Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/18/01. The contractual start date was in August 2011. The draft report began editorial review in September 2012 and was accepted for publication in April 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Note
this is a ‘short report’ that has been produced with only one-third of the resources that would be available for a full technology assessment report and it cannot be as comprehensive as a full report would be.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Waugh et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Type 2 diabetes mellitus
People can have type 2 diabetes mellitus (T2DM) with none of the classical symptoms, such as the passing of larger volumes of urine (polyuria) and thirst. Some may have had some symptoms when diagnosed, but have not recognised them as due to diabetes.
Sometimes by the time people are diagnosed with diabetes, they have developed complications such as retinopathy, due to an effect of diabetes on small blood vessels (microvascular disease). About half of the UK Prospective Diabetes Study (UKPDS) recruits had some sign of complications at entry, mostly retinopathy in 36%,1 although the proportion who have retinopathy at diagnosis is now lower than in past studies, with only about 2% having referable retinopathy at first screening. 2 (Note that patients who had established macrovascular complications, such as angina, were excluded from UKPDS.)
However, the main risk to health in undiagnosed T2DM is an increased risk of cardiovascular disease (CVD), in particular ischaemic heart disease (IHD), because of damage to the arteries (macrovascular disease). Indeed, the first manifestation of diabetes could be a heart attack, often fatal.
Early detection of diabetes would lead to measures to reduce the risk of heart disease, such as the use of statins to lower cholesterol, and treatment for raised blood pressure (BP), as well as reduction of blood glucose levels, initially by diet and exercise, and supplemented with glucose-lowering drugs if necessary.
It is known that a proportion of people with T2DM are undiagnosed. In the age group of 52–79 years, the English Longitudinal Study of Ageing (ELSA)3 in 2004–5 found that almost 20% of those with diabetes were undiagnosed, with a higher percentage in men (22%) than women (12%). The overall prevalence was 9.1%, with 1.7% undiagnosed. Predictors of undiagnosed diabetes included body mass index (BMI), waist circumference, systolic blood pressure (SBP) and triglycerides. Diagnosis was based on a single fasting plasma glucose (FPG) value of ≥ 7.0 mmol/l and would therefore miss those whose diabetes is manifested mainly by postprandial hyperglycaemia.
The authors of the ELSA study3 note that the proportion undiagnosed has fallen, and attribute this to increased opportunistic screening in general practice.
The results from the pilot screening programme in England support this, with an overall prevalence of 4.08%, including 0.54% undiagnosed. However, uptake of screening was only 61%4 and the non-responders might have had a higher proportion with undiagnosed diabetes.
Prevalence
The increase in reported prevalence of T2DM depends on a number of factors, including:
-
An increase in the incidence of T2DM, related to rising levels of overweight and obesity. Data from the Framingham study show that almost all of the increase in the USA in diabetes prevalence is in the obese category. 5
-
Demographic change – half of all people with diabetes are > 65 years of age, so an increase in the number of people over that age will increase the prevalence of diabetes.
-
A fall in the age at onset of T2DM – people contracting diabetes earlier in life, probably because of earlier weight gain and reduced physical activity compared with previous generations. 6
-
Changes in the definition of diabetes, with the diagnosis made at a lower level of FPG.
-
Better survival with diabetes because of improved control of blood glucose level, hypertension and cholesterol level.
-
More complete recording of diabetes on general practitioner (GP) computer systems.
-
Better detection of undiagnosed diabetes by opportunistic case-finding or practice-based screening, linked with greater public awareness of diabetes.
The York and Humber Public Health Observatory (YHPHO) diabetes report7 estimated that 40% of the rise in crude prevalence was due to changing age and ethnic group structure, and 60% to obesity and overweight.
The prevalence of T2DM is closely linked with that of overweight. The proportion of the population that is overweight or obese (BMI of ≥ 30 kg/m2) has been increasing in recent years.
The importance of BMI in the incidence of T2DM is shown in Figure 1. There is a close relationship between BMI and the incidence of T2DM, and it is worth noting that it starts well below the obesity range.
FIGURE 1.
Age-adjusted incidence rates of diabetes as a function of baseline BMI in 30- to 55-year-olds (both sexes), based on data from Ford et al. (1997). 8
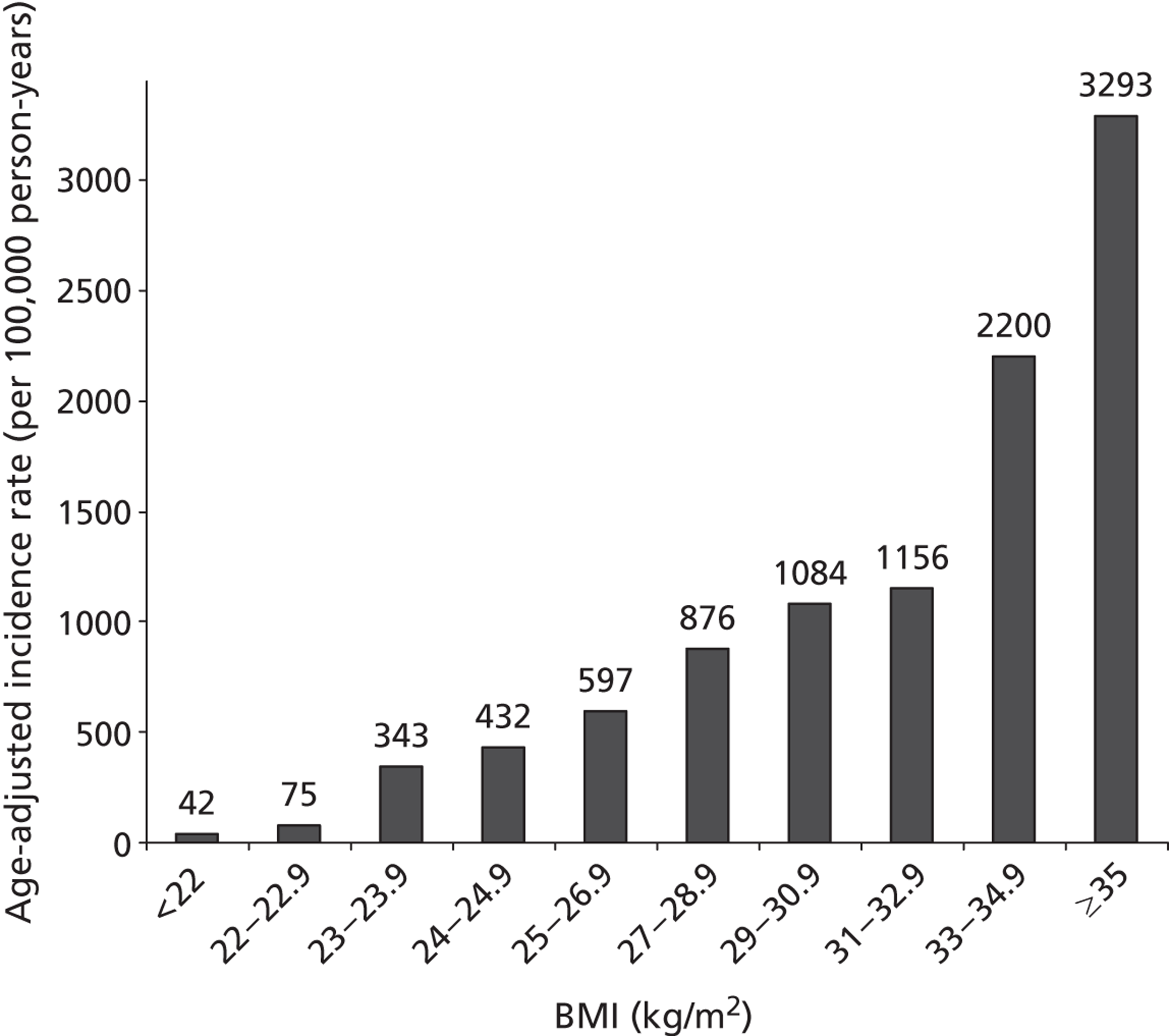
In the Cambridge centre of the ADDITION (Anglo-Danish-Dutch Study in General Practice of Intensive Treatment and Complication Prevention in Type 2 Diabetic Patients Identified by Screening) study, high proportions of people with screen-detected diabetes had risk factors for CVD. 9 Almost all were overweight or obese (mean BMI = 32.5 kg/m2); 86% had hypertension, 75% had dyslipidaemia, and many of those with hypertension and dyslipidaemia did not have good control of BP or lipids. Hence, those detected by screening form a group in which CVD risk can be reduced by combined treatment. The ADDITION study is described in detail in Chapter 4. Another implication is that first-stage screening based on weight would identify most people with undiagnosed diabetes.
Screening
The UK National Screening Committee (NSC) has reviewed its policy on screening for T2DM at intervals. A review in 2006 was underpinned by a Health Technology Assessment (HTA) report that included a systematic literature review and economic modelling of the case for population screening. 10 The HTA report found that the case for screening for undiagnosed diabetes and for IGT was becoming stronger because of greater options for the reduction of CVD and because of the rising prevalence of obesity, and hence of T2DM. (Details of methods are given in Appendix 1.)
The National Screening Committee criteria
The NSC has a list of criteria for assessing possible population screening programmes. It is usually expected that all should be met before a screening programme is introduced. 11
The last HTA report10 concluded that some criteria had not been met, including:
-
criterion 12, on optimisation of existing management of the condition
-
criterion 13, which requires that there should be evidence from high-quality randomised controlled trials (RCTs) showing that a screening programme would reduce mortality or morbidity
-
criterion 18, that there should be adequate staffing and facilities for all aspects of the programme
-
and possibly criterion 19, that all other options, including prevention, should have been considered; the issue here is whether or not all methods of improving lifestyles in order to reduce obesity and increase physical activity have been sufficiently tried; the rise in overweight and obesity suggests that health promotion interventions have not so far been effective.
There have been several developments since the last review that affect the case for screening.
Criteria 12 and 18
There have been some improvements that affect criterion 12. The introduction of the Quality and Outcomes Framework as part of changes to the GP contract in 2004 provided incentives to improve selected aspects of clinical care, including diabetes and hypertension. For example, 79% of practices were meeting the BP 5 target of the Quality and Outcomes Framework, of getting a recent BP value of < 150/90 mmHg. The audits of diabetes care in England and Wales showed that 67% (England) and 68% (Wales) of patients with T2DM had glycated haemoglobin (HbA1c) levels of < 7.5% [note: the National Institute for Health and Care Excellence (NICE) guidelines target for T2DM was 6.5%]; 69% and 61%, respectively, had BPs of ≤ 140/8 mmHg. In both countries, 78% of patients were meeting the total cholesterol target of ≤ 5.0 mmol/l, and 41% were meeting the more stringent target of ≤ 4.0 mmol/l.
However, the National Audit Office (NAO) report on adult diabetes services identifies shortcomings in care in many primary care trusts (PCTs), as reported in the following extracts. 12
7. In 2009–10, national clinical audit data found that only half of the increasing number of people with diabetes received all the recommended care processes that could reduce their risk of developing diabetes-related complications
8. Less than one in five people with diabetes are achieving recommended treatment standards that reduce their risk of developing diabetes-related complications
9. There is significant variation in the quality of care received by people with diabetes across the NHS
And:
The Department of Health has failed to deliver diabetes care to the standard it set out as long ago as 2001. This has resulted in people with diabetes developing avoidable complications, in a high number of preventable deaths and in increased costs for the NHS.
The expected 23 per cent increase by 2020 in the number of people in England with diabetes will have a major impact on NHS resources unless the efficiency and effectiveness of existing services are substantially improved.
Amyas Morse, head of the NAO, 23 May 2012
Hence, criterion 12 is far from met and the audit report also implies that criterion 18 is also not met.
Criterion 13
One trial13 of screening for diabetes and cardiovascular risk factors has been carried out in Ely in England. In this study, one-third of people aged 40–65 years, not known to have diabetes, were randomly selected and invited for screening, using the oral glucose tolerance test (OGTT), in 1990; 68% attended, of whom 4.4% were found to be diabetic. They were invited for rescreening for diabetes in 1994–6 and 2000–2. The results of the OGTT, and of cholesterol and BP levels, were fed back to GPs, who took whatever action they deemed appropriate. No standardised advice on diabetes management was given.
In 2000–2, half of those not invited for screening at baseline (i.e. another third) were randomly invited for screening. Uptake was lower, at 45%. The remaining third were not invited.
All participants, whether or not invited, were flagged at the Office of the Registrar General, now the Office for National Statistics, so that mortality data could be obtained.
In the 1990–9 period, the relative risk (RR) of mortality for the invited group compared with those non-invited, was 0.96 [95% confidence interval (CI) 0.77 to 1.20] before adjustment for age, sex and deprivation, and 0.79 (95% CI 0.63 to 1.00) afterwards.
In the 2000–8 period, the RR for the invited group was 1.20 (95% CI 0.95 to 1.51) before adjustment and similar afterwards.
The only significant differences in mortality were between those invited who attended and those invited who did not attend – about a threefold increase in the latter. This is as expected, as those who accept screening invitations have other protective health behaviours.
In terms of morbidity, at the 13-year follow-up, health status and cardiovascular morbidity were assessed in the screened group (43% attended) and in a randomly selected and invited sample of half of those previously not screened (42% attended). There were no differences in health status or cardiovascular morbidity.
Hence, criterion 13 is not met – we have a RCT but no benefit was shown. It should be noted that the numbers of people found to have diabetes were low, so it would be difficult to show any effect of intervention in them. The ADDITION study addresses this issue and is described in detail later in this review.
Criterion 19
This criterion is about prevention.
A recent study14 examined the incidence of T2DM and concluded that about 90% could be avoided by adherence to five lifestyle factors:
-
physical activity
-
a healthy diet
-
BMI of < 25 kg/m2
-
not smoking
-
moderate alcohol consumption.
That study14 was in people aged > 65 years, but similar findings have been seen in all age groups. Similar findings were reported from the Finnish Diabetes Prevention Study (DPS). 15 Participants were divided into six groups according to how many lifestyle goals were achieved, so that group 5 achieved all and group 0 none.
Table 1 shows the incidence of diabetes for each group, expressed as a ratio to group 0.
| Success score | HR (95% CI) |
|---|---|
| 0 | 1.00 |
| 1 | 0.85 (0.57 to 1.28) |
| 2 | 0.66 (0.40 to 1.09) |
| 3 | 0.69 (0.38 to 1.26) |
| 4–5 | 0.23 (0.10 to 0.52) |
| Test for trend p = 0.0004 |
Hence, we know what people should do to avoid getting T2DM. The problem is that we do not know how to persuade them to do it. The rising trends in obesity and overweight show that health promotion measures have so far failed.
Developments
In 2008, the NSC recommended the introduction of a Vascular Risk Management Programme in which ‘the whole population would be offered a risk assessment that could include, among other risk factors, measurement of BP, cholesterol and glucose’. The NSC concluded that: ‘targeted screening for T2DM was feasible but should be undertaken as part of an integrated programme to detect and manage vascular risk factors in certain subgroups of the population who are at high risk of T2DM’ (UK NSC, DPH Newsletter, 2008, March). 17 This policy acknowledges that the relationship between blood glucose levels and CVD is a continuous one and, therefore, the detection of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), in addition to the detection of diabetes, could be part of a programme of CVD prevention.
That programme is for England only. One effect of devolution is that policy and practice have been diverging in the four territories. Hence, different approaches to diabetes screening may emerge.
In Wales, there has been a pilot of screening in community pharmacies. This lasted for only 2 weeks but more than 17,500 people had their risk of diabetes assessed, of whom 8.4% were identified as high risk. They were referred to their GPs for further testing. 18
If we screen for diabetes, we will identify, depending on the screening strategy used and cut-offs chosen, more people with lesser degrees of hyperglycaemia, such as IGT, than with T2DM. 10 Before a screening programme is started, we should therefore consider how best to manage such people. Another HTA review19 was commissioned to examine the clinical effectiveness and cost-effectiveness of non-pharmacological interventions to prevent or reduce progression to diabetes in people with IGT. It is summarised in Chapter 5, with details of methods provided in Appendix 1.
This report builds on and updates these two HTA reports, produced for the Department of Health (England) and the NSC by the Aberdeen Health Technology Assessment Group and colleagues in the School of Health and Related Research (ScHARR) in Sheffield. It also draws on a research review, carried out by the former Diabetes and Health Technology Assessment Group in the Department of Public Health in the University of Aberdeen, to support a working group of the Scottish Public Health Network (SPHN) set up to review screening and prevention of T2DM. The report of the working group is available on the SPHN website (www.scotphn.net) and its remit and conclusions are given in Appendix 1. We have reviewed studies published since these reports were undertaken, to update the evidence base.
What should we be screening for?
Diabetes is defined on the basis of high blood glucose levels. The key feature of the classification is that the diagnosis is based on the level at which the risk of retinopathy starts. At the risk of a little oversimplification, people with glucose levels below the threshold do not get retinopathy; those with levels above the threshold are at risk of retinopathy, with the risk increasing as glucose levels rise further. This was based on three studies, described in the report of the expert committee of the American Diabetes Association (ADA). 20 A very large recent study from the DETECT-2 (Evaluation of Screening and Early Detection Strategies for Type 2 Diabetes and Impaired Glucose Tolerance) Group21 also found clear thresholds for moderate retinopathy of 6.5 mmol/l for FPG and 6.5% for HbA1c. The threshold for the 2-hour plasma glucose (PG) level was less distinct, at around 10.1–11.2 mmol/l.
The DETECT-2 Group21 used ‘moderate retinopathy’. Some retinopathy, such as microaneurysms only, has been reported in IGT from the Diabetes Prevention Program. 22
However, the risk of heart disease increases at lower levels of hyperglycaemia than diabetes. So for public health purposes, there could be advantages in having screening for diabetes done as part of assessment of cardiovascular risk and the English vascular risk assessment programme provides an opportunity for that.
Impaired fasting glucose and impaired glucose tolerance
In IFG, FPG is above the upper limit of normal but below the diabetes level of 7.0 mmol/l. The European definition uses a cut-off for FPG of 6.1 mmol/l. In the USA the cut-off for IFG is 5.5 mmol/l. The European definition omits a group with FPG above normal (up to 5.4 mmol/l) but < 6.1 mmol/l.
Impaired glucose tolerance is defined as a postload level of > 7.8 mmol/l but < 11.1 mmol/l.
These conditions are often referred to as ‘pre-diabetes’, but this term is somewhat misleading because under half go on to get diabetes. However, those with IGT are at increased risk of vascular disease compared with people with normal glucose tolerance (NGT).
One issue is about the value of categorisation. With continuous variables such as PG, subdividing the range into categories such as IGT is always somewhat arbitrary. Chamnan et al. (2011)23 from the Medical Research Council (MRC) Epidemiology Unit in Cambridge have made a convincing case for using hyperglycaemia as a continuous variable as part of overall cardiovascular risk assessment. They point out that someone with HbA1c in the IGT range (6.0–6.4%) but no other raised CVD risk factors, will have a much lower risk than someone with a HbA1c level ≤ 5.5% but high values for other risk factors such as hypertension and hyperlipidaemia.
Microvascular disease, such as retinopathy, is specific to diabetes. However, the macrovascular disease seen in diabetes is broadly the same disease as seen in people without diabetes. The difference in diabetes is an increased risk and a more diffuse distribution of arterial disease. They are more likely to die after a heart attack than people without diabetes. This is particularly important as the heart attack may occur without any prior warning of heart disease, such as angina.
The increase in cardiovascular risk starts below the level of blood glucose used to define diabetes, so if reduction of heart disease is one of the aims of screening, we should consider screening not just for diabetes, but also for IGT. The risk of CVD in IGT is slightly less than with T2DM, but the number of people with IGT is much higher than those with undiagnosed diabetes, and so the cardiovascular population impact of IGT is much greater than for undiagnosed diabetes [for a review, see Waugh et al. (2007)10].
The importance of large vessel disease can be seen in the end points reported in the UKPDS. 24 The majority of adverse events were due to large vessel disease.
If we are considering screening based on risk factors then those in the UKPDS who were overweight (defined as > 120% ideal body weight for height) may be more similar to those who would be found by screening. As in the total UKPDS group, the end points in the control group were dominated by large vessel disease, with 157 macrovascular end points and 19 microvascular ones in the control group of 411 patients. 25
The risks of CVD in those with IFG and IGT have been reported to be higher than in people with normal glucose levels. Table 2 shows results from the DECODE (Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe) meta-analysis.
| FPG and 2-hour PG levels both normal | 1.0 |
| IFG – raised FPG but 2-hour PG levels normal | 1.18 (95% CI 0.99 to 1.42) |
| IGT alone – raised 2-hour PG levels but normal FPG | 1.56 (95% CI 1.33 to 1.83) |
Hence IFG alone, without IGT, is associated with a slight increase in mortality [although CIs overlap with no increase], but IGT carries more risk, possibly as a consequence of stronger associations with hypertension and dyslipidaemia than for IFG. The risk of CVD is also more closely related to the 2-hour PG than FPG, even in people with normal glucose levels. 27
Similar findings were reported from a meta-analysis by Coutinho et al. (1999)28 of 20 studies examining cardiovascular mortality (19 studies) or morbidity (four studies). A FPG level of 6.1 mmol/l carried 1.3 times the risk of the reference one of 4.2 mmol/l; a 2-hour glucose level of 7.8 mmol/l carried a RR of 1.6 compared with a 2-hour PG level of 4.2 mmol/l.
The Emerging Risk Factors Collaboration study,29 which had data from 698,782 people, also found that IFG had little effect on cardiovascular risk, with RRs of 1.11 for the 5.6 to < 6.0 mmol/l range and 1.17 for the 6.0–6.9 mmol/l range.
More recent work has suggested that the excess risk from IGT is lower than previously thought. A meta-analysis by Sarwar et al. (2010)30 reported a RR of 1.05 for every 1-mmol/l increase in postload glucose. They found a stronger link between HbA1c level and coronary heart disease (CHD), with a RR of 1.2 for every 1% rise in HbA1c.
The meta-analysis included early data from the Australian Diabetes, Obesity and Lifestyle (AusDiab) study, but at a time when there were only 31 CHD cases. A later paper from AusDiab, by Barr et al. (2009)31 reported a linear relationship between HbA1c level and CHD mortality, with the risk at HbA1c 6% being double that at 4.5%. The Edinburgh Artery Study, reported by Wild et al. (2005)32 was not included in the meta-analysis. Wild et al. (2005)32 reported that isolated postload hyperglycaemia conferred little increase in cardiovascular risk.
The Hoorn study33 from the Netherlands found the reverse – postload hyperglycaemia in the IGT range was associated with an RR of 1.48, but the number of events was small and the 95% CI was 0.7 to 3.2. FPG in the IFG range was associated with a RR of 1.4, but, after adjustment for factors including hypertension and lipids, the RR was reduced to 1.07 (the same adjustment reduced the IGT RR from 1.9 to 1.48).
In the Rancho Bernardo study, Barrett-Connor et al. (1998)34 found that the risk of cardiovascular mortality was increased in women with isolated postchallenge hyperglycaemia [age-adjusted hazard ratio (HR) 2.6, 95% CIs 1.5 to 4.8] but not in men (age-adjusted HR 0.7, 95% CI 0.3 to 1.6). The point estimates for the Edinburgh Artery Study32 participants were similar, with odds ratios (ORs) for cardiovascular mortality of 2.7 (95% CI 0.6 to 11.6) for women and 0.8 (95% CI 0.09 to 6.7) for men. However, a Paris study35 found that the heart disease mortality rate in men with normal fasting glucose but IGT was three times that of those with NGT.
IGT is common – it affects 17% of Britons aged 40–65 years. 36
Unlike with retinopathy, there is no sudden inflexion in the risk curve for CVD according to blood glucose levels, but rather a continuum of risk. Indeed, even within what is regarded as being the normal range, higher blood glucose levels have higher IHD rates. In the EPIC (European Prospective Investigation into Cancer and Nutrition) study in Norfolk,37 the relationship between HbA1c and cardiovascular risk started well within the non-diabetic range (Table 3).
| HbA1c band (%) | RR of CVD | |
|---|---|---|
| Men | Women | |
| < 5 | 1.0 | 1.0 |
| 5–5.4 | 1.23 | 0.89 |
| 5.5–5.9 | 1.56 | 0.98 |
| 6.0–6.4 | 1.79 | 1.63 |
| 6.5–6.9 | 3.03 | 2.37 |
| > 7 (newly diagnosed diabetes) | 5.01 | 7.96 |
| Prior diabetes | 3.32 | 3.36 |
However, in a more recent paper from the EPIC-Norfolk study, Chamnan et al. (2011)23 report that the risk associated with hyperglycaemia across the whole range, from just above normal to diabetes, depends more on associated risk factors. They analysed data from a subgroup of 10,144 subjects whose had HbA1c level had been measured. The subgroup arose because funding of HbA1c testing was not initially available so no particular bias arises. All subjects were free of CVD at baseline, and were followed up for 10 years through linkage with mortality and hospital admission data. The CVD event rates per 1000 person-years were for bands of baseline HbA1c:
-
< 5.5% 7.0
-
5.5–5.9% 12.3
-
6.0–6.4% 16.5
-
diabetes 28.9.
The authors report that each 1% increase in HbA1c level increased the risk of CVD by 27%, and that individuals with HbA1c levels of ≥ 6.5% were more than twice as likely to have an event as those with HbA1c levels of < 5.5%. These figures do not appear to match the event rates above.
Chamnan et al. (2001)23 give RRs for different bands, taking a HbA1c level of < 5.5% as the reference of 1.0. RRs were 1.31 for HbA1c band 5.5–5.9%, 1.50 for 6.0–6.4%, 2.19 for 6.5–6.9% and 3.21 for ≥ 7%. These are somewhat different from the Khaw et al. (2004)37 results.
Notably, the risk associated with different bands was determined more by the presence of other CVD risk factors, so that people with a low HbA1c level but other risk factors had higher CVD risk than someone with a high HbA1c level but no other raised CVD factors. This is reminiscent of an earlier study from this group,38 which showed that adding HbA1c value to the Framingham score added little to the predictive value.
Chamnan et al. (2001)23 therefore argue against basing management on defined categories such as IGT, and suggest that risk factors such as PG, BP and lipid levels are continuous variables. They recommend that management should be based on an overall assessment of cardiovascular risk.
In a 2009 paper, Chamnan et al. (2009)39 reported the findings of a high-quality systematic review of risk scores for CVD in people with diabetes. Their main findings were, in brief:
-
Scores were developed for different purposes: ranking of risk to determine priority for treatment (especially when statins were introduced); assessing the potential absolute benefit of treatment; and motivating patients to change behaviour.
-
They identified 17 scoring systems, but that number included five variants of the Framingham score and two of a Hong Kong system.
-
Eight scores were developed in diabetes populations, and five of these [UKPDS, DARTS (Diabetes Audit and Research in Tayside Scotland), Swedish National Diabetes Register, ARIC (Atherosclerosis Risk in Communities Study) and Hong Kong] included HbA1c level.
-
Most scores developed in general population groups had diabetes as a binary variable.
-
Most of the scores developed in general populations underestimated CVD risk in diabetic groups, with underestimates ranging from 11% to 64%.
-
However, in populations with low CVD risk, scores overestimated CVD risk.
As noted above, the addition of diabetes or HbA1c to the score had only modest effect, because it carries less weight than age, cholesterol level, BP and smoking.
These findings have an implication when considering screening for T2DM. Much of the benefit in detecting undiagnosed diabetes comes from treatment of associated metabolic conditions, such as hyperlipidaemia and hypertension. So the better controlled those conditions are in the general population, including those with undiagnosed diabetes, the less there is to gain by detecting diabetes.
