Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/29/01. The contractual start date was in May 2008. The draft report began editorial review in November 2011 and was accepted for publication in May 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
JE has received payment for lectures from MSD and for travel expenses from Novartis; his institution has received grant funding from Pfizer. CK has received payment for consultancy and lectures from Astellas, Gilead, MSD and Pfizer. RB has been a paid advisory board member or received payment for lectures and/or travel expenses from Astellas, Gilead, MSD, and Pfizer; her institution has received grant funding from Gilead and Pfizer.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Harrison et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
In the past, invasive fungal disease (IFD) was more commonly found in patients who were neutropenic, had received a solid organ transplant or had been treated with corticosteroids or cytotoxic agents. Increasingly, IFD is now more likely to occur in non-neutropenic patients in critical care units. 1 The majority of IFDs in the critical care setting are due to Candida species. 2,3 In 2006, the Health Protection Agency (HPA) estimated that over 5000 cases of invasive Candida species infections occur in the UK each year and around 40% of these occur in critical care units. 4 An epidemiological survey in six UK sentinel hospitals reported that 45% of Candida bloodstream infections occurred in the critically ill. 5 IFD in critically ill patients is associated with increased morbidity and mortality at a cost to both the individual and the NHS. 6,7
A number of randomised controlled trials (RCTs) have evaluated antifungal prophylaxis in non-neutropenic, critically ill patients, predominantly evaluating either fluconazole8–12 or ketoconazole. 13–16 Several systematic reviews and meta-analyses of these studies have been performed,17–22 including a Cochrane systematic review. 19 These reviews reveal that patient groups selected for the individual RCTs were very heterogeneous, ranging from high-risk, surgical patients8,11,15 to those with septic shock10 or with acute respiratory distress syndrome. 13,14,16 All seemed to represent groups that were at high risk of IFD, with rates of IFD in the control arms of included studies typically over 10%. Despite this heterogeneity in patient groups, the RCTs demonstrated a remarkably homogeneous effect of antifungal prophylaxis on the risk of proven IFD [relative risk (RR) 0.46; 95% confidence interval (CI) 0.31 to 0.68] and suggested a reduction in mortality assessed at varying time points (RR 0.76; 95% CI 0.59 to 0.97). 19 The question, therefore, is not whether or not antifungal prophylaxis is effective but rather how to select an appropriate group of patients at high risk of IFD in which to use it, given that indiscriminate use of antifungal drugs is likely to promote increased resistance and drive up costs.
In 2007, a systematic review of the risk of resistance associated with fluconazole prophylaxis concluded that the evidence from RCTs indicated an increased risk of colonisation with both fluconazole-susceptible, dose-dependent and fluconazole-resistant fungi. 23 There was also some suggestion of increased breakthrough infections with non-albicans Candida including Candida krusei, which has innate resistance to fluconazole, and strains of Candida glabrata with acquired resistance to fluconazole.
Given that the effectiveness of antifungal prophylaxis has been demonstrated only in groups at high risk of IFD and that more widespread use of antifungal drugs may promote resistance, it is necessary to establish a method to identify those patients who are at highest risk of IFD and at whom to target antifungal prophylaxis, therefore targeting use to those who stand to benefit most from any antifungal prophylaxis strategy. 24
Several models for identifying patients at high risk of IFD have been proposed. 25–28 These models, however, are limited. The populations included have typically been selected based on the length of stay (LOS) in the critical care unit, for example to those staying 2,25 427,28 or 726 days in the unit, and are therefore not appropriate for making treatment decisions earlier in the stay. The populations have been restricted in other ways, for example to only post-surgical patients25,28 or to only those with Candida colonisation. 26 These again limit the generalisability of the resultant model to a mixed UK critical care population. Furthermore, no models have been developed or validated in UK NHS adult critical care patients.
A clinical decision rule is a tool that quantifies the contributions that medical history, physical examination and laboratory results make towards the diagnosis, prognosis or likely response to treatment for a patient. McGinn et al. 29 define four levels of evidence for clinical decision rules:
-
Level 1 Rules that can be used in a wide variety of settings with confidence that they can change clinical behaviour and improve patient outcomes – this requires at least one prospective validation in a different population and one impact analysis demonstrating change in clinical behaviour with beneficial consequences.
-
Level 2 Rules that can be used in various settings with confidence in their accuracy – this requires demonstrated accuracy in either one large prospective study including a broad range of patients and clinicians or validation in several smaller and varied settings.
-
Level 3 Rules that clinicians may consider using with caution and only if patients in the study are similar to the clinician's setting – this requires validation on only one narrow prospective sample.
-
Level 4 Rules that need further evaluation before they can be applied clinically – these are rules that have been derived but either not validated or validated only in split samples, large retrospective databases or by statistical techniques.
Currently, no existing clinical decision rule for antifungal prophylaxis in non-neutropenic, critically ill adult patients could be considered to achieve higher than level 3.
In 2007, the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme put out a call for primary research to identify risk factors and develop algorithms for the prospective identification of critically ill patients at increased risk of IFD who would most benefit from antifungal prophylaxis (see Appendix 1). The Intensive Care National Audit & Research Centre (ICNARC) responded to this call with a proposal for a study – the Fungal Infection Risk Evaluation (FIRE) Study – with the overall aim to develop and validate risk models to identify non-neutropenic, critically ill adult patients at high risk of invasive Candida infection who would benefit from antifungal prophylaxis. The study was designed with six objectives:
-
to undertake a systematic literature review to identify risk factors for IFD (see Chapter 2)
-
to undertake data collection on risk factors and IFD in patients admitted to UK NHS adult general critical care units (see Chapters 3 and 4)
-
to develop, and internally validate, risk models for invasive Candida infection using both classical statistical methods and machine learning techniques (see Chapters 5 and 6)
-
to externally validate the risk models for invasive Candida infection (see Chapters 5 and 6)
-
to assess the cost-effectiveness of targeting antifungal prophylaxis to admissions identified as high risk based on the risk models for invasive Candida infection (see Chapter 7)
-
to make recommendations for future research, based on value of information analysis (see Chapter 8).
Chapter 2 Systematic review of the literature to identify risk factors for invasive fungal disease in critically ill adult patients
Introduction
A systematic review of the literature was performed to identify and summarise the important risk factors from published multivariable analyses, risk models and clinical decision rules for IFD in critically ill adult patients to inform the data set for primary data collection in the FIRE Study.
Methods
An electronic search was performed using MEDLINE (SilverPlatter WebSPIRS; 1950–2008); EMBASE (SilverPlatter WebSPIRS; 1947–2008); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost; 1960–2008) in order to identify published English-language articles that (1) investigated the predictive value of risk factors for IFD in critically ill adult patients; (2) developed or evaluated a risk score or risk model for IFD in critically ill adult patients; or (3) developed or evaluated a clinical decision rule or patient algorithm for use of antifungal prophylaxis in critically ill adult patients. Three search themes were combined: ‘fungal disease and treatment’; ‘patient population’; and ‘risk factors/risk models/clinical rules’ (see Appendix 2 for search strategy).
Articles were identified in a staged process whereby titles were initially screened for potential eligibility by a single reviewer (GE). Abstracts and full texts of those determined to be potentially eligible were then assessed by two reviewers (HM, JS), independently, and included if the following criteria were all met: (1) evaluation of multiple risk factors, a scoring system or a clinical decision rule for IFD in critically ill patients; (2) control group consisting of patients without IFD or any other systemic infection; and (3) study in adult (> 18 years) humans. Any disagreements between the reviewers were resolved by a third (DH). Full texts were obtained for all eligible articles. Finally, members of the FIRE Study Steering Group, as clinical experts in the field, were contacted to determine if any relevant articles were missed.
Data were extracted onto standard data extraction sheets, independently, by two reviewers (HM, JS). The following data were abstracted for each article: study design, method of data collection, setting, population characteristics, method of analysis, risk factors reported, outcome (types/definitions of IFD), and strength of association reported. For the last of these, data were abstracted for any adjusted odds ratios, 95% CIs and p-values reported for each of the studied risk factors.
The methods of development and validation in each of the articles reporting the development of a risk model or clinical decision rule were described in more detail. Performance measures of the risk models and clinical decision rules were extracted, when reported. For risk models, the c-index,30 or equivalently the area under the receiver operating characteristic (ROC) curve,31 was extracted. The ideal value of the c-index is 1, representing perfect discrimination where every patient with IFD has a higher predicted risk than every patient without IFD. A c-index of 0.5 represents discrimination that is no better than chance. For clinical decision rules, the sensitivity, specificity, positive predictive value (PPV) and negative predicted value (NPV) were extracted. Sensitivity represents the proportion of patients with IFD who were identified as high risk by the clinical decision rule. Specificity represents the proportion of patients without IFD who were identified as low risk by the clinical decision rule. PPV represents the proportion of patients identified as high risk by the clinical decision rule who went on to develop IFD. NPV represents the proportion of patients identified as low risk by the clinical decision rule who did not go on to develop IFD. The ideal value for all of these measures is 100%.
The methodological quality of reporting for the eligible articles was assessed, independently, by two reviewers (HM, JS) using a set of questions addressing both general and statistical methodology. Given that no gold standard method exists for the methodological assessment of risk factor studies, questions were drawn from research from a published quality assessment method for randomised and non-randomised studies,32 and from research on reporting of prognostic models in the oncology field. 33,34
Eight questions assessed the general methodology: study objectives, number of centres, patient characteristics, definition of risk factors, outcome description, existence of an a priori analysis plan, rationale behind risk factor inclusion, and adjustment for known risk factors. In assessing whether or not a study was adjusted for known risk factors, the factors considered were acute severity of illness, LOS, diabetes, renal dysfunction, major surgery, antibiotics use, receipt of total parenteral nutrition (TPN), immunosuppressant use, renal replacement therapy and central venous catheter (CVC) use. These known risk factors were selected based on expert clinical opinion. A study was recorded as adjusting for the majority of known risk factors if six or more of the nine risk factors were accounted for.
Three questions assessed the statistical methodology: adequacy of sample size, risk factor selection, and model strategy. Adequacy of sample size was established using the generally held rule of 10 events per variable (EPV). 34 All risk factors included in the statistical modelling, including those excluded from multivariable modelling following univariable analysis, were included in the calculation of EPV. Risk factor selection referred to how risk factors were entered into the multivariable model. The selection process was based on either univariable analysis, previous literature/investigator choice or no selection strategy whereby all risk factors were entered into the model. Model strategy consisted of either forward selection, backwards elimination or no stepwise process whereby all risk factors were kept in the model. If detail on the risk factor selection and model strategy was absent, then it was labelled as unclear.
Results
The electronic search identified a total of 1864 citations (Figure 1). After screening of titles, 165 articles were selected for abstract and full-text review and 152 of these potentially eligible articles were excluded because they failed to meet the inclusion criteria: 109 did not assess multiple risk factors, a scoring system and/or a clinical decision rule, 122 articles had a control group with a systemic infection, and five were not studies in adult humans. Some articles were excluded for multiple reasons. No additional articles were identified by the clinical experts consulted.
FIGURE 1.
Article flow through different stages of review. a, Articles may have more than one reason for exclusion.
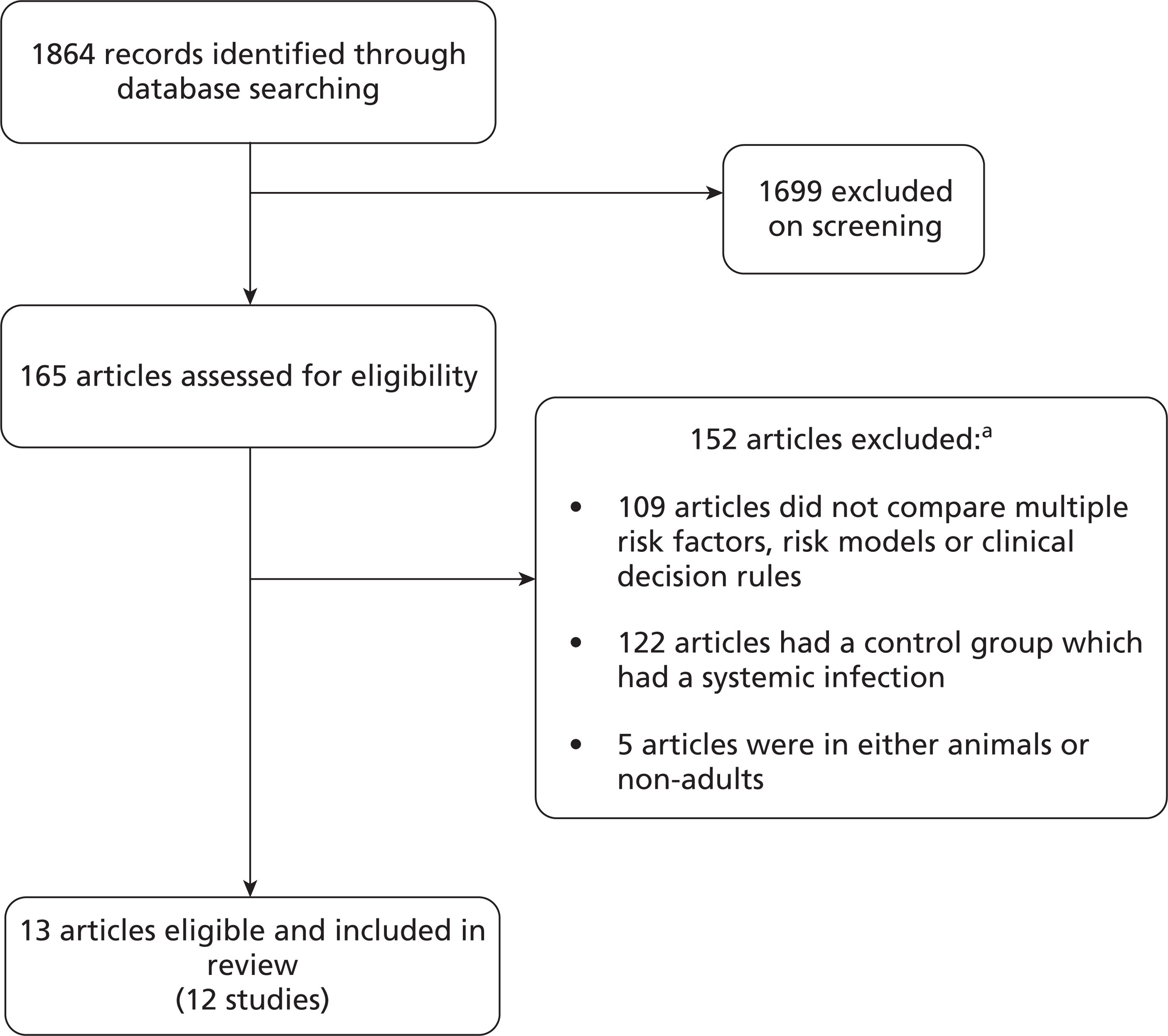
The 13 articles that met the inclusion criteria fell into three groups: eight articles examined risk factors, four developed a risk model or clinical decision rule, and one was an evaluation of a clinical decision rule. Two of the articles utilised data from the same study: the EPCAN Study. 26,35 There were three case–control and nine cohort studies, with varying inclusion criteria, including age and LOS in the critical care unit. The studies were conducted in various countries: Brazil, France, Greece, Sweden, Spain, Switzerland and the USA. Six were based on general critical care patients, whereas the rest were based on selected patients in specialised units, including surgical, cardiac and trauma units. Studies varied greatly in terms of defining outcome(s). Four studies reported solely on Candida infections in blood, four used European Organisation for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) criteria or modifications of this, and the rest used other definitions. Given the heterogeneity of the studies, no meta-analysis was performed. The general characteristics of the selected studies are shown in Table 1.
| Article | Study type | Selection criteria | Study design | No. of centres (n) | Total patients | Patients with outcomea/casesb (n) | Outcome/case definition |
|---|---|---|---|---|---|---|---|
| Agvald-Ohman et al. 200836 | Risk factor analysis | Any multidisciplinary ICU patients LOS ≥ 7 days | Prospective cohort | 1 | 59 | 10 | Blood and/or sterile body site culture-positive for Candida species |
| Blumberg et al. 200125 | Risk factor analysis | SICU patients LOS > 48 hours | Prospective cohort | 6 | 4276 | 42 | Blood cultures collected > 48 hours after admission to the SICU positive for Candida species |
| Borzotta and Beardsley 199937 | Risk factor analysis | Trauma ICU patients | Case–control | 1 | 656 | 20 | Blood culture positive for yeast |
| Cases selected if LOS > 4 days and age ≥ 16 years and any evidence of fungal infection or treatment for fungal infection | Yeast from any sterile area | ||||||
| Control subjects (2 : 1): matched on sex, mechanism of injury, age, Injury Scale Score | Funguria with signs of sepsis and no bacterial pathogen source if > 105 colonies per ml of yeast | ||||||
| Candida growth at two sites with fever and white blood cell count > 12 and no bacterial isolates within 48 hours | |||||||
| Chow et al. 200838 | Risk factor analysis | Medical or SICU patients | Case–control | 2 | 926 | 146 | One or more blood cultures positive for Candida species |
| Cases selected if blood culture positive for Candida species after first 48 hours following admission | |||||||
| Control subjects (5 : 1): matched on hospital, ICU type and admission date | |||||||
| Control subjects selected in 5 : 1 ratio | |||||||
| Ibanez-Nolla et al. 200439 | Risk factor analysis | Any multidisciplinary ICU patients | Prospective cohort | 1 | 145 | 120 | Multifocal candidiasis – simultaneous isolation of Candida species in two or more of the following locations: respiratory, digestive, urinary or other locations; or |
| Candida species in culture or on histology during ICU stay or post mortem | Disseminated candidiasis – yeast in fuids from sterile sites or histologic samples from deep organs or diagnosis of endophthalmitis or candidaemia with negative catheter-tip cultures | ||||||
| Neutrophil count ≥ 500/mm3 | Also used EORTC/MSG guidelines | ||||||
| cJorda-Marcos et al. 200735 | Risk factor analysis | Any multidisciplinary ICU patients | Prospective cohort | 73 | 1765 | 63 | At least one blood culture-positive for Candida species |
| Age > 18 years | |||||||
| LOS ≥ 7 days | |||||||
| cLeon et al. 200626 | Development of risk model | Multidisciplinary ICU patients | Prospective cohort | 73 | 1699 | 97 | Candidaemia |
| Age > 18 years | Candidal endophthalmitis in a patient with clinical sepsis | ||||||
| LOS ≥ 7 days | Candida species from sterile sites | ||||||
| Only patients with fungal colonisation included in risk factor analysis/model development | Histologically documented candidiasis | ||||||
| McKinnon et al. 200140 | Risk factor analysis | SICU patients | Prospective cohort | 3 | 301 | 27 | Colonisation of two or more sites or candidaemia |
| LOS ≥ 5 days | |||||||
| Age > 18 years | |||||||
| Michalopoulos et al. 200341 | Risk factor analysis | CICU patients | Case–control | 1 | 150 | 30 | At least one blood culture-positive for Candida species |
| Cases selected if at least one blood culture-positive for Candida species detected | |||||||
| Control subjects (4 : 1): matched on admission date, sex, BMI, sedatives, CPB technique, cardioplegia type | |||||||
| Ostrosky-Zeichner et al. 200727 | Development of clinical decision rule | Multidisciplinary ICU patients | Retrospective cohort | 12 | 2890 | 88 | EORTC/MSG criteria |
| Age ≥ 19 years | |||||||
| LOS ≥ 4 days | |||||||
| No evidence of invasive candidiasis or systemic antifungal use in week prior to ICU admission through to first 3 days of admission | |||||||
| Paphitou et al. 200528 | Development of clinical decision rule | SICU patients LOS ≥ 4 days | Retrospective cohort | 1 | 327 | 36 | Based on proven, probable or possible cases |
| Criteria modelled on EORTC/MSG criteria | |||||||
| Piarroux et al. 200442 | Evaluation of clinical decision rule | SICU patients | Prospective and retrospective cohorts | 1 | 933 | 50 | EORTC/MSG criteria |
| LOS ≥ 5 days | |||||||
| Excluded liver transplants | |||||||
| Pittet et al. 199443 | Risk factor analysis and development of clinical decision rule | Surgical/neonatal ICU patients | Prospective cohort | 2 | 29 | 11 | Candidaemia – one blood culture with one histologically documented invasive candidiasis or ophthalmic examination consistent with candidal endophthalmitis; or at least two blood cultures taken at different times; or one peripheral blood culture and one central line blood culture showing identical Candida species, or |
| Candida colonisation in three or more samples on two consecutive days | Severe non-bloodstream Candida species infection – Candida species in normally sterile site and at least one of fever or hypothermia, unexplained prolonged hypotension, or no response to adequate antibiotic treatment for a suspected bacterial infection |
Analysis of risk factors
Eight articles examined risk factors for IFD, each of which is described briefly below.
Agvald-Ohman et al.36
Invasive candidiasis in long-term patients at a multidisciplinary intensive care unit: Candida colonisation index, risk factors, treatment and outcome
A prospective cohort study to investigate Candida colonisation pattern and colonisation index, in combination with other risk factors, and in relation to invasive Candida infection. Patients on a multidisciplinary intensive care unit (ICU) with a LOS of ≥ 7 days were included in the study over a 17-month period. Samples for surveillance cultures were taken on day 7 and then weekly throughout the ICU stay. High colonisation index and recent extensive gastroabdominal surgery were shown to be significantly correlated with invasive Candida infection.
Blumberg et al.25
Risk factors for candidal bloodstream infections in surgical intensive care patients: the NEMIS prospective multicentre study
A prospective multicentre cohort study to assess risk factors for the development of Candida bloodstream infections. Patients on the surgical ICU (SICU) admitted for > 48 hours were included, over a 2-year period. Fungal surveillance cultures were taken on admission to SICU and then weekly throughout SICU stay. Prior surgery, acute renal failure, receipt of TPN and presence of a triple-lumen catheter (for patients who had undergone surgery) were all found to be independently associated with increased risk of Candida bloodstream infections. Receipt of an antifungal agent was found to be associated with a decreased risk.
Borzotta and Beardsley37
Candida infections in critically ill trauma patients
A case–control study to determine whether or not the classic risk factors for fungal infection were applicable to trauma patients. Patients aged ≥ 16 years, with a LOS in ICU of 4 days were considered for the study, over a 3-year period. Patients infected with Candida species were identified and two control subjects were selected from the remaining patients. Data on risk factors were abstracted from medical records. TPN was found to be significantly associated with Candida infection.
Chow et al.38
Risk factors for albicans and non-albicans candidaemia in the intensive care unit
A case–control study to determine risk factors for bloodstream infections with Candida albicans and non-albicans species, in critically ill patients. Medical or SICU patients were selected for the case group if they had a blood culture-positive for Candida species after the first 48 hours following admission to the unit. Control subjects (non-candidaemia) were matched at a ratio of 5 : 1, for data collected over a 10-year period. Demographic and clinical data for ICU stay were collected by chart review. Multiple common risk factors for both albicans and non-albicans species bloodstream infections were found, but no risk factors were found that could differentiate between the two species.
Ibanez-Nolla et al.39
Early diagnosis of candidiasis in non-neutropenic, critically ill patients
A prospective cohort study to determine a method for the early diagnosis of candidiasis in non-neutropenic critically ill patients, in a multidisciplinary ICU. Non-neutropenic patients with Candida species in any sample during ICU stay or on post-mortem were included in the study, over a 7-year period. Once enrolled in the study, a screening of standardised cultures was carried out for each patient, and a post-mortem study of microbiological and histological analyses performed when consent was given. Invasive candidiasis was found to be related to digestive and respiratory foci and the presence of non-Candida albicans species.
Jorda-Marcos et al.35
Risk factors for candidaemia in critically ill patients: a prospective surveillance study
A prospective cohort study to assess the risk factors for candidaemia in critically ill patients with prolonged stay in a multidisciplinary ICU (EPCAN Study). Patients from 70 tertiary care hospitals in Spain, aged ≥ 18 years, with an ICU LOS of ≥ 7 days were included, over a 9-month period. Cultures for Candida species were obtained 7 days after admission to ICU, and once a week thereafter. Candida colonisation, TPN, elective surgery and haemofiltration were found to be independently associated with candidaemia.
McKinnon et al.40
Temporal assessment of Candida risk factors in the surgical intensive care unit
A prospective cohort study to determine whether or not risk factors for Candida infection in patients in SICUs change over time and the degree to which this progression influences Candida colonisation and infection in patients aged > 18 years with an SICU LOS of 5 days, over a period of 7 months. Patients were assessed for risk factors, Candida colonisation and antifungal use on days 1, 3, 4, 6 and 8 in the SICU. Risk factors for Candida infection were shown to change over time. Mechanical ventilation after day 3, multiple surgical procedures, CVCs, diarrhoea and peripheral catheter use were all found to be associated with Candida infection.
Michalopoulos et al.41
Determinants of candidaemia and candidaemia-related death in cardiothoracic intensive care unit patients
A case–control study to develop and prospectively validate risk models of independent predictors of candidaemia and candidaemia-related death in patients in cardiothoracic ICUs (CICUs). Patients with at least one blood culture that was positive for Candida species were included in the model development study, and control subjects were matched in a 4 : 1 ratio, over a 2.5-year period. Model validation study was carried out prospectively over a subsequent 2.5-year period. Follow-up culture samples were taken from those patients with at least one positive initial culture, and cultures were repeated at least twice until CICU discharge. Invasive mechanical ventilation for ≥ 10 days, hospital-acquired bacterial infection and/or bacteraemia, cardiopulmonary bypass (CBP) duration of > 120 minutes and diabetes mellitus were all found to be independently predictive of candidaemia.
Risk factors explored between the studies varied. Table 2 reports all the risk factors that were identified as statistically significantly associated with IFD in one or more of the 10 studies (11 articles) that conducted a multivariable analysis. Table 3 reports all significant risk factors that were examined and the number of studies in which these were associated with IFD, on univariable and multivariable analysis. Candidate risk factors are described below. All results are presented in descending order of the number of studies for which the risk factor was significantly associated on multivariable analysis.
| Risk factor | Article | Odds ratio (95% CI) | p-value |
|---|---|---|---|
| Surgery | |||
| General abdominal surgery | Agvald-Ohman et al. 200836 | 60.7 (7.3 to infinity) | 0.0013 |
| Any surgery | Blumberg et al. 200125 | 7.3 (1 to 53.8) | 0.05 |
| Gastrointestinal procedure | Chow et al. 200838 | 2.24 (1.49 to 3.38)a | < 0.001a |
| Major operation during ICU stay | Chow et al. 200838 | 1.26 (1.01 to 1.58)b | 0.04b |
| Major pre-ICU operation | Chow et al. 200838 | 2.12 (1.14 to 3.97)a | 0.02a |
| Elective surgery | cJorda-Marcos et al. 200735 | 2.75 (1.17 to 6.45) | 0.02 |
| Surgery on ICU admission | cLeon et al. 200626 | 2.71 (1.45 to 5.06) | < 0.001 |
| Multiple surgical procedures | McKinnon et al. 200140 | Not recorded | ≤ 0.05 |
| TPN | |||
| TPN | Blumberg et al. 200125 | 3.6 (1.8 to 7.5) | < 0.001 |
| TPN | Borzotta and Beardsley 199937 | Not recorded | < 0.001 |
| TPN duration/days at risk | Chow et al. 200838 | 11.0 (5.52 to 21.7)b | < 0.01b |
| 2.87 (1.4 to 5.9)a | < 0.01a | ||
| TPN | cJorda-Marcos et al. 200735 | 3.89 (1.73 to 8.78) | 0.001 |
| TPN | cLeon et al. 200626 | 2.48 (1.16 to 5.31) | < 0.001 |
| Fungal colonisation | |||
| Colonisation index ≥ 0.5 | Agvald-Ohman et al. 200836 | 19.1 (2.38 to 435) | 0.017 |
| Digestive focus | banez-Nolla et al. 200439 | 20.24 (6.11 to 67.03) | < 0.001 |
| Non-Candida albicans at screening | banez-Nolla et al. 200439 | 11.68 (1.93 to 70.63) | 0.007 |
| Respiratory focus | banez-Nolla et al. 200439 | 6.55 (1.25 to 34.3) | 0.026 |
| Candida colonisation | cJorda-Marcos et al. 200735 | 4.12 (1.82 to 9.33) | 0.001 |
| Candida colonisation | cLeon et al. 200626 | 3.04 (1.45 to 6.39) | < 0.001 |
| Candida species corrected colonisation index | Pittet et al. 199443 | 4.01 (2.16 to 7.45) | < 0.001 |
| Renal replacement therapy | |||
| Haemodialysis duration/days at risk | Chow et al. 200838 | 3.84 (1.75 to 8.4)b | < 0.001b |
| 6.2 (2.67 to 14.4)a | < 0.0001a | ||
| Haemofiltration | cJorda-Marcos et al. 200735 | 1.96 (1.06 to 3.62) | 0.032 |
| New-onset haemodialysis | Paphitou et al. 200528 | 5.4 (2.5 to 11.8) | 0.029 |
| Infection | |||
| Enteric bacteraemia | Chow et al. 200838 | 3.45 (1.38 to 8.63)b | < 0.01b |
| 3.43 (1.39 to 8.48)a | < 0.01a | ||
| Severe sepsis | cLeon et al. 2060626 | 7.68 (4.14 to 14.22) | < 0.001 |
| Hospital acquired | Michalopoulos et al. 200341 | 9.4 (2.5 to 48.3) | < 0.001 |
| Mechanical ventilation | |||
| Mechanical ventilation after day 3 | McKinnon et al. 200140 | Not recorded | ≤ 0.05 |
| Mechanical ventilation > 10 days | Michalopoulos et al. 200341 | 28.2 (3.6 to 119.5) | < 0.001 |
| Diabetes | |||
| Diabetes | Michalopoulos et al. 200341 | 2.4 (1.3 to 13.5) | < 0.01 |
| Diabetes | Paphitou et al. 200528 | 2.8 (1.6 to 4.7) | 0.053 |
| Acute severity score | |||
| APACHE II | Pittet et al. 199443 | 1.03 (1.01 to 1.05) | 0.007 |
| APACHE III | banez-Nolla et al. 200439 | 1.03 (1.00 to 1.06) | 0.004 |
| Other | |||
| Acute renal failure | Blumberg et al. 200125 | 4.2 (2.1 to 8.3) | < 0.001 |
| Antifungal medication | Blumberg et al. 200125 | 0.3 (0.1 to 0.6) | < 0.001 |
| Red blood cell transfusion | Chow et al. 200838 | 1.97 (0.98 to 3.99)b | 0.06b |
| 2.72 (1.33 to 5.58)a | < 0.01a | ||
| CVCs | McKinnon et al. 200140 | Not recorded | ≤ 0.05 |
| Diarrhoea | McKinnon et al. 200140 | Not recorded | ≤ 0.05 |
| Peripheral catheter use | McKinnon et al. 200140 | Not recorded | ≤ 0.05 |
| CBP time > 120 minutes | Michalopoulos et al. 200341 | 8.1 (2.9 to 23.6) | < 0.01 |
| Broad-spectrum antibiotics | Paphitou et al. 200528 | 3.0 (1.8 to 5.0) | 0.028 |
| Risk factor | No. of studies examining risk factor | No. of studies for which risk factor significantly associated with IFD on univariable/multivariable analysis |
|---|---|---|
| Surgery | 7 | 5/5 |
| TPN | 6 | 6/4 |
| Fungal colonisation | 5 | 4/4 |
| Renal replacement therapy | 7 | 5/3 |
| Infection/sepsis | 5 | 3/3 |
| Mechanical ventilation | 5 | 2/2 |
| Diabetes | 4 | 3/2 |
| Acute severity score | 8 | 2/2 |
| CVCs | 7 | 4/1 |
| Broad-spectrum antibiotics | 8 | 5/1 |
| CPB > 120 minutes | 1 | 1/1 |
| Red blood cell transfusions | 3 | 3/1 |
| Antifungal medication | 4 | 2/1 |
| Acute renal failure | 2 | 1/1 |
| Diarrhoea | 1 | 1/1 |
| Peripheral catheter | 1 | 1/1 |
Surgery
Seven studies25,26,35,36,38–40,43 examined the association between surgery and IFD. The type and timing of surgery varied across the studies, with two36,38 looking at abdominal surgery and the others looking at any surgical procedure. Five of the seven studies25,26,35,36,38,40 reported a significant association between surgery and IFD on both univariable and multivariable analysis.
Total parenteral nutrition
Six studies25,26,28,35,37,38,40 examined the association between TPN and IFD. All six found a significant association with IFD on univariable analysis. Four of the six studies25,26,35,37,38 also found a significant association on multivariable analysis.
Fungal colonisation
Five studies25,26,35,36,39,43 examined the association between fungal colonisation and IFD. Four of the five studies26,35,36,39,43 reported an association on both univariable and multivariable analysis. Sites of fungal colonisation examined and modelling approaches varied across the studies.
Renal replacement therapy
Seven studies26,28,35,37,38,40,41,43 examined renal replacement therapy as a risk factor for IFD. Five of the seven studies26,28,35,37,38,40 found a significant association on univariable analysis. Three of the seven studies28,35,38 demonstrated a significant association on multivariable analysis. Only one of the two EPCAN articles demonstrated a significant result on multivariable analysis. The type and exposure time to dialysis varied across the studies; some looked at pre-admission dialysis, whereas others examined haemofiltration in the unit.
Infection
Five studies25,26,38,41,43 examined the relationship between infections/sepsis and IFD. Three of the five studies26,38,41 demonstrated an association on multivariable analysis. The source and site of infection varied across the studies. One examined bacterial infection/bacteraemia without specifying type and source of infection. 41 Another examined enteric bacteraemia, which included enterococcal, Bacteroides and other Gram-negative bacilli bloodstream infections. 38 One demonstrated an association with severe sepsis, although the infection source was not specified. 26
Mechanical ventilation
Five studies25,26,35,37,40,41 examined the association between receipt of mechanical ventilation and IFD. Two of the five studies40,41 reported a significant association on multivariable analysis. Both timing and duration of mechanical ventilation varied across the studies, with one study finding presence of mechanical ventilation was significant after day 3 of critical care unit admission,40 and the other finding that a duration of mechanical ventilation > 10 days was significant. 41
Diabetes
Four studies26,28,36,41 examined whether or not a past medical history of diabetes mellitus was a risk factor for IFD. Two of the four studies28,41 demonstrated a significant association on both univariable and multivariable analysis.
Acute severity score
Eight studies25,26,28,35–37,39,43 examined whether either the APACHE II or APACHE III (Acute Physiology And Chronic Health Evaluation) score was a risk factor for IFD. Two of the eight studies39,43 demonstrated a significant association on both univariable and multivariable analysis.
Other risk factors
A number of other risk factors were identified to be significantly associated with IFD on multivariable analysis in single studies. These included CBP time, acute renal failure, broad-spectrum antibiotic use, red blood cell transfusions, antifungal medication use, CVC use, diarrhoea and peripheral catheter use (see Table 2). Of note, two studies27,35 examined the association between neutropenia and IFD, neither of which demonstrated a significant association. Similarly, none of the five studies26,28,36,37,43 looking at immunosuppressant use demonstrated an association with IFD.
Risk models and clinical decision rules
Four of the studies26–28,43 developed a risk model or clinical decision rule for IFD (Table 4) and one evaluated a clinical decision rule for IFD, all in the critical care setting.
| Study | Development | Validation | Model(s)/rule(s) | c-index | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Leon et al. 200626 | Risk factors significant (p < 0.05) on univariable analysis of full sample included in multivariable logistic regression model fitted in 65% development sample. Final model chosen by backwards elimination; stopping criterion unclear. Simplified to bedside score by rounding coefficients | ROC and sensitivity/specificity at cutoff values in 35% validation sample | 0.908 × (TPN) + 0.997 × (surgery) + 1.112 × (multifocal Candida species colonisation) + 2.038 × (severe sepsis) | 0.847 (0.800 to 0.894) | NA | NA | NA | NA |
| 1 × (TPN) + 1 × (surgery) + 1 × (multifocal Candida species colonisation) + 2 × (severe sepsis) | NR | NA | NA | NA | NA | |||
| Bedside score ≥ 3, or equivalently: (severe sepsis plus at least one other risk factor) or (all three other risk factors) | NA | 81 | 74 | NR | NR | |||
| Ostrosky-Zeichner et al. 200727 | All rule development in 75% development sample. Univariable analysis of risk factors. Clinical decision rules constructed for ‘all possible combinations of risk factors and time points’ in ‘several different formats (with different weights for the risk factors)’. ‘Best’ rules selected on sensitivity, PPV, PPV/(1 − NPV), and proportion of patients identified as high risk in development sample | Chi-squared test of association, sensitivity, specificity, PPV and NPV in 25% validation sample | (any antibiotic day 1 to 3) and (CVC days 1 to 3) | NA | 89 | 38 | 4 | 99 |
| (any antibiotic days 1 to 3) and (CVC days 1 to 3) and (at least one of: any surgery day −7 to 0; immunosuppressive use days −7 to 0; pancreatitis days −7 to 0; TPN days 1 to 3; dialysis days 1 to 3; steroid use days −7 to 3) | NA | 66 | 69 | 6 | 98 | |||
| [(any antibiotic days 1 to 3) or (CVC days 1 to 3)] and (at least two of: any surgery days −7 to 0; immunosuppressive use days −7 to 0; pancreatitis days −7 to 0; TPN days 1 to 3; dialysis days 1 to 3; steroid use days −7 to 3) | NA | 34 | 90 | 9 | 97 | |||
| Paphitou et al. 200528 | All rule development in full sample. Univariable analysis of risk factors; unclear whether or not used to select factors for multivariable model. Multivariable logistic regression model with stepwise procedure; unclear whether forward selection or backwards elimination and unclear stopping criterion. Clinical decision rules constructed ‘using a combination of inspection of the data and results of the multivariable analysis’ | Sensitivity and PPV in full sample. NNT assuming 50% relative reduction in IFD associated with therapy. Cost to prevent one case assuming prophylaxis costs of US$100/day | At least one of: diabetes mellitus; TPN days −7 to 0; new-onset haemodialysis days −7 | NA | 39-39 | NR | 17-26 | NR |
| At least one of: diabetes mellitus; TPN days −7 to 0; new-onset haemodialysis days −7 to 3; broad-spectrum antibiotics day −7 to 3 | NA | 78-83 | NR | 11-17 | NR | |||
| (at least one of: diabetes mellitus; TPN days −7 to 0; new-onset haemodialysis days −7 to 3) AND (broad-spectrum antibiotics days −7 to 3) | NA | 30-33 | NR | 20-34 | NR | |||
| Pittet et al. 199443 | All rule development in full sample. Clinical decision rules constructed from colonisation parameters only (no. of sites, colonisation index, corrected colonisation index – derived post hoc); methods unclear. Risk factors with p < 0.15 on univariable analysis included in multivariable logistic regression model. Only those with p < 0.05 in multivariable model reported; unclear if stepwise procedure used | Clinical decision rules validated by sensitivity, specificity, PPV and NPV in full sample | Colonisation at two or more sites | NA | 100 | 22 | 44 | 100 |
| Colonisation at three or more sites | NA | 73 | 56 | 50 | 77 | |||
| Colonisation at four or more sites | NA | 45 | 72 | 50 | 68 | |||
| Candida colonisation index ≥ 0.5 | NA | 100 | 69 | 66 | 100 | |||
| Candida-corrected colonisation index ≥ 0.4 | NA | 100% | 100% | 100% | 100% |
Leon et al. 26 developed and validated a risk model from which they derived a bedside scoring system to inform early antifungal therapy in non-neutropenic, critically ill patients. The study was a prospective cohort of 1699 patients, of whom 980 with colonisation or infection were included in the model development with 97 cases of IFD. Multifocal Candida colonisation, surgery directly prior to critical care unit admission, severe sepsis and TPN were included in the final risk model. The optimal score from the model gave a sensitivity of 81% and a specificity of 74%.
