Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 08/70/01. The contractual start date was in May 2009. The draft report began editorial review in September 2010 and was accepted for publication in February 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Soares et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Definitions of severe sepsis and septic shock
Sepsis is a syndrome characterised by a systemic inflammatory response to infection that leads to rapid acute organ failure and potentially rapid decline to death. Sepsis, severe sepsis and septic shock are generic terms and do not represent a single homogeneous disease; rather they are terms for a common syndrome.
In an attempt to formalise a definition for the sepsis syndrome, in 1991, a consensus conference was convened by the American College of Chest Physicians (ACCP) and the Society of Critical Care Medicine (SCCM). 1 This conference defined the concept of the systemic inflammatory response syndrome (SIRS) – a systemic activation of the innate immune response, regardless of cause. SIRS could be triggered by multiple insults, including infection, trauma, burns and pancreatitis. SIRS was considered to be present if two or more of the following four specific conditions were satisfied:
-
temperature > 38°C or < 36°C
-
heart rate > 90/min
-
respiratory rate > 20/min or partial pressure of carbon dioxide (PaCO2) < 4.3 kPa, and
-
white blood cell count > 12 × 109/l or < 4 × 109/l (or > 10% immature neutrophils – ‘bands’).
Sepsis was defined as SIRS (above) in response to infection, severe sepsis as sepsis associated with organ dysfunction, hypoperfusion or hypotension and septic shock as sepsis with hypotension despite adequate fluid resuscitation (Figure 1). These definitions have formed the basis of entry criteria to the majority of recent studies investigating sepsis.
FIGURE 1.
Definitions of sepsis, severe sepsis and septic shock.
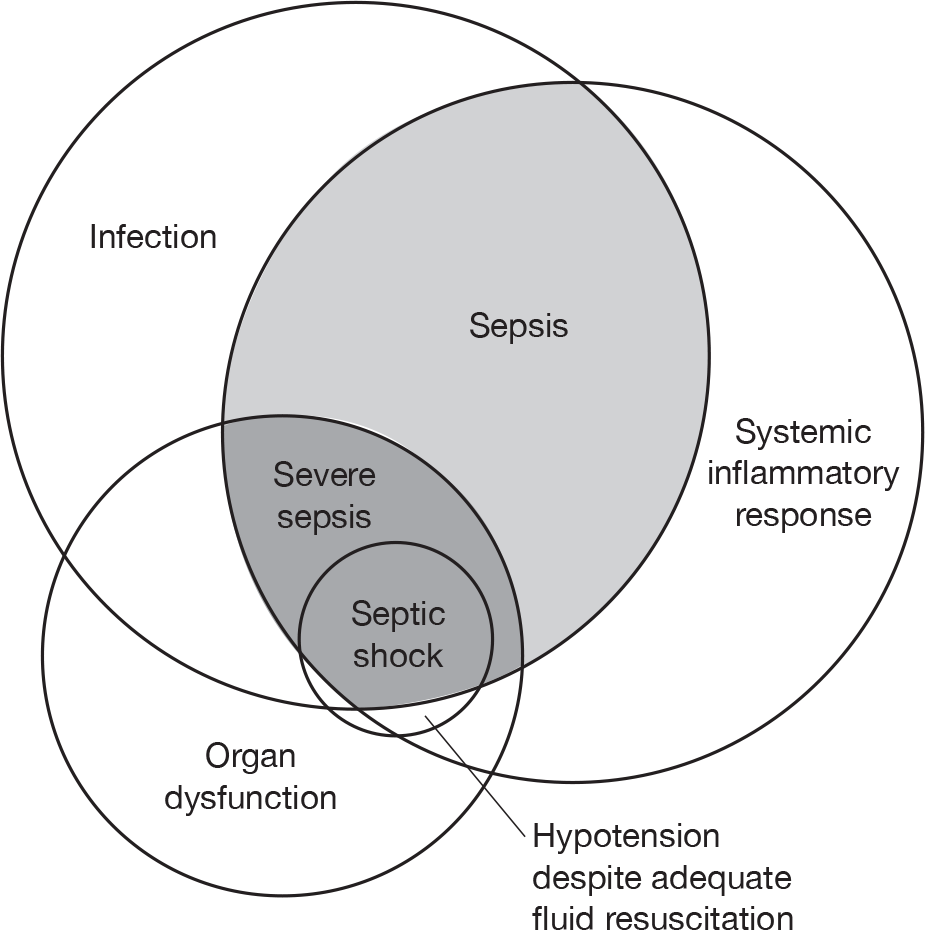
In 2001, another consensus conference was convened, sponsored by the SCCM, the European Society of Intensive Care Medicine (ESICM), ACCP, the American Thoracic Society and the Surgical Infection Society. 2 This consensus conference agreed the concept of SIRS, but considered the 1991 definition too non-specific to be useful. The basic definition of sepsis as ‘the clinical syndrome defined by the presence of both infection and a systemic inflammatory response’ remained unchanged but, in place of the SIRS criteria, the 2001 consensus definitions recommend a wider list of ‘possible signs of systemic inflammation in response to infection’. The definitions of severe sepsis as sepsis associated with organ dysfunction, and septic shock as sepsis associated with hypotension despite adequate fluid resuscitation, remained unchanged.
Epidemiology of severe sepsis in the UK NHS
Estimates of severe sepsis in the UK NHS derive from adult critical-care units in the Intensive Care National Audit & Research Centre (ICNARC) Case Mix Programme (CMP) Database. These indicate an increasing treated incidence of severe sepsis in critical care, rising from 50 to 70 cases per 100,000 population per year over the last decade. 3 This now represents approximately 31,000 critical-care unit patient episodes per year. Similarly high incidence rates have been reported elsewhere. 4 Overall, 29% of all admissions to adult, general critical-care units were associated with severe sepsis in the first 24 hours following admission and had an in-hospital mortality of 45%, corresponding to approximately 15,000 deaths per year. These estimates may underestimate the overall burden of severe sepsis within critical-care units in the UK, because of the limitation of the available data restricting analysis to severe sepsis present during the first 24 hours following admission to the critical-care unit.
Severity of severe sepsis has often been summarised by the number of organ dysfunctions (i.e. the number of distinct organ systems with dysfunction). However, although the number of organ dysfunctions is strongly associated with mortality (rising from 22% for single organ dysfunction to 86% for five organ dysfunctions), the particular combination of organ dysfunctions is also important, with the combination of both cardiovascular and renal organ dysfunction associated with particularly high mortality. 5
Intravenous immunoglobulin
Intravenous immunoglobulin (IVIG) is a blood product derived from human donor blood. The serum from around 1000 to 15,000 donors is required for each batch. 6 The mechanisms of action of IVIG are complex, but are increasingly being understood. 7 IVIG is predominantly used in neurology, haematology, immunology and dermatology, but also in nephrology, rheumatology, ophthalmology and other specialties. However, new uses are emerging and off-label use is increasing. 8
Intravenous immunoglobulin has been proposed as an adjuvant therapy for severe sepsis/septic shock since the 1980s and a number of (predominantly small) randomised controlled trials (RCTs) have been conducted. The Cochrane systematic review of the use of IVIG in severe sepsis/septic shock describes the clinical rationale for this as follows: ‘The cascade of harmful effects from sepsis and septic shock has been postulated to be largely due to the lipid A component of the endotoxin molecule in Gram-negative bacteria. Thus the use of antibodies against different components of the endotoxin molecule has been the target of various investigations’. 9 Numerous systematic reviews and meta-analyses of IVIG in severe sepsis/septic shock have been performed. 9–15 As a result of the heterogeneity across studies and inconsistencies in results, the majority of authors have concluded that there is insufficient evidence to recommend IVIG as an adjuvant therapy for severe sepsis/septic shock and that more evidence, in the form of a large, well-conducted RCT, is required.
Current policy and practice with intravenous immunoglobulin for severe sepsis and septic shock in the UK
Intravenous immunoglobulin is a scarce resource worldwide. Costs have escalated, associated with a reduced demand for plasma-derived factor VIII and albumin. In addition, there are supply issues, unique to the UK, that further limit the availability of IVIG. Where IVIG was previously produced in the UK using plasma sourced from within the UK as a by-product of blood donations, plasma must now be imported owing to the risk of variant Creutzfeldt–Jakob disease. In addition, the closure of one UK manufacturer (the Scottish National Blood Transfusion Service) and withdrawal of batches of IVIG because of safety concerns have led to both local and national, transient and longer-term shortages.
In response to this, the Department of Health implemented a Demand Management Programme for IVIG. The programme consists of three components: the Demand Management Plan for Immunoglobulin Use,16 Clinical Guidelines for Immunoglobulin Use17 and the National Immunoglobulin Database. Indications for IVIG use are colour-coded in the following way:
-
red – a disease for which treatment is considered the highest priority because of a risk to life without treatment
-
blue – a disease for which there is a reasonable evidence base, but where other treatment options are available
-
grey – a disease for which the evidence base is weak, in many cases because the disease is rare; treatment should be considered on a case-by-case basis, prioritised against other competing demands, and
-
black – a disease for which there is evidence to suggest that IVIG is not an appropriate treatment and treatment is not recommended.
‘Sepsis in the intensive care unit not related to specific toxins or Clostridium difficile’ is currently a black indication and, consequently, IVIG should not be used under any circumstances. The Clinical Guidelines for Immunoglobulin Use do, however, make a research recommendation that, ‘there is a need for adequately powered high-quality RCTs to assess the impact of IVIG in severe sepsis in the general (intensive care unit). 17
In view of the heterogeneity of results from existing RCTs and the unique supply and demand issues for IVIG (especially in the UK), a research priority was identified to establish if such a trial was necessary and feasible and if the costs of carrying out the trial were outweighed by the potential benefit of the resulting information.
Aims and objectives
The aim of this study was to evaluate the feasibility, cost and value of information of conducting a large, high-quality, multicentre RCT to assess the clinical effectiveness and cost-effectiveness of IVIG for adult patients severely ill with sepsis (severe sepsis or septic shock) in the UK.
The specific objectives were:
-
to describe current practice in the management of adult patients severely ill with sepsis (severe sepsis or septic shock) in the UK
-
to assess the clinical effectiveness of IVIG for severe sepsis and septic shock, and to obtain the appropriate inputs for the relative efficacy parameters and the key uncertainties associated with these parameters, required to populate the decision model
-
to develop a decision-analytic model structure and identify key parameter inputs consistent with the decision problem and relevant to an NHS setting
-
to populate the decision model and determine the cost-effectiveness of IVIG and to estimate the value of additional primary research.
Chapter 2 Survey of the management of severe sepsis in UK critical-care units
Objective
To describe current practice in the management of adult patients severely ill with sepsis (severe sepsis or septic shock) in the UK.
Background
Most clinicians look to international guidelines for guidance on the management and treatment of patients with sepsis. The Surviving Sepsis Campaign (SSC), an initiative of the ESICM, the International Sepsis Forum and the SCCM, was developed (and updated in 2008) to improve the diagnosis, management and treatment of sepsis. 18
The SSC partnered with the Institute for Healthcare Improvement (IHI) to incorporate its ‘bundle concept’ into the management and treatment of sepsis. A bundle was defined by the SSC/IHI as a group of interventions related to a disease process that, when implemented together, result in better outcomes than when implemented individually. 19 The SSC claim that ‘the science behind the elements of the bundle is so well-established that their implementation should be considered a generally accepted practice’. 20 They also indicate that bundle components can be easily measured as completed or not completed and, as such, the overall bundle (all of the elements taken together) can also be measured as completed or not completed.
Two bundles were developed: the resuscitation bundle (which must be completed within 6 hours) and the management bundle (which must be completed within 24 hours). 19 The SSC describes the bundles as a distillation of the concepts and recommendations found in the first set of international clinical guidelines were originally published in 2004. 21
Resuscitation bundle
-
Measure serum lactate.
-
Obtain blood cultures prior to antibiotic administration.
-
Administer broad-spectrum antibiotic within 3 emergency department (ED) hours/1 non-ED hour of admission.
-
In the event of hypotension and/or serum lactate > 4 mmol/l:
-
– deliver initial minimum of 20 ml/kg of crystalloid or equivalent
-
–apply vasopressors for hypotension not responding to initial fluid resuscitation to maintain mean arterial pressure (MAP) ≥ 65 mmHg.
-
-
In the event of persistent hypotension despite fluid resuscitation (septic shock) and/or lactate > 4 mmol/l:
-
–achieve a central venous pressure (CVP) ≥ 8 mmHg
-
–achieve a central venous oxygen saturation (ScvO2) ≥ 70% or mixed venous oxygen saturation (SvO2) ≥ 65%.
-
Management bundle
-
Administer low-dose steroids for septic shock in accordance with a standardised critical-care policy (if not administered, document why the patient did not qualify for low-dose steroids).
-
Administer recombinant human activated protein C (rhAPC) in accordance with a standardised critical-care policy (if not administered, document why the patient did not qualify for rhAPC).
-
Maintain glucose control ≥ 3.9 mmol/l, but ≤ 8.3 mmol/l.
-
Maintain a median inspiratory plateau pressure < 30 cmH2O for mechanically ventilated patients.
Methods
To describe current practice in the management of adult patients severely ill with sepsis (severe sepsis or septic shock), a national survey of clinical directors of adult, general critical-care units in the NHS in the UK was conducted in February 2010. The survey was designed and set up online using the online survey software, S[sc]mart[/sc]-S[sc]urvey[/sc]™ version 4 (Smartline International Ltd, Tewkesbury, Gloucestershire, UK). The SSC guidelines were reviewed and items were selected for inclusion in the survey if ranked as 1A or 1B based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system, which classifies quality of evidence as high (A), moderate (B), low (C) or very low (D) and recommendations as strong (1) or weak (2). 22 In addition, items that are components of the resuscitation and management bundles (listed above) and not 1A or 1B were also included.
The 25 items selected for inclusion in the survey were reviewed by the Expert Group (see Acknowledgements) for content and clarity and grouped into six core domains as follows:
-
domain 1: resuscitation practices in the ED: critical-care clinicians’ perceptions of management of severe sepsis/septic shock in their ED
-
domain 2: resuscitation practices in the critical-care unit
-
domain 3: use of adjuvant therapy in the critical-care unit
-
domain 4: use of IVIG in the critical-care unit
-
domain 5: safety interventions in the critical-care unit
-
domain 6: uptake of bundles-based management of severe sepsis/septic shock.
The layout of the survey was organised such that clinicians were first asked about specific aspects of patient care relating to resuscitation (domains 1 and 2) and management (domains 3–5) of patients with severe sepsis/septic shock, which included questions about the preferred choice of fluids and vasopressors, target levels for blood pressure, CVP and other physiological parameters, and administration of antibiotics and adjunctive therapies (including IVIG), prior to being asked about bundles-based management (domain 6).
Survey questions were further refined following piloting by the Expert Group and Clinical Research Associates working with ICNARC.
UK adult, general critical-care units (n = 231) were identified from a database of all UK critical-care units maintained by ICNARC. An e-mail was sent to the clinical director of each unit containing the online link for the survey (see Appendix 1). An e-mail reminder was sent to all non-responders after 4 weeks and repeated on a weekly basis for 3 months. As part of the ICNARC CMP, there is regular telephone contact with units, and this was used to facilitate reminders about the survey.
Statistical analysis
A descriptive analysis was conducted reporting proportion, mean with standard deviation (SD) or median with interquartile range (IQR), as appropriate. Given that for a future RCT of patients with severe sepsis, the recommendation for the control arm would be usual clinical care based on the best available evidence. Adoption of elements from the SSC guidelines ranked level 1A (indicating high-quality evidence and strongly recommended), but which are not included in the resuscitation and management bundles (described above), were examined and reported. These were:
-
use of a ventilation weaning protocol
-
use of either low-dose unfractionated heparin or low-molecular-weight heparin, unless contraindicated
-
use of a mechanical prophylaxis device such as a compression stocking or an intermittent device when heparin is contraindicated
-
provision of stress ulcer prophylaxis using an H2 blocker
-
contraindicated use of a pulmonary artery catheter (PAC) for routine monitoring of patients with acute lung injury (ALI)/acute respiratory distress syndrome (ARDS).
Components of the resuscitation and management bundles (described above) were also examined and reported. Although not included by the SSC, with strong evidence to support the use of selective decontamination of the digestive tract (SDD), SDD was also examined and reported.
Finally, current use of IVIG was examined and reported.
Results
Survey response
Of the 231 adult, general critical-care units, a senior clinician to complete the survey could not be identified for 14 of the units. Of the remaining 217 units, respondents at four (2%) units refused to complete the survey and completed surveys were received for 123 (57%) units.
Surviving Sepsis Campaign recommendations: level 1A (not included in the bundles)
Responses to the survey for each level 1A item in the SSC guidelines not included in the bundles are reported below.
-
Use of a ventilation weaning protocol.
Sixty-three (51%) respondents reported using a ventilation weaning protocol for mechanically ventilated patients in their unit. Overall, respondents estimated that the median proportion of mechanically ventilated patients who were managed using a ventilation weaning protocol was 80% (IQR 50–100%).
-
Use of either low-dose unfractionated heparin or low-molecular-weight heparin, unless contraindicated, or a mechanical prophylaxis device such as a compression stocking or an intermittent device when heparin is contraindicated.
All but one of the respondents (n = 122, 99%) reported that they used prophylaxis for deep-vein thrombosis.
-
Provision of stress ulcer prophylaxis using an H2 blocker.
All but two of the respondents (n = 121, 98%) reported that they provided stress ulcer prophylaxis.
-
Contraindicated use of a PAC for routine monitoring of patients with ALI/ARDS.
A small number of respondents (n = 5, 4%) reported using a PAC.
Resuscitation bundle
The elements that constitute the SSC resuscitation bundle are listed below, along with the strength of the recommendation (1 = strong or 2 = weak) and the quality of evidence (A = high, B = moderate or C = low) assigned by the SSC. 18
-
Obtain blood cultures prior to antibiotic administration (1C).
-
Administer broad-spectrum antibiotic within 3 ED hours/1 non-ED hour of admission (1B).
-
In the event of hypotension and/or serum lactate > 4 mmol/l:
-
– deliver initial minimum of 20 ml/kg of crystalloid or equivalent (1B)
-
– apply vasopressors for hypotension not responding to initial fluid resuscitation to maintain MAP ≥ 65 mmHg (1C).
-
-
In the event of persistent hypotension despite fluid resuscitation (septic shock) and/or lactate > 4 mmol/l:
-
– achieve a CVP of ≥ 8 mmHg (1C)
-
– achieve an ScvO2 ≥ 70% or SvO2 ≥ 65% (1C).
-
Responses to the survey are reported for each component of the bundle below.
-
Obtain blood cultures prior to antibiotic administration (1C).
Nearly all respondents reported that blood cultures are taken in the ED (95%) and in the critical-care unit (98%; Table 1). Respondents estimated that this is carried out for a high proportion of patients presenting at the ED (median 80%, IQR 60–90%) and in almost all patients who are admitted to the critical-care unit (median 100%, IQR 98–100%).
| Initial treatment | ED | Critical-care unit |
|---|---|---|
| Blood cultures, n (%) | 117 (95.1) | 121 (98.4) |
| Imaging studies, n (%) | 112 (91.1) | 120 (97.6) |
| Antibiotics within 1 hour, n (%) | 108 (87.8) | 114 (92.7) |
| Preferred i.v. fluid for volume resuscitation: | ||
| • crystalloid, % patients – mean (SD) | 77.7 (2.1) | 55.6 (2.9) |
| • colloid, % patients – mean (SD) | 31.4 (2.5) | 58.8 (2.7) |
In addition, a high proportion of respondents reported that imaging studies are carried out in the ED and critical-care unit. Although not part of the resuscitation bundle, they are recommended in the SSC guidelines as level 1C (see Table 1).
-
Administer broad-spectrum antibiotic within 3 ED hours/1 non-ED hour of admission (1B).
Respondents reported that intravenous antibiotics are given within 1 hour of presentation to the ED (88%) and/or admission to the critical-care unit (93%) (see Table 1). However, they estimated that, on average, a higher proportion of patients receive intravenous antibiotics in the critical-care unit (median 90%, IQR 80–100%) than in the ED (median 60%, IQR 50–80%).
The remaining elements of the resuscitation bundle require specific goals for serum lactate, MAP, CVP and either ScvO2 or SvO2. Goals require action that usually translates to the existence of a protocol. Therefore, the survey first asked whether or not the ED and critical-care unit have resuscitation protocols and, if yes, an indication of the clinical parameters included in the protocols.
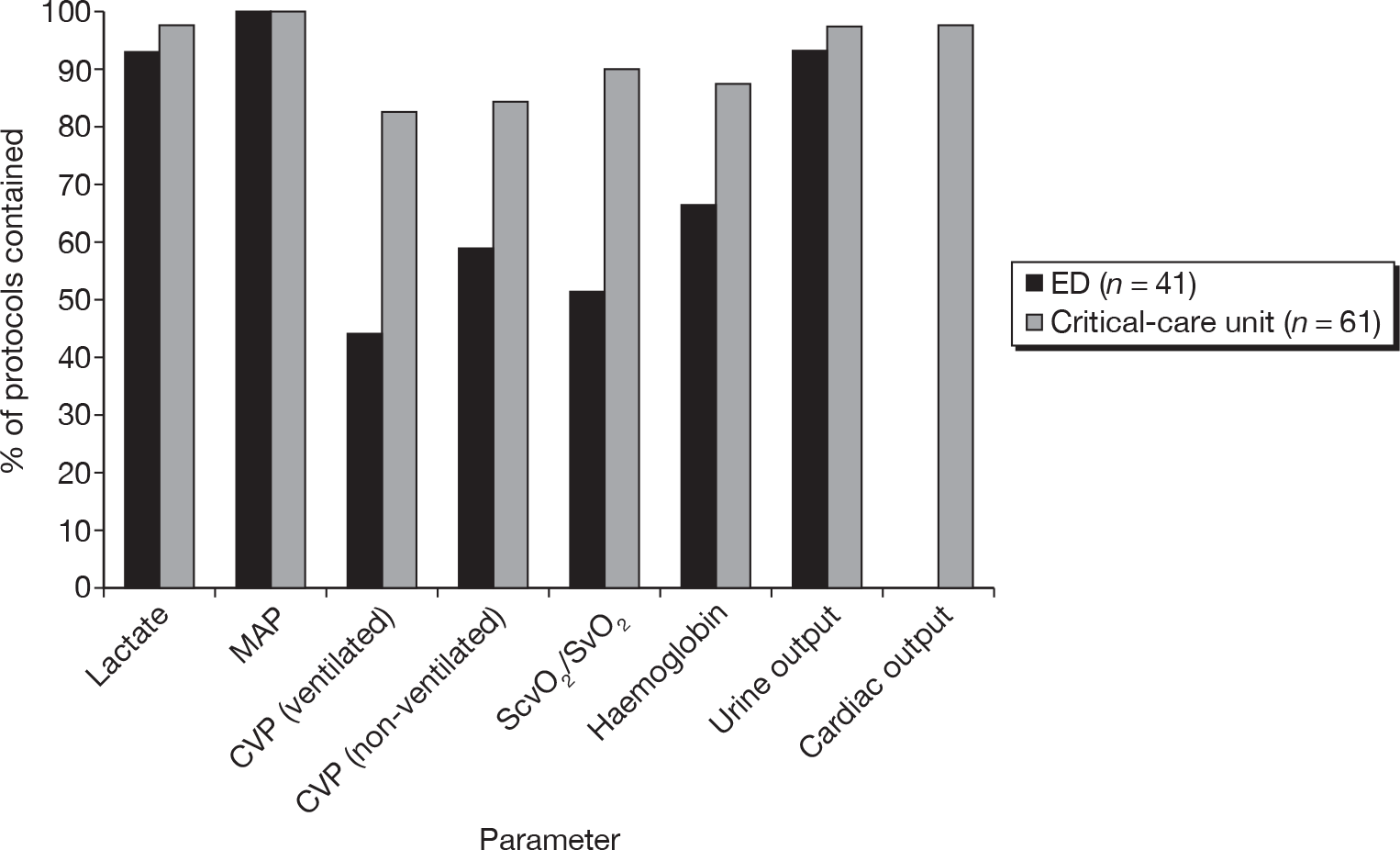
Forty-one (33%) respondents reported using a resuscitation protocol in the ED and 61 (50%) in the critical-care unit. For the latter, nearly half (n = 29, 48%) of respondents reported that the protocol commenced in the ED and transitioned to the critical-care unit. Although there was variation across hospitals, estimated compliance with the critical care resuscitation protocols was higher (median 77.5%, IQR 60–90%) than with the ED resuscitation protocols (median 60%, IQR 40–70%). The proportions of ED and critical-care unit resuscitation protocols that were reported to include MAP, CVP and ScvO2/SvO2 are shown in Figure 2. In addition, respondents reported that ED and critical-care resuscitation protocols also included targets for other parameters that are recommended in the SSC guidelines, but not included in the bundles, e.g. urine output (level 1C) and haemoglobin (level 1B). Nearly all of the critical-care unit resuscitation protocols included targets for cardiac output; however, this was not included in any of the ED resuscitation protocols (see Figure 2).
FIGURE 2.
Reported components of the resuscitation bundle included in ED and critical-care resuscitation protocols.
-
In the event of hypotension and/or serum lactate > 4 mmol/l:
-
– deliver initial minimum of 20 ml/kg of crystalloid or equivalent (1B)
-
– apply vasopressors for hypotension not responding to initial fluid resuscitation to maintain MAP ≥ 65 mmHg (1C).
-
Most ED and critical-care resuscitation protocols include serum lactate (see Figure 2) and, although all respondents who answered the question reported aiming to keep serum lactate levels < 4 mmol/l, many reported aiming for ≤ 2 mmol/l.
Both crystalloid and colloid intravenous fluids are used for volume resuscitation; however, respondents reported greater use of crystalloid in the ED than in the critical-care unit, where colloid is used as much as crystalloid (see Table 1).
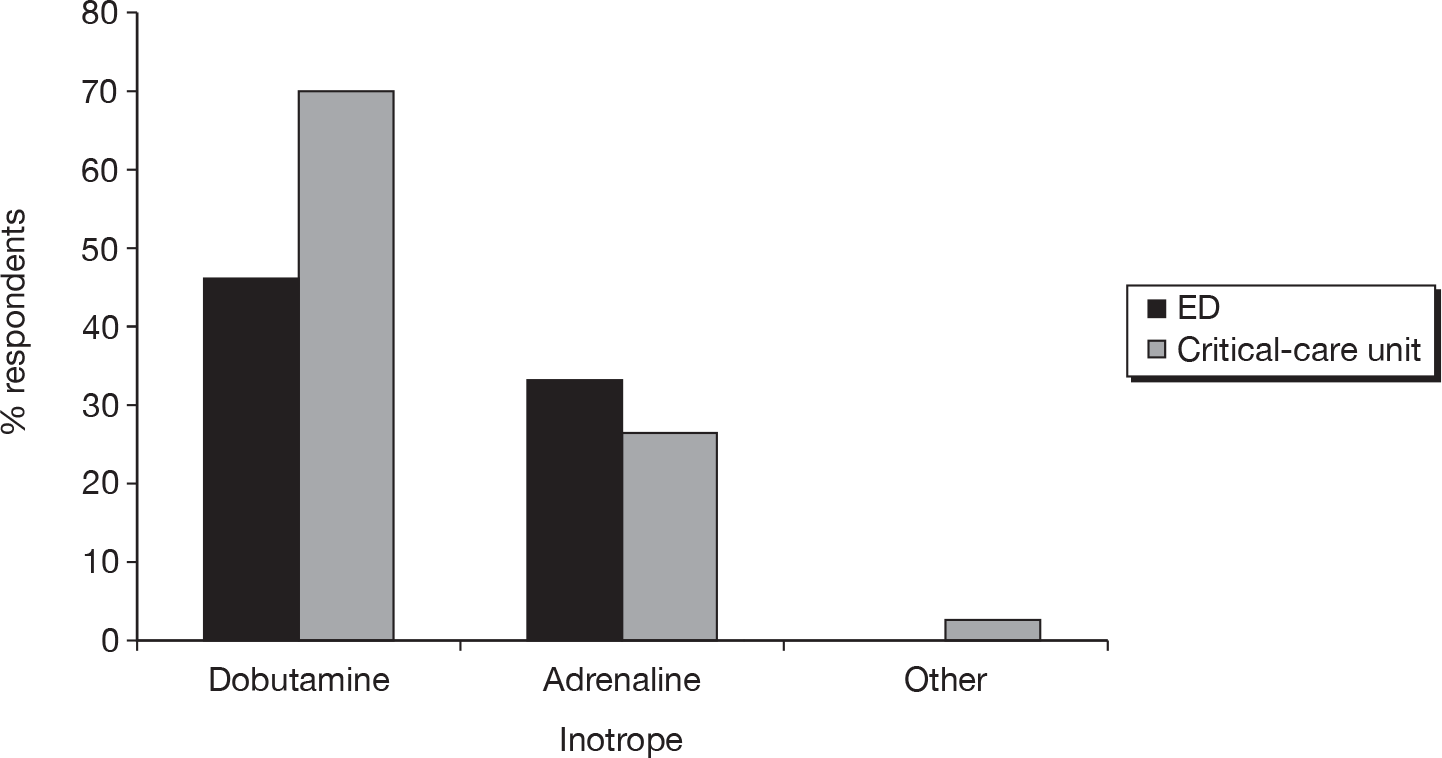
All respondents reported that MAP is included in the both ED and critical-care unit resuscitation protocols and the majority reported aiming to keep the MAP > 65 mmHg. The reported preferred choice of ‘first-line’ vasopressor in both the ED and critical-care unit was noradrenaline (Figure 3) and the preferred choice of ‘first-line’ inotrope was either dobutamine or adrenaline, although dobutamine was more frequently used in the critical-care unit than in the ED (Figure 4). A small number of respondents (n = 10 and n = 13, respectively) reported that vasopressors and/or inotropes were not given in the ED or were used only with the involvement of critical-care clinicians.
FIGURE 3.
Reported preferred choice of ‘first-line’ vasopressor in the ED and the critical-care unit.
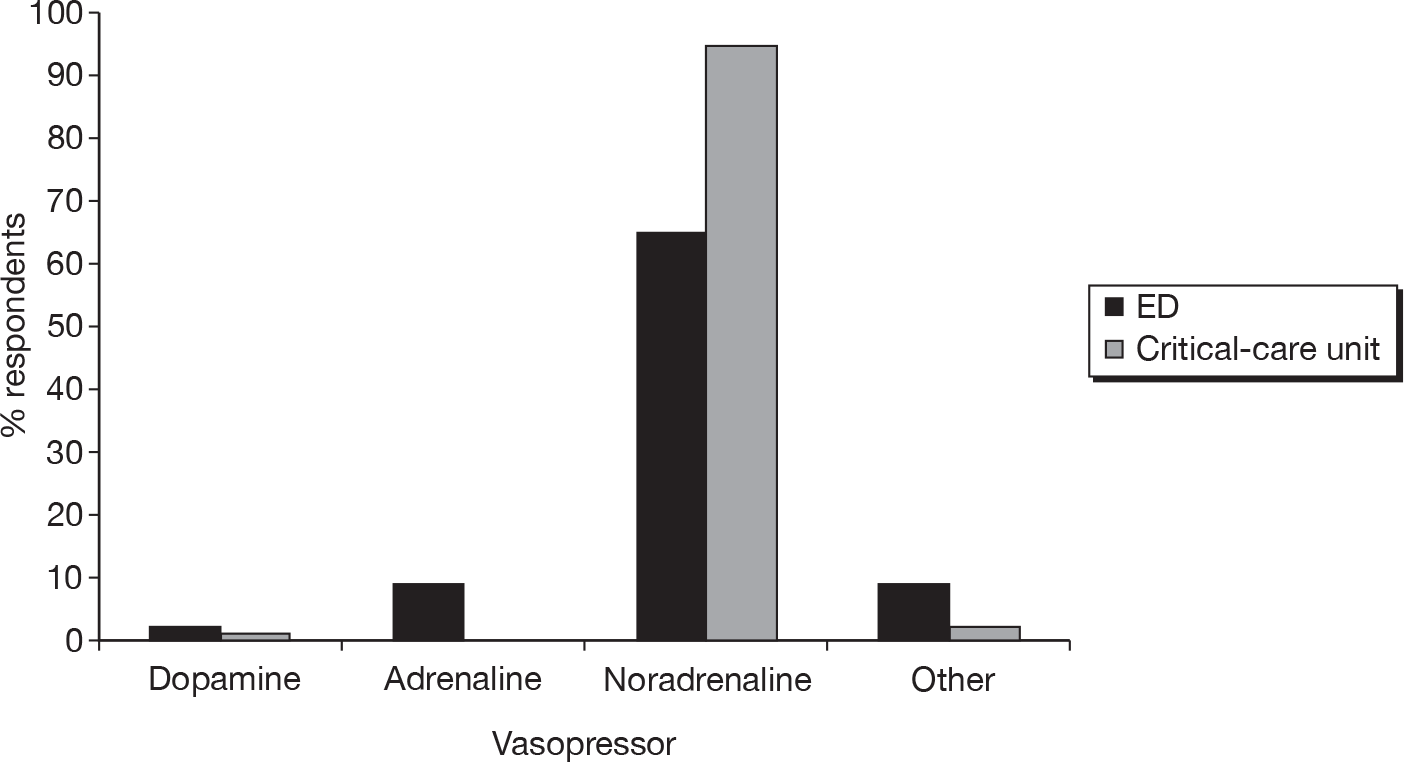
FIGURE 4.
Reported preferred choice of ‘first-line’ inotrope in the ED and the critical-care unit.
-
In the event of persistent hypotension despite fluid resuscitation (septic shock) and/or lactate > 4 mmol/l:
-
– achieve a CVP of ≥ 8 mmHg (1C)
-
– achieve an ScvO2 ≥ 70% or SvO2 ≥ 65% (1C).
-
Central venous pressure and ScvO2/SvO2 were reported less likely to be included in ED than in critical-care resuscitation protocols (see Figure 2). Although there was some variation, most respondents reported aiming for a non-ventilated CVP of ≥ 8 mmHg and a ventilated CVP of around 10–15 mmHg. All respondents reported that they aimed to achieve ScvO2 of ≥ 70%.
Management bundle
The elements that constitute the management bundle are listed below, along with the strength of the recommendation (1 = strong or 2 = weak) and the quality of evidence (A = high, B = moderate, C = low or D = very low) assigned by the SSC. 18
-
Administer low-dose steroids for septic shock in accordance with a standardised critical-care policy (if not administered, document why the patient did not qualify for low-dose steroids) (2C).
-
Administer rhAPC in accordance with a standardised critical-care policy (if not administered, document why the patient did not qualify for rhAPC). [2B or 2C for postoperative patients; SSC guidelines state that adult patients with severe sepsis and low risk of death – typically, Acute Physiology and Chronic Health Evaluation (APACHE) II score < 20 or one organ failure – should not receive rhAPC (1A).]
-
Maintain glucose control ≥ 3.9 mmol/l but ≤ 8.3 mmol/l (2C).
-
Maintain a median inspiratory plateau pressure < 30 cmH2O for mechanically ventilated patients (1C).
Responses to the survey are reported for each component of the management bundle below.
-
Administer low-dose steroids for septic shock in accordance with a standardised critical-care policy (if not administered, document why the patient did not qualify for low-dose steroids) (2C).
A high proportion of respondents (n = 116, 94%) reported that steroids were given in their units for persistent hypotension in septic shock. Although there was variation across units, it was estimated that a high proportion of patients with severe sepsis were given steroids (median 75%, IQR 43–99%).
-
Administer rhAPC in accordance with a standardised critical-care policy (if not administered, document why the patient did not qualify for rhAPC). [2B or 2C for postoperative patients; SSC guidelines state that adult patients with severe sepsis and low risk of death – typically, APACHE II score < 20 or one organ failure – should not receive rhAPC (1A).]
A high proportion of respondents (n = 105, 85%) reported that rhAPC was administered to some patients in their unit with severe sepsis. There was variation across units in the proportion of patients who were estimated to receive rhAPC; however, overall, the median proportion was estimated to be 10% (IQR 5–21%).
-
Maintain glucose control ≥ 3.9 mmol/l, but ≤ 8.3 mmol/l (2C).
Nearly all respondents (n = 119, 97%) reported that blood glucose control formed part of their unit’s management of patients with severe sepsis. Respondents indicated that blood glucose levels were maintained somewhere within the range of 4–10 mmol/l, although there was variation as to how tightly clinicians aimed to control levels. For example, 35% of respondents reported aiming to keep blood glucose within the range 6–8 mmol/l and 31% within the range 8–10 mmol/l, the latter, higher range resulting from more recent results from a large, multicentre RCT of glucose control. 23
-
Maintain a median inspiratory plateau pressure < 30 cmH2O for mechanically ventilated patients (1C).
Of the 123 respondents, 110 (89%) reported that they aimed to keep the inspiratory plateau pressure < 30 cmH2O for mechanically ventilated patients. Overall, respondents estimated that this was done for a high proportion of their patients (mean 87.1%, SD 1.4).
Use of selective decontamination of the digestive tract
Only 11 (9%) respondents reported that their unit delivered SDD.
Use of intravenous immunoglobulin
Seventy (56.9%) respondents reported that they used IVIG for advanced management of patients. The clinical reasons given for administering IVIG included neurological diseases, e.g. myasthenia gravis and Guillain–Barré syndrome; toxin-mediated illnesses, e.g. invasive group A streptococcal disease, toxic shock syndrome, necrotising fasciitis, Clostridium difficile colitis, Panton–Valentine leukocidin toxin-producing staphylococcal infection; and other indications, e.g. severe sepsis, liver disease, haematological disease, bronchospasm and immunocompromised patients.
Adoption of resuscitation and management bundles
Overall, 91 (74%) respondents reported that they had adopted a resuscitation bundle and 97 (79%) respondents reported that they had adopted a management bundle. These were mostly the SSC bundles. In addition, 21 respondents reported using the Survive Sepsis UK Sepsis Six24 (Table 2).
| Bundle | n (%) |
|---|---|
| Resuscitation | |
| SSC | 73 (59.3) |
| Own bundle | 18 (14.6) |
| Management | |
| SSC | 76 (61.8) |
| Own bundle | 21 (17.1) |
| Survive Sepsis UK Sepsis Six | 21 (17.1) |
Discussion
The survey indicated that there has been high uptake (> 70%) of bundles for the resuscitation and management of patients with severe sepsis, predominantly those recommended by the SSC. The responses to the survey indicated that, despite variation across units, usual clinical practice for patients with severe sepsis can be broadly summarised into immediate resuscitation and advanced management, as follows.
Resuscitation
-
Take blood cultures.
-
Give intravenous antibiotics within 1 hour.
-
Maintain serum lactate < 4 mmol/l.
-
Fluid resuscitate using a combination of crystalloids and colloids.
-
Maintain MAP ≥ 65 mmHg.
-
Maintain CVP ≥ 8 mmHg (or 10–15 mmHg for mechanically ventilated patients).
-
Give noradrenaline for hypotension not responding to initial fluid resuscitation.
-
Maintain ScvO2 or SvO2 ≥ 70%.
Management
-
Administer low-dose steroids in accordance with standardised critical-care protocol.
-
Administer rhAPC in accordance with standardised critical-care protocol.
-
Maintain blood glucose levels within the range 4–10 mmol/l.
-
Maintain inspiratory plateau pressure < 30 cmH2O for mechanically ventilated patients.
-
Give prophylaxis for deep-vein thrombosis.
-
Give stress ulcer prophylaxis.
These results suggest that a protocolised/bundle approach to immediate resuscitation and advanced management would need to be considered for the usual-care arm in any future multicentre RCT of IVIG as an adjunctive therapy in the advanced management of patients acutely ill with severe sepsis. However, specifically with regard to advanced management, a degree of clinical discretion would need to be maintained, illustrated by the high level of variation in compliance with bundle elements in the survey. This variation most likely relates to the heterogeneous nature of the severe sepsis population.
It should be noted that the main limitation of this survey is, despite regular follow-up of non-responders via e-mail and telephone, the low response rate. A major reason for the poor response, based on anecdotal evidence from critical-care clinicians, was the H1N1 swine influenza pandemic. Logistical and management issues took priority over research activities as senior clinicians were required to plan for the pandemic, such as extending critical care areas to be able to cope with additional demands for critical-care services. However, despite the poor response, data from the survey provide useful information on the now widespread adoption, initially resisted, of a protocolised approach to care for patients with severe sepsis in the UK.
Finally, these survey data provide the context for the case mix and outcome data, from the ICNARC CMP Database, used to inform the cost-effectiveness modelling.
Chapter 3 Clinical effectiveness of intravenous immunoglobulin for severe sepsis and septic shock
Objective
To assess the clinical effectiveness of IVIG for severe sepsis and septic shock, and to obtain the appropriate inputs for the relative efficacy parameters and the key uncertainties associated with these parameters, required to populate the decision model.
Methods
Literature searching
The search strategy was divided into four stages.
Stage 1: previous systematic reviews
Previous systematic reviews evaluating the effectiveness of IVIG were identified by one of the authors (PP). Individual studies, identified from these systematic reviews, were assessed against the inclusion criteria for the current review.
Stage 2: updating existing systematic review
A literature search was conducted to update Alejandria et al. ,9 a previous Cochrane review most relevant to our current review. Literature searching was conducted for the dates 1 January 2002 to 2 October 2009 and the search strategy employed is presented in Appendix 2. The following search terms were employed: immunoglobulin*, IVIG, sepsis, septic shock, septicaemia and septicemia. The following databases were searched; the Cochrane Infectious Diseases Group Specialized Trials Register, the Cochrane Trials Register, MEDLINE and EMBASE. No language restrictions were applied. All studies identified from these searches were assessed against the inclusion criteria for the current review. A check was conducted to ensure that all studies and systematic reviews, identified from stage 1, were also identified from the literature searching for stage 2.
Stage 3: review of excluded studies from existing systematic review
The Alejandria et al. 9 review focused on placebo-controlled trials and excluded any studies evaluating active-versus-active comparisons. Our review of the clinical effectiveness of IVIG included these active-versus-active studies and, to this end, all studies excluded from Alejandria et al. 9 as an active-versus-active comparison were considered for potential inclusion for the current review. In addition, any studies evaluating active-versus-active comparisons published since Alejandria et al. 9 were also identified from the literature searching in stage 2.
Stage 4: final comparison with update of existing systematic review
Towards the end of the current review, Alejandria et al. published an update to their existing Cochrane review. 15 This update was checked to ensure that no further studies, not already identified by us, had been identified by these authors.
The titles for all the studies, identified from the literature searching, were screened for potential inclusion and, of those identified as potentially relevant, the abstracts were obtained and screened for inclusion. Full-text copy was obtained for all studies identified as potentially relevant from screening the abstract. Translation of the abstract, methods section and tables of results was conducted for those studies published in non-English-language journals.
Inclusion criteria
Inclusion criteria covered design, setting, participants, intervention and outcome measures, as follows:
-
design: RCT
-
setting: critical-care setting
-
participants: adult patients with severe sepsis or septic shock
-
intervention: any standard polyclonal IVIG or immunoglobulin (IgM)-enriched polyclonal IVIG (IVIGAM) compared with no intervention, placebo or another standard polyclonal IVIG or IVIGAM preparation
-
outcome measures: all-cause mortality, all-cause mortality reported by subgroup and adverse events.
For design, studies that used alternative (rather than randomly generated) allocation sequence were excluded. For participants, studies were included if the majority of patients were aged ≥ 18 years. Clinical judgement was used to determine if the population studied had severe sepsis or septic shock. Studies were assessed by a clinician member of our study team (MSH) and the decision was verified by a clinician member of the Expert Group (MS).
Data extraction
Data were extracted from studies by two independent reviewers (NJW and JJM) using a standardised data extraction spreadsheet. Duplicate extraction was performed for 9/17 (53%) of the studies and any differences were resolved through discussion. Extracted data from all studies were compared with extracted data reported in the previous systematic reviews identified from stage 1 of the literature searching (see Stage 1: previous systematic reviews). Finally, all clinical data were double-extracted by a clinician on the study team (MSH) and any queries addressed and confirmed through discussion with clinical experts on the Expert Group (WACS and MS).
Data extraction covered details, quality, population, intervention and outcomes for each study. Data were extracted for all, where available.
-
Details: date recruitment started; study duration; publication date; critical-care setting reported; whether or not multicentre and, if so, the number of centres.
-
Quality: whether or not concealment of allocation to treatment was adequate/unclear/inadequate; whether or not blinding to treatment was adequate/unclear/inadequate; whether or not randomisation procedure was adequate/unclear/inadequate; whether or not an intention-to-treat analysis was performed; whether or not the trial received funding from industry sponsors; and the Jadad score,25 which is based on a composite score for adequacy of randomisation (0–2 points), blinding (0–2 points) and presence or absence of attrition information (0–1 points), yielding a score from 0 to 5, where 5 represents the best-quality score.
-
Population: study inclusion and exclusion criteria; proportion of male/female patients; mean age; proportion of patients with septic shock; baseline severity scores [APACHE II score26; Simplified Acute Physiology Score (SAPS) II27; Sequential Organ Failure Assessment (SOFA)28; Sepsis Score29]; multiorgan dysfunction/organ failure/number and type of organ failures.
-
Intervention: IVIG product used in the intervention arm(s); information on dosing, including daily dose (g/kg day); volume of fluid given (ml/kg day); duration of treatment (days); total dose (g/kg); description of the control intervention.
-
Outcomes: number of events (deaths) out of total number of patients per trial arm; follow-up duration; any reported adverse events; duration of mechanical ventilation; duration of critical-care unit stay; and duration of acute hospital stay.
Data analysis
Data analysis was divided into descriptive analyses and modelling.
Descriptive analyses
Summary tables describing the studies, identified from the literature searching and meeting the inclusion criteria and the data extracted from each study, were presented. The primary outcome measure was mortality, which was summarised on the odds ratio (OR) scale. Forest plots were produced to display results across studies. Both fixed- and random-effects models were considered and results presented for both, using inverse variance weights for both models. The I2 measure and Cochrane Q-statistic were used to describe and test for heterogeneity. Potential sources of heterogeneity were explored descriptively by plotting fixed-effects meta-analyses categorised by the following possible explanatory factors:
-
whether or not IVIG or IVIGAM used
-
whether albumin or no treatment used as control
-
duration of treatment (days)
-
quartiles of daily dose (g/kg/day)
-
quartiles of volume of fluid (ml/kg/day)
-
quartiles of total dose (g/kg)
-
whether or not an intention-to-treat analysis performed
-
whether or not concealment of allocation to treatment adequate/unclear/inadequate
-
whether or not blinding to treatment adequate/unclear/inadequate
-
whether or not randomisation procedure adequate/unclear/inadequate
-
Jadad score
-
whether or not industry sponsorship was acknowledged
-
quartiles by publication date
-
quartiles of sample size (intervention arm)
-
whether or not the study clearly took place in a critical-care setting
-
quartiles of baseline risk (control arm log-odds of mortality)
-
follow-up period (weeks).
Relationships between the potential explanatory factors were presented using scatterplots. Publication bias was investigated by inspecting a funnel plot for asymmetry,30 as well as by using the descriptive results categorised by quartiles of sample size (above).
Stata version 11.0 (StataCorp LP, College Station, TX, USA) was used for all the descriptive analyses except for the scatterplots, which were produced in Microsoft Excel version 2007 (Microsoft Corporation, Redmond, WA, USA).
Modelling
More formal modelling selection processes were performed to identify the key covariates (listed above) responsible for heterogeneity and for considering combinations of covariates to adjust for potential confounding. The descriptive analyses were restricted by having to combine all the IVIG preparations into a single ‘intervention’ whereas, in the modelling work, consideration of the type of IVIG preparation was an important explanatory factor for the treatment effect. For the modelling, the evidence forms a network of treatment comparisons, often termed mixed-treatment comparisons, multiple treatments meta-analysis or network meta-analysis. 31–34 A Bayesian approach to model estimation was conducted using Markov chain Monte Carlo simulation in WinBUGS version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK). 35,36
The posterior mean residual deviance (D¯res) was used to measure model fit and the deviance information criterion (DIC), a composite measure of model fit and model complexity, was used to choose between competing models. 37 For the random-effects models, the posterior mean of the between-study SD parameter (τ¯) was used to investigate the impact of the inclusion of the covariates on explaining (reducing) heterogeneity. Model selection proceeded as follows.
First, a simple two-treatment model, grouping all IVIG preparations into a single IVIG treatment category and all controls into a single control category, was used. Fixed- and random-effects models were fitted and model fit statistics (D¯res, DIC and τ¯) were compared to investigate evidence of heterogeneity. Where evidence of heterogeneity was identified, this was explored by fitting a fixed-effects model with each of the potential covariates (listed above) individually. Key covariates that explained some of the heterogeneity using model fit statistics (D¯res, DIC and τ¯) were identified. In addition, combinations of key potential covariates were explored to identify which of the covariates best explained the heterogeneity, after having adjusted for other covariates.
Second, the above modelling was repeated for other treatment models, in which the type of IVIG preparation and type of control were not grouped together. However, this modelling was restricted to investigating solely the key covariates identified from the simple two-treatment model above, to keep the set of models fitted realistic and feasible.
All treatment and covariate models were compared using the model fit statistics (D¯res, DIC and τ¯). Results were reported for the best-fitting, competing models.
Results
Literature searching/inclusion criteria
Stage 1: previous systematic reviews
Table 3 lists and describes the six previous systematic reviews that were identified as relevant to our current review. 9–15 All the previous systematic reviews reported all-cause mortality as their primary outcome. The previous systematic reviews differed, however, in the age of the populations considered (adults, children, neonates or no age restriction), the population included (sepsis, severe sepsis, septic shock) and the IVIG preparations included.
| Systematic review | Outcomes measured | Population studied | Intervention(s)/control | Study design included | Databases searched |
|---|---|---|---|---|---|
| Kreymann et al. (2007)10 | 28-day mortality (if reported), critical-care unit or hospital mortality | Adults, children or neonates with proven sepsis or septic shock (equivalent to ACCP/SCCM guidelines) | Polyclonal IVIG (excluded older 5S IVIG preparations) | RCTs, any language | MEDLINE, EMBASE, Cochrane Library (to 14 August 2006) |
| Laupland et al. (2007)11 | All-cause mortality | Adults admitted to critical-care units with severe infection, sepsis or septic shock | Polyclonal IVIG vs placebo or no treatment | RCTs, intention to treat, any language | MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, other sources (to 24 March 2006) |
| Turgeon et al. (2007)12 | Mortality, duration of critical-care unit stay, duration of mechanical ventilation | Adults (majority ≥ 18 years) critically ill with sepsis | IVIG vs placebo or no treatment | RCTs, any language | MEDLINE, Cochrane Central Register of Controlled Trials, other sources (to May 2006) |
| Norrby-Tegland et al. (2006)13 | All-cause mortality | No age restriction, sepsis patients | IVIGAM vs placebo or no treatment | Prospective, controlled studies | MEDLINE, published Cochrane reviews (search dates not reported) |
| Pildal and Gøtzsche (2004)14 | 30-day mortality, duration of acute hospital stay, complications, adverse events | No age restriction, suspected or proven sepsis or septic shock | Polyclonal IVIG vs placebo or no treatment | RCTs, any language | PubMed, EMBASE, Cochrane Library (to 21 January 2004) |
| Alejandria et al. (2002)9 [Update Alejandria et al. (2010)15] | All-cause mortality, mortality from septic shock, bacteriological failure rates, duration of acute hospital stay | No age restriction, sepsis or septic shock caused by bacteria | Any monoclonal or polyclonal IVIG vs placebo or no treatment | RCTs, any language | Cochrane Infectious Diseases Group Specialized Trials Register, Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE (to October 2002) [to October 2008] |
To this end, all the previous systematic reviews9–15 included a slightly different set of studies (Table 4). 38–58 There were 21 studies of adults;38–58 of these, two were excluded as they were duplicate studies,38,39 one study was excluded because only a proportion of the patients were determined to have had severe sepsis40 and one study was excluded because the IVIG preparation used was a mixture of a commercially available immunoglobulin G (IgG) preparation with an unspecified, locally produced IgM preparation that was not generally available. 41 This trial was terminated early because of a lack of availability of the intervention-arm treatment and included peritonitis patients, diagnosed during operation, without any further clinical diagnosis of severe sepsis. The remaining 17 studies42–58 met our inclusion criteria (see Table 4).
| Study | Included in previous systematic reviews? | Include in current review? | Comments | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Kreymann et al. (2007)10 | Laupland et al. (2007)11 | Turgeon et al. (2007)12 | Norrby-Tegland et al. (2006)13 | Pildal and Gøtzsche (2004)14 | Alejandria et al. (2002)9 | Alejandria et al. (2010)15 [update] | |||
| Rodriguez et al. (2005)42 | X | X | X | X | X | Yes | |||
| Hentrich et al. (2006)43 | X | X | X | X | Yes | ||||
| Karatzas et al. (2002)44 | X | X | X | X | X | Yes | |||
| Tugrul et al. (2002)45 | X | X | X | X | X | X | Yes | ||
| Behre et al. (1995)46 | X | X | X | X | Yes | ||||
| Schedel et al. (1991)47 | X | X | X | X | X | X | X | Yes | |
| Wesoly et al. (1990)48 | X | X | X | X | X | X | Yes | Included after consultation with clinicians (MSH and MS) | |
| Vogel et al. (1988)38 | X | X | No | From book chapter – reported data same as Spannbrucker et al.49 | |||||
| Spannbrucker et al. (1987)49 | X | Yes | |||||||
| Just et al. (1986)40 | X | X | X | X | X | No | Excluded after consultation with clinicians (MSH and MS) | ||
| Dominioni et al. (1996)50 | X | X | X | X | X | Yes | |||
| Burns et al. (1991)51 | X | X | X | X | Yes | Included after consultation with clinicians (MSH and MS) | |||
| Dominioni et al. (1991)39 | X | X | No | Reported data interim analysis for Dominioni et al.50 | |||||
| De Simone et al. (1988)52 | X | X | X | X | X | X | Yes | ||
| Werden et al. (2007)53 | X | X | X | X | X | Yes | |||
| Grundmann and Hornung (1988)54 | X | X | X | X | X | X | Yes | ||
| Yakut et al. (1998)55 | X | X | X | X | X | Yes | |||
| Darenberg et al. (2003)56 | X | X | X | X | X | Yes | |||
| Jesdinsky et al. (1987)41 | X | No | Intervention mixture of commercially available IgG with unspecified, locally produced IgM preparation, not generally available. Trial terminated early because of the lack of supply of intervention. Population not relevanta | ||||||
| Lindquist et al. (1981)57 | X | X | X | Yes | Included after consultation with clinicians (MSH and MS) | ||||
| Masaoka et al. (2000)58 | X | X | Yes | ||||||
Stage 2: updating existing systematic review
The literature search initially identified 215 references (available from the authors on request). Titles of the 215 references were scanned for relevance, based on the inclusion criteria, and this identified 48 references as potentially relevant. Abstracts of the 48 references (available from the authors on request) were scanned for relevance, again based on the inclusion criteria, and this identified 12 references42–45,53,56,59,60–64 as potentially relevant. Full-text copy was obtained for each of the 12 references and, of these, six42–45,53,56 were already identified from the six previous systematic reviews. Of the remaining six,59–64 one60 was a duplicate reference and five59,61–64 failed to meet the inclusion criteria for our current review (Table 5). In summary, no new studies were identified for inclusion to update the existing systematic review.
| Study | Include in current review? | Reasons for exclusion |
|---|---|---|
| Raphael et al. (2001)59 | No | Guillain–Barré syndrome not sepsis patients |
| Tugrul et al. (2001)60 | No | Same study as Tugrul et al.45 |
| Tugrul et al. (2002)45 | Yes, identified by previous systematic review (Table 4) | |
| Karatzas et al. (2002)44 | Yes, identified by previous systematic review (Table 4) | |
| Darenberg et al. (2003)56 | Yes, identified by previous systematic review (Table 4) | |
| Bellomo et al. (2004)61 | No | Review – provided useful information on Masaoka et al.58 |
| Reith et al. (2004)62 | No | Preoperative intervention – sepsis/organ damage listed as exclusion |
| Buda et al. (2005)63 | No | Retrospective, case–control study |
| Rodriguez et al. (2005)42 | Yes, identified by previous systematic review (Table 4) | |
| Hentrich et al. (2006)43 | Yes, identified by previous systematic review (Table 4) | |
| Khan and Sewell (2007)64 | No | Letter citing non-randomised study of adverse events of IVIG |
| Werdan et al. (2007)53 | Yes, identified by previous systematic review (Table 4) |
Stage 3: review of excluded studies from existing systematic review
Titles of excluded studies from Alejandria et al. 9 were scanned for possible inclusion in the current review on the basis that active-versus-active comparisons, which were excluded from Alejandria et al. ,9 were included in the current review. This identified two references65,66 as potentially relevant. Full-text copy was obtained and the citations in these identified a further reference as potentially relevant. Of these three additional studies,65–67 none satisfied our inclusion criteria (Table 6).
| Study | Include in current review? | Reasons for exclusion |
|---|---|---|
| Calandra et al. (1988)65 | No | Comparison of standard preparation IVIG with specific IgG antibody to Escherichia coli J5 (J5-IVIG) – not considered standard IVIG product and deemed not relevant |
| Pilz et al. (1997)66 | No | Prophylactic use of IVIG in patients at high risk of sepsis |
| Keane et al. (1991)67 | No | Protracted septic states (> 5 days) listed as exclusion – only 5/17 in the intervention group had severe sepsis/septic shock |
Stage 4: final comparison with update of existing systematic review
There were no further studies identified from Alejandria et al. 15 that were not already identified in stage 1.
In summary, 17 studies were identified that met our inclusion criteria. 42–58 The studies included in our review were very similar to those in the recently updated Cochrane review;15 however, Just et al. 40 was omitted and Spannbrucker et al. 49 included.
Data extraction
The data extracted from the 17 included studies42–55 are summarised in Tables 7–13.
Study characteristics
Table 7 presents basic characteristics of the included studies. Initiation of recruitment ranged from 1977 to 1999. Of particular note is the long delay from the start of recruitment (1991) to full publication (2007) for one of the largest studies, Werdan et al. 53 Seven42,43,46,50,53,56,58 out of the 17 studies (41%) were multicentre trials.
| Study number | Study | Initiation of recruitment (year) | Date of publication (year) | Total centres, n | Total patients, n | Reported critical-care unit setting? |
|---|---|---|---|---|---|---|
| 1 | Rodriguez et al. (2005)42 | 1996 | 2005 | 7 | 56 | Yes |
| 2 | Hentrich et al. (2006)43 | 1992 | 2006 | 6 | 206 | No |
| 3 | Karatzas et al. (2002)44 | NR | 2002 | 1 | 68 | No |
| 4 | Tugrul et al. (2002)45 | NR | 2002 | 1 | 42 | No |
| 5 | Behre et al. (1995)46 | 1992 | 1995 | 2 | 52 | No |
| 6 | Schedel et al. (1991)47 | 1985 | 1991 | 1 | 55 | No |
| 7 | Wesoly et al. (1990)48 | NR | 1990 | 1 | 35 | Yes |
| 8 | Spannbruker et al. (1987)49 | NR | 1987 | 1 | 50 | Yes |
| 9 | Dominioni et al. (1996)50 | 1986 | 1996 | 4 | 113 | Yes |
| 10 | Burns et al. (1991)51 | NR | 1991 | 1 | 38 | No |
| 11 | De Simone et al. (1988)52 | 1984 | 1988 | 1 | 24 | Yes |
| 12 | Werdan et al. (2007)53 | 1991 | 2007 | 23 | 624 | Yes |
| 13 | Grundmann and Hornung (1988)54 | NR | 1988 | 1 | 46 | Yes |
| 14 | Darenberg et al. (2003)56 | 1999 | 2003 | 17 | 21 | No |
| 15 | Lindquist et al. (1981)57 | 1977 | 1981 | 1 | 148 | No |
| 16 | Masaoka et al. (2000)58 | 1993 | 2000 | 141 | 682 | No |
| 17 | Yakut et al. (1998)55 | 1992 | 1998 | 1 | 40 | Yes |
Only 842,48–50,52–55 out of the 17 studies (47%) explicitly reported that they were carried out in a critical-care unit setting; however, our assessment of the patient characteristics for inclusion in the other studies indicated a population with severe sepsis in each case, and so it was inferred that these studies would have been conducted in a critical-care unit setting. Many of the studies were small, with as few as 20 patients in total randomised to treatment arms in one trial. There are two large studies: Werdan et al. ,53 with 624 patients randomised, and Masaoka et al. ,58 with 682 patients randomised.