Further evidence on the associations between hyperglycaemia and cardiovascular risk comes from the Telde study from Spain,40 which compared risk factors in four groups – NGT, IFG, IGT and combined IFG and IGT – recruited from a population-based study. Novoa et al. (2005)40 reported that, compared with the NGT group, the other three had higher proportions with hypertension, high BMI, higher triglycerides and higher insulin levels, reflecting insulin resistance.
Even when fasting and 2-hour postload glucose levels are within the normal range, there are associations with cardiovascular mortality. Ning et al. (2005)27 from the DECODE study group showed that those with the highest (but still normal) 2-hour glucose levels had insulin resistance and increased cardiovascular mortality.
There is also a relationship between HbA1c level and peripheral vascular disease. Muntner et al. (2005)41 report data from the 1999–2002 NHANES (National Health and Nutrition Examination Survey). The data shown in Table 4 follow multivariate adjustment. Peripheral vascular disease (PVD) was defined as an ankle–brachial BP ratio of < 0.9.
| HbA1c band (%) | RR of peripheral arterial disease |
|---|---|
| < 5.3 | 1.0 |
| 5.3–5.4 | 1.41 |
| 5.5–5.6 | 1.39 |
| 5.7–6.0 | 1.57 |
However, CIs were wide and only the last figure had a 95% CI that did not overlap with 1.0.
Decision point
Hence, if one aim of screening is to reduce heart disease, we should look not only for diabetes, but also for IGT. Even if we did look only for diabetes, we would identify many with IFG and/or IGT, depending on which test was used and what cut-offs were chosen.
The aims of treatment might be:
-
For those with definite diabetes, reduction of the risk of retinopathy and nephropathy, by reduction of PG, initially trying diet and exercise, but using drug therapy when indicated.
-
For those with PG levels in the IFG and IGT ranges, prevention of progression to diabetes, by diet and exercise, or by drug therapy (metformin) if indicated.
-
For all of the above, measures to reduce cardiovascular risk, by measures other than the glucose control ones already mentioned, such as qualitative improvements in diet, cholesterol-lowering measures (such as statins), BP control and anti-obesity measures.
There could be large implications for workload and costs. About 0.5% of the population may have undiagnosed diabetes, but there may be 10% with IGT. Before any screening was started, there would need to be careful planning of workload, involved in both screening and follow-up. Screening might be introduced in a phased manner in order to avoid overload.
Chapter 2 Screening strategies and tests
The strategies to be considered will vary according to what is done at present.
Should screening be selective or universal over the age of 40 years?
It is assumed that screening will be selective by age because T2DM is much less common in younger age groups. The first question is whether or not other selection criteria should be applied. Given the link between T2DM and overweight, the obvious next criterion is BMI.
Organised screening could be a three-stage process, with the first stage being selection from the general population (using general practice registers or self-completed questionnaires) of those likely to be more at risk than average, the second being testing of blood glucose levels, and the third being confirmation (or not) of raised blood glucose level.
Testing only people who are at higher than average risk means that a higher proportion of those who will be tested for glucose will be positive. The number needed to screen to detect each true-positive will be lower, and the whole programme will be more cost-effective. However, the distribution of risk needs to be considered. As the risk threshold is raised, the proportion of those with diabetes will rise, but the absolute number detected may fall. Because of the shape of the distribution curve, most people with undiagnosed diabetes may be in the middle risk band.
Many risk-scoring systems have been used around the world5,42 based on different questionnaires. Risk factors were listed by Paulweber et al. (2010)43 in an analysis to support development of European guidelines. This analysis identified both non-modifiable and modifiable factors, listed in Table 5.
| Non-modifiable risk factors | Modifiable risk factors |
|---|---|
| Age | Overweight and obesity |
| Family history/genetic predisposition | Physical inactivity |
| Ethnicity | Disturbances in intrauterine development/prematurity |
| History of GDM | IFG/IGT |
| POS | Metabolic syndrome |
| Dietary factors | |
| Diabetogenic drugs | |
| Depression | |
| Obesigenic/diabetogenic environment | |
| Low socioeconomic status |
Different risk scores seem to have different specificity and sensitivity. The commonly used Finnish Diabetes Risk Score (FINDRISC) score is based on a sample from the population in Finland. This scorecard predicts the probability of T2DM development over the following 10 years. It uses information such as age, BMI, sex, antihypertensive medication history and some lifestyle factors. This risk score has high sensitivity towards undiagnosed diabetes. It has been validated both in Germany44 and Italy. 45
Risk factors
Age has been mentioned above. The cost-effectiveness of screening will be lower at younger ages, as the number needed to be screened to find each case will increase and also because the event rate from CVD will be lower. However, although the prevalence of diabetes is greater in the older age groups, the excess mortality may fall. Tan et al. (2004)46 found that in men diagnosed with T2DM, aged > 65 years, in Tayside, there was no excess mortality compared with the general population. The situation in women was different, with a RR of death of 1.29 (95% CI 1.15 to 1.45). The implication of this might be that if the main aim of screening is to reduce heart disease mortality and morbidity, screening for diabetes in men should not include the over-65s. However, if the aim is to detect undiagnosed diabetes, we should screen older age groups – perhaps to the age of 75 years.
The age at which screening should start has been debated. Kahn et al. (2010)47 modelled a range of screening strategies based on a US population, starting at ages 30, 45 and 60 years, or at diagnosis of hypertension, and found that the lowest costs per quality-adjusted-life year (QALY) were obtained by starting at age 45 years or at diagnosis of hypertension, and screening at 3-or 5-yearly intervals. In practice, the age at which screening will start will be determined by each health department, with the age threshold in England being determined by the Government's decision on the vascular screening programme.
Body mass index is the second factor. The risk of T2DM is greatly increased by excess weight. But there is also a link with the distribution of body fat, with abdominal (especially visceral) fat distribution carrying a higher risk. Waist measurement could be used as a risk factor – for example, > 40 inches in men or > 35 inches in women. However, waist data are unlikely to be held on GP computer systems.
Comorbidities affect risk. The risk of diabetes is associated with other metabolic conditions, such as hypertension and hyperlipidaemia, and with the presence of vascular disease, such as PVD or IHD. There will be data on comorbidities on GP systems, even if just the fact of prescriptions for antihypertensive or lipid-lowering drugs or steroids.
A family history of diabetes, or of premature vascular disease or hypertension, also increases the risk.
Some ethnic groups have a higher risk of T2DM than others, although this is less if adjustments are made for BMI and fat distribution. In the Manchester survey48 the prevalence of known diabetes in a poor inner city area was 8% and 3.7% in European men and women, and 14% and 18.2% in Pakistani men and women. The Pakistani women had higher BMI than the Europeans – 29.6 kg/m2 compared with 27.2 kg/m2, respectively – and a higher waist–hip ratio – 0.88 compared with 0.81, respectively. Pakistani and European men had similar BMIs (27.4 and 27.5 kg/m2, respectively) but the Pakistani waist–hip ratio was higher [0.96 (95% CI 0.94 to 0.97) vs 0.92 (95% CI 0.92 to 0.94), respectively]. However, the most striking differences were in physical activity. The proportions taking at least 20 minutes of exercise three times a week were 38% and 29% for European men and women, and 7% and 5% for Pakistani men and women, respectively. Physical activity reduces insulin resistance even if there is little or no weight loss.
Risk-scoring systems
There are various scoring systems. The Finnish questionnaire-based system, FINDRISC, includes age, BMI, waist measurement, physical activity, diet (vegetable, fruit and berry consumption), treatment for hypertension, any previous hyperglycaemia and family history. 49
It might be easier if we could use a smaller set of indicators, and there would be little difference in predictive power, as age and BMI provide most of that. 50
One advantage of using a smaller set of risk indicators is that computer systems in general practices will usually have the necessary data – certainly age, drug treatment, comorbidities and, usually, BMI. They are less likely to have family history, and probably will not have waist measurements. But it means that the first stage of any screening system could use existing data at little extra cost.
One scoring system using data that should be available on GP systems was developed by Hippisley-Cox et al. (2009),51 and comprises the following factors: ethnicity, age, sex, BMI, smoking, family history of diabetes, Townsend deprivation score, treated hypertension, CVD and current use of corticosteroids. It was developed using the QResearch database (Version 19; University of Nottingham, Nottingham, UK ) and is known as the QD score. The authors report receiver operating characteristic (ROC) statistics of 0.85 for women and 0.83 for men for detection of diabetes. The results indicate that people from South Asian ethnic groups have different risk factors. These include different lifestyle habits, particularly smoking, along with a family history of diabetes. These factors make them more vulnerable to getting diabetes earlier in life than Caucasians, with a four- to fivefold variation in risk among different ethnicities. So ethnicity should be included in the risk evaluation.
There has been an independent validation of the QD score by Collins and Altman,52 which reported that it performed well in distinguishing between those who develop diabetes and those who do not.
Other scoring systems include the Cambridge Risk Score (CRS), again based on data available in GP systems (see Appendix 2). 53 Those in the highest quintile of the CRS had 22 times the risk of diabetes as those in the lowest quintile, and 54% of incident cases were in the top quintile. 50 It takes into account ethnicity. This score is also validated in the Danish population. 54
Body mass index is probably the single most powerful factor, and other factors may add much less to the detection rate. One review of risk scores by Witte et al. (2010)55 for predicting undiagnosed diabetes concluded that a combination of age and BMI was as good as more complex scores. However, if all of the data are on GP systems, then they may as well be used.
A very thorough systematic review of risk-scoring systems by Noble et al. (2011)56 identified 145 risk prediction models, and described 94 in detail. They noted that almost 7 million people had been in risk-scoring studies, with ages ranging from 18 to 98 years, and follow-up ranging from 3 to 28 years. They estimated that risk scores for diabetes were appearing at a rate of one every 3 weeks. Noble et al. (2011)56 set out to look for scores that were ‘sufficiently simple, plausible, affordable and widely implementable in clinical practice’. They noted that some scores included expensive laboratory biomarkers.
Noble et al. (2011)56 applied a set of quality criteria to the scores. These were:
-
generalisability external validation by a separate research team on a different population
-
statistically significant calibration i.e. that predictions match observations
-
discrimination that the score reliably distinguishes high-risk people from low-risk people
-
usability defined as the score having 10 or fewer components.
They noted that there were two broad categories of score: those that were completed by people themselves, based on questionnaires, and those that were based on data held by health-care providers such as GPs.
Noble et al. (2011)56 did not recommend any one system, making the point that the choice would depend on local circumstances. However, they did identify seven very good systems. These included two from the UK, the CRS and the QD score. In terms of area under ROC curves (AUROCs), the QD score came out better, with external validation AUROCs of 0.80 for men and 0.81 for women, compared with the Cambridge AUROC of 0.72. It should be noted that AUROCs can be altered by changing thresholds for positivity. The Cambridge score of 0.72 uses a threshold of 0.38.
Both of these scores include age, sex, family history of diabetes, BMI, current use of corticosteroids, treatment for hypertension and smoking. The QD score adds ethnicity, deprivation score and CVD. All of the QD components should be on GP computer systems.
Hence, if screening for T2DM in the UK was to be based in primary care and to use a first stage of selection by risk, the QD score seems best.
Another of the Noble et al. (2011)56 ‘top seven’ scores was FINDRISC, which has been validated in a UK population. So if screening in the UK or part thereof was to be based on self-scoring by completion of questionnaires, then FINDRISC could be used. The cut-off might be 12, based on ROC curves from a study by Martin et al. (2011)57 of Munich, Germany. Martin et al. (2011)57 measured HbA1c levels in 12,773 blood donors of whom almost 8% scored 12 points or more in the FINDRISC questionnaire and had HbA1c values of ≥ 5.6%. The 8% and age- and sex-matched controls were invited for an OGTT. Unfortunately, only about 10%, 671 participants, came for the OGTT, some citing reasons such as time costs.
Using a FINDRISC score of 12 as the cut-off, over half had some degree of impaired glucose regulation (IGR) in the OGTT: 9.7% diabetes, 32.6% IFG, 5.7% IGT, 11.8% both IFG and IGT, and 40% having normal glucose levels. Martin et al. (2011)57 compared the percentages of those with diabetes in two FINDRISC subgroups: medium risk, with scores 12–14, and high risk, with scores ≥ 15. Half (31) of the newly diagnosed group were in the high-risk group and 40% in the medium-risk group. The proportions with IFG, IGT or the combination were similar in the medium- and high-risk groups.
Martin et al. (2011)57 state that:
The high acceptance of the FINDRISC questionnaire and the HbA1c testing contrasted with the rather low number of participants that were willing to participate in the OGTT. This finding indicates that simple screening procedures are necessary to achieve compliance and that the OGTT is not attractive for people who would otherwise be accessible for diabetes screening.
They conclude that a combination of FINDRISC cut-off of 12 and HbA1c value of 5.9% would be optimal, as that would identify 56 of the 65 people diagnosed with diabetes.
Abbasi et al. (2012)58 used data from the European Prospective Investigation into Cancer and Nutrition (EPIC-NL) study to validate 25 models for predicting future T2DM. Two approaches were used, depending on what data were available, with data from 38,379 people being used for validating ‘basic’ models (with no blood test data) and data from 2506 individuals used for validating prediction models that included biochemical data. Most models performed well, but most overestimated the risk. The measure of capture used was the c-statistic, which is comparable with the AUROC. FINDRISC had a c-statistic of 0.81 at 7.5 years, in both full and concise form. QD score was lower, at 0.76. AUSDRISK scored 0.84 and EPIC-Norfolk37 0.81.
One issue has been raised by Griffin et al. (2000)53 and the Dutch Hoorn group. 59 Griffin et al. (2000)53 wondered about the dangers of reassurance in those who have high-risk scores but who do not have hyperglycaemia – will they feel they are able to persist with unhealthy lifestyles? And in the Hoorn study, Spijkerman et al. (2002)59 found that the group with high-risk scores, but who did not have diabetes on glucose testing, had a CVD risk that was almost as high as those who were glycaemia-positive. As there were more of the risk-positive but glucose-negatives, they had more cardiac events, leading the authors to comment that:
It may be of greater public health benefit to intervene in the screen positive group as a whole rather than only in the relatively small group who on subsequent biochemical testing have an increased glucose concentration.
However, a recent study by Paddison et al. (2009)60 from the Cambridge MRC group found that people with negative diabetes screening tests were not so reassured that they would have an adverse shift in health behaviours.
In the EPIC-Norfolk study,38 adding a measure of hyperglycaemia, in this case HbA1c, to the Framingham risk score, added little to the predictive value for CHD. That might imply that glucose testing would not be necessary. However, their focus was on CVD, and detection of diabetes would also lead to reduction of microvascular events, for example by screening for retinopathy.
The tests for blood glucose include:
-
casual (non-fasting) blood glucose
-
FPG
-
glucose tolerance testing, combining fasting and 2-hour levels (OGTT)
-
the 50-g glucose challenge test (GCT), which has been used mainly for screening for gestational diabetes
-
HbA1c, which reflects blood glucose over the previous 3 months [assuming red blood cells (RBCs) of normal longevity and the absence of haemoglobin variants].
Casual blood glucose is usually discounted because of its variability and poor sensitivity (at levels which give acceptable specificity). 61
The OGTT is expensive, inconvenient (and sometimes unpleasant – in some people the glucose load causes nausea) and has poor reproducibility. It may also not fit easily into the primary care setting in which most T2DM is diagnosed,62 although some practices have carried out oral glucose tolerance testing. 63
The choice of test depends on what we are screening for. FPG is reliable, in the sense of showing less day-to-day variability than OGTTs, and will identify people with diabetes and IFG. However, it will miss those people with IGT, who have a higher IHD risk than those with IFG.
As with all tests, there is a trade-off between sensitivity and specificity, nicely shown by Hu et al. (2010)64 (Table 6).
| Cut-off | Sensitivity (%) | Specificity (%) | Likelihood ratio | |
|---|---|---|---|---|
| Positive | Negative | |||
| FPG ≥ 5.6 mmol/l | 92.5 | 54.3 | 2.02 | 0.14 |
| FPG ≥ 7.0 mmol/l | 54.5 | 100 | Infinite | 0.46 |
| FPG ≥ 6.1 mmol/l | 81.5 | 80.5 | 4.18 | 0.23 |
| HbA1c ≥ 6.1% | 81.0 | 81.0 | 4.26 | 0.23 |
| FPG > 6.1 mmol/l and HbA1c > 6.1% | 66.0 | 96.3 | 17.84 | 0.35 |
Note that although the cut-offs of FPG 6.1 mmol/l and HbA1c 6.1% give the same sensitivity (they were chosen from ROC curves) they will not identify exactly the same people.
Glycated haemoglobin
An expert group in the UK65 has issued a position statement on the implementation of the World Health Organization (WHO) 2011 guidance on the use of HbA1c levels in the diagnosis of diabetes. The WHO guidance was that, given good quality assurance (QA) testing, a HbA1c value of 48 mmol/mol (6.5%) was recommended as the cut-off point for diagnosing diabetes but that a lower value did not exclude diabetes.
The main points in the UK expert group statement are:
-
UK laboratories meet QA requirements.
-
HbA1c testing should be based on laboratory measurement of a venous blood sample. Point of care results should always be confirmed in a laboratory.
-
One positive HbA1c result is enough in the presence of symptoms, but should be repeated in the absence of symptoms. (The implication for screening is that repeats would be required.)
-
People with HbA1c values in the range 6.0–6.4% (42–47 mmol/mol) were at high risk of diabetes and should receive lifestyle advice, be warned to report symptoms and should have their HbA1c level checked annually.
-
Some people with HbA1c values of < 6.0% (42 mmol/mol) may be at high risk of diabetes, and they should be monitored as above.
The ADA Expert Committee66 on the diagnosis and classification of diabetes mellitus summarised the advantages and disadvantages of HbA1c for the diagnosis of diabetes. The Committee listed the advantages as:
-
HbA1c testing measures average glycaemic levels over a period of 10 weeks or so, and is therefore more stable than FPG testing, and especially 2-hour OGTT.
-
Fasting is not required and the test can be carried out at any time of day.
-
The precision of HbA1c testing can be as good as that of PG testing.
-
HbA1c level is the test used for monitoring control of diabetes and correlates well with the microvascular complications; it may be useful to use the same test for diagnosis and monitoring.
-
It has been shown by meta-analysis that when using a statistical cut-point of two standard deviations (SDs) above the non-diabetic mean value, HbA1c testing is as good as FPG testing and 2-hour PG in terms of sensitivity (66%) and specificity (98%).
The disadvantages were identified as:
-
Internationally, there had been a profusion of assay methods and reference ranges. However, this can be overcome by standardisation to the DCCT (Diabetes Control and Complications Trial) assay.
-
HbA1c level may be affected by other conditions that affect the life of the RBC; results may then be misleading. This could be a particular problem in ethnic groups in which haemoglobinopathy is common.
-
A chemical preparation for uniform calibration standards had become available only recently and was not universally available.
However, with the exception of the other conditions, these disadvantages need not apply in a national screening system that would include quality control measures. Therefore, there is a case for using HbA1c as the screening test, particularly in view of its correlation with cardiovascular risk across a wide spectrum. As mentioned above, Khaw et al. (2004)37 noted that the rise in cardiovascular events with rising HbA1c level starts well below the diabetic range. Indeed, they point out that when both diabetes and HbA1c level are included in the statistical analysis HbA1c dominates; as Gerstein (2004)67 argues in an editorial:
. . . the glycosylated haemoglobin level is an independent progressive risk factor for cardiovascular events, regardless of diabetes status.
The glucose levels for the diagnosis of diabetes were based on the relationship between PG and retinopathy. Recently, a similar study by Sabanayagam et al. (2009)68 has examined the relationship between HbA1c level and retinopathy. Using the presence of moderate retinopathy as the indicator of diabetes, the authors suggest a HbA1c threshold of 6.6%. This is very close to the 6.5% suggested by the DETECT-2 Group. 21
The ADA position statement69 in January 2010 recommended a cut-off of 6.5% for diagnosing diabetes, based on retinopathy risk. They recommended a cut-off of 5.7%, and hence a range of 5.7% to < 6.5%, for identifying those at high risk of diabetes. The arguments in favour of the 5.7% cut-off were that:
-
The 6.0% to < 6.5% range misses a lot of patients who have IFG or IGT and who are at increased risk of diabetes. They cite studies reporting that people in the 5.5% to < 6.0% range have a 5-year incidence of diabetes of 12–25%.
-
That unpublished NHANES data show that a HbA1c value of 5.7% corresponds with a FPG of 6.1 mmol/l (i.e. IFG).
-
That other unpublished NHANES data show that a HbA1c cut-off of 5.7% has modest sensitivity (about 40%) but good specificity (81–91%) for IFG and IGT
-
That other unpublished analyses indicate that a HbA1c value of 5.7% is associated with a similar risk of diabetes to the high-risk group in the Diabetes Prevention Program.
As always, there is a trade-off between sensitivity and specificity. If we used a low cut-off of HbA1c of 5.7%, there would be more false-positives. However, they are at higher risk of CVD than the rest of the population and would benefit from lifestyle measures. The only harm might be from the labelling as ‘pre-diabetic’.
In July 2009, an expert committee appointed by the European Association for the Study of Diabetes (EASD), International Diabetes Federation (IDF) and ADA published a report70 on the role of the HbA1c assay in the diagnosis of diabetes. The key recommendations of this report were that:
-
HbA1c assay should be used as a diagnostic test for diabetes with a threshold of ≥ 6.5% for defining diabetes.
-
That HbA1c measurement has several advantages (both logistical and technical) over fasting glucose.
-
Individuals whose HbA1c values are close to the 6.5% threshold for diabetes (i.e. ≥ 6.0%) should receive demonstrably effective interventions aimed at preventing progression to diabetes.
-
Testing should be by clinical laboratory instruments, not point-of-care instruments.
A cut-off level of 6.0% might pick up most people with IFG, but not all. Selvin et al. (2010)71 (from the ARIC study) reported that a HbA1c cut-off level of ≥ 6.5% would detect 49% of those with FPG of ≥ 7.0 mmol/l, and a cut-off of 6.0% would detect 75% of those diabetic by FPG. In the band below (HbA1c level of 5.5% to < 6.0%), only 3% were diabetic by FPG. In this band, the mean HbA1c level was 5.7% and mean FPG was 5.8 mmol/l. A cut-off of 5.5% would detect 91% of those with diabetic FPGs.
In Germany, Peter et al. (2001)72 examined the value of HbA1c testing using a cut-off of 6.5% in patients shown to be diabetic by OGTTs. Sensitivity was only 47% and specificity was 98.7%. Using a lower cut-off of 6.1% gave sensitivity of 71% and specificity of 92%.
In Wales, Morrison et al. (2011)73 reported a sensitivity of 67% and specificity of 57% using a 6.5% threshold.
A very useful study by Schottker et al. (2011)74 compared FPG and HbA1c testing in the ESTHER (Epidemiologische Studie zu Chancen der Verhütung, Früherkennung und optimierten Therapie chronischer Erkrankungen in der älteren Bevölkerung) study in Germany among almost 10,000 subjects aged 50–74 years. They excluded people with diabetes at baseline and re-tested the others at 2 and 5 years. They classified those who had pre-diabetes at baseline using FPG levels of 100–125 mg/dl and HbA1c levels of 5.7–6.4%. In this group, 23% had pre-diabetes only by FPG testing; 23% had pre-diabetes by both FPG and HbA1c testing, and 54% had it only by HbA1c testing. Hence, in total, FPG testing detected 46% and HbA1c testing detected 77%. They did not carry out OGTTs.
Schottker et al. (2011)74 determined the risks of incident diabetes over the next 5 years. Compared with those who had neither raised FPG or HbA1c levels, the RRs were:
-
IFG alone 4.23
-
pre-diabetic HbA1c alone 3.05
-
both FPG and HbA1c pre-diabetic 7.81.
They concluded that screening for future diabetes should use both FPG and HbA1c testing. They did not report incidence of CVD.
Moves towards global standardisation of HbA1c measurement will help. 75 HbA1c testing has advantages of not requiring people to be fasting, and its diagnostic accuracy now rivals that of PG. However, it should be noted that any cut-off will be arbitrary because for vascular disease there is a continuum of risk, unlike the dichotomy seen with moderate retinopathy.
There are ethnic differences in HbA1c levels and the cut-off may have to be adjusted for different groups. A study from China by Bao et al. (2010)76 suggested a cut-off of 6.3%.
We need to distinguish the use of HbA1c testing for diagnosing diabetes from its value in predicting vascular risk. In the latter case, it is correct to say that HbA1c level is a good predictor of vascular risk on its own, but that once other traditional markers of vascular risk – such as hypertension, smoking, lipid level – are added, HbA1c testing gives limited marginal benefit. 38
Reservations about the use of glycated haemoglobin
Various concerns about reliance on HbA1c level as the diagnostic test for diabetes have been raised. Some of these come from the clinical biochemists and, therefore, need to be recognised.
The Association of British Clinical Diabetologists (ABCD) position statement77 on using HbA1c testing for diagnosis (not screening) lists the advantages and disadvantages (Table 7).
| Advantages | Disadvantages |
|---|---|
| No need for fasting | Abnormal haemoglobins |
| Low biological variability | Anaemias |
| Measure of glycaemia over a period of months | Ageing and ethnicity |
| Analytical standardisation | Residual analytical variations |
Each of the first three disadvantages leads to misleading results.
The ABCD choose a HbA1c range of 5.8–7.2% for intermediate hyperglycaemia and recommend another test, such as FPG or an OGTT, to confirm or exclude diabetes. They suggest that combined HbA1c and FPG testing could be used for diagnosis.
Schindhelm et al. (2010)78 also warn that laboratory assays for HbA1c level still show significant variability, noting that coefficients of variance ranged among methods from 1.7% to 7.6%. However, if there was a national screening system with QA systems, this should be less of a problem.
There has been debate about the lower cut-off level for HbA1c. The SPHN working group79 noted that some groups advocate a HbA1c range of 5.7–6.4% for defining non-diabetic hyperglycaemia (NDH), whereas others suggest 6.0% as the lower limit. Unfortunately, most studies simply report results for the whole band, whereas what we need is a comparison of the 5.7–5.9% range with the 6.0–6.4% range.
Cederberg et al. (2010)80 used the 5.7% cut-off and compared it with IGT and IFG as revealed by OGTTs. Diabetes was preceded by raised HbA1c, IGT and IFG levels in 33%, 41% and 22% of cases, respectively, after 10 years. The converse may be more important – diabetes was not preceded by raised HbA1c in 67%, although if screening were to be introduced in the UK, the repeat testing interval would most likely be 5 years.
Mostafa et al. (2010)81 from Leicester used data on OGTTs and HbA1c from a cohort of 8696 subjects to compare proportions with abnormal results. Using the OGTT, 3.3% were diabetic, and of these about one-third had a HbA1c level of < 6.5%. Using a HbA1c level of 6.5% as the threshold for diabetes increased the prevalence to 5.8%, but on OGTT over half had IGT or IFG. Of 595 people, 198 were identified as diabetic by both OGTT and HbA1c testing, 93 by only OGTT, and 304 only by HbA1c. The paper does not give details of how many who were diabetic by OGTT, had the diagnosis made by the FPG or the 2-hour PG, or both. All of those who were recognised as diabetic on OGTT had the OGTT repeated – one-third were not diabetic on the second OGTT. Mostafa et al. (2010)81 noted that a HbA1c cut-off level of 5.7% would identify 51% of their cohort as abnormal.
A later abstract from the same group82 reported that HbA1c testing and the OGTT gave similar numbers for incident diabetes – but not the same people.
Borg et al. (2010)83 from Denmark also compared the characteristics of those diagnosed by OGTT and HbA1c but, again, give no details of the OGTT time points responsible for diagnosis. Using a HbA1c cut-off level of ≥ 6.5%, 6.6% were identified as diabetic compared with 4.1% using the OGTT. Almost 58% of those recognised as diabetic using the OGTT were not picked up by HbA1c testing. In terms of cardiovascular risk profile, those people considered diabetic by HbA1c testing, but not using the OGTT, had as high a risk (actually higher, but not statistically significantly so). Hence, OGTT and HbA1c testing appear to be detecting groups that only partly overlap.