Ostrosky-Zeichner et al. 27 developed a number of clinical decision rules for IFD in the critical care setting. The study was a retrospective chart review of 2890 patients from 12 participating centres with 88 cases of IFD. Several clinical decision rules, with varying combinations of risk factors, were developed and tested. The best performing rule consisted of the following risk factors: any systemic antibiotic, presence of a CVC, and at least two of the following – TPN, any dialysis, any major surgery, pancreatitis, and use of steroids or other immunosuppressants. The model gave a sensitivity of 34% and a specificity of 90%.
Paphitou et al. 28 developed and validated a number of clinical decision rules from a single centre, retrospective cohort study of 327 critically ill patients. There were nine cases of proven IFD with 27 probable/possible cases.
Several combinations of risk factors were evaluated, of which any combination of diabetes mellitus, new-onset haemodialysis, use of TPN or receipt of broad-spectrum antibiotics was considered the most useful. The model gave a sensitivity of 78–83% and specificity of approximately 50%.
Pittet et al. 43 developed a number of clinical decision rules based on intensity of Candida colonisation from a single-centre, prospective cohort study of 29 critically ill patients with significant Candida colonisation of whom 11 had severe Candida infection. The best-performing rule, developed post hoc to give perfect discrimination in the small data set, was a Candida-corrected colonisation index (ratio of highly positive fungal screening samples to the total number of samples) of 0.4 or more.
Finally, Piarroux et al. 42 evaluated the clinical decision rule developed by Pittet et al. 43 whereby patients admitted to a single SICU were screened for fungal colonisation and pre-emptively treated with fluconazole if the Candida-corrected colonisation index was ≥ 0.4. Using same centre, historical control subjects, from a time period prior to offering prophylaxis, a reduction of unit-acquired IFD from 2.2% to 0% (p < 0.001) was reported.
Reporting of methodological assessment
The included studies varied with respect to their methodological quality (Table 5a and b). All 12 studies reported objectives, main outcome and characteristics of the selected study patients (see Table 5a). The majority of the studies were carried out in at least two critical care units. The analysis was defined a priori in 10 of the 12 studies (83%) and the majority of known risk factors were accounted for in 10 of the 11 studies that conducted multivariable analyses (91%). The study by Piarroux et al. 42 evaluated a clinical decision rule and therefore did not carry out a risk factor analysis. Risk factors were poorly defined in over half of the studies and the rationale for inclusion was missing in over two-thirds of studies.
| Article | Is the study objective clearly described? | Are the main outcomes measured clearly described? | Are the characteristics of the patients clearly described? | Was the study performed in multiple centres (> 2)? | Was the analysis defined a prior? | Did the analysis account for the majority of known risk factors? | Was rationale behind inclusion of risk factors included? | Were the risk factors clearly defined? |
|---|---|---|---|---|---|---|---|---|
| Agvald-Ohman et al. 200836 | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Blumberg et al. 200125 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ |
| Borzotta and Beardsley 199937 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
| Chow et al. 200838 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ |
| banez-Nolla et al. 200439 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ |
| Jorda-Marcos et al. 200735 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
| Leon et al. 200626 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
| McKinnon et al. 200140 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Michalopoulos et al. 200341 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ostrosky-Zeichner et al. 200727 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ |
| Paphitou et al. 200528 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ |
| Piarroux et al. 200442 | ✓ | ✓ | ✓ | ✗ | ✓ | NA | NA | NA |
| Pittet et al. 199443 | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✓ |
| Article | Adequacy of sample size | Risk factor selectiona | Model strategya | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Variables | Events per variable | ≥ 10 events per risk factor? | All candidate risk factors used | Risk factor selection on previous literature/investigator choice | Risk factor selection on univariable analysis | Risk factor selection unclear | All potential risk factors retained in final model | Backwards elimination | Forward selection | Unclear selection | |
| Agvald-Ohman et al. 200836 | 10 | > 10 | < 1 | ✗ | ✓ | ✓ | ||||||
| Blumberg et al. 200125 | 42 | 49 | 0.9 | ✗ | ✓ | ✓ | ||||||
| Borzotta and Beardsley 199937 | 20 | > 21 | < 1 | ✗ | ✓ | ✓ | ||||||
| Chow et al. 200838 | 67b | 35 | 1.9b | ✗ | ✓ | ✓ | ||||||
| 79c | 2.3c | |||||||||||
| Ibanez-Nolla et al. 200439 | 120 | 30 | < 4 | ✗ | ✓ | ✓ | ||||||
| Jorda-Marcos et al. 200735 | 63 | 15 | 4.2 | ✗ | ✓ | ✓ | ||||||
| Leon et al. 200626 | 97 | 22 | 4.4 | ✗ | ✓ | ✓ | ||||||
| McKinnon et al. 200140 | 27 | 23 | 1.2 | ✗ | ✓ | ✓ | ||||||
| Michalopoulos et al. 200341 | 30 | 29 | 1.0 | ✗ | ✓ | ✓ | ||||||
| Ostrosky-Zeichner et al. 200727 | 88 | 27 | 3.3 | ✗ | ✓ | ✓ | ||||||
| Paphitou et al. 200528 | 36 | > 49 | < 0.8 | ✗ | ✓ | ✓ | ||||||
| Piarroux et al. 200442 | 50 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Pittet et al. 199443 | 11 | 9 | 1.2 | ✗ | ✓ | ✓ | ||||||
Reporting of the statistical modelling was generally poor (see Table 5b), and it was usually impossible to determine, exactly, the number of variables that were considered as candidate risk factors in each article. Reported methods often stated, ‘risk factors examined included …’ but it was not clear whether or not the subsequent list was exhaustive and risk factors could often only be determined from those reported in the results, which, in some cases, were only those selected by a modelling process or only those that were statistically significant.
The numbers of risk factors reported in Table 5b are therefore approximate and, in many cases, a minimum. Some studies split data into development and validation samples but did not report how many of the events were in the development sample. However, even assessing the models on the minimum number of variables included, as indicated by the article and the number of events in the full sample (and therefore the maximum EPV), all of the papers had a strong likelihood of presenting results that were overfitted to the data. Taking into account all variables considered in the statistical modelling (including those screened out on univariable analysis), the largest studies had around four EPV and a number of studies had examined at least as many risk factors as there were events in the data set, giving values of one EPV or below. No studies reached the predefined threshold of 10 EPV. Roughly half of the studies based their decision of which risk factors to include in the multivariable analysis on the results of the univariable analysis, whereas the reporting in the remaining studies was insufficient to determine risk factor selection. In terms of modelling strategies, one-third of the studies used a backwards elimination process, one-third a forwards selection process, and for the remaining third it was unclear from the reporting what modelling strategy was used.
Discussion
Thirteen articles exploring risk factors, risk models or clinical decision rules for IFD in critically ill adult patients were identified. Of these, eight examined risk factors specifically, four developed risk models or clinical decision rules and one evaluated a clinical decision rule.
In this systematic review, the following risk factors were found in multiple studies to be significantly associated with IFD: surgery, TPN, fungal colonisation, renal replacement therapy, infection, mechanical ventilation, diabetes and acute severity score. CBP time, acute renal failure, broad-spectrum antibiotics, red blood cell transfusion, antifungal medication, CVCs, diarrhoea and peripheral catheter use were also found to be statistically significant but each solely in a single study. The risk model and clinical decision rule studies used all of the risk factors that were found to be significant in multiple studies reported above, apart from mechanical ventilation and acute severity scores, and, in addition, included pancreatitis and immunosuppressant use.
Risk factor definitions varied across studies, with many studies offering no definition at all. Risk factor selection process and modelling strategy also varied across studies, and no studies had an adequate sample size for the multivariable analyses. None of the selected studies described the degree of missing data or how missing data were handled in the analysis. Some reported numbers of patients included in each model but reasons for any exclusions were not reported.
The risk models and clinical decision rules identified in this review have a number of factors that limit their usefulness for guiding early decision-making regarding antifungal prophylaxis. First, the patient populations studied. The models and rules developed and evaluated used data from patients staying 4,27,28 542 or 7 days26 in the critical care unit. These help to identify high-risk populations; however, the performance of these models or rules, applied at an earlier time point in the critical care unit stay, cannot be determined. Some models and rules were developed using patients with Candida colonisation only26,43 and, consequently, they could be used to guide only empiric therapy and not true prophylaxis. Second, the statistical modelling. Models are likely to be overfitted owing to the small numbers of events in the data used for model development. Stepwise selection of risk factors is likely to have resulted in model coefficients that are too large and measures of model performance that are optimistic. 44 Finally, despite being developed in higher-risk populations identified by longer ICU stays, the specificity of the rules was generally low and, hence, their use to guide treatment could result in overuse of antifungal drugs with costs both financial and in terms of increased resistance. No studies have adequately addressed the cost-effectiveness of using clinical decision rules to guide delivery of antifungal therapy. The only study to give any consideration to costs was Paphitou et al. ,28 who estimated the number needed to treat and associated cost to prevent one case of IFD assuming a RR of 0.5 and a cost of US$100/day for antifungal prophylaxis. The most promising rule on these criteria had a number needed to treat of 6–10 and associated cost of US$12,000–21,000 per case prevented.
Since the end date of our systematic review, three studies have been published validating risk models or clinical decision rules identified in this review. Leon et al. 26 validated their risk model, the Candida score, among a new prospective cohort of 892 admissions with Candida colonisation staying at least 7 days in one of 36 multidisciplinary ICUs in Spain, Argentina and France. 45 As expected, the performance of the score was not as good in the validation sample with an area under the ROC curve of 0.77 compared with 0.85 in the development data. Based on a cut-off of a score of 3, the sensitivity was 78% (81% in development data), specificity was 66% (74% in development data), and PPVs and NPVs were 14% and 98%, respectively (not reported in development data). Playford et al. 46 validated four clinical decision rules – the best rule from Ostrosky-Zeichner et al. 27 and a subsequent revision to this, published in abstract form, and the two best rules from Pittet et al. 43 – in a prospective cohort of 615 patients admitted for at least 72 hours to four multidisciplinary ICUs in Australia. Performance of the clinical prediction rules was worse than in the development data sets and the authors recommended that to identify a sufficiently high-risk population to consider for antifungal therapy would require a combination of the clinical risk factors from Ostrosky-Zeichner et al. 27 with measures of colonisation from Pittet et al. 43 Most recently, Hermsen et al. 47 set out to validate the clinical decision rules of Paphitou et al. 28 and Ostrosky-Zeichner et al. 27 in a case–control study of 88 cases and 264 matched control subjects staying at least 4 days in a single multidisciplinary ICU in the USA. Rather than validate the rules as published, Hermsen et al. 47 fitted new conditional logistic regression models using the risk factors from these rules, rendering their results incomparable with the original publications. It is, however, worth noting that a number of the risk factors included in the rules (surgery, pancreatitis, haemodialysis and diabetes) were not subsequently found to be significantly associated with risk of IFD.
This is the first literature review to systematically evaluate and assess the quality of the literature on risk factors for IFD. Rigorous search methods and a tailored quality assessment tool were combined to produce a high-quality systematic review. As search strategies are designed for identifying RCTs rather than risk factor studies, a comprehensive search strategy, including multiple medical subject heading (MeSH) terms and keywords describing risk, risk models and clinical decision rules, was employed. Furthermore, abstracts and full text articles were reviewed and data extracted, by two investigators independently, to ensure that all relevant articles and data were captured. There is currently no validated gold standard or single recommended instrument for methodological assessment of risk factor studies, and so a combined methodological assessment was developed for this review and tailored to assess the specific areas for risk factor studies that were considered to be important.
One limitation of this systematic review was that the heterogeneity of the included studies precluded any meta-analysis. Objectives differed between the studies, with some assessing a specific clinical decision rule and some examining a range of risk factors. The way in which the risk factors and outcomes were defined also differed and different inclusion criteria were imposed across the studies making combining results inappropriate. The existence of publication bias is always a possibility in systematic reviews but many risk factors were shown to be non-significant on multivariable analysis indicating that negative, as well as positive, results were represented in the studies. In the univariable analysis, however, it was difficult to identify which risk factors were non-significant as the full list of factors examined was not always made clear.
In conclusion, this review has shown a number of risk factors to be significantly associated with the development of IFD in critically ill adults. However, this review has highlighted numerous methodological limitations in the design and conduct of studies in this area and, as such, it is suggested that caution should be used in their interpretation.
Chapter 3 Data collection for risk factors and outcomes of invasive fungal disease
Introduction
The FIRE Study collected data on risk factors and outcomes of IFD in UK critical care. This chapter reports the methods used to develop and refine the data set, the data collection tools and the recruitment of the participating adult general critical care units.
Methods
Design and development of data set and protocol
A list of the key risk factors for IFD, identified from the systematic review of the literature, was compiled. Through consultation with the clinical experts on the FIRE Study Steering Group, the list was added to and refined after comprehensive discussion, to produce a final data set and definitions.
Data were collected at three different decision time points: on admission to the critical care unit; at the end of the first 24 hours; and at the end of the third calendar day. Outcomes data were collected until discharge from critical care or death. The rationale for the time points is as follows:
-
On admission Allowed a record of the risk factors to which the patient was exposed in the period up to 7 days prior to admission and provided the first decision point for antifungal prophylaxis.
-
At the end of the first 24 hours Given the interventions performed in the first 24 hours of care in the critical care unit, this allowed an updated record of the risk factors to which the patient was exposed and provided a second decision point for antifungal prophylaxis. Data are routinely collected in the first 24 hours for the Case Mix Programme (CMP), the national clinical audit for adult critical care, which reduced duplication of data collection effort.
-
At the end of calendar day 3 With the median stay in adult general critical care of 53 hours, patients still on the critical care unit at this time point are expected to be long-stay patients and at higher risk of IFD. This allowed an updated records of the risk factors to which the patient was exposed and provided the third and final decision point for antifungal prophylaxis. End of calendar day 3 was selected to coincide with data collection in adult general critical care units for the Critical Care Minimum Data Set (CCMDS).
Primary outcome
The primary outcome for the FIRE Study was IFD, defined as a blood culture or sample from a normally sterile site (including, but not restricted to, cerebrospinal fluid, peritoneal fluid, pleural fluid and pericardial fluid, and excluding bronchoalveolar lavage, urine and sputum) that was positive for yeast/mould cells in a microbiological or histopathological report. This definition was chosen to best capture Candida IFD and was recognised to be likely to under-represent IFD due to other species.
For statistical and economic modelling, the primary outcome was invasive Candida infection, defined as IFD (as above)-positive for Candida species in a microbiological or histopathological report.
Data
Data collected for each patient:
-
CMP admission number (for data linkage with the CMP; see below)
-
hospital number (for local retrieval)
-
NHS/Community Health Index (CHI) number
-
date of birth
-
sex
-
date of admission to hospital
-
date and time of admission to the critical care unit.
Data collected at each of the three time points:
-
lines in arteries (number)
-
major intra-arterial devices (any)
-
catheters in central veins (number, position)
-
peripheral lines (any)
-
intracranial devices/perineural lines (number)
-
drains (number)
-
enteral feeding tube
-
urinary catheter
-
organ support (advanced respiratory support, renal support)
-
TPN
-
steroids (high or low dose)
-
immunosuppressives
-
existing diagnosis of diabetes mellitus (admission only)
-
antimicrobial drugs (last antimicrobials prior to admission and first antimicrobials following admission)
-
neutropenic status (end of 24 hours only).
Data collected on admission and at any time up to discharge from the critical care unit or death:
-
surgery (condition requiring surgery, urgency of surgery, unexpected complications and open abdomen following surgery)
-
fungal colonisation (numbers of samples reported and numbers positive)
-
IFD (date/time, organism and site)
-
antifungal drug use (topical and systemic, initial regimen and date/time of first administration).
Fungal colonisation was defined as the presence of yeast colonisation in any sample reported on a microbiology system and was recorded as the date that a positive report was received, i.e. the point at which a treatment decision could be made based on this knowledge.
Research governance
The FIRE Study was sponsored by ICNARC. An application was made to the Bolton NHS Research Ethics Committee (REC) following confirmation of funding, and a favourable opinion was received on 15 December 2008. The Scotland A REC reviewed the protocol on 26 October 2009 and concluded that the project could be conducted as an extension to existing audit and was not classified as research.
The FIRE Study was piggybacked on to the CMP in England, Wales and Northern Ireland, and linked with data provided by the Scottish Intensive Care Society Audit Group (SICSAG) in Scotland. The CMP is the national clinical audit of adult general critical care units in England, Wales and Northern Ireland, established in 1995. Trained data collectors collect the raw data to precise rules and definitions. The data then undergo extensive local and central validation for completeness, illogicalities and inconsistencies prior to pooling. SICSAG is the national clinical audit of adult general critical care units in Scotland, established in 1995. Data are collected on local software and undergo logical checks on data entry. Monthly case note validation is undertaken on a random sample of 10% of records. Both the CMP and SICSAG databases have been independently assessed against 10 criteria for coverage and accuracy by the Directory of Clinical Databases (DoCDat; www.icapp.nhs.uk/docdat/) and achieved mean quality scores of 3.7 and 3.8 (on a scale of 1 = worst to 4 = best).
The CMP has approval under Section 251 of the NHS Act 2006 (originally enacted as Section 60 of the Health and Social Care Act 2001) to hold limited patient identifiable data (date of birth, sex, postcode, NHS number) without consent (approval number: PIAG 2–10(f)/2005). No additional patient identifiable data were required for the FIRE Study. In June 2008, the Patient Information Advisory Group (PIAG), since superseded by the National Information Governance Board for Health and Social Care (NIGB) Ethics and Confidentiality Committee, approved the extension of the Section 251 approval for the CMP to cover the FIRE Study.
Each participating critical care unit in England, Wales and Northern Ireland completed local research and development (R&D) approvals prior to commencing recruitment. In accordance with the guidance given by the Scotland A REC, each participating critical care unit in Scotland obtained approval from their local Caldicott Guardian prior to commencing recruitment.
Patient information sheets (see Appendix 3) and posters were displayed in participating critical care units so that patients and families/close friends would be aware that the unit was taking part in the FIRE Study, which would not affect their care. Patients or families/close friends were able to opt out from participation and their data were removed from the FIRE Study database. Patient information sheets and posters to be displayed in critical care units in Scotland were adapted to reflect the classification as an extension to existing audit rather than as research.
Critical care unit recruitment
All adult general critical care units in England, Wales and Northern Ireland participating in the CMP were initially invited to take part in the FIRE Study. Subsequently all adult general critical care units in Scotland were also invited to participate. Separate, standalone, high dependency units (HDUs) and specialist units (neurosciences, cardiothoracic, etc.) were not eligible for participation in the FIRE Study. Staff in participating critical care units collected data on consecutive admissions to their unit.
Dataset familiarisation courses
Regional Dataset Familiarisation Courses were held across England and Scotland. Staff from critical care units who were unable to attend on any of these days were provided training via teleconference.
The Dataset Familiarisation Courses were one-day events where the background, aims and rationale for the FIRE Study were discussed with the collaborating clinicians, research nurses and data clerks. This was followed by a detailed explanation of the definition for each field in the data set with opportunities for questions and examples. Each delegate was given a FIRE Study Data Collection Manual to take back with them to their unit for reference.
The Data Collection Manual contained precise, standardised definitions for each field, along with data collection forms and flows (see Appendices 4 and 5) to guide them through the data collection process. From the data collection forms, data were entered on to a dedicated, secure web-based data entry system developed and hosted by ICNARC. Data collection manuals, flows and forms, frequently asked questions (FAQs), definitions and error checking were also available, either for download or built into the design for the web portal. Web portal pages were regularly reviewed and new versions released to ensure up-to-date clarity and to answer common queries.
Maintenance and motivation of units
During the course of the study, quarterly newsletters were sent to all participating critical care units. Newsletters were used as an opportunity to clarify any data issues, as well as to maintain motivation and encourage involvement through regular updates.
The Study Coordinator maintained close contact with all units by telephone and e-mail throughout the study.
Support costs
The FIRE Study was set up at the same time as the new system for supporting research in the NHS through Comprehensive Local Research Networks (CLRNs) was evolving. Initially, funding for NHS support costs was sought through local systems in each CLRN. In June 2010, support for data collection was centrally approved by the Central and East London CLRN to an equivalent of a 0.5 WTE (whole-time equivalent) Band 6 Research Nurse for a critical care unit with 800 admissions per year.
Sample size calculation
Assuming a 1% incidence of invasive Candida infection among non-neutropenic, adult patients admitted to UK critical care units,4,5 a sample size of 40,000 patients in the development sample was selected to give 20 EPV for consideration of 20 candidate variables in the risk model. This sample size was also sufficient to give 80% power to detect, as statistically significant (p < 0.05), a risk factor present in 10% of the population associated with a 50% increase in the risk of invasive Candida infection. Simulation modelling indicated that this sample size calculation was robust to clustering of both risk factors and outcomes at the critical care unit level. An additional 20,000 admissions were recruited for the validation sample. The 60,000 admissions target was based on the assumption that 80 critical care units would participate in, and complete, data collection and validation.
Data management
Data management was an ongoing process. Data were monitored and validated throughout the data collection period in order to ensure that the database was as complete and accurate as possible during the study, and to minimise the time between the end of data collection and start of data analysis.
Data linkage between the FIRE and CMP databases was performed regularly, to ensure complete capture of admissions. Data collectors were notified of the missing records and asked to update the portal with this information.
Data validation reports
Data validation checks were run periodically on each record on the web portal. These checks identified any incomplete data (missing values) and inconsistent data (unusual, although not impossible, data) both within and across data fields. Following receipt of a Data Validation Report (DVR), data collectors either updated/corrected the data on the web portal or responded to the FIRE Study Team to confirm data were correct.
Data linkage with the Case Mix Programme and Scottish Intensive Care Society Advisory Group
FIRE data were linked with the corresponding CMP/SICSAG data and any discrepancies in patient identifiable data (date of birth, sex, NHS number, date of admission to hospital and critical care) raised and resolved.
Reliability study
At the end of data collection, a reliability study was conducted to confirm that all cases of IFD were correctly recorded. Each critical care unit received a mixed, blinded list of all of the reported IFD-positive cases from the unit, along with a 2% random sample of non-IFD cases. For each patient, the local principal investigator was required to recheck the original hospital notes and microbiology records and make an independent decision on the IFD status originally recorded. Reliability study results were completed and signed off by the local principal investigator at each unit and returned centrally to the FIRE Study Team for verification against the original IFD data. The reliability study was conducted following all other validation, once access to the web portal had been disabled, to ensure that the data on it were not used to complete the Reliability Study. Where there were discrepancies between original and reliability study data, the IFD status provided by the local principal investigator was accepted as final. Any diagnosis of IFD in a sample from a non-sterile site was followed up with the local principal investigator to determine whether or not IFD had also been found in a sterile site as per the definition.
Results
Critical care unit recruitment
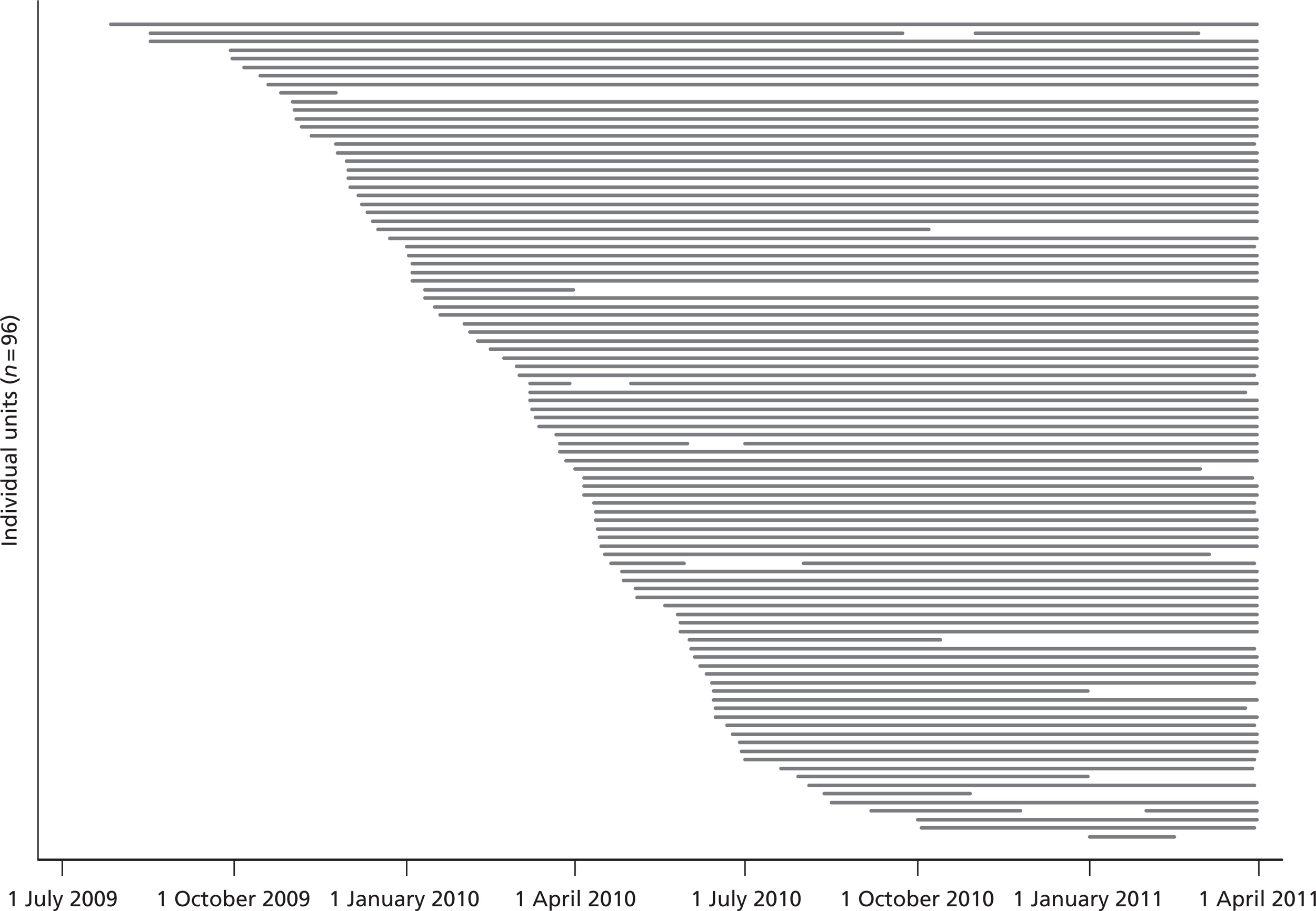
Recruitment of critical care units took place between April 2008 and December 2010 (England, Wales and Northern Ireland) and February 2010 and October 2010 (Scotland). One hundred and three critical care units expressed an interest in taking part in the FIRE Study and were sent a Principal Investigator Details Form and Site-Specific Information (SSI) Form to complete. Of these, 100 (83 in England, Wales and Northern Ireland, and 17 in Scotland) critical care units sought and gained approval from either their local R&D Departments (England, Wales and Northern Ireland) or Caldicott Guardians (Scotland) and commenced data collection. R&D approval took a median of 45 days [interquartile range (IQR) 28.5 to 79.5] from submission of SSI Form. R&D approval to start of FIRE Study data collection took a median of 24 days (IQR 8.0 to 52.5). Four critical care units withdrew from the study owing to local staffing and data collection issues, giving a final total of 96 participating units. Eleven critical care units stopped data collection early and the remaining 85 continued data collection to 31 March 2011 (Figure 2). Five critical care units had periods of 1–2 months' data excluded from the final data set owing to failure to capture all consecutive admissions as a result of temporary local staffing issues.
FIGURE 2.
Participation timeline.
Each critical care unit was represented at a Dataset Familiarisation Course. Representatives from 90 units attended one of the nine regional Dataset Familiarisation Courses and a further 10 units, who were unable to attend one of these courses, were provided individual or group Dataset Familiarisation by teleconference.
The final 96 participating critical care units were representative of all UK adult general critical care units in terms of geographical distribution, teaching status of hospital located within and number of critical care unit beds (Table 6).
| Characteristic | No. of critical care units (% of all adult general critical care units) |
|---|---|
| Geographical region | |
| England | 76 (36) |
| Strategic Health Authority | |
| East Midlands | 4 (29) |
| East of England | 13 (68) |
| London | 6 (16) |
| North East | 4 (24) |
| North West | 11 (35) |
| South Central | 5 (38) |
| South East Coast | 5 (28) |
| South West | 11 (58) |
| West Midlands | 10 (43) |
| Yorkshire and The Humber | 7 (32) |
| Wales | 2 (13) |
| Northern Ireland | 2 (22) |
| Scotland | 16 (89) |
| Teaching status | |
| University hospital | 21 (29) |
| University affiliated | 17 (44) |
| Non-University hospital | 58 (40) |
| No. of beds | |
| 2–5 | 9 (38) |
| 6–10 | 44 (34) |
| 11–20 | 35 (40) |
| 21+ | 8 (50) |
Recruitment of admissions
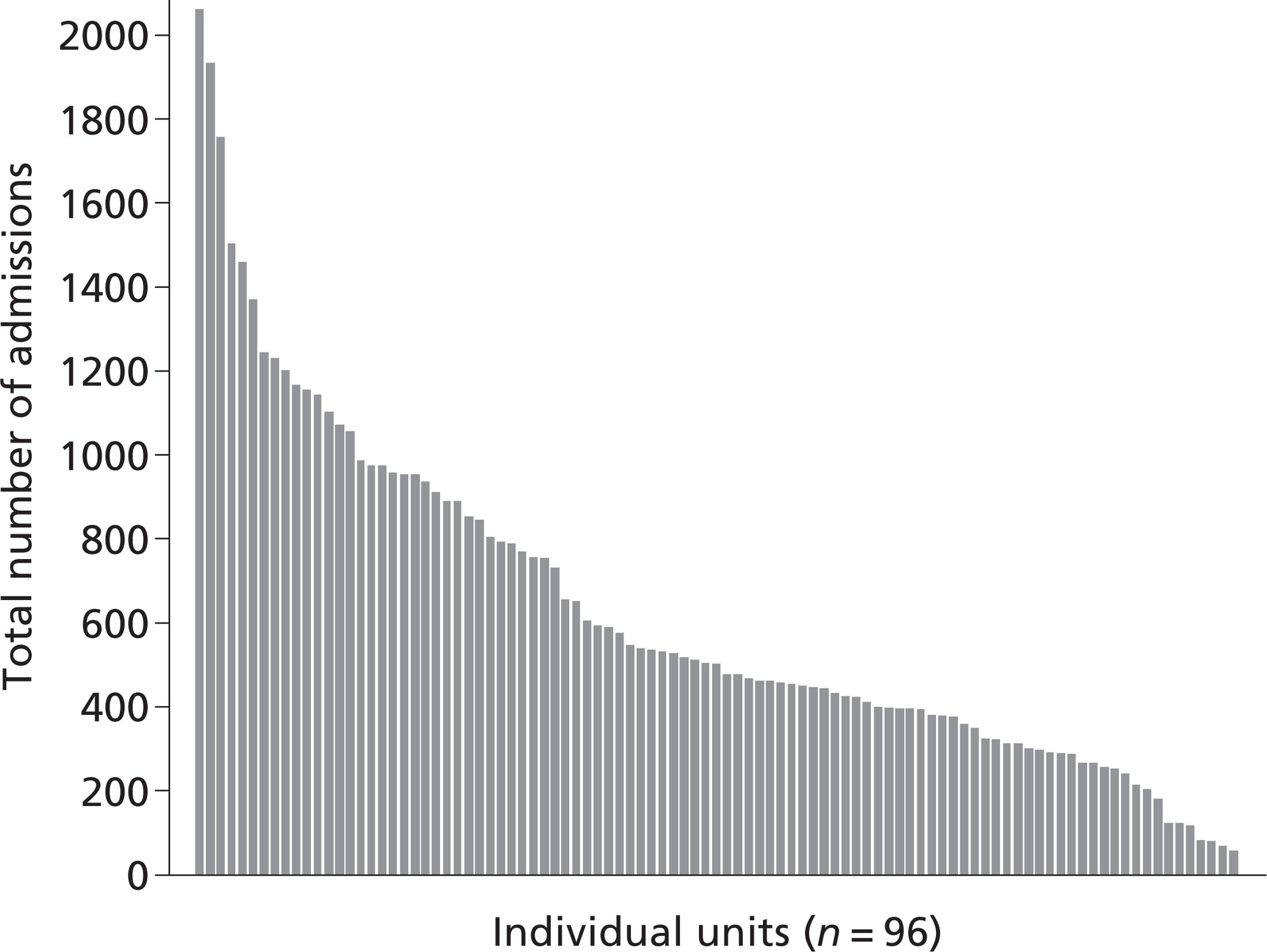
The final data set contained a total of 60,778 admissions. Individual critical care units recruited between 58 and 2061 admissions (median 503, IQR 368 to 890; Figure 3).
FIGURE 3.
Recruitment by critical care unit.
Reliability study
Ninety-six reliability study reports were sent out for a total of 1293 cases originally reported with a status of IFD and 1289 reported as non-IFD. A total of 917 discrepancies were identified; 913 of these were cases originally reported as IFD not confirmed as IFD by the principal investigator in the reliability study. In the majority of these cases, the positive sample was not from a normally sterile site and this had been recorded incorrectly in the original data. These cases were amended in the final data set. Only four cases which were originally recorded as non-IFD were amended in the final data set, following the reliability study, to IFD.
Discussion
The FIRE Study successfully recruited over 60,000 admissions to 96 critical care units that were representative of all adult general critical care units in the UK. However, recruitment took longer than originally planned due to slower start-up of units. The main barrier to recruitment was the length of time between initial registration of interest in the study and submission for R&D approvals and from receipt of R&D approval to start of data collection. These extended times, plus anecdotal information from units, suggested that other more pressing considerations were dominant in critical care units and the wider hospital setting, including the actual and anticipated impact of the 2009 influenza A (H1N1) pandemic. Support from the NIHR Critical Care Specialty Group and the CLRNs was instrumental in encouraging wider uptake of the study and ensuring its successful completion. Following initial slow uptake from units in England, Wales and Northern Ireland, participation in the FIRE Study was opened up to units in Scotland, with the support of SIGSAG to provide linked national audit data. This approach proved very successful and had a very positive impact on the study; the FIRE Study received extremely strong support from critical care units in Scotland, resulting in Scotland having the highest participation rate among all regions of the UK.
The reliability study identified substantial over-reporting of IFD in the original data submissions suggesting a difficulty in correctly applying the IFD definitions at sites. A large number of cases that had originally been recorded as IFD were amended on the database after verification from the principal investigator that the original data were incorrect. Consequently, the event rate for IFD in the final data set was substantially lower than anticipated, and also lower than had been suggested by previous literature.
Chapter 4 Epidemiology of invasive fungal disease in UK critical care units
Introduction
This chapter reports an analysis of the FIRE Study database with the aim of describing the epidemiology of IFD across UK critical care units, with a specific focus on Candida infections.
Methods
Descriptive analysis
Admissions with IFD were classified according to species, site(s) of IFD and timing of IFD (pre-admission or day of admission). Case mix was summarised in terms of age, sex, past medical history (severe comorbidities, diabetes mellitus and neutropenia), classification of surgery within up to 7 days prior to admission, acute severity of illness and primary reason for admission to the critical care unit. The following were reported as therapies received during the first 24 hours following admission to the critical care unit: TPN, systemic antimicrobial use, immunosuppressive use and CVC use. Corticosteroids were included as immunosuppressives. Organ support was reported if received at any time during the critical care unit stay. Fungal colonisation was reported according to the time of the report (pre-admission or identified in unit). Outcomes reported were mortality in the original critical care unit and at ultimate discharge from acute hospital, length of stay in the original critical care unit and total length of stay in acute hospital, stratified by survival status. Use of topical and systemic antifungal therapy was reported by time of initiation (pre-admission or in unit). For admissions with IFD identified in the unit, the timing of the first systemic antifungal was also reported relative to the timing of IFD.