Study quality and publication bias
Table 8 reports the assessment of study quality metrics. Concealment of allocation to treatments was considered adequate in 5/17 (29.4%) studies,42,43,47,53,58 inadequate in 2/17 (11.8%) studies,52,57 and it was not declared and was therefore unclear from the published paper for the remaining 10/17 (58.8%) studies. 44–46,48–51,54–56 For blinding of patients and assessors to treatment received was considered adequate in 5/17 (29.4%) studies,42,50,51,53,56 inadequate in 5/17 (29.4%) studies,43,47,52,57,58 and it was unclear in the published paper for the remaining 7/17 (41.2%) studies. 44–46,48,49,54,55 The majority of the studies, 9/17 (52.9%),42,43,45,47–49,53,54,58 used an appropriate method of randomisation, although this was unclear in the published paper in the remaining 8/17 (47.1%) studies. 44,46,50–52,55–57 An intention-to-treat analysis was performed in 12/17 (70.6%) studies,42,43,45,46,48,49,52–56,58 was not performed in 3/17 (17.6%) studies,47,50,51 and it was unclear from the published paper for the remaining 2/17 (11.8%) studies. 44,57 The Jadad score, a composite measure of study quality ranging from 0 to 5 (where 5 represents best study quality). This analysis revealed that only 4/17 (23.5%) studies achieved a Jadad score of 5,42,51,53,56 7/17 (41.2%) achieved a Jadad score of 3,43,45,47,50,55,57,58 and the remaining 6/17 (35.3%) studies achieved a Jadad score of ≤ 2. 44,46,48,49,52,54 Industry sponsorship was acknowledged in 7/17 (41.2%) studies. 42,43,47,51,53,56,58 For the remaining studies it was unclear if there was industry sponsorship. 44–46,48–50,52,54,55,57
| Study number | Study | Allocation concealment | Blinding | Randomisation | Intention-to-treat analysis? | Jadad score | Industry sponsorship declared? |
|---|---|---|---|---|---|---|---|
| 1 | Rodriguez et al. (2005)42 | Adequate | Adequate | Adequate | Yes | 5 | Yes |
| 2 | Hentrich et al. (2006)43 | Adequate | Inadequate | Adequate | Yes | 3 | Yes |
| 3 | Karatzas et al. (2002)44 | Unclear | Unclear | Unclear | Unclear | 2 | No |
| 4 | Tugrul et al. (2002)45 | Unclear | Unclear | Adequate | Yes | 3 | No |
| 5 | Behre et al. (1995)46 | Unclear | Unclear | Unclear | Yes | 1 | No |
| 6 | Schedel et al. (1991)47 | Adequate | Inadequate | Adequate | No | 3 | Yes |
| 7 | Wesoly et al. (1990)48 | Unclear | Unclear | Adequate | Yes | 1 | No |
| 8 | Spannbruker et al. (1987)49 | Unclear | Unclear | Adequate | Yes | 1 | No |
| 9 | Dominioni et al. (1996)50 | Unclear | Adequate | Unclear | No | 3 | No |
| 10 | Burns et al. (1991)51 | Unclear | Adequate | Unclear | No | 5 | Yes |
| 11 | De Simone et al. (1988)52 | Inadequate | Inadequate | Unclear | Yes | 1 | No |
| 12 | Werdan et al. (2007)53 | Adequate | Adequate | Adequate | Yes | 5 | Yes |
| 13 | Grundmann and Hornung (1988)54 | Unclear | Unclear | Adequate | Yes | 2 | No |
| 14 | Darenberg et al. (2003)56 | Unclear | Adequate | Unclear | Yes | 5 | Yes |
| 15 | Lindquist et al. (1981)57 | Inadequate | Inadequate | Unclear | Unclear | 3 | No |
| 16 | Masaoka et al. (2000)58 | Adequate | Inadequate | Adequate | Yes | 3 | Yes |
| 17 | Yakut et al. (1998)55 | Unclear | Unclear | Unclear | Yes | 3 | No |
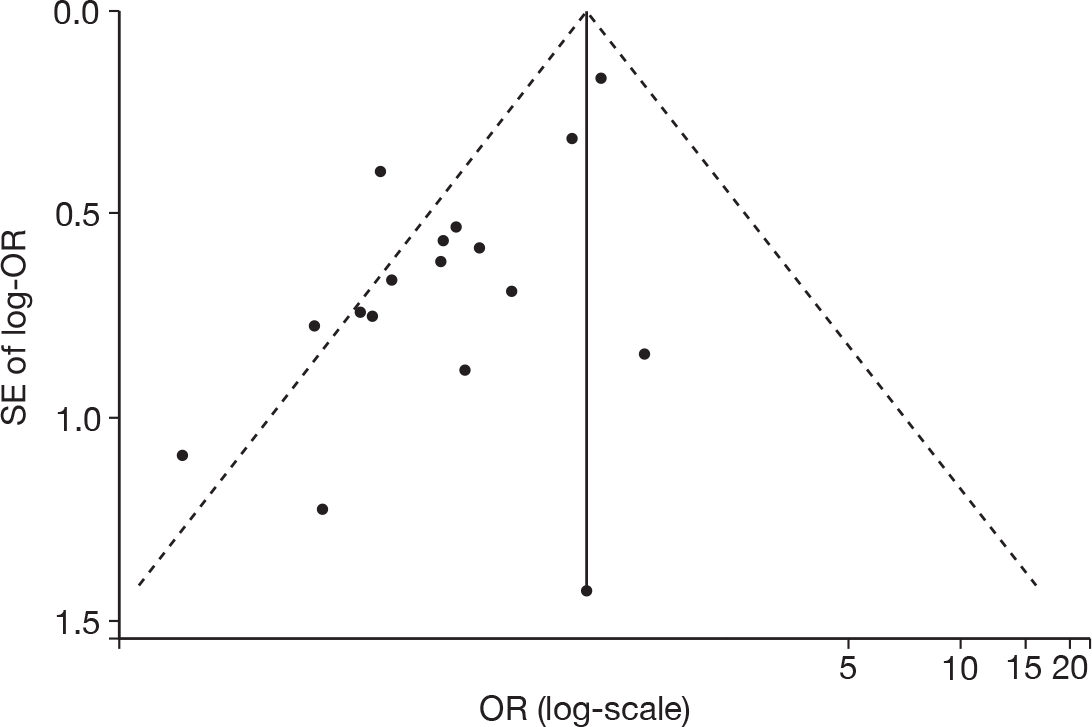
Figure 5 shows a funnel plot of the standard error (SE) of the effect size (log-OR) plotted against study effect size (OR on the log-scale). From this plot, it can be seen that there does appear to be funnel-plot asymmetry, where there are studies ‘missing’ from the right-hand-side of the plot when the SE is high (bottom right of plot), and this is supported by the Peters test for small-study effects (p = 0.0008). This suggests that there may potentially be an issue with publication bias with this evidence.
FIGURE 5.
Publication bias – funnel plot (with pseudo 95% confidence limits) of mortality for IVIG and IVGAM versus control.
Baseline patient characteristics
Participants in the studies are described in Table 9. The baseline patient characteristics were used to identify if studies met the severe sepsis/septic shock eligibility criterion for inclusion in our review.
| Study number | Study | Sepsis definition | Additional enrolment criteria and definitions |
|---|---|---|---|
| 1 | Rodriguez et al. (2005)42 | ACCP/SCCM criteria | Severe sepsis/septic shock of intra-abdominal origin admitted to a critical-care unit within 24 hours of onset of symptoms. Abdominal sepsis defined by the presence of SIRS and a surgically confirmed abdominal focus. Obtaining purulent material or detecting potential pathogens using Gram staining was mandatory. Appropriateness of the surgical procedure (successful eradication of focus), according to criteria of the attending surgical team and the intensivist, required for inclusion |
| 2 | Hentrich et al. (2006)43 | ACCP/SCCM criteria | Sepsis syndrome and:
|
| 3 | Karatzas et al. (2002)44 | ACCP/SCCM criteria | Severe sepsis |
| 4 | Tugrul et al. (2002)45 | ACCP/SCCM criteria | Severe sepsis |
| 5 | Behre et al. (1995)46 | ACCP/SCCM criteria | Sepsis syndrome and:
|
| 6 | Schedel et al. (1991)47 | ‘Septic shock’ | Detection of endotocaemia (> 12.5 pg/ml endotoxin) and at least five of the following criteria:
|
| 7 | Wesoly et al. (1990)48 | Sepsis score ≥ 12 | Postoperative |
| 8 | Spannbruker et al. (1987)49 | ‘Septic shock’ | |
| 9 | Dominioni et al. (1996)50 | Sepsis score ≥ 17 | Sepsis following surgery or trauma |
| 10 | Burns et al. (1991)51 |
|
Suspected infection documented by one or more of the following:
|
| 11 | De Simone et al. (1988)52 | ‘Severe sepsis’ | |
| 12 | Werdan et al. (2007)53 | At least four of nine ‘sepsis criteria’ |
|
| 13 | Grundmann and Hornung (1988)54 | Sepsis score > 12 | Postoperative Gram-negative bacterial infection with positive endotoxin in plasma for 2 subsequent days |
| 14 | Darenberg et al. (2003)56 | STSS consensus definition | Patients could be enrolled before results from bacteriological cultures were obtained if they had clinical symptoms of STSS and if a streptococcal infection was suspected |
| 15 | Lindquist et al. (1981)57 | Sepsis secondary to ‘septicaemia’ based on Svanbom criteria |
Purulent meningitis irrespective of aetiology Suspected or verified bacterial pneumonia (day-time admissions only) |
| 16 | Masaoka et al. (2000)58 | ACCP/SCCM criteria | Suspected sepsis, as defined by heart rate > 90/min, respiratory rate > 20/min, in addition to positive C-reactive protein and sustained fever ≥ 38°C with:
|
| 17 | Yakut et al. (1998)55 | Sepsis score > 16 | Post-surgical |
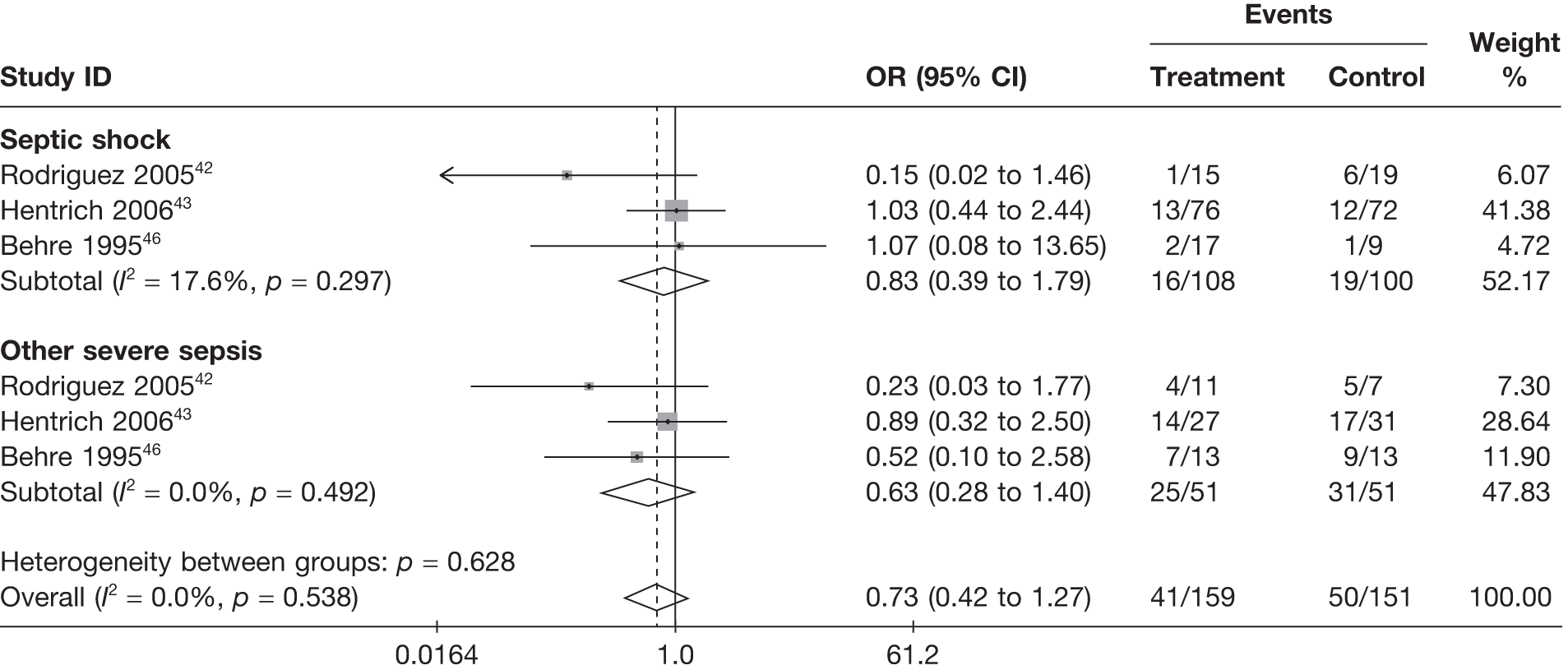
Summary patient baseline characteristics are reported in Table 10. Mean age was broadly comparable across treatment arms, both within and across studies. Mean severity was broadly comparable across treatment arms within studies but differed between studies, highlighting the heterogeneity in the severity of the severe sepsis/septic shock patients recruited into the different studies. The proportion of male patients randomised varied not just across studies, but also between treatment arms within studies. None of the studies reported mortality separately for men and women and, so, it is not possible to assess whether or not this baseline imbalance might introduce bias in the results. Similarly, where reported,42,43,46,52,53 the proportion of patients randomised with septic shock, rather than other severe sepsis, differed both across studies and across treatment arms within studies. Rodriguez et al. ,42 Hentrich et al. 43 and Behre et al. 46 reported results differentially by septic shock or other severe sepsis. These results showed that mortality rates were much lower for patients with septic shock than for those with severe sepsis; however, the treatment effects within these two subgroups did not differ substantially (Figure 6).
| Study number | Study | Age: mean (SD), years | Severity of illness: measure, mean (SD) | ||
|---|---|---|---|---|---|
| IVIG | Control | IVIG | Control | ||
| 1 | Rodriguez et al. (2005)42 | 61.3 (19.9) | 65.9 (18.2) | APACHE II 16.1 (5.9) | APACHE II 15.2 (6.1) |
| 2 | Hentrich et al. (2006)43 | 48.8 (NR) | 51.0 (NR) | NR | NR |
| 3 | Karatzas et al. (2002)44 | 50.5 (3.33) | 50.7 (7.4) | APACHE II 21.3 (7.2) | APACHE II 23.5 (7.9) |
| 4 | Tugrul et al. (2002)45 | 42 (18) | 49.3 (20.6) | APACHE II 10.5 (4.6) | APACHE II 14 (8.5) |
| 5 | Behre et al. (1995)46 | 50 (NR) | 55 (NR) | ||
| 6 | Schedel et al. (1991)47 | 46 (16) | 37 (18) | APACHE II 30a | APACHE II 24a |
| 7 | Wesoly et al. (1990)48 | 44.7 (19) | 54.8 (17) | Sepsis score 14.8 (2.5) | Sepsis score 16.3 (3.6) |
| 8 | Spannbruker et al. (1987)49 | 50.8 (15.5) | 54.5 (12) | NR | NR |
| 9 | Dominioni et al. (1996)50 | 55 (19) | 57 (19) | Sepsis score 23 (4) | Sepsis score 23 (4) |
| 10 | Burns et al. (1991)51 | 61.5 (NR) | 59.8 (NR) | NR | NR |
| 11 | De Simone et al. (1988)52 | 45 (4) | 45 (5) | NRb | NRb |
| 12 | Werdan et al. (2007)53 | 57.2 (13.7) | 57.7 (13.6) | APACHE II 27.6 (4.5) | APACHE II 28 (4.5) |
| 13 | Grundmann and Hornung (1988)54 | 46.9 (NR) | 52.8 (NR) | NR | NR |
| 14 | Darenberg et al. (2003)56 | 51.3 (NR) | 52.6 (NR) |
SAPS II 53 (NR) SOFA 11 (NR) |
SAPS II 51 (NR) SOFA 11 (NR) |
| 15 | Lindquist et al. (1981)57 | 48.3 (NR) | 39.2 (NR) | NR | NR |
| 16 | Masaoka et al. (2000)58 | NR | NR | NR | NR |
| 17 | Yakut et al. (1998)55 | 32 (16) | 31 (16) | APACHE II 16 (4) | APACHE II 16 (5) |
FIGURE 6.
Forest plot for fixed-effects model using inverse variance weights – IVIG/IVIGAM versus control for those studies reporting results by whether the patients have septic shock or severe sepsis.
Interventions
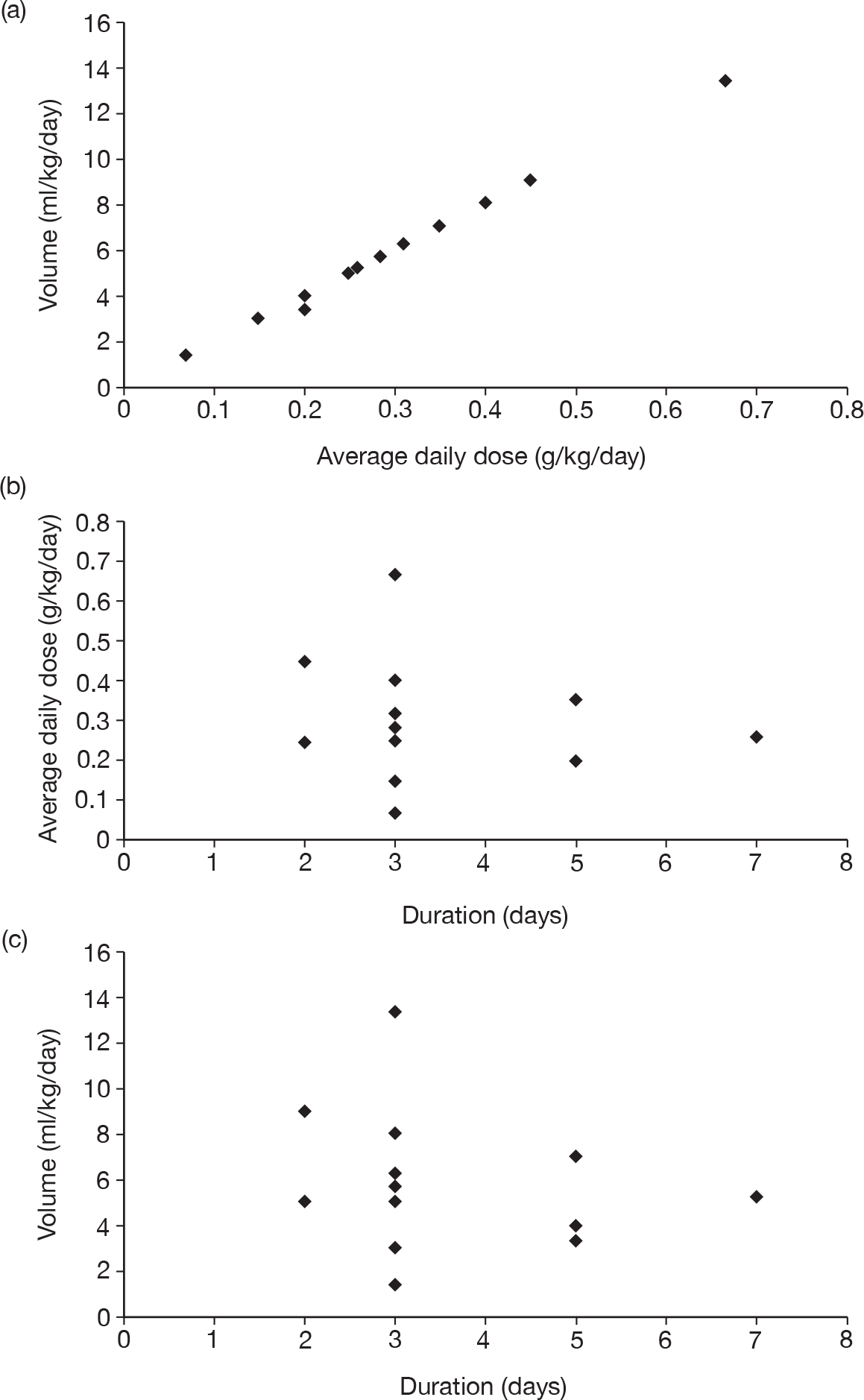
Table 11 describes the preparations used for the control and IVIG arms with the dosing regimes reported. In all cases, the control and IVIG arms were given as adjunct therapy to standard care, although standard care varied between studies. In 8/17 (47.1%) of the studies,42–49 Pentaglobin® (IVIGAM, Biotest Pharma, Germany), an IVIGAM, was used. In all other studies, standard preparations of IVIG were used; however, variation existed in the standard IVIG preparations used. The dosing regimes used also varied between the studies. Duration of treatment ranged from 2 days to 7 days (for some of the longer durations, treatment was not given on every day), with the majority of studies43–49,51,56–58 [11/17 (64.7%)] using a duration of 3 days. Average daily dose ranged from 0.07 g/kg/day to 0.67 g/kg/day, volume given ranged from 1.4 ml/kg/day to 13.34 ml/kg/day and total dose ranged from 0.45 g/kg to 2 g/kg. These dosing variables were inter-related as presented in Figure 7. There was a near perfect relationship between volume and average daily dose, which arose because nearly all studies used a 5% preparation. There was a negative relationship between volume (and average daily dose) and duration. These relationships may reflect the differences in dosing recommendations between different underlying disease conditions and the severity of sepsis.
| Study | Control | IVIG preparationa | IVIG dosing regime | Average daily doseb (g/kg/day) | Volume (ml/kg/day) | Duration of therapy (days) | Total dose (g/kg) |
|---|---|---|---|---|---|---|---|
| Rodriguez et al. (2005)42 | 5% HAS | Pentaglobin (Biotest Pharma, Germany) | 0.35 g/kg/day | 0.35 | 7 | 5 | 1.75 |
| Hentrich et al. (2006)43 | HAS | Pentaglobin (Biotest Pharma, Germany) | 1300 ml over 72 hours: 200 ml initially (0.5 ml/min) then 11 infusions of 100 ml every 6 hours | 0.31 | 6.2 | 3 | 0.93 |
| Karatzas et al. (2002)44 | No treatment | Pentaglobin (Biotest Pharma, Germany) | 5 ml kg/day over 6 hours | 0.25 | 5 | 3 | 0.75 |
| Tugrul et al. (2002)45 | No treatment | Pentaglobin (Biotest Pharma, Germany) | 5 ml kg/day over 6 hours | 0.25 | 5 | 3 | 0.75 |
| Behre et al. (1995)46 | 5% HAS | Pentaglobin (Biotest Pharma, Germany) | Loading dose 10 g then 5 g 6-hourly for 72 hours | 0.31 | 6.2 | 3 | 0.93 |
| Schedel et al. (1991)47 | No treatment | Pentaglobin (Biotest Pharma, Germany) | Loading dose 600 ml over 8 hours then two further doses of 300 ml every 24 hours | 0.285 | 5.7 | 3 | 0.855 |
| Wesoly et al. (1990)48 | No treatment | Pentaglobin (Biotest Pharma, Germany) | 0.25 g/kg/day | 0.25 | 5 | 3 | 0.75 |
| Spannbruker et al. (1987)49 | No treatment | Pentaglobin (Biotest Pharma, Germany) | 0.15 g/kg/day | 0.15 | 3 | 3 | 0.45 |
| Dominioni et al. (1996)50 | 5% HAS | Sandoglobulin (Sandoz Pharmaceutical Corp, Italy) |
0.4 g/kg on day 0 0.4 g/kg 24 hours later 0.2 g/kg 5 days later |
0.2 | 4 | 5 | 1 |
| Burns et al. (1991)51 | HAS | Sandoglobulin (Sandoz Pharmaceutical Corp, Italy) | 0.4 g/kg/day | 0.4 | 8 | 3 | 1.2 |
| De Simone et al. (1988)52 | No treatment | Sandoglobulin (Sandoz Pharmaceutical Corp, Italy) |
0.4 g/kg on day 0 0.2 g/kg 48 hours later 0.4 g/kg 5 days later |
0.2 | 3.33 | 5 | 1 |
| Werdan et al. (2007)53 | 0.1% HAS | Polyglobin N (Bayer Biological Products, Germany) |
0.6 g/kg on day 0 0.3 g/kg on day 1 or 2 |
0.45 | 9 | 2 | 0.9 |
| Grundmann and Hornung (1988)54 | No treatment | Intraglobin F (Biotest Pharma, Germany) | 0.25 g/kg/day | 0.25 | 5 | 2 | 0.5 |
| Darenberg et al. (2003)56 | 1% HAS | Endobulin SD (Baxter) | Loading dose of 1 g/kg then 0.5 g/kg every 24 hours for three doses | 0.667 | 13.34 | 3 | 2.001 |
| Lindquist et al. (1981)57 | No treatment | Pepsin-treated human gamma globulin – Gamma-venin | 0.15 g/kg over 1 hour | 0.15 | 3 | 3 | 0.45 |
| Masaoka et al. (2000)58 | No treatment | Not specified | 5 g/day for 3 consecutive days | 0.07 | 1.4 | 3 | 0.21 |
| Yakut et al. (1998)55 | 20% HAS | Gamimune N 10% (Miles Inc. Pharmaceutical Division, USA) |
0.4 g/kg on day 0 0.4 g/kg on day 1 0.2 g/kg on days 2–7 |
0.26 | 5.2 | 7 | 1.8 |
FIGURE 7.
Relationships between volume, average daily dose and duration of treatment.
For the analyses, several ways to allow for differences between the treatments and dosing regimes were considered. For the different IVIG and control preparations, five different possible treatment comparison models (numbered according to number of treatments) were considered:
-
model T2 – IVIG or IVIGAM versus albumin or no treatment
-
model T3a – IVIG versus IVIGAM versus albumin or no treatment
-
model T3b – IVIG or IVIGAM versus albumin versus no treatment
-
model T4 – IVIG versus IVIGAM versus albumin versus no treatment
-
model T10 – Sandoglobin® versus Intraglobin versus Gamma-Venin versus Polyglobin versus Endobulin versus Gamumin N versus IVIG unspecified versus IVIGAM versus albumin versus no treatment.
For the dosing regimes, extending the range of treatment comparison models according to dose was considered, but these models did not always result in a connected network of treatment comparisons. For those models that could be fitted, there was little to be gained from this approach. Dosing regime had multiple attributes and it was not clear how to define the treatments in this way. Instead, the attributes of the dosing regime (average daily dose, volume, duration and total dose) were considered as covariates for the five treatment comparison models described above.
Outcomes
The primary outcome for clinical effectiveness was all-cause mortality presented in Table 12. A range of follow-up periods were used across the studies. Mortality was highly variable between the studies. This was partly explained by the different follow-up periods, but mortality was still variable within the same follow-up period, reflecting the heterogeneous nature of the patient populations recruited to the different studies (different underlying diseases causing severe sepsis/septic shock and the acute severity of the illness).
| Study number | Study | All-cause mortality deaths/total (%) | Follow-up (days) | |
|---|---|---|---|---|
| IVIG | Control | |||
| 1 | Rodriguez et al. (2005)42 | 21/29 (72.4) | 13/27 (48.1) | Critical-care unit discharge |
| 2 | Hentrich et al. (2006)43 | 76/103 (73.8) | 29/103 (28.2) | 28 |
| 3 | Karatzas et al. (2002)44 | 26/34 (76.5) | 14/34 (41.2) | 28 |
| 4 | Tugrul et al. (2002)45 | 5/21 (23.8) | 7/21 (33.3) | 28 |
| 5 | Behre et al. (1995)46 | 9/30 (30.0) | 10/22 (45.5) | 28 |
| 6 | Schedel et al. (1991)47 | 1/27 (3.7) | 9/28 (32.1) | 42 |
| 7 | Wesoly et al. (1990)48 | 8/18 (44.4) | 13/17 (76.5) | Critical-care unit discharge |
| 8 | Spannbruker et al. (1987)49 | 6/25 (24.0) | 11/25 (44.0) | 12 |
| 9 | Dominioni et al. (1996)50 | 19/57 (33.3) | 36/56 (64.3) | Critical-care unit discharge |
| 10 | Burns et al. (1991)51 | 4/19 (21.1) | 3/19 (15.8) | 9 |
| 11 | De Simone et al. (1988)52 | 7/12 (58.3) | 9/12 (75.0) | 70 |
| 12 | Werdan et al. (2007)53 | 126/321 (39.3) | 113/303 (37.3) | 28 |
| 13 | Grundmann and Hornung (1988)54 | 15/24 (62.5) | 19/22 (86.4) | Critical-care unit discharge |
| 14 | Darenberg et al. (2003)56 | 1/10 (10.0) | 4/11 (36.4) | 28 |
| 15 | Lindquist et al. (1981)57 | 1/74 (1.4) | 1/74 (1.4) | 14 |
| 16 | Masaoka et al. (2000)58 | 3/339 (0.9) | 10/343 (2.9) | 7 |
| 17 | Yakut et al. (1998)55 | 3/21 (14.3) | 9/19 (47.4) | 28 |
Adverse events were reported in only six studies43,51,53,56–58 and these are presented in Table 13.
| Study | Mortality by subgroup | Adverse events | |||
|---|---|---|---|---|---|
| Subgroup | IVIG (%) | Control (%) | IVIG | Control | |
| Rodriguez et al. (2005)42 | Septic shock | 1/15 (6.7) | 6/19 (31.6) | NR | NR |
| Other severe sepsis | 4/11 (36.4) | 5/7 (71.4) | |||
| Hentrich et al. (2006)43 | Septic shock | 13/76 (17.1) | 12/72 (16.7) | Five events (WHO grade 1 allergic; grade 1 erythema; grade 2 nausea and vomiting; grade 4 allergic; grade 4 allergic) | None |
| Other severe sepsis | 14/27 (51.9) | 17/31 (54.8) | |||
| Karatzas et al. (2002)44 | NR | NR | NR | NR | NR |
| Tugrul et al. (2002)45 | NR | NR | NR | NR | NR |
| Behre et al. (1995)46 | Septic shock | 2/17 (11.8) | 1/9 (11.1) | NR | NR |
| Other severe sepsis | 7/13 (53.8) | 9/13 (69.2) | |||
| Schedel et al. (1991)47 | NR | NR | NR | NR | NR |
| Wesoly et al. (1990)48 | NR | NR | NR | NR | NR |
| Spannbruker et al. (1987)49 | NR | NR | NR | NR | NR |
| Dominioni et al. (1996)50 | Sepsis score > 25 | 8/14 (57.1) | 11/14 (78.6) | None | None |
| Sepsis score 20–25 | 11/33 (33.3) | 23/35 (65.7) | |||
| Sepsis score 17–19 | 0/10 (0.0) | 2/7 (28.6) | |||
| Burns et al. (1991)51 | NR | NR | NR | One event (clinically significant bleeding) | Four events (clinically significant bleeding) |
| De Simone et al. (1988)52 | NR | NR | NR | NR | NR |
| Werdan et al. (2007)53 | NR | NR | NR | Thirteen events in 11 patients, of which six were skin reactions. All patients experiencing adverse events were on antibiotics | Six events in six patients, of which six were skin reactions. All patients experiencing adverse events were on antibiotics |
| Grundmann and Hornung (1988)54 | NR | NR | NR | NR | NR |
| Darenberg et al. (2003)56 | NR | NR | NR | Six severe adverse events (deaths) and 12 adverse events or disease-related events. None of the events were reported to be related to the study drug | Six severe adverse events (deaths) and 12 adverse events or disease-related events. None of the events were reported to be related to the study drug |
| Lindquist et al. (1981)57 | NR | NR | NR | Nine events [shock (two); rigor, chills and somnolence (one); rigor, chills and elevation of temperature (five); and vomiting (one)] | None |
| Masaoka et al. (2000)58 | NR | NR | NR | Adverse events reported, but not broken down by treatment group | Adverse events reported, but not broken down by treatment group |
| Yakut et al. (1998)55 | NR | NR | NR | NR | NR |
Data analysis
Descriptive analyses
For all of the descriptive analyses, treatment model T2, comparing IVIG/IVIGAM versus albumin/no treatment, was used. All treatment effects are displayed as ORs with 95% confidence intervals (CIs). Figures 8 and 9 present forest plots for a fixed- and a random-effects meta-analysis, respectively. There is evidence of heterogeneity in the treatment effects (I2 = 46.9%, Q = 30.1, df = 16, p = 0.017). The pooled OR from the fixed-effects model is 0.68 (95% CI 0.54 to 0.84), showing a reduction in the odds of mortality with IVIG/IVIGAM compared with albumin/no treatment. The pooled OR from the random-effects model is 0.47 (95% CI 0.32 to 0.69), showing a stronger effect. Note that the large weight of the Werdan et al. 53 study drives the difference between the fixed- and random-effects models’ results because it is given less weight in the random-effects model.
FIGURE 8.
Forest plot for fixed-effects model using inverse variance weights – IVIG and IVIGAM treatments versus control.
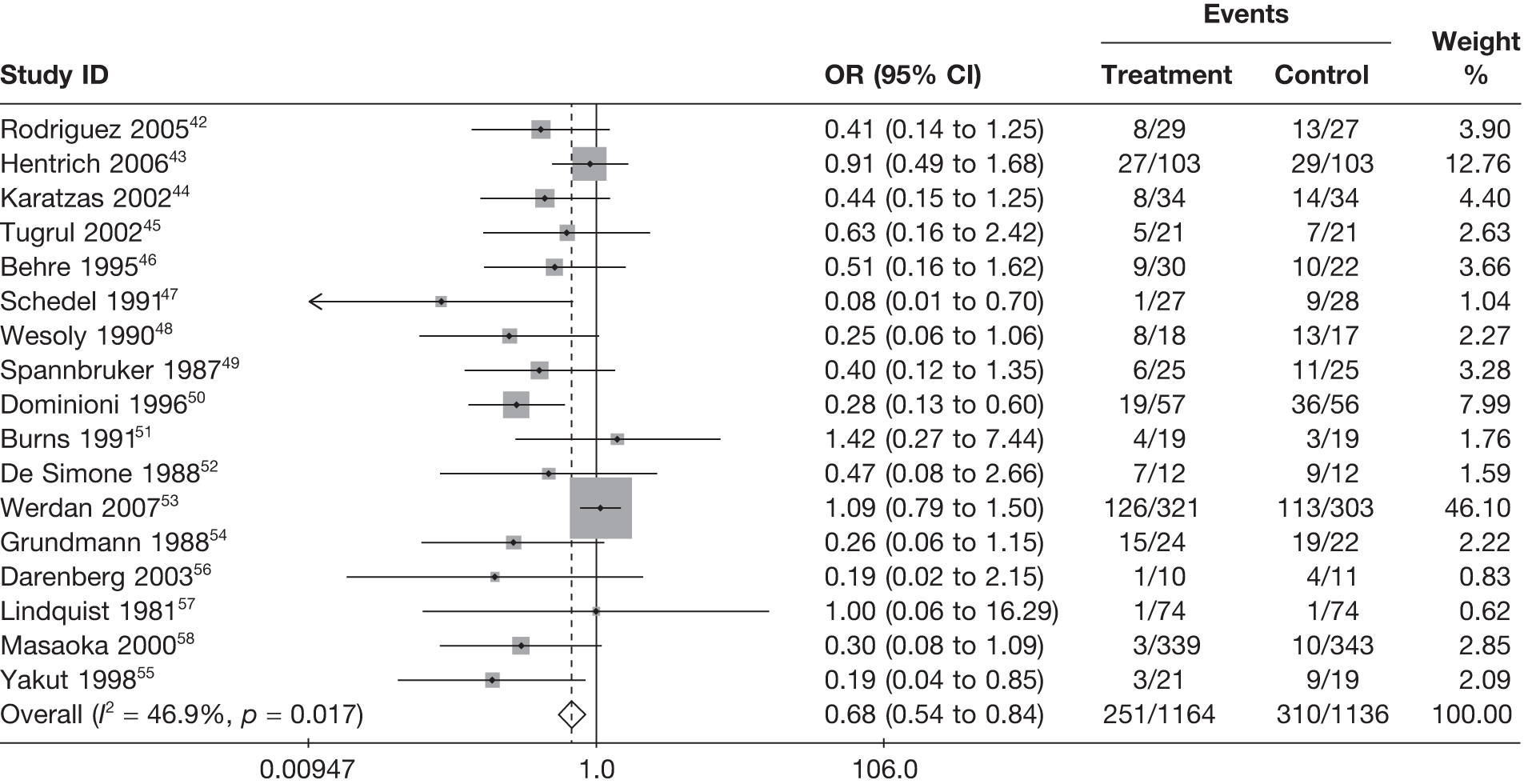
FIGURE 9.
Forest plot for random-effects model using inverse variance weights – IVIG and IVIGAM versus control.
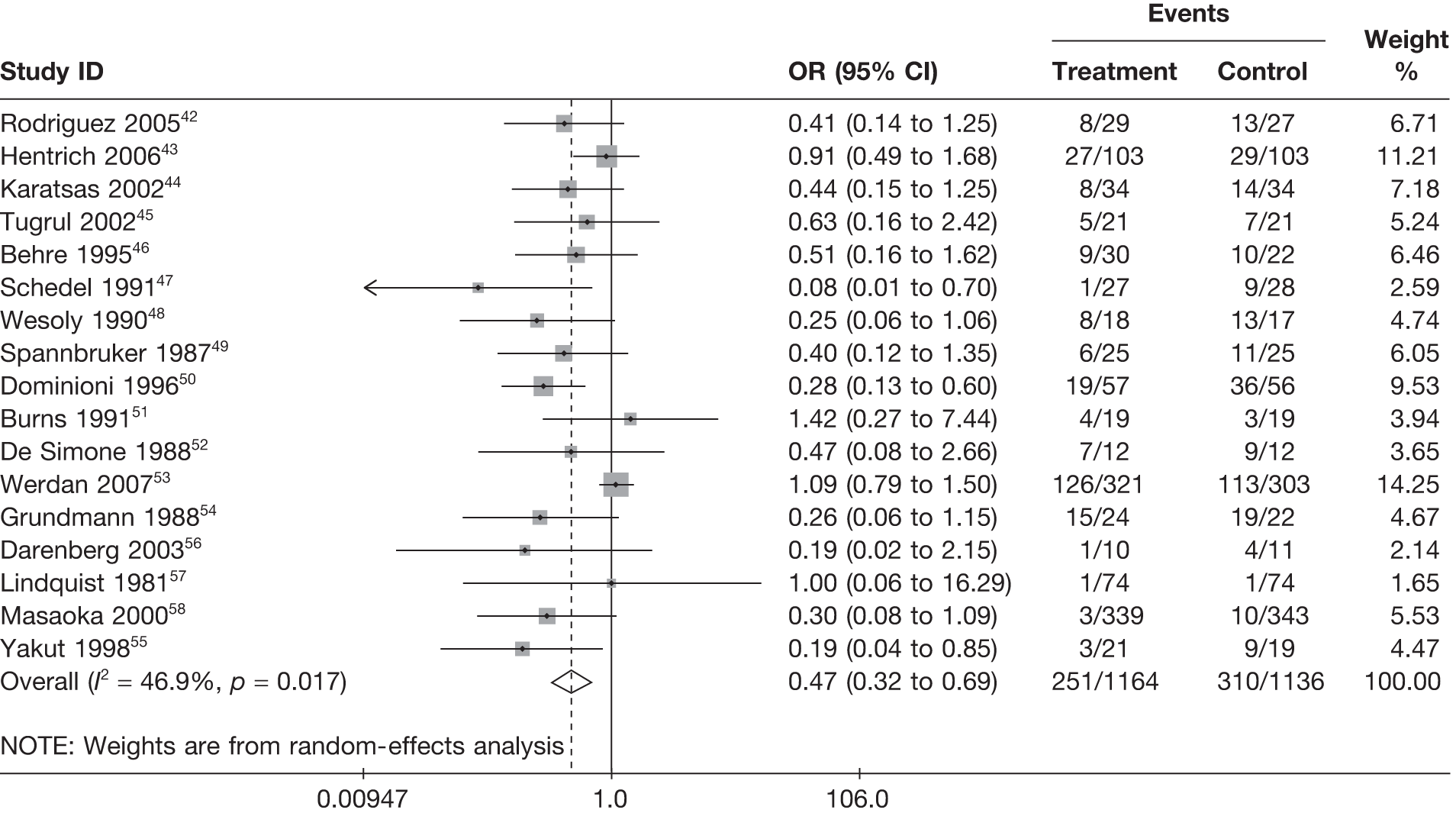
Heterogeneity in the study results was further explored by looking at the descriptive results for different values/subgroups of potential explanatory factors. In all cases, fixed-effects models are reported. Where the explanatory variable was a continuous measure (e.g. daily dose), studies were grouped into quartiles to explore trends in treatment effect over the continuous measure.
Type of intravenous immunoglobulin and control treatment
Figure 10 indicates that there is a slightly stronger effect for studies that used an enriched IVIGAM product (OR 0.54, 95% CI 0.37 to 0.79) than for studies that used a standard polyclonal IVIG (OR 0.76, 95% CI 0.58 to 0.99). However, this difference did not explain a large amount of the heterogeneity observed (p = 0.156 for heterogeneity between groups). Choice of control (albumin or no treatment) had a strong influence on the pooled treatment effect (Figure 11), with studies using albumin as control giving a pooled OR of 0.80 (95% CI 0.62 to 1.02) compared with an OR of 0.36 (95% CI 0.22 to 0.58) in studies that used no treatment as control. The choice of control explained some of the heterogeneity between studies (p = 0.004 for heterogeneity between groups). Possible reasons for this may relate to the fact that use of albumin introduces an intervention that may have biological effects. Two possible options are (1) albumin is an effective treatment for severe sepsis68 or (2) the use of albumin makes the control treatment appear similar to the IVIG treatment (i.e. slightly frothy) and the use of albumin as control is simply an indicator of appropriate blinding in these studies and could, therefore, possibly be a proxy for the risk of bias being lower in these studies.
FIGURE 10.
Fixed-effects model by IVIG preparation (IVIG or IVIGAM).
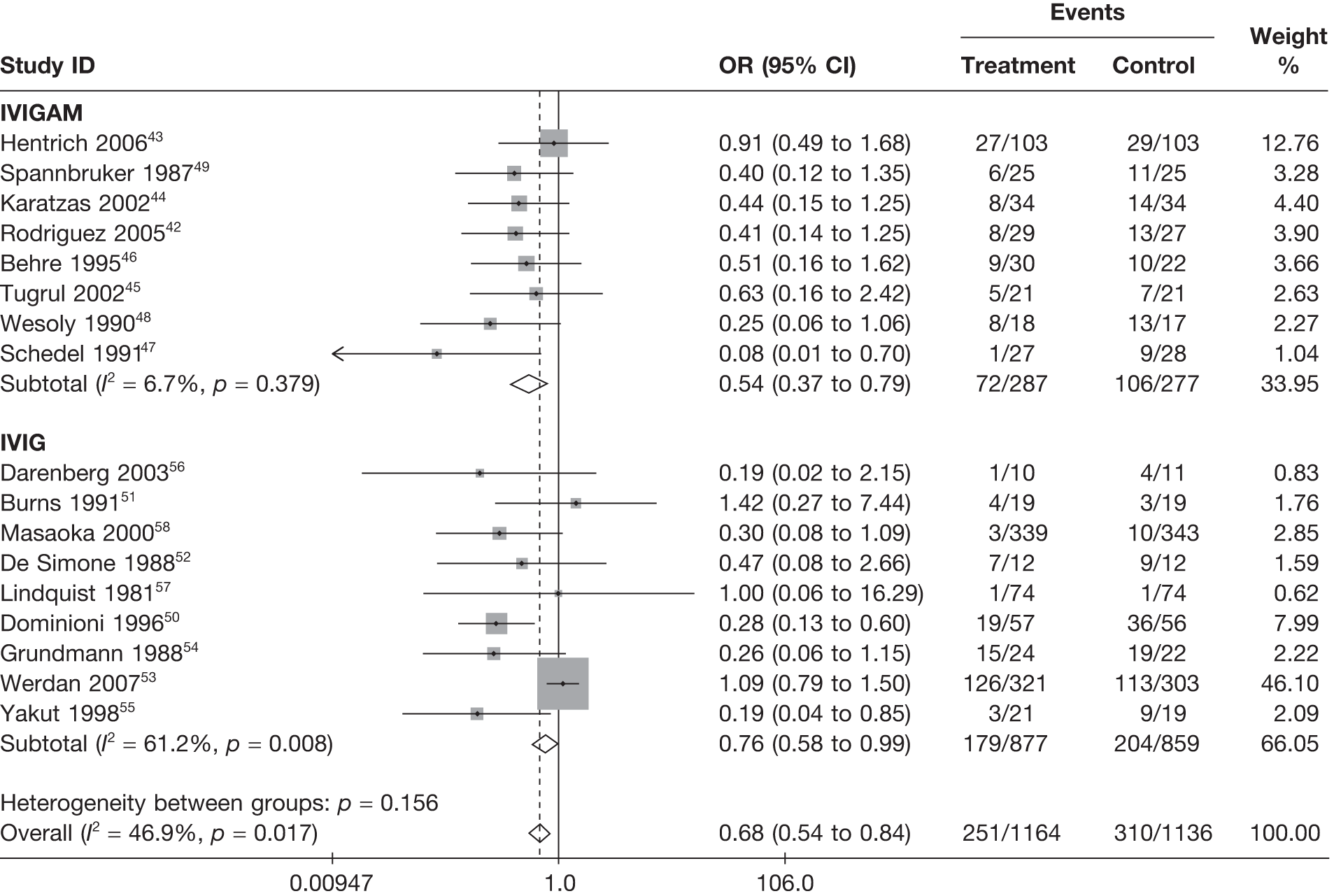
FIGURE 11.
Fixed-effects model by choice of control (no treatment or albumin).
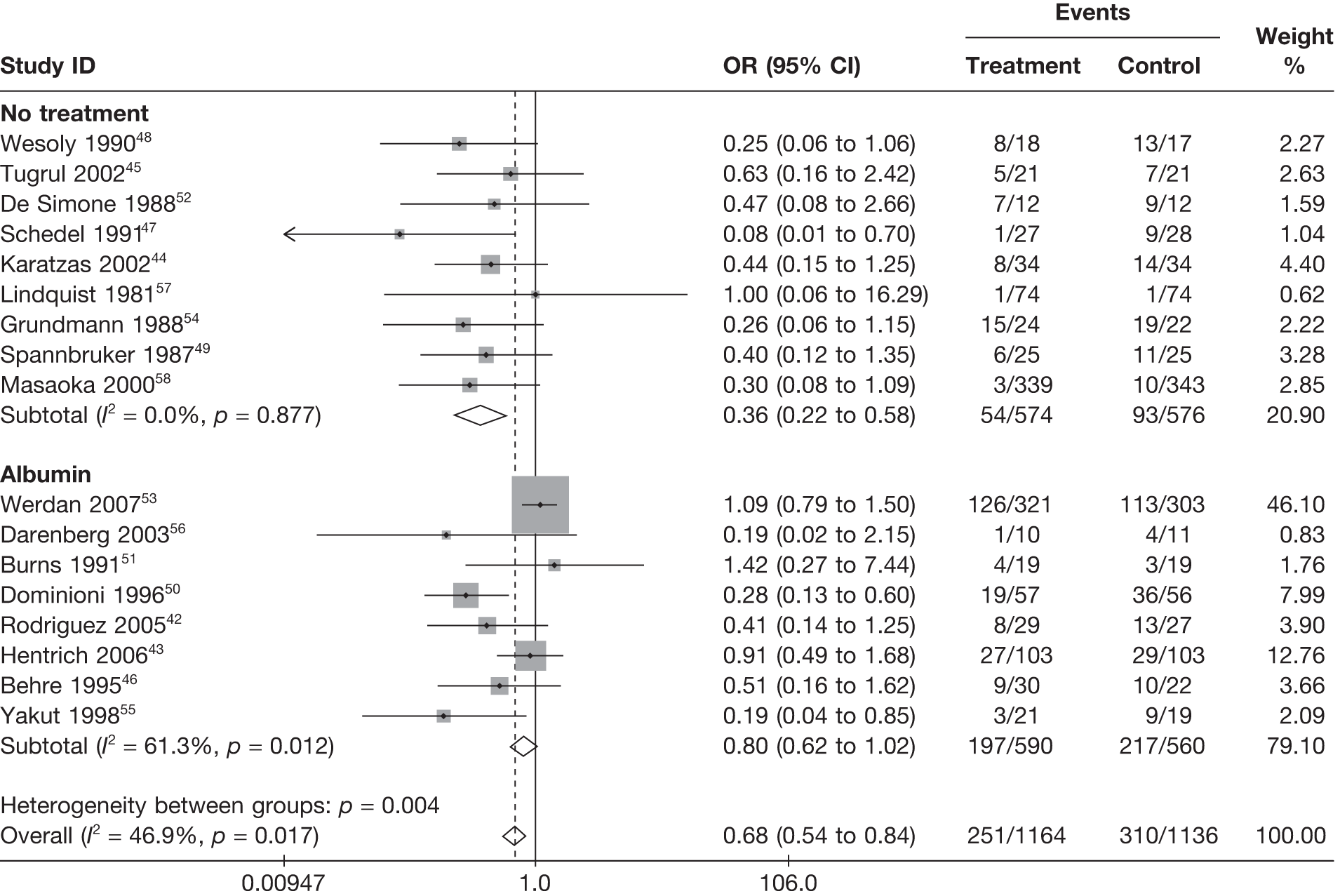
Dosing regimes
The pooled treatment effect becomes stronger (OR decreases) with increasing duration of treatment (p = 0.001 for heterogeneity between groups; Figure 12), becomes less strong (OR increases) with increasing daily dose and volume (p = 0.001 for heterogeneity between groups; Figures 13 and 14) and shows no clear pattern with total dose (Figure 15). Specific aspects of the dosing regime in the studies appeared to have a strong explanatory effect on the observed treatment effect. Whether the dosing regime itself was the cause of this difference or it was simply a measure that was confounded with other differences between the studies could not be determined from the available evidence. In particular, the absence of any dose-finding studies for IVIG preparations prevented us from drawing conclusions about the effect of dosing regime on treatment effect.
FIGURE 12.
Fixed-effects model by duration of treatment (days).
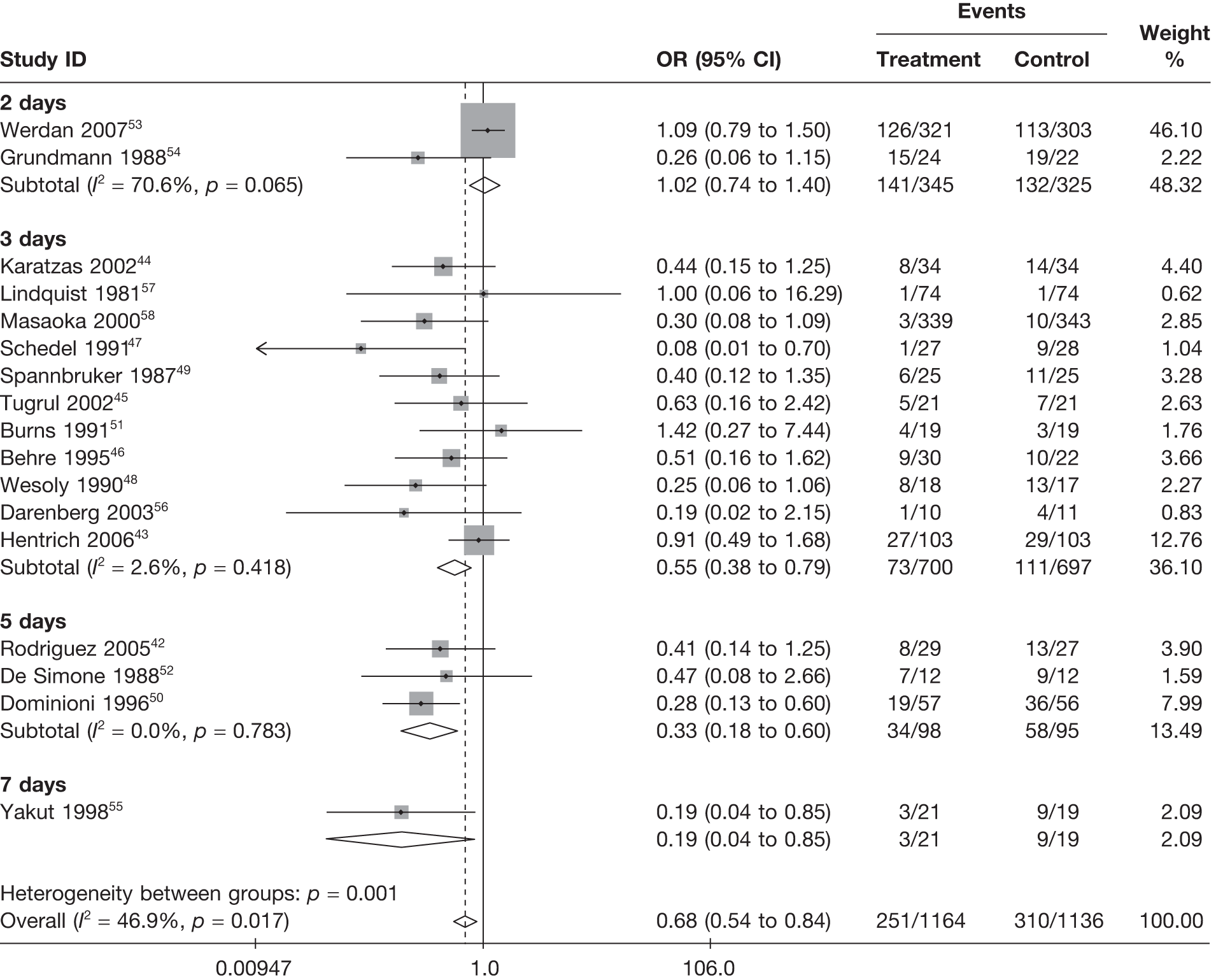
FIGURE 13.
Fixed-effects model by quartiles of daily dose (g/kg/day).
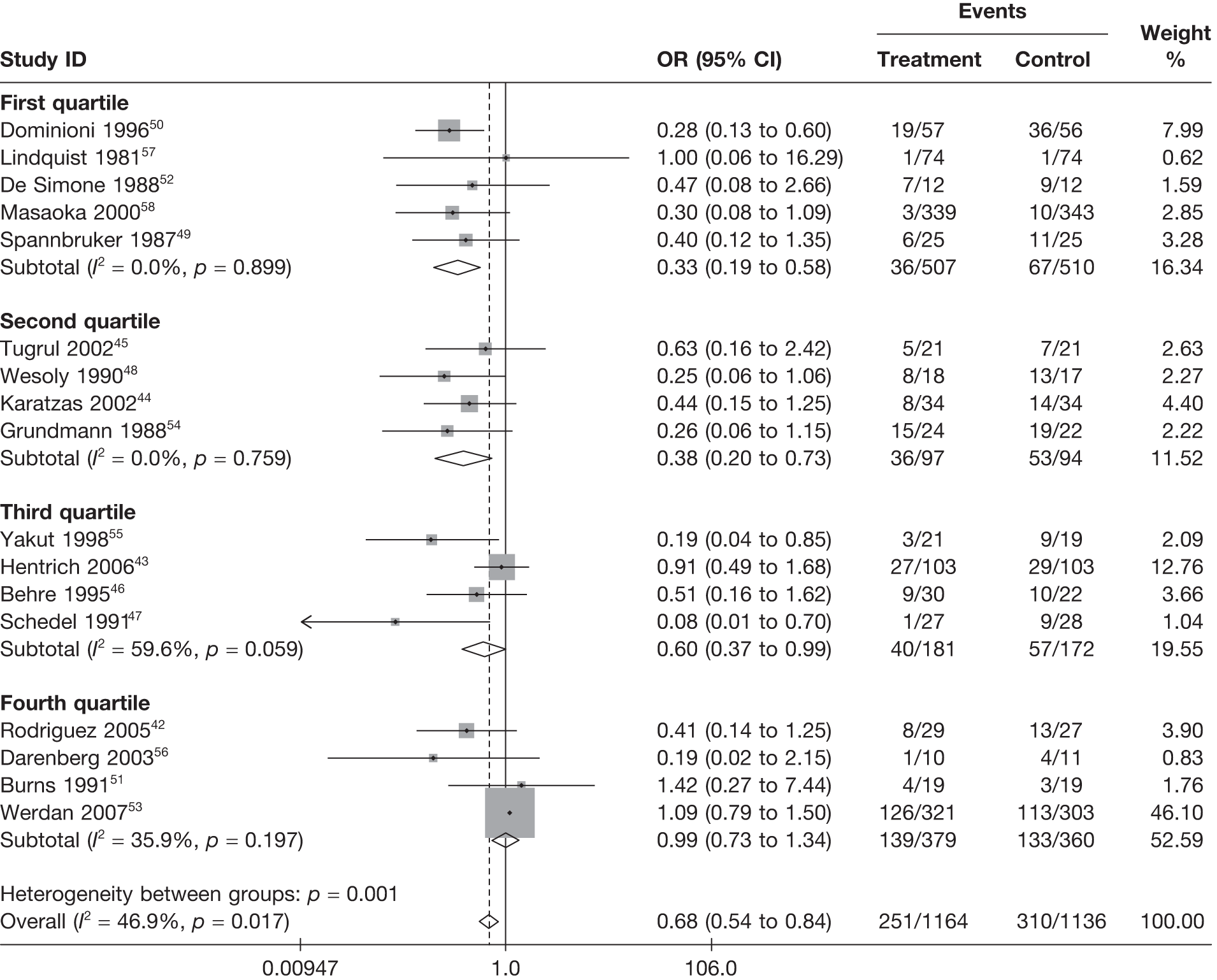
FIGURE 14.
Fixed-effects model by quartile of volume (ml/kg/day).
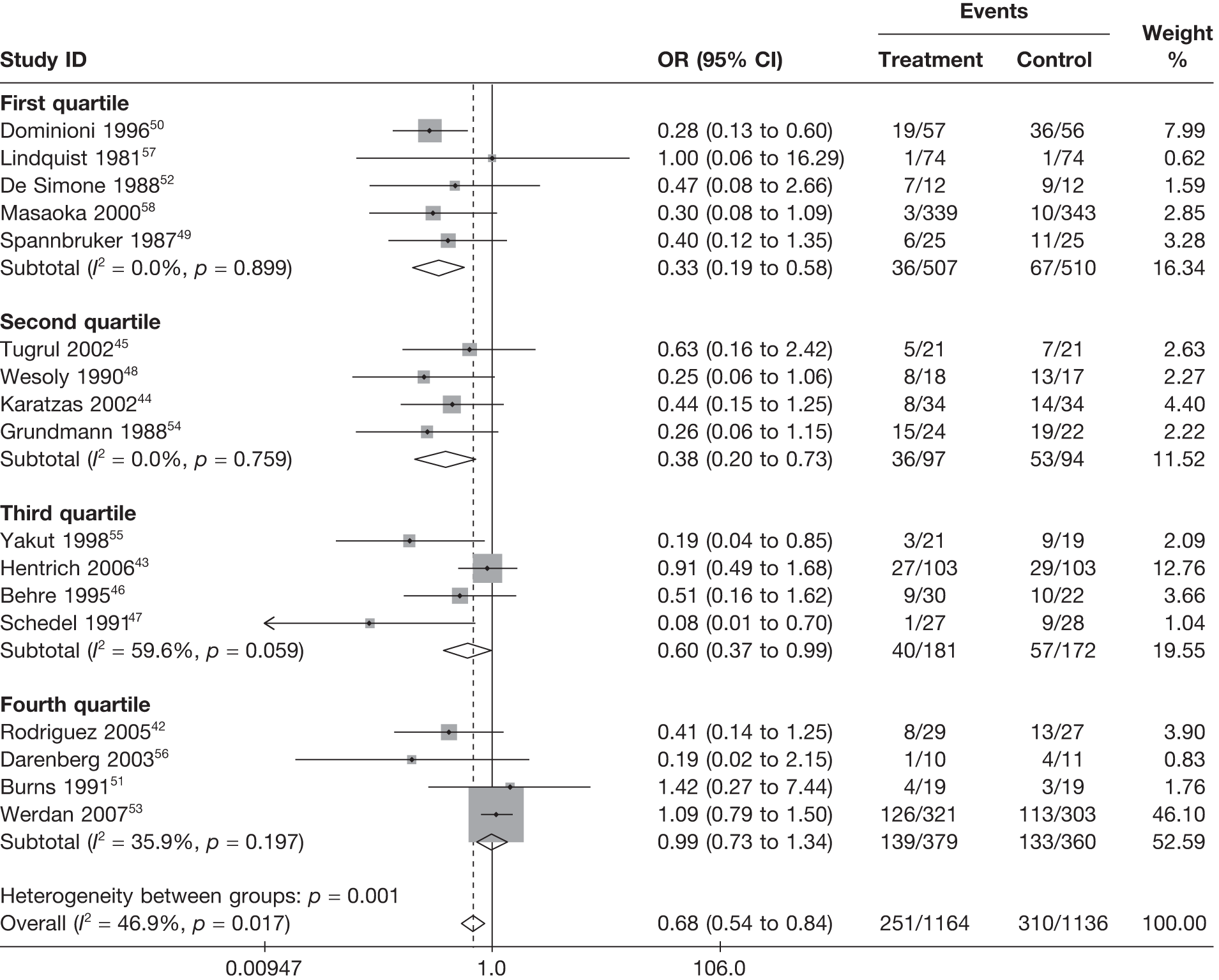
FIGURE 15.