Lorenzo et al. (2010)84 from the IRAS (Insulin Resistance Atherosclerosis Study) reported that HbA1c testing was less sensitive than IFG or IGT for detection of risk (not diabetes), but what was meant by this was that HbA1c testing classified fewer individuals as having abnormal glucose tolerance – it was not about diabetes. No specificity was reported.
Valdes et al. (2011)85 from Asturias found that for prediction of diabetes in those not diabetic when first screened, FPG (2-hour post challenge) and HbA1c testing had similar ROC curves [area under curve (AUC) 0.83, 0.79 and 0.80, respectively], but that a combination of HbA1c and FPG testing did better (AUC 0.88).
The incremental risks of higher HbA1c levels vary among outcomes. Selvin et al. (2010)86 took a HbA1c range of 5.0% to < 5.7% as the reference range (RR = 1.0). For diabetes, RRs for HbA1c levels of 5.7% to < 6.5% and ≥ 6.5% were 3.0 and 13.7, respectively, but for CHD the RRs were 1.6 and 1.9, respectively. 86
Skriver et al. (2010)87 from the Danish arm of the ADDITION trial, have provided data on the specificity of HbA1c testing. A high-risk group (identified by questionnaire and then by a second-stage casual blood glucose or HbA1c level) underwent OGTTs and then the HbA1c levels of those with NGT (defined by OGTT) were examined. Only 0.4% had HbA1c of ≥ 6.5%; 6.7% had HbA1c levels in the range 6.0–6.49%, and 93% had HbA1c levels of < 6.0%.
The sensitivity of HbA1c testing was assessed in a Paris study by Cosson et al. (2010). 88 In a group mainly composed of obese women, they compared FPG and HbA1c with the 2-hour OGTT. Using FPG testing alone, 70% of people with IGR would have been missed because most had isolated IGT. The sensitivity of HbA1c testing for detecting an abnormal 2-hour PG using a cut-off of ≥ 6% was only 37%.
From Brazil, Cavagnolli et al. (2011)89 also reported low sensitivity. They carried out OGTTs in 498 people of whom 115 were recognised as diabetic by the OGTT: 26 by FPG alone, 54 by 2-hour PG alone and 35 by both. But only 56 were identified as diabetic by HbA1c levels of ≥ 6.5%. HbA1c testing was stronger in specificity. Using a cut-off of ≥ 6.0% identified 167 people, of whom 65 had diabetes, 41 had IFG, 52 had IGT, and only nine had NGT.
Pajunen et al. (2010)90 (from the DPS) carried out two OGTTs to be sure of correctly diagnosing people with IGT. They then monitored participants for the development of diabetes. They found that the sensitivities of HbA1c levels of ≥ 6.5% for detecting new diabetes were 35% in women and 47% in men compared with two OGTTs. A cut-off level of 6.0% gave sensitivities of 67% and 68%. Specificities were better; using a cut-off HbA1c level of < 6.5% gave specificities of 90% in women and 91% in men.
Turning to the usefulness of HbA1c testing for detecting IGT at baseline, Pajunen et al. (2010)90 report that HbA1c levels in patients with IGT confirmed by two OGTTs were:
-
< 5% 8.5% of participants
-
5.0–5.9% 69.5% of participants
-
6.0–6.4% 14.3% of participants
-
6.5% or > 7.7% of participants.
So a HbA1c cut-off level of 6.0% missed most people with IGT.
Pajunen et al. (2010)90 compared those who developed diabetes and had a HbA1c level of ≥ 6.5% with those who developed diabetes and had a HbA1c level of < 6.5%. The former group had significantly higher weight, BMI and FPG, but there were no differences in BP or cholesterol levels. They then reclassified the DPS patients as diabetic or not, based on a HbA1c level of ≥ 6.5% and found that the results of the study, in terms of preventing progression to diabetes, would not have been statistically significant.
Older studies were reviewed by Bennett et al. (2006). 91 They identified nine studies that assessed the value of HbA1c testing in screening for diabetes, using the OGTT as the reference standard. They concluded that the HbA1c cut-off level should be 6.1%, which, for diabetes, had a sensitivity ranging from 78% to 81% and specificity of 79–84%. FPG testing, with a cut-off level of > 6.1 mmol/l, had poorer sensitivity (ranging from 48% to 64%) but better specificity (ranging from 94% to 98%). HbA1c and FPG testing had, at those cut-off levels, only 50% sensitivity for detecting IGT. Bennett et al. (2006)91 noted that the need for cut-off levels varied among different populations.
Glucose challenge test
The 50-g GCT has been used extensively in screening for gestational diabetes but has seldom been studied in screening for T2DM. It can be used for people who have not fasted, which makes it more convenient.
Abdul-Ghani and De Fronzo (2009)92 have reviewed the evidence on relationships of FPG and PG at all time points after the 75-g OGTT. They make a convincing case for the 1-hour PG being the strongest predictor of later diabetes. This would suggest that it would be worth researching the value of the 50-g 1-hour GCT in screening for IGT and diabetes.
A study published since that review provides further support for the superiority of the 1-hour PG, although it was based on the 75-g OGTT, not the 50-g GCT: Joshipura et al. (2011)93 reported that among a group of non-diabetic people aged 40–65 years in Puerto Rico, the 1-hour PG had stronger associations with metabolic factors – such as BMI, waist circumference and body fat percentage – than the 2-hour PG.
In a recent study, Abdul-Ghani et al. (2011)94 compared various measures of blood glucose for predicting future T2DM. A HbA1c level of 5.65% had an AUROC of 0.73, and FPG (126 mg/dl) had an AUROC of 0.75. However, the 1-hour PG (155-mg/dl cut-off) had a greater AUROC of 0.84. Adding HbA1c testing to the 1-hour PG increased the AUROC slightly, to 0.87. The 2-hour PG had an AUROC of 0.79.
Phillips et al. (2009)95 compared the non-fasting GCT with HbA1c testing and the OGTT (undertaken 1 week later) and found AUROCs of 0.90 for diabetes, and 0.79 for pre-diabetes (defined as IGT of IFG based on the 6.1-mmol/l threshold). For HbA1c testing (probably > 6.0%, although not clear) the AUROCs were 0.82 for diabetes and 0.68 for pre-diabetes.
From the same group, Chatterjee et al. (2010)96 examined the cost-effectiveness of screening by random PG, the 1-hour 50-g GCT and the 75-g OGTT, compared with no screening. Their model included costs of testing and treatment (with metformin). They concluded that the GCT would be the most cost-effective test and also that screening would be cost-effective compared with no screening. This study is discussed in more detail in Chapter 6.
Jones et al. (2013)97 from Exeter reported results from a group of 2253 people aged > 40 years and not known to have diabetes. They were participating in the ‘Exeter 10,000’ study. They had two risk scores applied: Leicester and Cambridge. The latter was more sensitive (69%) but less specific (57%) than the Leicester one (62% and 69%). However, the interest here is in the results of HbA1c and FPG testing; both result in diabetes being diagnosed in about 16%, but half of those diagnosed as having diabetes or at high risk by HbA1c testing were not diagnosed by FPG testing and vice versa.
Summary
There is no perfect screening test. HbA1c testing is accepted for diagnosis in people who are suspected to have diabetes, using a level of 6.5%, but is less reliable for screening because of its sensitivity. But it is convenient and a good marker of cardiovascular risk.
The OGTT has higher sensitivity but is inconvenient, and uptake is poorer.
The 50-g GCT looks good, and does not require fasting, but the evidence base is sparse.
The combination of HbA1c and FPG testing might be a compromise option, but will miss some people who diagnosed as diabetic on the 2-hour OGTT.
Suggested conclusions
The first step in screening for diabetes and IGT should be selection by risk factor score.
The second stage should use HbA1c level as the screening test, with 6.0% as the cut-off.
In the third stage, the diagnosis of diabetes should be confirmed by a second test of blood glucose: either FPG or HbA1c testing. Two HbA1c results of ≥ 6.5% would confirm the diagnosis.
Given the lack of agreement on the use of HbA1c testing alone, we recommend that if the first HbA1c value is in the 6.0–6.49% range then the third stage should meantime use both HbA1c testing and either FPG testing, with 7.0 mmol/l used as the diabetes cut-off as in the standard definitions, or a 2-hour OGTT. It is unlikely that people with HbA1c values in the range 6.0–6.49% will have FPG values of ≥ 7.0 mmol/l; this recommendation can be reviewed in the light of experience and FPG dropped if it does not contribute. However, more people may have postload hyperglycaemia and we might expect some with HbA1c values in the 6.0–6.49% range to have 2-hour PG in the diabetic range.
We recommend research into the usefulness of the 50-g non-fasting GCT as a screening test.
Chapter 3 Do different screening tests identify groups at different cardiovascular risk?
Concern has been raised that screening by HbA1c and FPG testing might pick up different groups. This was examined by Carson et al. (2010)98 using NHANES data, with cut-off levels of 6.5% for HbA1c and 7.0 mmol/l for FPG. There was some disagreement between the tests, but 96% were not diagnosed as diabetic by both, and 1.8% were diagnosed as diabetic by both. In 0.5% of people, diabetes was diagnosed by HbA1c testing but not FPG testing, but 82% of this group had IFG and would be treated correctly. In the 1.8% diagnosed as diabetic by FPG testing but not by HbA1c testing, almost half were in the HbA1c range 6.0% to < 6.5% and would also be treated.
However, there is less agreement between HbA1c and FPG testing when it comes to diagnosing ‘pre-diabetes’. Mann et al. (2010),99 also using NHANES data, compared results using a HbA1c cut-off level of 5.7% and FPG of 6 mmol/l among participants who also had an OGTT. The prevalence of pre-diabetes using the range 5.7% to 6.4% was 12.6%, of whom 4.9% were negative by FPG (PG level of < 100 mg/dl). However, almost 21% were identified as positive by FPG testing (in range 100–125 mg/dl) but negative by HbA1c testing. One could speculate that the last group had isolated IFG and hence were at lower risk of CVD, whereas the HbA1c-positive but FPG-negative group may have had IGT. There were some differences among the HbA1c-positive, FPG-negative groups and the FPG-positive, HbA1c-negative groups, with more of the former found to be hypertensive (41% vs 34%) and with slightly higher total cholesterol results (212 mg/dl vs 205 mg/dl).
Previous studies have reported that in pre-diabetes, HbA1c level has a stronger relationship with the 2-hour PG than the FPG,100 and that IFG is associated with beta cell dysfunction, whereas those with IGT and normal FPG levels have insulin resistance.
In monitoring people with pre-diabetes for progression to diabetes, there is a case for using both HbA1c and FPG testing. De Vegt et al. (2001)101 from the Hoorn study showed that having both IFG and IGT was associated with a much higher risk of progression to diabetes than either alone (rates of 40% vs 10% and 11%).
Recent papers: cohort studies
The ADDITION study102 followed up 20,916 participants for a median of 7 years, all of whom had a HbA1c level of > 5.8% or a random PG level of > 5.5 mmol/l. A HbA1c level of > 6.5% was associated with increased HRs for all-cause mortality in the NGT groups, and in groups with IGT but not IFG. This relationship was present in both the subsets, with high and low heart risk scores at baseline. In people with IFG but not IGT, increasing HbA1c level was not associated with significantly changing HR. Limited comparisons can be made between the effectiveness of HbA1c, FPG and IGT for predicting cardiovascular risk factors because the population investigated was selected partly on the basis of HbA1c risk score.
The ARIC Study71,103 followed a cohort of 11,057 people, without diabetes or heart failure at baseline, for a median of 14.1 years. This study found that a HbA1c level of between 6.0% and 6.4% is associated with a doubling of risk – adjusted for age, race and sex – for incident heart failure in comparison with a HbA1c level of between 5.0% and 5.4% (Table 8). The HR is reduced to 1.4 after adjustment for a range of CVD risk factors and fasting glucose levels. Compared with the group with HbA1c levels of 5.0–5.4%, the group with HbA1c levels of 6.0–6.4% were more likely to be smokers (31% vs 18%), had higher BMIs (29.7 kg/m2 vs 26.6 kg/m2), were more likely to be treated for hypertension (42% vs 23.5%) and had a much higher proportion of African American people (51% vs 12%).
A FPG of 6.1–6.9 mmol/l was not indicative of an increased risk of incident heart failure above a reference category of 5.0 to 5.5 when adjusted for covariates and HbA1c levels (see Table 8). Of the people with elevated HbA1c levels (6.0–6.4%), the greatest risk for incident heart disease was observed in those with lower FPG:
-
HR 3.57 (1.45–8.79) for FPG of < 5.0 mmol/l
-
HR 2.19 (1.40 to 3.43) for FPG of 5.0–5.5 mmol/l
-
HR 1.29 (0.86 to 1.94) for FPG of 5.6–6.0 mmol/l compared with reference category HbA1c 5.0–5.4%, FPG 5.0–5.5 mmol/l.
Each 1% increase in HbA1c level was associated with a 39% increase in heart failure risk after adjustment for covariates.
| HbA1c level (%) | HR adjusted for age, race and sex only (95% CI) | HR adjusted for age, race, sex and other covariates including FPG (95% CI) |
|---|---|---|
| 5.0–5.4 | Reference | Reference |
| 5.5–5.9 | 1.44 (1.24 to 1.68) | 1.16 (0.98 to 1.37) |
| 6.0–6.4 | 2.04 (1.63 to 2.54) | 1.40 (1.09 to 1.79) |
| FPG level (mmol/l) | HR adjusted for age, race and sex only (95% CI) | HR adjusted for age, race, sex and other covariates including HbA1c (95% CI) |
| 5.0–5.5 | Reference | Reference |
| 5.6–6.0 | 1.13 (0.95 to 1.34) | 1.00 (0.84 to 1.20) |
| 6.1–6.9 | 1.49 (1.23 to 1.79) | 1.11 (0.90 to 1.35) |
Hence, HbA1c level predicted heart failure but FPG level did not.
The Strong Heart Study104 followed a cohort of 4549 American Indian adults for a median of 15 years. There was a non-significant trend towards higher risk of CVD in people with elevated but non-diabetic HbA1c and FPG. This trend was stronger for HbA1c than FPG but both were non-significant. Elevated HbA1c of over 6.5% was associated with an increased HR for CVD 1.40 (95% CI 1.02 to 1.93) when correcting for a range of risk factors including FPG although the CI almost intersects zero (Table 9). Elevated FPG of over 126 mg/dl (7 mmol/l) was associated with a non-significant trend towards an increased HR for CVD 1.25 (95% CI 0.97 to 1.62) when correcting for a range of risk factors including HbA1c (see Table 9).
| HbA1c level (%) | HR for CVD adjusted for age and sex only (95% CI) | HR for CVD adjusted for age, race, sex and other covariates including FPG (95% CI) |
|---|---|---|
| < 5 | Reference | Reference |
| 5.0 to < 5.5 | 1.29 (1.04 to 1.61) | 1.17 (0.94 to 1.46) |
| 5.5 to < 6 | 1.21 (0.95 to 1.55) | 1.09 (0.85 to 1.39) |
| 6.0 to < 6.5 | 1.30 (0.91 to 1.85) | 1.13 (0.79 to 1.61) |
| ≥ 6.5 | 1.93 (1.43 to 2.59) | 1.40 (1.02 to 1.93) |
| FPG level: mg/dl (mmol/l) | HR adjusted for age and sex only (95% CI) | HR adjusted for age, race, sex and other covariates including HbA1c (95% CI) |
| < 100 (< 5.6) | Reference | Reference |
| 100 to < 126 (5.6 to 6.9) | 1.16 (0.96 to 1.41) | 1.10 (0.90 to 1.34) |
| ≥ 126 (7) | 1.51 (1.18 to 1.92) | 1.25 (0.97 to 1.62) |
These two cohort studies provide evidence that when considering cardiovascular risk, HbA1c is more predictive than FPG.
Other studies have reported different results. In the AusDiab cohort study, Barr et al. (2009)31 followed 10,026 people without diabetes for 7 years. They found that in a model with FPG the effect of a SD increase in HbA1c level (0.4%) on all-cause mortality of 1.1 (95% CI 0.9 to 1.2) and CVD mortality of 1.0 (95% CI 0.8 to 1.2) was not significant, but in a model containing HbA1c a SD increase in FPG level (0.7 mmol/l) was associated with a significant increase in CVD mortality 1.3 (95% CI 1.0 to 1.6) but not all-cause mortality 1.1 (95% CI 0.9 to 1.2). However, in terms of application to a population screening programme, comparing SD increases provides less useful information than making comparisons above and below a cut-point that may be implemented. The differences in results between the studies may also be associated with the differing length of follow-up time. Barr et al. (2009)31 found that one SD increase in 2-hour PG levels (2.2 mmol/l) increased the HRs for all-cause mortality even with FPG in the model 1.2 (95% CI 1.1 to 1.3) or with HbA1c in the model 1.2 (95% CI 1.1 to 1.3), suggesting that 2-hour PG may be the best predictor of all-cause mortality after 7 years' follow-up.
However, there are some curious features in this paper. The authors report that FPG, 2-hour PG and HbA1c levels were classified into quintiles, but the numbers of participants in each quintile were very different. The ‘quintiles’ just appear to be bands, and unequal ones, by level of PG or HbA1c.
Cardiovascular mortality was higher in the higher bands for all three measures. In most cases there were parallel increases in risk factors for CVD, such as hypertension, but the differences between lowest and highest band were different, being less for HbA1c than some other variables. For example, the difference in SBP between highest and lowest bands was 16 mmHg for FPG, 15 mmHg for 2-hour PG but only 6 mmHg for HbA1c. The differences in BMI between highest and lowest bands were 3.4 kg/m2 for FPG, 3.4 kg/m2 for 2-hour PG and 1.7 kg/m2 for HbA1c. The highest band was split in FPG and 2-hour PG so these figures use the lower half of the highest bands, which contained most of the participants.
Cross-sectional
The Danish Inter99 Study83 compared the cardiovascular risk profile of 6258 Danish participants with results of different measures of blood glucose. They used the PRECARD program to assess IHD risk. PRECARD is based on a suite of CVD risk factors. There was no significant difference between median PRECARD score for those identified as diabetic by the HbA1c test or the OGTT, but there was a trend towards higher risk score for those classified as diabetic by both tests, implying that they are associated with different but overlapping risk factors (Table 10). Those diagnosed as diabetic by OGTT only had higher BMI scores (33% with BMI of ≥ 30 kg/m2 compared with 27% diagnosed by HbA1c only), more hypertension (74% vs 48%), a greater proportion with raised triglyceride levels (29% vs 21%) and more central obesity. Conversely, those diagnosed by HbA1c only had a much higher proportion of smokers (64% vs 32%). So where there were differences in risk factors, in most cases they were worse in those diagnosed by OGTT only, with smoking being the exception.
Receiver operating characteristic analysis indicates that HbA1c testing performs better than FPG and 2-hour PG tests at discriminating between individuals at high and low risk of developing IHD over a 10-year period using the PRECARD tool (Centre for Preventive Medicine, Medical Department M, Glostrup University Hospital, Glostrup, Denmark), particularly at specificity of between 50% and 90%.
| Group | Median (IQR) PRECARD score for cardiovascular risk | BP ≥ 140/90 mmHg or antihypertensive medication (%) | Raised triglyceride level of > 2 mmol/l (%) | Current smokers (%) |
|---|---|---|---|---|
| No diabetes | 1.8 (0.7 to 3.7) | 35.9 (34.6 to 37.1) | 10.7 (10.0 to 11.6) | 34.2 (32.9 to 35.4) |
| (FPG < 7 mmol/l or 2-hour PG < 11.1 mmol/l and HbA1c < 6.5%) | ||||
| Diabetes by OGTT only | 5.7 (2.8 to 9.8) | 73.8 (66.0 to 80.7) | 29.3 (22.2 to 37.3) | 32.0 (24.6 to 40.1) |
| (FPG ≥ 7 mmol/l or 2-hour PG ≥ 11.1 mmol/l and HbA1c < 6.5%) | ||||
| Diabetes by HbA 1c only | 5.1 (2.7 to 7.7) | 47.9 (42.1 to 53.6) | 20.9 (16.4 to 25.9) | 64.4 (58.7 to 69.8) |
| (FPG < 7 mmol/l and 2-hour PG < 11.1 mmol/l and HbA1c ≥ 6.5%) | ||||
| Diabetes by HbA 1c and OGTT | 7.6 (4.2 to 14.5) | 83.5 (75.2 to 89.9) | 48.6 (38.9 to 58.4) | 41.3 (39.1 to 51.1) |
| (FPG ≥ 7 mmol/l or 2-hour PG ≥ 11.1 mmol/l and HbA1c ≥ 6.5%) |
Smoking has been linked with diabetes before Sargeant et al. (2001)105 from the EPIC-Norfolk study reported that mean HbA1c level was higher in smokers and hypothesised that smoking affected insulin sensitivity. One question is whether or not smoking affects HbA1c level rather than diabetes.
The ADDITION study81 compared blood tests and cardiovascular risk profile in 8696 individuals. As in the Danish Inter99 Study,83 individuals identified as diabetic by an OGTT, but not HbA1c testing, had significantly higher SBP, mean triglyceride level, total cholesterol level and mean Framingham 10-year cardiovascular risk profile than those identified as diabetic by HbA1c testing but not OGTT (Table 11). A screening programme first using HbA1c testing and subsequent oral glucose tolerance testing only in screen-positives may miss these high-risk individuals. There is clearly something about those identified by the OGTT that increases risk, possibly because the 2-hour PG correlates with insulin resistance.
| Diabetes identified by | Framingham 10-year cardiovascular risk: mean (SD) | Mean SBP: mmHg (SD) | Mean triglycerides: mmol/l (SD) | Total cholesterol > 5 mmol/l (%) |
|---|---|---|---|---|
| OGTT | 0.22 (0.16) | 148.1 (20.0) | 2.15 (1.57) | 65.4 |
| HbA1c | 0.19 (0.15) | 142.1 (19.8) | 1.89 (1.23) | 61.2 |
| OGTT only | 0.25 (0.16) | 150.4 (21.5) | 1.97 (1.45) | 71.7 |
| HbA1c only | 0.19 (0.14) | 138.9 (19.6) | 1.66 (0.82) | 58.6 |
| HbA1c and OGTT | 0.20 (0.15) | 147.0 (19.2) | 2.24 (1.62) | 65.5 |
The MET SIM (METabolic Syndrome In Men) study of 5836 Finnish men106 also found the least favourable lipid profiles in men with diabetes as defined by WHO OGTT 1999 criteria and HbA1c level of > 6.5%, with mean triglycerides (2.03 mmol/l) and high-density lipoprotein (HDL) cholesterol (1.21 mmol/l). Diabetes defined by HbA1c testing only was found to be associated with a trend towards lower triglycerides (1.55 mmol/l) but also lower HDL cholesterol (1.30 mmol/l) than diabetes, defined by WHO 1999 criteria triglycerides (1.67 mmol/l) and HDL cholesterol (1.37 mmol/l).
It appears that there may be a hierarchy of CVD risk prediction: OGTT stronger than HbA1c testing and HbA1c testing stronger than FPG testing. In the absence of details, this implies that OGTT positivity is driven by elevated 2-hour levels.
In a cross-sectional study of 1219 non-diabetic individuals,107 carotid intima media thickness was found to be correlated with HbA1c level and 1-hour glucose level but not with fasting glucose or 2-hour glucose, for the subgroup with normal PG. When adjusted for confounders of age, sex, waist, SBP, HDL and total cholesterol, only HbA1c testing was correlated to intima media thickness. Similarly analysis with all 1219 individuals (558 with NGT, 409 with IFG, 78 with IGT, and 174 with IFG and IGT) found that only HbA1c testing was significantly correlated to intima media thickness after adjustment for confounders. Hence, the predictive power of the 2-hour PG may be due to correlations with other aspects of the metabolic syndrome, such as hypertension and lipids, i.e. it is a correlate not a cause.
In the Rancho Bernardo study,34 the risk of cardiovascular mortality was increased in women with isolated postchallenge (2-hour) hyperglycaemia (age-adjusted HR 2.6; 95% CI 1.5 to 4.8) but not in men (age-adjusted HR 0.7; 95% CI 0.3 to 1.6). 34
The point estimates for Edinburgh Artery Study32 participants were similar with ORs for cardiovascular mortality of 2.7 (95% CI 0.6 to 11.6) for women and 0.8 (95% CI 0.09 to 6.7) for men.
However, a Paris study34 found that the heart disease mortality rate in men with normal fasting glucose, but IGT was three times that of those with NGT.
Ethnicity: Afro-Caribbean
A joint analysis of data from the Screening for Impaired Glucose Tolerance (SIGT) study and Third National Health and Nutrition Examination Survey (NHANES III)108 found that black people have higher HbA1c levels than white people, even after adjustment for covariates, including age, sex, BMI, BP, FPG and 2-hour PG. The increase in HbA1c level for black people with NGT was 0.13% and 0.21% (SIGT and NHANES, respectively), increasing to 0.26% and 0.30% for those with pre-diabetes, and 0.47% and 0.47% in the diabetic range. Furthermore,108 the HbA1c test identifies proportionally more black people than white people as both diabetic and pre-diabetic than the OGTT, using both International Expert Committee (IEC)70 and ADA criteria.
Similarly, in the ARIC study,71 black people accounted for just 15.5% of study participants with HbA1c levels of < 5%, 11.9% of participants with HbA1c levels of 5.0–5.5%, rising to 27.0% of those with HbA1c levels of 5.5–6.0%, 49.1% of those with HbA1c 6.0–6.5%, and the majority of participants (52.2%) with HbA1c levels of ≥ 6.5% were black. Ethnicity for all participants in this study was either black or white. There was no significant interaction between race and HbA1c value regarding the risk of CHD, ischaemic stroke or death from any cause (p > 0.80), suggesting that the raised HbA1c levels in the black population do not represent a higher risk in that population and, therefore, there need be no adjustment in recall thresholds for black people based on cardiovascular risk. There was an interaction between race and risk of diagnosed diabetes after 15 years (p = 0.007), based on data from follow-up telephone calls. There was no interaction between race and risk of diabetes at 6 years (p = 0.8) which was based on data from visits. Therefore, HbA1c score may be associated with different risks of diabetes according to race, or this effect may simply be driven by ethnic differences in reporting and health-care-seeking behaviour affecting rates of self-reported diabetes. For self-reported diabetes at 15 years of follow-up there was also a significant interaction between race and FPG level (p = 0.01). The NHANES99 in the USA also found that a greater proportion of black people than white people had a FPG level of < 5.5 mmol/l alongside higher HbA1c levels.
The UKPDS study109 found that in newly diagnosed people with diabetes between 1977 and 1991, the HR (adjusted for confounders) associated with Afro-Caribbean ethnicity for myocardial infarction (MI) was 0.3 (95% CI 0.2 to 0.6). Diagnosis of diabetes was by two FPG measurements of > 6 mmol/l, which is lower than currently accepted criteria.
The IRAS study84 found that African Americans made up 39% of those with HbA1c levels of ≥ 6.5% and only 24% of those with HbA1c levels of < 5.7%. In contrast, white Americans made up 25% of those with HbA1c levels of ≥ 6.5% and 45% of those with HbA1c levels of < 5.7% (the remainder were Hispanics). Taking all of those at increased risk of diabetes (IGT, IFG or HbA1c levels between 5.7% and 6.4%) as the gold standard, the sensitivity of HbA1c (5.7–6.4%) was higher in African Americans (31%) than White Americans (10%). The reverse was true for IGT (45% and 60% in African Americans and white Americans, respectively). IFG performance did not differ by ethnicity.