For the purpose of summarising case mix, outcomes and antifungal use, the cohort was divided into groups of admissions by fungal species. The groups consisted of admissions with IFD-positive for Candida albicans (Candida albicans IFD); admissions with IFD-positive for other Candida species (Candida non-albicans IFD); and admissions with no IFD either prior to or during the critical care unit stay (no IFD). Admissions with IFD-positive for Candida of unknown species or non-Candida species were excluded from these grouped comparisons owing to small numbers. Admissions with IFD-positive for both Candida albicans and Candida non-albicans were included in the Candida albicans subgroup.
Additional data definitions for linked data from the Case Mix Programme and the Scottish Intensive Care Society Audit Group
Severe comorbidities were defined using the APACHE II method48 and must have been evident in the 6 months prior to critical care unit admission. The following severe comorbidities were reported: very severe cardiovascular disease (fatigue, claudication, dyspnoea or angina at rest – New York Heart Association Functional Class IV); severe respiratory disease (permanent shortness of breath with light activity due to pulmonary disease or receipt of home ventilation); chronic renal failure (current requirement for chronic renal replacement therapy for irreversible renal disease); chronic liver disease (biopsy proven cirrhosis, portal hypertension or hepatic encephalopathy); haematological malignancy (acute or chronic myelogenous/lymphocytic leukaemia, multiple myeloma or lymphoma); metastatic disease (distant metastases, documented by surgery, imaging or biopsy); and immunocompromise due to disease or treatment (human immunodeficiency virus/acquired immunodeficiency syndrome, daily high-dose steroid treatment, radiotherapy, chemotherapy, or congenital immunohumoral or cellular immune deficiency state).
Acute severity of illness was measured using the APACHE II Score48 and the ICNARC Physiology Score49 assessed during the first 24 hours following admission to the critical care unit. The APACHE II score comprises the APACHE II Acute Physiology Score (weightings for 12 physiological variables: temperature, mean arterial pressure, heart rate, respiratory rate, oxygenation, pH, sodium, potassium, creatinine, haematocrit, white blood cell count and Glasgow Coma Score) plus weights for age and severe conditions in the past medical history. The ICNARC Physiology Score comprises objective weightings for 12 physiological variables (temperature, systolic blood pressure, heart rate, respiratory rate, oxygenation, pH, sodium, urea, creatinine, urine output, white blood cell count and Glasgow Coma Score).
The primary reason for admission to the critical care unit was recorded using the ICNARC Coding Method, a hierarchical system with five tiers: surgical status, body system, anatomical site, physiological or pathological process, and medical condition. 50 Admissions were grouped by medical and surgical primary reasons for admission according to body system.
Organ support consisted of advanced cardiovascular, advanced respiratory, renal, gastrointestinal and neurological support, defined according to the CCMDS. Advanced cardiovascular support was defined as receipt of multiple intravenous and/or rhythm-controlling drugs, of which at least one must be vasoactive, used simultaneously to support or control arterial pressure, cardiac output or organ/tissue perfusion; continuous cardiac output monitoring; intra-aortic balloon pump or ventricular assist device; or temporary cardiac pacemaker. Advanced respiratory support was defined as receipt of invasive mechanical ventilation; bilevel positive airway pressure (BiPAP) via a translaryngeal tracheal tube or tracheostomy; continuous positive airway pressure (CPAP) via a translaryngeal tracheal tube; or extracorporeal respiratory support. Renal support was defined as receipt of acute renal replacement therapy or chronic renal replacement therapy where other acute organ support was administered. Gastrointestinal support was defined as receipt of parenteral or enteral nutrition. Neurological support was defined as central nervous system depression sufficient to prejudice the airway and protective reflexes (not caused by intentional sedation or deliberate overdose) or receipt of invasive neurological monitoring or treatment (e.g. intracranial pressure monitoring, jugular bulb sampling, external ventricular drain); continuous intravenous medication to control seizures and/or for continuous cerebral monitoring; or therapeutic hypothermia.
Critical care unit mortality was defined as death in the original critical care unit. Ultimate acute hospital mortality was defined as death before final discharge from acute hospital and included deaths after direct transfer to another acute hospital from the hospital housing the critical care unit. Length of stay was reported as both critical care unit and total acute hospital stay. Total acute hospital stay included continuous stay in acute hospital, even if transferred from/to another acute hospital.
Statistical methods
Analyses were performed using Stata version 10.1 (StataCorp LP, College Station, TX, USA). Variables were summarised as either mean and standard deviation (SD) or median and IQR for continuous variables and as frequency and percentage for categorical variables. All analyses were univariable and no statistical testing was undertaken.
Results
Data on 60,778 admissions to 96 critical care units from July 2009 to March 2011 were analysed. In total, 383 patients (0.6%) were admitted with or developed IFD (Figure 4). The majority of IFD patients were positive for Candida species. Two-thirds of patients with Candida IFD were positive for Candida albicans. Candida glabrata was the most common Candida non-albicans species (16%) followed by Candida parapsilosis (3%), Candida tropicalis (3%) and Candida dubliniensis (1%). Of the non-Candida infections, six patients were positive for Aspergillus species, one patient for Geotrichum and two patients for Saccharomyces cerevisiae. Five patients were positive for multiple Candida species.
FIGURE 4.
Organogram of fungal species causing IFD. a, Percentages do not add up, as five admissions were infected by multiple Candida species.
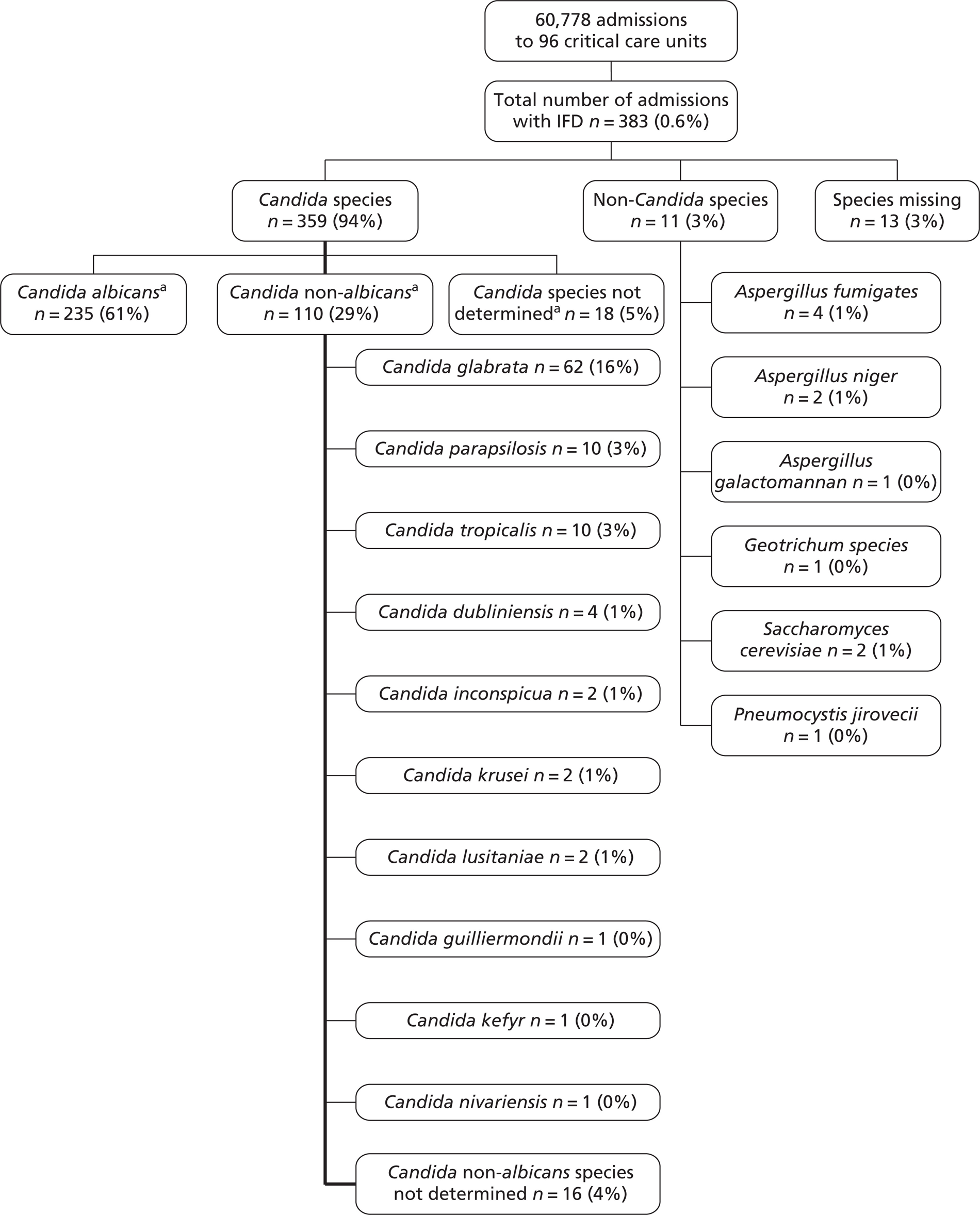
The most common IFD infection site was blood (55%) followed by peritoneal fluid (25%) and pleural fluid (10%) (Table 7). Seventy-five per cent of cases of IFD were identified in the critical care unit, with one-third of these being identified from samples taken during the first two calendar days (Figure 5). The median admission day for IFD identified in the critical care unit was day 4 (IQR 1 to 9), and for unit-acquired IFD (identified from a sample more than 48 hours after admission) was day 7 (IQR 4 to 12). The incidence of IFD identified in-unit was 4.7 cases per 1000 admissions, and of unit-acquired IFD was 3.2 cases per 1000 admissions. The incidence of IFD in blood was 3.5 cases per 1000 admissions.
| IFD infection sitesa | n (%) |
|---|---|
| Total number of IFD sites | 385 |
| Blood | 212 (55.4) |
| Peritoneal fluid | 96 (25.1) |
| Pleural fluid | 40 (10.4) |
| Tissue sample | 11 (2.9) |
| Intravascular catheter | 5 (1.3) |
| Pancreatic fluid | 3 (0.8) |
| Cerebrospinal fluid | 1 (0.3) |
| Pericardial fluid | 1 (0.3) |
| Other site | 16 (4.2) |
FIGURE 5.
Timing of IFD. 0, calendar day of admission; P, pre-admission. Values above bars indicate sample size.
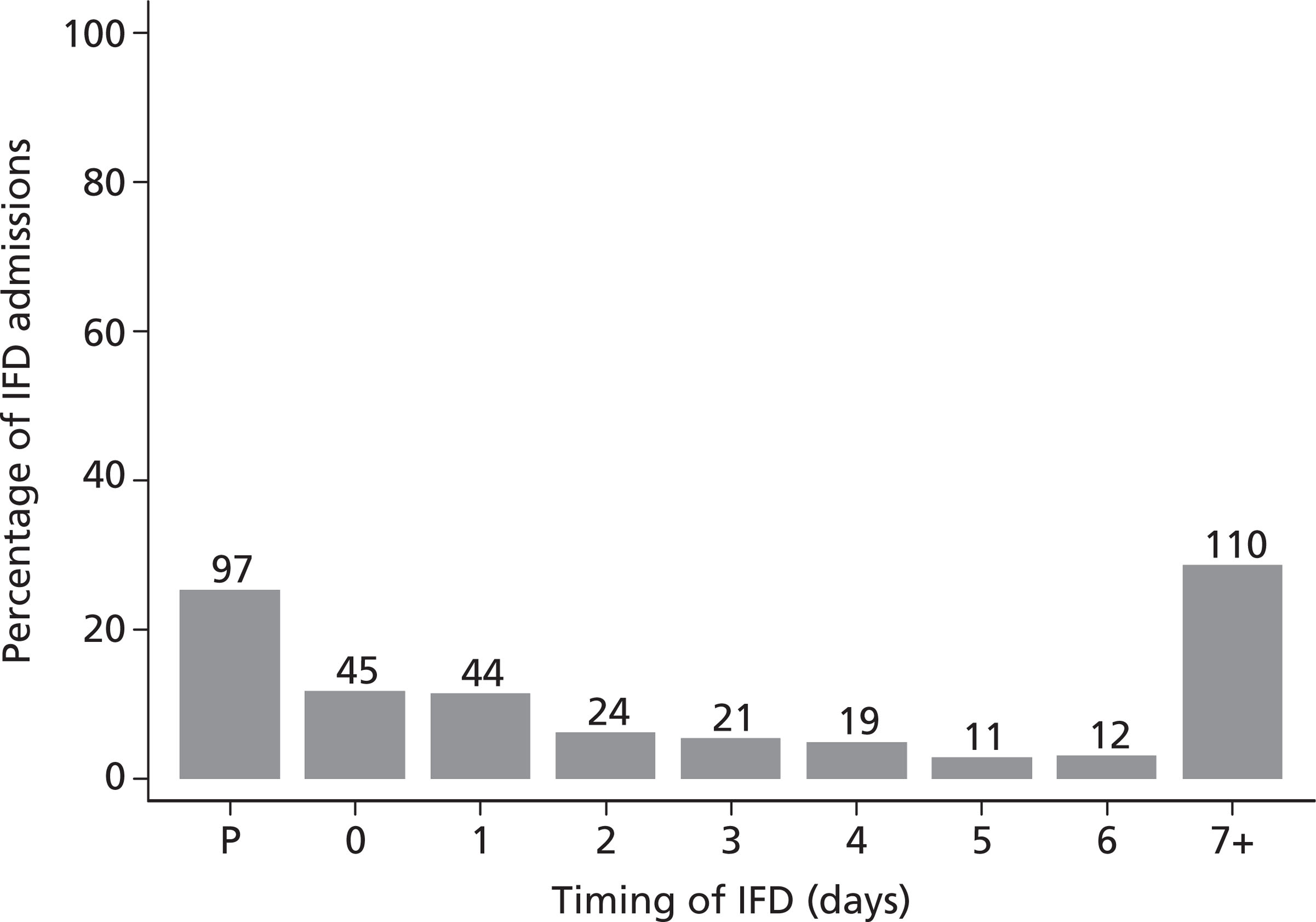
Patients admitted with Candida non-albicans IFD were similar to those with Candida albicans IFD in terms of age, sex and acute severity of illness on admission to the critical care unit (Table 8). The Candida non-albicans IFD group had more severe comorbidities, with 26% of admitted patients having a prior illness compared with only 15% in the Candida albicans IFD group. Specifically, the Candida non-albicans IFD group demonstrated higher rates of very severe cardiovascular disease, haematological malignancy and immunocompromise. Chronic renal failure was more common in the Candida albicans IFD group. The primary reasons for admission were also similar, with similar proportions of medical and surgical admissions. The main differences in case mix between patients with Candida species IFD and those with no IFD were the lower proportion of admissions following elective/scheduled surgery and higher severity of illness scores among patients with Candida species IFD.
| Case mix factors | Candida albicans IFD | Candida non-albicans IFD | No IFD |
|---|---|---|---|
| No. of admissions | 235 | 106 | 60,362 |
| Demographics | |||
| Median age (IQR) | 61 (49 to 71) | 62 (50, 73) | 64 (48 to 74) |
| Male sex (%) | 123 (52.3) | 60 (56.6) | 33,613 (55.7) |
| Past medical history, n (%) | |||
| Severe comorbidities | |||
| Any severe comorbidity | 34 (14.5) | 27 (25.5) | 10,142 (17.0) |
| Very severe cardiovascular disease | 2 (1.3) | 4 (5.1) | 1021 (2.5) |
| Severe respiratory disease | 5 (3.2) | 1 (1.3) | 1650 (4.0) |
| Chronic renal disease | 6 (3.8) | 2 (2.6) | 1122 (2.7) |
| Chronic liver disease | 9 (5.7) | 6 (7.6) | 1909 (4.7) |
| Metastatic disease | 2 (1.3) | 1 (1.3) | 1421 (3.5) |
| Haematological malignancy | 3 (1.9) | 5 (6.4) | 1064 (2.6) |
| Immunocompromise | 16 (10.2) | 14 (18.0) | 4092 (10.0) |
| Diabetes mellitus | 36 (15.3) | 20 (18.9) | 9608 (15.9) |
| Neutropenia | 7 (3.7) | 2 (2.5) | 930 (1.8) |
| Surgery within up to 7 days prior to admission, n (%) | |||
| Emergency/urgent | 91 (38.7) | 29 (27.4) | 13,127 (21.7) |
| Scheduled/elective | 27 (11.5) | 15 (14.2) | 14,987 (24.8) |
| No surgery | 117 (49.8) | 62 (58.5) | 32,230 (53.4) |
| Acute severity of illness, mean (SD) | |||
| APACHE II Score | 19.0 (6.6) | 19.6 (7.5) | 16.0 (7.0) |
| ICNARC Physiology Score | 23.1 (8.8) | 22.4 (9.2) | 17.3 (9.3) |
| Primary reason for admission to the critical care unit, n (%) | |||
| Medical | 126 (51.3) | 60 (62.5) | 32,114 (52.2) |
| Respiratory | 52 (22.4) | 24 (23.1) | 12,022 (21.0) |
| Cardiovascular | 28 (12.1) | 11 (10.6) | 5622 (9.8) |
| Gastrointestinal | 15 (6.5) | 13 (12.5) | 2519 (4.4) |
| Neurological | 6 (2.6) | 1 (1.0) | 4697 (8.2) |
| Other | 25 (10.8) | 11 (10.6) | 7254 (12.7) |
| Surgical | 118 (50.2) | 44 (41.5) | 28,131 (46.6) |
| Cardiovascular | 76 (32.8) | 37 (35.6) | 13,280 (23.2) |
| Gastrointestinal | 13 (5.6) | 5 (4.8) | 4201 (7.3) |
| Other | 17 (7.2) | 2 (1.9) | 7601 (13.3) |
With respect to therapies and outcomes, the Candida non-albicans IFD group was similar to the Candida albicans IFD group (Table 9). In comparison with patients admitted with no IFD, those with IFD were more likely to receive TPN, a systemic antimicrobial, immunosuppressives and a CVC. They were also more likely to receive organ support, with substantially higher rates of cardiovascular, respiratory, renal and gastrointestinal support. The critical care unit and acute hospital lengths of stay and mortality rates were similar between the Candida albicans and non-albicans IFD groups but substantially higher when compared with admissions with no IFD. Overall crude critical care unit and acute hospital mortality for admissions with any Candida species IFD were 29.9% and 39.6%, respectively.
| Therapies and outcomes | Candida albicans IFD | Candida non-albicans IFD | No IFD |
|---|---|---|---|
| No. of admissions | 235 | 106 | 60,362 |
| Therapies received,an (%) | |||
| TPN | 44 (18.7) | 18 (17.0) | 2153 (3.6) |
| Systemic antimicrobial use | 222 (94.5) | 104 (98.1) | 51,194 (84.8) |
| Immunosuppressive use | 71 (30.2) | 39 (36.8) | 12,476 (20.7) |
| CVC | 221 (94.0) | 95 (89.6) | 36,387 (60.3) |
| Organ support,bn (%) | |||
| Advanced cardiovascular support | 123 (52.3) | 52 (49.1) | 15,584 (25.8) |
| Advanced respiratory support | 199 (84.7) | 87 (82.1) | 31,380 (52.0) |
| Renal support | 96 (40.9) | 34 (32.1) | 7123 (11.8) |
| Gastrointestinal support | 201 (85.5) | 84 (79.3) | 23,139 (38.3) |
| Neurological support | 23 (9.8) | 9 (8.5) | 6612 (11.0) |
| Fungal colonisation,cn (%) | |||
| No (including no samples taken) | 17 (7.2) | 4 (3.8) | 54,193 (89.8) |
| Pre-admission | 39 (16.6) | 25 (23.6) | 765 (1.3) |
| Identified in unit | 179 (76.2) | 77 (72.6) | 5404 (9.0) |
| Mortality, deaths (%) | |||
| Critical care unit mortality | 82 (34.9) | 30 (28.3) | 10,047 (16.6) |
| Acute hospital mortality | 93 (49.5) | 42 (47.7) | 13,926 (24.5) |
| Length of stay (days), median (IQR) | |||
| Critical care unit stay | 12 (6 to 24) | 11 (5 to 25) | 2 (1 to 5) |
| Unit survivors | 12 (6 to 25) | 12 (6 to 26) | 2 (1 to 5) |
| Unit non-survivors | 12 (7 to 23) | 10 (3 to 25) | 2 (1 to 6) |
| Acute hospital stay | 33 (15 to 58) | 40 (20 to 73) | 13 (6 to 27) |
| Acute hospital survivors | 48 (31 to 79) | 51 (34 to 82) | 14 (7 to 29) |
| Acute hospital non-survivors | 19 (11 to 42) | 29 (10 to 63) | 8 (2 to 19) |
Use of topical and systemic antifungal agents was similar for the Candida albicans and non-albicans IFD groups (Table 10). Overall, 18% of IFD cases received topical antifungals and 80% received systemic antifungals, compared with 5% and 7% for those with no IFD. The most commonly prescribed antifungal agent was fluconazole (Table 11) with 74% of the Candida albicans cases and 58% of Candida non-albicans cases receiving the medication. The most common echinocandin, and second most commonly prescribed antifungal, was caspofungin. A greater proportion of Candida non-albicans cases than Candida albicans cases received caspofungin (37% vs 24%). The majority of admissions with IFD received a systemic antifungal agent either before or within 3 days of the sample from which IFD was first identified (Table 12). Use of antifungal prophylaxis was low (Figure 6). Overall, just under 5% of admissions received systemic antifungal therapy during the first 3 calendar days in the critical care unit either prior to, or in absence of, IFD. There were 44 cases of IFD among patients who received antifungal prophylaxis, suggesting that if there had been no use of antifungal prophylaxis there may have been an additional 44 cases (based on a RR of IFD associated with antifungal prophylaxis of 0.519), giving an estimated rate of IFD in the absence of prophylaxis of 0.70%.
| Use of topical and systemic antifungal therapy | Candida albicans IFD, n (%) | Candida non-albicans IFD, n (%) | No IFD, n (%) |
|---|---|---|---|
| Topical antifungal therapy | |||
| No | 190 (80.9) | 91 (85.9) | 57,405 (95.1) |
| Pre-admission | 13 (5.5) | 2 (1.9) | 678 (1.1) |
| In-unit | 32 (13.6) | 13 (12.3) | 2261 (3.8) |
| Systemic antifungal therapy | |||
| No | 48 (20.4) | 20 (18.9) | 56,063 (92.9) |
| Pre-admission | 33 (14.0) | 12 (11.3) | 1097 (1.8) |
| In-unit | 154 (65.5) | 74 (69.8) | 3185 (5.3) |
| Initial systemic antifungal regimen | Candida albicans IFD, n (%a) | Candida non-albicans IFD, n (%a) | No IFD, n (%a) |
|---|---|---|---|
| No. of admissionsb | 175 | 83 | 3894 |
| Azoles | |||
| Fluconazole | 130 (74.3) | 48 (57.8) | 3,174 (81.5) |
| Voriconazole | 2 (1.1) | 2 (2.4) | 121 (3.1) |
| Itraconazole | 0 | 0 | 77 (2.0) |
| Posaconazole | 0 | 0 | 8 (0.2) |
| Ketoconazole | 0 | 0 | 2 (0.1) |
| Echinocandins | |||
| Caspofungin | 42 (24.0) | 31 (37.3) | 417 (10.7) |
| Anidulafungin | 6 (3.4) | 2 (2.4) | 34 (0.9) |
| Micafungin | 0 | 0 | 4 (0.1) |
| Polyenes | |||
| Ambisome | 9 (5.1) | 6 (7.2) | 187 (4.8) |
| Amphotericin B | 3 (1.7) | 0 | 38 (1.0) |
| Other | |||
| Flucytosine | 0 | 1 (1.2) | 8 (0.2) |
| Timing of systemic antifungal therapy | Candida albicans IFD, n (%a) | Non-albicansCandida IFD, n (%a) |
|---|---|---|
| Before first IFD sample (including pre-admission) | 41 (29.3) | 24 (38.1) |
| Between 0 and 3 days of first positive sample | 70 (50.0) | 18 (28.6) |
| More than 3 days after first positive sample | 29 (20.7) | 21 (33.3) |
FIGURE 6.
Current use of antifungal prophylaxis and potential impact of antifungal prophylaxis on observed outcome. a, ‘Antifungal prophylaxis’ defined as use of systemic antifungal therapy prior to the end of calendar day 3 in the absence of IFD; b, assuming a RR of IFD of 0.5 associated with antifungal prophylaxis. 19
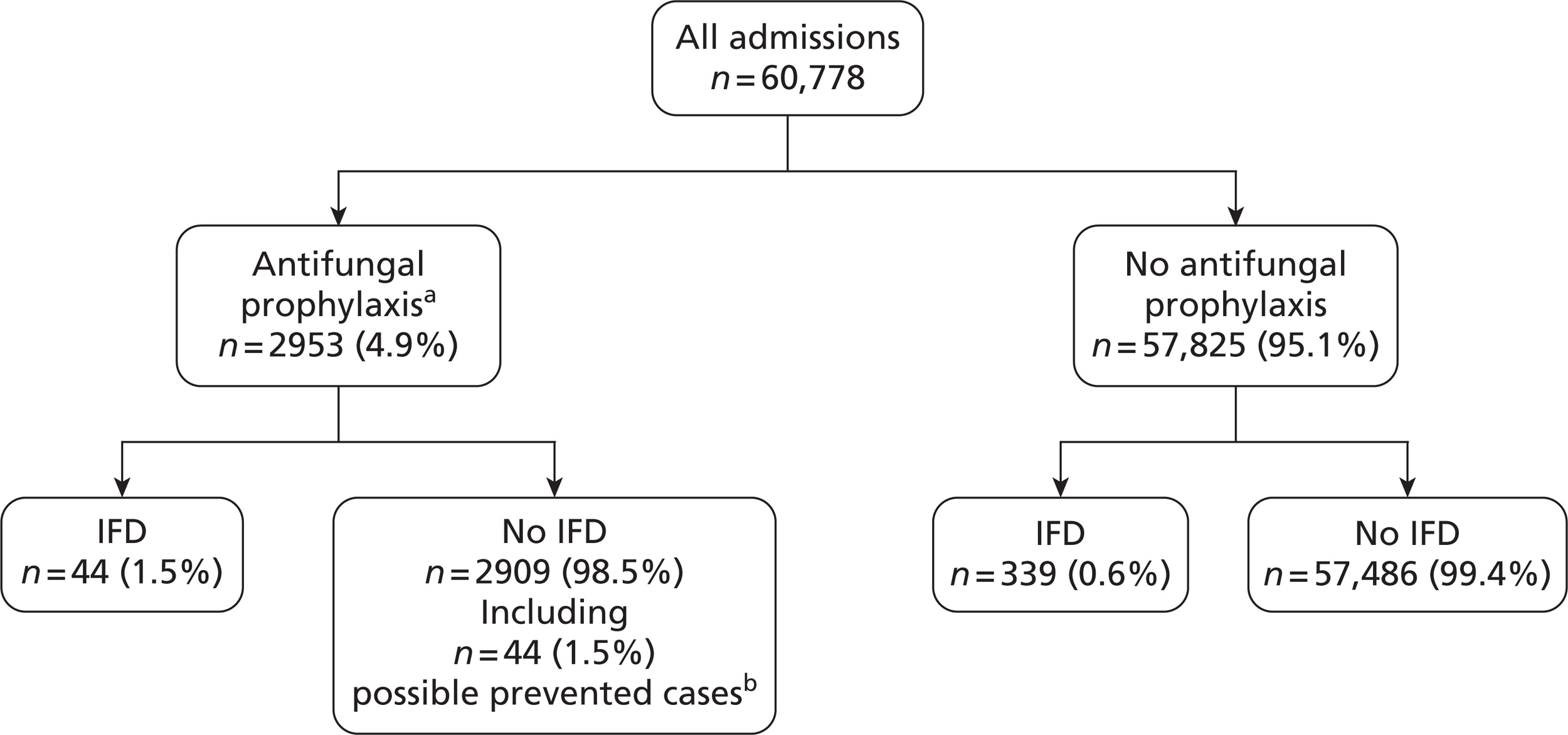
Discussion
These results describe the current epidemiological profile of IFD in UK critical care units. This is the first multicentre study, to our knowledge, to report specifically on IFD in UK critical care. Candida albicans accounted for 61% of all IFD. Blood was the most common infection site, accounting for more than half of all IFD. Case mix, therapies and outcomes were similar between Candida albicans and Candida non-albicans IFD. However, patients with Candida non-albicans IFD had more comorbidities than those with Candida albicans IFD. Compared with patients without IFD, patients with IFD experienced greater severity of illness scores, higher rates of therapies and organ support, longer length of unit and hospital stays, and almost double the mortality rates.
The strengths of this study are the following. First, the large sample of critical care units and admissions enrolled ensures that the analysis is unlikely to be biased by a particular unit's practice pattern or infection profile. Second, the inclusion of a control group (admissions without IFD) allows a better comparison and understanding of clinical characteristics associated with IFD. Third, the collection of prospective, clinical data from a cohort of admissions to critical care units, as opposed to data extracted from a laboratory database (a common study design in fungal epidemiological studies), allowed for the analysis of clinically orientated data.
The limitations of the study are mainly due to the difficulties surrounding diagnoses of fungal infections. The definitions chosen for IFD were aimed mainly at Candida infections, and were potentially insensitive to other IFD, which may be more difficult to identify from microbiological and histological samples. This would account for the low rates of non-Candida species, such as Aspergillus and Cryptococcus. The IFD definitions were chosen so as to best capture Candida IFD, as the primary focus of the FIRE study was to develop a risk model that could be used to identify non-neutropenic, critically ill, patients at high risk of invasive Candida infection. In addition, admissions were reported as having IFD based only on microbiological/histological samples taken while the patient was in the critical care unit. Other studies have often included samples taken for up to 48 hours following discharge from the critical care unit (as an infection identified during the subsequent 48 hours would be considered ‘unit acquired’). This approach was not considered practical for the very large numbers of admissions included in this study.
It is likely that the associations between IFD and therapy/outcomes identified in this analysis represent a combination of both risk factors for and consequences of IFD. For example, increased duration of critical care unit stay increases exposure and is therefore likely to be associated with higher rates of IFD; however, IFD is also likely to increase the subsequent length of stay in critical care. Analyses considering only risk factors measured prior to the diagnosis of IFD are included in subsequent chapters.
The incidence of IFD in FIRE Study was lower than had been anticipated in the sample size calculation (see Chapter 3) based on previous UK hospital-wide data. 4,5 However, the results of this study are consistent with the wider current literature on Candida infections. Incidence rates of critical care unit candidaemia in previous studies have varied from 1.1 to 94 cases per 1000 admissions, depending upon the geographic location of the unit, setting and inclusion criteria for the study. 25,51–56 The rate of IFD in blood in the present study, at 3.5 per 1000 admissions, is similar to that of other European critical care units. 51,54,57 A previous hospital-wide surveillance from six sentinel hospitals in the UK identified that 45% of candidaemia was reported from the critical care unit, corresponding with an incidence of 7.4 per 1000 admissions. 5 A recent prospective study in 24 French critical care units demonstrated a candidaemia incidence of 6.7 per 1000 admissions. 58 The lower incidence in the FIRE Study than anticipated from previous UK data may therefore simply be a reflection of more representative data than in the earlier studies. However, recent data from the HPA have suggested a decline in the number of Candida bloodstream infections,59 which may be the result of improved antibiotic stewardship and infection control practices in response to mandatory reporting of certain health care-associated infections. Such a trend would be further enhanced by the dilution effect of increasing critical care capacity and associated increasing numbers of critical care unit admissions. 60
The distribution of Candida species in the present study is also similar to that of other critical care units in Western Europe. 57 A recent retrospective analysis of the EPIC II study (Extended Prevalence of Infection in the ICU), examining Candida bloodstream infections in 14,414 patients to 1265 critical care units in 76 countries, demonstrated varying proportions of Candida albicans and Candida non-albicans infections. Seventy-two per cent of the Candida infections in Western European units were due to Candida albicans, compared with 69% in the present study and 79% in the previous UK sentinel hospital study. 5 Variations in proportions of Candida non-albicans infections may be due to differential use of fluconazole prophylaxis and subsequent emergence of resistant strains. 61 An analysis of Candida specimens to the Communicable Disease Surveillance Centre from England and Wales between 1990 and 1999 found that Candida albicans was responsible for 60% of all clinically significant specimens. 2 Annual reporting between 1990 and 1999 showed increasing rates of reported Candida specimens. However, more recent data from the HPA have shown a decline in both the total number of Candida bloodstream infections and the proportion of these due to Candida albicans (51% in 2010). 59
Crude mortality in the present study was generally lower than reported in the literature for admissions to a critical care unit with IFD. 51,54,57,62 The reported crude mortality varied from 48% to 58%, whereas the acute hospital mortality in the present study was 40%. The discrepancy in mortality rates may be accounted for by differences in case mix, secular variation in critical care therapies or rates of fungal prophylaxis.
In summary, incidence of IFD in UK critical care units in this study was consistent with the wide range reported from other European epidemiological studies, but lower than that suggested by previous hospital-wide surveillance in the UK during the 1990s.
Chapter 5 Development and validation of risk models for invasive Candida infection
Introduction
This chapter describes the development and validation of risk models for invasive Candida infection at three time points – admission to the critical care unit, 24 hours after admission, and the end of the third calendar day – using classical statistical approaches.
Methods
Development and validation samples
The FIRE Study data set was divided into the following groups to form the development and validation samples for the risk models:
-
Development sample All admissions to a random sample of participating critical care units in England, Wales and Northern Ireland, from July 2009 to December 2010 (approximately 40,000 admissions).
-
Random validation sample All admissions to the remaining participating critical care units in England, Wales and Northern Ireland (approximately 5000 admissions).
-
Temporal validation sample All admissions to critical care units in the development sample, from January to March 2011 (approximately 10,000 admissions).
-
Geographic validation sample All admissions to participating critical care units in Scotland (approximately 5000 admissions).
Patient inclusion/exclusion criteria
Data for the FIRE Study were collected on all admissions to participating critical care units. The following exclusions were applied to the data set for the development and validation of risk models.
For model 1, at admission to the critical care unit:
-
age < 18 years on admission;
-
second and subsequent admissions of the same patient during the same acute hospital stay
-
neutropenia (absolute neutrophil count < 1 × 109/l)
-
active haematological malignancy evident during the 6 months prior to admission to the critical care unit
-
admission to the critical care unit following solid organ transplant
-
IFD identified within up to 7 days prior to admission to the critical care unit; and
-
receipt of systemic antifungals within up to 7 days prior to admission to the critical care unit.
For model 2, at 24 hours following admission to the critical care unit, as model 1 plus:
-
death or discharge from the critical care unit within 24 hours
-
IFD identified during the first 24 hours following admission to the critical care unit; and
-
receipt of systemic antifungals during the first 24 hours following admission to the critical care unit.
For model 3, at the end of the third calendar day following admission to the critical care unit, as model 2, plus:
-
death or discharge from the critical care unit before the end of the third calendar day
-
IFD identified before the end of the third calendar day following admission to the critical care unit; and
-
receipt of systemic antifungals before the end of the third calendar day following admission to the critical care unit.
Selection of candidate variables
Variables considered for the three models were taken from the FIRE Study database having been selected based on their association with IFD in previous literature and on expert clinical opinion. Owing to the low number of events, and to maximise the number of EPV, each potential variable included in the data set was considered for inclusion in the modelling process with selection of candidate variables based on the strength of evidence in the existing literature (see Chapter 2), prevalence, data completeness, and consideration of whether or not the same concept was better captured by alternative variables. Risk factor data for interventions, organ support, therapies and fungal colonisation were updated using additional data available at the second and third time points (24 hours and the end of the third calendar day following admission to the critical care unit). At the second and third time points, candidate variables were included in the full model if they were either included or close to being included in the final model from the previous time point or if the prevalence of the risk factor had increased substantially from the previous time point. Physiological variables were only available from the first 24 hours following admission to the critical care unit; they were therefore introduced at the 24-hour time point and retained in the model at the end of the third calendar day (still using data from the first 24 hours) if included in the final model at 24 hours.
Handling of missing data
Extensive data validation was used to ensure that the data were as complete as possible (see Chapter 3). A complete case analysis was undertaken when the proportion of missing data was low (< 1%). Candidate predictors with substantial numbers of missing data were excluded if the problem was thought likely to be due to inherent difficulties in data collection, rendering any model using such variables difficult to apply in practice. When the proportion of missing data was larger than 1% but not considered to be a problem likely to recur then patients without evidence indicating the presence of the risk factor were assumed not to have it.
Model development
Logistic regression models were derived to model the risk of subsequently developing invasive Candida infection based on information available at the three time points. Robust standard errors were used to allow for clustering within critical care units. Candidate variables were identified and alternative approaches to modelling each individual risk factor were compared and evaluated in univariable analyses. All candidate variables were then included in a ‘full’ multivariable model and the model was progressively simplified using backwards stepwise selection with the least statistically significant being removed at each step.
Variables that remained in the final admission model were considered for inclusion in the full 24-hour model along with additional physiology variables measured only after admission and variables where lack of effect in the admission model might have been due to low prevalence. Variables updated at each time point were updated in each model. The surgery variable was recoded at this stage to combine the ‘elective with no complications’ and ‘elective with complications’ categories. A similar approach was taken to decide on candidate variables for the 3-day model.
