Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 08/60/01. The contractual start date was in April 2009. The draft report began editorial review in February 2011 and was accepted for publication in December 2011. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design.The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
PA has done consultancy and/or research on smoking cessation with McNeil, Pfizer and Xenova Biotechnology. MRM has received research funding for projects related to smoking cessation from Pfizer.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2012. This work was produced by Chen et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to NETSCC. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2012 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background and brief from the Health Technology Assessment programme
The published brief is as described below.
Introduction
The aim of the Health Technology Assessment (HTA) programme is to ensure that high-quality research information on the costs, effectiveness and broader impact of health technologies is produced in the most efficient way for those who use, manage, provide care in, or develop policy for, the NHS. Topics for research are identified and prioritised to meet the needs of the NHS. HTA forms a substantial portfolio of work within the National Institute for Health Research (NIHR) and each year about 50 new studies are commissioned to help answer questions of direct importance to the NHS. The studies include both primary research and evidence synthesis.
Research question
What is the effectiveness and cost-effectiveness of internet, PC and other electronic aids to help people stop smoking?
-
Technology Computer programs, internet sites, and other electronic media (such as mobile telephone texts) that help people stop smoking, or enhance the effectiveness of existing smoking cessation programmes by reducing relapse.
-
Patient group Adult smokers currently in NHS smoking cessation programmes.
-
Setting Those relevant to community-based programmes in the UK.
-
Control or comparator treatment No intervention or standard self-help material treatment.
-
Design An evidence synthesis of electronic aids that can be used in addition to standard NHS smoking cessation programmes to address the question that electronic media (and which aspects of the use of those media) have been shown to be effective in aiding smoking cessation and also to model the cost-effectiveness of using these media. Researchers should develop research recommendations for future HTA trials from their findings.
-
Primary outcomes Smoking cessation (ideally biochemically validated cessation rather than self-report).
-
Minimum duration of follow-up Six months.
Background to commissioning brief
Cessation services in the NHS achieve variable success rates with one-to-one and group sessions with smokers who want to quit, with wide variation across different age groups. Approaches to behaviour change can be supplemented with electronic aids, and this may significantly increase quit rates and prevent a proportion of cases that relapse.
In 2007, nearly 15 million households in Great Britain (61%) had internet access;1 many more people use mobile telephones. It is proposed that if computer and internet interventions to aid smoking cessation were shown to be effective, there would be potential for a large number of smokers to have access to this type of intervention at minimal cost to health services.
A recent systematic review showed some evidence that computer-based interventions changed smoking behaviour but identified a need for further research. 2 We are asking for further evidence synthesis because we want an estimate of cost-effectiveness and more precise specification of future research recommendations.
The harm of smoking and the benefits of cessation
Smoking is harmful to health. On average, lifelong smokers lose 10 years of life, and about half of all lifelong smokers have their lives shortened by smoking. 3,4 Half of these premature deaths occur before the age of retirement. 4 Fortunately, stopping smoking reverses or prevents many of these harms. Stopping smoking before the age of 40 years (when most smokers have smoked for at least 20 years) results in minimal loss of life expectancy. 2 In terms of population health, if no child ever took up smoking, the effect on mortality in the short to medium term would be relatively modest (although, of course, considerable over a longer period). 5 However, if smoking cessation rates were doubled, the effect in the short term would be considerable. Promoting smoking cessation, in conjunction with ongoing prevention efforts, is therefore a public health priority.
Most UK smokers are open to the idea of stopping smoking. More than 8 out of 10 smokers wish they had never started,6 7 out of 10 want to stop smoking,7 half of them think that they will be stopped in a year,7 and more than 4 out of 10 try to stop each year. 8 The problem of persistent smoking is therefore explained more by the failure of attempts to stop smoking because of addiction than it is by the desire of smokers to persist with their smoking.
Traditional interventions for promoting smoking cessation
Intervention for smoking cessation may be applied at a population level (e.g. taxation, restrictions on smoking in public places, health promotion campaigns, restrictions on sales or advertising, etc.) or at an individual level. Interventions for individual smokers to promote cessation fall into two broad categories, sometimes termed cessation induction and aid to cessation. 9 A typical cessation induction intervention is physician advice to stop smoking. Physician advice prompts smokers to make a quit attempt because they are concerned about the effect on their health, a concern that is reinforced by their doctors. 10 A cessation induction intervention might enhance cessation because it induces more people to try to stop smoking and a proportion of these people succeed. It may not enhance the success rates of those attempts to stop. An aid to cessation, in contrast, makes quitting more successful. Conceptually, there are three methods by which aids to cessation might work:
-
Enhance the motivation of the smoker to put up with the discomfort of withdrawal and to resist desires and needs to smoke. Behavioural support or counselling aims to do this.
-
Enhance the ability of a smoker to enact their intention to quit, for example by clarifying plans to cope with cravings. Behavioural support or counselling aims to do this.
-
Reduce the desire or need to smoke. Pharmacotherapy to support smoking cessation probably works in this way. 11
Thus, an aid to cessation intervention might not prompt individuals to attempt to stop, but would instead support individuals that do so. This will lead to improved population cessation rates.
The reach of traditional smoking cessation interventions
Smoking cessation interventions are typically given face to face. An archetypal intervention is advocated in current English and Welsh National Institute for Health and Clinical Excellence (NICE) guidelines. 12 A smoker attends her or his general practitioner (GP) (family physician) and is advised to stop smoking (cessation induction). A smoker who agrees to this is offered referral to the NHS Stop Smoking Services (SSS). There, the smoker will be given regular behavioural support and will usually be prescribed pharmacotherapy to support the quit attempt (aid to cessation).
US national guidelines recommend that physicians behave in this way with every smoker at every consultation. 13 NICE guidance is less explicit on the frequency. 12 However, there is physician resistance to giving advice frequently and routinely, and rates of brief advice are much lower than suggested in guidelines. All UK primary care physicians record smoking status;14 about 30% of smokers receive advice to quit annually15 and about 3% receive pharmacotherapy to support quitting. 16 Perhaps, as a consequence, most people who try to quit do so without support from the NHS. 8 Only about 5% of smokers use the NHS SSS annually, out of > 40% who try to stop. 8
Self-help interventions to reach smokers
Despite their past experience, many smokers believe that they can and should quit smoking without formal treatment programmes. 17 Self-help interventions can support smoking cessation, by acting either as a cessation induction intervention or as an aid to cessation intervention, or both. The typical intervention is a leaflet, or perhaps an advertisement, in which a smoker is confronted with good reasons to stop smoking, as the GP might give in consultation. Other leaflets aim to take smokers through the quitting process, essentially by writing down the kind of advice that is typically given in a smoking cessation clinic. A Cochrane review showed that overall self-help interventions increased cessation rates, with a risk ratio (RR) of 1.21 [95% confidence interval (CI) 1.05 to 1.39]. 18 The self-help materials did not show evidence of additional efficacy when added to other interventions, such as physician advice or pharmacotherapy.
Static, targeted and tailored interventions, and collaborative filtering
In a static information intervention a typical leaflet is produced for all smokers and it is likely that some information therein will not be relevant for many readers. A targeted information intervention uses some broad-brush information to ensure that more of the content is relevant to its reader. For example, leaflets could be geared towards broad groups, such as people planning to stop smoking and looking for tips and advice on the best way to do this, or to others who are wondering whether or not they should. Such a self-help intervention could be delivered by leaflet, using the title of the leaflet to signal its content. In principle, electronic aids such as websites can function as static leaflets. Many websites feature information about the harms of smoking and ways to stop smoking that a person could choose to read. There is no obvious reason why smoking cessation leaflets should not work on the web, and these can be static or targeted.
A tailored intervention is more individualised. A typical behavioural support session will open with the therapist asking the patient to describe previous attempts to stop smoking and the difficulties encountered. The therapist will typically assess tobacco dependence. Advice is then tailored to the individual based on these data, formed around a basic quit plan on which to draw. A key competency for stop smoking advisors is ‘building rapport’ in order that the client feels that the practitioner ‘cares’ about their quit attempt and thus does not want to let them down; it also makes the client more receptive to information. For experienced practitioners this is where tailoring is most likely to take place, and therefore tailoring of intervention style as well as information that may be critical. Clearly, only the latter aspect translates to computer and electronic aids. Tailoring requires some individual assessment and hence uses either a person or an ‘expert system’ (EXP) to decode questionnaire responses and provide material tailored to an individual. The Cochrane review18 showed some evidence that tailored materials were more effective than standard self-help materials, with an RR of 1.31 (95% CI 1.20 to 1.42). Computerised interventions are nearly always needed to produce tailored self-help interventions.
Websites such as Amazon or iTunes use collaborative filtering to suggest products that one might like to purchase based on the choices of similar individuals. However, collaborative filtering has not been used extensively in smoking cessation interventions or more generally in behaviour change contexts.
Computer and other electronic aids for smoking cessation
Computer and other electronic aids for cessation have been tested both as cessation induction and aid to cessation interventions, as well as a combination of the two. A typical study of the effect of electronic aids for cessation would enrol a mixed population of smokers and ask questions to segment the population into groups. Those who are deemed ready to stop would be prompted to do so and supported as in an aid to cessation study. Typically, the aids would involve a mixture of the first two ways to enhance cessation, namely enhancing motivation and enhancing planning. Most typically, these studies do not involve pharmacotherapy to support cessation because this would be inappropriate for most participants, most of whom would not be undertaking a quit attempt near the beginning of the intervention. Such interventions occasionally encourage the use of medication from other sources, for example a GP. Those who are not deemed ready to stop would typically be encouraged to work towards stopping by some means or other, typically by being encouraged to do various psychological or behavioural exercises. Many of these interventions are based on one or more theories of behavioural change.
A smaller group of interventions have been tested as ‘pure’ aids to cessation. Most typically these are interventions funded by the pharmaceutical companies to provide added value to people using their products. Most major manufacturers have these kinds of interventions and participants need a code from their medication to gain access to the computer program. Here participants have typically negotiated a quit day with their health-care provider, although with over-the-counter nicotine replacement therapy (NRT) this is usually not the case. Having a quit day usually means that a smoker smokes as normal until the day before the quit day. On the quit day, the person tries not to smoke at all, having typically removed all cigarettes from the house if possible. In such circumstances, the electronic aids are aiming to provide similar support as might be provided by face-to-face behavioural support or counselling. A major focus of these kinds of interventions is motivating adherence to medication, something that is usually not present or not so prominent in cessation induction or mixed cessation induction and aid to cessation studies. In addition to providing regular support advice, the electronic aids can sometimes respond to crises, such as overwhelming urges to smoke or a lapse. Some aid to cessation interventions can be very intensive, providing daily contact and support, because smokers have committed to a course of action that they would find difficult to complete without support. The cessation induction trials tend to have sporadic contact with participants. This is because it is not usually acceptable to attempt to pressure people into quitting through daily text messages, whereas a person trying to quit is engaging in a daily struggle and frequent contact can be helpful.
Potential public health importance
Computerised interventions have considerable potential in public health for at least three reasons. The first relates to the evidence that many people are ambivalent about smoking,18,19 and a good number are prepared to make quit attempts with only modest prompting. 10,20 Electronic aids could provide such a prompt and, although most quit attempts end in early failure, a small proportion succeed. 21 Thus prompting more quit attempts will improve population cessation rates. The second reason relates to the established efficacy of behavioural support. Although the most effective support for cessation is the NHS SSS, only about 5% of smokers use this annually, whereas > 40% try to stop. 8 It is possible that the behavioural support provided by electronic aids could reach many of these smokers who otherwise use no support and thus might have much higher reach than the NHS SSS, even if it is somewhat less effective than behavioural support. Third, medication adherence is also often poor in studies without behavioural support,22,23 and better adherence is associated with a higher likelihood of quitting smoking. 24,25 By supporting adherence to medication, computerised interventions may improve the success of already effective interventions that are widely used.
The question of whether or not electronic aids are important to public health depends on their efficacy relative to the comparator, their reach, and their costs. Most smoking cessation interventions are only modestly effective in the medium term in comparison with other medical interventions. However, the benefits of cessation are very great and nearly all smoking cessation interventions are very cheap. Consequently, smoking cessation interventions are among the most cost-effective of all health-care interventions,26 according to NICE, meaning that the cost per quality-adjusted life-year (QALY) saved is lower than for many other health-care interventions. At first glance, electronic aids are likely to be cost-effective if they are effective. 27 Electronic aids might be costly to set up but their delivery is either free or very cheap as they require little in the way of labour costs to deliver. It is as cheap to deliver 5000 interventions as 50, as these can exploit economies of scale. This feature of electronic aids means that we need to extrapolate from the costs incurred in a trial to a population in which it might be delivered when considering the scale of the benefits that might accrue.
Previous reviews on the efficacy of computer and electronic smoking cessation interventions
We found six relevant reviews, which are summarised in Tables 1–3. These reviews had a more restricted focus than we had in this report, typically focusing on subsets of electronic interventions. All of these studies conclude that electronic interventions have evidence of effectiveness, but the issue of which electronic interventions are most effective remains uncertain. None of the reviews clearly distinguished between types of interventions or explored whether effectiveness related to the medium used, intensity of intervention or the type of intervention delivered. We explore these questions in our review, which encompassed a much wider range of studies.
| Review | Search strategies | Focus/inclusion criteria | Exclusion | Quality assessment | Meta-analysis | Comments |
|---|---|---|---|---|---|---|
| Strecher 199928 (10 RCTs) | Not stated | First-generation tailored smoking cessation programme that used printed media | Not stated | No | No | Classified smoking cessation materials as general, targeted or tailored material |
| Walters et al. 20062 (19 RCTs: four adolescent and 15 adult) | Searched MEDLINE, CINAHL and PsycINFO between 1995 and August 2004; English language only |
1. Used computers (web-based, server-based or stand-alone programs) as a significant part of the intervention 2. Included at least one comparison or control condition 3. Included at least one intervention condition that was achieved without significant human contact 4. Reported at least one outcome directly related to smoking behaviour |
Non-automated e-mail, chat rooms, discussion boards, or direct personal contact as the primary delivery mode | No | No | No search of EMBASE and grey literature, and literature published before 1995 |
| Shahab and McEwen 200929 (15 RCTs) | PubMed, PsycINFO, CINAHL Plus, EconLit, ISI Web of Science, CENTRAL 1990–2008 | Internet-only interventions. Also interventions had to be interactive. At least 1 month of follow-up. English language only | Studies providing insufficient detail/data and those aimed at health professionals were excluded | Yes | Yes | Exclusion of studies due to inadequate reporting of data may aggravate publication bias |
| Myung et al. 200930 (22 RCTs) | MEDLINE, EMBASE, CENTRAL inception to August 2008 | RCTs that reported the effects of a web- or computer-based smoking cessation programme for current smokers if they included at least 3 months of follow-up data | Trials involving smokeless tobacco users and quasi-experimental trials were excluded | Yes (Jadad score) | Yes | Jadad score is not suitable for this type of intervention |
| Whittaker et al. 200931 (four RCTs) | MEDLINE, EMBASE, CINAHL, PsycINFO, The Cochrane Library, the National Research Register and the Clinical Trials register up to 10 December 2008 | Randomised or quasi-randomised trials that examined effects of any type of mobile telephone-based intervention on smoking abstinence | Trials where mobile telephones were seen as an adjunct to face-to-face or internet-based programmes such as to remind participants of appointments or where the effects of the various components of a multifaceted programme could not be separated were excluded | Yes (assessment of risk of bias) | Yes | |
| Krishna et al. 200932 (four RCTs) | MEDLINE 1950 to May 2008 |
RCTs or controlled studies that: 1. evaluated delivery of health information or educational intervention using mobile telephone or text messaging and measured change in the process of care and/or health outcomes 2. used wired internet to provide information through e-mail or the web in addition to wireless communication were included 3. published in a language other than English with a complete English abstract |
Studies that did not use a control group | No | No | Only MEDLINE was searched. Searches could have possibly missed some relevant studies |
| Review | Key findings | Research recommendations |
|---|---|---|
| Strecher 199928 | Most (6 out of 9) of the studies provided evidence that computer-based tailored interventions may be effective for smoking cessation. There is evidence from two RCTs that combined NRT with tailored materials had positive effect on smoking cessation | Future studies should examine ‘Why, and under what circumstances, do tailored materials have an influence on smoking cessation’ |
| Walters et al. 20062 | There is evidence from some studies (9 of the 19) that computer-based intervention had positive effect on smoking cessation at the longest follow-up | Future studies should identify patients’ subgroups that may benefit from computer-based programmes and clarify what types of programmes are most effective. Future studies should also examine whether or not there is evidence of differential effect of the intervention across ethnicity, sex and problem severity |
| Shahab and McEwen 200929 | Three RCTs provided evidence that web-based, tailored, interactive smoking cessation interventions were more effective than untailored booklet or e-mail interventions (pooled RR = 1.85, 95% CI 1.4 to 2.3). Results from seven RCTs provided evidence that only interventions targeted at smokers motivated to quit were effective for smoking cessation (pooled RR = 1.4, 95% CI 1.0 to 2.0) | There is a need for substantial further research on relative efficacy of static websites and face-to-face counselling with interactive online programmes. Futures studies should be reported in sufficient details to allow for proper evaluation of representativeness of participants and actual reach of the programmes |
| Myung et al. 200930 | Nine and 13 trials provided evidence that web-based intervention (pooled RR = 1.40, 95% CI 1.13 to 1.72) and computer-based intervention (pooled RR = 1.48, 95% CI 1.25 to 1.76) had significant effect on smoking cessation, respectively. The authors found that the effect of web- or computer-based was similar to that of counselling interventions. The authors concluded that there is sufficient evidence to support the use of web- and computer-based smoking cessation programmes for adult smokers | More studies with large sample sizes are needed to confirm the effectiveness of the smoking cessation interventions among adolescent or young adult smokers. Futures studies should evaluate overall socioeconomic status of the participants |
| Whittaker et al. 200931 | There is evidence from two RCTs that text messages programmes were effective in increasing self-reporting quit rate in the short term (pooled RR = 2.18, 95% CI 1.80 to 2.65). Results from two RCTs provided evidence that internet and mobile telephone programmes were effective in increasing self-reported quit rates in both short- and long-term (pooled RR = 2.03, 95% CI 1.40 to 2.94) | To allow for comparison, future studies should adhere to standard outcome measures of abstinence and be reported in sufficient detail |
| Krishna et al. 200932 | There is evidence from four studies that mobile telephone with voice or text messages for health information was effective in increasing smoking quit rates | The authors reported that because of small sample sizes the findings may not be generalisable to other populations and recommended that more controlled studies with larger sample sizes should be conducted in order to better understand the effectiveness of cell phone interventions in improving health outcomes. There is also need for more studies on the cost-effectiveness, technical and financial feasibility of adoption |
| No. | Study | Strecher 199928 | Walters et al.20062 | Krishna et al.200932 | Myung et al.200930 | Shahab and McEwen 200929 | Whittaker et al.200931 | Current HTA | Comment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Burling et al. 198933 | i | i | ||||||
| 2 | Owen et al. 198934 (n = 208) | i | i | Not identified by our search | |||||
| 3 | Schneider et al. 199035 | i | |||||||
| 4 | Curry et al. 199136 (n = 1217) | i | i | Not identified by our search | |||||
| 5 | Prochaska et al. 199337 | i | i | ||||||
| 6 | Strecher et al. 199438 (n = 72) | i | i | ||||||
| 7 | Strecher et al. 199438 (n = 297) | i | i | ||||||
| 8 | Curry et al. 199539 | i | i | i | |||||
| 9 | Kreuter and Strecher 199640 | i | |||||||
| 10 | Pallonen et al. 199841 | i | x | Adolescent population | |||||
| 11 | Dijkstra et al. 199842 (Health Educ Res) | i | i | i | |||||
| 12 | Dijkstra et al. 199843 (Health Psychol) | i | i | i | |||||
| 13 | Dijkstra et al. 199944 | i | i | ||||||
| 14 | Ershoff et al. 199945 | i | |||||||
| 15 | Velicer et al. 199946 | i | i | ||||||
| 16 | O’Neill et al. 200047 | i | i | ||||||
| 17 | Orleans et al. 200048 | ia | i | ||||||
| 18 | Shiffman et al. 200049,50 | ib | i | i | i | ||||
| 19 | Aveyard et al. 199951,52 | ic | i | x | Adolescent smoking prevention and cessation (age 13–14 years) | ||||
| 20 | Etter and Perneger 200153,54 | i | i | i | |||||
| 21 | Lennox et al. 200155 | i | i | ||||||
| 22 | Prochaska et al. 200156 (Addict Behav) | i | i | i | |||||
| 23 | Prochaska et al. 200157 (Prev Med) | i | i | ||||||
| 24 | Shiffman et al. 200158 | i | i | i | |||||
| 25 | Ausems et al. 200259 | i | x | Adolescent smoking prevention and cessation | |||||
| 26 | Riley et al. 200260 | i | |||||||
| 27 | Borland et al. 200361 | i | i | i | |||||
| 28 | Lawrence et al. 200362–64 | i | i | ||||||
| 29 | Ausems et al. 200465 | i | x | Adolescent smoking prevention and cessation | |||||
| 30 | Borland et al. 200466 | i | i | ||||||
| 31 | Clark et al. 200467 | Potentially eligible studyd | |||||||
| 32 | Lenert et al. 200468 | i | x | Not a RCT – historical control group | |||||
| 33 | Prochaska et al. 200469 | i | |||||||
| 34 | Etter et al. 200570,71 | i | |||||||
| 35 | Prochaska et al. 200572 | i | |||||||
| 36 | Rodgers et al. 200573,74 | ie | i | i | |||||
| 37 | Strecher et al. 200575 | i | |||||||
| 38 | Strecher et al. 200576,77 | i | i | i | |||||
| 39 | Wolfenden et al. 200578,79 | i | |||||||
| 40 | Hall et al. 200680,81 | i | |||||||
| 41 | Japuntich et al. 200682 | i | i | i | |||||
| 42 | Mermelstein and Turner 200683 | i | x | Adolescent population (mean age 16 years) | |||||
| 43 | Muñoz et al. 200684 | i | x | The trial actually tested mood management course | |||||
| 44 | Patten et al. 200685,86 | i | i | x | Adolescent population (age 12–18 years) | ||||
| 45 | Swartz et al. 200687 | i | i | i | |||||
| 46 | Velicer et al. 200688 | i | |||||||
| 47 | Vidrine et al. 200689,90 | i | x | Provision of free mobile telephone to increase telephone counselling | |||||
| 48 | Gilbert et al. 200791 | i | i | ||||||
| 49 | Pike et al. 200792 (Rabius et al. 2008),93 (Rabius et al. 2006)94 | i | i | i | |||||
| 50 | Reid et al. 200795 | i | |||||||
| 51 | Smeets et al. 200796 | i | |||||||
| 52 | Sutton and Gilbert 200797 | i | i | ||||||
| 53 | Abroms et al. 200898,99 | i | i | ||||||
| 54 | Al-Chalabi et al. 2008100 | i | |||||||
| 55 | An et al. 2008101–103 | i | i | i | |||||
| 56 | Brendryen and Kraft 2008104 (Addiction) | i | i | i | i | i | |||
| 57 | Brendryen et al. 2008105,106 (J Med Internet Res) | i | i | i | |||||
| 58 | McKay et al. 2008107,108 | i | i | ||||||
| 59 | Meyer et al. 2008109,110 | i | |||||||
| 60 | Oenema et al. 2008111 | i | |||||||
| 61 | Prochaska et al. 2008112 | i | i | ||||||
| 62 | Prokhorov et al. 2008113,114 | i | i | ||||||
| 63 | Schumann et al. 2008115 | i | i | ||||||
| 64 | Stoddard et al. 2008116 | i | |||||||
| 65 | Strecher et al. 2008117,118 | i | |||||||
| 66 | Etter 2009119 | i | |||||||
| 67 | Free et al. 2009120 | i | i | ||||||
| 68 | Haug et al. 2009121 | i | |||||||
| 69 | Te Poel et al. 2009122 | Potentially eligible studyd | |||||||
| 70 | Muñoz et al. 2009123 | i | |||||||
| 71 | Swan et al. 2010124 | i |
Key research questions
This review therefore asks the following key research questions:
-
What is the effectiveness of internet sites, computer programs, mobile telephone text messages, and other electronic aids (alone or in combination with other smoking cessation support), compared with alternative interventions or no intervention, in increasing the success rate of smoking cessation for adult smokers and/or reducing relapse for quitters?
We address other related questions in an exploratory way. These are:
-
Whether or not the intensity of intervention is associated with increased effectiveness. Intensity will be measured by number of contacts and whether or not these were interactive.
-
Whether or not the mode of delivery is associated with effectiveness. This includes issues such as whether or not the intervention is delivered by letter.
-
Whether or not the effect of the computerised interventions is modified by the presence or absence of co-interventions, such as pharmacotherapy or in-person behavioural support.
-
What is the cost-effectiveness of incorporating internet sites, computer programs, mobile telephone text messages, and other electronic aids into current NHS smoking cessation programmes, or offering these as an alternative to these programmes, in increasing the success rate of smoking cessation for adult smokers and/or reducing relapse for quitters?
The evidence base includes multiple electronic aids and conventional behavioural support interventions. The effectiveness review of Chapter 2 gives estimates of pair-wise treatment effects by carrying out a series of separate meta-analyses. This is not appropriate for the cost-effectiveness review, which requires a single set of coherent treatment effects for all possible comparisons of treatments relevant to the analysis. We therefore carried out additional evidence synthesis via a mixed-treatment comparison (MTC), also known as a network meta-analysis. This exercise is reported in Chapter 3.
To estimate the cost-effectiveness of different electronic smoking cessation aids, we first searched for cost-effectiveness estimates in the literature. This was done in parallel with the main effectiveness review, using a single search strategy. This part of the literature review is reported in Chapter 4. As the results did not fully answer this research question, we carried out an economic modelling exercise de novo (see Chapter 4: Decision-analytic model, Derivation of cost data for electronic interventions, Additional model inputs, and Results of the cost-effectiveness analysis). This drew on the results of the MTC for efficacy, and a range of sources in the literature for additional parameters (see Chapter 6: Derivation of cost data for electronic interventions and Additional model inputs).
-
What are the current gaps in existing research into the effectiveness of internet sites, computer programs, mobile telephone text messages and other electronic aids to help people stop smoking?
In addition to the effectiveness and cost-effectiveness review of electronic aids to help people stop smoking, we examine studies that do not meet the inclusion criteria for the main review but are useful for understanding the acceptability and usability of electronic aids and for identifying future research recommendations. Findings from this supplementary review are in the form of an additional narrative synthesis of evidence from studies using a range of research designs. This narrative should be considered alongside the clinical effectiveness and cost-effectiveness review findings to form a comprehensive overview of current evidence.
The review therefore consists of four components: (1) the main effectiveness review, which describes evidence from randomised controlled trials (RCTs) and quasi-RCTs, and focuses primarily on quantitative estimates of effectiveness; (2) the cost-effectiveness review, which summarises evidence from published economic evaluations; (3) the supplementary review, which draws evidence from studies of various designs including uncontrolled observational studies and qualitative studies, and focuses on factors that might influences effectiveness; and (4) evidence synthesis using mixed treatment comparison.
Although the published brief specified the inclusion of studies with at least 6-month follow-up data, we broadened this to include studies with any follow-up period, in an attempt to be fully inclusive.
Chapter 2 Review of effectiveness
This chapter describes the methods and results of the main effectiveness review, which focuses on evidence from RCTs (or quasi-RCTs) regarding the effectiveness of computer and electronic aids for smoking cessation. The chapter starts with a description of methods used for the effectiveness review, including an overarching literature search and sifting of studies that cover all three component reviews. An overview of quantitative evidence from included studies is then provided through meta-analyses and exploration of heterogeneity between the studies. This is followed by more detailed descriptions of individual studies, grouped according to their number of components and mode of delivery (see Grouping of studies and study arms). The methods and findings of further evidence synthesis modelling using MTC and of the cost-effectiveness review and supplementary review will be presented in subsequent chapters.
Methods
Search strategies
Searches were conducted in three phases: (1) the scoping searches to identify published and ongoing systematic reviews, which served as an additional source for identifying relevant primary studies and provided background information; (2) the main searches for primary studies to identify all relevant primary studies covering all of the three component reviews (effectiveness, supplementary and cost-effectiveness); and (3) the updated searches to identify relevant primary studies published during the preparation of this report. Detailed search strategies are described below.
Scoping searches
Completed and ongoing systematic reviews were sought from the following resources: The Cochrane Library Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE) and HTA database]; recent additions to DARE and HTA database via the Centre for Reviews and Dissemination (CRD) website; Aggressive Research Intelligence Facility (ARIF) Database of Reviews; TRIP database; MEDLINE (Ovid) and EMBASE (Ovid). Searches were based on index and text words that encompassed the population, smokers who wish to stop and the interventions, computers and other electronic aids. A search filter for systematic reviews was added to this strategy. Searches were conducted in April 2009. The results of the scoping searches were used as background references and a brief description of relevant systematic reviews identified can be found in Chapter 1.
Search strategies for primary studies
Relevant primary studies were sought from the following resources:
-
Bibliographic databases – The Cochrane Library [Cochrane Central Register of Controlled Trials (CENTRAL)] 2009, Issue 2, and updated in Issue 4; MEDLINE (Ovid) 1950 – May Week 4 2009 and updated December Week 5 2009; EMBASE (Ovid) 1980–2009 Week 21 and updated 2009 Week 53; PsycINFO (Ovid) 1967 – May Week 4 2009 and updated December Week 4 2009; Health Management Information Consortium (HMIC) (Ovid) March 2009 and updated November 2009; and Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost) 1980 – May 2009 and updated December 2009. Searches were based on index and text words that encompassed the population, smokers who wish to stop and the interventions, computers and other electronic aids.
-
Reference lists of included studies and of relevant systematic reviews were examined to identify further potentially relevant studies.
-
Research registries of ongoing studies including NIHR Clinical Research Network Portfolio Database, Current Controlled Trials and ClinicalTrials.gov.
-
Further information was sought from contacts with experts.
All study types were sought to enable each aspect of the systematic review to be informed (i.e. clinical effectiveness, cost-effectiveness and modelling). NHS EED and DARE were searched in addition to the databases already mentioned for information on cost-effectiveness and modelling. The databases were limited from 1980 to May 2009 and updated in December 2009. Searches were not limited by language.
Search strategies can be found in Appendix 1.
Sifting of records retrieved from searches of electronic databases
Records retrieved from searches were imported into Reference Manager (version 11; Thomson ResearchSoft, San Francisco, CA, USA), which automatically detected and excluded duplicate records between electronic databases. Further duplicated records were identified and deleted manually. The titles and abstracts of the remaining records were examined for relevance by one of three reviewers (EA, DW, YFC). In order to improve the consistency of the sifting process, the reviewers independently screened a common set of the first 200 records, compared the results and resolved any disagreement by discussion before sifting through the remaining records. The initial sifting aimed to exclude obviously irrelevant records and focused on whether or not a paper possibly met the intervention criterion (out of the full set of study selection criteria, described in the next section).
Study selection
The study selection criteria and algorithm are described in Appendix 2. Full-text publications were ordered for all the records that passed through the initial sifting stage. At least two reviewers independently assessed the full publications against the inclusion/exclusion criteria (listed below). Disagreements between reviewers were resolved by discussion and by seeking further advice from additional members of the project team to reach a consensus. Where full publications could not be obtained, the records/studies were excluded. Details of these records (predominantly conference abstracts) are presented in the list of excluded studies (see Appendix 3).
Inclusion criteria
The key criteria for a study to be included in one of the reviews (i.e. effectiveness review, supplementary review and cost-effectiveness review) were:
-
Population Predominantly adult smokers (mean age ≥ 18 years).
-
Intervention Any smoking cessation programme that utilises computer, internet, mobile telephone or other electronic aids (other than conventional mass media, such as TV or radio advertisements) to:
-
– generate tailored materials, and/or
-
– present or deliver information (which may not necessarily be tailored), and/or
-
– facilitate communication, for example chat rooms, blogs, e-mails (except telephone conversations), and/or
-
– increase recruitment.
-
A paper meeting the above criteria was then considered for inclusion in one of the reviews according to its study design and measurements of outcomes.
For inclusion in the main effectiveness review, a study needed to be either a RCT or quasi-RCT (using a method of allocation that is not strictly random but is less likely to introduce bias such as allocation of alternative options to consecutive participants enrolled) and report at least an outcome associated with smoking cessation (e.g. point prevalence abstinence and/or prolonged abstinence). We initially retained studies that reported only motivation to quit smoking for potential inclusion in the review, but these studies were subsequently excluded as we were unable to analyse the data related to motivation to quit owing to time constraint.
Economic evaluations (i.e. cost-effectiveness analyses, cost–utility analyses, cost–benefit analyses) meeting the population and intervention criteria were included in the cost-effectiveness review. In addition, studies that reported cost information were flagged for potential use in economic modelling.
Studies of other designs which met the population and intervention criteria were tagged and separately considered for inclusion in the supplementary review. Further details of the selection criteria and algorithm can be found in Appendix 2.
Exclusion criteria
Studies that met any of the following criteria were excluded:
-
Population Predominantly smokers < 18 years old (mean age < 18 years).
-
Intervention Interventions targeting solely at smokeless tobacco; interventions aiming exclusively at modifying the behaviour/enhancing the performance of the providers of a smoking cessation programme rather than aiming at smokers; the computer/electronic aids were used solely for passively monitoring smoking behaviour/collecting information (without using the information to generate further feedback).
-
Study design Commentaries, editorials, surveys, narrative reviews.
Data extraction and quality assessment for effectiveness review
Data from included RCTs/quasi-RCTs were extracted on to a data extraction form by one of the reviewers (DW, IY, EA, OU). The data extraction form (see Appendix 3) was designed ad hoc for this review and included details of the citation, study design (population, interventions, comparators and co-interventions, outcome measures, and statistical methods) and results. The data extraction form also included a quality assessment checklist, which assesses the following domains:
-
methods of randomisation
-
allocation concealment
-
similarity in baseline characteristics between groups
-
similarity in care provided between groups other than the intervention/comparator being tested
-
biochemical validation
-
extent of dropout
-
presence of differential dropout between groups, and methods for adjustment in analysis
-
use of intention-to-treat (ITT) analysis.
The main outcome measure of interest is prolonged abstinence. Data on point prevalence abstinence and other measures of motivation to quit (e.g. movement in the stages of change) were also recorded.
All of the extracted data and results of quality assessment were independently checked by another reviewer to ensure accuracy and consistency. Disagreements were resolved by discussion and/or consulting a third reviewer.
Grouping of studies and study arms
In an attempt to guide the report structure and to facilitate quantitative analysis, the components of the care provided in each study arm (irrespective of whether they are considered as an intervention, a control or a co-intervention) with regard to smoking cessation were coded using a coding scheme shown in Table 4. Components were categorised as either ‘electronic’ or ‘non-electronic’ and these were coded separately. The coding scheme was developed, piloted, and revised during the data extraction phase. Coding was undertaken independently by two reviewers (IY, YFC). Disagreements were resolved by discussion and by seeking further advice/arbitration from another team member (PA). The coding was finalised before data analysis took place.
| Code | Definitiona | Examples |
|---|---|---|
| Electronic interventions/components | ||
| e0 | Nothing (no electronic component) | Interventions with no electronic component, such as face-to-face counselling and NRT (which are coded separately); electronic reminders not related to the intervention itself;b control group without any intervention |
| e1 | Single generic component | Generic self-help material delivered by e-mails; static websites (websites containing generic information without providing tailored feedback to individuals) |
| e2 | Multiple generic components | Static websites + generic self-help material delivered by e-mails |
| e3 | Single tailored component | Computer-generated tailored feedback; interactive websites (websites providing stage-matched or other feedback tailored to individuals) |
| e4 | Single tailored component + generic component(s) | Interactive websites + e-mail reminders asking smokers to log on to the websites; stand-alone tailored computer program + printout of the same output posted to the smokers |
| e5 | Multiple tailored components (± generic components) | Interactive websites + additional computer-generated tailored feedback delivered by post; interactive website + chat room |
| Non-electronic interventions/components | ||
| 0 | Nothing (no non-electronic component) | Interventions that are fully automated; non-electronic reminders, telephone calls or questionnaires not related to intervention itself (e.g. for data collection); control group with no intervention |
| 1 | Generic self-help material | Self-help manuals, booklets |
| 2 | Brief advicec | Smoking cessation advice given during a GP consultation |
| 3 | Telephone or face-to-face counsellingc | Quitlines; one-to-one or group counselling |
| 4 | Pharmacotherapyc | NRT; bupropion (Zyban®, GSK), varenicline (Champix®, Pfizer) |
| 5 | Counselling + pharmacotherapyc | Smoking cessation clinic that offers NRT and one-to-one counselling |
The rationale behind our categorisation in Table 4 is as follows. Given the focus of this review, interventions (or parts of interventions) were firstly classified as either electronic or non-electronic. Electronic (or electronic part of) interventions were then grouped into five different categories (e1–5) according to the number and nature of ‘components’ included within an intervention. Components were defined according to both the mode of delivery (given that this review aims to determine which electronic media may be effective) and whether the contents within each mode of delivery are generic or individually tailored. We think the latter is important because previous reviews18 suggest that individually tailored materials appear to be more effective compared with generic, non-tailored (NT) material. Our categorisation therefore attempts to differentiate electronic interventions primarily on the basis of whether their contents are generic or tailored. The categorisation also aims to explore whether or not inclusion of more than one component (i.e. more than one mode of delivery and/or inclusion of both generic and tailored contents) enhance the effectiveness of interventions.
With respect to non-electronic interventions, our thinking was also informed by the relevant Cochrane review. 18 A simple self-help leaflet is marginally more effective on its own than no intervention (RR = 1.21, 95% CI 1.05 to 1.39) and thus they were coded separately. However, there is no evidence that self-help material adds to brief advice from a physician. The RR for brief advice is larger than for a self-help intervention. Likewise, behavioural support or counselling has been shown to be more effective than brief advice and the evidence indicates that proactive telephone counselling might be similarly effective as face-to-face counselling. Pharmacotherapy is also effective in enhancing smoking cessation. There is a common consensus that behavioural support and pharmacotherapy have additive effects. The coding scheme therefore groups each of these non-electronic interventions (and the combination of counselling and pharmacotherapy) separately, with ascending code numbers corresponding to potentially more effective interventions.
In addition to the coding scheme to categorise individual study arms, each study was also classified by one reviewer (YFC) with respect to the study population, type of electronic media, and comparisons made within each study based on the framework shown in Figure 1. The mapping of studies to the framework provided further guidance on report structure and meta-analysis within each major section.
FIGURE 1.
Classification of included studies in relation to study population, mode of delivery of the electronic intervention and comparisons made within each study.
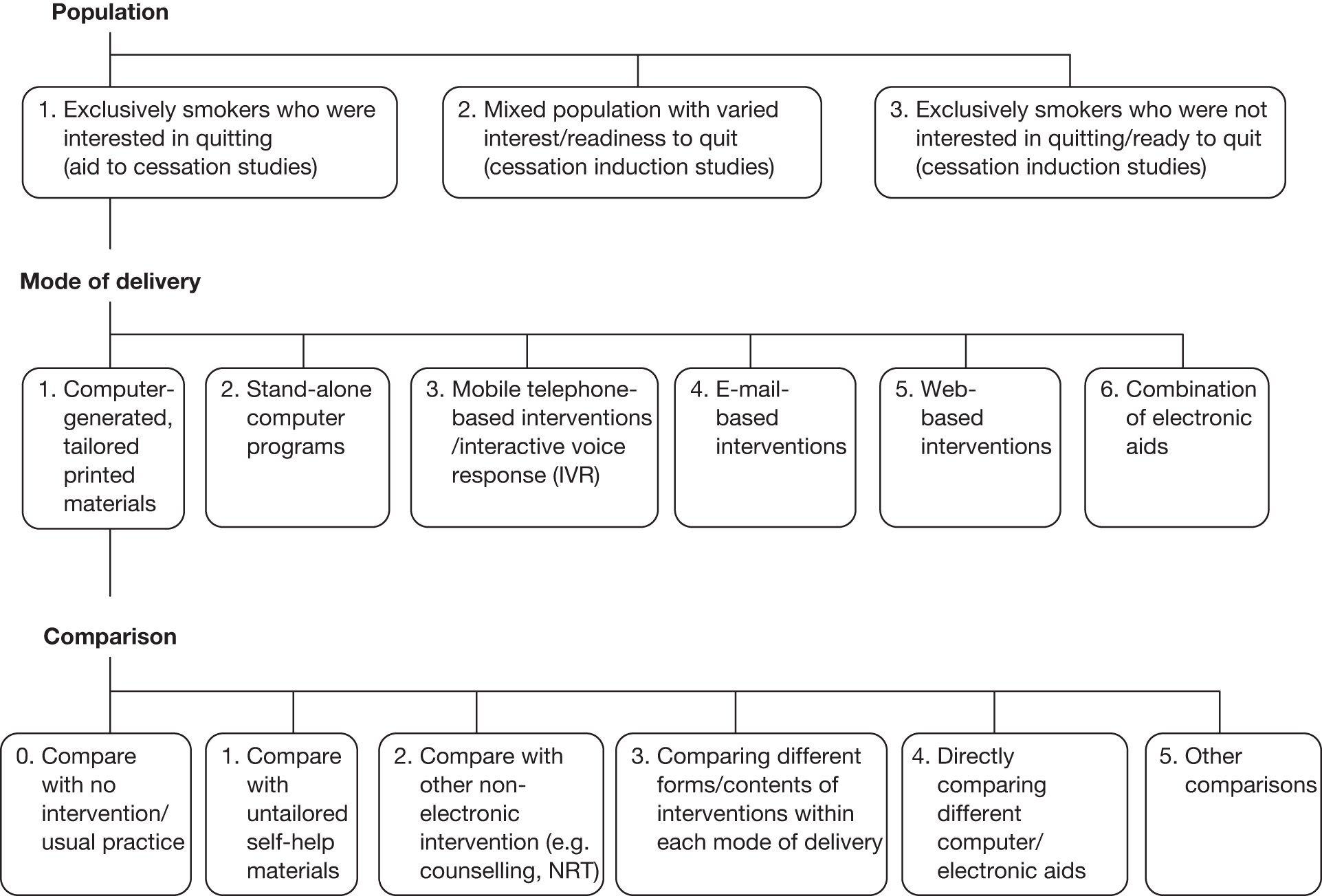
We have also attempted to apply a standardised coding with respect to the contents of each intervention using a taxonomy developed by Abraham and Michie. 126 However, our initial pilot indicated that information presented in published papers was often insufficient to allow accurate coding of each intervention/comparator. Detailed coding and analysis of the contents of the interventions were therefore not carried out.
Data handling and analysis
Data handling
Numerical data were entered into a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, WA, USA). Where possible, data including all randomised patients were used, with any patients lost to follow-up or with missing data counted as failing to achieve abstinence. Consequently, unless otherwise stated, meta-analyses presented in this review were undertaken according to the ITT principle irrespective of whether or not the analyses presented in the original article were based on ITT. In a few RCTs in which the exact number of participants randomised to each arm was not reported but total number of participants was known, it was assumed that the number of participants was distributed equally between trial arms.
Where prolonged abstinence was measured at multiple time points, the 6-month prolonged abstinence recorded approximately 6 months after the start of the intervention was used for ‘aid to cessation’ studies (i.e. in smokers who are prepared to quit at the beginning of the studies). For ‘cessation induction’ studies in which some of the smokers are not yet ready to quit at the start of the studies, the 6-month prolonged abstinence recorded approximately 6 months after the allowed ‘cessation induction period’ (i.e. from the start of the intervention to the expected quit day) was preferred if available.
Where more than one point prevalence abstinence rate based on different definitions (e.g. 24-hour, 7-day, etc.) were reported, the 7-day point prevalence abstinence was preferred. A few studies reported 30-day continuous abstinence. We considered this to be conceptually closer to point prevalence abstinence rather than prolonged abstinence, and thus 30-day abstinence was regarded as point prevalence abstinence in meta-analysis.
For studies in which both self-reported and biochemically validated abstinence were reported, data on self-reported abstinence were used in meta-analyses in order to maintain consistency across studies, given that biochemically validated abstinence was measured/reported only in less than one-third of included studies. However, data on biochemically validated abstinence were included in a sensitivity analysis.
Data analysis
Both a pair-wise meta-analysis and a Bayesian MTC were carried out. The methods used for meta-analysis are described here. Methods used for MTC will be described in Chapter 3.
Interventions in the included studies were categorised according to tailoring of contents and the number of electronic component(s) using the coding scheme described earlier (upper panel of Table 4). As only a small number of electronic interventions fell into category e2 (multiple generic components) and e4 (single tailored component + generic components), these categories were combined with category e1 (single generic component) and category e3 (single tailored component), respectively. The results section of the report therefore consists of three major sections, with increasing level of tailoring and/or number of different electronic media used in the interventions being evaluated:
-
interventions with single or multiple generic components
-
interventions with single tailored component (with or without a generic component)
-
interventions with multiple tailored components (with or without a generic component).
Different electronic interventions evaluated in a multiarm study could be mapped to more than one section and be analysed and presented in the relevant sections. As a large number of studies evaluated interventions with a single tailored component, this section is further divided into subsections according to the mode of delivery of the interventions (type of electronic media) as shown in Figure 1.
The comparison(s) made within each study was(were) coded according to the framework shown in Figure 1. Studies with multiple intervention arms could provide information on multiple comparisons. For example, a three-arm trial that compared A (electronic interventions with multiple tailored components), B (electronic interventions with a single tailored component) and C (control group with no intervention) could contribute to three pair-wise comparisons:
-
under ‘interventions with a single tailored component’ section:
-
– B versus C (comparison code 0, see Figure 1)
-
-
under ‘interventions with multiple tailored components’ section:
-
– A versus C (comparison code 0)
-
– A versus B (comparison code 3 if A and B use the same mode of delivery or comparison, code 4 if A and B use different mode of delivery).
-
Where sufficient data were available, pair-wise meta-analyses of relative risk (RR) of point prevalence abstinence and prolonged abstinence were carried out using Stata (version 10.0; StataCorp LP, College Station, TX) for each comparison under each section/subsection. Comparisons of electronic interventions with no intervention/usual care (comparison code 0) and with untailored printed self-help material (comparison code 1) were included in the same meta-analysis considering the possibly limited efficacy of the latter. Given the potential heterogeneity between studies in terms of participants, interventions, comparators, co-interventions and duration of follow-up, analyses of 6-month data using a random-effects model were considered as primary analyses. We chose the 6-month time frame as we considered it to be sufficiently long for estimating long-term success of smoking cessation, whereas losses to follow-up are likely to be reasonably low. Analyses using a fixed-effects model and using data from the longest follow-up of each study were also performed as sensitivity analyses. In order to maintain the clarity of the forest plots, results from fixed-effects model were not shown. Generally, they were very similar to results from the random-effects model but with narrower CIs. In a few cases the pooled results from the fixed-effects model suggested a larger intervention effect than results from the random-effects model. The random-effects model remains the more appropriate method, given the aforementioned heterogeneity between studies.
Within the summary tables of each section/subsection and within each meta-analysis, studies were grouped as either ‘aid to cessation’ studies or ‘cessation induction’ studies (see Figure 1) according to the readiness to quit of the study participants, which was closely related to the recruitment strategy of the trials. Within each of these subgroups, studies were then sorted according to concurrent co-intervention.
Statistical heterogeneity between studies was assessed using the I2-statistic, which ranges from 0% (no heterogeneity beyond what is expected by chance) to 100% (substantial heterogeneity). Considering the potential ‘clinical’ and methodological heterogeneity between studies included in this review in terms of study interventions, participants, co-interventions and assessment of outcomes among other features, many of the forest plots presented in this report were mainly used to display findings of results from individual studies and demonstrate the heterogeneity in the findings between the studies. Readers should interpret the displayed pooled results with great caution, particularly where I2 is high (≥ 50%). Funnel plots and Egger’s tests were used to examine potential publication bias or small study effects.
Results
Quantity and quality of the evidence
In total, 3969 records remained in the Reference Manager database after duplicate records were removed. Of these, 270 were considered potentially relevant, and full papers were ordered for further examination.
The searches for primary studies were updated in December 2009. An additional 151 records were retrieved from the updated searches, of which 34 were considered potentially relevant and were ordered. Three additional potentially relevant papers were identified from a reference list of previous reviews and from contact with experts.
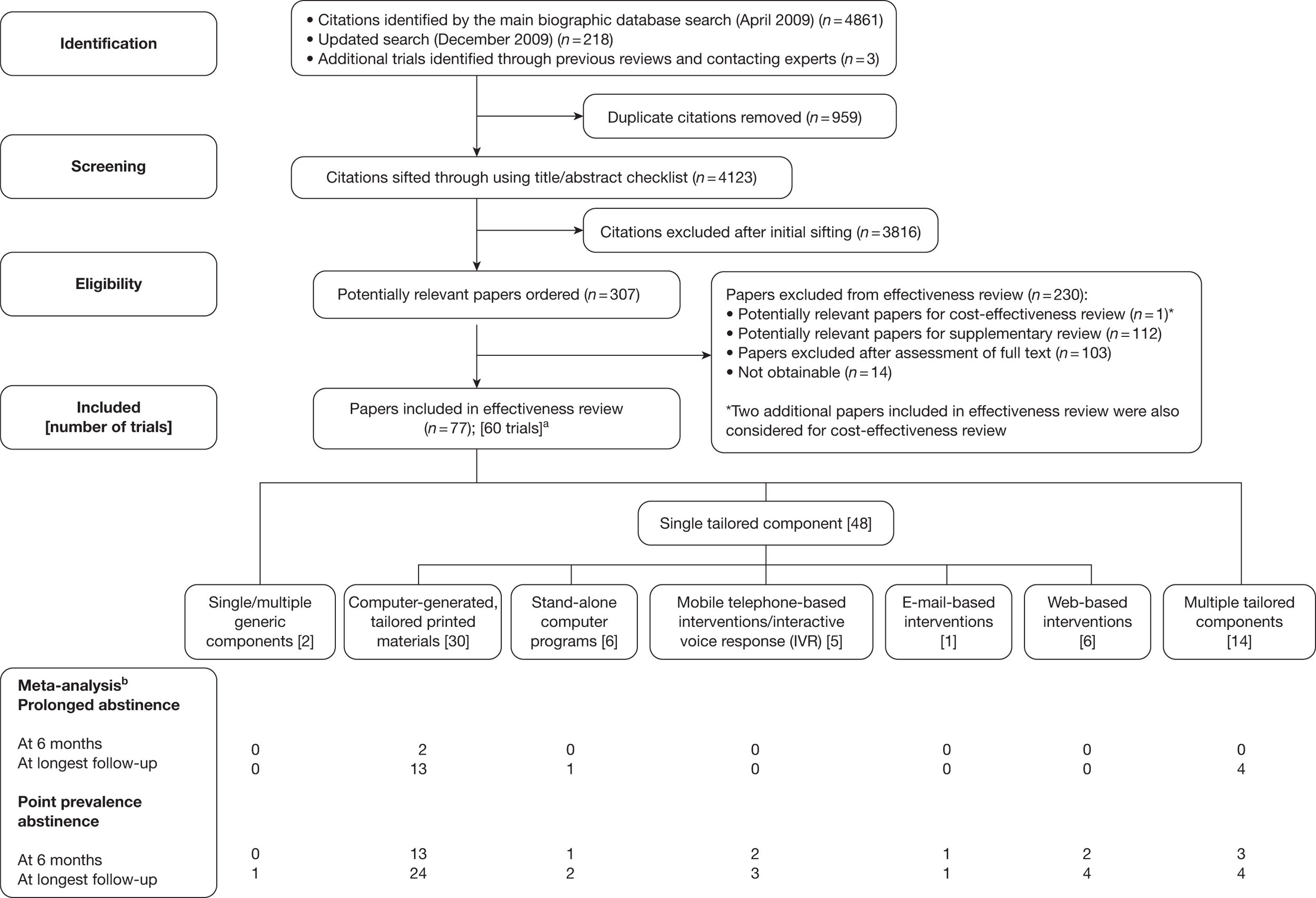
We could not obtain 14 of the 307 papers ordered – all of them conference abstracts. The remaining 293 papers were examined and 77 papers reporting results from 60 RCTs were selected for inclusion in the main effectiveness review. Papers potentially relevant for the cost-effectiveness review and supplementary review were forwarded to relevant team members for further considerations. A flow diagram for study selection process is shown in Figure 2 and a list of excluded studies and reasons for exclusion can be found in Appendix 4. Included studies are described in the following sections according to the categorisation of electronic interventions shown in Table 4.
FIGURE 2.
Flow diagram for study selection process. a, Some trials included multiple intervention arms with various levels of tailoring/numbers of components; b, meta-analysis only included comparisons of electronic interventions to minimal (generic self-help material) or no intervention control groups.
Overview of effectiveness
This section provides an overview of the effectiveness of computer and other electronic aids compared with no or minimal intervention (e.g. generic self-help material) across the different types of electronic interventions included in this review. Acknowledging the diverse nature of interventions, participants, methods and context across the included studies, the purpose of this section is to provide a panoramic view to answer the broad question of whether or not computer and other electronic aids are effective for smoking cessation. Quantitative findings from individual studies are presented in forest plots, along with pooled estimate of intervention effect across studies and measures of heterogeneity between studies. As stated above (see Data analysis), where the statistical heterogeneity between studies is high (e.g. I2 ≥ 50%), the forest plots are mainly used to graphically present results from individual studies and to illustrate the differences between study findings. The pooled estimates shown in the plots, if not discarded, need to be interpreted with extreme caution in these cases.
In addition to providing an overall quantitative estimate of effectiveness (where appropriate), the meta-analyses also offered opportunities to identify major study-level factors that may influence the estimated size of effects. Three factors were explored by subgroup analyses: (1) aid to cessation studies compared with cessation induction studies; (2) stratification of studies by mode of delivery of the interventions; and (3) stratification of studies by concurrent non-electronic co-interventions. The subgroup (1) was prespecified in the review protocol. Subgroups (2) and (3) are exploratory. In addition, subgroup/sensitivity analyses were carried out to explore the impact of quality of method/reporting (generation of random sequence, allocation concealment) and biochemical validation.
For each analysis, a forest plot for point prevalence abstinence measured at 6 months is first presented, followed by forest plots for point prevalence abstinence and for prolonged abstinence at longest follow-up of each study. As only a small number of studies reported 6-month prolonged abstinence, no separate forest plot is generated for this outcome measure. Funnel plots examining potential publication bias or small study effects are presented at the end of this chapter. More detailed descriptions of individual studies and results of other comparisons (e.g. direct comparison between different electronic interventions) are presented in subsequent sections (see Interventions with single or multiple generic components, Interventions with single tailored component and Interventions with multiple tailored components).
Aid to cessation compared with cessation induction
Results are presented in Figures 3–5. For point prevalence abstinence at 6 months (see Figure 3), substantial heterogeneity exists within each subgroup of studies (aid to cessation vs cessation induction) and this prevents valid comparison between the subgroups and meaningful interpretation of the pooled estimates within and across the subgroups. Point prevalence abstinence and prolonged abstinence measured at the longest follow-up of each study (see Figures 4 and 5, respectively) suggest that overall the computer and electronic aids are more effective than control (no intervention or generic self-help material). The effect sizes are small (point prevalence abstinence, pooled RR = 1.14, 95% CI 1.07 to 1.22) to moderate (prolonged abstinence, pooled RR = 1.32, 95% CI 1.21 to 1.45) and do not appear to differ significantly between the subgroups.
FIGURE 3.
Point prevalence abstinence measured at 6 months: aid to cessation studies vs cessation induction studies.
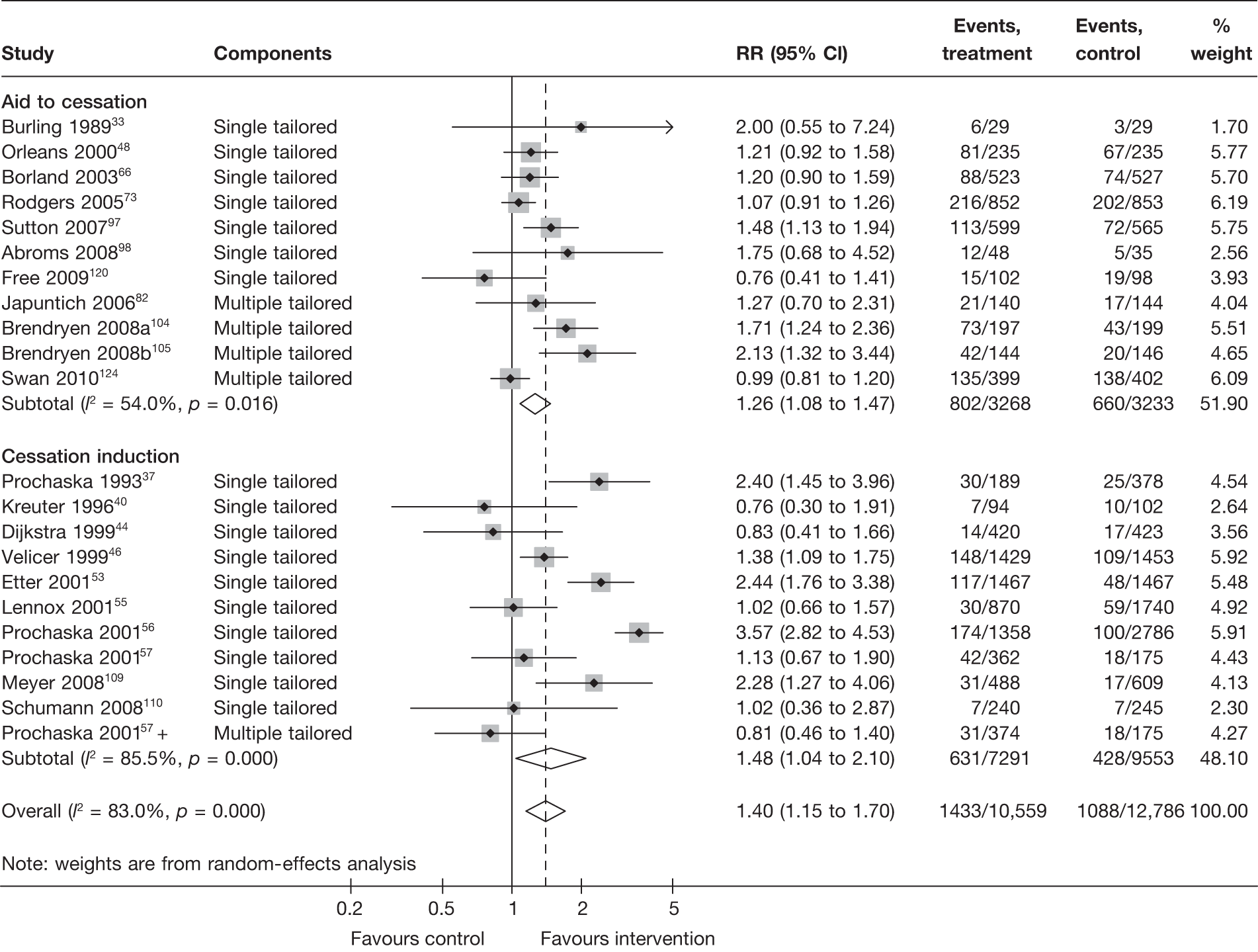
FIGURE 4.
Point prevalence abstinence measured at longest follow-up of each study: aid to cessation studies vs cessation induction studies.
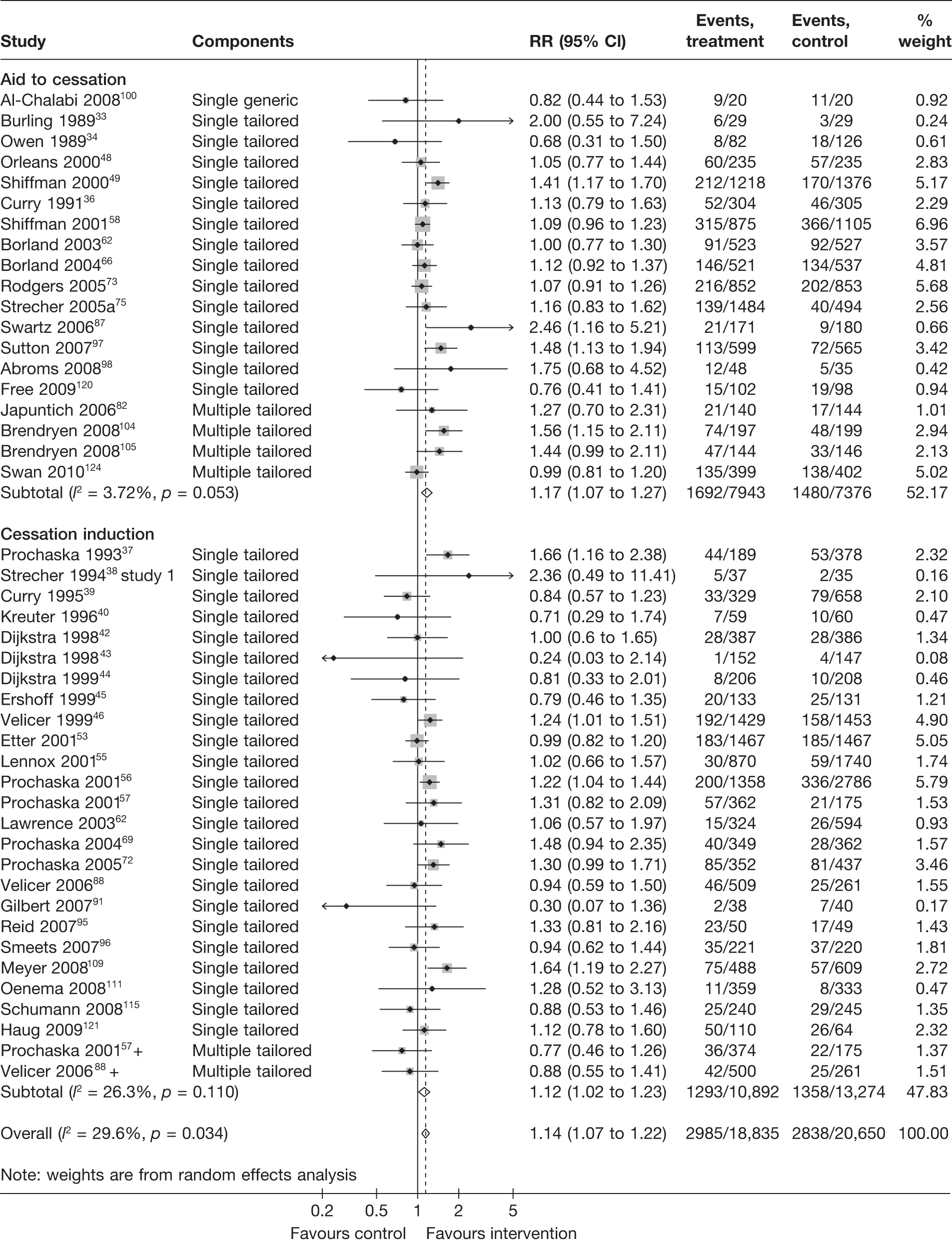
FIGURE 5.
Prolonged abstinence measured at longest follow-up of each study: aid to cessation studies vs cessation induction studies.