Fixed-effects model by quartiles of total dose (g/kg).
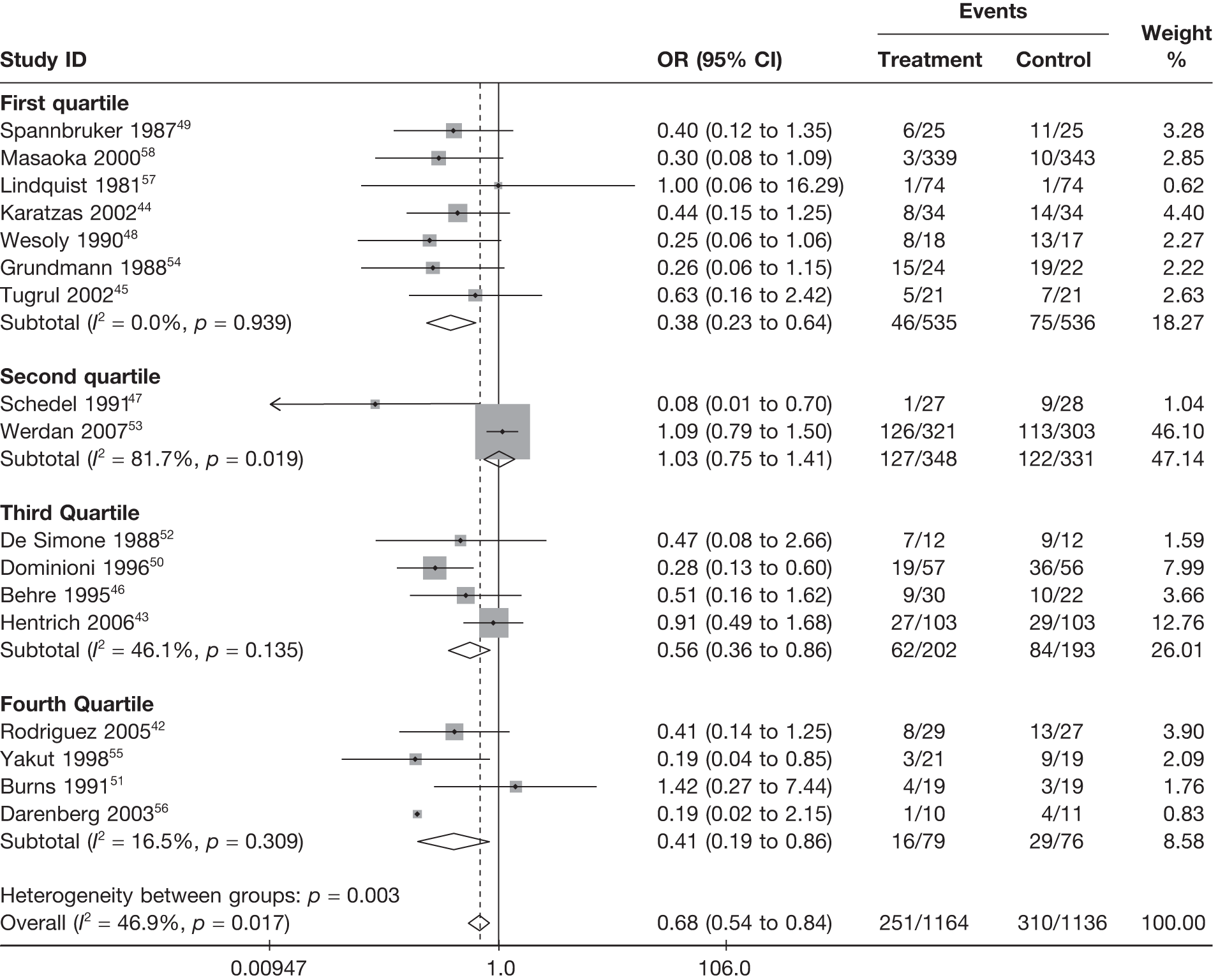
Study quality
Study quality indicators that were explored included intention-to-treat analysis, concealment of allocation, blinding to treatment and randomisation procedure.
In all cases (Figures 16–19), the pooled treatment effect was less strong (OR increased) when the study was considered adequate on each indicator. These study quality indicators explained a large amount of heterogeneity (p < 0.02 for heterogeneity between groups in all cases except intention-to-treat analysis, where p = 0.048).
FIGURE 16.
Fixed-effects model by whether or not intention-to-treat analysis performed.
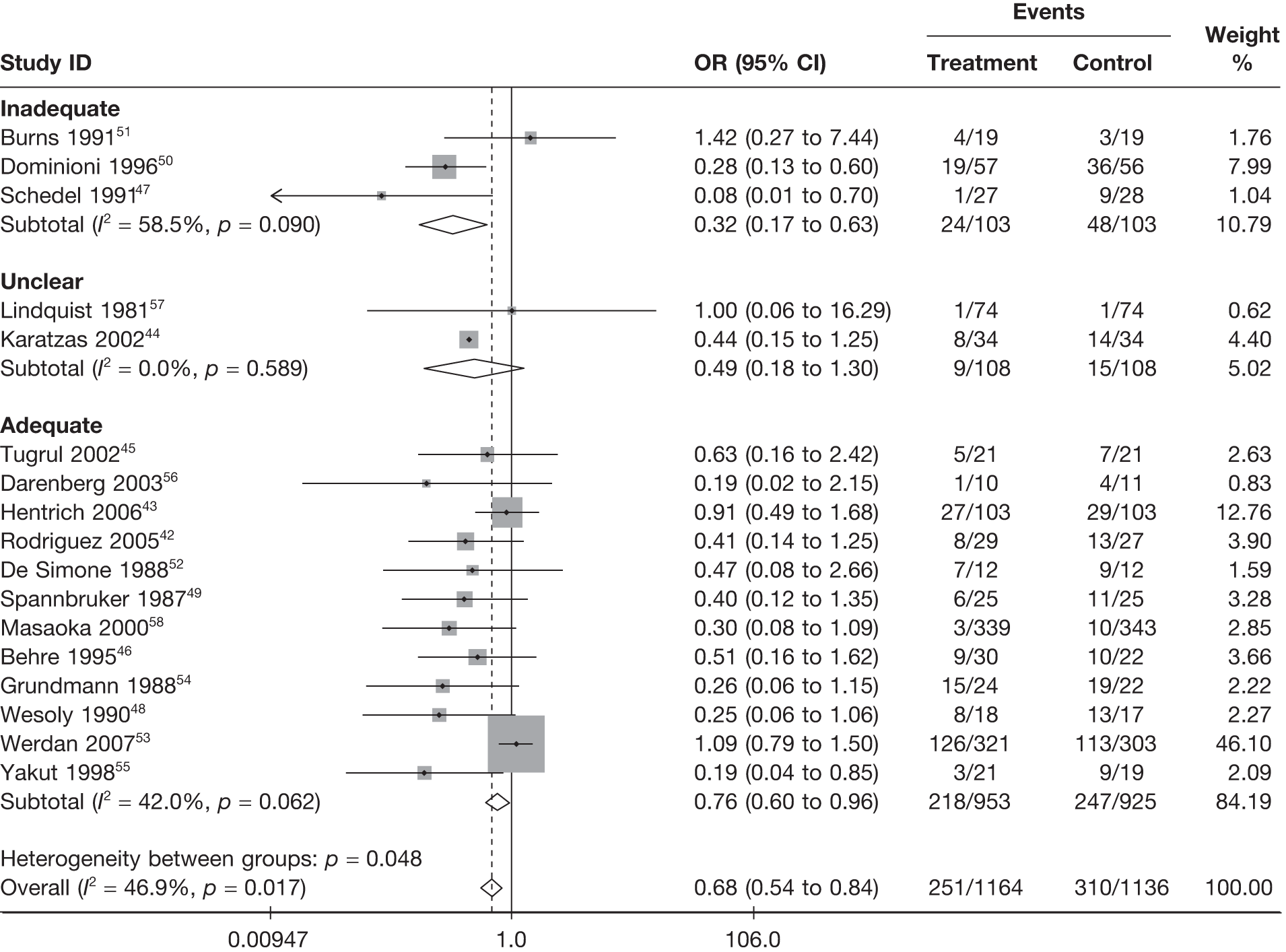
FIGURE 17.
Fixed-effects model by adequacy of concealment of allocation to treatment.
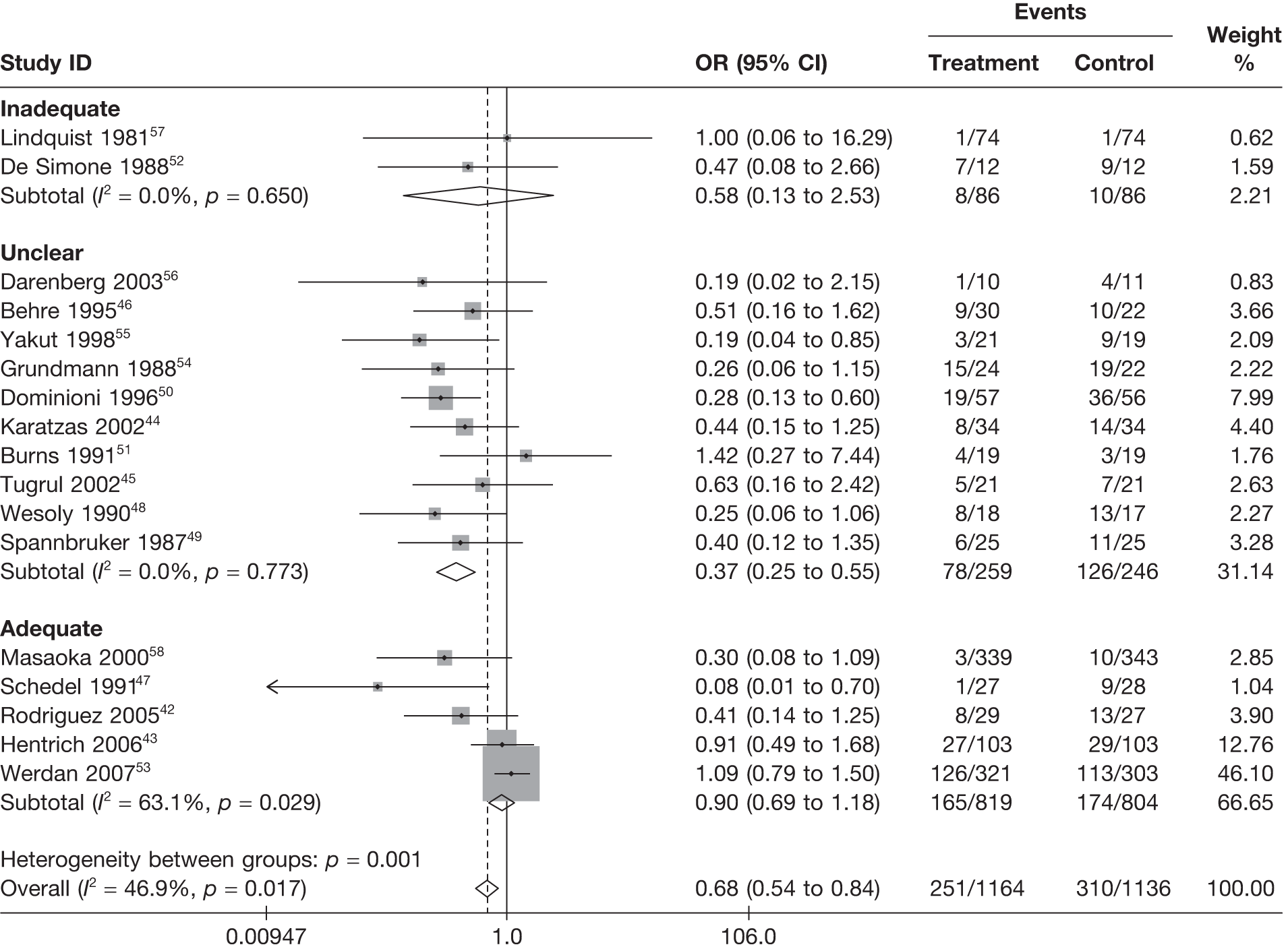
FIGURE 18.
Fixed-effects model by adequacy of blinding to treatment.
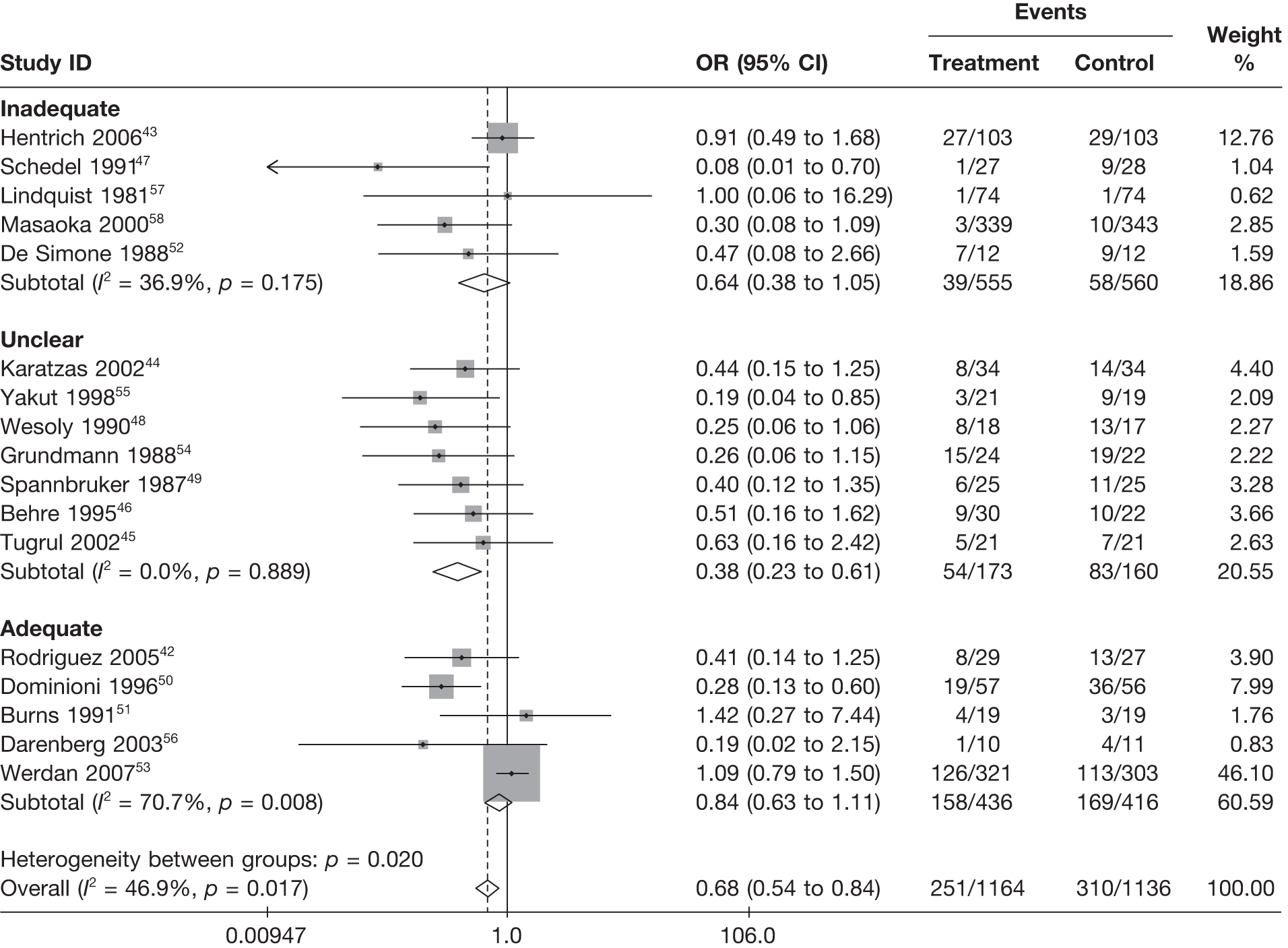
FIGURE 19.
Fixed-effects model by adequacy of randomisation procedure.
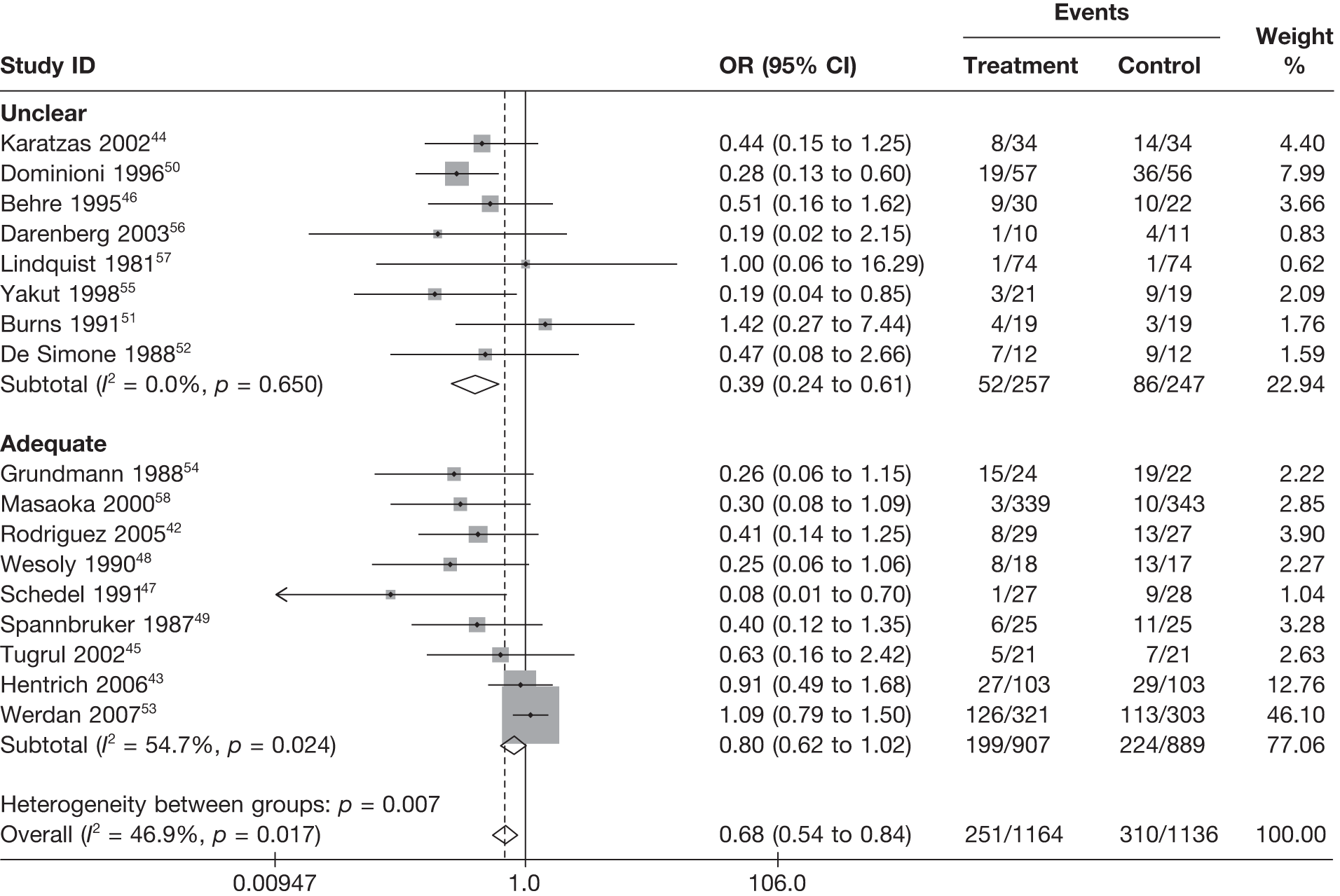
The Jadad score is a composite measure of study quality, and the pooled treatment effect was less strong (OR closer to 1) when the Jadad score was 5 (best quality score), compared with lower scores (Figure 20). Jadad score explained a large amount of the heterogeneity between studies (p = 0.004 for heterogeneity between groups).
FIGURE 20.
Fixed-effects model by Jadad score (where 5 represents the best quality).
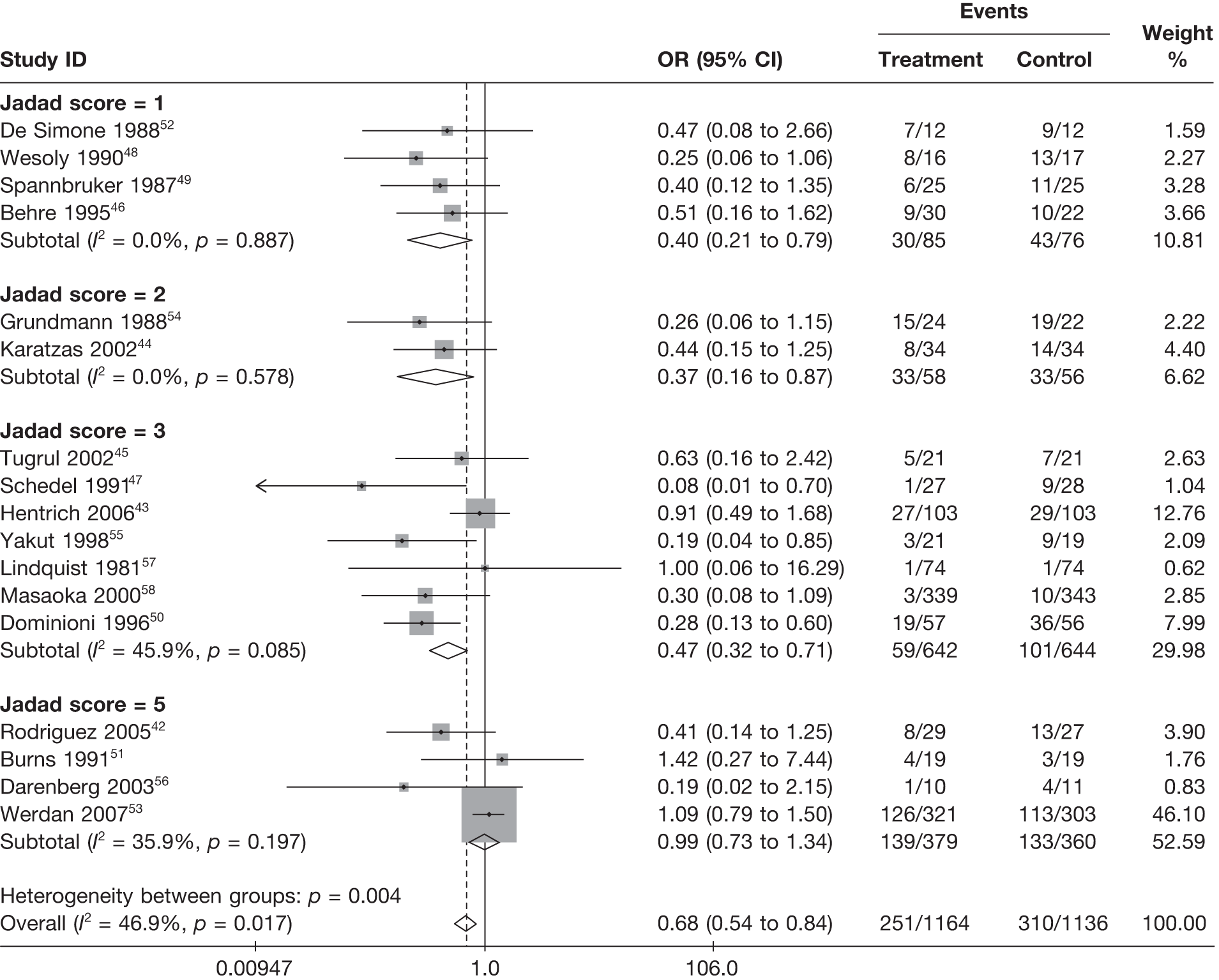
Studies that acknowledged industry sponsorship showed less strong treatment effect (OR closer to 1) than studies that did not acknowledge industry sponsorship (Figure 21) and this explained a large amount of heterogeneity (p < 0.001 for heterogeneity between groups). However, this result is dominated by the two very large studies41,51 acknowledging industry sponsorship. Because it was not clear if those studies that did not acknowledge industry sponsorship were sponsored or not (the information was essentially missing), the interpretation of this covariate is difficult and we, therefore, did not include this covariate in the modelling exercise.
FIGURE 21.
Fixed-effects model by whether industry sponsorship reported.
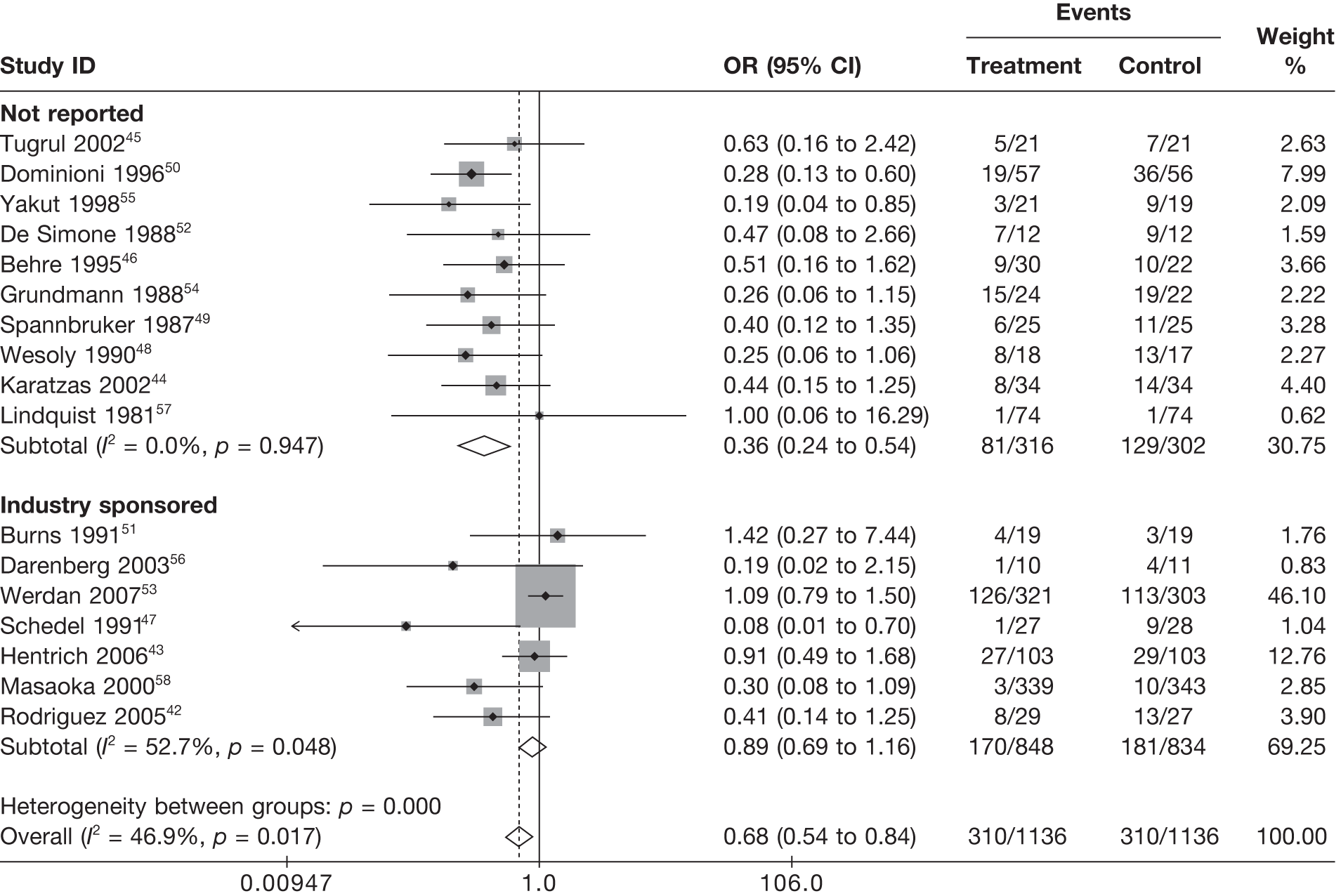
The pooled treatment effect was less strong (OR closer to 1) for studies published more recently than for older studies (Figure 22). This explained a large amount of heterogeneity (p = 0.001 for heterogeneity between groups). There was a trend across studies by quartile of sample size (Figure 23), indicating that pooled treatment effects were stronger (OR smaller) for studies with smaller sample sizes than for studies with larger sample sizes (p < 0.001 for heterogeneity between groups). Figure 24 presents the same pattern with sample size (N), but plotted for 1/N which is a useful way to model small-study effects. 69,70 This is because, as N gets large, 1/N becomes small, so the results for 1/N=0 can be interpreted as an effect estimate for ‘very large’ studies, i.e. adjusted for small study effects.
FIGURE 22.
Fixed-effects model by quartile of publication date.
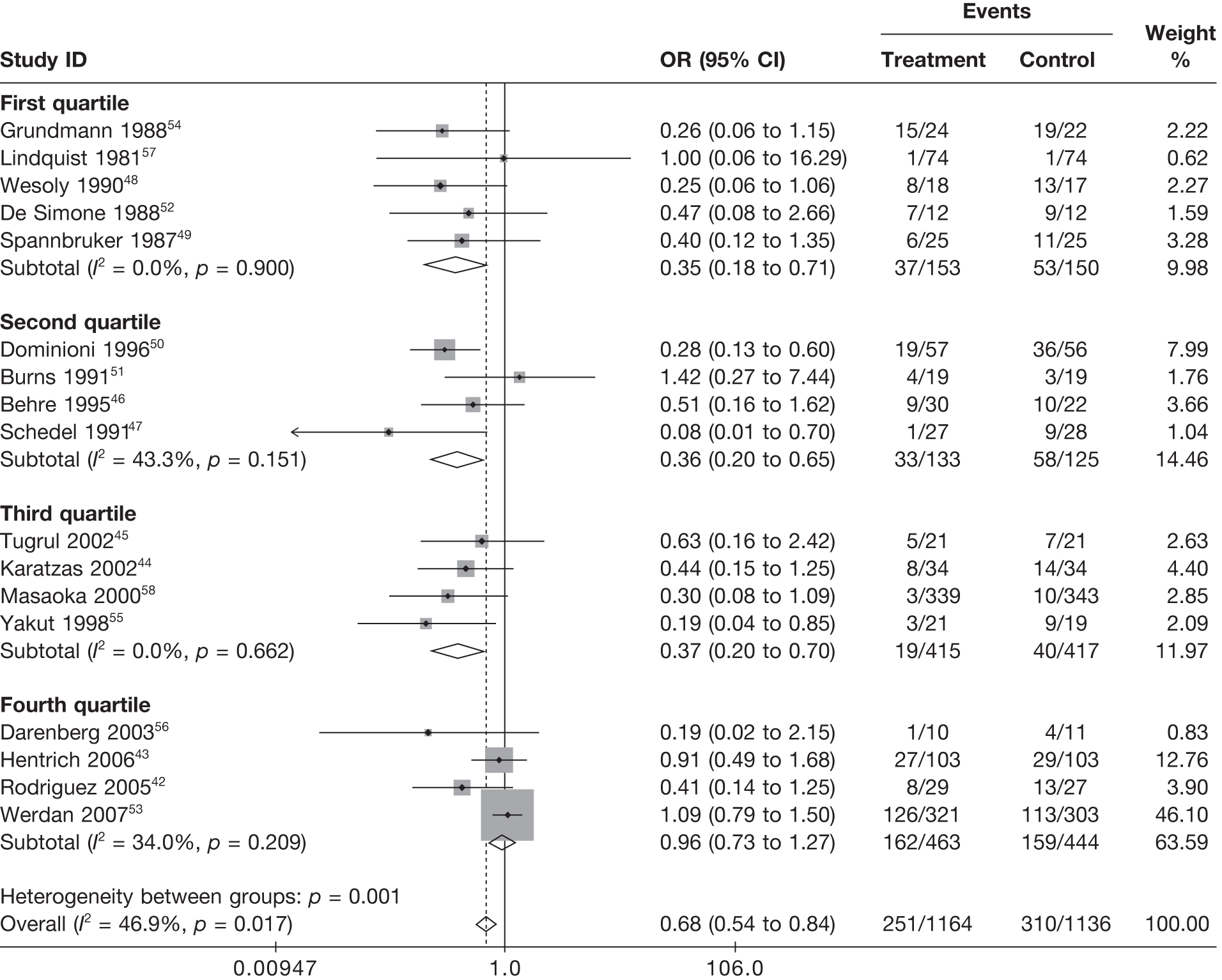
FIGURE 23.
Fixed-effects model by quartile of sample size for IVIG arm (first quartile = smallest to fourth quartile = largest).
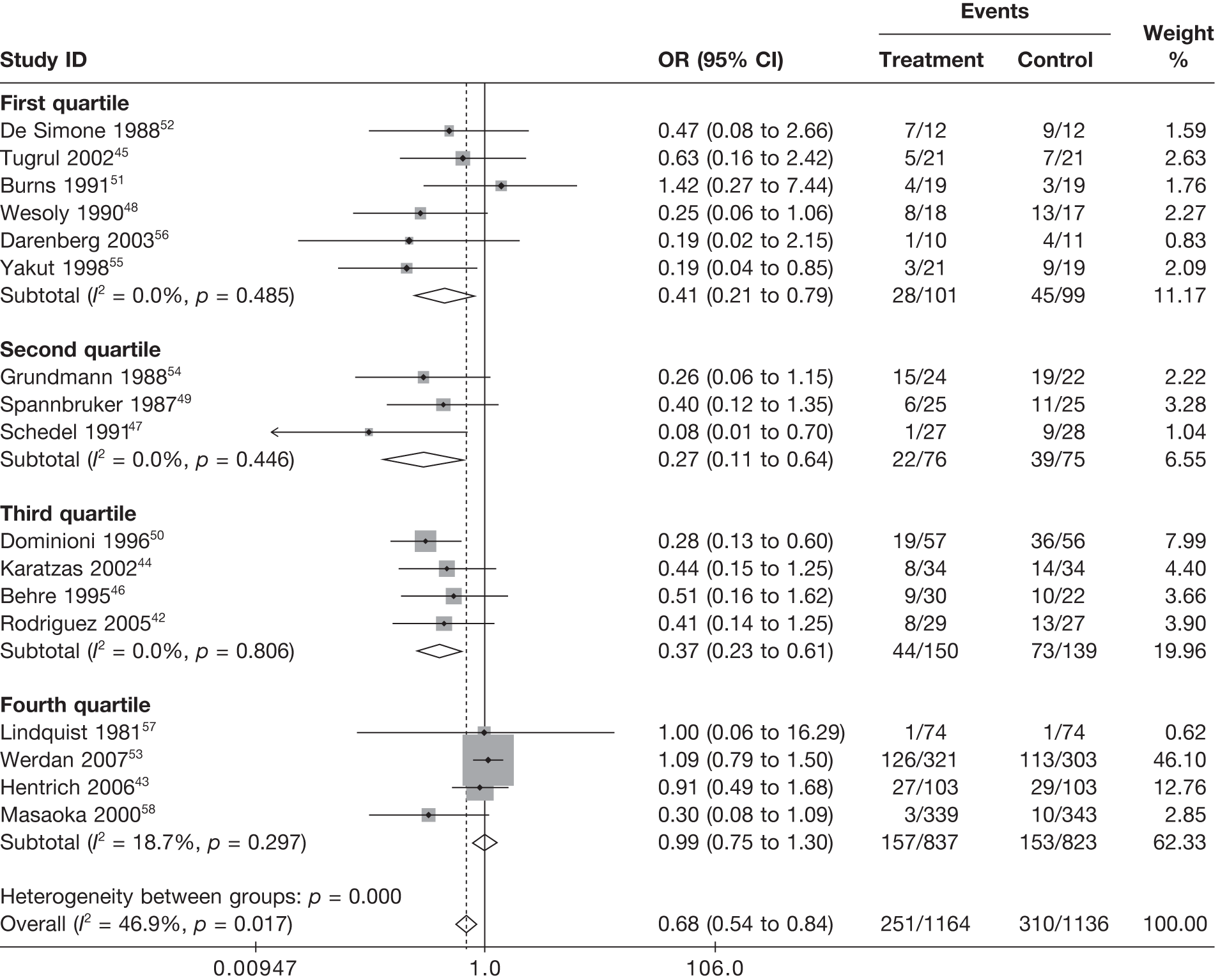
FIGURE 24.
Fixed-effects model by quartile of 1/N (first quartile = smallest to fourth quartile = largest).
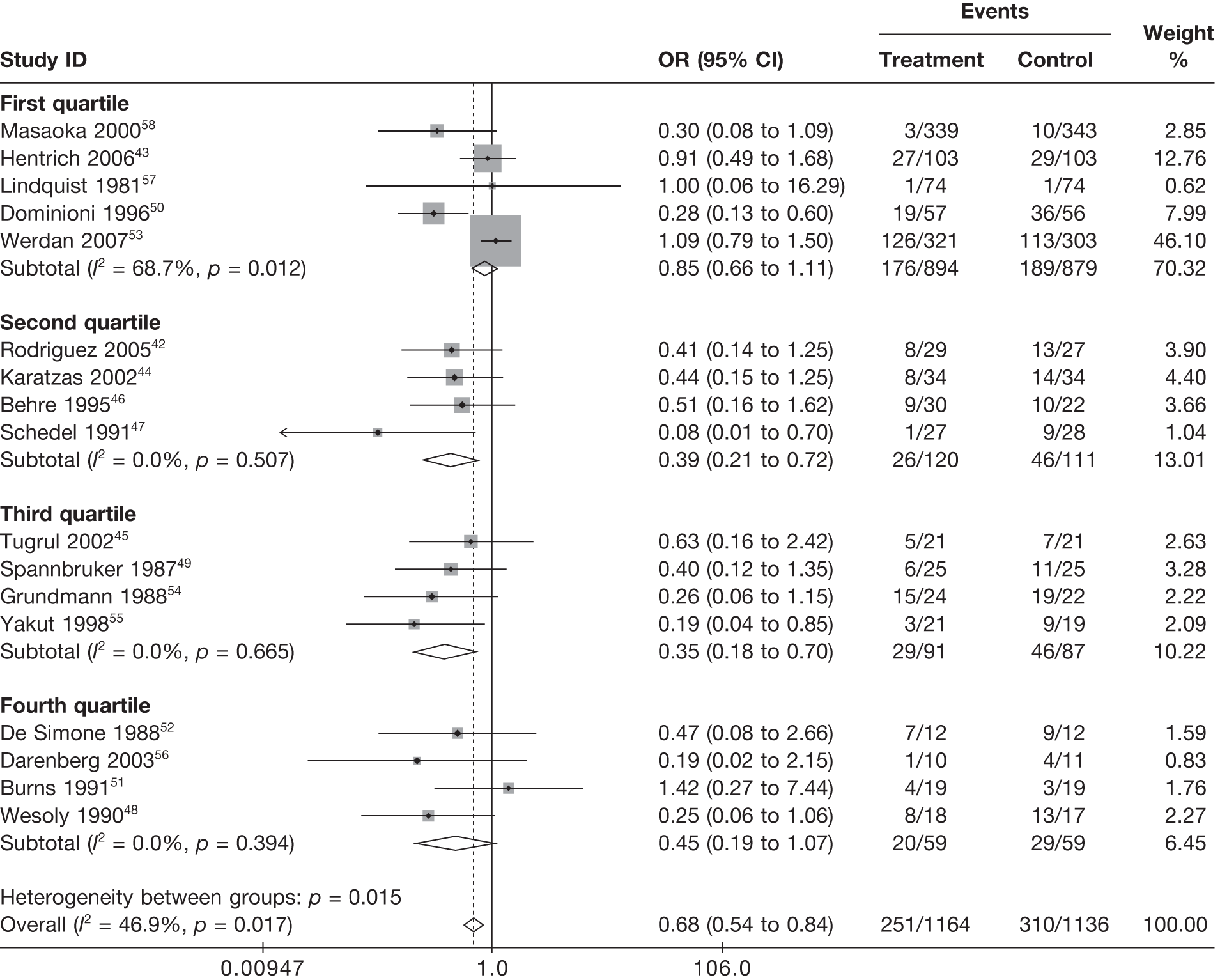
In summary, the exploration of study quality indicators identified that there appeared to be issues with study quality, potential publication bias and other small-study effects in the available evidence.
Other factors
Whether the study was clearly conducted in a critical-care setting, or not, did not have any effect on the pooled treatment effect (Figure 25). This was not surprising as our inclusion criteria limited selection to those studies that were deemed to be in a severe sepsis/septic shock population. It is highly likely that all of studies were conducted in a critical-care setting irrespective of whether or not this was reported.
FIGURE 25.
Fixed-effects model by whether or not the study clearly took place in a critical-care setting.
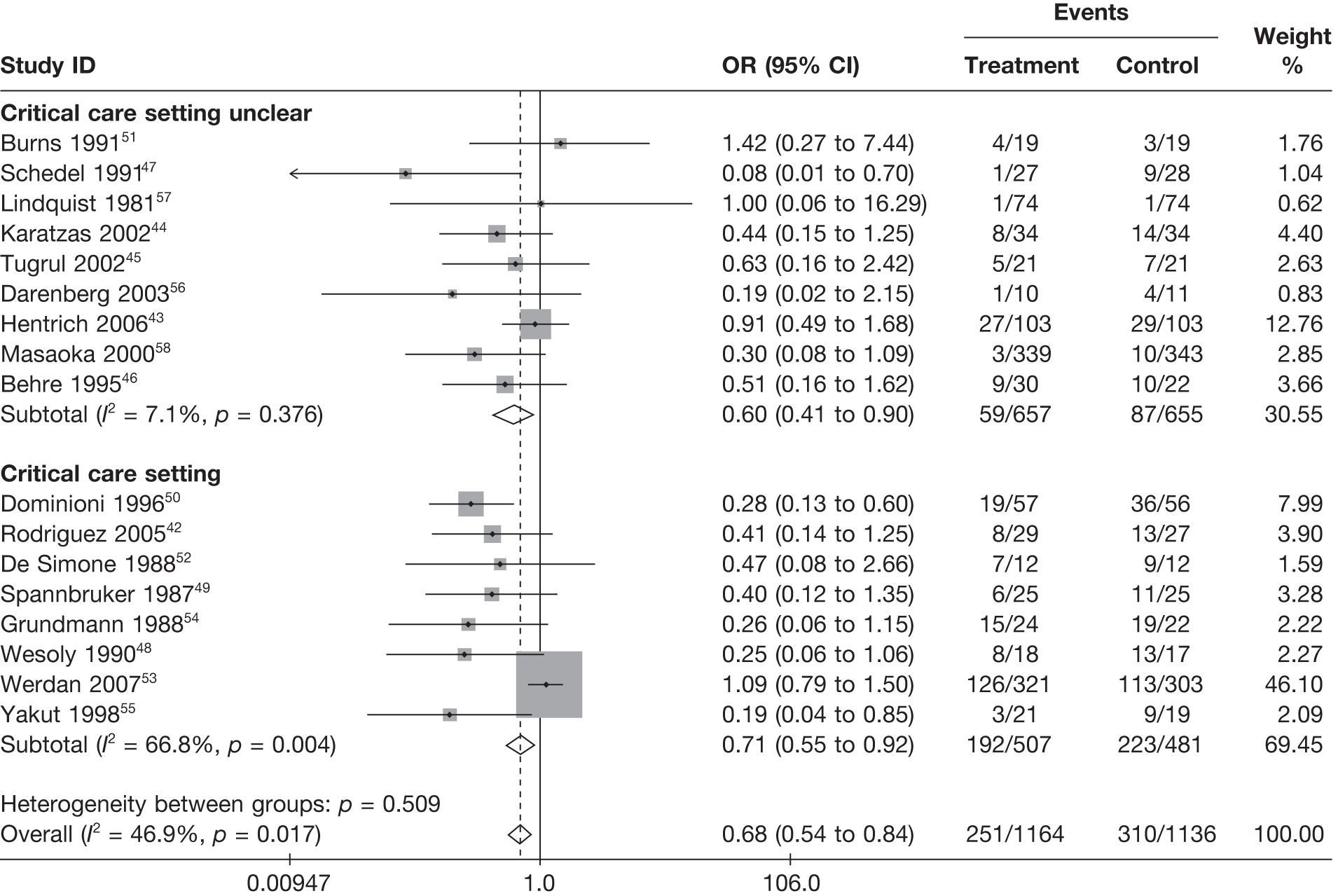
Figures 26 and 27 indicate that the pooled treatment effect was stronger (OR smaller) in studies conducted in populations with a higher baseline risk (third and fourth quartiles) than in studies conducted in populations with lower baseline risk (first and second quartiles). This explained a large amount of heterogeneity (p < 0.001 for heterogeneity between groups).
FIGURE 26.
Fixed-effects model by quartile of baseline risk (control arm log-odds of mortality) (first quartile = lowest risk to fourth quartile = highest risk).
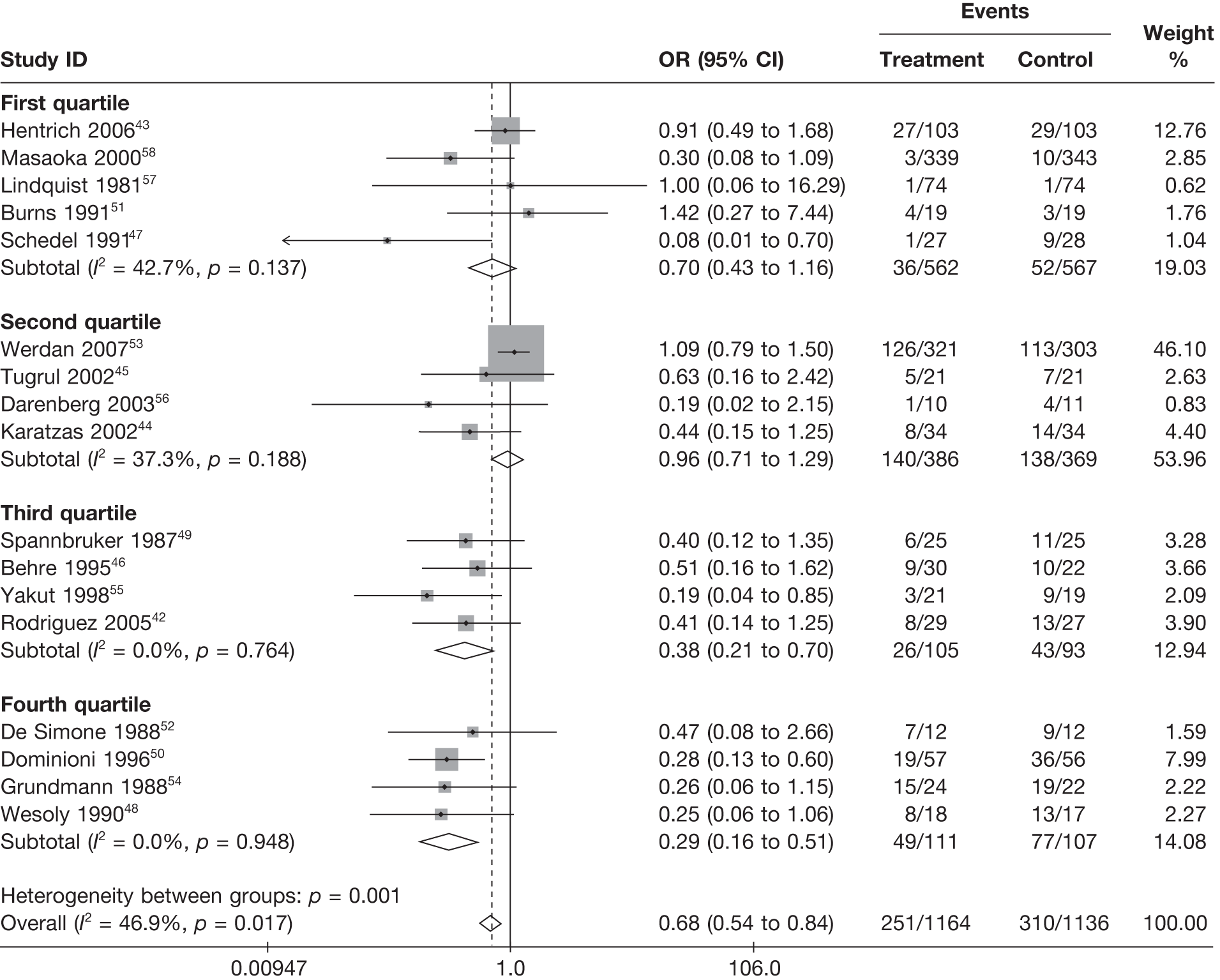
FIGURE 27.
Fixed-effects model by pooled quartiles of baseline risk (control arm log-odds of mortality) (first and second quartiles = low risk and third and fourth quartiles = high risk).
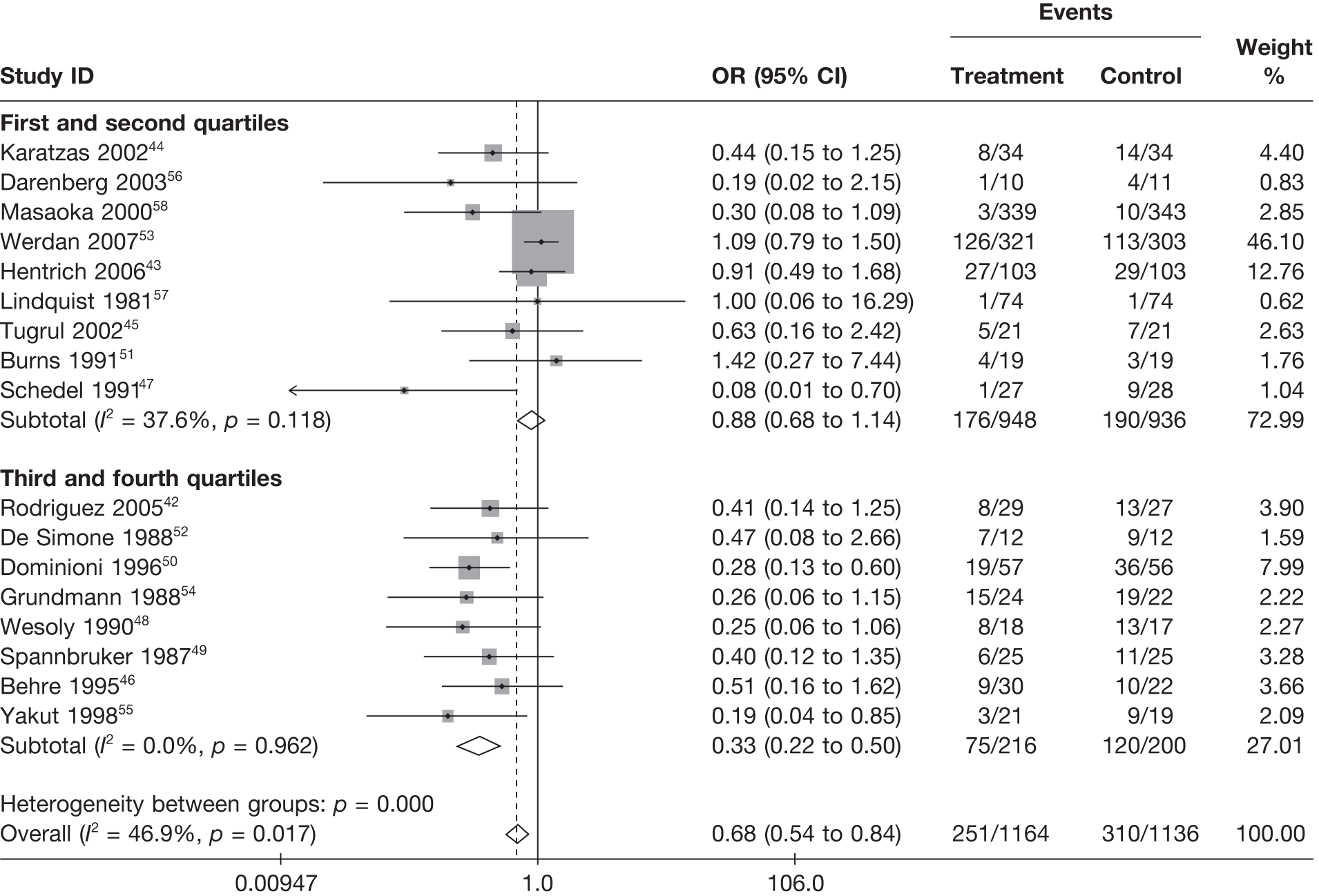
There was no clear pattern between the pooled treatment effect and the follow-up period used by the studies (Figure 28). Although there was clearly a relationship between mortality and follow-up period (see Table 12), the relative effects do not appear to depend strongly on follow-up period.
FIGURE 28.
Fixed-effects model by follow-up period.
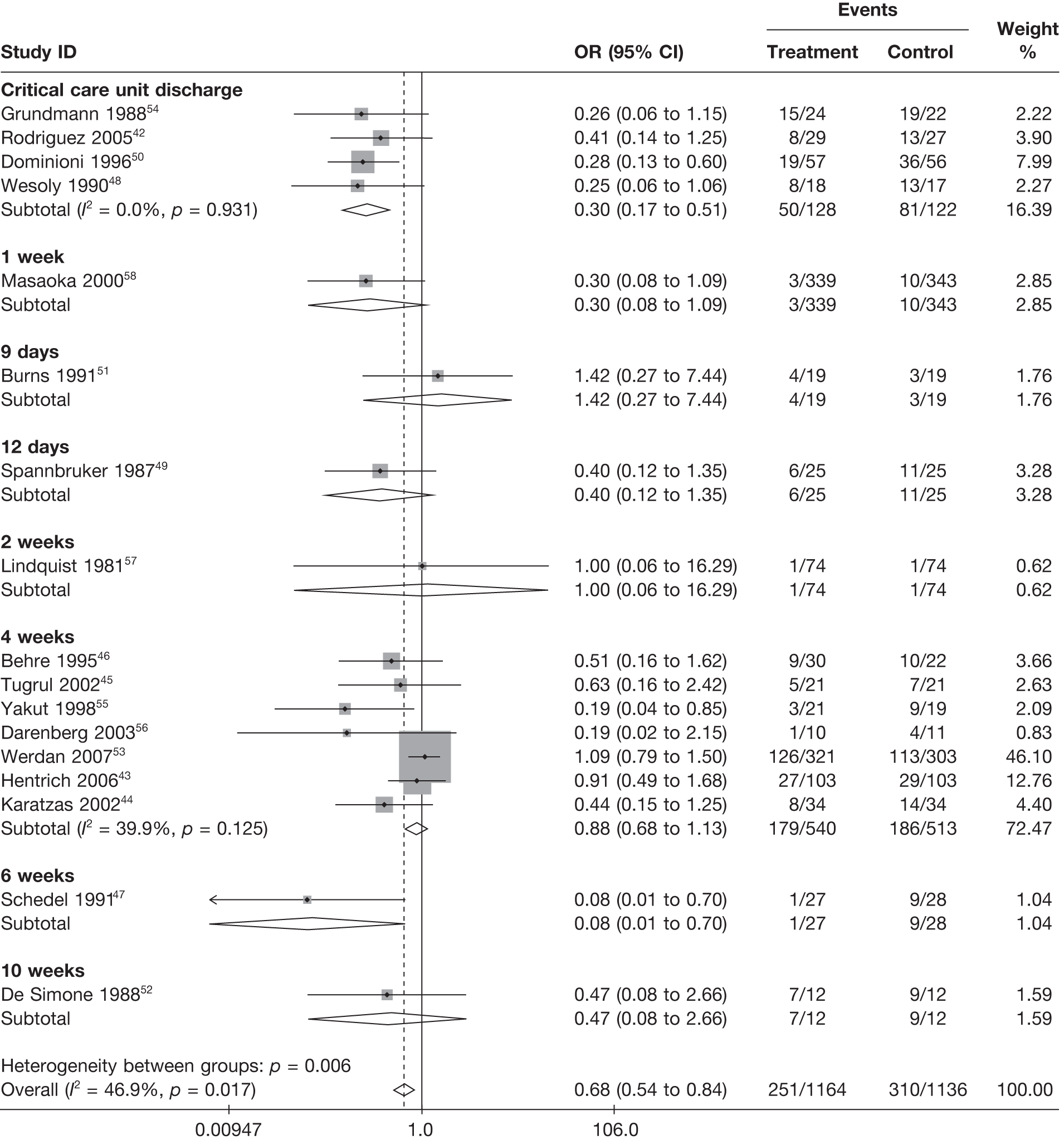
Modelling
Two-treatment comparison model – all-cause mortality
Table 14 presents model fit results from fixed- and random-effects models with no covariates for the two-treatment comparison model (model T2: IVIG or IVIGAM vs albumin or no treatment). For a well-fitting model, the posterior mean residual deviance, D¯res, would be expected to be approximately equal to the number of data points (e.g. 34 from 17 studies each with two arms). Models in which D¯res is much larger than this display evidence of lack of fit. The DIC provides a composite measure of model fit and complexity and models are preferred with lower DIC. Differences in D¯res and DIC of ≤ 2 are considered meaningful. 37 The fixed-effects model shows substantial lack of fit (D¯res=51.4), whereas the random-effects model fits well (D¯res=30.9). This highlights the heterogeneity present in the available evidence. The random-effects model is to be preferred on the basis of both model fit (D¯res) and DIC.
| Model | Posterior mean residual deviance, D¯resa | DICb | Posterior mean between trials heterogeneity (SD), τ¯ |
|---|---|---|---|
| No covariates | |||
| Random-effects model | 30.9 | 175.0 | 0.56 |
| Fixed-effects model | 51.4 | 188.2 | |
| Fixed-effects model adding covariates (individually) for dosing regime | |||
| Duration of treatment (days) | 37.1 | 175.0 | |
| Daily dose (g/kg/day) | 36.9 | 174.6 | |
| Volume (ml/kg/day) | 36.9 | 174.6 | |
| Total dose (g/kg) | 52.2 | 190.0 | |
| Fixed-effects model adding covariates (individually) for study quality | |||
| Whether or not intention-to-treat analysis performed | 45.0 | 182.7 | |
| Adequacy of concealment of allocation to treatment | 41.5 | 179.2 | |
| Adequacy of blinding to treatment | 48.8 | 186.5 | |
| Adequacy of randomisation procedure | 45.2 | 182.9 | |
| Jadad score | 39.2 | 176.9 | |
| Publication date | 35.9 | 173.7 | |
| 1/N (N = number of patients randomised to the IVIG arm) | 36.6 | 174.4 | |
| Fixed-effects model adding covariates (individually) for other factors | |||
| Critical-care setting | 51.6 | 189.4 | |
| Baseline risk (control arm log-odds of mortality) | 53.0 | 190.8 | |
| Follow-up period (linear relationship) | 46.5 | 184.3 | |
| Follow-up period (< 4 weeks or ≥ 4 weeks) | 48.5 | 186.3 | |
| Fixed-effects model adding combinations of key covariates (i.e. results in bold above) | |||
| Duration of treatment + daily dose + volume | 34.3 | 173.6 | |
| Jadad score + publication date + 1/N | 35.7 | 175.4 | |
| Duration of treatment + Jadad score | 33.4 | 172.3 | |
| Duration of treatment + publication date | 31.4 | 170.2 | |
| Duration of treatment + 1/N | 33.7 | 172.5 | |
| Daily dose + Jadad score | 37.4 | 176.2 | |
| Daily dose + publication date | 34.6 | 173.3 | |
| Daily dose + 1/N | 32.2 | 171.0 | |
| Volume + Jadad score | 37.5 | 176.3 | |
| Volume + publication date | 34.7 | 173.4 | |
| Volume + 1/N | 32.4 | 171.2 | |
| Random-effects model adding key covariates (individually) (i.e. results in bold above) | |||
| Duration of treatment (days) | 32.5 | 175.3 | 0.38 |
| Daily dose (g/kg/day) | 33.0 | 175.2 | 0.36 |
| Volume (ml/kg/day) | 33.2 | 175.3 | 0.36 |
| Jadad score | 32.2 | 175.6 | 0.45 |
| Publication date | 33.1 | 174.8 | 0.31 |
| 1/N (N = number of patients randomised to the IVIG arm) | 32.7 | 174.6 | 0.33 |
Table 14 also presents model fit statistics for the fixed-effects model for the two-treatment comparison model (Model T2: IVIG or IVIGAM vs albumin or no treatment) with a range of covariates included individually (i.e. univariate analyses). Results for covariates that substantially improve model fit are highlighted in bold (see Table 14). The key covariates that appeared to explain the heterogeneity in these studies were dosing regime covariates [duration of treatment (days), daily dose (g/kg/day) and volume (ml/kg/day)] and study quality covariates (Jadad score, publication date and a measure of sample size; 1/N). Including any one of these key covariates resulted in a model that fitted adequately and, on the basis of the DIC, was comparable with the random-effects model with no covariates. In other words, these key covariates explained the majority of the heterogeneity (as indicated by the reduction in the posterior mean between study SD, τ¯, when the random-effects models was fitted with these covariates). Follow-up period showed a mild effect, but this disappeared when any of the above key covariates were included (results not shown). Including all three dosing regime covariates did not improve model fit (see Table 14) and, therefore, the conclusion was that, as long as one of these three aspects of dosing regime was included, it was not necessary to include the other two. In other words, these three dosing regime covariates were explaining the same aspects of heterogeneity in treatment effect across the studies. Similar results were observed for the three key study quality covariates and it was only considered necessary to include one of these three covariates in further models.