At each stage the model was fitted in the development sample and the performance of the model was assessed. Model discrimination was assessed with the c-index,30 equivalent to the area under the ROC curve,31 calibration by graphical plots of observed against expected risk, and overall fit by Brier's score. 63
Internal validation
Bootstrapping with 200 bootstrap samples was used on the development sample to internally validate the final selected model at each time point and to estimate optimism adjusted measures of the model discrimination and overall fit. 64 Overfitting is a phenomenon whereby the process of selecting variables for a risk model and estimating the coefficients results in a model that has very good fit in the data set used to develop the model but will fit less well when applied to other data. The bootstrap procedure refits the model in each bootstrap sample and compares the performance of the refitted model in the bootstrap sample with that of the refitted model in the original development data set. This gives, for each bootstrap sample, an estimate of the optimism in each of the measures of model performance that can be averaged and subtracted from the original values to give optimism-adjusted measures.
External validation
The final selected model at each time point was evaluated in the three external validation samples, chosen to test different aspects of future performance. Evaluation in the random validation sample, drawn from a random selection of critical care units withheld from the development sample, tested the performance of the model when applied to different settings within the same health system and time period. The temporal validation sample, drawn from the final 3 months' data collection from the critical care units in the development sample, tested the performance of the model in the same critical care units over time. Finally, the geographical validation sample, drawn from critical care units in Scotland, tested the performance of the model when applied in a different geographical region with a different (but similar) health-care system. The models were evaluated in each validation sample separately and then in all three samples combined. Model performance in the validation samples was assessed using the same measures as in the development sample.
Comparison with existing clinical decision rules
The performance of the risk models at the end of calendar day 3 was compared with that of existing clinical decision rules identified from the systematic literature review (see Chapter 2) using the full validation data set. Models at earlier time points were not considered, as all existing rules had been developed using only patients with stays in the critical care unit of at least 3 days. For comparison with the clinical decision rules, three alternative thresholds were applied to the risk predictions FIRE Study models: 0.5%, 1% and 2%. The corresponding clinical decision rules have been identified as F1, F2 and F3, respectively. The following existing clinical decision rules were included in the comparison:
-
Three rules presented in Ostrosky-Zeichner et al. :27
-
(any antibiotic use days 1 to 3) and (central venous catheter days 1 to 3) OZ1
-
(any antibiotic use days 1 to 3) and (central venous catheter days 1 to 3) and (at least one of surgery days −7 to 0, immunosuppressive use days −7 to 0, pancreatitis, TPN days 1 to 3, renal support days 1 to 3, steroid use days −7 to 3) OZ2
-
[(any antibiotic use days 1 to 3) or (central venous catheter days 1 to 3)] and (at least two of surgery days −7 to 0, immunosuppressive use days −7 to 0, pancreatitis, TPN days 1 to 3, renal support days 1 to 3, steroid use days −7 to 3) OZ3
-
-
Three rules presented in Paphitou et al. :28
-
(at least one of diabetes, TPN days −7 to 0, renal support days 1 to 3) P1
-
(at least one of diabetes, TPN days –7 to 0, renal support days 1 to 3, broad-spectrum antibiotic use days –7 to 3) P2
-
(at least one of diabetes, TPN days –7 to 0, renal support days 1 to 3) and (broad-spectrum antibiotic use days –7 to 3) P3
-
Here days –7 to 0 denotes the time period within up to 7 days prior to admission to the critical care unit, days 1 to 3 denotes the time period from admission to the end of the third calendar day, and days –7 to 3 denotes the entire time period from up to 7 days prior to admission until the end of the third calendar day following admissions. For applying these rules in the FIRE Study data set, antibiotic use within up to 7 days prior to admission to the critical care unit was approximated by the last antibiotic use prior to admission, and antibiotic use by the end of calendar day 3 following admission to the unit was approximated by the first antibiotic use following admission.
Clinical decision rules were assessed for sensitivity (the proportion of admissions with subsequent invasive Candida infection identified as being high risk), specificity (the proportion of admissions without subsequent invasive Candida infection identified as being low risk), PPV (the proportion of those identified as high risk who subsequently developed invasive Candida infection) and NPV (the proportion of those identified as low risk who did not subsequently develop invasive Candida infection).
Results
Development and validation samples
The data set was divided into development and validation samples as follows:
-
development sample 39,685 admissions to 70 units
-
random validation sample 4669 admissions to 10 units
-
temporal validation sample 11,051 admissions to 66 units
-
geographic validation sample 5373 admissions to 16 units.
Patient inclusion/exclusion criteria
Figure 7 illustrates the selection of admissions for inclusion in the risk models at the three time points. Following exclusions, validated data on 35,455 admissions were available for the development of the admission model with data available on a further 18,834 for validation. Similarly data were available on 26,540 admissions for the development of the 24-hour model with data on a further 13,832 for validation. Finally, data were available on 16,405 for the development of the 3-day model with 8488 for validation. Table 13 describes the populations included in the development and validation samples for each of the three models.
FIGURE 7.
Flow diagram of patient inclusion/exclusion for model development and validation. D, development sample; V, full validation sample; VG, geographical validation sample; VR, random validation sample; VT, temporal validation sample.
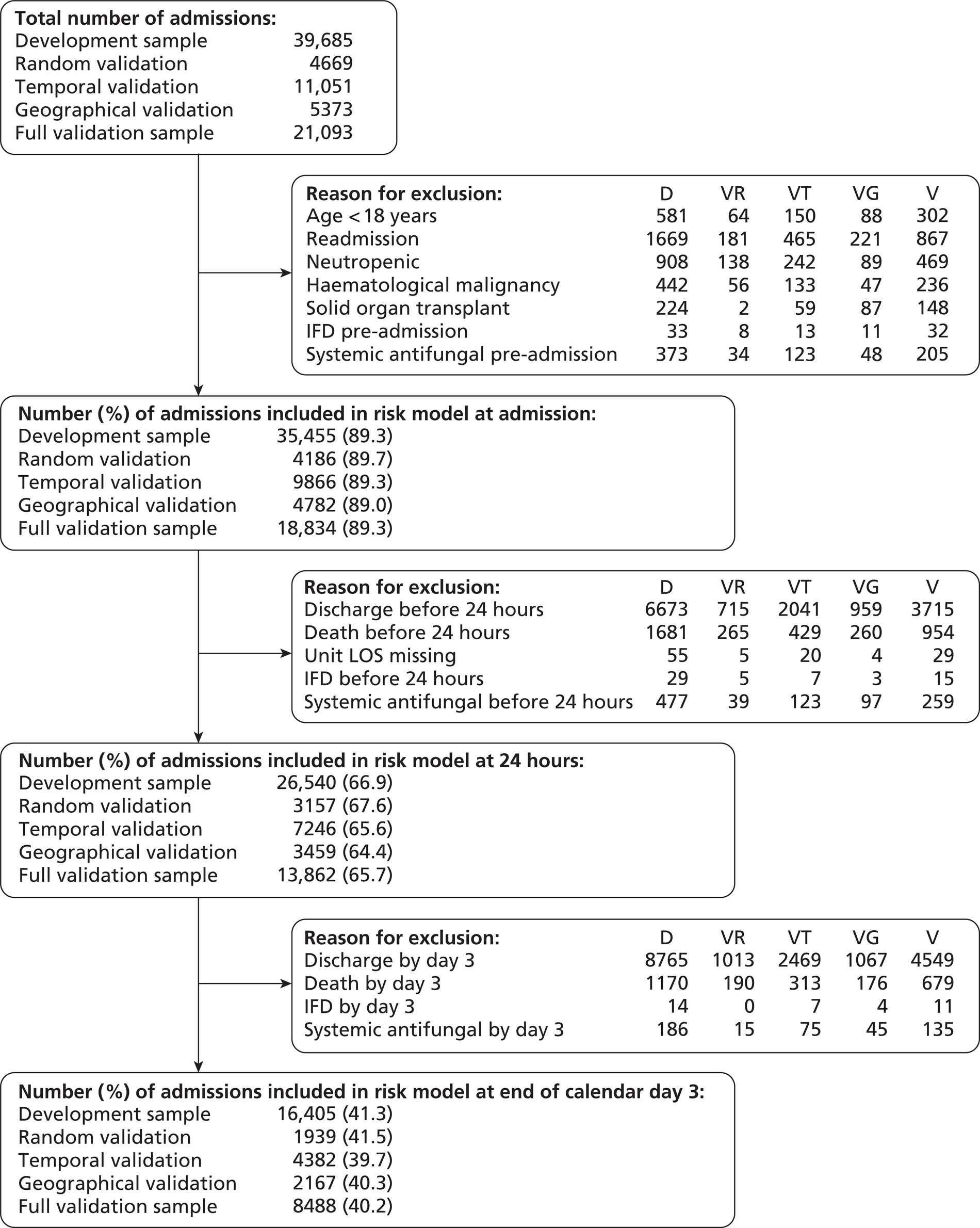
| Characteristics | Development sample | Random validation sample | Temporal validation sample | Geographical validation sample | Full validation sample |
|---|---|---|---|---|---|
| Patients | 35,455 | 4186 | 9866 | 4782 | 18,834 |
| Critical care units | 70 | 10 | 66 | 16 | 92 |
| Age (years), mean (SD) | 61.4 (17.6) | 61.4 (18.1) | 61.4 (17.4) | 57.3 (17.4) | 60.4 (17.7) |
| Male, n (%) | 19,648 (55.4) | 2247 (53.4) | 5474 (55.5) | 2720 (56.9) | 10,441 (55.4) |
| Surgery within up to 7 days prior to admission | |||||
| Elective/scheduled | 9699 (27.4) | 738 (17.6) | 2700 (27.4) | 905 (18.9) | 4343 (23.1) |
| Emergency/urgent | 7651 (21.6) | 883 (21.1) | 2088 (21.2) | 1136 (23.8) | 4107 (21.8) |
| No surgery | 18,095 (51.1) | 2564 (61.3) | 5069 (51.4) | 2741 (57.3) | 10,374 (55.1) |
| ICNARC Physiology Score,a mean (SD) | 17.0 (9.3) | 19.2 (9.8) | 17.0 (9.3) | 13.9 (7.8) | 16.7 (9.2) |
| APACHE II Score,a mean (SD) | 15.8 (6.9) | 16.4 (7.1) | 15.6 (6.8) | 15.2 (6.9) | 15.7 (6.9) |
| Invasive Candida infection, n (%) | 144 (0.41) | 11 (0.26) | 42 (0.43) | 19 (0.40) | 72 (0.38) |
| Characteristics | Development sample | Random validation sample | Temporal validation sample | Geographical validation sample | Full validation sample |
|---|---|---|---|---|---|
| Patients | 26,540 | 3157 | 7246 | 3459 | 13,862 |
| Critical care units | 70 | 10 | 66 | 16 | 92 |
| Age (years), mean (SD) | 61.7 (17.4) | 61.8 (17.7) | 61.5 (17.1) | 58.1 (16.8) | 60.7 (17.2) |
| Male, n (%) | 14,893 (56.1) | 1726 (54.7) | 4015 (55.4) | 1995 (57.7) | 7736 (55.8) |
| Surgery within up to 7 days prior to admission | |||||
| Elective/scheduled | 6494 (24.5) | 496 (15.7) | 1659 (22.9) | 602 (17.4) | 2757 (19.9) |
| Emergency/urgent | 5829 (22.0) | 659 (20.9) | 1581 (21.8) | 831 (24.0) | 3071 (22.2) |
| No surgery | 14,211 (53.6) | 2001 (63.4) | 3999 (55.2) | 2026 (58.6) | 8026 (57.9) |
| ICNARC Physiology Score,a mean (SD) | 17.5 (8.5) | 19.5 (8.9) | 17.7 (8.5) | 14.6 (7.2) | 17.3 (8.5) |
| APACHE II Score,a mean (SD) | 16.3 (6.6) | 16.7 (6.8) | 16.2 (6.5) | 15.3 (6.9) | 16.1 (6.7) |
| Invasive Candida infection, n (%) | 104 (0.39) | 6 (0.19) | 31 (0.43) | 15 (0.43) | 52 (0.38) |
| Characteristics | Development sample | Random validation sample | Temporal validation sample | Geographical validation sample | Full validation sample |
|---|---|---|---|---|---|
| Patients | 16,405 | 1939 | 4382 | 2167 | 8488 |
| Critical care units | 70 | 10 | 66 | 16 | 92 |
| Age, mean (SD) | 62.2 (16.9) | 62.8 (16.7) | 61.7 (16.6) | 59.3 (15.9) | 61.3 (16.5) |
| Male, n (%) | 9387 (57.2) | 1105 (57.0) | 2488 (56.8) | 1278 (59.0) | 4871 (57.4) |
| Surgery within up to 7 days prior to admission | |||||
| Elective/scheduled | 3101 (18.9) | 226 (11.7) | 708 (16.2) | 358 (16.5) | 1292 (15.2) |
| Emergency/urgent | 3789 (23.1) | 412 (21.2) | 973 (22.2) | 504 (23.3) | 1889 (22.3) |
| No surgery | 9514 (58.0) | 1301 (61.7) | 2698 (61.6) | 1305 (60.2) | 5304 (62.5) |
| ICNARC Physiology Score,a mean (SD) | 19.1 (8.2) | 20.9 (8.3) | 19.3 (8.2) | 15.7 (6.9) | 18.8 (8.1) |
| APACHE II Score,a mean (SD) | 17.1 (6.4) | 17.5 (6.4) | 17.0 (6.5) | 15.9 (6.7) | 16.8 (6.5) |
| Invasive Candida infection, n (%) | 85 (0.52) | 5 (0.26) | 22 (0.50) | 10 (0.46) | 37 (0.44) |
Selection of candidate variables
Table 14 lists all of the variables considered and the rationale for inclusion or not as candidate variables in the modelling process.
| Variables considered | Outcome/rationale |
|---|---|
| Age | Excluded as not supported by existing literature |
| Sex | Excluded as not supported by existing literature |
| Ethnicity | Excluded as not supported by existing literature |
| Diabetes mellitus | Included |
| Severe conditions in past medical history | Excluded as not sufficiently supported by existing literature |
| Surgery | Included |
| Condition requiring surgery | Included as gastrointestinal or not |
| Urgency of surgery | Included |
| Unexpected complications in surgery | Included |
| Open abdomen following surgery | Included |
| Admission for presurgical preparation | Included |
| Admission to the critical care unit from another acute hospital | Included |
| Pancreatitis | Included |
| Burns | Excluded as very low prevalence (0.3%) and not strongly supported by existing literature |
| No. of lines in arteries | Included |
| Major intra-arterial devices | Excluded as strongly collinear with arterial lines |
| No. of catheters in central veins | Included |
| Location(s) of catheters in central veins | Excluded as insufficient evidence that risk varies by location |
| Peripheral lines | Excluded as very high prevalence (93%) and not strongly supported by existing literature |
| No. of intracranial devices | Included |
| No. of drains | Included |
| Enteral feeding tube | Included |
| Urinary catheter | Included |
| Advanced respiratory support | Included |
| Renal support | Included |
| TPN | Included |
| Steroids | Included |
| Immunosuppressives | Included |
| Antimicrobials | Included |
| Fungal colonisation | Included |
| Temperature | Highest central temperature included (or non-central if no central temperature recorded) |
| Blood pressure | Lowest systolic blood pressure included |
| Heart rate | Highest included |
| Respiratory rate | Highest included |
| Oxygenation/pH | Excluded, as not sufficiently supported by existing literature |
| Serum sodium | Excluded, as not sufficiently supported by existing literature |
| Serum potassium | Excluded, as not sufficiently supported by existing literature |
| Serum glucose | Excluded, as not sufficiently supported by existing literature |
| Serum urea | Excluded, as associated risk most directly captured by receipt of renal support |
| Serum creatinine | Excluded, as associated risk most directly captured by receipt of renal support |
| Urine output | Excluded, as associated risk most directly captured by receipt of renal support and presence of urinary catheter |
| Haemoglobin | Excluded, as not sufficiently supported by existing literature |
| Platelet count | Lowest included |
| White blood cell count | Lowest included (note that patients with neutropenia excluded) |
| Severe sepsis | Excluded, as combination of physiological response (derived from above parameters) and evidence of infection (captured by receipt of antimicrobials) |
| APACHE II Score/ICNARC Physiology Score | Excluded, as considered more relevant to focus on specific physiological parameters |
Model development
The following modelling approaches were selected. Variables relating to surgery within up to 7 days prior to admission were combined and modelled as a single categorical variable with the following categories: no surgery, elective/scheduled surgery with no unexpected complications, elective/scheduled surgery with unexpected complications, and emergency/urgent surgery. An additional categorical variable was created representing gastrointestinal surgery with and without an open abdomen following surgery. Categorical variables were created for the number of lines in arteries (none, one, two or more), number of catheters in central veins (none, one, two or more), number of intercranial devices (none, one, two or more), number of drains (none, one to three, four or more) and number of microbiological samples positive for fungal colonisation (none, one, two or more).
For the model at 24 hours, highest central temperature (or non-central temperature if no central temperature available), highest respiratory rate, lowest systolic blood pressure, and highest heart rate were all included in the full model as continuous variables. However, those remaining in the final model (highest heart rate and lowest systolic blood pressure) were categorised to aid implementation of the model in practice. Highest heart rate was categorised above or below 100 beats per minute and lowest systolic blood pressure > and < 90 mmHg. This simplification resulted in a slight improvement in calibration. Lowest platelet count and lowest white blood cell count both had a higher proportion of missing data which was considered acceptable for a complete case analysis and were therefore categorised, before inclusion in the full model, to categories of lowest platelet count (above and below 190 × 109/l) and lowest white blood cell count (above and below 12 × 109/l). Patients with missing data for these variables were then assumed to be in the low-risk category (i.e. unmeasured laboratory values were expected to be normal). In the final models, the number of microbiological samples positive for fungal colonisation were recategorised as < 2 and ≥ 2 for the model at admission and as none and ≥ 1 for the models at 24 hours and the end of the third calendar day.
The full admission model (19 candidate variables with 27 parameters) had a c-index of 0.720 when fitted in the development sample. Following backwards elimination, the final admission model contained seven variables with 11 parameters (c-index 0.705). The full 24-hour model (16 candidate variables with 19 parameters) had a c-index of 0.828 when fitted in the development sample. Following backwards elimination, the final 24-hour model contained seven variables with 10 parameters (c-index 0.824). The full 3-day model (nine candidate variables with 13 parameters) had a c-index of 0.837 when fitted in the development sample. Following backwards elimination the final 3-day model contained five variables with seven parameters (c-index 0.835). Full details of the model selection process are reported in Table 15 and the final risk models are presented in Table 16. The distribution of predicted risk from the final risk models is shown in Figure 8, and the calibration of the final risk models based on three equal-sized groups on predicted risk is shown in Figure 9.
| Model | c-index | Brier's score |
|---|---|---|
| Full model | 0.720 | 0.0040 |
| Variables removed | ||
| Admission to the critical care unit from another acute hospital | 0.720 | 0.0040 |
| Diabetes mellitus | 0.720 | 0.0040 |
| TPN (pre-admission) | 0.720 | 0.0040 |
| Steroids (pre-admission) | 0.720 | 0.0040 |
| Immunosuppressives (pre-admission) | 0.719 | 0.0040 |
| Antimicrobials (pre-admission) | 0.716 | 0.0040 |
| Renal support (pre-admission) | 0.718 | 0.0040 |
| Urinary catheter (pre-admission) | 0.709 | 0.0040 |
| Arterial lines (pre-admission) | 0.710 | 0.0040 |
| Respiratory support (pre-admission) | 0.711 | 0.0040 |
| Intracranial devices (pre-admission) | 0.708 | 0.0040 |
| Gastrointestinal surgery/open abdomen (pre-admission) | 0.706 | 0.0040 |
| Final reduced modela | 0.705 | 0.0040 |
| Model | c-index | Brier's score |
|---|---|---|
| Full model | 0.827 | 0.0038 |
| Variables removed | ||
| Lowest platelet count (first 24 hours) | 0.827 | 0.0038 |
| Renal support (first 24 hours) | 0.828 | 0.0038 |
| Highest central temperature (first 24 hours) | 0.827 | 0.0038 |
| Lowest white blood cell count (first 24 hours) | 0.827 | 0.0038 |
| Enteral feeding tube (first 24 hours) | 0.824 | 0.0038 |
| Admission for presurgical preparation | 0.821 | 0.0038 |
| TPN (first 24 hours) | 0.820 | 0.0038 |
| Highest respiratory rate (first 24 hours) | 0.819 | 0.0038 |
| Final reduced modela | 0.824 | 0.0038 |
| Model | c-index | Brier's score |
|---|---|---|
| Full model | 0.837 | 0.0049 |
| Variables removed | ||
| TPN (admission to end of calendar day 3) | 0.836 | 0.0050 |
| Surgery (admission to end of calendar day 3) | 0.830 | 0.0050 |
| Renal support (admission to end of calendar day 3) | 0.832 | 0.0050 |
| Systolic blood pressure (first 24 hours) | 0.832 | 0.0050 |
| Final reduced modela | 0.835 | 0.0050 |
| Risk factor | Coefficient | 95% CI |
|---|---|---|
| Admission for presurgical preparation | 1.61 | 0.80 to 2.42 |
| Surgery within up to 7 days prior to admission | ||
| Elective/scheduled surgery with no unexpected complications | 0 | – |
| Elective/scheduled surgery with unexpected complications | 0.92 | −0.12 to 1.95 |
| Emergency/urgent surgery | 1.28 | 0.77 to 1.79 |
| No surgery | 1.72 | 1.07 to 2.37 |
| Pancreatitis | 1.39 | 0.56 to 2.21 |
| No. of catheters in central veins (pre-admission) | ||
| None | 0 | |
| 1 | 0.40 | 0.04 to 0.75 |
| 2 | 1.43 | 0.59 to 2.26 |
| No. of drains (pre-admission) | ||
| None | 0 | |
| 1–3 | 0.64 | 0.21 to 1.08 |
| 4 | 1.63 | 0.26 to 3.00 |
| Enteral feeding tube (pre-admission) | 0.42 | 0.04 to 0.79 |
| No. of samples positive for fungal colonisation (pre-admission) | ||
| None or 1 | 0 | |
| 2 | 2.06 | 0.43 to 3.68 |
| Constant | −7.47 | −8.05 to −6.89 |
| Risk factor | Coefficient | 95% CI |
|---|---|---|
| Surgery within up to 7 days prior to admission | ||
| Elective/scheduled surgery | 0 | |
| Emergency/urgent surgery | 0.89 | 0.25 to 1.53 |
| No surgery | 0.89 | 0.11 to 1.67 |
| Pancreatitis | 1.22 | 0.29 to 2.15 |
| No. of catheters in central veins (first 24 hours) | ||
| None | 0 | |
| 1 | 1.35 | 0.42 to 2.28 |
| 2 | 2.60 | 1.70 to 3.51 |
| No. of drains (first 24 hours) | ||
| None | 0 | |
| 1–3 | 0.71 | 0.34 to 1.08 |
| 4 | 2.09 | 0.85 to 3.32 |
| Lowest systolic blood pressure (first 24 hours) < 90 mmHg | 0.55 | 0.19 to 0.90 |
| Highest heart rate (first 24 hours) ≥ 100 beats per minute | 0.85 | 0.39 to 1.32 |
| No. of samples positive for fungal colonisation (first 24 hours) | ||
| None | 0 | |
| 1 | 1.87 | 1.45 to 2.29 |
| Constant | −8.96 | −10.04 to −7.89 |
| Risk factor | Coefficient | 95% CI |
|---|---|---|
| Pancreatitis | 1.10 | 0.28 to 1.92 |
| No. of catheters in central veins (days 1–3) | ||
| None | 0 | |
| 1 | 1.29 | 0.10 to 2.49 |
| 2 | 2.69 | 1.49 to 3.90 |
| No. of drains (days 1–3) | ||
| None | 0 | |
| 1–3 | 0.66 | 0.23 to 1.08 |
| 4 | 2.03 | 0.96 to 3.10 |
| Highest heart rate (first 24 hours) ≥ 100 beats per minute | 0.79 | 0.22 to 1.36 |
| No. of samples positive for fungal colonisation (days 1–3) | ||
| None | 0 | |
| 1 | 2.11 | 1.41 to 2.80 |
| Constant | −8.02 | –9.14 to –6.89 |
FIGURE 8.
Distribution of predicted risk from the final risk models in the development and validation samples. Patients with predicted risk of > 5% not shown: 65 (0.1%) at admission (44 development, 21 validation); 198 (0.5%) at 24 hours (119 development, 79 validation); 296 (1.2%) at end of calendar day 3 (170 development, 126 validation).
FIGURE 9.
Calibration of the final risk models in the development and validation samples.
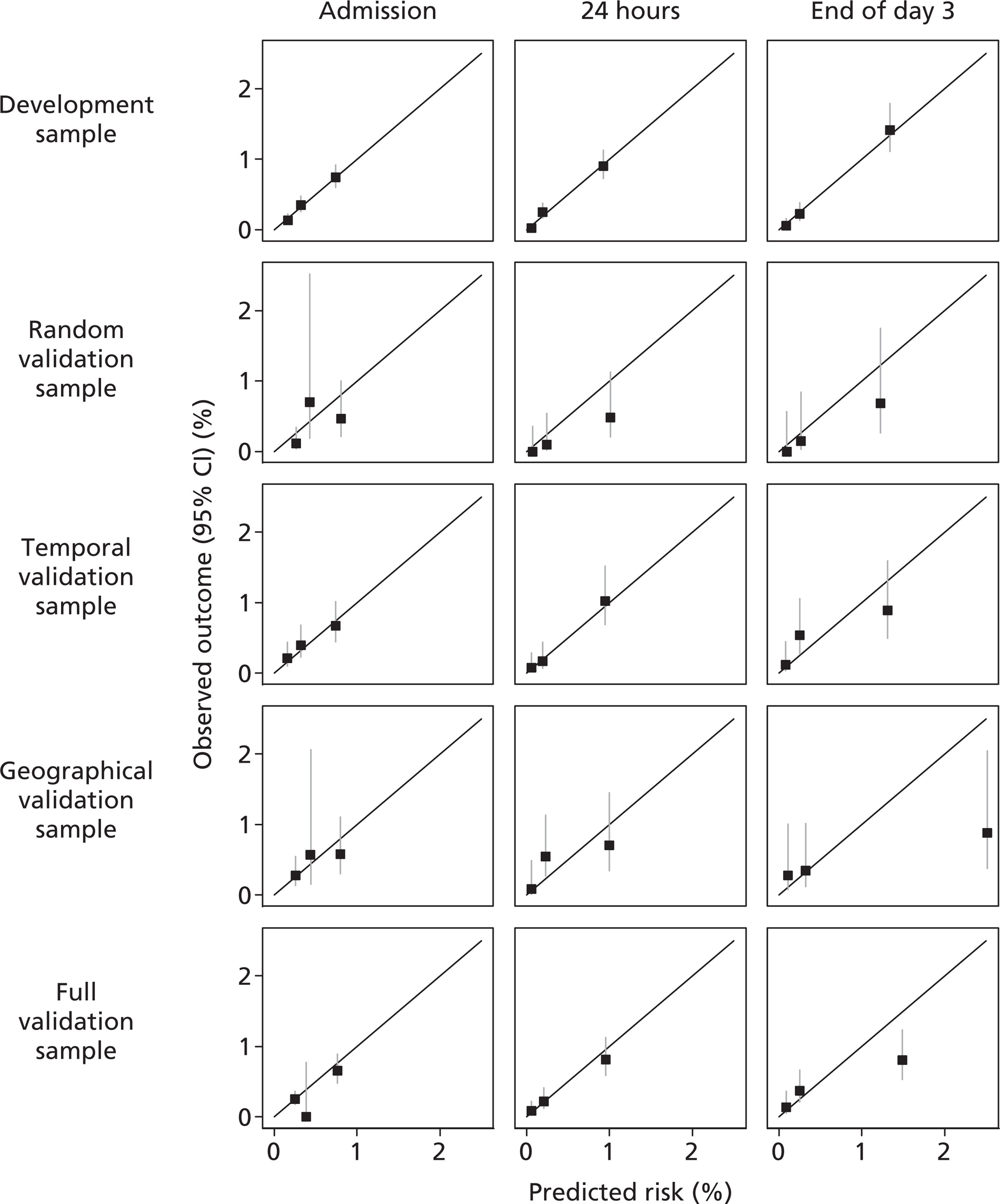
Internal validation
Optimism adjusted estimates of the c-index and Brier's score are reported in Table 17. As anticipated, some overfitting was present, resulting in small reductions in the estimated model performance.
| Measures of model performance | Development sample | Validation sample | ||||
|---|---|---|---|---|---|---|
| Original | Optimism adjusted | Random validation sample | Temporal validation sample | Geographical validation sample | Combined validation sample | |
| At admission | ||||||
| c-index | 0.705 | 0.688 | 0.721 | 0.650 | 0.640 | 0.655 |
| Brier's score | 0.0040 | 0.0041 | 0.0026 | 0.0043 | 0.0040 | 0.0038 |
| 24 hours | ||||||
| c-index | 0.824 | 0.810 | 0.840 | 0.759 | 0.650 | 0.732 |
| Brier's score | 0.0038 | 0.0038 | 0.0019 | 0.0042 | 0.0044 | 0.0037 |
| End of calendar day 3 | ||||||
| c-index | 0.835 | 0.825 | 0.803 | 0.720 | 0.661 | 0.709 |
| Brier's score | 0.0050 | 0.0050 | 0.0026 | 0.0049 | 0.0048 | 0.0043 |
External validation
There was a further loss of model performance in the validation samples (see Table 17), reflecting the fact that the bootstrap procedure applied in the development sample did not account for all aspects of the modelling procedure that may have introduced overfitting (in particular, the stepwise selection of variables for the final models).
Comparison with existing clinical decision rules
Table 18 reports the sensitivity, specificity, PPV and NPV in the full validation sample for clinical decision rules based on risk predictions from the FIRE Study model at the end of the third calendar day following admission to the critical care unit compared with existing clinical decision rules. The comparison of sensitivity and specificity is illustrated in Figure 10. The most promising of the existing rules was that identified as the ‘best performing’ by Ostrosky-Zeichner et al. 27 (OZ3). This rule had better sensitivity, specificity, PPV and NPV than rules P1 and P3 from Paphitou et al. ,28 whereas the remaining rules (OZ1, OZ2 and P2) gave higher sensitivity but at the cost of treating 43–83% of admissions. Applying cut-points of 0.5% (F1) or 1% (F2) to risk predictions from the FIRE Study model gave similar performance to rule OZ1, with rule F1 giving slightly higher sensitivity but lower specificity, and rule F2 giving slightly lower sensitivity but higher specificity (see Figure 10).
| Rule | Percentage ‘high risk’ | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|
| FIRE Study | |||||
| F1 | 27.1 | 54.1 (36.9 to 70.5) | 73.0 (72.0 to 73.9) | 0.87 (0.53 to 1.34) | 99.7 (99.6 to 99.8) |
| F2 | 15.7 | 40.5 (24.8 to 57.9) | 84.5 (83.7 to 85.2) | 1.13 (0.63 to 1.85) | 99.7 (99.5 to 99.8) |
| F3 | 6.4 | 24.3 (11.8 to 41.2) | 93.6 (93.1 to 94.2) | 1.65 (0.76 to 3.11) | 99.6 (99.5 to 99.8) |
| Ostrosky-Zeichner et al. 200727 | |||||
| 0Z1 | 58.5 | 86.5 (71.2 to 95.5) | 41.6 (40.5 to 42.7) | 0.64 (0.44 to 0.91) | 99.9 (99.7 to 100) |
| 0Z2 | 43.4 | 81.1 (64.8 to 92.0) | 56.7 (55.7 to 57.8) | 0.81 (0.55 to 1.16) | 99.9 (99.7 to 99.9) |
| 0Z3 | 21.1 | 51.4 (34.4 to 68.1) | 79.1 (78.2 to 79.9) | 1.06 (0.64 to 1.65) | 99.7 (99.6 to 99.8) |
| Paphitou et al. 200528 | |||||
| P1 | 29.0 | 43.2 (27.1 to 60.5) | 71.0 (70.1 to 72.0) | 0.65 (0.37 to 1.05) | 99.7 (99.5 to 99.8) |
| P2 | 83.2 | 97.3 (85.8 to 99.9) | 16.8 (16.0 to 17.7) | 0.51 (0.36 to 0.71) | 99.9 (99.6 to 100) |
| P3 | 23.3 | 43.2 (27.1 to 60.5) | 76.8 (75.9 to 77.7) | 0.81 (0.46 to 1.31) | 99.7 (99.5 to 99.8) |
FIGURE 10.
Comparison of sensitivity and specificity of clinical decision rules at the end of the third calendar day following admission to the critical care unit in the full validation sample (n = 8488).
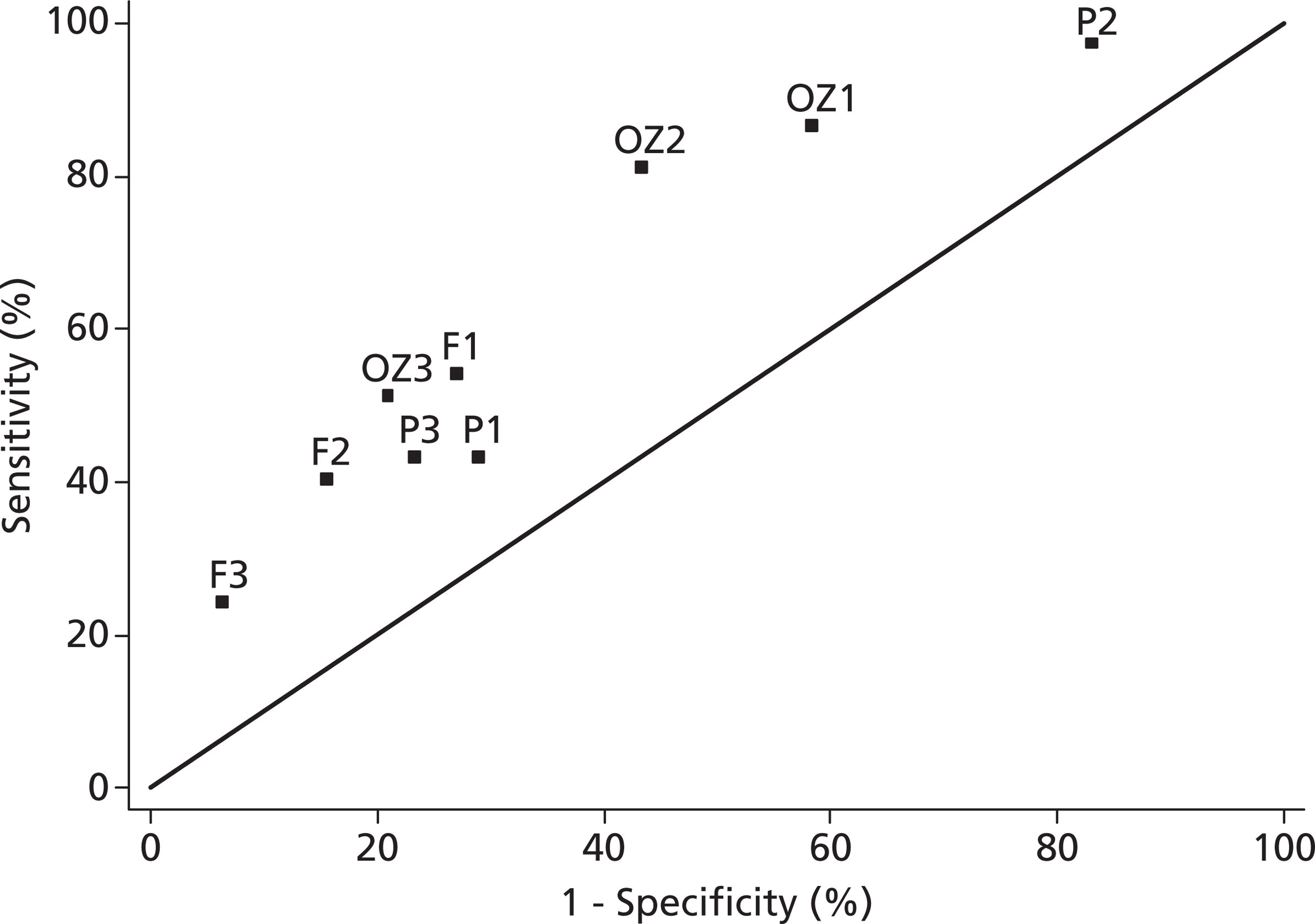
Discussion
Using data from the FIRE Study database, we have derived simple models to predict the risk of invasive Candida infection at three time points: at admission to the critical care unit, at 24 hours following admission, and at the end of the third calendar day following admission. The final model at admission had fair discrimination (c-index ∼0.7). When additional information from the first 24 hours following admission became available, discrimination improved (c-index ∼0.8) and this level of discrimination was maintained at the end of the third calendar day.
Despite the huge sample size of admissions from a substantial proportion of all critical care units in the UK, the low rate of invasive Candida infection observed, although clearly a good thing for the patients, made robust statistical modelling difficult. Consequently, the resulting EPV of the models was low (EPV = 5 for the full model at admission). This leaves the possibility that the models may have been overfitted to the data in the development sample,65 and this may contribute to the drop in model performance when assessed in the validation samples. Model performance was worst when applied in the geographical validation sample, suggesting that particular care should be taken in transferring the models to different geographical settings.
When clinical decision rules were defined based on cut-points of predicted risk at the end of the third calendar day following admission to the critical care unit, the performance of these rules was similar to the best performing rule from the literature. The comparison with existing rules was limited to this time point, as no previous studies have produced risk models or clinical decision rules to aid decisions earlier in the critical care stay. By providing risk predictions at three time points, the models developed from the FIRE Study can support more flexible decision strategies for commencing antifungal prophylaxis with consideration given to both when and at what risk threshold to commence treatment. Evaluating the cost-effectiveness of such strategies is the focus of Chapter 7.