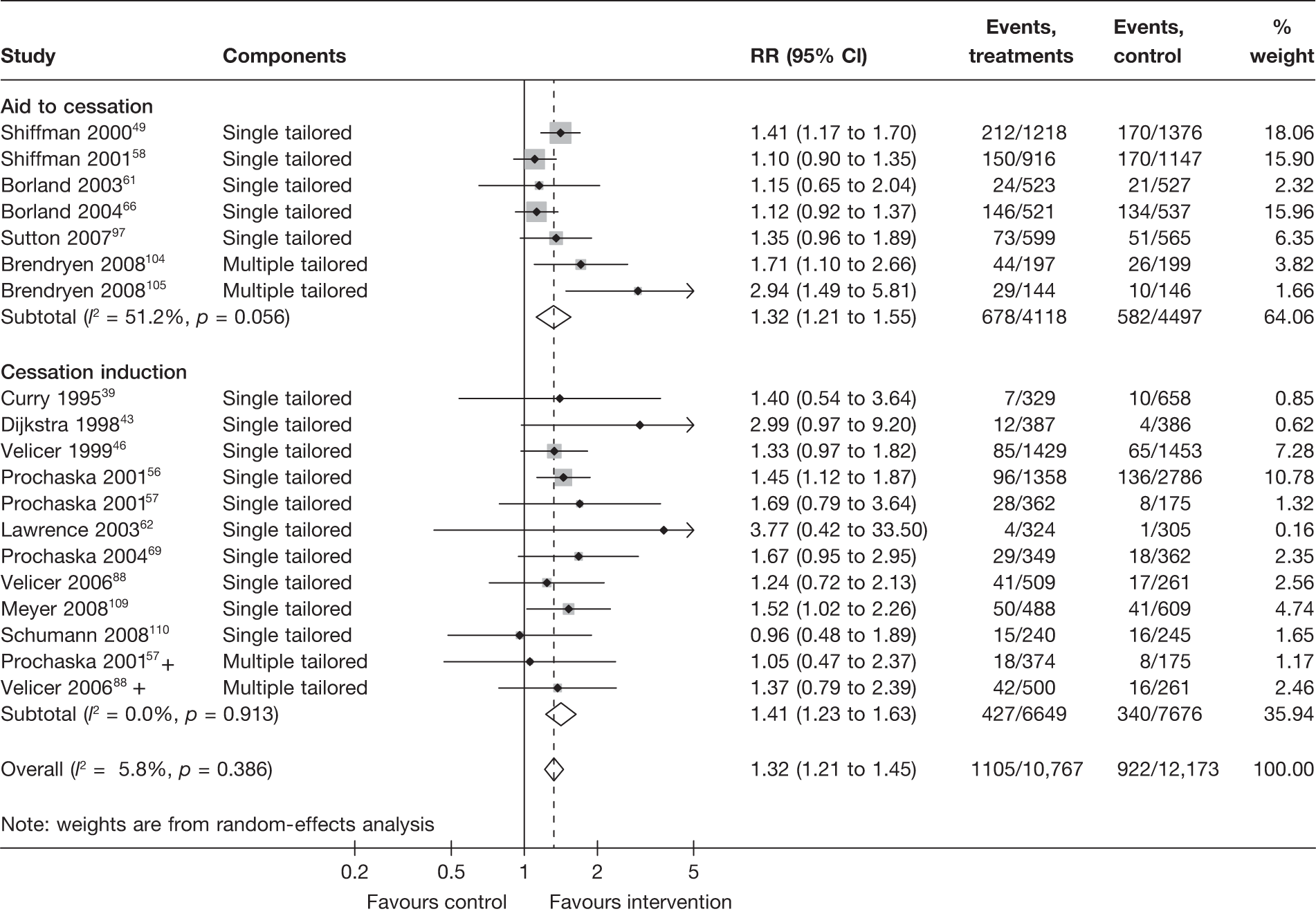
There is substantial heterogeneity (I2 = 51%) among aid to cessation studies for the outcome of prolonged abstinence (see Figure 5). Further analysis reveals that the heterogeneity may partly be attributed to the difference between the five studies with single tailored component (RR = 1.22, 95% CI 1.10 to 1.37, I2 = 6%) and the two studies with multiple tailored components (RR = 2.10, 95% CI 1.25 to 3.53, I2 = 42%), but a large part of the heterogeneity is attributed to the difference between the two studies with multiple tailored components as exemplified by the I2 value of 42%.
Mode of delivery
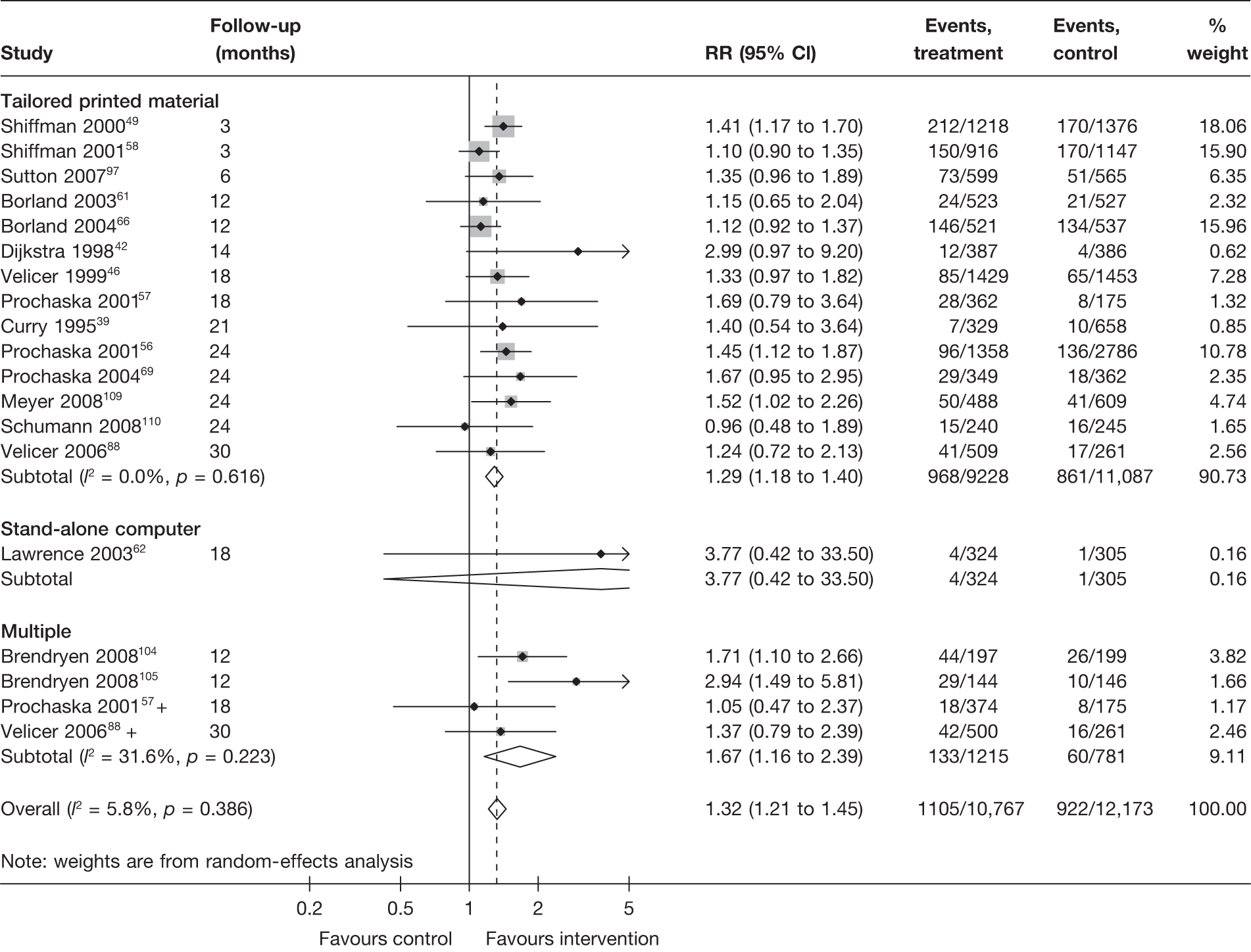
Stratification of studies according to the mode of delivery of electronic interventions does not reveal a clear pattern of effect among electronic interventions using different modes of delivery. For point prevalence abstinence measured at 6 months (Figure 6), significant heterogeneity was observed among cessation induction studies using computer-generated tailored printed materials, and among studies using multiple modes of delivery. For point prevalence abstinence measured at longest follow-up (Figure 7), substantial heterogeneity was also observed within the subgroups of e-mail-based interventions, web-based interventions and interventions using multiple modes of delivery. Results for prolonged abstinence (Figure 8) were relatively homogeneous and suggested that, overall, tailored printed materials (RR = 1.29, 95% CI 1.18 to 1.40, I2 = 0%) and interventions utilising multiple channels of delivery (RR = 1.67, 95% CI 1.16 to 2.39, I2 = 31.6%) are effective. The effectiveness of web-based intervention appears to vary between studies/contexts. The number of studies utilising stand-alone computers, mobile telephone text messages, interactive voice response (IVR) and e-mails was small. Their effectiveness particularly in terms of prolonged abstinence has not been demonstrated.
FIGURE 6.
Point prevalence abstinence measured at 6 months, stratified by mode of delivery of interventions.
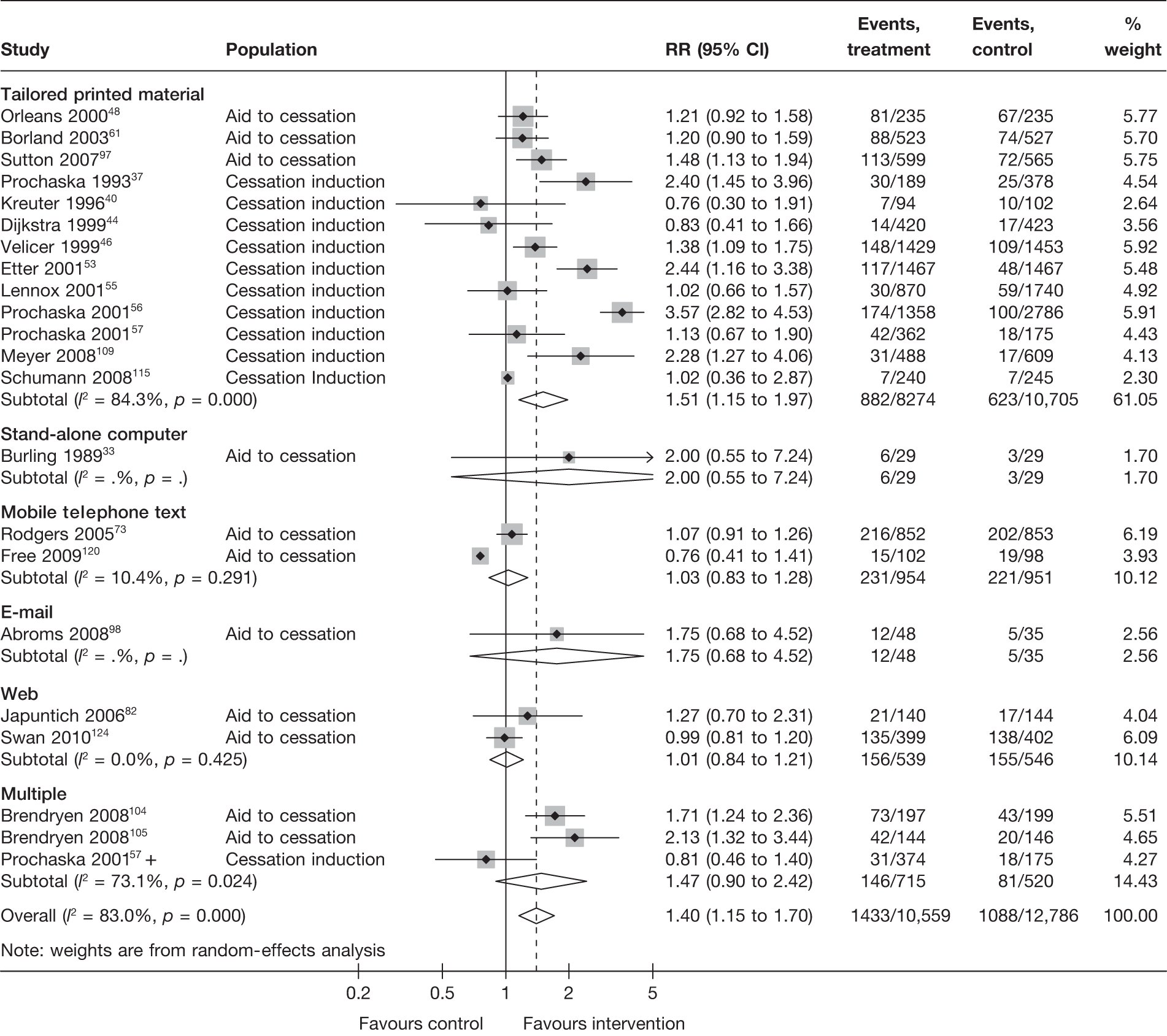
FIGURE 7.
Point prevalence abstinence measured at longest follow-up of each study, stratified by mode of delivery of interventions.
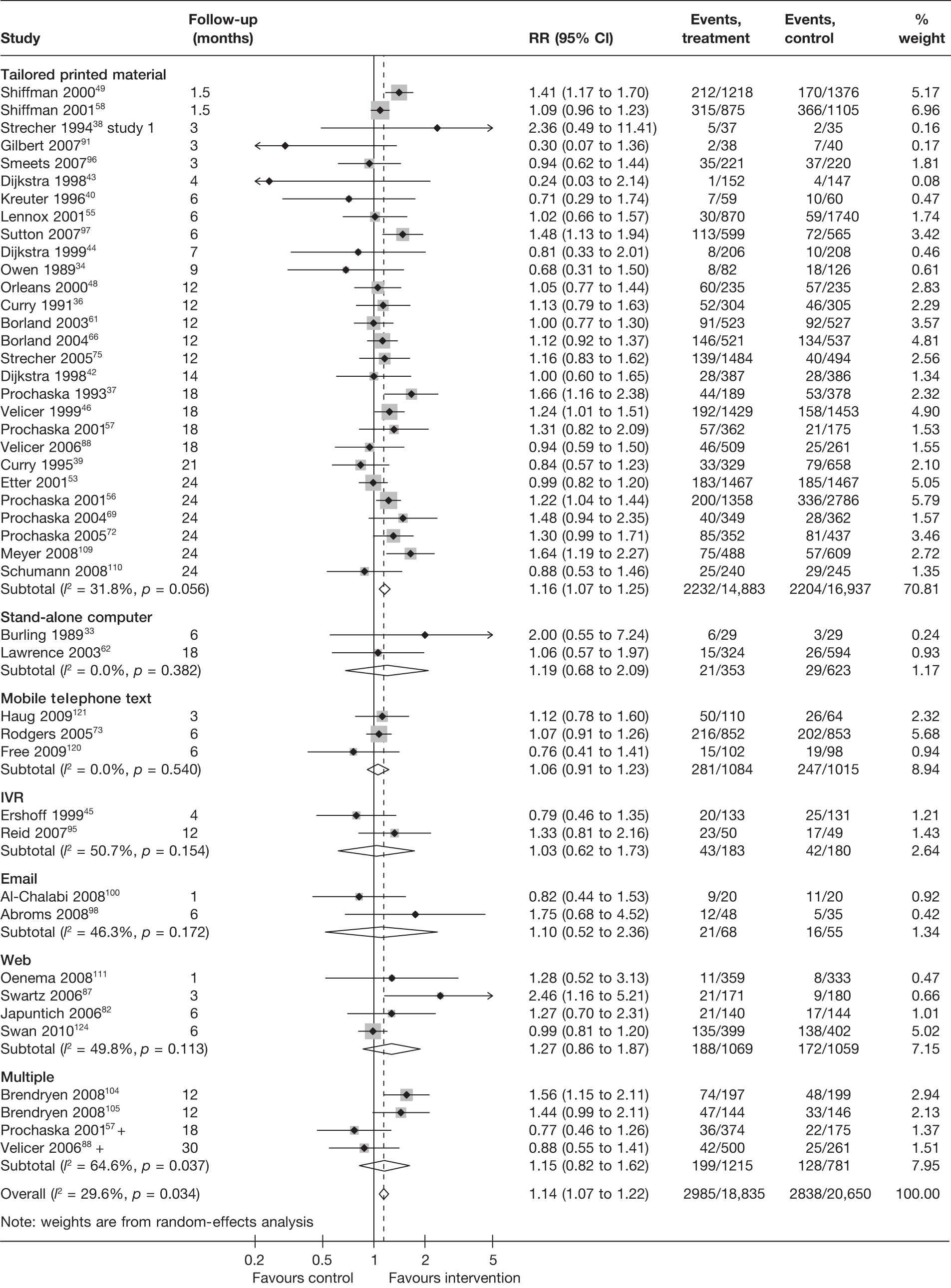
FIGURE 8.
Prolonged abstinence measured at longest follow-up of each study, stratified by mode of delivery of interventions.
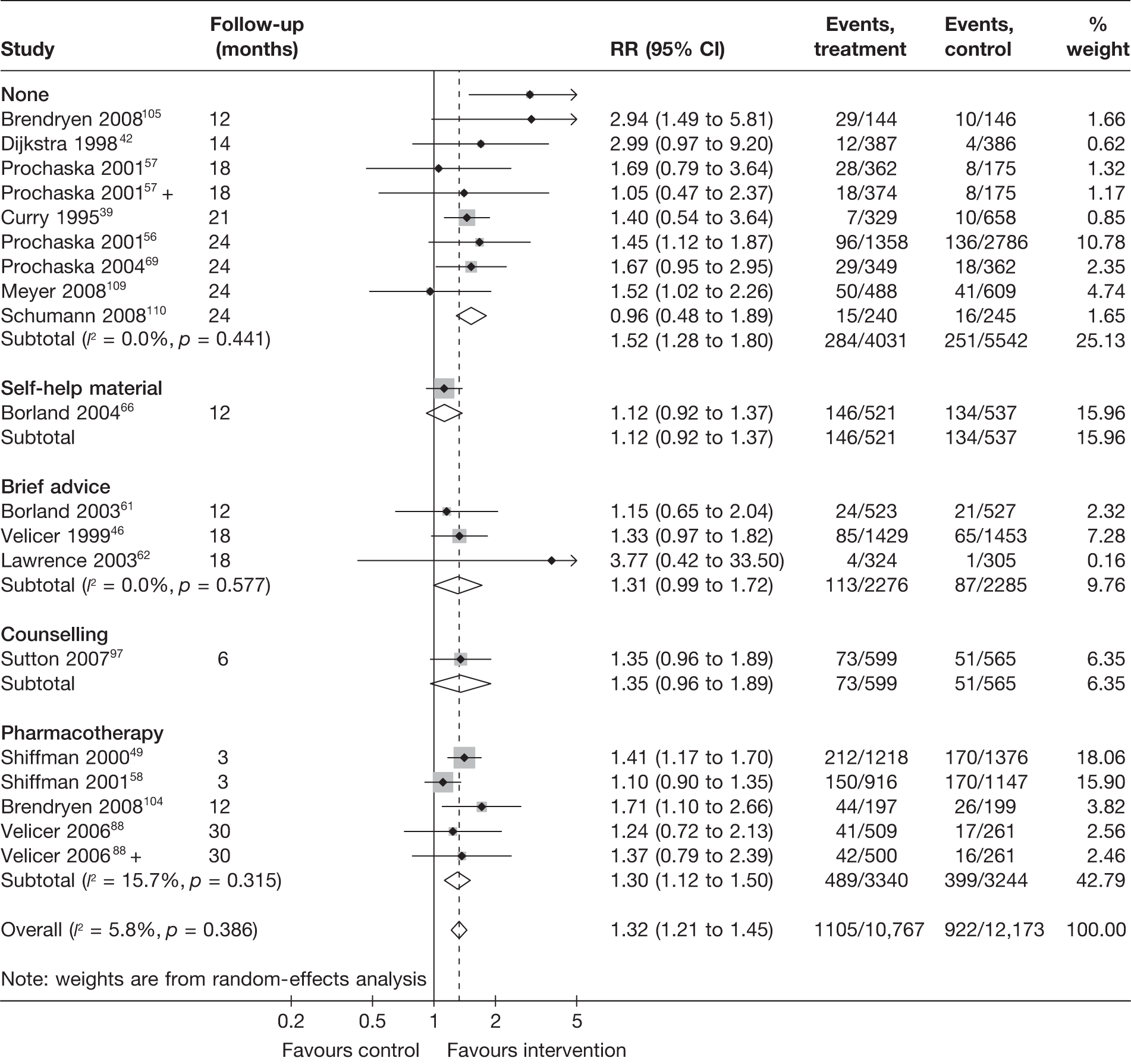
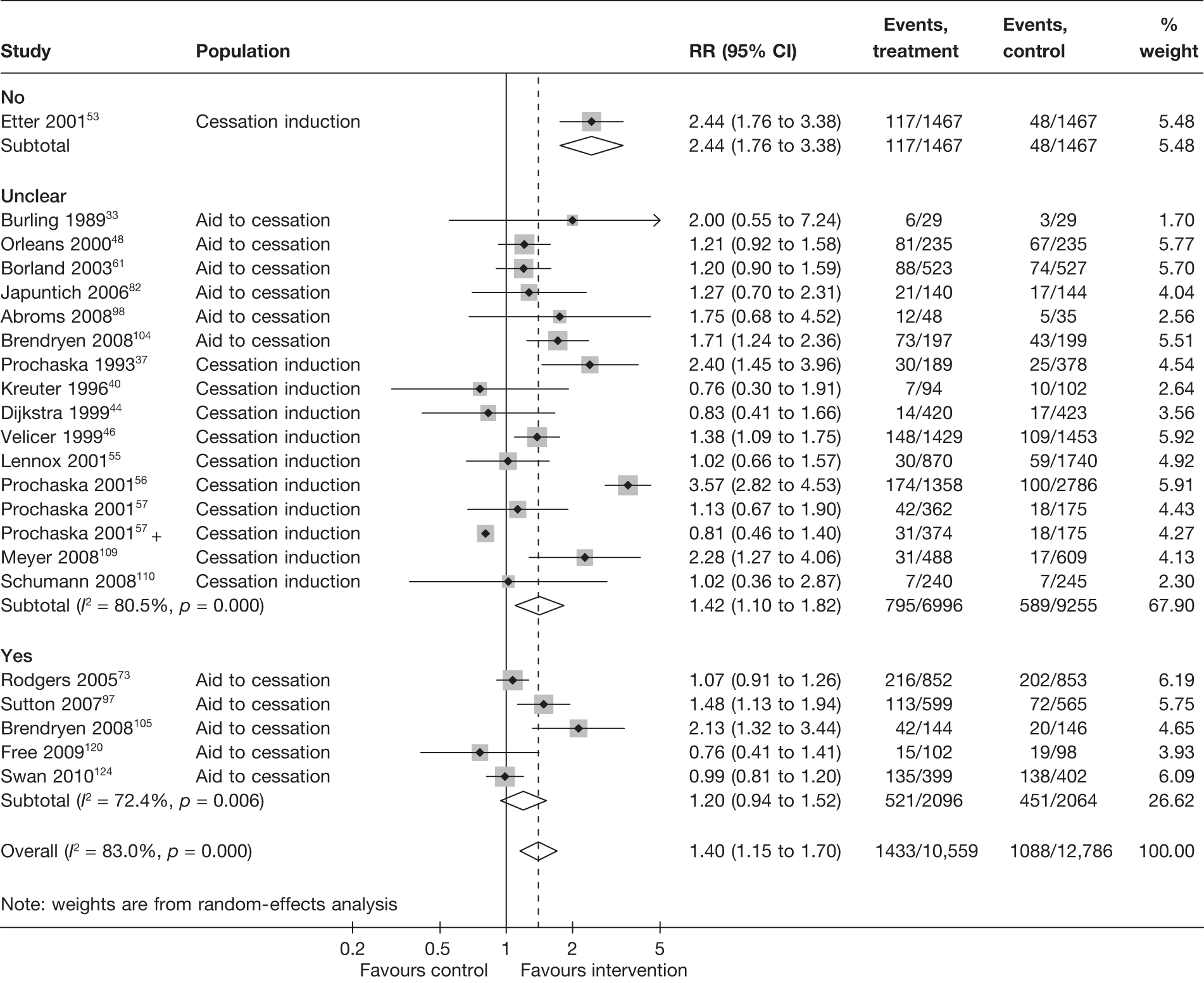
Non-electronic co-interventions
Non-electronic co-interventions that were used in conjunction with electronic interventions were classified into the following categories: none, self-help material, brief advice, counselling, pharmacotherapy, and pharmacotherapy plus counselling. Based on point prevalence abstinence (Figures 9 and 10), computer and electronic aids appear to be effective when used in conjunction with other non-electronic co-interventions, except when added to a combination of pharmacotherapy and counselling. Substantial heterogeneity in point prevalence abstinence measured at 6 months was observed when computer and electronic aids were used without any non-electronic co-interventions. Results for prolonged abstinence (Figure 11) are more homogeneous and are broadly consistent with the findings according to point prevalence abstinence.
FIGURE 9.
Point prevalence abstinence measured at 6 months, stratified by non-electronic co-intervention.

FIGURE 10.
Point prevalence abstinence measured at longest follow-up of each study, stratified by non-electronic co-intervention.
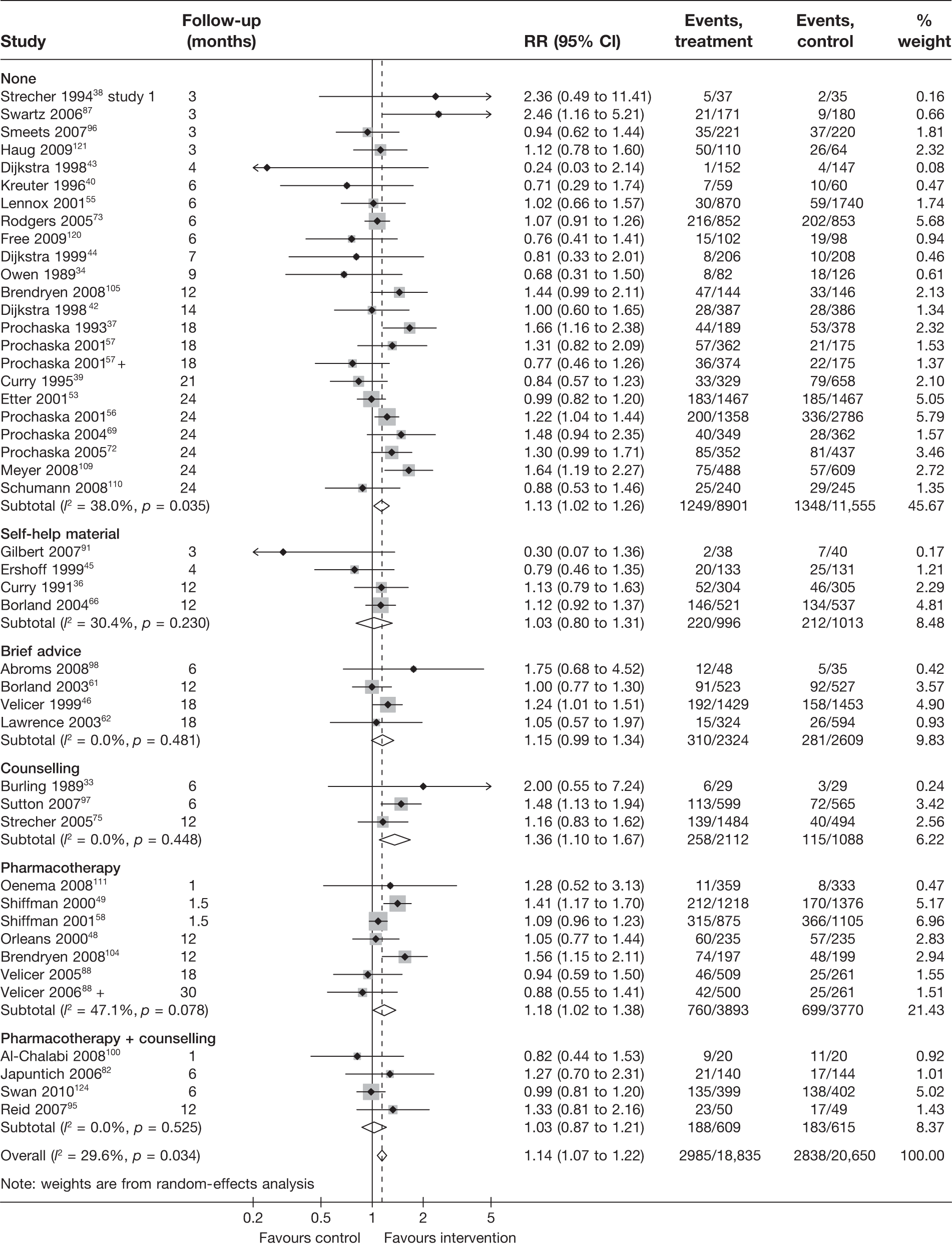
FIGURE 11.
Prolonged abstinence measured at longest follow-up of each study, stratified by non-electronic co-intervention.
Biochemical validation
Seventeen studies adopted some methods of biochemical validation of abstinence, such as measuring cotinine level in saliva or carbon monoxide level in exhaled air. Four studies39,62,98,120 presented data for both self-reported and biochemically validated abstinence, whereas six studies33,42,45,55,82,100 provided data on only biochemically validated abstinence. A further three studies34,36,73 conducted biochemical validation on a proportion of (usually randomly selected) participants and thus data for biochemically validated abstinence were not available for ITT analysis. The remaining four studies60,80,101,113 were not included in meta-analyses, as their control groups were neither ‘no intervention’ nor ‘non-electronic generic self-help material’.
This section explores the impact of biochemical validation in two subgroup/sensitivity analyses. First, a subgroup analysis was carried out comparing studies that reported biochemically validated abstinence to studies that only reported self-reported abstinence. Where both biochemically validated and self-reported data were available, the former was used in this analysis (in contrast with the main analysis, for which self-reported abstinence took preference). As mentioned above, in a few studies34,36,39,73 biochemical validation was conducted in a small proportion of participants. These studies were included in the ‘biochemically validated’ group in this analysis, although only self-reported abstinence was available and was included in the analysis. In these cases the limited biochemical validation was more akin to bogus pipeline and thus this sensitivity analysis explores, in part, the effect of biochemical validation and/or bogus pipeline compared with no validation at all. Second, a sensitivity analysis was conducted using the four studies39,62,98,120 in which both self-reported and biochemically validated abstinence was reported. This analysis allows comparison and contrast between these two types of data without being confounded by other study characteristics.
Figure 12 shows the results of studies with and without biochemical validation for point prevalence abstinence at 6 months. The pooled RR (1.09, 95% CI 0.94 to 1.27) for studies with biochemical validation suggests a much smaller and statistically insignificant effect than for the pooled RR for studies without biochemical validation. The statistical heterogeneity among the latter studies was very high (I2 = 85.5%) and thus the pooled estimate is difficult to interpret and further investigation of source of heterogeneity is required.
FIGURE 12.
Findings from studies with biochemical validation vs studies without biochemical validation: point prevalence abstinence at 6 months.
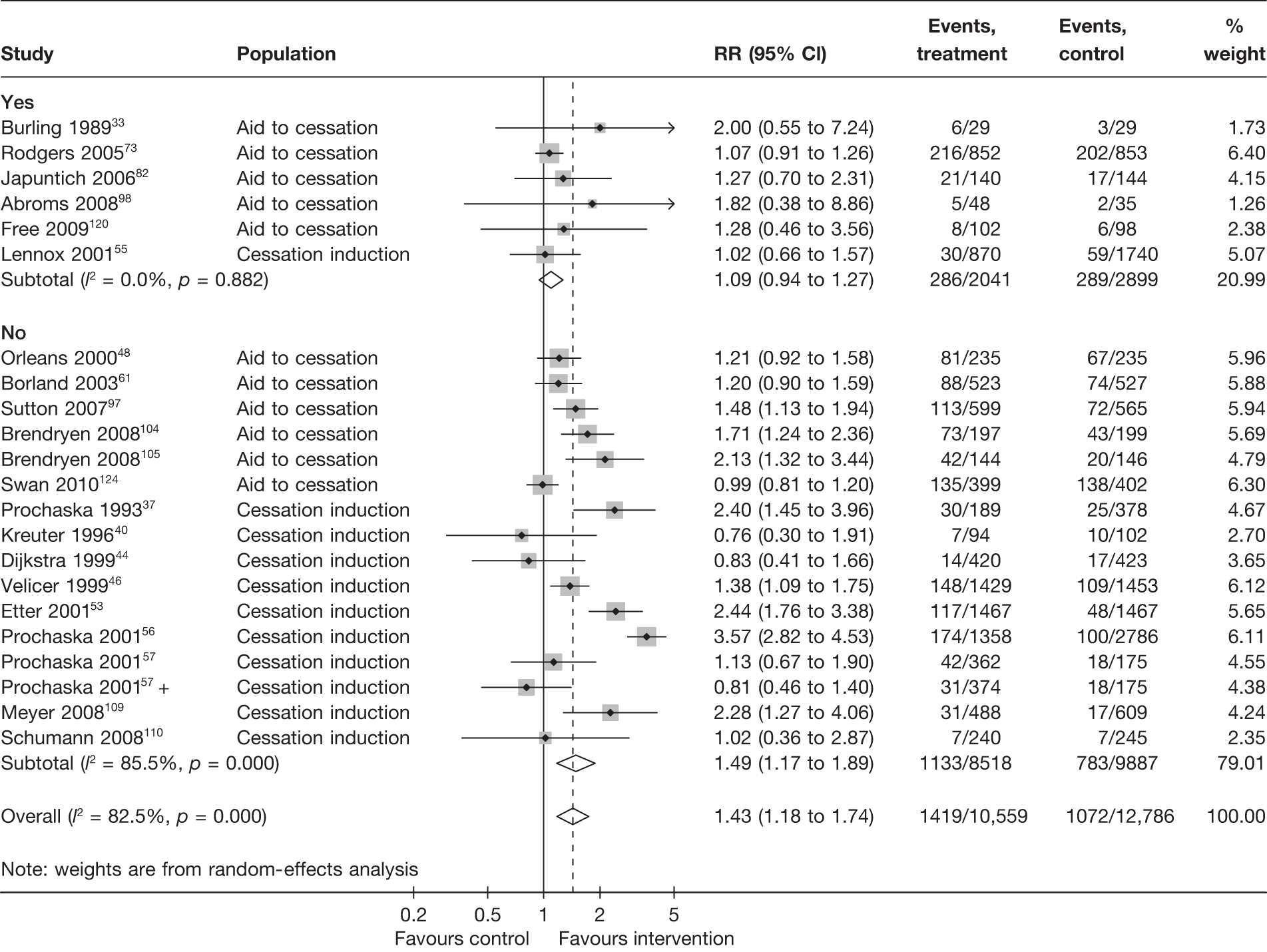
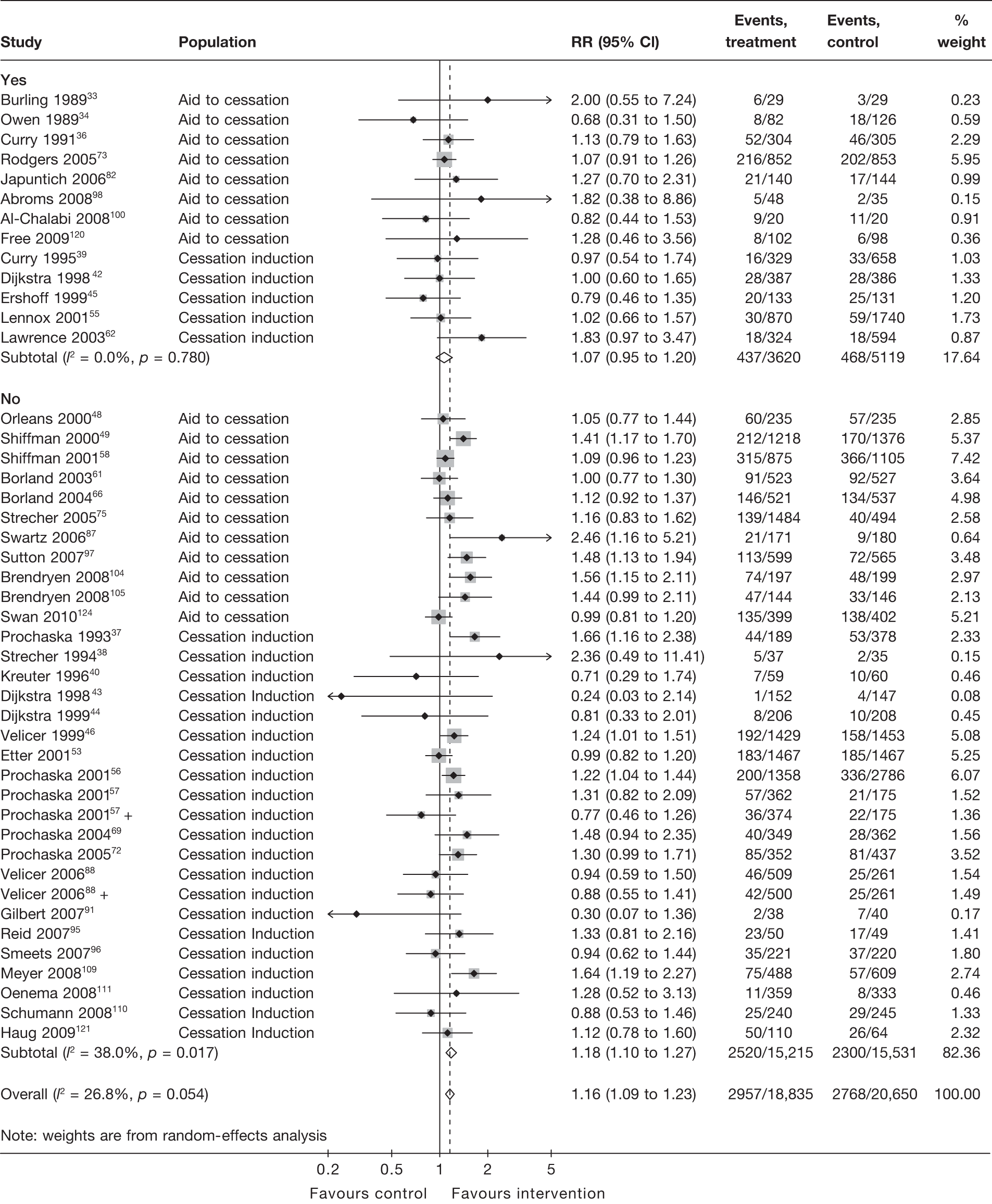
Figure 13 shows the results for point prevalence abstinence at longest follow-up of each study. Again the pooled RR (1.07, 95% CI 0.95 to 1.20) for studies with biochemical validation suggest a smaller and statistically insignificant effect compared with that for studies without biochemical validation (RR 1.18, 95% CI 1.10 to 1.27), which shows a small but significant intervention effect. Test for interaction indicates the difference between subgroups is not statistically significant (p = 0.157), but the result of the test needs to be interpreted with caution, given that there is still moderate heterogeneity (I2 = 38.0%) in the ‘no biochemical validation’ group.
FIGURE 13.
Findings from studies with biochemical validation vs studies without biochemical validation: point prevalence abstinence at longest follow-up.
Comparison of self-reported and biochemically validated abstinence using data from studies that reported both outcomes is shown in Figure 14. The pooled RR for biochemically validated abstinence suggests slightly larger effect than that for self-reported abstinence, but test for interaction indicates the difference is not statistically significant (p = 0.455).
FIGURE 14.
Comparison of self-reported abstinence and biochemically validated abstinence.
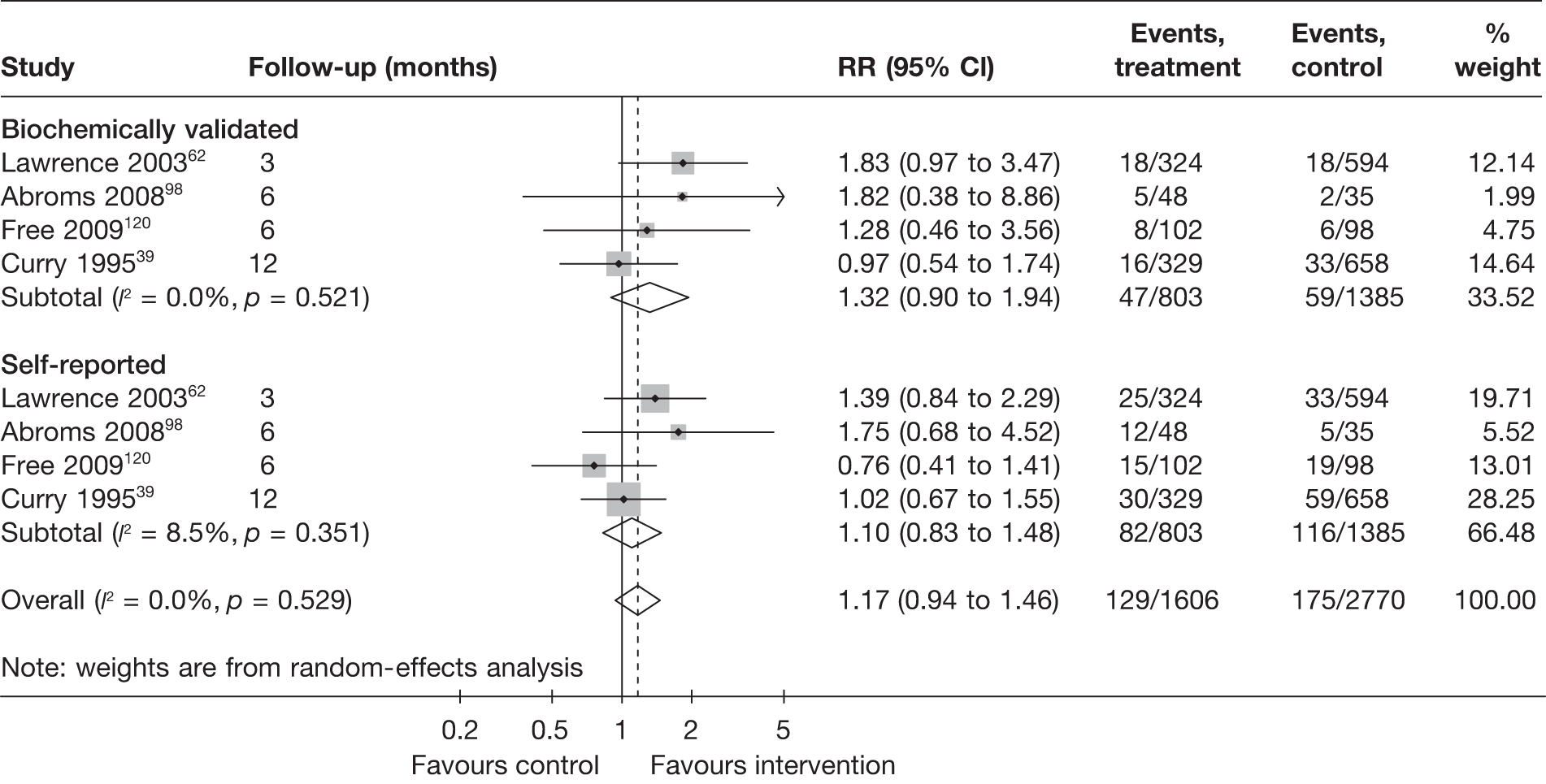
It is worth noting that in studies such as these, in which there is little or no therapeutic relationship between the patient and the individual offering the intervention, the scope for misreporting of smoking status is lower. In addition, collection of biochemical validation in such studies may be difficult, leading to a low response rate. If non-responders are rated as smoking, as is typical, this may serve to attenuate treatment effects disproportionately. This should be borne in mind when interpreting these results.
Methods of randomisation
This section explores whether or not methods of randomisation, including the generation of random sequence and concealment of allocation, had significant impact on the estimates of intervention effects.
Figure 15 shows prolonged abstinence at longest follow-up for each study, grouped according to the adequacy of methods for generating random sequence. Intervention effects estimated from studies with truly random methods appear to be similar to those estimated from studies with unclear description of the methods. Only one study109 clearly stated a quasi-randomised design. The results for point prevalence abstinence at 6 months and point prevalence abstinence at longest follow-up are generally similar to that of prolonged abstinence, although there is high level (I2 > 70%) of heterogeneity within the subgroups (‘truly random’ and ‘unclear’) for point prevalence abstinence at 6 months (data not shown).
FIGURE 15.
Prolonged abstinence at longest follow-up according to whether or not the methods for generating sequence were truly random.
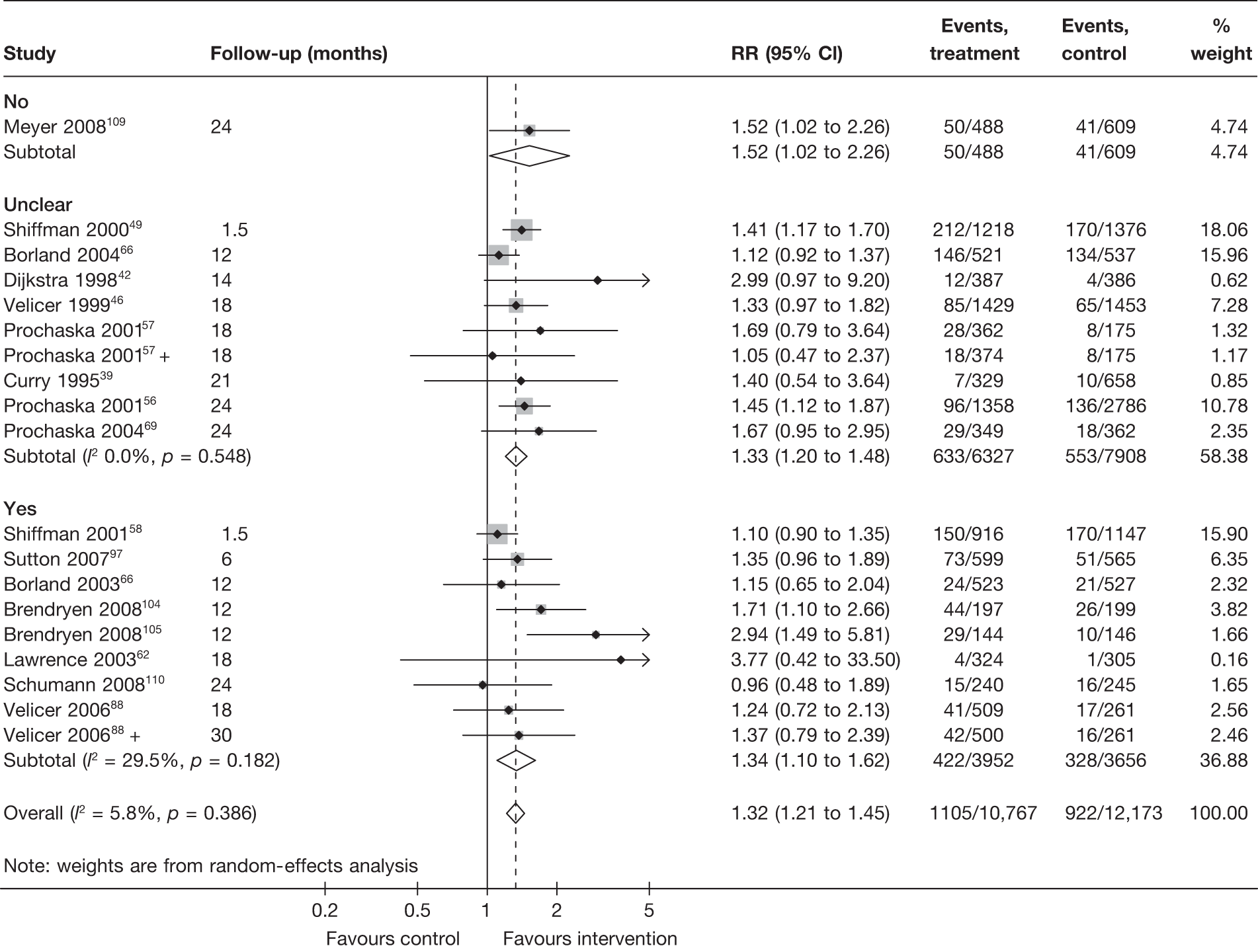
Figure 16 shows prolonged abstinence at longest follow-up, grouped according to the adequacy of allocation concealment. Allocation concealment was unclear in the majority of cases and was judged to be clearly adequate in only two trials with very heterogeneous results.
FIGURE 16.
Prolonged abstinence at longest follow-up according to whether or not allocation concealment was adequate.
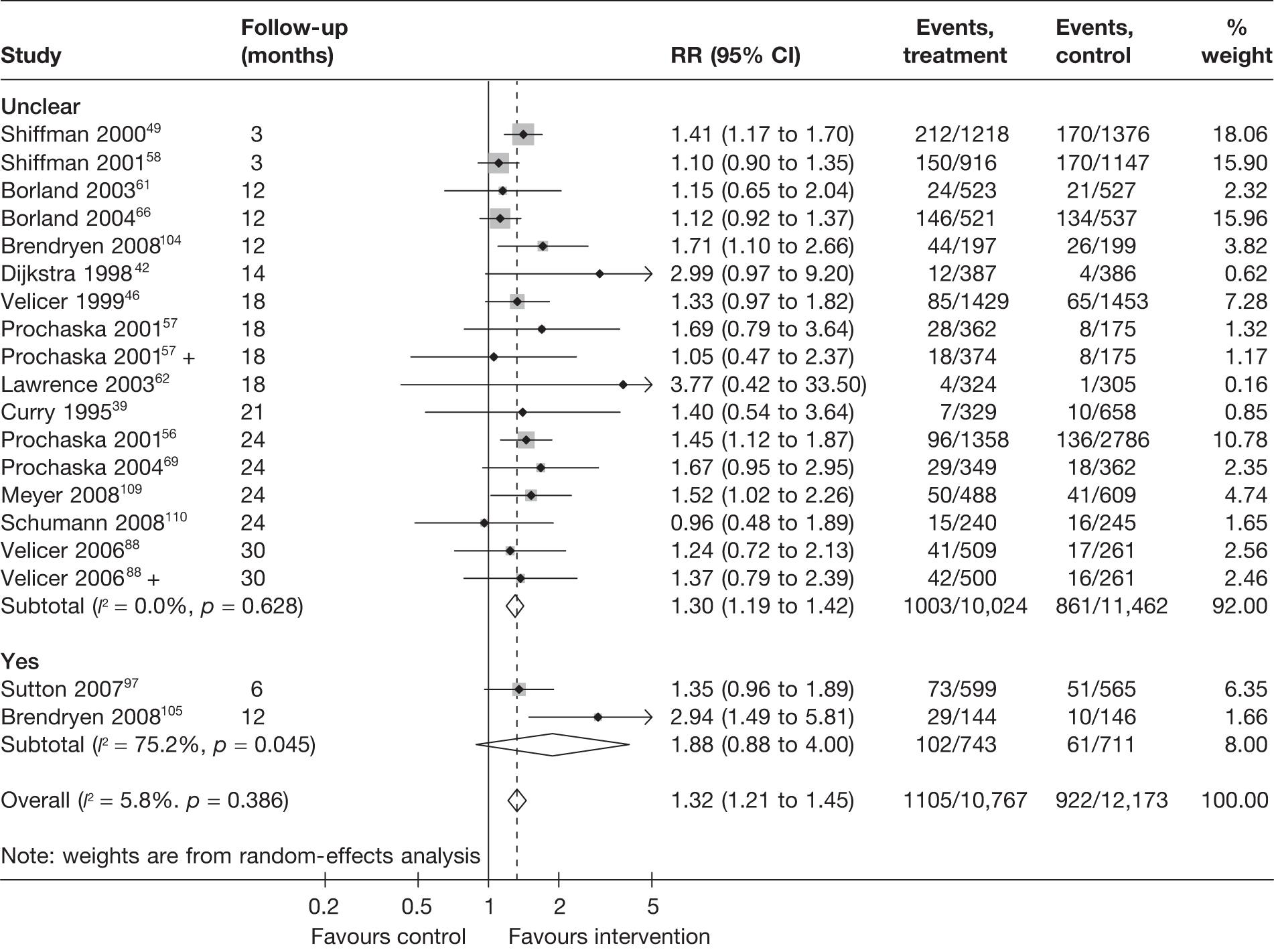
Figure 17 shows the results for point prevalence abstinence at longest follow-up. Pooled RRs are very similar between the subgroups, with either adequate or unclear allocation concealment. There is moderate heterogeneity (I2 = approximately 30%) within each of the two subgroups. Results for point prevalence abstinence at 6 months suggest that studies with adequate allocation concealment tended to produce smaller effect sizes (Figure 18). However, very high levels of heterogeneity (I2 > 70%) were observed within each subgroups and this precludes a valid test for interaction to be conducted and any firm conclusions to be drawn.
FIGURE 17.
Point prevalence abstinence at longest follow-up according to whether or not allocation concealment was adequate.
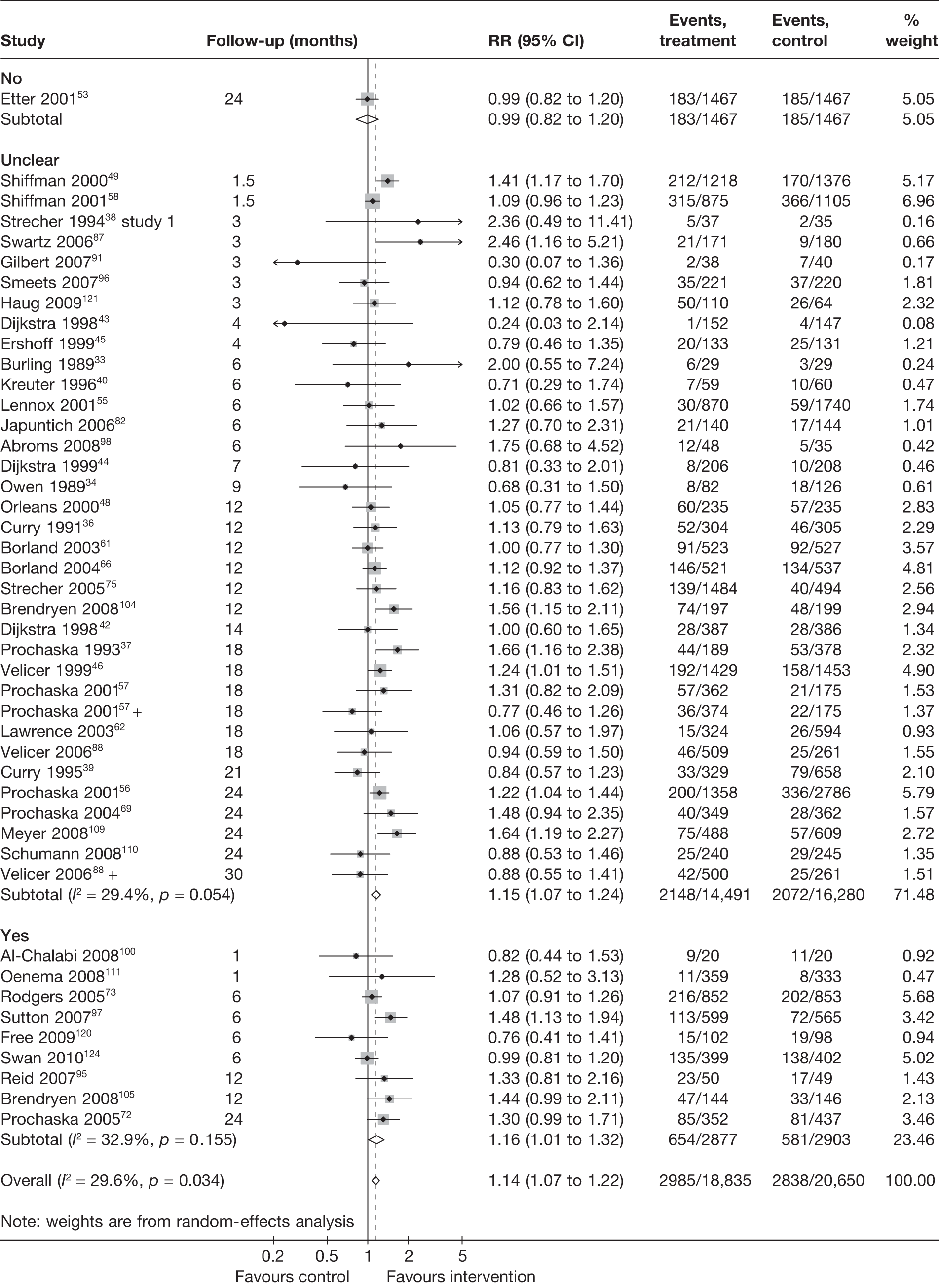
FIGURE 18.
Point prevalence abstinence at 6 months according to whether or not allocation concealment was adequate.
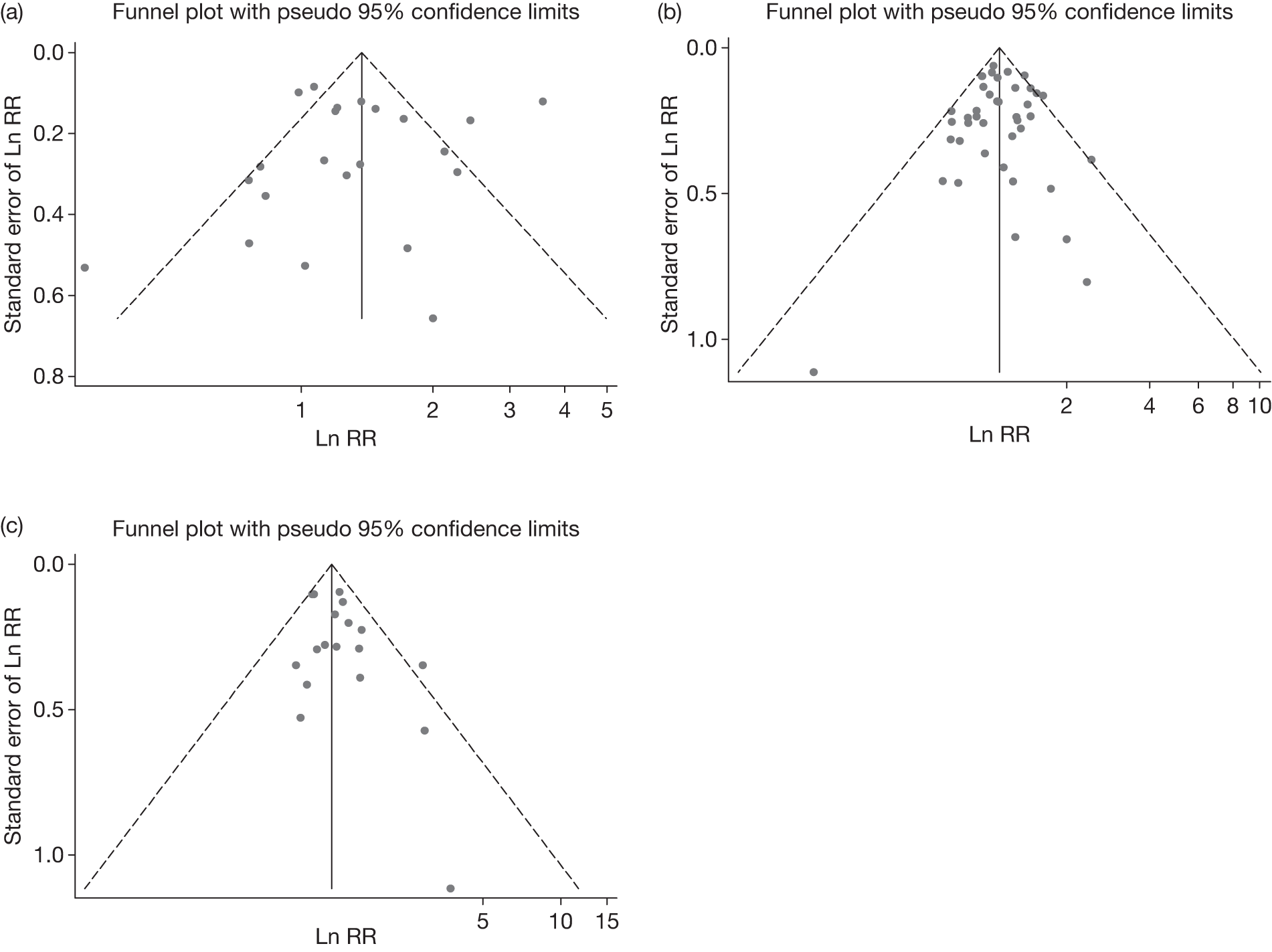
Funnel plots
Funnel plots were generated for point prevalence abstinence at 6 months, point prevalence abstinence at longest follow-up, and prolonged abstinence at longest follow-up. They are shown in Figure 19. Consistent with the above findings, substantial heterogeneity is observed for point prevalence abstinence at 6 months. Egger’s test for all the three outcome measures does not suggest significant funnel plot asymmetry, although the possibility of some missing studies with negative outcomes cannot be ruled out by inspection of the funnel plots for point prevalence abstinence and prolonged abstinence at longest follow-up.
FIGURE 19.
Funnel plots showing all studies comparing computer and electronic aids to control of no or minimal intervention. (a) Point prevalence abstinence at 6 months, Egger’s test p = 0.15; (b) point prevalence abstinence at longest follow-up, Egger’s test p = 0.30; (c) prolonged abstinence at longest follow-up, Egger’s test p = 0.25. Ln, natural log.
Interventions with single or multiple generic components
The previous section (see Overview of effectiveness) provided an overview of quantitative findings from included studies in terms of the effectiveness of the interventions compared with no or minimal intervention. This section and the following two sections (see Interventions with single tailored component and Interventions with multiple tailored components) describe individual studies in more detail, including the assessment of their methodological quality. Studies were grouped according to the categorisation described previously (see Grouping of studies and study arms). Each of the sections includes descriptions of interventions and co-interventions, study design and characteristics of participants, quality assessment of included studies, and comparisons and findings. The latter present different types of comparison (see Figure 1) in the following order:
-
electronic interventions compared with no intervention, usual care or untailored printed self-help material
-
comparison of electronic interventions with other non-electronic interventions
-
comparisons between electronic interventions
-
other comparisons.
This section summarises two studies100,123 that evaluated interventions with single or multiple generic components; both were aid to cessation studies. The characteristics of these studies and their participants are presented in Table 5 and are briefly described below.
| Study and country | Co-intervention (non-electronic) | Population, key criteria related to smoking history and method of recruitment | Mean age (years); % female | Intervention, comparators and sample size (n) | Outcome measure and length of follow-up; comparison code [0–5] (see Figure 1 for the coding scheme) |
|---|---|---|---|---|---|
| Aid to cessation studies | |||||
|
Muñoz et al. 2009123 Based in the USA; participants were from 68 countries |
None | Adults smoking ≥ 5 cigarettes/day and intending to quit in the next month. Recruited online using Google AdWords campaigns targeted at users worldwide | 37.9; 45 |
Guía: static website (247) vs Guía + ITEMs (251) vs Guía + ITEMs + plus eight-lesson cognitive behaviour mood management course (251) vs Guía + ITEMs + mood management + virtual group (an asynchronous bulletin board) (251) |
7-day point prevalence abstinence Follow-up: 1, 3, 6, 12 months [4] |
|
Al-Chalabi et al. 2008100 UK |
NRT + counselling (drop-in clinic) | Smokers attending NHS stop smoking clinics were recruited in the clinics by the researchers. No criteria related to smoking history were used | 34.5; 53 | E-mail instructions for doing body scan and isometric exercises (20) vs no intervention (20) |
4-week prolonged abstinence Follow-up: 4 weeks after quit day [0] |
Interventions and co-interventions
Interventions tested were different between the studies. Al-Chalabi et al. 100 conducted a pilot RCT in smokers attending NHS stop-smoking clinics to evaluate the feasibility of delivering, by e-mail, instructions for performing activities (isometric exercises and body scan) that might help reduce the urges to smoke. Muñoz et al. 123 compared a static, online cessation guide with three interventions with incremental features added to this guide, including automated e-mails with links to sections of the guide, an eight-lesson cognitive–behavioural mood management course and an asynchronous bulletin board for mutual support between smokers. The static online guide, the additional automated e-mails, and mood management course were considered generic components and thus the three interventions including these components (incrementally) are described in this section. The intervention with all additional features, including a bulletin board (considered as a tailored component in our coding scheme), will be described below (see Summary and discussion).
Study design and characteristics of participants
Both studies were RCTs that randomised participants individually. Al-Chalabi et al. 100 reported continuous abstinence during a 4-week follow-up period and Muñoz et al. 123 reported 7-day point prevalence abstinence at various points during the follow-up of 1 year. Prolonged abstinence was not reported.
Participants in Al-Chalabi et al. 100 were users of NHS stop smoking clinics. Muñoz et al. 123 recruited smokers through online advertisements, links and media stories, etc. In addition, participants needed to have logged the number of cigarettes smoked on 3 days within a week and have subsequently set a quit date before they were randomised. The mean age of participants ranged from 34 to 38 years among these trials, and 45% to 53% of participants were female.