The Health Aging and Body Composition study110 of 70- to 79-year-olds in the USA found similar trends in HbA1c levels, detecting a greater proportion of black than white people. A HbA1c score of > 6.5% was observed in 5.7% of black and 1.8% of white participants, and a score of 5.7–6.5% was found in 37% of black people in comparison with 15% of white people; 3.5% of black people and 2.4% of white people had FPG levels in excess of 126 mg/dl, and 19% of black people and 22% of white people had FPG levels of between 100 and 125 mg/dl.
Ethnicity: South Asian
The term ‘South Asian’ is used to refer to people with ancestry in India, Pakistan, Nepal, Sri Lanka and Bangladesh, even if that ancestry is quite distant. For example, many South Asians in the UK moved here from Uganda but had ancestors in places such as Gujarat. In studies from the USA, ‘Asian’ often refers to people of Chinese or Korean ancestry.
The LEADER (Leicester Ethnic Atherosclerosis and Diabetes Risk) study81 found that using the HbA1c cut-off level of 6.5% rather than the 1999 WHO criteria resulted in a 1.4- and 2.2-fold increase in detection rates for white Europeans and South Asians, respectively, indicating that the HbA1c test may be relatively more sensitive in the South Asian population.
A small cross-sectional study111 in 139 subjects – 26% South Asian – found that South Asian origin was associated with higher HbA1c levels than in white subjects (6.1% vs 5.9%, p = 0.02), even although the South Asians were younger and weighed less. There were no significant differences in FPG and 2-hour PG levels between the two groups.
Similarly, in 3819 US participants with IGT,112 after correcting for confounders, ethnicity affected HbA1c levels, with means of 5.8% for white people, 6.0% for Asian people and 6.2% for black people.
In the INTERHEART (A Study Of Risk Factors For First Myocardial Infarction In 52 Countries And Over 27,000 Subjects) study,113 the increase in OR of having an MI for every 1% increase in HbA1c level differed significantly by self-identified ethnicity (p < 0.0001) and region (p < 0.0001). A 1% increase in HbA1c level corresponded with an OR increase for MI, which was higher in western Europe (OR 1.85, 95% CI 1.52 to 2.26) in comparison with China (OR 1.22, 95% CI 1.14 to 1.30), suggesting that the predictive value for MI is greater in western Europe. Note, however, that T2DM in China is less associated with overweight than in Europeans, having more of an insulin deficiency pattern than an insulin resistance one.
Similarly, a 1% increase in HbA1c level had differing effects on the odds of MI for different ethnicities, the greatest effect was in the ‘other Asian’ subjects (OR 1.80 95%, CI 1.54 to 2.09) alongside European (OR 1.50 95%, CI 1.37 to 1.66), South Asian (OR 1.47 95%, CI 1.34 to 1.62) and Arab subjects (OR 1.54, 95% CI 1.41 to 1.67) in comparison with Chinese (OR 1.22, 95% CI 1.14 to 1.30) and Latin American subjects (OR 1.28, 95% CI 1.17 to 1.41). The effect of a 1% increase in HbA1c level on the odds of MI in black subjects is unclear, with those classified as black African (OR 1.44, 95% CI 1.12 to1.84) being higher than for coloured African subjects (OR 0.86, 95% CI 0.61 to 1.22) but with very large overlapping CIs.
In the ADDITION study,114 South Asians with T2DM (FPG of ≥ 7.0 mmol/l or 2-hour PG of ≥ 11.1 mmol/l) were at higher estimated risk of CVD over 10 years using the ETHRISK score* in comparison with Western Europeans [risk score = 27.7% (95% CI 25.2 to 30.2); risk score = 18.3% (95% CI 16.5 to 20.1), respectively]. (*ETHRISK is a web-based calculator for assessing risk in ethnics groups in the UK. 115) A significantly higher ETHRISK score was also observed in those with IFG exclusively [FPG level of 6.1–6.9 mmol/l and (if measured) 2-hour PG of < 7.8 mmol/l or IGT exclusively (FPG of < 7.0 mmol/l and 2-hour PG of ≥ 7.8 and < 11.1 mmol/l). ETHRISK score assesses risk according to smoking, sex, age, SBP, HDL and total cholesterol levels, adjusted for ethnicity. South Asians were also more likely to test positive for IGT exclusively (OR 1.66, 95% CI 1.33 to 2.06) or T2DM (OR 2.18, 95% CI 1.56 to 3.06), but not IFG exclusively (OR 1.03, 95% CI 0.67 to 1.60), suggesting that the FPG test at this threshold may be a less sensitive test in the South Asian population with regard to predicting risk of CVD. Changing the criteria for detecting diabetes from FPG level of ≥ 7.0 mmol/l or 2-hour PG of ≥ 11.1 mmol/l to HbA1c level of ≥ 6.5% would double the prevalence of diabetes in this data set and the increase would be greater in South Asians than in white Europeans (2.1-fold vs 1.4-fold increase, respectively), suggesting that HbA1c testing may be more sensitive than the OGTT in the South Asian population. This result is similar to the LEADER study. 81
The reasons for the much higher prevalence of T2DM are complex and have been reviewed recently by Bhopal. 116 He hypothesises a four-stage model starting at birth, with small, relatively fat babies with low lean body mass and fewer beta cells, continuing through childhood and early adult life with energy intake excessive compared with low physical activity levels, followed by the development of insulin resistance and fatty livers, and ending in beta cell failure.
Others have noted the difference between BMI and risk in South Asians, with ‘overweight’ being defined as BMI of > 23 kg/m2 and obesity as BMI of > 25 kg/m2, and the need for lifestyle intervention across the entire life course. For a review, see Szczepura (2011). 117
Summary
In people of African or South Asian ancestry, HbA1c level tends to be a little higher, meaning that it is more sensitive when used in screening, at standard cut-offs.
How often should screening be done?
There is a shortage of evidence to answer this question. Takahashi et al. (2010)118 carried out annual OGTTs in Japan and found that the cumulative incidence of diabetes after 3 years by band of HbA1c was:
-
Baseline < 5.0% CI 0.05%
-
5.0–5.4% CI 0.05%
-
5.5–5.9% CI 1.2%
-
5.5–5.9% CI 1.2%
-
6.0–6.4% CI 20%.
This not only suggests that the screening interval should not be < 3 years for those with initial HbA1c level of < 6.0%, but also supports the case for using the cut-off level of 6.0% HbA1c.
Davies and Day (1994)119 suggested a repeat interval of 30 months, based on postprandial glycosuria screening.
Chapter 4 The ADDITION trial
In the previous HTA review of screening, it was noted that one of the NSC criteria that was not met was the need for a RCT of screening and intervention. This has now changed following the ADDITION trial. 120,121
It should be noted that ADDITION was not a trial of screening. The two arms were:
-
screening plus intensive intervention
-
screening plus standard care.
However, it addresses a key question: once diabetes has been diagnosed by screening, does intervention reduce cardiovascular morbidity and mortality? The Ely study14 of screening reported in Chapter 1 was not powered to answer this question.
Background and aims
The aims of the ADDITION study were to assess whether screening for undiagnosed T2DM is feasible, and to determine whether intensive treatment of people with screen-detected T2DM can reduce cardiovascular mortality and prevent or delay the macrovascular and microvascular complications of diabetes.
The trial was carried out in Denmark, the UK (Cambridge/East Anglia and Leicester) and the Netherlands. It was conducted in two phases: screening and intervention.
Design
Screening study
The first phase screened people for diabetes, using a variety of different approaches in different centres. (Note: the trial was not set up to compare the effectiveness of different approaches.)
The screening methods were reported in the paper by van Donk et al. (2011)122 Screening was conducted in four steps:
-
identification of those at high risk using risk scores
-
capillary random blood glucose (RBG) testing with or without HbA1c
-
capillary FBG testing
-
OGTT.
Not all study centres followed these four steps (Figure 2). In the Leicester centre, the screening moved directly to step 4 from step 1. One centre in the Netherlands and those using one of the two types of opportunistic strategies in Denmark skipped step 2.
FIGURE 2.
Screening stages used in the ADDITION study.
Treatment study
In the treatment phase, participating practices were randomly assigned in a 1 : 1 ratio to provide routine diabetes care according to local and national guidelines, or to provide an intensive intervention protocol, which included structured lifestyle advice (dietary modification – low fat, 600 g of fruit and vegetables/day; adherence to medication; increased physical activity – 30 minutes/day – and smoking cessation), prophylactic aspirin, with or without motivational interviewing. 121 Primary outcomes included mortality, macrovascular and microvascular disease. Secondary outcomes included increases in pharmacological interventions to control blood glucose, BP and lipids. The aim was to achieve a HbA1c level of < 7.0%, total cholesterol level of < 5.0 mmol/l (with a lower target of 4.5 mmol/l if CVD was present) and a BP of < 135/85 mmHg (in Leicester, < 130/80 mmHg). Aspirin in the dose of 75–150 mg/day was prescribed to everyone on antihypertensive treatment if there were no contraindications. There was a provision to intensify treatment targets during the study according to results of other clinical trials published during the study period. Initially, in order to achieve the target total cholesterol level, the recommendation was to prescribe statins if the total cholesterol levels reached > 5 mmol/l (> 4.5 mmol in those with CVD). However, after the publication of Heart Protection Study, the investigators changed this threshold to ≥ 3.5 mmol/l for all patients, i.e. with or without CVD. 123 The study also explored the impact of treatment on health status, treatment satisfaction and health service costs.
In the intervention group, 50% of the patients were allocated to country-specific interventions concerned with motivating adherence to lifestyle changes and medication. This intervention, including the use of motivational interviewing, was delivered either by a trained facilitator (UK and the Netherlands) or through training of practitioners (Denmark).
The treating clinicians decided which drugs to use to achieve the targets. The treatment was adjusted at 2–4 week intervals until targets were reached.
Outcomes
The primary end point was a composite of cardiovascular mortality, cardiovascular morbidity (non-fatal MI and non-fatal stroke), revascularisation and amputations.
Secondary end points included all-cause mortality, development of renal impairment, development of eye complications (progression of retinopathy, macular oedema, reduced visual acuity and blindness), diabetic foot ulcers, patient and health service costs, and perceived health (health status, quality of life, patient satisfaction and health utility).
Intermediate end points included changes in smoking status, diet, physical activity, medication adherence, HbA1c level, total cholesterol, low-density lipoprotein (LDL) cholesterol, HDL cholesterol, triglycerides, BP and cardiovascular risk score.
Results of screening study
The results of the screening phase of the ADDITION trial were reported by van den Donk et al. (2011). 122
Details of screening centres and uptake of screening programme
Cambridge, UK
Forty-nine general practices participated. The electronic medical records were searched using an automated computerised technique to identify patients at risk. A validated diabetes risk score was used to determine the risk status. Information on age, sex, prescription of steroid or antihypertensive medication, parent and/or sibling with diabetes, smoking status and BMI was used to calculate the risk of undiagnosed diabetes.
Patients with a diabetes score of ≥ 0.17, corresponding to the 25% of the population, were invited for capillary RBG and HbA1c testing at their general practice. Patients who did not attend the general practice were sent one reminder letter. The next step depended on their RBG levels. Patients with RBG levels of ≥ 11.1 mmol/l were invited directly for an OGTT. Patients with RBG levels of between 5.5 and 11.0 mmol/l were invited for capillary FBG testing. Patients with FBG levels of ≥ 6.1 mmol/l alone or FBG levels of 5.5–6.0 mmol/l and a HbA1c level of ≥ 6.1% were invited for an OGTT at a local outpatient clinic. Those patients with a FBG level of 5.5–6.0 mmol/l, a HbA1c level of ≥ 6.1% and a positive OGTT underwent a second confirmatory OGTT. No data comparing the performance of HbA1c compared with OGTT are included.
From a target population of 135,825 aged between 40 and 69 years, 35,297 patients were found to be at high risk of undiagnosed diabetes. A total of 33,539 patients were invited for further analysis, of whom 73.5% attended the general practice for measurement of RBG and HbA1c level. Approximately 94% and 82% of those invited for FBG and OGTT, respectively, attended the general practice.
Leicester, UK
Twenty general practices participated. As one of the aims of this centre was to develop a risk score for a multiethnic population, patients were not stratified using a risk score. The electronic medical records were searched to identify patients at high risk of diabetes. Out of 20 practices, six invited 20% of the eligible patients randomly, whereas the remaining practices invited all eligible patients. All patients were invited for an OGTT at a screening clinic. A target population of 47,806 aged between 40 and 69 years was identified. However, only about 20% of the total of 23,060 individuals invited for an OGTT attended.
Denmark
The screening study was carried out in general practices of five counties, namely Copenhagen, Aarhus, Ringkøbing, Ribe and South Jutland. The eligible patients were invited using postal invitation or via opportunistic screening strategies.
In the postal invitation, a letter and a modified Danish Diabetes Risk Score Questionnaire was sent to eligible patients from 148 practices. The questionnaire asked for information regarding age, sex, BMI, known hypertension, family history of T2DM, gestational diabetes and leisure time physical activity. Patients were also asked to calculate their own diabetes risk. Patients with a risk score of ≥ 5 points were asked to contact their GP and book an appointment for measurement of RBG and HbA1c levels. Those patients with a RBG level of ≥ 5.5 mmol/l or HbA1c level of ≥ 5.8% were further assessed for FBG level. If their FBG level was ≥ 6.1 mmol/l then they were considered to have met one diabetic value and, thus, invited for another FBG test and OGTT if necessary. Patients with FBG level of 5.5–6.0 mmol/l or HbA1c level of ≥ 5.8% were invited for an OGTT. Patients with OGTT value of ≥ 11.1 mmol/l were invited for another FBG test and OGTT if necessary.
In regions not using postal invitation to identify patients at risk, two opportunistic screening strategies were used. In the first, all four steps were followed, whereas in the second, patients skipped step 2, i.e. measurement of RBG and HbA1c levels. In the four-step programme, patients were invited for RBG testing on the same day, whereas in the three-step programme, patients were invited for FBG on another occasion. Sixteen general practices followed the four-step programme and 11 practices followed the three-step one. The protocol for entering into the next step is similar to that of the postal invitation method.
A total of 124,588 patients aged 40–69 years were invited via post to complete a risk questionnaire, out of which 22,645 attended the practice for a blood test. Of patients invited for an OGTT (6666), approximately 62% (4124) attended their general practice.
In the four-step screening programme, 4599 patients were invited to complete the risk questionnaire. A total of 2625 individuals were invited for their first blood test out of which 2496 (95.1%) attended the practice. Of those patients invited for an OGTT, approximately 73% attended.
In the three-step screening programme, 2546 individuals were invited to complete the risk questionnaire. Approximately 1400 individuals were found to be at risk and were invited for blood test, out of which 81% attended. Out of 300 individuals invited for an OGTT, 82% attended.
The Netherlands
The centres in the Netherlands used two types of screening programme: a four-step programme and a three-step screening programme. Centres in the Netherlands screened individuals aged 50–69 years, whereas the other participating countries screened those aged 40–69 years. A total of 41 practices used the four-step screening programme and 38 practices used the three-step screening programme. In both types of programme, eligible patients were sent a Hoorn Symptom Risk Questionnaire to identify those at high risk of T2DM. The questionnaire asked about: age; sex; BMI; antihypertensive medication; parent or sibling with diabetes; history of frequent thirst; pain during walking, with need to slow down; shortness of breath when walking with people of the same age; or any reluctance to use a bicycle for transportation.
In the four-step screening programme, a slightly modified questionnaire was used. Patients scoring ≥ 4 points were invited for further tests. Those with a RBG level of ≥ 5.5 mmol/l were further analysed in 1 week's time for FBG level. Patients with a RBG level of ≥ 11.1 mmol/l and a FBG level of ≥ 6.1 mmol/l were categorised as having T2DM. If patients had a FBG level of ≥ 6.1 mmol/l and a RBG level of < 11.1 mmol/l then they were invited for an OGTT using venous blood. Similarly, patients with FBG level of between 5.2 and 6.0 mmol/l were invited for an OGTT. If a 2-hour OGTT level was ≥ 11.1 mmol/l then patients were invited for a second OGTT. No data on correlation between the two OGTTs is provided.
In the three-step screening programme, patients scoring ≥ 6 points on the risk questionnaire were invited for measurement of FBG level. For those patients with a FBG level of ≥ 6.1 mmol/l, an OGTT was undertaken. Patients with at least one diabetic value during an OGTT were considered diabetic.
For the four-step programme, a target population of 29,251 aged 50–69 years was identified. All were invited to complete the risk questionnaire. The number of patients invited for blood testing was not known. Approximately 11,000 (38% of the target population) attended the screening clinic for RBG testing. Of the patients invited for FBG, 91% attended the clinic. Out of 1209 patients invited for an OGTT, 75% attended.
A target population of 27,727 aged between 50 and 69 years was identified for the three-step screening programme. All were invited to complete a risk questionnaire. Again the number of patients invited for blood testing was not known; however, as per the target population, approximately 25% attended the clinic for FBG testing. Of those invited for an OGTT, approximately 81% attended.
Prevalence of undiagnosed diabetes
See van den Donk et al. (2011). 122
Cambridge, UK
A total of 867 patients were diagnosed with T2DM, which was 0.64% of the target population. The overall median prevalence of known T2DM in the participating practices in Cambridge was 3.1%. Among those attending blood testing, approximately 3.5% patients were found to have diabetes. It was estimated that to detect a person with T2DM, 39 patients had to be invited for blood testing and the number of OGTTs required was 1.6.
Leicester, UK
The median prevalence of known diabetes in the participating practices in Leicester was 4.4%. However, only approximately 0.3% (n = 159) of the target population was diagnosed with T2DM. The authors suggest that the low prevalence of diabetes found in Leicester compared with Cambridge and other countries could be because of low participation. The inconvenient and lengthy nature of the OGTT may have led to a lower attendance rate.
Denmark
In Denmark, the prevalence of known diabetes in 2001 was 3.3%. Using the postal invitation screening method, 1179 patients, i.e. 0.9% of the target population, were diagnosed with T2DM. In the four-step opportunistic screening programme, 130 (1.06% of the target population) patients were found to have T2DM, whereas in the three-step programme, 66 (0.57% of the target population) patients were diagnosed with T2DM.
The Netherlands
The prevalence of known diabetes in the participating practices in the Netherlands was 3.1%. Using the four-step postal screening strategy, 285 patients were diagnosed with T2DM, which was 0.97% of the target population. A total of 301 (1.09% of the target population) patients were found to have T2DM using the three-step screening method.
The ADDITION-Leicester study
This study reported uptake of screening by racial group (white European vs South Asian) in the ADDITION-Leicester cohort. 114 The study also compared the cardiovascular risks among white European people and South Asian people, and then predicted the potential cardiovascular risk reduction using systematic multifactorial interventions.
In total, 20 local practices participated in the screening programme. Patients were selected randomly. An invitation to attend a single session of glucose and cardiovascular risk assessment was sent between 2005 and 2008 to this random sample of patients. The age of the invited participants ranged between 40 and 70 years in the white European population and between 25 and 75 years in South Asian population. The assessment included a 75-g OGTT, plasma lipid profile and standardised BP and anthropometric measurements. Participants also filled in a self-completed questionnaire that explored their medical history, smoking status and ethnicity. Deprivation was also measured using the Index of Multiple Deprivation.
According to the WHO criteria, participants were divided into three categories: diabetic, IFG and IGT. IGR refers to a composite outcome of IFG and/or IGT.
From 20 practices within Leicester, 66,320 patients were found to be eligible, out of which 30,950 patients (white European 18,113 and South Asian 12,837) were randomly invited. A total of 6749 (22%) [white European 4687 (26%) and South Asians 1684 (13%)] attended the screening programme. To make comparison between the two groups possible, participants aged under 40 years in the South Asian group were excluded (n = 331) and, thus, the revised number of patients included in the analysis from this group was 1353. The different ethnic groups within the South Asian population were Indians (94%), Pakistanis (2.2%), Bangladeshis (0.4%) and 46 from other unspecified ethnic groups.
The South Asian population was younger than the white European population [mean age: white Europeans 58.6 years (SD 9.5 years); South Asians 53.0 years (SD 8.7 years)]. More than half of the patients were female in both groups (male–female ratio: white Europeans 47 : 53; South Asians 49 : 52). South Asian patients were more deprived and had lower BMI, waist circumference, and total cholesterol, LDL cholesterol and HDL cholesterol levels compared with the white Europeans. South Asians were also less likely to smoke and were less physically active compared with the white Europeans. However, South Asian patients had higher HbA1c levels, medication use, 10-year modelled CVD risk and a greater frequency of previous CVD. There was no difference between the two groups in terms of body fat, BP and aspirin use.
Approximately 18% of patients had abnormal glucose tolerance tests (GTTs). Using the WHO criteria, 3.3%, 2.6%, 9.7% and 2.0% were categorised as T2DM, IFG, IGT, and both IFG and IGT, respectively. The South Asian patients had significantly higher prevalences of IGT (adjusted OR 1.66, 95% CI 1.33 to 2.06), IGT or IFG (adjusted OR 1.53, 95% CI 1.26 to 1.87), IGT and IFG (adjusted OR 1.78, 95% CI 1.12 to 2.81) and T2DM (adjusted OR 2.18, 95% CI 1.56 to 3.06). The odds of having any glucose disorder were 1.8 times higher in the South Asians compared with the white Europeans.
The 10-year CVD risk was raised in those with abnormal glucose levels. It ranged between 15.2% and 18.9% for white Europeans. For South Asians, the risk of CVD was higher in all categories and ranged from 23.5% to 27.7%.
Results of intervention study
Overall, 3233 individuals had screen-detected diabetes by the end of the screening phase of the ADDITION study,123,124 and 3057 individuals (male 1771, female 1286) were recruited to the second phase (the intervention study) of the study: 1533 in Denmark, 867 in Cambridge, 498 in Netherlands and 159 in Leicester. 123 The patients were followed up for a mean period of 5.3 years. 123
The baseline characteristics of the individuals participating in this phase of the study are given in Table 12. 124
| Baseline characteristics, mean (SD) | No. of participants with complete information | Overall | Male | Female |
|---|---|---|---|---|
| Age at diagnosis (years) | 3057 | 59.6 (6.8) | 59.2 (7.0) | 60.4 (6.6) |
| HbA1c (%) | 2888 | 7.0 (1.6) | 7.1 (1.7) | 6.9 (1.4) |
| SBP (mmHg) | 2961 | 151 (23) | 152 (22) | 151 (23) |
| DBP (mmHg) | 2962 | 87 (12) | 88 (12) | 86 (11) |
| BMI (kg/m2) | 2960 | 31.6 (5.6) | 31.0 (5.0) | 32.3 (6.2) |
| Total cholesterol (mmol/l) | 2892 | 5.6 (1.1) | 5.4 (1.1) | 5.7 (1.1) |
| Total triglycerides (mmol/l) | 2873 | 2.0 (1.5) | 2.1 (1.7) | 1.9 (1.1) |
| HDL cholesterol (mmol/l) | 2856 | 1.3 (0.4) | 1.2 (0.4) | 1.4 (0.4) |
| LDL cholesterol (mmol/l) | 2762 | 3.4 (1.0) | 3.3 (1.0) | 3.5 (1.0) |
The baseline characteristics differed significantly among centres. 124 In the Leicester centre, patients were younger than in other centres (mean age of 57.2 years vs 59.9 years in other centres, p < 0.001). This centre also had a larger proportion of non-white patients (41.3% vs 3.9% in other centres, p < 0.001) and approximately 8% of the patients were unemployed (2.2% in other centres, p < 0.001). The proportion of patients smoking cigarettes was highest in Denmark (35%), followed by the Netherlands (26%), Cambridge (18%) and Leicester (16%). Similarly, the number of patients drinking a large amount of alcohol per week was higher in Denmark than in other countries. However, they had the lowest mean HbA1c level compared with patients from other centres (6.8% vs 7.3%, p < 0.001). 124
Of the 73% patients who had BP of > 140/90 mmHg at baseline, 58% had not been prescribed any antihypertensive drug. The mean SBP and diastolic blood pressure (DBP) in these patients was 152 mmHg (SD 23 mmHg) and 88 mmHg (SD 12 mmHg), respectively. In those patients already on antihypertensive agents, the mean SBP was 151 mmHg (SD 22 mmHg) and the DBP was 86 mmHg (SD 12 mmHg). 124
Approximately 86% of patients were not on lipid-lowering drugs and in these patients the mean total cholesterol level was 5.6 mmol/l (SD 1.1 mmol/l). In those patients receiving lipid-lowering drugs, the mean total cholesterol level was 4.9 mmol/l (SD 1.0 mmol/l). In the whole ADDITION trial, approximately 64% of patients had a total cholesterol level of > 5 mmol/l. 124
Although the practices were randomised to two groups, some imbalances in allocation of participants in Denmark were seen. More patients were assigned to intensive treatment than to routine care (n = 910 vs n = 623). The group assigned to intensive therapy had by chance more IHD. The authors suggested that the imbalance between the two groups could have occurred because of awareness of the staff in the intervention arm about the potential benefits of early detection and treatment, in those at high risk of CVD, might have led to the staff testing only those at risk. The authors noted that, at diagnosis, patients overall showed high levels of untreated cardiovascular risk factors. 123
Data on the primary composite outcome were available for 3055 out of 3057 patients (99.9%) at 5 years. The primary end point included cardiovascular death, MI, stroke, revascularisation and amputation. The proportions of patients suffering from first cardiovascular events were similar – approximately 8.5% (n = 117) and 7.2% (n = 121) patients in the routine care and intensive treatment, respectively. Although there was a reduction in the occurrence of first cardiovascular event by 17% in the intensive treatment group (HR 0.83, 95% CI 0.65 to 1.05), the difference between the two groups was not significant (p = 0.12). Out of the 117 patients in the routine care arm in whom first cardiovascular events occurred, 32 had a MI, 19 had a stroke, 22 died and 44 patients had revascularisation. In the intensive treatment group, 26 died due to CVD, 29 had a non-fatal MI, 22 had a stroke and 44 had revascularisation. In the intensive treatment group, there was reduction of cardiovascular death, non-fatal MIs and revascularisation by 12% (HR 0.88, 95% CI 0.51 to 1.51), 30% (HR 0.70, 95% CI 0.41 to 1.21) and 21% (HR 0.79, 95% CI 0.53 to 1.18), respectively. However, there was no difference between the two groups for stroke (HR 0.98, 95% CI 0.57 to 1.71) and all-cause mortality (HR 0.91, 95% CI 0.69 to 1.21). None of the patients had amputation as first cardiovascular event. 123
There were 196 deaths in total: 92 in the routine care and 104 in the intensive treatment group. Sixty deaths were due to cardiovascular events, 97 due to cancer and 39 to other causes. 123
After 5 years, 84% of the 2859 patients still alive returned for follow-up assessment. 123 The clinical and biochemical findings of another 328 patients were collected from the general practices. No information could be found on remaining 131 patients. When the patients who had no information were compared with patients that had follow-up, the authors found that the patients with no information were more likely to come from an ethnic minority group, and their baseline, total and LDL cholesterols were high. 123
The changes seen during mean follow-up of 5.3 years are given in Table 13. 123
| Characteristics | Routine care | Intensive treatment | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change | Baseline | Follow-up | Change | |
| Current smoker | 375 (27.8%) | 187 (18.4%) | – 188 | 444 (26.9%) | 261 (20.2%) | – 183 |
| Units of alcohol per week: median (IQR) | 4 (1–13) | 3 (0–10) | – 1 | 4 (1–13) | 3 (0 to 10) | – 1 |
| BMI (kg/m2): mean (SD) | 31.6 (5.6) | 31.0 (5.6) | – 0.6 | 31.6 (5.6) | 31.1 (5.7) | – 0.5 |
| Weight (kg): mean (SD) | 90.3 (17.6) | 88.4 (17.8) | – 1.9 | 90.9 (17.5) | 89.1 (18.2) | – 1.8 |
| HbA1c (%): mean (SD) | 7.0 (1.5) | 6.7 (0.95) | – 0.3 | 7.0 (1.6) | 6.6 (0.95) | – 0.4 |
| SBP (mmHg): mean (SD) | 149.8 (21.3) | 138.1 (17.6) | – 11.7 | 148.5 (22.1) | 134.8 (16.8) | – 13.7 |
| DBP (mmHg): mean (SD) | 86.5 (11.3) | 80.7 (10.8) | – 5.8 | 86.1 (11.1) | 79.5 (10.7) | – 6.6 |
| Total cholesterol (mmol/l): mean (SD) | 5.6 (1.2) | 4.4 (0.9) | – 1.2 | 5.5 (1.1) | 4.2 (0.9) | – 1.3 |
| HDL cholesterol (mmol/l): mean (IQR) | 1.2 (1.0 to 1.5) | 1.3 (1.1 to 1.6) | 0.1 | 1.2 (1.0 to 1.5) | 1.2 (1.0 to 1.5) | 0 |
| LDL cholesterol (mmol/l): mean (SD) | 3.5 (1.0) | 2.3 (0.8) | – 1.2 | 3.4 (1.0) | 2.1 (0.8) | – 1.3 |
| Triglycerides (mmol/l): mean (IQR) | 1.7 (1.2 to 2.4) | 1.6 (1.1 to 2.3) | – 0.1 | 1.6 (1.2 to 2.3) | 1.5 (1.0 to 2.1) | – 0.1 |
| Use of any glucose-lowering drug | 7 (0.5%) | 681 (56.4%) | 55.9% | 8 (0.5%) | 990 (65.0%) | 64.5% |
| Metformin | 5 (0.4%) | 583 (48.3%) | 47.9% | 6 (0.4%) | 835 (54.8%) | 54.4% |
| Sulphonylurea | 2 (0.1%) | 215 (17.8%) | 17.7% | 2 (0.1%) | 291 (19.1%) | 19% |
| Thiazolidinedione | 0 | 50 (4.1%) | 4.1% | 0 | 69 (4.5%) | 4.5% |
| Insulin | 0 | 43 (3.6%) | 3.6% | 0 | 96 (6.3%) | 6.3% |
| Other | 0 | 31 (2.6%) | 2.6% | 0 | 81 (5.3%) | 5.3% |
| Any hypertensive drugs | ||||||
| ACE inhibitors | 248 (18.5%) | 721 (59.7%) | 41.2% | 345 (21.4%) | 1126 (73.9%) | 52.5% |
| Beta-blockers | 252 (18.8%) | 285 (23.6%) | 4.8% | 366 (22.7%) | 462 (30.3%) | 7.6% |
| Calcium channel blockers | 166 (12.4%) | 326 (27.0%) | 14.6% | 202 (12.6%) | 446 (29.3%) | 16.7% |
| Diuretic | 330 (24.6%) | 529 (43.8%) | 19.2% | 415 (25.8%) | 767 (50.3%) | 24.5% |
| Other | 23 (1.7%) | 49 (4.1%) | 2.4% | 32 (2.0%) | 70 (4.6%) | 2.6% |
| Any cholesterol-lowering drugs | 206 (15.4%) | 889 (73.6%) | 58.2% | 274 (17.0%) | 1241 (81.4%) | 64.4% |
| Statins | 200 (14.9%) | 864 (71.5%) | 56.6% | 271 (16.8%) | 1217 (79.9%) | 63.1% |
| Aspirin | 169 (12.6%) | 504 (41.7%) | 29.1% | 249 (15.5%) | 1078 (70.7%) | 55.2% |
There were similar changes in SBP, DBP, and in total and LDL cholesterol levels. Use of glucose-lowering drugs increased in both groups, but more in the intensive treatment group. More patients in the intensive treatment group received aspirin, angiotensin-converting enzyme (ACE) inhibitors and lipid-lowering drugs. The proportion of patients smoking decreased by the end of the study. 123
The proportion of patients achieving the target HbA1c level of < 7%, BP of < 135/85 mmHg, and cholesterol of < 5 mmol/l (if no CVD) or < 4.5 mmol/l (if CVD) was higher in the intensive treatment than in the routine care group (Table 14: read from the published figure no. 4). 123
| HbA1c < 7% | Cholesterol < 5 mmol/l (no CVD) or < 4.5 mmol/l (CVD) | BP < 135/85 mmHg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Routine care | Intensive treatment | Routine care | Intensive treatment | Routine care | Intensive treatment | ||||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up |
| 65% | 71% | 67% | 77% | 28% | 87% | 31% | 84% | 20% | 38% | 24% | 45% |
The proportions of patients reporting hypoglycaemia as assessed by the diabetes treatment satisfaction questionnaire were similar between the groups, although the proportions are not given (χ2 = 4.44, p = 0.62). 123
The 10-year results were disappointing. 102 After a median follow-up of 9.6 years [interquartile range (IQR) 8.9 to 9.9 years; 184,057 person-years], there was no significant difference in all-cause mortality between the two groups (HR 1.06, 95% CI 0.90 to 1.25; p = 0.46). Cancer was the commonest cause of death. Intervention caused no significant reduction in cardiovascular mortality (HR 1.02, 95% CI 0.75 to 1.38) or diabetes-related mortality (HR 1.26, 95% CI 0.75 to 2.10). It should be noted that in the 10-year results, only 466 patients from 33 practices were included and, of these, five practices were randomised to no screening.