Combining a dosing regime covariate with a study quality covariate improved model fit and led to reductions in DIC (see Table 14). This suggested that these two types of covariates were both measuring different aspects of heterogeneity across the studies. The fixed-effects models that gave the lowest DIC are highlighted in bold in Table 14 and listed below:
-
duration of treatment + Jadad score
-
duration of treatment + publication date
-
duration of treatment + 1/N
-
daily dose + 1/N
-
volume + 1/N.
Discussions with the Expert Group highlighted that there was no clear clinical rationale why duration of treatment, daily dose or volume would affect treatment efficacy or effectiveness. For this reason, random-effects models with solely study quality covariates were considered. The heterogeneity that can be explained with the dosing regime covariates was left unexplained in these models, reflecting a belief that these covariates were a proxy for other, unmeasured, differences between the studies.
Comparing treatment comparison models T2, T3a, T3b, T4 and T10
Table 15 presents model fit summaries for the key covariates for the treatment comparison models T2, T3a, T3b, T4 and T10. For the fixed-effects model with no covariates, model fit was improved for models T3b and T4 that included albumin and no treatment as separate treatments, compared with models T2 and T3a that did not distinguish between the treatments given in the control arm. Further improvement in model fit was seen by treating each IVIG preparation as a separate treatment (treatment comparison model T10); however, the DIC was higher for this model than for the treatment comparison models T3a and T3b owing to the increased complexity (number of parameters). On the basis of DIC, model T3b (IVIG or IVIGAM vs albumin vs no treatment) was preferred as the model providing the most parsimonious compromise between model fit and complexity.
| Model | T2 | T3a | T3b | T4 | T10 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| D¯resa | DICb | D¯resa | DICb | D¯resa | DICb | D¯resa | DICb | D¯resa | DICb | |
| No covariates | ||||||||||
| Random-effects model | 30.9 | 175.0 | 31.6 | 175.3 | ||||||
| Fixed-effects model | 51.4 | 188.2 | 50.1 | 187.9 | 42.8 | 180.5 | 43.6 | 182.3 | 36.2 | 180.7 |
| Fixed-effects model adding covariates (individually) for dosing regime | ||||||||||
| Duration of treatment (days) | 37.1 | 175.0 | 37.6 | 176.4 | 29.8 | 168.6 | 30.7 | 170.5 | 34.5 | 180.1 |
| Daily dose (g/kg/day) | 36.9 | 174.6 | 38.0 | 176.7 | 37.4 | 176.2 | 38.3 | 178.1 | 36.5 | 182.1 |
| Volume (ml/kg/day) | 36.9 | 174.6 | 38.0 | 176.8 | 37.5 | 176.3 | 38.4 | 178.3 | 36.5 | 181.9 |
| Fixed-effects model adding covariates (individually) for study quality | ||||||||||
| Jadad score | 39.2 | 176.9 | 39.7 | 178.4 | 39.3 | 178.1 | 39.7 | 179.5 | 37.1 | 182.6 |
| Publication date | 35.9 | 173.7 | 36.5 | 175.3 | 36.4 | 175.2 | 37.2 | 179.5 | 36.3 | 182.6 |
| 1/N | 36.6 | 174.4 | 37.3 | 176.0 | 36.1 | 174.9 | 36.8 | 176.6 | 37.3 | 182.9 |
| Fixed-effects model adding combinations of key covariates | ||||||||||
| Duration of treatment + Jadad score | 33.4 | 172.3 | 30.7 | 170.5 | ||||||
| Duration of treatment + publication date | 31.4 | 170.2 | 30.1 | 169.8 | ||||||
| Duration of treatment + 1/N | 33.7 | 172.5 | 30.7 | 170.5 | ||||||
| Daily dose + Jadad score | 37.4 | 176.2 | 38.0 | 177.7 | ||||||
| Daily dose + publication date | 34.6 | 173.3 | 35.6 | 175.4 | ||||||
| Daily dose + 1/N | 32.2 | 171.0 | 33.1 | 172.9 | ||||||
| Volume + Jadad score | 37.5 | 176.3 | 38.1 | 177.8 | ||||||
| Volume + publication date | 34.7 | 173.4 | 35.7 | 175.5 | ||||||
| Volume + 1/N | 32.4 | 171.2 | 33.3 | 173.1 | ||||||
Including covariates, the best-fitting model (and lowest DIC) was obtained for treatment model T3b with duration of treatment as a covariate. Note that, for this treatment comparison model, the study quality covariates did not yield a big improvement in model fit. This suggested that the choice of control treatment was confounded with other study quality covariates. In other words, this suggested that treatment effects were smaller when albumin was used as a control, indicating adequate blinding to treatment, plus also acting as a proxy for other aspects of study quality rather than albumin necessarily having a clinical effect on mortality as compared with no treatment.
Considering treatment comparison models T2 and T3b, the fixed-effects models giving the lowest DIC are highlighted in bold in Table 15 and are as follows:
-
T3b with duration of treatment
-
T2 with duration of treatment + Jadad score
-
T2 with duration of treatment + publication date
-
T2 with duration of treatment + 1/N
-
T2 with daily dose + 1/N
-
T2 with volume + 1/N.
There is little to choose between these models on the basis of model fit and DIC.
As described above, dropping dosing regime covariates from these models (owing to the lack of clinical interpretability) and replacing the heterogeneity explained by these covariates with a random-effects model with a between-study heterogeneity parameter was considered (below all random-effects models):
-
T3b
-
T2 with Jadad score
-
T2 with publication date
-
T2 with 1/N.
Fixed-effects model T3b with duration of treatment was selected as our best-fitting model, but results from the other nine models, listed above, as a sensitivity analysis to assess robustness of conclusions on clinical efficacy to model choice are also presented.
Best-fitting model – all-cause mortality
Our best-fitting treatment comparison model was model T3b with duration of treatment. Treatment comparison model T3b compares IVIG/IVIGAM versus albumin versus no treatment. As discussed previously, choice of control appeared to be a proxy for study quality. Therefore, the estimate of relative treatment effect used was for IVIG/IVIGAM versus albumin. Figure 29 shows the OR for IVIG/IVIGAM versus albumin, plotted against duration of treatment, with 95% credible intervals (figures for ORs are provided in Table 16). The treatment effect was stronger for longer durations of treatment; the majority of studies used a duration of treatment of 3 days. For a duration of treatment of 3 days, the OR of mortality for IVIG/IVIGAM versus albumin was 0.75 (95% credible interval 0.58 to 0.96), indicating that there was some evidence that IVIG/IVIGAM was effective in reducing all-cause mortality.
FIGURE 29.
Treatment comparison model T3b: IVIG/IVIGAM versus albumin with duration of treatment – OR (posterior mean and 95% credible intervals) by duration of treatment (days).
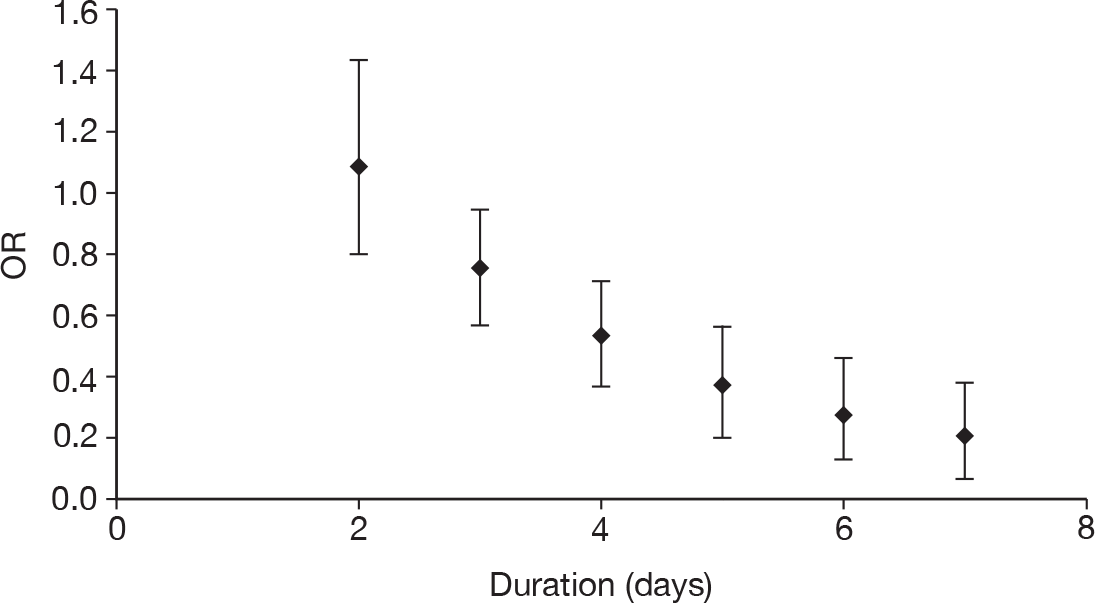
| Model | Covariate | Predicted OR (95% credible intervals) |
|---|---|---|
|
Fixed-effects treatment comparison model T3b Covariate: duration of treatment |
Duration (days): | |
| 2 | 1.10 (0.79 to 1.44) | |
| 3 | 0.75 (0.58 to 0.96) | |
| 4 | 0.52 (0.37 to 0.72) | |
| 5 | 0.37 (0.22 to 0.58) | |
| 6 | 0.26 (0.13 to 0.47) | |
| 7 | 0.19 (0.07 to 0.39) | |
|
Fixed-effects treatment comparison model T2 Covariates: duration of treatment and Jadad score |
Duration (days): | |
| 2 | 1.07 (0.79 to 1.43) | |
| 3 | 0.81 (0.59 to 1.11) | |
| 4 | 0.63 (0.39 to 0.95) | |
| 5 | 0.49 (0.24 to 0.85) | |
| 6 | 0.38 (0.15 to 0.77) | |
| 7 | 0.30 (0.09 to 0.70) | |
|
Fixed effect Treatment comparison model T2 Covariates: duration of treatment and publication date |
Duration (days): | |
| 2 | 1.05 (0.78 to 1.38) | |
| 3 | 0.82 (0.60 to 1.08) | |
| 4 | 0.64 (0.41 to 0.96) | |
| 5 | 0.51 (0.26 to 0.89) | |
| 6 | 0.41 (0.17 to 0.84) | |
| 7 | 0.33 (0.10 to 0.79) | |
|
Fixed-effects treatment comparison model T2 Covariates: duration of treatment and 1/N |
N → ∞ | |
| Duration (days): | ||
| 2 | 1.33 (0.84 to 2.01) | |
| 3 | 1.07 (0.61 to 1.73) | |
| 4 | 0.86 (0.41 to 1.58) | |
| 5 | 0.71 (0.27 to 1.49) | |
| 6 | 0.59 (0.17 to 1.43) | |
| 7 | 0.50 (0.11 to 1.41) | |
| N = 339 | ||
| Duration (days): | ||
| 2 | 1.04 (0.76 to 1.39) | |
| 3 | 0.83 (0.58 to 1.14) | |
| 4 | 0.67 (0.39 to 1.05) | |
| 5 | 0.54 (0.25 to 1.00) | |
| 6 | 0.45 (0.16 to 0.98) | |
| 7 | 0.38 (0.10 to 0.97) | |
|
Fixed-effects treatment comparison model T2 Covariates: daily dose and 1/N |
Daily dose (g/kg/day): | |
| 0.1 | 0.48 (0.22 to 0.89) | |
| 0.2 | 0.60 (0.34 to 0.97) | |
| 0.3 | 0.76 (0.52 to 1.07) | |
| 0.4 | 1.00 (0.72 to 1.30) | |
| 0.5 | 1.29 (0.87 to 1.83) | |
| 0.6 | 1.70 (0.97 to 2.78) | |
| 0.7 | 2.27 (1.04 to 4.38) | |
|
Fixed-effects treatment comparison model T2 Covariates: volume and 1/N |
Volume (ml kg–1 day–1) | |
| 2 | 0.48 (0.22 to 0.91) | |
| 3 | 0.54 (0.28 to 0.94) | |
| 4 | 0.61 (0.34 to 0.98) | |
| 5 | 0.68 (0.43 to 1.02) | |
| 6 | 0.77 (0.52 to 1.08) | |
| 7 | 0.87 (0.63 to 1.17) | |
| 8 | 0.99 (0.72 to 1.30) | |
| 9 | 1.12 (0.81 to 1.52) | |
| 10 | 1.28 (0.87 to 1.81) | |
| Random-effects treatment comparison model T3b | 0.68 (0.16 to 1.83) | |
|
Random-effects treatment comparison model T2 Covariate: Jadad score |
0.83 (0.18 to 2.13) | |
|
Random-effects treatment comparison model T2 Covariate: publication date |
0.83 (0.24 to 1.72) | |
|
Random-effects treatment comparison model T2 Covariate: 1/N |
N → ∞ | 1.27 (0.25 to 3.17) |
| N = 339 | 0.92 (0.23 to 2.10) |
Sensitivity analyses for remaining nine treatment comparison models with covariates
In models with covariates, we need to choose a specific value of each covariate in order to obtain estimated treatment effects. First consider the fixed effect treatment comparison model T2 with covariates duration of treatment and Jadad score. Jadad score is an indicator for quality (Jadad score of 5 indicating best quality or lowest risk of bias).The Jadad score was fixed to 5 to produce the treatment estimates from this model, which gives a treatment effect estimate that can be interpreted as adjusting for bias introduced by study quality. Figure 30 presents the OR for IVIG/IVIGAM versus albumin/no treatment for 3 days, the OR of mortality for IVIG/IVIGAM versus albumin/no treatment was 0.81 (95% credible intervals 0.59 to 1.11), suggesting only weak evidence that IVIG/IVIGAM was effective in reducing all-cause mortality with the 95% credible intervals containing 1.00 (i.e. no effect).
FIGURE 30.
Treatment comparison model T2: IVIG/IVIGAM versus albumin/no treatment with duration of treatment and Jadad score = 5 – OR (posterior mean and 95% credible intervals) by duration of treatment (days).
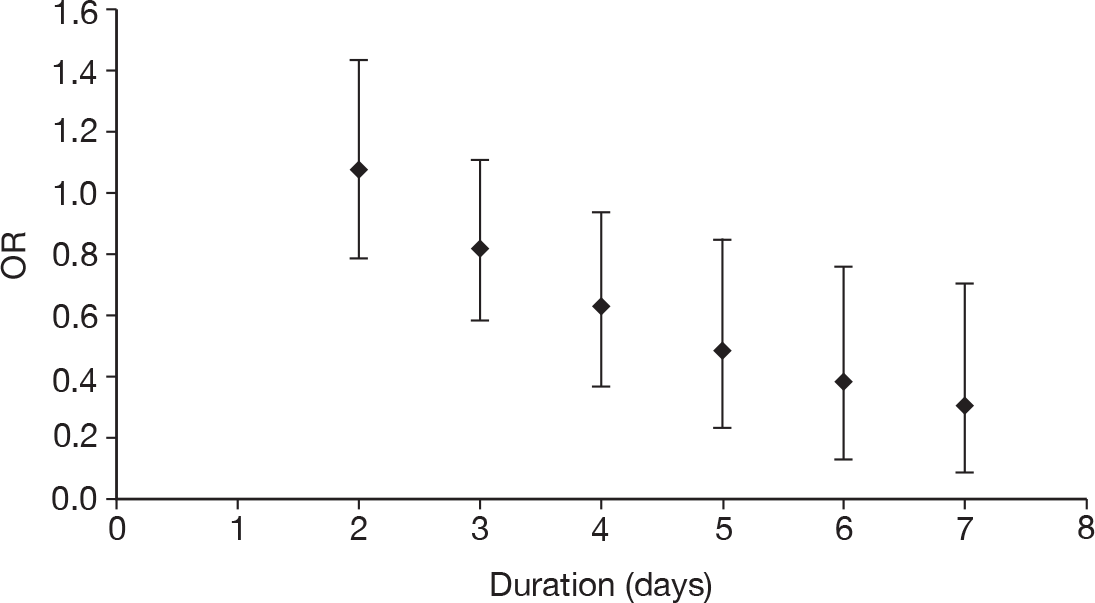
For the fixed effect treatment comparison model T2 with covariates duration of treatment and publication date we need to specify a value for publication date. As publication date reflects changes in clinical practise over time, the treatment effect estimate from the most recent studies in our evidence should be used. Publication date was fixed to 2007 to produce the treatment estimates from this model. This can be interpreted as controlling for changes in clinical practice over time. Figure 31 presents the OR for IVIG/IVIGAM versus albumin/no treatment plotted against duration of treatment for publication date of 2007. For duration of treatment of 3 days, the OR of mortality for IVIG/IVIGAM versus albumin/no treatment was 0.82 (95% credible interval 0.60, 1.08), suggesting only weak evidence that IVIG/IVIGAM was effective in reducing all-cause mortality with the 95% credible intervals containing 1.00 (i.e. no effect).
FIGURE 31.
Treatment comparison model T2: IVIG/IVIGAM versus albumin/no treatment with duration of treatment and publication date of 2007 – OR (posterior mean and 95% credible intervals) by duration of treatment (days).
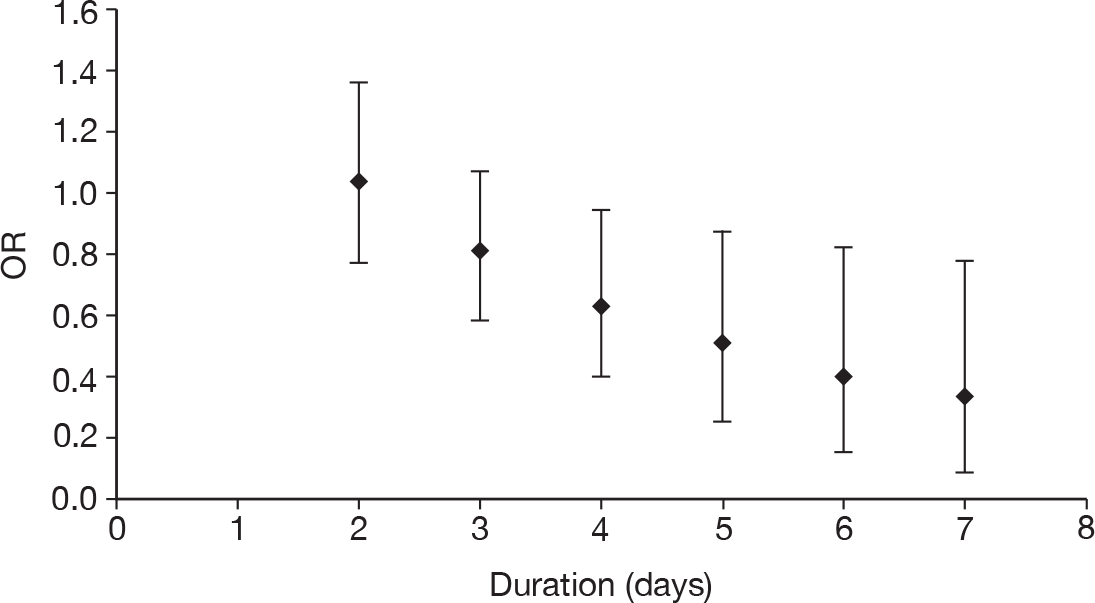
For the fixed-effects treatment comparison model T2 with covariates duration of treatment and 1/N, as sample size is an indicator for publication bias and other small-study effects,69,70 the treatment effect estimate from larger studies in/from our evidence should be used. Letting N → ∞ can be interpreted as representing the treatment effect estimated from an infinitely large study. This can be interpreted as adjusting for publication bias and other small-study effects. However, letting N → ∞ may lead to extrapolation well outside the limits of the data set with which the model was fitted. Results are also presented for N = 339, the largest treatment arm sample size in the set of studies included in our evidence synthesis. Figure 32a and b presents the ORs for IVIG/IVIGAM versus albumin/no treatment plotted against duration of treatment for N → ∞ and N = 339, respectively. For duration of treatment of 3 days and letting N → ∞, the OR of mortality for IVIG/IVIGAM versus albumin/no treatment was 1.07 (95% credible interval 0.61 to 1.73), showing no evidence that IVIG/IVIGAM was effective in reducing all-cause mortality. However, this estimate extrapolated the effects of sample size beyond the limits in the data set. For duration of treatment of 3 days and letting N = 339, the OR of mortality for IVIG/IVIGAM versus albumin/no treatment was 0.83 (95% credible interval 0.58 to 1.14), suggesting only weak evidence that IVIG/IVIGAM was effective in reducing all-cause mortality with the 95% credible interval containing 1.00 (i.e. no effect). Although N = 339 may appear an arbitrary choice, this should be interpreted as adjusting for publication bias/small-study effects based on the assumption that the largest trial published in this area was not subject to such bias. The results from using N = 339 are comparable to the other study quality adjustment results; therefore, this value was used for presenting the results of further models with 1/N as a covariate.
FIGURE 32.
Treatment comparison model T2: IVIG/IVIGAM versus albumin/no treatment with duration of treatment (a) for N ≥ ∞ (b) for N = 339 – OR (posterior mean and 95% credible intervals) by duration of treatment (days).
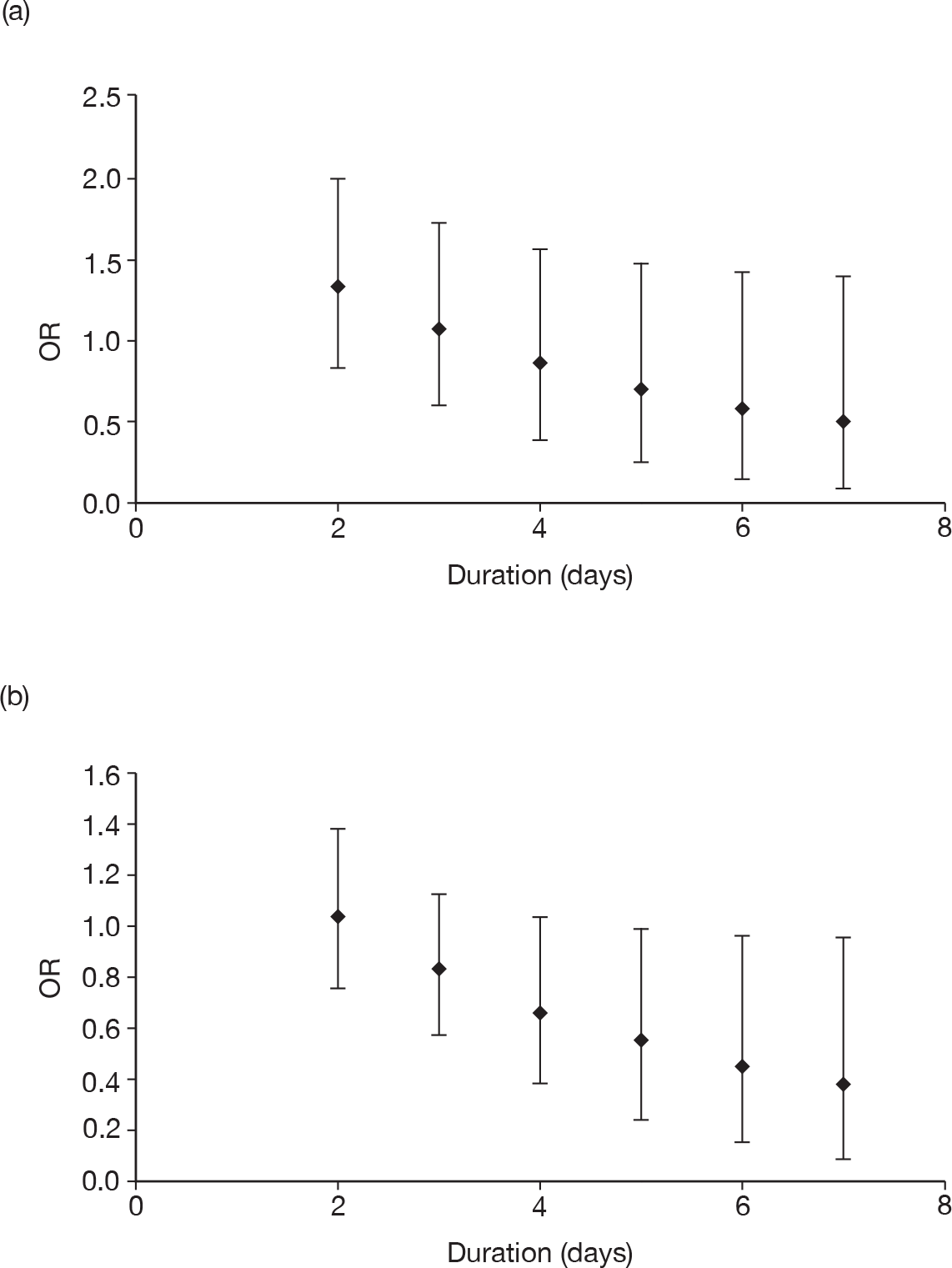
For the fixed-effects treatment comparison model T2 with covariates daily dose + 1/N, Figure 33 presents the OR for IVIG/IVIGAM versus albumin/no treatment plotted against daily dose of IVIG for N = 339. Treatment effect was stronger with lower daily doses. Average daily dose was approximately 0.3 g/kg/day. For a daily dose of 0.3 g/kg/day, the OR of mortality for IVIG/IVIGAM versus albumin/no treatment was 0.76 (95% credible interval 0.52 to 1.07), suggesting only weak evidence that IVIG/IVIGAM was effective in reducing all-cause mortality with the 95% credible intervals containing 1.00 (i.e. no effect).
FIGURE 33.
Treatment comparison model T2: IVIG/IVIGAM versus albumin/no treatment with daily dose for N = 339 – OR (posterior mean and 95% credible intervals) by daily dose (g/kg/day).
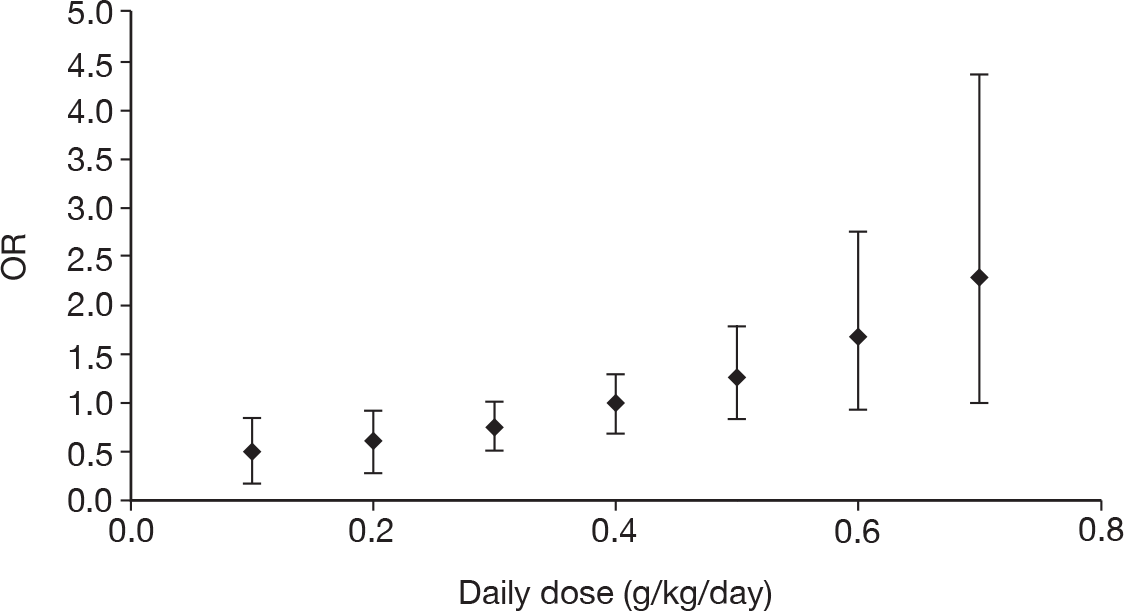
For the fixed-effects treatment comparison model T2 with covariates volume and 1/N, Figure 34 presents the OR for IVIG/IVIGAM versus albumin/no treatment plotted against volume of IVIG therapy for N = 339. Treatment effect was stronger with lower volumes. Average volume was approximately 5 ml/kg/day. For a volume of 5 ml/kg/day, the OR of mortality for IVIG/IVIGAM versus albumin/no treatment was 0.68 (95% credible interval 0.43 to 1.02), suggesting only weak evidence that IVIG/IVIGAM was effective in reducing all-cause mortality with the 95% credible interval containing 1.00 (i.e. no effect).
FIGURE 34.
Treatment comparison model T2: IVIG/IVIGAM versus albumin/no treatment with volume for N = 339 – OR (posterior mean and 95% credible intervals) by volume (ml/kg/day).
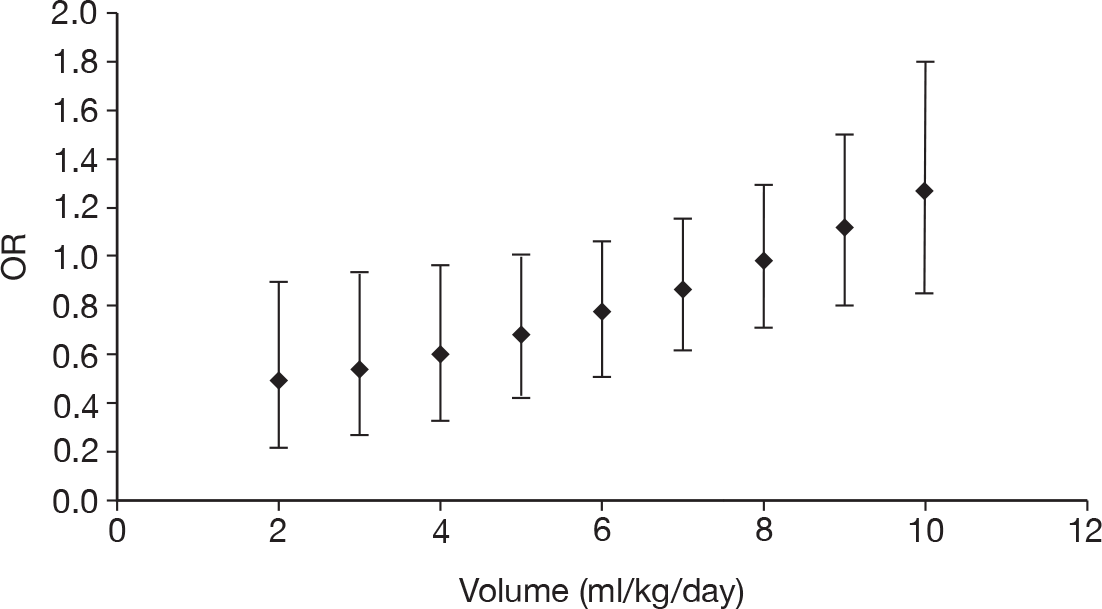
For the random-effects models it was assumed that the treatment effects for the different studies come from a common population of treatment effects. The predicted effect in a ‘new’ or ‘typical’ study, drawn from this common distribution, was used to summarise the treatment effect from these models. Note that this produced wider credible intervals than for the fixed-effects models because there were two components of uncertainty: one in the estimate of the pooled mean and the second in where the distribution of effects the population of interest might lie.
For the random-effects treatment comparison model T3b, the OR of mortality for IVIG/IVIGAM versus albumin was 0.68 (95% credible interval 0.16 to 1.83), suggesting a large degree of uncertainty that IVIG/IVIGAM was effective in reducing all-cause mortality.
For the random-effects treatment comparison model T2 with covariate Jadad score, for the Jadad score of 5, the OR of mortality for IVIG/IVIGAM versus albumin was 0.83 (95% credible interval 0.18 to 2.13), suggesting a large degree of uncertainty that IVIG/IVIGAM was effective in reducing all-cause mortality.
For the random-effects treatment comparison model T2 with covariate publication date, for a publication date of 2007, the OR of mortality for IVIG/IVIGAM versus albumin was 0.83 (95% credible interval 0.24 to 1.72), suggesting a large degree of uncertainty that IVIG/IVIGAM was effective in reducing all-cause mortality.
For the random-effects treatment comparison model T2 with covariate 1/N, for N → ∞, the OR of mortality for IVIG/IVIGAM versus albumin was 1.27 (95% credible interval 0.25 to 3.17) and for N = 339, the OR of mortality for IVIG/IVIGAM versus albumin was 0.92 (95% credible intervals 0.23 to 2.10). Both of these suggested a large degree of uncertainty that IVIG/IVIGAM was effective in reducing all-cause mortality. However, when N = 339, the results were in line with other results from the random-effects models, whereas when N → ∞, the posterior mean OR was > 1 and the 95% credible intervals were very wide.
Summary of sensitivity analyses
The treatment effect estimates were robust to the choice of method to adjust for study quality, publication bias and small-study effects (when N was set equal to the maximum arm size in the studies in our review – in those models that depended on sample size – avoiding extrapolation beyond the data set).
Treatment effect estimates were, however, sensitive to the assumed dose regime. It was not clear which values these should take. Robust results were obtained by setting these covariates equal to their average value. From the studies in our review, however, in the absence of any clinical rationale why these covariates should have a causative relationship with treatment efficacy, these relationships can only be considered as association. For this reason, the random-effects models that omitted these covariates were explored.
The results from the different random-effects models were fairly comparable, but provided wider credible intervals than the results from the fixed-effects models with the dosing regime covariates included. This was because the dosing covariates were not being used to explain heterogeneity but, instead, the heterogeneity present was acknowledged and a prediction made for a population drawn from the distribution of study effects.
Discussion
Key findings
There is evidence that there are issues with bias associated with trial methodology and publication/small-study effects and these were, therefore, explored by adjusting treatment effect using measures of trial methodology or publication bias/small-study effects (Jadad score, publication date, sample size, choice of control). Results were found to be fairly robust to whichever measure of study quality was adjusted for (note, a marginally significant result can become a marginally non-significant result). The conclusion is that there is a borderline significant (at the 5% level) effect of IVIG on reducing all-cause mortality for patients with severe sepsis/septic shock.
There was a large degree of heterogeneity in the treatment effects between studies. However, some measure of dosing regime, together with a measure of study quality or study size, could explain the between-study heterogeneity in treatment effect results. The estimates of treatment effect were therefore sensitive to the dosing regime; however, there was no clear clinical rationale for this result.
The best-fitting model adjusted for study quality by incorporating an effect for the choice of control (albumin or no treatment) and included duration of IVIG therapy as a treatment effect modifying covariate. The resulting treatment effect estimates, therefore, depended on duration of IVIG therapy. The most commonly used duration of therapy reported in the studies was 3 days and so this was chosen to report the results. This gave an OR of 0.75 with a 95% credible interval of 0.58 to 0.96, showing a reduction in the odds of all-cause mortality in patients with severe sepsis/septic shock using IVIG compared with albumin, a result that was just marginally significant at the 5% significance level.
If the heterogeneity explained by duration of IVIG therapy was treated as unexplained heterogeneity (i.e. a random-effect models), the results still showed a reduction in the odds of all-cause mortality in patients with severe sepsis using IVIG compared with albumin (OR 0.68), but the 95% credible intervals were widened (0.16 to 1.83) such that this result was no longer statistically significant.
Comparison with previous meta-analyses
There have been several previous meta-analyses conducted on IVIG for severe sepsis/septic shock (see Tables 3 and 4) and conflicting conclusions have been drawn. 71 The different meta-analyses produce slightly different results owing to the included studies (see Table 4), the type of IVIG (or IVIGAM) included, whether and how ‘high-quality’ trials have been defined and how heterogeneity has been accounted for (whether with fixed- or random-effects models and whether or not treatment moderating covariates have been adjusted for).
Previous meta-analyses that have estimated treatment effects separately for IVIG and IVIGAM10,15 have found a strong treatment effect for IVIGAM and a borderline significant effect for IVIG. However, although Kreymann et al. 10 reported that this result was robust to including high-quality evidence only, Alejandria et al. 15 found that when they restricted their analysis to studies at low risk of bias only, then neither of the treatment effects for IVIG or IVIGAM were significant at the 5% level. Most of the previous meta-analyses that explored the effects of trial quality11,14,15 found significant treatment effects when all evidence was included, but non-significant results when the analyses were restricted to ‘high-quality’ trials, however defined.
Although all previous meta-analyses tested for heterogeneity, all (with the exception of Turgeon et al. 12) performed a fixed-effects meta-analysis. Turgeon et al. 12 fitted a random-effects model, to allow for the heterogeneity between studies, and also explored factors that may explain the heterogeneity in treatment effects between studies. They found that the following factors were associated with treatment effect: dosage regime, duration of IVIG therapy, trial quality, publication date and whether patients had septic shock or other forms of severe sepsis. These results are all in line with our findings. Laupland et al. 11 demonstrated that treatment effects were stronger when no treatment was used as the control compared with when albumin was used, again in line with our findings.
Our meta-analysis is the first to simultaneously allow for type of IVIG/IVIGAM, control treatment, study quality/publication bias, dosing regime and other potential covariates. When some measure of study quality (e.g. choice of control) and some measure of dosing regime (e.g. duration of IVIG therapy) were controlled for, there appeared to be no difference between the type of IVIG/IVIGAM therapy.
Limitations of available evidence
As has been identified in previous meta-analyses, there are issues with the methodological quality of the available evidence. Although the treatment effect results are fairly robust to various different approaches to adjust for these, because the treatment effect measure is ‘borderline significant at the 5% level’, the choice of method to adjust for study quality/publication bias can lead to either significant or non-significant results. Although we do not place too much focus on statistical significance and focus more on the credible intervals, this sensitivity to the method for adjusting for study quality/publication bias causes some concern with the interpretation of the results based on this evidence.
There is substantial heterogeneity in the treatment effects from the different studies. Although this heterogeneity can be explained using aspects of the dosing regime, on detailed discussion with the Expert Group, it is not clear if there is any clinical rationale for these effects. These results should therefore be interpreted with caution, and we should note that these effects are only associations and should not be interpreted as necessarily causative in the absence of well-designed, dose-ranging studies.
Recommendations for models to be used in sensitivity analyses for cost-effectiveness modelling
There is no clear one best-fitting model that makes clinical sense. Sensitivity analyses to model results were therefore recommended for the cost-effectiveness modelling. The sensitivity analyses performed in the clinical effectiveness work suggested that the method used to adjust for study quality was not important (as long as one approach was used). The exception to this was letting in models that adjusted for sample size. Either one of the dosing covariates should be included or a random-effects model fitted. Results were sensitive to this choice and this should be explored in sensitivity analyses.
Chapter 4 Cost-effectiveness and value of information analysis – informing the model structure and identifying relevant data sources and inputs
Objectives
The assessment of cost-effectiveness and the value of information were conducted in two related phases of work.
-
Phase I
The objective of phase I was to develop the structure of a decision-analytic model and identify key parameter inputs consistent with the decision problem and relevant to an NHS setting.
Phase I was based on a review of existing cost-effectiveness studies and other relevant literature, to help develop a decision-analytic model structure consistent with the stated decision problem and to identify appropriate assumptions and input parameters required to populate it.
The review of existing cost-effectiveness studies was used to identify alternative structural assumptions and data sources used in existing studies to estimate resource use, survival and quality of life estimates associated with the initial episode of severe sepsis and septic shock and the longer-term prognostic implications. The review also served to identify key issues and potential data gaps that needed to be addressed within phase I, using additional focused systematic reviews and analyses of primary data.
-
Phase II
The objective of phase II was to populate the decision model, determine the cost-effectiveness of IVIG and to estimate the value of additional primary research.
The findings from phase I were used to inform the final structure of a new decision-analytic model and to identify appropriate parameter inputs required to determine the potential cost-effectiveness of IVIG in the NHS. Formal quantitative methods, based on expected value of information (EVI) approaches, were also used to inform future research priorities and to consider if investment in a multicentre randomised trial for sepsis (severe sepsis and septic shock) is likely to be worthwhile. The methods and results of the cost-effectiveness and value of information analyses are reported in Chapters 5 and 6, respectively.
This chapter describes the separate stages of work, methods and results from phase I.
Overview
The search strategies and associated work was planned in two separate stages.
-
Stage 1
Previous studies evaluating the cost-effectiveness of IVIG for the management of severe sepsis and septic shock were assessed against the inclusion criteria for the current review. A scoping search undertaken at the start of the project indicated that there was likely to be very limited published evidence specifically related to the use of IVIG in this population. As a result, the final searches and inclusion criteria were extended to include cost-effectiveness studies of other (non-IVIG) interventions for the management of severe sepsis and septic shock in adults.
-
Stage 2
From the initial review in Stage 1, the following key issues were prioritised for further systematic reviews and primary data analysis:
-
baseline mortality rates
-
long-term life expectancy of survivors of severe sepsis
-
health-related quality of life of survivors of severe sepsis.
Methods for the cost-effectiveness literature review
A systematic literature search was undertaken to identify existing evidence on the cost-effectiveness of IVIG and other interventions for the treatment of adult patients with severe sepsis and septic shock.
The specific aims of the review were to:
-
critically appraise the existing evidence on the cost-effectiveness of IVIG in the treatment of adult patients with sepsis or septic shock
-
evaluate published decision-analytic models in detail (both IVIG and other interventions) to identify important structural assumptions and data sources for parameter inputs and to highlight key areas of uncertainty and potential data gaps
-
identify key parameter inputs requiring additional systematic reviews and/or analyses of primary data
-
inform the development and population of our own decision model, relevant to the NHS.
Inclusion criteria
A broad range of studies were considered for inclusion in the assessment of cost-effectiveness, including economic evaluations conducted alongside trials, modelling studies and analyses of administrative databases. Only full economic evaluations that considered both costs and consequences (including cost-effectiveness, cost–utility and cost–benefit analysis) and compared two or more treatment options were included in the literature review.
Studies were identified by electronically searching the NHS Economic Evaluation Database (NHS EED) via the Cochrane Library (searched 2 October 2009). No date or language restrictions were used. The full search strategy is reported in Appendix 2. The reference lists from identified studies were also screened.
Two reviewers (PP and SJP) independently assessed all titles and abstracts. Full texts of titles/abstracts deemed relevant were retrieved and the full text was used for the final selection. Reasons for excluding the full-text studies were recorded.
All studies of IVIG for the treatment of severe sepsis were critically reviewed with the assistance of a quality assessment checklist for cost-effectiveness studies (see Appendix 3). 72 For those studies evaluating non-IVIG interventions, information was extracted on the comparators, study population, main analytical approaches (e.g. patient-level analysis/decision-analytic modelling), primary outcome specified for the economic analysis, details of adjustment for quality of life and costing approaches. This information is tabulated and summarised in the following sections.
The differences in approaches, assumptions and data sources were explored to inform the need for additional systematic reviews and/or further primary or secondary analyses. The findings from these reviews and analyses provided the basis for the development and population of the new decision model reported in Chapter 5.
Results of the cost-effectiveness literature review
The systematic literature review identified 149 potential references, of which 16 studies subsequently met the inclusion criteria. 73–89 Only one of these studies specifically evaluated the cost-effectiveness of IVIG for the management of sepsis and septic shock in adult patients. 73 The remaining 15 studies evaluated the cost-effectiveness of other health-care interventions for the treatment of severe sepsis and septic shock. 74–89 Five published abstracts were also identified; however, only limited information on the methods employed was reported and so these studies were excluded from the final literature review. A flow chart of studies included in the final cost-effectiveness review is shown in Figure 35.
FIGURE 35.
Flow chart of studies included in the cost-effectiveness literature review.
Cost-effectiveness of intravenous immunoglobulin
The single published study of IVIG evaluated the cost-effectiveness of IVIGAM (Pentaglobin) as an adjunct to standard care, compared with standard care alone, in an adult critical-care unit population with severe sepsis or septic shock. 73 The analysis was undertaken from a German hospital perspective.
The study was based on a decision-analytic approach using a simple decision tree structure (Figure 36) to assess the costs and effects of IVIGAM added to standard care compared with standard care alone. Although the time horizon was not explicitly stated in the analysis, the model structure was restricted to the initial period in the critical-care unit only. Conditional upon the treatment strategy, the model estimates the associated probability of critical-care unit mortality/survival. These estimates were subsequently used to derive the number needed to treat (NNT) with IVIGAM to avoid one case of critical-care unit mortality being compared with standard care alone. Cost-effectiveness was assessed by estimating the incremental cost per additional life saved. No discounting of future costs or benefits was carried out given the focus on the initial period in the critical-care unit.
FIGURE 36.
Structure of the decision tree used for the evaluation of IVIGAM. ICU, intensive care unit.

Clinical evidence for the risk of critical-care unit mortality for IVIGAM and standard care alone was based on the results of a separate meta-analysis that updated a previously published analysis. 90 Studies were restricted to the nine RCTs90 in adult populations comparing IVIGAM with placebo (435 patients; control group, n = 212; IVIGAM, n = 223).
The overall critical-care unit mortality in the pooled control arms of the placebo studies was reported to be 44% (95% CI 33% to 52%). In the model this was assumed to represent the baseline risk of critical-care unit mortality for standard care alone. Owing to heterogeneity in the relative treatment effect reported across the individual RCTs, the authors performed a random-effects meta-analysis to estimate the relative effectiveness of IVIGAM in reducing critical-care unit mortality (relative risk 0.57; 95% CI 0.43 to 0.74). Applying the relative risk to the baseline mortality risk resulted in an absolute risk reduction of 19% (95% CI 14% to 22%) for IVIGAM compared with standard care alone. From this, the NNT with IVIGAM to save one life was estimated to be 5.2 (95% CI 4.0 to 9.0).
Only direct medical costs incurred in the critical-care unit were included in the evaluation. These included: length of critical-care unit stay, use of ‘block’ therapies (i.e. sepsis therapy, blood therapy, ventilation and renal therapy) and the acquisition costs of IVIGAM. The length of critical-care unit stay and unit costs were assumed to be different for survivors and non-survivors of the critical-care unit stay. The difference in the length of critical-care unit stay between IVIGAM and standard care was, therefore, based on the difference in the proportion of survivors and non-survivors between the two strategies. The acquisition costs of IVIGAM were assumed to be the same for survivors and non-survivors. As only short-term costs associated with the initial critical-care unit stay were considered, discounting was not applied. The price year for costs was not stated. The results of the base-case analysis showed that the use of IVIGAM resulted in incremental costs of €2037 compared with standard care alone.
The incremental cost-effectiveness ratio (ICER) for IVIGAM compared with standard therapy was €10,565 per life saved (i.e. €2037/0.1928). In a univariate sensitivity analysis (i.e. varying estimates for single input parameters), the ICER varied between €7231 and €28,443 per life saved. The results of the probabilistic sensitivity analysis (i.e. varying estimates for all input parameters simultaneously) suggested that at a willingness-to-pay level of €15,000 per life saved, the probability that IVIGAM is cost-effective was 83.9%.
Discussion and key issues
Only one published cost-effectiveness analysis of IVIG was identified,73 which evaluated the short-term cost-effectiveness of IVIGAM compared with standard care for severe sepsis/septic shock. The results of the quality assessment of this study are reported in Appendix 3. The quality assessment highlighted several important issues that potentially limit the generalisability of the findings from this study to UK clinical practice.
-
The analysis has a short-term time horizon, restricted to the critical-care unit stay, and the long-term impact of IVIG has not been considered. Any assessment of the cost-effectiveness of IVIG should allow for the long-term cost and outcome implications of the short-term effects of the intervention. This ‘extrapolation’ is needed for two reasons. First, many patients who are treated for severe sepsis will continue to consume health-service resources beyond the initial critical-care unit stay and the cost-effectiveness of IVIG may influence these costs. Second, to compare the cost-effectiveness of IVIG with other uses of health-service resources (inside and outside of critical care), it is necessary to express the benefits of the drug in terms of a generic measure of health gain that can be compared across treatment areas. The most frequently used generic measure for this purpose is the quality-adjusted life-year (QALY). To provide a realistic estimate of the QALY impact of IVIG, the long-term implications for survival and health-related quality of life arising from the short-term effects of the intervention need to be incorporated.
-
IVIGAM (Pentaglobin) is a specific IgM-enriched immunoglobulin product that is not available in the UK and the results of the cost-effectiveness analysis may not be generalisable to non-IgM-enriched immunoglobulin products. Consequently, it is not clear if IVIGAM is potentially more or less cost-effective than the alternative IVIG products available in the UK, owing to differences in clinical effectiveness and/or acquisition costs.
-
Standard care has been used as comparator technology in the analysis. However, the authors do not attempt to define the components of standard care or to explore whether or not the placebo arms of the trials are likely to provide an appropriate source for this. As a result, it is difficult to assess the generalisability of the estimate of critical-care unit mortality to a UK setting.
-
Both the baseline risk assigned to standard care and the relative effectiveness estimates of IVIGAM have been derived from the same studies included in the meta-analysis. Using the pooled estimate from the placebo arms of these studies to inform the baseline mortality risk for standard care arm, raises a couple of issues. First, as the studies included in the meta-analysis enrolled a small number of patients from various settings and had highly selected participants, it is unclear if the pooled baseline risk appropriately reflects standard care in Germany or the UK. Second, the heterogeneity noted across the studies in terms of the relative treatment effect may also exist for the baseline mortality risk in the placebo arms across the studies. Instead of using an ‘average’ measure of baseline risk, it might have been more appropriate to consider if some of the variation in mortality could be explained by study-level characteristics (e.g. severity of illness, setting) or if particular studies more closely related to the specific population and setting under consideration could have been used. Alternatively, external epidemiological evidence relevant to the setting and populations considered in routine clinical practice could have been more appropriate in informing the baseline risk of critical-care unit mortality (i.e. from cohort or registry data).
Cost-effectiveness of non-intravenous immunoglobulin interventions
The review of the cost-effectiveness evidence for IVIG identified several major limitations with the existing study and the results are unlikely to be relevant to informing the use of IVIG in the NHS for adult patients with severe sepsis and septic shock. Furthermore, the simple model structure, and the exclusion of long-term survival, quality of life and costs meant that this study did not provide much insight into the key areas required to develop our own model. Published decision-analytic models of other interventions for the management of severe sepsis were therefore examined.
The 15 studies74–89 identified evaluating the cost-effectiveness of other interventions for the management of adult patients with severe sepsis and septic shock are summarised in Table 17.
| Study | Country | Interventions | Analysis | Perspective | Time horizon |
|---|---|---|---|---|---|
| Manns et al. (2002)74 | Canada | rhAPC vs standard care alone | CEA, CUA |
Base case: purchaser of health-care services Sensitivity analysis: broader societal perspective |
Lifetime |
| Angus et al. (2003)75 | USA | rhAPC vs standard care alone | CEA, CUA | Societal |
Base case: 28 days Reference case: lifetime |
| Betancourt et al. (2003)76 | USA | rhAPC vs standard care alone | CEA | Hospital | 28 days |
| Fowler et al. (2003)77 | USA | rhAPC vs standard care alone | CEA, CUA | Societal | Lifetime |
| Neilson et al. (2003)78 | Germany | rhAPC vs standard care alone | CEA | German health-care setting | Lifetime |
| Sacristán et al. (2004)79 | Spain | rhAPC vs standard care alone | CEA | Health-care payer | Lifetime |
| Davies et al. (2005)80 | UK | rhAPC vs standard care alone | CEA, CUA | NHS | Lifetime |
| Hjelmgren et al. (2005)81 | Sweden | rhAPC vs standard care alone | CEA, CUA | NR | Lifetime |
| Green et al. (2005),82 (2006)83 | UK | rhAPC vs standard care alone | CEA, CUA | NHS | Lifetime |
| Franca et al. (2006)84 | France | rhAPC vs standard care alone | CEA, CUA | NR | Lifetime |
| Dhainaut et al. (2007)85 | France | rhAPC (pre-licence patients/post-licence patients) | CEA, CUA | Health-care provider | Lifetime |
| Costa et al. (2007)86 | Canada | rhAPC vs standard care alone | CEA, CUA | Public health-care provider |
Base case: 20 years Sensitivity analysis: 10–30 years |
| Guidet et al. (2007)87 | France | Systemic albumin infusion vs standard care alone | CEA | NHS | Lifetime |
| Huang et al. (2007)88 | USA | EGDT vs standard care alone | CEA, CUA |
Hospital case: hospital Reference case: societal |
Hospital case: hospital stay Reference case: lifetime |
| Talmor et al. (2008)89 | USA | EGDT-based treatment pathway vs historical controls | CEA, CUA | Health-care system | Lifetime |
Twelve of these studies evaluated the cost-effectiveness of adding rhAPC to standard care compared with standard care alone. 74–86 Two studies evaluated the cost-effectiveness of early goal-directed therapy (EGDT)88,89 and one study evaluated the cost-effectiveness of systemic albumin infusion compared with standard medical care. 87
Two of the 12 rhAPC studies were from the UK. 80,82,83 The remainder were from the USA (n = 3),75–77 Canada (n = 2),74,86 France (n = 2),84,85 Germany (n = 1),78 Spain (n = 1)79 and Sweden (n = 1). 81 Both EGDT analyses were from the USA88,89 and the one albumin study was from France. 87 Eleven of the 15 studies reported results in terms of the incremental cost per QALY gained. 74,75,77,80–86,88,89
The perspective assumed by each of the studies is also reported in Table 17. The majority of studies considered the perspective of the providing institution (hospital) or of the health-care sector more generally. Four studies stated that a societal perspective was considered either in the base case or in a sensitivity analysis. 74,75,77,88 However, only one of these analyses74 actually attempted to incorporate the impact of productivity losses.
Model structures, time horizon and approaches to extrapolation
The studies used a range of different model structures and assumptions to model the costs and benefits of the interventions considered. Typically, studies used either (1) a simple decision tree; or (2) a combination of a decision tree to capture the short-term mortality of the initial episode and a Markov model to extrapolate survival and costs over a longer-term time horizon; or (3) a single Markov model to characterise both the short- and longer-term time horizons.
Markov models (Markov chains evaluated in discrete time) are useful when a decision problem involves modelling risk over time, when the timing of events is important and when these events may recur over time. 91 In contrast to a decision tree, where the full range of mutually exclusive pathways representing a patient’s prognosis are represented schematically by the individual tree ‘branches’, Markov models are based on a finite (or countable) number of discrete health states, called Markov states. In a Markov structure, hypothetical individuals reside in one out of the set of mutually exclusive health states at particular points in time. During discrete time intervals of equal length (normally referred to as Markov cycles), individuals can either remain in a particular health state or move to a separate health state (e.g. because of a patient experiencing a particular clinical event). The movements between states represent the potential clinical pathways that a patient may follow at different time points and over his or her remaining lifetime. The likelihood that an individual remains in a particular health state, or moves to a separate state, in the next Markov cycle is represented in terms of transition probabilities. Defining and subsequently estimating these transition probabilities represent both key structural and analytical elements of the decision model. The use of Markov model structure allows a more sophisticated approach to modelling the annual risk of death after the survival of the initial episode, allowing the annual risk of death to be altered over the longer term. Hence, the choice of model structure in the published studies relates closely to the study time horizon and the assumptions made to extrapolate short-term survival to mid- and long-term survival.
The majority of the studies used a decision tree approach to model short-term costs and outcomes of the alternative strategies. The short-term period varied in the studies between the critical-care unit stay, a fixed period of 28 days and/or the overall hospital stay. This period typically reflected the short-term nature of the relevant RCT evidence (and outcomes reported therein) used to inform the relative effectiveness of the specific interventions under investigation.
An example of a typical decision-tree structure is provided in Figure 37. Survival beyond the initial short-term period was estimated either by applying average age- and sex-specific estimates of the remaining years of life for short-term survivors or by adding a separate Markov model structure to the end of the short-term decision tree to characterise the longer-term prognosis of a sepsis survivor. Generally, a Markov model structure was used to estimate the duration over which a sepsis patient faced an increased risk of death compared with the general population (mid-term survival), after which the longer-term mortality risk was subsequently assumed to match that of the general population.
FIGURE 37.
Example of a short-term decision tree.
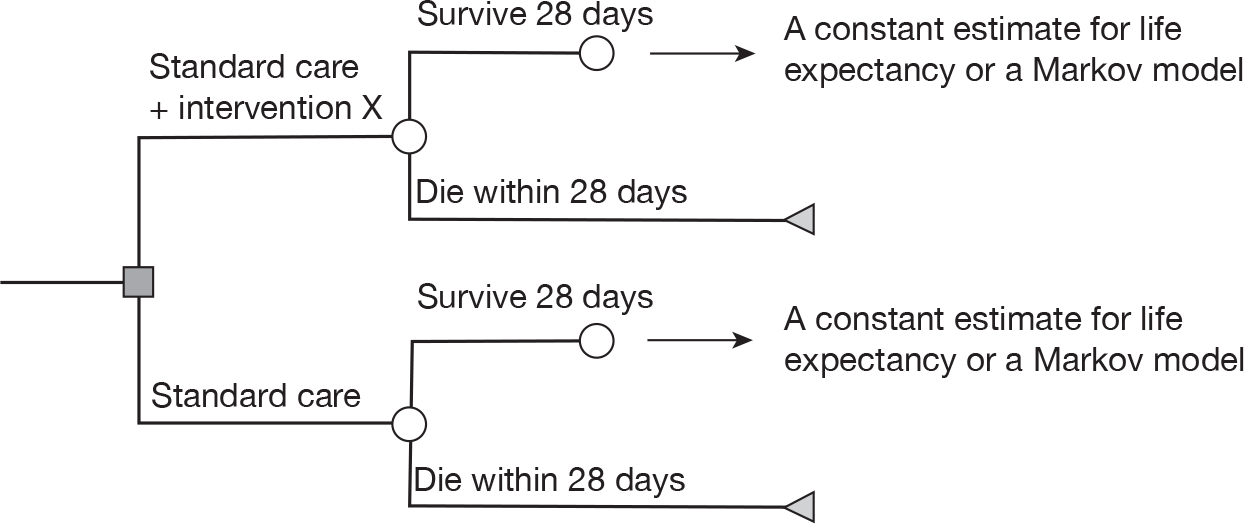
The majority of studies used the placebo arm of the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study92 to estimate the baseline mortality risk in the short-term period assigned to standard care. Only two studies74,82,83 used epidemiological evidence from non-RCT sources to reflect the specific setting of the cost-effectiveness analysis. One study82,83 from the UK used national audit data from the ICNARC CMP Database and the authors of another study74 conducted a separate cohort study in critical-care units of three local tertiary care hospitals.
All but one76 of the studies considered time horizons beyond the short-term period and the majority of these used a lifetime horizon to estimate the remaining life-years and QALYs for a survivor of the short-term sepsis period. The remaining life expectancy for survivors of severe sepsis was calculated using two alternative approaches in the studies considered:
-
the remaining life expectancy of survivors of severe sepsis was calculated in relation to the general population life expectancy by applying a single adjustment factor to represent the additional long-term mortality risk for sepsis survivors (e.g. relative survival over a lifetime was assumed to be approximately half that of the general population); or
-
short-term survivors were assumed to have an increased risk of mortality for a specified time period ranging from 30 months to 8 years (mid-term survival), after which the mortality of survivors was generally assumed to match the mortality rates of the general population (long-term survival).