Quality assessment of included studies
Results of quality assessment are presented in Table 6. The methods of randomisations were adequate, and ITT analyses were performed in both Al-Chalabi et al. 100 and Muñoz et al. 123 Biochemical validation was undertaken in only Al-Chalabi et al. 100 Approximately one-third of participants were lost to follow-up at 12 months in Muñoz et al. 123
| Study | Generation of random sequence | Allocation concealment | Blinding | Biochemical validation | Baseline characteristics similar between groups | Lost to follow-up | ITT analysis |
|---|---|---|---|---|---|---|---|
| Muñoz et al. 2009123 | Yes | Yes | Outcome assessors | No | Unclear | 30.8% (308/1000) at 12 months | Yes |
| Al-Chalabi et al. 2008100 | Yes | Yes | Outcome assessors | Yes | No (minor imbalance in favour of intervention arm) | 20% (4/20) at 4 weeks for intervention arm. Unclear for control arm | Yes |
Comparisons and findings
Electronic interventions compared with no intervention, usual care or untailored printed self-help material
This comparison was made in only Al-Chalabi et al. 100 At 4 weeks, continuous abstinence was achieved by 45% (9/20) of participants in the intervention (isometric exercise and body scan) group and 55% (11/20) in the control group (RR = 0.82, 95% CI 0.44 to 1.53). This pilot study was too small to allow adequate assessment of effectiveness.
Comparison of electronic interventions with other non-electronic interventions
No study addressed this comparison.
Comparisons between electronic interventions
The study by Muñoz et al. 123 allowed comparison between an intervention with a single generic component (i.e. a static online cessation guide) and interventions with multiple generic components (i.e. with either the addition of automated e-mails referring to the guide, or the addition of both automated e-mails and a generic online mood management course). No significant difference in 7-day point prevalence abstinence was found between the three groups at 6 months (14.5% vs 16.7% vs 14.3% for guide only vs guide + e-mails vs guide + e-mails + mood course, respectively) and 12 months (19.8% vs 19.1% vs 20.7%).
Summary and discussion
Two aid to cessation studies evaluated interventions with single or multiple generic components. 100,123
The effectiveness of electronic interventions with single or multiple generic components compared with minimum/no intervention cannot be adequately quantified, as the sample size was too small in one RCT (Al-Chalabi et al. 100) and there was no ‘inactive control’ group in the other (Muñoz et al. 123).
The point prevalence abstinence rates (approximately 15% at 6 months and 20% at 12 months) reported by Muñoz et al. 123 for a static online cessation guide with or without other generic component seem to be higher than those observed in no-intervention control groups in other studies, hence suggest their possible effectiveness. However, this trial required participants to have logged the number of cigarettes smoked on 3 days within a week and to have set a quit date prior to study enrolment. Therefore, it is likely that the participants were relatively motivated/compliant smokers and the abstinence rates may not be directly comparable to other studies.
Results of the trial by Muñoz et al. 123 suggest that the addition of further generic component(s) does not enhance the effectiveness of a static online smoking cessation guide.
Interventions with single tailored component
The vast majority of included studies evaluated interventions with a single tailored component, with or without additional generic component(s). This section is therefore further subdivided according to the mode of delivery of the interventions.
Computer-generated, tailored printed materials
Thirty studies34,36–40,42–44,46,48,49,53,55–58,61,66,69,72,75,80,88,91,96,97,109,110 evaluated electronic interventions with computer-tailored printed materials, 1034,36,38,48,49,58,61,66,75,97 of which were aid to cessation studies and 2037–40,42–44,46,53,55–57,69,72,80,88,91,96,109,110 of which were cessation induction studies. The study characteristics, results of quality assessment and key findings are described below.
Interventions and co-interventions
Table 7 shows the characteristics of included studies and their participants. The interventions consisted of individualised feedback/printed letters generated by special software or ‘expert systems’. Most of the letters were tailored based on conceptual models on relevant theories of smoking cessation and behaviour change (e.g. transtheoretical model or social cognitive theory).
| Study and country | Co-intervention (non-electronic) | Population, key criteria related to smoking history and method of recruitment | Mean age (years); % female | Intervention, comparators and sample size (n) | Outcome measure and length of follow-up; comparison code [0–5] (see Figure 1 for the coding scheme) |
|---|---|---|---|---|---|
| Aid to cessation studies | |||||
|
Owen et al. 198934 Australia |
None | Inclusion/exclusion criteria were not clearly stated. Smokers were recruited by public advertisements in newspapers and local radio and television | 42; 45 |
Quit kit control (40) vs standard correspondence course (86) vs personalised correspondence (82) |
7–10 days point prevalence (not clearly defined) Follow-up: 3 and 9 months [1] |
|
Curry et al. 199136 USA |
Self-help material | Smokers recruited through advertisement in View magazine | 44.1; 65 |
Intrinsic group (304) vs extrinsic (304) vs both (304) vs control (305) |
7-day point prevalence abstinence Follow-up: 3 and 12 months [0] |
|
Strecher et al. 199438 – study 2 USA |
None | Adult patients from 12 community-based group family practice physicians’ office, aged 18–75 years | 36.7; 67 |
Tailored smoking letters (NR) vs no messages (NR) Total sample size = 296 |
7-day point prevalence Follow-up: 6 months [0] |
|
Borland et al. 200466 Australia |
Self-help material | Callers to the Victorian Quitline (a community service providing information and advice on all aspects of smoking cessation), who are adult smokers or recent quitters who requested written materials only. English speakers with no obvious psychiatry or neurological problems | 32.3; 54 |
Tailored advice (521) vs control (537) |
7-day point prevalence and 6-month prolonged abstinence Follow-up: 12 months [0] |
|
Borland et al. 200361 Australia |
Brief advice (plus self-help material) | Adult smokers who called to the Victorian Quitline telephone counselling and advice service | NR; 54 |
Computer-generated, tailored advice + call-back telephone counselling (528) vs computer-generated, tailored advice only (523) vs control ( 527) |
Not smoked for 3 months or 9 months Follow-up: 3, 6 and 12 months [0][5] |
|
Strecher et al. 200575 USA |
Telephone counselling | Callers to National Cancer Institute’s Cancer Information Service enquiring about smoking cessation. English speakers, ≥ 18 years of age, who smoke at least five cigarettes per day, interested in quitting smoking, not involved in another cessation programmes | 41; 70 |
Single untailored (NR) vs single tailored (NR) vs multiple tailored (NR) vs multiple retailored materials (NR) Total sample size = 1978 |
7-day abstinence Follow-up: 5 and 12 months [1][3] |
|
Sutton and Gilbert 200797 UK |
Telephone counselling (+ self-help material) | Current and recent ex-smokers/participants, who smoked on average 20.5 cigarettes per day/via callers to the Quitline | 38.1; 66 |
Computer-generated, tailored advice letter + usual care (765) vs usual care (562) |
3-month prolonged abstinence; 24-hour and 7-day point-prevalence abstinence Follow-up: 24 hours, 7 days, and 1 and 3 months [0] |
|
Orleans et al. 200048 USA |
Transdermal nicotine | Smokers aged ≥ 65 years, who filled a transdermal nicotine prescription | 72; NR |
Tailored treatment group = NR Control group = NR |
7-day point prevalence Follow-up: 6 and 12 months [0] |
|
Shiffman et al. 200049 USA |
NRT (+ self-help material) | Adults/current cigarette smoker/smokers who purchased nicotine polacrilex gum and called the CQP programme through freephone | 41.5; 54 |
CQP (1217) vs CQP + C (1207) vs UG (1203) |
28-day abstinence at week 6 10-week continuous abstinence at week 12 Follow-up: 6 and 12 weeks [0] |
|
Shiffman et al. 200158 USA |
NRT (+ self-help material) | US purchasers of NicoDerm CQ/smoke almost 30 cigarettes per day/calling into a freephone number | 40; 64 |
CQP (1865) vs UG (2344) |
28-day and 10-week continuous abstinence Follow-up: 6 and 12 weeks [0] |
| Cessation induction studies | |||||
|
Prochaska et al. 199337 USA |
None | Adult smokers recruited by newspaper advertisements. No criteria related to smoking history were used | 43; 62 |
Counsellor calls + computer-generated, tailored reports + MAN vs tailored reports + MAN vs MAN vs standard self-help manual Total sample randomised = 756 |
24-hour point prevalence abstinence; not smoking at two consecutive follow-ups Follow-up: 6, 12 and 18 months [1][5] |
|
Strecher et al. 199438 – study 1 USA |
None | Adult patients of a large family practice, aged 40–65 years, with telephone, who do not share a household with someone in the same sample list | 49.5; 68 |
Tailored smoking letters (NR) vs standardised smoking letters (NR) Total sample size = 72 |
7-day point prevalence abstinence Follow-up: 4 months [1] |
|
Curry et al. 199539 USA |
None | Enrolees in a health maintenance organisation/17.3 cigarettes/day/telephone survey | 41.1; 52 |
Booklet (327) vs feedback (323) vs telephone (150) vs control (324) |
7-day abstinence Follow-up: 3, 12 and 21 months [0][1][5] |
|
Kreuter and Stretcher 199640 USA |
None | Adult family practice patients | 40; 65 |
Enhanced health risk appraisal (427) vs typical health risk appraisal (427) vs control (463) |
Smoking prevalence Follow-up: 6 months [0][3] |
|
Dijkstra et al. 199842 The Netherlands |
None | Adult smokers/average of 20.3 cigarettes/day; smoked for an average of 21.8 years/advertisement in a local newspaper | 39.7; 59 |
Only OC (386) vs Only SE (387) vs BO (387) vs no information at all (CO) (286) |
7-day abstinence, not smoking for at least 24 hours, 12-month prolonged abstinence Follow-up: 3 and 14 months [0][3] |
|
Dijkstra et al. 199843 The Netherlands |
None | Adult smokers who were not planning to quit within the next 6 months. Recruited by advertisements in local newspapers | 39.3; 60 |
A tailored letter three times (multiple) with SHG (MT-plus condition; n = 140) vs three times (multiple) a tailored letter only (MT-only condition; n = 156) vs a ST with a SHG (ST-plus condition; n = 157) vs a ST only (ST-only condition; n = 152) vs single NT letter once (NT condition; n = 147) |
24-hour quit attempt, 7-day abstinence, intention to quit, stage transition, self-efficacy expectations, smoking behaviours Follow-up: 4 months [1][3] |
|
Dijkstra et al. 199944 The Netherlands |
None | Adult smokers who were not planning to quit within the next 6 months. Recruited by advertisements in local newspapers | 41.7; 63 |
MT (214) vs ST (206) vs SHG (215) vs CO (208) |
7-day point prevalence abstinence Follow-up: 7 months [0][1][3] |
|
Velicer et al. 199946 USA |
None | Adult smokers of a managed care system identified through mail and telephone surveys. No criteria relating to smoking history or readiness to quit were applied | 38; 56 |
EXP+ MAN – one contact (357) vs EXP + MAN – two contacts (353) vs EXP+ MAN – three contacts (362) vs EXP+ MAN – six contacts (357) vs MAN – one contact (359) vs MAN – two contacts (368) vs MAN – three contacts (366) vs MAN – six contacts (360) |
24-hour and 7-day point prevalence abstinence 30-days and 6-month prolonged abstinence Follow-up: 6, 12 and 18 months [1][3] |
|
Switzerland |
None | Residents aged 18–60 of the French-speaking part of Switzerland/daily cigarette smokers/randomly selected from a general population register | 36.3; NR |
Computer-generated tailored counselling letter (1467) vs no intervention (1467) |
7-day point prevalence abstinence and 1-month abstinence Follow-up: 7 months [0] |
|
Lennox et al. 200155 UK |
None | Smokers aged 17–65 years registered at six general practices in Aberdeen | NR |
Computer-tailored letter (870) vs standard letter (869) vs no intervention (871) |
7-day point abstinence, stage of change Follow-up: 6 months [0][1] |
|
Prochaska et al. 200156 USA |
None | Rhode Island smokers/20.5 cigarettes per day/random digit-dialling telephone calls | 41.3; 55 |
EXP – computer report (1358) vs assessment only (2786) |
24-hour and 7-day abstinence 30-day and 6-month prolonged abstinence rates Follow-up: 6, 12, 18 and 24 months [0] |
|
Prochaska et al. 200157 USA |
None | Adults/criteria related to smoking history not reported/contacted via telephone and mail | 38.1; 56 |
EXP (368) vs EXP + counselling (359) vs EXP + LifeSign computer (366) vs Assessment only (359) |
24-hr and 7-day point prevalence abstinence 30 days and 6 months prolonged abstinence Follow-up: 6, 12 and 18 months [0] |
|
Prochaska et al. 200469 USA |
None | Parents/criteria related to smoking history not reported/list of parents who had a 9th grader | 42.5; 75 |
Computer-generated tailored materials (1209) vs assessment only (1251) |
24-hour and 7-day point prevalence abstinence 6-month prolonged abstinence Follow-up: 12 and 24 months [0] |
|
Prochaska et al. 200572 USA |
None | Primary care patients/17.2 cigarettes/day/recruited at home via telephone | 44.7; 70 |
EXP intervention (2667) vs assessment only (2740) |
7-day abstinence, not smoking for at least 24 hours, 6-month prolonged abstinence Follow-up: 12 and 24 months [0] |
|
USA |
None | Adult patients receiving outpatient treatment for depression; having smoked one or more cigarettes per day during the week before recruitment. Recruited by provider referral, invitation letters, and flyers in the participating clinics | 41.8; 61 |
Computer-generated tailored materials + one-to-one face-to-face counselling + nicotine patches + bupropion (163) vs a folder containing a list of referral to smoking cessation programmes + a stop smoking guide (159) |
7-day point prevalence abstinence Follow-up: 3, 6, 12 and 18 months [5] |
|
Smeets et al. 200796 The Netherlands |
None | Volunteers ages 18–65 years/15.6% smoked at baseline/contacted by telephone | 47; 57 |
Computer-generated tailored materials (1417) vs printed untailored materials (1410) |
Smoking behaviour was measured by asking smokers to state the number of cigarettes they smoked a day and whether or not they had made any quit attempts during the last 3 months Follow-up: 3 months [1] |
|
Meyer et al. 2008109 Germany |
None (usual practice) | Primary care patients aged 18–70 years who smoked cigarettes daily in the past 4 weeks. Patients who attended study practices were screened for smoking status and those who fulfilled the inclusion criteria were invited to participate | 34.0; 48 |
Computer-tailored letters (488) vs practitioners’ brief advice (402) vs assessment only (609) |
24-hour and 7-day point prevalence abstinence; 4-week and 6-month prolonged abstinence Follow-up: 6, 12, 18 and 24 months [0][2] |
|
Schumann et al. 2008110,115,127 Germany |
None | Adults/former and current smokers/from an existing general population health examination survey | 44.5; 47 |
Computer-generated, tailored materials (302) vs no intervention (309) |
7-day abstinence rate, prolonged abstinence of at least 6-months Follow-up: 6, 12, 18 and 24 months [0] |
|
Gilbert et al. 200791 UK |
Self-help material (+ standard care from GP) | Smokers between 18 and 65 years/93.6% smoked everyday/selected from GP practice using EMIS | 42.1; 54 |
Computer-tailored feedback report (38) vs no intervention (40) |
24-hour and 7-day point prevalence abstinence; 1- and 3-month prolonged abstinence Follow-up: 3 months [0] |
|
Velicer et al. 200688 USA |
NRT | Smokers from a New England Veterans Affairs Medical Center/smoked on average 24.5 cigarettes per day/via letter | 50.5; 23 |
MAN (523) vs NRT plus MANs (522) vs EXP + NRT + MANs ( 509) vs TEL + EXP + NRT + MANs (500) |
24-hour and 7-day point-prevalence abstinence 6-month prolonged abstinence Follow-up: 10, 20 and 30 months [0] |
Four studies48,49,58,88 included pharmacotherapy as a co-intervention in each of the study arms; two studies75,97 included telephone counselling as a co-intervention, while another study61 included brief advice. Seven studies,36,46,49,58,61,66,97 including four mentioned above,49,58,61,97 had generic self-help material as a co-intervention in each of the study arms. Twenty studies34,37–40,42–44,53,55–57,69,72,80,91,96,109,110 do not have non-electronic co-interventions.
Study design and characteristics of participants
All the 30 studies, except Meyer et al. ,109 which was a quasi-RCT, were RCTs that randomised participants individually. 34,36–40,42–44,46,48,49,53,55–58,61,66,69,72,75,80,88,91,96,97,109,110 Follow-up ranged from 3 months to 30 months88 and was shorter than 12 months in 10 studies. All studies reported point prevalence, whereas 1639,42,46,49,56–58,61,66,69,72,88,91,97,109,110 reported prolonged abstinence.
Participants were recruited into trials as advertised through various media. Three studies66,97,115 included both current smokers and ex-smokers; only current smokers at study entry were included in the analysis in this section.
Mean age of study participants ranged from 32.366 to 72 years. 48 The proportion of females varies between 23%88 and 76%. 69
Quality assessment of included studies
Results of quality assessment of included studies are presented in Table 8. The methods of generation of random sequence, allocation concealment and blinding are unclear in the vast majority of the studies. Only seven studies34,36,39,42,55,62,80 included biochemical validation of smoking status in respondents. Loss to follow-up ranged from as low as 11% at 6 months44 to 56% at 12 months. 75 Six studies39,43,49,55,57,115 reported significant difference between intervention groups at baseline.
| Study | Generation of random sequence | Allocation concealment | Blinding | Biochemical validation | Baseline characteristics similar between groups | Lost to follow-up | ITT analysis |
|---|---|---|---|---|---|---|---|
| Schumann et al. 2008110,115,128 | Yes | Unclear | Unclear | No | No (difference in intention to quit within 6 months and past quit attempts) |
Overall: 175/611 (28.6%) Computer-generated, tailored materials: 101/302 (33.4%) No intervention: 74/309 (23.9%) |
Yes |
| Meyer et al. 2008109 | No (based on the time of practice attendance) | Unclear | Outcome assessors | No | Yes | 558/1499 (37.2%) | Yes |
| Gilbert et al. 200791 | Yes | Unclear | Unclear | No | No | 26/72 (36.1%) | Yes |
| Sutton and Gilbert 200797 | Yes | Yes | Outcome assessors | No | Yes |
Computer-generated, tailored advice + usual care: 159/765 (20.8%) Usual care: 181/743 (24.4%) |
Yes |
| Smeets et al. 200796 | Unclear | Unclear | Unclear | No | Yes | NR | Unclear |
| Velicer et al. 200688 | Yes | Unclear | Participants | No | Yes |
MAN: 126/523 (24.1%) NRT + MAN: 142/522 (27.2%) EXP + NRT + MAN: 153/509 (30.0%) TEL + EXP + NRT + MAN: 155/500 (31.0%) |
Yes |
| Hall et al. 200680 | Yes | Unclear | Outcome assessors | Yes | Yes |
Computer-generated, tailored material + counselling +nicotine patched + bupropion: 41/163 (25.2%) List of referrals to smoking cessation programmes: 49/159 (30.8%) |
Yes |
| Strecher et al. 200575 | Unclear | Unclear | Unclear | No | Yes | 34% at 5 months and 56% at 12 months | Yes |
| Prochaska et al. 200572 | Unclear | Yes | Outcome assessors | No | Yes |
EXP: 784/2667 (29.3%) Assessment only: 595/2740 (21.7%) |
Unclear |
| Prochaska et al. 200469 | Unclear | Unclear | Unclear | No | Yes |
Computer-generated, tailored materials: 273/1209 (22.6%) Assessment only: 272/1251 (21.7%) |
Yes |
| Borland et al. 200366 | Unclear | Unclear | Unclear | No | Yes |
Tailored advice = 170/521 Control = 146/537 |
Yes |
| Borland et al. 200361 | Yes | Unclear | Participants | No | Yes | 369/1578 (23.4%) | Yes |
| Etter and Perneger 200153,54 | Yes | No | Unclear | No | Yes |
Computer-generated, tailored counselling letter: 357/1467 (24.3%) No intervention: 121/1467 (8.2%) |
Yes |
| Shiffman et al. 200158 | Yes | Unclear | Unclear | No | Yes |
Overall: 2194/4209 (52.1%) Computer-tailored program: 860/1865 (46.1%) UG: 1334/2344 (56.9%) |
No |
| Prochaska et al. 200156 | Unclear | Unclear | Unclear | No | No (proportions for ethnic and racial subgroups) |
Overall: 1573/4144 (38.0%) EXP: 556/1358 (40.9%) Assessment only: 1017/2786 (36.5%) |
No |
| Lennox et al. 200155 | Yes | Unclear | Unclear | Yes | No (proportion of heavy smokers higher in tailored group) |
Computer-tailored = 213/870 Standard letter = 236/869 Control = 166/871 |
Yes |
| Prochaska et al. 200157 | Unclear | Unclear | Unclear | No | No (for number of cigarettes per day) | NR | Yes |
| Shiffman et al. 200049 | Unclear | Unclear | Open label | No | Yes |
CQP: 347/1217 (28.5%) CQP + C: 349/1207 (28.9%) UG: 303/1203 (25.2%) |
Yes |
| Orleans et al. 200048 | Unclear | Unclear | Unclear | No | Unclear | 22% at 12 months | No |
| Dijkstra et al. 199944 | Unclear | Unclear | Unclear | No | Yes |
At 6 months: MT: 27/214 (12.6%) ST: 26/206 (12.2%) SHG: 15/215 (7.0%) CO: 26/208 (12.5%) |
Yes |
| Velicer et al. 199946 | Unclear | Unclear | Unclear | No | Yes | NR | Unclear |
| Dijkstra et al. 199843 | Unclear | Unclear | Unclear | No | No (higher proportion classified as emotive in non-tailored group) | 147/752 (19.5%) at 4 months | No |
| Dijkstra et al. 199842 | Unclear | Unclear | Unclear | Yes | Yes | 555/1546 (35.9%) | Unclear |
| Kreuter et al. 199640 | Unclear | Unclear | Unclear | No | Yes | 186/1317 (14.1%) | Unclear |
| Curry and Strecher 199539 | Unclear | Unclear | Unclear | Yes | No (previous quit attempt) | 272/1137 (23.9%) | Yes |
| Strecher et al. 199438 – study 1 | Unclear | Unclear | Unclear | No | Yes | 29.2% | No |
| Strecher et al. 199438 – study 2 | Unclear | Unclear | Unclear | No | Yes | 33% | No |
| Prochaska et al. 199337 | Unclear | Unclear | Unclear | No | Unclear | 13% at 6 months; 14% at 12 months; 20% at 18 months | No |
| Curry et al. 199136 | Unclear | Unclear | Unclear | Yes | Yes | Unclear | Yes |
| Owen et al. 198934 | Unclear | Unclear | Unclear | Yes | Yes | 12% | Unclear |
Comparisons and findings
This comparison was addressed by 27 out of the 31 studies comparing computer-generated, tailored printed materials to either no intervention, usual care or untailored printed self-help materials, with or without concurrent co-interventions. Figure 20 shows the results of point prevalence abstinence at 6 months, which was reported in 13 studies. 37,40,44,46,48,53,55–57,61,97,109,110 Pooled result showed that computer-generated, tailored printed materials, when added to varied co-interventions, increase the likelihood of cessation in studies in which smokers are prepared to quit at the beginning of the studies (RR = 1.29, 95% CI 1.10 to 1.51). Tailored printed materials also appear to be effective in studies in which the smokers were not ready to quit at the start of the study, but there was significant heterogeneity in effect estimates (I2 = 85%, p < 0.001). Overall, only five studies53,56,88,97,109 demonstrated a statistically significant increase in abstinence rates in the intervention groups compared with the control groups.
FIGURE 20.
Electronic interventions with computer-generated, tailored printed materials vs control: point prevalence abstinence at 6 months.
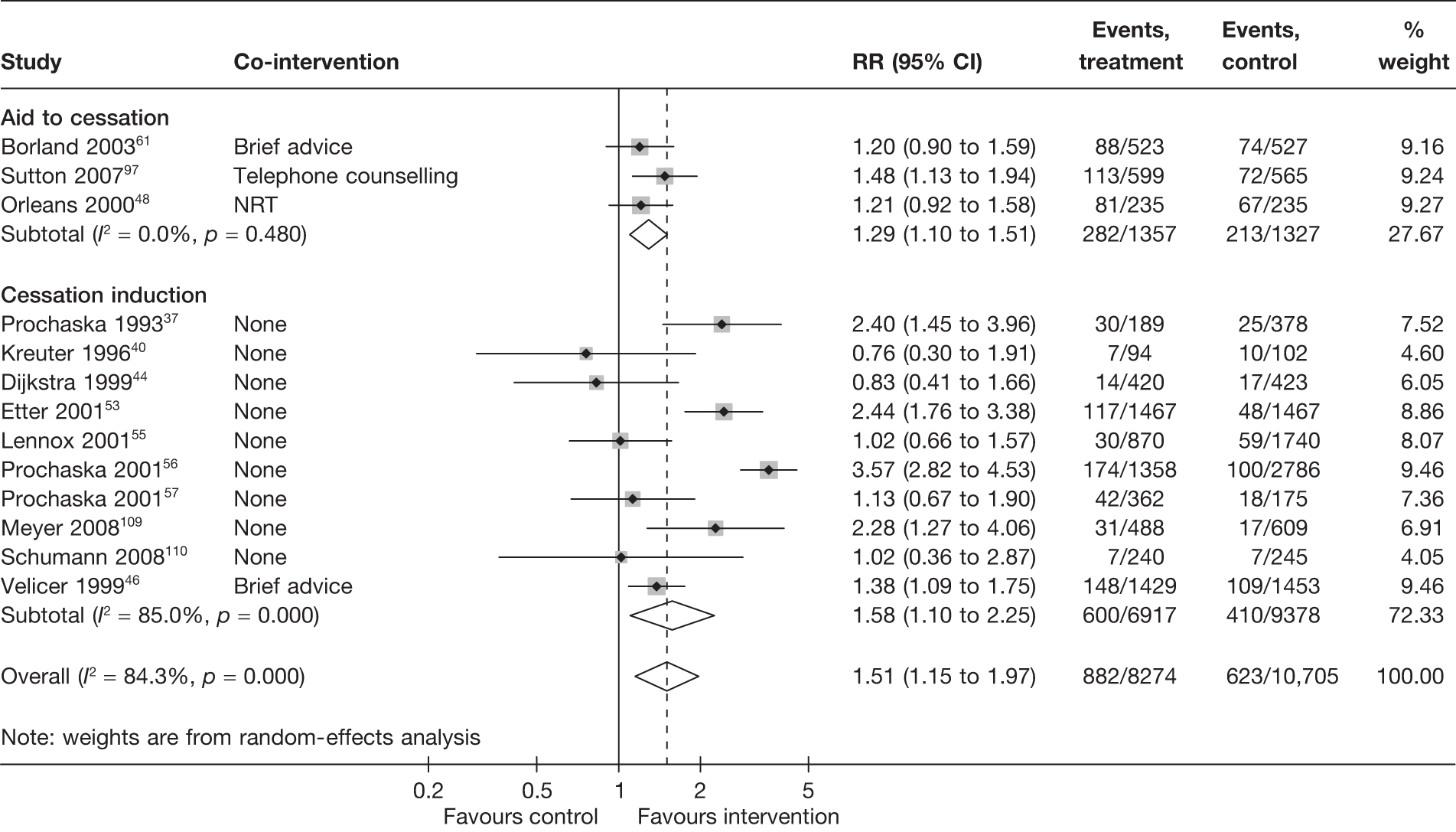
Figure 21 shows the results of point prevalence abstinence measured at the last follow-up of each study. Twenty-eight studies contributed to the overall summary estimates. There was some heterogeneity (I2 = ∼30%) between the studies. Pooled results showed a similar increase in abstinence from the use of computer-generated, tailored printed materials compared with control group regardless of whether a study was a cessation induction study or aid to cessation study. Only six studies37,49,56,88,97,109 demonstrated a statistically significant increase in abstinence rates in the intervention groups compared with control groups. Of these studies, three56,88,109 reported smaller effect size at maximum follow-up compared with 6 months.
FIGURE 21.
Electronic interventions with computer-generated, tailored printed materials vs control: point prevalence abstinence at maximum follow-up.
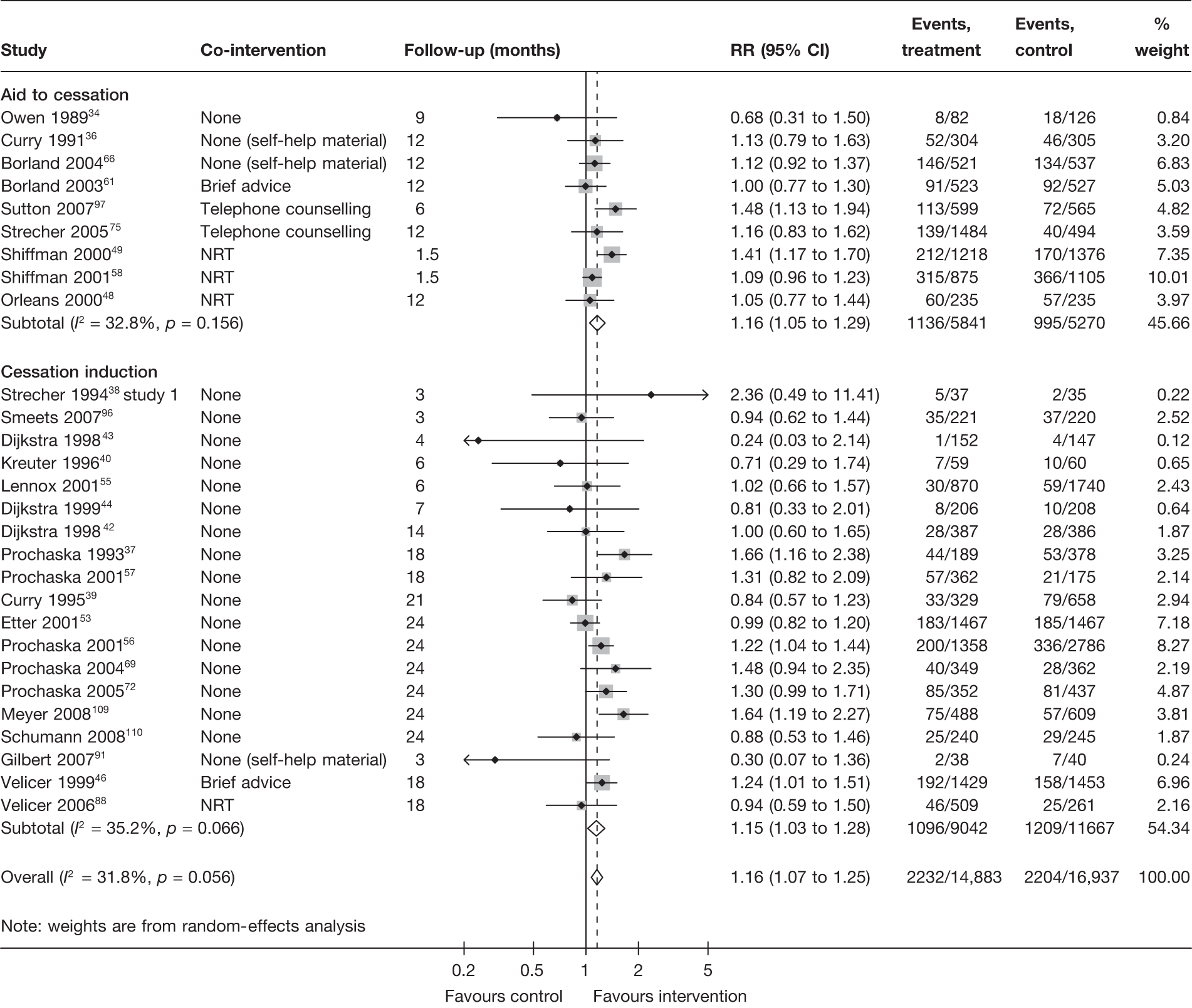
Figure 22 presents the results for prolonged abstinence at 6 months and are represented by two studies,61,97 which are aid to cessation studies. The pooled result (RR = 1.31, 95% CI 1.00 to 1.71) just reaches statistical significance and is in line with point prevalence abstinence. Similarly, the results of prolonged abstinence at maximum follow-up are also in line with point abstinence at maximum follow-up and are shown in Figure 23.
FIGURE 22.
Electronic interventions with computer-generated, tailored printed materials vs control: prolonged abstinence at 6 months.
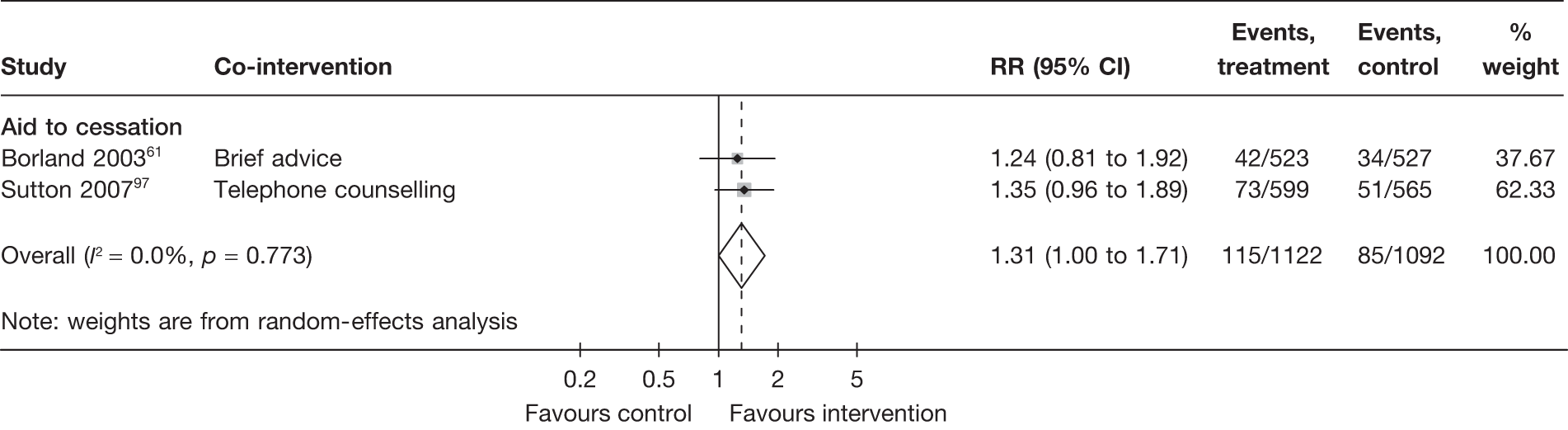
FIGURE 23.
Electronic interventions with computer-generated, tailored printed materials vs control: prolonged abstinence at maximum follow-up.
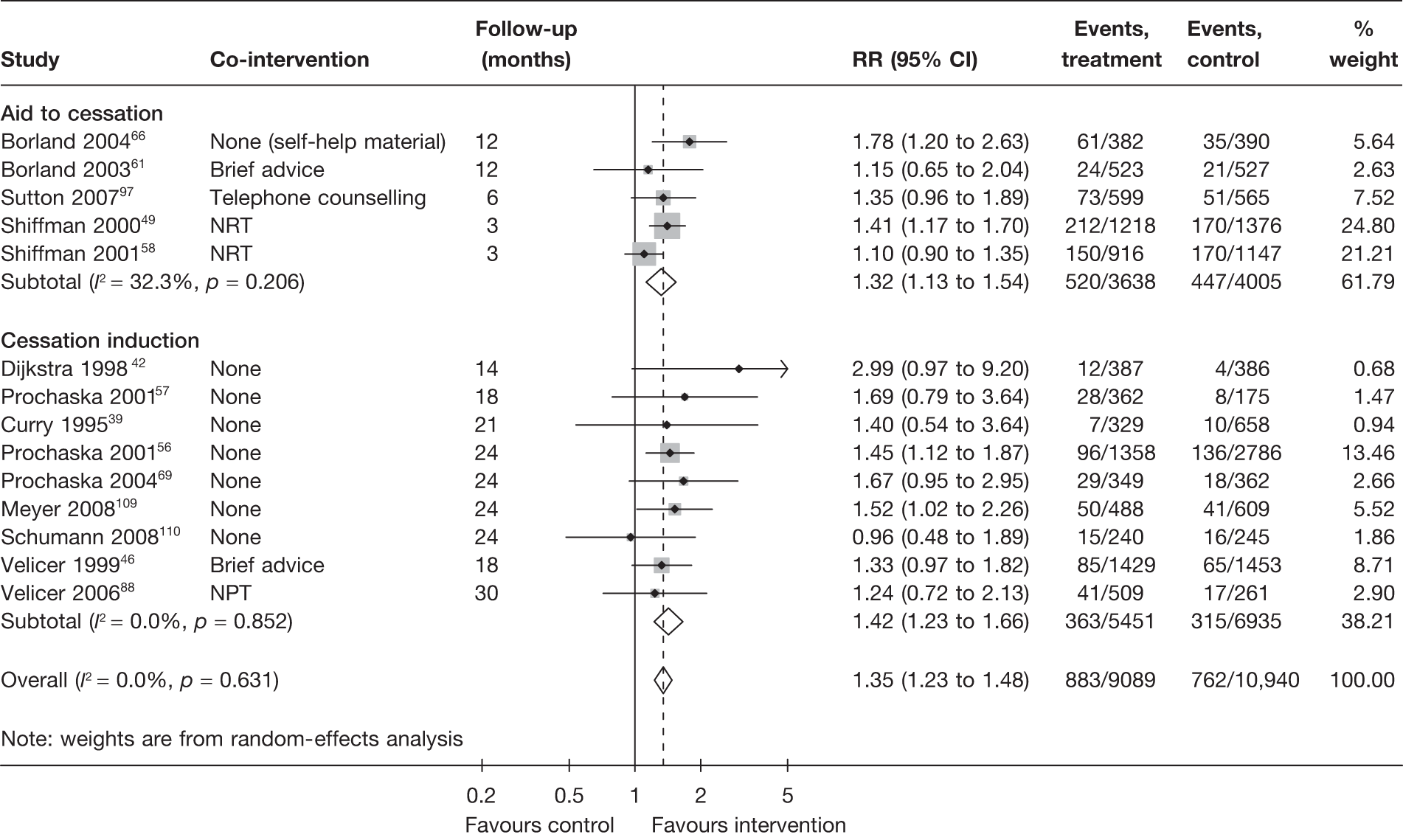
This comparison was addressed only in one cessation induction study. Meyer et al. 109 compared (up to three) computer-tailored letters with brief advice delivered by GPs, who received 2-hour on-site training in counselling techniques. Similar rates of point prevalence abstinence (tailored letters vs brief advice, 6.4% vs 4.0%, RR = 1.60, 95% CI 0.89 to 2.88 at 6 months; 15.4% vs 14.2%, RR = 1.08, 95% CI 0.79 to 1.49 at 24 months) and prolonged abstinence (tailored letters vs brief advice, 10.2% vs 9.7%, RR = 1.06, 95% CI 0.71 to 1.57) were observed between the groups. The abstinence rates in both intervention groups were significantly higher than an ‘assessment only’ control group within the same study (the ‘tailored letters’ vs ‘assessment only’ comparison has been described in the previous section).
Six studies40,42–44,46,75 compared different electronic interventions with a single tailored component against each other. The comparisons and findings are summarised in Table 9 and each study is briefly described below. As settings and contents of the electronic interventions being assessed were different, it was not possible to include them in a meta-analysis and compare the electronic interventions with each other.
| Study | Study type | Co-intervention | Comparisons | Key findingsa |
|---|---|---|---|---|
| Comparing different forms/contents/provision of interventions within each mode of delivery (comparison code 3, see Figure 1) | ||||
| Strecher et al. 200575 | Aid to cessation | One initial telephone counselling cessation |
A series of four printed materials initially tailored to baseline data and subsequently retailored to follow-up data at 5 months vs a series of four printed materials tailored only to baseline data vs a single tailored smoking cessation guide (Total n for the trial = 1978) |
Point prevalence abstinence (at 12 months): Multiple retailored 10.5% vs multiple tailored 10.3% vs single tailored 7.2% RR (multiple retailored + multiple tailored vs single tailored) = 1.43, 95% CI 0.99 to 2.06b Prolonged abstinence: NR |
| Kreuter and Strecher 199640 | Cessation induction | None |
‘Enhanced HRA’ providing tailored feedback on risk information + behaviour-change information (n = 94) vs ‘typical HRA’ providing feedback on risk information only (n = 102) |
Point prevalence abstinence (at 6 months): Enhanced HRA vs typical HRA 7.4% vs 7.8% RR = 0.95, 95% CI 0.36 to 2.52 Prolonged abstinence: NR |
| Dijkstra et al. 199842 | Cessation induction | None |
2 × 2 factorial design. One-off tailored material containing: (information on outcomes of quitting vs no information) × (information on self-efficacy enhancement vs no information) Total n = 1546 |
Point prevalence abstinence (at 14 months): Outcomes information 6.3% vs no information 7.2% RR = 0.88, 95% CI 0.60 to 1.27 Self-efficacy information 7.2% vs no information 6.3% RR = 1.14, 95% CI 0.79 to 1.65 Prolonged abstinence (at 14 months): Outcomes information 2.3% vs no information 1.6% RR = 1.50, 95% CI 0.73 to 3.09 Self-efficacy information 2.6% vs no information 1.3% RR = 2.00, 95% CI 0.94 to 4.23 |
| Dijkstra et al. 199843 | Cessation induction | None |
2 × 2 factorial design: (multiple tailored letters vs ST) × (self-help manual vs no manual) Total n = 605 |
Point prevalence abstinence (at 4 months): Multiple tailored letters 4.1% vs ST 0.6% RR = 6.26, 95% CI 1.41 to 27.75 Self-help manual 1.0% vs no manual 3.6% RR = 0.28, 0.08 to 1.00 Prolonged abstinence: NR |
| Dijkstra et al. 199944 | Cessation induction | None | Three tailored letters (n = 214) vs single tailored letter (n = 206) |
Point prevalence abstinence (at 6 months): Three tailored letters 2.8% vs ST 3.9% RR = 0.72, 95% CI 0.26 to 2.05 Prolonged abstinence: NR |
| Velicer et al. 199946 | Cessation induction | None |
Different number of mailings of tailored reports: one (n = 357) vs two (n = 353) vs three (n = 362) vs six (n = 357) |
Point prevalence abstinence (at 6 months): One mailing 11.2% vs two mailings 9.1% vs three mailings 12.2% vs six mailings 9.0% Prolonged abstinence (at 12 months): One mailing 2.5% vs two mailings 3.4% vs three mailings 5.5% vs six mailings 3.4% |
Strecher et al. 75 compared the effectiveness of the following four interventions when added to a brief cognitive–behavioural cessation telephone counselling: (a) a single, untailored smoking cessation guide; (b) a single, computer-tailored smoking cessation guide; (c) a series of four printed materials tailored only to baseline data; and (d) a series of four printed materials initially tailored to baseline data and subsequently retailored to follow-up data at 5 months. Point prevalence abstinence at 12 months was 8.1%, 7.2%, 10.3% and 10.5% for groups a, b, c and d, respectively. A global test for a trend of increasing effectiveness with increasing intensity and tailoring was not significant. In a possibly post hoc analysis, the authors found that the abstinence rates for the two multiple tailored material groups were significantly higher than the two single materials groups (either untailored or tailored). The reported odds ratio (groups c + d vs groups a + b) was 1.41 (95% CI 1.04 to 1.99).
Kreuter and Strecher40 compared two versions of health risk appraisal (HRA), which provided printed feedback to participants by post and covered seven health-related behaviours, including smoking, exercise, seat belt use, alcohol consumption, dietary fat intake, fruit and vegetable consumption, and safe gun storage. The ‘typical HRA’ provided personal feedback on risk information and the ‘enhanced HRA’ provided, additionally, behaviour-change information that was tailored individually. Similar point prevalence abstinence rates were observed at 6 months among adult family practice patients (enhanced HRA vs typical HRA using baseline smokers as denominators, 7.4% vs 7.8%, RR = 0.95, 95% CI 0.36 to 2.52). These abstinence rates were slightly lower than the 9.8% observed in the control group of no feedback within the same study (this comparison was covered in the previous section).
Dijkstra et al. 42 compared the effectiveness of three one-off tailored materials that contained different contents: (a) information on outcomes of quitting; (b) information on self-efficacy enhancement; and (c) information on both. A ‘no-information’ control group was also included. The study could be analysed as a factorial trial (outcomes information vs no information) × (self-efficacy information vs no information), as there appeared to be no significant interaction between the two factors. The inclusion of either information did not increase point prevalence abstinence at 14 months. Prolonged abstinence was increased slightly by inclusion of either information in the tailored material, but the increase was not statistically significant.
Dijkstra et al. 43 incorporated a factorial design of [multiple tailored letters vs single tailored letter (ST)] × (self-help manual vs no manual). Tests for interaction were not significant. The study specifically recruited smokers who were not planning to quit within the next 6 months. The point prevalence abstinence at 4 months was significantly higher in the two groups receiving multiple tailored letters (4.1%) than in the two groups receiving a ST (0.6%). The addition of a self-help manual appeared to have negative impact on point prevalence abstinence.
Dijkstra et al. 44 compared an intervention with three tailored letters to an intervention with a ST in a further study of smokers with low readiness to quit. Contrary to the findings of the previous study, no significant difference in point prevalence abstinence between groups receiving multiple tailored letters and a ST was found.
Velicer et al. 46 conducted a trial with a factorial design of (tailored report + manual vs manual only) × (one vs two vs three vs six contacts). They found no dose–response relationship between the number of contacts/mailings and abstinence rates in both the ‘tailored report + manual’ group and the ‘manual-only’ group.
In an aid to cessation trial conducted by Borland et al. 61 among callers to a quitline service in Australia, the combination of computer-tailored letters plus call-back telephone counselling generally produced higher abstinence rates than computer-tailored letters alone, although the differences were not statistically significant when data were analysed on an ITT basis assuming that participants who were lost to follow-up did not quit. Point prevalence abstinence (not clearly defined) was 20.8% compared with 16.8% (RR = 1.24, 95% CI 0.96 to 1.59) at 6 months and 18.9% compared with 17.3% (RR = 1.09, 95% CI 0.84 to 1.41) at 12 months for the tailored letters + telephone counselling group compared with the tailored letters group. Three-month prolonged abstinence measured at 6 months (11.2% vs 8.0%, RR = 1.39, 95% CI 0.96 to 2.03) and 9-month prolonged abstinence measured at 12 months (6.1% vs 4.6%, RR = 1.32, 95% CI 0.79 to 2.21) showed a similar trend.
Prochaska et al. 37 compared a combined intervention incorporating four proactive counsellor calls, computer-tailored reports and stage-matched manuals (MANs) with a similar intervention without the counsellor calls. The intervention with added counsellor calls in this study produced lower abstinence rates compared with the intervention with tailored reports only. Reported point prevalence abstinence rates were 14% vs 16% at 6 months and 18% vs 25% at 18 months for the intervention with and without the addition of counsellor calls. Reported prolonged abstinence rates were also higher in the group without counsellor calls. The number of participants for individual arms was not reported in this study and the abstinence rates mentioned above appeared to have been calculated using available data (i.e. not ITT).
Curry et al. 39 investigated the effect of adding three telephone counselling calls to an intervention that consisted of computer-tailored feedback and a self-help manual among smokers in a large health maintenance organisation identified through a telephone survey. Higher abstinence rates were observed in the counselling + feedback group compared with the feedback-only group. The difference was statistically significant for point prevalence abstinence at 3 months (11% vs 4%, RR = 2.87, 1.43 to 5.75) but not at 12 months (11% vs 9%) and 21 months (15% vs 10%). No significant difference was found for continuous abstinence during 3–12 months (counselling + feedback 5% vs feedback alone 3%) and 3–21 months (4% vs 2%).
Hall et al. 80 conducted a trial comparing a staged care intervention that incorporated computer-tailored feedback, psychological counselling and pharmacotherapy to a brief contact control in which self-help materials and a list of local providers of smoking cessation programmes were provided. Participants were adult smoking patients receiving treatment for depression in an outpatient setting. Patients who received the staged care intervention reported higher 7-day point prevalence abstinence than the patients in the control group at 3 months (13.5% vs 9.4%, RR = 1.43, 95% CI 0.77 to 2.66), 12 months (14.1% vs 9.4%, RR = 1.50, 95% CI 0.81 to 2.76) and 18 months (18.4% vs 13.2%, RR = 1.39, 95% CI 0.83 to 2.33), but not at 6 months (14.1% vs 15.7%, RR = 0.90, 95% CI 0.53 to 1.51).
Summary and discussion
Overall, 30 studies34,36–40,42–44,46,48,49,53,55–58,61,66,69,72,75,80,88,91,96,97,109,110 that randomised between 72 and 3627 participants and followed up between 3 and 30 months evaluated the effect of tailored printed materials. In the meta-analysis, computer-generated, tailored printed materials were effective in increasing smoking cessation compared with no intervention or generic self-help materials. This finding was consistent irrespective of the study type (aid to cessation study or cessation induction study), type of outcome associated with smoking cessation measured (point prevalence abstinence or prolonged abstinence), the time at which follow-up was measured (6 months’ follow-up or maximum follow-up).
Six studies40,42–44,46,75 assessed comparisons between various electronic interventions. As settings and contents of the electronic interventions being assessed were different, it was not possible to include them in a meta-analysis and compare the electronic interventions with each other. However, four studies43,44,46,75 compared the effect of multiple tailored printed materials over a single tailored letter. There was no clear evidence to suggest multiple interventions was better than a single intervention as two studies43,75 favoured multiple interventions, whereas in the other two studies44,46 there was no significant difference between multiple tailored printed letters and a single letter.
Stand-alone computer program
Six studies (two aid to cessation studies,33,113 four cessation induction studies47,60,62,78) evaluated electronic interventions with stand-alone computer programs. The characteristics of the studies and participants, results of quality assessment and key findings are described below.
Interventions and co-interventions
Table 10 presents the characteristics of the included studies and their participants. In five studies33,60,62,78,113 the interventions contained stand-alone computer programs only. Of the other two studies, one had telephone hotline, stop-smoking contest and self-help material as a co-intervention in each of the study arms,33 whereas the other had brief advice by midwives and self-help manual as a co-intervention. 62
| Study | Co-intervention | Population, key criteria related to smoking history and method of recruitment | Mean age (years); % female | Intervention, comparators and sample size (n) | Outcome measure and length of follow-up; comparison code [0–5] (see Figure 1 for the coding scheme) |
|---|---|---|---|---|---|
| Aid to cessation studies | |||||
| Prokhorov et al. 2008 USA113,114 | None? | Young adult/participants began smoking regularly at 16 years of age and smoked 12.5 cigarettes per day/announcements by college instructors, public service announcements in student newsletters and newspapers, school marquee announcements, and flyers | 22.8; 59 | ‘Look At Your Health’ LAYH computer-assisted, smoking cessation counselling (219) vs standard care (207) |
7-day abstinence rate Follow-up: 10 months [5] |
|
Burling et al. 1989 USA33 |
Telephone hotline + stop-smoking contest + self-help material | Veterans’ administration employees/recruitment? | 43.6; 57 | Computer + contest (29) vs contest only (29) |
Abstinence (details NR) Follow-up: 3 and 6 months [0] |
| Cessation induction studies | |||||
|
Lawrence et al. 2003 |
Brief advice by midwives + self-help manual | Pregnant women/via antenatal clinics | NR; 100 | Computer (324) vs manuals (305) vs control subjects (289) |
Sustained abstinence assessed at 28–30 weeks and 10 days postnatally [0] [5] |
|
Riley et al. 2002 USA60 |
None | Adult smokers/regular smokers (15–50 cigarettes per day for ≥ 1 year)/television advertisements | 44.8; 44 | Stand-alone computer program (44) vs selective elimination via manual instructions (49) |
7-day point prevalence abstinence Follow-up: 6 and 12 months [5] |
|
O’Neill et al. 2000 USA47 |
None | Young adult smokers/daily smokers/via telephone | 19.7; 63 | Computer-administered intervention on cigarette smoking (31) vs computer-administered intervention on other health behaviours (34) |
1 ° outcome: stage of change 2 ° outcome: abstinence rate Follow-up: 7 months [3] |
|
Wolfenden et al. 2005 |
None | Surgical patients having non-cardiac elective surgery with pre-existing health problems, who are smokers aged 18 years and above, could read English, had booked day for surgery | 43; 62 | Interactive, tailored counselling delivered by computer + tailored self-help material + telephone counselling (124) vs usual care (86) |
Abstinence (not defined) Follow-up: 3 months [5] |
Study design and characteristics of participants
All of the six studies were RCTs that randomised participants individually. Length of follow-up ranged from 378 to 12 months. 60 Three studies47,60,113 evaluated point prevalence abstinence. Two studies33,78 did not define the abstinence. The study by Lawrence et al. 62 reported sustained abstinence assessed at 28–30 weeks of gestation and 10 days postnatally. The study by O’Neill et al. 47 reported stage of change and abstinence rate.
Participants included responders to invitations to participate in the trial advertised through various media: pregnant women via antenatal clinics, surgical patients having non-cardiac elective surgery with pre-existing health problems, and patients undergoing outpatient surgery or invasive screening in a large managed care organisation.
Mean age ranged from 19.7 years47 to just over 44.8 years. 60 Proportion of females varied from 44%60 to 100%. 62
Quality assessment of included studies
The results of quality assessment are presented in Table 11. None of the studies provided information on blinding. Four studies33,60,62,113 included biochemical validation of smoking status. Loss to follow-up varied significantly and ranged from 7% (at 6 months)33 to 45% 60 (at 12 months). Three studies60,62,78 included ITT analysis.
| Study | Generation of random sequence | Allocation concealment | Blinding | Biochemical validation | Baseline characteristics similar between groups | Lost to follow-up | ITT analysis |
|---|---|---|---|---|---|---|---|
| Aid to cessation studies | |||||||
|
USA |
Yes | Unclear | Unclear | Yes | Yes |
Overall: 100/426 (23.5%) Computer-assisted counselling: 61/219 (27.9%) Standard care: 39/207 (18.8%) |
No |
|
Burling et al. 1989 USA33 |
Unclear | Unclear | Unclear | Yes | Unclear | 4/58 (6.9%) | Unclear |
| Cessation induction studies | |||||||
|
Lawrence et al. 2003 |
Yes | Unclear | Unclear | Yes | Yes |
Postnatal follow-up: Computer: 249/324 (77%) Manual: 219/305 (72%) Control: 185/289 (64%) |
Yes |
|
Riley et al. 2002 USA60 |
Unclear | Unclear | Unclear | Yes | Yes |
Stand-alone computer program: 14/44 (31.8%) Selective elimination via manual instruction: 22/49 (44.9%) |
Yes |
|
O’Neill et al. 2000 USA47 |
Unclear | Unclear | Unclear | No | Unclear |
Overall: 9/65 (13.8%) Computer-intervention on smoking cessation: 4/31 (12.9%) Computer-intervention on other health behaviours: 5/34 (14.7%) |
No |
|
Wolfenden et al. 2005 |
Yes | Unclear | No | No | Yes |
Smoking cessation programme = 16/124 Usual care = 10/86 |
Yes |
Comparisons and findings
One aid to cessation study (Burling et al. 33) and one cessation induction study (Lawrence et al. 62) compared stand-alone computer programs with either no intervention, usual care or untailored printed self-help material, with or without concurrent co-interventions.
Figure 24 shows the results of point prevalence abstinence measured at the last follow-up of each study. The results for the study (aid to cessation) by Burling et al. 33 at 6 months showed that the stand-alone computer program intervention increased cessation compared with control but the sample size was small (n = 58) and the difference was not statistically significant (RR = 2.00, 95% CI 0.55 to 7.24). The results for the study (cessation induction) by Lawrence et al. 62 showed that the stand-alone computer program intervention was not effective at 18 months (RR = 1.06, 95% CI 0.57 to 1.97).
FIGURE 24.
Stand-alone program vs control: point prevalence abstinence at maximum follow-up.
One study by O’Neill et al. 47 reported point prevalence rate of 29% (9/31) for intervention and 21% (7/34) for control at 3 months, and 30% (9/31) for intervention and 32% (11/34) for control at 6 months. It also reported prolonged abstinence rate of 19% (6/31) for intervention and 15% (5/34) for control at 6 months. However, both point prevalence rate and prolonged abstinence rate were not defined and none of the differences reached statistical significance.
Four studies60,62,78,113 estimated the effect of the combined electronic and non-electronic intervention compared with control. However, the effects of the electronic component cannot be separated out. The studies by Prokhorov et al. 113,114 compared computer-assisted motivational counselling and tailored material with brief counselling. The studies by Lawrence et al. 62–64 compared computer programs, printout by post and stage-based brief advice with non-stage-based brief. The study by Riley et al. 60 compared computerised scheduled gradual reduction (LifeSign) and programme guide with manual-based selective elimination. The studies by Wolfenden et al. 78,79 compared computer counselling program, telephone counselling and NRT with brief advice.
Summary and discussions
Six studies33,47,60,62,78,113 evaluated the use of a stand-alone computer program for smoking cessation. Only two of them33,62 compared the intervention with no intervention, usual care or untailored printed self-help material. Stand-alone computer program did not significantly increase cessation, but the number of trials and sample sizes were too small to allow a firm conclusion to be drawn.
Mobile telephone/interactive voice response-based intervention
Five studies (two aid to cessation studies,73,120 three cessation induction studies45,95,121) evaluated electronic interventions with mobile telephone or IVR. The characteristics of the studies and participants, results of quality assessment and key findings are described below.
Interventions and co-interventions
Table 12 presents the characteristics of the included studies and their participants. The interventions of three studies73,120,121 contained mobile telephone text messaging only. One study45 had an intervention of IVR and a co-intervention of self-help material. The other study95 had an intervention of IVR and a co-intervention of inhospital brief advice, NRT, counselling and self-help material.
| Study | Co-intervention | Population, key criteria related to smoking history and method of recruitment | Mean age (years); % female | Intervention, comparators and sample size (n) | Outcome measure and length of follow-up; comparison code [0–5] (see Figure 1 for the coding scheme) |
|---|---|---|---|---|---|
| Aid to cessation studies | |||||
|
Free et al. 2009120 UK |
None | Aged ≥ 16 years, currently smoking cigarettes daily and interested in quitting, currently owns a mobile telephone, living within an hour of London and familiar with text messaging | 36; 38 |
The txt2stop intervention (102) vs control (98) |
7-day point prevalence and 28-day continuous abstinence Follow-up: 4 weeks and 6 months [1] |
|
Rodgers et al. 200573 New Zealand |
None | Aged ≥ 16 years, smokers (smoking cigarettes daily) who wanted to quit in next month (i.e. in contemplative stage of change) and owned a mobile telephone and able to send/receive texts | 22; 59 |
Regular personalised text messages providing advice, support and distraction (852) vs one text message every 2 weeks, which only meant to thank them for being in the study, provide study contact details and inform them of the rewards for participants (853) |
7-day point prevalence and 24-week continuous abstinence Follow-up: 6 months [1] |
| Cessation induction studies | |||||
|
Haug et al. 2009121 Germany |
None | Young adults/daily smokers/at the university cafeteria | 25.0; 57 | Three SMS (60) vs one SMS (50) vs no SMS (64) |
7-day point prevalence abstinence Follow-up: 3 months [0][3] |
|
Reid et al. 200795 Canada |
In-hospital brief advice, NRT, counselling and self-help material | Current smokers (five or more cigarettes per day) aged > 18 years, hospitalised for acute coronary syndrome, percutaneous coronary intervention or diagnostic catheterisation related to coronary heart disease | 54; 32 | IVR group (50) vs usual care (49) |
7-day point prevalence abstinence Follow-up: 3 and 12 months [0] |
|
Ershoff et al. 199945 USA |
Self-help material | English-speaking pregnant women/smoked average 17 cigarettes/day/during antenatal booking | 29.4; 100 |
Booklet only (131) vs motivational interview (126) vs IVR (133) |
7-day abstinence (biochemically confirmed) Follow-up: 4 weeks [0][2] |
Study design and characteristics of participants
All the five studies45,73,95,120,121 were RCTs that randomised participants individually. Length of follow-up ranged from 4 weeks45 to 12 months. 95 All the studies evaluated 7-day point prevalence abstinence. The study by Rodgers et al. 73 also measured 24 weeks’ continuous abstinence.
Participants included those aged ≥ 16 years: (1) currently smoking cigarettes daily and interested in quitting, currently owning a mobile telephone; (2) currently smokers who were hospitalised for acute coronary syndrome, percutaneous coronary intervention or diagnostic catheterisation related to coronary heart disease; or (3) presenting as English-speaking pregnant women smokers during antenatal booking.
The mean age ranged from 2273 to 54 years. 95 The proportion of females varied from 32%95 to 58–59%. 73
Quality assessment of included studies
The results of the quality assessment are presented in Table 13. Most of the studies were single-blind for care providers. 45,73,120 The study by Haug et al. 121 was blinded for participants, and the study by Reid et al. 95 did not report blinding information.
| Study | Generation of random sequence | Allocation concealment | Blinding | Biochemical validation | Baseline characteristics similar between groups | Lost to follow-up | ITT analysis |
|---|---|---|---|---|---|---|---|
| Aid to cessation studies | |||||||
| Free et al. 2009120 | Unclear | Yes | Single blind | Yes | Unclear |
Control = 8/98 Intensive group = 8/102 |
Yes |
| Rodgers et al. 200573 | Yes | Yes | Care providers | Yes | Yes |
Intervention 261/852 (30.6%) Control 179/853 (21%) |
Yes |
| Cessation induction studies | |||||||
| Haug et al. 2009121 | Yes | Unclear | Participants | No | Yes |
Three SMS – 9/60 (15%) One SMS – 5/50 (10%) No SMS – 5/64 (7.8%) |
Yes |
| Reid et al. 200795 | Yes | Yes | Unclear | No | No (education level) |
UC group = 83.7% IVR group = 86% |
Yes |
| Ershoff et al. 199945 | Unclear | Unclear | Care providers | Yes | Yes | 58/390 (14.9%) | No |
Three studies45,73,120 included biochemical validation of smoking status. Loss to follow-up varied significantly and ranged from 8% (at 4 weeks, 3 months)120,121 to 86–87%95 (at 12 months). Four studies73,95,120,121 included ITT analysis.
Comparisons and findings
Two aid to cessation studies73,120 and three cessation induction studies45,95,121 compared mobile telephone text messaging or IVR with either no intervention, usual care or untailored printed self-help material, with or without concurrent co-interventions.
Figure 25 shows the results of point prevalence abstinence at 6 months. This outcome was reported in two aid to cessation studies,73,120 neither of which gave a significant result. The pooled results showed that the mobile text messaging intervention was not effective (RR = 1.03, 95% CI 0.83 to 1.28, I2 = 10.4%).
FIGURE 25.
Mobile telephone text messaging vs control: point prevalence abstinence at 6 months.
Figure 26 shows the results of point prevalence abstinence measured at the last follow-up of each study. In addition to the two aid to cessation studies73,120 shown in Figure 25, three cessation induction studies reported this outcome at various durations of follow-up,45,95,121 two of which showed increased cessation in the intervention group but neither of which gave a significant result (see lower part of Figure 26). The pooled result did not reach statistical significance (RR = 1.08, 95% CI 0.83 to 1.40, I2 = 4%).
FIGURE 26.
Mobile telephone text messaging vs control: point prevalence abstinence at maximum follow-up.

The study by Ershoff et al. 45 compared the effectiveness of IVR with motivational interview having self-help booklet as a co-intervention in each arm. The result showed no significant difference for IVR compared with motivational interview (RR = 0.81, 95% CI 0.39 to 1.69).
The study by Haug et al. 121 compared three short message service (SMS) with one SMS. The result showed that three SMS did not perform significantly better than one SMS (RR = 1.25, 95% CI 0.82 to 1.91).
Summary and discussion
Five studies45,73,95,120,121 evaluated the use of mobile telephone text messaging or IVR for smoking cessation. No significant increase in abstinence rates was observed when these interventions were compared with no intervention, usual care or untailored printed self-help material, and to other non-electronic interventions. However, the number of RCTs is too small to allow a firm conclusion to be drawn.