Barakat et al. (2013)125 reported that recruits to ADDITION in the Cambridge centre, made little change in their physical activity. This paper was based on 867 participants from 49 general practices. It is not clear whether or not the results are from both intervention and control arms, but the numbers suggest both. The advice given was to undertake 35 minutes of brisk walking a day. There was some variation among participants, but even those who reported increased recreational activity had little change in HbA1c: reductions of 0.093% in men and 0.022% in women. The reduction in men was statistically significant (p = 0.02) but of doubtful clinical significance. So even volunteers in a trial, who were told that they have newly diagnosed T2DM, made little change in physical activity.
Summary and discussion
The findings from the ADDITION study suggest that:
-
Screening for T2DM and early intensive multifactorial treatment of the screen-detected patients are feasible.
-
Uptake of screening was relatively low in the South Asian population.
-
The identified patients are at high risk of developing CVD.
-
The uptake of screening varied according to which test was used.
-
Informing patients that they were at higher risk of diabetes seemed to improve uptake of blood glucose testing.
-
Most of the risk factors identified were modifiable.
-
At mean follow-up of 5.3 years, there was no difference in the incidence of cardiovascular events and all-cause mortality between the routine care arm and the intensive treatment arm.
-
Patients in both arms lost weight and there was no difference in BMI. The reductions in BMI were small.
-
There were reductions in SBP, DBP, and total and LDL cholesterol levels in both groups but the difference between the two groups was not significant.
-
The finding confirms that implementing interventions aimed at reducing HbA1c level to 6.5% does not increase mortality.
-
If the difference had been statistically significant, to prevent one cardiovascular event, 1824 people at high risk would need to be screened and 76 treated for 5.3 years.
The uptake was considerably lower in the South Asian population in the Leicester centre. Although the investigators used culturally sensitive recruitment methods, the uptake of screening programme was low. 114
The attendance rate appeared to be affected by the blood testing method used. The OGTT, which involves overnight fasting and 2–3 hours' glucose challenge, is inconvenient to patients, and was associated with a lower attendance rate. The attendance rate in Leicester where patients were directly invited for an OGTT was low (20%) compared with those invited directly for RBG testing after risk assessment (95%). The authors believed other screening methods, such as FBG or HbA1c testing, might have increased the number of participants. The attendance for an OGTT was also found to be lower in other centres: 82% in Cambridge (following 94% for FBG), 62–82% in Denmark (following 81–95% for RBG) and 75–81% in Netherlands. The investigators thought that a convenient first blood test might increase participation.
The unexpectedly low prevalence of undiagnosed diabetes in a high-risk South Asian population was attributed by the authors to the low attendance rate.
It was also found that the attendance was lower if the patient had to attend for blood testing on another occasion. For example, the attendance rate was higher in those invited for RBG testing immediately after appointment with their GP (opportunistic screening) than in those invited for FPG testing at another time (95% vs 81%).
Prestratification of population as high or low risk, and then inviting the high-risk population for blood testing, was found to increase attendance rate. In Denmark, where patients were stratified as high risk and then invited for blood testing, the attendance rate was high (95%). The attendance of patients in ADDITION-Leicester study, where pre-stratification was not carried out, was low. The authors suggested that this group of patients might be less motivated to attend for blood testing as they were unaware of their risk status. Sending out risk questionnaires, instead of a simple mail invitation to participate, increased attendance rate. In Denmark where mail invitation was used, approximately 17% attended for blood testing whereas in Netherlands where a risk questionnaire was sent out, the attendance rate in the four-step and three-step screening programme was 38% and 25%, respectively. The attendance rate for confirmatory tests was high (e.g. in Cambridge 75% attended for RBG and HbA1C, whereas 94% attended for subsequent FBG testing). The authors believed that this could be due to those at risk overcoming psychological barriers to screening. The ADDITION investigators conducted a prospective qualitative interview study of the experiences and perceptions of patients at different stages of the screening programme. 126 Twenty-three patients from seven general practices in Cambridge participated in this study. It was found that the patients underwent psychological adjustments as the stepwise screening programme progressed. As these tests were not carried out on a single day, the patients found to be positive on their first test, had time to obtain information on diabetes before they came back for a confirmatory test. And during this time, most of them were found to have accepted that they could be diagnosed with T2DM. However, in Denmark, the attendance rate for subsequent testing was not high (four-step screening 95% for first RBG testing but 73% for an OGTT). The authors suggested that the patients may not have been keen to re-attend for a second test, following an easier first blood testing, which was carried out immediately following their GP appointment. 114 The authors then highlight the importance of attention to follow-through and also to first attendance in optimising the screening programme. 114
After screening, a considerable number of patients were found at baseline to have high BP, high glucose level and high cholesterol level. Some of them were on medication, whereas others were yet to be treated. Some risk factors identified were modifiable.
There was no significant difference between the two groups in terms of the primary end point. The authors argued that this could be because of improvements in the routine care of diabetes in all the participating countries during the study period. The authors noted that, as the study progressed, routine care became very close to the intensive treatment arm.
There were some notable improvements from baseline to end of the study in cardiovascular risk factors, such as BP and cholesterol levels, and these changes were noted also in the routine care group, who received no additional support. Patients in neither group gained weight. HbA1c level did not deteriorate over time, suggesting that early detection and implementation of intervention in screen-detected diabetic patients had some benefit.
The finding that intensive treatment targeting tight HbA1c level control of < 7% did not increase mortality contradicted the findings of the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial. 127 In the ADDITION trial, the mortality rates between groups receiving routine care and those receiving intensive treatment were similar.
Conclusions
The ADDITION trial was the first RCT of screening and intensive intervention to reduce cardiovascular risk. The main findings were that screening and intervention are feasible, but that intervention did not improve cardiovascular outcomes compared with routine care. This may have been due to unintended improvements in routine care over the course of the trial.
Use of the OGTT as the screening test was associated with a lower uptake.
Chapter 5 Prevention of type 2 diabetes in people with impaired glucose tolerance
As noted earlier, if screening for T2DM is implemented, many people with lesser degrees of impaired glucose metabolism will be found. Consideration needs to be given to what care they would receive.
This chapter summarises the published HTA monograph19 on the clinical effectiveness and cost-effectiveness of non-pharmacological prevention of T2DM in people with IGT, commissioned by the Health Technology Assessment programme on behalf of the Department of Health for England. Only brief details are given here.
Clinical effectiveness
The review of clinical effectiveness was based primarily on RCTs. Nine RCTs comparing lifestyle interventions (predominantly diet and physical activity advice, with regular reinforcement and frequent follow-up) with standard lifestyle advice or placebo were identified. They included 5875 people randomised to receive lifestyle advice, exercise programmes or combinations thereof. The trials varied in design and quality. The primary outcome for the trials was progression to T2DM.
Participants had IGT. In most of the trials, lifestyle interventions reduced progression to diabetes (RR range 0.33 to 0.96).
The Diabetes Prevention Program (DPP) from North America (which had higher risk recruits than most other trials) reported that the prevalence of diabetes at 3 years was 29% in the control group compared with 14% in the lifestyle intervention arm. 128
The DPS15 had the longest follow-up, to 7 years, which included the 4 years of intervention and then 3 years of postintervention follow-up. After 4 years, 4% of the lifestyle group and 7.4% of the control group had developed diabetes, roughly a halving of risk. At 7 years, the difference had diminished slightly but the intervention group retained most of the benefit, suggesting that 4 years of the lifestyle intervention had resulted in a sustained change in lifestyle habits. 16
The benefits of the lifestyle intervention were greatest in those with the highest compliance and who achieved more of the targets (such as weight loss and dietary change). For example, in the Finnish study, those who achieved four or five of the five targets had a risk of developing diabetes which was only 23% of those who achieved none. However, even among the volunteers in the trials, many did not succeed, and others succeeded in the short term (such as the first 6 months) but not in the longer term. The key to success is sustained lifestyle change, especially weight loss.
The DPS involved quite intensive lifestyle intervention. A subsequent study from Finland used low-intensity intervention (six sessions of counselling by public health nurses) over an 8-week period, and found that the effects persisted for 3 years. 129 However, the effects were much less than in DPS: 0.8 kg weight loss compared with 4.5 kg in the DPS. The most successful prevention studies had more intensive interventions with more frequent contacts. 130
Perry et al. (2005)131 from Cork in Ireland identified factors from previous studies, which protected against diabetes: BMI < 25 kg/m2; waist–hip ratio of < 0.85 for women and 0.90 for men; never smoking; medium- to high-level physical activity; light drinking (three to five, to seven units per week); and a prudent diet. In their sample of middle-aged Irish men and women drawn from general practice populations, 7.5% had none of these protective factors. Insulin resistance was calculated using the homeostasis model analysis (HOMA) score (based on fasting levels of both insulin and glucose). Taking the 7.5% with no protective factors as the reference group, multivariate analysis gave ORs for insulin resistance of 0.59 with one protective factor, 0.48 with two, 0.14 with three, and 0.04 with four or more. About 13% had four or more protective factors.
Hence, there is little doubt that lifestyle measures could prevent most cases of T2DM. Weight loss would also benefit those who already have diabetes, or hypertension, and improvements can follow even modest weight loss. Goldstein reviewed studies in which large and small amounts of weight were lost, and concluded that even modest weight reductions of 10% or less, resulted in significant benefit in a substantial subset. 132 Even loss of a few kilograms can provide benefit.
One of the key factors in cost-effectiveness analysis is adherence to lifestyle changes. Even among the volunteers in the trials, a large proportion did not adhere. It was also noticeable that in many trials, initial gains were lost after the intervention ceased.
A more radical option, bariatric surgery for obesity, reduces progression from IGT to diabetes (and also reverses diabetes in many cases).
Cost-effectiveness
The modelling in the HTA review examined the cost-effectiveness of preventing or delaying T2DM in people with IGT, including the effects of interventions on CVD.
Modelling based on data from the trials may not reflect what would happen in routine care. Trials are protocol driven and patients are supposed to stay on the treatments to which they are randomised. In normal care, if an intervention is not working, it should be stopped. The modelling assumed that people with IGT would initially be treated with a structured lifestyle intervention similar to that in the Finnish trial, but that those who did not comply would be switched to metformin after 12 months. Metformin is now a very cheap drug, and reduces the risk of progression to diabetes, although not by as much as adherence to lifestyle measures does. Applying an early switch to metformin in the non-adherers means that the adherers remaining on diet and physical activity will do better than seen in the lifestyle arms of the trials. It was assumed that the non-adherers to lifestyle modifications will have better adherence to metformin, so that they will also do better than if left on the lifestyle interventions.
Using the switching assumption, intervention is highly cost-effective and, in certain scenarios, cost-saving.
The main conclusion of the review was that in people with IGT, lifestyle change (diet and physical activity) is clinically effective and cost-effective in reducing progression to diabetes.
Chapter 6 Review of cost-effectiveness studies
This chapter provides an update of a previous review10 that examined the cost-effectiveness of screening for T2DM among the UK population, and another (summarised in Chapter 5) that reviewed the cost-effectiveness of intervention to reduce progression to diabetes in people with IGT, published in a second HTA monograph. 19 New modelling was not possible within the constraints of this short report.
The key questions are:
-
Assuming that screening might start at the age of 40 years, should it thereafter be universal or selective?
-
If screening was selective, how should risk be assessed and how much do risk scores improve detection over a simple age and BMI approach?
-
Should the approach to screening vary with ethnicity? Should people belonging to South Asian ethnicity (India, Pakistan, Nepal, Bangladesh and Sri Lanka) be screened at younger ages or at lower BMIs?
-
Which test of blood glucose should be used?
-
Should screening be just for diabetes or for IGT as well?
Study selection
The databases searched were MEDLINE, EMBASE, The NHS Economic Evolution Database (NHS EED), HTA, Cost-effectiveness Analysis (CEA) Registry, Web of Science, EASD and ADA for studies published since 2008. Titles and abstracts were reviewed and if considered potentially relevant; full papers were reviewed. The sifting was carried out independently by two reviewers. Any discrepancies were resolved by discussion. The flow chart (Figure 3) illustrates the search results.
Inclusion criteria Studies were included if they assessed the costs and/or the outcomes (long or short term) of screening strategies for either undiagnosed T2DM or IGT/IFG.
FIGURE 3.
Flow chart of economic search and filtering.
Exclusion criteria – studies were excluded if they:
-
did not assess the costs and or the outcomes of screening strategies for either undiagnosed diabetes or IGT/IFG
-
reported in languages other than English
-
were assessing screening tests for complications of diabetes
-
were assessing screening tests for gestational diabetes
-
were targeting an incorrect age group of the population (either teenagers or very old individuals)
-
were based on data recorded in non-westernised countries.
The costs [US dollars (US$), Australian dollars (AU$) and euros (€)] in the texts have been converted into UK pounds sterling (£) on the basis of purchasing power parity. 133
Screening for diabetes could produce benefit in two ways. First, identification of those with pre-diabetes and lifestyle intervention, could prevent or delay the onset of diabetes. Second, intervention in those found to have diabetes could reduce the morbidity and the costs owing to the complications, primarily cardiovascular, associated with diabetes.
It is known that diabetes reduces average survival, possibly by as much as 15 years. 134 However, the excess mortality among people with diabetes has been falling over recent decades135 and may be less in those diagnosed more recently.
Most previous studies have examined screening for diabetes alone, but incorporating it into a broader vascular risk assessment programme, as being introduced in England, would reduce costs. The issue then becomes whether adding a test of blood glucose to all or selected individuals is cost-effective.
Universal screening compared with selective screening
Age
We know that diabetes prevalence rises with age. 43
Kahn et al. (2010)47 examined a range of screening strategies from a US perspective, which differed in the age at which screening was started and the frequency of repeat screening. They concluded that the most cost-effective screening strategy would be to initiate screening at between 30 and 45 years, with screening follow-ups occurring every 3–5 years. The costs per QALY for these strategies were around £7245 (US$10,500) starting at the age of 30 years and £6714 (US$9731) starting at the age of 45 years. Almost all of the screening strategies were assumed to reduce MI and other diabetes-related macrovascular complications by 3–9 events per 1000 screened individuals. There was a non-significant reduction in stroke occurrence of 0–1 events per 1000 screened. The decrease in the occurrence of diabetes and associated events was assumed to reduce total health-care costs. It should be noted that Kahn et al. (2010)47 assumed that the diagnosis of diabetes would lead to checking for hypertension at each clinic visit and annual screening for hyperlipidaemia (especially LDL). So, as with the ADDITION study, screening identifies people at increased cardiovascular risk and benefits accrue not only from glycaemic control. Costs per QALY were much lower in those with hypertension. Both the costs and the QALYs have been discounted. The data for disutilities are extracted from Sullivan et al. (2006). 136 A time horizon of 50 years was selected for modelling purposes.
Kahn et al. (2010)47 used the Archimedes model that was used by Eddy et al. (2004)137 in the only economic study in our previous review of screening and prevention that did not considered that combination to be cost-effective. The difference between Eddy et al. (2004)137 and Kahn is that Kahn et al. (2010)47 showed that detection and treatment of diabetes is cost-effective, whereas Eddy et al. (2004)137 were modelling detection of IGT and interventions to prevent T2DM.
Although costs were expressed in US$, and reflect a different form of health care, it is the relative merits of the various options that matter, not the absolute costs.
Feenstra et al. (2011)138 compared interventions in two groups, the general population and those with diabetes, with three age groups of 20–44, 45–64 and ≥ 65 years. Of the 17 lowest-cost-per-QALY scenarios (all < €10,000 per QALY), 11 were for interventions in the diabetes group because they were higher risk with more to gain. The lowest costs per QALY were seen in the youngest age groups and ranged from £1050 to £14,925 (€1400–19900). This arises because of an assumption that intervention at a young age allows the biggest effect of prevention. It also seems to be assumed that interventions at younger ages are inexpensive but effective. Another assumption is that quality of life declines with age. Details of most underlying assumptions are not provided. The costs may not be applicable to the UK but the relativities are.
Colagiuri et al. (2008)139 modelled screening at different ages in a complex model with 15 diabetes states, ranging from no diabetes and low risk of future diabetes, through no diabetes but high risk, to diabetes and early complications, and, finally, to diabetes with late complications and then death. Screening was carried out in three stages: risk scoring; FPG in high risk; and OGTT for borderline FPGs. However, their age comparison was confounded in that they compared all people aged 55–74 years with obese people aged 45–54 years. Nevertheless, the cost per disability-adjusted life-year (DALY) avoided was somewhat lower in the 55- to 74-year age range, implying that had they included all people aged 45–54 years then the difference would have been more marked. The difference was partly because the prevalence of undiagnosed diabetes was lower in obese 45- to 54-year-olds (5.4%) than in all 55- to 74-year-olds (7.3%). The costs per DALY avoided were quite high, AU$54,000 (about £24,000) in 45- to 54-year-olds and AU$48,000 (about £22,000) in 55- to 74-year-olds.
Their modelling involves reductions in the incidence of MI, eye and kidney disease, and death.
Chamnan et al. (2012)140 published a thorough modelling study that examined a range of screening strategies, including comparing the age ranges of 40–74 years and 50–74 years. The marginal benefit of starting at age 40 years was that all incident cases in the age range over a 3-year period would be detected, compared with only 88% when starting at the age of 50 years. Their results were based on the EPIC-Norfolk study141 of 5788 participants who were free of diabetes at baseline, of whom 77 developed diabetes in the 3-year follow-up period. So the 88% capture represents nine fewer people diagnosed, but also represents a reduction in those screened by 1473, 25%. In terms of number needed to screen (NNS), screening from the age of 40 years required 77 to be screened per case detected, and screening from the age of 50 years had a NNS of 65 years. (Our calculations, based on data from Chamnan study, table 4. 140) However, in terms of marginal gain, the NNS to detect each of the nine extra cases was 164.
They also showed that adding overweight as a selection factor reduced the NNS by about one-third but reduced the number diagnosed by only 14%. They used a BMI of 25 kg/m2 to define overweight.
Chamnan et al. (2012)140 also examined selection by risk factors. 140 They estimated that screening from the age of 50 years only in those who were overweight (BMI ≥ 25 kg/m2) would identify 57 of the 77 who became diabetic, but at a cost of screening only 2977 people, giving a NNS of 52. Using a Cambridge risk score of ≥ 0.15 or a FINDRISC score of ≥ 9 identified an extra seven cases, with a NNS of 48 and 50, respectively. Chamnan et al. (2012)140 in these scenarios used HbA1c levels of 6.0–6.4% to identify those at high risk, who were then followed for 3 years.
Hence, selection makes screening more cost-effective in terms of cost per case detected but will inevitably miss some cases. Chamnan et al. (2102)140 reported that the proportions of the non-diabetic population with baseline HbA1c levels in the range of 6.0–6.4% and 5.5–5.9% were 6.7% and 24.2%, respectively, making it much more cost-effective to apply preventative interventions in the former group. However, only 38% of those who developed diabetes were in the 6.0–6.4% HbA1c range – the risk in the 5.5–5.9% range is much lower but, given the distribution curve, their numbers are much higher.
Chamnan et al. (2012)140 did not carry out an economic evaluation, such as producing costs per QALY of the marginal costs and benefits of extending intervention to the 5.5–5.9% range of HbA1c.
The IDF consensus approved both universal and target screening for diabetes prevention. 43
The NSC in UK has produced guidance on screening linked to the vascular risk assessment programme. 142 The guidelines recommend selective or opportunistic screening of individuals in the high-risk category. 114
Screening for diabetes or for impaired glucose tolerance as well
Gillies et al. (2008)143 compared three different screening interventions: (1) screening for diabetes; (2) screening for diabetes and IGT followed by lifestyle interventions; and (3) screening for diabetes and IGT followed by pharmacological interventions against no screening.
The base-case scenario was one-off screening was at the age of 45 years, in people with above-average risk of diabetes. Risk was based on known history of IHD, hypertension, dyslipidaemia, family history of T2DM and a BMI of > 25 kg/m2. They based their effectiveness estimates for the prevention of diabetes on a previous review of pharmacological and non-pharmacological interventions of their own. They assumed that lifestyle intervention would cost about £400 in the first year, and £280 per year thereafter. These costs were derived from a review of interventions in obesity rather than from the diabetes trials.
The results confirmed that all interventions are cost-effective compared with a no-screening strategy. The estimated costs per QALY were (1) £14,150; (2) £6242; and (3) £7023 for the aforementioned strategies. So screening for, and intervention in, both diabetes and IGT was more cost-effective than screening for diabetes alone.
A fuller account of this excellent study is included in Gillett et al. (2012). 19
In Germany, Schaufler et al. (2010)144 also concluded that screening for both diabetes and IGT was worthwhile. They examined the cost-effectiveness of two screening and intervention strategies, for T2DM and pre-diabetes, and with lifestyle and metformin interventions. The target population covered individuals aged 35–75 years. Screening was yearly with the OGTT.
A Markov Monte Carlo/TreeAge Pro Suite 2007 (TreeAge Software, Inc., Williamstown, MA, USA) microsimulation model was used. Sensitivity analysis was also carried out to assess the robustness of the results. The outcomes reported included quality of life, lifetime costs, incidence and age at occurrence of diabetes-related complications and age at diabetes diagnosis. National databases were used for retrieving the population data. A lifelong horizon period was used for the modelling purpose. Other information pertaining to modelling was collected from relevant articles and previous studies. This evaluation was carried out from the perspective of German Statutory Health Insurance's (SHI) perspectives. Sensitivity analysis was also carried out for validating the different scenarios, keeping in mind the private health-care infrastructure of the German settings.
Screening for diabetes allowed diagnosis 3.8 years earlier and a gain of 0.8 life-years. Intervention proved cost-effective and reduced the average cost of illness by £605 (€807) per detected T2DM case in the screening programme when compared with no screening at all. Intervening in pre-diabetic individuals gave average savings per detected case of £4861 (€6481). Costs per QALY showed that screening was cost-effective for T2DM (€563 per QALY with lifestyle) and could even be cost-saving in pre-diabetes.
Webb et al. (2011)114 suggested that there is a CVD risk reduction of 6% and 13% in diabetic and IGR screen-detected patients, respectively.
Should the approach to screening vary with ethnicity?
The question here is whether people belonging to South Asian ethnicity (India, Pakistan, Nepal, Bangladesh and Sri Lanka) be screened at younger ages or at lower BMIs.
The European guideline by Paulweber et al. (2010)43 suggests that prevalence rates vary two- to fivefold among people from different ethnicities. This difference is explained by the different lifestyle or disposition to a particular disease owing to genetic reasons.
