in terms of forgone paid employment, leisure activity and personal health. 54 Inevitably, in a disease characterised by chronic pain, discomfort and physical impairment, the burden on individuals and families is increased. Medication costs, especially in those treated with biologic agents such as TNF inhibitors, account for a majority of the direct costs of RA. 55 Some drug intervention studies have shown reduced work absence with aggressive treatment strategies,56 although only one-third of employed patients cease work because of disease and, unsurprisingly, manual workers are much more likely to stop work. 57 It is estimated that the total costs of RA to the UK economy is between £3.8 and £4.8 billion. 6
Current service provision
Services for patients with RA have been reviewed in detail in a recent report by the NAO. 6 Diagnosis and management of RA is led primarily by consultant rheumatologists employed by acute hospital trusts. People who may have RA often seek help late and may suffer owing to delayed treatment and referral. There are around 460 consultant rheumatologists in England, giving a ratio of 1 : 100,000 rheumatologists per head of population (the ratio in Wales is 1 : 106,000). Consultants are supported by specialist nurses and the NAO census identified 377 specialist rheumatology nurses in England. Considerable variations and deficiencies in service provision were identified by the NAO. Specific recommendations for improving services were made by the NAO in the following areas:
-
timely diagnosis and treatment
-
better integration between primary and secondary care services
-
improved holistic care including strategies to improve self-management and providing support for maintaining employment.
Description of the technologies
Five intervention technologies are considered in this report. Three are TNF inhibitors (ADA, ETN and IFX), and one each a T-cell costimulation modulator (ABT) and a selective CD20 B-cell depleting agent (RTX). The technologies are described below. Licensed indications and relevant NICE guidance are detailed in Table 1.
| Drug | Indications and population | Doses and routes of administration | Synopsis of relevant NICE guidance |
|---|---|---|---|
| ABT | Moderate-to-severe RA – in combination with MTX. Patients with insufficient response to DMARDs including at least one TNF inhibitor | Intravenous infusion over 30 minutes. Dose according to weight, range 500–1,000 mg. Infusions at 0, 2 and 4 weeks followed by 4-weekly maintenance infusions indefinitely |
TA141 Not recommended |
| ADA | Moderate-to-severe RA – in combination with MTX (unless MTX inappropriate). Patients with insufficient response to DMARDs including MTX | Subcutaneous injection of 40 mg every other week indefinitely. Dose may be increased to 40 mg weekly if patients experience a decrease in their response (monotherapy) |
TA130 and TA36 (for ADA, ETN and IFX) DAS28 score of > 5.1 measured on at least two occasions, 1 month apart Previous trial of two DMARDs including MTX (unless contraindicated) necessary Normally used in combination with MTX – unless intolerant or inappropriate when monotherapy with ADA and ETN may be given Only continue after 6 months if DAS28 improves by > 1.2 Alternative TNF inhibitor may be considered if treatment is withdrawn due to an adverse effect before the initial 6-month assessment of efficacy Dose escalation above licensed starting dose is not recommended TA36 does not recommend the consecutive use of TNF inhibitors. This recommendation is not reproduced in the NICE RA guideline. TA130 does not report on consecutive use |
| ETN | Moderate-to-severe RA – monotherapy or in combination with MTX in those with an inadequate response to DMARDs. Patients with severe RA not previously treated with MTX may also be treated | Subcutaneous injection of 25 mg twice a week or 50 mg weekly given indefinitely | |
| IFX | Moderate-to-severe RA – in combination with MTX (unless contraindicated) in those with an inadequate response to DMARDs. Patients with severe RA not previously treated with MTX or other DMARDs may also be treated | Intravenous infusion over 2 hours at a dose of 3 mg/kg at 0, 2 and 6 weeks followed by 8-weeky maintenance infusions indefinitely. If response lost or inadequate, stepwise increases in dose by 1.5 mg/kg every 8 weeks may given up to a maximum of 7.5 mg/kg. Alternatively, dosing at 3 mg/kg may be given as frequently as 4-weekly | |
| RTX | Severe RA in combination with MTX in patients who have had an inadequate response or intolerance to other DMARDs including one or more TNF inhibitor | Intravenous infusion given as a course of two infusions (1,000 mg each) 2 weeks apart. Further infusions may be given but a precise limit is not given. Repeat course of treatment must not be given within 16 weeks |
TA126 Use in combination with MTX in severe RA not responding to DMARDs including at least one TNF inhibitor Continue only if DAS28 improves by > 1.2 Repeat courses to be given no more frequently than every 6 months |
Tumour necrosis factor inhibitors
Adalimumab
Adalimumab is a recombinant monoclonal antibody, made from human peptide sequences, which neutralises the biological functions of tumour necrosis factor alpha (TNFα) by binding to TNF cell-surface receptors. ADA is licensed for use in RA, juvenile idiopathic arthritis, psoriasis, psoriatic arthritis, ankylosing spondylitis and Crohn’s disease.
Etanercept
Etanercept is a combination protein consisting of the extracellular portion of two TNFα receptors (75-kDa TNF receptors) combined with a human fragment crystallisable (Fc) portion of the human immunoglobulin G1 (IgG1). ETN inhibits TNFα activity by binding soluble and cell-bound TNFα with high affinity and by competing with natural TNFα receptors. ETN is licensed for use in RA, psoriatic arthritis, psoriasis and ankylosing spondylitis.
Infliximab
Infliximab is a recombinant chimeric human–murine monoclonal antibody that binds soluble and membrane-bound TNFα thereby, inhibiting the functions of TNFα. IFX is licensed for use in RA, Crohn’s disease, ulcerative colitis, ankylosing spondylitis, psoriatic arthritis and psoriasis.
Other tumour necrosis factor inhibitors
Certolizumab pegol has been granted a marketing authorisation in the European Union (EU) for the treatment of moderate-to-severe RA. It is administered by subcutaneous injection. Certolizumab pegol was the subject of a separate NICE single technology appraisal (STA),58 with guidance published in February 2010. Golimumab is currently being assessed by the European Medicines Agency. A positive opinion has been given for the granting of marketing authorisation in RA. Golimumab has been referred to NICE, but the appraisal has been suspended because the manufacturer is not in a position to submit evidence to NICE.
Special precautions for use of tumour necrosis factor inhibitors
TNFα is a key component of host defence against Mycobacterium tuberculosis (MTB), especially by forming granulomas and preventing dissemination of mycobacteria. 59,60 Inhibition of TNFα increases the risk of MTB and other granulomatous diseases, such as those due to Listeria monocytogenes (a bacterium associated with food-borne diseases) and Histoplasma capsulatum (a fungus which, in endemic areas, causes lung disease in people with a compromised immune system). Recommendations for screening patients for tuberculosis (TB) before treatment have been published. 61 In the UK this is done most commonly by taking a medical history focusing on TB and a pre-treatment chest radiograph. Some centres also perform a tuberculin skin test,62 although interpretation of such tests is complicated by the UK’s previous vaccination programme for TB prevention and also the fact that many patients with RA respond poorly to tuberculin (possibly because of current immunosuppressive therapy but also because of the disease). 63
Routine monitoring of blood tests is not necessary for patients taking TNF inhibitors, but is needed for concomitantly used DMARDs such as MTX. TNF inhibitors can induce anti-nuclear and anti-double-stranded DNA antibodies in the blood of some patients treated with TNF inhibitors. These antibodies are associated with systemic lupus erythematosus (SLE), a potentially serious rheumatic disease. Cases of drug-induced SLE have been reported with TNF inhibitors, but are rare. 64
Other technologies
Rituximab
Rituximab is a chimeric monoclonal antibody which binds the CD20 cell surface marker found on B lymphocytes and depletes these cells. CD20 occurs on normal and malignant B lymphocytes (as in non-Hodgkin’s lymphomas). Normal plasma cells, an important component of host defence, and haematopoietic stem cells do not carry CD20. RTX is licensed for use in RA, non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. A small number of cases of progressive multifocal leucoencephalopathy, a rare but usually fatal demyelinating brain disease, have been reported in RA patients following RTX treatment. 65
Abatacept
Abatacept is a fusion protein consisting of CTLA-4 (cytotoxic T-lymphocyte-associated antigen-4) linked to a modified Fc portion of the human IgG1. ABT works by blocking activation of certain populations of T lymphocytes. ABT is currently licensed for use only in RA.
Tocilizumab
Tocilizumab was the subject of a separate NICE STA,66 with guidance published in August 2010. This guidance is likely to have a key impact on the treatment pathways considered in this review. TOC is a humanised monoclonal antibody that inhibits the activity of the cytokine interleukin-6 (IL-6). In the EU it is licensed for use only in moderate-to-severe RA patients who are intolerant, or have responded inadequately, to one or more DMARDs or TNF inhibitors. The drug is recommended for use in combination with MTX, but may be used alone in patients intolerant of MTX or for whom it is contraindicated. TOC is given by intravenous (i.v.) infusion over 1 hour once a month indefinitely.
Disease-modifying antirheumatic drugs, biologics, treatment sequences and combinations
Rheumatoid arthritis is characterised, in many patients, by an excellent initial response to a DMARD with subsequent loss of response with time. Most randomised trials are of a relatively short duration (typically less than 12 months) and do not study a treatment pathway. Trials of DMARDs sequences are increasingly common. 25,67,68 Remission is possible in early disease with MTX alone or in combination with other agents such as sulfasalazine, HCQ, CyA and TNF inhibitors. The optimal sequence is yet to be determined, and perhaps the choice of drug is not relevant, but the key to successful management appears to be regular patient review with a focus on optimal disease control.
The NICE RA guidance is consistent with this approach, although recent trials indicate that early use of MTX in combination with a TNF inhibitor provides better outcomes. 25,69 NICE recommends that TNF inhibitors are used only in those not responding to MTX and another DMARD. Delayed addition of a TNF inhibitor need not necessarily compromise medium-term outcomes23,25,69 and may be justified on health-economic grounds.
What steps should be taken when a first TNF inhibitor and several DMARDs including MTX fail? This technology assessment report sets out to examine clinical effectiveness and cost-effectiveness evidence from available randomised controlled trials (RCTs), observational studies and economic evaluations. A small survey conducted as part of this technology assessment on a convenience sample of consultant rheumatologists in the West Midlands indicated considerable variability in approach for patients who fail a first TNF inhibitor. The most common suggested approaches were to consider a second TNF inhibitor or RTX (in combination with MTX). Further details of this survey can be found in Appendix 11.
There are many and increasing permutations of treatment sequences. Combinations of biologic agents are not licensed and where combinations have been tried there is an increased risk of serious infections. Potential drug toxicity of newly licensed agents is an important unknown. Other considerations include practical matters to do with drug delivery such as i.v. or subcutaneous administration and availability of infusion facilities. Patients with RA tend to be risk averse70 and strategies mandating targeted disease control in late ‘stable’ RA are commonly resisted by doctors and patients. 71 However, in those with active and progressive disease new therapies are needed. This review seeks to explore some aspects of these uncertainties as determined by a protocol agreed with NICE and interested parties.
Degree of diffusion and anticipated costs
The number of RA patients currently being treated with TNF inhibitors is unknown. By July 2009, 12,626 patients who started treatment with a TNFα inhibitor were registered with the British Society for Rheumatology Biologics Registry (BSRBR). This register has stopped recruiting patients with RA starting ADA, ETN and IFX. So far 2,876 (23%) have ceased taking the first prescribed TNFα inhibitor and switched to a second TNFα inhibitor [1,881 switched owing to the lack of efficacy and 995 because of an an adverse event (AE)]. Of these the mean and maximum observed duration of treatment with a second TNFα are currently 18 months and 64 months, respectively. By August 2009 the BSRBR had registered 442 patients treated with RTX from a target of 1,100. 72
The drug costs of biologic agents are similar for the agents given by subcutaneous injection at around £9,000 per annum. Costs of i.v. administered drugs vary depending on patient weight and frequency of treatments courses (with RTX). Likely drug costs for these agents range between £7,000 and £10,000 per annum.
Chapter 2 Definition of the decision problem
Decision problems
According to the final scope issued by NICE for this technology appraisal, the decisions to be made are:
-
Decision problem 1: whether there are significant differences in clinical effectiveness and cost-effectiveness between ADA, ETN, IFX, RTX and ABT (referred to as ‘the interventions’ hereafter), when used within their licensed indications in adults with active RA who have had an inadequate response to a first TNF inhibitor prescribed according to current NICE guidance.
-
Decision problem 2: whether the interventions are clinically effective and cost-effective compared with previously untried conventional DMARDs (such as LEF and CyA).
-
Decision problem 3: whether the interventions are clinically effective and cost-effective compared with other biologic agents (including TOC, golimumab and certolizumab pegol).
-
Decision problem 4: whether the interventions are clinically effective and cost-effective compared with supportive care (including conventional DMARDs to which patients have had inadequate response).
-
Decision problem 5: whether the clinical effectiveness and cost-effectiveness of the interventions differ significantly between certain subgroups of patients (see Definition of the interventions).
The assessment report set out to address these decision problems as they apply to potential patient pathways in the UK. The nature of evidence and the timelines for this technology appraisal constrain the focus of the assessment report to key clinically relevant questions.
Definition of the interventions
The interventions being considered are:
-
Adalimumab: a TNF inhibitor administered by subcutaneous injection and usually prescribed in combination with MTX, except in cases where MTX is not tolerated or is contraindicated.
-
Etanercept: a TNF inhibitor administered by subcutaneous injection in combination with MTX, except in cases where MTX is not tolerated or is contraindicated.
-
Infliximab: a TNF inhibitor administered by i.v. infusion in combination with MTX.
-
Rituximab: a monoclonal antibody directed at CD20+ B cells, administered by i.v. infusion in combination with MTX.
-
Abatacept: a T-cell costimulation modulator, administered by i.v. infusion in combination with MTX.
Population and relevant subgroups
The population being considered is adults with active RA who have had an inadequate response to a first TNF inhibitor.
Potentially relevant subgroups are numerous and include:
-
patients having had primary or secondary (had initial response, but subsequently lost the response over time) failure of response to the first TNF inhibitor or having withdrawn from the first TNF inhibitor mainly owing to adverse effects
-
subgroups defined by autoantibody status [e.g. presence or absence of RF and/or anti-cyclic citrullinated peptide (anti-CCP) antibodies]
-
subgroups defined by different doses of the intervention (within licence)
-
patients with comorbidities for which some treatments may be contraindicated (e.g. heart failure).
The specific subgroups examined in the effectiveness review of this report were determined in light of available evidence and in consultation with clinical experts. Subgroups were not considered in the economic modelling as compelling evidence of differential effectiveness between subgroups was lacking from the effectiveness review.
Clarification of population of interest
The NICE guidance states that an alternative (second) TNF inhibitor may be considered for patients in whom treatment is withdrawn because of an AE before the initial 6-month assessment of efficacy. This group of patients (withdrawal because of an early AE) is strictly speaking outside the remit of this technology appraisal and should ideally be excluded from the technology assessment. However, in practice, the reason for the withdrawal of a TNF inhibitor may not be clear-cut as a decision to withdraw may be related to both efficacy and adverse effects (and the balance of risk and benefit for the patient).
Relevant comparators
Potential comparators include:
-
supportive care (including corticosteroids and ongoing or reinstated conventional DMARDs, such as MTX, sulfasalazine to which the patients have had inadequate response previously)
-
conventional DMARDs which have not been tried prior to trying a TNF inhibitor for example AZA, CyA and GST, either as monotherapy or combined with other DMARDs or corticosteroids
-
biologic agents including TOC, golimumab and certolizumab pegol
-
the interventions being considered compared with each other.
Clarification of comparators
The assessment report focuses on key clinically relevant questions, including, where data allow, comparing each of the interventions with supportive care and comparing each of the interventions against each other. This was based on the following considerations:
-
The majority of patients considered in this technology appraisal may have already had inadequate response to at least two conventional DMARDs, including MTX tried for an adequate length of time and at adequate doses, as indicated in the current NICE guidance. These DMARDs may still be continued in the comparator (and intervention) arm(s) of trials in patients who have responded inadequately to these options. In such cases continued use of these DMARDs was regarded as supportive care rather than as a credible alternative treatment option. Therefore, a clear distinction was made between conventional DMARDs depending on whether the patients had tried them before and if there was a history of inadequate response to the DMARD tried.
-
Only conventional DMARDs to which the patients have not had inadequate response or have not tried were to be regarded as separate comparators. The evidence for use of conventional DMARDs in patients who have failed to respond to TNF inhibitors was expected to be very limited.
-
Although conventional DMARDs which are continued and to which the patients had an inadequate response were regarded as supportive care, subgroup analysis was considered (where relevant and evidence permits) to assess whether the presence or absence of these (failed) DMARDs in the control and intervention groups influenced the estimated treatment effects of the interventions.
-
Tocilizumab, golimumab and certolizumab pegol were potentially relevant comparators. These drugs are not yet available in the UK, but all are (or are potentially) the subject of STAs by NICE. The inclusion of these three drugs in the final scope as comparators means that there were no formal submissions from their manufacturers for this technology appraisal. This may have had implications with regard to the acquisition of evidence for these comparators. It was proposed that TOC, golimumab and/or certolizumab pegol could have been reviewed in the assessment report as a comparator if marketing authorisation of the technology was obtained before the submission of the protocol for this assessment report. This condition was not met.
Relevant outcomes
Key outcomes considered appropriate to the decision problem were:
-
withdrawals (with reason)
-
treatment response (ACR)
-
disease activity (DAS)
-
physical function (HAQ)
-
joint damage/radiological progression
-
pain
-
fatigue
-
serious AEs (including death)
-
other AEs potentially associated with treatment
-
health-related QoL (HRQoL).
Key issues
Key issues have been mentioned, where relevant, earlier in this section and also in the background section of this report.
Further key issues predominantly concern the limited availability of evidence from controlled trials and the impact this has on the assessment of clinical effectiveness and cost-effectiveness of each of the interventions compared with the potential comparators (and the other interventions), and the ability to identify relevant subgroups in whom the technologies are more or less beneficial.
Place of the intervention in the treatment pathway(s)
Based on the final scope, the interventions are to be used when patients have had an inadequate response to a TNF inhibitor.
Overall aims and objectives of assessment
The overall aims and objectives were to address the decision questions outlined in section Decision problems. These aims were to be achieved by:
-
A systematic review of RCTs of the efficacy, tolerability and safety of ADA, ETN, IFX, RTX and ABT for the treatment of RA in adults who have had an inadequate response to a first TNF inhibitor.
-
As the volume of RCT evidence was expected to be relatively small, relevant non-randomised comparative studies and uncontrolled studies were also reviewed.
-
A systematic review of published studies on the cost and cost-effectiveness of the technologies in the treatment of RA in adults who have had an inadequate response to a first TNF inhibitor.
-
A review of economic evaluations included in any manufacturer’s submissions (MSs) for this appraisal.
-
A focused, model-based economic evaluation of the cost-effectiveness of the technologies from the perspective of the UK NHS.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
Search strategy
The following resources were searched for relevant studies:
-
Bibliographic databases: Cochrane Library (CENTRAL) 2009 Issue3, MEDLINE (Ovid) 1,950 to July week 1 2009, MEDLINE In-Process & Other Non-Indexed Citations (Ovid) 13 July 2009, EMBASE (Ovid) 1980–2009 week 28. Searches were based on index and text words that encompassed the condition, RA, and the interventions ADA, IFX, ETN, RTX and ABT.
-
Citations of included studies were examined.
-
Reference lists of identified systematic reviews were checked.
-
Further information was sought from contacts with experts.
-
Research registries of ongoing trials including the National Institute for Health Research (NIHR) Clinical Research Network Portfolio Database, Current Controlled Trials and Clinical Trials.gov using terms for the particular drugs.
-
Manufacturer submissions.
The searches were not limited by date of publication or language.
Search strategies can be found in Appendix 2.
Study selection
All articles identified in the searches were imported into a reference manager database (reference manager v.11, Thomson ResearchSoft). Duplicate entries were allowed to be removed by the inbuilt feature in reference manager and removed when encountered by reviewers. Titles and abstracts were independently checked for relevance based on the population and intervention by two reviewers. If articles were considered relevant by at least one of the reviewers a full paper copy was ordered.
Full papers were assessed for relevance by two independent reviewers using an inclusion/exclusion checklist (see Appendix 6) based on the following criteria:
-
population: a majority of adults with active RA who have had an inadequate response to a TNF inhibitor
-
intervention: ADA, ETN, IFX, RTX, or ABT
-
outcomes: clinical outcomes related to efficacy, safety or tolerability
-
study design: primary study (except case reports) or a systematic review
-
study duration: at least 12 weeks
-
participant numbers: for non-randomised studies – at least 20 patients in one arm.
Disagreements were resolved by discussion with the involvement of a third reviewer when necessary.
Conference abstracts were not sought. If they were identified as relevant in the first stage of study selection, an attempt was made to match them with journal publications. If this was not possible, contact with authors was not attempted owing to time constraints and they were not included in the analysis.
A list of excluded studies and the reason for exclusion were recorded (see Appendix 4).
Included systematic reviews were not themselves systematically reviewed, but were utilised to identify further primary studies.
Additional references identified from systematic reviews or industry submissions were entered into the reference manager database. The same process was applied to additional the references as to the references identified from initial searches.
Data extraction
Data were extracted into a standard form (see Appendix 8) for all included studies by one reviewer. A second reviewer checked the accuracy of the extracted information. Disagreements were resolved by consensus or by referral to a third reviewer if necessary.
Information regarding study design and characteristics of study participants was extracted. Data on the following outcomes were sought from included studies:
-
treatment withdrawal (and reasons for withdrawal)
-
ACR20, ACR50, ACR70
-
disease activity (e.g. DAS28 or DAS)
-
physical function (e.g. HAQ)
-
joint damage/radiological progression (measured by a scoring system)
-
pain
-
fatigue
-
extra-articular manifestations of the disease
-
serious AEs (including death)
-
other adverse effects potentially associated with treatments
-
HRQoL.
Data for any outcomes other than those listed above were also extracted if they were considered relevant to this report.
Additional data from industry submissions were extracted by only one reviewer owing to time constraints.
Quality assessment
The quality of included studies was assessed independently by two reviewers. Any disagreements were resolved by discussion and if necessary a third reviewer was consulted.
For randomised trials the following criteria were considered:
-
Randomisation: whether allocation was truly random. Randomisation using a computer or a random number table was considered adequate, whereas the use of alternation, case record numbers, or dates of birth and day of the week was considered inadequate.
-
Allocation concealment: whether allocation concealment was adequate. Any of the following methods was considered adequate: centralised (e.g. allocation by a central office unaware of subject characteristics) or pharmacy-controlled randomisation; pre-numbered or coded identical containers which are administered serially to participants; on-site computer system combined with allocations kept in a locked unreadable computer file that can be accessed only after the characteristics of an enrolled participant have been entered; or sequentially numbered, sealed, opaque envelopes.
-
Blinding: use of blinding and who was blinded (patients, study investigators/outcome assessors, data analysts).
-
Patients withdrawn: what was the percentage of patients withdrawn from the study?
-
Intention-to-treat (ITT) analysis: whether ITT analysis was used.
For non-randomised studies the following criteria were considered:
-
Study design: if the study was controlled or uncontrolled, prospective or retrospective.
-
Inclusion criteria: if inclusion criteria were clearly stated.
-
Consecutive patients: if consecutive patients were included in the study.
-
Patients withdrawn: what was the percentage of patients withdrawn from the study?
The results of quality assessments are reported in relevant sections of the report.
Data analysis/synthesis
Outcomes of interest
Selected outcomes of interest were specified in the review protocol, based upon the final scope issued by NICE for this technology appraisal. These were:
-
treatment withdrawal (and reasons for withdrawal)
-
ACR20, ACR50, ACR70
-
disease activity (e.g. DAS28 or DAS)
-
physical function (e.g. HAQ)
-
joint damage/radiological progression (measured by a valid scoring system)
-
pain
-
fatigue
-
extra-articular manifestations of the disease
-
serious AEs (including death)
-
other adverse effects potentially associated with treatment
-
HRQoL.
Handling of data and presentation of results
Comparisons with supportive care
Studies were considered to compare interventions with supportive care if they:
-
had an arm receiving supportive care
-
had a placebo arm.
Owing to the paucity of evidence from controlled studies of TNF inhibitors, evidence from uncontrolled studies (i.e. single-group before-and-after studies) is also considered in this section.
Studies were considered separately for each of the interventions. In addition, TNF inhibitors were discussed together as a class of drugs. Results were presented in figures and discussed in the main text of the report for the following outcomes:
-
withdrawals (for any reason, owing to the lack of efficacy and owing to AEs)
-
ACR20, ACR50 and ACR70
-
DAS
-
European League Against Rheumatism (EULAR) response
-
HAQ
-
QoL
-
joint damage
-
serious AEs
-
infections and serious infections
-
injection/infusion reaction.
For other outcomes only figures were created, and these can be found in Appendix 10.
Dichotomous measures data are presented as relative risks (RRs) (for RCTs) and percentages (for other study designs). For continuous outcomes, mean differences (for RCTs) and means (for other study designs) were used.
Where available, data were analysed for 3, 6, 9, 12, etc. months’ duration of follow-up. They were assumed to be 3-month data if they were collected between 3 and 4 months from the initiation of treatment, 6-month data if they were collected between 5 and 7 months from the initiation of treatment. If more than one estimate was available for a time interval, the value nearest to the assumed follow-up was used.
Pooling of results was not attempted for the assessment of effectiveness of individual technologies because the majority of included studies had no control group and there was substantial methodological and clinical heterogeneity between included studies. Given the relatively small number of patients that can be analysed in subgroup analyses, some pooling of data using a random-effects model was attempted. The results were presented with I2 statistics mainly for demonstrating consistency of findings between studies (see Subgroup analyses).
Comparisons with newly initiated and previously untried conventional disease-modifying antirheumatic drugs
No studies were identified and therefore analyses were not undertaken.
Comparisons with other biologic agents
No studies were identified and therefore analyses were not undertaken.
Comparisons between technologies (head-to-head comparisons)
No studies were identified and therefore direct comparisons were not undertaken.
Indirect comparison (IC) was undertaken when data were available from RCTs. It was conducted using the method by Bucher et al. 73 The results of the analyses were presented in tabular format.
Subgroup analyses
The following subgroups were specified in the review protocol:
-
patients having withdrawn from the first TNF inhibitor owing to the lack of response (primary failure), loss of response (secondary failure) or AEs/intolerance
-
subgroups defined by autoantibody status (e.g. presence or absence of RF or anti-CCP antibodies)
-
subgroups defined by different doses of the intervention (within licence)
-
patients with comorbidities for which some treatments may be contraindicated (e.g. heart failure).
No subgroup data concerning the last two categories (varied doses; comorbidities) were identified, and thus no subgroup analysis was performed for these. Subgroup analyses relating to the reasons of withdrawal from the first TNF inhibitor were carried out as two separate comparisons:
-
withdrawal owing to lack of response versus withdrawal due to loss of response
-
withdrawal owing to lack of efficacy (which includes both lack of response and loss of response) versus withdrawal due to AEs/intolerance.
In addition to the above, subgroup data in relation to the identity of the first TNF inhibitor which the patients received before discontinuation and the number of prior TNF inhibitor(s) that the patients had tried before switching were reported in some studies. These were considered potentially of clinical relevance and thus subgroup analyses on these were also performed where data were available [commercial-in-confidence information (or data) removed].
Ongoing studies
Ongoing primary studies were identified in the searches. They were not included in the systematic review, but discussed in Ongoing studies.
Assessment of publication bias
All manufacturers of the interventions provided a list of all company-sponsored RCTs and other non-randomised or uncontrolled studies that are relevant for this appraisal. Requests of clarification of trial data that are potentially available but not reported in published papers were also made to the manufacturers of RTX and ABT.
The number of relevant studies for individual technology was too small to allow a formal assessment of publication bias.
Sensitivity analyses
The protocol specified that if evidence permits sensitivity analyses may be carried out taking into account the following factors:
-
quality measures of studies such as blinding and randomisation
-
factors associated with the characteristics of the study population
-
factors associated with study design such as study duration and drug doses
-
exclusion of data supplied as commercial/academic in confidence.
However, sensitivity analyses were not performed as no pooling of study results was undertaken.
Changes to the original protocol
During the study selection process, several potentially relevant studies including mixed proportion of patients with or without prior treatment with a TNF inhibitor were identified. No criterion relating to inclusion or exclusion of these studies was specified in the original protocol. It was agreed by consensus within the project team that studies that included less than 50% of patients with RA who have failed a TNF inhibitor were excluded, unless results from these patients were described separately and the number of these patients was greater than or equal to 20.
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Results: quantity and quality of research available
The searches resulted in the identification of 10,281 records and an additional 17 were identified from industry submissions and 15 from reference lists of included studies.
Nine relevant systematic reviews74–82 were identified in addition to the reports conducted for previous NICE appraisals in RA. Examination of these nine reviews did not identify any further primary studies that met all the criteria for inclusion in either the clinical effectiveness or cost-effectiveness sections of this report.
Duplicates had been removed, leaving 7,486 records. Screening of the title and abstract of these articles indicated that 174 were directly relevant to the clinical effectiveness section of this report. Full paper copies of these articles were ordered. Five of them were unobtainable. 83–87 Inclusion criteria were applied to the remaining 169 articles. Of these, 113 were excluded for not meeting at least one of the inclusion criteria. Three articles were identified as conference abstracts88–90 and, as these could not be matched to full publications, they were excluded. Details of excluded studies together with reasons for exclusion can be found in Appendix 4.
A flow diagram presenting the process of identification of relevant studies can be found in Appendix 3.
There were 35 studies described in 45 papers meeting the inclusion criteria. Five of the studies were RCTs, one was a comparative study, one was a non-randomised controlled study and 28 were uncontrolled studies [including one long-term extension (LTE) of an RCT].
A randomised study on RTX [study for understanding rituximab safety and efficacy (SUNRISE)91] that was not yet published in full was identified. Data from this study were requested from the manufacturer; however, the clinical study report was received too late to be included in the analyses.
Table 2 presents mapping of studies to relevant interventions and comparators.
| Comparators | Interventions (newly initiated) | |||||
|---|---|---|---|---|---|---|
| ADA | ETN | IFX | TNF inhibitors | RTX | ABT | |
| Nonea |
Bennett 200592 (n = 26, 52 weeks) Wick 200593 (n = 27, 24 weeks) Nikas 200694 (n = 24, 52 weeks) Bombardieri 200795,96 (n = 899, 12 weeks) van der Bijl 200897 (n = 41, 16 weeks) |
Haraoui 200498 (n = 25, 12 weeks) Buch 200599 (n = 207, 12 weeks) Cohen 2005100 (n = 24, 13 weeks) Buch 2007101 (n = 95, 12 weeks) Iannone 2007102 (n = 37, 24 weeks) Laas 2008103 (n = 49, >36 weeks) Bingham 2009104 (n = 201, 16 weeks) |
Ang 2003105 (n = 24, unclear) Hansen 2004106 (n = 20, unclear) Yazici 2004107 (n = 21, unclear) |
Gomez-Reino 2006108 (n = 488, 104 weeks) Solau-Gervais 2006109 (n = 70, > 13 weeks) Hjardem 2007110 (n = 235, 13 weeks) Duftner 2008111 (n = 109, up to 208 weeks) Karlsson 2008112 (n = 337, 13 weeks) Blom 2009113 (n = 197, 48 weeks) |
Bokarewa 2007114 (n = 48, 52 weeks) Jois 2007115 (n = 20, 26 weeks)b Keystone 2007116 (n = 158, 24 weeks) Assous 2008117 (n = 50, 26 weeks) Thurlings 2008118 (n = 30, 24 weeks) |
ATTAIN LTE119 (n = 317, < 260 weeks) ARRIVE120 (n = 1,046, 24 weeks) |
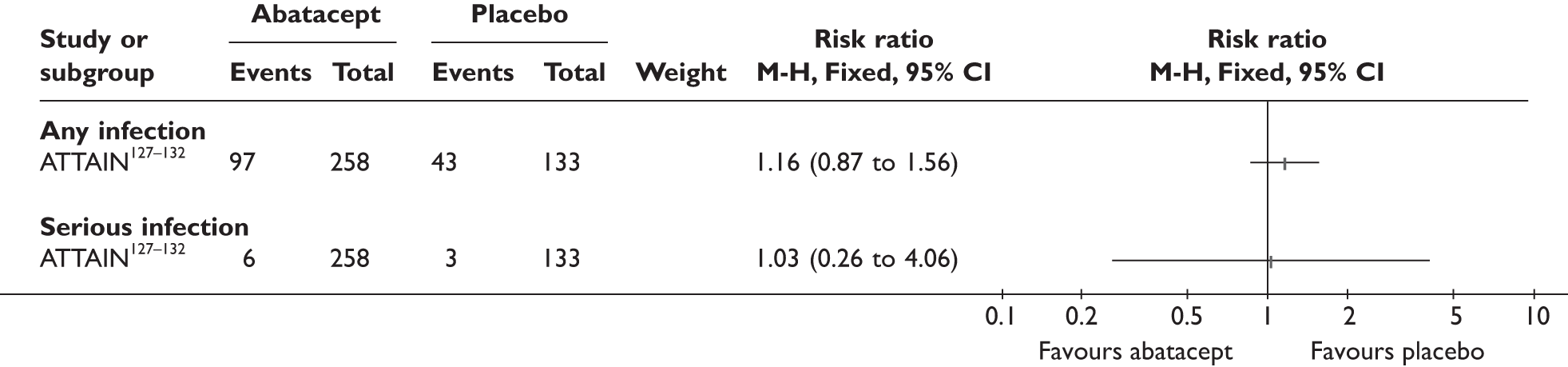
| Supportive carec | Hyrich 2009121–123 (n = 736, > 24 weeks) |
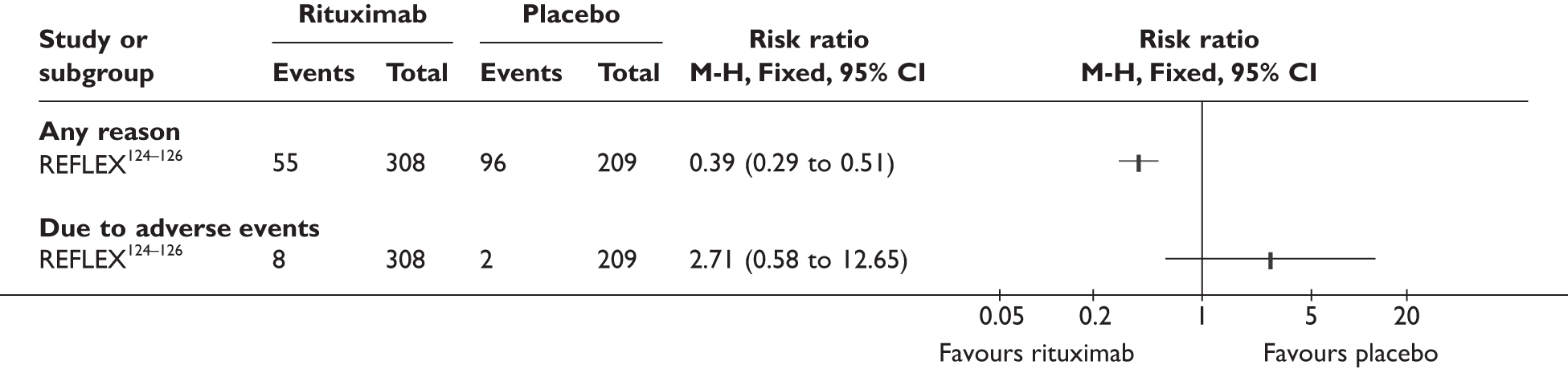
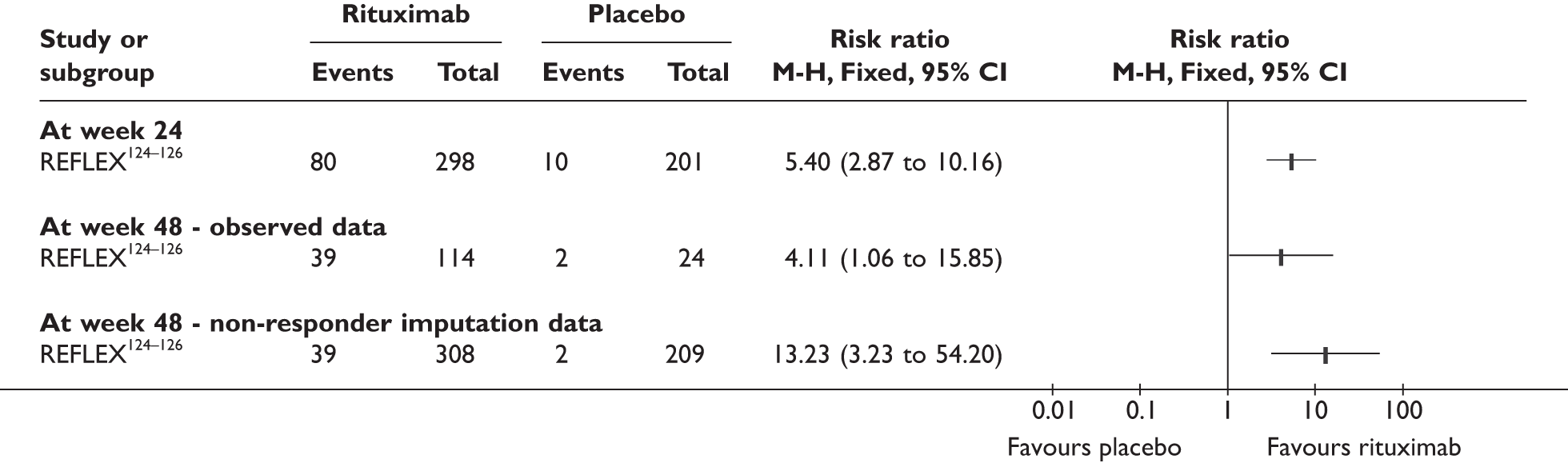
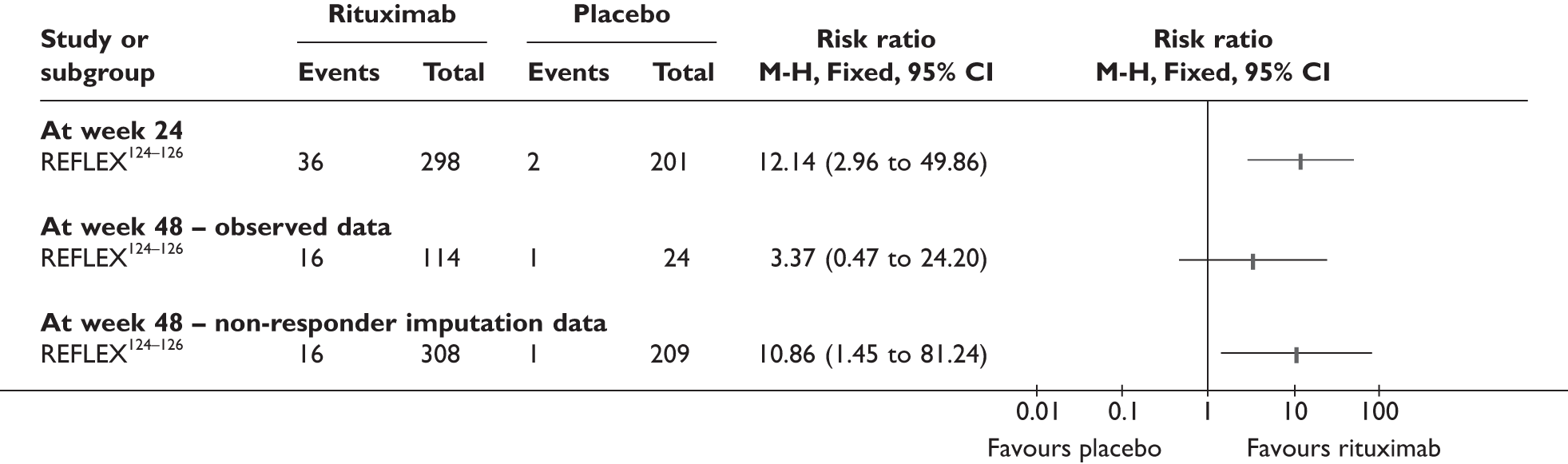
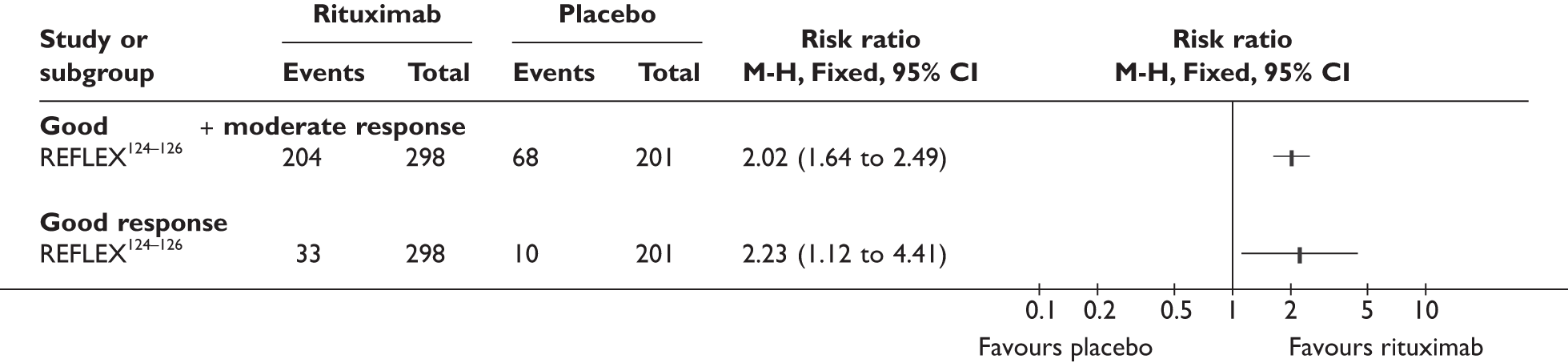
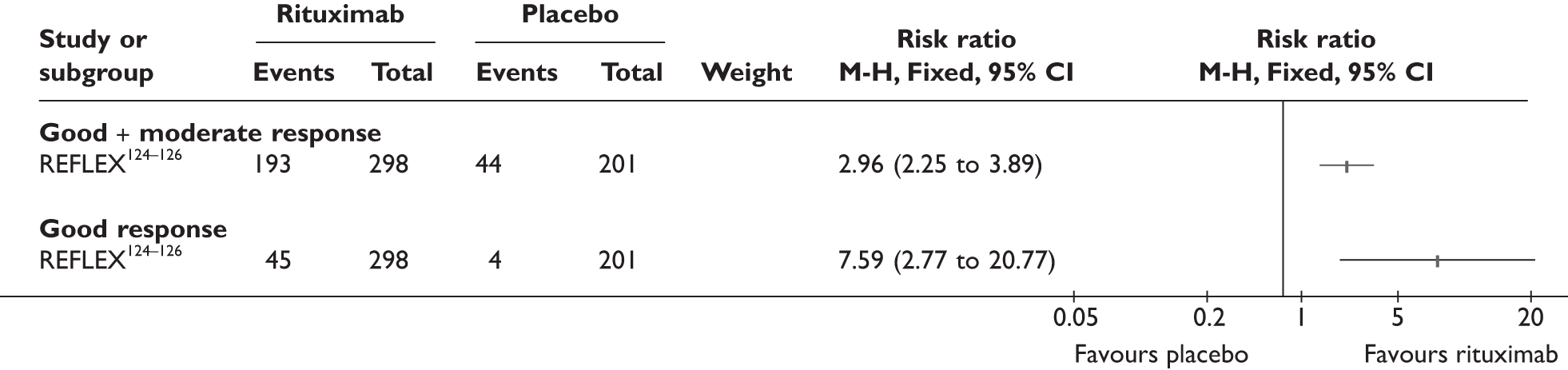
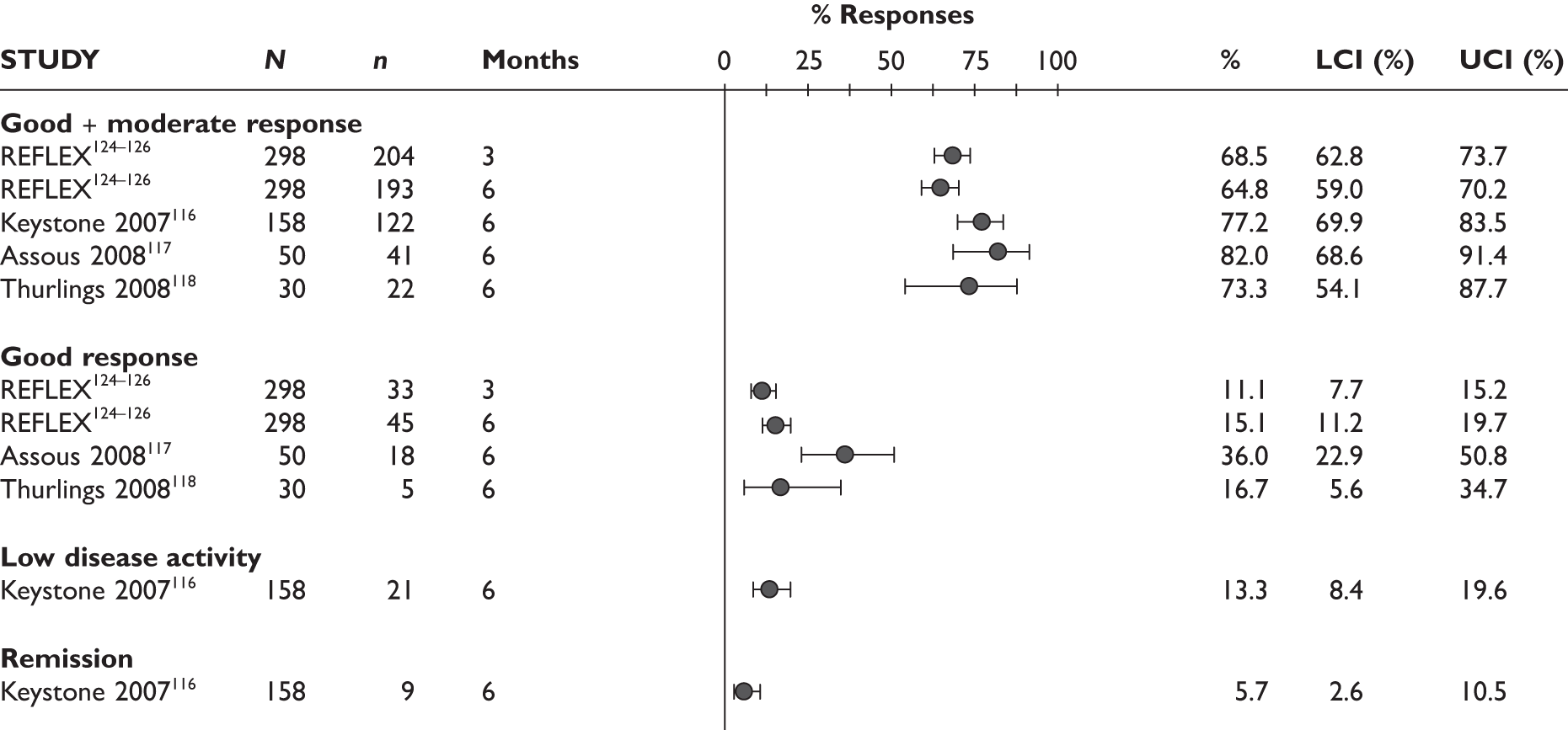
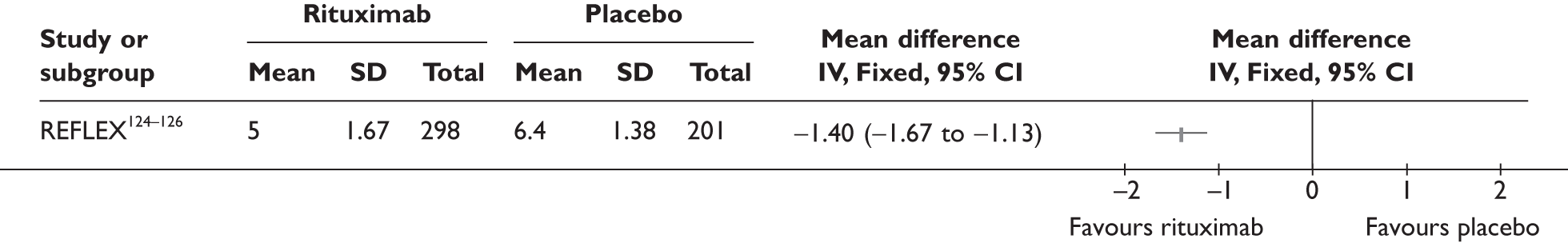
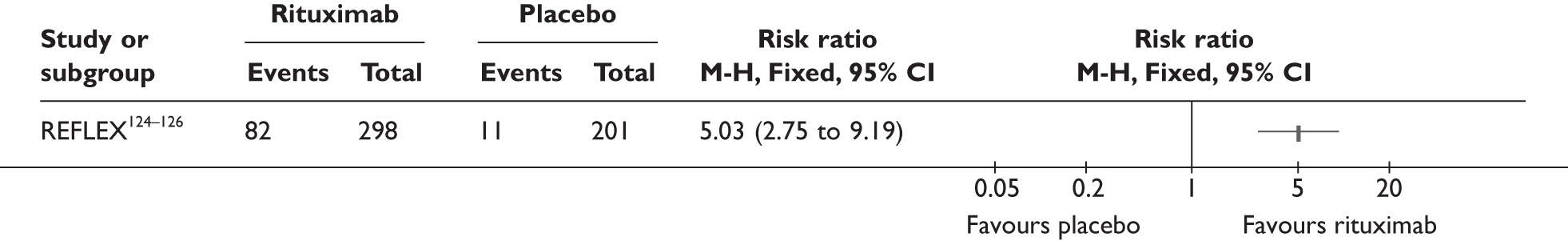
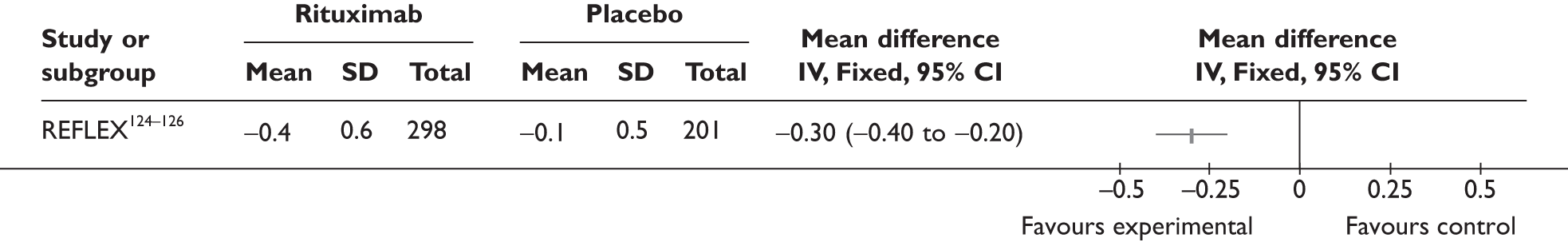
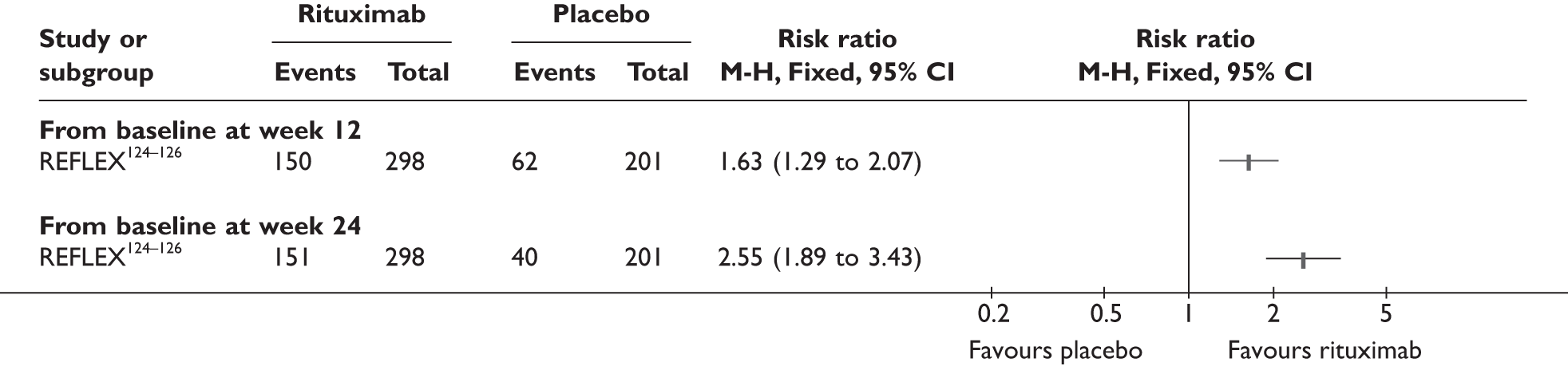
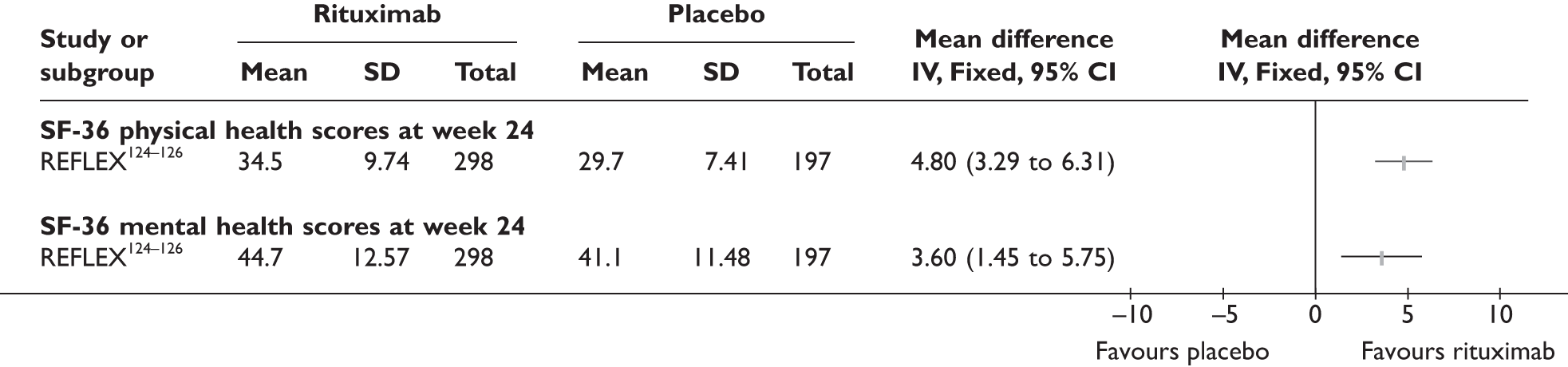
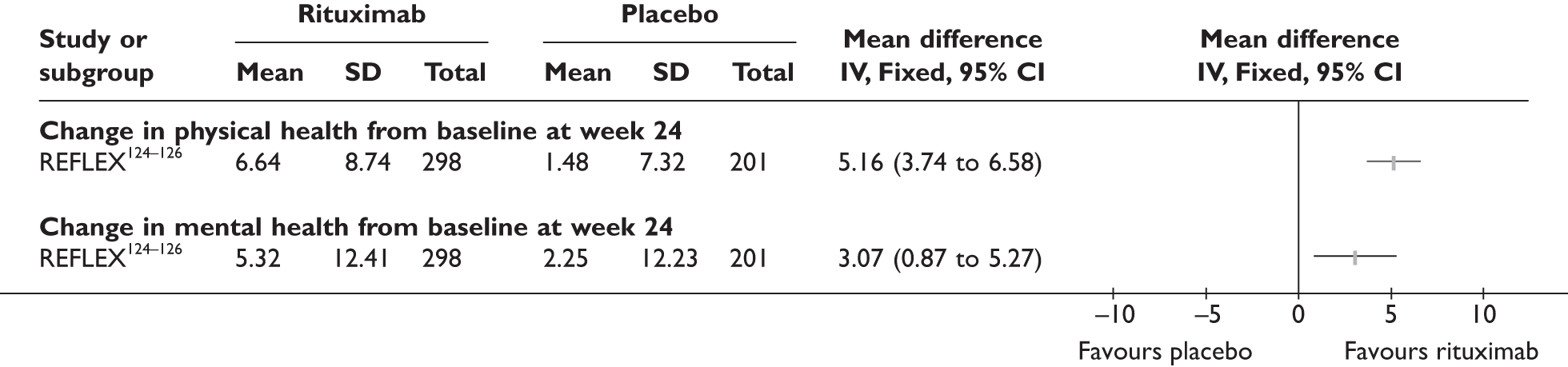
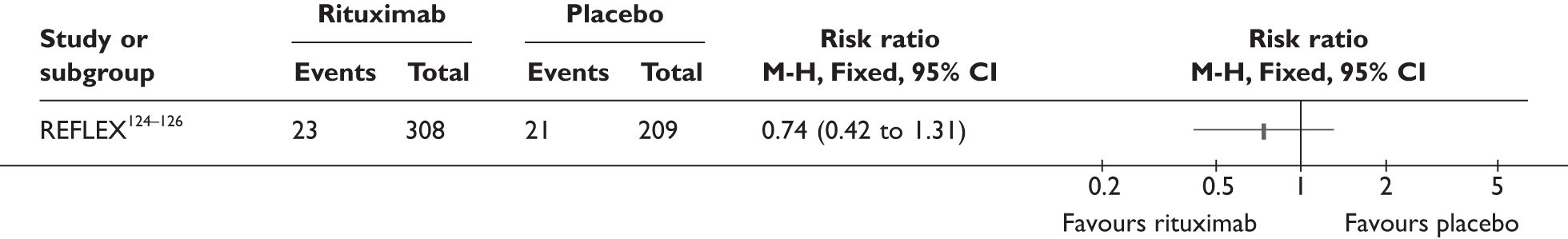
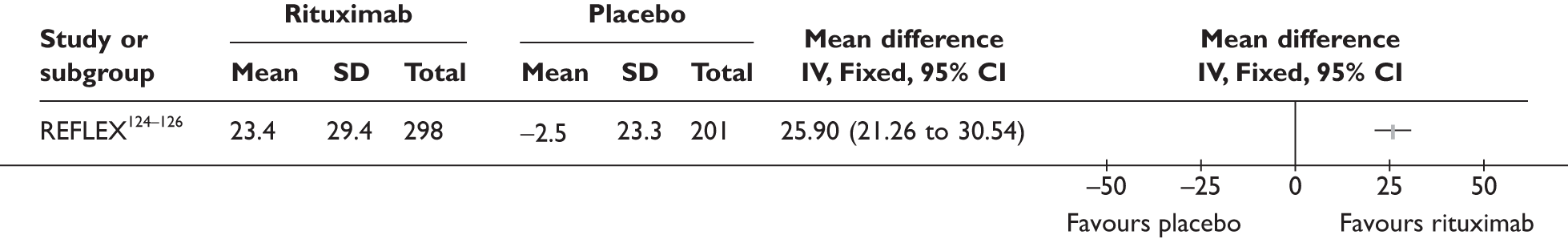
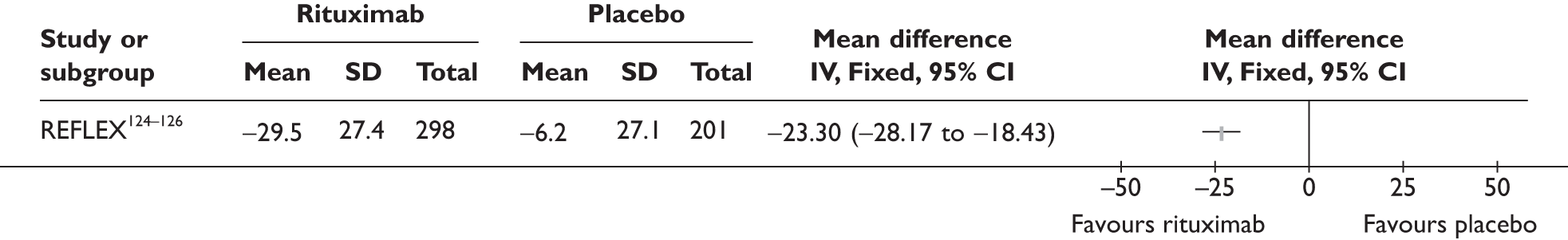
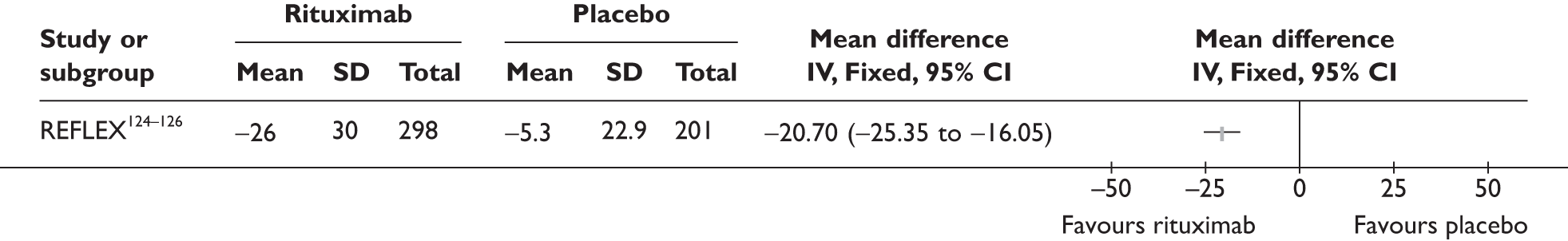
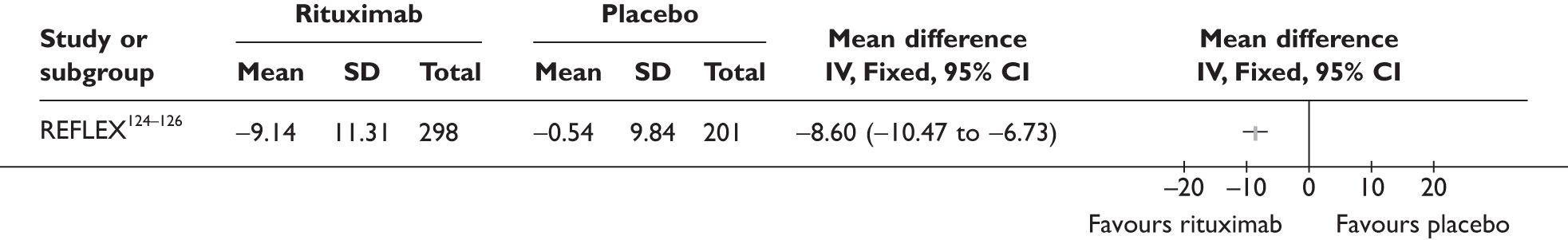
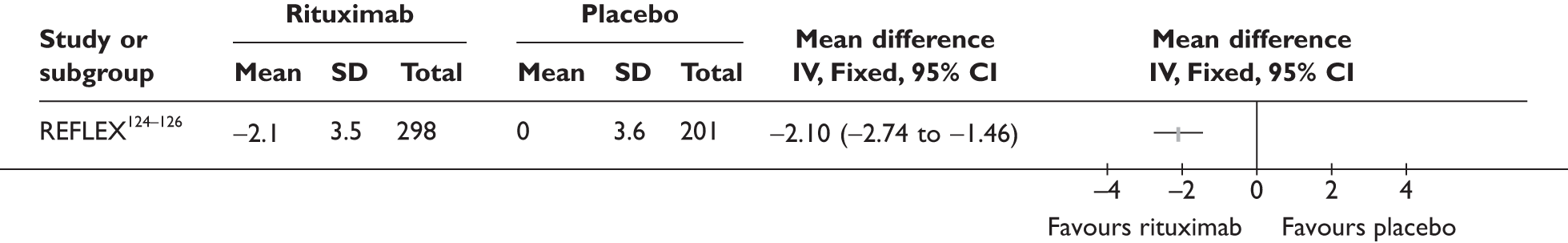
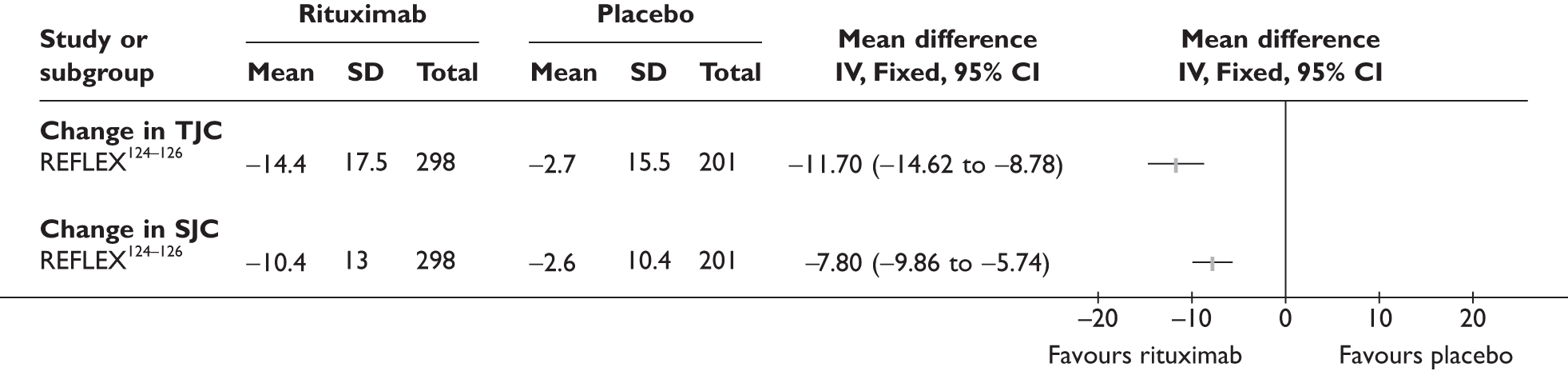
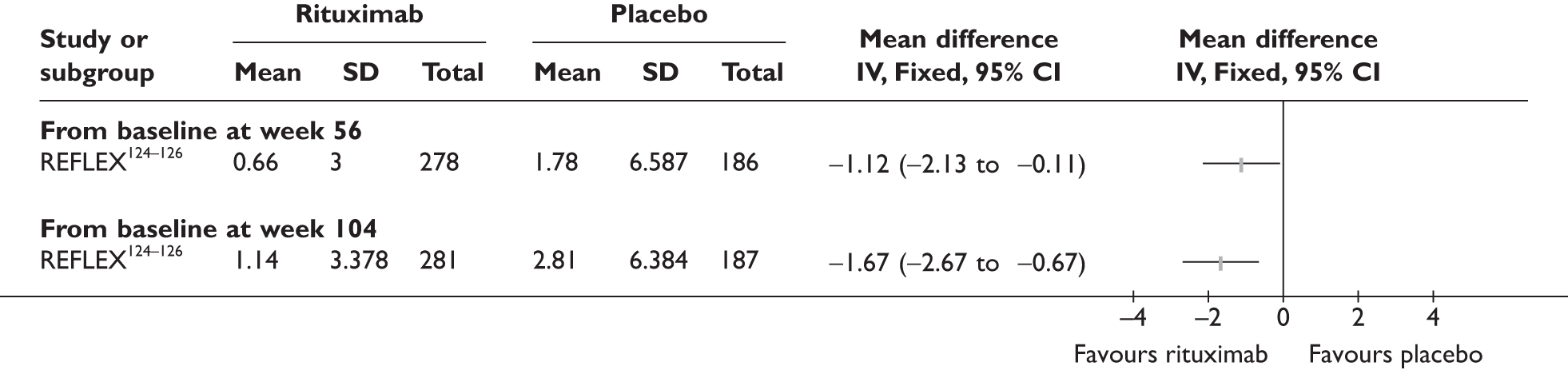
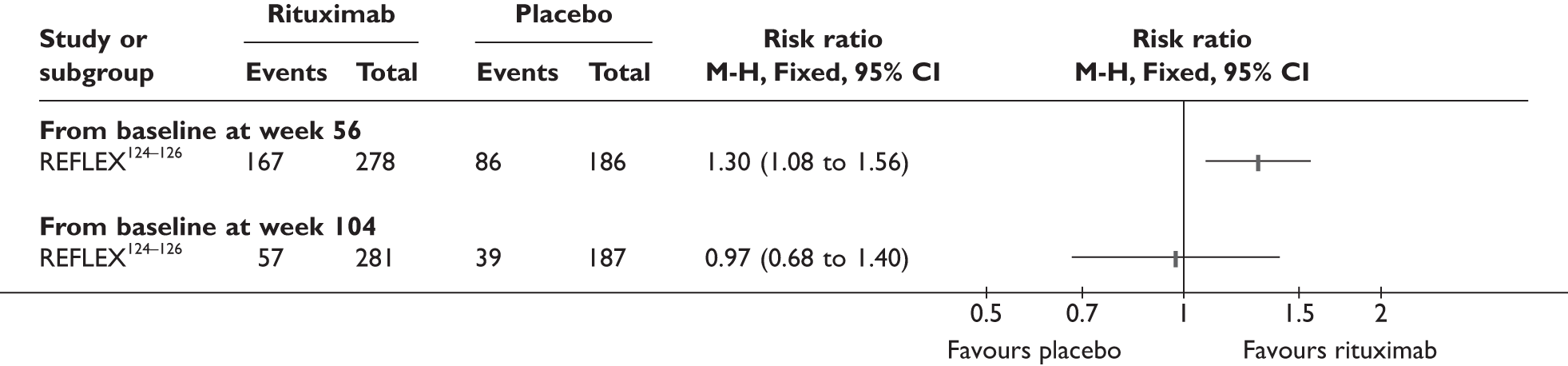
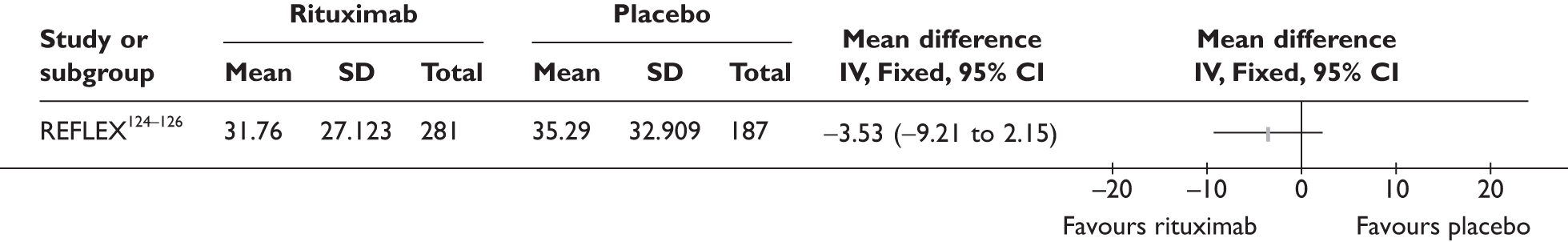
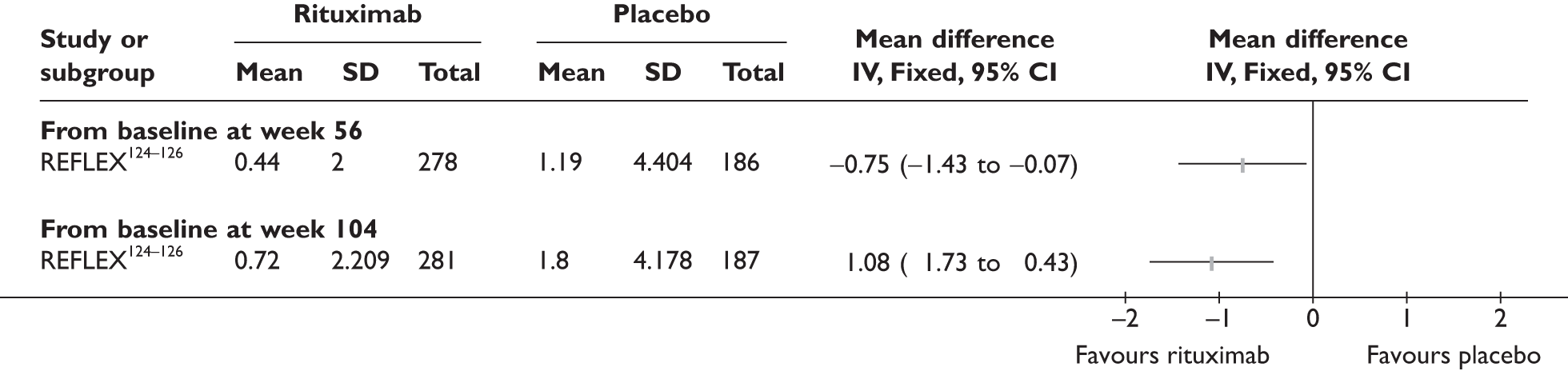
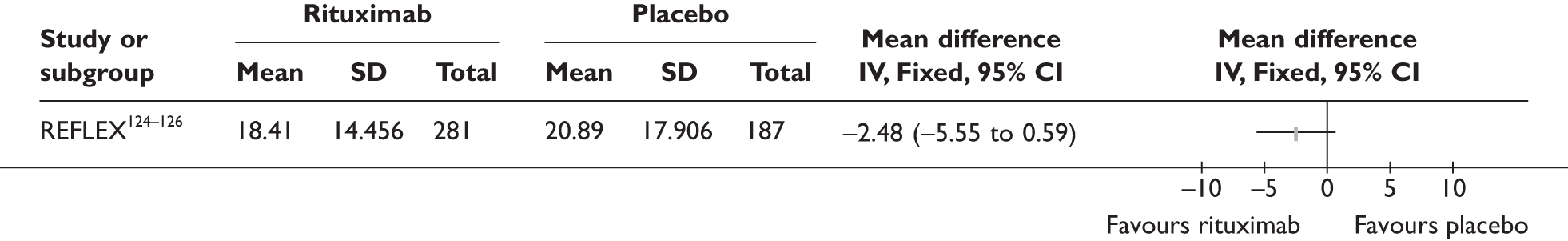
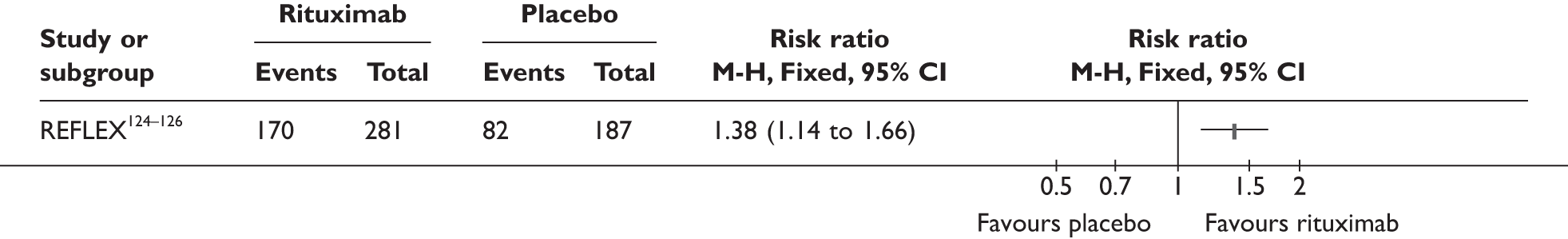
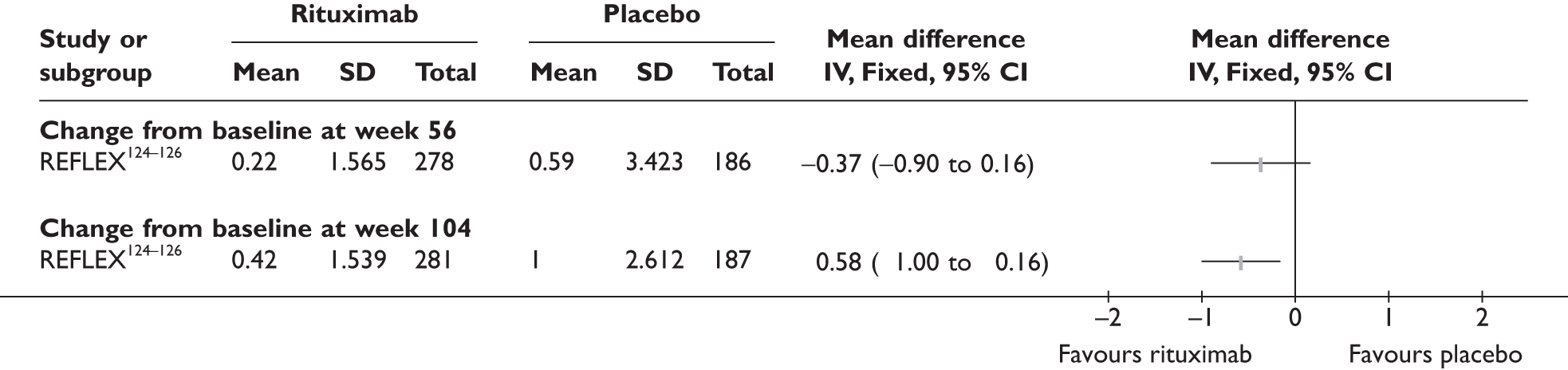
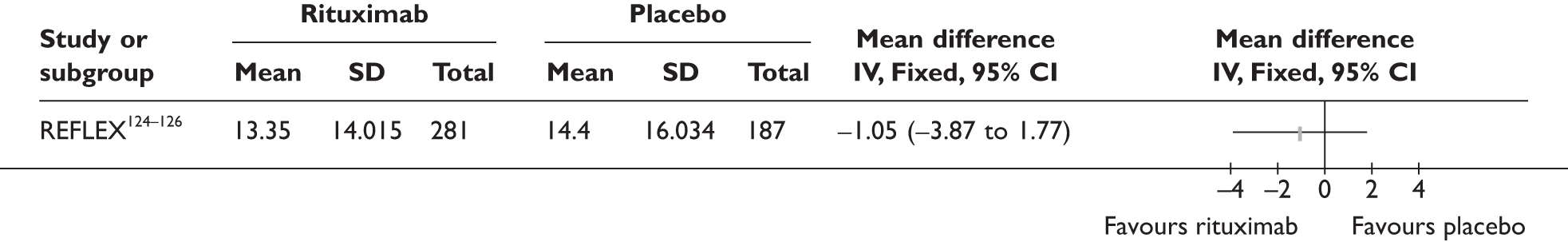
REFLEX124–126 (n = 517, 48 weeks) SUNRISE91 (n = 559, > 48 weeks) |
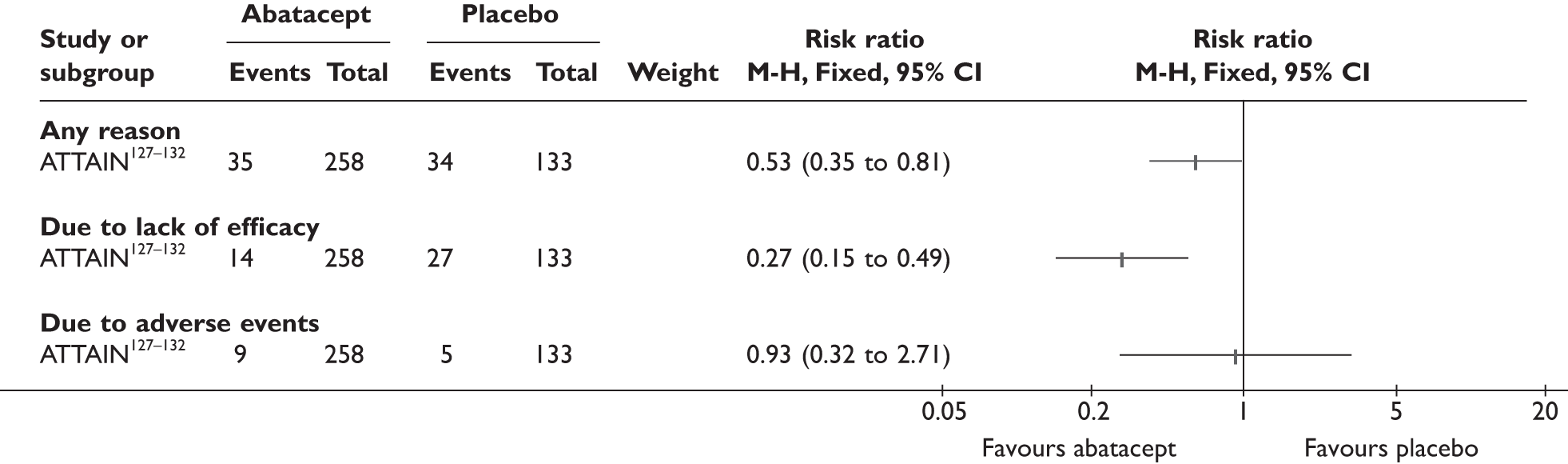
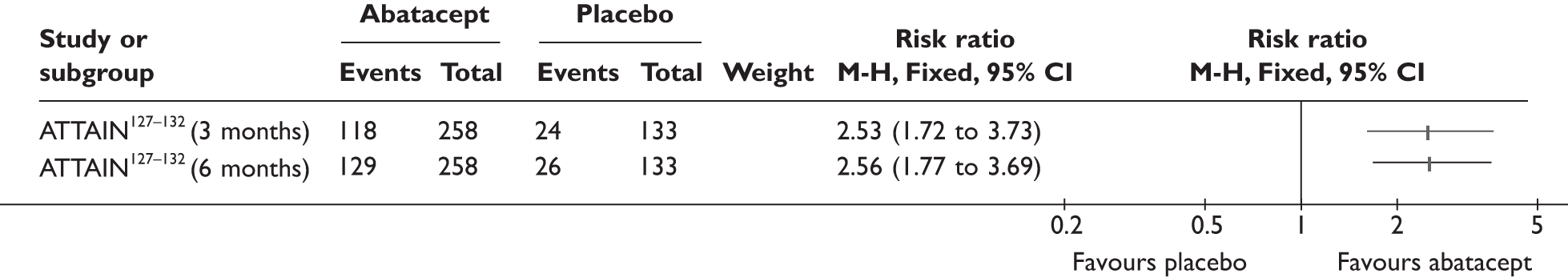
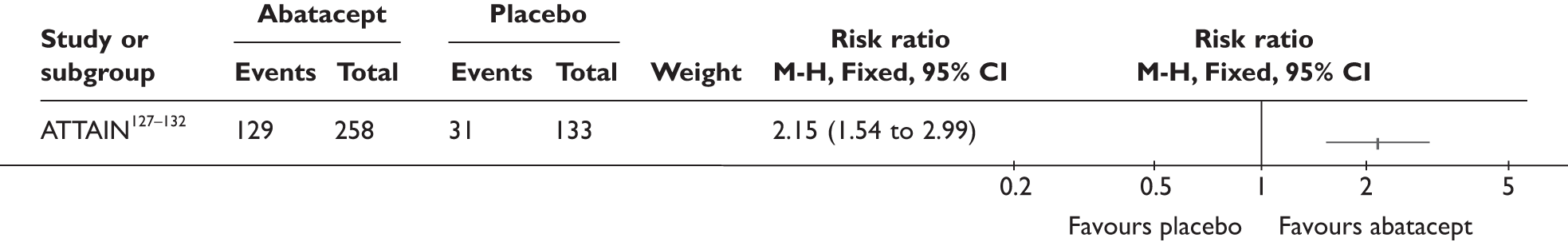
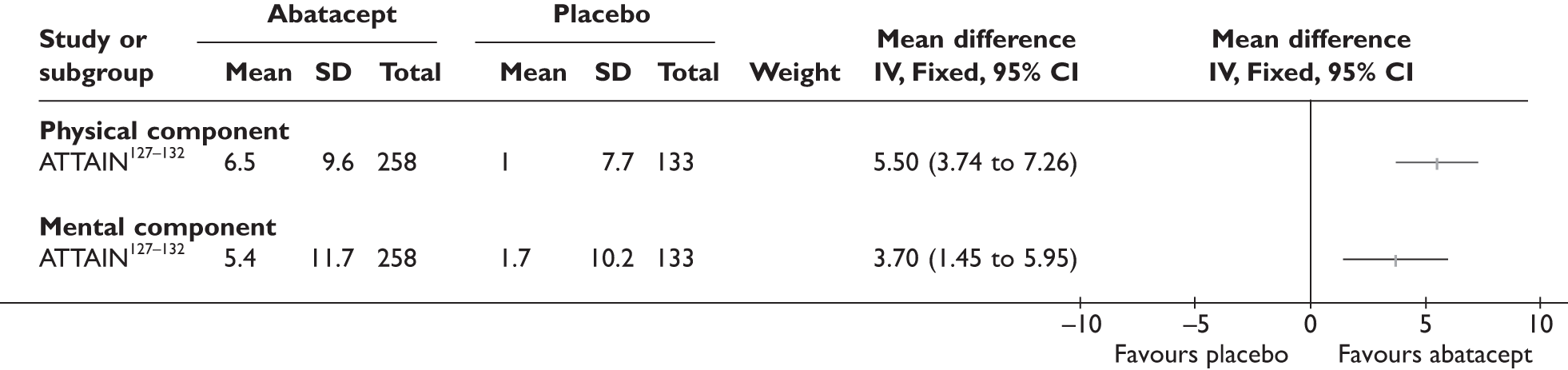
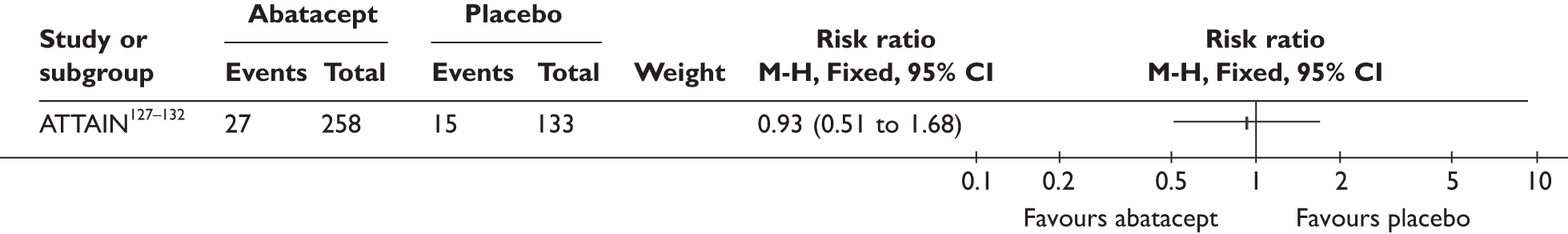
ATTAIN127–132 (n = 391, 26 weeks) | |||
| Ongoing biologicsd | OPPOSITE133 (n = 27, 16 weeks) |
Weinblatt 2007134 (n = 121, 52 weeks) ASSURE135 (n = 167, 52 weeks) |
||||
| Newly initiated DMARD | ||||||
| ADA | ||||||
| ETN | ||||||
| IFX | ||||||
| TNF inhibitors | ||||||
| RTX | Finckh 2009136,137 (n = 318, > 44 weeks) | |||||
| ABT | ||||||
| TOC | ||||||
| Golimumab | ||||||
| Certolizumab pegol | ||||||
The assessment of effectiveness of the technologies is reported below in six sections, one for each of the technologies and one for TNF inhibitors as a class (see Effectiveness of the technologies compared with supportive care). Studies directly comparing the technologies and ICs are reported in Evidence from comparative studies and Indirect comparisons sections respectively.
Effectiveness of the technologies compared with supportive care
This section describes evidence relating to each of the technologies compared with supportive care, which includes treatments received by the placebo group in placebo-controlled trials and ongoing conventional DMARDs or biologics to which the patients had had inadequate response. Owing to the paucity of evidence from controlled studies for TNF inhibitors, evidence from uncontrolled studies (i.e. single-group before-and-after studies) is also considered in this section.
Adalimumab
Overview of evidence
Five studies in six publications92–97 met the inclusion criteria. No RCT was found. Four studies had comparator arms in which the patients were TNF inhibitor naive. 92–94,96 These arms were excluded here. One of the four studies93 also had a small comparator arm of nine patients, which did not meet the inclusion criteria of this report of greater than or equal to 20 patients for an arm to be included; thus, data from this arm were excluded.
One multicentre study was conducted in 12 countries, 11 of which were European, including the UK. Other studies were conducted in the UK, Sweden and Greece. It was unclear in which country one of the studies was conducted.
Sample sizes were small, ranging from 24 to 41 patients, that are relevant to the review in four studies; in one study there were 899 patients. Patients included all had previous treatment with either one or two TNF inhibitors, most frequently IFX. Reasons for switching TNF inhibitors were lack of efficacy only in one study,93 lack of efficacy or intolerance in two studies96,97 and lack of efficacy or AEs in two studies. 92,94 Details on ADA treatment were not reported in one study; in all the other studies ADA was given 40 mg subcutaneously every other week. Study duration ranged from 12 weeks to over 1 year. Further details are outlined in Table 3.
| Study | Country | Design | Reason for switching (n) | Prior TNF inhibitors (n) | Treatment arms (no. of patients) | Duration of follow-up | Comments |
|---|---|---|---|---|---|---|---|
| Bennett 200592 | UK | Uncontrolled prospective | Primary (8) and secondary (13) failure, AEs, other | IFX, ETN, anakinra (1) | ADA, (26) | > 52 weeks | Primary and secondary failures – all IFX |
| Wick 200593 | Sweden | Uncontrolled retrospective | Secondary failure | IFX (1) | ADA, (27) | 3, 6 months | |
| Nikas 200694 | Greece | Uncontrolled prospective | Lack of efficacy, AEs | IFX (1) | ADA, (24) | 12 months | Possibly one or two active TB patients (outside study inclusion criteria) |
| Bombardieri 2007 (ReAct)95,96 | Australia, Austria, Belgium, France, Germany, Greece, Italy, the Netherlands, Portugal, Spain, Switzerland, UK | Uncontrolled prospective | Primary and secondary failure, intolerance | IFX, ETN, or both (≥ 1) | ADA, (899) | 12 weeks | |
| van der Bijl 200897 | Unclear | Uncontrolled prospective | Primary and secondary failure, intolerance | IFX (1) | ADA, (41) | 16 weeks (follow-up to 56 weeks; treatment for and efficacy measured at 16 weeks) | Pre-existing antirheumatic therapy (in about 12 patients) was continued and remained stable until week 16 |
Patient characteristics
Data on patient characteristics can be found in Table 4. Characteristics of the patients included in the five studies varied in some aspects:
| Study | Number of patients/% female | Age (years), mean (SD) | RA duration (years), mean (SD) | RF positive (%) | % of patients on concomitant DMARDs and steroids | Number of previous DMARDs, mean (SD) | Number of previous TNF inhibitors, mean (SD) | HAQ, mean (SD) | DAS28, mean (SD) | TJC/SJC, mean (SD) | ESR (mm/hour), mean (SD) | CRP (mg/dl), mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bennett 200592,a | 26/87 | 54 (range 19–77) | NR | NR | MTX (37); LEF (3); HCQ (3); AZA (1). All above with or without low-dose prednisone | 3.4 (range 2–7) | 1 (IFX, ETN, anakinra) | 2.07 (NR) | 6.3 (NR) | NR | NR | NR |
| Wick 200593 | 27/84b | 50 (15) | NR | NR | MTX (85); steroids (NR) | 2.0 (NR) | 1 (all IFX) | 1.39 (0.52)c | 5.5 (1.6)c | Tender 8 (5)c; swollen 10 (5)c | 41.7 (27.5)c | 43.9 (45.2)c |
| Nikas 200694 | 24/92 | 57 (11) | 16.6 (7.0) | 63 | MTX (83); CyA (4); LEF (13); steroids (100) | NR | 1 (all IFX) | NR | 5.6 (0.8) | Given graphically only | Given graphically only | Given graphically only |
| Bombardieri 200795,96 | 899/81 | 53 (13) | 12.0 (8.0) | 72 | DMARDs (31), steroids (77) | 5.0 (1.9) | ≥ 1 (IFX and/or ETN) | 1.85 (0.66) | 6.3 (1.1) | Tender 15 (7); swollen 11 (6) | NR | NR |
| van der Bijl 200897 | 41/88 | 55 (NR) | 11.6 (7.4) | NR | One DMARD (66); steroids (NR) | NR | 1 (all IFX) | 1.85 (0.49) | 6.1 (0.9) | Tender 6 (1); swollen 8 (5) | NR | 25.1 (32.0) |
-
Where reported 81%–92% were female.
-
The mean age of the patients ranged from 50 to 57 years.
-
The mean RA duration ranged from 11.6 to 16.6 years, but was not reported in two studies.
-
The percentage of RF-positive patients was reported only in two studies (63% and 72%).
-
Concomitant DMARDs: where reported 37%–85% patients were on MTX other DMARDs included CyA (4%), leflunonide (3%–13%), HCQ (3%) and AZA (1%).
-
The percentage of patients on concurrent steroids was reported in two studies and ranged from 77% to 100%.
-
Where reported the mean number of previous DMARDs used ranged from 2 to 5.
-
The mean number of previous TNF inhibitors was greater than or equal to 1 in the biggest study, and it was exactly 1 in all the other studies.
-
The HAQ scores ranged from 1.29 to 2.07 in four studies, but were not reported in one study.
-
The mean DAS28 scores were very similar, ranging from 5.5 to 6.3.
-
The mean number of tender and swollen joints at baseline was reported in three studies and ranged from 6 to 15 and from 8 to 11, respectively.
-
Baseline ESR was reported in only one study (41.7 mm/hour) and CRP in only two studies (25.1 mg/dl and 43.9 mg/dl).
Quality assessment
The studies were all uncontrolled; four of them were prospective and one was retrospective. 93 Criteria for patient inclusion were clearly stated in four studies; however, in three of these it was unclear whether consecutive patients were included. The highest percentage of patients withdrawn from a study was 26.8%. There were no withdrawals from the retrospective study. In general, the higher withdrawal rates occurred with the longer follow-up durations. Further details on the quality assessment of the studies are given in Table 5.
| Study | Study design | Inclusion criteria clearly defined? | Were consecutive patients included in the study? | Patients withdrew (%) | Comments |
|---|---|---|---|---|---|
| Bennett 200592 | Prospective uncontrolled | Yes | Yes | NRa | |
| Wick 200593 | Retrospective uncontrolled | No | NA | 0 | |
| Nikas 200694 | Prospective uncontrolled | Yes | Unclear | 16.7 | |
| Bombardieri 200795,96 | Multicentre, uncontrolled open-label | Yes | Unclear | 9.9 | |
| van der Bijl 200897 | Pilot uncontrolled open-label prospective | Yes | Unclear | 26.8 |
Results
Tables 6 and 7 show what outcomes were measured in each study. Outcomes in Table 6 are reported and discussed in the main text of this report and those in Table 7 are reported in Appendix 10 only.
| Study | Total withdrawal | Withdrawal by reason | ACR (20/50/70) | DAS28 | EULAR response | HAQ | QoL | Joint damage | Serious AEs | Infection/serious infection | Injection/infusion reaction |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bennett 200592 | ✓ | ✓ time range | ✓ time range | ||||||||
| Wick 200593 | ✓ | ✓ | ✓ | ✓ | |||||||
| Nikas 200694 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Bombardieri 200795,96 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| van der Bijl 200897 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Study | Other measures of disease activity | Fatigue | Pain | TJC/SJC | CRP/ESR |
|---|---|---|---|---|---|
| Bennett 200592 | |||||
| Wick 200593 | |||||
| Nikas 200694 | ✓a | ✓a | ✓a | ||
| Bombardieri 200795,96 | ✓ | ||||
| van der Bijl 200897 | ✓ |
Withdrawals
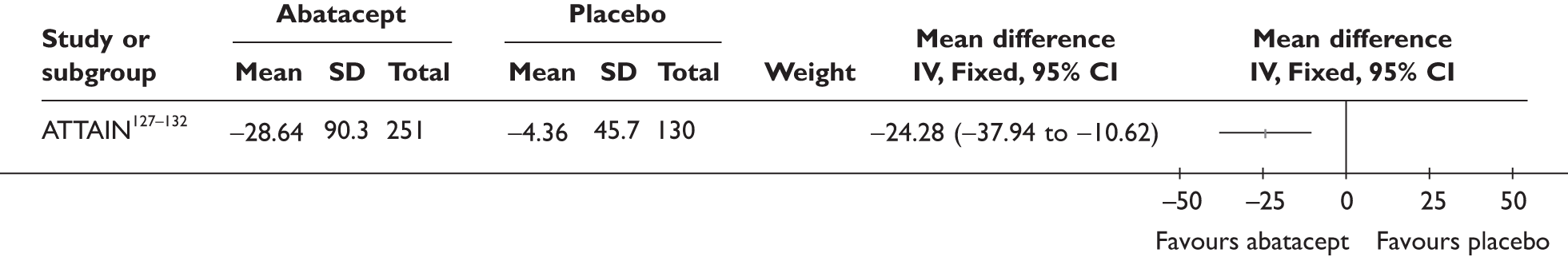
Withdrawal rates are presented in Figure 1. At 3 months, the percentage of patients withdrawn was very similar in the two studies that reported this outcome (9.9% and 9.8%). No patients withdrew in a retrospective study during 6 months. Withdrawal rates reported at 1 year were 12.5% and 26.8% in the two studies that reported this outcome. The percentage of patients withdrawn owing to lack of efficacy and owing to AEs at 3 months was reported only in the biggest study and was 2.9% and 5.6%, respectively. The percentage of patients withdrawn owing to lack of efficacy and owing to AEs at 12 months was measured in two studies: 8.3% and 17.1% withdrew because of lack of efficacy and 8.3% and 14.6% withdrew because of AEs.
FIGURE 1.
Adalimumab: withdrawals from studies by reason. LCI, lower confidence interval; UCI, upper confidence interval.
One study92 reported withdrawal data based on all 70 patients, including 44 patients who received a prior TNF inhibitor as well as TNF inhibitor-naive patients; the withdrawal data were not included in this report.
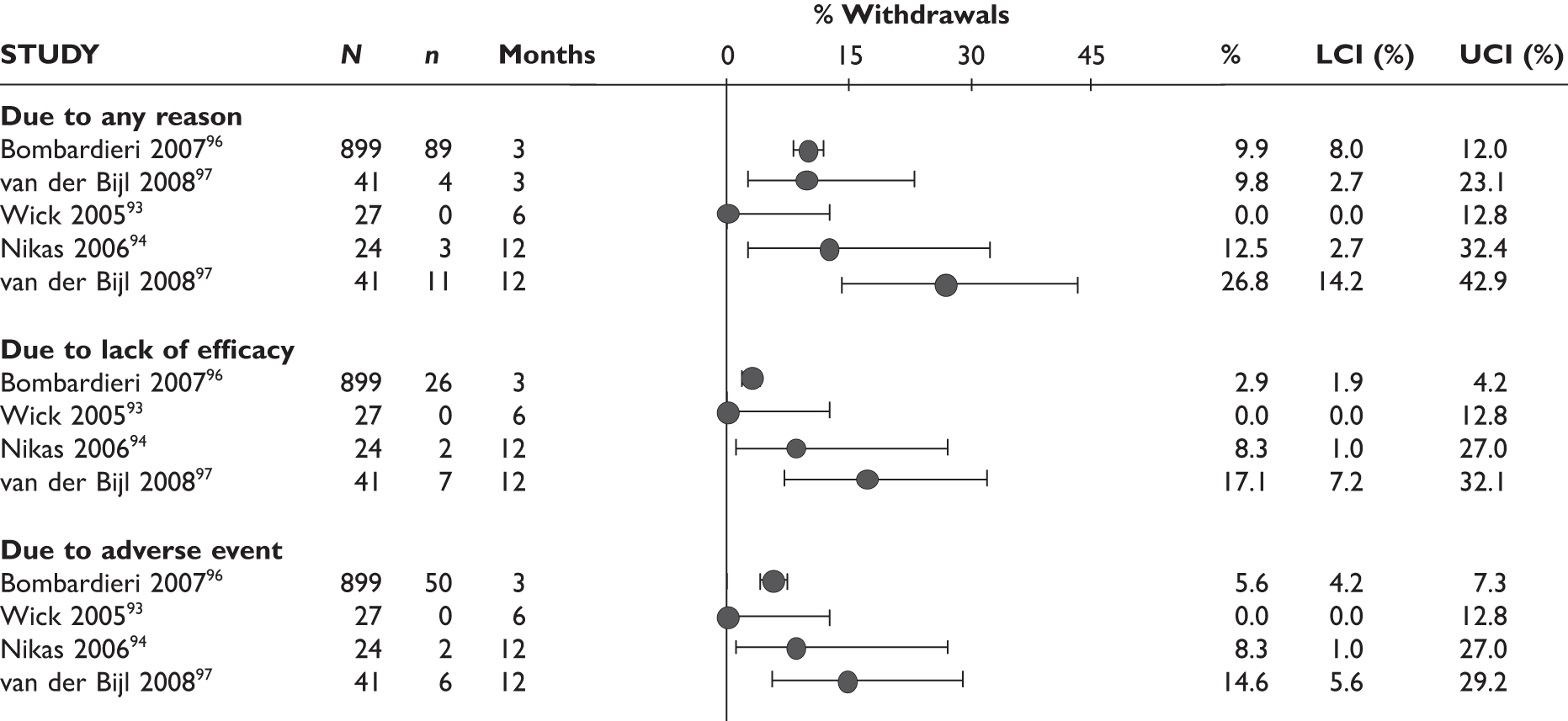
ACR20 response
The ACR20 response was assessed in four studies (Figure 2). Two studies assessed it at 3 months and the response was achieved by around half of the patients (46% and 60%). In the other two studies, the percentage of patients who achieved ACR20 response was 70% at 6 months and 75% at 12 months.
FIGURE 2.
Adalimumab: ACR (20, 50, 70) responses. LCI, lower confidence interval; UCI, upper confidence interval.
ACR50 response
The ACR50 response was measured in three studies (Figure 2): 26.8%–33% of patients achieved ACR50 response at 3 months. When measured at 12 months in the other study, half of the patients achieved this response.
ACR70 response
The ACR70 response was measured in three studies (Figure 2). ACR70 response at 3 months was similar in two studies that measured this outcome (13% and 12%). ACR70 response at 12 months was reported in one study, with 33% of the patients achieving this response.
A similar pattern was seen for ACR20, ACR50 and ACR70, with a relatively higher percentage of patients achieving a response with longer duration of treatment.
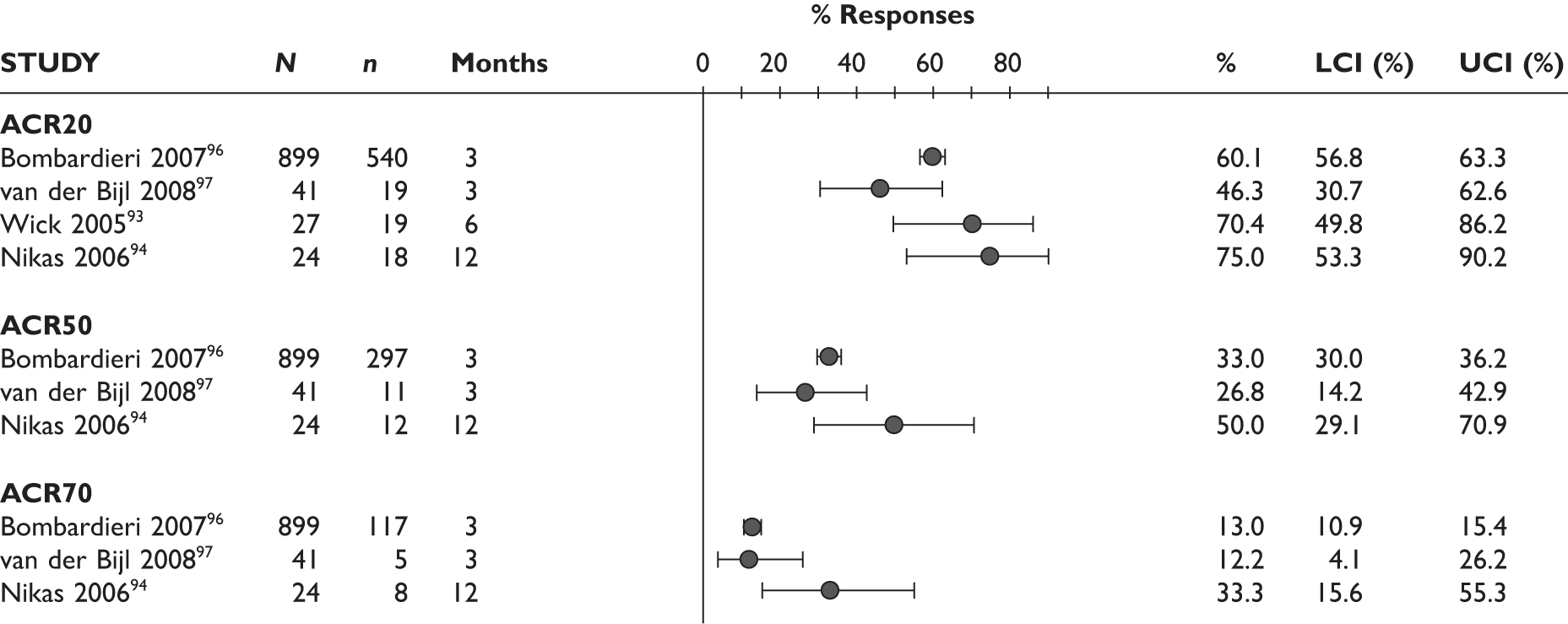
DAS28
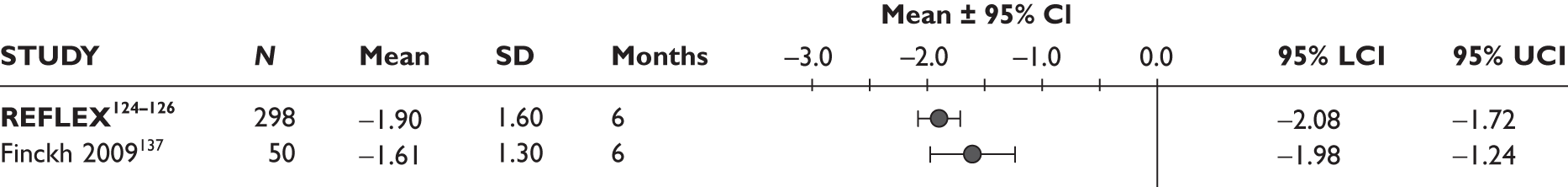
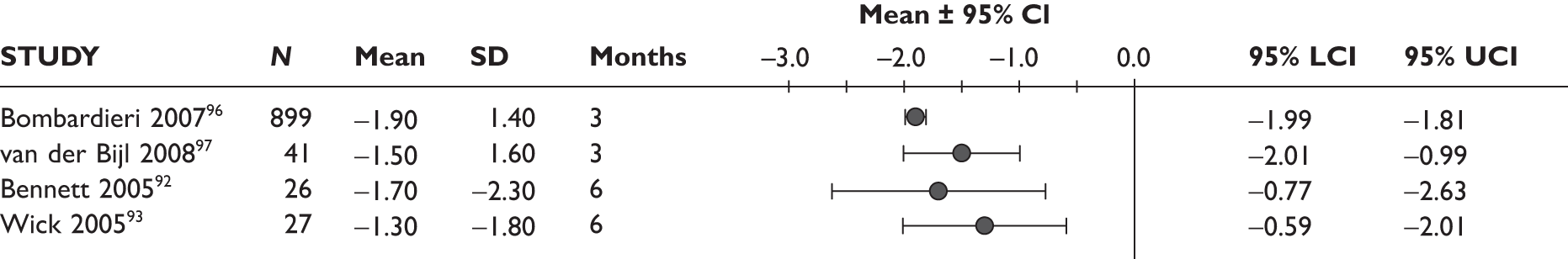
One study measured DAS28 at 3 and 6 months and another study at 12 months; the mean scores were 4.5, 4.2 and 3.2, respectively. See Figure 3 for details. The mean changes from baseline to 3 months and to 6 months [note: in the Bennett et al. study92 it was measured after mean treatment duration of 8.5 (range 1–19) months], were reported in four studies including the biggest study. They all showed that treatment with ADA significantly improved DAS28 scores (mean changes ranged from –1.30 to –1.90). See Figure 4 for details.
FIGURE 3.
Adalimumab: DAS28 scores. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.
FIGURE 4.
Adalimumab: mean changes in DAS28 scores. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.
EULAR response
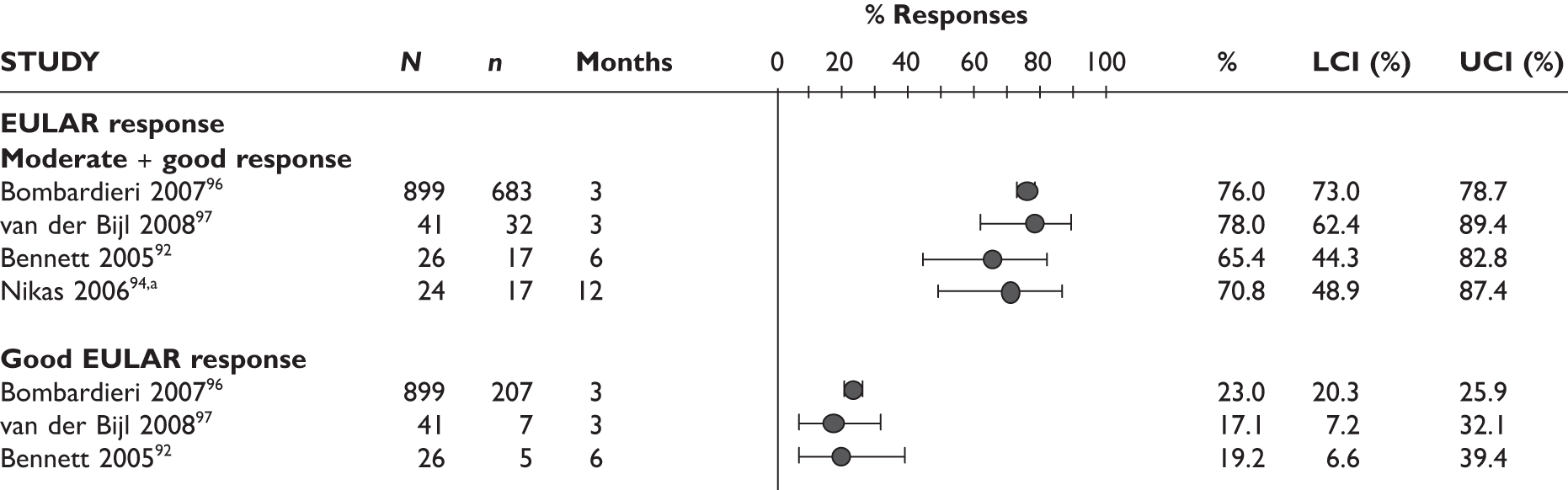
Two studies reported EULAR response at 3 months; most of the patients had a good/moderate response (76% and 78%) and 17%–23% had a good response. The Bennett et al. study92 measured EULAR response after a mean treatment duration of 8.5 months (range 1–19 months); the response rate was 65%, of whom 46% had a moderate response and 19% had a good response. See Figure 5 for details.
FIGURE 5.
Adalimumab: EULAR response. (a) Nikas et al. 94 only reported ‘EULAR response’ without providing further detail. LCI, lower confidence interval; UCI, upper confidence interval.
Health Assessment Questionnaire
Mean change in HAQ score was reported in three studies. Figure 6 shows that the mean HAQ score measured at 3 months in two studies, including the biggest study, and at mean 8.5 months (range 1–19 months) in the Bennett et al. study92 in all cases showed a significant decrease, ranging from –0.21 to –0.48, with the largest improvement observed in the biggest study.
FIGURE 6.
Adalimumab: mean change in HAQ scores. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.