E-mail-based intervention
Only one aid to cessation study, by Abroms et al. ,98 evaluated e-mail-based intervention. The characteristics of the study and participants, results of quality assessment and key findings are described below.
Interventions and co-interventions
Table 14 presents the characteristics of the included study and its participants. The intervention X-pack programme in the study by Abroms et al. 98 consisted of an initial in-person session to introduce the programme to the participant and to encourage setting a quit date, a self-help kit (the X-pack), which included, among other things, a smoking cessation guide and various products for use as a substitute for cigarettes, and 10–12 counselling e-mails written by staff counsellors over the course of 6 months. The control group received an initial in-person session to introduce the self-help materials (Clearing the Air) and to encourage setting a quit date, but was not provided with further assistance in quitting.
| Study, country | Co-intervention | Population, key criteria related to smoking history and method of recruitment | Mean age (years); % female | Intervention, comparators and sample size (n) | Outcome measure and length of follow-up; comparison code [0–5] (see Figure 1 for the coding scheme) |
|---|---|---|---|---|---|
| Aid to cessation studies | |||||
|
Abroms et al. 200898 USA |
Brief advice (an initial in-person session to introduce the programme/material and to encourage setting a quit date) | Undergraduate students aged 18–24 years smoking at least one cigarette per day and interested in quitting smoking in the next 6 months; recruited using flyers, advertisements in the college newspaper and a study-staff table outside the student centre | 19.8; 46 |
X-pack programme consisted of a self-help kit and 10–12 counselling e-mails (48) vs Clearing the Air self-help materials (35) |
7-day point prevalence abstinence Follow-up: 3 and 6 months [1] |
Study design and characteristics of participants
The Abroms et al. study98 was a RCT that randomised participants individually. Length of follow-up was 6 months. It evaluated 7-day point prevalence abstinence.
Participants were undergraduate students aged 18–24 years interested in quitting smoking in the next 6 months. The mean age was 19.8 years and the proportion of female participants was 46%.
Quality assessment of included studies
The result of the quality assessment is presented in Table 15. Blinding information was not reported. It included biochemical validation of smoking status. Loss to follow-up was 31% (at 6 months). Analysis was ITT.
| Study | Generation of random sequence | Allocation concealment | Blinding | Biochemical validation | Significant difference between groups at baseline | Lost to follow-up | ITT analysis |
|---|---|---|---|---|---|---|---|
| Aid to cessation studies | |||||||
| Abroms et al. 200898 | Yes | Unclear | Unclear | Yes | No | 31.3% | Yes |
Comparisons and findings
The aid to cessation study by Abroms et al. 98 compared the e-mail-based X-pack programme with Clearing the Air self-help materials as described above. The result showed that the X-pack programme increased point prevalence abstinence compared with the control condition but the differences were not statistically significant, with RR = 1.56, 95% CI 0.71 to 3.42 measured at 3 months, and RR = 1.75, 95% CI 0.68 to 4.52 measured at 6 months.
Summary and discussion
Only one trial98 compared e-mail-based interventions with printed generic self-help material. A higher point prevalence abstinence was observed among participants receiving e-mail-based interventions, but the study was underpowered to allow firm conclusions to be drawn.
Internet sites
Six studies (four aid to cessation studies,35,87,117,123 two cessation induction studies111,112) evaluated internet sites with/without co-interventions. The characteristics of the studies and participants, results of quality assessment and key findings are described below.
Interventions and co-interventions
Table 16 presents the characteristics of included studies and their participants. The interventions of three studies35,87,111 contained internet sites only. The studies by Oenema et al. 111 and Swartz et al. 87 evaluated the effectiveness of the internet sites compared with no intervention or delayed intervention. The study by Schneider et al. 35 evaluated the effectiveness of the internet sites with different contents. The other three studies had NRT,117 Health Risk Intervention,112 and automated e-mails plus online mood management course123 as a co-intervention in each of the study arms. The study by Strecher et al. 117 evaluated the effectiveness of interventions with a variation of each of the five two-level intervention factors related to the depth of tailoring of different components, personalisation of introductory messages and time schedule of the interventions. These factors will be further described later in this section under ‘comparison between electronic interventions’. The study by Prochaska et al. 112 evaluated the effectiveness of Online Transtheoretical Model and Motivational Interviewing with the adjunct of Health Risk Intervention. The study by Muñoz et al. 123 evaluated the effectiveness of Guía (static website) with or without individually timed educational messages (ITEM) compared with Guía with ITEM and an eight-lesson cognitive behaviour mood management course.
| Study, country | Co-intervention | Population, key criteria related to smoking history and method of recruitment | Mean age (years); % female | Intervention, comparators and sample size (n) | Outcome measure and length of follow-up; comparison code [0–5] (see Figure 1 for the coding scheme) |
|---|---|---|---|---|---|
| Aid to cessation studies | |||||
|
Swartz et al. 200687 USA |
None | Adults currently smoking cigarettes on daily basis/worksites advertisements and links to websites, electronic newsletter, e-mails | NR; 52 |
1–2–3 Smokefree website (171) vs delayed access (180) |
7-day abstinence Follow-up: 90 days [0] |
|
Schneider et al. 199035 USA |
None | Adult subscribers to the CompuServe Information System who smoked cigarettes daily; recruited via articles in magazines and online newsletter | NR (≥ 18); 16 |
2 × 2 factorial design (1158) Interactive computer program vs non-interactive program (both delivered via internet) × internet forum vs no forum |
Abstinence (not defined) Follow-up: 1, 3 and 6 months [3] |
|
Strecher et al. 2008 |
NRT | Members of two HMOs; smoking at least 10 cigarettes per day and considering quitting within the next 30 days; invitation letter and HMO newsletter | 46.3; 60 | Study participants (1866) received a variation of each of the five two-level intervention factors: (1) depth of tailored outcome expectation feedback; (2) depth of efficacy expectations; (3) depth of success stories; (4) personalisation of source; and (5) exposure schedule |
7-day point prevalence abstinence Follow-up: 6 months [3] |
|
Muñoz et al. 2009123 Multicountry |
None | Young adults/smoking at least five cigarettes/day/recruited online | 37.9; 45 |
Guía: static website (247) vs Guía ITEMs (251) vs Guí + ITEMs + plus eight-lesson cognitive behaviour MM course (251) vs Guí + ITEM + MM + VG (251) |
7-day point prevalence abstinence Follow-up: 1, 3, 6 and 12 months [4] |
| Cessation induction studies | |||||
|
Oenema et al. 2008111 The Netherlands |
None | Adults ≥ 30 years of age from a pool of an online research panel, recruited by e-mail invitation | 43.6; 54 |
A website with tailored information modules on saturated fat intake, physical activity and smoking cessation (1080) vs delayed intervention (1079) |
Self-reported smoking cessation (not defined) and forward stage transition Follow-up: 1 month [0] |
|
Prochaska et al. 2008112 USA |
Online health risk assessment and HRI | Employees at a major medical university/9.7% current smoker/sent persuasive letter and e-mail, some given incentives to participate. Some received follow-up telephone call | 41.6; 79 |
HRI (464) vs online: TTM + HRI (504) vs MI + HRI (433) |
Stage of change, point prevalence abstinence Follow-up: 6 months [2][3] |
Study design and characteristics of participants
All the six studies35,87,111,112,117,123 were RCTs that randomised participants individually. Length of follow-up ranged from 1111 to 12 months. 123 Four studies87,112,117,123 evaluated point prevalence abstinence. The study by Oenema et al. 111 reported the smoking prevalence of the current, ex-smokers or never smokers. The study by Schneider et al. 35 reported the outcome of abstinence, which was not defined. Participants were responders to invitations to participate in the trial advertised through various media. Mean age ranged from 37.9 years123 to just over 46.3 years. 117 One study focused specifically on young adults. 123 The proportion of female participants varied from 16%35 to 79%. 112
Quality assessment of included studies
The results of the quality assessment are presented in Table 17. Studies varied in the adequacy of methods of blinding. None of the studies included biochemical validation of smoking status. Loss to follow-up varied significantly and ranged from 16% (at 1 month)111 to 56% (at 6 months). 35 The study by Prochaska et al. 112 did not report loss to follow-up. Three studies87,111,117 included ITT analysis.
| Study | Generation of random sequence | Allocation concealment | Blinding | Biochemical validation | Significant difference between groups at baseline | Lost to follow-up | ITT analysis |
|---|---|---|---|---|---|---|---|
| Aid to cessation studies | |||||||
| Swartz et al. 200687 | Yes | Unclear | Open label | No | No |
Overall: 154/351 (43.9%) 1–2–3 Smokefree website: 84/171 (49.1%) Delayed access: 70/180 (38.9%) |
Yes |
| Schneider et al. 199035 | Yes | Unclear | Unclear | No | No | Overall: 56.3% | Unclear |
| Strecher et al. 2008117,118 | Yes | Yes | Participants | No | Yes | 451/1866 (24.2%) | Yes |
| Muñoz et al. 2009123 | Yes | Yes | Outcome assessors | No | Unclear |
Guía:a 72/247 (29.1%) Guía + ITEMs: 78/251 (31.1%) Guía + ITEMs + MM:b 78/251 (31.1%) Guía + ITEMs + MMb + VG: 80/251 (31.9%) |
Unclear |
| Cessation induction studies | |||||||
| Oenema et al. 2008111 | Yes | Yes | Outcome assessors | No | Yes |
Static website: 231/1080 (21.4%) No intervention: 171/1079 (15.9%) |
Yes |
| Prochaska et al. 2008112 | Unclear | No | Unclear | No | No | NR | Unclear |
Comparisons and findings
One aid to cessation study (Swartz et al. 87 and one cessation induction study (Oenema et al. 111) compared internet site interventions with either no intervention, usual care or untailored printed self-help material, with or without concurrent co-interventions.
Figure 27 shows the results of point prevalence abstinence measured at the last follow-up of each study. The results for the study by Swartz et al. 87 at 3 months showed that the internet site intervention is significantly effective, with RR = 2.46, 95% CI 1.16 to 5.21. The results for the study by Oenema et al. 111 at 1 month showed that the internet site intervention is not effective, with RR = 1.28, 95% CI 0.52 to 3.13. Pooled estimate just failed to reach statistical significance (RR = 1.86, 95% CI 0.98 to 3.50).
FIGURE 27.
Internet site interventions vs control: point prevalence abstinence at maximum follow-up.
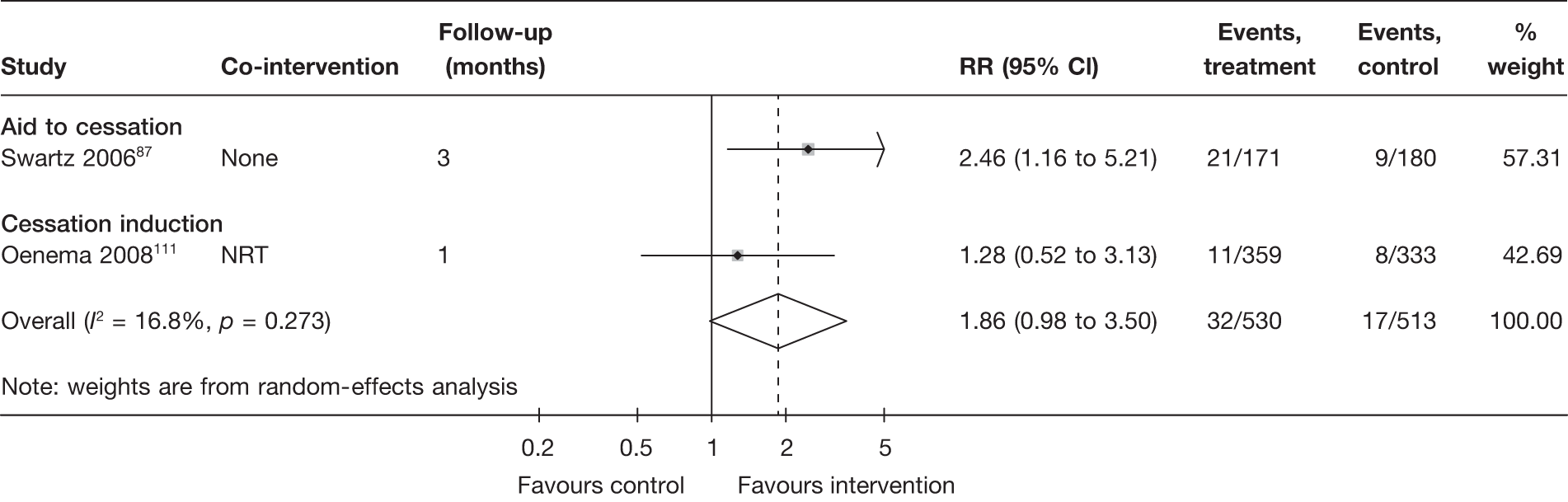
One cessation induction study (Prochaska et al. 112) provided information on the relative effectiveness of an internet site intervention (online transtheoretical model) and a non-electronic intervention (three sessions of motivational interviewing conducted face to face or over the telephone) when added to an online health risk intervention. The point prevalence abstinence at 6 months was 21% and 35% for online transtheoretical model and motivational interviewing, respectively. The difference was, however, not statistically significant owing to small sample size, as the study was conducted in a worksite population with a variety of health risk factors (i.e. not just smoking) and only 9.8% of the study participants were smoking at baseline and were included in this comparison.
Two aid to cessation studies (Strecher et al. ,117 Schneider et al. 35) and two cessation induction studies (Prochaska et al. ,112 Muñoz et al. 2009123) made direct comparisons between different electronic interventions.
In a RCT with factorial design, Schneider et al. 35 evaluated two intervention components: (tailored vs untailored online programme) × (online forum vs no forum). The results of this study are described in the next section (see Interventions with multiple tailored components) (as the tailored programme-plus-online forum arm is considered as having multiple tailored components).
Using a fractional factorial design, Strecher et al. 117,118 conducted a RCT to examine the impact of five intervention components on 7-day point prevalence abstinence at 6 months. They found that two of the intervention components, namely depth of success stories (hypothetic stories of successful cessation created using information on individual characteristics and personal circumstances to address smokers’ motives and barriers to quitting) and personalisation of source (using photographs, signatures and friendly, personal-manner texts in the introduction to the programme) were associated with increased cessation. The other three components including depth of outcome expectations (tailored feedback and advice related to smokers’ motives for quitting), depth of efficacy expectations (tailored feedback and advice related to smokers’ barriers to quitting), and exposure schedule (single instalment vs a series of instalments over 5 weeks) were not significantly associated with cessation.
The aforementioned study by Prochaska et al. 112 also allowed comparison between different electronic interventions. It was found that the point prevalence abstinence at 6 months was 21% for the online transtheoretical model plus health risk intervention and was 17% for the online health risk intervention only. The difference was not statistically significant owing to the small sample size. Comparisons between generic components of the electronic interventions (static online cessation guide vs static guide + automated e-mails vs static guide + automated e-mails + an eight-lesson cognitive–behavioural mood management course) in the study by Muñoz et al. 124 have been described above (see Interventions with single or multiple generic components, Comparisons and findings, Comparisons between electronic interventions). The addition of an asynchronous bulletin board (considered as a tailored component) to these generic components did not significantly increase point prevalence abstinence (with and without bulletin board, 12.7% vs 14.3% at 6 months and 22.7% vs 20.7% at 12 months).
Summary and discussion
Six studies35,87,111,112,117,123 evaluated web-based interventions with a single tailored component. Two of them87,111 compared the interventions with a no-intervention control. Pooled estimate of point prevalence abstinence based on short-term follow-up (≤ 3 months) favours the web-based interventions. The difference reached statistical significance with a fixed-effects model but not a random-effects model.
Interventions with multiple tailored components
Fourteen studies (11 aid to cessation studies,35,70,76,82,92,104,105,107,116,119,124 three cessation induction studies57,88,101) evaluated electronic interventions with multiple tailored components. The characteristics of the studies and participants, results of quality assessment and key findings are described below.
Interventions and co-interventions
Table 18 presents the characteristics of included studies and their participants. The majority of the interventions (10 studies35,70,76,82,92,101,107,116,119,124) consisted of an interactive, web-based smoking cessation programme combined with some form of social interaction/support through internet forum (bulletin board), chat rooms, and/or tailored e-mails. Four studies57,88,104,105 assessed a variety of other combinations of electronic interventions: Brendryen et al. 104,105 conducted two studies evaluating the fully automated ‘Happy Ending’ programme, which incorporated web pages, e-mails, IVR and SMS. Prochaska et al. 57 assessed tailored printed material generated by an EXP plus the LifeSign program (a hand-held computer that initially records the smoker’s smoking pattern then accustoms the smoker to smoke only when cued by the machine and gradually decreases the number of cues hence the amount of smoking over time). Velicer et al. 88 evaluated an automated counselling intervention plus tailored printed material generated by an EXP.
| Study, country | Co-intervention (non-electronic) | Population, key criteria related to smoking history and method of recruitment | Mean age (years); % female | Interventions/comparators and sample size (n) | Outcome measure; length of follow-up; comparison code [0–5] (see Figure 1 for the coding scheme) |
|---|---|---|---|---|---|
| Aid to cessation studies | |||||
|
Schneider et al. 199035 USA |
None | Adult subscribers to the CompuServe Information System who smoked cigarettes daily; recruited via articles in magazines and online newsletter | NR (≥ 18 years); 16 |
2 × 2 factorial design (1158) Interactive computer program vs non-interactive program (both delivered via internet) × internet forum vs no forum |
Abstinence (not defined) Follow-up: 1, 3 and 6 months [3] |
|
Switzerland |
None | Visitors of Stop-tabac.ch website who were current smokers or ex-smokers |
OP: 34.1 vs MP: 33.8 61% vs 62% |
OP (5966) vs MP (6003); both programmes were run within a common interactive website (Stop-tabac.ch) |
7-day point prevalence Follow-up: 2.5 months [3] |
|
USA |
None | English-speaking daily smokers residing in the USA/21 cigarettes per day/self-referral through the American Cancer Society website | 41.0; 70 | Five interactive websites vs one static website |
7-day abstinence Follow-up: 3 months [3] |
|
Brendryen et al. 2008105 Norway |
None | Young adult smokers/currently smoking five cigarettes or more on a daily basis/internet advertisement | 39.6; 50 |
Happy Ending – internet + telephone (144) vs self-help booklet (146) |
7-day abstinence rate Follow-up: 1, 3, 6 and 12 months [1] |
|
McKay et al. 2008107 USA |
None | Young adult smokers/current smokers/internet-based recruitment campaign | NR; 71 |
Web-based smoking cessation (1159) vs web-based exercise enhancement (1159) |
7-day point prevalence abstinence Follow-up: 3 and 6 months [3] |
|
Stoddard et al. 2008116 USA |
None | Adult employees and contractors/18.3 cigarettes per day (average)/e-mail invitation | 43.6; 54 |
Intervention: www.smokefree.gov website + bulletin board (691) vs control: www.smokefree.gov (684) |
7-day abstinence, adherence, satisfaction with the resources Follow-up: 3 months. [3] |
|
Etter et al. 2009119 Switzerland |
None | Adults/ criteria related to smoking history not reported via e-mail | 36.1; 64 | Computer-generated tailored materials (1086) vs Computer-generated untailored materials (1082); both arms had access to an interactive website (Stop-tabac.ch) |
24-hour point prevalence abstinence Follow-up: 48 hours [3] |
|
UK and Ireland |
Nicotine patch | Adult smokers who purchased a nicotine patch (NiQuitin® CQ 21-mg patch, GlaxoSmithKline); had smoked ≥ 10 cigarettes per day and had a target quit date within 7 days from enrolment; recruited while the smokers connected to a website for free behavioural support material | 36.9; 57 | Web-based tailored behavioural intervention (1991) vs web-based NT materials (1980) |
7-day point prevalence 28-days and 10 weeks prolonged abstinence Follow-up: 6 and 12 weeks [4] |
|
Brendryen and Kraft 2008104 Norway |
NRT | Adult smokers/smoked ≥ 10 cigarettes daily/internet advertisements, self-selection | 36.2; 50 | Happy Ending (197) vs booklet only (199) |
7-day abstinence Follow-up: 1, 3, 6 and 12 months [1] |
|
Swan et al. 2010124 USA |
Varenicline | 18 years old, smoked at least 10 cigarettes per day over the past year and five cigarettes per day within the past week. Had dependable telephone and internet access, eligible for smoking cessation services and medically appropriate to use varenicline | 47.3; 67 |
Web-based counselling (401) vs proactive telephone-based counselling (402) vs web-based counselling + proactive telephone-based counselling (399) |
7- or 30-day point prevalence abstinence Follow-up: 3 and 6 months [0][2] |
|
Japuntich et al. 200682 USA |
Bupropion + counselling | Adults/smoking at least 10 cigarettes/via billboards, bus interior posters, flyers, television advertisements and press releases | 40.8; 55 |
Website + one-to-one face-to-face counselling + bupropion (140) vs one-to-one face-to-face counselling + bupropion (144) |
7-day point prevalence abstinence Follow-up: 3 and 6 monthsa [0] |
| Cessation induction studies | |||||
|
Prochaska et al. 200157 USA |
None | Adults/nr/contacted via telephone and mail | 38.1; 56 |
EXP (368) vs EXP + counselling (359) vs EXP + LifeSign computer (366) vs assessment only (359) |
24-hour and 7-day point prevalence abstinence 30 days and 6 months prolonged abstinence Follow-up: 6, 12 and 18 months [0][2][4] |
|
USA |
Stop smoking contest | College smokers/smoked cigarettes in the past 30 days/e-mail invitations | 19.9; 73 |
RealU interactive website + e-mails from peer coaches (257) vs an e-mail with links to QuitNet.com and other online health resources (260) |
30-day abstinence, 6-month prolonged abstinence Follow-up: 8, 20 and 30 weeks [4] |
|
Velicer et al. 200688 USA |
NRT | Smokers from a New England Veterans Affairs Medical Center/smoked on average 24.5 cigarettes per day/via letter | 50.5; 23 |
MANs (523) vs NRT + MAN (522) vs EXP + NRT + MAN (509) vs TEL + EXP + NRT + MAN (500) |
24-hour and 7-day point prevalence abstinence 6-month prolonged abstinence Follow-up:10, 20 and 30 months [0][4] |
Five studies had pharmacotherapy [NRT,76,88,104 varenicline124 or bupropion82] as a co-intervention in each of the study arms. Two of the studies also had face-to-face82 or telephone counselling124 in some of the study arms. The remaining nine studies35,57,70,92,101,105,107,116,119 do not have non-electronic co-interventions.
Study design and characteristics of participants
All of the 14 studies35,57,70,76,82,88,92,101,104,105,107,116,119,124 were RCTs that randomised participants individually.
Length of follow-up ranged from 2 days119 to 30 months. 88 The study by Etter119 aimed to evaluate the immediate effect of a single tailored feedback report on quit attempts and thus follow-up was carried out 48 hours after baseline. Owing to the very short follow-up, this study is not included in meta-analysis. Five studies57,76,88,104,105 followed up participants for 1 year or longer. Most studies reported point prevalence abstinence. Prolonged abstinence was reported only in four studies. 57,76,88,101
Participants were either self-referred to smoking cessation websites or were responders to invitations to participate in the trial advertised through various media. Two studies70,119 included both current smokers and ex-smokers; only current smokers at study entry were included in the analysis in this section.
The mean age ranged from 20101 to just over 50 years. 88 Three studies101,105,107 focused specifically on young adults. The proportion of female participants varied from 16%35 to 73%. 107
Quality assessment of included studies
The results of the quality assessment are presented in Table 19. Studies varied in the adequacy of methods of randomisation and blinding. Only two studies82,101 included biochemical validation of smoking status. Loss to follow-up varied significantly and ranged from 14% (at 12 months)104 to over 60% (at 3–6 months). 76,107,116 All studies included ITT analysis.
| Study | Generation of random sequence | Allocation concealment | Blinding | Biochemical validation | Significant difference between groups at baseline | Loss to follow-up | ITT analysis |
|---|---|---|---|---|---|---|---|
| Aid to cessation studies | |||||||
| Schneider et al. 199035 | No (used customer account number) | Unclear | Unclear | No | No | Overall: 56.3% (at 6 months) | Yes |
| Etter 200570,71 | Yes | Yes | Unclear | No | No |
At 2.5 months OP: 60.8% MP: 68.4% |
Yes |
| Pike et al. 200792–94 | Unclear | Unclear | Unclear | No | Unclear | 2645/6145 (43%) | Yes |
| Brendryen et al. 2008105 | Yes | Yes | Participants | No | No |
Overall: 64/290 (22.1%) Happy Ending (internet) + telephone: 26/144 (18.1%) Assessment only: 38/146 (26.0%) |
Yes |
| McKay et al. 2008107 | Unclear | Unclear | Unclear | No | No |
Overall: 1409/2318 (60.8%) Web-based smoking cessation: 711/1159 (61.3%) Web-based exercise enhancement: 698/1159 (60.2%) |
Yes |
| Stoddard et al. 2008116 | Yes | Yes | Outcome assessors | No | No |
www.smokefree.gov+bulletin board: 417/684 (70%) www.smokefree.gov: 412/691 (59.6%) |
Yes |
| Etter 2009119 | Yes | Unclear | Unclear | No | No |
Tailored report: 24% (346/1444) Untailored report: 21% (300/1428) at 2 days |
Yes |
| Strecher et al. 200576,77 | Unclear | Unclear | Participants | No | No | 62.5% (2480/3971) at 3 months | Yes |
| Brendryen and Kraft 2008104 | Yes | Yes | Participants | No | No |
Overall: 55/396 (13.9%) Happy Ending: 24/197 (12.2%) Booklet only: 31/199 (15.6%) |
Yes |
| Swan et al. 2010124 | Yes | Yes | No | No | No | 25.6% | Yes |
| Japuntich et al. 200682 | Unclear | Unclear | Unclear | Yes | Unclear |
Website + counsellinga + bupropion: 24/140 (17.1%) Counselling + bupropion: 30/144 (20.8%) |
Yes |
| Cessation induction studies | |||||||
| Prochaska et al. 200157 | Unclear | Unclear | Unclear | No | Yes | NR | Yes |
| An et al. 2008101–103 | Yes | Yes | No | Yes | Yes |
Overall: 103/517 (19.9%) RealU website: 57/257 (22.2%) QuitNet.com: 46/260 (17.7%) |
Yes |
| Velicer et al. 200688 | Yes | Unclear | Participants | No | No |
MAN: 126/523 (24.1%) NRT + MAN: 142/522 (27.2%) EXP + NRT + MAN: 153/509 (30.1%) TEL + EXP + NRT + MAN: 155/500 (31.0%) |
Yes |
Comparisons and findings
A variety of comparisons were made in the included studies. Relevant pair-wise comparisons were categorised according to the scheme shown in Figure 1 and the findings for each type of comparison are described below.
Electronic interventions compared with no intervention, usual care or untailored printed self-help material
Only six57,82,88,104,105,124 out of the 14 studies compared electronic interventions with multiple tailored components with either no intervention, usual care or untailored printed self-help material, with or without concurrent co-interventions.
Figure 28 shows the results of point prevalence abstinence at 6 months. This outcome was reported in five studies57,82,104,105,124 with varied interventions. Significant heterogeneity in effect estimates was observed. Only the two studies by Brendryen et al. 104,105 demonstrated a statistically significant increase in abstinence rates in the intervention groups compared with control groups. Both studies compared the multimedia Happy Ending programme (web + e-mails + IVR + text messages) with self-help manuals, one with concurrent NRT, the other without. Two of the studies82,124 assessing interactive websites incorporating some form of social interaction/support failed to show a significant increase in abstinence rates when these interventions were added to counselling plus pharmacotherapy. In the study by Prochaska et al. ,57 the combination of EXP reports and LifeSign program did not increase abstinence rates compared with the assessment only control.
FIGURE 28.
Electronic interventions with multiple tailored components vs control: point prevalence abstinence at 6 months.
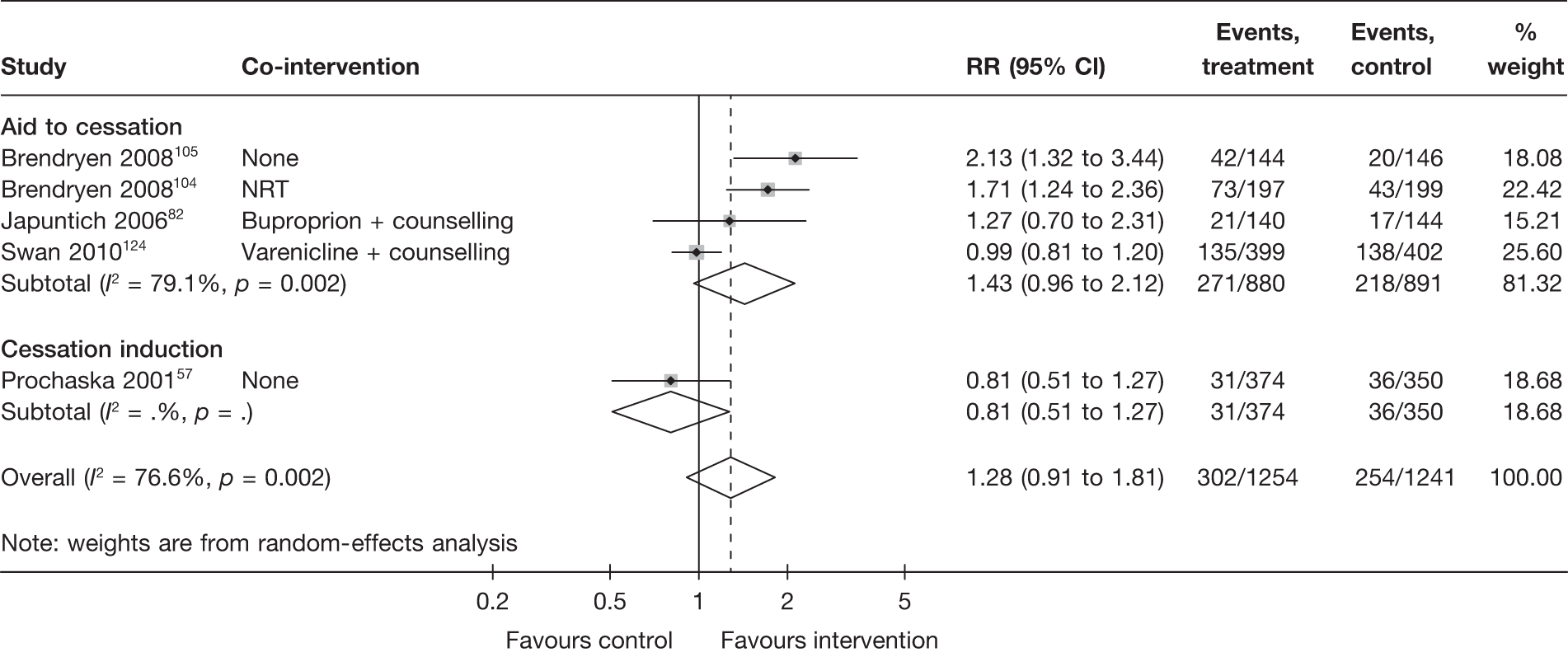
Figure 29 shows the results of point prevalence abstinence measured at the last follow-up of each study. The effect size for the two studies by Brendryen et al. 104,105 at 12 months appears to become smaller compared with 6 months, mainly owing to increased abstinence in the control groups. One additional study88 showed that the combination of an EXP report and automated telephone counselling did not increase abstinence rates when they were added to NRT and stage-matched self-help manual.
FIGURE 29.
Electronic interventions with multiple tailored components vs control: point prevalence abstinence at maximum follow-up.
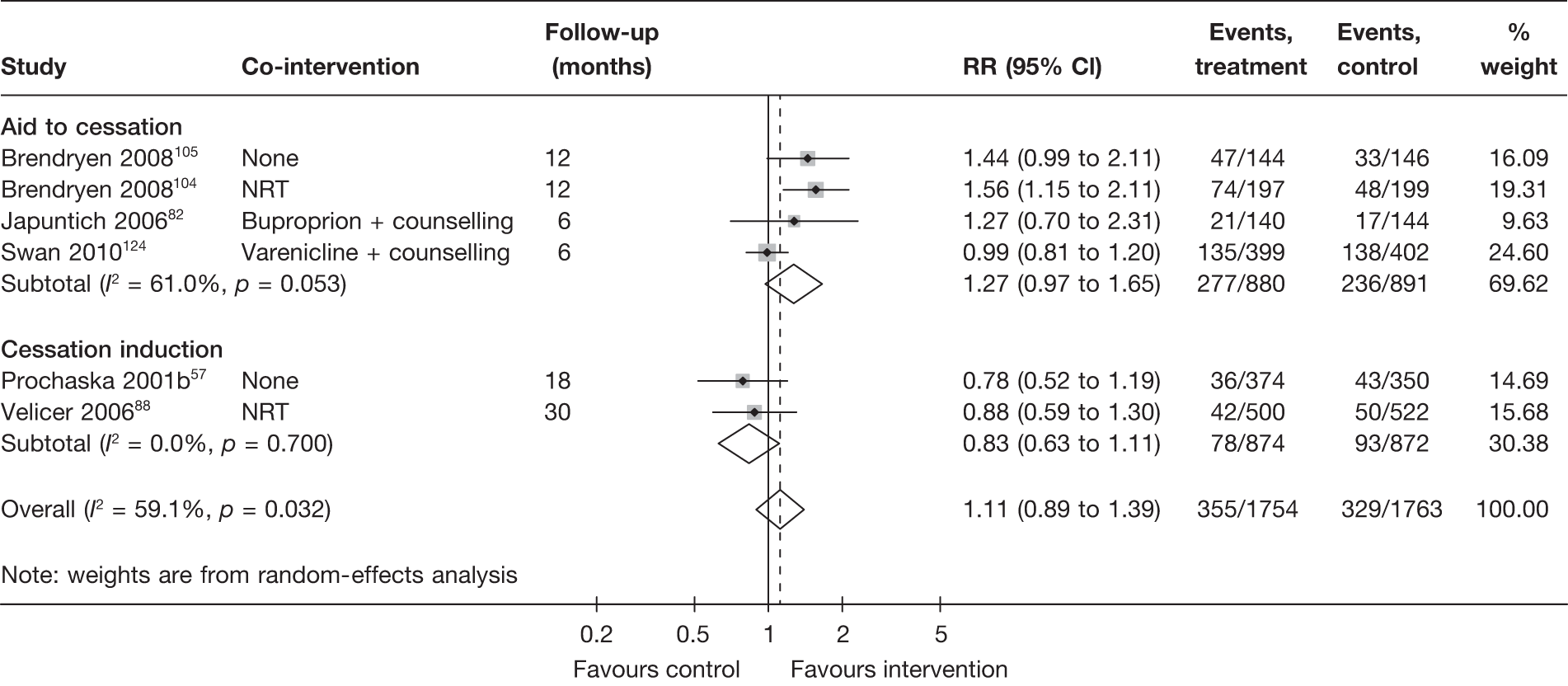
Results for prolonged abstinence are shown in Figure 30. The results are broadly in line with point prevalence abstinence.
FIGURE 30.
Electronic interventions with multiple tailored components vs control: 6-month prolonged abstinence at maximum follow-up.
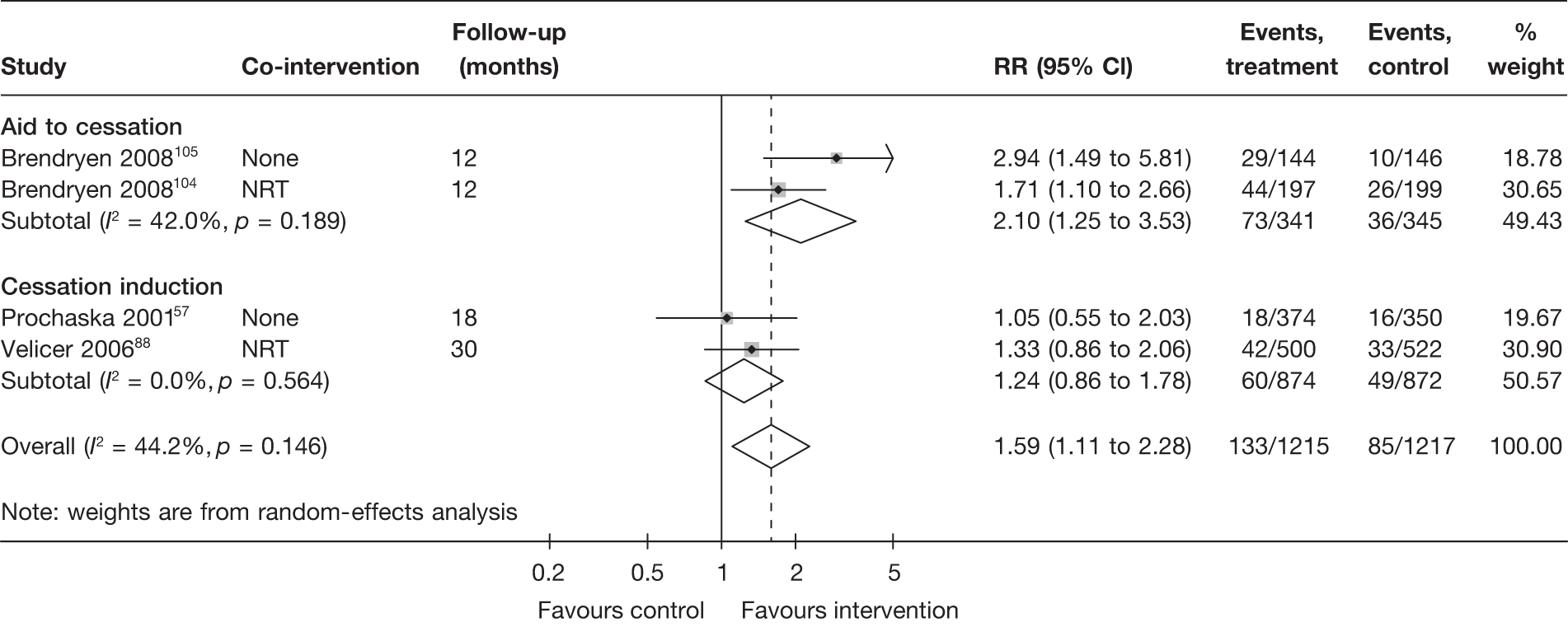
Comparison of electronic interventions with other non-electronic interventions
Two studies (one aid to cessation study124 and one cessation induction study57) provided information on the relative effectiveness of an electronic intervention with multiple tailored components (or one component of the intervention) compared with telephone counselling.
Swan et al. 124 compared an interactive web-based programme (including a discussion forum) with multiple (up to five) proactive telephone counselling in treatment seeking smokers from a large health-care organisation in the USA. All of the participants received concurrent varenicline. Seven-day point prevalence abstinence was lower among smokers receiving the web-based programme than those receiving proactive telephone counselling. The difference between groups was statistically significant at 3 months but not at 6 months (30.7% vs 34.3%, RR = 0.89, 95% CI 0.73 to 1.09).
Prochaska et al. 57 evaluated the effect of adding either LifeSign program or counsellor calls to an EXP intervention that included three computer-tailored reports and a stage-matched self-help manual. Abstinence rates were similar or lower in the LifeSign + EXP group compared with counsellor calls + EXP group but the difference was not statistically significant (7-day point prevalence abstinence at 6 months 8.3% vs 11.6%, RR = 0.71, 95% CI 0.46 to 1.11; prolonged abstinence at 12 months 5.1% vs 5.3%, RR = 0.97, 95% CI 0.52 to 1.79). Neither study arms appeared to demonstrate additional benefit compared with a third arm of the trial in which smokers received the EXP alone. The comparison between LifeSign + EXP versus EXP alone is described in the next section.
Comparisons between electronic interventions
Ten studies35,57,70,76,88,92,101,107,116,119 allowed direct comparisons between different electronic interventions. These comparisons were further classified according to whether the studies compared different provision of contents/intensity/level of tailoring for interventions that were delivered through the same type of media (comparison code 3, see Figure 1), or whether the studies compared different combinations of electronic intervention delivered through different types of media (comparison code 4, see Figure 1).
Table 20 summarises the comparisons made and key findings from these studies. As settings and contents of the electronic interventions being assessed were different, it was not possible to include them in a meta-analysis and compare the electronic interventions with each other. A brief description of each study is provided below.
| Study | Study type | Co-intervention | Comparison | Key findingsa |
|---|---|---|---|---|
| Comparing different forms/contents/provision of interventions within each mode of delivery (comparison code 3; see Figure 1) | ||||
| Schneider et al.199035 | Aid to cessation | None | 2 × 2 factorial design: (tailored online programme vs untailored online programme) × (forum vs no forum) (total n = 1158) |
Point prevalence abstinence: Tailored programme 10.5% vs untailored programme 8.4% (RR = 1.24, 95% CI 0.87 to 1.78b) Forum 9.6% vs no forum 9.2% (RR = 1.06, 95% CI 0.74 to 1.51b) Prolonged abstinence: NR |
| Etter et al. 200570 | Aid to cessation | Interactive smoking cessation website Stop-tabac.ch | Tailored web-based programme (n = 4346) vs modified web-based programme focusing more on NRT and nicotine dependence and less on health risks and coping strategies (n = 4336) |
Point prevalence abstinence (at 2.5 months): OP 10.9% vs MP 8.9% (RR = 1.21, 95% CI 1.07 to 1.38) Prolonged abstinence: NR |
| Pike et al. 200792,93 | Aid to cessation | None | Five interactive websites (n = 5404) vs one static website (n = 1047) |
Point prevalence abstinence (at 3 months): Interactive websites 11.0% vs static website 10.9% (RR = 1.01, 95% CI 0.84 to 1.22) Prolonged abstinence: NR |
| Mckay et al. 2008107 | Aid to cessation | None | Tailored web-based smoking cessation programme (QSN) (n = 1159) vs tailored web-based programme for promoting physical activity (Active Lives) (n = 1159) |
Point prevalence abstinence: QSN 9.7% vs Active Lives 10.4% (RR = 0.93, 95% CI 0.73 to 1.19) Prolonged abstinence: c QSN 3.9% vs Active Lives 3.8% (RR = 1.02, 95% CI 0.68 to 1.54) |
| Stoddard et al. 2008116 | Aid to cessation | Interactive smoking cessation website smokefree.gov | BB (n = 684) vs no BB (n = 691) |
Point prevalence abstinence (at 3 months): With BB 6.6% vs without BB 6.9% (RR = 0.95, 95% CI 0.64 to 1.40) Prolonged abstinence: NR |
| Etter 2009119 | Aid to cessation | Interactive smoking cessation website Stop-tabac.ch | One-off tailored report vs one-off untailored report |
24-hour point prevalence abstinence (at 2 days): Tailored report 12.1% vs untailored report 12.1% (RR = 1.00, 95% CI 0.79 to 1.25) Prolonged abstinence: NR |
| Comparing different (combinations of) electronic interventions (comparison code 4; see Figure 1) | ||||
| Strecher et al. 2005b76,77 | Aid to cessation | NRT | Tailored programme (website + e-mails) vs untailored website |
10-week prolonged abstinence (at 3 months): Tailored programme 20.1% vs untailored website 15.9% (RR = 1.26, 95% CI 1.11 to 1.44) Point prevalence abstinence: NR |
| An et al. 2008101 | Cessation induction | Stop smoking contest | RealU (tailored website + multiple tailored e-mails by peer coaches) vs one-off untailored e-mail control |
Prolonged abstinence (at 7 months): Stated no difference between groups (overall 6%) Point prevalence abstinence (at 7 months): RealU 59.1% vs one-off untailored e-mail 38.5% (RR = 1.54, 95% CI 1.28 to 1.85) |
| Prochaska et al. 200157 | Cessation induction | Stage-matched self-help manual | Three EXP tailored reports + LifeSign (n = 374) vs EXP (n = 362) |
Prolonged abstinence (at 12 months): ES + LifeSign 5.1% vs EXP 6.1% (RR = 0.84, 95% CI 0.46 to 1.52) Point prevalence abstinence: EXP + LifeSign 8.3% vs EXP 11.6% (RR = 0.71, 95% CI 0.46 to 1.11) |
| Velicer et al. 200688 | Cessation induction | Stage-matched self-help manual + NRT | EXP report + TEL (n = 500) vs EXP (n = 509) |
Prolonged abstinence (at 10 months): EXP + TEL 6.6% vs EXP 5.4% (RR = 1.24, 95% CI 0.76 to 2.04) Point prevalence abstinence (at 10 months): EXP + TEL 11.1% vs EXP 9.7% (RR = 1.16, 95% CI 0.81 to 1.67) |
Schneider et al. 35 conducted a trial evaluating one of the earliest internet-based smoking cessation programmes in the late 1980s. The trial adopted a factorial design that allowed comparison in a full tailored version of the programme compared with an untailored version, and between the provision of an internet forum compared with no forum. Test for interaction between the two factors was not reported. Calculation assuming equal sample size between treatment arms suggested no significant interaction, and thus effect estimates assuming no interaction are presented in Table 20. Smokers who received the untailored version and had no access to the forum achieved the lowest abstinence, but the differences between groups and between the tested factors were not statistically significant at 6 months.
Etter70 compared an internet-based, tailored programme designed according to psychological and addiction theory with a modified programme (MP) that was designed for users of nicotine replacement products (but NRT was not part of the intervention within this trial). Both programmes were run within an interactive website (Stop-tabac.ch), from which the visitors were recruited to the study. The results suggested the original programme was more effective than the MP.
Pike et al. 92,93 compared five interactive websites (combined as a group) with a static website and found no significant difference in point prevalence abstinence between them. The contents, features and utilisation by smokers varied between the five interactive websites, and the authors suggested in a post hoc analysis that point prevalence abstinence was higher among two highly utilised sites compared with three less-utilised sites (12.2% vs 10.2%, RR = 1.18, 95% CI 1.01 to 1.38).
Mckay et al. 107 compared two web-based smoking cessation programmes: the Quit Smoking Network, which included tailored web pages, a peer-to-peer web forum and a professionally moderated forum; and the Active Lives programme, which was a web-based exercise enhancement programme that was ‘adapted somewhat to encourage smoking cessation’ and included tailored web pages and peer support forum. No significant difference in abstinence rates was observed between the groups.
Stoddard et al. 116 found that the addition of a bulletin board to an interactive website (www.smokefree.gov) did not further improve the effectiveness of the website, and the utilisation of the bulletin board was low. Only 12% (81/684) of smokers allocated to this arm actually viewed or posted individual messages.
Etter119 compared the immediate effect (2 days after enrolment) of a single tailored report with a single untailored report among visitors of an aforementioned interactive smoking cessation website (Stop-tabac.ch) and found no difference between the two groups.
Strecher et al. 76,77 compared a web-based computer-tailored programme with web-based non-tailored materials as a supplement to nicotine patch therapy. The tailored programme included a web-based cessation guide and tailored newsletters, behaviour support messages delivered via e-mails and tailored advice delivered by e-mail to a supportive person identified by the smoker. Results indicated that the tailored programme was more effective than the untailored web materials.
An et al. 101 assessed the RealU online cessation intervention at the University of Minnesota. The RealU intervention consisted of an interactive website, online magazine and peer e-mail support. Compared with a control group which received an one-off e-mail containing links to online cessation, health and academic support websites, smokers in the RealU group reported a significantly higher point prevalence abstinence at 30 weeks. However, there was no difference in prolonged abstinence between groups. The observed point prevalence abstinence rates in both RealU and control groups were higher than those observed in other studies. The authors attributed the finding to the high rates of occasional smoking at baseline. A campus-wide Quit and Win contest that took place around the 30-week assessment could also have had some impact. Given the nature of the intervention (cessation induction), the follow-up at 30 weeks was likely to be too short to capture any longer-term impact of the intervention.
The study by Prochaska et al. 57 mentioned in the previous section allowed the comparison of the combination of LifeSign program and an EXP intervention compared with the EXP intervention alone. The combined intervention achieved lower abstinence rates compared with the EXP intervention alone, but the differences between the two arms were not statistically significant.
In a study by Velicer et al. ,88 the addition of regular automated telephone counselling (which utilised a series of pre-recorded voice files to form a conversation that was tailored to the smoker’s responses to assessment questions entered using a telephone keypad) to one tailored report generated by an EXP produced slightly higher abstinence rates than the tailored report alone, but the differences between the two arms were not statistically significant. Smokers in both arms also received stage-based self-help manuals and NRT (when the smokers progressed to the appropriate stages).
Summary and discussion
Fourteen studies35,57,70,76,82,88,92,101,104,105,107,116,119,124 evaluated electronic interventions with multiple tailored components. Six57,82,88,104,105,124 of them compared the interventions with either no intervention, usual care or untailored printed self-help material, with or without concurrent co-interventions. Substantial heterogeneity in effectiveness was observed between studies. The interventions have been shown to be effective in aid to cessation studies but not in cessation induction studies.
Ten studies35,57,70,76,88,92,101,107,116,119 directly compared electronic interventions incorporating multiple tailored components with other electronic interventions. Statistically significant findings were observed in three of them, which showed that a MP was less effective than its original form,70 a multicomponent tailored programme was more effective than an untailored website, and a multicomponent tailored programme (RealU) was more effective than an one-off untailored e-mail. Meta-analysis was not carried out owing to the diverse nature of comparisons between studies.
Discussion
Summary of findings
This effectiveness review includes 60 RCTs/quasi-RCTs reported in 77 publications that evaluate the use of computers and other electronic aids for smoking cessation. The results of meta-analyses show that computer and other electronic aids increase the likelihood of cessation compared with no intervention or generic self-help materials, but the effect is small. The effectiveness does not appear to vary with respect to mode of delivery and concurrent non-electronic co-interventions. Overall, similar sizes of effect are observed in both aid to cessation studies (in smokers who are ready to quit) and cessation induction studies (in smokers who are not yet ready to quit), but there is substantial heterogeneity among the latter (cessation induction studies), particularly when 6-month point prevalence abstinence is used as the outcome measure.
Our review has several points of strength:
-
Compared with previously published reviews that have focused on specific types of computer and/or other electronic aids, this review is wider in its scope and encompasses all interventions that make use of automated features brought by the advance in information technology and telecommunication in the past couple of decades. The broader scope allows us to include a larger evidence base in this review and to examine the potential impact of different computer/electronic tools on the effectiveness of the interventions.
-
Comprehensive searches were undertaken. Study selection, data extraction and coding followed standardised procedure and explicit criteria.
-
Where sufficient data were available, meta-analyses were carried out following a stringent ITT principle, which minimises one of the major threats on the validity of effect estimates owing to loss to follow-up.
-
The effectiveness review which focuses on evidence from RCTs/quasi-RCTs is complemented by a supplementary review and a cost-effectiveness review examining other types of relevant evidence.
This review has some limitations which should be considered:
-
The review focuses on smoking cessation programmes in the adult population. It does not cover smoking cessation in adolescents, which, in our view, is a distinct population warranting a separate review.
-
Although we have used explicit criteria based on evidence from previous reviews to categorise electronic interventions with respect to the nature of tailoring and the number of components, the bulk of included evidence falls within one specific category (interventions with a single tailored component) and this has somewhat limited the utility of the categorisation.
-
Similar to the above point, although we are able to examine the effectiveness of electronic interventions utilising different mode of delivery, the volume of evidence for many of the delivery modes, for example e-mails and mobile telephone text messaging, remains limited. Therefore, the findings of lack of sufficient evidence for proving or refuting effectiveness should not be regarded as evidence of ineffectiveness.
-
We have examined only a small number of factors that could potentially influence the effectiveness of the interventions. A comprehensive evaluation of potential effect modifiers at study level in a systematic review of complex interventions remains challenging. For example, in addition to the categorisation of interventions with respect to tailoring and numbers of components, we have also attempted to apply a standardised coding with respect to the contents of each intervention. However, our initial pilot indicated that information presented in published papers is often insufficient to allow accurate coding of each intervention/comparator.
Chapter 3 Evidence synthesis modelling
Chapter 2 provides a detailed overview of the evidence base obtained through our systematic review of the literature. Studies are organised into groups according to the interventions being compared and the outcomes used to measure efficacy, and separate meta-analyses are presented for each group. Although this yields a detailed description of the comparative efficacy of different pairs of electronic interventions, when there are multiple interventions there is no guarantee that the estimated intervention effects are consistent (i.e. the effect of C vs B must equal the effect of C vs A minus the effect of B vs A). The economic model requires consistent estimates of all of the intervention comparisons for 12-month continuous abstinence. There is also a requirement that the economic model be informed by all of the relevant evidence to correctly reflect the uncertainty in the optimal decision. In particular, not all studies report 12-month continuous abstinence. A model for relapse rates over time is required to be able to pool information from studies with different follow-up times.
In this chapter, we present a comprehensive evidence synthesis specifically designed to combine as much of the evidence as possible in a consistent way, in order to make comparisons across multiple interventions and to inform the cost-effectiveness analysis (presented in Chapter 4). We use a method known as MTCs (or network meta-analysis) to combine multiple complex interventions129 and explore time-to-event survival models for relapse rates to enable us to combine studies with different (and multiple) follow-up times. The methods are described later in the chapter (see Methods) and the results are presented (see Results) and discussed (see Discussion). Table 25 gives a complete summary of the results that will be used as estimates of efficacy in the cost-effectiveness model described later in Chapter 4 (see Decision-analytic model). We also explored an expanded model that was able to synthesise evidence from studies reporting point prevalence outcomes together with studies reporting continuous abstinence; however, we found that results were largely unaffected by the inclusion of studies reporting point abstinence (see Discussion). Therefore, for simplicity, only models based on the 28 studies reporting continuous abstinence are described in Methods, and only results based on these studies are reported in Results.
Overview of evidence and the multiple treatment comparison approach
The results reported in the clinical effectiveness chapter perform separate pair-wise comparisons for:
-
electronic interventions compared with control
-
electronic interventions compared with non-electronic interventions
-
electronic interventions compared with other electronic interventions.
However, the classification scheme we have developed (Table 21) identified five different types of electronic interventions and five different types of non-electronic interventions. Viewing the interventions in this way, the evidence identified in the systematic review forms a network of treatment comparisons (see Figure 31). It is therefore possible to combine all of this evidence in a single coherent analysis in order to draw inference on any pair of interventions. Note that some of the trial evidence compared two interventions that differed only in the control scenario. This evidence does not provide information on the relative efficacy of the electronic interventions, but can still be included in a network meta-analysis and provide information on between-trial variability.
| Code | Definitiona | Examples |
|---|---|---|
| Electronic interventions/components | ||
| e0 | Nothing (no electronic component) | Interventions with no electronic component, such as face-to-face counselling and NRT (which are coded separately); electronic reminders not related to the intervention itself;b control group without any intervention |
| e1 | Single generic component | Generic self-help material delivered by e-mails; static websites (websites containing generic information without providing tailored feedback to individuals) |
| e2 | Multiple generic components | Static websites + generic self-help material delivered by e-mails |
| e3 | Single tailored component | Computer-generated tailored feedback; interactive websites (websites providing stage-matched or other feedback tailored to individuals) |
| e4 | Single tailored component + generic component(s) | Interactive websites + e-mail reminders asking smokers to log on to the websites; stand-alone tailored computer program + printout of the same output posted to the smokers |
| e5 | Multiple tailored components (± generic components) | Interactive websites + additional computer-generated tailored feedback delivered by post; interactive website + chat room |
| Non-electronic interventions/components | ||
| c0 | Nothing (no non-electronic component) | Interventions that are fully automated; non-electronic reminders, telephone calls or questionnaires not related to intervention itself (e.g. for data collection); control group with no intervention |
| c1 | Generic self-help material | Self-help manuals, booklets |
| c2 | Brief advicec | Smoking cessation advice given during a GP consultation |
| c3 | Telephone or face-to-face counsellingc | Quitlines; one-to-one or group counselling |
| c4 | Pharmacotherapyc | NRT; bupropion |
| c5 | Counselling + pharmacotherapyc | Smoking cessation clinic, which offers NRT and one-to-one counselling |
FIGURE 31.
Network of treatment comparisons evaluated in the studies forming the evidence base. Only studies reporting continuous abstinence are included. Interventions are defined as eAcB, where A and B are the index for the electronic and conventional components, respectively (see Table 23). Each connector represents at least one study and is labelled with the number of studies making that comparison (if > 1).
In order to derive a coherent set of estimates of intervention efficacy to inform the economic analysis, we developed a Bayesian MTC. 130–133 This statistical method allows for a single analysis incorporating all treatment comparisons to be performed. Such an analysis is also known as network meta-analysis or multiple treatments meta-analysis. Suppose we have a network of evidence for treatments A, B and C consisting of head-to-head trials of A versus B, A versus C, and B versus C. The MTC approach assumes that the mean intervention effect in the B versus C trials is equal to the difference between the mean intervention effect in the A versus C and A versus B trials. As with ordinary pair-wise meta-analysis we can either assume that the studies estimate a common intervention effect (fixed-effects MTC) or that the study-specific intervention effects can be considered to come from a distribution of study intervention effects with common mean intervention effect and between-study variance (random-effects MTC). Given the considerable heterogeneity in the available evidence, as identified in Chapter 2, we fit random-effects models in our analysis.
The synthesis is complicated by the fact that the included studies heterogeneously report outcomes at different (and multiple) follow-up times. In this chapter we describe the MTC models explored that allow us to combine evidence reported at different (and multiple) follow-up times. The models allow us to compare the effects of the different types of electronic interventions, and investigate whether the different types of interventions are associated with increased likelihood of quitting, whether they act additively with the type of control intervention, or whether they interact with the type of control intervention (e.g. if there is synergy obtained from combining one type of intervention with a particular control). We compare the fit of the various different models and present the results for the models that make predictions that best fit with the observed data. These results for intervention efficacy are those required as inputs to the cost-effective analysis (see Chapter 4).
Methods
We constructed a number of models for the MTC, in order to explore different assumptions about the intervention effects and the pattern of relapse over time. These models are described in this section.
Time-to-relapse models for continuous abstinence
A number of studies report continuous abstinence of some duration as an outcome measure. Some studies record this result at multiple durations. To synthesise this range of outcomes and allow our desired intervention effect (change in 12-month continuous abstinence) to be estimated, we base our model on the time from initiation of the quit attempt to relapse. This approach is possible, as some trials report continuous abstinence at multiple time points.
The model interprets data on continuous abstinence as samples from a binomial distribution, where success is defined as maintaining abstinence up to the observation time. The probability of avoiding relapse is derived from a survival model in which the time-to-relapse for participants in a given trial and arm are assumed to be independent and identically distributed. We explore two parametric models for this distribution – the Weibull and exponential distributions. The models are described fully in Appendix 6.
Incorporating intervention effects
Interventions were assumed to have an effect on the scale parameter of the time-to-relapse model but not (in the Weibull model) the shape – this is equivalent to fitting a proportional-hazard survival model. Given the considerable heterogeneity in the data, as described in Chapter 2, a random-effects treatment model was assumed, where intervention effects were assumed to vary between studies but be drawn from a common distribution of intervention effects. This model for study-level intervention effect is given by:
where:
-
λi,j = the scale parameter in the time-to-relapse distribution for arm j of trial i
-
µi = the log-scale in the control arm (j = 1) of trial i
-
δi,j = the effect of the intervention given in arm j of trial i
-
I(j,1) = 1 if j = 1, 0 otherwise to indicate the baseline arm.
The study-specific intervention effects on the log-scale δ i,j were assumed to be normally distributed (random-effects assumption) with mean µDi,j, where:
-
treati,j = the intervention used in arm j of trial i
-
= the mean intervention effect for studies of intervention treat against reference intervention [in this case no electronic (e0, see Table 21) or non-electronic components (c0, see Table 21)].
The assumption made is that the indirect evidence between two different interventions is consistent with the direct head-to-head evidence on those interventions. By imposing this assumption across all interventions in the evidence base, we ensure that the correct intervention comparison is made for the evidence from each trial, and a coherent set of estimates of intervention effects is generated from the evidence that respects the randomisation in the original trials, as described in Chapter 2.
As described in Chapter 2, the interventions given consist of an electronic component and/or a conventional intervention. Both types consisted of six classes, including nothing (listed in Table 21), and so this pairing gives 36 intervention combinations (a system for labelling these combinations, used in Table 21, is to define an intervention consisting of conventional intervention A and electronic intervention B as eAcB. The interventions then range from e0c0 (no support) to e5c5 (professional face-to-face counselling and pharmacotherapy combined with a complex electronic intervention, such as an interactive website with a bulletin board).
We could assume that the effects of these 36 interventions are completely independent from each other (Assumption A1). This would involve fitting 35 treatment parameters (as one of the 36 interventions is placebo), which is the most general model possible. However, this ignores the fact that the interventions share components, and also it may not be possible to fit this many treatment parameters given the available data. We also fitted a more restrictive model that assumed that the intervention effect of combining types was additive, so that the impact of an intervention with both active electronic and conventional interventions was equal to the sum of the effect of each intervention given in isolation (Assumption A2), giving 10 treatment parameters, five electronic and five conventional. We then explored the impact of assuming that the effect of each electronic intervention was the same irrespective of its category (Assumption A3), reducing the total number of treatment parameters to six (five conventional and one electronic). Finally, we fitted a more relaxed version of this model, still assuming that the effect of an electronic intervention did not depend on its category, but allowing for that effect to vary depending on the conventional co-therapy.
To illustrate the impact of these alternatives, consider four interventions: e0c3, e4c0, e4c3 and e2c3. If D(x) is defined as the effect of intervention x, then the three different intervention effect models imply respectively:
-
Assumption A1 D(treat) is different for different interventions. So, for example, D(e0c3), D(e4c0) and D(e4c3) are independent.
-
Assumption A2 D(eAcB) = D(eA) + D(cB). So, for example, D(e4c3) = D(e4) + D(c3), and D(e2c3) = D(e2c0) + D(e0c3), etc.
-
Assumption A3 D(eAcB) = D(e) + D(cB), where D(e) = D(e0) = D(e1) = … = D(e6). So, for example, D(e4c3) = D(e2c3) = D(e) + D(c3), etc.
It can be seen that Assumption A1 allows for an interaction between the effect of an electronic intervention and the choice of conventional co-therapy, whereas Assumption A2 and Assumption A3 assume that there is no such interaction.
Incorporating readiness to quit
One hypothesis regarding the value of electronic aids to smoking cessation is that they might encourage those not actively seeking to quit to make an attempt to do so. A number of trials include such participants. If this effect exists, the impact of electronic interventions will be higher in this population. We explore this hypothesis within the model assuming a single intervention effect across electronic interventions (Assumption A3), by amending it to include two separate treatment parameters, one for studies restricted to those actively seeking to quit, and one for those not stating such a restriction, giving seven intervention effect parameters.
Model comparison
To explore the alternative assumptions described above, we compared the fit of models with the data:
-
Model 1 35 intervention effects (Assumption A1), exponential model for time to relapse.
-
Model 2 35 intervention effects (Assumption A1), Weibull model for time to relapse.
-
Model 3 10 intervention effects (Assumption A2), Weibull model for time to relapse.
-
Model 4 6 intervention effects (Assumption A3), Weibull model for time to relapse.
-
Model 5 7 intervention effects (Assumption A3 incorporating readiness to quit), Weibull model for time to relapse.
The choice of time-to-relapse distribution was made by comparing models 1 and 2. Model choice was assessed using the deviance information criterion (DIC). 134 This statistic measures a compromise between model fit (measured by D¯) and model complexity (measured by pD), and is calculated as follows:
D¯ is the posterior mean of the deviance, a measure of model fit. A model is considered to provide an adequate fit to the data when D¯ is approximately equal to the number of data points. pD is interpreted as the effective number of parameters in the model. It reflects the flexibility with which the model can adjust to fit the data. The lower the value of DIC, the better, as this reflects improved model fit and/or a more parsimonious model.