Chapter 6 Machine learning as an alternative approach to developing risk models for invasive Candida infection
Introduction
This chapter reports an investigation of alternative approaches to the analysis of the FIRE Study database using machine learning techniques.
Data handling
Missing data
For the model on admission to the critical care unit, 74 candidate variables were identified. Of 54,289 admissions in the data set, only 15 had complete data for all of these variables (although it should be noted that many of these missing values were missing for structural reasons, e.g. data items related to surgery that were only collected on those who underwent surgery).
An attempt was made to estimate the missing values in the admission data set using multiple imputation66 via the Amelia algorithm,67 as provided by the amelia{Amelia} function written in R (www.r-project.org) (this report uses the R convention of writing the name of a function and its parent package as functionName{packageName}); however, the algorithm did not converge after 2500 iterations within the first imputation.
As a result of the difficulty in implementing multiple imputation, default-orientated cold-deck imputation was used, in which missing values were filled either with values corresponding to default values or, when a default value was not obvious for a variable, with a value indicating ‘not known’. For example, missing values of severe conditions in the past medical history and of fungal colonisation were set to ‘No’, and those of ethnicity were set to ‘Not reported’. The only continuous numerical variable was age, which was complete, and so hot-deck imputation was not required.
A similar approach was applied to the data sets for the models at 24 hours (36,943 admissions, 140 candidate variables) and the end of calendar day 3 (22,726 admissions, 148 candidate variables). Application of the Amelia multiple imputation algorithm was again unsuccessful owing to non-convergence. Cold-deck imputation was applied to categorical variables, as for the admission model. Imputation was then concluded for the numerical variables, such as the physiological measurements from the first 24 hours, by using k-NN hot-deck imputation (with k = 1) through the imputation{rminer} function in R.
Unbalanced data
The FIRE data set was extremely unbalanced (i.e. one outcome was much more common than the other). In the admission data set, following imputation, only 216 of 54,289 admissions (0.4%) had invasive Candida infection. A consequence of the extreme imbalance is that the application of a machine learning method such as tree induction or a neural network will result in a model that will be heavily biased toward correctly classifying the majority class (i.e. no invasive Candida infection) at the expense of the minority class (invasive Candida infection).
A standard approach to an unbalanced data set is to use undersampling, in which majority-class records are randomly selected from the data set until the number selected is equal to the number of minority-class records present in the data. If this is done repeatedly, a set of pseudo-samples is created from which a corresponding set of models can be induced;68 however, a problem with this approach is that, if the number of pseudo-samples does not adequately cover the majority-class cases, the resulting model may be far from optimal.
Another approach to an imbalanced data set is to create a set of n records from the majority class, equal in size to the set of minority-class records, which is somehow representative of the majority class. One way of attempting this is to apply learning vector quantisation69 to the majority-class cases. 70 If n vectors are used, they will converge towards becoming n centroids that collectively represent the distribution of the majority-class records. This was attempted with n = 216 using the lvq3{class} function, but the running time was too slow.
Deepa and Punithavalli71 proposed using a genetic algorithm72 to evolve a near-optimal pseudo-sample. This is done by defining a chromosome to be a collection of n genes, each gene corresponding to a majority-class record row number. In other words, each chromosome corresponds to a random selection of majority-class records. This approach was attempted using the galgo package, but more work was required to determine the minimal size of the genetic algorithm population to evolve a representative chromosome of cases.
Chawla et al. 73 describe the synthetic minority over-sampling technique (SMOTE) data rebalancing algorithm, which uses a combination of undersampling and oversampling to produce a near-balanced pseudo-sample. The SMOTE method is available from the SMOTE{DMwR} function, which was applied to the post-imputation data set. This quickly created a balanced pseudo-sample with 2808 records from the minority class (invasive Candida infection) and 2592 records from the majority class (no invasive Candida infection).
The data sets for the models at 24 hours and the end of calendar day 3 were similarly unbalanced, with 141 (0.4%) and 112 (0.5%) admissions with invasive Candida infection. The SMOTE method was applied to the imputed data sets, which resulted in a pseudo-sample for the 24-hour model consisting of 2115 records with and 1974 records without invasive Candida infection, and a pseudo-sample for the end of calendar day 3 model consisting of 1120 records with and 1008 records without invasive Candida infection.
Feedforward neural networks
The term ‘artificial neural network’ covers a wide variety of classification and regression models,74,75 and these networks have been applied to a variety of medical problems. 76,77
Logistic regression analysis is an established classical statistical technique for developing predictive models for critical care. 78 Feedforward neural networks (FFNNs) are regression models that consist of nested regression functions, and this creates highly flexible non-linear models. 79 Jaimes et al. 80 compared logistic regression models with neural networks for predicting mortality in ICUs, and they found that the FFNN gave better discrimination, as measured by the area under the ROC curve (0.7517 and 0.8782 for the logistic regression model and the neural network, respectively, p = 0.037). Clermont81 states that FFNNs do have benefits, such as adaptability to unforeseen interactions, so long as issues such as overfitting82 are addressed. Lukaszewski et al. 83 were able to develop an FFNN for the prediction of sepsis in intensive care patients based on biomarkers, such as cytokine and chemokine with high sensitivity and specificity (91.4% and 80.2%, respectively).
In the FIRE Study protocol, the use of FFNNs was proposed as an alternative to logistic regression modelling; however, there are some potential issues with the use of FFNNs. First, the issue of overfitting; however, this can be controlled by ‘early stopping’ and regularisation. 74 Second, and more importantly, the existence of local error minima. The training of a FFNN involves a search across an error surface that starts at some point in the space of all possible weights for a FFNN and hopefully finishes at the global minimum. Standard optimization algorithms such as gradient descent always aim to decrease error at every step during training, but this creates a potential problem. Error surfaces often consist of many minima, and the attempt by an algorithm to always decrease the error function at each step can result in a vector of weights reaching a minimum that is not the global minimum, with the weight vector remaining stuck at the local minimum. Consequently, the optimum weight vector is not reached. This problem can be reduced by restarting the training at many different starting points on the error surface, but this will substantially increase the training time of a FFNN.
The local minima problem with FFNNs led to the consideration of two alternative approaches to developing accurate classification models for the FIRE Study: support vector machines and random forests.
Support vector machines
A support vector machine maps the set of feature vectors in a training set to a corresponding set of points in a higher-dimensional space such that the associated classes are linearly separable by a hyperplane. Moreover, the classes are separated by a gap (margin) that is as wide as possible and which is defined by support vectors. 84
Verplancke et al. 85 compared the use of support vector machines with logistic regression for the prediction of hospital mortalities among patients with haematological malignancies, but they did not find the discrimination of support vector machines to be statistically better than that of logistic regression. In contrast, Van Looy et al. 86 compared the ability of support vector machines to predict tacrolimus blood concentrations with that of linear regression and found the support vector machines to be significantly better.
An approach to feature selection with support vector machines is as follows. A support vector machine (M1) with feature vector x1 is trained and tested on data using cross-validation. The total misclassification rate ψ1 is recorded. The sensitivity of each x in x1 is determined, where the sensitivity of x is the change in the output of the support vector machine for a unit change in x, whereas all of the other variables in x1 are held at their median values. This gives the relative importance of the variables in x1 with respect to M1. If x• is the least important variable, a support vector machine (M2) with feature vector x1 − {x•} is trained and tested on the same data and its misclassification rate ψ2 is recorded. If ψ2 ≤ ψ1 then (M2) becomes the current model and the above comparison is repeated. This continues until either ψ2 > ψ1 or |x1| = 1, whereupon x1 is the selected feature subset.
The above approach was tested on a number of test data sets, such as the Hosmer and Lemeshow87 low-birthweight data, with the fit{rminer} function in R, which utilises Gaussian radial basis functions, but the results varied. It is believed that this is due to the support vector machine parameters being non-optimal, an area in which more work is required.
The svm.fs{penalizedsupport vector machine} function was also investigated. This function provides both the LASSO (least absolute shrinkage and selection operator)88 and SCAD (smoothly clipped absolute deviation)89 support vector machine feature selection techniques; however, the results varied with the test data sets, presumably because only linear kernel functions are provided by the function.
As a number of issues with the implementation of support vector machines for the FIRE Study were unable to be resolved, attention turned to the use of random forests to model the FIRE Study data.
Random forests
Tree induction is an established method for modelling data. It is based on the greedy, recursive partitioning of a feature space into disjoint rectangular regions, the optimal split at each successive partition being based on minimising a measure of class heterogeneity, such as the Gini index:90
where p^υ,k is the relative frequency of class k at node υ. Alternatively, splits can be based on maximising the reduction in a measure of tree deviance. 75
The larger the tree, the more likely that it will overfit the data; therefore, some form of pruning is used to obtain a subtree that generalises beyond the training data. Essentially, there is a trade-off between tree complexity (size) and goodness of fit to the data (tree node purity) according to a cost complexity function.
Breiman91 showed that significant improvements in accuracy could be achieved by using a collection of trees called a random forest. Formally, a random forest can be defined as a learning ensemble consisting of a bagging of unpruned decision tree learners with a randomised selection of features at each split.
Random forests go beyond the standard tree-induction process by introducing two additional types of randomisation. First, random-forest construction uses the concept of bootstrap aggregation (or bagging) in which ‘learners’ are trained independently on distinct bootstrap samples. Classification is determined by majority vote from the learners. Breiman92 proved that bagging decreases misclassification rates by reducing prediction variance. Second, random-forest construction selects a random subset of candidate predictor variables at each node split. This further reduces variance,91 as well as reducing search times for variable selections at the nodes.
Misclassification errors can be determined while a random forest is grown. When the training set for a tree is drawn by sampling with replacement during bootstrapping, about one-third of the cases are left out of the sample. The remaining out-of-bag cases are used as a test set for the tree. This approach provides an estimated misclassification error for a random forest that is very accurate in practice.
The random forest algorithm is summarised below:
Let D be the data set with N records and set of candidate predictors V. Let Ntrees be the number of trees required for random forest F.
-
F ← ∅
-
for i ∈ {1, … ,Ntrees} do
-
Select bootstrap sample x* of size N from D. Let d be the set of cases in D not in x*.
-
Grow an unpruned tree Ti on x* during which, at each node, randomly select mtry (< |V|) candidate predictors from V and determine the best split with respect to the Gini index using only the selected predictors. Do not prune Ti.
-
Classify the records in d using Ti.
-
F ← F ∪ {Ti}
-
-
Let k be the class that got most of the votes every time record x was out-of-bag. The proportion of times that k is not equal to the true class of x, averaged over all records, is the out-of-bag error estimate.
-
return F and the out-of-bag error estimate.
Random forests can be induced by a number of packages written in R, one of which is randomForest. The function randomForest{randomForest} was applied to the balanced pseudo-sample for the admission model (following imputation and balancing with the SMOTE algorithm) to create a random forest consisting of 100 trees. The number of variables randomly sampled as candidates at each split of a tree node (mtry) was set at √(v), where v = 74 was the number of candidate variables.
The out-of-bag estimated misclassification rate was 4.13%. This error rate as a function of the number of trees used in the random forest is shown in Figure 11. From the out-of-bag data, the estimated sensitivity, specificity and PPV were 99.5%, 92.4% and 92.5%, respectively.
FIGURE 11.
Out-of-bag estimated misclassification error, positive predictive error (1 − PPV) and negative predictive error (1 − NPV) as a function of the number of trees added to a random forest for the FIRE Study data set at admission.
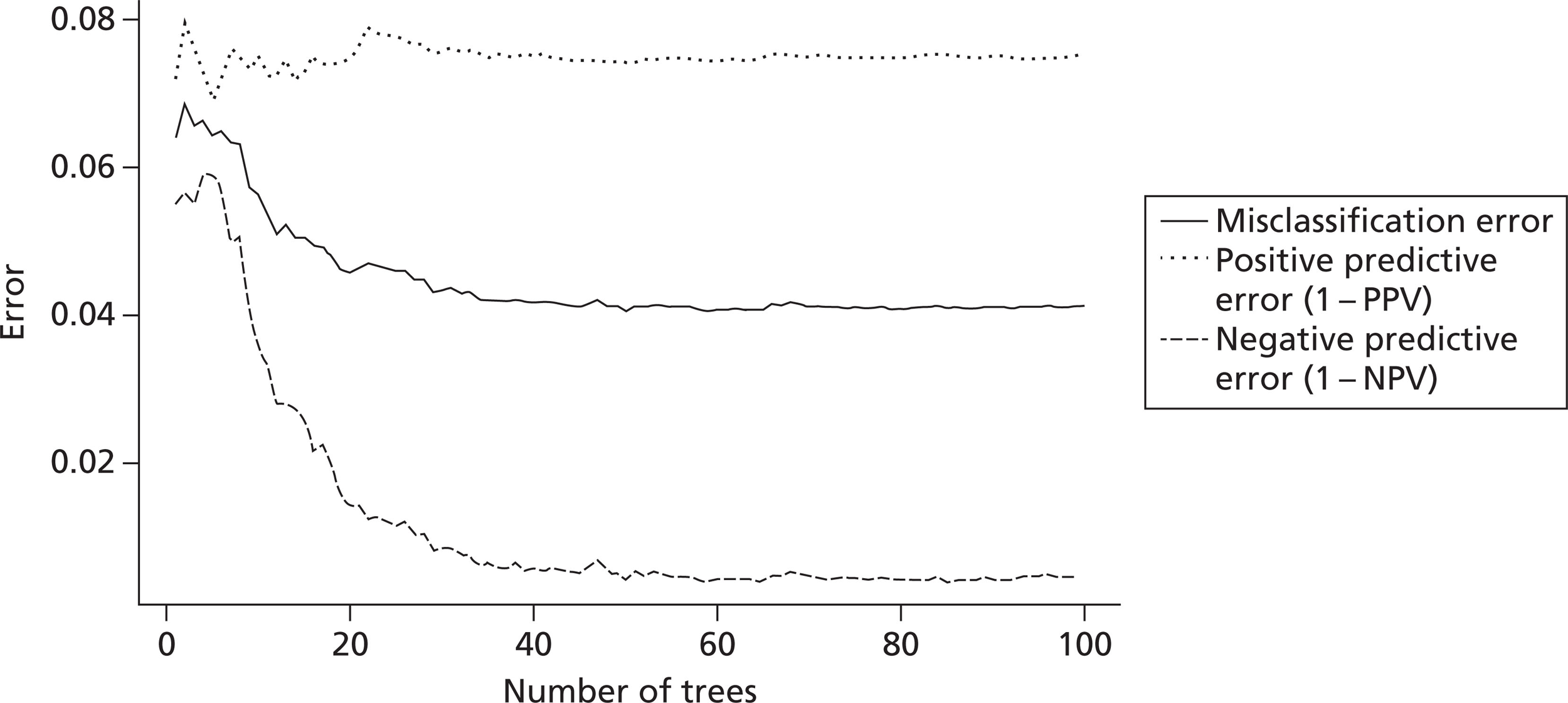
The relative importance of the variables present amongst the trees of a random forest was determined by measuring, for each variable, the mean decrease in the Gini index of that variable across the nodes of all the trees (Table 19).
| Variable | Mean Gini index decrease |
|---|---|
| No. of drains pre-admission | 205 |
| LOS in acute hospital pre-admission | 203 |
| No. of drains pre-admission (0, 1, 2, 3, 4 or more) | 199 |
| Fungal colonisation pre-admission (yes, no) | 171 |
| No. of samples positive for fungal colonisation pre-admission (0, 1, 2 or more) | 167 |
| No. of microbiological samples reported pre-admission | 151 |
| No. of samples positive for fungal colonisation pre-admission (0, 1 or more; missing recoded as 0) | 125 |
| No. of samples positive for fungal colonisation pre-admission (0, 1, 2 or more; missing recoded as 0) | 125 |
| Classification of surgery pre-admission (none, elective/scheduled, emergency/urgent) | 122 |
| Body system of condition requiring surgery pre-admission | 111 |
| No. of samples positive for fungal colonisation pre-admission | 107 |
| Surgery pre-admission (yes, no) | 74 |
| Age | 65 |
| Enteral feeding tube pre-admission (yes, no) | 56 |
| No. of catheters in central veins pre-admission | 55 |
| No. of lines in arteries pre-admission | 54 |
| Location of catheter in central vein pre-admission: internal jugular | 45 |
| No. of lines in arteries pre-admission (0, 1, 2 or more) | 44 |
| No. of catheters in central veins pre-admission (0, 1, 2, 3 or more) | 39 |
| Classification of surgery pre-admission (none, elective, scheduled, urgent, emergency) | 35 |
The technique described above for the creation of a random forest for the admission model was repeated for models at 24 hours and the end of calendar day 3 following admission to the critical care unit. The out-of-bag estimated overall misclassification rate for the 24-hour model was 2.86% (Figure 12). From the out-of-bag data, the estimated sensitivity, specificity and PPV for this model were 99.8%, 94.6% and 94.7%, respectively. The out-of-bag estimated overall misclassification rate for the end-of-calendar-day 3 model was 4.98% (Figure 13). The estimated sensitivity, specificity and PPV for this model were 97.7%, 92.4% and 92.8%, respectively. The relative importance of the variables for the 24-hour and end-of-calendar-day 3 models were determined from the mean decrease in the Gini index of those variables, as for the admission model. Tables 20 and 21 list the 20 most important variables for each model.
FIGURE 12.
Out-of-bag estimated misclassification error, positive predictive error (1 − PPV) and negative predictive error (1 − NPV) as a function of the number of trees added to a random forest for the FIRE Study data set at 24 hours.
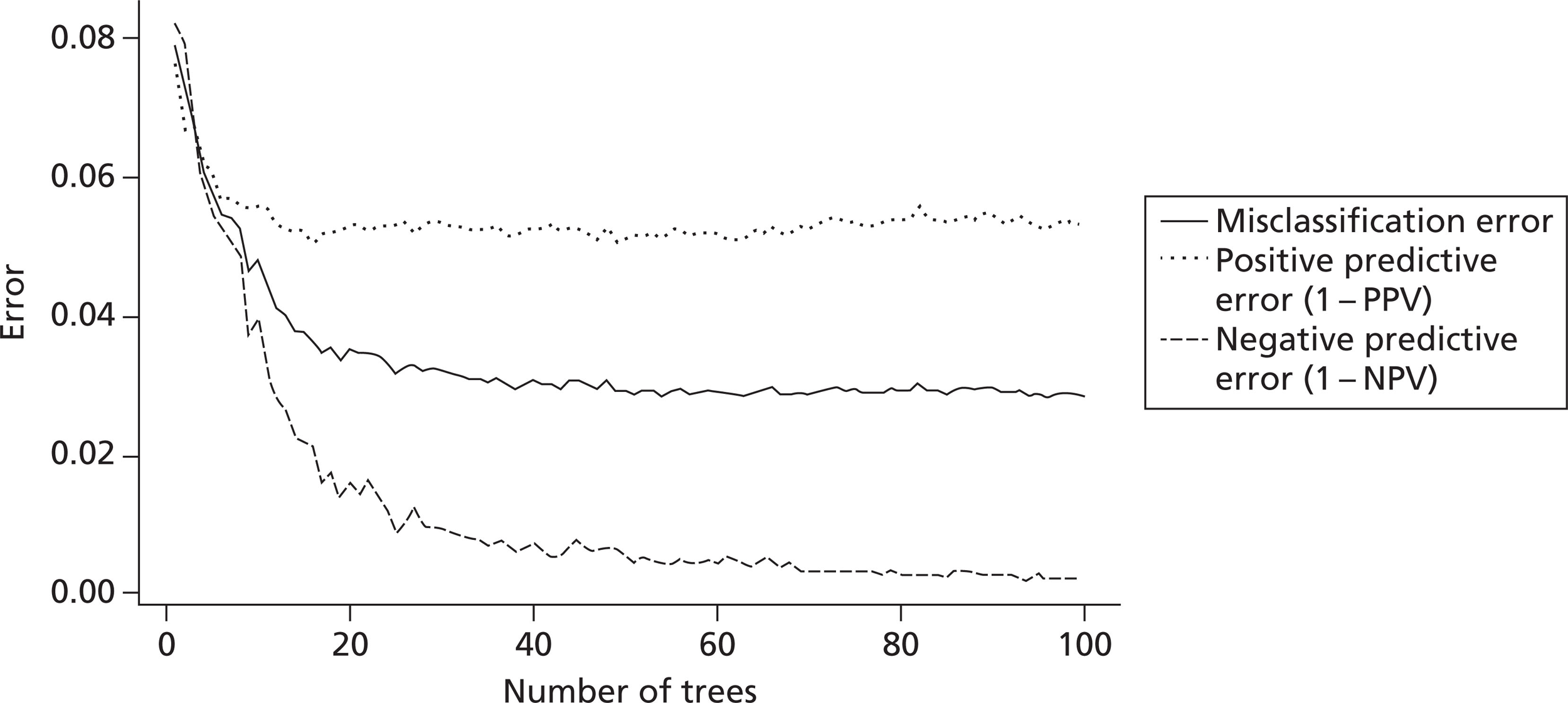
FIGURE 13.
Out-of-bag estimated misclassification error, positive predictive error (1 − PPV) and negative predictive error (1 − NPV) as a function of the number of trees added to a random forest for the FIRE Study data set at the end of calendar day 3.
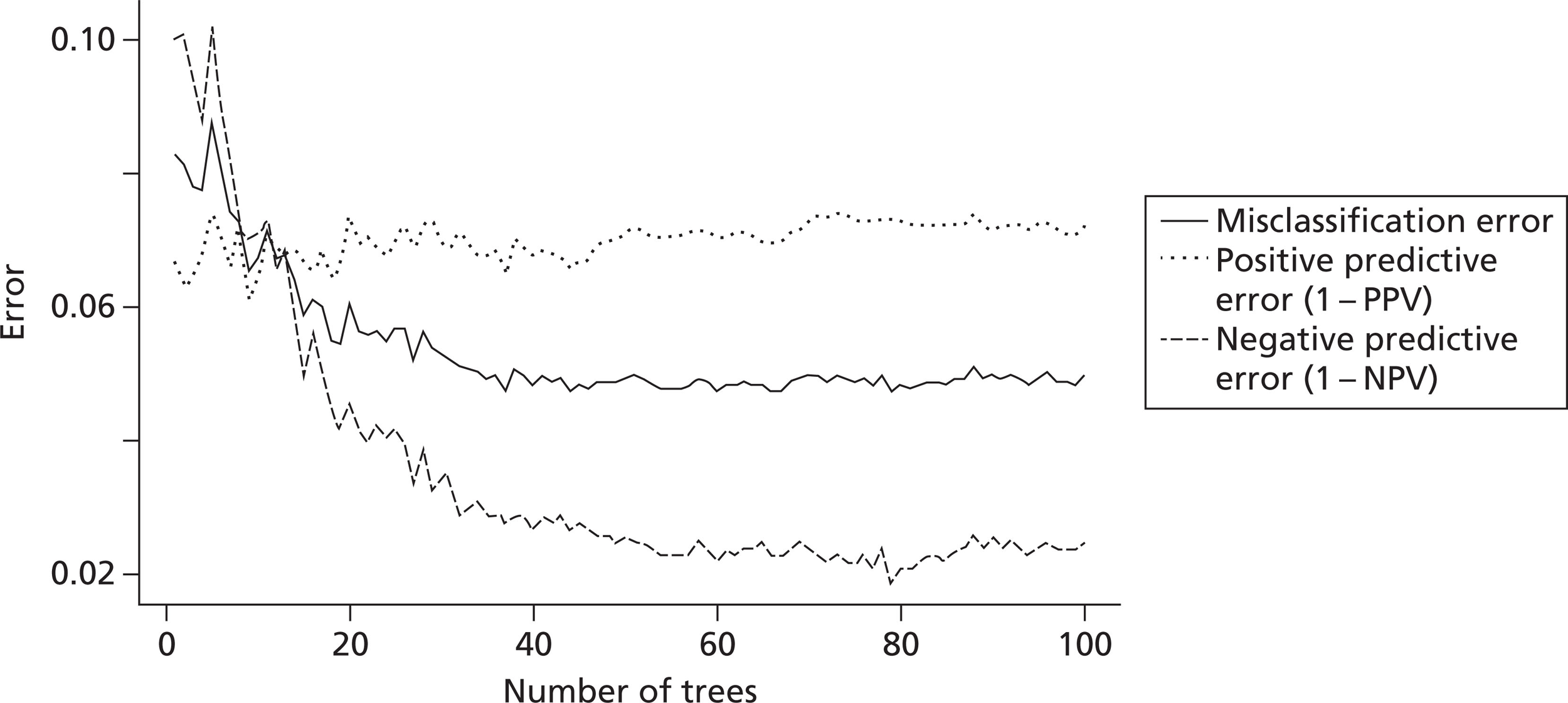
| Variable | Mean Gini index decrease |
|---|---|
| CPR within 24 hours prior to admission (yes, no) | 348 |
| Advanced respiratory support pre-admission (yes, no) | 179 |
| Surgery during first 24 hours (yes, no) | 155 |
| Advanced respiratory support during first 24 hours (yes, no) | 122 |
| Enteral feeding tube during first 24 hours (yes, no) | 99 |
| No. of microbiological samples reported during first 24 hours | 99 |
| Location of catheter in central vein during first 24 hours: internal jugular | 76 |
| No. of lines in arteries pre-admission | 68 |
| Highest non-ventilated respiratory rate during first 24 hours | 61 |
| No. of catheters in central veins during first 24 hours | 58 |
| No. of lines in arteries pre-admission (0, 1, 2 or more) | 41 |
| Lowest non-ventilated respiratory rate during first 24 hours | 32 |
| Sedation/paralysis during first 24 hours (sedated for whole of first 24 hours, paralysed and sedated for whole of first 24 hours, sedated and/or paralysed for some of first 24 hours, never sedated or paralysed at any time during first 24 hours) | 31 |
| CPR within 24 hours prior to admission (no, in hospital, out of hospital) | 29 |
| No. of catheters in central veins during first 24 hours (0, 1, 2, 3 or more) | 27 |
| Highest central temperature during first 24 hours | 22 |
| Surgery pre-admission (yes, no) | 22 |
| Lowest heart rate during the first 24 hours | 21 |
| FiO2 from the arterial blood gas with the lowest PaO2 during first 24 hours | 20 |
| Admission from another acute hospital | 19 |
| Variable | Mean Gini index decrease |
|---|---|
| No. of microbiological samples reported by end of calendar day 3 | 97 |
| No. of drains during first 24 hours (0, 1, 2, 3, 4 or more) | 62 |
| No. of drains pre-admission (0, 1, 2, 3, 4 or more) | 62 |
| No. of drains by end of calendar day 3 | 48 |
| No. of samples positive for fungal colonisation by end of calendar day 3 (0, 1, 2 or more; missing recoded as 0) | 39 |
| No. of drains pre-admission | 38 |
| No. of drains by end of calendar day 3 (0, 1, 2, 3, 4 or more) | 34 |
| No. of samples positive for fungal colonisation by end of calendar day 3 (0, 1, 2 or more) | 30 |
| Body system of condition requiring surgery pre-admission | 29 |
| No. of samples positive for fungal colonisation by end of calendar day 3 | 26 |
| Advanced respiratory support by end of calendar day 3 (yes, no) | 24 |
| FiO2 from the arterial blood gas with the lowest PaO2 during first 24 hours | 23 |
| Classification of surgery pre-admission (none, elective/scheduled, emergency/urgent) | 21 |
| Location of catheter in central vein by end of calendar day 3: internal jugular | 18 |
| Surgery by end of calendar day 3 (yes, no) | 17 |
| Surgery pre-admission (yes, no) | 17 |
| Renal support by end of calendar day 3 (yes, no) | 15 |
| Location of catheter in central vein pre-admission: internal jugular | 15 |
| Enteral feeding tube by end of calendar day 3 (yes, no) | 13 |
| Highest heart rate during the first 24 hours | 13 |
Discussion
The random forest approach revealed a number of possible risk factors for invasive Candida infection and was seen to be a fairly accurate predictor within the balanced pseudo-samples created for model development. The performance of these models cannot be directly compared with that reported from the classical statistical models (see Chapter 5) as a result of the rebalancing procedure. In particular, PPV and NPV are highly dependent on the prevalence of the outcome, which has been artificially inflated by the rebalancing. Sensitivity and specificity (which would be less affected by rebalancing) were also very high in the out-of-bag data, suggesting the potential for this approach to yield more accurate clinical decision rules than those developed in Chapter 5 or in the existing literature. The cost of these more accurate rules, however, is the complexity of their application at the bedside. These ‘black box’ models would need to be implemented in a computer-driven approach, for example on patient information systems or personal digital assistants, and require substantially more data items than the simple reduced models developed in Chapter 5.
With regard to data handling, the SMOTE algorithm was found to be an efficient technique for producing balanced pseudo-samples. The attempted multiple imputations did not converge, which may be due to the presence of skewed numerical data; therefore, variable transformations should be considered.
In the absence of multiple imputation, a combination of hot-deck and cold-deck imputations provided an alternative.
Further work that may improve the performance of the random forest approach include optimising the choice of the number of variables randomly sampled as candidates at each split of a tree node (mtry) and investigating alternative measures of relative variable importance, such as random perturbation of predictors. 91 Given the high classification accuracies achievable with support vector machines in general, further work on this approach should also be considered so as to provide models that are more compact than random forests but at least as accurate.
Chapter 7 Economic modelling to assess the cost-effectiveness of prophylaxis based on the risk models for invasive Candida infection
Introduction
Invasive fungal disease is associated with increased mortality, morbidity and use of critical care and hospital beds. 6,7 About half of IFD occurs in non-neutropenic patients in critical care units,1 and most of these infections are due to the Candida species. 2,3 In the USA, candidaemia has been estimated to lead to excess costs of US$44,000 per episode. 93 RCTs have established that antifungal prophylaxis with either fluconazole or ketoconazole is effective in reducing mortality in non-neutropenic, critically ill patients. 19 However, patients included in the RCTs were at high risk of IFD, with baseline risk in the control arm typically exceeding 10%.
The previous chapters have highlighted that the incidence of invasive Candida infection in unselected, non-neutropenic adult patients admitted to NHS critical care units is relatively low (see Chapter 4). Given this low incidence, the costs of prophylaxis and concerns about resistance, it is unclear at what levels of baseline risk prophylaxis is cost-effective for critically ill patients. Indeed, current usual practice in the NHS is not to provide prophylaxis, irrespective of baseline risk. The relative gains and costs of administering antifungal prophylaxis are also anticipated to differ according to the time at which the prophylaxis is administered. In particular, providing prophylaxis to those patients who are judged to be high risk on admission to critical care would entail providing prophylaxis to more patients than waiting and only providing prophylaxis to those patients who were still in critical care, and high risk, after 3 days. Furthermore, it is unclear whether or not it is more cost-effective to consider prophylaxis at single or at multiple time points. Although current usual practice in the NHS is not to provide prophylaxis, this standpoint has not been informed by a careful assessment of the relative cost-effectiveness of alternative prophylaxis strategies for invasive Candida infection. Indeed, no previous study has assessed the cost-effectiveness of using a risk model to define a risk threshold above which to initiate antifungal prophylaxis for preventing invasive Candida infection in non-neutropenic, critically ill adult patients. 46
This chapter therefore presents an economic evaluation with the aim to report the relative cost-effectiveness of alternative strategies to prevent invasive Candida infection for non-neutropenic, critically ill adult patients admitted to NHS critical care units. The objectives of the economic evaluation were to establish the relative cost-effectiveness of risk assessment using the FIRE Study risk models, followed by initiation of prophylaxis at different thresholds of baseline risk and at different time points; and to assess the relative value of further research to reduce uncertainty about the optimum strategy to adopt.
Methods: overview
The economic evaluation assessed the cost-effectiveness of alternative strategies to risk assessment followed by prophylaxis using the risk models developed for invasive Candida infection. The study compared alternative treatment protocols for providing antifungal prophylaxis to patients identified as high risk (‘interventions’) with providing no prophylaxis (‘current practice’). The prophylaxis treatment regimen evaluated followed current recommendations and is for 400 mg of fluconazole per day. 4,42 There is no specific guideline on the duration of prophylaxis with fluconazole in critical care. A previous systematic review suggested that, across studies, prophylaxis was generally administered until discharge from critical care. 19 For the FIRE Study economic evaluation, prophylaxis was assumed to be applied for 10 days, which is the mean LOS in critical care for the study population. The economic evaluation used a decision-analytical approach to project lifetime cost-effectiveness. The decision model was populated with estimates of PPV (the proportion of those identified as high risk that subsequently developed invasive Candida infection) and NPV (the proportion of those identified as low risk that did not subsequently develop invasive Candida infection) from the FIRE Study risk models at each time point (see Chapter 5), and estimates of the effectiveness of antifungal prophylaxis from systematic reviews of published RCTs. 19 The input parameters were all estimated for patients aged 60 years, the mean age of patients who met the inclusion criteria for the FIRE Study. A probabilistic sensitivity analysis was undertaken to recognise the sampling uncertainty surrounding the input parameters, following general recommendations in the choice of distribution for each parameter. 94 The main structural assumptions were subjected to sensitivity analyses. Finally, the value of further research was established both overall and for specific parameters.
Methods: base case
Population of interest
The population of interest, represented by the development and validation samples described in Chapter 5, was defined as non-neutropenic adult patients admitted to NHS critical care units, and excluded those with IFD prior to a decision time point and those receiving systemic antifungal therapy as part of routine clinical practice prior to a decision time point. This last criterion excluded a number of patients that received systemic antifungal therapy in the absence of IFD, and may therefore have represented use of antifungal prophylaxis. Figure 14 reports the proportion of patients that were excluded at each time point due to receiving systemic antifungal therapy that would otherwise have met the inclusion criteria for the models. Overall, 1635 patients (3.0%) otherwise eligible for the models were excluded for this reason at any of the three time points. There were 26 cases of invasive Candida infection among these patients, and assuming a RR of invasive Candida infection associated with antifungal prophylaxis of approximately 0.519 we may anticipate that a further 26 cases may have been prevented.
FIGURE 14.
Current use of antifungal prophylaxis and potential impact of antifungal prophylaxis on observed outcome. a, Assuming a RR of invasive Candida infection of 0.5 associated with antifungal prophylaxis;19 b, prior to 24 hours; c, prior to end of calendar day 3.
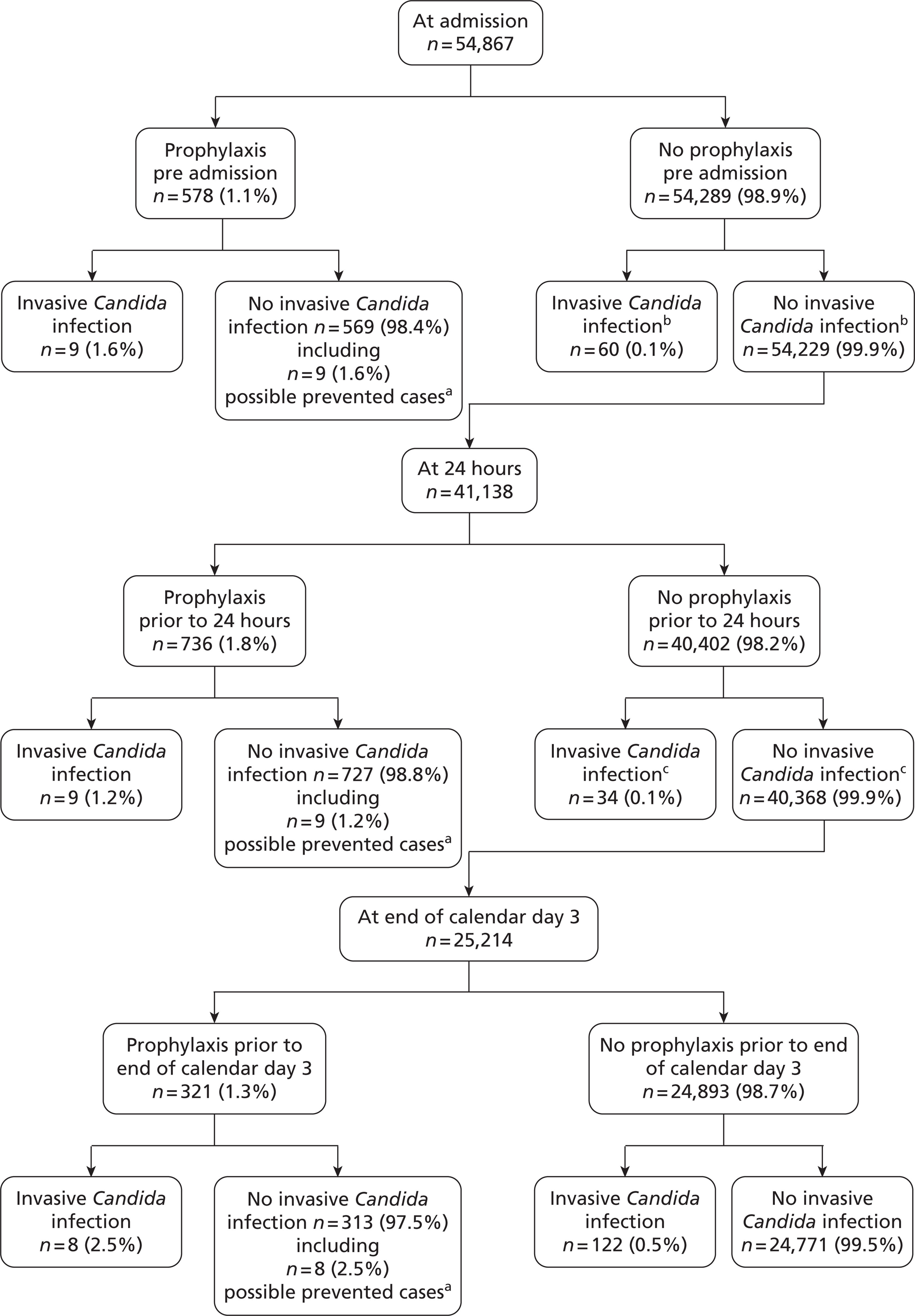
Strategies under comparison
The economic evaluation defined three decision time points at which to consider assessment of the risk of invasive Candida infection. These were:
-
on admission
-
at the end of the first 24 hours, and
-
at the end of calendar day 3.