Joint damage
None of the studies reported this outcome measure.
Quality of life
None of the studies reported this outcome measure.
Serious adverse events
One study (the largest) reported that 18% of the patients experienced serious AEs and 13% withdrew because of AEs; none of these was lupus related or a demyelinating disorder. 96,97
Any infections/serious infections
The largest study reported that the serious infection rate was 10.0/100 patient-years. The prevalence of TB infection was 0.4/100 patient-years in this study. In another study97 one patient developed pulmonary TB at 11 months. In the latter study, serious infection with cellulitis was also reported in one patient. One patient in a 12-month study by Nikas et al. 94 had to stop the study because of herpes zoster infection; it was not reported at which time point the treatment was stopped.
Injection site reaction/infusion reaction
The largest study stated that none of the patients experienced a serious anaphylactic response during the study period of 3 months. In a 12-month study,94 one patient had to stop the study because of an immediate hypersensitivity reaction; it was not reported at which time point it was stopped.
Summary
Five uncontrolled studies were identified for the assessment of effectiveness of ADA in comparison with standard care. Follow-up duration ranged from 3 months to over 1 year. All patients included in the studies were generally similar. The main results are summarised in Table 8.
| Outcome | 3 months | 6 months | ≥ 9 months |
|---|---|---|---|
| Withdrawals (%): | |||
| • for any reason | 9.8–9.9 | 0 | 12.5–26.8 |
| • due to lack of efficacy | 2.9 | 0 | 8.3–17.1 |
| • due to AEs | 5.6 | 0 | 8.3–14.6 |
| ACR20 response (%) | 46.3–60.1 | 70.4 | 75.0 |
| ACR50 response (%) | 26.8–33.0 | NR | 50.0 |
| ACR70 response (%) | 12.2–13.0 | NR | 33.3 |
| EULAR response (%): | |||
| • good/moderate response | 76.0–78.0 | 65.4 | 70.8 |
| • good response | 17.1–23.0 | 19.2 | NR |
| remission | NR | 7.7 | NR |
| DAS28: | |||
| • mean change from baseline | –1.50 to –1.90 (significant improvement) | –1.30 to –1.70 (significant improvement) | NR |
| • mean at time point | 4.50 | 4.20 | 3.20 |
| HAQ: mean change from baseline | –0.21 to –0.48 (significant improvement) | –0.31 (significant improvement) | NR |
| QoL | NR | NR | NR |
| Joint damage | NR | NR | NR |
| Serious AEs | From one study:95,96 18% had serious AE (no lupus-related or demyelinating disorder) and 13% withdrew because of AE | NR | NR |
| Any infections/serious infections | From one study:95,96 serious infections rate 10.0/100 patient years; TB infection rate 0.4/100 patient-years | NR |
From one study:97 one patient developed pulmonary TB; one with serious cellulitis From one study:94 one herpes zoster infection led to withdrawal |
| Infusion reaction | From one study:95,96 allergic AEs 6.5/100 patient-years (no serious anaphylactic response) | NR | From one study:94 one withdrawal because of an immediate hypersensitivity reaction |
Etanercept
Overview of evidence
No RCT was found. Seven uncontrolled observational studies98–104 were identified that assessed efficacy of ETN.
In the studies by Buch et al. 99 and Bingham et al. 104 lack of efficacy was the primary reason for switching to ETN. In studies by Haraoui et al. ,98 Cohen et al. 100 and Buch et al. 101 patients discontinued IFX owing to a lack of efficacy or safety. In Iannone et al. ,102 patients had to have responded to prior IFX treatment but later switched to ETN due to side effects. The patient population in this study was therefore different from the other studies. In Laas et al. ,103 patients discontinued IFX owing to a lack of efficacy, safety or non-medical reasons. The group of patients who discontinued IFX owing to non-medical reasons (46%, 23/49) had responded well to IFX, but switched to ETN for practical reasons such as convenience (e.g. no need for hospital visit to receive infusion). Two studies99,101 were carried out at the same centre (Leeds Teaching Hospitals) in the UK. These studies were described separately in this section although it is possible that patients included in Buch et al. 200599 were a subgroup of the cohort included in Buch et al. 2007. 101 The other studies were carried out in France,100 Italy,102 Finland103 and the USA. 98 One study104 was a multicentre study that enrolled patients from both the USA and Canada. The length of follow-up varied from 12 weeks to more than 9 months. Further details are provided in Table 9.
| Study | Country | Design | Reason for switching | Prior TNF inhibitor | Treatment arms (no. of patients) | Duration of follow-up | Comments |
|---|---|---|---|---|---|---|---|
| RCTs | |||||||
| None were identified | |||||||
| Non-randomised comparative studies | |||||||
| None were identified | |||||||
| Uncontrolled studies | |||||||
| Haroui 200498 | USA | Uncontrolled prospective | Inefficacy and AEs | IFX | ETN (25) | 12 weeks | |
| Buch 200599 | UK | Uncontrolled prospective | Inefficacy | IFX | ETN (25) | 12 weeks | This study had other subgroups not relevant to this review |
| Cohen 2005100 | France | Uncontrolled retrospective | Inefficacy and AEs | IFX | ETN (24) | 3 months | Included an arm with 14 patients on IFX (switched from ETN) |
| Buch 2007101 | UK | Uncontrolled prospective | Inefficacy and AEs | IFX | ETN (95) | 12 weeks | |
| Iannone 2007102 | Italy | Uncontrolled retrospective | AEs | IFX | ETN (37) | 24 weeks | |
| Laas 2008103 | Finland | Uncontrolled prospective | Inefficacy, AEs, non-medical reasons | IFX | ETN (49) | > 9 months | Results > 9 months reported but duration of follow-up unclear |
| Bingham 2009104 | USA and Canada | Uncontrolled prospective | Inefficacy | IFX | ETN (201) | 16 weeks | |
Patient characteristics
Full details of patients’ characteristics are reported in Table 10. The number of patients included in the studies varied from 24 to 201. Patient characteristics differed across the seven studies:
| Study | Number of patients/% female | Age (years), mean (SD) | RA duration (years), mean (SD) | RF positive (%) | % of patients on concomitant DMARDs and steroids | Number of previous DMARDs, mean (SD) | Number of previous TNF inhibitors, mean (SD) | HAQ, mean (SD) | DAS28, mean (SD) | TJC/SJC, mean (SD) | ESR (mm/hour), mean (SD) | CRP (mg/dl), mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haraoui 200498 | 25/84 | 50 (39) | 10.0 (25.2) | NR | MTX (88), oral corticosteroid (48) | 4.8 (3.7) | 1 | 1.53 (NR) | NR | 10.0 (NR)/8.6 (NR) | NR | 1.7 (NR) |
| Buch 200599,a | 34/71 | 56 (NR) | NR | 44 | NR | NR | 1 | NR | 6.4 (NR) | NR | NR | 3.8–4.2a |
| Cohen 2005100 | 24/88 | 54 (11) | 12.2 (9.6) | NR | MTX (NR) | 4.1 (1.8) | 1 | NR | 5.6 (1.1) | NR | NR | NR |
| Buch 2007101 | 95/NR | 57 (14) | NR | 71 | NR | NR | 1 |
2.16 (0.64) |
6.4 (1.3) |
14.0 (1.0)/9.0 (0.9) | NR | 6.0 (NR) |
| Iannone 2007102 | 37/81 | 49(12) | 8.3 (6.0) | 75 | MTX (NR), prednisone (NR) | NR | 1 | 0.90 (NR) | 2.7 (NR) (DAS44) | NR | 21 (NR) | 0.6b (NR) |
| Laas 2008103 | 49/88 | NR | 12.2 (NR) | 65 | MTX (NR), prednisone (88) | 6.0–7.0b | 1 | NR | NR | NR | NR | NR |
| Bingham 2009104 | 201/60 | 57 (13) | 9.1 (9.5) | 58 | MTX (99), sulfasalazine (5), HCQ (9), prednisone (40) | NR | 1 | 1.60 (0.50) | 6.6 (1.0) | 17.8 (7.1)/14.3 (6.3) | 30b (range 2–125) | 6.2 (NR) |
-
Where reported, the percentage of female patients ranged from 60% to 88%.
-
Where reported, the mean age ranged from 49 to 57 years.
-
Where reported, the mean disease duration ranged from 8.3 to 12.2 years.
-
Where reported, the percentage of RF-positive patients ranged from 44% to 75%.
-
Where reported concomitant DMARDs were: 88–99% MTX, other DMARDs included HCQ (9%) and sulfasalazine (5%).
-
Where reported, 40%–88% of patients were receiving corticosteroids.
-
Where reported, the mean/median number of previously used conventional DMARDs varied from 4.1 to 7.0.
-
All the studies included patients previously treated with IFX.
-
Where reported the mean baseline HAQ ranged from 0.90 to 2.16.
-
The mean baseline DAS28 score ranged from 5.6 to 6.6.
-
One study102 reported baseline DAS44 (mean value was 2.7).
-
Where reported, the mean number of tender and swollen joints was variable (tender: 10.0–17.8 and swollen: 8.6–14.3).
-
Baseline ESR was reported only in two studies (21 mm/hour and 30 mm/hour).
-
Where reported, CRP ranged from 0.6 (median) to 6.2 (mean) mg/dl.
The baseline values listed in Table 10 for Iannone et al. 102 were measured 8 weeks before patients switched from IFX to ETN (while they were still responding to IFX) and thus the values may not be comparable with those from the other studies.
Quality assessment
All the seven studies were uncontrolled studies. Five were prospective98,99,101,103,104 and two were retrospective. 100,102 Full details of the quality assessment are reported in Table 11. With the exception of Laas et al. ,103 studies stated clearly their inclusion criteria. Only Buch et al. 200599 and Buch et al. 2007101 clearly stated that consecutive patients were included in the studies; this information was unclear in Bingham et al. 104 and Haraoui et al. 100 Only one study104 reported the percentage of patients lost to follow-up (0.5%).
| Study | Study design | Inclusion criteria clearly defined? | Were consecutive patients included in the study? | Patients withdrawn (%) | Comments |
|---|---|---|---|---|---|
| Haraoui 200498 | Uncontrolled prospective | Yes | Unclear | Unclear | |
| Buch 200599 | Uncontrolled prospective | Yes | Yes | Unclear | |
| Cohen 2005100 | Uncontrolled retrospective | Yes | NA | Unclear | |
| Buch 2007101 | Uncontrolled prospective | Yes | Yes | Unclear | |
| Iannone 2007102 | Uncontrolled retrospective | Yes | NA | Unclear | |
| Laas 2008103 | Uncontrolled prospective | No | NR | Unclear | |
| Bingham 2009104 | Uncontrolled prospective | Yes | Unclear | 0.5 |
Results
Table 12 and Table 13 show what outcomes were measured in each study. Outcomes in Table 12 are reported and discussed in the main text and in Table 13 are reported in Appendix 10 only.
| Study | Total withdrawal | Withdrawal by reason | ACR (20/50/70) | DAS28 | EULAR response | HAQ | QoL | Joint damage | Serious AEs | Infection/serious infection | Injection/infusion reaction |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Haraoui 200498 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Buch 200599 | ✓ | ||||||||||
| Cohen 2005100 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Buch 2007101 | ✓ | ✓ | ✓ | ||||||||
| Iannone 2007102 | ✓a | ✓ | ✓ | ||||||||
| Laas 2008103 | ✓ | ✓ | ✓ | ✓ | |||||||
| Bingham 2009104 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Study | Other measures of disease activity | Fatigue | Pain | TJC/SJC | CRP/ESR |
|---|---|---|---|---|---|
| Buch 200599 | ✓ | ||||
| Haroui 200498 | ✓ | ✓ | ✓ | ✓ | |
| Cohen 2005100 | ✓ | ✓ | ✓ | ||
| Buch 2007101 | ✓ | ||||
| Iannone 2007102 | ✓ | ✓ | |||
| Laas 2008103 | ✓ | ||||
| Bingham 2009104 | ✓ | ✓ | ✓ | ✓ |
Withdrawals
Five out of seven studies reported withdrawals and the reasons for withdrawing from treatment. The percentages and reasons for withdrawing from the study after commencing ETN are shown in Figure 7. The percentage of patients who withdrew for any reason ranged from as low as 6.5% (at 3 months) to as high as 58.3% (at 12 months). The percentage of patients who withdrew because of AEs and lack of efficacy ranged from 0% to 16.3% and from 0% to 29.2%, respectively.
FIGURE 7.
Etanercept: withdrawals in the studies by reason. LCI, lower confidence interval; UCI, upper confidence interval.
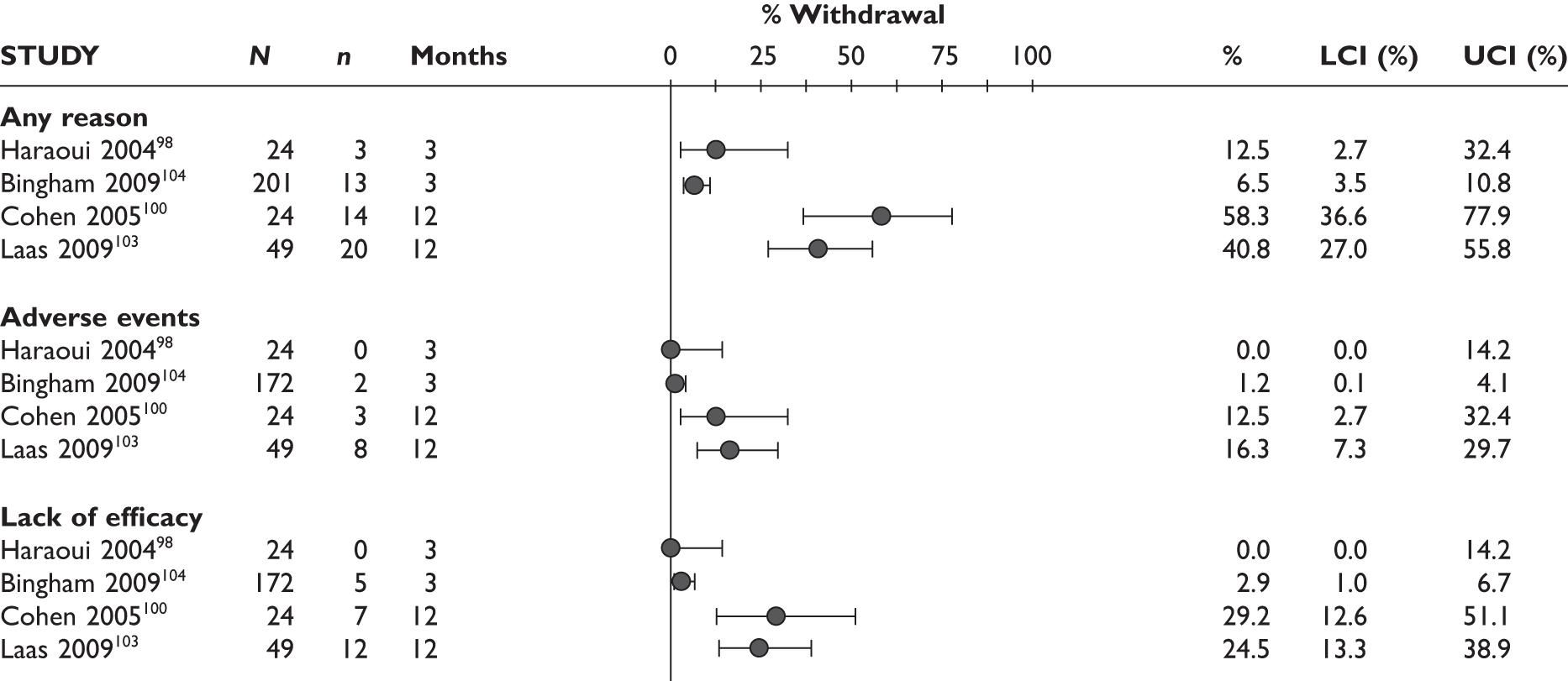
ACR20 response
The ACR20 response was assessed in four studies (Figure 8). The percentage of patients treated with ETN after IFX failure that achieved ACR20 response after 3 months ranged from 37.5% to 72.0%.
FIGURE 8.
ACR20: responses in patients receiving etanercept. LCI, lower confidence interval; UCI, upper confidence interval.
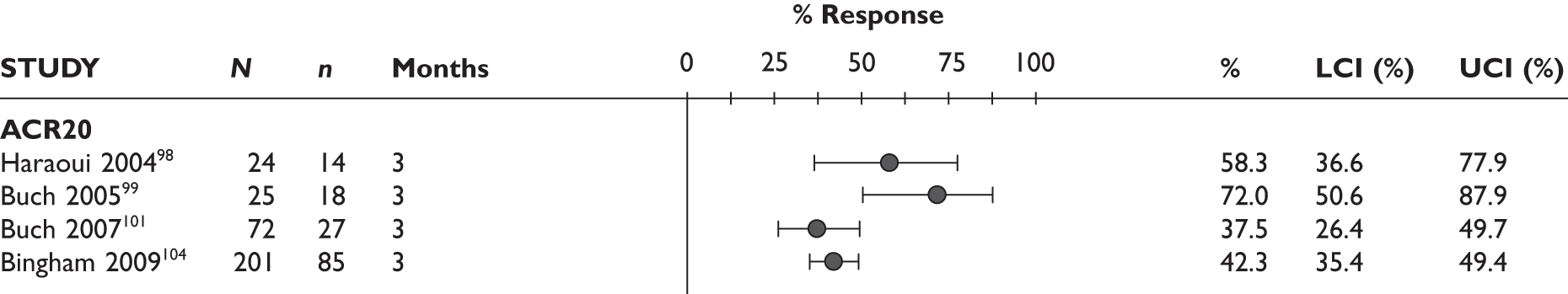
ACR50 response
The ACR50 response was assessed in five studies, but results from Iannone et al. 102 are not presented here, as explained above (Figure 9). The proportion of patients achieving a ACR50 response after 3 months ranged from 18.4% to 64.0%.
FIGURE 9.
ACR50: responses in patients receiving etanercept. LCI, lower confidence interval; UCI, upper confidence interval.
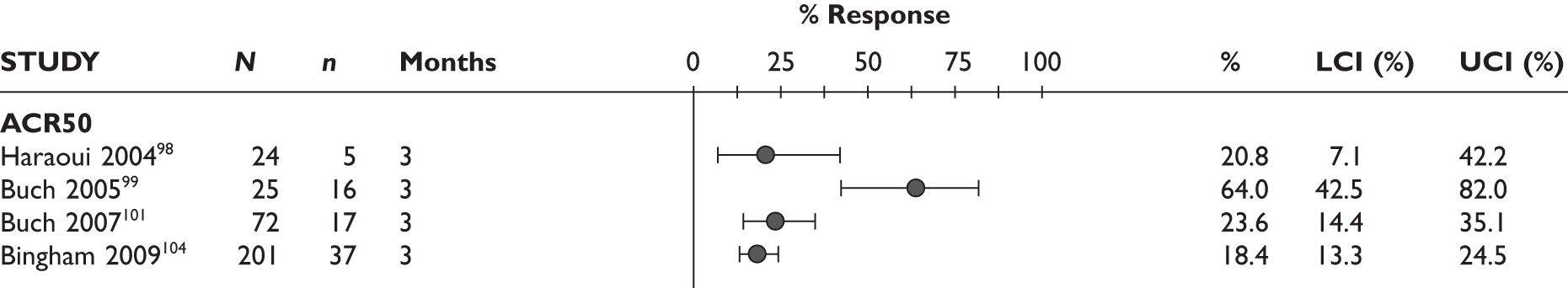
ACR70 response
The ACR70 response was assessed in five studies, but results from Iannone et al. 102 are not presented here, as explained above (Figure 10). The proportion of patients achieving a ACR70 response after 3 months ranged from 4.2% to 20.0%.
FIGURE 10.
ACR70: responses in patients receiving etanercept. LCI, lower confidence interval; UCI, upper confidence interval.
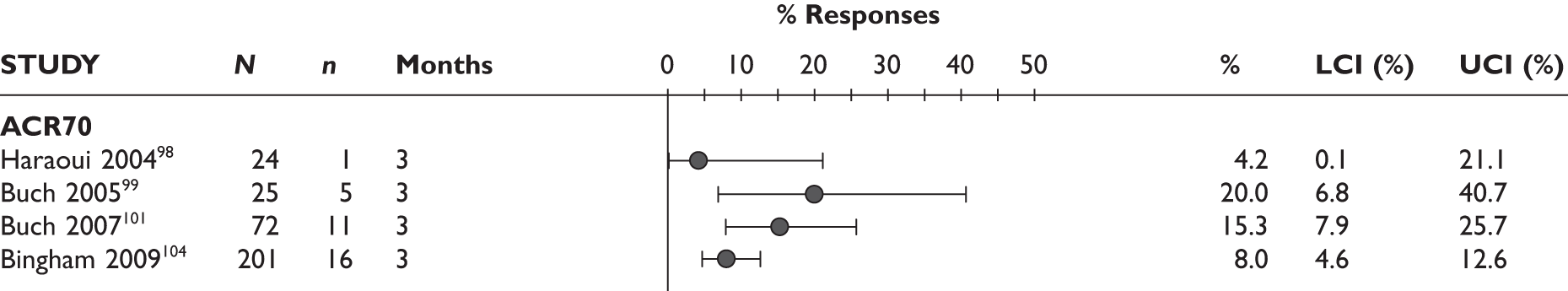
DAS
Figure 11 presents the mean changes from baseline in DAS. Four studies100,101,103,104 reported using DAS28. The mean decrease in DAS28 ranged from 1.47 to 1.80 at 3 months. One study102 reported no statistically significant decrease in DAS28 score from baseline at 12 months [mean change = –0.47, 95% confidence interval (CI) –1.06 to 0.12]. One study102 reported DAS calculated based on 44 joints (DAS44). Iannone et al. 102 found no statistically significant differences in DAS44 scores when results for 16 and 24 weeks were compared with the baseline value.
FIGURE 11.
Etanercept: mean changes from baseline in DAS. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.
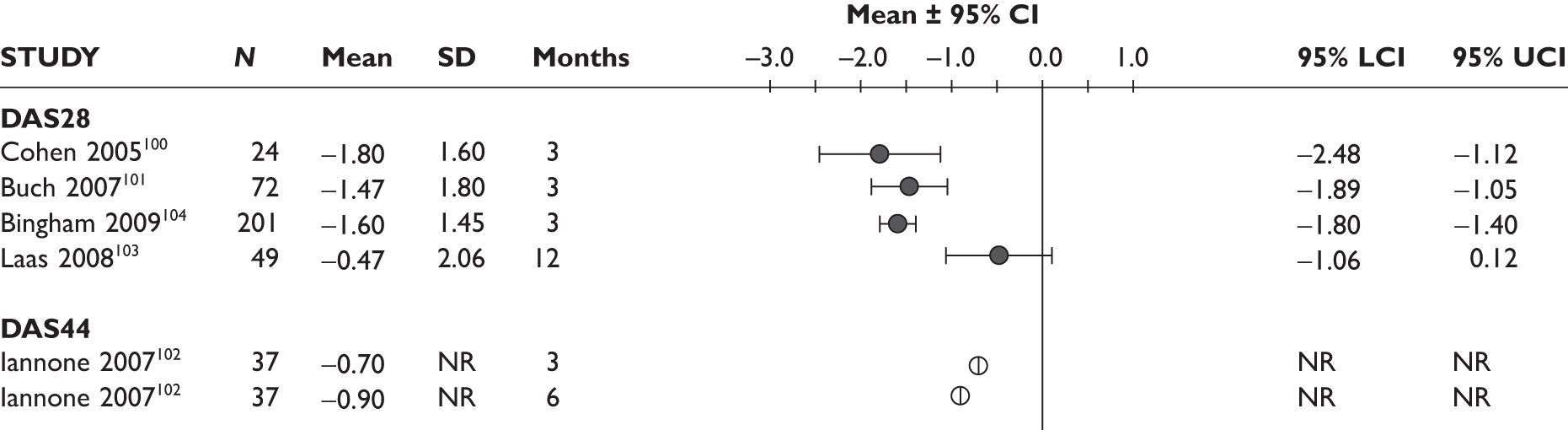
EULAR response
Three studies reported EULAR responses. Figure 12 shows the proportion of patients treated with ETN who achieved a good and good-to-moderate EULAR response after IFX failure. The percentage of patients who achieved a good score EULAR ranged from 12.5% to 45.8% at 3 months. The percentage of patients who achieved a good-to-moderate EULAR response ranged from 58.2% to 61.1% at 3 months.
FIGURE 12.
Etanercept: EULAR. LCI, lower confidence interval; UCI, upper confidence interval.
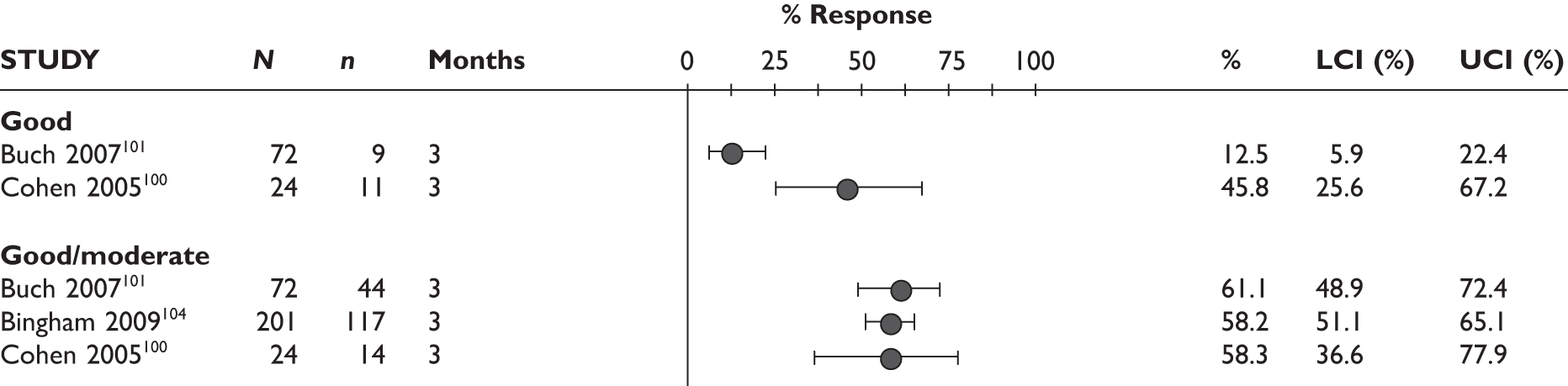
Health Assessment Questionnaire
Three studies reported mean changes from baseline in HAQ score (Figure 13). In Haraoui et al. ,98 the change in HAQ score was –0.45. However, it was not reported whether this change was statistically significant. For Iannone et al. ,102 the value of HAQ remained largely unchanged at 16 weeks (0.90) and 24 weeks (0.75) compared with the baseline value (0.75). In Bingham et al. ,104 there was a mean decrease in HAQ score of 0.35 at 3 months; this corresponds to a 22% decrease from baseline. This change was statistically significant.
FIGURE 13.
Etanercept: mean change from baseline in HAQ. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.
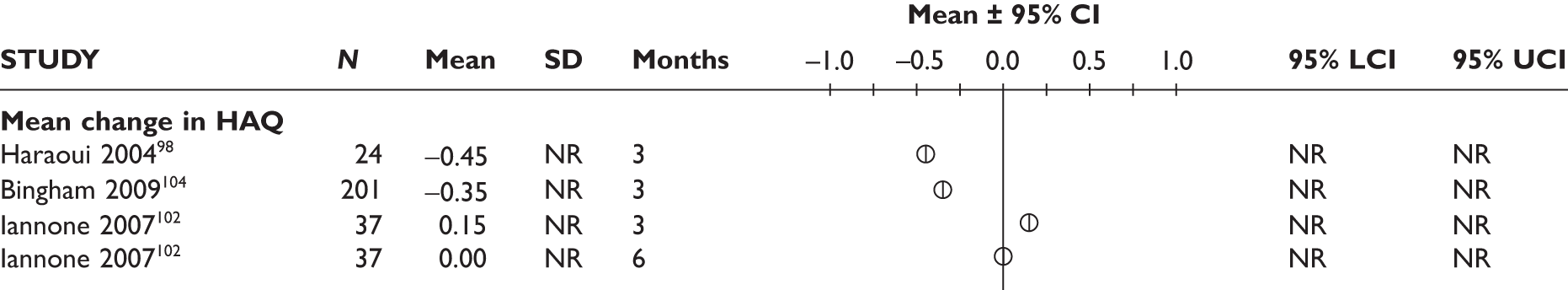
One study98 reported the percentage of patients who achieved minimal clinically important difference (MCID) in physical function (Figure 14). MCID was defined as a change of at least 0.22 in HAQ score. The percentage of patients who achieved MCID was 52%. Forty per cent of patients experienced an improvement in physical function of at least twice the value considered to represent MCID.
FIGURE 14.
Etanercept: MCID physical function. LCI, lower confidence interval; UCI, upper confidence interval.
Quality of life
None of the studies assessed QoL.
Joint damage
None of the studies assessed joint damage.
Serious adverse events
Two studies reported serious AEs (Figure 15). Haraoui et al. 98 reported that no serious AEs occurred during the study. Bingham et al. 104 found that 5% of the patients experienced a serious AE during the study period.
FIGURE 15.
Etanercept: reported serious AEs. LCI, lower confidence interval; UCI, upper confidence interval.
Infection and serious infection
Three studies reported infection and serious infection (Figure 16). One study104 reported that two patients (1%) experienced serious infections. The percentage of patients treated with ETN who reported any infection ranged from 4.1% to 8.3%.
FIGURE 16.
Etanercept: reported infection or serious infection. LCI, lower confidence interval; UCI, upper confidence interval.
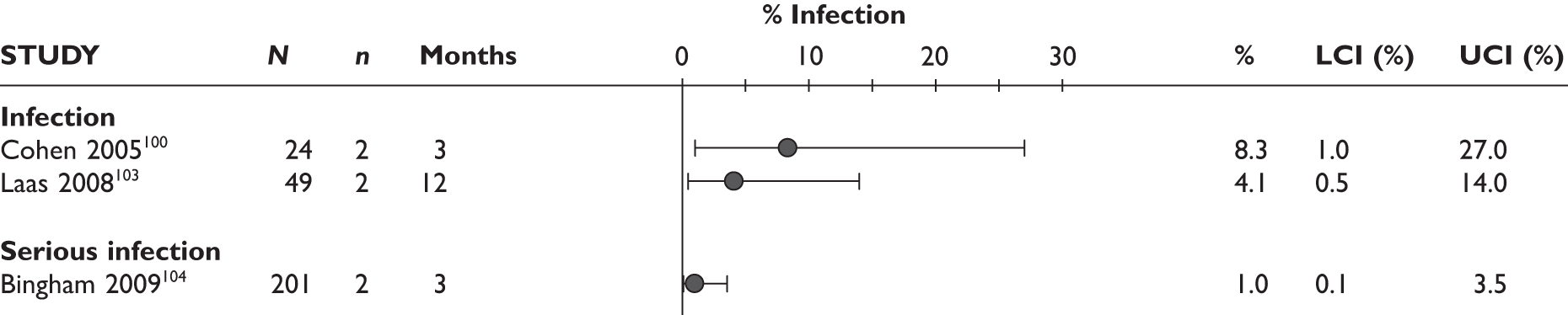
Injection/infusion reaction
No study reported injection or infusion reaction.
Summary
For the assessment of effectiveness of ETN, seven uncontrolled studies were identified. Follow-up duration ranged from 12 weeks to over 9 months. Patients included in the studies were generally similar. The main results are summarised in Table 14.
| Outcome | 3 months | 6 months | ≥ 9 months |
|---|---|---|---|
| Withdrawals (%): | |||
| • for any reason | 6.5–12.5 | NR | 40.8–58.3 |
| • due to lack of efficacy | 0.0–2.9 | NR | 24.5–29.2 |
| • due to AEs | 0.0–1.2 | NR | 12.5–16.3 |
| ACR20 response (%) | 37.5–72.0 | NR | NR |
| ACR50 response (%) | 18.4–64.0 | NR | NR |
| ACR70 response (%) | 4.2–20.0 | NR | NR |
| EULAR response (%): | |||
| • good/moderate response | 58.2–61.1 | NR | NR |
| • good response | 12.5–45.8 | NR | NR |
| • remission | NR | NR | NR |
| DAS28 | |||
| • mean change from baseline | –1.47 to –1.80 | NR | –0.47 |
| DAS44 | |||
| • mean change from baseline | –0.70 | –0.90 | NR |
| HAQ: mean change from baseline | –0.45 to 0.15 | 0.00 | NR |
| QoL | NR | NR | NR |
| Joint damage | NR | NR | NR |
| Serious AEs (%) | 0.0–5.0 | NR | NR |
| Any infections/serious infections (%) | 8.3/1.0 | NR/NR | 4.1/NR |
| Infusion reaction | NR | NR | NR |
Infliximab
Overview of evidence
Three studies were identified that assessed IFX in comparison with standard care: one uncontrolled prospective study107 and two uncontrolled retrospective studies. 105,106 (Note: the study by Yazici et al. 107 had a control group consisting of patients who were given their first biologic drug. This control group was not relevant to this report and, therefore, the study was utilised as uncontrolled.)
All included patients had tried one TNF inhibitor (ETN) before. Reasons for discontinuation included lack of efficacy, toxicity drug shortage, patient concerns about safety and thrombocytopenia.
All studies were conducted in the USA. Duration of follow-up was unclear in all the three studies.
Further details are provided in Table 15.
| Study | Country | Design | Reason for switching | Prior TNF inhibitors; n | Treatment arms (no. of patients) | Duration of follow-up | Comments |
|---|---|---|---|---|---|---|---|
| RCTs | |||||||
| None were identified | |||||||
| Non-randomised comparative studies | |||||||
| None were identified | |||||||
| Uncontrolled studies | |||||||
| Ang 2003105 | USA | Uncontrolled retrospective | Inadequate response, toxicity | ETN; 1 | IFX (24) | Unclear | Average treatment duration 8.2 months |
| Hansen 2004106 | USA | Uncontrolled retrospective | Lack of efficacy, drug shortage, patient concerns about safety, thrombocytopenia | ETN; 1 | IFX (20) | Unclear | |
| Yazici 2004107 | USA | Uncontrolled prospective | Inefficacy | ETN; 1 | IFX (21); IFX (41) | Unclear | Group with 41 patients received IFX as first TNF inhibitor |
Patient characteristics
All three studies were rather small, with the number of patients treated with IFX ranging from 20 to 24. They provided very little information about the baseline characteristics of included patients. However, based on the available information there might have been some baseline differences between study populations (Table 16).
| Study | Number of patients/% female | Age (years), mean (SD) | RA duration (years), mean (SD) | RF positive (%) | % of patients on concomitant DMARDs and steroids | Number of previous DMARDs, mean (SD) | Number of previous TNF inhibitors, mean (SD) | HAQ, mean (SD) | DAS28, mean (SD) | TJC/SJC, mean (SD) | ESR (mm/hour), mean (SD) | CRP (mg/dl), mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ang 2003105 | 24/90a | NR | NR | 34a | MTX (62), LEF (31)a | 0 to > 5a | 1 | NR | NR | NR | NR | NR |
| Hansen 2004106 | 20/60 | 48 (NR) | 9.3 (NR) | 65 | LEF (100); AZA (5); sulfasalazine (5); MTX (10); prednisone (75) | NR | 1 | NR | NR | 14 (NR)/14 (NR) | 13 (NR) | 23.8 (NR) |
| Yazici 2004107 | 21/NR | 61 (12)b | 13.4 (9.8)b | NR | NR | 2b | 1 | NR | NR | NR | NR | NR |
| Study | Study design | Inclusion criteria clearly defined? | Were consecutive patients included in the study? | Patients withdrawn (%) | Comments |
|---|---|---|---|---|---|
| Ang 2003105 | Uncontrolled retrospective | No | NA | Unclear | |
| Hansen 2004106 | Uncontrolled retrospective | No | NA | Unclear | |
| Yazici 2004107 | Uncontrolled prospective | No | Unclear | 28.6 |
-
In two studies the percentage of female participants ranged from 60% to 90%; Yazici et al. 107 did not provide any information.
-
In two studies the mean age was 48 years and 61 years; it was not reported in Ang et al. 105
-
In two studies disease duration was 9.3 years and 13.4 years; it was not reported in Ang et al. 105
-
In two studies 34%–65% of patients were RF positive; no information was provided in Yazici et al. 107
-
In Ang et al. 105 62% of patients were receiving MTX and 31% LEF; in Hansen et al. 106 all patients were receiving LEF and some of them also other DMARDs (AZA, sulfasalazine, MTX and prednisone); Yazici et al. 107 did not report concomitant DMARDs.
-
Only one study (Hansen et al. 106) reported that 75% of patients were receiving concomitant prednisone.
-
Two studies reported the number of previous DMARDs – it ranged from 0 to over 5; it was not reported in Hansen et al. 106
-
Patients had tried one previous TNF inhibitor (ETN) in all three studies.
-
None of the studies reported the baseline HAQ or DAS score.
-
Only one study106 reported that patients had a mean of 14 tender and 14 swollen joints at baseline.
-
Only one study106 reported the baseline ESR (mean 13 mm/hour) and CRP (mean 23.8 mg/dl).
Quality assessment
Of the three identified studies, two were uncontrolled retrospective analyses. One study was uncontrolled and prospective. None of the studies reported inclusion criteria clearly. It was unclear if consecutive patients were included in Yazici et al. 107 and this item was not applicable to retrospective studies. A total of 28.6% were withdrawn from Yazici et al. 107 and this percentage was unclear in the remaining two studies. Details of the quality assessment are reported in Table 7.
Results
Table 18 indicates which of the outcomes reported in the main text of the report were assessed in individual studies and Table 19 provides similar information for outcomes described in Appendix 10 only.
| Study | Total withdrawal | Withdrawal by reason | ACR (20/50/70) | DAS28 | EULAR response | HAQ | QoL | Joint damage | Serious AEs | Infection/serious infection | Injection/infusion reaction |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ang 2003105 | |||||||||||
| Hansen 2004106 | ✓ | ✓ | ✓ | ||||||||
| Yazici 2004107 | ✓ | ✓ |
| Study | Other measures of disease activity | Fatigue | Pain | TJC/SJC | CRP/ESR |
|---|---|---|---|---|---|
| Ang 2003105 | |||||
| Hansen 2004106 | ✓ | ✓ | ✓ | ||
| Yazici 2004107 | ✓ |
Ang et al. 105 reported results in a way that made it impossible to utilise them in this report (correlations between response to IFX and ETN).
Withdrawals
Withdrawal for any reason was assessed only in Yazici et al. ,107 withdrawal because of lack of efficacy only in Hansen et al. 106 and withdrawal because of AEs was not assessed in any of the studies. Details are reported in Figure 17. Yazici et al. 107 reported that 28.6% of patients were withdrawn from the study for any reason (follow-up unclear). Ten per cent of patients were withdrawn from Hansen et al. 106 owing to lack of efficacy (follow-up unclear).
FIGURE 17.
Infliximab: withdrawals. LCI, lower confidence interval; NR, not reported; UCI, upper confidence interval.
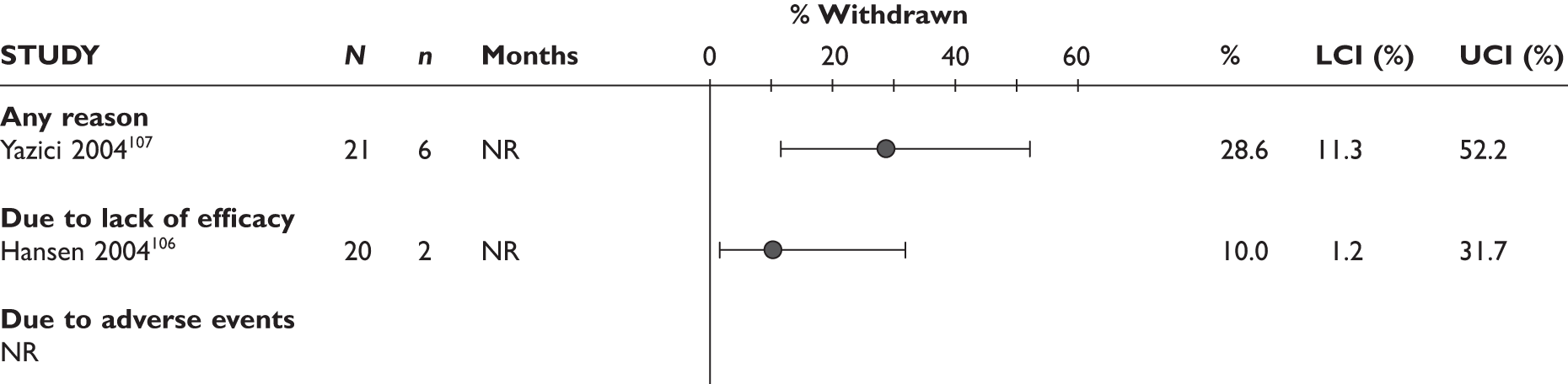
ACR20 response
None of the studies assessed ACR20 response.
ACR50 response
None of the studies assessed ACR50 response.
ACR70 response
None of the studies assessed ACR70 response.
DAS28
The only information on DAS28 change came from Yazici et al. 107 and the authors claimed that at 12 months patients ‘improved significantly’.
EULAR response
The EULAR response was not assessed in any of the studies.
Health Assessment Questionnaire
The only information on HAQ change came from Yazici et al. 107 and the authors claimed that at 12 months patients ‘improved significantly’.
Quality of life
Quality of life was not assessed in any of the studies.
Joint damage
Joint damage was not assessed in any of the studies.
Serious adverse events
Serious AEs were not assessed in any of the studies.
Infections/serious infections
Details of infections are reported in Figure 18. Fifteen percent of patients in Hansen et al. 106 experienced an infection (follow-up was unclear). No other studies reported infections. Serious infections were not reported in any of the studies.
FIGURE 18.
Infliximab: infections. LCI, lower confidence interval; NR, not reported; UCI, upper confidence interval.
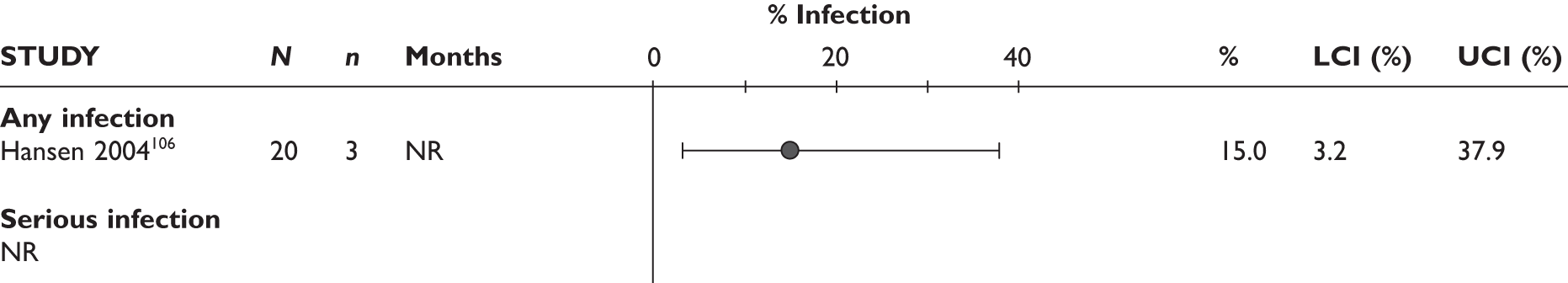
Injection/infusion reaction
There were no infusion reactions in Hansen et al. 106 Other studies did not report this outcome.
Infliximab in comparison with an ongoing biologic agent
One RCT [open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept (OPPOSITE133)] was identified that compared IFX with ongoing ETN. Although the study met the inclusion criteria of the systematic review, this comparison was not considered relevant to this report and, therefore, the study was not analysed.
It was a multicentre randomised trial and included 27 patients who had active RA and had an ‘incomplete response to etanercept’. Patients were randomised either to discontinue ETN and receive IFX (13 patients) or to continue ETN treatment (14 patients). The follow-up duration was 30 weeks. Data were collected on outcomes including ACR response, HAQ, radiological progression, serum biomarker levels and safety.
Summary
Three studies compared IFX with standard care: two uncontrolled retrospectiv105,106 and one uncontrolled prospective Yazici et al. studies. 107 They included small numbers of patients ranging from 20 to 24. Follow-up was unclear in all of them. There was little information about baseline characteristics; however, it seems that there may be some, if small, differences between studies. The main results of included studies are summarised in Table 20.
| Outcome | Uncontrolled studies Unclear follow-up |
|---|---|
| Withdrawals (%): | |
| • for any reason | 28.6 (reported in one study) |
| • due to lack of efficacy | 10 (reported in one study) |
| • due to AEs | NR |
| ACR20 response | NR |
| ACR50 response | NR |
| ACR70 response | NR |
| DAS28 | Only one study included a statement that at 12 months patients ‘improved significantly’ |
| EULAR response | NR |
| HAQ | Only one study included a statement that at 12 months patients ‘improved significantly’ |
| QoL | NR |
| Joint damage | NR |
| Serious AEs | NR |
| Any infections/serious infections (%) | 15 (reported in one study)/NR |
| Infusion reaction | 0 (reported in one study) |
Tumour necrosis factor inhibitors as a class
Overview of evidence
This section reports on studies that evaluated the use of TNF inhibitors as a class after the failure of the first one. No RCT was found. One controlled121–123 and six uncontrolled observational studies108–113 were identified. In Finckh et al. 136,137 lack of efficacy was the primary reason for switching TNF inhibitors. In Hyrich et al. ,121–123 Gomez-Reino et al. 108 and Blom et al. 113 patients switched to another TNF inhibitor because of a lack of efficacy or AEs. In Hjardem et al. ,110 Duftner et al. 111 and Karlsson et al. 112 patients switched TNF inhibitorsowing to lack of efficacy or AEs or for other reasons. The reason for changing from one TNF inhibitor to another was unclear in Solau-Gervais et al. 109 Hyrich et al. 121–123 used data from the BSRBR. The other studies were carried out in Switzerland, Spain, France, Denmark, Austria, Sweden and the Netherlands. The length of follow-up ranged from 3 months to up to 4 years. Further details are provided in Table 21.
| Study | Country | Design | Reason for switching | Prior TNF inhibitors (n) | Treatment arms (no. of patients) | Duration of follow-up | Comments |
|---|---|---|---|---|---|---|---|
| RCTs | |||||||
| None were identified | |||||||
| Non-randomised controlled studies | |||||||
| Hyrich 2009121–123 | UK | Cohort | Inefficacy, AEs | ETN, IFX, ADA | TNF inhibitor (all switchers: n = 534; stoppers: n = 202) | > 6 months | |
| Uncontrolled studies | |||||||
| Gomez-Reino 2006108 | Spain | Uncontrolled prospective | AEs, lack of efficacy | IFX, ETN | TNF inhibitor (n = 448) | 2 years | Including other forms of arthritis (ankylosing spondylitis, psoriatic arthritis and other chronic arthritis; n = 385 for RA) |
| Solau-Gervais 2006109 | France | Uncontrolled prospective | Unclear | Any | TNF inhibitor (n = 70) | > 3 months | |
| Hjardem 2007110 | Denmark | Uncontrolled retrospective | Inefficacy, AE, other | ETN, IFX, ADA | TNF inhibitor (n = 235) | 3 months | |
| Duftner 2008111 | Austria | Uncontrolled retrospective | Inefficacy, AE, other | IFX, ETN, ADA | TNF inhibitor (n = 109) | < 4 years | Length of follow-up including first line; reported 12-month drug continuation rate for second, third and fourth line |
| Karlsson 2008112 | Sweden | Uncontrolled retrospective | Inefficacy, AE, other | Any | TNF inhibitor (n = 337) | 3 months | Second and third line separately |
| Blom 2009113 | Netherlands | Uncontrolled retrospective | Non-response, loss of response, and AEs | IFX, ETN, ADA | IFX, ETN, ADA (n = 197) | 6 months | |
| Finckh 2009136,137 | Switzerland | Prospective cohort | Inadequate response | Any (≥ 1) | RTX (n = 155); alternative TNF inhibitor (n = 163) | 11 months (median) | Based on the Swiss Clinical Quality Management program for Rheumatoid Arthritis (SCQM-RA) |
Patient characteristics
Full details of patients’ characteristics are reported in Table 22. The number of patients included in the studies ranged from 70 to 818. Patient characteristics were generally similar across the eight studies:
| Study | Number of patients/% female | Age (years), mean (SD) | RA duration (years), mean (SD) | RF positive (%) | % of patients on concomitant DMARDs and steroids | Number of previous DMARDs, mean (SD) | Number of previous TNF inhibitors, mean (SD) | HAQ, mean (SD) | DAS28, mean (SD) | TJC/SJC, mean (SD) | ESR (mm/hour), mean (SD) | CRP (mm/dl), mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyrich 2009121–123 | 818/80 | 58 (11) | 10.0 (8.9) | NR | NR | 4.0 (1.5) | NR | 1.90 (0.63) | 6.5 (1.0) | NR | NR | NR |
| Gomez-Reino 2006108 | 448/67 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Solau-Gervais 2006109 | 70/86 | 57 (NR) | 14.7 (NR) | 81 | NR | 4.3 (NR) | ≥ 1 | NR | 6.0 (NR) | NR | NR | NR |
| Hjardem 2007110 | 235/75 | 55 (12) | 8.0 (9.6) | NR | MTX (75), prednisone, corticosteroids | 4.0 (1.5) | ≥ 1 | NR | 5.2 (1.3) | NR | NR | NR |
| Duftner 2008111 | 109/89 | 51 (12) | 8.0 (7.5) | NR | NR | NR | ≥ 1 | NR | NR | NR | NR | NR |
| Karlsson 2008112 | 337/82 | 56 (13) | 14.0 (10.0) | NR | MTX, corticosteroid (68) | 4.7 (1.9) | 1–3 | 1.40 (0.60) | 5.5 (1.3) | 9.3 (6.8)/8.4 (5.9) | 36 (25) | 28 (35) |
| Blom 2009113 | 197/71 | 55 (NR) | 7.9 (NR) | 52 | MTX, steroids | NR | NR | NR | 5.1 (1.2) | NR | NR | NR |
| Finckh 2009136,137 | 163/78 | 55 (13) | 11.0 (7.0) | 77 | MTX 61, steroids 55 | NR | 1 | 1.42 (0.96–1.85)a | 4.1 (1.3) | NR | NR | NR |
-
The percentage of female patients ranged from 67% to 89%.
-
Where reported, the mean age ranged from 51 years to 58 years.
-
Where reported, the mean disease duration ranged from 8.0 years to 14.7 years.
-
Where reported, the percentage of RF-positive patients ranged from 51.5% to 81%.
-
Where reported, 61%–75% patients were on MTX; 55%–68% of patients were receiving corticosteroids.
-
Where reported, the mean number of previously used conventional DMARDs varied from 4.0 to 4.7.
-
Where reported, studies included patients who previously tried IFX, ETN and ADA.
-
Where reported, the mean baseline HAQ ranged from 1.4 to 1.9.
-
Where reported, the mean DAS28 score ranged from 4.1 to 6.5.
-
The mean number of tender and swollen joints was reported only in one study (tender 9.3 and swollen 8.4).
-
The mean baseline ESR was reported in one study and was 36 mm/hour.
-
The baseline CRP was reported in one study and was 2.8 mg/dl.
Quality assessment
One study was controlled. 121–123 Two studies108,109 were uncontrolled and prospective. Four studies were uncontrolled and retrospective. 110–113 Finckh et al. 136,138 was a non-randomised comparative study (TNF inhibitors vs RTX). This section presents data only for TNF inhibitors. Full details of the quality assessment are reported in Table 23. Most studies stated clearly their inclusion criteria. The inclusion criteria were unclear in two studies. 108,110 It was unclear in most studies whether consecutive patients were included in the study. Nearly one-third (140/477) of patients who met the study inclusion criteria were excluded from Karlsson et al. 112 because of dropouts/missing response data at 3 months. The exclusion of these patients may partly account for the higher rates of EULAR responses observed in this study compared with other studies (see Figure 25). The percentage of patients withdrawn was clearly reported in two studies.
| Study | Design | Inclusion criteria clearly defined? | Were consecutive patients included in the study? | Patients withdrawn (%) | Comments |
|---|---|---|---|---|---|
| Hyrich 2009121–123 | Controlled prospective | Yes | Unclear | Unclear | |
| Gomez-Reino 2006108 | Uncontrolled prospective | Unclear | Unclear | Unclear | |
| Solau-Gervais 2006109 | Uncontrolled retrospective | Yes | NA | Unclear | |
| Hjardem 2007110 | Uncontrolled retrospective | Unclear | Unclear | 34.5 | |
| Duftner 2008111 | Uncontrolled retrospective | Yes | NA | NA | |
| Karlsson 2008112 | Uncontrolled prospective | Yes | No | NA | 140/477 excluded (see text) |
| Blom 2009113 | Uncontrolled retrospective | Yes | NA | 38.6 | |
| Finckh 2009136,137 | Prospective cohort | Yes | Unclear | NA |
Results
Tables 24 and 25 state which outcomes were measured in each study and whether they are reported in the main text or Appendix 10 of this report.
| Study | Total withdrawal | Withdrawal by reason | ACR (20/50/70) | DAS28 | EULAR response | HAQ | QoL | Joint damage | Serious AEs | Infection/serious infection | Injection/infusion reaction |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyrich 2009121–123 | ✓ | ||||||||||
| Gomez-Reino 2006108 | |||||||||||
| Solau-Gervais 2006109 | |||||||||||
| Hjardem 2007110 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Duftner 2008111 | ✓ | ✓ | |||||||||
| Karlsson 2008112 | ✓ | ✓ | ✓ | ||||||||
| Blom 2009113 | ✓ | ✓ | ✓ | ✓ | |||||||
| Finckh 2009136,137 | ✓ |
| Study | Other measures of disease activity | Fatigue | Pain | TJC/SJC | CRP/ESR |
|---|---|---|---|---|---|
| Hyrich 2009121–123 | |||||
| Gomez-Reino 2006108 | |||||
| Solau-Gervais 2006109 | |||||
| Hjardem 2007110 | |||||
| Duftner 2008111 | |||||
| Karlsson 2008112 | |||||
| Blom 2009113 | |||||
| Finckh 2009136,137 | ✓ | ✓ |
Withdrawals
Two studies reported withdrawals together with reasons for withdrawing treatment (Figure 19). The percentage of patients who withdrew for any reason ranged from 7.6% (at 3 months) to 38.6% (at 12 months). The percentage of patients who withdrew because of AEs ranged from 6.1% (at 3 months) to 10.2% (at 6 months). At 12 months, the percentage of patients who withdrew because of AEs ranged from 6.0% to 14.7%. The percentage of patients who withdrew because of lack of efficacy ranged from 1.5% (at 3 months) to 22.6% (at 12 months).
FIGURE 19.
Tumour necrosis factor inhibitor as a class: withdrawals from the studies by reason. LCI, lower confidence interval; UCI, upper confidence interval.
One study reported 1-year drug survival108 (probability of staying on treatment at 12 months) of 0.79 (95% CI 0.74 to 0.83). Two studies reported median drug survival. 110,111 Hjardem et al. 110 and Duftner et al. 111 reported that the median drug survival was 37 weeks and 8.0 months (range 0–43.7 months), respectively.
ACR20 response
The ACR20 response was assessed in one study (Figure 20). Karlsson et al. 112 reported that at 3 months ACR20 response rate was 49.0% (95% CI 43.5% to 54.4%).
FIGURE 20.
TNF inhibitors as a class: ACR20 response. LCI, lower confidence interval; UCI, upper confidence interval.
ACR50 response
The ACR50 response was assessed in one study (Figure 21). Karlsson et al. 112 reported that at 3 months ACR50 response rate was 25.8% (95% CI 21.2% to 30.8%).
FIGURE 21.
TNF inhibitors as a class: ACR 50 response. LCI, lower confidence interval; UCI, upper confidence interval.
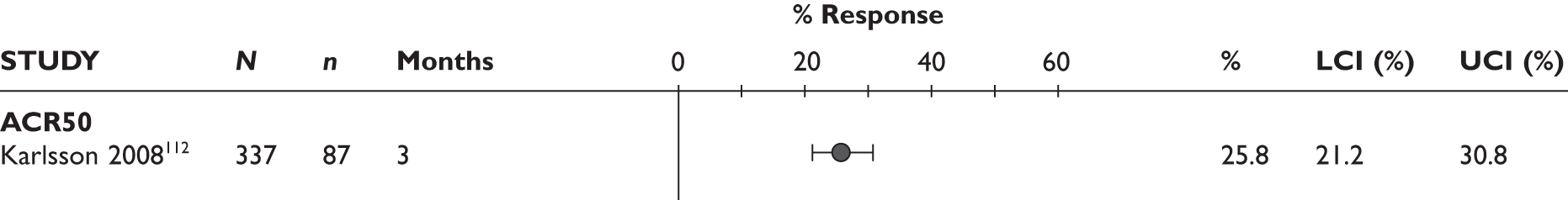
ACR70 response
The ACR70 response was assessed in one study (Figure 22). Karlsson et al. 112 reported that at 3 months ACR70 response rate was 7.1% (95% CI 4.6% to 10.4%).
FIGURE 22.
TNF inhibitor as a class: ACR70 response. LCI, lower confidence interval; UCI, upper confidence interval.
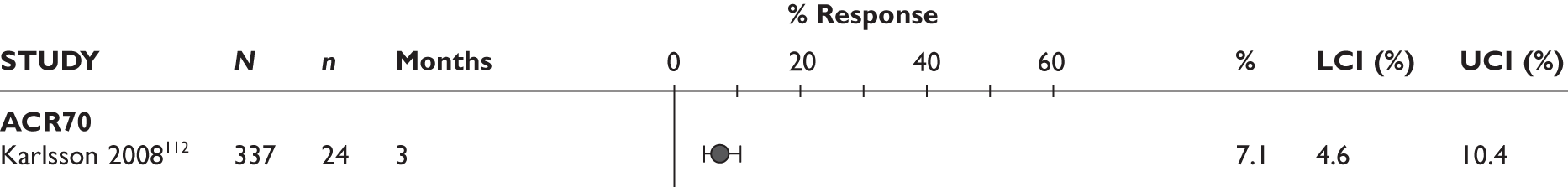
DAS28
Three studies reported mean changes from baseline in the DAS28 score (Figure 23). The mean decrease in DAS28 ranged from 0.86 to 1.00 at 3 months and from 0.88 to 0.92 at 6 months. Two studies112,113 reported low disease activity (DAS28 less than 3.2) (Figure 24). At 3 months the percentage of patients with low disease activity ranged from 14.2% to 29.1%. One study reported DAS28 remission (DAS28 less than 2.6) (Figure 24). Karlsson et al. 112 reported that 15.4% (95% CI 11.7% to 19.7%) of patients were in remission.
FIGURE 23.
TNF inhibitors as a class: mean changes from baseline in DAS28. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.
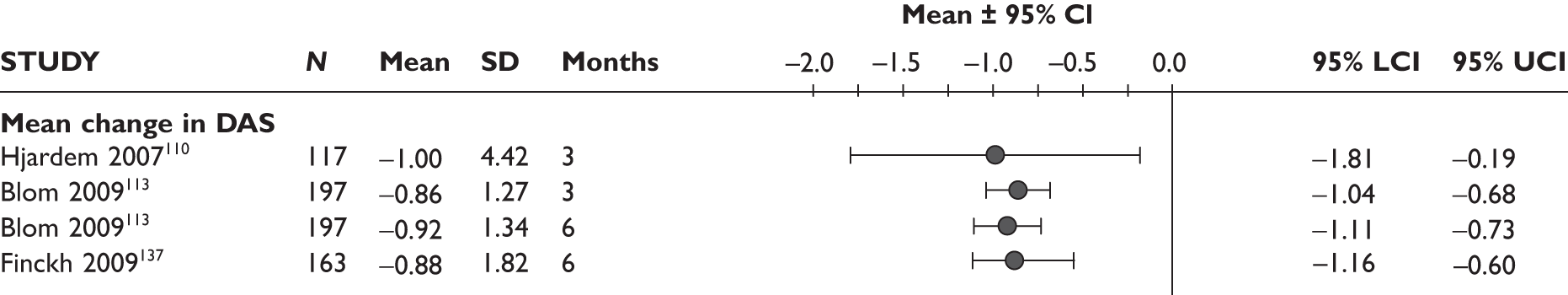
FIGURE 24.
TNF inhibitors as a class: low disease activity (DAS28 < 3.2) and remission (DAS28 < 2.6). LCI, lower confidence interval; UCI, upper confidence interval.
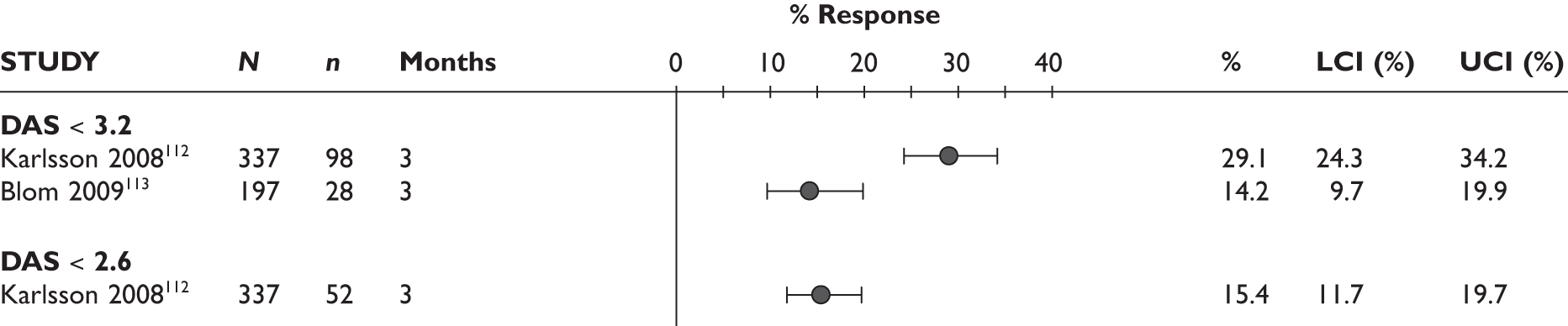
EULAR response
Three studies110,112,113 reported the percentage of patients who achieved good and good-to-moderate EULAR responses (Figure 25). The percentage of patients who achieved good EULAR response ranged from 8.6% to 22.8% at 3 months and was 9.1% at 6 months. The percentage of patients who achieved good-to-moderate EULAR response ranged from 31.5% to 64.7% at 3 months. Only one study reported good-to-moderate EULAR response at 6 months (32.5%).
FIGURE 25.
TNF inhibitors as a class: EULAR response rates. LCI, lower confidence interval; UCI, upper confidence interval.

Health Assessment Questionnaire
Only one study reported mean changes from baseline in HAQ score (Figure 26). Hyrich et al. 121–123 compared patients who discontinued TNF inhibitor within the first 12 months and did not start a subsequent TNF inhibitor or other biologic drug during the next 12 months (‘stoppers’) with patients who stopped their first TNF inhibitor within the first 12 months of therapy because of the lack of efficacy, but started a second TNF inhibitor during the subsequent 12 months (‘switchers’). The mean change in HAQ score was adjusted for differences in age, gender, disease duration, HAQ score at first failure, DAS28 at start of first TNF inhibitor and DAS28 score at first failure. ‘Switchers’ (adjusted mean change = –0.11, 95% CI –0.18 to –0.04) had significantly greater improvement in HAQ score than ‘stoppers’ (Figure 26).
FIGURE 26.
TNF inhibitors as a class: adjusted mean change from baseline in HAQ score. Adjusted for age, gender, disease duration, HAQ score at first failure, DAS28 at start of first TNF inhibitor and DAS28 score at first failure. LCI, lower confidence interval; UCI, upper confidence interval.

Quality of life
None of the studies reported QoL.
Joint damage
None of the studies reported joint damage.
Serious adverse events
Only one study reported serious AEs (Figure 27). Hjardem et al. 110 reported that 6.0% (95% CI 3.3% to 9.8%) of the patients experienced a serious AE during the study period.
FIGURE 27.
TNF inhibitors as a class: serious adverse events. LCI, lower confidence interval; UCI, upper confidence interval.
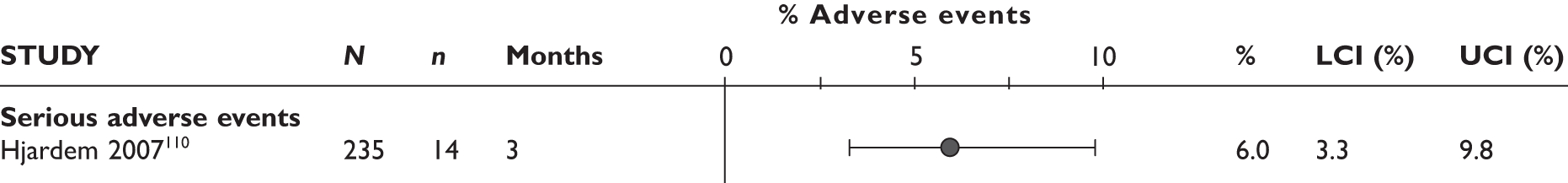
Infection and serious infection
Two studies reported infection and serious infection (Figure 28). At 3 months the percentage of patients who experienced infection ranged from 27.2% to 28.1%. One study111 reported that 13.9% (95% CI 9.1% to 19.9%) of the patients experienced serious infections at 3 months.
FIGURE 28.
TNF inhibitors as a class: infections and serious infections. LCI, lower confidence interval; UCI, upper confidence interval.
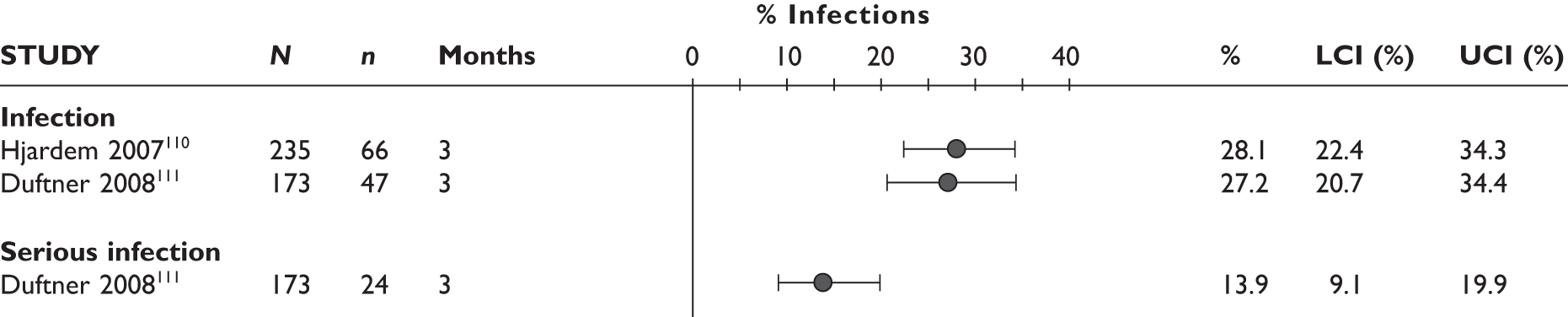
Injection/infusion reaction
None of the studies reported injection or infusion reactions.
Summary
For the assessment of effectiveness of TNF inhibitors as a class after failure of the first TNF inhibitor, one non-randomised comparative and seven uncontrolled studies were identified. Follow-up duration ranged from 3 months to 4 years. Patients included in the studies were generally similar. The main results are summarised in Table 26.
| Outcome | 3 months | 6 months | ≥ 9 months |
|---|---|---|---|
| Withdrawals (%): | |||
| ■ for any reason | 7.6 | 19.8 | 34.5–38.6 |
| ■ due to lack of efficacy | 1.5 | 9.1 | 20.3–22.6 |
| ■ due to AEs | 6.1 | 10.2 | 6.0–14.7 |
| ACR20 response (%) | 49.0 | NR | NR |
| ACR50 response (%) | 25.8 | NR | NR |
| ACR70 response (%) | 7.1 | NR | NR |
| EULAR response (%): | |||
| ■ good/moderate response | 31.5–64.7 | 32.5 | NR |
| ■ good response | 8.6–22.8 | 9.1 | NR |
| ■ remission | NR | NR | NR |
| DAS28: mean change from baseline | |||
| –1.00 to –0.86 | –0.92 to –0.88 | NR | |
| DAS28 < 3.2 (%) | 14.2–29.1 | NR | NR |
| DAS28 < 2.6 (%) | 15.4 | NR | NR |
| HAQ: mean change from baseline | NR | NR | –0.11a |
| QoL | NR | NR | NR |
| Joint damage | NR | NR | NR |
| Serious AEs | 6.0% | NR | NR |
| Any infections/serious infections (%) | 27.2–28.1/13.9 | NR | NR |
| NR | NR | ||
| Infusion reaction | NR | NR | NR |
Rituximab
Overview of evidence
Seven studies were identified that assessed RTX: one RCT [randomised evaluation of long-term efficacy of rituximab in rheumatoid arthritis (REFLEX)124–126] and six uncontrolled studies. 114–118,137,139 One of these (Finckh et al. 136,137) contained a comparative arm with an alternative TNF inhibitor; the comparative data are described in the section Evidence from comparative studies. One study116 included data from patients of whom nearly half were previously TNF inhibitor naive. Only data reported separately for those who had a previous TNF inhibitor were included in this report. In another study,118 at 6 months, 17 patients (including five who were TNF inhibitor naive at original baseline) started a second course of TNF inhibitor; data for this group of patients were excluded from the report.
Data from one cohort analysis of the REFLEX RCT extension139 and one pooled analysis of all RTX development studies from the MS are also described. The REFLEX extension139 was a long-term follow-up analysis of repeated treatment data of the original RCT: it included patients who had responded to an initial course of RTX during the RCT and received open-label treatment with the same RTX regimen for up to three repeat treatment courses. (Note: responding patients in the initial REFLEX RCT124–126 after reaching the primary end point at week 24 requiring further courses of RTX treatment entered the open-extension study.) Patients from the placebo arm of the RCT were also included and received their first course of RTX within the extension study. A total of 480 patients from the RCT (308 from the RTX arm and 172 from the placebo arm) entered the extension phase.
The manufacturer’s pooled analysis combined data from patients of the REFLEX RCT,124–126 together with data from its open-label extension study, and from other studies in manufacturer’s RTX development programme. (Note: data were pooled for patients who only received the expected licensed dose of RTX two × 1,000 mg plus MTX regimen for first and subsequent courses and who received prior TNF inhibitor therapy.) It is unclear how many patients from the REFLEX trial124–126 were included in the pooled analysis.
The Keystone et al. uncontrolled study116 also reported data for up to two treatment courses; these data are presented with those from the REFLEX extension139 and the RTX pooled analysis.
The REFLEX trial was a multicentre RCT conducted in 114 counties in the USA, Europe, Canada and Israel. Of the six uncontrolled studies, one was conducted in Switzerland, one in the UK, one in Sweden, one in the Netherlands and one in France. For the studies included in the Keystone et al. analysis,116 and for those included in the manufacturer’s pooled analysis, except the REFLEX trial,139 it is unclear in which country the studies were conducted.
Further details are provided in Table 27.
| Study | Country | Design | Reason for switching | Prior anti-TNFs (no.) | Treatment arms (n of patients) | Duration of follow-up | Comments |
|---|---|---|---|---|---|---|---|
| RCTs | |||||||
| REFLEX 124 – 126 | North America, Europe, Israel | Prospective randomised controlled parallel | Inadequate response or intolerance | Any (≥ 1) |
RTX (n = 308) PL (n = 209) |
24 weeks; 48 weeksa | Pivotal trial for anti-TNF inadequate responders |
| Uncontrolled studies | |||||||
| Bokarewa 2007114 | Sweden | Prospective uncontrolled | Lack of response | Any biologic (n unclear) | RTX (n = 48) | 12 months | Dosing schedule different from licence; not only TNF inhibitor failures; a few patients tried other biologics (anti-thymocyte globulin treatment, IL-1 receptor antagonist); 64% had experienced more than one biologic drug prior to RTX treatment |
| Jois 2007115 | UK | Prospective uncontrolled | Lack of response | Any (≥ 2) | RTX (n = 20) | 6 months |
All patients had failed at least two TNF inhibitors (10 had failed three TNF inhibitors, five also failed anakinra) Patients were offered retreatment with a second cycle of RTX if they had responded to the earlier one but flared |
| Keystone 2007116 | Unclear | Retrospective uncontrolled | Unclear | All had TNF inhibitor (n unclear) | RTX (n = 155 to 158b) | 6 monthsb | A pooled analysis of 1,039 patients who received ≥ 1 courses of RTX, 427 (41%) of whom were previously TNF inhibitor naive. 570 of these patients had ≥ 2 courses of RTX, 255 (45%) of whom were previously TNF inhibitor naive. Only data that were reported separately for those who had prior TNF inhibitor were included in this report |
| Assous 2008117,c | France | Retrospective uncontrolled | Lack of response; contraindication | Any (n unclear) | RTX (n = 50) | 6 months | 20/50 patients had contraindications to TNF inhibitors; previous exposure to TNF inhibitor treatment was not clear in these patients |
| Thurlings 2008118 | Netherlands | Prospective uncontrolled | Side effects; inefficacy | Any (= 1; > 1?) | RTX (n = 30) | 6 months | Five patients were TNF inhibitor naive; at 6 months 17 patients including the five who were TNF inhibitor naive were retreated with a second RTX course, seven patients (unclear how many of them were TNF inhibitor naive at the beginning of the study) were retreated later with a third RTX course |
| Finckh 2009136,137 | Switzerland | Prospective cohort | Inadequate response | Any (≥ 1) | RTX (n = 155) | 11 months (median) | Based on the SCQM-RA |
| REFLEX extension139 | North America, Europe, Israel | Uncontrolled retrospective | Inadequate response or intolerance | Any (≥ 1) | RTX (n = 480) | 308 were from the RTX arm and 172 were from the placebo arm. Of these, 307 received two courses, 235 received three courses, 146 received four courses and 58 received five courses | |
| RTX pooled analysis139,d | NA | Uncontrolled retrospective | NR | Any (≥ 1) | RTX | A pooled analysis of data derived primarily from REFLEX RCT124–126 and its extension study,139 and also other studies in Roche’s RTX development programme. Data included only for patients who received previous TNF inhibitor treatment and who had received the licensed dose of RTX for first and subsequent courses | |
Patient characteristics
Data on patient baseline characteristics can be found in Table 28. Patient characteristics were not reported for the manufacturer’s pooled analysis and were not reported separately for the patients who had previously received a TNF inhibitor in the Keystone et al. analysis. 116
| Study | Number of patients/% female | Age (years), mean (SD) | RA duration (years), mean (SD) | RF positive (%) | % of patients on concomitant DMARDs and steroids | Number of previous DMARDs, mean (SD) | Number of previous TNF inhibitors, mean (SD) | HAQ, mean (SD) | DAS28, mean (SD) | TJC/SJC, mean (SD) | ESR (mm/hour), mean (SD) | CRP (mg/dl), mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| REFLEX124–126 | 517/81 | 52 (12) | 12 (8) | 79 | MTX 100; steroids 63 | 2.5 (1.8) | 1.5 (0.7) | 1.90 (0.60) | 6.9 (1.0) | 34 (15)/23 (12) | 48 (27) | 3.7 (3.9) |
| Bokarewa 2007114 | 48/79 | 58 (11) | 10 (7) | 83 | MTX 77; steroids 42 | 4.2 (range 3.0–8.0) | 64% had had > 1 biologic previously | NR | 6.1 mean (range 4.0 to 7.8) | NRb | NRb | NRb |
| Jois 2007115 | 20/80 | 54 (33–80)d | 16 (5–39)d | 90 | MTX 30; steroids 60 | 3.0 (2.0–8.0)d | 2 (2–4)b | 2.60 (0.75–3.00)d | 7.2 (5.3–9.0)d | 26 (2–28)d/13 (0–26)d | 56 (14–125)d | 3.2 (0.3–17.4) |
| Keystone 2007116,e | NR | NR | NR | NR | MTX 100; steroids 100 | NR | NR | NR | NR | NR | NR | NR |
| Assous 2008117 | 50/86 | 58 (10) | 15 (9) | 90 | NR | 3.5 (1.4)c | NR | NR | 5.7 (4.2–8.7)d | NR | NR | 1.9 (0.1–29.2)d |
| Thurlings 2008118 | 24/80 | 55 (22–75)d | 12 (1–50)d | NR | MTX 100; steroids 100 | 4.0 (2.0–9.0)d | 1 (≥ 1?) (ETN; ADA; IFX) | NR | 6.5 (1.1) | NR | 37 (22–52)a | 2.9 (1.2–6.4)a |
| Finckh 2009136,137 | 155/77 | 55 (13) | 12 (9) | 88 | MTX 67; steroids 58 | NR | 2 (1–2)a | 1.60 (1.10–2.00)a | 5.0 (1.3) | NR | NR | NR |
| REFLEX extension139 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| RTX pooled analysis139 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
The number of patients included in the REFLEX RCT124–126 was 517 and ranged from 20 to 155 in the six uncontrolled studies. Where reported, characteristics of the patients included in the studies varied in some aspects, but were generally similar:
-
The percentage of female patients ranged from 77% to 86%.
-
The mean age ranged from 52 to 58 years in four studies and the median age in two studies was 54–55 years.
-
The mean disease duration ranged from 10 to 15 years in four studies and the median age in two studies was 12–16 years.
-
The percentage of RF-positive patients ranged from 79% to 90% and was lowest in the REFELX study; one study and both analyses from the MS did not report this.
-
Concomitant DMARDs were reported in five studies: 30%–100% patients were on MTX; all the patients in the REFLEX RCT124–126 were on concomitant DMARDs.
-
The proportion of patients who were receiving concurrent steroids ranged from 55% to 100%; one study did not report this.
-
The mean number of previously used conventional DMARDs reported in three studies ranged from 2.5 to 4.2 and median reported in the other two ranged from 3 to 4.
-
Where reported, the mean number of previous TNF inhibitors was 1 or greater than 1 and the median number reported in two uncontrolled studies was 2.
-
The mean baseline HAQ was reported only in the REFELX study was 1.9 and the median baseline HAQ reported in two uncontrolled studies ranged from 1.6 to 2.6.
-
Where reported, the mean DAS28 score ranged from 5.0 to 6.9 and it was the highest in the REFELX study.
-
The mean number of tender joints was 34 and swollen joints was 23 in the REFLEX trial; 124–126 the median number was 26 and 13 respectively in Jois et al. ;115 other studies did not report the baseline number of tender and swollen joints.
-
The baseline mean ESR was 48 mm/hour in REFLEX124–126 and the median value 37 mm/hour and 56 mm/hour in other two studies.
-
The mean CRP was 3.7 mg/dl in the REFELEX trial and 3.2 mg/dl in another study; median CRP was 1.9 and 2.9 in the other two studies.
Quality assessment
Randomised controlled trial
The only RCT (REFLEX124–126) was of good quality. Full details of the quality assessment are reported in Table 29. Randomisation was appropriate and allocation concealment was not described in the paper. Patients and outcome assessors were blinded. It was not clear if data analysts were aware to which group patients were assigned. Withdrawal rate from the RTX group and the placebo group was 18% and 46%, respectively, at week 24, and 63% and 89%, respectively, at week 48. ITT analysis was not used, as 21 patients were excluded from analysis owing to protocol violations.
| Study | Was method of randomisation appropriate? | Was allocation adequately concealed? | Blinding | Patients withdrawn (%) | Was ITT used? | Comments | ||
|---|---|---|---|---|---|---|---|---|
| Patients | Investigators/outcome assessors | Data analysts | ||||||
| REFLEX124–126 | Yes | Uncleara | Yes | Yesb | Unclear |
Week 24: RTX 18; placebo 46 Week 48:c RTX 63; placebo 89 |
Yesd | Twenty-one of the randomised patients were excluded from the ITT populationd |
Non-randomised controlled trials
All the non-RCTs were uncontrolled; four of these were prospective and two were retrospective. Full details of the quality assessment are reported in Table 30. All stated clearly their inclusion criteria; however, only in one study was it clear that consecutive patients were included. The percentage of patients withdrawn reported in one study was 25% (at 6 months), the percentage was unclear in two studies and was not applicable in two retrospective studies as only patients with follow-up assessment were included.
| Study (duration of follow-up) | Study design | Inclusion criteria clearly defined? | Were consecutive patients included in the study? | Patients withdrawn (%) | Comments |
|---|---|---|---|---|---|
| Bokarewa 2007114 | Prospective uncontrolled | Yes | Unclear | NR | |
| Jois 2007115 | Prospective uncontrolled | Yes | Unclear | 25% at 6 months | |
| Keystone 2007116 | Retrospective uncontrolled | Yes | NR | NA | |
| Assous 2008117 | Retrospective uncontrolled | Yes | Yes | Unclear | |
| Thurlings 2008118 | Prospective uncontrolled | Yes | NR | Unclear | Unclear for those who had subsequent courses at what time point the outcomes were assessed |
| Finckh 2009136,137 | Prospective uncontrolled | Yes | NR | NA (only those with follow up assessment were included) | |
| REFLEX extension139 | Prospective uncontrolled | Yes | NA | NA | |
| RTX pooled analysis139 | Retrospective uncontrolled | Unclear | NR | NR |
REFLEX extension and rituximab pooled analyses
Although some inclusion criteria were stated, in both analyses information on the study characteristics, patient characteristics and methodological appropriateness was insufficient, in particular in the pooled analysis. Details of the quality assessment are reported in Table 30.
Results
Tables 31 and 32 present what outcomes were measured in the studies. Outcomes in Table 31 are reported and described in the main text of this report and those in Table 32 are reported in Appendix 10 only. Outcome data from the RTX arm in the RCT are also included in the section on uncontrolled studies for comparison purposes. As data from the REFLEX extension cohort139 and the RTX pooled analyses were analysed according to RTX treatment courses, the results of these analyses are described separately from the results of the uncontrolled studies.
| Study | Total withdrawal | Withdrawal by reason | ACR (20/50/70) | DAS28 | EULAR response | HAQ | QoL | Joint damage | Serious AEs | Infection/serious infection | Injection/infusion reaction |
|---|---|---|---|---|---|---|---|---|---|---|---|
| REFLEX 124 – 126 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Bokarewa 2007114 | ✓ | ✓ | |||||||||
| Jois 2007115 | ✓ | ✓ | ✓ | ||||||||
| Keystone 2007116 | ✓ | Reported graphically | ✓ | ✓ | |||||||
| Assous 2008117 | ✓ | ✓ | |||||||||
| Thurlings 2008118 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Finckh 2009136,139 | ✓ | ✓ | |||||||||
| REFLEX extension139 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| RTX pooled analysis139 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Study | Other measures of disease activity | Fatigue | Pain | TJC/SJC | CRP/ESR |
|---|---|---|---|---|---|
| REFLEX 124 – 126 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Bokarewa 2007114 | Reported graphically | Reported graphically | |||
| Jois 2007115 | ✓ | ✓ | ✓ | ||
| Keystone 2007116 | |||||
| Assous 2008117 | ✓ | ||||
| Thurlings 2008118 | |||||
| Finckh 2009136,137 | ✓ | ✓ | |||
| REFLEX extension139 | |||||
| RTX pooled analysis139 |
Withdrawals
Withdrawal rates are presented in Figure 29. At week 24, there were significantly fewer withdrawals for any reason in the RTX arm than in the placebo arm of the REFLEX RCT124–126 (RR = 0.39, 95% CI 0.29 to 0.51). Risk of withdrawal because of AEs tended to be higher in the RTX than in the placebo group; however, the difference was not statistically significant (RR = 2.71, 95% CI 0.58 to 12.65).
Withdrawal rate for any reason at 6 months was reported in only one uncontrolled study115 and it was 10%. For comparison, 17.9% of patients in the RTX arm in the REFLEX RCT124–126 withdrew at 6 months for any reason and 2.6% withdrew because of AEs (Figure 30). In one study114 the total number of patients withdrawn by reason was not reported, but it was stated that one patient discontinued RTX treatment after a second infusion (week 4) because of severe headache and stomach pain. Two patients who had a medical history of chronic myocardial ischaemia died of myocardial infarction, one within the first month and the other at 13 months.
FIGURE 30.
Rituximab: withdrawals in uncontrolled studies by reason. LCI, lower confidence interval; UCI, upper confidence interval.