The estimates for the long-term increased risk of mortality for these approaches were typically taken from one of four studies reporting long-term survival rates for a cohort of sepsis patients or a general critical-care population. 74,93–95 The four studies and the methods used in the cost-effectiveness analysis to extrapolate the data are briefly described in Table 18.
| Study | Brief study description | Extrapolation methods used in the cost-effectiveness analysis (reference to studies that have used this method) |
|---|---|---|
| Quartin et al. (1997)93 |
Aim: to evaluate the magnitude and duration of the effect of sepsis on survival Setting: 10 Department of Veterans Affairs Medical Centers in the USA Population: patients with sepsis (n = 1505) compared with non-psychiatric and non-infected patients (n = 19,830) Follow-up: 8 years after the index hospitalisation |
Quartin et al. reports that sepsis reduces the mean remaining life expectancy from 8.03 years to 4.08 years in 30-day survivors Method I: age- and sex-specific life expectancy for every short-term survivor adjusted by a factor 0.51 (= 4.08/8.03) for the additional mortality due to severe sepsis75,78,79,81,88,89 Method II: increased risk of death applied for years 1–8 after sepsis episode; outcomes beyond 8 years assumed to follow age-specific mortality rates of relevant surviving population77 |
| Wright et al. (2003)94 |
Aim: to compare the long-term survival of critically ill patients with survival of age- and sex-matched general population Setting: critical-care unit of a teaching hospital in Glasgow Patients: patients [n = 2104, of whom 202 (9.6%) had septic shock] admitted to the critical-care unit; expected mortality for the control group was established from general population of Scotland (published by the office of the Registrar General) Follow-up: ≥ 5 years, but ≤ 12 years |
Method I: annual mortality rates from the cohort study (n = 2104) applied for 28-day survivors through years 1–4 following critical-care unit discharge. Beyond 4 years, survival assumed to follow age- and sex-specific survival of general population extracted from life tables82,83 Method II: results from Cox proportional hazard model (n = 202) were used to adjust the life expectancy of general population (taken from life tables) for the additional mortality due to severe sepsis80 |
| Manns et al. (2002)74 |
Aim: to estimate subsequent mortality and direct health-care costs of surviving patients hospitalised with severe sepsis Setting: three tertiary care hospitals in Canada Patients: patients hospitalised with severe sepsis (n = 787) Follow-up: 3 years |
Method I: annual mortality rates from the cohort study applied for sepsis survivors through years 1–3 following hospital discharge. Beyond 3 years, mortality rates of general population adjusted with age-specific increment calculated from the cohort study (at year 3)74 Method II: survival from hospital discharge to 30 months, calculated using long-term data from the PROWESS trial. 95 Long-term benefits beyond 30 months estimated from population life tables and adjusted for the higher mortality risk for sepsis patients reported in the cohort study as the year 3 risk of death after hospital discharge97 |
| Angus et al. (2004)95 |
Aim: to report the long-term survival of patients with severe sepsis enrolled in PROWESS trial of rhAPC compared with placebo Setting: multinational Patients: patients enrolled in the PROWESS trial (n = 1690, of whom 1220 were alive 28 days after enrolment) Follow-up: ≤ 3.6 years |
Method I: survival from hospital discharge to 30 months, calculated using long-term data from the PROWESS trial.95 Long-term benefits beyond 30 months estimated from population life tables and adjusted for the higher mortality risk for sepsis patients reported in the cohort study by Manns et al.,74 using the year 3 risk of death after hospital discharge86 |
Quality of life
Eleven66–75,77,80–85,88,89 of the 15 studies conducted cost–utility analysis and included an adjustment for quality of life of the remaining life expectancy estimates to estimate QALYs. Three80,81,89 of these 11 studies used utility values from a published abstract by Drabinski et al. 96 in adult sepsis survivors and four studies80,82–85 used values from a cohort study of ARDS by Angus et al. 97 Brief details of these two sources and the methods used by the cost-effectiveness studies are reported in Table 19. Typically, the remaining years of life were simply weighted by a single utility value (either 0.60 or 0.69) from one of these sources, to estimate lifetime QALYs for the survivors of severe sepsis.
| Study | Brief study description | Methods used in the cost-effectiveness analysis (reference to studies that have used this method) |
|---|---|---|
| Angus et al. (2001)97 |
Aim: to provide an estimate of quality-adjusted survival after ARDS to explore the extent to which quality-adjusted survival is associated with particular baseline characteristics, and to compare results in ARDS survivors with healthy and sick control subjects Setting: 35 hospitals in the USA Population: patients with ARDS (n = 200) Instrument: quality of well-being scale Results: mean (SD) scores 0.59 (0.015) and 0.60 (0.015) at 6 months and 12 months after study enrolment, respectively |
A utility value of 0.6 was used to estimate quality of life in all remaining life-years74,82-85 |
| Drabinski et al. (2001)96 |
Aim: to assess change in health status in sepsis survivors over a 6-month period Setting: 53 hospitals in the USA Population: survivors of severe sepsis (n = 93) Instrument: EQ-5D and visual analogue scale Results: average EQ-5D scores 0.53, 0.62, 0.68 and 0.69 at days 30, 60, 90 and 180, respectively |
A utility value of 0.69 was assumed to represent quality of life in all remaining life-years80,81,89 |
In the remaining cost-effectiveness studies, two separate approaches were used to calculate the quality-adjusted survival of sepsis patients. Two cost-effectiveness analyses75,88 used the average quality-adjusted survival of someone in the general population with the same estimated remaining life expectancy and one study77 used published utility values for other non-sepsis health states, which were judged by the authors to be comparable to sepsis.
Resource use and costs
The key short-term and long-term costs included in the published cost-effectiveness studies are summarised in Table 20. All studies included the costs of study drug and the initial hospitalisation. Most of these studies separated the costs of the initial hospitalisation into critical-care unit and non-critical-care unit hospital ward costs. A limited number of studies also included the cost of treating adverse events, cost of other therapies (ventilation support, vasodilator support, renal support, blood therapy) and post-hospital costs up to day 28 (subsequent acute hospital care, nursing home, formal or informal supportive care at home).
| Study | Short-term costs | Long-term costs | ||||
|---|---|---|---|---|---|---|
| Hospitalisation | Adverse events | Other | Health care | Nursing home | Other | |
| Manns et al. (2002)74 | X | X | X (years 1–3) | X | ||
| Angus et al. (2003)75 | X | X | X | X | ||
| Betancourt et al. (2003)76 | X | X | ||||
| Fowler et al. (2003)77 | X | X | X | X | ||
| Neilson et al. (2003)78 | X | X | ||||
| Sacristán et al. (2004)79 | X | |||||
| Davies et al. (2005)80 | X | |||||
| Hjelmgren et al. (2005)81 | X | |||||
| Green et al. (2005),82 (2006)83 | X | X | ||||
| Franca et al. (2006)84 | X | |||||
| Dhainaut et al. (2007)85 | X | |||||
| Costa et al. (2007)86 | X | X | X (years 1–3) | |||
| Guidet et al. (2007)87 | X | |||||
| Huang et al. (2007)88 | X | X | X | |||
| Talmor et al. (2008)89 | X | |||||
Only 674,75,77,82,86,88 of the 15 studies modelled longer-term costs for survivors beyond the initial hospitalisation. Within these studies there was variation in both the types of costs included and the duration over which these costs were modelled. Although all of these studies included the cost of subsequent health care for survivors, fewer studies included other costs such as annual nursing home costs, productivity losses and death from any cause. Furthermore, only four75,77,82,83,88 of the six studies74–76,82,83,86,88 incorporated costs incurred over the remaining time horizon of the analysis. The two remaining studies restricted the analysis of long-term costs to a fixed period of 3 years. 74,86
Methods and results of systematic reviews and additional primary data analysis for priority issues
The 16 identified studies73–89 used a range of alternative methods, assumptions and data sources for several key aspects. From this initial review, three specific issues were subsequently prioritised, which were considered to require additional investigation to assist in extrapolating the short-term results from the studies included in the clinical effectiveness review into lifetime QALY estimates.
These issues were:
-
the baseline mortality risk of critical-care unit/hospital mortality with standard care alone
-
long-term life expectancy for severe sepsis survivors
-
health-related quality of life after survival of severe sepsis.
Given the variation in approaches used in existing studies and the lack of a clear consensus emerging on appropriate data sources and assumptions, these specific issues were identified as priority areas for further focused systematic reviews and additional primary data analyses. The central consideration of these reviews was to help identify and inform the most appropriate data relevant to our decision problem and to the NHS.
Baseline mortality of severe sepsis/septic shock
Of the 16 studies73–89 considered in the cost-effectiveness review, the majority used data from the control arms of RCTs to estimate the baseline mortality risk during the critical-care unit or overall hospital stay. However, these RCTs were mainly or wholly undertaken outside the UK. In many respects, treatment patterns and resource use in the UK can be expected to differ from those in centres involved in the trials. One implication of these differences in UK practice is that the baseline event rates observed in the trials (i.e. in the control groups) are unlikely to provide reliable estimates for UK practice. For this reason, baseline mortality rates in our analysis were informed by additional primary data analysis of an alternative data source, the ICNARC Case Mix Programme (CMP) Database.
The CMP is the national comparative audit of patient outcomes from adult critical-care units in England, Wales and Northern Ireland, co-ordinated by ICNARC. The CMP is a voluntary performance assessment programme using high-quality clinical data to facilitate local quality improvement through routine feedback of comparative outcomes and key quality indicators to clinicians/managers in adult critical-care units. The CMP recruits predominantly adult general critical-care units, either standalone intensive care units or combined intensive care/high-dependency units. Currently, approximately 90% of adult, general critical-care units in England, Wales and Northern Ireland are participating in the CMP.
Case Mix Programme specified data are recorded prospectively and abstracted retrospectively by trained data collectors according to precise rules and definitions. Data collectors from each unit are trained prior to commencing data collection with retraining of existing staff, or training of new staff, also available. Data are collected on consecutive admissions to each participating critical-care unit and are submitted to ICNARC quarterly. Data are validated locally, on data entry, and then undergo extensive central validation for completeness, illogicalities and inconsistencies, with data validation reports returned to the units for correction and/or confirmation. The validation process is repeated until all queries have been resolved and then the data are incorporated into the CMP Database. The CMP Database has been evaluated according to the quality criteria of the Directory of Clinical Databases98 and scored highly. 99
Admissions with severe sepsis during the first 24 hours following admission to the critical-care unit were identified and extracted from the CMP Database using physiological criteria derived from those used in the PROWESS study of rhAPC. 3,5 Briefly, severe sepsis in the CMP is defined as evidence of infection (identified from the primary and/or secondary reason for admission to the critical-care unit), plus three or more SIRS criteria and at least one organ dysfunction (cardiovascular, respiratory, renal, haematological or metabolic) at any time during the 24-hour period. Severity of illness was summarised by the ICNARC physiology score,100 the APACHE II score26 and the number of organ dysfunctions. Outcome was measured by mortality at discharge from the original critical-care unit and mortality at ultimate discharge from an acute hospital. Activity was measured by length of stay in the critical-care unit (stratified by survival status at acute hospital discharge) and the total length of stay in acute hospital (stratified by survival status at acute hospital discharge). Both the outcome and activity data provide important sources relevant to informing parameter estimates for cost-effectiveness analysis in the UK. The database has been previously used to establish baseline epidemiology for severe sepsis in the UK3,5 and baseline event rates in one of the UK cost-effectiveness studies of rhAPC. 82,83
Data collected between 1995 and 2009 from the CMP Database were available for analysis. However, owing to changes in the management of patients in the UK, the mortality risk may have changed over time. This was explored descriptively by comparing critical-care unit and hospital mortality rates between 2002 and 2009 (Table 21). Given the trend towards lower mortality rates over time, analyses were restricted to data collected from 2007 to 2009.
| Financial year (April–March) | Number of admissions | Critical-care unit mortality (%) | Hospital mortality (%) | APACHE II score (mean) | ICNARC physiology score (mean) | Number of organ dysfunctions (mean) |
|---|---|---|---|---|---|---|
| 2002–3 | 16,605 | 31.6 | 44.8 | 20.10 | 23.54 | 2.47 |
| 2003–4 | 19,536 | 31.7 | 45.0 | 19.96 | 23.59 | 2.51 |
| 2004–5 | 20,539 | 30.4 | 43.2 | 20.07 | 23.56 | 2.53 |
| 2005–6 | 21,502 | 29.6 | 42.2 | 20.21 | 23.55 | 2.51 |
| 2006–7 | 20,651 | 29.6 | 41.9 | 20.21 | 23.60 | 2.51 |
| 2007–8 | 19,636 | 29.0 | 41.0 | 20.00 | 23.37 | 2.49 |
| 2008–9 | 18,345 | 28.3 | 39.6 | 19.57 | 23.16 | 2.46 |
Table 22 reports the mean sample characteristics used to inform the baseline mortality estimates in the decision model. The mean critical-care unit and overall hospital mortality for all admissions were 29.1% (95% CI 28.6% to 29.7%) and 40.6% (95% CI 40.0% to 41.2%), respectively. Variation in the baseline mortality risk in different subgroups may also have important implications in terms of cost-effectiveness assessments. A more detailed presentation of the baseline mortality across a range of subgroups is presented in Appendix 4. The use of these data within the cost-effectiveness model is discussed further in Chapter 5.
| Sample characteristic | Mean (SD) |
|---|---|
| Age (years) | 62.6 (17.19) |
| ICNARC physiology score | 23.3 (9.50) |
| APACHE II score | 19.7 (6.96) |
| Number of organ dysfunctions | 2.5 (1.07) |
Long-term life expectancy
The assumptions used to estimate long-term survival of sepsis patients in the existing cost-effectiveness studies were typically based on three (non-UK) studies74,93,95 reporting long-term outcomes in severe sepsis patients and one UK study94 assessing long-term outcomes in general critical-care unit patients. As the assumptions and data sources were considered a key aspect of the development and population of our own cost-effectiveness model, an additional systematic search was conducted updating a previous review by Green et al. 82 In contrast to Green et al. ,82 cohort studies of general critical-care populations were excluded. These cohorts constitute a heterogeneous population and long-term mortality rates have been reported to vary significantly between patient subgroups. 101 Consequently, the assumption that average life expectancy of general critical-care unit patients reflects the life expectancy of a severe sepsis/septic shock patient admitted to a critical-care unit may not be appropriate. This review was, therefore, restricted to cohort studies of severe sepsis and septic shock patients.
Primary data analysis was also undertaken using an unpublished cohort study of severe sepsis from the UK. The results of the review and the primary data analysis are reported below.
Systematic review
Studies were included in the update review if they fulfilled all of the following inclusion criteria: (1) adult patients with severe sepsis or septic shock, (2) mortality data reported beyond hospitalisation reported and (3) a follow-up time of ≥ 1 year.
Studies were identified by searching MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (4 November 2009). As this was an update search, the MEDLINE searches were limited to studies published after 2004. The full search strategy is reported in Appendix 2. In addition, three specialist journals (Critical Care Medicine, Intensive Care Medicine and American Journal of Respiratory and Critical Care Medicine) were also hand searched following input from the Expert Group. Finally, the reference lists of identified studies were also checked to identify other relevant studies. No date restrictions were placed on these additional searches.
Studies included in the systematic review
In addition to the two severe sepsis studies74,93 previously identified by Green et al. ,82,83 our updated MEDLINE search identified four new studies. 95,102–104 Two further studies were also identified in the hand search of relevant journals74,105 and three studies106–108 were identified from the reference lists of the included studies. The flow chart of studies included in the review is reported in Figure 38. Ten individual publications74,93,95,102,103,105–109 were identified from nine separate studies (with two publications coming from the same study).
FIGURE 38.
Flow chart of studies included in the long-term life expectancy review.
Overview of included studies
The characteristics of identified studies are summarised in Table 23. Although the studies were undertaken in a variety of countries, no published study of the long-term survival of severe sepsis specific to the UK was identified. The majority of cohorts were from the 1980s or 1990s, although the most contemporary cohort was the Finnsepsis study from 2004–5. 103,109 The follow-up time across the studies varied from 1 year to 9.6 years.
| Study | Country | Time of selection | Follow-up (years) | n | Male (%) | Age (years) | Severity | |
|---|---|---|---|---|---|---|---|---|
| APACHE II score (mean) | Multiple organ dysfunction (%) | |||||||
| Leibovici et al. (1995)106 | Israel | 1992–8 | 1.25 | 1991 | 52 | 72 | NR | NR |
| Sasse et al. (1995)108 | USA | 1987–91 | 1 | 153 | 58.2 | 56.8 | 23.4 | NR |
| Perl et al. (1995)107 | USA | 1986–90 | 2–6 | 100 | 55 | 57 | 23.1 | NR |
| Quartin et al. (1997)93 | USA | 1983–6 | 8 | 1505 | 99.3 | 61.7 | NR | NR |
| Manns et al. (2002)74 | Canada | 1996–9 | 3 | 787 | 55.8 | 61.1 | 20.9 | NR |
| Weycker et al. (2003)105 | USA | 1991–2000 | ≤ 5 | 16,019 | 53.4 | NR | NR | NR |
| Angus et al. (2004)95 | Multinational | 1998–2000 | 1.3–3.8 | 1690 | 57 | 60.5 | 24.8 | 75.2 |
| Karlsson et al. (2007),103 (2009)109 | Finland | 2004–5 | 2 | 470 | 67 | 59.6 | 24.1 | NR |
| Ghelani et al. (2009)102 | Australia | 1993–9 | 4.2–9.6 | 191 | 58 | 62.5 | 22.1 | NR |
A comparison of baseline characteristics across the studies is difficult owing to limitations in the reported data. Generally, the study populations included more males than females and the mean age of the cohorts ranged from 57 years to 63 years. Most of the studies measured severity of illness by using the APACHE II score74,95,102,103,107–109 and the mean score varied between 20.9 and 24.8 across the studies. Only limited information was reported on the proportion of patients with multiple organ dysfunction.
The results of these studies74,93,95,103,105–109 were presented in terms of absolute survival estimates and/or relative survival estimates compared with a reference population (i.e. a non-sepsis cohort or general population survival estimates). This distinction is a potentially important factor when considering the generalisability of these data to the UK and their appropriateness for informing parameter estimates in our decision model. As previously noted in relation to baseline mortality, differences in UK practice and the characteristics of severe sepsis patients could also impact the generalisability of absolute survival estimates from long-term studies of non-UK cohorts to our specific decision problem. Hence, in the absence of published UK data, it may be more appropriate to consider the use of relative survival estimates compared with a reference population, assuming that the relative survival estimates may be more transferable than the absolute estimates themselves. This assumption has been widely applied in previous cost-effectiveness analysis where the relative survival estimate has been applied as an adjustment factor to life expectancy data from a reference population from the setting of interest. Indeed, seven of the cost-effectiveness studies75,77–79,81,88,89 reviewed used data from Quartin et al. 93 (see Table 18) to estimate the adjustment factor (0.51).
Absolute survival estimates
The absolute mortality rates from these studies are reported in Table 24. The mortality ranged from 21% to 51% at hospital discharge, from 41% to 72% at 1 year and from 49% to 65% at 3 years. Differences in the setting, characteristics of the study populations and the statistical analyses make a direct comparison between the mortality rates of these studies difficult.
Several factors limit the generalisability and appropriateness of using these estimates directly to inform the survival inputs to inform our stated decision problem: (1) all studies were undertaken outside of the UK, (2) only limited data were reported in terms of the case mix of the cohorts and (3) there appeared marked variation across the studies for the survival estimates.
| Study | Critical-care unit (%) | Hospital (%) | Time point after hospital discharge (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 month | 3 months | 6 months | 1 year | 2 years | 2.5 years | 3 years | 4 years | 5 years | |||
| Leibovici et al. (1995)106 | 25.8 | 26 | 43 | 48 | 54 | 63 | |||||
| Sasse et al. (1995)108 | 37.3 | 51 | 40.5 | 64.7 | 71.9 | ||||||
| aPerl et al. (1995)107 | 32 | 39 | 43 | 47 | 55 | 59a | |||||
| bQuartin et al. (1997)93 | |||||||||||
| Manss et al. (2002)74 | 36 | 44 | 47 | 49 | |||||||
| Weycker et al. (2003)105 | 21.2 | 39.4 | 45.1 | 51.4 | 64.8 | 74.2 | |||||
| Angus et al. (2004)95 | |||||||||||
| Treatment group | 29.7 | 33.9 | 37.8 | 41.1 | 47.4 | ||||||
| Placebo group | 34.9 | 37.6 | 39.7 | 42.8 | 50.7 | ||||||
| Karlsson et al. (2007),103 (2009)109 | 15.5 | 28.3 | 40.9 | ||||||||
| cGhelani et al. (2009)102 | 30.3 | 42 | |||||||||
| Range | 15.5–37.3 | 21.2– 51 | 26–40.5 | 33.9– 39.4 | 37.8– 64.7 | 40.9– 71.9 | 47–55 | 47.4–50.7 | 49–64.8 | 63 | 74.2 |
Relative survival estimates
As previously outlined, the use of relative survival estimates compared with a reference population may be more transferable to a separate setting and, hence, may provide a more appropriate methodology (compared with the application of absolute survival estimates from non-UK studies) to apply within a decision modelling approach. However, significant differences were identified in our review of cost-effectiveness studies in relation to both the magnitude of any excess mortality assumed and the time point at which the relative survival is assumed to equal 1, i.e. the time point at which the mortality rate of the sepsis cohort is assumed to be the same as the mortality rate of the comparator population (typically the general population). The variation in approaches across published cost-effectiveness studies appeared largely driven by the particular cohort study chosen (general critical care or sepsis), the statistical conclusions derived from these studies and the assumptions of the authors.
In our own review of long-term life expectancy following severe sepsis, two studies93,102 comparing the relative survival of sepsis patients to a non-septic control population were identified.
-
Quartin et al. 93 compared 1505 patients with uncomplicated sepsis, severe sepsis and septic shock with a control group of 91,830 non-psychiatric, non-infected, discharged hospitalised patients from 10 Department of Veterans Affairs Medical Centers in the USA, over an 8-year period. The mortality risk of patients with sepsis exceeded the equivalent risk of the control group for 5 years and the risk rose with increasing severity of the septic episode throughout the first year (p < 0.05). After 5 years, the mortality among survivors of severe sepsis or septic shock was not statistically significantly different from that of the control population of non-psychiatric, non-infected, discharged hospitalised patients.
-
Ghelani et al. 102 compared the relative survival of septic (n = 224) and non-septic (n = 1798) critical-care cohorts from a single tertiary-level adult critical-care unit with survival in general hospital patient cohorts (infected, non-infected) and the Australian general population. Follow-up was until death or for a minimum of 4.2 years to a maximum of 9.6 years. Survival of all cohorts was shorter than in the Australian general population; for the two critical-care cohorts, a progressive decline in the relative survival suggested an excess mortality over the entire follow-up compared with the general population. Although the survival difference between the critical-care unit sepsis and critical-care unit non-sepsis cohort was not statistically significant, the number of patients with sepsis was relatively small and there appeared to be a trend towards lower relative survival among the sepsis patients.
Although both studies clearly demonstrate an excess mortality risk of severe sepsis significantly beyond the initial episode itself, the duration of this excess mortality appears to differ between the studies. These differences may be owing to differences in case mix, underlying treatment patterns and/or the different comparator populations (i.e. comparison with a general population or a non-septic hospitalised population). Although the use of a general population control within Ghelani et al. 102 provides a potentially suitable basis to link in a decision model to a UK general population control, the relatively small numbers of sepsis patients (n = 224) and recruitment from a single tertiary centre represent potentially important limitations.
Given the heterogeneity in approaches and comparator populations, it was not considered appropriate to combine the separate studies using formal pooling. Furthermore, in the absence of any single study that was considered representative of the population in our decision problem, the availability of other primary data sources in the UK was explored. An unpublished UK severe sepsis cohort with 5-year follow-up was identified (Brian Cuthbertson, Sunnybrook Health Sciences Centre, Toronto, ON, 2010, personal communication) and additional primary data analysis was undertaken to inform the cost-effectiveness model.
Additional primary data analysis
The cohort used was taken from a case–control study of the use of rhAPC in severe sepsis and septic shock. The data used were from the control group who did not receive rhAPC. This included 345 subjects from the Scottish Intensive Care Society (SICS) prospective, observational, multicentre, epidemiological study of sepsis in the Scottish critical-care database collected in a 5-month period in 2002. From these 345, only those patients (n = 271) for whom data on organ dysfunction were clearly reported were selected. The characteristics of this cohort (at admission to the critical-care unit) are shown in Table 25. Average follow-up for survival for this cohort was 787 (range 0–2062) days.
| Characteristic | Control (n = 271) |
|---|---|
| Age (years) on admission to the critical-care unit, mean (SD) | 57.7 (14.1) |
| Male gender (%) | 52.4 |
| APACHE II score, mean (SD) | 23.1 (8.4) |
| Quartiles of APACHE II score | |
| First quartile | 0–20 |
| Second quartile | 21–24 |
| Third quartile | 25–28 |
| Fourth quartile | 29–48 |
| APACHE II score ≥ 25 (%) | 47.6 |
| Organ dysfunctions | |
| Metabolic acidosis (%) | 46.5 |
| Haematological (%) | 24.7 |
| Renal (%) | 41.3 |
| Respiratory (%) | 78.2 |
| Cardiovascular (%) | 56.8 |
| Cardiovascular and renal OD (%) | 26.9 |
| Number of organ dysfunctions | |
| One (%) | 28.0 |
| Two (%) | 28.4 |
| Three (%) | 18.8 |
| Four (%) | 17.3 |
| Five (%) | 7.4 |
| Length of stay (days) in the critical-care unit, mean (SD) | 11.4 (12.9) |
| Mortality | |
| Critical-care unit (%) | 41.7 |
| Original hospital (%) | 47.6 |
| Any hospital (%) | 50.0 |
| Overall follow-up period (%) | 65.7 |
| Follow-up (days) , mean (min–max) | 787 (0–2062) |
After hospital discharge, 144 subjects were alive and followed up. Figure 39 reports the Kaplan–Meier curve (and 95% CI) for survival after hospital discharge. The analytical approach used to populate the cost-effectiveness model with these data is discussed in Chapter 5.
FIGURE 39.
Observed survival after discharge from hospital (Kaplan–Meier).
Health-related quality of life after survival of severe sepsis (utilities)
Green et al. 82 have previously reported the findings from a literature search of health-state utilities associated with severe sepsis. However, this review identified only a single published abstract relating to patients with severe sepsis and septic shock. In the absence of robust data from the previous review, a separate systematic review was undertaken to update these findings.
Methods
Studies were included in the present review if they assessed the health utilities associated with severe sepsis using either multi-attribute health-state classification systems [e.g. European Quality of Life-5 Dimensions (EQ-5D), Health Utilities Index (HUI), Short Form questionnaire-6 Dimensions, etc.] or other choice-based approaches (e.g. time trade-off, standard gamble).
Studies were identified by searching MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations and EMBASE. The full search strategy is reported in Appendix 2. Only studies published after 2004 were included in the review. The lists of references included in the identified studies were checked to identify any further relevant studies.
Results
Four studies (including one abstract) were identified in our update review. 96,109–111 The flow chart of the search results and inclusion/exclusion of the studies is provided in Figure 40. A summary of these studies is reported in Table 26.
FIGURE 40.
Flow chart of studies included in the literature review for health-related quality of life (utilities). SF-36, Short Form questionnaire-36 items.
| Study | Country | Setting | n | Assessment times (number of respondents) | Male (%) | Mean age (years) | Mean APACHE II score |
|---|---|---|---|---|---|---|---|
| Drabinski et al. (2001)96 | USA | 53 hospitals | 703 | Day 30 (93) | 52a | 60a | NR |
| Day 60 (93) | |||||||
| Day 90 (93) | |||||||
| Day 180 (93) | |||||||
| Granja et al. (2004)110 | Portugal | 1 critical-care unit | 305 | 6 months (104) | 64b | 52b,c | 17b,c |
| Korošec Jagodic et al. (2006)111 | Slovenia | 1 critical-care unit | 66 | 2 years (10) | 49d | 64.4d | 15.5d |
| Karlsson et al. (2009)109 | Finland | 24 critical-care units | 470 | Before critical illness (252) | 64.7e | 60.4c,e | 24c,e |
| ≈ 17 months (156) |
In all four studies,96,109–111 utilities were derived using the EQ-5D instrument. The EQ-5D is a standardised instrument for use as a measure of health outcome and is applicable to a wide range of health conditions and treatments. 112 It provides a simple descriptive profile and a single index value (utility) for health status. It is the measure currently recommended by the National Institute for Health and Clinical Excellence (NICE) to be used as part of its ‘reference case’ approach to undertaking cost-effectiveness analyses. 113
The follow-up period in the studies ranged from 6 months to 2 years. None of the studies reported data beyond 2 years’ follow-up. The utility values are reported in Table 27. Anchor points for these values are perfect health (1) and death (0). Differences in the patient characteristics, country, centres and assessment times across the studies again make a direct comparison problematic.
| Study | Follow-up point | ||||||
|---|---|---|---|---|---|---|---|
| Before critical illness | 1 month | 2 months | 3 months | 6 months | 17 months | 2 years | |
| Drabinski et al. (2001)96 | 0.53 | 0.62 | 0.68 | 0.69 | |||
| Granja et al. (2004)110 | 0.84 | ||||||
| Korošec Jagodic et al. (2006)111 | 0.72 | ||||||
| Karlsson et al. (2009)109 | 0.70 | 0.86 | |||||
Three109–111 of the four96,109–111 studies compared the utility values of sepsis patients with those of non-septic populations. Two110,111 of these studies compared the utility values of sepsis survivors with those of another critical-care unit population (patients admitted without sepsis110 or trauma patients111). Only one study109 compared the utility values of severe sepsis patients with age- and sex-adjusted general population estimates. In this study, the utility values of severe sepsis patients were reported to be lower than those of the age- and sex-adjusted general population, both before the onset of the clinical illness and at approximately 1.5 years after discharge from intensive care.
Two studies96,109 reported patients’ utility values for multiple time points, informing how the quality of life of sepsis survivors might alter over time. The abstract by Drabinski et al. 96 (previously identified by Green et al. 82) assessed changes in health status at days 30, 60, 90 and 180, suggesting that quality of life appears to improve within the first few months after a sepsis episode and then appears to plateau between 3 months and 6 months after the episode. Karlsson et al. 109 assessed patients’ health status within 1 week from the study entry and approximately 1.5 years after the study entry. Again, improvements in the quality of life were reported over the follow-up period. However, the majority (61.9%) of the initial assessments were completed by the patients’ next of kin to assess the patients’ quality of life before the sepsis episode. Consequently, the study does not directly inform the impact of the initial episode, nor does it provide an appropriate basis for assessing how quality of life may alter over the longer term.
Summary and key issues
Our updated review identified three additional studies109–111 to those previously reported96 by Green et al. 82 These additional studies provided evidence that (1) the quality of life of sepsis patients appears to be lower than that of the age- and sex-adjusted general population (even before the clinical illness),109 (2) the utility values of sepsis survivors appear to improve over time96,109 (with much of this improvement incurring during the first months after sepsis episode)96 and (3) surviving patients have a lower utility value than the general population even 1.5 years after the initial episode. 109
Although these studies provide useful information to assist in drawing general conclusions about the potential long-term impact of severe sepsis on health utility, several limitations were identified in relating the findings from these studies to appropriate parameter values and assumptions to be applied in our own decision model. Given the differences in the patient characteristics and follow-up times reported, it was not considered appropriate to pool the results from the separate studies. Although no single study was ideal, the abstract by Drabinski et al. 96 was considered the most relevant to informing our own decision problem by providing evidence at multiple follow-up points after the initial sepsis episode. This study was therefore used to inform the parameter inputs for quality of life reported in Chapter 5.
Chapter 5 Cost-effectiveness analysis – analytic methods and results
Overview
The objective of phase II was to determine the cost-effectiveness of IVIG and to estimate the value of additional primary research.
Phase II comprised two related elements: cost-effectiveness analysis and value of information analysis.
Cost-effectiveness analysis
The decision model was developed and populated using data identified during phase I and the results from the clinical effectiveness review. All stages of the work were also informed by discussions with the Expert Group to provide feedback on specific aspects of the analysis including the model structure, data inputs and assumptions.
The model evaluated costs from the perspective of the NHS and Personal Social Services, expressed in UK pounds sterling at a 2009 price base. Outcomes were expressed in terms of QALYs. Both costs and outcomes were discounted using a 3.5% annual discount rate, in line with current guidelines. 113
The model was developed in the statistical programming package R114 and is probabilistic in that input parameters are entered into the model as probability distributions to reflect parameter uncertainty, i.e. uncertainty in the expected value of the inputs. 115 Monte Carlo simulation was used (5000 iterations) to propagate uncertainty in input parameters through the model in such a way that the results of the analysis can also be presented with their associated uncertainty. The probabilistic analysis also provided a formal approach to quantifying the consequences associated with the uncertainty surrounding the model results and can be used to identify priorities for future research. 116
The expected cost and QALYs for each of the strategies were estimated and compared, using ICERs where appropriate. The ICER represents the incremental cost per additional QALY associated with a more costly and effective strategy. The ICER was then compared against thresholds used by NICE to establish value for money in the NHS (currently in the region of £20,000–£30,000 per additional QALY). 113 These thresholds can be used to identify the optimal strategy in terms of cost-effectiveness considerations based on existing evidence.
A range of separate scenarios were also undertaken to assess the impact of key uncertainties related to input parameters and assumptions. Consistent with available evidence, the model also explored variability in the cost-effectiveness estimates for specific subgroups of patients.
Value of information analysis
Formal methods, based on EVI approaches, were used to identify potential research priorities and to establish whether or not investment in a multicentre RCT is likely to be a cost-effective use of resources. 116–118 The EVI approaches were also extended to consider a range of sources of uncertainty in the model to help identify and prioritise specific research questions that could also be addressed with other (non-RCT) research designs. The methods and results of this analysis are reported in Chapter 6.
The following sections outline the decision problem and the structure of the model, and report the key assumptions and data used to populate the model.
Methods for the cost-effectiveness analysis
Treatment strategies/comparators
The decision problem addressed by the model relates to the cost-effectiveness of IVIG as an adjunctive treatment to standard care for the management of adults with severe sepsis and septic shock, compared with standard care alone. The base-case population in the model reflects the baseline characteristics of the population in the ICNARC CMP Database, under the assumption that this population is more representative of current NHS practice than the populations recruited into the RCTs. The impact of patient heterogeneity (e.g. owing to different clinical characteristics) was explored in separate analyses. This approach ensures that uncertainty in the decision because of the imprecision in parameter inputs can be separated from uncertainty in whether or not an intervention is cost-effective for particular subgroups of the population.
Model structure
The model evaluated the lifetime prognosis of severe sepsis in order to capture the long-term costs and consequences associated with the natural history of these patients in the absence of IVIG. The findings from the clinical effectiveness review were then employed to model the effect of using IVIG as an adjunctive treatment to standard care. The model structure was informed by the series of reviews described in previous chapters and is used to estimate lifetime costs and benefits associated with the primary outcome of the clinical effectiveness review: short-term all-cause mortality.
A simplified schematic of the decision model structure is shown in Figure 41 and a full technical description is provided in Appendix 5.
FIGURE 41.
Structure of the decision model representing the progression of patients diagnosed with severe sepsis or septic shock.
In common with many of the existing model structures, two related elements were considered reflecting short- and long-term consequences.
-
Short term The short-term consequences of the initial sepsis episode reflect the initial hospitalisation period (critical-care unit and non-critical-care unit). The decision tree quantifies the probability of surviving or dying during the initial hospitalisation for the sepsis episode. Baseline mortality data from the ICNARC CMP Database were used to estimate the risk of mortality associated with standard care and the results of the clinical effectiveness review were applied to estimate the risk with IVIG.
-
Long term Conditional on having survived the initial hospitalisation, a Markov structure was used to characterise the long-term prognosis over the remainder of a patient’s lifetime. Annual cycles were employed to reflect the annual probability of death for each year after the initial episode. Hence, the extent to which the use of IVIG reduces the risk of mortality during the initial hospitalisation period is translated into differences in long-term costs and QALYs on the basis of the long-term model.
In developing and populating the decision model there were two important considerations applied to inform the approaches and methods employed:
-
the requirement to extrapolate outcomes beyond the time horizon of the main RCTs to ensure that differences in QALYs were appropriately quantified
-
the need to ensure that the data inputs and assumptions were relevant to the specific population and setting to inform decision-making in the context of the NHS.
The use of decision analysis provides a number of advantages in exploring these issues in more detail: (1) it provides a framework to model both the short- and long-term costs and benefits associated with IVIG; (2) it makes each of these assumptions explicit and can highlight where the current uncertainties exist; (3) it provides a quantitative approach to combining evidence from separate sources and the use of probabilistic analysis means that the degree of uncertainty surrounding particular inputs can be reflected; (4) the potential impact of the assumptions on the cost-effectiveness of IVIG can be considered; and (5) the value of additional research to inform the decision problem can be established.
The following sections provide an overview of the model inputs and the methods used to inform the cost-effectiveness of IVIG. This information is summarised in Appendix 9 for the overall severe sepsis and septic shock population.
Model inputs
Baseline event rates for standard care (initial hospitalisation)
As previously reported in Chapter 4, data from the ICNARC CMP Database (2007–9, n = 26,249) were used to inform the baseline risk of mortality applied to standard care during the initial hospitalisation. The probability of mortality during this period was estimated to be 40.6% (95% CI 40.0% to 41.2%). Variation in the baseline risk of mortality was explored for a range of separate subgroups, defined by age and gender, APACHE II score, ICNARC physiology score and number of organ dysfunctions.
For the subgroup analyses, estimates of the baseline probability of mortality were obtained by conditioning on specific patient or severity of illness characteristics (at presentation). Separate logistic regression models were used and are described in Table 28. All models were fitted with robust (Huber–White) SEs adjusted for clustering on critical-care unit. The full results of these regressions are reported separately in Appendix 6.
| Subgroup analysis | Description | Variable type |
|---|---|---|
| A | Age at admission | Continuous variable |
| Gender | One dummy variable | |
| B | APACHE II score | Continuous variable |
| C | ICNARC physiology score | Continuous variable |
| Age at admission | Continuous variable | |
| Source of admission | Set of five dummy variables for ‘clinic or home’, ‘critical-care unit (same or other hospital)’, ‘theatre (elective/scheduled surgery)’, ‘theatre (emergency/urgent surgery)’, ‘ward or intermediate care (same hospital)’, relative to reference category of ‘ED or other hospital (not critical care)’ | |
| CPR within 24 hours prior to admission | One dummy variable | |
| D | Number of organ dysfunctions during first 24 hours | Set of four dummy variables indicating the number of organ dysfunctions (from two to five), relative to reference category of one organ dysfunction |
| Age at admission | Continuous variable | |
| CV organ dysfunction | One dummy variable | |
| Renal organ dysfunction | One dummy variable | |
| CV and renal organ dysfunctions | One dummy variable |
Clinical effectiveness of intravenous immunoglobulin
The results of the clinical effectiveness review (see Chapter 3) were used to model the effect of IVIG for all-cause mortality during the initial hospitalisation. Based on the conclusions from this review, separate analyses were undertaken using the best-fitting model (model T3b with covariate duration of IVIG therapy) and a range of other models as a sensitivity analysis to assess the robustness of conclusions on the cost-effectiveness of IVIG to model choice. The clinical effectiveness models considered within the sensitivity analysis were restricted to the random-effects models given the lack of a clear causative relationship for the covariates with treatment efficacy, replacing the heterogeneity explained by these covariates with a between-study heterogeneity parameter. Within the random-effects models, a range of approaches were considered to adjust for potential bias associated with trial methodology or publication bias.
In the short-term model, the relative treatment effect measure for all-cause mortality (OR) was applied to the baseline event rates (estimated as the odds of an event) and then converted to probabilities in order to obtain absolute probability estimates for IVIG. The ORs applied in the separate scenarios are summarised in Table 29.
| Clinical effectiveness model | OR (95% credible intervals) |
|---|---|
| Fixed-effects model T3b with covariate: duration of IVIG therapy (duration = 3 days and relative to albumin) | 0.75 (0.58 to 0.96) |
| Random-effects model T3b (relative to albumin) | 0.68 (0.16 to 1.83) |
| Random-effects model T2 with covariate: Jadad score (Jadad score = 5) | 0.83 (0.18 to 2.13) |
| Random-effects model T2 with covariates: 1/N (N = 339) | 0.92 (0.23 to 2.10) |
| Random-effects model T2 with covariates: daily dose + 1/N (N → ∞) | 1.27 (0.25 to 3.17) |
Long-term survival for sepsis survivors
UK data were used to estimate long-term survival for sepsis survivors from the cohort of the SICS prospective, observational, multicentre, epidemiological study of sepsis in Scottish critical care (Brian Cuthbertson, Sunnybrook Health Sciences Centre, 2010, personal communication) reported in Chapter 4. Parametric survival analyses were undertaken to estimate the long-term mortality estimates applied in the model using alternative distributions (Weibull, exponential and log-normal). To assess goodness of fit, the Akaike Information Criterion (AIC) was utilised along with a graphical inspection of the fit of the data and plausibility of longer-term predictions beyond the 5-year follow up period of the cohort study, before selecting the most appropriate curve for the final model.
Three separate models were fitted including additional covariates for:
-
age at admission
-
APACHE II score at admission, and
-
organ dysfunction (and age at admission).
The covariates were included to consider whether or not subgroup-specific estimates for the long-term survival were appropriate and to adjust for any potential imbalance between the baseline characteristics of the CMP data (used to estimate short-term mortality data) and the SICS cohort. Additional covariates considered were explored, but only age and APACHE II score were identified as significant predictors of long-term mortality (p < 0.05).
The full results from the parametric survival analysis are reported in Appendix 7. The distribution with the lowest AIC and representing the best statistical goodness of fit (sustained across different covariate sets) was the Weibull function. A graphical comparison of the predicted survival from the different parametric functions compared with the observed Kaplan–Meier survival curve (with 95% confidence bounds) is presented in Figure 42.
FIGURE 42.
Comparison of predicted survival from alternative parametric survival functions and observed Kaplan–Meier survival curve (with 95% confidence bounds).
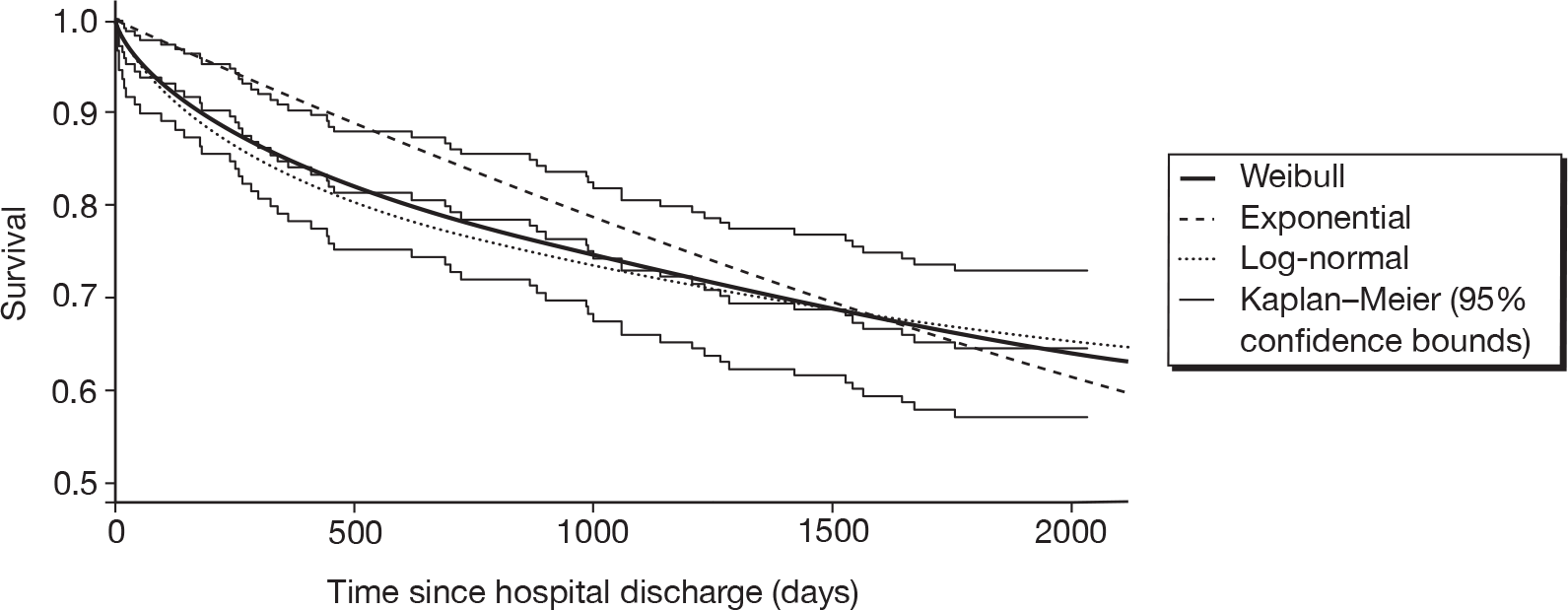
The plausibility of the different parametric predictions beyond the 5 years of observed data was also explored by comparing these with age-adjusted estimates from the general population. This comparison is shown in Figure 43. It was considered implausible that the long-term mortality estimate of sepsis patients would become lower than that of the general population. Consequently, in the model, it was further assumed that the probability of mortality would be the maximum of that predicted from the parametric distributions and the observed yearly probability of mortality for the general population (age and sex adjusted). The time point at which the model switches from predictions from the parametric distributions to the estimates from the general population represents the point at which the mortality of the sepsis cohort is assumed to be the same as the mortality of the general population. For the overall population, the switch points were 9, 13 and 22 years for the log-normal, Weibull and exponential distributions, respectively. The ‘modified’ parametric survival functions are reported in Figure 44.
FIGURE 43.
Comparison of parametric survival functions over time with general population (GP) estimates.
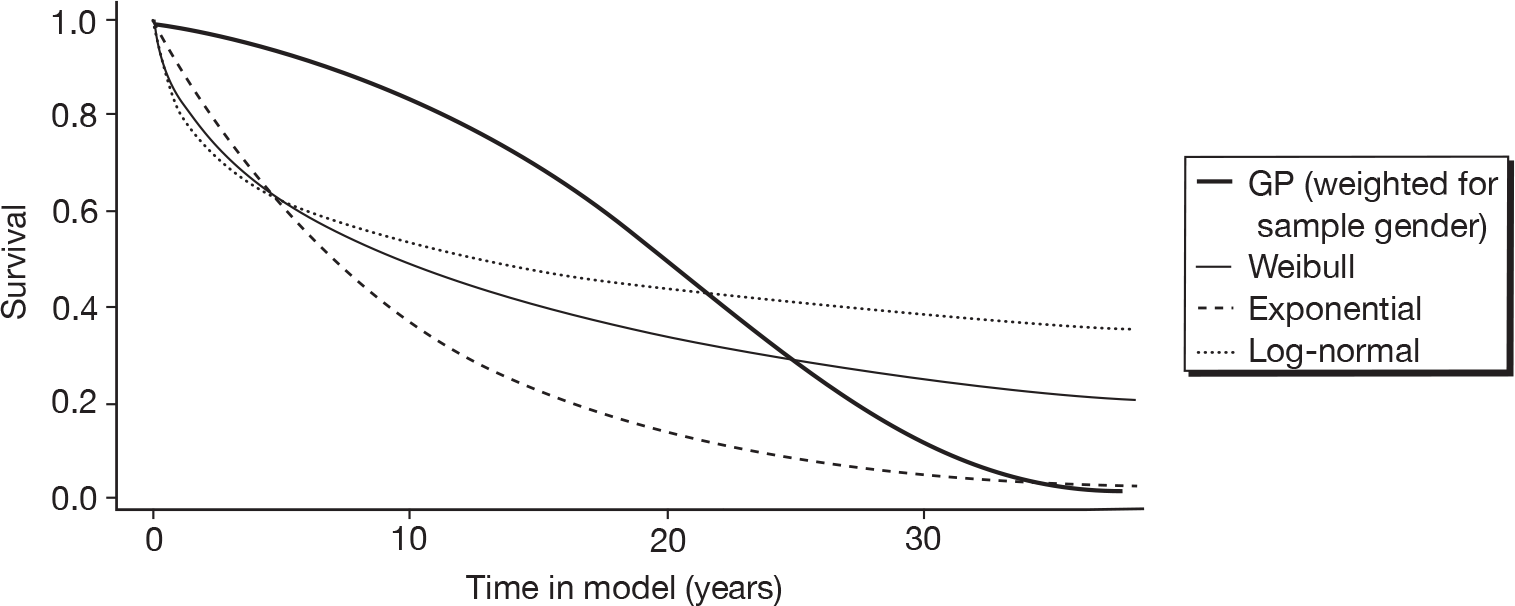
FIGURE 44.
Comparison of ‘modified’ parametric survival functions over time with general population (GP) estimates.
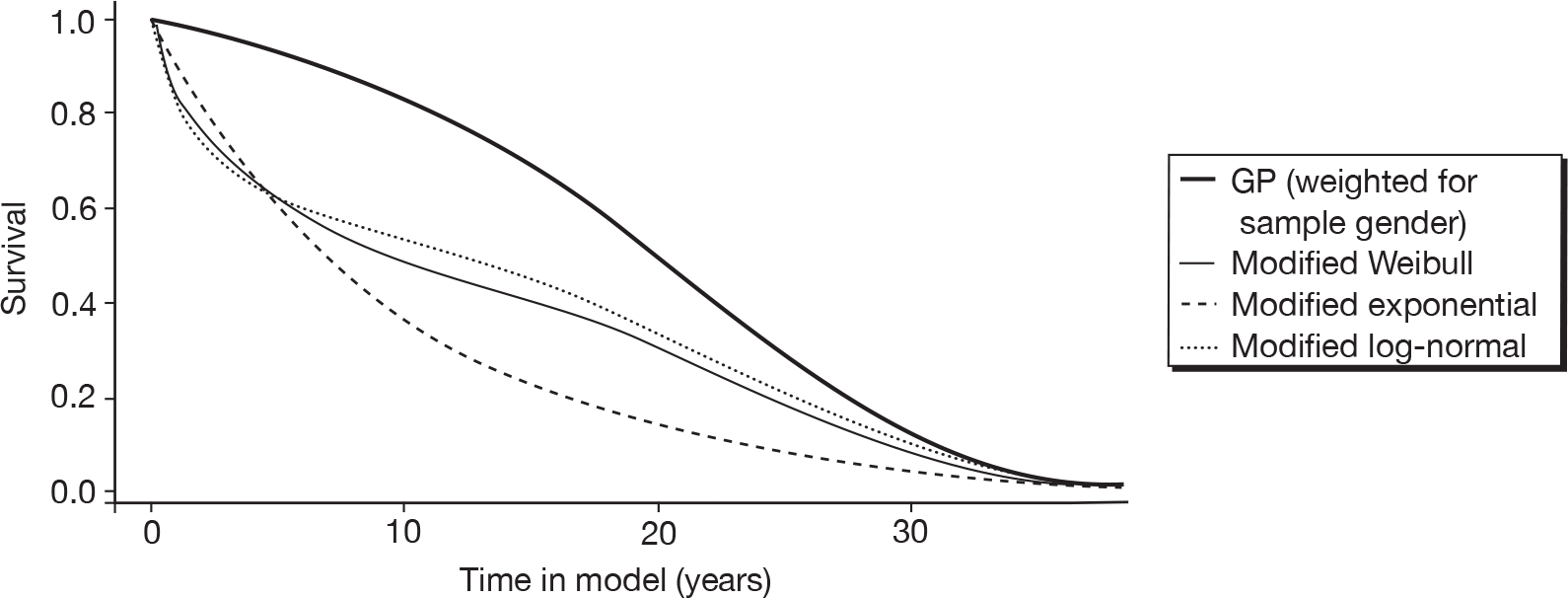
Given the inevitable uncertainty about the longer-term survival extrapolation, the robustness of the results was explored using a range of scenario analyses in which we varied the time point at which patients switched from the predicted survival distributions to the corresponding estimates from the general population (varied between 5 years and 25 years).
Quality of life
Utility estimates applied in the model were based on estimates reported by Drabinski et al. ,96 reported previously in the review in Chapter 4. This was the only study that reported utility values at multiple time points following an episode of severe sepsis. A single utility value of 0.69 was assigned to represent the quality of life of long-term survivors of sepsis. In the absence of any reported measure of uncertainty around this estimate, a SE of 0.028 was assigned based on an estimate reported by Cuthbertson et al. 119 in general critical-care patients reporting similar absolute quality of life values. Additional decrements were assigned to the within-hospital period (0.09) and for the first month after hospitalisation (0.06) based on the 1-month and 2-month follow-up data reported in Drabinski et al. 96
Resource use and unit costs
Resource use and costs were estimated both for the short-term hospitalisation period and for the longer-term extrapolation. Costs assigned in the short-term period of the model included the acquisition costs of IVIG treatment and length of stay in hospital (critical-care unit and other wards). Costs assigned in the longer-term extrapolation were based on an assessment of the continuing costs of managing survivors after the initial hospitalisation.
The acquisition costs of IVIG were estimated from the cost per gram of products (5% concentration) reported in the British National Formulary (BNF). 120 The products and average costs are reported in Table 30. The total number of grams used was based on a 2 g/g dose assuming a weight of 70 kg and a duration of 3 days, based on advice from the Expert Group. This was rounded down to the nearest whole vial based on the current guidelines for use. 17 The total acquisition cost of IVIG was estimated to be £5539.
| Product | Company | Vial sizes | Cost/g (£) |
|---|---|---|---|
| Vigam Liquid® (5%) | Bio Products Laboratory Ltd, Hertfordshire, UK | 2.5 g (50 ml), 5 g (100 ml), 10 g (200 ml) | 38.00 |
| Intratect® (5%) | Biotest, Dreieich, Germany | 1 g (20 ml), 2.5 g (50 ml), 5 g (100 ml), 10 g (200 ml) | 45.00 |
| Gammagard S/D® (5–10%) | Baxter, Dearfield, Illinois, USA | 0.5 g (with diluent), 2.5 g (with diluent), 5 g (with diluent), 10 g (with diluent) | 40.10 |
| Average cost per gram of 5% products | 41.03 |
The length of stay in hospital (critical-care unit/non-critical-care unit) was informed using activity data from the same CMP Database used to estimate baseline mortality. Estimates of the mean and SE length of stay for survivors and non-survivors of the initial hospitalisation were used to inform the model input parameters. The length of stay in non-critical care wards was assumed to be the difference between the length of the overall hospitalisation and the length of critical-care unit stay. Table 31 reports the descriptive statistics based on all admissions. Results for key subgroups are reported separately in Appendix 8.
| Population | Critical-care unita | Overall hospitalisation | ||||
|---|---|---|---|---|---|---|
| n | Mean (SE) | Median (IQR) | n | Mean (SE) | Median (IQR) | |
| All | 25,990 | 8.04 (0.067) | 4.25 (1.82–9.79) | 25,749 | 31.79 (0.233) | 20 (10–40) |
| Survivors | 15,446 | 8.48 (0.086) | 4.8 (2.22–10.24) | 15,215 | 39.07 (0.325) | 27 (15–49) |
| Non-survivors | 10,544 | 7.40 (0.108) | 3.42 (1.15–9.04) | 10,534 | 21.29 (0.292) | 12 (5–26) |
A per diem cost of £1293 was applied to the duration of the critical-care unit stay based on national unit cost estimates [National Schedule of Reference Costs 2007/08: NHS Trusts and Primary Care Trusts (PCTs) combined – Critical Care Services – Adult: Intensive Therapy Unit]. 121 For the remaining non-critical-care unit stay, a per diem estimate of £196 was used based on a general ward stay for septicaemia (National Schedule of Reference Costs 2007/08: NHS Trusts and PCTs combined Non-Elective Inpatient – Long Stay Excess Bed Day). 121
Existing cost-effectiveness studies were reviewed and additional searches were undertaken to identify potential sources of long-term cost data for the management of survivors of sepsis. Only one study74 was identified that reported estimates of the long-term costs for survivors of sepsis after the initial hospitalisation. This was a Canadian cohort study that reported costs in the first 3 years following the initial hospitalisation. Costs in the first year were reported to be considerably higher than those reported in years 2 and 3. Estimates reported in years 2 and 3 were very similar, suggesting that resource utilisation over the longer term was more stable. In the absence of any equivalent UK estimates, these estimates were converted to UK pounds sterling (and uprated to current prices). Given the similarity in the costs reported for years 2 and 3, these estimates were averaged and applied as an average cost incurred yearly in year 2 and beyond in the model. The specific estimates applied were £13,654 in the first year after discharge and £4467 for each year thereafter. In the absence of any reported measure of uncertainty around this estimate, a coefficient of variation of 2 was assumed. By using truncation, it was assumed that uncertainty could not lead to consider costs lower than the average annual per capita NHS cost of £1807.84.
Given the lack of UK cost data on the long-term management of sepsis survivors, additional scenarios were undertaken to explore the robustness of the model to alternative assumptions. For these scenarios alternative assumptions were explored regarding the magnitude of these estimates (± 50%). Given the lack of long-term UK cost data, the impact of alternative approaches using general population estimates of the average annual per capita NHS cost instead of using sepsis specific estimates was also explored.
Results of the cost-effectiveness analysis
The results of the decision model are presented in two ways. First, the mean lifetime costs and QALYs of the two strategies are presented and their cost-effectiveness compared, estimating ICERs where appropriate. The threshold cost per QALY estimates used by NICE (£20,000–£30,000) were used to provide an indication of whether or not the use of IVIG potentially represents good value for money in the NHS. Accordingly, if the ICER for IVIG is < £20,000 then IVIG should be considered potentially cost-effective. ICERs within the range £20,000–£30,000 are considered borderline and an ICER > £30,000 is not typically considered cost-effective.
Second, the results of the probabilistic analysis using Monte Carlo simulation were used to calculate the combined impact of the model’s various uncertainties on the overall uncertainty surrounding the cost-effectiveness results themselves. To present the uncertainty in the cost-effectiveness of the alternative strategies, cost-effectiveness acceptability curves (CEACs) were used. The CEAC shows the probability that IVIG is cost-effective using alternative values for the threshold cost per QALY. 122
Separate cost-effectiveness estimates are reported for different scenarios reflecting the uncertainty in several of the key inputs and assumptions. The scenarios consider alternative assumptions related to (1) clinical effectiveness of IVIG, (2) long-term survival estimates for survivors of the initial hospitalisation and (3) long-term costs. Finally, results are presented for separate subgroups to reflect clinical heterogeneity in the population under investigation.
Alternative clinical effectiveness scenarios
Table 32 reports the cost-effectiveness results using the best-fitting clinical effectiveness model for all-cause mortality [fixed-effects model T3b with covariate: duration of IVIG (3 days)]. The results show that the ICER of IVIG is £20,850 per QALY (i.e. incremental costs = £9308/incremental QALYs = 0.45), which is within the borderline region of estimates considered to be cost-effective in the NHS. At a threshold of £20,000 per QALY, the probability that IVIG is more cost-effective than standard care alone is 0.505. As the threshold cost per QALY increases, the probability that IVIG is cost-effective increases (i.e. increasing to 0.789 at a threshold of £30,000). The relationship between the threshold ICER and the probability that IVIG is cost-effective is shown more clearly in the CEAC reported in Figure 45.
| Fixed-effects model T3b with covariate: duration of IVIG therapy (3 days) | Probability of being cost-effective for cost-effectiveness threshold | ||||
|---|---|---|---|---|---|
| Treatment | Mean cost (£) | Mean QALY | ICER (£ per QALY) | £20,000 | £30,000 |
| IVIG | 54,901 | 4.35 | 20,850 | 0.505 | 0.789 |
| Standard care | 45,593 | 3.90 | 0.495 | 0.211 | |
FIGURE 45.
Cost-effectiveness acceptability curve using best-fitting model from the clinical effectiveness review.