The STAR (Screening Those At Risk) study145 reports that the rate of progression from IGT to diabetes is higher for South Asians living in the UK. 114 It suggests that screening for diabetes in South Asian people in the UK should use different threshold levels. Partly due to their sedentary lifestyle, many people from South Asian backgrounds have high BMI at a very young age. And, as reported earlier, at a given HbA1c, South Asians had higher BP and triglyceride levels, and higher incidence of CVD.
Davis et al. (2008)146 report that the Afro-Caribbean population also has high prevalence of T2DM.
There is, therefore, a case for starting screening from an earlier age in some ethnic groups.
Which test of blood glucose should be used?
Icks et al. (2004)147 compared the effectiveness for the combinations of either of HbA1c testing, fasting glucose testing or the OGTT. The most effective was the combination of the HbA1c testing followed by the OGTT, with a very high detection rate (54%) and cost per detected case, ranging from £16 to £26 (€21–34.5).
Chatterjee et al. (2010)96 used data from the SIGT study, in which 1259 adults without known diabetes had all of random PG, 50-g GCT, HbA1c testing and the OGTT. They modelled costs of screening and subsequent treatment of diabetes and IGT, and the costs of false-negatives and true-positives. Their focus was on screening for both pre-diabetes and undiagnosed diabetes; results are not given separately, and are for only a 3-year period. They provide costs but not cost-effectiveness data. Taking all costs into account, they conclude that the 50-g GCT provides the best strategy in terms of total health system costs.
Quality assessment
Table 15 shows the description of the various cost-effective studies that were included. The assessment of the selected studies was carried out using the British Medical Journal checklist modified form of the Drummond and Jefferson (1996)148 guidelines that are used for the evaluation of the economic studies. The studies have been assessed on three main aspects, namely study design, data collection (Table 16), and analysis and interpretation of results (Table 17).
| Study | Research question | Viewpoint of analysis mentioned | Form of economic evaluation is stated | Justification for choice of economic evaluation is mentioned |
|---|---|---|---|---|
| Gillies et al. (2008)143 | Yes. Comparison of four potential screening strategies and subsequent interventions: (a) screening for T2DM only, (b) screening for T2DM and IGT in those with a diagnosis of IGT, (c) as for (b) but with pharmacological intervention, and (d) no screening | NHS | Cost-effectiveness | Not clear |
| Schaufler et al. (2010)144 | Yes. To examine the cost-effectiveness of screening for T2DM from the perspective of German SHI | German SHI | Cost-effectiveness | Not clear |
| Bertram et al. (2010)149 | Yes. To evaluate the cost-effectiveness of a screening programme for pre-diabetes, followed by treatment with a) pharmaceutical intervention or b) lifestyle intervention in high-risk patients | Health care | Cost-effectiveness | Not clear |
| Hoerger et al. (2007)150 | Yes. To estimate the cost-effectiveness of screening overweight and obese individuals for pre-diabetes | Health System | Cost-effectiveness | Not clear |
| Kahn et al. 201047 | Yes. To estimate the cost-effectiveness of several screening strategies | Health service/delivery system | Cost-effectiveness | Not clear |
| Colagiuri et al. (2008)139 | Yes. To evaluate prevention and care strategies by using cost benefit model | Not clear | Cost-benefit | Yes |
| Glumer et al. (2006)42 | Yes. What determines the cost-effectiveness of diabetes screening? | Public health care | Cost-effectiveness | Not clear |
| Feenstra et al. (2011)138 | Yes. What is a better strategy: target the general population or diabetes patients? | Dutch health care | Cost-effectiveness | Yes |
| Chamnan et al. (2012)140 | Yes. To estimate the potential population impact of different stepwise screening strategies to identify individuals at high risk who might be offered preventative interventions | Department of Health | Not applicable | Not applicable |
| Study | Source of effectiveness estimates mentioned | Primary outcome measure for economic evaluation | Design and result of effectiveness study | Quantities of resource use and their costs | Currency and price data recorded | Details of currency inflation and price adjustment | Details of the model used given | Justification of choice of model used and key parameters |
|---|---|---|---|---|---|---|---|---|
| Gillies et al. (2008)143 | Yes | Yes; health-care costs and QALYs | Yes | Yes | Yes | Yes | Yes; hybrid decision tree and Markov | Yes |
| Schaufler et al. (2010)144 | Yes | Yes; cost/QALY | Yes | Yes | Yes | Yes | Yes; Markov microsimulation model | Yes |
| Bertram et al. (2010)149 | Yes | Yes; costs and DALYs | Yes | Yes | Yes | Yes | Yes; microsimulation model | Yes |
| Hoerger et al. (2007)150 | Not clear | Yes; cost/QALY | Yes | Yes | Yes | Yes | Yes; Markov simulation model | Not clear |
| Kahn et al. (2010)47 | Yes | Yes; cost/QALY | Yes | Yes | Yes | Yes | Yes; Archimedes model for simulation | Yes |
| Colagiuri et al. (2008)139 | Yes | Yes; costs/DALY | Yes | No | Yes | Not clear | Yes; microsimulation model | Yes |
| Glumer et al. (2006)42 | Yes | Yes; ICER | Yes | Not clear | Not clear | Not clear | Yes; UKPDS model | Not clear |
| Feenstra et al. (2011)138 | Yes | Yes; cost/QALY | Yes | No | Yes | Yes | Yes; simulation | Yes |
| Chamnan et al. (2012)140 | Yes | No | Yes | No | No | No | Unclear | No |
| Study | Time horizon of costs and benefits stated | Discount rate stated | Statistical tests and CIs for stochastic data | Approach to sensitivity analysis | Incremental analysis reported | Answer to study question reported | Conclusions follow from the data reported |
|---|---|---|---|---|---|---|---|
| Gillies et al. (2008)143 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Schaufler et al. (2010)144 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Bertram et al. (2010)149 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hoerger et al. (2007)150 | Yes | Yes | Not clear | Yes | Yes | Yes | Yes |
| Kahn et al. (2010)47 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Colagiuri et al. (2008)139 | Yes | Yes | Not clear | Yes | Yes | Yes | Yes |
| Glumer et al. (2004)42 | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Feenstra et al. (2011)138 | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Chamnan et al. (2012)140 | No | No | No | No | No | Yes | Yes |
All studies met most of the quality criteria. Most studies fulfilled the study design criteria. All the studies are well structured and address clear questions. The research question here relates to the feasibility of screening for T2DM. The studies differed from each other in terms of the screening interventions compared, lifetime horizon, evaluation perspective, country settings, target population and incremental cost-effectiveness ratios (ICERs). Only two studies138,139 had clear justification for the economic approach used.
Most of the studies followed systematic method of data collection, each study providing the source of effectiveness estimates and different primary outcome measure for the economic evaluations. However, these studies also differed in the outcomes used for quality evaluation. Some studies143,144 used QALY's as their primary outcomes, others47 used count of event incidents as primary outcomes. Two Australian studies139,149 used cost-per-DALY as outcome for comparison. All the studies have given adequate detail of design.
Costs have been discounted for all. Sensitivity analysis is carried out in all the studies by varying parameters for the validation of robustness of the economic model.
The study population remains a concern for few studies. The study of Hoerger et al. (2007)150 concerning cost-effectiveness of selective screening only looked at individuals with hypertension. Furthermore, the costs associated with selecting the hypertensive individuals were not accounted for. Similarly, the Gillies et al. (2008)143 analysis included only individuals with IGT.
A moderate participation rate was assumed in the Schaufler and Wolff study,144 although various participation rates were tested in the sensitivity analysis to verify the robustness of the model. The sensitivity analysis indicates that higher participation rates would lead to more cost-effective results. It is unrealistic to expect very high participation of individuals for screening when confirmation is by the OGTT. This study used a health-care insurance perspective.
A limitation of the Kahn study47 for our purposes is the way in which risk is assessed. First, there is no preliminary selection by risk factors. Second, the risk assessment is carried out using the FPG, which, as noted, has poor sensitivity.
Few studies included ethnicity. Gillies et al. (2008)143 included a sensitivity analysis. Not all of the studies considered all of the relevant and health outcomes. The Gillies et al. (2008)143 study did not consider both microvascular and macrovascular outcomes, which could actually show better results in terms of cost-effectiveness.
Conclusion
Most studies conclude that screening and intervention is cost-effective, for both diabetes and IGT.
Chapter 7 Discussion
Does screening for type 2 diabetes and impaired glucose tolerance meet the criteria of the National Screening Committee?
The UK NSC criteria for evaluating screening programmes were adapted from the WHO criteria published in 1966. The criteria are published by the NSC on their website (www.screening.nhs.uk/).
The HTA report on screening considered the case for it against the NSC criteria. Most criteria were met, but not all.
The condition
1. The condition should be an important health problem.
Met for both diabetes and IGT.
2. The epidemiology and natural history of the condition, including development from latent to declared disease, should be adequately understood and there should be a detectable risk factor, disease marker, latent period or early symptomatic stage.
Met, although with some reservations.
First, the terminology is not quite right, as rather than a latent period, there can be an asymptomatic period during which undiagnosed diabetes can be causing microvascular or macrovascular damage.
Second, there is uncertainty about the duration and speed of progression of the pre-diabetic stage, and about the duration of undiagnosed diabetes.
In IGT, we do not know why some people progress but others do not. Nor do we know if the progression is linear or initially slow with a rapid decline.
3. All of the cost-effective primary prevention interventions should have been implemented as far as practicable.
We know what people should do to avoid developing both conditions – lifestyle measures, such as maintaining a healthy weight and diet, and physical activity. But we do not know how to persuade them to do so. Given that current primary prevention measures do not appear to be effective, this criterion could be considered met.
4. If the carriers of a mutation are identified as a result of screening the natural history of people with this status should be understood, including the psychological implications.
Not applicable.
The test
5. There should be a simple, safe, precise and validated screening test.
Met for both diabetes and IGT, although there are several test options, now including HbA 1c , and no perfect test. The non-fasting 50-g GCT looks attractive but we need a trial of that.
6. The distribution of test values in the target population should be known and a suitable cut-off level defined and agreed.
Met for diabetes, though as usual there are trade-offs between sensitivity and specificity. The English Vascular Risk manual suggests a cut-off for HbA 1c level of < 6% as normal and a level of 6.5% as confirming diabetes if symptoms are present. The intermediate results ‘require further investigation’. The International Expert Group regards a HbA 1c level of 6.5% as diagnostic.
In the USA, a lower threshold of 5.7% is advocated.
For IGT, things are less certain, because most research into the use of HbA 1c testing has been for its use in screening for diabetes, and less is known about its performance in distinguishing IGT from normality. The EPIC Study and other results show a clear gradient of HbA 1c level and vascular disease.
7. The test should be acceptable to the population.
Given a fully informed public, one might expect screening to be acceptable, and bodies such as Diabetes UK support it. Those who did not wish to accept screening would not need to do so.
Met.
8. There should be an agreed policy on the further diagnostic investigation of individuals with a positive test result and on the choices available to those individuals.
Met for diabetes.
Those with HbA 1c levels of 6.0–6.4% would be given intensive lifestyle advice and rescreened 1 year later. If they had failed to improve on lifestyle alone, metformin would be added.
9. If the test is for mutations the criteria used to select the subset of mutations to be covered by screening, if all possible mutations are not being tested, should be clearly set out.
Not applicable.
The treatment
10. There should be an effective treatment or intervention for patients who are identified through early detection, with evidence of early treatment leading to better outcomes than late treatment.
Met.
Lifestyle change is effective in both conditions, and drug treatment with metformin is cost-effective if lifestyle fails or if people do not adhere. Note that treatment is not just to reduce glucose levels, as the diagnosis can trigger measures to reduce cardiovascular risk, such as statin treatment.
11. There should be agreed evidence-based policies covering which individuals should be offered treatment and the appropriate treatment to be offered.
Met.
12. Clinical management of the condition and patient outcomes should be optimised in all health-care providers prior to participation in a screening programme.
Not met – many people with T2DM do not have their condition optimally controlled, as reported in the Diabetes Audit for England. The Scottish Diabetes Survey shows that about 40% of all people with diabetes have HbA1c levels of > 7.5%. (But note that targets should be tailored to the individual, and for many elderly people, seeking to achieve the NICE target of 6.5% is undesirable. And in the wake of trials such as ADVANCE (Action in Diabetes and Vascular disease: preterAx and diamicroN mr Controlled Evaluation), ACCORD and VADT (Veterans Affairs Diabetes Trial), it may be that we should not aim to go < 7%).
The screening programme
13. There should be evidence from high-quality RCTs that the screening programme is effective in reducing mortality or morbidity.
Not met.
The Ely and ADDITION trials showed no benefit in terms of reducing CVD.
14. There should be evidence that the complete screening programme (test, diagnostic procedures, treatment/intervention) is clinically, socially and ethically acceptable to health professionals and the public.
Screening would be offered, and this criterion would be met by those who accepted.
15. The benefit from the screening programme should outweigh the physical and psychological harm (caused by the test, diagnostic procedures and treatment).
The Hoorn study 101 suggests that those diagnosed were not unduly anxious because they felt they could deal with the condition. The other issue – would those, screened on the grounds of being higher risk, who are screen-negative and reassured, feel so reassured that they continued unhealthy lifestyles – seems to have been addressed and resolved.
Met.
16. The opportunity cost of the screening programme (including testing, diagnosis and treatment, administration, training and QA) should be economically balanced in relation to expenditure on medical care as a whole (i.e. value for money).
Met – see HTA report on screening.
17. There should be a plan for managing and monitoring the screening programme and an agreed set of QA standards.
Not yet applicable.
18. Adequate staffing and facilities for testing, diagnosis, treatment and programme management should be available prior to the commencement of the screening programme.
Screening would be done in general practice and GPs would no doubt request additional resources. There would be some minor administrative costs for selecting the high-risk people; then the HbA 1c test. The main costs would follow – informing patients of the results and their implications, and then providing lifestyle intervention including dietetic time. Dietetic services may not be able to cope.
Could be met if there was political will to fund the service.
19. All other options for managing the condition should have been considered (e.g. improving treatment, providing other services) to ensure that no more cost-effective intervention could be introduced or current interventions increased within the resources available.
Uncertain.
In theory, an effective health education campaign to encourage people to keep weight down and take exercise would prevent much of the cases. However, health education appears to be ineffective. Should we try harder? Is there a danger of ‘medicalising’ unhealthy lifestyles and discouraging people from taking personal responsibility for their own health?
20. Evidence-based information, explaining the consequences of testing, investigation and treatment, should be made available to potential participants to assist them in making an informed choice.
Would be met.
21. Public pressure for widening the eligibility criteria for reducing the screening interval, and for increasing the sensitivity of the testing process, should be anticipated. Decisions about these parameters should be scientifically justifiable to the public.
Uncertain. If we introduced screening using an HbA 1c cut-off of 6.0% for abnormality, and then people with lower levels developed diabetes, as would be inevitable (only 38% of those who developed diabetes in the EPIC-Norfolk study had HbA 1c of 6.0–6.4%) there might be pressure to lower the threshold.
22. If screening is for a mutation, the programme should be acceptable to people identified as carriers and to other family members.
Not applicable.
As reported in Chapter 1, the last HTA report on screening for T2DM10 identified four criteria that had not been met.
Criterion 12 has not been met, as outlined in Chapter 1, with the report of the NAO giving details of shortcomings.
Criterion 13 requires evidence of benefit from high-quality RCTs. This has not been met. The only RCT of screening50 showed no benefit. The ADDITION trial was not a trial of screening but is relevant because it (at present) shows no benefit from intervention to reduce CVD. It may do so with longer follow-up. The failure to show any benefit compared with standard follow-up in general practice appears to have been due to improvements in standard care.
Criterion 18 on staffing and facilities does not appear to have been met, according to the NAO report, which gives details (Appendix 2 of full report) of very marked variations in care among PCTs.
Criterion 19 requires that all other options, including prevention, should have been considered. This is a difficult area because of the difference between what is theoretically possible and what can be achieved in reality.
About 80–90% of T2DM could be prevented by lifestyle measures. The key problem is that we know what people should do to avoid T2DM but not how to persuade them to do it.
One issue is what balance to strike between the ‘medical model’ of screening, detection and treatment of individuals, and the ‘public health’ model of changing behaviour in the entire population. The latter could include not only health education measures, but also other interventions such as those to make physical activity easier (e.g. good-quality cycle lanes entirely separate from traffic) and weight control easier (changes to taxation of foodstuffs, legislation on fat content, taxation by unit of alcohol).
Part of the balance problem is that there is a good evidence base for the medical model, whereas the effectiveness and cost-effectiveness of many health promotion measures are not proven.
As criterion 19 merely states that all other measures ‘should have been considered’ it could be regarded as having been met.
Harms of screening
One issue has been raised by Griffin et al. (2000)53 and the Dutch Hoorn group. 59 Griffin et al. (2000)53 wondered about the dangers of reassurance in those who have high-risk scores but who do not have hyperglycaemia – will they feel they are able to persist with unhealthy lifestyles?53 And in the Hoorn study, Spijkerman et al. (2002)59 found that the group with high-risk scores, but who did not have diabetes on glucose testing, had a CVD risk almost as high as those who were glycaemia-positive. And as there were more of the risk-positive but glucose-negatives, they had more cardiac events, leading the authors to comment that:
It may be of greater public health benefit to intervene in the screen-positive group as a whole rather than only in the relatively small group who on subsequent biochemical testing have an increased glucose concentration.
However, a recent study by Paddison et al. (2009),60 Griffin et al. (2000)53 from the Cambridge MRC group found that people with negative diabetes screening tests were not so reassured that they would have an adverse shift in health behaviours.
National Institute for Health and Care Excellence guidance
Guidance issued by NICE on risk identification and interventions to prevent T2DM in adults at high risk, summarised in the British Medical Journal of 28 July 2012. 151
It should be noted that the introductory statement on the NICE website said:
The guidance is not advocating a national screening programme for type 2 diabetes.
Much of the guidance was about how to prevent T2DM, but there were some recommendations on screening. A two-stage approach was recommended, as in this report. The first stage would use either a self-administered questionnaire or a computerised system based on data in GP records to identify those at high risk. No particular tools were recommended.
The second stage was a measure of blood glucose, using either HbA1c or FPG testing, with cut-offs of 6.5% and 7.0 mmol/l, respectively. Abnormal levels should be confirmed by FPG testing, HbA1c testing or the OGTT.
Those with FPG levels of 5.5–6.9 mmol/l or HbA1c 42–47 mmol/mol (6.0–6.4%) should be offered an intensive lifestyle change programme, but no repeat blood glucose test is suggested at this stage. Repeat blood testing should be offered a year later.
It is recommended by NICE that those at high risk, but with HbA1c level of < 6.0%, should have risk assessed every 3 years.
One concern with this approach is that, although those with HbA1c levels of 6.0–6.4% are at higher risk, most of those who develop diabetes may come from the large proportion with HbA1c levels of < 6.0%. In the EPIC-Norfolk study, two-thirds of those who developed diabetes had baseline HbA1c levels of < 6.0%. 152
An alternative: opportunistic screening
The emphasis in this review has been on systematic population-based screening. Another option would be opportunistic screening in general practice. Such a system has been described by Pereira Gray et al. (2012)153 from Exeter. They report the results from 3 years of opportunistic screening of patients who attend for other reasons, but have one or more risk factors for diabetes, including CVD, hypertension, hyperlipidaemia, obesity, recurrent skin infections or a family history of diabetes. Screening was done using mainly FPG testing (two FPG values of ≥ 7.0 mmol/l) but some OGTTs were carried out, using a 2-hour threshold of ≥ 11.1 mmol/l for diagnosis. In a practice with around 6400 adults, 5720 screening tests were carried out over a 3-year period, of which 190 were OGTTs. A total of 2763 patients were screened at least once – 43% of the practice population. Of the 86 new cases of T2DM diagnosed during the period, 54 (63%) were detected by screening. There were 333 screening tests reported as abnormal but most (279) did not turn out to be in people with diabetes.
The cost per screen-detected case was £377 and 51 patients had to be screened to detect one case of diabetes.
The screening was undertaken as patients attended for other purposes. The authors do not report how many patients with one or more risk factors did not attend during the period, or how many attended but were not screened. It would be useful if they would check the practice computer records for all people who would be invited for systematic screening to see how many were not screened opportunistically. It would also be useful to retest a sample of the FPG-negatives, to assess sensitivity.
Bertram et al. (2010)149 produced a cost-effectiveness model based on opportunistic screening of patients attending Australian general practices for other reasons, using as selection criteria age > 55 years or age > 45 years plus one or more of high BMI, hypertension, and family history of T2DM; ethnicity; previous gestational diabetes. They concluded that a combined package of opportunistic screening and lifestyle intervention would be cost-effective.
Another option?
Maynard et al. (2007)154 reported an entirely different method of screening – spectroscopic measurement of advanced glycation end-products (AGEs) in the skin using a fluorescent technique. The authors report that within a minute, skin AGEs (SAGEs) can be measured. Fasting is not required.
They studied 351 subjects comparing FPG, HbA1c and OGTT data with SAGE results. The comparison was mainly with FPG and HbA1c testing, with OGTT as the reference standard.
The OGTT found IGT in 55 subjects and T2DM in 29. Sensitivities are reported as 75% for SAGE, 58% for FPG at a threshold of 100 mg/dl and 64% for HbA1c (at 5.8%); these thresholds being chosen to all provide 77% specificity. In ROC analysis, SAGEs had an AUC of 79.7% compared with HbA1c 79.2% and FPG 72%.
This technology does not appear to have come into use. A literature search (September 2012) found that the original paper had been cited 18 times, mainly in reviews, with only a few studies producing new data. It does sound as if further research would be worthwhile.
Other views and contributions to the debate about screening
Those in favour of screening for T2DM point to rising prevalence, the proportion undiagnosed, the significant presence of complications at diagnosis and the range of effective treatments for T2DM. 155 They also point to the effective and cost-effective methods of reducing or delaying progression to diabetes from IGT.
Those against note that the additional information provided by knowledge of glucose status may not affect advice or management:
Changes to diet or physical activity levels will always be advisable for people who are overweight or sedentary whatever their overall diabetes or cardiovascular risk score and whatever their glucose result. 156
They also note that changes in lifestyle and overweight render most of the population at risk and, therefore, argue that a population-level approach is required, than that targeting individuals. 156
The 2008 statement from the US Preventive Services Task Force recommended screening for diabetes in people with sustained BP of ≥ 135/80 mmHg, but made no recommendation for other people on the basis of lack of evidence.
Table 18 gives details of a few guidelines, including those from NICE, and shows a fair amount of variation around the world.
| Country | |||||
|---|---|---|---|---|---|
| USA | Canada | New Zealand | UK | Australia | |
| Source | |||||
| ADA157 | Canadian Diabetes Association Clinical Practice Guidelines Expert Committee158 | NZSSD159 | NICE 2012160 | RACGP guidelines for preventative activities in general practice161 | |
| Screening selective or universal above a certain age | |||||
| Age 45 years, particularly in those with BMI of ≥ 25 kg/m2 (performed within health-care setting) | Screening from 40 years in family physicians' office | Opportunist screening and undertaken in conjunction with cardiovascular risk assessment | Selective based on questionnaire from participants or data from GP computer systems | From 40 years of age using the AUSDRISK | |
| Aboriginal people or Torres Strait Islanders – those 18 years of age and with risk scores of ≥ 15 or more should be tested by FPG | |||||
| Selection criteria | |||||
| Age (years) | ≥ 45 | ≥ 40 | 35–55 | > 40 if white, or 25–39 in people of Chinese or South Asian ancestry | All high-risk patients > 40 |
| BMI (kg/m2) | ≥ 25 | Overweight | ≥ 30 or ≥ 27 for South Asians, especially in those with a family history of T2DM | Overweight or obese; in South Asian or Chinese, use BMI of 23 kg/m2 as cut-off | NR |
| Waist circumference | NR | NR | NR | Not mentioned | Included in AUSDRISK |
| Family history | Diabetes | First-degree relative with T2DM | T2DM in more than one first-degree relative (including adolescents) | First-degree family history of diabetes | Included in AUSDRISK |
| Adjustment for ethnicity | African American | People of Aboriginal, Hispanic, South Asian, Asian or African descent | Black, Asian and Chinese groups | Aboriginal people and Torres Strait Islandera | |
| Past GDM | Yes | Yes | Yes | Yesb | |
| Other metabolic problem | Hypertension, dyslipidaemia | Hypertension, POS | Those with IGT test or IFG (not limited by age)b | ||
| Others | POS, abdominal obesity, history of IGT or IFG | IHD, cerebrovascular disease, PVD | Any CVD | Previous history of cardiovascular event (acute MI or stroke)b | |
| Women with POSb | |||||
| Frequency of screening | |||||
| 3-year intervals beginning at age 45 years | Every 3 years in individual ≥ 40 years of age | FPG of < 5.5 mmol/l; 5-yearly screening or earlier if risk factors for diabetes present | Every 3 years | Every 3 years | |
| Earlier and more frequent screening is need in people with additional risk of diabetes | |||||
| Test | |||||
| FPG and 75-g OGTT | FPG and 2-hour PG in a 75-g OGTT | FPG or non-fasting HbA1c | FPG or HbA1c | FPG, OGTT | |
| FPG ≥ 126 mg/dl (7.0 mmol/l) is an indication for retesting | |||||
| Cut-off points used in each test | |||||
| FPG < 126 mg/dl (7.0 mmol/l) | FPG (6.1–6.9 mmol/l) or ≥ 7.0 mmol/l | Fasting glucose of ≥ 5.5 mmol/l or a HbA1c > 6% requires further diagnostic test | FPG > 5.5 mmol/l | FPG < 5.5 mmol/l – diabetes | |
| OGTT: 2-hour PG ≥ 200 mg/dl (11.1 mmol/l) is positive test for diabetes and should be confirmed on an alternative day | 75-g OGTT | FPG ≥ 7 mmol/l or 2-hour post glucose load ≥ 11.1 mmol/l or both indicates diabetes | HbA1c > 42 mmol/mol (6.0%) | If 5.5–6.9 mmol/l may need to perform an OGTT | |
| HbA1c is currently not recommended | Fasting value ≥7.0 mmol/l or 2-h value ≥11.1 mmol/l | If 7.0 mmol/l or > 11.1 non-fasting – diabetes likely, repeat fasting blood sugar to confirm on a separate day | |||
| HbA1c not currently recommended | |||||
Recent evidence from the NHS Health Check programme
A study from Birmingham by Smith et al. (2013)162 has examined the performance of the diabetes filter in the Health Check programme. The aim of the filter is to identify those people in whom a measure of PG is recommended. The filter is based on ethnicity, BMI and BP. The measure of PG can be FPG or HbA1c. The Heart of Birmingham PCT has a high-risk population for T2DM, largely because of the ethnic mix, and so it was decided to test all those attending with HBA1c, without selection by the filter. The performance of the filter in identifying those at risk of diabetes could then be checked.
The results showed that the sensitivity of the filter was only 67%. One-third of those with diabetes or at high risk of it (as determined by HbA1c of ≥ 42 mmol/mmol or greater) would have been missed. Conversely, of those deemed positive by the filter, over half would have been false-positives (positive predictive value 41%).
Further refinement of the filter seems to be indicated.
Research needs
These include:
-
How to motivate people to adopt healthier lifestyles.
-
Cost-effectiveness modelling of selection for screening at different risk scores.
-
The use of the non-fasting 50-g GCT in screening.
-
If screening were to be approved in principle, modelling of different screening scenarios.
-
Determining the incidence of T2DM in 10-year age bands and, hence, the extent of earlier onset.
The highest priority is how to motivate people to adopt healthier lifestyles. The rising prevalence of T2DM could be largely prevented if people led healthier lifestyles and, in particular, avoided obesity. One of our referees suggested that public health interventions to reduce the incidence of T2DM should be compared with screening to detect it.
Further work is needed on modelling the cost-effectiveness of selection for screening by risk factors and scores, including refining the Health Check diabetes filter, or replacing it with QDiabetes Risk Score or FINDRISC.