ACR20
In the REFLEX trial,124–126 the percentage of patients who achieved ACR20 response at week 24 in the RTX group was nearly three times that in the placebo group and the difference was statistically significant (RR = 2.85, 95% CI 2.08 to 3.91). At week 48, the response rate based on observed data (of a smaller number of patients) favoured the RTX group, but the difference was not significant (RR = 1.53, 9% CI 0.84 to 2.76); when analysed based on non-responder imputation data, the response rate in the RTX group was nearly five times of that in the placebo group and the difference was significant (RR = 4.92, 95% CI 2.40 to 10.09). Details can be found in Figure 31.
In the Keystone et al. 116 pooled analysis, 24 weeks after the first course of RTX, 65.2% patients had an ACR20 response, while in the RTX arm of the REFLEX trial124–126 the figure was 51% (Figure 32). None of the other uncontrolled studies reported ACR20 responses.
FIGURE 32.
Rituximab: ACR20 response in cohorts 24 weeks after first course of RTX. LCI, lower confidence interval; UCI, upper confidence interval.
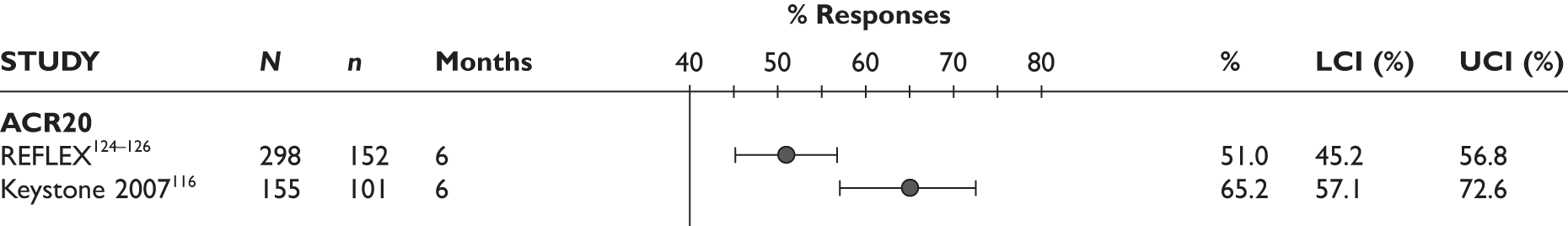
ACR50
At week 24 in the REFLEX trial,124–126 the percentage of ACR50 responders in the RTX group was nearly five and a half times that of the placebo group and the difference was statistically significant (RR = 5.40, 95% CI 2.87 to 10.16). The effect persisted at week 48, analysed based on either observed data (RR = 4.11, 95% CI 1.06 to 15.85) or non-responder imputation data, and based on non-responder imputation data the response rate in the RTX group was over 13 times that of the placebo group (RR = 13.23, 95% CI 3.23 to 54.20). Details are presented in Figure 33.
In the Keystone et al. 116 pooled analysis, 24 weeks after the first course of RTX, ACR50 response was observed in 32.9% patients, while in the RTX arm of the REFLEX trial124–126 it was 26.8% (Figure 34). None of the other uncontrolled studies reported ACR50 response.
FIGURE 34.
Rituximab: ACR50 response in uncontrolled studies 24 weeks after the first course of RTX. LCI, lower confidence interval; UCI, upper confidence interval.
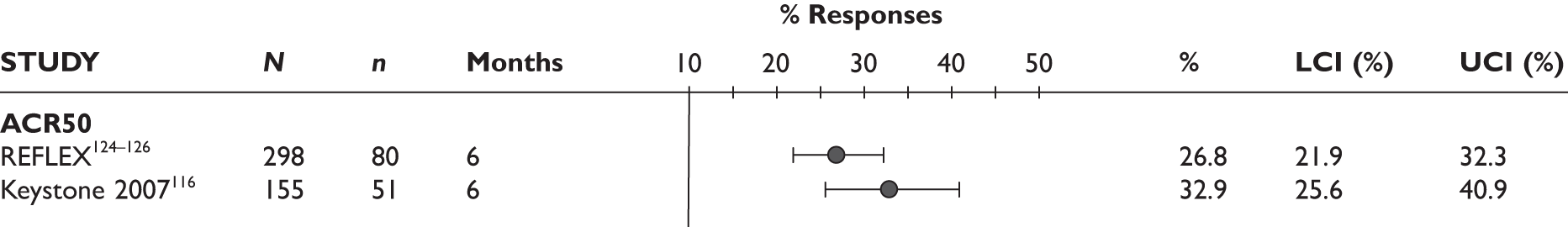
ACR70
At week 24 the percentage of patients achieving ACR70 response in the RTX group in the REFLEX trial124–126 was over 12 times of that of the placebo group and the difference was statistically significant (RR = 12.14, 95% CI 2.96 to 49.86). At week 48 the beneficial effect of RTX was not significant based on observed data for a much smaller patient group (RR = 3.37, 95% CI 0.47 to 24.2), but was significant based on non-responder imputation data (RR = 10.86, 95% CI 1.45 to 81.24). See Figure 35 for details.
In the Keystone et al. pooled analysis the percentage of ACR70 responders 24 weeks after the first course of RTX was 12.3%; it was similar to that reported in the RTX arm of the REFLEX trial124–126 (12.1%) (Figure 36). No other uncontrolled study reported ACR70 responses.
FIGURE 36.
Rituximab: ACR70 response in uncontrolled studies 24 weeks after the first course of RTX.
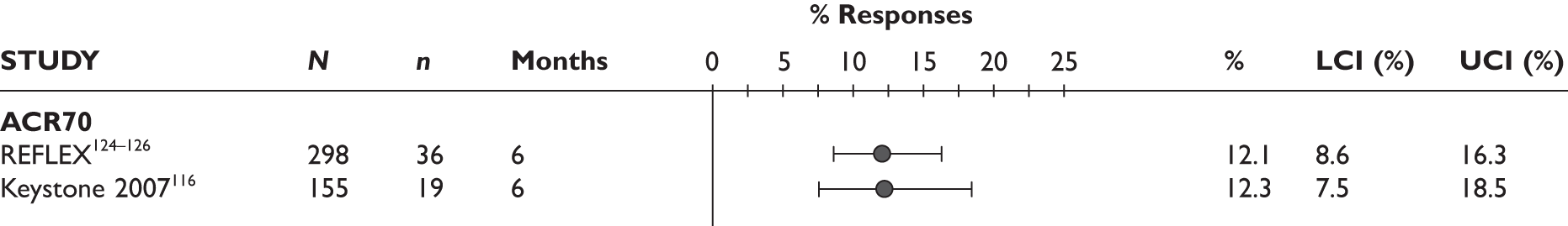
EULAR response
EULAR responses are presented in Figures 37 and 38. In the REFLEX trial,124–126 at week 12 the percentage of patients achieving good or moderate response in the RTX group was over twice that of the placebo group, as was the percentage achieving a good response; the effects were statistically significant (RR = 2.02, 95% CI 1.64 to 2.49 and RR = 2.23, 95% CI 1.12 to 4.41, respectively). At week 24 the percentage of patients achieving a EULAR good or moderate response in the RTX group was nearly three times that of the placebo group and the effect was significant (RR = 2.96, 95% CI 2.25 to 3.89); the rate of achieving a good response was also higher in the RTX group and the difference was statistically significant (RR = 7.59, 95% CI 2.77 to 20.77).
None of the uncontrolled studies reported EULAR response at 3 months, whereas three reported it at 6 months. At 3 months in the RTX arm of the REFLEX RCT,124–126 68.5% of patients had moderate or good response, with 11.1% having achieved a good response; at 6 months the rates remained similar (64.8% and 15.1%, respectively). At 6 months the percentage of good or moderate EULAR responders in four uncontrolled studies including the RTX arm of the REFLEX trial124–126 ranged from 64.8% to 82%, and the good response rate ranged from 15.1% to 36%. The REFLEX trial124–126 had the lowest percentage of responders in both categories. One study also reported EULAR low disease activity and remission at 6 months (13.3% and 5.7%, respectively). See Figure 39 for details.
DAS28
In the REFLEX trial,124–126 at week 24, the RTX arm had a significantly smaller mean DAS28 score and significantly greater reduction in the mean DAS28 score from baseline than the placebo arm (–1.40, 95% CI –1.67 to –1.13, and – 1.50, 95% CI –1.74 to –1.26, respectively). At week 24, the proportion of patients with DAS28 improvement in the RTX group was over five times that in the placebo group and the difference was statistically significant. See Figures 40–42 for details.
DAS28 score at 3 months was available in only one uncontrolled study (median DAS28 = 5.60). DAS28 score at 6 months was measured in three studies. The mean score was 5.0 in one study and it was the same as that of the RTX arm of the REFLEX trial. 124–126 Two studies provided a median score and it was 5.50 and 3.97. See Figure 43 for details. (Note: for the Jois et al. 115 and Assous et al. 117 studies scores were reported as medians.)
FIGURE 43.
Rituximab: mean DAS28 in uncontrolled studies. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval. Bold type indicates that the study was an RCT.
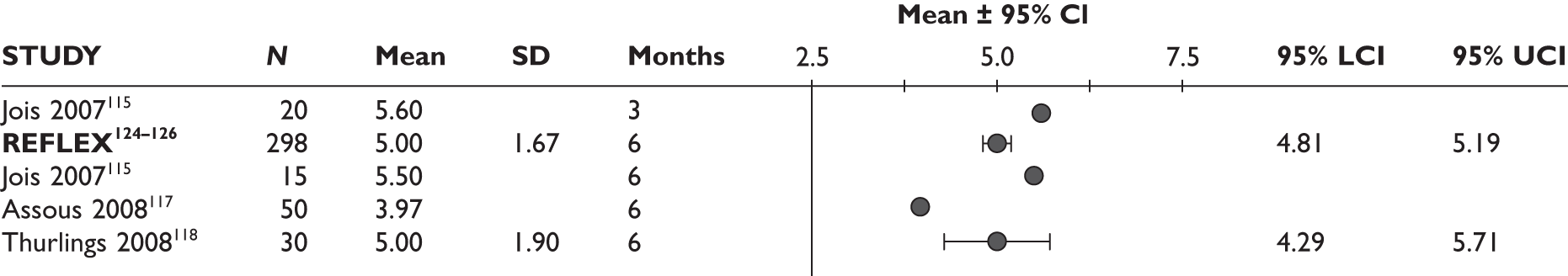
In Finckh et al. 136 the change in mean DAS28 score from baseline at 6 months was reported only for a subgroup of 50 patients. It was similar to that reported for the RTX arm of the REFLEX trial124–126 and both showed significant improvement (–1.90, 95% CI –2.08 to –1.72, and –1.61, 95% CI –1.98 to –1.24, respectively). See Figure 44 for details.
Health Assessment Questionnaire
In the REFLEX trial,124–126 the RTX group had significantly more reduction in mean HAQ score from baseline at week 24 compared with the placebo group (mean difference = –0.30, 95% CI –0.40 to –0.20; Figure 45).
The percentage of patients who showed HAQ improvement, defined as a decrease in score from baseline of greater than 0.25, in the RTX group of the REFLEX trial124–126 was nearly twice that of the placebo group at week 12, and over two and a half times as high at week 24; both effects were statistically significant (RR = 1.63, 95% CI 1.29 to 2.07, and RR = 2.55, 95% CI 1.89 to 3.43, respectively). See Figure 46 for details.
At week 24, the observed percentage of patients with minimal clinically meaningful improvement in HAQ, defined as a decrease in HAQ score of 0.22, in the RTX group of the REFLEX trial,124–126 was over 1.6 times that of the placebo groups and the difference was significant; whereas observed at week 48 there was no significant difference (Figure 47).
FIGURE 47.
Rituximab: percentage of patients with a clinically meaningful improvement in HAQ, 24 and 48 weeks after the first course of RTX (observed data from MS).
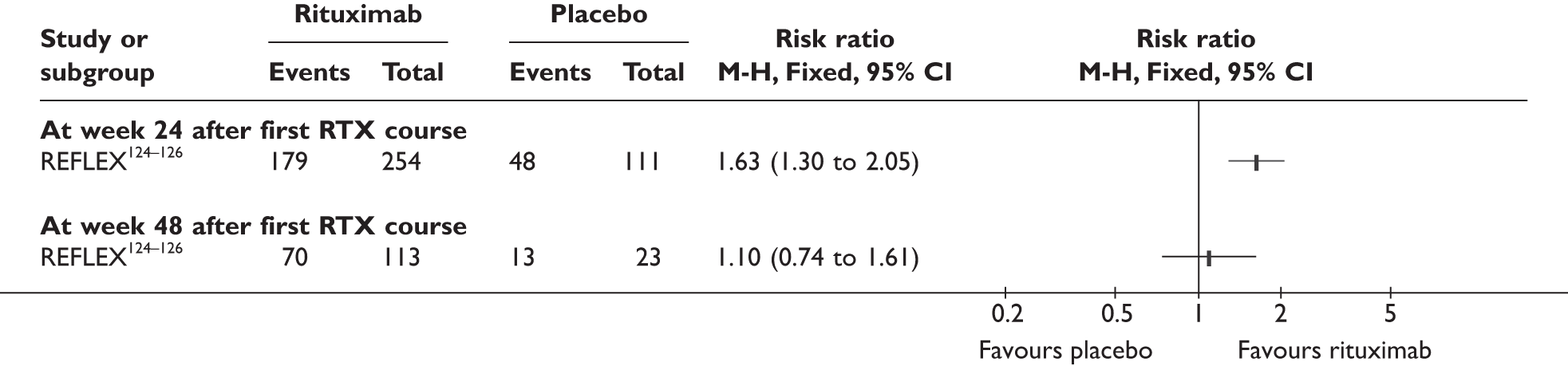
When analysed based on non-responder imputation data, the percentage of patients with minimal clinically meaningful improvement in HAQ at week 24 and week 48 in the RTX group was over two and a half and over three and a half times that of the placebo group (58% vs 23% and 23% vs 6%, respectively) and both differences were statistically significant (Figure 48).
FIGURE 48.
Rituximab: percentage of patients with a clinically meaningful improvement in HAQ, 24 and 48 weeks after the first course of RTX (non-responder imputation data from MS).

Two uncontrolled studies reported HAQ score. The median HAQ score in one study115 was 2.13 (range 0.63–2.88) at 3 months and decreased to 1.86 (range 1–3) at 6 months; however, in both cases, the reduction compared with baseline was not significant. In the Keystone et al. study,116 the percentage of patients with a decrease in the mean HAQ score of greater than or equal to 0.22 from baseline at week 24 (after one course of RTX treatment) was 71.8%, which is very similar to the observed rate reported in the RTX arm of the REFLEX trial124–126 (70.5%) (Figure 49).
FIGURE 49.
Rituximab: percentage of patients with clinically meaningful improvement in HAQ score from baseline at week 24. LCI, lower confidence interval; UCI, upper confidence interval. Bold type indicates that the study was an RCT.
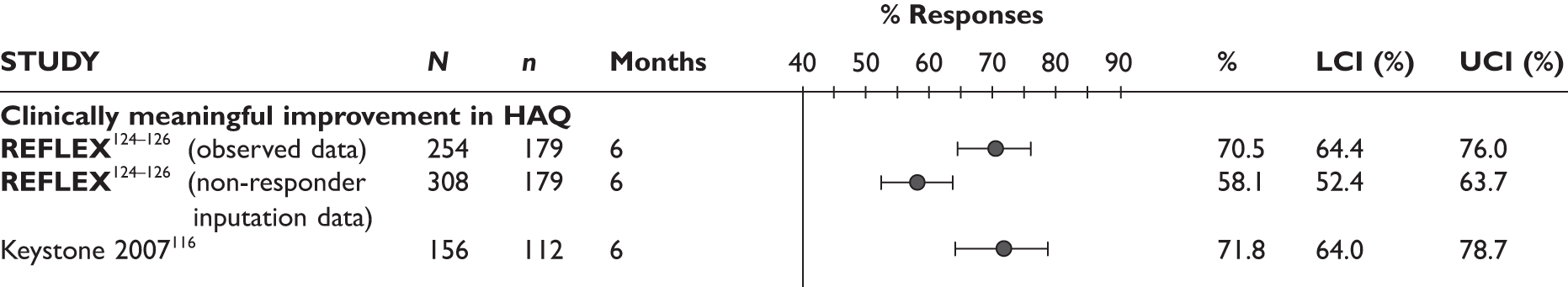
Joint damage
The RTX group of the REFLEX trial124–126 had significantly less changes in Sharp-Genant total score from baseline than the placebo group at both week 56 (mean difference = –1.12, 95% CI –2.13 to –0.11) and week 104 (mean difference = –1.67, 95% CI –2.67 to –0.67). At week 56 the percentage of patients with no worsening of Sharp-Genant total score from baseline in the RTX group was nearly one and a half times that in the placebo group and the difference was statistically significant. Sharp-Genant total score measured at week 104 favoured the RTX group but the difference was not statistically significant (mean difference = –3.53, 95% CI –9.21 to 2.15). See Appendix 10 for details.
There was significantly less change from baseline in the erosion score in the RTX group than in the placebo group at week 56 (mean difference = –0.75, 95% CI –1.43 to –0.07), and at week 104 the significant difference became larger (mean difference = –1.08, 95% CI –1.73 to –0.43). The erosion score at week 104 favoured the RTX arm, but the difference was not statistically significant (mean difference = –2.48, 95% CI–5.55 to 0.59). The percentage of patients with no erosive progression from baseline at week 104 in the RTX group was nearly one and a half times that of the placebo group and the difference was statistically significant (RR = 1.38, 95% CI 1.14 to 1.66).
Joint space narrowing score change from baseline was smaller in the RTX group than in the placebo group both at week 56 and week at 104; the difference was not statistically significant at week 56 but became significant at week 104, though at week 104 the joint space narrowing score was not significantly lower in the RTX group than in the placebo group.
Non-randomised controlled trials
None of the uncontrolled studies reported joint damage.
Quality of life
Mean Short Form questionnaire-36 items (SF-36) mental and physical health scores measured at week 24 in the REFLEX trial124–126 were both significantly higher in the RTX group than in the placebo group (Figure 50). The RTX group increased mean SF-36 physical health score by 5.16 and mean SF-36 mental health score by 3.07 higher than in the placebo group, and the differences were statistically significant (Figure 51).
None of the uncontrolled studies reported QoL.
Serious adverse events
In the REFLEX trial,124–126 the percentage of patients with serious AEs was lower in the RTX group than in the placebo group; the difference was not statistically significant (RR = 0.74, 95% CI 0.42 to 1.31). See Figure 52 for details.
In one 12-month study,114 one patient (2%) had severe headache and stomach pain 1 day after RTX infusion and this led to a discontinuation of treatment. A 6-month study,115 stated that no major side effects were found during the study. During a 6-month period the Thurlings et al. 118 study reported five serious AEs (16.7%): two severe infusion reactions, one arterial embolism, one pulmonary embolism and one toxic hepatitis. The other studies did not report information on serious AEs.
Any infection/serious infection
In the REFLEX trial124–126 both the percentage of patients with any infections and the percentage of patients with serious infections were greater in the RTX group than in the placebo group; however, none of the differences was statistically significant (RR = 1.08, 95% CI 0.87 to 1.35 and RR = 1.58, 95% CI 0.41 to 6.05, respectively). See Figure 53 for details.
In the Bokarewa et al. study114 3 months after the treatment with RTX, pneumonia requiring hospitalisation was reported in one patient (2.0%). In Thurlings et al. 118 the incidence of infection per patient-year was 0.9: 48 infections requiring antibiotic, antimycotic, or antiviral treatment and one serious infection requiring i.v. antibiotics occurred among 30 patients over 2 years of follow-up. One serious infection requiring i.v. antibiotics was observed in this study.
Injection site reaction/infusion reaction
In the REFLEX trial,124–126 the percentage of patients with acute infusion reactions did not differ significantly between groups (RR = 1.24, 95% CI 0.90 to 1.83, for the first course and RR = 0.74, 95% CI 0.43 to 1.24, for the second course). See Figure 54 for details.
FIGURE 54.
Rituximab: percentage of patients who had acute infusion reactions after the first and second infusion.
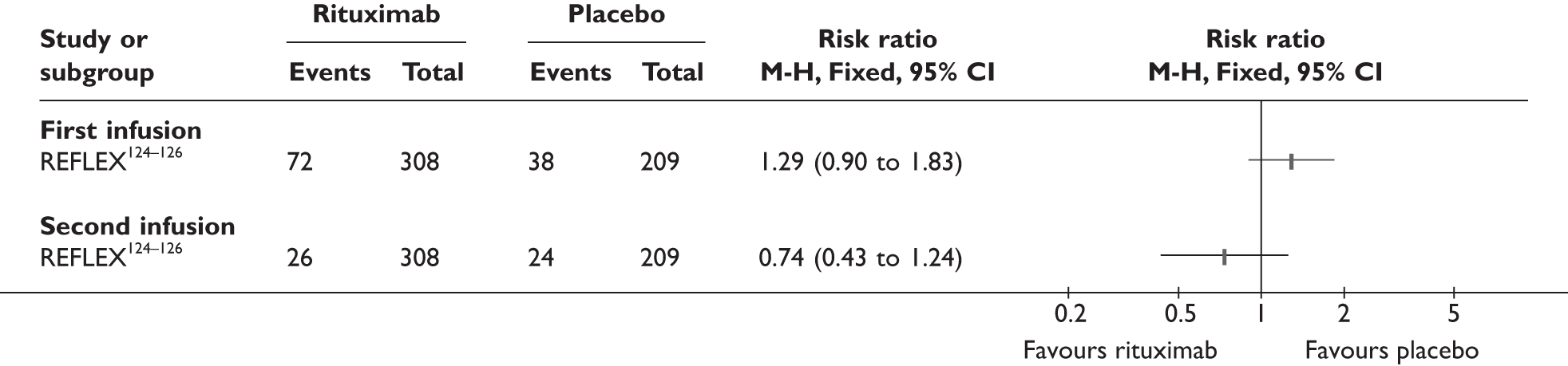
Data reported by treatment course
Pooled analysis (data from Keystone et al.)
In the Keystone et al. study,116 based on evaluable data, the percentage of patients achieving ACR responses increased from course 1 to course 2 of RTX measured 24 weeks after each course (Figure 55). A similar pattern was seen for the percentage of patients with EULAR response 24 weeks after course 1 and course 2 (Figure 56).
FIGURE 55.
Percentage of patients achieving ACR responses 24 weeks after course 1 and course 2 – based on evaluable patients who had prior TNF inhibitor. LCI, lower confidence interval; UCI, upper confidence interval.
FIGURE 56.
Percentage of patients with EULAR responses 24 weeks after course 1 and course 2 – based on evaluable patients who had prior TNF inhibitor. LCI, lower confidence interval; UCI, upper confidence interval.
The percentage of patients who achieved meaningful improvement in HAQ, i.e. had a decrease of HAQ scores at least 0.22 from baseline, were similar 24 weeks after course 1 and course 2 of RTX treatment (Figure 57).
FIGURE 57.
Percentage of patients with a decrease in HAQ score of ≥ 0.22 at week 24 after course 1 and course 2 – based on evaluable patients who had prior TNF inhibitor. LCI, lower confidence interval; UCI, upper confidence interval.
Data from manufacturer’s submission
Data analysis based on the MS can be found together with all additional analyses in Appendix 10.
Summary
For the assessment of effectiveness of RTX in comparison with standard care, one RCT and six uncontrolled studies were identified. Follow-up duration ranged from 3 months to 24 months. Patients included in the studies were generally similar. The main results of the seven studies are summarised in Table 33.
| Outcome | RCT [result (95% CI)] | Uncontrolled studies | ||
|---|---|---|---|---|
| 6 months (RTX vs placebo) | 6 months (RTX arm) | 3 months | 6 months | |
| Withdrawals (%): | ||||
| ■ for any reason | RR = 0.39 (0.29 to 0.51), favours RTX | 17.9 | NR | 10.0 |
| ■ due to lack of efficacy | NR | NR | NR | NR |
| ■ due to AEs | RR = 2.71 (0.58 to 12.65), NS | 2.6 | NR | NR |
| ACR20 response (%) | RR = 2.85 (2.08 to 3.91), favours RTX | 51.0 | NR | 65.2 |
| ACR50 response (%) | RR = 5.40 (2.87 to 10.16), favours RTX | 26.8 | NR | 32.9 |
| ACR70 response (%) | RR = 12.14 (2.96 to 49.86), favours RTX | 12.1 | NR | 12.3 |
| EULAR response (%): | ||||
| ■ good and moderate response | RR = 2.96 (2.25 to 3.89), favours RTX | 64.8 | NR | 73.3–82.0 |
| ■ good response | RR = 0.76 (0.52 to 1.12), NS | 15.1 | NR | 16.7–36.0 |
| DAS28: mean change from baseline | Mean difference = –1.40 (–1.67 to –1.13), favours RTX | –1.90 | NR | –1.61 |
| HAQ: mean change from baseline | Mean difference = –0.30 (–0.40 to –0.20), favours RTX | –0.40 | NR | NR |
| Patients with an improvement in HAQ > 0.25 from baseline (%) | RR = 2.55 (1.89 to 3.43), favours RTX | 50.7 | NR | 71.8 |
| Joint damage (Sharp–Genant total score) | Mean difference (week 56) = –1.12 (–2.13 to –0.11), favours RTX |
0.66 (week 56) |
NR | NR |
| QoL | ||||
| Change from baseline in SF-36 physical health score | Mean difference = 5.16 (3.74 to 6.58), favours RTX | 6.64 | NR | NR |
| Change from baseline in SF-36 mental health score | Mean difference = 3.07 (0.87 to 5.27) | 5.32 | NR | NR |
| Serious AEs (%) | RR = 0.74 (0.42 to 1.31), NS | 7.5 | NR |
0–16.7 (2% for 12 months) |
| Any infections (%) | RR = 1.08 (0.87 to 1.35), NS | 40.9 | NR | Infections (requiring antibiotic, antimycotic or antiviral treatment) per patient-year = 0.9 (over 2 years) |
| Serious infections (%) | RR = 1.58 (0.41 to 6.05), NS | 2.3 | 2 | NR |
| Infusion reaction (%) | ||||
| First infusion reaction | RR = 1.29 (0.90 to 1.83), NS | 23.4 | NR | NR |
| Second infusion reaction | RR = 0.74 (0.43 to 1.24), NS | 8.4 | NR | NR |
Abatacept
Overview of evidence
Three studies were identified that assessed ABT: one RCT [abatacept trial in treatment of anti-TNF inadequate responders (ATTAIN127–132)], an extension of this RCT (ATTAIN LTE119) and an uncontrolled study [abatacept researched in rheumatoid arthritis patients with an inadequate anti-TNF response to validate effectiveness (ARRIVE)120].
Patients were included in the ATTAIN LTE119 after completing 6 months of the RCT. It was reported that in total 74.4% of the placebo group and 86.4% of the ABT group were included in the extension.
Patients in the studies were non-responders to at least one TNF inhibitor. In the ATTAIN RCT127–132 and LTE119 lack of efficacy was the primary reason for switching biologic agents. In ARRIVE120 patients discontinued the previous TNF inhibitor because of lack of efficacy, safety concerns or intolerability.
All studies were carried out in North America and Europe. ARRIVE120 additionally included Mexican patients. No information was provided if these studies included UK patients. Follow-up was 6 months for the ATTAIN RCT127–132 and ARRIVE study. 120 In the ATTAIN LTE119 patients were followed up for up to 5 years; however, there was no published data beyond 2 years. Further details are provided in Table 34.
| Study | Country | Design | Reason for switching | Prior TNF inhibitors; n | Treatment arms (no. of patients) | Duration of follow-up | Comments |
|---|---|---|---|---|---|---|---|
| RCTs | |||||||
| ATTAIN 127 – 132 | North America and Europe | Parallel prospective | Primarily lack of efficacy | Any; 1–2 |
ABT (258) PL (133) |
6 months | |
| Non-randomised comparative studies | |||||||
| None were identified | |||||||
| Uncontrolled studies | |||||||
| ATTAIN LTE119 | North America and Europe | Uncontrolled prospective LTE of RCT | Primarily lack of efficacy | Any; 1–2 | ABT (317) | Up to 5 years | Some patients have not yet completed the 5-year follow-up; published data only up to 2 years; data beyond that from MS |
| ARRIVE120 | USA, EU, Mexico | Uncontrolled prospective | Lack of efficacy, safety, intolerability | Any; 1–3 | ABT (1,046) | 6 months | Two main subgroups: patients switched to ABT after a washout period and those who switched directly |
Patient characteristics
Full details of patient characteristics are reported in Table 35.
The number of patients included in the studies was 391 in the ATTAIN RCT,127–132 317 in its LTE119 and 1,046 in the ARRIVE study. 120 Patient characteristics were generally similar across studies and study arms:
| Study | Number of patients/% female | Age (years), mean (SD) | RA duration (years), mean | RF positive (%) | % of patients on concomitant DMARDs and steroids | Number of previous DMARDs, mean (SD) | Number of previous TNF inhibitors, mean (SD) | HAQ, mean (SD) | DAS28, mean (SD) | TJC/SJC, mean (SD) | ESR (mm/hour), mean (SD) | CRP (mg/dl), mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATTAIN 127 – 132 | 391/78 | 53.2 (12.0) | 11.9 (8.6) | 73.2 | MTX (77.8); HCQ (8.9); LEF (8.7); sulfasalazine (8.0); corticosteroids (68.3) | NR | 1–2 | 1.8 (0.6) | 6.5 (0.9) | 31.7 (13.1) of 68/22.2 (10.1) of 66 | NR | 4.4 (3.9) |
| ATTAIN LTE119 | 317/78 | 53.0 (11.7) | 11.8 (8.6) | NR | Continued MTX, DMARDs and corticosteroids allowed | NR | 1–2 | 1.8 (0.6) | 6.5 (0.8) | 31.8 (13.4)/22.3 (10.4) | NR | 4.2 (3.7) |
| ARRIVE120 | 1,046/81 | 54.4 (12.4) | 11.6 (9.5) | 61.3 | MTX (69.8), AZA (4.1), GST (0.5), HCQ/chloroquine (15.0), LEF (12.8), sulfasalazine (8.8), corticosteroids (58.4) | NR | 1–3 | 1.7 (0.6) | 6.2 (0.7) | 17.8 (6.0)/13.6 (5.5) | NR | 2.1 (3.0) |
-
The percentage of female patients ranged from 78% to 81%.
-
The mean age ranged from 53.0 to 54.4 years.
-
The mean disease duration ranged from 11.6 to 11.9 years.
-
In two studies the percentage of RF-positive patients ranged from 61.3% to 73.2%; it was not reported in the ATTAIN LTE. 119
-
Concomitant DMARDs were reported in detail in ATTAIN127–132 and ARRIVE:120 69.8%–77.7% patients were on MTX; other DMARDs included HCQ (8.9%–15.0%), LEF (8.7%–2.8%) and sulfasalazine (8.0%–8.8%). In the ARRIVE study,120 AZA (4.1%) and gold (0.5%) were also used.
-
In two studies 58.4%–68.3% of patients were receiving corticosteroids; this information was not reported in detail in the ATTAIN LTE. 120
-
The number of previously used conventional DMARDs was not reported in any of the studies.
-
The number of previous TNF inhibitors ranged from one to two in the ATTAIN127–132 and ATTAIN LTE119 studies and from one to three in the ARRIVE study. 120
-
The mean baseline HAQ ranged from 1.7 to 1.8.
-
The mean DAS28 score ranged from 6.2 to 6.5.
-
The mean number of tender and swollen joints ranged from 17.8 to 31.8 and from 13.6 to 22.3, respectively.
-
Baseline ESR was not reported in any of the studies.
-
CRP ranged from 2.1 mg/dl to 4.4 mg/dl.
Quality assessment
The only RCT (ATTAIN127–132) was of high quality. Full details of the quality assessment are reported in Table 36. Randomisation and allocation concealment were appropriate. Patients and investigators/outcome assessors were blinded. It was not clear if data analysts knew to which group patients were assigned. A total of 13.6% of patients were withdrawn from the ABT group and 25.6% from the placebo group. ITT analysis was not used, as only data from patients who received at least one dose of the study drug were analysed. Two patients were excluded from analysis because of protocol violations, possibly post hoc. The potential impact on the results is likely to be small.
| Study | Was randomisation appropriate? | Was allocation adequately concealed? | Blinding | Patients withdrawn (%) | Was ITT used? | Comments | ||
|---|---|---|---|---|---|---|---|---|
| Patients | Investigators/outcome assessors | Data analysts | ||||||
| ATTAIN 127 – 132 | Yes | Yes | Yes | Yes | Unclear | ABT 13.6; PL 25.6 | No; modified ITT used (patients who were given at least one dose of the drug) | Two patients excluded from analysis because of protocol violation |
Both non-randomised studies were uncontrolled and prospective. Full details of the quality assessment are reported in Table 37. Both studies stated clearly their inclusion criteria; however, it was not clear if consecutive patients were included in ARRIVE. 120 The percentage of patients withdrawn from the study was 18% in the ARRIVE study120 at 6 months and 30% in the ATTAIN LTE119 at 2 years.
| Study | Study design | Inclusion criteria clearly defined? | Were consecutive patients included in the study? | Patients withdrawn (%) | Comments |
|---|---|---|---|---|---|
| ATTAIN LTE119 | Uncontrolled long-term open-label extension of RCT | Yes | NA | 30 | Data for 2-year follow-up |
| ARRIVE120 | Uncontrolled prospective | Yes | Unclear | 18 |
Results
The RCT and non-randomised studies were analysed separately. Data from the ABT arm of the ATTAIN RCT are included in all figures referring to uncontrolled studies for comparison.
Table 38 indicates which of the outcomes reported in the main text of the report were assessed in individual studies and Table 39 provides similar information for outcomes described in Appendix 10 only.
| Study | Total withdrawal | Withdrawal by reason | ACR (20/50/70) | DAS28 | EULAR response | HAQ | QoL | Joint damage | Serious AEs | Infection/serious infection | Injection/infusion reaction |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ATTAIN 127 – 132 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| ATTAIN LTE119 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| ARRIVE120 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Study | Other measures of disease activity | Fatigue | Pain | TJC/SJC | CRP/ESR |
|---|---|---|---|---|---|
| ATTAIN 127 – 132 | ✓ | ||||
| ATTAIN LTE119 | ✓ | ✓ | ✓ | ||
| ARRIVE120 |
Withdrawals
There were significantly fewer withdrawals for any reason in the ABT arm than in the placebo arm of the ATTAIN RCT127–132 (RR = 0.53, 95% CI 0.35 to 0.81). There were also significantly fewer withdrawals in the ABT group because of lack of efficacy (RR = 0.27, 95% CI 0.15 to 0.49). The risk of withdrawal because of AEs was similar in both groups (RR = 0.93, 95% CI 0.32 to 2.71). Details of the analysis are presented in Figure 58.
At 6 months 17.8% patients withdrew from the ARRIVE study. 120 This percentage was slightly higher than in the ABT-treated arm of the RCT. At 2 years, 30% of patients had withdrawn from the ATTAIN LTE. 119 In both studies, more patients withdrew because of lack of efficacy than because of AEs. A similar relationship was observed in the ABT arm of the RCT. Full details are presented in Figure 59.
FIGURE 59.
Abatacept: withdrawals in uncontrolled studies by reason. LCI, lower confidence interval; UCI, upper confidence interval. Bold type indicates that the study was an RCT.
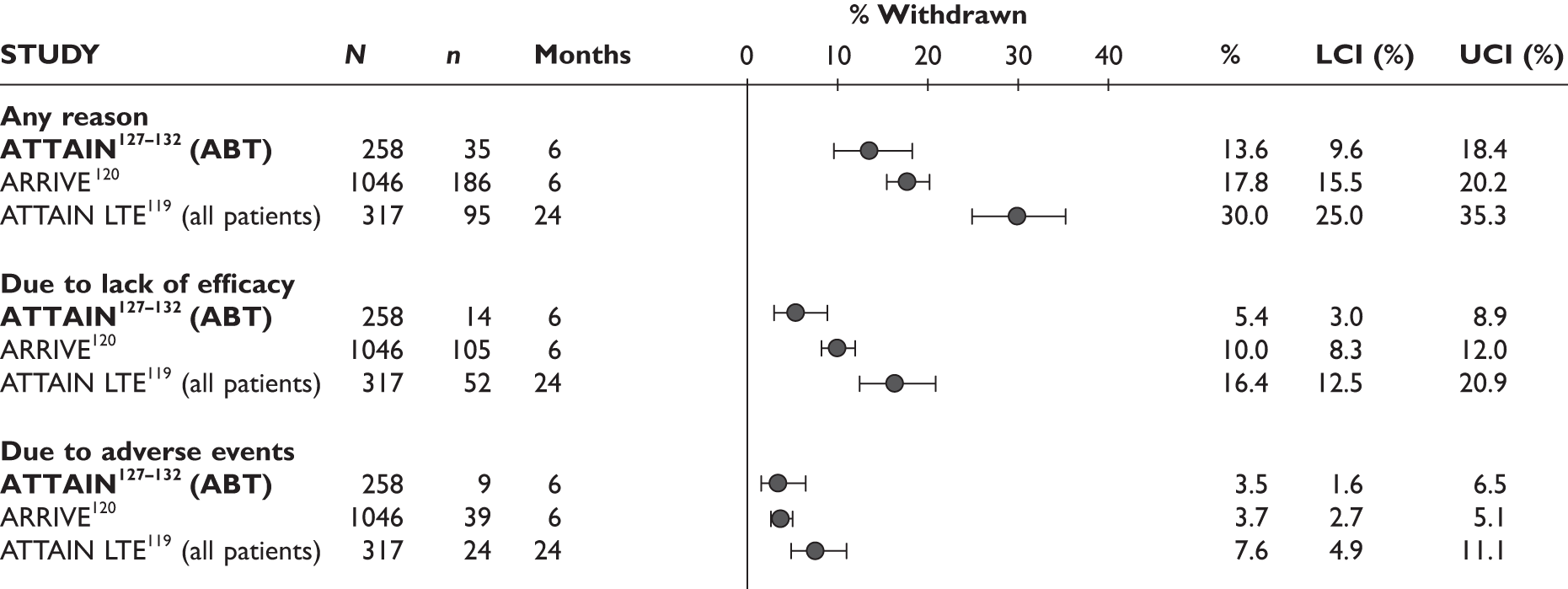
ACR20 response
ATTAIN127–132 reported ACR20 response at 3 and 6 months. At both follow-up times the risk of an ACR20 response was over two and a half times higher in the ABT group than in the placebo group and the difference was statistically significant (for 3 months, RR = 2.53, 95% CI 1.72 to 3.73; for 6 months, RR = 2.56, 95% CI 1.77 to 3.69). Details can be found in Figure 60.
Of the uncontrolled studies, only the ATTAIN LTE119 reported ACR20 response. Results are reported by subgroup based on whether patients were originally randomised to ABT or placebo in the randomised phase (see Figure 61). After 6 months of ABT treatment, 57.3% patients in the group initially randomised to ABT and 63.6% in the group initially randomised to placebo achieved an ACR20 response. This was slightly more than in the ABT arm of the RCT (50.0%). After 6 months, there was a further increase in the percentage of ACR20 responders at 12 months in those initially randomised to ABT followed by a decrease up to 5 years (30.3%). Among those initially randomised to placebo, there was a decrease in the percentage of responders from 12 months onwards, and at 54 months 30.3% of patients were ACR20 responders.
FIGURE 61.
Abatacept: ACR20 response in non-RCTs. LCI, lower confidence interval; n/a, not available; PL, placebo; UCI, upper confidence interval. Bold type indicates that the study was an RCT.
If only patients for whom data were available at different time points were analysed, the increase in percentage of ACR20 responders continued to 3 years (82.1%) and then decreased to 65.6% at 5 years for patients initially randomised to ABT. In the same analysis, among patients initially randomised to placebo there was an increase in the percentage of ACR20 responders up to 42 months (82.0%), and at 54 months 78.9% were ACR20 responders.
ACR50 response
At 6 months the percentage of ACR50 responders was over five times higher in the ABT group than in the placebo group of the ATTAIN trial127–132 and the difference was statistically significant (RR = 5.36, 95% CI 2.19 to 13.10). Details are presented in Figure 62.
Of the uncontrolled studies, only the ATTAIN LTE119 reported ACR50 response. Results are reported by subgroup based on whether patients were originally randomised to ABT or placebo in the randomised phase (see Figure 63). This outcome was achieved at 6 months by 22.9% patients in the arm initially randomised to ABT and 37.4% in the arm initially randomised to placebo. For comparison, this outcome was achieved by 20.2% of patients in the ABT arm of the RCT. In the arm initially randomised to ABT, the percentage of ACR50 responders increased up to 18 months (33.9%) and then decreased to 20.6% at 5 years. In the arm initially randomised to placebo, there was a decrease after 6 months to 21.2% achieving ACR50 response at 48 months.
FIGURE 63.
Abatacept: ACR50 response in non-RCTs. LCI, lower confidence interval; n/a, not available; PL, placebo; UCI, upper confidence interval. Bold type indicates that the study was an RCT.
In the analysis based on the observed data, only the percentage of ACR50 responders among those initially randomised to ABT increased up to 3 years (51.1%) and then it was 46.1% at 4 years and 51.1% at 5 years. Among those initially randomised to placebo there was an almost constant increase up to 48 months (53.8%).
ACR 70 response
In the ATTAIN RCT,127–132 the percentage of patients achieving ACR70 response at 6 months was almost seven times higher in the ABT group than in the placebo group (RR = 6.70, 95% CI 1.62 to 27.8). This difference was statistically significant; however, it needs to be highlighted that the CIs were very wide (see Figure 64).
Of the uncontrolled studies, only the ATTAIN LTE119 reported ACR70 response. After 6 months of treatment the percentage of ACR70 responders was 11.5% among patients initially treated with ABT and 13.1% among patients initially treated with placebo. For comparison, it was 10.1% in the ATTAIN RCT. 127–132 In the arm initially randomised to ABT, there was a further increase to 17.0% at 12 months followed by a decrease to 9.6% at 5 years. In the arm initially randomised to placebo, there was an increase up to 15.2% at 30 months followed by a decrease to 7.1% at 54 months. Analysis based on observed data only provided more favourable results, with the highest percentage of ACR70 responders being 23.4% at 36 months in the arm initially randomised to ABT and 25.9% at 30 months in the arm initially randomised to placebo. See Figure 65 for details.
FIGURE 65.
Abatacept: ACR70 response in non-RCTs. LCI, lower confidence interval; n/a, not available; PL, placebo; UCI, upper confidence interval. Bold type indicates that the study was an RCT.
DAS28
The mean change from baseline in DAS28 was –1.98 in the ABT group and –0.71 in the placebo group. The difference between these values was –1.27 (95% CI –1.62 to –0.93, p < 0.001). These data were provided in the industry submission only. No further information was provided and therefore analyses could not be undertaken.
As indicated in Figure 66, over twice as many patients achieved a clinically meaningful DAS28 improvement (defined as greater than or equal to 1.2) in the ABT arm as in the control arm (RR = 2.15, 95% CI 1.54 to 2.99).
The ATTAIN study127–132 also reported percentages of patients who, based on DAS28, achieved a low score (DAS28 less than or equal to 3.2) or remission (DAS28 less than 2.6). At 6 months, patients in the ABT arm were over five times more likely to have a DAS28 less than or equal to 3.2 than those in the placebo arm and the difference was statistically significant (RR = 5.67, 95% CI 2.08 to 15.44). They were also over 13 times more likely to have a DAS28 less than 2.6 than the placebo group and the difference was statistically significant (RR = 13.40, 95% CI 1.84 to 97.69); however, the CIs were wide. See Figure 67 for details.
Change in the DAS28 score was assessed in both uncontrolled studies. Details are presented in Figure 68. After 6 months of treatment, there was a mean change of –1.99 in the arm initially randomised to ABT and of –2.14 in the arm initially randomised to placebo in the ATTAIN LTE,119 and of –2.00 in the ARRIVE study. 120 This was similar in the RCT. 127–132 In the ATTAIN LTE,119 DAS28 further decreased with time and the mean change was –2.90 at 5 years in the arm initially randomised to ABT and –2.96 at 54 months in the arm initially randomised to placebo.
FIGURE 68.
Abatacept: DAS28 change from baseline in uncontrolled studies. LCI, lower confidence interval; n/a, not available; PL, placebo; SD, standard deviation; UCI, upper confidence interval.
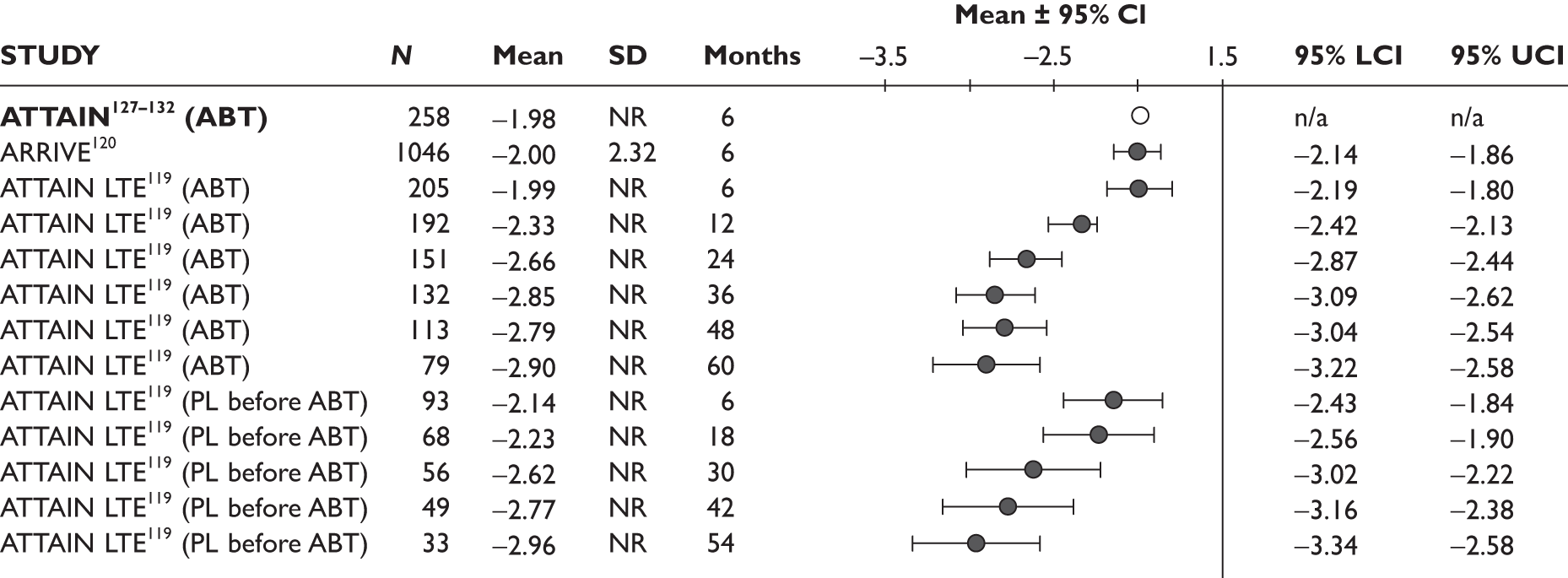
ARRIVE120 measured clinically meaningful DAS28 improvement. It was defined as a decrease of greater than or equal to 1.2 or a score of less than or equal to 3.2. At 6 months, 56.1% of patients in ARRIVE120 achieved this outcome. This was slightly more than in the ABT group of the RCT127–132 (although in ATTAIN127–132 this was defined as a decrease of greater than or equal to 1.2 only). See Figure 69 for details.
FIGURE 69.
Abatacept: clinically meaningful DAS28 improvement in non-randomised studies at 6 months.
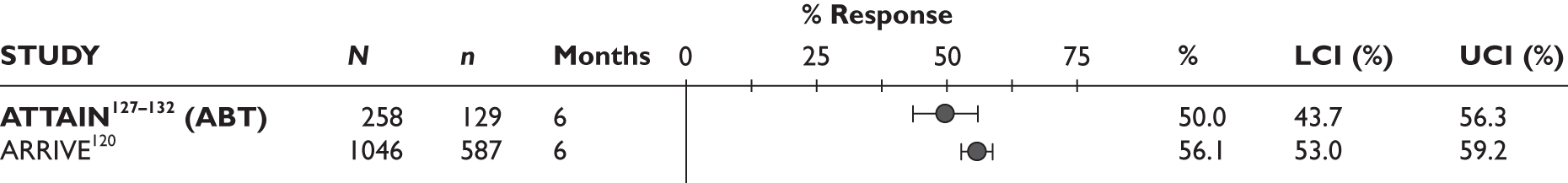
Both uncontrolled studies reported percentages of patients who, based on DAS28, achieved a low score (DAS28 less than or equal to 3.2) or remission (DAS28 less than 2.6). Full details are reported in Figure 70.
FIGURE 70.
Abatacept: patients with final DAS28 values ≤ 3.2 and < 2.6 in uncontrolled studies. LCI, lower confidence interval; n/a, not available; PL, placebo; UCI, upper confidence interval. Bold type indicates that the study was an RCT.
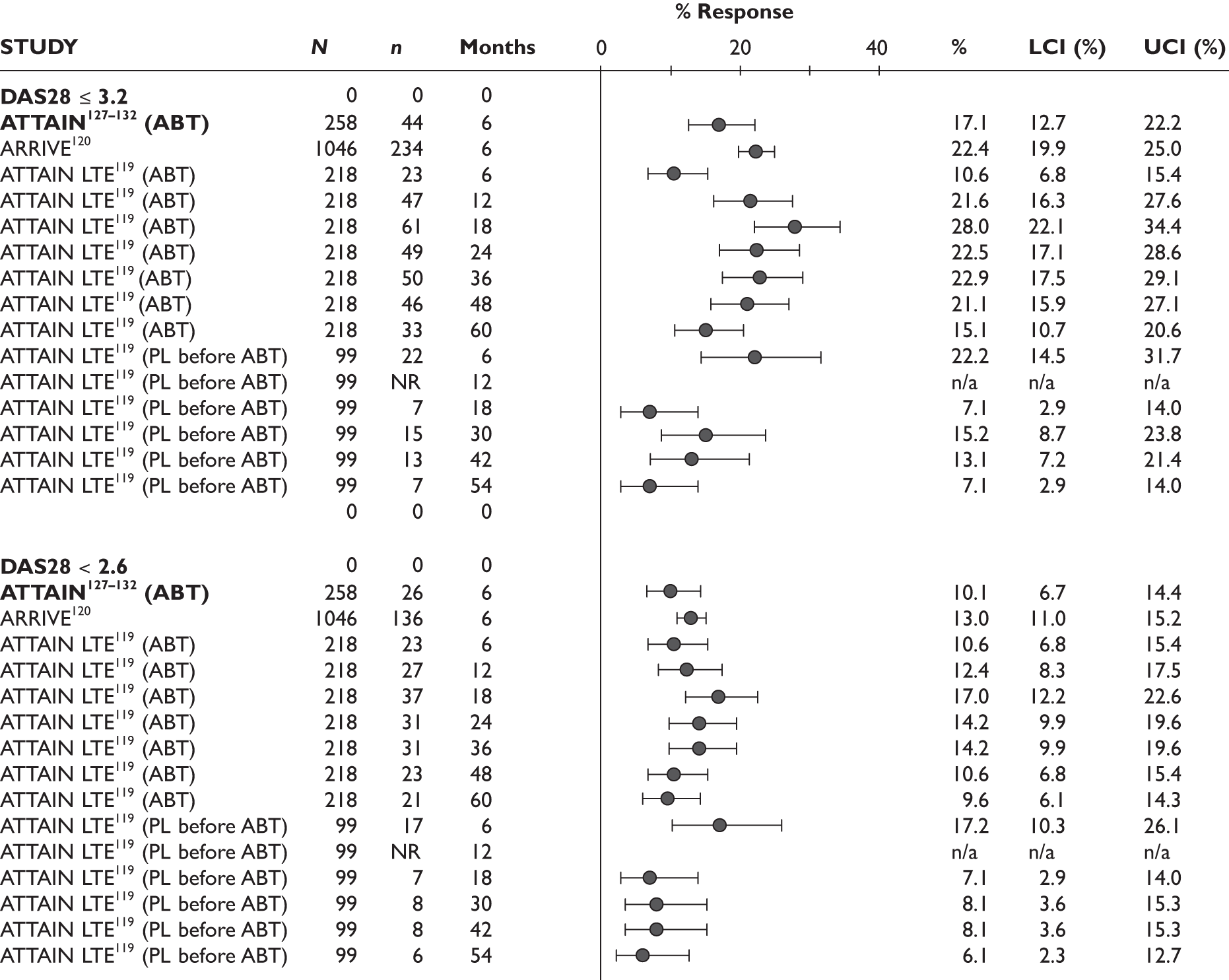
At 6 months a DAS28 score of less than or equal to 3.2 was achieved by 10.6% of patients initially randomised to ABT in the ATTAIN LTE,119 by 22.2% of patients initially randomised to placebo in the ATTAIN LTE119 and by 22.4% of patients in ARRIVE. 120 For comparison, this was 17.1% of patients in the ABT arm of ATTAIN. 127–132 The percentage of patients initially randomised to ABT in ATTAIN LTE119 who achieved a DAS28 of less than or equal to 3.2 increased up to 18 months (28%) and then decreased up to 5 years (15.1%). In the arm initially randomised to placebo, the percentage of patients with low DAS28 decreased up to 54 months (7.1%).
A DAS28 of less than 2.6 was achieved at 6 months by 10.6% and 17.2% in the ATTAIN LTE119 (initial ABT and placebo, respectively) and by 13.0% in ARRIVE. 120 For comparison, 10.1% of the ABT arm of the RCT achieved this outcome. In the ATTAIN LTE119 arm initially randomised to ABT, the highest percentage of patients with DAS28 less than 2.6 was recorded at 18 months (17.0%), following which it decreased to 9.6% at 5 years. In the arm initially randomised to placebo, the highest percentage of patients with DAS28 less than 2.6 was recorded after 6 months of treatment, and at 54 months it was 6.1%.
EULAR response
EULAR response was not assessed in any of the studies.
Health Assessment Questionnaire
Randomised controlled trial
At 6 months, the HAQ change from baseline in the ATTAIN RCT127–132 was –0.45 in the ABT group and –0.11 in the placebo group and the difference between the two groups was reported to be statistically significant (p < 0.001). No data on uncertainty of individual assessments were provided in the study and therefore further analyses could not be undertaken.
This study also assessed clinically meaningful HAQ improvement, defined as a decrease in HAQ score of at least 0.3 (details are reported in Figure 71). Clinically meaningful HAQ improvement was over two times more frequent in the ABT group than in the placebo group and the difference was statistically significant (RR = 2.01, 95% CI 1.44 to 2.81).
FIGURE 71.
Abatacept: clinically meaningful improvement (≥ 0.3) in HAQ score.
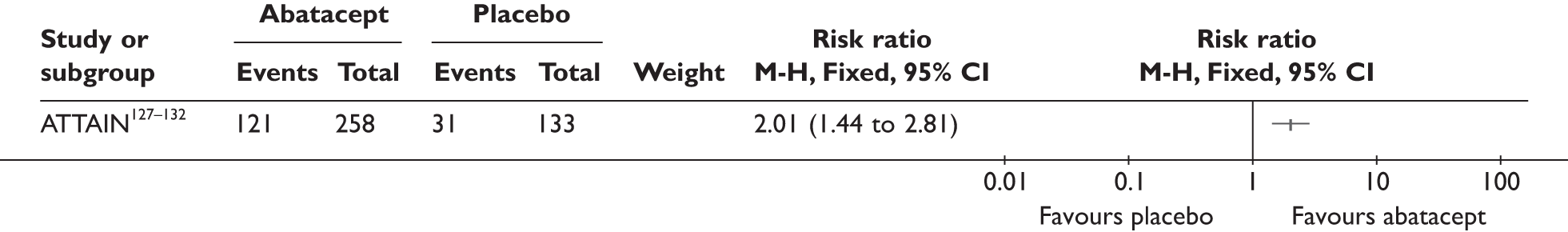
Change in HAQ score was assessed in both uncontrolled studies (however, in the case of for ARRIVE120 only data for a subgroup of 43 US patients receiving monotherapy were reported; ABT monotherapy is licensed in the USA but not in Europe). Figure 72 presents the mean changes from baseline in HAQ score. The mean change from baseline at 6 months was –0.51 in the arm of ATTAIN127–132 initially randomised to ABT, –0.40 in the arm of ATTAIN127–132 initially randomised to placebo and –0.38 in the monotherapy subgroup of ARRIVE. 120 The results for the ABT arm of the RCT were similar. In the arm initially randomised to ABT in the ATTAIN LTE,119 the change decreased up to 3 years (–0.65) and then started slowly increasing (to –0.58 at 4 years and to –0.56 at 5 years). In the group initially randomised to placebo, there was a decrease up to 54 months of treatment (–0.71).
FIGURE 72.
Abatacept: mean changes from baseline in HAQ score. LCI, lower confidence interval; PL, placebo; SD, standard deviation; UCI, upper confidence interval.
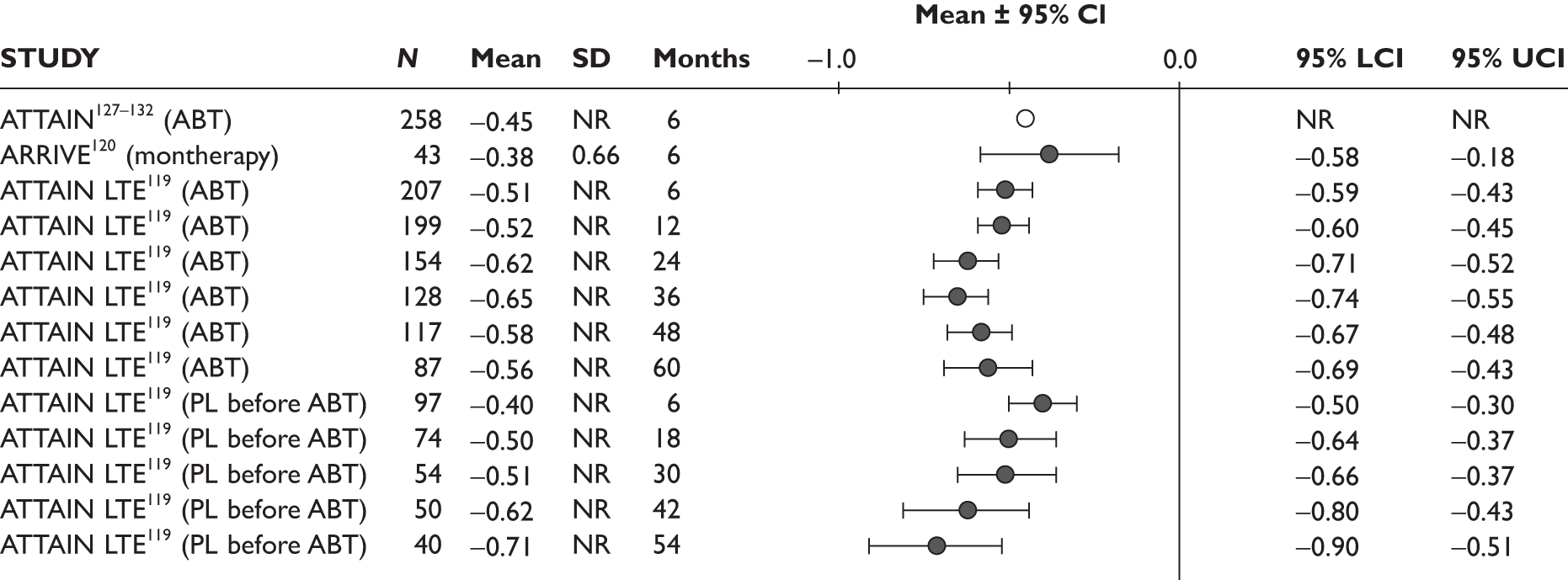
Both uncontrolled studies reported the number of patients who achieved a clinically meaningful improvement in HAQ (details are provided in Figure 73). The ATTAIN LTE119 defined this outcome as an improvement of at least 0.3 in the HAQ score, while in ARRIVE120 it was an improvement of at least 0.22. After 6 months of treatment with ABT, the percentage of patients who achieved this outcome was 52.8% in the ATTAIN LTE119 arm that comprised patients initially randomised to ABT, 49.5% in the ATTAIN LTE119 arm comprising patients initially randomised to placebo and 46.7% in the ARRIVE study. 120 For comparison, it was 46.9% in the ABT arm of the RCT. Analysis of the data from the ATTAIN LTE119 using a non-responder imputation showed a decrease in the percentage of patients who achieved a clinically meaningful HAQ over time, with 24.8% of patients initially randomised to ABT achieving clinically meaningful HAQ improvement at 5 years and 27.3% of patients initially randomised to placebo achieving clinically meaningful HAQ improvement at 54 months. When the analysis in both groups included only patients in whom HAQ improvement was measured at different time points, there was a slight increase in the percentage over time, with a decrease in the last outcome measurement.
Quality of life
Randomised controlled trial
The ATTAIN RCT127–132 assessed patients’ QoL using the SF-36 scale. Patients in the ABT arm improved significantly more in both the physical component (mean difference = 5.50, 95% CI 3.74 to 7.26) and the mental component (mean difference = 3.70, 95% CI 1.45 to 5.95). Details are presented in Figure 74.
For all individual SF-36 items there was a significantly higher improvement in the ABT arm than in the placebo arm. Details for each item are presented in Figure 75.
Of the uncontrolled studies, change in SF-36 was assessed only in the ARRIVE study120 (however, it was reported only for a subgroup of 43 patients receiving monotherapy; ABT monotherapy is licensed in the USA but not in Europe). For the physical component of the SF-36 scale, there was improvement of 4.80 for the monotherapy subgroup of ARRIVE. 120 For the mental component, the improvement was 7.34. For comparison, in the ABT arm of ATTAIN127–132 it was 6.50 and 5.40, respectively. Further details are provided in Figure 76. Data for individual items were not reported in ARRIVE. 120
FIGURE 76.
Abatacept: SF-36 items changes from baseline in components. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval. Bold type indicates that the study was an RCT.
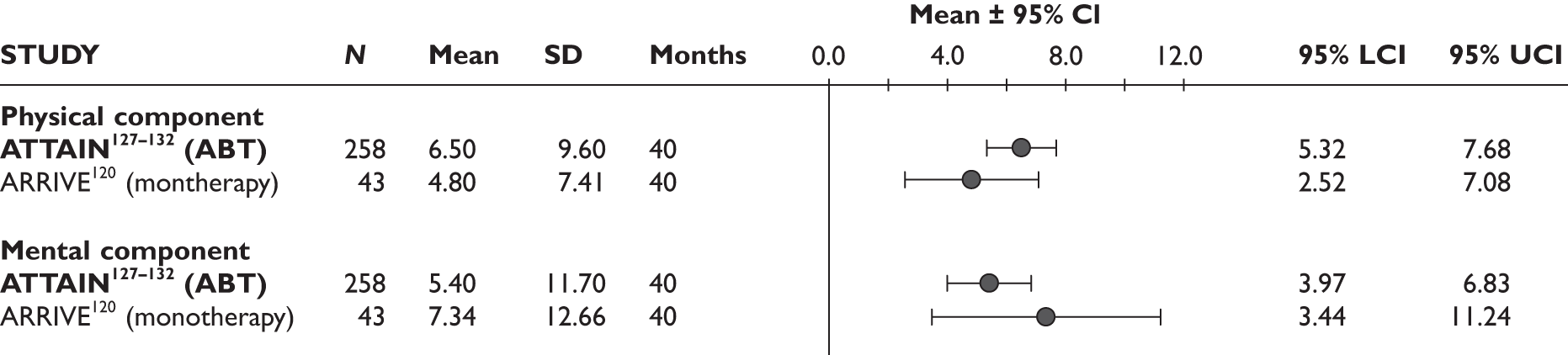
Joint damage
Joint damage was not assessed in any of the studies.
Serious adverse events
In ATTAIN,127–132 there was no significant difference at 6 months between ABT and placebo in the risk of experiencing a serious AE (RR = 0.93, 95% CI 0.51 to 1.68). Details are presented in Figure 77.
Serious AEs were assessed in both uncontrolled studies. At 6 months the percentage of patients who had experienced a serious AE was 10.4% in ARRIVE. 120 It was similar in the ABT arm of the ATTAIN RCT127–132 (10.5%). At 2 years, 32.5% of patients in the ATTAIN LTE119 had experienced a serious AE. Full details are presented in Figure 78.
FIGURE 78.
Abatacept: serious adverse events in non-randomised studies. LCI, lower confidence interval; UCI, upper confidence interval. Bold type indicates that the study was an RCT.
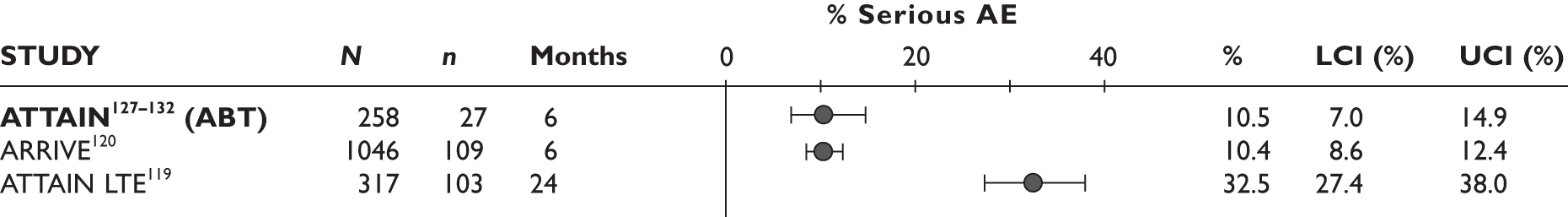
Infections/serious infections
At 6 months there was no statistically significant difference between ABT and placebo in the risk of infection (RR = 1.16, 95% CI 0.87 to 1.56) or serious infection (RR = 1.03, 95% CI 0.26 to 4.06). Details are presented in Figure 79.
Both uncontrolled studies reported infections. The percentages of patients who experienced any infection were similar at 6 months in the ABT arm of ATTAIN127–132 and in the ARRIVE study120 (37.6% and 38.9%, respectively). Of these 2.3% and 2.4% were serious. At 2 years 73.8% of patients in the ATTAIN LTE119 experienced an infection of any kind and 7.9% a serious infection. Details are reported in Figure 80.
FIGURE 80.
Abatacept: infections in non-randomised studies.
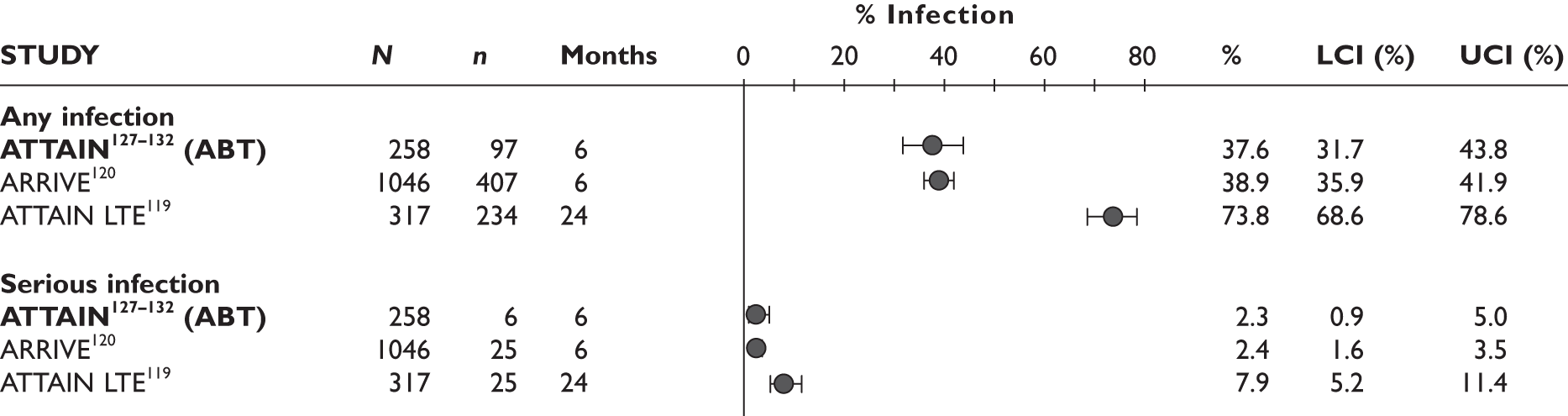
Injection/infusion reaction
At 6 months there was no statistically significant difference between ABT and placebo in the risk of infusion reaction (RR = 1.68, 95% CI 0.56 to 5.04). Details are reported in Figure 81.
FIGURE 81.
Abatacept: infusion reactions. LCI, lower confidence interval; UCI, upper confidence interval. Bold type indicates that the study was an RCT.