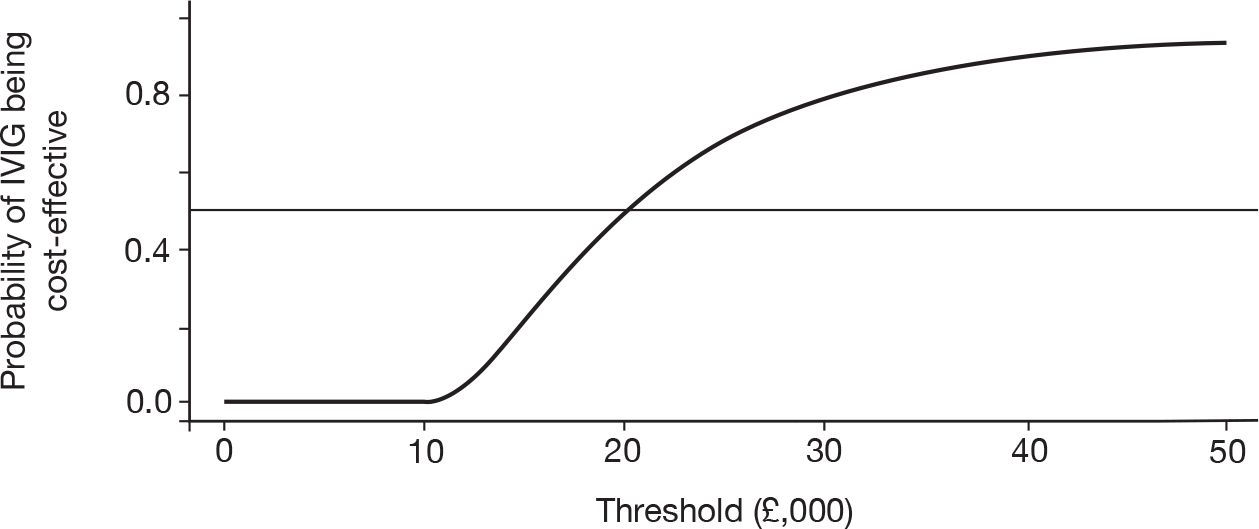
Table 33 reports the results using each of the alternative models from the clinical effectiveness review considered within the sensitivity analysis. The ICER estimates vary between £16,177 per QALY to IVIG being dominated by standard care alone (i.e. IVIG being both less effective and more costly).
| Random-effects model | Probability of being cost-effective for cost-effectiveness threshold | ||||
|---|---|---|---|---|---|
| Treatment | Mean cost (£) | Mean QALY | ICER (£ per QALY) | £20,000 | £30,000 |
| Random-effects model T3b (compared with albumin) | |||||
| IVIG | 57,200 | 4.62 | 16,177 | 0.597 | 0.707 |
| Standard care | 45,593 | 3.90 | 0.403 | 0,295 | |
| Random-effects model T2 with covariate: Jadad score (Jadad score = 5) | |||||
| IVIG | 55,238 | 4.39 | 19,968 | 0.502 | 0.611 |
| Standard care | 45,593 | 3.90 | 0.498 | 0.389 | |
| Random-effects model T2 with covariate: 1/N (N = 339) | |||||
| IVIG | 53,518 | 4.18 | 28,520 | 0.404 | 0.514 |
| Standard care | 45,593 | 3.90 | 0.596 | 0.486 | |
| Random-effects model T2 with covariate: 1/N (N → ∞) | |||||
| IVIG | 50,024 | 3.76 | Dominated | 0.275 | 0.348 |
| Standard care | 45,593 | 3.90 | 0.725 | 0.652 | |
These results clearly demonstrate that any conclusions regarding the cost-effectiveness of IVIG are highly sensitive to the choice of model used for clinical effectiveness. The most favourable ICER estimate (£16,177) is obtained using a random-effects model (comparing IVIG with albumin). However, IVIG appears dominated when a random-effects model is used with an adjustment for publication bias using sample size (N) and setting N to infinity. As noted in the clinical review, setting N to infinity involves extrapolation beyond the data set; when N was restricted to 339 (i.e. equivalent to the largest existing study), then the ICER of IVIG was £28,520 per QALY.
Alternative long-term survival scenarios
Given the uncertainty surrounding the long-term survival extrapolation required to estimate lifetime QALY gains, the robustness of the results to alternative assumptions was explored. Two separate scenarios were considered.
-
Alternative time horizons Our main analysis was based on a lifetime time horizon (30 years) requiring extrapolation beyond the 5-year follow-up from our cohort study. The impact of restricting the analysis to shorter time horizons of between 5 years and 30 years was explored. This provides an indication of the importance of the period of extrapolation beyond the observed data in determining the overall cost-effectiveness of IVIG.
-
Long-term survival of sepsis patients compared with the general population The time point at which we assumed patients revert from the predicted survival distributions from the long-term cohort data to survival estimates from the general population was varied. In our main analysis, this time point was determined by the time the mortality predictions from the parametric survival analysis became lower than the equivalent age- and sex-matched estimates from the general population. In the separate scenarios, this switch was assumed to happen at fixed time points between 5 years and 25 years after the initial episode.
Both scenarios were undertaken using the estimate of short-term clinical effectiveness from the fixed-effect model T3b with covariate: duration of IVIG (3 days).
Table 34 reports the cost-effectiveness results based on alternative time horizons. Restricting the time horizon to 5 years increased the ICER of IVIG to £43,717 per additional QALY, well above the conventional threshold considered to represent value for money to the NHS. As the time horizon increased, the cost-effectiveness of IVIG became more favourable. The results clearly demonstrate that the cost-effectiveness of IVIG is dependent upon the additional QALY gains predicted as part of the longer-term extrapolation.
| Time horizon | Probability of being cost-effective for cost-effectiveness threshold | ||||
|---|---|---|---|---|---|
| Treatment | Mean cost (£) | Mean QALY | ICER (£ per QALY) | £20,000 | £30,000 |
| 5 years | |||||
| IVIG | 37,633 | 1.69 | 47,717 | 0 | 0.129 |
| Standard care | 30,115 | 1.52 | 1 | 0.871 | |
| 10 years | |||||
| IVIG | 44,366 | 2.73 | 29,450 | 0.138 | 0.533 |
| Standard care | 36,150 | 2.45 | 0.862 | 0.467 | |
| 15 years | |||||
| IVIG | 49,030 | 3.45 | 24,637 | 0.317 | 0.684 |
| Standard care | 40,330 | 3.09 | 0.683 | 0.316 | |
| 20 years | |||||
| IVIG | 52,201 | 3.94 | 22,374 | 0.430 | 0.748 |
| Standard care | 43,172 | 3.53 | 0.570 | 0.252 | |
| 25 years | |||||
| IVIG | 54,052 | 4.22 | 21,296 | 0.484 | 0.777 |
| Standard care | 44,832 | 3.79 | 0.516 | 0.223 | |
| 30 years | |||||
| IVIG | 54,901 | 4.35 | 20,850 | 0.505 | 0.789 |
| Standard care | 45,593 | 3.90 | 0.495 | 0.211 | |
Table 35 reports the cost-effectiveness results based on varying the time point at which sepsis survivors are assumed to revert back to general population mortality rates. The ICER estimates improved marginally when it was assumed that patients reverted back to general population mortality rates earlier than in our main analysis. When it was assumed that patients reverted back to the general population mortality rate immediately after the 5-year follow-up period of the separate cohort study, the ICER improved to £19,974 per QALY, just under the lower bound of current cost-effectiveness thresholds. However, the ICER estimate increased to over £20,000 for all other time points. The ICER varied between £19,974 and £20,164 across these scenarios, indicating that the assumption that the prognosis of severe sepsis patients remains worse than that of the general population after 5 years is not a key driver of cost-effectiveness.
| Time point at which patients revert to general population mortality rates | Probability of being cost-effective for cost-effectiveness threshold | ||||
|---|---|---|---|---|---|
| Treatment | Mean cost (£) | Mean QALY | ICER (£ per QALY) | £20,000 | £30,000 |
| 5 years | |||||
| IVIG | 56,733 | 4.63 | 19,974 | 0.545 | 0.813 |
| Standard care | 47,234 | 4.16 | 0.455 | 0.187 | |
| 10 years | |||||
| IVIG | 55,053 | 4.37 | 20,773 | 0.508 | 0.791 |
| Standard care | 45,729 | 3.93 | 0.492 | 0.209 | |
| 15 years | |||||
| IVIG | 54,993 | 4.36 | 20,803 | 0.507 | 0.791 |
| Standard care | 45,675 | 3.92 | 0.493 | 0.209 | |
| 20 years | |||||
| IVIG | 55,572 | 4.45 | 20,515 | 0.519 | 0.797 |
| Standard care | 46,194 | 4.00 | 0.481 | 0.203 | |
| 25 years | |||||
| IVIG | 56,311 | 4.57 | 20,164 | 0.535 | 0.806 |
| Standard care | 46,857 | 4.10 | 0.465 | 0.194 | |
Alternative long-term cost scenarios
Given the lack of UK cost data on the long-term management of sepsis survivors, additional scenarios were undertaken to explore the robustness of the model to alternative costing assumptions. The impact of the following approaches was considered: (1) altering the magnitude of these estimates (± 50%) and (2) using general population estimates of the average annual per capital NHS cost, instead of sepsis-specific estimates from a non-UK source. Again, both scenarios were undertaken using the estimate of short-term clinical effectiveness from the fixed-effects model T3b with covariate duration of IVIG (3 days).
Table 36 reports the cost-effectiveness results varied the estimates applied in the main analysis by ± 50%. The ICER across this range varied between £19,418 and £22,282 per QALY, suggesting that the results were relatively robust to this magnitude of change. However, the ICER estimates appeared more sensitive to the assumption that the long-term costs of managing sepsis survivors would be higher than the annual NHS costs incurred by the general population in the longer term. Table 36 presents the ICER results assuming that the long-term costs of sepsis patients were (1) the same the general population after 3 years (reflecting the time horizon of the study used to estimate the long-term costs) or (2) the same as the general population immediately after the initial hospitalisation period. The ICERs for these separate analyses were £17,962 and £15,792 per QALY, respectively. Both of these estimates were well within the threshold range considered to represent value for money to the NHS, suggesting that the assumption that patients continue to incur higher costs than the general population over the longer-term extrapolation period is an important consideration.
| Long-term cost estimates | Probability of being cost-effective for cost-effectiveness threshold | ||||
|---|---|---|---|---|---|
| Treatment | Mean cost (£) | Mean QALY | ICER (£ per QALY) | £20,000 | £30,000 |
| Base-case costs +50% | |||||
| IVIG | 48,750 | 4.35 | 19,418 | 0.562 | 0.825 |
| Standard care | 40,081 | 3.90 | 0.438 | 0.175 | |
| Base-case costs –50% | |||||
| IVIG | 61,052 | 4.35 | 22,282 | 0.451 | 0.753 |
| Standard care | 51,104 | 3.90 | 0.549 | 0.247 | |
| NHS costsa applied to survivors after 3 years | |||||
| IVIG | 42,448 | 4.35 | 17,962 | 0.611 | 0.845 |
| Standard care | 34,429 | 3.90 | 0.389 | 0.155 | |
| NHS costsa applied to survivors after discharge from initial hospitalisation | |||||
| IVIG | 33,125 | 4.35 | 15,792 | 0.697 | 0.875 |
| Standard care | 26,075 | 3.90 | 0.303 | 0.125 | |
Subgroups
The results of the scenarios presented have been based on the average baseline characteristics of patients in the ICNARC CMP Database. However, the cost-effectiveness results may also vary according to different patient characteristics. Heterogeneity in patient characteristics and the impact on the ICER estimates were explored using a series of separate scenarios based on:
-
APACHE II score
-
ICNARC physiology score
-
organ dysfunctions.
These scenarios were explored by varying the baseline hospital mortality rate according to the particular characteristics considered (using predictions from the logistic regressions detailed in Chapter 4 and Appendix 6). Long-term mortality was also varied according to APACHE II score as this score was demonstrated to be a significant predictor of long-term mortality in our long-term cohort data (using predictions from the parametric survival regressions detailed in Chapter 5 and Appendix 6). Subgroup estimates of long-term mortality were not used for either the analyses based on ICNARC physiology score, as these data were not collected within the long-term cohort, or those based on organ dysfunctions, as these covariates were not identified as significant predictors of long-term mortality. All scenarios were undertaken using the estimate of short-term clinical effectiveness from the fixed-effects model T3b with covariate duration of IVIG (3 days).
Detailed results based on the APACHE II and ICNARC physiology scores are presented in Appendix 10. A clear non-linear relationship is apparent in the cost-effectiveness estimates. That is, ICER estimates were markedly higher for very low- and very high-risk patients compared with our main results. However, these higher estimates were reported for relatively extreme scores, which were not considered representative of the majority of patients in the CMP Database. The results indicated that the cost-effectiveness results were relatively robust to variation in the scores actually observed in the database.
Table 37 presents the cost-effectiveness results for different subgroups defined according to the number of organ dysfunctions during the first 24 hours following admission to the critical-care unit. The ICER estimates for IVIG were more favourable for patients with two or more organ dysfunctions (£20,706) than for those with only one (£26,049). However, simply dichotomising the population in this manner ignored the potential heterogeneity that existed within the group with two or more organ dysfunctions. Within this subgroup, the ICER varied between £20,611 (three organ dysfunctions) and £26,268 (five organ dysfunctions).
| Number of organ dysfunctions during first 24 hours | Probability of being cost-effective for cost-effectiveness threshold | ||||
|---|---|---|---|---|---|
| Treatment | Mean cost (£) | Mean QALY | ICER (£ per QALY) | £20,000 | £30,000 |
| One | |||||
| IVIG | 61,034 | 5.44 | 26,049 | 0.264 | 0.648 |
| Standard care | 55,370 | 5.15 | 0.736 | 0.352 | |
| Two or more | |||||
| IVIG | 53,726 | 4.11 | 20,706 | 0.511 | 0.793 |
| Standard care | 44,133 | 3.65 | 0.489 | 0.207 | |
| Two | |||||
| IVIG | 59,031 | 4.81 | 21,817 | 0.457 | 0.763 |
| Standard care | 50,356 | 4.41 | 0.543 | 0.237 | |
| Three | |||||
| IVIG | 53,870 | 4.04 | 20,611 | 0.515 | 0.795 |
| Standard care | 44,236 | 3.57 | 0.485 | 0.205 | |
| Four | |||||
| IVIG | 44,859 | 2.93 | 22,163 | 0.430 | 0.756 |
| Standard care | 34,497 | 2.46 | 0.570 | 0.244 | |
| Five | |||||
| IVIG | 34,527 | 1.92 | 26,268 | 0.220 | 0.633 |
| Standard care | 24,667 | 1.54 | 0.780 | 0.367 | |
Summary of the cost-effectiveness analysis results
The results demonstrate that the cost-effectiveness of IVIG is subject to several key assumptions and uncertainties. At best, the cost-effectiveness case for IVIG currently appears borderline in terms of representing value for money to the NHS, with several scenarios reporting ICER results close to the lower bound of acceptable thresholds. However, the ICER results appeared particularly sensitive to the clinical effectiveness model used to estimate the relative effectiveness of IVIG, with ICER estimates ranging from > £20,000 per QALY to IVIG being dominated by standard care across the different scenarios considered. This degree of variation suggests that the cost-effectiveness is difficult to determine without additional information to help interpret and understand the existing clinical effectiveness data for IVIG.
Chapter 6 Value of information analysis – analytic methods and results
Overview
In the previous chapter, the expected cost-effectiveness of IVIG in adults with severe sepsis and septic shock was assessed given the existing evidence available. Evidence on a number of key inputs and assumptions was demonstrated to be uncertain, and there is a need to identify whether or not further research would be potentially worthwhile and to help prioritise areas where this research would appear to be most valuable in terms of informing decision-making in the NHS concerning the appropriate use of IVIG. An analysis of EVI is presented to help to inform and prioritise potential areas where further research is needed.
Methods for the expected value of perfect information
Decisions based on existing information for IVIG are clearly uncertain and there will always be a chance that the wrong decision will be made. If the wrong decision is made, there will be costs in terms of health benefit and resources forgone. The maximum amount the NHS should be willing to invest to reduce uncertainty in the decision can be informed by the expected value of perfect information (EVPI). 116 The EVPI evaluates the expected cost of current uncertainty by accounting for both the probability that a decision based on existing evidence is wrong and for the magnitude of the consequences of making the wrong decision.
The EVPI can then be used as a necessary requirement for determining the potential efficiency of further primary research. Applying this decision rule, additional research should be considered only if the EVPI exceeds the expected cost of the research. EVPI can also be estimated for individual parameters (or groups of parameters) contained in the model, termed partial EVPI or expected value of partial perfect information (EVPPI). EVPPI considers particular elements of the decision problem in order to direct and focus research towards the specific areas where the elimination of uncertainty has the most value. This can be particularly relevant to the design of any future research. On the basis of EVPI and EVPPI calculations, the potential value of a future trial, or other research designs, can be evaluated.
As information can be of value to more than one individual, EVPI can also be expressed for the total population who stand to benefit over the expected lifetime of the programme/technology. If the EVPI for the population of current and future patients exceeds the expected costs of additional research, then it is potentially cost-effective to conduct further research. Population EVPI is determined by applying the individual EVPI estimate to the number of people who would be affected by the information over the anticipated lifetime of the technology:
where It is the incidence in the tth year, T is the total number of years for which information from research would be useful and r is the discount rate.
The yearly incidence of critical-care admission with severe sepsis during the first 24 hours has been reported to be 66 per 100,000 adult population. 3 The yearly incident cases of severe sepsis in the UK are therefore estimated to be 33,160. Our analysis assumes that the information would be valuable for 10 years. A 3.5% annual rate of discount is applied.
Results for the expected value of perfect information
Table 38 provides a summary of the population EVPI estimates based on a cost-effectiveness threshold of £20,000 per QALY. The results demonstrate a considerable range in the population EVPI estimates depending on the clinical effectiveness model applied to estimate the relative effectiveness of IVIG. As expected, the random-effects model gave higher EVPI estimates given the additional between-study heterogeneity that is included. For a time horizon (T) of 10 years, population EVPI varies between approximately £393M and £1.4B. These results suggest that further primary research appears to be potentially worthwhile given the high cost of current decision uncertainty across all scenarios.
| Clinical effectiveness model | EVPI per patient (£) | Population EVPI (£) (T = 10 years) |
|---|---|---|
| Fixed-effects model T3b with covariate: duration of IVIG (3 days) | 1377 | 392,994,216 |
| Random-effects model T3 (compared with albumin) | 3563 | 1,017,023,732 |
| Random-effects model T2 with covariate: Jadad score (Jadad score = 5) | 4791 | 1,367,426,550 |
| Random-effects model T2 with covariate: 1/N (N = 339) | 3146 | 897,945,285 |
| Random-effects model T2 with covariate: 1/N (N → ∞) | 2113 | 603,018,958 |
The value of reducing the uncertainty surrounding particular input parameters in the model can be informed by estimating EVPPI. There are five groups of uncertain parameters considered in the partial EVPI analysis. These relate to:
-
baseline mortality rate during the initial hospitalisation with standard care
-
clinical effectiveness of IVIG
-
long-term mortality estimates for survivors of severe sepsis
-
long-term costs for survivors of severe sepsis
-
quality of life of sepsis survivors.
The groups of parameters also reflect potentially different research designs. For example, although a RCT would ideally be required to further inform the clinical effectiveness of IVIG, evidence on the other parameters could be generated using record linkage with existing data sets (e.g. linking the existing ICNARC CMP Database with national mortality registers to inform long-term survival of sepsis survivors) or by establishing new cohort studies (e.g. to estimate the costs and long-term quality of life impact of survivors of severe sepsis) where issues of bias may be less important in terms of study design.
Table 39 reports the EVPPI estimates for the five groups of uncertain parameters for each of the clinical effectiveness models. The EVPPI associated with the relative treatment effect of IVIG consistently emerges as having significant influence on the overall decision uncertainty, having the highest estimate across the different groups of parameters in four of the five scenarios. Indeed, the lowest estimate of EVPPI for the relative effect of IVIG was £173.7M. The long-term costs of severe sepsis also emerge as an important driver of uncertainty, with significant value related to current decision uncertainty in all except one of the scenarios. A less consistent story emerged for the remaining three groups of parameters. However, in one of the five scenarios considered, these parameters also reported relatively high values (£2.2M for short-term mortality data, £14.6M for longer-term mortality data in survivors of severe sepsis and £39.2M for quality-of-life data in sepsis survivors). Although estimates for these three parameters appear considerably lower than those reported for the relative effectiveness estimates, it should also be appreciated that the costs of undertaking research would also be significantly lower than those required to undertake a multicentre RCT.
| Scenario | EVPPI per patient (£) | Population EVPPI (£) (T = 10 years) |
|---|---|---|
| Fixed-effects model T3b with covariate: duration of IVIG (3 days) | ||
| Baseline mortality (short term) | 0 | 0 |
| Relative treatment effect of IVIG | 609 | 173,736,363 |
| Long-term mortality | 0 | 0 |
| Long-term costs | 876 | 249,956,670 |
| Quality of life | 28 | 7,919,499 |
| Random-effects model T3 (compared with albumin) | ||
| Baseline mortality (short term) | 0 | 0 |
| Relative treatment effect of IVIG | 2514 | 717,558,633 |
| Long-term mortality | 0 | 0 |
| Long-term costs | 1205 | 344,184,097 |
| Quality of life | 0 | 0 |
| Random-effects model T2 with covariate: Jadad score (Jaded score = 5) | ||
| Baseline mortality (short term) | 8 | 2,240,224 |
| Relative treatment effect of IVIG | 3582 | 1,022,413,680 |
| Long-term mortality | 51 | 14,628,446 |
| Long-term costs | 1224 | 349,373,644 |
| Quality of life | 137 | 39,189,945 |
| Random-effects model T2 with covariate: 1/N (N = 339) | ||
| Baseline mortality (short term) | 0 | 0 |
| Relative treatment effect of IVIG | 2173 | 620,201,792 |
| Long-term mortality | 0 | 0 |
| Long-term costs | 0 | 0 |
| Quality of life | 0 | 0 |
| Random-effects model T2 with covariate: 1/N (N → ∞) | ||
| Baseline mortality (short term) | 0 | 0 |
| Relative treatment effect of IVIG | 1335 | 381,161,822 |
| Long-term mortality | 0 | 0 |
| Long-term costs | 9 | 2,616,609 |
| Quality of life | 0 | 0 |
We now focus in more detail on the value of obtaining further evidence on the relative treatment effect of IVIG and the potential value of undertaking a further RCT.
Methods for the expected value of sample information
In the previous sections, EVPI and EVPPI set an upper limit on the returns to further research. However, to fully inform the research decision the most efficient research design needs to be established, for example the type of study to be conducted, the optimal sample size, the optimal allocation of patients within a clinical trial, the appropriate follow-up time and which end points should be included. To establish the most appropriate design, the marginal benefits and marginal costs of gathering sample information need to be considered. 117,118 The same framework of EVI analysis can be extended to establish the expected value of sample information (EVSI) for a particular research design. The difference between the EVSI and the costs of sampling gives the expected net benefit of sampling (ENBS). The ENBS provides a measure of the payoff from research and can be calculated for a range of sample sizes and alternative designs of research. This provides both a necessary and sufficient condition for deciding to conduct more research, i.e. if the ENBS is > 0 then the marginal benefits of gathering the sample information exceed the marginal costs. The optimal design and sample size can then be determined from the ENBS.
Further details on the methods used are presented in Appendix 5.
Results for the expected value of sample information
Analogous to population EVPI, the overall value of sample information is estimated for a population of patients who could potentially benefit from IVIG. The EVSI in Table 40 provides the upper limit on the cost of conducting a new trial for a given sample size, based on one of the clinical effectiveness models considered – the fixed-effects model T3b with covariate duration of IVIG (3 days).
| Sample size (per arm assuming equal allocation) | EVSI per patient (£) | Population EVSI (£) (T = 10 years) |
|---|---|---|
| 50 | 114 | 32,632,619 |
| 100 | 194 | 55,397,599 |
| 200 | 287 | 82,022,999 |
| 500 | 416 | 118,818,304 |
| 1000 | 498 | 142,225,815 |
| 2000 | 552 | 157,462,313 |
| 5000 | 589 | 168,164,299 |
To obtain the societal payoff for the proposed research, the population EVSI needs to be compared with the costs of sampling. This provides a sufficient condition for deciding to conduct more research. If the ENBS is > 0 for any sample size, then further research is potentially justified. The ENBS also provides a framework for the efficient design of the clinical trial, where the optimal sample size, n*, for the proposed trial is where the ENBS reaches its maximum. This optimal sample size indicates how many patients should be enrolled for the trial to provide the highest payoff.
Figure 46 presents the trial costs, population EVSI and ENBS estimates for different sample sizes assuming equal allocation between arms. The costs of the trial are based on a fixed-cost component (£2M) and variable costs for each patient recruited (£2000 + £5500 for patients receiving IVIG). At a threshold of £20,000 per QALY, the ENBS reaches an optimal sample size of 1900 subjects for each arm.
FIGURE 46.
Trial costs, population EVSI and ENBS based on the fixed-effects model T3b with covariate duration of IVIG (3 days).
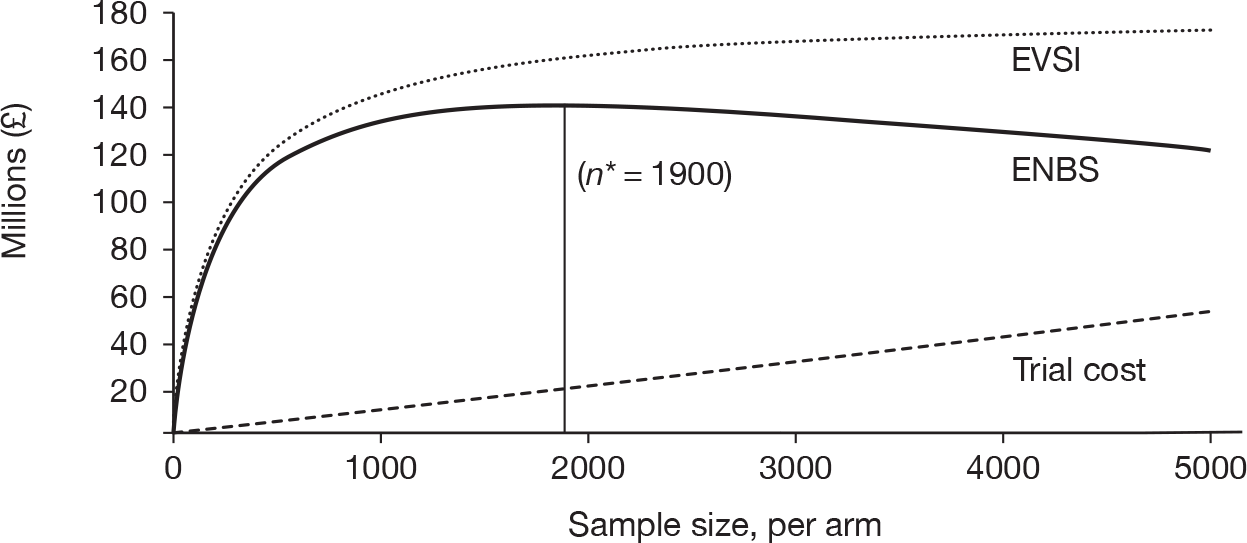
Figure 47 presents the same estimates assuming different per patient costs (between £2000 and £35,000). The maximum payoff from conducting this research (the ENBS) decreases as the per patient trial costs increase. The optimal sample size also decreases to a minimum of 500 subjects for each arm when per patient costs are assumed to be £35,000.
FIGURE 47.
Expected net benefit of sampling assuming alternative per patient trial costs based on the fixed-effects model T3b with covariate duration of IVIG (3 days).
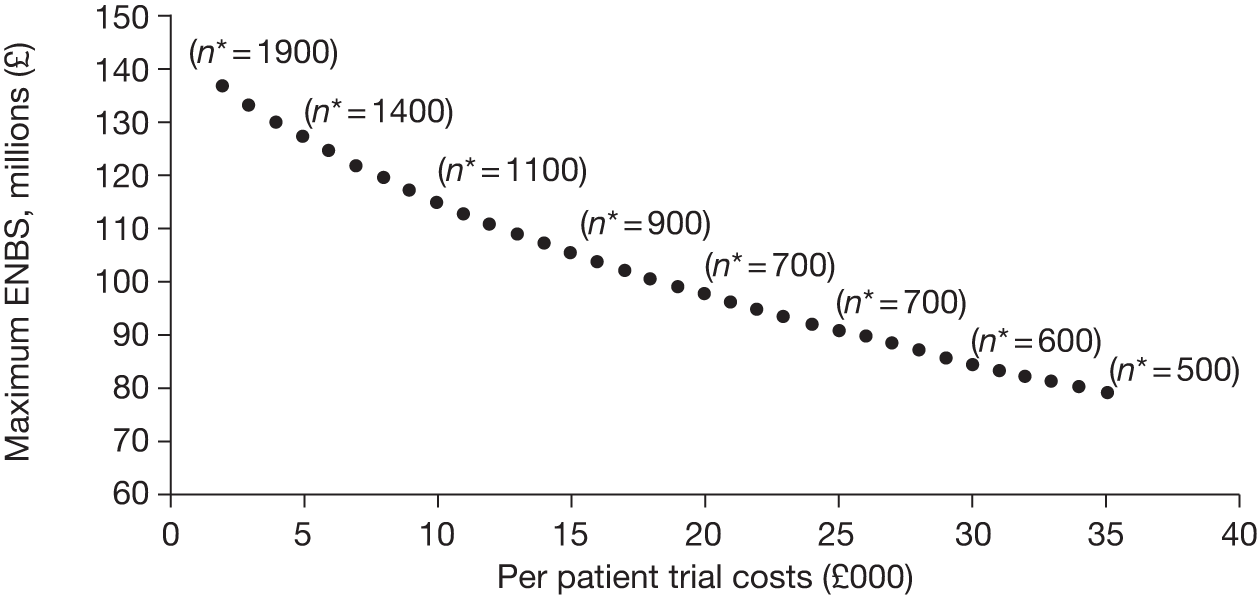
The impact of the different clinical effectiveness models in estimating the optimal sample size of a future trial is depicted in Table 41. Across scenarios, the maximum payoff from conducting this research varied between £137M and £1011M. The optimal sample size always exceeded 800 subjects for each arm.
| Scenario | Maximum ENBS (£) | Optimal sample size (n*) |
|---|---|---|
| Fixed-effect model T3b with covariate: duration of IVIG (3 days) | 136,703,882 | 1900 |
| Random-effects model T3 (compared with albumin) | 687,441,146 | 1200 |
| Random-effect model T2 with covariate: Jadad score (Jadad score = 5) | 1,010,953,361 | 800 |
| Random-effect model T2 with covariate: 1/N (N = 339) | 605,931,859 | 900 |
| Random-effect model T2 with covariate: 1/N (N → ∞) | 365,050,246 | 800 |
Summary of the value of information analysis results
The value of information analysis showed further primary research to be worthwhile in resolving the uncertainty on whether or not to adopt IVIG as an adjunctive treatment for severe sepsis or septic shock. However, the consequences of the existing uncertainty are important because we may not be recommending the treatment that is cost-effective and because the net consequences of making the wrong choice are relevant.
Across the majority of scenarios explored, a study collecting data on the relative effectiveness of IVIG (in relation to standard care alone) appeared the most efficient research design to invest in. However, results on the value of conducting such research are sensitive to the clinical effectiveness model used – current clinical effectiveness evidence is very heterogeneous and it is not clear whether or not there is any clinical rationale for the effects explored within each of the clinical effectiveness models. So, although the need for a further RCT exists, designing this study is complex when uncertainties at this level exist.
Although the required RCT would be challenging and expensive, with design aspects requiring careful thought, research informing other parameters may be worthwhile, especially regarding the long-term survival and costs of severe sepsis survivors. However, research over these parameters was not consistently highlighted in our results, but only in scenarios in which IVIG was deemed borderline cost-effective. Whether or not conducting this research is relevant is thus still dependent on clarifying the results on clinical effectiveness by possibly further understanding the heterogeneous nature of the severe sepsis syndrome and the mechanistic role of IVIG. If this research was to be conducted, then it could be undertaken using a non-RCT design. Record linkage between existing databases or a prospective cohort study may be alternative specifications for such a study, provided that the period for which patients are observed is sufficiently long to capture the impact on costs for several years after the initial episode. Whereas a prospective study may allow a more detailed collection of relevant resource use data, it may be more costly to implement and results may only be available much later on.
Extensive EVI analyses have been conducted evaluating multiple alternative representations of the effectiveness of IVIG. However, the scenarios presented reflect only a small set of all the possible alternatives and, hence, the true cost of decision uncertainty for some of the parameters evaluated may not be captured.
Chapter 7 Conclusions
Implications for health care
Our meta-analysis, the first to simultaneously allow for type of IVIG (IVIG or IVIGAM), choice of control (no treatment or albumin), study quality/publication bias and other potential covariates, indicated that the treatment effect of IVIG on mortality for patients with severe sepsis is borderline significant with a large degree of heterogeneity in treatment effect between individual studies. Based on the results of combining the available evidence, and until further evidence becomes available, the immediate implications for health care are as per current policy and practice for off-label use of IVIG in severe sepsis (i.e. colour-coded black as treatment not recommended).
Recommendations for research
Although the EVI analyses suggested substantial expected net benefit from a large multicentre RCT evaluating the clinical effectiveness of IVIG, the remaining uncertainties around the design of such a study mean that we are unable to recommend it at this time. Our recommendations for research focus on filling the knowledge gaps to inform a future multicentre RCT prior to recommending its immediate design and conduct.
Modelling indicated that there were issues with bias associated with trial methodology, publication and small-study effects with the current evidence. The large degree of heterogeneity in treatment effects between studies, however, could be explained (best-fitting model) by a measure of study quality (i.e. use of albumin as control – as an indicator of proper blinding to treatment as a proxy for study quality – associated with decreased effect) and duration of IVIG therapy (longer duration associated with increased effect). In-depth discussion within the Expert Group on duration of IVIG therapy, with daily dose and total dose also clearly inter-related, indicated no clear clinical rationale for this association and exposed a lack of evidence on the understanding of the mechanism of action of IVIG in severe sepsis (evidence also being weak on how IVIG works in toxic states, such as toxic syndrome).
Intravenous immunoglobulin as an adjunctive treatment can be a physiological replacement and/or a pharmacological treatment (immunomodulation) and, with marked differences in the immunological profile during severe sepsis, the Expert Group identified research to better understand the mechanism(s) of action of IVIG preparations (10 products are licensed for use in the UK with few evaluated in previous RCTs) in the severe sepsis population, and dose-ranging/finding studies to inform the dose, duration and timing of intervention(s) for a future multicentre RCT, as the highest priority. Note that IVIGAM (Pentaglobin) has been evaluated most in the severe sepsis population, but is not licensed for use in the UK. The response in children may be very different from that in adults. Modern IVIG preparations are more concentrated. Though an adjunctive treatment, evidence in severe sepsis suggests that early treatment is beneficial. Sufficient supplies of IVIG for a future RCT would require consideration.
Recommendation 1
Research on the mechanism(s) of action of IVIG preparation(s) in the severe sepsis population commencing with a thorough review of existing research prior to embarking on any new research.
Recommendation 2
Informed by recommendation 1, dose-ranging/finding studies to identify dose, timing of dose and safety data (tolerability/side-effects) to inform the intervention(s) for a future multicentre RCT.
There was a dearth of long-term outcome and cost/resource data on severe sepsis survivors to inform the cost-effectiveness analyses. Either by exploiting existing databases, through record linkage, or by initiating a prospective cohort study, long-term survival, including quality of survival and costs of survival for several years after the initial severe sepsis episode, should be explored.
Recommendation 3
Research to inform the long-term survival, including quality and costs of survival for the severe sepsis population.
Recommendation 4
Results of recommendations 1–3 should be re-evaluated for their impact on our EVI analyses.
The primary target population is adult patients with severe sepsis. There is increasing awareness that the syndrome described as severe sepsis represents a large and extremely heterogeneous group of patients. The heterogeneity of the severe sepsis population has plagued large, multicentre RCTs and there is a realisation that the focus should be on more homogeneous, specific, severe sepsis subpopulations. Heterogeneity appears to exist at the genetic, biochemical and clinical level, all of which may be associated. The current focus of research on severe sepsis has been in the identification of relevant genetic, biochemical and clinical markers with the aim better describing more homogeneous severe sepsis subpopulations, providing more rapid bedside markers for the early identification of sepsis and establishing which patients are most likely to benefit from therapy. Such advancements should inform the final design of a multicentre RCT of IVIG.
Recommendation 5
Recommendations 1–3 require knowledge of, and design of the definitive RCT for IVIG in severe sepsis requires a comprehensive review of, the emerging evidence surrounding the heterogeneity of the severe sepsis population at the genetic, biochemical and clinical level.
In summary, although the results highlight the value for money obtained in conducting further primary research in this area, the biggest limitation for such research regards the uncertainties over the heterogeneous nature of severe sepsis and the mechanism of action of IVIG. Resolving these would allow for better definition of the plausibility of the effectiveness scenarios presented and, consequently, a better understanding of the cost-effectiveness of this treatment. This information would also inform the design of future, primary evaluative research.
Acknowledgements
We acknowledge both the time and input from our Expert Group members: Maureen Dalziel (service user representative); Carrock Sewell (Consultant Immunologist, Northern Lincolnshire and Goole Hospitals NHS Foundation Trust and Visiting Professor of Immunology, University of Lincoln, UK); Mervyn Singer (Professor of Intensive Care Medicine, University College London, UK); Richard Beale (Head of Perioperative, Critical Care and Pain Services and Consultant Intensivist, Guy’s and St Thomas’ NHS Foundation Trust, London, UK); and Graham Ramsay (Chief Executive, Mid Essex Hospital Services NHS Trust, Chelmsford, UK). We also acknowledge Phil Restarick who co-ordinated the survey and Expert Group meetings.
Contributions of authors
Marta Soares (Research Fellow, Health Economics) contributed to acquisition, analysis and interpretation of the data (cost-effectiveness and value of information), drafted and revised the manuscript and provided final approval of the version to be published.
Nicky Welton (Senior Lecturer in Biostatistics) contributed to the design of the study, acquisition, analysis and interpretation of the data (clinical effectiveness), drafted and revised the manuscript and provided final approval of the version to be published.
David Harrison (Senior Statistician and Honorary Senior Lecturer in Medical Statistics) contributed to the design of the study, acquisition, analysis (analyses of the ICNARC CMP Database) and interpretation of the data, drafted and revised the manuscript and provided final approval of the version to be published.
Piia Peura (Visiting Researcher, Health Economics) contributed to the acquisition of the data (cost-effectiveness and value of information), revised the manuscript and provided final approval of the version to be published.
Manu Shankar Hari (Consultant, Intensive Care Medicine) contributed to the acquisition, analysis (survey of critical-care units) and interpretation of the data, drafted and revised the manuscript and provided final approval of the version to be published.
Sheila Harvey (Senior Research Fellow, Health Services Research) contributed to the acquisition, analysis and interpretation of the data (survey of critical-care units), drafted and revised the manuscript and provided final approval of the version to be published.
Jason Madan (Research Associate, Social and Community Medicine) contributed to the acquisition and analysis of the data (systematic reviews of clinical effectiveness), revised the manuscript and provided final approval of the version to be published.
A E Ades (Professor of Public Health Science) contributed to design of the study and interpretation of the data (clinical effectiveness), revised the manuscript and provided final approval of the version to be published.
Stephen Palmer (Professor of Health Economics) contributed to the design of the study, acquisition, analysis (cost-effectiveness and value of information) and interpretation of the data, drafted and revised the manuscript and provided final approval of the version to be published.
Kathryn Rowan (Director of ICNARC and Honorary Professor of Health Services Research) conceived, designed and led the study, contributed to acquisition, analysis and interpretation of the data, drafted and revised the manuscript and provided final approval of the version to be published.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644-55.
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250-6.
- Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care 2006;10.
- Linde-Zwirble WT, Angus DC. Severe sepsis epidemiology: sampling, selection, and society. Crit Care 2004;8:222-6.
- Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med 2003;31:2332-8.
- Jolles S, Sewell WAC, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol 2005;142:1-11.
- Sewell WA, Jolles S. Immunomodulatory action of intravenous immunoglobulin. Immunology 2002;107:387-93.
- Wallington T. New uses for IVIgG immunoglobulin therapies. Vox Sang 2004;87:155-7.
- Alejandria MM, Lansang MA, Dans LF, Mantaring JB. Intravenous immunoglobulin for treating sepsis and septic shock. Cochrane Database Syst Rev 2002;1.
- Kreymann KG, de Heer G, Nierhaus A, Kluge S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med 2007;35:2677-85.
- Laupland KB, Kirkpatrick AW, Delaney A. Polyclonal intravenous immunoglobulin for the treatment of severe sepsis and septic shock in critically ill adults: a systematic review and meta-analysis. Crit Care Med 2007;35:2686-92.
- Turgeon AF, Hutton B, Fergusson DA, McIntyre L, Tinmouth AA, Cameron DW, et al. Meta-analysis: intravenous immunoglobulin in critically ill adult patients with sepsis. Ann Intern Med 2007;146:193-20.
- Norrby-Teglund A, Haque KN, Hammarstrom L. Intravenous polyclonal IgM-enriched immunoglobulin therapy in sepsis: a review of clinical efficacy in relation to microbiological aetiology and severity of sepsis. J Intern Med 2006;260:509-16.
- Pildal J, Gøtzsche PC. Polyclonal immunoglobulin for treatment of bacterial sepsis: a systematic review. Clin Infect Dis 2004;39:38-46.
- Alejandria MM, Lansang MA, Dans LF, Mantaring JB. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock (review). Cochrane Library 2010.
- Department of Health . Demand Management Plan for Immunoglobulin Use 2008.
- Department of Health . Clinical Guidelines for Immunoglobulin Use 2008.
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008;36:296-327.
- Levy MM, Pronovost PJ, Dellinger RP, Townsend S, Resar RK, Clemmer TP, et al. Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome. Crit Care Med 2004;32:S595-7.
- Surving Sepsis Campain . Severe Sepsis Bundles n.d. www.survivingsepsis.com/node/89 (accessed 18 August 2010).
- Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004;32:858-73.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6.
- Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283-97.
- Survive Sepsis n.d. www.survivesepsis.org.uk (accessed 18 August 2010).
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized trials: is blinding necessary?. Control Clin Trials 1996;17.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29.
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270:2957-63.
- Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10.
- Elebute EA, Stoner HB. The grading of sepsis. Br J Surg 1983;70:29-31.
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34.
- Higgins JPT, Whitehead A. Borrowing strength from external trials in a meta-analysis. Stat Med 1996;15:2733-49.
- Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900.
- Ades AE, Sculpher M, Sutton A, Abrams K, Copper N, Welton NJ, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics, PharmacoEcon 2006;24:1-19.
- Ades A, Welton N, Lu G. Introduction to Mixed Treatment Comparisons 2005. www.bris.ac.uk/cobm/research/mpes/mtc.html (accessed 17 October 2011).
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions. Stat Med 2009;28:3049-67.
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37.
- Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Ser B 2002;64:583-616.
- Vogel F, Exner M, Franke P, Gien C, Deicher H, Schoppe W. Klinisch angewandte immunologie-sepsis therapie mit IgM-angereichertem immunoglobulin. Berlin: Springer-Verlag; 1988.
- Dominioni L, Dionigi R, Zanello M, Chiaranda M, Dionigi R, Acquarolo A, et al. Effects of high-dose IgG on survival of surgical patients with sepsis scored of 20 or greater. Arch Surg 1991;126:236-40.
- Just H-M, Metzger M, Vogel W, Penack O. Einfluss einer adjuvanten immunglobulintherapie auf infektionen bei patienten einer operativen intensiv-therapie-station. Klin Wochenschr 1986;64:245-56.
- Jesdinsky HJ, Tempel G, Castrup HJ, Seifert J. Cooperative group of additional immuinoglobulin therapy in severe bacterial infections: results of a multicenter randomized controlled trial in cases of diffuse fibrinpurulent peritonitis. Klin Wochenschr 1987;65:1132-8.
- Rodriguez A, Rello J, Neira J, Maskin B, Ceraso D, Vasta L, et al. Effects of high-dose of intravenous immunoglobulin and antibiotics on survival for severe sepsis undergoing surgery. Shock 2005;23:298-304.
- Hentrich M, Fehnle K, Ostermann H, Kienast J, Cornely O, Salat C, et al. IgMA-enriched immunoglobulin in neutropenic patients with sepsis syndrome and septic shock: a randomized, controlled, multiple-center trial. Crit Care Med 2006;34:1319-25.
- Karatzas S, Boutzouka E, Venetsanou K, Myrianthefs P, Fildisis G, Baltopoulos G. The effects of IgM-enriched immunoglobulin preparations in patients with severe sepsis: another point of view. Crit Care 2002;6:543-4.
- Tugrul S, Ozcan PE, Akinci O, Seyhun Y, Cagatay A, Cakar N, et al. The effects of IgM-enriched immunoglobulin preparations in patients with severe sepsis. Crit Care 2002;6:357-62.
- Behre G, Ostermann H, Schedel I, Helmerking M, Schiel X, Rothenburger M, et al. Endotoxin concentrations and therapy with polyclonal IgM-enriched immunoglobulins in neutropenic cancer patients with sepsis syndrome: pilot study and interim analysis of a randomized trial. Anti Infect Drugs Chemother 1995;13:129-34.
- Schedel I, Dreikhausen U, Nentwig B, Hockenschnieder M, Rauthmann D, Balikcioglu S, et al. Treatment of Gram-negative septic shock with an immunoglobulin preparation: a prospective, randomized clinical trial. Crit Care Med 1991;19:1104-13.
- Wesoly C, Kipping N, Grundmann R. Immunglobulintherapie der postoperativen sepsis. Z Exp Chir Transplant Kunstliche Organe 1990;23:213-16.
- Spannbrucker N, Munch HG, Kunze R, Vogel F. Auswirkungen von immunglobulinsubstitution bei sepsis. Intensivmedizin 1987;6.
- Dominioni L, Bianchi V, Imperatori A, Minoia G, Dionigi R, Abakumov MM. High-dose intravenous IgG for treatment of severe surgical infections. Dig Surg 1996;13:430-4.
- Burns ER, Lee V, Rubinstein A. Treatment of septic thrombocytopenia with immune globulin. J Clin Immunol 1991;11:363-8.
- De Simone C, Delogu G, Corbetta G. Intravenous immunoglobulins in association with antibiotics: a therapeutic trial in septic intensive care unit patients. Crit Care Med 1988;16:23-6.
- Werdan K, Pilz G, Bujdoso O, Fraunberger P, Neeser G, Schmieder RE, et al. Score-based immunoglobulin G therapy of patients with sepsis: the SBITS study. Crit Care Med 2007;35:2693-701.
- Grundmann R, Hornung M. Immunoglobulin therapy in patients with endotoxemia and postoperative sepsis – a prospective randomized study. Prog Clin Biol Res 1988;272:339-49.
- Yakut M, Cetiner S, Akin A, Tan A, Kaymakcioglu N, Simsek A, et al. Sepsisdeki hastalarda immunglobulin G (IgG) kullaniminin mortalite oranina etkisi. GATA Bulteni 1998;40:76-81.
- Darenberg J, Ihendyane N, Sjolin J, Aufwerber E, Haidl S, Follin P, et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2003;37:333-40.
- Lindquist L, Lundbergh P, Maasing R. Pepsin-treated human gamma globulin in bacterial infections: a randomized study in patients with septicaemia and pneumonia. Vox Sang 1981;40:329-37.
- Masaoka T, Hasegawa H, Takaku F. The efficacy of intravenous immunoglobulin in combination therapy with antibiotics for severe infections. Jpn J Chemother 2000;48:199-217.
- Raphael JC, Chevret S, Harboun M, Jars-Guincestre MC. French Guillain–Barré Syndrome Cooperative G . Intravenous immune globulins in patients with Guillain–Barré syndrome and contraindications to plasma exchange: 3 days versus 6 days. J Neurol Neurosurg Psychiatry 2001;71:235-8.
- Tugrul S, Ozcan PE, Akinci O, Cagatay A, Cakar N, Esen F. [The effect of pentaglobin therapy on prognosis in patients with severe sepsis]. Ulus Travma Derg 2001;7:219-23.
- Bellomo R, Uchino S, Naka T, Wan L. Hidden evidence to the West: multicentre, randomised, controlled trials in sepsis and systemic inflammatory response syndrome in Japanese journals. Intensive Care Med 2004;30:911-17.
- Reith HB, Rauchschwalbe SK, Mittelkotter U, Engemann R, Thiede A, Arnold A, et al. IgM-enriched immunoglobulin (pentaglobin) positively influences the course of post-surgical intra-abdominal infections. Eur J Med Res 2004;9:479-84.
- Buda S, Riefolo A, Biscione R, Goretti E, Cattabriga I, Grillone G, et al. Clinical experience with polyclonal IgM-enriched immunoglobulins in a group of patients affected by sepsis after cardiac surgery. J Cardiothorac Vasc Anesth 2005;19:440-5.
- Khan S, Sewell WAC. Risks of intravenous immunoglobulin in sepsis affect trial design. Ann Intern Med 2007;147.
- Calandra T, Glauser MP, Schellekens J, Verhoef J. Swiss-Dutch J5 Immunoglobulin Study Group . Treatment of Gram-negative septic shock with human IgG antibody to Escherichia coli J5: a prospective, double-blind randomized trial. J Infect Dis 1988;158:312-19.
- Pilz G, Appel R, Kreuzer E, Werdan K. Comparison of early IgM-enriched immunoglobulin vs polyvalent IgG administration in score-identified postcardiac surgical patients at high risk of sepsis. Chest 1997;111:419-26.
- Keane W, Hirata-Dulas CAI, Bullock ML, Ney AL, Guay DRP, Kalil RSN, et al. Adjunctive therapy with intravenous human immunoglobulin G improved survival of patients with acute renal failure. J Am Soc Nephrol 1991;2:841-7.
- Vincent J-L, Navickis RJ, Wilkes MM. Morbidity in hospitalized patients receiving human albumin: a meta-analysis of randomized, controlled trials. Crit Care Med 2004;32:2029-38.
- Moreno SG, Sutton AJ, Ades AE, Stanley TD, Abrams KR, Peters JL, et al. Assessment of regression-based methods to adjust for publication bias through a comprehensive simulation study. BMC Med Res Methodol 2009;9.
- Moreno SG, Sutton AJ, Turner EH, Abrams KR, Cooper NJ, Palmer TM, et al. Novel methods to deal with publication biases: secondary analysis of antidepressant trials in the FDA trial registry database and related journal publications. BMJ 2009;339.
- Werdan K. Mirror, mirror on the wall, which is the fairest meta-analysis of all?. Crit Care Med 2007;35:2852-4.
- Drummond MF. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Neilson AR, Burchardi H, Schneider H. Cost-effectiveness of immunoglobulin M-enriched immunoglobulin (Pentaglobin) in the treatment of severe sepsis and septic shock. J Crit Care 2005;20:239-49.
- Manns BJ, Lee H, Doig CJ, Johnson D, Donaldson C. An economic evaluation of activated protein C treatment for severe sepsis. N Engl J Med 2002;347:993-1000.
- Angus DC, Linde-Zwirble WT, Clermont G, Ball DE, Basson BR, Ely EW, et al. Cost-effectiveness of drotrecogin alfa (activated) in the treatment of severe sepsis. Crit Care Med 2003;31:1-11.
- Betancourt M, McKinnon PS, Massanari RM, Kanji S, Bach D, Devlin JW. An evaluation of the cost effectiveness of drotrecogin alfa (Activated) relative to the number of organ system failures. PharmacoEconomics 2003;21:1331-40.
- Fowler RA, Hill-Popper M, Stasinos J, Petrou C, Sanders GD, Garber AM. Cost-effectiveness of recombinant human activated protein C and the influence of severity of illness in the treatment of patients with severe sepsis. J Crit Care 2003;18:181-91.
- Neilson AR, Burchardi H, Chinn C, Clouth J, Schneider H, Angus D. Cost-effectiveness of drotrecogin alfa (activated) for the treatment of severe sepsis in Germany. J Crit Care 2003;18:217-27.
- Sacristán JA, Prieto L, Huete T, Artigas A, Badia X, Chinn C, et al. [Cost-effectiveness of drotrecogin alpha [activated] in the treatment of severe sepsis in Spain]. Gac Sanit 2004;18:50-7.
- Davies A, Ridley S, Hutton J, Chinn C, Barber B, Angus DC. Cost effectiveness of drotrecogin alfa (activated) for the treatment of severe sepsis in the United Kingdom. Anaesthesia 2005;60:155-62.
- Hjelmgren J, Persson U, Tennvall GR. Local treatment pattern versus trial-based data: a cost-effectiveness analysis of drotrecogin alfa (activated) in the treatment of severe sepsis in Sweden. Am J Ther 2005;12:425-30.
- Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al. Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris) for the treatment of severe sepsis in adults: a systematic review and economic evaluation. Health Technol Assess 2005;9.
- Green C, Dinnes J, Takeda AL, Cuthbertson BH. Evaluation of the cost-effectiveness of drotrecogin alfa (activated) for the treatment of severe sepsis in the United Kingdom. Int J Technol Assess Health Care 2006;22:90-100.
- Franca LR, Launois R, Le Lay K, Aegerter P, Bouhassira M, Meshaka P, et al. Cost-effectiveness of drotrecogin alfa (activated) in the treatment of severe sepsis with multiple organ failure. Int J Technol Assess Health Care 2006;22:101-8.
- Dhainaut JF, Payet S, Vallet B, Franca L, Annane D, Bollaert PE, et al. Cost-effectiveness of activated protein C in real-life clinical practice. Crit Care 2007;11.
- Costa V, Brophy JM. Drotrecogin alfa (activated) in severe sepsis: a systematic review and new cost-effectiveness analysis. BMC Anaesthesiol 2007;7.
- Guidet B, Mosqueda GJ, Priol G, Aegerter P. The COASST study: cost-effectiveness of albumin in severe sepsis and septic shock. J Crit Care 2007;22:197-203.
- Huang DT, Clermont G, Dremsizov TT, Angus DC. Implementation of early goal-directed therapy for severe sepsis and septic shock: a decision analysis. Crit Care Med 2007;35:2090-100.
- Talmor D, Greenberg D, Howell MD, Lisbon A, Novack V, Shapiro N. The costs and cost-effectiveness of an integrated sepsis treatment protocol. Crit Care Med 2008;36:1168-74.
- Alejandria MM, Lansang MA, Dans LF, Mantaring JB. Intravenous immunoglobulin for treating sepsis and septic shock. Cochrane Database Syst Rev 2001;2.
- Briggs A, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- Bernard GR, Vincent JL, Laterre P, Larosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001;344:699-70.
- Quartin AA, Schein RMH, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival. JAMA 1997;277:1058-63.
- Wright JC, Plenderleith L, Ridley SA. Long-term survival following intensive care: subgroup analysis and comparison with the general population. Anaesthesia 2003;58:637-42.
- Angus DC, Laterre PF, Helterbrand J, Ely EW, Ball DE, Garg R, et al. The effect of drotrecogin alfa (activated) on long-term survival after severe sepsis. Crit Care Med 2004;32:2199-206.
- Drabinski A, Williams G, Formica C. Observational evaluation of health state utilities. Value Health 2001;4.
- Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1389-94.
- n.d. www.icapp.nhs.uk/docdat/ (accessed 18 August 2010).
- Harrison DA, Brady AR, Rowan K. Case mix, outcome and length of stay for admissions to adult, general critical-care units in England, Wales and Northern Ireland: the Intensive Care National Audit & Research Centre Case Mix Programme Database. Crit Care 2004;8:R99-111.
- Harrison DA, Parry GJ, Carpenter JR, Short A, Rowan K. A new risk prediction model for critical care: the Intensive Care National Audit & Research Centre (ICNARC) model. Crit Care Med 2007;35:1091-8.
- Williams TA, Dobb GJ, Finn JC, Webb SAR. Long-term survival from intensive care: a review. Intensive Care Med 2005;31:1306-15.
- Ghelani D, Moran JL, Sloggett A, Leeson RJ, Peake SL. Long-term survival of intensive care and hospital patient cohorts compared with the general Australian population: a relative survival approach. J Eval Clin Pract 2009;15:425-35.
- Karlsson S, Varpula M, Ruokonen E, Pettila V, Parviainen I, Ala-Kokko TI, et al. Incidence, treatment, and outcome of severe sepsis in ICU-treated adults in Finland: the Finnsepsis study. Intensive Care Med 2007;33:435-43.
- Varpula M, Karlsson S, Parviainen I, Ruokonen E. Community-acquired septic shock: early management and outcome in a nationwide study in Finland. Acta Anaesthesiol Scand 2007;51:1320-6.
- Weycker D, Akhras KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med 2003;31:2316-23.
- Leibovici L, Samra Z, Konigsberger H, Drucker M, Ashkenazi S, Pitlik SD. Long-term survival following bacteremia or fungemia. JAMA 1995;274:807-12.
- Perl TM, Dvorak LA, Hwang T, Wenzel RP. Long-term survival and function after suspected Gram-negative sepsis. JAMA 1995;274:338-45.
- Sasse KC, Nauenberg E, Long A, Anton B, Tucker HJ, Hu TW. Long-term survival after intensive-care unit admission with sepsis. Crit Care Med 1995;23:1040-7.
- Karlsson S, Ruokonen E, Varpula T, Ala-Kokko TI, Pettila V. Long-term outcome and quality-adjusted life years after severe sepsis. Crit Care Med 2009;37:1268-74.
- Granja C, Dias C, Costa-Pereira A, Sarmento A. Quality of life of survivors from severe sepsis and septic shock may be similar to that of others who survive critical illness. Critical Care 2004;8:R91-8.
- Korošec Jagodic H, Jagodic K, Podbregar M. Long-term outcome and quality of life of patients treated in surgical intensive care: a comparison between sepsis and trauma. Crit Care 2006;10.
- Williams A. Euroqol - a new facility for the measurement of health-related quality-of-life. Health Policy 1990;16:199-208.
- National Institute for Health and Clinical Excellence . Guidance to the Methods of Technology Appraisal 2008.
- R Development Core Team . The R Project for Statistical Computing 2010. www.R-project.org (accessed 17 October 2011).
- Briggs AH, Goeree R, Blackhouse G, O’Brien BJ. Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Making 2002;22:290-308.
- Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S. A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme. Health Technol Assess 2004;8.
- Ades AE, Lu G, Claxton K. Expected value of sample information calculations in medical decision modeling. Med Decis Making 2004;24:207-27.
- Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ 1996;5:513-24.
- Cuthbertson BH, Roughton S, Jenkinson D, Maclennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care 2010;14.
- British Medical Association amd Royal Pharmaceutical Society of Great Britain . British National Formulary 2009.
- Department of Health . NHS Reference Costs 2007 08 n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_098945 (accessed 17 October 2011).
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87.
Appendix 1 Questionnaire for the survey of the management of severe sepsis in UK critical-care units
Appendix 2 Search strategies
Randomised controlled trials
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations
EMBASE
2001 onwards
Human only
MEDLINE
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) < 1950 to present >
Searched via Ovid interface: 2 October 2009
-
immunoglobulins/ (37,225)
-
immunoglobulin$.tw. (105,959)
-
ivig.tw. (3259)
-
1 or 2 or 3 (125,909)
-
sepsis/ (34,958)
-
sepsis.tw. (48,066)
-
septic shock/ (15,622)
-
septic shock.tw. (10,633)
-
septicemia/ (34,958)
-
septicaemia.tw. (4736)
-
septicemia.tw. (9296)
-
5 or 6 or 7 or 8 or 9 or 10 or 11 (91,020)
-
4 and 12 (1290)
-
randomized controlled trial.pt. (283,692)
-
controlled clinical trial.pt. (80,983)
-
randomized.ab. (201,142)
-
placebo.ab. (120,675)
-
drug therapy.fs. (1,358,908)
-
randomly.ab. (148,294)
-
trial.ab. (208,511)
-
groups.ab. (997,172)
-
14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (2,559,489)
-
exp animals/not humans.sh. (3,473,795)
-
22 not 23 (2,179,816)
-
13 and 24 (388)
-
limit 25 to yr=“2001 -Current” (160)
EMBASE
Database: EMBASE < 1996 to 2009 week 39 >
Searched via Ovid interface: 2 October 2009
-
immunoglobulins/ (28,604)
-
immunoglobulin$.tw. (40,750)
-
ivig.tw. (2552)
-
1 or 2 or 3 (58,658)
-
sepsis/ (31,978)
-
sepsis.tw. (26,319)
-
septic shock/ (10,171)
-
septic shock.tw. (6530)
-
septicemia/ (5658)
-
septicaemia.tw. (1560)
-
septicemia.tw. (2717)
-
5 or 6 or 7 or 8 or 9 or 10 or 11 (53,621)
-
4 and 12 (1367)
-
random.tw. (61,491)
-
placebo.mp. (123,372)
-
double-blind.tw. (47,097)
-
14 or 15 or 16 (196,237)
-
17 and 13 (111)
-
limit 18 to yr=“2001 -Current” (94)
-
animals/not (animals/and humans/) (2161)
-
19 not 20 (95)
Cost-effectiveness studies (for intravenous immunoglobulin and sepsis, and for all sepsis)
NHS EED
No date or language restrictions
NHS Economic Evaluation Database
Searched via The Cochrane Library (www.mrw.interscience.wiley.com/cochrane/cochrane_search_fs.html): 2 October 2009
#1 MeSH descriptor Immunoglobulins, this term only
#2 (immunoglobulin*)
#3 (ivig)
#4 (#1 OR #2 OR #3)
#5 MeSH descriptor Sepsis, this term only
#6 (sepsis)
#7 MeSH descriptor Shock, Septic, this term only
#8 (septic shock)
#9 MeSH descriptor Hemorrhagic Septicemia, this term only
#10 (septicaemia)
#11 (septicemia)
#12 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11)
#13 (#4 AND #12)
Results for all sepsis (line #12) and for sepsis and IVIG (line #13) saved.