Results
Overview of evidence
Of the studies identified in the main effectiveness review, only 28 reported continuous abstinence. 36,37,39,43,46,47,49,53,56–58,62,63,66,69,73,76,88,91,97,100,101,104,105,109,110,120,124
Table 22 shows how many arms provided information on each of the types of electronic intervention. There are many studies evaluating class three and class five electronic interventions; far fewer evaluating interventions of class one, two or four. Figure 31 illustrates the network of evidence on continuous abstinence, by depicting each pair-wise comparison contained within the evidence base reporting this measure. It can be seen that only 19 of the 36 possible comparisons are evaluated. However, the comparisons do form a single connected network for those 19 interventions, so that an intervention effect can be estimated for any pair of comparisons of these interventions. For example, although no trials have compared e3c0 and e4c2 directly, trials exist comparing the former to e0c0 and the latter to e0c2. There is also a head-to-head trial of e0c0 and e0c2, so if the assumption of consistency is made, the intervention effect of e4c2 compared with e3c0 can be calculated.
| Outcome reported | e1 | e2 | e3 | e4 | e5 |
|---|---|---|---|---|---|
| Any outcome | 6 | 2 | 40 | 5 | 14 |
| Continuous abstinence | 2 | 1 | 21 | 1 | 7 |
Table 23 shows the variation between studies in terms of the readiness-to-quit of participants. Only two studies explicitly state that they excluded participants actively seeking to quit. 73,104
| Readiness-to-quit stage | No. of studies |
|---|---|
| Actively seeking to quit | 7 |
| Contemplating quitting | 2 |
| Not wanting to quit | 0 |
| Mixed | 12 |
| NR/assessed | 7 |
Comparison of fit for evaluated models
Table 24 gives the model fit statistics D¯, pD and DIC. The first conclusion to draw from comparing the models is that the exponential is an extremely poor fit with the data, and that the more flexible Weibull distribution improves model fit considerably. The model fitted assumes that the shape parameter α is treatment invariant, and its posterior mean was found to be around 0.14 in models 2–5. This suggests that the hazard of relapse decreases sharply over time. Although the fit was improved, none of the Weibull models achieved a value for that was close to 89 or less (based on the rule of thumb mentioned earlier, which compares with the number of data points), suggesting that there is still some lack of fit. A comparison of observed and fitted values found that the observations with the largest deviance were related to arms from two studies105,109 with repeated observations, where a very high relapse rate at the first observation was followed by a relapse rate in subsequent observations at or close to 0%.
| Model | Time-to-relapse distribution | No. of treatment parameters | D¯ | pD | DIC |
|---|---|---|---|---|---|
| 1 | Exponential | 35 | 3727.3 | –271.3 | 3456.0 |
| 2 | Weibull | 35 | 106.6 | 62.3 | 168.9 |
| 3 | 10 | 108.7 | 57.5 | 166.2 | |
| 4 | 6 | 108.0 | 51.9 | 159.9 | |
| 5 | 7 | 108.6 | 51.1 | 159.7 |
Reducing the number of parameters from 35 to 10 (Model 3) did have an impact on model fit, but the DIC suggests that this impact is not strong enough to justify the model with full interactions (Model 2). Assuming that the intervention effect was identical across intervention classes had little further impact on model fit (although using fewer parameters), reflected in a reduction in the DIC statistic for Model 4. Adapting this model to include separate intervention effects according to readiness to quit, or interaction effects, led to a slightly worse model fit.
The conclusion from this comparative exercise is that, based on the DIC, the preferred model is one in which time to relapse has a Weibull distribution, and the intervention effect is assumed identical across electronic intervention classes, without interaction effects with the conventional co-intervention. The fit of such models with or without a differentiation of intervention effect between those ready to quit, and those not, is comparable, suggesting no evidence that there is a difference in intervention effect on continuous abstinence between these different populations.
Intervention effect estimates
The models give intervention effects that can be interpreted as hazard ratios (HRs) for the time to relapse. As described in section 5.1.2, the basic parameters of each model are the effects of each intervention relative to reference intervention e0c0. Using the consistency assumption we can then form the HR for any head-to-head comparison, calculated by dividing the HR of the active intervention (vs reference) by the HR of the control (vs reference). As the hazard is that of time to relapse, a lower HR represents an improvement.
Based on model fit alone, the preferred assumption is that of a single intervention effect across different classes of electronic intervention, with (Model 5) or without (Model 4) a modifier for readiness to quit. However, we were also interested in the impact of allowing separate intervention effects for each of the five classes of electronic intervention (Model 3) as providing the inputs required in the cost-effectiveness analysis. Table 25 gives the intervention effects for these three models. Although the inclusion criteria restricted trials to those with an electronic intervention arm, the network allows for estimates of intervention effects against reference intervention (e0c0) for each of the conventional interventions. These estimates are given in Table 25 for Model 3 – models 4 and 5 produce very similar results. They suggest that there is considerable uncertainty in the effectiveness of these interventions, and whether or not they are more effective than e0c0 at all. The result arises because of the limited and largely indirect nature of the evidence included in this review to base such comparisons – evaluations specifically concerned with established interventions have drawn on a larger evidence base to derive estimates of these intervention effects that are more precise and more positive. The estimates of the effectiveness of conventional interventions is not the focus of this review, are therefore essentially ‘nuisance parameters’ in our model, and we do not use them in the cost-effectiveness analysis described in Chapter 4 (see Decision-analytic model).
| Model and intervention | Intervention effect (mean and 95% credible interval for HR) | |
|---|---|---|
| Full evidence base | Excluding Meyer et al.109 and Brendryen et al.105 | |
| Model 3 (separate electronic intervention effects) | ||
| Conventional interventions | ||
| c1 | 1.04 (0.94 to 1.14) | 1.02 (0.92 to 1.13) |
| c2 | 0.99 (0.84 to 1.17) | 1.08 (0.87 to 1.32) |
| c3 | 0.95 (0.79 to 1.12) | 0.98 (0.81 to 1.18) |
| c4 | 1.00 (0.75 to 1.30) | 1.06 (0.77 to 1.40) |
| c5 | 0.85 (0.59 to 1.17) | 0.93 (0.64 to 1.30) |
| Electronic interventions | ||
| e1 | 0.89 (0.66 to 1.16) | 0.94 (0.71 to 1.22) |
| e2 | 0.98 (0.78 to 1.21) | 1.05 (0.85 to 1.27) |
| e3 | 0.88 (0.83 to 0.93) | 0.89 (0.84 to 0.94) |
| e4 | 1.02 (0.78 to 1.32) | 1.03 (0.79 to 1.30) |
| e5 | 0.85 (0.75 to 0.96) | 0.91 (0.80 to 1.02) |
| Model 4 (single electronic intervention effect) | ||
| epooled | 0.87 (0.83 to 0.92) | 0.89 (0.84 to 0.94) |
| Model 5 (separate electronic intervention effects by motivation) | ||
| eNQ | 0.88 (0.83 to 0.93) | 0.89 (0.83 to 0.95) |
| eQ | 0.88 (0.81 to 0.96) | 0.91 (0.84 to 0.98) |
Table 25 gives intervention effect estimates for electronic interventions vs nothing. Model 4 gives a single intervention effect for all electronic interventions showing a benefit that is significant, although not strong (mean HR 0.87, 95% credible interval 0.83 to 0.92). Model 5 allows for the intervention effect to depend on whether or not the study explicitly restricts participation to smokers actively seeking to quit, but the resultant HRs are virtually identical. For Model 3, five separate intervention effects are generated. The mean HRs vary from 0.85 (e5) to 1.02 (e4). There is no discernable trend (either positive or negative) in mean effectiveness as intervention complexity increases. Reflecting the available evidence, there is considerable uncertainty in the intervention effect for e1 (mean HR 0.89, 95% credible interval 0.66 to 1.16), e2 (mean HR 0.98, 95% credible interval 0.78 to 1.21) and e4 (mean HR 1.02, 95% credible interval 0.78 to 1.32). Only interventions e3 (mean HR 0.88, 95% credible interval 0.83 to 0.93) and e5 (mean HR 0.85, 95% credible interval 0.75 to 0.96) have an intervention effect whose credible interval excludes 1, which is not surprising given the larger number of studies falling into this category.
Figure 32 illustrates the intervention effects (mean and 95% credible intervals) generated by the three models, so that the findings can be compared simultaneously. It illustrates the considerable uncertainty around the five separate intervention effects from Model 3, relative to the differences between the means, explaining why the DIC favoured Model 4. The resulting single estimate from that model, epooled, is clearly influenced most by those interventions with the most evidence (e3 and e5). It can also be seen that there is almost no difference in intervention effect between trials that restrict inclusion to those actively seeking to quit, and those that do not.
FIGURE 32.
Caterpillar plot of HRs (mean and 95% credible intervals) derived from synthesis of evidence using models 3–5. ‘e1–e5’ are the separate intervention effects for each class, estimated by Model 3. ‘epooled’ is the intervention effect from Model four, which assumes the intervention effect is independent of class. ‘eNQ’ is the intervention effect for those not actively seeking to quit and ‘eQ’ is the intervention effect for those who are seeking to quit (both estimated by Model 5).
Discussion
We chose a time-to-relapse survival model to synthesise the evidence on continuous abstinence. This allowed us to account for variability in follow-up between studies and repeated measures reported by some studies. We found that assuming an exponential survival model led to an extremely poor fit with the data, and that this fit was improved considerably by the use of a Weibull model, with any resulting lack of fit explained by two studies39,80 with an unusual pattern of repeated observations over multiple time points. We explored the impact of excluding these studies and found that estimates of treatment effect were not sensitive to their inclusion (see Table 25). As we were unaware of any other reasons to exclude these studies, we based further analyses on the full set of trials. The results indicated that the shape parameter of the time-to-relapse distribution was around 0.14 (an exponential distribution would be implied if the shape parameter was 1), indicating that the chance of sustaining a quit attempt is far higher once the first month or two have been negotiated successfully.
Figure 33 illustrates a Weibull time-to-relapse model with shape = 0.14 for two interventions: a control with a 6-month continuous abstinence rate of 10% (as might be found with smokers given counselling alone) and an active intervention with HR 0.87 (reflecting the mean for electronic interventions found in our analysis).
FIGURE 33.
Example of a Weibull time-to-relapse model for a control intervention with 6-month continuous abstinence of 10%, assuming a HR for the active intervention of 0.85 and a shape of 0.14.
Our overview of the available data shows that evaluations have concentrated on electronic interventions that fit our categories 3 and 5 (single tailored component and multiple components, at least two of which are tailored). Comparing these two categories suggests that there is little additional benefit from the latter, which we would expect to be more resource intensive. Given the lack of information on other categories, it is unsurprising that the model assuming a single intervention effect fits the data equally well. We also failed to find a difference in intervention effect between trials restricted to those actively seeking to quit and all other trials, although this may well be due to a lack of studies explicitly targeting populations not contemplating a quit attempt.
The analysis above is restricted to those studies that report continuous outcomes, which represent less than half of the studies in the main review. Many of these studies also report point prevalence outcomes. During the model development process, we explored models that allowed for a correlation between the two outcome types and then used that correlation to draw on studies that only reported point abstinence to inform estimates of continuous abstinence. However, despite observing a strong correlation between intervention effects on the two types of outcome, including the additional studies had little effect on the posterior mean or credible intervals of the estimated pooled intervention effects. This may be because the additional studies largely evaluated the two intervention types well represented in the studies reporting continuous abstinence only. Given that the results were unchanged by including point abstinence, and that our analysis is based on continuous abstinence, we have not included the results from this exercise in this chapter.
Given that the DIC is minimised by Model 4, it might be argued that once a decision has been made to implement an electronic intervention, the choice of that intervention comes down to minimising cost. This would imply recommending a class one (single generic component) intervention over anything more expensive. However, such a recommendation would fail to allow for the considerable uncertainty around several of the intervention classes. To account for this, we take a two-step approach in the cost-effectiveness analysis. We first explore the cost-effectiveness of a generic electronic intervention, taking the result from Model 4 as our measure of intervention effect. Then, we explore which category of electronic intervention should be chosen, if a decision is made to implement this type of intervention. We base the latter analysis on the intervention effects from Model 3. The results from this exercise are given in Chapter 4 (see Decision-analytic model).
Limitation of the analysis and recommendations for future research
A limitation of the system used to categorise electronic interventions was that it does not allow us to categorise the impact of mode of delivery. This was because there is a wide range of possible modes of delivery, with only limited data to compare them. In order to simplify the analysis and derive meaningful results, we developed a categorisation system based on expert advice which identified underlying factors (such as interaction and intensity) that might explain the relative efficacy of different electronic interventions. The additional impact of alternative modes of delivery should be explored, once data are available to allow this. We were unable to explore the impact of interventions on the quit–relapse cycle and the impact of factors such as age and motivational status on the optimal intervention due to a lack of evidence. There is a clear need for research designed to support these further analyses.
Summary of findings
-
There is no evidence from the trials available that there is a differential intervention effect across categories of electronic intervention (see Tables 24 and 25 and Figure 32).
-
When viewed as a single category, the addition of an electronic intervention of some sort to conventional smoking cessation aids improves continuous abstinence (mean HR 0.87, 95% credible interval 0.83 to 0.92).
-
The available evidence does not show any difference in intervention effect for electronic interventions between trials restricted to smokers actively seeking to quit and those not imposing this inclusion criterion.
-
There is considerable uncertainty in the estimates for e1 (single generic component, mean HR 0.89, 95% credible interval 0.66 to 1.16), e2 (multiple generic components, mean HR 0.98, 95% credible interval 0.78 to 1.21) and e4 (single generic component plus multiple tailored components, mean HR 1.02, 95% credible interval 0.66 to 1.16), reflecting the lack of trials assessing these categories of intervention.
Given these findings, we consider two sets of results for use in the cost-effectiveness analysis. When considering the cost-effectiveness of electronic interventions as a class, we will use the pooled estimate from Model 4. When exploring the question of which type of intervention to use, we will use the separate estimates from Model 3, as shown in Table 25 and Figure 32.
Chapter 4 Economic analysis
Chapters 4 and 5 present detailed evidence on the efficacy of electronic aids to smoking cessation. In this chapter, our aim is to assess the cost-effectiveness of these interventions. We address this with two questions. The first is whether or not an electronic intervention of some kind is likely to be cost-effective compared with conventional support alone. The second considers the comparative cost-effectiveness of different types of electronic intervention. The sections Methods and Results outline the existing literature informing these questions. Studies exploring the cost-effectiveness of electronic aids for smoking cessation were identified alongside the review of literature on effectiveness. This identified three studies, which are described in Results. As the existing evidence base on cost-effectiveness was inconclusive, we carried out an economic analysis de novo. The decision problem for this economic analysis is specified in the section Decision-analytic model and is used to construct an economic model. Chapter 3, Methods described how Bayesian MTC models were developed to synthesise the evidence identified in the main effectiveness review (as described in Chapter 2) and generate efficacy estimates for the economic model. The economic model is described in this chapter in the section Derivation of cost data for electronic interventions, and a description of sources for parameters other than efficacy is given in Additional model inputs. Results from the model are also presented (see Results of the cost-effectiveness analysis).
Methods
Search strategy
The search strategy was identical to the strategy used to identify effectiveness studies (described in Appendix 1), which was designed to also capture any studies reporting outcomes relevant to the cost-effectiveness of electronic aids for smoking cessation.
Inclusion criteria
All studies, of any design, that reported a full economic evaluation (i.e. cost-effectiveness analysis, cost-utility analysis, or cost–benefit analysis) were eligible for inclusion. The same criteria on the basis of the intervention were used as for inclusion in the effectiveness review (described in Appendix 2).
Data extraction
Data were extracted by one reviewer (JJM) and independently checked for accuracy by a second reviewer (NJW). Disagreements were resolved by discussion. Outcome measures extracted were intervention costs, incremental cost per quitter, incremental cost per life-year saved, incremental cost per QALY saved, for each intervention compared with the control scenario. Descriptions of the model used and economic data reported are given below.
Quality assessment
Quality assessment of cost-effectiveness studies was performed using standard criteria. 135 The results of this process are documented in Appendix 5.
Results
Three studies were identified that appeared to meet our inclusion criteria for cost-effectiveness analyses of interventions satisfying the scope of this review: Lennox et al. ,55 Smith et al. 136 and Barnett et al. 81
Description of retrieved studies
Lennox et al.
This study55 was carried out at six general practices in Aberdeen, Scotland. The intervention evaluated was a letter tailored by a specialist computer software package to answers given by smokers to a questionnaire. The intervention was compared with a default NT letter and with a control group who received no letter at all.
The measure of effectiveness was point prevalence abstinence at 6 months. The cost for each arm was divided by the number of quitters to give a cost per quitter. A value for the number of life-years gained by quitting was taken from Oster et al. ,137 a study of nicotine gum published in 1986, which used the American Cancer Society Cancer Prevention Study to estimate the life-years (undiscounted and discounted at 5%) gained by quitters as a function of age and gender. Oster et al. 137 calculated that the increase in life expectancy would be around 0.8–1.1 discounted life-years for men aged 35–64 years and 0.54–0.65 discounted life-years gained for women aged 35–64 years.
Of those who received a tailored letter, 30/857 (3.5%; 95% CI 2.3% to 4.7%) were abstainers at 6 months compared with 37/846 (4.4%; 95% CI 3.0% to 5.8%) of those who received NT letters and 22/850 (2.6%; 95% CI 1.5% to 3.7%) of those who received no letter. Therefore, NT letters achieved the highest quit rate, although differences between the arms were not statistically significant (after adjusting for confounders, the comparison p-values were 0.25 for tailored letter vs no letter, and 0.07 for NT letter vs no letter). It is stated that the 15 extra quitters gained by issuing a NT letter rather than no letter were gained at a cost of £464, based on the actual number of participants recruited. The cost-effectiveness ratio is given as £37 per quitter if mistargeted participants behaved similarly to the 846 included smokers, or £89 per quitter if none of the mistargeted participants benefitted from the letter in any way. Using the discounted life-years saved (LYS) from Oster et al. ,137 the authors translate this into a LYS of between £50 and £122, although this implies the number of discounted LYS as 1.4. Further information on costs/resource use is not given. It is not possible to derive information on the cost of the tailored letter, and so this study is not helpful in determining intervention costs relevant to this review.
Smith et al.
This study136 presents a cost-effectiveness analysis to evaluate the incremental cost-effectiveness of a staged-based, computerised smoking intervention relative to standard care in an urban managed care network of primary care physicians. The model is based on patient outcomes and cost estimates that had been collected alongside a clinical trial.
The trial setting was primary care practices in New York City. One physician was chosen from each participating practice and randomly allocated to one of the study arms (along with all of their patients who were current smokers). The intervention was targeted at both physicians and patients. Ten smokers were enrolled from each participating physician’s list. Each smoker completed a 20-minute computer-based assessment of their smoking history. A tailored report was generated for each participant by an EXP. Each physician in the intervention arm received 30 minutes of training in smoking cessation counselling techniques and instructions in interpreting the report, before a medical consultation took place. Participants in the control arm completed the assessment and received the consultation, but no report was generated and their physicians received no additional training.
Analysis was carried out from the perspective of the primary care practice. The resources used in delivering the intervention and control were recorded, and included staff costs (physician, nurse, administrator and computer technician), computing costs (hardware and software) and the costs of pharmacological aids to quitting smoking. Quit rates in each arm were recorded in terms of point abstinence at 18 months. An assumption was made by Smith et al. 136 that 55% of those reporting point abstinence at 18 months would sustain abstinence permanently. Quitters were assigned a gain in life expectancy and QALYs based on values reported by Fiscella and Franks138 of 1.46 life-years and 1.97 QALYs (discounted at 3%).
Installation for the computer system required 60 minutes of technician time and a further 40 minutes of physician training was required. It was assumed that the lifetime of the system was 10 years. For the intervention, 2.5 minutes of office administrator time and 13 minutes of physician time were required per smoker, compared with 7 minutes of physician time per smoker in the control group. The upfront costs of installation and training were US$2514. Based on this pattern of resource use, it was found that the intervention involved extra costs of US$40.83 per smoker. These consisted of US$8.82 in extra workstation and primary care physician training costs, US$21.41 in additional costs for office support staff and the initial and follow-up counselling visits, and US$6.89 in additional adjuvant therapy costs.
The point abstinence rate was 12.2% in the intervention group compared with 7.9% in the control group, which translated to 2.4% difference in long-term quit rates after adjusting for relapse. For those in the pre-preparation stage, the 6-month rates were 7.9% compared with 6.1%, and for those in the preparation stage, the rates were 18.3% compared with 10.3%. This implied an incremental cost-effectiveness ratio (ICER) of US$4797 per net quitter for those in the pre-preparation stage and US$735 for those in preparation stage. This translated into an overall ICER of US$869 per QALY.
Barnett et al.
The article81 presents analysis of a RCT of counselling to assist smoking cessation offered to patients receiving outpatient treatment for depression. The intervention involved a computer-mediated evaluation reviewed by a counsellor. This determined whether or not the smoker was ready to quit, in which case six sessions of counselling and up to 10 weeks of NRT were offered. If still smoking, two further counselling sessions were offered, along with bupropion. Those in the control arm were offered a stop-smoking guide, and a list of smoking cessation programmes.
Computer evaluation sessions lasted 15 minutes, and counselling sessions lasted 45 minutes for the first visit, 30 minutes for subsequent sessions. For the intervention group, the average number of evaluations per smoker was three, and the average number of cessation sessions was 1.3. The average number of weeks’ nicotine patches dispensed to this group was 2.7. There was also a small, non-significant difference in health care utilisation outside the study, and inpatient mental health treatment days received. This gave a net difference in health-care costs of US$341 between the intervention and control groups. There was a difference in point prevalence of abstinence at 18 months of 5.5%, which gives an ICER of US$6204 per quitter. The probability that the intervention was not cost-effective at US$20,000/QALY was 25%. A review of nine studies investigating counselling for smoking cessation by Ronckers et al. 139 found that the average number of life-years gained used was 1.2. This would imply an ICER of US$5170 per LYS.
Although there was a computer-based aspect to the study, it related to the stratification of participants rather than the delivery of an intervention. The study was essentially an evaluation of counselling delivered by therapists, and related to a narrowly focused population. Therefore, it cannot appropriately inform our economic analysis.
Decision-analytic model
Our review has identified a variety of interventions and two distinct populations for whom these interventions have been used. Previous cost-effectiveness analyses have been developed for specific interventions and populations. Here, we therefore develop a de novo decision-analytic model, as described below. We adopt a health service cost perspective for our analysis, and we assume that the costs of electronic interventions would be borne by the health service.
Population
The review identified two distinct populations for whom these interventions have been used:
-
Those making a committed quit attempt using the electronic aid as an adjunct to pharmacological interventions.
-
Those at an earlier stage in the quitting process who are less motivated and not using pharmacological interventions. For these smokers, we would expect successful quit rates to be lower. However, the progression to making a committed quit attempt in the future is a relevant secondary outcome.
A simplified model of the quit process is illustrated in Figure 34.
FIGURE 34.
Decision model for electronic aid interventions for those who are about to undertake a quit attempt (committed quit attempt) and those who are not intending to make such an attempt (non-committed quit attempt) at the time the intervention is provided.
Interventions
The need for, and derivation of, a classification system for electronic interventions is presented in the main effectiveness review. The five-level classification system developed for this purpose is set out in Table 21. However, the evidence synthesis exercise of Chapter 3 suggested that there is considerable uncertainty around the comparative benefits of different classes of intervention. Following the results of that exercise, we also consider ‘lumping’ together all electronic aid interventions as a single intervention. This does raise issues in identifying a single cost for this ‘lumping’ of such heterogeneous interventions.
Control scenarios
The decision problem for our economic analysis is defined as whether or not some form of electronic aid should be added to conventional behavioural support. This raises the question of what exactly the conventional intervention used for the control arm should be. A variety of control interventions were used in the identified effectiveness studies. Current guidance provided by NICE on technology appraisals140 defines the relevant comparators as routine practice in the NHS and best practice (where this is different to routine care). Regarding non-electronic behavioural support for smoking cessation, current NICE guidance recommends pharmacotherapy in conjunction with brief opportunistic advice or professional counselling. 12 In line with this recommendation, we define the control scenario for the decision analysis as pharmacotherapy with opportunistic advice, and explore the control scenario of counselling plus NRT in sensitivity analysis. In each case, the intervention is defined as electronic interventions as well as, rather than instead of, the conventional control.
Decision questions
Given the description of the decision context, the decision problem consists of several separate questions:
-
Are electronic aids, as adjunct to current best conventional practice, cost-effective interventions for smoking cessation in committed quitters?
-
If so, what type of electronic aid is most cost-effective in this population (i.e. which components as defined above)?
-
Are electronic aids cost-effective for smoking cessation in smokers not yet attempting to quit?
-
If so, what type of electronic aid is most cost-effective in this population?
-
Is there value in carrying out further research into electronic aids as adjunct therapy for smoking cessation (either in committed quitters or those not actively seeking to quit)?
Our intention was to answer each of these questions. However, the evidence synthesis exercise found considerable uncertainty in differentiating between classes of intervention, and between populations, which will have an impact on the extent to which this aim can be achieved.
Model specification
We use a decision tree model to assess the cost-effectiveness of electronic aid interventions for smoking cessation (see Figure 34). Decision tree models are well established in HTA for smoking cessation;141,142 our model draws from this previous work. This avoids duplication of effort and also ensures consistency with previous HTAs in the area of smoking cessation.
The appropriate intervention to support behaviour change in smokers will depend on their intentions. Pharmacological interventions, such as bupropion and NRT, assist those who are actively trying to quit. Some interventions are targeted instead at smokers who feel they are unwilling or unable to quit, and aim to encourage them to make an attempt to quit that otherwise they would not consider. Electronic smoking cessation aids can potentially be effective in both populations, depending on the specific details of the intervention. Figure 34 illustrates the decision tree we have developed to represent this distinction. For those smokers actively trying to quit, we split the process into a first stage of success or failure. There are a number of ways to define success – along with previous HTAs141,142 we define this as continuous abstinence over a period of 12 months. For those who achieve this, we assume only some will remain ex-smokers indefinitely. This is also in line with previous evaluations. We assume only those who avoid relapse gain health benefits as a result of the intervention, and draw on the literature to estimate the size of these benefits (see Life-years saved, on long-term health benefits of quitting smoking). Again, this is in line with previous economic analyses of smoking cessation interventions. 55,81,141
For interventions aimed at those not yet actively attempting to quit, we extend the base of the decision tree so that an intermediate end point is the proportion who go on to make such an attempt. Those who do can then progress through the decision tree for those attempting to quit, if we assume that the treatment effect on quit rates in this group is similar to that for those actively attempting to quit prior to receiving an electronic intervention.
Derivation of cost data for electronic interventions
The key additional inputs required for our model are the costs/resource use of the different categories of electronic aid interventions. It is possible to use the cost data from Smith et al. 136 to help populate this aspect of our decision model; however, this study refers to only one category of intervention. We therefore performed supplementary searches to identify any cost-effectiveness studies of similar types of interventions in different public health settings.
Review of additional evidence
Search methods
To identify such studies, several databases were interrogated:
-
All published HTA monographs up to volume 13(49) (published November 2009) were reviewed initially by title. This identified monographs where the use of relevant interventions may have been investigated. For each monograph, the executive summary was reviewed to determine whether or not any behaviour support interventions delivered electronically had been reviewed.
-
A similar review was carried out for all NICE technology appraisals, published up to November 2009 (nos. 1–187).
-
A search was carried out of the NHS EED using the query (internet OR electronic) AND (cost* OR resource*) to identify any economic evaluations or cost studies of the intervention type of interest.
Search results
The full search of HTA monographs revealed 26 publications that might potentially contain cost information on electronic health behaviour interventions (see Appendix 9). Further review of these reports identified two HTA monographs that described relevant interventions:
-
A review of the use of the ‘stages of change’ model in smoking cessation programmes. 143 This identified one trial of computer-based smoking cessation interventions but that trial did not report cost data.
-
A review of computerised cognitive behavioural therapy (CCBT). 144 This looked at a range of commercially available software packages that could deliver cognitive behaviour therapy. The cost per person depended on the throughput that could be achieved, as a large proportion of the costs were not directly tied in to individual clinical episodes. These included licensing costs (£34,000 per year for a 20-machine licence), hardware (£700 per machine) and staff training (£280 per machine over 2 years). Assuming that 750 patients per year could be treated by a set of 20 machines, this gave a cost per patient of £400.
The search of NICE technology appraisals revealed two reviews that might potentially contain relevant information:
-
TA102, which provided guidance on parent education and training regarding conduct disorder in children. The report looked at programmes comprising support by trained professionals alongside audiovisual and printed materials, but did not include an electronic element.
-
TA097, which provided guidance on CCBT, and reported the findings of Kaltenthaler et al. ,144 discussed above.
The search of the NHS EED found 187 articles that satisfied the search criteria. After a review of the descriptions provided on the database for each reference, this was reduced to eight articles for full paper review (see Appendix 10). Of them, five were found to include potentially relevant information:
-
An overview145 of the issues involved in internet-based delivery of intervention for long-term conditions. This identified eight reviews of internet-based interventions for conditions such as depression and cardiovascular disease. Four of the reviews mentioned in the overview investigated cost-effectiveness, one of which was the review of CCBT by Kaltenthaler et al. 144 Most of the costs associated with such interventions can be divided into two categories: development and maintenance. These costs tend to be fixed, so that the intervention is more likely to be cost-effective the greater the number of users. Murray145 concludes that such interventions have the potential to be highly cost-effective, but there is an absence of good health economic data in the field to confirm this.
-
Meenan et al. 146 describe a cost analysis of telephone and internet-based interventions to encourage the maintenance of weight loss. All resource use was costed in 2006 US dollars. The internet-based service included weekly weight-loss tips, a bulletin board, and a personal profile with an action plan that could be modified at any time. Tailored e-mail reminders encouraged regular use of the website, and there was also an IVR feature. The entire system cost US$840,000 to develop (US$805 per participant-year), of which US$750,000 were labour costs related to specification, programming and project management. Implementation costs were US$215,000 ($257 per participant-year), of which US$202,000 were labour costs related largely to additional programming and user support. These are significant costs, reflecting the complexity of the service provided.
-
Graham et al. 147 report a study of online advertising campaigns to increase demand for smoking cessation interventions. Advertising on the six websites used cost between US$470 and US$10,000 and delivered registrations to a smoking cessation service at a cost per registrant of between US$7 (Google) and US$476 (WebMD).
-
Runge et al. 148 report a study of a web-based patient education programme for asthmatic children and adolescents. Their website, which comprised an online quiz/game and information pages, was reported to cost 44 euros (2001 prices) per participant.
-
Southard et al. 149 describe an intensive internet-based case-management system for patients with cardiovascular disease. The system allowed patients to communicate with their nurse case manager over the web, and record personal information such as exercise completed and blood pressure. An online discussion site was also hosted, and the total costs per patient of the system was US$453, of which US$236 were labour costs for the nurse case manager.
The studies identified cover a range of situations and intervention types, and are not an exhaustive survey of such programmes. However, they do provide an indication of the range of costs that might be incurred by such programmes. The work by Graham et al. 147 illustrates the point made by Murray145 around the relationship between cost-effectiveness and take-up. 145 A comparison of the costs reported by the studies also suggests that increasing the complexity of the intervention, as might result from developing an interactive service, can add to costs substantially.
Development of cost scenarios
Given the heterogeneous nature of the interventions and the lack of information on resource use, it is difficult to provide precise cost estimates for the five classes of electronic intervention. In response, we developed five scenarios for these costs. The primary source for this was the study by Meenan et al. ,146 corroborated by findings from Runge et al. ,148 Smith et al. 136 and Southard et al. 149 These scenarios are presented in Table 26 and their impact explored in sensitivity analyses.
| Scenario | Electronic intervention class: costs (£) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Base case | 7.70 | 30.10 | 14.70 | 44.80 | 77.00 |
| High cost to tailoring | 7.70 | 30.10 | 32.20 | 62.30 | 77.00 |
| Limited savings | 30.10 | 44.80 | 14.70 | 59.50 | 77.00 |
| Intensive support | 7.70 | 30.10 | 14.70 | 44.80 | 270.20 |
| Mass uptake | 0.70 | 0.70 | 0.28 | 0.98 | 2.10 |
Base-case scenario
The Meenan et al. study146 involves substantial interactivity, the use of multiple channels of communication (website, e-mail, bulletin board, IVR) and some tailoring of responses. It can therefore be seen as mirroring the most resource-intensive intervention within the classification system. Estimates of the costs for less intensive interventions can be generated by removing or down-weighting components from the costings provided by Meenan et al. 146 A total of 348 participants used the service for 30 months, a level set by the design of the study. The authors state that they were advised that the system could comfortably cope with at least 3500 simultaneous participants. Assuming that participants use the system for a maximum of 12 months, and that it has a life span of 3 years, gives a maximal user load of around 10,000 participants. The total cost of the system was US$1.1M, which therefore implies a cost per user of a system of this type of around US$110.
We used this in our base case as the cost of the most intensive class of electronic intervention (class five). For the costs of system with multiple generic contacts (class two), we removed from this the cost of the IVR (US$191,000), user support (US$68,000) and 50% of the labour costs (US$411,000). This gave a remaining cost of US$430,000 or US$43 per user. For the most basic type of intervention (class one) we assume that the labour costs would be a further 40% lower (US$329,000). This gives a cost of US$101,000 or US$10 per user.
One remaining issue is how to cost the addition of tailoring. This is effectively included in the full cost given by Meenan et al. ,146 but could be seen as part of the cost reduction applied in calculating costs for the less-intensive interventions. The Smith et al. study136 examined a pure tailoring intervention, and gave a cost of US$46 per smoker, based on 136 smokers participating per year. This reflected the cost of installing expert computer-based systems in participating GP practices, a resource-intensive delivery method. If added to a web-based system, the cost of tailoring responses is likely to be lower. A reduction of 50% in non-user-support labour costs was applied to derive the costs for an intermediate NT intervention. If we assume that 50% of this value relates to the development and implementation of tailoring algorithms, the result is a cost per user for tailoring of 0.5 × 411,000/10,000 = US$21 per smoker.
This gives the cost for the class three electronic intervention. We assume the cost for the remaining intervention, class four, is equal to the sum of class two and class three costs. All costs are converted to 2006 GBP (British pound sterling) using Organisation for Economic Co-operation and Development (OECD) purchasing power indices150 and then inflated to 2009 GBP using a health-care cost inflation index. 151
Additional scenarios
We have been required to make assumptions without adequate supporting evidence to derive base-case costs. We explore alternative cost assumptions in four scenarios:
-
High cost to tailoring The base-case estimates this as US$21 per person. This scenario assumes the value to be that given by Smith et al. 136 (US$46 per user). As a result, the costs are increased for intervention classes four and five.
-
Limited savings case The base case assumes that a less complex intervention will lead to substantial savings in development time and reduced labour costs of 50% (intermediate) or 90% (basic). This case assumes the relevant values are 25% and 50%.
-
Intensive support case This case assumes that intensive support from a trained professional will be included in the complex intervention, and costs it at the rate given by Southard et al. 149 (US$236 per patient).
-
Mass uptake case Meenan et al. 146 show that the fixed costs of electronic interventions form a high percentage of the total cost, and that scaling up capacity is often quite cheap to achieve. Trials may therefore overstate the costs of such interventions without any adjustment for increased use. The base case allows for this to some extent, with 10,000 users assumed. Expert opinion suggests that the potential uptake of any intervention could be up to 10% of UK smokers, approximately 1 million users, and the maintenance costs for a website reaching this size of population would be around £100,000 per year (Robert West, University College London, 2010, personal communication). This assumes that uptake is 500,000, and that economies of scale reduce total cost accordingly.
Table 26 illustrates the impact of varying the assumptions as described.
Long-term medical costs
On the one hand it has been argued that smokers increase health-care costs because of increased incidence of smoking-related diseases. 152 On the other hand, it has also been argued that smokers have a lower life expectancy and so the health-care burden in the elderly is subsequently reduced in the long term. 153 Some have suggested that these two factors may in effect cancel out, so that the net change in long-term cost is small. 154 As there is no consensus on this issue, we make the assumption that there are no long-term cost implications as a result of quitting, only health benefits. This is in line with previous economic evaluations141 of aids to smoking cessation.
Additional model inputs
Twelve-month continuous cessation rates for baseline interventions
The evidence synthesis provides estimates of treatment effects as HRs from a Weibull time-to-relapse model. In that exercise we compared a model with independent treatment effects with a model where the effect of intervention, on the log-hazard scale, was additive (see Chapter 3, Overview of evidence and the mixed-treatment comparison approach). As the additive model was found to provide a better fit than either of the models allowing for interactions, we assume that the effectiveness of the electronic aid interventions is the same regardless of the control arm intervention. In other words, the effect of the electronic aid intervention is additive when used as an adjunct to pharmacological and/or counselling control interventions. We convert the HR to a RR of 12-month continuous abstinence using the Weibull model. If the probability of 12-month continuous abstinence for the control and the control plus intervention are defined as pc(12) and pi(12):
From the time-to-relapse model:
where h is the HR for the intervention vs placebo. This gives
We represent uncertainty around the baseline probability of 12-month continuous abstinence by assigning the parameter a normal distribution, truncated at 0, with mean 6% and standard deviation 1.6% (brief advice), or mean 12% and standard deviation 1.4% (counselling). The odds ratio for nicotine therapy is assumed to be normally distributed with mean 1.67 and standard deviation 0.06. These estimates were taken from previous economic evaluations of conventional therapies. 141,142
Lifetime cessation rates
The quit process is not always straightforward – some may relapse after a considerable period of abstinence, whereas others may achieve permanent cessation after several failed short-term attempts. A common approach in economic evaluations of smoking cessation interventions is to define the initial outcome in terms of the proportion achieving continuous abstinence for 12 months, but then to assume that a proportion will subsequently relapse. Furthermore, it is often assumed that long-term relapse rates are similar across intervention, and that health benefits only accrue to those that avoid relapse. We follow this approach in our base case.
Trials commonly follow participants and report results up to a fixed point in time, so that observational data are required to extrapolate their outcomes to permanent quit rates. Woolacott et al. 141 carried out a review of estimates of the proportion who maintain continuous abstinence for 12 months but subsequently relapse. The mean reported rate was 40%, and the estimates ranged from 30% to 50%. In an analysis of the use of genetic information to target pharmacotherapy, Welton et al. 155 capture this information by assigning to it a beta(38,57) distribution, which has a mean of 40% and a 95% CI of 30% to 50%. 155 We use this distribution in our cost-effectiveness analyses.
Life-years saved
To fully inform decision-making, an estimate of the long-term health impact of quitting is required. This is a complex parameter and difficult to assess. 141 At an individual level, the risk reduction achieved by quitting will depend on several factors. These include the age at which the smoker quits, the length of time they had been a smoker, the number of cigarettes smoked and the gender of the smoker. 156 The average benefit for the relevant population will further depend on its composition. The calculation is complicated by the wide range of conditions for which smoking is a risk factor.
Woolacott et al. 141 identified 17 economic evaluations of smoking cessation therapies, of which 12 use a value for life-years or QALYs gained by quitting. The average discounted QALY gain reported was 2.7, with a range of 1.35–4.07 QALYs gained per quitter. Welton et al. 155 capture this range of estimates for the discounted QALY gain by assigning it a normal distribution, truncated at 0, with mean 2.7 and variance 0.47, and we use this distribution for the parameter in our model. This approach allows us to represent the uncertainty around this parameter, although it does have limitations which are discussed below (see Limitations of the analysis and recommendations for further research).
Discounting and time horizon
We take a lifetime perspective for the economic analysis. However, for reasons given above (see Long-term medical costs), we limit our costs to the immediate resource requirements of the intervention, which are short term. The only long-term component of the costs and benefits arising in the model relate to the long-term health benefits of quitting. As described above (see Life-years saved), the distribution used for this benefit is based on a range of estimates from the literature, all of which discount health benefits. The distribution therefore reflects a discounting of these future health gains. The source studies for this distribution do not use a consistent rate for discounting health benefits – an attempt to adjust each estimate for the rate used was considered, but given the considerable uncertainty around this measure, such an adjustment would be unlikely to materially alter the distribution we use to represent this uncertainty.
Results of the cost-effectiveness analysis
Summary of analyses
As outlined above (see Decision questions), there are several questions that arise in determining the cost-effectiveness of electronic interventions. We began by considering the population of those actively seeking to quit. We first explored whether or not electronic interventions were cost-effective in this group when considered as a single class of intervention. To analyse this, we used the results from the evidence synthesis arising when all classes are assumed to be equally effective (Model 4 of Table 25). Given the heterogeneity of these interventions, we carried out a cost threshold analysis. This involved deriving the distribution for QALYs gained, and determining the maximum cost at which the intervention was cost-effective at commonly quoted thresholds (£20,000–30,000 per QALY). As described above (see Control scenarios), we consider electronic intervention as an adjunct to two possible conventional interventions: (1) pharmacotherapy plus brief advice and (2) pharmacotherapy plus professional counselling.
We then explore the question of which class of intervention should be chosen, based on cost-effectiveness, if a decision is made to implement an electronic intervention of some type. For this, we draw on the results of the evidence synthesis arising when separate effects are assumed for the different classes of electronic intervention (see Model 3 of Table 25). As with the single intervention case, we carry out separate analyses for the two possible conventional best practice interventions. We also carry out separate analyses exploring the five cost scenarios developed above (see Derivation of cost data for electronic interventions).
Having explored cost-effectiveness in those actively seeking to quit, we consider those who are not committed to such an attempt. Our approach to this question is based on the result found in the evidence synthesis, that there is no evidence to suggest the relative effect of electronic interventions in this group. We allow for the difference by adjusting the conventional intervention used in the control arm. As these are participants who are not seeking to quit, we assume that they will not turn to the two control interventions described above. Instead, we reperform our analysis, based on spontaneous quit rates, which are estimated at 1% over 12 months. 141 We explore the cost-effectiveness of electronic interventions as a single class in this population, as there is insufficient evidence to comment on what type of electronic intervention might be particularly suitable for this population.
Results for those actively seeking to quit
Cost-effectiveness of electronic intervention compared with no electronic intervention
Table 27 gives an analysis of the economic impact of implementing an electronic intervention alongside a conventional control. The mean QALY gain depends on the choice of conventional therapy (0.053 for brief advice, 0.069 for counselling). The lower bound of the 95% credible interval only just includes negative values, reflecting a small possibility that the addition would actually reduce long-term quit rates. As we are considering a broad class of interventions with a wide range of plausible costs, we carry out a threshold analysis to explore cost-effectiveness. This involves multiplying any desired willingness-to-pay (WTP) threshold by the mean QALY gain to determine the cost above which the therapy is no longer cost-effective. Table 27 gives the results of these calculations at WTP thresholds of £20,000 and £30,000 per QALY – the results in every case are well above the costs explored in the scenarios of Table 26.
| Control | QALY gain from electronic intervention | Maximum acceptable cost (£) | ||
|---|---|---|---|---|
| Mean | Credible interval | £30,000/QALY | £20,000/QALY | |
| Pharmacotherapy + brief advice | 0.053 | –0.016 to 0.152 | 1579 | 1053 |
| Pharmacotherapy + counselling | 0.069 | –0.023 to 0.190 | 2081 | 1387 |
To illustrate the uncertainty around the cost-effectiveness results, we construct cost-effectiveness acceptability curves (CEACs) for the two control scenarios. This requires some assumption to be made around the cost of the intervention. We present CEACs based on assuming this cost to be either £10, £50 or £90 per user in Figure 35, as this reflects the range of values included in our base case for the costs of the electronic intervention types. In all cases considered, the probability of cost-effectiveness reaches 90% before the WTP reaches £20,000/QALY, and remains below 95%, reflecting the probability that the electronic intervention reduces long-term quit rates.
FIGURE 35.
(a) CEACs for electronic intervention vs NRT plus brief advice. (b) CEACs for electronic intervention vs NRT plus counselling. Three CEACs are shown based on different assumptions for the cost of the intervention.
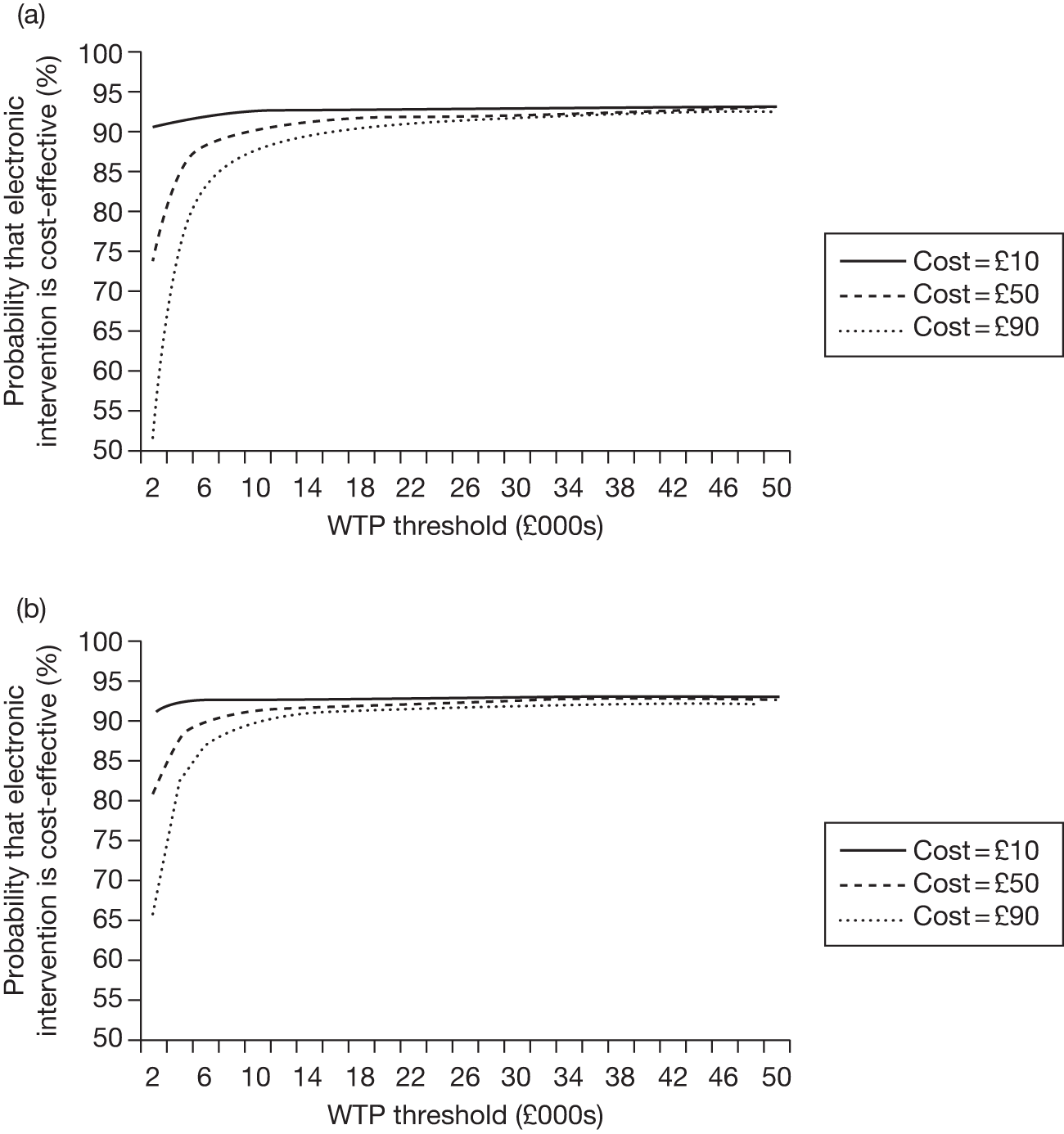
Comparative cost-effectiveness of electronic interventions
We also carried out an analysis of the comparative cost-effectiveness of the five electronic interventions. The results of this exercise are given in Table 28 (where the conventional intervention is pharmacotherapy plus brief advice) and Table 29 (where the conventional intervention is pharmacotherapy plus counselling). Results are given for each of the five cost scenarios listed in Table 26. They show that for all scenarios except high cost to tailoring, e3 interventions (single tailored component) dominate (provide more QALYs at less cost) e4 (multiple components including a single tailored component) and e2 (multiple generic components) interventions. Even in the exceptional scenario, the ICERs for e3 (single tailored component) interventions compared with e2 (multiple generic components) interventions are very favourable (£55/QALY as an adjunct to brief advice, £40/QALY as an adjunct to counselling). The e3 (single tailored component) interventions compare unfavourably with e1 (single generic component) interventions – the latter either dominates or provides additional benefits at an ICER of ≤ £4400/QALY). In turn, the e5 (multiple tailored components) interventions appear cost-effective compared with e1 (single generic component) interventions in most scenarios when added to either conventional intervention. The least favourable scenario for e5 (multiple tailored components) is the intensive support scenario, under which the ICER for e5 compared with e1 is £28,000/QALY when added to brief support, and £18,000 when added to counselling.
| Class of electronic intervention | Cost of intervention (£) | Mean QALYs gained | Cost (£)/QALY vs conventional only | ICER (£ per QALY) |
|---|---|---|---|---|
| e4 | 44.80 | 0.0004 | 112,000 | Dominated |
| e2 | 30.10 | 0.0145 | 2076 | Dominated |
| e3 | 14.70 | 0.0525 | 280 | Dominated |
| e1 | 7.70 | 0.0576 | 134 | 134 |
| e5 | 77.00 | 0.0669 | 1151 | 7452 |
| Class of electronic intervention | Cost of intervention (£) | Mean QALYs gained | Cost (£)/QALY vs conventional only | ICER (£ per QALY) |
|---|---|---|---|---|
| e4 | 62.30 | 0.0004 | 155,750 | Dominated |
| e2 | 30.10 | 0.0145 | 2076 | Dominated |
| e3 | 32.20 | 0.0525 | 613 | Dominated |
| e1 | 7.70 | 0.0576 | 134 | 134 |
| e5 | 77.00 | 0.0669 | 1151 | 7452 |
| Class of electronic intervention | Cost of intervention (£) | Mean QALYs gained | Cost (£)/QALY vs conventional only | ICER (£ per QALY) |
|---|---|---|---|---|
| e4 | 59.50 | 0.0004 | 148,750 | Dominated |
| e2 | 44.80 | 0.0145 | 3090 | Dominated |
| e3 | 14.70 | 0.0525 | 280 | 280 |
| e1 | 30.10 | 0.0576 | 523 | 3020 |
| e5 | 77.00 | 0.0669 | 1151 | 5043 |
| Class of electronic intervention | Cost of intervention (£) | Mean QALYs gained | Cost (£)/QALY vs conventional only | ICER (£ per QALY) |
|---|---|---|---|---|
| e4 | 44.80 | 0.0004 | 112,000 | Dominated |
| e2 | 30.10 | 0.0145 | 2076 | Dominated |
| e3 | 14.70 | 0.0525 | 280 | Dominated |
| e1 | 7.70 | 0.0576 | 134 | 134 |
| e5 | 270.20 | 0.0669 | 4039 | 28,226 |
| Class of electronic intervention | Cost of intervention (£) | Mean QALYs gained | Cost (£)/QALY vs conventional only | ICER (£ per QALY) |
|---|---|---|---|---|
| e4 | 0.98 | 0.0004 | 2450 | Dominated |
| e2 | 0.70 | 0.0145 | 48 | Dominated |
| e3 | 0.28 | 0.0525 | 5 | 5 |
| e1 | 0.70 | 0.0576 | 12 | 82 |
| e5 | 2.10 | 0.0669 | 31 | 151 |
| Class of electronic intervention | Cost of intervention (£) | Mean QALYs gained | Cost (£)/QALY vs conventional only | ICER (£ per QALY) |
|---|---|---|---|---|
| e4 | 44.80 | –0.0034 | Dominated | Dominated |
| e2 | 30.10 | 0.0171 | 1760 | Dominated |
| e3 | 14.70 | 0.0693 | 212 | Dominated |
| e1 | 7.70 | 0.0728 | 106 | 106 |
| e5 | 77.00 | 0.0874 | 881 | 4756 |
| Class of electronic intervention | Cost of intervention (£) | Mean QALYs gained | Cost (£)/QALY vs conventional only | ICER (£ per QALY) |
|---|---|---|---|---|
| e4 | 62.30 | –0.0034 | Dominated | Dominated |
| e2 | 30.10 | 0.0171 | 1760 | Dominated |
| e3 | 32.20 | 0.0693 | 465 | Dominated |
| e1 | 7.70 | 0.0728 | 106 | 106 |
| e5 | 77.00 | 0.0874 | 881 | 4756 |
| Class of electronic intervention | Cost of intervention (£) | Mean QALYs gained | Cost (£)/QALY vs conventional only | ICER (£ per QALY) |
|---|---|---|---|---|
| e4 | 59.50 | –0.0034 | Dominated | Dominated |
| e2 | 44.80 | 0.0171 | 2620 | Dominated |
| e3 | 14.70 | 0.0693 | 212 | 212 |
| e1 | 30.10 | 0.0728 | 413 | Dominated (extended) |
| e5 | 77.00 | 0.0874 | 881 | 3442 |
| Class of electronic intervention | Cost of intervention (£) | Mean QALYs gained | Cost (£)/QALY vs conventional only | ICER (£ per QALY) |
|---|---|---|---|---|
| e4 | 44.80 | –0.0034 | Dominated | Dominated |
| e2 | 30.10 | 0.0171 | 1760 | Dominated |
| e3 | 14.70 | 0.0693 | 212 | Dominated |
| e1 | 7.70 | 0.0728 | 106 | 106 |
| e5 | 270.20 | 0.0874 | 3093 | 18,016 |
| Class of electronic intervention | Cost of intervention (£) | Mean QALYs gained | Cost (£)/QALY vs conventional only | ICER (£ per QALY) |
|---|---|---|---|---|
| e4 | 0.98 | –0.0034 | Dominated | Dominated |
| e2 | 0.70 | 0.0171 | 41 | Dominated |
| e3 | 0.28 | 0.0693 | 4 | 4 |
| e1 | 0.70 | 0.0728 | 10 | Dominated (extended) |
| e5 | 2.10 | 0.0874 | 24 | 101 |
These results relate to the mean estimates of cost-effectiveness. As described in the main effectiveness review (see Chapter 2) and in the mixed-treatment comparison (see Chapter 3), there is considerable uncertainty around the comparative efficacy of the different classes of electronic intervention, and this translates into uncertainty around the mean estimates given in Tables 28 and 29.
Table 28 shows the cost-effectiveness results (under five cost scenarios) for electronic interventions as an adjuvant to pharmacotherapy plus brief advice.
Table 29 shows cost-effectiveness results (under five cost scenarios) for electronic interventions as an adjuvant to pharmacotherapy plus counselling.
Figure 36 gives the CEACs illustrating the likelihood that each intervention class is the most effective, depending on the value chosen for the WTP threshold. CEACs are shown for each scenario, assuming that the therapy is added to pharmacotherapy and brief advice (curves for the alternative control scenario are almost identical).
FIGURE 36.
Cost-effectiveness acceptability curves illustrating the probability that each class of electronic intervention is the most cost-effective, given different assumed WTP thresholds.
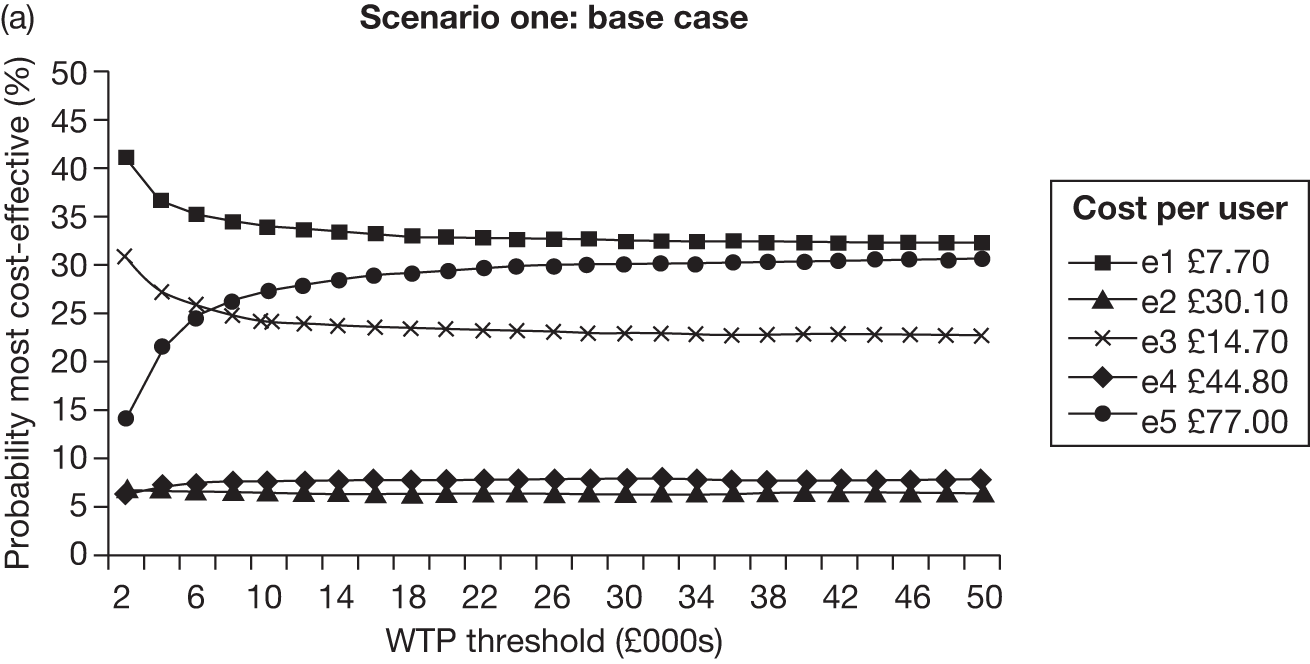
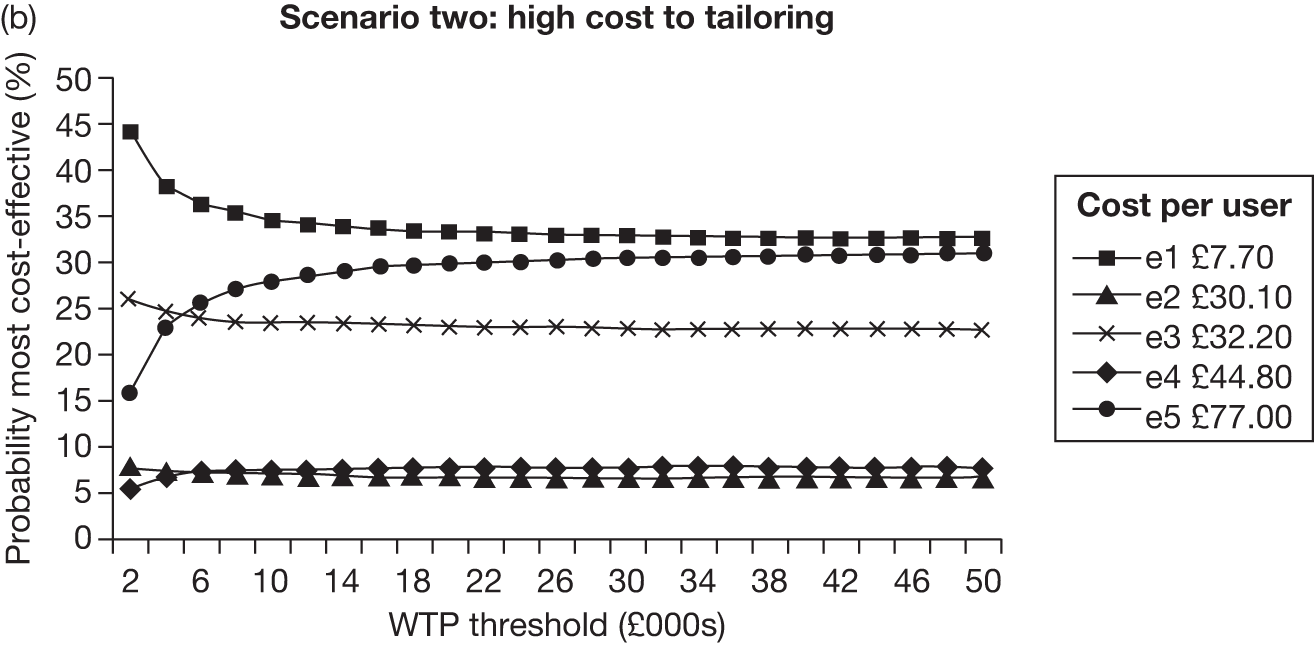
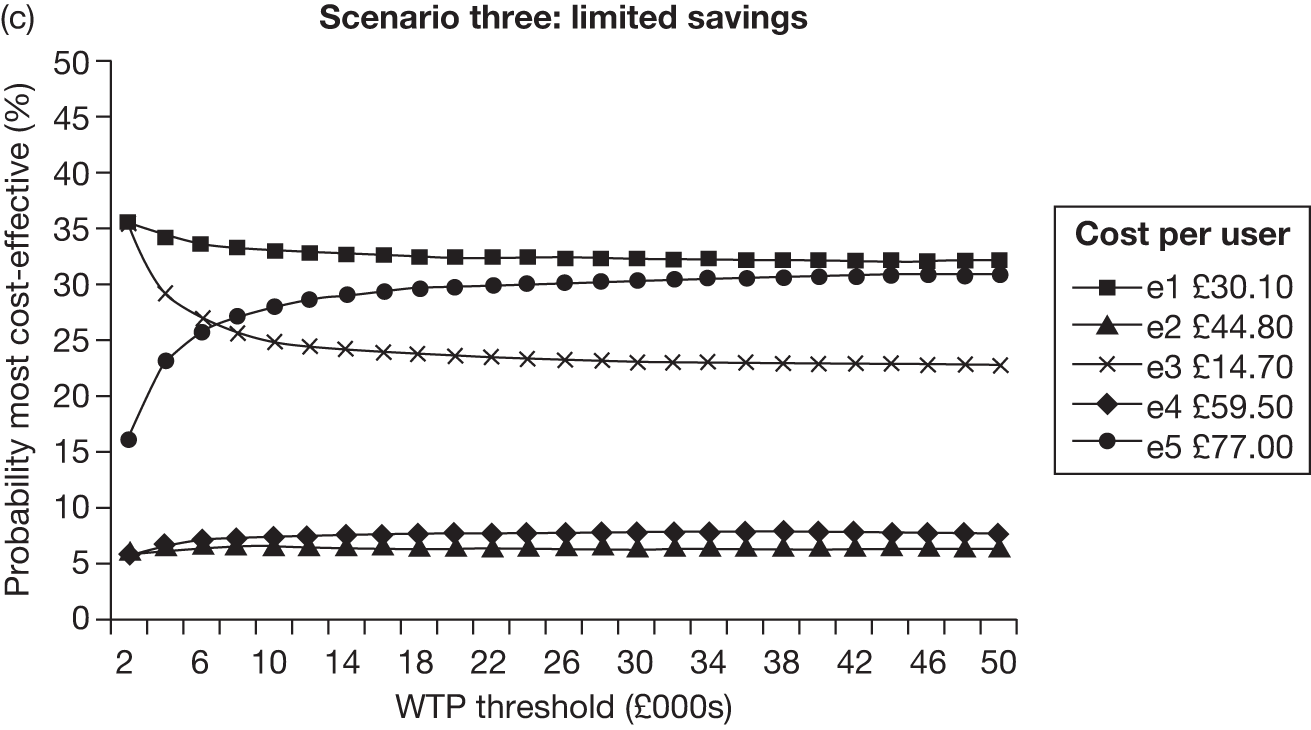
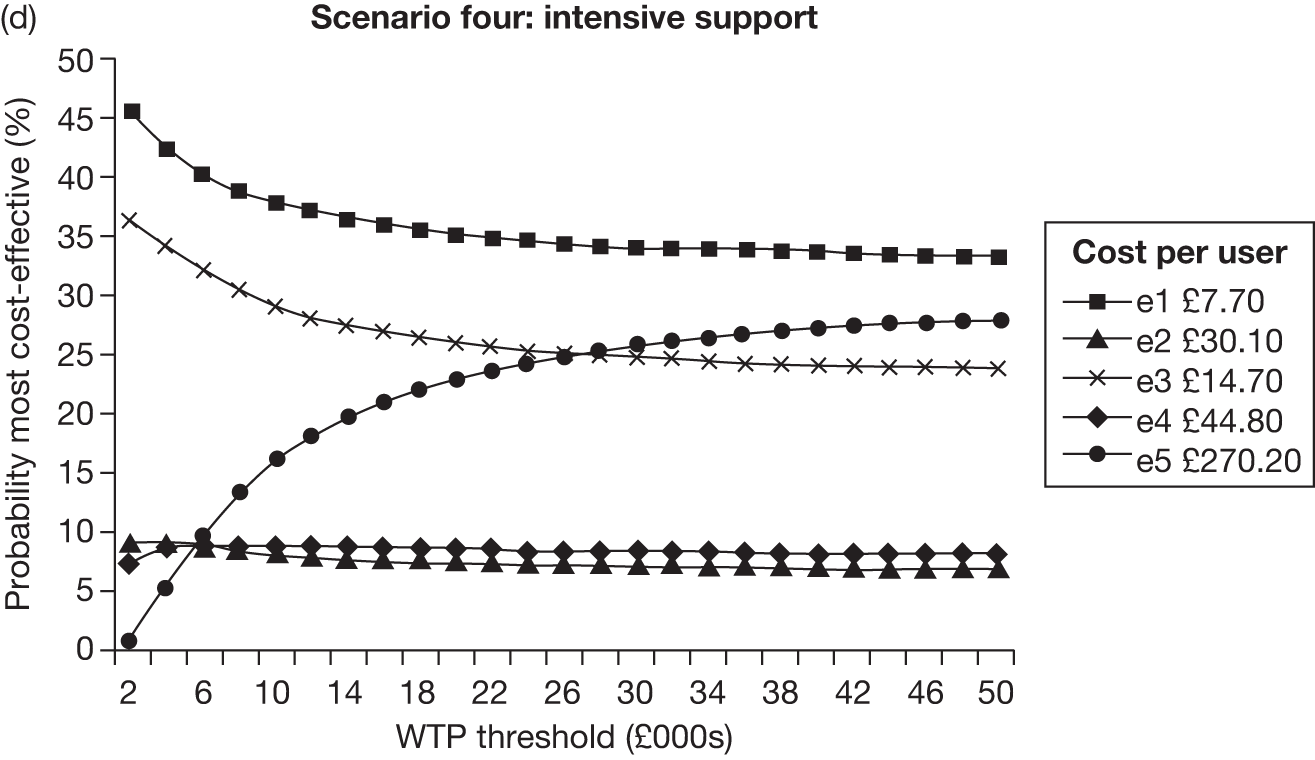
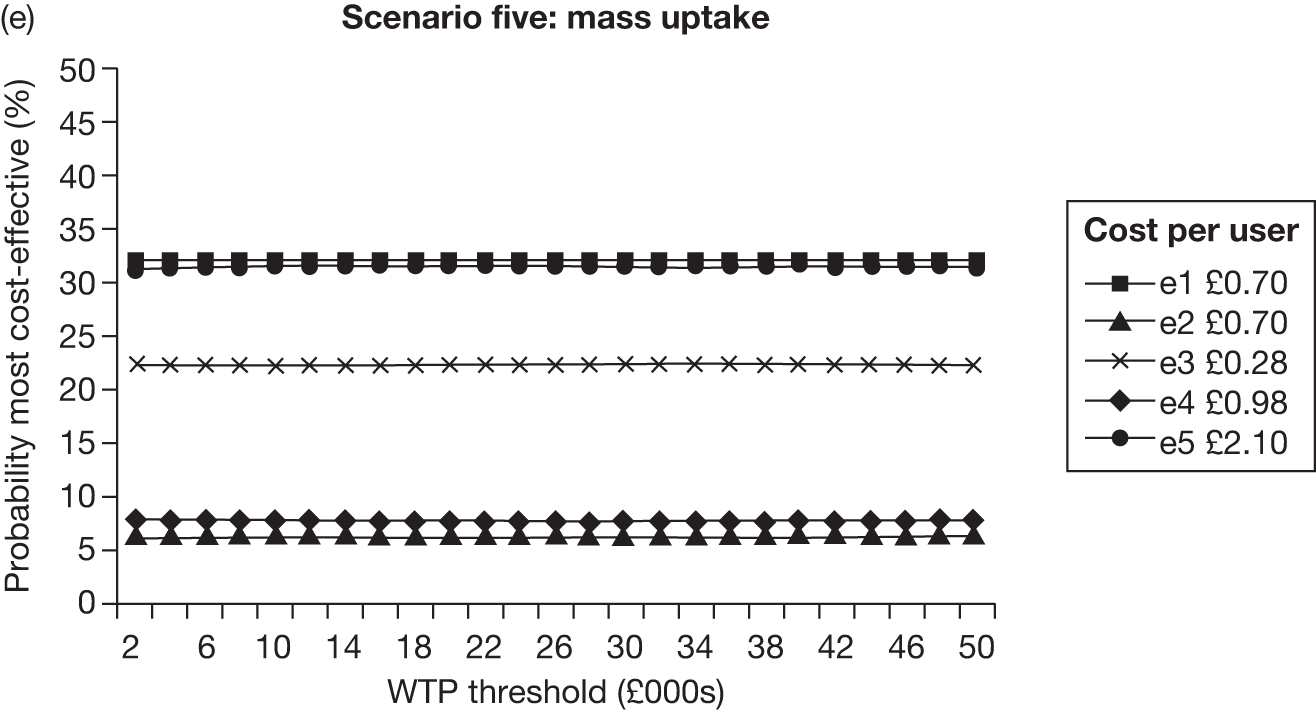
The CEACs illustrate the considerable uncertainty around which of the five classes of intervention should be preferred on cost-effectiveness grounds. This uncertainty is reflected across the cost scenarios, suggesting that it is the lack of information on efficacy that drives the uncertainty. Although class five interventions had the most favourable mean cost-effectiveness, the probability that they are indeed the most cost-effective class is around 30–35%. The probability that class one interventions are the most cost-effective tends to be similar, if not higher, and is favoured by lower WTP thresholds and the intensive support scenario. The probability that class three interventions are the most cost-effective tends to be lower, but still considerable, at 20–30%. Even class two and class four interventions, which are dominated by the other classes in almost every scenario at the mean, have a probability of cost-effectiveness of between 5% and 10%.