The alternative strategies recognised these time points (Table 22). Each time point defined a decision node, with two possibilities: either assessment of the risk of invasive Candida infection was not undertaken (no risk assessment) or risk assessment was undertaken (risk assessment). For example, under risk assessment on admission, the patients' risk was assessed according to the predicted risk from the on admission FIRE Study risk model. Those patients whose predicted risk of invasive Candida infection during the critical care unit stay exceeded the specified threshold (PT) were designated ‘high risk’. These high-risk patients were then assumed to receive a single course of prophylaxis with fluconazole.
| Strategy | Decision node | ||
|---|---|---|---|
| On admission | At end of 24 hours | At end of calendar day 3 | |
| 1 | Do not assess risk | Do not assess risk | Do not assess risk |
| 2 | Assess risk | Do not assess risk | Do not assess risk |
| Prophylaxis if risk > PT | |||
| 3 | Do not assess risk | Assess risk | Do not assess risk |
| Prophylaxis if risk > PT | |||
| 4 | Do not assess risk | Do not assess risk | Assess risk |
| Prophylaxis if risk > PT | |||
| 5 | Assess risk | Assess risk | Do not assess risk |
| Prophylaxis if risk > PT | Prophylaxis if risk > PT | ||
| 6 | Do not assess risk | Assess risk | Assess risk |
| Prophylaxis if risk > PT | Prophylaxis if risk > PT | ||
| 7 | Assess risk | Do not assess risk | Assess risk |
| Prophylaxis if risk > PT | Prophylaxis if risk > PT | ||
| 8 | Assess risk | Assess risk | Assess risk |
| Prophylaxis if risk > PT | Prophylaxis if risk > PT | Prophylaxis if risk > PT | |
Strategy 1 assumed that there was no assessment of the risk of invasive Candida infection, as is usual practice in UK critical care units for the majority of patients (see Chapter 4). Strategies 2–4 assumed that risk assessment was performed using a single FIRE Study risk model at a single point in time – either on admission, at the end of 24 hours or at the end of calendar day 3. So, for example, strategy 4 assumed that patients' risk was only assessed at the end of calendar day 3 with prophylaxis initiated for those patients defined by the end of calendar day 3 FIRE Study risk model as having high risk of invasive Candida infection. Strategies 5–8 allowed for risk assessment at multiple time points. At any time point, risk assessment was only considered for the subgroup who were still in critical care, not already receiving systemic antifungal therapy, and without IFD. It was assumed that prophylaxis was initiated for those newly defined as high risk at the particular time point.
The risk thresholds (PT) defined a priori according to the literature and expert opinion were 0.5%, 1%, 2%, 5% and 10%. However, the low incidence of Candida infection in the FIRE Study meant that under some of the strategies there were no infections in those designated high risk, and so 2% was the highest risk threshold considered in the analysis. In strategies involving risk assessment at multiple time points, the same risk threshold was applied at all time points.
Model structure
The model included a hypothetical cohort of 1000 cases with characteristics defined by the patients who met the FIRE Study inclusion criteria. The model structure (Figure 15) recognised the alternative strategies and time points described in Table 22.
FIGURE 15.
Structure of the model comparing alternative strategies for assessing risk and initiating prophylaxis for non-neutropenic, critically ill adult patients.
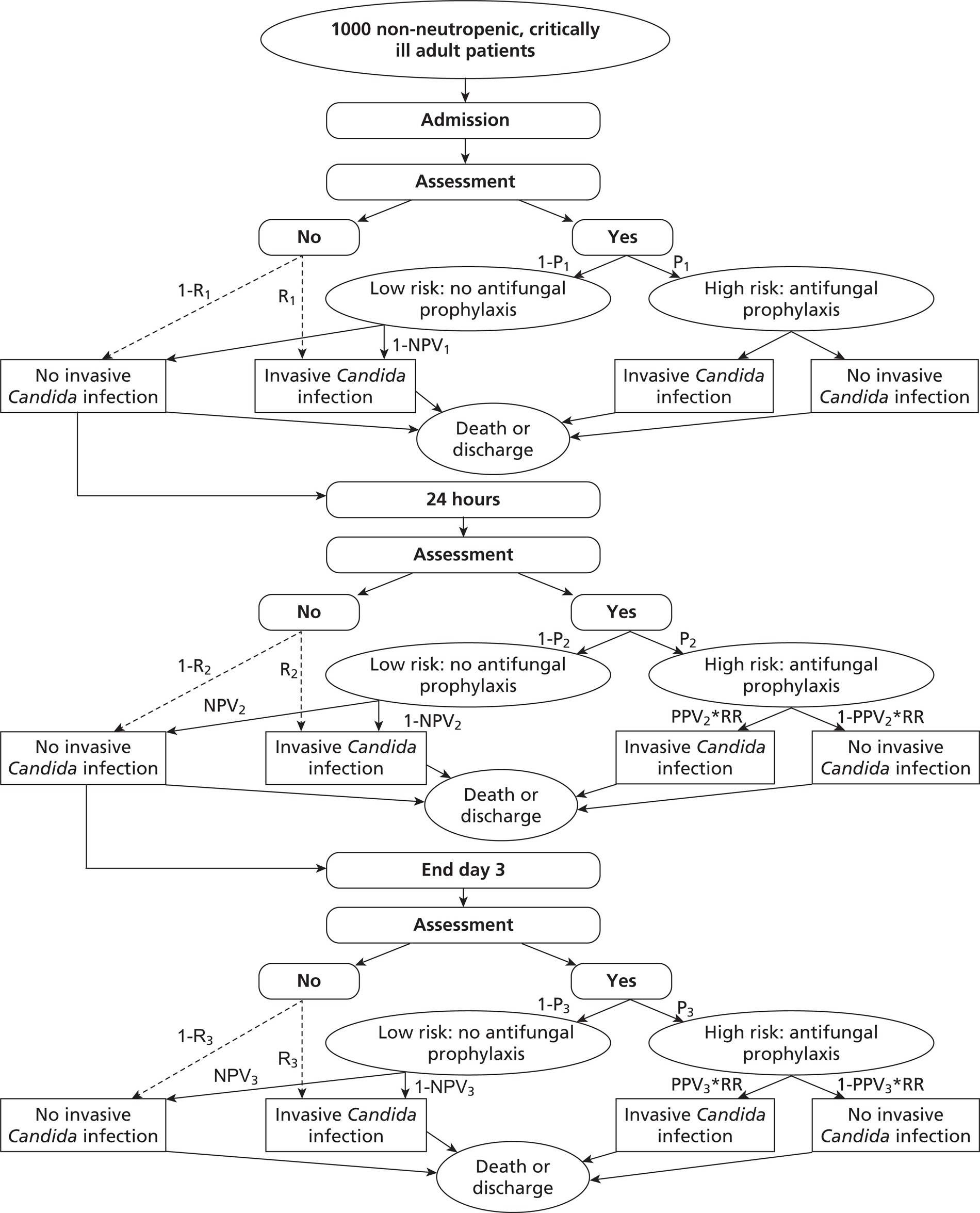
If, on admission, no risk assessment was undertaken (strategies 1, 3, 4 and 6), patients faced the risk of either having (R1) or not having (1 − R1) invasive Candida infection within the first 24 hours in the critical care unit. From the ‘no invasive Candida infection’ health state, patients faced a baseline risk of all cause death. For patients predicted to develop invasive Candida infection, an excess risk of death was applied. Under the strategies where risk assessment was undertaken on admission (strategies 2, 5, 7 and 8), the predicted probability of invasive Candida infection at any time during the critical care stay was estimated from the on-admission FIRE Study risk model. The proportion of patients (P1) whose predicted risk of infection was higher than the risk threshold (e.g. 2%) were judged ‘high risk’ and assumed to receive prophylaxis. For these patients, the probability of developing invasive Candida infection at any time during critical care was estimated by multiplying the PPV from the ‘on-admission’ FIRE model (PPV1) by the RR of invasive Candida infection associated with receiving antifungal prophylaxis versus no prophylaxis. 19 The proportion of patients (1 − P1) whose predicted risk of infection was lower than the risk threshold (e.g. 2%) were judged ‘low risk’ and assumed not to receive prophylaxis. [For these patients, the probability of developing invasive Candida infection prior to the next decision time point in the strategy under consideration (or at any time during critical care, if admission was the only decision time point, i.e. strategy 2) was estimated as one minus the corresponding NPV from the ‘on admission’ FIRE model (1 − NPV1). Note that the NPVs were calculated for each time point because the patients that did not receive antifungal prophylaxis may be reconsidered for risk assessment and prophylaxis at subsequent time points. By contrast, as antifungal prophylaxis was a ‘one-off’ treatment, those that received antifungal prophylaxis had a subsequent risk of invasive Candida infection at any time during the critical care stay.]
The risk of death, conditional on presence or absence of invasive Candida infection, was assumed the same whether or not patients received antifungal prophylaxis.
At the second assessment time point, the model considered those patients still on the critical care unit, not receiving systemic antifungal therapy and without IFD at the end of 24 hours. The strategies with no assessment at this time point (strategies 1, 2, 4 and 7) allowed for patients to face a baseline risk of invasive Candida infection (R2) before the end of calendar day 3. For strategies with risk assessment at this time point (strategies 3, 5, 6 and 8), prophylaxis was initiated for the proportion of patients (P2) whose predicted risk from the 24-hour FIRE Study risk model exceeded the risk threshold. The subsequent probability of invasive Candida infection was then calculated by multiplying the PPV of invasive Candida infection from the 24-hour FIRE Study risk model (PPV2) by the RR of invasive Candida infection. For the proportion of patients (1 − P2) whose predicted risk from the 24-hour FIRE Study risk model was below the risk threshold, the subsequent probability of invasive Candida infection prior to the next decision time point was estimated as one minus the corresponding NPV from the 24-hour FIRE Study risk model (1 − NPV2).
At the third assessment time point, the model considered those patients still on the critical care unit, not receiving systemic antifungal therapy and without IFD at the end of the third calendar day. The strategies with no assessment at this time point (strategies 1, 2, 3 and 5), allowed for patients to face a baseline risk of invasive Candida infection (R3) over the remaining critical care stay. For strategies with risk assessment at this time point (strategies 4, 6, 7 and 8), prophylaxis was initiated for the proportion of patients (P3) whose predicted risk from the end of calendar day 3 FIRE Study risk model exceeded the risk threshold. The subsequent probability of invasive Candida infection was then calculated by multiplying the PPV of invasive Candida infection from the end of calendar day 3 FIRE Study risk model (PPV3) by the RR of invasive Candida infection. For the proportion of patients (1 − P3) whose predicted risk from the end of calendar day 3 FIRE Study risk model was below the risk threshold, the subsequent probability of invasive Candida infection at any subsequent time during critical care was estimated as one minus the corresponding NPV from the end of calendar day 3 FIRE Study risk model (1 − NPV3).
At each decision node, a proportion of patients left the model because they died or were discharged from critical care. After the last decision node (end of calendar day 3), remaining patients were assigned the mean LOS and cost of those remaining in critical care in the FIRE Study for > 3 days, according to whether or not they had invasive Candida infection. These patients were then assigned lifetime quality-adjusted life-years (QALYs) according to the assumptions detailed below.
Model input parameters
The decision problem required information on the following input parameters: transition probabilities (risk of invasive Candida infection without prophylaxis, PPV and NPV following risk assessment, probabilities of death with and without invasive Candida infection, RR of invasive Candida infection after prophylaxis); costs; and lifetime QALYs. The estimation and sources for each set of input parameters are detailed below.
Transition probabilities
Risk of invasive Candida infection
The risk of invasive Candida infection (R1, R2, R3) for each decision node without prophylaxis was predicted within each time period (admission to 24 hours, 24 hours to day 3, and after day 3). These baseline risks of infection were estimated from the combined FIRE Study development and validation samples. The probabilistic sensitivity analysis assumed that each probability was drawn from a beta distribution. 94Table 23 presents the risks of invasive Candida infection for each time period.
| Time period (hours) | n | Point estimate (%) | Distributiona |
|---|---|---|---|
| Within 24 | 60,778 | 0.082 | beta (50; 60,728) |
| 24–48 | 36,943 | 0.087 | beta (32; 36,911) |
| After 72 | 22,726 | 0.449 | beta (102; 22,624) |
Positive and negative predictive value The decision model required PPV and NPV for each strategy and for each risk threshold. Estimates of PPV were required to predict the risk of invasive Candida infection at any subsequent time in critical care (as once prophylaxis was initiated, it was assumed that no further risk assessments took place), whereas estimates of NPV only considered the risk of invasive Candida infection prior to the next time point at which risk assessment would take place. For the strategies where multiple risk assessments were undertaken, PPV and NPV were calculated depending on the decision at the previous time points, i.e. they were conditional on the previous predicted risk and assumed risk threshold. To avoid concerns about overfitting from use of the FIRE Study development data set, PPVs and NPVs were estimated from the validation sample only. Table 24 presents PPVs and NPVs for each strategy and risk threshold. The PPVs were low for all strategies; even at the 2% risk threshold and with prophylaxis at each time point, the PPVs remained below 2%. By contrast, the NPVs all exceeded 99%. To recognise the uncertainties in estimating the PPVs and NPVs given the small number of infections, we assumed vague priors with uniform distributions (range from 0 to 1). 95 Parameter values for the PPVs and NPVs were sampled from the resultant posterior distributions.
| Scenario | Time point | PPV by risk threshold (%) | NPV by risk threshold (%) | ||||
|---|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 2.0 | 0.5 | 1.0 | 2.0 | ||
| 2 | On admission | 0.85 | 1.94 | 1.32 | 99.95 | 99.94 | 99.92 |
| 3 | At end of 24 hours | 0.88 | 1.60 | 1.35 | 99.92 | 99.93 | 99.92 |
| 4 | At end of calendar day 3 | 0.95 | 1.21 | 1.26 | 99.79 | 99.73 | 99.65 |
| 5 | On admission | 0.85 | 1.94 | 1.32 | 99.95 | 99.94 | 99.92 |
| At end of 24 hours | 0.70 | 1.31 | 1.71 | 99.92 | 99.93 | 99.92 | |
| 6 | At end of 24 hours | 0.88 | 1.60 | 1.35 | 99.92 | 99.93 | 99.92 |
| At end of calendar day 3 | 0.98 | 0.79 | 1.62 | 99.78 | 99.72 | 99.66 | |
| 7 | On admission | 0.85 | 1.94 | 1.32 | 99.95 | 99.94 | 99.92 |
| At end of calendar day 3 | 0.70 | 0.99 | 1.38 | 99.78 | 99.73 | 99.64 | |
| 8 | On admission, | 0.85 | 1.94 | 1.32 | 99.95 | 99.94 | 99.92 |
| At end of 24 hours | 0.70 | 1.31 | 1.71 | 99.92 | 99.93 | 99.92 | |
| At end of calendar day 3 | 0.57 | 0.85 | 1.70 | 99.77 | 99.73 | 99.66 | |
Probabilities of death and relative risk of invasive Candida infection
For patients who did not have invasive Candida infection, the probabilities of death were estimated for the three time periods (admission to 24 hours, 24 hours to day 3, and after day 3) using the combined FIRE Study development and validation samples (Table 25). For patients with invasive Candida infection, the excess risk of death was estimated from the combined data set. The same excess risk of death for patients was applied for each time point and irrespective of whether or not patients had received prophylaxis. The effectiveness of prophylaxis was recognised by taking the RR of invasive Candida infection after prophylaxis from the Cochrane systematic review by Playford et al. 19 The systematic review reported similar RRs across different levels of baseline risk. Hence, we applied the same RR for all time points and all risk thresholds.
| Parameter | Time period | Point estimate | Distribution |
|---|---|---|---|
| Probability of death, no invasive Candida infection | Admission to 24 hours | 4.57% | beta (2777; 57,999) |
| 24 hours to end of calendar day 3 | 5.82% | beta (2151; 34,790) | |
| After end of calendar day 3 | 8.78% | beta (3057; 31,737) | |
| RR of death, invasive Candida infection vs no invasive Candida infection | During critical care | 2.14 | log-normal (0.57 to 0.95) |
| RR of invasive Candida infection, prophylaxis vs no prophylaxis | During critical care | 0.46 | log-normal (−1.17 to −0.39) |
Costs
Risk assessment was assumed (based on expert opinion) to require 10 minutes of nurse time, giving a cost of £8.67. 96 Prophylaxis costs were calculated assuming a standard regimen of 400 mg for 10 days, with unit costs taken from the British National Formulary (BNF)97 (Table 26). This unit cost recognises that, according to the BNF, non-proprietary fluconazole intravenous infusion was available from September 2011. The recommended dose of 400 mg per day would be given as two 100 ml (200 mg) infusions. This would be an appropriate regimen to use for non-neutropenic, critically ill patients when the source of infection is unknown and there is a very high suspicion of invasive Candida infection. Once the patient can absorb, they may, even within critical care, be switched to the lower-cost oral formulation and this, together with the possibility of local discounts, is considered in the sensitivity analysis. The resultant unit cost for intravenous fluconazole of £7.78 per day replaces the unit cost of £45.74 per day used in a previous analysis of the FIRE Study, which was taken from a 2006 HPA report,4 inflated to 2010–2011 prices. The previous unit cost did not reflect the price reductions for the generic indication. Note also that, unlike the previous version, the base case assumes a more realistic treatment duration of 10 days rather than 14 days. The net effect is that in the base case the unit cost of antifungal prophylaxis is £77.80 per day, not the £640 assumed previously. Morbidity costs were included from critical care admission until ultimate discharge from acute hospital. These costs were calculated by estimating LOS both within and after critical care. Each day of critical care was classified according to Healthcare Resource Group 4 (HRG4) category derived from organ support data in the CCMDS, which forms part of the routine CMP data collection. Each bed-day was costed with the corresponding cost per bed-day from the UK ‘Payment by Results’ database. 98 Costs per bed-day in hospital after discharge from critical care were taken from the literature. 99 No costs after the initial hospital episode were considered. All costs were adjusted to 2010–11 price levels. 96
| Parameter | Point estimate/mean | Distributiona | Source |
|---|---|---|---|
| Cost of course of prophylaxis (£) | 77.80 | gamma (0.98, 79.00) | BNF 201397 |
| Critical care unit LOS (days): no invasive Candida infection | 10.16 | gamma (0.83, 12.18) | Combined data set |
| Critical care unit LOS (days): invasive Candida infection | 24.95 | gamma (1.82, 13.74) | Combined data set |
| Hospital LOS after critical care (days): no invasive Candida infection | 22.72 | gamma (0.57, 39.53) | Combined data set |
| Hospital LOS after critical care (days): invasive Candida infection | 36.60 | gamma (1.07, 34.22) | Combined data set |
| Unit cost of critical care bed-day (£): no invasive Candida infection | 1085 | gamma (22.3, 48.63) | Reference cost by HRG |
| Unit cost of critical care bed-day (£): invasive Candida infection | 1351 | gamma (31.06, 43.48) | Reference cost by HRG |
Lifetime quality-adjusted life-years
The main outcome measure was the lifetime QALY. This measure required using data on mortality from the original hospital episode (for patients with and without invasive Candida infection), and all-cause mortality after hospital discharge to project life-years following each strategy. These estimated life-years were combined with estimates of health-related quality of life (HRQOL) to project lifetime QALYs for each patient. 100 It was recognised that critical care survivors have a higher risk of death than the age-/ sex-matched general population. 101,102 There is a lack of work defining the size and duration of excess mortality following critical care survival, both generally and specifically for non-neutropenic adult patients. For example, for adult patients with severe sepsis (including sepsis shock) the strongest evidence is in support of an excess mortality of approximately 20% for up to 4 years after discharge from critical care,103 although some previous work has applied excess mortality for up to 25 years. 104 In this evaluation, the base-case analysis followed previous studies in taking a conservative approach and applied excess mortality of 20% for up to 4 years (see subsequent sensitivity analysis). 100,102,105 Future costs and outcomes were discounted at the recommended rate of 3.5%. 106
Base-case analysis
The probabilistic sensitivity analysis recognised parameter uncertainty by resampling the input parameters 5000 times from the designated distributions. Each iteration processed the patient cohort through each of the eight strategies described. For each strategy the model reported process measures and short-term end points, including the proportion of patients predicted to receive antifungal prophylaxis, the proportion having invasive Candida infection, and the mortality within critical care. The model also reported final end points including lifetime costs (£) and QALYs per patient for each strategy. Incremental costs, QALYs and incremental net benefits (INBs), at a threshold of £20,000 per QALY, were calculated as the differences in mean end points following each prophylaxis strategy compared with current practice. Across the 5000 runs, means were reported together with the 2.5 and 97.5 percentiles to give the limits of the 95% credible intervals. Cost-effectiveness acceptability curves (CEACs) were calculated according to the proportion of replications for which each strategy was the most cost-effective, i.e. had the maximum net monetary benefits across all eight strategies, at different levels of willingness to pay for a QALY gain (£0 to £50,000 per QALY gained). The analyses were repeated for the risk thresholds of 0.5%, 1% and 2%.
Results: base case
The model predicted that following risk assessment with the threshold set to 0.5%, the proportion of patients receiving prophylaxis ranged from 17% to 30% (Table 27). The strategies that had risk assessment at multiple time points were predicted to result in a higher proportion of patients receiving antifungal prophylaxis; if, for example, risk assessment was only undertaken at the end of calendar day 3, then around 23% of patients were predicted to have prophylaxis versus 30% if risk assessment was performed at admission and the end of calendar day 3 or at all three time points. At the higher risk thresholds of 1% and 2%, the predicted proportions receiving prophylaxis ranged from 4% to 14% and from 1% to 5%, respectively (Tables 28 and 29). The current practice of no risk assessment and prophylaxis was predicted to have an incidence of invasive Candida infection during the critical care stay of 0.57%. The prophylaxis strategies were predicted to somewhat reduce the incidence of infection, for example to 0.47% if prophylaxis was provided for patients whose risk at the end of day 3 exceeded 2%. The lowest incidences of invasive Candida infection were following the strategies which required risk assessment at all three time points. The proportion who died after invasive Candida infection was slightly lower following prophylaxis than under current practice, but the reductions in overall mortality during the critical care stay were small (see Tables 27–29).
| Strategy | Percentage treated | Percentage invasive Candida infection | Mortality from invasive Candida infection | Overall mortality | Mean LOS in critical care | Assessment costa | Mean prophylaxis costa | Mean hospitalization costa |
|---|---|---|---|---|---|---|---|---|
| No risk assessment | 0.00 | 0.57 | 0.13 | 18.08 | 27.78 | 0 | 0 | 16,772 |
| Risk assessment | ||||||||
| On admission | 17.53 | 0.51 | 0.12 | 18.07 | 27.77 | 9 | 14 | 16,760 |
| At end of 24 hours | 15.65 | 0.55 | 0.13 | 18.08 | 27.78 | 8 | 12 | 16,767 |
| At end of calendar day 3 | 22.57 | 0.41 | 0.10 | 18.06 | 27.78 | 8 | 18 | 16,740 |
| Risk assessment (at multiple time points) | ||||||||
| On admission and at end of 24 hours | 24.29 | 0.47 | 0.12 | 18.07 | 27.77 | 15 | 19 | 16,756 |
| At end of 24 hours and at end of calendar day 3 | 21.74 | 0.36 | 0.10 | 18.06 | 27.74 | 15 | 17 | 16,735 |
| On admission and at end of calendar day 3 | 30.37 | 0.32 | 0.09 | 18.06 | 27.74 | 15 | 24 | 16,728 |
| On admission, at end of 24 hours and at end of calendar day 3 | 29.79 | 0.31 | 0.09 | 18.06 | 27.74 | 21 | 23 | 16,723 |
| Strategy | Percentage treated | Percentage invasive Candida infection | Mortality from invasive Candida infection | Overall mortality | Mean LOS in critical care | Assessment costa | Mean prophylaxis costa | Mean hospitalization costa |
|---|---|---|---|---|---|---|---|---|
| No risk assessment | 0.00 | 0.57 | 0.13 | 18.08 | 27.78 | 0 | 0 | 16,772 |
| Risk assessment | ||||||||
| On admission | 4.12 | 0.56 | 0.13 | 18.08 | 27.78 | 9 | 3 | 16,769 |
| At end of 24 hours | 5.76 | 0.57 | 0.13 | 18.08 | 27.78 | 8 | 4 | 16,771 |
| At end of calendar day 3 | 11.76 | 0.44 | 0.11 | 18.06 | 27.78 | 8 | 9 | 16,748 |
| Risk assessment (at multiple time points) | ||||||||
| On admission and at end of 24 hours | 8.37 | 0.52 | 0.13 | 18.08 | 27.78 | 17 | 7 | 16,767 |
| At end of 24 hours and at end of calendar day 3 | 11.30 | 0.41 | 0.11 | 18.06 | 27.75 | 16 | 9 | 16,743 |
| On admission and at end of calendar day 3 | 14.31 | 0.37 | 0.10 | 18.06 | 27.75 | 16 | 11 | 16,742 |
| On admission, 24 hours and at end of calendar day 3 | 13.54 | 0.36 | 0.10 | 18.06 | 27.75 | 24 | 11 | 16,738 |
| Strategy | Percentage treated | Percentage invasive Candida infection | Mortality from invasive Candida infection | Overall mortality | Mean LOS in critical care | Assessment costa | Mean prophylaxis costa | Mean hospitalization costa |
|---|---|---|---|---|---|---|---|---|
| No risk assessment | 0.00 | 0.57 | 0.13 | 18.08 | 27.78 | 0 | 0 | 16,772 |
| Risk assessment | ||||||||
| On admission | 1.21 | 0.56 | 0.13 | 18.08 | 27.78 | 9 | 1 | 16,771 |
| At end of 24 hours | 2.06 | 0.56 | 0.13 | 18.08 | 27.78 | 8 | 2 | 16,771 |
| At end of calendar day 3 | 4.54 | 0.49 | 0.12 | 18.07 | 27.78 | 8 | 4 | 16,756 |
| Risk assessment (at multiple time points) | ||||||||
| On admission and at end of 24 hours | 2.83 | 0.55 | 0.13 | 18.08 | 27.78 | 17 | 2 | 16,770 |
| At end of 24 hours and at end of calendar day 3 | 4.72 | 0.46 | 0.11 | 18.07 | 27.77 | 16 | 4 | 16,754 |
| On admission and at end of calendar day 3 | 5.37 | 0.47 | 0.12 | 18.07 | 27.77 | 16 | 4 | 16,757 |
| On admission, at end of 24 hours and at end of calendar day 3 | 5.37 | 0.44 | 0.11 | 18.07 | 27.77 | 24 | 4 | 16,754 |
Prophylaxis was predicted to slightly reduce mean hospitalisation costs. For example, at all three risk thresholds, a strategy of providing prophylaxis at the end of calendar day 3 was predicted to reduce mean hospitalisation costs by around £25 (see Tables 27–29). For some risk assessment and prophylaxis strategies, for example risk assessment at admission or the end of 24 hours, the reduction in hospitalisation costs was offset by higher assessment and prophylaxis costs, leading to higher total costs than current practice (Tables 30–32). For other risk assessment strategies, for example risk assessment at the end of day 3, the costs of assessment and prophylaxis were exceeded by the reduction in hospitalisation costs, leading to lower total costs than that of current practice at each risk threshold.
| Strategy | Total costs (£) | Life-years | QALY | Net monetary benefit (£)a |
|---|---|---|---|---|
| No risk assessment | 16,772 | 11.797 | 8.628 | 155,791 |
| Risk assessment | ||||
| On admission | 16,782 | 11.798 | 8.629 | 155,790 |
| At end of 24 hours | 16,787 | 11.797 | 8.628 | 155,775 |
| At end of calendar day 3 | 16,765 | 11.800 | 8.630 | 155,832 |
| Risk assessment (at multiple time points) | ||||
| On admission and at end of 24 hours | 16,790 | 11.798 | 8.629 | 155,784 |
| At end of 24 hours and at end of calendar day 3 | 16,766 | 11.800 | 8.630 | 155,830 |
| On admission and at end of calendar day 3 | 16,767 | 11.800 | 8.630 | 155,837 |
| On admission, at end of 24 hours and at end of calendar day 3 | 16,767 | 11.800 | 8.630 | 155,838 |
| Strategy | Total costs (£) | Life-years | QALY | Net monetary benefit (£)a |
|---|---|---|---|---|
| No risk assessment | 16,772 | 11.797 | 8.628 | 155,791 |
| Risk assessment | ||||
| On admission | 16,781 | 11.798 | 8.628 | 155,784 |
| At end of 24 hours | 16,784 | 11.797 | 8.628 | 155,777 |
| At end of calendar day 3 | 16,765 | 11.799 | 8.629 | 155,825 |
| Risk assessment (at multiple time points) | ||||
| On admission and at end of 24 hours | 16,791 | 11.798 | 8.628 | 155,774 |
| At end of 24 hours and at end of calendar day 3 | 16,767 | 11.799 | 8.630 | 155,823 |
| On admission and at end of calendar day 3 | 16,769 | 11.800 | 8.630 | 155,824 |
| On admission, at end of 24 hours and at end of calendar day 3 | 16,772 | 11.800 | 8.630 | 155,823 |
| Strategy | Total costs (£) | Life-years | QALY | Net monetary benefit (£)a |
|---|---|---|---|---|
| No risk assessment | 16,772 | 11.797 | 8.628 | 155,791 |
| Risk assessment | ||||
| On admission | 16,780 | 11.798 | 8.628 | 155,784 |
| At end of 24 hours | 16,781 | 11.797 | 8.628 | 155,783 |
| At end of calendar day 3 | 16,768 | 11.799 | 8.629 | 155,812 |
| Risk assessment (at multiple time points) | ||||
| On admission and at end of 24 hours | 16,789 | 11.798 | 8.628 | 155,775 |
| At end of 24 hours and at end of calendar day 3 | 16,774 | 11.799 | 8.629 | 155,808 |
| On admission and at end of calendar day 3 | 16,777 | 11.799 | 8.629 | 155,802 |
| On admission, at end of 24 hours and at end of calendar day 3 | 16,782 | 11.799 | 8.629 | 155,800 |
The incremental analysis compared each prophylaxis strategy with current practice (Tables 33–35). These results showed that, irrespective of the risk threshold, the incremental QALYs of the prophylaxis strategies compared with current practice were positive, but small. The incremental costs of the risk assessment strategies were negative for strategies including risk assessment at the end of day 3, whether at single or at multiple time points. The INB at a risk threshold of 0.5% was highest when assessment and prophylaxis were administered at all time points. For risk threshold of 1% and 2%, the highest INB was associated with risk assessment and prophylaxis at the end of calendar day 3.
| Strategy | Incremental cost (£) | Incremental QALYa | INB (95% credible intervals)b |
|---|---|---|---|
| No risk assessment | – | – | – |
| Risk assessment | |||
| On admission | 10 | 0.0004 | −2 (−60 to 65) |
| At end of 24 hours | 15 | 0.0000 | −16 (−90 to 58) |
| At end of calendar day 3 | −7 | 0.0017 | 41 (−81 to 162) |
| Risk assessment (at multiple time points) | |||
| On admission and end of 24 hours | 18 | 0.0005 | −7 (−92 to 78) |
| At end of 24 hours and at end of calendar day 3 | −6 | 0.0016 | 38 (−89 to 165) |
| On admission and at end of calendar day 3 | −5 | 0.0020 | 46 (−81 to 172) |
| On admission, at end of 24 hours and at end of calendar day 3 | −4 | 0.0021 | 47 (−100 to 194) |
| Strategy | Incremental cost (£) | Incremental QALYa | INB (95% credible intervals)b |
|---|---|---|---|
| No risk assessment | – | – | – |
| Risk assessment | |||
| On admission | 9 | 0.0001 | −8 (−59 to 44) |
| At end of 24 hours | 12 | 0.0001 | −15 (−79 to 50) |
| At end of calendar day 3 | −7 | 0.0013 | 33 (−90 to 157) |
| Risk assessment (at multiple time points) | |||
| On admission and at end of 24 hours | 19 | 0.0001 | −17 (−103 to 69) |
| At end of 24 hours and at end of calendar day 3 | −5 | 0.0014 | 32 (−109 to 173) |
| On admission and at end of calendar day 3 | −3 | 0.0015 | 33 (−105 to 171) |
| On admission, at end of 24 hours and at end of calendar day 3 | 0 | 0.0016 | 32 (−132 to 195) |
| Strategy | Incremental cost (£) | Incremental QALYa | INB (95% credible intervals)b |
|---|---|---|---|
| No risk assessment | – | – | – |
| Risk assessment | |||
| On admission | 8 | 0.0001 | −7 (−22 to 8) |
| At end of 24 hours | 9 | 0.0000 | −8 (−29 to 12) |
| At end of calendar day 3 | −4 | 0.0008 | 20 (−23 to 64) |
| Risk assessment (at multiple time points) | |||
| On admission and at end of 24 hours | 18 | 0.0001 | −16 (−42 to 9) |
| At end of 24 hours and at end of calendar day 3 | 2 | 0.0009 | 16 (−32 to 64) |
| On admission and at end of calendar day 3 | 6 | 0.0008 | −10 (−36 to 57) |
| On admission, at end of 24 hours and at end of calendar day 3 | 10 | 0.0009 | 8 (−47 to 63) |
The CEACs are plotted for each risk threshold in Figures 16–18. They show that at the 1% and 2% risk thresholds, risk assessment and prophylaxis at the end of calendar day 3 was the strategy most likely to be cost-effective at the recommended cost-effectiveness threshold of £20,000 per QALY gain. For the lower risk threshold (0.5%), the strategy with the highest probability of being cost-effective at £20,000 per QALY was to assess risk at all three time points. At each risk threshold there was considerable uncertainty surrounding the relative cost-effectiveness of the alternative strategies, and at the £20,000 per QALY threshold the probability that any particular strategy would in fact be the most cost-effective did not exceed 30%.
FIGURE 16.
Cost-effectiveness acceptability curves at risk threshold of 0.5%. Note: the strategies for ‘admission’ and ‘admission and 24 hours’ are indistinguishable from ‘24 hours’.
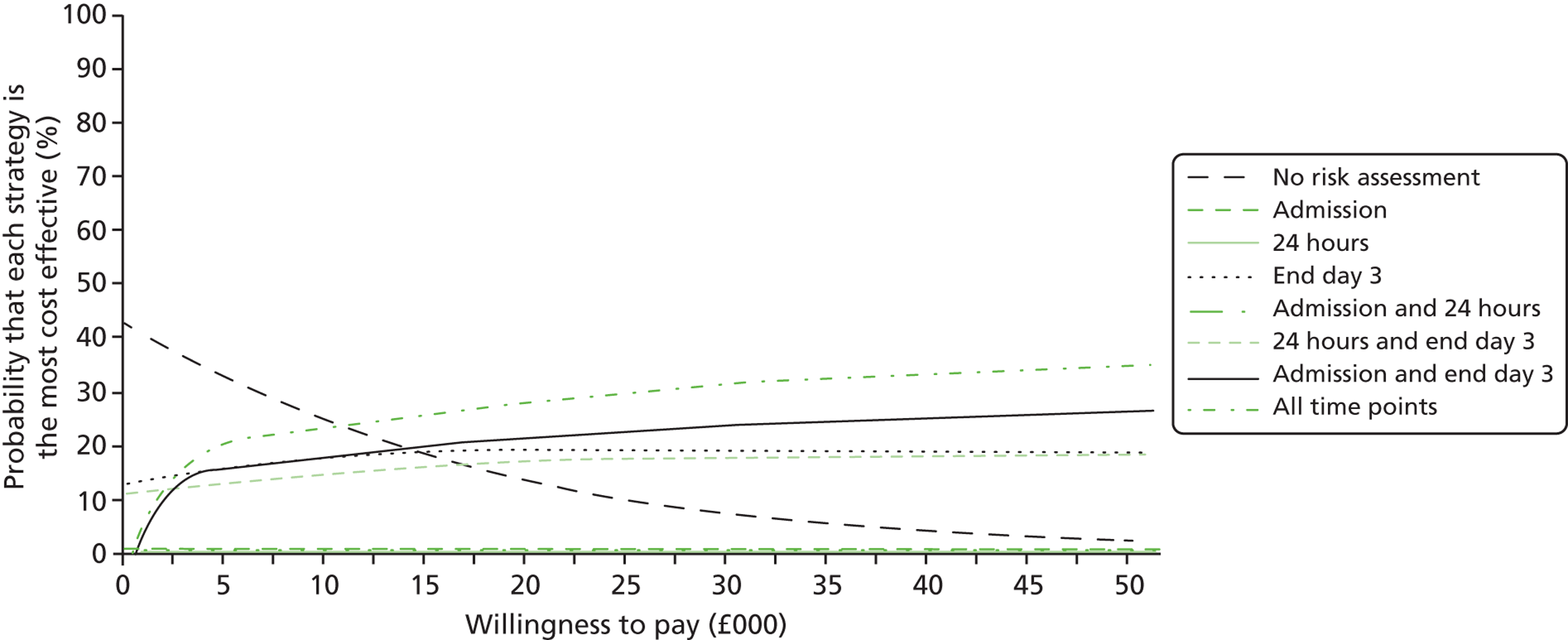
FIGURE 17.