Of the uncontrolled studies, infusion reactions were reported only in ARRIVE. 120 At 6 months, 5.4% patients had experienced infusion reactions. For comparison, this figure was 5.0% in the ABT arm of ATTAIN. 127–132 Details are provided in Figure 82.
FIGURE 82.
Abatacept: infusion reactions. LCI, lower confidence interval; UCI, upper confidence interval. Bold type indicates that the study was an RCT.
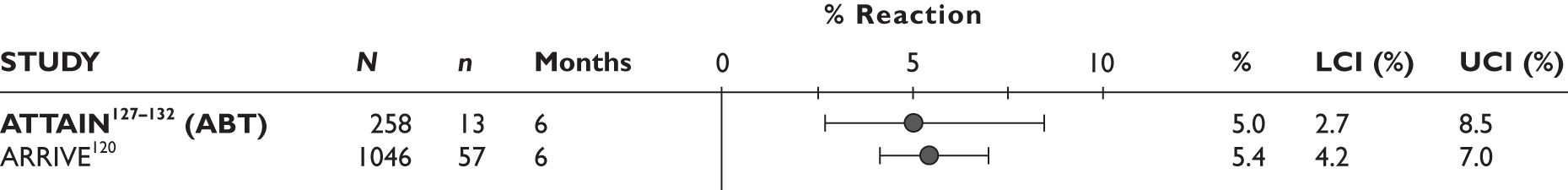
Abatacept in combination with other biologic drugs
Two RCTs [Weinblatt et al. 134 and abatacept study of safety in use with other rheumatoid arthritis therapies (ASSURE)135] were identified that assessed ABT in combination with previously tried biologic drugs. Although both studies met the inclusion criteria of the systematic review, combination therapy was not considered relevant to this report and, therefore, they were not analysed.
The study by Weinblatt et al. 134 was a multicentre placebo-controlled randomised trial and included 121 patients who had active RA despite treatment with ETN. Patients were randomised to receive ETN and ABT or ETN and placebo and were followed up for 1 year. Afterwards they could enter a LTE (data provided for 2 years of the extension study). Data were collected on outcomes including ACR response, HAQ, SF-36 and safety.
ASSURE135 was a multicentre placebo-controlled randomised trial and included 167 patients who had active RA in spite of receiving therapy with biologic agents (ETN, IFX, ADA and anakinra), ‘warranting additional therapy at the discretion of the investigator’. (Note: it also included 1,274 patients who received background DMARDs and were probably biologic naive.) Patients continued their treatment and in addition to that were randomised to receive ABT or placebo. They were followed up for 1 year. The study assessed outcomes including HAQ Disability Index, pain, patient and physician global assessment and safety.
Summary
Three studies assessed ABT in comparison with standard care: one RCT (ATTAIN127–132) and two uncontrolled studies (ATTAIN LTE119 and ARRIVE120). Follow-up ranged from 6 months to 5 years. All studies included patients with similar baseline characteristics. The main results of the included studies are summarised in Table 40.
| Outcome | RCT [result (95% CI)] | Uncontrolled studies | ||
|---|---|---|---|---|
| 6 months (ABT vs placebo) | 6 months (ABT arm) | 6 months | 4.5–5 years | |
| Withdrawals: | 24 months (longer follow-up NA) | |||
|
■ for any reason ■ due to lack of efficacy ■ due to AEs |
RR = 0.53 (0.35 to 0.81); less in ABT RR = 0.27 (0.15 to 0.49), less in ABT RR = 0.93 (0.32 to 2.71), no difference |
13.6% 5.4% 3.5% |
17.8% 10% 3.7% |
30% 16.4% 7.6% |
| ACR20 response | RR = 2.56 (1.77 to 3.69), favours ABT; similar results for 3 months | 50.0% | 57.3%–63.6% | 30.3% |
| ACR50 response | RR = 5.36 (2.19 to 13.10), favours ABT | 20.2% | 22.9%–37.4% | 20.6%–21.2% |
| ACR70 response | RR = 6.70 (1.62 to 27.81), favours ABT | 10.1% | 11.5%–13.1% | 7.1%–9.6% |
| DAS28: | ||||
|
■ change from baseline ■ clinically meaningful ■ ≤ 3.2 ■ < 2.6 |
Mean difference = –1.27 (–1.62 to –0.93), favours ABT RR = 2.15 (1.54 to 2.99), favours ABT RR = 5.67 (2.08 to 15.44), favours ABT RR = 13.40 (1.84 to 97.69), favours ABT |
–1.98 50.0% 17.1% 10.1% |
–1.99 to –2.14 56.1% 10.6%–22.4% 13.0%–17.2% |
–2.00 to –2.90 NA 7.1%–15.1% 6.1%–9.6% |
| EULAR response | NA | NA | NA | NA |
| HAQ: | ||||
|
■ change from baseline ■ clinically meaningful |
Mean difference = –0.34, favours ABT (p < 0.001) RR = 2.01 (1.44 to 2.81), favours ABT |
–0.45 46.9% |
–0.38 to –0.51 46.7%–52.8% |
–0.56 to –0.71 24.8%–27.3% |
| QoL (SF-36) | ||||
|
■ physical component, change from baseline ■ mental component, change from baseline |
Mean difference = 5.50 (3.74 to 7.26), favours ABT Mean difference = 3.70 (1.45 to 5.95), favours ABT |
6.50 5.40 |
7.41 12.66 |
NA NA |
| Joint damage | NA | NA | NA | NA |
| Serious AEs | RR = 0.93 (0.51 to 1.68), NS | 10.5% | 10.4% | 32.5% |
|
Any infections Serious infections |
RR = 1.16 (0.87 to 1.56), NS RR = 1.03 (0.26 to 4.06), NS |
37.6% 2.3% |
38.9% 2.4% |
73.8% 7.9% |
| Infusion reaction | RR = 1.68 (0.56 to 5.04), NS | 5.0% | 5.4% | NA |
Effectiveness of the technologies compared with newly initiated and previously untried conventional disease-modifying antirheumatic drug
No study addressing the comparison was found.
Effectiveness of the technologies compared with other biologic agents
No study addressing this comparison was found.
Comparison of effectiveness between technologies (head-to-head comparisons)
Evidence from comparative studies
Overview of evidence
One prospective cohort study was identified to compare RTX with TNF inhibitors as a class. 136,137
Included patients had tried at least one TNF inhibitor (ADA, ETN or IFX) before and discontinued treatment owing to inadequate response. The study was conducted in Switzerland and the median duration of follow-up was 11 months. Full details of this study are provided in Table 41.
| Study | Country | Design | Reason for switching | Prior TNF inhibitors; n | Treatment arms (no. of patients) | Duration of follow-up | Comments |
|---|---|---|---|---|---|---|---|
| RCTs | |||||||
| None were identified | |||||||
| Non-randomised comparative studies | |||||||
| Finckh 2009136,137 | Switzerland | Prospective cohort | Inadequate response | Any (≥ 1) | TNF (163); RTX (155) | 11 months (median) | Based on the Swiss Clinical Quality Management program for Rheumatoid Arthritis (SCQOM-RA) |
| Uncontrolled studies | |||||||
| Not applicable | |||||||
Patient characteristics
Full details baseline characteristics are reported in Table 42. The study included 318 patients and:
| Study | Number of patients/% female | Age (years), mean (SD) | RA duration (years), mean (SD) | RF positive (%) | % of patients on concomitant DMARDs and steroids | Number of previous DMARDs, mean | Number of previous TNF inhibitors, mean (SD) | HAQ, mean (SD) | DAS28, mean (SD) | TJC/SJC, mean (SD) | ESR (mm/hour), mean (SD) | CRP (mg/dl), mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Finckh 2009136,137 | 318/77.5 | 55 (12.85) | 11.3 (8.1) | 82.4 | MTX (63.9), LEF (18.0), other (4.5), steroids (56.5) | NR | 1 to > 2 | 1.5 (2.9) | 4.5 (1.3) | NR | NR | NR |
-
The proportion of women was 77.5%.
-
The mean age was 55 years.
-
The mean disease duration was 11.3 years.
-
The proportion of RF-positive patients was 82.4%.
-
Concomitant DMARDs used were MTX (63.9%), LEF (18%) and other (4.5%).
-
The proportion of patients receiving steroids was 56.5%.
-
The number of previous DMARDs was not reported.
-
The number of previous TNF inhibitors ranged from one to over two.
-
The mean baseline HAQ score was 1.5.
-
The mean baseline DAS28 score was 4.5.
-
No information was provided on CRP and ESR.
Quality assessment
Full details of quality assessment are reported in Table 43. The study was a prospective cohort. It had clearly defined inclusion criteria. It was; however, unclear if consecutive patients were included in the study and what percentage of patients were withdrawn.
Results
Table 44 indicates which of the outcomes reported in the main text of the report were assessed in the Finckh et al. study. 136,137 No outcomes apart from the ones reported in Table 44 were assessed.
| Total withdrawal | Withdrawal by reason | ACR (20/50/70) | DAS28 | EULAR response | HAQ | QoL | Joint damage | Serious AEs | Infection/serious infection | Injection/infusion reaction | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Finckh 2009136,137 | ✓ | ✓ |
Withdrawals
Withdrawals were not assessed in this study.
ACR20/50/70 response
ACR response was not assessed in this study.
DAS28
There was a trend favouring TNF inhibitors over RTX for change from baseline in DAS28; however, this difference was not statistically significant (mean difference = –0.35, 95% CI –0.71 to 0.01). The follow-up for this outcome was unclear. See Figure 83 for details.
FIGURE 83.
Tumour necrosis factor inhibitors versus rituximab: DAS28 change from baseline. SD, standard deviation.
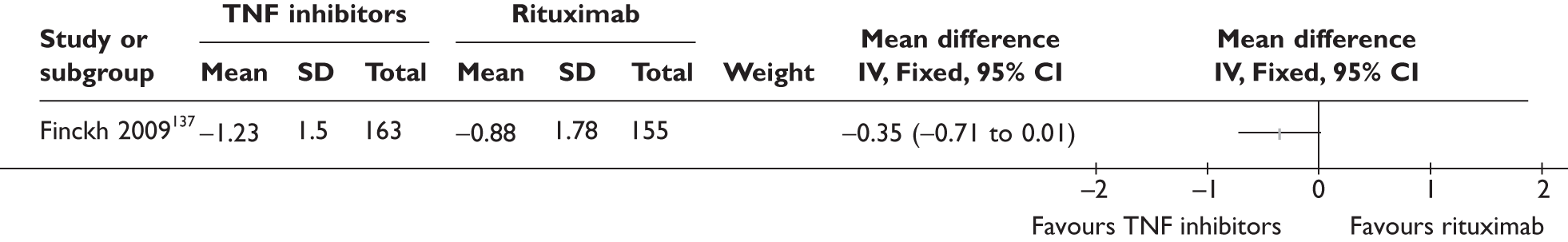
EULAR response
EULAR response was not reported in this study.
Health Assessment Questionnaire
Health Assessment Questionnaire score was reported only for baseline in this study.
Quality of life
Quality of life was not reported in this study.
Joint damage
Joint damage was not reported in this study.
Serious adverse events
Serious AEs were not reported in this study.
Infections/serious infections
Infections were not reported in this study.
Injection/infusion reaction
Data for injection/infusion reactions were reported only for a subgroup of 116 patients. 136 Dermatological complications (mainly injection site reactions) occurred in one RTX patient and nine TNF inhibitor patients. Infusion reactions were reported in three RTX and none of the TNF inhibitor patients. Data from both categories were analysed together to compare AEs associated with drug administration (Figure 84). There was no statistically significant difference between groups (RR = 1.70, 95% CI 0.56 to 5.22).
FIGURE 84.
Tumour necrosis factor inhibitors versus rituximab: injection/infusion site reactions.
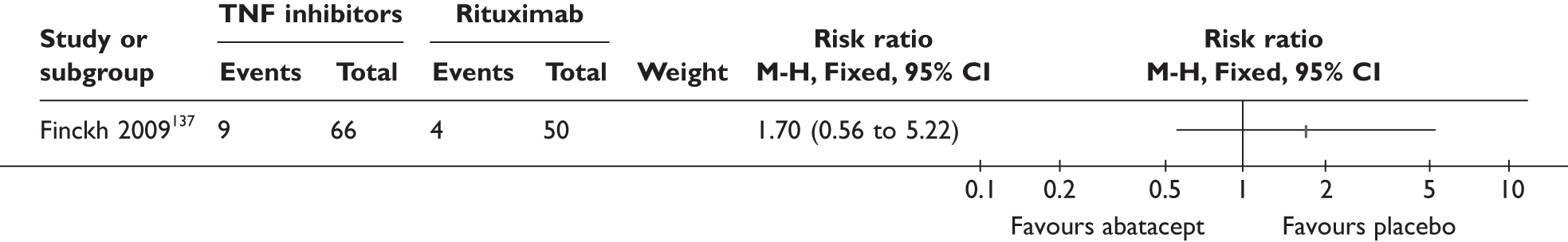
Summary
One prospective cohort study136,137 compared TNF inhibitors as a class with RTX. The median follow-up was 11 months; however, it was not clearly stated when outcomes were assessed. The main results of the study are summarised in Table 45.
| Outcome | Results (TNF inhibitors vs RTX) Unclear follow-up |
|---|---|
| Withdrawals | NR |
| ACR20 response | NR |
| ACR50 response | NR |
| ACR70 response | NR |
| DAS28 – change from baseline | Mean difference = –0.35, 95% CI –0.71 to 0.01, NS |
| EULAR response | NR |
| HAQ | NR |
| QoL | NR |
| Joint damage | NR |
| Serious AEs | NR |
|
Any infections Serious infections |
NR NR |
| Injection/infusion reactions | RR = 1.70, 95% CI 0.56 to 5.22, NS |
Indirect comparisons
Two placebo-controlled RCTs were identified that were considered amenable for an IC of effectiveness of two of the drugs of interest. These trials were REFLEX124–126 and ATTAIN127–132 which investigated RTX and ABT, respectively, in similar populations with similar follow-up and outcome measures.
Indirect comparison was conducted (RTX vs ABT) using the method of Bucher et al. 73 The following binary outcomes were examined: ACR20, ACR50 and ACR70 responses and ‘withdrawal for any reason’. The results are summarised in Table 46.
| Comparison | RR | LCI | UCI | Comment |
|---|---|---|---|---|
| ACR20 | ||||
| RTX vs placebo | 2.85 | 2.08 | 3.91 | Favours RTX |
| ABT vs placebo | 2.55 | 1.74 | 3.76 | Favours ABT |
| RTX vs ABT | 1.12 | 0.68 | 1.84 | Favours RTX, wide CIs |
| ACR50 | ||||
| RTX vs placebo | 5.40 | 2.87 | 10.16 | Favours RTX |
| ABT vs placebo | 5.40 | 2.21 | 13.20 | Favours ABT |
| RTX vs ABT | 1.00 | 0.33 | 2.98 | No difference |
| ACR70 | ||||
| RTX vs placebo | 12.14 | 2.96 | 49.86 | Favours RTX |
| ABT vs placebo | 6.75 | 1.63 | 28.02 | Favours ABT |
| RTX vs ABT | 1.80 | 0.24 | 13.35 | Favours RTX, wide CIs |
| Withdrawal any reason | ||||
| RTX vs placebo | 0.39 | 0.29 | 0.52 | Favours RTX |
| ABT vs placebo | 0.53 | 0.35 | 0.81 | Favours ABT |
| RTX vs ABT | 0.73 | 0.44 | 1.21 | Favours RTX, wide CIs |
No IC approached statistical significance; however, the IC point estimates slightly favoured RTX for ACR20, ACR70 and withdrawal for any reason.
Indirect comparison for change in HAQ score from baseline to 6 months of treatment was of potential interest. However, data reporting was incomplete in REFLEX124–126 and the uncertainty in the reported estimates could not be computed reliably. The change in HAQ score was almost the same in the two trials (see Table 47) so that it is unlikely that an IC would indicate a difference between the treatments for this outcome measure.
Subgroup analyses
This section summarises results from subgroup analyses. Data from RCTs and observational studies were reported separately. Planned subgroup analyses from placebo-controlled RCTs provide the least biased information with regard to whether effectiveness (i.e. the effects of treatment over and above what could be expected without the treatment) varies significantly between the subgroups of interest. Subgroup analyses performed post hoc were highlighted and need to be interpreted with caution.
Owing to the relatively small number of data from RCTs, results from non-randomised, uncontrolled studies were also included but were reported separately from RCT data. Because of the lack of control groups in these studies, any observed differences in the observed response (i.e. not corrected for what would happen without treatment) between the subgroups can be due to differences in baseline characteristics before switching, (and the natural course of the disease that follows) as well as genuine differences in the effectiveness between the subgroups.
In accordance with the study selection criteria for non-randomised studies, subgroup analyses were included only if the number of patients was greater than or equal to 20 in at least one of the subgroups being compared. For studies in which some patients were excluded owing to missing data, ‘non-responder imputations’ were performed and presented for binary outcomes assuming patients with missing data did not achieve the favourable outcomes such as ACR20. ‘Observed data’ analyses based on actually observed/reported data were presented only when the statistical significance of the results and/or the direction of effect differ from non-responder imputation analyses. For continuous outcomes, results were presented as reported in the original papers and no imputation of missing data was carried out. Where data were available from more than one study for a given outcome/time point, pooled estimates using the random-effects model were presented. Given the potential differences in the populations and methods between studies, the main aim is to illustrate the existence or absence of heterogeneity between studies using the I2 statistic.
Reasons for withdrawal of the previous tumour necrosis factor inhibitor
Lack of response (primary failure) versus loss of response (secondary failure)
Randomised controlled trials
No evidence from RCTs was reported.
Non-randomised controlled trials
Subgroup data were available for switching to ADA, ETN, an unspecified TNF inhibitor and ABT. No subgroup data were identified for switching to IFX and RTX.
Two uncontrolled studies reported data separately for patients who switched because of lack of response and those who had initial treatment response but later switched because of loss of response. 96,97 Results comparing these two subgroups of patients are summarised in Tables 48 and 49.
| Study | Switched due to lack of response | Switched due to loss of response | RRa (95% CI) | RD (95% CI) | ||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| Withdrawal for any reasons at 3 months | ||||||
| Bombardieri 2007 (ReAct study)96 | 14/173 | 8 | 24/306 | 8 | 1.03 (0.55 to 1.94) | 0.00 (–0.05 to 0.05) |
| Withdrawal due to lack of efficacy at 3 months | ||||||
| Bombardieri 2007 (ReAct study)96 | 5/173 | 3 | 5/306 | 2 | 1.77 (0.52 to 6.02) | 0.01 (–0.02 to 0.04) |
| Withdrawal due to intolerance/AE at 3 months | ||||||
| Bombardieri 2007 (ReAct study)96 | 5/173 | 3 | 16/306 | 5 | 0.55 (0.21 to 1.48) | –0.02 (–0.06 to 0.01) |
| ACR20 at 3 months | ||||||
| Bombardieri 2007 (ReAct study)96 | 91/173 | 53 | 205/306 | 67 | 0.79 (0.67 to 0.92) | –0.14 (–0.24 to –0.05) |
| van der Bijl 200897 | 4/15 | 27 | 13/21 | 62 | 0.43 (0.17 to 1.06) | –0.35 (–0.66 to –0.05) |
| Pooled estimates | 0.69 (0.42 to 1.12) | –0.20 (–0.37 to –0.02) | ||||
| (random effects) | I2 = 40% | I2 = 39% | ||||
| ACR50 at 3 months | ||||||
| Bombardieri 2007 (ReAct study)99 | 44/173 | 25 | 111/306 | 36 | 0.70 (0.52 to 0.94) | –0.11 (–0.19 to –0.02) |
| van der Bijl 200897 | 2/15 | 13 | 8/21 | 38 | 0.35 (0.09 to 1.42) | –0.25 (–0.52 to 0.02) |
| Pooled estimates (random effects) | 0.68 (0.51 to 0.91) | –0.12 (–0.20 to –0.04) | ||||
| I2 = 0% | I2 = 0% | |||||
| ACR70 at 3 months | ||||||
| Bombardieri 2007 (ReAct study)96 | 15/173 | 9 | 41/306 | 13 | 0.65 (0.37 to 1.13) | –0.05 (–0.10 to 0.01) |
| van der Bijl 200897 | 1/15 | 7 | 4/21 | 19 | 0.35 (0.04 to 2.83) | –0.12 (–0.33 to 0.09) |
| Pooled estimates (random effects) | 0.62 (0.36 to 1.07) | –0.05 (–0.11 to 0.00) | ||||
| I2 = 0% | I2 = 0% | |||||
| EULAR moderate/good response | ||||||
| Bombardieri 2007 (ReAct study)96 | 127/173 | 73 | 243/306 | 79 | 0.92 (0.83 to 1.03) | –0.06 (–0.14 to 0.02) |
| van der Bijl 200897 | 7/15 | 47 | 14/21 | 67 | 0.70 (0.38 to 1.30) | –0.20 (–0.52 to 0.12) |
| Pooled estimates (random effects) | 0.92 (0.83 to 1.02) | –0.07 (–0.15 to 0.01) | ||||
| I2 = 0% | I2 = 0% | |||||
| EULAR good response | ||||||
| Bombardieri 2007 (ReAct study)96 | 33/173 | 19 | 68/306 | 22 | 0.86 (0.59 to 1.24) | –0.03 (–0.11 to 0.04) |
| van der Bijl 200897 | 1/15 | 7 | 5/21 | 24 | 0.28 (0.04 to 2.16) | –0.17 (–0.39 to 0.05) |
| Pooled estimates (random effects) | 0.78 (0.42 to 1.44) | –0.06 (–0.17 to 0.05) | ||||
| I2 = 11% | I2 = 28% | |||||
| Study | Switch owing to lack of response | Switch owing to loss of response | Mean differencea (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| DAS28 change from baseline at 3 months | |||||||
| Bombardieri 2007 (ReAct study)96 | 173 | –1.87 | 1.48 | 306 | –2.03 | 1.36 | 0.16 (–0.11 to 0.43) |
| van der Bijl 200897 | 15 | –1.0 | 0.9 | 21 | –1.8 | 2.0 | 0.80 (–0.17 to 1.77) |
| Pooled estimates (random effects) | 0.30 (–0.22 to 0.83) | ||||||
| I2 = 36% | |||||||
| HAQ change from baseline at 3 months | |||||||
| Bombardieri 2007 (ReAct study)96 | 173 | –0.44 | 0.54 | 306 | –0.51 | 0.62 | 0.07 (–0.04 to 0.18) |
| van der Bijl 200897 | 15 | –0.13 | 0.53 | 21 | –0.36 | 0.48 | 0.23 (–0.11 to 0.57) |
| Pooled estimates (random effects) | 0.08 (–0.02 to 0.19) | ||||||
| I2 = 0% | |||||||
Overall there was no significant difference in treatment withdrawal between the two subgroups. Patients who switched to ADA because of loss of response had significantly higher response rates for ACR20 and ACR50.
Two uncontrolled studies reported subgroup data. 101,104 The results are summarised in Tables 50 and 51. Overall the results were similar between the subgroups and no significant difference was observed.
| Study | Switched owing to lack of response | Switch owing to loss of response | RRa (95% CI) | RDa (95% CI) | ||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| Total withdrawal at 3 months | ||||||
| Bingham 2009104 | 1/29 | 3 | 12/172 | 7 | 0.49 (0.07 to 3.66) | –0.04 (–0.11 to 0.04) |
| ACR20 at 3 months – non-responder imputation | ||||||
| Buch 2007101 | 14/34 | 41 | 13/38 | 34 | 1.20 (0.66 to 2.19) | 0.07 (–0.15 to 0.29) |
| Bingham 2009104 | 12/29 | 41 | 73/172 | 42 | 0.97 (0.61 to 1.56) | –0.01 (–0.20 to 0.18) |
| Pooled estimates (random effects) | 1.06 (0.73 to 1.53) | 0.02 (–0.12 to 0.17) | ||||
| I2 = 0% | I2 = 0% | |||||
| ACR20 at 3 months – observed data | ||||||
| Buch 2007101 | 14/34 | 41 | 13/38 | 34 | 1.20 (0.66 to 2.19) | 0.07 (–0.15 to 0.29) |
| Bingham 2009104 | 12/28 | 43 | 73/160 | 46 | 0.94 (0.59 to 1.49) | –0.03 (–0.23 to 0.17) |
| Pooled estimates (random effects) | 1.03 (0.72 to 1.48) | 0.02 (–0.13 to 0.16) | ||||
| I2 = 0% | I2 = 0% | |||||
| ACR50 at 3–4 months – non-responder imputation | ||||||
| Buch 2007101 | 10/34 | 29 | 8/38 | 21 | 1.40 (0.62 to 3.13) | 0.08 (–0.12 to 0.28) |
| Bingham 2009104 | 4/29 | 14 | 33/172 | 19 | 0.72 (0.28 to 1.88) | –0.05 (–0.19 to 0.08) |
| Pooled estimates (random effects) | 1.06 (0.55 to 2.02) | –0.00 (–0.14 to 0.13) | ||||
| I2 = 9% | I2 = 21% | |||||
| ACR50 at 3 months – observed data | ||||||
| Buch 2007101 | 10/34 | 29 | 8/38 | 21 | 1.40 (0.62 to 3.13) | 0.08 (–0.12 to 0.28) |
| Bingham 2009104 | 4/28 | 14 | 33/160 | 21 | 0.69 (0.27 to 1.80) | –0.06 (–0.21 to 0.08) |
| Pooled estimates (random effects) | 1.03 (0.52 to 2.05) | –0.01(–0.15 to 0.14) | ||||
| I2 = 19% | I2 = 29% | |||||
| ACR70 at 3 months – non-responder imputation | ||||||
| Buch 2007101 | 5/34 | 15 | 5/38 | 13 | 1.12 (0.35 to 3.53) | 0.02 (–0.14 to 0.18) |
| Bingham 2009104 | 1/29 | 3 | 15/172 | 9 | 0.40 (0.05 to 2.88) | –0.05 (–0.13 to 0.03) |
| Pooled estimate (random effects) | 0.86 (0.32 to 2.33) | –0.04 (–0.11 to 0.03) | ||||
| I2 = 0% | I2 = 0% | |||||
| ACR70 at 3 months – observed data | ||||||
| Buch 2007101 | 5/34 | 15 | 5/38 | 13 | 1.12 (0.35 to 3.53) | 0.02 (–0.14 to 0.18) |
| Bingham 2009104 | 1/28 | 4 | 15/160 | 9 | 0.38 (0.05 to 2.77) | –0.06 (–0.14 to 0.02) |
| Pooled estimate (random effects) | 0.85 (0.32 to 2.31) | –0.04 (–0.12 to 0.03) | ||||
| I2 = 0% | I2 = 0% | |||||
| EULAR good/moderate response at 3 months – non-responder imputation | ||||||
| Buch 2007101 | 23/34 | 68 | 21/38 | 55 | 1.22 (0.85 to 1.77) | 0.12 (–0.10 to 0.35) |
| Bingham 2009104 | 17/29 | 59 | 100/172 | 58 | 1.01 (0.72 to 1.40) | 0.00 (–0.19 to 0.20) |
| Pooled estimates (random effects) | 1.10 (0.86 to 1.41) | 0.06 (–0.09 to 0.20) | ||||
| I2 = 0% | I2 = 0% | |||||
| EULAR good/moderate response at 3 months – observed data | ||||||
| Buch 2007101 | 23/34 | 68 | 21/38 | 55 | 1.22 (0.85 to 1.77) | 0.12 (–0.10 to 0.35) |
| Bingham 2009104 | 17/28 | 61 | 100/160 | 63 | 0.97 (0.70 to 1.34) | –0.02 (–0.21 to 0.18) |
| Pooled estimate (random effects) | 1.07 (0.84 to 1.37) | 0.04 (–0.10 to 0.19) | ||||
| I2 = 0% | I2 = 0% | |||||
| EULAR good response at 3 months | ||||||
| Buch 2007101 | 4/34 | 12 | 5/38 | 13 | 0.89 (0.26 to 3.06) | –0.01 (–0.17 to 0.14) |
| Serious AEs | ||||||
| Bingham 2009104 | 0/29 | 0 | 10/172 | 6 | 0.27 0.02 to 4.56) | –0.06 (–0.12 to 0.00) |
| Serious infection | ||||||
| Bingham 2009104 | 0/29 | 0 | 2/172 | 1 | 1.15 (0.06 to 23.43) | –0.01 (–0.06 to 0.04) |
| Study | Switch due to lack of response | Switch due to loss of response | Mean differencea (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| DAS28 change from baseline at 3 months | |||||||
| Buch 2007101 | 34 | –1.49 | 2.25 | 38 | –1.53 | 2.16 | 0.04 (–0.98 to 1.06) |
No studies of switching to IFX provided subgroup data.
One observational study reported data separately for patients who switched because of lack of response and those who had initial treatment response but later switched because of loss of response. 113 Outcomes for the second TNF inhibitor were reported as an aggregated group and were not reported separately for individual TNF inhibitors. The results from the study are shown in Tables 52 and 53.
| Study: Blom 2009113 | Switched owing to lack of response | Switch owing to loss of response | RRa (95% CI) | RDa (95% CI) | ||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| Withdrawal for any reasons at 3 and 6 months | ||||||
| 3 months | 2/49 | 4 | 5/75 | 7 | 0.61 (0.12 to 3.03) | –0.03 (–0.10 to 0.05) |
| 6 months | 6/49 | 12 | 16/75 | 21 | 0.57 (0.24 to 1.37) | –0.09 (–0.22 to 0.04) |
| Withdrawal due to lack of efficacy at 3 and 6 months | ||||||
| 3 months | 0/49 | 0 | 2/75 | 3 | 0.30 (0.01 to 6.20) | –0.03 (–0.08 to 0.02) |
| 6 months | 4/49 | 8 | 10/75 | 13 | 0.61 (0.20 to 1.84) | –0.05 (–0.16 to 0.06) |
| Withdrawal due to intolerance/AE at 3 and 6 months | ||||||
| 3 months | 2/49 | 4 | 3/75 | 4 | 1.02 (0.18 to 5.89) | 0.00 (–0.07 to 0.07) |
| 6 months | 2/49 | 4 | 6/75 | 8 | 0.51 (0.11 to 2.43) | –0.04 (–0.12 to 0.04) |
| EULAR moderate/good response at 3 and 6 months | ||||||
| 3 months – non-responder imputation | 25/49 | 51 | 16/75 | 21 | 2.39 (1.43 to 4.00) | 0.30 (0.13 to 0.46) |
| 3 months – observed data | 25/44 | 57 | 16/38 | 42 | 1.35 (0.86 to 2.12) | 0.15 (–0.07 to 0.36) |
| 6 months – non-responder imputation | 22/49 | 45 | 21/75 | 28 | 1.60 (0.99 to 2.58) | 0.17 (0.00 to 0.34) |
| EULAR good response at 3 and 6 months | ||||||
| 3 months – non-responder imputation | 7/49 | 14 | 3/75 | 4 | 3.57 (0.97 to 13.15) | 0.10 (0.00 to 0.21) |
| 6 months – non-responder imputation | 4/49 | 8 | 7/75 | 9 | 0.87 (0.27 to 2.83) | –0.01 (–0.11 to 0.09) |
| DAS28 ≤ 3.2 at 3 and 6 months | ||||||
| 3 months – non-responder imputation | 8/49 | 16 | 7/75 | 9 | 1.75 (0.68 to 4.52) | 0.07 (–0.05 to 0.19) |
| 6 months – non-responder imputation | 5/49 | 10 | 11/75 | 15 | 0.70 (0.26 to 1.88) | –0.04 (–0.16 to 0.07) |
| Study: Blom 2009113 | Switch due to lack of response | Switch due to loss of response | Mean differencea (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| DAS28 change from baseline at 3 and 6 months | |||||||
| 3 months (observed data) | 44 | –1.2 | 1.0 | 38 | –0.7 | 1.3 | –0.50 (–1.01 to 0.01) |
| 6 months (observed data) | 33 | –1.3 | 1.3 | 41 | –0.6 | 1.3 | –0.70 (–1.30 to –0.10) |
There were no significant differences between the subgroups in withdrawal and treatment response, except for the analysis with non-responder imputation for good/moderate EULAR response at 3 months. A significantly higher proportion of patients who switched owing to lack of response achieved a good/moderate EULAR response compared with those who switched owing to loss of response. Data were missing for nearly half of the patients in the ‘switching owing to loss of response’ for several outcomes, which may compromise the reliability of the results.
No studies of switching to RTX provided subgroup data.
Subgroup data from the LTE of the ATTAIN trial (ATTAIN LTE119) were reported in the MS. As patients had to complete 6 months of treatment in the ATTAIN trial127–132 in order to enter ATTAIN LTE,119 the included patients were no longer representative of the randomised cohort. The results are shown in Table 54. A significant difference between the subgroups was found only in an observed data analysis of HAQ improvement greater than or equal to 0.3 at 6 months. Significantly more patients who switched owing to loss of response achieved this criterion than those who switched owing to lack of response.
| Resultsa at 6 months (unless otherwise stated) | Switched due to lack of response | Switched due to loss of response | RRb (95% CI) | RDb (95% CI) | ||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| ACR20 (non-responder imputation) | 73/130 | 56 | 50/84 | 60 | 0.94 (0.75 to 1.19) | –0.03 (–0.17 to 0.10) |
| ACR50 (non-responder imputation) | 30/130 | 23 | 20/84 | 24 | 0.97 (0.59 to 1.59) | –0.01 (–0.12 to 0.11) |
| ACR70 (non-responder imputation) | 13/130 | 10 | 12/84 | 14 | 0.70 (0.34 to 1.46) | –0.04 (–0.13 to 0.05) |
| HAQ improvement ≥ 0.3 (non-responder imputation) | 77/130 | 59 | 60/84 | 71 | 0.83 (0.68 to 1.01) | –0.12 (–0.25 to 0.01) |
| HAQ improvement ≥ 0.3 (observed data) | 77/126 | 61 | 60/79 | 76 | 0.80 (0.67 to 0.97) | –0.15 (–0.28 to –0.02) |
| DAS28 ≤ 3.2 (non-responder imputation) 3 months | 11/130 | 8 | 11/84 | 13 | 0.65 (0.29 to 1.42) | –0.05 (–0.13 to 0.04) |
| DAS28 ≤ 3.2 (non-responder imputation) 6 months | 21/130 | 16 | 17/84 | 20 | 0.80 (0.45 to 1.42) | –0.04 (–0.15 to 0.07) |
| DAS28 < 2.6 (non-responder imputation) 3 months | 8/130 | 6 | 3/84 | 4 | 1.72 (0.47 to 6.31) | 0.03 (–0.03 to 0.08) |
| DAS28 < 2.6 (non-responder imputation) 6 months | 11/130 | 8 | 12/84 | 14 | 0.59 (0.27 to 1.28) | –0.06 (–0.15 to 0.03) |
Summary
-
No conclusion can be made with regard to whether the effectiveness of the five technologies varies according to lack of response or loss of response to the prior TNF inhibitor because of the lack of RCT evidence.
-
Evidence from two uncontrolled studies96,97 of switching to ADA showed significant differences in favour of patients who switched because of loss of response for ACR20 and ACR50.
-
Evidence from two uncontrolled studies101,104 of switching to ETN indicated that there was no significant difference in treatment withdrawal and response between the subgroups.
-
Evidence from a Dutch study (DREAM113) of switching to an unspecified alternative TNF inhibitor did not find a significant difference between the subgroups.
-
Evidence from the ATTAIN LTE119 of switching to ABT did not find a significant difference between the subgroups except in an analysis based on observed data in which more patients who switched due to loss of response achieved HAQ improvement greater than or equal to 0.3 at 6 months than due to lack of response.
-
No evidence from observational studies was identified for switching to IFX and RTX.
-
Discussion: there is lack of RCT evidence. It has been speculated that patients who withdrew from a TNF inhibitor owing to lack of response may not respond as well to another TNF inhibitor as those who withdrew owing to loss of response. This was observed in studies of switching to ADA, but not in studies of switching to ETN or an unspecified alternative TNF inhibitor. Of note, a similar trend (higher response rates for patients who withdrew owing to loss of response) was seen in the ATTAIN LTE119 for switching to ABT, which is not a TNF inhibitor. These observational studies were insufficiently powered to identify clinically important differences and thus the findings require further confirmation.
Switching due to lack of efficacy (lack or loss of response) versus switching due to intolerance (adverse events)
Randomised controlled trials
RCT evidence was available only for RTX. Data were provided in the MS as commercial-in-confidence information.
Commercial-in-confidence information (or data) removed.
FIGURE 85.
Commercial-in-confidence information (or data) removed.
FIGURE 86.
Commercial-in-confidence information (or data) removed.
Non-randomised controlled trials
Subgroup data were available for switching to ADA, ETN and an alternative, unspecified, TNF inhibitor.
Subgroup data were reported in two uncontrolled studies96,97 and were summarised in Tables 55 and 56. The results, mainly driven by the Research in Active Rheumatoid Arthritis (ReAct) study,96 showed significant differences for EULAR response and change in DAS28 in favour of patients who switched because of intolerance/AEs.
| Study | Switched owing to lack of efficacy | Switched owing to intolerance/AE | RRa (95% CI) | RD (95% CI) | ||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| Withdrawal for any reasons at 3 months | ||||||
| Bombardieri 2007 (ReAct)96 | 38/479 | 8 | 18/179 | 10 | 0.79 (0.46 to 1.35) | –0.02 (–0.07, 0.03) |
| Withdrawal due to lack of efficacy at 3 months | ||||||
| Bombardieri 2007 (ReAct)96 | 10/479 | 2 | 3/179 | 2 | 1.25 (0.35 to 4.47) | 0.00 (–0.02 to 0.03) |
| Withdrawal due to intolerance/AE at 3 months | ||||||
| Bombardieri 2007 (ReAct)96 | 21/479 | 4 | 12/179 | 7 | 0.65 (0.33 to 1.30) | –0.02 (–0.06 to 0.02) |
| ACR20 at 3 months | ||||||
| Bombardieri 2007 (ReAct)96 | 296/479 | 62 | 120/179 | 67 | 0.92 (0.81 to 1.04) | –0.05 (–0.13 to 0.03) |
| van der Bijl 200897 | 17/36 | 47 | 2/5 | 40 | 1.18 (0.38 to 3.65) | 0.07 (–0.39 to 0.53) |
| Pooled estimates (random effects) | 0.92 (0.82 to 1.05) | –0.05 (–0.13 to 0.03) | ||||
| I2 = 0% | I2 = 0% | |||||
| ACR50 at 3 months | ||||||
| Bombardieri 2007 (ReAct)96 | 155/479 | 32 | 68/179 | 38 | 0.85 (0.68 to 1.07) | –0.06 (–0.14 to 0.03) |
| van der Bijl 200897 | 10/36 | 28 | 1/5 | 20 | 1.39 (0.22 to 8.66) | 0.08 (–0.30 to 0.46) |
| Pooled estimates (random effects) | 0.86 (0.68 to 1.08) | –0.05 (–0.13 to 0.03) | ||||
| I2 = 0% | I2 = 0% | |||||
| ACR70 at 3 months | ||||||
| Bombardieri 2007 (ReAct)96 | 56/479 | 12 | 30/179 | 17 | 0.70 (0.46 to 1.05) | –0.05 (–0.11 to 0.01) |
| van der Bijl 200897 | 5/36 | 14 | 0/5 | 0 | 1.78 (0.11 to 28.28) | 0.14 (–0.11 to 0.39) |
| Pooled estimates (random effects) | 0.71 (0.47 to 1.07) | 0.00 (–0.17 to 0.17) | ||||
| I2 = 0% | I2 = 53% | |||||
| EULAR good/moderate response | ||||||
| Bombardieri 2007 (ReAct)96 | 370/479 | 77 | 151/179 | 84 | 0.92 (0.85 to 0.99) | –0.07 (–0.14 to –0.01) |
| van der Bijl 200897 | 21/36 | 58 | 4/5 | 80 | 0.73 (0.43 to 1.22) | –0.22 (–0.60 to 0.17) |
| Pooled estimates (random effects) | 0.91 (0.84 to 0.99) | –0.08 (–0.14 to –0.01) | ||||
| I2 = 0% | I2 = 0% | |||||
| EULAR good response | ||||||
| Bombardieri 2007 (ReAct)96 | 101/479 | 21 | 51/179 | 28 | 0.74 (0.55 to 0.99) | –0.07 (–0.15 to 0.00) |
| van der Bijl 200897 | 6/36 | 17 | 1/5 | 20 | 0.83 (0.12 to 5.57) | –0.03 (–0.40 to 0.34) |
| Pooled estimates (random effects) | 0.74 (0.56 to 0.99) | –0.07 (–0.15 to 0.00) | ||||
| I2 = 0% | I2 = 0% | |||||
| Study | Switch due to lack of efficacy | Switch due to intolerance/AE | Mean differencea (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| DAS28 change from baseline at 3 months | |||||||
| Bombardieri 2007 (ReAct)96 | 479 | –1.97 | 1.40 | 179 | –2.22 | 1.28 | 0.25 (0.02 to 0.48) |
| van der Bijl 200897 | 36 | –1.47 | 1.64 | 5 | –1.40 | 0.60 | –0.07 (–0.82 to 0.68) |
| Pooled estimate (random effects) | 0.22 (0.01 to 0.44) | ||||||
| I2 = 0% | |||||||
| HAQ change from baseline at 3 months | |||||||
| Bombardieri 2007 (ReAct)96 | 479 | –0.49 | 0.59 | 179 | –0.55 | 0.64 | 0.06 (–0.05 to 0.17) |
| van der Bijl 200897 | 36 | –0.26 | 0.50 | 5 | –0.15 | 0.34 | –0.11 (–0.45 to 0.23) |
| Pooled estimate (random effects) | 0.04 (–0.06 to 0.15) | ||||||
| I2 = 0% | |||||||
Subgroup data were available from one uncontrolled study. 103 The results are presented in Table 57. No significant difference between subgroups was found.
| Study | Switch due to lack of efficacy | Switch due to intolerance/AE | Mean differencea (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| DAS28 change from baseline (time not specified; between 3 months to 9 months/last observed value on treatment) | |||||||
| Laas 2008103 | 20 | –1.19 | 2.09 | 6 | –1.30 | 1.25 | 0.11 (–1.25 to 1.47) |
Subgroup data were available from three observational studies. 110,112,113 The results are shown in Tables 58 and 59. Patients who withdrew from the previous TNF inhibitors because of intolerance/AEs were more likely to withdraw because of intolerance/AEs again compared with those who withdrew from the previous TNF inhibitors because of lack of efficacy. On the other hand, patients who withdrew from the previous TNF inhibitors because of intolerance/AEs were more likely to achieve various ACR, EULAR and other DAS28-based response criteria.
| Study | Switched owing to lack of efficacy | Switched owing to intolerance/AE | RRa (95% CI) | RDa (95% CI) | ||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| Withdrawal for any reason at 3 and 6 months (non-responder imputation) | ||||||
| Blom 2009113 – 3 months | 7/124 | 6 | 8/73 | 11 | 0.52 (0.19 to 1.36) | –0.05 (–0.14 to 0.03) |
| Blom 2009113 – 6 months | 22/124 | 18 | 17/73 | 23 | 0.76 (0.43 to 1.34) | –0.06 (–0.17 to 0.06) |
| Withdrawal due to lack of efficacy at 3 and 6 months (non-responder imputation) | ||||||
| Blom 2009113 – 3 months | 2/124 | 2 | 1/73 | 1 | 1.18 (0.11 to 12.76) | 0.00 (–0.03 to 0.04) |
| Blom 2009113 – 6 months | 14/124 | 11 | 4/73 | 5 | 2.06 (0.70 to 6.02) | 0.06 (–0.02 to 0.13) |
| Withdrawal due to intolerance/AE at 3 and 6 months (non-responder imputation) | ||||||
| Blom 2009113 – 3 months | 5/124 | 4 | 7/73 | 10 | 0.42 (0.14 to 1.28) | –0.06 (–0.13 to 0.02) |
| Blom 2009113 – 6 months | 8/124 | 6 | 12/73 | 16 | 0.39 (0.17 to 0.92) | –0.10 (–0.20 to 0.00) |
| ACR20 at 3 months (non-responder imputation) | ||||||
| Karlsson 2008112 | 61/137 | 45 | 78/138 | 57 | 0.79 (0.62 to 1.00) | –0.12 (–0.24 to 0.00) |
| ACR50 at 3 months (non-responder imputation) | ||||||
| Karlsson 2008112 | 28/137 | 20 | 44/138 | 32 | 0.64 (0.43 to 0.97) | –0.11 (–0.22 to –0.01) |
| ACR70 at 3 months (non-responder imputation) | ||||||
| Karlsson 2008112 | 8/137 | 6 | 10/138 | 7 | 0.81 (0.33 to 1.98) | –0.01 (–0.07 to 0.04) |
| EULAR good/moderate response at 3 months (non-responder imputation) | ||||||
| Hjardem 2007110 | 38/109 | 35 | 19/72 | 26 | 1.32 (0.83 to 2.10) | 0.08 (–0.05 to 0.22) |
| Karlsson 2008112 | 80/137 | 58 | 100/138 | 72 | 0.81 (0.68 to 0.96) | –0.14 (–0.25 to –0.03) |
| Blom 2009113 | 41/124 | 33 | 21/73 | 29 | 1.15 (0.74 to 1.78) | 0.04 (–0.09 to 0.18) |
| Pooled estimate | 1.02 (0.72 to 1.45) | –0.01 (–0.15 to 0.13) | ||||
| (random effects) | I2 = 67% | I2 = 74% | ||||
| EULAR good/moderate response at 6 months (non-responder imputation) | ||||||
| Blom 2009113 | 43/124 | 35 | 21/73 | 29 | 1.21 (0.78 to 1.86) | 0.06 (–0.07 to 0.19) |
| EULAR good response at 3 months (non-responder imputation) | ||||||
| Hjardem 2007110 | 14/109 | 13 | 5/72 | 7 | 1.85 (0.70 to 4.91) | 0.06 (–0.03 to 0.14) |
| Karlsson 2008112 | 24/137 | 18 | 42/138 | 30 | 0.58 (0.37 to 0.90) | –0.13 (–0.23 to –0.03) |
| Blom 2009113 | 10/124 | 8 | 7/73 | 10 | 0.84 (0.33 to 2.11) | –0.02 (–0.10 to 0.07) |
| Pooled estimate | 0.87 (0.44 to 1.70) | –0.03 (–0.13 to 0.08) | ||||
| (random effects) | I2 = 58% | I2 = 77% | ||||
| EULAR good response at 6 months (non-responder imputation) | ||||||
| Blom 2009113 | 11/124 | 9 | 7/73 | 10 | 0.93 (0.38 to 2.28) | –0.01 (–0.09 to 0.08) |
| DAS28 ≤ 3.2 at 3 months (non-responder imputation) | ||||||
| Karlsson 2008112 | 33/137 | 24 | 51/138 | 37 | 0.65 (0.45 to 0.94) | –0.13 (–0.24 to –0.02) |
| Blom 2009113 | 15/124 | 12 | 13/73 | 18 | 0.68 (0.34 to 1.35) | –0.06 (–0.16 to 0.05) |
| Pooled estimate | 0.66 (0.48 to 0.91) | –0.09 (–0.17 to –0.02) | ||||
| (random effects) | I2 = 0% | I2 = 0% | ||||
| DAS28 ≤ 3.2 at 6 months (non-responder imputation) | ||||||
| Blom 2009113 | 16/124 | 13 | 11/73 | 15 | 0.86 (0.42 to 1.74) | –0.02 (–0.12 to 0.08) |
| DAS28 < 2.6 at 3 months (non-responder imputation) | ||||||
| Karlsson 2008112 | 16/137 | 12 | 25/138 | 18 | 0.64 (0.36 to 1.15) | –0.06 (–0.15 to 0.02) |
| Study: Blom 2009113 | Switch due to lack of efficacy | Switch due to intolerance/AE | Mean differencea (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| DAS28 change from baseline at 3 and 6 months | |||||||
| 3 months | 82 | –0.97 | 1.15 | 46 | –0.80 | 1.40 | –0.17 (–0.65 to 0.31) |
| 6 months | 74 | –0.91 | 1.30 | 40 | –1.00 | 1.40 | 0.09 (–0.44 to 0.62) |
Summary
-
Evidence [commercial-in-confidence information (or data) removed]. No subgroup data from RCT were identified for the other technologies.
-
Evidence from observational studies was available for switching to ADA, ETN and an alternative, unspecified, TNF inhibitor. Evidence was not available for switching to IFX and ABT.
-
Evidence from two observational studies of switching to ADA showed significant differences for EULAR response and change in DAS28 in favour of patients who switched because of intolerance/AEs.
-
No significant difference between subgroups was found in a small, uncontrolled study of switching to ETN.
-
Evidence from three observational studies110,112,113 of switching to an unspecified, alternative TNF inhibitor suggested that patients who withdrew from the previous TNF inhibitor because of intolerance/AE were more likely to withdraw because of intolerance/AEs and more likely to achieve ACR, EULAR and DAS28-related response criteria than patients who withdrew from the previous TNF inhibitor because of lack of efficacy.
-
Discussion: it is suggested that the effectiveness of a TNF inhibitor may differ between patients who have withdrawn from the previous TNF inhibitor because of lack of efficacy and those who have withdrawn because of AEs, but the effectiveness of other technologies with different mechanism of action may not. There is a lack of RCT evidence to confirm the former. RCT evidence suggests that [commercial-in-confidence information (or data) removed]. RCT evidence for ABT is also lacking. Data from observational studies appear to agree with what is expected in terms of treatment withdrawal and treatment response.
Autoantibody status
Randomised controlled trial
RCT data for subgroups stratified by autoantibody status were available only from the REFLEX trial124–126 of RTX.
Rituximab
Subgroup data stratified by RF status from the REFLEX trial124–126 were reported in the MS. Randomisation in this trial was stratified by RF status (RF +, defined as a value of RF greater than or equal to 20 IU/ml at screening; or RF–, defined as RF less than 20 IU/ml at screening) and region (US or non-US). The results for ACR20 at 6 months are shown in Figure 87 (RR) and Figure 88 [risk difference (RD)] and for all the ACR response criteria are shown in Table 60. Although the proportion of patients achieving ACR criteria was generally lower in RF– patients than in RF + patients, there was no significant difference in treatment effect between the subgroups.
FIGURE 87.
Subgroup analysis (switching to rituximab) by RF status: ACR20 at 6 months (relative risk).
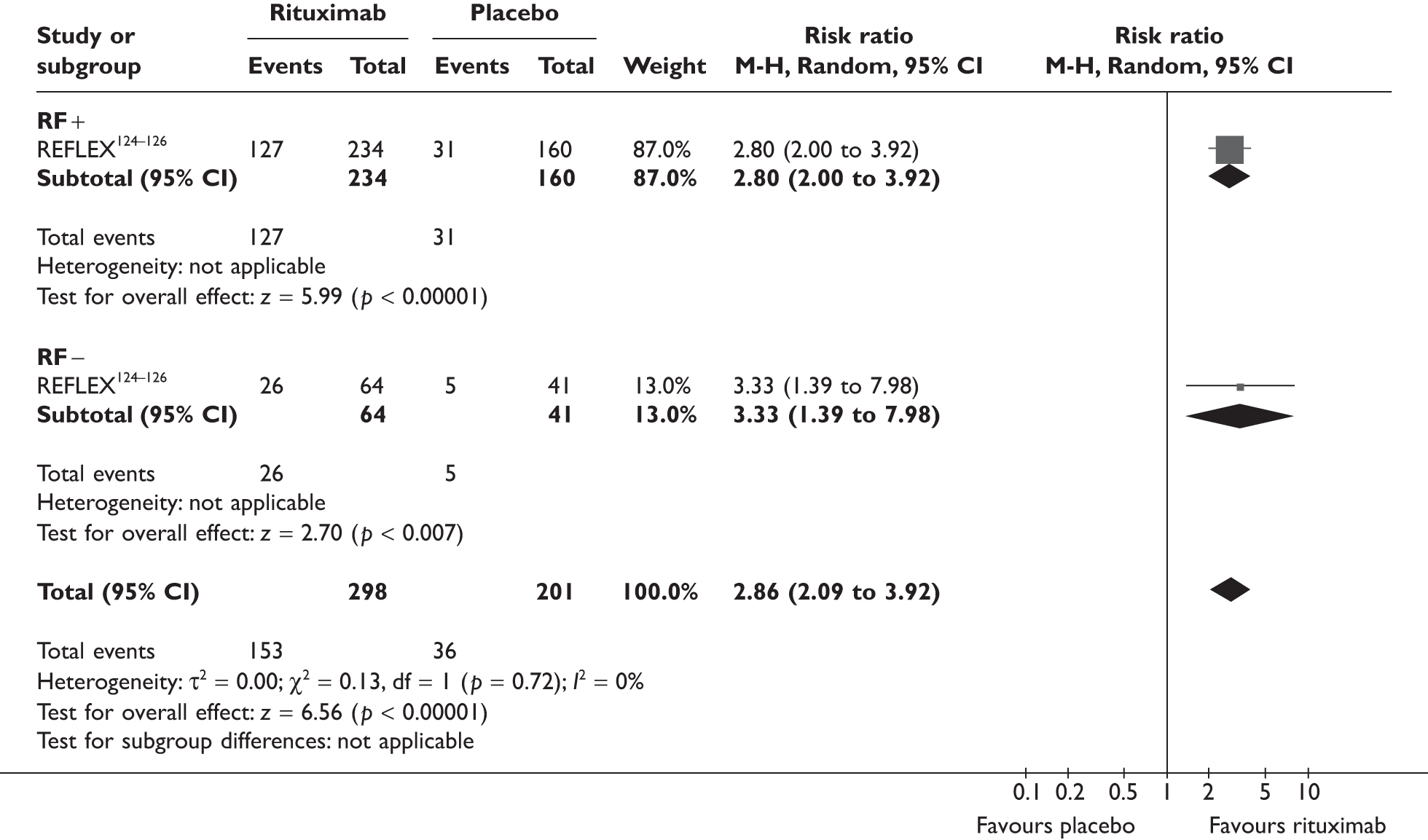
FIGURE 88.
Subgroup analysis (switching to rituximab) by RF status: ACR20 at 6 months (risk difference).
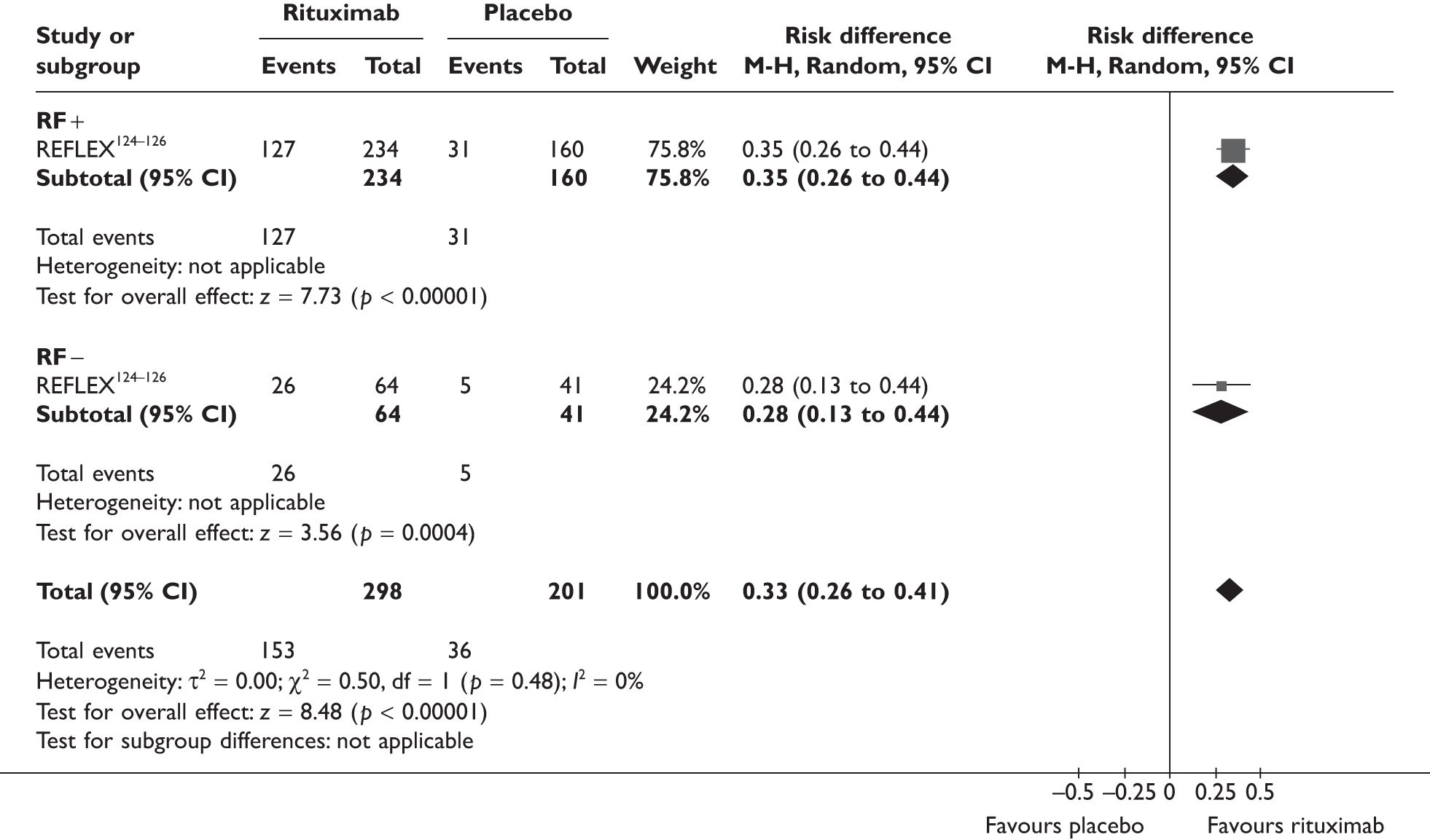
| Study: REFLEX124–126 | RTX | Placebo | RRa (95% CI) | RD (95% CI) | ||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| ACR20 at 6 months | ||||||
| RF + | 127/234 | 54 | 31/160 | 19 | 2.80 (2.00 to 3.92) | 0.35 (0.26 to 0.44) |
| RF– | 26/64 | 41 | 5/41 | 12 | 3.33 (1.39 to 7.98) | 0.28 (0.13 to 0.44) |
| Test for interaction | p = 0.72 | p = 0.48 | ||||
| ACR50 at 6 months | ||||||
| RF + | 69/234 | 29 | 9/160 | 6 | 5.24 (2.70 to 10.19) | 0.24 (0.17 to 0.31) |
| RF– | 11/64 | 17 | 2/41 | 5 | 3.52 (0.82 to 15.09) | 0.12 (0.01 to 0.24) |
| Test for interaction | p = 0.63 | p = 0.08 | ||||
| ACR70 at 6 months | ||||||
| RF + | 31/234 | 13 | 3/160 | 2 | 7.07 (2.20 to 22.72) | 0.11 (0.07 to 0.16) |
| RF– | 6/64 | 9 | 0/41 | 0 | 8.40 (0.49 to 145.24) | 0.09 (0.01 to 0.17) |
| Test for interaction | p = 0.91 | p = 0.67 | ||||
Further subgroup data stratified by baseline RF and anti-CCP status from the REFLEX trial124–126 were also reported in the MS and are summarised in Table 61. Although test for interaction was significant for RD in ACR50, suggesting a greater treatment effect in patients who were either RF or anti-CCP positive than in those with both RF and anti-CCP negative, the number of patients in the latter subgroup was too small to allow firm conclusion to be drawn. This subgroup analysis was performed post hoc and needs to be interpreted with caution.
| Study: REFLEX124–126 | RTX | Placebo | RRa (95% CI) | RDa (95% CI) | ||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| ACR20 at 6 months | ||||||
| RF and/or anti-CCP positive | 79/157 | 50 | 19/107 | 18 | 2.83 (1.83 to 4.38) | 0.33 (0.22 to 0.43) |
| RF/anti-CCP negative | 8/29 | 28 | 1/16 | 6 | 4.41 (0.61 to 32.20) | 0.21 (0.01 to 0.41) |
| Test for interaction | p = 0.67 | p = 0.33 | ||||
| ACR50 at 6 months | ||||||
| RF and/or anti-CCP positive | 46/157 | 29 | 8/107 | 7 | 3.92 (1.93 to 7.97) | 0.22 (0.13 to 0.31) |
| RF/anti-CCP negative | 2/29 | 7 | 1/16 | 6 | 1.10 (0.11 to 11.25) | 0.01 (–0.14 to 0.16) |
| Test for interaction | p = 0.31 | p = 0.01 | ||||
| ACR70 at 6 months | ||||||
| RF and/or anti-CCP positive | 20/157 | 13 | 2/107 | 2 | 6.82 (1.63 to 28.55) | 0.11 (0.05 to 0.17) |
| RF/anti-CCP negative | 1/29 | 3 | 0/16 | 0 | 1.70 (0.07 to 39.47) | 0.03 (–0.08 to 0.15) |
| Test for interaction | p = 0.43 | p = 0.24 | ||||
Non-randomised controlled trials
No subgroup data from observational studies was identified.
Summary
-
Evidence from the REFLEX trial124–126 did not suggest a significant difference in the effectiveness of RTX according to the presence or absence of RF, although the trial may be underpowered for ruling out a clinically relevant difference between subgroups. There is lack of evidence for other technologies.
-
Discussion: in the REFLEX trial,124–126 the proportion of patients achieving ACR criteria was generally lower in RF– patients than in RF + patients irrespective of treatment group. The treatment effects in terms of RDs between RTX and placebo group were generally larger in RF + patients than in RF– patients, but this does not hold true when RR is used as the measure of effect. Differences between subgroups were not statistically significant according to test for interaction, but the test may be underpowered due to the sample size. Post hoc analysis according to RF and anti-CCP status needs to be interpreted with caution.
Number of tumour necrosis factor inhibitors previously tried
Randomised controlled trials
Randomised controlled trial data stratified by the number of TNF inhibitors the patients had tried before switching were available from the REFLEX trial124–126 of RTX and the ATTAIN trial127–132 of ABT.
Rituximab
Subgroup data from the REFLEX trial124–126 stratified by the number of prior TNF inhibitors (one prior TNF inhibitor vs two or more prior TNF inhibitors) were reported in the MS and are presented in Table 62. The results show that RTX was more effective than placebo in both subgroups and there is no significant difference in treatment effects between the subgroups.
| Study: REFLEX124–126 | RTX | Placebo | RRa (95% CI) | RDa (95% CI) | ||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| ACR20 at 6 months | ||||||
| 1 prior TNF inhibitor | 104/179 | 58 | 25/121 | 21 | 2.81 (1.94 to 4.07) | 0.37 (0.27 to 0.48) |
| ≥ 2 prior TNF inhibitors | 50/119 | 42 | 11/80 | 14 | 3.06 (1.70 to 5.50) | 0.28 (0.17 to 0.40) |
| Test for interaction | p = 0.81 | p = 0.24 | ||||
| ACR50 at 6 months | ||||||
| 1 prior TNF inhibitor | 54/179 | 30 | 8/121 | 7 | 4.56 (2.25 to 9.24) | 0.24 (0.16 to 0.32) |
| ≥ 2 prior TNF inhibitors | 26/119 | 22 | 2/80 | 3 | 8.74 (2.13 to 35.80) | 0.19 (0.11 to 0.28) |
| Test for interaction | p = 0.41 | p = 0.46 | ||||
| ACR70 at 6 months | ||||||
| 1 prior TNF inhibitor | 25/179 | 14 | 1/121 | 1 | 16.90 (2.32 to 123.06) | 0.13 (0.08 to 0.18) |
| ≥ 2 prior TNF inhibitors | 12/119 | 10 | 2/80 | 3 | 4.03 (0.93 to 17.54) | 0.08 (0.01 to 0.14) |
| Test for interaction | p = 0.23 | p = 0.19 | ||||
Abatacept
Subgroup data from the ATTAIN trial127–132 stratified by prior TNF inhibitor (ETN, IFX or both) were reported in the MS. For this subgroup analysis, data from patients who had received either ETN or IFX were combined and then were compared with data from patients who had received both ETN and IFX before switching to ABT. The trial was conducted before ADA became widely available and thus few patients had tried more than two TNF inhibitors.
The results are shown in Table 63. Irrespective of the nu–mber of prior TNF inhibitor(s), a higher proportion of patients in the ABT group than in the placebo group achieved ACR20 and a HAQ improvement of greater than or equal to 0.3. The difference was larger and statistically significant in the subgroup of patients who had one prior TNF inhibitor, and was smaller and not statistically significant in the subgroup of patients who had two prior TNF inhibitors. The results of tests for interaction do not suggest differential treatment effects between the subgroups, although the tests may be underpowered as the number of patients in the subgroup of two prior TNF inhibitors is relatively small.
| Study: ATTAIN127–132 | ABT | Placebo | RRa (95% CI) | RDa (95% CI) | ||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| ACR20 at 6 months | ||||||
| 1 prior TNF inhibitor | 108/201 | 54 | 22/111 | 20 | 2.71 (1.83 to 4.03) | 0.34 (0.24 to 0.44) |
| 2 prior TNF inhibitors | 21/55 | 38 | 4/22 | 18 | 2.10 (0.81 to 5.42) | 0.20 (–0.01 to 0.41) |
| Test for interaction | p = 0.63 | p = 0.23 | ||||
| HAQ improvement from baseline ≥ 0.3 at 6 months | ||||||
| 1 prior TNF inhibitor | 102/201 | 51 | 26/111 | 23 | 2.17 (1.51 to 3.11) | 0.27 (0.17 to 0.38) |
| 2 prior TNF inhibitors | 19/55 | 35 | 5/22 | 23 | 1.52 (0.65 to 3.56) | 0.12 (–0.10 to 0.33) |
| Test for interaction | p = 0.45 | p = 0.20 | ||||
Non-randomised controlled trials
Subgroup data stratified by the number of prior TNF inhibitors (or prior biologics) were available for switching to an unspecified TNF inhibitor and to ABT.
Tumour necrosis factor inhibitors as a class
Subgroup data (one prior TNF inhibitor vs two prior TNF inhibitors) were reported in Karlsson et al. 112 and the results are presented in Table 64. A higher proportion of patients who previously tried one TNF inhibitor achieved various ACR and EULAR response criteria than those who previously tried two TNF inhibitors, although the differences were not statistically significant except for the difference in achieving good EULAR response (25% vs 8%).
| Study: Karlsson 2008112 | 1 prior TNF inhibitor | 2 prior TNF inhibitors | RRa (95% CI) | RDa (95% CI) | ||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| ACR20 at 3 months | ||||||
| 172/337 | 51 | 13/36 | 36 | 1.41 (0.90 to 2.21) | 0.15 (–0.02 to 0.32) | |
| ACR50 at 3 months | ||||||
| 91/337 | 27 | 7/36 | 19 | 1.39 (0.70 to 2.76) | 0.08 (–0.06 to 0.21) | |
| ACR70 at 3 months | ||||||
| 24/337 | 7 | 1/36 | 3 | 2.56 (0.36 to 18.40) | 0.04 (–0.02 to 0.10) | |
| EULAR moderate/good response at 3 months | ||||||
| 240/337 | 71 | 21/36 | 58 | 1.22 (0.92 to 1.62) | 0.13 (–0.04 to 0.30) | |
| EULAR good response at 3 months | ||||||
| 84/337 | 25 | 3/36 | 8 | 2.99 (1.00 to 8.98) | 0.17 (0.06 to 0.27) | |
In addition to the above, Duftner et al. 111 reported a 12-month discontinuation rate of 53.5%, 66.7% (18/27) and 28.6% for the first, second and third biologics (ADA, ETN, IFX and anakinra) in Austrian RA patients. This study included a mixed patient population of those with RA (63%, 109/173) and other rheumatic diseases (37%). The exact number of patients from whom the above RA-specific discontinuation rates were derived was not clearly stated except for the second biologic.
Abatacept
Subgroup data stratified by the number of prior TNF inhibitors (one, two or three) were reported by Schiff et al. (ARRIVE study). 120 The results are presented in Figures 89 and 90. The results indicate that the proportion of patients achieving DAS28-related response criteria decreases as the number of prior TNF inhibitor(s) that the patients have tried increases (χ2 test for linear trend, p = 0.009 for DAS28 less than or equal to 3.2 and p = 0.005 for DAS28 less than 2.6). The change in DAS28 from baseline at 6 months was the same for patients who had previously tried one or two TNF inhibitors, but was significantly lower for patients who had previously tried three TNF inhibitors (–2.1 vs –1.7, test for interaction, p = 0.001).
FIGURE 89.
Switching to abatacept: DAS28 responses at 6 months stratified by the number of prior TNF inhibitors in the ARRIVE study. 120 LCI, lower confidence interval; UCI, upper confidence interval.
FIGURE 90.
Switching to abatacept: DAS28 change from baseline at 6 months stratified by the number of prior TNF inhibitors in the ARRIVE study. 120 LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.
Summary
-
Evidence from the REFLEX trial124–126 did not show a significant difference in the effectiveness of RTX (measured as RRs of achieving ACR responses over placebo) between the subgroup of patients who had tried one TNF inhibitor and those who had tried more than one TNF inhibitor. However, the trial may be underpowered for ruling out a clinically relevant difference between the subgroups. The response rates tend to be higher among patients who had tried one TNF inhibitor than among with those who had tried more than one TNF inhibitor irrespective of treatments (i.e. RTX or placebo) received.
-
Evidence from the ATTAIN trial127–132 did not show a significant difference in the effectiveness of ABT (measured as RRs of achieving ACR20 response and HAQ improvement over placebo) between the subgroup of patients who had tried one TNF inhibitor and those who had tried more than one TNF inhibitor. The number of patients in the latter subgroup was small and the difference between ABT and placebo did not reach statistical significance. The trial is likely to be underpowered for ruling out a clinically relevant difference between the subgroups.
-
No evidence from RCTs and observational studies was available for the individual TNF inhibitors.
-
In an observational study112 of switching to an unspecified, alternative TNF inhibitor, higher response rates to ACR and EULAR response criteria were reported in patients who tried one TNF inhibitor than in those who tried two TNF inhibitors.
-
One observational study120 of switching to ABT showed that the proportion of patients achieving DAS28-related response criteria decreases as the number of prior TNF inhibitors increases.
-
Discussion: many of the studies included in this review included patients who had previously tried more than one TNF inhibitor. Determining whether the effectiveness of the technologies varies depending on the number of TNF inhibitors previously tried is useful to inform the applicability of findings from these studies to the main population of interest for this appraisal, i.e. patients who had previously had inadequate response to one TNF inhibitor. Results from the REFLEX124–126 and ATTAIN127–132 trials suggested that the effectiveness of RTX and ABT (measured as RRs of achieving various improvement criteria over placebo) does not differ significantly between patients who have tried one TNF inhibitor compared and those who have tried more than one. The subgroup analyses; however, were limited by the relatively small number of patients, and thus the possibility of differential treatment effect, particularly in terms of RD, cannot be ruled out. Findings from observational studies for switching to an alternative TNF inhibitor and to ABT agree with an inverse relationship between treatment response and number of prior TNF inhibitors. To what extent the effectiveness of the technologies (in particular the TNF inhibitors) varies by the number of prior TNF inhibitors remains unclear owing to the small volume or complete lack of evidence from RCTs.
Prior tumour necrosis factor inhibitor
Randomised controlled trials
RCT data stratified by the TNF inhibitor from which the patients had switched were available only from the ATTAIN trial127–132 of ABT.
Abatacept
Subgroup data stratified by prior TNF inhibitor (ETN vs IFX) from the ATTAIN trial127–132 were reported in the MS and are presented in Table 65. The results of the subgroup analyses show that ABT is more effective than placebo in both patients who have previously had inadequate response to ETN and those who have previously had inadequate response to IFX. Tests for interaction do not suggest differential treatment effects between subgroups, although the tests may be underpowered.
| Study: ATTAIN127–132 | ABT | Placebo | RRa (95% CI) | RDa (95% CI) | ||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| ACR20 at 6 months | ||||||
| Prior ETN | 28/61 | 46 | 8/43 | 19 | 2.47 (1.25 to 4.88) | 0.27 (0.10 to 0.44) |
| Prior IFX | 80/140 | 57 | 14/68 | 21 | 2.78 (1.70 to 4.52) | 0.37 (0.24 to 0.49) |
| Test for interaction | p = 0.78 | p = 0.39 | ||||
| HAQ improvement from baseline of ≥ 0.3 at 6 months | ||||||
| Prior ETN | 25/61 | 41 | 11/43 | 26 | 1.60 (0.89 to 2.90) | 0.15 (–0.03 to 0.33) |
| Prior IFX | 77/140 | 55 | 15/68 | 22 | 2.49 (1.56 to 3.99) | 0.33 (0.20 to 0.46) |
| Test for interaction | p = 0.25 | p = 0.12 | ||||
Non-randomised controlled trials
Adalimumab
Subgroup data stratified by patients who switched from either ETN or IFX to ADA were available from one study (ReAct). 96 The results are shown in Tables 66 and 67. No significant difference between the subgroups was found.
| Study: Bombardieri 2007 (ReAct)96 | Switched from ETN | Switched from IFX | RRa (95% CI) | RDa (95% CI) | ||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| Withdrawal for any reasons at 3 months | ||||||
| 20/188 | 11 | 50/591 | 8 | 1.26 (0.77 to 2.06) | 0.02 (–0.03 to 0.07) | |
| Withdrawal due to lack of efficacy at 3 months | ||||||
| 5/188 | 3 | 12/591 | 2 | 1.31 (0.47 to 3.67) | 0.01 (–0.02 to 0.03) | |
| Withdrawal due to intolerance/AE at 3 months | ||||||
| 10/188 | 5 | 33/591 | 6 | 0.95 (0.48 to 1.90) | 0.00 (–0.04 to 0.03) | |
| ACR20 at 3 months | ||||||
| 107/188 | 57 | 378/591 | 64 | 0.89 (0.77 to 1.02) | –0.07 (–0.15 to 0.01) | |
| ACR50 at 3 months | ||||||
| 64/188 | 34 | 201/591 | 34 | 1.00 (0.80 to 1.26) | 0.00 (–0.08 to 0.08) | |
| ACR70 at 3 months | ||||||
| 24/188 | 13 | 77/591 | 13 | 0.98 (0.64 to 1.50) | 0.00 (–0.06 to 0.05) | |
| EULAR moderate/good response | ||||||
| 149/188 | 79 | 460/591 | 78 | 1.02 (0.94 to 1.11) | 0.01 (–0.05 to 0.08) | |
| EULAR good response | ||||||
| 40/188 | 21 | 154/591 | 26 | 0.82 (0.60 to 1.11) | –0.05 (–0.12 to 0.02) | |
| Study: Bombardieri 2007 (ReAct)96 | Switched from ETN | Switched from IFX | Mean differencea (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| DAS28 change from baseline at 3 months | |||||||
| 188 | –2.0 | 1.4 | 591 | –2.0 | 1.4 | 0.00 (–0.23 to 0.23) | |
| HAQ change from baseline at 3 months | |||||||
| 188 | –0.43 | 0.61 | 591 | –0.51 | 0.60 | 0.08 (–0.02 to 0.18) | |
In addition to the above, Gomez-Reino et al. 108 reported 12-month retention on treatment of 0.75 (95% CI 0.31 to 0.93) for patients who switched from ETN to ADA (n = 33) and 0.69 (95% CI 0.43 to 0.85) for patients who switched from IFX to ADA (n = 14). No statistical comparisons were made.
Abatacept
Subgroup data stratified by the TNF inhibitor from which the patients switched were reported by Schiff et al. (ARRIVE study). 120 The results are presented in Figures 91 and 92. At 6 months, there was no significant difference in the proportion of patients who achieved DAS28 less than or equal to 3.2 (χ2 test, p = 0.67) and DAS28 less than 2.6 (χ2 test, p = 0.34). The mean changes from baseline in DAS28 were also similar between the groups (test for interaction, p = 0.21).
FIGURE 91.
Switching to abatacept: DAS28 responses at 6 months stratified by prior TNF inhibitor in the ARRIVE study. 120 LCI, lower confidence interval; UCI, upper confidence interval.
FIGURE 92.
Switching to abatacept: DAS28 change from baseline at 6 months stratified by prior TNF inhibitor in the ARRIVE study. 120 LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.
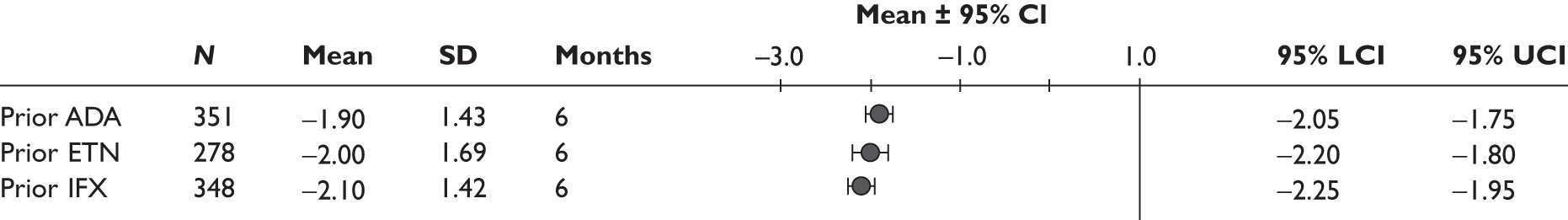
Summary
-
Evidence from the ATTAIN trial127–132 suggested that the effectiveness of ABT did not vary significantly according to the TNF inhibitor (ETN or IFX) from which the patients had switched, although the subgroup analysis may be underpowered. No RCT evidence was identified for the other technologies.
-
Evidence from observational studies of switching to ADA96 and to ABT120 suggested that treatment response does not vary significantly according to the TNF inhibitor that the patients had previously tried.
-
Assuming no interaction between the technologies that have been used sequentially, the results of this subgroup analysis provide an indication of whether patients previously treated with different TNF inhibitors represented distinctly different populations when they switch. Limited data do not suggest this is the case although the evidence is very limited in view of possible combinations of treatment sequence.
Other subgroups
Commercial-in-confidence information (or data) removed.
Ongoing studies
Electronic searches
Electronic searches for ongoing studies identified only two relevant studies. One of these is looking at extended treatment with RTX in patients who have had an inadequate response (due to toxicity or inadequate efficacy) to previous or current treatment with ETN, IFX or ADA are being entered into an open-label study of two doses RTX and subsequently randomised to a third dose or placebo (if still having B cells). The study acronym is EXTRRA and it is being conducted in the UK. It has a target sample size of 60. The study appears to have been completed in 2010 but has not yet been published. Parts of this study are relevant to the decision problem in this report.
The second study is a ‘multicentre clinical observation real-life study’ of RTX in patients with active RA whose current treatment with TNF inhibitors in combination with MTX is insufficient. The study acronym is RIRA, and it has a target sample size of 20. It appears to have been undertaken in Austria and to have been completed. This study does not as yet appear to have been published.
Manufacturer’s submissions
Mentions of ongoing studies in the MSs were as follows:
-
Adalimumab: no explicit statements are provided in the MS about ongoing studies on ADA. Data from large registries are included.
-
Etanercept: no explicit statements are provided in the MS about ongoing studies on ETN. Data from registries and LTEs are included.
-
Infliximab: the MS provides details on an ongoing multicentre open-label RCT (RE-START; C0168Z05) which aims to assess the efficacy and safety of IFX in patients with active RA who inadequately respond to ETN or ADA. The primary outcome is EULAR response at week 10. Other outcomes will include ACR, tender/swollen joints, HAQ and HRQoL using the SF-36 instrument. Evaluations will be made up to 26 weeks. The study is being conducted in North America, the EU and Israel. The sample size is indicated as ∼ 200.
-
Rituximab: the MS lists eight ongoing studies (REFLEX open-label extension, SERENE, IMAGE, MIRROR, SUNRISE, SIERRA, DANCER open-label extension, WA16291 and its open-label extension) and various data are presented from these studies in the submission.
-
Abatacept: no explicit statements are provided in the MS about ongoing studies on ABT. Data from registries and LTEs are included.
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
Methods
Search strategy
Articles on the cost and cost-effectiveness of drugs for RA after the failure of a TNF inhibitor were identified from the searches for clinical effectiveness. In addition, the NHS Economic Evaluation Database (NHS EED), Cochrane Library 2009 (Issue 3) and the internet sites of national economic units were searched.
Study selection
All articles identified in the searches were imported into the same reference manager database (reference manager v.11, Thomson ResearchSoft) as for clinical effectiveness. Titles and abstracts were independently checked for relevance based on the population and intervention by two reviewers alongside selection of papers for clinical effectiveness. If articles were considered relevant by at least one of the reviewers, a full paper copy was ordered. A flow chart presenting the process of selection of studies for the systematic review can be found in Appendix 3.
One reviewer applied the inclusion and exclusion criteria using a standard checklist (see Appendix 7). Data was extracted by one reviewer using a pre-designed data extraction form and were independently checked by a second reviewer. Data on the following were extracted:
-
study characteristics, such as form of economic analysis, population, interventions, comparators, perspective, time horizon and modelling used
-
clinical effectiveness and cost parameters, such as effectiveness data, health-state valuations (utilities), resource-use data, unit cost data and key assumptions
-
results and sensitivity analyses.
These characteristics and the main results of included economic evaluations are summarised in subsequent tables. The study population and question, selection of alternatives, form of evaluation, effectiveness data, costs, benefit measurement and valuation, decision modelling, discounting, allowance for uncertainty and presentation of results were all evaluated as part of this process.
In addition, all five manufacturers submitted economic analyses. These submissions are reviewed in detail in Critique of manufacturers’ submissions.
Results
Thirty-eight papers were potentially relevant and ordered. One paper140 was unobtainable. Four studies met the inclusion criteria and the key features of these studies are summarised in Table 69. Further details of the four studies are presented in Appendix 9. Their quality was assessed using a simplified version of the Drummond and Jefferson checklist. 141 A summary of the strategies compared and incremental cost-effectiveness ratios (ICERs) reported from these studies is provided in Table 70. A list of the excluded papers with reasons for exclusion is presented in Appendix 5.
| Study | Drug considered | Population (patients with RA who failed to respond adequately to) | Form of economic analysis | Model used | Time horizon |
|---|---|---|---|---|---|
| Vera-Llonch 2008142 | ABT | TNF inhibitors | Cost–utility | Patient-level simulation |
10 years Lifetime |
| Russell 2009143 | ABT | ETNa | Cost-effectiveness | Decision tree | 2 years |
| Kielhorn 2008144 | RTX | Two non-biologic DMARDs and one TNF inhibitor | Cost–utility | Markov | Lifetime |
| Lindgren 2009145 | RTX | One or more TNF inhibitors | Cost–utility | Patient-level simulation | Lifetime |
| Drug | Study | Time horizon | Strategies compared | ICER |
|---|---|---|---|---|
| ABT | Vera-Llonch 2008142 | 10 years | ABT + MTX vs MTX | US$50,576 per QALY |
| Lifetime | US$45,979 per QALY | |||
| Russell 2009143 | 2 years | ABT → IFX → DMARDs vs IFX → ADA → DMARDs | CAN$12,514 per additional case of ‘low disease-activity state’ gained | |
| CAN$16,829 per additional remission gained | ||||
| RTX | Kielhorn 2008144 | Lifetime | RTX → DMARDs vs DMARDs | £14,690 per QALY |
| RTX → ADA → IFX → DMARDs vs ADA → IFX → DMARDs | £11,601 per QALY | |||
| Lindgren 2009145 | Lifetime | RTX → TNF inhibitors vs TNF inhibitors | RTX dominates TNF inhibitors |
The review identified two ABT studies, and these differed in how ABT was modelled. Vera-Llonch et al. 142 considered ABT with MTX compared with MTX alone while Russell et al. 143 considered ABT first, then switch to IFX if there was no response, then switch to conventional DMARDs compared with IFX first, then switch to ADA if there was no response, then switch to conventional DMARDs.
The review also identified two RTX economic evaluations, and these differ in how RTX was modelled. Kielhorn et al. 144 considered two different RTX pathways (RTX followed by traditional DMARDs compared with traditional DMARDs only and RTX first, then switch to ADA if there was no response, then switch to IFX if there was no response, then switch to traditional DMARDs, compared with ADA first, then switch to IFX, then switch to conventional DMARDs). Lindgren et al. 145 considered RTX first, followed by a series of TNF inhibitors compared with a series of TNF inhibitors.
Data source
Both ABT studies142,143 used the ATTAIN trial127–132 as their source for ABT effectiveness. Russell et al. 144 also extracted the effectiveness of TNF inhibitors in patients with an inadequate response to TNF inhibitors from the ATTAIN trial,127–132 assuming a 10% reduction after each switch. The same study also used the TEMPO (Trial of Etanercept and Methotrexate with radiographic Patient Outcomes) trial as the source for ETN effectiveness, when ETN appears in the sequence for the first time in patients with an inadequate response to DMARDs.
The two RTX studies144,145 used data from the REFLEX trial as their source for RTX effectiveness. Kielhorn et al. 144 calculated the mean drop in HAQ score for each of the responder groups from the REFLEX trial. 124–126 Utilities were mapped from the HAQ score and their model uses the equation as estimated by Bansback et al. 146 (QoL = 0.76 – 0.28 × HAQ + 0.05 × Female). Lindgren and colleagues146 in their model mapped utilities from an equation as estimated by patient-level data from the Southern Swedish Arthritis Treatment Group Registry (SSTAG) (QoL = 0.915 – 0.252 × HAQ – 0.05 × Male – 0.107 × DAS28). The SSATG data were also used to estimate the HAQ progression [HAQ progression = 0.106 + 0.241 × (HAQ at treatment start) + 0.002 × (Months on treatment) – 0.087 × (second line) – 0.192 × (third line) – 0.007 × (Disease duration)]. It is unclear though what type of regression was used; the text suggests linear whereas the table suggests logistic.
Study type
Three studies were cost–utility analyses, with the cost-effectiveness ratio reported as cost per QALY gained. 142,144,145 Russell et al. 143 used the DAS28 response and reported results in cost per additional case of ‘low disease activity state’ gained (DAS28 less than 2.6) and cost per additional remission gained (DAS28 less than or equal to 3.2).
Perspective
Kielhorn et al. 144 carried out the analysis from the UK health-care perspective. Lindgren et al. 145 carried out the analysis from a societal perspective, including direct and indirect costs as well as informal care, therefore, results are not directly relevant to a UK health-care perspective. Vera-Llonch et al. 142 carried out the analysis from a third-party payer perspective, including medical treatment only. Finally, Russell et al. 143 carried out the analysis from the Swedish health-care perspective. Therefore, results from Russell et al. 143 cannot be applied directly to the UK.
Modelling approach
Each study used a different modelling approach. Russell et al. 143 used a simple decision-tree structure and modelled cost and outcomes over 2 years. Vera-Llonch et al. 142 used a patient simulation model exploring two time horizons: 10 years and lifetime. Kielhorn et al. 144 used a Markov model structure with a lifetime time horizon and a 6-month cycle length. Lindgren et al. 145 used a patient-level simulation model. The time horizon of the model appears to be lifetime, although this was not explicitly stated in the paper. The model runs for continuous time with no fixed cycle length.
Findings
Russell et al. 143 conclude that ABT (followed by IFX, then switch to DMARDs) is a cost-effective strategy in patients with an inadequate response to ETN when compared with IFX (followed by ADA, then switch to DMARDs). The ICER was CAN$12,514 per additional case of ‘low disease activity state’ gained and CAN$16,829 per additional remission gained. Vera-Llonch et al. 142 concluded that ABT (combined with MTX) is cost-effective when compared with MTX alone, with an ICER of US$50,576 per QALY in the 10-year time horizon analysis and an ICER of US$45,979 per QALY in the lifetime time horizon. The results of the ABT studies are not comparable as one study143 is a cost-effectiveness analysis whereas the other is a cost–utility analysis,142 the studies do not have the same time horizon and, finally, do not apply the same perspective.
Kielhorn et al. 144 concluded that RTX is highly cost-effective for patients who have failed to respond adequately to one biologic DMARD. The ICER for RTX followed by DMARDs was £14,690 per QALY compared with conventional DMARDs only, while the ICER for RTX first, then switch to ADA, then to IFX, then to DMARDs, compared with ADA first, then switch to IFX, then to DMARDs, was £11,601 per QALY. Lindgren et al. 145 concluded that the RTX strategy (followed by a series of TNF inhibitors) was dominant (i.e. cheaper and provided a QALY gain) when compared with a TNF inhibitor strategy. This was explained by the lower price and better effect of RTX than the mix of second-line TNF inhibitors. Both studies favour RTX and their results could be comparable as both studies are cost–utility analyses with a lifetime horizon. However, the study by Lindgren et al. 145 uses a societal perspective, which could give a more favourable ICER (in this instance the RTX strategy dominates the TNF inhibitors strategy) as the difference in costs is driven by the indirect costs and the costs of informal care.
Summary
-
A direct comparison of ICERs between studies is not possible because of the different approaches to modelling, in particular time horizon, country of origin and perspective chosen.
-
All studies used a decision-analytic model. Published models vary in some important aspects: the type of model used, the sequence of drugs, comparator therapies and time horizon.
-
Incremental analyses, to which appropriate sensitivity analyses had been applied, were reported without exception.
-
All but one study carried out a cost–utility analysis and reported results in ‘cost per QALY’. One study carried out a cost-effectiveness analysis and reported results in cost per additional case of ‘low disease activity state’ gained (DAS28 less than 2.6) and cost per additional remission gained (DAS28 less than or equal to 3.2).
-
There was disparity in the selection of perspectives chosen for the analyses. One study reported costs that included both those from a health-care perspective as well as indirect costs and costs of informal care; inclusion of these costs improves the cost-effectiveness of the drug.
Critique of manufacturers’ submissions
A submission was received from each company, all including a model-based economic analysis. Table 71 provides a brief summary of the five economic analyses provided, based on the companies’ written submissions.
| Submission features | ADA (Abbott) | ETN (Wyeth Pharmaceuticals) |
IFX (Schering-Plough Ltd) |
RTX (Roche) |
ABT (Bristol-Myers Squibb Ltd) |
|---|---|---|---|---|---|
| Population | Adult patients with active RA who have had an inadequate response to MTX, sulfasalazine, HCQ and one TNF inhibitor | Adult patients with active RA who have had an inadequate response to ETN | Adult patients with active RA who have had an inadequate response to two non-biologic DMARDs and one TNF inhibitor | Adult patients with active RA who have had an inadequate response to a TNF inhibitor | Adult patients with moderate-to-severe RA who have had an inadequate response to at least one TNF inhibitor |
| Interventions and comparators |
GST → LEF → CyA → rescue vs ADA/ETN → GST → LEF → CyA → rescue vs IFX → GST → LEF → CyA → rescue vs RTX → GST → LEF → CyA → rescue vs ABT → GST → LEF → CyA → rescue vs ADA/ETN → RTX → GST → LEF → CyA → rescue |
ETN/IFX/ADA → DMARDs → ‘salvage therapy’ vs DMARDs → DMARDs → ‘salvage therapy’ vs RTX → DMARDs → ‘salvage therapy’ |
ADA → DMARDs vs ETN → DMARDs vs IFX → DMARDs vs ABT → DMARDs vs RTX → DMARDs vs ADA → RTX → DMARDs vs ETN → RTX → DMARDs vs IFX → RTX → DMARDs vs DMARDs |
RTX → LEF → GST → CyA → palliative care vs ETN → LEF → GST → CyA → palliative care vs ADA → LEF → GST → c CyA → palliative care vs IFX → LEF → GST → CyA → palliative care vs ABT → LEF → GST → CyA → palliative care vs LEF → GST → CyA → palliative care |
ABT → IFX → LEF → GST →AZA → CyA → penicillamine → palliative care vs RTX → IFX → LEF → GST → AZA → CyA → penicillamine → palliative care ABT → TNF inhibitors → LEF → GST → AZA → CyA → penicillamine → palliative care vs TNF inhibitors → TNF inhibitors → LEF → GST → AZA → CyA → penicillamine → palliative care |
| Form of analysis | Cost–utility analysis | Cost–utility analysis | Cost–utility analysis | Cost–utility analysis | Cost–utility analysis |
| Model used | Discrete event simulation model | Markov model | Patient simulation | Patient-level simulation | Patient-level simulation |
| Cycle length | Continuous | 6 months | 1 month | 6 months | Continuous |
| Time horizon | Lifetime | Lifetime | Lifetime | Lifetime | Lifetime |
| Base-case results presented: ICERs (£/QALY) |
ADA/ETN vs DMARDs: 15,962 IFX vs DMARDs: 21,529 RTX (9 months) vs DMARDs: 10,986 ABT vs DMARDs: 30,104 ADA/ETN → RTX vs DMARDs: 13,797 |
TNF inhibitors vs DMARDs: 14,501 TNF inhibitors vs RTX: 16,225 |
ADA vs DMARDs: 35,138 ETN vs DMARDs: 35,898 IFX vs DMARDs: 28,661 ABT vs DMARDs: 44,769 RTX vs DMARDs: 17,422 (9-month dose of RTX); 27,161 (6-month dose of RTX) ADA + RTX vs DMARDs: 27,998 (9-month dose of RTX); 32,345 (6-month dose of RTX) ETN + RTX vs DMARDs: 27,936 (9-month dose of RTX); 32,412 (6-month dose of RTX) IFX + RTX vs DMARDs: 24,236 (9-month dose of RTX); 28,617 (6-month dose of RTX) |
RTX vs ETN: RTX dominates RTX vs IFX: RTX dominates RTX vs ABT: RTX dominates RTX vs ADA: 310,771 RTX vs DMARDs: 5,311 |
ABT → IFX vs RTX → IFX: 20,438 ABT → TNF inhibitors vs TNF inhibitors → TNF inhibitors: 23,019 |
| PSA results |
ADA/ETN → RTX vs DMARDs: 100% cost-effective at £30,000/QALY RTX vs DMARDs probability of being cost-effective ~ 60% at £20,000/QALY ADA/ETN vs DMARDs probability of being cost-effective ~ 40% at £20,000/QALY |
Not presented |
RTX (9-month dose) vs DMARDs: probability of being cost-effective > 90% IFX vs DMARDs: probability of being cost-effective ~ 60% at £30,000/QALY IFX + RTX vs RTX: probability of being cost-effective > 40% at £30,000/QALY |
RTX vs ETN: RTX is 100% cost-effective, dominating 74% of iterations RTX vs IFX: RTX is 100% cost-effective, dominating 70% of iterations RTX vs ADA: RTX is 100% cost-effective, dominating 37% of iterations RTX vs ABT: RTX is 100% cost-effective, dominating 70% of iterations RTX vs DMARDs: RTX is 100% cost-effective |
Probability of ABT being cost-effective at £30,000/QALY: 99% when compared with RTX 97% when compared with TNF inhibitors |
| HAQ → QoL | EQ-5D = 0.82 − 0.11 × HAQ–0.07 × HAQ2 | EQ-5D = 0.76 − 0.28 × HAQ | NA | EQ-5D = 0.82 − 0.11 × HAQ − 0.07 × HAQ2 | HUI3 = 0.76 − 0.28 × HAQ + 0.05 × female |
| AEs |
Included Rates of TB (for TNF inhibitors) from BSRBR Rates of mild, moderate and serious AEs of ETN, IFX and LEF from an observational study LEF was used as a proxy for all traditional DMARDs ETN was used as a proxy for ADA, ABT and RTX |
Included Serious AEs were modelled at £1,181 AEs of conventional DMARDs assumed to be more frequent that those of TNF inhibitors |
Although the submission provides background evidence on AEs, they have not been included in the model |
Not included The clinical section of the submission indicates that the incidence of AEs is very similar across all treatments in the appraisal |
Included Sources were mainly published sources ABT has the lowest rates in all AEs apart from sinusitis |
| Mortality | Applied a treatment-specific mortality effect. Produced a parametric version of the mortality risk, with adjustments for treatment and HAQ | Used a baseline mortality of 1.63 times general population mortality, with an adjustment for change in HAQ (not clear how they have implemented this as they did not supply their model electronically) | Used mortality ratios dependent on age and gender but no variation by HAQ or treatment | Started from general population mortality and applied a multiplier of 1.33 to the power of the HAQ score, with the parameter 1.33 varied in sensitivity analysis | Started from general population mortality and applied a multiplier of 1.33 to the power of the HAQ score, with the parameter 1.33 varied in sensitivity analysis |
Abbott submission (adalimumab)
A discrete event simulation model was built to evaluate the cost-effectiveness of ADA. The type of evaluation undertaken was a cost–utility analysis with outcomes measured in QALYs.
Adalimumab was compared with all interventions included in the scope: ETN, IFX, RTX and ABT, all combined with MTX. In each of these five strategies, each drug was followed by GST, then LEF, then CyA, then rescue therapy. A comparison was also made with a strategy of traditional DMARDs only (GST, then LEF, then CyA, then rescue therapy) and also a strategy in which ADA (or ETN) is followed by RTX, GST, then LEF, then CyA, then rescue therapy.
It is assumed that the population has already had an inadequate response to at least two traditional DMARDs, as these are patients who have had an inadequate response to a TNF inhibitor. Therefore, MTX, sulfasalazine and HCQ are not considered as comparators in the economic evaluation.
Response rates are assumed to be equal across all the TNF inhibitors. In addition, drug, administration and monitoring costs of ADA and ETN are assumed to be equal. Therefore, ADA and ETN are evaluated in the same treatment sequence and results for these two drugs are considered similar throughout the submission.
New biologic agents (TOC, golimumab and certolizumab pegol) were excluded from the analysis as these drugs were considered not yet available in the UK.
Adverse events
Adverse events were included in the economic analysis. Rates of TB associated with each of the TNF inhibitors (ADA, ETN, IFX) were based on data from the BSRBR. 147 Rates of mild, moderate and serious AEs were estimated from an observational study in Sweden, which evaluated the safety of patients receiving ETN, IFX or LEF. 148 Values for these drugs were used as proxies for other drugs. The effect of this was that the rate of AEs was higher for conventional DMARDs than for biologics.
Health Assessment Questionnaire to utility
A quadratic mapping mechanism was used in order to convert HAQ scores to European Quality of Life-5 Dimensions (EQ-5D) scores (EQ-5D = 0.82 – 0.11 × HAQ – 0.07 × HAQ2). This equation was estimated through EQ-5D data collected in TOC trials [OPTION (tOcilizumab Pivotal Trial in methotrexate Inadequate respONders) and LITHE (tociLIzumab safety and THE prevention of structural joint damage)]. 149 The linear mapping mechanism reported in the same study (EQ-5D = 0.89 – 0.28 × HAQ) was explored in a sensitivity analysis.
Results
The base-case results show that all drugs (ADA/ETN, IFX, RTX and ABT, all followed by traditional DMARDs) may represent cost-effective treatment options when compared with a sequence of traditional DMARDs. RTX had the lowest ICER (£10,986) while ABT had the highest (£30,104). The strategy of introducing RTX after ADA/ETN (i.e. as a third-line biologic) had an ICER of £13,797 per QALY when compared with traditional DMARDs. The ICERs are as follows:
-
ADA/ETN versus DMARDs: £15,962 per QALY
-
IFX versus DMARDs: £21,529 per QALY
-
RTX (9-month dose) versus DMARDs: £10,986 per QALY
-
ABT versus DMARDs: £30,104 per QALY
-
ADA/ETN + RTX versus DMARDs: £13,797 per QALY.
Incremental cost-effectiveness ratios of ADA/ETN (followed by DMARDs) versus DMARDs presented in the sensitivity analyses varied from £11,191 per QALY to £26,456 per QALY, with ADA/ETN being cost-effective in the vast majority of the scenarios explored.
The probabilistic sensitivity analysis (PSA) results for 100 replications (for a cohort of 20,000 patients per replication) showed that at a willingness to pay (WTP) of £30,000 per QALY, ADA/ETN followed by RTX is the most cost-effective strategy, with the probability of being cost-effective being close to 1. At a WTP of £20,000 per QALY, RTX followed by conventional DMARDs is cost-effective, with a probability of being cost-effective at around 60%, while there is a 40% (approximate) chance of ADA/ETN followed by conventional DMARDs being cost-effective. The submission; however, states: ‘although the cost-effectiveness acceptability curve (CEAC) shows the probability that a treatment sequence is the most cost-effective option at various willingness-to-pay thresholds, it does not show all treatment strategies which can be considered cost-effective at these threshold(s)’. Therefore, the submission concludes that although the strategy of ADA/ETN followed by conventional DMARDs is never shown to be cost-effective (submission Figure 3.3.2.1),150 the deterministic results showed that it is cost-effective, with an ICER of under £16,000 per QALY. The MS fails to point out though that both RTX followed by conventional DMARDs and ADA/ETN followed by RTX had lower ICERs (£10,986 and £13,797, respectively).
Wyeth Pharmaceuticals submission (etanercept)
A Markov model (6-month cycle) was built to evaluate the cost-effectiveness of ETN. The type of evaluation undertaken was a cost–utility analysis with outcomes measured in QALYs. However, Wyeth Pharmaceuticals did not provide the model that produced the results presented in the submission.
Patients in the model were assumed to receive initial treatment with MTX, then to switch to sulfasalazine, then to switch to a ‘1st TNF inhibitor’. It is unclear which TNF inhibitor this was. However, cost data suggest that it is ETN in all strategies compared. Therefore, it is assumed that the population modelled were patients whose first failed TNF inhibitor was ETN.
The three strategies compared are second TNF inhibitor, DMARDs and ‘rituximab’, all followed by traditional DMARDs, and then the ‘best supportive care’ (salvage therapy). It is unclear; however, which TNF inhibitor is compared in the ‘second TNF inhibitor’ strategy. Cost data suggest that it was an average of ETN, ADA and IFX combined with MTX. Similarly, in the ‘DMARDs’ strategy, it was unclear which DMARD was compared: cost data suggest that it was MTX. Finally, the DMARD following a TNF inhibitor seems to be sulfasalazine (again based on cost data).
Cost-effectiveness results were presented for a range of assumed HAQ changes of both the TNF inhibitor (ETN/IFX/ADA) and the conventional DMARDs.
Adverse events
Adverse events were included in the economic analysis. For simplicity, only serious AEs were modelled, assuming that they last for one cycle (6 months) only. The cost of a serious AE was estimated at £1,181, which included two general practitioner (GP) visits and 7 inpatient days. The text (submission p. 33) suggests that various published sources were used for the rates of AEs for each drug. AE rates for all TNF inhibitors were assumed to be the same for ETN. Data in the table suggest that rates of AEs are higher in traditional DMARDs than in biologics.
Health Assessment Questionnaire to utility
A linear mapping mechanism was used in order to convert HAQ scores to EQ-5D scores during each model cycle (EQ-5D = 0.76 – 0.28 × HAQ). 151 It was assumed that patients experiencing serious AEs would lose 0.05 units of utility (or 10% of a QALY) over 1 year.
Results
Results were presented for a range of assumed HAQ changes of both TNF inhibitor (ETN/IFX/ADA) and conventional DMARDs. The ICER for TNF inhibitors versus conventional DMARDs was £14,501, when a HAQ drop of 0.55 was assumed for the TNF inhibitors and no change was assumed for the conventional DMARDs. The ICER for TNF inhibitors versus RTX was £16,225, when a HAQ drop of 0.55 was assumed for the TNF inhibitors and a HAQ drop of 0.40 was assumed for RTX.
Probabilistic sensitivity analysis results were not presented in the submission.
Schering-Plough Ltd submission (infliximab)
A patient-simulation/individual sampling model was used to evaluate the cost-effectiveness of IFX. The type of evaluation undertaken was a cost–utility analysis with outcomes measured in QALYs.
Nine treatment sequences were compared in the cost-effectiveness analysis:
-
ADA/ETN/IFX/RTX/ABT, each followed by a sequence of traditional DMARDs
-
ADA/ETN/IFX, each followed by RTX and then a sequence of traditional DMARDs
-
a sequence of traditional DMARDs.
Patients in the model could receive a maximum of two biologic DMARDs followed by a maximum of three non-biologic DMARDs and were limited to a maximum of five treatments within each of the nine sequences. New biologic agents (TOC, golimumab and certolizumab pegol) are excluded from the analysis as these drugs were considered not yet available in the UK.
The baseline characteristics of patients in the GO-AFTER (GOlimulab After Former anti-tumour necrosis factor Therapy Evaluated in Rheumatoid arthritis) trial, in which treatment with a TNF inhibitor (golimumab) following withdrawal from one or more previous TNF inhibitors (ADA, ETN or IFX) was investigated, were considered for the start of the model.
Adverse events
Adverse events were not included in the model although evidence on AEs was included in the efficiency part of the submission.
Health Assessment Questionnaire to utility
There was no mapping mechanism applied on EQ-5D scores. Utility gains or losses were modelled directly using a QoL measure. Each treatment was associated with an initial utility gain, which was estimated from BSRBR data.
Results
The base-case results showed that ADA, ETN, IFX and RTX (followed by traditional DMARDs) might represent cost-effective treatment options, whereas ABT (followed by traditional DMARDs) did not represent a cost-effective treatment option, when all strategies are compared with a sequence of traditional DMARDs. The ICERs were as follows:
-
ADA versus DMARDs: £35,138 per QALY
-
ETN versus DMARDs: £35,898 per QALY
-
IFX versus DMARDs: £28,661 per QALY
-
ABT versus DMARDs: £44,769 per QALY
-
RTX (9-month dose) versus DMARDs: £17,422 per QALY
-
RTX (6-month dose) versus DMARDs: £27,161 per QALY.
Further analysis, adding RTX after the TNF inhibitors (ADA, ETN, IFX), was performed. IFX had the lowest ICER for both doses of RTX explored (6-month dose/9-month dose) when compared with both traditional DMARDs and RTX (both followed by traditional DMARDs). The ICERs were as follows:
-
Versus DMARDs:
-
– ADA + RTX (9-month dose): £27,998 per QALY
-
– ADA + RTX (6-month dose): £32,345 per QALY
-
– ETN + RTX (9-month dose): £27,936 per QALY
-
– ETN + RTX (6-month dose): £32,412 per QALY
-
– IFX + RTX (9-month dose): £24,236 per QALY
-
– IFX + RTX (6-month dose): £28,617 per QALY.
-
-
Versus RTX:
-
– ADA + RTX (9-month dose): £41,747 per QALY
-
– ADA + RTX (6-month dose): £39,084 per QALY
-
– ETN + RTX (9-month dose): £42,477 per QALY
-
– ETN + RTX (6-month dose): £39,673 per QALY
-
– IFX + RTX (9-month dose): £33,274 per QALY
-
– IFX + RTX (6-month dose): £30,549 per QALY.
-
Overall, when compared with DMARDs, RTX had the lowest ICER for both 9-month (£17,422 per QALY) and 6-month doses (£27,161 per QALY). Among TNF inhibitors (ETN, IFX, ADA), IFX had the lowest ICER (£28,661 per QALY).
Incremental cost-effectiveness ratios in the sensitivity analyses varied from £16,752 per QALY (RTX vs DMARDS, when a HAQ improvement of 0.01 per annum was assumed for all biologic DMARDS) to £58,850 per QALY (IFX + RTX vs RTX, when the weight of the patient was assumed to be 120 kg).
The PSA results showed that, when compared with traditional DMARDs, the probability of RTX (9-month dose) being cost-effective was greater than 90% at a range of WTP thresholds greater than £20,000 per QALY. When a 6-month dose was assumed for RTX, the probability of RTX being cost-effective was marginally greater than the probability of IFX being cost-effective, at WTP greater than £20,000 per QALY. The probability of IFX (vs DMARDs) being cost-effective was ∼ 60% at £30,000 per QALY. When compared with RTX, the probability of IFX followed by RTX being cost-effective was greater than 40% at £30,000 per QALY.
Roche submission (rituximab)
A patient-level simulation was built to evaluate the cost-effectiveness of RTX. The type of evaluation undertaken was a cost–utility analysis with outcomes measured in QALYs.
Rituximab was compared with all interventions included in the scope: ADA, ETN, IFX and ABT. In addition, RTX was compared with a strategy of traditional DMARDs. In all strategies compared, the first active treatment was followed by salvage therapy consisting of LEF, GST and CyA followed by palliative care. Response rates of LEF, GST and CyA were assumed to be equivalent to MTX for this population. Comparison of RTX against the new biological agents (TOC, golimumab and certolizumab pegol) was not performed as these treatments were considered not used in routine clinical practice in the NHS.
Adverse events
Adverse events were not included in the economic analysis. The clinical section of the submission indicates that the incidence of AEs was very similar across all treatments in the appraisal. Given that RTX was compared head-to-head with each of the interventions in the scope, it was assumed that the costs of treating an AE would be the same in all strategies compared and therefore the cost-effectiveness ratios would not be affected by these costs.
Health Assessment Questionnaire to utility
A quadratic mapping mechanism was used in order to convert HAQ scores to EQ-5D scores during each model cycle (EQ-5D = 0.82 – 0.11 × HAQ – 0.07 × HAQ2). This equation was estimated through EQ-5D data collected in two Roche phase III trials [DMARD-inadequate response (IR)] for TOC. The linear mapping mechanism used by Bansback et al. 146 [Health Utilities Index Mark 3 (HUI3) = 0.76 – 0.28 × HAQ + 0.05 × female] was explored in a scenario analysis.
The model also assumed that the relationship of HAQ score to patient-reported utility was independent of the number of previous biologics used. Moreover, for the base-case analysis, the model allowed for estimates of QALYs being less than zero, when patients progress to very high HAQ scores. However, this relationship was not explored in the sensitivity analysis by adding a restriction to the negative QALY values.
Results
The base-case results showed that RTX dominates ETN (incremental costs –£13,246; incremental QALYs 0.0168), IFX (incremental costs –£10,490; incremental QALYs 0.0699) and ABT (incremental costs –£16,075; incremental QALYs 0.0606). When compared with ADA, RTX was less costly (incremental costs –£13,551) but also less effective (incremental QALYs –0.0436) with an ICER of £310,771 per QALY. When compared with the traditional DMARDs strategy, RTX was more costly (incremental costs £6,323) but also more effective (incremental QALYs 1.0705), with an ICER of £5,311 per QALY.
Overall, TNF inhibitors (ETN, IFX, ADA) were dominated by RTX, i.e. RTX was more effective and less costly. ADA was marginally more effective but also more costly than RTX, resulting in an ICER of £310,771 per QALY. When compared with traditional DMARDs, RTX was cost-effective at £5,311 per QALY.
Incremental cost-effectiveness ratios in the sensitivity analyses varied from £4,898 per QALY (vs traditional DMARDs when a 9-month time to retreatment was assumed for RTX) to £326,397 per QALY (vs ADA when a linear mapping mechanism was assumed for the HAQ to QoL conversion), while in most of the scenarios RTX dominated the other strategies (i.e. RTX was less costly and more effective).
The PSA results for 1,000 Monte Carlo simulations showed that the probability of RTX being cost-effective is 100% at a wide range of WTP thresholds (£5,000–400,000 per QALY).
Bristol-Myers Squibb Ltd submission (abatacept)
A patient-level simulation model was built to evaluate the cost-effectiveness of ABT. The type of evaluation undertaken was a cost–utility analysis with outcomes measured in QALYs. Baseline patient characteristics were from the ATTAIN trial. 127–132 Data from ATTAIN,127–132 REFLEX124–126 and BSRBR were used for the treatment efficacy of the drugs modelled.
Abatacept was compared with all interventions included in the scope: ADA, ETN, IFX and RTX. However, TNF inhibitors were also grouped under a ‘basket’ of TNF inhibitors and these were the base-case comparator. The rationale was reported as based on the conclusions from the NICE appraisal of the sequential use of TNF inhibitors. 152 In addition, the submission argued that TNF inhibitors were grouped because no data were available to draw conclusions about the efficacy of different TNF inhibitors, after a failure of a first TNF inhibitor.
The ‘basket’ labelled TNF inhibitors was defined through use of market share data estimated through survey data (Bristol-Myers Squibb Ltd data on file). These were 22% ETN, 52% ADA, 24% IFX and 2% RTX for the second-line treatment, and 15% ETN, 9% ADA, 37% IFX and 38% RTX for the third-line treatment, as presented on p. 134 of the submission. Patients in the model were randomly assigned to one of the three ‘basket’ treatments, based on these data, after excluding RTX. Efficacy, costs and other parameters related to that therapy were applied to the proportion of patients receiving that therapy. Total costs and outcomes of the ‘basket’ treatment are the sum of the three ‘basket’ therapies.
There were two main comparisons. In the first comparison ABT was compared with RTX, both followed by IFX, then traditional DMARDs, then palliative care. In the second comparison, ABT was compared with a ‘basket’ of TNF inhibitors, both followed by another ‘basket’ of TNF inhibitors, then traditional DMARDs, then palliative care.
Traditional DMARDs were not considered as comparators in the economic analysis on the basis that the target population (RA patients with an inadequate response to TNF inhibitors) should have tried multiple traditional DMARDs, and so it was assumed that clinicians were unlikely to revert to these therapies. DMARDs were included as part of the sequence of treatments only after an insufficient response or intolerance to multiple biological therapies (after failure of three biologic DMARDs). After failing DMARDs, patients received NSAIDs only (palliative care).
Other new biologic agents were not considered as comparators for two reasons. Firstly, price information for the new biological therapies was not available at the time of writing. Secondly, new biological therapies were considered not routinely used in the NHS.
In summary, this submission did not consider a ‘non-biologic’ strategy. All strategies compared included at least two biologic DMARDs (patients with an inadequate response to one TNF inhibitor).
Adverse events
Adverse events were assumed to reduce QoL as well as increasing costs. The following AEs were included in the economic analysis: infusion-related reaction, injection site reactions, upper respiratory tract infection and urinary tract infection, rash, nausea, neutropenia, hypotension, leucopenia, severe allergic reaction and sinusitis. The sources for the rates of the AEs were mainly published data. 126,130 ABT was associated with the lowest rates of all AEs apart from sinusitis.
Health Assessment Questionnaire to utility
A linear mapping mechanism was used in order to convert HAQ scores to HUI3 scores during each model cycle (HUI3 = 0.76 – 0.28 × HAQ + 0.05 × female). 153 The submission discussed the available sources for conversion of HAQ to utility, and selected the formula above for the base-case analysis, on the basis that this formula was used in previous RA appraisals and models144,146,154 and was preferred over other algorithms155,156 by the Evidence Review Group (ERG) in the original ABT appraisal. The submission acknowledged that the average baseline HAQ score of 1.5 from the formula selected might not be appropriate for a population with an inadequate response to one TNF inhibitor, and therefore explored the EQ-5D approach157 in sensitivity analysis.
Results
The base-case results showed that ABT was cost-effective when compared with RTX (both followed by IFX as the third biologic) with an ICER of £20,438 per QALY. ABT was also cost-effective when compared with a ‘basket’ of TNF inhibitors (both followed by another ‘basket’ of TNF inhibitors) with an ICER of £23,019 per QALY. Overall, the results showed that the ICERs for ABT were all below £30,000 whether compared with single or a ‘basket’ of TNF inhibitors, or RTX.
Incremental cost-effectiveness ratios for ABT in the sensitivity analyses varied from £14,145 per QALY (vs RTX, when a 1.5% discount rate was assumed for QALYs) to £40,534 (vs RTX, when the ABT HAQ progression rate was assumed to be 0.012 rather than –0.013 in the base case).
The PSA results showed that the probability of ABT being cost-effective was 99% at £30,000 per QALY when compared with RTX. When compared with a ‘basket’ of TNF inhibitors, the probability of ABT being cost-effective was 97% at £30,000 per QALY. However, the submission failed to report any other PSA results (particularly below the £30,000 per QALY threshold). From the presented figures it seems that at £20,000 per QALY, both RTX and the ‘basket’ of TNF inhibitors were cost-effective when compared with ABT, with the probabilities being greater than 50% and greater than 95%, respectively.
Summary
A key issue is the appropriate comparator to be used. All but one submission chose conventional DMARDs as their base-case comparator. One submission did not consider a strategy of conventional DMARDs at all, assuming a switch to a third biologic in all strategies compared.
All submissions used the same type of economic evaluation, with cost per QALY being offered as efficiency measure.
There is some variation in the methods used and sources of data for important model inputs such as QoL scores or baseline population characteristics. Three submissions considered AEs in their model; however, methods and sources of rates and costs of AEs varied.
Critique of indirect comparisons and mixed-treatment comparisons included in manufacturers’ submissions
Four of the manufacturers (Abbott, Schering-Plough Ltd, Roche and Bristol-Myers Squibb Ltd) used results from ICs and/or mixed-treatment comparisons (MTCs) to inform their model. This section provides a critical appraisal of these analyses and highlights issues that may impact on the validity of their results.
Before commencing on the critique of IC/MTC, it is pertinent to clarify the definition of these terms. NICE’s Methods guide (2008) states that ‘a mixed treatment comparison (MTC) includes trials that compare the interventions head-to-head and indirectly’, whereas an IC is a ‘synthesis of data from a network of trials’. These two terms have been used inconsistently and sometimes inter-changeably in some of the MSs. In this section of the assessment report, all the syntheses of data from a network of trials without incorporating evidence from head-to-head trials are referred to as ICs in line with the methods guidance. This also avoids creating a false impression that direct evidence from head-to-head trials was included in these analyses. Only analyses that incorporated both direct and indirect evidence were referred to as MTCs.
For the RA population defined in the scope of this appraisal (patients who had inadequate response to a TNF inhibitor), no head-to-head trial between the five technologies under assessment was identified by the assessment group and the manufacturers, and thus it was not possible to carry out an MTC. IC was possible between RTX and ABT through placebo-controlled trials of respective drugs. This was conducted by Roche and Bristol-Myers Squibb Ltd.
Owing to the lack of head-to-head trials and a complete absence of placebo-controlled trials for the three TNF inhibitors under assessment in the population defined by the scope, three manufacturers have attempted to carry out ICs/MTCs by extending the inclusion criteria to the RA population outside the scope (e.g. patients who had not been treated with a TNF inhibitor and/or patients who had not been treated with MTX). One head-to-head trial exists in this broader population and thus an MTC combining direct and indirect evidence is possible. The key issue for this approach is whether basic assumptions with regard to clinical and methodological homogeneity and exchangeability of estimated treatment effects between trials held.
Indirect comparisons in patient population specified in the scope
Roche and Bristol-Myers Squibb Ltd performed ICs for the RA population defined in the scope using network meta-analyses/Bayesian methods (see Table 72, Roche TNF-IR IC and Bristol-Myers Squibb Ltd IC). The ICs were based on the same placebo-controlled RCTs for RTX (REFLEX trial124–126) and ABT (ATTAIN trial127–132), and additionally included a placebo-controlled RCT for TOC [RADIATE (Research on Actemra Determining effIcacy after Anti-TNF failurEs)]. A further golimumab RCT (GO-AFTER) was also in the Bristol-Myers Squibb Ltd analysis. No placebo-controlled trial for the patient population defined in the scope was identified for ADA, ETN and IFX, and thus it was not possible to include the three TNF inhibitors. The selection and inclusion of TOC and golimumab trials in the IC seemed arbitrary as they provided no evidence regarding the relative effectiveness of RTX versus ABT. The inclusion of these trials had little impact on the estimates of relative effectiveness (expressed as response rates to ACR response criteria and RRs/odds ratios) between RTX and ABT compared with results from a pair-wise IC conducted by the assessment group based on the same RTX and ABT trials (see bottom of Table 73).
| Summary item | Abbott (ADA) MTC | Schering-Plough Ltd (IFX) MTC | Roche (RTX): TNF-IR IC and DMARD-IR MTC |
Bristol-Myers Squibb Ltd (ABT) IC |
|---|---|---|---|---|
| Literature search | Based on a number of previous studies/reports (Nixon et al. 2007,158 Wailoo 2008,159 Bristol-Myers Squibb Ltd submission160 and ERG report for TA141161) plus an updated search of PUBMED from 1 January 2005 to 31 May 2009 | Search of EMBASE, MEDLINE, MEDLINE In Process and Cochrane Library from inception to April 2009; bibliographies of identified studies | Search of MEDLINE and EMBASE from 1990 through 2007 | Search of multiple databases from 1 January 1990 to 8 May 2009, conference abstracts, manufacturers and NICE web sites, bibliographies of identified studies |
| Inclusion criteria |
Design: clinical trial Population: broader than scope (including patients not previous treated with TNF inhibitors and/or MTX) Intervention: broader than scope (including anakinra, certolizumab pegol, golimumab, and TOC) Outcome: need to report ACR response Other: at least 6-month follow-up time |
Design: double-blind RCTs ≥ 24 weeks (except RTX trials) Population: broader than scope (including RA patients of any stage) Intervention: broader than scope (including certolizumab pegol, golimumab and TOC) Outcome: need to report ACR response criteria or mean change in HAQ score Other: published as full papers in English |
Design: RCTs of duration ≥ 6 months Population: two analyses were performed: TNF inadequate response (TNF-IR) IC: same as scope DMARD inadequate response (DMARD-IR) MTC: Population: outside scope (including patients who had inadequate response to DMARD but predominantly not previously treated with a TNF inhibitor) Intervention: broader than scope (including TOC) Outcome: need to report ACR response criteria/ACR core disease parameters Other: published as full papers in English, German, French and Dutch |
Design: RCTs Population: same as scope intervention: broader than scope (including certolizumab pegol, golimumab and TOC) Outcome: clinically relevant outcomes Other: published as full papers in English and conducted in Europe or America |
| Included studies |
29 RCTs, plus one open-label randomised study, three prospective cohort study, one study based on registry Within scope (2): ABT (1), RTX (1), plus five other studies of TNF inhibitors Outside scope (27)c: ABT (4), ADA (5) ETN (5), IFX (2), anakinra (3), certolizumab pegol (1), golimumab (3), TOC (5) |
34 RCTs Within scope (2): RTX (1), ABT (1) Outside scope (32)c: ADA (7), ETN (5), IFX (4), RTX (2), ABT (4), certolizumab pegol (3), golimumab (3), TOC (5) |
TNF-IR IC : Three RCTs Within scope (2): RTX (1), ABT (1) Outside scope (1): TOC (1) DMARD-IR MTC: 18 RCTs Within scope (0): none Outside scope (18):a,c ADA (4), ETN (4), IFX (3), RTX (2),b ABT (3), TOC (3) |
Four RCTs Within scope (2): RTX (1), ABT (1) Outside scope (2): TOC (1) and golimumab (1) |
| Assessment of homogeneity and similarityd between included studies | Not stated | Not stated. Plots of the treatment effect on ACR response against baseline HAQ and disease duration were used to selected covariables into the analyses | Homogeneity at each ACR response level was assessed using Q-statistics. Stated that ‘baseline characteristics across the trials were comparable with respect to ACR core parameters’ | Not stated |
| Outcome analysed | ACR20, ACR50 and ACR70 | ACR20, ACR50 and ACR70 | ACR20, ACR50 and ACR70 | Multiple outcomes including ACR responses; response criteria derived from DAS HAQ scores; withdrawal, DAS and HAQ change from baseline; various outcomes on AEs, component outcomes of ACR criteria; SF-36 component summary scores |
| Analytical methods | Bayesian hierarchical models estimated with winbugs. ACR responses were modelled on a log-odds ratio scale. Log-odds ratios of responses were adjusted for addition of MTX, disease duration and baseline HAQ among other variables. Also used ‘Fully conditional predictive mean matching’ to impute data | Network meta-analyses conducted on an ordered logit scale. Analyses were performed both with and without adjustment of disease duration | Analyses were performed with winbugs and conducted with non-informative priors. Results for TNF inhibitors were pooled | Models were fitted using winbugs, employing Markov chain Monte Carlo (MCMC) simulation. Both fixed-effects and random-effects estimation was conducted for all analyses |
| Input into the manufacturer model | Using Bayesian hierarchical models, posterior mean predicted treatment response rates (predicted for a patient with a disease duration of 11 years and an average HAQ score of 2.1) | Odds ratios (adjusted for disease duration) for ACR responses derived from IC were used in the model | For RTX and ABT, ACR response rates from TNF-IR ICs were used. For TNF inhibitors, ACR response rates from DMARD-IR MTC were firstly discounted by 30% and then used in the model | Results from IC for HAQ change were used in the model, but only for RTX and ABT. Data from registry (BSRBR) on HAQ change were used for TNF inhibitors |
| Comments |
Included trials of both early and late RA populations with very different treatment history (e.g. patients who had inadequate response to a TNF inhibitor vs patients who were naive to TNF inhibitors vs patients who were naive to MTX). The basic requirement for ICs with regard to exchangeability of relative treatment effect between trials cannot be assumed and thus the validity of the results is questionable Also the IC included evidence from multiple study design (i.e. RCTs and observational studies). RCT evidence did not appear to have been analysed separately from evidence from observational studies. The nature of randomised comparison therefore may not have been preserved. In addition, different search strategies and inclusion criteria were applied for different technologies |
Included trials of both early and late RA populations with very different treatment history (e.g. patients who had inadequate response to a TNF inhibitor vs patients who were naive to TNF inhibitors vs patients who were naive to MTX). The basic requirement for ICs with regard to exchangeability of relative treatment effect between trials cannot be assumed. The validity of the results is questionable particularly because the IC used MTX as the reference standard (i.e. the hub of the evidence network) for comparison The proportional odds assumption of the ordered logit model (i.e. treatment effect was constant across ACR20, 50 and 70) did not seem to be consistent with observations from REFLEX124–126 and ATTAIN127–132 trials |
Patient populations included in TNF-IR IC were in line with the scope. The major limitation of the analysis was that only one trial each was available for RTX and ABT and no trial was available for the three TNF inhibitors The inclusion of the TOC trial appeared arbitrary as it provided no information regarding relative effectiveness of RTX and ABT. The inclusion of the trial had little impact on the estimates of relative effectiveness (in terms of ACR responses) between RTX and ABT compared with a pair-wise adjusted IC conducted by the assessment group based on the same trials (see bottom of Table 73) RRs were translated into response rates using the pooled placebo response as baseline. Given the substantial heterogeneity between studies (e.g. placebo response rates for ACR20 ranged from 15% to 72% according to Figure 35 of Roche submission), the validity of pooling placebo response across studies and consequently the RRs derived from it was questionable |
Patient populations included in the IC were in line with the scope. The major limitation of the analysis was that only one trial each was available for RTX and ABT and no trial was available for the three TNF inhibitors The inclusion of the TOC and golimumab trials appeared arbitrary as they provided no information regarding relative effectiveness of RTX and ABT. The inclusion of these trials had little impact on the estimates of relative effectiveness (in terms of ACR responses) between RTX and ABT compared with a pair-wise adjusted IC conducted by the assessment group based on the same trials (see bottom of Table 73) |
| Interventions/comparatorsa | ACR20 | ACR50 | ACR70 | |
|---|---|---|---|---|
| ACR responses | ||||
| Control (traditional DMARD/placebo/none) | % | |||
| Data from RCTs | GO-AFTER (week 14) | 18 | 6 | 2 |
| REFLEX (week 24) | 18 | 5 | 1 | |
| ATTAIN (week 24) | 20 | 4 | 2 | |
| Results from IC/MTC | Abbott MTC (model input) | 25 | 10 | 4 |
| Roche DMARD-IR MTC | 32 | 12 | 4 | |
| Roche TNF-IR IC | 15 | 4 | 1 | |
| TNF inhibitors | % | |||
| Data from RCT | GO-AFTER (golimumab 50 mg) week 24 | 34 | 18 | 12 |
| GO-AFTER (golimumab 100 mg) week 24 | 44 | 20 | 10 | |
| Results from IC/MTC | Abbott MTC (model input) | 64 | 40 | 21 |
| Roche DMARD-IR MTC | ||||
| ADA | 66 | 44 | 18 | |
| ETN | 64 | 36 | 14 | |
| IFX | 60 | 33 | 14 | |
| 30% degradation of Roche DMARD-IR MTC (model input) | % | |||
| ADA | 46 | 31 | 13 | |
| ETN | 45 | 25 | 10 | |
| IFX | 42 | 23 | 10 | |
| RTX | % | |||
| Data from RCT | REFLEX (RTX) week 24 | 51 | 27 | 12 |
| Results from IC/MTC | Abbott MTC (model input) | 62 | 38 | 20 |
| Roche DMARD-IR MTC | 60 | 35 | 18 | |
| Roche TNF-IR IC (model input) | 46 | 23 | 14 | |
| ABT | % | |||
| Data from RCT | ATTAIN (ABT) week 24 | 50 | 20 | 10 |
| Results from IC/MTC | Abbott MTC (model input) | 55 | 31 | 15 |
| Roche DMARD-IR MTC | 59 | 33 | 15 | |
| Roche TNF-IR IC (model input) | 43 | 22 | 8 | |
| Estimates of relative effectiveness | ||||
| TNF inhibitors vs control (odds ratios) | ||||
| Data from RCT | GO-AFTER (golimumab 50 mg) week 24 | 2.55 | 4.12 | 4.0 |
| GO-AFTER (golimumab 100 mg) week 24 | 3.87 | 4.67 | 3.5 | |
| Results from IC/MTC | Schering-Plough Ltd MTC, ADA | Commercial-in-confidence information (or data) removed | ||
| Schering-Plough Ltd MTC, ETN | Commercial-in-confidence information (or data) removed | |||
| Schering-Plough Ltd MTC, IFX | Commercial-in-confidence information (or data) removed | |||
| Bristol-Myers Squibb Ltd IC, golimumab 50 mg | 2.55 | 4.30 | NA | |
| Bristol-Myers Squibb Ltd IC, golimumab 100 mg | 3.90 | 4.89 | NA | |
| RTX vs control (odds ratios) | ||||
| Data from RCT | REFLEX (RTX) week 24 | 4.77 | 7.00 | 13.67 |
| Results from IC/MTC | Schering-Plough Ltd MTC | Commercial-in-confidence information (or data) removed | ||
| Bristol-Myers Squibb Ltd IC | 4.84 | 7.27 | 16.38 | |
| ABT vs control (odds ratios) | ||||