Results for those not actively seeking to quit
The evidence synthesis found that the relative treatment effect of electronic interventions did not differ between trials in those actively seeking to quit and trials in other populations. To explore the cost-effectiveness of electronic aids in this group, we repeated the analysis described in Results of the cost-effectiveness analysis, Summary of analyses. As in that section, we use the relative treatment effect arising from the assumption that electronic aids form a single class of intervention. The only difference is that we assume that smokers not actively seeking to quit would not seek out conventional interventions. Therefore, we apply the same treatment effect, but use a different baseline quit rate, reflecting spontaneous unsupported quitting. The 12-month quit rate in this situation has been estimated at 1%,141 and we apply the HR for electronic intervention to this rate as described in the section Twelve-month continuous cessation rates for baseline interventions.
We found the mean QALY gain from implementing an electronic intervention in this population to be 0.014 (95% credible interval 0 to 0.04). This is considerably less than we predict in the population of smokers actively seeking to quit. We carried out a cost-threshold analysis as described in the section Results for those actively seeing to quit, above, and found that the electronic intervention would not be cost-effective if it cost more than £271 (assuming a WTP of £30,000/QALY) or £406 (assuming a WTP of £20,000/QALY). This is considerably less than the threshold for smokers willing to quit but still well above the values in the various cost scenarios.
As in Results for those actively seeing to quit, above, we developed CEACs to represent the uncertainty around this finding. The CEAC for this population is presented in Figure 37. The probability of cost-effectiveness at a WTP threshold of £20,000/QALY is 77% if the cost per user is £90, rising to 91% if this cost is £10.
FIGURE 37.
Cost-effective acceptability curve for electronic intervention vs placebo in a population of smokers not actively seeking to quit. A 1% spontaneous quit rate is assumed for the comparator. CEACs are presented assuming costs of the electronic intervention of £10, £50 and £90.
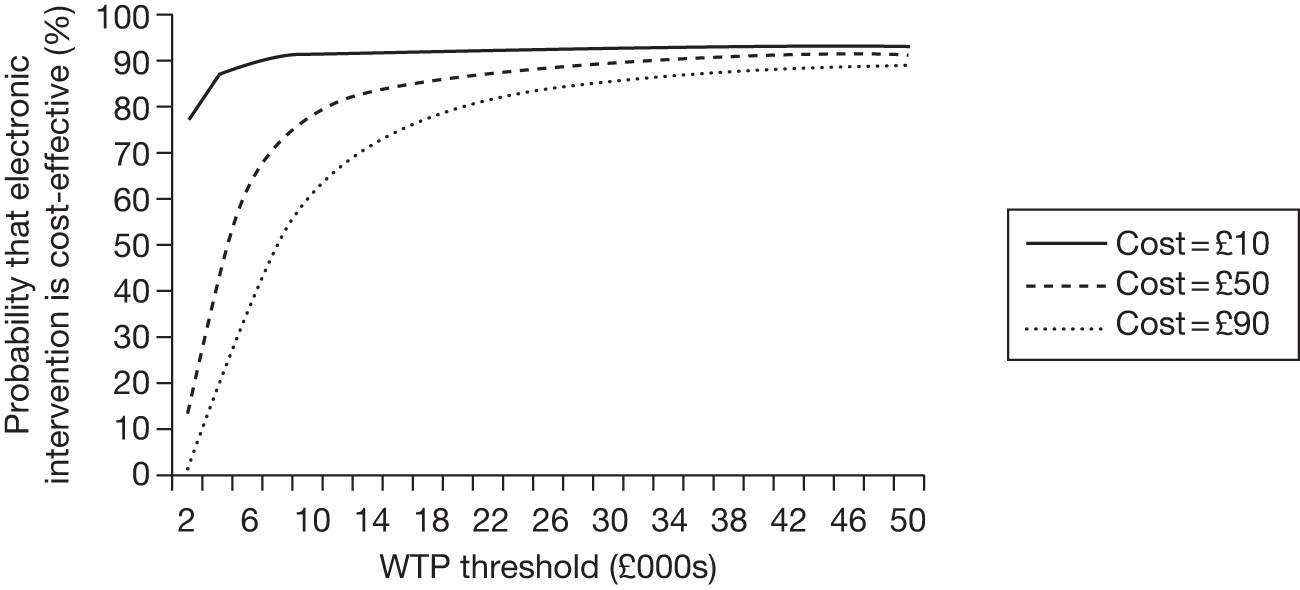
Estimated value of additional information
The results shown above suggest that adding an electronic aid of some sort to current conventional support for smokers attempting to quit would be cost-effective, and that the intensive class five intervention would be expected to maximise cost-effectiveness. We have shown that there is considerable uncertainty around this finding, particularly regarding the choice of class of intervention. One response to this uncertainty would be to delay the choice until further information was available. Value-of-information (VOI) methods provide a framework for determining whether or not this is an advisable course of action. 157
Value-of-information methods assess the likelihood that additional information will lead to a change in the decision, and provide an estimate of the expected loss from acting without that information. The expected value of perfect information (EVPI) is this estimate when the additional information provides exact values for all model parameters and eliminates all uncertainty. We calculate the EVPI first assuming that the treatment effect is identical across electronic intervention classes, and used a uniform (0,200) distribution to represent our uncertainty about cost. The resulting estimate, in terms of benefit per person, depends on the threshold uses to assign a monetary value to health benefits. At a value of £20,000 per QALY, the EVPI was £31 per person if the comparator includes brief advice, or £41 per person if the comparator included counselling.
We repeated the exercise based on the evidence synthesis model allowing separate treatment effects across electronic intervention classes. We explored the impact of different conventional comparators and cost scenarios, and also compared the EVPI assuming the current decision would be in favour of class one interventions or class five interventions. We found that the EVPI was much higher in this case, as we would expect given the additional uncertainty around the comparative efficacy of intervention classes. The strongest factor affecting the EVPI was the choice of comparator: when it included brief advice, estimates (given a threshold of £20,000 per QALY) ranged from £2047 to £2132 per person; when it included counselling, this rose to £2643–2802. As can be seen in these ranges, the other factors had comparatively little impact on EVPI.
The EVPI provides an upper bound on the benefit of conducting future research, as such research is unlikely to reduce uncertainty completely. The benefit is also proportional to the relevant population affected by the decision, and the choice of timescale to assess the decision. Given the large numbers that are eligible for smoking cessation support, this suggests that even a large study would be cost-effective. In 2009–10, over 750,000 smokers in England used NHS SSS (reference statistics on NHS SSS England 2009–10, The NHS Information Centre; lifestyle statistics available at www.ic.nhs.uk/webfiles/publications/Health%20and%20Lifestyles/SSS_2009_10_revised.pdf). This indicates the potential reach of electronic intervention, although it is not possible to predict how extensive their uptake would be. Assuming that electronic interventions would reach 20% of these users, that the decision time horizon is 5 years, and applying a discount rate of 3.5%, the population EVPI would be approximately 700,000 times higher than the EVPI per person results quoted above. Therefore, population EVPI would lie between £1.4B and £2B. Even if it is assumed that all electronic interventions are equally effective, the population EVPI would be approximately £20–30M. Table 30 further illustrates the sensitivity of population EVPI to assumptions regarding uptake and the time horizon. These are EVPI estimates, so the expected benefit of a specific study would be lower. Also, these estimates are sensitive to assumptions around the decision population and time horizon that must be based on limited robust evidence. Nevertheless, they illustrate the potential value in conducting further research, particularly on the relationship between the design of electronic interventions and their efficacy.
| Electronic treatment effects | Time horizon (years) | EVPI, assuming treatment reaches x users per year | |||
|---|---|---|---|---|---|
| x = 50,000 | x = 100,000 | x = 250,000 | x = 500,000 | ||
| Single | 5 | £7.2M | £14.4M | £36.1M | £72.3M |
| 10 | £14.4M | £29.0M | £72.3M | £144.5M | |
| Multiple | 5 | £490M | £979M | £2.5B | £4.9B |
| 10 | £979M | £2.0B | £4.9B | £9.8B | |
Discussion
Our results suggest that making some form of electronic support available to smokers actively seeking to quit is highly likely to be cost-effective (Table 27). This is true whether the electronic intervention is delivered alongside brief advice or more intensive counselling. It is less clear, from the available evidence, what form that electronic support should take. The key source of uncertainty is that around the comparative effectiveness of different types of electronic interventions. Although there were also challenges in costing different types of electronic intervention, our sensitivity analyses suggest that these are unlikely to drive cost-effectiveness results unless additional evidence were to show that the design of an electronic intervention has close to zero impact on its efficacy.
Our results also suggest that such aids may be cost-effective in populations of smokers not actively looking to quit, based on the finding that the efficacy of electronic interventions is similar in such populations compared with those actively seeking to quit. However, this is only a tentative finding, based on the information available at present. There are few studies that have specifically explored efficacy in smokers not willing to quit. Also, an approach we would have preferred to take to answer this question is to estimate the impact of electronic aids in causing a quit attempt to be made where the motivation did not previously exist, and then compare the success rate in this case with that for smokers already actively looking to quit. Further information is required for such an approach to be viable and useful.
Limitations of the analysis and recommendations for further research
A limitation of the cost-effectiveness analysis, shared with several previous cost-effectiveness analyses of smoking cessation interventions,104,105 is that intervention benefit is restricted to the first quit attempt. Thus even if those who fail at the first attempt go on to quit permanently at some future attempt, such attempts do not need to be accounted for, as we are assuming that the probability of success in those attempts is unchanged by exposure to the intervention. This assumption simplifies the model considerably, as it allows us to ignore subsequent quit attempts following relapse. However, the numbers of those who sustain their first quit attempt to permanent abstinence is small. If some of those who fail their first quit attempt go on to make a future quit attempt that is successful and interventions increase the success rate of these subsequent attempts then we will have underestimated the benefits of effective treatments. We would have liked to explore this issue using a more realistic model that included the impact of interventions on subsequent attempts. Unfortunately, such models require more detailed information on patient event histories than is available from the evidence base currently available.
To translate the efficacy estimates of Chapter 3 into estimates of cost-effectiveness, the model requires information that cannot be obtained from the trials identified in our systematic review. This includes information on the long-term relapse rate, and the QALY gain associated with permanently quitting smoking. A detailed investigation of the evidence around these parameters was beyond the scope of this work. Instead, we have based our estimates on evidence from previous evaluations of smoking cessation interventions, and used distributions to capture the strength (or weakness) of evidence in that work on the parameters of interest. There are limitations to this approach. A more extensive analysis might have allowed us to uncover new evidence, or identify biases and other factors driving divergent estimates in the existing literature. The studies underpinning estimates of the QALY gain from quitting, for example, vary in terms of the data used and the choice of discount rate for health benefits (which range from 0–6%). It may well be, therefore, that our approach overestimates the uncertainty around these parameters. However, our results already show that electronic interventions of some type are so likely to be cost-effective that it is unlikely that better estimates of long-term relapse and QALY gains would change that conclusion.
A further limitation of our analysis is that we have not included reductions in smoking-related health costs in our analysis. This is in line with previous evaluations (Woolacott et al. 141) found that most of the cost-effectiveness studies identified in their review of the literature on smoking cessation interventions excluded such costs. The justification for this was that there is uncertainty as to whether or not such costs might be offset by the increased costs of providing other health services, pension costs and reduced tax revenue. However, it is not appropriate to include these offsetting costs – the costs of unrelated diseases are generally excluded in economic evaluations, and tax revenues are merely transfer payments. Our main reason for excluding any reductions in smoking-related health-care costs for quitters was the lack of robust estimates of this parameter in the literature, and the fact that the present value of such savings will be reduced substantially by discounting. We may therefore have underestimated the cost-effectiveness of effective interventions. However, the threshold analysis presented above (see Cost-effectiveness of electronic intervention compared with no electronic intervention) suggests that the costs of electronic interventions are already likely to be well below the level at which such interventions are cost-effective. The inclusion of additional cost savings will only strengthen this conclusion. Once further effectiveness data are available to allow the analysis of the incremental cost-effectiveness of alternative types of electronic intervention then the analysis may become more sensitive to the values used for these additional parameters (smoking related health-care costs, relapse rates, health benefits of quitting).
Although our analysis has focused on the health benefits accruing directly to the ex-smoker, it is important to note that there are others who may gain. Passive smoking, smoking during pregnancy and the influence of behaviour on other smokers are all means by which a decision to quit may impact on others. These effects are difficult to quantify, but if they were to be included, effective interventions would become even more cost-effective than our results suggest.
The EVPI analysis gives some guidance on the benefits of conducting further research to inform parameters in the model. The estimates of EVPI quoted above (see Estimated value of additional information) should be interpreted by considering the number of people affected by, and the time horizon of, the decision being informed by the cost-effectiveness analysis. Estimates of EVPI are several orders of magnitude lower when it is assumed that all electronic interventions are equally effective, compared with estimated EVPI without this assumption. This suggests that there is substantial value in research on the comparative short-term efficacy of alternative classes of intervention. However, given the numbers who are potentially affected by this decision, research into other model parameters (such as the rate of long-term relapse and the benefits of quitting) is also likely to be worthwhile.
Expected value of perfect information methods can estimate only the value of information related to parameters included in the economic model. Further comparative research on alternative methods for electronic interventions would allow analysis of the impact on cost-effectiveness of aspects of interventions not evaluated in the current analysis (e.g. mode of delivery). Research on issues such as the influence of the age and motivational state of the smoker would allow the issue of the most cost-effective design of electronic intervention for specific groups to be explored.
Summary of findings
-
If we assume that the treatment effect is identical across classes of electronic intervention then some sort of electronic intervention is likely to be cost-effective (when added to non-electronic behavioural support).
-
Based on mean cost-effectiveness, e5 interventions (multiple tailored components, such as an interactive website and chat room) have the highest cost-effectiveness in most scenarios. Estimates of incremental cost-effectiveness of e5 [multiple tailored components + generic component(s)] vs e1 (single generic component), which is usually the next best option, range from £100–28,000 per QALY. However, probabilistic sensitivity analysis suggests that there is substantial uncertainty around this estimate, and the probability that e5 is indeed the most cost-effective type of intervention is 30–35%.
-
Given this uncertainty, further effectiveness research is likely to be cost-effective, particularly around the most effective type of electronic intervention. EVPI calculations suggest the upper limit for the benefit of this research is around £2000–3000 per person. Given the large numbers that are eligible for smoking cessation support, this suggests that even a large study would be cost-effective.
-
There is some support in the evidence for the cost-effectiveness of electronic interventions in smokers not actively seeking to quit but there is very little evidence available in this population on which to base any conclusions.
Chapter 5 Supplementary review
In addition to the effectiveness and cost-effectiveness review of electronic aids to help people stop smoking, a supplementary review was conducted. This review examines studies that did not meet the inclusion criteria for the main reviews but may be useful for understanding factors that may influence the reach or effectiveness of electronic aids. In particular, this review aimed to explore the acceptability and usability of aids – including who uses electronic aids, how acceptable these aids are to particular groups of smokers, how feasible delivery is to smokers in different settings, and aspects of usability. Findings from this supplementary review should be considered alongside the effectiveness and cost-effectiveness review findings to form a comprehensive overview of current evidence.
Methods
As outlined in Chapter 2 (see Sifting of records received from searches of electronic databases) an over-arching literature search and sifting of studies that covered all three component reviews was initially conducted. After the combined search, screening and selection process, 112 papers were identified that potentially met the inclusion criteria for the supplementary review. Of these, 18 were found to be systematic reviews of trials or commentaries on systematic reviews. These are listed in Appendix 12 but were not included in this supplementary review. After these papers were excluded, 94 papers remained. The abstracts for these papers were screened by two reviewers. A total of 26 papers were selected for inclusion in the review and full paper copies of each of these papers were obtained. A list of the papers that were rejected at the final screening stage and the reasons for exclusion are outlined in Appendix 11.
Data were extracted from the included studies and summarised in a series of evidence tables. Because of the range of study designs included in the supplementary review, data were extracted to inform a narrative synthesis of key themes and issues rather than to inform a meta-analysis.
Following the effectiveness review, components of the care provided in each study in the supplementary review with regard to smoking cessation were coded using the coding scheme described in the effectiveness review. Components were categorised as either ‘electronic’ or ‘non-electronic’ and these were separately coded. The coding scheme was developed, piloted and revised during the data extraction phase by two reviewers (YFC and IY) and then applied to the supplementary review studies by a third reviewer (LB). Where possible, the type of intervention (based on the coding scheme) is described in the narrative summary of findings below.
Results
The 26 papers99,103,158–181 included in the review used the following designs: four non-randomised trials,103,162,166,175 18 cross-sectional,99,158–160,163–165,167–170,173,174,176,178–181 two cohort,161,177 and two qualitative studies. 171,172
Two studies99,177 described electronic aids with single or multiple generic components, 18 studies103,158–171,176,180,181 described electronic aids with a single tailored component (with or without a generic component) and six studies171–175,178 described interventions that had multiple tailored components (with or without generic components). Thus, the balance of studies in the supplementary review is similar to the effectiveness review in that the majority involved interventions with a single tailored component.
The supplementary review is primarily concerned with exploring the acceptability and usability of aids. As the studies in the review did not use a randomised controlled design, their outcomes are unlikely to be as robust or as replicable as the findings of those studies included in the main effectiveness review. This review seeks to increase our understanding of how electronic aids reach and treat smokers and what they tell us about research gaps. The review is organised around four main themes:
-
profile of users (exploring who uses these aids)
-
acceptability of interventions to particular groups of smokers
-
feasibility of delivery in different settings
-
usability of interventions.
Profile of users
Interventions delivered via electronic aids, particularly the internet, have the potential to reach large numbers of smokers. 29,104 This review aimed to examine whether or not electronic interventions were able to reach smokers who were unwilling or unable to attend face-to-face interventions, or to contact a telephone quit line. One limitation of controlled studies of electronic aids (such as those included in the effectiveness review component of this report) is that the inclusion criteria they apply may mean that the smokers recruited to the trial are not necessarily representative of those who would choose to use an intervention in practice. Alternative study designs, particularly cross-sectional studies that examine the profile of users of an existing intervention, can therefore shed some light on who might use electronic aids and how the reach and acceptability of such aids can be enhanced.
This supplementary review included four studies that described the characteristics of existing users of already available internet sites (with or without a tailored component) for smoking cessation. 158–161 It also included three pilot studies that examined the profile of those who volunteered to test electronic aids. 162–164
Profile of existing online intervention users
Saul et al. 159 aimed to follow up all smokers (n = 607) who had accessed a state-wide internet cessation programme in Minnesota (Quitplan.com) during a 10-week period in 2004. They contacted 471 (77.6%) at 6-month follow-up. Most users of the site were 25–40 years old (57.3%, 270/471), female (66%, 311/471), had some further or higher education (82.8%, 358/432) and were employed (74.9%).
Wang and Etter160 conducted a cross-sectional study of clients accessing the website Stop-tabac. The website included tailored and generic components. The study involved analysing data from 18,361 users of the programme who accessed the site from 15 different countries between June 1998 and March 2001. The website was available in French (accessed by 77% of users) and English. Users were fairly evenly split in terms of sex (51% male, 49% female) with an average age of 36 years and with higher levels of education (average 15 years’ schooling, meaning some further or higher education for most clients). Smokers who accessed the site smoked on average 20 cigarettes per day and the median time to first cigarette was 20 minutes. The site was also accessed by ex-smokers, half of whom had quit in the past 12 days.
Wang and Etter160 examined who made more than one visit to the site providing some insight into the determinants of adherence to a web-based programme for smoking cessation. Just 19.5% of users made more than one visit. Women were slightly more likely to return than men (20.7% vs 18.5%, p < 0.0001). Those aged < 20 years were least likely to return, followed by those in their twenties (11.3% vs 17.9% vs 20.8%, p < 0.0001). Respondents with < 13 years’ education were less likely to return than those with more education (16.8% vs 17.9% vs 20.8%). There were some country differences in return rates. There were differences related to intention and behaviour to stop smoking: people already making a quit attempt were most likely to return (27.5%), whereas those with no imminent intention to stop smoking were least likely (14%). Ex-smokers were most likely to return (27.7%, p < 0.0001) and it was those who had quit in the last 3 days who returned quickest but only through the first 75 days. Among current smokers, neither the number of cigarettes smoked per day nor time to first cigarette was related to return rates. Use of NRT was positively associated with rates of return: 25.6% among daily users, 21.3% among occasional users and 18.8% among non-users of NRT (p < 0.0001).
Zbikowski et al. 161 conducted a cross-sectional study of QuitNet – a cessation intervention in the USA combining a telephone quitline with a website that had a single tailored component and generic components. Website support was triggered by smokers accessing the telephone quitline and providing information that was then used to produce tailored online material. The study examined the experience of 11,143 enrollees of the programme over 18 months in 2006–7. Typical participants were middle-aged (mean 43 years, standard deviation = 10.8) and were fairly evenly distributed between sexes (54% female, 46% male); on average participants smoked 12.5 cigarettes per day. The authors observed that QuitNet clients were, overall, less dependent than the general population of smokers in the USA. Almost all participants (91.7%) reported planning to stop smoking in the next 30 days. Women used web and telephone services significantly more than men. They were also more likely than men to use online discussion forums and complete a larger number of calls.
Some potentially useful common findings emerge from this set of studies that explored the profile of smokers and recent ex-smokers who accessed existing online aids for smoking cessation. The first is that the assumption that younger people are more likely to be willing to use new technology of the kind used in electronic aids – and therefore may be reached by online interventions – is not necessarily true, particularly in recent studies. For example, the Wang and Etter study160 in particular showed poor uptake and lower rates of return among smokers aged < 20 years compared with all other age groups. Zbikowski et al. 161 found that smokers aged < 26 years logged on to their online intervention less often than older smokers. There is some suggestion from these studies that male smokers may be just as likely as females to use websites to access information or support about smoking cessation – this differs from some face-to-face interventions, for which more users are female. Cessation website users also appear to have fairly high levels of education, although this finding may not be sustained in more recent studies as internet use becomes more widespread. An additional finding from these studies is that even smokers who declare no imminent plans to stop smoking still log on to smoking cessation websites. Many studies have argued that electronic aids reach smokers who are ambivalent about quitting – but some of the studies included here show that although these smokers may look at these websites they are unlikely to continue using them. Recent quitters access these websites and therefore internet-based interventions may have some role to play in relapse prevention.
Profile of pilot online intervention users
Five studies described the process of piloting electronic aids for smoking cessation and outlined the characteristics of those who have volunteered to participate in these pilots. This includes some early studies of computer-generated materials and computer-aided telephone interventions. 165–167 Findings from these early studies include some comparisons with existing face-to-face interventions and assert that these aids may attract smokers who would not normally access face-to-face treatment.
For example, Schneider et al. 167 piloted a computerised, telephone-based interactive smoking cessation programme and provide some limited comparisons of the uptake of this programme with previous interventions. Two of the worksites where the pilot telephone-based programme was offered had previously run face-to-face programmes. This included an advertising agency that had provided four stop-smoking groups over 2 years with a mean of 16.5 participants per group. By comparison the computer-tailored telephone intervention attracted 20 people. A second employer, a manufacturing company, had held three face-to-face groups in 2 years, attracting a mean of nine people per group. The computer-generated telephone intervention recruited 11 people who called at least once.
Other pilot studies including more recent research suggest that, overall, women are more likely to volunteer to participate in this type of research even when the groups targeted are primarily male. For example, Schneider et al. 166 conducted a case–control study that piloted computer-generated tailored materials with American veterans. Women constituted just 27.5% of those recruited but 40% of those who chose to participate once assigned. Lenert et al. 162 conducted a pilot comparing two web-based cessation interventions, one of which included a tailored e-mail component. Participants were recruited via web advertisements on search engines and 78% of those who volunteered to participate were female. Other research not included in this review has found that reactively recruited samples (i.e. relying on volunteers) are fairly consistently more likely to be female and also more highly educated and more motivated to stop smoking. 182 One of the studies included here aimed to overcome this by using random digit dialling to recruit smokers to a study of a computer-generated tailored intervention. 168 This ‘cold-calling’ approach was fairly successful: 83.5% of identified smokers agreed to participate and the sample was more balanced than previous studies by the same team that had applied a reactive sampling method. 168,169
Overall, the data indicate that computerised interventions seem to be used by more affluent smokers and more likely to be used by women.
Acceptability of interventions to groups of smokers
Previous reviews of electronic aids for behaviour change have suggested that receptivity to such aids varies significantly between patient groups or populations. 183 The supplementary review therefore examined the extent to which electronic aids were acceptable to different groups of smokers, namely:
-
young adults
-
lower-income groups
-
black and minority ethnic (BME) communities.
Acceptability was not always directly measured but was assessed by uptake or continued use, for example return visits to a website.
Young adults
Four studies in the review described interventions targeted at young adults. 103,164,170,171
An et al. 103 compared a pilot and refined (RealU) version of a cessation website for American university students. They reported results from the piloting and testing of the website170 and then went on to conduct a RCT that is included in the effectiveness review. 85 Participants were recruited via internet health screening on campus and paid to participate. The study found that the tailored website (that also offered peer support e-mails) was more effective in retaining participation in the study and intervention (93% at the end of the tailored intervention compared with 26% for the more basic intervention).
The cross-sectional studies by Obermayer et al. 170 and Riley et al. 164 involved the same research team with the first study describing results of an initial pilot and the second pilot aiming to improve some elements of the intervention tested in the first study. Both studies involved developing and testing an integrated web and test messaging programme for smoking cessation with American university students (aged 18–25 years). In the first study, 46 students agreed to participate in the pilot but only 29 of them registered on the website. On the site participants completed questions about their smoking that triggered a series of tailored text messages and an optional social support (web and text) element. 170 The study included an end-of-programme questionnaire that asked about:
-
ease of using programme
-
comfort in using the programme components
-
overall satisfaction with the programme.
Overall, participants who used the programme rated it highly on acceptability, satisfaction and subjective ratings of success. Unsurprisingly, those who quit (22%, n = 10, 7-day point prevalence at 6 weeks) were more satisfied than those that did not. The subsequent study with 31 students164 also found positive levels of satisfaction with the improved programme and the refinements introduced (described below – see Usability of interventions) resulted in lower rates of dropout between recruitment and intervention use.
Abroms et al. 99 explored the efficacy of e-mail support for smoking cessation amongst college students as part of a trial, with a separate article (included in this review) exploring feasibility and acceptability in an article including only the intervention group from the trial. Twelve e-mails on average were sent to student participants over the course of 6 months. Almost all (91%) of the intervention group read the e-mails. Overall, the students were positive about the e-mails and particularly valued the encouragement and social support that they provided.
Whittaker et al. 171 describe a multimedia cessation intervention delivered to young people by mobile telephone. The same team went on to conduct a trial of the intervention that is included in the effectiveness review. 69 The aim of this primarily qualitative study was to develop and pilot the intervention and seek young people’s views about content and acceptability. Young people participated in three content development phases, and the video and text messaging intervention was then developed based on their feedback. Views were sought via four focus groups with 16- to 18-year-old smokers (n = 27) and an online survey of a larger sample (number not specified) via a radio station website. Whittaker et al. 171 describe findings from the focus groups regarding the acceptability of such an intervention (p. 5):
Findings from the focus groups discussions demonstrated that all of the participants used mobile telephones regularly, and all groups expressed an interest in the idea of a mobile phone programme to support them in dealing with any particular issues they may face. Text messaging was considered to be potentially useful for positive reinforcement messages and providing information.
The intervention, once developed, was piloted by 17 young people, although only 15 completed the full registration over a 5-week period, and just 13 could be contacted at the 4-week follow-up. Of these 13, all but one stated that they liked the programme. In the section below on usability (see Usability of interventions), further details on which elements were particularly well-received are provided.
Overall, therefore, findings from these four pilot studies suggest that electronic aids for smoking cessation can be used with, and are probably accepted by, young people. However, they also highlight the challenges in retention with this group – with relatively high rates of dropout or loss to follow-up.
Lower-income smokers
Although access to computers among low-income populations has increased, it has been argued that relatively little is known about the acceptability and efficacy of electronic aids for influencing behaviour change in this group. 184 The supplementary review identified just two studies that explored the acceptability of electronic aids for smoking cessation with disadvantaged smokers, and no studies that compared the views of, or differences in outcomes between, more- and less-deprived groups.
Gilbert et al. 172 conducted a qualitative study that compared UK smokers’ views of self-help booklets for smoking cessation with computer-generated tailored material. Members of the same research team had previously conducted a trial of individually tailored smoking cessation advice letters as an adjunct to telephone counselling and generic self-help materials that is included in the effectiveness review. 32 Four focus groups were convened (n = 19), stratified by social class. Overall, the groups preferred the tailored material (a three-page feedback letter) to the generic self-help booklets. Men were more sceptical about the letters than women, but the researchers did not identify any differences between those from different socioeconomic backgrounds who participated. However, the small sample size, even for a qualitative study, makes it difficult to conclude much from this research.
McDaniel et al. 173,174 aimed to assess the usability and impact of an interactive computer-mediated cessation program for inner-city women in Indianapolis, USA, and described their results in two separate but related articles. The program was intended to increase readiness to quit rather than result in cessation. The research team sought the women’s views about the acceptability of the computer program and used these views to inform the design. They held focus groups with 15 women and asked about preferences for source and content of information on quitting. The women said that they wanted information on diagnosis and treatment options from a health professional, but, in contrast, would prefer if information on behaviour change came from ‘real people’. Thus, in the design of the programme the developers included video clips of health professionals conveying the risks of smoking. Material on attitudes to smoking or motivation to quit was presented as vignettes of former smokers who were similar in age and ethnicity to the intended audience. All textual information that appeared on screen was simultaneously presented in audio to decrease difficulty for low literacy participants. The programme was then piloted with 100 women (110 initially recruited, 10 dropped out before initiating the programme). The pilot primarily explored how the interactive computer program functioned in practice, but also measured satisfaction levels. Satisfaction with the programme was high: the mean score on the satisfaction measure was 60.2, with a possible range of 14–70.
The articles by McDaniel et al. 173,174 are potentially useful as they not only describe an intervention targeted at low-income smokers but also provide some evidence of the benefits of involving the target group in intervention design. This type of involvement is not necessarily unusual, but is rarely reported. We return to this issue in the final section of the review (see Usability of interventions).
Black and minority ethnic communities
This review identified just one study175 that examined the acceptability of electronic interventions with BME groups. Hoffman et al. 175 conducted a non-randomised trial of a stand-alone tailored computer program for smoking cessation with African American smokers (n = 98) in a community health clinic. The first group received two sessions with the ‘computer expert system’, a stage-based manual and audiotapes providing information on stress and smoking. The second group received the same intervention but the audiotapes provided specific advice on how to address potential problems with stress while quitting smoking. In terms of applicability to this review, the most useful element of this study was its exploration of acceptability in terms of the electronic element of the interventions. The computerised element (a program that provided information on cessation and tailored the information to participants’ responses) was delivered once at baseline and again at 3 months only. The researchers found that only 28 of 79 participants who completed both stages knew how to use a mouse. After instruction on use was provided, participants were given the choice of using the computer alone or with the help of a research assistant controlling the mouse. Around one-third (32%) chose the research assistant option. Participants (80 of the original 98) were followed up at 6 months and satisfaction levels were high, with 90–96% of subjects agreeing or strongly agreeing with the six items on the follow-up survey that described the sessions as interesting, not too long, delivering new information and that they would recommend the sessions to other smokers. However, it is difficult to determine to what extent these satisfaction levels related to the computer program element of the intervention rather than the other components.
Feasibility of delivery in different settings
Three cross-sectional studies focus on the delivery of electronic aids for smoking cessation in different settings. One study176 examines the uptake of and outcomes from a web-based intervention offered from the workplace. Two related studies177,178 describe the development, piloting and outcomes from a tailored computerised cessation programme delivered in a presurgical secondary care setting.
Workplace interventions to support behaviour change can provide a useful way of improving the health of adults of working age. Reviews of workplace interventions for smoking cessation have concluded that, although they are likely to yield only a modest impact on smoking prevalence owing to low participation rates, they can be effective in supporting individual smokers to quit. 185,179 Graham et al. 176 report results from an internet-based workplace smoking cessation intervention delivered to IBM employees in the USA in 2003. During online enrolment for the company’s health-care benefits programme, employees were asked about smoking status. Those willing to identify themselves as current smokers (just 6.6%; n = 8688) were given a choice of a self-help printed cessation intervention or an internet-based intervention with tailored and interactive elements. Those who agreed to take up either option were given a benefits premium discount (worth US$132 per year). Although 72% of smokers took up one of the two options (n = 6235), just 28.5% of these (1776) chose to use the website and 1746 registered on the site.
Most of those that registered were male (65%; average age 44 years) and, as in the studies described above under ‘profile of users’, a proportion who chose to use the site (11.5%) had recently stopped smoking. Around one-third (32%) of those who registered were successfully followed up at 12 months, and it was found that the 7-day point prevalence abstinence rate was still 12.8% using an ITT approach. 176
Haile et al. 177 initially piloted a computerised interactive cessation programme in a pre-surgical admission clinic in a hospital in Australia in 1999. They reported results from this pilot in 2002 including assessing the acceptability of this electronic aid with patients. The intervention was brief, involving smokers attending the clinic (n = 56) completing a baseline questionnaire on a computer at the clinic and then receiving a short (completion time 12–43 minutes) computer-delivered cessation intervention during the same visit. Nine months later these smokers were followed up by telephone to examine cessation outcomes. Patients reported that they found the programme acceptable and had used the information learned in the programme. The authors also examined the cost of the programme and concluded that its principal advantage was to convey ‘expert’ smoking cessation counselling and feedback that clinic staff did not have the time or capacity to provide. 177
Members of the same research team then went on to refine and expand the intervention in the same clinic setting with results reported in 2009. 178 The expanded intervention integrated the computer-based programme into both preoperative and postoperative care. In this study the team also reviewed relevant literature and interviewed preoperative clinic staff (number of interviews not reported) regarding the barriers to the provision of effective smoking cessation care. They identified the following barriers:
-
lack of organisational support
-
perceived patient objection
-
lack of systems to identify smokers
-
lack of staff time and skill
-
perceived inability to change care practices
-
perceived lack of efficacy of cessation care
-
cost of providing care.
The expanded programme involved completion of the same preoperative computerised questionnaire for smokers and delivery of tailored counselling via a computer in the clinic. Additions included brief advice from clinic nursing and anaesthetist staff that was guided by computer-generated prompts, followed by preoperative and postoperative provision of NRT. Just before admission, patients were telephoned and a computer-administered telephone interview providing further cessation counselling was delivered. Postdischarge patients were referred to a telephone quitline.
The authors concluded that the computer-based intervention provided a way to systematically and accurately identify smokers, did not require much clinical staff time or skill, was viewed by staff and patients as an acceptable form of care, and was inexpensive to deliver compared with other surgical costs. They also noted that the programme continued to be offered in the preoperative clinic after the research had ended. At the time the authors wrote the paper, the programme had been in place for 3 years since the end of the study and continued to be offered despite caseload expansion and restructuring of services in the hospital where the study took place.
Usability of interventions
A number of studies describe the features of electronic aids that can enhance user acceptability and potentially efficacy. These observations are not usually the main focus of articles but are reported in the description of the intervention and then commented on as part of the results. A number of themes relating to usability were identified from studies in the supplementary review. These themes were identified following review and data extraction (into narrative tables) of the main findings of each included study:
-
involving users in the development of electronic aids can enhance usability
-
enrolment in programmes involving electronic aids should be as easy as possible in order to retain smokers
-
aids that include interactive tools or social support elements may be more acceptable and/or effective
-
more frequent use of electronic aids may enhance efficacy.
User involvement
Three studies171,173,174 describe the process of involving users in the development and piloting of electronic aids and assert that this process improved the acceptability and efficacy of these aids. McDaniel et al. 173,174 conducted a cross-sectional pilot study of an interactive computer-based programme designed to increase readiness to quit and report the results in two separate but related articles. The target group was disadvantaged women accessing an inner-city health clinic. They tested a prototype of the aid with the women and describe in the articles a number of problems identified during this process, including the provision of information that was in some cases inappropriate to the woman using it at the time. They described the benefits of this usability testing:
In this study we tested the program with a sample of end users under ‘real world’ conditions in the clinic setting as opposed to using simulations. As a result of this process, we were able to discover and revise previously undetected errors in the algorithm so that users could successfully navigate the program and receive meaningful tailored feedback.
(McDaniel et al.,173 p. 512)
Whittaker et al. 171 describe in some detail how they consulted with and involved young people in the design and development of a multimedia mobile telephone intervention for smoking cessation. Focus groups with young people identified the type of media that they felt would be most effective in communicating cessation information (videos and cartoons of characters of a similar age and ethnicity to the target group). An online survey with young people identified preferences for programme content, in particular that the person in video clips (their credibility and appeal) was more important than the style of the clip. In the subsequent pilot study, the authors incorporated this feedback into the design of the intervention and reported positive results in terms of satisfaction with the programme.
Facilitating enrolment
Three other studies observe that smokers may be deterred from using electronic aids, particularly websites, by a delay between expressing an interest in receiving support and access to resources to support a quit attempt. This observation is not unique to electronic aids and probably applies to any cessation programme. However, given the potential of the internet for instant access it is possibly a valuable observation in terms of improving the usability of interventions.
Schneider et al. 167 mention this issue in an example of a relatively early study of electronic aids. In their study of a computer-aided telephone intervention they observe that a substantial proportion of those who initially expressed an interest in the programme never called the programme quitline. As this was in the context of a pilot study, the smokers were required to return a consent form and then after this had been sent they could ring the computer-aided helpline. The authors observe that many smokers lost interest in the programme in the time between consenting and making the effort to call. As they state (p. 147):
Perhaps a much larger proportion of those who expressed interest would have called if they had been allowed to start the programme immediately … It may be that the desire to join a smoking cessation programme is often transitory, and frequently diminishes even during a brief delay. The programme was free of charge and entailed little inconvenience. It is likely that many of the participants called more out of curiosity than out of a strong desire to quit smoking.
In two pilot studies of a web and text messaging intervention for university students in the USA, Obermayer et al. 170 and Riley et al. 164 describe how they learned from uptake problems in their first study and aimed to resolve them in the second. In the first study,170 they had asked participants to initiate the programme on their own after providing them with a URL. Only 31 of 46 participants did this. In the second study,164 participants were assisted in initiating the programme and began receiving text messages immediately after baseline assessment. Both follow-up and abstinence rates were improved in the second study compared with the first and the authors asserted that adding this element had improved uptake and also increased satisfaction. 164
Interactive tools
Some of the more recent studies in this supplementary review included electronic aids with multiple components and commented on which of these components were favourably received by participants. A consistent finding was that interactive tools were well-received by those who used them, as were aids that had a social support element. This finding (although drawn from small non-controlled studies) is potentially useful as neither the effectiveness nor cost-effectiveness reviews were able to identify what form electronic aids should take or what form of delivery channel is most effective.
An et al. 103 conducted a cohort study that compared a generic website with a revised interactive programme for university students in the USA. The generic website was developed first, and then modified to appear like a college magazine (the idea being that if the intervention appeared like something already used by the target group, it would have more appeal) and enhanced by the addition of proactive e-mail support to students from peer ‘coaches’. The authors concluded that these improvements enhanced adherence to the programme and improved cessation outcomes. Members of the same research team conducted a subsequent study with adults (rather than students) and concluded that the use of interactive quitting tools and one-to-one messaging with other smokers using the website increased abstinence rates. 180 This positive finding relating to social support mechanisms (i.e. one-to-one e-mails or text messaging with other smokers) was also identified by Graham et al. 176 in their study of a worksite intervention.
Whittaker et al. ,171 in the study mentioned above, also examined which features of the mobile telephone intervention were most popular among the New Zealand young people in their study. The features liked the most were the support provided, reminders, information, encouragement, the fact that the young people knew that the messages were coming, advice and the relevance of messages to them personally. The ability to request messages on demand (to deal with cravings) was popular among those who used them, as were text messages. Polosa et al. 181 explored the addition of tailored e-mails and an adjunct to face-to-face behavioural support in a small pilot study involving 30 smokers. Accepting e-mails from the counsellor was voluntary but the study found that this additional element of support was feasible and effective and merited further evaluation.
Repeat usage
Four cross-sectional studies report that more frequent use of electronic aids is associated with increased abstinence in participants. 161,162,167,176 This finding can be linked to the concept of intensity reported in the main effectiveness review above.
Schneider et al. 167 in their study of a computerised, telephone-based smoking cessation programme found that subjects who were abstinent at the 6-month follow-up period tended to make more use of the programme than those who were smoking at 6-month follow-up [mean 17.6 calls (standard deviation = 24.37) vs 7.65 calls (standard deviation = 12.66); p > 0.001]. Similar results were obtained at the 1- and 3-month follow-up periods. Zbikowski et al. ,161 in their study of a telephone and web-based intervention, found that greater adherence to the programme, as defined by using both the telephone and the web components, was associated with higher quit rates.
Graham et al. 176 who described the provision of an internet-based cessation programme for IBM employees found that those who used the website four or more times were more likely to be abstinent at 12-month follow-up.
Finally, Lenert et al. 162 outline in some detail the different components of a web-based, eight-stage intervention with additional tailored e-mails that was piloted with 40 smokers. Those viewing zero to two lessons had a 29% chance of quitting (at 30+ days), those viewing three or four lessons had a 82% chance, and those viewing five or more lessons had a 45% chance of quitting (p = 0.012). The authors asked participants for feedback regarding the site and describe the factors that may inhibit repeat usage and therefore restrict efficacy. These factors included:
-
Relatively complex design The design focused on browsing as the primary activity by which participants would acquire information; the authors concluded that website users needed a more linear structure or step-by-step instructions to guide participants through the site.
-
Overemphasis on the use of text for delivery of content Alternative media (videos, pictures, etc.) could be used to convey information and may have more appeal.
-
Use of e-mail reminders to redirect participants back to the site to complete lessons had limited success For some this was effective, whereas others saw it as spam.
Lenert et al. ’s observations162 on improving usability of internet-based interventions are consistent with the descriptions of elements of interventions described as popular or well received by participants in some of the other studies listed above. Clearly, elements of usability are intervention specific and will vary dependent on the type of electronic aid being used and the group of smokers being targeted. However, some common themes emerge across articles in this supplementary review.
Discussion
The main reviews in this report describe the effectiveness and cost-effectiveness of electronic aids for smoking cessation. They found that electronic aids increase the likelihood of cessation compared with no intervention or self-help materials, and that electronic aids are highly likely to be cost-effective. However, neither of the main reviews was able to determine, from the available evidence, what form electronic aids should take or how the content of interventions may affect outcomes. Evidence from the supplementary review does not directly fill these research gaps but it does highlight some of the factors that may affect the usability and acceptability of interventions and suggests who is most likely to use electronic aids for smoking cessation.
Evidence from non-controlled studies suggests that smokers who choose to use electronic aids, particularly internet sites, are likely to have a similar profile to smokers who access face-to-face interventions but may have higher levels of education and may be less nicotine dependent than the general population of smokers. There is little evidence to suggest that electronic aids are likely to encourage younger smokers to quit in larger numbers despite assumptions about the appeal of these types of technologies to younger smokers. Likewise it is difficult to determine from the studies here if these aids, particularly internet sites, will have a particular appeal to smokers who are not yet motivated to quit, although there is some evidence from the effectiveness review that electronic aids can increase the likelihood of cessation among these smokers.
There is limited evidence regarding the acceptability (measured by uptake or continued use) of different forms of electronic aids among subpopulations of smokers, in particular disadvantaged and black and ethnic minority groups. Interventions that are specifically designed for young people appeared to be well received but, as in most studies of cessation interventions with youth, rates of dropout or loss to follow-up were high. One study included here reported that internet-based interventions were accepted and used by employees who smoke, and two studies demonstrated that a stand-alone computer program can provide a useful addition to support for smoking cessation before and after surgery.
The supplementary review did not examine the content of interventions in any detail but some studies did point to particular design features that may enhance usability, including involving users in intervention design, simplifying enrolment procedures in programmes to reduce dropout and adding interactive or social support elements to aids, particularly internet sites. There is also some suggestion that more frequent use of aids (repeat visits, for example) may enhance efficacy in non-controlled studies, but this is not supported by the effectiveness review where no clear effects by intensity of intervention were identified.
In terms of research gaps, the effectiveness and cost-effectiveness reviews suggest that further research is needed on the relative benefits of different forms of delivery for electronic aids (internet, mobile telephone) and the content of delivery (including more research on the efficacy of interactive electronic aids). The supplementary review, in addition, points to the additional further research gaps.
In particular, there is a need for further research on the acceptability of these technologies for smoking cessation with subpopulations of smokers. For example, studies in the review suggest that younger smokers are not more likely to use electronic aids than other groups, but there is limited evidence available to suggest how more younger smokers can be encouraged to access these forms of support or what elements of electronic aids will appeal to this group. In addition, we could find no studies that explored outcomes for more or less affluent smokers using internet or other electronic cessation programmes and very little evidence on uptake or cessation among disadvantaged groups. Research with poorer smokers, who increasingly constitute the largest group of smokers in developed countries, is required now.
Research gaps also exist relating to the usability and acceptability of electronic aids for smoking cessation in particular settings. Studies in this review suggest, for example, that they may have particular promise when used as an adjunct to treatment in health care settings including secondary care. More evidence is also required on the relationship between involving users in the design of interventions and the impact this has on effectiveness, and finally on how electronic aids developed and tested in research settings are applied in routine practice and in the community. Finally, there are additional research questions not included in this review that may merit future exploration, including the potential for electronic aids to help prevent relapse to smoking either as an adjunct to face-to-face or telephone behavioural support or as part of a longer term stand-alone intervention.
Summary of findings
-
Profile of users:
-
– Computerised interventions seem to be used by more affluent smokers and more likely to be used by women.
-
-
Acceptability of interventions:
-
– Electronic aids for smoking cessation can be used with, and are probably accepted by, young people, although there are challenges in retention with this group. There is little evidence to suggest that electronic interventions are likely to appeal in particular to younger smokers.
-
-
Feasibility of delivery:
-
– Computerised interventions provide a way to systematically and accurately identify smokers, do not require much clinical staff time or skill, are viewed by staff and patients as an acceptable form of care, and are inexpensive to deliver compared with other surgical costs.
-
-
Usability of interventions:
-
– A number of themes relating to usability were identified: involving users in the development of electronic aids can enhance usability; enrolment in programmes involving electronic aids should be as easy as possible in order to retain smokers; aids that include interactive tools or social support elements may be more acceptable and/or effective; and more frequent use of electronic aids may enhance efficacy.
-
Chapter 6 General discussion
Effectiveness review
Our effectiveness review included 60 RCTs/quasi-RCTs reported in 77 publications that evaluated the use of computers and other electronic aids for smoking cessation. The results of meta-analyses show that computer and other electronic aids increase the likelihood of cessation compared with no intervention or generic self-help materials, but the effect is small. The effectiveness does not appear to vary with respect to mode of delivery and concurrent non-electronic co-interventions. Overall, similar sizes of effect are observed in both aid to cessation studies (in smokers who are ready to quit) and cessation induction studies (in smokers who are not yet ready to quit), but there is substantial heterogeneity among the latter (cessation induction studies), particularly when 6-month point prevalence abstinence is used as the outcome measure.
Compared with previously published reviews that have focused on specific types of computer and/or other electronic aids, this review is wider in its scope and encompasses all interventions that make use of automated features brought by the advances in information technology and telecommunication in the past couple of decades. The broader scope allows us to include a larger evidence base in this review and to examine the potential impact of different computer/electronic tools on the effectiveness of the interventions. The effectiveness review was supplemented by a cost-effectiveness review and a supplementary review.
Cost-effectiveness review
We chose a time-to-relapse survival model to synthesise the evidence on continuous abstinence. This allowed us to account for variability in follow-up between studies and repeated measures reported by some studies. We found that assuming an exponential survival model led to an extremely poor fit with the data, and that this fit was improved considerably by the use of a Weibull model. The results indicated that the chance of sustaining a quit attempt is far higher once the first month or two have been negotiated successfully.
Our overview of the available data shows that evaluations have concentrated on electronic interventions that fit two of our categories (single tailored component and multiple components, at least two of which are tailored). Comparing these two categories suggests that there is little additional benefit from the latter, which we would expect to be more resource intensive. We also failed to find a difference in intervention effect between trials restricted to those actively seeking to quit and all other trials, although this may well be owing to a lack of studies explicitly targeting populations not contemplating a quit attempt.
The analysis above is restricted to those studies that report continuous outcomes, which represent less than half of the studies in the main review. Many of these studies also report point prevalence outcomes. During the model development process, we explored models that allowed for a correlation between the two outcome types, and then used that correlation to draw on studies that only reported point abstinence to inform estimates of continuous abstinence. However, despite observing a strong correlation between intervention effects on the two types of outcome, including the additional studies had little effect on the posterior mean or credible intervals of the estimated pooled intervention effects. This may be because the additional studies largely evaluated the two treatment types well represented in the studies reporting continuous abstinence only. Given that the results were unchanged by including point abstinence, and that our analysis is based on continuous abstinence, we have not included the results from this exercise.
It might be argued that once a decision has been made to implement an electronic intervention, the choice of that intervention comes down to minimising cost. This would imply recommending a class one (single generic component) intervention over anything more expensive. However, such a recommendation would fail to allow for the considerable uncertainty around several of the intervention classes. To account for this, we took a two-step approach in the cost-effectiveness analysis. We first explored the cost-effectiveness of a generic electronic intervention. Then, we explored which category of electronic intervention should be chosen, if a decision were made to implement this type of intervention.
Our results suggest that making some form of electronic support available to smokers actively seeking to quit is highly likely to be cost-effective. This is true whether the electronic intervention is delivered alongside brief advice or more intensive counselling. It is less clear, from the available evidence, what form that electronic support should take. What the analysis does suggest, however, is that the decision is not very sensitive to the cost differentials between electronic interventions. Instead, the key source of uncertainty is that around the comparative effectiveness of different types of electronic interventions.
Our results also suggest that such aids may be cost-effective in populations of smokers not actively looking to quit, based on the finding that the efficacy of electronic interventions is similar in such populations compared with those actively seeking to quit. However, this is only a tentative finding based on the information available at present. There are few studies that have specifically explored efficacy in smokers who are not willing to quit. Also, an approach we would have preferred to take to answer this question is to estimate the impact of electronic aids in causing a quit attempt to be made where the motivation did not previously exist, and then compare the success rate in this case with that for smokers already actively looking to quit. Further information is required for such an approach to be viable and useful.
Supplementary review
Neither of the main reviews was able to determine, from the available evidence, what form electronic aids should take or how the content of interventions may affect outcomes. Evidence from the supplementary review does not directly fill these research gaps but it does highlight some of the factors that may affect the usability and acceptability of interventions, and suggests who is most likely to use electronic aids for smoking cessation.
Evidence from non-controlled studies suggests that smokers who choose to use electronic aids, particularly internet sites, are likely to have a similar profile to smokers who access face-to-face interventions but may have higher levels of education and may be less nicotine dependent than the general population of smokers. There is little evidence to suggest that electronic aids are likely to encourage younger smokers to quit in larger numbers despite assumptions about the appeal of these types of technologies to younger smokers. Likewise it is difficult to determine from the studies here if these aids, particularly internet sites, will have a particular appeal to smokers who are not yet motivated to quit, although there is some evidence from the effectiveness review that electronic aids can increase the likelihood of cessation among these smokers.
There is limited evidence regarding the acceptability (measured by uptake or continued use) of different forms of electronic aids among subpopulations of smokers, in particular disadvantaged and BME groups. Interventions specifically designed for young people appeared to be well received but, as in most studies of cessation interventions with youth, rates of dropout or loss to follow-up were high. One study included here reported that internet-based interventions were accepted and used by employees who smoke and two studies demonstrated that a stand-alone computer program can provide a useful addition to support for smoking cessation before and after surgery.
The supplementary review did not examine the content of interventions in any detail but some studies did point to particular design features that may enhance usability, including involving users in intervention design, simplifying enrolment procedures in programmes to reduce dropout and adding interactive or social support elements to aids, particularly internet sites. There is also some suggestion that more frequent use of aids (repeat visits, for example) may enhance efficacy in non controlled studies, but this is not supported by the effectiveness review, in which no clear effects by intensity of intervention were identified.
In terms of research gaps, the effectiveness and cost-effectiveness reviews suggest that further research is needed on the relative benefits of different forms of delivery for electronic aids (internet, mobile telephone) and the content of delivery (including more research on the efficacy of interactive electronic aids). The supplementary review, in addition, points to the need for further research on the acceptability of these technologies for smoking cessation with subpopulations of smokers, particularly disadvantaged groups. More evidence is also required on the relationship between involving users in the design of interventions and the impact this has on effectiveness, and finally on how electronic aids developed and tested in research settings are applied in routine practice and in the community.
Acknowledgements
Contribution of authors
Paul Aveyard, Linda Bauld and Marcus R Munafò conceived the original project, contributed to the design of the effectiveness and cost-effectiveness reviews, and assisted with the writing of the final report.
Linda Bauld was responsible for the supplementary review.
Yen-Fu Chen, Ismail Yahaya, Dechao Wang and Anne Fry-Smith were responsible for the effectiveness review and drafting relevant sections of the report, and assisted with the supplementary review and the writing of the final report.
Jason Madan and Nicky Welton were responsible for the evidence synthesis modelling and cost-effectiveness review and analyses and drafting relevant sections of the report, and assisted with the supplementary review and the writing of the final report.
We are grateful to Dr Esther Albon for her help in study selection and data extraction during the early phase of the project, Dr Olalekan Uthman for his help in the compilation of tables and data checking, Fay Beck for her assistance with data extraction, and Shelley Smith and Linda Briscoe for their administrative support. We thank anonymous referees for their helpful comments on a draft of this report.
Paul Aveyard, Linda Bauld and Marcus R Munafò are members of the UK Centre for Tobacco Control Studies, a UKCRC Public Health Research: Centre of Excellence. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- The Office for National Statistics . Internet Access 2007: Households and Individuals 2007.
- Walters ST, Wright JA, Shegog R. A review of computer and Internet-based interventions for smoking behavior. Addict Behav 2006;31:264-77.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328.
- Peto R, Doll R, Bock G, Goode J. Understanding nicotine and tobacco addiction. Chichester: John Wiley & Sons; 2006.
- Peto R, Lopez AD, Koop CE, Pearson CE, Schwarz MR. Critical issues in global health. San Francisco, CA: Wiley (Jossey-Bass); 2001.
- Jarvis MJ, McIntyre D, Bates C, Foulds J. Effectiveness of smoking cessation initiatives. BMJ 2002;324.
- Lader D. Smoking-related behaviour and attitudes, 2008/09. London: Office for National Statistics; 2009.
- West R, Brown J, Fidler J. Key Findings from the Smoking Toolkit Study n.d. http://smokinginengland.info/ (accessed 29 May 2012).
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res 2003;5:13-25.
- Russell MA, Wilson C, Taylor C, Baker CD. Effect of general practitioners’ advice against smoking. Br Med J 1979;2:231-5.
- West R, Shiffman S. Effect of oral nicotine dosing forms on cigarette withdrawal symptoms and craving: a systematic review. Psychopharmacology 2001;155:115-22.
- National Institute for Health and Clinical Excellence (NICE) . Smoking Cessation Services in Primary Care, Pharmacies, Local Authorities and Workplaces, Particularly for Manual Working Groups, Pregnant Women and Hard to Reach Communities n.d. www.nice.org.uk/PH010.
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating Tobacco Use and Dependence: 2008 Update n.d. http://www.ahrq.gov/clinic/tobacco/treating_tobacco_use08.pdf (accessed 24 July 2012).
- McEwen A, West R. Smoking cessation activities by general practitioners and practice nurses. Tob Control 2001;10:27-32.
- Coleman T, Lewis S, Hubbard R, Smith C. Impact of contractual financial incentives on the ascertainment and management of smoking in primary care. Addiction 2007;102:803-8.
- Wilson A, Hippisley-Cox J, Coupland C, Coleman T, Britton J, Barrett S. Smoking cessation treatment in primary care: prospective cohort study. Tob Control 2005;14:242-6.
- Jarvis MJ, McIntyre D, Bates C, Foulds J. Effectiveness of smoking cessation initiatives. BMJ 2002;324.
- Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database Syst Rev 2005;3.
- Jarvis MJ, McIntyre D, Bates C, Foulds J. Effectiveness of smoking cessation initiatives. BMJ 2002;324.
- Russell MA, Merriman R, Stapleton J, Taylor W. Effect of nicotine chewing gum as an adjunct to general practitioner’s advice against smoking. Br Med J (Clin Res Ed) 1983;287:1782-5.
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004;99:29-38.
- Leischow SJ, Muramoto ML, Cook GN, Meikke EP, Castellini SM, Otte PS. OTC nicotine patch: effectiveness alone and with brief physician intervention. Am J Health Behav 1999;23:61-9.
- Leischow SJ, Ranger-Moore J, Muramoto ML, Matthews E. Effectiveness of the nicotine inhaler for smoking cessation in an OTC setting. Am J Health Behav 2004;28:291-30.
- Shiffman S. Use of more nicotine lozenges leads to better success in quitting smoking. Addiction 2007;102:809-14.
- Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther 2008;30:1852-8.
- National Institute for Health and Clinical Excellence (NICE) . Guidance on the Use of Nicotine Replacement Therapy (NRT) and Bupropion for Smoking Cessation n.d. http://guidance.nice.org.uk/TA39 (accessed 16 February 2008).
- West R. The clinical significance of ‘small’ effects of smoking cessation treatments. Addiction 2007;102:506-9.
- Strecher VJ. Computer-tailored smoking cessation materials: a review and discussion. Patient Educ Couns 1999;36:107-17.
- Shahab L, McEwen A. Online support for smoking cessation: a systematic review of the literature. Addiction 2009;104:1792-804.
- Myung SK, McDonnell DD, Kazinets G, Seo HG, Moskowitz JM. Effects of web- and computer-based smoking cessation programs: meta-analysis of randomized controlled trials. Arch Int Med 2009;169:929-37.
- Whittaker R, Borland R, Bullen C, Lin RB, McRobbie H, Rodgers A. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev 2009;4.
- Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemed J E Health 2009;15:231-40.
- Burling TA, Marotta J, Gonzalez R, Moltzen JO, Eng AM, Schmidt GA, et al. Computerized smoking cessation program for the worksite: treatment outcome and feasibility. J Consult Clin Psychol 1989;57:619-22.
- Owen N, Ewins A-L, Lee C. Smoking cessation by mail: a comparison of standard and personalized correspondence course formats. Addict Behav 1989;14:355-63.