Cost-effectiveness acceptability curves at risk threshold of 1%. Note: the strategies for ‘admission’ and ‘admission and 24 hours’ are indistinguishable from ‘24 hours’.
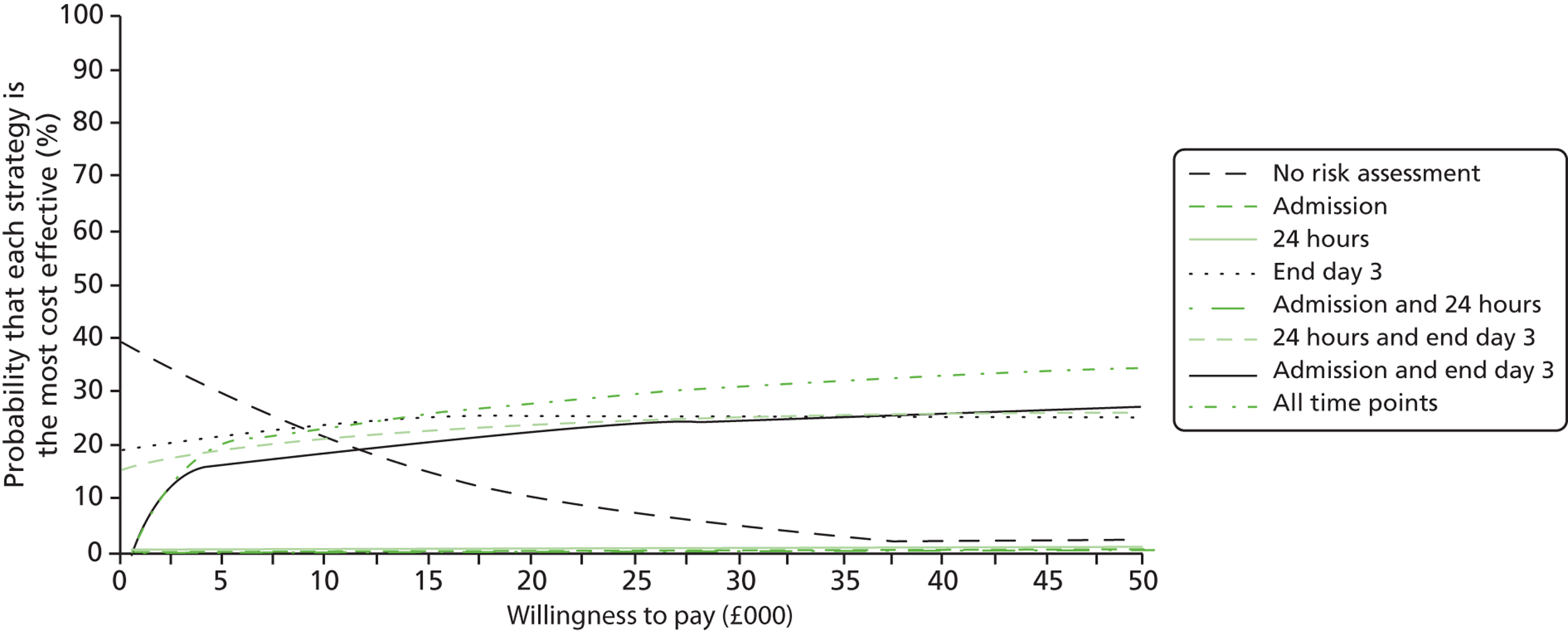
FIGURE 18.
Cost-effectiveness acceptability curves at risk threshold of 2%. Note: the strategies for ‘admission’ and ‘admission and 24 hours’ are indistinguishable from ‘24 hours’.
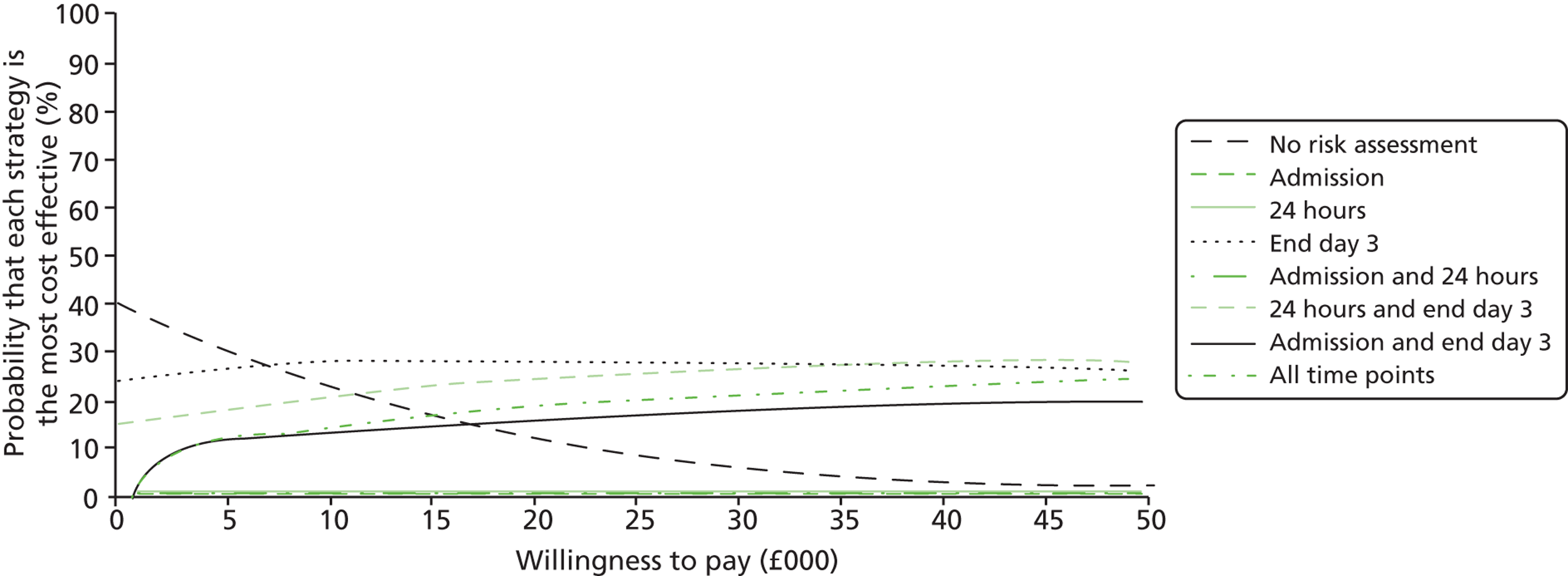
Methods: scenario analyses
The main assumptions made in the base-case analysis were challenged in the following scenario analyses, which repeated the above analyses but with alternative assumptions.
-
The base-case analysis assumed the cost of risk assessment was based on 10 minutes of nursing time. The sensitivity analysis considered alternative scenarios assuming 5 or 20 minutes of nursing time to undertake the risk assessment.
-
The base-case analysis assumed each patient had the recommended dose of fluconazole (400 mg per day), administered in an intravenous form. However, the systematic review reported that some RCTs have reported similar levels of effectiveness, with doses as low as 100 mg. 19 We therefore undertook a sensitivity analysis in which the dose of fluconazole was reduced to 100 mg, and the costs lowered accordingly by 75%, while the RR of invasive Candida infection remained the same as in the base case. Another rationale for considering a 75% reduction in the unit costs of fluconazole is that even if the dose is maintained at 400 mg, local discounts in the range 50–70% may be available. Also, once the patient is able to absorb orally, some hospitals may administer the lower-cost oral formulation of fluconazole. Therefore, it is relevant to consider a reduction in the unit cost of 75% even for the same dose.
-
The base case analysis assumed a duration of prophylaxis of 10 days based on the average LOS in critical care. The sensitivity analysis considered alternative scenarios assuming 5 days and 14 days of prophylaxis as informed by systematic review. 19
-
The base-case analysis assumed a duration of excess mortality for critical care survivors of 4 years after discharge from critical care, but excess mortality could continue for up to 25 years. 104 The sensitivity analysis assumed the magnitude of excess death rates for an extended period of time (25 years) taken from a previous study. 104 These excess death rates, relative to age- and sex-matched mortality in the general population, were applied beyond 4 years for up to 25 years.
-
The base-case analysis assumed the HRQOL of critical care survivors was 80% of that of the general population. The sensitivity analysis assumed a lower figure of 70%.
The scenario analysis also examined best-case and worst-case scenarios as defined below:
-
Best-case scenario: 10 minutes of nursing time, low dose of prophylaxis/75% discount on cost of prophylaxis, prophylaxis administered for 5 days, excess mortality for four years and HRQOL of survivors 80% that of general population.
-
Worst-case scenario: 20 minutes of nursing time, standard dose of prophylaxis/no discount on cost of prophylaxis, prophylaxis administered for 14 days, excess mortality for 25 years and HRQOL of survivors 70% that of general population.
Results: scenario analyses
The results of the scenario analyses showed that the base-case results were generally robust to each of the alternative assumptions (Tables 36–38). Across risk thresholds and prophylaxis strategies, the scenarios that considered increased costs of assessment and prophylaxis (e.g. higher nursing time, increased duration of prophylaxis) led to lower INB for the risk assessment strategies than for the base case, assuming lower costs of assessment and prophylaxis (e.g. lower nursing time, shorter duration of prophylaxis) led to higher INB. Excess mortality for 25 years and decrement in HRQOL weights showed small effects on INB. The general conclusion that the strategies that included risk assessment at the end of calendar day 3 were relatively cost-effective was robust to the alternative best-case and worst-case scenarios considered.
| Strategy | Base case | Assessment time | Low dose of prophylaxis/75% discounted price | Excess mortality up to 25 years | HRQOL 70% of general population | Prophylaxis | Best case | Worst case | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 minutes of nursing time | 20 minutes of nursing time | 5 days | 14 days | |||||||
| No risk assessment | – | – | – | – | – | – | – | – | – | – |
| Risk assessment | ||||||||||
| On admission | −2 | 2 | −11 | 8 | −2 | −2 | 5 | −7 | 16 | −18 |
| At end of 24 hours | −16 | −12 | −24 | −7 | −16 | −16 | −10 | −21 | 0 | −29 |
| At end of calendar day 3 | 41 | 44 | 33 | 54 | 40 | 40 | 49 | 34 | 61 | 17 |
| Risk assessment (at multiple time points) | ||||||||||
| On admission and at end of 24 hours | −7 | 0 | −23 | 7 | −8 | −7 | 2 | −14 | 19 | −33 |
| At end of 24 hours and at end of calendar day 3 | 38 | 46 | 24 | 51 | 37 | 38 | 47 | 32 | 62 | 8 |
| On admission and at end of calendar day 3 | 46 | 53 | 31 | 64 | 44 | 45 | 57 | 37 | 76 | 11 |
| On admission, at end of 24 hours and at end of calendar day 3 | 47 | 58 | 26 | 64 | 46 | 46 | 58 | 38 | 80 | 5 |
| Strategy | Base case | Assessment time | Low dose of prophylaxis/75% discounted price | Excess mortality up to 25 years | HRQOL 70% of general population | Prophylaxis | Best case | Worst case | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 minutes of nursing time | 20 minutes of nursing time | 5 days | 14 days | |||||||
| No risk assessment | – | – | – | – | – | – | – | – | – | – |
| Risk assessment | ||||||||||
| On admission | −8 | −3 | −16 | −5 | −8 | −8 | −6 | −9 | 0 | −18 |
| At end of 24 hours | −15 | −10 | −23 | −11 | −14 | −14 | −12 | −16 | −6 | −24 |
| At end of calendar day 3 | 33 | 37 | 26 | 40 | 33 | 32 | 38 | 30 | 46 | 15 |
| Risk assessment (at multiple time points) | ||||||||||
| On admission and at end of 24 hours | −17 | −9 | −34 | −12 | −17 | −17 | −14 | −19 | −2 | −36 |
| At end of 24 hours and at end of calendar day 3 | 32 | 40 | 16 | 39 | 31 | 31 | 36 | 29 | 48 | 6 |
| On admission and at end of calendar day 3 | 33 | 41 | 17 | 41 | 32 | 32 | 39 | 29 | 51 | 5 |
| On admission, at end of 24 hours and at end of calendar day 3 | 32 | 43 | 8 | 40 | 31 | 30 | 37 | 28 | 53 | −5 |
| Strategy | Base case | Assessment time | Low dose of prophylaxis/75% discounted price | Excess mortality up to 25 years | HRQOL 70% of general population | Prophylaxis | Best case | Worst case | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 minutes of nursing time | 20 minutes of nursing time | 5 days | 14 days | |||||||
| No risk assessment | – | – | – | – | – | – | – | – | – | – |
| Risk assessment | ||||||||||
| On admission | −7 | −3 | −16 | −6 | −7 | −7 | −7 | −7 | −2 | −16 |
| At end of 24 hours | −8 | −4 | −17 | −7 | −8 | −8 | −8 | −9 | −3 | −17 |
| At end of calendar day 3 | 20 | 24 | 13 | 23 | 20 | 20 | 22 | 19 | 28 | 7 |
| Risk assessment (at multiple time points) | ||||||||||
| On admission and at end of 24 hours | −16 | −8 | −33 | −15 | −16 | −17 | −15 | −17 | −6 | −34 |
| At end of 24 hours and at end of calendar day 3 | 16 | 24 | 0 | 19 | 16 | 16 | 18 | 15 | 28 | −6 |
| On admission and at end of calendar day 3 | −10 | 19 | −6 | 14 | 10 | 10 | 13 | 9 | 22 | −12 |
| On admission, at end of 24 hours and at end of calendar day 3 | 8 | 20 | −16 | 11 | 8 | 7 | 10 | 7 | 24 | −23 |
Methods: value of information analysis
The decision as to which prophylaxis strategy to adopt based on the current evidence available remains uncertain. There is always the possibility that the strategy that appears the most cost-effective from current evidence would not be the optimal approach if perfect information was available. The expected costs of choosing the wrong strategy can be considered in terms of lost resources, but also health gain forgone. The expected costs of this decision uncertainty can be quantified according to the expected value of perfect information (EVPI). 107 EVPI can inform whether or not further research is worthwhile, by reporting whether or not the EVPI exceeds the anticipated research costs. 108,109 EVPI was calculated for the total population anticipated to benefit from the strategies considered, assuming that the eligible population of interest was 100,000 admissions to critical care each year, and that the relevant life cycle for the technology was 5 years.
To establish where further research might be best targeted, EVPI can also be reported for groups of parameters termed EVPI for parameters or expected value of partial perfect information (EVPPI). 110 This approach can direct research towards those parameters and research designs that have most value. The groups of parameters considered were baseline probability of invasive Candida infection, mortality with and without invasive Candida infection, PPV and NPV, RRs of infection after prophylaxis, morbidity costs, long-term HRQOL, and survival.
Results: value of information analysis
Figures 19 and 20 summarise the population EVPI for the three threshold levels of risk. At a threshold of £20,000 per QALY the EVPI estimates for the population ranged between £12M (0.5% risk) and £14M (1% risk) at £20,000 per QALY. The corresponding EVPI per patient ranged from around £120 to £140.
FIGURE 19.
Expected value of perfect information for the population.
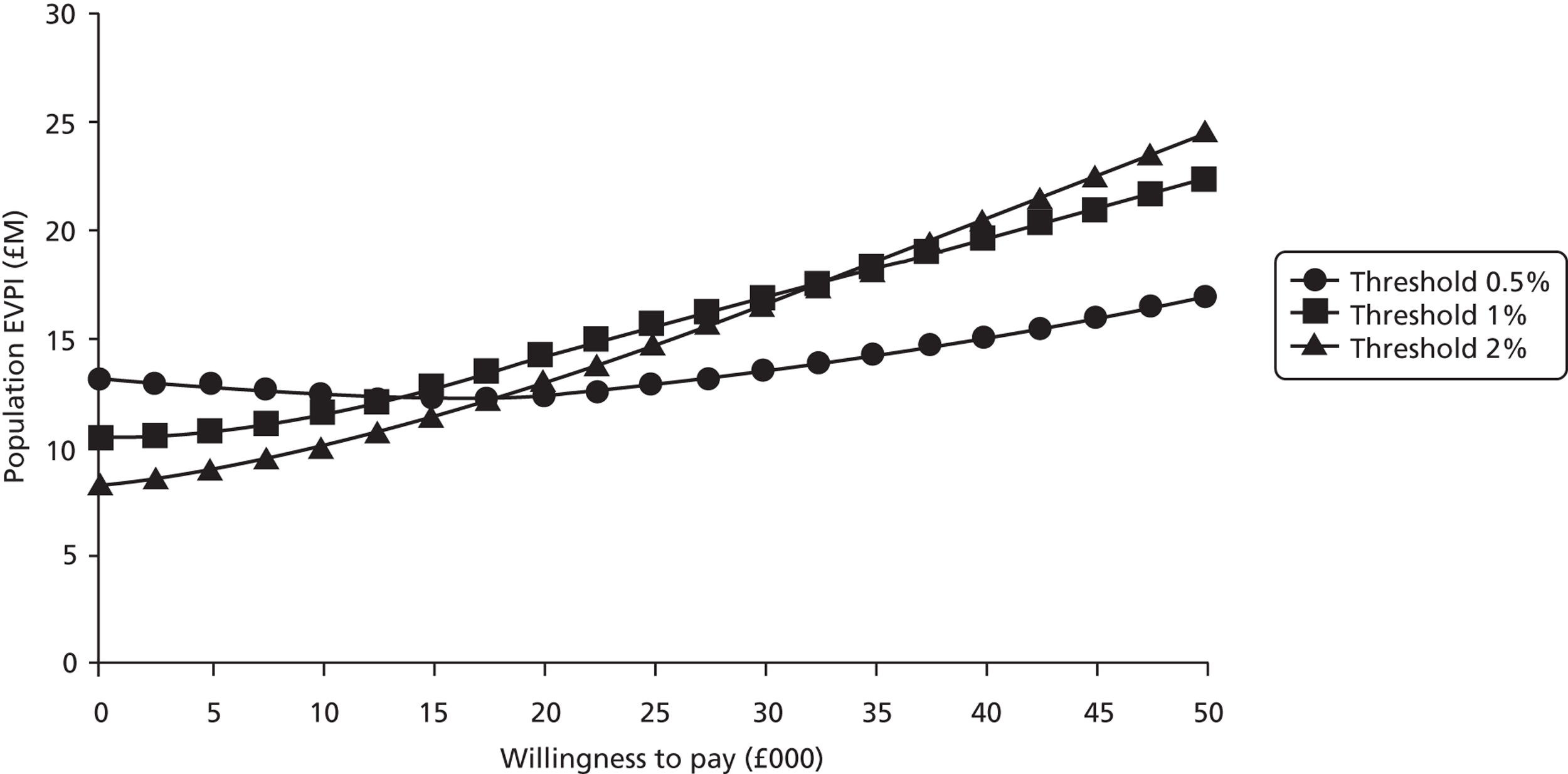
FIGURE 20.
Expected value of perfect information per patient.

These results indicate that across all parameters in the decision model, the value of further research for the whole population of interest is high relative to the likely research costs, and that the value is similar across risk thresholds.
Figure 21 reports for the overall population EVPPI estimates for each group of parameters according to risk threshold. The results highlight that the value of information for each group of parameters is similar across risk thresholds. The results also suggest that even after the FIRE Study, given the large population of interest for this decision problem (100,000 per year), there is still high value in acquiring more information on parameters such as PPV and NPV.
FIGURE 21.
Expected value of partial perfect information for the population at £20,000 per QALY, according to alternative risk thresholds.
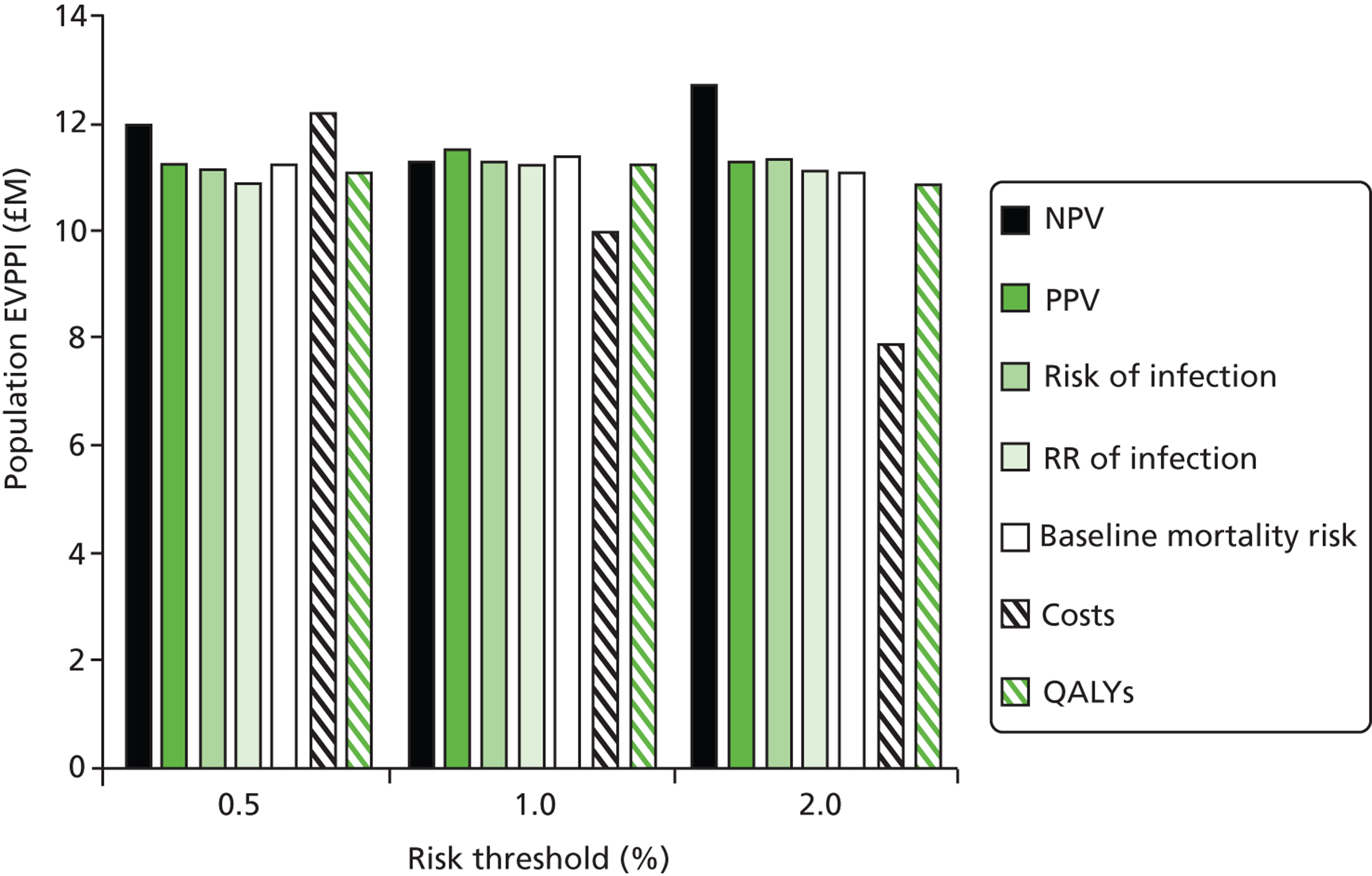
The EVPPI per patient (Figure 22) suggests the value of further research for most parameters is from £80 to £127 per patient. The decison whether or not to fund further research must then be weighed against the additional costs.
FIGURE 22.
Expected value of partial perfect information per patient, at £20,000 per QALY, for alternative risk thresholds.
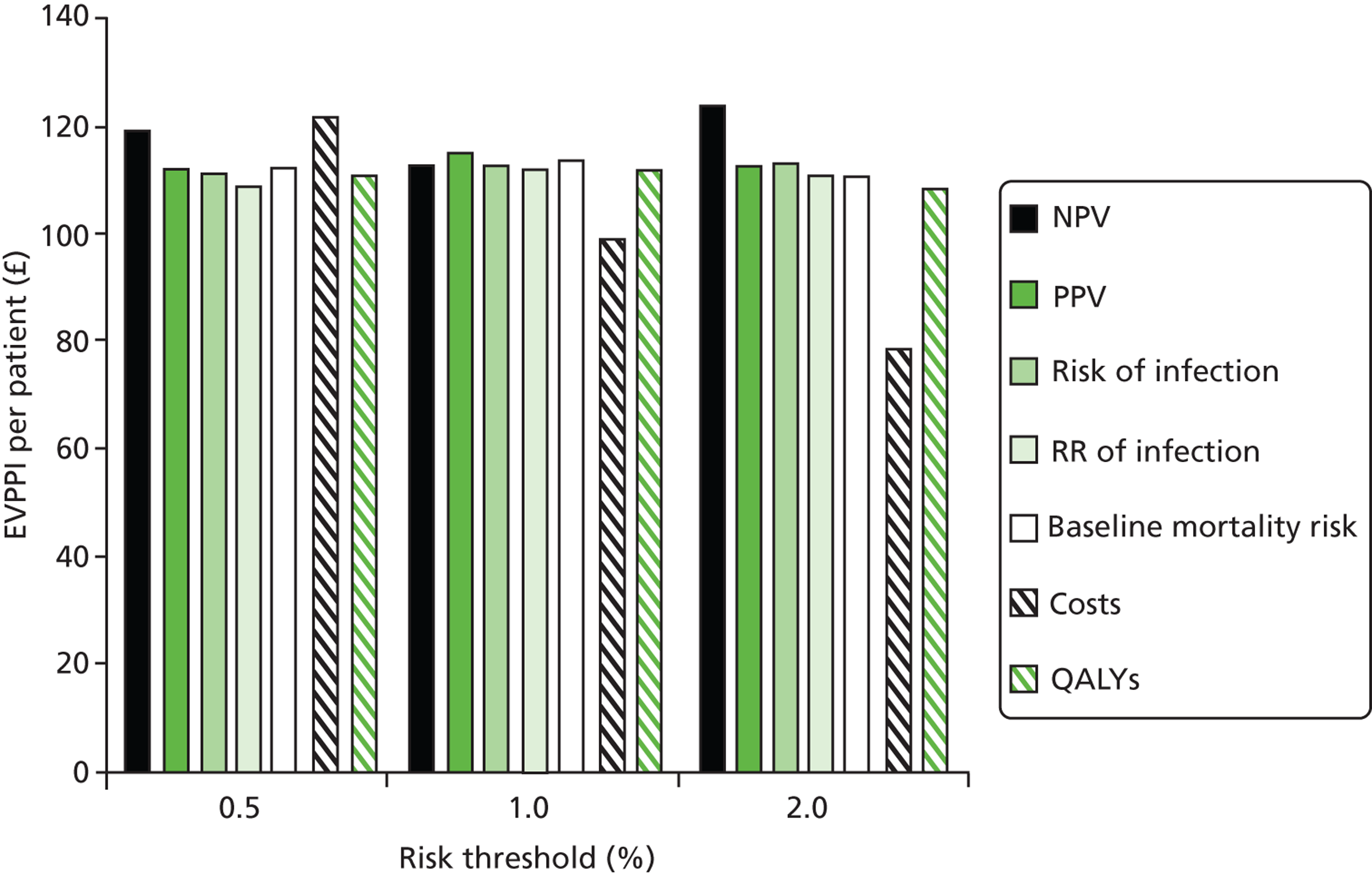
Discussion
This economic evaluation assessed the relative cost-effectiveness of alternative prophylaxis strategies for preventing invasive Candida infection for patients admitted to critical care who do not currently receive antifungal prophylaxis. The main finding was that, at a threshold risk for invasive Candida infection of 1% or 2%, the most cost-effective strategy was risk assessment and prophylaxis at the end of calendar day 3, which would require approximately 5–12% of eligible patients to receive antifungal prophylaxis. With a lower threshold risk for invasive Candida infection (0.5%), risk assessment and prophylaxis at all time points was the most cost-effective prophylaxis strategy, but would require around 30% of eligible patients to receive antifungal prophylaxis, which raises concerns about the impact on resistance.
In this general population of non-neutropenic, critically ill adult patients, the incidence of invasive Candida infection was low. As a result, the cost-effectiveness model predicted that prophylaxis prevented relatively few invasive Candida infections leading to small reductions in mortality and gains in QALYs. However, the costs of risk assessment and prophylaxis were also low relative to other hospitalisation costs, and for some risk assessment strategies this led to a net reduction in hospitalisation costs. The risk assessment strategies only lead to small gains in net monetary benefits compared with current practice, however, and so, given the large uncertainties, the probability that any particular risk assessment strategy was most cost-effective did not exceed 30% at the recommended threshold willingness to pay for a QALY gain.
This is the first cost-effectiveness analysis (CEA) to compare alternative strategies for preventing invasive Candida infection. The study has several important strengths. First, the decision model is populated mainly by parameters (e.g. PPV, NPV, baseline risk of infection and death, cost with and without infection) estimated from patient-level data collected prospectively in the FIRE Study. The PPVs and NPVs were taken from the FIRE Study validation sample, rather than from the development sample, to avoid any concerns about overfitting the models. Second, the RR of invasive Candida infection after prophylaxis was taken from a systematic review of published RCTs. The review suggested that the RRs were similar irrespective of the level of baseline risk, and were applicable to the low-risk population considered here. Third, the CEA followed methodological recommendations and fully considered both parameter uncertainty and structural uncertainty emanating from the assumptions made in constructing and populating the model. Fourth, the initial proposal was for a CEA limited to comparing current practice with risk assessment and prophylaxis at a single time point, but instead we followed expert clinical advice and broadened the range of strategies to allow risk assessment at several time points. Fifth, the CEA quantified the expected value of further research both overall and for specific groups of parameters.
Cost-effectiveness analyses that estimate relative costs and outcomes over the lifetime inevitably make assumptions. 111 Here we made plausible assumptions, for example about life expectancy for critical care survivors by drawing on the previous literature. 101,102 The base-case finding that a strategy of risk assessment and prophylaxis was relatively cost-effective was robust to the base-case assumptions concerning the costs of risk assessment and the long-term prognosis for critical care survivors. However, the results were relatively more sensitive to the worst- and best-case scenarios in which a number of parameters such as duration of prophylaxis, nursing time, discounted price, dose of prophylaxis, duration of excess mortality and quality of life were varied jointly.
The decision model also reported the expected value of further research. The EVPI for the entire population relevant to this decision problem (100,000 critical care admissions per year) may be as high as £14M. The reasons why further research may have high overall value are that the differences in costs and outcomes across strategies were small, the parameter uncertainty was relatively high and the overall target population is large. It should be recognised, however, that these estimates of the upper bound on the value of research in this area are lower than previous estimates for other interventions in critical care, although these interventions would affect substantially smaller populations. 104 The estimates of EVPPI provide an upper bound on the relative value of further research on each group of parameters such as the RRs after prophylaxis, baseline risks of infection and mortality, PPVs and NPVs, costs and lifetime QALYs. For RRs, further research would imply additional RCTs, but it could be argued that the anticipated research would be justified by the value (£11M). For other parameters such as baseline risk of infection and death, where further research is of similar value, such parameters could be collected at relatively low cost, for example alongside existing national clinical audit through the CMP. Parameters such as PPV and NPV also have high value but they would require a new prospective study, and so would be costly to estimate. Such parameters are specific to this decision problem. Instead, it might be more worthwhile to invest in further research on estimating parameters, such as lifetime QALYs after critical care survival, which would be useful for all decision problems in this area and would inform subsequent economic evaluations both for ongoing and future research studies in critical care.
This CEA does have some limitations. First, the model ignored any impact on resistance from increased use of prophylaxis. Including the effects of resistance on the costs and health outcomes of future patients would reduce the relative cost-effectiveness of the risk assessment strategies compared with current practice. Second, no consideration is given to prevention of onward transmission. Hence, the gain from prophylaxis could have been understated. That said, given the model predicted that prophylaxis prevented few invasive Candida infections, it can be anticipated that moving to a dynamic model structure and including the effect on onward transmission would be unlikely to change the conclusions. When an additional variable was added to the FIRE Study risk models indicating the presence of another patient with invasive Candida infection in the critical care unit this was not significantly associated with increased risk of invasive Candida infection, which suggests that onwards transmission was not a major factor. Third, the CEA took a narrow perspective to costing, and included hospitalisation costs only for the initial hospital episode. Hence, any additional costs attributable to invasive Candida infection that fell on community health services or came from hospital readmissions were excluded. It should be noted that the model did incorporate a relatively large increase in average hospitalisation costs following invasive Candida infection but, given the low incidence, it would seem unlikely that including a broader range of costs would alter the conclusions. Finally, it should be noted that the results of this CEA do not apply to those patients (approximately 3% of those otherwise eligible for the decision problem) who are currently prescribed prophylaxis according to clinical judgement.
The study suggests that a strategy of risk assessment and prophylaxis within three calendar days of admission to critical care may be cost-effective. However, it should be recognised that this could increase the risk of resistance, leading to higher costs and increased morbidity for future patients. Emergence of resistance has only been directly linked to fluconazole usage in cases of prolonged treatment in HIV-associated candidosis or in patients with chronic mucocutaneous disease and, as such, is relatively unlikely in this patient population. 112 However, fluconazole usage has been linked to the pathogen shifts away from Candida albicans towards fluconazole-resistant species such as Candida glabrata. 113 The possible consequences of resistance to antifungal prophylaxis could include increased lengths of stay in critical care and in hospital, the additional diagnostic tests and treatment costs for a patient infected with a resistant organism. 114 Studies to date have not fully assessed the cost of resistance to antifungal prophylaxis. In a related context, Smith and Coast115 highlighted that published studies underestimated the true costs of antibacterial resistance. Further research is required to consider the full costs of antifungal prophylaxis in terms of the additional burden to future patients whose treatment with antifungal agents becomes inappropriate due to increased resistance, and the consequent increased use of newer, and more costly, next generation antifungals. 116 Incorporating these effects of resistance in decision analytic modelling is challenging as it requires estimates of additional parameters, such as the resistance rate, the ensuing effect on morbidity and mortality, and a broader model structure to consider future populations who may be affected by increased resistance.
In conclusion, this CEA found that, for non-neutropenic, critically ill adult patients who met the inclusion criteria for the FIRE Study, the most cost-effective strategy at a 1% or 2% risk threshold was to assess the risk of invasive Candida infection at the end of calendar day 3, which would lead to 5–12% of patients receiving antifungal prophylaxis. Although risk assessment at all three time points was the most cost-effective strategy at a 0.5% risk threshold, this would lead to antifungal prophylaxis for around 30% of patients and thus raise concerns about resistance. The incremental costs and QALYs for each of the prophylaxis strategies compared with current practice were relatively small, and there is considerable uncertainty surrounding the cost effectiveness of the alternative strategies. Hence, even after the FIRE study the expected value of further research for this population appears large, but any further research recommendations pertaining to this decision problem should recognise the cost of further research and also consider whether or not the value of such research can be transferred to other decision problems of high clinical relevance. In particular, further research could take the approach followed in this study to assess whether or not a diagnostic test, such as a quantitative polymerase chain reaction (PCR) test117 or (1→3)-β-d-glucan assay,118 would be worthwhile for those patients who according to the FIRE Study risk models are at high risk of invasive Candida infection.
Chapter 8 Conclusions
Implications for health care
The results of the FIRE Study, derived from a highly representative sample of adult general critical care units across the UK, indicated that the rate of IFD among non-neutropenic, critically ill adult patients was lower than had been anticipated from previous, smaller sentinel studies. The ‘true’ incidence of IFD is difficult to determine owing to current use of systemic antifungal therapy in around 5% of admissions in the absence of IFD.
The low incidence of IFD is clearly good news for patients but must not engender complacency, as IFD, although rare, was associated with substantially higher mortality, more intensive organ support and longer LOS within both the critical care unit and acute hospital.
The systematic review of the literature to identify potential risk factors for IFD found that previous work developing risk models or clinical decision rules was generally lacking in statistical power and rigour, and was not focused on making decisions regarding use of antifungal prophylaxis early during the critical care unit stay. Current practice is therefore based on poor evidence and existing clinical decision rules should be used with caution.
Risk modelling using classical statistical methods produced relatively simple risk models, and associated clinical decision rules, which provided acceptable discrimination for identifying patients at ‘high risk’ of invasive Candida infection. Validation of the models within the geographical validation sample (admissions to critical care units in Scotland) indicated that care should be taken when translating the models to a different health-care system/setting. The utility of these models, however, is primarily dependent on their incorporation into a cost-effective treatment strategy.
The results of the economic model suggested that the current most cost-effective treatment strategy for prophylactic use of systemic antifungal agents among non-neutropenic, critically ill adult patients admitted to NHS adult general critical care units is a strategy of risk assessment and antifungal prophylaxis at the end of calendar day 3 at a risk threshold of 1% or 2%. When threshold risk is low (0.5%), risk assessment and prophylaxis at all time points appears to be the most cost effective strategy, but this ignores any additional costs and adverse outcomes resultant from the increase in resistance due to providing antifungal prophylaxis to large numbers of patients (30% of those eligible).
The exploratory work involving machine learning showed some potential to produce more accurate clinical decision rules, but a number of barriers, both technical (e.g. identifying the optimum methods for model development) and practical (e.g. delivering these ‘black box’ models at the bedside in an efficient manner), would need to be overcome before such an approach had any direct impact on health-care delivery and the economic implications would need to be considered.
Recommendations for research
The value of information analysis indicated that an upper bound for future research spending to obtain a perfect answer to this decision problem is around £140 per patient. However, owing to the large number of potential future patients considered in the decision problem (approximately 100,000 non-neutropenic adult patients admitted to NHS adult general critical care units every year), the population total EVPI over a 10-year time horizon is estimated to be around £14M.