The non-fasting 50-g GCT might be the best way of screening, but the evidence base is sparse. Trials are needed.
If screening were to be approved in principle, there should be modelling of different screening scenarios to how to implement screening and intervention in a way with which the NHS could cope. That might involve screening only those at highest risk in the first year or two of the programme, and then gradually extending it.
We need to monitor the age at onset of T2DM to determine the extent of earlier onset.
The age at which screening for diabetes and IGT should start in different ethnic groups needs further study. It may be worthwhile starting at a younger age in some populations.
Further research is needed into opportunistic screening in general practice, and the implications for population screening. In practices such as in Exeter, which have had systematic opportunistic screening, there may be little to gain from further screening. This needs investigation.
Conclusions
The case for population screening for T2DM remains unproven. It does not meet all of the NSC criteria.
Acknowledgements
We thank Dr Tara Gurung for producing Table 18.
Contributions of authors
Beth Hall undertook literature searches.
Sian Taylor-Phillips drafted Chapter 2, with help from Norman Waugh and Gaurav Suri.
Deepson Shyangdan wrote Chapter 4 on the ADDITION trial, checked and formatted the report, and created the bibliography.
Gaurav Suri drafted Chapter 6.
Norman Waugh wrote the remaining chapters, edited the whole document and is guarantor.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- UKPDS Study Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53.
- Looker HC, Nyangoma SO, Cromie D, Olson JA, Leese GP, Black M, et al. Diabetic retinopathy at diagnosis of type 2 diabetes in Scotland. Diabetologia 2012;55:2335-42.
- Pierce MB, Zaninotto P, Steel N, Mindell J. Undiagnosed diabetes: data from the English longitudinal study of ageing. Diabet Med 2009;26:679-85. http://dx.doi.org/10.1111/j.1464-5491.2009.02755.x.
- Goyder E, Wild S, Fischbacher C, Carlisle J, Peters J. Evaluating the impact of a national pilot screening programme for type 2 diabetes in deprived areas of England. Fam Pract 2008;25:370-5. http://dx.doi.org/10.1093/fampra/cmn054.
- Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RB. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med 2007;167:1068-74. http://dx.doi.org/10.1001/archinte.167.10.1068.
- Grant I, Fischbacher C, Whyte B. Scottish Public Health Observatory (ScotPHO) collaboration. Obesity in Scotland: An Epidemiology Briefing 2007. www.scotpho.org.uk/home/Publications/scotphoreports/pub_obesityinscotland.asp (accessed 15 May 2012).
- Yorkshire & Humber Public Health Observatory (YHPHO) . Diabetes Key Facts 2006. www.yhpho.org.uk/resource/item.aspx?RID=8872 (accessed 18 June 2012).
- Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol 1997;146:214-22. http://dx.doi.org/10.1093/oxfordjournals.aje.a009256.
- Echouffo-Tcheugui JB, Sargeant LA, Prevost AT, Williams KM, Barling RS, Butler R, et al. How much might cardiovascular disease risk be reduced by intensive therapy in people with screen-detected diabetes?. Diabet Med 2008;25:1433-9. http://dx.doi.org/10.1111/j.1464-5491.2008.02600.x.
- Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al. Screening for type 2 diabetes: literature review and economic modelling. Health Technol Assess 2007;11.
- UK National Screening Committee . Programme Appraisal Criteria. Criteria for Appraising the Viability, Effectiveness and Appropriateness of a Screening Programme 2012. www.screening.nhs.uk/criteria (accessed 20 February 2012).
- National Audit Office (NAO) . The management of adult diabetes services in the NHS. Department of Health 2012. www.nao.org.uk/publications/1213/adult_diabetes_services.aspx (accessed 18 June 2012).
- Rahman M, Simmons RK, Hennings SH, Wareham NJ, Griffin SJ. Effect of screening for type 2 diabetes on population-level self-rate health outcomes and measures of cardiovascular risk: 13 year follow-up of the Ely cohort. Diabet Med 2012;29:886-92.
- Mozaffarian D, Kamineni A, Carnethon M, Djousse L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med 2009;169:798-807. http://dx.doi.org/10.1001/archinternmed.2009.21.
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-50. http://dx.doi.org/10.1056/NEJM200105033441801.
- Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673-9. http://dx.doi.org/10.1016/S0140-6736(06)69701-8.
- UK National Screening Committee . DPH Newsletter. 2008. www.screening.nhs.uk/publications-archive (accessed 15 May 2012).
- Diabetes UK Cymru . Welsh Pharmacies Offered Type 2 Diabetes Assessments 2011. www.diabetes.org.uk/In_Your_Area/Wales/Campaigning/Pharmacy-campaign (accessed 16 February 2012).
- Gillett MF, Royle PF, Snaith AF, Scotland GF, Poobalan AF, Imamura MF, et al. Non-pharmacological interventions to reduce the risk of diabetes in people with impaired glucose regulation: a systematic review and economic evaluation. Health Technol Assess 2012;16.
- Gavin II, Alberti KGMM, Davidson MB, DeFronzo RA, Drash A, Gabbe SG, et al. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183-97.
- Colagiuri S, Lee CM, Wong TY, Wong TY, Balkau B, Shaw JE, et al. Glycemic thresholds for diabetes-specific retinopathy: implications for diagnostic criteria for diabetes. Diabetes Care 2011;34:145-50. http://dx.doi.org/10.2337/dc10-1206.
- Diabetes Prevention Program Research Group . The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med 2007;24:137-44.
- Chamnan P, Simmons RK, Jackson R, Khaw KT, Wareham NJ, Griffin SJ. Non-diabetic hyperglycaemia and cardiovascular risk: moving beyond categorisation to individual interpretation of absolute risk. Diabetologia 2011;54:291-9. http://dx.doi.org/10.1007/s00125-010-1914-6.
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-12. http://dx.doi.org/10.1136/bmj.321.7258.405.
- UKPDS Study Group . Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854-65.
- Balkau B. Prediction of the risk of cardiovascular mortality using a score that includes glucose as a risk factor. The DECODE Study. Diabetologia 2004;47:2118-28.
- Ning F, Tuomilehto J, Pyorala K, Onat A, Soderberg S, Qiao Q. Cardiovascular disease mortality in Europeans in relation to fasting and 2-h plasma glucose levels within a normoglycemic range. Diabetes Care 2010;33:2211-16. http://dx.doi.org/10.2337/dc09-2328.
- Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999;22:233-40. http://dx.doi.org/10.2337/diacare.22.2.233.
- Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di AE, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215-22.
- Sarwar N, Aspelund T, Eiriksdottir G, Gobin R, Seshasai SR, Forouhi NG, et al. Markers of dysglycaemia and risk of coronary heart disease in people without diabetes: Reykjavik prospective study and systematic review. PLoS Medicine 2010;7. http://dx.doi.org/10.1371/journal.pmed.1000278.
- Barr EL, Boyko EJ, Zimmet PZ, Wolfe R, Tonkin AM, Shaw JE. Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia 2009;52:415-24. http://dx.doi.org/10.1007/s00125-008-1246-y.
- Wild SH, Smith FB, Lee AJ, Fowkes FG. Criteria for previously undiagnosed diabetes and risk of mortality: 15-year follow-up of the Edinburgh Artery Study cohort. Diabet Med 2005;22:490-6. http://dx.doi.org/10.1111/j.1464-5491.2004.01433.x.
- de Vegt F, Dekker JM, Ruhe HG, Stehouwer CD, Nijpels G, Bouter LM, et al. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia 1999;42:926-31. http://dx.doi.org/10.1007/s001250051249.
- Barrett-Connor E, Ferrara A. Isolated postchallenge hyperglycemia and the risk of fatal cardiovascular disease in older women and men. The Rancho Bernardo Study. Diabetes Care 1998;21:1236-9. http://dx.doi.org/10.2337/diacare.21.8.1236.
- Charles MA, Balkau B, Vauzelle-Kervroedan F, Thibult N, Eschwege E. Revision of diagnostic criteria for diabetes. Lancet 1996;348:1657-8. http://dx.doi.org/10.1016/S0140-6736(05)65719-4.
- Davies MJ, Gray IP. Impaired glucose tolerance. BMJ 1996;312:264-5. http://dx.doi.org/10.1136/bmj.312.7026.264.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004;141:413-20. http://dx.doi.org/10.1016/j.accreview.2004.11.074.
- Simmons RK, Sharp S, Boekholdt SM, Sargeant LA, Khaw KT, Wareham NJ, et al. Evaluation of the Framingham risk score in the European Prospective Investigation of Cancer-Norfolk cohort: does adding glycated hemoglobin improve the prediction of coronary heart disease events?. Arch Intern Med 2008;168:1209-16. http://dx.doi.org/10.1001/archinte.168.11.1209.
- Chamnan P, Simmons RK, Sharp SJ, Griffin SJ, Wareham NJ. Cardiovascular risk assessment scores for people with diabetes: a systematic review. Diabetologia 2009;52:2001-14. http://dx.doi.org/10.1007/s00125-009-1454-0.
- Novoa FJ, Boronat M, Saavedra P, Diaz-Cremades JM, Varillas VF, La RF, et al. Differences in cardiovascular risk factors, insulin resistance, and insulin secretion in individuals with normal glucose tolerance and in subjects with impaired glucose regulation: the Telde Study. Diabetes Care 2005;28:2388-93. http://dx.doi.org/10.2337/diacare.28.10.2388.
- Muntner P, Wildman RP, Reynolds K, Desalvo KB, Chen J, Fonseca V. Relationship between HbA1c level and peripheral arterial disease. Diabetes Care 2005;28:1981-7. http://dx.doi.org/10.2337/diacare.28.8.1981.
- Glumer C, Carstensen B, Sandbaek A, Lauritzen T, Jorgensen T, Borch-Johnsen K. A Danish diabetes risk score for targeted screening: the Inter99 study. Diabetes Care 2004;27:727-33. http://dx.doi.org/10.2337/diacare.27.3.727.
- Paulweber B, Valensi P, Lindstrom J, Lalic NM, Greaves CJ, McKee M, et al. A European evidence-based guideline for the prevention of type 2 diabetes. Horm Metab Res 2010;42:S3-36. http://dx.doi.org/10.1055/s-0029-1240928.
- Bergmann A, Li J, Wang L, Schulze J, Bornstein SR, Schwarz PE. A simplified Finnish diabetes risk score to predict type 2 diabetes risk and disease evolution in a German population. Horm Metab Res 2007;39:677-82. http://dx.doi.org/10.1055/s-2007-985353.
- Franciosi M, De BG, Rossi MC, Sacco M, Belfiglio M, Pellegrini F, et al. Use of the diabetes risk score for opportunistic screening of undiagnosed diabetes and impaired glucose tolerance: the IGLOO (Impaired Glucose Tolerance and Long-Term Outcomes Observational) study. Diabetes Care 2005;28:1187-94. http://dx.doi.org/10.2337/diacare.28.5.1187.
- Tan HH, McAlpine RR, James P, Thompson P, McMurdo MET, Morris AD, et al. Diagnosis of type 2 diabetes at an older age: Effect on mortality in men and women. Diabetes Care 2004;27:2797-9. http://dx.doi.org/10.2337/diacare.27.12.2797.
- Kahn R, Alperin P, Eddy D, Borch-Johnsen K, Buse J, Feigelman J, et al. Age at initiation and frequency of screening to detect type 2 diabetes: a cost-effectiveness analysis. Lancet 2010;375:1365-74. http://dx.doi.org/10.1016/S0140-6736(09)62162-0.
- Riste L, Khan F, Cruickshank K. High prevalence of type 2 diabetes in all ethnic groups, including Europeans, in a British inner city: relative poverty, history, inactivity, or 21st century Europe?. Diabetes Care 2001;24:1377-83. http://dx.doi.org/10.2337/diacare.24.8.1377.
- Lindstrom J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725-31. http://dx.doi.org/10.2337/diacare.26.3.725.
- Rahman M, Simmons RK, Harding AH, Wareham NJ, Griffin SJ. A simple risk score identifies individuals at high risk of developing Type 2 diabetes: a prospective cohort study. Family Practice 2008;25:191-6. http://dx.doi.org/10.1093/fampra/cmn024.
- Hippisley-Cox J, Coupland C, Robson J, Sheikh A, Brindle P. Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b880.
- Collins GS, Altman DG. External validation of QDSCORE((R)) for predicting the 10-year risk of developing type 2 diabetes. Diabet Med 2011;28:599-607.
- Griffin SJ, Little PS, Hales CN, Kinmonth AL, Wareham NJ. Diabetes risk score: towards earlier detection of type 2 diabetes in general practice. Diabetes Metab Res Rev 2000;16:164-71. http://dx.doi.org/10.1002/1520-7560(200005/06)16:3<164::AID-DMRR103>3.3.CO;2-I.
- Heldgaard PE, Griffin SJ. Routinely collected general practice data aids identification of people with hyperglycaemia and metabolic syndrome. Diabet Med 2006;23:996-1002. http://dx.doi.org/10.1111/j.1464-5491.2006.01929.x.
- Witte DR, Shipley MJ, Marmot MG, Brunner EJ. Performance of existing risk scores in screening for undiagnosed diabetes: an external validation study. Diabet Med 2010;27:46-53. http://dx.doi.org/10.1111/j.1464-5491.2009.02891.x.
- Noble D, Mathur R, Dent T, Meads C, Greenhalgh T. Risk models and scores for type 2 diabetes: systematic review. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d7163.
- Martin E, Ruf E, Landgraf R, Hauner H, Weinauer F, Martin S. FINDRISK questionnaire combined with HbA1c testing as a potential screening strategy for undiagnosed diabetes in a healthy population. Horm Metab Res 2011;43:782-7. http://dx.doi.org/10.1055/s-0031-1286333.
- Abbasi A, Peelen LM, Corpeleijn E, van der Schouw YT, Stolk RP, Spijkerman AM, et al. Prediction models for risk of developing type 2 diabetes: systematic literature search and independent external validation study. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e5900.
- Spijkerman A, Griffin S, Dekker J, Nijpels G, Wareham NJ. What is the risk of mortality for people who are screen positive in a diabetes screening programme but who do not have diabetes on biochemical testing? Diabetes screening programmes from a public health perspective. J Med Screen 2002;9:187-90. http://dx.doi.org/10.1136/jms.9.4.187.
- Paddison CA, Eborall HC, Sutton S, French DP, Vasconcelos J, Prevost AT, et al. Are people with negative diabetes screening tests falsely reassured? Parallel group cohort study embedded in the ADDITION (Cambridge) randomised controlled trial. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b4535.
- Qiao Q, Keinanen-Kiukaanniemi S, Rajala U, Uusimaki A, Kivela SL. Random capillary whole blood glucose test as a screening test for diabetes mellitus in a middle-aged population. Scand J Clin Lab Invest 1995;55:3-8.
- Wareham NJ, Pfister R. Diabetes: glycated hemoglobin is a marker of diabetes and CVD risk. Nat Rev Cardiol 2010;7:367-8. http://dx.doi.org/10.1038/nrcardio.2010.84.
- Evans P, Langley P, Gray DP. Diagnosing type 2 diabetes before patients complain of diabetic symptoms: clinical opportunistic screening in a single general practice. Fam Pract 2008;25:376-81. http://dx.doi.org/10.1093/fampra/cmn052.
- Hu Y, Liu W, Chen Y, Zhang M, Wang L, Zhou H, et al. Combined use of fasting plasma glucose and glycated hemoglobin A1c in the screening of diabetes and impaired glucose tolerance. Acta Diabetol 2010;47:231-6. http://dx.doi.org/10.1007/s00592-009-0143-2.
- John WG, Hillson R, Alberti SG. Use of haemoglobin A1c (HbA1c) in the diagnosis of diabetes mellitus. The implementation of World Health Organization (WHO) guidance 2011. Practical Diabetes 2012;29:12-12a.
- American Diabetes Association . Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26:S5-20.
- Gerstein HC. Glycosylated hemoglobin: finally ready for prime time as a cardiovascular risk factor. Ann Intern Med 2004;141:475-6. http://dx.doi.org/10.7326/0003-4819-141-6-200409210-00014.
- Sabanayagam C, Liew G, Tai ES, Shankar A, Lim SC, Subramaniam T, et al. Relationship between glycated haemoglobin and microvascular complications: is there a natural cut-off point for the diagnosis of diabetes?. Diabetologia 2009;52:1279-89. http://dx.doi.org/10.1007/s00125-009-1360-5.
- American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33:62-9.
- The International Expert Committee . International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327-34.
- Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800-11. http://dx.doi.org/10.1056/NEJMoa0908359.
- Peter A, Fritsche A, Stefan N, Heni M, Haring HU, Schleicher E. Diagnostic value of haemoglobin A1c for type 2 diabetes mellitus in a population at risk. Exp Clin Endocrinol Diabetes 2011;119:23-7. http://dx.doi.org/10.1055/s-0030-1270440.
- Morrison G, Morrison CL, Purewal TS, Weston PJ. Evaluating the use of HbA1c in the diagnosis of diabetes compared with the oral glucose tolerance test in a primary care setting. Diabet Med 2011;28.
- Schottker B, Raum E, Rothenbacher D, Muller H, Brenner H. Prognostic value of haemoglobin A1c and fasting plasma glucose for incident diabetes and implications for screening. Eur J Epidemiol 2011;26:779-87. http://dx.doi.org/10.1007/s10654-011-9619-9.
- John WG. Haemoglobin A1c towards global standardization. Diabet Med 2010;27:733-4. http://dx.doi.org/10.1111/j.1464-5491.2010.03044.x.
- Bao Y, Ma X, Li H, Zhou M, Hu C, Wu H, et al. Glycated haemoglobin A1c for diagnosing diabetes in Chinese population: cross sectional epidemiological survey. BMJ 2010;340.
- Kilpatrick ES, Winocour PH. ABCD position statement on haemoglobin A1c for the diagnosis of diabetes. Practical Diabetes Int 2010;27:306-10.
- Schindhelm RK, Lenters-Westra E, Slingerland RJ. Glycated haemoglobin A(1c) (HbA1c) in the diagnosis of diabetes mellitus: don't forget the performance of the HbA1c assay. Diabet Med 2010;27:1214-15.
- Scottish Public Health Network (SPHN) . Type 2 Diabetes Needs Assessment. 2012. www.scotphn.net/projects/previous_projects/type_2_diabetes_needs_assessment (accessed 11 February 2012).
- Cederberg H, Saukkonen T, Laakso M, Jokelainen J, Harkonen P, Timonen M, et al. Postchallenge glucose, A1C, and fasting glucose as predictors of type 2 diabetes and cardiovascular disease: a 10-year prospective cohort study. Diabetes Care 2010;33:2077-83. http://dx.doi.org/10.2337/dc10-0262.
- Mostafa SA, Davies MJ, Webb D, Gray LJ, Srinivasan BT, Jarvis J, et al. The potential impact of using glycated haemoglobin as the preferred diagnostic tool for detecting Type 2 diabetes mellitus. Diabet Med 2010;27:762-9. http://dx.doi.org/10.1111/j.1464-5491.2010.03015.x.
- Mostafa SA, Srinivasan BT, Webb D, Sehmi S, Gray LJ, Yates T, et al. A comparison of incident Type 2 diabetes using WHO 1999 and the proposed HbA1cdiagnostic criteria in people with impaired glucose regulation (pre-diabetes): a prospective longitudinal follow-up study of a UK multi-ethnic cohort. Diabet Med 2011;28.
- Borg R, Vistisen D, Witte DR, Borch-Johnsen K. Comparing risk profiles of individuals diagnosed with diabetes by OGTT and HbA1c. The Danish Inter99 study. Diabet Med 2010;27:906-10.
- Lorenzo C, Wagenknecht LE, Hanley AJG, Rewers MJ, Karter AJ, Haffner SM. A1C between 5.7 and 6.4% as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2010;33:2104-9. http://dx.doi.org/10.2337/dc10-0679.
- Valdes S, Botas P, Delgado E, Alvarez F, Diaz-Cadorniga F. HbA1c in the prediction of type 2 diabetes compared with fasting and 2-h post-challenge plasma glucose: the Asturias study (1998–2005). Diabetes Metab 2011;37:27-32.
- Selvin E, Steffes MW, Bergenstal R, Coresh J, Brancati FL. 2010 American Diabetes Association (ADA) Cut-Points for Glycated Hemoglobin (A1c) and the Risk of Diabetes, Kidney, and Cardiovascular Disease. American Diabetes Association 70th Scientific Sessions, 2010 n.d.
- Skriver MV, Borch-Johnsen K, Lauritzen T, Sandbaek A. HbA1c as predictor of all-cause mortality in individuals at high risk of diabetes with normal glucose tolerance, identified by screening: a follow-up study of the Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen-Detected Diabetes in Primary Care (ADDITION), Denmark. Diabetologia 2010;53:2328-33. http://dx.doi.org/10.1007/s00125-010-1867-9.
- Cosson E, Hamo-Tchatchouang E, Banu I, Nguyen MT, Chiheb S, Ba H, et al. A large proportion of prediabetes and diabetes goes undiagnosed when only fasting plasma glucose and/or HbA1c are measured in overweight or obese patients. Diabetes Metab 2010;36:312-18. http://dx.doi.org/10.1016/j.diabet.2010.02.004.
- Cavagnolli G, Comerlato J, Comerlato C, Renz PB, Gross JL, Camargo JL. HbA1c measurement for the diagnosis of diabetes: is it enough?. Diabet Med 2011;28:31-5.
- Pajunen P, Peltonen M, Eriksson JG, Ilanne-Parikka P, Aunola S, Keinanen-Kiukaanniemi S, et al. HbA1c in diagnosing and predicting type 2 diabetes in impaired glucose tolerance: the Finnish Diabetes Prevention Study. Diabet Med 2010;28:36-42.
- Bennett CM, Guo M, Dharmage SC. HbA1c as a screening tool for detection of type 2 diabetes: a systematic review. Diabet Med 2007;24:333-43.
- Abdul-Ghani MA, DeFronzo RA. Plasma glucose concentration and prediction of future risk of type 2 diabetes. Diabetes Care 2009;32:194-8. http://dx.doi.org/10.2337/dc09-S309.
- Joshipura KJ, Andriankaja MO, Hu FB, Ritchie CS. Relative utility of 1-h Oral Glucose Tolerance Test as a measure of abnormal glucose homeostasis. Diabetes Res Clin Pract 2011;93:268-75. http://dx.doi.org/10.1016/j.diabres.2011.05.035.
- Abdul-Ghani MA, Abdul-Ghani T, Muller G, Bergmann A, Fischer S, Bornstein S, et al. Role of glycated hemoglobin in the prediction of future risk of T2DM. J Clin Endocrinol Metab 2011;96:2596-600. http://dx.doi.org/10.1210/jc.2010-1698.
- Phillips LS, Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, et al. Glucose challenge test screening for prediabetes and undiagnosed diabetes. Diabetologia 2009;52:1798-807. http://dx.doi.org/10.1007/s00125-009-1407-7.
- Chatterjee R, Narayan KM, Lipscomb J, Phillips LS. Screening adults for pre-diabetes and diabetes may be cost-saving. Diabetes Care 2010;33:1484-90. http://dx.doi.org/10.2337/dc10-0054.
- Jones AG, Knight BA, Baker GC, Hattersley AT. Practical implications of choice of test in National Institute for Health and Clinical Excellence (NICE) guidance for the prevention of type 2 diabetes. Diabet Med 2013;30:126-7. http://dx.doi.org/10.1111/dme.12025.
- Carson AP, Reynolds K, Fonseca VA, Muntner P. Comparison of A1C and fasting glucose criteria to diagnose diabetes among U.S. adults. Diabetes Care 2010;33:95-7. http://dx.doi.org/10.2337/dc09-1227.
- Mann DM, Carson AP, Shimbo D, Fonseca V, Fox CS, Muntner P. Impact of A1C screening criterion on the diagnosis of pre-diabetes among U.S. adults. Diabetes Care 2010;33:2190-5. http://dx.doi.org/10.2337/dc10-0752.
- Davies MJ, Raymond NT, Day JL, Hales CN, Burden AC. Impaired glucose tolerance and fasting hyperglycaemia have different characteristics. Diabet Med 2000;17:433-40. http://dx.doi.org/10.1046/j.1464-5491.2000.00246.x.
- de Vegt F, Dekker JM, Jager A, Hienkens E, Kostense PJ, Stehouwer CD, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA 2001;285:2109-13. http://dx.doi.org/10.1001/jama.285.16.2109.
- Simmons RK, Echouffo-Tcheugui JB, Sharp SJ, Sargeant LA, Williams KM, Prevost AT, et al. Screening for type 2 diabetes and population mortality over 10 years (ADDITION-Cambridge): a cluster-randomised controlled trial. Lancet 2012;380:1741-8. http://dx.doi.org/10.1016/S0140-6736(12)61422-6.
- Matsushita K, Blecker S, Pazin-Filho A, Bertoni A, Chang PP, Coresh J, et al. The association of HbA1c with incident heart failure among people without diabetes: the atherosclerosis risk in communities study. Diabetes 2010;59:2020-6.
- Wang W, Lee ET, Howard BV, Fabsitz RR, Devereux RB, Welty TK. Fasting plasma glucose and HbA1c in identifying and predicting diabetes: the strong heart study. Diabetes Care 2011;34:363-8.
- Sargeant LA, Khaw KT, Bingham S, Day NE, Luben RN, Oakes S, et al. Cigarette smoking and glycaemia: the EPIC-Norfolk Study. European Prospective Investigation into Cancer. Int J Epidemiol 2001;30:547-54.
- Haffner S. M., Lorenzo C., Laakso M. Performance characteristics of the 6.5% HbA1c threshold for detecting undiagnosed diabetes in Finnish men: the METabolic Syndrome In Men (METSIM) study. Proceedings of the American Diabetes Association 71st Scientific Sessions 1364-P 2011.
- Bobbert T, Mai K, Fischer-Rosinsky A, Pfeiffer AF, Spranger J. A1C is associated with intima-media thickness in individuals with normal glucose tolerance. Diabetes Care 2010;33:203-4. http://dx.doi.org/10.2337/dc09-1009.
- Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, Phillips LS. Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care 2010;33:2184-9. http://dx.doi.org/10.2337/dc10-0433.
- UKPDS Study Group . Ethnicity and cardiovascular disease. The incidence of myocardial infarction in white, South Asian, and Afro-Caribbean patients with type 2 diabetes (U.K. Prospective Diabetes Study 32). Diabetes Care 1998;21:1271-7.
- Lipska KJ, De RN, Van Ness PH, Johnson KC, Kanaya A, Koster A, et al. Identifying dysglycemic states in older adults: implications of the emerging use of hemoglobin A1c. J Clin Endocrinol Metab 2010;95:5289-95. http://dx.doi.org/10.1210/jc.2010-1171.
- Likhari T, Gama R. Ethnic differences in glycated haemoglobin between white subjects and those of South Asian origin with normal glucose tolerance. J Clin Pathol 2010;63:278-80. http://dx.doi.org/10.1136/jcp.2009.065821.
- Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007;30:2453-7. http://dx.doi.org/10.2337/dc06-2003.
- Gerstein HC, Islam S, Anand S, Almahmeed W, Damasceno A, Dans A, et al. Dysglycaemia and the risk of acute myocardial infarction in multiple ethnic groups: an analysis of 15,780 patients from the INTERHEART study. Diabetologia 2010;53:2509-17. http://dx.doi.org/10.1007/s00125-010-1871-0.
- Webb DR, Gray LJ, Khunti K, Srinivasan B, Taub N, Campbell S, et al. Screening for diabetes using an oral glucose tolerance test within a western multi-ethnic population identifies modifiable cardiovascular risk: the ADDITION-Leicester study. Diabetologia 2011;54:2237-46. http://dx.doi.org/10.1007/s00125-011-2189-2.
- Brindle P, May M, Gill P, . ETHRISK: A modified Framingham CHD and CVD risk calculator for British black and minority ethnic groups. UK: University of Bristol; n.d.
- Bhopal RS. A four-stage model explaining the higher risk of type 2 diabetes mellitus in South Asians compared with European populations. Diabet Med 2013;30:35-42. http://dx.doi.org/10.1111/dme.12016.
- Szczepura A. Nutrition in an ethnically diverse society: what are some of the key challenges?. Proc Nutr Soc 2011;70:252-62. http://dx.doi.org/10.1017/S0029665111000085.
- Takahashi O, Farmer AJ, Shimbo T, Fukui T, Glasziou PP. A1C to detect diabetes in healthy adults: when should we recheck?. Diabetes Care 2010;33:2016-17. http://dx.doi.org/10.2337/dc10-0588.
- Davies M, Day J. Screening for non-insulin-dependent diabetes mellitus (NIDDM): how often should it be performed?. J Med Screen 1994;1:78-81.
- The ADDITION Study Group . Protocol, ADDITION (Anglo-Danish-Dutch Study in General Practice of Intensive Treatment and Complication Prevention in Type 2 Diabetic Patients Identified by Screening) Study 2005. www.addition.au.dk/Protocol%20-%20ADDITION.pdf (accessed 16 February 2012).
- Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BH, Rutten G. The ADDITION study: proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with type 2 diabetes detected by screening. Int J Obes Relat Metab Dis 2000;24:6-11. http://dx.doi.org/10.1038/sj.ijo.0801420.
- van den Donk M, Sandbaek A, Borch-Johnsen K, Lauritzen T, Simmons RK, Wareham NJ, et al. Screening for type 2 diabetes. Lessons from the ADDITION-Europe study. Diabet Med 2011;28:1416-24. http://dx.doi.org/10.1111/j.1464-5491.2011.03365.x.
- Griffin SJ, Borch-Johnsen K, Davies MJ, Khunti K, Rutten GE, Sandbaek A, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet 2011;378:156-67. http://dx.doi.org/10.1016/S0140-6736(11)60698-3.
- Sandbaek A, Griffin SJ, Rutten G, Davies M, Stolk R, Khunti K, et al. Stepwise screening for diabetes identifies people with high but modifiable coronary heart disease risk. The ADDITION study. Diabetologia 2008;51:1127-34. http://dx.doi.org/10.1007/s00125-008-1013-0.
- Barakat A, Williams KM, Prevost AT, Kinmonth AL, Wareham NJ, Griffin SJ, et al. Changes in physical activity and modelled cardiovascular risk following diagnosis of diabetes: 1-year results from the ADDITION-Cambridge trial cohort. Diabet Med 2013;30:233-8. http://dx.doi.org/10.1111/j.1464-5491.2012.03765.x.
- Eborall H, Davies R, Kinmonth AL, Griffin S, Lawton J. Patients' experiences of screening for type 2 diabetes: prospective qualitative study embedded in the ADDITION (Cambridge) randomised controlled trial. BMJ 2007;335. http://dx.doi.org/10.1136/bmj.39308.392176.BE.
- The Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545-59.
- Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-40.
- Absetz P, Oldenburg B, Hankonen N, Valve R, Heinonen H, Nissinen A, et al. Type 2 diabetes prevention in the real world: three-year results of the GOAL lifestyle implementation trial. Diabetes Care 2009;32:1418-20. http://dx.doi.org/10.2337/dc09-0039.
- Norris SL, Zhang X, Avenell A, Gregg E, Schmid CH, Lau J. Long-term non-pharmacological weight loss interventions for adults with prediabetes. Cochrane Database Syst Rev 2005;2. http://dx.doi.org/10.1002/14651858.CD005270.
- Perry IJ, Villegas R, Salim A, Flynn A. Clustering of protective factors for glucose intolerance and insulin resistance: a cross-sectional study. Diabet Med 2005;22:1091-7. http://dx.doi.org/10.1111/j.1464-5491.2005.01617.x.
- Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes 1992;16:397-415.
- United Nations Statistics Division . Millennium Development Goals Indicators. The Official United Nations Site for the MDG Indicators 2012. http://unstats.un.org/unsd/mdg/SeriesDetail.aspx?srid=699 (accessed 20 June 2012).
- Donnelly R, Emslie-Smith AM, Gardner ID, Morris AD. ABC of arterial and venous disease: vascular complications of diabetes. BMJ 2000;320:1062-6. http://dx.doi.org/10.1136/bmj.320.7241.1062.
- Gulliford MC, Charlton J. Is relative mortality of type 2 diabetes mellitus decreasing?. Am J Epidemiol 2009;169:455-61. http://dx.doi.org/10.1093/aje/kwn342.
- Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006;26:410-20. http://dx.doi.org/10.1177/0272989X06290495.
- Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med 2005;143:251-64. http://dx.doi.org/10.7326/0003-4819-143-4-200508160-00006.
- Feenstra TL, van Baal PM, Jacobs-van der Bruggen MO, Hoogenveen RT, Kommer GJ, Baan CA. Targeted versus universal prevention. a resource allocation model to prioritize cardiovascular prevention. Cost Eff Resour Alloc 2011;9. http://dx.doi.org/10.1186/1478-7547-9-14.
- Colagiuri S, Walker AE. Using an economic model of diabetes to evaluate prevention and care strategies in Australia. Health Affairs 2008;27:256-68. http://dx.doi.org/10.1377/hlthaff.27.1.256.
- Chamnan P, Simmons RK, Khaw KT, Wareham NJ, Griffin SJ. Estimating the potential population impact of stepwise screening strategies for identifying and treating individuals at high risk of type 2 diabetes: a modelling study. Diabet Med 2012;29:893-904. http://dx.doi.org/10.1111/j.1464-5491.2012.03609.x.
- Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, et al. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer 1999;80:95-103.
- University of Leicester . UK National Screening Committee. Handbook for Vascular Risk Assessment, Risk Reduction and Risk Management 2008. www.screening.nhs.uk/getdata.php?id=9051 (accessed 16 February 2012).
- Gillies CL, Lambert PC, Abrams KR, Sutton AJ, Cooper NJ, Hsu RT, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ 2008;336:1180-5. http://dx.doi.org/10.1136/bmj.39545.585289.25.
- Schaufler TM, Wolff M. Cost effectiveness of preventive screening programmes for type 2 diabetes mellitus in Germany. Appl Health Econ Health Policy 2010;8:191-202. http://dx.doi.org/10.2165/11532880-000000000-00000.
- Davies MJ, Tringham JR, Jarvis J, Skinner TC, Farooqi AM, Khunti K. Systematic screening for type 2 diabetes mellitus: results of a large population based study targeting those with conventional risk factors. Diabet Med 2005;22.
- Davis TM. Ethnic diversity in type 2 diabetes. Diabet Med 2008;25:52-6. http://dx.doi.org/10.1111/j.1464-5491.2008.02499.x.
- Icks A, Haastert B, Gandjour A, John J, Lowel H, Holle R, et al. Cost-effectiveness analysis of different screening procedures for type 2 diabetes: the KORA Survey 2000. Diabetes Care 2004;27:2120-8. http://dx.doi.org/10.2337/diacare.27.9.2120.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Bertram MY, Lim SS, Barendregt JJ, Vos T. Assessing the cost-effectiveness of drug and lifestyle intervention following opportunistic screening for pre-diabetes in primary care. Diabetologia 2010;53:875-81. http://dx.doi.org/10.1007/s00125-010-1661-8.
- Hoerger TJ, Hicks KA, Sorensen SW, Herman WH, Ratner RE, Ackermann RT, et al. Cost-effectiveness of screening for pre-diabetes among overweight and obese U.S. adults. Diabetes Care 2007;30:2874-9. http://dx.doi.org/10.2337/dc07-0885.
- Chatterton H, Younger T, Fischer A, Khunti K. Risk identification and interventions to prevent type 2 diabetes in adults at high risk: summary of NICE guidance. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e4624.
- Chamnan P, Simmons RK, Forouhi NG, Luben RN, Khaw KT, Wareham NJ, et al. Incidence of type 2 diabetes using proposed HbA1c diagnostic criteria in the European prospective investigation of cancer-Norfolk cohort: implications for preventive strategies. Diabetes Care 2011;34:950-6. http://dx.doi.org/10.2337/dc09-2326.
- Pereira Gray DJ, Evans PH, Wright C, Langley P. The cost of diagnosing type 2 diabetes mellitus by clinical opportunistic screening in general practice. Diabet Med 2012;29:863-8. http://dx.doi.org/10.1111/j.1464-5491.2012.03607.x.
- Maynard JD, Rohrscheib M, Way JF, Nguyen CM, Ediger MN. Noninvasive type 2 diabetes screening: superior sensitivity to fasting plasma glucose and A1C. Diabetes Care 2007;30:1120-4. http://dx.doi.org/10.2337/dc06-2377.
- Khunti K, Davies M. Should we screen for type 2 diabetes? Yes. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e4514.
- Goyder E, Irwig L, Payne N. Should we screen for type 2 diabetes? No. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e4516.
- American Diabetes Association . Screening for type 2 diabetes. Diabetes Care 2004;27:11-4.
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee . Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2008;32:1-201.
- New Zealand Society for the Study of Diabetes . NZSSD Position Statement on Screening and Type 2 Diabetes 2009. www.nzssd.org.nz/position_statements/screening.html (accessed 1 September 2012).
- National Institute for Health and Clinical Excellence (NICE) . Preventing Type 2 Diabetes: Risk Identification and Interventions for Individuals at High Risk. NICE Public Health Guidance 38. 2012. www.nice.org.uk/nicemedia/live/13791/59951/59951.pdf (accessed 1 September 2012).
- Guidelines for preventive activities in general practice. 2012.
- Smith S, Waterall J, Burden AC. An evaluation of the performance of the NHS Health Check programme in identifying people at high risk of developing type 2 diabetes. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-002219.
- Scottish Intercollegiate Guidelines Network . Risk estimation and the prevention of cardiovascular disease. Guideline No. 97 2007.
Appendix 1 Objectives, methods and conclusions of the previous reports
Previous review of screening for type 2 diabetes
Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al. Screening for type 2 diabetes: literature review and economic modelling. Health Technol Assess 2007;11(17).
The objectives of this review were as follows:
-
to consider the aims of screening for undiagnosed diabetes, and whether screening should be for other abnormalities of glucose metabolism, such as IGT, or the ‘metabolic syndrome’
-
to update the previous review for the NSC on screening for diabetes, including reviewing choice of screening test
-
to consider what measures would be taken if IGT and IFG were identified by screening and, in particular, to examine evidence on treatment to prevent progression to diabetes in these groups
-
to examine the cost-effectiveness of screening, by a review of previous economic models, and by new modelling to take account of recent developments in treatment, such as the use of statins
-
as part of the economic analysis, to consider groups at higher risk at which screening might be targeted.
Methods
The literature searches (carried out up to the end of June 2005) and review concentrated on evidence published since the last review of screening for NSC in 2001. Both reviews and primary studies were included. The 2001 review was:
Wareham NJ, Griffin SJ. Should we screen for type 2 diabetes? Evaluation against the National Screening Committee criteria. BMJ 2001;322:986–8.
MEDLINE
-
exp *Diabetes Mellitus, Type 2/
-
exp Mass Screening/
-
(diabetes and screening).m_titl.
-
1 and 2
-
1 and 3
-
4 or 5
-
limit 6 to (english language and yr = “2000 –June 2005”)
EMBASE
-
exp *Non Insulin Dependent Diabetes Mellitus/
-
exp Mass Screening/
-
(diabetes and screening).m_titl.
-
1 and 2
-
1 and 3
-
4 or 5
-
limit 6 to (english language and yr = “2000 –June 2005”)
The Cochrane Library 2005
Issue 2: all sections
#1 Medical subject heading (MeSH) descriptor Diabetes Mellitus, Type 2 explode all trees in MeSH products
#2 MeSH descriptor Mass Screening explode all trees in MeSH products
#3 (#1 AND #2)
#4 screening in Record Title and diabetes in Record Title in all products
#5 (#3 OR #4)
The review of economic studies included only those models that covered screening. Databases searched were MEDLINE, EMBASE, NHS EED, and the Science and Social Science Citation Index.
The aim was to identify and appraise economic studies relevant to the decision of whether or not to screen for undiagnosed diabetes and or IGT. Three principal questions were addressed:
-
Is it a cost-effective use of resources to screen for and treat people with undiagnosed diabetes?
-
Is it a cost-effective use of resources to screen for and treat people with IGT?
-
If a screening programme were to be implemented, what screening tests and cut-off points should be used?
The review covered:
-
modelling studies that assess the long-term cost and consequences of screening for T2DM
-
models that assess the long-term costs and consequences of treating people with IGT or IFG
-
studies that consider the short-terms costs and outcomes of alternative screening tests (and cut-off points) for diabetes and IGT/IFG.
Articles cited by other relevant studies were also retrieved for review. In addition, a broader search was conducted for any economic models within the area of T2DM, to ascertain if any such models had been used to address any of the questions of interest.
The new modelling extended the existing Sheffield diabetes treatment model by developing a screening module.
The NSC criteria for evaluating population screening cover the condition, the screening test or tests, treatment and the screening programme. Screening for diabetes was therefore considered using these criteria.
Conclusions The case for screening for undiagnosed diabetes was somewhat stronger than it had been at the 2001 review, because of the greater options for reduction of CVD, principally through the use of statins, and because of the rising prevalence of obesity and, hence, T2DM. However, there was also a good case for screening for IGT, with the aim of preventing some future diabetes and reducing CVD.
Further research was needed into the duration of undiagnosed diabetes, and whether the rise in blood glucose levels is linear throughout or whether there may be a slower initial phase followed by an acceleration around the time of clinical diagnosis. This has implications for the interval after which screening would be repeated. Further research was also needed into the natural history of IGT and, in particular, what determines progression to diabetes.
Previous review of prevention of diabetes
Gillett M, Royle P, Snaith A, Scotland G, Poobalan A, Imamura M, et al. Non-pharmacological interventions to reduce the risk of diabetes in people with impaired glucose regulation; systematic review and economic evaluation. Health Technol Assess 2012;16(33).
Objective
To review the clinical effectiveness and cost-effectiveness of non-pharmacological treatments, principally diet and physical activity, for the prevention of T2DM in people with intermediate hyperglycaemia.
Methods
Clinical effectiveness
Electronic databases were searched for systematic reviews, RCTs and other relevant literature on the effectiveness of diet and/or exercise for IGT or IFG. Searches were undertaken up to October 2007. Auto-alerts were kept running, and updating searches were carried out in February 2011, with selective ones in January 2012. Some more recent studies were added to the final version.
The review of clinical effectiveness was based primarily on RCTs, which were critically appraised for internal and external validity. We also searched for recent systematic reviews and for longer-term follow-up from the RCTs.
Cost-effectiveness
The review of screening for T2DM (mentioned above) had included a review of five studies on the long-term costs and health outcomes associated with delaying or preventing diabetes in high-risk groups. Most of these studies concluded that screening and intervention would be cost-effective.
We, therefore, searched for more recent studies in order to update the previous review.
Electronic databases were searched for relevant published literature on the cost-effectiveness of diet and/or exercise for IGT or IFG, and a critical review was undertaken. We further developed the Sheffield economic model of T2DM. The model examined the cost-effectiveness of preventing or delaying T2DM in people with IGT, including the effects of interventions on CVD.
Modelling based on data from the trials may not reflect what would happen in routine care. Trials are protocol driven, and patients are supposed to stay on the treatments to which they are randomised. In normal care, if an intervention is not working then it should be stopped. We therefore created a ‘real-life’ scenario, whereby people who did not benefit from lifestyle measures (usually because they did not adhere to diet and exercise, and, in particular, did not achieve sufficient weight loss) would be switched to alternative treatment, usually metformin.
The cost to the NHS of the implementation of any recommendations on screening and intervention would depend on the extent to which those are already provided. We, therefore, used data from the General Practice Research Database (GPRD) to assess the extent to which IGT and IFG were diagnosed at present, and how they were managed. We were interested not only in interventions to reduce progression to diabetes, but also those to reduce CVD, such as statins.
Conclusion In people with IGT, dietary change to ensure weight loss, coupled with physical activity, is clinically effective and cost-effective in reducing progression to diabetes.
The Scottish Public Health Network report
The SPHN set up a working group, chaired by Professor Helen Colhoun, to review the case for screening for T2DM in Scotland. The report of the working group was to the Scottish Directors of Public Health. The SPHN is accountable to the Directors of Public Health. Its remit is to undertake prioritised national pieces of work for which there is a clearly identified need; to facilitate information exchange between all those working in public health, link with other networks and share learning; and to create effective communication among professionals and the public to allow efficient coordination of public health activity.
The summary of the SPHN report is reproduced below. The full report is available on the SPHN website. 79
On the basis of the current evidence, this Health Care Need Assessment (HCNA) has concluded that a case can, and should, be made for screening for T2DM within the context of vascular risk profiling programmes.
Recommendations on screening (based on current evidence)
-
In Scotland, screening for diabetes and NDH should be integrated into population-based vascular risk profiling programmes carried out by NHS Boards. A clear implementation plan for vascular risk profiling in Scotland is needed and the diabetes screening element should be included in that plan.
-
All of those being profiled for CVD risk should have HbA1c level measured.
-
The upper age limit of such screening would be set by the upper age limit for the vascular profiling programme as a whole.
-
HbA1c testing should be used as the preferred screening test for diabetes and NDH. The best alternative when this is not suitable is FPG testing. Random blood glucose is not recommended for screening for diabetes and the Scottish Intercollegiate Guidelines Network (SIGN) 97 guideline163 should be updated accordingly. Random glucose of ≥ 11.1 mmol/l remains a satisfactory way of confirming a clinical diagnosis in a symptomatic patient.
-
Those patients known to have clinical conditions that interfere with the validity of HbA1c testing should be screened by fasting glucose instead.
-
In those people with an initial HbA1c value of < 6% (42 mmol/mol), screening with HbA1c should be repeated every 3 years. However, earlier repetition of HbA1c testing may be warranted for individuals with significant risk factors such as family history of diabetes and obesity.
-
In those individuals with an initial HbA1c level of ≥ 6% (42 mmol/mol) a subsequent visit should be arranged to assess who has diabetes and who has non-diabetic hyperglycaemia. At this subsequent visit, both fasting glucose and HbA1c level should be measured.
-
Asymptomatic individuals with an initial HbA1c value of ≥ 6.5% (48 mmol/mol) should be diagnosed with diabetes if this repeat HbA1c value is also ≥ 6.5%.
Appendix 2 Cambridge Risk Score
-
Based on data routinely available from the UK general practices.
-
Makes use of combination of six characteristics for generating the risk scores: age, sex, family history of diabetes, smoking status, prescription of steroid or antihypertensive medication and BMI.
-
Validated in the Danish population.
-
Absence of family history and smoking would have only modest effect on CRS.
-
The CRS is a simple, yet effective, tool for the detection of T2DM and could be used for identification for the detection of high-risk individuals with symptoms of diabetes.
Appendix 3 Protocol
Screening for type 2 diabetes: updating review for National Screening Committee
HTA project number 2011/18.
Project lead:
Professor Norman Waugh
Warwick Evidence
Health Sciences Research Institute
Gibbett Hill Campus
University of Warwick
Coventry CV4 7AL
Background
The prevalence of type 2 diabetes has been increasing, linked to trends in overweight and obesity, to other lifestyle changes such as reduced physical activity, and to an ageing population. About 4% of the UK population is known to have diabetes.
However, it is estimated that around another 0.5% or more have undiagnosed diabetes (YHPHO model). Type 2 diabetes (T2DM) can have an insidious onset with few or no symptoms. By the time of diagnosis, people with T2DM may already have damage to eyes (retinopathy) or to kidneys (nephropathy). More seriously, they may have accelerated arterial disease, most notably coronary artery disease. Diabetes increases the risk of arterial disease. They may have myocardial infarction (heart attacks) with no prior warning, and some of these will be fatal.
In addition to diabetes, there are two other conditions which are characterised by hyperglycaemia;
-
Impaired glucose tolerance (IGT) wherein blood glucose levels are raised, though not to diabetic levels, after meals. IGT is usually diagnosed by means of a oral glucose tolerance test (OGTT)
-
Impaired fasting glucose
Both may progress to diabetes. IGT is also important because it increases the risk of arterial disease.
There is therefore a case for screening for type 2 diabetes. The last HTA review of screening (Waugh et al.) noted that not all the NSC criteria were met, but that the case for screening was becoming stronger.
Current and planned screening services for diabetes
One effect of devolution is that the four UK territories have different policies for screening. Early in the review, we will contact each of the health departments to ascertain their current plans.
In England, screening for diabetes would be done, in those over 40, as part of the vascular risk programme. This will involve face to face contact. It appears that screening would be universal.
In Scotland, there are no plans to introduce a vascular risk screening programme. Instead, the Scottish Executive is considering using questionnaires on lifestyle and health, possibly via NHS 24. This provides an opportunity for selective screening based on questionnaire data, possibly akin to the Finnish system.
Wales – plans to be ascertained.
Northern Ireland – plans to be ascertained.
Previous reports
These include:
-
HTA report on screening for type 2 diabetes, published 2007, written 2006.
That report noted that if screening for T2DM were to be introduced, there would, depending on test used and thresholds chosen, be more people detected with IGT or IFG than with diabetes. The HTA Programme therefore commissioned a complementary review of non-pharmacological prevention of type 2 diabetes in people with IGT or IFG. (In editorial process, due for submission of revised report in August 2011).
More recently, the SPHN has produced a report on screening for type 2 diabetes (not yet published but will be on SPHN website in due course).
An economic assessment of screening options by Gillies and colleagues was published in 2008 (BMJ). It concluded that screening for both diabetes and IGT would be cost-effective, but that “The cost-effectiveness of a policy of screening for diabetes alone, which offered no intervention for those with impaired glucose tolerance, is still uncertain.”
Current questions
The issues to be addressed in this review are:
-
Should screening for type 2 diabetes be universal at age, say, 40 years and over, or should only people at higher risk be screened?
-
If screening were to be selective, how should risk be assessed? There are various risk scores, all of which involve age and BMI. Some can be derived from data held on GP computer systems, but others require questionnaires to be sent or administered to patients. The question here is how much risk scores improve detection over a simple age and BMI approach.
-
Should the approach to screening vary with ethnicity? The prevalence of T2DM is higher those with South Asian ethnicity (meaning India, Pakistan, Bangladesh, Sri Lanka, Nepal). Should they be screened at younger ages or at lower BMIs?
-
Which test of blood glucose should be used? Glycated haemoglobin, or HbA1c, is now being used for diagnosing diabetes. One issue here is whether uptake of screening varies amongst tests. The most sensitive test may not be the best option if uptake is low. Another important issue is that different screening tests detect over-lapping but different groups, with different risk of cardiovascular disease. Since the main aim of screening is to reduce CVD, the choice of test is important. It may be that detection of cardiovascular risk should take precedence over detection of diabetes.
-
Should screening be just for diabetes, or for IGT as well?
Structure of report
Chapter 1 will review the background and summarise past reports.
Chapter 2 will review methods for selection based on risk scoring, including a systematic review of different scoring systems.
Chapter 3 will review the tests for blood glucose, including sensitivity and specificity. It will also review the evidence on acceptability and uptake.
Chapter 4 will consider the issues around vascular risk and their implications for the screening programme.
Chapter 5 will review recent studies of the economics of screening, and may include de novo modelling.
Chapter 6 will summarise the options for NSC, and will identify research needs.
Methods
The review will be done using the usual methods:
-
Systematic searches of MEDLINE, EMBASE and all sections of the Cochrane Library for reviews and primary studies of both clinical and cost-effectiveness, and of current trial databases for ongoing research.
-
Existing guidelines of screening from the UK and other relevant countries will be summarised. If recommendations differ, reasons for differences will be explored.
-
Lists of retrieved abstracts will be checked by two reviewers with full papers obtained in cases in doubt.
-
Studies will be quality assessed by appropriate methods as per CRD 4 or NICE.
-
Data will be extracted by one researcher and checked by another using predefined data extraction forms.
-
Data from screening studies will be used to produce sensitivity, specificity, PPV and NPV, if sufficient data are given to construct 2 × 2 tables. This will apply to the first stage of screening (risk scoring) and to the second stage (blood glucose).
-
The case for screening will be assessed against the NSC criteria.
8th August 2011.
List of abbreviations
- ABCD
- Association of British Clinical Diabetologists
- ACCORD
- Action to Control Cardiovascular Risk in Diabetes
- ACE
- angiotensin-converting enzyme
- ADA
- American Diabetes Association
- ADDITION
- Anglo-Danish-Dutch Study in General Practice of Intensive Treatment and Complication Prevention in Type 2 Diabetic Patients Identified by Screening
- ADVANCE
- Action in Diabetes and Vascular disease: preterAx and diamicroN mr Controlled Evaluation
- AGE
- advanced glycation end-product
- ARIC
- Atherosclerosis Risk in Communities Study
- AUC
- area under curve
- AUROC
- area under receiver operating characteristic
- AusDiab
- Australian Diabetes, Obesity and Lifestyle
- BMI
- body mass index
- BP
- blood pressure
- CHD
- coronary health disease
- CI
- confidence interval
- CRS
- Cambridge Risk Score
- CVD
- cardiovascular disease
- DALY
- disability-adjusted life-year
- DARTS
- Diabetes Audit and Research in Tayside Scotland
- DBP
- diastolic blood pressure
- DCCT
- Diabetes Control and Complications Trial
- DECODE
- Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe
- DPP
- Diabetes Prevention Program
- DPS
- Diabetes Prevention Study
- EASD
- European Association for the Study of Diabetes
- ELSA
- English Longitudinal Study of Ageing
- EPIC
- European Prospective Investigation into Cancer and Nutrition
- FBG
- fasting blood glucose
- FINDRISC
- The Finnish Diabetes Risk Score
- FPG
- fasting plasma glucose
- GCT
- glucose challenge test
- GDM
- gestational diabetes mellitus
- GP
- general practitioner
- GTT
- glucose tolerance test
- HbA1c
- glycated haemoglobin
- HDL
- high-density lipoprotein
- HOMA
- homeostasis model analysis
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IDF
- International Diabetes Federation
- IFG
- impaired fasting glucose
- IGR
- impaired glucose regulation
- IGT
- impaired glucose tolerance
- IHD
- ischaemic heart disease
- IQR
- interquartile range
- IRAS
- Insulin Resistance Atherosclerosis Study
- LDL
- low-density lipoprotein
- LEADER
- Leicester Ethnic Atherosclerosis and Diabetes Risk
- MeSH
- medical subject heading
- MI
- myocardial infarction
- MRC
- Medical Research Council
- NAO
- National Audit Office
- NDH
- non-diabetic hyperglycaemia
- NGT
- normal glucose tolerance
- NHANES
- National Health and Nutrition Examination Survey
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NNS
- number needed to screen
- NSC
- National Screening Committee
- OGTT
- oral glucose tolerance test
- OR
- odds ratio
- PCT
- primary care trust
- PG
- plasma glucose
- PVD
- peripheral vascular disease
- QA
- quality assurance
- QALY
- quality-adjusted life-year
- RBC
- red blood cell
- RBG
- random blood glucose
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- RR
- relative risk
- SAGE
- skin advanced glycation end-product
- SBP
- systolic blood pressure
- ScHARR
- School of Health and Related Research
- SD
- standard deviation
- SHI
- statutory health insurance
- SIGT
- Screening for Impaired Glucose Tolerance
- SPHN
- Scottish Public Health Network
- STAR
- Screening Those At Risk
- T2DM
- type 2 diabetes mellitus
- UKPDS
- UK Prospective Diabetes Study
- VADT
- Veterans Affairs Diabetes Trial
- WHO
- World Health Organization
- YHPHO
- York and Humber Public Health Observatory
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.