- Schneider SJ, Walter R, O’Donnell R. Computerized communication as a medium for behavioral smoking cessation treatment: controlled evaluation. Comput Hum Behav 1990;6:141-51.
- Curry SJ, Wagner EH, Grothaus LC. Evaluation of intrinsic and extrinsic motivation interventions with a self-help smoking cessation program. J Consult Clin Psychol 1991;59:318-24.
- Prochaska JO, DiClemente CC, Velicer WF, Rossi JS. Standardized, individualized, interactive, and personalized self-help programs for smoking cessation. Health Psychol 1993;12:399-405.
- Strecher VJ, Kreuter M, Den Boer DJ, Kobrin S, Hospers HJ, Skinner CS. The effects of computer-tailored smoking cessation messages in family practice settings. J Fam Pract 1994;39:262-70.
- Curry SJ, McBride C, Grothaus LC, Louie D, Wagner EH. A randomized trial of self-help materials, personalized feedback, and telephone counseling with nonvolunteer smokers. J Consult Clin Psychol 1995;63:1005-14.
- Kreuter MW, Strecher VJ. Do tailored behavior change messages enhance the effectiveness of health risk appraisal? Results from a randomized trial. Health Educ Res 1996;11:97-105.
- Pallonen UE, Velicer WF, Prochaska JO, Rossi JS, Bellis JM, Tsoh JY, et al. Computer-based smoking cessation interventions in adolescents: description, feasibility, and six-month follow-up findings. Subst Use Misuse 1998;33:935-65.
- Dijkstra A, de VH, Roijackers J. Long-term effectiveness of computer-generated tailored feedback in smoking cessation. Health Educ Res 1998;13:207-14.
- Dijkstra A, de VH, Roijackers J, van BG. Tailoring information to enhance quitting in smokers with low motivation to quit: three basic efficacy questions. Health Psychol 1998;17:513-19.
- Dijkstra A, de VH, Roijackers J. Targeting smokers with low readiness to change with tailored and nontailored self-help materials. Prev Med 1999;28:203-11.
- Ershoff DH, Quinn VP, Boyd NR, Stern J, Gregory M, Wirtschafter D. The Kaiser Permanente prenatal smoking-cessation trial: when more isn’t better, what is enough?. Am J Prev Med 1999;17:161-8.
- Velicer WF, Prochaska JO, Fava JL, Laforge RG, Rossi JS. Interactive versus noninteractive interventions and dose–response relationships for stage-matched smoking cessation programs in a managed care setting. Health Psychol 1999;18:21-8.
- O’Neill HK, Gillispie MA, Slobin K. Stages of change and smoking cessation: a computer-administered intervention program for young adults. Am J Health Promot 2000;15:93-6.
- Orleans CT, Boyd NR, Noll E, Crosette L, Glassman B. Computer tailored intervention for older smokers using transdermal nicotine. Tob Control 2000;9.
- Shiffman S, Paty JA, Rohay JM, Di Marino ME, Gitchell J. The efficacy of computer-tailored smoking cessation material as a supplement to nicotine polacrilex gum therapy. Arch Int Med 2000;160:1675-81.
- Shiffman S, Gitchell JG, Strecher VJ. Real-World Efficacy of Computer-Tailored Smoking Cessation Material As a Supplement to Nicotine Replacement n.d.
- Aveyard P, Cheng KK, Almond J, Sherratt E, Lancashire R, Lawrence T, et al. Cluster randomised controlled trial of expert system based on the transtheoretical (‘stages of change’) model for smoking prevention and cessation in schools. BMJ 1999;319:948-53.
- Aveyard P, Sherratt E, Almond J, Lawrence T, Lancashire R, Griffin C, et al. The change-in-stage and updated smoking status results from a cluster-randomized trial of smoking prevention and cessation using the transtheoretical model among British adolescents. Prev Med 2001;33:313-24.
- Etter JF, Perneger TV. Effectiveness of a computer-tailored smoking cessation program: a randomized trial. Arch Int Med 2001;161:2596-601.
- Etter JF, Perneger TV. Post-intervention effect of a computer tailored smoking cessation programme. J Epidemiol Comm Health 2004;58:849-51.
- Lennox AS, Osman LM, Reiter E, Robertson R, Friend J, McCann I, et al. Cost effectiveness of computer tailored and non-tailored smoking cessation letters in general practice: randomised controlled trial. BMJ 2001;322.
- Prochaska JO, Velicer WF, Fava JL, Rossi JS, Tsoh JY. Evaluating a population-based recruitment approach and a stage-based expert system intervention for smoking cessation. Addict Behav 2001;26:583-602.
- Prochaska JO, Velicer WF, Fava JL, Ruggiero L, Laforge RG, Rossi JS, et al. Counselor and stimulus control enhancements of a stage-matched expert system intervention for smokers in a managed care setting. Prev Med 2001;32:23-32.
- Shiffman S, Paty JA, Rohay JM, Di Marino ME, Gitchell JG. The efficacy of computer-tailored smoking cessation material as a supplement to nicotine patch therapy. Drug Alcohol Depend 2001;64:35-46.
- Ausems M, Mesters I, van BG, de VH. Short-term effects of a randomized computer-based out-of-school smoking prevention trial aimed at elementary schoolchildren. Prev Med 2002;34:581-9.
- Riley W, Jerome A, Behar A, Weil J. Computer and manual self-help behavioral strategies for smoking reduction: initial feasibility and one-year follow-up. Nicotine Tob Res 2002;4:183-8.
- Borland R, Balmford J, Segan C, Livingston P, Owen N. The effectiveness of personalized smoking cessation strategies for callers to a Quitline service. Addiction 2003;98:837-46.
- Lawrence T, Aveyard P, Evans O, Cheng KK. A cluster randomised controlled trial of smoking cessation in pregnant women comparing interventions based on the transtheoretical (stages of change) model to standard care. Tob Control 2003;12:168-77.
- Lawrence T, Aveyard P, Cheng KK, Griffin C, Johnson C, Croghan E. Does stage-based smoking cessation advice in pregnancy result in long-term quitters? 18-month postpartum follow-up of a randomized controlled trial. Addiction 2005;100:107-16.
- Aveyard P, Lawrence T, Croghan E, Evans O, Cheng KK. Is advice to stop smoking from a midwife stressful for pregnant women who smoke? Data from a randomized controlled trial. Prev Med 2005;40:575-82.
- Ausems M, Mesters I, van BG, de VH. Effects of in-school and tailored out-of-school smoking prevention among Dutch vocational school students. Health Educ Res 2004;19:51-63.
- Borland R, Balmford J, Hunt D. The effectiveness of personally tailored computer-generated advice letters for smoking cessation. Addiction 2004;99:369-77.
- Clark MM, Cox LS, Jett JR, Patten CA, Schroeder DR, Nirelli LM, et al. Effectiveness of smoking cessation self-help materials in a lung cancer screening population. Lung Cancer 2004;44:13-21.
- Lenert L, Munoz RF, Perez JE, Bansod A. Automated e-mail messaging as a tool for improving quit rates in an internet smoking cessation intervention. J Am Med Informat Assoc 2004;11:235-40.
- Prochaska JO, Velicer WF, Rossi JS, Redding CA, Greene GW, Rossi SR, et al. Multiple risk expert systems interventions: impact of simultaneous stage-matched expert system interventions for smoking, high-fat diet, and sun exposure in a population of parents. Health Psychol 2004;23:503-16.
- Etter JF. Comparing the efficacy of two Internet-based, computer-tailored smoking cessation programs: a randomized trial. J Med Int Res 2005;7.
- Etter JF. Internet-based smoking cessation programs. Int J Med Informat 2006;75:110-16.
- Prochaska JO, Velicer WF, Redding C, Rossi JS, Goldstein M, DePue J, et al. Stage-based expert systems to guide a population of primary care patients to quit smoking, eat healthier, prevent skin cancer, and receive regular mammograms. Prev Med 2005;41:406-16.
- Rodgers A, Corbett T, Bramley D, Riddell T, Wills M, Lin RB, et al. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tob Control 2005;14:255-61.
- Bramley D, Riddell T, Whittaker R, Corbett T, Lin RB, Wills M, et al. Smoking cessation using mobile phone text messaging is as effective in Maori as non-Maori. N Z Med J 2005;118.
- Strecher VJ, Marcus A, Bishop K, Fleisher L, Stengle W, Levinson A, et al. A randomized controlled trial of multiple tailored messages for smoking cessation among callers to the cancer information service. J Health Comm 2005;10:105-18.
- Strecher VJ, Shiffman S, West R. Randomized controlled trial of a web-based computer-tailored smoking cessation program as a supplement to nicotine patch therapy. Addiction 2005;100:682-8.
- Strecher VJ, Shiffman S, West R. Moderators and mediators of a web-based computer-tailored smoking cessation program among nicotine patch users. Nicotine Tob Res 2006;8:95-101.
- Wolfenden L, Wiggers J, Knight J, Campbell E, Rissel C, Kerridge R, et al. A programme for reducing smoking in pre-operative surgical patients: Randomised controlled trial. Anaesthesia 2005;60:172-9.
- Wolfenden L, Wiggers J, Knight J, Campbell E, Spigelman A, Kerridge R, et al. Increasing smoking cessation care in a preoperative clinic: a randomized controlled trial. Prev Med 2005;41:284-90.
- Hall SM, Tsoh JY, Prochaska JJ, Eisendrath S, Rossi JS, Redding CA, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Publ Health 2006;96:1808-14.
- Barnett PG, Wong W, Hall S. The cost-effectiveness of a smoking cessation program for out-patients in treatment for depression. Addiction 2008;103:834-40.
- Japuntich SJ, Zehner ME, Smith SS, Jorenby DE, Valdez JA, Fiore MC, et al. Smoking cessation via the internet: a randomized clinical trial of an internet intervention as adjuvant treatment in a smoking cessation intervention. Nicotine Tob Res 2006;8:59-67.
- Mermelstein R, Turner L. Web-based support as an adjunct to group-based smoking cessation for adolescents. Nicotine Tob Res 2006;8:69-76.
- Muñoz RF, Lenert LL, Delucchi K, Stoddard J, Perez JE, Penilla C, et al. Toward evidence-based Internet interventions: A Spanish/English Web site for international smoking cessation trials. Nicotine Tob Res 2006;8:77-8.
- Patten CA, Croghan IT, Meis TM, Decker PA, Pingree S, Colligan RC, et al. Randomized clinical trial of an Internet-based versus brief office intervention for adolescent smoking cessation. Patient Educ Couns 2006;64:249-58.
- Patten CA, Rock E, Meis TM, Decker PA, Colligan RC, Pingree S, et al. Frequency and type of use of a home-based, Internet intervention for adolescent smoking cessation. J Adolesc Health 2007;41:437-43.
- Swartz LH, Noell JW, Schroeder SW, Ary DV. A randomised control study of a fully automated internet based smoking cessation programme. Tob Control 2006;15:7-12.
- Velicer WF, Friedman RH, Fava JL, Gulliver SB, Keller S, Sun X, et al. Evaluating nicotine replacement therapy and stage-based therapies in a population-based effectiveness trial. J Consult Clin Psychol 2006;74:1162-72.
- Vidrine DJ, Arduino RC, Gritz ER. Impact of a cell phone intervention on mediating mechanisms of smoking cessation in individuals living with HIV/AIDS. Nicotine Tob Res 2006;8:103-8.
- Vidrine DJ, Arduino RC, Lazev AB, Gritz ER. A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS 2006;20:253-60.
- Gilbert H, Nazareth I, Sutton S. Assessing the feasibility of proactive recruitment of smokers to an intervention in general practice for smoking cessation using computer-tailored feedback reports. Fam Pract 2007;24:395-400.
- Pike KJ, Rabius V, McAlister A, Geiger A. American Cancer Society’s QuitLink: randomized trial of Internet assistance. Nicotine Tob Res 2007;9:415-20.
- Rabius V, Pike KJ, Wiatrek D, McAlister AL. Comparing internet assistance for smoking cessation: 13-month follow-up of a six-arm randomized controlled trial. J Med Int Res 2008;10.
- Rabius V, Pike J, Geiger A, Hunter J, McAlister A. The American Cancer Society’s Quitlink: A Randomized Clinical Trial Evaluating Internet-Based Smoking Cessation Programs and Medication Use (RPOS 3–62) 2006.
- Reid RD, Pipe AL, Quinlan B, Oda J. Interactive voice response telephony to promote smoking cessation in patients with heart disease: a pilot study. Patient Educ Couns 2007;66:319-26.
- Smeets T, Kremers SP, Brug J, de VH. Effects of tailored feedback on multiple health behaviors. Ann Behav Med 2007;33:117-23.
- Sutton S, Gilbert H. Effectiveness of individually tailored smoking cessation advice letters as an adjunct to telephone counselling and generic self-help materials: randomized controlled trial. Addiction 2007;102:994-1000.
- Abroms LC, Windsor R, Simons-Morton B. Getting young adults to quit smoking: a formative evaluation of the X-Pack Program. Nicotine Tob Res 2008;10:27-33.
- Abroms LC, Windsor R, Simons-Morton B. A Formative Evaluation of an Email-Based Program for Smoking Cessation in Young Adults (POS1–115) 2007.
- Al-Chalabi L, Prasad N, Steed L, Stenner S, Aveyard P, Beach J, et al. A pilot randomised controlled trial of the feasibility of using body scan and isometric exercises for reducing urge to smoke in a smoking cessation clinic. BMC Publ Health 2008;8.
- An LC, Klatt C, Perry CL, Lein EB, Hennrikus DJ, Pallonen UE, et al. The RealU online cessation intervention for college smokers: a randomized controlled trial. Prev Med 2008;47:194-9.
- Klatt C, Berg CJ, Thomas JL, Ehlinger E, Ahluwalia JS, An LC. The role of peer e-mail support as part of a college smoking-cessation website. Am J Prev Med 2008;35:471-8.
- An LC, Perry CL, Lein EB, Klatt C, Farley DM, Bliss RL, et al. Strategies for increasing adherence to an online smoking cessation intervention for college students. Nicotine Tob Res 2006;8:7-12.
- Brendryen H, Kraft P. Happy ending: a randomized controlled trial of a digital multi-media smoking cessation intervention. Addiction 2008;103:478-4.
- Brendryen H, Drozd F, Kraft P. A digital smoking cessation program delivered through internet and cell phone without nicotine replacement (happy ending): randomized controlled trial. J Med Int Res 2008;10.
- Brendryen H, Kraft P. A RCT of an internet and cell-phone based smoking cessation intervention. Psychol Health 2006;21:23-4.
- McKay HG, Danaher BG, Seeley JR, Lichtenstein E, Gau JM. Comparing two web-based smoking cessation programs: randomized controlled trial. J Med Int Res 2008;10.
- Danaher BG, Lichtenstein E, McKay HG, Seeley JR. Use of non-assigned smoking cessation programs among participants of a Web-based randomized controlled trial. J Med Int Res 2009;11.
- Meyer C, Ulbricht S, Baumeister SE, Schumann A, Ruge J, Bischof G, et al. Proactive interventions for smoking cessation in general medical practice: a quasi-randomized controlled trial to examine the efficacy of computer-tailored letters and physician-delivered brief advice. Addiction 2008;103:294-30.
- Schumann A, John U, Ulbricht S, Ruge J, Bischof G, Meyer C. Computer-generated tailored feedback letters for smoking cessation: theoretical and empirical variability of tailoring. Int J Med Informat 2008;77:715-22.
- Oenema A, Brug J, Dijkstra A, de Weerdt I, de Vries H. Efficacy and use of an internet-delivered computer-tailored lifestyle intervention, targeting saturated fat intake, physical activity and smoking cessation: a randomized controlled trial. Ann Behav Med 2008;35:125-35.
- Prochaska JO, Butterworth S, Redding CA, Burden V, Perrin N, Leo M, et al. Initial efficacy of MI, TTM tailoring and HRI’s with multiple behaviors for employee health promotion. Prev Med 2008;46:226-31.
- Prokhorov AV, Yost T, Mullin-Jones M, de Moor C, Ford KH, Marani S, et al. ‘Look at your health’: outcomes associated with a computer-assisted smoking cessation counseling intervention for community college students. Addict Behav 2008;33:757-71.
- Prokhorov AV, Fouladi R, deMoore C, Warneke C, Luca M, Mullin Jones M, et al. Computer-assisted, counselor-delivered smoking cessation counseling for community college students: intervention approach and sample characteristics. J Child Adolesc Subst Abuse 2007;16:35-62.
- Schumann A, John U, Baumeister SE, Ulbricht S, Rumpf HJ, Meyer C. Computer-tailored smoking cessation intervention in a general population setting in Germany: outcome of a randomized controlled trial. Nicotine Tob Res 2008;10:371-9.
- Stoddard JL, Augustson EM, Moser RP. Effect of adding a virtual community (bulletin board) to smokefree.gov: randomized controlled trial. J Med Int Res 2008;10.
- Strecher VJ, McClure J, Alexander G, Chakraborty B, Nair V, Konkel J, et al. The role of engagement in a tailored web-based smoking cessation program: randomized controlled trial. J Med Int Res 2008;10.
- Strecher VJ, McClure JB, Alexander GL, Chakraborty B, Nair VN, Konkel JM, et al. Web-based smoking-cessation programs: results of a randomized trial. Am J Prev Med 2008;34:373-81.
- Etter JF. Comparing computer-tailored, internet-based smoking cessation counseling reports with generic, untailored reports: a randomized trial. J Health Comm 2009;14:646-57.
- Free C, Whittaker R, Knight R, Abramsky T, Rodgers A, Roberts IG. Txt2stop: a pilot randomised controlled trial of mobile phone-based smoking cessation support. Tob Control 2009;18:88-91.
- Haug S, Meyer C, Schorr G, Bauer S, John U. Continuous individual support of smoking cessation using text messaging: a pilot experimental study. Nicotine Tob Res 2009;11:915-23.
- Te Poel F, Bolman C, Reubsaet A, de Vries H. Efficacy of a single computer-tailored e-mail for smoking cessation: results after 6 months. Health Educ Res 2009;24:930-40.
- Muñoz RF, Barrera AZ, Delucchi K, Penilla C, Torres LD, Perez-Stable EJ. International Spanish/English Internet smoking cessation trial yields 20% abstinence rates at 1 year. Nicotine Tob Res 2009;11:1025-34.
- Swan GE, McClure JB, Jack LM, Zbikowski SM, Javitz H, Catz SL, et al. Behavioural counselling and varenicline treatment for smoking cessation. Am J Prev Med 2010;38:482-90.
- Civljak M, Koshy E, Marlais M, Car J. Internet-based interventions for smoking cessation (protocol). Cochrane Database Syst Rev 2009;2.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions 2008;27:379-87.
- Schumann A, John U, Rumpf HJ, Hapke U, Meyer C. Changes in the ‘stages of change’ as outcome measures of a smoking cessation intervention: a randomized controlled trial. Prev Med 2006;43:101-6.
- Bornhauser A, McCarthy J, Glantz SA. German tobacco industry’s successful efforts to maintain scientific and political respectability to prevent regulation of secondhand smoke. Tob Control 2006;15.
- Welton NJ, Caldwell DM, Adamopoulos E, Vedhara K. Mixed treatment comparison meta-analysis of complex interventions: psychological interventions in coronary heart disease. Am J Epidemiol 2009;169:1158-65.
- Higgins JP, Whitehead A. Borrowing strength from external trials in a meta-analysis. Stat Med 1996;15:2733-49.
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900.
- Ades AE, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. PharmacoEconomics 2006;24:1-19.
- Welton NJ, Caldwell DM, Adamopoulos E, Vedhara K. Mixed treatment comparison meta-analysis of complex interventions: psychological interventions in coronary heart disease. Am J Epidemiol 2009;169:1158-65.
- Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Series B-Stat Methodol 2002;64:583-616.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83.
- Smith MY, Cromwell J, DePue J, Spring B, Redd W, Unrod M. Determining the cost-effectiveness of a computer-based smoking cessation intervention in primary care. Manag Care 2007;16:48-55.
- Oster G, Huse DM, Delea TE, Colditz GA. Cost-effectiveness of nicotine gum as an adjunct to physician’s advice against cigarette smoking. JAMA 1986;256:1315-8.
- Fiscella K, Franks P. Cost-effectiveness of the Transdermal Nicotine Patch as an Adjunct to Physicians’ Smoking Cessation Counselling. JAMA 1996;275:1247-51.
- Ronckers ET, Groot W, Ament AJ. Systematic review of economic evaluations of smoking cessation: standardizing the cost-effectiveness. Med Decis Making 2005;25:437-48.
- National Institute for Health and Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2008. http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (accessed 24 July 2012).
- Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al. The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: A systematic review and economic evaluation. Health Technol Assess 2002;6.
- Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D. ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis. Health Technol Assess 2008;12.
- Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al. Systematic review of the effectiveness of stage based interventions to promote smoking cessation. BMJ 2003;326:1175-7.
- Kaltenthaler E, Brazier J, De NE, Tumur I, Ferriter M, Beverley C, et al. Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation. Health Technol Assess 2006;10.
- Murray E. Internet-delivered treatments for long-term conditions: strategies, efficiency and cost-effectiveness. Exp Rev Pharmacoecon Outcomes Res 2008;8:261-72.
- Meenan RT, Stevens VJ, Funk K, Bauck A, Jerome GJ, Lien LF, et al. Development and implementation cost analysis of telephone- and Internet-based interventions for the maintenance of weight loss. Int J Technol Assess Health Care 2009;25:400-10.
- Graham AL, Milner P, Saul JE, Pfaff L. Online advertising as a public health and recruitment tool: comparison of different media campaigns to increase demand for smoking cessation interventions. J Med Int Res 2008;10.
- Runge C, Lecheler J, Horn M, Tews JT, Schaefer M. Outcomes of a Web-based patient education program for asthmatic children and adolescents. Chest 2006;129:581-93.
- Southard BH, Southard DR, Nuckolls J. Clinical trial of an Internet-based case management system for secondary prevention of heart disease. J Cardiopulm Rehabil 2003;23:341-8.
- Organisation for Economic Co-operation and Development (OECD) . Stat Extracts: PPPs and Exchange Rates n.d. http://stats.oecd.org/Index.aspx?themetreeid=-200 (accessed 1 August 2012).
- Curtis L. Unit Costs of Health and Social Care n.d. http://www.pssru.ac.uk/project-pages/unit-costs/2009/index.php (accessed 1 August 2012).
- Hodgson TA. N Engl J Med 1998;338.
- Barendregt JJ, Bonneux L, van der Maas PJ. The health care costs of smoking. N Engl J Med 1997;337:1052-7.
- Cohen DR, Fowler GH. Economic implications of smoking cessation therapies: a review of economic appraisals. Pharmacoeconomics 1993;4:331-44.
- Welton NJ, Johnstone EC, David SP, Munafo MR. A cost-effectiveness analysis of genetic testing of the DRD2 Taq1A polymorphism to aid treatment choice for smoking cessation. Nicotine Tob Res 2008;10:231-40.
- Taylor DH, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of smoking cessation for longevity. Am J Public Health 2002;92:990-6.
- Ades AE, Lu G, Claxton K. Expected value of sample information calculations in medical decision modeling. Med Decis Making 2004;24:207-27.
- Balmford J, Borland R, Benda P. Patterns of use of an automated interactive personalized coaching program for smoking cessation. J Med Int Res 2008;10.
- Saul JE, Schillo BA, Evered S, Luxenberg MG, Kavanaugh A, Cobb N, et al. Impact of a statewide Internet-based tobacco cessation intervention. J Med Int Res 2007;9.
- Wang J, Etter JF. Administering an effective health intervention for smoking cessation online: the international users of Stop-Tabac. Prev Med 2004;39:962-8.
- Zbikowski SM, Hapgood J, Smucker BS, McAfee T. Phone and web-based tobacco cessation treatment: real-world utilization patterns and outcomes for 11,000 tobacco users. J Med Int Res 2008;10.
- Lenert L, Munoz RF, Stoddard J, Delucchi K, Bansod A, Skoczen S, et al. Design and pilot evaluation of an internet smoking cessation program. J Am Med Informat Assoc 2003;10:16-20.
- Ebbert JO, Montori V, Vickers KS, Erwin PC, Dale LC, Stead LF. Interventions for smokeless tobacco use cessatio. Cochrane Database Syst Rev 2007;4.
- Riley W, Obermayer J, Jean-Mary J. Internet and mobile phone text messaging intervention for college smokers. J Am Coll Health 2008;57:245-8.
- Schneider SJ. Trial of an on-line behavioral smoking cessation program. Comput Human Behav 1986;2:277-86.
- Schneider SJ, Benya A, Singer H. Computerized direct mail to treat smokers who avoid treatment. Comput Biomed Res 1984;17:409-18.
- Schneider SJ, Schwartz MD, Fast J. Computerized, telephone-based health promotion: I. Smoking cessation program. Comput Human Behav 1995;11:135-48.
- Velicer WF, Prochaska JO. An expert system intervention for smoking cessation. Patient Educ Couns 1999;36:119-29.
- Velicer WF, Prochaska JO, Bellis JM, DiClemente CC, Rossi JS, Fava JL, et al. An expert system intervention for smoking cessation. Addict Behav 1993;18:269-90.
- Obermayer JL, Riley WT, Asif O, Jean-Mary J. College smoking-cessation using cell phone text messaging. J Am Coll Health 2004;53:71-8.
- Whittaker R, Maddison R, McRobbie H, Bullen C, Denny S, Dorey E, et al. A multimedia mobile phone-based youth smoking cessation intervention: findings from content development and piloting studies. J Med Int Res 2008;10.
- Gilbert H, Nazareth I, Sutton S. Perception of computer-tailored feedback for smoking cessation: qualitative findings from focus groups. J Appl Biobehav Res 2009;14:15-29.
- McDaniel AM, Hutchison S, Casper GR, Ford RT, Stratton R, Rembusch M. Usability testing and outcomes of an interactive computer program to promote smoking cessation in low income women. AMIA Annu Symp Proc 2002:509-13.
- McDaniel AM, Casper GR, Hutchison SK, Stratton RM. Design and testing of an interactive smoking cessation intervention for inner-city women. Health Educ Res 2005;20:379-84.
- Hoffman AM, Redding CA, Goldberg D, Anel D, Prochaska JO, Meyer PM, et al. Computer expert systems for African-American smokers in physicians offices: a feasibility study. Prev Med 2006;43:204-11.
- Graham AL, Cobb NK, Raymond L, Sill S, Young J. Effectiveness of an internet-based worksite smoking cessation intervention at 12 months. J Occup Environ Med 2007;49:821-8.
- Haile MJ, Wiggers JH, Spigelman D, Knight J, Considine RJ, Moore K. Novel strategy to stop cigarette smoking by surgical patients: pilot study in a preadmission clinic. ANZ J Surg 2002;72:618-22.
- Wolfenden L, Wiggers J, Campbell E, Knight J, Kerridge R, Spigelman A. Providing comprehensive smoking cessation care to surgical patients: the case for computers. Drug Alcohol Rev 2009;28:60-5.
- Moher M, Hey K, Lancaster T. Workplace interventions for smoking cessation. Cochrane Database Syst Rev 2005;2.
- An LC, Schillo BA, Saul JE, Wendling AH, Klatt CM, Berg CJ, et al. Utilization of smoking cessation informational, interactive, and online community resources as predictors of abstinence: cohort study. J Med Int Res 2008;10.
- Polosa R, Russo C, Di Maria A, Arcidiacono G, Morjaria JB, Piccillo GA. Feasibility of using E-mail counseling as part of a smoking-cessation program. Respir Care 2009;54:1033-9.
- Riesma RP, Pattenden J, Bridle C, Sowden AJ, Mather J, Watt IS, et al. A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change 2002;6.
- Fava J, Velicer W, Prochaska J. Applying the transtheoretical model to a representative sample of smokers. Addict Behav 2010;20:189-203.
- Revere D, Dunbar PJ. Review of computer-generated outpatient health behavior interventions: clinical encounters ‘in absentia’. J Am Med Informat Assoc 2001;8:62-79.
- Stanley LD. Beyond access: psychosocial barriers to computer literacy. Inform Soc 2003;19:407-16.
- Glasgow R, McCaul K, Fisher K. Participation in worksite health promotion: a critique of the literature and recommendations for future practice. Health Educ Q 1993;3:391-408.
Appendix 1 Search strategies
Ovid MEDLINE 1950 to May week 4 2009 (updated December week 5 2009)
-
(smoker or smokers or smoking).mp.
-
(tobacco or cigarette$ or nicotine$).mp.
-
smoking cessation.mp.
-
“tobacco use cessation”/
-
(prevent$ or abstain$ or abstin$ or discourag$ or cease$ or cessation or quit or quits or quitting).mp.
-
(stop or stops or stoppping).mp.
-
health behavior/
-
behavior therapy/
-
or/1-2
-
or/3-4
-
computer$.ti,ab.
-
expert systems/
-
computer aided design/
-
therapy, computer assisted/
-
internet.mp.
-
computer communication networks/
-
communications media/
-
cellular phone$.mp.
-
mobile phone$.mp.
-
text messag$.mp.
-
sms.mp.
-
web.mp.
-
electronic mail/
-
email$.mp.
-
blog$.mp.
-
(chat room$ or chatroom$).mp.
-
podcast$.mp.
-
video recording/
-
video$.mp.
-
or/11-29
-
or/5-8
-
31 and 10
-
30 and 11
-
30 and 32
-
33 or 34
-
limit 35 to (humans and yr = “1980 - current”)
EMBASE (Ovid) 1980 to 2009 week 21 (updated 2009 week 53)
-
(smoker or smokers or smoking).mp.
-
(tobacco or cigarette$ or nicotine$).mp.
-
smoking cessation.mp.
-
smoking cessation program/
-
(prevent$ or abstain$ or abstin$ or discourag$ or cease$ or cessation or quit or quits or quitting).mp.
-
(stop or stops or stopping).mp.
-
health behavior/
-
behavior therapy/
-
1 or 2
-
or/3-4
-
computer$.ti,ab.
-
computer/
-
expert systems/
-
online system/
-
computer program/
-
computer assisted therapy/
-
internet.mp.
-
(cellular phone$ or mobile phone$).mp.
-
mobile phone/
-
(text messag$ or sms or web or email$ or blog$).mp.
-
e-mail/
-
(chat room$ or chatroom$).mp.
-
(podcast$ or video$).mp.
-
videorecording/
-
or/11-24
-
or/5-8
-
9 and 26
-
10 and 25
-
25 and 27
-
28 or 29
-
limit 30 to (human and yr = “1980 - current”)
PsycINFO (Ovid) 1967 to May week 4 2009 (updated December week 4 2009)
-
(smoker or smokers or smoking).mp.
-
(tobacco or cigarette$ or nicotine$).mp.
-
smoking cessation.mp.
-
or/1-2
-
(prevent$ or abstain$ or abstin$ or discourag$ or cease$ or cessation or quit or quits or quitting).mp.
-
(stop or stops or stopping).mp.
-
health behavior/
-
behavior therapy/
-
or/5-8
-
computer$.ti,ab.
-
expert systems/
-
exp Computer Assisted Design/
-
computer assisted therapy/ or online therapy/
-
internet.mp.
-
cellular phones/
-
cellular phone$.mp.
-
mobile phone$.mp.
-
text message$.mp.
-
(sms or web).mp.
-
computer mediated communication/
-
email$.mp.
-
blog$.mp.
-
(chat room$ or chatroom$).mp.
-
(podcast$ or video$).mp.
-
exp videotapes/
-
or/10-25
-
4 and 9
-
26 and 3
-
26 and 27
-
28 or 29
-
limit 30 to yr = “1980 - current”
Health Management Information Consortium (Ovid) March 2009 (updated November 2009)
-
(smoker or smokers or smoking).mp.
-
(tobacco or cigarette$ or nicotine$).mp.
-
smoking cessation.mp.
-
or/1-2
-
(prevent$ or abstain$ or abstin$ or discourag$ or cease$ or cessation or quit or quits or quitting).mp.
-
(stop or stops or stopping).mp.
-
health behaviour/
-
behaviour therapy/ or behaviour modification/
-
8 or 6 or 7 or 5
-
computer$.ti,ab.
-
internet.mp.
-
cellular phone$.mp.
-
mobile telephones/
-
mobile phone$.mp.
-
text messag$.mp.
-
sms.mp.
-
web.mp.
-
email$.mp.
-
electronic mail.mp.
-
blog$.mp.
-
(chat room$ or chatroom$).mp.
-
podcast$.mp.
-
video$.mp.
-
or/10-23
-
9 and 4
-
3 and 24
-
and 25
-
26 or 27
-
limit 28 to yr = “1980 - current”
The Cochrane Library (all databases) 2009 Issue 2 (updated Issue 4)
#1 (smoker or smokers or smoking)
#2 (tobacco or cigarette* or nicotine*)
#3 smoking next cessation
#4 MeSH descriptor Tobacco Use Cessation explode all trees
#5 (#1 OR #2)
#6 (#3 OR #4)
#7 computer*
#8 MeSH descriptor Expert Systems, this term only
#9 MeSH descriptor Computer-Aided Design, this term only
#10 MeSH descriptor Therapy, Computer-Assisted, this term only
#11 internet
#12 MeSH descriptor Computer Communication Networks, this term only
#13 MeSH descriptor Communications Media, this term only
#14 cellular next phone*
#15 mobile next phone*
#16 text next messag*
#17 sms
#18 web
#19 MeSH descriptor Electronic Mail, this term only
#20 email*
#21 blog*
#22 ((chat next room*) OR (chatroom))
#23 podcast*
#24 MeSH descriptor Video Recording, this term only
#25 video*
#26 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25)
#27 prevent* or abstain* or abstin* or discourag* or ceases* or cessation or quit or quits or quitting
#28 stops or stops or stopping
#29 MeSH descriptor Health Behavior, this term only
#30 MeSH descriptor Behavior Therapy, this term only
#31 (#27 OR #28 OR #29 OR #30)
#32 (#5 AND #31)
#33 (#6 AND #26)
#34 (#26 AND #32)
#35 (#33 OR #34)
#36 #35 from 1980 to 2009
Cumulative Index to Nursing and Allied Health Literature (EBSCOhost) 1980: May 2009 (updated December 2009)
S1 smoker or smokers or smoking
S2 tobacco* or cigarette* or nicotine*
S3 (MH “smoking cessation”) or (MH “smoking cessation programs)
S4 S1 or S2
S5 prevent* or abstain* or abstin* or discourag* or cease* or cessation or quit or quit or quitting
S6 stop or stops or stopping
S7 (MH “health behavior”)
S8 behavior therapy
S9 S5 or S6 or S7 or S8
S10 TI computer* or AB computer*
S11 (MH “therapy, computer assisted”)
S12 internet
S13 (MH “computer communication networks”)
S14 (MH “communications media”)
S15 cellular phone*
S16 mobile phone*
S17 text message*
S18 sms
S19 web
S20 (MH “electronic mail”)
S21 email*
S22 blog* or podcast* or video*
S23 chatroom* or chat room*
S24 (MH “videorecording”)
S25 S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24
S26 S4 and S9
S27 S3 and S25
S28 S26 and S25
S29 S27 or S28
S30 Limiters – publication year from 1980 - 2009
Appendix 2 Study selection criteria and algorithm (full paper)
| Question number | Criteria | Answer | Action | Reference Manager tag (user defined field 2) |
|---|---|---|---|---|
| Q1 |
Intervention: did the intervention utilise computer, internet, mobile telephone or other electronic aids (other than conventional mass media such as TV or radio advertisements) to: |
Yes | Go to Q2 | |
| No | Exclude | Exclude owing to intervention | ||
| Q2 |
Check if: |
Yes | Exclude | Exclude owing to intervention |
| No | Go to Q3 | |||
| Q3 | Population: Are the study participants predominantly adults (age ≥ 18 years)? | Yes | Go to Q4 | |
| No | Exclude | Exclude owing to population | ||
| Q4 | Study design: Is the study a RCT, quasi-randomised controlled study,a or an interrupted time series? | Yes | Go to Q7 | |
| No | Go to Q5 | |||
| Q5 | Study design: Is the study a full economic evaluation (i.e. cost-effectiveness analysis, cost–utility analysis, cost–benefit analysis)? | Yes | Include | Include for economic review |
| No | Go to Q6 | |||
| Q6 |
Study design: Does the paper describe: |
Yes | Tag Supplementary |
To be considered for supplementary review Studies describing intervention costs flagged FAO NJW/JJM |
| No | Exclude | Exclude owing to study design | ||
| Q7 | Comparison: Was the same computer and/or electronic aid(s) used in both/all the groups being compared (and thus the study was actually assessing the effectiveness of something else, e.g. mood management course + e-mails vs e-mails)? | Yes | Exclude | Exclude owing to irrelevant comparison |
| No | Go to Q8a | |||
| Q8a | Outcome: Was an outcome associated with smoking cessationb (e.g. point prevalence or prolonged abstinence) and/or motivation to quit smoking reported? | Yes | Include. Go to Q8b | Include for effectiveness review |
| No | Go to Q9 | |||
| Q8b | Outcome: Does the study also include a full economic evaluation (i.e. cost-effectiveness analysis, cost–utility analysis, cost–benefit analysis)? | Yes | Include | Include also for economic review |
| No | No further action | |||
| Q9 | Check: Was the smoking cessation outcome(s) of the study reported in another paper? | Yes | Include (and append to the main paper that reported the outcome) | Include for effectiveness review (see main paper [Ref ID]) |
| No |
(1) Contact author and if data not available then (2) Exclude |
(1) Query – contact author (2) Exclude owing to outcome |
||
Appendix 3 Data extraction form
| Author year | Trial name | |||
| Ref Man ID | Trial ID | |||
| Citation | ||||
| Related references | ||||
| Reviewer | Double checked by | |||
| Last updated | ||||
| Note (e.g. action to be taken; specific query to be clarified) | ||||
Summary tick boxes
To fill in during or at the end of data extraction; put ‘x’ against a suitable category or categories.
| Population (readiness to quit if given) |
|||||
|---|---|---|---|---|---|
| Pre-contemplation (not currently considering stopping smoking) | |||||
| Contemplation (willing to consider a quit attempt) | |||||
| Preparation (interested in stopping smoking) | |||||
| Other (please state) | |||||
| Age | |||||
| Adults | |||||
| Adolescents | |||||
| Other (please state) | |||||
| Intervention(s) and comparator(s) | |||||
| Intervention | Comparator 1 | Comparator 2 | Comparator 3 | ||
| Computer-generated tailored materials | |||||
| Stand-alone computer programs (i.e. not web based) | |||||
| Mobile telephone text messages | |||||
| E-mails | |||||
| Newsletters | |||||
| Websites (static) | |||||
| Websites (interactive) | |||||
| Web-based questionnaires | |||||
| Chat rooms | |||||
| Blogs | |||||
| Other electronic aids (please state) | |||||
| Printed, untailored material | |||||
| One-to-one face-to-face counselling | |||||
| One-to-one telephone counselling | |||||
| Group counselling | |||||
| NRT | |||||
| Supporter | |||||
| Other | |||||
| Comparisons (possible programme effects) | |||||
| Tailoring effect – as oppose to general, untailored material | |||||
| Media effect (audiovisual effect) – as opposed to printed material | |||||
| Immediate feedback (‘interactive’ or ‘give and take’) – as opposed to delayed feedback delivered by post | |||||
| Time/location effect (+ anonymous + autonomy?) – website, blogs, chat room, etc., offer the advantages of being accessible at any time and from any location (obviously still subject to availability of internet connection) and other potential advantages such as being able to be anonymous and to access the intervention only when someone feels like it | |||||
| Low cost/high volume – e.g. e-mails, SMS could simply be used to replace mailed or telephone reminders or ‘boosters’ because of the advantage of cost and coverage | |||||
Study design
| Population | ||||
|---|---|---|---|---|
| No. approached | ||||
| No. enrolled | ||||
|
No. randomised Intervention/ comparator |
||||
| No. available at further time points | ||||
| Method of recruitment | ||||
| Inclusion/exclusion criteria | ||||
| Intervention(s) | ||||
| Details e.g. Setting; contents and ways of delivery; duration and frequency | ||||
| Comparator(s) | ||||
| Details e.g. Setting; contents and ways of delivery; duration and frequency | ||||
| Common to both interventions and comparators | ||||
| Outcome measures [state whether not reported (NR), do not leave blank] | ||||
| Point prevalence abstinence | Definition: | |||
| Prolonged (population-based) or continuous abstinence | Definition: | |||
| Biochemical validation? |
Yes/no /unclear If yes, give details: |
|||
| Other outcome measures (please list) e.g. Intention to quit, self-efficacy, other possible mediators, process evaluation | ||||
| Time points at which outcomes were measured | ||||
| Trial duration/last follow-up | ||||
| Statistical methods | ||||
| 1. Power calculation done? |
Yes/no/unclear If yes, give details: |
|||
| 2. Methods of analysis (briefly describe): | ||||
| 3. Methods of dealing with missing data (briefly describe): | ||||
Quality assessment
|
1. Was randomisation adequate? Adequate: computer, random number table Inadequate: alternation; the use of case record numbers, dates of birth or day of the week |
Yes/no/unclear Give details: |
|
2. Was allocation adequately concealed? Adequate: Inadequate: |
Yes/no/unclear Give details: |
|
3. Were the groups similar at baseline? If dissimilar, was this explained or adjusted for? |
Yes/no/unclear Give details: |
| 4. Was care received by the groups similar other than the intervention? |
Yes/no/unclear Give details: |
| 5. Was contamination between groups acceptably low? |
Yes/no/unclear Give details: |
| 6. Were there significant dropouts (> 20%) |
Yes/no/unclear Give details: |
|
7. Were there any imbalances in dropouts between groups? 7.1 If so, were they explained or adjusted for? |
Yes/no/unclear Give details: |
| 8. Did the analysis include an ITT analysis? |
Yes/no/unclear Give details: |
Results
| Patient characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Whole study | Intervention | Comparator 1 | Comparator 2 | |||||||
| Mean age (SD) [range] | ||||||||||
| % male | ||||||||||
| Smoking history | ||||||||||
| Mean no. cigarettes smoked/day (SD) | ||||||||||
| Mean no. years smoking (SD) | ||||||||||
| Stage of change (n) | ||||||||||
| Other | ||||||||||
| Outcomes | ||||||||||
| Intervention ( n ) | Comparator 1 ( n ) | Comparator 2 ( n ) | OR or RR | 95% CI | p -value | |||||
| Point prevalence abstinence | ||||||||||
| Prolonged abstinence | ||||||||||
| Other | ||||||||||
Appendix 4 List of excluded studies
Excluded owing to intervention
For example, interventions targeting smokeless tobacco or aiming at enhancing the performance of providers of smoking cessation interventions, and other interventions not meeting the inclusion criteria with regard to intervention.
-
Anonymous. Testing online smoking cessation programs. CA-Cancer J Clin 2005;55:6.
-
Buller DB, Borland R, Woodall WG, Hall JR, Hines JM, Burris-Woodall P, et al. Randomized trials on consider this, a tailored, internet-delivered smoking prevention program for adolescents. Health Educ Behav 2008;35:260–81.
-
Dallery J, Glenn IM, Raiff BR. An Internet-based abstinence reinforcement treatment for cigarette smoking. Drug Alcohol Depend 2007;86:230–8.
-
Danaher BG, Boles SM, Akers L, Gordon JS, Severson HH. Defining participant exposure measures in web-based health behavior change programs. J Med Internet Res 2006;8:e15.
-
Dijkstra A. Working mechanisms of computer-tailored health education: evidence from smoking cessation. Health Educ Res 2005;20:527–39.
-
Donelle L, Hoffman-Goetz L. An exploratory study of Canadian aboriginal online health care forums. Health Comm 2008;23:270–81.
-
Durdle HE. Computerized motivational intervention and contingency management for smoking cessation in methadone-maintained opiate-dependent individuals. Dis Abst Int B Sci Eng 2009;70(3-B).
-
Etter J-F, Perneger TV. A comparison of cigarette smokers recruited through the internet or by mail. Int J Epidemiol 2001;30:521–5.
-
Etter J-F, le Houezec J, Landfeldt B. Impact of messages on concomitant use of nicotine replacement therapy and cigarettes: a randomized trial on the Internet. Addiction 2003;98:941–50.
-
Etter JF, le HJ, Huguelet P, Etter M. Testing the Cigarette Dependence Scale in 4 samples of daily smokers: psychiatric clinics, smoking cessation clinics, a smoking cessation website and in the general population. Addict Behav 2009;34:446–50.
-
Fu SS, Okuyemi KS, Partin MR, Ahluwalia JS, Nelson DB, Clothier BA, et al. Menthol cigarettes and smoking cessation during an aided quit attempt. Nicotine Tob Res 2008;10:457–62.
-
Gala S, Pesek F, Murray J, Kavanagh C, Graham S, Walsh M. Design and pilot evaluation of an Internet spit tobacco cessation program. J Dent Hyg 2008;82:11.
-
Graham AL, Papandonatos GD, Bock BC, Cobb NK, Baskin-Sommers A, Niaura R, et al. Internet- vs telephone-administered questionnaires in a randomized trial of smoking cessation. Nicotine Tob Res 2006;8(Suppl. 1):49–57.
-
Gwaltney CJ, Shiffman S, Balabanis MH, Paty JA. Dynamic self-efficacy and outcome expectancies: prediction of smoking lapse and relapse. J Abnorm Psychol 2005;114:661–75.
-
Hickcox ME. Non-drug substitute reinforcers for smoking: analysis of dimensions of similarity. Dis Abst Int B Sci Eng 2000;61(1-B).
-
Linder JA, Rigotti NA, Schneider LI, Kelley JHK, Brawarsky P, Haas JS. An electronic health record-based intervention To improve tobacco treatment in primary care a cluster-randomized controlled trial. Arch Int Med 2009;169:781–7.
-
Mallen MJ, Mallen MJ. Using technology to serve patients and practitioners: A comprehensive tobacco-cessation program for cancer patients. Counsell Psychother Res 2006;6:196–201.
-
Marks IM, Cavanagh K, Gega L. Hands-on help: computer-aided psychotherapy. New York, NY: Psychology Press; 2007.
-
O’Brien CP. Webcast video editorials: medical education and treatment of addictive disorders. MedGenMed 2006;8:21.
-
O’Connell KA, Hosein VL, Schwartz JE, Leibowitz RQ. How does coping help people resist lapses during smoking cessation? Health Psychol 2007;26:77–84.
-
O’Gara C, Munafò M. Psychiatric patients and gene-based smoking cessation packages. Psychiatr Bull 2006;30:1–2.
-
Partin MR, Partin MR. Randomized trial of an intervention to facilitate recycling for relapsed smokers. Am J Prev Med 2006;31:293–9.
-
Pletsch PK. Reduction of primary and secondary smoke exposure for low-income black pregnant women. Nurs Clin N Am 2002;37:315–29, viii.
-
Price JH, Krol RA, Desmond SM, Losh DP, Roberts SM, Snyder FF. Comparison of three antismoking interventions among pregnant women in an urban setting: a randomized trial. Psychol Rep 1991;68:595–604.
-
Rowan PJ, Cofta-Woerpel L, Mazas CA, Vidrine JI, Reitzel LR, Cinciripini PM, et al. Evaluating reactivity to ecological momentary assessment during smoking cessation. Exp Clin Psychopharmacol 2007;15:382–9.
-
Royer L. Dear sir… INTERVENE … computer assisted instructional programme … nicotine addiction and how one may coach individuals in tobacco cessation. J Subst Misuse Nurs Health Soc Care 1998;3:247.
-
Schinke S, Schwinn T. Gender-specific computer-based intervention for preventing drug abuse among girls. Am J Drug Alcohol Abuse 2005;31:609–16.
-
Schneider NG, Cortner C, Justice M, Gould JL, Amor C, Hartman N, et al. Preferences among five nicotine treatments based on information versus sampling. Nicotine Tob Res 2008;10:179–86.
-
Severson HH, Akers L, Andrews JA, Lichtenstein E, Jerome A. Evaluating two self-help interventions for smokeless tobacco cessation. Addict Behav 2000;25:465–70.
-
Severson HH, Gordon JS, Danaher BG, Akers L. ChewFree.com: evaluation of a Web-based cessation program for smokeless tobacco users. Nicotine Tob Res 2008;10:381–91.
-
Severson HH, Peterson AL, Andrews JA, Gordon JS, Cigrang JA, Danaher BG, et al. Smokeless tobacco cessation in military personnel: a randomized controlled trial. Nicotine Tob Res 2009;11:730–8.
-
Strayer SM, Rollins LK, Martindale JR. A handheld computer smoking intervention tool and its effects on physician smoking cessation counseling. JABFM 2006;19:350–7.
-
Taylor CB, Chang VY. Issues in the dissemination of cognitive-behavior therapy. Nordic J Psychiatr 2008;62(Suppl. 47):37–44.
-
Ulbricht S, Baumeister SE, Meyer C, Schmidt CO, Schumann A, Rumpf HJ, et al. Does the smoking status of general practitioners affect the efficacy of smoking cessation counselling? Patient Educ Couns 2009;74:23–8.
-
Unrod M, Smith M, Spring B, DePue J, Redd W, Winkel G. Randomized controlled trial of a computer-based, tailored intervention to increase smoking cessation counseling by primary care physicians. J Gen Internal Med 2007;22:478–84.
-
Vidrine DJ, Arduino RC, Lazev AB, Gritz ER. A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS 2006;20:253–60.
-
Vidrine DJ, Arduino RC, Gritz ER. Impact of a cell phone intervention on mediating mechanisms of smoking cessation in individuals living with HIV/AIDS. Nicotine Tob Res 2006;8(Suppl. 1):103–8.
Excluded owing to population
For example, interventions targeted at school children or adolescents.
-
Ausems M, Mesters I, van BG, de VH. Short-term effects of a randomized computer-based out-of-school smoking prevention trial aimed at elementary schoolchildren. Prev Med 2002;34:581–9.
-
Ausems M, Mesters I, van BG, de VH. Effects of in-school and tailored out-of-school smoking prevention among Dutch vocational school students. Health Educ Res 2004;19:51–63.
-
Aveyard P, Cheng KK, Almond J, Sherratt E, Lancashire R, Lawrence T, et al. Cluster randomised controlled trial of expert system based on the transtheoretical (‘stages of change’) model for smoking prevention and cessation in schools. BMJ 1999;319:948–53.
-
Aveyard P, Sherratt E, Almond J, Lawrence T, Lancashire R, Griffin C, et al. The change-in-stage and updated smoking status results from a cluster-randomized trial of smoking prevention and cessation using the transtheoretical model among British adolescents. Prev Med 2001;33:313–24.
-
Bernhardt AM, Dalton MA, Sargent JD, Stevens MM. E-mail communication between medical students and schoolchildren: a model for medical education. Arch Pediatr Adolesc Med 2000;154:1258–62.
-
Fritz DJ. An intervention for adolescent smoking cessation. PhD thesis. Saint Louis, MI: University of Missouri; 2003.
-
Fritz DJ, Hardin SB, Gore PA, Jr, Bram D. A computerized smoking cessation intervention for high school smokers. Pediatr Nurs 2008;34:13–17.
-
Hawkins RP. Reaching hard-to-reach populations: interactive computer programs as public information campaigns for adolescents. J Comm 1982;37:8–28.
-
Hollis JF, Polen MR, Whitlock EP, Lichtenstein E, Mullooly JP, Velicer WF, et al. Teen reach: outcomes from a randomized, controlled trial of a tobacco reduction program for teens seen in primary medical care. Pediatrics 2005;115:981–9.
-
Kaiser NC, Owen JE, Winzelberg AJ. Technological advances in modifying adolescent health risk behaviors. In Diclemente RJ, Santelli JS, Crosby RA, editors. Adolescent health: understanding and preventing risk behaviors. San Francisco, CA: Jossey-Bass; 2009.
-
Kingston D. Clinician advice, an interactive computer program, and motivational counselling during routine medical visits increased reported smoking abstinence among teens. Evid Base Nurs 2005;8:105.
-
Kolinek M, Cizek L, Janout V. Efficiency Evaluation of Web Pages on Smoking Prevention in High School Students. Hygiena 2003;48:194–201.
-
Mermelstein R, Turner L. Web-based support as an adjunct to group-based smoking cessation for adolescents. Nicotine Tob Res 2006;8(Suppl. 1):S69–76.
-
Norman CD, Maley O, Li X, Skinner HA. Using the internet to assist smoking prevention and cessation in schools: a randomized, controlled trial. Health Psychol 2008;27:799–810.
-
Pallonen UE, Velicer WF, Prochaska JO, Rossi JS, Bellis JM, Tsoh JY, et al. Computer-based smoking cessation interventions in adolescents: description, feasibility, and six-month follow-up findings. Subst Use Misuse 1998;33:935–65.
-
Patten CA, Croghan IT, Meis TM, Decker PA, Pingree S, Colligan RC, et al. Randomized clinical trial of an Internet-based versus brief office intervention for adolescent smoking cessation. Patient Educ Couns 2006;64:249–58.
-
Patten CA, Rock E, Meis TM, Decker PA, Colligan RC, Pingree S, et al. Frequency and type of use of a home-based, Internet intervention for adolescent smoking cessation. J Adolesc Health 2007;41:437–43.
-
Prokhorov AV, Kelder SH, Shegog R, Murray N, Peters R, Jr, Gurcia-Parker C, et al. Impact of A Smoking Prevention Interactive Experience (ASPIRE), an interactive, multimedia smoking prevention and cessation curriculum for culturally diverse high-school students. Nicotine Tob Res 2008;10:1477–85.
-
Schinke SP, Fang L, Cole KC. Preventing substance use among adolescent girls: 1-year outcomes of a computerized, mother-daughter program. Addict Behav 2009;34:1060–4.
-
Severson HH, Arthur C, Widdop C, Shaw T, Christiansen S, Sarnoff-Wood A. Tobacco world: an interactive computer program for tobacco prevention with middle school students (RPOS3–104). Society for Research on Nicotine and Tobacco 13th Annual Meeting, 21–24 February 2007, Austin, TX.
-
Turner L, Mermelstein R. Web-based support as an adjunct to group-based smoking cessation for adolescents (POS2–50). Society for Research on Nicotine and Tobacco 12th Annual Meeting, 15–18 February 2006, Orlando, FL.
-
Woodruff SI, Conway TL, Edwards CC, Elliott SP, Crittenden J. Evaluation of an Internet virtual world chat room for adolescent smoking cessation. Addict Behav 2007;32:1769–86.
Excluded owing to study design
For example, commentary, letters, narrative reviews, systematic reviews superseded by later versions, surveys, use of historical control.
-
Anonymous. Internet-based smoking cessation schemes focused on individual patients work the best. Pharmaceut J 2005;274:536.
-
Anonymous. A computerized smoking-cessation program helped 23% of participating teens quit. Am J Nurs 2008;108:18.
-
Barrera AZ, Perez-Stable EJ, Delucchi KL, Muñoz RF. Global reach of an internet smoking cessation intervention among Spanish- and English-speaking smokers from 157 countries. Int J Environ Res Publ Health 2009;6:927–40.
-
Bialous SA, Sarna L, Wells M, Elashoff D, Wewers ME, Froelicher ES. Characteristics of nurses who used the Internet-based nurses QuitNet for smoking cessation. Publ Health Nurs 2009;26:329–38.
-
Bitton A. Outcomes research in review. Web- and computer-based smoking cessation programs are effective for adult smokers. J Clin Outcome Manag 2009;16;301–3.
-
Cunningham JA. Internet-based interventions for alcohol, tobacco and other substances of abuse. In Miller PM, Kavanagh DJ, editors. Translation of addictions science into practice. Amsterdam: Elsevier; 2007. pp. 399–416.
-
Farvolden P, Cunningham J, Selby P. Using E-health programs to overcome barriers to the effective treatment of mental health and addiction problems. J Tech Hum Serv 2009;27:5–22.
-
Fletes I, Sanchez B, Quesada M, Carreras JM, Maldonado B, Sanchez LE-MA, et al. New psychological treatments in tobacco dependence: new non face-to-face smoking cessation interventions. Psicooncologia 2006;3:337–45.
-
Jones T. Smoking and use of mobile phones. Data have been wrongly interpreted. BMJ 2001;322:616.
-
Lancaster T, Stead LF. Self-help interventions for smoking cessation. [Update of Cochrane Database Syst Rev 2002;3:CD001118; PMID: 12137618.] Cochrane Database Syst Res 2005;3:CD001118.
-
Lancaster T. Clinician advice, an interactive computer program, and motivational counseling increased smoking cessation in teens: commentary. Evid Base Med 2005;10:144.
-
Meyer C, Ulbricht S, Baumeister S, Schumann A, Ruge J, Bischof G, et al. Supporting GPS in the provision of smoking cessation intervention: from research to daily routine. Addiction 2008;103:309.
-
Myung S-K, McDonnell DD, Kazinets G, Seo HG, Moskowitz JM. Concerns about a meta-analysis of computer smoking cessation programs: reply. Arch Int Med 2009;169:1814–15.
-
Norman CD, McIntosh S, Selby P, Eysenbach G. Web-assisted tobacco interventions: empowering change in the global fight for the public’s (e)Health. J Med Internet Res 2008;10:e48.
-
Op’t Holt TB. E-mail communication in a smoking-cessation program. Respir Care 2009;54:1024–5.
-
Prokhorov AV, Fouladi R, deMoore C, Warneke C, Luca M, Mullin Jones M, et al. Computer-assisted, counselor-delivered smoking cessation counseling for community college students: Intervention approach and sample characteristics. J Child Adolesc Subst Abuse 2007;16:35–62.
-
Ramelson HZ, Friedman RH, Ockene JK. An automated telephone-based smoking cessation education and counseling system. Patient Educ Couns 1999;36:131–44.
-
Sanchez AL, Carreras Castellet JM. [Smoking cessation treatment on the internet.] Arch Bronconeumol 2007;43:1–3.
-
Schumann A, John U, Ulbricht S, Ruge J, Bischof G, Meyer C. Variability of tailoring of a smoking cessation intervention based on the transtheoretical model. Addict Behav 2007;32:3083–7.
-
Stitzer MA. Brief interventions for tobacco cessation: comment. Addiction 2008:103:306.
-
Strecher VJ. Computer-tailored smoking cessation materials: a review and discussion. Patient Educ Couns 1999;36:107–17.
-
Strecher VJ. The internet: Just another smoking cessation tool? Addiction 2008;103:485–6.
-
Webb TL. Commentary on Shahab & McEwen: Understanding and preventing attrition in online smoking cessation interventions: a self-regulatory perspective. Addiction 2009;104:1805–6.
-
Wolfenden L, Wiggers J, Campbell E, Knight J. Pilot of a preoperative smoking cessation intervention incorporating post-discharge support from a Quitline. Health Promot J Aust 2008;19:158–60.
Excluded owing to irrelevant comparison
For example, the comparison made in the study actually evaluated the effectiveness of other (non-electronic) interventions rather than the effectiveness of the electronic interventions of interest.
-
Etter JF, Huguelet P, Perneger TV, Cornuz J. Nicotine gum treatment before smoking cessation: a randomized trial. Arch Int Med 2009;169:1028–34.
-
Muñoz RF, Lenert LL, Delucchi K, Stoddard J, Perez JE, Penilla C, et al. Toward evidence-based Internet interventions: a Spanish/English Website for international smoking cessation trials. Nicotine Tob Res 2006;8:77–87.
-
Stoops WW, Dallery J, Fields NM, Nuzzo PA, Schoenberg NE, Martin CA, et al. An internet-based abstinence reinforcement smoking cessation intervention in rural smokers. Drug Alcohol Depend 2009;105:56–62.
Excluded owing to study ongoing: outcome data not available
-
Claes N, Jacobs N. The PreCardio-study protocol: a randomized clinical trial of a multidisciplinary electronic cardiovascular prevention programme. BMC Cardiovasc Dis 2007;7:27.
-
Gilbert H, Nazareth I, Sutton S, Morris R, Godfrey C. Effectiveness of computer-tailored Smoking Cessation Advice in Primary Care (ESCAPE): a randomised trial. Trials 2008;9:23.
-
Graham A. Internet and telephone treatment for smoking cessation: the iQUITT study. Brown Univ Dig Addict Theory Appl 2006;25:8.
-
Kramer JJ, Willemsen MC, Conijn B, van Emst AJ, Brunsting S, Riper H. Effectiveness of a web-based self-help smoking cessation intervention: protocol of a randomised controlled trial. BMC Publ Health 2009;9:32.
Excluded owing to outcome
For example, smoking cessation outcomes not reported; methodological paper evaluating recruitment or reliability of measurements.
-
Aveyard P, Lawrence T, Croghan E, Evans O, Cheng KK. Is advice to stop smoking from a midwife stressful for pregnant women who smoke? Data from a randomized controlled trial. Prev Med 2005;40:575–82.
-
Aveyard P, Lawrence T, Evans O, Cheng KK. The influence of in-pregnancy smoking cessation programmes on partner quitting and women’s social support mobilization: a randomized controlled trial [ISRCTN89131885]. BMC Publ Health 2005;5:80.
-
Graham AL, Bock BC, Cobb NK, Niaura R, Abrams DB. Characteristics of smokers reached and recruited to an internet smoking cessation trial: a case of denominators. Nicotine Tob Res 2006;8(Suppl. 1):43–8.
-
Koo M, Skinner H. Challenges of internet recruitment: a case study with disappointing results. J Med Internet Res 2005;7:e6.
Non-English-language papers not assessed owing to time constraint
-
Baena A, Quesada M. [The integrating and complementary role of the new information and communication technologies in the control and treatment of smoking habit.] Trastornos Adictivos 2007;9:46–52.
-
Del P, I, Esteban A, Nuez C, Gonzalez A. [Smoking cessation treatment in the regional community of La Rioja.] Trastornos Adictivos 2007;9:6–13.
-
Dijkstra A, de Vries H, Roijackers J, van Breukelen G. [Individualized interventions in smoking cessation: a field experimental trial.] Gedrag Gezondheid: Tijdschr Psychol Gezondheid24:322.
-
Etter J-F. [An expert system for smoking cessation.] Rev Med Suisse Romande 1998;118:515–16.
-
Martin-Diener E, Gehring TM, Somaini B. [Computer-assisted smoking cessation.] Ther Umsch 1997;54:463–7.
-
Meyer C, Ulbricht S, Schumann A, Hannover W, Hapke U, Rumpf H-J, et al. [Interventions fostering the motivation to quit for smokers in general practice.] SMP 2003;5:134–6.
Conference abstracts unobtainable
-
Cobb NK, Graham AL, Stoddard JL, Rabius V, Saul JE. Evaluating Internet based cessation programs Part 1. National Conference on Tobacco or Health, 4–6 May 2005, Chicago, IL.
-
Strecher V, Shiffman S, West R. Evaluation of a web-based computer-tailored smoking cessation program among nicotine patch users. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20–23 March 2005, Prague, Czech Republic.
-
Buller D, Borland R, Woodall G, Hall J, Hines J, Burris-Woodall P, et al. Randomized trials on ‘Consider This’, an internet smoking prevention program for adolescents. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20–23 March 2005, Prague, Czech Republic.
-
Strecher V, Shiffman S, West R. Evaluation of a web-based computer-tailored smoking cessation program among nicotine patch users. Society for Research on Nicotine and Tobacco 10th Annual Meeting, 18–21 February 2004, Phoenix, AZ.
-
Strecher V, Shiffman S, West R. Evaluation of a web-based computer-tailored smoking cessation program among nicotine patch users. Society for Research on Nicotine and Tobacco 5th European Meeting, 20–22 November 2003, Padua, Abstract book 2003.
-
Borland R, Hunt R, Balmford J. A randomised trial of a program of computer-generated personalized advice focussing on relapse prevention. Society for Research on Nicotine and Tobacco 8th Annual Meeting, 20–23 February 2002, Savannah, GA.
-
An L, Hennrikus D, Baker RD, Ehlinger E, Lando H. Recruiting college smokers to a web-based smoking cessation resource (RP-34). Society for Research on Nicotine and Tobacco 8th Annual Meeting, Rapid Communications Posters 20–23 February 2002, Savannah, GA.
-
Gilljam H. Office based sequential computer tailoring as an aid for smoking cessation (PO3 74). Society for Research on Nicotine and Tobacco, 7th Annual Meeting, 23 March 2001, Seattle, WA.