Analysis of EVPPI suggested potential benefit from further research on most parameters within the decision model. Future research to inform this decision problem should therefore be focused on parameters for which additional information can be obtained at a relatively low cost. In addition, a number of parameters, for which there is considerable uncertainty, relate not only to this specific decision problem, but also would be applicable more generally across all decision models and economic evaluations in the UK critical care setting, and these would therefore appear to represent the highest priority for future research.
Recommendation 1: Further research is required to consider the full costs of antifungal prophylaxis
The economic analysis of the FIRE Study identified potentially cost-effective treatment strategies that would involve a substantial proportion of patients receiving antifungal prophylaxis. Few data currently exist on the potential downstream impact of such strategies on the management of future patients and the consequent impact on costs and outcomes as a result of the potential to promote antifungal resistance. Future research should consider the additional burden to patients whose treatment with antifungal agents becomes inappropriate owing to increased resistance. This research can inform future decision analytic models required to incorporate additional parameters such as the resistance rate and the ensuing effect on patient morbidity and mortality.
Recommendation 2: Further research should be conducted to inform the long-term survival, including quality and costs of survival, for the population of patients admitted to UK adult general critical care units
Very limited information was available to inform the estimates of long-term survival and quality of life in the decision model and many assumptions needed to be made. Increasing the available information on these important parameters would inform all future economic evaluations in this area. Further information on the long-term survival of critically ill patients may be obtained at low cost through record linkage between the CMP and the NHS Central Register. To date, linkage has been prevented by the lack of algorithms at the NHS Information Centre to link on NHS Number alone, without the need for more detailed patient identifiable data (the collection of which cannot be justified in the context of an ongoing national clinical audit using patient data without individual patient consent). Such algorithms are in the process of being developed, and we are working with the NHS Information Centre to conduct such record linkage in the future. More detailed information on the quality and costs of survival would require specific data collection, with patient consent, in the form of long-term follow-up studies.
Recommendation 3: Future research into treatment strategies for selecting patients for antifungal prophylaxis should consider combining clinical risk estimates, such as those from the FIRE Study risk models, with novel diagnostic tests based on biomarkers
The treatment strategies considered within the FIRE Study consisted of either giving or not giving antifungal prophylaxis to patients identified as high risk by the risk models at one or more of the time points. More complex, but potentially more beneficial, treatment strategies may incorporate a two-stage process, whereby the risk models are used to identify patients as being at high risk of IFD based on their clinical characteristics, and that these patients (rather than receiving antifungal prophylaxis directly as a result of the risk model) are provided a diagnostic test such as quantitative-PCR117 or (1→3)-β-d-glucan assay. 118 Alternatively, patient groups for application of rapid diagnostic tests may be selected based on individual clinical criteria, such as fever. Once suitable biomarkers are identified and appropriately evaluated, such treatment strategies should be evaluated using a similar decision model structure to that used in the FIRE Study.
Recommendation 4: Further research should be considered to inform estimates of the positive predictive value and negative predictive value of the FIRE Study risk models among non-neutropenic, critically ill adult patients admitted to UK adult general critical care units
Given the low event rate observed in the FIRE Study, the estimates of PPV and NPV were uncertain. In addition, the threshold levels of risk that could be considered in the decision model were limited by the sparsity of the data. Obtaining more information on these parameters would improve the decision model and may allow consideration of strategies involving use of prophylaxis at higher estimates of risk; however, such a study would be more costly to undertake as it would involve further primary data collection on risk factors as well as outcomes (although not the number of potential risk factors considered in the FIRE Study) and may therefore be best focused only on admissions remaining in the critical care unit at the end of calendar day 3, the decision time point at which treatment strategies appeared most likely to be cost-effective.
Recommendation 5: Further research should be considered to inform estimates of baseline risk of invasive fungal disease and associated outcomes among non-neutropenic, critically ill adult patients admitted to UK adult general critical care units
Owing to the low event rate, estimates of the baseline risk of IFD and associated outcomes were uncertain. Further data collection on these parameters would both provide a larger sample size to give more accurate estimates and permit monitoring over time in order to observe and evaluate trends. Research to inform estimates of the baseline risk of IFD and associated outcomes could be conducted at low cost by collecting a very small amount of additional information (at the minimum, fields for the presence of IFD and the date/time) either within or alongside the CMP. However, in considering such a study, it would be necessary to take note of the difficulty in applying the definitions of IFD that was highlighted through the reliability study. It may therefore be best to focus such work on monitoring rates of IFD in blood, for which the definitions may be most reliably applied.
Recommendation 6: Results of recommendations 1, 2, 4 and 5 (above) should be re-evaluated for their impact on the decision model and value of information analyses
As more information becomes available on any of the parameters in the decision model, the results of the model should be updated to evaluate whether or not the conclusions and/or the estimates of the value of further research have changed.
Recommendation 7: Further research into machine learning techniques should be considered to establish whether or not current barriers to their implementation at the bedside can be overcome
A number of technical and practical barriers were identified in the application of machine learning techniques to this decision problem. However, the random forests method showed considerable promise in producing potentially highly accurate models, and further consideration should be given to support vector machines, as the literature suggests that this approach may produce equally accurate but simpler models. Research looking to overcome these barriers and provide reliable, accurate models that can be delivered simply at the bedside should therefore be considered.
Acknowledgements
We wish to thank SICSAG for providing access to their database and the staff at participating hospitals (shown below).
We would also like to thank the ICNARC staff, with special thanks to Ruth Canter, Petra Selmer and Suzy Taljard, and Dr Manuel Gomes (Research Assistant in Health Economics, London School of Hygiene and Tropical Medicine) for his contribution to the economic analysis.
Contribution of authors
Dr David Harrison (Senior Statistician, ICNARC) conceived and designed the study, conducted and analysed data for the systematic review, conducted the analysis and interpretation of data for the study and drafted the manuscript. Hannah Muskett (Study Coordinator, ICNARC) conducted and analysed data for the systematic review and drafted the manuscript. Dr Sheila Harvey (Clinical Trials Unit Manager, ICNARC) interpreted the data for the study and drafted the manuscript. Dr Richard Grieve (Reader in Health Economics, London School of Hygiene and Tropical Medicine) designed the economic evaluation, analysed and interpreted the data and drafted the manuscript. Dr Jason Shahin (Research Associate, ICNARC) conducted the analysis and interpretation of data for the study, conducted the analysis and interpretation of data for the study and drafted the manuscript. Krishna Patel (Statistical Research Assistant, ICNARC) conducted the analysis and interpretation of data for the study and assisted with drafting the manuscript. Dr Zia Sadique (Research Fellow, Health Economics, London School of Hygiene and Tropical Medicine) designed the economic evaluation, analysed and interpreted the data and drafted the manuscript. Dr Elizabeth Allen (Senior Lecturer, Medical Statistics, London School of Hygiene and Tropical Medicine) conducted the risk model analysis and interpretation of data for the study and drafted the manuscript. Dr Richard Dybowski (Principal, Inferspace) conducted the neural network analysis and interpretation of data for the study and drafted the manuscript. Dr Mark Jit (Mathematical Modeller and Health Economist, HPA Centre for Infections) assisted with the economic evaluation, analysed and interpreted the data, and reviewed the manuscript. Dr Jonathan Edgeworth (Consultant Microbiologist, St Thomas' Hospital) conceived and designed the study. Professor Christopher Kibbler (Clinical Lead Consultant for Microbiology, Royal Free Hospital) conceived and designed the study. Professor Rosemary Barnes (Professor/Honorary Consultant in Medical Microbiology, Cardiff University) conceived and designed the study. Dr Neil Soni (Consultant Anaesthetist, Chelsea and Westminster Hospital) conceived and designed the study. Professor Kathy Rowan (Co-investigator, ICNARC) conceived and designed the study, interpreted the data for the systematic review, interpreted the data for the study and drafted the manuscript. All authors revised the manuscript critically for important intellectual content.
Study management
Hannah Muskett (Study Coordinator); Andrew Craven (previous Study Administrator); Gavin Eyres (previous Study Coordinator); Dr David Harrison (Chief Investigator); Dr Sheila Harvey (CTU/Research Manager); Rahi Jahan (Study Administrator); Phil Restarick (previous Research Coordinator); Professor Kathy Rowan (Director); Andrew Stenson (previous Research Manager); and Emma Walmsley (previous Study Administrator).
Steering Group
Dr Bernard Riley (chairperson); Professor Rosemary Barnes; Dr Jonathan Edgeworth; Dr Richard Grieve; Dr David Harrison; Dr Mark Jit; Professor Christopher Kibbler; Dr Ronan McMullan; Hannah Muskett; Professor Kathy Rowan; Dr Neil Soni; and Dr Thomas Stambach.
Research staff at participating critical care units
V Navapurkar, H Brown (Addenbrooke's Hospital); L Gilfeather, S O'Kane (Altnagelvin Hospital); H Black, R Jacob, C Smalley (Arrowe Park Hospital); I Taylor (Ayr Hospital); D Gilmour, M Henry, M Lee, F Wright (Ayrshire & Arran); M Sun Wai, H Chitty, K Hayden (Basildon Hospital); G Perkins, J Hull, L Blythe, K Couper, T Melody (Birmingham Heartlands and Good Hope Hospitals); J Cupitt, S Baddeley, L Rice (Blackpool Victoria Hospital); J Bewley, H Dunderdale, L Flintoff, K Pollock, K Sweet (Bristol Royal Infirmary); B Campbell, L Harrison (Bronglais Hospital); N Spittle, J Toms (Chesterfield Royal Hospital); S Ridler, H Jeffrey, N Robin (Countess of Chester Hospital); R Simmons (Crosshouse Hospital); P Wakefield, C Cripps, V Currie (Darent Valley Hospital); S Dickson, S Read (Derriford Hospital); S Dharmarajah, J Plume, M Wain (Diana, Princess of Wales Hospital); D Williams, C Jardine (Dumfries & Galloway Royal Infirmary); S Wright, H Chohan, C Higham (Freeman Hospital); J Brown, S Grier, E Hall (Frenchay and Southmead Hospitals); A Szymczakowski, J Craig, L Rice (Furness General Hospital); V Susarla, M Alarcon, G Ward (George Eliot Hospital); M Booth (Glasgow Royal Infirmary); E Cant, K Duffy, C Horn, C Johnston, C Keenan, L McGregor, B McLaren, M MacLeod, K Ocker, V Paterson (Greater Glasgow & Clyde); C Roberts (Gloucestershire Royal Hospital); T McLennan, V Watson (Hairmyres Hospital); D Earl, A Fairclough, J Featherstone (Harrogate District Hospital); R Harding, P Matheson, G Ward (Hereford Country Hospital); I Hille, S Bovan (Hinchingbrooke Hospital); A Gratrix, N Smith (Hull Royal Infirmary); F Munroe (Inverclyde Royal Hospital); J Millo, M Frise (John Radcliffe Hospital); D Higgs, R English, C Wilson (Kent and Canterbury; Queen Elizabeth, the Queen Mother; and William Harvey Hospitals); S Elliot, Z Beardow (Leeds General Infirmary); N Flint, S Bowrey, N Griffin-Teall, N Rich (Leicester Royal Infirmary); P Board, P Chilton (Leighton Hospital); S Gowrie-Mohan, E Ekanem, C Merrill (Lister and Queen Elizabeth II Hospitals); M Patten, K Sivagnanam (Luton and Dunstable Hospital); I Dave, A Williams (Macclesfield District General Hospital); D Simpson, N Pinto (Medway Maritime Hospital); H Manji, J Adderley (Milton Keynes General Hospital); J Ruddy, M Harkins (Monklands District General Hospital); S Gopal, R Cunningham, H Lewis, S Shaw (New Cross Hospital); A Sweenie, H Chohan, C Higham (Newcastle General Hospital); B Hussain, E Kalogianni, J Barry (Newham University Hospital); S Hutchinson, M Rosbergen (Norfolk and Norwich University Hospital); A Walder, K Jones (North Devon District Hospital); A Sharman, B Whysall (Nottingham City Hospital); P Sadler, A Hyson, J Mouland, J Rickards (Queen Alexandra Hospital); T de Beer, S Finucane (Queen's Medical Centre); J Whiteside, J Matheson, A Skene (Raigmore Hospital); D Alcorn (Royal Alexandra Hospital); C Danbury, E Bowley, N Jacques, A Pells, H Prowse (Royal Berkshire Hospital); L Donohue, K Burt (Royal Cornwall Hospital); D Rogerson, A Bunting, S Hamandishe, L Thomas (Royal Derby Hospital); C Day, A Causley, T Palmer [Royal Devon and Exeter Hospital (Wonford)]; C Kibbler, B Agarwal, C Stewart (Royal Free Hospital); A Goldsmith, J Martin, R Petch, D Trodd, J Wilson (Royal Hampshire County Hospital); D Highley, J Craig, L Rice (Royal Lancaster Infirmary); S Laha, J Baldwin, N Doherty, L Rice (Royal Preston Hospital); S Laver (Royal United Hospital); D McAuley, S Derbyshire, D Hendron, D Trainor, G White (Royal Victoria Hospital); I Clement, H Chohan, C Higham (Royal Victoria Infirmary); S Murdoch, Z Beardow (St James's University Hospital); J Jaidev, S Dyer, L Tindall (Scarborough Hospital); R Sharawi, A Day (Scunthorpe Hospital); M Grocott, K de Courcy-Golder, L Hawkins, K Linford (Southampton General Hospital); D Higgins, S Andrews (Southend University Hospital); G Imrie (Southern General Hospital); M Cecconi, S Keeling, J Mellinghoff (St George's Hospital); L Morrison, J Bruce, K McGuigan (St Johns Hospital at Howden); M Stotz, C Gibbs (St Mary's Hospital London); G Chandan, C Jackson, L Welham (Stafford Hospital); C Cairns, J Grant (Stirling Royal Infirmary); B Miles (Stobhill General Hospital); M Watters, K Mayell (The Great Western Hospital); R Lloyd, A Kong, S Bell (The Ipswich Hospital); J Wright, R Clarkson (The James Cook University Hospital); P Bothma, L Everett (The James Paget Hospital); D Dutta, C McClements, J Power (The Princess Alexandra Hospital); J Gibson, K Tarsey (The Queen Elizabeth Hospital Norfolk); A Krige, M Bland, L Bullock, D Harrison (The Royal Blackburn Hospital); D Swann, C McCulloch (The Royal Infirmary of Edinburgh); M Buddhavarapu, S Rowe, G Ward (University Hospital Coventry); S Nagaiyan, R Ahern, D Cartlidge (University Hospital of North Staffordshire); A Davidson (Victoria Infirmary); J Little, G Delaney-Segar (Warrington Hospital); T Stambach, S Sundayi (Watford General Hospital); A Binning, M Pollock (Western Infirmary); F Keane, I Bird (Whipps Cross University Hospital); C Jones, S Dowling, A McCairn (Whiston Hospital); M Kuper, M Bianchi, S Pahary (Whittington Hospital); D Dalchow, J Campbell (Wishaw General Hospital); G Sellors, A Jakeman, P Matheson (Worcestershire Royal Hospital); C Edmondson, C Davies (Wrexham Maelor Hospital); J Reid, D McCall, B Williams-Yesson (Yeovil District Hospital); and R Pretorius, C Plows (York Hospital).
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
Muskett H, Shahin J, Eyres G, Harvey S, Rowan K, Harrison D. Risk factors for invasive fungal disease in critically ill adult patients: a systematic review. Crit Care 2011;15:R287.
References
- Kauffman CA. Fungal infections. Proc Am Thorac Soc 2006;3:35-40.
- Lamagni TL, Evans BG, Shigematsu M, Johnson EM. Emerging trends in the epidemiology of invasive mycoses in England and Wales (1990–9). Epidemiol Infect 2001;126:397-414.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004;39:309-17.
- Fungal Diseases in the UK – The current provision of support for diagnosis and treatment: assessment and proposed network solution. Report of a working group of the Health Protection Agency Advisory Committee for Fungal Infection and Superficial Parasites. London: HPA; 2006.
- Kibbler CC, Seaton S, Barnes RA, Gransden WR, Holliman RE, Johnson EM, et al. Management and outcome of bloodstream infections due to Candida species in England and Wales. J Hosp Infect 2003;54:18-24.
- Gudlaugsson O, Gillespie S, Lee K, Vande BJ, Hu J, Messer S, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis 2003;37:1172-7.
- Pittet D, Li N, Woolson RF, Wenzel RP. Microbiological factors influencing the outcome of nosocomial bloodstream infections: a 6-year validated, population-based model. Clin Infect Dis 1997;24:1068-78.
- Eggimann P, Francioli P, Bille J, Schneider R, Wu MM, Chapuis G, et al. Fluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patients. Crit Care Med 1999;27:1066-72.
- Garbino J, Lew DP, Romand JA, Hugonnet S, Auckenthaler R, Pittet D. Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: a randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med 2002;28:1708-17.
- Jacobs S, Price Evans DA, Tariq M, Al Omar NF. Fluconazole improves survival in septic shock: a randomized double-blind prospective study. Crit Care Med 2003;31:1938-46.
- Pelz RK, Hendrix CW, Swoboda SM, Diener-West M, Merz WG, Hammond J, et al. Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann Surg 2001;233:542-8.
- Sandven P, Qvist H, Skovlund E, Giercksky KE. Significance of Candida recovered from intraoperative specimens in patients with intra-abdominal perforations. Crit Care Med 2002;30:541-7.
- ARDS Network . Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. The ARDS Network. JAMA 2000;283:1995-2002.
- Savino JA, Agarwal N, Wry P, Policastro A, Cerabona T, Austria L. Routine prophylactic antifungal agents (clotrimazole, ketoconazole, and nystatin) in nontransplant/nonburned critically ill surgical and trauma patients. J Trauma 1994;36:20-5.
- Slotman GJ, Burchard KW. Ketoconazole prevents Candida sepsis in critically ill surgical patients. Arch Surg 1987;122:147-51.
- Yu M, Tomasa G. A double-blind, prospective, randomized trial of ketoconazole, a thromboxane synthetase inhibitor, in the prophylaxis of the adult respiratory distress syndrome. Crit Care Med 1993;21:1635-42.
- Cruciani M, de Lalla F, Mengoli C. Prophylaxis of Candida infections in adult trauma and surgical intensive care patients: a systematic review and meta-analysis. Intensive Care Med 2005;31:1479-87.
- Ho KM, Lipman J, Dobb GJ, Webb SA. The use of prophylactic fluconazole in immunocompetent high-risk surgical patients: a meta-analysis. Crit Care 2005;9:R710-17.
- Playford EG, Webster AC, Sorrell TC, Craig JC. Antifungal agents for preventing fungal infections in non-neutropenic critically ill patients. Cochrane Database Syst Rev 2006;1.
- Playford EG, Webster AC, Sorrell TC, Craig JC. Antifungal agents for preventing fungal infections in non-neutropenic critically ill and surgical patients: systematic review and meta-analysis of randomized clinical trials. J Antimicrob Chemother 2006;57:628-38.
- Shorr AF, Chung K, Jackson WL, Waterman PE, Kollef MH. Fluconazole prophylaxis in critically ill surgical patients: a meta-analysis. Crit Care Med 2005;33:1928-35.
- Vardakas KZ, Samonis G, Michalopoulos A, Soteriades ES, Falagas ME. Antifungal prophylaxis with azoles in high-risk, surgical intensive care unit patients: a meta-analysis of randomized, placebo-controlled trials. Crit Care Med 2006;34:1216-24.
- Brion LP, Uko SE, Goldman DL. Risk of resistance associated with fluconazole prophylaxis: systematic review. J Infect 2007;54:521-9.
- Rex JH, Sobel JD. Prophylactic antifungal therapy in the intensive care unit. Clin Infect Dis 2001;32:1191-200.
- Blumberg HM, Jarvis WR, Soucie JM, Edwards JE, Patterson JE, Pfaller MA, et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin Infect Dis 2001;33:177-86.
- Leon C, Ruiz-Santana S, Saavedra P, Almirante B, Nolla-Salas J, Alvarez-Lerma F, et al. A bedside scoring system (‘Candida score’) for early antifungal treatment in non-neutropenic critically ill patients with Candida colonization. Crit Care Med 2006;34:730-7.
- Ostrosky-Zeichner L, Sable C, Sobel J, Alexander BD, Donowitz G, Kan V, et al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis 2007;26:271-6.
- Paphitou NI, Ostrosky-Zeichner L, Rex JH. Rules for identifying patients at increased risk for candidal infections in the surgical intensive care unit: approach to developing practical criteria for systematic use in antifungal prophylaxis trials. Med Mycol 2005;43:235-43.
- McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’; guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA 2000;284:79-84.
- Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA 1982;247:2543-6.
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29-36.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84.
- Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87.
- Mallett S, Royston P, Dutton S, Waters R, Altman DG. Reporting methods in studies developing prognostic models in cancer: a review. BMC Med 2010;8.
- Jorda-Marcos R, Alvarez-Lerma F, Jurado M, Palomar M, Nolla-Salas J, Leon MA, et al. Risk factors for candidaemia in critically ill patients: a prospective surveillance study. Mycoses 2007;50:302-10.
- Agvald-Ohman C, Klingspor L, Hjelmqvist H, Edlund C. Invasive candidiasis in long-term patients at a multidisciplinary intensive care unit: Candida colonization index, risk factors, treatment and outcome. Scand J Infect Dis 2008;40:145-53.
- Borzotta AP, Beardsley K. Candida infections in critically ill trauma patients: a retrospective case–control study. Arch Surg 1999;134:657-64.
- Chow JK, Golan Y, Ruthazer R, Karchmer AW, Carmeli Y, Lichtenberg DA, et al. Risk factors for albicans and non-albicans candidemia in the intensive care unit. Crit Care Med 2008;36:1993-8.
- Ibanez-Nolla J, Nolla-Salas M, Leon MA, Garcia F, Marrugat J, Soria G, et al. Early diagnosis of candidiasis in non-neutropenic critically ill patients. J Infect 2004;48:181-92.
- McKinnon PS, Goff DA, Kern JW, Devlin JW, Barletta JF, Sierawski SJ, et al. Temporal assessment of Candida risk factors in the surgical intensive care unit. Arch Surg 2001;136:1401-8.
- Michalopoulos AS, Geroulanos S, Mentzelopoulos SD. Determinants of candidemia and candidemia-related death in cardiothoracic ICU patients. Chest 2003;124:2244-55.
- Piarroux R, Grenouillet F, Balvay P, Tran V, Blasco G, Millon L, et al. Assessment of preemptive treatment to prevent severe candidiasis in critically ill surgical patients. Crit Care Med 2004;32:2443-9.
- Pittet D, Monod M, Suter PM, Frenk E, Auckenthaler R. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg 1994;220:751-8.
- Steyerberg EW, Eijkemans MJ, Habbema JD. Stepwise selection in small data sets: a simulation study of bias in logistic regression analysis. J Clin Epidemiol 1999;52:935-42.
- Leon C, Ruiz-Santana S, Saavedra P, Galvan B, Blanco A, Castro C, et al. Usefulness of the ‘Candida score’; for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit Care Med 2009;37:1624-33.
- Playford EG, Lipman J, Kabir M, McBryde ES, Nimmo GR, Lau A, et al. Assessment of clinical risk predictive rules for invasive candidiasis in a prospective multicentre cohort of ICU patients. Intensive Care Med 2009;35:2141-5.
- Hermsen ED, Zapapas MK, Maiefski M, Rupp ME, Freifeld AG, Kalil AC. Validation and comparison of clinical prediction rules for invasive candidiasis in intensive care unit patients: a matched case–control study. Crit Care 2011;15.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29.
- Harrison DA, Parry GJ, Carpenter JR, Short A, Rowan K. A new risk prediction model for critical care: the Intensive Care National Audit & Research Centre (ICNARC) model. Crit Care Med 2007;35:1091-8.
- Young JD, Goldfrad C, Rowan K. Development and testing of a hierarchical method to code the reason for admission to intensive care units: the ICNARC Coding Method. Intensive Care National Audit & Research Centre. Br J Anaesth 2001;87:543-8.
- Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Effects of nosocomial candidemia on outcomes of critically ill patients. Am J Med 2002;113:480-5.
- Garbino J, Kolarova L, Rohner P, Lew D, Pichna P, Pittet D. Secular trends of candidemia over 12 years in adult patients at a tertiary care hospital. Medicine 2002;81:425-33.
- Hung CC, Chen YC, Chang SC, Luh KT, Hsieh WC. Nosocomial candidemia in a university hospital in Taiwan. J Formos Med Assoc 1996;95:19-28.
- Nolla-Salas J, Sitges-Serra A, Leon-Gil C, Martinez-Gonzalez J, Leon-Regidor MA, Ibanez-Lucia P, et al. Candidemia in non-neutropenic critically ill patients: analysis of prognostic factors and assessment of systemic antifungal therapy. Study Group of Fungal Infection in the ICU. Intensive Care Med 1997;23:23-30.
- Petri MG, Konig J, Moecke HP, Gramm HJ, Barkow H, Kujath P, et al. Epidemiology of invasive mycosis in ICU patients: a prospective multicenter study in 435 non-neutropenic patients. Paul-Ehrlich Society for Chemotherapy, Divisions of Mycology and Pneumonia Research. Intensive Care Med 1997;23:317-25.
- Yap HY, Kwok KM, Gomersall CD, Fung SC, Lam TC, Leung PN, et al. Epidemiology and outcome of Candida bloodstream infection in an intensive care unit in Hong Kong. Hong Kong Med J 2009;15:255-61.
- Leleu G, Aegerter P, Guidet B. Systemic candidiasis in intensive care units: a multicenter, matched-cohort study. J Crit Care 2002;17:168-75.
- Bougnoux ME, Kac G, Aegerter P, d’;Enfert C, Fagon JY. Candidemia and candiduria in critically ill patients admitted to intensive care units in France: incidence, molecular diversity, management and outcome. Intensive Care Med 2008;34:292-9.
- Health Protection Agency . Health Protection Report 2011;5. http://www.hpa.org.uk/hpr/archives/2011/hpr3711.pdf (accessed 3 December 2012).
- Hutchings A, Durand MA, Grieve R, Harrison D, Rowan K, Green J, et al. Evaluation of modernisation of adult critical care services in England: time series and cost effectiveness analysis. BMJ 4353.
- Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis 2003;3:685-702.
- Voss A, le Noble JL, Verduyn Lunel FM, Foudraine NA, Meis JF. Candidemia in intensive care unit patients: risk factors for mortality. Infection 1997;25:8-11.
- Brier GW. Verification of forecasts expressed in terms of probability. Mon Weather Rev 1950;75:1-3.
- Efron B. Estimating the error rate of a prediction rule: improvement on cross-validation. J Am Stat Assoc 1983;78:316-31.
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373-9.
- Rubin D. Multiple imputation for nonresponse in surveys. New York, NY: John Wiley; 1987.
- King G, Honaker J, Joseph A, Scheve K. Analyzing incomplete political science data: An alternative algorithm for multiple imputation. Am Polit Sci Rev 2001;95:46-9.
- Easterbrooks A, Jo T, Japkowicz N. A multiple resampling method for learning from imbalanced datasets. Comput Intell 2004;20:18-36.
- Kohonen T. Self-organizing maps. Berlin: Springer; 1997.
- Lebrun G, Charrier C, Cardot H. SVM training time reduction using vector quantization. ICPR 2004;1:160-3.
- Deepa A, Punithavalli M. A new sampling technique and SVM classification for feature selection in high-dimensional imbalanced dataset n.d.
- Goldberg D. Genetic algorithms in search, optimization and machine learning. Reading, MA: Addison-Wesley; 1989.
- Chawla N, Bowyer K, Hall L, Kegelmeyer W. SMOTE: Synthetic minority over-sampling technique. J Artif Intell Res 2002;16:321-57.
- Bishop C. Neural networks for pattern recognition. Oxford: Clarendon Press; 1995.
- Ripley B. Pattern recognition and neural networks. Cambridge: Cambridge University Press; 1996.
- Dybowski R, Gant V. Clinical applications of artificial neural networks. Cambridge: Cambridge University Press; 2001.
- Dybowski R, Roberts S, Husmeier D, Dybowski R, Roberts S. Probabilistic modelling in bioinformatics and medical informatics. London: Springer; 2005.
- Bewick V, Cheek L, Ball J. Statistics review 14: Logistic regression. Crit Care 2005;9:112-18.
- Dybowski R, Husmeier D, Dybowski R, Roberts S. Probabilistic modelling in bioinformatics and medical informatics. London: Springer; 2005.
- Jaimes F, Farbiarz J, Alvarez D, Martinez C. Comparison between logistic regression and neural networks to predict death in patients with suspected sepsis in the emergency room. Crit Care 2005;9:R150-6.
- Clermont G. Artificial neural networks as prediction tools in the critically ill. Crit Care 2005;9:153-4.
- Tu JV. Advantages and disadvantages of using artificial neural networks versus logistic regression for predicting medical outcomes. J Clin Epidemiol 1996;49:1225-31.
- Lukaszewski RA, Yates AM, Jackson MC, Swingler K, Scherer JM, Simpson AJ, et al. Presymptomatic prediction of sepsis in intensive care unit patients. Clin Vaccine Immunol 2008;15:1089-94.
- Russel S, Norvig P. Artificial intelligence: a modern approach. Boston, MA: Pearson; 2010.
- Verplancke T, Van Looy S, Benoit D, Vansteelandt S, Depuydt P, De Turck F, et al. Support vector machine versus logistic regression modeling for prediction of hospital mortality in critically ill patients with haematological malignancies. BMC Med Inform Decis Making 2008;8.
- Van Looy S, Verplancke T, Benoit D, Hoste E, Van Maele G, De Turck F, et al. A novel approach for prediction of tacrolimus blood concentration in liver transplantation patients in the intensive care unit through support vector regression. Crit Care 2007;11.
- Hosmer D, Lemeshow S. Applied logistic regression. New York, NY: Wiley; 1989.
- Bradely P, Mangasarian O. Feature selection via concave minimization and support vector machines 1998.
- Zhang HH, Ahn J, Lin X, Park C. Gene selection using support vector machines with non-convex penalty. Bioinformatics 2006;22:88-95.
- Breiman L, Friendman J, Olshen R, Stone C. Classification and regression trees. New York, NY: Chapman & Hall; 1984.
- Breiman L. Random forests. Machine Learning 2001;45:5-32.
- Breiman L. Bagging predictors. Machine Learning 1996;24:123-40.
- Rentz AM, Halpern MT, Bowden R. The impact of candidemia on length of hospital stay, outcome, and overall cost of illness. Clin Infect Dis 1998;27:781-8.
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- Lambert PC, Sutton AJ, Burton PR, Abrams KR, Jones DR. How vague is vague? A simulation study of the impact of the use of vague prior distributions in MCMC using WinBUGS. Stat Med 2005;24:2401-28.
- Curtis L. Unit costs of health social care 2010. Canterbury: PSSRU, University of Kent; 2010.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2013. www.bnf.org/bnf/index.htm (accessed 11 July 2013).
- NHS reference costs 2009–2010. London: DoH; 2011.
- Davies A, Ridley S, Hutton J, Chinn C, Barber B, Angus DC. Cost effectiveness of drotrecogin alfa (activated) for the treatment of severe sepsis in the United Kingdom. Anaesthesia 2005;60:155-62.
- Leventhal H, Colman S. Quality of life: a process review. Psychol Health 1997;12:753-67.
- Cuthbertson BH, Roughton S, Jenkinson D, Maclennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care 2010;14.
- Wright JC, Plenderleith L, Ridley SA. Long-term survival following intensive care: subgroup analysis and comparison with the general population. Anaesthesia 2003;58:637-42.
- Sadique MZ, Grieve R, Harrison DA, Cuthbertson BH, Rowan KM. Is drotrecogin alfa (activated) for adults with severe sepsis, cost-effective in routine clinical practice?. Crit Care 2011;15.
- Soares MO, Welton NJ, Harrison DA, Peura P, Shankar HM, Harvey SE, et al. An evaluation of the feasibility, cost and value of information of a multicentre randomised controlled trial of intravenous immunoglobulin for sepsis (severe sepsis and septic shock): incorporating a systematic review, meta analysis and value of information analysis. Health Technol Assess 2012;16.
- Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al. Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris) for the treatment of severe sepsis in adults: a systematic review and economic evaluation. Health Technol Assess 2005;9.
- Guide to the methods of technology appraisal. London: NICE; 2008.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64.
- Fenwick E, Marshall DA, Blackhouse G, Vidaillet H, Slee A, Shemanski L, et al. Assessing the impact of censoring of costs and effects on health-care decision-making: an example using the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Value Health 2008;11:365-75.
- Willan AR. Clinical decision making and the expected value of information. Clin Trials 2007;4:279-85.
- Ades AE, Lu G, Claxton K. Expected value of sample information calculations in medical decision modeling. Med Decis Making 2004;24:207-27.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. Pharmacoeconomics 2006;24:355-71.
- Johnson EM, Warnock DW, Luker J, Porter SR, Scully C. Emergence of azole drug resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. J Antimicrob Chemother 1995;35:103-14.
- Snydman DR. Shifting patterns in the epidemiology of nosocomial Candida infections. Chest 2003;123:500S-3S.
- McGowan JE. Economic impact of antimicrobial resistance. Emerg Infect Dis 2001;7:286-92.
- Smith R, Coast J. The true cost of antimicrobial resistance. BMJ 2013;346.
- Cataldo MA, Petrosillo N. Economic considerations of antifungal prophylaxis in patients undergoing surgical procedures. Ther Clin Risk Manag 2011;7:13-20.
- Dark PM, Dean P, Warhurst G. Bench-to-bedside review: the promise of rapid infection diagnosis during sepsis using polymerase chain reaction-based pathogen detection. Crit Care 2009;13.
- Posteraro B, De Pascale G, Tumbarello M, Torelli R, Pennisi MA, Bello G, et al. Early diagnosis of candidemia in intensive care unit patients with sepsis: a prospective comparison of (1→3)-beta-D-glucan assay, Candida score, and colonization index. Crit Care 2011;15.
Appendix 1 National Institute for Health Research Health Technology Assessment programme call
National Institute for Health Research Health Technology Assessment programme call (PDF download)
Appendix 2 Search strategy for systematic review
-
Mycoses in MeSH
-
Antifungal Agents in MeSH
-
fung* in AB/TI/KW
-
Candida in AB/TI/KW
-
candidemia in AB/TI/KW
-
candidaemia in AB/TI/KW
-
candidiasis in AB/TI/KW
-
candidal in AB/TI/KW
-
fluconazole in AB/TI/KW
-
Diflucan in AB/TI/KW
-
itraconazole in AB/TI/KW
-
sporanox in AB/TI/KW
-
ketocanazole in AB/TI/KW
-
nizoral in AB/TI/KW
-
voriconazole in AB/TI/KW
-
amphotericin in AB/TI/KW
-
ambisome in AB/TI/KW
-
amphotec in AB/TI/KW
-
abelcet in AB/TI/KW
-
flucytosine in AB/TI/KW
-
Nystatin in AB/TI/KW
-
miconazole in AB/TI/KW
-
echinocandin* in AB/TI/KW
-
caspofungin in AB/TI/KW
-
(select* NEAR decontam*) in AB/TI/KW
-
OR/1-25
-
Intensive Care Units in MeSH
-
Critical Care in MeSH
-
intensive care in AB/TI/KW
-
critical care in AB/TI/KW
-
critical illness in AB/TI/KW
-
critically ill in AB/TI/KW
-
OR/27-32
-
26 and 33
-
Risk in MeSH
-
Models, Statistical in MeSH
-
Regression Analysis in MeSH
-
Sensitivity and Specificity in MeSH
-
Survival Analysis in MeSH
-
Operations Research in MeSH
-
Decision Support Techniques in MeSH
-
Clinical Protocols in MeSH
-
Practice Guidelines in MeSH
-
Patient Selection in MeSH
-
risk* in AB/TI/KW
-
predict* in AB/TI/KW
-
model* in AB/TI/KW
-
rule* in AB/TI/KW
-
((decision OR algorithm) NEAR (clinical or treatment or prophyla*)) in AB/TI/KW
-
OR/35-49
-
34 and 50
Appendix 3 Patient information sheet
Appendix 4 Data collection forms
Appendix 5 Flows
Appendix 6 Protocol
List of abbreviations
- APACHE
- Acute Physiology And Chronic Health Evaluation
- BiPAP
- bilevel positive airway pressure
- BNF
- British National Formulary
- CBP
- cardiopulmonary bypass
- CCMDS
- Critical Care Minimum Data Set
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CICU
- cardiothoracic intensive care unit
- CLRN
- Comprehensive Local Research Network
- CMP
- Case Mix Programme
- CPAP
- continuous positive airway pressure
- CPB
- cardiopulmonary bypass
- CPR
- cardiopulmonary resuscitation
- CVC
- central venous catheter
- EORTC/MSG
- European Organisation for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group
- EPV
- events per variable
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- FFNN
- feedforward neural network
- FIRE
- Fungal Infection Risk Evaluation
- HDU
- high-dependency unit
- HPA
- Health Protection Agency
- HRG
- Healthcare Resource Group
- HRQOL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICNARC
- Intensive Care National Audit & Research Centre
- ICU
- intensive care unit
- IFD
- invasive fungal disease
- INB
- incremental net benefit
- IQR
- interquartile range
- LOS
- length of stay
- MeSH
- medical subject heading
- NA
- not applicable
- NIGB
- National Information Governance Board
- NIHR
- National Institute for Health Research
- NPV
- negative predictive value
- NR
- not reported
- PCR
- polymerase chain reaction
- PIAG
- Patient Information Advisory Group
- PPV
- positive predictive value
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- ROC
- Receiver operating characteristic
- RR
- relative risk
- SD
- standard deviation
- SICSAG
- Scottish Intensive Care Society Advisory Group
- SICU
- surgical intensive care unit
- SMOTE
- synthetic minority over-sampling technique
- SSI
- site-specific information
- TPN
- total parenteral nutrition
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.