| Data from RCT | ATTAIN (ABT) week 24 | 4.18 | 6.53 | 7.40 |
| Results from IC/MTC | Schering-Plough Ltd MTC | Commercial-in-confidence information (or data) removed | ||
| Bristol-Myers Squibb Ltd IC | 4.20 | 6.98 | 9.28 | |
| RTX vs ABT (RRs) | ||||
| Results from ICs | Assessment group IC | 1.12 | 1.00 | 1.80 |
| Roche TNF-IR IC | 1.06 | 1.05 | 1.75 | |
| Bristol-Myers Squibb Ltd IC (ratio of odds ratios) | 1.14 | 1.07 | 1.85 | |
Roche and Bristol-Myers Squibb Ltd used results from ICs described above to inform their model (ACR responses for Roche; HAQ changes for Bristol-Myers Squibb Ltd). However, this was restricted to the estimates of effectiveness for RTX and ABT and was not applicable for the estimates of effectiveness for TNF inhibitors. For TNF inhibitors, Roche used results from a separate MTC based on different patient populations outside the scope (described below), whereas Bristol-Myers Squibb Ltd used observational data from the BSRBR. The comparisons of effectiveness between TNF inhibitors and RTX/ABT in their models were therefore not based on an IC or MTC.
Mixed-treatment comparisons in patient population outside the scope
Three manufacturers have carried out MTCs based on RCTs of an RA population outside the scope [e.g. patients who had not been treated with a TNF inhibitor and/or patients who had not been treated with MTX; see Table 72 Abbott (MTC), Schering-Plough Ltd (MTC) and Roche (DMARD-IR MTC)].
Owing to the broad inclusion criteria beyond the scope of the appraisal, substantial clinical and statistical heterogeneity exists between the RCTs included in the MTCs. The basic requirement for ICs/MTCs regarding the exchangeability of relative treatment effects between the included studies could not be assumed and thus the validity of the results was questionable. The violation of the basic requirement was particularly prominent in the MTCs conducted by Abbott and Schering-Plough Ltd, in which RCTs of early RA patients who were naive to MTX treatment were included in the analyses along with RCTs of late RA patients who had inadequate response to MTX and/or TNF inhibitors.
Despite the broad inclusion criteria for the MTCs, clinical and methodological similarity/difference between the included studies was only briefly described or not mentioned at all. Statistical heterogeneity between included studies was either not assessed or (where assessed) only dealt with by using random-effects models without further exploration of the potential source of heterogeneity. All the MTCs included a head-to-head trial [ATTEST (Abatacept or infliximab vs placebo, a Trial for Tolerability, Efficacy and Safety in Treating RA), comparing IFX with ABT], but did not examine the direct evidence separately from indirect evidence. Consistency between direct and indirect evidence was not examined.
There is an appreciable difference between the results obtained from the three MTCs (which were based on population outside the scope) and the actual results (where available) observed in RCTs conducted in relevant populations defined in the scope (see Table 73). For ACR response criteria, results from these MTCs tend to overestimate the response rates (for both intervention and control arms but to a different extent) compared with the response rates observed in relevant RCTs.
The substantial heterogeneity among studies included in these MTCs and the discrepancy between the results from these analyses and those actually observed in RCTs raise serious concern with regard to the validity of the MTCs as well as the validity of economic evaluations that utilised data from them.
Further critique of manufacturers’ models
A description of the models included in each of the MSs and a summary of results from this modelling is provided in section Critique of manufacturers’ submissions. A critique of ICs and/or MTCs that were used to inform the models is given in section Critique of manufacturers’ submissions. Building upon the Critique of manufacturers’ submissions, this section aims to provide further critique of the manufacturers’ models by highlighting issues and uncertainties related to data input and assumptions used.
Data input and assumptions used in the manufacturer models are summarised in Table 74. Key issues relating to characteristics of starting population, estimates of clinical effectiveness (short term and long term), mapping of effectiveness data to utility, discontinuation rule(s) and treatment duration, handling of AEs and mortality, estimates of costs and other relevant factors are discussed below for each of the models.
| BRAM | Abbott | Wyeth Pharmaceuticals | Schering-Plough Ltd | Roche | Bristol-Myers Squibb Ltd | |
|---|---|---|---|---|---|---|
| Baseline characteristics | Based on BSRBR:163 mean age 58 years, 81% female, baseline mean HAQ 2.00 | Based on BSRBR:122 median age 58 years, 79% female, baseline mean HAQ 2.10 | Based on TEMPO trial (a trial in TNF inhibitor naive patients): mean age 53 years, 74% female, baseline mean HAQ 1.60a | Based on GO-AFTER (golimumab trial in patients who had inadequate response to TNF inhibitors): mean age 54 years, 79% female, baseline mean HAQ 1.61 | Based on REFLEX trial:124–126 mean age 52.2 years, 81% female, baseline mean HAQ 1.88b | Based on the ATTAIN trial:127–132 mean age 53.4 years, 77% female, baseline mean HAQ 1.80 |
| Treatment sequence (after the failure of one TNF inhibitor) | Compared each of the five technologies against conventional DMARDs | Compared each of the five technologies against conventional DMARDs and a strategy of TNF inhibitor followed by RTX | Compared TNF inhibitor (as a class) with conventional DMARD and RTX | Compared each of the five technologies against conventional DMARDs. Also compared each of the three TNF inhibitors followed by RTX against conventional DMARDs | Compared each of the five technologies against conventional DMARDs | Compared various strategies of sequential use of two biologics |
| Estimates of clinical effectiveness – short term |
Based on HAQ changes For biologics, data was obtained from the systematic review (where available – IFX was assumed to be the same as ETN due to lack of data); for DMARDs halved effectiveness in early RA was used due to lack of data for the relevant population |
Based on ACR response rates mapped to HAQ changes Estimated ACR response rates were obtained from MTC of trials outside the scope (see section Critique of indirect comparisons and mixed-treatment comparisons included in manufacturers’ submissions) Response rates were assumed to be equal for the three TNF inhibitors. Predicted response rates for the placebo/control arms were used as the response rates for conventional DMARDs ACR response rates were then mapped to HAQ changes using data from DE019 trial (ADA as first biologic therapy, outside scope) |
Based on HAQ changes Short-term HAQ improvement for TNF inhibitors (as a class) was based on data from ReAct study,96 an observational study of switching to ADA after failing a TNF inhibitor. Data from REFLEX trial124–126 were used for RTX. ABT was not included in the model Assumed zero HAQ improvement for patients switched to conventional DMARDs according to BSRBR data122 |
Based on mapping between EULAR response and ACR response using an algorithm derived from the GO-AFTER trial data For biologics, effectiveness was estimated as odds ratios of ACR responses obtained from a network meta-analysis of RCTs largely outside the scope. The validity of the network meta-analysis was questionable (see section Critique of indirect comparisons and mixed-treatment comparisons included in manufacturers’ submissions) The odds ratios were applied to baseline ACR response rates for conventional DMARDs, which were converted from EULAR response rates based on BSRBR data173 The results were then converted back to EULAR responses using the aforementioned algorithm |
Based on ACR response rates mapped to HAQ changes For RTX and ABT, ACR response rates from TNF-IR ICs (see section Critique of indirect comparisons and mixed-treatment comparisons included in manufacturers’ submissions) were used. For TNF inhibitors, ACR response rates from DMARD-IR MTC were firstly discounted by 30% and then used in the model For conventional DMARDs, ACR response rates for placebo groups from TNF-IR ICs (see section Critique of indirect comparisons and mixed-treatment comparisons included in manufacturers’ submissions) were used ACR responses were then mapped to HAQ changes using data from REFLEX trial124–126 |
Based on HAQ changes Short-term HAQ improvement was based on IC for ABT and RTX (see section Critique of indirect comparisons and mixed-treatment comparisons included in manufacturers’ submissions), and on an analysis of BSRBR data by NICE Decision Support Unit196 for TNF inhibitors For conventional DMARDs data from early RA trials were used179–181 |
| Estimates of clinical effectiveness – long term | HAQ progression – base case:
|
HAQ progression – base case:
|
HAQ progression – base case:
|
Utility progression – base case:
|
HAQ progression – base case:
|
HAQ progression – base case:
|
| Discontinuation rule and treatment duration |
No formal withdrawal rule, but based on available data For long-term survival on treatment Weibull curves were fitted to the available data:DMARDs – General Practice Research Database (GPRD) data |
The minimal response required for continuation of treatment after the initial 6-month period is ACR50 Withdrawal rates used in the base-case analysis for TNF inhibitors are based on a shared frailty model previously developed by the Decision Support Unit using BSRBR data for patients receiving second TNF inhibitor. Withdrawal rates for ABT and RTX were assumed to be the same as for TNF inhibitors |
Mentioned ‘switching thresholds’ based on relationship between HAQ and DAS28, but discontinuation rule was not clearly stated |
Withdrawal data for TNF inhibitors (assumed the same for RTX and ABT) were taken from a BSRBR analysis. 173 Patients receiving biologics who do not achieve a moderate or good EULAR response were discontinued from treatment at 6 months Withdrawal data for conventional DMARDs were taken from Barton et al. 175 The utility rebound following treatment discontinuation was equal to the initial utility gain |
Continuation of treatment (for all drugs) was subject to achieving an ACR20 or higher at the end of first 6 months. Subsequently, the same annual withdrawal rate (9.5%) for all biologics was assumed. This was based on Geborek et al. :148 an average of two estimates for ETN (8%) and IFX (12%) used as the first biologic therapy Assumed the same annual withdrawal rate (27%) for all traditional DMARDs. This was based on Bansback et al. ,146 which cited Wolfe177 as the source |
The treatment duration was based on data from ATTAIN LTE119 for ABT (clinical study report 029). For all other treatments data for first biologic use from Barton et al. 175 were utilised Discontinuation rates due to AEs in the first 6 months for ABT and RTX were based on an IC (see section Critique of indirect comparisons and mixed-treatment comparisons included in manufacturers’ submissions). For all other treatments data from studies and reviews in TNF inhibitor-naive patients were used179,184–188 |
| Mapping of effectiveness data to utility | Quadratic equation using dataset supplied by Hurst and reported in Hurst et al.155 in the absence of any more recent dataset available to the assessment group | HAQ scores were converted to EQ-5D scores according to equations (linear and non-linear) developed by Ducournau et al.165 using data from TOC trials. The non-linear equation was used for the base-case analysis, while the linear equation was examined in sensitivity analyses | HAQ scores were converted to EQ-5D scores according to a linear equation developed by Brennan et al.150 | Utility was estimated to be a function of EULAR response, treatment (on biologic treatment or not), health-state utility at time of treatment initiation, age, disease duration, number of previous DMARDs and gender | HAQ scores were converted to EQ-5D scores according to the non-linear equation developed by Ducournau et al.165 using data from TOC trials |
HUI 3 utilities were calculated from the HAQ based on a conference abstract153 EQ-5D utilities calculated from HAQ were used in a sensitivity analysis |
| AEs | Not incorporated into the model |
Data on the occurrence of mild, moderate and serious AEs for ETN, IFX and LEF were estimated from Gebroek et al. 148 AEs for ADA, RTX and ABT were assumed to be the same as for ETN Rates of TB associated with each of the TNF inhibitors were based on data from the BSRBR198 |
Data were obtained from various literature sources (however, references seem to be wrong) | Not included in the model | Not included in the model |
Occurrence of AEs was based on MTC (please see comments) Utility decrements for AEs based on ERG report on erlotinib for relapsed non-small cell lung cancer STA |
| Mortality | Basic mortality was taken from standard life tables. A RR (1.33) per unit HAQ was applied based on Wolfe et al.199 For PSA a log-normal distribution was assumed (95% CI 1.10 to 1.61) | Assumed a reduction in mortality for patients receiving TNF inhibitors based on Jacobsson et al.166 The reduction also applies to RTX or ABT | At baseline 1.63 times standard mortality from UK life tables. Adjusted based on HAQ Δ(mortality) = current mortality × [0.375 Δ(HAQ)] | No impact of treatment on mortality was assumed in the model | Mortality was adjusted according HAQ score based on Barton et al.,175 which in turn was based on Wolfe et al.199 | HAQ mortality HR based on Wolfe et al.199 |
| Drug costs and other costs |
Costs are made up of drug and monitoring costs. A ‘start-up’ cost reflects higher dosage and additional monitoring, as appropriate for each treatment Unit costs were based on:For drugs – BNF 58 accessed online |
Based on Monthly Index of Medical Specialties (July 2009) assuming an average patient weight of 70 kg Disease-related hospital costs (inpatient days and joint replacement procedures) were estimated based on HAQ band using data from the NOAR database150 |
Unit drug costs from BNF. Other costs from Curtis 2007 (Table 10 MS) | It was assumed that where possible the monitoring and administration for biologics and MTX was carried out concurrently. This seems appropriate. Two cost assumptions are presented for RTX based on a 6-month or 9-month dosing frequency. The 6-month dosing frequency was based on market research | Drug acquisition, administration and monitoring costs were estimated based on a 5-year average. This may not accurately reflect the costs of drugs with higher start-up costs |
Drug costs were based on MIMS. Drug administration costs were based on Chen et al. 179 and an ERG report on RTX. 183 Monitoring costs were based on Barton et al. 175 and Curtis191 Hospitalisation resource use was based mainly on data from the Barbieri study192 The cost of joint replacement was assumed to be around £6000195 NHS Reference costs for 2007–8 were used for AEs [as stated in the manufacturer’s submission;205 no citation provided] |
| Vial sharing | No vial sharing assumed | No vial sharing assumed | Not stated | Vial sharing for IFX was assumed | Not stated | The stated number of ABT vials used (2.85) implies vial sharing. See section Bristol-Myers Squibb Ltd (abatacept) |
| Time to retreatment for RTX | Base case: 8.7 months (based on Roche submission). Sensitivity analysis: 6 months | Base case: 9 months. Sensitivity analyses: 3, 6 or 12 months | Unclear | Both 6 months and 9 months were tested in base cases | Base case: 8.7 months (based on UK market research data, GfK HealthCare, January 2008; Roche data on file). Sensitivity analyses: 6, 7, 8 or 9 months | Base case: 6 months. Sensitivity analysis: 9 months |
Abbott (adalimumab)
Characteristics of starting population
The characteristics of the starting population were based on data from the BSRBR122 that is appropriate. These published data were collected in 2006 and are slightly dated. The starting population in the Abbott model had a slightly higher HAQ score at baseline than the equivalent population described in the current British Society for Rheumatology (BSR) submission (2.1 vs 2.0). The current BSR submission to NICE (Section 4, Table 4–1)163 highlights a trend over the past 8 years that patients treated more recently have shorter disease durations, lower DASs, and lower HAQ scores and have tried fewer conventional DMARDs before starting a TNF inhibitor.
Treatment sequence
The stated assumptions that patients will have tried MTX, sulfasalazine and HCQ (and thus these drugs are not evaluated) are clinically appropriate. The evaluated sequences include gold as the comparator or first traditional DMARD after failing biologics (see Table 71). Sequences that consider GST early are increasingly unlikely. GST is now likely to be used much later during treatment (for example, Survey of West Midlands rheumatologists, Appendix 11). In addition, although AZA has limited efficacy, this drug would still be tried in patients with resistant disease. This drug should therefore be used late in the sequence.
Estimates of clinical effectiveness – short term
Clinical effectiveness was estimated according to ACR response rates obtained from the manufacturer’s MTC, which included RCTs of very heterogeneous patient populations outside the scope of this appraisal as well as a few selected observational studies of relevant populations within the scope. As described in section Critique of indirect comparisons and mixed-treatment comparisons included in manufacturers’ submissions, the validity of the MTC was questionable. The ACR responses estimated from the MTC for control groups (i.e. placebo or DMARDs for which patients had had inadequate response) were used for conventional DMARDs in the model. These response rates, if estimated correctly, would not have reflected the response rates for a conventional DMARD that patients had not previously tried.
Mapping of ACR responses to HAQ change was based on an RCT (DE019) of ADA used as the first biologic therapy. Mapping using alternative data from PREMIER (an RCT of ADA in early RA, MTX-naive patients) suggested that the relationship between ACR response to treatment and changes in HAQ score will differ depending on the population being treated. Therefore, mapping based on data from a subgroup of patients in DE019 with a HAQ score greater than 2 was used by the manufacturer in a sensitivity analysis.
Estimates of clinical effectiveness – long term
The base case assumed that HAQ progression on biologics is the same as that of the general population (0.03 per year). An annual increase of 0.045 for conventional DMARDs and 0.06 for non-responders was assumed. Zero HAQ progression on biologic treatment was explored in sensitivity analyses. While previous analyses have considered the possibility that HAQ does not progress at all in a population of patients treated with a TNF inhibitor this assumption lacks face validity. Remission was achieved by 7% of patients in a large cohort of RA patients and minimum disease activity was achieved by around 20%, including those on a TNF inhibitor. 164 On the basis that a majority of RA patients treated with a TNF inhibitor have continued disease activity, it is not credible that HAQ does not change with time in this population.
The model assumed that, following treatment withdrawal, the HAQ score would immediately worsen by an exactly equivalent amount to the initial improvement. A sensitivity analysis was conducted in which the HAQ score worsens by 75% of the initial gain. It seems appropriate to explore several possible scenarios. Patients experiencing a severe flare of disease are unlikely to be left in this state and unlikely to suffer a prolonged worsening of function because of the short-term use of corticosteroids combined with other DMARDs and/or a biologic as appropriate.
Mapping of effectiveness data to utility
Health Assessment Questionnaire scores were converted to EQ-5D scores according to equations developed by Ducournau et al. ,159 using data from TOC trials [OPTION (tOcilizumab Pivotal Trial in methotrexate Inadequate respONders) and LITHE (tociLIzumab safety and THE prevention of structural joint damage)] in patients who had had inadequate response to MTX. Two equations (linear and non-linear) were available. The non-linear equation was used for the base-case analysis, while the linear equation was examined in sensitivity analyses.
Discontinuation rule and treatment duration
The model demands for an ACR50 response at 6 months in order that patients are eligible to continue treatment. This threshold appears too high compared with clinical practice. It is clear from the BSRBR and other data that patients continue treatment with a TNF inhibitor despite not meeting NICE-stipulated DAS28 criteria (so called ‘stayers’ in BSRBR analyses). This suggests that there is worthwhile clinical benefit despite a failure to meet thresholds (which are derived from populations and have limitations when applied to individual patients; see Chapter 1, Disease Activity Score response criteria).
Withdrawal rates used in the base-case analysis for TNF inhibitors are based on a shared frailty model previously developed by the Decision Support Unit using BSRBR data for patients receiving their second TNF inhibitor. Withdrawal rates for ABT and RTX were assumed to be the same as for TNF inhibitors.
Handling of adverse events and mortality
A reduction in mortality (independent of age, HAQ and comorbidity) for patients on TNF inhibitors was assumed based on Jacobsson et al. 166 This assumption was also applied for RTX and ABT. A hazard ratio (HR) of 0.92 for males and 0.52 for females was used. The reported mortality advantages for patients on TNF inhibitor treatment compared with conventional DMARDs need great care in interpretation because of selection biases involved in treating patients with a TNF inhibitor which may not be sufficiently adjusted for. Sicker individuals, those with cardiac failure and those with previous malignancies are much less likely to be treated.
Estimates of costs
Abbott states that the drug costs of ADA and ETN are similar but fails to acknowledge that this applies only to ADA used every other week. The licence for ADA permits dose increases so the drug may be administered every week (potentially doubling drug costs). European data, including from the UK, suggest that around 8% of patients need an increase in their dose of ADA. This figure may be an underestimate as many investigators reported that financial constraints inhibited dose increases. 167
The dose of LEF is 20 mg per day not 25 mg as stated. The stated dose for CyA was 2.5 mg per kg. In practice, this can range from 2.5 mg to 4 mg per kg.
The stated six outpatient visits and 11 nurse visits during the first 6 months for patients starting a TNF inhibitor are excessive for ETN and ADA. For IFX the necessary assessments can be done on the day a patient receives an infusion though it may be appropriate to include a nurse visit at other times to ensure that MTX safety is maintained. So, there will be five visits for infusions during the first 6 months. Blood and other monitoring can be done at these visits and an additional two nurse visits would be needed to ensure that a minimum of monthly checks were made.
Two outpatient visits and six nurse visits were assumed for monitoring after the first 6 months. An outpatient visit every 3 months is appropriate for a period of around 18 months, but after this, in stable patients with well-controlled disease, monitoring by a rheumatologist can be reduced to every 6 months. Frequency of blood testing for concomitant MTX can be done at nurse visits or in GP practices where there are shared care agreements.
Disease-related hospital costs (inpatient days and joint replacement procedures) were estimated based on HAQ band using data from the Norfolk Arthritis Register (NOAR) database. 168 Higher costs are more likely with higher HAQ scores, but for items such as joint replacement this is likely to apply only to those with persistently raised HAQ scores (i.e. those with more fixed damage) rather than in those whom HAQ scores rise as a result of flares of inflammatory disease. The latter group have a higher risk of hospitalisation because of this but rates in contemporary practice are low because of the use of corticosteroids.
Wyeth Pharmaceuticals (etanercept)
Wyeth Pharmaceuticals did not submit an electronic version of the model. Overall, the description with regard to methods for identifying data and justification for the selection of data was very limited.
Characteristics of starting population
The mean age of the starting population was 53 years and was based on the TEMPO trial. This is an RCT of TNF-naive patients (mean disease duration 6.6 years) who had not experienced treatment failure with MTX. The rationale for choosing this trial is not described. The modelling appears to start when patients first receive RA treatment (MTX), so it is not clear why a starting cohort of early RA patients was not chosen. The starting population in TEMPO was younger than the BSRBR cohort at study entry (mean age 56 years), but it is difficult to ascertain whether patients’ age would be similar to the BSRBR data (i.e. reflecting UK population and practice) when the patients reached the point of failing a TNF inhibitor. Other characteristics of the starting population were not described, including baseline HAQ score.
Treatment sequence
The identity of drugs in the treatment sequence was not clearly described. For example, the terms ‘first TNF-α inhibitor’, ‘second TNF-α inhibitor’ and ‘DMARD after TNF’ were used without further clarification. The costs for the second TNF inhibitor (the intervention under evaluation) were assumed to be the average of ADA, ETN and IFX + MTX. The assumed costs for the second TNF inhibitor (£4,159.68), therefore, do not reflect the (higher) costs for ETN + MTX (£4,687.83) according to the table of unit costs provided in the Wyeth Pharmaceuticals submission.
Estimates of clinical effectiveness – short term
Short-term HAQ improvement for TNF inhibitor (average –0.48; varied between –0.55 to –0.41 depending on reasons for withdrawal of previous TNF inhibitor) was based on data from the ReAct study,96 an observational study of switching to ADA after failing a TNF inhibitor. Short-term HAQ improvement for conventional DMARD was assumed to be zero according to the BSRBR data. In contrast with the –0.48 observed in ReAct study,96 short-term HAQ improvement for TNF inhibitor observed in BSRBR was only –0.11, but these data were not used in the model. The estimates of effectiveness for the model were therefore taken from studies using different methods of data collection and thus inappropriate for comparison.
Various sources have been cited for HAQ improvements on other treatments but the citations may be incorrect (e.g. the cited references for DMARDs before first TNF inhibitor appears to be uncontrolled studies of second-line biologics).
Estimates of clinical effectiveness – long-term
Long-term HAQ progression for patients on TNF inhibitors (and RTX) was assumed to be zero according to Wick et al. 93 Various levels of HAQ progression were applied for patients on conventional DMARDs based on assumption.
Mapping of effectiveness data to utility
The HAQ score was converted to EQ-5D score using the equation reported by Brennan et al. 151
Discontinuation rule and treatment duration
This was not described.
Handling of adverse events and mortality
Various probabilities of experiencing a serious AE were assigned for each treatment. The cited references included a systematic review including probably first-line biologic use, narrative reviews and methodological papers discussing HAQ and QoL (possibly incorrectly cited). Mortality rates were adjusted according to change in HAQ score using an equation, but the source of the equation was not cited.
Estimates of costs
Resource use was based on HAQ score according to Taylor et al. 169
Schering-Plough Ltd (infliximab)
Characteristics of starting population
The characteristics of starting population were based on GO-AFTER (a golimumab trial in patients who had inadequate response to TNF inhibitors): mean age 54, female 79%, baseline HAQ score of 1.61. The starting population was younger and had much lower baseline HAQ score than corresponding patients in the BSRBR. Baseline utility (EQ-5D and SF-6D) was imputed from baseline HAQ using simple linear regression (lower HAQ corresponding to higher utility). The consequence is that the estimated baseline utility may have been higher than it should be.
Treatment sequence
The model compared the five technologies against conventional DMARDs. It also compared each of the three TNF inhibitors followed by RTX against conventional DMARDs.
Estimates of clinical effectiveness – short term
Effectiveness of biologics was measured using ACR response, which was then mapped to EULAR response using an algorithm derived from GO-AFTER data.
Effectiveness data for biologics was obtained from a network meta-analysis of RCTs largely outside the scope. The validity of the network meta-analysis was questionable (see section Critique of indirect comparisons and mixed-treatment comparisons included in manufacturers’ submissions). Effectiveness data for conventional DMARDs were obtained from EULAR response estimated by Brennan et al. 170 using regression analysis based on the BSRBR data. It appears that EULAR response for corresponding patients who switched to a second TNF inhibitor (rather than conventional DMARDs) was available from the same analysis, but these data were not used in the model. Instead, estimates of effectiveness for TNF inhibitors were taken from the MTC and thus the data for comparative effectiveness were obtained from different sources that may not be comparable.
Estimates of clinical effectiveness – long term
For patients receiving biologics, the base-case analysis assumed zero utility progression. A sensitivity analysis was carried out assuming that utility progression was equal to that observed in the BSRBR (by EULAR response), which suggests that utility worsens for EULAR good responders, is close to zero for moderate responders and improves marginally for non-responders. 171 This seems counterintuitive.
A further assumption was made that patients have the same radiological damage at the end of biologic treatment as at the start and therefore their ability to improve on further treatment was also retained. This was implemented in the model by holding age and disease duration constant for the time on biologic. The impact of this assumption is unclear and does not seem to have been explored in sensitivity analyses.
Mapping of effectiveness data to utility
For the base case, utility was estimated to be a function of EULAR response, treatment (on biologic treatment or not), health-state utility at time of treatment initiation, age, disease duration, number of previous DMARDs and gender according to an analysis of BSRBR data. 172
Discontinuation rule and treatment duration
Withdrawal data for TNF inhibitors were taken from the BSRBR analysis of patients receiving a second TNF inhibitor. 173 All patients receiving biologics who did not achieve a moderate or good EULAR response were discontinued from treatment at 6 months. Treatment withdrawals were assumed to be the same for RTX and ABT. This assumption may overestimate the proportions of people who continue with these therapies although data are limited. For RTX, in the German registry [RABBIT (Rheumatoid Arthritis oBservation of BIologic Therapy); Stangfeld et al. 174], 39% of people had no response after 6 months. However, at 12 months 68% of patients had gone on to receive a second infusion. What proportion of the remaining 32% goes on to receive a further infusion is not yet known. Further attrition with subsequent courses is likely but difficult to estimate.
Withdrawal data for conventional DMARDs was taken from Barton et al. 175
Handling of adverse events and mortality
No impact of treatment on mortality was assumed in the model.
Estimates of costs
It was assumed that where possible the monitoring and administration for biologics and MTX were carried out concurrently. This seems appropriate. Two cost assumptions are presented for RTX based on a 6-month or 9-month dosing frequency. The 6-month dosing frequency was based on market research rather than on actual data from systematically collected data and may not be appropriate.
Vial optimisation was assumed in the base case. The assumptions are based on a questionnaire survey of rheumatology units (33% response rate). In many institutions vial sharing is achieved by central (pharmacy) preparation of infusions in advance of patient arrival. This can lead to drug wastage where patients are deemed not fit for infusion or fail to turn up. In any case, any savings from vial sharing are dwarfed by dose escalation. 176 In the cited systematic review, 44% of patients treated with IFX had the drug dose increased.
Roche (rituximab)
Characteristics of starting population
The starting population was based on the REFLEX trial:124–126 mean age 52.4 years, 81% female, disease duration 11.9 years, prior DMARDs 2.5 (excluding MTX). Over half of the patients in REFLEX124–126 were recruited from the USA and thus the cohort does not reflect UK population/practice, as exemplified in the much younger age compared with the BSRBR cohort.
Treatment sequence
The treatment sequence did not contain AZA.
As mentioned before, while AZA has limited efficacy, this drug would still be tried in patients with resistant disease and thus should be used late in the sequence.
Estimates of clinical effectiveness – short term
For RTX and ABT, ACR response rates from TNF-IR ICs (based on trials of patients who had failed one or more TNF inhibitor) were used. For TNF inhibitors, ACR response rates from DMARD-IR MTC (based on trials of patients naive to TNF inhibitors) were firstly discounted by 30% and then used in the model. The validity for the DMARD-IR MTC was questionable (see section Critique of indirect comparisons and mixed-treatment comparisons included in manufacturers’ submissions). The estimates of effectiveness for TNF inhibitors and RTX/ABT were therefore taken from a different set of analyses that are not comparable.
Estimates of clinical effectiveness – long term
Long-term HAQ progression for patients staying on treatment was set at zero (and also assumed to be zero for other biologics) according to observation from the LTE arm of the REFLEX trial. 139 A 6-monthly progression of 0.0225 was assumed for conventional DMARDs and 0.03 for palliative care. These were slightly lower than figures used in other manufacturer models.
Mapping of effectiveness data to utility
The HAQ scores were converted to EQ-5D scores according to the non-linear equation developed by Ducournau et al. 165 using data from TOC trials. An additional analysis that included age as a covariate in the non-linear model was also performed.
Discontinuation rule and treatment duration
Continuation of treatment (for all drugs) was subject to achieving an ACR20 or higher at the end of the first 6-month cycle. Subsequently, the same annual withdrawal rate (9.5%) for all biologics was assumed. This was based on Geborek et al. :148 an average of two estimates for ETN (8%) and IFX (12%) used as the first biologic therapy. The same annual withdrawal rate (27%) was assumed for all traditional DMARDs. This was based on Bansback et al. ,146 which cited Wolfe177 as the source. The data are likely to be outdated for some of the DMARDs.
Handling of adverse events and mortality
Adverse events were not included in the model.
Estimates of costs
Drug acquisition, administration and monitoring costs were estimated based on a 5-year average. This may not accurately reflect the costs of drugs with higher start-up costs.
Bristol-Myers Squibb Ltd (abatacept)
Characteristics of starting population
The characteristics of the starting population were based on the ATTAIN RCT. 130 Using data from a recent UK cohort (BSRBR122) might have been a more appropriate approach. Compared with the BSRBR data, patients in the ATTAIN trial130 were on average slightly younger (58.0 years vs 53.4 years), and had a longer disease duration (9.0 years vs 12.2 years) and more patients were receiving glucocorticoids (44%–52% vs 70.2%). The mean HAQ score was slightly lower in the ATTAIN trial130 than in BSRBR122 data (1.8 vs 2.0) and the DAS28 score was slightly higher (6.5 vs 6.4).
Treatment sequence
It was assumed that a conventional DMARD is not likely to be used after a failure of the first TNF inhibitor. This is arguable and it is likely that at least a proportion of rheumatologists may seek to try drugs such as LEF, GST or CyA in this circumstance.
Penicillamine is included although it is used rarely today. The treatment sequences described, which were based on Barton et al. ,175 are credible.
Estimates of clinical effectiveness – short term
Clinical effectiveness in the first 6 months was estimated using HAQ scores. For RTX and ABT these were obtained from an MTC (see section Critique of indirect comparisons and mixed-treatment comparisons included in manufacturers’ submissions). For TNF inhibitors the estimate was based on a BSRBR data analysis by the Decision Support Unit for NICE178 and it used the adjusted result for switchers with long duration of second treatment (the report concluded that this is a good estimate for a year of treatment). For conventional DMARDs, data from early RA patients were used. 179–181 These data do not come from the population relevant to the scope (patients who failed a TNF inhibitor), but it was probably not possible to identify more relevant data.
Estimates of clinical effectiveness – long term
For long-term HAQ progression there were two sets of data: one versus RTX and one versus TNF inhibitors. For ABT there was a further HAQ reduction on treatment based on an analysis of ATTAIN and an extension of RTX trials130,176 (–0.0729 and –0.013, respectively). For all other treatments (biologic drugs and conventional DMARDs) an annual increase in HAQ score of 0.012 was assumed based on an ERG STA report on RTX (calculation was actually based on non-biologic data). 183 It is unclear why only patients on ABT were assumed to further improve after the initial effect of the treatment, while all the other treatments are associated with deterioration.
Mapping of effectiveness data to utility
The algorithm mapping HAQ to utility was based on a conference abstract. 152 A linear equation (intercept 0.76, slope –0.28, female + 0.05) was used for that purpose.
Discontinuation rule and treatment duration
The treatment duration was based on data from ATTAIN LTE119 for ABT (clinical study report 029). For all other treatments data, for first biologic use from Barton et al. 175 were utilised. As there were no data for ADA and RTX, an average for all biologics was assumed. These may not be directly applicable to the present decision problem.
The data used in the model differ from those in the BSRBR, but it is unclear if these parameters affect the results.
Discontinuation rates due to AEs in the first 6 months for ABT and RTX were based on a MTC (see section Critique of indirect comparisons and mixed-treatment comparisons included in manufacturers’ submissions). For all other treatments, data from studies and reviews in TNF inhibitor naive patients were used. 179,184–188 The applicability of their results might be limited, although for conventional DMARDs probably no data in the relevant population were available. The proportion of patients discontinuing because of AEs was the lowest for ABT (2.3%) and ADA (2.8%) and was the highest for conventional DMARDs (12%–20%).
Handling of adverse events and mortality
The submission states that ‘The event rates for ABT and RTX were derived from the mixed treatment comparison [please see comments]. The event rates for etanercept, adalimumab and infliximab were derived from individual trials and the event rates for conventional DMARDs were based on the literature (as used in Chen et al. 179)’.
The utility loss due to AEs was based on data from an ERG STA report on erlotinib for relapsed non-small cell lung cancer. 189 Neutropenia and leucopenia were associated with a utility loss of 0.15 and all other AEs with a utility loss of 0.05. The applicability of these estimates to RA patients might be limited.
For mortality, a HAQ mortality HR of 1.33 (95% CI 1.10 to 1.61) was used based on Wolfe et al. 190
Estimates of costs
The submission states that drug costs were based on the doses recommended in the drugs’ summary of product characteristics. Drug treatment costs were taken from the Monthly Index of Medical Specialties (MIMS). The number of ABT vials used is assumed to be 2.85. This implies vial sharing. Currently less than 200 patients have been treated with ABT in the UK. Presently, it is unlikely that significant vial sharing can occur unless many more patients are treated. As dose wastage for IFX is assumed it would also be appropriate to model dose wastage with ABT.
Drug administration costs were based on Chen et al. 179 and an ERG STA on RTX. 183 Monitoring costs were based on Barton et al. 175 and Curtis. 191 These sources seem to be credible.
Hospitalisation resource use was based mainly on data from the NOAR Database (which included joint replacement). 197 Joint replacement surgery was included in the model separately and therefore it was deduced from the NOAR data assuming that two-thirds of RA hospitalisations are due to joint replacement (as stated in Pugner et al. 193).
Time to joint replacement was assumed to be the same as in Barton et al. 175 and its impact on HAQ score was based on Wolfe and Zwillich. 194 The cost of joint replacement was assumed to be around £6,000. 195
NHS Reference costs for 2007–8 were used for AEs [as stated in the manufacturer’s submission 205; no citation provided].
Discussion
A few common issues were identified in the critique of manufacturer models:
-
starting population might not reflect UK population and practice
-
validity and uncertainty in translating effectiveness measures into utility
-
validity of ICs/MTCs carried out in trials of a heterogeneous population
-
uncertainty in the relative effectiveness between individual TNF inhibitors and between these drugs and RTX/ABT
-
uncertainty related to the effectiveness of conventional DMARDs
-
uncertainty in long-term disease progression on various treatments
-
different discontinuation rules
-
different assumptions with regard to dosing interval or vial optimisation.
One particular challenge for this technology assessment/appraisal was an absence of RCTs for the three TNF inhibitors. It is the assessment group’s view that evidence for technologies other than ABT and RTX is not appropriate for MTC or IC. Different approaches have been used by the assessment group and the manufacturers in this circumstance. The assessment group evaluated evidence from observational studies in detail in the absence of relevant RCTs for ADA, ETN and IFX, which is an unusual situation. The most appropriate data from either RCTs or observational studies for each of the technologies under assessment were then selected for economic modelling.
In order to conduct a valid IC, a network of RCTs that are comparable with respect to patient population and study design is needed. As stated above, no RCT conducted in a relevant patient population was found for the three TNF inhibitors. In order to perform ICs beyond ABT and RTX, one or more assumptions have to be made (as the manufacturers did):
-
Assumption (1) – the effectiveness and safety of different TNF inhibitors are the same (e.g. evidence from trials of golimumab is applicable to the three TNF inhibitors under assessment).
-
Assumption (2) – treatment effects are comparable between trials conducted in patients with different treatment history (DMARDs and biologics) and duration of RA, among other characteristics.
No evidence currently allows verification of assumption (1). To confirm or refute assumption (2) requires a systematic and comprehensive review far beyond the scope of this technology assessment/appraisal. Based on limited information provided in the MTCs included in the MSs, it appears substantial clinical, methodological and statistical heterogeneity exists among trials conducted in populations beyond the scope of this appraisal. The validity of analyses based on this assumption is thus questionable. It should therefore be borne in mind that potential uncertainties relating to these assumptions may not have been adequately reflected in the results of ICs/MTCs and the economic evaluations based on them.
Independent economic assessment
The assessment group’s own independent analysis was carried out using the Birmingham Rheumatoid Arthritis Model (BRAM), which has been further updated to allow for a non-linear relationship between HAQ and utility. Additional coding has been added to the model to facilitate the use of PSA. This means putting a distribution around all parameters in the model. Unless there is a good reason to treat a parameter as fixed, some distribution has been used. Fixed parameters were: life tables, discount rates, treatment costs and times at which early withdrawal of treatment was assessed.
The BRAM is an individual sampling model. A large number of virtual patient histories are simulated with the accumulation of costs and QALYs. The basic model structure is shown in Figure 93. A complete description of the model follows here. A list of the assumptions in the model is given in Appendix 15.
FIGURE 93.
Basic structure of the BRAM individual sampling model.
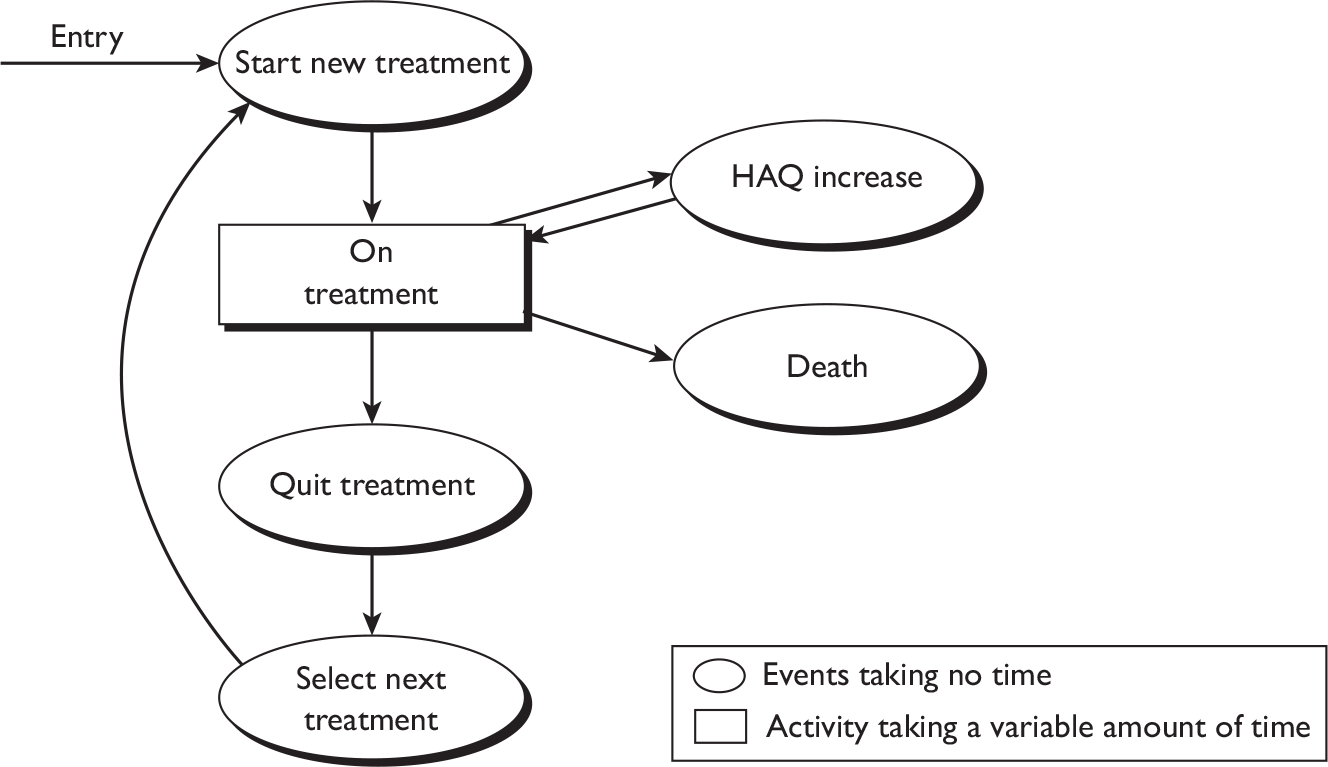
Methods
Patients are assumed to follow a sequence of treatments. This involves starting a treatment, spending some time on that treatment, quitting a treatment if it is toxic or ineffective and starting the next treatment. The pattern is then repeated as long as active treatments are available. The final treatment in any strategy is palliation (Pall).
The HAQ DI (see Appendix 1) is used as the marker for disease severity. Scores on this scale range from 0 (best) to 3 (worst) in multiples of 0.125. Patients’ HAQ scores are assumed to improve (decrease) on starting a treatment and this improvement is lost on quitting the treatment regardless of reason for quitting. While on treatment, a patient’s condition is assumed to decline slowly over time. This is modelled by occasional increases of 0.125 in HAQ score. The mean time between such increases in HAQ is allowed to vary by treatment; see Figure 94 for a possible HAQ trajectory. In the reference case analysis, HAQ is assumed to remain constant while a patient is successfully treated with a biological agent: this is modelled by a very large mean time to increase in HAQ.
FIGURE 94.
Possible trajectory of HAQ score over time. Initial improvement on a biological agent (AB) is lost on quitting the treatment (CD). A smaller improvement (DE) on starting LEF is similarly lost on quitting (FG) and followed by a gain (GH) on starting GST. In this case the patient dies of other causes (J) while still responding to GST. There is a gradual deterioration in HAQ from E to F and from H to J, but not from B to C in the reference case analysis. In some cases, the time spent on a conventional DMARD is not long enough for any deterioration in HAQ to occur.
Strategies to be compared
The current appraisal is concerned solely with the decision to be made at the point of failure of a first TNF inhibitor. Accordingly, the starting population consists of patients who have reached that point in a sequence of treatments. Table 75 shows the treatment sequences compared in this appraisal.
| Strategy name | ADA | ETN | IFX | RTX | ABT | DMARDs |
|---|---|---|---|---|---|---|
| First | ADA | ETN | IFX | RTX | ABT | LEF |
| Second | LEF | LEF | LEF | LEF | LEF | GST |
| Third | GST | GST | GST | GST | GST | CyA |
| Fourth | CyA | CyA | CyA | CyA | CyA | AZA |
| Fifth | AZA | AZA | AZA | AZA | AZA | Pall |
| Sixth | Pall | Pall | Pall | Pall | Pall |
Note, that previous versions of the BRAM used a starting population of DMARD-naive patients, and generated a range of different decision populations within the model. Strategies compared also allowed different choices of treatment options depending on the toxicity of previous treatments. While the coding to allow this flexibility remains within the model, such flexibility is not required within the present appraisal.
The choice of DMARDs following biologic therapy has been made in line with expected practice and excludes any DMARDs that are likely to have been used before biologic therapy.
Data used in the Birmingham Rheumatoid Arthritis Model
What follows is a detailed description of the data and sources thereof. Updated literature reviews have been used wherever possible.
Tables 76 and 77 show the information about the initial population. As stated earlier, the initial population is a population immediately following failure of a first TNF inhibitor. The values are based on the BSRBR submission to NICE. 163
| Gender | Age (years) | |||||||
|---|---|---|---|---|---|---|---|---|
| 15–24 | 25–34 | 35–44 | 45–54 | 55–64 | 65–74 | 75–84 | Total | |
| Male | 0.0 | 0.4 | 1.9 | 5.2 | 6.5 | 3.8 | 1.2 | 19 |
| Female | 0.1 | 1.5 | 8.2 | 22.1 | 27.7 | 16.3 | 5.1 | 81 |
| HAQ | 0.125 | 0.25 | 0.375 | 0.5 | 0.625 | 0.75 | 0.875 | 1 |
|---|---|---|---|---|---|---|---|---|
| % | 0.0 | 0.1 | 0.2 | 0.5 | 0.7 | 1.2 | 1.7 | 2.2 |
| HAQ | 1.125 | 1.25 | 1.375 | 1.5 | 1.625 | 1.75 | 1.875 | 2 |
| % | 2.9 | 3.6 | 4.3 | 5.1 | 5.8 | 6.6 | 7.2 | 7.7 |
| HAQ | 2.125 | 2.25 | 2.375 | 2.5 | 2.625 | 2.75 | 2.875 | 3 |
| % | 8.1 | 8.4 | 8.3 | 8.0 | 7.1 | 5.9 | 3.7 | 0.7 |
As in the previous version of the BRAM, the change in HAQ on starting a new DMARD is sampled on an individual basis and takes the form of a multiplier applied to the HAQ score on starting treatment. This multiplier is sampled from a beta distribution. The method used to estimate the parameters of the beta distribution is the same as in a previous report. 179
To illustrate the method, consider the calculations used in the previous report for LEF. The data available were baseline HAQ [mean 1.03, standard deviation (SD) 0.62] and HAQ improvement [mean 0.48, (SD) 0.5]. 1,100 An excel spreadsheet was set up to create a starting population of 10,000 virtual patients with HAQ scores drawn from a normal distribution with mean and SD supplied by the user. Each generated HAQ score was converted to the nearest legitimate value (multiples of 0.125 in the range 0–3). The parameters supplied were adjusted to compensate for the effect of this conversion, so that the mean and SD of the population generated corresponded to the data. In this case, this involved adjusting the mean of the underlying distribution to 1.01 and the SD to 0.66. The sample mean and SD then agreed with the data.
A beta distribution was found to match the given mean and SD for HAQ improvement. In this case the parameters were a = 0.57 and b = 0.65. Figure 95 shows the simulated population in this case. Each square within the graph represents a possible pair of values of starting HAQ and HAQ on treatment: the darker the square, the larger the number of simulated patients with that pair of HAQ values. It can be seen that there was a high proportion of patients with equal HAQ on treatment compared with before treatment. In this example, the sampled population contained a large number of zero initial HAQ values. These are omitted from the graphs, but included in the calculations relating to HAQ improvement.
FIGURE 95.
Modelled distribution of HAQ score change on starting leflunomide (from previous report). 177
In the current report, for biologic DMARDs, the parameters have been re-estimated using the best available data for use immediately after a first TNF inhibitor. For conventional DMARDs to be used after biologics, the only available data were from trials in early RA. The effectiveness was halved for use in late RA.
When a patient starts a new treatment in the model, a random number is drawn to determine the HAQ improvement for that patient. Consider, for example, a patient about to start LEF with a HAQ score of 2 and suppose that the random number drawn is 0.5. The value of 0.5 indicates that the improvement multiplier should be at the median of the relevant distribution. In the case of LEF, using the values from Table 78, the median is 0.358 so the HAQ should improve by 0.358 × 2 = 0.716. However, because HAQ is measured on a discrete scale, the improvement must be rounded to the nearest multiple of 0.125, which in this case is 0.75. The HAQ score on treatment would then be 2 – 0.75 = 1.25, and the 0.75 improvement (reduction) would be lost on quitting treatment. Had the starting HAQ score been 1, the improvement would have been 0.375 to give a HAQ on treatment of 0.625.
| Treatment | a | b | Mean | HAQ improvement on starting treatment/baseline HAQ; source |
|---|---|---|---|---|
| ADA | 0.32 | 0.92 | 0.26 | 0.48/1.85; Bombardieri 200795,96 |
| ETN | 0.21 | 0.75 | 0.22 | 0.35/1.60; Bingham 2009104 |
| IFX | 0.21 | 0.75 | 0.22 | Assume same as ETN |
| RTX | 0.20 | 0.75 | 0.21 | 0.40/1.90; REFLEX124–126 |
| ABT | 0.33 | 0.85 | 0.28 | 0.50/1.80; ATTAIN127–132 |
| LEF | 0.285 | 0.935 | 0.23 | Effectiveness halved from values used in previous report179 |
| GST | 0.225 | 0.925 | 0.20 | |
| CyA | 0.065 | 0.325 | 0.17 | |
| AZA | 0.10 | 0.90 | 0.10 |
Table 78 shows the point estimates for the parameters of the beta distributions used. However, these values are not known with certainty, so some variation must be included in the PSA. In the absence of any obvious way of measuring the uncertainty around the parameters, an assumption was made that each could be independently sampled from a normal distribution with an SD equal to 0.1 times the point estimate. This is still likely to underestimate the uncertainty in these parameters, but is preferable to using fixed values. Note that, although the same point estimates have been used for ETN and IFX, separate and independent samples have been used for the two drugs in the PSA. This principle has been applied throughout the model. In such cases, it is not known in which direction the difference between the treatments should be, but it is not a reasonable assumption that the treatments should take identical values.
Added in response to consultees’ comments: the values here give LEF a higher immediate effectiveness than any of the biologics. This is offset in part by the assumption described below about changes in HAQ score while on treatment. However, it is stressed that these values are not being used for a comparison in which the biologic treatments replace LEF in a sequence of treatments. Additional scenario analyses have been added to consider alternative assumptions.
The model allows for two stages of early quitting of treatment. For conventional DMARDs, this facility has been used with parameters preserved from Chen et al. 179 For TNF inhibitors and ABT, a single stage of early quitting has been included in line with available data, while for RTX no early quitting can be allowed, because it is necessary to model the full costs of each cycle of treatment. The values used are in Table 79. For long-term survival on treatment, Weibull curves were fitted to the available data.
| Treatment | Parameter | Point estimate (%) | Distribution | Source |
|---|---|---|---|---|
| ADA | Withdrawal at 12 weeks | 9.9 | Beta (89, 810) | Bombardieri 200795,96 |
| Toxicity if above | 56.2 | Beta (50, 39) | ||
| ETN | Withdrawal at 13 weeks | 5.2 | Beta (21, 385) | Bingham 2009104 and Buch 200599 |
| Toxicity if above | 16.7 | Beta (2, 10) | Bingham 2009104 | |
| IFX | Withdrawal at 16 weeks | 23 | Beta (3, 10) | OPPOSITE133 |
| Toxicity if above | 66.7 | Beta (2, 1) | ||
| RTX | No early withdrawal (see text) | |||
| ABT | Withdrawal at 6 months | 13.6 | Beta (35, 223) | ATTAIN127–132 |
| Toxicity if above | 25.7 | Beta (9, 26) | ||
| LEF | Withdrawal at 6 weeks | 13 | Beta (13, 87) | Geborek 2002148 |
| Withdrawal 6–24 weeks | 30 | Beta (30, 70) | ||
| Toxicity if above | 33.3 | Beta (10, 20) | ||
| GST | Withdrawal at 6 weeks | 14 | Beta (10, 62) | Hamilton 20011101 |
| Withdrawal 6–24 weeks | 27.1 | Beta (19.5, 52.5) | ||
| Toxicity if above | 66.7 | Beta (6.5, 13) | ||
| CyA | Withdrawal at 6 weeks | 8 | Beta (16, 184) | Yocum 2000202 |
| Withdrawal 6–24 weeks | 24 | Beta (48, 152) | ||
| Toxicity if above | 50 | Beta (24, 24) | Marra 2001203 | |
| AZA | Withdrawal at 6 weeks | 15 | Beta (15, 85) | Willkens 1995204 |
| Withdrawal 6–24 weeks | 25 | Beta (25, 75) | ||
| Toxicity if above | 50 | Beta (12.5, 12.5) |
In the form used, a random variable X has a Weibull distribution with shape parameter a and scale parameter b if:
has an exponential distribution with unit mean. If a = 1 the Weibull reduces to the exponential distribution with mean b; in any case b is the time until:
of the original population remains. If a < 1 then the hazard decreases with time; if a > 1 the hazard increases. The values used are shown in Table 80. For convenience, the mean of the distribution is also shown for the point estimates of the parameters.
| Treatment | a | 95% CI | b (years) | 95% CI | Mean (years) | Source |
|---|---|---|---|---|---|---|
| TNF inhibitors | 0.701 | 0.634 to 0.768 | 3.211 | 3.022 to 3.412 | 4.06 | BSRBR submission123 |
| RTX | 0.474 | 0.403 to 0.545 | 5.1 | 3.742 to 6.951 | 11.31 | REFLEX LTE139 |
| ABT | 0.81 | 0.734 to 0.886 | 5.49 | 5.166 to 5.834 | 6.17 | BMS submission205 |
| LEF | 1 | 0.905 to 1.095 | 5.98 | 5.627 to 6.355 | 5.98 | GPRD database206 |
| GST | 0.48 | 0.434 to 0.526 | 1.81 | 1.703 to 1.923 | 3.91 | |
| CyA | 0.5 | 0.452 to 0.548 | 4.35 | 4.094 to 4.623 | 8.70 | |
| AZA | 0.39 | 0.353 to 0.427 | 4.35 | 4.094 to 4.623 | 15.53 |
For TNF inhibitors, the same principle as for initial effectiveness has been applied: independent samples were drawn each time from the same distribution. For RTX, the time sampled is then taken up to the nearest multiple of the assumed time between treatment cycles.
Details of the implementation are as follows. For conventional DMARDs, the survival time is assumed to follow a distribution of the type shown in Figure 96, which is based on the data for LEF. The first step represents cessation of treatment after 6 weeks, which is assumed to be for toxicity. The second step represents cessation between 6 and 24 weeks after starting treatment, which could be for toxicity or inefficacy. At each appropriate stage in the running of the model, two variables, u1 and u2, are each drawn from a uniform distribution between 0 and 1. Figure 97 shows how these numbers are used. The value of u1 is first used in the beta distribution to determine the HAQ improvement described earlier. Then u2 is used to determine the time on treatment.
FIGURE 96.
Survival time on a treatment (based on LEF data).
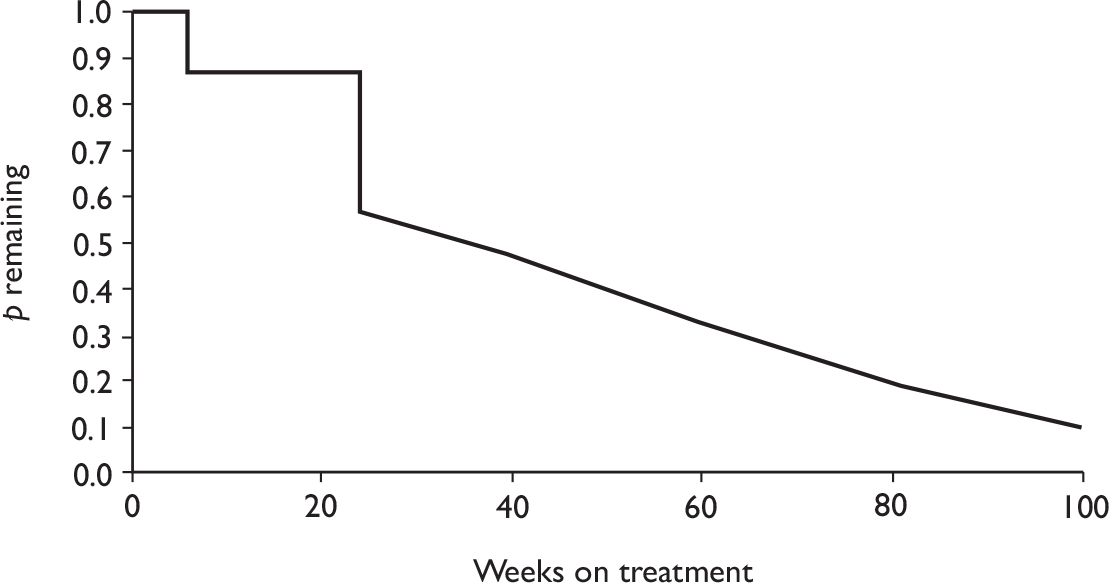
FIGURE 97.
Early cessation of treatment. The four zones represent the following: A, withdrawal within 6 weeks (assumed due to toxicity); B, withdrawal between 6 and 24 weeks for inefficacy; C, withdrawal between 6 and 24 weeks for toxicity; and D, remaining on the treatment after 24 weeks.
In implementation, critical values are calculated each time the population parameters are sampled for each treatment, so that the areas of the four zones in Figure 97 correspond to the probabilities sampled from the distributions indicated in Table 79. Then, for each individual, the values of u1 and u2 are compared with those critical values in the following ways:
-
If u2 is below its lower critical value, then the individual is in Zone A, and withdraws because of toxicity after 6 weeks.
-
Otherwise, u1 is compared with its critical value. If u1 is below the critical value, then the individual is in Zone B, and withdraws because of ineffectiveness after 24 weeks.
-
Otherwise, u2 is compared with its higher critical value. If u2 is below this value, then the individual is in Zone C, and withdraws because of toxicity after 24 weeks.
-
Otherwise, the individual is in Zone D, and remains on treatment beyond 24 weeks. The value of u2 is converted to a value from the appropriate Weibull distribution to determine the time on treatment.
For TNF inhibitors and ABT, the 6-week quitting was not used and the time shown in Table 79 was used in place of the 24-week limit used for conventional DMARDs. The implication of this is that for all modelled treatments except RTX, those individuals with the lowest HAQ improvement on starting treatment all quit early.
In the reference case analysis, it is assumed that HAQ remains constant while on any biologic treatment. Mean rates of HAQ increase of 0.045/year on conventional DMARDs and 0.06/year on Pall are modelled as mean times to increase (by 0.125) of 2.7 years and 2 years, respectively. In the PSA these times are sampled from normal distributions with SDs 0.27 years and 0.2 years, respectively. Again, the times for the conventional DMARDs are sampled independently each time.
Costs are made up of drug costs plus monitoring costs. As in previous versions, the model includes an annual usage cost for each treatment, together with a ‘start-up’ cost reflecting higher dosage and additional monitoring early in treatment, as appropriate for each treatment. Table 81 shows the unit costs for tests and visits and Table 82 the unit costs for drugs, leading to annual costs in Table 83.
| Test | Cost (£) | Source |
|---|---|---|
| FBC | 4.55 | Values from Chen 2006179 inflated to 2008 prices using the Hospital and Community Health Services inflation index (Curtis 2008)207 |
| ESR | 3.51 | |
| BCP | 4.39 | |
| CXR | 17.82 | |
| Urinalysis | 0.09 | |
| Visit | ||
| GP | 36 | Curtis 2008207 |
| Hospital outpatient | 71 | |
| Specialist nurse visit | 35.50 | Assumed half of outpatient visit |
| Administration of infusion | 141.83 | Chen 2006179 inflated to 2008 prices |
| Treatment | Cost | Assumptions |
|---|---|---|
| ADA | £357.50 per dose | 26 doses per year |
| ETN | £178.75 per dose | 52 doses of 50 mg per year |
| INF | £419.62 per vial | 70-kg patient; drug wastage |
| RTX | £873.15 per 500-mg vial | Dosage of two × 1,000 mg every 8.7 months in base case |
| ABT | £242.17 per 250 mg | 750 mg every 4 weeks |
| MTX | 11.7p per tablet | 15 mg per week |
| LEF | £1.70 per day | 20 mg per day |
| GST | £11.23 per dose | 50-mg ampoule administered at GP visit |
| CyA | £5.37 per day | 225 mg per day |
| AZA | 40.3p per day | 150 mg per day |
| Treatment | Cost (£) (steady-state yearly) | Cost (£) (additional in first year) | Assumptions |
|---|---|---|---|
| ADA | 9,295 | 0 | Twenty-six doses per year |
| ETN | 9,295 | 0 | Fifty-two doses of 50 mg per year |
| INF | 7,553.16 | 1,258.86 | Six doses per year; one additional dose in first year; three vials per dose |
| RTX | 4,817.38 | 0 | Each course is four 500-mg vials; multiply by 12/8.7 for annual cost |
| ABT | 9,444.63 | 726.51 | Thirteen doses of 750 mg = 39 times unit cost; one additional dose in first year |
| MTX | 36.50 | 0 | Six tablets per week for 52 weeks |
| LEF | 620.50 | 0 | 365 times daily cost |
| GST | 134.76 | 224.60 | Steady-state 12 doses per year; additional 20 doses in first year |
| CyA | 1,960.05 | 0 | 365 times daily cost |
| AZA | 147.10 | 0 | 365 times daily cost |
An administration cost of £141.83 is assumed for each dose of IFX, RTX, and ABT. This figure is inflated from the figure of £124.00 used in earlier versions of the BRAM. Annual administration costs are shown in Table 84. Monitoring assumptions for conventional DMARDs are shown in Table 85. Annual cost for tests and administration are shown in Tables 86 and 87, respectively. It is assumed that monitoring for biologic therapies is included within the monitoring for MTX or administration costs, so no additional monitoring cost is included for these. Combining the monitoring assumptions with the unit costs then leads to start-up and annual usage costs as shown in Table 88. Note, that as these costings are based on fixed prices and monitoring rules, rather than measured resource use, the prices are not varied in the PSA. All costs were discounted at 3.5% per annum from the start of the model.
| Treatment | Cost (£) (steady-state yearly) | Cost (£) (additional in first year) | Assumptions |
|---|---|---|---|
| ADA | 0 | 106.50 | Three visits to nurse specialist |
| ETN | 0 | 106.50 | Three visits to nurse specialist |
| INF | 850.98 | 141.83 | Six doses per year; one additional dose in first year |
| RTX | 391.26 | 0 | Two infusions per course; multiply by 12/8.7 for annual cost |
| ABT | 1,843.79 | 141.83 | Thirteen infusions per year; one additional infusion in first year |
| MTX | 0 | 0 | |
| LEF | 0 | 0 | |
| GST | 432 | 720 | Steady-state 12 doses per year; additional 20 doses in first year; GP visit for each dose |
| CyA | 0 | 0 | |
| AZA | 0 | 0 |
| Treatment | Pre-treatment | On treatment |
|---|---|---|
| MTX | FBC, ESR, BCP, CXR | FBC and BCP every 2 weeks for 4 months then monthly |
| LEF | FBC, ESR, BCP, urinalysis | FBC every 2 weeks for 6 months, every 8 weeks thereafter. BCP monthly for 6 months, every 8 weeks thereafter |
| GST | FBC, ESR, BCP, urinalysis | FBC and BCP, urinalysis every week for up to 21 injections, then every 2 weeks for 3 months, then every 3 weeks for 3 months, then monthly. Treatment given by i.m. injections |
| CyA | FBC, 2 × BCP, ESR, urinalysis | FBC and BCP every 2 weeks for 4 months, then BCP monthly |
| AZA | FBC, ESR, BCP | FBC and BCP weekly for 6 weeks, then every 2 weeks for three visits, then monthly |
| Pall | Outpatient visit every 3 months |
| Treatment | Cost (£) (pre-treatment) | Cost (£) (steady-state yearly) | Cost (£) (additional in first year) |
|---|---|---|---|
| MTX | 30.27 | 107.28 | 35.76 |
| LEF | 12.54 | 53.64 | 54.12 |
| GST | 12.54 | 108.36 | 180.60 |
| CyA | 16.93 | 52.68 | 53.96 |
| AZA | 12.45 | 107.28 | 53.64 |
| Treatment | Cost (£) (pre-treatment) | Cost (£) (steady-state yearly) | Cost (£) (additional in first year) |
|---|---|---|---|
| MTX | 71 | 852 | 284 |
| LEF | 71 | 426 | 639 |
| GST | 71 | 852 | 1,420 |
| CyA | 71 | 852 | 142 |
| AZA | 71 | 852 | 426 |
| Treatment | Start-up (£) | Annual use (£) |
|---|---|---|
| ADA | 527.53 | 10,290.78 |
| ETN | 527.53 | 10,290.78 |
| IFX | 1,821.72 | 9,399.92 |
| RTX | 421.03 | 6,204.42 |
| ABT | 1,289.37 | 12,284.20 |
| LEF | 776.66 | 1,100.14 |
| GST | 2,628.74 | 1,527.12 |
| CyA | 283.89 | 2,864.73 |
| AZA | 563.09 | 1,106.38 |
| Pall | 0.00 | 284.00 |
Costs for hospitalisation and joint replacement are estimated by a cost per unit HAQ score. In the base-case analysis, this was set at £1,120.00 per unit HAQ. This was inflated from the previous figure of £860.00 per unit included in previous versions of the BRAM. Scenario analysis includes various alternative costings here based on industry submissions.
Basic mortality was taken from standard life tables. A RR per unit HAQ was applied. The point estimate for this RR was set to 1.33, sampling in the PSA from a log-normal distribution with 95% CI (1.10 to 1.61).
In the reference case analysis, a quadratic equation was used to relate HAQ score to QoL score. This was of the form QoL = a – b1 × HAQ – b2 × HAQ2 where the coefficients are shown in Table 89. It is noted that this equation gives negative values (indicating a state worse than death) for high HAQ scores. While this reflects the fact that individual patients in the dataset used to generate the equation gave EQ-5D responses that map to scores below zero on the standard UK tariff, it is acknowledged that the use of negative QoL scores is controversial. Accordingly, coding was added to allow such scores to be adjusted to zero in the model. This coding was used in scenario analysis.
| Coefficient | Point estimate | 95% CI |
|---|---|---|
| a | 0.804 | 0.711 to 0.897 |
| b 1 | 0.203 | 0.054 to 0.351 |
| b 2 | 0.045 | –0.007 to 0.096 |
It was assumed that start and end effects could be modelled as one-off deductions proportional to the change in QoL score. The multiplier was set to a base-case value of 0.2 (years), sampled from a normal distribution with an SD 0.02 (separately for start and end).
Accumulated QALYs were discounted at 3.5% per annum from the starting point of the model.
Results
When an individual sampling model is run with a fixed parameter set, it must be run with a large number of patients to produce a precise estimate of the population mean cost and QALY differences between strategies. When such a model is run using PSA, the aim is to produce a distribution for the population outcomes that reflects the parameter uncertainty. This is done by sampling repeatedly from the joint distribution of parameters, and then for any parameter set, sampling a sufficient number of individuals.
Figure 98 shows the overall design of such a model run.
FIGURE 98.
Running an individual sampling model under PSA.
Note that a new set of patients is sampled for each parameter set, but the same patients are run through each of the possible strategies. Trial runs were made with different numbers of patients per parameter set. At fewer than 2,000 patients, the distribution of points in the cost-effectiveness plane became visibly wider. For safety, we used 5,000 patients per parameter set. For the reference case analysis, 2,000 parameter sets were sampled from the parameter distributions as described in the previous section. For each parameter set, 5,000 individual patient attributes were sampled and these patients were run through each of the six strategies defined in Table 75.
Reference case
The discounted lifetime costs and QALYs for each patient were calculated and the mean results for each parameter set output. The overall mean of these results forms the reference case estimate for the mean cost and QALY of each strategy: the 2.5 and 97.5 percentiles give the limits of the 95% credible interval. Note that these percentiles are likely to come from different parameter sets not just between strategies, but also for costs and QALYs for any particular strategy. These results are shown in Table 90. In each case, the lower credible limit for QALYs is negative, reflecting the use of an equation that allowed negative QoL scores; see the Scenario analysis for the effect of changing this assumption.
| Treatment | Mean cost (£) | 95% credible interval | Mean QALY | 95% credible interval | ||
|---|---|---|---|---|---|---|
| ADA | 74,800 | 68,800 | 81,000 | 2.89 | –2.12 | 7.87 |
| ETN | 75,100 | 68,700 | 81,500 | 2.80 | –2.21 | 7.84 |
| IFX | 73,000 | 66,100 | 79,700 | 2.80 | –2.24 | 7.82 |
| RTX | 69,400 | 62,700 | 76,400 | 3.10 | –1.78 | 7.95 |
| ABT | 93,000 | 86,200 | 100,100 | 3.28 | –1.46 | 8.05 |
| DMARDs | 49,000 | 43,300 | 54,900 | 2.13 | –3.27 | 7.46 |
Incremental results were obtained by subtraction for each parameter set, thus producing a sample of 2,000 points from the incremental cost-effectiveness distribution between any pair of strategies. Again, the 95% credible interval can be found for cost and QALY differences: note that, although the mean results can be inferred from Table 90 (subject to rounding effects), the relevant percentiles cannot. The results are shown in Table 91, which shows all the pair-wise comparisons. Scatter plots for the comparisons between the biologic strategies and conventional DMARDs alone are shown in Figure 99, together with the CEACs for these five comparisons: the remaining scatter plots are shown in Appendix 13.
| Comparison | Diff cost (£) | 95% credible interval | Diff QALY | 95% credible interval | ||
|---|---|---|---|---|---|---|
| ADA–DMARDs | 25,800 | 24,100 | 27,500 | 0.75 | 0.33 | 1.23 |
| ETN–DMARDs | 26,100 | 24,200 | 27,900 | 0.67 | 0.30 | 1.10 |
| IFX–DMARDs | 24,000 | 19,500 | 26,800 | 0.67 | 0.29 | 1.12 |
| RTX–DMARDs | 20,400 | 17,500 | 23,200 | 0.96 | 0.41 | 1.61 |
| ABT–DMARDs | 44,000 | 41,300 | 46,700 | 1.15 | 0.52 | 1.88 |
| ADA–RTX | 5,400 | 2,200 | 8,700 | –0.21 | –0.52 | 0.03 |
| ETN–RTX | 5,700 | 2,400 | 9,100 | –0.29 | –0.63 | –0.04 |
| IFX–RTX | 3,600 | –1,600 | 7,600 | –0.30 | –0.62 | –0.05 |
| ABT–RTX | 23,600 | 19,800 | 27,400 | 0.18 | –0.10 | 0.50 |
| ADA–ABT | –18,200 | –21,300 | –15,200 | –0.39 | –0.77 | –0.12 |
| ETN–ABT | –18,000 | –21,200 | –14,600 | –0.47 | –0.88 | –0.17 |
| IFX–ABT | –20,000 | –25,100 | –16,200 | –0.48 | –0.88 | –0.17 |
| ADA–ETN | –300 | –2,800 | 2,100 | 0.08 | –0.09 | 0.29 |
| ADA–IFX | 1,800 | –1,400 | 6,500 | 0.09 | –0.10 | 0.29 |
| ETN–IFX | 2,000 | –1,200 | 6,800 | 0.00 | –0.17 | 0.19 |
FIGURE 99.
Cost-effectiveness scatter plots for main comparisons in the reference case. Diff, difference.
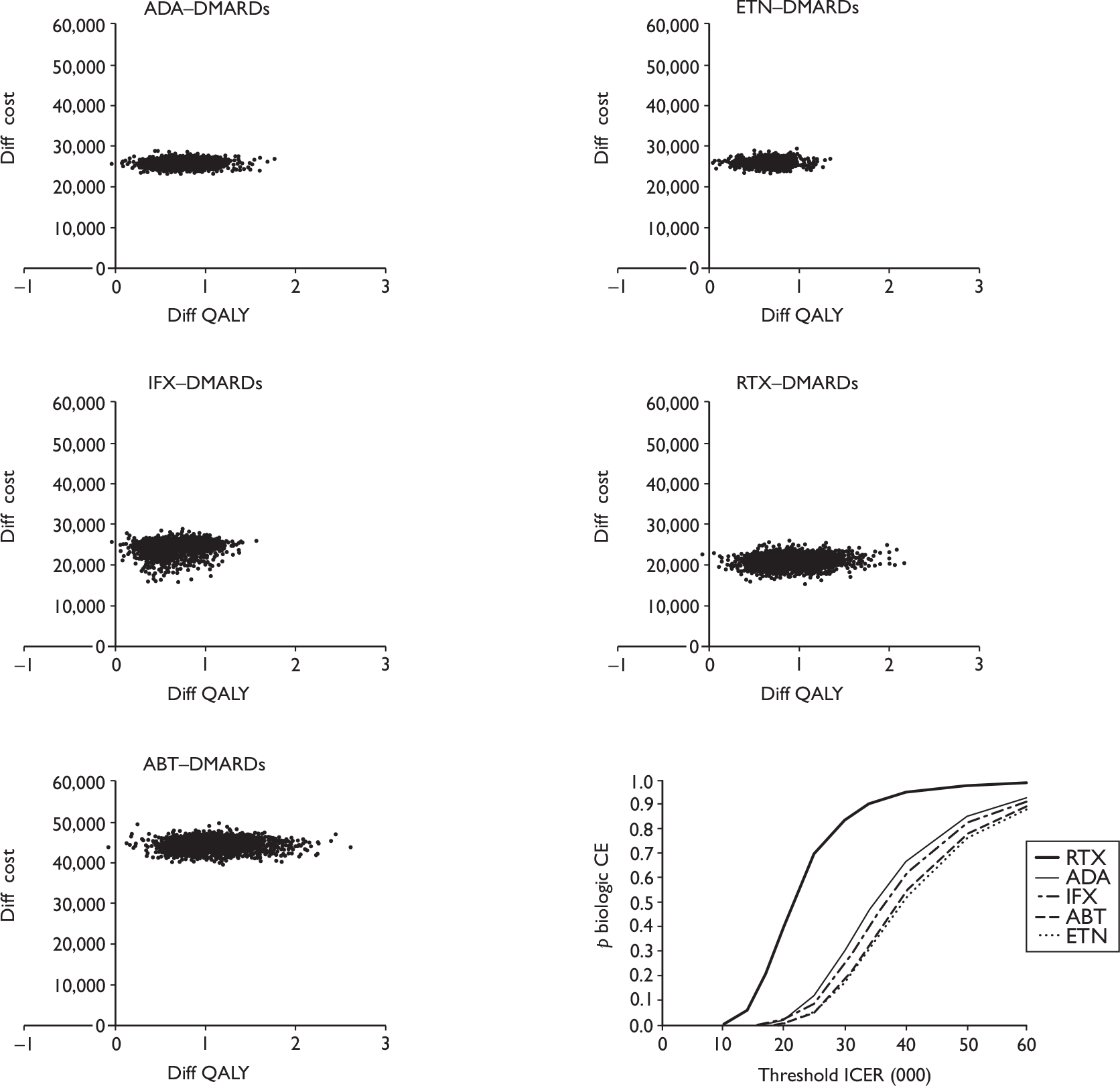
Similar remarks apply to the ICER, which is found by dividing the difference in mean cost by the difference in mean QALY. Finally, the proportion of model replications for each biologic strategy appears cost-effective compared with any other is shown, using a threshold ICER of £20,000/QALY and £30,000/QALY. These results are shown in Table 92.
| Comparison | ICER (£/QALY) | 95% credible interval | Proportion of cases cost-effective at | ||
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| ADA–DMARDs | 34,300 | 20,900 | 79,100 | 0.02 | 0.30 |
| ETN–DMARDs | 38,900 | 23,500 | 89,000 | 0.00 | 0.17 |
| IFX–DMARDs | 36,100 | 21,200 | 82,000 | 0.02 | 0.24 |
| RTX–DMARDs | 21,100 | 12,800 | 49,700 | 0.40 | 0.84 |
| ABT–DMARDs | 38,400 | 23,000 | 84,700 | 0.00 | 0.17 |
| ADA–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ETN–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| IFX–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ABT–RTX | 130,600 | 47,900 | RTX | 0.00 | 0.00 |
| ADA–ABT | 46,400 | 23,100 | 152,100 | 0.99 | 0.90 |
| ETN–ABT | 37,800 | 20,100 | 102,300 | 0.98 | 0.77 |
| IFX–ABT | 41,700 | 22,000 | 113,500 | 0.99 | 0.84 |
| ADA–ETN | ADA | Not meaningful | 0.84 | 0.84 | |
| ADA–IFX | 20,500 | Not meaningful | 0.50 | 0.61 | |
| ETN–IFX | 456,700 | Not meaningful | 0.20 | 0.24 | |
Scenario analysis
A number of different scenarios have been run. Details of each scenario and the results are given in Appendix 14, and a summary is provided in Tables 93–95. It should be noted that, although it is always possible to give a result based on the mean of the probabilistic analysis, the results for comparison between TNF inhibitors almost invariably are from a distribution covering all four quadrants of the cost-effectiveness plane, and thus the mean results are subject to enormous uncertainty in that case. The sole exception to this is the scenario ‘Vary time on TNF inhibitors’.
| Scenario | ADA–DMARDs | ETN–DMARDs | IFX–DMARDs | RTX–DMARDs | ABT–DMARDs |
|---|---|---|---|---|---|
| Reference | 34,300 | 38,900 | 36,100 | 21,100 | 38,400 |
| Vary time on TNF inhibitors | 34,300 | 38,400 | 37,700 | 21,200 | 38,500 |
| Same time on all biologics | 34,400 | 38,700 | 35,900 | 21,100 | 39,500 |
| RTX cycle time 6 months | 34,300 | 38,900 | 35,900 | 32,600 | 38,400 |
| RTX cycle time 11.6 months | 34,200 | 38,800 | 35,900 | 11,400 | 38,400 |
| Poor late DMARDs | 28,100 | 31,100 | 28,800 | 16,300 | 32,100 |
| HAQ change on biologics | 61,300 | 76,300 | 68,900 | 46,000 | 63,300 |
| AE costs included | 34,700 | 39,900 | 36,800 | 22,500 | 38,800 |
| No offset costs | 36,900 | 41,400 | 38,600 | 23,600 | 41,000 |
| Extra cost for Pall | 33,400 | 37,800 | 35,000 | 20,100 | 37,600 |
| No negative QoL scores | 48,600 | 56,500 | 52,100 | 30,700 | 52,800 |
| Linear equation HAQ to QoL | 38,600 | 43,800 | 40,600 | 23,700 | 42,300 |
| Scenario | ADA–RTX | ETN–RTX | IFX–RTX | ABT–RTX |
|---|---|---|---|---|
| Reference | RTX | RTX | RTX | 130,600 |
| Vary time on TNF inhibitors | RTX | RTX | 4,100 | 131,800 |
| Same time on all biologics | 206,000 | RTX | RTX | 131,200 |
| RTX cycle time 6 months | 430 | RTX | 14,700 | 51,500 |
| RTX cycle time 11.6 months | RTX | RTX | RTX | 861,100 |
| Poor late DMARDs | RTX | RTX | RTX | 158,600 |
| HAQ change on biologics | RTX | RTX | RTX | 96,400 |
| AE costs included | RTX | RTX | RTX | 126,100 |
| No offset costs | RTX | RTX | RTX | 134,100 |
| Extra cost for Pall | RTX | RTX | RTX | 131,000 |
| No negative QoL scores | RTX | RTX | RTX | 140,700 |
| Linear equation HAQ to QoL | RTX | RTX | RTX | 130,900 |
| Scenario | ADA–ABT | ETN–ABT | IFX–ABT | ADA–ETN | ADA–IFX | ETN–IFX |
|---|---|---|---|---|---|---|
| Reference | 46,400 | 37,800 | 41,700 | ADA | 20,500 | 456,700 |
| Vary time on TNF inhibitors | 47,700 | 38,900 | 39,100 | 72,800 | 28,700 | 39,300 |
| Same time on all biologics | 84,100 | 42,700 | 53,700 | ADA | 21,600 | 351,500 |
| RTX cycle time 6 months | 46,300 | 37,800 | 42,000 | ADA | 21,700 | 1,325,400 |
| RTX cycle time 11.6 months | 46,400 | 37,800 | 41,800 | ADA | 20,700 | 591,000 |
| Poor late DMARDs | 40,100 | 33,500 | 36,900 | ADA | 20,600 | 316,000 |
| HAQ change on biologics | 66,500 | 50,600 | 57,600 | ADA | 24,300 | IFX |
| AE costs included | 46,700 | 37,400 | 41,700 | ADA | 19,000 | 502,600 |
| No offset costs | 49,000 | 40,500 | 44,400 | ADA | 23,500 | 460,000 |
| Extra cost for Pall | 45,800 | 37,300 | 41,200 | ADA | 20,300 | 452,000 |
| No negative QoL scores | 60,300 | 48,300 | 53,700 | ADA | 25,300 | 7,430,000 |
| Linear equation HAQ to QoL | 49,100 | 40,300 | 44,600 | ADA | 23,100 | 667,000 |
Summary of model results
The reference case model results show similar costs and QALYs for the TNF inhibitors, with somewhat lower costs and QALYs for RTX and higher costs and QALYs for ABT. Compared with conventional DMARDs alone, the ICER for RTX is somewhat lower than for the other biologics. RTX dominates the TNF inhibitors (lower cost and more QALYs). The ICER for ABT compared with RTX is over £100,000/QALY. These results are subject to considerable uncertainty. Important drivers of that uncertainty were found in the scenario analysis to include:
-
the assumptions about HAQ progression on biologic treatments
-
the equation relating HAQ to QoL – in particular whether negative QoL scores can be allowed
-
for comparisons involving RTX, the assumed time between treatments.
The results were fairly sensitive to the assumptions on efficacy of conventional DMARDs given after biologic therapy. The inclusion of AE costs for biologic therapy made little difference to the results. The mean time on RTX was considerably longer than for other biologics. This parameter was varied downwards in the scenario analysis ‘Same time on all biologics’ and the results were not generally sensitive to this parameter: this makes sense because the costs and QALYs in the RTX strategy were both reduced when the mean time on RTX was reduced.
Additional sensitivity analysis to assess impact of differences in assumptions between models
The main aim of this analysis was to explore the differences between the results of the various models. Two of the industry submissions (Abbott and Schering-Plough Ltd) contained ICERs that are directly comparable with the main BRAM results. Roche gave ICERs for RTX against DMARDs and against other biologics. As the mean costs and QALYs for RTX were the same in each comparison (Tables 101–105 from MS, pp. 226–8 of their report), it is possible to infer the ICERs for other biologics against DMARDs. Table 96 shows the results from the various models.
| Model | ADA–DMARDs | ETN–DMARDs | IFX–DMARDs | RTX–DMARDs | ABT–DMARDs |
|---|---|---|---|---|---|
| BRAM reference | 34,300 | 38,900 | 36,100 | 21,100 | 38,400 |
| BRAM with poor late DMARDs | 28,100 | 31,100 | 28,800 | 16,300 | 32,100 |
| Abbott | 16,000 | 16,000 | 21,500 | 11,000 | 30,100 |
| Rochea | 14,600 | 18,000 | 16,200 | 5,300 | 21,500 |
| Schering-Plough Ltd | 35,100 | 35,900 | 28,700 | 17,400 | 44,800 |
As well as the BRAM reference case, the scenario analysis with reduced efficacy for conventional DMARDs has been quoted above. This scenario is sufficient to account for cases where the Schering-Plough model gave a more favourable result than the BRAM reference case. Accordingly the main focus of further analysis is the assumptions in the Abbott and Roche models. Two aspects of the modelling have been considered: the short-term change in HAQ on starting treatment and the proportion of early quitters. The aim was to apply the industry assumptions to the BRAM. The process for doing this is described below.
Short-term change in Health Assessment Questionnaire on starting treatment
The Abbott and Roche models each had HAQ change based on ACR response using values shown in the tables below. To compare with the BRAM, it is necessary to convert this HAQ change pattern into a set of figures in the same structure as the BRAM. This means estimating a and b parameters for the beta distribution of HAQ change multipliers used in the BRAM. As the purpose of this exercise is to assess the impact of the difference in the effectiveness assumption, the mean HAQ change multiplier was estimated from the two company submissions. The value of a + b used in the BRAM reference case was preserved and the a and b parameters were inferred using this value.
For Abbott, the relevant figures were taken to be the ACR response rates (Table 3.2.3.1 of MS, p. 51) and the relative change in HAQ score based on ACR response by treatment from baseline to 6 months (Table 3.2.5.1 of MS, p. 52).
These are repeated for convenience (Tables 97 and 98).
| Treatment | ACR < 20 | ACR20–50 | ACR50–70 | ACR > 70 |
|---|---|---|---|---|
| TNF inhibitor | 0.3574 | 0.2414 | 0.1958 | 0.2054 |
| RTX | 0.3822 | 0.2337 | 0.1858 | 0.1983 |
| ABT | 0.4531 | 0.2355 | 0.1631 | 0.1483 |
| DMARDs | 0.7474 | 0.1486 | 0.0631 | 0.0409 |
| Treatment | ACR < 20 | ACR20–50 | ACR50–70 | ACR > 70 |
|---|---|---|---|---|
| Biologics | 0.110 | 0.405 | 0.588 | 0.806 |
| DMARDs | 0.016 | 0.300 | 0.565 | 0.735 |
The mean change in HAQ score for each type of treatment is then found from using the probabilities in Table 97 as weights to calculate a weighted average of the changes in Table 98. For example, for TNF inhibitors, the calculation is 0.3574 × 0.110 + 0.2414 × 0.405 + 0.1958 × 0.588 + 0.2054 × 0.806 = 0.418.
For ETN and IFX we have a +b = 0.96 from the reference case in the BRAM, from which a = 0.418 × 0.96 = 0.401 and hence b = 0.559. Similar principles apply to the other DMARDs and the results are shown in Table 99.
| Treatment | Mean | a + b | a | b |
|---|---|---|---|---|
| ETN/IFX | 0.418 | 0.96 | 0.401 | 0.559 |
| ADA | 0.418 | 1.24 | 0.518 | 0.722 |
| RTX | 0.406 | 0.95 | 0.385 | 0.565 |
| ABT | 0.361 | 1.18 | 0.426 | 0.754 |
| LEF | 0.122 | 1.22 | 0.149 | 1.071 |
| GST | 0.122 | 1.15 | 0.141 | 1.009 |
| CyA | 0.122 | 0.39 | 0.048 | 0.342 |
| AZA | 0.122 | 1.00 | 0.122 | 0.878 |
Similarly, using the Roche parameters, Table 100 shows the probability of responses. For HAQ change, Roche give absolute falls in HAQ. These have been converted in Table 101 to relative changes by dividing by 2, which is the mean starting HAQ in the BRAM reference case. Then the same system of calculations gives the results in Table 102.
| Treatment | ACR < 20 | ACR20–50 | ACR50–70 | ACR > 70 |
|---|---|---|---|---|
| ADA | 0.538 | 0.154 | 0.182 | 0.126 |
| ETN | 0.552 | 0.196 | 0.154 | 0.098 |
| IFX | 0.58 | 0.189 | 0.133 | 0.098 |
| RTX | 0.54 | 0.23 | 0.09 | 0.14 |
| ABT | 0.57 | 0.21 | 0.14 | 0.08 |
| DMARDs | 0.85 | 0.11 | 0.03 | 0.01 |
| ACR<20 | ACR20–50 | ACR50–70 | ACR70+ |
|---|---|---|---|
| 0.05 | 0.225 | 0.405 | 0.555 |
| Treatment | Mean | a + b | a | b |
|---|---|---|---|---|
| ADA | 0.205 | 1.24 | 0.254 | 0.986 |
| ETN | 0.188 | 0.96 | 0.181 | 0.779 |
| IFX | 0.180 | 0.96 | 0.173 | 0.787 |
| RTX | 0.193 | 0.95 | 0.183 | 0.767 |
| ABT | 0.177 | 1.18 | 0.209 | 0.971 |
| LEF | 0.085 | 1.22 | 0.104 | 1.116 |
| GST | 0.085 | 1.15 | 0.098 | 1.052 |
| CyA | 0.085 | 0.39 | 0.033 | 0.357 |
| AZA | 0.085 | 1.00 | 0.085 | 0.915 |
Changing the proportion of early quitters
Another potentially important difference between the models is the proportion of people withdrawing from the treatment early. The Abbott model reference case used failure to achieve ACR50 response as the criterion for early withdrawal. Therefore, 59.88% of those starting a TNF inhibitor would not continue beyond 6 months (Abbott submission, p. 51). In the BRAM reference case, the corresponding figure (for ADA) is just under 24%, made up of the short-term withdrawals at 13 weeks (9.9%) and the first 13 weeks of the long-term survival curve (15.4% of the remaining 90.1%). Similar remarks apply to all other drugs.
Continuing to use ADA as the example, the BRAM reference case is based on a data set of 899 patients of whom 89 had withdrawn from treatment by 12 weeks, 50 of these for toxicity. For this exploratory analysis, the withdrawal time is changed to 26 weeks, and parameters for beta distributions are calculated on the basis of still having 899 patients of whom 0.5988 × 899 = 538.3 withdrew by 26 weeks. In the absence of any obvious alternative figure, the number withdrawing from because of toxicity is kept at 50. While rounding to the nearest integer would make little difference, the beta distributions can be used with non-integer parameters and so unrounded figures have been used. With regard to conventional DMARDs, the proportions withdrawing for toxicity either side of the 6-week cut-off have been maintained, the additional withdrawal rate being assigned to those withdrawal because of loss of effectiveness at 26 weeks.
As with the reference case, the structure of the model does not allow early withdrawal for RTX. Table 103 shows the revised parameters.
| Treatment | Parameter | Point estimate (%) | Distribution |
|---|---|---|---|
| ADA | Withdrawal at 26 weeks | 59.88 | Beta (538.3, 360.7) |
| Toxicity if above | 9.29 | Beta (50, 488.3) | |
| ETN | Withdrawal at 26 weeks | 59.88 | Beta (243.1, 162.9) |
| Toxicity if above | 1.45 | Beta (2, 136.2) | |
| IFX | Withdrawal at 26 weeks | 59.88 | Beta (7.8, 5.2) |
| Toxicity if above | 25.61 | Beta (2, 5.8) | |
| RTX | No early withdrawal (see text) | ||
| ABT | Withdrawal at 26 weeks | 68.86 | Beta (177.7, 80.3) |
| Toxicity if above | 5.08 | Beta (9, 168.2) | |
| LEF | Withdrawal at 6 weeks | 13 | Beta (13, 87) |
| Withdrawal 6–26 weeks | 76.6 | Beta (76.6, 23.4) | |
| Toxicity if above | 13.05 | Beta (10, 66.6) | |
| GST | Withdrawal at 6 weeks | 14 | Beta (10.1, 61.9) |
| Withdrawal 6–26 weeks | 75.6 | Beta (54.4, 17.6) | |
| Toxicity if above | 23.81 | Beta (13, 41.6) | |
| CyA | Withdrawal at 6 weeks | 8 | Beta (16, 184) |
| Withdrawal 6–26 weeks | 81.6 | Beta (163.2, 36.8) | |
| Toxicity if above | 14.71 | Beta (24, 139.2) | |
| AZA | Withdrawal at 6 weeks | 15 | Beta (15, 85) |
| Withdrawal 6–26 weeks | 74.6 | Beta (74.6, 25.4) | |
| Toxicity if above | 16.76 | Beta (12.5, 62.1) | |
For the Roche model, the early withdrawal rates were taken as the failure to achieve an ACR20 response and are therefore shown in Table 100. The same method was used to produce the figures in Table 104.
| Treatment | Parameter | Point estimate (%) | Distribution |
|---|---|---|---|
| ADA | Withdrawal at 26 weeks | 53.8 | Beta (483.7, 415.3) |
| Toxicity if above | 10.34 | Beta (50, 433.6) | |
| ETN | Withdrawal at 26 weeks | 55.2 | Beta (224.1, 181.9) |
| Toxicity if above | 1.57 | Beta (2, 125.4) | |
| IFX | Withdrawal at 26 weeks | 58 | Beta (7.5, 5.5) |
| Toxicity if above | 26.44 | Beta (2, 5.6) | |
| RTX | No early withdrawal (see text) | ||
| ABT | Withdrawal at 26 weeks | 57 | Beta (147.1, 110.9) |
| Toxicity if above | 6.13 | Beta (9, 137.7) | |
| LEF | Withdrawal at 6 weeks | 13 | Beta (13, 87) |
| Withdrawal 6–26 weeks | 72 | Beta (72, 28) | |
| Toxicity if above | 13.89 | Beta (10, 62) | |
| GST | Withdrawal at 6 weeks | 14 | Beta (10.1, 61.9) |
| Withdrawal 6–26 weeks | 71 | Beta (51.1, 20.9) | |
| Toxicity if above | 25.35 | Beta (13, 38.3) | |
| CyA | Withdrawal at 6 weeks | 8 | Beta (16, 184) |
| Withdrawal 6–26 weeks | 77 | Beta (154, 46) | |
| Toxicity if above | 15.58 | Beta (24, 130) | |
| AZA | Withdrawal at 6 weeks | 15 | Beta (15, 85) |
| Withdrawal 6–26 weeks | 70 | Beta (70, 30) | |
| Toxicity if above | 17.86 | Beta (12.5, 57.5) | |
Results
For comparison with the Abbott model, the parameters in Table 99 (short-term HAQ increase) were used in place of the BRAM reference case parameters in one analysis, all other parameters remaining as in the BRAM reference case. Separately, the parameters in Table 103 (early withdrawal) were used, keeping the short-term HAQ increase as in the BRAM reference case. Finally, both sets of parameters were changed at the same time. The results are shown in Table 105. For comparison with the Roche model, the results of a similar analysis using the parameters in Tables 102 and 104 are shown in Table 106.
| Model | ADA–DMARDs | ETN–DMARDs | IFX–DMARDs | RTX–DMARDs | ABT–DMARDs |
|---|---|---|---|---|---|
| BRAM reference | 34,300 | 38,900 | 36,100 | 21,100 | 38,400 |
| Changing HAQ increase | 21,700 | 21,900 | 20,100 | 11,100 | 28,700 |
| Changing short-term withdrawal rate | 22,200 | 23,400 | 26,200 | 19,500 | 24,100 |
| Changing both | 16,200 | 15,700 | 16,500 | 11,500 | 33,400 |
| Abbott model | 16,000 | 16,000 | 21,500 | 11,000 | 30,100 |
| Model | ADA–DMARDs | ETN–DMARDs | IFX–DMARDs | RTX–DMARDs | ABT–DMARDs |
|---|---|---|---|---|---|
| BRAM reference | 34,300 | 38,900 | 36,100 | 21,100 | 38,400 |
| Changing HAQ increase | 31,900 | 33,500 | 32,000 | 17,200 | 41,500 |
| Changing short-term withdrawal rate | 23,800 | 25,100 | 27,400 | 20,500 | 26,400 |
| Changing both | 24,500 | 24,400 | 26,900 | 17,900 | 30,900 |
| Rochea | 14,600 | 18,000 | 16,200 | 5,300 | 21,500 |
Conclusion
The differences between the reference case results in the BRAM and those produced by Abbott and Schering-Plough Ltd can be explained by changing a small number of parameters in the model. There are some differences with the Roche model that remain unexplained in this analysis. It should be stressed that the purpose of this analysis is to compare the models and this is a separate matter from the discussion of the appropriateness of the various parameters.
Chapter 5 Assessment of factors relevant to the NHS and other parties
Wide use of biologic agents, NICE guidance on RA and the recent NAO report on services for patients with RA have profound implications for specialist rheumatology services. The NAO report suggests that acute trusts and primary care trusts (PCTs) have not yet met all the challenges they face. For example, monthly review in patients with active disease, as recommended in NICE guidance, is achieved by only 15% of acute trusts surveyed by the NAO. The main barriers reported by trusts were staffing, limited outpatient capacity and pressures to improve the ratio of follow-up to new patients. A majority of the acute trusts reported that they were unable to provide adequate follow-up for RA patients. 6 Models of shared care between primary care and secondary care exist, but only around half of the GPs in the NAO survey said that they had a shared care agreement with their local acute trust. 207 Good shared-care schemes with appropriate patient selection71,208 could reduce the burden on specialists and meet some of the objectives set out in Lord Darzi’s review. 209
Increasing use of biologics, different mechanisms for obtaining funding (including appeals processes and inconsistency of response) for different PCTs and collection and submission of audit data have increased the administrative burden on specialist departments. PCTs have parallel demands with a need to monitor high-cost drug use and manage the implications of burgeoning NICE guidance while facing increasing demands from patients and hospital doctors with varying approaches to disease management. Expert teams remain vital to the delivery of services for RA patients, but pressures to provide community clinics in many locations risk fragmenting small teams and diluting expertise. The increasing complexity of care driven by new agents and more aggressive disease management means that primary care physicians are less able to take a lead role in the management of individual patients. 207 Also, the fact that prescriptions for biologics can be issued only by a specialist means that even better links between primary and secondary care colleagues are needed to co-ordinate care and avoid drug interactions.
Abatacept and TOC both require monthly i.v. infusions. Currently, such treatment is delivered largely in a hospital day-case unit. Capacity is under pressure as newer agents arrive and indications for existing agents widen. Solutions to improve capacity are needed. It seems likely that periodic i.v. infusions, required long term, will be administered away from acute hospitals and within patients’ homes or other community settings. Pilot studies exploring IFX infusions at home in stable clients are under way.
In summary, it is imperative that acute trusts and PCTs are better placed to meet the challenges of therapeutic innovations in RA and the deficiencies of care identified by the NAO.
Chapter 6 Discussion
Statement of principal findings
Quantity and quality of evidence
Thirty-five studies described in 44 papers met the inclusion criteria. These included five RCTs, three comparative studies and 28 uncontrolled studies. Comparisons made in the included RCTs were switching to IFX (from ongoing ETN) versus ongoing ETN (OPPOSITE trial, n = 27);133 RTX versus placebo with ongoing traditional DMARDs (REFLEX trial, n = 517);124–126 ABT versus placebo with ongoing traditional DMARDs (ATTAIN trial, n = 391);127–132 ABT added to ongoing ETN versus ongoing ETN (Weinblatt et al. ,134 n = 121);40 and ABT added to ongoing biologics or non-biologic DMARDs versus ongoing biologics or non-biologic DMARDs (ASSURE trial, n = 167). 134 No directly relevant head-to-head trial directly comparing any of the five technologies against each other or directly comparing any of the technologies against other biologics or previously untried, newly initiated DMARDs, was found.
Effectiveness of adalimumab
No RCT was identified. Five uncontrolled studies with duration of follow-up ranging from 3 to 12 months showed that between 46% and 75% of patients achieved ACR20 and between 13% to 33% patients achieved ACR70. Mean reductions of 1.3–1.9 in DAS28 score and of 0.21–0.48 in HAQ score were observed. Results were not pooled owing to substantial clinical and statistical heterogeneity.
Effectiveness of etanercept
No RCT was found. Seven uncontrolled studies with duration of follow-up ranging from 3 to over 9 months showed that ACR20 was achieved in 37%–71% of patients after switching to ETN, ACR70 in 4%–21% of patients. Mean reductions of 0.47 to 1.80 in DAS28, and of 0.35 to 0.45 in HAQ score were observed. Results were not pooled due to substantial clinical and statistical heterogeneity between studies.
Effectiveness of infliximab
One RCT (OPPOSITE trial133) compared switching to IFX (n = 13) versus staying on ETN (n = 14) in patients who had an incomplete response to ETN. The study was considered not directly relevant to this report. Three uncontrolled studies with unclear length of follow-up were found, but none of these reported ACR response criteria or quantitative results of changes in DAS28 and HAQ scores.
Effectiveness of tumour necrosis factor inhibitors as a class
Some of the included studies assessed switching to an alternative TNF inhibitor, but did not provide data separately for individual TNF inhibitors. Two non-randomised comparative studies and six uncontrolled studies with duration of follow-up ranging from 3 months to 4 years were identified. ACR responses were reported in only one study, with response rates of 49% for ACR20 and 7% for ACR70 being observed. Reported mean reductions in DAS28 score ranged from –0.88 to –1.00. Only one study (using data from BSRBR) reported mean reduction in HAQ score of –0.11.
Effectiveness of rituximab
One good-quality RCT (REFLEX)124–126 compared RTX with placebo (with ongoing DMARDs in both groups) in patients who had had inadequate response to one or more TNF inhibitors. At 6 months significantly more patients treated with RTX achieved ACR20 (RR = 2.85, 95% CI 2.08 to 2.91) and ACR70 (RR = 12.14, 95% CI 2.96 to 49.86) than those treated with the placebo. Significant differences between groups in favour of RTX were observed at 6 months for mean change from baseline in DAS28 score (mean difference –1.50, 95% CI –1.74 to –1.26) and mean change from baseline in HAQ score (mean difference –0.30, 95% CI –0.40 to –0.20). No significant difference in the risk of serious AEs and serious infections was observed. One non-randomised comparative study, five uncontrolled studies and two further analyses of data from RTX RCTs were also identified. Results generally supported findings from the REFLEX trial. 124–126
Effectiveness of abatacept
One good-quality RCT (ATTAIN127–132) compared ABT with placebo (with ongoing DMARDs in both groups) in patients who had had inadequate response to one or more TNF inhibitors. At 6 months significantly more patients treated with ABT achieved ACR20 (RR = 2.56, 95% CI 1.77 to 3.69) and ACR70 (RR = 6.70, 95% CI 1.62 to 27.80) than those treated with the placebo. Significant differences between groups in favour of ABT were observed at 6 months for mean change from baseline in DAS28 score (mean difference –1.27, 95% CI –1.62 to –0.93) and mean change from baseline in HAQ score (mean difference –0.34, insufficient data for calculating 95% CI). No significant difference in the risk of serious AEs and serious infections was observed. Further data from the LTE of the ATTAIN trial119 and a large prospective uncontrolled study (ARRIVE) generally supported findings from the ATTAIN trial. 127–132 Two further RCTs (Weinblatt et al. 133 and ASSURE135) were identified that compared ABT added to ongoing TNF inhibitors/biologics versus ongoing TNF inhibitors/biologics. The results from these trials showed patients who received a combination of ABT and a TNF inhibitor had an increased risk of infection and serious infection. This is reflected in the licensed indication, which advises against the use of such combination therapy, and thus further data from combination therapy were not assessed in this report.
Comparative effectiveness
No RCT provided evidence on genuine head-to-head comparisons between the technologies, other biologics and newly initiated, previously untried DMARDs. One non-randomised controlled study136,137 compared switching to RTX versus switching to an alternative TNF inhibitor. The mean change in DAS28 score was greater in the RTX group than in the TNF inhibitor group (mean difference –0.35, 95% CI –0.71 to 0.01; median follow-up 11 months) but the difference just failed to reach statistical significance.
It was possible to carry out adjusted IC between RTX and ABT using data from placebo-controlled trials that included similar patient populations. The results showed no evidence of significant difference in their effectiveness (ACR20 for RTX vs ABT, RR = 1.12, 95% CI 0.68 to 1.84). No further analyses for comparative effectiveness were performed owing to limitation in available data.
Subgroup analyses
Evidence from the REFLEX trial124–126 suggested that the effectiveness of RTX does not vary significantly according to reasons of withdrawal, baseline RF status and number of prior TNF inhibitors tried (one vs more than one).
No significant differences in the effectiveness of ABT between subgroups defined by the number of prior TNF inhibitor (one vs two) and the identity of the prior TNF inhibitor received (ETN vs infliximab) were observed in the ATTAIN trial. 127–132 However, some of these subgroup analyses may be underpowered.
Evidence from observational studies showed that the proportion of patients responding to a subsequent TNF inhibitor might vary according to reason for withdrawal of the previous TNF inhibitor (higher response in patients who withdrew due to intolerance/AEs than in those who withdrew due to lack of efficacy). The proportion of patients who respond to a subsequent treatment (including TNF inhibitors, RTX and ABT) decreases as the number of prior TNF inhibitor(s) that the patients have tried increases.
Review of cost-effectiveness studies
Four studies met the inclusion criteria. All studies used a decision-analytic model. Published models vary in some important aspects: the type of model used, the sequence of drugs, comparator therapies and time horizon. All but one study carried out a cost–utility analysis and reported results in ‘cost per QALY’. One study carried out a cost-effectiveness analysis and reported results in cost per additional case of ‘low disease activity state’ gained (DAS28 less than 2.6) and cost per additional remission gained (DAS28 less than or equal to 3.2). Appropriate sensitivity analyses were carried out in all studies. A comparison of ICERs between studies is not possible because of the different approaches to modelling, in particular time horizon, country of origin and perspective chosen. There was disparity in the selection of perspectives chosen for the analyses. One study reported costs that include both those from a health-care perspective as well as indirect costs and costs of informal care; inclusion of these costs improves the cost-effectiveness of the drug.
Independent modelling
The reference case model results show similar costs and QALYs for the TNF inhibitors, with somewhat lower costs and QALYs for RTX and higher costs and QALYs for ABT. Compared with conventional DMARDs alone, the ICER for RTX is somewhat lower than for the other biologics. RTX dominates the TNF inhibitors and the ICER for ABT compared with RTX is over £100,000/QALY. These results are subject to considerable uncertainty. Important drivers of that uncertainty were found in scenario analysis to include:
-
the assumptions used about HAQ progression on biologic treatments
-
the equation relating HAQ to QoL – in particular whether negative QoL scores can be allowed
-
for comparisons involving RTX, the assumed time between treatments.
The inclusion of AE costs for biologic therapy made little difference to the results.
Strengths and limitations of the assessment
Strengths of the assessment
The strengths of this assessment include:
-
A comprehensive literature review was undertaken which went beyond RCT evidence. Studies were selected and assessed according to a pre-specified protocol. Additional data from MSs were included.
-
Key data were graphically presented in a systematic way to allow easy inspection of the variations between studies.
-
Detailed subgroup analyses were carried out to examine factors that may influence the effectiveness of the technologies.
-
The BRAM model has been further improved and modelling was carried out on various scenarios to explore uncertainties.
Limitation of the assessment
The limitations predominantly relate to factors outside the control of the assessment group. The major limitation of the assessment was the paucity of evidence from RCTs for assessing the clinical effectiveness of the three TNF inhibitors, and a complete absence of genuine head-to-head trials comparing the five technologies against each other, against other biologics or against newly initiated, previously untried DMARDs.
Given the paucity of RCT evidence, this report assessed data from observational studies that are more prone to potential bias. Most of the included studies were uncontrolled studies, which allow only the assessment of treatment response post-intervention compared with before intervention. Such comparisons do not adjust for the natural course of the disease; hence any observed responses could be attributed to possible effects of the treatment as well as other factors such as different methods of follow-up and data collection, data imputation and regression to the mean.
As registration of observational study is not mandated, they are more prone to publication bias. In addition, the reporting of outcomes varies widely between studies, and the scope for selective reporting of outcomes is substantial. These biases are difficult to assess.
The focus of this assessment was on the patient population who have had an inadequate response to a first TNF inhibitor. Many existing studies have included patient populations who withdrew from the previous TNF inhibitor due to AEs/intolerance and/or who had already tried more than one TNF inhibitor. The subgroup analysis suggests these factors may influence the proportion of patients who respond to subsequent treatments, but this does not necessarily translate into differential effectiveness measured as RR or RD. Furthermore, there is much less evidence to allow assessment of whether the magnitude of effects varies between subgroups in those patients who do respond. These require further research.
Uncertainties
Lack of good-quality evidence on effectiveness of the use of an alternative TNF inhibitor after patients had an inadequate response is the source of major uncertainty for this assessment. For the assessment of cost-effectiveness, lack of evidence assessing the effectiveness of previous untried traditional DMARDs in this patient population is also an important source of uncertainty.
Additional areas of uncertainty identified in the independent modelling include assumptions about HAQ progression on biologic treatments; whether negative QoL scores can be allowed when estimating QoL from HAQ score, and treatment interval between courses of RTX.
Chapter 7 Conclusions
Implications for service provision
In relation to the decision problems described in Chapter 2, the findings of this assessment report suggest:
-
There is a lack of good-quality evidence directly comparing the effectiveness of the five technologies against each other. This imposes significant uncertainties with regard to any assessment of their relative cost-effectiveness. Adjusted IC suggests that there is no significant difference in the effectiveness between RTX and ABT, both of which are supported by good-quality RCT evidence. Existing data do not allow reliable quantification of the effectiveness of TNF inhibitors compared with RTX and ABT. Independent modelling comparing each of the other four technologies with RTX (recommended in current NICE guidance) suggests RTX dominating ADA, ETN and infliximab, and an estimated ICER of £131,000 (per QALY) for ABT compared with RTX.
-
There is a lack of evidence comparing the effectiveness of the five technologies with newly initiated, previously untried DMARDs. Independent modelling based on certain assumptions suggests the following ICERs: £34,300 (per QALY) for ADA, £38,800 for ETN, £36,200 for infliximab, £21,200 for RTX and £38,600 for ABT.
-
There is a lack of evidence directly comparing the effectiveness of the five technologies with other biologic agents.
-
Good-quality evidence from RCTs suggests that RTX and ABT are more effective than supportive care (including ongoing DMARDs which had provided inadequate control of the disease). Data from observational studies suggest that the use of an alternative TNF inhibitor after patients had inadequate response to a first TNF inhibitor may offer some benefit, but there remain significant uncertainties with regard to the magnitude of treatment effects and how these translate into cost-effectiveness.
-
Good-quality evidence from RCTs does not suggest differential effectiveness between various subgroups for RTX and ABT.
Suggested research priorities
The following research priorities are suggested in view of findings of this assessment:
-
Head-to-head trials of adequate size and duration comparing the clinical effectiveness and cost-effectiveness of the technologies against each other and emerging biologics.
-
Good-quality studies collecting information on the clinical effectiveness and cost-effectiveness of the technologies compared with previously untried conventional DMARDs in this patient population.
-
Further analysis and synthesis of existing and future RCT data to quantify the potential impact of reasons for withdrawal of first TNF inhibitor, the history of prior exposure to TNF inhibitor(s) and autoantibody status (e.g. RF and anti-CCP antibody) on the effectiveness of the technologies.
-
An overarching synthesis of evidence for the effectiveness of treatment modalities that can be used in various places of the treatment pathway for RA.
-
Development of technologies/methods for identifying patients who are likely to respond to a biologic with a particular mode of action.
-
Assessment of different methods and tariffs of utility valuations in RA and the impact of different methods on economic evaluation.
Acknowledgements
We thank Karen Biddle for providing administrative support.
Contributions of authors
Kinga Malottki was the main reviewer on this report and maintained day-to-day running of the review. She participated in study selection, data extraction and analyses. She drafted the methods results (template) and sections and edited the report. She conducted the clinical analyses for infliximab, abatacept and comparative studies.
Dr Pelham Barton constructed and revised the Birmingham Rheumatoid Arthritis Model (BRAM) and carried out de novo modelling using the revised BRAM. He wrote the sections of the report relating to modelling and also provided senior support for all economic sections.
Angelos Tsourapas conducted the cost-effectiveness review and critique of MSs.
Abdulrahman Uthman participated in data extraction and data checking and conducted analyses for etanercept and TNF inhibitors as a class.
Zulian Liu participated in data extraction and data checking and conducted analyses for adalimumab and rituximab.
Dr Kristina Routh participated in data extraction and data checking.
Dr Martin Connock provided support for statistical analyses and conducted indirect analyses.
Dr Paresh Jobanputra provided clinical advice, conducted the survey of the West Midlands Rheumatologists and drafted the background and factors relevant to the NHS sections.
Dr David Moore participated in study selection, edited various sections of the report and provided senior support.
Anne Fry-Smith devised and implemented search strategies for bibliographic databases and drafted the searching methods section.
Dr Yen-Fu Chen was the senior reviewer on this report and provided project management and advice on all aspects of the report. He compiled the study protocol, participated in study selection, data extraction and analyses, conducted subgroup analyses, drafted the summary and discussion and takes responsibility for the whole report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Jacoby R, Jayson M, Cosh J. Onset, early stages, and prognosis of rheumatoid arthritis: a clinical study of 100 patients with 11-year follow-up. Br Med J 1973;ii:96-100.
- Wiles NJ, Scott DGI, Barrett EM, Merry P, Arie E, Gaffney K, et al. Benchmarking: the five year outcome of rheumatoid arthritis assessed using a pain score, the Health Assessment Questionnaire and the Short Form-36 in a community and a clinic based sample. Ann Rheum Dis 2001;60:956-61.
- Arnett FC, Edworthy S, Bloch D. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315-24.
- Wiles N, Symmons D, Harrison B, Barrett E, Barrett J, Scott D. Estimating the incidence of rheumatoid arthritis. Trying to hit a moving target?. Arthritis Rheum 1999;42:1339-46.
- Symmons D, Turner G, Webb R, Asten P, Barrett E, Lunt M, et al. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology 2002;41:793-800.
- National Audit Offices . Services for People With Rheumatoid Arthritis n.d. www.nao.org.uk/publications/0809/services_for_people_with_rheum.aspx (accessed November 2009).
- MacGregor A, Snieder H, Rigby A, Koskenvuo M, Kapiro J, Aho K. Characterising the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum 2000;43:30-7.
- Jones MA, Silman AJ, Whiting S, Barrett EM, Symmons DP. Occurrence of rheumatoid arthritis is not increased in the first degree of relatives of a population based inception cohort of inflammatory polyarthritis. Ann Rheum Dis 1996;55:89-93.
- Barton P, Worthington J. Genetic susceptibility to rheumatoid arthritis: an emerging picture. Arthritis Rheum 2009;61:1441-6.
- Newton JL, Harvey SM, Wordsworth BP, Brown MA. A review of the MHC genetics of rheumatoid arthritis. Genes Immun 2004;5:151-7.
- Brennan F, McInnes I. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 2008;118:3537-45.
- Firestein GS. Pathogensis of rheumatoid arthritis: how early is early?. Arthritis Res Ther 2005;7:157-9.
- Edwards JCW, Cambridge G. Prospects for B-cell-targeted therapy in autoimmune disease. Rheumatology 2005;44:151-6.
- Waldburger JM, Firestein GS. Garden of therapeutic delights: new targets in rheumatic diseases. Arthritis Res Ther 2009;11.
- National Collaborating Centre for Chronic Conditions . Rheumatoid Arthritis: National Clinical Guideline for Management and Treatment in Adults 2009.
- Kirwan JR, Power LL. Glucocorticoids in Rheumatic Disease n.d. www.arc.org.uk/arthinfo/medpubs/6633/6633.asp (accessed November 2009).
- Jones G, Halbert J, Crotty M, Shanahan EM, Batterham M, Ahern M. The effect of treatment on radiological progression in rheumatoid arthritis: a systematic review of randomized placebo-controlled trials. Rheumatology 2003;42:6-13.
- Jobanputra P, Wilson J, Douglas K, Burls A. A survey of British rheumatologists’ DMARD preferences for rheumatoid arthritis. Rheumatology 2004;43:206-10.
- Pincus T, Marcum SB, Callahan LF. Long-term drug therapy for rheumatoid arthritis in seven rheumatology private practices: II. Second line drugs and prednisone. J Rheumatol 1992;19.