Long-term prognostic studies (for life expectancy estimates)
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations
2004 onwards
English-language only
Human only
MEDLINE
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) < 1950 to present >
Searched via Ovid interface: 20 October 2009
-
sepsis/ (35,031)
-
sepsis.tw. (48,315)
-
shock, septic/ (15,639)
-
septic shock.tw. (10,675)
-
septicaemia.tw. (4752)
-
septicemia.tw. (9308)
-
1 or 2 or 3 or 4 or 5 or 6 (91,334)
-
prognos$.tw. (269,223)
-
first episode.tw. (5305)
-
cohort.tw. (141,155)
-
8 or 9 or 10 (405,343)
-
7 and 11 (5309)
-
limit 12 to yr=“2004 -Current” (2154)
-
exp animals/not humans/ (3,478,640)
-
13 not 14 (2097)
Quality of life studies (for utility studies)
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations
EMBASE
2004 onwards
English-language only
Human only
MEDLINE
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) < 1950 to present >
Searched via Ovid interface: 20 October 2009
-
sepsis/ (35,031)
-
sepsis.tw. (48,315)
-
shock, septic/ (15,639)
-
septic shock.tw. (10,675)
-
septicaemia.tw. (4752)
-
septicemia.tw. (9308)
-
1 or 2 or 3 or 4 or 5 or 6 (91,334)
-
quality adjusted life year/ (4132)
-
quality adjusted life.tw. (3524)
-
(qaly$or qald$or qale$or qtime$).tw. (2923)
-
disability adjusted life.tw. (694)
-
daly$.tw. (734)
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. (1020)
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. (1451)
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. (20)
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. (300)
-
(euroqol or euro qol or eq5d or eq 5d).tw. (1875)
-
(hql or hqol or h qol or hrqol or hr qol).tw. (4200)
-
(hye or hyes).tw. (50)
-
health$year$equivalent$.tw. (37)
-
health utilit$.tw. (660)
-
(hui or hui1 or hui2 or hui3).tw. (610)
-
disutili$.tw. (126)
-
rosser.tw. (63)
-
quality of wellbeing.tw. (3)
-
quality of well being.tw. (248)
-
qwb.tw. (137)
-
willingness to pay.tw. (1286)
-
standard gamble$.tw. (564)
-
time trade off.tw. (509)
-
time tradeoff.tw. (178)
-
tto.tw. (384)
-
or/8-32 (17,318)
-
7 and 33 (52)
-
limit 34 to yr=“2004 -Current” (32)
-
limit 35 to english language (32)
-
exp animals/not humans/ (3,478,640)
-
36 not 37 (32)
EMBASE
Database: EMBASE < 1980 to 2009 week 42 >
Searched via Ovid interface: 20 October 2009
-
sepsis/ (43,984)
-
sepsis.tw. (40,106)
-
septic shock/ (14,047)
-
septic shock.tw. (9145)
-
septicemia/ (9193)
-
septicaemia.tw. (3523)
-
septicemia.tw. (6151)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 (81,698)
-
quality adjusted life year/ (4481)
-
quality adjusted life.tw. (3029)
-
(qaly$or qald$or qale$or qtime$).tw. (2491)
-
disability adjusted life.tw. (503)
-
daly$.tw. (529)
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. (880)
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. (1141)
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. (14)
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. (198)
-
(euroqol or euro qol or eq5d or eq 5d).tw. (1594)
-
(hql or hqol or h qol or hrqol or hr qol).tw. (3519)
-
(hye or hyes).tw. (32)
-
health$year$equivalent$.tw. (28)
-
health utilit$.tw. (566)
-
(hui or hui1 or hui2 or hui3).tw. (451)
-
disutili$.tw. (101)
-
rosser.tw. (55)
-
quality of wellbeing.tw. (5)
-
quality of well being.tw. (206)
-
qwb.tw. (119)
-
willingness to pay.tw. (1108)
-
standard gamble$.tw. (478)
-
time trade off.tw. (452)
-
time tradeoff.tw. (155)
-
tto.tw. (353)
-
or/9-33 (15,078)
-
8 and 34 (90)
-
limit 35 to yr=“2004 -Current” (73)
-
limit 36 to english language (72)
-
exp animals/not humans/ (14,494)
-
37 not 38 (72)
Appendix 3 Quality assessment of published cost-effectiveness evidence for intravenous immunoglobulin
| Item | Question | Response | Comment |
|---|---|---|---|
| 1 | Was a well-defined question posed in answerable form? | Yes | The aim was to compare the cost-effectiveness of Pentaglobin with standard therapy in adult patients treated for sepsis and septic shock in Germany |
| 2 | Was a comprehensive description of the competing alternatives given (i.e. can you tell who did what to whom, where and how often)? | No | The approach of standard care for sepsis patients was not described |
| 3 | Was the effectiveness of the programme or services established? | Yes | A previously published review was updated. The results of the nine identified RCTs were pooled with meta-analysis |
| 4 | Were all the important and relevant costs and consequences for each alternative identified? | No | The study was conducted from the hospital perspective and considered only the costs and consequences of the critical-care unit stay. However, episode of severe sepsis is likely to impact patient’s health and resource use after the initial critical-care unit stay |
| 5 | Were costs and consequences measured accurately in appropriate physical units (e.g. hours of nursing time, number of physician visits, lost work-days, gained life-years)? | ? | Critical-care unit resource use (measured in length of stay) was multiplied by mean daily unit costs of critical care (basic critical care + hotel costs + personnel). The cost of ‘block’ therapies (sepsis, blood, ventilation, renal) was added to the critical care costs. The costs were weighted averages of surgical and nonsurgical patients. The length of stay and unit costs were assumed to be different for survivors and non-survivors |
| 6 | Were the cost and consequences valued credibly? | Yes | The critical-care unit resource use and unit cost were based on a German severe sepsis costing study |
| 7 | Were costs and consequences adjusted for differential timing? | No | The time horizon was the critical-care unit inpatient episode. Since time horizon was < 1 year, discounting was not needed |
| 8 | Was an incremental analysis of costs and consequences of alternatives performed? | Yes | Cost-effectiveness was measured in incremental cost per life saved |
| 9 | Was allowance made for uncertainty in the estimates of costs and consequences? | Yes | Parameter uncertainty was addressed using univariate sensitivity analysis and probabilistic sensitivity analysis |
| 10 | Did the presentation and discussion of study results include all issues of concern to users? | No |
The ICER was compared with cost-effectiveness of various health-care interventions in Germany The generalisability of the results to other settings or patient groups was not discussed The study did not discuss the feasibility of Pentaglobin in the treatment of severe sepsis in Germany |
Appendix 4 Critical-care unit and hospital mortality data from the Intensive Care National Audit & Research Centre Case Mix Programme Database
| Characteristic | Critical-care unit mortality | Hospital mortality | ||
|---|---|---|---|---|
| Frequency, n (%) | Mortality, % (95% CI) | Frequency, n (%) | Mortality, % (95% CI) | |
| All admissions | 26,249 (100) | 29.1 (28.6 to 29.7) | 25,991 (100) | 40.6 (40.0 to 41.2) |
| Quartiles of age (years) | ||||
| 0–53 | 6731 (25.6) | 17.3 (16.4 to 18.2) | 6645 (25.6) | 22.9 (21.9 to 23.9) |
| 54–66 | 6746 (25.7) | 27.8 (26.7 to 28.9) | 6675 (25.7) | 37.2 (36.1 to 38.4) |
| 67–76 | 6764 (25.8) | 33.4 (32.3 to 34.5) | 6698 (25.8) | 46.8 (45.7 to 48.0) |
| 77–100 | 6008 (22.9) | 38.9 (37.7 to 40.2) | 5973 (23.0) | 56.9 (55.6 to 58.1) |
| Gender | ||||
| Female | 12,257 (46.7) | 27.8 (27.0 to 28.6) | 12,142 (46.7) | 38.9 (38.0 to 39.8) |
| Male | 13,992 (53.3) | 30.2 (29.5 to 31.0) | 13,849 (53.3) | 42.0 (41.2 to 42.9) |
| Number of ODs | ||||
| One | 5262 (20.0) | 10.6 (9.8 to 11.5) | 5211 (20.0) | 21.5 (20.4 to 22.6) |
| Two | 9128 (34.8) | 19.0 (18.2 to 19.8) | 9036 (34.8) | 31.3 (30.3 to 32.2) |
| Three | 7020 (26.7) | 33.7 (32.6 to 34.9) | 6943 (26.7) | 46.0 (44.8 to 47.1) |
| Four | 3969 (15.1) | 59.1 (57.5 to 60.6) | 3939 (15.2) | 69.0 (67.6 to 70.5) |
| Five | 870 (3.3) | 73.2 (70.2 to 76.1) | 862 (3.3) | 80.2 (77.4 to 82.7) |
| Two or more ODs | ||||
| No | 5262 (20.0) | 10.6 (9.8, 11.5) | 5211 (20.0) | 21.5 (20.4 to 22.6) |
| Yes | 20,987 (80.0) | 33.7 (33.1, 34.4) | 20,780 (80.0) | 45.4 (44.7 to 46.0) |
| Three or more ODs | ||||
| No | 14,390 (54.8) | 15.9 (15.3 to 16.5) | 14,247 (54.8) | 27.7 (26.9 to 28.4) |
| Yes | 11,859 (45.2) | 45.1 (44.2 to 46.0) | 11,744 (45.2) | 56.2 (55.3 to 57.1) |
| Four or more ODs | ||||
| No | 21,410 (81.6) | 21.8 (21.2 to 22.3) | 21,190 (81.5) | 33.7 (33.0 to 34.3) |
| Yes | 4839 (18.4) | 61.6 (60.2 to 63.0) | 4801 (18.5) | 71.0 (69.7 to 72.3) |
| Cardiovascular OD | ||||
| No | 4907 (18.7) | 18.5 (17.4 to 19.6) | 4859 (18.7) | 29.9 (28.6 to 31.2) |
| Yes | 21,342 (81.3) | 31.5 (30.9 to 32.2) | 21,132 (81.3) | 43.0 (42.4 to 43.7) |
| Renal OD | ||||
| No | 19,326 (73.6) | 20.4 (19.8 to 20.9) | 19,121 (73.6) | 31.6 (30.9 to 32.2) |
| Yes | 6923 (26.4) | 53.5 (52.3 to 54.7) | 6870 (26.4) | 65.6 (64.5 to 66.8) |
| Cardiovascular and renal ODs | ||||
| No | 20,212 (77.0) | 20.9 (20.3 to 21.4) | 19,995 (76.9) | 32.2 (31.6 to 32.9) |
| Yes | 6037 (23.0) | 56.7 (55.5 to 58.0) | 5996 (23.1) | 68.4 (67.2 to 69.5) |
| Quartiles of APACHE II score | ||||
| 1–15 | 7371 (28.1) | 9.0 (8.4 to 9.7) | 7292 (28.1) | 16.3 (15.5 to 17.2) |
| 16–19 | 5978 (22.8) | 20.4 (19.4 to 21.4) | 5919 (22.8) | 33.4 (32.2 to 34.6) |
| 20–24 | 6242 (23.8) | 32.4 (31.3 to 33.6) | 6174 (23.8) | 47.7 (46.4 to 48.9) |
| 25–52 | 5787 (22.0) | 54.1 (52.8 to 55.4) | 5738 (22.1) | 66.2 (64.9 to 67.4) |
| APACHE II score ≥ 25 | ||||
| No | 19,591 (74.6) | 19.9 (19.4 to 20.5) | 19,385 (74.6) | 31.5 (30.9 to 32.2) |
| Yes | 5787 (22.0) | 54.1 (52.8 to 55.4) | 5738 (22.1) | 66.2 (64.9 to 67.4) |
| Quartiles of ICNARC physiology score | ||||
| 1–16 | 6710 (25.6) | 6.4 (5.9 to 7.1) | 6671 (25.7) | 15.0 (14.1 to 15.9) |
| 17–22 | 6656 (25.4) | 17.1 (16.2 to 18.0) | 6584 (25.3) | 30.4 (29.3 to 31.5) |
| 23–29 | 6506 (24.8) | 31.4 (30.3 to 32.5) | 6422 (24.7) | 46.4 (45.2 to 47.7) |
| 30–75 | 6377 (24.3) | 63.2 (62.0 to 64.4) | 6314 (24.3) | 72.3 (71.2 to 73.4) |
| Septic shock | ||||
| No | 4395 (16.7) | 16.8 (15.7 to 17.9) | 4354 (16.8) | 28.2 (26.9 to 29.6) |
| Yes | 21,854 (83.3) | 31.6 (31.0 to 32.2) | 21,637 (83.2) | 43.1 (42.4 to 43.7) |
| CPR within 24 hours prior to admission | ||||
| No | 25,320 (96.5) | 28.0 (27.5 to 28.6) | 25,068 (96.4) | 39.5 (38.9 to 40.1) |
| Yes | 929 (3.5) | 58.5 (55.3 to 61.6) | 923 (3.6) | 70.7 (67.7 to 73.6) |
| Source of admission | ||||
| A&E or other hospital | 4632 (17.6) | 29.8 (28.5 to 31.1) | 4580 (17.6) | 38.5 (37.1 to 39.9) |
| Clinic or home | 135 (0.5) | 29.6 (22.6 to 37.8) | 132 (0.5) | 34.8 (27.3 to 43.3) |
| Critical-care unit | 2095 (8.0) | 31.1 (29.1 to 33.1) | 2043 (7.9) | 44.6 (42.4 to 46.8) |
| Theatre – elective/scheduled | 1133 (4.3) | 11.7 (10.0 to 13.7) | 1132 (4.4) | 20.8 (18.5 to 23.2) |
| Theatre – emergency/urgent | 5858 (22.3) | 20.3 (19.3 to 21.4) | 5812 (22.4) | 32.5 (31.3 to 33.7) |
| Ward or other intermediate area | 12,396 (47.2) | 34.2 (33.4 to 35.1) | 12,292 (47.3) | 46.4 (45.5 to 47.3) |
Appendix 5 Technical description of decision model and methods for the expected value of sample information
Appendix 6 Logistic regression results and predictions for short-term mortality
Data from the ICNARC CMP Database (2007–9; n = 26,249) were used to inform the baseline risk of mortality applied to standard care during the initial hospitalisation. Although the overall mortality observed in these data was used to represent the overall severe sepsis and septic shock population, regression analyses were used in exploring subgroups. Variation in the baseline risk of mortality was explored for a range of separate subgroups, defined by (A) age and gender; (B) components of the ICNARC model [age, ICNARC physiology score, source of admission, cardiopulmonary resuscitation (CPR) within 24 hours prior to admission]; (C) APACHE II score; and (D) age, number of organ dysfunctions, cardiovascular organ dysfunction, renal organ dysfunction and the combination of cardiovascular and renal organ dysfunction.
For the subgroup analyses, estimates of the baseline probability of mortality were obtained by conditioning on specific patient or severity of illness characteristics (at presentation). Separate logistic regression models were used and are described in Table 28. All models were fitted with robust (Huber–White) SEs adjusted for clustering on critical-care unit. The full results of each of the models implemented are reported next (Tables 42–45 and Figures 48–50). Alongside the results of the logistic regressions, the predicted probability of death was plotted for a range of values of the characteristics of interest (other variables were set at their mean values).
| Subgroup analysis A | Critical-care unit mortality | Hospital mortality | ||
|---|---|---|---|---|
| Coefficient (SE) | 95% CI | Coefficient (SE) | 95% CI | |
| Agea | 0.026 (0.001) | 0.024 to 0.028 | 0.035 (0.001) | 0.032 to 0.037 |
| Male | 0.118 (0.029) | 0.060 to 0.175 | 0.133 (0.027) | 0.080 to 0.186 |
| Constant | –2.633 (0.082) | –2.793 to –2.473 | –2.660 (0.088) | –2.836 to –2.490 |
| Subgroup analysis B | Critical-care unit mortality | Hospital mortality | ||
|---|---|---|---|---|
| Coefficient (SE) | 95% CI | Coefficient (SE) | 95% CI | |
| Agea | 0.025 (0.001) | 0.023 to 0.028 | 0.035 (0.001) | 0.032 to 0.037 |
| IMscoreb | 0.133 (0.002) | 0.128 to 0.138 | 0.112 (0.002) | 0.108 to 0.117 |
| IMsource2c | –0.128 (0.205) | –0.531 to 0.274 | –0.317 (0.213) | –0.734 to 0.100 |
| IMsource3c | 0.131 (0.068) | –0.002 to 0.264 | 0.355 (0.069) | 0.219 to 0.490 |
| IMsource4c | –0.481 (0.091) | –0.659 to –0.303 | –0.311 (0.084) | –0.476 to –0.147 |
| IMsource5c | –0.230 (0.055) | –0.337 to –0.122 | –0.059 (0.05) | –0.157 to 0.039 |
| IMsource6c | 0.403 (0.045) | 0.314 to 0.491 | 0.475 (0.042) | 0.393 to 0.557 |
| CPRd | 0.460 (0.083) | 0.298 to 0.621 | 0.636 (0.086) | 0.467 to 0.804 |
| Constant | –6.034 (0.114) | –6.257 to –5.811 | –5.545 (0.109) | –5.759 to –5.331 |
| Subgroup analysis C | Critical-care unit mortality | Hospital mortality | ||
|---|---|---|---|---|
| Coefficient (SE) | 95% CI | Coefficient (SE) | 95% CI | |
| AP2scorea | 0.140 (0.003) | 0.134 to 0.147 | 0.136 (0.003) | 0.130 to 0.141 |
| Constant | –3.894 (0.072) | –4.036 to –3.752 | –3.165 (0.059) | –3.281 to –3.050 |
| Subgroup analysis D | Critical-care unit mortality | Hospital mortality | ||
|---|---|---|---|---|
| Coefficient (SE) | 95% CI | Coefficient (SE) | 95% CI | |
| Agea | 0.023 (0.001) | 0.021 to 0.026 | 0.033 (0.001) | 0.030 to 0.035 |
| orgdys2b | 0.747 (0.056) | 0.638 to 0.856 | 0.504 (0.044) | 0.419 to 0.590 |
| orgdys3b | 1.454 (0.061) | 1.335 to 1.572 | 1.041 (0.047) | 0.949 to 1.132 |
| orgdys4b | 2.233 (0.080) | 2.076 to 2.390 | 1.712 (0.070) | 1.575 to 1.848 |
| orgdys5b | 2.880 (0.101) | 2.682 to 3.078 | 2.344 (0.117) | 2.115 to 2.574 |
| ODcardioc | –0.362 (0.065) | –0.491 to –0.234 | –0.226 (0.052) | –0.327 to –0.124 |
| ODrenald | 0.097 (0.102) | –0.102 to 0.296 | 0.323 (0.090) | 0.146 to 0.500 |
| ODcke | 0.403 (0.111) | 0.185 to 0.620 | 0.247 (0.101) | 0.049 to 0.446 |
| Constant | –3.432 (0.094) | –3.617 to –3.248 | –3.256 (0.089) | –3.431 to –3.081 |
FIGURE 48.
Predicted probability of dying in hospital by age at admission (subgroup analysis A: age at admission and gender).
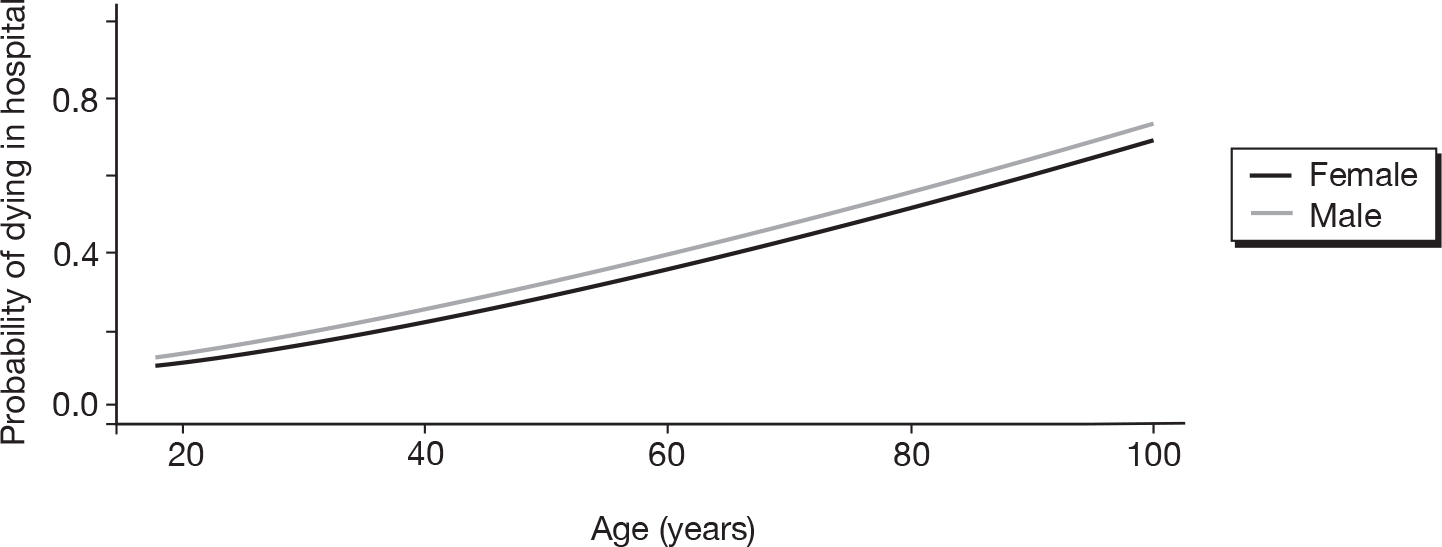
FIGURE 49.
Predicted probability of dying in hospital by ICNARC physiology score at admission (subgroup analysis B: components of the ICNARC model).
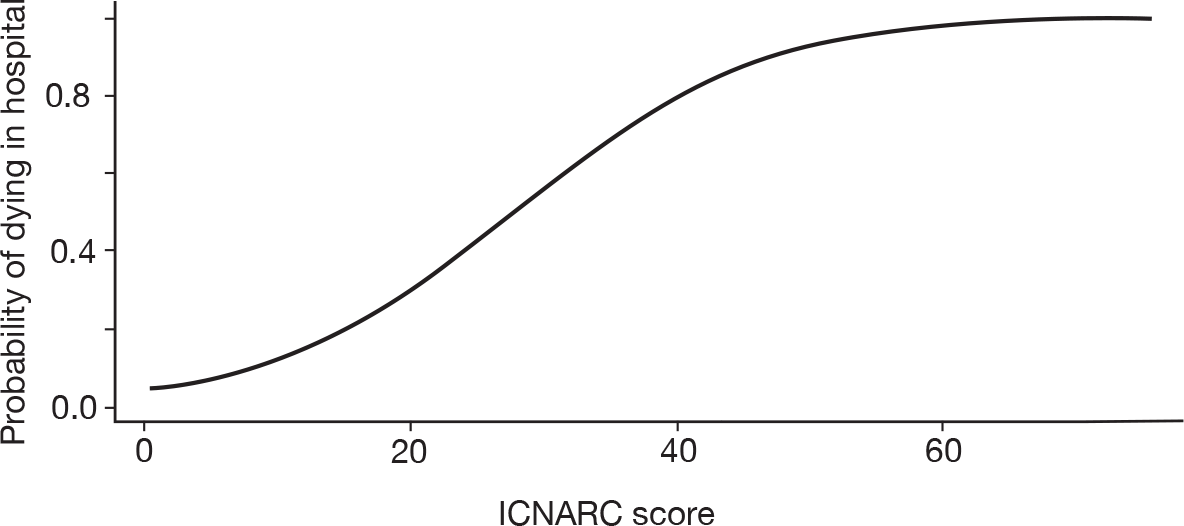
FIGURE 50.
Predicted probability of dying in hospital by APACHE II score at admission (subgroup analysis C: APACHE II score).
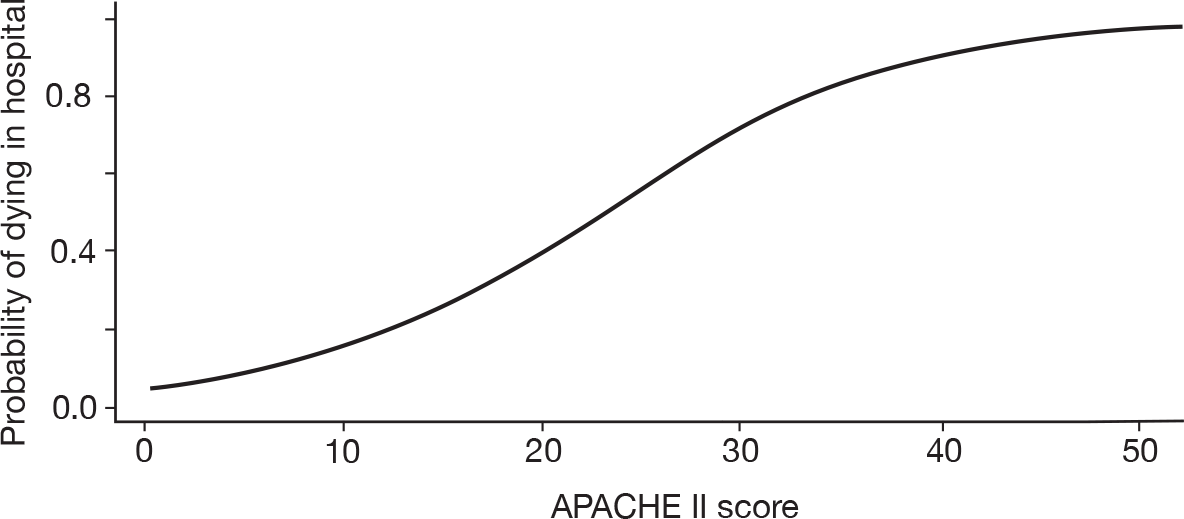
Appendix 7 Results of parametric survival models for long-term survival
| Distribution | Age model (model i) | APACHE II model (model ii) | OD model (model iii) |
|---|---|---|---|
| Exponential | 949.7 | 951.3 | 952.5 |
| Weibull | 932.8 | 934.4 | 936.2 |
| Log-normal | 934.9 | 937.3 | 938.4 |
| Coefficient (SE) | Age model (model i) | APACHE II model (model ii) | OD model (model iii) |
|---|---|---|---|
| Intercept | 10.6 (1.05)a | 9.91 (0.795)a | 10.59 (1.133)a |
| Age at admission | –0.029 (0.017)a | –0.029 (0.017)a | |
| APACHE II score | –0.041 (0.032)a | ||
| Two or more ODs | 0.328 (0.674) | ||
| Renal OD | 0.640 (0.906) | ||
| CV OD | –0.230 (0.724) | ||
| CV and renal OD | –1.537 (1.093) | ||
| Log(scale) | 0.527 (0.133)a | 0.526 (0.133)a | 0.516 (0.132)a |
Appendix 8 Length of stay in the critical-care unit and in hospital from the ICNARC Case Mix Programme Database
| Subgroup | Critical-care unita | Hospitalisation | ||||
|---|---|---|---|---|---|---|
| n | Mean (SE) | Median (IQR) | n | Mean (SE) | Median (IQR) | |
| All admissions | ||||||
| All | 25,990 | 8.04 (0.067) | 4.25 (1.82–9.79) | 25,749 | 31.79 (0.233) | 20 (10–40) |
| Survivors | 15,446 | 8.48 (0.086) | 4.80 (2.22–10.24) | 15,215 | 39.07 (0.325) | 27 (15–49) |
| Non-survivors | 10,544 | 7.40 (0.108) | 3.42 (1.15–9.04) | 10,534 | 21.29 (0.292) | 12 (5–26) |
| Quartiles of age | ||||||
| 0–53 | ||||||
| All | 6645 | 8.12 (0.143) | 4.13 (1.82–9.98) | 6525 | 31.71 (0.502) | 20 (10–38) |
| Survivors | 5122 | 8.17 (0.158) | 4.33 (1.95–9.99) | 5006 | 34.48 (0.593) | 22 (12–41) |
| Non-survivors | 1523 | 7.97 (0.331) | 3.34 (1.18–9.90) | 1519 | 22.56 (0.868) | 12 (4–27) |
| 54–66 | ||||||
| All | 6675 | 8.85 (0.139) | 4.92 (1.98–11.23) | 6612 | 33.86 (0.469) | 22 (11–43) |
| Survivors | 4189 | 9.28 (0.170) | 5.38 (2.57–11.72) | 4129 | 40.67 (0.625) | 28 (16–51) |
| Non-survivors | 2486 | 8.11 (0.237) | 3.80 (1.16–10.71) | 2483 | 22.54 (0.633) | 12 (5–27) |
| 67–76 | ||||||
| All | 6698 | 8.56 (0.137) | 4.52 (1.89–10.50) | 6658 | 33.5 (0.477) | 22 (10–43) |
| Survivors | 3560 | 8.96 (0.181) | 4.97 (2.49–10.90) | 3523 | 42.73 (0.685) | 30 (17–54) |
| Non-survivors | 3138 | 8.10 (0.208) | 3.89 (1.19–10.05) | 3135 | 23.12 (0.608) | 12 (5–28) |
| 77–100 | ||||||
| All | 5972 | 6.45 (0.112) | 3.68 (1.62–7.75) | 5954 | 27.68 (0.386) | 19 (9–36) |
| Survivors | 2575 | 7.10 (0.169) | 4.27 (2.22–8.13) | 2557 | 40.4 (0.678) | 30 (19–50) |
| Non-survivors | 3397 | 5.96 (0.148) | 2.96 (1.11–7.32) | 3397 | 18.11 (0.369) | 11 (5–23) |
| Sex | ||||||
| Female | ||||||
| All | 12,141 | 7.66 (0.093) | 4.03 (1.79–9.14) | 12,032 | 31.51 (0.339) | 20 (10–39) |
| Survivors | 7418 | 8.08 (0.116) | 4.59 (2.15–9.71) | 7315 | 38.61 (0.47) | 26 (15–47) |
| Non-survivors | 4723 | 7 (0.155) | 3.08 (1.10–8.21) | 4717 | 20.5 (0.417) | 11 (4–25) |
| Male | ||||||
| All | 13,849 | 8.37 (0.097) | 4.52 (1.86–10.29) | 13,717 | 32.04 (0.32) | 21 (10–40) |
| Survivors | 8028 | 8.84 (0.127) | 4.93 (2.31–10.77) | 7900 | 39.49 (0.45) | 27 (15–50) |
| Non-survivors | 5821 | 7.72 (0.149) | 3.73 (1.21–9.73) | 5817 | 21.93 (0.406) | 12 (5–26) |
| Number of organ dysfunctions | ||||||
| One | ||||||
| All | 5210 | 6.21 (0.123) | 3.29 (1.69–6.96) | 5144 | 34.11 (0.577) | 21 (12–41) |
| Survivors | 4093 | 5.53 (0.115) | 3.08 (1.60–6.11) | 4028 | 34.85 (0.652) | 22 (13–42) |
| Non-survivors | 1117 | 8.71 (0.376) | 4.76 (2.09–10.38) | 1116 | 31.42 (1.241) | 19 (10–38) |
| Two | ||||||
| All | 9036 | 8.35 (0.115) | 4.80 (2.22–9.93) | 8938 | 34.41 (0.398) | 23 (12–42) |
| Survivors | 6211 | 8.06 (0.136) | 4.61 (2.20–9.33) | 6117 | 38.09 (0.503) | 26 (15–47) |
| Non-survivors | 2825 | 9 (0.214) | 5.26 (2.30–11.22) | 2821 | 26.43 (0.608) | 16 (8–32) |
| Three | ||||||
| All | 6943 | 9.11 (0.138) | 5.13 (2.11–11.89) | 6876 | 31.26 (0.42) | 21 (10–40) |
| Survivors | 3751 | 10.24 (0.184) | 6.24 (3.00–13.51) | 3688 | 40.49 (0.621) | 29 (17–51) |
| Non-survivors | 3192 | 7.79 (0.205) | 3.74 (1.35–9.79) | 3188 | 20.58 (0.488) | 12 (5–25) |
| Four | ||||||
| All | 3939 | 8.1 (0.187) | 3.63 (1.00–10.29) | 3930 | 26.05 (0.557) | 13 (4–34) |
| Survivors | 1220 | 13.79 (0.394) | 9.28 (4.81–17.83) | 1212 | 51.02 (1.221) | 39 (23–64) |
| Non-survivors | 2719 | 5.55 (0.186) | 1.76 (0.72–6.25) | 2718 | 14.92 (0.452) | 7 (3–18) |
| Five | ||||||
| All | 862 | 6.77 (0.352) | 1.89 (0.77–8.85) | 861 | 21.18 (1.134) | 8 (3–27) |
| Survivors | 171 | 17.35 (1.04) | 12.68 (7.53–23.96) | 170 | 57.63 (3.487) | 45 (27–72) |
| Non-survivors | 691 | 4.16 (0.277) | 1.23 (0.65–3.80) | 691 | 12.22 (0.822) | 5 (3–14) |
| Two or more organ dysfunctions | ||||||
| No | ||||||
| All | 5210 | 6.21 (0.123) | 3.29 (1.69–6.96) | 5144 | 34.11 (0.577) | 21 (12–41) |
| Survivors | 4093 | 5.53 (0.115) | 3.08 (1.60–6.11) | 4028 | 34.85 (0.652) | 22 (13–42) |
| Non-survivors | 1117 | 8.71 (0.376) | 4.76 (2.09–10.38) | 1116 | 31.42 (1.241) | 19 (10–38) |
| Yes | ||||||
| All | 20,780 | 8.5 (0.078) | 4.66 (1.88–10.64) | 20,605 | 31.21 (0.252) | 20 (9–40) |
| Survivors | 11,353 | 9.54 (0.108) | 5.61 (2.74–11.90) | 11,187 | 40.58 (0.374) | 28 (16–51) |
| Non-survivors | 9427 | 7.24 (0.112) | 3.23 (1.09–8.92) | 9418 | 20.09 (0.289) | 11 (4–25) |
| Three or more organ dysfunctions | ||||||
| No | ||||||
| All | 14,246 | 7.57 (0.086) | 4.12 (1.96–8.86) | 14,082 | 34.3 (0.329) | 22 (12–42) |
| Survivors | 10,304 | 7.06 (0.095) | 3.91 (1.92–8.07) | 10,145 | 36.81 (0.399) | 24 (14–45) |
| Non-survivors | 3942 | 8.92 (0.187) | 5.09 (2.22–11.00) | 3937 | 27.85 (0.561) | 17 (9–33) |
| Yes | ||||||
| All | 11,744 | 8.6 (0.106) | 4.49 (1.55–11.15) | 11,667 | 28.76 (0.323) | 18 (7–37) |
| Survivors | 5142 | 11.32 (0.169) | 7.21 (3.49–14.87) | 5070 | 43.59 (0.555) | 32 (18–55) |
| Non-survivors | 6602 | 6.49 (0.13) | 2.41 (0.89–7.81) | 6597 | 17.37 (0.315) | 9 (4–21) |
| Four or more organ dysfunctions | ||||||
| No | ||||||
| All | 21,189 | 8.08 (0.074) | 4.45 (2.00–9.74) | 20,958 | 33.3 (0.261) | 22 (12–41) |
| Survivors | 14,055 | 7.91 (0.086) | 4.40 (2.09–9.32) | 13,833 | 37.79 (0.337) | 26 (15–47) |
| Non-survivors | 7134 | 8.41 (0.138) | 4.54 (1.79–10.48) | 7125 | 24.6 (0.381) | 15 (7–30) |
| Yes | ||||||
| All | 4801 | 7.86 (0.166) | 3.22 (0.94–10.06) | 4791 | 25.18 (0.501) | 13 (4–32) |
| Survivors | 1391 | 14.22 (0.37) | 9.83 (5.08–18.59) | 1382 | 51.84 (1.154) | 40 (23–65) |
| Non-survivors | 3410 | 5.27 (0.159) | 1.63 (0.71–5.79) | 3409 | 14.37 (0.397) | 7 (3–17) |
| Cardiovascular organ dysfunction | ||||||
| No | ||||||
| All | 4858 | 7.71 (0.145) | 4.26 (1.98–9.12) | 4809 | 32.37 (0.514) | 21 (12–40) |
| Survivors | 3407 | 7.19 (0.157) | 4.05 (1.95–8.47) | 3360 | 35.16 (0.609) | 24 (14–43) |
| Non-survivors | 1451 | 8.93 (0.317) | 4.79 (2.07–11.65) | 1449 | 25.88 (0.937) | 16 (7–31) |
| Yes | ||||||
| All | 21,132 | 8.11 (0.076) | 4.25 (1.79–9.90) | 20,940 | 31.66 (0.261) | 20 (10–40) |
| Survivors | 12,039 | 8.84 (0.101) | 4.96 (2.36–10.86) | 11,855 | 40.17 (0.379) | 28 (15–50) |
| Non-survivors | 9093 | 7.15 (0.114) | 3.15 (1.07–8.76) | 9085 | 20.55 (0.303) | 11 (4–25) |
| Renal organ dysfunction | ||||||
| No | ||||||
| All | 19,120 | 8.24 (0.078) | 4.64 (2.10–9.91) | 18,899 | 33.43 (0.276) | 22 (12–41) |
| Survivors | 13,086 | 8.00 (0.090) | 4.47 (2.13–9.46) | 12,873 | 37.49 (0.351) | 25 (14–46) |
| Non-survivors | 6034 | 8.78 (0.152) | 4.97 (2.02–10.98) | 6026 | 24.74 (0.408) | 15 (7–30) |
| Yes | ||||||
| All | 6870 | 7.47 (0.134) | 3.14 (0.97–9.39) | 6850 | 27.29 (0.427) | 15 (5–35) |
| Survivors | 2360 | 11.14 (0.259) | 6.95 (3.09–14.67) | 2342 | 47.73 (0.838) | 36 (20–61) |
| Non-survivors | 4510 | 5.54 (0.144) | 1.79 (0.72–6.11) | 4508 | 16.67 (0.399) | 8 (3–20) |
| Cardiovascular and renal organ dysfunctions | ||||||
| No | ||||||
| All | 19,994 | 8.2 (0.076) | 4.59 (2.06–9.88) | 19,772 | 33.36 (0.269) | 22 (12–41) |
| Survivors | 13,550 | 7.98 (0.088) | 4.46 (2.11–9.46) | 13,336 | 37.59 (0.345) | 25 (15–46) |
| Non-survivors | 6444 | 8.67 (0.146) | 4.85 (1.96–10.91) | 6436 | 24.6 (0.397) | 15 (7–30) |
| Yes | ||||||
| All | 5996 | 7.49 (0.146) | 3.04 (0.93–9.41) | 5977 | 26.6 (0.452) | 14 (5–35) |
| Survivors | 1896 | 12 (0.298) | 7.79 (3.67–15.77) | 1879 | 49.53 (0.937) | 38 (22–63) |
| Non-survivors | 4100 | 5.4 (0.151) | 1.71 (0.71–5.92) | 4098 | 16.09 (0.405) | 8 (3–19) |
| Quartiles of APACHE II score | ||||||
| 1–15 | ||||||
| All | 7292 | 7.03 (0.114) | 3.75 (1.84–8.07) | 7172 | 31.48 (0.41) | 20 (12–38) |
| Survivors | 6103 | 6.42 (0.113) | 3.5 (1.75–7.33) | 5986 | 32.04 (0.444) | 21 (12–39) |
| Non-survivors | 1189 | 10.17 (0.376) | 5.96 (2.77–12.90) | 1186 | 28.66 (1.056) | 18 (9–33) |
| 16–19 | ||||||
| All | 5919 | 8.69 (0.134) | 5.07 (2.49–10.72) | 5861 | 35.61 (0.538) | 24 (13–43) |
| Survivors | 3944 | 8.51 (0.162) | 5.02 (2.54–10.38) | 3889 | 40.8 (0.72) | 28 (16–49) |
| Non-survivors | 1975 | 9.03 (0.239) | 5.23 (2.38–11.55) | 1972 | 25.39 (0.679) | 16 (8–31) |
| 20–24 | ||||||
| All | 6173 | 9.35 (0.152) | 5.35 (2.42–11.83) | 6141 | 34.21 (0.48) | 23 (11–44) |
| Survivors | 3231 | 10.48 (0.218) | 6.12 (3.17–13.49) | 3200 | 44.21 (0.711) | 32 (19–55) |
| Non-survivors | 2942 | 8.11 (0.208) | 4.28 (1.75–10.00) | 2941 | 23.32 (0.574) | 14 (6–29) |
| 25–52 | ||||||
| All | 5738 | 8.36 (0.155) | 4.04 (1.32–10.50) | 5709 | 28.61 (0.484) | 16 (5–37) |
| Survivors | 1942 | 12.36 (0.286) | 7.98 (4.08–15.94) | 1916 | 50.49 (0.971) | 39 (23–62) |
| Non-survivors | 3796 | 6.31 (0.175) | 2.24 (0.92–7.18) | 3793 | 17.56 (0.442) | 9 (3–21) |
| APACHE II score ≥ 25 | ||||||
| No | ||||||
| All | 19,384 | 8.27 (0.077) | 4.65 (2.11–9.99) | 19,174 | 33.62 (0.273) | 22 (12–42) |
| Survivors | 13,278 | 8.03 (0.09) | 4.48 (2.13–9.57) | 13,075 | 37.62 (0.346) | 25 (15–46) |
| Non-survivors | 6106 | 8.81 (0.147) | 4.97 (2.09–11.20) | 6099 | 25.03 (0.409) | 15 (7–30) |
| Yes | ||||||
| All | 5738 | 8.36 (0.155) | 4.04 (1.32–10.50) | 5709 | 28.61 (0.484) | 16 (5–37) |
| Survivors | 1942 | 12.36 (0.286) | 7.98 (4.08–15.94) | 1916 | 50.49 (0.971) | 39 (23–62) |
| Non-survivors | 3796 | 6.31 (0.175) | 2.24 (0.92–7.18) | 3793 | 17.56 (0.442) | 9 (3–21) |
| Quartiles of ICNARC physiology score | ||||||
| 1–16 | ||||||
| All | 6671 | 5.22 (0.091) | 2.90 (1.58–5.78) | 6568 | 31.17 (0.454) | 20 (11–37) |
| Survivors | 5672 | 4.73 (0.087) | 2.76 (1.52–5.16) | 5570 | 30.6 (0.477) | 19 (11–37) |
| Non-survivors | 999 | 8.03 (0.347) | 4.50 (2.11–9.60) | 998 | 34.36 (1.361) | 22 (11–40) |
| 17–22 | ||||||
| All | 6583 | 8.17 (0.123) | 4.87 (2.52–9.76) | 6507 | 35.31 (0.482) | 24 (13–43) |
| Survivors | 4584 | 7.93 (0.142) | 4.75 (2.52–9.21) | 4511 | 38.92 (0.604) | 26 (15–48) |
| Non-survivors | 1999 | 8.73 (0.243) | 5.26 (2.53–10.98) | 1996 | 27.14 (0.748) | 17 (9–32) |
| 23–29 | ||||||
| All | 6422 | 10.45 (0.159) | 6.43 (2.96–13.07) | 6373 | 35.26 (0.475) | 24 (12–45) |
| Survivors | 3440 | 11.63 (0.223) | 7.66 (4.04–14.46) | 3394 | 45.76 (0.71) | 34 (20–57) |
| Non-survivors | 2982 | 9.08 (0.224) | 4.86 (2.06–11.20) | 2979 | 23.29 (0.536) | 14 (6–29) |
| 30–75 | ||||||
| All | 6314 | 8.42 (0.151) | 3.52 (0.89–11.65) | 6301 | 25.3 (0.436) | 13 (4–33) |
| Survivors | 1750 | 15.86 (0.32) | 12.12 (6.66–21.36) | 1740 | 53.44 (1.038) | 42 (25–65) |
| Non-survivors | 4564 | 5.57 (0.149) | 1.59 (0.67–6.36) | 4561 | 14.56 (0.338) | 7 (3–17) |
| Septic shock | ||||||
| No | ||||||
| All | 4353 | 7.67 (0.153) | 4.26 (1.99–9.04) | 4308 | 32.69 (0.548) | 21 (12–40) |
| Survivors | 3125 | 7.04 (0.161) | 4.00 (1.94–8.27) | 3080 | 34.95 (0.641) | 23 (14–43) |
| Non-survivors | 1228 | 9.28 (0.353) | 5.07 (2.29–12.00) | 1228 | 27.04 (1.036) | 17 (9–32) |
| Yes | ||||||
| All | 21,637 | 8.11 (0.075) | 4.25 (1.79–9.91) | 21,441 | 31.61 (0.257) | 20 (10–40) |
| Survivors | 12,321 | 8.84 (0.100) | 4.96 (2.36–10.86) | 12,135 | 40.11 (0.373) | 28 (15–50) |
| Non-survivors | 9316 | 7.15 (0.112) | 3.15 (1.07–8.76) | 9306 | 20.53 (0.300) | 11 (4–25) |
| CPR | ||||||
| No | ||||||
| All | 25,067 | 8.04 (0.068) | 4.31 (1.85–9.80) | 24,831 | 32.05 (0.238) | 21 (10–40) |
| Survivors | 15,176 | 8.39 (0.086) | 4.75 (2.19–10.11) | 14,950 | 38.88 (0.327) | 26 (15–49) |
| Non-survivors | 9891 | 7.51 (0.111) | 3.58 (1.21–9.23) | 9881 | 21.71 (0.305) | 12 (5–27) |
| Yes | ||||||
| All | 923 | 7.91 (0.408) | 3.02 (0.88–9.67) | 918 | 24.82 (1.121) | 13 (4–30) |
| Survivors | 270 | 13.44 (0.871) | 7.80 (3.60–18.68) | 265 | 49.35 (2.635) | 37 (20–62) |
| Non-survivors | 653 | 5.63 (0.419) | 1.71 (0.60–6.12) | 653 | 14.87 (0.903) | 6 (3–18) |
| Source of admission | ||||||
| A&E | ||||||
| All | 4580 | 7.87 (0.164) | 4.06 (1.68–9.70) | 4488 | 23.08 (0.478) | 14 (6–28) |
| Survivors | 2816 | 8.78 (0.219) | 4.91 (2.42–10.77) | 2727 | 29.96 (0.678) | 19 (11–35) |
| Non-survivors | 1764 | 6.42 (0.240) | 2.54 (0.83–7.69) | 1761 | 12.42 (0.528) | 5 (2–14) |
| Clinic or home | ||||||
| All | 132 | 8.36 (1.211) | 3.89 (1.29–10.21) | 130 | 26.31 (2.408) | 18 (6.8–35) |
| Survivors | 86 | 8.80 (0.982) | 6.30 (1.98–12.03) | 84 | 33.92 (2.895) | 24 (15–46) |
| Non-survivors | 46 | 7.52 (2.971) | 1.38 (0.80–5.88) | 46 | 12.41 (3.481) | 4 (2–9.3) |
| Critical-care unit | ||||||
| All | 2043 | 10.62 (0.262) | 6.82 (2.84–14.36) | 2038 | 42.99 (0.946) | 31 (15–57) |
| Survivors | 1132 | 11.26 (0.341) | 7.39 (3.79–15.02) | 1128 | 53.53 (1.373) | 40 (24–68) |
| Non-survivors | 911 | 9.82 (0.405) | 5.49 (1.75–13.23) | 910 | 29.92 (1.121) | 18 (8–39) |
| Theatre – elective/scheduled | ||||||
| All | 1132 | 5.98 (0.254) | 2.88 (1.33–6.71) | 1124 | 36.88 (1.300) | 34 (12–46) |
| Survivors | 897 | 5.36 (0.244) | 2.85 (1.30–5.55) | 889 | 38.46 (1.524) | 25 (14–47) |
| Non-survivors | 235 | 8.35 (0.776) | 3.50 (1.41–9.56) | 235 | 30.89 (2.298) | 17 (9–38) |
| Theatre – emergency/urgent | ||||||
| All | 5812 | 7.16 (0.131) | 3.76 (1.75–8.31) | 5792 | 33.79 (0.465) | 23 (12–43) |
| Survivors | 3925 | 7.13 (0.158) | 3.79 (1.83–8.08) | 3907 | 39.46 (0.575) | 28 (16–50) |
| Non-survivors | 1887 | 7.21 (0.232) | 3.61 (1.44–8.71) | 1885 | 22.02 (0.717) | 13 (6–27) |
| Ward or other intermediate area | ||||||
| All | 12,291 | 8.27 (0.1) | 4.51 (1.88–10.13) | 12,177 | 31.77 (0.343) | 21 (10–39) |
| Survivors | 6590 | 9.09 (0.134) | 5.26 (2.64–11.05) | 6480 | 40.29 (0.517) | 28 (16–50) |
| Non-survivors | 5701 | 7.33 (0.149) | 3.44 (1.17–8.96) | 5697 | 22.08 (0.399) | 13 (6–27) |
Appendix 9 Summary of input parameters and sources
| Parameter | Description | Source | Base case |
|---|---|---|---|
| Cohort characteristics | Mean age of a severe sepsis patient at admission to hospital | ICNARC CMP Database | 63 years |
| Proportion of males in a severe sepsis population at admission to hospital | ICNARC CMP Database | 0.53 | |
| Short-term outcome | Probability of dying in hospital when SC is used in the treatment of sepsis (baseline risk) | ICNARC CMP Database | 40.6% (95% CI 40.0% to 41.2%) |
| Log-odds ratio, when IVIG is used to complement SC in the treatment of sepsis [based on model T3b with covariate: duration of IVIG therapy (3 days)] | Evidence synthesis, Chapter 3 | Normal (mean = –0.2978; SD = 0.1279) | |
| Long-term outcomes | Predicted probability of dying in yearly intervals, conditional on patients having survived up to the start of the year | Cuthbertson Database |
Varies with time Calculations based on Weibull regression of survival time from hospital discharge |
| Age-specific yearly probability of dying, conditional on patients having survived up to the start of the year | General population life tables | Varies with time | |
| Cost-related parameters | Costs of overall IVIG therapy | Non-stochastic, BNF | £5539.05 |
| Costs of SC, when only SC is used in the treatment of sepsis | Non-stochastic | £0.00 | |
| Critical-care unit LOS for patients remaining alive until discharge from hospital | ICNARC CMP Database | 8.48 (SE = 0.086) | |
| Critical-care unit LOS for patients dying in hospital | ICNARC CMP Database | 7.40 (SE = 0.108) | |
| Costs associated with a day in a critical-care unit for a patient with severe sepsis | Non-stochastic, Reference costs | £1393.00 | |
| Overall hospital LOS for patients remaining alive until discharge from hospital | ICNARC CMP Database | 21.29 (SE = 0.292) | |
| Overall hospital LOS for patients dying in hospital | ICNARC CMP Database | 39.07 (SE = 0.325) | |
| Costs associated with a day in wards other than a critical-care unit, for a patient with a severe sepsis episode | Non-stochastic, Reference costs | £196.00 | |
| Costs incurred between year t–1 and year t after hospital discharge | Manns et al. (2002)74 | t = 1: £13,654.00; t > 1: £4466.50 per year | |
| Utilities | In-hospital utility associated with severe sepsis patients | Drabinsky et al. (2001)96 | 0.53 |
| Utility associated with severe sepsis patients at year t | Cuthbertson et al.,119 Drabinsky et al. (2001)96 | t = 1: 0.62; t > 1: 0.6833 | |
| Discount rates | Discount rate for future benefits | Non-stochastic, NICE | 0.035 |
| Discount rate for future costs | Non-stochastic, NICE | 0.035 |
Appendix 10 Cost-effectiveness results for subgroups
| APACHE II score | Standard care | IVIG | Incremental | ICER (£) | Probability of IVIG being cost-effective | ||||
|---|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALY | Costs (£) | QALY | Costs (£) | QALY | £20,000 | £30,000 | ||
| 1 | 72,756 | 7.92 | 78,936 | 8.01 | 6179 | 0.09 | 67,522 | 0.000 | 0.000 |
| 2 | 72,068 | 7.82 | 78,329 | 7.92 | 6261 | 0.10 | 60,718 | 0.000 | 0.001 |
| 3 | 71,328 | 7.71 | 77,679 | 7.83 | 6351 | 0.12 | 54,768 | 0.000 | 0.010 |
| 4 | 70,530 | 7.59 | 76,981 | 7.72 | 6451 | 0.13 | 49,565 | 0.000 | 0.039 |
| 5 | 69,673 | 7.47 | 76,233 | 7.61 | 6560 | 0.15 | 45,016 | 0.000 | 0.094 |
| 6 | 68,750 | 7.33 | 75,430 | 7.49 | 6680 | 0.16 | 41,041 | 0.001 | 0.175 |
| 7 | 67,759 | 7.18 | 74,570 | 7.37 | 6811 | 0.18 | 37,569 | 0.009 | 0.276 |
| 8 | 66,693 | 7.03 | 73,645 | 7.23 | 6952 | 0.20 | 34,540 | 0.031 | 0.373 |
| 9 | 65,550 | 6.86 | 72,653 | 7.08 | 7103 | 0.22 | 31,899 | 0.074 | 0.461 |
| 10 | 64,327 | 6.68 | 71,591 | 6.93 | 7263 | 0.25 | 29,601 | 0.129 | 0.536 |
| 11 | 63,021 | 6.49 | 70,454 | 6.76 | 7432 | 0.27 | 27,604 | 0.197 | 0.601 |
| 12 | 61,630 | 6.29 | 69,238 | 6.58 | 7608 | 0.29 | 25,874 | 0.267 | 0.654 |
| 13 | 60,154 | 6.07 | 67,943 | 6.39 | 7788 | 0.32 | 24,380 | 0.337 | 0.693 |
| 14 | 58,595 | 5.85 | 66,566 | 6.19 | 7971 | 0.35 | 23,097 | 0.398 | 0.729 |
| 15 | 56,955 | 5.61 | 65,107 | 5.98 | 8152 | 0.37 | 22,001 | 0.453 | 0.758 |
| 16 | 57,764 | 5.36 | 66,427 | 5.76 | 8663 | 0.40 | 21,923 | 0.456 | 0.763 |
| 17 | 55,806 | 5.11 | 64,660 | 5.52 | 8854 | 0.42 | 21,158 | 0.492 | 0.780 |
| 18 | 53,780 | 4.84 | 62,809 | 5.28 | 9029 | 0.44 | 20,532 | 0.520 | 0.798 |
| 19 | 51,698 | 4.57 | 60,883 | 5.03 | 9184 | 0.46 | 20,035 | 0.542 | 0.809 |
| 20 | 50,819 | 4.29 | 60,440 | 4.76 | 9621 | 0.47 | 20,304 | 0.532 | 0.807 |
| 21 | 48,521 | 4.01 | 58,254 | 4.50 | 9733 | 0.49 | 20,049 | 0.542 | 0.812 |
| 22 | 46,212 | 3.73 | 56,022 | 4.23 | 9810 | 0.49 | 19,904 | 0.547 | 0.814 |
| 23 | 43,914 | 3.46 | 53,762 | 3.95 | 9848 | 0.50 | 19,868 | 0.549 | 0.814 |
| 24 | 41,645 | 3.18 | 51,490 | 3.68 | 9845 | 0.49 | 19,939 | 0.543 | 0.812 |
| 25 | 39,102 | 2.92 | 49,402 | 3.41 | 10,301 | 0.49 | 21,146 | 0.486 | 0.786 |
| 26 | 36,724 | 2.66 | 46,937 | 3.14 | 10,213 | 0.48 | 21,457 | 0.470 | 0.774 |
| 27 | 34,437 | 2.42 | 44,521 | 2.88 | 10,083 | 0.46 | 21,891 | 0.445 | 0.760 |
| 28 | 32,256 | 2.19 | 42,173 | 2.63 | 9917 | 0.44 | 22,456 | 0.413 | 0.743 |
| 29 | 30,191 | 1.97 | 39,911 | 2.39 | 9719 | 0.42 | 23,167 | 0.375 | 0.721 |
| 30 | 28,255 | 1.76 | 37,750 | 2.16 | 9496 | 0.40 | 24,038 | 0.333 | 0.696 |
| 31 | 26,451 | 1.57 | 35,705 | 1.94 | 9253 | 0.37 | 25,089 | 0.285 | 0.661 |
| 32 | 24,784 | 1.40 | 33,783 | 1.74 | 8998 | 0.34 | 26,343 | 0.232 | 0.619 |
| 33 | 23,254 | 1.24 | 31,991 | 1.55 | 8737 | 0.31 | 27,830 | 0.177 | 0.568 |
| 34 | 21,857 | 1.10 | 30,332 | 1.38 | 8475 | 0.29 | 29,583 | 0.130 | 0.510 |
| 35 | 20,591 | 0.97 | 28,807 | 1.23 | 8216 | 0.26 | 31,643 | 0.084 | 0.438 |
| 36 | 19,449 | 0.85 | 27,415 | 1.08 | 7966 | 0.23 | 34,059 | 0.047 | 0.361 |
| 37 | 18,423 | 0.75 | 26,151 | 0.96 | 7727 | 0.21 | 36,887 | 0.024 | 0.284 |
| 38 | 17,507 | 0.65 | 25,010 | 0.84 | 7502 | 0.19 | 40,196 | 0.010 | 0.199 |
| 39 | 16,692 | 0.57 | 23,984 | 0.74 | 7292 | 0.17 | 44,067 | 0.004 | 0.131 |
| 40 | 15,970 | 0.50 | 23,068 | 0.65 | 7098 | 0.15 | 48,592 | 0.001 | 0.073 |
| 41 | 15,331 | 0.44 | 22,251 | 0.57 | 6920 | 0.13 | 53,885 | 0.000 | 0.035 |
| 42 | 14,769 | 0.38 | 21,527 | 0.49 | 6758 | 0.11 | 60,076 | 0.000 | 0.014 |
| 43 | 14,275 | 0.33 | 20,887 | 0.43 | 6612 | 0.10 | 67,321 | 0.000 | 0.005 |
| 44 | 13,842 | 0.29 | 20,323 | 0.38 | 6481 | 0.09 | 75,804 | 0.000 | 0.001 |
| 45 | 13,464 | 0.25 | 19,828 | 0.33 | 6364 | 0.07 | 85,741 | 0.000 | 0.000 |
| 46 | 13,134 | 0.22 | 19,393 | 0.29 | 6260 | 0.06 | 97,386 | 0.000 | 0.000 |
| 47 | 12,846 | 0.20 | 19,013 | 0.25 | 6167 | 0.06 | 111,042 | 0.000 | 0.000 |
| 48 | 12,596 | 0.17 | 18,682 | 0.22 | 6086 | 0.05 | 127,063 | 0.000 | 0.000 |
| 49 | 12,378 | 0.15 | 18,393 | 0.19 | 6015 | 0.04 | 145,868 | 0.000 | 0.000 |
| 50 | 12,190 | 0.13 | 18,142 | 0.17 | 5952 | 0.04 | 167,953 | 0.000 | 0.000 |
| 51 | 12,026 | 0.12 | 17,923 | 0.15 | 5897 | 0.03 | 193,903 | 0.000 | 0.000 |
| 52 | 11,885 | 0.11 | 17,734 | 0.13 | 5849 | 0.03 | 224,407 | 0.000 | 0.000 |
FIGURE 51.
Estimated probability that IVIG is cost-effective assuming specific values of APACHE II score.