-
Burling AS, Burling TA. A work in progress: effectiveness of a comprehensive internet-delivered interactive multimedia stop smoking program. Association for the Advancement of behavior Therapy, 34th Annual Meeting, November 2000, New Orleans, LA.
-
Aboussafy D. Behavioral smoking cessation: effectiveness, challenges, and development of an internet stop smoking program. 11th World Conference on Tobacco or Health, 6–11 August 2000, Chicago, IL, Abstract book 2000. 1:177.
-
McDaniel AM. Computer-assisted smoking cessation counseling for hospitalized smokers. Society for Research on Nicotine and Tobacco, Sixth Annual Meeting, 18–20 February 2000, Arlington VI.
-
Etter JF, Perneger TV. Effectiveness of a computer-tailored smoking cessation program. 11th World Conference on Tobacco or Health, 6–11 August 2000, Chicago, IL, Abstract book 2000.
-
Jordan JC, Reynolds RV, Myers DL, Tobin TL, Jones JR, Hall LK, et al. Comparison of internet delivered and printed self-help smoking cessation programs with hospital employees. Society for Research on Nicotine and Tobacco, Fifth Annual Meeting, 5–7 March 1999, San Diego, CA.
-
Shiffman S, Gitchell JG, Strecher VJ. Real-world efficacy of computer-tailored smoking cessation material as a supplement to nicotine replacement. 10th World Conference on Tobacco or Health, 24–28 August 1997, Beijing, China, Abstract book 1997:125.
Further duplicate record not previously identified
-
Muñoz RF, Barrera AZ, Delucchi K, Penilla C, Torres LD, Perez-Stable EJ. International Spanish/English internet smoking cessation trial yields 20% abstinence rates at 1 year. Nicotine Tob Res 2009;11:1025–34.
Appendix 5 Results of quality assessment of cost-effectiveness papers based on the Drummond checklist
| Question from checklist | Lennox et al.55 | Smith et al.136 | Barnett et al.81 |
|---|---|---|---|
| 1. Was a well-defined question posed in answerable form? | |||
| 1.1 Did the study examine both costs and effects of the services(s) or programme(s)? |
Effects: Point abstinence at 6 months Costs: Additional cost per quitter of tailored letter is given, but no information is presented on how this was derived |
The trial end point was point abstinence at 6 months. Data from the literature were used to translate this into QALYs/LYS. Costs included fixed upfront system costs, allocated across smokers, time taken by doctors to review reports, administration and report production costs |
Effects: 7-day point abstinence measured four times over 18 months Costs: Health-care utilisation in 2003 US$ |
| 1.2. Did the study involve a comparison of alternatives? | Computer-tailored letter, generic letter, no letter | The intervention was a computer system to generate reports for physicians tailored to individual smoker patients, to guide the provision of appropriate advice. This was compared with usual care |
Control: Printed stop-smoking guide and list of smoking cessation programmes Intervention: Step 1 – three assessments of readiness to quit using a computer-mediated evaluation reviewed by counsellor Step 2 – if contemplating quitting, or requested treatment, then get six sessions of counselling and up to 10 weeks of NRT patch. If still smoking, bupropion and two additional sessions |
| 1.3. Was a view point for the analysis stated and was the study placed in any particular decision-making context? | The interventions were evaluated in a general practice setting. However, a specific view point or decision context was not specified | The view point was the primary care practice | Costs were estimated from the perspective of the health-care payer. The intervention was targeted at smokers receiving treatment for depression |
| 2. Was a comprehensive description of the competing alternatives given (i.e. can you tell who did what to whom, where and how often)? | |||
| 2.1. Were there any important alternatives omitted? | No | A generic system was not evaluated | Targeted computer-generated support could have been included in the evaluation |
| 2.2. Was (should) a do-nothing alternative be considered? | The option of sending no letter was included | Usual care was the comparator | The control did involve some resource use but this could be considered the minimum acceptable level of support |
| 3. Was the effectiveness of the programme or services established? | |||
| 3.1. Was this done through a RCT? If so, did the trial protocol reflect what would happen in regular practice? | The evaluation was based on results of a RCT. The protocol could be the basis of actual practice without amendment | The evaluation was based on results of a RCT. The protocol could be the basis of actual practice without amendment | A randomised trial was performed to generate data for the analysis. The trial protocol could be implemented as service delivery, although changes in staff delivering counselling might have an impact on costs and effects. Payments were made for each research assessment, which will have influenced follow-up rates |
| 3.2. Was effectiveness established through an overview of clinical studies? | No | No | No |
| 3.3. Were observational data or assumptions used to establish effectiveness? If so, what are the potential biases in results? | LYS from quitting from Oster et al.138 | Quit rate 45% ‘from the literature’. LYS by quitting from Fiscella and Franks139 | Nine studies are identified of smoking cessation, reporting an average of 1.2 LYS per quitter |
| 4. Were all the important and relevant costs and consequences for each alternative identified? | |||
| 4.1. Was the range wide enough for the research question at hand? | Point abstinence at 6 months is insufficient for decision-making. Information is lacking on the costs included. There may have been additional resources used resulting from the intervention beyond those involved in generating the letters themselves (e.g. advice services, NRT), and these may not have been included | Point abstinence at 6 months is insufficient for decision-making. NRT and bupropion costs per smoker were included, but not the use of any support services | Costs included the resources involved in the computer-mediated evaluations (including patient reminder letters), counselling sessions and pharmacological support. Effectiveness was measured as the quit rate (point abstinence) at 18 months. Medical care costs were not included, and quality-of-life adjustments not performed |
| 4.2. Did it cover all relevant viewpoints? (Possible viewpoints include the community or social viewpoint, and those of patients and third-party payers. Other viewpoints may also be relevant depending upon the particular analysis) | This is difficult to answer, particularly regarding costs, given the lack of information on how they were derived | The viewpoint was specifically restricted to the individual clinic. Patient or third-party viewpoints were not fully included, even though they may be more appropriate to the decision being made. However, adjuvant therapy costs were included | The viewpoint was that of the health-care payer. Participant self-incurred costs were not included |
| 4.3. Were the capital costs, as well as operating costs, included? | Cannot say from the information given | Capital costs for the computer system were included and amortised over 10 years | Labour costs included an overheads component. The capital costs of the computer hardware and software do not appear to have been included |
| 5. Were costs and consequences measured accurately in appropriate physical units (e.g. hours of nursing time, no. of physician visits, lost work days, gained life-years)? | |||
| 5.1. Were any of the identified items omitted from measurement? If so, does this mean that they carried no weight in the subsequent analysis? | No items were specifically identified and then omitted | All identified items are quantified | No identified items appear to have been omitted |
| 5.2. Were there any special circumstances (e.g. joint use of resources) that made measurement difficult? Were these circumstances handled appropriately? | The development of the tailoring software is a fixed cost that would need to be spread over the total no. of users – it is not stated if or how this was done | The system cost is fixed and up front. It was spread over 136 smokers per year, based on US routine data on the average size of a practice, the rate of new joiners, and the percentage of smokers in the population | Any computer support system will have generated hardware and software costs that need to be spread over the lifetime of the system and the no. of patients benefitting. It is not clear if or how this has been done |
| 6. Were the cost and consequences valued credibly? | |||
| 6.1. Were the sources of all values clearly identified? (Possible sources include market values, patient or client preferences and views, policy-makers’ views and health professionals’ judgements) | No specific values used to derive costs for the cost-effectiveness analysis were listed | Staffing costs were taken from Medicare allowable fees. Source of IT costs NR | Costs were based on staff reimbursement, Medicare rates for in-patient care, and the Pharmacy Red Book |
| 6.2. Were market values employed for changes involving resources gained or depleted? | Cannot tell from the analysis | Medicare may not represent true market cost of time. Not possible to be sure that IT costs reflect true market value | Yes, as far as standard tariffs do so |
| 6.3. Where market values were absent (e.g. volunteer labour), or market values did not reflect actual values (such as clinic space donated at a reduced rate) were adjustments made to approximate market values? | No such adjustments are referred to | No such adjustments are referred to | Not applicable |
| 6.4. Was the valuation of consequences appropriate for the question posed (i.e. has the appropriate type or types of analysis – cost-effectiveness, cost–benefit, cost–utility – been selected)? | If the comparison is to be with other means of achieving smoking cessation, then cost-effectiveness analysis was appropriate | Not clear whether or not cost–utility analysis is appropriate at the individual clinic level | |
| 7. Were costs and consequences adjusted for differential timing? | |||
| 7.1. Were costs and consequences that occur in the future ‘discounted’ to their present values? | When extending the results to estimate costs per LYS, a discount rate of 5% was used | A discount rate of 3% was used, although it is not obvious that it was used to amortise IT system costs | No discount rate is referred to. For cost-effectiveness, this may be appropriate as resource use is immediate. For cost-utility analysis, the discounted value for LYS should be used (the sources for LYS need to be checked to determine whether or not the 1.2 LYS is discounted) |
| 7.2. Was there any justification given for the discount rate used? | No | No | NA |
| 8. Was an incremental analysis of costs and consequences of alternatives performed? | |||
| 8.1. Were the additional (incremental) costs generated by one alternative over another compared with the additional effects, benefits or utilities generated? | The incremental cost per quitter for generic compared with no letter was presented | The incremental costs and LYS/QALYs were calculated of replacing usual care with the computer system for guiding physicians in interacting with potential quitters | An ICER for the stepped programme compared with the control option was presented |
| 9. Was allowance made for uncertainty in the estimates of costs and consequences? | |||
| 9.1. If data on costs and consequences were stochastic (randomly determined sequence of observations), were appropriate statistical analyses performed? | Logistic regression modelling was carried out to evaluate potential explanatory variables for abstinence rates. No statistical analyses of costs are presented | CIs were presented around effectiveness results. Only point estimates were given for ICERs | Statistically significant differences in resource utilisation/cost were identified using non-parametric (rank sum) tests. Bootstrapping was used to estimate the probability of cost-effectiveness |
| 9.2. If a sensitivity analysis was used, was justification provided for the range of values (or for key study parameters)? | Sensitivity analysis on the number of non-smokers receiving the control letter unnecessarily explored a best-case and worst-case scenario. No other sensitivity analyses were presented | Four sensitivity analyses were calculated – time spent by doctors reviewing the system outputs, distribution of smokers within ranges, computer costs, underlying quit rate. A few illustrations are provided of the impact of varying these parameters. Threshold analyses are not presented | The impact of adding mental health care costs on the ICER was explored. The impact of assuming that mental health outpatients realise only 50% of the gain from quitting |
| 9.3. Were the study results sensitive to changes in the values (within the assumed range for sensitivity analysis, or within the CI around the ratio of costs to consequences)? | The sensitivity analysis presented suggested that generic letters were cost-effective across the range considered for mistargeting | Not enough information is presented to determine this | Including these costs increased the ICER from US$5170 per LYS to US$9580 per LYS. Assuming that mental health outpatients gain 50% of the benefit from quitting increased the ICER to US$19,160 per LYS |
| 10. Did the presentation and discussion of study results include all issues of concern to users? | |||
| 10.1. Were the conclusions of the analysis based on some overall index or ratio of costs to consequences (e.g. cost-effectiveness ratio)? If so, was the index interpreted intelligently or in a mechanistic fashion? | Cost-effectiveness ratios were presented for the comparison of generic vs no letter. These were compared with similar ratios generated for other interventions with the same goal | Conclusions compare cost-effectiveness ratio – US$869 per QALY – with commonly used thresholds. Cost-effectiveness looked also at stages of change, and compared results with a counselling-based intervention | The ICER was calculated in terms of both cost per quitter and cost per LYS |
| 10.2. Were the results compared with those of others who have investigated the same question? If so, were allowances made for potential differences in study methodology? | A comparison was made, which highlighted a lack of biochemical verification of smoking status as a potential source of discrepancies | Results compared with a range of evaluations of similar interventions. Although comparison was favourable to the intervention being evaluated, differences in study methodology were not explored | The results were compared with nine studies of smoking cessation programmes that included counselling. One important difference was that this study specifically looked at a depressed population |
| 10.3. Did the study discuss the generalisability of the results to other settings and patient/client groups? | The study was based on a RCT set in north-east Scotland, and no reference is made to how well the results might generalise beyond this area. However, logistic regression analysis is presented which would allow some adaptation of the results to populations with different characteristics | Study carried out in NYC, USA. It is argued that gains were smaller here than might be observed elsewhere, as the base smoking rate is low | No |
| 10.4. Did the study allude to, or take account of, other important factors in the choice or decision under consideration (e.g. distribution of costs and consequences or relevant ethical issues)? | The impact of social deprivation on outcome was modelled. No other such analyses were presented | One issue raised was how quality adjustment might impact the results. If mental health outpatients have an average quality of life that is lower than the general population, this might reduce the cost-effectiveness of the intervention for the subgroup. The ethical implications of this were not explored | |
| 10.5. Did the study discuss issues of implementation, such as the feasibility of adopting the ‘preferred’ programme given existing financial or other constraints, and whether or not any freed resources could be redeployed to other worthwhile programmes? | The discussion argues that the intervention could be easily implemented in a range of situations, as it does not require input from trained health professionals to deliver the letters. This would allow scarce time to be freed up for other uses | It is suggested that the programme would take advantage of the growing computerisation of physicians’ offices | It is suggested that the mental health treatment setting is a convenient place to deliver the intervention |
Appendix 6 Description of the time-to-relapse model
We had data on continuous abstinence from n = 28 trials, each with between two and four arms. The duration of abstinence varied from 1 to 12 months, and some trials reported abstinence at several durations. We therefore have the following information reported:
-
ri,j,k = number of people reporting continuous abstinence at the kth observation for arm j of trial i
-
ti,j,k = time of reporting at the kth observation for arm j of trial i
-
ni,j = number of participants in arm j of trial i.
This observed number of people reporting continuous abstinence is assumed to have a binomial likelihood:
where:
-
pi,j,k = probability of continuous abstinence on interval [ti,j(k – 1),ti,j,k] given continuous abstinence up until time ti,j(k–1)
-
nbi,j,k = ri,j,k – 1 if k > 1 and nbi,j,k = ni,j if k = 1.
The reason for defining nbi,j,k in this way is to account for the fact that where multiple observations of continuous abstinence are made, these observations are not independent, as a participant can be abstinent only at a later observation if they were abstinent at an earlier one. By conditioning on abstinence at the previous time point we allow for this lack of independence.
We assume that the times to relapse Ti,j for each participant in arm j of trial i are independent and identically distributed. Then:
giving:
where:
i.e. Si,j(t) is the survival function for our time-to-relapse model.
We considered two different distributions for time-to-relapse, the Exponential and the Weibull distributions. The survival functions for these two distributions are:
and
We assume that treatment has an additive effect on the log of the scale parameter λ in both the Exponential and Weibull models, but not the shape parameter α of the Weibull distribution. This results in a proportional hazard treatment effect model, as is shown below.
The Weibull survival model gives the following functional form for pi,j,k:
so that:
The equivalent for the exponential survival model can be derived by setting α to equal 1 giving:
so that:
The shape parameter for the Weibull model depends on study, and we assume that the log of the shape parameters are exchangeable across studies (i.e. come from a common Normal distribution of shape parameters).
Appendix 7 WinBUGS code for synthesis models
The code for the evidence synthesis models is given below.
Appendix 8 Search of health technology appraisals for cost information on relevant electronic aid interventions
The complete list of NIHR HTA monographs up to Vol. 13(49) (published November 2009) was reviewed by title to identify potential sources of information on the resources required to provide electronic interventions supporting behavioural change. Twenty-six articles were selected for executive summary review – these are given in Appendix 9. On review of the executive summary of each monograph (and parts of the main report, if required), two were found to have assessed potentially relevant interventions (Riemsma et al. ,186 Kaltenthaler et al. 144). These two articles are described in more detail in Chapter 4.
Appendix 9 NIHR Health Technology Assessment monographs potentially informative for resource use of behavioural change interventions
| Volume and no. | Title and lead author | Description of intervention |
|---|---|---|
| v1n6 | Systematic review of outpatient services for chronic pain control (McQuay) | CBT delivered by professionally trained therapists |
| v3n23 | Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee: a review (Lord) | Nurse-led group sessions |
| v4n14 | Systematic review of the determinants of screening uptake and interventions for increasing uptake (Jepson) | Audiovisual educational materials, telephone counselling, educational home visits |
| v4n19 | RCT of non-directive counselling, cognitive-behaviour therapy and usual GP care in the management of depression as well as mixed anxiety and depression in primary care (King) | CBT/counselling delivered by professionally trained therapists |
| v4n27 | Treatments for fatigue in multiple sclerosis: a rapid and systematic review (Branas) | Behavioural advice |
| v5n10 | Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review (Hampson) | Expert or peer-led education programmes |
| v5n35 | A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression (Churchill) | CBT and other forms of psychotherapy delivered by professionally trained therapists |
| v6n22 | A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety (Kaltenthaler) | CBT software packages |
| v6n24 | A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change in health care settings (Riemsma) | Smoking cessation support based on stages-of-change |
| v7n22 | The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation (Loveman) | Expert-led education on self-management |
| v7n28 | A RCT to assess the impact of a package comprising a patient-orientated, evidence-based self-help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease (Kennedy) | Training for consultants and a patient guidebook |
| v8n8 | Psychological treatment for insomnia in the regulation of long-term hypnotic drug use (Morgan) | CBT delivered by professionally trained therapists |
| v9n23 | A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma (Smith) | Intervention(s) – education delivered by health professionals |
| v9n42 | Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland (Durham) | CBT delivered by professionally trained therapists |
| v9n50 | The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children (Dretzke) | Professional or self directed parent training programmes (the latter involving workbooks or videos) |
| v10n19 | Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: a RCT (Kennedy) | CBT delivered by professionally trained therapists |
| v10n26 | A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use (Robinson) | Music therapy, special care units, etc. |
| v10n33 | Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation (Kaltenthaler) | CBT software packages |
| v10n37 | Cognitive behavioural therapy in chronic fatigue syndrome: a RCT of an outpatient group programme (O’Dowd) | Group CBT run by professionally trained therapists |
| v11n39 | A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder (Soares-Weiser) | Pharmacological agents and CBT delivered by professionally trained therapists |
| v12n9 | The clinical effectiveness of diabetes education models for type 2 diabetes: a systematic review (Loveman) | Health-professional led education programmes |
| v12n14 | A RCT of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial (Goodyer) | CBT delivered by professionally trained therapists |
| v13n30 | Psychological interventions for postnatal depression: cluster randomised trial and economic evaluation. The PONDER trial (Morrell) | Training for health visitors |
Appendix 10 Search of the NHS Electronic Evaluations Database for studies reporting for cost information on relevant electronic aid interventions
The NHS EED is maintained by the CRD of the University of York. It contains details of around 24,000 health economic evaluations.
A search was carried out of the NHS EED using the query (internet OR electronic) AND (cost* OR resource*) to identify any economic evaluations or cost studies of the intervention type of interest. The search strategy identified 187 articles Following a review of the description of each article in the database, this was reduced to the eight references for full-paper review. Of these, three did not provide any information on costing/resource use for electronic interventions:
-
Ref. A2.1 Block G, Sternfeld B, Block C H, Block T J, Norris J, Hopkins D, et al. Development of Alive! (a lifestyle intervention via email), and its effect on health-related quality of life, presenteeism, and other behavioral outcomes: randomized controlled trial. J Med Internet Res 2008;10:e43.
-
Ref. A2.2 Hurley S F, Matthews J P. The Quit Benefits Model: a Markov model for assessing the health benefits and health care cost savings of quitting smoking. CERA 2007;32(2).
-
Ref. A2.3 Kaldo V, Levin S, Widarsson J, Buhrman M, Larsen H C, Andersson G. Internet versus group cognitive-behavioral treatment of distress associated with tinnitus: a randomized controlled trial. Behav Ther 2008;39:348–59.
The remaining five references were considered to provide information in line with the aims of the search, and their findings are discussed in the main body of the report. These references are listed below.
-
Ref A2.4 Graham A L, Milner P, Saul J E, Pfaff L. Online advertising as a public health and recruitment tool: comparison of different media campaigns to increase demand for smoking cessation interventions. J Med Internet Res 2008;10:e50.
-
Ref A2.5 Runge C, Lecheler J, Horn M, Tews J T, Schaefer M. Outcomes of a web-based patient education program for asthmatic children and adolescents. Chest 2006;129:581–93.
-
Ref A2.6 Murray E. Internet-delivered treatments for long-term conditions: strategies, efficiency and cost-effectiveness. Expert Rev of Pharmacoeconomics Outcomes Res 2008;8:261–72.
-
Ref A2.7 Meenan RT, Stevens VJ, Funk K, Bauck A, Jerome GJ, Lien LF, et al. Development and implementation cost analysis of telephone- and Internet-based interventions for the maintenance of weight loss. Int J Technol Assess Health Care 2009;25:400–10.
-
Ref A2.8 Southard BH, Southard DR, Nuckolls J. Clinical trial of an Internet-based case management system for secondary prevention of heart disease. J Cardiopulm Rehabil 2003;23:341–4.
Appendix 11 Supplementary review excluded articles
| Article | Reason for exclusion |
|---|---|
| An LC, Hennrikus DJ, Perry CL, Lein EB, Klatt C, Farley DM, et al. Feasibility of internet health screening to recruit college students to an online smoking cessation intervention. Nicotine Tob Res 2007;9(Suppl. 1):11–18. | Recruitment only |
| Anderson C, Mair A. Pro-change adult smokers program: Northumberland pilot. Int J Pharm Pract 2002;10:281–7. | Pharmacy-based smoking cessation intervention, no relevant outcomes included |
| Balmford J, Borland R, Li L, Ferretter I. Usage of an Internet smoking cessation resource: the Australian QuitCoach. Drug Alcohol Rev 2009;28:66–72. | Results from same study included in an article already in the review |
| Becona E, Becona, E. Smoking cessation at home: ‘Programa 2001 para dejar de%%fumar’ (Program to stop smoking). Psicooncologia 2006;3:2336. | Article in Spanish |
| Bock B, Graham A, Sciamanna C, Krishnamoorthy J, Whiteley J, Carmona-Barros R, et al. Smoking cessation treatment on the Internet: content, quality, and usability. Nicotine Tob Res 2004;6:207–19. | Review of content of internet smoking cessation websites |
| Brandon TH, Copeland AL, Saper ZL. Programmed therapeutic messages as a smoking treatment adjunct: reducing the impact of negative affect. Health Psychol 1995;14:41–7. | Message content only |
| Burling TA, Seidner AL, Gaither DE. A computer-directed program for smoking cessation treatment. J Subst Abuse 1994;6:427–31. | No relevant outcomes |
| Carpenter KM, Watson JM, Raffety B, Chabal C. Teaching brief interventions for smoking cessation via an interactive computer-based tutorial. J Health Psychol 2003;8:149–60. | Focus is on teaching professionals only |
| Chen HH, Yeh ML. Developing and evaluating a smoking cessation program combined with an internet assisted instruction program for adolescents with smoking. Patient Educ Couns 2006;61:411–18. | Children included in sample |
| Chen HH, Yeh ML, Chao YH. Comparing effects of auricular acupressure with and without an internet assisted programme on smoking cessation and self-efficacy of adolescents. J Alternative Compl Med 2006;12:147–52. | Children included in sample |
| Cobb NK, Graham AL. (2006). Characterizing internet searchers of smoking cessation information. J Med Int Res 2006;8:e17. | Limited to description of characteristics of smokers who search for online cessation advice |
| Cobb NK, Graham AL, Bock BC, Papandonatos, G, Abrams DB. (2005). Initial evaluation of a real-world Internet smoking cessation system. Nicotine Tob Res 2006;7:207–16. | No relevant outcomes |
| Coffay AO. Smoking cessation: Tactics that make a big difference. J Fam Pract 2007;56:824. | No relevant outcomes |
| Dallery J, Glenn IM. Effects of an Internet-based voucher reinforcement program for smoking abstinence: a feasibility study. J Appl Behav Anal 2005;38:349–57. | Limited study: includes a sample of four smokers |
| Dallery J, Meredith S, Glenn IM. A deposit contract method to deliver abstinence reinforcement from smoking. J Appl Behav Anal 2008;41:609–15. | Not an electronic aid |
| Danaher BG, Hart LG, McKay HG, Severson HH. Measuring participant rurality in Web-based interventions. BMC Publ Health 2007;7:228. | Focus on where participants live only |
| Escoffery C, McCormick L, Bateman K. Development and process evaluation of a web-based smoking cessation program for college smokers: innovative tool for education. Patient Educ Couns 2004;53:217–25. | Commentary on development of a tool |
| Finkelstein J, Lapshin O, Cha E. Feasibility of promoting smoking cessation among methadone users using multimedia computer-assisted education. J Med Int Res 2008;10:e33. | Primary focus drug misuse clients and on tool promotion not use |
| Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med 2009;36:165–73. | Describes an electronic aid but no outcomes |
| French D. Influence smoking cessation with computer-assisted instruction. AAOHN J 1986;34:391–4. | No abstract |
| Gilbert H, Nazareth I, Sutton SE, Gilbert H. ‘Some of these words I can’t pronounce’. A qualitative exploration of the readability of generic and tailored self-help material for quitting smoking. J Appl Biobehav Res 2009;14:3620. | Findings from the same study included in another article in the review |
| Glasgow RE, Hollis JF, McRae SG, Lando HA, LaChance P. Providing an integrated program of low intensity tobacco cessation services in a health maintenance organization. Health Educ Res 1991;6:87–99. | No relevant outcomes: electronic aids only and a small part of an intervention |
| Graham AL, Abrams DB. Reducing the cancer burden of lifestyle factors: opportunities and challenges of the internet. J Med Int Res 2005;7:e26. | Commentary only |
| Graham AL, Papandonatos GD. Reliability of internet versus telephone administered questionnaires in a diverse sample of smokers. J Med Int Res 2008;10:e8. | Testing reliability of a tool |
| Graham AL, Milner P, Saul JE, Pfaff L. Online advertising as a public health and recruitment tool: comparison of different media campaigns to increase demand for smoking cessation interventions. J Med Int Res 2008;10:e50. | No relevant outcomes: primarily a mass media intervention not treatment |
| Hailstone S, Wyndham A, Mitchell E. Delivering smoking cessation information in the workplace using Quit Online. NSW Publ Health Bull 2005;16:18–22. | Commentary/magazine article |
| Hareva DH, Okada H, Kitawaki T, Oka H. Supportive intervention using a mobile phone in behaviour modification. Acta Med Okayama 2009;63:113–20. | Article in Japanese |
| Haug S, Meyer C, Gross B, Schorr G, Thyrian JR, Kordy H, et al. Continuous individual support of smoking cessation in socially deprived young adults via mobile phones: results of a pilot study. Gesundheitswesen 2008;70:364–71. | Article in German |
| Hirdes BAP. The role of motivational design in health education: an examination of computer-based eking and health education on women. Dis Abst Int A Hum Soc Sci 2005;65(10-A). | Dissertation abstract |
| Hotta K, Kinumi K, Naito K, Kuroki K, Sakane H, Imai A, et al. An intensive group therapy programme for smoking cessation using nicotine patch and internet mailing supports in a university setting. Int J Clin Pract 2007;61:1997–2001. | Not an electronic aid |
| Houston TK, Ford DE. A tailored internet-delivered intervention for smoking cessation designed to encourage social support and treatment seeking: usability testing and user tracing. Inform Health Soc Care 2008;33:5–19. | No relevant outcomes: primarily trying to get smokers to access face-to-face treatment |
| Japuntich SJ, Zehner ME, Smith SS, Jorenby DE, Valdez JE, Fiore MC, et al. Smoking cessation via the internet: two randomized clinical trials of an internet intervention for smoking cessation (PA11–2). Society for Research on Nicotine and Tobacco, 12th Annual Meeting, 15–18 February 2006, Orlando, FL. | Conference abstract |
| Johs Artisensi JL. The effect of web-based support as an adjunct to a self-help smoking cessation program. Dis Abst Int B Sci Eng 2003;63:4138. | Dissertation abstract |
| Konstantinidis ST, Konstantinidis E, Nikolaidou MM, Boutou AK, Havouzis N, Argyropoulou P, et al. The use of open source and Web 2.0 in developing an integrated EHR and e-learning system for the Greek Smoking Cessation Network. Stud Health Tech Informat 2009;150:354–8. | No relevant outcomes: primarily e-learning/training |
| Krebs PM. Computerized, tailored, theory-based interventions for health behavior change: A comprehensive meta-analysis. Dis Abstr Int B Sci Eng 2008;68(8-B). | Dissertation abstract |
| Krist AH, Woolf SH, Frazier CO, Johnson RE. Rothemich SF, Wilson DB, et al. An electronic linkage system for health behavior counseling effect on delivery of the 5A’s. Am J Prev Med 2008;35(Suppl. 5):350–8. | No relevant outcomes |
| Lazev A, Vidrine D, Arduino R, Gritz E. Increasing access to smoking cessation treatment in a low-income, HIV-positive population: the feasibility of using cellular telephones. Nicotine Tob Res 2004;6:281–6. | Telephone intervention |
| Ma M. It design for sustaining virtual communities: An identity-based approach. Dis Abst Int A Hum Soc Sci 2006;66(7-A). | Dissertation abstract |
| Martin RA. Latent Transition Analysis as a sensitive outcome analysis for longitudinal data. Dis Abst Int B Sci Eng 2004;64(8-B). | Dissertation abstract |
| McClure JB, Greene SM, Wiese C, Johnson KE, Alexander G, Strecher V. Interest in an online smoking cessation program and effective recruitment strategies: results from Project Quit. J Med Int Res 2006;8:e14. | No relevant outcomes: recruitment only |
| McDaniel AM, Benson PL, Roesener GH, Martindale J. An integrated computer-based system to support nicotine dependence treatment in primary care. Nicotine Tob Res 2005;7(Suppl .1):57–66. | IVR technology |
| Meis TM, Gaie MJ, Pingree S, Boberg EW, Patten CA, Offord KP, et al. Development of a tailored, Internet-based smoking cessation intervention for adolescents. JCMC 2002;7(3). | Sample includes children |
| Moreno Arnedillo JJ. The programme to stop smoking ‘on line’ of Madrid City Council. An exploratory research. Adicciones 2006;18:345–58. | Article in Spanish |
| Omenn GS, Thompson B, Sexton M, Hessol N, Breitenstein B, Curry S, et al. A randomized comparison of Worksite sponsored smoking cessation programs. Am J Prev Med 1988;4: 261–7. | Not an electronic aid |
| Ota A, Takahashi Y. Factors associated with successful smoking cessation among participants in a smoking cessation program involving use of the internet, e-mails, and mailing-list. Nippon Koshu Eisei Zasshi Jpn J Publ Health 2005;52:999–1005. | Article in Japanese |
| Pearson Hirdes BA. The role of motivational design in health education: An examination of computer-based education on women, smoking and health. Dis Abst Int A Hum Soc Sci 2005;65(10-A). | Dissertation abstract |
| Pederson LL, Blumenthal DS, Dever A, McGrady G. A web-based smoking cessation and prevention curriculum for medical students: why, how, what, and what next. Drug Alcohol Rev 2006;25:39–47. | Use of the internet to teach medical students |
| Polosa R, Russo C, Di MA, Arcidiacono G, Piccillo G. Smoking cessation and reduction through e-mail counselling. Respir Med 2008;102:632. | No relevant outcomes |
| Reynolds B, Dallery J, Shroff P, Patak M, Leraas K. A web-based contingency management program with adolescent smokers. J Appl Behav Anal 2008;41:597–601. | Sample includes children |
| Rice VH, Fotouhi F, Burn E, Hoyer P, Ayers M. Exemplary program development: hypermedia interactive smoking cessation intervention program for pregnant women. J Perinat Educ 1997;6:47–61. | No relevant outcomes |
| Riley W, Jerome A, Behar A, Zack S. Feasibility of computerized scheduled gradual reduction for adolescent smoking cessation. Subst Use Misuse 2002;37:255–63. | Sample includes children |
| Ruggiero L, Redding CA, Rossi JS, Prochaska JO. A stage-matched smoking cessation program for pregnant smokers. Am J Health Promot 1997;12:31–3. | Not an electronic aid |
| Ruggiero KJ, Resnick HS, Acierno R, Coffey SF, Carpenter MJ, Ruscio AM, et al. Internet-based intervention for mental health and substance use problems in disaster-affected populations : a pilot feasibility study. Behav Ther 2006;37:190–205. | Primary focus not smoking cessation |
| Sarna L, Bialous SA, Wewers ME, Froelicher ES, Wells M, Balbach ED. Web log analysis of the first two years of the tobacco free nurses website. OJNI 2007;11(3). | No relevant outcomes: looking at website utilisation of health professionals |
| Sarna L, Bialous S, Wewers ME, Froelicher ES, Wells MJ, Kotlerman J, et al. Nurses trying to quit smoking using the Internet. Nurs Outlook 2009;57:246–56. | Magazine article, no outcomes |
| Schneider SJ, Tooley J. Self-help computer conferencing. Comput Biomed Res 1986;19: 274–81. | Computer conferencing only |
| Sciamanna CN, Ford DE, Flynn JA, Langford C. An evidence-based interactive computer program to assist physicians in counseling smokers to quit. M Comput 1999;16:54–60. | No relevant outcomes: computer print outs of patients records |
| Shegog R, McAlister AL, Hu S, Ford KC, Meshack AF, Peters RJ. Use of interactive health communication to affect smoking intentions in middle school students: a pilot test of the ‘Headbutt’ risk assessment program. Am J Health Promot 19:334–8. | Sample includes children |
| Skewes MC. Utep women kick butt! development, implementation, and evaluation of a web-based smoking cessation intervention targeted to Hispanic female college students (Texas). Dis Abst Int B Sci Eng 2007;67(11-B). | Dissertation abstract |
| Strecher V, Wang C, Derry H, Wildenhaus K, Johnson C. Tailored interventions for multiple risk behaviors. Health Educ Res 2002;17:619–26. | No relevant outcomes: a methods paper |
| Strecher V, Shiffman S, West R, McClure J, Greene S, Davis R. Web-based tailored smoking cessation (SYM4B). Society for Research on Nicotine and Tobacco, 12th Annual Meeting, 15–18 February 2006, Orlando, FL. | Conference abstract |
| Takahashi, Y, Satomura, K, Miyagishima, K, Nakahara, T, Higashiyama, A, Iwai, K, et al. A new smoking cessation programme using the Internet. Tob Control 1999;8:109–10. | No relevant outcomes (brief commentary) |
| Tossmann P, Jonas B, Tensil M, Nowotny G, Lang PE, Tossmann PE. Smokefree: an internet based smoking cessation programme for adolescent smokers. Z Wiss Prax 54:42. | Article in German |
| Van%%OL, Lechner L, Reubsaet A, Steenstra M, Wigger S, De%%VH. Optimizing the efficacy of smoking cessation contests: an exploration of determinants of successful quitting. Health Educ Res 2009;24:54–63. | Not an electronic aid |
| West R, Gilsenan A, Coste F, Zhou X, Brouard R, Nonnemaker J, et al. The ATTEMPT cohort: a multi-national longitudinal study of predictors, patterns and consequences of smoking cessation;introduction and evaluation of internet recruitment and data collection methods. Addiction 2006;101:1352–61. | No relevant outcomes: primarily about recruitment |
| Wetter D, Cinciripini PM. Palmtop computer delivered treatments for smoking cessation (SYM4C). Society for Research on Nicotine and Tobacco 12th Annual Meeting, 15-18 February 2006, Orlando, FL. | Conference abstract only |
| Woodruff SI, Edwards CC, Conway TL, Elliott, SP. Pilot test of an Internet virtual world chat room for rural teen smokers. J Adolesc Health 2001;29:239–43. | Sample includes children |
| Woolf SH. A Practice-Sponsored Website to Help Patients Pursue Healthy Behaviors: an ACORN Study: Corrections. Ann Fam Med 2006;371(4). | Corrections to article only |
Appendix 12 List of systematic reviews of electronic aids for smoking cessation and commentaries on systematic reviews
These articles were identified during the search and screening process. They served as a context for this report but were not included in any of the reviews.
-
Aveyard P, West R. Managing smoking cessation. BMJ 2007;335:37–41.
-
Civljak M, Koshy E, Marlais M, Car J. Internet-based interventions for smoking cessation (protocol). Cochrane Database Syst Rev 2009;2:CD007078.
-
Hyde J, Hankins M, Deale A, Marteau TM. Interventions to increase self-efficacy in the context of addiction behaviours: a systematic literature review. J Health Psychol 2008;13:607–23.
-
Krebs PM. Computerized tailored theory-based interventions for health behavior change: a comprehensive meta-analysis. Dis Abst Int B Sci Eng 2008;68(8-B).
-
Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemed J E Health 2009;15:231–40.
-
Lancaster T, Stead LF. Self-help interventions for smoking cessation.[Update in Cochrane Database Syst Rev 2005;3:CD001118;PMID: 16034855.][Update of Cochrane Database Syst Rev 2000;2:CD001118;PMID: 10796601.] Cochrane Database Syst Rev 2002;3:CD001118.
-
Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database Syst Rev 2005;3:CD001118.
-
Myung SK, McDonnell DD, Kazinets G, Seo HG, Moskowitz JM. Effects of web- and computer-based smoking cessation programs: meta-analysis of randomized controlled trials. [Erratum appears in Arch Intern Med 2009;169:1194]. Arch Int Med 2009;169:929–37.
-
Neubeck L, Redfern J, Fernandez R, Briffa T, Bauman A, Freedman SB. Telehealth interventions for the secondary prevention of coronary heart disease: a systematic review. Eur J Cardiovasc Prev Rehabil 2009;16:281–9.
-
Portnoy DB, Scott-Sheldon LA, Johnson BT, Carey MP. Computer-delivered interventions for health promotion and behavioral risk reduction: a meta-analysis of 75 randomized controlled trials 1988–2007. Prev Med 2008;47:3–16.
-
Revere D, Dunbar PJ. Review of computer-generated outpatient health behavior interventions: clinical encounters ‘in absentia’. J Am Med Informat Assoc 2001;8:62–79.
-
Sanchez Meca J, Olivares Rodrigues J, Alcazar AIR. The problem of tobacco addiction: meta-analysis of behavioural treatments in Spain. Psychol Spain 1999;3:36–45.
-
Shahab L, McEwen A. Online support for smoking cessation: a systematic review of the literature. Addiction 2009;104:1792–804.
-
Strecher VJ. Computer-tailored smoking cessation materials: a review and discussion. Patient Educ Couns 1999;36:107–17.
-
Velicer WF, Prochaska JO, Redding CA. Tailored communications for smoking cessation: past successes and future directions. Drug Alcohol Rev 2006;25:49–57.
-
Walters ST, Wright JA, Shegog R. A review of computer and Internet-based interventions for smoking behavior. Addict Behav 2006;31:264–77.
-
Whittaker R, Borland R, Bullen C, Lin R, McRobbie H, Rodgers A. Mobile phone-based interventions for smoking cessation (protocol). Cochrane Database Syst Rev 2007;3:CD006611.
-
Whittaker R, Borland R, Bullen C, Lin RB, McRobbie H, Rodgers A. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev 2009;4:CD006611.
Appendix 13 Final protocol
Project title
Effectiveness and Cost-Effectiveness of Computer and Other Electronic Aids for Smoking Cessation: a systematic review and network meta-analysis
Project number
NIHR HTA programme 08/60/01
Principal investigator
Professor Marcus Munafò
School of Experimental Psychology, University of Bristol, 12a Priory Road, Bristol BS8 1TU, United Kingdom
Tel: +44.117.9546841
Fax: +44.117.9288588
E-mail: marcus.munafo@bristol.ac.uk
Background
Smoking is harmful to health. On average, lifelong smokers lose 10 years of life, and about half of all lifelong smokers have their lives shortened by smoking. 1,2 Half of these premature deaths occur before the age of retirement. 2 Fortunately, stopping smoking reverses or prevents many of these harms. Stopping smoking before the age of 40 years (when most smokers have smoked for at least 20 years) results in minimal loss of life expectancy. 3 Computerised interventions have considerable potential in public health because many people are ambivalent about smoking,4,5 and a good number are prepared to make quit attempts with only modest prompting. 6,7 Electronic aids could provide such a prompt, and although most quit attempts end in early failure, a small proportion succeed. 8 It is possible that the behavioural support provided by electronic aids could reach many of these smokers who otherwise use no support and thus might have much higher reach than the NHS Stop Smoking Services.
This project is commissioned research by the NIHR Health Technology Assessment Programme. The primary research question we seek to answer is: What is the effectiveness and cost effectiveness of internet, PC and other electronic aids to help people stop smoking?
Specifically, we will address the following three questions:
-
What is the effectiveness of internet sites, computer programs, mobile telephone text messages, and other electronic aids (alone or in combination with other smoking cessation support), compared with alternative interventions or no intervention, in increasing the success rate of smoking cessation for adult smokers and/or reducing relapse for quitters?
-
What is the cost-effectiveness of incorporating internet sites, computer programs, mobile telephone text messages, and other electronic aids into current NHS smoking cessation programmes, or offering these as an alternative to these programmes, in increasing the success rate of smoking cessation for adult smokers and/or reducing relapse for quitters?
-
What are the current gaps in existing research into the effectiveness of internet sites, computer programs, mobile telephone text messages and other electronic aids to help people stop smoking?
Method
Overall study design
This project consists of four main components:
-
main review on effectiveness
-
evidence synthesis modelling using mixed-treatment comparison (MTC) approach
-
economic analysis, including a review of cost-effective literature and a de novo analytic model
-
supplementary review.
Search strategies
Searches are conducted in three phases: (1) the scoping searches to identify published and ongoing systematic reviews, which served as an additional source for identifying relevant primary studies and provided background information; (2) the main searches for primary studies to identify all relevant primary studies covering all the three component reviews (effectiveness, supplementary and cost-effectiveness); and (3) the updated searches to identify relevant primary studies published during the preparation of this report. Detailed search strategies are described below.
Scoping searches
Completed and on-going systematic reviews were sought from the following resources: The Cochrane Library (CDR, DARE and HTA database); recent additions to DARE and HTA database via the CRD website; ARIF Database of Reviews; TRIP Database; MEDLINE (Ovid) and EMBASE (Ovid). Searches were based on index and text words that encompassed the population: smokers who wish to stop and the interventions: computers and other electronic aids. A search filter for systematic reviews was added to this strategy. Searches were conducted in April 2009 and the results were used to inform the development of the protocol.
Search strategies for primary studies
Relevant primary studies will be sought from the following resources:
-
Bibliographic databases: The Cochrane Library (CENTRAL); MEDLINE (Ovid); EMBASE (Ovid); PsycINFO (Ovid); HMIC (Ovid; and CINAHL (EBSCOhost). Searches will be based on index and text words that encompassed the population: smokers who wish to stop and the interventions: computers and other electronic aids.
-
Reference lists of included studies and of relevant systematic reviews were examined to identify further potentially relevant studies.
-
Research registries of ongoing studies including NIHR Clinical Research Network Portfolio Database, Current Controlled Trials and Clinical Trials.gov.
-
Further information will be from contacts with experts.
All study types will be sought to enable each aspect of the systematic review to be informed (i.e. clinical effectiveness, cost effectiveness and modelling). EED and DARE will be searched in addition to the databases already mentioned for information on cost effectiveness and modelling. The databases will be limited from 1980 onwards. Searches will not be limited by language
A sample search strategy for MEDLINE can be found in Appendix 1.
Updated searches
Searches for main databases will be updated towards the end of data extraction phase.
Sifting of records retrieved from searches of electronic databases
Records retrieved from searches will be imported into Reference Manager (Version 11, Thomson ResearchSoft) which automatically detects and excludes duplicate records between electronic databases. Further duplicated records will be identified and deleted manually. The titles and abstracts of the remaining records will be examined for relevance by one of the three reviewers within the project team. In order to improve the consistency of the sifting process, the reviewers will independently screen a common set of the first 200 records, compare the results and resolve any disagreement by discussion before sifting through the remaining records. The initial sifting aims to exclude obviously irrelevant records and focuses on whether a paper possibly meets the intervention criterion (out of the full set of study selection criteria, described in the next section).
Study selection
The study selection criteria and algorithm are described in Appendix 2. Full-text publications will be ordered for all the records that passed through the initial sifting stage. Two reviewers will independently assess the full publications against the inclusion/exclusion criteria (listed below). Disagreements between reviewers will be resolved by discussion and by seeking further advice from additional members of the project team to reach a consensus. Where full publications cannot be obtained, the records/studies will be excluded. Details of these records will be recorded.
Inclusion criteria
The key criteria for a study to be included in one of the reviews (i.e. effectiveness review, supplementary review and cost-effectiveness review) are:
-
Population: predominantly adult smokers (mean age_ 18 years).
-
Intervention: any smoking cessation program that utilises computer, internet, mobile telephone or other electronic aids (other than conventional mass media such as TV or radio advertisements) to:
-
– generate tailored materials; and/or
-
– present or deliver information (which may not necessarily be tailored); and/or
-
– facilitate communication, e.g. chatrooms, blogs, e-mails (except telephone conversations); and/or
-
– increase recruitment.
-
A paper meeting the above criteria will then be considered for inclusion in one of the reviews according to its study design and measurements of outcomes.
For inclusion in the main effectiveness review, a study needs to be either a RCT or quasi-RCT (using a method of allocation that is not strictly random but is less likely to introduce bias such as allocation of alternative options to consecutive participants enrolled) and reports at least an outcome associated with smoking cessation (e.g. point prevalence abstinence and/or prolonged abstinence).
[Protocol amendment] We initially planned to include studies that reported only motivation to quit smoking for potential inclusion in the review, but a decision was made during the course of the project to exclude these studies in view of time constraint.
Economic evaluations (i.e. cost-effectiveness analyses, cost-utility analyses, cost-benefit analyses) meeting the population and intervention criteria will be included in the cost-effectiveness review. In addition, studies that reported cost information will be flagged for potential use in economic modelling.
Studies of other designs which meet the population and intervention criteria will be tagged and separately considered for inclusion in the supplementary review.
Exclusion criteria
Studies that meet any of the following criteria will be excluded:
-
Population: predominantly smokers younger than 18 years old (mean age < 18 years)
-
Intervention: interventions targeting solely at smokeless tobacco; interventions aiming exclusively at modifying the behaviour/enhancing the performance of the providers of a smoking cessation programme rather than aiming at smokers; the computer/electronic aids were used solely for passively monitoring smoking behaviour/collecting information (without using the information to generate further feedback)
-
Study design: commentaries, editorials, surveys, narrative reviews.
Data extraction and quality assessment for effectiveness review
Data from included RCTs/quasi-RCTs will be extracted on to a data extraction form by one of the reviewers. The data extraction form (see Appendix 3) is designed ad hoc for this review and includes details of the citation, study design (population, interventions, comparators and co-interventions, outcome measures, and statistical methods) and results. The data extraction form also includes a quality assessment checklist, which assesses the following domains:
-
methods of randomisation
-
allocation concealment
-
similarity in baseline characteristics between groups
-
similarity in care provided between groups other than the intervention/comparator being tested
-
biochemical validation
-
extent of drop-out
-
presence of differential drop-out between groups, and methods for adjustment in analysis
-
use of intention to treat analysis.
The main outcome measure of interest is prolonged abstinence. Data on point prevalence abstinence and other measures of motivation to quit (e.g. movement in the stages of change) will also be recorded.
All the extracted data and results of quality assessment will be independently checked by another reviewer to ensure accuracy and consistency. Disagreements will be resolved by discussion and/or consulting a third reviewer.
Grouping of studies and study arms
In order to guide the report structure and to facilitate quantitative analysis, the components of the care provided in each study arm (irrespective of whether they are considered as an intervention, a control or a co-intervention) with regard to smoking cessation will be coded using a coding scheme to be developed and piloted during the course of the project. Components will be categorised as either ‘electronic’ or ‘non-electronic’ and these will be coded separately. Coding will be undertaken independently by two reviewers. Disagreements will be resolved by discussion and by seeking further advice/arbitration from another team member. The coding will be finalised before data analysis takes place.
In addition to the coding scheme to categorise individual study arms, each study will also be classified with respect to the study population, type of electronic media, and comparisons made within each study. The classification of studies will provide further guidance on report structure and meta-analysis within each major section.
Data handling and analysis
Data handling
Numerical data will be entered on to an Excel spreadsheet. Where possible, data including all randomised patients will be used, with any patients lost to follow-up or with missing data counted as failing to achieve abstinence.
Where prolonged abstinence was measured at multiple time points, the 6-month prolonged abstinence recorded approximately 6 months after the start of the intervention will be used for ‘aid to cessation’ studies (i.e. in smokers who are prepared to quit at the beginning of the studies). For ‘cessation induction’ studies in which some of the smokers are not yet ready to quit at the start of the studies, the 6-month prolonged abstinence recorded approximately 6 months after the allowed ‘cessation induction period’ (i.e. from the start of the intervention to the expected quit day) is preferred if available. Where more than one point prevalence abstinence rate based on different definitions (e.g. 24-hour, 7-day, etc.) were reported, the 7-day point prevalence abstinence is preferred.
For studies in which both self-reported and biochemically validated abstinence were reported, data on self-reported abstinence will be used in meta-analyses in order to maintain consistency across studies. However, data on biochemically validated abstinence will be included in a sensitivity analysis.
Data analysis
Both pair-wise meta-analysis and Bayesian mixed-treatment comparison (MTC) will be carried out. The methods used for meta-analysis are described here. Methods used for MTC will be described in a separate section.
Each study will be classified with respect to the study population, type of electronic media, and comparisons made (as mentioned above). Where sufficient data are available, pair-wise meta-analyses of relative risk (risk ratio) of point prevalence abstinence and prolonged abstinence will be carried out using STATA (Version 10.0) for each comparison. Given the potential heterogeneity between studies in terms of participants, interventions, comparators, co-interventions and duration of follow-up, analyses of 6-month data using a random effects model will be considered as primary analyses. We choose the six-month time frame as we consider it to be sufficiently long for estimating long-term success of smoking cessation, while losses to follow-up are likely to be reasonably low. Analyses using a fixed-effect model and using data from the longest follow-up of each study will also be performed as sensitivity analyses.
Statistical heterogeneity between studies will be assessed using I2 statistic, which ranges from 0% (no heterogeneity beyond what is expected by chance) to 100% (substantial heterogeneity). Funnel plots and Egger’s tests will be used to examine potential publication bias or small study effects.
Evidence synthesis modelling
The aim of the Bayesian Mixed-treatment comparisons (MTC) Evidence Synthesis was to combine as much of the evidence as possible in a consistent way, in order to make comparisons across multiple interventions and to inform the cost-effectiveness analysis. Mixed-treatment comparisons (or Network Meta-Analysis) is a statistical method which combines multiple complex interventions in a single coherent analysis incorporating all relevant pairwise treatment comparisons. Time-to-event models for relapse rates are embedded with the MTC to enable us to combine studies with different (and multiple) follow-up times.
Cost-effectiveness modelling
Our aim was to assess the comparative cost-effectiveness of the range of interventions lying with the scope of our analysis. We address this as two questions. The first is whether an electronic intervention of some kind is likely to be cost-effective compared with conventional support alone. The second considers the comparative cost-effectiveness of different types of electronic intervention. We begin by reviewing the existing literature on this topic, identified in the main literature review (which was designed to identify economic studies). As the existing evidence base on cost-effectiveness was inconclusive, we carried out an economic analysis de novo. A series of decision problems were set out, and decision-tree models were constructed to support these decisions. The decision models were populated with the results of the Bayesian MTC Evidence Synthesis, and supplementary information as required.
Supplementary review
This review aims to examine studies that do not meet the inclusion criteria for the main reviews but may be useful for understanding factors that may influence the reach or effectiveness of electronic aids. In particular, this review aims to explore the acceptability and usability of aids – including who uses electronic aids, how acceptable these aids are to particular groups of smokers, how feasible delivery is to smokers in different settings, and aspects of usability.
Study selection
The over-arching literature search and sifting of studies for the main review outlined above will cover relevant literature for the supplementary review. Records identified as potentially relevant for the supplementary review will be forwarded to relevant team members for further assessment and selection. Study selection for the supplementary review will be carried out by two reviewers.
Data extraction
Data will be extracted from the included studies and summarised in a series of evidence tables. Because of the range of study designs to be included in the supplementary review, data will be extracted to inform a narrative synthesis of key themes and issues rather than to inform a meta-analysis.
References
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328.
- Peto R, Doll R, Bock G, Goode J. Understanding nicotine and tobacco addiction. Chichester: John Wiley & Sons; 2006.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328.
- Jarvis MJ, McIntyre D, Bates C, Foulds J. Effectiveness of smoking cessation initiatives. BMJ 2002;324.
- Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database Syst Rev 2005;3.
- Russell MA, Wilson C, Taylor C, Baker CD. Effect of general practitioners’ advice against smoking. Br Med J 1979;2:231-35.
- Russell MA, Merriman R, Stapleton J, Taylor W. Effect of nicotine chewing gum as an adjunct to general practitioner’s advice against smoking. BMJ (Clin Res Educ) 1983;287:1782-5.
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004;99:29-38.
Appendix 1: Sample search strategy for MEDLINE (Ovid)
-
(smoker or smokers or smoking).mp.
-
(tobacco or cigarette$ or nicotine$).mp.
-
smoking cessation.mp.
-
“tobacco use cessation”/
-
(prevent$ or abstain$ or abstin$ or discourag$ or cease$ or cessation or quit or quits or quitting).mp.
-
(stop or stops or stoppping).mp.
-
health behavior/
-
behavior therapy/
-
or/1-2
-
or/3-4
-
computer$.ti,ab.
-
expert systems/
-
computer aided design/
-
therapy, computer assisted/
-
internet.mp.
-
computer communication networks/
-
communications media/
-
cellular phone$.mp.
-
mobile phone$.mp.
-
text messag$.mp.
-
sms.mp.
-
web.mp.
-
electronic mail/
-
email$.mp.
-
blog$.mp.
-
(chat room$ or chatroom$).mp.
-
podcast$.mp.
-
video recording/
-
video$.mp.
-
or/11-29
-
or/5-8
-
31 and 10
-
30 and 11
-
30 and 32
-
33 or 34
-
limit 35 to (humans and yr = “1980 - current”)
Appendix 2: Study selection criteria and algorithm
| Criteria | Answer | Action | Reference Manager Tag (user defined field 2) | |
|---|---|---|---|---|
| Q1 |
Intervention: did the intervention utilise computer, internet, mobile telephone or other electronic aids (other than conventional mass media such as TV or radio advertisements) to: of/for a smoking cessation programme? |
Yes | Go to Q2 | |
| No | Exclude | Exclude owing to intervention | ||
| Q2 |
Check if: The intervention targeted solely on smokeless tobacco The intervention aimed at modifying the behaviour/enhancing the performance of the providers of a smoking cessation programme rather than aiming at smokers The computer/electronic aids were used solely for monitoring smoking behaviour/collect information (without using the information to generate further feedback) |
Yes | Exclude | Exclude owing to intervention |
| No | Go to Q3 | |||
| Q3 | Population: are the study participants predominantly adults (age ≥ 18 years)? | Yes | Go to Q4 | |
| No | Exclude | Exclude owing to population | ||
| Q4 | Study design: is the study a randomised controlled trial, quasi-randomised controlled study*, or an interrupted time series? | Yes | Go to Q7 | |
| No | Go to Q5 | |||
| Q5 | Study design: is the study a full economic evaluation (i.e. cost-effectiveness analysis, cost-utility analysis, cost-benefit analysis)? | Yes | Include | Include for economic review |
| No | Go to Q6 | |||
| Q6 |
Study design: does the paper describe: (1) a systematic review; (2) a non-randomised or uncontrolled study of an intervention; or (3) the methodological design, process evaluation and/or qualitative research of an intervention; or (4) cost of an intervention in the UK? |
Yes | Tag Supplementary |
To be considered for supplementary review Studies describing intervention costs flagged FAO NJW/JJM |
| No | Exclude | Exclude owing to study design | ||
| Q7 | Comparison: was the same computer and/or electronic aid(s) used in both/all the groups being compared (and thus the study was actually assessing the effectiveness of something else, e.g. mood management course + e-mails versus e-mails)? | Yes | Exclude | Exclude owing to irrelevant comparison |
| No | Go to Q8a | |||
| Q8a | Outcome: was an outcome associated with smoking cessation** (e.g. point prevalence or prolonged abstinence) and/or motivation to quit smoking reported? | Yes | Include. Go to Q8b | Include for effectiveness review |
| No | Go to Q9 | |||
| Q8b | Outcome: does the study also include a full economic evaluation (i.e. cost-effectiveness analysis, cost-utility analysis, cost-benefit analysis)? | Yes | Include | Include also for economic review |
| No | No further action | |||
| Q9 | Check: was the smoking cessation outcome(s) of the study reported in another paper? | Yes | Include (& append to the main paper which reported the outcome) | Include for effectiveness review (see main paper [Ref ID]) |
| No |
(1) Contact author and if data not available (2) Exclude |
(1) Query -contact author (2) Exclude owing to outcome |
||
Appendix 3
Data extraction form
| Author year | Trial name | |||
| Ref Man ID | Trial ID | |||
| Citation | ||||
| Related references | ||||
| Reviewer | Double checked by | |||
| Last updated | ||||
| Note (e.g. action to be taken; specific query to be clarified) | ||||
Summary tick boxes
(To fill in during or at the end of data extraction; put ‘x’ against a suitable category or categories.)
| Population (readiness to quit if given) |
|||||
|---|---|---|---|---|---|
| Pre-contemplation (not currently considering stopping smoking) | |||||
| Contemplation (willing to consider a quit attempt) | |||||
| Preparation (interested in stopping smoking) | |||||
| Other (please state) | |||||
| Age | |||||
| Adults | |||||
| Adolescents | |||||
| Other (please state) | |||||
| Intervention(s) and comparator(s) | |||||
| Intervention | Comparator 1 | Comparator 2 | Comparator 3 | ||
| Computer-generated tailored materials | |||||
| Stand-alone computer programs (i.e. not web based) | |||||
| Mobile phone text messages | |||||
| E-mails | |||||
| Newsletters | |||||
| Websites (static) | |||||
| Websites (interactive) | |||||
| Web-based questionnaires | |||||
| Chat rooms | |||||
| Blogs | |||||
| Other electronic aids (please state) | |||||
| Printed, untailored material | |||||
| One-to-one face-to-face counselling | |||||
| One-to-one telephone counselling | |||||
| Group counselling | |||||
| NRT | |||||
| Supporter | |||||
| Other | |||||
| Comparisons (possible programme effects) | |||||
| Tailoring effect – as oppose to general, untailored material | |||||
| Media effect (audiovisual effect) – as opposed to printed material | |||||
| Immediate feedback (‘interactive’ or ‘give and take’) – as opposed to delayed feedback delivered by post | |||||
| Time/location effect (+ anonymous + autonomy?) – website, blogs, chat room, etc., offer the advantages of being accessible at any time and from any location (obviously still subject to availability of internet connection) and other potential advantages such as being able to be anonymous and to access the intervention only when someone | |||||
| Low cost/high volume – e.g. e-mails, SMS could simply be used to replace mailed or telephone reminders or ‘boosters’ because of the advantage of cost and coverage | |||||
Study design
| Population | ||||
|---|---|---|---|---|
| No. approached | ||||
| No. enrolled | ||||
|
No. randomised Intervention/ comparator |
||||
| No. available at further time points | ||||
| Method of recruitment | ||||
| Inclusion/exclusion criteria | ||||
| Intervention(s) | ||||
| Details e.g. Setting; contents and ways of delivery; duration and frequency | ||||
| Comparator(s) | ||||
| Details e.g. Setting; contents and ways of delivery; duration and frequency | ||||
| Common to both interventions and comparators | ||||
| Outcome measures (state whether not reported (NR), do not leave blank) | ||||
| Point prevalence abstinence | Definition: | |||
| Prolonged (population-based) or continuous abstinence | Definition: | |||
| Biochemical validation? |
Yes/no /unclear If yes, give details: |
|||
|
Other outcome measures (please list) e.g. Intention to quit, self-efficacy, other possible mediators, process evaluation |
||||
| Time points at which outcomes were measured | ||||
| Trial duration/last follow-up | ||||
| Statistical methods | ||||
| 1. Power calculation done? |
Yes/no/unclear If yes, give details: |
|||
| 2. Methods of analysis (briefly describe): | ||||
| 3. Methods of dealing with missing data (briefly describe): | ||||
Quality assessment
|
1. Was randomisation adequate? Adequate: computer, random number table Inadequate: alternation; the use of case record numbers, dates of birth or day of the week |
Yes/no/unclear Give details: |
|
2. Was allocation adequately concealed? Adequate: Inadequate: |
Yes/no/unclear Give details: |
|
3. Were the groups similar at baseline? If dissimilar, was this explained or adjusted for? |
Yes/no/unclear Give details: |
| 4. Was care received by the groups similar other than the intervention? |
Yes/no/unclear Give details: |
| 5. Was contamination between groups acceptably low? |
Yes/no/unclear Give details: |
| 6. Were there significant dropouts (> 20%) |
Yes/no/unclear Give details: |
|
7. Were there any imbalances in dropouts between groups? 7.1 If so, were they explained or adjusted for? |
Yes/no/unclear Give details: |
| 8. Did the analysis include an ITT analysis? |
Yes/no/unclear Give details: |
Results
| Patient characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Whole study | Intervention | Comparator 1 | Comparator 2 | |||||||
| Mean age (SD) [range] | ||||||||||
| % male | ||||||||||
| Smoking history | ||||||||||
| Mean no. cigarettes smoked/ day (SD) | ||||||||||
| Mean no. years smoking (SD) | ||||||||||
| Stage of change (n) | ||||||||||
| Other | ||||||||||
| Outcomes | ||||||||||
| Intervention ( n ) | Comparator 1 ( n ) | Comparator 2 ( n ) | OR or RR | 95% CI | p -value | |||||
| Point prevalence abstinence | ||||||||||
| Prolonged abstinence | ||||||||||
| Other | ||||||||||
List of abbreviations
- CCBT
- computerised cognitive behavioural therapy
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- DIC
- deviance information criterion
- EVPI
- expected value of perfect information
- EXP
- expert system
- GBP
- British pound sterling
- GP
- general practitioner
- HR
- hazard ratio
- HRA
- health risk appraisal
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ITEM
- individually timed educational messages
- ITT
- intention to treat
- IVR
- interactive voice response
- LYS
- life-years saved
- MAN
- stage-matched manual
- MP
- modified programme
- MTC
- mixed-treatment comparison
- NICE
- National Institute for Health and Clinical Excellence
- NIHR
- National Institute for Health Research
- NR
- not reported
- NRT
- nicotine replacement therapy
- NT
- non-tailored
- OECD
- Organisation for Economic Co-operation and Development
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk (risk ratio)
- SMS
- short message service
- SSS
- Stop Smoking Services
- ST
- single tailored letter
- VOI
- value of information
- WTP
- willingness to pay
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of Bio-Statistics, Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, Department of Pharmacology and Therapeutics, University of Liverpool
-
Professor Zarko Alfirevic, Head of Department for Women’s and Children’s Health, Institute of Translational Medicine, University of Liverpool
-
Professor Judith Bliss, Director of ICR-Clinical Trials and Statistics Unit, The Institute of Cancer Research
-
Professor David Fitzmaurice, Professor of Primary Care Research, Department of Primary Care Clinical Sciences, University of Birmingham
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, Department of Specialist Radiology, University College Hospital, London
-
Professor Angela Harden, Professor of Community and Family Health, Institute for Health and Human Development, University of East London
-
Dr Joanne Lord, Reader, Health Economics Research Group, Brunel University
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Dion Morton, Professor of Surgery, Academic Department of Surgery, University of Birmingham
-
Professor Gail Mountain, Professor of Health Services Research, Rehabilitation and Assistive Technologies Group, University of Sheffield
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Director, Centre for Healthcare Randomised Trials, Health Services Research Unit, University of Aberdeen
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Peter Tyrer, Professor of Community Psychiatry, Centre for Mental Health, Imperial College London
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Caroline Watkins, Professor of Stroke and Older People’s Care, Chair of UK Forum for Stroke Training, Stroke Practice Research Unit, University of Central Lancashire
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health