- Klarenbeek NR, Güler-Yüksel M, Gerards AH, Kerstens PJSM, Molenaar ETH, Huizinga TW, et al. Clinical and Radiological Outcomes of Four DAS Driven Treatment Strategies: 6-Year Results of the BeSt Study 1019.
- Grigir C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263-9.
- Goekoop-Ruiterman YPM, De Vries-Bouwstra JK, Allaart CF, Van ZD, Kerstens PJSM, Hazes JMW, et al. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med 2007;146:406-15.
- Moreland LW, O’Dell JR, Paulus H, Curtis JR, Bridges SL, Zhang X, et al. Treatment of Early Aggressive Ra; A Randomized, Double-Blind, 2-Year Trial Comparing Immediate Triple DMARD Versus MTX Plus Etanercept to Step-up from Initial MTX Monotherapy n.d.
- Wiles N, Scott DGI, Barrett EM, Merry P, Arie E, Gaffney K, et al. Benchmarking: the five year outcome of rheumatoid arthritis assessed using a pain score, the Health Assessment Questionnaire, and the Short Form-36 (SF-36) in a community and a clinic based sample. Ann Rheum Dis 2001;60:956-61.
- Van Der Kooij SM, Goekoop-Ruiterman YP, De Vries-Bouwstra JK, Guler-Yuksel M, Zwinderman AH, Kerstens PJ, et al. Drug-free remission, functioning and radiographic damage after 4 years of response-driven treatment in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis 2009;68:914-21.
- Smolen JS, Aletaha D, Keystone E. Superior efficacy of combination therapy for rheumatoid arthritis fact or fiction?. Arthritis Rheum 2005;52:2975-83.
- Saag KG, Gim GT, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2008;59:762-84.
- Combe B, Landewe R, Lukas C, Bolosiu HD, Breedveld F, Dougados M, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007;66:34-45.
- National Institute for Health and Clinical Excellence . Rheumatoid Arthritis: Consultation Table. n.d. www.nice.org.uk/guidance/index.jsp?action=download%26o=43340 (accessed November 2009).
- Treharne GJ, Lyons AC, Hale ED, Douglas KMJ, Kitas GD. ‘Compliance’ is futile but is ‘concordance’ between rheumatology patients and health professionals attainable?. Rheumatol 2006;45:1-5.
- Scott D, Shipley M, Dawson A, Edwards S, Symmons D, Woolf A. The clinical management of rheumatoid arthritis and osteoarthritis: strategies for improving clinical effectiveness. Br J Rheumatol 1998;37:546-54.
- Scottish Intercollegiate Guidelines Network (SIGN) . Management of Early Rheumatoid Arthritis n.d. www.sign.ac.uk/guidelines/fulltext/48/index.html (accessed 17 May 2005).
- Wolfe F, Zwillich SH. The long-term outcomes of rheumatoid arthritis. Arthritis Rheum 1998;41:1072-82.
- van Gestel AM, Haagsma CJ, Van Riel PLCM. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum 1998;41:1845-50.
- Fransen J, Creemers MCW, Van Riel PLCM. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology 2004;43:1252-55.
- Jerram S, Butt S, Gadsby K, Deighton C. Discrepancies between the EULAR response criteria and the NICE guidelines for continuation of anti-TNF therapy in RA: a cause for concern?. Rheumatology 2008;47:180-2.
- Wolfe F, Michaud K, Pincus T, Furst D, Keystone E. The disease activity score is not suitable as the sole criterion for initiation and evaluation of anti-tumor necrosis factor therapy in the clinic. Arthritis Rheum 2005;52:3873-9.
- Uhlig T, Kvien TK, Pincus T. Test retest reliability of disease activity core set measures and indicies in rheumatoid arthritis. Ann Rheum Dis 2009;68:972-5.
- Wolfe F. Which HAQ is best? A comparison of the HAQ, MHAQ and RA-HAQ, a difficult 8 item HAQ (DHAQ), and a rescored 20 item HAQ (HAQ20). J Rheumatol 2001;28:982-9.
- Kirwan JR. Links between radiological change, disability and pathology in rheumatoid arthritis. Rheumatology 2001;28:881-6.
- Landewe R, Boers M, van der Heijde D. How to interpret radiological progression in randomised clinical trials?. Rheumatology 2003;42:2-5.
- Young A, Dixey J, Cox N, Davies P, Devlin J, Emery P. How does disability in early rheumatoid arthritis (RA) affect patients and their lives? Results of 5 years follow-up in 732 patients from the Early RA Study (ERAS). Rheumatology 2000;39:603-11.
- Barrett EM, Scott DGI, Wiles N, Symmons D. The impact of rheumatoid arthritis on employment status in the early years of disease: a UK community-based study. Rheumatology 2000;39:1403-9.
- Chorus AMJ, Miedem HS, Wevers CJ, van der Linden S. Labour force participation among patients with rheumatoid arthritis. Ann Rheum Dis 2000;59:549-54.
- Goodson N, Wiles N, Lunt M, Barrett EM, Silman A, Symmons DPM. Mortality in early inflammatory polyarthritis. Cardiovascular disease is increased in seropositive patients. Arthritis Rheum 2002;46:2010-19.
- Boers M, Dijkmans B, Gabriel S, Maradit-Kremers H, O’Dell J, Pincus T. Making an impact on mortality in rheumatoid arthritis. Targeting cardiovascular disease. Arthritis Rheum 2004;50:1734-9.
- Navarro-Cano G, del Rincon I, Pogosian S, Roldan JF, Escalante A. Association of mortality with disease severity in rheumatoid arthritis, independent of comorbidity. Arthritis Rheum 2003;48:2425-33.
- Ward MM. Recent improvements in survival in patients with rheumatoid arthritis: better outcomes or different study designs?. Arthritis Rheum 2001;44:1467-9.
- Gabriel SE, Crowson CS, Kremers HM, Doran MF, Turesson C, O’Fallon WM, et al. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum 2003;48:54-8.
- Menue C, Touze E, Ludovic Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology 2009;48:1309-13.
- Gabriel S. Why do people with rheumatoid arthritis still die prematurely?. Ann Rheum Dis 2008;67:iii30-iii34.
- Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum 2002;46:2294-300.
- Hallert E, Husberg M, Jonsson D, Skogh T. Rheumatoid arthritis is already expensive during the first year of the disease (the Swedish TIRA project). Rheumatology 2004;43:1374-82.
- Brouwer WB, van Exel NJ, Van de Berg B, Dinant HJ, Koopmanschap MA. Burden of caregiving: evidence of objective burden, subjective burden, and quality of life impacts on informal caregivers of patients with rheumatoid arthritis. Arthritis Rheum 2004;51:570-7.
- Michaud K, Messer J, Choi HK, Wolfe F. Direct medical costs and their predictors in patients with rheumatoid arthritis: a three-year study of 7,257 patients. Arthritis Rheum 2003;48:2750-62.
- Puolakka K, Kautiainen H, Mottonen T, Hannonen P, Korpela M, Julkunen H, et al. Impact of initial aggressive drug treatment with a combination of disease-modifying antirheumatic drugs on the development of work disability in early rheumatoid arthritis: a five-year randomized follow-up trial. Arthritis Rheum 2004;50:55-62.
- Young A, Dixey J, Kulinskay E, Cox N, Davies P, Devlin J, et al. Which patients stop working because of rheumatoid arthritis? Results of five years’ follow up in 732 patients from Early RA Study (ERAS). Ann Rheum Dis 2002;6:335-40.
- National Institute for Health and Clinical Excellence . Certolizumab Pegol for the Treatment of Rheumatoid Arthritis 2010. http://guidance.nice.org.uk/TA186/Guidance/pdf/English (accessed 15 February 2011).
- Ehlers S. Why does tumour necrosis factor targeted therapy reactivate tuberculosis?. J Rheumatol 2005;32:35-9.
- Kindler V, Sappiro AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 1989;56:731-40.
- British Thoracic Society Standards of Care Committee . BTS recommendations for assessing risk, and for managing M.tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 2005;60:800-5.
- Ledingham J, Wilkinson C, Deighton C. British Thoracic Society (BTS) recommendations for assessing risk and managing tuberculosis in patients due to start anti-TNF-a treatments. Rheumatology 2005;44:1205-6.
- Gardam MA, Keystone EC, Menzies R, Manners S, Skamene E, Long R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. Lancet 2003;3:148-55.
- Charles PJ, Smeenk RJT, De Jong J, Feldmann M, Maini R. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumour necrosis factor a: findings in open-label and randomised placebo controlled trials. Arthritis Rheum 2000;43:2383-90.
- Fleischmann RM. Progressive multifocal leukoencephalopathy following rituximab treatment in a patient with rheumatoid arthritis. Arthritis Rheum 2009;60:3225-8.
- National Institute for Health and Clinical Excellence . Tocilizumab for the Treatment of Rheumatoid Arthritis 2010. http://guidance.nice.org.uk/TA198/Guidance/pdf/English (accessed 15 February 2011).
- Hetland ML, Stengaard-Pedersen K, Junker P, Lottenburger T, Hansen I, Andersen LS, et al. Aggressive combination therapy with intra-articular glucocorticoid injections and conventional disease-modifying anti-rheumatic drugs in early rheumatoid arthritis: second-year clinical and radiographic results from the CIMESTRA study. Ann Rheum Dis 2008;67:815-22.
- Van Vollenhoven RF, Ernestam S, Geborek P, Petersson IF, Coster L, Waltbrand E, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet 2009;374:459-66.
- Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 2008;372:375-82.
- Fraenkel L, Bogardus ST, Concato J, Felson DT, Wittink DR. Patient preferences for treatment of rheumatoid arthritis. Ann Rheum Dis 2004;63:1372-8.
- Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL. The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis. Health Technol Assess 2005;9.
- The British Society for Rheumatology Biologics Register . Newsletter n.d. www.medicine.manchester.ac.uk/images/File/bsrbr_newsletter_oct_2009.pdf (accessed November 2009).
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91.
- Suarez-Almazor M, Ortiz Z, Lopez-Olivo M, Pak C, Skidmore B, Kimmel B, et al. Infliximab and etanercept in rheumatoid arthritis: timing, dose escalation, and switching. Ottawa: Canadian Agency for Drugs and Technologies in Health (CADTH) 2007;47:1-34.
- Anonymous . Rituximab (in rheumatoid arthritis): for a few patients, with close monitoring. Prescrire Int 2007;16:186-8.
- Carmona L, Ortiz A, Abad MA. How good is to switch between biologics? A systematic review of the literature. Acta Reumatol Port 2007;32:113-28.
- Unit of Health Economics and Technology Assessment . Rituximab in Patients With Rheumatoid Arthritis: Systematic Review and Economic Evaluation 2006.
- Kaine JL. Abatacept for the treatment of rheumatoid arthritis: a review. Curr Ther Res Clin Exp 2007;68:379-99.
- Brodszky V, Czirjak L, Geher P, Hodinka L, Karpati K, Pentek M, et al. Rituximab in patients with rheumatoid arthritis: systematic review. Orvosi Hetilap 2007;148:1883-93.
- Sherrer Y. Abatacept in biologic-naive patients and TNF inadequate responders: clinical data in focus. Curr Med Res Opin 2008;24:2283-94.
- Lutt JR, Deodhar A. Rheumatoid arthritis: strategies in the management of patients showing an inadequate response to TNFalpha antagonists. Drugs 2008;68:591-606.
- Coughlin M. Improving patient outlook in rheumatoid arthritis: experience with abatacept. J Am Acad Nurse Pract 2008;20:486-95.
- Calvet FJ, Maymo J. What can be done in inadequate response to anti-TNF inhibitors? Review of the evidence. Reumatologia Clinica Suplementos 2008;3:24-31.
- De Lara MGM, Balsa A. Efficacy of rituximab in rheumatoid arthritis. Medicina Clinica Monografias 2008;9:20-6.
- Fernandez-Lopez C, Blanco FJ. ATTAIN study: efficacy of abatacept in patients with rheumatoid arthritis and inadequate response to anti-TNF-alpha. Reumatologia Clinica Suplementos 2006;1:34-43.
- Maymo-Guarch J. Rituximab in patients with rheumatoid arthritis and inadequate response to anti-TNF therapy. Reumatologia Clinica Suplementos 2006;1:31-5.
- Morovic-Vergles J. Rituximab (Mabthera)--treatment of rheumatoid arthritis patients with inadequate response to TNF inhibitors--when to change therapy?. Reumatizam 2008;55:70-2.
- Kristensen LE, Saxne T, Geborek P. Switching between anti-tnf therapies does not affect level of adherence to therapy in rheumatoid arthritis but response rates seem to decline. Ann Rheum Dis 2006;65.
- Kafka SP, Hinkle K, Reed G. Discontinuing or switching TNF antagonists in patients with rheumatoid arthritis: data collected from the corrona database. Ann Rheum Dis 2005;64.
- Naumann J, Detert J, Buttgereit F, Burmester G. A second anti-tnfa therapy after treatment failure of the first anti-tnfa therapy results in a significant decrease of concomitant glucocorticoid treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2006;65.
- Genentech . A Study of Retreatment With Rituximab in Patients With Rheumatoid Arthritis Receiving Background Methotrexate (SUNRISE) n.d. http://clinicaltrials.gov/ct2/show/NCT00266227 (accessed July 2009).
- Bennett AN, Peterson P, Zain A, Grumley J, Panayi G, Kirkham B. Adalimumab in clinical practice. Outcome in 70 rheumatoid arthritis patients, including comparison of patients with and without previous anti-TNF exposure. Rheumatology 2005;44:1026-31.
- Wick MC, Ernestam S, Lindblad S, Bratt J, Klareskog L, Van Vollenhoven RF. Adalimumab (Humira) restores clinical response in patients with secondary loss of efficacy from infliximab (Remicade) or etanercept (Enbrel): results from the STURE registry at Karolinska University Hospital. Scand J Rheumatol 2005;34:353-8.
- Nikas SN, Voulgari PV, Alamanos Y, Papadopoulos CG, Venetsanopoulou AI, Georgiadis AN, et al. Efficacy and safety of switching from infliximab to adalimumab: a comparative controlled study. Ann Rheum Dis 2006;65:257-60.
- Burmester GR, Mariette X, Montecucco C, Monteagudo-Saez I, Malaise M, Tzioufas AG, et al. Adalimumab alone and in combination with disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis in clinical practice: the Research in Active Rheumatoid Arthritis (ReAct) trial. Ann Rheum Dis 2007;66:732-9.
- Bombardieri S, Ruiz AA, Fardellone P, Geusens P, McKenna F, Unnebrink K, et al. Effectiveness of adalimumab for rheumatoid arthritis in patients with a history of TNF-antagonist therapy in clinical practice. Rheumatology 2007;46:1191-9.
- van der Bijl AE, Breedveld FC, Antoni CE, Kalden JR, Kary S, Burmester GR, et al. An open-label pilot study of the effectiveness of adalimumab in patients with rheumatoid arthritis and previous infliximab treatment: relationship to reasons for failure and anti-infliximab antibody status. Clin Rheumatol 2008;27:1021-8.
- Haraoui B, Keystone EC, Thorne JC, Pope JE, Chen I, Asare CG, et al. Clinical outcomes of patients with rheumatoid arthritis after switching from infliximab to etanercept. J Rheumatol 2004;31:2356-9.
- Buch MH, Seto Y, Bingham SJ, Bejarano V, Bryer D, White J, et al. C-reactive protein as a predictor of infliximab treatment outcome in patients with rheumatoid arthritis: defining subtypes of nonresponse and subsequent response to etanercept. Arthritis Rheum 2005;52:42-8.
- Cohen G, Courvoisier N, Cohen JD, Zaltni S, Sany J, Combe B. The efficiency of switching from infliximab to etanercept and vice-versa in patients with rheumatoid arthritis. Clin Exp Rheumatol 2005;23:795-800.
- Buch MH, Bingham SJ, Bejarano V, Bryer D, White J, Emery P, et al. Therapy of patients with rheumatoid arthritis: outcome of infliximab failures switched to etanercept. Arthritis Care Res (Hoboken) 2007;57:448-53.
- Iannone F, Trotta F, Monteccuco C, Giacomelli R, Galeazzi M, Matucci-Cerinic M. Erratum: Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects (Ann Rheum Dis 2007; 66: 249– 52). Ann Rheum Dis 2007;66.
- Laas K, Peltomaa R, Kautiainen H, Leirisalo-Repo M. Clinical impact of switching from infliximab to etanercept in patients with rheumatoid arthritis. Clin Rheumatol 2008;27:927-32.
- Bingham CO, Ince A, Haraoui B, Keystone EC, Chon Y, Baumgartner S. Effectiveness and safety of etanercept in subjects with RA who have failed infliximab therapy: 16-week, open-label, observational study. Curr Med Res Opin 2009;25:1131-42.
- Ang HTS, Helfgott S. Do the clinical responses and complications following etanercept or infliximab therapy predict similar outcomes with the other tumor necrosis factor-alpha antagonists in patients with rheumatoid arthritis?. J Rheumatol 2003;30:2315-18.
- Hansen KE, Hildebrand JP, Genovese MC, Cush JJ, Patel S, Cooley DA, et al. The efficacy of switching from etanercept to infliximab in patients with rheumatoid arthritis. J Rheumatol 2004;31:1098-102.
- Yazici Y, Erkan D, Van Vollenhoven RF. Do etanercept-naive patients with rheumatoid arthritis respond better to infliximab than patients for whom etanercept has failed?. Ann Rheum Dis 2004;63:607-8.
- Gomez-Reino JJ, Carmona L. Biobadaser group . Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther 2006;8.
- Solau-Gervais E, Laxenaire N, Cortet B, Dubucquoi S, Duquesnoy B, Flipo R-M. Lack of efficacy of a third tumour necrosis factor alpha antagonist after failure of a soluble receptor and a monoclonal antibody. Rheumatology 2006;45:1121-4.
- Hjardem E, Ostergaard M, Podenphant J, Tarp U, Andersen LS, Bing J, et al. Do rheumatoid arthritis patients in clinical practice benefit from switching from infliximab to a second tumor necrosis factor alpha inhibitor?. Ann Rheum Dis 2007;66:1184-9.
- Duftner C, Dejaco C, Larcher H, Schirmer M, Herold M. Biologicals in rheumatology: Austrian experiences from a rheumatic outpatient clinic. Rheumatol Int 2008;29:69-73.
- Karlsson JA, Kristensen LE, Kapetanovic MC, Gulfe A, Saxne T, Geborek P. Treatment response to a second or third TNF-inhibitor in RA: results from the South Swedish Arthritis Treatment Group Register. Rheumatology 2008;47:507-13.
- Blom M, Kievit W, Fransen J, Kuper IH, Den Broeder AA, De Gendt CM, et al. The reason of discontinuation of the first tumor necrosis factor (TNF) blocking agent does not influence effectiveness of a second TNF blocking agent in patients with rheumatoid arthritis. J Rheumatol 2009;36:2171-7.
- Bokarewa M, Lindholm C, Zendjanchi K, Nadali M, Tarkowski A. Efficacy of anti-CD20 treatment in patients with rheumatoid arthritis resistant to a combination of methotrexate/anti-TNF therapy. Immunol 2007;66:476-83.
- Jois RN, Masding A, Somerville M, Gaffney K, Scott DGI. Rituximab therapy in patients with resistant rheumatoid arthritis: real-life experience. Rheumatology 2007;46:980-2.
- Keystone E, Fleischmann R, Emery P, Furst DE, van Vollenhoven R, Bathon J, et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum 2007;56:3896-908.
- Assous N, Gossec L, Dieude P, Meyer O, Dougados M, Kahan A, et al. Rituximab therapy in rheumatoid arthritis in daily practice. J Rheumatol 2008;35:31-4.
- Thurlings RM, Vos K, Gerlag DM, Tak PP. Disease activity-guided rituximab therapy in rheumatoid arthritis: the effects of re-treatment in initial nonresponders versus initial responders. Arthritis Rheum 2008;58:3657-64.
- Genovese MC, Schiff M, Luggen M, Becker J-C, Aranda R, Teng J, et al. Efficacy and safety of the selective costimulation modulator abatacept following 2 years of treatment in patients with rheumatoid arthritis and an inadequate response to anti-tumour necrosis factor therapy. Ann Rheum Dis 2008;67:547-54.
- Schiff M, Pritchard C, Huffstutter JE, Rodriguez-Valverde V, Durez P, Zhou X, et al. The 6-month safety and efficacy of abatacept in patients with rheumatoid arthritis who underwent a washout after anti-tumour necrosis factor therapy or were directly switched to abatacept: the ARRIVE trial. Ann Rheum Dis 2009;68:1708-14.
- Hyrich KL, Lunt M, Watson KD, Symmons DPM, Silman AJ. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum 2007;56:13-20.
- Hyrich KI, Lunt M, Dixon WG, Watson KD, Symmons DPM. Effects of switching between anti-TNF therapies on HAQ response in patients who do not respond to their first anti-TNF drug. Rheumatology 2008;47:1000-5.
- NICE Decision Support Unit . Effect of a Second Course of Anti-TNF Therapy on HAQ Following Lack of Response to the First Course n.d. www.nice.org.uk/nicemedia/live/11702/37296/37296.pdf (accessed January 2010).
- Keystone E, Burmester GR, Furie R, Loveless JE, Emery P, Kremer J, et al. Improvement in patient-reported outcomes in a rituximab trial in patients with severe rheumatoid arthritis refractory to anti-tumor necrosis factor therapy. Arthritis Care Res (Hoboken) 2008;59:785-93.
- Keystone E, Emery P, Peterfy CG, Tak PP, Cohen S, Genovese MC, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis 2009;68:216-21.
- Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006;54:2793-806.
- Hassett AL, Li T, Buyske S, Savage SV, Gignac MAM. The multi-faceted assessment of independence in patients with rheumatoid arthritis: preliminary validation from the ATTAIN study. Curr Med Res Opin 2008;24:1443-53.
- Emery P. Abatacept has beneficial effects in rheumatoid arthritis patients with an inadequate response to anti-TNFalpha therapy. Clin Exp Rheumatol 2005;23:767-8.
- Westhovens R, Cole JC, Li T, Martin M, Maclean R, Lin P, et al. Improved health-related quality of life for rheumatoid arthritis patients treated with abatacept who have inadequate response to anti-TNF therapy in a double-blind, placebo-controlled, multicentre randomized clinical trial. Rheumatology 2006;45:1238-46.
- Genovese MC, Becker J-C, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 2005;353:1114-23.
- Bristol-Meyers Squibb . A Phase III, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of BMS-188667 in Subjects With Active Rheumatoid Arthritis on Background DMARDS Who Have Failed Anit-TNF Therapy 2004. http://ctr.bms.com/pdf//IM101029LT.pdf (accessed October 2009).
- Erratum: Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition (N Engl J Med 2005; 353: 1114– 23). N Engl J Med 2005;353.
- Furst DE, Gaylis N, Bray V, Olech E, Yocum D, Ritter J, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis 2007;66:893-9.
- Weinblatt M, Schiff M, Goldman A, Kremer J, Luggen M, Li T, et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann Rheum Dis 2007;66:228-34.
- Weinblatt M, Combe B, Covucci A, Aranda R, Becker JC, Keystone E. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: a one-year randomized, placebo-controlled study. Arthritis Rheum 2006;54:2807-16.
- Finckh A, Ciurea A, Brulhart L, Kyburz D, Moller B, Dehler S, et al. B cell depletion may be more effective than switching to an alternative anti-tumor necrosis factor agent in rheumatoid arthritis patients with inadequate response to anti-tumor necrosis factor agents. Arthritis Rheum 2007;56:1417-23.
- Finckh A, Ciurea A, Brulhart L, Moller B, Walker UA, Courvoisier D, et al. Which subgroup of rheumatoid arthritis patients benefits from switching to rituximab versus alternative anti-TNF agents after previous failure to anti-TNF agent?. Ann Rheum Dis 2010;69:387-93.
- Kievit W, Adang EM, Fransen J, Kuper HH, Van De Laar MAFJ, Jansen TL, et al. The effectiveness and medication costs of three anti-tumour necrosis factor alpha agents in the treatment of rheumatoid arthritis from prospective clinical practice data. Ann Rheum Dis 2008;67:1229-34.
- Roche . MabThera® (rituximab) for the treatment of rheumatoid arthritis after the failure of a TNF inhibitor. Submission to NICE 2009.
- Triggiani M. Cost-effectiveness analysis of two biological treatments (Adalimumab vs. Etanercept) in moderate-severe rheumatoid arthritis. Ital J Allergy Clin Immunol 2006;16:47-50.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83.
- Vera-Llonch M, Massarotti E, Wolfe F, Shadick N, Westhovens R, Sofrygin O, et al. Cost-effectiveness of abatacept in patients with moderately to severely active rheumatoid arthritis and inadequate response to tumor necrosis factor-alpha antagonists. J Rheumatol 2008;35:1745-53.
- Russell A, Beresniak A, Bessette L, Haraoui B, Rahman P, Thorne C, et al. Cost-effectiveness modeling of abatacept versus other biologic agents in DMARDS and anti-TNF inadequate responders for the management of moderate to severe rheumatoid arthritis. Clin Rheumatol 2009;28:403-12.
- Kielhorn A, Porter D, Diamantopoulos A, Lewis G. Uk cost-utility analysis of rituximab in patients with rheumatoid arthritis that failed to respond adequately to a biologic disease-modifying antirheumatic drug. Curr Med Res Opin 2008;24:2639-50.
- Lindgren P, Geborek P, Kobelt G. Modeling the cost-effectiveness of treatment of rheumatoid arthritis with rituximab using registry data from Southern Sweden. Int J Technol Assess Health Care 2009;25:181-9.
- Bansback NJ, Brennan A, Ghatnekar O. Cost effectiveness of adalimumab in the treatment of patients with moderate to severe rheumatoid arthritis in Sweden. Ann Rheum Dis 2005;64:995-1002.
- Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, Ustianowski A, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis 2010;69:522-8.
- Geborek P, Crnkic M, Petersson IF, Saxne T. Etanercept, infliximab, and leflunomide in established rheumatoid arthritis: clinical experience using a structured follow up programme in southern Sweden. Ann Rheum Dis 2002;61:793-8.
- Ducornau P, Kielhorn A, Wintfeld N. Comparison of Linear and Non-Linear Utility Mapping Between HAQ and EQ-5D Using Pooled Data from the Tolicizumab Trials OPTION and LITHE n.d.
- Abbott . Adalimumab, etanercept, infliximab, abatacept and rituximab for the treatment of rheumatoid arthritis after failure of an anti-TNF agent. Submission to NICE 2009.
- Brennan A, Bansback N, Reynolds A, Conway P. Modelling the cost-effectiveness of etanercept in adults with rheumatoid arthritis in the UK. Rheumatology 2004;43:62-7.
- National Coordinating Centre for Health Technology Assessment (NCCHTA) . Abatacept for the Treatment of Refractory Rheumatoid Arthritis, HTA Ref 06 52 01, Evidence Review Group Report for NICE (Project) (Project Record) 2007.
- Boggs R, Sengupta N, Ashraf T. Estimating Health Utility from a Physical Function Assessment in Rheumatoid Arthritis Patients Treated With Adalimumab n.d.
- Davies A, Cifaldi MA, Segurado OG, Weisman MH. Cost-effectiveness of sequential therapy with tumor necrosis factor antagonists in early rheumatoid arthritis. J Rheumatol 2009;36:16-25.
- Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol 1997;36:551-9.
- Hawthorne G, Richardson J, Day N, Osborne R, McNeil H. Construction and utility scaling of the assessment of quality of life (AQOL) instrument. Melbourne, VIC: Centre for Health Program Evaluation; 2000.
- Michaud K, Wolfe F. EQ5D Changes Rheumatoid Arthritis (RA) Quality of Life in United States: Study of 11,289 RA Patients n.d.
- Nixon RM, Bansback N, Brennan A. Using mixed treatment comparisons and meta-regression to perform indirect comparisons to estimate the efficacy of biologic treatments in rheumatoid arthritis. Stat Med 2007;26:1237-54.
- Wailoo A. The Sequential Use of TNF-Alpha Inhibitors. Update to a Report by the Decision Support Unit. Evaluations n.d. www.nice.org.uk/guidance/index.jsp?action=folder%2626o=40507.
- Bristol-Myers Squibb . Abatacept (Orencia®) for the treatment of rheumatoid arthritis. Submission to NICE 2007.
- Liverpool Reviews and Implementation Group . Abatacept for the treatment of rheumatoid arthritis. ERG Report for NICE 2007.
- Song F, Loke YK, Walsh T, Glenny A-M, Eastwood AJ, Altman DG. Methodological problems in the use of indirect comparisons for evaluation healthcare interventions: survey of published systematic reviews. BMJ 2009;338.
- Hyrich KL, Lunt M, Davies R, Harrison M, Watson K, Symmons D. The British society for rheumatology biologics register: updated analysis on sequential use of anti-TNF therapies in patients with rheumatoid arthritis. Submission to NICE 2009:1-30.
- Wolfe F, Rasker JJ, Boers M, Wells GA, Michaud K. Minimal disease activity, remission, and the long-term outcomes of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2007;57:935-42.
- Ducournau P, Kielhorn A, Wintfeld N. Comparison of Linear and Non-Linear Utility Mapping Between HAQ and EQ-5D Using Pooled Data from the Tocilizumab Trials OPTION and LITHE n.d.
- Jacobsson LT, Turesson C, Nilsson J-A. Treatment with TNF blockers and mortality risk in patients with rheumatoid arthritis. Ann Rheum Dis 2007;66:670-5.
- Moots RJ, Riel PV, Kekow J. Patterns of dose escalation and DMARD intensification in 739 patients with rheumatoid arthritis (RA) treated with anti-TNF agents (ATAS): results from the DART study. Rheumatology 2008.
- Krishnan E, Sokka T, Hakkinen A, Hubert H, Hannonen P. Normative values for the health assessment questionnaire disability index. Arthritis Rheum 2004;50:953-60.
- Taylor M, Saxby R, Ganderton M, Conway P, Lebmeier P, Peckham J. The relationship between Health Assessment Questionnaire score and resource use in the management of rheumatoid arthritis. Paris: European League Against Rheumatism (EULAR); 2008.
- Brennan A, Bansback N, Nixon R, Madan J, Harrison M, Watson K, et al. Modelling the cost effectiveness of TNF-alpha antagonists in the management of rheumatoid arthritis: results from the British Society for Rheumatology Biologics Registry. Rheumatology 2007;46:1345-54.
- Schering-Plough Limited . Remicade® for the treatment of rheumatoid arthritis after the failure of a TNFá inhibitor in England and Wales. Submission to NICE 2009.
- Bansback N, Marra C, Tsuchiya A, Anis A, Guh D, Hammond T, et al. Using the health assessment questionnaire to estimate preference-based single indices in patients with rheumatoid arthritis. Arthritis Rheum 2007;57:963-71.
- Brennan A, Madan J, Bansback N, Nixon R, Symmoins D, Lunt M. Modelling the cost-effectiveness of sequential use of TNF-alpha inhibitors in the management of rheumatoid arthritis: an update. HEDS Discussion Paper Series 6/12; 2006.
- Strangfeld A, Eveslage M, Kekow J, Gräßler A, Kaufmann J, Listing J, et al. Effectiveness of Treatment With Rituximab Depends on Autoantibody Status – Results from 2 Years of Experience in the German Biologics Register RABBIT n.d.
- Barton P, Jobanputra P, Wilson J, Bryan S, Burls A. The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis. Health Technol Assess 2004;8.
- Ariza-Ariza R, Navarro-Sarabia F, Hernandez-Cruz B, Rodriguez-Arboleya L, Navarro-Compan V, Toyos J. Dose escalation of the anti-TNF- agents in patients with rheumatoid arthritis. A systematic review. Rheumatology 2007;46:529-32.
- Wolfe F. The epidemiology of drug treatment failure in rheumatoid arthritis. Baillieres Best Pract Res Clin Rheumatol 1995;9:619-32.
- National Institute for Health and Clinical Excellence . Effect of a Second Course of Anti-TNF Therapy on HAQ Following Lack of Response to the First Course 2008.
- Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess 2006;10.
- Fumagalli M, Incorvaia C, Nitti F. The assessment of quality of life as a measure of gold salts treatment efficacy in rheumatoid arthritis. Minerva Med 2002;93:199-202.
- Poor G, Strand V. Efficacy and safety of leflunomide 10 mg versus 20 mg once daily in patients with active rheumatoid arthritis: multinational double-blind, randomized trial. Rheumatology 2004;43:744-9.
- Keystone E, Fleischmann R, Emery P, Furst DE, Van VR, Bathon J, et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum 2007;56:3896-908.
- NIHR Coordinating Centre for Health Technology Assessment . Rituximab for the Treatment of Rheumatoid Arthritis. NICE Evidence Review Group Report (ERG) in Support of NICE’s Single Technology Appraisal Process [1645] n.d. www.nice.org.uk/nicemedia/live/11719/36074/36074.pdf (accessed November 2009).
- Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999;340:253-9.
- Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). J Rheumatol 2003;30:2563-71.
- Westhovens R, Yocum D, Han J, Berman A, Strusberg I, Geusens P, et al. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum 2006;54:1075-86.
- Kruger K, Schattenkirchner M. Comparison of cyclosporin A and azathioprine in the treatment of rheumatoid arthritis – results of a double-blind multicentre study. Clin Rheumatol 1994;13:248-55.
- van Rijthoven AW, Dijkmans BA, The HS. Comparison of cyclosporine and D-penicillamine for rheumatoid arthritis: a randomized, double blind, multicenter study. J Rheumatol 1991;18:815-20.
- Liverpool Reviews and Implementation Group . Erlotinib for the Treatment of Relapse Non-Small Cell Lung Cancer 2006. www.nice.org.uk/nicemedia/live/11714/35177/35177.pdf (accessed January 2011).
- Wolfe F, Mitchell DM, Sibley JT. The mortality of rheumatoid arthritis. Arthritis Rheum 1994;37:481-94.
- Curtis L. Unit Costs of Community Care. Personal Social Services Research Unit 2008. www.pssru.ac.uk (accessed November 2009).
- Barbieri M, Wong JB, Drummond M. The cost effectiveness of infliximab for severe treatment-resistant rheumatoid arthritis in the UK. Pharmacoeconomics 2005;23:607-18.
- Pugner KM, Scott DI, Holmes JW, Hieke K. The costs of rheumatoid arthritis: an international long-term view. Semin Arthritis Rheum 2000;29:305-20.
- Wolfe F, Zwillich SH. The long-term outcomes of rheumatoid arthritis: a 23-year prospective, longitudinal study of total joint replacement and its predictors in 1,600 patients with rheumatoid arthritis. Arthritis Rheum 1998;41:1072-82.
- Hamilton P, Lemon M, Field R. Cost of total hip and knee arthroplasty in the UK. A comparison with the current reimbursement system in the NHS. J Bone Joint Surg Br 2009;91-B.
- NICE Decision Support Unit . Sequential Use of TNF-a Inhibitors for the Treatment of Rheumatoid Arthritis n.d. www.nice.org.uk/nicemedia/pdf/DSUReportWithAppendicesForConsultation.pdf (accessed 11 June 2010).
- Scott DL, Garrood T. Quality of life measures: use and abuse. Baillieres Best Pract Res Clin Rheumatol 2000;14:663-87.
- Dixon W, Hyrich K, Watson K, Lunt M. Drug-Specific Risk of Tuberculosis in Patients With Rheumatoid Arthritis Treated With Anti-TNF Therapy: Results from the BSRBR 2008.
- Wolfe F, Mitchell DM, Sibley JT. The mortality of rheumatoid arthritis. Arthritis Rheum 1994;37:481-94.
- Emery P, Breedveld FC, Lemmel EM, Kaltwasser JP, Dawes PT, Gomor PA. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology 2000;39:655-65.
- Hamilton J, McInnes IB, Thomson EA, Porter D, Hunter JA, Madhok R, et al. Comparative study of intramuscular gold and methotrexate in a rheumatoid arthritis population from a socially deprived area. Ann Rheum Dis 2001;60:566-72.
- Yocum DE, Allard S, Cohen SB, Emery P, Flipo RM, Goobar J, et al. Microemulsion formulation of cyclosporin (Sandimmun Neoral) vs Sandimmun: comparative safety, tolerability and efficacy in severe active rheumatoid arthritis. On behalf of the OLR 302 Study Group. Rheumatology 2000;39:156-64.
- Marra CA, Esdaile JM, Guh D, Fisher JH, Chalmers A, Anis AH. The effectiveness and toxicity of cyclosporin A in rheumatoid arthritis: longitudinal analysis of a population-based registry. Arthritis Rheum 2001;45:240-5.
- Willkens RF, Sharp JT, Stablein D, Marks C, Wortmann R. Comparison of azathioprine, methotrexate, and the combination of the two in the treatment of rheumatoid arthritis. A forty-eight-week controlled clinical trial with radiologic outcome assessment. Arthritis Rheum 1995;38:1799-806.
- Bristol-Myers Squibb Pharmaceuticals Ltd . Abatacept (Orencia) for the Treatment of Rheumatoid Arthritis. Submission to NICE 2009.
- Edwards CJ, Arden NK, Fisher DSJC, Reading I, van Staa TP. The changing use of disease-modifying anti-rheumatic drugs in individuals with rheumatoid arthritis from the United Kingdom General Practice Research Database. Rheumatology 2005;44.
- National Audit Office . Survey of General Practitioners about the Diagnosis and Management of Rheumatoid Arthritis n.d. www.nao.org.uk/idoc.ashx?docId=de0440fa-a1a9-4d6c-9975-f04a74011030%26version=-1 (accessed November 2009).
- Royal College of Physicians . Teams Without Walls. The Value of Medical Innovation and Leadership n.d. www.rcplondon.ac.uk/professional-Issues/Documents/teams-without-walls.pdf (accessed November 2009).
- Department of Health . High Quality Care for All: NHS Next Stage Review Final Report n.d. www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/DH_085825 (accessed November 2009).
- Fries JF, Spitz PW, Kraines RG, Holman HR. Measurement of patient outcomes in arthritis. Arthritis Rheum 1980;23:137-45.
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727-35.
- Siegel JN, Zhen B-G. Use of the American College of Rheumatology N (ACR-N) index of improvement in rheumatoid arthritis: argument in favour. Arthritis Rheum 2005;52:1637-41.
- Fransen J, Van Riel PL. The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol 2005;23:S93-S99.
- Scott DL. Radiographs in rheumatoid arthritis. Int J of Adv Rheumatol 2003;1:2-8.
- Sharp JT, Young DY, Bluhm GB, Brook A, Brower AC, Corbett M. How many joints in the hands and wrists should be included in a score of radiologic abnormalities use to assess rheumatoid arthritis?. Arthritis Rheum 1985;28:26-34.
- Van Der Heijde D, van Leeuwen MA, Riel PLCM, Koster AM, van T Hof MA, van Rijswijk MH. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum 1992;35:26-34.
- Scott DL, Housseien DA, Laasonen L. Proposed modification to Larsen’s scoring methods of hand and wrist radiographs. Rheumatology 1995;34.
- The United Kingdom Government Actuary’s Department. GAD; 2005.
- Nuijten MJ, Engelfriet P, Duijn K, Bruijn G, Wierz D, Koopmanschap M. A cost-cost study comparing etanercept with infliximab in rheumatoid arthritis. Pharmacoeconomics 2001;19:1051-64.
- Kobelt G, Eberhardt K, Jönsson L, Jönsson B. Economic consequences of the progression of rheumatoid arthritis in Sweden. Arthritis Rheum 1999;42:347-56.
- Kobelt G, Lindgren P, Singh A, Klareskog L. Cost effectiveness of etanercept (Enbrel) in combination with methotrexate in the treatment of active rheumatoid arthritis based on the TEMPO trial. Ann Rheum Dis 2005;64:1174-9.
- National Institute for Health and Clinical Excellence . Guidance on the Use of Etanercept and Infliximab for the Treatment of Rheumatoid Arthritis 2002.
- Hyrich KL, Silman AJ, Lunt M. Influence of response and adverse events rates to a first anti-TNF alpha agent on the outcome from switching to a second agent: results from British Society for Rheumatology Biologics Register. BSR Biologics Register Newsletter; 2006.
- Kielhorn A, Tony H, Jost F, Adultman R. Rituximab in rheumatoid arthritis: translating ACR responses into benefit for the patients. Ann Rheum Dis 2006;65.
- Scott DL, Garrood T. Quality of life measures: use and abuse. Bailliere’s Best Prac Res Clin Rheumatol 2000;14:663-87.
- Wyeth Pharmaceuticals . Etanercept (ENBREL) 2009.
- Hetland ML, Chistensen JL, Tarp U, Dreyer L, Hansen A, Hansen IT, et al. Direct Comparison of 4 years’ Drug Survival of Adalimumab, Etanercept and Infliximab in Rheumatoid Arthritis Patients. An Observational Study from the DANBIO Registry n.d.
- Curtis JR, John A, Baser O. Determinants of Tumor Necrosis Factor Alpha Inhibitor Switching in Patients With Rheumatoid Arthritis n.d.
- Zink A, Listing J, Kary S, Ramlau P, Stoyanova-Scholz M, Babinsky K, et al. Treatment continuation in patients receiving biological agents or conventional DMARD therapy. Ann Rheum Dis 2005;64:1274-9.
- Wolfe F, Michaud K. Duration of Use of Anti-TNF Therapy in Rheumatoid Arthritis n.d.
- Finckh A, Simard JF, Gabay C, Guerne P-A. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis 2006;65:746-52.
- Duclos M, Gossec L, Ruyssen-Witrand A, Salliot C, Luc M, Guignard S, et al. Retention rates of tumor necrosis factor blockers in daily practice in 770 rheumatic patients. J Rheumatol 2006;33:2433-9.
- Van Vollenhoven RF, Carli CC, Bratt J, Klareskog L. Secondary loss of efficacy with TNF-alpha antagonists. Data from the STURE registry. Rheumatology 2005;52.
- Kristensen LE, Saxne T, Nilsson JA, Geborek P. Impact of concomitant DMARD therapy on adherence to treatment with etanercept and infliximab in rheumatoid arthritis. Results from a six-year observational study in southern Sweden. Arthritis Res Ther 2006;8.
Appendix 1 Details of key outcomes used in rheumatoid arthritis trials
The Health Assessment Questionnaire
The HAQ now comprises a family of questionnaires designed to assess the functional capacity of patients with musculoskeletal complaints and specifically RA. The most widely used HAQ is derived from the Stanford Health Assessment Questionnaire210 and consists of two or three questions in eight categories:
-
dressing and grooming: dress yourself, including doing shoelaces, and shampooing your hair
-
rising: from an armless chair and in and out of bed
-
eating: being able to cut meat, lift a full cup or glass to mouth, and open a new carton of milk
-
walking: outdoors on flat ground and climb five steps
-
hygiene: wash and dry entire body, take a bath, get on and off the toilet
-
reaching: reach and get down a 5-lb object, bend down and pick up clothing
-
grip: open car doors, open previously unopened jars, turn taps on and off
-
activities: run errands and shop, get in and out of car, do chores.
The score from the most limited activity in each category is obtained. Each category is scored 0 (without any difficulty), 1 (with some difficulty), 2 (with much difficulty) or 3 (unable to do). Use of aids or devices to help with function is taken into account so that need for such assistance automatically scores 2 (unless 3 has been ticked). The maximum score in each of the eight categories is added to give a maximum possible score of 24. This total score may be divided by 8 to give an average value in the range 0–3.
The HAQ has several modifications:39
-
Modified HAQ (MHAQ) is a shortened version of HAQ which uses only one question in each of the eight categories and does not consider the use of aids and devices to assist function. It is simpler to score and has the same range as HAQ (0–3).
-
RA-HAQ is another shortened version of HAQ designed to overcome some of the metric limitations of MHAQ.
-
DHAQ this uses the original eight categories of HAQ, but is based on the most difficult items in each of the categories. Neither the RA-HAQ nor DHAQ have been widely used, unlike MHAQ.
American College for Rheumatology response criteria209
In order to achieve an ACR20 response a 20% improvement in the score for tender joints and a 20% improvement in swollen joints is necessary and 20% improvement in at least three of the following:
-
global disease activity assessed by observer
-
global disease activity assessed by patient
-
patient assessment of pain
-
physical disability score (e.g. HAQ)
-
acute phase response (e.g. ESR or CRP).
Responses may also be defined as ACR50 (50%) or ACR70 (70%) depending on degree of benefit.
The ACR-N is an extension of the ACR response criteria, and is defined as the lowest of the following three values:
-
percentage change in the number of swollen joints
-
percentage change in the number of tender joints
-
the median of the percentage change in the other five measures listed above.
It is thus a continuous variable. For example, an ACR-N score of 38 means an improvement of at least 38% in tender joint counts (TJCs) and swollen joint counts (SJCs) and an improvement of at least 38% in three of the five other parameters. 212
DAS
Original DAS
DAS = 0.54(√RAIa) + 0.065(total number of swollen joints out of 44) + 0.33(In ESRb) + 0.0072 (patient general health score where 0 = best, 100 = worst).
(a) RAI refers to a graded score of joint tenderness for 53 joints known as the Ritchie Articular Index and (b) the ESR.
DAS based on 28 joint evaluations
DAS28 – 4 = 0.56(√TJC28) + 0.28(√SJC28) + 0.7ln(ESR) + 0.014(patient general health score where 0 = best, 100 = worst).
Where scores for general health are not available, or not measured, the following formula is used:
EULAR response criteria
The EULAR response criteria213 are based on the DAS score. They incorporate both change from baseline and DAS or DAS28 at end point and, based on both, classify patients as good or moderate responders or non-responders (Table 107).
| DAS at end point | DAS28 at end point | Improvement in DAS or DAS28 from baseline | ||
|---|---|---|---|---|
| ≥ 1.2 | > 0.6 and ≤ 1.2 | ≤ 0.6 | ||
| ≤ 2.4 | ≤ 3.2 | Good | ||
| > 2.4 and ≤ 3.7 | > 3.2 and ≤ 5.1 | Moderate | ||
| > 3.7 | > 5.1 | None | ||
Radiographic assessment methods212
Sharp Score
The simplified Sharp system,215 which evaluates hand and wrist images, assesses 17 areas for erosions and 18 areas for joint space narrowing. Each joint is scored on a 6-point scale as follows: 0 = no erosion; 1 = discrete erosion; 2 = two separate quadrants with erosions or 20%–40% joint involvement; 3 = three separate quadrants with erosions or 41%–60% joint involvement; 4 = all four quadrants with joint erosion or 61%–80% joint involvement; and 5 = extensive destruction with greater than 80% joint involvement. The range of erosion scores for a patient with two hands and wrists is 0–170. For joint space narrowing each joint is scored using a 5-point scale as follows: 0 = no narrowing; 1 = up to 25% narrowing; 2 = 26%–65% narrowing; 3 = 66%–99% narrowing; 4 = complete narrowing. The range for joint space narrowing is therefore 0–144. This gives a total joint score in the range 0–314.
Van der Heijde-modified Sharp score
In this case 16 joints are assessed in each hand and wrist and six joints in each foot. Erosions are scored 0–5 and depending on the affected surface area and 0–10 in the fee, yielding possible erosion scores of 0–160 for hands/wrists and 0–120 for feet (total 0–280). Joint space narrowing is assessed in 15 joints for each hand/wrist and six joints in each foot on a scale of 0–4. The range of possible joint space narrowing scores is in the range 0–168. This yields a possible total score in the range 0– 448. 216
The Larsen score
In this method standard films are used to classify each joint into one of six possible categories (0 = normal, 5 = severely damaged). Any joint may be scored but the focus is on hands and feet. In the hands each proximal interphalangeal joint and each metacarpophalangeal joint scores 0–5; each wrist joint scores 0–25 (the basic score is multiplied by 5): this gives a maximum score of 150 for two hands and wrists. In the feet each metatarsophalangeal joint is scored 0–5, giving a total score of 50 for two feet. This yields a possible total score in the range 0–200.
Scott-modified Larsen
Scott et al. 217 suggested minor modifications to the scale in order to improve correlation between scorers. It was proposed that grade 1 included erosions and cysts of less than 1 mm diameter and grade included one or more erosions of greater than 1 mm diameter.
Appendix 2 Literature search strategies
Source – Cochrane Library (CENTRAL, DARE and NHS EED) 2009 Issue 3
-
#1 rheumatoid next arthritis
-
#2 MeSH descriptor Arthritis, Rheumatoid explode all trees
-
#3 (#1 OR #2)
-
#4 adalimumab or humira
-
#5 etanercept or enbrel
-
#6 infliximab or remicade
-
#7 rituximab or mabthera
-
#8 abatacept or orencia
-
#9 (#4 OR #5 OR #6 OR #7 OR #8)
-
#10 (#3 AND #9)
Source – MEDLINE (Ovid) 1950 – July Week 1 2009
-
rheumatoid arthritis.tw. (58,668)
-
arthritis rheumatoid/ (68,937)
-
or/1-2 (83,478)
-
(adalimumab or humira).mp. (1,199)
-
(etanercept or enbrel).mp. (2,138)
-
(rituximab or mabthera).mp. (5,052)
-
(abatacept or orencia).mp. (1,779)
-
(infliximab or remicade).mp. (4,830)
-
or/4-8 (13,083)
-
3 and 9 (2,759)
Source – MEDLINE(Ovid) In-Process & Other Non-Indexed Citations 13 July, 2009
-
(adalimumab or humira).mp. (129)
-
(etanercept or enbrel).mp. (203)
-
(rituximab or mabthera).mp. (455)
-
(abatacept or orencia).mp. (39)
-
(infliximab or remicade).mp. (346)
-
or/1-5 (990)
-
rheumatoid arthritis.tw. (1,987)
-
6 and 7 (220)
Source – EMBASE (Ovid) 1980 to 2009 Week 28
-
(adalimumab or humira).ti,ab,sh. (4,120)
-
(etanercept or enbrel).ti,ab,sh. (8,362)
-
(rituximab or mabthera).ti,ab,sh. (12,634)
-
(abatacept or orencia).ti,ab,sh. (1,014)
-
(infliximab or remicade).ti,ab,sh. (12,117)
-
or/1-5 (26,879)
-
rheumatoid arthritis/ (59,837)
-
rheumatoid arthritis.tw. (47,871)
-
7 or 8 (68,003)
-
6 and 9 (6,262)
Appendix 3 Flow diagram
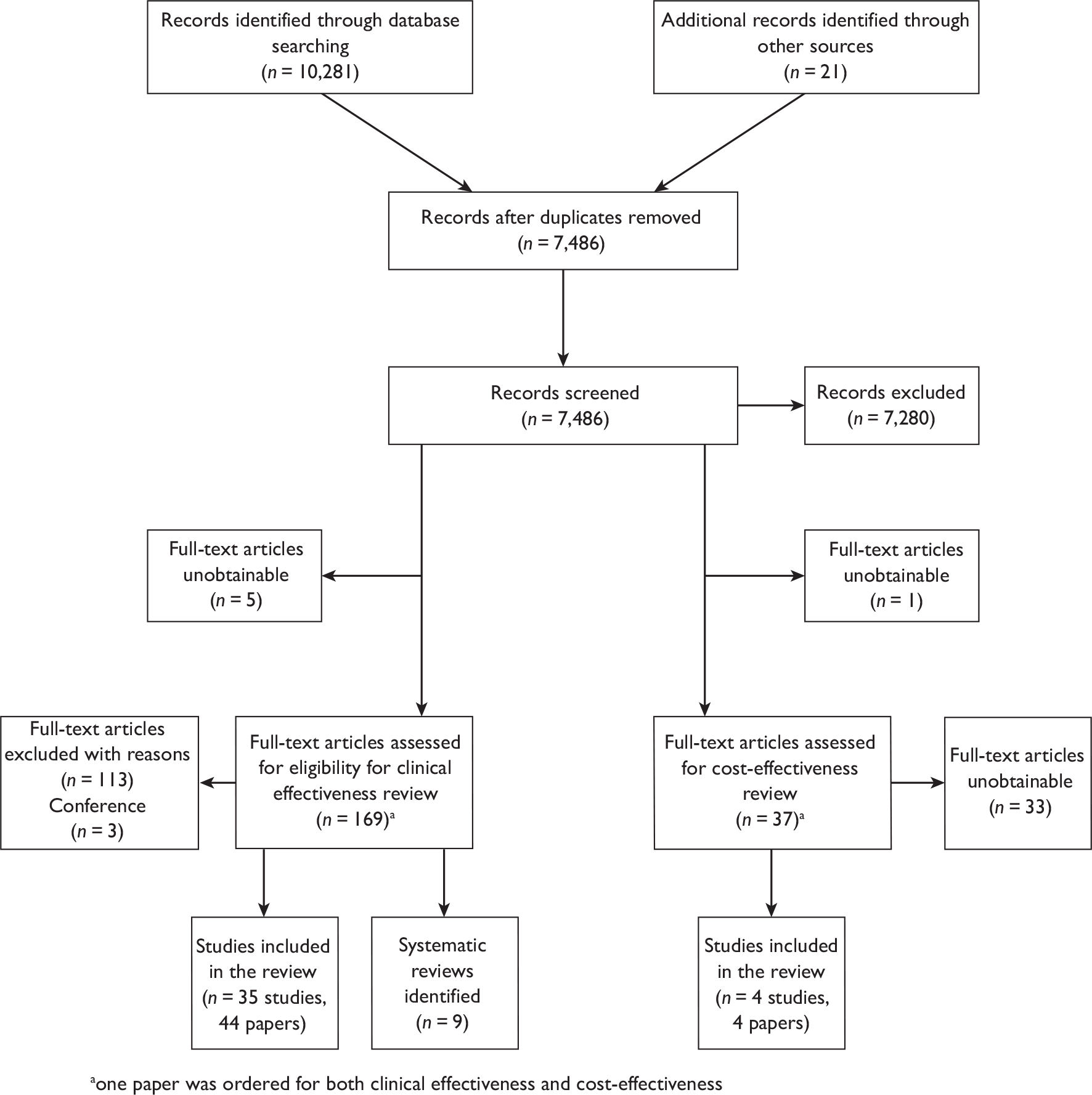
Appendix 4 Clinical effectiveness: table of excluded studies with rationale
| Article | Reason for exclusion |
|---|---|
| Prior lack of efficacy with etanercept does not predict lack of efficacy with infliximab. Formulary 2005;40:93. | Design |
| Abatacept: rheumatoid arthritis: after failure of TNF alpha antagonists and rituximab. Prescrire Int 2008;17:232. | Design |
| [Fusion protein abatacept. Remission in every 5th TNF-alpha refractory patient]. [German]. MMW Fortschritte der Medizin 2008;150:56–7. | Design |
| The COMET study: high remission rate through the use of etanercept in early rheumatoid arthritis. [German]. Arzneimitteltherapie 2008;26:434–5. | Population |
| Alexander W, Han C, Giles J. American College of Rheumatology Scientific Meeting. ASPIRE: Infliximab (Remicade) plus methotrexate for rheumatoid arthritis. P T 2009;34:37. | Population |
| Allison C. Abatacept as add-on therapy for rheumatoid arthritis. Ottawa: Canadian Coordinating Office for Health Technology Assessment (CCOHTA) 2005;4. | Design |
| Allison C. Abatacept as add-on therapy for rheumatoid arthritis. Issues Emerg Health Technol 2005;73:1–4. | Design |
| Alonso-Ruiz A, Pijoan JI, Ansuategui E, Urkaregi A, Calabozo M, Quintana A. Tumour necrosis factor alpha drugs in rheumatoid arthritis: systematic review and metaanalysis of efficacy and safety. BMC Musculoskelet Disord 2008;9:52. | Population |
| Alten R. Costimulation with abatacept – a new and successful therapeutic principle in rheumatoid athritis. Part 2: efficacy and safety of abatacept. Aktuelle Rheumatologie 2007;32:271–7. | Design |
| Alten R, Musch A. Abatacept in patients with rheumatoid arthritis. Arzneimitteltherapie 2008;26:9–16. | Design |
| Arenere MM, Navarro AH, Cilveti SU, Allende BM, Rabanaque HM, Arrieta NR, et al. Etanercept use in rheumatoid arthritis patients treated previously with infliximab. Atencion Farmaceutica 2005;7:465–9. | Participant number |
| Assous N, Gossec L, Dougados M, Kahan A, Allanore Y. Efficacy of rituximab in patients with rheumatoid arthritis refractory or with contra-indication to anti-tumour necrosis factor-alpha drugs in daily practice: an open label observational study. Clin Exp Rheumatol 2007;25:504. | Participant number |
| Baumgartner SW, Fleischmann RM, Moreland LW, Schiff MH, Markenson J, Whitmore JB. Etanercept (Enbrel) in patients with rheumatoid arthritis with recent onset versus established disease: improvement in disability. J Rheumatol 2004;31:1532–7. | Population |
| Bazzani C, Filippini M, Caporali R, Bobbio-Pallavicini F, Favalli EG, Marchesoni A, et al. Anti-TNFalpha therapy in a cohort of rheumatoid arthritis patients: clinical outcomes. Autoimmun Rev 2009;8:260–5. | Population |
| Bernal RL, Guerrero A, Monzon MA, Beltran GM, Hernandez CB, Colmenero MA. Effectiveness and safety of adalimumab and etanercept for rheumatoid arthritis in a third-level hospital. Farm Hosp 2006;30:223–9. | Population |
| Blank N, Max R, Schiller M, Briem S, Lorenz H-M. Safety of combination therapy with rituximab and etanercept for patients with rheumatoid arthritis. Rheumatology 2009;48:440–1. | Participant number |
| Blumenauer Barbara BTB, Judd M, Wells GA, Burls A, Cranney A, Hochberg MC, et al. Infliximab for the treatment of rheumatoid arthritis. Reviews. Cochrane Database of Systematic Reviews 2002 Issue 3. Chichester (UK): John Wiley and Sons, Ltd; 2002. | Population |
| Blumenauer Barbara BTB, Cranney A, Burls A, Coyle D, Hochberg MC, Tugwell P, et al. Etanercept for the treatment of rheumatoid arthritis. Cochrane Database of Systematic Reviews 2003 Issue 3. Chichester (UK): John Wiley and Sons, Ltd; 2003. | Population |
| Braun-Moscovici Y, Markovits D, Rozin A, Toledano K, Nahir AM, Balbir-Gurman A. Anti-tumour necrosis factor therapy: 6 year experience of a single centre in northern Israel and possible impact of health policy on results. Isr Med Assoc J 2008;10:277–81. | Population |
| Brocq O, Plubel Y, Breuil V, Grisot C, Flory P, Mousnier A, et al. Etanercept – infliximab switch in rheumatoid arthritis 14 out of 131 patients treated with anti TNFalpha. Presse Med 2002;31:1836–9. | Participant number |
| Brocq O, Plubel Y, Breuil V, Grisot C, Flory P, Mousnier A, et al. Switch etanercept – infliximab dans la polyarthrite rhumatoide. Presse Med 2002;31:1836–9. | Participant number |
| Brocq O, Albert C, Roux C, Gerard D, Breuil V, Ziegler LE. Adalimumab in rheumatoid arthritis after failed infliximab and/or etanercept therapy: experience with 18 patients. Joint Bone Spine 2004;71:601–3. | Participant number |
| Buch MH, Marzo-Ortega H, Bingham SJ, Emery P. Long-term treatment of rheumatoid arthritis with tumour necrosis factor alpha blockade: outcome of ceasing and restarting biologicals. Rheumatology 2004;43:243–4. | Population |
| Buch MH, Bingham SJ, Seto Y, McGonagle D, Bejarano V, White J, et al. Lack of response to Anakinra in rheumatoid arthritis following failure of tumour necrosis factor alpha blockade. Arthritis Rheum 2004;50:725–8. | Intervention |
| Buch MH, Boyle DL, Rosengren S, Saleem B, Reece RJ, Rhodes LA, et al. Mode of action of abatacept in rheumatoid arthritis patients having failed tumour necrosis factor blockade: a histological, gene expression and dynamic magnetic resonance imaging pilot study. Ann Rheum Dis 2009;68:1220–7. | Participant number |
| Burmester GR, Mariette X, Montecucco C, Monteagudo-Saez I, Malaise M, Tzioufas AG, et al. Adalimumab alone and in combination with disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis in clinical practice: the Research in Active Rheumatoid Arthritis (ReAct) trial. Ann Rheum Dis 2007;66:732–9. | Population |
| Burr ML, Malaviya AP, Gaston JH, Carmichael AJ, Ostor AJK. Rituximab in rheumatoid arthritis following anti-TNF-associated tuberculosis. Rheumatology 2008;47:738–9. | Design |
| Carmona L. Changes in anti-TNF: is this always justified? Reumatologia Clinica 2008;4:87–9. | Design |
| Combe B. Switching between anti-TNFalpha agents: what is the evidence? Joint Bone Spine 2004;71:169–71. | Design |
| Coyle D, Judd M, Blumenauer B, Cranney A, Maetzel A, Tugwell P, et al. Infliximab and etanercept in patients with rheumatoid arthritis: a systematic review and economic evaluation (DARE structured abstract). Ottawa: Canadian Coordinating Office for Health Technology Assessment (CCOHTA) 2006;45. | Population |
| Davies A, Cifaldi MA, Segurado OG, Weisman MH. Cost-effectiveness of sequential therapy with tumour necrosis factor antagonists in early rheumatoid arthritis. J Rheumatol 2009;36:16–25. | Design |
| Di PE, Perin A, Morassi MP, Del FM, Ferraccioli GF, De VS. Switching to etanercept in patients with rheumatoid arthritis with no response to infliximab. Clin Exp Rheumatol 2007;25:85–7. | Participant number |
| Donahue KE, Gartlehner G, Jonas DE, Lux LJ, Thieda P, Jonas BL, et al. Systematic review: comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis. Ann Intern Med 2008;148:124–34. | Population |
| Emery P. Abatacept has beneficial effects in rheumatoid arthritis patients with an inadequate response to anti-TNFalpha therapy. Clin Exp Rheumatol 2005;23:767–8. | Design |
| Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIb randomised, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum 2006;54:1390–400. | Population |
| Emery P, Keystone E, Tony HP, Cantagrel A, Van VR, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008;67:1516–23. | Intervention |
| Erickson AR, Mikuls TR. Switching anti-TNF-alpha agents: what is the evidence? Curr Rheumatol Rep 2007;9:416–20. | Design |
| Favalli EG, Arreghini M, Arnoldi C, Panni B, Marchesoni A, Tosi S, et al. Anti-tumour necrosis factor alpha switching in rheumatoid arthritis and juvenile chronic arthritis. Arthritis Rheum 2004;51:301–2. | Participant number |
| Fernandez Lison LC, Vazquez DB, Luis FJ, Moreno AP, Fruns GI, Liso RJ. Quality of life of patients with rheumatoid arthritis undergoing out-patient treatment with TNF inhibitors. Farm Hosp 2008;32:178–81. | Population |
| Filippini M, Bazzani C, Zingarelli S, Ziglioli T, Nuzzo M, Vianelli M, et al. Anti-TNF alpha agents in elderly patients with rheumatoid arthritis: a study of a group of 105 over sixty five years old patients. Reumatismo 2008;60:41–9. | Population |
| Gartlehner G, Hansen RA, Jonas BL, Thieda P, Lohr KN. The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol 2006;33:2398–408. | Population |
| Genta MS, Kardes H, Gabay C. Clinical evaluation of a cohort of patients with rheumatoid arthritis treated with anti-TNF-alpha in the community. Joint Bone Spine 2006;73:51–6. | Population |
| Gomez-Puerta JA, Sanmarti R, Rodriguez-Cros JR, Canete JD. Etanercept is effective in patients with rheumatoid arthritis with no response to infliximab therapy. Ann Rheum Dis 2004;63:896. | Participant number |
| Gomez CT. Rituximab and abatacept in rheumatoid arthritis. Reumatologia Clinica 2009;5:77–81. | Design |
| Gonzalez-Juanatey C, Llorca J, Sanchez AA, Garcia-Porrua C, Martin J, Gonzalez-Gay MA. Short-term adalimumab therapy improves endothelial function in patients with rheumatoid arthritis refractory to infliximab. Clin Exp Rheumatol 2006;24:309–12. | Participant number |
| Gonzalez-Juanatey C, Llorca J, Vazquez-Rodriguez TR, az-Varela N, Garcia-Quiroga H, Gonzalez-Gay MA. Short-term improvement of endothelial function in rituximab-treated rheumatoid arthritis patients refractory to tumour necrosis factor alpha blocker therapy. Arthritis Care Res (Hoboken) 2008;59:1821–4. | Participant number |
| Haraoui B. Is there a rationale for switching from one anti-tumour necrosis factor agent to another? J Rheumatol 2004;31:1021–2. | Design |
| Hay EM, Thomas E, Paterson SM, Dziedzic K, Croft PR. Do etanercept-naqive patients with rheumatoid arthritis respond better to infliximab than patients for whom etanercept has failed? Ann Rheum Dis 2004;63:607–12. | Design |
| Health Q, I, Scotland. Adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis. Glasgow: NHS Quality Improvement Scotland (NHS QIS); 2007. | Population |
| Health Q, I, Scotland. Rituximab for the treatment of rheumatoid arthritis. Glasgow: NHS Quality Improvement Scotland (NHS QIS); 2007. | Design |
| Heiberg MS, Rodevand E, Mikkelsen K, Kaufmann C, Didriksen A, Mowinckel P, et al. Adalimumab and methotrexate is more effective than adalimumab alone in patients with established rheumatoid arthritis: results from a 6-month longitudinal, observational, multicentre study. Ann Rheum Dis 2006;65:1379–83. | Population |
| Higashida J, Wun T, Schmidt S, Naguwa SM, Tuscano JM. Safety and efficacy of rituximab in patients with rheumatoid arthritis refractory to disease modifying antirheumatic drugs and anti-tumour necrosis factor-alpha treatment. J Rheumatol 2005;32:2109–15. | Population |
| Hoff M, Kvien TK, Kalvesten J, Elden A, Haugeberg G. Adalimumab therapy reduces hand bone loss in early rheumatoid arthritis: explorative analyses from the PREMIER study. Ann Rheum Dis 2009; 68:1171–6. | Population |
| Iking-Konert C. Therapy-refractive rheumatoid arthritis: effectiveness and reliability of abatecept and infliximab. Aktuelle Rheumatologie 2008;33:239–40. | Population |
| Jamal S, Patra K, Keystone EC. Adalimumab response in patients with early versus established rheumatoid arthritis: DE019 randomised controlled trial subanalysis. Clin Rheumatol 2009;28:413–9. | Population |
| Kavanaugh A, Rosengren S, Lee SJ, Hammaker D, Firestein GS, Kalunian K, et al. Assessment of rituximab’s immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results. Ann Rheum Dis 2008;67:402–8. | Participant number |
| Kielhorn A, Porter D, Diamantopoulos A, Lewis G. Uk cost-utility analysis of rituximab in patients with rheumatoid arthritis that failed to respond adequately to a biologic disease-modifying antirheumatic drug. Curr Med Res Opin 2008;24:2639–50. | Design |
| Kievit W, Adang EM, Fransen J, Kuper HH, Van De Laar MAFJ, Jansen TL, et al. The effectiveness and medication costs of three anti-tumour necrosis factor alpha agents in the treatment of rheumatoid arthritis from prospective clinical practice data. Ann Rheum Dis 2008;67:1229–34. | Population |
| Koike T, Harigai M, Inokuma S, Inoue K, Ishiguro N, Ryu J, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol 2009;36:898–906. | Population |
| Kristensen LE, Saxne T, Geborek P. The LUNDEX, a new index of drug efficacy in clinical practice: results of a five-year observational study of treatment with infliximab and etanercept among rheumatoid arthritis patients in Southern Sweden. Arthritis Rheum 2006;54:600–6. | Population |
| Laas K, Peltomaa R, Puolakka K, Kautiainen H, Leirisalo-Repo M. Early improvement of health-related quality of life during treatment with etanercept and adalimumab in patients with rheumatoid arthritis in routine practice. Clin Exp Rheumatol 2009;27:315–20. | Population |
| Li S, Kaur PP, Chan V, Berney S. Use of tumour necrosis factor-alpha (TNF-alpha) antagonists infliximab, etanercept, and adalimumab in patients with concurrent rheumatoid arthritis and hepatitis B or hepatitis C: a retrospective record review of 11 patients. Clin Rheumatol 2009;28:787–91. | Population |
| Lopez-Olivo MA, Amezaga M, McGahan L, Suarez-Almazor ME. Rituximab for rheumatoid arthritis. Cochrane Database Syst Rev 2008. | Design |
| Mease PJ, Revicki DA, Szechinski J, Greenwald M, Kivitz A, Barile-Fabris L, et al. Improved health-related quality of life for patients with active rheumatoid arthritis receiving rituximab – results of the dose-ranging assessment: international clinical evaluation of rituximab in rheumatoid arthritis (DANCER) trial. J Rheumatol 2008;35:20–30. | Population |
| Miyasaka N. Clinical investigation in highly disease-affected rheumatoid arthritis patients in Japan with adalimumab applying standard and general evaluation: the CHANGE study. Mod Rheumatol 2008;18:252–62. | Population |
| Moreland L. Efficacy of costimulation blockade with abatacept in rheumatoid arthritis patients refractory to tumour necrosis factor-alpha inhibition. Curr Rheumatol Rep 2006;8:367. | Design |
| Navarra SV, Raso A-A, Lichauco JJ, Tan PP. Clinical experience with infliximab among Filipino patients with rheumatic diseases. APLAR J Rheumatol 2006;9:150–6. | Population |
| Navarro-Sarabia F, riza-Ariza R, Hernandez-Cruz B, Villanueva I. Adalimumab for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews 2005 Issue 3. Chichester (UK): John Wiley and Sons, Ltd; 2005. | Population |
| NHS Quality Improvement Scotland (NHS QIS). Abatacept for the treatment of rheumatoid arthritis. Glasgow: NHS Quality Improvement Scotland (NHS QIS); 2008. | Design |
| Nixon R, Bansback N, Brennan A. The efficacy of inhibiting tumour necrosis factor alpha and interleukin 1 in patients with rheumatoid arthritis: a meta-analysis and adjusted indirect comparisons. Rheumatology 2007;46:1140–7. | Population |
| Olsen N. Anti-TNF switching: effect on outcomes in patients with RA: commentary. Nat Clin Pract Rheumatol 2007;3:430–1. | Design |
| Ostergaard M, Unkerskov J, Linde L, Krogh NS, Ravn T, Ringsdal VS, et al. Low remission rates but long drug survival in rheumatoid arthritis patients treated with infliximab or etanercept: results from the nationwide Danish DANBIO database. Scand J Rheumatol 2007;36:151–4. | Population |
| Ostor AJK. Abatacept: a T-cell costimulation modulator for the treatment of rheumatoid arthritis. Clin Rheumatol 2008;27:1343–53. | Design |
| Owczarczyk KM, Hellmann M, Fliedner G, Rohrs T, Maizus K, Passon D, et al. Clinical outcome and B cell depletion in patients with rheumatoid arthritis receiving rituximab monotherapy in comparison with patients receiving concomitant methotrexate. Ann Rheum Dis 2008;67:1648–50. | Population |
| Palylyk-Colwell E, McGahan L. Rituximab for rheumatoid arthritis. Ottawa: Canadian Agency for Drugs and Technologies in Health (CADTH) 2006;4. | Design |
| Parker CT, Rennie T, Yocum DE, Furst DE, Kaine JL, Baldassare A, et al. Failure to report previously used drugs and dosages in pharmaceutical company-sponsored rheumatoid arthritis trials: comment on the article by Yocum et al. Arthritis Rheum 2004;50:3051–2. | Population |
| Pavelka K, Gatterova J, Vencovsky J, Sedova L, Chroust K. Radiographic progression of rheumatoid arthritis in real clinical practice results in national registry attra. Rheumatologia 2009;23:7–11. | Population |
| Pedersen SJ, Hetland ML, Ostergaard M, Navarro-Sarabia F, riza-Ariza R, Hernandez-Cruz B, et al. Adalimumab for treating rheumatoid arthritis. Ugeskr Laeger 2006;168:2899–902. | Population |
| Pisetsky DS. A landmark study on treatment strategies for rheumatoid arthritis. Arthritis Rheum 2008;58:S123–S125. | Population |
| Reynolds J, Shojania K, Marra CA. Abatacept: a novel treatment for moderate-to-severe rheumatoid arthritis. Pharmacotherapy 2007;27:1693–701. | Design |
| Rubbert-Roth A, Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther 2009;11. | Design |
| Russell A, Beresniak A, Bessette L, Haraoui B, Rahman P, Thorne C, et al. Cost-effectiveness modelling of abatacept versus other biologic agents in DMARDS and anti-TNF inadequate responders for the management of moderate to severe rheumatoid arthritis. Clin Rheumatol 2009;28:403–12. | Design |
| Salliot C, Gossec L, Ruyssen-Witrand A, Luc M, Duclos M, Guignard S, et al. Infections during tumour necrosis factor-alpha blocker therapy for rheumatic diseases in daily practice: a systematic retrospective study of 709 patients. Rheumatology 2007;46:327–34. | Population |
| Sanmarti R, Gomez-Puerta JA, Rodriguez-Cros JR, Albaladejo C, Munoz-Gomez J, Canete JD. Etanercept in rheumatoid arthritis patients with a poor therapeutic response to infliximab. Medicina Clinica 2004;122:321–4. | Participant number |
| Sheitanov I. Our experience with Remicade (infliximab) in patients with early and refractory rheumatoid arthritis. Rheumatology 2005;13:66–73. | Population |
| Silman AJ. Available therapeutic options following failure of a first anti-TNF agent. Nat Clin Pract Rheumatol 2009;5:115. | Design |
| Singh A, Ghazvini P, Honeywell M, Treadwell P. Rituximab for the treatment of refractory rheumatoid arthritis: new information from clinical trials. P T 2006;31:321 + 343. | Design |
| Smolen JS, Weinblatt ME. When patients with rheumatoid arthritis fail tumour necrosis factor inhibitors: what is the next step? Ann Rheum Dis 2008;67:1497–8. | Design |
| Strand V, Balbir-Gurman A, Pavelka K, Emery P, Li N, Yin M, et al. Sustained benefit in rheumatoid arthritis following one course of rituximab: improvements in physical function over 2 years. Rheumatology 2006;45:1505–13. | Population |
| Suarez-Almazor M, Ortiz Z, Lopez-Olivo M, Moffett M, Pak C, Skidmore B, et al. Infliximab and etanercept in rheumatoid arthritis: systematic review of long-term clinical effectiveness, safety, and cost-effectiveness. Ottawa: Canadian Agency for Drugs and Technologies in Health (CADTH) 2007;32. | Population |
| Summers KM, Kockler DR. Rituximab treatment of refractory rheumatoid arthritis. Ann Pharmacother 2005;39:2091–5. | Population |
| Taylor PC. Is abatacept an effective treatment for patients with RA who do not respond to other anti-TNF treatments? Commentary. Nat Clin Pract Rheumatol 2006;2:128–9. | Design |
| Van De Putte LBA, Atkins C, Malaise M, Sany J, Russell AS, Van Riel PLCM, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis 2004;63:508–16. | Population |
| Van Der Kooij SM, De Vries-Bouwstra JK, Goekoop-Ruiterman YP, Ewals JA, Han KH, Hazes JM, et al. Patient-reported outcomes in a randomised trial comparing four different treatment strategies in recent-onset rheumatoid arthritis. Arthritis Rheum 2009;61:4–12. | Population |
| Van Der Kooij SM, De Vries-Bouwstra JK, Goekoop-Ruiterman YPM, Ewals JAPM, Han KH, Hazes JMW, et al. Patient-reported outcomes in a randomised trial comparing four different treatment strategies in recent-onset rheumatoid arthritis. Arthritis Care Res (Hoboken) 2009;61:4–12. | Population |
| Van Vollenhoven R, Harju A, Brannemark S, Klareskog L. Treatment with infliximab (Remicade) when etanercept (Enbrel) has failed or vice versa: data from the STURE registry showing that switching tumour necrosis factor alpha blockers can make sense. Ann Rheum Dis 2003;62:1195–8. | Participant number |
| Van Vollenhoven RF. Switching between biological agents. Clin Exp Rheumatol 2004;22:S115–S121. | Design |
| Van Vollenhoven RF. Switching between anti-tumour necrosis factors: trying to get a handle on a complex issue. Ann Rheum Dis 2007;66:849–51. | Design |
| Van VR, Harju A, Brannemark S, Klareskog L. Treatment with infliximab (Remicade) when etanercept (Enbrel) has failed or vice versa: data from the STURE registry showing that switching tumour necrosis factor alpha blockers can make sense. Ann Rheum Dis 2003;62:1195–8. | Participant number |
| Venkateshan SP, Sidhu S, Malhotra S, Pandhi P. Efficacy of biologicals in the treatment of rheumatoid arthritis: a meta-analysis. Pharmacology 2009;83:1–9. | Population |
| Vera-Llonch M, Massarotti E, Wolfe F, Shadick N, Westhovens R, Sofrygin O, et al. Cost-effectiveness of abatacept in patients with moderately to severely active rheumatoid arthritis and inadequate response to tumour necrosis factor-alpha antagonists. J Rheumatol 2008;35:1745–53. | Design |
| Villamayor BL, Moreno Ramos MJ, Urbieta SE, Martinez PM, Jorge V, Gonzalez Perez-Crespo C, et al. Study of adalimumab’s use in rheumatoid arthritis. Atencion Farmaceutica 2006;8:157–62. | Population |
| Vital EM, Dass S, Buch MH, Rawstron AC, Ponchel F, McGonagle D, et al. Re-treatment of rheumatoid arthritis patients who were initial nonresponders to rituximab: comment on the article by Thurlings et al. Arthritis Rheum 2009;60:1867. | Design |
| Voulgari PV, Alamanos Y, Nikas SN, Bougias DV, Temekonidis TI, Drosos AA. Infliximab therapy in established rheumatoid arthritis: an observational study. Am J Med 2005;118:515–20. | Population |
| Walsh CAE, Minnock P, Slattery C, Kennedy N, Pang F, Veale DJ, et al. Quality of life and economic impact of switching from established infliximab therapy to adalimumab in patients with rheumatoid arthritis. Rheumatology 2007;46:1148–52. | Population |
| Weaver AL, Lautzenheiser RL, Schiff MH, Gibofsky A, Perruquet JL, Luetkemeyer J, et al. Real-world effectiveness of select biologic and DMARD monotherapy and combination therapy in the treatment of rheumatoid arthritis: results from the RADIUS observational registry. Curr Med Res Opin 2006;22:185–98. | Population |
| Weisman MH, Paulus HE, Burch FX, Kivitz AJ, Fierer J, Dunn M, et al. A placebo-controlled, randomised, double-blinded study evaluating the safety of etanercept in patients with rheumatoid arthritis and concomitant comorbid diseases. Rheumatology 2007;46:1122–5. | Population |
| Witte F. How beneficial is switching from one anti-TNF-alpha agent to a second anti-TNF-alpha agent in patients with rheumatoid arthritis? Aktuelle Rheumatologie 2007;32:182. | Design |
| Yazici Y, Yazici H. Tumour necrosis factor alpha inhibitors, methotrexate or both? An inquiry into the formal evidence for when they are to be used in rheumatoid arthritis. Clin Exp Rheumatol 2008;26:449–52. | Population |
| Yazici Y, Krasnokutsky S, Barnes JP, Hines PL, Wang J, Rosenblatt L. Changing patterns of tumour necrosis factor inhibitor use in 9074 patients with rheumatoid arthritis. J Rheumatol 2009;36:907–13. | Population |
| Yukawa N, Mimori T. [B cell depletion therapy using anti-CD20 antibodies in rheumatoid arthritis.] Clin Calcium 2007;17:569–76. | Design |
| Zhang W, Bansback N, Guh D, Li X, Nosyk B, Marra CA, et al. Short-term influence of adalimumab on work productivity outcomes in patients with rheumatoid arthritis. J Rheumatol 2008;35:1729–36. | Population |
| Zintzaras E, Dahabreh IJ, Giannouli S, Voulgarelis M, Moutsopoulos HM. Infliximab and methotrexate in the treatment of rheumatoid arthritis: a systematic review and meta-analysis of dosage regimens. Clin Ther 2008;30:1939–55. | Population |
Appendix 5 Cost-effectiveness: table of excluded studies with rationale
| Article | Reason for exclusion |
|---|---|
| Bansback N, Ara R, Karnon J, Anis A. Economic evaluations in rheumatoid arthritis: a critical review of measures used to define health states. Pharmacoeconomics 2008;26:395–408. | Review of clinical measures in rheumatoid arthritis |
| Bansback NJ, Brennan A, Ghatnekar O. Cost effectiveness of adalimumab in the treatment of patients with moderate to severe rheumatoid arthritis in Sweden. Ann Rheum Dis 2005;64:995–1002. | Population |
| Barton P, Jobanputra P, Wilson J, Bryan S, Burls A. The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis. Health Technol Assess 2004;8(11). | Population |
| Bullano MF, McNeeley BJ, Yu YF, Quimbo R, Burawski LP, Yu EB, et al. Comparison of costs associated with the use of etanercept, infliximab, and adalimumab for the treatment of rheumatoid arthritis. Manag Care Interface 2006;19:47–53. | Population |
| Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess 2006;10(42). | Population |
| Chiou C-F, Choi J, Reyes CM. Cost-effectiveness analysis of biological treatments for rheumatoid arthritis. Expert Rev Pharmacoecon Outcomes Res 2004;4:307–15. | Population |
| Davies A, Cifaldi MA, Segurado OG, Weisman MH. Cost-effectiveness of sequential therapy with tumor necrosis factor antagonists in early rheumatoid arthritis. J Rheumatol 2009;36:16–25. | Population |
| Doan QV, Chiou C-F, Dubois RW. Review of eight pharmacoeconomic studies of the value of biologic DMARDs (adalimumab, etanercept, and infliximab) in the management of rheumatoid arthritis. J Manag Care Pharm 2006;12:555–69. | Review of TNF inhibitors in rheumatoid arthritis |
| Kamal KM, Miller L-A, Kavookjian J, Madhavan S. Alternative decision analysis modeling in the economic evaluation of tumur necrosis factor inhibitors for rheumatoid arthritis. Semin Arthritis Rheum 2006;36:50–60. | Review of decision modelling in economic evaluations of TNF inhibitors in rheumatoid arthritis |
| Kobelt G, Jonsson L, Young A, Eberhardt K. The cost-effectiveness of infliximab (Remicade) in the treatment of rheumatoid arthritis in Sweden and the United Kingdom based on the ATTRACT study. Rheumatology 2003;42:326–35. | Population |
| Kobelt G, Eberhardt K, Geborek P. TNF inhibitors in the treatment of rheumatoid arthritis in clinical practice: costs and outcomes in a follow up study of patients with Ra treated with etanercept or infliximab in southern Sweden. Ann Rheum Dis 2004;63:4–10. | Population |
| Launois R, Payet S, Saidenberg-Kermanac’h N, Francesconi C, Franca LR, Boissier M-C. Budget impact model of rituximab after failure of one or more TNFalpha inhibitor therapies in the treatment of rheumatoid arthritis. Joint Bone Spine 2008;75:688–95. | Design |
| Lyseng-Williamson KA, Foster RH. Infliximab: a pharmacoeconomic review of its use in rheumatoid arthritis. Pharmacoeconomics 2004;22:107–132. | Population |
| Lyseng-Williamson KA, Plosker GL. Etanercept: a pharmacoeconomic review of its use in rheumatoid arthritis. Pharmacoeconomics 2004;22:1071–95. | Population |
| Merkesdal S, Ruof J, Mittendorf T, Zeidler H. Cost-effectiveness of TNF-A-blocking agents in the treatment of rheumatoid arthritis. Expert Opin Pharmacother 2004;5:1881–6. | Review of TNF inhibitors in rheumatoid arthritis |
| Monteiro RDC, Zanini AC. Cost analysis of drug therapy in rheumatoid arthritis. Braz J Pharm Sci 2008;44:25–33. | Population |
| Muller-Ladner U. Cost effectiveness of biologics in the treatment of rheumatoid arthritis. Internist 2004;45:1402–6. | Population |
| Nuijten MJ, Engelfriet P, Duijn K, Bruijn G, Wierz D, Koopmanschap M. A cost-cost study comparing etanercept with infliximab in rheumatoid arthritis. Pharmacoeconomics 2001;19:1051–64. | Population |
| Prokes M. Effectiveness of TNF antagonists in routine clinical practice and costs. Vnitr Lek 2009;55:45–53. | Population |
| Ravasio R, Lucioni C. Economic evaluation of etanercept in AR. Pharmacoeconomics – Italian Research Articles 2006;8:129–40. | Review of etanercept |
| Regier DA, Bansback N, Dar SA, Marra CA. Cost-effectiveness of tumor necrosis factor-alpha antagonist in rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. Expert Rev Pharmacoecon Outcomes Res 2007;7:155–69. | Review of TNF inhibitors in rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis |
| Rubio-Terres C, Ordovas Baines JP, Pla PR, Martinez NC, Sanchez Garre MJ, Rosado Souviron MA. Use and cost of biological disease -modifying anti-rheumatic drugs in Spain (PRAXIS study). Farm Hosp 2007;31:78–92. | Population |
| Rubio-Terres C, Ordovas Baines JP, Pla PR. Critical analyis of the article: Use and cost of biological disease-modifying anti-rheumatic drugs in Spain (PRAXIS study). Farm Hosp 2008;32:190–3. | Population |
| Suka M, Yoshida K. [Economic evaluation of a new treatment for rheumatoid arthritis.] Nippon Rinsho 2007;65:1327–30. | Population |
| Tsutani K, Igarashi A. [Anti-rheumatoid biologics and pharmacoeconomic evaluation.] Nippon Rinsho 2005;63:711–18. | Design |
| Unit of Health Economics and Technology Assessment. Rituximab in patients with rheumatoid arthritis:systematic review and economic evaluation. Budapest: Unit of Health Economics and Technology Assessment in Health Care (HUNHTA); 2006. | Population |
| Van Den Hout WB, Goekoop-Ruiterman YPM, Allaart CF, Vries-Bouwstra JKD, Hazes JMM, Kerstens PJSM, et al. Cost-utility analysis of treatment strategies in patients with recent-onset rheumatoid arthritis. Arthritis Care Res (Hoboken) 2009;61:291–9. | Population |
| Virkki LM, Konttinen YT, Peltomaa R, Suontama K, Saario R, Immonen K, et al. Cost-effectiveness of infliximab in the treatment of rheumatoid arthritis in clinical practice. Clin Exp Rheumatol 2008;26:1059–66. | Population |
| Wailoo AJ, Bansback N, Brennan A, Michaud K, Nixon RM, Wolfe F. Biologic drugs for rheumatoid arthritis in the medicare program: a cost-effectiveness analysis. Arthritis Rheum 2008;58:939–46. | Population |
| Walsh CAE, Minnock P, Slattery C, Kennedy N, Pang F, Veale DJ, et al. Quality of life and economic impact of switching from established infliximab therapy to adalimumab in patients with rheumatoid arthritis. Rheumatology 2007;46:1148–52. | Population |
| Wong JB, Singh G, Kavanaugh A. Estimating the cost-effectiveness of 54 weeks of infliximab for rheumatoid arthritis. Am J Med 2002;113:400–8. | Population |
| Wong JB. Cost-effectiveness of anti-tumor necrosis factor agents. Clin Exp Rheumatol 2004;22:S65–S70. | Review of TNF inhibitors in rheumatoid arthritis |
| Wu EQ, Chen L, Birnbaum H, Yang E, Cifaldi M. Cost of care for patients with rheumatoid arthritis receiving TNF-antagonist therapy using claims data. Curr Med Res Opin 2007;23:1749–59. | Population |
Appendix 6 Clinical effectiveness: full paper inclusion/exclusion checklist
Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor – full-text inclusion checklist for clinical effectiveness
| Question | Yes | No | |
|---|---|---|---|
| Q1 |
Population Did the study include a majority (> 50%) of adults with active rheumatoid arthritis who have had an inadequate response to a TNF inhibitor? |
Go to Q2 |
Exclude UD4 = excluded pop |
| Q2 |
Interventions Did the interventions include at least one of the following drugs: Adalimumab? Etanercept? Infliximab? Rituximab? Abatacept? |
Go to Q3 |
Exclude UD4 = excluded int |
| Q3 |
Outcomes Did the study report any clinical outcomes related to efficacy, safety or tolerability? |
Go to Q4 |
Exclude UD4 = excluded out |
| Q4 |
Study design Was it a primary study (except case reports) or a systematic review? |
For primary study: go to Q5 For systematic review: include; UD4 = SR |
Exclude UD4 = excluded des |
| Q5 |
Study duration Was the study at least 12 weeks duration? |
Go to Q6 |
Exclude UD4 = excluded dur |
| Q6 |
Participant numbers If the study was not an RCT, did it include at least 20 patients in at least one of the treatment arms (if there was more than one arm)? |
Include UD4 = included |
Exclude UD4 = excluded num |
Appendix 7 Cost-effectiveness: full paper inclusion/exclusion checklist
Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor – full-text inclusion checklist for cost-effectiveness
| Question | Yes | No | |
|---|---|---|---|
| Q1 |
Population Did the study include a majority of adults with active rheumatoid arthritis who have had an inadequate response to a TNF inhibitor? |
Go to Q2 |
Exclude UD5 = excluded pop |
| Q2 |
Interventions Did the interventions include at least one of the following drugs: Adalimumab? Etanercept? Infliximab? Rituximab? Abatacept? |
Go to Q3 |
Exclude UD5 = excluded int |
| Q3 |
Outcomes Did the study report any quality of life estimates, cost estimates or cost-effectiveness results? |
Go to Q4 |
Exclude UD5 = excluded out |
| Q4 |
Study design Was it a cost–consequence analysis, cost–benefit analysis, cost-effectiveness analysis, cost–utility analysis, cost study (UK only), or quality of life study? |
Include UD5 = included |
Exclude UD5 = excluded des |
Appendix 8 Clinical effectiveness review: data extraction form
Adalimumab/Etanercept/Infliximab/Rituximab/Abatacept (delete as appropriate)
RCT/Controlled study (concurrent)/Controlled study (historical)/Uncontrolled study (delete as appropriate)
| First author and year | Reference no. | |||
| Trial name/protocol no. | Reviewer | |||
| Citation | Date of abstraction | |||
| Country and no. of centres | Sponsorship | |||
| Related references | ||||
| Inclusion criteria | General comments and comments on exclusions | |||
| Age: | ||||
| Duration of RA ≥ | ||||
|
Prior TNF inhibitor treatment: Reason for discontinuation of TNF inhibitor: |
||||
| Disease activity parameters | ||||
| Tender joint count ≥ | ||||
| Swollen joint count ≥ | ||||
| ESR ≥ | ||||
| CRP ≥ | ||||
| Morning stiffness > | ||||
| Other inclusion/exclusion criteria: | ||||
|
Concomitant treatments during the trial Methotrexate: allowed/not allowed/unclear/conditional: Other DMARDs: allowed/not allowed/unclear/conditional: Steroids: allowed/not allowed/unclear/conditional: Other treatments allowed: Other treatments not allowed: |
||||
|
Previous TNF inhibitor(s) Eligibility for the previous anti-TNF: Doses and treatment duration of previous TNF inhibitor (and concomitant DMARDs): Washout period from the previous TNF inhibitor: |
||||
Randomised controlled trial study design and quality
| Was randomisation adequate: Yes/No/Unclear | |
| Was allocation adequately concealed: Yes/No/Unclear | |
| Blinding: | |
| Were patients blinded from the study interventions: Yes/No/Unclear | |
| Were study investigators/outcome assessors blinded from the study interventions: Yes/No/Unclear | |
| Were data analysts blinded from the study interventions: Yes/No/Unclear | |
| Was lost to follow-up stated for each treatment groups: Yes/No/Unclear | |
| Was ITT analysis used: Yes/No/Unclear | |
| Duration of treatment: | Duration of follow-up (if different): |
| Study visits (outcome data available): | |
| Comments on study design and quality (problem in study design; power of study; potential bias): | |
Non-randomised controlled trial study design and quality
| What was the study design: | |
| Were criteria for including patients into the study stated? | |
| Were consecutive patients meeting the inclusion criteria (if any) entered into the study? | |
| Was lost to follow-up stated for each treatment groups: Yes/No/Unclear | |
| Duration of treatment: | Duration of follow-up (if different): |
| Study visits (outcome data available): | |
| Comments on study design and quality (problem in study design; power of study; potential bias): | |
Interventions and comparators
| State drug name(s), dose, frequency, route of administration |
| A) |
| B) |
| C) |
| D) |
| E) |
| F) |
Baseline characteristics
| Tx arm | A) | B) | C) | D) | E) | F) | All patients |
| Patient number | |||||||
| Age (mean, yrs) | |||||||
| Female % | |||||||
| Disease duration (yrs) | |||||||
| Auto antibody status | |||||||
| (Comorbidity) % | |||||||
| (Comorbidity) % | |||||||
| (Comorbidity) % | |||||||
| Previous TNF inhibitor | |||||||
| No. of previous DMARDs | |||||||
| (Previous DMARD) % | |||||||
| (Previous DMARD) % | |||||||
| On steroids (%) | |||||||
| On NSAIDs (%) | |||||||
| If on MTX – dose? | |||||||
| % joint replm | |||||||
|
Comments on the presence or absence of significant differences between treatment arms: No. of patients screened: No. of patients randomised: No. of patients received at least one dose of study drug: |
|||||||
Outcomes
ITT population/efficacy population (delete as appropriate)
| Measure of activity | Values (SD or IQR) |
Intervention – A
n = |
Intervention – B
n = |
Intervention – C
n = |
Intervention – D
n = |
Intervention – E
n = |
Intervention – F
n = |
|---|---|---|---|---|---|---|---|
| 1. Withdrawal – lack of efficacy |
No. eval. No. withdrew |
||||||
| 2. Withdrawal –adverse events |
No. eval. No. withdrew |
||||||
| 3. Withdrawal – any reason |
No. eval. No. withdrew |
||||||
| 4. ACR20 % |
No. eval. No. improved |
||||||
| 5. ACR50 % |
No. eval. No. improved |
||||||
| 6. ACR70 % |
No. eval. No. improved |
||||||
|
7. Swollen joint count ( ) Specify week |
No. eval. | ||||||
| Pre-Rx | |||||||
| Post | |||||||
|
Chge p-value |
|||||||
|
8. Tender joint count ( ) Specify week |
No. eval. | ||||||
| Pre-Rx | |||||||
| Post | |||||||
|
Chge p-value |
|||||||
|
9. Pain – patient ( ) Specify week |
No. eval. | ||||||
| Pre-Rx | |||||||
| Post | |||||||
|
Chge p-value |
|||||||
|
10. Phys. Global ( ) Specify week |
No. eval. | ||||||
| Pre-Rx | |||||||
| Post | |||||||
|
Chge p-value |
|||||||
|
11. Patient global ( ) Specify week |
No. eval. | ||||||
| Pre-Rx | |||||||
| Post | |||||||
|
Chge p-value |
|||||||
|
12. CRP Specify week |
No. eval. | ||||||
| Pre-Rx | |||||||
| Post | |||||||
|
Chge p-value |
|||||||
|
13. ESR Specify week |
No. eval. | ||||||
| Pre-Rx | |||||||
| Post | |||||||
|
Chge p-value |
|||||||
|
14. HAQ Specify week |
No. eval. | ||||||
| Pre-Rx | |||||||
| Post | |||||||
|
Chge p-value |
|||||||
|
15. DAS Specify week |
No. eval. | ||||||
| Pre-Rx | |||||||
| Post | |||||||
|
Chge p-value |
|||||||
| 16. Joint damage | No. eval. | ||||||
| (scale: | Pre-Rx | ||||||
| Post | |||||||
| Specify week |
Chge p-value |
||||||
| (scale: | Pre-Rx | ||||||
| Post | |||||||
| Specify week |
Chge p-value |
||||||
|
Comments: Which is/are the primary end point(s)? How were missing data handled (e.g. LOCF)? Were any outcome evaluation planned but not reported? Results of subgroup analysis |
|||||||
Adverse events
| Interventions: | ||||||
|---|---|---|---|---|---|---|
| A n = |
B n = |
C n = |
D n = |
E n = |
F n = |
|
| Deaths | ||||||
| Serious adverse events | ||||||
|
Serious infection (definition: |
||||||
| Infections needing antibiotics | ||||||
| Any infection | ||||||
| Malignancy | ||||||
| Injection site reaction | ||||||
| Infusion reaction | ||||||
| Others: | ||||||
| Comments: | ||||||
Appendix 9 Cost-effectiveness review: data extraction of included studies
| Author | Lindgren | Date | 2009 | Study population | Patients with active RA and an inadequate response to one or more TNF inhibitor agents | Type of economic evaluation | Cost–utility analysis |
| Intervention | RTX | ||||||
| Clinical effectiveness | |||||||
|---|---|---|---|---|---|---|---|
| Source of effectiveness data |
Effectiveness of treatment with TNF inhibitors is based on patient level data from the Southern Swedish Arthritis Treatment Group Registry (SSATG) (1997–2007) This data set contains baseline demographic data, disease information (all available HAQ and DAS28 scores), treatment data (biologics and DMARDs) and utility scores EQ-5D The data set used for this analysis contained 1,903 patients with sufficient data on up to three lines of treatment Source for RTX effectiveness was the REFLEX trial,124–126 where patients with active RA and an inadequate response to one or more TNF inhibitors were randomised to receive i.v. RTX (one course, two infusions of 1,000 mg each) or placebo, both with MTX as background therapy |
Clinical outcomes measured and methods of valuation used |
REFLEX primary efficacy point was ACR20 response at 6 months. Secondary end points were ACR50 and ACR70 response, DAS28, and EULAR response criteria at 6 months Mean HAQ scores declined from 1.9 to 1.4 at the 4-week measurement and remained constant up to 6 months of treatment Mean DAS28 scores declined from 6.9 to 5.4 after 4 weeks and to 5.0 after 6 months. Assuming normal distribution of the scores, 5.9% of patients would achieve a DAS28 below 3.2 at week 4, but no further change to low disease activity thereafter Utilities are mapped from the HAQ score. The model uses the equation as estimated by SSATG data (6,860 observations for 1,787 patients) Quality of life (QoL) = 0.915 – 0.252 × HAQ – 0.05 × male – 0.107 × DAS28 HAQ progression was estimated through the SSATG data. It is unclear though what type of regression was used; text suggests linear while Table 2 suggests logistic. Also, Table 2 should have a clearer indication of which variable is the dependent one on all functions used HAQ progression = 0.106 + 0.241 × (HAQ at treatment start) + 0.002 × (months on treatment) – 0.087 × (second line) – 0.192 × (third line) 0.007 × (disease duration) |
||||
| Cost data | |||||||
| Currency used | Costs estimated in Swedish krona (SEK) and presented in euro (€) (1€ = 9.45 SEK) | Years to which costs apply | 2008 | Perspective(s) | Societal perspective (direct and indirect costs included as well as informal care) | ||
| Cost data handled appropriately |
Yes Source for resource consumption was a survey carried out at regular intervals by the department of rheumatology at the Malmö University Hospital (southern Sweden). The survey covers an estimated 90% of the patient population in the area and includes all costs, direct medical and non-medical, as well as productivity losses Costs were calculated as a function of HAQ and DAS28 The cost of TNF inhibitor treatment was a weighted mean based on usage of each drug Unit costs were obtained from standard national (Swedish) sources The cost of RTX was based on the dose used in REFLEX124–126 (two infusions of 1,000 mg each per course). Retreatment could take place between 4 and 12 months, at a 6-month interval Costs of AEs (such as hospitalisation due to severe infections or clinical investigations) were excluded from the analysis as such costs would occur in both arms Costs are discounted at 3% |
||||||
| Cost-effectiveness | |||||||
| Modelling summary |
A discrete event simulation model was developed Patients in the model can be in three states: on treatment, off treatment or dead. On treatment, a difference is made between the first, second or third TNF inhibitors, but not between the different agents. The treatment state is further divided into high or low disease activity, with the cut-off point defined as DAS28 = 3.2 Simulation starts when patients start on second-line treatment, either with a second TNF inhibitor or with RTX. Patients will stay on these treatments until discontinuation of the second-line TNF inhibitor (according to SSATG data) or withdrawal from RTX (according to data from REFLEX124–126). Patients previously on RTX will receive their second TNF inhibitor. When patients fail again, they will switch to another TNF inhibitor again. In the absence of sufficient data to estimate the event rates for the fourth (or subsequent) TNF treatment lines, these are assumed to be the same as for the third line Improvement in HAQ score was assumed to occur immediately and HAQ levels thereafter were assessed using linear regression (as indicated in text – not clear on the table) on the difference compared with the initial HAQ response. At treatment discontinuation, patients return to the initial HAQ score and progress at the rate of 0.03 per year while off treatment Base case is for a 52-year-old female patient with a HAQ score of 1.9 at the start of the second biologic and disease duration of 12 years |
||||||
| Outcome measures used in economic evaluations | Incremental QALYs and ICERs | Statistical analysis for patient-level stochastic data |
A Cox proportional hazards model was estimated to identify covariates (age, gender, disease duration, current HAQ, current disease activity, treatment line) with a possible impact on times to event Bootstrapping was used for parameters where patient-level data were available |
Appropriateness of statistical analysis | Yes | ||
| Uncertainty | |||||||
| Uncertainty around cost-effectiveness expressed |
Yes Model uncertainty was explored using PSA with 1,000 samples by Monte Carlo simulation using all available data and patient characteristics |
Appropriateness of method dealing with uncertainty around cost-effectiveness | Yes | ||||
| Sensitivity analysis |
Sensitivity analysis for the key variables was performed For parameters relating to RTX and the progression of HAQ, normal distribution was assumed |
Modelling inputs and techniques appropriate | Yes | ||||
| Author’s conclusions |
The strategy including RTX in second line dominates current treatment Total costs were €401,000 for the RTX arm and €403,600 for current treatment Patients in the RTX arm gain 0.20 additional QALYs, owing in part to the absence of lag-time in restarting a TNF inhibitor at withdrawal of RTX Changes in the individual key parameters do not affect these results Only if RTX was administered every 4 months or less, then costs for this strategy are higher The results from the PSA indicate that all but one of the 1,000 simulations fall below a theoretical threshold of 500,000 SEK (€53,000) |
||||||
| Author | Russell | Date | 2009 | Study population | Patients with moderate to severe RA and with an inadequate response to one or more DMARDs and/or TNF inhibitors | Type of economic evaluation | Cost-effectiveness analysis |
| Intervention | ABT | ||||||
| Clinical effectiveness | |||||||
|---|---|---|---|---|---|---|---|
| Source of effectiveness data |
DAS data are from various published sources, including the ATTAIN127–132 and TEMPO trials The AIM trial was the source for patients’ inadequate response to DMARDs; safety and effectiveness of ABT when appearing in the sequence for the first time (TNF inhibitor inadequate responders); effectiveness of ABT maintained after the first cycle and for one or more subsequent 6-month cycles The ATTAIN trial127–132 was the source for patients’ inadequate response to TNF inhibitor therapies; safety of ABT, effectiveness of ABT maintained after the first cycle and for one or more subsequent 6-month cycles The TEMPO trial was the source for effectiveness of ETN when appearing in the sequence for the first time (DMARD inadequate responders); effectiveness of ETN maintained after the first cycle and for one or more subsequent 6-month cycles |
Clinical outcomes measured and methods of valuation used |
Treatment effectiveness was defined as either achieving disease remission (DAS28 < 2.6) or low disease activity rate (DAS28 ≤ 3.2) The effectiveness of TNF inhibitors in TNF inhibitor inadequate responders was extracted from the ATTAIN trial,127–132 assuming a 10% reduction after each switch |
||||
| Cost data | |||||||
| Currency used | CAN$ | Years to which costs apply | 2006 | Perspective(s) | Public payer | ||
| Cost data handled appropriately |
ABT is administered over a 30-minute i.v. infusion at 2 and 4 weeks after the first infusion, and every 4 weeks thereafter The analysis assumes an average dose of 750 mg (3 × 250 mg vials) per infusion However, infusion costs were not included because in Canada, IFX and ABT were administered in participating rheumatology and infusion clinics or at home for ABT Direct medical costs per DAS score categories were assessed based on a Canadian cost survey. Data were collected from 253 adult patients and the following cost categories were collected: visits to health professionals [family physician, specialist (non-surgical reported separately from surgical visits), allied health, dentist], laboratory tests or investigation (X-ray, computerised tomography, magnetic resonance imaging, ultrasound, electrocardiogram, other laboratory, bone density), hospitalisations, prescribed drugs [arthritis (not including TNF inhibitor or costimulation modulator), antihypertensive, gastroprotective, other], home care, transportation services, adaptive aids/other devices The estimated annual costs of therapy were: ABT (250-mg vial): $18,480 (year 1), $17,160 (year 2) ADA (40-mg pre-filled syringe): $17,680 (year 1), $17,680 (year 2) ETN (25-mg vial): $18,200 (year 1), $18,200 (year 2) IFX (100-mg vial): $20,445 (year 1), $18,330 (year 2) |
||||||
| Cost-effectiveness | |||||||
| Modelling summary |
Fourteen decision trees (for the various strategies) were designed and analysed as simulation models in decisionpro software Patients with moderate-to-severe RA with an inadequate response to DMARDs, eligible for biologic therapy are entering the model |
||||||
|
Patients achieving treatment success (defined as achieving either a low disease activity rate or remission) are maintained on existing therapy for up to 2 years. Those with an inadequate response to a biologic therapy are switched to a subsequent biologic agent, with decision to switch made at 6-month intervals in case of an inadequate response The model assesses the cost-effectiveness of ABT used as first biologic therapy in patients with an inadequate response to DMARDs and as second biologic therapy in patients with an inadequate response to a first TNF inhibitor The comparator was defined as a successive trial of TNF inhibitor therapies based on the most established treatment pattern in Canada at time of model development. RTX was not reimbursed for RA in Canada at that time, therefore it was not considered as a valid comparator The same treatment continues as long as it is efficacious; decision to switch treatment for all causes (lack or loss of efficacy, adverse events, intolerance, etc.); the model allows switches to occur every 6 months The model calculates the overall effectiveness of each entire sequence of biologic strategies as an effectiveness outcome Reference case was a 2-year treatment with up to three successive biologic agents (in case of an inadequate response to the previous biologic agent) ETN → IFX → ADA → DMARDs The following strategies were simulated: ABT → ETN → IFX → DMARDs ETN → ABT → IFX → DMARDs |
|||||||
| Outcome measures used in economic evaluations |
Cost per additional case of LDAS gained Cost per additional remission gained |
Statistical analysis for patient-level stochastic data | Not undertaken | Appropriateness of statistical analysis | NA | ||
| Uncertainty | |||||||
| Uncertainty around cost-effectiveness expressed |
PSA using 5,000 Monte Carlo simulations was used to explore uncertainty in the model Beta distribution was used for transition probabilities; log-normal distribution was used for costing variability |
Appropriateness of method dealing with uncertainty around cost-effectiveness | Yes | ||||
| Sensitivity analysis | One-way sensitivity analyses (scenario based) was undertaken | Modelling inputs and techniques appropriate | Yes | ||||
| Author’s conclusions |
Inadequate response to DMARDs – cost per additional case of LDAS gained The lowest cost biologic strategy was ABT used as the first biologic agent. This strategy dominated the other two, providing 13.8% greater probability (29.4% vs 15.6%) of achieving LDAS than sequential TNF inhibitor therapy with an overall RA-related cost saving of $730 ($39,759 vs $40,489) over 2 years ABT used as a second biologic after an inadequate response to one TNF inhibitor (ETN) was cost-effective, providing 3.7% greater probability of achieving LDAS (19.3% vs 15.6%) at an additional cost of $463 ($40,952 vs $40,489) over the 2-year period, with an ICER of $12,514 per additional case of LDAS gained Thus, ABT used as first biologic appears to be less costly and to provide greater probability of achieving LDAS than using ABT as second biologic agent Inadequate response to DMARDs – cost per additional remission gained The lowest cost biologic strategy was ABT used as the first biologic agent. This strategy dominated the other two, providing 9.6% greater probability (14.8% vs 5.2%) of remission than sequential TNF inhibitor therapy with an overall RA-related cost-saving of $504 ($38,061 vs $38,565) over 2 years ABT used as a second biologic after an inadequate response to one TNF inhibitor (ETN) was cost-effective, providing 3.5% greater probability of achieving remission (8.7% vs 5.2%) at an additional cost of $589 ($39,154 vs $38,565) over the 2-year period, with an ICER of $16,829 per additional remission gained Thus, ABT used as first biologic appears to be less costly and to provide greater probability of achieving remission than using ABT as second biologic agent Inadequate response to ETN After an initial 6-months treatment failure to ETN, all patients were switched to either ABT or IFX as the second biologic option, followed by IFX and ADA, respectively ABT used as second biologic agent was cost-effective, providing 6.9% additional treatment success rate for achieving LDAS (17.1% vs 10.2%) and 3.5% additional treatment success rates for achieving remission (7.4% vs 3.9%) at an ICER of £20,377 per additional case of LDAS and $26,400 per additional remission, respectively |
||||||
| Author | Kielhorn | Date | 2008 | Study population | Patients with RA who failed to respond adequately to two non-biologic DMARDs and one TNF inhibitor | Type of economic evaluation | Cost–utility analysis |
| Intervention | RTX | ||||||
| Clinical effectiveness | |||||||
|---|---|---|---|---|---|---|---|
| Source of effectiveness data | The mean drop in HAQ for each of the responder groups is calculated from the REFLEX trial124–126 | Clinical outcomes measured and methods of valuation used |
Utilities are mapped from the HAQ score. The model uses the equation as estimated by Bansback et al. 146 QoL = 0.76 – 0.28 × HAQ + 0.05 × female All-cause mortality is derived by GAD218 and adjusted with an RA risk multiplier related to each individual’s HAQ score (Barton et al. 175) |
||||
| Cost data | |||||||
| Currency used | British £ | Years to which costs apply | 2004 (not explicitly stated) | Perspective(s) | NHS and Personal Social Services | ||
| Cost data handled appropriately |
For each treatment, drug cost, administration cost and monitoring cost were considered Drug costs were obtained from British National Formulary 50 Administration costs are generated by biological DMARDs requiring infusion or injection For RTX, 5 hours of administration was assumed on average, including pre-medication For IFX, a 3-hour infusion time for the 225 mg of active substance was assumed including post-infusion observation time A weight of 78 kg was assumed (Cohen et al. 126) No drug wastage or increase in dose was included in the calculation Healthcare personnel attendance time was estimated according to Nuijten et al. ,219 and personnel salaries were obtained from Personal Social Services Research Unit (PSSRU) 2004 Monitoring costs include an outpatient visit or a GP visit, and certain examination and tests. Costs for these were obtained from NHS, PSSRU or Barton et al. 175 Costs are linked to functional status, as measured by the HAQ score, by grouping HAQ scores into six categories (Kobelt et al. 1999,220 2004221). Each HAQ score category was assigned an average cost. Direct costs included the cost of the drug, drug administration, medical resource consumption (comedication, surgery, etc.) All costs accruing after the first year of the evaluation were discounted at 3.5% |
||||||
| Cost-effectiveness | |||||||
| Modelling summary |
A microsimulation Markov model was designed and analysed in Microsoft excel. A cycle length of 6 months was used. Patients either follow the current standard treatment sequence reflecting real-life clinical practice in the UK or an alternative sequence, which is identical, except for the introduction of RTX as an additional treatment within the sequence. If patients respond they remain on the drug for a predetermined period of time. If they do not respond, they continue to the next treatment in the sequence. They remain in palliative care (MTX) until they reach 100 years of age or death Analysis A assumes non-sequential use of bDMARDs (NICE 36222) Analysis B assumed sequential use of bDMARDs; (based on data from the BSRBR and Hyrich et al. 223) Patients enter the model and are allocated to either of the two treatment sequences. The patients are then exposed to the first treatment in the sequence and are allocated to one of the three responder groups ACR 20–49, 50–69, 70 + or to the non-responder group The HAQ score is assumed to drop by 0.1 for non-respondents, 0.45 for ACR20–49, 0.85 for ACR50–69 and 1.11 for ACR70 + respondents (Kielhorn et al. 224). While on treatment, patient HAQ scores are assumed to progress by 0.017 during each cycle of the model (Scott et al. 225). HAQ progression for patients on palliative care is assumed to be 0.065 (Bansback et al. 146) Time on treatment in the sequence was derived from Barton et al. ,175 and was 4.25 years for all bDMARDs apart from IFX, for which, driven by a higher drop-out of patients, 2.46 years was assumed. bDMARDs treatment duration was 1.7 years for ciclosporin A, 3.85 years for gold and 4.1 years for LEF. For RTX a course of 2 × 1,000 mg every 9 months over the course of 4.25 years was assumed. For all other drugs the licensed dose as per the EU label was assumed Once treatment stops, the entire initial gain in HAQ score is assumed to be lost instantly (100% rebound effect). Patients are then allocated to the next available treatment option until the treatment sequence is exhausted. At this point, all patients receive palliative care, defined as single agent MTX, until death Patients leave the model when they reach the age of 100 years or die |
||||||
| Outcome measures used in economic evaluations | Incremental QALYs and ICERs | Statistical analysis for patient-level stochastic data | Not undertaken | Appropriateness of statistical analysis | NA | ||
| Uncertainty | |||||||
| Uncertainty around cost-effectiveness expressed |
Yes Model uncertainty was explored using PSA with 1,000 samples by Monte Carlo simulation. Owing to lack of data it was not possible to run a PSA on all variables. For these variables, one-way sensitivity analysis was applied instead A Dirichlet distribution was fit for response rate parameters, a Weibull distribution into the time on treatment parameters and a normal distribution was fit into the inpatient days, trimmed for values [0, + ∞) |
Appropriateness of method dealing with uncertainty around cost-effectiveness | Yes | ||||
| Sensitivity analysis |
Yes One-way sensitivity analysis was applied to determine the relative importance of different parameters to the primary outcome The model was not sensitive with respect to changes to assumed time on treatment, or changes between adjusted and unadjusted response rates Larger variability was observed in changes to RTX dosing retreatment from 9 months to 6 months and when changing the HAQ long-term progression Variability was also observed when baseline age is increased |
Modelling inputs and techniques appropriate | Yes | ||||
| Author’s conclusions |
Both analyses showed higher treatment cost in the sequence containing RTX Analysis A Total discounted QALYs were 3.051 and 2.324 for the RTX arm and the standard of care arm, respectively, resulting in a QALY gain of 0.727. The ICER based on total direct medical costs was £14,690 Analysis B QALY gain was 0.526; the ICER based on total direct medical costs was £11,601 |
||||||
| Author | Vera-Llonch | Date | 2008 | Study population | Women with moderate-to-severe RA with inadequate response to TNF inhibitors | Type of economic evaluation | Cost–utility analysis |
| Intervention | ABT | ||||||
| Clinical effectiveness | |||||||
|---|---|---|---|---|---|---|---|
| Source of effectiveness data | Source for effectiveness data was the ATTAIN trial127–132 | Clinical outcomes measured and methods of valuation used |
Improvement in HAQ scores during the first 6 months of therapy For patients continuing to receive ABT beyond 6 months, the improvement at 6 months was assumed to persist over time. For patients discontinuing ABT, the HAQ score was assumed to return to a value equal to what it would have been in the absence of such treatment (oral DMARD only) Initial HAQ scores are randomly assigned to each patient entering the model from an assumed initial probability distribution. Future values of the HAQ score were estimated based on the assumed initial value, the expected rate of disease progression and the expected effect of treatment The estimated mean percentage HAQ change 3 months after therapy initiation in ATTAIN127–132 was 21%; at 6 months it was 25.5% The distribution of the HAQ change with ABT was assumed to be truncated normal, based on visual inspection of the data in ATTAIN127–132 Among patients continuing to receive ABT, the percentage reduction in the HAQ score was assumed to remain constant at the level prevailing at 6 months. However, the HAQ value against which this percentage reduction was applied was increased by 0.015 annually Health-state utility values were mapped from the HAQ score. Although mean utilities corresponding to the appropriate HAQ score are presented in a table, the exact formula that was using for this mapping is not provided For patients receiving oral DMARD only, the HAQ score was assumed to increase by 0.065 annually to reflect disease progression Mortality risk was estimated through age and the expected value of the HAQ score Health-state utilities were similarly estimated based on the expected future values of the HAQ score |
||||
| Cost data | |||||||
| Currency used | US$ | Years to which costs apply | 2006 | Perspective(s) | Third-party payer (medical treatment only – direct non-medical costs or loss productivity were excluded) | ||
| Cost data handled appropriately |
Following an initial infusion, ABT was assumed to be administered on days 14 and 29, and every 4 weeks thereafter, for a total of 15 infusions during the first year and 13 infusions every year thereafter Patients weighing < 60 kg were assumed to receive two vials (500 mg) per infusion; 60–100 kg, three vials (750 mg); and > 100 kg, four vials (1 g) The cost of ABT was assumed to be $450 per 250-mg vial The cost of each 30-minute infusion was assumed to be $129 Oral DMARD therapy was assumed to consist of MTX. The annual cost of treatment with MTX was assumed to be $600, based on an assumed dose of 15 mg weekly Estimates of the cost of baseline and routine monitoring for patients receiving ABT were based on product labelling, published guidelines and Medicare payment rates Tests for ABT patients were assumed to cost $9 (one-off cost) while tests for the DMARD patients were at $181 per year Costs were discounted at 3% |
||||||
| Cost-effectiveness | |||||||
| Modelling summary |
A simulation model of a hypothetical cohort of 1,000 women aged 55–64 years was developed. The model cycle was 3 months Patients enter the model at either the ‘oral DMARD’ state or the ‘oral DMARD state plus ABT’ Patients on ABT are assumed to initiate treatment on day 1 [500–1,000 mg (based on body weight) i.v. infusion over 30 minutes], and receive additional infusions on day 14, day 29 and every 4 weeks thereafter Patients with HAQDI improvements of –0.50 or greater at 6 months were assumed to continue to receive ABT Patients failing to achieve this improvement are assumed to discontinue treatment Patients also discontinue treatment for other reasons such as side effects, intercurrent illness and surgery All patients discontinuing ABT are assumed to continue to receive ‘oral DMARDs’ Authors justify this assumption (assuming no switch from ABT to another biologic DMARD) on the bases that there are no data on the efficacy of the latter agents given prior failure with ABT Time horizons were 10 years and lifetime |
||||||
| Outcome measures used in economic evaluations | Incremental cost per QALY | Statistical analysis for patient-level stochastic data | Not undertaken | Appropriateness of statistical analysis | NA | ||
| Uncertainty | |||||||
| Uncertainty around cost-effectiveness expressed | Expressed through 100 Monte Carlo simulations | Appropriateness of method dealing with uncertainty around cost-effectiveness | Yes | ||||
| Sensitivity analysis |
Yes Selected assumptions and parameter estimates were varied, including: Discontinuation of ABT therapy for lack of efficacy or other reasons Timing of therapy discontinuation due to lack of efficacy (3 months vs 6 months) Odds ratio for mortality associated with each 1-point increase in the HAQ score Assumption of mortality benefit with ABT Expected rate of disease progression Threshold for clinical meaningful improvement in HAQ Women aged other than 55–64 years Male population |
Modelling inputs and techniques appropriate | Yes | ||||
| Author’s conclusions |
Over a 10-year time horizon, the cost-effectiveness of ABT was estimated to be $50,576 per QALY gained On a lifetime basis, cost-effectiveness was $45,979 per QALY gained At a threshold of $100,000 per QALY, the probability that ABT would be cost-effective was 1 At a threshold of $20,000 per QALY, ABT would be unlikely to be cost-effective (probability = 0) At a threshold of $50,000 per QALY, the probability that ABT would be cost-effective was 0.39 over a 10-year time horizon and 1 over lifetime |
||||||
Appendix 10 Outcomes not reported in the main text of the report
Adalimumab
FIGURE 100.
Adalimumab: SJC, change from baseline. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.
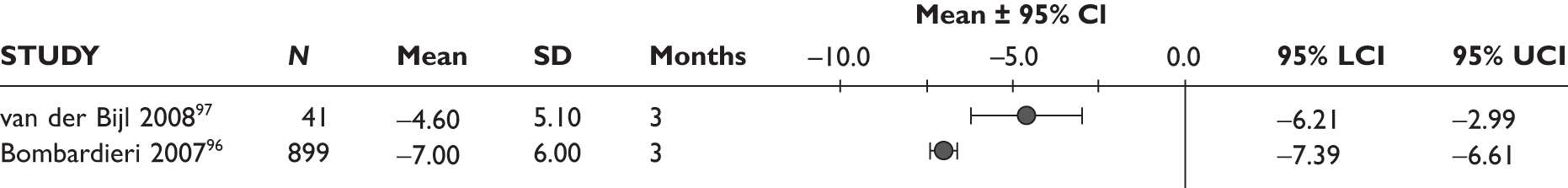
FIGURE 101.
Adalimumab: TJC, change from baseline. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.
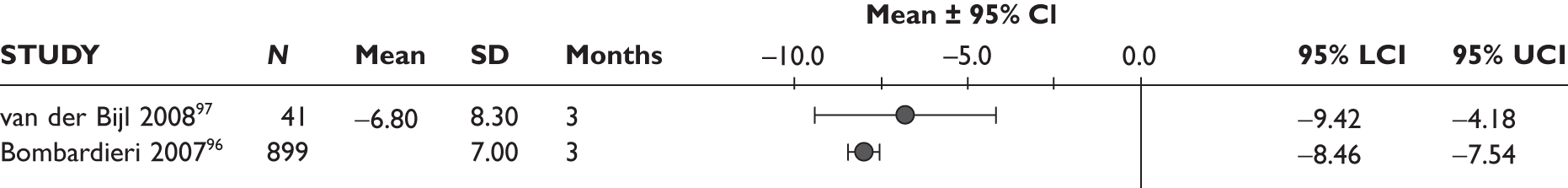
FIGURE 102.
Adalimumab: pain (visual analogue scale), change from baseline. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.

FIGURE 103.
Adalimumab: physician global assessment (visual analogue scale), change from baseline. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.

FIGURE 104.
Etanercept: SJC. LCI, lower confidence interval; NR, not reported; SD, standard deviation; UCI, upper confidence interval.
FIGURE 105.
Etanercept: TJC. LCI, lower confidence interval; NR, not reported; SD, standard deviation; UCI, upper confidence interval.

FIGURE 106.
Etanercept: patient pain. LCI, lower confidence interval; NR, not reported; SD, standard deviation; UCI, upper confidence interval.
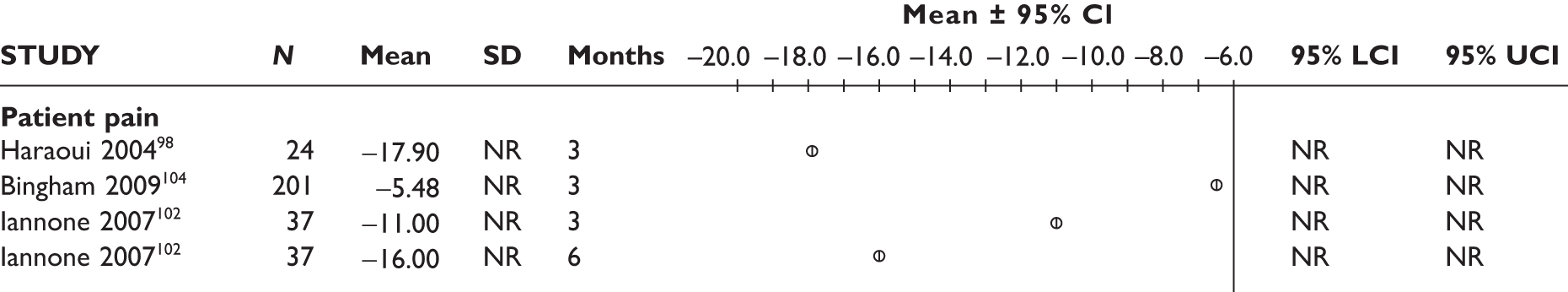
FIGURE 107.
Etanercept: physician global function. LCI, lower confidence interval; NR, not reported; UCI, upper confidence interval.
FIGURE 108.
Etanercept: patient global function. LCI, lower confidence interval; NR, not reported; SD, standard deviation; UCI, upper confidence interval.
FIGURE 109.
Etanercept: mean change from baseline in CRP. LCI, lower confidence interval; NR, not reported; SD, standard deviation; UCI, upper confidence interval.
FIGURE 110.
Etanercept: mean change from baseline in ESR. LCI, lower confidence interval; NR, not reported; SD, standard deviation; UCI, upper confidence interval.
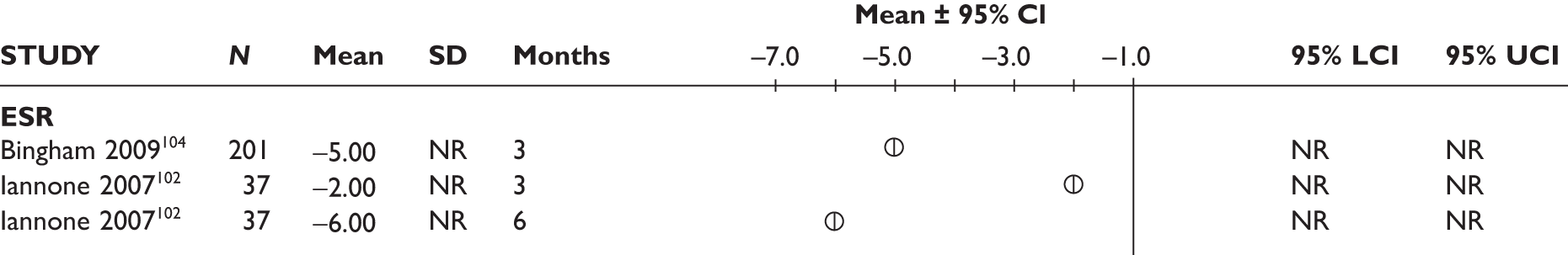
Etanercept
FIGURE 111.
Infliximab: physician global assessment. LCI, lower confidence interval; NR, not reported; UCI, upper confidence interval.
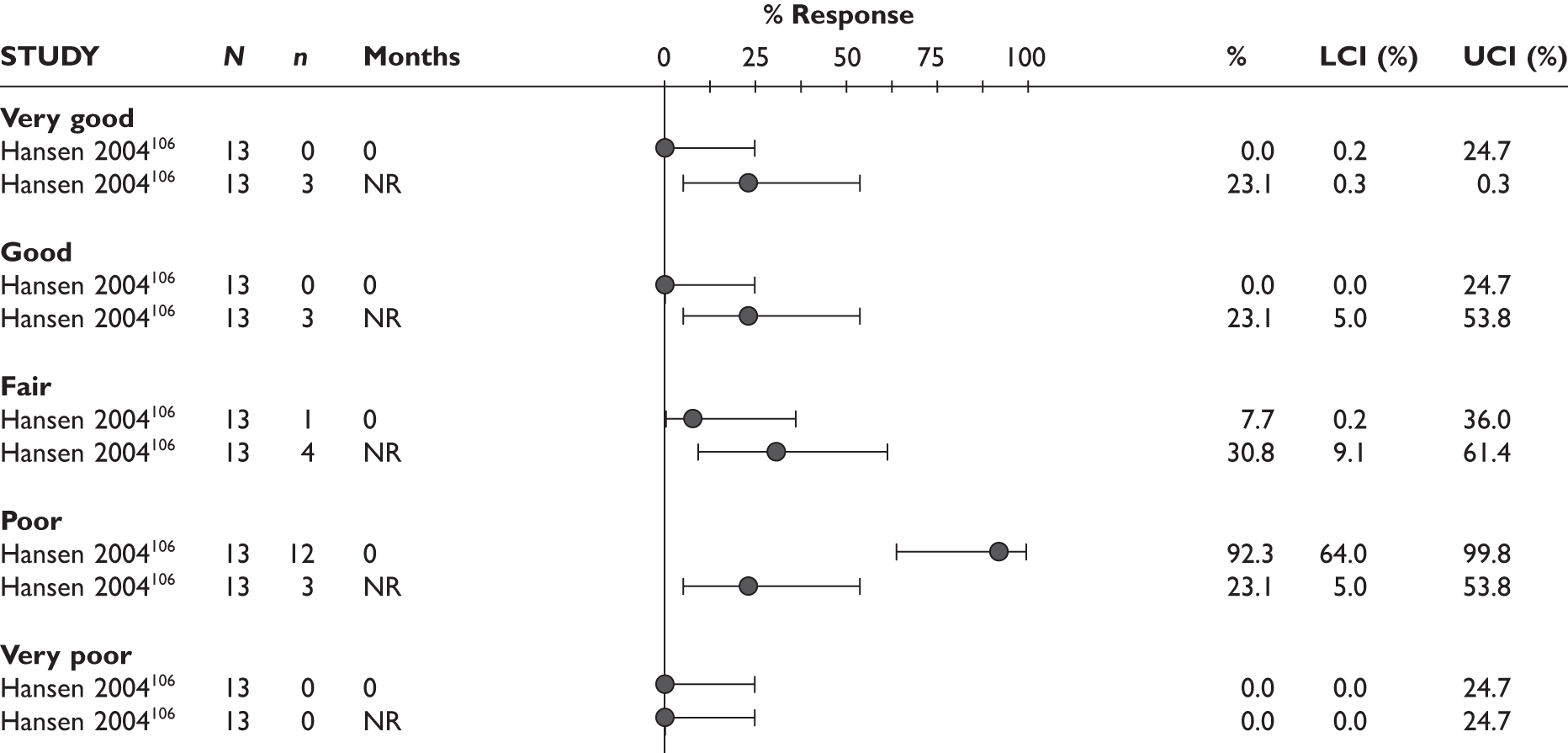
FIGURE 112.
Infliximab: patient global assessment. LCI, lower confidence interval; NR, not reported; UCI, upper confidence interval.
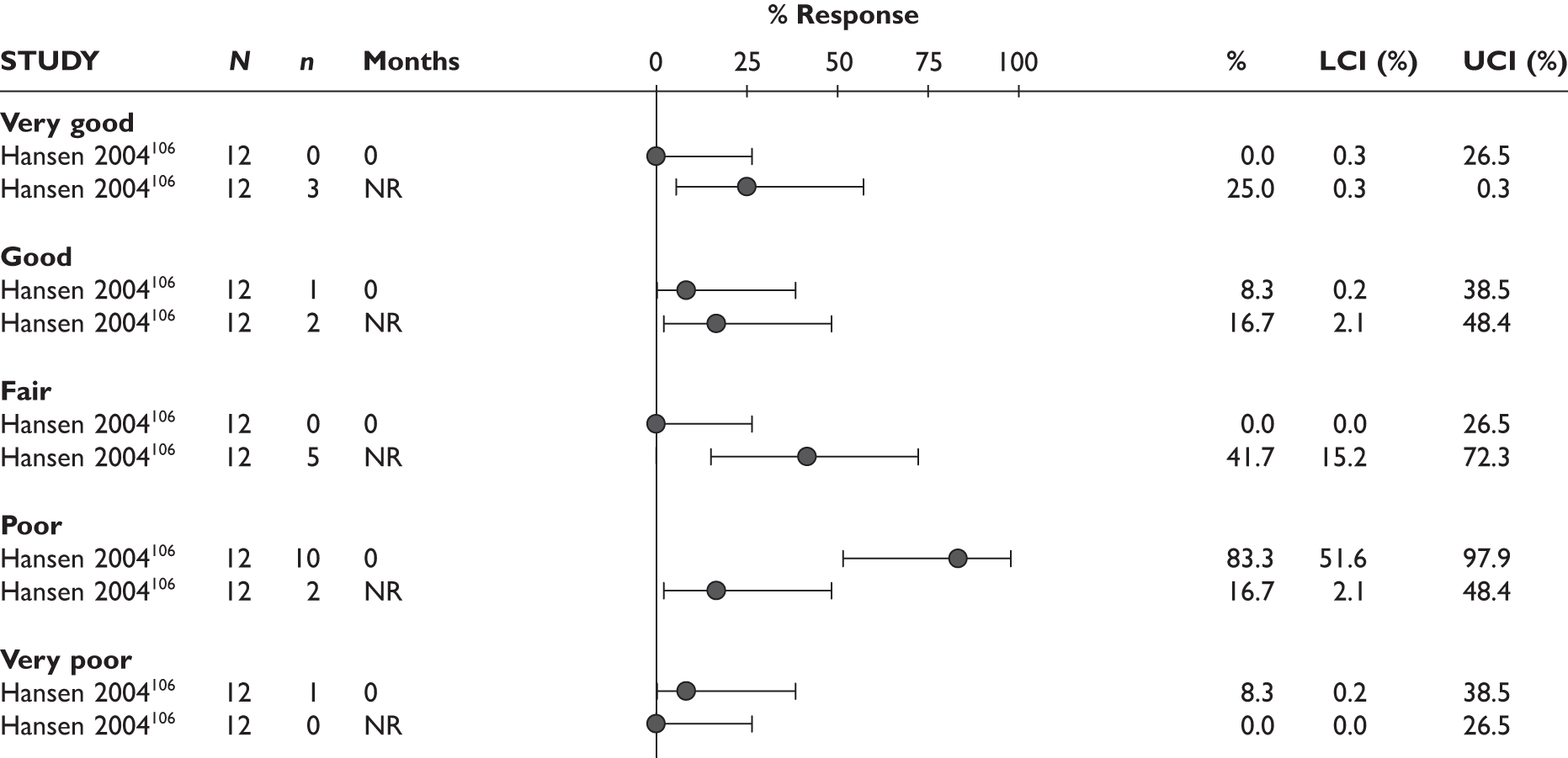
FIGURE 113.
Infliximab: percentage change from baseline in SJC and TJC. LCI, lower confidence interval; n/a, not applicable; NR, not reported; SD, standard deviation; UCI, upper confidence interval.

FIGURE 114.
Infliximab: mean change from baseline in CRP. LCI, lower confidence interval; n/a, not applicable; NR, not reported; SD, standard deviation; UCI, upper confidence interval.
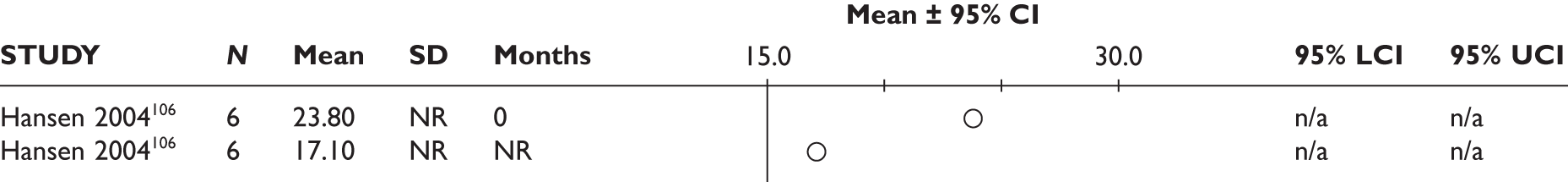
FIGURE 115.
Infliximab: mean change from baseline in ESR. LCI, lower confidence interval; n/a, not applicable; NR, not reported; SD, standard deviation; UCI, upper confidence interval.
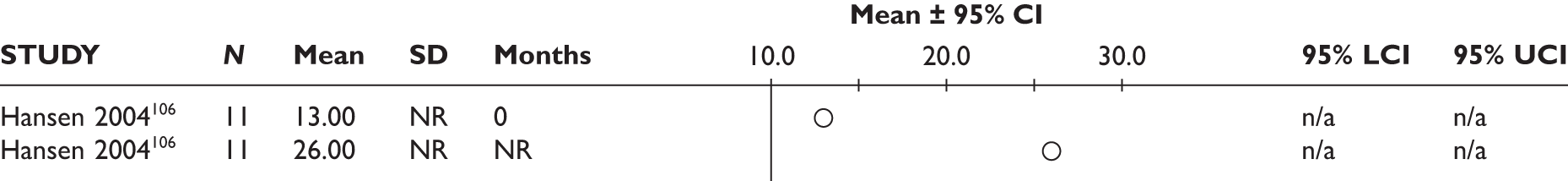
Infliximab
FIGURE 116.
TNF inhibitor: SJC. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.
FIGURE 117.
TNF inhibitor: TJC. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.
FIGURE 118.
TNF inhibitor: mean change from baseline in ESR. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.
Tumour necrosis factor inhibitors as a class
FIGURE 125.
Rituximab: mean ESR (mm/h) in uncontrolled studies. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.

FIGURE 126.
Rituximab: median ESR (mm/h) in uncontrolled studies. (p < 0.0001 for both at 3 months and 6 months.) SD, standard deviation.
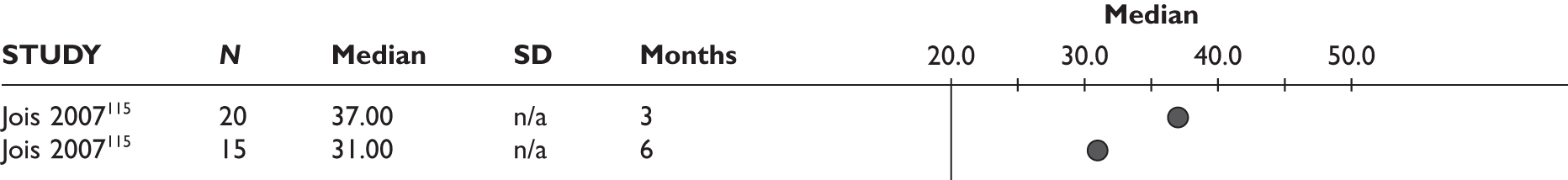
FIGURE 128.
Rituximab: median TJC/SJC count in uncontrolled studies (p< 0.0001 for 3 months vs baseline and p< 0.05 for 6 months vs baseline). SD, standard deviation.
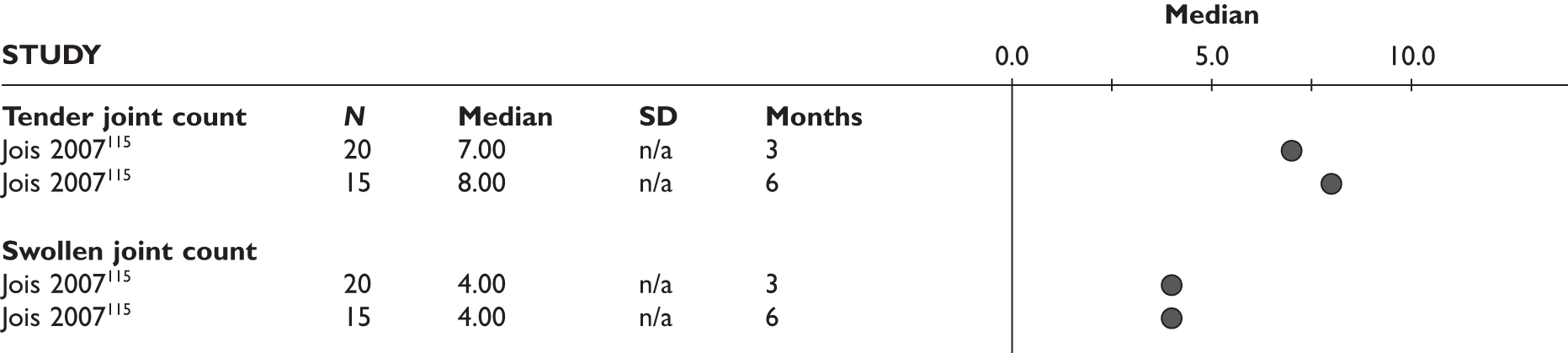
FIGURE 129.
Rituximab: mean TJC/SJC count in uncontrolled studies. Bold type idicates that the study was an RCT. LCI, lower confidence interval; SD, standard deviation; UCI, upper confidence interval.
FIGURE 130.
Rituximab: median patient global score (0–100 mm visual analogue scale) in uncontrolled studies. SD, standard deviation.
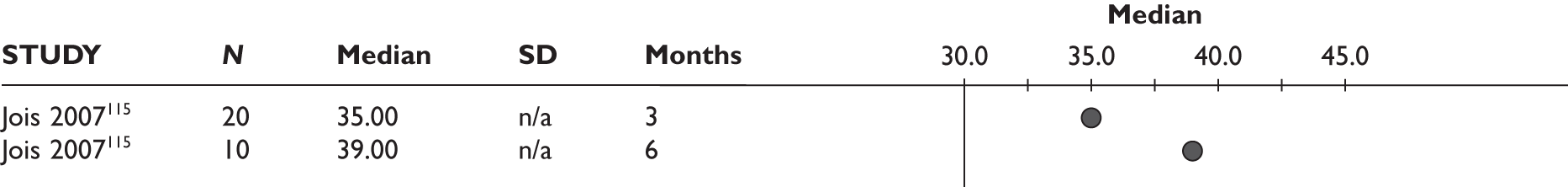
Rituximab
Joint damage data from the manufacturers’ submissions
REFLEX extension
Figure 139 presents ACR response at week 24 after one, two and three RTX treatment courses versus original baseline in the REFLEX trial. 124–126 A similar pattern was seen for each ACR response 24 weeks after each course, with the ACR responses following each course slightly increased with subsequent courses.
FIGURE 139.
Rituximab: ACR response at week 24 after each course versus original baseline (data from MS). LCI, lower confidence interval; UCI, upper confidence interval.
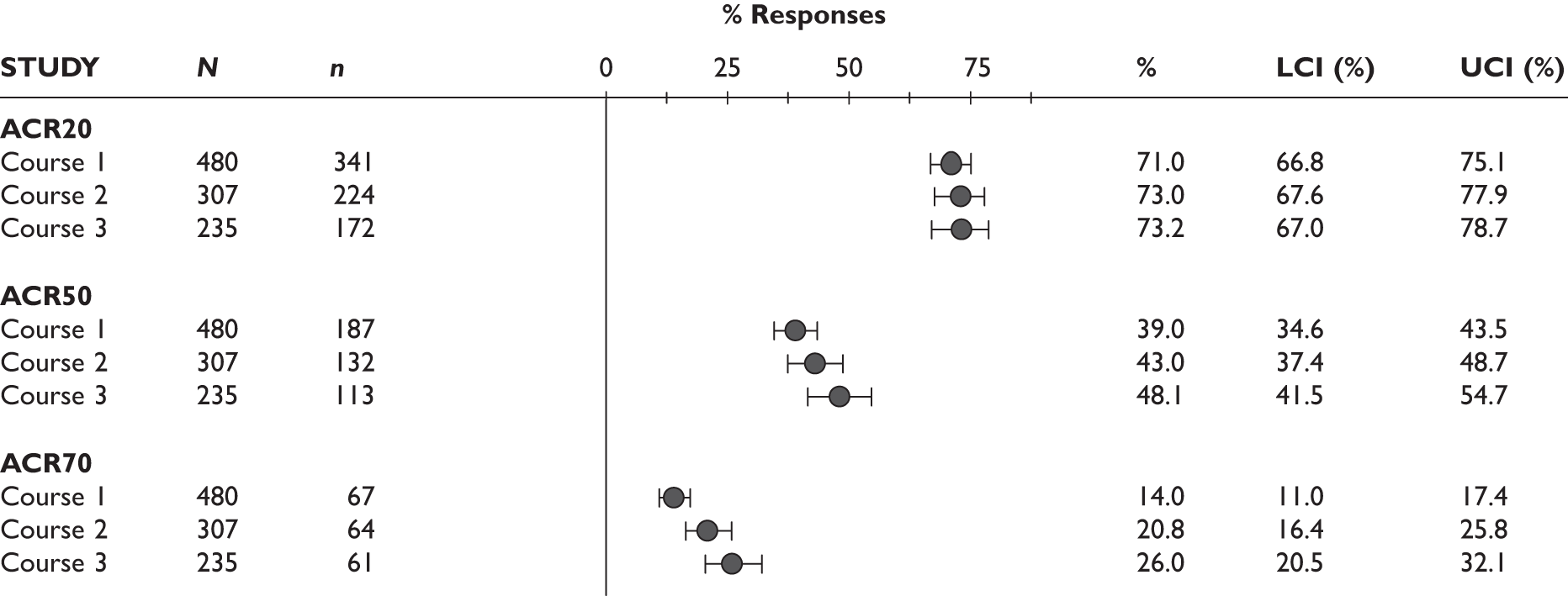
Figure 140 presents EULAR response at week 24 after one, two and three courses of RTX versus original baseline of the REFLEX trial. 124–126 The percentage of patients achieving moderate plus good response and good response alone increased with each treatment course (from 84% to 87.9% to 88.9% and from 17.1% to 26.1% to 28%, respectively).
FIGURE 140.
Rituximab: EULAR responses 24 weeks after each course versus original baseline (data from MS). LCI, lower confidence interval; UCI, upper confidence interval.