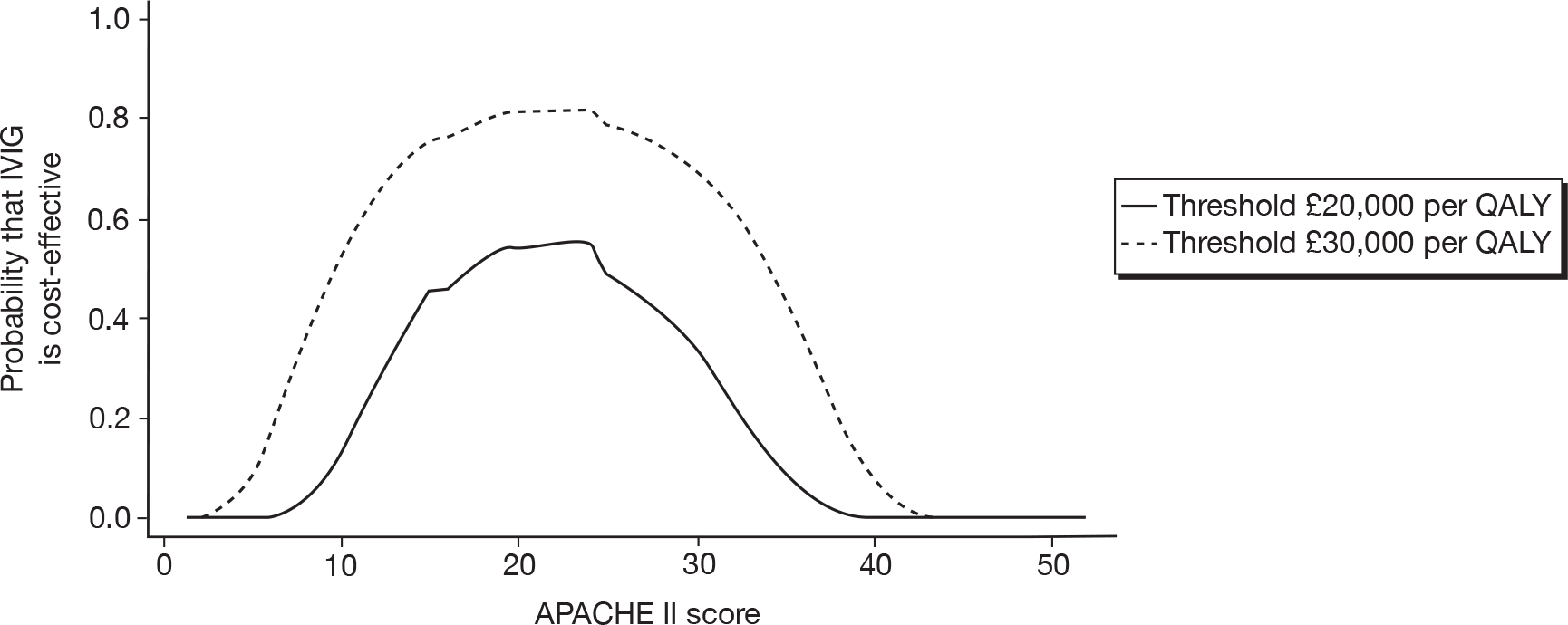
| ICNARC II score | Standard care | IVIG | Incremental | ICER (£) | Probability of IVIG being cost-effective | ||||
|---|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALY | Costs (£) | QALY | Costs (£) | QALY | £20,000 | £30,000 | ||
| 1 | 59,556 | 6.24 | 65,609 | 6.32 | 6053 | 0.07 | 82,273 | 0.000 | 0.000 |
| 2 | 59,316 | 6.21 | 65,425 | 6.29 | 6109 | 0.08 | 74,846 | 0.000 | 0.000 |
| 3 | 59,050 | 6.17 | 65,220 | 6.26 | 6171 | 0.09 | 68,220 | 0.000 | 0.000 |
| 4 | 58,755 | 6.13 | 64,993 | 6.23 | 6238 | 0.10 | 62,307 | 0.000 | 0.001 |
| 5 | 58,430 | 6.08 | 64,742 | 6.19 | 6312 | 0.11 | 57,033 | 0.000 | 0.006 |
| 6 | 58,072 | 6.03 | 64,464 | 6.15 | 6392 | 0.12 | 52,330 | 0.000 | 0.022 |
| 7 | 57,678 | 5.97 | 64,157 | 6.11 | 6479 | 0.13 | 48,135 | 0.000 | 0.059 |
| 8 | 57,245 | 5.91 | 63,818 | 6.06 | 6573 | 0.15 | 44,397 | 0.000 | 0.108 |
| 9 | 56,771 | 5.84 | 63,445 | 6.01 | 6674 | 0.16 | 41,066 | 0.002 | 0.179 |
| 10 | 56,253 | 5.77 | 63,035 | 5.95 | 6782 | 0.18 | 38,099 | 0.009 | 0.263 |
| 11 | 55,688 | 5.69 | 62,586 | 5.88 | 6897 | 0.19 | 35,458 | 0.027 | 0.343 |
| 12 | 55,074 | 5.60 | 62,094 | 5.81 | 7020 | 0.21 | 33,110 | 0.057 | 0.420 |
| 13 | 54,409 | 5.50 | 61,557 | 5.73 | 7148 | 0.23 | 31,023 | 0.097 | 0.488 |
| 14 | 53,690 | 5.40 | 60,973 | 5.65 | 7283 | 0.25 | 29,172 | 0.149 | 0.548 |
| 15 | 52,916 | 5.29 | 60,338 | 5.56 | 7422 | 0.27 | 27,531 | 0.203 | 0.602 |
| 16 | 56,271 | 5.17 | 64,105 | 5.46 | 7834 | 0.29 | 27,007 | 0.215 | 0.618 |
| 17 | 55,267 | 5.04 | 63,266 | 5.35 | 7999 | 0.31 | 25,728 | 0.270 | 0.658 |
| 18 | 54,201 | 4.91 | 62,366 | 5.24 | 8165 | 0.33 | 24,604 | 0.322 | 0.689 |
| 19 | 53,073 | 4.76 | 61,402 | 5.12 | 8330 | 0.35 | 23,620 | 0.371 | 0.716 |
| 20 | 51,885 | 4.61 | 60,376 | 4.99 | 8491 | 0.37 | 22,764 | 0.411 | 0.739 |
| 21 | 50,641 | 4.46 | 59,287 | 4.85 | 8646 | 0.39 | 22,024 | 0.447 | 0.756 |
| 22 | 49,346 | 4.29 | 58,138 | 4.70 | 8793 | 0.41 | 21,392 | 0.481 | 0.772 |
| 23 | 51,500 | 4.12 | 60,828 | 4.55 | 9328 | 0.43 | 21,794 | 0.456 | 0.767 |
| 24 | 49,955 | 3.95 | 59,418 | 4.39 | 9463 | 0.44 | 21,352 | 0.481 | 0.779 |
| 25 | 48,375 | 3.77 | 57,954 | 4.22 | 9579 | 0.46 | 20,997 | 0.499 | 0.788 |
| 26 | 46,768 | 3.58 | 56,441 | 4.05 | 9673 | 0.47 | 20,723 | 0.510 | 0.792 |
| 27 | 45,145 | 3.40 | 54,887 | 3.88 | 9743 | 0.47 | 20,527 | 0.518 | 0.797 |
| 28 | 43,515 | 3.22 | 53,302 | 3.70 | 9787 | 0.48 | 20,407 | 0.524 | 0.800 |
| 29 | 41,890 | 3.03 | 51,695 | 3.51 | 9805 | 0.48 | 20,360 | 0.525 | 0.799 |
| 30 | 39,762 | 2.85 | 50,478 | 3.33 | 10,716 | 0.48 | 22,302 | 0.427 | 0.760 |
| 31 | 37,830 | 2.67 | 48,503 | 3.15 | 10,672 | 0.48 | 22,403 | 0.421 | 0.755 |
| 32 | 35,941 | 2.49 | 46,538 | 2.96 | 10,597 | 0.47 | 22,578 | 0.408 | 0.749 |
| 33 | 34,103 | 2.32 | 44,594 | 2.78 | 10,492 | 0.46 | 22,831 | 0.394 | 0.741 |
| 34 | 32,325 | 2.16 | 42,685 | 2.60 | 10,359 | 0.45 | 23,164 | 0.376 | 0.730 |
| 35 | 30,617 | 2.00 | 40,820 | 2.43 | 10,203 | 0.43 | 23,582 | 0.352 | 0.717 |
| 36 | 28,984 | 1.84 | 39,009 | 2.26 | 10,026 | 0.42 | 24,089 | 0.327 | 0.699 |
| 37 | 27,430 | 1.70 | 37,262 | 2.10 | 9832 | 0.40 | 24,694 | 0.296 | 0.680 |
| 38 | 25,961 | 1.56 | 35,585 | 1.94 | 9624 | 0.38 | 25,402 | 0.263 | 0.657 |
| 39 | 24,577 | 1.43 | 33,984 | 1.79 | 9407 | 0.36 | 26,224 | 0.228 | 0.630 |
| 40 | 23,280 | 1.31 | 32,464 | 1.65 | 9184 | 0.34 | 27,170 | 0.192 | 0.595 |
| 41 | 22,069 | 1.20 | 31,028 | 1.52 | 8959 | 0.32 | 28,252 | 0.157 | 0.556 |
| 42 | 20,942 | 1.10 | 29,676 | 1.39 | 8734 | 0.30 | 29,484 | 0.123 | 0.512 |
| 43 | 19,899 | 1.00 | 28,411 | 1.27 | 8512 | 0.28 | 30,882 | 0.090 | 0.466 |
| 44 | 18,936 | 0.91 | 27,231 | 1.16 | 8295 | 0.26 | 32,464 | 0.063 | 0.412 |
| 45 | 18,049 | 0.83 | 26,135 | 1.06 | 8086 | 0.24 | 34,250 | 0.040 | 0.356 |
| 46 | 17,235 | 0.75 | 25,120 | 0.97 | 7885 | 0.22 | 36,264 | 0.024 | 0.295 |
| 47 | 16,490 | 0.68 | 24,183 | 0.88 | 7693 | 0.20 | 38,531 | 0.013 | 0.238 |
| 48 | 15,810 | 0.62 | 23,322 | 0.80 | 7512 | 0.18 | 41,080 | 0.007 | 0.178 |
| 49 | 15,190 | 0.56 | 22,531 | 0.73 | 7342 | 0.17 | 43,945 | 0.003 | 0.126 |
| 50 | 14,626 | 0.51 | 21,808 | 0.66 | 7182 | 0.15 | 47,161 | 0.000 | 0.083 |
| 51 | 14,115 | 0.46 | 21,148 | 0.60 | 7034 | 0.14 | 50,772 | 0.000 | 0.049 |
| 52 | 13,651 | 0.42 | 20,547 | 0.54 | 6897 | 0.13 | 54,822 | 0.000 | 0.027 |
| 53 | 13,231 | 0.38 | 20,001 | 0.49 | 6770 | 0.11 | 59,365 | 0.000 | 0.013 |
| 54 | 12,852 | 0.34 | 19,505 | 0.44 | 6653 | 0.10 | 64,458 | 0.000 | 0.006 |
| 55 | 12,510 | 0.31 | 19,056 | 0.40 | 6546 | 0.09 | 70,169 | 0.000 | 0.003 |
| 56 | 12,202 | 0.28 | 18,650 | 0.36 | 6448 | 0.08 | 76,569 | 0.000 | 0.000 |
| 57 | 11,924 | 0.25 | 18,283 | 0.33 | 6358 | 0.08 | 83,742 | 0.000 | 0.000 |
| 58 | 11,674 | 0.23 | 17,952 | 0.30 | 6277 | 0.07 | 91,779 | 0.000 | 0.000 |
| 59 | 11,450 | 0.21 | 17,653 | 0.27 | 6203 | 0.06 | 100,786 | 0.000 | 0.000 |
| 60 | 11,248 | 0.19 | 17,384 | 0.25 | 6136 | 0.06 | 110,876 | 0.000 | 0.000 |
| 61 | 11,067 | 0.17 | 17,143 | 0.22 | 6076 | 0.05 | 122,182 | 0.000 | 0.000 |
| 62 | 10,904 | 0.16 | 16,925 | 0.20 | 6021 | 0.04 | 134,847 | 0.000 | 0.000 |
| 63 | 10,759 | 0.15 | 16,730 | 0.19 | 5971 | 0.04 | 149,036 | 0.000 | 0.000 |
| 64 | 10,628 | 0.13 | 16,555 | 0.17 | 5927 | 0.04 | 164,932 | 0.000 | 0.000 |
| 65 | 10,511 | 0.12 | 16,398 | 0.16 | 5887 | 0.03 | 182,738 | 0.000 | 0.000 |
| 66 | 10,406 | 0.11 | 16,257 | 0.14 | 5851 | 0.03 | 202,685 | 0.000 | 0.000 |
| 67 | 10,312 | 0.10 | 16,130 | 0.13 | 5818 | 0.03 | 225,030 | 0.000 | 0.000 |
| 68 | 10,228 | 0.10 | 16,017 | 0.12 | 5789 | 0.02 | 250,059 | 0.000 | 0.000 |
| 69 | 10,153 | 0.09 | 15,916 | 0.11 | 5763 | 0.02 | 278,097 | 0.000 | 0.000 |
| 70 | 10,086 | 0.08 | 15,825 | 0.10 | 5739 | 0.02 | 309,504 | 0.000 | 0.000 |
| 71 | 10,026 | 0.08 | 15,744 | 0.09 | 5718 | 0.02 | 344,684 | 0.000 | 0.000 |
| 72 | 9972 | 0.07 | 15,671 | 0.09 | 5699 | 0.01 | 384,092 | 0.000 | 0.000 |
| 73 | 9924 | 0.07 | 15,606 | 0.08 | 5682 | 0.01 | 428,234 | 0.000 | 0.000 |
| 74 | 9881 | 0.06 | 15,548 | 0.08 | 5667 | 0.01 | 477,680 | 0.000 | 0.000 |
| 75 | 9843 | 0.06 | 15,496 | 0.07 | 5654 | 0.01 | 533,066 | 0.000 | 0.000 |
FIGURE 52.
Estimated probability that IVIG is cost-effective assuming specific values of ICNARC II score.
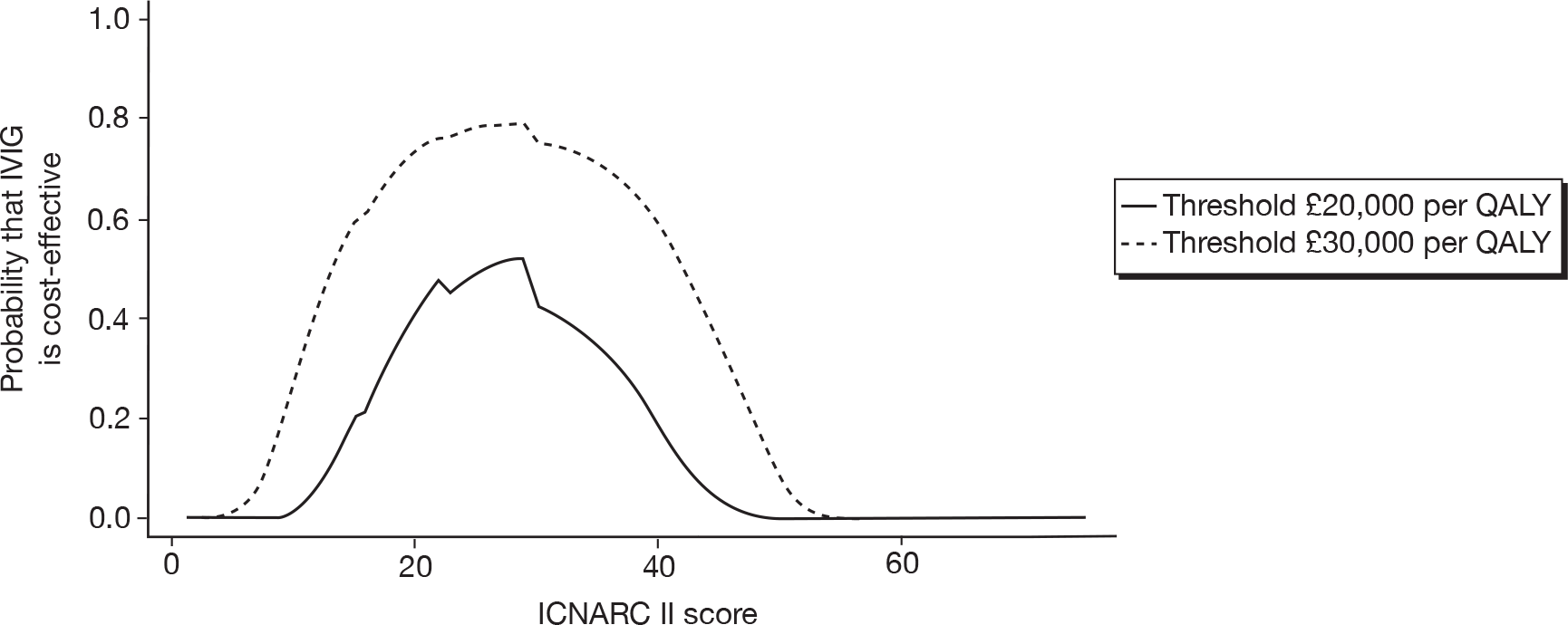
Appendix 11 Study protocol
An evaluation of the feasibility, cost and value of information of a multicentre randomised controlled trial of intravenous immunoglobulin for sepsis (severe sepsis and septic shock)
Study Protocol
Version 1.0
29 July 2008
Protocol Number: ICNARC/02/02/09
1. Project title
08/70: An evaluation of the feasibility, cost and value of information of a multicentre randomised controlled trial of intravenous immunoglobulin for sepsis (severe sepsis and septic shock)
2. How the project has changed since the outline proposal was submitted
No outline stage was required.
3. Planned investigation
Research objectives
The aim of this project is to evaluate the feasibility, cost and value of information of conducting a multicentre randomised controlled trial (RCT) to assess the clinical and cost-effectiveness of intravenous immunoglobulin (IVIg) for adult patients severely ill with sepsis in the UK.
This aim will be achieved by addressing the following research questions:
-
What is the existing evidence for the benefit of IVIg for adult patients with sepsis?
-
What are the key sources of heterogeneity within this evidence and are existing results subject to potential publication bias or any other sources of bias?
-
What is existing practice within the NHS with regard to management and treatment of adult patients with sepsis, and how does this relate to current best practice according to research evidence and international guidelines?
-
What is the current usage of, and demand for, IVIg for sepsis?
-
What is the expected value of perfect information for the decision problem of treating adult patients with sepsis using IVIg both versus existing practice and versus best practice without IVIg?
-
What would be the anticipated research costs, treatment costs and NHS support costs for conducting an RCT of IVIg for adult patients with sepsis?
-
What is the feasibility of being able to conduct an RCT of IVIg for adult patients with sepsis within the NHS, with regard to the availability of IVIg and availability of eligible patients?
-
What would be the optimal design for a new RCT of IVIg for adult patients with sepsis?
-
What is the expected value of sample information from this RCT?
Existing research
Sepsis is a major public health problem
Sepsis is a syndrome characterised by a systemic inflammatory response to infection that leads to rapid acute organ failure and potentially rapid decline to death. 1 In 2006, we reported an increasing incidence of severe sepsis (sepsis resulting in organ dysfunction) in UK adult critical care units, rising from 50 to 70 cases per 100,000 population per year over the last decade. 2 This now represents approximately 31,000 patient episodes per year. Similarly high incidence rates have been reported elsewhere. 3 We found 29% of all admissions to adult, general critical care units were associated with severe sepsis in the first 24 hours following admission and had an in-hospital mortality of 45% (approximately 15,000 deaths per year). 2
International guidelines for management of sepsis (severe sepsis and septic shock)
Most clinicians look to the international guidelines for guidance on the management and treatment of patients with sepsis.
In early 2008, the current, third edition of clinical practice guidelines, building on two previous editions in 2001 and 2004 were published. 4 The 2001 publication incorporated literature from the preceding ten years, the 2004 publication incorporated the evidence available to the end of 2003 and the current guidelines were based on an updated search into early 2007.
The 2008 guidelines process included a modified Delphi method, a consensus conference, several subsequent meetings/teleconferences/electronic discussions among subgroups and members of the entire committee and two follow-up nominal group meetings in 2007. Differences of opinion among committee members about interpretation of evidence, wording of proposals, or strength of recommendations were resolved using a specifically developed set of rules.
The scope of the guidelines was wide and subgroups were formed, each charged with updating recommendations in specific areas. Initial resuscitation and infection issues covered: initial resuscitation; diagnosis; antibiotic therapy; and source identification and control. Haemodynamic support and adjunctive therapy covered: fluid therapy; vasopressors; inotropic therapy; steroids; and recombinant human activated protein C. Other supportive therapy covered: blood product administration; mechanical ventilation; sedation, analgesia and neuromuscular blockade; glucose control; renal replacement; bicarbonate therapy; deep-vein thrombosis prophylaxis; stress ulcer prophylaxis; selective digestive tract decontamination; and consideration for limitation of support. IVIg, however, was neither considered nor was the evidence reviewed (personal communication: G Ramsay).
For the 2008 guidelines, quality of evidence was judged by pre-defined Grades of Recommendation, Assessment, Development and Evaluation (GRADE) criteria – a structured system for rating quality of evidence and grading strength of recommendation in clinical practice. 5 The GRADE system is based on a sequential assessment of the quality of evidence – as high (Grade A), moderate (Grade B), low (Grade C), or very low (Grade D) – and the strength of the recommendation – as strong (Grade 1) or weak (Grade 2). The rating of quality of evidence and strength of recommendation is explicitly separate and constitutes a crucial and defining feature of the GRADE approach. The grade of recommendation, strong or weak, is considered of greater clinical importance than a difference in level of quality of evidence. For example, RCTs begin as high quality evidence, but may be downgraded due to limitations in implementation, inconsistency or imprecision of the results, indirectness of the evidence, and possible reporting bias.
Of 62 recommendations, only 23 (37%) were strong (Grade 1) recommendations based on high/moderate (Grade A/B) evidence and only eight (13%) were strong recommendations on high-quality evidence (1A), listed below:
Vasopressors
-
Do not use low-dose dopamine for renal protection.
Steroids
-
Hydrocortisone dose should be ≤ 300 mg/day.
Recombinant human activated protein C (rhAPC)
-
Adult patients with severe sepsis and low risk of death (e. g.: APACHE II < 20 or one organ failure) should not receive rhAPC.
Mechanical ventilation of sepsis-induced acute lung injury (ALI)/ARDS
-
Use a weaning protocol and a spontaneous breathing trial (SBT) regularly to evaluate the potential for discontinuing mechanical ventilation.
-
SBT options include a low level of pressure support with continuous positive airway pressure 5 cm H2O or a T-piece.
-
Before the SBT, patients should:
-
be arousable
-
be haemodynamically stable without vasopressors
-
have no new potentially serious conditions
-
have low ventilatory and end-expiratory pressure requirement
-
require FiO2 levels that can be safely delivered with a face mask or nasal cannula.
-
Do not use a pulmonary artery catheter for the routine monitoring of patients with ALI/ARDS.
Deep-vein thrombosis (DVT) prophylaxis
-
Use either low-dose unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH), unless contraindicated.
-
Use a mechanical prophylactic device, such as compression stockings or an intermittent compression device, when heparin is contraindicated.
Stress ulcer prophylaxis
-
Provide stress ulcer prophylaxis using H2 blocker. Benefits of prevention of upper GI bleed must be weighed against the potential for development of ventilator-associated pneumonia.
Surviving Sepsis Campaign
Most clinicians look to the Surviving Sepsis Campaign (SSC) for guidance on the translation and implementation of the international guidelines into practice. The SSC, an initiative of the European Society of Intensive Care Medicine, the International Sepsis Forum, and the Society of Critical Care Medicine, was developed to improve the management, diagnosis, and treatment of sepsis.
The SSC partnered with the Institute for Healthcare Improvement (IHI) to incorporate its ‘bundle concept’. A bundle was defined by the SSC/IHI as a group of interventions related to a disease process that, when implemented together, result in better outcomes than when implemented individually. The SSC claim that ‘the science behind the elements of the bundle is so well-established that their implementation should be considered a generally accepted practice’. They also indicate that bundle components can be easily measured as completed or not completed and, as such, the overall bundle—all of the elements taken together—can also be measured as completed or not completed.
Two bundles were developed: the resuscitation bundle that must be completed within six hours and the management bundle that must be completed within 24 hours. The SSC describe the bundles as a distillation of the concepts and recommendations found in the second set of international clinical guidelines published in 2004.
Resuscitation bundle
-
Measure serum lactate.
-
Obtain blood cultures prior to antibiotic administration.
-
Administer broad-spectrum antibiotic within three (emergency department)/one (non-emergency department) hours of admission.
-
In the event of hypotension and/or serum lactate > 4 mmol/L:
-
deliver initial minimum of 20 ml/kg of crystalloid or equivalent
-
apply vasopressors for hypotension not responding to initial fluid resuscitation to maintain mean arterial pressure (MAP) > 65 mmHg.
-
In the event of persistent hypotension despite fluid resuscitation (septic shock) and/or lactate > 4 mmol/L:
-
achieve a central venous pressure (CVP) of ≥ 8 mmHg
-
achieve a central venous oxygen saturation (ScvO2) ≥ 70% or mixed venous oxygen saturation (SvO2) ≥ 65%.
Management bundle
-
Administer low-dose steroids for septic shock in accordance with a standardized ICU policy. If not administered, document why the patient did not qualify for low-dose steroids based upon the standardized protocol;
-
Administer recombinant human activated protein C (rhAPC) in accordance with a standardized ICU policy. If not administered, document why the patient did not qualify for rhAPC;
-
Maintain glucose control ≥ 70, but ≤ 150 mg/dL;
-
Maintain a median inspiratory plateau pressure (IPP) < 30 cm H2O for mechanically ventilated patients.
UK practice in the management and treatment of sepsis
Little information on current practice in the management and treatment of sepsis in the UK exists; and especially prior to the inception of the SSC.
The SSC was formally launched in the UK in June 2005 with a Steering Group formed in September 2005 to aid the introduction of the SSC bundles into hospitals. The Steering Group was composed of representatives from critical care organisations including: the European Society of Intensive Care Medicine; the Intensive Care Society; the British Association of Critical Care Nurses; the Royal College of Nurses; the College of Emergency Medicine; and clinical and managerial staff from Critical Care Networks across the UK. However, despite the claim from the SSC, the fact that many of the bundle elements lacked a rigorous evidence base and that there was no prospective evaluation of bundles per se resulted in low adoption and poor compliance, in large part due to substantial clinical equipoise.
To address low adoption/poor compliance with the bundles, Survive SEPSIS (www.survivesepsis.org), an education programme developed in the UK and approved by the SSC, was launched in September 2007. 6 The launch was designed to bring about the creation of a national network of centres with the aim of raising compliance with the resuscitation bundle (which was 11%) and the management bundle (which was 36%). Compliance targets of 25% for the resuscitation bundle and 50% for the management bundle have been set for April 2009.
In the UK as elsewhere, major challenges lie in placing central venous catheters, starting vasoactive infusions, and measuring central venous oxygen saturation outside the critical care environment. This contributes to further non-compliance with the resuscitation bundle and, in view of the timing (there is an extra 18 hours available in which to complete the management bundle), to around three times as many patients receiving the management bundle as the resuscitation bundle, despite compliance with both being low. 7
This led to the creation of a UK concept of the Sepsis Six – six tasks to be completed by non-specialist staff within the first hour (give 100% oxygen, take blood cultures, give IV antibiotics, start IV fluid resuscitation, check haemoglobin and lactate, place and monitor urinary catheter) – and the need for close and early liaison with critical care to complete the elements for early goal-directed therapy (the last two elements of the resuscitation bundle).
Data from a web-based survey of UK emergency physicians, acute care physicians and intensivists in 2007 (personal communication: Dr Michael Reade) indicated that more than 90% of respondents were aware of the concept of early goal-directed therapy, the basis of the resuscitation bundle, and yet very few delivered this in routine practice.
Data from an audit of rhAPC (one of the elements in the management bundle), conducted by ICNARC between 2002 and 2006, indicate that only one in sixteen (approximately 6%) of admissions with severe sepsis receive this. 8
Intravenous immunoglobulin
IVIg is a blood product derived from human donor blood. The serum from around 1000 to 15,000 donors is required for each batch. 9 The mechanism of action of IVIg is complex, but is increasingly being understood. 10 IVIg is predominantly used in neurology, haematology, immunology and dermatology, but also in nephrology, rheumatology, ophthalmology and other specialties. 9 New uses are emerging and off-label use increasing. 11
IVIg has been proposed as an adjuvant therapy for sepsis since the 1980s, and a number of (predominantly small) RCTs have been performed. Numerous systematic reviews and meta-analyses of IVIg in sepsis have been performed. These have predominantly included the same trials, but have reached differing conclusions. 12
A Cochrane systematic review in 2002 concluded that polyclonal IVIg had a stronger effect than monoclonal IVIg,13 and subsequent systematic reviews have focussed on polyclonal preparations only,14–18 with one review restricted to Immunoglobulin M-enriched IVIg only. 15 Pooled treatment effects in these reviews varied from an odds ratio of 0.35 to a relative risk of 0.79 for all-cause mortality, and all primary analyses were statistically significant. Four of the meta-analyses, when repeated in subsets of high-quality trials (varying from selection of three to eight trials), produced results that were more variable and, in three of the four, were not statistically significant.
Differences between the meta-analyses conducted to date include: the age groups studied – some studies pooled adult, paediatric and neonatal results together, whereas others analysed different age groups separately or restricted to studies in adults only; different inclusion criteria for the severity of infection/sepsis; different definitions of ‘high quality’; and different choices of effect estimate (odds ratio or relative risk) and model (fixed or random effects).
Evaluation of subgroup effects in the different systematic reviews suggested treatment effects may vary by type of IVIg preparation (IgM-enriched versus standard), dose, and duration, as well as by methodological quality, although again these effects were not consistent across the different meta-analyses. In addition, the meta-analysis of Laupland et al. examined funnel plots and found evidence of significant publication bias. 17
As a result of the heterogeneity across studies and inconsistencies in results, the majority of authors concluded that there was insufficient evidence to recommend IVIg as an adjuvant therapy for sepsis and that more evidence, in the form of a large, well-conducted RCT, was required.
Issues and debate on the use of IVIg for sepsis
IVIg is a scarce resource worldwide. Costs have escalated, associated with a reduced demand for plasma-derived factor VIII and albumin. In addition, there are supply issues unique to the UK, that further limit the availability of IVIg. Where IVIg was previously produced in the UK using plasma sourced from within the UK as a by-product of blood donations, plasma must now be imported due to the risk of variant Creuzfeldt Jakob disease (vCJD). In addition, the closure of one UK manufacturer (the Scottish National Blood Transfusion Service) and withdrawal of batches of IVIg due to safety concerns have led to both local and national, transient and longer-term, shortages.
In response to this, the Department of Health implemented a Demand Management Programme for IVIg. The Programme consists of three components: the Demand Management Plan for Immunoglobulin Use;19 Clinical Guidelines for Immunoglobulin Use;20 and the National Immunoglobulin Database. Revised editions of both the Demand Management Plan and Clinical Guidelines were launched in May 2008. Indications for IVIg use are colour-coded in the following way:19
-
red: a disease for which treatment is considered the highest priority because of a risk to life without treatment
-
blue: a disease for which there is a reasonable evidence base but where other treatment options are available
-
grey: a disease for which the evidence base is weak, in many cases because the disease is rare; treatment should be considered on a case-by-case basis, prioritised against other competing demands
-
black: a disease for which there is evidence to suggest that IVIg is not an appropriate treatment and treatment is not recommended.
‘Sepsis in the intensive care unit not related to specific toxins or Clostridium difficile’ is currently a black indication, and consequently IVIg should not be used under any circumstances. 20 The Clinical Guidelines do, however, make a research recommendation that, ‘there is a need for adequately powered high quality RCTs to assess the impact of IVIg in severe sepsis in the general ICU’. 20
In view of the heterogeneity of results of existing RCTs, and the unique supply and demand issues for IVIg, there is an urgent need to establish whether such a trial is necessary and feasible, and whether the costs of carrying out the trial are outweighed by the potential benefit of the resulting information.
Research methods
The study will be conducted in four related phases of work:
Phase I
Objective: To define the appropriate decision problem and to develop a provisional decision-analytic model structure consistent with this and relevant to an NHS setting. To define the requirements for the subsequent phases of work.
Phase I will be based on a review of previous systematic reviews, recent national and international guidelines for the management of sepsis, high quality epidemiological studies and existing cost-effectiveness studies (including any previous decision-analytic models). Initial high-level searches for systematic reviews and guidelines will be conducted by searching major databases including MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Pascal, Science Citation Index (SCI), BIOSIS, Latin American and Caribbean Health Sciences (LILACS), Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE) and Health Technology Assessment Database (HTA). These will be combined with more focused searches in relation to epidemiological and cost-effectiveness studies. For the cost-effectiveness review, additional searches of NHS EED and HEED will also be carried out, along with a search of the Economics Working Papers archive (IDEAS). These reviews will be supplemented by discussion with key individuals involved in service provision and policy.
A key element of this phase will be to identify relevant population subgroups, alternative treatment strategies and outcomes to be considered in the decision-analytic model. Our preliminary review of the literature suggests that there are a number of potentially relevant subgroups who are readily identifiable and differ in their underlying mortality risk. Subgroups may be based on: age; ethnicity; underlying condition; pre-existing organ insufficiency; immunocompromised state; acute severity of illness; infectious organism; site of infection; presence of septic shock; and the number and type of organs failing. 2,21 These subgroups will be revised during this phase of the work. Relevant strategies will comprise both different types of IVIg preparations (including different doses and duration) as well as alternative comparators to IVIg, including current NHS practice as well as alternative strategies proposed for improving the current management and treatment of sepsis (e.g. the resuscitation and management bundles proposed by the SSC). Relevant outcomes will include morbidity, short- and long-term mortality, health-related quality of life, and time course of return to premorbid function. 22
The aim of this phase will be to define the appropriate decision problem relating to the relevant patient populations, alternative interventions (including IVIg) and outcomes to be considered. These will be used to develop a provisional decision-analytic model structure consistent with these issues. This will also serve to identify relevant data sources to be considered in more detail in subsequent phases and to identify the key questions that need to be addressed therein.
At the end of this phase, the results will be presented to the Expert Group in order to obtain feedback on the relevance of the decision problem and the provisional model structure and to ensure that key issues have been appropriately identified at an early stage.
Phase II
Objective: To obtain appropriate inputs for the decision model parameters based on evidence synthesis approaches employing meta-analysis, primary data analysis and other published evidence. To establish current NHS practice based on a national survey and re-analysis of existing audit data, and also to reflect potential anticipated changes to current practice.
Phase I will be used to identify both the range of parameters required to populate the decision model as well as key uncertainties in model parameters themselves. Through this process, we will determine which parameters require more detailed consideration of the primary literature or analysis of locally applicable primary or secondary data, and those which can be populated from existing reviews based on update searches. Phase II will thus comprise a more in-depth review and synthesis of the different inputs required to populate the proposed model.
Although the detailed specification of this phase will be determined by the results of Phase I, our initial expectations are that this work will entail a number of distinct elements including: (i) establishing the clinical effectiveness of IVIg; (ii) defining the current standard of care in the NHS by establishing current practice and associated outcomes, as well as anticipating potential changes to current practice and/or potential barriers to change; (iii) establishing the relative effectiveness of alternative comparators; (iv) estimating resource use and quality of life considerations (attributed to both morbidity and also premature mortality). It will also be essential to consider how these elements relate to the relevant and important population subgroups identified during Phase I.
(i) The clinical effectiveness of IVIg
The application of appropriate methods of evidence synthesis to the existing RCTs of IVIg represents a major element of the proposed work and represents an important extension to the ‘critical appraisal of existing systematic reviews’ outlined in the commissioning brief. As previously stated, our initial review of the various systematic reviews and accompanying meta-analyses have identified a number of important differences between studies. These differences arise in terms of the studies included, the application of separate inclusion/exclusion criteria as well as differences in the subsequent methodologies and analytical approaches employed therein. Consequently, despite the comparatively high-number of previous systematic reviews in this area, the subsequent interpretation and conclusions drawn have often been quite different. These differences also reflect the different approaches employed with respect to evaluation of subgroups and approaches to dealing with heterogeneity more generally across the existing studies. In addition, despite evidence of significant publication bias, there appear to have been few formal attempts to attempt to account for this within existing studies.
These issues are likely to be important factors that need to be adequately understood and reflected in the inputs into the decision model, in order to ensure that subsequent research recommendations are not compromised by potential confounders. Appropriate methods of synthesis are thus required to deal with the heterogeneity both within and between individual RCTs, as well as accounting for potential publication bias. These methods will need to consider the different subgroups, outcomes, comparators and follow-up times reported across the various studies.
It should be emphasised that, in considering the need for and design of a future randomised trial, it is essential that the main causes of heterogeneity in the existing evidence base are understood as far as is possible. Otherwise, there is a danger that a new trial will be just as difficult to interpret as the existing RCT evidence. Apart from the factors mentioned above, we anticipate that much of the heterogeneity arises from differences between trials in the extent to which patients can benefit from IVIg treatment, and thus from differences in patient populations. The evidence synthesis will, therefore, combine the available trial evidence with data on background mortality rates in the underlying conditions.
The review of existing meta-analyses will be used as the main source for identifying relevant RCTs of IVIg. However, additional update searches will also be conducted by searching the major databases considered in Phase I to ensure that any more recent studies are also included.
(ii) Defining the current standard of care in the NHS
The second major element of work will be used to define the current standard of care in the NHS, establishing current practice and associated outcomes, as well as anticipating changes to current practice and/or potential barriers to change. This element will provide the contextual basis for informing the potential improvements that may be achieved through the use of IVIg as well as potentially providing a source of baseline data for the decision model itself.
We anticipate that this element will be principally informed by: (a) a national survey of current UK practice; (b) re-analysis of existing national audit data; and (c) analysis of available data on current usage of, and demand for, IVIg for severe sepsis.
(a) A survey of Clinical Directors for all adult, general critical care units in the UK will be conducted. To maximise response rate, both electronic and paper questionnaires will be used and followed up by direct telephone contact with non-responders at 2–4 weeks. Other evidence based strategies for increasing response rates will also be employed. 23 ICNARC has an established network of contacts in critical care units in the UK and in a recently-conducted (December 2007) survey on ventilator bundle compliance achieved an 84% response rate.
The survey instrument will encompass aspects of the management and treatment of sepsis, both related to the SSC bundles (individual elements within the resuscitation and management bundles) and to other interventions for which a strong evidence base exists (e.g. selective decontamination of the digestive tract). Barriers to bundle elements/important interventions will be explored, estimated future uptake and compliance determined, and views of the potential role for IVIg elicited.
(b) Analyses will be conducted using data from the Case Mix Programme, the voluntary, national comparative audit of patient outcome from adult, general critical care units in England, Wales and Northern Ireland ongoing in 207 units (over 80% coverage). In units participating in the Case Mix Programme, prospective, raw, clinical data are abstracted retrospectively, to precise rules and definitions, by trained, local data collectors and undergo extensive validation, locally and centrally. 24 The Case Mix Programme Database has been independently assessed to be of high quality (www.docdat.org).
Using the Case Mix Programme Database (over 720,000 admissions) and once relevant population subgroups have been established (Phase I), baseline event rates, outcomes, resource utilisation etc associated with usual sepsis care will be estimated, both overall and the variation compared across subgroups.
(a) and (b) Current practice data from the survey will be linked to outcome data (crude and risk-adjusted) from the Case Mix Programme Database to further inform the model.
(c) Any off-licence use of IVIg for sepsis, and declined requests for use, will be identified from the National Immunoglobulin Database. The National Immunoglobulin Database records data on all uses of IVIg and declined requests to use IVIg in the NHS. The process for obtaining permissions to access these data has been commenced.
(iii) The relative effectiveness of alternative comparators
Based on the material examined in Phase I and on advice from the Expert Group, we will have identified what constitute best current practice and alternative comparators that should be considered alongside IVIg. Existing audit and other published data from existing meta-analyses and guidelines will be used to identify baseline outcomes and event rates. These sources will also be relied on to provide data on the relative effectiveness of any alternative comparators that require consideration.
(iv) Resource utilisation, costs and quality of life
Additional data on resource utilisation, costs and quality of life will also be required in order to determine the potential cost-effectiveness of IVIg. Data on resource utilisation will be derived from national audit data and other relevant evidence identified during Phase I. These estimates will provide the basis for estimating the overall costs of managing sepsis, together with the potential impact of the alternative interventions. Resource utilisation will reflect the inputs associated with the interventions themselves as well as the resources associated with sepsis related events (e.g. length of ICU stay, overall length of hospital stay, etc.). These data will be combined with national sources of cost data (e.g. NHS Reference Costs, British National Formulary, etc.) in order to estimate the total costs associated with each strategy considered.
In order to estimate Quality-Adjusted Life Years (QALYs) required for the cost-effectiveness analysis, it will be necessary to systematically search for appropriate published utility or preference scores related to different patient groups and the impact of sepsis. Additional evidence will also need to be considered to quantify potential life-years lost due to premature mortality.
Resource utilisation, costs and quality of life data related to potential complications and side-effects of IVIg will also be considered (e.g. infection by contaminated blood, pulmonary oedema, allergic/anaphylactic reactions, etc.). Safety aspects of IVIg need careful consideration, as it is generally considered poor practice to give IVIg to patients that have a co-existing infection.
At the end of this phase, the Expert Group will meet to provide interpretation to the sources of information identified above and inform the final inputs to the decision model.
Phase III
Objective: To determine the cost-effectiveness of IVIg and to estimate the value of additional primary research.
Phase III comprises two related aspects:
(i) Cost-effectiveness analysis
The decision model will be populated using the most appropriate data identified during Phase II. The mean cost-effectiveness of IVIg compared with current NHS practice and other relevant comparators will be determined based on an assessment of NHS and Personal Social Service costs and QALYs. Consistent with available evidence, the model will also report the cost-effectiveness of alternative treatments for specific subgroups of patients. This may include cost-effectiveness by patients’ underlying risk of particular clinical events.
The model will be probabilistic in order to appropriately characterise the uncertainty in the data used to populate the model and to present the uncertainty in these results to decision makers. 25 Each parameter input in the model will thus be entered as an uncertain, rather than a fixed, parameter by assigning probability distributions to reflect the precision of their estimation. Using Monte Carlo simulation, this parameter uncertainty, is translated into uncertainty in the overall results. This ultimately helps decision makers understand the probability that, in choosing to fund an intervention, they are making the wrong decision – that is, decision uncertainty. This is presented using cost-effectiveness acceptability curves which show the probability that each intervention is cost-effective conditional on a range of possible threshold values which NHS decision makers attach to an additional QALY. 26
The expected cost and QALYs for each of the strategies will be estimated. Strategies will be compared by estimating incremental cost-effectiveness ratios (ICERs), where appropriate. Conventional decision rules will be used to identify strategies which are either dominated or subject to extended dominance. 27 The remaining, non-dominated, strategies will be compared in terms of their ICERs (representing the incremental cost per additional QALY gained). The ICERs will be compared against thresholds representing the incremental cost per QALY used by the National Institute for Health and Clinical Excellence (NICE) to establish value for money in the NHS (in the region of £20,000-£30,000). These thresholds will be used to identify the optimal strategy in terms of cost-effectiveness considerations.
Variability in cost-effectiveness will be investigated by clinical subgroups. For each subgroup, separate ICERs and cost-effectiveness acceptability curves will be presented, and an optimal strategy will be identified using the threshold cost per QALY estimates.
(ii) Value of information analysis
To evaluate future research priorities and to establish whether investment in a large scale randomised trial is likely to be cost-effective, we will use formal methods based on value of information approaches. These approaches will assess the need for major investment in future research and also prioritise the potential research questions. 28
The expected value of perfect information (EVPI) will be estimated for the overall decision problem and for key parameters. 29 EVPI represents the expected costs of decision uncertainty since perfect information would eliminate the possibility of making the wrong decision. Hence, EVPI for the overall decision problem represents the value of eliminating all uncertainty and EVPI for key parameters (termed partial EVPI) represents the value of eliminating uncertainties in particular subsets of parameters. Separate analyses will be undertaken to reflect the variability considered in the decision model itself. Per patient EVPI estimates will be scaled up to reflect the relevant UK population size and will adopt an appropriate time horizon.
EVPI also represents the maximum amount that a decision-maker should be willing to pay for additional evidence to inform this decision in the future. EVPI provides an upper bound on the value of additional research. This valuation provides an initial hurdle, acting as a necessary requirement for determining the potential efficiency of further primary research. Applying this decision rule, additional research should only be considered if the EVPI exceeds the expected cost of the research. In addition to providing a global estimate of the total cost of uncertainty related to all inputs in the model, EVPI can also be estimated for individual parameters (and groups of parameters) contained in the model. The objective of this analysis (termed partial EVPI) is to identify the model parameters where it would be most worthwhile obtaining more precise estimates.
At the end of this phase, the results will be presented to the Expert Group to obtain their feedback and to identify key issues related to the potential design, feasibility and costs of a subsequent trial. If this phase establishes that it could be cost-effective and feasible to carry out further research, separate value of information approaches will be used to identify the optimal design and sample size as part of Phase IV.
Phase IV
Objective: To develop a draft protocol outlining the optimal design, sample size, potential costs and value of commissioning a substantive trial using expected value of sample information (EVSI) approaches.
Phase IV will use EVSI calculations in order to determine the appropriate design, optimal sample size and allocation rate for a future trial. 30 Information from the evidence synthesis on possible differences in treatment effectiveness in different patient groups will be used to generate EVSI per group. EVSI calculations will be set against the potential costs of obtaining such a sample. The difference between the value of the sample (EVSI) and the costs of obtaining the sample are the expected net benefit of sampling and reflect the societal return to the proposed research. The costs themselves comprise both the direct resource costs (representing the fixed costs of further research and the marginal reporting/treatment costs) and opportunity costs including those attributed to different sample sizes and/or longer follow-up periods.
The results from the EVSI approaches will provide the basis for a draft proposal for the trial itself. The draft proposal will be discussed with the Expert Group to discuss feasibility and obtain final feedback and input into the proposal and overall report.
Results of the project will be disseminated to the critical care community through the Intensive Care Society and the Annual Meeting of the Case Mix Programme (attended by representatives of around 200 UK critical care units), and to the wider research community and service users via the ICNARC website.
Ethical arrangements
This study combines evidence synthesis from existing literature, a survey of organisational practice and analysis of existing audit data. The study does not require approval from an NHS Research Ethics Committee.
Analyses of existing data will make use of data collected for the Case Mix Programme. Support for the collection and use of patient identifiable data has been approved for the Case Mix Programme by the Patient Information Advisory Group (PIAG) under Section 251 of the NHS Act 2006 (originally enacted under Section 60 of the Health and Social Care Act 2001) – Approval Number: PIAG 2-10(f)/2005. Section 251 support is reviewed annually by PIAG and covers all aspects of data management including data security. ICNARC is also registered under the Data Protection Act.
Research Governance
The project will be managed according to the Medical Research Council’s Guidelines for Good Research Practice (http://www.mrc.ac.uk/pdf-good_research_practice.pdf) and Procedure for Inquiring into Allegations of Scientific Misconduct (http://www.mrc.ac.uk/pdf-mis_con.pdf). ICNARC has developed its own policies and procedures based on these MRC guidelines, which are adhered to for all research activities at ICNARC. In addition, ICNARC has contractual confidentiality agreements with all members of staff. Policies regarding alleged scientific misconduct and breach of confidentiality are reinforced by disciplinary procedures.
Day-to-day running of the project will be overseen by a Project Management Group (KMR, DAH, SJP, AEA, NJW, Research Fellow), which will meet face-to-face at the start and end of each phase of the project and will maintain contact throughout the phases by telephone and electronic conferencing. An Expert Group, consisting of the other co-applicants plus the service user representative (see below), will meet at pre-defined, regular intervals throughout the study.
Project timetable and milestones
See Appendix 1 for project timetable.
Milestones
-
Month 1 (Apr 2009): Project Management Group meet.
-
Month 2 (May 2009): Provisional model structure presented to Expert Group.
-
Month 6 (Sep 2009): Literature searches/evidence synthesis complete; Survey complete; Analysis of Case Mix Programme Database and National Immunoglobulin Database complete.
-
Month 7 (Oct 2009): Expert Group meet to interpret above results.
-
Month 10 (Jan 2010): Cost-effectiveness analysis and value of information analysis results presented to Expert Group.
-
Month 12 (Mar 2010): Draft protocol and costs for multicentre RCT presented to Expert Group for final input; Final report to HTA.
Expertise
ICNARC, and KMR and DAH as senior researchers within ICNARC, have a track record in the conduct and dissemination of results of large, multicentre research studies and methodological studies in both adult and paediatric intensive care (e.g. PAC-Man – 1014 patients in 65 units – the first, academic, multicentre RCT in UK adult critical care funded by NIHR HTA). KMR has extensive experience as Principal Investigator for both methodological and evaluative research studies in critical care. DAH has considerable experience of designing, conducting and analysing multicentre studies, and has particular expertise in risk adjustment and analysis of observational data. Further details of ICNARC’s research can be seen at http://www.icnarc.org.
SJP is a senior researcher at the Centre for Health Economics and currently leads the Technology Appraisal Programme of work for NICE within the centre. He is also a lead member and manager of the NICE Decision Support Unit. SJP has extensive experience related to the methodology and application of decision-analytic modelling, evidence synthesis and value of information approaches, including previous pilot work for the HTA Programme using value of information to inform commissioning decisions. Further details of the work of the Centre for Health Economics can be seen at http://www.york.ac.uk/inst/che.
AEA is the PI of an MRC-funded research programme ‘Multi-parameter evidence synthesis in epidemiology and decision making’, formerly within the Health Services Research Collaboration and now transferred to University of Bristol. NJW is a Senior Research Fellow within the programme. They are internationally recognised for their extensive expertise in advanced evidence synthesis methods, and particular experience with synthesis for disease natural history and with comparisons of multiple treatment alternatives, in a cost-effectiveness setting. NJW and AEA have also contributed landmark publications on EVI analysis, several in collaboration with the Centre for Health Economics in York. Further details of their work can be seen at http://www.bris.ac.uk/cobm/research/mpes.
GR, RB and MS are internationally renowned opinion leaders in the field of severe sepsis and sepsis trials. GR is a member of the SSC Executive Committee, and both GR and RB are members of the SSC Guidelines Committee and were authors on the recently updated international guidelines for the management of severe sepsis and septic shock. MS is an expert in the basic science relating to severe sepsis and was the Intensive Care Society representative to the Department of Health IVIg Guideline Development Group. WACS is a leading expert in the mechanism of action of IVIg and is a member of the Department of Health IVIg Expert Working Group, involved with the development of the Demand Management Plan and Clinical Guidelines.
Service Users
ICNARC has a history of involving and listening to users’ views and experiences and has access to a wide range of users (patients and their families and close friends) from its recent funding of two DIPEx modules (http://www.dipex.org/intensivecare and http://www.dipex.org/relativesofintensivecare).
All involvement of service users in this study will follow the guidelines and recommendations for good practice from INVOLVE (http://www.invo.org.uk). Maureen Dalziel will join the Expert Group as a service user representative. Maureen is a public health physician by training, and a member of ICNARC’s Board of Trustees, and has held senior board appointments within the NHS and the Department of Health. However, of specific relevance to this project, Maureen also has personal experience of critical care, having previously been admitted to a critical care unit with severe sepsis.
Justification of support required
KMR (5%, 12 months) will oversee the running of the project and chair the Expert Group. DAH (10%, 12 months) will undertake analyses of the Case Mix Programme Database to inform the decision model. SJP (10%, 12 months) and AEA (5%, 12 months) will oversee the evidence synthesis and decision analysis work, which will primarily be carried out by NJW (50%, 12 months) and a Research Fellow (50%, 12 months) based in the Centre for Health Economics, York (to be recruited). An Administrative Assistant at ICNARC (25%, 12 months) will co-ordinate the administrative aspects of the project, including arranging the Project Management Group and Expert Group meetings, and will administer and follow up the survey of current practice. No costs have been included for clinical co-applicants on the basis of time commitment to the project, but all members of the Expert Group will receive an honorarium for meetings attended.
Project infrastructure costs (Project Management Group and Expert Group meetings) will ensure proper governance of the project. To maximise dissemination of the project results, costs have been included for one researcher to attend an international conference to present the results. Consumables required to administer and follow-up the survey of current practice have been based on actual figures from previous national surveys administered by ICNARC. Costs for literature searching and document retrieval were provided by the Centre for Reviews and Dissemination, York. Indirect costs for staff based at ICNARC have been included as 46% of Direct Staff Costs as agreed with HTA Finance Manager, Kim Wherry, 24 July 2008.
Flow diagram.
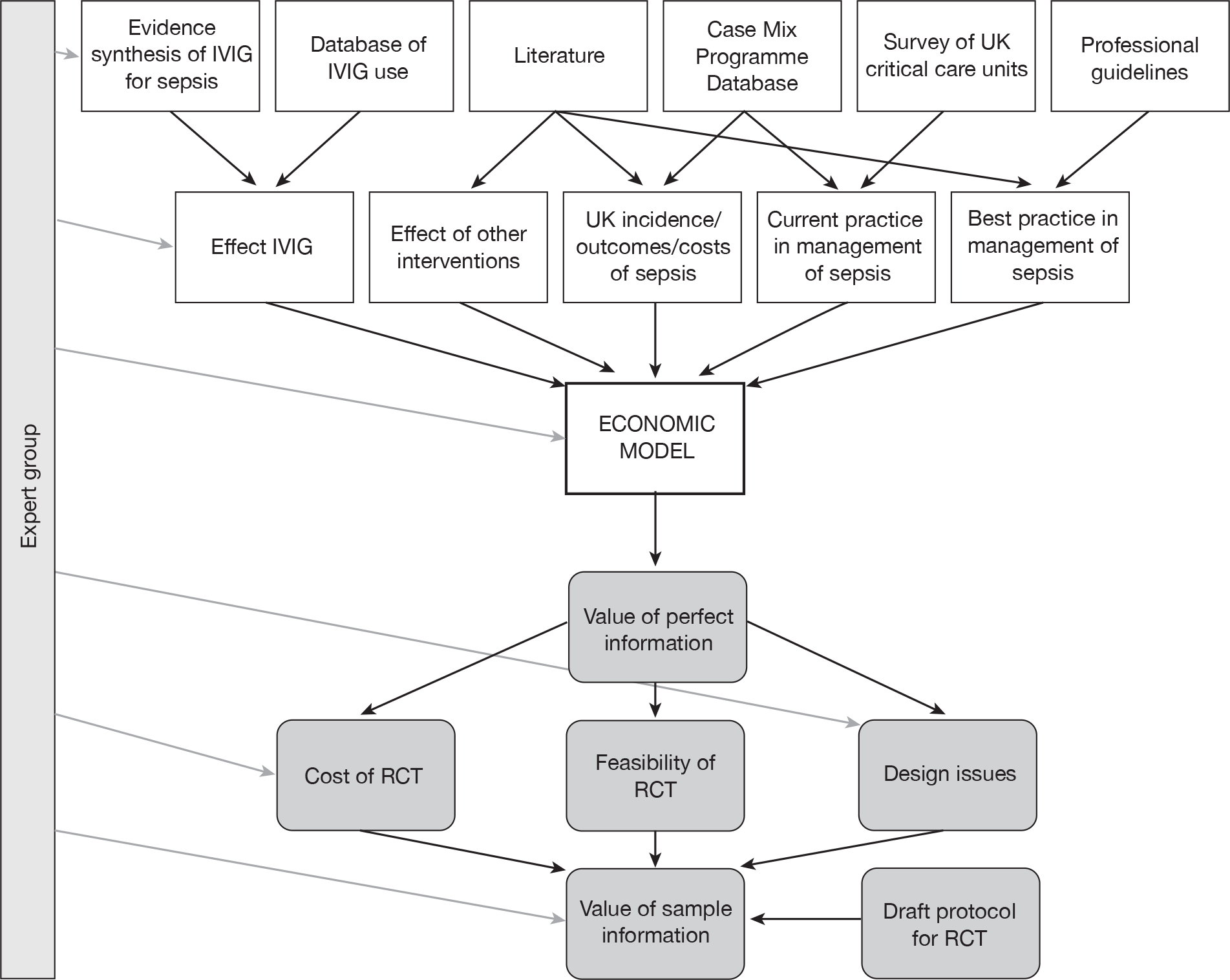
References
- Levy MM, Fink MP, Marshall JC, . 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250-6.
- Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care 2006;10.
- Linde-Zwirble WT, Angus DC. Severe sepsis epidemiology: sampling, selection, and society. Crit Care 2004;8:222-6.
- Dellinger RP, Levy MM, Carlet JM, . Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008;36:296-327.
- Atkins D, Best D, Briss PA, . Grading quality of evidence and strength of recommendations. BMJ 2004;328.
- Daniels R. Survive SEPSIS Launch in UK n.d. URL: http://www.survivingsepsis.com/node/155.
- Daniels R. Severe Sepsis Care Bundles n.d. URL: http://www.library.nhs.uk/Emergency/Page.aspx?pagename=SEPSIS.
- Rowan KM, Welch CA, North E, Harrison DA. Drotrecogin alfa (activated): real-life use and outcomes for the UK. Crit Care 2008;12.
- Jolles S, Sewell WA, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol 2005;142:1-11.
- Sewell WA, Jolles S. Immunomodulatory action of intravenous immunoglobulin. Immunology 2002;107:387-93.
- Wallington T. New uses for IVIgG immunoglobulin therapies. Vox Sang 2004;87:155-7.
- Werdan K. Mirror, mirror on the wall, which is the fairest meta-analysis of all?. Crit Care Med 2007;35:2852-4.
- Alejandria MM, Lansang MA, Dans LF, Mantaring JB. Intravenous immunoglobulin for treating sepsis and septic shock. Cochrane Database Syst Rev 2002.
- Pildal J, Gøtzsche PC. Polyclonal immunoglobulin for treatment of bacterial sepsis: a systematic review. Clin Infect Dis 2004;39:38-46.
- Norrby-Teglund A, Haque KN, Hammarstrom L. Intravenous polyclonal IgM-enriched immunoglobulin therapy in sepsis: a review of clinical efficacy in relation to microbiological aetiology and severity of sepsis. J Intern Med 2006;260:509-16.
- Turgeon AF, Hutton B, Fergusson DA, . Meta-analysis: intravenous immunoglobulin in critically ill adult patients with sepsis. Ann Intern Med 2007;146:193-20.
- Laupland KB, Kirkpatrick AW, Delaney A. Polyclonal intravenous immunoglobulin for the treatment of severe sepsis and septic shock in critically ill adults: a systematic review and meta-analysis. Crit Care Med 2007;35:2686-92.
- Kreymann KG, de Heer G, Nierhaus A, Kluge S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med 2007;35:2677-85.
- Department of Health . Demand Management Plan for Immunoglobulin Use 2008.
- Clinical Guidelines for Immunoglobulin Use. London: Department of Health; 2008.
- Padkin A, Goldfrad C, Brady AR, . Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med 2003;31:2332-8.
- Marshall JC, Vincent JL, Guyatt G, . Outcome measures for clinical research in sepsis: A report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Critical Care Medicine 2005;33:1708-16.
- Edwards P, Roberts I, Clarke M, . Increasing response rates to postal questionnaires: systematic review. BMJ 2002;324.
- Harrison DA, Brady AR, Rowan K. Case mix, outcome and length of stay for admissions to adult, general critical care units in England, Wales and Northern Ireland: the Intensive Care National Audit & Research Centre Case Mix Programme Database. Crit Care 2004;8:R99-111.
- Briggs AH, Goeree R, Blackhouse G, O’Brien BJ. Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Making 2002;22:290-308.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87.
- Johannesson M, Weinstein MC. On the decision rules of cost-effectiveness analysis. J Health Econ 1993;12:459-67.
- Claxton K, Ginnelly L, Sculpher M, . A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme. Health Technol Assess 2004;8:1-103.
- Felli JC, Hazen GB. Sensitivity analysis and the expected value of perfect information. Med Decis Making 1998;18:95-109.
- Ades AE, Lu G, Claxton K. Expected value of sample information calculations in medical decision modeling. Med Decis Making 2004;24:207-27.
APPENDIX 1. Study timeline
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apr-09 | May-09 | Jun-09 | Jul-09 | Aug-09 | Sep-09 | Oct-09 | Nov-09 | Dec-09 | Jan-10 | Feb-10 | Mar-10 | |
| Phase I | ||||||||||||
| Initial development of model | ||||||||||||
| Phase II | ||||||||||||
| Update searches for CEA/QOL | ||||||||||||
| Evidence synthesis | ||||||||||||
| Survey of current practice | ||||||||||||
| Analyses of CMPD | ||||||||||||
| Phase III | ||||||||||||
| Economic modelling/VOI | ||||||||||||
| Phase IV | ||||||||||||
| Development of trial protocol | ||||||||||||
| Final report to HTA | ||||||||||||
| Project Management Group meetings | X | X | X | X | ||||||||
| Expert Group meetings | X | X | X | X | ||||||||
List of abbreviations
- ACCP
- American College of Chest Physicians
- AIC
- Akaike Information Criterion
- ALI
- acute lung injury
- APACHE
- Acute Physiology and Chronic Health Evaluation
- ARDS
- acute respiratory distress syndrome
- BNF
- British National Formulary
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CMP
- Case Mix Programme
- CPR
- cardiopulmonary resuscitation
- CVP
- central venous pressure
- DIC
- deviance information criterion
- ED
- emergency department
- EGDT
- early goal-directed therapy
- ENBS
- expected net benefit of sampling
- EQ-5D
- European Quality of Life-5 Dimensions
- ESICM
- European Society of Intensive Care Medicine
- EVI
- expected value of information
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- EVSI
- expected value of sample information
- HUI
- health utilities index
- ICER
- incremental cost-effectiveness ratio
- ICNARC
- Intensive Care National Audit & Research Centre
- Ig G
- immunoglobulin G
- Ig M
- immunoglobulin M
- IHI
- Institute for Healthcare Improvement
- IQR
- interquartile range
- IVIG
- intravenous immunoglobulin
- IVIGAM
- IgM-enriched polyclonal IVIG
- MAP
- mean arterial pressure
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Clinical Excellence
- NNT
- number needed to treat
- OR
- odds ratio
- PAC
- pulmonary artery catheter
- PCT
- primary care trust
- PROWESS
- Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis study
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- rhAPC
- recombinant human activated protein C
- SAPS
- Simplified Acute Physiology Score
- SCCM
- Society for Critical Care Medicine
- ScvO2
- central venous oxygen saturation
- SD
- standard deviation
- SDD
- selective decontamination of the digestive tract
- SE
- standard error
- SICS
- Scottish Intensive Care Society
- SIRS
- systemic inflammatory response syndrome
- SOFA
- Sequential Organ Failure Assessment
- SSC
- Surviving Sepsis Campaign
- SvO2
- mixed venous oxygen saturation
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington