Notes
Article history paragraph text
The research reported in this issue of the journal was commissioned by the HTA programme as project number 09/22/01. The contractual start date was in May 2008. The draft report began editorial review in November 2011 and was accepted for publication in May 2012. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Goodacre et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2013
Chapter 1 Background
Description of health problem
Acute coronary syndrome (ACS) typically occurs when coronary artery disease (CAD) leads to obstruction of a patient's coronary arteries. This can lead to myocardial infarction (MI), heart failure (HF), arrhythmia, cardiac arrest and death. ACS has a 6-month mortality of up to 20%,1 and one-fifth of patients are rehospitalised within 6 months of their initial admission. 2
Acute coronary syndrome usually presents as chest pain and must be differentiated from other common causes of chest pain, such as muscular pain, gastro-oesophageal pain and anxiety. Differentiation is difficult because clinical assessment is unreliable and the electrocardiogram may be normal in the presence of ACS. Patients with suspected ACS therefore constitute a large and varied population, many of whom will not have ACS or CAD, but have non-cardiac causes for their chest pain. Accurate identification of ACS and CAD is therefore required to guide subsequent intervention.
The health-care burden of suspected acute coronary syndrome
Suspected ACS represents a substantial health-care problem and investigation represents a substantial challenge. Chest pain is responsible for around 700,000 emergency department (ED) attendances in England and Wales,3 with the main reason for attendance being suspected ACS. Hospital Episodes Statistics for England (1998–2010)4 report 253,765 emergency admissions with chest pain (code R07), 63,082 with angina (I20) and 50,386 with MI. Table 1 shows how emergency admission rates, length of stay (LoS) and bed-days for these three codes have changed over the last 10 years and Figure 1 shows the change in admission rates.
| Year | Chest pain | Angina | MI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | LoS | Days | n | LoS | Days | n | LoS | Days | |
| 1998–9 | 114,828 | 3.0 | 352,706 | 98,198 | 5.3 | 573,135 | 67,116 | 8.2 | 571,257 |
| 1999–2000 | 127,379 | 2.9 | 373,162 | 99,562 | 5.2 | 564,750 | 63,397 | 8.2 | 546,357 |
| 2000–1 | 144,148 | 2.9 | 426,269 | 98,772 | 5.4 | 580,097 | 61,760 | 8.6 | 559,324 |
| 2001–2 | 152,721 | 2.8 | 436,342 | 92,332 | 5.4 | 551,913 | 61,716 | 9.0 | 591,917 |
| 2002–3 | 161,931 | 2.6 | 430,799 | 89,435 | 5.5 | 541,421 | 64,415 | 9.5 | 657,104 |
| 2003–4 | 176,887 | 2.0 | 425,389 | 85,066 | 5.0 | 501,108 | 62,032 | 10 | 666,788 |
| 2004–5 | 205,306 | 2.1 | 431,440 | 81,331 | 5.0 | 452,282 | 61,423 | 9.7 | 687,331 |
| 2005–6 | 224,086 | 1.9 | 414,174 | 77,510 | 4.6 | 401,562 | 59,067 | 9.0 | 638,397 |
| 2006–7 | 236,028 | 1.6 | 379,968 | 73,790 | 4.0 | 331,029 | 56,889 | 8.4 | 587,450 |
| 2007–8 | 233,736 | 1.4 | 345,857 | 69,707 | 3.7 | 292,519 | 54,759 | 8.0 | 538,996 |
| 2008–9 | 246,854 | 1.3 | 332,739 | 67,998 | 3.5 | 272,921 | 53,333 | 7.9 | 510,633 |
| 2009–10 | 253,765 | 1.3 | 331,284 | 63,082 | 3.3 | 234,897 | 50,386 | 7.6 | 461,573 |
FIGURE 1.
Hospital admissions for chest pain, angina and MI in England, 1998–2010.
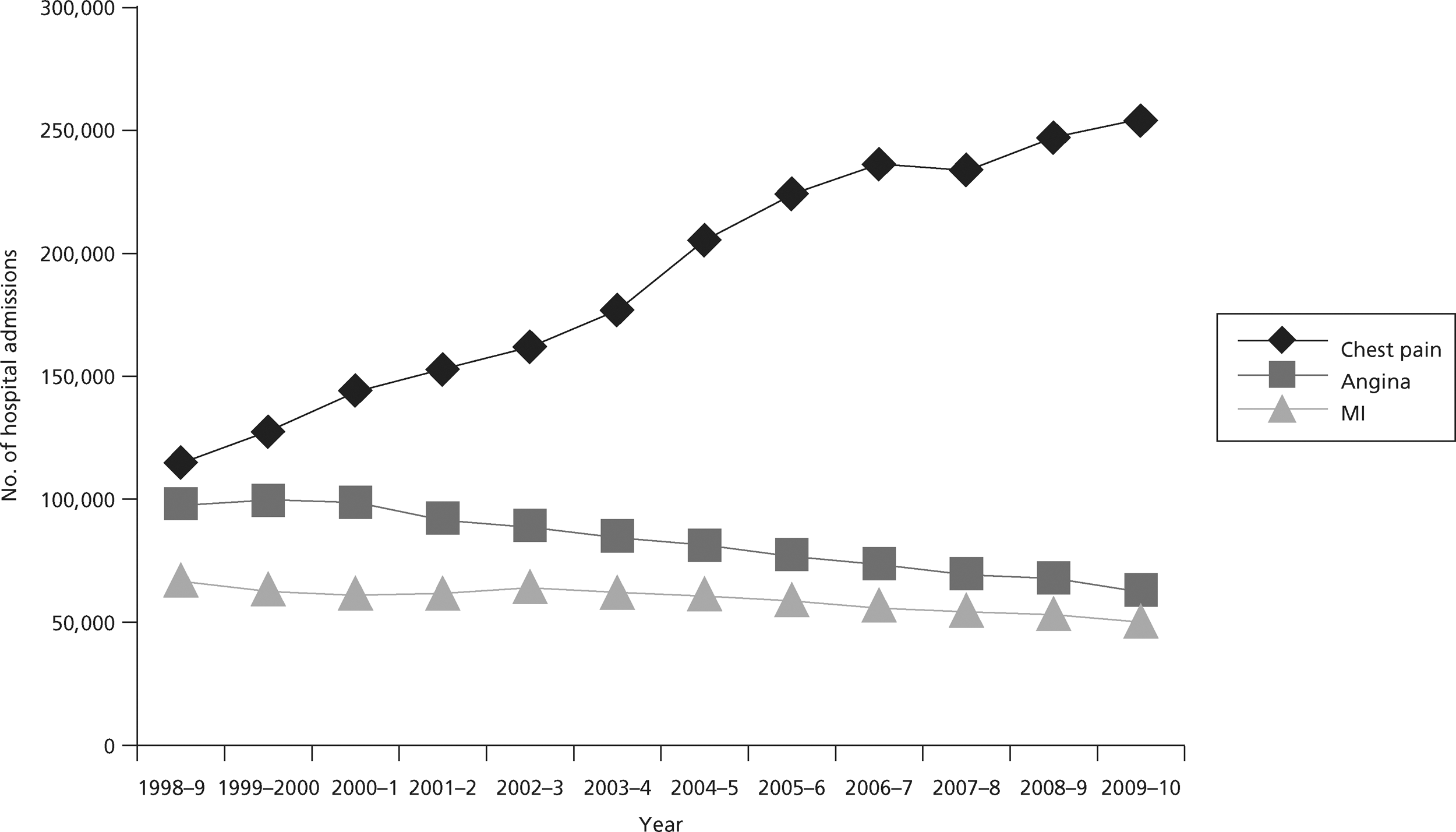
Hospital Episodes Statistics for England4 show that emergency admission rates have been falling for angina and MI, but more than doubled for chest pain between 1998 and 2010. This was accompanied by falls in LoS for chest pain and angina, and, since 2004, for MI. As a result, bed-days are falling for all three conditions. The changes in admissions and LoS for angina and MI probably reflect the decreasing incidence of these conditions and changes in practice that have resulted in shorter hospital stay. 5 The changes in admissions and LoS for chest pain probably reflect changes in service delivery to promote emergency hospital attendance with chest pain1 and changing threshold for decision-making, leading to more admissions with chest pain and a low risk of ACS for diagnostic assessment. 6
Investigation for suspected acute coronary syndrome
Investigation for suspected ACS has two main elements: (1) diagnosis of MI and (2) diagnosis of underlying CAD. Diagnosis of unstable angina is another consideration but of decreasing importance for reasons outlined below.
In the context of investigating suspected ACS the term MI usually refers to non-ST elevation MI (NSTEMI). Although ST-elevation MI (STEMI) is included in the definition of ACS it can usually be identified on the presenting electrocardiogram and thus does not form part of the typical diagnostic challenge of suspected ACS, although electrocardiography (ECG) interpretation and differentiation from other causes of ST elevation may present separate challenges.
Clinical diagnosis of NSTEMI, according to the universal definition of MI,7 is based on a troponin elevation above the 99th percentile of the upper reference limit for the normal population. Patients with elevated troponin levels have an increased risk of adverse outcome8 and are more likely to benefit from treatments usually provided in hospital. 9 However, testing troponin does not achieve optimal sensitivity for MI until several hours after the symptoms of MI,10 so guidelines typically recommend delaying sampling until 10–12 hours after symptom onset. 11 Patients with suspected ACS typically present to hospital within a few hours of symptom onset,12 so delaying blood sampling usually incurs costs of hospital observation and/or admission. Earlier blood sampling is cheaper but may miss cases of MI, so the timing of sampling and tests used involve a trade-off between cost and accuracy.
Many patients with suspected ACS are known to have CAD and are receiving secondary preventative treatment. However, a substantial proportion of patients have not previously been investigated for CAD. Once MI has been ruled out these patients may be investigated for underlying CAD by either provocative cardiac testing to identify symptoms of CAD induced by exertional or pharmacological stress or anatomical imaging of the coronary arteries. Identification of CAD allows treatment with aspirin, statins and angiotensin-converting enzyme inhibitors to be commenced and consideration of coronary revascularisation for high-risk cases. The benefits of diagnosing CAD relate to the opportunity to reduce subsequent major adverse cardiac events (MACEs), particularly cardiac death and non-fatal MI. Technologies used to diagnose CAD thus also need to predict risk of adverse events to allow targeting of treatment. It could be argued that prediction of adverse events is of more practical value than the diagnosis of CAD in determining management decisions.
Investigation of suspected ACS also involves identification and treatment of patients with unstable angina. These patients have CAD and worsening symptoms, but no evidence of cardiac damage. Previously they constituted the majority of patients with suspected ACS. However, the increasing sensitivity of biochemical tests for myocardial damage, and the redefinition of MI to include all patients with evidence of myocardial damage, means that patients with unstable angina and no myocardial damage are fewer in number and have a relatively low risk of adverse outcome. Furthermore, in the absence of ECG changes there are substantial difficulties defining which patients have unstable angina, as the diagnosis is based on unreliable clinical features. These factors make it difficult to define the population with unstable angina and estimate any benefits from treatment, beyond secondary prevention for underlying CAD.
Current service provision
Acute chest pain due to possible ACS is managed in the NHS according to guidance issued by the National Institute for Health and Clinical Excellence (NICE). 11 These guidelines recommend measurement of troponin levels at presentation to hospital and 10–12 hours after the onset of symptoms. This is based on evidence that troponin levels predict subsequent risk of adverse outcome8 and response to treatment,9 but do not achieve optimal sensitivity until 10–12 hours after symptom onset. 10 However, delaying blood testing until 10–12 hours after symptom onset is inconvenient for patients and often incurs additional health-care costs associated with hospital admission and/or observation. As a result, various alternative strategies have been proposed for earlier diagnosis of MI using combinations of biomarkers, measuring biomarker gradients and using newer, more sensitive troponin assays. A survey undertaken prior to NICE guidance being issued13 suggested substantial variation in the biomarker strategies used. It is not known whether or not NICE guidance has reduced this variation.
The NICE guidance for chest pain of recent onset recommends that patients with an elevated troponin level are treated for ACS according to the NICE guidance for unstable angina and NSTEMI. 14 Those with a negative troponin level should be reassessed and if myocardial ischaemia is suspected then patients are managed as an outpatient according to the guidance for stable chest pain. 11 This involves coronary artery calcium (CAC) scoring and computed tomographic coronary angiography (CTCA) for selected cases. Exercise testing is not recommended in NICE acute chest pain guidance, although it is recommended in European Society of Cardiology guidance. 15 A survey of the management of troponin-negative patients with acute chest pain, undertaken prior to publication of NICE guidance, showed variability in the use of risk stratification methods and subsequent use of other investigations, such as the exercise tolerance test (ETT). 16
The NICE guidelines11 identified areas of uncertainty where further research is required. These are:
-
evaluation of new, high-sensitivity troponin assay methods in low-, medium- and high-risk groups with acute chest pain, and evaluation of other putative biomarkers in comparison with the diagnostic and prognostic performance of the most clinically effective and cost-effective troponin assays
-
investigation of the cost-effectiveness of multislice CTCA as a first-line test for ruling out obstructive CAD in patients with suspected troponin-negative acute coronary syndromes.
Description of technology under assessment
High-sensitivity troponin and alternative biomarkers
The cardiac troponins form part of the cardiac contractile apparatus, the troponin–tropomyosin complex, and comprise three troponins [troponin C (TnC), troponin I (TnI) and troponin T (TnT)] plus tropomyosin. As they have unique structures, immunoassays to measure TnT and TnI were developed, and preliminary studies demonstrated that the measurement of cardiac troponin was both more sensitive and more specific for myocardial injury than previously used biomarkers [creatine kinase (CK) and creatine kinase MB isoenzyme (CK-MB)]. TnT or TnI is now the recommended biomarker for MI. 7
The original redefinition of acute MI suggested that the analytical imprecision of the assay should allow measurement with a low analytical imprecision within the reference interval of the assay. This quality specification was not met by the assays available at the time and resulted in progressive improvement in assay quality to produce the current generation of sensitive troponin assays. Sensitive troponin assays are capable of measuring troponin in healthy individuals with a high degree of analytical imprecision, typically < 10% imprecision at the 99th percentile of a reference population.
In addition to meeting the quality specification stipulated in the universal definition of acute MI, the new sensitive assays can detect myocardial injury substantially earlier than the previous generation of assays. Progressive improvement in the analytical performance of troponin assays demonstrated that the analytical performance of second- and third-generation assays was already beginning to outstrip that of other markers of myocardial injury, such as myoglobin and CK-MB,17,18 and studies of new high-sensitivity assays suggest that they are superior to all of the conventional markers of myocardial injury. 19,20
Systematic reviews have established the diagnostic10 and prognostic8 accuracy of troponin testing in suspected ACS, and a systematic review of the diagnostic accuracy of troponin, CK, CK-MB and myoglobin21 established that troponin has the highest accuracy for MI. Measurement of troponin levels at 10–12 hours after symptom onset is now standard diagnostic practice for suspected ACS. 11 There is effectively no potential for alternative biomarkers to improve on the diagnostic accuracy of a 10- to 12-hour troponin assay for MI, as this forms the reference standard. 7 However, alternative biomarkers may have a role in addressing two limitations of troponin measurement. First, the limited early sensitivity of troponin means that there is the potential for biomarkers with better early sensitivity for MI to improve care. Second, although a negative 10- to 12-hour troponin assay stratifies patients to a low risk of adverse outcome, this does not equate to a negligible risk. Thus alternative biomarkers may have a useful role in further risk stratifying patients with a negative 10- to 12-hour troponin assay result.
The relative insensitivity of the early generation of cardiac troponin assays led to the suggestion that small cytoplasmic proteins that would leak earlier through the ischaemic myocardial cell membrane would provide early sensitive diagnostic information in patients presenting with acute chest pain. Myoglobin is a single-chain globular protein containing a haem prosthetic group and is the primary oxygen storage protein of muscle tissues that could be an early marker for MI.
An alternative approach was to find markers that would be released when myocardial ischaemia occurred. Ischaemia-modified albumin (IMA) is a form of human serum albumin in which the N-terminal amino acids have been affected by ischaemia so as to be unable to bind transition metals. Fatty acid-binding proteins are relatively small proteins, of 126–137 amino acids in length, present in tissues with an active fatty acid metabolism, such as heart, liver and intestine. The myocardial isoform, heart-type fatty acid-binding protein (H-FABP), is present predominantly in the heart, but is also found in other tissues including skeletal muscle and the distal tubal cells in the kidney.
In addition to the measurement of cardiac troponin, other markers of the atherothrombotic process could be measured to allow earlier diagnosis. Markers of atheromatous plaque destabilisation or rupture have been proposed, including inflammatory markers [C-reactive protein (CRP), interleukin 6, interleukin 33/ST2 and growth differentiation factor 15 (GDF-15)] and biomarkers considered to be associated with the plaque itself [myeloperoxidase (MPO), matrix metalloproteinases and pregnancy-associated plasma protein A (PAPP-A)]. Alternatively, markers of myocardial dysfunction could be used, such as B-type natriuretic peptide (BNP), N-terminal pro-B-type natriuretic peptide (NT-pro-BNP), copeptin and adrenomedullin.
A systematic review of 22 novel biomarkers, including CRP, MPO, BNP and H-FABP,22 concluded that there was insufficient evidence to support the use of these biomarkers in ED assessment of suspected ACS. As this analysis was published, further studies have been undertaken to estimate the diagnostic and prognostic accuracy of alternative biomarkers, whereas other studies have suggested that modern troponin assays have much improved early sensitivity. We therefore planned to synthesise the evidence relating to the role of early biomarkers (including troponin) for identifying MI before 10–12 hours and the role of alternative biomarkers in providing additional risk stratification for troponin-negative patients with suspected ACS.
Exercise electrocardiography testing
Exercise ECG testing involves using exercise, typically walking on a treadmill or static cycling, to provoke physiological stress, thus increasing heart rate and myocardial oxygen demand. Continuous ECG monitoring is used to identify changes that indicate myocardial ischaemia due to underlying CAD. Development of cardiac-type pain on exercise, and other measurements such as blood pressure recording, can also be used to indicate CAD or other heart disease. A conclusive test result requires the patient to achieve 85% of their predicted maximal heart rate. This may not be achievable if the patient has neurological or musculoskeletal comorbidities. As a result, a proportion of exercise ECG tests are inconclusive.
Exercise ECG has been widely used in the investigation of patients with stable chest pain due to suspected CAD. Most studies of prognostic accuracy and all studies of diagnostic accuracy have involved patients with stable symptoms and until recently suspected ACS was considered a contraindication to exercise testing. The most recent meta-analysis23 of the diagnostic accuracy of exercise ECG reported that the main diagnostic criterion (ST depression) performed only moderately well, with a positive likelihood ratio (PLR) of 2.79 for a 1-mm cut-off and 3.85 for a 2-mm cut-off. The negative likelihood ratios were 0.44 and 0.72, respectively. Exercise ECG would therefore be expected to miss a significant proportion of patients with CAD, while subjecting others with normal coronary arteries to an unnecessary invasive coronary angiogram.
The role of exercise ECG has only recently developed in patients with suspected ACS. Biomarker testing with a 10- to 12-hour troponin assay or alternative strategy is used to rule out MI before exercise testing, so it is effectively used only on those with troponin-negative suspected ACS. Also, as patients with known CAD are unlikely to benefit from diagnostic assessment for CAD, use in those without known CAD is limited to providing prognostic information.
Exercise ECG testing is not currently widely used in suspected ACS. When used it is typically in the context of a standardised assessment alongside biomarker testing on a chest pain unit. These units are widespread in the USA but have been established in only a few centres in the UK in the light of a cluster randomised trial that failed to show evidence of benefit. 24 European Society of Cardiology guidelines recommend using a stress test (typically exercise ECG) to select patients for further investigation with coronary angiography,15 whereas NICE guidance does not recommend using exercise ECG in the context of suspected ACS. 11 The role of exercise ECG testing in suspected ACS therefore remains unclear and involves extrapolating evidence from other settings. We therefore planned to synthesise the evidence relating to the role of exercise ECG in assessing patients with suspected ACS.
Computed tomographic coronary angiography
Computed tomographic coronary angiography uses computerised tomography (CT) scanning to allow non-invasive imaging of the coronary arteries. CT scanning involves an X-ray source and sensors mounted on opposite sides of a gantry that rotates around the patient to provide a computer-generated three-dimensional image of the heart. Modern scanners have an array of X-ray detectors that collect data from multiple ‘slices’ on each rotation of the scanner (multislice CT). Initially, scanners with four slices were developed. Currently available scanners commonly use 16 or 64 slices.
Computerised tomography can be used without intravenous contrast to quantify CAC (CT CAC scoring) and thus estimate the extent of coronary atheroma. Patients with a calcium score of zero are unlikely to have CAD, whereas the higher the score the greater the probability of CAD. It can be used in conjunction with clinical assessment of CAD risk to select patients for invasive coronary angiography (ICA) or CTCA. However, CT coronary artery scoring does not determine whether or not coronary atheroma is obstructive. When patients present with suspected ACS it is usually considered more important to determine whether their symptoms are due to obstructive CAD than estimate the probability of CAD, so evaluation of the role of CT in suspected ACS has focused on CTCA rather than CT coronary artery scoring.
Computed tomographic coronary angiography involves injection of intravenous contrast medium with CT scanning timed to coincide with circulation of contrast through the coronary arteries. The scans are then interpreted to determine the extent of coronary artery stenosis. As intravenous contrast is required, the procedure is contraindicated in renal failure and allergy to contrast media, and is used with caution in pregnancy. The quality of imaging can be impaired by artefact due to inability to breath hold, tachycardia or arrhythmia. Artefact may be reduced by using beta-blocking drugs to slow the patient's heart rate.
Computed tomographic coronary angiography may provide a more accurate and cost-effective alternative to exercise ECG in troponin-negative patients with suspected ACS. As with exercise ECG, most studies have evaluated CTCA in patients with stable symptoms rather than suspected ACS. A recent systematic review of 21 diagnostic accuracy studies of CTCA reported a pooled sensitivity of 99% and specificity of 89% for detection of CAD. 25 On the basis of this and similar analyses, NICE guidance has recommended that CT calcium scoring with CTCA for selected patients should replace exercise ECG for patients with stable symptoms. 11 There has been less research into the use of CTCA in suspected ACS. NICE guidance for chest pain of recent onset suggests that patients with suspected ACS in whom MI has been ruled out should be risk stratified and those considered to be at risk of myocardial ischaemia managed according to the guidance for patients with stable symptoms. 11 The guidance highlighted that this contrasts with European Society of Cardiology guidelines recommending stress testing,15 and identified evaluation of the cost-effectiveness of CTCA in troponin-negative patients with suspected ACS as being a research priority. We therefore planned to synthesise the evidence for the use of CTCA in patients with suspected ACS.
Chapter 2 Research questions
Rationale for the study
This study aimed to reduce uncertainty around two issues highlighted in NICE guidance:
-
The use of troponin and other biomarkers to diagnose MI at presentation to hospital.
-
The use of other biomarkers, exercise ECG and CTCA to risk-stratify patients with acute chest pain and a negative troponin.
Troponin measured at least 10–12 hours after symptom onset and using the 99th percentile as a diagnostic threshold, accurately diagnoses MI and identifies patients who are at high risk of adverse outcome and who will benefit from hospital treatment. However, patients awaiting delayed testing are currently detained in hospital until 10–12 hours after symptom onset. This incurs health services costs and inconvenience for the patient. An earlier diagnostic assessment could allow earlier hospital discharge, thus decreasing costs, but would risk missed MI and opportunity to benefit from treatment if sensitivity were suboptimal. High-sensitivity troponin assays, either alone or in combination with other biomarkers, can be used to diagnose MI before 10–12 hours, but the cost savings of this approach need to be weighed against the missed benefit (or, more rationally, the additional benefits of 10- to 12-hour troponin sampling need to be weighed against the additional costs, compared with earlier diagnostic assessments). We therefore need to undertake evidence synthesis to estimate (1) the diagnostic accuracy of early biomarkers and (2) the cost-effectiveness of alternative diagnostic strategies for MI.
Biomarkers may also provide benefits by risk-stratifying troponin-negative patients. A negative troponin assay at 10–12 hours (and potentially earlier) stratifies patients with acute chest pain to a low but not negligible risk of subsequent MACEs. However, because the risk remains non-negligible there may still be some benefit in measuring other biomarkers that predict increased risk independent of troponin level. These biomarkers could be used to select higher risk troponin-negative patients for further investigation and treatment to reduce the risk of adverse outcome. We therefore need to undertake evidence synthesis to estimate (1) the prognostic accuracy of biomarkers other than troponin and (2) the cost-effectiveness of using these biomarkers to select patients for hospital treatment.
Troponin-negative patients may also be investigated by exercise ECG or CTCA to identify those with CAD and an increased risk of adverse outcome who may benefit from coronary intervention and medical treatment to reduce the risk. We therefore need to undertake evidence synthesis to estimate (1) the diagnostic accuracy of exercise ECG and CTCA for CAD, and the prognostic accuracy of exercise ECG and CTCA for MACEs and (2) the cost-effectiveness of using exercise ECG or CTCA to select patients for hospital treatment.
Overall aims and objectives of assessment
The overall aim was to evaluate the cost-effectiveness of various strategies for diagnosing MI and CAD in unselected populations with suspected ACS. More specifically, the objectives were:
-
to undertake systematic reviews to determine:
-
the diagnostic accuracy of early biomarkers (including troponin) for MI in patients with suspected ACS
-
the prognostic accuracy of biomarkers for predicting MACEs in troponin-negative patients
-
the diagnostic accuracy of CTCA and exercise ECG for CAD in patients with suspected ACS
-
the prognostic accuracy of CTCA and exercise ECG for predicting MACEs in patients with suspected ACS
-
-
to develop an economic model to:
-
estimate the cost-effectiveness [measured as the cost per quality-adjusted life-year (QALY) gained by each strategy] of using various early biomarker strategies to diagnose MI
-
estimate the cost-effectiveness of using biomarkers, CTCA and exercise ECG to risk-stratify patients with troponin-negative suspected ACS
-
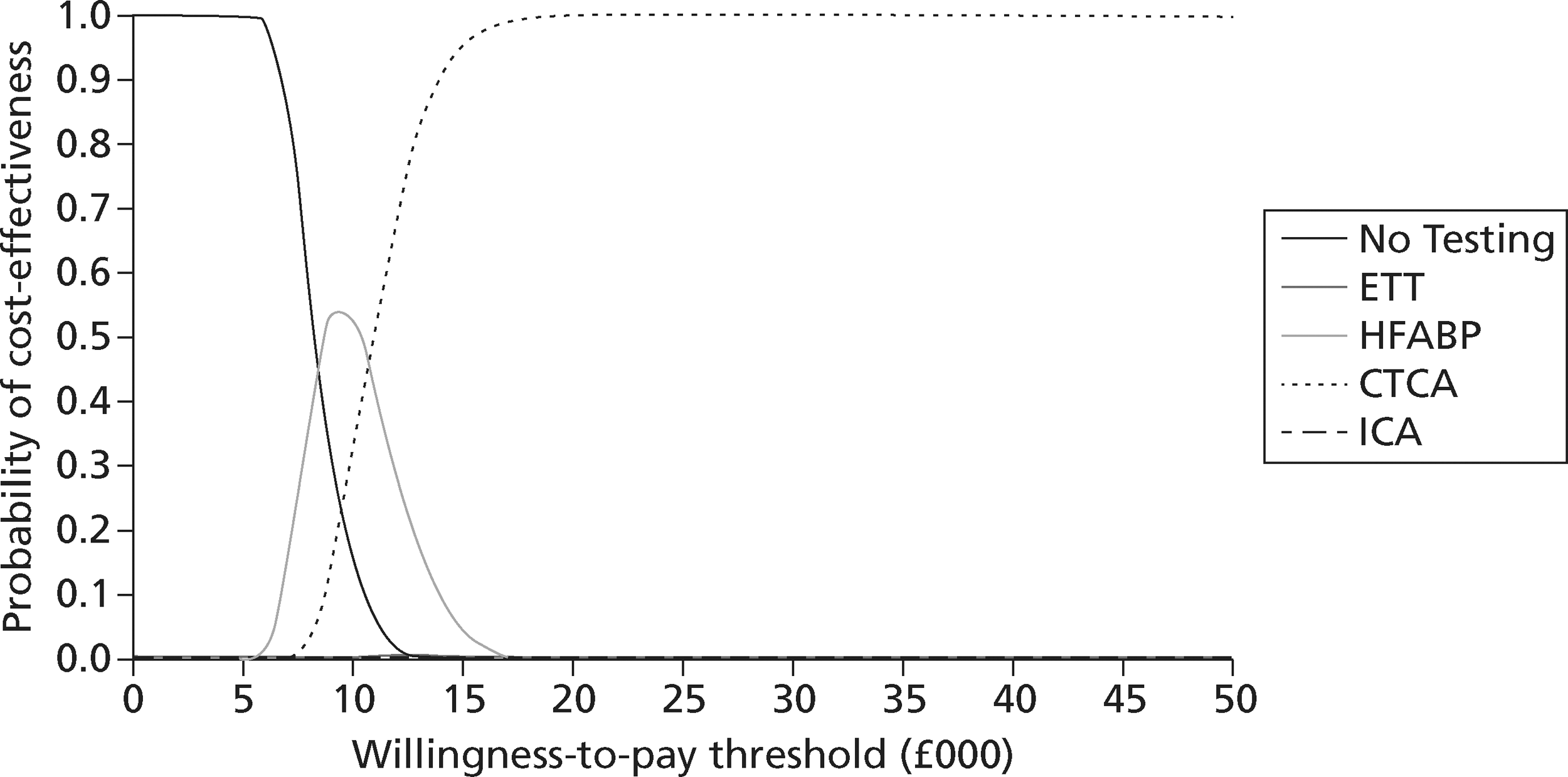
identify the optimal strategies for diagnosing MI and investigating troponin-negative patients in the NHS, defined as the most cost-effective strategy at the NICE threshold for willingness to pay per QALY gained
-
identify the critical areas of uncertainty in the management of suspected ACS and where future primary research would produce the most benefit.
-
Chapter 3 Assessment of diagnostic and prognostic accuracy
We conducted two systematic reviews of the literature, and meta-analysis (where appropriate), to evaluate the diagnostic accuracy of biochemical markers for MI, and of CTCA and exercise ECG for CAD, as well as two further reviews to evaluate the prognostic performance of both approaches for predicting MACEs. The population in all reviews was unselected patients with suspected ACS.
The systematic reviews and meta-analysis were undertaken in accordance with the guidelines published by the Centre for Reviews and Dissemination for undertaking systematic reviews26 and the Cochrane Diagnostic Test Accuracy Working Group on the meta-analysis of diagnostic tests. 27
Methods for reviewing diagnostic accuracy
Identification of studies
Electronic databases
All searches were undertaken by an information specialist (PE) in November 2010. Studies were identified by searching the following electronic databases:
-
MEDLINE (via Ovid SP) 1950–
-
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid SP) 1950–
-
Cumulative Index of Nursing and Allied Health Literature (CINAHL) (via EBSCO) 1981–
-
EMBASE (via Ovid SP) 1980–
-
Web of Science (WoS) (includes Science Citation Index and Conference Proceedings Citation Index) [via Web of Knowledge (WoK)] 1899–
-
Cochrane Central Register of Controlled Trials (CENTRAL)
-
Cochrane Database of Systematic Reviews (CDSR)
-
NHS Database of Abstracts of Reviews of Effects (DARE)
-
Health Technology Assessment database (HTA).
Sensitive keyword strategies using free text and, where available, thesaurus terms using Boolean operators and database-specific syntax were developed to search the electronic databases. For the biochemical markers reviews, synonyms relating to the population (e.g. chest pain, ACS or MI) were combined with terms for the biochemical markers of interest, and the reference standard (troponin), and a search filter aimed at restricting results to studies of either diagnostic accuracy or prognosis (used in the searches of MEDLINE, CINAHL and EMBASE). For the CTCA and exercise ECG review, synonyms relating to the population (e.g. chest pain, ACS or MI) were combined with terms for the diagnostic tests, and the reference standard (coronary angiography) or outcomes (e.g. MACE), and a search filter aimed at restricting results to studies of either diagnostic accuracy or prognosis (used in the searches of MEDLINE, CINAHL and EMBASE). Date limits or language restrictions were not used on any database for either review. All resources were searched from 1985 (CTCA review) or 1995 (biomarkers review) to November 2010. Examples of the MEDLINE search strategy for each review is provided in Appendix 1.
Other resources
To identify additional published, unpublished and ongoing studies, the reference lists of all relevant studies (including existing systematic reviews) were checked. In addition, key experts in the field were approached to identify any relevant citations missed by the search methods applied.
All identified citations from the electronic searches and other resources were imported into and managed using the Reference Manager bibliographic software (version 12.0; Thomson Reuters, Philadelphia, PA, USA).
Study selection and inclusion/exclusion criteria
The selection of potentially relevant articles was undertaken across both reviews by an experienced reviewer (CC) and the principal investigator, a clinical expert (SG). An acceptable inter-rater reliability was achieved from a test screen of a sample of citations retrieved for each set of reviews: k = 0.71 for 700 citations for the biochemical markers review and k = 0.61 for 400 from the CTCA/exercise ECG review. The remaining citations were then divided between the reviewers (CC and SG) and each independently screened their respective sample against the inclusion criteria and excluded any citations that clearly did not meet these criteria. The full manuscript of all potentially eligible citations that were considered relevant by either reviewer was then obtained, where possible. One reviewer (CC) then independently assessed the full-text articles for inclusion and this decision was double-checked by the principal investigator (SG). Blinding of journal, institution and author was not performed. Any disagreement in the selection process was resolved through discussion. The relevance of each article to the two diagnostic or prognostic reviews was assessed according to the following criteria.
Study design
All prospective diagnostic cohort studies comparing a relevant index test (biochemical markers or CTCA/ exercise ECG) to the required reference standard for the relevant outcome (MI or CAD) were included in their relevant review. All studies examining the prognostic value of a relevant index text (biochemical markers or CTCA) for at least 30 days' follow-up for MACEs were included, regardless of the reference standard used. Case–control studies (i.e. studies in which patients were selected on the basis of the results of their reference standard test) were excluded.
Population
To be included, a study had to assess adults presenting with suspected ACS. Studies were excluded if patients were selected on the basis of having a clinical diagnosis of ACS (rather than a clinical suspicion of ACS) or positive diagnostic test for ACS, such as ST deviation on the ECG or an elevated biomarker. Studies of patients selected on the basis of a negative diagnostic test were included [e.g. studies that excluded patients with ST elevation myocardial infarction (STEMI)].
Index tests
For the biochemical markers review, the index test included any test assessing the following markers individually or in combination:
-
adrenomedullin
-
BNP or NT-pro-BNP
-
copeptin
-
CRP
-
galectin-15
-
H-FABP
-
interleukin 33
-
IMA
-
matrix metalloproteinase 9 (MMP9)
-
MPO
-
myoglobin
-
PAPP-A
-
ST-2
-
TnI or TnT.
Studies were only included in the diagnostic accuracy review if the index test was measured at or before patient arrival at hospital. We excluded prognostic studies that only evaluated troponin (or other biomarkers not included in the review, such as CK and CK-MB).
For the second diagnostic review, the index test was either CTCA, regardless of sensitivity (e.g. 64 or 16 slices) or exercise ECG.
Target condition
The target conditions or outcomes of the reviews of biochemical markers were:
-
Diagnostic review Acute MI defined according to the universal definition. 7
-
Prognostic review MACE, defined as including at least cardiac death and non-fatal MI (individually or as a composite).
The target conditions or outcomes of the review of CTCA and exercise ECG were:
-
Diagnostic review CAD identified on ICA.
-
Prognostic review MACE, defined as including at least cardiac death and non-fatal MI (individually or as a composite).
Reference standards
Acute MI was defined according to the universal definition and required TnI or TnT measurement for at least 80% of the population at least 6 hours after symptom onset. If the reference standard was any biomarker other than troponin the study was excluded from the diagnostic review. Many studies reported composite diagnostic reference standards or a diagnostic standard of ACS, which included clinically diagnosed ACS, development of ECG changes or a subsequent MACE. Where possible we attempted to extract data for MI according to our definition. If this was not possible we made a judgement whether or not the reference standard approximated to our definition of MI. We included studies that used only new diagnostic ECG changes or outcome-based MACE (e.g. death, non-fatal MI or life-threatening arrhythmia) alongside a troponin-based reference standard. We excluded studies that used clinically diagnosed ACS (i.e. by history and examination findings alone), undefined or any ECG changes, or process-based MACE (e.g. coronary reperfusion) in the reference standard.
Coronary artery disease was determined by ICA and defined in accordance with the primary study. Studies were excluded if coronary angiography was performed only in selected patients, such as those with positive CTCA or exercise ECG. The definition of MACEs required that at least 80% of the cohort be followed for at least 30 days and that a MACE included, at least, cardiac death and non-fatal MI.
Outcomes
Sufficient data were required to construct tables of diagnostic test performance, i.e. numbers of true-positives (TPs), false-negatives (FNs), false-positives (FPs) and true-negatives (TNs). If raw numbers were not reported we attempted to calculate these data from sensitivity and specificity, using prevalence and total number analysed to calculate the denominators. Studies were excluded from analysis as ‘unable to extract data’ if these calculations were not possible or yielded markedly inconsistent data.
Data abstraction strategy
Data abstraction of each study was performed by one reviewer (CC, MK or JL) into a standardised data extraction form and independently checked for accuracy by a second reviewer (CC, MK, JL or SG). Discrepancies were resolved by discussion between the two reviewers and if agreement could not be reached, the principal investigator was consulted (SG). Where multiple publications of the same study were identified, data were extracted and reported as a single study. Where there was possible overlap between cohorts reported from the same author group or study centre we excluded data from one of the cohorts to avoid duplication.
For the review of biochemical markers, the following information was extracted for all studies when reported: study characteristics (author, year of publication, journal, country, study design, setting); participant details (age, sex, presenting condition, inclusion and exclusion criteria); index test details (including time from pain onset to presentation or blood test, diagnostic threshold and assay); reference standard details (including diagnostic threshold and assay, and timing of test); prevalence of MI and data for a two-by-two table (TP, FN, FP, TN); sensitivity; specificity; and any additional potential relevant citations from the reference list. Where a study presented prognostic data, the following additional information was extracted: whether the participants were TP or TN; duration of follow-up; method of data collection; mortality data; and data on non-fatal MI.
For the review of CTCA and exercise ECG, the following information was extracted for all studies when reported: study characteristics (author, year of publication, journal, country, study design, setting); participant details (age, sex, presenting condition, inclusion and exclusion criteria); index test details (including diagnostic threshold); reference standard details (including diagnostic threshold); prevalence of CAD and data for a two-by-two table (TP, FN, FP, TN); sensitivity; specificity; and any additional potential relevant citations from the reference list. Where a study presented prognostic data, the following additional information was extracted: duration of follow-up; method of data collection; mortality data; data on non-fatal MI and any other MACE.
Quality assessment strategy
The methodological quality of each diagnostic study in the review of biochemical markers was assessed by one reviewer (CC or MK) but checked by a second (CC or MK) using a modified version of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool28 (a generic, validated, quality assessment instrument for diagnostic accuracy studies). The methodological quality of each included study in the review of CTCA and exercise ECG was assessed by one reviewer (JL) but checked by a second (CC) using the same modified version of the QUADAS tool. In all cases of doubt in either review, the principal investigator (SG) was consulted.
The quality assessment items included from QUADAS28 were the following: whether or not patients were representative of those who would receive the test in practice, i.e. patients presenting to the emergency services or department with chest pain and suspected ACS; whether or not the reference standard was likely to correctly classify the condition, i.e. was it based on the universal definition of MI; whether or not the time period between onset of symptoms and reference standard and index test was clear enough to be reasonably sure that index and reference tests are meaningful, i.e., were the two tests both conducted within the 12-hour time frame required for the reference standard; whether or not patients received same reference standard regardless of index test result; whether or not the reference standard was independent of the index test (i.e. index test did not form part of reference standard); whether or not the whole sample (or a random selection of the sample) received verification using a reference standard of diagnosis; whether or not the index test was interpreted without knowledge of reference standard results; and whether or not the reference standard was interpreted without knowledge of index test results (blinding).
The following elements from the original QUADAS checklist were omitted either because they did not apply (e.g. inclusion criteria for the reviews was that all studies had to be prospective and patients unselected, i.e. consecutive) or because they were not likely to impact on results in this case (e.g. descriptions of selection criteria or the tests): whether the study was prospective or retrospective; whether or not selection criteria were clearly described; whether or not the reference standard was likely to correctly classify the condition; whether or not the execution of the reference standard was described in sufficient detail to permit its replication; the relevance of index test to clinical practice; and whether or not the execution of the index test was described in sufficient detail to permit its replication. The criterion concerning whether or not there were any interpretable/intermediate test results and whether these were reported was only included in the CTCA/exercise ECG review as there was a risk of loss of data due to uninterpretable results from imaging in this review, which did not apply to the biomarkers review. Study quality was assessed with each item scored as ‘yes’, ‘no’ or ‘unclear’. Further details on the modified version of the QUADAS tool are provided in Appendix 2.
The quality assessment for prognostic studies of biomarkers, exercise ECG and CTCA was conducted using an adapted version of the framework described by Altman. 29 The assessment asked the following seven questions of each study:
-
Sample of patients Are inclusion criteria defined?
-
Sample of patients Are characteristics described (age and sex)?
-
Outcome Is a MACE defined in the methods section?
-
Outcome Is a MACE identification and definition independent of the index test?
-
Outcome Is a MACE outcome recorded for at least 80% of the cohort from baseline episode?
-
Analysis Was a multivariate analysis undertaken (were other variables, other than our variable of interest, included in the analysis)?
-
Analysis Is troponin measured and included in the multivariate analysis, or is analysis stratified by troponin or limited to those with a negative troponin?
Questions 1 and 2 assessed adequacy of reporting. Question 3 aimed to determine whether or not the outcome of interest (MACE) appeared to have been defined a priori by the researchers (i.e. in the methods section rather than the results section). Question 4 aimed to determine whether or not a presenting diagnosis (such as MI) that could have been associated with a positive index test was incorporated in the definition of MACEs. Question 5 assessed adequacy of follow-up. Although this was an inclusion criterion for the review, 80% follow-up was not always clearly reported or achieved at all time points. Question 6 assessed whether or not the study had explored beyond an association between the index test and MACEs to determine whether or not the biomarker added prognostic value beyond routine assessment. Question 7 assessed whether this analysis was stratified by or adjusted for troponin, to determine whether the biomarker added prognostic value to that provided by troponin.
The methodological quality of each prognostic study in the review of biochemical markers was assessed by one reviewer (SG or CC) but checked by a second (SG or CC) using this modified version of the Altman criteria. 29 The methodological quality of each included study in the review of CTCA and exercise ECG was assessed by one reviewer (JL) but checked by a second (FM) using these same criteria. In all cases of doubt in either review, the principal investigator (SG) was consulted.
Methods of data synthesis
The analysis was conducted using Bayesian Markov chain Monte Carlo simulation. In general, there are advantages of the Bayesian approach over a Classical approach, including the ability to (1) analyse complex models exactly; (2) incorporate external evidence in addition to sample data; and (3) make probabilistic statements about parameters. In particular, the approach allowed the direct use of a binomial likelihood for the sample data, including for studies with very small or zero counts; the ability to incorporate uncertainty in the estimate of the between-study standard deviation (SD), including in studies with relatively few studies;30 and the ability to generate probability distributions that represent parameter uncertainty about inputs to the economic model.
The use of a random-effects model is motivated a priori by the assumption that the true sensitivities and specificities vary according to the study but that they arise from a common (bivariate) population distribution. Heterogeneity is common in meta-analyses of diagnostic test data and the results of these analyses are no exception. The pooled effects presented in the forest plots represent the means of the population of sensitivities and specificities, and these are the parameters that are commonly presented as the results of a meta-analysis. Also presented with the forest plots are predictive effects; these represent the range of estimates that we might expect to see in the population taking into account uncertainty in both the estimate of the mean sensitivity and specificity and the uncertainty in the estimates of the variability between studies. The predictive effect can be thought of as providing an estimate of the effect of a randomly selected new study in the population. 30,31
A meta-analysis of diagnostic test accuracy was undertaken for selected biomarkers, assays and decision thresholds. Data were selected and categorised post hoc on the basis of combining data for similar assays at a similar decision threshold. Patients were classified with respect to the index test as being either a TP or a FN if they had the condition, and a FP or TN if they did not have the condition. The model used to summarise the data was a random-effects model in which the true study-specific sensitivities and specificities on the logit scale were assume to be exchangeable across studies and arising from a bivariate normal distribution with common mean and variance-covariance matrix across studies to allow for correlation within studies. Given the observed (or sample) data, the application of Bayes' theorem provides estimates of the mean and variance for the true study-specific sensitivities and specificities that are functions of the weight given to the prior mean. The weights depend on the variability between studies and the precision of individual studies. The random-effects model leads to estimates of the true sensitivities and specificities for each study with narrower intervals than if the studies were assumed to be independent but shrunk towards the prior mean sensitivities and specificities. The extent of the shrinkage is greatest when there is relatively little information in the sample data relative to the prior distribution. 32
We let:
We completed the model by giving the uncertain parameters the following prior distributions:
The data were analysed using the freely available software WinBUGS version 1.4.1 (MRC Biostatistics Unit, Cambridge, UK). 33 Convergence was assessed using the Gelman-Rubin convergence statistic. 34 Convergence occurred after 15,000 iterations. We used a burn-in of 15,000 and generated a further 20,000 iterations to estimate the parameters.
In one analysis (Abbott troponin I) the model failed to fit using the weak prior specified in the analyses of the other diagnostic accuracy data. In this case, we used the following prior distributions:
The impact of this is mainly on the prior estimates of the between-study SDs, which are reduced from 1.5 [95% credible interval (CrI) 0.4 to 33.1] to 0.5 (95% CrI 0.3 to 1.4) when R is increased from ‘2’ to ‘5’ in the inverse Wishart distribution.
Meta-analysis of prognostic test accuracy Data were available from studies in which patients were classified as either having an event or not having an event, depending on whether the index test was positive, inconclusive or negative. Not all studies reported inconclusive tests separately; some reported inconclusive results with the positives, others with the negatives and in others it was not clear whether or not there were any inconclusive tests. Furthermore, some studies reported outcomes only for those with a positive or negative index test. We excluded studies that reported outcomes only for positive or negative index tests. If no inconclusive tests were reported, we included the data in the analyses by assuming that there were no inconclusive results.
Relative risks (RRs) were calculated by comparing (1) positive compared with inconclusive and negative and (2) positive and inconclusive compared with negative. The data were meta-analysed using a Bayesian random-effects model as follows. 34
We let rij represent the number of events in category j in study i and Nij represent the total number of individuals in category j in study i. We assumed that the data followed a Binomial distribution such that:
where Pij represents the probability of an event category j in study I.
We let:
so that the μi are study-specific baselines representing the log of the absolute risk of an event in the baseline category and the second term is the log-RR in study i.
We assumed a random-effects model in which the study-specific RRs are assumed to come from a population of effects that are normally distributed such that:
We completed the model by giving the uncertain parameters the following prior distributions:
The data were analysed using the freely available WinBUGS software. 33 Convergence was assessed using the Gelman-Rubin convergence statistic. 35 Convergence occurred after 50,000 iterations. There was some evidence of high autocorrelation between successive iterations of the Markov chains. We used a burn-in of 50,000 and generated a further 60,000 iterations after thinning the chains every 10 iterations to estimate the parameters.
Results of the reviews
This section presents the results of the following systematic reviews separately:
-
the diagnostic accuracy of biomarkers measured at presentation, including troponin, compared with the universal definition of MI, and the prognostic accuracy of biomarkers, excluding troponin, for predicting MACEs, in unselected patients presenting with chest pain and suspected ACS (see Studies included in the biochemical markers review, below)
-
the diagnostic accuracy and prognostic performance of exercise ECG and CTCA compared with ICA for identifying CAD or predicting MACEs in unselected patients presenting with chest pain and suspected ACS (see Studies included in the computed tomographic coronary angiography and exercise electrocardiography review, below).
Studies included in the biochemical markers review
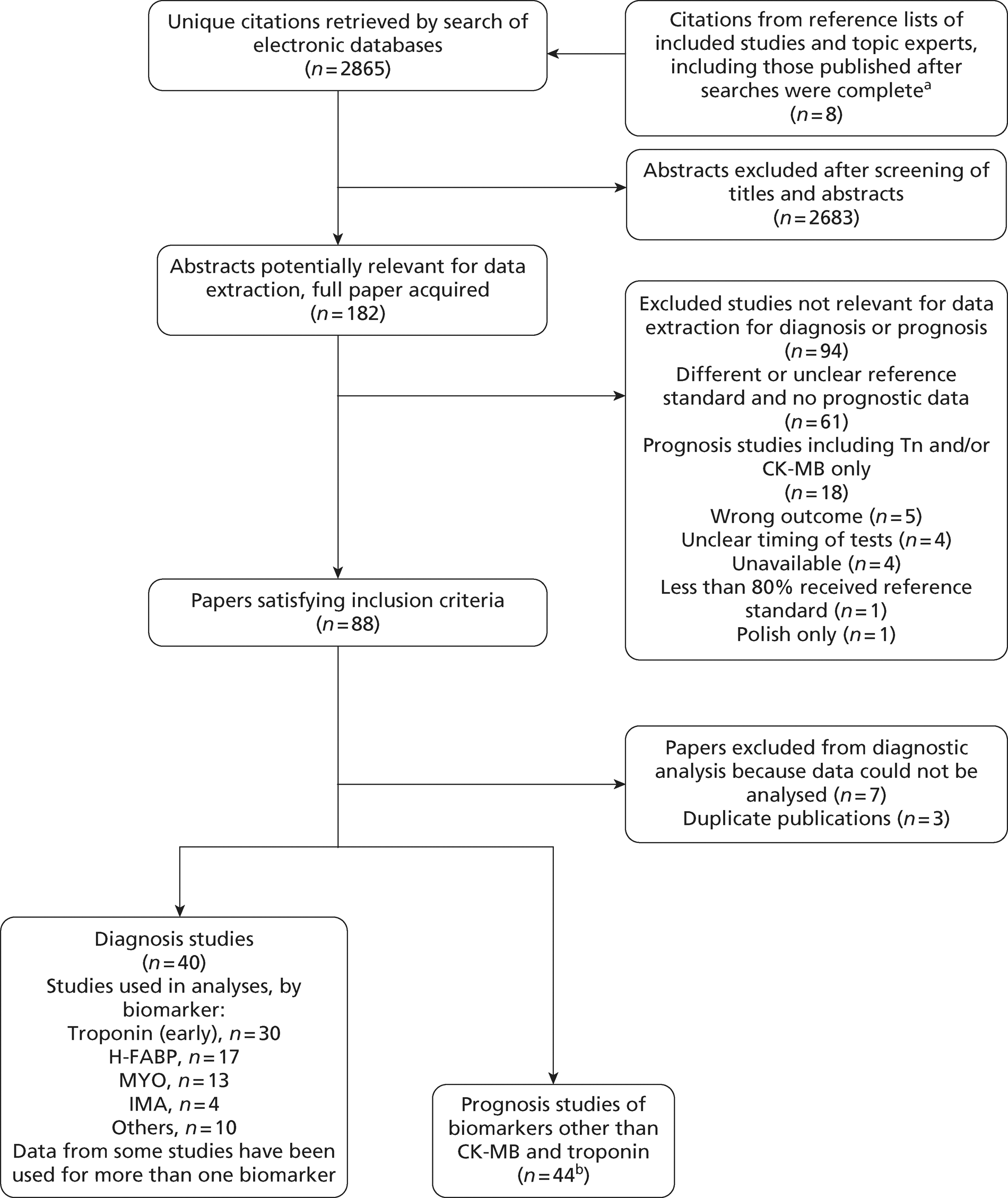
Overall, the literature searches identified 2865 citations. A flow chart describing the process of identifying relevant literature can be found in Figure 2. Of the titles and abstracts screened, 182 relevant full papers were retrieved and assessed in detail. Studies excluded from the review, with reasons, are listed in Appendix 3. A total of 88 papers evaluating the diagnostic accuracy or prognostic performance of biochemical markers met the inclusion criteria. Of these, we were unable to extract appropriate data from seven studies36–42 and identified three43–45 in which there seemed to be duplication of data with other included studies. A total of 40 studies reported data on diagnostic accuracy and 44 studies reported data on prognostic performance, with six of these studies reporting both prognostic and diagnostic data. 46–51
FIGURE 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart biochemical markers review. a, we would like to thank Professor Paul Collinson and Rick Body for these studies; b, n = 6 studies report usable diagnostic and prognostic data for the same cohort.
Overview of biomarker studies included in the diagnostic review
Table 2 lists all the studies included in the diagnostic accuracy review and the biomarkers that were evaluated with extractable data. We were not able to extract data for all the biomarkers reported in each study. Table 2 lists only the biomarkers with extractable data.
| Study | Relevant index test biomarkers in study |
|---|---|
| Amodio 200752 | TnI, myoglobin |
| Apple 200853 | TnI |
| Apple 200854 | TnI |
| Apple 200955 | TnI, CD40L, NT-pro-BNP, CRP, MMP9, MPO |
| Bassan 200556 | TnI, BNP |
| Body 201157 | TnI, H-FABP, myoglobin, BNP, MPO |
| Body 201146 | TnT, PAPP-A, CD40L |
| Brown 200747 | ST2 |
| Cete201058 | TnT, H-FABP, myoglobin |
| Charpentier 201059 | TnI, H-FABP, IMA |
| Christ 201060 | TnT |
| Christenson 200161 | IMA |
| Collinson 200662 | TnI, TnT |
| Collinson 200648 | TnI, IMA |
| Di Serio 200563 | TnI, H-FABP, myoglobin |
| Ecollan 200764 | TnI, H-FABP, myoglobin |
| Eggers 200418 | Myoglobin |
| Esporcatte 200765 | MPO |
| Garcia-Valdecasas 201149 | TnI, H-FABP, myoglobin |
| Guo 200666 | TnI |
| Haltern 201067 | TnT, H-FABP |
| Hjortshoj 201067 | H-FABP, myoglobin, IMA |
| Ilva 200950 | TnI, H-FABP |
| Ilva 200569 | Myoglobin |
| Keating 200670 | TnI, IMA |
| Keller 200920 | TnI |
| Keller 201071 | TnT, myoglobin, copeptin |
| Lefevre 200772 | TnI, H-FABP |
| Li 201073 | TnT, H-FABP |
| Liao 200974 | TnI, H-FABP, myoglobin |
| Mad 200775 | H-FABP |
| McCann 200876 | TnT, H-FABP |
| Mion 200777 | TnI, H-FABP, myoglobin |
| Naroo 200978 | TnT, H-FABP |
| Penttilä 200279 | Myoglobin |
| Potsch 200651 | CRP |
| Reichlin 200980 | Copeptin |
| Reichlin 200919 | TnI, TnT |
| Rudolf 201081 | TnI, MPO |
| Valle 200882 | TnT, H-FABP |
Description of diagnostic studies of presentation troponin
We identified 21 diagnostic studies19,20,48–50,52–57,59,62–64,66,70,72,74,77,81 of presentation TnI and 11 studies19,46,58,60,62,67,71,73,76,78,82 of TnT for inclusion in the review. Two studies19,62 evaluated TnI and T. The characteristics of the study populations are outlined in Tables 3 and 4, whereas details of the index and reference standard test definitions are provided in Tables 5 and 6. Some studies evaluated more than one assay, so assays are reported separately in Tables 3 and 4. Reporting of inclusion and exclusion criteria were variable and several studies excluded patients with a diagnostic ECG. Prevalence of MI varied from 5% to 73% and was relatively high, suggesting that patient cohorts may have been subject to implicit selection processes. Time delay from symptoms to presentation varied from 1.2 hours (mean) to 6 hours (median). Several studies reported data using different diagnostic thresholds for the index test. Where this was done we extracted data for threshold based on the 99th percentile, 10% coefficient of variation (CV) and limit of detection (LoD). In accordance with our inclusion criteria, all studies used the universal definition of MI as the reference standard, and most reported using some form of adjudication, taking into account the results of troponin testing. In most cases the troponin used for the reference standard was a standard (i.e. not high sensitivity) assay using the 10% CV or 99th percentile as a diagnostic threshold. However, the study by Christ et al. 60 reported the use of a reference standard based on high-sensitivity TnT (HsTnT) alongside a reference standard based on the standard assay. For this study we extracted data based on the standard assay reference standard.
| Study | Study type | Population: age (years) and sex | Inclusion criteria | Exclusion criteria | Time from symptoms (hours) | No. of patients |
|---|---|---|---|---|---|---|
| Amodio 200752 | Single centre, Italy | Mean age 61, 308 (60%) male | Chest pain with suspected clinical angina or AMI | STEMI, new-onset LBBB | 5.0 (median) | 516 |
| Apple 200853 | Multicentre, USA and France | NR | Symptoms suggestive of ACS | NR | NR | 545 |
| Apple 200854 | Single centre, USA | Mean age 57, 223 (60%) male | Symptoms indicative of ACS | NR | 5.1 (median) | 371 |
| Apple 200955 | Single centre, USA | Mean age 54, 260 (57%) male | Symptoms suggestive of ACS within 12hours | NR | 3.1 (median) | 457 |
| Bassan 200556 | Single centre, Brazil | Mean age 67, 343 male | Chest pain < 12 hours due to possible acute cardiac ischaemia | ST segment elevation | 2.0 (median) | 631 |
| Body 201157 | Single centre, UK | Mean age 59, 430 (61%) male | Suspected cardiac chest pain occurring within the previous 24 hours | Chest trauma, renal failure requiring dialysis,medical condition necessitating admission, pregnancy | 3.5 (median) | 705 |
| Charpentier 201059 | Single centre, France | Mean age 57, 454 (67%) male | Chest pain due to suspected within 12 hours | ST elevation, traumatic cause, previous severerenal impairment or severe communicationproblems | 2.9 (median) | 677 |
| Collinson 200662 | Multicentre, UK | Median age 60, 150 (70%) male | Chest pain due to suspected ACS within 24 hours | STEMI | 3 (median) | 213 |
| Collinson 200648 | Single centre, UK | Median age 52, 335 (62%) male | Undifferentiated chest pain | ECG changes of ACS, unstable angina, comorbidity requiring admission, clearly non-cardiac pain | 6 (median) | 539 |
| Di Serio 200563 | Single centre, Italy | Mean age: female 79, male 65; 23 (77%) male | Not specified | ST elevation | 3.4 (mean) | 30 |
| Ecollan 200764 | Mobile units, France | Mean age 68, 68 (63%) male | Consecutive emergencies with chest pain | Patients with cardiogenic shock or those with any evidence of a recent chest trauma | 2.3 (median) | 108 |
| Garcia-Valdecasas 201149 | Single centre, France | Mean age 67, 114 (69%) male | Chest pain > 20 minutes' duration within 6 hours | Chest trauma | NR | 165 |
| Guo 200666 | Single centre, China | Median age 72, 237(47%) male | Chest pain within 0.5–24 hours | NR | 4 (median) | 502 |
| Ilva 200950 | Single centre, Finland | Mean age 67, 181 (62%) male | Chest pain suggesting myocardial ischaemia | Uncertain or > 24-hour delay from symptom onset | 4.7 (median) | 293 |
| Keating 200670 | Multicentre, UK | Median age 61, 251/399 eligible (63%) male | Possible ischaemic cardiac chest pain and normal ECG | Pain for > 8 hours on admission, pain ceased > 2 hours previously, pregnant, renal replacement therapy, jaundice | Not stated | 277 |
| Keller 200920 | Multicentre, Germany | Mean age 61, 1208 (66.4%) male | New-onset chest pain presenting at chest pain unit | Major surgery or trauma within the previous 4weeks, pregnancy, obvious intravenous drug abuse and anaemia with haemoglobin level of < 10g/dl | 59.5% < 6 hours | 1818 |
| LeFevre 200772 | Multicentre, France | Mean age 61, 71/100 male | Not specified | Not specified | 5.9 (median) | 75 |
| Liao 200974 | Single centre, China | Mean age 69, 54 (73%) male | Chest pain and/or dyspnoea lasting for at least 20 minutes within the last 3 hours | None reported | 2.2 (median) | 74 |
| Mion 200777 | Single centre, Italy | Mean age 63, 88 (67%) male | Non-consecutive patients with chest pain | None reported | 3.8 (median) | 132 |
| Reichlin 200919 | Multicentre, international | Median age 64 471 (66%) male | Chest pain within 12 hours | Terminal kidney failure requiring dialysis | NR | 718 |
| Rudolph 201081 | Single centre, Germany | Mean age 64, 192 (70%) male | Chest pain presenting to the ED | NR | 4.5 (median) | 274 |
| Study | Study type | Population: age (years) and sex | Inclusion criteria | Exclusion criteria | Time from symptoms (hours) | No. of patients |
|---|---|---|---|---|---|---|
| Body 201146 | Single centre, UK | Mean age 59, 434 (61%) male | Suspected cardiac chest pain occurring within the previous 24 hours | Chest trauma, renal failure requiring dialysis,medical condition necessitating admission, pregnancy | 3.5 (median) | 713 |
| Cete 201058 | Single centre, Turkey | Mean age 57, 163 (73%) male | Aged > 18 years presenting to the ED with typical chest pain | Atypical chest pain, musculoskeletal trauma, electrical cardioversion within the last 24 hours, musculoskeletal disease, acute or chronic renal failure, liver disease | NR | 224 |
| Christ 201160 | Single centre, Germany | Mean age 66, 87 (64%) male | Acute chest pain of possible coronary origin | No other criteria reported | 48% < 6 hours | 137 |
| Collinson 200662 | Multicentre, UK | Median age 60, 150 (70%) male | Chest pain due to suspected ACS within 24 hours | STEMI | 3 (median) | 213 |
| Haltern 201067 | Single centre, Germany | Mean age 69, 27/49 (55%) male | Ischaemic-type chest pain | Age < 18 years,interhospital transfer | 4 (median) | 94 |
| Keller 201071 | Multicentre, Germany | Mean age 61, 920 (66%) male | Aged 18–85 years, with angina pectoris or equivalent symptoms | Trauma or major surgery within the last 4 weeks, pregnancy, intravenous drug abuse, and anaemia | 57.6% < 6 hours | 1386 |
| Li 201073 | Multicentre, China | Mean age 64, 163 (72%) male | Chest pain for > 30 minutes and < 12 hours, suspected of MI | No other criteria reported | 4 (median) | 227 |
| McCann 200876 | Multicentre, Northern Ireland | Mean age 63, 281 (68%) male | Ischaemic-type chest pain within 24 hours | Age < 18 years,interhospital transfer, and previous participation in thestudy | 5.3 (median) | 415 |
| Naroo 200978 | Single centre, United Arab Emirates | Age not reported, 627 (79%) male | Typical chest pain within 12 hours | STEMI, known renal disease | NR | 791 |
| Reichlin 200919 | Multicentre, international | Median age 64, 471 (66%) male | Chest pain within 12 hours | Terminal kidney failure requiring dialysis | NR | 718 |
| Valle 200882 | Multicentre, Spain | Mean age 65, 287 (68%) male | Suspected ACS with symptoms between 20 minutes and 180 minutes of presentation | No other criteria reported | 1.2 (mean) | 419 |
| Study | Index test assay | Index test threshold (µg/l) | 99th percentile, 10% CV and LoD (µg/l) | Reference troponin assay and timinga | Reference troponin threshold (µg/l) | Prevalence MI (%) |
|---|---|---|---|---|---|---|
| Amodio 200752 | Dade Behring Stratus CS | 0.03 | 0.03 | TnT, timing and assay not specified | 0.07 | 21 |
| 0.07 | 0.07 | |||||
| 0.015 | ||||||
| Apple 200853 | BioMérieux Vidas TnI-Ultra | 0.01 | 0.01 | Dade Behring Dimension TnI at 4–12 hours after presentation | 0.15 | 29 |
| 0.11 | 0.11 | |||||
| <0.01 | ||||||
| Apple 200854 | Bayer (now Siemens) ADVIA Centaur Ultra | 0.04 | 0.04 | Dade Behring Dimension or Stratus CS, timing not stated | 0.1 | 13 |
| 0.006 | 0.03 | |||||
| 0.006 | ||||||
| Apple 200955 | Dade Behring Stratus CS and Dimension RxL | 0.1 | 0.1 | Dade Behring Dimension or Stratus CS TnI at 8 hours after presentation | 0.1 | 5 |
| 0.15 | ||||||
| Not stated | ||||||
| Bassan 200556 | Biosite immunofluorescence BNP assay | 1 | Not stated | Dade Behring TnI; within 9 hours post admission | 1.0 | 11 |
| Not stated | ||||||
| Not stated | ||||||
| Body 201157 | Alere fluorescence immunoassay | 0.055 | 0.055 | Roche fourth-generation TnT at 12 hours | 0.035 | 18 |
| Not stated | ||||||
| 0.055 | ||||||
| Charpentier 201059 | ADVIA Centaur system (Bayer Diagnostics) | 0.1 | 0.1 | ADVIA Centaur TnIc system (Bayer Diagnostics) at 6 hours after presentation | 0.1 | 15 |
| 0.1 | ||||||
| Not stated | ||||||
| Collinson 200662 | Euro/DPC Immulite | 0.2 | <1.0 | Roche third-generation TnT at 24 hours | 0.05 | 29 |
| 0.2 | ||||||
| 0.1 | ||||||
| Collinson 200648 | Beckman Coulter AccuTnI assay | 0.03 | Not stated | Roche Elecsys TnT at 6 and 72 hours | 0.05 | 7 |
| 0.03 | ||||||
| Not stated | ||||||
| Di Serio 200563 | Randox Evidence Investigator | 1 | 1.1 | ESC/ACC, not further specified | Not specified | 53 |
| Not stated | ||||||
| Not stated | ||||||
| Ecollan 200764 | Biosite Triage (pre-hospital) | 0.4 | Not stated | Dade Behring TnI up to 24 hours | 0.07 | 51 |
| Not stated | ||||||
| 0.05 | ||||||
| Garcia-Valdecasas 201149 | ELISA (Dainippon Pharmaceutical, Japan) | 0.6 | Not stated | TnI Dimension Analyser (Dade), timing not stated | 0.6 | 39 |
| Guo 200666 | Roche CARDIAC reader | 0.1 | Not stated | Beckman Coulter AccuTnI every 6 hours on first day and every 24 hours for 6 days | 0.33 | 30 |
| Not stated | ||||||
| Not stated | ||||||
| Ilva 200950 | Abbott Architect | 0.032 | 0.032 | Abbott Architect up to 24 hours | 0.032 | 46 |
| Not stated | ||||||
| 0.01 | ||||||
| Keating 200670 | Beckman Access | 0.06 | 0.04 | TnI; Beckman Access; taken at least 8 hours after pain onset | 0.06 | 15 |
| Not stated | ||||||
| Not stated | ||||||
| Keller 200920 | Siemens TnI-Ultra (ADVIA Centaur immunoassay) | 0.04 | 0.04 | Roche Troponin T or Siemens Dimension RxL TnI within 6 hours of admission | 0.3 (cTnT) | 23 |
| 0.03 | 0.14 (cTnI) | |||||
| Not stated | ||||||
| LeFevre 200772 | (1) Dade Behring RxL | (1) 0.14 | (1) 0.07 | (1) Dade Behring RxL or (2) Siemens Centaur TnI, timing not stated | (1) 0.14 | 48 |
| (2) Siemens Centaur | (2) 0.33 | 0.14 | (2) 0.33 | |||
| Not stated | ||||||
| (2) 0.1 | ||||||
| 0.33 | ||||||
| Not stated | ||||||
| Liao 200974 | Not specified | 0.5 | Not stated | No details | 0.5 | 73 |
| Mion 200777 | Evidence® Cardiac Panel | 0.47 | Not stated | Dimension | 0.15 | 32 |
| Not stated | RxL TnI, timing not specified | |||||
| Not stated | ||||||
| Reichlin 200919 | Siemens TnI-Ultra (ADVIA Centaur immunoassay) | 0.04 | 0.04 | Abbott AxSYM TnI, Beckman Coulter | 99th percentile | 17 |
| 0.006 | 0.03 | AccuTnI or Roche TnT at 6–9 hours after presentation | ||||
| 0.006 | ||||||
| Reichlin 200919 | Abbott Architect TnI | 0.028 | 0.028 | Abbott AxSYM TnI, Beckman Coulter | 99th percentile | 17 |
| 0.032 | 0.032 | AccuTnI or Roche TnT at 6–9 hours after presentation | ||||
| 0.01 | 0.01 | |||||
| Reichlin 200919 | Roche TnI | 0.16 | 0.16 | Abbott AxSYM TnI, Beckman Coulter | 99th percentile | 17 |
| 0.3 | 0.3 | AccuTnI or Roche TnT at 6–9 hours after presentation | ||||
| 0.1 | 0.1 | |||||
| Rudolph 201081 | Abbott Architect | 0.032 | Not stated | Abbott Architect, timing not stated | 0.032 | 36 |
| 0.032 | ||||||
| 0.032 |
| Study | Index test assay | Index test threshold (µg/l) | 99th percentile, 10% CV and LoD (µg/l) | Reference troponin assay andtiminga | Reference troponin threshold (µg/l) | Prevalence MI (%) |
|---|---|---|---|---|---|---|
| Body 201146 | Roche Diagnostics Elecsys fourth-generation TnT | 0.01 | 0.01 | Roche fourth-generation TnT at 12 hours | 0.035 | 18 |
| 0.035 | ||||||
| Not stated | ||||||
| Cete 201058 | Not stated | 0.1 | Not stated | Unspecified TnT at 6 hours | 0.1 | 33 |
| Christ 201060 | Roche Diagnostics Elecsys fourth-generation TnT | 0.01 | 0.01 | Roche fourth-generation TnT at 6 hours after presentation | 0.04 | 15 |
| 0.04 | 0.035 | |||||
| Not stated | ||||||
| Christ 201060 | Roche Diagnostics Elecsys HsTnT | 0.003 | 0.014 | Roche fourth-generation TnT at 6 hours after presentation | 0.04 | 15 |
| 0.014 | 0.013 | |||||
| 0.002 | ||||||
| Collinson 200662 | Roche Diagnostics Elecsys third-generation TnT | 0.03 | 0.01 | Roche third-generation TnT at 24 hours | 0.05 | 29 |
| 0.03 | ||||||
| 0.01 | ||||||
| Haltern 201067 | Roche Diagnostics Elecsys TnT | 0.03 | 0.01 | Roche TnT at 12 hours | 0.03 | 33 |
| 0.03 | ||||||
| 0.01 | ||||||
| Keller 200920 | Roche Diagnostics Elecsys TnT | 0.01 | 0.01 | Roche TnT or Siemens Dimension RxL TnI within 6 hours of admission | 0.3 (TnT) | 29 |
| 0.03 | 0.03 | 0.14 (TnI) | ||||
| 0.01 | ||||||
| Li 201073 | Not stated | 0.1 | Not stated | Unspecified TnT at 12 hours | 0.1 | 50 |
| McCann 200876 | Roche Diagnostics Elecsys TnT | 0.03 | 0.01 | Roche TnT at 12 hours | 0.03 | 48 |
| 0.03 | ||||||
| 0.01 | ||||||
| Naroo 200978 | Electrochemiluminescence immunoassay | 0.03 | Not stated | Electrochemiluminescence immunoassay TnT at 6–12 hours after presentation | 1 | 13 |
| Reichlin 200919 | Roche Diagnostics Elecsys HsTnT | 0.014 | 0.014 | Abbott AxSYM TnI, Beckman Coulter | 99th percentile | 17 |
| 0.002 | 0.013 | AccuTnI or Roche TnT at 6–9 hours after presentation | ||||
| 0.002 | ||||||
| Reichlin 200919 | Roche Diagnostics, Elecsys fourth-generation TnT | 0.035 | 0.01 | Abbott AxSYM TnI, Beckman Coulter | 99th percentile | 17 |
| 0.01 | 0.035 | AccuTnI or Roche TnT at 6–9 hours after presentation | ||||
| 0.01 | ||||||
| Valle 200882 | Not stated | Not stated | Not stated | Unspecified TnT at 6–12 hours | Not stated | 35 |
Quality assessments of diagnostic studies of presentation troponin
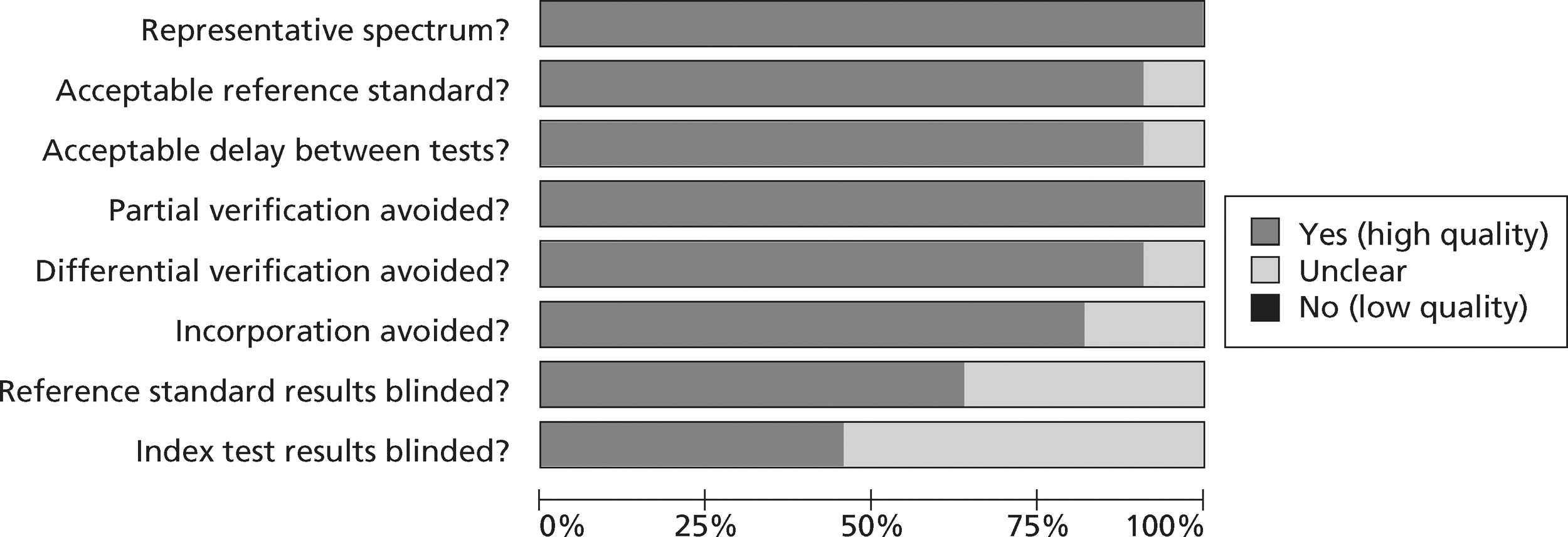
Figures 3 and 4 show the quality assessments for studies of TnI and TnT, respectively, whereas Figures 5 and 6 show the methodological quality summaries. The studies were generally high quality, perhaps reflecting exclusion of lower-quality studies by our selection criteria. Presentation troponin measurement is obviously not independent of a troponin-based reference standard, so our assessment of verification bias focused on whether or not the index and reference standard troponin were measured on different samples. There was some uncertainty about whether index and reference standard tests were assessed blind. This is not likely to have influenced reporting of the index test as in most cases this was a mechanised process producing a quantitative result. However, bias could have resulted if reference standard adjudicators were aware of the presentation troponin result (detection bias). The only other possible issue was the timing of the reference standard, which was not always explicit.
FIGURE 3.
Quality assessment of diagnostic studies of TnI.

FIGURE 4.
Quality assessment of diagnostic studies of TnT.
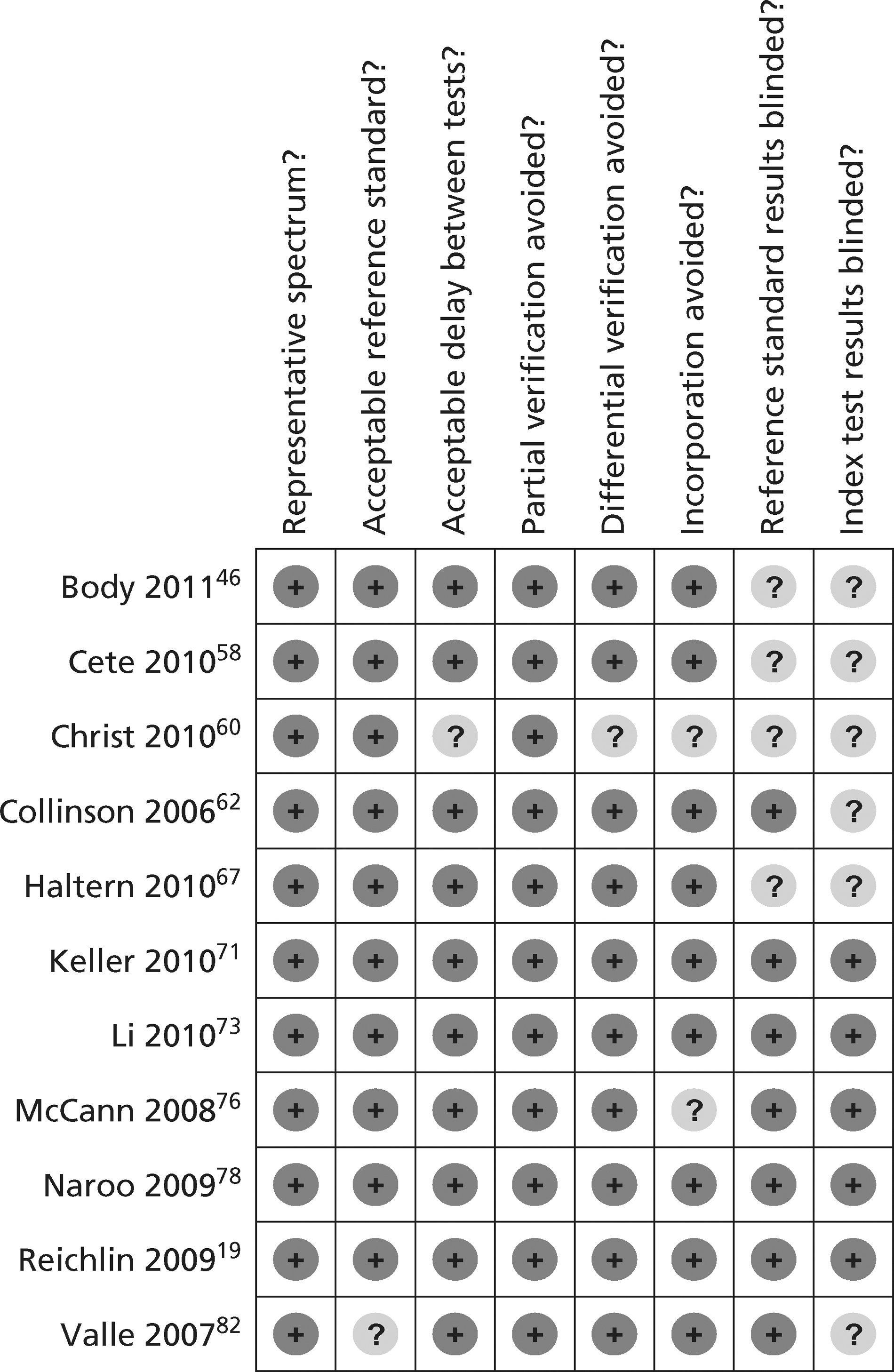
FIGURE 5.
Methodological quality summary of diagnostic studies of TnI.
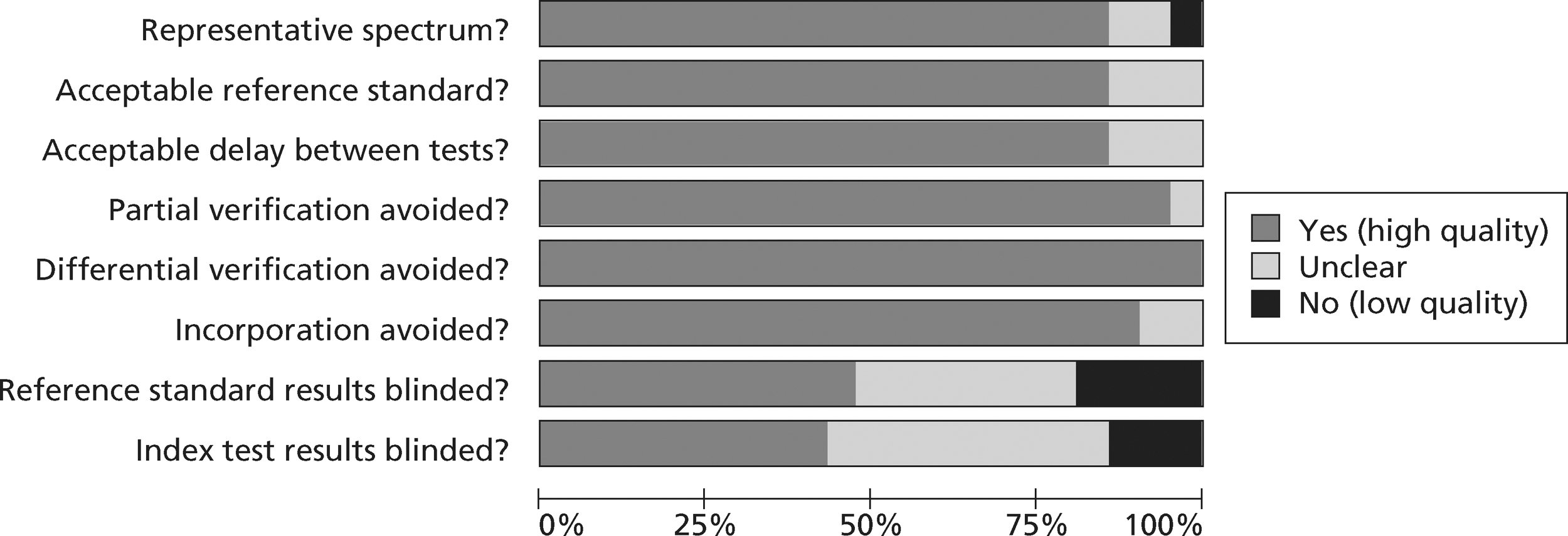
FIGURE 6.
Methodological quality summary of diagnostic studies of TnT.
Analysis of diagnostic accuracy studies of presentation troponin
Tables 7 and 8 show the reported sensitivity and specificity of each assay at key thresholds in each study of TnI and TnT, respectively. The studies used a variety of different assays and thresholds for positivity. In consequence, there is a wide range of reported values for sensitivity and specificity.
| Study | Biomaker | Threshold value | Threshold definition | Reported sensitivity | Reported specificity |
|---|---|---|---|---|---|
| Amodio 200752 | Dade Behring Stratus CS | 0.03 | 99th percentile | 0.773 | 0.84 |
| Amodio 200752 | Dade Behring Stratus CS | 0.07 | 10% CV | 0.636 | 0.931 |
| Apple 200853 | BioMérieux VIDAS TnI-Ultra | 0.01 | 99th percentile | 0.882 | 0.799 |
| Apple 200853 | BioMérieux VIDAS TnI-Ultra | 0.11 | 10% CV | 0.763 | 0.944 |
| Apple 200854 | ADVIA Centaur Ultra | 0.006 | LoD | 0.96 | 0.33 |
| Apple 200854 | ADVIA Centaur Ultra | 0.04 | 99th percentile | 0.74 | 0.84 |
| Apple 200955 | Dade Behring Stratus CS and Dimension RxL | 0.1 | 99th percentile | 0.72 | 0.89 |
| Bassan 200556 | Dade Behring | 1 | Not stated | 0.507 | 0.988 |
| Body 201157 | Alere | 0.055 | 99th percentile | 0.42 | 0.96 |
| Charpentier 201059 | ADVIA Centaur Ultra | 0.1 | 99th percentile | 0.561 | 0.986 |
| Collinson 200662 | EuroDPC Immulite | 0.2 | 10% CV | 0.9 | NR |
| Collinson 200648 | Beckman Coulter AccuTnI assay | 0.03 | 10% CV | 0.946 | NR |
| Di Serio 200563 | Randox Evidence Investigator | 1 | Not stated | 0.687 | 0.93 |
| Ecollan 200764 | Biosite Triage | 0.4 | Not stated | 0.218 | 1 |
| Garcia-Valdecasas 201149 | ELISA (Dainippon Pharmaceutical, Japan) | 0.6 | Not stated | 0.25 | 0.91 |
| Guo 200666 | Roche Cardiac Reader | 0.1 | Not stated | 0.952 | 0.938 |
| Ilva 200950 | Abbot Architect | 0.032 | 99th percentile | 0.784 | 1 |
| Keating 200670 | Beckman Access | 0.06 | Not stated | 0.74 | 0.99 |
| Keller 200920 | ADVIA Centaur Ultra | 0.04 | 99th percentile | 0.907 | 0.902 |
| LeFevre 200772 | Dade Behring RxL or Siemens Centaur | 0.14 or 0.33 | 10% CV | 0.66 | 0.95 |
| Liao 200974 | Not stated | 0.5 | Not stated | 0.648 | 0.5 |
| Mion 200777 | Evidence Cardiac Panel | 0.47 | Not stated | 0.548 | 0.978 |
| Reichlin 200919 | ADVIA Centaur Ultra | 0.04 | 99th percentile | 0.89 | 0.92 |
| Reichlin 200919 | ADVIA Centaur Ultra | 0.006 | LoD | 0.97 | 0.68 |
| Reichlin 200919 | Abbot Architect | 0.028 | 99th percentile | 0.88 | 0.92 |
| Reichlin 200919 | Abbot Architect | 0.032 | 10% CV | 0.85 | 0.93 |
| Reichlin 200919 | Abbot Architect | 0.01 | LoD | 0.94 | 0.87 |
| Reichlin 200919 | Roche | 0.16 | 99th percentile | 0.84 | 0.94 |
| Reichlin 200919 | Roche | 0.3 | 10% CV | 0.75 | 0.97 |
| Reichlin 200919 | Roche | 0.1 | LoD | 0.92 | 0.88 |
| Rudolph 201081 | Abbot Architect | 0.032 | 10% CV | 0.859 | 0.897 |
| Study | Biomaker | Threshold value | Threshold definition | Reported sensitivity | Reported specificity |
|---|---|---|---|---|---|
| Body 201146 | Fourth-generation TnT | 0.01 | 99th percentile | 0.748 | 0.937 |
| Cete 201058 | Not stated | 0.1 | Not stated | 0.452 | 1 |
| Christ 201060 | Fourth-generation TnT | 0.01 | 99th percentile | 0.9 | 0.812 |
| Christ 201060 | Fourth-generation TnT | 0.04 | 10% CV | 0.65 | 0.906 |
| Christ 201060 | HsTnT | 0.003 | LoD | 1 | 0.214 |
| Christ 201060 | HsTnT | 0.014 | 99th percentile | 0.95 | 0.615 |
| Collinson 200662 | Third-generation TnT | 0.03 | 10% CV | NR | NR |
| Haltern 201067 | Roche TnT | 0.03 | 10% CV | 0.74 | 1 |
| Keller 200920 | Fourth-generation TnT | 0.03 | 10% CV | 0.637 | 0.972 |
| Keller 200920 | Fourth-generation TnT | 0.01 | 99th percentile | 0.727 | 0.921 |
| Li 201073 | Not stated | 0.1 | Not stated | 0.693 | 0.9754 |
| McCann 200876 | Roche TnT | 0.03 | 10% CV | 0.75 | 0.94 |
| Naroo 200978 | Not stated | 0.03 | Not stated | 0.586 | 0.989 |
| Reichlin 200919 | HsTnT | 0.014 | 99th percentile | 0.95 | 0.8 |
| Reichlin 200919 | HsTnT | 0.002 | LoD | 1 | 0.14 |
| Reichlin 200919 | Fourth-generation TnT | 0.035 | 10% CV | 0.72 | 0.97 |
| Reichlin 200919 | Fourth-generation TnT | 0.01 | 99th percentile | 0.83 | 0.93 |
| Valle 200882 | Not stated | Unclear | Not stated | 0.19 | 0.99 |
We did not undertake meta-analysis across all studies because of variation in the assays and thresholds used, with some studies using high thresholds with no clear basis. Instead, we undertook separate analyses for TnI and TnT using the 99th percentile or 10% CV threshold, when these data were reported (Figures 7–10). The studies by Christ and Popp60 and Reichlin et al. 19 reported data for more than one assay in each potential analysis, so we selected data from one assay in each analysis. We also analysed the following high-sensitivity assays using the 99th percentile (Figures 11–13):
-
ADVIA Centaur Ultra troponin I (Siemens Healthcare, Basel, Switzerland)
-
Abbott Architect troponin I (Abbott Laboratories, IL, USA)
-
Roche hsTnT. (Roche Diagnostics, Basel, Switzerland).
FIGURE 7.
Meta-analysis of studies of Tnl using the 99th percentile.
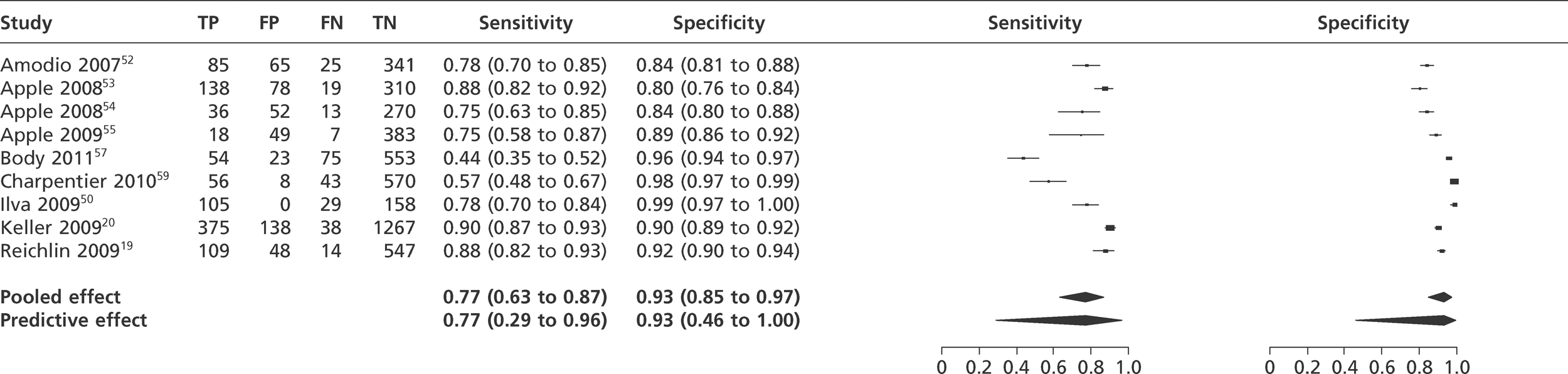
FIGURE 8.
Meta-analysis of studies of Tnl using 10% CV.
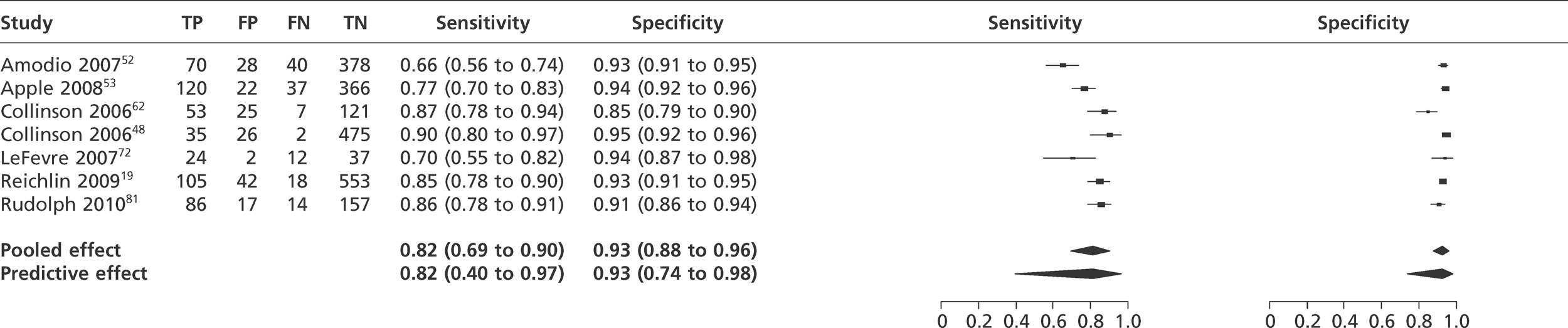
FIGURE 9.
Meta-analysis of studies of TnT using the 99th percentile.
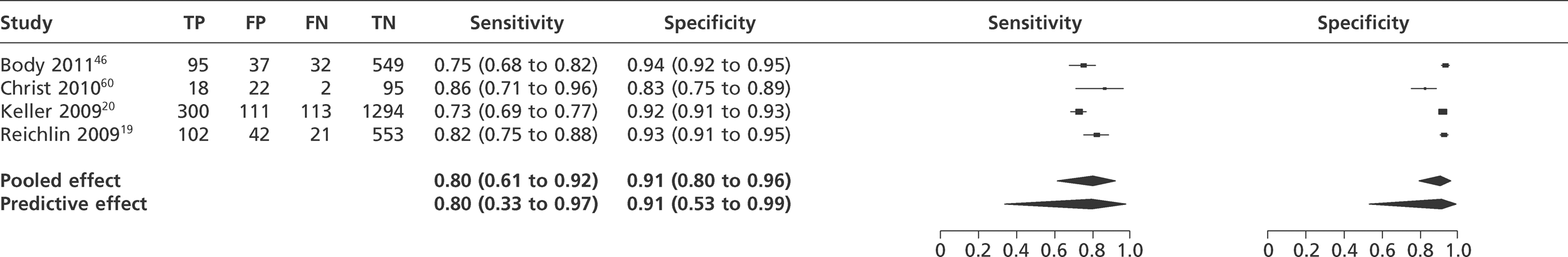
FIGURE 10.
Meta-analysis of studies of TnT using 10% CV.
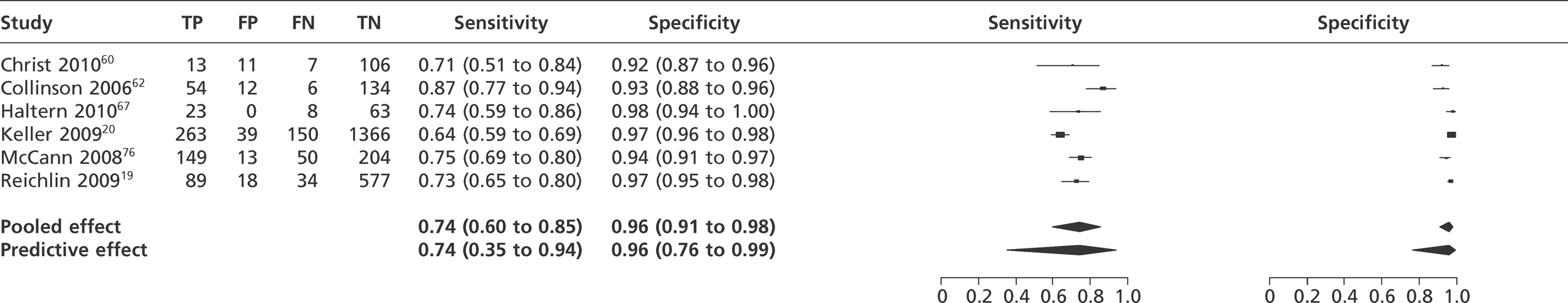
FIGURE 11.
Meta-analysis of studies of ADVIA Centaur Ultra Tnl.
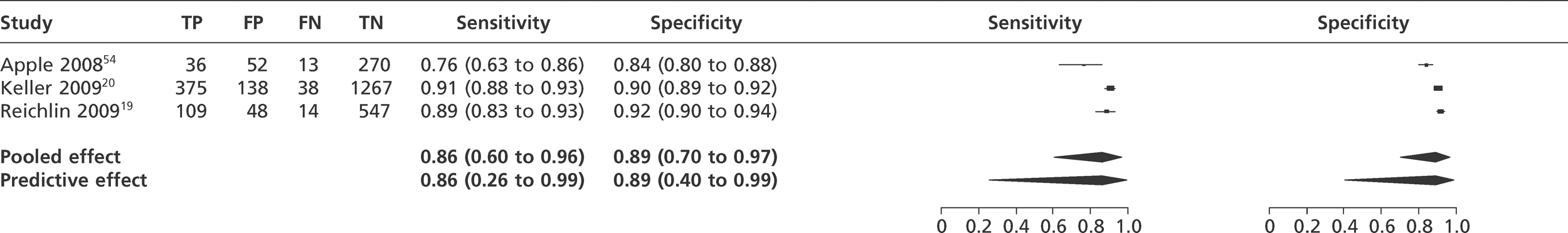
FIGURE 12.
Meta-analysis of studies of Abbott Architect Tnl.
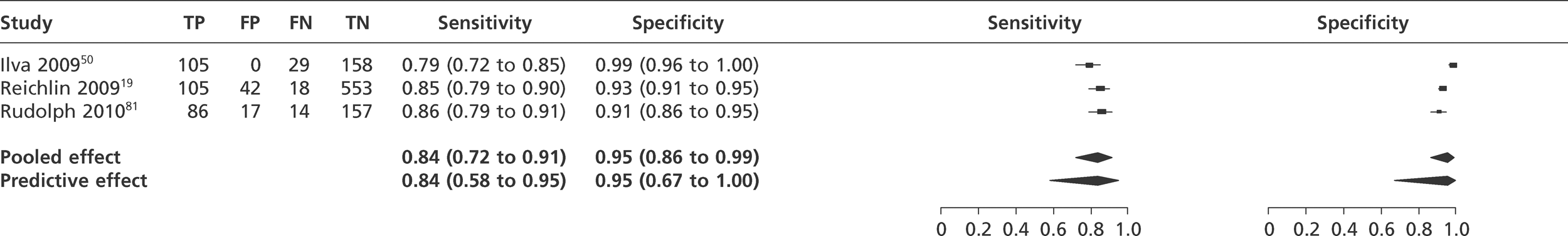
FIGURE 13.
Meta-analysis of studies of Roche HsTnT.
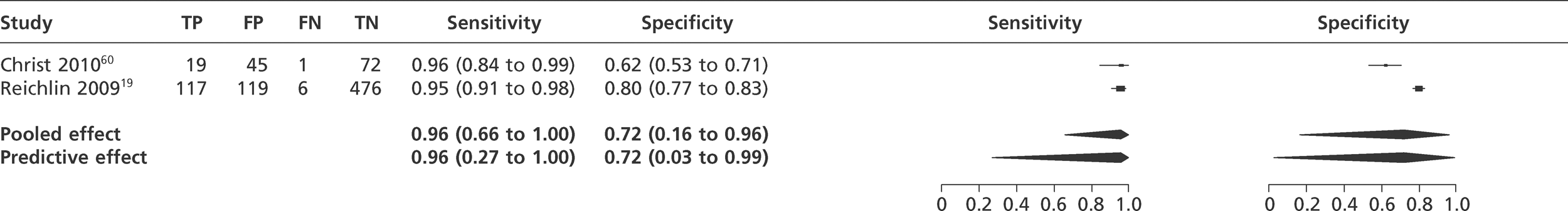
The results show that sensitivity and specificity for TnI were 77% (95% predictive interval 29–96%) and 93% (95% predictive interval 46–100%), respectively, when the 99th percentile was used and 82% (95% predictive interval 40–97%) and 93% (95% predictive interval 74–98%) when the 10% CV was used. This apparently counterintuitive finding (lower sensitivity at a lower diagnostic threshold) is probably explained by either random error or differences in the study populations or assays included in the two analyses. The corresponding results for TnT were 80% (95% predictive interval 33–97%) and 91% (95% predictive interval 53–99%) when the 99th percentile was used and 74% (95% predictive interval 34–94%) and 96% (95% predictive interval 76–99%) when the 10% CV was used, suggesting that different thresholds provide a trade-off between sensitivity and specificity. The differences between point estimates of sensitivity and specificity for TnI and TnT probably reflect differences in the assays or thresholds evaluated, the constituent study populations or random error, rather than a systematic difference between TnI and TnT. The credible ranges for the estimates differed markedly depending upon whether the pooled effect or predictive effect was estimated, reflecting the marked heterogeneity between the studies. The predictive distribution is likely to provide the most appropriate reflection of uncertainty and is used in the cost-effectiveness modelling.
The high-sensitivity assays unsurprisingly had higher sensitivity but lower specificity, although with considerable uncertainty reflected in the wide predictive intervals for their estimates. The Roche HsTnT assay had a sensitivity of 96% (95% predictive interval 27–100%) and a specificity of 72% (95% predictive interval 3–96%). The ADVIA Centaur Ultra-TnI assay had a sensitivity of 86% (95% predictive interval 22–96%) and a specificity of 89% (95% predictive interval 40–97%). The Abbot Architect troponin I assay had a sensitivity of 83% (95% predictive interval 58–95%) and a specificity of 95% (95% predictive interval 67–100%).
These analyses compared high-sensitivity troponin index tests with a reference standard based on a standard troponin assay. We identified one study60 that compared HsTnT at presentation with a reference standard based on HsTnT, as well as a standard TnT assay. The sensitivity and specificity of HsTnT were 95.0% and 61.5%, respectively, compared with a reference standard based on the standard assay, and were 94.3% and 69.6%, respectively, compared with a reference standard based on the high-sensitivity troponin assay. These findings suggest that the higher sensitivity and lower specificity of high-sensitivity assays compared with standard troponin assays are not simply due to different assays being used for index test and reference standard, but represent a genuine improvement in early sensitivity at the expense of specificity for a final diagnosis of MI. The lower specificity of high-sensitivity assays may be due to a greater ability to detect myocardial injury secondary to other clinical conditions. 83,84
Description of diagnostic studies of heart-type fatty acid-binding protein
We identified 17 diagnostic studies49,50,57–59,63,64,67,68,72–78,82 of H-FABP for inclusion in the review. Table 9 shows the population characteristics and Table 10 shows the index and reference standard test characteristics. As with the troponin studies, reporting of exclusion criteria were variable, with some studies excluding patients with diagnostic ECG changes. The prevalence of MI varied from 15% to 73% and was relatively high, suggesting some selection of higher risk cases. The time from symptom onset to sampling varied from 1.2 hours (mean) to 5.9 hours (median). Around half of the studies evaluated qualitative assays, most specifying that this was the CardioDetect assay with a diagnostic threshold of 7 µg/l. The threshold used by quantitative assay was variable. Most of the studies used reference standards based on a standard modern troponin assay, using the 10% CV or 99th percentile as a diagnostic threshold, although not all gave details of the assay and threshold.
| Study | Study type | Population: age (years) and sex | Inclusion criteria | Exclusion criteria | Time from symptoms (hours) | No. of patients |
|---|---|---|---|---|---|---|
| Body 201157 | Single centre, UK | Mean age 59; 430/705 (61%) male | Suspected cardiac chest pain occurring within the previous 24 hours | Chest trauma, renal failure requiring dialysis, medical condition necessitating admission, pregnancy | 3.5 (median) | 705 |
| Cete 201058 | Single centre, Turkey | Mean age 57; 163 (73%) male | Aged > 18 years presenting to the ED with typical chest pain | Atypical pain, trauma, electrical cardioversion within 24 hours, musculoskeletal disease, acute or chronic renal failure, liver disease | NR | 224 |
| Charpentier 201059 | Single centre, France | Mean age 57; 454 (67%) male | Chest pain due to suspected within 12 hours | ST elevation, traumatic cause, previous severe renal impairment, or severe communication problems | 2.9 (median) | 677 |
| Di Serio 200563 | Single centre, Italy | Mean age 79 (women), 65 (men); 23 (77%) male | Not specified | ST elevation | 3.4 (mean) | 30 |
| Ecollan 200764 | Mobile units, France | Mean age 68; 68 (63%) male | Consecutive emergencies with chest pain | Patients with cardiogenic shock or those with any evidence of a recent chest trauma | 2.3 (median) | 108 |
| Garcia-Valdecasas 201149 | Single centre, France | Mean age 67; 114 (69%) male | Chest pain > 20 minutes' duration within 6 hours | Chest trauma | NR | 165 |
| Haltern 201067 | Single centre, Germany | Mean age 69; 27/49 (55%) male | Ischaemic-type chest pain | Age < 18 years,interhospital transfer | 4 (median) | 94 |
| Hjortshoj 201068 | Single centre, UK | Median range across three groups (60–63); 70 men, 37 women | Chest pain and suspected of ACS | None reported | NR | 107 |
| Ilva 200950 | Single centre, Finland | Mean age 67; 181 (62%) male | Chest pain suggesting myocardial ischaemia | Uncertain or > 24-hour delay from symptom onset | 4.7 (median) | 293 |
| Lefevre 200772 | Multicentre, France | Mean age 61; 71/100 eligible (71%) male | Not specified | None reported | 5.9 (median) | 75 |
| Li 201073 | Multicentre, China | Mean age 64; 163 (72%) male | Chest pain > 30 minutes and < 12 hours suspected of AMI | None reported | 4 (median) | 227 |
| Liao 200974 | Single centre, China | Mean age 69; 54 (73%) male | Onset of acute chest pain and/or dyspnoea lasting for at least 20 minutes within the last 3 hours, age > 18 years | None reported | 2.2 (IQR 1.5–2.9) (median) | 74 |
| Mad 200775 | Single centre, Austria | Mean age 58; 213 (76%) male | Onset of acute chest pain and/or dyspnoealasting for at least 20 minutes within the last 24 hours, age > 18 years | None reported | 3 (IQR 2–6) (median) | 280 |
| McCann 200876 | Multicentre, Northern Ireland | Mean age 63; 281 (68%) male | Ischaemic type chest pain within 24 hours | Age < 18 years,interhospital transfer, and previous participation in the study | 5.3 (median) | 415 |
| Mion 200777 | Single centre, Italy | Mean age 63; 88 (67%) male | Non-consecutive patients with chest pain | None reported | 3.8 (range, 1.5–13.0) (median) | 132 |
| Naroo 200978 | Single centre, United Arab Emirates | Age not reported; 627 (79%) male | Typical cardiac chest pain within 20 minutes to12 hours | STEMI, renal disease, age < 20 years | NR | 791 |
| Valle 200882 | Multicentre, Spain | Mean age 65; 287 (68%) male | Suspected ACS with symptoms between 20 and 180 minutes after presentation | None reported | 1.2 (mean) | 419 |
| Study | Index test analyser | Threshold (µg/l) | Reference test assay and timing | Reference Tn diagnostic threshold (µg/l) | MI prevalence in sample (%) |
|---|---|---|---|---|---|
| Body 201157 | Alere Fluorescence immunoassay | 58 | Roche Elecsys TnT at least 12 hours after symptom onset | 0.01 | 18 |
| Cete 201058 | CardioDetect® | 6.2 | TnT; assay not reported; 6 hours | 0.1 | 33 |
| Charpentier 201059 | CardioDetect® | 7 | ADVIA Centaur TnI system (Bayer Diagnostics) at 6 hours | 0.1 | 15 |
| Di Serio 200563 | Randox Evidence Investigator | 6.4 | ESC/ACC, not further specified | Not specified | 53 |
| Ecollan 200764 | CardioDetect® | 7 | Dade Behring TnI over 24 hours | 0.07 (99th percentile) | 51 |
| Garcia-Valdecasas 201149 | Dainippon Pharmaceutical ELISA | 6.2 | Dade Dimension TnI; timing not stated | 0.6 | 39 |
| Haltern 201067 | HyCult Biotechnology ELISA | 7 | Roche Elecsys TnT at 12 hours | 0.03 | 33 |
| Hjortshoj 201068 | HyCult Biotechnology ELISA | 6.0 | Roche Elecsys TnT at 6–9 hours, and 12–24 hours | 0.03 | 33 |
| Ilva 200950 | Innotrac Aio! Immunoanalyzer | 10.4 | Abbott Architect TnI up to 24 hours | 0.032 | 46 |
| Lefebvre 200772 | CardioDetect® | 6.2 | Dade Behring RxL TnI or Siemens Centaur TnI | 0.14 and 0.33 | 48 |
| Li 201073 | Wuhan EasyDiagnosis Biomedicine Co | 7 | TnT; assay not specified; 12 hours | 0.1 | 50 |
| Liao 200974 | CardioDetect® | 7 | NR; NR; assumed to be10–12 hours | 0.5 | 73 |
| Mad 200775 | CardioDetect® | 7 | TnT; no details of assay; ACC and ESC guidelines (10–12 hours) | 0.04 | 39 |
| McCann 200876 | HyCult Biotechnology ELISA | 1.2 | Roche Elecsys TnT at 12 hours | 0.03 | 48 |
| Mion 200777 | Evidence® Cardiac Panel | 6.02 | Dimension RxL TnI; timing NR (as ESC/ACC guidelines) | 0.15 | 32 |
| Naroo 200978 | CardioDetect® | 7 | Assay not specified; 6–12 hours after initial sampling | 1 | 13 |
| Valle 200882 | CardioDetect® | 7 | TnT at 6–12 hours, assay not reported | Not stated | 35 |
Quality assessment of diagnostic studies of heart-type fatty acid-binding protein
Figure 14 shows the quality assessments for studies of H-FABP, while Figure 15 shows the methodological quality summary. The overall quality and the issues raised were similar to those for the studies of troponin.
FIGURE 14.
Quality assessment of diagnostic studies of H-FABP.
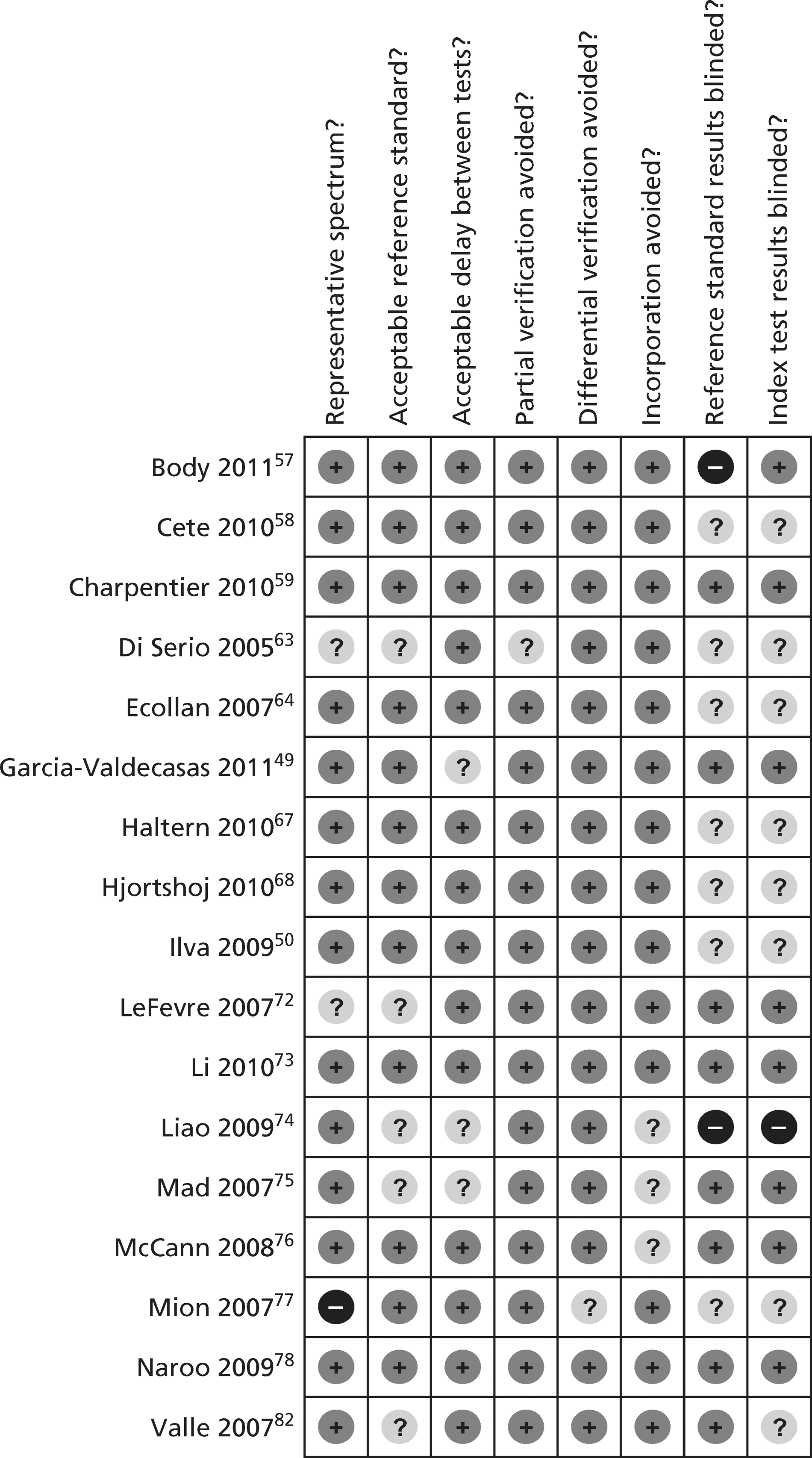
FIGURE 15.
Methodological quality summary of diagnostic studies of H-FABP.
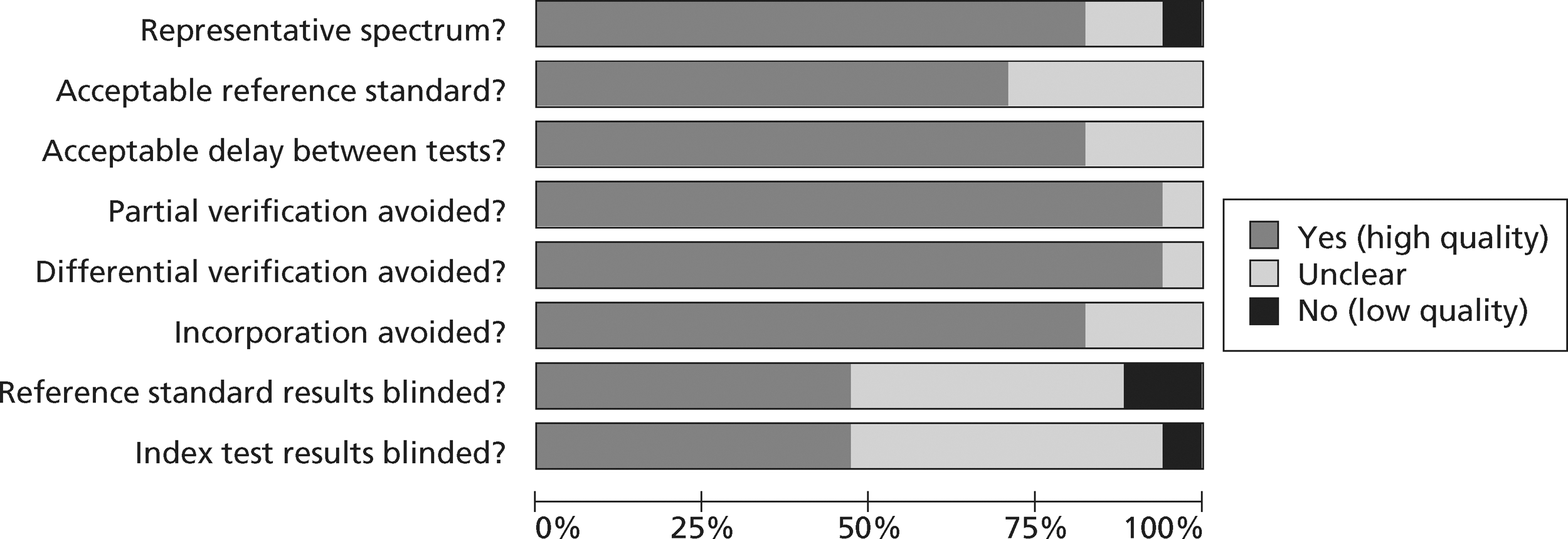
Analysis of diagnostic studies of heart-type fatty acid-binding protein
Figure 16 shows the meta-analysis of the studies of quantitative H-FABP and Figure 17 shows the meta-analysis of qualitative assays. The summary estimates of sensitivity and specificity were 81% (95% predictive interval 50–95%) and 80% (95% predictive interval 26–98%), respectively, for the quantitative assays and 68% (95% predictive interval 11–97%) and 92% (95% predictive interval 20–100%), respectively, for the qualitative assays.
FIGURE 16.
Meta-analysis of quantitative H-FABP assays.
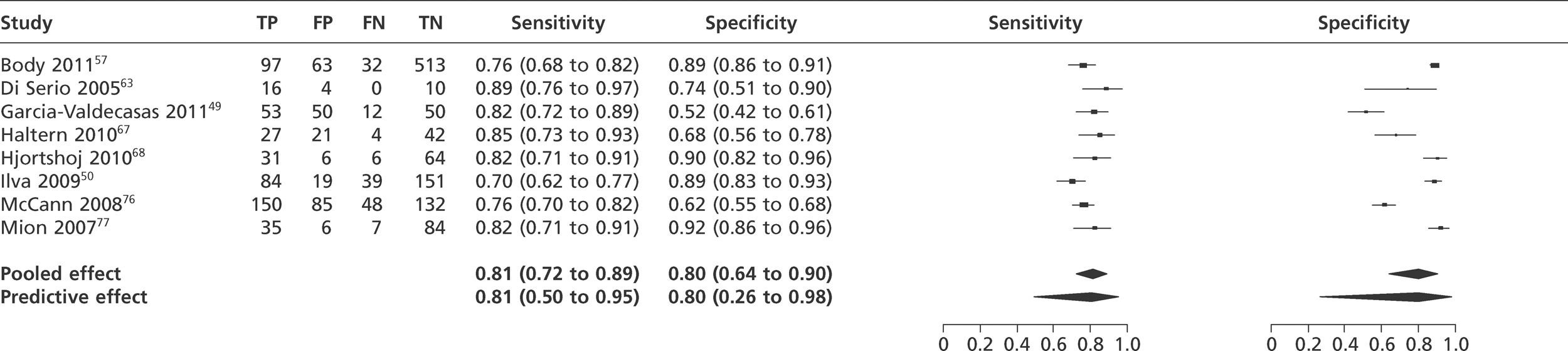
FIGURE 17.
Meta-analysis of qualitative H-FABP assays.
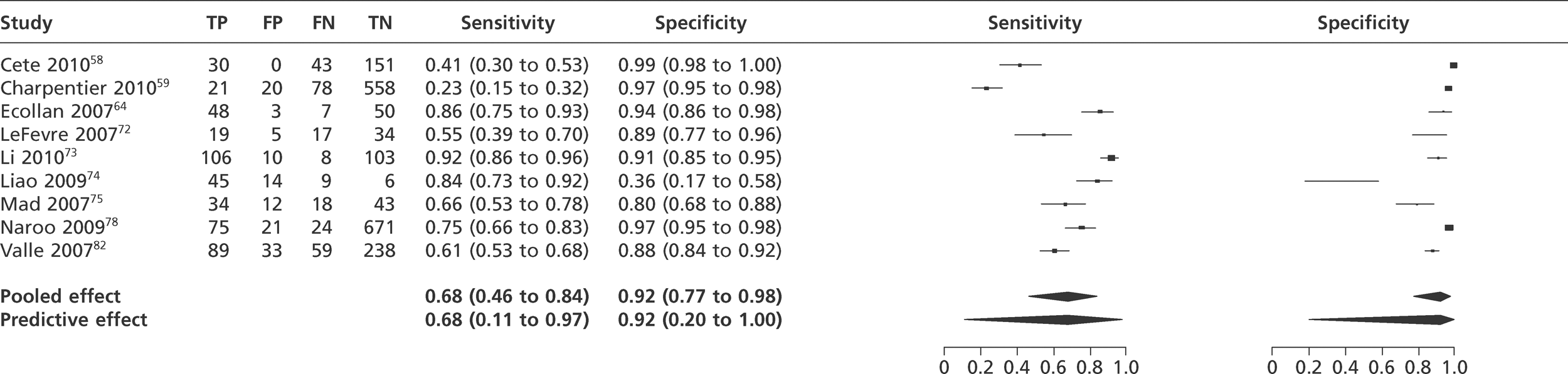
Description of diagnostic studies of ischaemia-modified albumin
We identified four studies48,61,68,70 that were eligible for inclusion in the review (Tables 11 and 12). A number of other studies of IMA were excluded because the reference standard was ACS, based on clinical criteria, and cases with MI were not reported separately. Two studies restricted recruitment to patients presenting within 370 and 8 hours61 of symptom onset. Only one study48 reported the median time delay from symptom onset. Thresholds of between 75 and 91 were used for IMA. Three studies used a modern standard troponin assay for the reference standard, whereas the older study from Christensen et al. 61 inevitably used an older troponin reference standard with a higher threshold for positivity.
| Study | Study type | Population: age (years); sex | Inclusion criteria | Exclusion criteria | Time from symptoms (hours) | No. of patients |
|---|---|---|---|---|---|---|
| Christenson 200161 | Multicentre, USA | Mean age 59.1 (SD 14.8); 112 (50%) male | Arrival at ED within 3 hours of clinical signs and symptoms of ACS | MI > 3 hours before presentation, inconsistencies between cTnI and other biochemical marker data in the 6- to 24-hour time frame | < 3hours | 224 |
| Collinson 200648 | Single centre, UK | Median age 51.9; 335 (62%) male | Admissions to the ED with undifferentiated chest pain | Significant new ECG changes, requiring hospital admission, known CAD with unstable angina, clearly non-cardiac chest pain | 6 (median) | 538 |
| Hjortshoj 201068 | Single centre, UK | Median range across three groups (60–63); 70 (65%) male | Chest pain and suspected of ACS | None reported | Not stated | 107 |
| Keating 200670 | Multicentre, UK | Median age 61; 251/399 eligible (63%) male | Possible ischaemic cardiac chest pain and normal ECG | Pain > 8 hours on admission, pain ceased > 2 hours previously, pregnant, renal replacement therapy, jaundice | Not stated | 277 |
| Study | Index test analyser | Threshold | Reference test assay and timing | Reference Tn diagnostic threshold (µg/l) | MI prevalence in sample (%) |
|---|---|---|---|---|---|
| Christenson 200161 | Albumin Cobalt Binding (ACBTM) Test (Ischemia Technologies, Denver, CO, USA) on a Cobas MIRA Plus instrument | 75 U/ml | Vitros ECi, Abbott AxSYM or Dimension RxL TnI at 6–24 hours | 0.8, 1.6 and 1.5, respectively | 13 |
| Collinson 200648 | ACB assay (Ischemia Technologies, Denver, CO, USA) | 85 U/ml | Roche Elecsys TnT or Beckman Coulter TnI up to72 hours | 0.05 | 7 |
| Hjortshoj 201068 | ACB test (Inverness Medical Innovations Inc., Stirling, UK) on a Cobas MIRA Plus instrument | 88.2 U/ml and 91 U/ml | Roche Elecsys TnT after 6–9 hours, and 12–24 hours | 0.03 | 33 |
| Keating 200670 | Beckman LX 20 | 86 U/ml | TnI; Beckman Access; taken at least 8 hours after pain onset | 0.06 | 15 |
Quality assessment of diagnostic studies of ischaemia-modified albumin
Figure 18 shows the quality assessments for studies of IMA, whereas Figure 19 shows the methodological quality summary.
FIGURE 18.
Quality assessment of diagnostic studies of IMA.
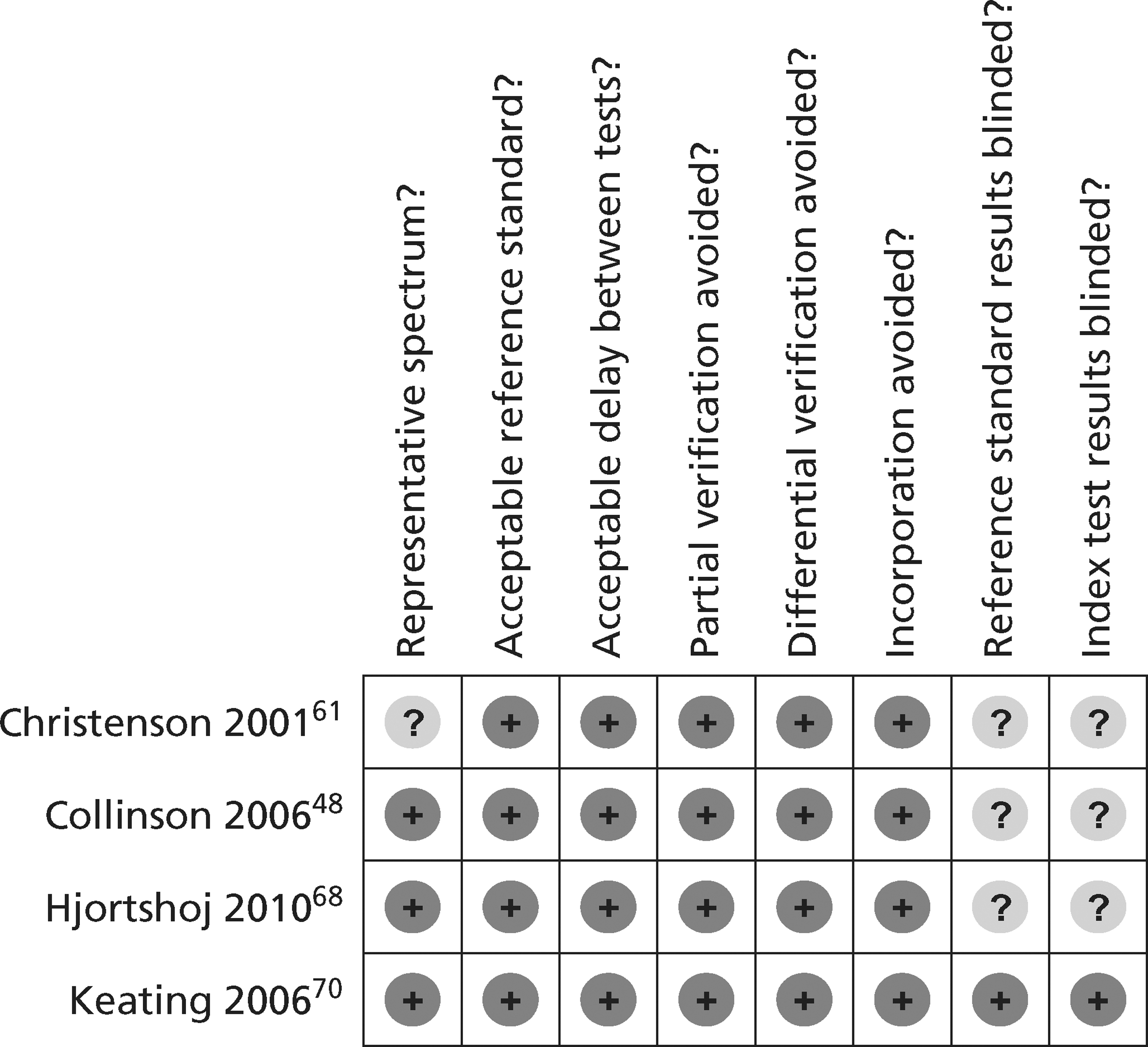
FIGURE 19.
Methodological quality summary of diagnostic studies of IMA.
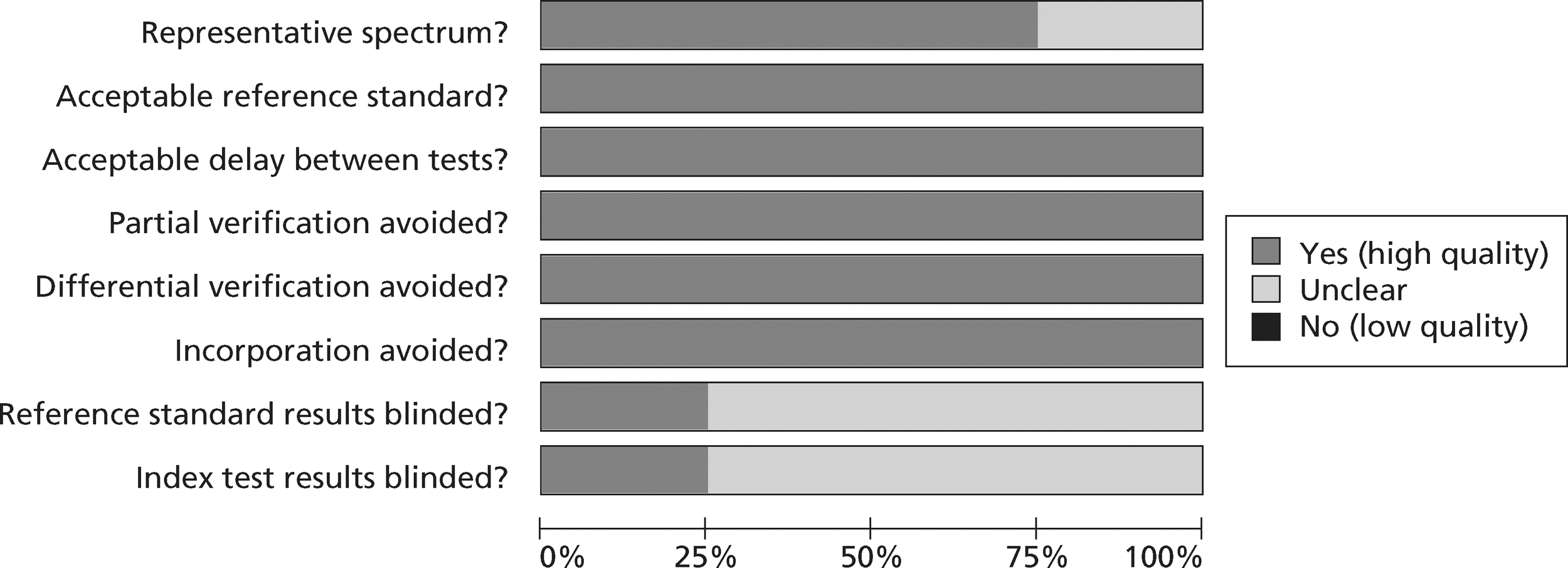
Analysis of diagnostic studies of ischaemia-modified albumin
Figure 20 shows the results of meta-analysis of studies of IMA. The summary estimates of sensitivity and specificity were 77% (95% predictive interval 19–98%) and 39% (95% predictive interval 2–95%), respectively.
FIGURE 20.
Meta-analysis of studies of IMA.
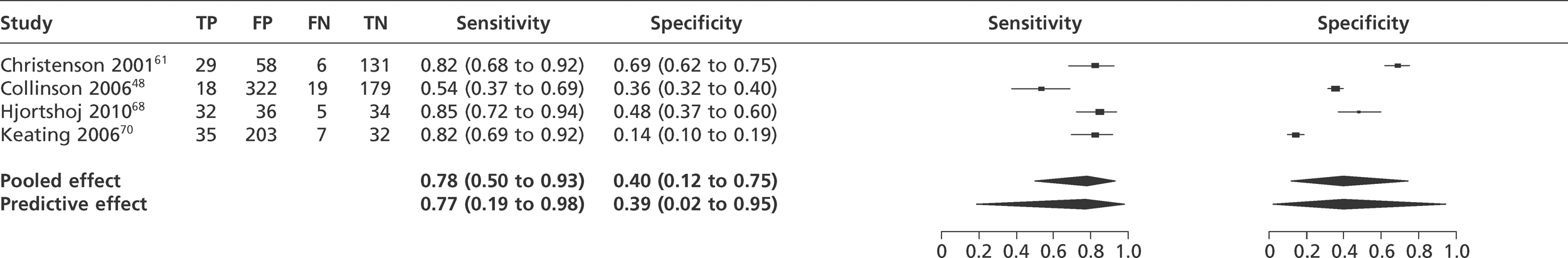
Description of diagnostic studies of myoglobin
We identified 13 diagnostic studies18,49,52,57,58,63,64,68,69,71,74,77,79 of myoglobin for inclusion in the review. Table 13 shows the population characteristics and Table 14 shows the index and reference standard test characteristics. Reporting of exclusion criteria was variable and some studies excluded patients with diagnostic ECG changes. The prevalence of MI was generally high and varied from 18% to 73%. The median time from symptom onset to sampling varied from 2.2 to 7.8 hours. There was no consistency in the diagnostic threshold used. It ranged from 51 to 150 µg/l and 5 out of 13 studies used different thresholds for men and women, whereas 8 out of 13 studies used the same threshold. Several studies used relatively high thresholds for positivity for the reference standard troponin or did not report the timing of sampling or the assay used.
| Study | Study type | Population: age (years); sex | Inclusion criteria | Exclusion criteria | Time from symptoms (hours) | No. of patients |
|---|---|---|---|---|---|---|
| Amodio 200752 | Single centre, Italy | Mean age 61; 308 (60%) male | Suspected clinical angina or AMI | STEMI, new-onset LBBB | 5.0 (median) | 516 |
| Body 201157 | Single centre, UK | Mean age 59; 434 (61%) male | Suspected cardiac chest pain occurring within the previous 24 hours | Chest trauma, renal failure, needing hospital admission, pregnancy | 3.5 (median) | 713 |
| Cete 201058 | Single centre, Turkey | Mean age 57; 163 (73%) male | Aged > 18 years presenting to the ED with typical chest pain | Atypical pain, trauma, recent electrical cardioversion, musculoskeletal disease, renal failure, liver disease | NR | 224 |
| Di Serio 200563 | Single centre, Italy | Mean age 79 (women), 65 (men); 23 (77%) male | Not specified | ST elevation | 3.4 (mean) | 30 |
| Ecollan 200764 | Mobile units, France | Mean age 68; 68 (63%) male | Consecutive emergencies with chest pain | Cardiogenic shock, recent chest trauma | 2.3 (median) | 108 |
| Eggers 200418 | Single centre, Sweden | Median age 66; 130 (66%) male | Chest pain within 24 hours, suspicious of unstable angina or AMI | STEMI | 5.5 (median) | 180 |
| Garcia-Valdecasas 201149 | Single centre, France | Mean age 67; 114 (69%) male | Chest pain > 20 minutes' duration within 6 hours | Chest trauma | NR | 165 |
| Hjortshoj 201068 | Single centre, UK | Median range across three groups (60–63); 70 (65%) male | Chest pain and suspected of ACS | None reported | NR | 107 |
| Ilva 200569 | Single centre, Finland | Mean age 67; 314 (59%) male | Suspected MI | None reported | 7.8 hours (median) | 531 |
| Keller 201071 | Multicentre, Germany | Mean age 61; 920 (66%) male | Aged 18–85 years with angina pectoris or equivalent symptoms | Trauma or major surgery within the last 4 weeks, pregnancy, intravenous drug abuse, anaemia | 57.6% < 6 hours | 1386 |
| Liao 200974 | Single centre, China | Mean age 69; 54 (73%) male | Onset of acute chest pain and/or dyspnoea within the last 3 hours | None reported | 2.2 (median) | 74 |
| Mion 200777 | Single centre, Italy | Mean age 63; 88 (67%) male | Non-consecutive patients with chest pain | None reported | 3.8 (median) | 132 |
| Penttilä 200279 | Single centre, Finland | Mean age 68; 246 (56%) male | Chest pain within 12 hours | None reported | < 12 hours | 440 |
| Study | Index test analyser and timing | Threshold (µg/l) | Reference test assay and timing | Reference Tn diagnostic threshold (µg/l) | MI prevalence in sample (%) |
|---|---|---|---|---|---|
| Amodio 200752 | Dade Behring Stratus CS | 68 and 86 | TNT; assay and timing not specified | 0.07 | 21 |
| Body 201157 | Alere Fluorescence immunoassay | 107 | Roche Diagnostics, Elecsys at 12 hours from symptoms | 0.01 | 18 |
| Cete 201058 | Not stated | 52 (women), 81 (men) | TnT; assay not reported; 6 hours | 0.1 | 33 |
| Di Serio 200563 | Evidence Investigator™ | 73.4 | ESC/ACC, not further specified | Not specified | 53 |
| Ecollan 200764 | Biosite Triage® | 150 | Dade Behring TnI test at any time point over 24 hours | 0.07 | 51 |
| Eggers 200418 | Dade Behring Stratus CS | 98 (men), 56 (women) | Abbott AxSYM TnI; within 24 hours | 0.1 | 22 |
| Garcia-Valdecasas 201149 | Dade Dimension | 92 (men), 76 (women) | Dimension Analyser TnI, timing not stated | 0.6 | 39 |
| Hjortshoj 201068 | Roche Elecsys | 51 (women), 72 (men) | Roche Elecsys TnT after 6 to 9 hour and 12–24 hours | 0.03 | 33 |
| Ilva 200569 | Innotrac Aio | 150 | Bayer Diagnostics TnI up to 24 hours | 0.3 | 37 |
| Keller 201071 | Not stated | 107 | Roche TnT or Siemens Dimension RxL TnI within 6 hours of admission | 0.3 (TnT) | 29 |
| 0.14 (TnI) | |||||
| Liao 200974 | Not stated | 70 | NR | 1 | 73 |
| Mion 200777 | Evidence® Cardiac Panel | 87 | Dimension | 0.15 | 32 |
| RxL TnI, timing not specified | |||||
| Penttilä 200279 | Hitachi 717 | 65 (men), 55 (women) | Abbott IMx TnT at two different time points during 72 hours | 0.1 | 30 |
Quality assessment of diagnostic studies of myoglobin
Figure 21 shows the quality assessments for studies of myoglobin, whereas Figure 22 shows the methodological quality summary. The quality assessment of acceptability of the reference standard was limited to whether or not the reference standard criteria were reported clearly and met the universal definition. Although most studies had an acceptable reference standard by these criteria, it is debatable whether the troponin assays and threshold used represented best current practice in some cases.
FIGURE 21.
Quality assessment of diagnostic studies of myoglobin.
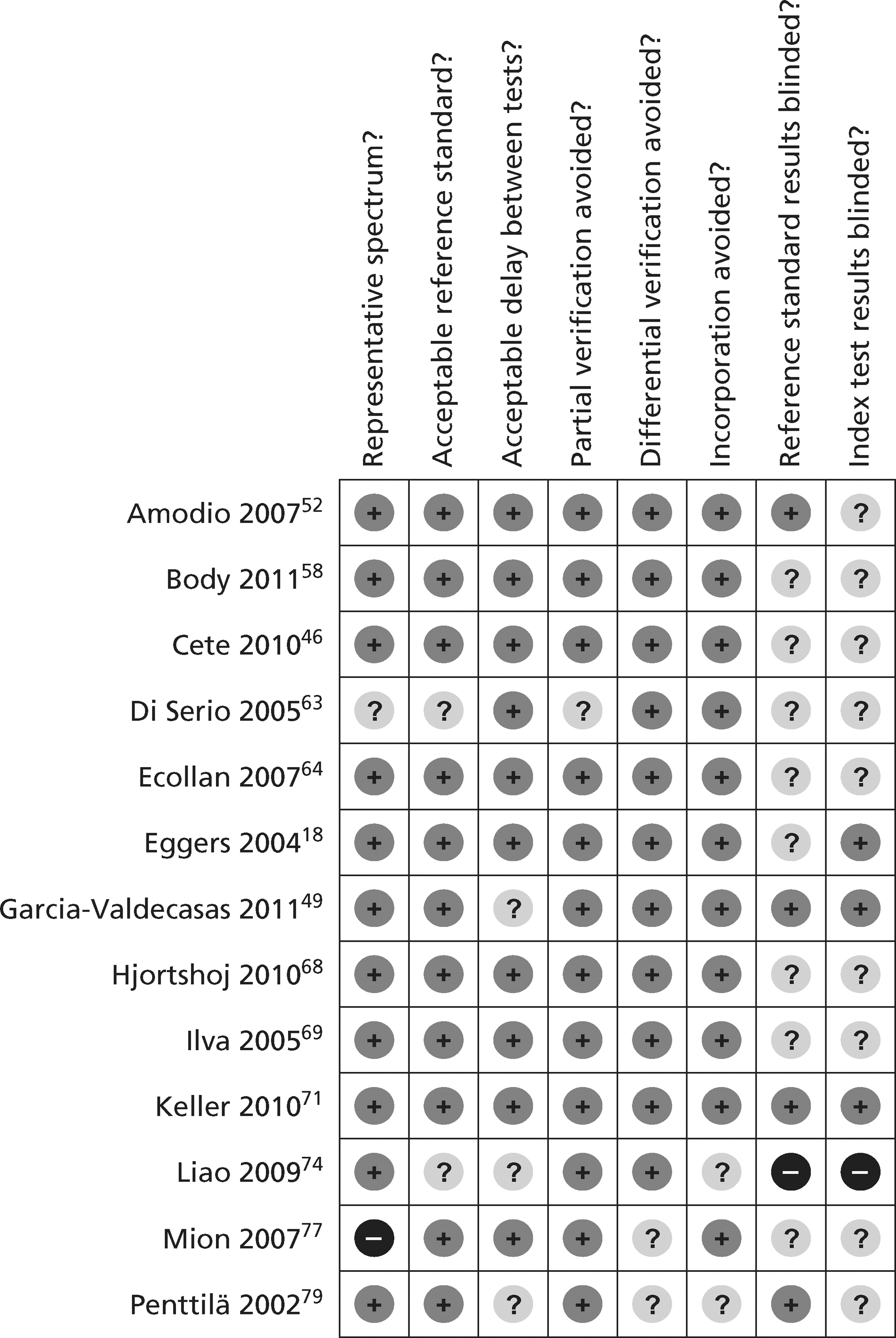
FIGURE 22.
Methodological quality summary of diagnostic studies of myoglobin.
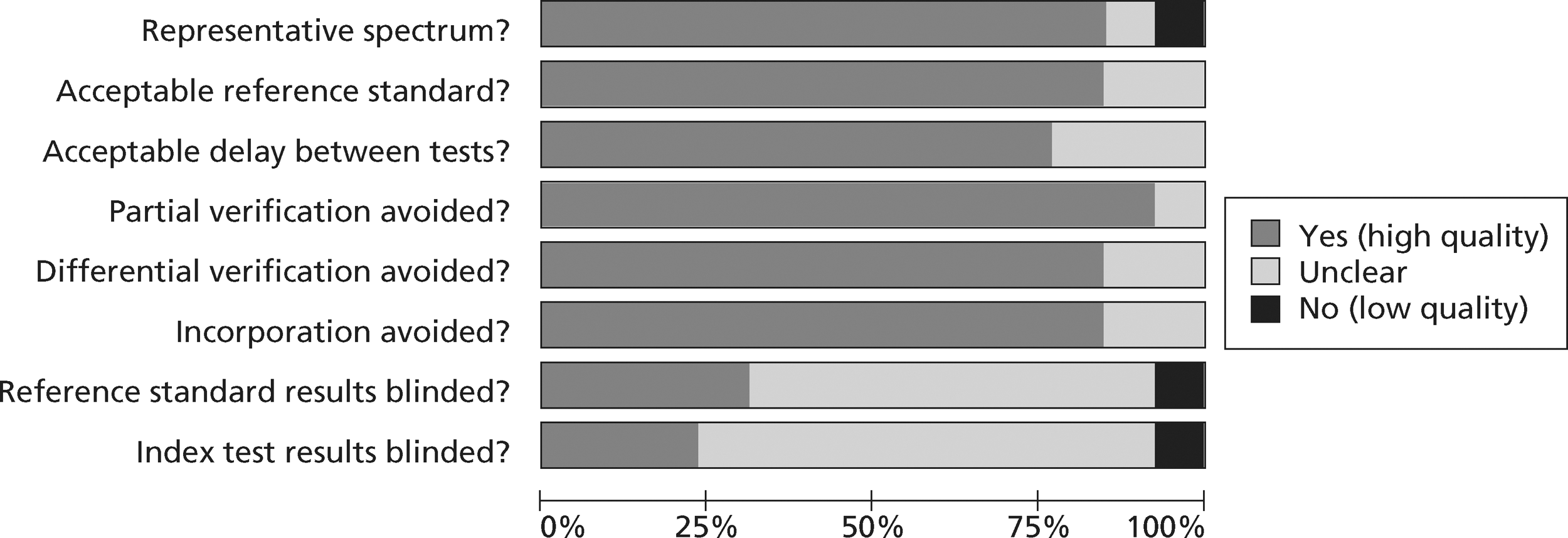
Analysis of diagnostic studies of myoglobin
Figure 23 shows the results of meta-analysis of studies of myoglobin. The summary estimates of sensitivity and specificity were 62% (35–83%) and 83% (35–98%), respectively.
FIGURE 23.
Meta-analysis of studies of myoglobin.
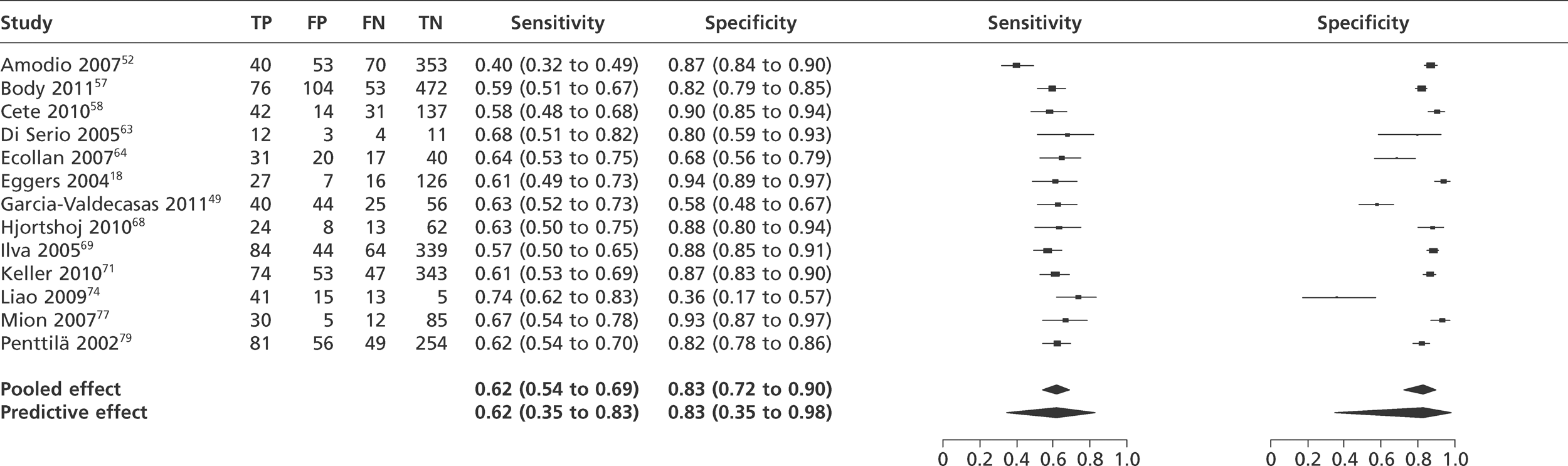
Description of diagnostic studies of other biomarkers
We identified 10 diagnostic studies46,47,51,55–57,65,71,80,81 of other biomarkers. Table 15 shows the population characteristics and Table 16 shows the index and reference standard test characteristics. The prevalence of MI was lower than the studies of troponin, H-FABP and myoglobin, and varied from 5% to 29%. The median time from symptom onset to sampling varied from 2 to 4.5 hours. Most of the studies used a modern troponin assay with an acceptable threshold for the reference standard.
| Study | Biomarker | Study type | Population: age (mean in years, unless stated); sex | Inclusion criteria | Exclusion criteria | Time from symptoms (hours) | No. of patients |
|---|---|---|---|---|---|---|---|
| Apple 200955 | CD40L, MPO, MMP9, CRP, NT-pro-BNP | Single centre, USA | Mean age 54; 260 (57%) male | Symptoms suggestive of ACS within 12 hours | NR | 3.1 (median) | 457 |
| Bassan 200556 | BNP | Single centre, Brazil | Mean age 67.3; 343 male | Chest pain < 12 hours due to possible acute cardiac ischaemia | ST segment elevation | 2.0 (median) | 631 |
| Body 201157 | BNP, MPO | Single centre, UK | Mean age 59; 430 (61%) male | Suspected cardiac chest pain occurring within the previous 24 hours | Chest trauma, renal failure requiring dialysis, medical condition necessitating admission, pregnancy | 3.5 (median) | 705 |
| Body 201146 | PAPP-A, CD40L | Single centre, UK | Mean age 59; 434 (61%) male | Suspected cardiac chest pain occurring within the previous 24 hours | Chest trauma, renal failure requiring dialysis, medical condition necessitating admission, pregnancy | 3.5 (median) | 713 |
| Brown 200747 | ST2 | Single centre, US | Mean age 49.8; 160 (46%) male | Chest pain prompting an ECG | NR | 4.3 (median) | 348 |
| Esporcatte 200765 | MPO | Single centre, Brazil | Mean age 63.0; 76 (54%) male | Chest pain within 24 hours | STEMI, inflammatory or infectious syndrome, neoplasia or the use of drugs affecting the immune system | Within 24 hours | 140 |
| Keller 201071 | Copeptin | Multicentre, Germany | Mean age 61; 920 (66%) male | Aged 18–85 years with angina pectoris or equivalent symptoms | Trauma or major surgery within the last 4 weeks, pregnancy, intravenous drug abuse, anaemia | 57.6% < 6 hours | 1386 |
| Potsch 200651 | CRP | Single centre, Brazil | Mean age 64.9; 54.6% male | Chest pain presenting to ED | ST elevation | Within 12 hours | 980 |
| Reichlin 200980 | Copeptin | Single centre, Switzerland | Mean age 62; 321 male | Symptoms suggestive of MI within the last 12 hours | Terminal renal failure requiring dialysis | Within 12 hours | 487 |
| Rudolph 201081 | MPO | Single centre, Germany | Mean age 64; 192 (70%) male | Chest pain presenting to the ED | NR | 4.5 (median) | 274 |
| Study | Index test analyzer (timing is at presentation unless otherwise stated) | Threshold | Reference test assay and timing | Reference Tn diagnostic threshold (µg/l) | MI prevalence in sample (%) |
|---|---|---|---|---|---|
| Apple 200955 | CD40L, R&D Systems ELISA | 1.08 ng/l | Dade Behring Dimension or Stratus CS TnI at 8 hours after presentation | 0.1 | 5 |
| Apple 200955 | MPO, Assay Designs ELISA | 125 µg/l | Dade Behring Dimension or Stratus CS TnI at 8 hours after presentation | 0.1 | 5 |
| Apple 200955 | MMP9, Assay Designs ELISA | 233 µg/l | Dade Behring Dimension or Stratus CS TnI at 8 hours after presentation | 0.1 | 5 |
| Apple 200955 | CRP, Dade Behring Dimension | 1.0 and 3.0 mg/l | Dade Behring Dimension or Stratus CS TnI at 8 hours after presentation | 0.1 | 5 |
| Apple 200955 | NT-pro-BNP, Roche Elecsys | 125 ng/l age < 75 years, 450 ng/l age > 75 years | Dade Behring Dimension or Stratus CS TnI at 8 hours after presentation | 0.1 | 5 |
| Bassan 200556 | Biosite immunofluorescence BNP assay | 100 pg/m | Dade Behring TnI; within 9 hours post-admission; | 1.0 | 11 |
| Body 201157 | BNP Alere Fluorescence Immunoassay | 73 ng/m | cTnT; Roche Elecsys; at least 12 hours after symptom onset | 0.01 | 18 |
| Body 201157 | MPO Alere Fluorescence Immunoassay | 510 pM | cTnT; Roche Elecsys; at least 12 hours after symptom onset | 0.01 | 18 |
| Body 201146 | PAPP-A, Demeditec Diagnostics ultrasensitive ELISA | 4.4 µg/l | Roche fourth generation TnT at 12 hours | 0.035 | 18 |
| Body 201146 | CD40L, R&D Systems Quantikine kit | 17.2 ng/l | Roche fourth-generation TnT at 12 hours | 0.035 | 18 |
| Brown 200747 | Medical & Biological Laboratories ELISA | None reported | First-generation Abbott AxSym | 2.0 | 4.9 |
| Esporcatte, 200765 | ELISA assay, Oxis Research International, Inc. | ≥100pM | Dade Behring TnI within 12 hours | 1.0 | 9 |
| Keller 201071 | Copeptin; BRAHMS. LUMItest CT-proAVP | 13, 18.9 pmol/l | Roche TnT or Siemens Dimension RxL TnI within 6 hours of admission | 0.3 (TnT) | 29 |
| 0.14 (TnI) | |||||
| Potsch 200651 | t-CRP: immunochemical titrated CRP | 1.0 mg/l | Dade Behring TnI at hour 9 | 0.1 | 13 |
| hs-CRP: immunonephelometric, high-sensitivity CRP | |||||
| Reichlin 200980 | Copeptin; BRAHMS LUMItest CT-proAVP | 9, 14, 20, 24 pmol/l | Roche TnT at 6–9 hours after presentation | 0.04 | 17 |
| Rudolph 201081 | MPO, Abbott ARCHITECT system | Sample median | Abbott Architect, timing not stated | 0.032 | 3 |
Quality assessment of diagnostic studies of other biomarkers
Figure 24 shows the quality assessments for studies of other biomarkers, whereas Figure 25 shows the methodological quality summary.
FIGURE 24.
Quality assessment of diagnostic studies of other biomarkers.
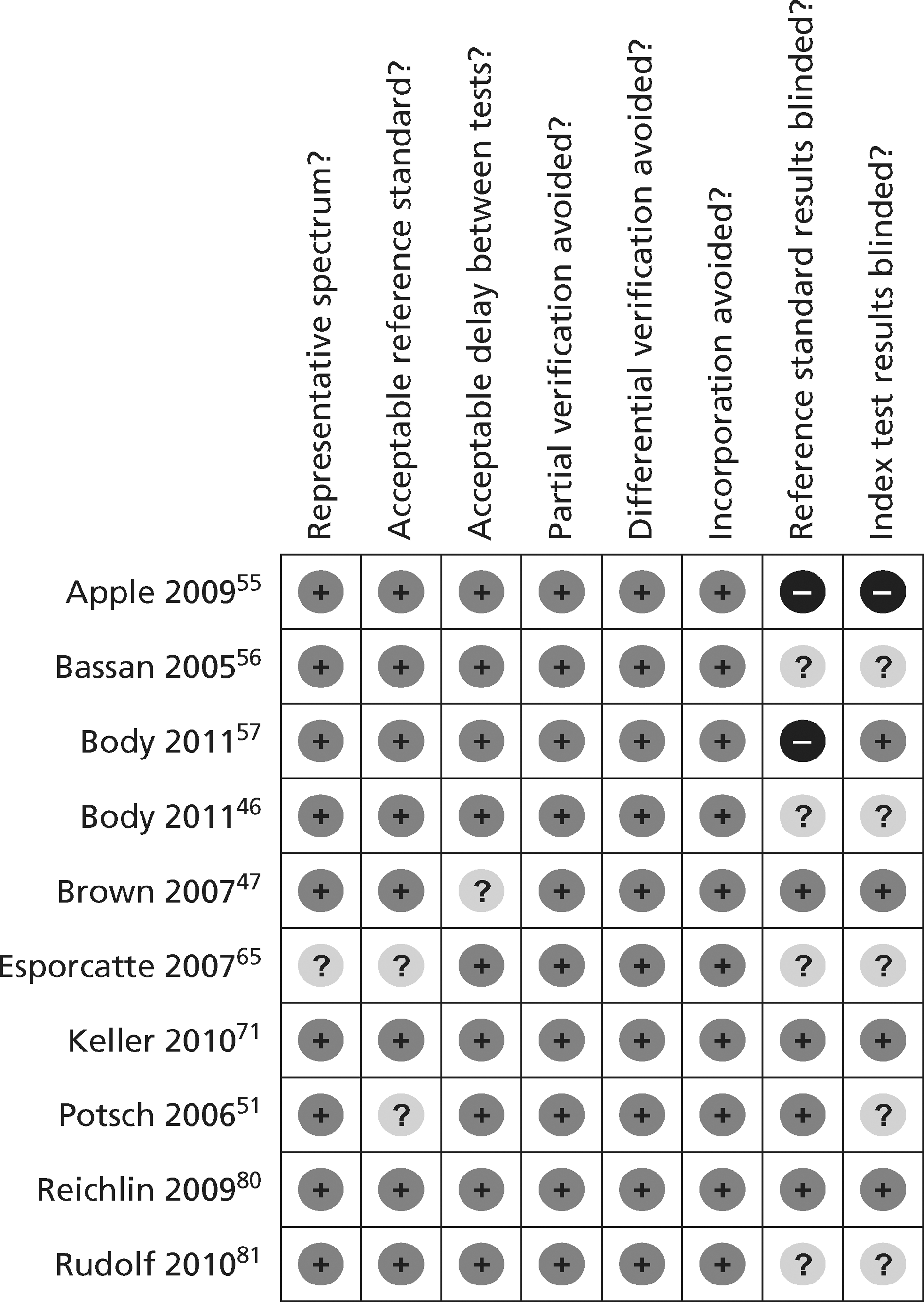
FIGURE 25.
Methodological quality summary of diagnostic studies of other biomarkers.
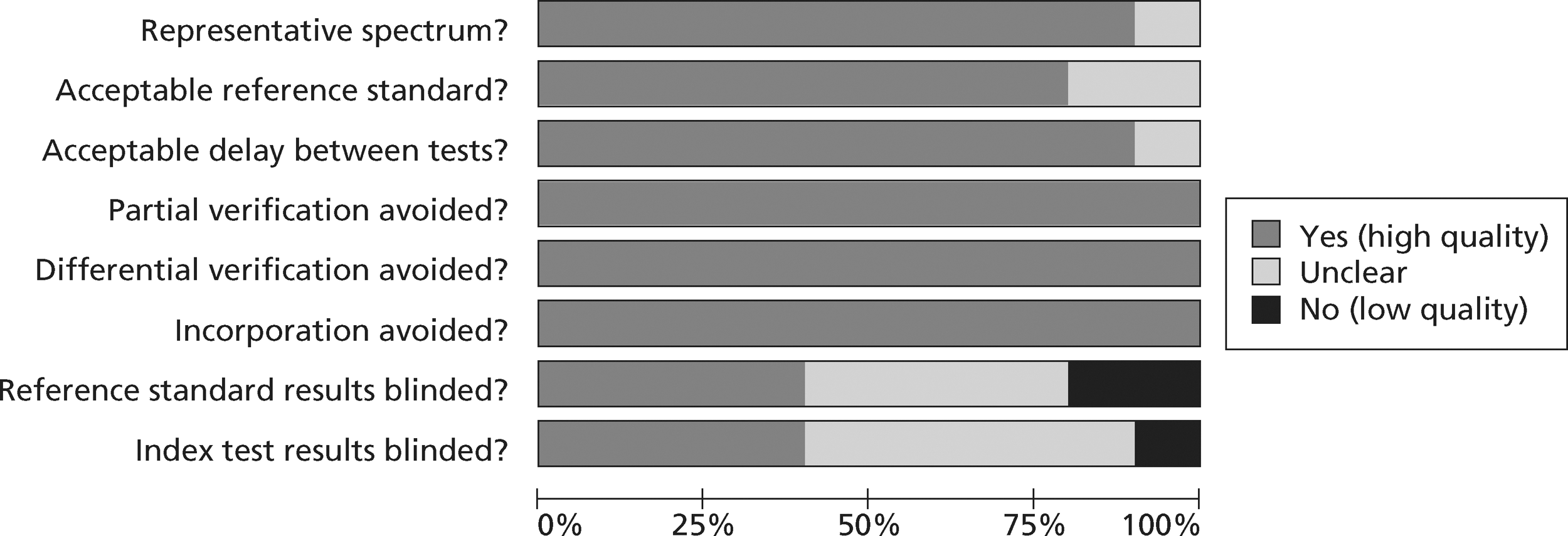
Analysis of diagnostic studies of other biomarkers
The studies reported four analyses of MPO,55,57,65,81 two each of BNP,55,56 CD40L,46,55 copeptin71,80 and CRP,51,55 and one each of MMP9,55 NT-pro-BNP55 and PAPP-A. 46 No two analyses evaluated the same biomarker at the same threshold. The data were therefore insufficient for meaningful meta-analysis. Sensitivity and specificity of each biomarker in each analysis are reported in Table 17. Overall, diagnostic accuracy was modest. Sensitivity exceeding 0.8 was achieved only at the expense of specificity. None of these analyses suggests that the biomarker in question could be used as a single test for early diagnosis of MI.
| Study | Biomarker | Threshold | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|
| Bassan 200556 | BNP | 100 pg/m | 0.71 (0.67 to 0.74) | 0.69 (0.65 to 0.72) |
| Body 201157 | BNP | 73 ng/m | 0.35 (0.26 to 0.44) | 0.85 (0.82 to 0.88) |
| Apple 200955 | CD40L | 1.08 ng/l | 0.72 (0.51 to 0.88) | 0.23 (0.19 to 0.27) |
| Body 201146 | CD40L | 17.2 ng/l | 0.67 (0.58 to 0.75) | 0.25 (0.21 to 0.28) |
| Keller 201071 | Copeptin | 9.8 pmol/l | 0.66 (0.6 to 0.71) | 0.70 (0.67 to 0.73) |
| 13 pmol/l | 0.57 (0.52 to 0.63) | 0.78 (0.75 to 0.8) | ||
| 18.9 pmol/l | 0.49 (0.43 to 0.55) | 0.84 (0.82 to 0.87) | ||
| Reichlin 200980 | Copeptin | 9 pmol/l | Reported only in combination with troponin | Reported only in combination with troponin |
| 14 pmol/l | ||||
| 20 pmol/l | ||||
| 24 pmol/l | ||||
| Apple 200955 | CRP | 125 ng/l age < 75 years, | 0.79 (0.54 to 0.94) | 0.47 (0.42 to 0.53) |
| 450 ng/l age > 75 years | 0.50 (0.12 to 0.88) | 0.28 (0.17 to 0.40) | ||
| Potsch 200651 | CRP | 1.0 mg/l | 0.30 (0.22 to 0.38) | 0.80 (0.78 to 0.83) |
| Apple 200955 | MMP9 | 125 µg/l | 0.96 (0.80 to 0.99) | 0.19 (0.15 to 0.23) |
| Apple 200955 | MPO | 233µg/l | 0.76 (0.55 to 0.91) | 0.38 (0.34 to 0.43) |
| Body 201157 | MPO | 510 pM | 0.60 (0.51 to 0.68) | 0.58 (0.54 to 0.62) |
| Esporcatte 200765 | MPO | ≥100 pM | 0.92 (0.67 to 1.0) | 0.40 (0.32 to 0.49) |
| Rudolph 201081 | MPO | Sample median | 0.80 (0.76 to 0.84) | 0.68 (0.65 to 0.71) |
| Apple 200955 | NT-pro-BNP | 1.0 mg/l | 0.80 (0.59 to 0.93) | 0.39 (0.35 to 0.46) |
| 3.0 mg/l | 0.88 (0.69 to 0.97) | 0.19 (0.15 to 0.23) | ||
| Body 201146 | PAPP-A | 4.4 µg/l | 0.49 (0.4 to 0.58) | 0.67 (0.63 to 0.71) |
| Brown 200747 | ST2 | NR | NRa | NR |
Diagnostic studies of biomarkers in combination with troponin
Nine studies48,55,57,67,70,71,76,77,80 reported 11 analyses of the sensitivity and specificity of biomarkers in combination with troponin, compared with troponin alone. These are outlined in Table 18. We did not undertake meta-analysis because no combination was evaluated in more than two studies. In most cases, troponin and the alternative biomarker were combined by classifying the combination as positive if either test was positive. However, the study by Apple et al. 55 classified the combination as positive only if both tests were positive. Thus, in most studies the combination had higher sensitivity and lower specificity than troponin alone, whereas the combinations tested by Apple et al. 55 had lower sensitivity and higher specificity than troponin alone.
| Study | Combination | Troponin alone | Combination | ||
|---|---|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | ||
| Body 201157 | TnI or H-FABP | 0.42 (0.33 to 0.51) | 0.96 (0.94 to 0.97) | 0.82 (0.74 to 0.88) | 0.88 (0.83 to 0.88) |
| Haltern 201067 | TnT or H-FABP | 0.74 (0.66 to 0.74) | 1.00 (0.96 to 1.0) | 0.97 (0.86 to 0.99) | 0.65 (0.60 to 0.66) |
| McCann 200876 | TnT or H-FABP | 0.75 (0.69 to 0.81) | 0.94 (0.9 to 0.96) | 0.93 (0.89 to 0.96) | 0.93 (0.89 to 0.96) |
| Mion 200777 | TnI or H-FABP | 0.55 (0.39 to 0.70) | 0.98 (0.92 to 1.00) | 0.76 (0.60 to 0.87) | 0.93 (0.86 to 0.97) |
| Keller 201071 | TnT or copeptin | 0.62 (0.56 to 0.67) | 0.97 (0.96 to 0.98) | 0.88 (0.83 to0.91)a | 0.76 (0.73 to0.79)a |
| Reichlin 200980 | TnT or copeptin | 0.75 (0.65 to 0.83) | 0.94 (0.91 to 0.96) | 0.99 (0.92 to1.00)b | 0.77 (0.73 to0.81)b |
| Keller 201071 | TnT or myoglobin | 0.62 (0.56 to 0.67) | 0.97 (0.96 to 0.98) | 0.81 (0.76 to 0.85) | 0.85 (0.82 to 0.87) |
| Mion 200777 | TnI or myoglobin | 0.55 (0.39 to 0.70) | 0.98 (0.92 to 1.00) | 0.83 (0.68 to 0.92) | 0.92 (0.84 to 0.97) |
| Collinson 200648 | TnT or IMA | 0.95 (0.8 to 0.99) | 0.95 (0.92 to 0.97) | 1.00 (0.88 to 1.00) | 0.35 (0.31 to 0.40) |
| Keating 200670 | TnI or IMA | 0.74 (0.58 to 0.86) | 0.99 (0.97 to 1.00) | 0.98 (0.86 to 1.00) | 0.14 (0.10 to 0.19) |
| Apple 200955 | TnI and CRP | 0.72 (0.51 to 0.88) | 0.89 (0.85 to 0.92) | 0.56 (0.35 to 0.76) | 0.95 (0.92 to 0.97) |
| Apple 200955 | TnI and MMP9 | 0.72 (0.51 to 0.88) | 0.89 (0.85 to 0.92) | 0.68 (0.47 to 0.85) | 0.91 (0.88 to 0.94) |
These studies show that combining troponin with another biomarker at presentation, with elevation of either biomarker producing a positive test, results in markedly improved sensitivity but with a loss in specificity that can be substantial. None of these analyses uses a high-sensitivity troponin assay. The results of the troponin meta-analysis suggest that a similar improvement in sensitivity at the expense of specificity can be achieved if a lower threshold for troponin positivity is used.
Summary of the findings of the diagnostic biomarker review
The sensitivity and specificity of troponin measurement at presentation depends on the assay used and the threshold for positivity. High-sensitivity assays using the 99th percentile as the threshold for positivity can achieve sensitivity at presentation close to, or exceeding, 90%. However, maximising early sensitivity involves some loss of specificity. Only one study60 compared presentation testing with a high-sensitivity assay with a reference standard based on a high-sensitivity assay and showed that the loss of specificity did not seem to be explained by using a standard troponin assay in the reference standard.
Many other biomarkers have been tested for their ability to detect MI at presentation but of those we set out to investigate only myoglobin and H-FABP have been evaluated against an acceptable reference standard in a large number of studies. In general, the alternative biomarkers had inadequate diagnostic accuracy to act as a single diagnostic test for MI at presentation. When used in combination with troponin a number of biomarkers (H-FABP, copeptin, IMA and myoglobin) improved sensitivity for MI at presentation, but at the expense of loss of specificity. Similar changes in sensitivity and specificity can be achieved with troponin as a single test by using a high-sensitivity assay.
Overview of biomarker studies included in the prognostic review
We identified 44 studies46–51,85–122 for inclusion in the prognostic accuracy review. These are listed along with the relevant biomarkers in Table 19. We have only listed the biomarkers identified for our review. Some studies evaluated additional biomarkers. Five studies46,48–51 reported both prognostic and diagnostic data. Two studies44,45 were subsequently excluded because data could have overlapped with other included studies. 85,86
| Study | Included index test biomarkers in study |
|---|---|
| Apple 200787 | BNP, hsCRP, MMP9, MPO |
| Apple 201188 | MPO |
| Bholasingh 200389 | CRP |
| Blum 200390 | CRP |
| Body 201146 | PAPP-A |
| Brennan 200391 | CRP, MPO |
| Brown 200792 | BNP, myoglobin |
| Brown 200747 | ST-2 |
| Brugger-Anderson 200893 | BNP, hsCRP |
| Cameron94 | BNP, hsCRP, myoglobin |
| Collinson 200648 | IMA |
| Consuegra-Sanchez 200895 | IMA |
| De Winter 199696 | Myoglobin |
| Eggers 200885 | NT-pro-BNP, CRP |
| Eggers 200897 | NT-pro-BNP, CRP |
| Fromm 200198 | Myoglobin |
| Garcia-Valdecasas 201149 | H-FABP, myoglobin |
| Green 200099 | Myoglobin |
| Hillis 2003100 | Myoglobin |
| Ilva 200950 | H-FABP |
| Jaffery 2008101 | Myoglobin |
| Jernberg 2002102 | NT-pro-BNP |
| Kavsak 2009103 | PAPP-A |
| Kontos 2007104 | Myoglobin |
| Laterza 2004105 | PAPP-A |
| Lim 2002106 | Myoglobin |
| Lund 2003107 | PAPP-A |
| Manini 2009108 | IMA |
| Markovic 2010109 | BNP, hsCRP |
| Mathew 1999110 | Myoglobin |
| McCann 2009111 | BNP, H-FABP, hsCRP, MMP9, MPO, PAPP-A |
| McCord 2003112 | Myoglobin |
| Menown 2003113 | hsCRP, interleukin 6 |
| Mockel 200886 | NT-pro-BNP, hsCRP |
| Newby 2001114 | Myoglobin |
| Ordonez-Llanos 2006115 | Myoglobin |
| Pontiz 2009116 | BNP |
| Potsch 200651 | CRP |
| Sonel 2000117 | Myoglobin |
| Svensson 2004118 | Myoglobin |
| Szymanski 2007119 | Myoglobin |
| Van Domburg 2000120 | Myoglobin |
| Viswanathan 2010121 | H-FABP |
| Yamashita 2010122 | NT-pro-BNP, H-FABP |
Description of studies included in the prognostic biomarker review
The characteristics of the included studies are outlined in Table 20. Most studies did not report selection criteria beyond those needed to define acute chest pain or suspected ACS. However, some studies excluded patients with high clinical risk, ECG changes of ischaemia or positive admission troponin or CK-MB. The duration of follow-up ranged from the duration of inpatient stay to 5 years. Definitions of MACEs varied between studies, with some studies predicting only mortality, whereas others predicted a range of outcomes. Where more than one definition of a MACE was used or more than one time point for follow-up was reported, we used the most inclusive definition and the longest duration of follow-up.
| Study | Population | n | Age (years) and sex | Follow-up | MACE |
|---|---|---|---|---|---|
| Apple 200752 | All | 457 | NR | 4 months | Cardiac death, MI, revascularisation |
| Apple 201188 | All | 400 | Mean age 56, 228 (57%) men | 6 months | Cardiac death, MI, revascularisation |
| Bholasigh 200389 | < 6 hours, ECG, TnI –ve | 382 | Mean age 57, 215 (56%) male | 6 months | Cardiac death, MI, admission |
| Blum 200390 | Age < 55 years, ECG, CK-MB –ve | 40 | Mean age 45, 38 (95%) men | 6 months | Cardiac death, MI, revascularisation |
| Body 201146 | <24 hours | 713 | Mean age 59, 434 (61%) men | 30 days | Death, MI, revascularisation |
| Brennan 2003 | <24 hours | 604 | Mean age 63, 354 (57%) men | 6 months | Death, MI, revascularisation |
| Brown 200792 | All | 359 | Mean age 55, 203 (48%) men | 30 days | Death, MI, LTA, HF, revascularisation |
| Brown 200747 | All | 348 | Mean age 50, 160 (46%) men | 30 days | Death, MI, revascularisation |
| Brugger-Anderson 200893 | All | 871 | Mean age 69, 548 (63%) men | 24 months | Death, MI |
| Cameron 200794 | All | 422 | Mean age 57, 203 (48%) men | 30 days | Death, MI, UA, revascularisation |
| Consuegra-Sanchez 200895 | < 3 hours | 207 | Mean age 61, 142 (69%) men | 30 days | Cardiac death, MI, UA |
| Collinson 200648 | ECG | 539 | Median age 52, 335 (62%) male | 6 months | Cardiac death, MI, revascularisation |
| De Winter 199696 | < 12 hours, ECG, CK-MB –ve | 128 | Mean age 63, 78 (61%) men | 6 months | Cardiac death, MI, revascularisation |
| Eggers 200885 | < 24 hours, ECG | 452 | Mean age 65, 298 (66%) men | 6 months | Death, MI |
| Eggers 200897 | < 24 hours, ECG | 479 | Mean age 66, 311 (65%) men | 6 months | Death, MI |
| Fromm 200198 | < 24 hours | 955 | NR | 6 months | Death, revascularisation |
| Garcia-Valdecasas 201149 | < 6 hours | 165 | Mean age 67, 114 (69%) men | 6 months | Death, MI, angina, revascularisation, HF |
| Green 200099 | All | 396 | Mean age 61, 199 (50%) men | 14 days | Death, MI, UA, LTA, HF |
| Hillis 2003100 | < 24 hours, low Goldman risk | 501 | Median age 58, 243 (49%) men | 1–49 months | Death, MI |
| Ilva 200950 | < 24 hours | 351 | Mean age 67, 181 (62%) men | 6 months | Death, MI |
| Jaffery 2008101 | ECG | 951 | Median age 65, 434 (46%) men | 5 years | Death |
| Jernberg 2002102 | ECG | 775 | Median age 69, 468 (60%) men | 35–47 months | Death |
| Kavsak 2009103 | All | 320 | Median age 64, 192 (60%) men | 2 years | Death |
| Kontos 2007104 | Low clinical risk, ECG | 3461 | Mean age 59, 1737 (50%) men | 1 year | Death |
| Laterza 2004105 | All | 346 | Mean age 57, 166 (48%) men | 1 month | Death, MI, revascularisation |
| Lim 2002106 | < 8 hours, ECG | 37 | Mean age 58, 17 (73%) men | 3 months | Death, stroke, hospitalisation, MI, revascularisation |
| Lund 2003107 | Tn –ve | 136 | Mean age 66, 69 (50%) men | 6 months | Death, MI, revascularisation |
| Manini 2009108 | ECG | 106 | Mean age 60, 57 (54%) men | 30 days | Death, MI, revascularisation |
| Markovic 2010109 | ECG | 102 | Mean age 63, 70 (70%) men | 30 days | Death, MI |
| Mathew 1999110 | < 24 hours, ECG | 214 | Mean age 59, 151 (71%) men | 3 months | Death, MI, UA, revascularisation |
| McCann 2009111 | Cardiac-type chest pain | 555 | Mean age 62, 386 (70%) men | 1 year | Death, MI |
| McCord 2003112 | ECG | 764 | Mean age 64, 345 (45%) men | 30 days | Death, MI |
| Menown 2003113 | Cardiac-type chest pain | 391 | Mean age 63 | 1 year | Death, MI |
| Mockel 200886 | All | 432 | Mean age 60, 261 (60%) men | 42 days | Cardiac death, MI, UA, HF, revascularisation |
| Newby 2001114 | ECG | 1005 | Mean age 51, 423/851 (50%) men | 30 days | Death, MI |
| Ordonez-Llanos 2006115 | < 24 hours | 1410 | Mean age 63, 906 (64%) men | 1 year | Death, MI, UA, revascularisation |
| Ponitz116 | Strongly suspected ACS | 870 | Mean age 70, 531 (61%) men | 2 years | Death, MI |
| Potsch 200651 | < 12 hours | 980 | Mean age 65, 535 (55%) men | Inpatient stay | Cardiac death, MI, revascularisation |
| Sonel 2000117 | All | 247 | Mean age 52, 133 (54%) men | 6 months | Death, MI, UA, revascularisation |
| Svensson 2004118 | < 6 hours | 511 | Mean age 69, 293/500 (50%) men | 1 year | Death |
| Symanski 2007119 | High clinical probability ACS | 336 | Mean age 66, 180 (54%) men | 30 day | Death |
| Van Domberg 2000120 | All | 163 | Mean age 62, 124 (76%) men | 3 years | Death |
| Viswanathan 2010121 | All | 955 | Mean age 60, 577 (60%) men | > 1 year | Death, MI |
| Yamashita 2010122 | All | 162 | Mean age 64, 107 (66%) men | Inpatient stay | Cardiac death |
Quality assessment of studies included in the prognostic biomarkers review
Table 21 shows the results of quality assessment. Nearly all the studies reported adequately, defined MACEs in the methods section, did not incorporate presenting diagnosis in the definition of a MACE and achieved adequate follow-up. However, only around half undertook analysis that went beyond testing or estimating the association between the biomarker and a MACE, and only a minority tested whether or not the biomarker added prognostic value to that provided by troponin.
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 |
|---|---|---|---|---|---|---|---|
| Apple 200787 | Y | Y | Y | Y | Y | Y | N |
| Apple 201188 | Y | Y | Y | Y | Y | Y | N |
| Bholasigh 200389 | Y | Y | Y | Y | Y | N | N |
| Blum 200390 | Y | Y | Y | Y | Y | N | N |
| Body 201146 | Y | Y | Y | Y | Y | Y | Y |
| Brennan 200391 | Y | Y | Y | Y | Y | Y | Y |
| Brown 200792 | Y | Y | Y | Y | Y | N | N |
| Brown 200747 | Y | Y | Y | U | Y | N | N |
| Brugger-Anderson 200893 | Y | Y | Y | Y | Y | Y | Y |
| Cameron 200794 | Y | Y | Y | U | Y | N | N |
| Consuegra-Sanchez 200895 | Y | Y | Y | Y | Y | Y | Y |
| Collinson 200648 | Y | Y | Y | Y | Y | N | N |
| De Winter 199696 | Y | Y | Y | Y | Y | N | N |
| Eggers 200885 | Y | Y | Y | Y | Y | Y | Y |
| Eggers 200897 | Y | Y | Y | Y | Y | Y | Y |
| Fromm 200198 | Y | N | N | Y | Ya | N | N |
| Garcia-Valdecasas 201149 | Y | Y | Y | Y | Y | Y | Y |
| Green 200099 | Y | Y | Y | Y | Y | N | N |
| Hillis 2003100 | Y | Y | Y | Y | Y | N | N |
| Ilva 200950 | Y | Y | Y | Y | Y | Y | Y |
| Jaffery 2008101 | Y | Y | Y | Y | Y | Y | Y |
| Jernberg 2002102 | Y | Y | Y | Y | U | Y | Y |
| Kavsak 2009108 | Y | Y | Y | Y | Y | Y | Y |
| Kontos 2007104 | Y | Y | Y | Y | Y | Y | Y |
| Laterza 2004105 | Y | Y | Y | Y | Y | N | N |
| Lim 2002106 | Y | Y | Y | Y | U | N | N |
| Lund 2003107 | Y | Y | Y | Y | Y | Y | Y |
| Manini 2009108 | Y | Y | Y | Y | Y | N | N |
| Markovic 2010109 | Y | Y | Y | y | Y | Yb | N |
| Mathew 1999110 | Y | Y | Y | Y | Y | N | N |
| McCann 2009111 | Y | Y | Y | Y | Y | Nc | N |
| McCord 2003112 | Y | Y | Y | Y | Y | N | N |
| Menown 2003113 | Y | N | Y | Y | Y | Nd | N |
| Mockel 200886 | Y | Y | Y | Y | Y | Y | Y |
| Newby 2001114 | Y | Y | Y | Y | Y | N | N |
| Ordonez-Llanos 2006115 | Y | Y | Y | Y | Y | N | N |
| Ponitz 2009116 | Y | Y | Y | Y | Y | Y | Y |
| Potsch 200651 | Y | Y | Y | Y | Y | Y | N |
| Sonel 2000117 | Y | Y | Y | Y | Y | Y | Y |
| Svensson 2004118 | Y | Y | Y | Y | Y | Y | Y |
| Symanski 2007119 | Y | Y | Y | Y | Y | N | N |
| Van Domberg 2000120 | Y | Y | Y | Y | Y | Y | Y |
| Viswanathan 2010121 | Y | Y | Y | Y | Y | Y | Y |
| Yamashita 2010122 | Y | Y | Y | Y | Y | Y | Y |
Analysis of prognostic biomarker studies
Table 22 shows the main univariate analyses reported in the prognostic biomarker studies, i.e. any analysis that tested or estimated the association between a biomarker and a MACE. There was substantial variation in the analyses reported. Some only used a hypothesis test for the association between a biomarker and a MACE, others estimated parameters [sensitivity, specificity or area under receiver operating characteristic (AUROC)] for discriminating between patients with and without MACEs, and others estimated the odds ratio (OR), RR or hazard ratio (HR) for MACEs for quartiles of the biomarker or a biomarker level above a specified threshold. Many of these analyses report a significant association but they are of limited value because they do not tell us whether or not the biomarker in question provides prognostic information beyond that already available from clinical assessment, ECG and troponin measurement.
| Study | Biomarker | Threshold | Biomarker selection | Analysis | Finding |
|---|---|---|---|---|---|
| Brown 200792 | BNP | 31 pg/ml | None | AUROC | 0.675 |
| Brugger-Anderson 200893 | BNP | Quartiles | None | Log-rank test | p = 0.001 |
| Ponitz 2009116 | BNP | Quartiles | Tn –ve | Univariate HR | Q1: Reference |
| Q2: 7.2 (95% CI 1.6 to 31.9) | |||||
| Q3: 9.3 (95% CI 2.1 to 40.3) | |||||
| Q4: 11.9 (95% CI 2.8 to 50.7) | |||||
| Apple 200787 | CD40 ligand | 1.081 ng/l | None | Univariate RR | 1.3 (95% CI 0.6 to 2.9) |
| McCann 2009111 | CD40 ligand | 462 pg/m | None | Univariate OR | 0.9 (95% CI 0.4 to 1.7) |
| Body 201146 | CD40 ligand | Tertiles | Tn –ve | Mantel–Haenszel test | p = 0.453 |
| Apple 200787 | CRP | 3 mg/l | None | Univariate RR | 0.5 (95% CI 0.2 to 1.3) |
| Bholasingh 200389 | CRP | 0.3 mg/dl | Tn –ve | Univariate HR | 4.5 (95% CI 1.2 to 17.0) |
| Blum 200390 | CRP | 15 mg/dl | CK-MB –ve | Sensitivity and specificity | 67% and 97% |
| Brennan 200391 | CRP | Quartiles | TN –ve | Univariate OR | Q1: Reference |
| Q2: 1.6 (95% CI 0.9 to 2.7) | |||||
| Q3: 0.9 (95% CI 0.5 to 1.7) | |||||
| Q4: 1.0 (95% CI 0.6 to 1.9) | |||||
| Brugger-Anderson 200893 | CRP | Quartiles | None | Log-rank test | p < 0.001 |
| Cameron 200794 | CRP | 13.6 mg/dl | None | Univariate RR | 1.9 (95% CI 1.1 to 3.4) |
| Eggers 200885 | CRP | 3.7 mg/l | None | Chi-squared test | p = 0.01 |
| Eggers 200897 | CRP | Not stated | None | Univariate OR | 1.4 (95% CI 1.1 to 1.8) |
| Markovic 2010109 | CRP | 10 mg/l | None | AUROC | 0.626 (95% CI 0.525 to 0.720) |
| McCann 2009111 | CRP | 12.0 mg/l | None | Univariate OR | 1.4 (95% CI 0.7 to 2.6) |
| Menown 2003113 | CRP | 7.1 mg/l | TN –ve and CK-MB –ve | Univariate OR | 2.5 (95% CI 0.6 to 9.8) |
| Mockel 200886 | CRP | 10 mg/l | None | Univariate OR | 1.9 (95% CI 1.0 to 3.5) |
| Potsch 200651 | CRP | Quartiles | None | Linear trend | p = 0.003 |
| Eggers 200897 | Cystatin-C | Not stated | None | Univariate OR | 9.0 (95% CI 3.4 to 23.6) |
| Apple 200787 | eGFR | 60 ml/minute | None | Univariate RR | 1.1 (95% CI 0.6 to 2.2) |
| Markovic 2010 | eGFR | 60 ml/minute | None | AUROC | 0.630 (95% CI 0.529 to 0.723) |
| Body 201146 | E-selectin | Tertiles | Tn –ve | Mantel–Haenszel test | p = 0.816 |
| McCann 2009111 | GPBB | 7 ng/m | None | Univariate OR | 1.9 (95% CI 1.0 to 3.5) |
| Eggers 200897 | GRF-15 | Not stated | None | Univariate OR | 4.5 (95% CI 2.5 to 8.1) |
| Garcia-Valdecasas 201149 | H-FABP | 6.2 ng/m | None | Breslow test | p < 0.01 |
| Ilva 200950 | H-FABP | 10.4 µg/l | None | NR | – |
| McCann 2009121 | H-FABP | 5 ng/m | None | Univariate OR | 5.4 (95% CI 2.4 to 12.2) |
| Viswanathan 2010121 | H-FABP | Quartiles | Tn –ve | Univariate HR | Q1: Reference |
| Q2: 3.5 (95% CI 1.7 to 7.1) | |||||
| Q3: 11.2 (95% CI 4.9 to 25.4) | |||||
| Q4: 16.6 (95% CI 2.2 to 125.5) | |||||
| Yamashita 2010122 | H-FABP | None: continuous | None | Univariate OR | 1.003 (95% CI 1.002 to 1.005) |
| Menown 2003113 | Interleukin 6 | 10.7 pg/m | TN –ve and CK-MB –ve | Univariate OR | 3.2 (95% CI 0.6 to 16.8) |
| Collinson 200648 | IMA | 85 kU/l | TnI –ve | Univariate RR | 1.3 (95% CI 1.0 to 1.6) |
| Consuegra-Sanchez 200895 | IMA | 93.3 U/ml | None | Univariate HR | 1.04 (95% CI 1.01 to 1.07) |
| Manini 2009108 | IMA | 75 kU/l | None | Univariate RR | 2.4 (95% CI 0.8 to 7.9) |
| Apple 200787 | MMP9 | 233.7 µg/l | None | Univariate RR | 1.8 (95% CI 0.6 to 5.2) |
| McCann 2009111 | MMP9 | 1599 ng/m | None | Univariate OR | 1.1 (95% CI 0.6 to 2.1) |
| Apple 200787 | MPO | 125.6 µg/l | None | Univariate RR | 1.9 (95% CI 0.9 to 4.0) |
| Apple 201188 | MPO | 633 pmol/l | None | Univariate HR | 2.8 (95% CI 1.5 to 5.3) |
| Brennan 200391 | MPO | Quartiles | Tn –ve | Univariate OR | Q1: Reference |
| Q2:1.9 (95% CI 1.0 to 3.8) | |||||
| Q3: 4.4 (95% CI 2.3 to 8.4) | |||||
| Q4: 3.9 (95% CI 2.0 to 7.7) | |||||
| McCann 2009111 | MPO | 421 ng/m | None | Univariate OR | 0.8 (95% CI 0.4 to 1.6) |
| Cameron 200794 | Myoglobin | 61 ng/m | None | Univariate RR | 3.1 (95% CI 1.7 to 5.7) |
| De Winter 199696 | Myoglobin | 90 ng/m | None | Univariate RR | 1.0 (95% CI 0.3 to 3.2) |
| Fromm 200198 | Myoglobin | 85 ng/m | None | Univariate RR | 1.6 (95% CI 1.1 to 2.9) |
| Green 200099 | Myoglobin | 69 ng/m | None | Univariate RR | 3.4 (95% CI 2.2 to 5.1) |
| Hills 2003100 | Myoglobin | 100 ng/m | None | Univariate RR | 2.2 (95% CI 1.3 to 4.0) |
| Jaffery 2008101 | Myoglobin | 200 ng/m | None | NR | – |
| Kontos 2007104 | Myoglobin | 90 ng/m | None | Univariate RR | 3.7 (95% CI 2.8 to 4.7) |
| Lim 2002106 | Myoglobin | 116 ng/m | All | Univariate OR | 12.5 (95% CI 2.1 to 71) |
| Mathew 1999110 | Myoglobin | 92 µg/l | None | Univariate RR | 2.5 |
| McCord 2003112 | Myoglobin | 200 ng/m | None | Sensitivity and specificity | 74.8 (95% CI 65 to 83) and 70.4 (95% CI 67 to 74) |
| Newby 2001114 | Myoglobin | 105 ng/m | None | NR | – |
| Ordonez-Llanos 2006115 | Myoglobin | Quartiles | None | Univariate OR | 5.2 (95% CI 3.0- 9.2) |
| Sonel 2000117 | Myoglobin | 100 µg/m | None | Univariate OR | 2.5 (95% CI 1.3 to 4.6) |
| Svensson 2004118 | Myoglobin | 50 ng/m | None | Fishers exact test | p = 0.07 |
| Symanski 2007119 | Myoglobin | 82 ng/m | None | AUROC | 0.78 (95% CI 0.72 to 0.83) |
| Van Domberg 2000120 | Myoglobin | 64µg/ml (women), 76µg/ml (men) | None | NR | |
| Cameron 200794 | NT-pro-BNP | 280 ng/m | None | Univariate RR | 3.0 (95% CI 1.6 to 5.7) |
| Mockel 200886 | NT-pro-BNP | 145 ng/m | None | Univariate OR | 3.8 (95% CI 1.9 to 7.5) |
| Apple 200787 | NT-pro-BNP | < 75 years, 125 ng/l ≥ 75 years, 450 ng/l | None | Univariate RR | 2.9 (95% CI 1.1 to 7.4) |
| Eggers 200885 | NT-pro-BNP | 550 ng/l | None | Chi-squared test | p < 0.001 |
| Eggers 200897 | NT-pro-BNP | Not stated | None | Univariate OR | 1.8 (95% CI 1.4 to 2.3) |
| Jernberg 2002102 | NT-pro-BNP | Quartiles | None | Log-rank test | Q1: Reference |
| Q2: p = 0.005 | |||||
| Q3: p < 0.001 | |||||
| Q4: p < 0.001 | |||||
| McCann 2009111 | NT-pro-BNP | 1371 ng/l | None | Univariate OR | 5.4 (95% CI 3.0 to 9.7) |
| Yamashita 2010122 | NT-pro-BNP | None: continuous | None | Univariate OR | 1.0 (95% CI 1.0 to 1.0) |
| Body 201146 | PAPP-A | Tertiles | Tn –ve | Mantel–Haenszel test | p = 0.619 |
| Kavsak 2009103 | PAPP-A | Tertiles | None | Log-rank test | p = 0.05 |
| Laterza 2004105 | PAPP-A | 0.22 mlU/l | None | Univariate RR | 4.7 (95% CI 2.2 to 9.8) |
| Lund 2003107 | PAPP-A | 2.9 mlU/l | Tn –ve | Univariate RR | 2.3 (95% CI 1.1 to 5.0) |
| McCann 2009111 | PAPP-A | 12.4 ng/m | None | Univariate OR | 1.1 (95% CI 0.6 to 2.2) |
| Apple 200787 | PIGF | 17 ng/m | None | Univariate RR | 0.8 (95% CI 0.4 to 1.6) |
| Markovic 2010109 | PIGF | 13.2 ng/l | None | AUROC | 0.713 (95% CI 0.615 to 0.799) |
| Body 201146 | P-selectin | Tertiles | Tn –ve | Mantel–Haenszel test | p = 0.006 |
| Menown 2003113 | P-selectin | 152 ng/m | TN –ve and CK-MB –ve | Univariate OR | 3.2 (95% CI 0.9 to 11.6) |
| Brown 200747 | ST2 | None: continuous | None | AUROC | 0.579 |
Some of the studies used multivariate analysis to adjust for known predictors of MACEs and determine whether or not the biomarkers predicted a MACE when other variables were taken into account. These are shown in Table 23. If troponin was included as a covariate then this analysis could potentially show whether the biomarker provided additional prognostic information to troponin. The findings showed some evidence that BNP, NT-pro-BNP, MPO and H-FABP can provide prognostic value when other predictor variables are taken into account, whereas results for CRP and myoglobin were mixed.
| Study | Biomarker | Threshold | Biomarker selection | Analysis | Finding |
|---|---|---|---|---|---|
| Brugger-Anderson 200893 | BNP | Quartiles | None | Multivariate HR (with Tn) | Q1: Reference |
| Q2: 0.9 (95% CI 0.5 to 1.6) | |||||
| Q3: 1.7 (95% CI 0.9 to 3.0) | |||||
| Q4: 2.3 (95% CI 1.3 to 4.2) | |||||
| Ponitz 2009116 | BNP | Quartiles (highest vs 1–3) | Tn –ve | Multivariate HR | 1.5 (95% CI 1.1 to 2.0) |
| Apple 200787 | CD40 ligand | 1.081 ng/l | None | Multivariate RR | 1.4 (95% CI 0.6 to 3.2) |
| Apple 200787 | CRP | 3 mg/l | None | Multivariate RR | 0.8 (95% CI 0.4 to 1.9) |
| Brennan 200391 | CRP | Quartiles | Tn –ve | Multivariate OR | Q1: Reference |
| Q2: 1.6 (95% CI 0.9 to 2.7) | |||||
| Q3: 0.9 (95% CI 0.5 to 1.7) | |||||
| Q4: 1.0 (95% CI 0.6 to 1.9) | |||||
| Brugger-Anderson 200893 | CRP | Quartiles | None | Multivariate HR (with Tn) | Q1: Reference |
| Q2: 1.1 (95% CI 0.7 to 1.8) | |||||
| Q3: 1.1 (95% CI 0.7 to 1.8) | |||||
| Q4: 1.3 (95% CI 0.8 to 2.0) | |||||
| Eggers 200885 | CRP | 3.7 mg/l | None | Multivariate OR (with Tn) | Non-significant |
| Eggers 200897 | CRP | Not stated | None | Multivariate OR (with Tn) | 1.2 (95% CI 0.9 to 1.7) |
| Lund 2003107 | CRP | 2.0 mg/l | Tn –ve | Multivariate RR | 4.6 (95% CI 1.8 to 11.8) |
| Mockel 200886 | CRP | 10 mg/l | None | Multivariate OR (with Tn) | Non-significant |
| Ponitz 2009116 | CRP | Quartiles | Tn –ve | Multivariate HR | Not significant |
| Potsch 200651 | CRP | 1 mg/l | None | Multivariate OR | 2.2 (1.1 to 4.5) |
| Eggers 200897 | Cystatin-C | Not stated | None | Multivariate OR (with Tn) | 2.7 (95% CI 0.7 to 10.4) |
| Apple 200787 | eGFR | 60 ml/minute | None | Multivariate RR | 0.8 (95% CI 0.4 to 1.7) |
| Eggers 200897 | GRF-15 | Not stated | None | Multivariate OR (with Tn) | 2.7 (95% CI 1.0 to 6.0) |
| Garcia-Valdecasas 201149 | H-FABP | 6.2 ng/m | None | Multivariate HR (with Tn) | 2.5 (95% CI 1.3 to 4.8) |
| Ilva 200950 | H-FABP | 10.4 µg/l | None | Multivariate OR (with Tn) | Non-significant |
| McCann 2009111 | H-FABP | 5 ng/m | None | Multivariate OR | 2.7 (95% CI 1.1 to 6.4) |
| Viswanathan 2010122 | H-FABP | Quartiles | Tn –ve | Multivariate HR | Q1: Reference |
| Q2: 1.5 (95% CI 0.7 to 3.4) | |||||
| Q3: 3.1 (95% CI 1.1 to 8.8) | |||||
| Q4: 16.7 (95% CI 2.2 to 127.1) | |||||
| Yamashita 2010122 | H-FABP | None: continuous | None | Multivariate OR (with Tn) | 1.001 (95% CI 0.998 to 1.003) |
| Consuegra-Sanchez 200895 | IMA | 93.3 U/ml | None | Multivariate HR (with Tn) | 1.04 (95% CI 1.01 to 1.07) |
| Apple 200787 | MMP9 | 233.7 µg/l | None | Multivariate RR | 1.6 (95% CI 0.6 to 4.6) |
| Apple 200787 | MPO | 125.6 µg/l | None | Multivariate RR | 1.7 (95% CI 0.8 to 3.7) |
| Apple 201188 | MPO | 633 pmol/l | None | Multivariate HR | 2.4 (95% CI 1.3 to 4.6) |
| Brennan 200391 | MPO | Quartiles | Tn –ve | Multivariate OR | Q1: Reference |
| Q2: 1.9 (95% CI 1.0 to 3.8) | |||||
| Q3: 4.4 (95% CI 2.3 to 8.4) | |||||
| Q4: 3.9 (95% CI 2.0 to 7.7) | |||||
| Jaffery 2008101 | Myoglobin | 200 ng/m | None | Multivariate HR (with Tn) | 1.60 (95% CI 1.21 to 2.11) |
| Kontos 2007104 | Myoglobin | 90 ng/m | None | Multivariate OR (with Tn) | 2.8 (95% CI 2.1 to 3.7) |
| Sonel 2000117 | Myoglobin | 100 µg/m | None | Multivariate OR (with Tn) | Non-significant |
| Svensson 2004118 | Myoglobin | 50 ng/m | None | Multivariate OR (with Tn) | Non-significant |
| Van Domberg 2000120 | Myoglobin | 64 µg/ml (women), 76 µg/ml (men) | None | Multivariate OR (with Tn) | 2.2 (95% CI 0.7 to 6.7) |
| Mockel 200886 | NT-proBNP | 145 ng/m | None | Multivariate OR (with Tn) | 2.6 (95% CI 1.2 to 5.7) |
| Apple 200787 | NT-pro-BNP | < 75 years, 125 ng/l; ≥ 75 years, 450 ng/l | None | Multivariate RR | 2.4 (95% CI 0.9 to 6.3) |
| Eggers 200885 | NT-pro-BNP | 550 ng/l | None | Multivariate OR (with Tn) | 2.7 (95% CI 1.0 to 7.3) |
| Eggers 200897 | NT-pro-BNP | Not stated | None | Multivariate OR (with Tn) | 1.0 (95% CI 0.7 to 1.5) |
| Jernberg 2002102 | NT-pro-BNP | Quartiles | None | Multivariate RR (with Tn) | Q1: Reference |
| Q2: 1.8 (95% CI 0.7 to 5.1) | |||||
| Q3: 3.0 (95% CI 1.1 to 7.8) | |||||
| Q4: 5.4 (95% CI 2.0 to 14.4) | |||||
| McCann 2009111 | NT-pro-BNP | 1371 ng/l | None | Multivariate OR | 2.7 (95% CI 1.4 to 5.2) |
| Yamashita 2010122 | NT-pro-BNP | None: continuous | None | Multivariate OR (with Tn) | 1.0 (95% CI 1.0 to 1.0) |
| Kavsak 2009103 | PAPP-A | Tertiles | None | Multivariate HR (with Tn) | T1: Reference |
| T2: 1.8 (95% CI 0.8 to 4.1) | |||||
| T3: 2.1 (95% CI 1.0 to 4.6) | |||||
| Lund 2003107 | PAPP-A | 2.9 mlU/l | Tn –ve | Multivariate RR | 2.6 (95% CI 1.1 to 6.5) |
| Apple 200787 | PIGF | 17 ng/m | None | Multivariate RR | 0.7 (95% CI 0.3 to 1.5) |
| Markovic 2010109 | PIGF | 13.2 ng/l | None | Multivariate HR | 2.1 (95% CI 1.1 to 4.2) |
| Body 201146 | P-selectin | 60µg/l | None | Multivariate OR (with Tn) | 1.8 (95% CI 1.1 to 3.1) |
| Menown 2003113 | P-selectin | 152 ng/m | TN –ve and CK-MB –ve | Multivariate OR | 4.0 (95% CI 1.0 to 15.7) |
Table 24 shows whether or not the biomarker predicts MACEs in troponin-negative patients. This is probably the most useful analysis because troponin measurement is likely to be routine practice in most settings. Unfortunately, only a few studies reported this analysis so it is difficult to draw conclusions. However, there is some evidence that CRP, PAPP-A and H-FABP can predict MACEs in troponin-negative patients.
| Study | Biomarker | Threshold | Biomarker positive | Biomarker negative | RR |
|---|---|---|---|---|---|
| Ponitz 2009116 | BNP | Quartiles | NR | NR | NRa |
| Bholasingh 200389 | CRP | > 0.3 mg/dl | 8/135 | 3/236 | 4.7 (95% CI 1.3 to 17.3) |
| Brennan 200391 | CRP | Quartiles | NR | NR | NRb |
| Menown 2003113 | CRP | Quartiles | NR | NR | NRc |
| Ponitz 2009116 | CRP | Quartiles | NR | NR | NRa |
| Eggers 200897 | GRF-15 | 1200 ng/l | 8/201 | 1/117 | 4.7 (95% CI 0.6 to 36.8) |
| 1800 ng/l | 8/104 | 1/204 | 15.6 (95% CI 2.0 to 124) | ||
| Ilva 200950 | H-FABP | 10.4 µg/l | 6/28 | 11/159 | 3.1 (95% CI 1.2 to 7.7) |
| Viswanathan 2010121 | H-FABP | 6.48 µg/l | 10/35 | 30/721 | 6.9 (95% CI 3.7 to 12.9) |
| Menown 2003113 | Interleukin 6 | Quartiles | NR | NR | NRc |
| Collinson 200648 | IMA | 85 kU/l | 11/279 | 2/139 | 2.7 (95% CI 0.6 to 12.2) |
| Apple 200787 | MPO | 125.6 µg/l | 9/240 | 6/150 | 0.9 (95% CI 0.3 to 2.6) |
| Apple 201188 | MPO | 633 pmol/l | Unable to extract | Unable to extract | Unable to extractd |
| Brennan 200391 | MPO | Quartiles | NR | NR | NRb |
| Apple 200787 | NT-pro-BNP | < 75 years, 125 ng/l; ≥ 75 years, 450 ng/l | 13/245 | 2/142 | 3.8 (95% CI 0.9 to 16.5) |
| Lund 2003107 | PAPP-A | 2.9 mlU/l | 20/61 | 6/75 | 4.1 (95% CI 1.8 to 9.6) |
Summary of the findings of the prognostic biomarker review
A variety of different biomarkers have been studied and an association shown between increased levels and risk of MACEs, but it is not clear in most cases whether or not this adds useful prognostic information beyond that available from clinical assessment, ECG and troponin. There is some evidence that BNP, NT-pro-BNP, MPO and H-FABP can provide additional prognostic value beyond troponin, whereas CRP, PAPP-A and H-FABP can predict MACEs in troponin-negative patients. However, these findings are based on a small number of heterogeneous studies and the utility of this prognostic value is unclear.
Studies included in the computed tomographic coronary angiography and exercise electrocardiography review
Overall, the literature searches identified 2667 citations. A flow chart describing the process of identifying relevant literature is shown in Figure 26. Of the titles and abstracts screened, 173 relevant full papers were retrieved and assessed in detail. A total of 29 papers evaluating the diagnostic accuracy or prognostic performance of CTCA or exercise ECG met the inclusion criteria. Studies excluded from the review are listed in Appendix 4. The principal reasons for exclusion were that the population was not suspected ACS and the reference standard was not coronary angiography. The included studies consisted of eight diagnostic studies of CTCA,123–130 seven prognostic studies of CTCA,131–137 no diagnostic studies of exercise ECG and 13 prognostic studies of exercise ECG. 138–150 We also identified a prognostic study of CT CAC scoring without angiography. 151 Two of the prognostic studies of CTCA reported different follow-up for the same cohort,132,133 and two of the prognostic studies of exercise ECG reported some patients in common. 138,140 The lack of any diagnostic studies comparing exercise ECG with ICA is not surprising, as exercise ECG only started to be used in patients presenting to hospital with acute pain many years after its diagnostic accuracy for CAD had been evaluated in patients with stable chest pain.
FIGURE 26.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart CTCA and ETT review. Ex ECG, exercise ECG.
Diagnostic studies of computed tomographic coronary angiography
Table 25 shows the characteristics of the diagnostic studies that compared CTCA with a reference standard of ICA for CAD. The studies were relatively small (n = 31 to 113). Mean age varied from 53 to 62 years, and men outnumbered women in all studies. Most studies explicitly excluded patients with diagnostic ECG changes. The threshold for diagnosing obstructive CAD was 50% stenosis in all studies, except for the study of Sato et al. ,129 which used a threshold of 75% for both tests.
| Paper | Technology | Beta-blocker | n | Mean age (years) and sex | Inclusion criteria | Exclusion criteriaa | CTCA diagnostic criteria | ICA diagnostic criteria |
|---|---|---|---|---|---|---|---|---|
| Casciani 2008123 | 16 slice | Ye s | 37 | 62; 29/37 (78%) male | Chest pain compatible with myocardial ischaemia | Diagnostic ECG changes, elevated biomarkers | > 50% stenosis | > 50% stenosis |
| Coles 2007124 | 16 slice | Ye s | 113 | 62; 78/113 (65%) male | Acute chest pain suggesting ACS within 24 hours | STEMI, haemodynamically unstable | ≥ 50% stenosis | ≥ 50% stenosis |
| Ghersin 2006125 | 16 slice | No | 66 | 57; 52/66 (79%) male | Acute chest pain | Arrhythmia | ≥ 50% stenosis | ≥ 50% stenosis |
| Henneman 2008126 | 64 slice | Ye s | 40 | 57; 26/40 (65%) male | Suspected ACS | STEMI | 50% stenosis + calciumscore | ≥ 50%stenosis |
| Minocha 2006127 | 16 slice | Unclear | 70 | 53; 63/70 (90%) male | Acute chest pain | Definite ACS, previous CABG | > 50% stenosis | > 50% stenosis |
| Olivetti 2006128 | 16 slice | Ye s | 31 | 59; 19/31 (61%) male | Medium/low-risk chest pain within 24 hours | Arrhythmia, previous cardiac disease | > 50% stenosis | > 50% stenosis |
| Sato 2005129 | 4 slice | Ye s | 34 | 56; 29/31 (93%) male | > 30 minutes of chest pain within 24 hours | Diagnostic ECG changes, elevated biomarkers | ≥ 75% stenosis | ≥ 75% stenosis |
| Tsai 2007130 | Unclear | Yes | 78 | 61; 55/78 (71%) male | Low-risk suspected ACS | Prolonged symptoms, age > 70 years, diagnostic ECG changes, positive biomarkers, previous MI or CABG | ≥ 50% stenosis | ≥ 50% stenosis |
Figure 27 shows the quality assessment and Figure 28 the methodological quality summary of diagnostic studies of CTCA. Study quality was generally high, although blinding of interpretation of the index or reference standard test was unclear or absent in around half of the studies.
FIGURE 27.
Quality assessment of diagnostic studies of CTCA.
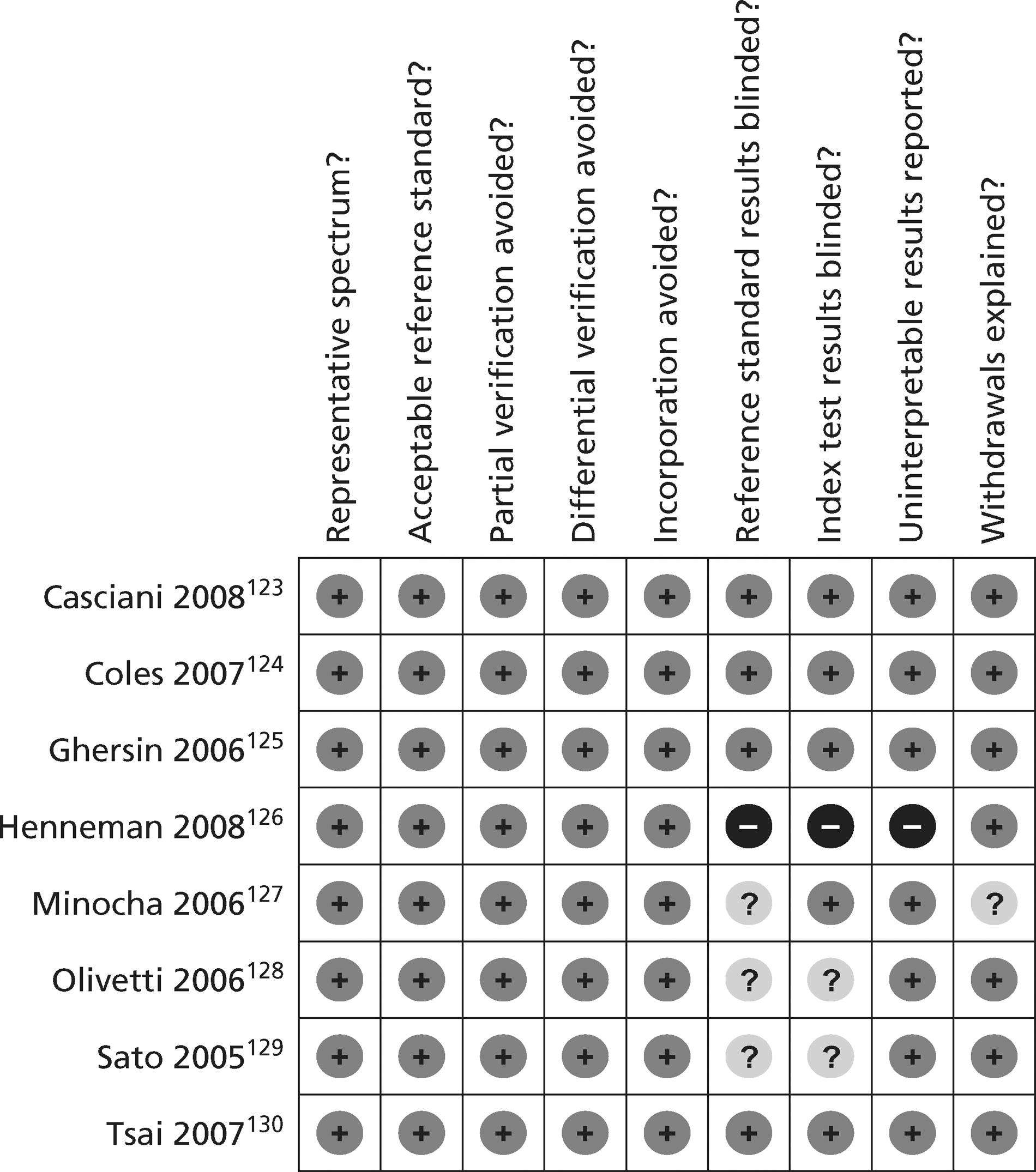
FIGURE 28.
Methodological quality summary of diagnostic studies of CTCA.
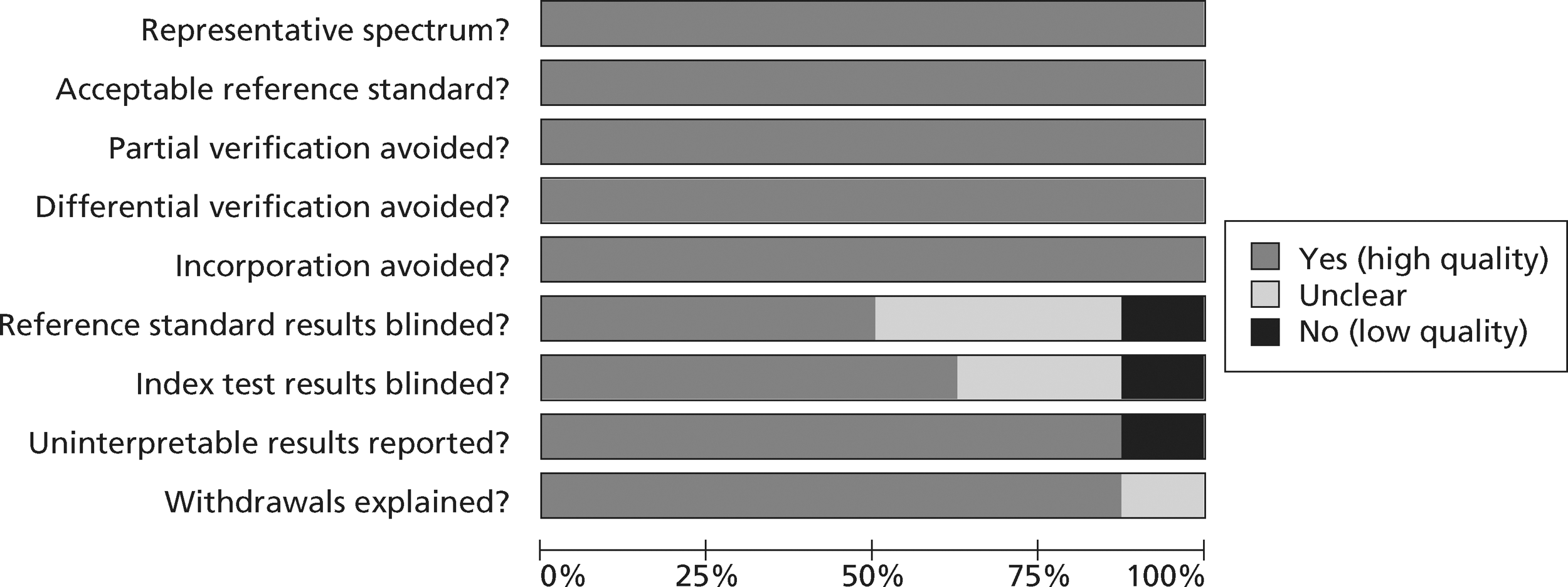
Figure 29 shows the result of meta-analysis of CTCA diagnostic studies. The summary estimates of sensitivity and specificity were 93% (95% predictive interval 61% to 99%) and 87% (16% to 100%), respectively. The highest sensitivity and specificity was achieved in the only study of 64-slice C T. 126 Two studies124,125 reported markedly lower specificity. The variation in specificity may be explained by artifact due to calcification, movement or heart rate, which may be more common or more variable in patients presenting with acute symptoms.
FIGURE 29.
Meta-analysis of CTCA diagnostic studies.
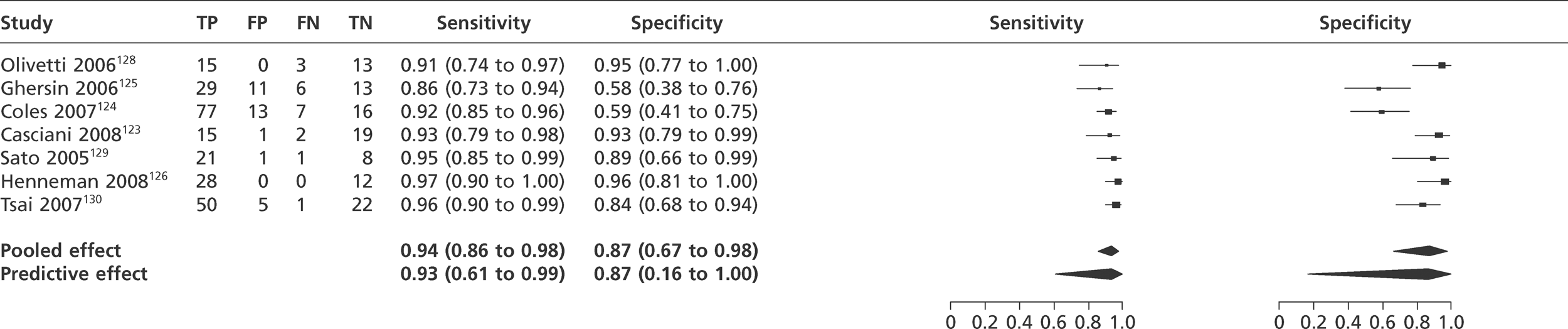
Prognostic studies of computed tomographic coronary angiography
Table 26 shows the characteristics of the seven prognostic studies of CTCA and one study of CAC scoring. Three of the cohorts (four studies) were compared with control groups in a trial,131–134 whereas the others were single cohort studies. All of the CTCA studies used 64-slice CT. The cohorts were generally larger (n = 30–588) and the mean age (46–56 years) younger than the diagnostic studies. This reflects the inclusion criteria that generally selected low- to intermediate-risk patients. Those with ECG changes and positive biomarkers were usually explicitly excluded. The diagnostic classification for CTCA either dichotomised scans into obstructive (> 50% stenosis) or non-obstructive (< 50%), or limited positive scans to those with stenosis > 70% and used an intermediate category for stenosis of 26–69% or 50–70%. Duration of follow-up ranged from 30 days to 2 years. Definitions of MACEs varied, with most studies including revascularisation in the definition but two limiting MACEs to death and MI,133 or death, MI and unstable angina. 131 Most cases of MACEs were revascularisation rather than death or MI.
| Paper | Technology | Beta-blocker | n a | Age (years) and sex | Inclusion criteria | Exclusion criteriab | Diagnostic criteria for CTCA | Duration of follow-up | MACE |
|---|---|---|---|---|---|---|---|---|---|
| Goldstein 2007131 | 64-slice CTCA | Yes | 99 | 48, 42/99 (43%) male | Low-risk chest pain within 12 hours | Diagnostic ECG changes, known CAD, positive biomarkers | > 70% = positive | 6 months | Death, MI, revascularisation |
| < 26% = negative | |||||||||
| 26–70% = inter | |||||||||
| Hollander 2009132 | 64-slice CTCA | Yes | 588 | 46, 193/588 (40%) male | Low-risk chest pain | Comorbidity, known cardiac disease, cocaine use | ≥70% = positive | 1 year | Cardiovascular death, MI |
| < 50% = negative | |||||||||
| 50–69% = inter | |||||||||
| Hollander 2009133 | 64-slice CTCA | Yes | 568 | 47, 252/568 (44%) male | Low-risk chest pain | Comorbidity, known cardiac disease, cocaine use | ≥ 70% = positive | 30 days | Cardiac death, MI, revascularisation, stroke and hospitalisation for angina |
| < 50% = negative | |||||||||
| 50–69% = inter | |||||||||
| Laudon 2010151 | CTCAC scoring | No | 263 | 48, 159/263 (60%) male | Low-/intermediate-risk chest pain | Ischaemic ECG, elevated biomarkers,haemodynamically unstable | Calcium score>0 | 30 days, 1 + 5 years | MI, PCI, CABG, death |
| Miller 2011134 | 64-slice CTCA | Yes | 30 | 51, 13/30 (43%) male | Low-/intermediate-risk chest pain within 12 hours | Diagnostic ECG changes, positive biomarkers, needing hospital admission | Normal, non-obstructive, obstructive | 90 days | MI, death, unplanned revascularisation |
| Rubinshtein 2007135 | 64-slice CTCA | Yes | 58 | 56, 21/58 (64%) male | Intermediate-risk suspected ACS | Diagnostic ECG changes, positive biomarkers, comorbidities | ≥ 50% stenosis | 15 months | Death, MI, PCI, CABG |
| Schlett 2011136 | 64-slice CTCA | Yes | 368 | 53, 226/368 (61%) male | > 5 minutes of chest pain within 24 hours | Abnormal ECG, elevated troponin | > 50% stenosis | 2 years | Not stated |
| Shuman 2010137 | 64-slice CTCA | Yes | 81 | 55, 49/81 (60%) male | Low-/moderate-risk chest pain | Diagnostic ECG changes, positive biomarkers, TIM score of >4, known cardiac disease | ≥ 50% stenosis | 3, 6, 12 months | Cardiac death, MI, revascularisation |
Table 27 shows the quality assessment of the CTCA and CAC scoring prognostic studies. All the studies described patient characteristics in terms of age and sex, but the description of times to presentation was inconsistent. All but one study134 defined MACEs in their methods section. In all studies the identification and definition of MACEs was independent of the index test and, in accordance with the inclusion criteria, MACEs were reported for at least 80% of the cohort. However, only one study136 used multivariate analysis to determine if CTCA provided additional prognostic value beyond routine assessment with ECG and biomarkers.
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 |
|---|---|---|---|---|---|---|---|
| Goldstein 2007131 | Y | Y | Y | Y | Y | N | N |
| Hollander 2009132 | N | Y | Y | Y | Y | N | N |
| Hollander 2009133 | N | Y | Y | Y | Y | N | N |
| Laudon 2010151 | N | Y | Y | Y | Y | N | N |
| Miller 2011134 | Y | Y | N | Y | Y | N | N |
| Rubinshtein 2007135 | N | Y | Y | Y | Y | N | N |
| Schlett 2011136 | Y | Y | Y | Y | Y | Y | Y |
| Shuman 2010137 | N | Y | Y | Y | Y | N | N |
Table 28 summarises the results of the prognostic studies of CTCA. It was not always clear whether patients with positive CTCA had been followed up and whether there had been any events in these patients. MACE rates were generally very low in patients with a negative CTCA. The only adverse event in a patient with negative CTCA was a death in the long-term follow-up cohort of Hollander et al. 132 However, these low event rates may reflect selection of low-risk patients rather than accurate risk stratification by CTCA. Most of the events reported in patients with positive CTCA findings were process events [i.e. percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG)], which in an unblinded study may simply reflect physicians acting upon CTCA findings. No patient with positive or intermediate CTCA died on follow-up. There were 2 out of 43 and 12 out of 185 non-fatal MIs among those patients with positive or intermediate CTCA in the cohorts of Rubinshtein135 and Schlett. 136 The cohorts of Miller134 (n = 18), Goldstein131 (n = 32) and Hollander133 (n = 54, follow-up to 30 days) reported no cases of death or non-fatal MIs among those patients with positive CTCA. It could be argued that the process outcomes (PCI and CABG) prevented subsequent death or non-fatal MI in those with positive CTCA, but this is difficult to determine.
| Paper | Positive CTCAa | Intermediate CTCAa | Negative CTCAa |
|---|---|---|---|
| Goldstein 2007131 | 0/8 | 0/24 | 0/67 |
| Hollander 2009132 | NR | NR | 1/481 (death) |
| Hollander 2009133 | 0/13 | 0/41 | 0/508 |
| Shuman 2010137 | NR | NR | 0/70 |
| Rubinshtein 2007135 | 13/23 (two MI, eight PCI, three CABG) | 1/20 (PCI) | 0/15 |
| Miller 2011134 | 0/18 | – | 0/10 |
| Schlett 2011136 | 20/68b | 5/117b | 0/183 |
In the study of Schlett et al. 136 patients and carers were blind to CTCA findings, so any association between CTCA findings and process events (PCI and CABG) was not simply due to physicians acting on CTCA findings. Schlett et al. found that CTCA predicted MACEs, even after adjustment using a clinical risk score incorporating ECG and troponin measurement. Thus, this study provides the best evidence that CTCA provides independent prognostic value beyond routine clinical assessment.
The study of CT CAC scoring151 reported that 9 out of 91 patients with a CAC score of > 0 had MACEs (two MI and nine PCI), compared with 0 out of 82 with a CAC score = 0.
The results of meta-analysis of CTCA prognostic studies are shown in Figure 30 (positive and intermediate vs negative) and Figure 31 (positive vs intermediate and negative). Only studies that definitely reported data from patients with positive and negative CTCA are included in this analysis. Meta-analysis of the five studies with analysable data showed a RR for MACEs of 3.1 (95% CrI 0.3 to 18.7) for positive and intermediate scans compared with negative scan and 5.8 CrI (95% Crl 0.6 to 24.5) for positive scan compared with intermediate or negative scans. These estimates are subject to considerable uncertainty, with the CrI including one (i.e. no association) for both estimates. Taken alongside the limitations relating to patient selection and process outcomes suggests that there is currently only weak evidence that CTCA provides prognostically useful information in patients with suspected ACS.
FIGURE 30.
Meta-analysis of CTCA prognostic studies: positive and intermediate vs negative.
FIGURE 31.
Meta-analysis of CTCA prognostic studies: positive vs intermediate and negative.
Prognostic studies of exercise electrocardiography
Table 29 shows the characteristics of the prognostic studies of exercise ECG. Sample sizes ranged from 28 to 1000. The mean age (30–60 years) was relatively young, reflecting the selection of low-risk patients in many of the cohorts. Follow-up ranged from 30 days to > 12 months. There was no consistency in the definitions and reporting of MACEs, with some studies reporting composite outcomes only and others reporting outcomes separately with no indication of whether or not some patients had suffered multiple different adverse outcomes.
| Paper | n tested | n follow-up | Mean age (years) | Sex | Inclusions | Exclusions | Duration of follow-up | MACE |
|---|---|---|---|---|---|---|---|---|
| Amsterdam 2002138 | 1000 | 1000 | 50 | 520/1000 male | Resting ECG that was normal, only minor ST-T changes | ECG ischaemia or infarction | 30 days | Revascularisation, death |
| De Filippi 2001139 | 125 | 110 | 48 | 59/125 | Low probability (≤7%) of acute MI (Goldman et al.,152 low risk), ability to exercise, no prior history of CAD | ECG ischaemia | Median 374 days | Revascularisation, death, MI |
| Diercks 2000140 | 958 | 742 | 43 | 522/958 male | Non-diagnostic ECG for MI and ischaemia | ECG MI | 12 months | Revascularisation, shock, cardiac death, MI, HF, life-threatening arrhythmia |
| Gomez 1996141 | 50 | 50 | 50 | 31/50 male | Low risk | > 7% probability of having an AMI (Goldman et al.,152 high risk), ECG ischaemia, arrhythmia, HF, high blood pressure | 30 days | Death, MI |
| Goodacre 2005142 | 422 | 422 | 54 | 461/706 male | Acute chest pain, normal or non-diagnostic ECG, no prior history of CAD | Recent diagnostic testing for coronary heart disease, unable to exercise | 6 months | Revascularisation, MI, death |
| Jeetley 2006143 | 154 | 151 | 60 | 87/154 male | Normal or non-diagnostic ECG, and≥ 2 risk factors for CAD | ECG ischaemia, contraindications to perform exercise testing | 8.5 months | Revascularisation, MI, death |
| Kerns 1993144 | 32 | 32 | 35 | 20/32 male | Atypical chest pain, normal ECG, low-risk CAD | Moderate suspicion of AMI or ischaemic heart disease, high risk, history of CAD, physical limitations precluding performance of ETT | 6 months | MI, death |
| Kirk 1998145 | 212 | 200 | 49 | 121/212 male | Low-risk patients (based on electrocardiographic and clinical findings) | ECG suggestive of MI or ischaemia, unable to perform a treadmill test | 30 days | Revascularisation |
| Lewis 1994146 | 93 | 82 | 50 | 48/93 male | Low-risk patients (based on electrocardiographic and clinical findings). | ECGs diagnostic of infarction or ischaemia | 6–37 months | MI |
| Polananczyk 1998147 | 276 | 276 | 59 | 130/276 male | Lower risk for major cardiac events | High risk (Goldman et al). ECG ischaemia | 6 months | PTCA, CABG or MI |
| Ramakrishna 2005148 | 125 | 125 | 55 | 71/125 male | Intermediate-risk profile for cardiovascular events | ECG ischaemia | 6 months | MI or HF |
| Sarullo 2000149 | 190 | 190 | 57 | 127/190 male | Low-risk patients (Pryor monogram) | ECG diagnostic of infarction or ischaemia | Minimum 12 months | Cardiac death, MI, PTCA, CABG |
| Tsakonis 1991150 | 28 | 28 | 45 | 23/28 male | Unstable CAD. No prior cardiac history, normal or near normal ECG, able to exercise on a treadmill | Prior cardiac history, ECG ischaemia | 1–12 months | Cardiac events |
Table 30 shows the quality assessment of the exercise ECG studies. The population age and sex were always well described but most studies did not clearly define their inclusion criteria. MACEs were defined in the methods section in all but one study and was defined and identified independent to the index test in all studies. No study undertook multivariate analysis to determine the independent prognostic value of exercise ECG.
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 |
|---|---|---|---|---|---|---|---|
| Amsterdam 2002138 | N | Y | Y | Y | Y | N | N |
| De Filippi 2001139 | Y | Y | Y | Y | Y | N | N |
| Diercks 2000140 | N | Y | Y | Y | Ya | N | N |
| Gomez 1996141 | N | Y | Y | Y | Y | N | N |
| Goodacre 2005142 | Y | Y | Y | Y | Y | N | N |
| Jeetley 2006143 | N | Y | Y | Y | Y | N | N |
| Kerns 1993144 | N | Y | Y | Y | Y | N | N |
| Kirk 1998145 | N | Y | Y | Y | Y | N | N |
| Lewis 1994146 | N | Y | Y | Y | Y | N | N |
| Polanczyk 1998147 | N | Y | Y | Y | Y | N | N |
| Ramakrishna 2005148 | N | Y | Y | Y | Y | N | N |
| Sarullo 2000149 | N | Y | Y | Y | Y | N | N |
| Tsakonis 1991150 | N | Y | N | Y | Y | N | N |
Table 31 shows the outcomes of the studies of exercise ECG. Most of the studies reported inconclusive results separately from positives and negatives but three studies142,147,148 reported them with positives, one with negatives,139 and it was unclear in one study whether or not there were any inconclusive results. 150 Overall, MACE rates varied between the studies, reflecting variation in patient selection criteria and the definition of MACEs. Rates were generally low among patients with negative ETT results and there was some evidence that positive tests identified higher-risk patients. However, higher rates of revascularisation among patients with positive ETT may reflect physician awareness and expectation of a need for revascularisation. There was evidence from some studies that death and MI rates were higher among patients with positive ETT, although the modest numbers limit the conclusions that may be drawn.
| Paper | Outcomes of interest | Positive ETT | Inconclusive ETT | Negative ETT |
|---|---|---|---|---|
| Amsterdam 2002138 | Revascularisation | 12/114 | 7/192 | 0/582 |
| Death | 4/114 | 0/192 | 1/582 | |
| De Filippi 2001139 | Revascularisation, death, MI | 5/9 | Reported with negatives | 1/110 |
| Diercks 2000140 | Revascularisation, cardioshock, cardiac death, MI, HF, LTA | 7/19 | 9/267 | 5/456 |
| Gomez 1996141 | Death, MI | 0/2 | 0/1 | 0/41 |
| Goodacre 2005142 | Revascularisation, MI, LTA, death | 9/37 | Reported with positives | 4/385 |
| MI, LTA, death only | 2/37 | 3/385 | ||
| Jeetley 2006143 | Revascularisation, MI, death MI | 9/27 | 11/79 | 0/39 |
| Death/MI | 1/27 | 2/79 | 2/39 | |
| Kerns 1993144 | MI, death | 0 | 0 | 0/32 |
| Kirk 1998145 | Revascularisation | 6/28 | 0/55 | 0/118 |
| Lewis 1994146 | MI | 1/12 | 0/22 | 0/59 |
| Polanczyk 1998147 | PTCA, CABG or MI | 12/81 | Reported with positives | 4/195 |
| Ramakrishna 2005148 | MI or HF | 3/37 | Reported with positives | 0/88 |
| Sarullo 2000149 | Cardiac death | 0/57 | 0/22 | 0/111 |
| MI | 1/57 | 0/22 | 0/111 | |
| PTCA | 29/57 | 0/22 | 0/111 | |
| CABG | 15/57 | 0/22 | 0/111 | |
| Tsakonis 1991150 | Cardiac events | 0/4 | 0/19 |
The results of meta-analysis of prognostic studies of exercise ECG are shown in Figure 32 (positive and inconclusive vs negative) and Figure 33 (positive vs inconclusive and negative). Meta-analysis showed a RR for MACEs of 8.4 (95% CrI 3.1 to 17.3) for positive and inconclusive compared with negative and 8.0 (95% Crl 2.3 to 22.7) for positive compared with inconclusive or negative test. The CrIs around these estimates were relatively wide but did not include one (i.e. no association). We therefore identified evidence that exercise ECG predicts MACEs in patients with suspected ACS, although this finding may be limited by the inclusion of process outcomes (revascularisation procedures) in the definition of MACEs in some studies.
FIGURE 32.
Meta-analysis of prognostic studies of exercise ECG: positive and inconclusive vs negative.
FIGURE 33.
Meta-analysis of prognostic studies of exercise ECG: positive vs inconclusive and negative.
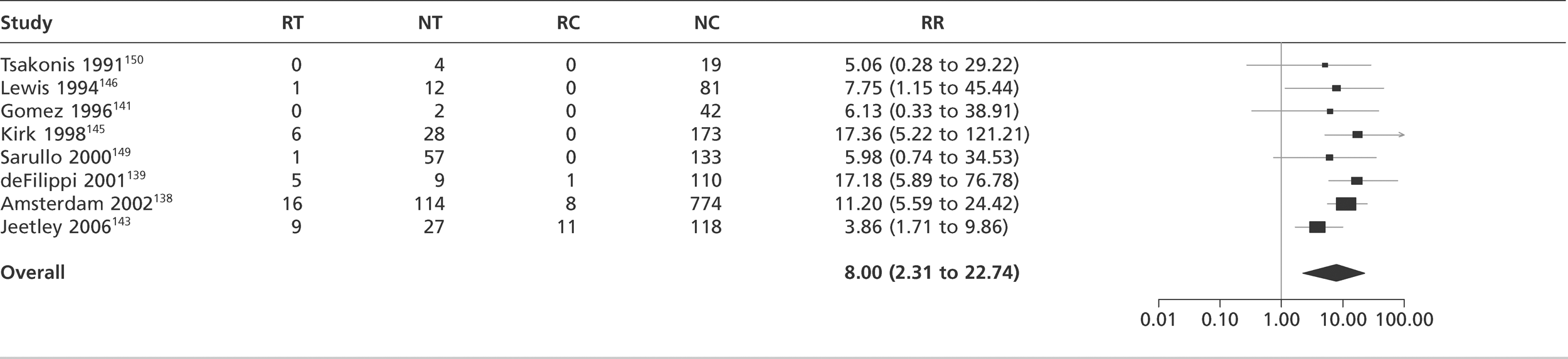
Chapter 4 Assessment of cost-effectiveness evidence
This section details the methods and results of our health economic model, constructed to compare investigation strategies for patients with suspected ACS. We developed a decision-analysis model to evaluate the cost-effectiveness of using (1) early biomarker strategies to diagnose MI before a 10- to 12-hour troponin assay and (2) biomarkers, CTCA or ETT to risk-stratify patients with a negative troponin. The model applied diagnostic strategies to a hypothetical cohort of patients with suspected ACS to determine the costs and outcomes associated with each strategy. The model involved two phases:
-
The diagnostic phase tested biomarker strategies for MI. Early biomarker strategies (involving troponin alone or in combination with sensitive early biomarkers) were compared with the most effective and expensive strategy of 10- to 12-hour troponin assays (specified in our model as being 10 hours) and the least effective and cheapest strategy of no testing or treatment. Early biomarkers were assumed to incur costs and miss cases due to suboptimal sensitivity compared with a 10-hour troponin test (thus worsening outcomes) but could save costs by reducing length of hospital stay.
-
The prognostic phase tested biomarkers and other investigations (CTCA and exercise ECG) that could stratify patients with a negative troponin for subsequent risk of MACEs. The potential benefit of additional biomarkers, CTCA or exercise ECG was assumed to relate to identifying which troponin-negative patients have a higher risk of MACEs, which could be reduced by investigation and intervention.
The diagnostic phase model
This section details the methods and results of our health economic model constructed to compare diagnostic strategies for identifying MI in patients with suspected ACS. We developed a decision-analysis model to estimate the costs and QALYs accrued by each potential management strategy for diagnosing patients with MI. A theoretical ‘zero option’ strategy of discharging all patients home without investigation was also included. The key aim was to determine the optimal diagnostic strategy in terms of cost-effectiveness. We also aimed to use the model to estimate the effect of different diagnostic strategies upon subsequent event rates.
Objectives
The objectives of the cost-effectiveness analysis were to:
-
estimate the cost-effectiveness of diagnostic strategies for ACS, in terms of the cost per QALY gained by each strategy compared with the next most effective
-
identify the optimal strategy for diagnosing ACS in the NHS, defined as the most cost-effective strategy at a willingness-to-pay threshold of £20,000–30,000 per QALY gained
-
estimate subsequent rates of death and non-fatal MI among the whole study population and among those with negative diagnostic tests according to the various diagnostic strategies
-
identify the critical areas of uncertainty in the diagnosis of ACS, where future research would produce the most benefit.
The costs and benefits of diagnostic management of suspected acute coronary syndrome
The main benefits of diagnostic management relate to rapid identification and treatment of patients with risk of MI and death. The direct costs of diagnostic management include the costs of investigation, hospital stay for diagnosis, and the subsequent costs of providing treatment, intensive care and reinfarction. The assumed gold standard for diagnosis, troponin measured 10 hours after worst symptoms is the most effective, but also the most expensive strategy because patients are admitted to hospital until results are available. Presentation biomarkers incur costs and may miss cases due to suboptimal sensitivity (thus worsening outcomes), but save costs by reducing length of hospital stay. We built a model to allow us to analyse the effect of different diagnostic management strategies on these costs and benefits.
The decision-analysis model structure
The different diagnostic strategies were applied to a hypothetical cohort of patients attending the ED with suspected, but not proven, ACS. We assumed that the diagnostic strategy would determine which patients had MI and that the probability of detecting an MI was determined by the sensitivity of the diagnostic strategy. We assumed that patients with detected MI would be managed promptly by treatment. The model assigned each patient a probability of reinfarction or death depending on their characteristics and whether or not they had treatment. Each patient then accrued lifetime QALYs and health-care costs according to their age, sex, reinfarction and treatment status. Costs were also accrued through measuring biomarkers, hospital stay for diagnosis, further investigation, treatment and/or reinfarction depending on the strategy and the patient characteristics. Details of each of these processes are outlined below.
Population
The population consisted of a hypothetical cohort of patients attending the ED with suspected but not proven ACS, i.e. a history compatible with ACS but no diagnostic ECG changes (ST deviation of > 1 mm or T-wave inversion > 3 mm), and who had no major comorbidities requiring inpatient treatment (such as HF or arrhythmia). We ran the diagnostic phase model separately for patients with and without a known history of CAD. Different characteristics were used for the populations with and without known CAD.
Each patient entering the model had the following characteristics defined: age, sex, MI present or not, time delay between onset of worst pain and arrival at hospital, and time of day. We estimated population characteristics using data from a large recent trial of point-of-care markers in patients with suspected but not proved MI, the RATPAC (Randomised Assessment of Treatment using Panel Assay of Cardiac markers) trial. 153Table 32 shows the population characteristics used in the model.
| Estimate | Distribution | |
|---|---|---|
| Population without known CAD | ||
| Mean age (SD), years | 53.0 (13.5) | SE = 0.30 |
| % male | 58.1% | n/N = 1138/1958 |
| MI prevalence | 7.0% | n/N = 137/1958 |
| Median (IQR) time delay (minutes) | 132 (80 to 255) | |
| Time of day | See Table 33 | |
| Population with known CAD | ||
| Mean age (SD), years | 65.5 (13.4) | SE = 0.82 |
| % male | 59.5% | n/N = 160/269 |
| MI prevalence | 7.8% | n/N = 21/248 |
| Median (IQR) time delay (minutes) | 101 (67 to 170) | |
| Time of day | See Table 33 | |
The arrival time of patients is an important factor when considering the optimal cost-effectiveness strategy because outside the ED medical staff may be available only at certain times of the day to make disposition decisions (e.g. ward rounds at specific times of the day). We analysed the arrival times of 2240 patients from the RATPAC trial153 to estimate the arrival distribution used in the model and the results are shown in Table 33. Patients in the RATPAC trial153 presented across six hospitals over a 15-month period, so the table is intended to demonstrate relative differences in arrival rates at different times of the day, rather than providing any meaningful estimate of absolute arrival rates at a particular hospital.
| Time period | No. of hours | Inter-arrival time in minutes | Arrival rate per hour | Arrivals in this period | Cumulative arrivals |
|---|---|---|---|---|---|
| 12 midnight to 7 am | 7 | 2 | 28 | 195 | 195 |
| 7 am to 9 am | 2 | 0.7 | 88 | 175 | 370 |
| 9 am to 2 pm | 5 | 0.3 | 212 | 1060 | 1430 |
| 2 pm to 6 pm | 4 | 0.5 | 118 | 470 | 1900 |
| 6 pm to 12midnight | 6 | 1 | 57 | 340 | 2240 |
The results are also shown in the form of a histogram in Figure 34. It can be seen that between midnight and 7 am, there are small numbers of patients. The patients arrive at a faster rate between 7 am and 9 am but between 9 am and 2 pm is the peak time, which sees the fastest arrival rate of patients. There is a steady decrease in the patient arrival rate between 2 pm and 6 pm and the finally, patients arrive in a constant slow stream between 6 pm and midnight.
FIGURE 34.
Histogram of the patient arrival data.
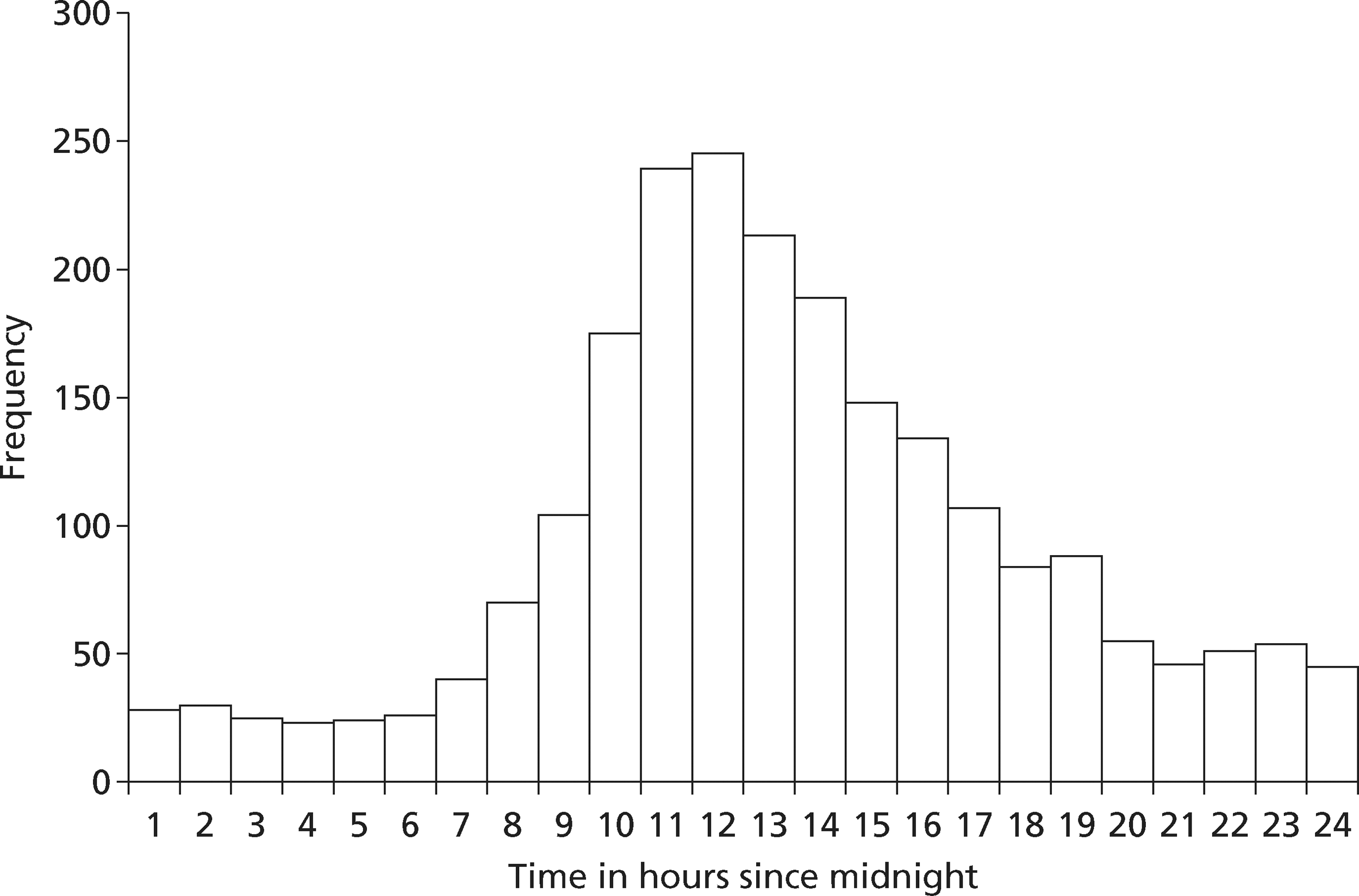
Selection of strategies
We tested several strategies to explore the trade-off between sensitivity and specificity. Each potential strategy was applied to each patient. The strategy determined:
-
what tests each patient received and when
-
how long each patient spent in hospital
-
what treatments each patient received.
The following strategies were tested in the main analysis:
-
Cheapest and least effective Discharge all patients home immediately without testing or treatment.
-
Most effective and expensive Measure troponin level after 10 hours has elapsed from the worst symptoms, admit to hospital and treat if troponin assay is positive, discharge home without treatment is troponin assay is negative.
-
Troponin testing on arrival Measure troponin level on arrival, manage according to strategy 2 if positive (i.e. measure troponin level again after 10 hours from worst symptoms), discharge home without treatment if negative. This strategy was tested using different initial troponin assays and thresholds for positivity.
In each strategy we assumed that there was a 2-hour delay from the time at which sampling could be performed to the time at which results became available and a decision made. If the results were available within 4 hours of patient presentation to hospital we assumed that the patient was still in the ED and a decision could be made immediately. If not, we assumed that they had moved to another location (a ward or clinical decision unit) and managed according to one of the three scenarios outlined below. We also assumed that there was a 1-hour delay between arrival at hospital and biomarker assessment commencing. This effectively meant that only decisions made on presentation biomarkers could be acted on in the ED.
With regard to patient management after the ED, we tested the model in three different scenarios:
-
The ‘doctor on demand’ scenario, in which medical staff were available 24 hours a day to make a disposition decision within 1 hour of the results being available.
-
The twice-daily ward round scenario, in which medical staff were only available at twice-daily ward rounds (9 am and 6 pm) to make disposition decisions.
-
The once-daily ward round scenario, in which medical staff were only available at one daily ward round (2 pm) to make disposition decisions.
We took this approach because it was possible that different strategies may have different levels of cost-effectiveness in different settings. For example, early discharge strategies may be less cost-effective if the LoS associated with delayed testing strategies is controlled by efficient patient review. Users of the results are thus able to decide which scenario best reflects their local practice.
We also undertook a secondary analysis that involved adding other biomarkers to troponin at presentation to determine whether adding an alternative biomarker was cost-effective compared with troponin alone at presentation or a 10-hour troponin test. This analysis was undertaken using data from primary studies that compared the sensitivity and specificity of troponin alone to troponin with the biomarker (with elevation of either biomarker being considered positive). We assumed that the additional biomarker would incur an additional cost, but otherwise the model would follow the main analysis. For each study the model compared the following strategies:
-
discharge without testing or treatment
-
presentation troponin alone
-
presentation troponin in combination with the other biomarkers
-
10-hour troponin test.
Diagnostic parameters of each strategy
Each strategy specified how the biomarker(s) should be interpreted and what decision would be made on the basis of each biomarker result. The options were:
-
MI ruled out: discharge with no further testing
-
MI ruled in: admit for MI treatment
-
MI uncertain: wait and repeat biomarker testing.
Option 1 relates to strategy sensitivity. Although the strategy may define MI as having been ruled out, the patient may actually have MI that is missed owing to suboptimal sensitivity.
We stipulated that option 2 could only be applied on the basis of a standard modern troponin assay result above the 99th percentile. We assumed that this provided definitive evidence of MI and that strategies would only recommend MI treatment on the basis of this evidence. Every strategy, (except no testing or treatment), therefore had to include troponin at some point to diagnose MI.
For option 3, the strategy defined when further testing was performed, what test would be performed and how this test would be interpreted. In most strategies the next test was a 10-hour troponin and in all strategies the MI uncertain option ended when a 10-hour troponin test was performed. We stipulated that the 10-hour troponin test would use a standard modern assay with the 99th percentile as the threshold for positivity, thus allowing MI to be definitively ruled in or ruled out.
Table 34 shows the estimates of sensitivity and specificity for MI for each strategy tested and the sources for these estimates. We selected meta-analysis data for TnT because the point estimates of sensitivity and specificity varied in the expected manner when different thresholds and assays were used, i.e. a lower threshold and/or high-sensitivity assay had higher sensitivity and lower specificity. This allowed us to explore the influence of varying the diagnostic threshold upon cost-effectiveness. The median values of the posterior distributions for sensitivity and specificity were used in the deterministic analysis.
| Strategy | Sensitivity (95% predictive interval) | Specificity (95% predictive interval) | Source |
|---|---|---|---|
| Discharge without testing or treatment | 0 | 1 | Theoretical |
| 10-hour troponin test | 1 | 1 | Theoretical |
| Presentation TnT using 10% CV threshold (0.03 µg/l) | 0.74 (0.35 to 0.94) | 0.96 (0.76 to 0.99) | Meta-analysis |
| Presentation TnT using 99th percentile threshold (0.01 µg/l) | 0.80 (0.30 to 0.97) | 0.91 (0.53 to 0.99) | Meta-analysis |
| Presentation HsTnT using 99th percentile threshold (0.014 µg/l) | 0.96 (0.27 to 1.00) | 0.72 (0.03 to 0.99) | Meta-analysis |
We also undertook two sensitivity analyses:
-
Replacing presentation HsTnT with the ADVIA Centaur Ultra troponin I assay. Our meta-analysis suggested that this assay has lower sensitivity and higher specificity than HsTnT, so this analysis tested whether or not findings were dependent on the high estimated sensitivity of TnT. The estimates for sensitivity and specificity for the ADVIA Centaur Ultra troponin I assay were 0.86 (95% predictive interval 0.26 to 0.99) and 0.89 (95% predictive interval 0.40 to 0.99), respectively.
-
Additional inclusion of a strategy using measurement of high-sensitivity TnI at presentation and 3 hours later. Recent analysis154 has suggested that this provides better sensitivity than presentation testing. We assumed that additional costs were incurred providing care until 3-hour results were available but that a doctor would be available on demand to act on the results. The estimates of sensitivity and specificity were 0.982 [95% confidence interval (CI) 0.959 to 0.994] and 0.904 (95% CI 0.884 to 0.922), respectively. 154
For the secondary analysis evaluating the cost-effectiveness of adding other biomarkers to troponin at presentation we used estimates of sensitivity and specificity from primary studies that compared the combination of the biomarker and troponin (i.e. test positive if either troponin or biomarker is positive) with troponin alone (see Chapter 3, Diagnostic studies of biomarkers in combination with troponin). We used primary studies to estimate the sensitivity and specificity of troponin alone rather than meta-analysis estimates because there was substantial heterogeneity between the studies with resulting heterogeneity in estimates of troponin sensitivity. Using estimates from the primary studies allowed us to evaluate the relative effect of adding another biomarker. Table 35 shows the sensitivity and specificity of troponin alone and the biomarker plus troponin combination for each analysis. These strategies were only tested in the population without known CAD in the twice-daily ward round scenario.
| Study | Combination | Troponin alone | Combination | ||
|---|---|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | ||
| Body 201157 | TnI or H-FABP | 0.42 (0.33 to 0.51) | 0.96 (0.94 to 0.97) | 0.82 (0.74 to 0.88) | 0.88 (0.83 to 0.88) |
| Haltern 201067 | TnT or H-FABP | 0.74 (0.66 to 0.74) | 1.00 (0.96 to 1.00) | 0.97 (0.86 to 0.99) | 0.65 (0.60 to 0.66) |
| McCann 200876 | TnT or H-FABP | 0.75 (0.69 to 0.81) | 0.94 (0.90 to 0.96) | 0.93 (0.89 to 0.96) | 0.93 (0.89 to 0.96) |
| Mion 200777 | TnI or H-FABP | 0.55 (0.39 to 0.70) | 0.98 (0.92 to 1.00) | 0.76 (0.60 to 0.87) | 0.93 (0.86 to 0.97) |
| Keller 201071 | TnT or copeptin | 0.62 (0.56 to 0.67) | 0.97 (0.96 to 0.98) | 0.88 (0.83 to 0.91) | 0.76 (0.73 to 0.79) |
| Reichlin 200980 | TnT or copeptin | 0.75 (0.65 to 0.83) | 0.94 (0.91 to 0.96) | 0.99 (0.92 to 1.00) | 0.77 (0.73 to 0.81) |
| Keller 201020 | TnT or myoglobin | 0.62 (0.56 to 0.67) | 0.97 (0.96 to 0.98) | 0.81 (0.76 to 0.85) | 0.85 (0.82 to 0.87) |
| Mion 200777 | TnI or myoglobin | 0.55 (0.39 to 0.70) | 0.98 (0.92 to 1.00) | 0.83 (0.68 to 0.92) | 0.92 (0.84 to 0.97) |
| Collinson 200648 | TnT or IMA | 0.95 (0.80 to 0.99) | 0.95 (0.92 to 0.97) | 1.00 (0.88 to 1.00) | 0.35 (0.31 to 0.40) |
| Keating 200670 | TnI or IMA | 0.74 (0.58 to 0.86) | 0.99 (0.97 to 1.00) | 0.98 (0.86 to 1.00) | 0.14 (0.10 to 0.19) |
Outcomes
We assumed that after the strategy had been applied and any treatments given the subsequent progress of each patient would depend on whether or not they had MI, and if they had MI whether or not it was identified and treated. Patients with MI risked reinfarction or death dependent on whether or not they received treatment. The risk of reinfarction and death (with and without treatment) was determined using data from a study by Mills. 155 This cohort of patients with suspected ACS allows comparison between those with recognised and treated MI, and those with untreated MI, because the threshold for reporting positive results was changed after an initial validation phase when low positive results were recorded but not reported. We selected patients from this study who matched our inclusion criteria of having a non-diagnostic ECG. Table 36 shows the estimates of the risk of reinfarction and death among relevant patients in the Mills155 cohort.
| Source | Estimate (%) | Distribution | |
|---|---|---|---|
| Death | |||
| Treated MI | Mills155 | 11 | n/N = 9/80 |
| Untreated MI | Mills155 | 21 | n/N = 19/90 |
| Patients with no MI | Mills155 | 1 | n/N = 4/402 |
| Reinfarction | |||
| Treated MI | Mills155 | 11 | n/N =9/80 |
| Untreated MI | Mills155 | 29 | n/N = 26/90 |
| Patients with no MI | Mills155 | 3.9 | n/N = 17/440 |
After this we assumed that survivors accrued QALYs according to their age and sex, whether or not they had MI, and whether or not they suffered reinfarction. The lifetime QALYs are estimated based on patients' life expectancy and their corresponding annual utilities. The discounted life expectancy of patients with MI, and MI with reinfarction was captured from Polanczyk et al. ,156 whereas the utility of MI patients was estimated from Ward et al. 157 The utility of patients with reinfarction was estimated by using a multiplicative factor of 0.8 for patients with MI based on the input from clinicians. Life expectancy of general population (without MI) was estimated from the Office for National Statistics158 and the general population utilities are estimated from Ara et al. 15 which included different utilities for men and women. Sensitivity analysis was also performed using utility values from Ara et al.,159 which included different utilities for men and women. It should also be noted that the utilities were not capped at the population means as this was a minor issue and is only relevant for people aged > 90 years. The estimated QALY pay-offs for patients with MI and reinfarction are outlined in Table 37, whereas the age-specific QALYs for the general population are reported in Appendix 5.
| Age (years) | QALYs | |
|---|---|---|
| MI | MI with reinfarction | |
| 30–44 | 12.20 | 9.76 |
| 45–54 | 9.47 | 7.58 |
| 55–64 | 6.73 | 5.39 |
| 65–74 | 4.65 | 3.72 |
| >75 | 2.43 | 1.95 |
Costs
The costs included in the model are:
-
all biomarker measurement costs
-
hospital stay as determined by the strategy
-
treatments administered
-
subsequent cardiac events
-
lifetime costs of care for patients with CAD.
We assumed that patients would incur costs whenever a test was performed and the costs of biomarkers were estimated from the RATPAC trial data, 160 with all of them around £20. In the case of multiple biomarker strategies, the costs of each biomarker are added.
The patients also accrued costs proportional to their length of hospital stay. It was assumed that any time spent in hospital incurred costs at the rate for admission to a general medical ward, regardless of their location in the hospital. This was because per diem costs for different locations reflected different types of patients managed in those locations, whereas patients with suspected ACS were likely to incur the same true costs regardless of their location within the hospital.
The cost of index admission and treatment for MI and the costs of reinfarction were estimated as one-off costs of £3587, based on national tariff for non-elective acute MI without complications. Length of hospital stay was determined from appropriate data sources, such as the RATPAC trial. 160
Lifetime costs of survivors were estimated according to their age and sex, whether or not they had MI, and whether or not they suffered reinfarction . The lifetime costs for MI patients are estimated using the annual costs from Ward et al. 157 and the discounted life expectancy of patients with MI were captured from Polanczyk et al. 156 The cost of reinfarction was estimated as a one-off cost of £3587, based on national tariff for non-elective acute MI without complications. 161 The costs are outlined in Tables 38 and 39.
| Strategy | Source | Estimate (£) | 95% CI (£) |
|---|---|---|---|
| Admission for MI or reinfarction treatment | NHS reference costs161 | 3587 | 3000 to 4000 |
| Hospital stay (per hour) for testing | NHS reference costs for general medical ward161 | 22 | 20 to 30 |
| Troponin | RATPAC160 | 20 | 18 to 25 |
| Other biomarkers | RATPAC160 | 20 | 18 to 25 |
| Age (years) | MI cost (£) |
|---|---|
| 30–44 | 4012.5 |
| 45–54 | 3115 |
| 55–64 | 2215 |
| 65–74 | 1530 |
| >75 | 800 |
Modelling methodology
A model was developed using SIMUL8 software (SIMUL8 Corporation, Boston, MA, USA) to explore the costs and health outcomes associated with different diagnostic strategies. The analysis was conducted for patients aged 40–75 years when presenting to the ED. The model takes a lifetime horizon with mean life expectancy based on UK interim lifetables. 158 The economic perspective of the model is the NHS in England and Wales with the structure of the model shown in Figure 35. Figure 36 shows the diagnostic pathway associated with the 10-hour troponin test in the model, whereas Figure 37 shows the pathway associated with the combination of presentation biomarkers and 10-hour troponin testing.
FIGURE 35.
Structure of the diagnostic model.
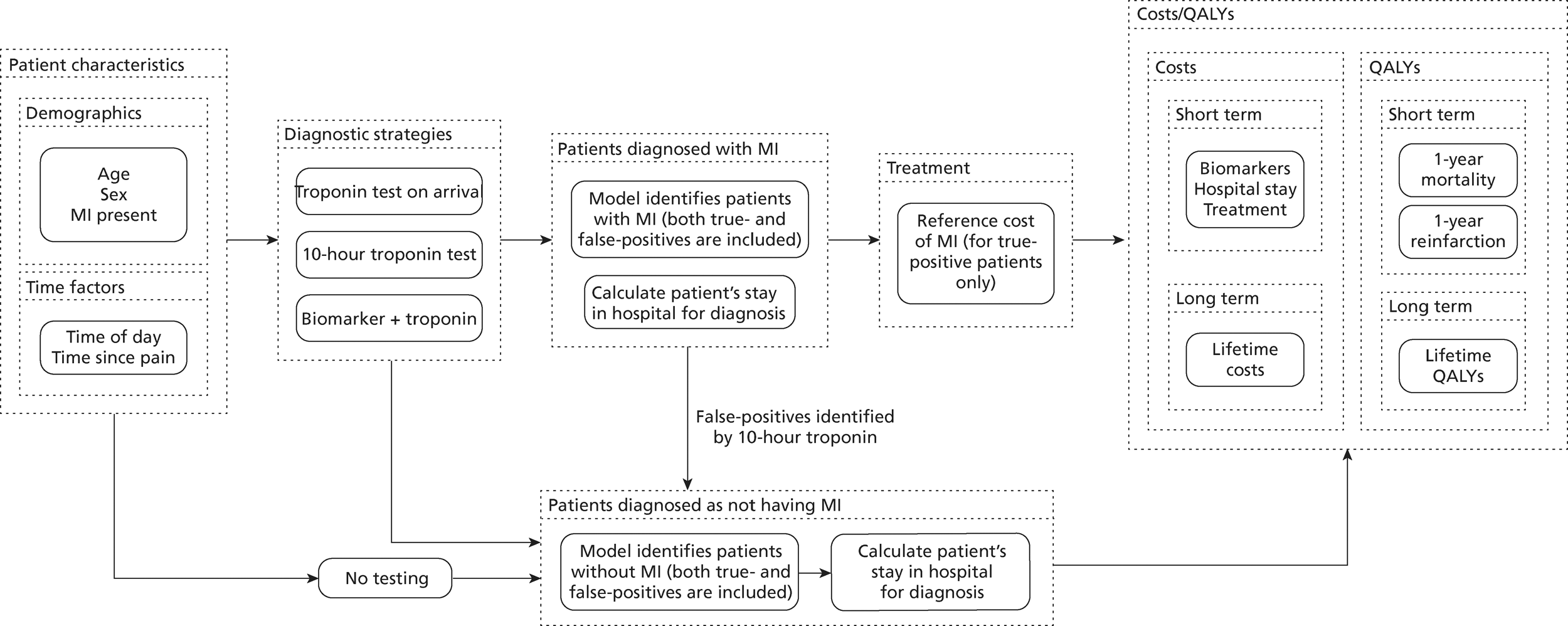
FIGURE 36.
Ten-hour troponin diagnostic strategy.
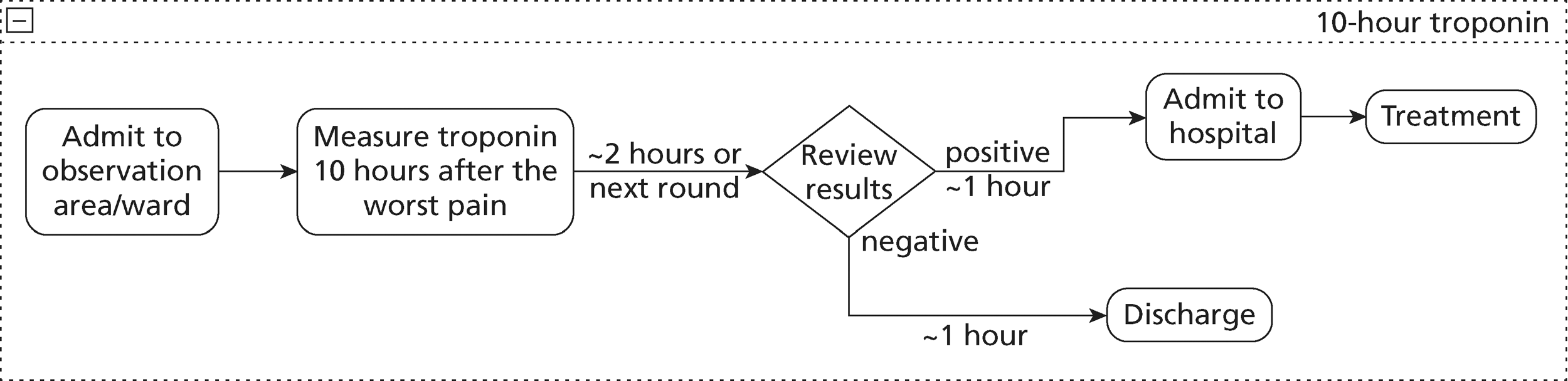
FIGURE 37.
Combined biomarker and 10-hour troponin testing diagnostic strategies.
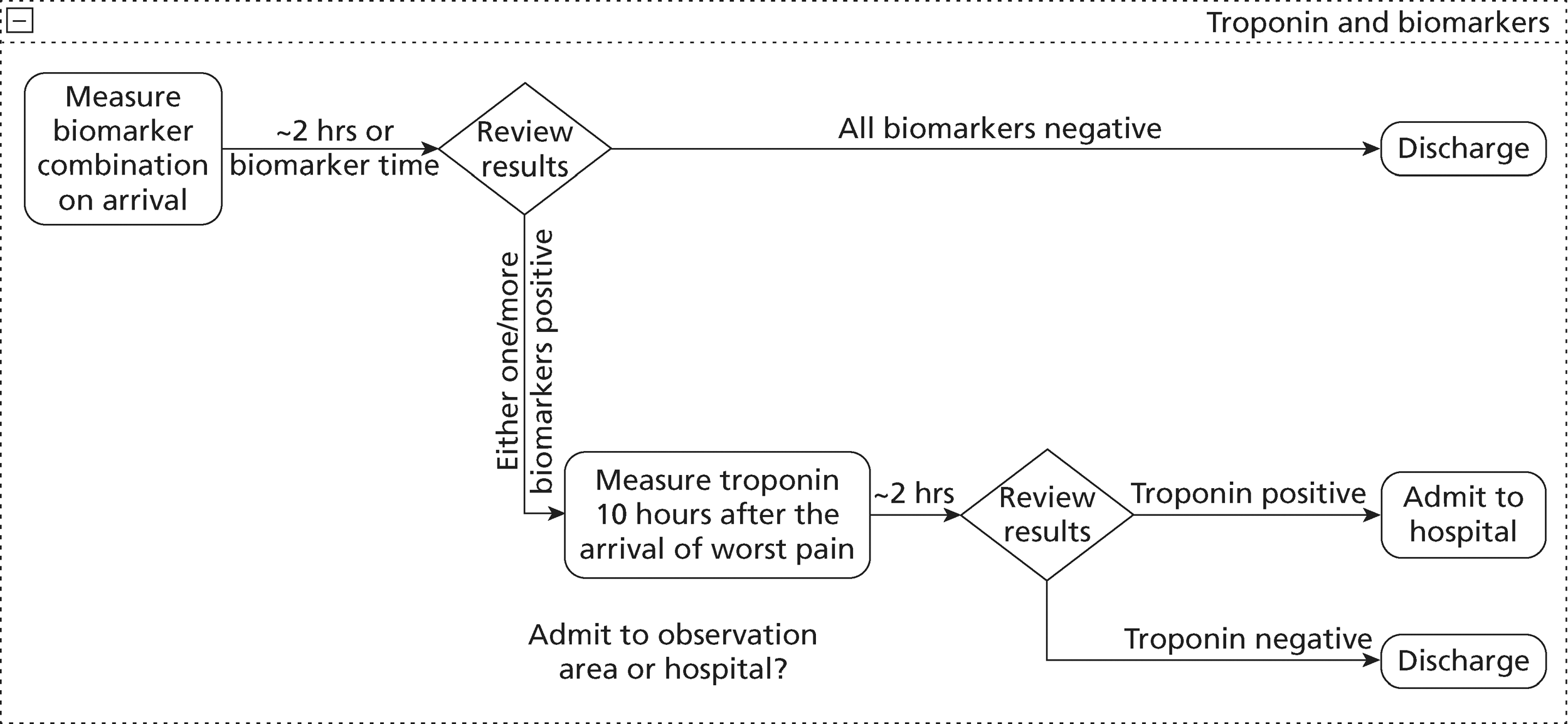
Model stability
The number of model runs determines the accuracy of the results for estimating the optimal management strategy. This uncertainty is a result of the random nature of some events (reinfarction and death) and accuracy can only be achieved by having sufficient numbers of model runs to account for these random occurrences. We ran the model 100 times to estimate the costs and QALYs along with their 95% CIs for each diagnostic strategy.
Main analysis deterministic results
The main analysis compared the presentation troponin strategies in two different populations (no known CAD and known CAD) and three different scenarios (doctor on demand, twice-daily ward round and once-daily ward round), so a total of six analyses are presented in Tables 40–45.
| Strategy | Total costs, £ (95% CI) | Total QALYs (95% CI) | ICER (£/QALY) |
|---|---|---|---|
| No testing | 965,994 (957,259 to 974,730) | 26,226.68 (26,196.77 to 26,256.60) | – |
| Presentation TnT, 10% CV | 1,560,351 (1,548,935 to 1,571,768) | 26,344.84 (26,317.49 to 26,374.19) | 5030 |
| Presentation TnT, 99th percentile | 1,609,760 (1,597,955 to 1,621,564) | 26,352.42 (26,323.70 to 26,382.13) | 6518 |
| Presentation HsTnT, 99th percentile | 1,806,910 (1,794,447 to 1,819,373) | 26,378.75 (26,350.16 to 26,406.94) | 7487 |
| 10-hour troponin test | 2,016,540 (2,004,601 to 2,028,749) | 26,386.36 (26,358.57 to 26,414.16) | 27,546 |
| Strategy | Total costs, £ (95% CI) | Total QALYs (95% CI) | ICER (£) |
|---|---|---|---|
| No testing | 965,994 (957,259 to 974,730) | 26,226.68 (26,196.77 to 26,256.60) | – |
| Presentation TnT, 10% CV | 1,595,955 (1,584,418 to 1,607,492) | 26,344.84 (26,317.49 to 26,374.19) | 5331 |
| Presentation TnT, 99th percentile | 1,655,424 (1,653,855 to 1,676,933) | 26,352.42 (26,323.70 to 26,382.13) | 7845 |
| Presentation HsTnT, 99th percentile | 1,936,718 (1,924,723 to 1,948,713) | 26,378.75 (26,350.16 to 26,406.94) | 10,683 |
| 10-hour troponin test | 2,416,409 (2,404,435 to 2,428,383) | 26,386.36 (26,358.57 to 26,414.16) | 63,034 |
| Strategy | Total costs, £ (95% CI) | Total QALYs (95% CI) | ICER (£/QALY) |
|---|---|---|---|
| No testing | 965,994 (957,259 to 974,730) | 26,226.68 (26,196.77 to 26,256.60) | – |
| Presentation TnT, 10% CV | 1,621,152 (1,609,727 to 1,632,576) | 26,344.84 (26,317.49 to 26,374.19) | 5544 |
| Presentation TnT, 99th percentile | 1,705,989 (1,694,089 to 1,717,888) | 26,352.42 (26,323.70 to 26,382.13) | 11,192 |
| Presentation HsTnT, 99th percentile | 2,030,901 (2,018,511 to 2,043,290) | 26,378.75 (26,350.16 to 26,406.94) | 12,340 |
| 10-hour troponin test | 2,705,696 (2,693,761 to 2,717,630) | 26,386.36 (26,358.57 to 26,414.16) | 88,672 |
| Strategy | Total costs, £ (95% CI) | Total QALYs (95% CI) | ICER (£/QALY) |
|---|---|---|---|
| No testing | 895,440 (887,764 to 903,117) | 20,122.36 (20,098.26 to 20,146.46) | – |
| Presentation TnT, 10% CV | 1,526,705 (1,515,468 to 1,537,942) | 20,221.03 (20,196.30 to 20,243.76) | 6397 |
| Presentation TnT, 99th percentile | 1,580,066 (1,569,186 to 1,590,946) | 20,229.36 (20,205.28 to 20,253.43) | 6405 |
| Presentation HsTnT, 99th percentile | 1,791,928 (1,780,253 to 1,803,603) | 20,249.14 (20,224.45 to 20,273.83) | 10,710 |
| 10-hour troponin test | 2,024,269 (2,012,991 to 2,035,547) | 20,255.68 (20,230.45 to 20,280.91) | 35,526 |
| Strategy | Total costs, £ (95% CI) | Total QALYs (95% CI) | ICER (£/QALY) |
|---|---|---|---|
| No testing | 895,440 (887,764 to 903,117) | 20,122.36 (20,098.26 to 20,146.46) | – |
| Presentation TnT, 10% CV | 1,565,347 (1,553,759 to 1,576,935) | 20,221.03 (20,196.30 to 20,243.76) | 6790 |
| Presentation TnT, 99th percentile | 1,634,789 (1,623,585 to 1,645,992) | 20,229.36 (20,205.28 to 20,253.43) | 8336 |
| Presentation HsTnT, 99th percentile | 1,923,076 (1,911,130 to 1,935,023) | 20,249.14 (20,224.45 to 20,273.83) | 14,575 |
| 10-hour troponin test | 2,423,332 (2,412,088 to 2,434,575) | 20,255.68 (20,230.45 to 20,280.91) | 76,492 |
| Strategy | Total costs, £ (95% CI) | Total QALYs (95% CI) | ICER (£/QALY) |
|---|---|---|---|
| No testing | 895,440 (887,764 to 903,117) | 20,122.36 (20,098.26 to 20,146.46) | – |
| Presentation TnT, 10% CV | 1,591,876 (1,580,221 to 1,603,532) | 20,221.03 (20,196.30 to 20,243.76) | 7058 |
| Presentation TnT, 99th percentile | 1,671,994 (1,662,038 to 1,683,950) | 20,229.36 (20,205.28 to 20,253.43) | 9618 |
| Presentation HsTnT, 99th percentile | 2,012,040 (1,999,995 to 2,024,084) | 20,249.14 (20,224.45 to 20,273.83) | 17,191 |
| 10-hour troponin test | 2,689,319 (2,678,062 to 2,700,577) | 20,255.68 (20,230.45 to 20,280.91) | 103,560 |
For each scenario the table shows the total costs and total QALYs accrued by the population of 2240 patients when each potential strategy is used. As expected, the effectiveness of the strategies (as measured by the total QALYs) increases in accordance with the strategy sensitivity, whereas the cost of each strategy increases as specificity decreases. The incremental cost-effectiveness ratio (ICER) reports the additional cost required using the strategy to accrue one additional QALY compared with the next most effective alternative. NICE decision-making suggests that a threshold of £20,000–30,000 per QALY is usually used, so if the ICER exceeds £20,000–30,000 per QALY then the strategy is unlikely to be considered cost-effective.
The analysis shows that the strategies based on presentation troponin are likely to be considered cost-effective compared with no testing or the next most effective alternative. Of these strategies, the one using presentation HsTnT gains the most QALYs and still has an acceptable ICER, so it appears to be the optimal strategy. In five out of six scenarios, the ICER for 10-hour troponin testing, compared with presentation HsTnT, exceeds £20,000–30,000 per QALY, so it is unlikely to be considered cost-effective. In one scenario (patients without known CAD and with doctor available on demand) the ICER for 10-hour TnT is £27,546/QALY, so the 10-hour troponin strategy may be cost-effective for patients without known CAD if a decision can be made and the patient discharged as soon as the 10-hour troponin result is available.
The effects of suboptimal diagnosis by the biomarkers were also estimated. The number of adverse events (reinfarctions and deaths) and their proportions for each of the biomarker strategies are shown in Table 46 (population without known CAD) and Table 47 (population with known CAD). These tables show the effect of different testing strategies on clinically relevant outcomes across the whole presenting population. However, clinicians and patients are often more interested to know the risk of adverse outcome in those discharged after negative testing. These estimates are given in Table 48 (population without known CAD) and Table 49 (population with known CAD). It should be recognised that the differences in event rates between strategies shown in Tables 46 and 47 and Tables 48 and 49 are, in part, explained by differences in the populations compared, i.e. lower event rates are in part achieved by positive tests removing those at risk from the reported population rather than actually preventing adverse events.
| Strategy | MI | Deaths | MIs avoided compared with no testing | Lives saved compared with no testing |
|---|---|---|---|---|
| No testing | 56.57 | 24.00 | – | – |
| Presentation TnT, 10% CV | 47.22 | 18.80 | 9.45 | 5.20 |
| Presentation TnT, 99th percentile | 46.50 | 18.40 | 10.07 | 5.60 |
| Presentation HsTnT, 99th percentile | 44.49 | 17.29 | 12.08 | 6.71 |
| 10-hour troponin test | 43.97 | 17.00 | 12.60 | 7.00 |
| Strategy | MI | Deaths | MIs avoided compared with no testing | Lives saved compared with no testing |
|---|---|---|---|---|
| No testing | 58.57 | 25.60 | – | – |
| Presentation TnT, 10% CV | 48.16 | 19.81 | 10.41 | 5.79 |
| Presentation TnT, 99th percentile | 47.36 | 19.36 | 11.21 | 6.24 |
| Presentation HsTnT, 99th percentile | 45.12 | 18.12 | 13.45 | 7.48 |
| 10-hour troponin test | 44.53 | 17.80 | 14.04 | 7.80 |
| Strategy | Proportion of MI in discharged patients without treatmenta | Proportion of deaths in discharged patients without treatmenta | Improvement in proportion of MI in discharged patients over no testing | Improvement in proportion of deaths in discharged patients over no testing |
|---|---|---|---|---|
| No testing | 0.0566 | 0.0240 | – | – |
| Presentation TnT, 10% CV | 0.0438 | 0.0138 | 0.0128 | 0.0102 |
| Presentation TnT, 99th percentile | 0.0427 | 0.0130 | 0.0139 | 0.0110 |
| Presentation HsTnT, 99th percentile | 0.0398 | 0.0106 | 0.0168 | 0.0134 |
| 10-hour troponin test | 0.0390 | 0.0100 | 0.0176 | 0.0140 |
| Strategy | Proportion of MI in discharged patients without treatmenta | Proportion of deaths in discharged patients without treatmenta | Improvement in proportion of MI in discharged patients over no testing | Improvement in proportion of deaths in discharged patients over no testing |
|---|---|---|---|---|
| No testing | 0.0586 | 0.0256 | – | – |
| Presentation TnT, 10% CV | 0.0444 | 0.0143 | 0.0142 | 0.0113 |
| Presentation TnT, 99th percentile | 0.0432 | 0.0133 | 0.0154 | 0.0123 |
| Presentation HsTnT, 99th percentile | 0.0399 | 0.0107 | 0.0187 | 0.0149 |
| 10-hour troponin test | 0.0390 | 0.0100 | 0.0196 | 0.0156 |
Tables 46 and 47 show that if patients are discharged without testing, their risk of death and non-fatal MI over the following year are estimated to be around 2.5% and 6%, respectively. We estimated that the various testing strategies could reduce these risks by 0.5–0.7% and 0.9–1.3%, respectively, in patients without known CAD and by marginally more in patients with known CAD.
Tables 48 and 49 show that the various testing strategies reduce the estimated risk of adverse outcome after discharge with a negative assessment but, based on the Mills data,155 the rate of death and non-fatal MI remained 1.0% and 3.9%, respectively, even after a negative 10-hour troponin result.
Table 50 shows the results for the sensitivity analysis using high-sensitivity TnI instead of HsTnT at presentation. Only the ICERs for presentation TnI and 10-hour troponin testing are shown because the other strategies are all less effective than these two strategies so, using the £20,000/QALY or £30,000/QALY threshold, one or other of these two strategies would always be optimal. The point estimates from our meta-analysis suggested that the ADVIA Ultra high-sensitivity TnI assay had lower sensitivity than the Roche HsTnT assay. This may be due to random error, patient selection or choice of threshold, but the difference in point estimates provides the opportunity to explore whether the 10-hour troponin strategy is more cost-effective than a less-sensitive presentation strategy. The ICERs in Table 50 suggest that this is the case, although the 10-hour troponin strategy would still only be optimal in one scenario (doctor on demand, patients without known CAD) if the £20,000/QALY were used and would be optimal in three of the six scenarios if the £30,000/QALY threshold were used.
| Scenario | ICER for presentation ADVIA Centaur Ultra troponin I assay (£/QALY) | ICER for 10-hour troponin testing (£/QALY) |
|---|---|---|
| Doctor on demand, patients without known CAD | 5029/QALY | 15,255/QALY |
| Twice-daily ward round, patients without known CAD | 6774/QALY | 29,064/QALY |
| Once-daily ward round, patients without known CAD | 7981/QALY | 39,116/QALY |
| Doctor on demand, patients with known CAD | 6483/QALY | 20,775/QALY |
| Twice-daily ward round, patients with known CAD | 7947/QALY | 38,387/QALY |
| Once-daily ward round, patients with known CAD | 9175/QALY | 49,902/QALY |
Table 51 shows the results for the sensitivity analysis in which a strategy of measuring high-sensitivity troponin at presentation and 3 hours later is included alongside the other strategies used in the main analysis. Again, only the ICERs for the 3-hour strategy and 10-hour troponin testing are shown because the other strategies are all less effective than these two strategies and will not be optimal if either a £20,000/QALY or £30,000/QALY threshold is used. The ICERs in Table 51 show that the 3-hour strategy is the most cost-effective strategy at either the £20,000/QALY or £30,000/QALY threshold, whereas the ICER for the 10-hour troponin strategy substantially exceeds both thresholds in all scenarios.
| Scenario | ICER for high-sensitivity troponin at presentation and 3 hours (£/QALY) | ICER for 10-hour troponin testing (£/QALY) |
|---|---|---|
| Doctor on demand, patients without known CAD | 5596 | 405,312 |
| Twice-daily ward round, patients without known CAD | 6247 | 1,008,159 |
| Once-daily ward round, patients without known CAD | 6727 | 1,444,659 |
| Doctor on demand, patients with known CAD | 7735 | 128,640 |
| Twice-daily ward round, patients with known CAD | 8189 | 296,754 |
| Once-daily ward round, patients with known CAD | 8710 | 408,536 |
The final deterministic diagnostic analysis estimated the cost-effectiveness of adding an alternative biomarker to troponin alone. This analysis was limited to patients without known CAD using the twice-daily ward round scenario. Estimates of presentation troponin sensitivity and specificity were based on the primary study that evaluated the relevant biomarker. This provided the best estimate of the effect of adding the biomarker but means that we are not always comparing the biomarker combination to the optimal presentation troponin strategy. Table 52 shows the ICERs for each strategy compared with the next most effective alternative. Details of costs and QALYs are presented in Appendix 6.
| Study | Biomarkers | Presentation troponin alone (£/QALY) | Presentation troponin and biomarker (£/QALY) | 10-hour troponin testing (£/QALY) |
|---|---|---|---|---|
| Body 201157 | TnI or H-FABP | 6596 | 5120 | 24,147 |
| Haltern 201067 | TnT or H-FABP | 4849 | 14,615 | 58,330 |
| McCann 200876 | TnT or H-FABP | 5296 | 5945 | 54,820 |
| Mion 200777 | TnI or H-FABP | 5785 | 6125 | 18,904 |
| Keller 201071 | TnT or copeptin | 5545 | 9606 | 23,222 |
| Reichlin 200980 | TnT or copeptin | 5295 | 9244 | 117,176 |
| Keller 201071 | TnT or myoglobin | 5545 | 7769 | 22,733 |
| Mion 200777 | TnI or myoglobin | 5785 | 5877 | 23,048 |
| Collinson 200648 | TnT or IMA | 4874 | 99,948 | Dominated |
| Keating 200670 | TnT or IMA | 4876 | Extendedly dominated | 23,658 |
Table 52 shows that, compared with troponin alone, the addition of H-FABP, copeptin or myoglobin appears to be cost-effective with ICERs of < £20,000–30,000/QALY. However, at this threshold, the 10-hour troponin strategy may also be cost-effective according to some of the studies. If the presentation biomarker and troponin combination increased sensitivity to over 90%67,76,80 then 10-hour troponin testing was unlikely to be cost-effective in comparison. If the presentation biomarker and troponin combination did not achieve 90% sensitivity,57,71,77 then 10-hour troponin testing may be considered cost-effective. Neither of the strategies involving IMA appeared to be cost-effective, presumably because both involved substantial losses in specificity with only modest gains in sensitivity compared with troponin alone.
Probabilistic results of the diagnostic model
Probabilistic analysis incorporated uncertainty in the parameter estimates to provide estimates of the probability that each strategy would be cost-effective at different thresholds for willingness to pay for health gain. Figures 38–40 show the probabilistic analysis for patients without known CAD according to the doctor-on-demand, twice-daily ward and once-daily ward scenarios. The tables containing the probabilities at different willingness-to-pay thresholds are in Appendix 7. The probabilistic results were similar to those of the deterministic analysis, with the conclusions identical for both methodologies.
FIGURE 38.
Probability of cost-effectiveness of strategies in doctor-on-demand scenario.
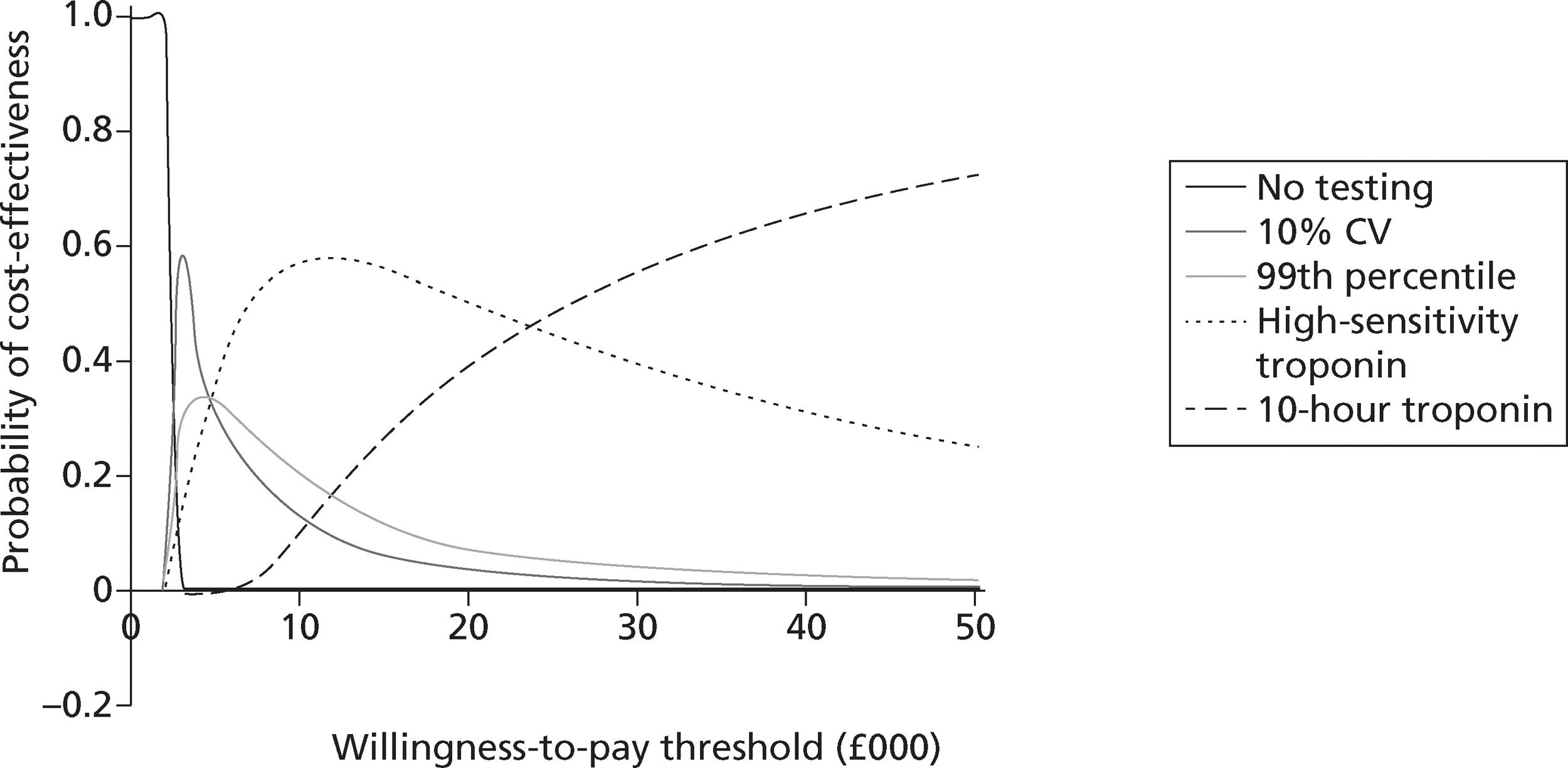
FIGURE 39.
Probability of cost-effectiveness of strategies in twice-daily ward scenario.
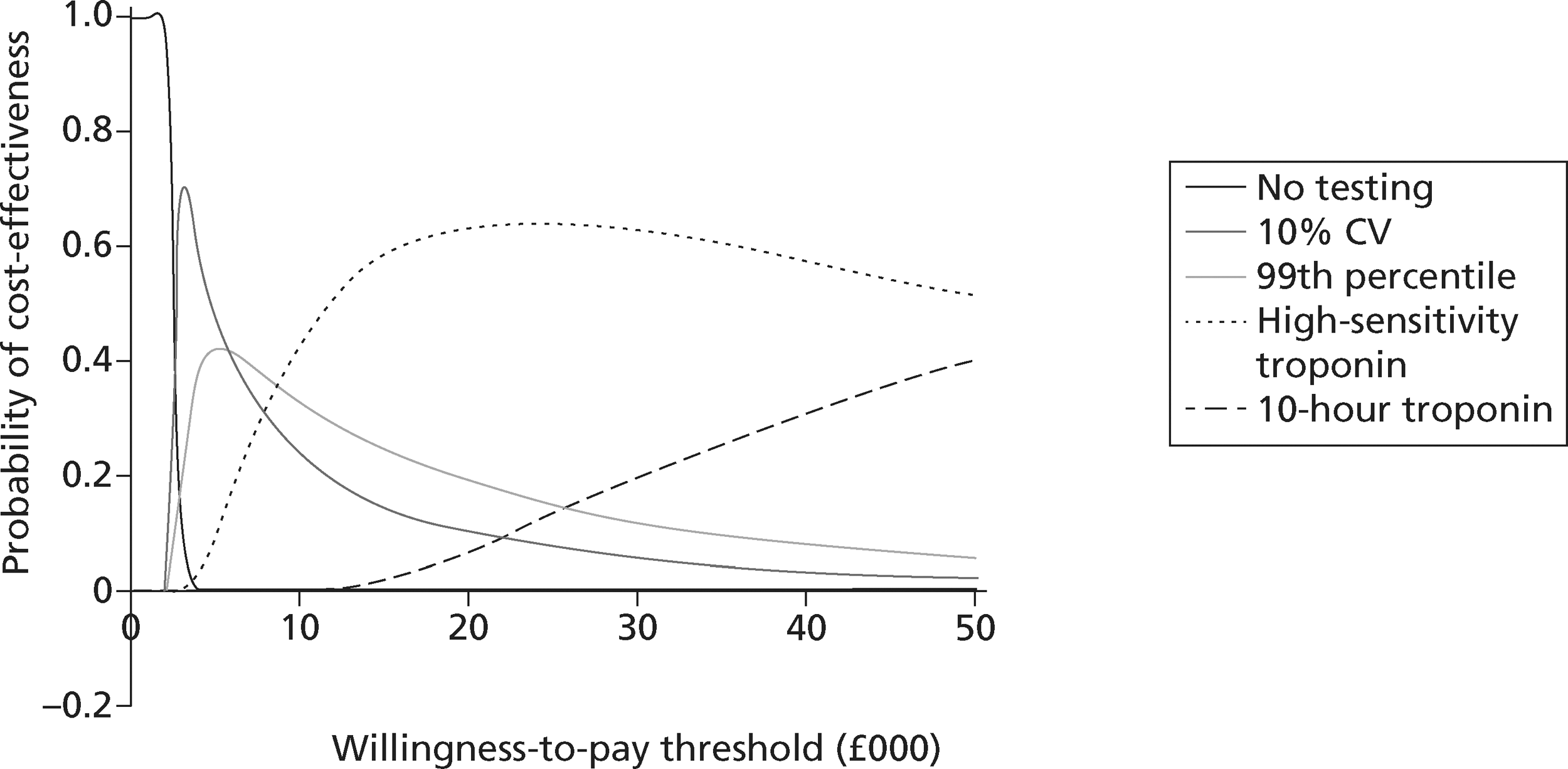
FIGURE 40.
Probability of cost-effectiveness of strategies in once-daily ward scenario.
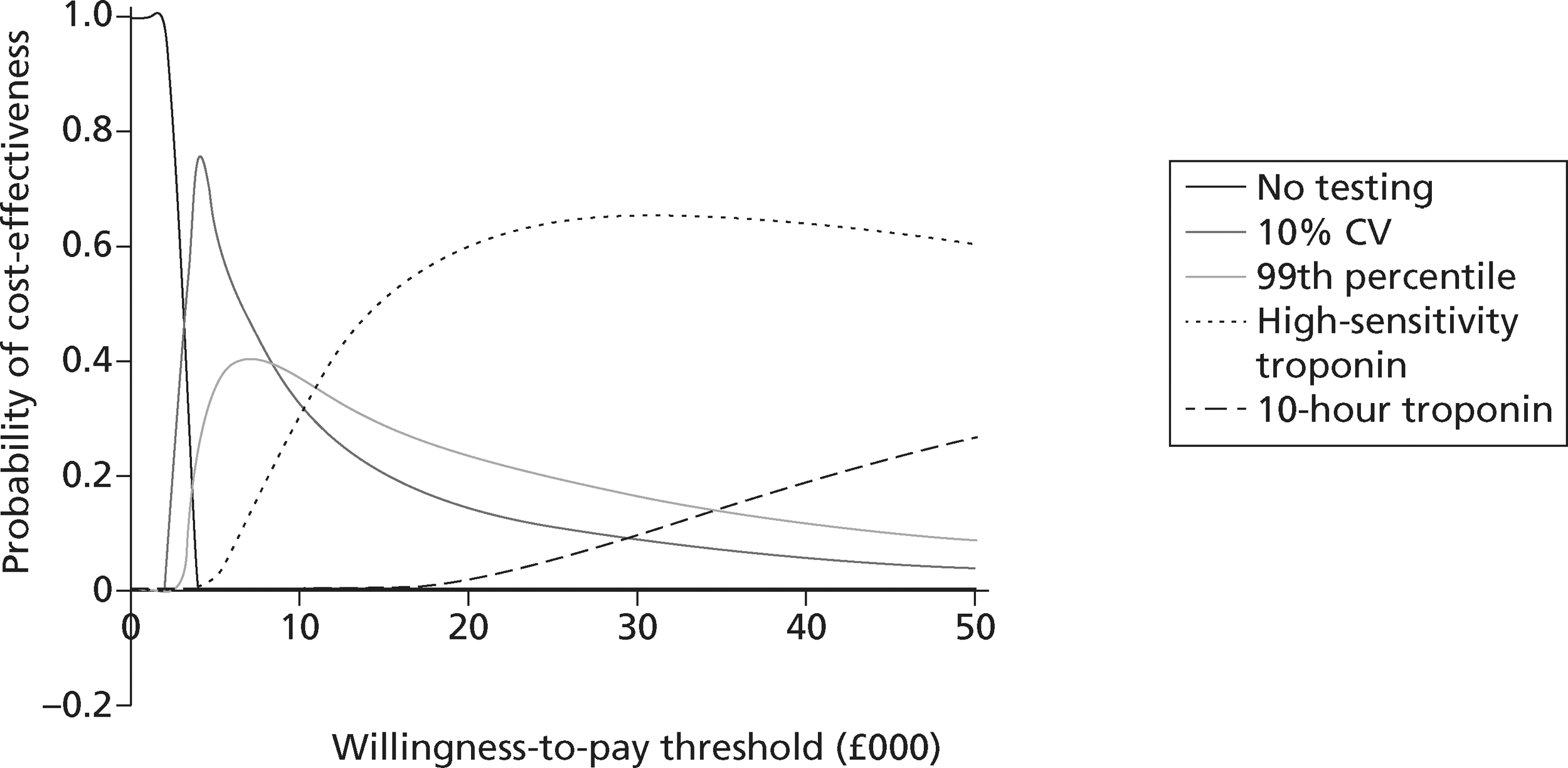
These analyses show that the strategy based on measuring high-sensitivity troponin at presentation had the highest probability of being cost-effective for thresholds of between around £5000 and £23,000/QALY in the doctor-on-demand strategy and for thresholds exceeding around £10,000/QALY for the other two strategies. For thresholds exceeding around £23,000/QALY in the doctor-on-demand scenario the 10-hour troponin strategy had the highest probability of being cost-effective. These results reflect the deterministic analysis and suggest that high-sensitivity troponin on presentation has the highest probability of being cost-effective in most scenarios and at typically used thresholds for willingness to pay.
The prognostic phase model
This section details the methods and results of the health economic model constructed to compare prognostic strategies for troponin-negative patients without known CAD. We developed a decision-analytic model to estimate the costs and QALYs accrued by each potential management strategy for identifying patients with subsequent risk of MACEs. The strategies involved using CTCA, exercise ECG or a biomarker (H-FABP) to select patients for further investigation with ICA. We also included a ‘perfect’ strategy of ICA for all patients and a no-testing strategy. We assumed that patients who were discharged without testing would ultimately present with further symptoms and receive appropriate testing if they did not die in the meantime. The key aim was to determine the optimal strategy in terms of cost-effectiveness.
Objectives
The objectives of the prognostic cost-effectiveness analysis were to:
-
estimate the cost-effectiveness of prognostic strategies for troponin-negative patients, in terms of the cost per QALY gained by each strategy
-
identify the optimal strategy, defined as the most cost-effective strategy at a willingness to pay per QALY gained threshold of £20,000–30,000
-
identify the critical areas of uncertainty in the prognosis of troponin-negative patients with unknown CAD, where future research would produce the most benefit.
The costs and benefits of prognostic testing
The main benefits of prognostic testing relate to identification and intervention of patients with risk of non-fatal MI and death. The direct costs of prognostic testing include the costs of investigation, hospital stay for diagnosis, the subsequent costs of providing intervention and also reinfarction, if any. ICA, assumed as gold standard for diagnosing CAD, is the most effective but is also the most expensive and invasive strategy. CTCA, exercise ECG or biomarkers incur costs and may miss cases owing to suboptimal sensitivity (thus worsening outcomes) but save costs by reducing the number of ICA performed. We built a model to allow us to analyse the effect of different prognostic testing strategies on these costs and benefits.
The decision-analysis model structure
The different prognostic strategies were applied to a hypothetical cohort of troponin-negative patients who initially presented with suspected ACS. The model used the estimated probability of non-fatal MI or death for troponin-negative patients from the study of Mills155 to determine a proportion of the cohort who would die or suffer non-fatal MI without early investigation and treatment. The sensitivity of each prognostic strategy for predicting MACEs would then determine which of these patients would have a positive test according to the strategy. We assumed that patients with a TP strategy would be investigated and treated promptly, and a proportion of those who would have died or suffered non-fatal MI without treatment would avoid this outcome. Meanwhile those with a FP strategy would undergo investigation and treatment without any change to their prognosis. Each patient then accrued lifetime QALYs and health-care costs according to their age, sex, reinfarction and treatment status. Costs were also accrued for biomarker costs and hospital stay for prognosis; costs were also accrued for further investigation, treatment and/or reinfarction, depending on the strategy and the patient characteristics.
Population
Patients with a positive 10-hour troponin result were assumed to be admitted for treatment and only those with a negative 10-hour troponin result were eligible for additional testing in the prognostic model. Moreover, the model was only tested on the population without known CAD because patients with known CAD are already known to be at higher risk and will be receiving appropriate treatment.
The population age and sex parameters were assumed to be the same as the population without known CAD in the diagnostic model (see Table 32). We assumed that the prevalence of (unknown) CAD was 10% in this population, based on the prevalence of positive non-invasive tests in the studies of Hollander and Goodacre. 132,142 These tests have suboptimal accuracy for CAD, but the potential bias from suboptimal accuracy was felt to be much less than the potential selection bias in studies in which all patients received invasive testing. The parameters relating to MI prevalence and timing of symptoms were not relevant to this phase.
Selection of strategies
The following strategies were tested:
-
discharge all patients home without testing or treatment
-
CTCA for all patients, admit for ICA if occlusive coronary disease (i.e. > 50% stenosis in any vessel), discharge if negative
-
exercise ECG for all patients, admit for coronary angiography if positive (i.e. > 1 mm horizontal or down-sloping ST-segment depression, > 1 mm ST elevation or ventricular arrhythmia), discharge if negative
-
biomarker (H-FABP) for all patients, admit for ICA if positive, discharge if negative
-
ICA for all patients.
Prognostic parameters of each strategy
We selected appropriate studies from the systematic review to estimate the sensitivity and specificity of the test for predicting MACEs and the RR for MACEs with a positive test compared with a negative test. Studies were selected on the basis of providing data relevant to the population of interest, i.e. patients attending the ED with chest pain, a non-diagnostic ECG and a negative troponin. We selected H-FABP and the study of Viswanathan et al. ,121 as this provides the best estimate of prognostic value in troponin-negative patients.
Table 53 shows the estimates of sensitivity and specificity for each strategy tested and the sources for these estimates. The sources for sensitivity and specificity estimates were selected by identifying studies with sufficient numbers of relevant patients that reported relevant data.
| Test | Study | Follow-up | Proportion positive | Sensitivity for MACEs | Specificity for MACEs | RR |
|---|---|---|---|---|---|---|
| CTCA | Schlett136 | 12 months | 68/368 (18.5%) | 18/22 (81.8%) | 296/346 (85.5%) | 19.9 |
| ETT | Goodacre142 | 6 months | 37/422 (8.8%) | 2/5 (40.0%) | 382/417 (91.6%) | 6.6 |
| H-FABP | Viswanathan121 | > 12 months | 40/756 (5.3%) | 10/35 (28.6%) | 691/721 (95.8%) | 6.9 |
| ICA | Mowatt25 | – | – | 100% | 100% | – |
Outcomes
We considered only adverse cardiac outcomes in the model and assumed that these would all occur in patients with CAD. The estimated risk of death and non-fatal MI following diagnostic strategy was crucial in this analysis because this defined the baseline risk against which alternative strategies might improve outcomes. We estimated this parameter by selecting patients in the Mills155 and RATPAC12 cohorts who had a non-diagnostic ECG, no known CAD and no MI at presentation. Patients in these studies did not routinely receive immediate investigation with other biomarkers, CTCA or exercise ECG if troponin testing was negative, so they provide a pragmatic estimate of the baseline risk. The rates of death and non-fatal MI are shown in Table 54. The RATPAC cohort12 is probably lower risk because it selected patients who gave consent participate in a trial and followed up for 3 months, whereas the Mills cohort155 is probably higher risk because it selected only patients who were admitted to hospital and followed up for 12 months. We tested both estimated rates in the model to explore the importance of the baseline rates and in determining cost-effectiveness. We assumed that the testing strategy could only influence adverse events up to the end of the relevant follow-up period.
| Study | Follow-up (months) | Deaths | Non-fatal MI |
|---|---|---|---|
| Mills155 | 12 | 4/402 (1%) | 17/440 (3.9%) |
| RATPAC12 | 3 | 4/2085 (0.19%) | 5/2085 (0.24%) |
Each patient was assumed to have a baseline risk of death or non-fatal MI up to 3 months or 1 year, determined by the Mills155 or RATPAC data. 12 We applied the sensitivity and specificity of each test to determine whether the patient would effectively be TP (i.e. correctly predicted to suffer an event unless treated), TN (correctly predicted not to suffer an event), FP (incorrectly predicted to suffer an event) and FN (incorrectly predicted not to suffer an event). We assumed that the TNs and FPs would not suffer an event and that FNs would suffer an event. For the TPs we needed to estimate the effect of intervention in reducing the risk of death or non-fatal MI. There are very limited relevant data to estimate this so we estimated that intervention would approximately halve the risk of both events, in line with our estimate from the diagnostic model of the effect of treatment on adverse outcome after MI.
Some of the investigations also carried risks to patient health. These were modelled by estimating a QALY loss that was applied each time the investigation was performed. The following disbenefits were estimated and are shown in Table 55.
| Test | Risk | Estimate | Source | QALY loss per test |
|---|---|---|---|---|
| ETT | Death | 0.5 in 10,000 | Stuart 1980,162 Mowatt 200825 | 0.0012 |
| MI | 3.58 in 10,000 | Stuart 1980162 | ||
| CTCA | Malignancy | 1 in 10,000 | Stein 2008163 | 0.0015 |
| Fatal contrast reaction | 1 in 55,000 | Shehadi 1975,164 Cashman 1991165 | ||
| ICA | Death | 11 in 10,000 | Johnson 1993,166 Mowatt 200825 | 0.0145 |
| MI | 6 in 10,000 | Johnson 1993166 | ||
| Stroke | 5 in 10,000 | Johnson 1993166 | ||
| Fatal contrast reaction | 1 in 55,000 | Shehadi 1975,164 Cashman 1991165 |
Risk of:
-
death or MI induced by exercise treadmill testing
-
developing radiation-related malignancy as a consequence of CTCA
-
fatal anaphylactic reaction to contrast media associated with ICA and CTCA
-
MI caused by ICA.
Costs
Costs were assumed to be incurred in a similar manner to the diagnostic model. TPs and FPs incurred the costs of hospital admission and coronary angiography. TPs then incurred the costs of coronary intervention. All patients who suffered a non-fatal MI incurred an associated unit cost. TPs and FNs that did not die incurred lifetime costs of treatment for CAD. The costs included in the prognostic model are:
-
all biomarker measurement costs
-
coronary intervention costs
-
subsequent cardiac events
-
lifetime costs of care for patients with CAD.
Lifetime costs were estimated according to patient age and sex, whether or not they had MI, and whether or not they suffered reinfarction . The lifetime costs for MI patients are estimated using the annual costs from Ward et al. 157 and the discounted life expectancy of patients with MI captured from Polanczyk et al. 156
The cost of reinfarction was estimated as a one-off cost of £3587 from NHS reference costs. 161 The costs are outlined in Tables 56 and 57.
| Diagnostic test | Source | Estimate (£) | 95% CI (£) |
|---|---|---|---|
| CTCA | NHS ReferenceCosts161 | 109 | 90 to 206 |
| Exercise ECG | Mowatt25 | 69 | 66 to 107 |
| H-FABP | RATPAC160 | 20 | 18 to 22 |
| ICA | Mowatt25 | 1032 | 850 to 1100 |
| Age (years) | MI cost (£) |
|---|---|
| 30–44 | 4012.5 |
| 45–54 | 3115 |
| 55–64 | 2215 |
| 65–74 | 1530 |
| >75 | 800 |
Modelling methodology
A model was developed using DecisionPro (Vanguard Software Corporation, Cary, NC, USA) to explore the costs and health outcomes associated with different prognostic strategies. The analysis was conducted for troponin-negative patients aged 40–75 years after initial hospital assessment. The model takes a lifetime horizon with mean life expectancy based on UK interim lifetables. 158 The economic perspective of the model is the NHS in England and Wales.
Deterministic results of the prognostic model
The main deterministic analysis for the prognostic model, using the 1-year event rates from Mills,155 is shown in Table 58. The total costs increase in proportion to the cost of the test involved and the QALYs in proportion to the prognostic value of the test. Although we assumed ICA had perfect prognostic value it incurred a significant QALY loss due to procedure-related adverse events. Exercise ECG was subject to extended domination. H-FABP and CTCA would both be considered cost-effective compared with the NICE threshold of £20,000–30,000/QALY. CTCA is the more effective of these two strategies and would therefore be considered optimal. Although ICA is slightly more effective than CTCA, the ICER of £219,532/QALY substantially exceeds the usual NICE threshold for decision-making.
| Strategy | Total costs (£) | Total QALYs | ICER (£/QALY) |
|---|---|---|---|
| No testing | 374,040 | 11,891.14 | – |
| Exercise ECG | 678,120 | 11,917.75 | Extendedly dominated |
| H-FABP | 544,340 | 11,911.26 | 8464 |
| CTCA | 937,426 | 11,946.86 | 11,041 |
| ICA | 1,705,790 | 11,950.36 | 219,532 |
The analysis was repeated using 3-month event rates from the RATPAC trial12 and the implicit assumption that events were only influenced by testing up to 3 months. The results are shown in Table 59. Changing the assumed baseline rate of adverse events and the time horizon over which initial diagnostic testing could influence event rates markedly reduced the estimated QALY gains from diagnostic testing strategies compared with no testing. ICA even appeared to be less effective than no testing, presumably because the negative effect of procedure-related events outweighed the benefit of reducing subsequent adverse outcome in a low risk population. Although the other strategies gained a small number of QALYs compared with no testing, exercise ECG was dominated by H-FABP and both H-FABP and CTCA accrued QALYs at with a very high ICER. Therefore, assuming the adverse event rate from the RATPAC trial,12 the no-testing strategy appeared to be optimal.
| Strategy | Total costs (£) | Total QALYs | ICER (£/QALY) |
|---|---|---|---|
| No testing | 260,901 | 12,180.71 | – |
| Exercise ECG | 590,601 | 12,181.78 | Dominated |
| H-FABP | 449,520 | 12,182.57 | 101,408 |
| CTCA | 876,680 | 12,184.20 | 262,061 |
| ICA | 1,656,701 | 12,176.07 | Dominated |
The cost-effectiveness of CTCA therefore appears to depend on the assumed rate of subsequent death and non-fatal MI. Given the uncertainty in these risks, we performed ‘goal-seeking’ analysis to identify the level of risk at which the ICER for CTCA crosses the NICE threshold of £20,000–30,000/QALY compared with either H-FABP, ETT or no testing. We assumed a proportional relationship that risk of non-fatal MI is four times the risk of death. The results are shown in Table 60. Depending on the threshold used, CTCA is likely to be cost-effective if the combined risk of death and non-fatal MI within the time period assumed to be influenced by initial diagnostic testing exceeds 2% (£30,000/QALY threshold) or 2.9% (£20,000/QALY threshold).
| Threshold (£/QALY) | Risk of non-fatal MI | Risk of death |
|---|---|---|
| 20,000 | 0.023 | 0.0057 |
| 30,000 | 0.016 | 0.0041 |
Probabilistic results of the prognostic model
The main probabilistic analysis for the prognostic model, using the 1-year event rates from Mills,155 is shown in Figure 41. CTCA had the highest probability of being cost-effective at thresholds above £10,000/QALY. Around £10,000 H-FABP had the highest probability, and below this level no testing had the highest probability of being cost-effective.
The main probabilistic analysis for the prognostic model, using the 1-year event rates from RATPAC,12 is shown in Figure 42. No testing was highly likely to be the most cost-effective strategy for all thresholds of < £100,000/QALY.
FIGURE 42.
Probability of cost-effectiveness of strategies using RATPAC data. 12 MAICER, maximum acceptable incremental cost-effectiveness ratio.
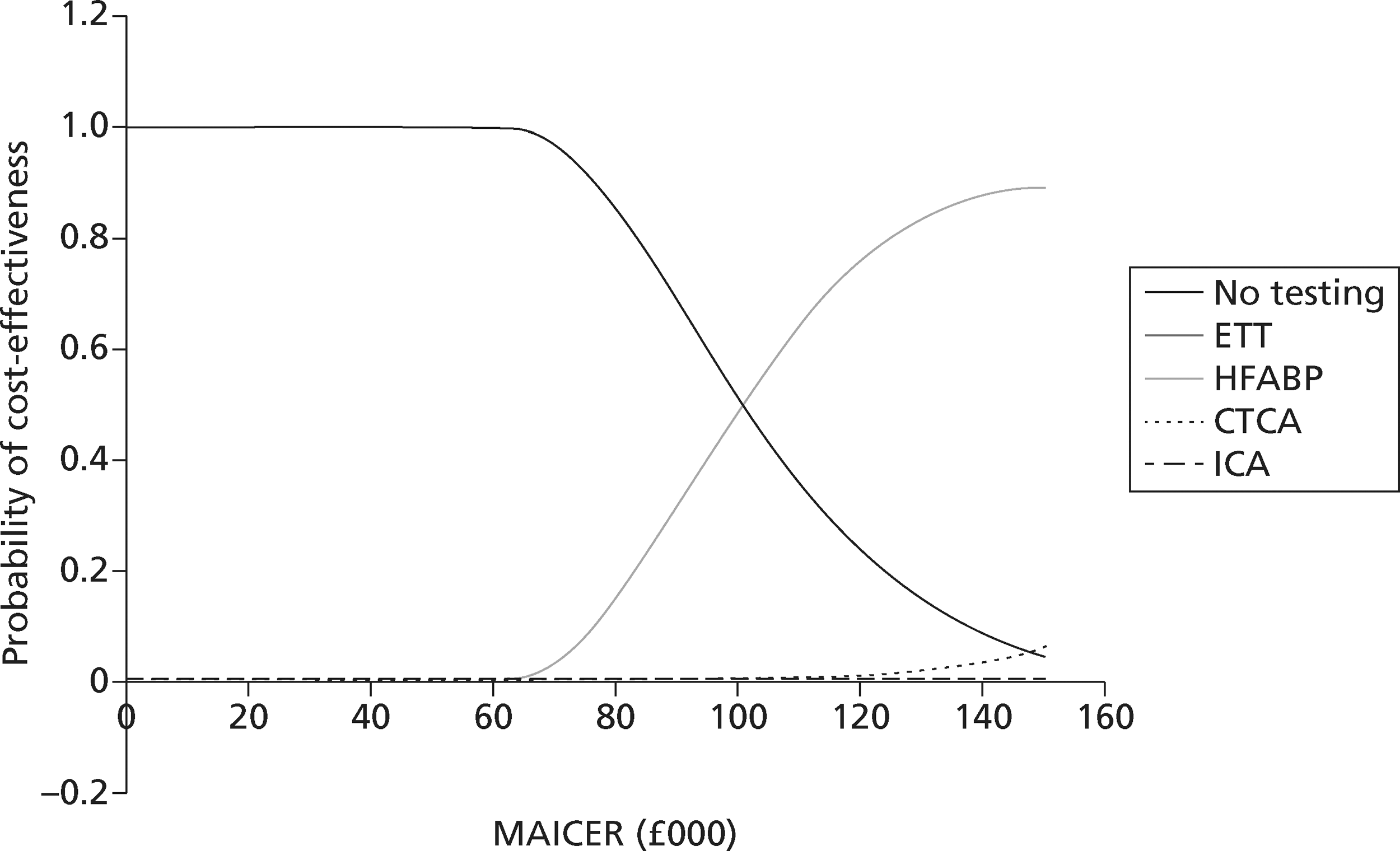
Value of information analyses
There is always a chance that the wrong decision will be made as a result of the uncertainty in the existing information and the costs in terms of health benefit and resources forgone owing to this uncertainty can be interpreted as expected value of perfect information (EVPI). Perfect information would eliminate the possibility of making the wrong decision and therefore EVPI is determined jointly by the probability that a decision based on existing information will be wrong and the consequences of a wrong decision.
The EVPI, although calculated for individual patients, can also be expressed for the total population of patients who stand to benefit, based on prevalence and the lifetime of the technology. This can also be thought as the maximum that the health-care system should be willing to pay for additional evidence to inform the decision in the future and thus is an upper bound on the value of conducting further research, i.e. if the population EVPI exceeds the expected costs of additional research then it is potentially cost-effective to conduct further research.
Partial EVPI provides the value of reducing the uncertainty surrounding particular input parameters in the decision model and this can be used to identify the parameters for which more precise estimates would be most valuable to focus further research. However, this is computationally expensive for complex models.
Expected value of perfect information
The individual patient EVPI for the prognostic model is illustrated in Figure 43. At low and high thresholds for cost-effectiveness, additional information is unlikely to change that decision. The EVPI reaches maximum when there is most uncertainty about whether to adopt or reject the technology based on existing evidence, i.e. at a threshold of £19,000/QALY.
FIGURE 43.
Individual patient EVPI.
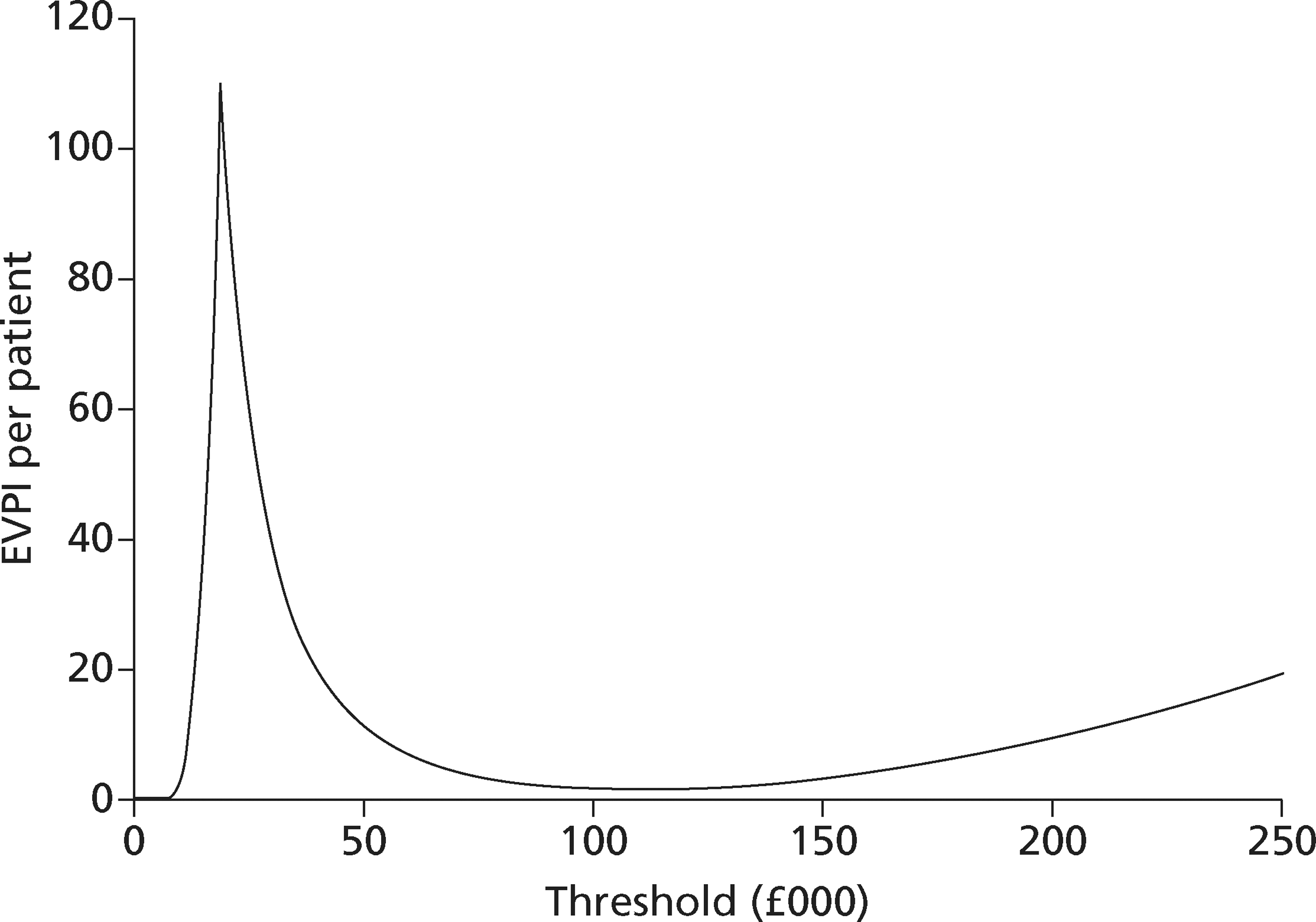
The EVPI for the whole population can be estimated as ‘EVPI per patient multiplied by the number of patients affected by the decision over the lifetime of the technology’. Assuming an incidence of 1000 patients of the disease per year and a lifetime of 10 years for the technology, the undiscounted population EVPI at the threshold of £19,000/QALY is £1.09M.
Expected value of partial perfect information
The expected value of partial perfect information (EVPPI) details associated with the parameters are illustrated in Figures 44 and 45. At the threshold of £20,000/QALY, EVPPIs associated with baseline risk of MI and relative reduction in risk of adverse events after treatment are higher than the EVPPIs associated with the rest of the parameters. However, at the threshold of £30,000/QALY, only the EVPPI associated with relative reduction in risk of adverse events is significant.
FIGURE 44.
Individual patient EVPPI at £20,000/QALY.
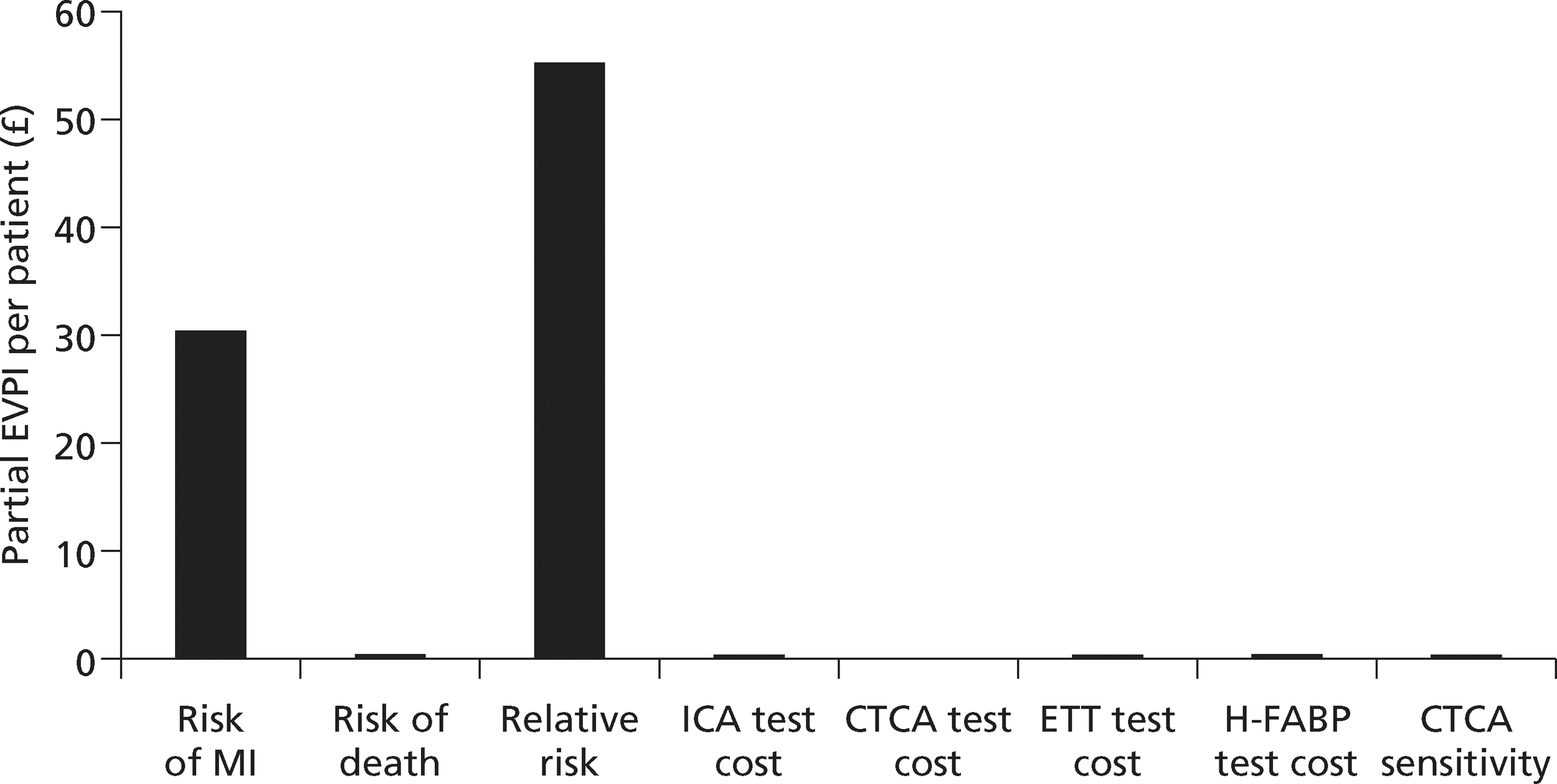
FIGURE 45.
Individual patient EVPPI at £30,000/QALY.
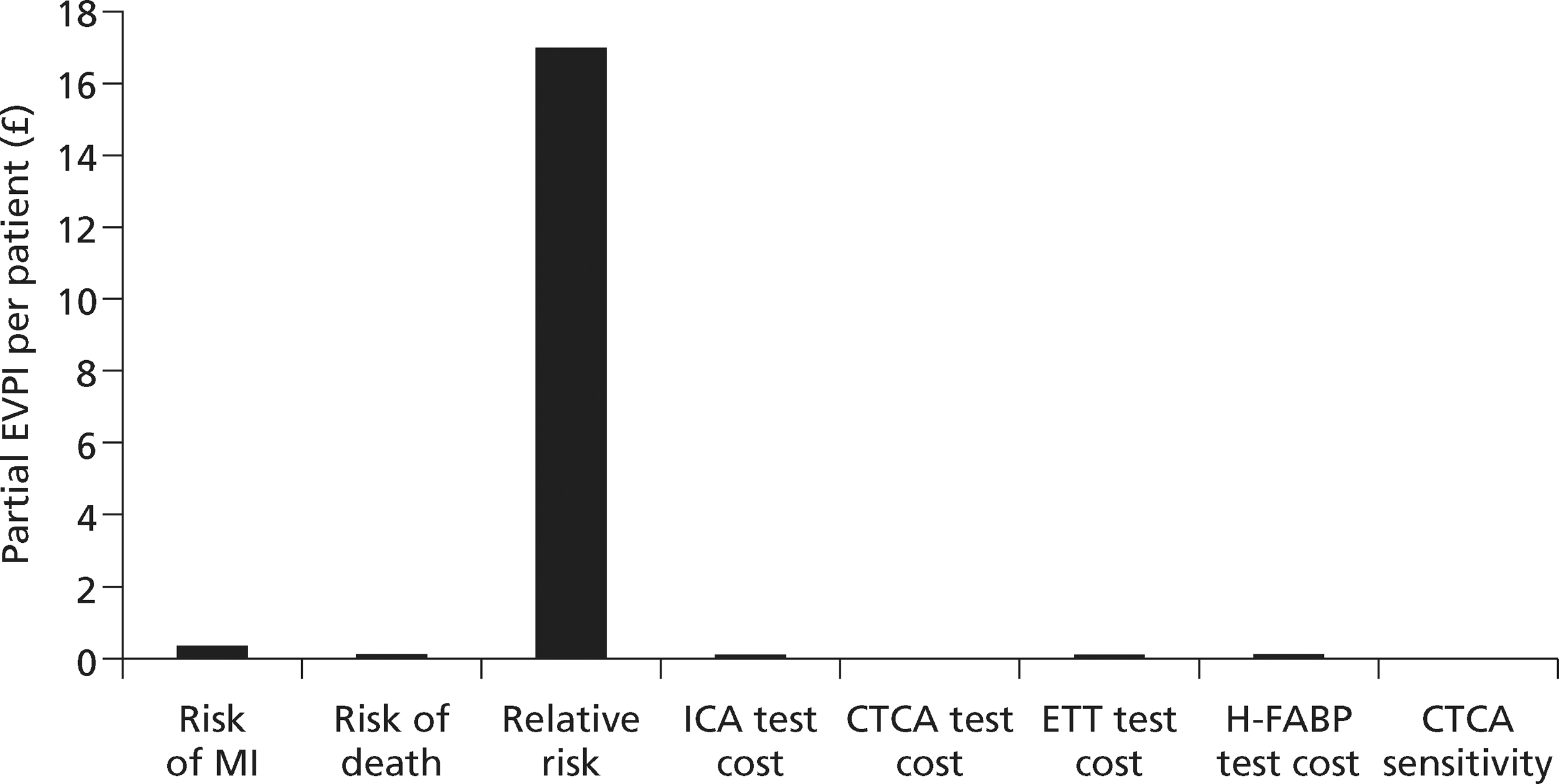
Around the NICE threshold, assumed to be between £20,000 and £30,000/QALY, the EVPPIs associated with both these parameters are relatively high suggesting that further experimental research will potentially be cost-effective.
Chapter 5 Discussion
Statement of principal findings
Diagnostic accuracy of presentation biomarkers for myocardial infarction
A large number of studies have estimated the accuracy of troponin at presentation for diagnosing MI, compared with a reference standard based on the universal definition using delayed troponin testing. Many of these are limited by inadequacies of the troponin assay used as the index test or reference standard, whereas differences in the assays and threshold used limited our ability to compare and synthesise data from different studies. We restricted meta-analysis to studies using similar or the same assay at a comparable diagnostic threshold and using a reference standard based on a modern troponin assay with an acceptable diagnostic threshold. Even in these analyses there was substantial heterogeneity between results, which is reflected in the wide predictive intervals around each estimate.
Our meta-analysis showed that the sensitivity and specificity of TnI at presentation were 77% (95% predictive interval 29% to 96%) and 93% (95% predictive interval 46% to 100%), respectively, when the 99th percentile was used and 82% (95% predictive interval 40% to 97%) and 93% (95% predictive interval 74% to 98%) when the 10% CV was used. The corresponding results for TnT were 80% (95% predictive interval 33% to 97%) and 91% (95% predictive interval 53% to 99%) when the 99th percentile was used and 74% (95% predictive interval 34% to 94%) and 96% (95% predictive interval 76% to 99%) when the 10% CV was used. When analysis was restricted to high-sensitivity assays we found that the Roche HsTnT assay had a sensitivity of 96% (95% predictive interval 27% to 100%) and a specificity 72% (95% predictive interval 3% to 96%), the ADVIA Centaur Ultra troponin I assay had a sensitivity of 86% (95% predictive interval 22% to 96%) and a specificity 89% (95% predictive interval 40% to 97%), and the Abbott Architect troponin I assay had a sensitivity of 83% (95% predictive interval 58% to 95%) and a specificity 95% (95% predictive interval 67% to 100%).
The differences in estimates of sensitivity and specificity for different assays may reflect differences in study methods and populations, but they suggest that using a lower threshold for positivity and high-sensitivity assay improves sensitivity at the expense of specificity. It is not entirely clear whether this loss of specificity represents the expected loss of specificity that is seen whenever the threshold for positivity is lowered for an imperfect test, or whether the apparent FPs may actually be TPs misclassified by an inadequate reference standard. We identified one study60 that seemed to suggest the former, but further data are required an address this issue. Such data would also determine whether the estimates of sensitivity for troponin at presentation are lower when compared with a high-sensitivity reference standard.
The findings suggest that high-sensitivity assays have sufficient sensitivity at presentation to identify most cases of MI that would subsequently be identified by a standard 10-hour troponin test, but there is substantial uncertainty around these estimates and a significant proportion with MI will be missed by presentation troponin testing. Whether or not this means that 10-hour troponin testing should be undertaken depends on the costs and benefits of detecting additional cases and is explored in detail in the economic analysis.
We also sought studies of other biomarkers measured at presentation to determine their accuracy for MI either alone or in combination with troponin. Only myoglobin and H-FABP had been evaluated against an acceptable reference standard in a large number of studies. Our meta-analysis of H-FABP showed that the summary estimates of sensitivity and specificity were 81% (95% predictive interval 50 % to 95%) and 80% (95% predictive interval 26% to 98%), respectively, for the quantitative assays and 68% (95% predictive interval 11% to 97%) and 92% (95% predictive interval 20% to 100%), respectively, for the qualitative assays. Our meta-analysis of myoglobin showed that the summary estimates of sensitivity and specificity were 62% (95% predictive interval 35% to 83%) and 83% (95% predictive interval 35% to 98%), respectively. These findings suggest inadequate diagnostic accuracy to act as a single diagnostic test for MI at presentation.
A few studies reported the accuracy of alternative biomarkers in combination with troponin at presentation, with the combination being positive if either marker were positive. H-FABP, copeptin, IMA and myoglobin improved sensitivity for MI at presentation but at the expense of loss of specificity. However, the estimates of diagnostic accuracy for presentation troponin alone varied substantially in these studies and used an unclear threshold for positivity in some cases. Our meta-analysis suggests that high-sensitivity troponin assays can achieve similar sensitivity to the biomarker and troponin combination with a similar loss of specificity. Future evaluations of alternative biomarkers at presentation should include measurement of a high-sensitivity troponin assay to determine whether or not the biomarker still produces an incremental improvement in sensitivity.
Prognostic accuracy of biomarkers for predicting major adverse cardiac events
The prognostic value of troponin is well established8 and elevated troponin levels is associated with increased potential to benefit from treatment. 9,155 As a result, troponin is established as an essential biomarker in the assessment of suspected ACS. We identified a large number studies evaluating the ability of other biomarkers to predict MACEs in patients with suspected ACS. However, many of these simply evaluated whether there was an association between biomarker levels and risk of MACEs. In clinical assessment, the ECG and troponin are already established in routine practice on the basis of value in predicting adverse outcome, so any new biomarker would need to demonstrate additional prognostic value beyond routine assessment. We found some evidence that BNP, NT-pro-BNP, MPO and H-FABP could predict MACEs even after adjustment for troponin and other variables in multivariate analysis. However, results were sometimes inconsistent and it was not always clear whether or not all potentially important covariates had been included in analysis. We also found evidence that CRP, PAPP-A and H-FABP could predict MACEs in troponin-negative patients. These findings were based on a small number of heterogeneous studies with differing methods of analysis and there was some inconsistency in the findings. Meta-analysis was not possible so the estimates of RR were based on single studies and should be interpreted with caution.
Diagnostic accuracy of computed tomographic coronary angiography and exercise electrocardiography for coronary artery disease
The diagnostic accuracy of CTCA and exercise ECG for identifying CAD in patients with stable symptoms has been extensively studied and summarised in previous meta-analyses. We aimed to determine whether or not similar estimates existed in patients presenting to hospital with suspected ACS.
We identified eight studies comparing CTCA to conventional coronary angiography in patients presenting with suspected ACS, reporting sensitivities ranging from 83% to 100% and specificities ranging from 54% to 100%. The summary estimates for sensitivity and specificity were 93% (95% predictive interval 61% to 99%) and 87% (95% predictive interval 16% to 100%), respectively. The studies were relatively small, evaluated various different techniques and used different methods of analysis, so there are a number of potential explanations for the variation in results. Only one study126 used 64-slice CT and this reported the highest sensitivity and specificity (both 100%). The other studies used 16- or 4-slice CT and reported lower sensitivity and specificity.
Our findings are similar to other published reviews. Mowatt et al. 25 sought all diagnostic studies of CTCA and included 18 studies with 1286 patients in the meta-analysis. Most of the included studies were of patients with stable symptoms rather than suspected ACS. Sensitivity ranged from 94% to 100%, with a pooled sensitivity of 99% (95% CrI 97% to 99%). Specificity ranged from 50% to 100%, with a pooled specificity of 89% (95% CrI 83% to 94%). Athappan et al. 167 included 16 studies of CTCA in acute chest pain. The pooled sensitivity and specificity for ACS were 0.96 (95% CI 0.93 to 0.98) and 0.92 (95% CI 0.89 to 0.94), respectively. There was surprisingly little overlap between this review and ours. The studies of Sato et al. ,129 Tsai et al. 130 and Olivetti et al. 128 were included in both reviews. The other five studies we identified123–127 were not included in the Athappan review. We excluded four studies131,135,168,169 because only those with positive CTCA underwent ICA as the reference standard test, two studies170,171 because the reference standard was not based on ICA, and two studies172,173 because the study population were not patients with suspected ACS. We excluded studies that used reference standards other than CAD on ICA and studies that confirmed only CAD on ICA in those with a positive CTCA result because these studies will be prone to work-up bias and will overestimate diagnostic parameters. This probably explains why our estimates of sensitivity and specificity (albeit for CAD rather than ACS) were lower than those reported by Athappan et al. 167
The most recent meta-analysis23 of the diagnostic accuracy of exercise ECG reported that the main diagnostic criterion (ST depression) performed only moderately well, with a PLR of 2.79 for a 1-mm cut-off and 3.85 for a 2-mm cut-off. The negative likelihood ratios were 0.44 and 0.72, respectively. All of the included studies were of patients with chronic chest pain. We identified no studies that compared exercise ECG to ICA for the diagnosis of CAD in patients presenting with acute symptoms due to suspected ACS. We are therefore unable to determine whether the diagnostic accuracy of exercise ECG estimated in patients with stable symptoms can be extrapolated to those presenting with suspected ACS.
Prognostic accuracy of computed tomographic coronary angiography and exercise electrocardiography for predicting major adverse cardiac events
We identified seven studies that evaluated the prognostic accuracy of CTCA for major cardiac events in patients with suspected ACS. MACE rates were generally very low in patients with a negative CTCA but this may reflect selection of low-risk patients rather than accurate risk stratification by CTCA. Most of the events reported in patients with positive CTCA findings were process events (i.e. PCI or CABG), which, in an unblinded study, may simply reflect physicians acting on CTCA findings. However, one study136 reported an association between positive CTCA and MACEs (including revascularisation) despite patients and carers being blind to CTCA results. Furthermore, this study used multivariate analysis to show that CTCA findings predicted MACEs even after adjustment for a clinical risk score incorporating ECG and troponin. This study therefore shows that CTCA can provide potentially useful additional prognostic information, beyond routine clinical assessment with ECG and troponin. Despite this, the overall findings of our review suggested only weak evidence that CTCA findings predicted MACEs in patients with suspected ACS. The 95% CrIs of estimates of the RR of MACEs associated with positive CTCA were wide and included the possibility of no association.
A previous systematic review and meta-analysis by Hulten et al. 174 sought all prognostic studies of CTCA rather than just studies of patients with suspected ACS. Most studies included patients with stable symptoms rather than suspected ACS. Only the study by Rubinshtein et al. 135 was included in this review and ours. Hulten et al. 174 included 18 studies evaluating 9592 patients with a median follow-up of 20 months. The pooled annualised event rate for obstructive (any vessel with 50% luminal stenosis) compared with normal CTCA was 8.8% compared with 0.17% per year for MACEs (p < 0.05) and 3.2% compared with 0.15% for death or MI (p < 0.05). These findings suggest that abnormalities on CTCA predict an increased risk of a MACE in patients with suspected CAD and that the risk of MACEs is very low if CTCA is normal. Our review confirms that the low risk of a MACE associated with CTCA is also seen in patients with suspected ACS but the low overall rate of adverse outcome means that we cannot be sure whether this reflects low-risk patient selection or effective risk stratification by CTCA.
We identified 13 studies reporting risk of MACEs after ETT for patients presenting to hospital with suspected ACS. Overall, MACE rates were generally low among patients with negative ETT results. There was some evidence that positive tests identified higher-risk patients and were associated with an eightfold increase in the risk of a MACE. However, as with CTCA, in unblinded studies higher rates of revascularisation among patients with positive ETT may reflect physician awareness and expectation of a need for revascularisation. There was evidence from some studies that death and MI rates were higher among patients with positive ETT, although the modest numbers limit the conclusions that may be drawn. No studies reported multivariate analysis to determine whether exercise ECG added to the prognostic value of routine clinical assessment, including ECG and troponin, although most of the studies excluded patients with diagnostic ECG changes.
Cost-effectiveness of presentation biomarker strategies for myocardial infarction
We developed a decision-analysis model to compare different strategies for using biomarkers at presentation with a no testing (discharge all home) and delayed troponin (admit and measure troponin at 10 hours) strategies. We tested presentation TnT using either the 10% CV or 99th percentile as the diagnostic threshold and using a high-sensitivity assay with the 99th percentile as the diagnostic threshold. We selected these strategies because the estimates from our meta-analysis would allow us to investigate the effect of varying the diagnostic threshold on sensitivity and specificity, and thus on cost-effectiveness. We tested the strategies in various scenarios to examine whether (1) the presence or absence of known CAD and (2) the inpatient management, in terms of access to a decision-making doctor, influenced cost-effectiveness. We also tested presentation high-sensitivity TnI instead of HsTnT, because the point estimate of sensitivity was lower and specificity higher in our meta-analysis, and a 3-hour high-sensitivity troponin strategy, because recent analysis suggests that this improves sensitivity but provides a strategy that can be applied without hospital admission.
The results showed that, as expected, effectiveness (QALYs) increased with increasing sensitivity and costs increased with decreasing specificity. In all but one scenario a strategy of measuring HsTnT at presentation (with admission for a 10-hour troponin testing if positive and discharge home if negative) was the optimal strategy. It was the most effective strategy among those with an ICER of < £20,000–30,000/QALY. The 10-hour troponin testing was more effective, but had an ICER that exceeded the £30,000/QALY threshold. In one scenario the 10-hour troponin strategy may have been optimal, i.e. if the patient did not have known CAD, a doctor was available on demand to discharge the patient when the 10-hour troponin level was measured and the £30,000/QALY threshold was used.
These findings suggest that in most circumstances delaying troponin measurement until 10 hours is unlikely to represent a cost-effective use of NHS resources. The exception to this may be a setting where the decision-making is efficient enough to ensure that patient discharge can occur as soon as the 10-hour troponin result is available. However, there are a number of assumptions in the model that need to be taken into account when interpreting these findings, two of which were explored in sensitivity analysis.
Our meta-analysis suggested that presentation HsTnT has sensitivity of 96%, but this was based on only two studies. The uncertainty around the estimate was reflected in the wide predictive interval around this estimate, which was used in the cost-effectiveness modelling. If this is an overestimate of sensitivity, then we will have underestimated the comparative cost-effectiveness of the 10-hour troponin strategy. This is supported by our sensitivity analysis using estimates for the ADVIA Centaur Ultra troponin I assay instead of Roche HsTnT. When the lower estimate of sensitivity was used for presentation high-sensitivity troponin (and higher estimate of specificity), the 10-hour troponin strategy was more likely to be cost-effective. However, it was still likely to be optimal in only one scenario if the £20,000/QALY threshold were used and in three scenarios if the £30,000/QALY threshold were used.
Our main analysis also assumed that the only alternative strategies were presentation troponin or 10-hour troponin testing because these were the strategies with the best supporting data at the time the study was planned. However, a recent analysis suggested that measuring troponin at presentation and 3 hours later could optimise sensitivity yet still provide a strategy that does not require hospital admission in most cases. When we tested the 3-hour strategy in a sensitivity analysis, we found that it was optimal in all scenarios at both the £20,000/QALY and £30,000/QALY threshold, whereas the 10-hour strategy was not cost-effective in any scenario using either threshold. This suggests that high-sensitivity troponin measured at presentation and 3 hours later is the optimal strategy for MI diagnosis. However, this finding is based on data from a single study. The CI for sensitivity derived from this single study is unlikely to reflect the true extent of uncertainty in the way that the predictive interval from our meta-analysis does. Furthermore, if the study population characteristics differ from the UK population, particularly in terms of time delay before presentation, then the findings may not be generalisable to the UK.
We also assumed that the 10-hour troponin testing was diagnostically perfect (i.e. had 100% sensitivity and specificity). This assumption was necessary because the 10-hour troponin test is effectively the reference standard test for MI, so modelling outcomes following FN or FP 10-hour troponin testing would involve contentious and untestable assumptions. Although this assumption affects all the strategies, because they use the 10-hour troponin result to confirm MI, it favours the 10-hour strategy most.
Another assumption in our model that favours the 10-hour troponin strategy is that a patient with a FN troponin result at presentation was assumed to have the same prognosis (and thus the ability to benefit from treatment) as a patient with a TP troponin result at presentation. However, this assumption may not hold if those with a FN troponin result at presentation have a smaller infarct and better prognosis. We were unable to find adequate data to test this assumption.
Having compared presentation high-sensitivity troponin to 10-hour standard troponin, the obvious next question is whether or not the 10-hour troponin test should be of high sensitivity. We are unable to address this question because (1) our model assumes that the standard troponin assay at 10 hours is perfect for the reasons given above; (2) there are few data available to estimate presentation troponin accuracy in comparison with a high-sensitivity reference standard; and (3) the prognostic and therapeutic implications of a positive high-sensitivity troponin alongside a negative standard troponin are not clear. Our analysis only evaluated the role of high-sensitivity troponin in terms of an early biomarker rather than as an alternative to a 10-hour standard troponin.
Finally, our model assumes that patients awaiting troponin testing are cared for in hospital (even if not formally admitted) and therefore incur hospital costs. It could be argued that the benefits of delayed troponin testing could be accrued without most of the costs if patients were discharged home and asked to return for delayed testing. However, the feasibility and acceptability of this practice has not been tested and it is not routinely used.
The diagnostic decision-analysis model was also used to test the cost-effectiveness of H-FABP, copeptin, myoglobin and IMA measured at presentation alongside troponin, compared with troponin alone at presentation or 10 hours. There was substantial variation in estimates of troponin sensitivity at presentation in the sources studies for this analysis. This meant that we could not reasonably use our meta-analysis estimates of presentation troponin sensitivity and specificity in this particular analysis, as this would paradoxically result in the biomarker plus troponin sensitivity being lower than troponin alone in some analyses. We therefore used the individual studies to estimate the accuracy of troponin alone and undertook a separate analysis for each study. As a result, some of the analyses that were based on studies with low estimates of troponin sensitivity at baseline produced results that were inconsistent with our main analysis and suggested that a 10-hour troponin test would be cost-effective at the £30,000/QALY or even £20,000/QALY threshold. This is because we could not include the optimal strategy from the main analysis (high-sensitivity troponin at presentation) with our best estimate of sensitivity and specificity in the analysis.
The economic analysis of alternative biomarkers suggested that adding H-FABP, copeptin or myoglobin to troponin at presentation could be cost-effective, i.e. could improve sensitivity and thus QALYs at an acceptable cost per QALY. Adding IMA to troponin at presentation, in contrast, was unlikely to be cost-effective. These findings are obviously limited by our inability to include the optimal strategy with best estimates of sensitivity and specificity in the analysis. The findings of the meta-analyses suggest that the changes in sensitivity and specificity resulting from adding another biomarker at presentation are similar to the changes resulting from using a high-sensitivity troponin assay with a low threshold for positivity. If one assay can provide the same result as a combination, then it is likely to be more cost-effective.
We also used our economic model to produce estimates of 1-year rates of death and non-fatal MI among (1) all patients presenting with suspected ACS and (2) those discharged after negative assessment, for the main strategies tested. These estimates show how using more sensitive strategies decreases the expected risks of adverse outcome and could be used by clinicians attempting to weigh up the risks and benefits of different strategies for the individual patient. They could also be used, given a sufficiently interested and informed patient, to explain the potential risks and benefits of different strategies to the individual patient, potentially allowing them to participate in shared decision-making.
Cost-effectiveness of biomarkers, computed tomographic coronary angiography and exercise electrocardiography in troponin-negative patients
We developed a second (prognostic) decision-analysis model to evaluate the cost-effectiveness of using a biomarker (H-FABP), exercise ECG or CTCA to select troponin-negative patients for further investigation with ICA if positive or current standard care if negative. These strategies were compared with current standard care for all and ICA for all. We assumed that current standard care involved further investigation according to NICE guidance for stable chest pain11 if symptoms persisted or recurred. The benefit of investigation clearly depended on the subsequent risk of death and non-fatal MI, and we had two sources for this with contrasting estimates and implicit assumptions. Data from an observational study of patients admitted to hospital with suspected ACS155 produced an estimate of 1.0% for death and 3.9% for MI up to 1 year, whereas data from a randomised trial of ED chest pain assessment12 produced corresponding estimates of 0.19% and 0.24%. The difference in these estimates reflects patient selection and duration of follow-up. In using either data source in the model we make an implicit assumption about the duration of effect of initial testing. Using the Mills data155 we assumed that initial testing influences outcomes up to 1 year, whereas the RATPAC data12 assumes that initial testing only influences outcomes up to 3 months. There obviously is a limit to the effect of initial testing compared with current standard care as standard care involves subsequent investigation if symptoms recur or persist. However, it is not clear when this limit is.
The analysis showed that the estimate of the adverse event rate and associated implicit assumption regarding the duration of potential effect of initial testing on outcome were important in determining cost-effectiveness. If the higher estimates of adverse outcome and 1-year duration of effect were used, then CTCA was likely to be the optimal strategy at the NICE threshold for willingness to pay. If the lower estimates of adverse outcome and 3-month duration of effect were used, then the no-testing strategy was likely to be optimal. A threshold analysis suggested that CTCA was likely to be cost-effective if the estimated combined risk of death and non-fatal MI within the duration of effect of initial testing were > 2% or 3%, depending on the threshold used for willingness to pay (£20,000 or £30,000/QALY). It is important to note that this analysis was driven by the effectiveness of the strategies rather than costs, and outcomes associated with a high rate of referral for ICA were little better than no testing. This emphasises the importance of specificity in prognostic testing and the need to ensure that diagnostic thresholds are set and tests interpreted in a way that does not result in a large number of FP cases being referred for ICA.
The value of information analysis associated with this model showed that around the NICE threshold, assumed to be between £20,000/QALY and £30,000/QALY, the EVPPIs associated with the baseline risk of MI and the relative reduction in risk with treatment were relatively high, suggesting that further experimental research of these parameters will potentially be cost-effective. Research estimating the effect of treatment on patients identified as being at increased risk by CTCA is unlikely to be considered ethical, but research comparing a strategy of liberal compared with restrictive CTCA use (with treatment being consequent on CTCA findings) would be more likely to be considered ethical and would provide an estimate of the effect of treatment.
Strengths and limitations of the study
Systematic review and meta-analysis
The systematic review and meta-analysis was undertaken in accordance with the guidelines published by the Centre for Reviews and Dissemination for undertaking systematic reviews26 and the Cochrane Diagnostic Test Accuracy Working Group on the meta-analysis of diagnostic tests. 27 Our literature search was extensive and retrieved a large number of studies. We deliberately developed selection criteria that would limit the review to high-quality studies using a relevant and well-recognised reference standard. This involved excluding studies that used the old World Health Organization definition of MI as a reference standard and studies that used a composite outcome of ACS instead of MI alone (or did not report MI alone) as their reference standard. This had the advantage of ensuring a reasonable degree of homogeneity among the reference standard tests and excluded studies that risked having a reference standard (ACS) that included subjective clinical judgements and possibly elements of the index test. However, this approach could be criticised because it potentially excludes studies of important outcomes, such as unstable angina, that are not included in the reference standard.
Our meta-analysis did not include direct comparison of different biomarkers or assays (i.e. comparing different biomarkers or assays in the same cohorts). Our estimates of the diagnostic accuracy of different biomarkers or assays are therefore indirect (i.e. based on testing in different cohorts), so differences in accuracy may be explained by differences in cohort characteristics rather than the biomarker or assay performance. We did not undertake direct comparisons because, as Table 2 shows, most studies only analysed one or two biomarkers. Where multiple biomarkers were analysed in the same cohort there was little consistency between studies in terms of the biomarkers or assays tested.
Although we used a reasonably well-defined reference standard for the diagnostic biomarker review, there was still substantial variation in the tests used to confirm the reference standard, particularly the troponin assay used, threshold for positivity and the timing of sampling. Alongside variation in study populations and variation in index test assays, thresholds and timing, this probably explains the heterogeneity observed between the results of different studies.
We were unable to be as selective when defining the reference standard, or outcome, for the prognostic studies. There was substantial variation in the definition of MACEs and the duration and intensity of follow-up. In particular, some studies included process measures, such as revascularisation, in their definition of MACEs. If the decision to undertake a process is made by someone who is aware of the index test results then process measures are subject to bias and estimates of prognostic outcome will be consequently inflated. Given the limitations of the primary data it could be argued that summary estimates generated by our meta-analysis are misleading. We undertook meta-analysis because we felt that it would be helpful to have an overall estimate of prognostic value but urge caution in the interpretation of these estimates.
Although our literature search retrieved a large number of studies, it was limited by substantial variation in the terms used to describe tests and outcomes. As a result we retrieved a proportion of studies through expert contact, reviewing citation lists and other serendipitous means, rather than through the planned searches. This was particularly the case for the review of exercise ECG, where a wide range of different terms was used to classify studies that reported follow-up of cohorts of patients receiving exercise ECG after presentation with suspected ACS. Consequently, it is possible that despite our best efforts we have missed potentially eligible studies that could have contributed to the review.
Economic evaluation
The economic analysis used current best practice to develop the model and followed recommendations produced by NICE. 175 We used Bayesian methods to synthesise the data from the meta-analysis and generate probability distributions associated with the diagnostic accuracy in the model that fully reflect uncertainty about these parameters. We were also fortunate to have data from the Mills study155 to provide an estimate of the benefit of a positive reference standard test in the diagnostic model. Such estimates are unusual and modelling the benefit of diagnostic tests often involves relying on expert opinion to estimate treatment effects. We used expert opinion to build the model and develop our assumptions but did not need to draw upon expert opinion for parameter estimates.
As with any economic analysis, the model involved some important and influential assumptions. Most of these have been discussed alongside the summary of main findings above, as an appreciation of their impact is essential to understanding the model output. An additional assumption in the model is that medical decision-making flows in a predictable and consistent manner from the results of diagnostic testing. This obviously may not hold in practice and previous trials12 have been invaluable in testing assumptions that diagnostic test results will lead to predictable changes in patient care. Further research is required to test some of the assumptions in our model. For example, we assumed that the implication of a FP presentation biomarker was limited to the cost of keeping the patient in hospital until a definitive 10-hour troponin level was measured. We also assumed that the diagnostic testing strategy did not influence the location of patient admission (e.g. use of coronary care) and that patients would be discharged if tests were negative. These assumptions were justified on the basis of absence of evidence to challenge them and/or the practical difficulties of incorporating them into the model rather than available evidence to suggest they are not relevant or influential.
We only tested a limited range of potential strategies addressing specific issues in patient management. We typically limited the strategies tested to those with sufficient data to support them. This means that we did not test potentially worthwhile strategies with limited data, such as 6-hour strategies, or pragmatic strategies, such as selecting patients to delayed diagnostic testing or subsequent prognostic testing on the basis of clinical risk. In particular, we only tested using H-FABP as a prognostic marker in troponin-negative patients by assuming it would be used to select patients for ICA. A more logical approach might involve using H-FABP to select patients for CTCA. However, this would involve making an assumption about whether or not the prognostic value of H-FABP and CTCA are independent. We had no data to allow us to test this assumption, yet this interaction is crucial to determining the cost-effectiveness of the combination.
A substantial limitation of the prognostic model is that we had no data to directly estimate the benefit of treating positive cases, in the way that we had for the diagnostic model. 155 Therefore, we assumed that the effect of identifying and treating an increased risk of adverse outcome in the prognostic model was the same as the effect of identifying and treating MI in the diagnostic model. This assumption may not hold and, in combination with the uncertainty about the risk of subsequent adverse events discussed earlier, means that the benefit of identifying positive cases in the prognostic model is extremely uncertain. A further limitation of the prognostic model relates to limitations of the primary data. The heterogeneity in the definition of MACEs and follow-up procedures, and the potential for bias is discussed above, but other limitations of the primary data relate to implementation of the technology. Whereas, biomarkers are mostly quantitative tests with clear diagnostic thresholds, CTCA and exercise ECG require careful interpretation. Issues such as interobserver error and the training and expertise required for interpretation have not been extensively studied, creating more uncertainties about how these technologies will perform when put into practice.
Finally, the model assumed that all benefits from diagnostic testing were accrued as a result of the risk of adverse outcome. However, the testing process may have other benefits that are not captured in our model. Patients may benefit from the reassurance of negative testing or the opportunity to institute lifestyle changes stimulated by positive testing. The evidence for these benefits is limited and debatable but, if confirmed, could substantially alter the potential cost-effectiveness of diagnostic strategies.
Uncertainties
The main uncertainties identified in this report are:
-
The sensitivity and specificity of presentation high-sensitivity troponin compared with a delayed high-sensitivity troponin reference standard. Our analysis has provided estimates of the sensitivity and specificity of presentation high-sensitivity troponin compared with a delayed standard troponin reference standard, but it is not clear whether a delayed high-sensitivity troponin might (1) identify additional cases, thus reducing the sensitivity of presentation testing, and/or (2) demonstrate that apparently FP cases on presentation high-sensitivity testing are associated with a prognostically significant elevation on delayed high-sensitivity testing.
-
The prognostic and therapeutic importance of late troponin rises and low troponin rises on high-sensitivity testing. Our analysis assumed that all troponin rises above the 99th percentile have the same prognostic significance, but this assumption needs testing.
-
Diagnostic comparison of alternative biomarkers alongside troponin at presentation to high-sensitivity troponin alone. We found evidence that adding H-FABP, myoglobin or copeptin to troponin at presentation improves sensitivity but reduces specificity for MI. It is not clear whether a similar improvement can be achieved by using a high-sensitivity troponin assay and/or lower threshold for troponin positivity or whether alternative biomarkers can still improve sensitivity when a high-sensitivity troponin assay is used.
-
The independent prognostic value of alternative biomarkers in suspected ACS. Among a large number of studies of biomarkers we only found a limited number that estimated the prognostic value of the biomarker after taking all other potential predictors into account and reported results in troponin-negative patients separately. Studies that simply show an association between biomarker level and risk of a MACE have little value. Prognostic studies are required that measure and adjust for all potentially useful clinical predictors and biomarkers.
-
The prognostic and therapeutic value of CTCA in patients with suspected ACS but negative troponin. CTCA has a potentially valuable role to play in further investigation of troponin-negative patients but the evidence identified in our review was limited by small sample size, poor reporting of CTCA positive cases and low MACE rates. It is therefore unclear whether CTCA provides useful prognostic information in this circumstance and whether or not CTCA improves patient outcomes at acceptable cost. The economic analysis suggested CTCA could be cost-effective but with some important uncertainties around estimates of baseline MACE risk, prognostic value of CTCA and therapeutic benefit from detecting increased risk.
-
The interaction between different prognostic tests in troponin-negative patients, particularly H-FABP and CTCA. Our review suggested that the best evidence (albeit still very limited) of a prognostically useful test in troponin-negative patients related to H-FABP and CTCA. Logically, these two tests could be used in combination with H-FABP being used to select high-risk patients for CTCA. However, this would be only worthwhile if the two tests independently predicted risk. Further research is required to determine whether this is the case.
Assessment of factors relevant to the NHS and other parties
The NICE guidance for the management of chest pain of recent onset11 suggests that patients attending hospital with suspected ACS should receive troponin testing on initial assessment and 10–12 hours after the onset of symptoms. The guidance does not specify whether a high-sensitivity troponin assay should be used and other biomarkers are not recommended, but the use of high-sensitivity troponin and other biomarkers are highlighted as a research priority.
Our systematic review and meta-analysis provides estimates of the sensitivity and specificity of high-sensitivity troponin and other biomarkers at presentation. These estimates suggest that high-sensitivity troponin assays have better early sensitivity than standard troponin assays but are not perfect, so reliance on presentation testing alone will miss cases of MI. Our economic analysis suggests that troponin testing at 10 hours, compared with high-sensitivity troponin testing at presentation, is likely to be cost-effective only if patients can be discharged as soon as 10-hour results are available and if the £30,000/QALY threshold for willingness to pay is used. If rapid discharge is not achieved, then 10-hour troponin testing is unlikely to represent a worthwhile use of NHS resources.
Our analysis also suggests than H-FABP, myoglobin or copeptin could improve detection of MI at presentation in a cost-effective manner. However, these findings are based on a small number of studies using a variety of troponin assays as comparison, so further research is required to determine whether other biomarkers can consistently improve the early sensitivity of high-sensitivity troponin in a cost-effective manner. The evidence is currently insufficient to support their routine use in the NHS.
The NICE guidance suggests that patients with negative troponin samples should be reassessed and, if myocardial ischaemia is suspected, the guidance for stable chest pain should be followed. 11 In practice, this is likely to mean that patients presenting to hospital with suspected ACS but negative troponin are selected for further investigation, perhaps on the basis of recurrent symptoms that are considered consistent with myocardial ischaemia. The NICE guidance highlighted that the European Society for Cardiology guidelines recommend exercise treadmill testing for these patients despite evidence of limited sensitivity and specificity, and identified evaluation of CTCA in troponin-negative patients as being a research priority.
Our systematic review identified limited evidence to show that CTCA has reasonable diagnostic accuracy for CAD in patients with suspected ACS but no such evidence for exercise ECG. Both CTCA and exercise ECG had been evaluated in a number of studies that aimed to determine the prognostic value of testing in suspected ACS, but these studies were limited by low event rates, poor reporting and methodological limitations. As a result, the evidence that either exercise ECG or CTCA predicts adverse events in suspected ACS is weak and our economic model was based on limited data. The economic analysis showed that exercise ECG was unlikely to be cost-effective, whereas CTCA could be cost-effective if the risk of adverse events was sufficiently high (> 2–3% combined death and non-fatal MI rate within the period in which CTCA might be expected to influence outcome) and the estimates in the model were reliable. The cost-effectiveness of CTCA therefore appears to depend on being able to select patients with an increased risk of adverse outcome. Future research needs to explore this issue, but current evidence is insufficient to support routine investigation with CTCA for troponin-negative patients.
Chapter 6 Conclusions
Implications for service provision
The data cited in the introduction to this report show that chest pain due to suspected ACS is responsible for a large and growing number of emergency hospital attendances and admissions. Any recommendations relating to the management of suspected ACS therefore have substantial potential implications for service provision. Hospital Episode Statistics for England (see Chapter 1, The healthcare burden of suspected acute coronary syndrome) show that admissions for chest pain have been progressively rising, whereas LoS has been shortening. This probably reflects the increasing use of 10- to 12-hour troponin testing, allowing early discharge of patients with suspected MI and application of this test to increasing numbers of patients.
Our economic analysis suggests that hospital admission for 10-hour troponin testing is unlikely to be cost-effective compared with high-sensitivity troponin at presentation unless rapid decision-making and discharge is possible, although this conclusion may not hold in various scenarios if troponin sensitivity at presentation is < 90%. Our sensitivity analysis, admittedly based on data from one study, suggested that the 10-hour troponin strategy was very unlikely to be cost-effective compared with a strategy using a high-sensitivity assay at presentation and 3 hours later. Removing the recommendation for 10-hour troponin testing from NICE guidance could have substantial benefits for service provision. If patients were recommended for admission only if troponin level at presentation was positive then we would expect that fewer patients would need admission to hospital and the rise in chest pain admissions could be attenuated or even reversed. However, outpatient services for those discharged might need to be developed and/or a small increase in the risk of adverse outcome after discharge may be observed.
Increased use of high-sensitivity troponin assays has other potential implications for service provision. High-sensitivity assays produce more positive results than standard assays and the prognostic significance of these additional positive cases is not clear. Services have been developed on the assumption that patients with a positive troponin have an important risk of adverse outcome and will benefit from further investigation and intervention. This assumption may not hold for some patients if their troponin elevation indicates only a small increase or no significant increase in risk. Widespread use of high-sensitivity troponin testing has the potential to substantially increase demand for cardiology services. Further research is required to determine how and whether this demand should be met.
Similarly, any use of alternative biomarkers as an adjunct to troponin testing for ruling out MI at presentation may have implications for service provision. In our model we assumed that FP alternative biomarkers would be ignored once a 10-hour troponin test was found to be negative and would not result in additional testing or prolonged LoS. However, this assumption needs to be tested in practice.
Any recommendation that CTCA should be routinely used for troponin-negative patients with suspected ACS (even if selected on the basis of perceived risk) would have substantial service implications and would require rapid access to CT scanning and reporting in a way that is currently not available in most hospitals. Our analysis suggests that there is currently insufficient evidence to recommend CTCA but future research will need to take into account the potential impact upon service provision and explore potential knock-on implications, such as hospital admission being used for patients awaiting CTCA if it is not immediately available.
Suggested research priorities
The following suggested research priorities reflect the areas of uncertainty outlined in the previous chapter (see Chapter 5, Uncertainties) and are not listed in order of priority.
-
A diagnostic cohort study is required to estimate the sensitivity and specificity of presentation and 3-hour high-sensitivity troponin in patients presenting with suspected ACS compared with a 10-hour reference standard of MI based on high-sensitivity troponin.
-
A cohort study of patients presenting with suspected ACS is required to determine the prognostic importance of late troponin rises or troponin rises that are only detected by high-sensitivity assays. Alternatively a trial and economic evaluation could be used to evaluate the clinical effectiveness and cost-effectiveness of using high-sensitivity troponin compared with standard troponin, although the sample size required for such a trial may render it unfeasible.
-
A diagnostic cohort study is required to estimate the effect on sensitivity and specificity of adding alternative biomarkers to high-sensitivity troponin at presentation.
-
A cohort study is required to estimate the additional prognostic value of alternative biomarkers in suspected ACS. This study should measure all routinely available predictors (i.e. clinical assessment, ECG and troponin) to determine whether or not alternative biomarkers add worthwhile predictive information.
-
A cohort study is required to estimate the prognostic value of CTCA in patients with suspected ACS but negative troponin. As with biomarkers, this study should measure all routinely available clinical predictors to determine whether or not CTCA adds useful prognostic information. This study could be combined with the cohort study of biomarkers to determine whether biomarkers and CTCA are independent predictors, and thus whether biomarkers could be used to select patients for CTCA. Alternatively, a trial and economic evaluation could be undertaken to determine the clinical effectiveness and cost-effectiveness of early CTCA for all patients to selective delayed CTCA for those with persistent symptoms.
A single cohort study could be used to address many of these priorities. This would allow investigation of the interaction between different tests, investigation of the prognostic importance of different diagnostic references standards and ensure that the additional diagnostic or prognostic value of tests were estimated taking into account all available diagnostic and prognostic information. Thus the research priorities could be stated as follows:
-
A large multicentre cohort study of patients presenting with suspected ACS in which all receive multiple biomarker testing at presentation, 3 hours and 10 hours, CTCA and follow-up for at least 6 months. This study could potentially address all five research priorities above.
-
A clinical trial and economic evaluation comparing high-sensitivity troponin to standard troponin in the diagnostic assessment of suspected ACS, to determine the effect of using high-sensitivity troponin on event rates and health-care costs.
-
A clinical trial and economic evaluation comparing early CTCA for all patients to current standard practice (selective CTCA for those with persistent symptoms) for patients with troponin-negative ACS, to determine the effect of early CTCA on event rates and health-care costs. The value of information analysis undertaken for this project suggests that such a trial would represent a cost-effective use of NHS resources.
Acknowledgements
We would like to thank the following people for their help with this project:
Dave Newby, Nick Mills, Jason Kendall and Steve Thomas provided expert cardiology (DN, NM), emergency medicine (JK) and radiology (ST) advice to guide the literature reviews and develop the economic model. NM and DN provided the follow-up data used to estimate the benefit of detecting troponin positivity in the economic model. Matt Stevenson provided modelling expertise in the study design and advised PT in undertaking the economic analysis. Simon Dixon provided health economic expertise and Emma Simpson provided systematic reviewing expertise in developing the proposal. DN, JK, S T, MS, SD and ES were co-applicants on the funding application. Susan Proctor and Kathryn Paulucy provided clerical assistance to the project. Enid Hirst provided patient/public representation and advice in development of the project.
Contributions of authors
SG conceived, designed and led the project, assisted with reviewing and developing the economic model, and wrote the first and final drafts of the report.
PT developed the economic model, undertook the economic analysis and cowrote the economics section of the report.
CC developed the literature searches, undertook reviewing, quality assessment and data extraction mainly for the biomarker data, and cowrote the systematic reviews section of the report.
JWS oversaw and performed the Bayesian meta-analysis and provided posterior distributions for the economic model.
JL undertook reviewing, quality assessment and data extraction mainly for the CTCA/exercise ECG data.
MaK assisted reviewing, quality assessment and data extraction of the biomarker data.
PC provided expert advice, and assisted with the biomarker review and development of the economic model.
FM provided expert advice, and assisted with the CTCA/exercise ECG review and development of the economic model.
PE undertook the literature searches.
JW conducted the Bayesian meta-analyses under the guidance of JS.
All authors contributed to drafting the report and approved the final draft.
References
- The National Service Framework for Coronary Heart Disease. London: DoH; 2000.
- Goldberg RJ, Currie K, White K, Brieger D, Steg PG, Goodman SG, et al. Six-month outcomes in a multinational registry of patients hospitalized with an acute coronary syndrome (the Global Registry of Acute Coronary Events [GRACE]). Am J Cardiol 2004;93:288-93.
- Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart 2005;91:229-30.
- Hospital Episodes Statistics for England, 1998–2010. 2011.
- Walker L, Weston C, Birkhead J, van Leeven R, Dodd S, Dancy M, et al. Myocardial Infarction National Audit Project, 9th Public Report: How the NHS manages heart attacks. London: Royal College of Physicians; 2010.
- Blunt I, Bardsley M, Dixon J. Trends in emergency admissions in England 2004–2009: is greater efficiency breeding inefficiency?. London: The Nuffield Trust; 2010.
- Thygesen K, Alpert JS, White HD. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction . Universal definition of myocardial infarction. J Am Coll Cardiol 2007;50:2173-95.
- Ebell MH, White LL, Weismantel D, Ebell MH, White LL, Weismantel D. A systematic review of troponin T and I values as a prognostic tool for patients with chest pain. J Fam Pract 2000;49:746-53.
- Hamm CW, Heeschen C, Goldmann B, Vahanian A, Adgey J, Miguel CM, et al. Benefit of abciximab in patients with refractory unstable angina in relation to serum troponin T levels. N Engl J Med 1999;340:1623-9.
- Ebell MH, Flewelling D, Flynn CA. A systematic review of troponin T and I for diagnosing acute myocardial infarction. J Fam Pract 2000;49:550-6.
- Cooper A, Calvert N, Skinner J, Sawyer L, Sparrow K, Timmis A, et al. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. London: National Clinical Guideline Centre for Acute and Chronic Conditions; 2010.
- Goodacre S, Bradburn M, Cross E, Fitzgerald P, Collinson P, Gray A, et al. The RATPAC (Randomised Assessment of Treatment using Panel Assay of Cardiac Markers Trial: a randomised controlled trial of point-of-care cardiac markers in the emergency department. Health Technol Assess 2011;15.
- Cross E, How S, Goodacre S. Development of acute chest pain services in the UK. Emerg Med J 2007;24:100-2.
- Camm J, Gray H, Antoniou S, Cadman J, Crowe E, de Belder M, et al. Unstable angina and NSTEMI: the early management of unstable angina and non-ST-segment-elevation myocardial infarction. London: National Clinical Guideline Centre; 2010.
- Erhardt L, Herlitz J, Bossaert L, Halinen M, Keltai M, Koster R, et al. Task force on the management of chest pain. Eur Heart J 2002;23:1153-76.
- Dunham M, Challen K, Walter D. Risk stratification of patients with acute chest pain without a rise in troponin: current practice in England. Emerg Med J 2010;27:461-4.
- Collinson PO, Stubbs PJ, Kessler A. Multicentre evaluation of the diagnostic value of cardiac troponin T, CK-MB mass, and myoglobin for assessing patients with suspected acute coronary syndromes in routine clinical practice. Heart 2003;89:280-6.
- Eggers KM, Oldgren J, Id A, Lindahl B, Eggers KM, Oldgren J, et al. Diagnostic value of serial measurement of cardiac markers in patients with chest pain: limited value of adding myoglobin to troponin I for exclusion of myocardial infarction. Am Heart J 2004;148:574-81.
- Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009;27:858-67.
- Keller TZ. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med 2009;361:868-77.
- Balk EM. I. Accuracy of biomarkers to diagnose acute cardiac ischemia in the emergency department: A meta-analysis. Ann Emerg Med 2001;37:478-94.
- Mitchell AM, Brown MD, Menown IBA, Kline JA. Novel protein markers of acute coronary syndrome complications in low-risk outpatients: A systematic review of potential use in the emergency department. Clin Chem 2005;51:2005-11.
- Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol Assess 2009;13.
- Goodacre S, Cross E, Lewis C, Capewell S. ESCAPE Research Team . The ESCAPE cluster randomised trial: Effectiveness and Safety of Chest pain Assessment to Prevent Emergency admissions. BMJ 2007;335:659-62.
- Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al. Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease. Health Technol Assess 2008;12.
- CRD's guidance for undertaking reviews in health care. York: CRD, University of York; 2008.
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y, Deeks JJ, et al. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration; 2010.
- Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. Development and validation of methods for assessing the quality of diagnostic accuracy studies. Health Technol Assess 2004;8.
- Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224-8.
- Sutton A, Cooper N, Goodacre S, Stevenson M. Integration of meta-analysis and economic decision modelling for evaluating diagnostic tests. Med Dec Making 2008;28:650-68.
- Ades A, Lu G, Higgins JPT. The interpretation of random effects meta-analyses in decision models. Med Dec Making 2005;25:646-54.
- Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian approaches to clinical trials and health-care evaluation. London: John Wiley & Sons; 2004.
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS: a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37.
- Warn DE, Thompson SG, Spiegelhalter DJ. Bayesian random effects meta-analysis of trials with binary outcomes: methods for the absolute risk difference and relative risk scales. Stat Med 2002;21:1601-23.
- Brooks S. PGA . Alternative methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434-45.
- Alhashemi JA. Diagnostic accuracy of a bedside qualitative immunochromatographic test for acute myocardial infarction. Am J Emerg Med 2006;24:149-55.
- Eggers KM, Ellenius J, Dellborg M, Groth T, Oldgren J, Swahn E, et al. Artificial neural network algorithms for early diagnosis of acute myocardial infarction and prediction of infarct size in chest pain patients. Int J Cardiol 2007;114:366-74.
- Kurz K. Comparison of the new high sensitive cardiac troponin T with myoglobin, h-FABP and cTnT for early identification of myocardial necrosis in the acute coronary syndrome. Clin Res Cardiol 2011;100:209-15.
- Macdonald SP, Nagree Y, Macdonald SPJ, Nagree Y. Rapid risk stratification in suspected acute coronary syndrome using serial multiple cardiac biomarkers: a pilot study. Emerg Med Australas 2008;20:403-9.
- Ooi S. Incremental value of heart-type fatty acid-binding protein, myoglobin, troponin T and electrocardiogram in rapid bedside diagnosis of acute coronary syndrome in chest pain patients presenting to the emergency department. Society for Academic Emergency Medicine Annual Meeting 2007. Acad Emerg Med 2007;14.
- Rathore S, Knowles P, Mann AP, Dodds PA, Rathore S, Knowles P, et al. Is it safe to discharge patients from accident and emergency using a rapid point of care Triple Cardiac Marker test to rule out acute coronary syndrome in low to intermediate risk patients presenting with chest pain?. Eur J Intern Med 2008;19:537-40.
- Sinha MK, Roy D, Gaze DC, Collinson PO, Kaski J. Role of ‘Ischemia Modified Albumin’, a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg Med J 2004;21:29-34.
- Eggers KM, Oldgren J, Id A, Lindahl B, Eggers KM, Oldgren J, et al. Combining different biochemical markers of myocardial ischemia does not improve risk stratification in chest pain patients compared to troponin I alone. Coron Artery Dis 2005;16:315-19.
- Eggers KM, Kempf T, Venge P, Wallentin L, Wollert KC, Lindahl B, et al. Improving long-term risk prediction in patients with acute chest pain: the Global Registry of Acute Coronary Events (GRACE) risk score is enhanced by selected non-necrosis biomarkers. Am Heart J 2010;160:88-94.
- Mockel M, Muller R, Vollert JO, Muller C, Danne O, Gareis R, et al. Lipoprotein-associated phospholipase A for early risk stratification in patients with suspected acute coronary syndrome: a multi-marker approach. Clin Res Cardiol 2007;96:604-12.
- Body R, Phil P. Low soluble P-selectin may facilitate early exclusion of acute myocardial infarction. Clin Chim Acta 2011;412:614-18.
- Brown AM, Wu AH, Clopton. ST2 in emergency department chest pain patients with potential acute coronary syndromes. Ann Emerg Med 2007;50:153-8.
- Collinson PO, Gaze DC, Bainbridge K, Morris F, Morris B, Price A, et al. Utility of admission cardiac troponin and ‘ischemia modified albumin’ measurements for rapid evaluation and rule out of suspected acute myocardial infarction in the emergency department. Emerg Med J 2006;23:256-61.
- Garcia-Valdecasas S, Ruiz-Alvarez MJ, Garcia DT, De Pablo. Diagnostic and prognostic value of heart-type fatty acid-binding protein in the early hours of acute myocardial infarction. Acta Cardiol 2011;66:315-21.
- Ilva T, Lund J, Porela P, Mustonen H, Voipio-Pulkki LM, Eriksson S, et al. Early markers of myocardial injury: cTnI is enough. Clin Chim Acta 2009;400:82-5.
- Potsch AA, Siqueira Filho AG, Tura BR, Gamarski R, Bassan R, Nogueira MV, et al. C-reactive protein diagnostic and prognostic value in patients presenting at the emergency room with chest pain. Arq Bras Cardiol 2006;87:275-80.
- Amodio G, Antonelli G, Varraso L, Ruggieri V, Di SF, Amodio G, et al. Clinical impact of the troponin 99th percentile cut-off and clinical utility of myoglobin measurement in the early management of chest pain patients admitted to the Emergency Cardiology Department. Coron Artery Dis 2007;18:181-6.
- Apple FS, Smith SW, Pearce LA, Ler R, Murakami MM, Benoit MO, et al. Use of the bioMerieux VIDAS troponin I ultra assay for the diagnosis of myocardial infarction and detection of adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chim Acta 2008;390:72-5.
- Apple FS, Smith SW, Pearce LA, Ler R, Murakami MM. Use of the centaur TnI-Ultra assay for detection of myocardial infarction and adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem 2008;54:723-8.
- Apple FS, Smith SW, Pearce LA, Murakami MM, Apple FS, Smith SW, et al. Assessment of the multiple-biomarker approach for diagnosis of myocardial infarction in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem 2009;55:93-100.
- Bassan R, Potsch A, Maisel A, Tura B, Villacorta H, Nogueira MV, et al. B-type natriuretic peptide: a novel early blood marker of acute myocardial infarction in patients with chest pain and no ST-segment elevation. Eur Heart J 2005;26:234-40.
- Body R, McDowell H, Carley S, Wibberley C, Ferguson J, Mackway-Jones K. A FABP-ulous ‘rule out’ strategy? Heart fatty acid binding protein and troponin for rapid exclusion of acute myocardial infarction. Resuscitation 2011;82:1041-6.
- Cete YE. The value of point-of-care fatty acid binding protein in patients with chest pain in determining myocardial infarction in the emergency setting. Hong Kong J Emerg Med 2010;17:224-9.
- Charpentier S, Ducasse JL, Cournot M, Maupas-Schwalm F, Elbaz M, Baixas C, et al. Clinical assessment of ischemia-modified albumin and heart fatty acid-binding protein in the early diagnosis of non-ST-elevation acute coronary syndrome in the emergency department. Acad Emerg Med 2010;17:27-35.
- Christ M, Popp S, Pohlmann H, Poravas M, Umarov D, Bach R, et al. Implementation of High Sensitivity Cardiac Troponin T Measurement in the Emergency Department. Am J Med 2010;123:1134-42.
- Christenson RH, Duh SH, Sanhai WR, Wu AHB, Holtman V, Painter P, et al. Characteristics of an albumin cobalt binding test for assessment of acute coronary syndrome patients: a multicenter study. Clin Chem 2001;47:464-70.
- Collinson PO, Gaze DC, Stubbs PJ, Swinburn J, Khan M, Senior R, et al. Diagnostic and prognostic role of cardiac troponin I (cTnI) measured on the DPC Immulite. Clin Biochem 2006;39:692-6.
- Di Serio F, Amodio G, Ruggieri E, De Sario R, Varraso L, Antonelli G, et al. Proteomic approach to the diagnosis of acute coronary syndrome: preliminary results. Clin Chim Acta 2005;357:226-35.
- Ecollan P, Collet JP, Boon G, Tanguy ML, Fievet ML, Haas R, et al. Pre-hospital detection of acute myocardial infarction with ultra-rapid human fatty acid-binding protein (H-FABP) immunoassay. Int J Cardiol 2007;119:349-54.
- Esporcatte R, Rey HC, Rangel FO, Rocha RM, Filho HT, Dohmann HF, et al. Predictive value of myeloperoxidase to identify high risk patients admitted to the hospital with acute chest pain. Arq Bras Cardiol 2007;89:377-84.
- Guo XF. The predictive value of the bedside troponin T test for patients with acute chest pain. Exp Clin Cardiol 2006;11:298-301.
- Haltern G, Peiniger S, Bufe A, Reiss G, Iker H, Scheffold T. Comparison of usefulness of heart-type fatty acid binding protein versus cardiac troponin T for diagnosis of acute myocardial infarction. Am J Cardiol 2010;105:1-9.
- Hjortshoj S, Kristensen SR, Ravkilde J. Diagnostic value of ischemia-modified albumin in patients with suspected acute coronary syndrome. Am J Emerg Med 2010;28:170-6.
- Ilva T, Eriksson S, Lund J, Porela P, Mustonen H, Pettersson K, et al. Improved early risk stratification and diagnosis of myocardial infarction, using a novel troponin I assay concept. Eur J Clin Invest 2005;35:112-16.
- Keating L, Benger JR, Beetham R, Bateman S, Veysey S, Kendall J, et al. The PRIMA study: presentation ischaemia-modified albumin in the emergency department. Emerg Med J 2006;23:764-8.
- Keller T, Tzikas S, Zeller T, Czyz E, Lillpopp L, Ojeda FM, et al. Copeptin improves early diagnosis of acute myocardial infarction. J Am Coll Cardiol 2010;55:2096-106.
- Lefevre GF. Multicenter evaluation of h-FABP semi-quantitative assay (Cardio Detect in central laboratory: the point in acute myocardial infarction diagnosis. Ann Biol Clin 2007;65:377-84.
- Li C, Li J, Liang X, Li X, Cui J, Yang Z, et al. Point-of-care test of heart-type fatty acid-binding protein for the diagnosis of early acute myocardial infarction. Acta Pharmacol Sin 2010;31:307-12.
- Liao J, Chan CP, Cheung YC, Lu JH, Luo Y, Cautherley GW, et al. Human heart-type fatty acid-binding protein for on-site diagnosis of early acute myocardial infarction. Int J Cardiol 2009;133:420-3.
- Mad P, Domanovits H, Fazelnia C, Stiassny K, Iler G, Cseh A, et al. Human heart-type fatty-acid-binding protein as a point-of-care test in the early diagnosis of acute myocardial infarction. QJM 2007;100:203-10.
- McCann CJ, Glover BM, Menown IB, Moore MJ, McEneny J, Owens CG, et al. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur Heart J 2008;29:2843-50.
- Mion MM, Novello E, Altinier S, Rocco S, Zaninotto M, Plebani M, et al. Analytical and clinical performance of a fully automated cardiac multi-markers strategy based on protein biochip microarray technology. Clin Biochem 2007;40:1245-51.
- Naroo GYM. Elevated heart-type fatty acid-binding protein predicts early myocardial injury and aids in the diagnosis of non-ST elevation myocardial infarction. Hong Kong J Emerg Med 2009;16:141-7.
- Penttilä K, Koukkunen H, Halinen M, Rantanen T, Pyörälä K, . Myoglobin, creatine kinase MB isoforms and creatine kinase MB mass in early diagnosis of myocardial infarction in patients with acute chest pain. Clin Biochem 2002;35:647-53.
- Reichlin T, Hochholzer W, Stelzig C, Laule K, Freidank H, Morgenthaler NG, et al. Incremental value of copeptin for rapid rule out of acute myocardial infarction. J Am Coll Cardiol 2009;54:60-8.
- Rudolf V, Goldmann BU, Bös C, Rudolph TK, Klinke A, Friedrichs K, et al. Diagnostic value of MPO plasma levels in patients admitted for suspected myocardial infarction. Int J Cardiol 2011;153:267-71.
- Valle HA, Riesgo LG, Bel MS, Gonzalo FE, Sanchez MS, Oliva LI, et al. Clinical assessment of heart-type fatty acid binding protein in early diagnosis of acute coronary syndrome. Eur J Emerg Med 2008;15:140-4.
- Venge P, Johnston N, Lindahl B, James S. Normal plasma levels of cardiac troponin I measured by the high-sensitivity cardiac troponin I access prototype assay and the impact on the diagnosis of myocardial ischemia. J Am Coll Cardiol 2009;54:1165-72.
- Wallace TW, Abdullah SM, Drazner MH, Das SR, Khera A, McGuire DK, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation 2006;113:1958-65.
- Eggers KM, Dellborg M, Oldgren J, Swahn E, Venge P, Lindahl B, et al. Risk prediction in chest pain patients by biochemical markers including estimates of renal function. Int J Cardiol 2008;128:207-13.
- Mockel MD. Development of an optimized multimarker strategy for early risk assessment of patients with acute coronary syndromes. Clin Chim Acta 2008;393:103-9.
- Apple FS, Pearce LA, Chung A, Ler R, Murakami MM, Apple FS, et al. Multiple biomarker use for detection of adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem 2007;53:874-81.
- Apple FS, Smith SW, Pearce LA, Schulz KM, Ler R, Murakami MAM, et al. Myeloperoxidase improves risk stratification in patients with ischemia and normal cardiac troponin I concentrations. Clin Chem 2011;57:603-8.
- Bholasingh R, Cornel JH, Kamp O, Van Straalen JP, Sanders GT, Dijksman L, et al. The prognostic value of markers of inflammation in patients with troponin T-negative chest pain before discharge from the emergency department. Am J Med 2003;115:521-8.
- Blum AS. The prognostic value of high-sensitive C-reactive protein and cardiac troponin T in young and middle-aged patients with chest pain without ECG changes. Eur J Intern Med 2003;14:310-14.
- Brennan ML, Penn MS, Van LF, Nambi V, Shishehbor MH, Aviles RJ, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med 2003;349:1595-604.
- Brown AM, Sease KL, Robey JL, Shofer FS, Hollander JE. The impact of B-type natriuretic peptide in addition to troponin I, creatine kinase-MB, and myoglobin on the risk stratification of emergency department chest pain patients with potential acute coronary syndrome. Ann Emerg Med 2007;49:153-63.
- Brugger-Andersen TP. B-type natriuretic peptide is a long-term predictor of all-cause mortality, whereas high-sensitive C-reactive protein predicts recurrent short-term troponin T positive cardiac events in chest pain patients: A prognostic study. BMC Cardiovasc Disord 2008;8.
- Cameron SJ, Sokoll LJ, Laterza OF, Shah S, Green GB, Cameron SJ, et al. A multi-marker approach for the prediction of adverse events in patients with acute coronary syndromes. Clin Chim Acta 2007;376:168-73.
- Consuegra-Sanchez LB-M. Ischemia-modified albumin predicts short-term outcome and 1-year mortality in patients attending the emergency department for acute ischemic chest pain. Heart Vessels 2008;23:174-80.
- De Winter RJ, Koster RW, Schotveld JH, Sturk A, Van Straalen JP, Sanders GT, et al. Prognostic value of troponin T, myoglobin, and CK-MB mass in patients presenting with chest pain without acute myocardial infarction. Heart 1996;75:235-9.
- Eggers KM, Kempf T, Allhoff T, Lindahl B, Wallentin L, Wollert KC, et al. Growth-differentiation factor-15 for early risk stratification in patients with acute chest pain. Eur Heart J 2008;29:2327-35.
- Fromm R, Meyer D, Zimmerman J, Boudreaux A, Wun CC, Smalling R, et al. A double-blind, multicentered study comparing the accuracy of diagnostic markers to predict short- and long-term clinical events and their utility in patients presenting with chest pain. Clin Cardiol 2001;24:516-20.
- Green GB, Skarbek-Borowski GW, Chan DW, Kelen GD, Green GB, Skarbek-Borowski GW, et al. Myoglobin for early risk stratification of emergency department patients with possible myocardial ischemia. Acad Emerg Med 2000;7:625-36.
- Hillis GS, Taggart P, Hillis L, Zhao N, Dalsey WC, Mangione A, et al. Biochemical and clinical predictors of long-term outcome in patients with nonspecific chest pain and nondiagnostic electrocardiograms. Am Heart J 145:88-94.
- Jaffery Z, Nowak R, Khoury N, Tokarski G, Lanfear DE, Jacobsen G, et al. Myoglobin and troponin I elevation predict 5-year mortality in patients with undifferentiated chest pain in the emergency department. Am Heart J 2008;156:939-45.
- Jernberg T, Stridsberg M, Venge P, Lindahl B, Jernberg T, Stridsberg M, et al. N-terminal pro brain natriuretic peptide on admission for early risk stratification of patients with chest pain and no ST-segment elevation. J Am Coll Cardiol 2002;40:437-45.
- Kavsak PA, Wang XS, Henderson M, Ko DT, MacRae AR, Jaffe AS. PAPP-A as a marker of increased long-term risk in patients with chest pain. Clin Biochem 2009;42:1012-18.
- Kontos MC, Garg R, Anderson FP, Roberts CS, Ornato JP, Tatum JL, et al. Ability of myoglobin to predict mortality in patients admitted for exclusion of myocardial infarction. Am J Emerg Med 2007;25:873-9.
- Laterza OF, Cameron SJ, Chappell D, Sokoll LJ, Green GB, Laterza OF, et al. Evaluation of pregnancy-associated plasma protein A as a prognostic indicator in acute coronary syndrome patients. Clin Chim Acta 2004;348:163-9.
- Lim J, Hawkins RC, Ng K, Chan SP, Cheng A, Ng KS, et al. A preliminary study of the utility of combined cardiac markers in the evaluation of patients presenting early with suspected acute coronary syndrome. Ann Acad Med Singapore 2002;31:772-6.
- Lund J, Qin Q, Ilva T, Pettersson K, Voipio-Pulkki L, Porela P, et al. Circulating pregnancy-associated plasma protein A predicts outcome in patients with acute coronary syndrome but no troponin I elevation. Circulation 2003;108:1924-6.
- Manini AF, Ilgen J, Noble VE, Bamberg F, Koenig W, Bohan JS, et al. Derivation and validation of a sensitive IMA cutpoint to predict cardiac events in patients with chest pain. Emerg Med J 2009;26:791-6.
- Markovic M, Ignjatovic S, Dajak M, Majkic-Singh N, Markovic M, Ignjatovic S, et al. Utility of placental growth factor for prediction of 30-day adverse event in emergency department population with non-ST elevation acute coronary syndrome. Clin Lab 2010;56:215-22.
- Mathew T, Menown I, Smith B, Smye M, Nesbitt S, Young I, et al. Diagnosis and risk stratification of patients with anginal pain and non-diagnostic electrocardiograms. QJM 1999;92:565-71.
- McCann CJ, Glover BM, Menown IB, Moore MJ, McEneny J, Owens CG, et al. Prognostic value of a multimarker approach for patients presenting to hospital with acute chest pain. Am J Cardiol 2009;103:22-8.
- McCord J, Nowak RM, Hudson MP, McCullough PA, Tomlanovich MC, Jacobsen G, et al. The prognostic significance of serial myoglobin troponin I, and creatine kinase-MB measurements in patients evaluated in the emergency department for acute coronary syndrome. Ann Emerg Med 2003;42:343-50.
- Menown IB, Mathew TP, Gracey HM, Nesbitt GS, Murray P, Young IS, et al. Prediction of recurrent events by D-Dimer and inflammatory markers in patients with normal cardiac troponin I (PREDICT). Study. Am Heart J 2003;145:986-92.
- Newby LK, Storrow AB, Gibler WB, Garvey JL, Tucker JF, Kaplan AL, et al. Beside multimarker testing for risk stratification in chest pain units: the Chest Pain Evaluation by Creatine Kinase-MB, Myoglobin, and Troponin I (CHECKMATE) study. Circulation 2001;103:1832-7.
- Ordonez-Llanos JS-B. Risk stratification of chest pain patients by point-of-care cardiac troponin T and myoglobin measured in the emergency department. Clin Chim Acta 2006;365:93-7.
- Ponitz VB-A. Activated factor XII type A and B-type natriuretic peptide are complementary and incremental predictors of mortality in patients following admission with acute coronary syndrome. Blood Coagul Fibrinolysis 2009;20:652-60.
- Sonel A, Sasseen BM, Fineberg N, Bang N, Wilensky RL, Sonel A, et al. Prospective study correlating fibrinopeptide A, troponin I, myoglobin, and myosin light chain levels with early and late ischemic events in consecutive patients presenting to the emergency department with chest pain. Circulation 2000;102:1107-13.
- Svensson L, Axelsson C, Nordlander R, Herlitz J, Svensson L, Axelsson C, et al. Prognostic value of biochemical markers, 12-lead ECG and patient characteristics amongst patients calling for an ambulance due to a suspected acute coronary syndrome. J Intern Med 2004;255:469-77.
- Szymanski FM, Grabowski M, Filipiak KJ, Karpinski G, Hrynkiewicz A, Stolarz P, et al. Prognostic implications of myocardial necrosis triad markers' concentration measured at admission in patients with suspected acute coronary syndrome. Am J Emerg Med 2007;25:65-8.
- van Domburg RT, Cobbaert C, Kimman GJ, Zerback R, Simoons ML, van Domburg RT, et al. Long-term prognostic value of serial troponin T bedside tests in patients with acute coronary syndromes. Am J Cardiol 2000;86:623-7.
- Viswanathan K, Kilcullen N, Morrell C, Thistlethwaite SJ, Sivananthan MU, Hassan TB, et al. Heart-type fatty acid-binding protein predicts long-term mortality and re-infarction in consecutive patients with suspected acute coronary syndrome who are troponin-negative. J Am Coll Cardiol 2010;55:2590-8.
- Yamashita T, Seino Y, Ogawa A, Ogata K, Fukushima M, Tanaka K, et al. N-terminal pro-BNP is a novel biomarker for integrated cardio-renal burden and early risk stratification in patients admitted for cardiac emergency. J Cardiol 2010;55:377-83.
- Casciani E, Polettini E, Bertini L, Rotolo F, Truscelli G, Pittalis A, et al. 16-MDCT angiography coronary artery in the emergency department for patients with acute coronary syndrome (NSTEMI-UA). Clin Ter 2008;159:5-12.
- Coles DRW. Multislice computed tomography coronary angiography in patients admitted with a suspected acute coronary syndrome. Int J Cardiovasc Imag 2007;23:603-14.
- Ghersin E, Litmanovich D. 16-MDCT coronary angiography versus invasive coronary angiography in acute chest pain syndrome: a blinded prospective study. AJR Am J Roentgenol 2006;186:177-84.
- Henneman MM, Schuijf JD, Pundziute G, van Werkhoven JM, van der Wall EE, Jukema JW, et al. Noninvasive evaluation with multislice computed tomography in suspected acute coronary syndrome: plaque morphology on multislice computed tomography versus coronary calcium score. J Am Coll Cardiol 2008;52:216-22.
- Minocha G, Khurana P, Minocha VA, Manocha S, Srivastava S, Agarwal P, et al. Diagnostic accuracy of multislice CT coronary angiography in patients presenting with non diagnostic chest pain. J Am Coll Cardiol 2006;47.
- Olivetti L, Mazza G, Volpi D, Costa F, Ferrari O, Pirelli S. Multislice CT in emergency room management of patients with chest pain and medium-low probability of acute coronary syndrome. Radiol Med 2006;111:1054-63.
- Sato Y, Matsumoto N, Ichikawa M, Kunimasa T, Iida K, Yoda S, et al. Efficacy of multislice computed tomography for the detection of acute coronary syndrome in the emergency department. Circ J 2005;69:1047-51.
- Tsai IC, Lee T, Lee WL, Tsao CR, Tsai WL, Chen MC, et al. Use of 40-detector row computed tomography before catheter coronary angiography to select early conservative versus early invasive treatment for patients with low-risk acute coronary syndrome. J Comput Assist Tomogr 2007;31:258-64.
- Goldstein JAG. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol 2007;49:863-71.
- Hollander JE, Chang AM, Shofer FS, McCusker CM, Baxt WG, Litt HI. Coronary computed tomographic angiography for rapid discharge of low-risk patients with potential acute coronary syndromes. Ann Emerg Med 2009;53:295-304.
- Hollander JE, Chang AM, Shofer FS, Collin MJ, Walsh KM, McCusker CM, et al. One-year outcomes following coronary computerized tomographic angiography for evaluation of emergency department patients with potential acute coronary syndrome. Acad Emerg Med 2009;16:693-8.
- Miller AH, Pepe PE, Peshok R, Bhore R, Yancy CY, Xuan L, et al. Is Coronary Computed Tomography Angiography a Resource Sparing Strategy in the Risk Stratification and Evaluation of Acute Chest Pain? Results of a Randomized Controlled Trial. Acad Emerg Med 2011;18:458-67.
- Rubinshtein R, Halon DA, Gaspar T, Jaffe R, Karkabi B, Flugelman MY, et al. Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation 2007;115:1762-8.
- Schlett CL, Banerji D, Siegel E, Bamberg F, Lehman SJ, Brady TJ, et al. Prognostic value of CT angiography for major adverse cardiac events in patients with acute chest pain from the emergency department: 2-year outcomes of the ROMICAT trial. J Am Coll Cardiol Cardiovasc Imag 2011;4:481-91.
- Shuman WP, May JM, Branch KR, Mitsumori LM, Strote JN, Green DE, et al. Negative ECG-gated cardiac CT in patients with low-to-moderate risk chest pain in the emergency department: 1-year follow-up. AJR Am J Roentgenol 2010;195:923-7.
- Amsterdam EA, Kirk JD, Diercks DB, Lewis WR, Turnipseed SD. Immediate exercise testing to evaluate low-risk patients presenting to the emergency department with chest pain. J Am Coll Cardiol 2002;40:251-6.
- deFilippi CR, Rosanio S, Tocchi M, Parmar RJ, Potter MA, Uretsky BF, et al. Randomized comparison of a strategy of predischarge coronary angiography versus exercise testing in low-risk patients in a chest pain unit: in-hospital and long-term outcomes. J Am Coll Cardiol 2001;37:2042-9.
- Diercks DB, Gibler B, Liu T, Sayre MR, Storrow AB. Identification of patients at risk by graded exercise testing in an emergency department chest pain center. Am J Cardiol 2000;86:289-92.
- Gomez MA, Anderson JL, Karagounis LA, Muhlestein JB, Mooers FB. An emergency department-based protocol for rapidly ruling out myocardial ischemia reduces hospital time and expense: results of a randomized study. J Am Coll Cardiol 1996;28:25-33.
- Goodacre S, Locker T, Arnold J, Angelini K, Morris F. Which diagnostic tests are most useful in a chest pain unit protocol. BMC Emerg Med 2005;5.
- Jeetley P, Burden L, Senior R. Stress echocardiography is superior to exercise ECG in the risk stratification of patients presenting with acute chest pain with negative Troponin. Eur J Echocardiogr 2006;7:155-64.
- Kerns JR, Shaub TF. Emergency cardiac stress testing in the evaluation of emergency department patients with atypical chest pain. Ann Emerg Med 1993;22:794-8.
- Kirk JD, Turnipseed S, Lewis WR, Amsterdam EA. Evaluation of chest pain in low-risk patients presenting to the emergency department: the role of immediate exercise testing. Ann Emerg Med 1998;32:1-7.
- Lewis WR, Amsterdam EA. Utility and safety of immediate exercise testing of low-risk patients admitted to the hospital for suspected acute myocardial infarction. Am J Cardiol 1994;74:987-90.
- Polananczyk CA, Johnson PA, Hartley LH, Walls RM, Shaykevich S, Lee TH. Clinical correlates and prognostic significance of early negative exercise tolerance test in patients with acute chest pain seen in the hospital emergency department. Am J Cardiol 1998;81:288-92.
- Ramakrishna G, Milavetz JJ, Zinmeister AR, Farkouh ME, Evans RW, Allison TG, et al. Effect of exercise treadmill testing and stress imaging on the triage of patients with chest pain. Mayo Clin Proc 2005;80:322-9.
- Sarullo FM, Di Pasquale P, Orlando G, Buffa G, Cicero S, Schillaci AM. Utility and safety of immediate exercise testing of low-risk patients admitted to hospital with acute chest pain. Int J Cardiol 2000;75:239-43.
- Tsakonis JS, Shesser R, Rosenthal R, Bittar GD, Smith M, Wasserman AG. Safety of immediate treadmill testing in selected emergency department patients with chest pain: A preliminary report. Am J Emerg Med 1991;9:557-9.
- Laudon DA, Behrenbeck TR, Wood CM, Bailey KR, Callahan CM, Breen JF, et al. Computed tomographic coronary artery calcium assessment for evaluating chest pain in the emergency department: long-term outcome of a prospective blind study. Mayo Clinic Proc 2010;85:314-22.
- Goldman L, Cook EF, Brand DA, Lee TH, Rouan GW, Weisberg MC, et al. A computer protocol to predict myocardial infarction in emergency department patients with chest pain. N Engl J Med 1988;318:797-803.
- Goodacre SW, Bradburn M, Cross E, Collinson PO, Gray A, Hall AS. The Randomised Assessment of Treatment using Panel Assay of Cardiac Markers (RATPAC) trial: a randomised controlled trial of point-of-care cardiac markers in the emergency department. Heart 2011;97:190-6.
- Keller T, Zeller T, Ojeda FM, Tzikas S, Lillpopp L, Sinning CR, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011;306:2684-93.
- Mills NL. Implementation of a sensitive troponin I assay and risk of recurrent myocardial infarction and death in patients with suspected acute coronary syndrome. JAMA 2011;305:1210-16.
- Polanczyk CA, Kuntz KM, Sacks DB, Johnson PA, Lee TH. Emergency department triage strategies for acute chest pain using creatine kinase-MB and troponin I assays: a cost-effectiveness analysis. Ann Intern Med 1999;131:909-18.
- Ward S, Lloyd JM, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007;11.
- Interim Life Tables 2006–08. London: ONS; 2008.
- Ara R, Brazier J. Health related quality of life by age, gender or history of cardiovascular disease: results from the Health Survey for England (2003 and 2006). Sheffield: University of Sheffield; 2009.
- Fitzgerald P, Goodacre SW, Cross E, Dixon S. Cost-effectiveness of point-of-care biomarker assessment for suspected myocardial infarction: the randomized assessment of treatment using panel assay of cardiac markers (RATPAC) Trial. Acad Emerg Med 2011;18:488-95.
- Tariff information: confirmation of payment by results (PbR) arrangements for 2009–10. London: DoH; 2011.
- Stuart RJ, Ellestad MH. National survey of exercise stress testing facilities. Chest 1980;77:94-7.
- Stein SC, Hurst RW, Sonnad SS. Meta-analysis of cranial CT scans in children. A mathematical model to predict radiation-induced tumors. Pediatr Neurosurg 2011;44:448-57.
- Shehadi WH. Adverse reactions to intravascularly administered contrast media. A comprehensive study based on a prospective survey. Am J Roentgenol Radium Ther Nucl Med 1975;124:145-52.
- Cashman JD, McCredie J, Henry DA. Intravenous contrast media: use and associated mortality. Med J Aust 1991;155:618-23.
- Johnson LW, Krone R. Cardiac catheterization 1991: a report of the Registry of the Society for Cardiac Angiography and Interventions (SCA&I). Cathet Cardiovasc Diagn 1993;28:219-20.
- Atthappan G, Habib M, Ponniah T, Jeyaseelan L. Multi-detector computerized tomography angiography for evaluation of acute chest pain: a meta analysis and systematic review of literature. Int J Cardiol 2010;141:132-40.
- Savino G, Herzog C, Costello P, Schoepf UJ. 64 slice cardiovascular CT in the Emergency Department: concepts and first experiences. Emerg Radiol 2006;111:481-96.
- Gallagher MJ, Ross MA, Raff GL, Goldstein JA, O’Neill WW, O’Neil B. The diagnostic accuracy of 64-slice computed tomography coronary angiography compared with stress nuclear imaging in emergency department low-risk chest pain patients. Ann Emerg Med 2007;49:125-36.
- Hoffmann U, Nagurney JT, Moselewski F, Pena A, Ferencik M, Chae CU, et al. Coronary multidetector computed tomography in the assessment of patients with acute chest pain. Circulation 2006;114:2251-60.
- White CS, Kuo D, Keleman M, Jain V, Musk A, Zaidi E, et al. Chest pain evaluation in the emergency department: can MDCT provide a comprehensive evaluation?. AJR Am J Roentgenol 2005;185:533-40.
- Rubinshtein R, Halon DA, Gaspar T, Schliamser JE, Yaniv N, Ammar R, et al. Usefulness of 64-slice multidetector computed tomography in diagnostic triage of patients with chest pain and negative or nondiagnostic exercise treadmill test result. Am J Cardiol 2007;99:925-9.
- Meijboom WB, van Mieghem CAG, Mollet NR, Pugliese F, Weustinck AC, van Pelt N, et al. 64-slice computed tomography coronary angiography in patients with high, intermediate, or low pretest probability of significant coron artery disease. J Am Coll Cardiol 2007;50:1469-75.
- Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol 2011;57:1237-47.
- Guide to the methods of technology appraisals. London: NICE; 2008.
Appendix 1 Examples of search strategies used
Biomarkers review diagnostic accuracy search: a MEDLINE example
Chest pain population terms
-
exp Chest Pain/ (43,381)
-
(chest adj (pain or discomfort or tight* or pressure)).mp. (23,091)
-
chest-pain.mp. (21,852)
-
(cardiac adj pain).mp. (359)
-
(thora* adj pain).mp. (1021)
-
Acute Coronary Syndrome/(3223)
-
acute coronary syndrome.mp. (7783)
-
(acute adj coronary adj syndrome).mp. (7783)
-
Angina, Unstable/ (7731)
-
unstable angina.mp. (9934)
-
(unstable adj2 angina).mp. (13,058)
-
Myocardial Infarction/ (126,095)
-
myocardial.mp. (307,141)
-
infarct*.mp. (222,338)
-
(myocardial adj infarction).mp. (163,555)
-
heart attack.mp. (2540)
-
(heart adj (arrest$or attack*)).mp. (29,166)
-
(preinfarction or pre-infarction or (pre adj infarction)).mp. (408)
-
or/1–18 (417,505)
Biomarker terms
-
creatine kinase.mp. or Creatine Kinase/ (27,627)
-
((creatine adj kinase) or (creatine adj phosphokinase)).mp. (29,176)
-
creatine kinase MB.mp. (2589)
-
creatine kinase MB isoenzyme.mp. (288)
-
creatine kinase isoenzyme 2.mp. (8)
-
creatine kinase 2.mp. (40)
-
CK-2.mp. (161)
-
CK-MB.mp. (2903)
-
myoglobin.mp. or Myoglobin/ (11,827)
-
C-Reactive Protein/(22,195)
-
(c-reactive protein or c reactive protein).mp. (33,427)
-
CRP.mp. (18,869)
-
myeloperoxidase.mp. (12,827)
-
mpo.mp. (6279)
-
b-type natriuretic peptide.mp. (2624)
-
type b natriuretic peptide.mp. (47)
-
Natriuretic Peptides/ (847)
-
(brain adj natriuretic peptide).mp. (5237)
-
N terminal B type natriuretic peptide.mp. (36)
-
BNP.mp. (4976)
-
N-terminal-pro-natriuretic peptide.mp. (6)
-
NTproBNP.mp. (99)
-
NT-proBNP.mp. (1688)
-
heart type fatty acid binding protein.mp. (196)
-
heart-type fatty acid binding protein.mp. (196)
-
H-FABP.mp. (312)
-
(co-peptin or co?peptin or copeptin).mp. (116)
-
adrenomedullin.mp. or Adrenomedullin/ (2412)
-
ST-2.mp. (181)
-
interleukin 33.mp. (104)
-
galectin 3.mp. or Galectin 3/ (1455)
-
Matrix Metalloproteinase 9/or matrix metalloproteinase 9.mp. (10,074)
-
pregnancy-associated plasma protein.mp. (1164)
-
pregnancy associated plasma protein A.mp. (1145)
-
PAPP-A.mp. (853)
-
Ischaemia Modified Albumin.mp. (38)
-
early troponin.mp. (7)
-
troponin at presentation.mp. (0)
-
initial troponin.mp. (18)
-
or/20–58 (114,505)
Troponin and reference standard terms
-
Troponin T/or Troponin I/ (6449)
-
(troponin T or troponin I).mp. (9156)
-
cardiac troponin T.mp. (1573)
-
cardiac troponin I.mp. (2114)
-
ctnt.mp. (986)
-
reference standards/ (27,529)
-
reference standard$.mp. (32,855)
-
gold standard.mp. (22,828)
-
Major adverse event*.mp. (803)
-
(major cardiac adj events).mp. (497)
-
or/60–69 (65,727)
Human-only studies
-
human/ (11,609,100)
-
animal/ (4,746,079)
-
71 not (71 and 72) (10,378,731)
-
19 and 59 and 70 and 73 (2306)
-
exp “Sensitivity and Specificity”/ (327,463)
-
sensitivity.tw. (425,187)
-
specificity.tw. (267,581)
-
((pre-test or pretest) adj probability).tw. (932)
-
post-test probability.tw. (258)
-
predictive value$.tw. (52,063)
-
likelihood ratio$.tw. (6258)
-
or/75–81 (833,726)
-
74 and 82 (974)
Not case–control studies
-
Case-Control Studies/ (131,351)
-
83 not 84 (947)
Date limit
-
limit 85 to yr=“1995 -Current” (911)
Biomarkers review prognostic accuracy search: a MEDLINE example
Chest pain population terms
-
exp Chest Pain/ (43,381)
-
(chest adj (pain or discomfort or tight* or pressure)).mp. (23,091)
-
chest-pain.mp. (21,852)
-
(cardiac adj pain).mp. (359)
-
(thora* adj pain).mp. (1021)
-
Acute Coronary Syndrome/ (3223)
-
acute coronary syndrome.mp. (7783)
-
(acute adj coronary adj syndrome).mp. (7783)
-
Angina, Unstable/ (7731)
-
unstable angina.mp. (9934)
-
(unstable adj2 angina).mp. (13,058)
-
Myocardial Infarction/ (126,095)
-
myocardial.mp. (307,141)
-
infarct*.mp. (222,338)
-
(myocardial adj infarction).mp. (163,555)
-
heart attack.mp. (2540)
-
(heart adj (arrest$or attack*)).mp. (29,166)
-
(preinfarction or pre-infarction or (pre adj infarction)).mp. (408)
-
or/1–18 (417,505)
Biomarker terms
-
creatine kinase.mp. or Creatine Kinase/ (27,627)
-
((creatine adj kinase) or (creatine adj phosphokinase)).mp. (29,176)
-
creatine kinase MB.mp. (2589)
-
creatine kinase MB isoenzyme.mp. (288)
-
creatine kinase isoenzyme 2.mp. (8)
-
creatine kinase 2.mp. (40)
-
CK-2.mp. (161)
-
CK-MB.mp. (2903)
-
myoglobin.mp. or Myoglobin/ (11,827)
-
C-Reactive Protein/ (22,195)
-
(c-reactive protein or c reactive protein).mp. (33,427)
-
CRP.mp. (18,869)
-
myeloperoxidase.mp. (12,827)
-
mpo.mp. (6279)
-
b-type natriuretic peptide.mp. (2624)
-
type b natriuretic peptide.mp. (47)
-
Natriuretic Peptides/ (847)
-
(brain adj natriuretic peptide).mp. (5237)
-
N terminal B type natriuretic peptide.mp. (36)
-
BNP.mp. (4976)
-
N-terminal-pro-natriuretic peptide.mp. (6)
-
NTproBNP.mp. (99)
-
NT-proBNP.mp. (1688)
-
heart type fatty acid binding protein.mp. (196)
-
heart-type fatty acid binding protein.mp. (196)
-
H-FABP.mp. (312)
-
(co-peptin or co?peptin or copeptin).mp. (116)
-
adrenomedullin.mp. or Adrenomedullin/ (2412)
-
ST-2.mp. (181)
-
interleukin 33.mp. (104)
-
galectin 3.mp. or Galectin 3/ (1455)
-
Matrix Metalloproteinase 9/or matrix metalloproteinase 9.mp. (10,074)
-
pregnancy-associated plasma protein.mp. (1164)
-
pregnancy associated plasma protein A.mp. (1145)
-
PAPP-A.mp. (853)
-
Ischaemia Modified Albumin.mp. (38)
-
early troponin.mp. (7)
-
troponin at presentation.mp. (0)
-
initial troponin.mp. (18)
-
or/20–58 (114,505)
Troponin and reference standard terms 60 Troponin T/or Troponin I/ (6449)
-
(troponin T or troponin I).mp. (9156)
-
cardiac troponin T.mp. (1573)
-
cardiac troponin I.mp. (2114)
-
ctnt.mp. (986)
-
reference standards/ (27,529)
-
reference standard$.mp. (32,855)
-
gold standard.mp. (22,828)
-
Major adverse event*.mp. (803)
-
(major cardiac adj events).mp. (497)
-
or/60–69 (65,727)
Human-only studies
-
human/ (11,609,100)
-
animal/ (4,746,079)
-
71 not (71 and 72) (10,378,731)
-
19 and 59 and 70 and 73 (2306)
Not case–control studies
-
Case-Control Studies/ (131,351)
-
74 not 75 (2238)
Prognostics filter
-
prognosis.sh. (298,607)
-
diagnosed.tw. (261,406)
-
cohort:.mp. (226,523)
-
predictor:.tw. (148,838)
-
death.tw. (366,188)
-
exp models, statistical/ (191,474)
-
or/77–82 (1,290,944)
Date limits
-
limit 83 to yr=“1995 -Current” (969,679)
-
76 and 84 (876)
CTCA/ETT review diagnostic accuracy search: a MEDLINE example
Chest pain population terms
-
exp Chest Pain/ (43,388)
-
(chest adj (pain or discomfort or tight* or pressure)).mp. (23,109)
-
chest-pain.mp. (21,868)
-
(cardiac adj pain).mp. (359)
-
(thora* adj pain).mp. (1021)
-
Acute Coronary Syndrome/ (3225)
-
acute coronary syndrome.mp. (7796)
-
(acute adj coronary adj syndrome).mp. (7796)
-
Angina, Unstable/ (7731)
-
unstable angina.mp. (9938)
-
(unstable adj2 angina).mp. (13,062)
-
Myocardial Infarction/ (126,097)
-
myocardial.mp. (307,231)
-
infarct*.mp. (222,416)
-
(myocardial adj infarction).mp. (163,611)
-
heart attack.mp. (2541)
-
(heart adj (arrest$or attack*)).mp. (29,168)
-
(preinfarction or pre-infarction or (pre adj infarction)).mp. (408)
-
or/1–18 (417,635)
Coronary computed tomography angiography terms
-
Coronary computed tomography angiography.mp. (132)
-
CCTA.mp. (152)
-
((CT or comput$tomog$) adj3 coronary angiogra$).mp. (992)
-
((electrocard$or ecg) adj2 exercise).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] (2885)
-
(treadmill or stress or exercise adj2 test).tw
-
20 or 21 or 22 or 23 (4104)
Comparator terms
-
coronary angiography/ (40,511)
-
coronary angiogra$.mp. (49,249)
-
reference standards/ (27,529)
-
reference standard$.mp. (32,859)
-
gold standard.mp. (22,845)
-
Major adverse event*.mp. (805)
-
(major cardiac adj events).mp. (497)
-
or/25–31 (105,094)
-
24 and 33 (2029)
Human-only studies
-
human/ (11,609,245)
-
animal/ (4,746,107)
-
34 not (35 and 34) (10,378,871)
-
19 and 24 and 33 and 36 (1066)
Diagnostics filter
-
exp “Sensitivity and Specificity”/ (327,472)
-
sensitivity.tw. (425,433)
-
specificity.tw. (267,723)
-
((pre-test or pretest) adj probability).tw. (932)
-
post-test probability.tw. (258)
-
predictive value$.tw. (52,099)
-
likelihood ratio$.tw. (6262)
-
38 or 39 or 40 or 41 or 42 or 43 or 44 (834,065)
-
37 and 45 (529)
-
limit 46 to yr=“1985 -Current” (497)
Computed tomographic coronary angiography/exercise treadmill testing review prognostic accuracy search: a MEDLINE example
Chest pain population terms
-
exp Chest Pain/ (43,388)
-
(chest adj (pain or discomfort or tight* or pressure)).mp. (23,109)
-
chest-pain.mp. (21,868)
-
(cardiac adj pain).mp. (359)
-
(thora* adj pain).mp. (1021)
-
Acute Coronary Syndrome/ (3225)
-
acute coronary syndrome.mp. (7796)
-
(acute adj coronary adj syndrome).mp. (7796)
-
Angina, Unstable/ (7731)
-
unstable angina.mp. (9938)
-
(unstable adj2 angina).mp. (13,062)
-
Myocardial Infarction/ (126,097)
-
myocardial.mp. (307,231)
-
infarct*.mp. (222,416)
-
(myocardial adj infarction).mp. (163,611)
-
heart attack.mp. (2541)
-
(heart adj (arrest$or attack*)).mp. (29,168)
-
(preinfarction or pre-infarction or (pre adj infarction)).mp. (408)
-
or/1–18 (417,635)
Coronary computed tomography angiography terms
-
Coronary computed tomography angiography.mp. (132)
-
CCTA.mp. (152)
-
((CT or comput$tomog$) adj3 coronary angiogra$).mp. (992)
-
((electrocard$or ecg) adj2 exercise).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] (2885)
-
(treadmill or stress or exercise adj2 test).tw
-
20 or 21 or 22 or 23 (4104)
Comparator terms
-
coronary angiography/ (40,511)
-
coronary angiogra$.mp. (49,249)
-
reference standards/ (27,529)
-
reference standard$.mp. (32,859)
-
gold standard.mp. (22,845)
-
Major adverse event*.mp. (805)
-
(major cardiac adj events).mp. (497)
-
or/25–31 (105,094)
-
24 and 33 (2029)
Human-only studies
-
human/ (11,609,245)
-
animal/ (4,746,107)
-
34 not (35 and 34) (10,378,871)
-
19 and 24 and 33 and 36 (1066)
Prognotics filter
-
prognosis.sh. (298,609)
-
diagnosed.tw. (261,629)
-
cohort:.mp. (226,689)
-
predictor:.tw. (148,963)
-
death.tw. (366,380)
-
exp models, statistical/ (191,485)
-
85 or 86 or 87 or 88 or 89 or 90 (1,291,588)
-
19 and 24 and 34 and 37 and 91 (287)
Date limit
-
limit 92 to yr=“1985 -Current” (267)
Appendix 2 Methodology checklist: the modified QUADAS tool for studies of diagnostic test accuracy
| Checklist completed by: | ||||
|---|---|---|---|---|
| Circle one option for each question | ||||
| Was the spectrum of participants representative of the patients who will receive the test in practice? (i.e. patients presenting to the emergency services or department with chest pain and suspected ACS) | Yes | No | Unclear | N/A |
| Was the reference standard likely to classify the target condition correctly? (i.e. was it based on the universal definition of MI?) | Yes | No | Unclear | N/A |
| Was the period between performance of the reference standard and the index test short enough to be reasonably sure that the target condition did not change between the two tests? (i.e. were the two tests both conducted within the 12-hour time frame required for the reference standard?) | Yes | No | Unclear | N/A |
| Did the whole sample or a random selection of the sample receive verification using the reference standard? | Yes | No | Unclear | N/A |
| Did participants receive the same reference standard regardless of the index test result? | Yes | No | Unclear | N/A |
| Was the reference standard independent of the index test? (i.e. the index test did not form part of the reference standard) | Yes | No | Unclear | N/A |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | No | Unclear | N/A |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes | No | Unclear | N/A |
| Were uninterpretable, indeterminate or intermediate test results reported?a | Yes | No | Unclear | N/A |
Appendix 3 Studies excluded from the biomarkers review
Non-troponin or unclear reference standard (n = 59)
- Alansari SE, Croal BL, Alansari SE, Croal BL. Diagnostic value of heart fatty acid binding protein and myoglobin in patients admitted with chest pain. Ann Clin Biochem 2004;41:391-6.
- Anwaruddin S, Januzzi JL, Baggish AL, Lewandrowski EL, Lewandrowski KB, Anwaruddin S, et al. Ischemia-modified albumin improves the usefulness of standard cardiac biomarkers for the diagnosis of myocardial ischemia in the emergency department setting. Am J Clin Pathol 2005;123:140-5.
- Baxter MSB. Evaluation of a bedside whole-blood rapid troponin T assay in the emergency department. Acad Emerg Med 1997;4:1018-24.
- Brown A, George J, Murphy MJ, Struthers A, Brown A, George J, et al. Could BNP screening of acute chest pain cases lead to safe earlier discharge of patients with non-cardiac causes? A pilot study. QJM 2007;100:755-61.
- Chan CP, Sanderson JE, Glatz JF, Cheng WS, Hempel A, Renneberg R, et al. A superior early myocardial infarction marker. Human heart-type fatty acid-binding protein. Z Kardiol 2004;93:388-97.
- Chen L, Guo X, Yang F, Chen L, Guo X, Yang F. Role of heart-type fatty acid binding protein in early detection of acute myocardial infarction in comparison with cTnI, CK-MB and myoglobin. J Huazhong Univ Sci Technol Med Sci 2004;24:449-51.
- Collinson PO, Stubbs PJ, Kessler A. Multicentre evaluation of the diagnostic value of cardiac troponin T, CK-MB mass, and myoglobin for assessing patients with suspected acute coronary syndromes in routine clinical practice. Heart 2003;89:280-6.
- D’Costa M, Fleming E, Patterson MC, D’Costa M, Fleming E, Patterson MC. Cardiac troponin I for the diagnosis of acute myocardial infarction in the emergency department. Am J Clin Pathol 1997;108:550-5.
- De Winter RJ, Bholasingh R, Nieuwenthuijs AB, Koster RW, Peters RJG, Sanders GT. Ruling out acute myocardial infarction early with two serial creatine kinase-MB mass determinations. Eur Heart J 1999;20:967-72.
- De Winter RJ, Koster RW, Sturk A, Sanders GT, De Winter RJ, Koster RW, et al. Value of myoglobin, troponin T, and CK-MB mass in ruling out an acute myocardial infarction in the emergency room. Circulation 1995;92:3401-7.
- Dewinter RJ, Koster RW, Sturk A, Sanders GT. Value of Myoglobin, Troponin-T, and Ck-Mb(Mass) in Ruling Out An Acute Myocardial-Infarction in the Emergency Room. Circulation 1995;92:3401-7.
- Epelde F, Tomás S, Argilaga R, Martínez X, Sáenz L, . [Usefulness of troponin T and troponin I in the diagnosis and prognostic stratification of patients with ischemic cardiopathy attending an emergency service.]. An Med Interna 1999;16:511-14.
- Fernandez Portales JGR. Utility of the serum biochemical markers CPK, CPK MB mass, myoglobin, and cardiac troponin T in a chest pain unit. Which marker determinations should be requested and when?. Rev Esp Cardiol 2002;55:913-20.
- Fromm RM. Diagnostic accuracy of plasma markers for myocardial infarction: a randomized, double-blind, multicenter study. Cardiovasc Rev Rep 2001;22:273-6.
- Goldmann BUL. Quantitative bedside testing of troponin T: Is it equal to laboratory testing? The cardiac reader troponin T (CARE T) study. Clin Lab 2004;50:1-10.
- Grand A, Fournis Y, Siramy M, Ghadban W, Douieb A, Daboura A, et al. Value of mass measurement of the MB isoenzyme of creatinine phosphokinase in the diagnosis of recent myocardial infarction. Arch Mal Coeur Vaiss 1997;90:807-15.
- Haastrup B, Gill S, Kristensen SR, Jorgensen PJ, Glatz JF, Haghfelt T, et al. Biochemical markers of ischaemia for the early identification of acute myocardial infarction without St segment elevation. Cardiology 2000;94:254-61.
- Hawkins RC, Tan HL, Hawkins RC, Tan HL. Comparison of the diagnostic utility of CK, CK-MB (activity and mass), troponin T and troponin I in patients with suspected acute myocardial infarction. Singapore Med J 1999;40:680-4.
- Herren KR, Mackway-Jones K, Richards CR, Seneviratne CJ, France MW, Cotter L, et al. Is it possible to exclude a diagnosis of myocardial damage within six hours of admission to an emergency department? Diagnostic cohort study. BMJ 2001;323.
- Hetland O, Dickstein K, Hetland O, Dickstein K. Cardiac markers in the early hours of acute myocardial infarction: clinical performance of creatine kinase, creatine kinase MB isoenzyme (activity and mass concentration), creatine kinase MM and MB subform ratios, myoglobin and cardiac troponin T. Scand J Clin Lab Invest 1996;56:701-13.
- Hsu LF, Koh TH, Lim YL, Hsu LF, Koh TH, Lim YL. Cardiac marker point-of-care testing: evaluation of rapid on-site biochemical marker analysis for diagnosis of acute myocardial infarction. Ann Acad Med Singapore 2000;29:421-7.
- Juarez Herrera UL. The utility of rapid qualitative determination of troponin T, the MB fraction of creatine phosphokinase and myoglobin in acute ischemic coronary syndromes. Arch Inst Cardiol Mex 1998;68:473-81.
- Katz IA, Irwig L, Vinen JD, March L, Wyndham LE, Luu T, et al. Biochemical markers of acute myocardial infarction: strategies for improving their clinical usefulness. Ann Clin Biochem 1998;35:393-9.
- Lin RMH, Fatovich DM, Grasko JM, Vasikaran SD. Ischaemia modified albumin cannot be used for rapid exclusion of acute coronary syndrome. Emerg Med J 2010;27:668-71.
- Mair J, Smidt J, Lechleitner P, Dienstl F, Puschendorf B. A decision tree for the early diagnosis of acute myocardial infarction in nontraumatic chest pain patients at hospital admission. CHEST 1995;108:1502-9.
- Mair J, Smidt J, Lechleitner P, Dienstl F, Puschendorf B, Mair J, et al. Rapid accurate diagnosis of acute myocardial infarction in patients with non-traumatic chest pain within 1 h of admission. Coron Artery Dis 1995;6:539-45.
- McBride JH, OConnell JT. Assessment of creatine kinase MB, relative index calculation, troponin T and troponin I in the diagnoses of acute myocardial infarction. Clin Chem 1997;43.
- McCord J, Nowak RM, McCullough PA, Foreback C, Borzak S, Tokarski G, et al. Ninety-minute exclusion of acute myocardial infarction by use of quantitative point-of-care testing of myoglobin and troponin I. Circulation 2001;104:1483-8.
- McCord JN. Ninety-minute exclusion of acute MI using cardiac markers. Cardiol Rev 2002;19:22-5.
- Mills NL. Implementation of a sensitive troponin I assay and risk of recurrent myocardial infarction and death in patients with suspected acute coronary syndrome. JAMA 2011;305:1210-16.
- Mitchell AMG. Multimarker panel to rule out acute coronary syndromes in low-risk patients. Acad Emerg Med 2006;13:803-6.
- Mohler ER, Ryan T, Segar DS, Sawada SG, Sonel AF, Perkins L, et al. Clinical utility of troponin T levels and echocardiography in the emergency department. Am Heart J 1998;135:253-60.
- Nakata T, Hashimoto A, Hase M, Tsuchihashi K, Shimamoto K, Nakata T, et al. Human heart-type fatty acid-binding protein as an early diagnostic and prognostic marker in acute coronary syndrome. Cardiology 2003;99:96-104.
- Ooi SB, Lim YT, Lau TC, Chia BL, Pillai S, Liu T, et al. Value of troponin-T rapid assay, cardiac enzymes, electrocardiogram and history of chest pain in the initial diagnosis of myocardial infarction in the emergency department. Eur J Emerg Med 2000;7:91-8.
- Orak M, Ustundag M, Guloglu C, Ozhasenekler A, Alyan O, Kale E. The role of the heart-type fatty acid binding protein in the early diagnosis of acute coronary syndrome and its comparison with troponin I and creatine kinase-MB isoform. Am J Emerg Med 2010;28:891-6.
- Panteghini M, Cuccia C, Pagani F, Turla C, Panteghini M, Cuccia C, et al. Comparison of the diagnostic performance of two rapid bedside biochemical assays in the early detection of acute myocardial infarction. Clin Cardiol 1998;21:394-8.
- Penttilä K, Penttilä I, Bonnell R, Kerth P, Koukkunen H, Rantanen T, et al. Comparison of the troponin T and troponin I ELISA tests, as measured by microplate immunoassay techniques, in diagnosing acute myocardial infarction. Eur J Clin Chem Clin Biochem 1997;35:767-74.
- Pervae S, Anderson S. Comparative analysis of cardiac troponin I and creatine kinase-MB as markers of acute myocardial infarction. Clin Cardiol 1997;20:269-71.
- Pettersson T, Simonsson M, Mattsson E, Kerth P. Rapid bedside testing of CK-MB mass combined with myoglobin is more sensitive compared to troponin T alone for rule-out of acute myocardial infarction. Br J Anaesth 1996;76.
- Polanczyk CA, Johnson PA, Cook EF, Lee TH, Polanczyk CA, Johnson PA, et al. A proposed strategy for utilization of creatine kinase-MB and troponin I in the evaluation of acute chest pain. Am J Cardiol 1999;83:1175-9.
- Polanczyk CA, Lee TH, Cook EF, Walls R, Wybenga D, Printy-Klein G, et al. Cardiac troponin I as a predictor of major cardiac events in emergency department patients with acute chest pain. J Am Coll Cardiol 1998;32:8-14.
- Ravkilde J, Nissen H, rder M, Thygesen K, Ravkilde J, Nissen H, et al. Independent prognostic value of serum creatine kinase isoenzyme MB mass, cardiac troponin T and myosin light chain levels in suspected acute myocardial infarction. Analysis of 28 months of follow-up in 196 patients. J Am Coll Cardiol 1995;25.
- Roberts R, Roberts R. Early diagnosis of myocardial infarction with MB CK isoforms. Clin Chim Acta 1998;272:33-45.
- Ross G, Bever FN, Uddin Z, Hockman EM, Ross G, Bever FN, et al. Troponin I sensitivity and specificity for the diagnosis of acute myocardial infarction. J Am Osteopath Assoc 2000;100:29-32.
- Sayre MR, Kaufmann KH, Chen I, Sperling M, Sidman RD, Diercks DB, et al. Measurement of cardiac troponin T is an effective method for predicting complications among emergency department patients with chest pain. Ann Emerg Med 1998;31:539-49.
- Luscher MS, Ravkilde J, Thygesen K, scher MS, Ravkilde J, Thygesen K. Clinical application of two novel rapid bedside tests for the detection of cardiac troponin T and creatine kinase-MB mass/myoglobin in whole blood in acute myocardial infarction. Cardiology 1998;89:222-8.
- Schuchert A, Hamm C, Scholz J, Klimmeck S, Goldmann B, Meinertz T, et al. Prehospital testing for troponin T in patients with suspected acute myocardial infarction. Am Heart J 1999;138:45-8.
- Seino Y, Tomita Y, Takano T, Ohbayashi K, Seino Y, . Tokyo Rapid-Test Office Cardiologists (Tokyo-ROC) Study . Office cardiologists cooperative study on whole blood rapid panel tests in patients with suspicious acute myocardial infarction: comparison between heart-type fatty acid-binding protein and troponin T tests. Circ J 2004;68:144-8.
- Seino Y, Ogata K, Takano T, Ishii J, Hishida H, Morita H, et al. Use of a whole blood rapid panel test for heart-type fatty acid-binding protein in patients with acute chest pain: comparison with rapid troponin T and myoglobin tests. Am J Med 2003;115:185-90.
- Sobki SH, Saadeddin SM, Habbab MA, Sobki SH, Saadeddin SM, Habbab MA. Cardiac markers used in the detection of myocardial injury. Saudi Med J 2000;21:843-6.
- Stokke M, Gronvold T, Siebke AM, Skjaeggestad O, Stromme JH, Stokke M, et al. Serial measurements of cardiac markers to rule in or out acute myocardial damage less than 3 h after admission in acute chest pain patients without ECG-signs of acute myocardial infarction. Scand J Clin Lab Invest 1998;58:331-8.
- Stork TG. Suspected acute coronary syndrome in patients without ST-segment elevation: Rule-out infarction, value of clinical decision-making and non-coronary diagnoses. Dtsch Med Wochenschr 2002;127:260-5.
- Stork TV, Wu AHB, Muller-Bardorff M, Gareis R, Muller R, Hombach V, et al. Diagnostic and prognostic role of myoglobin in patients with suspected acute coronary syndrome. Am J Cardiol 2000;86.
- Suzuki M, Hori S, Fujishima S, Takatsuki S, Nakamura I, Kimura H, et al. Diagnostic value of a bedside test for cardiac troponin T in the patient with chest pain presenting to the emergency room. Keio J Med 2000;49:74-9.
- Walter S, Carlsson J, Cuneo A, Tebbe U, Walter S, Carlsson J, et al. [Leading symptoms of chest pain in the emergency room. Using cardiac markers for risk stratification.]. Dtsch Med Wochenschr 2001;126:771-8.
- Zarich S, Bradley K, Seymour J, Ghali W, Traboulsi A, Mayall ID, et al. Impact of troponin T determinations on hospital resource utilization and costs in the evaluation of patients with suspected myocardial ischemia. Am J Cardiol 2001;88:732-6.
- Zarich SW, Qamar AU, Werdmann MJ, Lizak LS, McPherson CA, Bernstein LH, et al. Value of a single troponin T at the time of presentation as compared to serial CK-MB determinations in patients with suspected myocardial ischemia. Clin Chim Acta 2002;326:185-92.
- Zhang PZ. A comparison of different combination regimens of biochemical markers in diagnosing acute myocardial infarction. Chin J Evid-Based Med 2006;6:777-82.
- Zimmerman J, Fromm R, Meyer D, Boudreaux A, Wun CC, Smalling R, et al. Diagnostic marker cooperative study for the diagnosis of myocardial infarction. Circulation 1999;99:1671-7.
Prognosis-only studies that analyse only troponin and/or creatine kinase MB isoenzyme as variables (n = 18)
- Christ M, Popp S, . Implementation of High Sensitivity Cardiac Troponin T Measurement in the Emergency Department. Am J Med 2010;123:1134-42.
- deFilippi CR, Tocchi M, Parmar RJ, Rosanio S, Abreo G, Potter MA, et al. Cardiac troponin T in chest pain unit patients without ischemic electrocardiographic changes: angiographic correlates and long-term clinical outcomes. J Am Coll Cardiol 2000;35:1827-34.
- deFilippi CR, Parmar RJ, Potter MA, Tocchi M, deFilippi CR, Parmar RJ, et al. Diagnostic accuracy, angiographic correlates and long-term risk stratification with the troponin T ultra sensitive Rapid Assay in chest pain patients at low risk for acute myocardial infarction. Eur Heart J 1998:42-7.
- Goodacre S, Locker T. Which diagnostic tests are most useful in a chest pain unit protocol?. BMC Emerg Med 2005;5.
- Herkner H, Waldenhofer U, Laggner AN, Ilner M, Oschatz E, Spitzauer S, et al. Clinical application of rapid quantitative determination of cardiac troponin-T in an emergency department setting. Resuscitation 2001;49:259-64.
- Kontos MC, Shah R, Fritz LM, Anderson FP, Tatum JL, Ornato JP, et al. Implication of different cardiac troponin I levels for clinical outcomes and prognosis of acute chest pain patients. J Am Coll Cardiol 2004;43:958-65.
- Kontos MCA. Ability of troponin I to predict cardiac events in patients admitted from the emergency department. J Am Coll Cardiol 2000;36:1818-23.
- Koukkunen H, Penttil K, Kemppainen A, Penttil I, . Ruling out myocardial infarction with troponin T and creatine kinase MB mass: diagnostic and prognostic aspects. Scand Cardiovasc J 2001;35:302-6.
- Limkakeng A, Gibler WB, Pollack C, Hoekstra JW, Sites F, Shofer FS, et al. Combination of Goldman risk and initial cardiac troponin I for emergency department chest pain patient risk stratification. Acad Emerg Med 2001;8:696-702.
- Meyer T, Binder L, Graeber T, Luthe H, Kreuzer H, Oellerich M, et al. Superiority of combined CK-MB and troponin I measurements for the early risk stratification of unselected patients presenting with acute chest pain. Cardiology 1998;90:286-94.
- Mills NL. Implementation of a sensitive troponin i assay and risk of recurrent myocardial infarction and death in patients with suspected acute coronary syndrome. JAMA 2011;305:1210-16.
- Muscholl MW, Oswald M, Mayer C, von SW, Muscholl MW, Oswald M, et al. Prognostic value of 2D echocardiography in patients presenting with acute chest pain and non-diagnostic ECG for ST-elevation myocardial infarction. Int J Cardiol 2002;84:217-25.
- Newby LK, Kaplan AL, Granger BB, Sedor F, Califf RM, Ohman EM. Comparison of cardiac troponin T versus creatine kinase-MB for risk stratification in a chest pain evaluation unit. Am J Cardiol 2000;85:801-5.
- Noeller TP, Meldon SW, Peacock WF, Emerman CL, McErlean ES, Vanlente F, et al. Troponin T in elders with suspected acute coronary syndromes. Am J Emerg Med 2003;21:293-7.
- Peacock WF, Emerman CL, McErlean ES, Deluca SA, Van LF, Rao JS, et al. Prediction of short- and long-term outcomes by troponin T levels in low-risk patients evaluated for acute coronary syndromes. Ann Emerg Med 2000;35:213-20.
- Rao SV, Ohman EM, Granger CB, Armstrong PW, Gibler WB, Christenson RH, et al. Prognostic value of isolated troponin elevation across the spectrum of chest pain syndromes. Am J Cardiol 2003;91:936-40.
- Solymoss BC, Bourassa MG, Fortier A, roux P, Solymoss BC, Bourassa MG, et al. Evaluation and risk stratification of acute coronary syndromes using a low cut-off level of cardiac troponin T, combined with CK-MB mass determination. Clin Biochem 2004;37:286-92.
- Wilcox G, Archer PD, Bailey M, Dziukas L, Lim CF, Schneider HG, et al. Measurement of cardiac troponin I levels in the emergency department: predictive value for cardiac and all-cause mortality. Med J Aust 2001;174:170-3.
Wrong outcomes for this review (n = 5)
- Kurata AW. Clinical usefulness of heart-type fatty acid binding protein as an early marker of myocardial damage. Jpn J Interv Cardiol 2003;18:463-9.
- Liyan C, Jie Z, Xiaozhou H. Prognostic value of combination of heart-type fatty acid-binding protein and ischemia-modified albumin in patients with acute coronary syndromes and normal troponin T values. J Clin Lab Anal 2009;23:14-8.
- Lozano T, Ena J, Almenar V, Graells M, Molina J, Antorrena I, et al. Evaluation of patients with acute chest pain of uncertain origin by means of serial measurements high-sensitivity C-reactive protein. Rev Esp Cardiol 2007;60:817-24.
- Ruzgar O, Bilge AK, Bugra Z, Umman S, Yilmaz E, Ozben B, et al. The use of human heart-type fatty acid-binding protein as an early diagnostic biochemical marker of myocardial necrosis in patients with acute coronary syndrome, and its comparison with troponin-T and creatine kinase-myocardial band. Heart Ves 2006;21:309-14.
- Sypniewska GS. The use of biochip cardiac array technology for early diagnosis of acute coronary syndromes. J Med Biochem 2009;28:293-9.
Not available (n = 4)
- Ellenius J, Groth T, Lindahl B, Ellenius J, Groth T, Lindahl B. Neural network analysis of biochemical markers for early assessment of acute myocardial infarction. Stud Health Technol Inform 1997;43:382-5.
- Higuchi K, Abe S, Matsuoka T, Nakajima H, Toda H, Akazaki Y, et al. [Usefulness of rapid quantitative cardiac troponin T and myoglobin assays for the diagnosis of acute myocardial infarction.]. J Cardiol 2003;41:55-62.
- Ogawa M, Abe S, Saigo M, Kozono T, Yamaguchi K, Toda H, et al. [Usefulness of rapid bedside cardiac troponin T assay for the diagnosis of acute myocardial infarction.]. J Cardiol 2000;35:157-64.
- Viggiano MS. Prehospital diagnosis and direction of patients suspected of acute coronary syndrome. Feasibility of the combined evaluation of a single blood sample value of cardiac troponin-I, myoglobin, and creatine phosphokinase MB. JEUR 2000;13:229-34.
Issues with timing of index and reference tests (n = 4)
- Collinson PO, Gaze DC, Morris F, Morris B, Price A, Goodacre S, et al. Comparison of biomarker strategies for rapid rule out of myocardial infarction in the emergency department using ACC/ESC diagnostic criteria. Ann Clin Biochem 2006;43:273-80.
- Goodacre S, Locker T, Arnold J, Angelin K, Morris F. Which diagnostic tests are most useful in a chest pain unit protocol?. BMC Emerg Med 2005;5.
- Januzzi JL, Bamberg F, Lee H, Truong QA, Nichols JH, Karakas M, et al. High-sensitivity troponin T concentrations in acute chest pain patients evaluated with cardiac computed tomography. Circulation 2010;121:1227-34.
- Kavsak PA, MacRae AR, Lustig V, Bhargava R, Vandersluis R, Palomaki GE, et al. The impact of the ESC/ACC redefinition of myocardial infarction and new sensitive troponin assays on the frequency of acute myocardial infarction. Am Heart J 2006;152:118-25.
Less than 80% of sample received tests (n = 1)
- Straface AL, Myers JH, Kirchick HJ, Blick KE, Straface AL, Myers JH, et al. A rapid point-of-care cardiac marker testing strategy facilitates the rapid diagnosis and management of chest pain patients in the emergency department. Am J Clin Pathol 2008;129:788-95.
Language issues (n = 1)
- Rembek M, Goch A, Chizynski K, Goch JH, Rembek M, Goch A, et al. [Estimation of clinical reliability and diagnostic usefulness of human fatty acid-binding protein in acute coronary syndromes.]. Pol Merkuriusz Lek 2006;21:418-22.
Appendix 4 Studies excluded from the computed tomographic coronary angiography and exercise electrocardiography review
References
- Achenbach S, Daniel WG. Cardiac imaging in the patient with chest pain: coronary CT angiography. Heart 2010;96:1241-6.
- Al-Mallah M, Alqaisi F, Arafeh A, Lakhdar R, Al-Tamsheh R, Ananthasubramaniam K. Long term favorable prognostic value of negative treadmill echocardiogram in the setting of abnormal treadmill electrocardiogram: a 95 month median duration follow-up study. J Am Soc Echocardiogr 2008;21:1018-22.
- Bamberg F, Truong QA, Blankstein R, Nasir K, Lee H, Rogers IS, et al. Usefulness of age and gender in the early triage of patients with acute chest pain having cardiac computed tomographic angiography. Am J Cardiol 2009;104:1165-70.
- Beigel R, Oieru D, Goitein O, Chouraqui P, Konen E, Shamiss A, et al. Usefulness of Routine Use of Multidetector Coronary Computed Tomography in the “Fast Track” Evaluation of Patients With Acute Chest Pain. Am J Cardiol 2009;103:1481-6.
- Bokhari S.Shahzad. Superiority of exercise myocardial perfusion imaging compared with the exercise ECG in the diagnosis of coronary artery disease. Coron Artery Dis 2008;19:399-404.
- Budoff MJD. Diagnostic performance of 64-multidetector Row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease. Results from the Prospective Multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) Trial. J Am Coll Cardiol 2008;52:1724-32.
- Butler J, Shapiro M, Reiber J, Sheth T, Ferencik M, Kurtz EG, et al. Extent and distribution of coronary artery disease: A comparative study of invasive versus noninvasive angiography with computed angiography. Am Heart J 2007;153:378-84.
- Carrascosa PM, Capunay CM, Parodi JC, Padilla LT, Johnson P, Carrascosa JM, et al. General utilities of multislice tomography in the cardiac field. Herz 2003;28:44-51.
- Chang AM, Shofer FS, Weiner MG, Synnestvedt MB, Litt HI, Baxt WG, et al. Actual financial comparison of four strategies to evaluate patients with potential acute coronary syndromes. Acad Emerg Med 2008;15:649-55.
- Chang SA, Il Choi S, Choi EK, Kim HK, Jung JW, Chun EJ, et al. Usefulness of 64-slice multidetector computed tomography as an initial diagnostic approach in patients with acute chest pain. Am Heart J 2008;156:375-83.
- Chen LC, Ding PYA, Chen JW, Wu MH, Liu JC, Lan GY, et al. Coronary artery calcium determined by electron beam computed tomography for predicting angiographic coronary artery disease in moderate- to high-risk Chinese patients. Cardiology 2001;95:183-9.
- Chow BJ, Wells GA, Chen L, Yam Y, Galiwango P, Abraham A, et al. Prognostic value of 64-slice cardiac computed tomography severity of coronary artery disease, coronary atherosclerosis, and left ventricular ejection fraction. J Am Coll Cardiol 2010;55:1017-28.
- Coles D, Wilde P, Rogers C, Oberhoff M, Karsch K, Baumbach A. The diagnostic accuracy of non-invasive multislice computed tomography coronary angiography in patients presenting with a suspected acute coronary syndrome. Circulation 2005;112.
- Coles DR, Wilde P, Rogers C, Oberhoff M, Karsch KR, Baumbach A. Diagnostic accuracy of non-invasive coronary angiography with multislice computed tomography in patients presenting with acute chest pain. Heart 2005;91:A15-16.
- Cox JL, Teskey RJ, Lalonde LD, Iles SE. Noninvasive Testing in Women Presenting with Chest Pain – Evidence for Diagnostic Uncertainty. Can J Cardiol 1995;11:885-90.
- Davin LL. Diagnostic accuracy of computed tomography coronary angiography in routine practice. Acta Cardiol 2007;62:339-44.
- Devine PJ. V. Real-world application of coronary computed tomography angiography and its potential effect on downstream resource utilization in evaluating angina. J Cardiovasc Comput Tomogr 2008;2:214-19.
- Feldman CL, MacCallum G, Hartley LH. Comparison of the Standard ECG With the EASIcardiogram (c) for Ischemia Detection During Exercise Monitoring. Conference Proceeding n.d.
- Flukinger T, White CS. Multidetector computed tomography in the evaluation of chest pain in the emergency department. Semin Roentgenol 2008;43:136-44.
- Gallagher MJR. The diagnostic accuracy of 64-Slice computed tomography coronary angiography compared with stress nuclear imaging in emergency department low-risk chest pain patients. Ann Emerg Med 2007;49:125-36.
- Geluk CA, Dikkers R, Perik PJ, Tio RA, Götte MJ, Hillege HL, et al. Measurement of coronary calcium scores by electron beam computed tomography or exercise testing as initial diagnostic tool in low-risk patients with suspected coronary artery disease. Eur Radiol 2008;18:244-52.
- Georgiou D, Budoff MJ, Kaufer E, Kennedy JM, Lu B, Brundage BH. Screening patients with chest pain in the emergency department using electron beam tomography: a follow-up study. J Am Coll Cardiol 2001;38:105-10.
- Halpern EJ, Levin DC, Zhang S, Takakuwa KM, Halpern EJ, Levin DC, et al. Comparison of image quality and arterial enhancement with a dedicated coronary CTA protocol versus a triple rule-out coronary CTA protocol. Acad Radiol 2009;16:1039-48.
- Hansen MG. The value of dual-source 64-slice CT coronary angiography in the assessment of patients presenting to an acute chest pain service. Heart Lung Circ 2010;19:213-18.
- Hendel RC, Hendel RC. Is computed tomography coronary angiography the most accurate and effective noninvasive imaging tool to evaluate patients with acute chest pain in the emergency department? CT coronary angiography is the most accurate and effective noninvasive imaging tool for evaluating patients presenting with chest pain to the emergency department: antagonist viewpoint. Circ Cardiovasc Imaging 2009;2:264-75.
- Hermann LK, Weingart SD, Duvall WL, Henzlova MJ. The limited utility of routine cardiac stress testing in emergency department chest pain patients younger than 40 years. Ann Emerg Med 2009;54.
- Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol 2009;53:1642-50.
- Hoffmann U, Nagurney JT, Moselewski F, Pena A, Ferencik M, Chae CU, et al. Coronary multidetector computed tomography in the assessment of patients with acute chest pain. Circ Cardiovasc Imaging 2006;114:2251-60.
- Hoffmann U, Bamberg F. CT Coronary angiography is the most accurate and effective noninvasive imaging tool for evaluating patients presenting with chest pain to the emergency department. Circ Cardiovascr Imaging 2009;2:251-63.
- Hollander JE, Litt HI, Chase M, Brown AM, Kim W, Baxt WG, et al. Computed tomography coronary angiography for rapid disposition of low-risk emergency department patients with chest pain syndromes. Acad Emerg Med 2007;14:112-16.
- Huber S, Huber M, Dees D, Redmond FA, Wilson JM, Flamm SD. Usefulness of multislice spiral computed tomography coronary angiography in patients with acute chest pain in the emergency department. J Cardiovasc Comput Tomogr 2007;1:29-37.
- Jeetley P, Burden L, Stoykova B, Senior R, Jeetley P, Burden L, et al. Clinical and economic impact of stress echocardiography compared with exercise electrocardiography in patients with suspected acute coronary syndrome but negative troponin: a prospective randomized controlled study. Eur Heart J 2007;28:204-11.
- Jeetley P, Burden L, Greaves K, Senior R, Jeetley P, Burden L, et al. Prognostic value of myocardial contrast echocardiography in patients presenting to hospital with acute chest pain and negative troponin. Am J Cardiol 2007;99:1369-73.
- Jeetley PB. Stress echocardiography is superior to exercise ECG in the risk stratification of patients presenting with acute chest pain with negative Troponin. Eur J Echocardiogr 2006;7:155-64.
- Jelinkova AH. New indexes in the diagnostics of coronary artery disease in females by means of exercise ECG. Scripta Medica 1997;70:45-50.
- Johnson TRC, Nikolaou K, Becker A, Leber AW, Rist C, Wintersperger BJ, et al. Dual-source CT for chest pain assessment. Eur Radiol 2008;18:773-80.
- Johnson TRC, Nikolaou K, Fink C, Becker A, Knez A, Rist C, et al. Dual-source CT in chest pain diagnosis. Radiologe 2007;47:301-9.
- Khare R, Lee T, Powell E, Courtney D. Cost effectiveness of CT coronary angiography compared to other strategies for evaluating chest pain in patients in the emergency department. Proceedings of the 2007 Society for Academic Emergency Medicine Annual Meeting. Acad Emerg Med 2007;14:S37-8.
- Khare RK, Courtney DM, Powell ES, Venkatesh AK, Lee TA. Sixty-four-slice computed tomography of the coronary arteries: cost-effectiveness analysis of patients presenting to the emergency department with low-risk chest pain. Acad Emerg Med 2008;15:623-32.
- Kim JL. Efficacy and safety of the computed tomography coronary angiography based approach for patients with acute chest pain at an emergency department: one month clinical follow-up study. J Korean Med Sci 2010;25:466-71.
- Kuntz KMF. Cost-effectiveness of diagnostic strategies for patients with chest pain. Ann Intern Med 1999;130:709-18.
- Langer C, Wiemer M, Peterschroder A, Franzke K, Mellwig KP, van Buuren F, et al. Stratification for noninvasive coronary angiography: patient preselection considering atypical angina pectoris, conventional cardiovascular risk assessment, and calcium scoring. Eur J Cardiovasc Prev Rehabil 2009;16:201-9.
- Lau J, Ioannidis JP, Balk E, Milch C, Chew P, Terrin N, et al. Evaluation of technologies for identifying acute cardiac ischemia in emergency departments. AHRQ 2001.
- Laudon DA, Vukov LF, Breen JF, Rumberger JA, Wollan PC, Sheedy PF. Use of electron-beam computed tomography in the evaluation of chest pain patients in the emergency department. Ann Emerg Med 1999;33:15-21.
- Laufer E, Wahi S, Lim YL. Cost-effectiveness and accuracy of exercise stress echocardiography in the non-invasive diagnosis of coronary heart disease. Aust NZ J Med 2000;30:660-7.
- Lazoura O, Kanavou T, Vassiou K, Gkiokas S, Fezoulidis IV, Lazoura O, et al. Myocardial bridging evaluated with 128-multi detector computed tomography coronary angiography. Surg Radiol Anat 2010;32:45-50.
- Lee HY, Yoo SM, White CS. Coronary CT angiography in emergency department patients with acute chest pain: triple rule-out protocol versus dedicated coronary CT angiography. Int J Cardiovasc Imaging 2009;25:319-26.
- Lehman SJ, Schlett CL, Bamberg F, Lee H, Donnelly P, Shturman L, et al. Assessment of coronary plaque progression in coronary computed tomography angiography using a semiquantitative score. JACC Cardiovasc Imaging 2009;2:1262-70.
- Lehman SJ, Abbara S, Cury RC, Nagurney JT, Hsu J, Goela A, et al. Significance of cardiac computed tomography incidental findings in acute chest pain. Am J Med 2009;122:543-9.
- Leischik R, Dworrak B, Littwitz H, Iker H, Leischik R, Dworrak B, et al. Prognostic significance of exercise stress echocardiography in 3329 outpatients (5-year longitudinal study). Int J Cardiol 2007;119:297-305.
- Leschka S, Scheffel H. Effect of decrease in heart rate variability on the diagnostic accuracy of 64-MDCT coronary angiography. Am J Roentgenol 2008;190:1583-90.
- Leschka S, Koepfli P, Husmann L, Plass A, Vachenauer R, Gaemperli O, et al. Myocardial bridging: Depiction rate and morphology at CT coronary angiography: comparison with conventional coronary angiography. Radiology 2008;246:754-62.
- Lessick J, Mutlak D, Rispler S, Ghersin E, Dragu R, Litmanovich D, et al. Comparison of multidetector computed tomography versus echocardiography for assessing regional left ventricular function. Am J Cardiol 2005;96:1011-15.
- Lewandowski MS. Searching for the optimal strategy for the diagnosis of stable coronary artery disease. Cost-effectiveness of the new algorithm. Cardiol J 2007;14:544-51.
- Lewandowski MS. The implication of probability analysis for diagnosis of stable coronary artery disease. Comparison of non-invasive diagnostic tests. Pol Arch Med Wewn 2004;111:171-82.
- Lin FY, Zentko SE, Min JK. The Prognostic Value of Cardiac Computed Tomographic Angiography. Cardiol Clin 2009;27.
- Ling Z-QZ. The application and advantages of multi-slice CT in the diagnosis of myocardial bridging. Chin J Radiol 2008;42:498-502.
- Lorenzoni R, Cortigiani L, Magnani M, Desideri A, Bigi R, Manes C, et al. Cost-effectiveness analysis of noninvasive strategies to evaluate patients with chest pain. J Am Soc Echocardiogr 2003;16:1287-91.
- Lu GM, Zhang LJ, Guo H, Huang W, Merges RD, Lu GM, et al. Comparison of myocardial bridging by dual-source CT with conventional coronary angiography. Circ J 2008;72:1079-85.
- Maffei E, Palumbo A. Diagnostic accuracy of computed tomography coronary angiography in a high risk symptomatic population. Ateneo Parmense Acta Biomed 2010;81:47-53.
- Marangelli V, Iliceto S, Piccinni G, Demartino G, Sorgente L, Rizzon P. Detection of coronary-artery disease by digital stress echocardiography: comparison of exercise, transesophageal atrial-pacing and dipyridamole-echocardiography. J Am Coll Cardiol 1994;24:117-24.
- Mark D. Contrast-enhanced cardiac computed tomographic angiography in the diagnosis of coronary artery stenosis or for evaluation of acute chest pain. Rockville, MD: Blue Cross and Blue Shield Association, Technology Evaluation Center, AHRQ; 2006.
- Marwick TH, Nemec JJ, Pashkow FJ, Stewart WJ, Salcedo EE. Accuracy and limitations of exercise echocardiography in a routine clinical setting. J Am Coll Cardiol 1992;19:74-81.
- Marwick TH, Shaw L, Case C, Vasey C, Thomas JD. Clinical and economic impact of exercise electrocardiography and exercise echocardiography in clinical practice. Eur Heart J 2003;24:1153-63.
- Marwick THA. Exercise echocardiography is an accurate and cost-efficient technique for detection of coronary artery disease in women. J Am Coll Cardiol 1995;26:335-41.
- Matsumoto N, Nagao K, Hirayama A, Sato Y. Non-Invasive assessment and clinical strategy of stable coronary artery disease by magnetic resonance imaging, multislice computed tomography and myocardial perfusion SPECT. Circ J 2010;74:34-40.
- Matsumoto N, Sato Y, Yoda S, Nakano Y, Kunimasa T, Matsuo S, et al. Prognostic value of non-obstructive CT low-dense coronary artery plaques detected by multislice computed tomography. Circ J 2007;71:1898-903.
- May JM, Shuman WP, Strote JN, Branch KR, Mitsumori LM, Lockhart DW, et al. Low-risk patients with chest pain in the emergency department: negative 64-MDCT coronary angiography may reduce length of stay and hospital charges. AJR Am J Roentgenol 2009;193:150-4.
- Medina HMR. What is the value of CT angiography for patients with acute chest pain?. Curr Treat Options Cardiovasc Med 2010;12:10-2.
- Meijboom WB. 64-Slice computed tomography coronary angiography in patients with high, intermediate, or low pretest probability of significant coronary artery disease. J Am Coll Cardiol 2007;50:1469-75.
- Meijboom WBM. 64-Slice CT coronary angiography in patients with non-ST elevation acute coronary syndrome. Heart 2007;93:1386-92.
- Mikheev NN, Zharikova MV. Value of various criteria of assessment of a test in stress Echocardiography in diagnosis of ischemic heart disease. Kardiologiya 2006;46:15-9.
- Min JK, Hachamovitch R, Rozanski A, Shaw LJ, Berman DS, Gibbons R, et al. Clinical benefits of noninvasive testing: coronary computed tomography angiography as a test case. JACC Cardiovasc Imaging 2010;3:305-15.
- Min JK, Gilmore A, Budoff MJ, Berman DS, O’Day K. Cost-effectiveness of coronary CT angiography versus myocardial perfusion SPECT for evaluation of patients with chest pain and no known coronary artery disease. Radiology 2010;254:801-8.
- Min JK, Kang N, Shaw LJ, Devereux RB, Robinson M, Lin F, et al. Costs and clinical outcomes after coronary multidetector CT angiography in patients without known coronary artery disease: comparison to myocardial perfusion SPECT (Provisional abstract). Radiology 2008;249:62-70.
- Min JK, Shaw LJ, Berman DS, Gilmore A, Kang N, Min JK, et al. Costs and clinical outcomes in individuals without known coronary artery disease undergoing coronary computed tomographic angiography from an analysis of Medicare category III transaction codes. Am J Cardiol 2008;102:672-8.
- Min JK, Robinson M, Shaw LJ, Lin F, Legorreta AP, Gilmore A, et al. Differences in episode-based care costs for multidetector computed tomographic coronary angiography versus myocardial perfusion imaging for the diagnosis of coronary artery disease. J Med Econ 2008;11:327-40.
- Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161-70.
- Min JKF. The prognostic value of multidetector coronary CT angiography for the prediction of major adverse cardiovascular events: a multicenter observational cohort study. Int J Cardiovasc Imaging 2010;26:721-8.
- Mitsumori LM, Wang E, May JM, Lockhart DW, Branch KR, Dubinsky TJ, et al. Triphasic Contrast Bolus for Whole-Chest ECG-Gated 64-MDCT of Patients With Nonspecific Chest Pain: Evaluation of Arterial Enhancement and Streak Artifact. Am J Roentgenol 2010;194:W263-71.
- Mollet NRC. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circ Cardiovasc Imaging 2005;112:2318-23.
- Mollet NRC. Improved diagnostic accuracy with 16-row multi-slice computed tomography coronary angiography. J Am Coll Cardiol 2005;45:128-32.
- Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49-57.
- Murphy JC, Scott PJ, Shannon HJ, Glover B, Dougan J, Walsh SJ, et al. ST elevation on the exercise ECG in patients presenting with chest pain and no prior history of myocardial infarction. Heart 2009;95:1792-7.
- Newman DH. Computed tomographic angiography for low risk chest pain: seeking passage. Ann Emerg Med 2009;53:305-8.
- Nicol EDS. Clinical management and short-term cost – 64-slice MDCT vs. myocardial perfusion scintigraphy. Int J Cardiol 2010;144:248-50.
- Nicol EDS. Sixty-four-slice computed tomography coronary angiography compared with myocardial perfusion scintigraphy for the diagnosis of functionally significant coronary stenoses in patients with a low to intermediate likelihood of coronary artery disease. J Nucl Cardiol 2008;15:311-18.
- Nieman K, Galema TW, Neefjes LA, Weustink AC, Musters P, Moelker AD, et al. Comparison of the value of coronary calcium detection to computed tomographic angiography and exercise testing in patients with chest pain. Am J Cardiol 2009;104:1499-504.
- Nikolaou K, Knez A, Sagmeister S, Wintersperger BJ, Boekstegers P, Steinbeck G, et al. Assessment of myocardial infarctions using multidetector-row computed tomography. J Comput Assist Tomogr 2004;28:286-92.
- Nikolaou K, Sanz J, Poon M, Wintersperger BJ, Ohnesorge B, Rius T, et al. Assessment of myocardial perfusion and viability from routine contrast-enhanced 16-detector-row computed tomography of the heart: preliminary results. Eur Radiol 2005;15:864-71.
- Nikolaou K, Sagmeister S, Knez A, Klotz E, Wintersperger BJ, Becker CR, et al. Multidetector-row computed tomography of the coronary arteries: predictive value and quantitative assessment of non-calcified vessel-wall changes. Eur Radiol 2003;13:2505-12.
- Nucifora G, Schuijf JD, van Werkhoven JM, Djaberi R, van der Wall EE, de RA, et al. Relation between Framingham risk categories and the presence of functionally relevant coronary lesions as determined on multislice computed tomography and stress testing. Am J Cardiol 2009;104:758-63.
- Ong TK, Chin SP, Liew CK, Chan WL, Seyfarth MT, Liew HB, et al. Accuracy of 64-row multicletector computed tomography in detecting coronary artery disease in 134 symptomatic patients: influence of calcification. Am Heart J 2006;151.
- Ouzan JH. A multivariate analysis of the diagnostic values of clinical examination, exercise electrocardiography and stress radionuclide angiography in coronary artery disease. Arch Mal Coeur Vaiss 1988;81:941-6.
- Ouzan JH. Role of clinical manifestations, the exercise test and exertion angioscintigraphy in the diagnosis of coronary disease. A multivariate study. Arch Mal Coeur Vaiss 1988;81:941-6.
- Patterson REE. Comparison of cost-effectiveness and utility of exercise ECG, single photon emission computed tomography, positron emission tomography, and coronary angiography for diagnosis of coronary artery disease. Circ Cardiovasc Imaging 1995;91:54-65.
- Pazhenkottil APH. Non-invasive assessment of coronary artery disease with CT coronary angiography and SPECT: A novel dose-saving fast-track algorithm. European Journal of Nuclear Medicine and Molecular Imaging 2010;37:522-7.
- Peteiro J, Bouzas A, Portillo P. Assessment of coronary artery disease by exercise echocardiography. Salud I Ciencia 2009;16:755-8.
- Placer Peralta LJ, Sanchez-Navarro F, Lomas FJ, Laperal Mur JR, Diarte de Miguel JA, Artal BA, et al. [Usefulness of exercise echocardiography for the diagnosis of ischemic heart disease.]. Rev Esp Cardiol 1994;47:12-6.
- Polizos G, Ghamsary M, Ellestad MH, Polizos G, Ghamsary M, Ellestad MH. The severity of myocardial ischemia can be predicted by the exercise electrocardiogram. Cardiology 2005;104:215-20.
- Pugliese FM. Diagnostic performance of coronary CT angiography by using different generations of multisection scanners: single-center experience. Radiology 2008;246:384-93.
- Pundziute G, Schuijf D, Jukema JW, de Roos A, van der Wall EE, Bax JJ. Advances in the noninvasive evaluation of coronary artery disease with multislice computer tomography. Expert Rev Med Dev 2006;3:441-51.
- Pundziute G, Schuijf JD, van Werkhoven JM, Nucifora G, EE, Wouter Jukema J, . Head-to-head comparison between bicycle exercise testing and coronary calcium score and coronary stenoses on multislice computed tomography. Coron Artery Dis 2009;20:281-7.
- Rigo F, Pratali L, Pálinkás A, Picano E, Cutaia V, Venneri L, et al. Coronary flow reserve and brachial artery reactivity in patients with chest pain and ‘false positive' exercise-induced ST-segment depression. Am J Cardiol 2002;89:1141-4.
- Rispler S, Keidar Z. Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol 2007;49:1059-67.
- Roach PJ, Hansen PS, Scott AM, Cooper RA, Hoschl R, Wiseman JC, et al. Comparison of optimised planar scintigraphy with SPECT thallium, exercise ECG and angiography in the detection of coronary artery disease. Aust NZ J Med 1996;26:806-12.
- Roberts WT, Bax JJ, Davies LC. Cardiac CT and CT coronary angiography: technology and application. Heart 2008;94:781-92.
- Rodriguez-Granillo GA. Non-invasive assessment of vulnerable plaque. Expert Opinion Med Diagn 2009;3:53-66.
- Roger VLP. Identification of multivessel coronary artery disease by exercise echocardiography. J Am Coll Cardiol 1994;24:109-14.
- Rubinshtein R, Halon DA, Gaspar T, Peled N, Lewis BS, Rubinshtein R, et al. Cardiac computed tomographic angiography for risk stratification and prediction of late cardiovascular outcome events in patients with a chest pain syndrome. Int J Cardiol 2009;137:108-15.
- Rubinshtein R, Halon DA, Gaspar T, Jaffe R, Goldstein J, Karkabi B, et al. Impact of 64-slice cardiac computed tomographic angiography on clinical decision-making in emergency department patients with chest pain of possible myocardial ischemic origin. Am J Cardiol 2007;100:1522-6.
- Rubinshtein R, Halon DA, Gaspar T, Peled N, Lewis BS. Predictive value of coronary CT angiography for late adverse cardiac events (MACE) in patients with chest pain syndromes. J Am Coll Cardiol 2007;49.
- Rubinshtein R, Halon DA, Gaspar T, Schliamser JE, Yaniv N, Ammar R, et al. Usefulness of 64-slice multidetector computed tomography in diagnostic triage of patients with chest pain and negative or nondiagnostic exercise treadmill test result. Am J Cardiol 2007;99:925-9.
- Runza G, La Grutta L, Alaimo V, Evola S, Lo Re F, Bartolotta TV, et al. Comprehensive cardiovascular ECG-gated MDCT as a standard diagnostic tool in patients with acute chest pain. Eur J Radiol 2007;64:41-7.
- Rychter K, Salanitri J, Edelman RR, Rychter K, Salanitri J, Edelman RR. Multifocal coronary artery myocardial bridging involving the right coronary and left anterior descending arteries detected by ECG-gated 64 slice multidetector CT coronary angiography. Int J Cardiovasc Imaging 2006;22:713-17.
- Santana CA, Garcia EV, Faber TL, Sirineni GK, Esteves FP, Sanyal R, et al. Diagnostic performance of fusion of myocardial perfusion imaging (MPI) and computed tomography coronary angiography. J Nucl Cardiol 2009;16:201-11.
- Santoro GM, Sciagra R, Buonamici P, Consoli N, Mazzoni V, Zerauschek F, et al. Head-to-head comparison of exercise stress testing, pharmacologic stress echocardiography, and perfusion tomography as first-line examination for chest pain in patients without history of coronary artery disease. J Nucl Cardiol 1998;5:19-27.
- Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffman U, Cury RC, et al. Diagnostic and prognostic value of absence of coronary artery calcification. Cardiol: Cardiovasc Imaging 2009;2:675-88.
- Sato A, Nozato T, Hikita H, Miyazaki S, Takahashi Y, Kuwahara T, et al. Incremental value of combining 64-slice computed tomography angiography with stress nuclear myocardial perfusion imaging to improve noninvasive detection of coronary artery disease. J Nucl Cardiol 2010;17:19-26.
- Sawada SG, Ryan T, Fineberg NS, Armstrong WF, Judson WE, McHenry PL, et al. Exercise echocardiographic detection of coronary artery disease in women. J Am Coll Cardiol 1989;14:1440-7.
- Schertler T, Scheffel H, Frauenfelder T, Desbiolles L, Leschka S, Stolzmann P, et al. Dual-source computed tomography in patients with acute chest pain: feasibility and image quality. Eur Radiol 2007;17:3179-88.
- Schertler T, Frauenfelder T, Stolzmann P, Scheffel H, Desbiolles L, Marincek B, et al. Triple Rule-Out CT in Patients with Suspicion of Acute Pulmonary Embolism: Findings and Accuracy. Acad Radiol 2009;16:708-17.
- Schmermund A, Stang A, Mohlenkamp S, Eggebrecht H, Baumgart D, Gilbert V, et al. Prognostic value of electron-beam computed tomography-derived coronary calcium scores compared with clinical parameters in patients evaluated for coronary artery disease: prognostic value of EBCT in symptomatic patients. Z Kardiol 2004;93:696-705.
- Schroeder S, Kuettner A. Usefulness of noninvasive MSCT coronary angiography as first-line imaging technique in patients with chest pain: Initial clinical experience. Int J Cardiol 2005;102:469-75.
- Schroeder S, Kuettner A, Wojak T, Janzen J, Heuschmid M, Athanasiou T, et al. Non-invasive evaluation of atherosclerosis with contrast enhanced 16 slice spiral computed tomography: results of ex vivo investigations. Heart 2004;90:1471-5.
- Schuijf JD, Bax JJ, Jukema JW, Lamb HJ, Salm LP, de Roos A, et al. Assessment of left ventricular volumes and ejection fraction with 16-slice multi-slice computed tomography; comparison with 2D-echocardiography. Int J Cardiol 2007;116:201-5.
- Schuijf JD, van Werkhoven JM, Pundziute G, Jukema JW, Decramer I, Stokkel MP, et al. Invasive versus noninvasive evaluation of coronary artery disease. Cardiol Cardiovasc Imaging 2008;1:190-9.
- Schuijf JD, Bax JJ, Salm LP, Jukema JW, Lamb HJ, van der Wall EE, et al. Noninvasive coronary imaging and assessment of left ventricular function using 16-slice computed tomography. Am J Cardiol 2005;95:571-4.
- Schussler JMG. Non-invasive coronary angiography using multislice computed tomography. Heart 2007;93:290-7.
- Scirica BM. Acute coronary syndrome emerging tools for diagnosis and risk assessment. J Am Coll Cardiol 2010;55:1403-15.
- Selker HP, Zalenski RJ, Antman EM, Aufderheide TP, Bernard SA, Bonow RO, et al. An evaluation of technologies for identifying acute cardiac ischemia in the emergency department: a report from a National Heart Attack Alert Program Working Group. Ann Emerg Med 1997;29:13-87.
- Seneviratne SK, Truong QA, Bamberg F, Rogers IS, Shapiro MD, Schlett CL, et al. Incremental Diagnostic Value of Regional Left Ventricular Function Over Coronary Assessment by Cardiac Computed Tomography for the Detection of Acute Coronary Syndrome in Patients With Acute Chest Pain From the ROMICAT Trial. Circ Cardiovasc Imaging 2010;3:375-83.
- Shabestari AA, Abdi S, Akhlaghpoor S, Azadi M, Baharjoo H, Pajouh MD, et al. Diagnostic performance of 64-channel multislice computed tomography in assessment of significant coronary artery disease in symptomatic subjects. Am J Cardiol 2007;99:1656-61.
- Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al. Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial. Health Technol Assess 2007;11.
- Shavelle DMB. Exercise testing and electron beam computed tomography in the evaluation of coronary artery disease. J Am Coll Cardiol 2000;36:32-8.
- Shinohara M, Yamashita T, Tawa H, Takeda M, Sasaki N, Takaya T, et al. Atherosclerotic plaque imaging using phase-contrast X-ray computed tomography. Am J Physiol Heart-Circ Physiol 2008;294:H1094-100.
- Shiran AH. Exercise testing in patients with chest pain and normal coronary arteries: Improving test specificity by use of a simple logistic model. Cardiology 1997;88:453-9.
- Shivalkar BG. Multislice cardiac computed tomography in symptomatic middle-aged women. Ann Med 2007;39:290-7.
- Shuman WP, Branch KR, May JM, Mitsumori LM, Strote JN, Warren BH, et al. Whole-chest 64-MDCT of Emergency Department Patients with nonspecific chest pain: radiation dose and coronary artery image quality with prospective ECG triggering versus retrospective ECG gating. Am J Roentgenol 2009;192:1662-7.
- Sievanen H, Karhumaki L, Vuori I, Malmivuo J. Improved diagnostic performance of the exercise ECG test by computerized multivariate ST-segment heart-rate analysis. J Electrocardiol 1991;24:129-43.
- Silber S, Richartz B. Evidence-based application of cardiac magnetic resonance and cardiac computed tomography for primary diagnosis of stable coronary artery disease with special attention to disease management programs and the German national medical care guidelines. Herz 2007;32:139-58.
- Ueno K, Anzai T, Jinzaki M, Yamada M, Kohno T, Kawamura A, et al. Diagnostic capacity of 64-slice multidetector computed tomography for acute coronary syndrome in patients presenting with acute chest pain. Cardiologia 2009;112:211-18.
- Van Lingen RK. Prognostic and accuracy data of multidetector CT coronary angiography in an established clinical service. Clin Radiol 2009;64:601-7.
- Walling ADC. Exercise testing in women with chest pain: applications and limitations of computer analysis. Coron Artery Dis 1993;4:783-9.
Appendix 5 Expected discounted quality-adjusted life-years of general population according to age and sex
| Age (years) | Male | Female |
|---|---|---|
| 20 | 23.84 | 23.27 |
| 21 | 23.69 | 23.12 |
| 22 | 23.45 | 22.89 |
| 23 | 23.29 | 22.73 |
| 24 | 23.12 | 22.57 |
| 25 | 22.95 | 22.40 |
| 26 | 22.78 | 22.23 |
| 27 | 22.50 | 21.96 |
| 28 | 22.32 | 21.78 |
| 29 | 22.13 | 21.59 |
| 30 | 21.93 | 21.40 |
| 31 | 21.73 | 21.20 |
| 32 | 21.53 | 21.00 |
| 33 | 21.19 | 20.68 |
| 34 | 20.98 | 20.46 |
| 35 | 20.75 | 20.24 |
| 36 | 20.53 | 20.02 |
| 37 | 20.14 | 19.65 |
| 38 | 19.90 | 19.41 |
| 39 | 19.65 | 19.16 |
| 40 | 19.39 | 18.91 |
| 41 | 18.95 | 18.48 |
| 42 | 18.68 | 18.21 |
| 43 | 18.40 | 17.94 |
| 44 | 18.11 | 17.66 |
| 45 | 17.61 | 17.16 |
| 46 | 17.30 | 16.86 |
| 47 | 16.98 | 16.55 |
| 48 | 16.41 | 16.00 |
| 49 | 16.08 | 15.66 |
| 50 | 15.73 | 15.32 |
| 51 | 15.09 | 14.70 |
| 52 | 14.71 | 14.33 |
| 53 | 14.33 | 13.96 |
| 54 | 13.93 | 13.57 |
| 55 | 13.53 | 13.18 |
| 56 | 13.12 | 12.77 |
| 57 | 12.33 | 12.00 |
| 58 | 12.26 | 11.93 |
| 59 | 11.81 | 11.50 |
| 60 | 11.35 | 11.05 |
| 61 | 10.88 | 10.59 |
| 62 | 10.40 | 10.13 |
| 63 | 9.91 | 9.64 |
| 64 | 9.40 | 9.15 |
| 65 | 9.34 | 9.09 |
| 66 | 8.83 | 8.59 |
| 67 | 8.30 | 8.07 |
| 68 | 8.24 | 8.02 |
| 69 | 7.70 | 7.49 |
| 70 | 7.15 | 6.95 |
| 71 | 7.10 | 6.90 |
| 72 | 6.54 | 6.35 |
| 73 | 6.49 | 6.31 |
| 74 | 5.91 | 5.75 |
| 75 | 5.87 | 5.70 |
| 76 | 5.28 | 5.13 |
| 77 | 5.24 | 5.09 |
| 78 | 4.64 | 4.51 |
| 79 | 4.61 | 4.47 |
| 80 | 4.00 | 3.88 |
| 81 | 3.97 | 3.85 |
| 82 | 3.94 | 3.82 |
| 83 | 3.90 | 3.79 |
| 84 | 3.29 | 3.19 |
| 85 | 3.26 | 3.17 |
| 86 | 3.24 | 3.14 |
| 87 | 2.62 | 2.54 |
| 88 | 2.60 | 2.52 |
| 89 | 2.57 | 2.49 |
| 90 | 2.55 | 2.47 |
| 91 | 2.53 | 2.44 |
| 92 | 1.92 | 1.86 |
| 93 | 1.90 | 1.84 |
| 94 | 1.88 | 1.82 |
| 95 | 1.86 | 1.80 |
| 96 | 1.84 | 1.78 |
| 97 | 1.82 | 1.76 |
| 98 | 1.23 | 1.19 |
| 99 | 0.62 | 0.60 |
Appendix 6 Cost-effectiveness of adding alternative biomarkers to troponin
| Study | Troponin alone | Biomarker combination | ||
|---|---|---|---|---|
| Costs, £ (95% CI) | QALYs (95% CI) | Costs, £ (95% CI) | QALYs (95% CI) | |
| Body201157 | 1,420,364 (1,409,967 to 1,430,762) | 26,295.56 (26,266.8 to 26,234.32) | 1,697,541 (1,686,216 to 1,708,865) | 26,358.44 (26,330.01 to 26,386.87) |
| Haltern 201067 | 1,560,158 (1,548,772 to 1,571,543) | 26,349.19 (26,321.78 to 26,376.60) | 1,953,948 (1,941,595 to 1,966,300) | 26,379.20 (26,350.30 to 26,408.11) |
| McCann 200876 | 1,614,259 (1,602,809 to 1,625,708) | 26,349.09 (26,322.15 to 26,376.03) | 1,720,365 (1,708,261 to 1,732,468) | 26,374.48 (26,346.03 to 26,402.93) |
| Mion 200777 | 1,473,403 (1,462,493 to 1,484,312) | 26,314.38 (26,286.22 to 26,342.54) | 1,628,844 (1,617,067 to 1,640,622) | 26,347.07 (26,138.76 to 26,375.39) |
| Keller 201071 | 1,522,637 (1,511,247 to 1,534,028) | 26,327.06 (26,298.69 to 26,335.43) | 1,820,502 (1,808,408 to 1,832,596) | 26,362.73 (26,332.55 to 26,392.92) |
| Reichlin 200980 | 1,614,259 (1,602,809 to 1,625,708) | 26,349.09 (26,322.15 to 26,376.03) | 1,874,782 (1,862,104 to 1,887,460) | 26,382.12 (26,353.68 to 26,410.56) |
| Keller 201071 | 1,522,637 (1,511,247 to 1,534,028) | 26,327.06 (26,298.69 to 26,335.43) | 1,713,718 (1,701,987 to 1,725,450) | 26,357.42 (26,329.10 to 26,385.73) |
| Mion 200777 | 1,473,403 (1,462,493 to 1,484,312) | 26,314.38 (26,286.22 to 26,342.54) | 1,673,692 (1,661,891 to 1,685,493) | 26,356.08 (26,326.76 to 26,385.40) |
| Collinson 200648 | 1,716,809 (1,704,484 to 1,729,134) | 26,380.72 (26,353.12 to 26,408.32) | 2,199,735 (2,187,170 to 2,212,300) | 26,386.00 (26,357.28 to 26,414.71) |
| Keating 200670 | 1,570,178 (1,558,422 to 1,581,935) | 26,350.59 (26,322.48 to 26,378.71) | 2,344,645 (2,332,475 to 2,356,815) | 26,378.56 (26,351.16 to 26,405.97) |
Appendix 7 Diagnostic model probabilistic sensitivity analysis results
Probability of cost-effectiveness in doctor-on-demand scenario
| Lambda (λ), £ | Prob NoT | Prob 10% | Prob 99th | Prob Hi Sens | Prob 10-hour Trop |
|---|---|---|---|---|---|
| 0 | 1 | 0 | 0 | 0 | 0 |
| 1000 | 1 | 0 | 0 | 0 | 0 |
| 2000 | 1 | 0 | 0 | 0 | 0 |
| 3000 | 0.0054 | 0.5724 | 0.2844 | 0.1378 | 0 |
| 4000 | 0 | 0.4074 | 0.3349 | 0.2577 | 0 |
| 5000 | 0 | 0.3186 | 0.3298 | 0.3510 | 0.0006 |
| 6000 | 0 | 0.2603 | 0.3076 | 0.4298 | 0.0023 |
| 7000 | 0 | 0.2155 | 0.2834 | 0.4903 | 0.0108 |
| 8000 | 0 | 0.1799 | 0.2574 | 0.5309 | 0.0318 |
| 9000 | 0 | 0.1515 | 0.2301 | 0.5585 | 0.0599 |
| 10,000 | 0 | 0.1266 | 0.2057 | 0.5751 | 0.0926 |
| 11,000 | 0 | 0.1075 | 0.1828 | 0.5813 | 0.1284 |
| 12,000 | 0 | 0.0924 | 0.1628 | 0.5792 | 0.1656 |
| 13,000 | 0 | 0.0782 | 0.1434 | 0.5771 | 0.2013 |
| 14,000 | 0 | 0.0682 | 0.1277 | 0.5701 | 0.2340 |
| 15,000 | 0 | 0.0609 | 0.1145 | 0.5628 | 0.2618 |
| 16,000 | 0 | 0.0539 | 0.1038 | 0.5501 | 0.2922 |
| 17,000 | 0 | 0.0481 | 0.0953 | 0.5395 | 0.3171 |
| 18,000 | 0 | 0.0409 | 0.0876 | 0.5281 | 0.3434 |
| 19,000 | 0 | 0.0369 | 0.0800 | 0.5167 | 0.3664 |
| 20,000 | 0 | 0.0335 | 0.0718 | 0.5041 | 0.3906 |
| 21,000 | 0 | 0.0309 | 0.0663 | 0.4933 | 0.4095 |
| 22,000 | 0 | 0.0272 | 0.0632 | 0.4815 | 0.4281 |
| 23,000 | 0 | 0.0246 | 0.0597 | 0.4678 | 0.4479 |
| 24,000 | 0 | 0.0225 | 0.0561 | 0.4568 | 0.4646 |
| 25,000 | 0 | 0.0210 | 0.0516 | 0.4454 | 0.4820 |
| 26,000 | 0 | 0.0195 | 0.0480 | 0.4363 | 0.4962 |
| 27,000 | 0 | 0.0177 | 0.0453 | 0.4258 | 0.5112 |
| 28,000 | 0 | 0.0162 | 0.0431 | 0.4158 | 0.5249 |
| 29,000 | 0 | 0.0151 | 0.0407 | 0.4070 | 0.5372 |
| 30,000 | 0 | 0.0144 | 0.0377 | 0.3944 | 0.5535 |
| 31,000 | 0 | 0.0135 | 0.0357 | 0.3848 | 0.5660 |
| 32,000 | 0 | 0.0131 | 0.0342 | 0.3750 | 0.5777 |
| 33,000 | 0 | 0.0128 | 0.0329 | 0.3659 | 0.5884 |
| 34,000 | 0 | 0.0127 | 0.0312 | 0.3562 | 0.5999 |
| 35,000 | 0 | 0.0122 | 0.0299 | 0.3480 | 0.6099 |
| 36,000 | 0 | 0.0113 | 0.0287 | 0.3409 | 0.6191 |
| 37,000 | 0 | 0.0103 | 0.0283 | 0.3319 | 0.6295 |
| 38,000 | 0 | 0.0093 | 0.0269 | 0.3252 | 0.6386 |
| 39,000 | 0 | 0.0089 | 0.0259 | 0.3192 | 0.6460 |
| 40,000 | 0 | 0.0089 | 0.0253 | 0.3130 | 0.6528 |
| 41,000 | 0 | 0.0085 | 0.0241 | 0.3067 | 0.6607 |
| 42,000 | 0 | 0.0083 | 0.0235 | 0.2999 | 0.6683 |
| 43,000 | 0 | 0.0078 | 0.0233 | 0.2915 | 0.6774 |
| 44,000 | 0 | 0.0075 | 0.0229 | 0.2847 | 0.6849 |
| 45,000 | 0 | 0.0072 | 0.0226 | 0.2790 | 0.6912 |
| 46,000 | 0 | 0.0070 | 0.0222 | 0.2738 | 0.6970 |
| 47,000 | 0 | 0.0068 | 0.0216 | 0.2685 | 0.7031 |
| 48,000 | 0 | 0.0066 | 0.0206 | 0.2639 | 0.7089 |
| 49,000 | 0 | 0.0064 | 0.0197 | 0.2580 | 0.7159 |
| 50,000 | 0 | 0.0060 | 0.0194 | 0.2527 | 0.7219 |
Probability of cost-effectiveness in twice-daily ward scenario
| Lambda (λ), £ | Prob NoT | Prob 10% | Prob 99th | Prob Hi Sens | Prob 10-hour Trop |
|---|---|---|---|---|---|
| 0 | 1 | 0 | 0 | 0 | 0 |
| 1000 | 1 | 0 | 0 | 0 | 0 |
| 2000 | 1 | 0 | 0 | 0 | 0 |
| 3000 | 0.1089 | 0.6946 | 0.1965 | 0 | 0 |
| 4000 | 0.0003 | 0.5894 | 0.3816 | 0.0287 | 0 |
| 5000 | 0 | 0.4845 | 0.4236 | 0.0919 | 0 |
| 6000 | 0 | 0.4093 | 0.4173 | 0.1734 | 0 |
| 7000 | 0 | 0.3519 | 0.3998 | 0.2483 | 0 |
| 8000 | 0 | 0.3060 | 0.3778 | 0.3160 | 0.0002 |
| 9000 | 0 | 0.2667 | 0.3548 | 0.3783 | 0.0002 |
| 10,000 | 0 | 0.2366 | 0.3349 | 0.4276 | 0.0009 |
| 11,000 | 0 | 0.2111 | 0.3117 | 0.4753 | 0.0019 |
| 12,000 | 0 | 0.1927 | 0.2928 | 0.5100 | 0.0045 |
| 13,000 | 0 | 0.1748 | 0.2768 | 0.5403 | 0.0081 |
| 14,000 | 0 | 0.1612 | 0.2585 | 0.5665 | 0.0138 |
| 15,000 | 0 | 0.1486 | 0.2453 | 0.5864 | 0.0197 |
| 16,000 | 0 | 0.1370 | 0.2345 | 0.6019 | 0.0266 |
| 17,000 | 0 | 0.1271 | 0.2238 | 0.6131 | 0.0360 |
| 18,000 | 0 | 0.1178 | 0.2129 | 0.6234 | 0.0459 |
| 19,000 | 0 | 0.1091 | 0.2037 | 0.6300 | 0.0572 |
| 20,000 | 0 | 0.1030 | 0.1932 | 0.6352 | 0.0686 |
| 21,000 | 0 | 0.0968 | 0.1820 | 0.6388 | 0.0824 |
| 22,000 | 0 | 0.0905 | 0.1734 | 0.6429 | 0.0932 |
| 23,000 | 0 | 0.0844 | 0.1648 | 0.6437 | 0.1071 |
| 24,000 | 0 | 0.0792 | 0.1552 | 0.6422 | 0.1234 |
| 25,000 | 0 | 0.0751 | 0.1464 | 0.6414 | 0.1371 |
| 26,000 | 0 | 0.0707 | 0.1386 | 0.6398 | 0.1509 |
| 27,000 | 0 | 0.0671 | 0.1321 | 0.6378 | 0.1630 |
| 28,000 | 0 | 0.0641 | 0.1273 | 0.6357 | 0.1729 |
| 29,000 | 0 | 0.0597 | 0.1208 | 0.6343 | 0.1852 |
| 30,000 | 0 | 0.0569 | 0.1169 | 0.6295 | 0.1967 |
| 31,000 | 0 | 0.0534 | 0.1124 | 0.6248 | 0.2094 |
| 32,000 | 0 | 0.0505 | 0.1082 | 0.6207 | 0.2206 |
| 33,000 | 0 | 0.0476 | 0.1051 | 0.6145 | 0.2328 |
| 34,000 | 0 | 0.0455 | 0.1015 | 0.6100 | 0.2430 |
| 35,000 | 0 | 0.0435 | 0.0979 | 0.6050 | 0.2536 |
| 36,000 | 0 | 0.0400 | 0.0947 | 0.5997 | 0.2656 |
| 37,000 | 0 | 0.0380 | 0.0909 | 0.5943 | 0.2768 |
| 38,000 | 0 | 0.0361 | 0.0874 | 0.5875 | 0.2890 |
| 39,000 | 0 | 0.0347 | 0.0840 | 0.5815 | 0.2998 |
| 40,000 | 0 | 0.0333 | 0.0814 | 0.5759 | 0.3094 |
| 41,000 | 0 | 0.0316 | 0.0776 | 0.5720 | 0.3188 |
| 42,000 | 0 | 0.0303 | 0.0743 | 0.5644 | 0.3310 |
| 43,000 | 0 | 0.0293 | 0.0724 | 0.5579 | 0.3404 |
| 44,000 | 0 | 0.0282 | 0.0697 | 0.5525 | 0.3496 |
| 45,000 | 0 | 0.0271 | 0.0674 | 0.5459 | 0.3596 |
| 46,000 | 0 | 0.0263 | 0.0656 | 0.5392 | 0.3689 |
| 47,000 | 0 | 0.0251 | 0.0643 | 0.5335 | 0.3771 |
| 48,000 | 0 | 0.0243 | 0.0623 | 0.5262 | 0.3872 |
| 49,000 | 0 | 0.0233 | 0.0597 | 0.5220 | 0.3950 |
| 50,000 | 0 | 0.0228 | 0.0589 | 0.5182 | 0.4001 |
Probability of cost-effectiveness in once-daily ward scenario
| Lambda (λ), £ | Prob NoT | Prob 10% | Prob 99th | Prob Hi Sens | Prob 10-hour trop |
|---|---|---|---|---|---|
| 0 | 1 | 0 | 0 | 0 | 0 |
| 1000 | 1 | 0 | 0 | 0 | 0 |
| 2000 | 1 | 0 | 0 | 0 | 0 |
| 3000 | 0.5785 | 0.4185 | 0.0030 | 0 | 0 |
| 4000 | 0.0014 | 0.7478 | 0.2489 | 0.0019 | 0 |
| 5000 | 0.0001 | 0.6277 | 0.3513 | 0.0209 | 0 |
| 6000 | 0 | 0.5445 | 0.3918 | 0.0637 | 0 |
| 7000 | 0 | 0.4735 | 0.4038 | 0.1227 | 0 |
| 8000 | 0 | 0.4191 | 0.3959 | 0.1850 | 0 |
| 9000 | 0 | 0.3693 | 0.3821 | 0.2486 | 0 |
| 10,000 | 0 | 0.3286 | 0.3677 | 0.3037 | 0 |
| 11,000 | 0 | 0.2926 | 0.3503 | 0.3569 | 0.0002 |
| 12,000 | 0 | 0.2657 | 0.3336 | 0.4005 | 0.0002 |
| 13,000 | 0 | 0.2412 | 0.3171 | 0.4408 | 0.0009 |
| 14,000 | 0 | 0.2216 | 0.3020 | 0.4750 | 0.0014 |
| 15,000 | 0 | 0.2041 | 0.2881 | 0.5051 | 0.0027 |
| 16,000 | 0 | 0.1894 | 0.2741 | 0.5317 | 0.0048 |
| 17,000 | 0 | 0.1757 | 0.2623 | 0.5548 | 0.0072 |
| 18,000 | 0 | 0.1639 | 0.2518 | 0.5735 | 0.0108 |
| 19,000 | 0 | 0.1549 | 0.2438 | 0.5873 | 0.0140 |
| 20,000 | 0 | 0.1457 | 0.2353 | 0.5997 | 0.0193 |
| 21,000 | 0 | 0.1377 | 0.2281 | 0.6099 | 0.0243 |
| 22,000 | 0 | 0.1300 | 0.2188 | 0.6206 | 0.0306 |
| 23,000 | 0 | 0.1223 | 0.2122 | 0.6291 | 0.0364 |
| 24,000 | 0 | 0.1163 | 0.2039 | 0.6365 | 0.0433 |
| 25,000 | 0 | 0.1112 | 0.1970 | 0.6414 | 0.0504 |
| 26,000 | 0 | 0.1058 | 0.1899 | 0.6464 | 0.0579 |
| 27,000 | 0 | 0.1004 | 0.1829 | 0.6493 | 0.0674 |
| 28,000 | 0 | 0.0964 | 0.1759 | 0.6517 | 0.0760 |
| 29,000 | 0 | 0.0916 | 0.1707 | 0.6521 | 0.0856 |
| 30,000 | 0 | 0.0883 | 0.1647 | 0.6547 | 0.0923 |
| 31,000 | 0 | 0.0840 | 0.1576 | 0.6553 | 0.1031 |
| 32,000 | 0 | 0.0805 | 0.1512 | 0.6529 | 0.1154 |
| 33,000 | 0 | 0.0770 | 0.1451 | 0.6521 | 0.1258 |
| 34,000 | 0 | 0.0739 | 0.1397 | 0.6520 | 0.1344 |
| 35,000 | 0 | 0.0712 | 0.1359 | 0.6508 | 0.1421 |
| 36,000 | 0 | 0.0685 | 0.1313 | 0.6486 | 0.1516 |
| 37,000 | 0 | 0.0657 | 0.1270 | 0.6484 | 0.1589 |
| 38,000 | 0 | 0.0629 | 0.1236 | 0.6466 | 0.1669 |
| 39,000 | 0 | 0.0605 | 0.1189 | 0.6426 | 0.1780 |
| 40,000 | 0 | 0.0582 | 0.1164 | 0.6390 | 0.1864 |
| 41,000 | 0 | 0.0553 | 0.1139 | 0.6367 | 0.1941 |
| 42,000 | 0 | 0.0534 | 0.1104 | 0.6324 | 0.2038 |
| 43,000 | 0 | 0.0518 | 0.1074 | 0.6290 | 0.2118 |
| 44,000 | 0 | 0.0500 | 0.1043 | 0.6257 | 0.2200 |
| 45,000 | 0 | 0.0478 | 0.1022 | 0.6228 | 0.2272 |
| 46,000 | 0 | 0.0458 | 0.0996 | 0.6196 | 0.2350 |
| 47,000 | 0 | 0.0444 | 0.0965 | 0.6166 | 0.2425 |
| 48,000 | 0 | 0.0424 | 0.0932 | 0.6143 | 0.2501 |
| 49,000 | 0 | 0.0402 | 0.0916 | 0.6111 | 0.2571 |
| 50,000 | 0 | 0.0383 | 0.0889 | 0.6067 | 0.2661 |
Appendix 8 Prognostic model probabilistic sensitivity analysis results
Probability of cost-effectiveness using RATPAC data
| Lambda (λ), £ | Prob NoT | Prob ETT | Prob HF | Prob CTCA | Prob CA |
|---|---|---|---|---|---|
| 0 | 1 | 0 | 0 | 0 | 0 |
| 5000 | 1 | 0 | 0 | 0 | 0 |
| 10,000 | 1 | 0 | 0 | 0 | 0 |
| 15,000 | 1 | 0 | 0 | 0 | 0 |
| 20,000 | 1 | 0 | 0 | 0 | 0 |
| 25,000 | 1 | 0 | 0 | 0 | 0 |
| 30,000 | 1 | 0 | 0 | 0 | 0 |
| 35,000 | 1 | 0 | 0 | 0 | 0 |
| 40,000 | 1 | 0 | 0 | 0 | 0 |
| 45,000 | 1 | 0 | 0 | 0 | 0 |
| 50,000 | 1 | 0 | 0 | 0 | 0 |
| 55,000 | 1 | 0 | 0 | 0 | 0 |
| 60,000 | 0.9999 | 0 | 0.0001 | 0 | 0 |
| 65,000 | 0.9932 | 0 | 0.0068 | 0 | 0 |
| 70,000 | 0.9709 | 0 | 0.0291 | 0 | 0 |
| 75,000 | 0.9217 | 0 | 0.0783 | 0 | 0 |
| 80,000 | 0.8542 | 0 | 0.1458 | 0 | 0 |
| 85,000 | 0.7755 | 0 | 0.2245 | 0 | 0 |
| 90,000 | 0.6850 | 0 | 0.3150 | 0 | 0 |
| 95,000 | 0.5974 | 0 | 0.4026 | 0 | 0 |
| 100,000 | 0.5115 | 0 | 0.4885 | 0 | 0 |
| 105,000 | 0.4336 | 0 | 0.5662 | 0.0002 | 0 |
| 110,000 | 0.3599 | 0 | 0.6389 | 0.0012 | 0 |
| 115,000 | 0.2920 | 0 | 0.7055 | 0.0025 | 0 |
| 120,000 | 0.2372 | 0 | 0.7562 | 0.0066 | 0 |
| 125,000 | 0.1863 | 0 | 0.8024 | 0.0113 | 0 |
| 130,000 | 0.1427 | 0 | 0.8398 | 0.0175 | 0 |
| 135,000 | 0.1129 | 0 | 0.8620 | 0.0251 | 0 |
| 140,000 | 0.0847 | 0 | 0.8820 | 0.0333 | 0 |
| 145,000 | 0.0618 | 0 | 0.8938 | 0.0444 | 0 |
| 150,000 | 0.0449 | 0 | 0.8943 | 0.0608 | 0 |
| 155,000 | 0.0322 | 0 | 0.8919 | 0.0759 | 0 |
| 160,000 | 0.0241 | 0 | 0.8808 | 0.0951 | 0 |
| 165,000 | 0.0178 | 0 | 0.8678 | 0.1144 | 0 |
| 170,000 | 0.0121 | 0 | 0.8564 | 0.1315 | 0 |
| 175,000 | 0.0073 | 0 | 0.8388 | 0.1539 | 0 |
| 180,000 | 0.0059 | 0 | 0.8198 | 0.1743 | 0 |
| 185,000 | 0.0042 | 0 | 0.7989 | 0.1969 | 0 |
| 190,000 | 0.0024 | 0 | 0.7763 | 0.2213 | 0 |
| 195,000 | 0.0014 | 0 | 0.7550 | 0.2436 | 0 |
| 200,000 | 0.0011 | 0 | 0.7337 | 0.2652 | 0 |
| 205,000 | 0.0006 | 0 | 0.7106 | 0.2888 | 0 |
| 210,000 | 0.0004 | 0 | 0.6879 | 0.3117 | 0 |
| 215,000 | 0.0002 | 0 | 0.6630 | 0.3368 | 0 |
| 220,000 | 0.0001 | 0 | 0.6401 | 0.3598 | 0 |
| 225,000 | 0.0001 | 0 | 0.6203 | 0.3796 | 0 |
| 230,000 | 0 | 0 | 0.5981 | 0.4019 | 0 |
| 235,000 | 0 | 0 | 0.5775 | 0.4225 | 0 |
| 240,000 | 0 | 0 | 0.5586 | 0.4414 | 0 |
| 245,000 | 0 | 0 | 0.5411 | 0.4589 | 0 |
| 250,000 | 0 | 0 | 0.5235 | 0.4765 | 0 |
Probability of cost-effectiveness using Mills data155
| Lambda (λ), £ | Prob NoT | Prob ETT | Prob HF | Prob CTCA | Prob CA |
|---|---|---|---|---|---|
| 0 | 1 | 0 | 0 | 0 | 0 |
| 5000 | 1 | 0 | 0 | 0 | 0 |
| 10,000 | 1 | 0 | 0 | 0 | 0 |
| 15,000 | 1 | 0 | 0 | 0 | 0 |
| 20,000 | 1 | 0 | 0 | 0 | 0 |
| 25,000 | 1 | 0 | 0 | 0 | 0 |
| 30,000 | 1 | 0 | 0 | 0 | 0 |
| 35,000 | 1 | 0 | 0 | 0 | 0 |
| 40,000 | 1 | 0 | 0 | 0 | 0 |
| 45,000 | 1 | 0 | 0 | 0 | 0 |
| 50,000 | 1 | 0 | 0 | 0 | 0 |
| 55,000 | 1 | 0 | 0 | 0 | 0 |
| 60,000 | 0.9999 | 0 | 0.0001 | 0 | 0 |
| 65,000 | 0.9932 | 0 | 0.0068 | 0 | 0 |
| 70,000 | 0.9709 | 0 | 0.0291 | 0 | 0 |
| 75,000 | 0.9217 | 0 | 0.0783 | 0 | 0 |
| 80,000 | 0.8542 | 0 | 0.1458 | 0 | 0 |
| 85,000 | 0.7755 | 0 | 0.2245 | 0 | 0 |
| 90,000 | 0.6850 | 0 | 0.3150 | 0 | 0 |
| 95,000 | 0.5974 | 0 | 0.4026 | 0 | 0 |
| 100,000 | 0.5115 | 0 | 0.4885 | 0 | 0 |
| 105,000 | 0.4336 | 0 | 0.5662 | 0.0002 | 0 |
| 110,000 | 0.3599 | 0 | 0.6389 | 0.0012 | 0 |
| 115,000 | 0.2920 | 0 | 0.7055 | 0.0025 | 0 |
| 120,000 | 0.2372 | 0 | 0.7562 | 0.0066 | 0 |
| 125,000 | 0.1863 | 0 | 0.8024 | 0.0113 | 0 |
| 130,000 | 0.1427 | 0 | 0.8398 | 0.0175 | 0 |
| 135,000 | 0.1129 | 0 | 0.8620 | 0.0251 | 0 |
| 140,000 | 0.0847 | 0 | 0.8820 | 0.0333 | 0 |
| 145,000 | 0.0618 | 0 | 0.8938 | 0.0444 | 0 |
| 150,000 | 0.0449 | 0 | 0.8943 | 0.0608 | 0 |
| 155,000 | 0.0322 | 0 | 0.8919 | 0.0759 | 0 |
| 160,000 | 0.0241 | 0 | 0.8808 | 0.0951 | 0 |
| 165,000 | 0.0178 | 0 | 0.8678 | 0.1144 | 0 |
| 170,000 | 0.0121 | 0 | 0.8564 | 0.1315 | 0 |
| 175,000 | 0.0073 | 0 | 0.8388 | 0.1539 | 0 |
| 180,000 | 0.0059 | 0 | 0.8198 | 0.1743 | 0 |
| 185,000 | 0.0042 | 0 | 0.7989 | 0.1969 | 0 |
| 190,000 | 0.0024 | 0 | 0.7763 | 0.2213 | 0 |
| 195,000 | 0.0014 | 0 | 0.7550 | 0.2436 | 0 |
| 200,000 | 0.0011 | 0 | 0.7337 | 0.2652 | 0 |
| 205,000 | 0.0006 | 0 | 0.7106 | 0.2888 | 0 |
| 210,000 | 0.0004 | 0 | 0.6879 | 0.3117 | 0 |
| 215,000 | 0.0002 | 0 | 0.6630 | 0.3368 | 0 |
| 220,000 | 0.0001 | 0 | 0.6401 | 0.3598 | 0 |
| 225,000 | 0.0001 | 0 | 0.6203 | 0.3796 | 0 |
| 230,000 | 0 | 0 | 0.5981 | 0.4019 | 0 |
| 235,000 | 0 | 0 | 0.5775 | 0.4225 | 0 |
| 240,000 | 0 | 0 | 0.5586 | 0.4414 | 0 |
| 245,000 | 0 | 0 | 0.5411 | 0.4589 | 0 |
| 250,000 | 0 | 0 | 0.5235 | 0.4765 | 0 |
Appendix 9 Final project description
Project title
Cost-effectiveness of diagnostic strategies for suspected acute coronary syndrome (ACS).
Planned investigation
Research objectives
-
To estimate the diagnostic accuracy for myocardial infarction and prognostic accuracy for cardiac events of biomarkers used to investigate suspected ACS.
-
To estimate the cost-effectiveness of biomarker strategies for investigating suspected ACS.
-
To estimate the diagnostic accuracy for coronary artery disease (CAD) and prognostic accuracy for cardiac events of multislice CT coronary angiography and exercise ECG in patients with suspected ACS.
-
To estimate the cost-effectiveness of multislice CT coronary angiography and exercise ECG for investigating patients with troponin-negative suspected ACS.
-
To identify the critical areas of uncertainty in the management of suspected ACS, where future primary research would produce the most benefit.
Existing research
ACS typically occurs when a patient with CAD develops obstruction of their heart arteries. This can lead to myocardial infarction (MI), heart failure, arrhythmia, cardiac arrest and death. ACS has 6-month mortality of up to 20% [2] and a fifth of patients are rehospitalised within 6 months of their initial admission [3].
ACS usually presents as chest pain and must be differentiated from other common causes of chest pain, such as muscular pain, gastro-oesophageal pain and anxiety. Differentiation is difficult because clinical assessment is unreliable and the ECG may be normal in the presence of ACS. Patients with suspected ACS therefore constitute a large and varied population, many of whom will not have ACS or CAD, but have non-cardiac causes for their chest pain. Accurate identification of ACS and CAD are therefore required to guide subsequent intervention.
Suspected ACS represents a substantial health-care problem and investigation represents a substantial challenge. Chest pain is responsible for around 700,000 emergency department attendances in England and Wales [4], with the main reason for attendance being suspected ACS. Hospital Episodes Statistics for England (2006–7) showed 158,342 emergency admissions with ischaemic heart disease, accounting for almost 1 million bed-days. In addition, many of the 351,716 emergency admissions classified as ‘signs and symptoms involving the circulatory or respiratory system’ will have been due to suspicion of ACS.
Investigation for suspected ACS has two main elements: (1) diagnosis of MI, and (2) diagnosis of underlying CAD. Diagnosis of unstable angina is another consideration but of decreasing importance for reasons outlined below.
Diagnosis of MI
The term MI usually refers to NSTEMI in the context of investigating suspected ACS. Although ST-elevation MI is included in the definition of ACS it can usually be identified on the presenting ECG and thus does not form part of the typical diagnostic challenge of suspected ACS, although ECG interpretation and differentiation from other causes of ST elevation may present separate challenges.
Clinical diagnosis of NSTEMI, according to the universal definition of MI [5], is based upon an elevation of cardiac biomarkers (preferably troponin) above the 99th percentile of the upper reference limit. Patients with an elevated troponin have an increased risk of adverse outcome and can benefit from hospital admission and treatment. However, troponin does not achieve optimal sensitivity for MI until several hours after the symptoms of MI [6] so guidelines typically recommend delaying sampling until 10–12 h after symptom onset. Most patients with suspected ACS present to hospital within a few hours of symptom onset, so delaying blood sampling usually incurs costs of hospital observation and/or admission. Earlier blood sampling is cheaper but may miss cases of MI, so the timing of sampling and tests used involve a trade-off between cost and accuracy.
Diagnosis of underlying CAD
Many patients with suspected ACS are known to have CAD and are receiving secondary preventative treatment. However, a substantial proportion of patients have not previously been investigated for CAD. Once MI has been ruled out these patients may be investigated for underlying CAD by either provocative cardiac testing to identify symptoms of CAD induced by exertional or pharmacological stress or anatomical imaging of the coronary arteries. Identification of CAD allows treatment with aspirin, statins and angiotensin converting enzyme inhibitors to be commenced and consideration of coronary revascularisation for high-risk cases.
Unstable angina
Investigation of suspected ACS also involves identification and treatment of patients with unstable angina. These patients have CAD and worsening symptoms but no evidence of cardiac damage. Previously they constituted the majority of patients with suspected ACS. However, the increasing sensitivity of biochemical tests for myocardial damage, and the redefinition of MI to include all patients with evidence of myocardial damage, means that patients with unstable angina and no myocardial damage are fewer in number and have a relatively low risk of adverse outcome. Furthermore, in the absence of ECG changes there are substantial difficulties defining which patients have unstable angina, since the diagnosis is based upon unreliable clinical features. These factors make it difficult to define the population with unstable angina and estimate any benefits from treatment, beyond secondary prevention for underlying CAD.
Uncertainties in the investigation of suspected ACS
There have been many published guidelines for the investigation of suspected ACS. Most recently the National Institute for Health and Clinical Excellence (NICE) has issued draft guidance for the management of patients with acute chest pain due to possible ACS [1]. These guidelines have identified areas of uncertainty where further research is required. These are:
-
Evaluation of new, high sensitivity troponin assay methods in low, medium and high-risk groups with acute chest pain, and evaluation of other putative biomarkers in comparison with the diagnostic and prognostic performance of the most clinically effective and cost-effective troponin assays.
-
Investigation of the cost-effectiveness of multislice CT coronary angiography as a first-line test for ruling out obstructive CAD in patients with suspected troponin-negative acute coronary syndromes.
Evaluation of new troponin assays and other biomarkers
The draft NICE guidelines recommend measurement of troponin levels at 10–12 h after the onset of symptoms to accurately identify cases of MI. This is based upon evidence that troponin levels predict subsequent risk of adverse outcome [7] and response to treatment [8], but do not achieve optimal sensitivity until 10–12 h after symptom onset [6]. However, delaying blood testing until 10–12 h after symptom onset is inconvenient for patients and often incurs additional health-care costs associated with hospital admission and/or observation. Various alternative strategies have been proposed for earlier diagnosis of MI using combinations of biomarkers, measuring biomarker gradients and using newer, more sensitive troponin assays.
Systematic reviews have established the diagnostic [6] and prognostic [7] accuracy of troponin testing in suspected ACS. A systematic review of the diagnostic accuracy of troponin, creatinine kinase, CK-MB and myoglobin [9] established that troponin has the highest accuracy for MI. Sensitivity and specificity of other markers were more modest but could be improved by serial testing, measurement of the gradient rise and using combinations of biomarkers. A systematic review of 22 novel biomarkers, including C-reactive protein, myeloperoxidase, B-type natriuretic peptide and heart-type fatty acid-binding protein [10], concluded that there was insufficient evidence to support the use of these biomarkers in emergency department assessment of suspected ACS. However, more data have emerged since these reviews were published suggesting that early biomarker testing with combinations of troponin, CK-MB and myoglobin may have comparable sensitivity to delayed troponin testing, and some novel biomarkers may provide additional prognostic information in patients with troponin negative suspected ACS. In addition, newer troponin assays capable of detecting changes within the reference interval and capable of significantly earlier detection have been developed and are entering, or have entered, routine clinical use.
Two economic analyses have examined the cost-effectiveness of biomarker strategies in the NHS. Goodacre [11] used a decision analysis model to compare five strategies for patients with undifferentiated chest pain and showed that rapid biomarker testing was most likely to be cost-effective in the NHS while hospital admission was unlikely to be cost-effective. This analysis only evaluated five potential strategies and did not explore uncertainty in estimates. Mant [12] used modelling to compare four strategies for identifying ST-elevation MI and to compare three models of care for patients presenting to primary care with possible angina. The modelling did not therefore evaluate the cost-effectiveness of biomarkers in patients with suspected ACS. Two studies from outside the UK have suggested that the use of troponin T is cost-effective compared with CK-MB [13,14], but neither evaluated other biomarker strategies in suspected ACS.
In summary, there is not yet convincing evidence that alternative biomarker strategies can match the diagnostic accuracy of a 10–12 h troponin. However, there are several reasons why a 10–12 h troponin may not be the optimal approach for the NHS:
-
Diagnostic data for alternative biomarker strategies have not to date been comprehensively and systematically summarised.
-
Alternative biomarkers may provide additional prognostic information beyond that provided by a 10–12 h troponin.
-
Selection of an optimal strategy is fundamentally an issue of cost-effectiveness. A 10–12 h troponin may not be cost-effective compared with earlier strategies, even if it is more accurate, if the benefit of more accurate diagnosis does not justify the additional costs associated with delayed testing.
Systematic reviews of potential biomarker strategies for suspected ACS need to be updated and include analysis of the additional prognostic value provided by these tests. Cost-effectiveness analysis is required to compare potential biomarker strategies in suspected ACS from the perspective of the NHS. This will allow us to determine what is the optimal strategy for the NHS on the basis of currently available data. It will also allow us to identify the most promising biomarker strategies for future evaluation and the key areas of uncertainty for primary research.
Multislice CT coronary angiography for troponin negative suspected ACS
Once MI has been ruled out by a negative 10–12 hour troponin (or alternative biomarker strategy) current European Society of Cardiology guidelines recommend using a stress test (typically exercise ECG) to select patients for further investigation with coronary angiography [15]. Most studies of the diagnostic accuracy of exercise ECG have been undertaken in patients with stable symptoms rather than suspected ACS. The most recent meta-analysis [12] of the diagnostic accuracy of exercise ECG reported that the main diagnostic criterion (ST depression) performed only moderately well, with a positive likelihood ratio of 2.79 for a 1-mm cutoff and 3.85 for a 2-mm cutoff. The negative likelihood ratios were 0.44 and 0.72 respectively. Exercise ECG would therefore be expected to miss a significant proportion of patients with CAD while subjecting others with normal coronary arteries to an unnecessary invasive coronary angiogram.
Multislice CT coronary angiography may provide a more accurate and cost-effective alternative to exercise ECG in troponin negative patients with suspected ACS. As with exercise ECG, most studies have evaluated CT coronary angiography in patients with stable symptoms rather than suspected ACS. A recent systematic review of 21 diagnostic accuracy studies of CT coronary angiography reported a pooled sensitivity of 99% and specificity of 89% for detection of CAD [16]. On the basis of this and similar analyses it has been recommended that CT calcium scoring with CT coronary angiography for selected patients replace exercise ECG [1].
It is not yet clear whether CT coronary angiography could have a similar role in suspected ACS. Four studies (N = 103, 120, 55 and 48) have evaluated it's use to detect CAD in patients with suspected ACS, yielding sensitivities of 92 to 100% and specificities of 46% to 92%, depending upon the diagnostic criteria used [17–20]. These studies suggest that CT coronary angiography may be used to rule out significant CAD in patients with troponin negative suspected ACS, but that limited specificity may increase unnecessary investigations and health-care costs.
Two economic analyses from the United States have used modelling to estimate the cost-effectiveness of CT coronary angiography in patients with suspected ACS [21,22]. Both models suggested that CT coronary angiography is cost-effective compared with exercise ECG or stress echocardiography. However, neither analysis involved comparison to a no further testing alternative. Exercise ECG is known to have limited diagnostic accuracy for CAD so it may represent an inefficient comparator. Furthermore, it is not clear whether findings from the high-cost North American health-care system will be reproduced in the NHS.
Cost-effectiveness analysis is required to compare CT coronary angiography and exercise ECG to each other and an alternative of no routine testing for patients with troponin negative suspected ACS. This will allow us to determine what is the optimal strategy for the NHS on the basis of currently available data. It will also allow us to identify whether primary research in the form of a trial is required and if so, what alternatives should be compared and outcomes measured.
Research methods
Design
We plan to undertake a cost-effectiveness analysis based on secondary research (systematic review, meta-analysis and decision-analysis modelling) to determine the most appropriate biomarker strategy for investigating patients with suspected ACS and determine whether CT coronary angiography or exercise ECG should be used to investigate troponin negative patients with suspected ACS.
Systematic reviews and meta-analysis
Systematic reviews and meta-analysis will be used to estimate the diagnostic and/or prognostic value of biomarkers, CT coronary angiography and exercise ECG in patients with suspected ACS. Systematic reviews will also be used to estimate the effectiveness of treatments for MI and CAD and estimate parameters required for the model.
There are a large number of published studies of biomarkers in suspected ACS but many are either of poor quality, due to lack of rigorous follow-up or an appropriate reference standard, or limited relevance because they have recruited a selected cohort of patients (for example, those with few or no co-morbidities or patients selected for coronary care admission). We plan to select studies for inclusion only if they have an appropriate reference standard and/or adequate follow-up, and only if they recruit unselected patients presenting to hospital with suspected ACS. Furthermore, we do not intend to repeat the existing systematic reviews of exercise ECG and CT coronary angiography in patients with stable symptoms and suspected CAD but will instead identify studies recruiting patients with suspected ACS.
We will search the literature for prospective cohort studies of biomarkers, CT coronary angiography and exercise ECG in unselected patients presenting to hospital with suspected ACS in which at least 80% of the cohort receives either:
-
Diagnostic testing for either MI using the universal definition or CAD using coronary angiography
-
Follow up to identify major adverse cardiac events up to at least 30 days after presentation
We will specifically search for studies of the following biomarkers: troponin, creatinine kinase MB, myoglobin, C-reactive protein, myeloperoxidase, B-type natriuretic peptide, heart-type fatty acid-binding protein, copeptin, ST-2 and galectin-15.
We will also use literature reviews to estimate the following parameters for the decision analysis model:
-
The effect of current treatments for MI upon mortality and adverse outcomes
-
The effect of secondary prevention upon long term CAD mortality and morbidity.
-
Quality-adjusted life expectancy after MI and with CAD.
-
The prevalence of MI and CAD and rate of adverse outcomes in a typical NHS population with suspected ACS.
-
Other characteristics of the typical population with suspected ACS: age, gender, prevalence of CAD and risk factors for CAD, clinical features, risk score profiles, and prevalence of abnormal test results (ECG, troponin, creatinine).
-
Long-term costs of care after event-free treatment for MI, after non-fatal adverse events and for CAD.
A hierarchical approach will be used so that the most valid and relevant estimates are given priority (i.e. randomised controlled trials for effectiveness data and prospective cohort studies for prognostic data), while data with low validity or relevance are excluded. Recent published systematic reviews will be used if they are of acceptable quality.
Search strategy
Relevant studies will be identified through electronic searches of key databases including MEDLINE, EMBASE, Science Citation Index and Biological Abstracts. Published empirical work will be used to identify optimal strategies for prognosis and diagnosis on MEDLINE and EMBASE [23–26]. A single search strategy will be used to identify all citations that include (a) a term or abbreviation for one of the technologies (including the named biomarkers above), (b) a term or abbreviation for ACS, MI or CAD, and (c) filter for cohort or diagnostic studies.
References will also be located through review of reference lists for relevant articles and through use of citation search facilities through the Web of Knowledge's Science Citation Index and Social Science Citation Index. Where existing systematic reviews already exist, these will be used both to identify relevant studies and to inform subsequent analysis. In addition systematic searches of the Internet using the Copernic meta-search engine will be used to identify unpublished materials and work in progress. Key authors and professional and academic research groups will also be contacted and asked for unpublished material.
Review strategy
The stages of the review will include:
-
Accumulation of references, entry and tagging on a Reference Manager database, enabling studies to be retrieved in each of the above categories by either keyword or textword searches.
-
Two reviewers will independently undertake preliminary review to identify any potentially relevant article based on titles, abstracts and subject indexing. All studies identified for inclusion, together with those where a decision on inclusion is not possible from these brief details, will be obtained for more detailed appraisal.
-
Two reviewers will make decisions on the final composition of included studies, assessed from a hard copy of the item. The decisions will be coded and recorded on the Reference Manager database by the Project Manager.
-
Authors will be contacted, if appropriate, to clarify details and obtain missing data.
-
The quality of each study will be assessed against recognised criteria [23–28].
-
Data extraction will be undertaken independently with discrepancies being discussed by the data extractors. Those that cannot be resolved at this stage will be referred to the rest of the project team.
Data extraction
The following data will be extracted from each study: population characteristics (age, gender, CAD risk factors, prevalence of known CAD), setting (emergency department, general ward, cardiology ward), characteristics of the index investigation (biomarker, exercise ECG or CT coronary angiography), characteristics of the reference standard and/or outcome measure, methods used to measure outcomes, duration of follow-up, study quality criteria (independence of the reference standard, blinding of the intervention and reference standard), prevalence of MI, CAD and adverse events, true positives, false positives, false negatives and true positives for each outcome. If raw data are not reported we will attempt to calculate these from the reported diagnostic parameters or, in the case of important recent studies, we will contact the authors for clarification.
Data synthesis
Where appropriate, we will combine data to provide pooled estimates of the accuracy of investigations for MI, CAD and adverse events. Where appropriate data exist we will use Bayesian evidence synthesis to characterise the uncertainty associated with the parameters of interest. Where possible, we will examine the use of baseline characteristics (i.e. covariates) to explain any heterogeneity between studies. We will then attempt to identify the study, or homogeneous studies, that most closely reflects the current typical NHS population and practice.
The model used to analyse the data will depend on characteristics of the data obtained. For example, if diagnostic thresholds can be assumed constant across studies then simple methods of pooling sensitivity and specificity will be conducted [29]. If there is implicit or explicit evidence that diagnostic thresholds differ between primary studies, then sensitivity and specificity cannot be considered independent and simultaneous modelling will be required [30]. A detailed assessment of heterogeneity will be conducted in all instances. If possible, meta-regression will be used to explore whether heterogeneity can be explained by study population characteristics, the characteristics of the intervention, the definition of the outcome or the study quality, although the feasibility of this will depend on the number of individual studies identified and the quality of reporting. Where exploration of covariates is not possible, or (unexplained) heterogeneity remains after the incorporation of covariates into the model(s), random effects will be incorporated to allow for such variability in results between studies.
Covariate effects, unexplainable variability and uncertainty in parameter estimates will all be reflected in the results using cutting-edge meta-analysis approaches. Since the outputs from these analyses will be used in the decision modelling all such sources of variation and uncertainty will be accurately reflected in the decision modelling [31].
Decision analysis modelling
We will develop our existing decision analysis models [11,32] to evaluate two specific decisions in the
investigation of suspected ACS:
-
Which biomarkers should be measured (and when) in patients presenting with suspected ACS?
-
Should exercise ECG or CT coronary angiography be used to identify CAD in patients with troponin negative suspected ACS?
MI diagnosis: biomarkers
We will test up to ten different biomarker strategies selected on the basis of the quality of supporting data from the literature review, the accuracy of the strategy for early MI diagnosis and/or the prognostic value of the strategy. We will also include a ‘zero option’ of discharging all patients without testing and the current recommended strategy of a 10–12 h troponin for all patients.
Each strategy will be applied in the model to a theoretical cohort of patients attending hospital with suspected ACS with a defined prevalence of MI and a defined prevalence of previously diagnosed and undiagnosed CAD. Estimates of diagnostic and prognostic accuracy from the systematic reviews will determine how many cases of MI are correctly identified, how many cases without MI require further testing and how many expected adverse events would be accurately predicted. We will assume that patients with positive biomarkers will receive hospital treatment while those with negative biomarkers will be discharged home. Adverse outcomes up to six months in patients with MI at presentation will be determined by whether the patient receives appropriate treatment. Adverse outcomes up to six months in patients without MI will be determined by whether the patient has CAD and whether a positive biomarker test predicts their risk of adverse outcome.
Initially we will assume that patients with negative biomarkers receive exercise ECG testing and subsequent coronary angiography if positive, according to current guidelines. We will then explore interactions between MI and CAD diagnosis.
CAD diagnosis: exercise ECG or CT coronary angiography
Initially we will assume that all patients receive diagnostic testing for MI with a 10–12 h troponin, before exploring interactions between MI and CAD diagnosis.
We will test strategies of using exercise ECG, CT coronary angiography and no CAD testing for biomarker negative patients. We will also test strategies based on these approaches but using different decision thresholds for undertaking coronary angiography and instituting secondary prevention on the basis of first-line tests. For the baseline analysis the decision threshold will be ≥ 50% luminal diameter stenosis in a major epicardial vessel for CT coronary angiography and greater than 2-mm ST depression on exercise ECG. We will estimate long-term outcomes depending upon whether each patient has CAD or not and whether they receive secondary prevention and/or percutaneous coronary intervention consequent upon positive findings at coronary angiography.
Long-term outcomes will be modelled as QALYs, determined by whether patients suffer death or adverse outcome up to six months, and whether they suffer subsequent CAD-related mortality or morbidity. Cohort study and registry data identified by the literature review or used in previous models [32,33] will be used to estimate QALYs after adverse events.
A societal costing perspective will be used and the following costs estimated from literature review and expert panel assessment: clinical assessment, tests, hospital admission, outpatient review, general practitioner review, treatments for MI or CAD, treatments for adverse outcomes, long-term costs of care and productivity losses. Where possible the modelling will adhere to the NICE reference case [34] with sensitivity analyses conducted on including further aspects such as productivity losses.
These costs, and the results of evidence synthesis, will be applied to the model and probabilistic modelling used to estimate the net benefit [35] of each strategy at varying thresholds of willingness to pay for health gain. The optimum strategy will be the one with the maximum expected net benefit at the NICE threshold of £20,000 per QALY gained. This will be the most appropriate strategy for the NHS. Modelling will be an iterative process with estimates of net benefit from the model being used to inform the development of new strategies until all potentially feasible alternatives have been explored.
The methodology used in the decision analytic model will be dependent on the data that are available and the number of health states following ACS that are necessary to incorporate, with the most appropriate technique selected. Probabilistic sensitivity analyses [36] in conjunction with jackknife techniques [37] will be conducted to formulate the mean cost-effectiveness and net benefit of each strategy, together with the probabilities of positive net benefit, cost-effectiveness acceptability curves and the cost-effectiveness frontier [38]. These analyses will facilitate the calculation of both full and partial expected value of perfect information [39], and if it is deemed appropriate an evaluation of the expected value of sample information will also be conducted [40].
Project timetable and milestones:
The project will commence on 1 April 2010 and complete by 30 June 2011. There will be three phases, although development of the model will begin during phase 1:
-
April to September 2010 systematic reviews and meta-analysis
-
October 2010 to March 2011 decision analysis modelling
-
April to June 2011 writing up and dissemination
We will provide one progress report by 30 September 2010 that will report progress with the systematic reviews and meta-analysis.
Expertise:
The core research team for this project previously worked together on a very successful HTA funded secondary research project evaluating diagnostic tests for deep vein thrombosis (DVT) [41]. This project was completed within the planned budget and has so far resulted in eight peer-reviewed publications, in addition to the HTA report. Methodological work arising from this project, undertaken by Alex Sutton and colleagues at the University of Leicester, has led to developments in the synthesis of data for decision-analysis modelling and acceptance of an article for publication in Medical Decision Making [31]. We anticipate that data from our current proposal will be suitable for use in further methodological work.
Steve Goodacre is a leading expert in emergency care research and is Principal Investigator for several major national evaluations. One of his main research interests is using decision analysis modelling and cost-effectiveness analysis to guide policy and practice in emergency care.
Matt Stevenson has a wide experience of different mathematical modelling techniques as has worked extensively for NICE and the NCCHTA. He is technical director of ScHARR-TAG (one of seven academic units contracted to work for NICE and the HTA) and a member of NICE appraisal committee C. In 2007 he was an invited expert to a NICE workshop to help formulate further the NICE reference case for evaluating the cost-effectiveness of diagnostic techniques.
Simon Dixon is a senior health economist who undertook economic analysis for the 3CPO and ESCAPE trials.
Emma Simpson is an expert in systematic reviewing who has extensive experience of reviewing for NICE and Health Technology Assessment. The Department of Information Resources has extensive experience of supporting evidence synthesis for NCCHTA and NICE.
John Stevens is Deputy Director of the Centre for Bayesian Statistics in Health Economics (CHEBS) and an expert in the application of Bayesian statistics to economic analysis.
Francis Morris and Jason Kendall are leading experts in the emergency management of chest pain and ACS. They are respectively members of the NICE Guideline Development Groups for acute chest pain and ACS.
David Newby is a leading academic cardiologist with research interest in the management of suspected ACS. He was vice chair for the Scottish Intercollegiate Guidelines Network (SIGN) guideline on the management of acute coronary syndromes.
Paul Collinson is a leading international expert in cardiac biomarkers. He acted as expert advisor on cardiac biomarkers to the NICE Guideline Development Groups on acute chest pain and on heart failure.
Steven Thomas is a clinical senior lecturer in Cardiovascular Radiology. As a Cardiovascular Radiologist he has clinical expertise in using CT coronary angiography in a range of clinical settings. He has previously collaborated with the Health Economics and Decision Science unit at ScHARR on a number of projects, including a HTA funded assessment of carotid stenosis, with collaborators in Edinburgh, and a HTA funded project evaluating diagnostic tests in DVT. He has also been involved in assessment of the cost-effectiveness of treatment in abdominal aortic aneurysm for a recent NICE appraisal, with the Centre for Health Economics at York.
Service users:
Enid Hirst is a member of the public who has previously provided and facilitated patient representation for evaluations led by SG. She established a Cardiac User Group for our recent evaluation of the National Infarct Angioplasty Project (NIAP). This group helped to develop the research plans, guided the development of patient and carer interview schedules, and reviewed the outputs of the project.
The opportunities for user involvement in this project are inevitably limited by the reliance upon secondary data sources. However, we plan to ask Enid and membersi of the NIAP Cardiac User Group to review the outputs from the project. We will present our findings to members of the User Group in order to identify ways of communicating our findings to the public and explore the potential acceptability of different strategies to patients.
Justification of support required:
The Project Manager (grade 7, 100% for 15 months) will undertake the survey, manage the literature searches, supervise quality assessment of selected papers, assist with meta-analysis and cost-effectiveness analysis, write reports and disseminate findings. An experienced full-time Project Manager for the duration of the project is crucial to success.
The Clerical Assistant (grade 4, 50% for 15 months) will assist with the survey, literature searches, photocopying, preparing papers and data management.
MS (Operational Researcher, 40% for 9 months) will undertake the decision analysis modelling and cost-effectiveness analysis.
SD (Health Economist, 20% for 6 months) will provide health economic expertise and assistance with QALY estimation and obtaining unit costs.
SG (Principal Investigator, 10% for 15 months) will supervise the Project Manager, co-ordinate the project and oversee all project planning, analysis and report writing.
JS (Statistician, 20% for 6 months) will undertake data synthesis.
ES (Information Resources, 5% for 6 months) will supervise systematic reviewing.
DN (University of Edinburgh, Cardiology, 2% for 15 months) and ST (University of Sheffield, Vascular Radiology, 2% for 15 months) will provide cardiology and vascular radiology expert input.
FM, JK and PC will provide emergency medicine and chemical pathology expertise, but will be funded through NIHR NHS support.
Other expenses will include:
-
Computing equipment, including licences for systematic review and decision analysis software = £1250.
-
Information resources support: literature searches, document retrieval, photocopying = £3000
-
Office expenses for the research team @ £1,500 per wte per year (total 2.5 wte years) = £4687
-
Travel for the expert panel and project management group, £2000, and for conference attendance, £1000.
The University of Sheffield has joined phase 3 of the Carbon Trust's Higher Education Carbon Management Programme. This programme is designed to deliver improved energy management of academic, accommodation and leisure buildings and vehicle feets. It also provides practical support to organisations by helping them identify carbon saving opportunities, providing software to analyse energy consumption and delivering workshop support for staff and senior managers to improve their awareness of energy efficiency.
Our proposal is a secondary research project that will be largely undertaken in a single centre, so greenhouse gas emissions directly related to the project will be relatively small. Indeed, this is another advantage of using modelling techniques. We will further minimise emissions by:
-
conducting project management and expert panel meetings by teleconference where possible
-
conducting meetings in a central location that is accessible by public transport
-
disseminating findings using electronic media where possible
-
using public transport to travel to conferences
References
- Chest pain of recent onset: assessment and investigation of recent onset chest pain or discomfort of suspected cardiac origin. n.d.
- London: Department of Health; 2000.
- Goldberg RJ, Currie K, White K, Brieger D, Steg PG, Goodman SG, et al. Six-month outcomes in a multinational registry of patients hospitalized with an acute coronary syndrome (the Global Registry of Acute Coronary Events [GRACE]). Am J Cardiol 2004;93:288-93.
- Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart 2005;91:229-30.
- Thygesen K, Alpert JS, White HD. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction . Universal definition of myocardial infarction. J Am Coll Cardiol 2007;50:2173-95.
- Ebell MH, Flewelling D, Flynn CA. A systematic review of troponin T and I for diagnosing acute myocardial infarction. J Fam Pract 2000;49:550-56.
- Ebell MH, White LL, Weismantel D. A systematic review of troponin T and I values as a prognostic tool for patients with chest pain. J Fam Pract 2000;49:746-53.
- Hamm CW, Heeschen C, Goldmann B, . Benefit of abciximab in patients with refractory unstable angina in relation to serum troponin T levels. N Engl J Med 1999;340:1623-9.
- Balk EM, Ioannidis JPA, Salem D, Chew PW, Lau J. Accuracy of cardiac biomarkers to diagnose acute cardiac ischaemia in the emergency department: a meta-analysis. Ann Emerg Med 2001;37:478-94.
- Mitchell AM, Brown MD, Menown IBA, Kline JA. Novel protein markers of acute coronary syndrome complications in low-risk outpatients: a systematic review of potential use in the emergency department. Clinical Chemistry 2005;51:2005-11.
- Goodacre S, Calvert N. Cost effectiveness of diagnostic strategies for patients with acute, undifferentiated chest pain. Emerg Med J 2003;20:429-33.
- Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al. Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care. Health Technol Assess 2004;8.
- Choi YF, Wong TW, Lau CC. The diagnostic value and cost effectiveness of creatine kinase-MB, myoglobin and cardiac troponin-T for patients with chest pain in emergency department observation ward. Hong Kong Journal of Emergency Medicine 2004;11:85-90.
- Zarich S, Bradley K, Seymour J, Ghali W, . Impact of troponin T determinations on hospital resource utilization and costs in the evaluation of patients with suspected myocardial ischemia. Am J Cardiol 2001;88:732-6.
- Task Force on the Management of Acute Coronary Syndromes of the European Society for Cardiology . Management of acute coronary syndromes in patients presenting without ST elevation. Eur Heart J 2002;23:1809-40.
- Mowatt G, Cummins E, Waugh N, . Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease. Health Technol Assess 2008;12.
- Hoffmann U, Nagurney JT, Moselewski F, Pena A, . Coronary multidetector computed tomography in the assessment of patients with acute chest pain. Circulation 2006;114:2251-60.
- Coles DR, Wilde P, Oberhoff M, Rogers CA, . Multislice computed tomography coronary angiography in patients admitted with a suspected acute coronary syndrome. Int J Cardiovasc Imaging 2007;23:603-14.
- Johnson TR, Nikolaou K, Wintersperger BJ, Knez A, . ECG-gated 64-MDCT angiography in the differential diagnosis of acute chest pain. AJR Am J Roentgenol 2007;188:76-82.
- Rubinshtein R, Halon DA, Gaspar T, Jaffe R, . Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation 2007;115:1762-8.
- Ladapo JA, Hoffmann U, Bamberg F, Nagurney JT, . Cost effectiveness of coronary MDCT in the triage of patients with acute chest pain. AJR Am J Roentgenol 2009;191:455-63.
- Khare RK, Courtney DM, Powell ES, Venkatesh AK, . Sixty-four slice computed tomography of the coronary arteries: cost effectiveness analysis of patients presenting to the emergency department with low-risk chest pain. Acad Emerg Med 2008;15:623-32.
- Westwood ME, Whiting PF, Kleijen J. How does study quality affect the results of a diagnostic meta-analysis?. BMC Med Res Methodol 2005;5.
- Whiting P, Harbord R, Kleijnen J. No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Med Res Methodol 2005;5.
- Whiting P, Rutjes AW, Dinnes J, Reitsma JB, Bossuyt PM, Kleijnen J. A systematic review finds that diagnostic reviews fail to incorporate quality despite available tools. J Clin Epidemiol 2005;58:1-12.
- Whiting P, Rutjes AW, Dinnes J, Reitsma J, Bossuyt PM, Kleijnen J. Development and validation of methods for assessing the quality of diagnostic accuracy studies. Health Technol Assess 2004;8.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD initiative, 2003. The Netherlands: Department of Clinical Epidemiology and Biostatistics Academic Medical Centre, University of Amsterdam; n.d.
- Lijmer JG, Mol JJ, Heisterkamp S, Bonsel GJ, van der Meulen JHP, Bossuyt PMM. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 1999;282:1061-6.
- Deeks JJ, Egger M, Davey Smith G, Altman DG. Systematic reviews in health care: meta-analysis in context. London: BMJ Publishing Group; 2001.
- Harbord RM, Deeks JJ, Egger M, . A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51.
- Sutton AJ, Cooper NJ, Goodacre S, Stevenson M. Integration of meta-analysis and economic decision modelling for evaluating diagnostic tests. Med Decis Making 2008;28:650-67.
- Oluboyede Y, Goodacre S, Wailoo AJ on behalf of the ESCAPE Research Team. Cost-effectiveness of chest pain unit care in the NHS. BMC Health Serv Res 2008;8.
- Wailoo AJ, Goodacre S, Sampson F, . Primary angioplasty versus thrombolysis for acute ST-elevation myocardial infarction: an economic analysis of the National Infarct Angioplasty Project. Heart 2009.
- Guide to the methods of technology appraisals. 2008.
- Stinnett A, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analyses. Med Dec Making 1998;18:S68-80.
- Claxton K, Sculpher M, McCabe C, Briggs AH, Akehurst R, Buxton M, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Economics 2005;14:339-47.
- Inglehart DL. Simulating stable stochastic systems, V: comparison of ratio estimators. Naval Res Logist Quart 1975;22:553-65.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ n.d.;10:779-87.
- Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ 1996;5:513-24.
- Ades AE, Lu G, Claxton K. Expected values of sample information calculation in medical decision making. Med Decis Making 2004;24:207-27.
- Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton AJ, Thomas S, et al. Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis. Health Technol Assess 2006;15.
Glossary
Technical terms and abbreviations are used throughout this report. The meaning is usually clear from the context, but a glossary is provided for the non-specialist reader. In some cases, usage differs in the literature, but the term has a constant meaning throughout this review.
- Cost-effectiveness acceptability curve
- A way of illustrating cost-effectiveness results by plotting the probability that the intervention is cost-effective (y-axis) against the maximum that society is willing to pay for an improvement in health (x-axis).
- Diagnostic cohort study
- Diagnostic accuracy study in which a group of individuals with a suspected disease undergo both the index test and the reference standard, and the results of the two tests are compared.
- False-negative
- A test result erroneously indicating that a patient with a condition does not have that condition.
- False-positive
- A test result erroneously indicating that a patient without a condition does have that condition.
- Incremental cost-effectiveness ratio
- The difference in costs between one intervention and an alternative, divided by the difference in outcomes.
- Quality-adjusted life-year
- A measure of benefit of health care combining the impact of both expected length of life and quality of life.
- Receiver operating characteristic
- A receiver operating characteristic curve represents the relationship between the ‘true-positive fraction’ (sensitivity) and the ‘false-positive fraction’ (1 – specificity). It displays the trade-offs between sensitivity and specificity as a result of varying the cut-off value for positivity in case of a continuous test result.
- Reference standard
- Established test(s) against which the accuracy of a new test for detecting a particular condition can be evaluated.
- Sensitivity (true-positive rate)
- The proportion of individuals with the target condition in a population who are correctly identified by a diagnostic test.
- Specificity (true-negative rate)
- The proportion of individuals free of the target condition in a population who are correctly identified by a diagnostic test.
- Test accuracy
- The proportion of test results that are correctly identified by the test.
- True-negative
- A test result correctly identifying that a patient without a condition does not not have that condition.
- True-positive
- A test result correctly identifying that a patient with a condition has that condition.
List of abbreviations
- ACS
- acute coronary syndrome
- AUROC
- area under receiver operating characteristic
- BNP
- B-type natriuretic peptide
- CABG
- coronary artery bypass graft
- CAC
- coronary artery calcium
- CAD
- coronary artery disease
- CI
- confidence interval
- CINAHL
- Cumulative Index of Nursing and Allied Health Literature
- CK
- creatine kinase
- CK-MB
- creatine kinase MB isoenzyme
- CrI
- credible interval
- CRP
- C-reactive protein
- CT
- computerised tomography
- CTCA
- computed tomographic coronary angiography
- CV
- coefficient of variation
- ECG
- electrocardiography
- ED
- emergency department
- ETT
- exercise tolerance test
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- FN
- false-negative
- FP
- false-positive
- H-FABP
- heart-type fatty acid-binding protein
- HF
- heart failure
- HR
- hazard ratio
- HsTnT
- high-sensitivity troponin T
- ICA
- invasive coronary angiography
- ICER
- incremental cost-effectiveness ratio
- IMA
- ischaemia-modified albumin
- LoD
- limit of detection
- LoS
- length of stay
- MACE
- major adverse cardiac event
- MI
- myocardial infarction
- MMP9
- matrix metalloproteinase 9
- MPO
- myeloperoxidase
- N/A
- not applicable
- NICE
- National Institute for Health and Clinical Excellence
- NR
- not reported
- NSTEMI
- non-ST elevation myocardial infarction
- NT-pro-BNP
- N-terminal pro-B-type natriuretic peptide
- OR
- odds ratio
- PAPP
- pregnancy-associated plasma protein
- PAPP-A
- pregnancy-associated plasma protein A
- PCI
- percutaneous coronary intervention
- PLR
- positive likelihood ratio
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- QUADAS
- Quality Assessment of Diagnostic Accuracy Studies
- RR
- relative risk
- SD
- standard deviation
- SE
- standard error
- STEMI
- ST elevation myocardial infarction
- TN
- true-negative
- TnC
- troponin C
- TnI
- troponin I
- TnT
- troponin T
- TP
- true-positive
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices in which case the abbreviation is defined in the figure legend or at the end of the table.