Figure 141 presents the percentage of patients achieving DAS28 low disease activity or remission at week 24 after course one, two and three versus original baseline of the REFLEX trial. 124–126 Improvement for both was observed following subsequent courses (from 17.1% to 26.1% to 34% and from 9% to 14% to 13.2%, respectively).
FIGURE 141.
Rituximab: percentage of patients achieving DAS28 low disease activity at week 24 after each course versus original baseline (data from MS). LCI, lower confidence interval; UCI, upper confidence interval.
Pooled analysis data (manufacturer’s submission)
Figure 142 presents ACR responses for four or five courses and Figure 143 presents ACR responses for three or four courses of RTX 24 weeks after each course. The overall pattern was that there was an improvement from the first to the second course and then maintained through the subsequent courses. Observed data on EULAR responses for four or five courses at 24 weeks after each course showed a similar pattern to ACR responses (Figure 144).
FIGURE 142.
Rituximab: ACR responses for five courses of treatment 24 weeks after each course (all patients; observed data; data from MS). LCI, lower confidence interval; UCI, upper confidence interval.
FIGURE 143.
Rituximab: ACR responses for three or four course (24 weeks) after each course (within-patients, within-visit comparisons, observed data, n = 146; data from MS). LCI, lower confidence interval; UCI, upper confidence interval.
FIGURE 144.
Rituximab: EULAR response rates for four or five courses (week 24 after each course, all patients, observed data; data from MS). LCI, lower confidence interval; UCI, upper confidence interval.
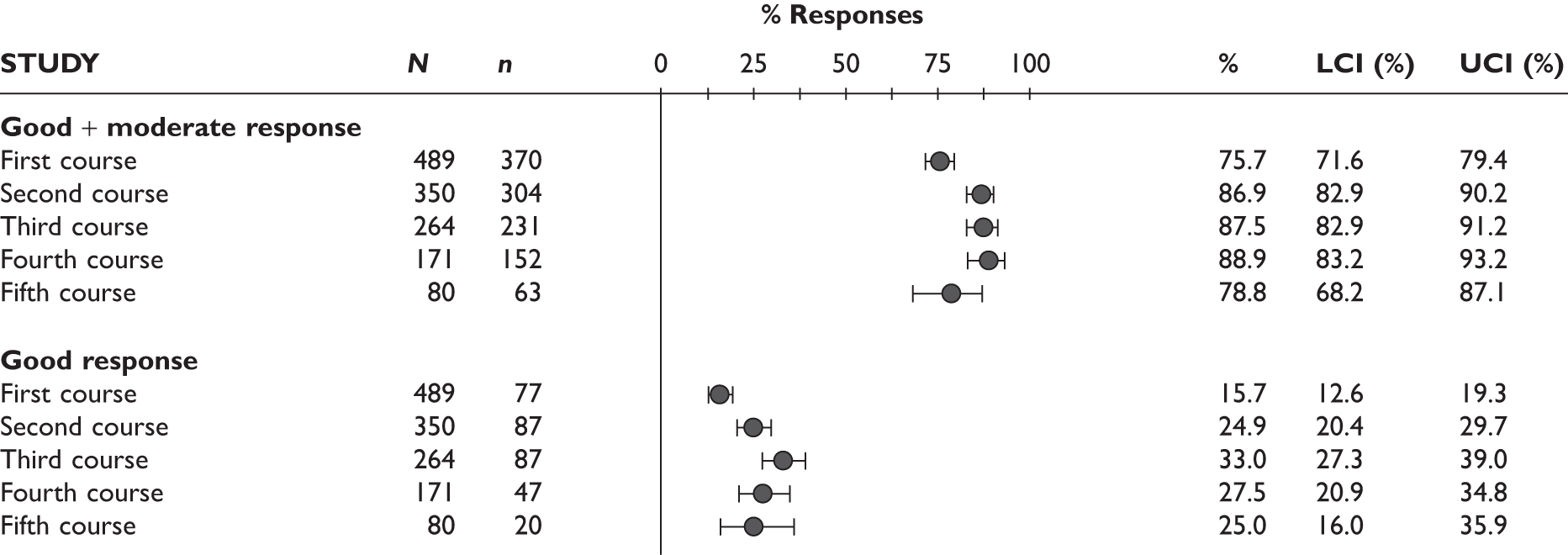
The patterns for the percentage of patients with low disease activity (defined as DAS28-ESR less than or equal to 3.2) and with remission (defined as DAS28-ESR less than 2.6) for four or five courses at week 24 after each course, and for data on three or four courses at week 24 after each course, were similar, with a improvement from first to second course and to third course and then generally maintained with subsequent courses (Figures 145 and 146).
FIGURE 145.
Rituximab: percentage of patients with low disease activity (DAS28-ESR ≤ 3.2) and with remission of disease activity (DAS28-ESR < 2.6) for four or five courses (week 24 after each course, all patients, observed data; data from MS). LCI, lower confidence interval; UCI, upper confidence interval.
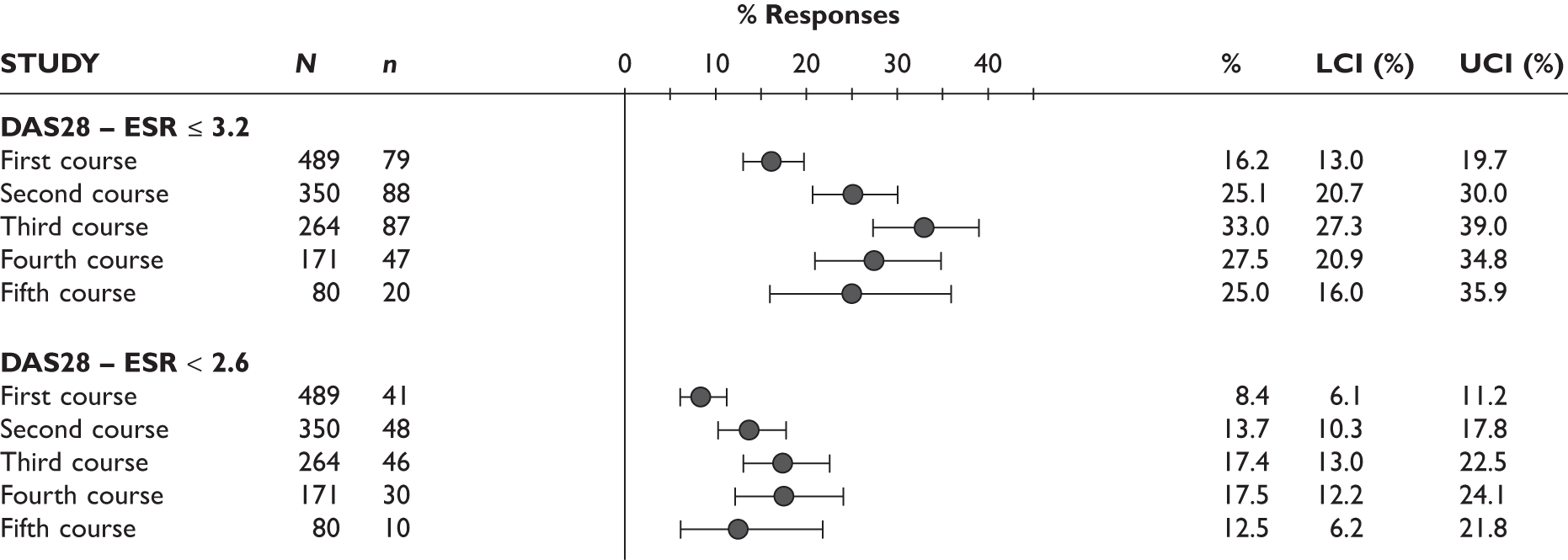
FIGURE 146.
Rituximab: percentage of patients with low disease activity (DAS28-ESR ≤ 3.2) and with remission of disease activity (DAS28-ESR < 2.6) for three or four course (week 24 after each course, all patients, observed data; data from MS). LCI, lower confidence interval; UCI, upper confidence interval.

Figure 147 presents the change from original baseline of the REFLEX trial124–126 in HAQ for four or five courses 24 weeks after each course and Figure 148 presents the percentage of patients achieving minimally important clinical difference, i.e. a decrease in HAQ score of greater than or equal to 0.22 from baseline, for four or five courses 24 weeks after each course. Both the change in HAQ score and the percentage of patients achieving a clinically meaningful decrease in HAQ score were maintained over treatment courses of RTX.
FIGURE 147.
Rituximab: change from original baseline in HAQ end points for four or five courses (week 24 after each course, all patients, observed data; data from MS). LCI, lower confidence interval; UCI, upper confidence interval.
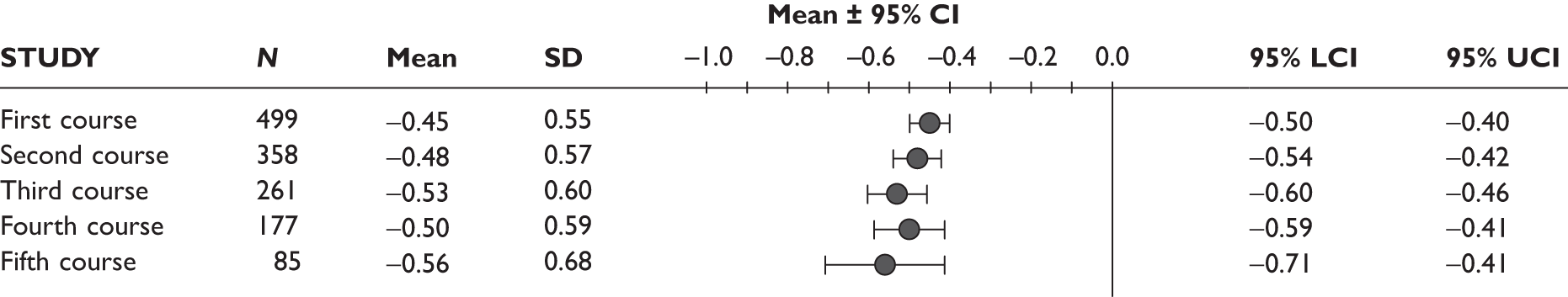
FIGURE 148.
Rituximab: percentage of patients with HAQ decrease ≥ 0.22 from original baseline for four or five courses (week 24 after each course, all patients, observed data; data from MS). LCI, lower confidence interval; UCI, upper confidence interval.
Abatacept
FIGURE 150.
Abatacept: change in pain score (visual analogue scale) in uncontrolled studies. LCI, lower confidence interval; n/a, not applicable; NR, not reported; SD, standard deviation; UCI, upper confidence interval.
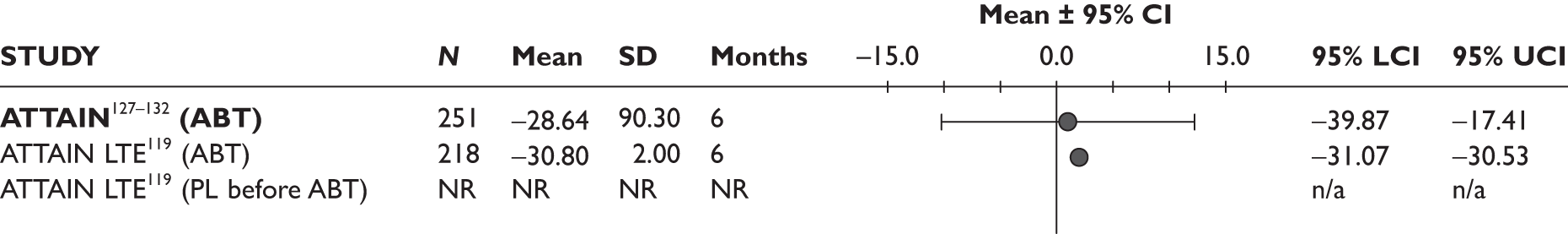
FIGURE 151.
Abatacept: change in sleep score in uncontrolled studies at 6 months. LCI, lower confidence interval; n/a, not applicable; NR, not reported; SD, standard deviation; UCI, upper confidence interval.

FIGURE 152.
Abatacept: change in fatigue score in uncontrolled studies at 6 months. LCI, lower confidence interval; n/a, not applicable; NR, not reported; SD, standard deviation; UCI, upper confidence interval.
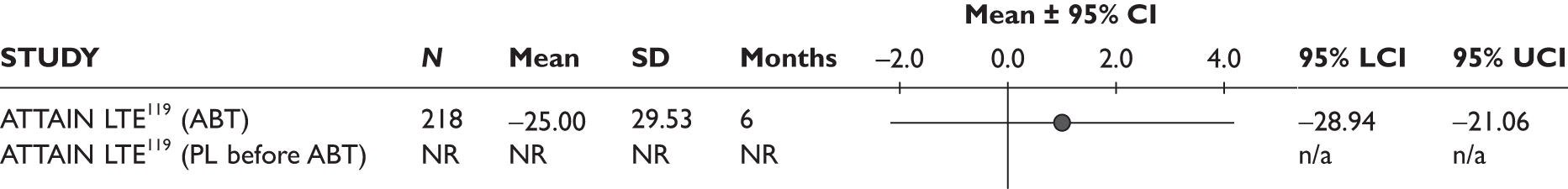
Appendix 11 Survey of West Midlands rheumatologists
A survey of rheumatologists in the West Midlands was conducted in June 2009 and July 2009 to investigate current practice and clinicians’ preferences for treatment options in rheumatoid arthritis.
Methods
In the beginning of June a questionnaire was sent to a convenience sample of 55 rheumatologists by email (Figure 153).
FIGURE 153.
Survey of West Midlands rheumatologists.

Responses were collected until early July, when a reminder together with the results of the survey so far was sent. Responses received afterwards were included in the results.
Owing to the overall variability it was not possible to determine in any way if the three responses received after the reminder were influenced by the knowledge of the early results.
Results
Twenty-four rheumatologists replied before the reminder email. Three additional responses were received after the reminder was sent out. The overall response rate was 49%.
For drugs used in addition to MTX before the initiation of the first TNF inhibitor responses often included combinations of multiple conventional DMARDs or different therapeutic options. Sulfasalazine alone or in combination with other DMARDs was the most frequently mentioned DMARD (in 22 responses) used before the initiation of the first TNF inhibitor. Leflunomide was mentioned in 17 responses and hydroxychloroquine in 10. Five respondents mentioned the use of steroids.
Results for the first TNF inhibitor and following treatment options are presented in Figure 154. The highest number of respondents (nine) left the choice of the first TNF inhibitor to the patient. Seven would chose ADA and one indicated that this drug was most often chosen by patients. Etanercept was the preferred first TNF inhibitor for six respondents; however, three would ultimately leave the choice to their patient. The remaining four would choose either ADA or ETN (two because of involvement in a clinical trial).
After the failure of the first TNF inhibitor, 17 respondents would try a second one (only six were specific and their preferences were – ADA in four and ETN in two cases). Nine respondents would try RTX as a second-line biologic agent and one TOC.
There was more variability in the following lines of treatment and preferences depended on what has been tried before. After the failure of a second TNF inhibitor, ten respondents would try RTX, five TOC, one ADA and one LEF. After the failure of RTX (following first TNF inhibitor) six respondents would try a second TNF inhibitor, two would try TOC and one ABT. One respondent who would try TOC after the failure of the first TNF inhibitor would choose RTX as the next therapeutic option.
For the next line of treatment see Figure 154. Results for the subsequent treatment options are not reported because of their high variability.
FIGURE 154.
Survey of West Midlands rheumatologists: results. Numbers in brackets are the number of respondents selecting and option.
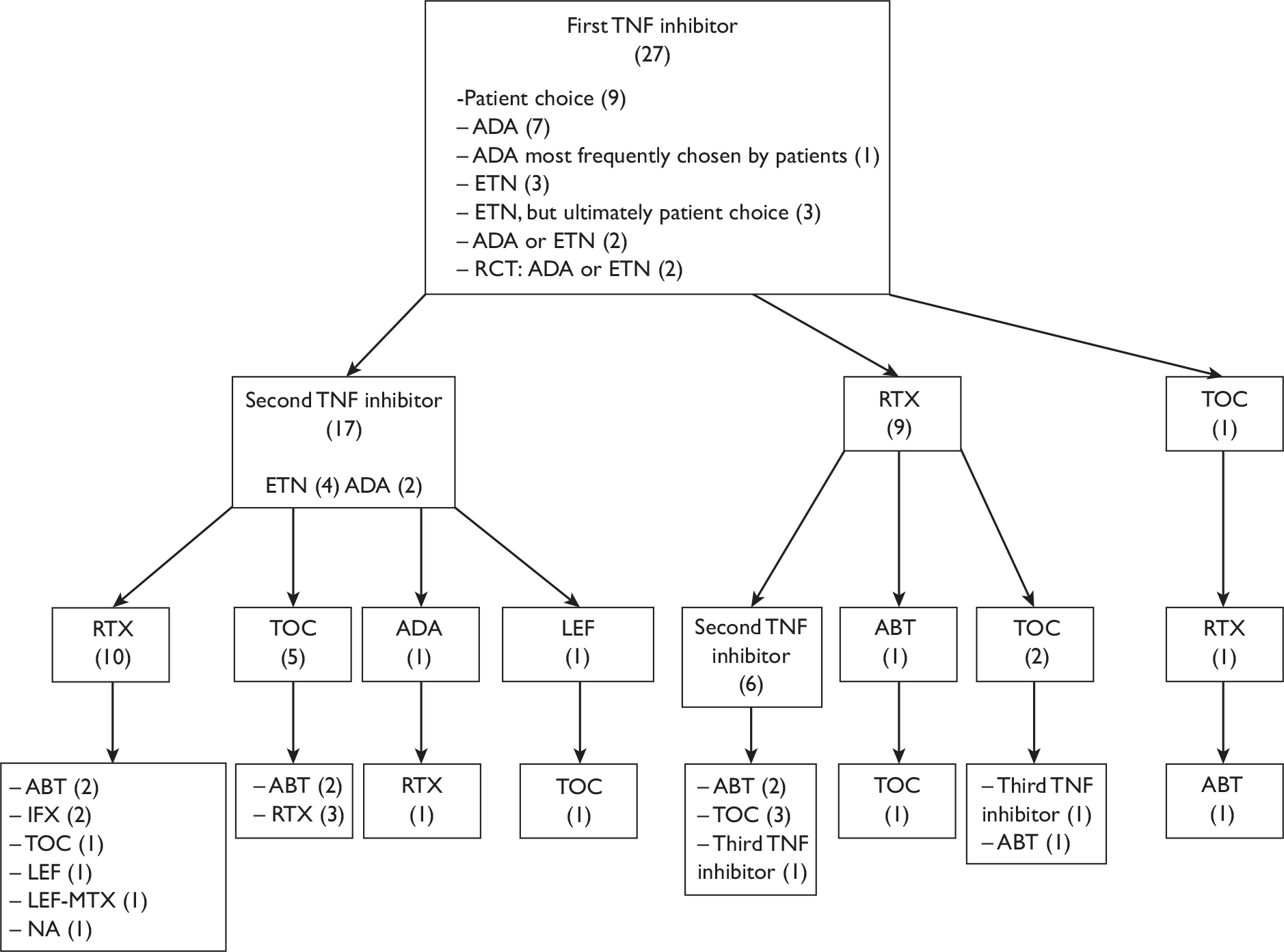
The comments from respondents included a number of issues referring both to current practice and to proposed research:
-
Different factors might influence choice of drug, such as:
-
– previous or possible tuberculosis
-
– risk of infection
-
– comorbidities
-
– primary versus secondary failure
-
– seropositive versus negative patients
-
– intolerance versus inefficacy
-
– ethnicity (ETN preferred in Asian patients)
-
– ‘needle-phobia’.
-
-
Practice is frequently tailored to the individual patient (pattern of disease, side-effect risks, etc.).
-
Going back to a TNF inhibitor already used could be considered.
-
For some patients receiving biologic treatments, adjunct DMARDs other than MTX could be considered.
-
Switching TNF inhibitors before the 3-month NICE deadline could be considered if the patient showed little response.
-
A combination of TNF inhibitors could be considered.
Appendix 12 Withdrawals from treatment with tumour necrosis factor inhibitors
Withdrawal from treatment with second-line tumour necrosis factor inhibitor (British Society for Rheumatology Biologics Registry data)
Updated BSRBR model data163 provided Kaplan–Meier (K–M) plots for survival in treatment for four groups of patients receiving second-line TNF inhibitors as follows: (i) withdrew from first-line TNF inhibitor for lack of efficacy and from second-line TNF inhibitor for lack of efficacy; (ii) withdrew from first-line TNF inhibitor for lack of efficacy and from second line TNF inhibitor for AEs; (iii) withdrew from first-line TNF inhibitor for AEs and from second-line TNF inhibitor for lack of efficacy; (iv) withdrew from first-line TNF inhibitor for AEs and from second-line TNF inhibitor for AEs.
The proportion lost to treatment at 3-month time points in each category was read from the graphs in the BSRBR submission and the absolute number lost calculated using N = 995 for first-line withdrawal through lack of efficacy and N = 1,882 for first-line withdrawal due to AEs. The proportion of patients withdrawing for any reason was then estimated and the proportion remaining in treatment plotted (data points in Figure 155). A Weibull distribution (time in years) was fitted to the data [scale parameter (lambda) 0.441555; standard error (SE) 0.00958300], shape parameter (gamma) 0.7008 (SE 0.033681) labelled BSRBR Weibull fit in Figure 155 (extrapolation to 25 years is shown in the inset).
FIGURE 155.
Continuation in second-line TNF inhibitor.

Comparison with manufacturers’ submissions
The Schering-Plough Ltd (infliximab) submission165 provided Weibull parameters for treatment withdrawal that were also based on BSRBR data; the parameters are shown below.
Log(scale) 3.529 (time in months)
Log(shape) –0.19 (time in months)
Assuming log(scale) in the table above refers to ‘log β’ where β = (1/λ) ^ [1/γ], and survival = exp[–(t × β) ^ γ], then lambda = 0.054 and gamma = 0.827 and the fitted curve labelled Schering-Plough Ltd in Figure 155 is generated (and can be seen to be very similar to the review group’s fit).
The Wyeth Pharmaceuticals submission226 modelled withdrawal from treatment using a ‘shared frailty’ model and this is also represented in Figure 155.
Withdrawal from second-line treatment according to tumour necrosis factor inhibitor
According to analysis of Danish Registry for Biologic Therapies in Rheumatology (DANBIO) data withdrawal from first line TNF inhibitor occurs at rates that are statistically significantly different between the three TNF inhibitors, Table 112 provides the reported HRs and 95% CIs (Hetland et al. 227).
| Comparison | HR | HR 95% CIs | Weibull fit HR |
|---|---|---|---|
| ADA vs ETN | 1.35 | 1.13 to 1.61 | 1.28 |
| IFX vs ETN | 2.10 | 1.70 to 2.59 | 1.80 |
| IFX vs ADA | 1.56 | 1.26 to 1.94 | 1.41 |
It may be reasonable to expect that similar differences might apply for second line TNF inhibitors.
Data were extracted from the K–M graph for each TNF inhibitor published for the Danish registry. 227 These were fitted with Weibull distributions (Figure 156) and survivors then combined for each drug (according to number of patients given each TNF inhibitor) so as to provide overall survival (N = 2,935), and this in turn was fitted with a Weibull distribution.
FIGURE 156.
Withdrawal from first-line TNF inhibitors (DANBIO data with Weibull fits).
The shape parameters for the Weibull fits were similar and therefore it was considered reasonable to average these and apply the same shape parameter for each drug and for overall survival. Because the BSRBR first-line withdrawal data were derived using equal numbers of patients (∼ 4,000) treated with each TNF inhibitor the shape parameters for the DANBIO data were combined to give an unweighted average. Using this ‘common’ shape parameter (0.5595) the data were again fitted with Weibull distributions, providing the fits shown in Figure 156; the overall survival then assumed that equal numbers received each of the three TNF inhibitors; this allows a comparison of DANBIO and BSRBR first-line withdrawal data (see below).
The HRs (ratio of scale parameters) for comparison of TNF inhibitors using these Weibull fits were within the HR 95% CIs reported for the Danish registry data (note: contact with the lead author confirmed that the published HRs were reversed for ADA versus ETN and IFX versus ADA; this has been corrected in Table 112. Relative to all patients (equal mixture) the HRs for each TNF inhibitor were calculated as follows: ETN versus all, 0.751; ADA versus all, 0.958; IFX versus all, 1.353.
When these HRs are applied to the Weibull fit of BSRBR data163 for continuation of second-line treatment, the drug-specific rates of withdrawal over 25 years are as shown in Figure 157.
FIGURE 157.
Estimated continuation of second-line treatment according to TNF inhibitor.
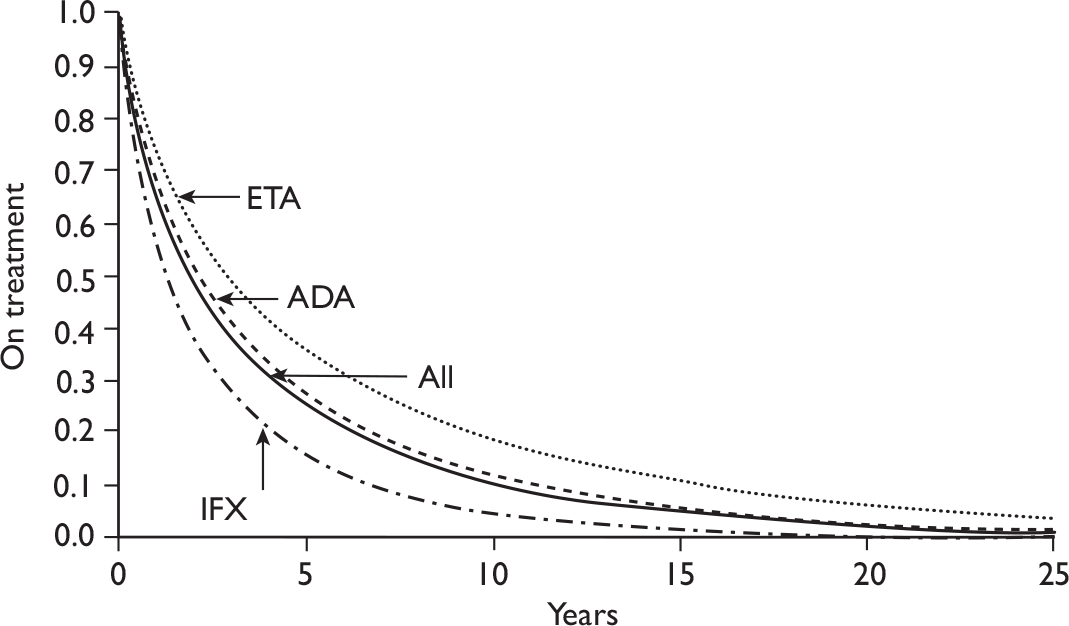
The Danish National Registry and the British Society for Rheumatology Biologics Registry withdrawal rates from first-line tumour necrosis factor inhibitor
Data for first-line withdrawal were extracted from the UK BSRBR submission163 and fitted with Weibull distributions in which the shape parameter was or was not fixed to that for overall survival derived from the DANBIO data (0.5595, see above). Extrapolations to 25 years were compared between UK and Danish first-line treatments and between first-line and second-line treatments (Figure 158).
FIGURE 158.
Modelled survival in treatment with first- and second-line TNF inhibitors.
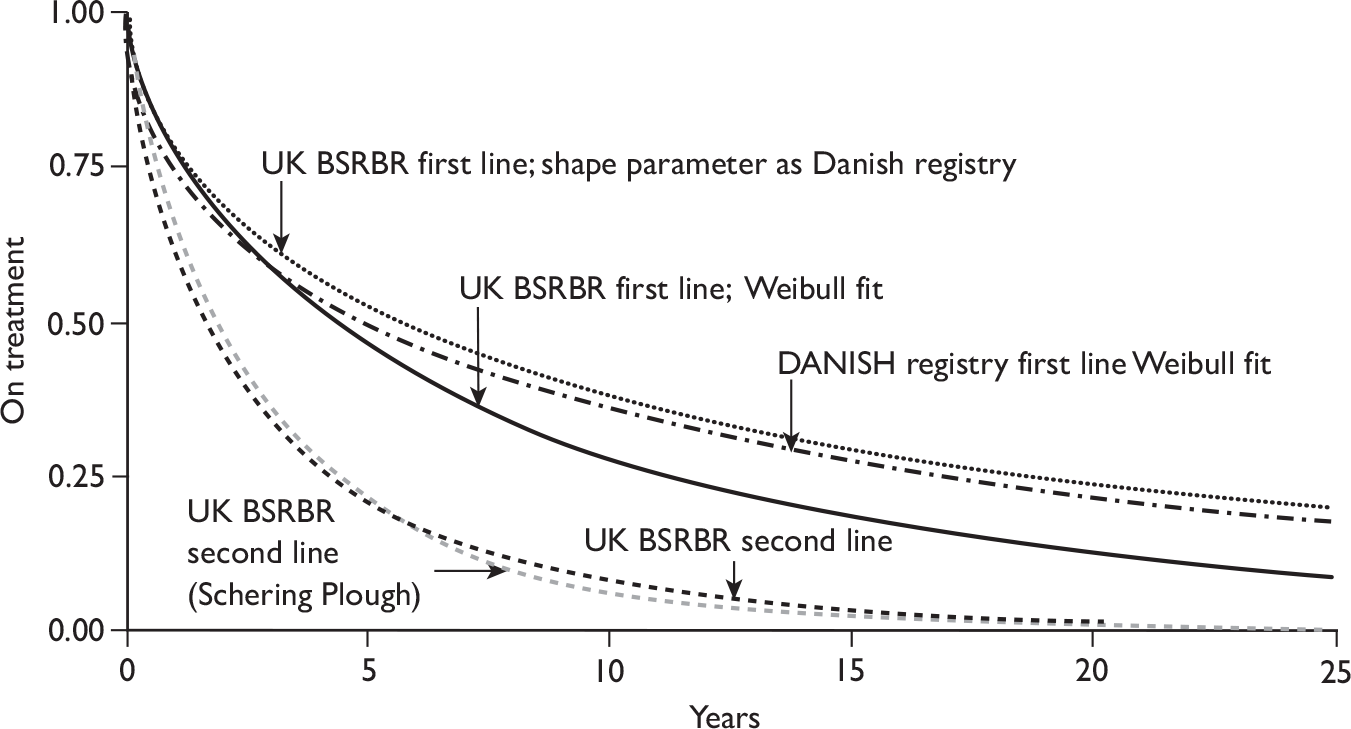
Additional sources of evidence
Several additional sources were identified with potentially relevant information on withdrawal from the different TNF inhibitors; these are listed in Table 113.
| Study country | Population (n) TNF inhibitors |
First-line/second-line withdrawal | Findings | Comment |
|---|---|---|---|---|
| DANBIO; Hetland 2009;227 Denmark |
RA [National registry] (2,935) IFX, ETN and ADA |
Withdrawal from first line | Withdrawal more likely for IFX than ADA and for ADA than ETN | Separate data for withdrawal from first-line treatment with each TNF inhibitor |
| Finckh 2006;231 Switzerland |
RA only (1,198) IFX, ETN and ADA |
Mixed, not differentiated | No difference between IFX, ETN and ADA after adjustment for RF positivity, baseline DAS28, HAQ, failure of previous TNF inhibitor | Not useful for first-line or second-line withdrawal for RA |
| Duclos 2006;232 France |
Mix of RA [57%] and SpA [one centre] (770) IFX, ETN and ADA |
Mixed, not differentiated | No difference between TNF inhibitors. Retention longer for first line vs second line (HR 2.17; 95% CI 1.82 to 2.58, p < 0.0001) and better if concomitant DMARD | Not useful for first-line or second-line withdrawal for RA |
| Gomez-Reino 2006;108 Spain |
Mixed [68% RA] (4,706) IFX, ETN and ADA |
Both first-line and second-line differentiated | Retention longer for first line vs second line, and for second line vs third line. Second-line retention better if first-line failure was for AEs rather than lack of efficacy. Retention n IFX influenced by availability of ETN. Second-line retention better after switch to ETN from IFX than if to switch to IFX from ETN | Not useful for first-line or second-line withdrawal for RA |
| Vollenhoven 2005;233 Sweden |
‘Rheumatic diseases’ (128) IFX, ETN and ADA |
Second-line withdrawal for lack of efficacy | Less withdrawal from ETN than from IFX; ADA data immature | Not useful for first-line or second-line withdrawal for RA |
| Kristensen 2006;234 Sweden |
RA only (1,161) IFX and ETN |
First line; separate analyses according to ± concomitant DMARD and ± MTX |
Retention better with ETN than IFX Better retention if patient also receives MTX |
K–M data for three subgroups; overall withdrawal from first line with each TNF inhibitor difficult to compute |
| Zink 2005;228 Germany |
RA (854) IFX and ETN |
First line | No statistically significant difference in retention at 12 months: 65.4% for IFX and 68.6% for ETN | Data too immature to draw conclusions |
| Curtis 2009;228 USA |
RA (11,903) IFX, ETN and ADA |
Withdrawal from first line or dose escalation |
Hazard ratio for switch from TNF inhibitor (to other DMARD) or dose escalation: IFX vs ETN 6.29 (5.82 to 6.81) ADA vs ETN 1.18 (1.08 to 1.30) |
Combines discontinuation and dose escalation |
| Wolfe and Michaud 2007;230 USA |
RA (4,915) IFX, ETN and ADA |
Mixed and second line |
Median continuation (years): For first and second line: ADA 3.0, ETN 5.5, IFX 4.5. For second line: ADA 2.0, ETN 2.5, IFX 2.5 |
K–M plots not supplied |
Except for the DANBIO registry data227 the studies do not provide the information required (K–M plots) to easily compare withdrawal rates between different TNF inhibitors, the main reasons being mixed analysis of first- and second-line withdrawal, mixed populations [rheumatoid arthritis (RA) only a subpopulation, or outcome measure a combination of switching and of dose escalation. 218 The German study229 does provide information for ETN and ADA but follow-up was insufficient to see any difference developing. Wolfe and Michaud230 reported median survival on second-line TNF inhibitor. These results (Table 114) compare reasonably well with the median survival for each TNF inhibitor calculated as described above and shown in Figure 157.
| TNF inhibitor | Median survival second line (years) | |
|---|---|---|
| Reported by Wolfe and Michaud 2007230 | Estimated (as Figure 157) | |
| ADA | 2 | 2.02 |
| ETN | 2.5 | 2.86 |
| IFX | 2.5 | 1.24 |
| Alla | 2.36 | 1.90 |
In general, the data from these studies are consistent with the DANBIO study in that continuation with ETN appears to be superior to that with infliximab and continuation with ADA treatment being intermediate.
Appendix 13 Scatter plots for comparisons among biologics in the reference case
This appendix (Figure 159) contains the cost-effectiveness scatter plots for the 10 comparisons between biologic treatments in the reference case. The comparisons between biologics and conventional DMARDs are shown in Chapter 4, Reference case.
FIGURE 159.
Cost-effectiveness scatter plots for comparisons between biologic treatments in the reference case. Diff, difference.
Appendix 14 Scenario analyses
The following scenarios were considered in addition to the reference case analysis. The section headings correspond to the abbreviated descriptions used in Chapter 4, Scenario analysis. In each case, any parameters not mentioned in the description of the scenario remain as in the reference case analysis.
Vary time on tumour necrosis factors inhibitors
In this case, the time to withdrawing TNF inhibitors treatments was changed to give the same relative risk as for their use as first biologic agents. The b parameters from Table 80 (for reference case) were changed as follows:
| Treatment | Reference case b parameter (point estimate) | New b parameter (point estimate) |
|---|---|---|
| ADA | 3.211 | 3.413 |
| ETN | 3.211 | 4.831 |
| IFX | 3.211 | 2.086 |
Results were as follows:
| Treatment | Mean cost | 95% credible interval | Mean QALY | 95% credible interval | ||
|---|---|---|---|---|---|---|
| ADA | 75,900 | 69,800 | 82,200 | 2.92 | –2.07 | 7.92 |
| ETN | 82,700 | 76,000 | 89,300 | 3.01 | –1.86 | 7.92 |
| IFX | 67,400 | 60,900 | 73,800 | 2.62 | –2.54 | 7.73 |
| RTX | 69,400 | 62,600 | 76,200 | 3.10 | –1.77 | 8.01 |
| ABT | 93,000 | 86,300 | 100,000 | 3.28 | –1.52 | 8.02 |
| DMARDs | 49,000 | 43,300 | 55,100 | 2.13 | –3.25 | 7.46 |
| Comparison | Diff cost | 95% credible interval | Diff QALY | 95% credible interval | ||
| ADA–DMARDs | 26,900 | 25,100 | 28,600 | 0.78 | 0.34 | 1.28 |
| ETN–DMARDs | 33,700 | 31,700 | 35,900 | 0.88 | 0.38 | 1.47 |
| IFX–DMARDs | 18,400 | 15,100 | 20,700 | 0.49 | 0.21 | 0.82 |
| RTX–DMARDs | 20,400 | 17,500 | 23,500 | 0.96 | 0.42 | 1.60 |
| ABT–DMARDs | 44,100 | 41,300 | 46,900 | 1.14 | 0.51 | 1.86 |
| ADA–RTX | 6,500 | 3,200 | 9,800 | –0.18 | –0.47 | 0.05 |
| ETN–RTX | 13,300 | 9,900 | 16,800 | –0.09 | –0.38 | 0.16 |
| IFX–RTX | –2,000 | –5,900 | 1,600 | –0.48 | –0.87 | –0.16 |
| ABT–RTX | 23,600 | 19,600 | 27,500 | 0.18 | –0.09 | 0.50 |
| ADA–ABT | –17,200 | –20,300 | –14,100 | –0.36 | –0.72 | –0.10 |
| ETN–ABT | –10,400 | –13,500 | –7,100 | –0.27 | –0.59 | –0.03 |
| IFX–ABT | –25,600 | –29,900 | –22,100 | –0.65 | –1.12 | –0.26 |
| ADA–ETN | –6,800 | –9,400 | –4,200 | –0.09 | –0.32 | 0.10 |
| ADA–IFX | 8,400 | 5,700 | 12,000 | 0.29 | 0.07 | 0.56 |
| ETN–IFX | 15,300 | 12,300 | 18,900 | 0.39 | 0.14 | 0.72 |
| Comparison | ICER | 95% credible interval | Proportion of cases cost-effective at | ||
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| ADA–DMARDs | 34,300 | 20,900 | 79,000 | 0.01 | 0.31 |
| ETN–DMARDs | 38,400 | 23,200 | 87,400 | 0.00 | 0.18 |
| IFX–DMARDs | 37,700 | 22,100 | 90,300 | 0.01 | 0.20 |
| RTX–DMARDs | 21,200 | 12,800 | 48,400 | 0.39 | 0.84 |
| ABT–DMARDs | 38,500 | 23,400 | 86,600 | 0.00 | 0.17 |
| ADA–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ETN–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| IFX–TX | 4,100 | RTX | 16,000 | 0.01 | 0.00 |
| ABT–RTX | 131,800 | 48,400 | RTX | 0.00 | 0.00 |
| ADA–ABT | 47,700 | 23,500 | 177,100 | 0.99 | 0.90 |
| ETN–ABT | 38,900 | 16,300 | 308,500 | 0.94 | 0.73 |
| IFX–ABT | 39,100 | 22,400 | 95,800 | 0.99 | 0.82 |
| ADA–ETN | 72,800 | 20,400 | ADA | 0.98 | 0.88 |
| ADA–IFX | 28,700 | 13,900 | 104,800 | 0.16 | 0.51 |
| ETN–IFX | 39,300 | 21,300 | 110,500 | 0.02 | 0.20 |
Same time on all biologics
In this scenario, the distribution of long-term survival time on all biologics was set to the value used for TNF inhibitors in the reference case. The results were as follows:
| Treatment | Mean cost | 95% credible interval | Mean QALY | 95% credible interval | ||
|---|---|---|---|---|---|---|
| ADA | 74,800 | 68,700 | 81,000 | 2.88 | –2.13 | 7.87 |
| ETN | 75,100 | 68,800 | 81,400 | 2.81 | –2.26 | 7.84 |
| IFX | 73,000 | 66,000 | 79,900 | 2.80 | –2.23 | 7.82 |
| RTX | 63,700 | 57,900 | 69,900 | 2.83 | –2.15 | 7.86 |
| ABT | 82,000 | 75,700 | 88,600 | 2.97 | –1.99 | 7.85 |
| DMARDs | 49,000 | 43,300 | 54,900 | 2.13 | –3.23 | 7.46 |
| Comparison | Diff cost | 95% credible interval | Diff QALY | 95% credible interval | ||
| ADA–DMARDs | 25,800 | 24,100 | 27,600 | 0.75 | 0.33 | 1.21 |
| ETN–DMARDs | 26,100 | 24,400 | 27,900 | 0.68 | 0.28 | 1.12 |
| IFX–DMARDs | 24,000 | 19,300 | 26,800 | 0.67 | 0.29 | 1.12 |
| RTX–DMARDs | 14,700 | 13,600 | 15,900 | 0.70 | 0.30 | 1.15 |
| ABT–DMARDs | 33,000 | 30,800 | 35,400 | 0.84 | 0.37 | 1.37 |
| ADA–RTX | 11,100 | 9,200 | 13,100 | 0.05 | –0.13 | 0.25 |
| ETN–RTX | 11,400 | 9,500 | 13,500 | –0.02 | –0.21 | 0.15 |
| IFX–RTX | 9,400 | 4,700 | 12,300 | –0.03 | –0.22 | 0.14 |
| ABT–RTX | 18,400 | 15,900 | 20,800 | 0.14 | –0.05 | 0.36 |
| ADA–ABT | –7,200 | –10,000 | –4,500 | –0.09 | –0.31 | 0.11 |
| ETN–ABT | –6,900 | –9,800 | –4,000 | –0.16 | –0.40 | 0.03 |
| IFX–ABT | –9,000 | –14,100 | –5,500 | –0.17 | –0.40 | 0.02 |
| ADA–ETN | –300 | –2,700 | 2,200 | 0.08 | –0.10 | 0.28 |
| ADA–IFX | 1,800 | –1,500 | 6,500 | 0.08 | –0.10 | 0.29 |
| ETN–IFX | 2,100 | –1,100 | 7,000 | 0.01 | –0.17 | 0.18 |
| Comparison | ICER | 95% credible interval | Proportion of cases cost-effective at | ||
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| ADA–DMARDs | 34,400 | 20,900 | 78,400 | 0.01 | 0.31 |
| ETN–DMARDs | 38,700 | 23,300 | 91,700 | 0.01 | 0.17 |
| IFX–DMARDs | 35,900 | 21,200 | 81,100 | 0.02 | 0.24 |
| RTX–DMARDs | 21,100 | 12,600 | 49,100 | 0.41 | 0.84 |
| ABT–DMARDs | 39,500 | 23,800 | 89,700 | 0.00 | 0.15 |
| ADA–RTX | 206,000 | 44,700 | RTX | 0.00 | 0.00 |
| ETN–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| IFX–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ABT–RTX | 131,200 | 49,700 | RTX | 0.00 | 0.00 |
| ADA–ABT | 84,100 | 22,500 | ADA | 0.99 | 0.92 |
| ETN–ABT | 42,700 | 15,900 | ETN | 0.92 | 0.76 |
| IFX–ABT | 53,700 | 20,800 | IFX | 0.98 | 0.88 |
| ADA–ETN | ADA | Not meaningful | 0.82 | 0.82 | |
| ADA–IFX | 21,600 | Not meaningful | 0.49 | 0.59 | |
| ETN–IFX | 351,500 | Not meaningful | 0.19 | 0.25 | |
Rituximab cycle time 6 months
In this case, it was assumed that cycles of RTX would be given every 6 months. The assumption was that withdrawal rates per cycle would be maintained from the reference case. The results are as follows:
| Treatment | Mean cost | 95% credible interval | Mean QALY | 95% credible interval | ||
|---|---|---|---|---|---|---|
| ADA | 74,800 | 68,500 | 81,100 | 2.89 | –2.16 | 7.81 |
| ETN | 75,100 | 69,000 | 81,500 | 2.80 | –2.25 | 7.80 |
| IFX | 73,000 | 65,800 | 79,900 | 2.80 | –2.27 | 7.81 |
| RTX | 74,800 | 67,200 | 82,400 | 2.93 | –2.06 | 7.89 |
| ABT | 93,000 | 86,400 | 100,100 | 3.28 | –1.52 | 8.05 |
| DMARDs | 49,000 | 43,400 | 55,000 | 2.13 | –3.25 | 7.46 |
| Comparison | Diff cost | 95% credible interval | Diff QALY | 95% credible interval | ||
| ADA–DMARDs | 25,800 | 24,000 | 27,600 | 0.75 | 0.33 | 1.24 |
| ETN–DMARDs | 26,100 | 24,300 | 27,900 | 0.67 | 0.27 | 1.11 |
| IFX–DMARDs | 24,000 | 19,200 | 26,800 | 0.67 | 0.30 | 1.12 |
| RTX–DMARDs | 25,800 | 21,800 | 30,000 | 0.79 | 0.33 | 1.34 |
| ABT–DMARDs | 44,000 | 41,300 | 46,800 | 1.15 | 0.50 | 1.88 |
| ADA–RTX | –18 | –4,500 | 4,500 | –0.04 | –0.29 | 0.18 |
| ETN–RTX | 300 | –4,100 | 4,700 | –0.12 | –0.38 | 0.09 |
| IFX–RTX | –1,800 | –7,500 | 3,200 | –0.12 | –0.38 | 0.08 |
| ABT–RTX | 18,200 | 13,200 | 23,100 | 0.35 | 0.07 | 0.73 |
| ADA–ABT | –18,200 | –21,300 | –15,200 | –0.39 | –0.78 | –0.12 |
| ETN–ABT | –17,900 | –21,100 | –14,700 | –0.47 | –0.87 | –0.18 |
| IFX–ABT | –20,000 | –25,400 | –16,200 | –0.48 | –0.88 | –0.16 |
| ADA–ETN | –300 | –2,800 | 2,100 | 0.08 | –0.09 | 0.29 |
| ADA–IFX | 1,800 | –1,500 | 6,500 | 0.08 | –0.11 | 0.29 |
| ETN–IFX | 2,100 | –1,200 | 7,200 | 0.00 | –0.18 | 0.19 |
| Comparison | ICER | 95% credible interval | Proportion of cases cost-effective at | ||
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| ADA–DMARDs | 34,300 | 20,600 | 78,900 | 0.02 | 0.31 |
| ETN–DMARDs | 38,900 | 23,400 | 95,200 | 0.00 | 0.17 |
| IFX–DMARDs | 35,900 | 21,500 | 81,700 | 0.02 | 0.26 |
| RTX–DMARDs | 32,600 | 19,900 | 74,300 | 0.03 | 0.37 |
| ABT–DMARDs | 38,400 | 23,300 | 88,800 | 0.00 | 0.17 |
| ADA–RTX | 430 | Not meaningful | 0.36 | 0.35 | |
| ETN–RTX | RTX | Not meaningful | 0.12 | 0.10 | |
| IFX–RTX | 14,700 | Not meaningful | 0.40 | 0.28 | |
| ABT–RTX | 51,500 | 25,400 | 229,200 | 0.00 | 0.07 |
| ADA–ABT | 46,300 | 23,400 | 150,600 | 1.00 | 0.90 |
| ETN–ABT | 37,800 | 20,300 | 95,700 | 0.98 | 0.77 |
| IFX–ABT | 42,000 | 22,500 | 117,700 | 0.99 | 0.86 |
| ADA–ETN | ADA | Not meaningful | 0.83 | 0.84 | |
| ADA–IFX | 21,700 | Not meaningful | 0.48 | 0.59 | |
| ETN–IFX | 1,325,400 | Not meaningful | 0.18 | 0.23 | |
Rituximab cycle time 11.6 months
In this case, it was assumed that cycles of RTX would be given every 11.6 months, which was the observed mean time in the REFLEX extension study (Roche submission, p. 200). The assumption was that withdrawal rates per cycle would be maintained from the reference case. The results were as follows:
| Treatment | Mean cost | 95% credible interval | Mean QALY | 95% credible interval | ||
|---|---|---|---|---|---|---|
| ADA | 74,800 | 68,600 | 81,200 | 2.89 | –2.15 | 7.84 |
| ETN | 75,100 | 68,900 | 81,500 | 2.81 | –2.23 | 7.87 |
| IFX | 73,000 | 66,000 | 80,000 | 2.80 | –2.30 | 7.83 |
| RTX | 61,700 | 55,800 | 67,900 | 3.25 | –1.58 | 8.11 |
| ABT | 93,100 | 86,100 | 100,100 | 3.28 | –1.53 | 8.09 |
| DMARDs | 49,000 | 43,300 | 55,100 | 2.13 | –3.27 | 7.49 |
| Comparison | Diff cost | 95% credible interval | Diff QALY | 95% credible interval | ||
| ADA–DMARDs | 25,800 | 24,100 | 27,600 | 0.75 | 0.32 | 1.24 |
| ETN–DMARDs | 26,100 | 24,300 | 28,000 | 0.67 | 0.29 | 1.10 |
| IFX–DMARDs | 24,000 | 19,300 | 26,800 | 0.67 | 0.28 | 1.10 |
| RTX–DMARDs | 12,700 | 11,000 | 14,500 | 1.11 | 0.49 | 1.81 |
| ABT–DMARDs | 44,000 | 41,300 | 46,800 | 1.15 | 0.52 | 1.89 |
| ADA–RTX | 13,100 | 10,800 | 15,500 | –0.36 | –0.74 | –0.07 |
| ETN–RTX | 13,400 | 10,900 | 15,700 | –0.44 | –0.84 | –0.14 |
| IFX–RTX | 11,300 | 6,600 | 14,600 | –0.44 | –0.85 | –0.14 |
| ABT–RTX | 31,300 | 28,200 | 34,500 | 0.04 | –0.27 | 0.33 |
| ADA–ABT | –18,300 | –21,400 | –15,200 | –0.39 | –0.76 | –0.11 |
| ETN–ABT | –17,900 | –21,100 | –14,600 | –0.48 | –0.89 | –0.17 |
| IFX–ABT | –20,000 | –25,400 | –16,100 | –0.48 | –0.89 | –0.17 |
| ADA–ETN | –300 | –2,700 | 2,100 | 0.08 | –0.09 | 0.28 |
| ADA–IFX | 1,800 | –1,600 | 6,300 | 0.09 | –0.09 | 0.29 |
| ETN–IFX | 2,100 | –1,400 | 6,900 | 0.00 | –0.18 | 0.20 |
| Comparison | ICER | 95% credible interval | Proportion of cases cost-effective at | ||
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| ADA–DMARDs | 34,200 | 20,800 | 79,900 | 0.02 | 0.32 |
| ETN–DMARDs | 38,800 | 23,300 | 90,500 | 0.00 | 0.17 |
| IFX–DMARDs | 35,900 | 21,400 | 84,800 | 0.01 | 0.25 |
| RTX–DMARDs | 11,400 | 6,800 | 25,600 | 0.92 | 0.98 |
| ABT–DMARDs | 38,400 | 23,300 | 85,200 | 0.00 | 0.17 |
| ADA–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ETN–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| IFX–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ABT–RTX | 861,100 | 95,700 | RTX | 0.00 | 0.00 |
| ADA–ABT | 46,400 | 23,600 | 150,400 | 1.00 | 0.90 |
| ETN–ABT | 37,800 | 20,100 | 103,800 | 0.98 | 0.77 |
| IFX–ABT | 41,800 | 22,600 | 120,500 | 0.99 | 0.85 |
| ADA–ETN | ADA | Not meaningful | 0.83 | 0.84 | |
| ADA–IFX | 20,700 | Not meaningful | 0.51 | 0.60 | |
| ETN–IFX | 591,000 | Not meaningful | 0.18 | 0.24 | |
Poor late disease-modifying antirheumatic drugs (additional analysis)
In this scenario, the efficacy of conventional DMARDs taken after biologic therapy was reduced. HAQ multipliers were inferred from the Abbott and Roche industry submissions, and the lower of these figures (0.085) was taken. Preserving a + b = 1.22 from the BRAM reference case for LEF gave a = 0.104, b = 1.116. These values were then used for all conventional DMARDs. The results were as follows:
| Treatment | Mean cost | 95% credible interval | Mean QALY | 95% credible interval | ||
|---|---|---|---|---|---|---|
| ADA | 76,400 | 69,600 | 83,100 | 2.30 | –3.02 | 7.59 |
| ETN | 76,600 | 70,000 | 83,300 | 2.23 | –3.06 | 7.55 |
| IFX | 74,600 | 67,100 | 81,800 | 2.22 | –3.12 | 7.51 |
| RTX | 70,700 | 63,800 | 78,000 | 2.61 | –2.58 | 7.72 |
| ABT | 94,400 | 87,300 | 101,800 | 2.76 | –2.33 | 7.81 |
| DMARDs | 51,000 | 44,900 | 57,300 | 1.40 | –4.38 | 7.15 |
| Comparison | Diff cost | 95% credible interval | Diff QALY | 95% credible interval | ||
| ADA–DMARDs | 25,400 | 23,700 | 27,200 | 0.90 | 0.41 | 1.47 |
| ETN–DMARDs | 25,600 | 23,900 | 27,400 | 0.82 | 0.36 | 1.33 |
| IFX–DMARDs | 23,600 | 19,000 | 26,400 | 0.82 | 0.36 | 1.35 |
| RTX–DMARDs | 19,700 | 16,900 | 22,400 | 1.21 | 0.53 | 1.95 |
| ABT–DMARDs | 43,400 | 40,700 | 46,200 | 1.35 | 0.62 | 2.17 |
| ADA–RTX | 5,700 | 2,500 | 8,800 | –0.30 | –0.66 | –0.03 |
| ETN–RTX | 6,000 | 2,800 | 9,100 | –0.38 | –0.77 | –0.08 |
| IFX–RTX | 3,900 | –1,100 | 7,800 | –0.39 | –0.77 | –0.09 |
| ABT–RTX | 23,700 | 19,900 | 27,500 | 0.15 | –0.14 | 0.48 |
| ADA–ABT | –18,100 | –21,300 | –15,000 | –0.45 | –0.83 | –0.17 |
| ETN–ABT | –17,800 | –21,000 | –14,600 | –0.53 | –0.96 | –0.21 |
| IFX–ABT | –19,800 | –25,100 | –16,000 | –0.54 | –0.94 | –0.20 |
| ADA–ETN | –300 | –2,600 | 2,100 | 0.08 | –0.10 | 0.28 |
| ADA–IFX | 1,800 | –1,500 | 6,500 | 0.09 | –0.09 | 0.29 |
| ETN–IFX | 2,000 | –1,300 | 7,000 | 0.01 | –0.17 | 0.20 |
| Comparison | ICER | 95% credible interval | Proportion of cases cost-effective at | ||
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| ADA–DMARDs | 28,100 | 17,200 | 62,300 | 0.10 | 0.57 |
| ETN–DMARDs | 31,100 | 19,200 | 70,400 | 0.04 | 0.43 |
| IFX–DMARDs | 28,800 | 17,200 | 63,400 | 0.08 | 0.54 |
| RTX–DMARDs | 16,300 | 10,100 | 36,100 | 0.73 | 0.94 |
| ABT–DMARDs | 32,100 | 20,000 | 71,600 | 0.02 | 0.39 |
| ADA–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ETN–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| IFX–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ABT–RTX | 158,600 | 51,500 | RTX | 0.00 | 0.00 |
| ADA–ABT | 40,100 | 21,400 | 106,200 | 0.99 | 0.82 |
| ETN–ABT | 33,500 | 18,400 | 81,400 | 0.95 | 0.67 |
| IFX–ABT | 36,900 | 20,500 | 95,800 | 0.98 | 0.76 |
| ADA–ETN | ADA | Not meaningful | 0.83 | 0.84 | |
| ADA–IFX | 20,600 | Not meaningful | 0.50 | 0.61 | |
| ETN–IFX | 316,000 | Not meaningful | 0.17 | 0.23 | |
Health Assessment Questionnaire change on biologics
In this scenario, a deterioration of 0.03/year in HAQ was assumed on biologic treatments. This was modelled as a mean time between 0.125-unit increases of 4 years. For each treatment separately, this figure was given a normal distribution with a SD of 0.4 years. The results were as follows:
| Treatment | Mean cost | 95% credible interval | Mean QALY | 95% credible interval | ||
|---|---|---|---|---|---|---|
| ADA | 75,500 | 69,200 | 81,900 | 2.53 | –2.56 | 7.75 |
| ETN | 75,800 | 69,500 | 82,200 | 2.44 | –2.69 | 7.74 |
| IFX | 73,700 | 66,500 | 80,700 | 2.45 | –2.71 | 7.73 |
| RTX | 70,400 | 63,600 | 77,500 | 2.56 | –2.50 | 7.81 |
| ABT | 93,900 | 86,900 | 101,200 | 2.80 | –2.17 | 7.91 |
| DMARDs | 49,100 | 43,500 | 54,700 | 2.09 | –3.17 | 7.50 |
| Comparison | Diff cost | 95% credible interval | Diff QALY | 95% credible interval | ||
| ADA–DMARDs | 26,400 | 24,600 | 28,400 | 0.43 | 0.16 | 0.78 |
| ETN–DMARDs | 26,700 | 24,800 | 28,700 | 0.35 | 0.12 | 0.64 |
| IFX–DMARDs | 24,600 | 20,300 | 27,300 | 0.36 | 0.12 | 0.66 |
| RTX–DMARDs | 21,300 | 18,200 | 24,400 | 0.46 | 0.15 | 0.85 |
| ABT–DMARDs | 44,800 | 42,000 | 47,700 | 0.71 | 0.29 | 1.22 |
| ADA–RTX | 5,100 | 1,600 | 8,500 | –0.03 | –0.30 | 0.20 |
| ETN–RTX | 5,400 | 2,000 | 8,700 | –0.11 | –0.41 | 0.11 |
| IFX–RTX | 3,300 | –2,000 | 7,400 | –0.11 | –0.39 | 0.12 |
| ABT–RTX | 23,500 | 19,600 | 27,400 | 0.24 | –0.03 | 0.61 |
| ADA–ABT | –18,400 | –21,400 | –15,300 | –0.28 | –0.59 | –0.02 |
| ETN–ABT | –18,100 | –21,100 | –15,000 | –0.36 | –0.72 | –0.09 |
| IFX–ABT | –20,100 | –25,000 | –16,300 | –0.35 | –0.72 | –0.08 |
| ADA–ETN | –300 | –2,700 | 2,100 | 0.08 | –0.11 | 0.32 |
| ADA–IFX | 1,800 | –1,500 | 6,300 | 0.07 | –0.12 | 0.29 |
| ETN–IFX | 2,100 | –1,300 | 6,700 | –0.01 | –0.21 | 0.18 |
| Comparison | ICER | 95% credible interval | Proportion of cases cost-effective at | ||
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| ADA–DMARDs | 61,300 | 33,600 | 168,600 | 0.00 | 0.01 |
| ETN–DMARDs | 76,300 | 42,500 | 228,200 | 0.00 | 0.00 |
| IFX–DMARDs | 68,900 | 36,200 | 200,000 | 0.00 | 0.00 |
| RTX–DMARDs | 46,000 | 24,600 | 134,400 | 0.00 | 0.09 |
| ABT–DMARDs | 63,300 | 36,700 | 151,700 | 0.00 | 0.00 |
| ADA–RTX | RTX | Not meaningful | 0.02 | 0.06 | |
| ETN–RTX | RTX | Not meaningful | 0.00 | 0.01 | |
| IFX–RTX | RTX | Not meaningful | 0.05 | 0.05 | |
| ABT–RTX | 96,400 | 38,900 | RTX | 0.00 | 0.00 |
| ADA–ABT | 66,500 | 29,400 | 718,000 | 1.00 | 0.97 |
| ETN–ABT | 50,600 | 24,300 | 205,800 | 0.99 | 0.91 |
| IFX–ABT | 57,600 | 27,400 | 250,500 | 1.00 | 0.96 |
| ADA–ETN | ADA | Not meaningful | 0.78 | 0.80 | |
| ADA–IFX | 24,300 | Not meaningful | 0.46 | 0.55 | |
| ETN–IFX | IFX | Not meaningful | 0.21 | 0.26 | |
Adverse event costs included
Additional annual costs based on the Bristol-Myers Squibb LTD submission as follows:
| Treatment | Additional cost (£) |
|---|---|
| ADA | 117.82 |
| ETN | 224.87 |
| IFX | 162.02 |
| RTX | 273.51 |
| ABT | 110.16 |
When these were included, the results were as follows:
| Treatment | Mean cost | 95% credible interval | Mean QALY | 95% credible interval | ||
|---|---|---|---|---|---|---|
| ADA | 75,100 | 69,200 | 81,400 | 2.89 | –2.12 | 7.87 |
| ETN | 75,700 | 69,400 | 82,200 | 2.80 | –2.21 | 7.84 |
| IFX | 73,500 | 66,500 | 80,300 | 2.80 | –2.24 | 7.82 |
| RTX | 70,700 | 63,800 | 77,700 | 3.10 | –1.78 | 7.95 |
| ABT | 93,500 | 86,600 | 100,600 | 3.28 | –1.46 | 8.05 |
| DMARDs | 49,000 | 43,300 | 54,900 | 2.13 | –3.27 | 7.46 |
| Comparison | Diff cost | 95% credible interval | Diff QALY | 95% credible interval | ||
| ADA–DMARDs | 26,100 | 24,500 | 27,900 | 0.75 | 0.33 | 1.23 |
| ETN–DMARDs | 26,800 | 24,900 | 28,700 | 0.67 | 0.30 | 1.10 |
| IFX–DMARDs | 24,500 | 19,800 | 27,300 | 0.67 | 0.29 | 1.12 |
| RTX–DMARDs | 21,700 | 18,600 | 24,700 | 0.96 | 0.41 | 1.61 |
| ABT–DMARDs | 44,500 | 41,700 | 47,200 | 1.15 | 0.52 | 1.88 |
| ADA–RTX | 4,500 | 1,200 | 8,000 | –0.21 | –0.52 | 0.03 |
| ETN–RTX | 5,100 | 1,600 | 8,700 | –0.29 | –0.63 | –0.04 |
| IFX–RTX | 2,800 | –2,500 | 7,000 | –0.30 | –0.62 | –0.05 |
| ABT–RTX | 22,800 | 18,800 | 26,800 | 0.18 | –0.10 | 0.50 |
| ADA–ABT | –18,300 | –21,500 | –15,300 | –0.39 | –0.77 | –0.12 |
| ETN–ABT | –17,700 | –21,100 | –14,300 | –0.47 | –0.88 | –0.17 |
| IFX–ABT | –20,000 | –25,200 | –16,100 | –0.48 | –0.88 | –0.17 |
| ADA–ETN | –600 | –3,200 | 1,800 | 0.08 | –0.09 | 0.29 |
| ADA–IFX | 1,600 | –1,600 | 6,400 | 0.09 | –0.10 | 0.29 |
| ETN–IFX | 2,200 | –1,100 | 7,100 | 0.00 | –0.17 | 0.19 |
| Comparison | ICER | 95% credible interval | Proportion of cases cost-effective at | ||
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| ADA–DMARDs | 34,700 | 21,200 | 80,200 | 0.01 | 0.29 |
| ETN–DMARDs | 39,900 | 24,200 | 91,400 | 0.00 | 0.14 |
| IFX–DMARDs | 36,800 | 21,700 | 83,700 | 0.01 | 0.22 |
| RTX–DMARDs | 22,500 | 13,700 | 52,800 | 0.32 | 0.80 |
| ABT–DMARDs | 38,800 | 23,300 | 85,600 | 0.00 | 0.17 |
| ADA–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ETN–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| IFX–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ABT–RTX | 126,100 | 46300 | RTX | 0.00 | 0.00 |
| ADA–ABT | 46,700 | 23,200 | 153,000 | 0.99 | 0.90 |
| ETN–ABT | 37,400 | 19,800 | 101,100 | 0.97 | 0.76 |
| IFX–ABT | 41,700 | 21,900 | 113,300 | 0.99 | 0.84 |
| ADA–ETN | ADA | Not meaningful | 0.87 | 0.87 | |
| ADA–IFX | 19,000 | Not meaningful | 0.53 | 0.63 | |
| ETN–IFX | 502,600 | Not meaningful | 0.17 | 0.22 | |
No offset costs (additional analysis)
In this case the ‘offset costs’ representing the estimates of joint replacement and hospitalisation costs were removed. The results were as follows:
| Treatment | Mean cost | 95% credible interval | Mean QALY | 95% credible interval | ||
|---|---|---|---|---|---|---|
| ADA | 47,200 | 44,500 | 49,800 | 2.89 | –2.12 | 7.87 |
| ETN | 47,200 | 44,400 | 50,000 | 2.80 | –2.21 | 7.84 |
| IFX | 45,100 | 40,100 | 48,700 | 2.80 | –2.24 | 7.82 |
| RTX | 42,100 | 38,200 | 46,100 | 3.10 | –1.78 | 7.95 |
| ABT | 66,400 | 62,500 | 70,400 | 3.28 | –1.46 | 8.05 |
| DMARDs | 19,400 | 17,900 | 20,900 | 2.13 | –3.27 | 7.46 |
| Comparison | Diff cost | 95% credible interval | Diff QALY | 95% credible interval | ||
| ADA–DMARDs | 27,800 | 26,000 | 29,600 | 0.75 | 0.33 | 1.23 |
| ETN–DMARDs | 27,800 | 25,800 | 29,800 | 0.67 | 0.30 | 1.10 |
| IFX–DMARDs | 25,700 | 21,000 | 28,500 | 0.67 | 0.29 | 1.12 |
| RTX–DMARDs | 22,700 | 19,500 | 26,000 | 0.96 | 0.41 | 1.61 |
| ABT–DMARDs | 47,000 | 44,000 | 50,100 | 1.15 | 0.52 | 1.88 |
| ADA–RTX | 5,000 | 1,600 | 8,500 | –0.21 | –0.52 | 0.03 |
| ETN–RTX | 5,100 | 1,500 | 8,800 | –0.29 | –0.63 | –0.04 |
| IFX–RTX | 3,000 | –2,400 | 7,100 | –0.30 | –0.62 | –0.05 |
| ABT–RTX | 24,300 | 20,200 | 28,400 | 0.18 | –0.10 | 0.50 |
| ADA–ABT | –19,200 | –22,400 | –16,200 | –0.39 | –0.77 | –0.12 |
| ETN–ABT | –19,200 | –22,500 | –16,000 | –0.47 | –0.88 | –0.17 |
| IFX–ABT | –21,300 | –26,500 | –17,400 | –0.48 | –0.88 | –0.17 |
| ADA–ETN | –33 | –2,400 | 2,400 | 0.08 | –0.09 | 0.29 |
| ADA–IFX | 2,000 | –1,200 | 6,700 | 0.09 | –0.10 | 0.29 |
| ETN–IFX | 2,100 | –1,300 | 6,900 | 0.00 | –0.17 | 0.19 |
| Comparison | ICER | 95% credible interval | Proportion of cases cost-effective at | ||
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| ADA–DMARDs | 36,900 | 22,800 | 84,200 | 0.01 | 0.22 |
| ETN–DMARDs | 41,400 | 25,400 | 95,100 | 0.00 | 0.11 |
| IFX–DMARDs | 38,600 | 23,100 | 89,600 | 0.01 | 0.17 |
| RTX–DMARDs | 23,600 | 14,600 | 55,300 | 0.27 | 0.76 |
| ABT–DMARDs | 41,000 | 24,900 | 90,700 | 0.00 | 0.11 |
| ADA–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ETN–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| IFX–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ABT–RTX | 134,100 | 50,100 | RTX | 0.00 | 0.00 |
| ADA–ABT | 49,000 | 25,100 | 153,200 | 1.00 | 0.92 |
| ETN–ABT | 40,500 | 22,100 | 109,500 | 0.99 | 0.83 |
| IFX–ABT | 44,400 | 24,000 | 118,000 | 1.00 | 0.89 |
| ADA–ETN | ADA | Not meaningful | 0.83 | 0.84 | |
| ADA–IFX | 23,500 | Not meaningful | 0.46 | 0.59 | |
| ETN–IFX | 460,000 | Not meaningful | 0.17 | 0.23 | |
Extra cost for palliation (additional analysis)
In this scenario, the cost for Pall was increased to the cost of methotrexate, including monitoring. This involved a start-up cost of £421.03 and an annual usage cost of £995.78. The results were as follows:
| Treatment | Mean cost | 95% credible interval | Mean QALY | 95% credible interval | ||
|---|---|---|---|---|---|---|
| ADA | 76,800 | 70,500 | 83,500 | 2.89 | –2.12 | 7.87 |
| ETN | 77,100 | 70,300 | 84,000 | 2.80 | –2.21 | 7.84 |
| IFX | 75,000 | 67,800 | 82,200 | 2.80 | –2.24 | 7.82 |
| RTX | 71,100 | 64,100 | 78,300 | 3.10 | –1.78 | 7.95 |
| ABT | 94,800 | 87,700 | 102,300 | 3.28 | –1.46 | 8.05 |
| DMARDs | 51,700 | 45,500 | 58,300 | 2.13 | –3.27 | 7.46 |
| Comparison | Diff cost | 95% credible interval | Diff QALY | 95% credible interval | ||
| ADA–DMARDs | 25,100 | 23,400 | 26,800 | 0.75 | 0.33 | 1.23 |
| ETN–DMARDs | 25,400 | 23,600 | 27,200 | 0.67 | 0.30 | 1.10 |
| IFX–DMARDs | 23,400 | 18,900 | 26,100 | 0.67 | 0.29 | 1.12 |
| RTX–DMARDs | 19,400 | 16,600 | 22,200 | 0.96 | 0.41 | 1.61 |
| ABT–DMARDs | 43,100 | 40,400 | 45,700 | 1.15 | 0.52 | 1.88 |
| ADA–RTX | 5,700 | 2,600 | 8,900 | –0.21 | –0.52 | 0.03 |
| ETN–RTX | 6,000 | 2,800 | 9,300 | –0.29 | –0.63 | –0.04 |
| IFX–RTX | 4,000 | –1,100 | 7,800 | –0.30 | –0.62 | –0.05 |
| ABT–RTX | 23,700 | 19,900 | 27,400 | 0.18 | –0.10 | 0.50 |
| ADA–ABT | –18,000 | –21,000 | –15,000 | –0.39 | –0.77 | –0.12 |
| ETN–ABT | –17,700 | –20,900 | –14,400 | –0.47 | –0.88 | –0.17 |
| IFX–ABT | –19,700 | –24,700 | –16,100 | –0.48 | –0.88 | –0.17 |
| ADA–ETN | –300 | –2,700 | 2,000 | 0.08 | –0.09 | 0.29 |
| ADA–IFX | 1,700 | –1,400 | 6,300 | 0.09 | –0.10 | 0.29 |
| ETN–IFX | 2,000 | –1,200 | 6,700 | 0.00 | –0.17 | 0.19 |
| Comparison | ICER | 95% credible interval | Proportion of cases cost-effective at | ||
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| ADA–DMARDs | 33,400 | 20,400 | 76,600 | 0.02 | 0.34 |
| ETN–DMARDs | 37,800 | 22,900 | 86,800 | 0.01 | 0.20 |
| IFX–DMARDs | 35,000 | 20,600 | 79,600 | 0.02 | 0.28 |
| RTX–DMARDs | 20,100 | 12,100 | 47,400 | 0.46 | 0.86 |
| ABT–DMARDs | 37,600 | 22,600 | 83,000 | 0.01 | 0.19 |
| ADA–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ETN–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| IFX–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ABT–RTX | 131,000 | 47,800 | RTX | 0.00 | 0.00 |
| ADA–ABT | 45,800 | 22,800 | 150,000 | 0.99 | 0.89 |
| ETN–ABT | 37,300 | 19,800 | 100,800 | 0.97 | 0.76 |
| IFX–ABT | 41,200 | 21,700 | 112,800 | 0.99 | 0.83 |
| ADA–ETN | ADA | Not meaningful | 0.84 | 0.84 | |
| ADA–IFX | 20,300 | Not meaningful | 0.50 | 0.61 | |
| ETN–IFX | 452,000 | Not meaningful | 0.19 | 0.25 | |
No negative quality of life scores
In this case, all QoL scores that were calculated as negative using the equation converting HAQ to QoL were replaced by zero. The results were as follows:
| Treatment | Mean cost | 95% credible interval | Mean QALY | 95% credible interval | ||
|---|---|---|---|---|---|---|
| ADA | 74,800 | 68,800 | 81,000 | 3.80 | 1.68 | 7.87 |
| ETN | 75,100 | 68,700 | 81,500 | 3.73 | 1.64 | 7.84 |
| IFX | 73,000 | 66,100 | 79,700 | 3.73 | 1.66 | 7.82 |
| RTX | 69,400 | 62,700 | 76,400 | 3.94 | 1.77 | 7.95 |
| ABT | 93,000 | 86,200 | 100,100 | 4.11 | 1.97 | 8.05 |
| DMARDs | 49,000 | 43,300 | 54,900 | 3.27 | 1.32 | 7.46 |
| Comparison | Diff cost | 95% credible interval | Diff QALY | 95% credible interval | ||
| ADA–DMARDs | 25,800 | 24,100 | 27,500 | 0.53 | 0.29 | 0.73 |
| ETN–DMARDs | 26,100 | 24,200 | 27,900 | 0.46 | 0.25 | 0.66 |
| IFX–DMARDs | 24,000 | 19,500 | 26,800 | 0.46 | 0.24 | 0.66 |
| RTX–DMARDs | 20,400 | 17,500 | 23,200 | 0.67 | 0.35 | 0.95 |
| ABT–DMARDs | 44,000 | 41,300 | 46,700 | 0.83 | 0.50 | 1.12 |
| ADA–RTX | 5,400 | 2,200 | 8,700 | –0.13 | –0.36 | 0.07 |
| ETN–RTX | 5,700 | 2,400 | 9,100 | –0.20 | –0.43 | 0.00 |
| IFX–RTX | 3,600 | –1,600 | 7,600 | –0.20 | –0.43 | 0.00 |
| ABT–RTX | 23,600 | 19,800 | 27,400 | 0.17 | –0.08 | 0.42 |
| ADA–ABT | –18,200 | –21,300 | –15,200 | –0.30 | –0.54 | –0.08 |
| ETN–ABT | –18,000 | –21,200 | –14,600 | –0.37 | –0.61 | –0.15 |
| IFX–ABT | –20,000 | –25,100 | –16,200 | –0.37 | –0.60 | –0.15 |
| ADA–ETN | –300 | –2,800 | 2,100 | 0.07 | –0.08 | 0.23 |
| ADA–IFX | 1,800 | –1,400 | 6,500 | 0.07 | –0.10 | 0.23 |
| ETN–IFX | 2,000 | –1,200 | 6,800 | 0.00 | –0.15 | 0.15 |
| Comparison | ICER | 95% credible interval | Proportion of cases cost-effective at | ||
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| ADA–DMARDs | 48,600 | 35,300 | 87,100 | 0.00 | 0.00 |
| ETN–DMARDs | 56,500 | 39,100 | 102,700 | 0.00 | 0.00 |
| IFX–DMARDs | 52,100 | 35,100 | 97,500 | 0.00 | 0.00 |
| RTX–DMARDs | 30,700 | 21,700 | 57,300 | 0.01 | 0.48 |
| ABT–DMARDs | 52,800 | 39,100 | 89,100 | 0.00 | 0.00 |
| ADA–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ETN–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| IFX–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ABT–RTX | 140,700 | 58,000 | RTX | 0.00 | 0.00 |
| ADA–ABT | 60,300 | 33,200 | 216,900 | 1.00 | 0.99 |
| ETN–ABT | 48,300 | 29,100 | 115,000 | 1.00 | 0.97 |
| IFX–ABT | 53,700 | 31,700 | 129,100 | 1.00 | 0.99 |
| ADA–ETN | ADA | Not meaningful | 0.82 | 0.84 | |
| ADA–IFX | 25,300 | Not meaningful | 0.46 | 0.57 | |
| ETN–IFX | 7,430,000 | Not meaningful | 0.18 | 0.22 | |
Linear equation Health Assessment Questionnaire to quality of life
In this scenario, the linear equation QoL = 0.862 – 0.327HAQ was used as in previous versions of the BRAM. For the probabilistic analysis, the coefficients were sampled from normal distributions with SDs 0.034 and 0.0201 respectively. 174 The results were as follows:
| Treatment | Mean cost | 95% credible interval | Mean QALY | 95% credible interval | ||
|---|---|---|---|---|---|---|
| ADA | 74,800 | 68,700 | 80,900 | 3.03 | 1.66 | 4.35 |
| ETN | 75,100 | 68,900 | 81,600 | 2.96 | 1.59 | 4.28 |
| IFX | 73,000 | 66,000 | 79,800 | 2.95 | 1.60 | 4.28 |
| RTX | 69,400 | 62,700 | 76,000 | 3.22 | 1.88 | 4.55 |
| ABT | 93,000 | 86,300 | 99,600 | 3.40 | 2.05 | 4.71 |
| DMARDs | 49,000 | 43,300 | 54,900 | 2.36 | 0.97 | 3.72 |
| Comparison | Diff cost | 95% credible interval | Diff QALY | 95% credible interval | ||
| ADA–DMARDs | 25,800 | 24,100 | 27,600 | 0.67 | 0.51 | 0.84 |
| ETN–DMARDs | 26,100 | 24,300 | 28,000 | 0.60 | 0.44 | 0.76 |
| IFX–DMARDs | 24,100 | 19,600 | 26,800 | 0.59 | 0.44 | 0.76 |
| RTX–DMARDs | 20,400 | 17,800 | 23,300 | 0.86 | 0.63 | 1.12 |
| ABT–DMARDs | 44,000 | 41,400 | 46,600 | 1.04 | 0.81 | 1.29 |
| ADA–RTX | 5,400 | 2,000 | 8,600 | –0.19 | –0.43 | 0.04 |
| ETN–RTX | 5,700 | 2,600 | 9,000 | –0.26 | –0.50 | –0.04 |
| IFX–RTX | 3,700 | –1,500 | 7,600 | –0.27 | –0.53 | –0.04 |
| ABT–RTX | 23,700 | 19,700 | 27,400 | 0.18 | –0.10 | 0.44 |
| ADA–ABT | –18,200 | –21,300 | –15,100 | –0.37 | –0.61 | –0.15 |
| ETN–ABT | –17,900 | –21,100 | –14,800 | –0.44 | –0.67 | –0.23 |
| IFX–ABT | –20,000 | –25,200 | –16,200 | –0.45 | –0.70 | –0.22 |
| ADA–ETN | –300 | –2,800 | 2,000 | 0.07 | –0.10 | 0.25 |
| ADA–IFX | 1,800 | –1,400 | 6,400 | 0.08 | –0.11 | 0.26 |
| ETN–IFX | 2,100 | –1,100 | 6,700 | 0.00 | –0.17 | 0.17 |
| Comparison | ICER | 95% credible interval | Proportion of cases cost-effective at | ||
|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | ||||
| ADA–DMARDs | 38,600 | 30,300 | 51,000 | 0.00 | 0.02 |
| ETN–DMARDs | 43,800 | 34,600 | 57,900 | 0.00 | 0.00 |
| IFX–DMARDs | 40,600 | 30,700 | 54,100 | 0.00 | 0.02 |
| RTX–DMARDs | 23,700 | 18,700 | 31,100 | 0.08 | 0.96 |
| ABT–DMARDs | 42,300 | 34,100 | 54,100 | 0.00 | 0.00 |
| ADA–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ETN–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| IFX–RTX | RTX | Not meaningful | 0.00 | 0.00 | |
| ABT–RTX | 130,900 | 54,400 | RTX | 0.00 | 0.00 |
| ADA–ABT | 49,100 | 29,300 | 120,000 | 1.00 | 0.97 |
| ETN–ABT | 40,300 | 26,100 | 77,500 | 1.00 | 0.90 |
| IFX–ABT | 44,600 | 27,900 | 92,400 | 1.00 | 0.95 |
| ADA–ETN | ADA | Not meaningful | 0.81 | 0.82 | |
| ADA–IFX | 23,100 | Not meaningful | 0.48 | 0.57 | |
| ETN–IFX | 667,000 | Not meaningful | 0.19 | 0.25 | |
Appendix 15 Assumptions in the Birmingham Rheumatoid Arthritis Model
| Item | Data source or assumption | Comments | |||||
|---|---|---|---|---|---|---|---|
| Baseline characteristics | Based on the British Society for Rheumatology (BSR) submission to the NICE | Most recent UK data reflecting characteristics of patients in actual clinical practice | |||||
| Sequence of treatments | Analysed strategies after the failure of a tumour necrosis factor (TNF) inhibitor are: | We restricted the analysis to a second biologic therapy and did not consider sequences including a third biologic therapy. We assumed that the effectiveness of the ‘late’ DMARDs (LEF, GST, CyA, AZA) would not be as good as when these drugs were used in early RA, but would be equally good between the strategies modelled | |||||
| ADA | ETN | IFX | RTX | ABT | DMARD | ||
| ADA | ETN | IFX | RTX | ABT | DMARD | ||
| LEF | LEF | LEF | LEF | LEF | GST | ||
| GST | GST | GST | GST | GST | CyA | ||
| CyA | CyA | CyA | CyA | CyA | AZA | ||
| AZA | AZA | AZA | AZA | AZA | Pall | ||
| Pall | Pall | Pall | Pall | Pall | |||
| DMARDs that are likely to have been used before biologic therapy are excluded from the sequence | |||||||
| Health Assessment Questionnaire (HAQ) change on initiation of treatment |
Biologics – data from randomised controlled trials and if not available from largest observational cohorts studies; we have assumed that IFX was equally effective to ETN DMARDs – no data for patients who have failed a TNF inhibitor were available; assumed to be half of the HAQ improvement in early RA. However, much lower responses are explored in sensitivity analyses |
The best available evidence was used | |||||
| Health Assessment Questionnaire change on treatment |
It is assumed that after initial improvement the HAQ score changes on treatment by: This is modelled as mean times to an increase in HAQ score of 0.125 (2.7 for DMARDs and 2.0 for Pall) In PSA times are sampled from normal distributions with SD of 0.27 (DMARD) and 0.2 (Pall) |
These assumptions are in line with those made in previous versions of the BRAM. However, it should be appreciated that even with the optimal treatment a majority of patients do not achieve remission. Therefore, because continuing disease activity is likely to have a detrimental effect on physical function, an assumption of zero HAQ progression on biologic treatments in models that span a lifetime is somewhat implausible | |||||
| Health Assessment Questionnaire increase on withdrawing from treatment | It is assumed to be the same as initial improvement on treatment | This has been an assumption in all versions of the BRAM and in other models | |||||
| Time on treatment – probability of early quitting | Data from the following sources is used: | The best available evidence was used. For biologic drugs the highest quality sources identified in the systematic review were used. For DMARDs no studies where DMARDs were used after failure of a TNF inhibitor were identified. Therefore, data from early RA were used. We are aware that disease duration can influence HAQ responses and taking values from studies in early RA is problematic. However, halving a HAQ response, for example for leflunomide from 0.38 to 0.19 (approaching the minimal clinical detectable difference), is plausible | |||||
| Time to withdrawal – long-term survival on treatment | For long-term survival on treatment Weibull curves were fitted to the available data: | The best available evidence was used. For TNF inhibitors most recent UK data were chosen and as it was not available for RTX and ABT data from clinical trials were utilised. For DMARDs no studies in the relevant population were identified and therefore data from GPRD were used | |||||
| Mortality | Basic mortality was taken from standard life tables. A RR (1.33) per unit HAQ was applied. For PSA a log-normal distribution was assumed (95% CI 1.10 to 1.61) | Based on Wolfe et al.199 Previous versions of the BRAM were not found to be sensitive to this parameter | |||||
| QoL scores |
The following equation was used to map HAQ onto QoL: QoL = a – b1 × HAQ – b2 × HAQ2 This allows negative utility values A scenario analysis that adjusted all negative utilities to zero is reported |
We used the results of a regression performed on data from Hurst et al.155 in the absence of any more recent data | |||||
| Costs |
Costs are made up of drug and monitoring costs. A ‘start-up’ cost reflects higher dosage and additional monitoring, as appropriate for each treatment Unit costs were based on: |
This simplifying assumption means that all patients incur the full additional ‘start-up’ costs even if quitting early. In most cases, the additional costs are complete within 3 months of starting; only in the case of GST do the additional costs extend beyond 6 months | |||||
| Monitoring assumptions | The data on monitoring was:
|
It was assumed that there will be no difference in the monitoring necessary for DMARDs between early and late RA and therefore data from Chen et al.179 were used. For biologics it was assumed that all of them are given with MTX and therefore MTX monitoring was assumed to be sufficient | |||||
Glossary
- American College of Rheumatology (ACR) 20
- Defined as a 20% improvement in the counts of the number of tender and swollen joints and at least three items from the following: observer evaluation of overall disease activity; patient evaluation of overall disease activity; patient evaluation of pain; a score of physical disability; and improvements in blood acute phase responses.
- ACR50
- Defined as a 50% improvement in the counts of the number of tender and swollen joints and at least three items from the following: observer evaluation of overall disease activity; patient evaluation of overall disease activity; patient evaluation of pain; a score of physical disability; and improvements in blood acute phase responses.
- ACR70
- Defined as a 70% improvement in the counts of the number of tender and swollen joints and at least three items from the following: observer evaluation of overall disease activity; patient evaluation of overall disease activity; patient evaluation of pain; a score of physical disability; and improvements in blood acute phase responses.
- Tumour necrosis factor (TNF) inhibitors
- Biological agents that block TNF activity.
- Health Assessment Questionnaire (HAQ)
- The Health Assessment Questionnaire is designed to assess the physical function of patients. Scores range from 0 (no functional impairment) to 3 (most impaired). Details are provided in Appendix 1.
- Disease Activity Score (DAS)
- The DAS is calculated using a formula that includes counts for tender (53 joints) and swollen joints (44 joints), an evaluation by the patient of general health and blood acute phase response. Scores range from 0 (best) to 10 (most active disease).
- DAS28
- Disease Activity Score 28, similar to DAS above but using only 28 joints for assessment. Scores range from 0 (best) to 10 (most active disease).
List of abbreviations
- ABT
- abatacept
- ACR
- American College of Rheumatology
- ADA
- adalimumab
- AE
- adverse event
- anti-CCP
- anti-cyclic citrullinated peptide
- ARRIVE
- abatacept researched in rheumatoid arthritis patients with an inadequate anti-TNF response to validate effectiveness
- ASSURE
- abatacept study of safety in use with other rheumatoid arthritis therapies
- ATTAIN
- abatacept trial in treatment of anti-TNF inadequate responders
- AZA
- azathioprine
- BRAM
- Birmingham Rheumatoid Arthritis Model
- BSRBR
- British Society for Rheumatology Biologics Registry
- CEAC
- cost-effectiveness acceptability cure
- CI
- confidence interval
- CRP
- C-reactive protein
- CyA
- ciclosporin A
- DANBIO
- Danish Registry for Biologic Therapies in Rheumatology
- DAS
- Disease Activity Score
- DAS28
- Disease Activity Score 28
- DMARD
- disease-modifying antirheumatic drug
- EQ-5D
- European Quality of Life-5 Dimensions
- ERG
- Evidence Review Group
- ESR
- erythrocyte sedimentation rate
- ETN
- etanercept
- EULAR
- European League Against Rheumatism
- Fc
- fragment crystallisable
- GO-AFTER
- GOlimulab After Former anti-tumour necrosis factor Therapy Evaluated in Rheumatoid arthritis
- GP
- general practitioner
- GST
- injectable gold
- HAQ
- Health Assessment Questionnaire
- HAQ DI
- Health Assessment Questionnaire Disability Index
- HCQ
- hydroxychloroquine
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HUI3
- Health Utilities Index Mark 3
- IC
- indirect comparison
- ICER
- incremental cost-effectiveness ratio
- IFX
- infliximab
- IgG1
- immunoglobulin G1
- IR
- inadequate response
- ITT
- intention to treat
- i.v.
- intravenous
- K–M
- Kaplan–Meier (curve)
- LEF
- leflunomide
- LTE
- long-term extension
- MCID
- minimal clinically important difference
- MS
- manufacturer’s submission
- MTC
- mixed-treatment comparison
- MTX
- methotrexate
- NAO
- National Audit Office
- NICE
- National Institute for Health and Clinical Excellence
- NOAR
- Norfolk Arthritis Register
- NSAID
- non-steroidal anti-inflammatory drug
- OPPOSITE
- open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept
- Pall
- palliation
- PCT
- primary care trust
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RA
- rheumatoid arthritis
- RCT
- randomised controlled trial
- RD
- risk difference
- ReAct
- Research in Active Rheumatoid Arthritis
- REFLEX
- randomised evaluation of long-term efficacy of rituximab in rheumatoid arthritis
- RF
- rheumatoid factor
- RR
- relative risk
- RTX
- rituximab
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SJC
- swollen joint count
- SSTAG
- Southern Swedish Arthritis Treatment Group Registry
- STA
- single technology appraisal
- SUNRISE
- study for understanding rituximab safety and efficacy
- TB
- tuberculosis
- TEMPO
- Trial of Etanercept and Methotrexate with radiographic Patient Outcomes
- TJC
- tender joint count
- TNF
- tumour necrosis factor
- TNFα
- tumour necrosis factor alpha
- TOC
- tocilizumab
- WTP
- willingness to pay
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Note
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed commercial-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of commercial-in-confidence data removed and replaced by the statement ‘commercial-in-confidence information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of General Practice, Department of Primary Health Care, University of Oxford Programme Director,
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics and Clinical Trials, Queen Mary, Department of Epidemiology and Public Health, Imperial College London
-
Professor Peter Brocklehurst, Director, National Perinatal Epidemiology Unit, University of Oxford
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire Country Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr David P Britt, Public contributor
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Professor Nicholas James, Professor of Clinical Oncology, School of Cancer Sciences, University of Birmingham
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Mr John Chapman, Public contributor
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Ms Kylie Gyertson, Oncology and Haematology Clinical Trials Manager, Guy’s and St Thomas’ NHS Foundation Trust London
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Martin Shelly, General Practitioner, Silver Lane Surgery, Leeds
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington