Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/130/01. The contractual start date was in July 2011. The draft report began editorial review in March 2012 and was accepted for publication in September 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Professor Khalid Khan has participated in research projects where pharmaceuticals (e.g. Ferring Pharmaceuticals) have contributed a grant. In addition, Professor Khalid Khan has received honoraria and has had travel/accommodation expenses covered/reimbursed for speaking at meetings from pharmaceuticals (e.g. Ferring Pharmaceuticals) and from various universities and societies interested in this research topic.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Deshpande et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
The World Health Organization (WHO) defines a preterm birth (PTB) as birth of an infant before 37 completed weeks of gestation. 1 In the UK, spontaneous PTB occurs in 7–12% of pregnancies before 37 weeks' gestation and in about 4% of pregnancies before the completion of 34 weeks' gestation. 2–6 According to the UK Office for National Statistics, in 2004, 1 in 13 live births in England and Wales was preterm. 7 The incidence of PTBs before 37 weeks' gestation has been reported to be greater in multiple pregnancies (61.9%) than in singleton pregnancies (11.1%). 4 In the majority of developed countries, PTB is one of the major causes of neonatal mortality and severe morbidities. 1 PTBs account for about 60–80% of the neonatal mortality and about 75% of severe morbidities. 8,9 These severe morbidities can cause significant psychological, sociological and financial burdens on parents and carers. 10
Recent developments in perinatal health care have not significantly reduced the incidence of spontaneous preterm labour. 4 However, timely intervention (e.g. the use of antenatal steroids) can significantly reduce the rate of neonatal mortality and morbidities in symptomatic women. 11 Antenatal corticosteroids are most effective in the infants who are delivered between 2 and 7 days after the administration of the drugs. 11 To maximise the effectiveness of antenatal steroid therapy and to plan other necessary management strategies (e.g. in utero transfer to neonatal intensive care facilities), it is therefore important to determine the likelihood of a PTB at an early stage after the appearance of signs and symptoms.
The inclusion of fetal fibronectin (fFN) testing in the diagnostic workup may help to predict which women displaying symptoms of premature labour will progress to preterm delivery and which do not require active intervention. fFN can be detected in cervicovaginal secretions in early pregnancy and just before birth; it is released into the cervix or vagina because of the mechanical damage caused to the fetal membrane before the onset of birth. However, in the normal course of pregnancy it is unusual to detect fFN between 22 and 37 weeks' gestational age. 12 Hence, the detection of elevated levels of fFN in cervicovaginal secretion between 22 and 37 weeks' gestation can be considered an indicator of preterm labour in symptomatic women. 13
The purpose of this project was to assess the clinical effectiveness and cost-effectiveness of adding fFN to conventional management, compared with conventional management alone, in women with symptoms of premature labour. The conventional methods of managing preterm labour in symptomatic women include hospitalisation for longer periods, antenatal steroid therapy and occasional in utero transfer. 14 However, only about 20% of women admitted for suspected preterm labour will actually progress to deliver the baby prematurely. The remaining 80% of admissions have normal delivery after 37 weeks' gestation; this means that there are many unnecessary and costly inpatient admissions and treatments for suspected preterm labour. 15 The addition of fFN testing to the diagnostic workup of women with suspected preterm labour may help to identify those 20% of women who require active management, and thus avoid unnecessary interventions, hospitalisations and associated costs.
Intervention
Fetal fibronectin is an extracellular matrix glycoprotein produced by amniocytes and by cytotrophoblast. 1 It is thought to be present mainly in the choriodecidual interface, which is a union between maternal and fetal tissues. 6 Normally, fFN is present in the cervicovaginal secretions of pregnant women until 22 weeks' gestation. However, the level of fFN in cervicovaginal secretions drops after 22 weeks' gestation (< 50 ng/ml). If the pregnancy is not normal, the level of fFN found in a cervicovaginal swab may be high (≥ 50 ng/ml) at or after 22 weeks' gestation; elevated levels of fFN may indicate early onset of labour. 1
The test is available in two formats: a quantitative solid-phase enzyme-linked immunosorbent assay (ELISA) or a qualitative membrane immunosorbent assay [rapid fFN for the TLi™ System (Adeza Biomedical, Sunnyvale, CA, USA), which has recently been renamed FullTerm™]. 16–18 Rapid fFN testing offers a more practical approach, as it gives the results instantly (30 minutes), unlike the laboratory-based ELISA which delivers the results 4–48 hours after sample collection. 17 This assessment, therefore, focuses on rapid fFN testing.
The FullTerm™ rapid fFN test is a lateral-flow, solid-phase immunosorbent assay designed to perform a qualitative detection (positive/negative) of fFN in cervicovaginal specimens collected in the Adeza Biomedical Collection Kit (Adeza Biomedical, Sunnyvale, CA, USA). 17 The cervicovaginal specimen (vaginal swab) is mixed with a liquid buffer in a collection tube, and a portion of this sample is pipetted to the lateral-flow, rapid fFN cassette in the TLi™ IQ Analyser. 17 The assay takes about approximately 30 minutes to process the sample and deliver the results. The TLi™ automatically prints and displays positive or negative results along with patient details (an fFN level of ≥ 50 ng/ml is positive result and an fFN level of < 50 ng/ml is negative result). 17
The intervention considered in this review is rapid fFN testing in addition to usual care.
Population
Data from England and Wales suggest that the estimated number of spontaneous PTBs before 37 weeks' gestation was 76,000 in 2004. 7 The majority of neonatal deaths occur in infants born before 34 weeks' gestation; surviving babies tend to suffer from serious morbidities such as bronchopulmonary dysplasia, respiratory distress syndrome (RDS), necrotising enterocolitis, intraventricular haemorrhage, retrolental fibroplasia, sepsis and long-term cognitive difficulties. 1,6 In addition, some premature infants who are classified as normal with respect to their development, or who have mild abnormalities, can have multiple health problems later in life. 10 PTBs not only affect the infant and family but also increase NHS resource use (e.g. longer hospital stays, or use of neonatal intensive care services). 19
The pathogenesis of preterm labour is unknown, but there are several risk factors which are believed to be predictive of PTB (e.g. non-white ethnicity, smoking, young/old maternal age, multiple pregnancy, stress, infection, low socioeconomic status and history of previous PTB). 20,21 Multiple pregnancies are more likely to be at risk of preterm labour than singleton pregnancies. In developed countries the incidence of multiple pregnancies has increased in the last 20–30 years, mainly because of advanced reproductive techniques such as drugs used to induce ovulation and in vitro fertilisation. 22 Most studies on fFN testing exclude women with multiple pregnancies because of the associated complications; however, in this review both singleton and multiple pregnancies will be considered.
This assessment will consider the population of women with singleton or multiple pregnancies displaying symptoms of labour before completing the 37-week gestational period (preterm labour). The clinical signs and symptoms that indicate onset of preterm labour are uterine contractions, low abdominal pain, dull backache, pelvic pressure, change in volume or consistency of vaginal discharge, and menstrual-like or intestinal cramping. 19,20,23 A further important sign of preterm labour is cervical effacement (80%) and dilation (< 3 cm).
Comparator (usual care)
Currently, the diagnosis of preterm labour is based mainly on signs and symptoms, clinical history and physical examination of the patient. Physical examination of the cervix indicating dilation of ≥ 3 cm and at least 80% effacement is indicative of the onset of preterm labour within 24 hours to 7 days. 17 If physical examination suggests that a woman is likely to experience preterm labour, treatment with tocolytic agents can be instituted with the aim of postponing delivery. However, in some cases, this is not possible and preparations have to be made for a preterm delivery. Clinicians need to take a number of key decisions before preparing for a preterm delivery (e.g. use of maternal intramuscular corticosteroid injection to facilitate fetal lung development and prevent RDS). 10 Antenatal corticosteroids are most effective in the infants who are delivered between 2 and 7 days after the administration of the drugs. 11 It is also important to check for the availability of neonatal intensive care unit space before in utero transfers. The arrangements for in utero transfers may take some time because of geographical constrains or long waiting periods. 24 Thus, considering the time required for the corticosteroid drugs to show maximum effectiveness (2–7 days) as well as the time required for making in utero transfer arrangements, it is very important for the clinicians to have advance timely knowledge of likely PTB in symptomatic women.
Where physical examination does not confirm preterm labour, symptomatic women are usually hospitalised under observation for longer periods to assess if the symptoms are subsiding or increasing. 20,25,26 During this period of hospitalisation, complete bed rest is suggested and clinicians may administer tocolytic drugs or antibiotics as required. The main concern for clinical assessment based on symptoms is that it is very unreliable, and leads to overdiagnosis of preterm labour. 27 The overdiagnosis of preterm labour incurs unnecessary hospitalisation, unnecessary interventions and wastage of resources; there is, therefore, a need for improved assessment.
Current evidence
A number of systematic reviews have previously evaluated the accuracy of the fFN testing. Honest et al. 10 conducted a Health Technology Assessment (HTA) review of screening to prevent spontaneous PTB in symptomatic and asymptomatic women. Honest et al. 10 evaluated several screening tests, including the rapid fFN test, which can be used to predict spontaneous PTB as well as interventions to prevent PTB. The accuracy of rapid fFN in symptomatic women for predicting PTB for the reference standards outcomes was as follows: within 7–10 days testing, the range of likelihood ratio for positive test result (LR+) was from 2.12 [95% confidence interval (CI) 1.05 to 4.28] to 9.29 (95% CI 5.06 to 17.06) with a summary LR+ of 4.10 95% CI 3.37 to 4.98) (chi-squared heterogeneity test, p = 0.00) and the range of likelihood ratio for negative test result (LR–) from 0.09 (95% CI 0.01 to 0.58) to 0.59 (95% CI 0.25 to 1.39) with a summary LR– of 0.35 (95% CI 0.27 to 0.46) (chi-squared heterogeneity test, p = 0.322);10 for predicting spontaneous PTB before 34 weeks' gestation, the range of LR+ was from 1.57 (95% CI 0.53 to 4.60) to 5.70 (95% CI 2.88 to 11.28) with a summary LR+ of 3.58 (95% CI 2.56 to 5.00) (chi-squared heterogeneity test, p = 0.05), and the range of LR– from 0.12 (95% CI 0.02 to 0.79) to 0.91 (95% CI 0.69 to 1.20) with summary LR– of 0.34 (95% CI 0.17 to 0.68) (chi-squared heterogeneity test, p = 0.00);10 for predicting spontaneous PTB before 37 weeks' gestation, the range of LR+ was from 1.00 (95% CI 0.44 to 2.30) to 14.36 (95% CI 5.81 to 35.47) with summary LR+ of 3.62 (95% CI 3.02 to 4.33) (chi-squared heterogeneity test, p = 0.00), and the range of LR– from 0.08 (95% CI 0.01 to 0.54) to 1.00 (95% 0.44 to 2.30) with a summary LR– of 0.50 (95% CI 0.43 to 0.59) (chi-squared heterogeneity test, p = 0.00). 10
A recent systematic review, exclusively evaluating the accuracy of fFN testing to predict the PTB in women with multiple pregnancies, concluded that fFN testing may be most accurate in predicting the spontaneous PTB within 7 days of testing (pooled sensitivity, specificity, and positive and negative likelihood ratios of 85%, 78%, 3.9 and 0.2, respectively) in women with twin pregnancies. 22 Similarly, an earlier review by Honest et al. 6 evaluated the accuracy of fFN testing in predicting spontaneous preterm labour and concluded that fFN testing is most accurate in predicting spontaneous PTB within 7–10 days of testing among symptomatic women. 6 This review evaluated the accuracy of 30 studies with quantitative solid-phase ELISA test and 11 studies using bedside testing. However, a metaregression analysis was carried out showing that the accuracy of test did not depend on method of testing. A systematic review by Sanchez-Ramos et al. ,28 in contrast to the studies detailed above, concluded that fFN has limited accuracy in predicting PTB within 7 days of sampling in symptomatic pregnant women.
Three previous systematic reviews have explored aspects of the clinical effectiveness of fFN testing other than accuracy for predicting PTB. The first study was carried out in Australia by the Medical Services Advisory Committee and determined the test to be safe but it did not determine the effectiveness in symptomatic preterm labour. 29 This review identified 41 studies: nine systematic reviews and 32 primary diagnostic accuracy studies. The results indicated that a negative fibronectin test result, in women with suspected preterm labour, provides moderate diagnostic value to assess preterm delivery risk within 7 or 14 days of testing. The second study was carried out by the Institute of Health Economics in Canada and did not include any accuracy studies, but concluded by supporting the previous findings that the rapid fFN test can be used to identify those symptomatic women who are at lower risk of preterm delivery, based on its higher negative predictive values. 17 A third systematic review explored the study designs used in randomised controlled trials (RCTs) of the clinical effectiveness of fFN testing with the aim of identifying possible reasons why they have failed to demonstrate benefits. 30 No previous systematic review identified has attempted to synthesise evidence from both RCTs assessing the clinical effectiveness of fFN testing and studies reporting the diagnostic accuracy of fFN testing for the prediction of PTB.
The report by Honest et al. 10 modelled four test–treat options to assess the relative cost-effectiveness of multiple tests and multiple treatments: (1) test no one and treat all; (2) test all and treat no one; (3) test all and treat only those with positive test; and (4) test all and treat all. Analyses were performed for both symptomatic and asymptomatic women. For the symptomatic women, fibronectin testing was either dominated or not considered in most analyses. In one analysis though (symptomatic women at 37 weeks), testing for fFN followed by indomethacin for those who tested positive was the least costly strategy. However, indomethacin for all without previous testing was the most cost-effective test and treat option in this group, at an incremental cost-effectiveness ratio (ICER) of £16,336 compared with fFN-testing and treating positives. Therefore, overall, fFN testing was not considered the preferred strategy from an economic perspective in any of the analyses.
Given the current evidence base and clinical imperative for rapid information, a rigorous, up-to-date evaluation of the clinical effectiveness and cost-effectiveness of rapid fFN testing to predict PTB in symptomatic women is needed. Some countries (Australia and Canada) have already assessed rapid fFN testing with respect to their health-care settings. However, to date, no similar assessment has been carried out for the UK setting; the current assessment will evaluate the clinical effectiveness and cost-effectiveness of fFN testing in suspected premature labour in the UK.
Chapter 2 Definition of decision problems
Aims and objectives
Aim
The aim of this project was to assess the impact of including fFN testing in the assessment of women with symptoms of preterm labour on NHS resource use and to propose possible changes in maternal management.
Objectives
-
To assess the clinical effectiveness and accuracy of the fFN test (commercial rapid test kit) in predicting PTB in symptomatic women.
-
To assess, from an NHS perspective, the cost-effectiveness of the use of fibronectin (rapid fFN testing) in the assessment of women with symptoms of threatened preterm labour, in comparison with no testing (current usual care).
The scope of this assessment did not include an evaluation of the effectiveness of treatment interventions to prevent PTB.
Chapter 3 Assessment of clinical effectiveness and test accuracy
Inclusion criteria
Population
Studies including pregnant women with singleton or twin gestations who have signs and symptoms of preterm labour (e.g. uterine contractions, dull backache, pelvic pressure, change in volume or consistency of vaginal discharge, and menstrual-like or intestinal cramping) before 37 weeks' gestation.
Setting
Secondary care.
Intervention
Studies assessing swab testing for fFN using a commercial rapid test kit before 37 weeks' gestation plus usual care, for the management of women with symptoms of preterm labour. Studies using rapid fFN test in participants after 37 weeks' gestation or studies assessing fFN for detecting any other risks than PTB were excluded from this review.
Comparator (clinical effectiveness studies only)
Usual care, without fibronectin testing, for managing PTB.
Reference standard (for test accuracy studies only)
Spontaneous PTBs which occur before 37 weeks' gestation, before 34 weeks' gestation, or within 7–10 days of testing.
Outcomes
-
Incidence of spontaneous PTB before 37 weeks' gestation, before 34 weeks' gestation, or within 24 hours, 48 hours, or 7–10 days of testing (time required for corticosteroids to exert beneficial effects and the potential for in utero transfer and tocolytic administration) – primary outcome measure.
-
Changes in maternal management.
-
admission to hospital
-
use of corticosteroids
-
changes in frequency of monitoring
-
changes from usual care.
-
-
Outcomes in the newborn, morbidity, mortality.
-
Outcomes of maternal health.
-
Diagnostic accuracy of the test.
-
Cost-effectiveness.
Study design
-
Randomised trials in which participants are assigned to the intervention group or comparator group, and which report patient-relevant outcomes (changes to maternal management, maternal health outcomes, newborn morbidity and mortality) and/or incidence of PTB (before 37 weeks).
-
Diagnostic cohort studies, published since the completion of the searches for the previous systematic review by Honest et al. ,10 in order to provide an updated estimate of test accuracy.
Included test accuracy studies were required to report sufficient data to construct 2 × 2 contingency tables [i.e. numbers of true-positive (TP), false-negative (FN), false-positive (FP) and true-negative (TN) test results].
The following study/publication types were excluded:
-
studies with < 10 participants
-
pre-clinical and animal studies
-
reviews, editorials, and opinion pieces
-
case reports and diagnostic case–control studies.
Search strategy
Search strategies were based on target condition and intervention, as recommended in the Centre for Reviews and Dissemination (CRD) guidance for undertaking reviews in health care and the Cochrane Handbook for diagnostic test accuracy reviews. 31–33
Literature searches were undertaken for eligible studies and evidence-based HTAs, systematic reviews and economic evaluations. Searches were not limited by language or publication status (unpublished or published). The MEDLINE strategy was independently peer reviewed by a second Information Specialist, using the Peer Review of Electronic Search Strategies (PRESS-EBC) checklist. 34
Clinical effectiveness
The clinical effectiveness searching was undertaken in two stages. In the first stage, RCTs and systematic reviews filters were applied to identify effectiveness studies. In the second stage, these filters were removed to allow identification of accuracy studies.
Effectiveness studies
These searches were an update of Honest et al. 6 and were limited by date from 2000 to September 2011. The following databases were searched for relevant studies:
-
MEDLINE (OvidSP): 2000 to September week 1 2011
-
MEDLINE In-Process & Other Non-Indexed Citations and Daily Update (OvidSP): 2000 to 15 September 2011
-
EMBASE (OvidSP): 2000 to week 36 2011
-
Cochrane Database of Systematic Reviews (CDSR) (Wiley): 2000 to Issue 9 2011
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley): 2000 to Issue 3 2011
-
Database of Abstracts of Reviews of Effects (DARE) (Wiley): 2000 to Issue 3 2011
-
HTA Database (Wiley): 2000 to Issue 3 2011
-
Science Citation Index (SCI) (Web of Knowledge): 2000 to 19 September 2011
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost): 2000 to 9 September 2011
-
Maternity and Infant Care (OvidSP): 2000 to August 2011
-
National Institutes of Health (NIH) ClinicalTrials.gov (URL: www.clinicaltrials.gov/): 2000 to 19 September 2011
-
Current Controlled Trials (URL: www.controlled-trials.com/): up to 19 September 2011
-
WHO International Clinical Trials Registry Platform (ICTRP) (URL: www.who.int/ictrp/en/): up to 19 September 2011
-
EU Clinical Trials Register (EUCTR) (URL: www.clinicaltrialsregister.eu/): up to 19 September 2011.
Accuracy studies
These searches were an update of Honest et al. 10,35 and were limited by date from 2005 to November 2011. Search strategies differed from those used by Honest et al. in that they did not include methodological terms for test accuracy studies.
The following databases were searched for relevant studies:
-
MEDLINE (OvidSP): 2005 to November week 3 2011
-
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP) 2005 to 28 November 2011
-
MEDLINE Daily Update (OvidSP): 2005 to 16 November 2011
-
EMBASE (OvidSP): 2005 to week 47 2011
-
Maternity and Infant Care (OvidSP): 2005 to November 2011
-
CDSR (Wiley): 2005 to Issue 11 2011
-
CENTRAL (Wiley): 2005 to Issue 4 2011
-
DARE (Wiley): 2005 to Issue 4 2011
-
HTA Database (Wiley): 2005 to Issue 4 2011
-
CINAHL (EBSCOhost): 2005 to 29 November 2011
-
SCI (Web of Knowledge): 2005 to 29 November 2011
-
NIH ClinicalTrials.gov (URL: www.clinicaltrials.gov/): 2005 to 29 November 2011.
Identified references were downloaded in EndNote X5 software (Thomson Reuters, CA, USA) for further assessment and handling.
The bibliographies of retrieved articles and relevant systematic reviews were checked for additional studies.
Full search strategies are reported in Appendix 1.
Inclusion screening and data extraction
Two reviewers independently screened titles and abstracts of all reports identified by searches and discrepancies were discussed. Full copies of all studies deemed potentially relevant, after discussion, were obtained and two reviewers independently assessed these for inclusion; any disagreements were resolved by consensus or discussion with a third reviewer.
Data relating to study details, participants, intervention, comparator tests or reference standard outcome (preterm delivery at various gestational ages and times from testing) for accuracy studies only and outcome measures and results were extracted by one reviewer, using a piloted, standard data extraction form. A second reviewer checked the data extraction and any disagreements were resolved by consensus or discussion with a third reviewer. Non-English-language articles were extracted by a native speaker, where available and limited data were extracted from the English-language abstract of one Turkish and one Italian publication.
Quality assessment
The methodological quality of included studies was assessed using standard tools. The Cochrane risk of bias tool was used to assess the quality of the included clinical effectiveness studies (RCTs). The evidence-based QUADAS tool is recommended for assessing the methodological quality of test accuracy studies. 31,36–39 A revised version of QUADAS (QUADAS-2) has recently been published. 40 QUADAS-2 more closely resembles the approach and structure of the Cochrane risk of bias tool. It is structured into four key domains covering participant selection, index test, reference standard, and the flow of patients through the study (including timing of tests). Each domain is rated for risk of bias (low, high, or unclear) and the tool provides signalling questions, in each domain, to aid reviewers in reaching a judgement. The participant selection, index test and reference standard domains are also, separately rated for concerns regarding the applicability of the study to the review question (low, high, or unclear). Thus, QUADAS-2 separates bias from external validity (applicability) and does not include any items which only assess reporting quality. A modified version of the QUADAS-2 tool was used in this assessment.
The version of QUADAS-2 used in this assessment included only the risk of bias components, as it was considered that the inclusion criteria matched the review question and that questions of applicability were, therefore, not relevant. The reference standard was the occurrence of PTB in all studies; we therefore considered that there were no issues of bias relating to the adequacy or application of the reference standard and the ‘reference standard’ domain of QUADAS-2 was omitted. Review-specific guidance was produced for the use of the modified version of QUADAS-2 in this assessment and is reported in Appendix 5.
The results of the quality assessment are summarised and presented in tables and graphs in the results of the systematic review (see Clinical effectiveness) and are presented in full, by study, in Appendices 3 and 6. The results of the quality assessment were also used to inform recommendations for future research.
All data extraction and quality assessment conducted for the update review of test accuracy was undertaken with consideration to consistency with the previous systematic review by Honest et al. 10
Methods of analysis/synthesis
The results of clinical effectiveness studies (RCTs) were summarised by outcome measure (e.g. incidence of PTB, incidence of hospital admissions, and administration of treatment). Individual study results were summarised in text and tables and, where appropriate, were illustrated using forest plots. Where three or more studies reported the same outcome, a DerSimonian and Laird random-effects model was used to generate pooled estimates of risk ratio (RR), with 95% CIs, for dichotomous outcomes (e.g. number of hospitalisations) and weighted mean difference, with 95% CIs, for continuous outcomes (e.g. gestational age at delivery). 41 Between study heterogeneity was assessed using the chi-squared test and inconsistency was quantified using the I2 statistic. 42 If clinical heterogeneity was apparent then the statistical heterogeneity was not quantified.
Test accuracy studies were grouped by reference standard outcome (delivery at < 37 weeks' gestation, < 34 weeks' gestation and within 7–10 days of testing); studies reporting delivery at < 38 weeks were grouped with the < 37 weeks outcome, and those reporting delivery at < 35 weeks were grouped with the < 34 weeks outcome. Absolute numbers of TP, FN, FP, and TN test results, as well as sensitivity and specificity values, with 95% CIs, were presented for each study and reference standard outcome reported. Pooled estimates of test performance were calculated by combining data extracted from studies included in this assessment with individual study results (numbers of TP, FN, FP, and TN test results) taken from the previous HTA by Honest et al. 10 Data taken from the previous HTA are reported in Appendix 7. Where groups of similar studies (same patient group and unit of analysis) included four or more data sets, summary estimates of sensitivity and specificity, with 95% CIs were calculated using the bivariate modelling approach; four data sets are the minimum requirement to fit models of this type. Analyses were conducted in Stata 10 (StataCorp LP, College Station, IL, USA), using the ‘metandi’ function. 31,43,44 Between-study heterogeneity was assessed using the chi-squared test and inconsistency was quantified using the I2 statistic. 42 Sensitivity analyses were undertaken to investigate the effect on accuracy estimates of excluding studies which used delivery at < 38 weeks' gestation or delivery at < 35 weeks' gestation as the reference standard from the analyses. The potential for exploration of possible sources of heterogeneity was limited by the numbers of studies available for each reference standard outcome and by the study details reported in the previous review. 10 Subgroup analyses were conducted for inclusion criteria (studies which excluded patients with multiple gestations vs. studies with mixed or unspecified populations) and for publication date (studies included in the earlier systematic review vs. studies identified by our update searches). A simple, exploratory regression analysis was also undertaken, using the summary receiver operating characteristic (SROC) model of Moses et al. extended to include the above factors and prospective, consecutive recruitment of participants compared with other study designs as independent variables; the dependent variable in this model is log- diagnostic odds ratio (DOR). 45,46 Initial univariate analyses showed no significant associations with log-DOR at the 10% level, therefore, no multivariate modelling was undertaken. This analysis was for exploratory purposes only and results are not reported.
A detailed commentary on the major methodological problems or biases that affected the studies was also included, together with a description of how this may have affected the individual study results.
Results
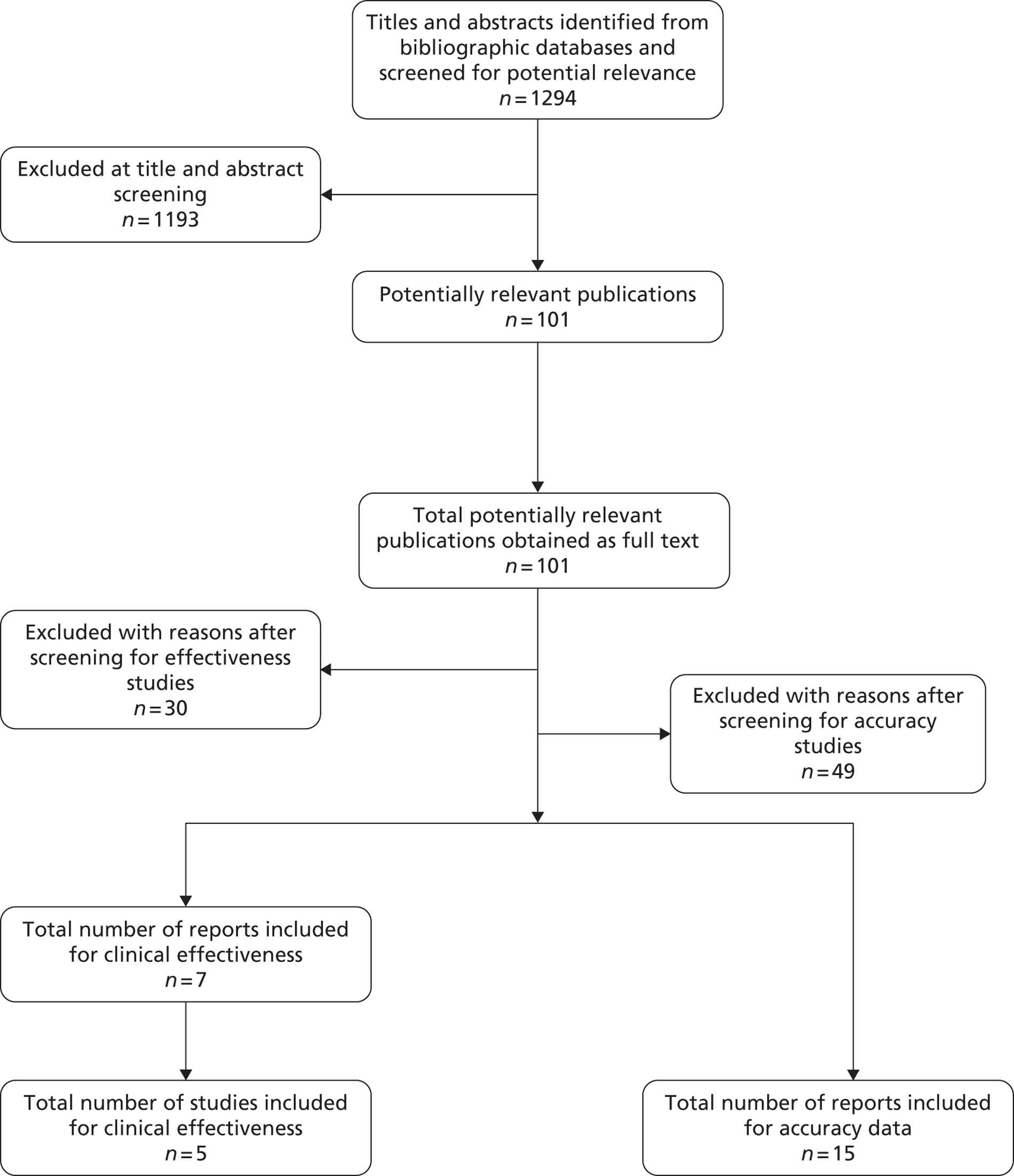
The literature searches of the bibliographic databases identified 1294 references. After initial screening of titles and abstracts, 101 were considered potentially relevant and ordered for full paper screening. Figure 1 shows the flow of studies through the review process, and Appendix 9 provides details, with reasons for exclusion, of all publications excluded at the full-paper screening stage.
Based on the searches and inclusion screening described above, 22 publications of 20 studies were included in the review; five of the included studies (seven publications47–53) were RCTs assessing the clinical effectiveness of fFN testing (changes to patient management and/or outcomes), and 15 were diagnostic test accuracy (DTA) studies. 54–68
FIGURE 1.
Flow of studies through the review process for studies.
Clinical effectiveness
Of the five included studies, four studies were published in full,47–50 whereas the remaining study was only published as a conference abstract. 51 Two studies (Lowe et al. 48 and Grobman et al. 50) were published as both full reports and conference abstracts;52,53 for these studies data extraction was based on the full reports. All the included studies were RCTs published 2002 or later. Four studies determined the impact of fFN testing on the maternal management. 47–51 Grobman et al. 50 also determined health care costs. Three studies were conducted in USA,48–50 one study was conducted in Portugal51 and Dutta and Norman47 was conducted in Scotland. Two studies were funded by Adeza Biomedical Corporation (Sunnyvale, CA, USA), which is the manufacturer of the fFN testing assay. 49,50 Participant recruitment was over a period of 1–2 years in all cases. An overview of the study design, objectives and outcomes reported by all studies is provided in Table 1. Further details of the inclusion/exclusion criteria, characteristics of study participants and details of the index test (fFN) are reported in the data extraction tables presented in Appendix 2.
| Study ID | Study design | Objective | Safety of newborn | Safety of mother | Costs | Maternal treatments | PTB | Estimated gestational age at birth | Length of stay |
|---|---|---|---|---|---|---|---|---|---|
| Dutta 201147 | Prospective RCT | The purpose of this study was to determine the role of fFN testing in women presenting to hospital with symptoms of preterm labour in reducing the hospital admissions, without significantly increasing the risk of PTB and neonatal RDS | ✓ | ✓ | ✓ | ✓ | |||
| From December 2007 to March 2009 | |||||||||
| Multicentre | |||||||||
| Country: Scotland | |||||||||
| Setting: Hospital (two large maternity units) | |||||||||
| Funding: Greater Glasgow Health Board North Glasgow Hospitals University Operating Division | |||||||||
| Grobman 200450 | Prospective RCT | The purpose of this study was to determine whether or not knowledge of fFN results affects patient treatment and health-care costs | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Duration 12 months (dates not mentioned) | |||||||||
| Single centre | |||||||||
| Country: Chicago, USA | |||||||||
| Setting: University hospital | |||||||||
| Funding: Adeza Biomedical Corporation | |||||||||
| Lowe 200448 | Prospective RCT | To investigate the effect of the rapid fFN on the length of hospital stay and the use of preterm labour interventions in a tertiary care centre | ✓ | ✓ | ✓ | ||||
| August 2000 to May 2002 | |||||||||
| Single centre | |||||||||
| Country: Iowa, USA | |||||||||
| Setting: Tertiary care | |||||||||
| Funding: Process Improvement grant, University of Iowa | |||||||||
| Osório 201051 | Prospective RCT | The purpose of this study was to determine whether or not knowledge of the results of a rapid fibronectin test affects treatment decision during the evaluation and treatment of women attending obstetric emergency because of preterm labour symptoms | ✓ | ||||||
| From April 2007 to December 2009 | |||||||||
| Single centre | |||||||||
| Country: Portugal | |||||||||
| Setting: Hospital | |||||||||
| Funding: Not reported | |||||||||
| Plaut 200349 | Prospective RCT | To determine whether or not knowledge of the results of a rapid fFN test affects treatment decisions during the evaluation and treatment of possible preterm labour | ✓ | ✓ | ✓ | ✓ | |||
| September 2000 to December 2001 | |||||||||
| Multicentre | |||||||||
| Country: USA | |||||||||
| Setting: Three community hospitals | |||||||||
| Funding: Supported by Adeza Biomedical Corporation | |||||||||
All studies followed standard methods for rapid fFN testing. Two studies reported the use of Adeza Tli™ to perform fFN testing. 49,50 These studies randomised patients, after rapid fFN testing, to the intervention group, in which case the physicians had knowledge of fFN test results, or the control group, in which case the physicians were unaware of the test results. 49,50 The test results were communicated to the treating physician by the resident physician50 or by laboratory personnel. 49 In the remaining studies, the participants were randomised to rapid fFN testing performed for managing preterm labour or to a control group in which rapid fFN testing was not performed.
The inclusion and exclusion criteria of the studies differed to some extent. All studies included women with signs and symptoms of preterm labour. All studies included women with an estimated gestation age within the range of 24–36 weeks, except for the study of Lowe et al. ,48 which recruited women with gestational ages between 23 and 24 weeks. The study by Lowe et al. 48 was the only one to allow the inclusion of multiparous women with cervical dilation of 3–4 cm. Three studies excluded women with cervical dilation ≥ 3 cm. 47,49,50 The remaining study was published as an abstract only and did not report the exclusion criteria. 51 Two studies included women with singleton or twin gestations. 48,49 However, Grobman et al. 50 and Dutta and Norman47 reported that they included only women with singleton gestations.
The five studies included a total of 541 women with symptoms of preterm labour; sample size ranged from 66 to 108 participants. All studies, with the exception of that by Osório et al. ,51 reported some form of sample size calculation for the primary outcome(s). Lowe et al. 48 calculated a sample size of 50 participants in each arm to detect a significant reduction in the length of stay of at least 1.3 days with 80% power. Dutta and Norman47 powered their study to detect a 40% reduction in the number of hospital admissions; the estimated sample size required for 85% power to detect a significant difference was 304 participants. Grobman et al. 50 was powered to detect 20% reduction in total health-care cost in the fFN group. Plaut et al. 49 estimated a required sample size of 500 women to detect a significant difference in transport to tertiary care centre; however, it is unclear how this estimate was calculated and the study was terminated prematurely because of low enrolments. All studies appeared to have made power calculations based on the whole study population, rather than on the index test (fFN) negative population; the latter option would be more appropriate as, if conservative management is the norm, only test-negative patients have the potential for changed management and outcomes. All the included studies were, therefore, likely to be underpowered. In all included trials, treatment decisions were at the discretion of clinicians, not based on fFN results alone. The trials may therefore provide important information about the consequences when fFN is used in clinical context. All studies reported that there was no significant difference in baseline demographic and clinical characteristics between patients in the index test and control groups.
None of the included studies was judged to be ‘high risk’ of bias overall (Figure 2). An overall rating of ‘high risk’ was defined as ‘high risk’ for any of three key domains: randomisation sequence, allocation concealment and blinded outcome assessment. Poor reporting resulted in a high number of ‘unclear’ risk of bias ratings across studies. Low et al. 's48 was the only study to be judged at ‘low risk’ of bias for two of the key domains. One study was a conference abstract so was judged to be at unclear risk of bias for all domains as the information reported was not sufficient to make any definitive judgement. 51 The complete risk of bias assessment along with relevant quotes from the included papers and review authors' judgements is provided in Appendix 3. The quality assessment of the included studies for all the domains of Cochrane risk of bias tool is summarised below. One study was judged to be at ‘high risk’ of bias for the ‘incomplete outcome data domain because drop outs and protocol violations were excluded from the analyses,47 and one study was judged to be at ‘high risk’ of bias for ‘selective outcome reporting’, focusing on the outcome measure where a significant effect was observed and, additionally, because it was stopped prematurely due to low enrolment. 49
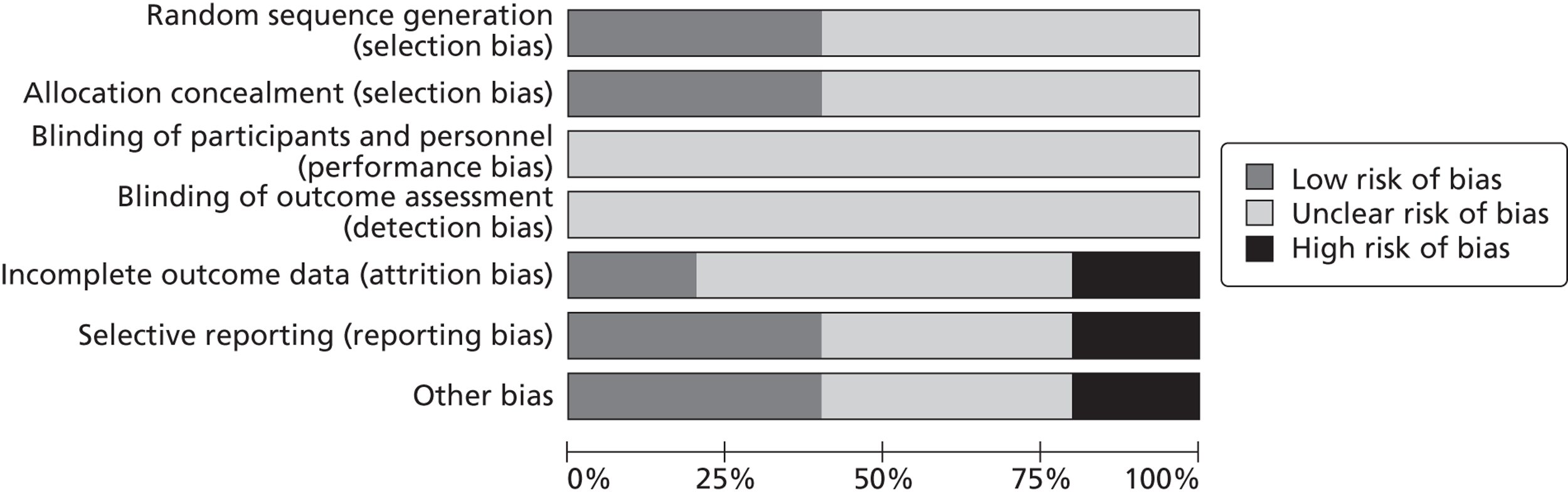
Overall, for all the domains across all the included studies, the majority of studies were rated ‘unclear risk’ of bias (Figure 3).
FIGURE 2.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
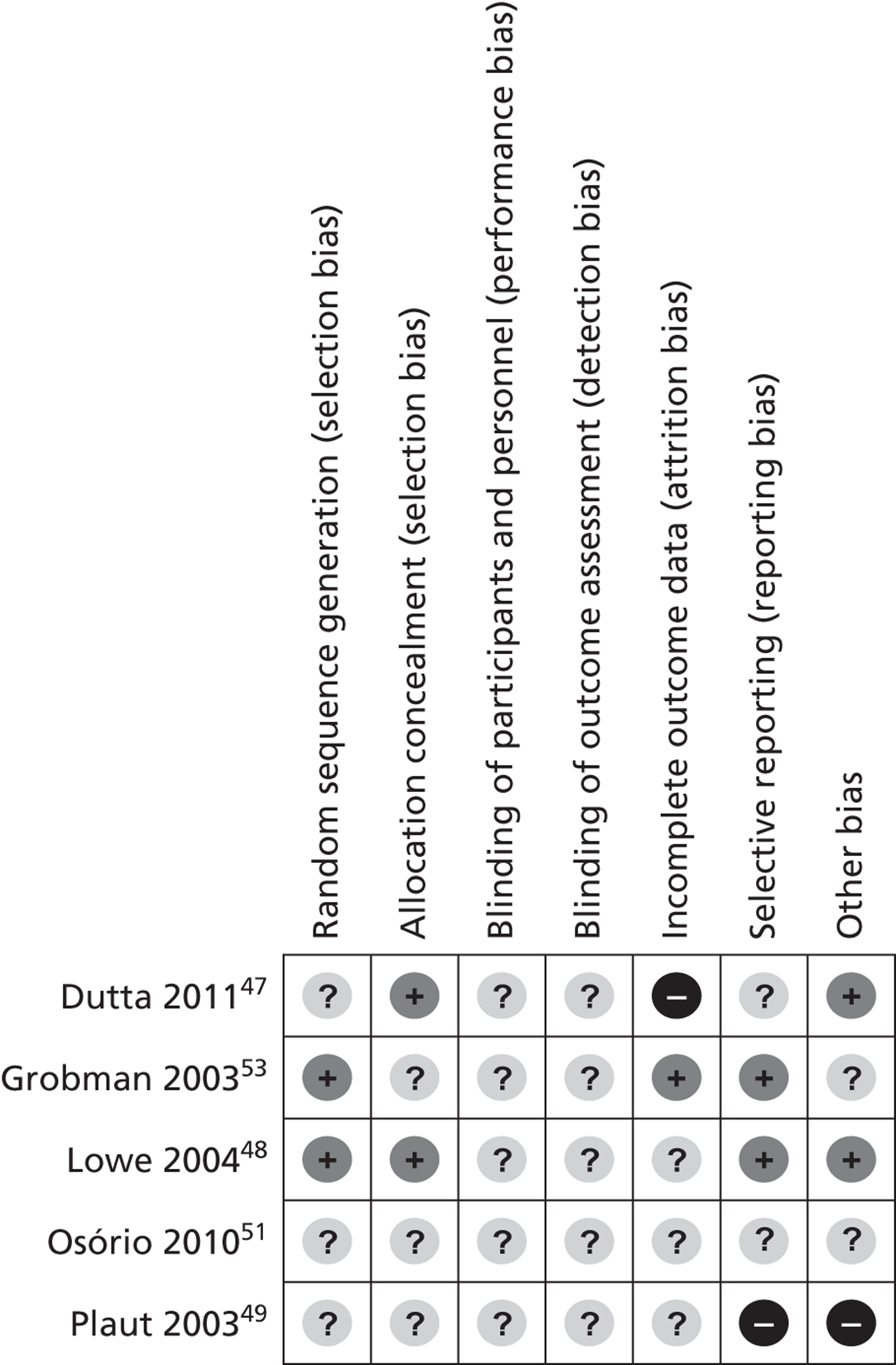
FIGURE 3.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Incidence of preterm birth
The primary outcome for this assessment was the incidence of spontaneous PTB before 37 weeks' gestation, before 34 weeks' gestation, or within 24 hours, 48 hours or 7–10 days of testing. Only two studies reported this outcome. 49,50 Plaut et al. 49 reported data for preterm delivery within 14 days of testing. Grobman et al. 50 reported the overall incidence of preterm delivery and hence it was not possible to have a pooled estimate of incidence of PTB. Neither study found a significant difference between fFN testing group and comparator (Table 2). We sought information on this outcome from the authors of two additional studies, but no responses were received.
Estimated gestational age at delivery
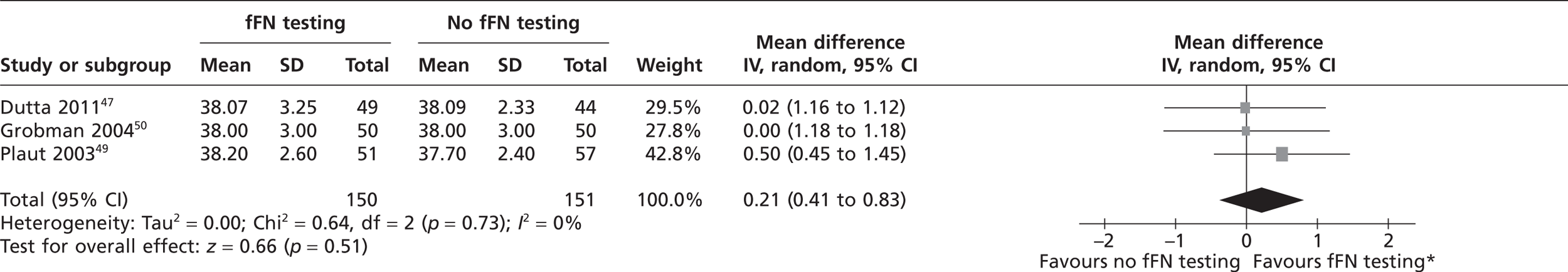
Three studies reported the mean gestational age at delivery in weeks. 47,49,50 No individual study found any significant difference in gestational age at delivery between the index test and control groups (see Table 2). Similarly, the pooled estimate showed no significant difference (Figure 4), indicating that the clinicians' knowledge of fFN test results did not affect gestational age at delivery. The study by Lowe et al. 48 was not included in the meta-analysis because they reported median gestational age at time of delivery; this study also found no significant difference between the test and control groups. 48
| Study ID | Main outcomes | fFN test: fFN testing done | Comparator: fFN testing not done/not known | p-value |
|---|---|---|---|---|
| Dutta 201147 | Gestational age at time delivery in weeks (mean) (± SD)a | 38.07 (3.25) (n = 43) | 38.09 (2.33) (n = 38) | 0.970 |
| Incidence of PTB/N (%) within 7 days | NR | NR | NR | |
| Grobman 200450 | Gestational age at time delivery in weeks (mean) (± SD) | 38 ± 3 | 38 ± 3 | 0.810 |
| Incidence of PTB/N (%) | 10 (20) | 13 (26) | 0.480 | |
| Lowe 200448 | Gestational age at time delivery in weeks (median) (IQR) | 38.3 (36.0–38.9) | 37.4 (35–39) | 0.258 |
| Incidence of PTB/N (%) | NR | NR | NR | |
| Osório 201051 | Gestational age at time delivery in weeks (mean) (± SD) | NR | NR | NR |
| Incidence of PTB/N (%) | NR | NR | NR | |
| Plaut 200349 | Gestational age at time delivery in weeks (mean) (± SD) | 38.2 ± 2.6 | 37.7 ± 2.4 | 0.860 |
| Incidence of PTB/N (%) within 14 days | 2 (4) | 1 (2) | NR |
FIGURE 4.
Forest plot of mean gestational ages at delivery in weeks. *The labels for the forest plot have been swapped because of the positive outcome.
Length of maternal hospital stay
Length of hospital stay was a common outcome, reported in all the included studies. None of the studies reported significant difference for this outcome among all the randomised patients (Table 3). Plaut et al. 49 compared the length of hospital stay in patients who tested negative for fFN testing and in whom the test result was known to clinicians (index test group) with test-negative patients for whom the result was not disclosed (control group); no significant difference was found. A subgroup analysis of women with negative fFN test observed for > 6 hours showed a significant reduction in the length of hospital stay when the test result was known to clinicians. The hospital stay was shortened by 40%, from 37.8 to 22.7 hours (p = 0.04) (see Table 3). However, the sample size for this analysis was very small and it was not clear whether or not the analysis had been planned a priori. The unit of measurement for length of hospital stay varied across studies; where possible, we standardised extracted data to number of days spent in hospital and the results of individual studies are presented in a forest plot (Figure 5). Grobman et al. 50 reported median length of stay and Osório et al. 51 reported a dichotomous outcome (number of women with hospital stays > 6 days); neither study found a significant difference between the index test and control groups.
| Study ID | fFN testing done | fFN testing not done/not known | p-value |
|---|---|---|---|
| Dutta 201147 | 0.736 (± 1.05) days (mean ± SD) (n =40) (interval of decision to admit to decision to discharge) | 0.699 (± 1.05) (mean ± SD) (n = 37) | 0.878 |
| Grobman 200450 | 2 days (1–5)a (during admission at study entry) | 2 days (1–5)a | 0.830 |
| 4 days (2–7)a (during admission after study entry) | 2 days (1–11)a | 0.620 | |
| Lowe 200448 | NR | NR | 0.224 |
| Osório 201051 | 33.3% (2/6) women stayed in hospital for > 6 daysb | 44.4% (4/9) stayed in hospital for > 6 daysb | 0.680 |
| Plaut 200349 | Women with negative fFN test: 0.28 (± 0.28) days mean (± SD) (n = 47) (observation period + any admissions) | Women with negative fFN test: 0.34 (± 0.28) days mean (± SD) (n = 51) | 0.350 |
| Women with negative fFN test: observed for > 6 hours 0.95 (± 0.6) days mean (± SD) (n = 10) | Women with negative fFN test: observed for > 6 hours 1.58 (± 0.6) days mean (± SD) (n = 8) | 0.040 |
FIGURE 5.
Forest plot of length of stay.
Incidence of hospital admissions
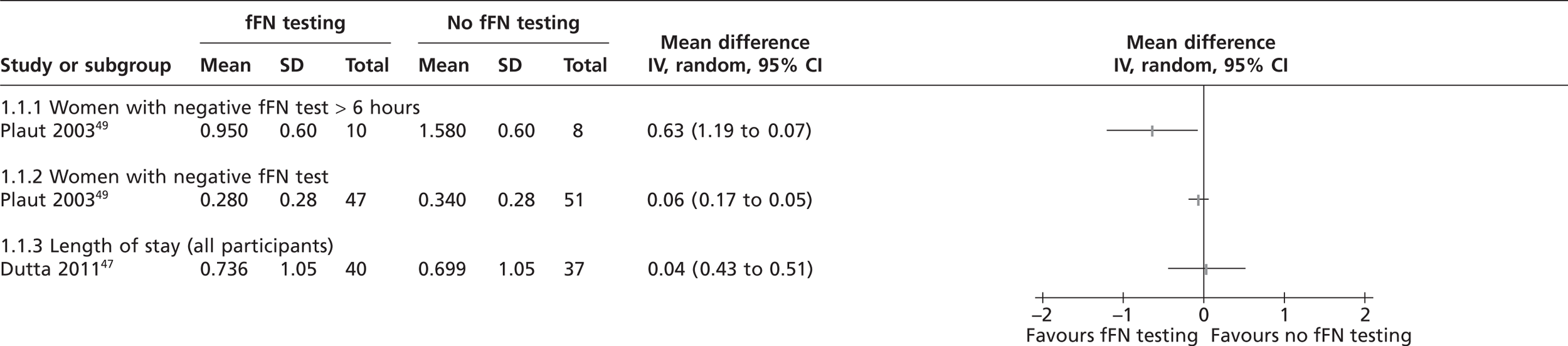
Four studies reported the number of hospital admissions before delivery (Table 4). 47,48,50,51 Grobman et al. 50 reported the number of hospital admissions at study entry and number of admissions for preterm contractions any time after study entry separately. Individual study results (see Figure 6 and Table 4) indicate a lower incidence of maternal admissions in the fFN test group than in the control group for three out of four studies;47,50,51 however, no study showed a statistically significant difference between groups. The study by Lowe et al. 48 was the only study that numerically favoured the no fFN testing group, but the authors reported that there were significantly fewer antepartum hospital admissions among the women with negative fFN test results (p = 0.032). The pooled RR for hospital admission showed no significant difference between the fFN test and control groups (RR 0.93%; CI 0.66% to 1.3%) (Figure 6). The study by Dutta and Norman47 was the only study evaluating the number of admissions to neonatal intensive care unit (NICU); although this study reported a higher number of NICU admissions in fFN test group, the difference was not statistically significant. 47 The conference abstract51 did not report the number of participants randomised to each group but after observing the data carefully we assumed that 33 women were allocated to each group. This assumption had to be made to calculate odds ratio for forest plot in Figure 6.
| Study ID | Hospital or NICU admissions | fFN testing done | fFN testing not done/not known | p-value |
|---|---|---|---|---|
| Dutta 201147 | Antepartum admissions (%) | 21 (42.9) | 22 (50) | 0.490 |
| Incidence of NICU admission (%) | 10 (33.3) | 3 (10) | 0.080 | |
| Grobman 200450 | Hospital admissions at study entry (%) | 13 (26) | 14 (28) | 0.440 |
| Hospital admission for preterm contractions at any time after study entry (%) | 5 (10) | 4 (8) | 0.780 | |
| Lowe 200448 | Antepartum admissions (%) | 16 (35) | 12 (24) | 0.265 |
| Osório 201051 | Hospital admissions for PTB (%) | 6/33 (18.2) | 9/33 (27.3) | 0.560 |
FIGURE 6.
Analysis on number of hospital admissions.
Treatments administered
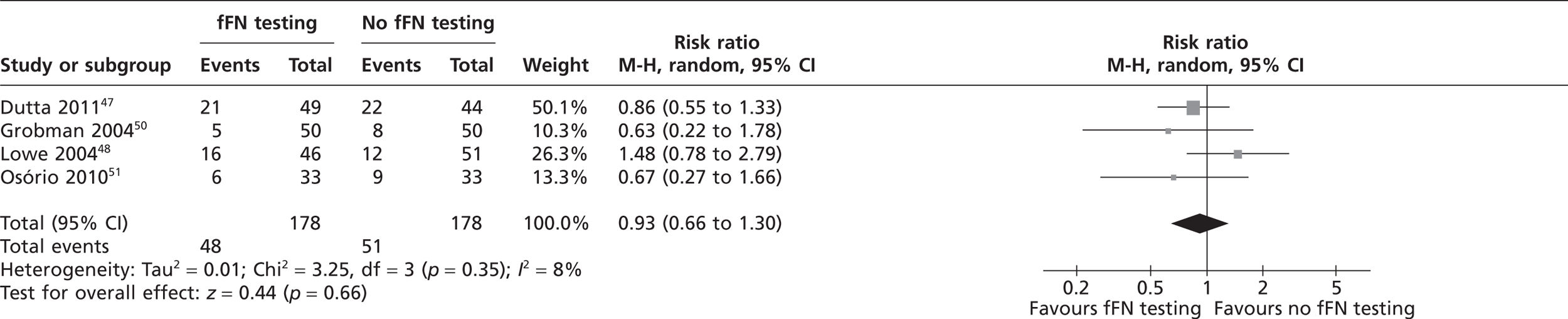
Three studies reported the use of tocolytic agents and corticosteroids (Table 5). 47,48,50 The meta-analysis in Figure 7 indicates that there was no significant difference in usage of tocolytic agents between two groups (pooled RR 1.0; 95% CI 0.69 to 1.44). Similarly, the meta-analysis in Figure 8 indicates that there was no significant difference in usage of corticosteroids between the two groups (pooled RR 0.93; 95% CI 0.68 to 1.27). Lowe et al. 48 also reported administration of an antibiotic therapy with no significant difference in usage between the groups. Plaut et al. 49 reported the administration of aggressive therapy which included use of tocolysis, corticosteroids and transfer to a tertiary care facility. Fourteen women were administered aggressive tocolytic therapy, of whom three delivered within 14 days and remaining 11 delivered after 14 days. 49 However, there was no significant difference in use of aggressive therapy between the two groups (see Table 5).
| Study ID | Treatments administered | fFN testing done | fFN testing not done/not known | p-value |
|---|---|---|---|---|
| Dutta 201147 | Tocolysis (%) | 3 (6.5) | 4 (8.7) | 1.000 |
| Corticosteroids (%) | 17 (37) | 21 (45.7) | 0.397 | |
| Grobman 200450 | Tocolysis (%) | 8 (16) | 9 (18) | 0.790 |
| Corticosteroids (%) | 8 (16) | 10 (20) | 0.600 | |
| Lowe 200448 | Tocolysis (%) | 22 (48) | 23 (45) | 0.840 |
| Corticosteroids (%) | 23 (50) | 22 (43) | 0.545 | |
| Antibiotics (%) | 17 (37) | 21 (41) | 0.683 | |
| Plaut 200349 | Aggressive therapy (%) | 8 (16) | 6 (11) | 0.430 |
FIGURE 7.
Analysis on usage of tocolytic agents.
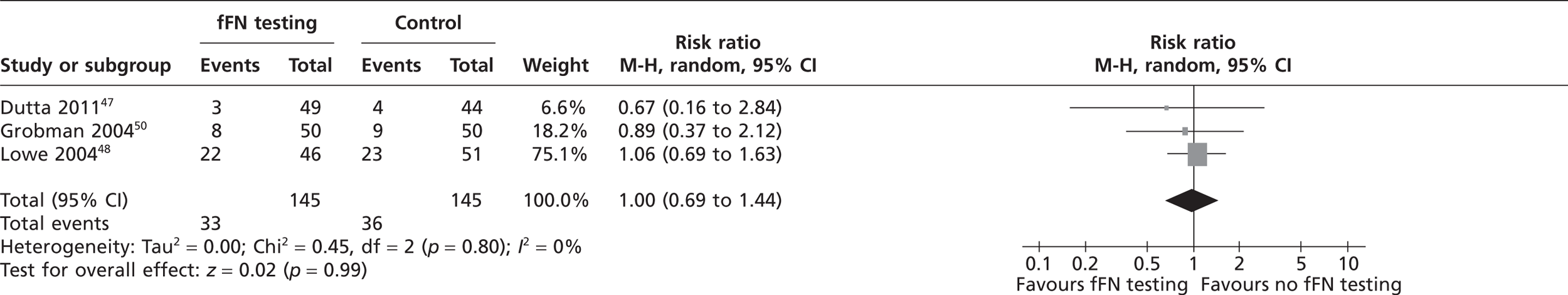
FIGURE 8.
Analysis on usage of corticosteroids.
Other outcomes
Two studies reported median duration of labour and delivery in hours with no significant difference between the two groups. 48,50 Only Dutta and Norman47 reported neonatal outcomes such as incidence of ventilator support and incidence of RDS. However, none of these outcomes showed a significant difference between groups. None of the included studies reported any adverse events.
Test accuracy
The 15 DTA studies identified by our update searches included a total of 2379 participants (range 38–516 participants). The majority of these studies reported data for more than one outcome (preterm delivery at various gestational ages and times from testing); 10 studies reported data for PTB within 7–10 days of testing,32,56–63 seven studies reported data for PTB before 34 weeks' gestation55,57,60,63–66 and seven studies reported data for PTB before 37 weeks' gestation. 54,55,60,63–65,67 In addition, four studies reported data for PTB before 35 weeks' gestation which were grouped with the 34 weeks category,54,58,59,61 and one study reported data for PTB before 38 weeks' gestation, which were grouped with the 37 weeks category. 61 Four studies included only women with singleton pregnancies,32,54,59,61 and the remainder either included both singleton and multiple pregnancies or did not report any inclusion/exclusion criteria for this factor. Eight studies reported the use of Adeza Biomedical Corporation fFN test kits,55–57,59,60,62,63,66 two studies used QuickCheck™ (Adeza Biomedical, Sunnyvale, CA, USA)/FullTerm fFN testing kits54,64 and one study used both. 58 Two studies did not specify the brand name of the kit used for fFN testing. 61,67 There were three non-English-language articles. For one Spanish article67 the data extraction and quality assessment was done by a native speaker. For remaining two articles, one Turkish68 and one Italian,65 limited data (results only) were taken from the English-language abstract; these studies are not included in Appendices 4 and 6. Further details of the inclusion/exclusion criteria, characteristics of study participants and details of the index test (fFN) are reported in the data extraction tables presented in Appendix 4.
The main risk of bias for these studies related to the ‘patient selection’ domain of our modified version of QUADAS-2; only three studies reported prospective, consecutive recruitment of participants. 54–56 The nature of the intervention meant that most included studies used commercial test kits, minimising the potential for bias arising from the conduct of the index test. Finally, the majority of included studies reported data for all participants. The results of QUADAS-2 assessment are summarised in Table 6 and full assessments for each study are provided in Appendix 6.
In addition to the 15 new studies described, data from 39 DTA studies included in the appendix of a previously published systematic review of fFN testing for the prediction of PTB were included in our meta-analysis. 35 Seventeen of these studies reported data for PTB within 7–10 days of testing, eight studies reported data for PTB before 34 weeks' gestation and 31 reported data for PTB before 37 weeks' gestation. Sixteen of the 39 studies included only women with singleton pregnancies and the remainder either included both singleton and multiple pregnancies or did not report any inclusion/exclusion criteria for this factor. The review from which these studies were taken used the authors' own, topic-specific, quality assessment tool; however, it was possible to determine from the data extraction tables that 10 of the 39 studies had reported prospective, consecutive recruitment of participants. The results and main characteristics of these studies are summarised in Appendix 7.
| Study ID | Risk of bias | ||
|---|---|---|---|
| Patient selection | Index test | Flow and timing | |
| Asakura 200955 | ☹ | ☺ | ☺ |
| Audibert 201064 | ☺ | ☺ | ☺ |
| Diaz 200954 | ☺ | ☺ | ☺ |
| Desjardins 200858 | ☹ | ☺ | ☺ |
| Eroglu 200759 | ? | ☺ | ☺ |
| Farfan 201167 | ☺ | ☺ | ☺ |
| Groom 200660 | ☹ | ☺ | ☺ |
| Henrich 201061 | ? | ☹ | ☺ |
| MacDonald 200762 | ☹ | ☺ | ☺ |
| Singer 200766 | ☹ | ☺ | ☺ |
| Skoll 200657 | ? | ☺ | ☺ |
| Swamy 200563 | ? | ☺ | ☺ |
| Tsoi 200656 | ☺ | ☺ | ☺ |
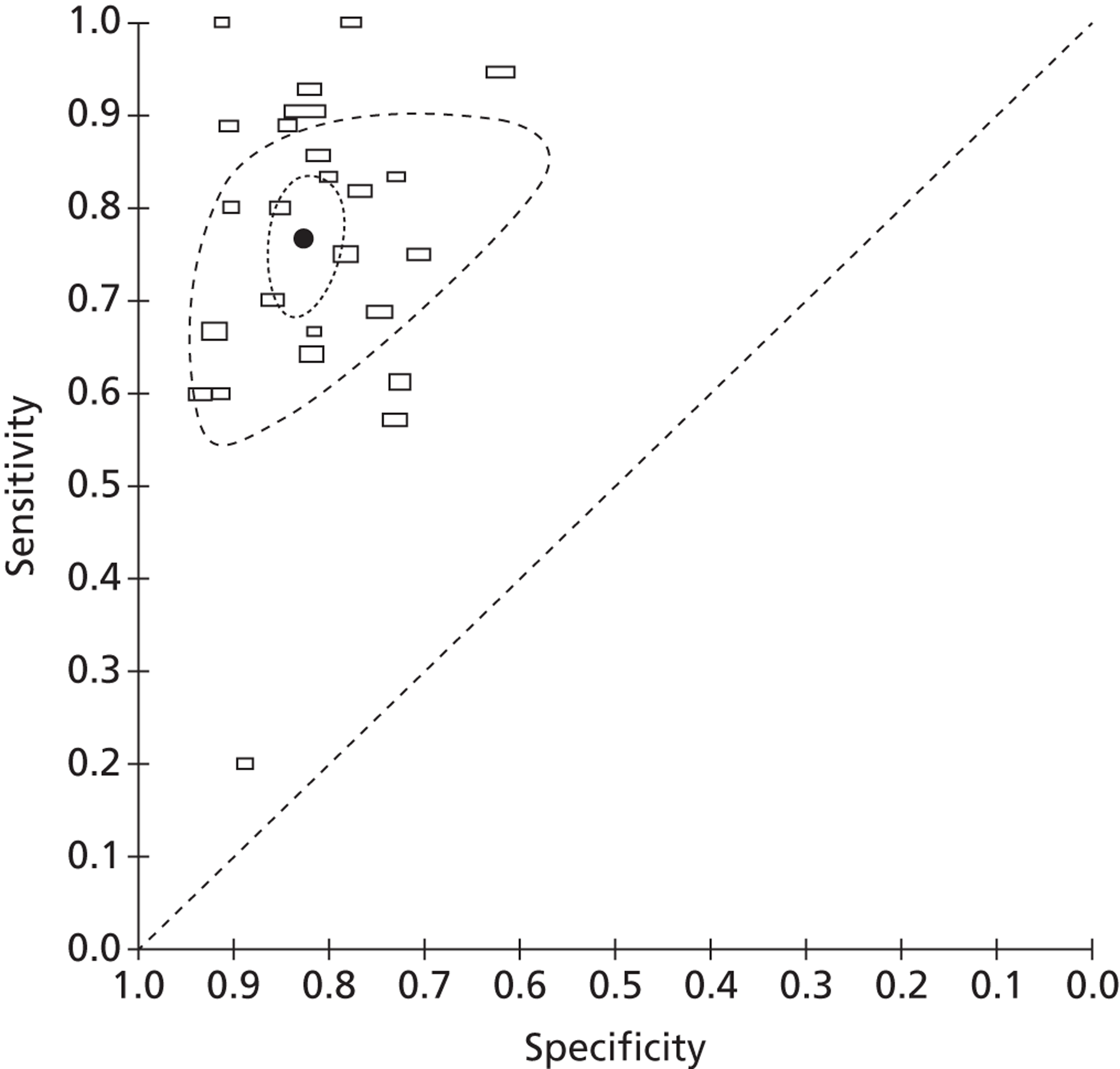
Accuracy of fetal fibronectin for the prediction of preterm birth within 7–10 days of testing
A total of 27 studies reported data on the accuracy of fFN testing to predict preterm delivery within 7–10 days of testing. Ten studies were identified by our update searches and 17 were taken from the previous systematic review, as described above. The results of the 10 new studies are summarised in Table 7. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 76.7% (95% CI 70.4% to 82.0%) and 82.7% (95% CI 79.4% to 85.5%), respectively. The I2 statistic indicated low between-study heterogeneity in the estimates of sensitivity (I2 = 24.8%) and high between-study heterogeneity in the estimates of specificity (I2 = 84.5%). Figure 9 shows individual studies, along with the summary estimate, plotted in receiver operating characteristic (ROC) space. Subgroup analyses, using a bivariate model, also showed similar estimates of test performance for studies that included only women with singleton pregnancies compared with unselected populations, and for studies identified by our update searches compared with studies from the previously published review (Table 8).
| Study ID | Description of arm and diagnostic threshold | TP | FN | FP | TN | Sensitivity (95% CI) | Specificity (95% CI) | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Desjardins 200858 | ≥ 50 ng/ml | 6 | 4 | 23 | 328 | 60.0% (26.2% to 87.8%)a | 93.4% (90.3% to 95.8%)a | NR |
| Diaz 200954 | ≥ 50 ng/ml | 18 | 6 | 34 | 122 | 75.0% (52.9% to 89.4%) | 78.2% (70.7% to 84.2%) | Women with positive fFN had higher rate of adverse neonatal outcomes |
| Eroglu 200759 | ≥ 50 ng/ml (from kit manual) | 5 | 1 | 9 | 36 | 83.3% (35.9% to 99.6%)a | 80.0% (65.4% to 90.4%)a | Bacterial vaginosis |
| Groom 200660 | ≥ 50 ng/ml | 7 | 3 | 24 | 145 | 70.0% (34.8% to 93.3%)a | 85.8% (79.6% to 90.7%)a | NR |
| Henrich 201061 | NR | 5 | 0 | 17 | 59 | 100.0% (47.8% to 100.0%)a | 77.6% (66.6% to 86.4%)a | NR |
| MacDonald 200762 | ≥ 50 ng/ml (from kit manual) | 4 | 0 | 3 | 31 | 100.0% (39.8% to 100.0%)a | 91.2% (76.3% to 98.1%)a | NR |
| Skoll 200657 | ≥ 50 ng/ml | 12 | 3 | 20 | 114 | 80.0% (51.4% to 94.7%) | 85.1% (77.6% to 90.4%) | NR |
| Swamy 200563 | > 50 ng/ml | 14 | 7 | 31 | 352 | 66.7% (43.0% to 85.4%)a,b | 91.9% (88.7% to 94.4%)a,b | NR |
| Sümer 201068 | ≥ 50 ng/ml | 1 | 4 | 7 | 55 | 20.0% (5.0% to 71.6%)a | 88.7% (78.1% to 95.3%)a | NR |
| Tsoi 200656 | ≥ 50 ng/ml (from kit manual) | 18 | 1 | 67 | 109 | 94.7% (74.0% to 99.9%)a | 61.9% (54.3% to 69.1%)a | NR |
FIGURE 9.
Receiver operating characteristic space plot of studies of fFN for the prediction of PTB within 7–10 days of testing.
| Data set | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| All studies (n = 27) | 76.7% (70.4% to 82.0%) | 82.7% (79.4% to 85.5%) |
| Studies of singleton pregnancies (n = 12) | 75.8% (63.2% to 85.1%) | 81.1% (75.8% to 85.6%) |
| Studies of unselected populations (n = 15) | 76.4% (68.6% to 82.8%) | 83.6% (79.6% to 87.0%) |
| Studies identified by update searches (n = 10) | 76.3% (63.8% to 85.4%) | 85.0% (78.8% to 89.6%) |
| Studies taken from previous systematic review10 (n = 17) | 77.1% (69.4% to 83.4%) | 81.7% (78.3% to 84.7%) |
Accuracy of fetal fibronectin for the prediction of preterm birth at < 34 weeks' gestation
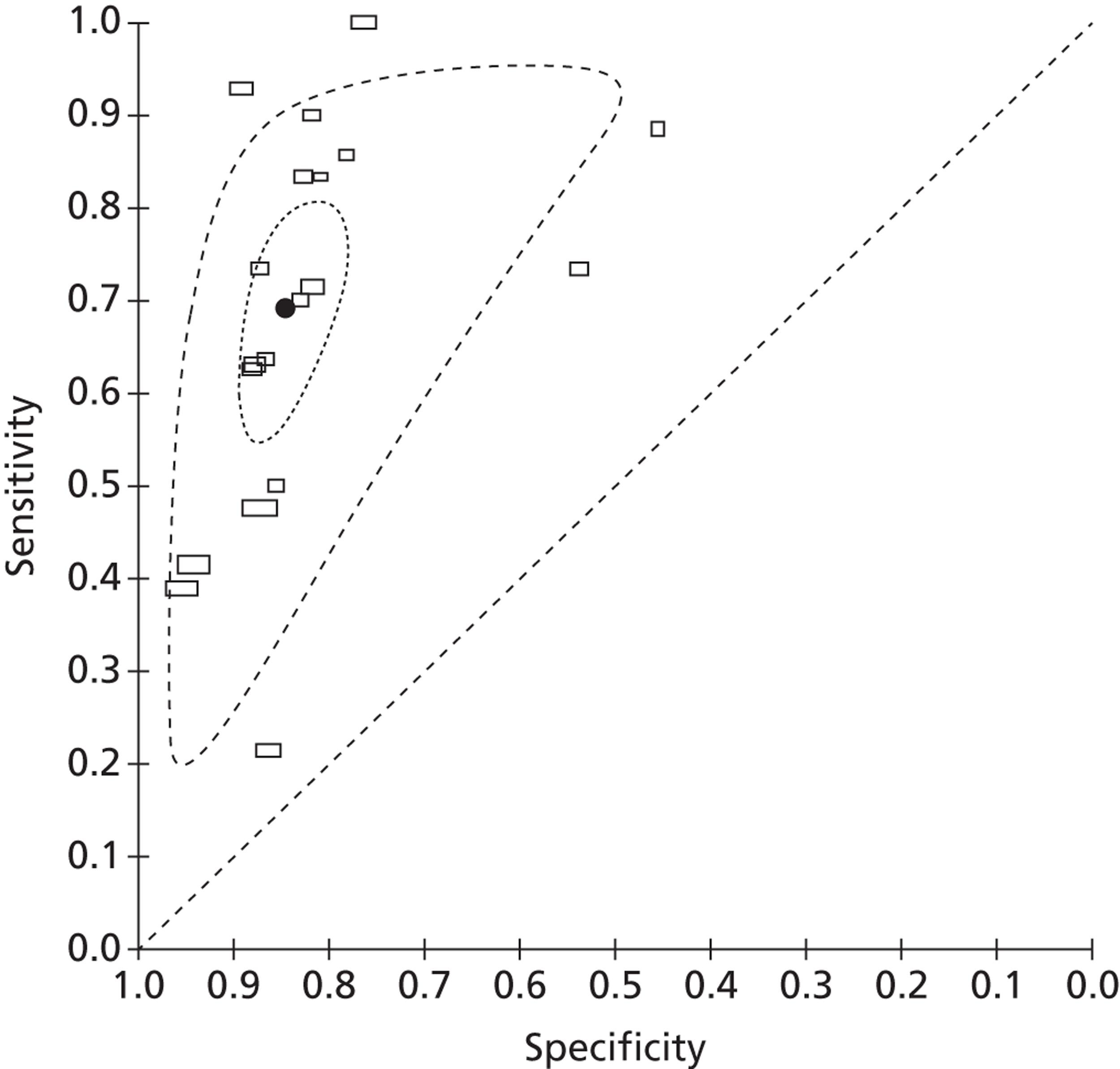
A total of 19 studies reported data on the accuracy of fFN testing to predict preterm delivery at < 34 weeks' gestation. Eleven studies were identified by our update searches and eight were taken from the previous systematic review, as described above. The results of the 11 new studies are summarised in Table 9. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 69.1% (95% CI 58.6% to 77.9%) and 84.4% (95% CI 79.8% to 88.2%), respectively. The I2 statistic indicated low between-study heterogeneity in the estimates of sensitivity (I2 = 75.5%) and high between-study heterogeneity in the estimates of specificity (I2 = 85.4%). Figure 10 shows individual studies, along with the summary estimate, plotted in ROC space. Subgroup analyses, using a bivariate model, also showed similar estimates of test performance for studies that included only women with singleton pregnancies versus unselected populations, and for studies identified by our update searches compared with studies from the previously published review (Table 10). A sensitivity analysis was carried out, which excluded four studies with a reference standard of PTB at < 35 weeks' gestation;54,58,59,61 there was no significant change in the results when these four studies were excluded.
| Study ID | Description of arm and diagnostic threshold | TP | FN | FP | TN | Sensitivity (95% CI) | Specificity (95% CI) | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Reference standard outcome: preterm delivery (< 34 weeks' gestation) | ||||||||
| Asakura 200955 | > 50 ng/ml | 10 | 6 | 11 | 81 | 62.5% (35.4% to 84.8%)a | 88.0% (79.6% to 93.9%)a | NR |
| Audibert 201064 | ≥ 50 ng/ml | 7 | 7 | 7 | 41 | 50.0% (23.0% to 77.0%) | 85.0% (72.0% to 94.0%) | NR |
| bDesjardins 200858 | ≥ 50 ng/ml | 14 | 22 | 15 | 310 | 38.9% (23.1% to 56.5%)a | 95.4% (92.5% to 97.4%)a | NR |
| bDiaz 200954 | ≥ 50 ng/ml | 12 | 0 | 40 | 128 | 100.0% (69.9% to 100.0%) | 76.2% (68.9% to 82.3%) | NR |
| Driul 200965 | ≥ 50 ng/ml | 11 | 4 | 31 | 36 | 73.3% (44.9% to 92.2%)a | 53.7% (41.1% to 66.0%)a | NR |
| bEroglu 200759 | NR | 7 | 3 | 7 | 34 | 70.0% (34.8% to 93.3%)a | 82.9% (67.9% to 92.8%)a | Bacterial vaginosis |
| Groom 200660 | ≥ 50 ng/ml | 13 | 1 | 18 | 147 | 92.9% (66.1% to 99.8%)a | 89.1% (83.3% to 93.4%)a | NR |
| bHenrich 201061 | NR | 10 | 2 | 12 | 57 | 83.3% (51.6% to 97.9%)a | 82.6% (71.6% to 90.7%)a | NR |
| Singer 200766 | ≥ 50 ng/ml | 19 | 21 | 61 | 415 | 47.5% (31.5% to 63.9%)a | 87.2% (83.8% to 90.1%)a | NR |
| Skoll 200657 | ≥ 50 ng/ml | 17 | 10 | 15 | 107 | 63.0% (42.4% to 79.9%) | 87.6% (80.1% to 92.7%) | NR |
| Swamy 200563 | > 50 ng/ml | 27 | 38 | 20 | 319 | 41.5% (29.4% to 54.4%)a | 94.1% (91.0% to 96.4%)a | NR |
FIGURE 10.
Receiver operating characteristic space plot of studies of fFN for the prediction of PTB at < 34 weeks' gestation.
| Data set | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| All studies (n = 19) | 69.1% (58.6% to 77.9%) | 84.4% (79.8% to 88.2%) |
| Studies of singleton pregnancies (n = 9) | 76.4% (57.7% to 88.5%) | 82.4% (78.9% to 85.3%) |
| Studies of unselected populations (n = 10) | 62.7% (49.6% to 74.2%) | 85.0% (75.8% to 91.1%) |
| Studies identified by up-date searches (n = 11) | 65.2% (51.8% to 76.5%) | 86.3% (80.1% to 90.8%) |
| Studies taken from previous systematic review10 (n = 8) | 74.0% (56.1% to 86.3%) | 82.1% (77.6% to 85.8%) |
| Sensitivity analysis excluding studies with reference standards < 35 weeks' gestation (n = 15) | 67.4% (56.3% to 76.8%) | 83.8% (78.5% to 88.0%) |
Accuracy of fetal fibronectin for the prediction of preterm birth at < 37 weeks' gestation
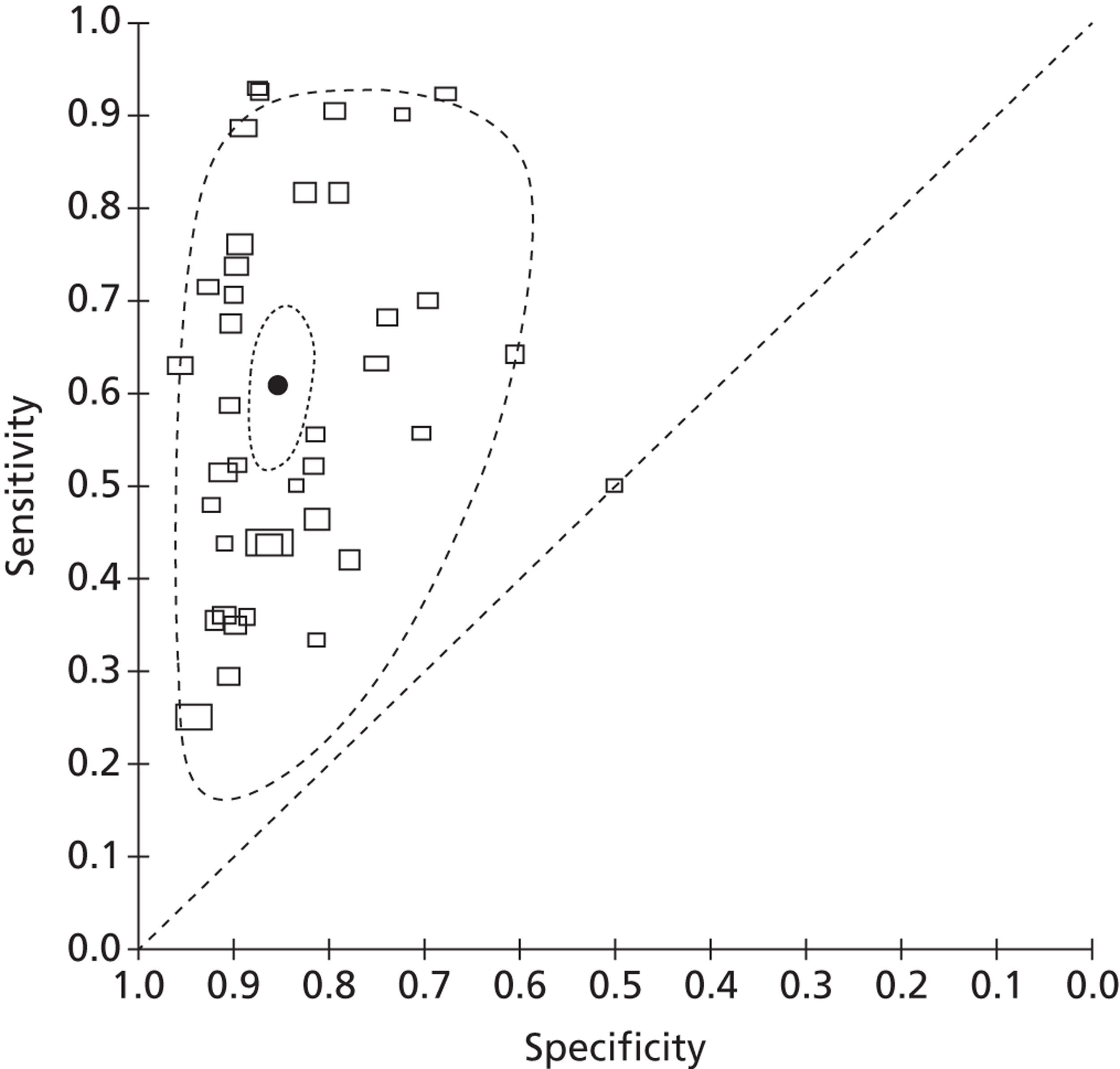
A total of 39 studies reported data on the accuracy of fFN testing to predict preterm delivery at < 37 weeks' gestation. Eight studies were identified by our update searches and 31 were taken from the previous systematic review, as described above. The results of the eight new studies are summarised in Table 11. The pooled estimates of sensitivity and specificity, derived from these data using a bivariate model, were 60.8% (95% CI 53.7% to 67.6%) and 85.3% (95% CI 82.5% to 87.7%), respectively. The I2 statistic indicated low between-study heterogeneity in the estimates of sensitivity (I2 = 83.7%) and high between-study heterogeneity in the estimates of specificity (I2 = 72.9%). Figure 11 shows individual studies, along with the summary estimate, plotted in ROC space. Subgroup analyses, using a bivariate model, also showed similar estimates of test performance for studies that included only women with singleton pregnancies versus unselected populations, and for studies identified by our update searches compared with studies from the previously published review (Table 12). A sensitivity analysis was performed, which excluded one study with a reference standard of PTB at < 38 weeks' gestation;61 there was no significant change in the results excluding this study.
| Study ID | Description of arm and diagnostic threshold | TP | FN | FP | TN | Sensitivity (95% CI) | Specificity (95% CI) | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Reference standard outcome: preterm delivery (< 37 weeks' gestation) | ||||||||
| Asakura 200955 | > 50 ng/ml | 14 | 26 | 7 | 61 | 35.0% (20.6% to 51.7%)a | 89.7% (79.9% to 95.8%)a | NR |
| Audibert 201064 | ≥ 50 ng/ml | 11 | 12 | 3 | 36 | 48.0% (35.0% to 60.0%) | 92.0% (86.0% to 99.0%) | NR |
| Diaz 200954 | ≥ 50 ng/ml | 38 | 12 | 14 | 116 | 76.0% (61.5% to 86.5%) | 89.2% (82.3% to 93.8%) | NR |
| Driul 200965 | ≥ 50 ng/ml | 25 | 14 | 17 | 26 | 64.1% (47.2% to 78.8%)a | 60.5% (44.4% to 75.0%)a | NR |
| Farfan 201167 | ≥ 50 ng/ml (from kit manual) | 25 | 2 | 5 | 34 | 92.6% (75.7% to 99.1%)a | 87.2% (72.2% to 95.7%)a | NR |
| Groom 200660 | ≥ 50 ng/ml | 18 | 17 | 13 | 131 | 51.4% (34.0% to 68.6%)a | 91.0% (85.1% to 95.1%)a | NR |
| bHenrich 201061 | NR | 17 | 12 | 5 | 47 | 58.6% (38.9% to 76.5%)a | 90.4% (79.0% to 96.8%)a | NR |
| Swamy 200563 | > 50 ng/ml | 30 | 90 | 17 | 267 | 25.0% (17.5% to 33.7%)a | 94.0% (90.6% to 96.5%)a | NR |
FIGURE 11.
Receiver operating characteristic space plot of studies of fFN for the prediction of PTB at < 37 weeks' gestation.
| Data set | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| All studies (n = 39) | 60.8% (53.7% to 67.6%) | 85.3% (82.5% to 87.7%) |
| Studies of singleton pregnancies (n = 16) | 66.4% (53.7% to 77.2%) | 85.6% (80.3% to 89.7%) |
| Studies of unselected populations (n = 23) | 57.3% (48.9% to 64.8%) | 85.0% (81.7% to 87.8%) |
| Studies identified by up-date searches (n = 8) | 57.1% (40.4% to 72.3%) | 88.7% (82.7% to 92.8%) |
| Studies taken from previous systematic review10 (n = 31) | 61.7% (53.9% to 69.0%) | 84.2% (81.1% to 86.9%) |
| Sensitivity analysis excluding studies with reference standards < 38 weeks' gestation (n = 38) | 60.9% (53.5% to 67.8%) | 85.4% (82.4% to 88.0%) |
Chapter 4 Economic evaluation
Identifying and reviewing published cost-effectiveness studies
Search strategy
Focused searches were undertaken to identify economic evaluations of the fFN test. No date limits were applied to these searches. The following resources were searched:
-
MEDLINE (OvidSP): 1946 to January week 4 2012
-
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP): up to 2 February 2012
-
MEDLINE Daily Update (OvidSP): up to 2 February 2012
-
EMBASE (OvidSP): 1980 to week 4 2012
-
DARE (Wiley): up to Issue 1 2012
-
HTA Database (Wiley): up to Issue 1 2012
-
NHS Economic Evaluation Database (NHS EED) (Wiley): up to Issue 1 2012
-
Paediatric Economic Database Evaluation (PEDE) (URL: http://pede.ccb.sickkids.ca/pede/search.jsp): 1980–2010.
Appendix 1 gives a full specification of search strategies.
All references were downloaded in EndNote X5 software and were further screened for inclusion.
Review of economic analyses on fibronectin
The objective of the review of extant economic evaluations was to summarise methods and findings of existing peer-reviewed studies. A total of 88 titles and abstracts were screened, from which we selected 12 studies. After a further full-text screening, only two studies were kept. These studies matched our criteria of a full economic analysis in which the fFN test was compared with an alternative option for predicting preterm labour. Studies that did not include ICERs were excluded from the review. A summary of the studies and the quality assessments is provided in Appendix 8.
Mozurkewich et al. 69 developed a decision-analytic model to compare nine different treatment strategies for the management of women presented with threatened preterm labour (i.e. regular uterine contractions at 24–34 weeks, no cervical dilation, and intact uterine membranes). The treatment consisted of the administration of one of the fibronectin tests (fFN or fibronectin rapid test) and parenteral corticosteroids and/or tocolytics for the prevention of RDS. The strategies compared were:
-
Treat all women with corticosteroids and tocolytics.
-
Treat all with tocolytics and corticosteroids until the results of fFN tests were available and discharge those with negative results.
-
Discharge only women with a cervical length measure > 26 mm on the (vaginal or transperineal) ultrasonography.
-
Discharge only women with a negative result on the rapid fibronectin test.
-
Discharge only women that have a negative rapid fibronectin test or a cervical length > 26 mm.
-
Do not treat any women with corticosteroids or tocolytics.
-
Treat all women with outpatient corticosteroids but not with tocolytics.
-
Treat all women with corticosteroids but administer tocolytics to those with abnormal results on rapid fibronectin test.
-
Treat all women with corticosteroids but give tocolytics only to women with an abnormal measure of the cervical length.
The health outcomes considered were neonatal death and RDS and time horizon was set until the time of hospital discharge. Accuracy data for fFN tests were obtained from Revah et al. 70 The cost data for the study came from statistical data of University of Michigan Hospital and the literature review. Total costing for each of the strategies was calculated by adding up the costs for outpatient treatments, fibronectin testing, cervical length measurement, hospitalisations and treatment, maternal delivery, and neonatal care (until death or discharge). The most cost-effective strategy (extended dominance) in terms of costs per neonatal death prevented were strategy 8 (rapid fibronectin plus corticosteroids and tocolysis only in those with abnormal fFN results), with an average cost of $13,000 (1999 prices in Canadian dollars) and 39 deaths/1000, and strategy 2 (treating all until results of fFN tests were available and discharge those with negative results) with an average cost of $13,600 (1999 prices in Canadian dollars) and 39 deaths/1000. The most cost-effective strategy for the prevention of RDS was strategy 2, with an average cost of $13,600 (1999 prices in Canadian dollars) and 53 RDS cases/1000.
Tsourapas et al. 71 developed a decision-analysing model to assess the cost-effectiveness of alternative ‘test-and-treat’ strategies in the prevention of PTB before weeks 34 and 37. The study compared the results of six models defined according to population and outcome as follows.
-
Model 1: Symptomatic women (with a viable PTB experiencing preterm labour) – giving birth before 37 weeks.
-
Model 2: Symptomatic women – giving birth before 34 weeks.
-
Model 3: Symptomatic women – giving birth within 7 days of treatment.
-
Model 4: Symptomatic women – giving birth within 48 hours of treatment.
-
Model 5: Asymptotic women – having threatened preterm labour before 37 weeks.
-
Model 6: Asymptotic women – having threatened preterm labour before 34 weeks.
The models combined all possible ‘test-and-treat’ combinations. The alternative tests consisted of fFN testing, highly phosphorylated insulin-like growth factor-binding proteins (phIGFBPs), C-reactive protein (CRP), absence of fetal breathing and previous history of PTB. The treatment consisted of administering progestational agents (i.e. atosiban, indomethacin, calcium channel blockers, magnesium sulphate and terbutaline). The comparators were no screening testing and no intervention.
The cost data for the study came from literature reviews and statistical data of the Birmingham Women's Hospital, which were adjusted to 2006 prices (pounds sterling). Test accuracy and treatment effectiveness data came from a meta-analysis of the systematic literature review carried out by the authors. 10
The results of model 1 show that the least expensive strategy is to conduct the fFN test and administer indomethacin to those who test positive. The cost of this strategy amounts to £2053. However, this is not the most cost-effective strategy. The ‘no test/administer indomethacin to all’ strategy costs £2609 but saves 34 cases of spontaneous PTB per 1000 women. The ICER for this strategy was estimated to be £16,336 per additional case of spontaneous PTB, making it the most cost-effective. Other noteworthy cost-effective strategies were:
-
In model 2 (avoiding premature births before week 34 for symptomatic women), testing with the amniotic fluid interleukin 6 test and providing hydration to those who tested positive (ICER £4976 per additional threatened PTB avoided).
-
In model 3 (avoiding premature births before 7 days of hospitalisation for symptomatic women), using the cervical length measurement < 15 mm test and administer indomethacin to those who tested positive (ICER £1703 per additional threatened PTB avoided).
-
In model 4 (avoiding premature births before 48 days of hospitalisation for symptomatic women) using the cervical length measurement < 15 mm test and administer indomethacin to those who tested positive (ICER £5268 per additional threatened PTB avoided).
Conclusions of review
Both of these studies showed that fibronectin testing could be cost-effective, depending on its place in the care pathway. However, whether or not testing was cost-effective depended on there being a difference in birth timing, which was not supported by the trial evidence from the systematic review. Therefore, we conducted a de novo analysis (see next section).
Evaluation of costs
Model structure and methodology
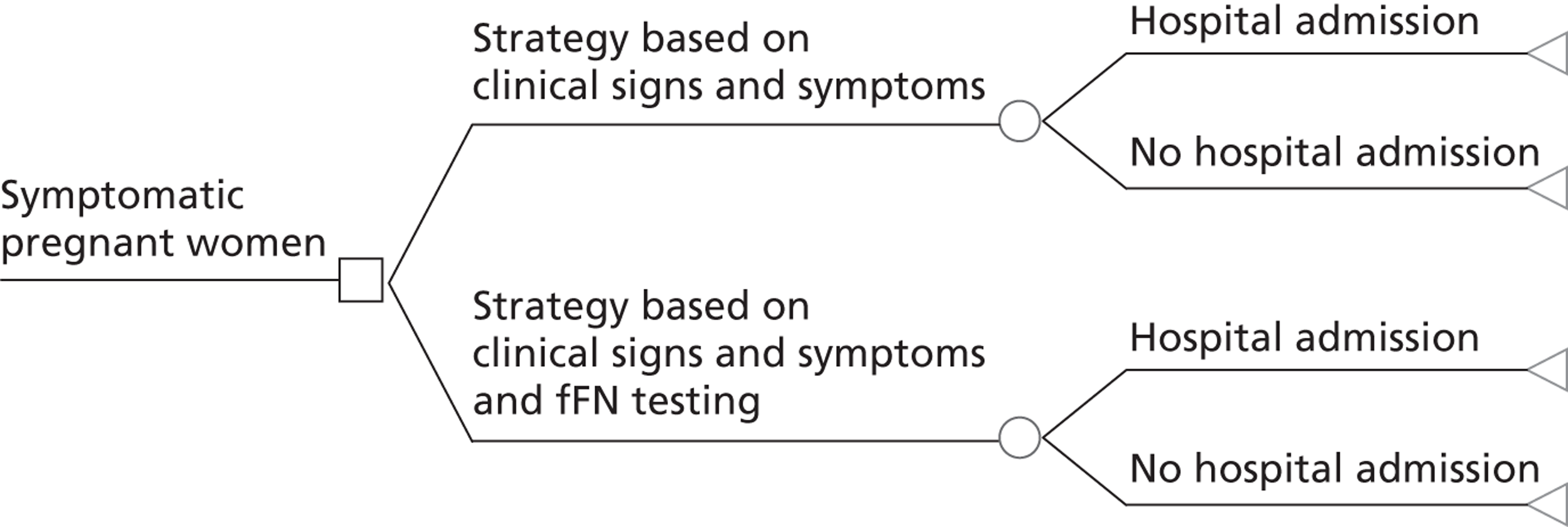
Full cost-effectiveness modelling was not feasible, as evidence from the systematic review indicated that fFN testing had no significant effect on outcome in terms of live births/PTBs/gestational age at delivery. Although the upper boundary of the CI of the pooled gestational age at delivery was positive (0.83, see Figure 4), it was decided that this was not clinically relevant as the studies were not designed or powered to detect a difference or equivalence in gestational age. In fact, they were all explicitly intended to detect a reduction in unnecessary treatment and/or hospital stay. It might have been possible to construct a model in which fFN testing was placed in the care pathway with all other tests (essentially history and examination). However, this would have required a review of the accuracy of all of these tests, which was beyond the scope of this project. Furthermore, we consider that the trial results, particularly from the Dutta and Norman study,47 do represent outcome given actual clinical practice. Therefore, it was decided to adjust the model structure, focusing on a reduction in admissions and costs. The decision tree is shown in Figure 12. This model cannot estimate an ICER as there is no measure of effectiveness included. Although a hospital admission could be interpreted as an outcome, it is mainly relevant from a cost perspective. Therefore, the outcome is cost difference of the fFN-testing strategy compared with the control strategy.
FIGURE 12.
Adjusted structure decision tree.
Structural assumptions:
-
The fFN-testing strategy, which is a combination of test-guided and clinical-guided care, will do no harm compared with the standard care strategy, which is based on clinical signs and symptoms alone. This means that it is assumed that in the fFN-testing strategy, there will be no patients deprived of care on the basis of test results when clinical signs and symptoms would indicate a hospital admission.
-
The time horizon of the model includes hospital admission for observational purposes, but not the delivery itself, since costs and consequences of delivery are considered not to be affected by the testing.
Model parameters
The study by Dutta and Norman47 was the only one that was performed in the UK. Therefore, the results from this study were used for the base-case input parameters, as, from an NHS perspective, they were considered more appropriate than the results from the non-UK-based studies. Results from the non-UK-based studies were used in sensitivity analyses.
Admission and treatment rates
Dutta and Norman47 reported 22 admissions out of 45 patients in the usual care group and 21 out of 46 patients in the treatment (with fFN) group. These values were taken as the base case. Incidence of steroid use and tocolysis were reported in a similar way, and are summarised in Table 13. The alpha value is the number of incident cases, and the beta value is its complement (i.e. the non-incident cases). These numbers are used to determine the base-case value, but also determine the distribution for the probabilistic sensitivity analysis.
| Parameter | Value | Alpha | Beta | Distribution |
|---|---|---|---|---|
| Control strategy | ||||
| Admission rate | 0.49 | 22 | 23 | Beta |
| Incidence of tocolysis | 0.18 | 4 | 18 | Beta |
| Incidence of steroid use | 0.95 | 21 | 1 | Beta |
| fFN-testing strategy | ||||
| Admission rate | 0.46 | 21 | 25 | Beta |
| Incidence of tocolysis | 0.14 | 3 | 18 | Beta |
| Incidence of steroid use | 0.81 | 17 | 4 | Beta |
As is apparent from the values in Table 13 and the results, which are reproduced in Table 14, there was a lack of clarity in the study by Dutta and Norman47 with respect to the total number of subjects. There were some differences between the total number of subjects (which is 44 in both groups) and the number which was used for reporting hospital admission and use of tocolysis and steroids. For the base case, we used the number as reported in the parameters of interest (which is either 45 or 46). However, from the results specified for positive and negative fFN test results (see Table 14, columns 5 and 6) it is clear that only 19 patients (instead of 21) were subjected to the fFN testing strategy. In the case of the control strategy, it is impossible to say what the true number of admissions was in the group of 44 subjects. In a sensitivity analysis, the influence of using the ‘original’ number of 44 and also reducing the number of incident cases was explored. In addition, the subject of sensitivity analyses was results from the other (non-UK-based) trials and a pooled average from all studies together.
| Parameter | Control | Treatment | p-value | fFN +ve | fFN −ve | p-valuea |
|---|---|---|---|---|---|---|
| Total number of subjects | 44 | 44 | – | 7 | 37 | – |
| Admission to hospital (%) | 22 (48.9%) (n = 45) | 21 (45.7%) (n = 46) | 0.757 | 7 (100.0%) (n = 7) | 12 (32.4%) (n = 37) | 0.002b |
| Transferred from hospital (%) | 4 (9.1%) (n = 44) | 3 (6.8%) (n = 44) | 1.000b | 1 (16.7%) (n = 6) | 2 (5.6%) (n = 36) | 0.441b |
| Incidence of steroid use (%) | 21 (45.7%) (n = 45) | 17 (37.0%) (n = 46) | 0.397 | 5 (71.4%) (n = 7) | 11 (29.7%) (n = 37) | 0.089b |
| Incidence of tocolysis (%) | 4 (8.7%) (n = 46) | 3 (6.5%) (n = 46) | 1.000b | 2 (28.6%) (n = 7) | 1 (2.7%) (n = 37) | 0.073b |
Treatment independent proportions applying to care in hospital
A number of parameters concerning care in hospital were assumed to be identical between treatment strategies. Although length of hospital stay was reported by Dutta and Norman47 (Table 15), it was calculated as an average among all study participants, not only the patients who were admitted. It was not possible to recalculate because of a large difference in total number (i.e. it was not possible to tell if all admitted patients were taken into account in this average). Therefore, it was decided to use a weighted (by activity) average length of stay of NHS reference costs:72 Healthcare Resource Group (HRG) NZ07 and NZ08 (both short and long stay). These HRGs include the code O60.X Preterm delivery, but not the delivery itself, since HRGs NZ11 through NZ15 represent deliveries. The weighted average length of stay was calculated at 1.63 days. This is substantially shorter than was reported in the HTA report by Honest et al. ,10 which can be explained by the fact that the delivery itself is not included here.
The other parameters that were considered to be equal between treatment strategies were hospital transfers, number of ultrasound examinations performed, and the proportion of tocolysis administered intravenously (i.v.) (as opposed to orally).
The number of ultrasound examinations was set at one per admission and the proportion of tocolysis administered by i.v. was set at 60%, both estimated by expert opinion (Professor Khalid Khan, University of London, 2012, personal communication).
As the number of hospital transfers, as reported by Dutta and Norman,47 was difficult to reliably recalculate into a parameter which would apply to only the admitted patients (as was the case for length of stay) and because there was a very small difference between treatment groups (see Table 15), it was assumed that the number of transfers would be equal for both strategies. The proportion was calculated at 16% (i.e. the total number of transfers for both groups, which is 7, divided by the total number of admissions, which is 43). Again, since there was a lack of clarity about the total number in the admission rate, the influence of this was explored in a sensitivity analysis.
When there was insufficient information to fit a beta or gamma distribution, we used a beta PERT distribution. 73 The beta PERT distribution is believed to be a useful form to express uncertainty, which is expressed in the form of most likely, highest and lowest. Each of these three values forms a parameter. Without a fourth parameter, this would imply a triangular distribution, but the beta PERT also has an additional ‘shape’ parameter, lambda, which, if set to 4, produces a shape similar to the normal distribution. 73
| Parameter | Value | Source | Distribution | |
|---|---|---|---|---|
| Control and fFN-testing strategy | ||||
| Length of stay (days) | 1.63 | HRGs72 | Beta PERTa | λ = 4 |
| Proportion of tocolysis i.v. | 0.60 | Expert opinion | Beta PERTa | λ = 4 |
| Proportion of transfers | 0.16 | Dutta and Norman4 | Beta | α = 7, β = 36 |
| Number of ultrasounds | 1 | Expert opinion | Beta PERTa | λ = 4 |
Costs
Costs of rapid fetal fibronectin test
The price of the fFN test was derived from the report by Honest et al. 10 and updated to the year 2011, resulting in a price of £21.29. 74 This price is, however, based on a pathology-based test, and includes lab costs, whereas the rapid test is a point of care test, and does not need any lab involvement. The rapid test does, however, require extra investment in the form of an analyser. We could not identify the costs of the analyser, or obtain an estimate of the utilisation rate. Therefore, we decided to use the price as reported by Honest et al. 10 and show the impact of varying the price, ranging between £0 and £300, in an additional analysis. The price of the fFN test was also varied in the regular probabilistic sensitivity analysis, with distribution beta PERT and λ = 4.
Costs of hospital stay and interventions
The costs of hospital stay and interventions are presented in Table 16. Costs of hospital stay (per day) were derived as a weighted (by activity) average from the NHS reference costs:72 HRGs NZ07 and NZ08, including short as well as long stay. Costs of tocolysis and corticosteroids were taken from British National Formulary 62 (BNF62),75 based on recommended doses as specified in the guidelines of the Royal College for Obstetricians and Gynaecologists. 77,78 The costs of an ultrasound examination were taken as an average from NHS reference costs:72 HRGs with code 501OU (antenatal ultrasound). The costs of hospital transfer is the reference cost for an emergency transfer, from the Department of Health, 2011. 74
Ranges used for specifying the beta PERT distribution were lower and upper boundaries as provided with the cost itself, or, if not available (in the case of tocolysis and corticosteroids), ± 20% of the base-case cost, since this was more or less comparable with the range for the parameters for which the information was known.
| Parameter | Value (£) | Source | Distribution | |
|---|---|---|---|---|
| Hospital day | 663.41 | HRGs72 | Beta PERTa | λ = 4 |
| Oral tocolysis | 0.27 | BNF6275 | Beta PERTa | λ = 4 |
| i.v. tocolysis | 484.79 | BNF6275 | Beta PERTa | λ = 4 |
| Corticosteroids | 4.46 | BNF6275 | Beta PERTa | λ = 4 |
| Ultrasound | 49.59 | NHS/HRG | Beta PERTa | λ = 4 |
| Hospital transfer | 253.00 | PSSRU76 | Beta PERTa | λ = 4 |
Additional analyses
First, one-way sensitivity analyses were performed for all parameters. Also, as has been indicated in the previous paragraphs, as there was uncertainty about the total number of subjects in certain parameters from the study by Dutta and Norman,47 these parameters were varied using a different number. Next, probabilistic sensitivity analyses were performed using parameter distributions instead of fixed values. The chosen distributions for each input parameter are presented in Tables 13, 15 and 16. In addition, we replaced the values taken from Dutta and Norman,47 where possible, with alternative values from other studies. We also performed an analysis exploring the effect of varying the price of the fFN test from £0 to £300. The last additional analysis was a scenario assuming that testing is not always necessary.
Results
Base-case analysis
In the base-case analysis, the costs of hospitalisations were higher in the control strategy. The difference was, however, partly offset by the costs of testing in the fFN-testing strategy. Total average costs of the control strategy were £599.53, whereas the costs of the fFN-testing strategy were £575.65. Therefore, the fFN-testing strategy saves £23.88.
Additional analyses
One-way sensitivity analyses
In the one-way sensitivity analyses, each parameter was varied between the lowest and highest values. The price of the fFN test itself is not included here, as it is subject of a separate sensitivity analysis. Results of the one-way sensitivity analyses are presented in Table 17.
| Parameter | Lowest value | Highest value | Incremental costs at | |
|---|---|---|---|---|
| Lowest value (£) | Highest value (£) | |||
| Costs of one hospital day (£) | 300.00 | 1000.00 | –4.75 | –41.59 |
| Costs of oral tocolysis (£) | 0.10 | 2.00 | –23.88 | –23.89 |
| Costs of i.v. tocolysis (£) | 200.00 | 700.00 | –19.83 | –26.93 |
| Costs of corticosteroids (£) | 2.00 | 7.00 | –23.64 | –24.12 |
| Costs of ultrasound (£) | 20.00 | 75.00 | –22.92 | –24.70 |
| Costs of transfer (£) | 100.00 | 500.00 | –23.07 | –25.18 |
| Number of transfers | 0.05 | 0.25 | –22.95 | –24.59 |
| Proportion of tocolysis i.v. | 0.1 | 0.9 | –18.14 | –27.32 |
| Number of ultrasounds | 0.5 | 3.0 | –23.08 | –27.07 |
| Admission rate fFN | 0.20 | 0.60 | –335.38 | 150.35 |
| Admission rate usual care | 0.20 | 0.60 | 330.39 | –160.13 |
| Incidence of tocolysis fFN | 0.10 | 0.40 | –29.57 | 10.28 |
| Incidence of tocolysis usual care | 0.10 | 0.40 | –12.24 | –54.91 |
| Incidence of corticosteroids fFN | 0.70 | 1.00 | –24.10 | –23.49 |
| Incidence of corticosteroids usual care | 0.70 | 1.00 | –23.32 | –23.97 |
Changing total numbers
As mentioned previously, the number of subjects, as reported in the study by Dutta and Norman,47 was unclear. Therefore, we performed a number of sensitivity analyses using an alternative number. For the hospital admission in the fFN-testing strategy there is only one alternative since from the table it was quite clear that the alternative could only go in one direction. For all other parameters, there are two alternatives: one which assumes the ‘extra’ subjects did experience the event (such as admission, tocolysis), and one which assumes they did not. For instance, admission to hospital in the control strategy counted 45 subjects, of whom 22 were admitted. However, the control strategy counted only 44 subjects overall. The extra patient was removed in calculating the parameter, so the first alternative, assuming the extra patient was admitted, will result in a 21/44 rate, whereas the second scenario, assuming the extra patient was not admitted, will result in a 22/44 rate. Therefore, in summary, the first alternative will lower the value and the second one will increase it. Table 18 shows the changed parameter values and results for these analyses.
| Parameter | Original value | Alternative value | Incremental costs (£) |
|---|---|---|---|
| Hospital admission fFN testing | 0.46 | 0.44 | –76.43 |
| Hospital admission usual care (1) | 0.49 | 0.48 | –13.08 |
| Hospital admission usual care (2) | 0.49 | 0.50 | –60.19 |
| Incidence of tocolysis fFN (1) | 0.14 | 0.05 | –22.44 |
| Incidence of tocolysis fFN (2) | 0.14 | 0.16 | –14.33 |
| Incidence of tocolysis usual care (1) | 0.18 | 0.14 | –36.02 |
| Incidence of tocolysis usual care (2) | 0.18 | 0.19 | –26.48 |
| Incidence of corticosteroids fFN (1) | 0.81 | 0.79 | –23.71 |
| Incidence of corticosteroids fFN (2) | 0.81 | 0.89 | –23.78 |
| Incidence of corticosteroids usual care (1) | 0.95 | 0.95 | –23.97 |
| Incidence of corticosteroids usual care (2) | 0.95 | 1.00 | –23.94 |
Probabilistic sensitivity analysis
The probabilistic sensitivity analysis (1000 simulations) was run using the distributions mentioned in the previous paragraphs. Total costs for the testing strategy were £606.11 as opposed to £631.69 for the control strategy, which means an incremental saving of £25.58.
Using alternative studies values
Because the study performed by Dutta and Norman47 was the only study identified by the systematic review that was UK-based, we used this study as input for the base-case analysis. However, there were several other studies which reported results on parameters such as hospital admission rate, incidence of tocolysis and corticosteroid use. Two studies48,50 reported admission rates as well as incidence of tocolysis and corticosteroid use. Therefore, in this additional analysis, we replaced the values derived from the study by Dutta and Norman47 by values from these two studies. Plaut et al. 49 also reported results on length of stay for patients admitted > 6 hours, which was significantly different between fFN testing and control strategies. Therefore, we additionally replaced the original length of stay, which in the base case was calculated according to HRG codes and assumed to be equal for both strategies, by these data. Table 19 shows which values were replaced and the corresponding results. Using the Lowe et al. 48 scenario resulted in an fFN-testing strategy that was more expensive than the control strategy. The Grobman et al. 50 scenario led to an incremental cost of close to zero. Using length of stay from Plaut et al. 49 favoured the fFN-testing strategy.
| Parameter | Original value | Alternative value | Incremental costs (£) |
|---|---|---|---|
| Using data from Lowe et al.48 | |||
| Admission rate fFN testing | 0.46 | 0.35 | 170.71 |
| Admission rate usual care | 0.49 | 0.24 | |
| Incidence of tocolysis fFN testing | 0.14 | 0.48 | |
| Incidence of tocolysis usual care | 0.18 | 0.45 | |
| Incidence of corticosteroids fFN testing | 0.81 | 0.50 | |
| Incidence of corticosteroids usual care | 0.95 | 0.43 | |
| Using data from Grobman et al.50 | |||
| Admission rate fFN testing | 0.46 | 0.26 | –4.72 |
| Admission rate usual care | 0.49 | 0.28 | |
| Incidence of tocolysis fFN testing | 0.14 | 0.16 | |
| Incidence of tocolysis usual care | 0.18 | 0.18 | |
| Incidence of corticosteroids fFN testing | 0.81 | 0.16 | |
| Incidence of corticosteroids usual care | 0.95 | 0.20 | |
| Using length of stay from Plaut et al.49 | |||
| Length of stay fFN testing | 1.63 | 0.95 | –213.33 |
| Length of stay usual care | 1.63 | 1.58 | |
Sensitivity analysis for price range
As it was difficult to obtain a reliable price for the rapid fFN test, in the base case we used a price which was derived from Honest et al. 10 In addition, we calculated incremental costs of the fFN-testing strategy for a range of prices for the test itself. The results of this analysis are shown in Figure 13. It is obvious that, all other costs held equal, the relation between test price and incremental costs is linear. Testing is cost-neutral at a test price of slightly over £45.
FIGURE 13.
Incremental costs of the fFN-testing strategy compared with control strategy at varying test prices.
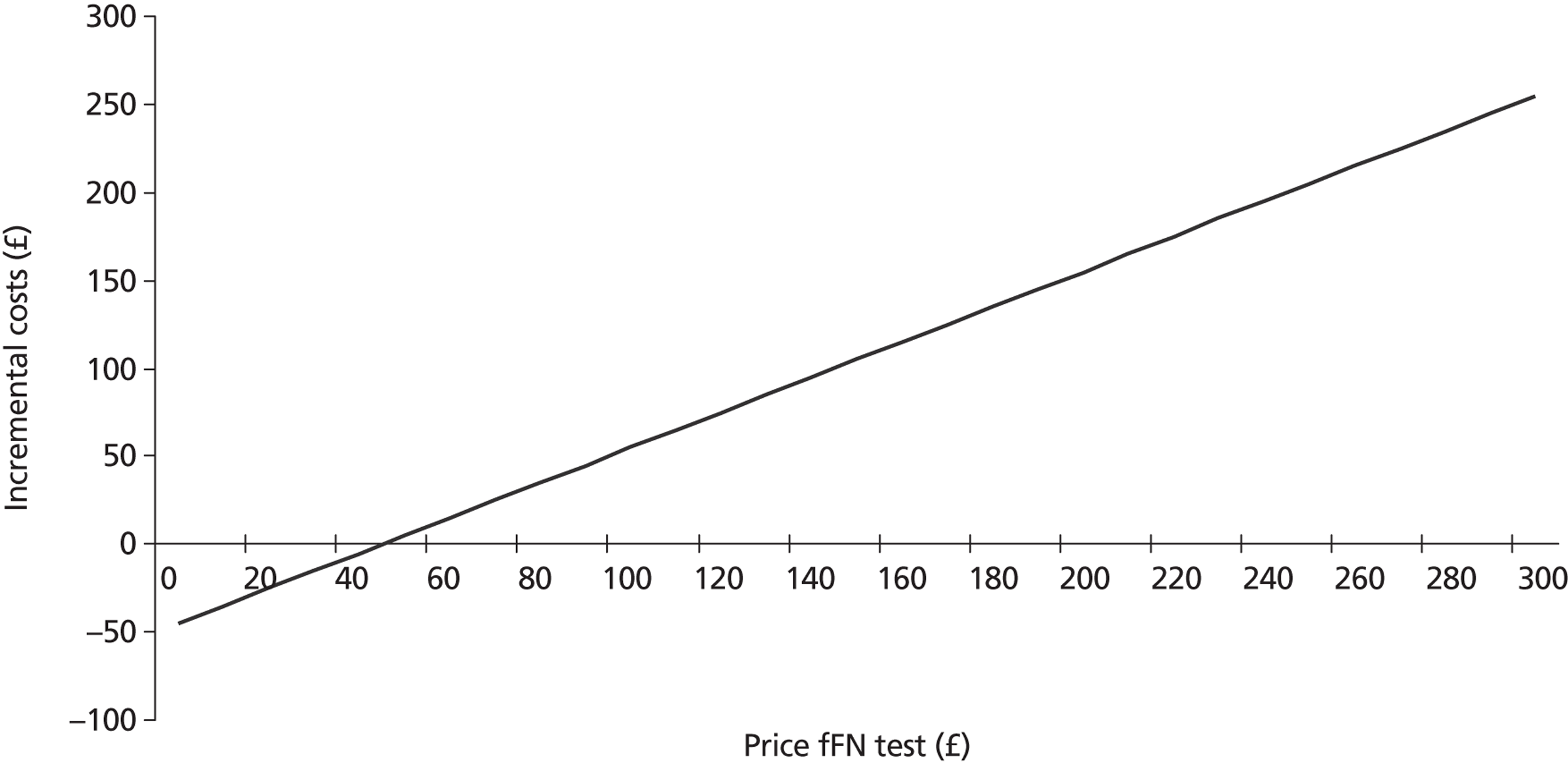
No test needed scenario
Dutta and Norman47 reported that, of the 37 patients who tested negative for fFN, 12 were admitted anyway for various reasons, such as previous history of preterm labour or stillbirth, or pyelonephritis. In these patients, an fFN test would not have been necessary, since they were admitted regardless of the test results. 47 We assumed an alternative scenario, reducing the test costs by this proportion of 12 out of 44 women (assuming that the test was indeed necessary in all of the seven patients who tested positive, which is probably a conservative estimate) for the admitted group. This reduced the incremental costs further to –£26.53.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness and test accuracy findings
Summary of results of effectiveness studies
The five studies included in this review were RCTs (four47–50 published in full and one51 published as a conference abstract). All studies randomly allocated the women with symptoms of preterm labour to a strategy of clinical management with or without the availability of fFN test results. All five reported measures of the duration of hospital stay and four studies reported the estimated gestational age at birth. However, incidence of PTB (our primary outcome measure) was reported by only two studies. 49,50 The outcome measure of maternal treatment was described by all studies except for the conference abstract. The maternal treatments administered were mainly prenatal corticosteroids and tocolytic agents. Three studies47,48,50 reported the use of both corticosteroids and tocolytic agents. One study49 reported the use of aggressive therapy that included administration of tocolytic agents, corticosteroids and transfer to a tertiary care facility. Four studies47,48,50,51 reported the incidence of hospital admissions for PTB and, of these, one47 also reported the incidence of NICU admissions. In line with previous observations, the majority of the included studies reported no significant benefits associated with the availability of fFN test results for any of the outcomes assessed. 30
The only significant benefit was reported by Plaut et al. ,49 who found that knowledge of fFN test results by clinicians significantly reduced the length of hospital stay for women with negative test results who were observed for > 6 hours (17% of the population). This study49 reported low enrolments. The original estimation of the required sample size was 500 women to detect a significant difference in transport to tertiary care centre; however, because of low enrolments this study was terminated prematurely. Hence, in our quality assessment this study was judged to be at ‘high risk’ of bias for two domains, because it was stopped early and also because of selective reporting of a secondary outcome of interest, which appeared to be based on significance. There were some quality issues concerning Dutta and Norman,47 it was judged to be at ‘high risk’ of bias for the domain incomplete data outcomes mainly because the study had some missing values. In addition, there was no intention-to-treat analysis (different sample sizes were reported for each outcome). The study by Lowe et al. 48 was the only study to be judged at low risk of bias for the three key domains. All the remaining studies were judged to be at ‘unclear risk’ of bias, because of poor reporting.
Summary of test accuracy results
A previous HTA report by Honest et al. 10 assessed various combinations of tests and treatments that aimed to predict and prevent spontaneous PTB. This report10 had wider inclusion criteria than the current assessment, as it assessed various tests to predict PTB in both symptomatic and asymptomatic women; however, it did not include evidence from RCTs of the clinical effectiveness of fFN testing. We have updated the section of the review which complied with our inclusion criteria involving symptomatic women (< 37 weeks' gestation) who underwent fFN testing, in order to provide a complete, up-to-date summary of all potentially relevant evidence (both on the clinical effectiveness and predictive accuracy of rapid fFN testing). The relevant searches from the HTA report covered the period up to 2005. Hence, we updated the searches from 2005 to present and identified new studies that matched our inclusion criteria.
We identified 15 new DTA studies in total from the updated search. In our analysis we combined data from these newly identified studies and the 39 relevant studies identified from the appendices of the previously published HTA report. 35 A modified version of QUADAS-2 was used to assess the quality of 13 new studies in this report (two non-English studies could not be assessed). Four of the 15 new studies reported prospective, consecutive recruitment of the participants. Thus, the majority of studies were rated at ‘high risk’ of bias for the patient selection domain. This was consistent with the findings of the previous HTA, in which 6 of the 39 studies reported prospective, consecutive recruitment. The threshold of the index test was known for all studies except one. 61 In some studies thresholds were not specifically mentioned, and in these cases the threshold was assumed to be the standard recommended by the commercial test kits used in the study. Missing data were found in only one study, which, as a result, was judged to be at ‘high risk’ of bias for QUADAS-2 domain ‘flow and timing’. 57
Test accuracy studies included in this review were grouped by reference standard outcome (preterm delivery within 7–10 days of testing, before 34 weeks' gestation and before 37 weeks' gestation). The accuracy of fFN testing to predict preterm delivery within 7–10 days of testing was reported by 10 studies from our update searches and data for 17 studies were taken from previous HTA report appendices. The overall sensitivity and specificity using a bivariate model were 76.7% (95% CI 70.4% to 82.0%) and 82.7% (95% CI 79.4% to 85.5%), respectively. The estimates of the test performance were similar across all the subgroups analysed (singleton gestations only vs. unselected populations and ‘new’ studies vs. studies included in the previous HTA). Accuracy data for PTB before 34 weeks' gestation were reported by 19 studies (11 new and eight from the previous systemic review). The overall sensitivity and specificity using bivariate model were 69.1% (95% CI 58.6% to 77.9%) and 84.4% (95% CI 79.8% to 88.2%), respectively. The subgroup analysis carried out using the bivariate model for studies that included only women with singleton pregnancies showed a trend towards increased sensitivity (76.4%) compared with an unselected population (62.7%). However, this difference was not statistically significant and estimates of test performances were similar in other subgroups analysed. The sensitivity analysis, excluding four studies which used PTB < 35 weeks' gestation as the reference standard, did not change the results significantly. In all, 39 studies reported the accuracy of fFN testing for predicting PTB before 37 weeks (eight new and 31 from the previous systematic review). The overall sensitivity and specificity using the bivariate model were 60.8% (95% CI 53.7% to 67.6%) and 85.3% (95% CI 82.5% to 87.7%), respectively. The estimates of the test performance were similar across all the subgroups analysed and for the sensitivity analysis excluding one study with reference standard < 38 weeks' gestation. 61
The sensitivity and specificity of fFN appeared similar in singleton gestation only and unselected populations. This finding was consistent with the findings of a previous review of the accuracy of fFN testing. 6 However, it should be noted that both this review and the current assessment compared accuracy in studies which excluded patients with multiple gestations with those that did not, rather than explicitly comparing accuracy in women with singleton with multiple gestations; none of the included studies exclusively included women with multiple gestations. Hence, it would seem likely that the unselected (mixed) population may have included more women with singleton than multiple gestations, thus masking any potential differences in accuracy between the two groups. We are not aware of any previous systematic review which has compared accuracy of fFN testing in women with singleton and multiple gestations; the results of a review assessing fFN testing in women with multiple gestations only appeared to indicate that sensitivity may be higher in this population. 22
In line with the findings of several previous systematic reviews, the results of this assessment suggest that the sensitivity of fFN testing may be highest for predicting PTB within 7–10 days of testing (specificity was similar for PTB within 7–10 days of testing, at < 34 weeks' gestation and at < 37 weeks' gestation). 6,22,29 Thus, fFN testing may be most useful as a component of the decision on whether or not to administer antenatal corticosteroids. However, the relatively low sensitivity estimates and correspondingly high numbers of FNs suggest that fFN testing alone would not be adequate to rule out intervention. The available evidence from clinical trials would appear to indicate that these FN results do not lead to an increase in negative clinical outcomes associated with testing (i.e. no evidence of an increased number of PTBs or adverse neonatal outcomes). This may be because fFN test results were only one component of the decision-making process; in most studies, treatment decisions were ‘at the clinicians' discretion’.
Cost-effectiveness findings
The cost-effectiveness analysis did not include an effectiveness measure, since no clear indication of improved effectiveness given fFN testing was found in the trial data. Instead, the decision tree aimed to give an assessment of the costs associated with fFN testing. The base-case analysis showed a small cost advantage (i.e. £23.88) for the fFN-testing strategy. This result is however surrounded by quite some uncertainty, as it leaned very heavily on data from the study by Dutta and Norman47 As we were aware of this uncertainty, a number of additional analyses were done. For instance, the base-case analysis was re-run with data from alternative studies. When applying data from the study by Lowe et al. ,48 which reported a higher admission rate in the fFN-testing strategy, running the model resulted in an incremental cost of £170.71 of fFN testing compared with control. When using data from the study by Grobman et al. ,50 results were more or less comparable with the base case. Plaut et al. 49 reported a statistically significant shorter length of stay for the fFN-testing strategy. When this fact was applied to the decision tree, fFN testing led to a cost saving of £213.33.
Performing a probabilistic sensitivity analysis resulted in an average cost saving of £25.58. The 2.5th and 97.5th percentiles of the simulated results were –£305 and £240, respectively, which indicates that although the absolute difference in costs between strategies may be modest, there is uncertainty about whether fFN testing will come at a cost or generate savings. All other additional analyses show results in the same range.
As there was also uncertainty about the actual price of the rapid fFN test itself, incremental costs of fFN testing compared with usual care was calculated for a large range of possible prices. A price of £45 turned out to be the point where costs between strategies break even. Although the base-case price, which was derived from Honest et al. ,10 is below this ‘threshold’ of £45, it is difficult to say whether or not this is a realistic price. An Australian report dating from 2006 calculated prices for two types of rapid tests, of which the cheapest amounted to around $100 (Australian dollars), which converted with exchange rates of 2011 would mean £68. 29
Strengths, limitations and uncertainties of the assessment
Strengths, limitations and uncertainties of the systematic review
The systematic review conducted for this assessment represents a step forward on previously published systematic reviews,6,10,17,22,28,29 in that we have included both up-to-date data on the accuracy of fFN testing for the prediction of PTB and data from clinical trials assessing the effectiveness of including fFN testing in the clinical decision-making process. We have synthesised evidence from both study types in an attempt to provide a more complete picture of how fFN testing might be used in clinical practice. Our assessment of test accuracy uses a bivariate modelling approach, as recommended by current methodological guidance. 31,39 In addition, we have used a combination of subgroup analyses, regression analyses and sensitivity analyses to explore the potential effects on test accuracy of selected population and study design characteristics, as well as publication date.
Extensive literature searches were conducted in an attempt to maximise retrieval of relevant studies. These included electronic searches of a variety of bibliographic databases, as well as screening of clinical trials registers and conference abstracts to identify unpublished studies. Because of the known difficulties in identifying test accuracy studies using study design-related search terms, search strategies were developed to maximise sensitivity at the expense of reduced specificity. 32 Thus, large numbers of citations were identified and screened, many of which did not meet the inclusion criteria of the review. However, it should be noted that our review of test accuracy studies was an update of the Honest et al. 10 review, which had a methodological search filter; it is therefore possible that some relevant studies published before 2005 may not have been included.
Clear inclusion criteria were specified in the protocol for this review. Eligibility of studies for inclusion is therefore transparent. In addition, we have provided specific reasons for excluding any of the studies considered potentially relevant at initial citation screening (see Appendix 9). The review process followed recommended methods to minimise the potential for error and/or bias;31 studies were independently screened for inclusion by two reviewers and data extraction and quality assessment were done by one reviewer and checked by a second. Any disagreements were resolved by consensus.
The studies included in the review were RCTs and DTAs. Methodological quality of the RCTs was assessed using Cochrane risk of bias tool and for DTAs the assessment was done using a modified version of QUADAS-2. The QUADAS tool is recommended for assessing the methodological quality of test accuracy studies,31,39 widely adopted by researchers and key organisations such as the Cochrane Collaboration, the National Institute for Health and Care Excellence (NICE) in the UK, and Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG) in Germany. The revised version of QUADAS (QUADAS-2) has recently been published. 43 We consider QUADAS-2 to be the most appropriate tool currently available for the quality assessment of test accuracy studies. However, the applicability of QUADAS-2 to the current assessment was somewhat limited. It was considered that the inclusion criteria matched the review question and that questions of applicability were, therefore, not relevant. In addition, because the reference standard was the occurrence of preterm or term birth in all studies, we considered that there were no issues of bias relating to the adequacy or application of the reference standard and the ‘reference standard’ domain of QUADAS-2 was omitted; this study design also meant that many of the signalling questions for the ‘flow and timing’ domain were not considered relevant. The usefulness of quality assessment was further limited by poor reporting of primary study methods. The review-specific guidance used in our QUADAS-2 assessment is reported in Appendix 5. The results of the risk of bias assessment are reported, in full, for all included studies (see Appendix 6) and in summary in Chapter 3, Results and Table 6. The extent to which we were able to explore the impact of the remaining, relevant components of study quality on test accuracy was also constrained by the extent to which comparable data were reported in the previous systematic review from which some of our data were drawn. 10
The possibility of publication bias remains a potential problem for all systematic reviews. Considerations may differ for systematic reviews of test accuracy studies. It is relatively simple to define a positive result for studies of treatment (e.g. a significant difference between the treatment and control groups which favours treatment). This is not the case for test accuracy studies, which measure agreement between index test and reference standard. It would seem likely that studies finding greater agreement (high estimates of sensitivity and specificity) will be published more often. In addition, test accuracy data are often collected as part of routine clinical practice, or by retrospective review of records; test accuracy studies are not subject to the formal registration procedures applied to RCTs and are therefore more easily discarded when results appear unfavourable. The extent to which publication bias occurs in studies of test accuracy remains unclear; however, simulation studies have indicated that the effect of publication bias on meta-analytic estimates of test accuracy is minimal. 79 Formal assessment of publication bias in systematic reviews of test accuracy studies remains problematic and reliability is limited. 79 We did not undertake a statistical assessment of publication bias for accuracy studies or effectiveness studies in this review. However, our search strategy included a variety of routes to identify unpublished studies and resulted in the inclusion of a number of conference abstracts.
Despite efforts to include evidence from both clinical trials and test accuracy studies, it should be noted that few RCTs were identified and the RCTs included in this review are of generally poor quality and are likely to be underpowered. All the included RCTs were rated to be at ‘unclear risk’ of bias except for one study which was judged to be at low risk of bias across the key domains of Cochrane risk of bias assessment tool. 48 The main methodological issue for the included RCTs was the lack of appropriate power calculations and hence the potential for underpowered studies. Power calculations were generally poorly reported and failed to take account of the proportion of patients in the study that had a negative fFN test result (those whose test results have the potential to change management). In addition, one of the studies was stopped early because of the low enrolments and did not achieve the desired sample size. 47 Hence, studies may have been inadequately powered to detect possible benefits of fFN testing. As has been previously discussed in a systematic review of the methodological issues associated with clinical trials in this field, no study used a discordancy design. 30 A discordancy study design aims to randomise only those patients whose test results indicate a different management strategy to that based on usual assessment, in this case symptomatic women who have a negative fFN test result (without testing all symptomatic women are assumed to receive treatment, e.g. tocolysis, antenatal corticosteroids, hospitalisation). Thus, symptomatic women with a negative fFN result would be randomised to treatment (management decision based on usual assessment) or no treatment (management decision based on fFN test results). Comparison of clinical outcomes between these randomised groups shows the effects of deciding not to treat on the basis of a negative fFN result. A further significant methodological problem was that none of the included studies used a fixed management protocol in women with known fFN test results; treatment was generally at the clinicians' discretion. Plaut et al. 49 and Grobman et al. 53 provided clinicians with information about the positive and negative predictive values of the fFN test, but did not mandate treatment protocols. Studies reported outcomes by randomised group, but not stratified by fFN test result. Thus, the extent to which clinicians incorporated the knowledge of test results in their decision making remained largely unclear and potential effects of fFN testing may have been missed because clinicians did not use the test results in their decision-making. This issue was also highlighted by the previous systematic review of methodology. 30 Grobman et al. 50 reported that if the test were used for longer period of time physicians would think that the test was more reliable.
Information of the effects of fFN testing on neonatal outcomes was particularly scarce; only one study reported the neonatal outcomes (number of NICU admissions). 47 This study had a very small sample size and found no significant effects of testing.
Strengths, limitations and uncertainties of the cost-effectiveness analysis
One of the main strengths of the model is that the evidence we used to inform the parameters was relevant for the UK and as up-to-date and high quality as possible. Where evidence was not available from published studies or databases, we used the most likely and plausible ranges based on expert opinion.
An important limitation, however, also lies in the parameters. As there was only one UK-based study (Dutta and Norman47), this was the main provider of the data. Dutta and Norman47 reported that the admission rate in the fFN-testing strategy was lower than the admission rate in the control strategy. If, in clinical practice, testing for fFN would indeed lead to lowered admission rates, then the costs of testing could quite easily be offset. 47 However, although Grobman et al. 50 and Osório et al. 51 also found a slightly lower admission rate in fFN-tested patients, and Plaut et al. 49 reported a length of stay for fFN testing that was statistically significantly shorter than in the control group, there is also evidence to the contrary, as Lowe et al. 48 found that patients in the fFN-testing strategy group were more likely to be admitted than patients in the control strategy group. Given the fact that almost all costs in the model (e.g. hospital stay, tocolysis, corticosteroids, ultrasound examinations and hospital transfers) are admission driven, being uncertain about the admission rate has major implications for the uncertainty of the model outcome as a whole.
Another limitation lies in the fact that we could not incorporate effectiveness in the model. However, assuming that fFN testing is mainly an instrument to safely select those patients who do not need treatment, one would not necessarily expect it to have an impact on pregnancy outcome. Also, we cannot be sure that fFN testing would not be useful for preventing PTBs. Although, of course, it would have been technically possible to include pregnancy outcome in the model, this would have required reliance on accuracy data both for the fFN test and for all other tests (essentially history and examination), which was beyond the scope of this project. Moreover, we would also argue that the evidence from trials, in particular that by Dutta and Norman,47 given that it was conducted in the UK, would, on balance, provide more reliable data than data from a combination of accuracy studies of probable low quality. We are informed that a large study assessing fFN and resource use, conducted at Guy's and St Thomas' NHS Foundation Trust, is currently being prepared for publication [Assessment of Fetal Fibronectin Testing to Improve Preterm Management (AFFIRM) study].
Chapter 6 Conclusions
Implications for service provision
The results of our systematic review suggest that fFN testing has a moderate accuracy for predicting PTB (with 7–10 days of testing, < 34 weeks' gestation, or < 37 weeks' gestation) and may be most sensitive for predicting PTB within 7–10 days of testing. The main potential role of fFN testing is likely to be to reduce health-care resource usage by identifying women who do not require active intervention (i.e. by ruling out likely PTB). The sensitivity estimates for fFN would suggest that, if considered in isolation, the test would be unlikely to be adequate to identify symptomatic women who do not need active intervention. However, because, in practice, clinical decision-making is multifactorial, FN results on fFN may not translate into an increase in adverse outcomes for mothers and neonates. It is highly unlikely that, in practice, fFN results would be considered in isolation, and it should be noted that none of the studies included in this review were optimally designed to fully assess the effectiveness of fFN testing as it would be likely to be used in clinical practice. The trials included in this review suggested that adverse outcomes do not increase as a result of including fFN in the diagnostic workup, where treatment decisions remain at the discretion of clinicians. There was also some, very limited, evidence that including fFN in the diagnostic workup may reduce resource use (e.g. maternal hospitalisation). There is no evidence to support the use of fFN testing in pursuit of improved maternal or neonatal outcomes. We did not identify any safety data, but since the test sample for fFN testing is obtained from a routine cervicovaginal swab after speculum examination, the risk to the mothers and their expected babies should be negligible. It should be noted that the studies identified by our review do not provide information on the effect of fFN testing on clinical decision-making.
The potential for the fFN to reduce health-care costs associated with management of women with clinical diagnosis of preterm labour appears to be dependent on a decision to admit. However, there is no evidence from RCTs that use of fFN test reduces admissions. Also, the effect of fFN test results on clinical decision-making in women to be admitted on clinical grounds is unclear. Larger, better-designed trials are required to confirm these findings.
The base-case analysis showed a small cost advantage (i.e. £23.88) associated with the fFN-testing strategy. However, this result was surrounded by considerable uncertainty and the conclusion of the cost analysis is largely dependent on whether or not fFN testing indeed reduces hospital admission. There was also uncertainty about the actual price of the rapid fFN test itself. For the base-case analysis, the price at which fFN testing is cost neutral lies at around £45.
Suggested research priorities
All the effectiveness studies included in our systematic review were RCTs. However, there were no high-quality studies, and studies were generally underpowered and of suboptimal design, as described in the previous sections. The existing evidence is extremely limited in terms of the impact of fFN testing on both clinical decision-making and patient outcomes. Hence, there is a need for high-quality, adequately powered trials, using appropriate study designs (e.g. discordancy), as described above, to confirm whether or not the use of fFN testing in clinical decision-making can reduce unnecessary interventions, and to assess how these treatment decisions relate to improved patient outcomes. There is also a need to investigate whether or not there is any increase in negative outcomes as a consequence of adding fFN testing to the triage of women with symptoms of preterm labour, particularly for neonatal outcomes, where currently there is lack of data.
As clinical decision-making is, in practice, multifactorial, more risk prediction modelling might provide an alternative, potentially informative approach to assessing the role of fFN in combination with other potential independent predictors (including components of the standard diagnostic workup) in predicting PTB outcomes. For example, such studies might focus on delivery within 7 days of testing as the dependent variable, since this is the treatment window for corticosteroids which are known to be effective in reducing neonatal morbidity/mortality.
Current evidence does not adequately assess potential variation in the accuracy of fFN testing between different clinical groups, particularly between singleton and multiple gestations. Therefore, large DTA studies, which report data separately for singleton and multiple gestations, might also be useful.
Acknowledgements
The authors acknowledge the screening and data extraction of clinical effectiveness studies done by Carla Truyers.
Contributions of authors
Marie Westwood and Sohan Deshpande planned and performed the systematic review and interpretation of evidence.
Thea van Asselt and Florian Tomini planned and performed the cost-effectiveness analyses and interpreted results.
Nigel Armstrong contributed to planning and interpretation of the cost-effectiveness analyses and acquisition of input data for modelling.
Alexander Allen and Caro Noake devised and performed the literature searches and provided information support to the project.
Khalid Khan provided the clinical advice and the expert opinion.
Johan Severens wrote the cost-effectiveness protocol and provided senior advice and support to the assessment.
Jos Kleijnen provided senior advice and support to the assessment.
All parties were involved in drafting and/or commenting on the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Berghella V, Hayes E, Visintine J, Baxter JK. Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database Syst Rev 2008;4. http://dx.doi.org/10.1002/14651858.CD006843.
- Morrison JJ, Rennie JM. Clinical, scientific and ethical aspects of fetal and neonatal care at extremely preterm periods of gestation. BJOG 1997;104:1341-50. http://dx.doi.org/10.1111/j.1471-0528.1997.tb11002.x.
- Byrne B, Morrison JJ. Preterm birth. Clin Evid 2004;11:1903-22. http://dx.doi.org/10.1016/j.ajog.2010.10.499.
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2003. Natl Vital Stat Rep 2005;54:1-116.
- McPheeters ML, Miller WC, Hartmann KE, Savitz DA, Kaufman JS, Garrett JM, et al. The epidemiology of threatened preterm labor: a prospective cohort study. Am J Obstet Gynecol 2005;192:1325-9. http://dx.doi.org/10.1016/j.ajog.2004.12.055.
- Honest H, Bachmann LM, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervicovaginal fetal fibronectin test in predicting risk of spontaneous preterm birth: systematic review. BMJ 2002;325. http://dx.doi.org/10.1136/bmj.325.7359.301.
- Live births in 2004, selected background data (final), England and Wales. Newport, Wales: ONS; 2004.
- Kramer MS, Demissie K, Yang H, Platt RW, Sauve R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. JAMA 2000;248:843-9. http://dx.doi.org/10.1001/jama.284.7.843.
- Mathews TJ, Menacker F, MacDorman MF. Infant mortality statistics from the 2002 period linked birth/infant death data set. Natl Vital Stat Rep 2004;53:1-30.
- Honest H, Forbes CA, Duree KH, Norman G, Duffy SB, Tsourapas A, et al. Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess 2009;13.
- Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database Syst Rev 2006;3. http://dx.doi.org/10.1002/14651858.CD000065.
- Ascarelli MH, Morrison JC. Use of fetal fibronectin in clinical practice. Obstet Gynecol Surv 1997;52:S1-12. http://dx.doi.org/10.1097/00006254-199704000-00024.
- Lockwood CJ, Senyei AE, Dische MR, Casal D, Shah KD, Thung SN, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med 1991;325:669-74. http://dx.doi.org/10.1056/NEJM199109053251001.
- Treatment of women presenting in preterm labour. Essex: Basildon and Thurrock University Hospital (BTUH); 2008.
- Morgan MA, Goldenberg RL, Schulkin J. Obstetrician-gynecologists' knowledge of preterm birth frequency and risk factors. J Matern Fetal Neonatal Med 2007;20:895-901. http://dx.doi.org/10.1080/14767050701750498.
- Andersen HF. Use of fetal fibronectin in women at risk for preterm delivery. Clin Obstet Gynecol 2000;43:746-58.
- Using fetal fibronectin to diagnose pre-term labour. Edmonton, AB: IHE; 2008.
- Ramsey PS, Andrews WW. Biochemical predictors of preterm labor: fetal fibronectin and salivary estriol. Clin Perinatol 2003;30:701-33. http://dx.doi.org/10.1016/S0095-5108(03)00109-X.
- Tucker J, McGuire W. Epidemiology of preterm birth. BMJ 2004;329:675-8. http://dx.doi.org/10.1136/bmj.329.7467.675.
- Haram K, Mortensen JH, Wollen AL. Preterm delivery: an overview. Acta Obstet Gynecol Scand 2003;82:687-704. http://dx.doi.org/10.1034/j.1600-0412.2003.00218.x.
- Logghe H, Walker JJ. Towards improved neonatal outcome: future strategies. Semin Fetal Neonatal Med 2004;9:491-8. http://dx.doi.org/10.1016/j.siny.2004.08.002.
- Conde-Agudelo A, Romero R. Cervicovaginal fetal fibronectin for the prediction of spontaneous preterm birth in multiple pregnancies: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2010;23:1365-76. http://dx.doi.org/10.3109/14767058.2010.499484.
- Koenn ME. Fetal fibronectin. Clin Lab Sci 2002;15:96-8.
- Lee SK, McMillan DD, Ohlsson A, Boulton J, Lee DS, Ting S, et al. The benefit of preterm birth at tertiary care centers is related to gestational age. Am J Obstet Gynecol 2003;188:617-22. http://dx.doi.org/10.1067/mob.2003.139.
- Iams JD. Prediction and early detection of preterm labor. Obstet Gynecol 2003;101:402-12. http://dx.doi.org/10.1016/S0029-7844(02)02505-X.
- Hole JW, Tressler TB. Management of preterm labor. J Am Osteopath Assoc 2001;101:S14-8.
- Terzidou V, Bennett PR. Preterm labour. Curr Opin Obstet Gynecol 2002;14:105-13. http://dx.doi.org/10.1097/00001703-200204000-00002.
- Sanchez-Ramos L, Delke I, Zamora J, Kaunitz AM. Fetal fibronectin as a short-term predictor of preterm birth in symptomatic patients: a meta-analysis. Obstet Gynecol 2009;114:631-40. http://dx.doi.org/10.1097/AOG.0b013e3181b47217.
- Fetal fibronectin test for preterm labour. Canberra: MSAC; 2006.
- Vis JY, Wilms FF, Oudijk MA, Bossuyt PMM, van der Post JAM, Grobman WA, et al. Why were the results of randomized trials on the clinical utility of fetal fibronectin negative? A systematic review of their study designs. Am J Perinatol 2011;28:145-50. http://dx.doi.org/10.1055/s-0030-1263297.
- Systematic reviews: CRD's guidance for undertaking reviews in health care. York: University of York; 2009.
- Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. J Clin Epidemiol 2011;64:602-7. http://dx.doi.org/10.1016/j.jclinepi.2010.07.006.
- Diagnostics Assessment Programme: interim methods statement (version 2). London: NICE; 2010.
- McGowan J, Sampson M, Lefebvre C. An evidence based checklist for the peer review of electronic search strategies (PRESS EBC). Evid Based Libr Inf Pract 2010;5:1-6.
- Honest H, Forbes CA, Duree KH, Norman G, Duffy SB, Tsourapas A, et al. [Appendices] Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess 2009;13.
- Zagorac D, Doll K, Schon JC, Jansen M. Ab initio structure prediction for lead sulfide at standard and elevated pressures. Physical Review B 2011;84. http://dx.doi.org/10.1103/PhysRevB.84.045206.
- Tang ZH, Tadesse S, Norwitz E, Mor G, Abrahams VM, Guller S. Isolation of hofbauer cells from human term placentas with high yield and purity. Am J Reprod Immunol 2011;66:336-48. http://dx.doi.org/10.1111/j.1600-0897.2011.01006.x.
- Taylor BK. Sonographic assessment of cervical length and the risk of preterm birth. J Obstet Gynecol Neonatal Nurs 2011;40:617-31. http://dx.doi.org/10.1111/j.1552-6909.2011.01284.x.
- Handbook for DTA reviews. Cochrane Collaboration; 2011.
- Solarino G, Piazzolla A, Mori CM, Moretti L, Patella S, Notarnicola A. Alumina-on-alumina total hip replacement for femoral neck fracture in healthy patients. BMC Musculoskelet Disord 2011;12.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. http://dx.doi.org/10.1016/0197-2456(86)90046-2.
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration; 2011.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. http://dx.doi.org/10.1016/j.jclinepi.2005.02.022.
- Harbord RM, Deek JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51. http://dx.doi.org/10.1093/biostatistics/kxl004.
- Moses LE, Shapiro D, Littenberg B. Combining independant studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993;12:1293-316. http://dx.doi.org/10.1002/sim.4780121403.
- Lijmer JG, Bossuyt PMM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med 2002;21:1525-37. http://dx.doi.org/10.1002/sim.1185.
- Dutta D, Norman JE. Pilot study into the efficacy of foetal fibronectin testing in minimising hospital admissions in women presenting with symptoms of preterm labour: a randomised controlled trial of obstetric and neonatal outcomes. Arch Gynecol Obstet 2011;284:559-65. http://dx.doi.org/10.1007/s00404-010-1712-x.
- Lowe MP, Zimmerman B, Hansen W. Prospective randomized controlled trial of fetal fibronectin on preterm labor management in a tertiary care center. Am J Obstet Gynecol 2004;190:358-62. http://dx.doi.org/10.1016/j.ajog.2003.08.041.
- Plaut MM, Smith W, Kennedy K, Nageotte M, DeCastro E, Steinke R, et al. Fetal fibronectin: the impact of a rapid test on the treatment of women with preterm labor symptoms. Am J Obstet Gynecol 2003;188:1588-95. http://dx.doi.org/10.1067/mob.2003.390.
- Grobman WA, Welshman EE, Calhoun EA. Does fetal fibronectin use in the diagnosis of preterm labor affect physician behavior and health care costs? A randomized trial. Am J Obstet Gynecol 2004;191:235-40. http://dx.doi.org/10.1016/j.ajog.2003.11.034.
- Osório M, Neiva R, Montes L, Silva J, Pinelo S, Pinho M, et al. The impact of fetal fibronectin assay on preterm labor management: a randomized controlled trial. Paper presented at 22nd European Congress of Perinatal Medicine; 26–29 May 2010; Granada, Spain. J Matern Fetal Neonatal Med 2010;23:304-5.
- Hansen W, Lowe MP, Zimmerman B. Effect of the fetal fibronectin assay on preterm labor management. Am J Obstet Gynecol 2001;185. http://dx.doi.org/10.1016/S0002-9378(01)80232-5.
- Grobman W, Welshman E, Calhoun E. Does fetal fibronectin use in the diagnosis of preterm labor affect physician behavior and health care costs. Paper presented at 23rd Annual Meeting of the Society for Maternal-Fetal Medicine; 3–8 Feb 2003; San Francisco, USA. Am J Obstet Gynecol 2003;187. http://dx.doi.org/10.1016/j.ajog.2003.11.034.
- Diaz J, Chedraui P, Hidalgo L, Medina M. The clinical utility of fetal fibronectin in the prediction of pre-term birth in a low socio-economic setting hospital in Ecuador. J Matern Fetal Neonatal Med 2009;22:89-93. http://dx.doi.org/10.1080/14767050802464551.
- Asakura H, Fukami T, Kurashina R, Tateyama N, Doi D, Takeshita T. Significance of cervical gland area in predicting preterm birth for patients with threatened preterm delivery: comparison with cervical length and fetal fibronectin. Gynecol Obstet Invest 2009;68:1-8. http://dx.doi.org/10.1159/000209394.
- Tsoi E, Akmal S, Geerts L, Jeffery B, Nicolaides KH. Sonographic measurement of cervical length and fetal fibronectin testing in threatened preterm labor. Ultrasound Obstet Gynecol 2006;27:368-72. http://dx.doi.org/10.1002/uog.2723.
- Skoll A, St Louis P, Amiri N, Delisle M-F, Lalji S. The evaluation of the fetal fibronectin test for prediction of preterm delivery in symptomatic patients. J Obstet Gynaecol Can 2006;28:206-13.
- Desjardins PR, Dansereau J, Hoag GN. Comparing the clinical effectiveness of Fetal Fibronectin and IGFBP-1 measurements in cervico-vaginal secretions, in predicting preterm deliveries. Clin Chem 2008;54:A39-40.
- Eroglu D, Yanik F, Oktem M, Zeyneloglu HB, Kuscu E. Prediction of preterm delivery among women with threatened preterm labor. Gynecol Obstet Invest 2007;64:109-16. http://dx.doi.org/10.1159/000100120.
- Groom KM, Liu E, Allenby K. The impact of fetal fibronectin testing for women with symptoms of preterm labour in routine clinical practice within a New Zealand population. Aust N Z J Obstet Gynaecol 2006;46:440-5. http://dx.doi.org/10.1111/j.1479-828X.2006.00631.x.
- Henrich W. Cervicometry and fibronectin role. Paper presented at 22nd European Congress of Perinatal Medicine; 26–29 May 2010; Granada, Spain. J Matern Fetal Neonatal Med 2010;23.
- MacDonald WA, Bender M, Saxton A. Use of fetal fibronectin in the management of preterm labour in Nunavut. Alaska Med 2007;49:215-17.
- Swamy GK, Simhan HN, Gammill HS, Heine RP. Clinical utility of fetal fibronectin for predicting preterm birth. J Reprod Med 2005;50:851-6.
- Audibert F, Fortin S, Delvin E, Djemli A, Brunet S, Dube J, et al. Contingent use of fetal fibronectin testing and cervical length measurement in women with preterm labour. J Obstet Gynaecol Can 2010;32:307-12.
- Driul L, Cacciaguerra G, Calcagno A, Vascotto L, Pontello D, Marchesoni D. Pretern delivery predition: use of fetal fribonectin in comparison and association with ultrasound measurement of cervical length and clinically evaluated cervical dilatation. Ital J Gynecol Obstet 2009;21:81-5.
- Singer E, Pilpel S, Bsat F, Plevyak M, Healy A, Markenson G. Accuracy of fetal fibronectin to predict preterm birth in twin gestations with symptoms of labor. Obstet Gynecol 2007;109:1083-7. http://dx.doi.org/10.1097/01.AOG.0000261896.20175.3a.
- Farfan JAL, Tovar HBS, del de Anda MRG, Guevara CG. Fetal fibronectin and cervical length as predictors of early preterm delivery. Ginecol Obstet Mex 2011;79:337-43.
- Sümer C, Yalvac S, Kandemir O, Karcaaltincaba D, Haberal A. The predictive value of sonographic cervical length measurement and fetal fibronection testing to determine true preterm labour. Turk Jinekoloji Ve Obstetrik Dernegi Dergisi 2010;7:189-95.
- Mozurkewich EL, Naglie G, Krahn MD, Hayashi RH. Predicting preterm birth: a cost-effectiveness analysis. Am J Obstet Gynecol 2000;182:1589-97. http://dx.doi.org/10.1067/mob.2000.106855.
- Revah A, Hannah ME, Sue AQAK. Fetal fibronectin as a predictor of preterm birth: an overview. Am J Perinatol 1998;15:613-21. http://dx.doi.org/10.1055/s-2007-994079.
- Tsourapas A, Roberts TE, Barton PM, Honest H, Forbes C, Hyde CJ, et al. An economic evaluation of alternative test-intervention strategies to prevent spontaneous pre-term birth in singleton pregnancies. Acta Obstet Gynecol Scand 2009;88:1319-30. http://dx.doi.org/10.3109/00016340903410873.
- Department of Health (DoH) . NHS Reference Costs 2009–10 n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111591 (accessed 15 March 2010).
- Beta distribution . Wikipedia: The Free Encyclopedia, Wikimedia Foundation 2012. http://en.wikipedia.org/wiki/Beta_distribution (accessed 7 March 2012).
- Curtis L. Unit costs of health and social care 2011. Canterbury: Personal Social Services Research Unit; 2011.
- British national formulary. No. 62, September 2011. London: BMA and RPS; 2011.
- Curtis L. Unit costs of health and social care 2010. Canterbury: Personal Social Services Research Unit; 2010.
- Duley L, Bennett P. Tocolysis for women in preterm labour. Green-Top Guideline No. 1b [Internet]: Royal College of Obstetricians and Gynaecologists; February 2011 n.d. www.rcog.org.uk/files/rcog-corp/GTG1b26072011.pdf (accessed 14 February 2012).
- Roberts D. Antenatal corticosteroids to reduce neonatal morbidity and mortality. Green-Top Guideline No. 7. Royal College of Obstetricians and Gynaecologists; 2010 n.d. www.rcog.org.uk/files/rcog-corp/GTG%207.pdf (accessed 14 February 2012).
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882-93. http://dx.doi.org/10.1016/j.jclinepi.2005.01.016.
- Lopez Farfan JA, Sanchez Tovar HB, Gutierrez de Anda MdR, Gamez Guevara C. Fetal fibronectin and cervical length as early predictors of preterm labor. Ginecol Obstet Mex 2011;79:337-43.
- Bartnicki J, Casal D, Kreaden US, Saling E, Vetter K. Fetal fibronectin in vaginal specimens predicts preterm delivery and very-low-birth-weight infants. Am J Obstet Gynecol 1996;174:971-4. http://dx.doi.org/10.1016/S0002-9378(96)70335-6.
- Benattar C, Taieb J, Fernandez H, Lindendaum A, Frydman R, Ville Y. Rapid fetal fibronectin swab-test in preterm labor patients treated by betamimetics. Eur J Obstet Gynecol Reprod Biol 1997;72:131-5. http://dx.doi.org/10.1016/S0301-2115(96)02673-5.
- Closset E, Dufour P, Coeugnet C, Subtil D, Valat AS, Puech F. Value of fetal fibronectin research for predicting premature delivery. Gynecol Obstet Fertil 2001;29:808-13.
- Foxman EF, Jarolim P. Use of the fetal fibronectin test in decisions to admit to hospital for preterm labor. Clin Chem 2004;50:663-5. http://dx.doi.org/10.1373/clinchem.2003.028720.
- Giles W, Bisits A, Knox M, Madsen G, Smith R. The effect of fetal fibronectin testing on admissions to a tertiary maternal-fetal medicine unit and cost savings. Am J Obstet Gynecol 2000;182:439-42. http://dx.doi.org/10.1016/S0002-9378(00)70236-5.
- Gomez R, Romero R, Medina L, Nien JK, Chaiworapongsa T, Carstens M, et al. Cervicovaginal fibronectin improves the prediction of preterm delivery based on sonographic cervical length in patients with preterm uterine contractions and intact membranes. Am J Obstet Gynecol 2005;192:350-9. http://dx.doi.org/10.1016/j.ajog.2004.09.034.
- Iams JD, Casal D, McGregor JA, Goodwin TM, Kreaden US, Lowensohn R, et al. Fetal fibronectin improves the accuracy of diagnosis of preterm labor. Am J Obstet Gynecol 1995;173:141-5. http://dx.doi.org/10.1016/0002-9378(95)90182-5.
- LaShay N, Gilson G, Joffe G, Qualls C, Curet L. Will cervicovaginal interleukin-6 combined with fetal fibronectin testing improve the prediction of preterm delivery?. J Matern Fetal Med 2000;9:336-41. http://dx.doi.org/10.3109/14767050009018422.
- Lopez RL, Francis JA, Garite TJ, Dubyak JM. Fetal fibronectin detection as a predictor of preterm birth in actual clinical practice. Am J Obstet Gynecol 2000;182:1103-6. http://dx.doi.org/10.1067/mob.2000.105411.
- Luzzi V, Hankins K, Gronowski AM. Accuracy of the rapid fetal fibronectin TLi system in predicting preterm delivery. Clin Chem 2003;49:501-2. http://dx.doi.org/10.1373/49.3.501.
- Malak TM, Sizmur F, Bell SC, Taylor DJ. Fetal fibronectin in cervicovaginal secretions as a predictor of preterm birth. Br J Obstet Gynaecol 1996;103:648-53. http://dx.doi.org/10.1111/j.1471-0528.1996.tb09832.x.
- McKenna DS, Chung K, Iams JD. Effect of digital cervical examination on the expression of fetal fibronectin. J Reprod Med 1999;44:796-800.
- Peaceman AM, Andrews WW, Thorp JM, Cliver SP, Lukes A, Iams JD, et al. Fetal fibronectin as a predictor of preterm birth in patients with symptoms: a multicenter trial. Am J Obstet Gynecol 1997;177:13-8. http://dx.doi.org/10.1016/S0002-9378(97)70431-9.
- Sakai M, Sasaki Y, Yamagishi N, Tanebe K, Yoneda S, Saito S. The preterm labor index and fetal fibronectin for prediction of preterm delivery with intact membranes. Obstet Gynecol 2003;101:123-8. http://dx.doi.org/10.1016/S0029-7844(02)02463-8.
- Senden IP, Owen P. Comparison of cervical assessment, fetal fibronectin and fetal breathing in the diagnosis of preterm labour. Clin Exp Obstet Gynecol 1996;23:5-9.
- Tekesin I, Marek S, Hellmeyer L, Reitz D, Schmidt S. Assessment of rapid fetal fibronectin in predicting preterm delivery. Obstet Gynecol 2005;105:280-4. http://dx.doi.org/10.1097/01.AOG.0000150557.00298.47.
- Burrus DR, Ernest JM, Veille JC. Fetal fibronectin, interleukin-6, and C-reactive protein are useful in establishing prognostic subcategories of idiopathic preterm labor. Am J Obstet Gynecol 1995;173:1258-62. http://dx.doi.org/10.1016/0002-9378(95)91366-1.
- Chuileannain FN, Bell R, Brennecke S. Cervicovaginal fetal fibronectin testing in threatened preterm labour – translating research findings into clinical practice. Aust N Z J Obstet Gynaecol 1998;38:399-402. http://dx.doi.org/10.1111/j.1479-828X.1998.tb03096.x.
- Cox S, Little B, Dax J, Leveno K. Fetal fibronectin and preterm delivery. Am J Obstet Gynecol 1996;174.
- Goffeng AR, Holst E, Milsom I, Lindstedt G, Lundberg PA, Andersch B. Fetal fibronectin and microorganisms in vaginal fluid of women with complicated pregnancies. Acta Obstet Gynecol Scand 1997;76:521-7. http://dx.doi.org/10.3109/00016349709024576.
- Musaad SMA, Melson CL, Boswell DR. Assessment of the impact of introducing fetal fibronectin assay in the management of preterm labour at Middlemore Hospital, New Zealand. Pathology 2005;37:226-30. http://dx.doi.org/10.1080/00313020500099056.
- Parker J, Bell R, Brennecke S. Fetal fibronectin in the cervicovaginal fluid of women with threatened preterm labour as a predictor of delivery before 34 weeks' gestation. Aust N Z J Obstet Gynaecol 1995;35:257-61. http://dx.doi.org/10.1111/j.1479-828X.1995.tb01976.x.
- Calda P, Cibula D, Doudova D, Binder T, Peterka T, Zizka Z, et al. Fetal fibronectin in cervico-vaginal secretions--an indicator of incipient premature labor using a membrane immunoassay. Ceska Gynekol 1995;60:293-5.
- Pieta-Dolinska A, Jaczewski B, Wozniak P, Oszukowski P. Evaluation of fetal fibronectin concentration in identifying patients at risk for preterm delivery. Ginekol Pol 2005;76:431-5.
- Grandi C, Perego M, Briozzo G, Cassini A. Fetal fibronectin (fFN) in cervical secretions as a predictor of premature delivery. Rev Hosp Mat Inf Ramon Sarda 1996;15:127-36.
- Hincz P, Wilczynski J, Kozarzewski M, Szaflik K. Two-step test: the combined use of fetal fibronectin and sonographic examination of the uterine cervix for prediction of preterm delivery in symptomatic patients. Acta Obstet Gynecol Scand 2002;81:58-63. http://dx.doi.org/10.1034/j.1600-0412.2002.810111.x.
- Inglis SR, Jeremias J, Kuno K, Lescale K, Peeper Q, Chervenak FA, et al. Detection of tumor necrosis factor-alpha, interleukin-6, and fetal fibronectin in the lower genital tract during pregnancy: relation to outcome. Am J Obstet Gynecol 1994;171:5-10. http://dx.doi.org/10.1016/S0002-9378(94)70069-9.
- Irion O, Matute J, Bischof P. Prediction of prematurity by oncofetal fibronectin. Prospective cohort study. J Gynecol Obstet Biol Reprod 1995;24:624-9.
- Langer B, Boudier E, Schlaeder G. Cervico-vaginal fetal fibronectin: predictive value during false labor. Acta Obstet Gynecol Scand 1997;76:218-21. http://dx.doi.org/10.1111/j.1600-0412.1997.tb07848.x.
- Mansouri A, Tadjerouni A, El Rabiey G, Baube S, Garnier G, Tribalat S. Is fetal fibronectin a valid test predictive of premature labor?. Fertilite Contraception Sexualite 1997;25:380-4.
- Morrison JC, Allbert JR, McLaughlin BN, Whitworth NS, Roberts WE, Martin RW. Oncofetal fibronectin in patients with false labor as a predictor of preterm delivery. Am J Obstet Gynecol 1993;168:538-42. http://dx.doi.org/10.1016/0002-9378(93)90488-5.
- Rizzo G, Capponi A, Vlachopoulou A, Angelini E, Grassi C, Romanini C. The diagnostic value of interleukin-8 and fetal fibronectin concentrations in cervical secretions in patients with preterm labor and intact membranes. J Perinat Med 1997;25:461-8. http://dx.doi.org/10.1515/jpme.1997.25.6.461.
- Rozenberg P, Goffinet F, Malagrida L, Giudicelli Y, Perdu M, Houssin I, et al. Evaluating the risk of preterm delivery: a comparison of fetal fibronectin and transvaginal ultrasonographic measurement of cervical length. Am J Obstet Gynecol 1997;176:196-9. http://dx.doi.org/10.1016/S0002-9378(97)80035-X.
- Stevens AO, Chauhan SP, Magann EF, Martin RW, Bofill JA, Cushman JL, et al. Fetal fibronectin and bacterial vaginosis are associated with preterm birth in women who are symptomatic for preterm labor. Am J Obstet Gynecol 2004;190:1582-7. http://dx.doi.org/10.1016/j.ajog.2004.03.059.
- Gomez-Bravo Topete E, Castillo-Lechuga C, Villegas-Su A, Briones-Garduno JC. Predictive value of fetal fibronectin for preterm labor. Cir Cir 2004;72:491-4.
- Vercoustre L, Sotter S, Bouige D, Walch R. Fibronectin as a predictor of labor, results and discussion about 206 samples. Gynecol Obstet Fertil 1996;4:23-32.
- Vetr M, Kudela M, Prasilova J. Fetal fibronectin in patients at increased risk for premature birth. Acta Univ Palacki Olomuc Fac Med 1996;140:55-7.
- Statistical abstracts of the United States US Census Bureau. Washington, DC: United States US Census Bureau; 1998.
- Haddix AC, Teutsch SM, Corso PS. Prevention effectiveness. New York, NY: Oxford University Press; 1996.
- Backhouse M, Mauskopf J, Jones D, Wold D, Schumacher R, Cotton R, et al. Economic outcomes of bolfosceril palmitate rescue therapy in infants weighing 1250 g or more with respiratory distress syndrome. Pharmacoeconomics 1194;6:358-69.
- Myers ER, Alvarez JG, Richardson DK, Ludmir J. Cost-effectiveness of fetal lung maturity testing in preterm labor. Obstet Gynecol 1997;90:824-9. http://dx.doi.org/10.1016/S0029-7844(97)00412-2.
Appendix 1 Search strategy
Clinical effectiveness studies
MEDLINE (OvidSP): 2000 to September week 1 2011
Searched 16 September 2011.
-
(f?etal adj2 fibronectin$).ti,ab,ot,hw. (410)
-
((oncofetal or oncofoetal) adj2 fibronectin$).ti,ab,ot,hw. (112)
-
(ffn or onfn or fdc-6).ti,ab,ot,hw. (149)
-
(tli system$ or (tli adj iq) or tliiq or quikcheck).ti,ab,ot,hw. (3)
-
or/1-4 (545)
-
fibronectins/ (19,207)
-
(86088-83-7 or fibronectin$).ti,ab,ot,rn. (31,130)
-
or/6-7 (31,130)
-
exp Obstetric Labor, Premature/ (14,939)
-
((Pre term or preterm or premature or early or immature) adj5 (labo?r or birth$ or childbirth$ or deliver$ or partu$ or ruptur$)).ti,ab,ot,hw. (40,748)
-
(PROM or PROM or PTB).ti,ab,ot. (3180)
-
((Short$ or reduced or multiple) adj4 gestation$).ti,ab,ot. (3111)
-
or/9-12 (45,249)
-
5 or (8 and 13) (643)
-
randomized controlled trial.pt. (316,345)
-
controlled clinical trial.pt. (83,446)
-
randomized.ab. (221,935)
-
placebo.ab. (128,225)
-
drug therapy.fs. (1,495,908)
-
randomly.ab. (160,028)
-
trial.ab. (229,549)
-
groups.ab. (1,062,145)
-
meta-analysis.mp,pt. or review.pt. or search:.tw. (1,809,563)
-
or/15-23 (4,234,913)
-
animals/ not (animals/ and humans/) (3,584,947)
-
24 not 25 (3,686,178)
-
26 and 14 (224)
-
limit 27 to yr="2000 -Current" (153)
Systematic reviews filter:
Montori VM, Wilczynski NL, Morgan D, Haynes RB. Optimal search strategies for retrieving systematic reviews from MEDLINE: analytical survey (top strategy minimising the difference between sensitivity and specificity). BMJ 2005;330(7482):68.
Randomised controlled trials filter:
Lefebvre C, Manheimer E, Glanville J. Chapter 6: searching for studies. Box 6.4.c: Cochrane Highly sensitive search strategy for identifying randomized controlled trials in Medline: sensitivity-maximizing version (2008 version); OVID format. In Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration; 2011. URL: www.cochrane-handbook.org
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP): 2000 to 15 September 2011, MEDLINE Daily Update (OvidSP): 2000 to 15 September 2011
Searched 16 September 2011.
-
(f?etal adj2 fibronectin$).ti,ab,ot,hw. (10)
-
((oncofetal or oncofoetal) adj2 fibronectin$).ti,ab,ot,hw. (3)
-
(ffn or onfn or fdc-6).ti,ab,ot,hw. (10)
-
(tli system$ or (tli adj iq) or tliiq or quikcheck).ti,ab,ot,hw. (0)
-
or/1-4 (18)
-
fibronectins/ (8)
-
(86088-83-7 or fibronectin$).ti,ab,ot,rn. (633)
-
or/6-7 (633)
-
exp Obstetric Labor, Premature/ (18)
-
((Pre term or preterm or premature or early or immature) adj5 (labo?r or birth$ or childbirth$ or deliver$ or partu$ or ruptur$)).ti,ab,ot,hw. (1347)
-
(PROM or PROM or PTB).ti,ab,ot. (193)
-
((Short$ or reduced or multiple) adj4 gestation$).ti,ab,ot. (105)
-
or/9-12 (1564)
-
5 or (8 and 13) (19)
-
randomized controlled trial.pt. (737)
-
controlled clinical trial.pt. (43)
-
randomized.ab. (10,394)
-
placebo.ab. (4201)
-
drug therapy.fs. (1213)
-
randomly.ab. (10,445)
-
trial.ab. (11147)
-
groups.ab. (60,889)
-
meta-analysis.mp,pt. or review.pt. or search:.tw. (16,499)
-
or/15-23 (94,506)
-
animals/ not (animals/ and humans/) (1599)
-
24 not 25 (94,179)
-
26 and 14 (4)
-
limit 27 to yr="2000 -Current" (4)
Systematic reviews filter:
Montori VM, Wilczynski NL, Morgan D, Haynes RB. Optimal search strategies for retrieving systematic reviews from MEDLINE: analytical survey (top strategy minimising the difference between sensitivity and specificity). BMJ 2005;330(7482):68.
Randomised controlled trials filter:
Lefebvre C, Manheimer E, Glanville J. Chapter 6: searching for studies. Box 6.4.c: Cochrane Highly sensitive search strategy for identifying randomized controlled trials in Medline: sensitivity-maximizing version (2008 version); OVID format. In Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration; 2011. URL: www.cochrane-handbook.org
EMBASE (OvidSP): 2000 to week 36 2011
Searched 16 September 2011.
-
(f?etal adj2 fibronectin$).mp. (524)
-
((oncofetal or oncofoetal) adj2 fibronectin$).mp. (126)
-
(ffn or onfn or fdc-6).mp. (212)
-
(tli system$ or (tli adj iq) or tliiq or quikcheck).mp. (4)
-
or/1-4 (681)
-
Fibronectin/ (27,775)
-
(86088-83-7 or fibronectin$).mp. (35,981)
-
or/6-7 (35,981)
-
exp "immature and premature labor"/ (76,650)
-
((Pre term or preterm or premature or early or immature) adj5 (labo?r or birth$ or childbirth$ or deliver$ or partu$ or ruptur$)).mp. (49,049)
-
(PROM or PROM or PTB).mp. (4030)
-
((Short$ or reduced or multiple) adj4 gestation$).mp. (4435)
-
or/9-12 (101,617)
-
5 or (8 and 13) (938)
-
Random$.tw. or clinical trial$.mp. or exp health care quality/ (2,536,239)
-
meta-analys:.mp. or search:.tw. or review.pt. (1,887,122)
-
or/15-16 (4,013,206)
-
14 and 17 (361)
-
animal/ or animal experiment/ (3,076,644)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).mp. (4,760,116)
-
or/19-20 (4,760,116)
-
exp human/ or human experiment/ (12,493,844)
-
21 not (21 and 22) (3,823,462)
-
18 not 23 (359)
-
limit 24 to (embase and yr="2000 -Current") (240)
Systematic reviews filter (best sensitivity and specificity) from:
Wilczynski NL, Haynes RB, the Hedges Team. EMBASE search strategies achieved high sensitivity and specificity for retrieving methodologically sound systematic reviews. J Clin Epidemiol 2007;60:29–33.
Randomised controlled trials (best sensitivity) from:
Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Libr Assoc 2006;94(1):41–7.
Cochrane Database of Systematic Reviews: 2000 to Issue 9 2011, Cochrane Central Register of Controlled Trials: 2000 to Issue 3 2011, Database of Abstracts of Reviews of Effects (Wiley): 2000 to Issue 3 2011, Health Technology Assessment Database (Wiley): 2000 to Issue 3 2011, NHS Economic Evaluation Database (Wiley): 2000 to Issue 3 2011, The Cochrane Library
Searched 19 September 2011.
The CDSR search retrieved 10 records.
The CENTRAL search retrieved 35 records.
The DARE search retrieved eight records.
The HTA search retrieved nine records.
The NHS EED search retrieved three records.
Science Citation Index Expanded (Web of Knowledge): 2000 to 19 September 2011
Searched 19 September 2011.
Date limit (time span) = 2000–11.
Cumulative Index to Nursing and Allied Health Literature (EBSCOhost): 2000 to 9 September 2011
Searched 19 September 2011.
Systematic reviews and randomised controlled trials filters based on:
Wong SS, Wilczynski NL, Haynes RB. Optimal CINAHL search strategies for identifying therapy studies and review articles. J Nurs Scholarsh 2006;38(2):194–9.
Maternity and Infant Care (OvidSP): 2000 to August 2011
Searched 19 September 2011.
-
(f?etal adj2 fibronectin$).af. (255)
-
((oncofetal or oncofoetal) adj2 fibronectin$).af. (10)
-
(ffn or onfn or fdc-6).af. (58)
-
(tli system$ or (tli adj iq) or tliiq or quikcheck).af. (2)
-
or/1-4 (262)
-
(86088-83-7 or fibronectin$).af. (342)
-
((Pre term or preterm or premature or early or immature) adj5 (labo?r or birth$ or childbirth$ or deliver$ or partu$ or ruptur$)).af. (13,953)
-
(PROM or PROM or PTB).af. (540)
-
((Short$ or reduced or multiple) adj4 gestation$).af. (1105)
-
or/7-9 (14,663)
-
5 or (6 and 10) (288)
-
(random$ or RCT or trial$ or systematic or placebo or groups or search).af. (33,697)
-
11 and 12 (79)
-
limit 13 to yr="2000 -Current" (57)
National Institutes of Health ClinicalTrials.gov (Internet)
Searched 19 September 2011.
Advanced search option
| Search terms | Received date | Results |
|---|---|---|
| 86088-83-7 OR fibronectin* OR ffn | 1 January 2000 to 1 January 2012 | 27 |
Total = 27 references.
International Clinical Trials Registry Platform (Internet)
URL: http://apps.who.int/trialsearch/
Searched 19 September 2011.
86088-83-7 OR fibronectin OR fibronectins OR fFN 34
Current Controlled Trials: metaRegister of Controlled Trials (Internet)
URL: www.controlled-trials.com/mrct/
Searched 19 September 2011.
86088-83-7 OR fibronectin OR fibronectins OR fFN 23
EU Clinical Trials Register (Internet): 2000 to 11 September 2011
URL: www.clinicaltrialsregister.eu/
Searched 19 September 2011.
86088-83-7 OR fibronectin OR fibronectins OR fFN 6
Accuracy studies
MEDLINE (OvidSP): 2005 to November week 3 2011
Searched 29 November 2011.
-
(f?etal adj2 fibronectin$).ti,ab,ot,hw. (412)
-
((oncofetal or oncofoetal) adj2 fibronectin$).ti,ab,ot,hw. (114)
-
(ffn or onfn or fdc-6).ti,ab,ot,hw. (150)
-
(tli system$ or (tli adj iq) or tliiq or quikcheck).ti,ab,ot,hw. (3)
-
or/1-4 (549)
-
fibronectins/ (19,529)
-
(86088-83-7 or fibronectin$).ti,ab,ot,rn. (31,753)
-
or/6-7 (31,753)
-
exp Obstetric Labor, Premature/ (15,259)
-
((Pre term or preterm or premature or early or immature) adj5 (labo?r or birth$ or childbirth$ or deliver$ or partu$ or ruptur$)).ti,ab,ot,hw. (41,550)
-
(PROM or PROM or PTB).ti,ab,ot. (3294)
-
((Short$ or reduced or multiple) adj4 gestation$).ti,ab,ot. (3162)
-
or/9-12 (46,172)
-
5 or (8 and 13) (648)
-
animals/ not (animals/ and humans/) (3,630,436)
-
14 not 15 (606)
-
limit 16 to yr="2005 -Current" (180)
-
remove duplicates from 17 (170)
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP): 2005 to 28 November 2011, MEDLINE Daily Update (OvidSP): 2005 to 16 November 2011
Searched 28 November 2011.
-
(f?etal adj2 fibronectin$).ti,ab,ot,hw. (13)
-
((oncofetal or oncofoetal) adj2 fibronectin$).ti,ab,ot,hw. (4)
-
(ffn or onfn or fdc-6).ti,ab,ot,hw. (11)
-
(tli system$ or (tli adj iq) or tliiq or quikcheck).ti,ab,ot,hw. (0)
-
or/1-4 (22)
-
fibronectins/ (5)
-
(86088-83-7 or fibronectin$).ti,ab,ot,rn. (648)
-
or/6-7 (648)
-
exp Obstetric Labor, Premature/ (15)
-
((Pre term or preterm or premature or early or immature) adj5 (labo?r or birth$ or childbirth$ or deliver$ or partu$ or ruptur$)).ti,ab,ot,hw. (1511)
-
(PROM or PROM or PTB).ti,ab,ot. (204)
-
((Short$ or reduced or multiple) adj4 gestation$).ti,ab,ot. (110)
-
or/9-12 (1738)
-
5 or (8 and 13) (22)
-
animals/ not (animals/ and humans/) (1559)
-
14 not 15 (22)
-
limit 16 to yr="2005 -Current" (18)
-
remove duplicates from 17 (18)
EMBASE (OvidSP): 2005 to week 47 2011
Searched 29 November 2011.
-
(f?etal adj2 fibronectin$).mp. (531)
-
((oncofetal or oncofoetal) adj2 fibronectin$).mp. (130)
-
(ffn or onfn or fdc-6).mp. (216)
-
(tli system$ or (tli adj iq) or tliiq or quikcheck).mp. (4)
-
or/1-4 (692)
-
Fibronectin/ (28,249)
-
(86088-83-7 or fibronectin$).mp. (36,580)
-
or/6-7 (36,580)
-
exp "immature and premature labor"/ (78,183)
-
((Pre term or preterm or premature or early or immature) adj5 (labo?r or birth$ or childbirth$ or deliver$ or partu$ or ruptur$)).mp. (50,214)
-
(PROM or PROM or PTB).mp. (4162)
-
((Short$ or reduced or multiple) adj4 gestation$).mp. (4532)
-
or/9-12 (103,743)
-
5 or (8 and 13) (951)
-
animal/ or animal experiment/ (3,123,853)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).mp. (4,830,062)
-
or/15-16 (4,830,062)
-
exp human/ or human experiment/ (12,738,143)
-
17 not (17 and 18) (3,870,815)
-
14 not 19 (898)
-
limit 20 to (embase and yr="2005 -Current") (330)
-
remove duplicates from 21 (328)
Maternity and Infant Care (OvidSP): 2005 to November 2011
Searched 29 November 2011.
-
(f?etal adj2 fibronectin$).af. (258)
-
((oncofetal or oncofoetal) adj2 fibronectin$).af. (10)
-
(ffn or onfn or fdc-6).af. (60)
-
(tli system$ or (tli adj iq) or tliiq or quikcheck).af. (2)
-
or/1-4 (265)
-
(86088-83-7 or fibronectin$).af. (346)
-
((Pre term or preterm or premature or early or immature) adj5 (labo?r or birth$ or childbirth$ or deliver$ or partu$ or ruptur$)).af. (14,202)
-
(PROM or PROM or PTB).af. (562)
-
((Short$ or reduced or multiple) adj4 gestation$).af. (1125)
-
or/7-9 (14,923)
-
5 or (6 and 10) (291)
-
limit 11 to yr="2005 -Current" (101)
Cochrane Database of Systematic Reviews (Wiley): 2005 to Issue 11 2011, Cochrane Central Register of Controlled Trials (Wiley): 2005 to Issue 4 2011, Database of Abstracts of Reviews of Effects (Wiley): 2005 to Issue 4 2011, Health technology Assessment Database (Wiley): 2005 to Issue 4 2011, NHS Economic Evaluation Database (Wiley): 2005 to Issue 4 2011, The Cochrane Library
Searched 29 November 2011.
The CDSR search retrieved 12 records.
The CENTRAL search retrieved 21 records.
The DARE search retrieved five records.
The HTA search retrieved six records.
The NHS EED search retrieved two records.
Cumulative Index to Nursing and Allied Health Literature (EBSCOhost): 2005 to 29 November 2011
Searched 29 November 2011.
Science Citation Index Expanded (Web of Knowledge): 2005 to 29 November 2011
Searched 29 November 2011.
Time span = 2005–11.
# 12 414 #11 OR #5
# 11 245 #10 AND #6
# 10 19,147 #9 OR #8 OR #7
# 9 1,520 TS=((Short* or reduced or multiple) near/4 gestation*)
# 8 2,140 TS=(PROM or PROM or PTB)
# 7 16,339 TS=(("Pre term" or preterm or premature or early or immature) near/5 (labo*r or birth* or childbirth* or deliver* or partu* or ruptur*))
# 6 9,532 TS=(86088-83-7 or fibronectin*)
# 5 383 #4 OR #3 OR #2 OR #1
# 4 32 TS=(tli system* or "tli iq" or tliiq or quikcheck)
# 3 94 TS=(ffn or onfn or fdc-6)
# 2 60 TS=((oncofetal or oncofoetal) near/2 fibronectin*)
# 1 263 TS=((fetal or foetal) near/2 fibronectin*)
National Institutes of Health ClinicalTrials.gov (Internet)
Searched 29 November 2011.
Advanced search option
| Search terms | Received date | Results |
|---|---|---|
| fibronectin* OR ffn | 1 January 2005 to 1 January 2012 | 26 |
Total = 26 references.
Cost-effectiveness studies
MEDLINE (OvidSP): 1946 to January week 4 2012
Searched 3 February 2012.
-
(f?etal adj2 fibronectin$).ti,ab,ot,hw. (407)
-
((oncofetal or oncofoetal) adj2 fibronectin$).ti,ab,ot,hw. (113)
-
(ffn or onfn or fdc-6).ti,ab,ot,hw. (150)
-
(tli system$ or (tli adj iq) or tliiq or quikcheck).ti,ab,ot,hw. (3)
-
or/1-4 (543)
-
fibronectins/ (18,968)
-
(86088-83-7 or fibronectin$).ti,ab,ot,rn. (30,769)
-
or/6-7 (30,769)
-
exp Obstetric Labor, Premature/ (15,100)
-
((Pre term or preterm or premature or early or immature) adj5 (labo?r or birth$ or childbirth$ or deliver$ or partu$ or ruptur$)).ti,ab,ot,hw. (40,957)
-
(PROM or PROM or PTB).ti,ab,ot. (3185)
-
((Short$ or reduced or multiple) adj4 gestation$).ti,ab,ot. (3121)
-
or/9-12 (45,449)
-
5 or (8 and 13) (637)
-
economics/ (26,147)
-
exp "costs and cost analysis"/ (160,560)
-
economics, dental/ (1834)
-
exp "economics, hospital"/ (17,605)
-
economics, medical/ (8423)
-
economics, nursing/ (3853)
-
economics, pharmaceutical/ (2283)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (348,948)
-
(expenditure$ not energy).ti,ab. (14,668)
-
(value adj1 money).ti,ab. (17)
-
budget$.ti,ab. (14,919)
-
or/15-25 (463,385)
-
((energy or oxygen) adj cost).ti,ab. (2361)
-
(metabolic adj cost).ti,ab. (623)
-
((energy or oxygen) adj expenditure).ti,ab. (13,506)
-
or/27-29 (15,862)
-
26 not 30 (459,766)
-
letter.pt. (729,121)
-
editorial.pt. (287,409)
-
historical article.pt. (279,013)
-
or/32-34 (1,282,430)
-
31 not 35 (434,835)
-
animals/ not (animals/ and humans/) (3,556,824)
-
36 not 37 (409,646)
-
14 and 38 (39)
-
remove duplicates from 39 (39)
Economics filter:
Centre for Reviews and Dissemination. NHS EED economics filter: MEDLINE (Ovid) monthly search. York: Centre for Reviews and Dissemination; 2010. URL: www.york.ac.uk/inst/crd/intertasc/nhs_eed_strategies.html (cited 28 September 2010).
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP): up to 2 February 2012, MEDLINE Daily Update (OvidSP): up to 2 February 2012
Searched 3 February 2012.
-
(f?etal adj2 fibronectin$).ti,ab,ot,hw. (11)
-
((oncofetal or oncofoetal) adj2 fibronectin$).ti,ab,ot,hw. (3)
-
(ffn or onfn or fdc-6).ti,ab,ot,hw. (11)
-
(tli system$ or (tli adj iq) or tliiq or quikcheck).ti,ab,ot,hw. (0)
-
or/1-4 (19)
-
fibronectins/ (7)
-
(86088-83-7 or fibronectin$).ti,ab,ot,rn. (629)
-
or/6-7 (629)
-
exp Obstetric Labor, Premature/ (22)
-
((Pre term or preterm or premature or early or immature) adj5 (labo?r or birth$ or childbirth$ or deliver$ or partu$ or ruptur$)).ti,ab,ot,hw. (1533)
-
(PROM or PROM or PTB).ti,ab,ot. (196)
-
((Short$ or reduced or multiple) adj4 gestation$).ti,ab,ot. (108)
-
or/9-12 (1751)
-
5 or (8 and 13) (21)
-
economics/ (2)
-
exp "costs and cost analysis"/ (97)
-
economics, dental/ (0)
-
exp "economics, hospital"/ (7)
-
economics, medical/ (0)
-
economics, nursing/ (0)
-
economics, pharmaceutical/ (3)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (26449)
-
(expenditure$ not energy).ti,ab. (729)
-
(value adj1 money).ti,ab. (3)
-
budget$.ti,ab. (1408)
-
or/15-25 (27,914)
-
((energy or oxygen) adj cost).ti,ab. (148)
-
(metabolic adj cost).ti,ab. (37)
-
((energy or oxygen) adj expenditure).ti,ab. (628)
-
or/27-29 (797)
-
26 not 30 (27,704)
-
letter.pt. (17,492)
-
editorial.pt. (11,069)
-
historical article.pt. (117)
-
or/32-34 (28,663)
-
31 not 35 (27,367)
-
animals/ not (animals/ and humans/) (1499)
-
36 not 37 (27,325)
-
14 and 38 (2)
-
remove duplicates from 39 (2)
Economics filter:
Centre for Reviews and Dissemination. NHS EED economics filter: MEDLINE (Ovid) monthly search. York: Centre for Reviews and Dissemination; 2010. URL: www.york.ac.uk/inst/crd/intertasc/nhs_eed_strategies.html (cited 28 September 2010).
EMBASE (OvidSP): 1980 to week 4 2012
Searched 3 February 2012.
-
(f?etal adj2 fibronectin$).mp. (535)
-
((oncofetal or oncofoetal) adj2 fibronectin$).mp. (130)
-
(ffn or onfn or fdc-6).mp. (220)
-
(tli system$ or (tli adj iq) or tliiq or quikcheck).mp. (5)
-
or/1-4 (698)
-
Fibronectin/ (28,581)
-
(86088-83-7 or fibronectin$).mp. (36,957)
-
or/6-7 (36,957)
-
exp "immature and premature labor"/ (79,241)
-
((Pre term or preterm or premature or early or immature) adj5 (labo?r or birth$ or childbirth$ or deliver$ or partu$ or ruptur$)).mp. (51,102)
-
(PROM or PROM or PTB).mp. (4300)
-
((Short$ or reduced or multiple) adj4 gestation$).mp. (4624)
-
or/9-12 (105,221)
-
5 or (8 and 13) (959)
-
health-economics/ (30,861)
-
exp economic-evaluation/ (176,566)
-
exp health-care-cost/ (169,283)
-
exp pharmacoeconomics/ (143,042)
-
or/15-18 (403,439)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (461,907)
-
(expenditure$ not energy).ti,ab. (18,337)
-
(value adj2 money).ti,ab. (1019)
-
budget$.ti,ab. (19,361)
-
or/20-23 (481,340)
-
19 or 24 (718,447)
-
letter.pt. (753,716)
-
editorial.pt. (390,154)
-
note.pt. (463,410)
-
or/26-28 (1,607,280)
-
25 not 29 (644,541)
-
(metabolic adj cost).ti,ab. (685)
-
((energy or oxygen) adj cost).ti,ab. (2628)
-
((energy or oxygen) adj expenditure).ti,ab. (15,957)
-
or/31-33 (18,582)
-
30 not 34 (640,392)
-
14 and 35 (64)
-
animal/ or animal experiment/ (3,141,087)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).mp. (4,869,368)
-
or/37-38 (4,869,368)
-
exp human/ or human experiment/ (12,876,181)
-
39 not (39 and 40) (3,897,091)
-
36 not 41 (63)
-
limit 42 to embase (52)
-
remove duplicates from 43 (52)
Economics filter:
Centre for Reviews and Dissemination. NHS EED economics filter: EMBASE (Ovid) weekly search. York: Centre for Reviews and Dissemination; 2010. URL: www.york.ac.uk/inst/crd/intertasc/nhs_eed_strategies.html (cited 17 March 2011).
Database of Abstracts of Reviews of Effects (Wiley): up to Issue 1 2012, Health technology Assessment Database (Wiley): up to Issue 1 2012, NHS Economic Evaluation Database (Wiley): up to Issue 1 2012
Searched 3 February 2012.
DARE search retrieved 12 records.
HTA search retrieved 10 records.
NHS EED search retrieved three records.
Paediatric Economic Database Evaluation (Internet): 1980–2010
URL: http://pede.ccb.sickkids.ca/pede/search.jsp
Searched 3 February 2012.
Searched ‘Title, Abstract, or Keywords’
| Search term: ‘Title, Abstract, or Keywords’ | Records retrieved |
|---|---|
| Fibronectin | 2 |
| Fibronectins | 2 |
| ffn | 1 |
| onfn | 0 |
| fdc-6 | 0 |
| Total before deduplication | 5 |
| Total | 2 |
Appendix 2 Data extraction tables for randomised controlled trials
| Study ID | fFN test details | Comparator test(s) details |
|---|---|---|
| Lowe 200448 | The following criteria were set to perform fFN test: Women with cervical examination, transvaginal ultrasound scanning or intercourse within 24 hours were also enrolled in the study. In some women, the fFN assay was delayed until these criteria were met. As soon as criteria were met, fFN was collected by a Dacron swab rolled against the posterior lip of the cervix. The collected specimen was the placed into a buffer solution and sent to the laboratory. The results were available within 1 hour. Results were reported as positive if assay measured > 50 ng/ml and negative if < 50 ng/ml | Preterm labour management without fFN test was left to the discretion of the treating physician |
| Grobman 200450 | Pre randomisation: After physical examination by the physician which included the examination of the cervix with speculum at which time a Dacron swab test was placed in the posterior vaginal fornix for 10 seconds to absorb cervicovaginal secretions. This was followed by digital cervical examination | Pre randomisation: Same as fFN test result group |
| Post randomisation: The Dacron swab from this group was sent immediately to the laboratory for an assessment of fFN | Post randomisation: The Dacron swab from the ‘no availability’ group was stored at –20 °C. No availability of test results | |
| Dutta 201147 | TLi IQ Analyser display screen result within 20 minutes as stating POSITIVE, NEGATIVE or INVALID. A copy of the result and details of the patient were recorded in a ‘fibronectin book’ and another copy of the result was kept in the patient’s notes. The results were revealed to the clinician who performed the test. The clinician used this test in his/her decision-making about how to manage the patients. In case of positive fFN test, women should be admitted and the patient should be managed. For negative fFN results, it was recommended to discharge the patient unless there was a clinical indication or previous preterm labour history (clinician’s decision on discharge) | No fFN testing done. The patients in the control group were managed according to the hospital protocol. Where PTB was strongly suspected, admission, steroid administration, possible use of tocolytic and if needed in utero transfer were normally involved |
| Plaut 200349 | A rapid fFN immunoassay test (Adeza Biomedical) making the results available within hours of performance. During admission speculum examination (before digital examination), a Dacron swab was rotated in the posterior fornix for 10 seconds; the swabs for the eligible patients who consented to enrolment were sent to the laboratory for rapid analysis with the Adeza TLi qualitative method, with results reported as either positive or negative | Same test but results not know to the physicians |
| Osório 201051 | Abstract only, no details reported | Abstract only, no details reported |
| Study ID | Participant (number) | Inclusion criteria | Exclusion criteria | Participant baseline characteristics | |||
|---|---|---|---|---|---|---|---|
| Lowe 200448 | 97 | Women with signs and symptoms of preterm labour (uterine contractions and/or cervical change) or women who were transferred already receiving tocolytic medications, gestational ages between 23 and 24 weeks, > 16 years of age, cervical dilation of ≤ 3 cm for primigravid women, and ≤ 4 cm for multiparous women | Higher order multifetal gestations (more than twins), cerclage, preterm premature rupture of membranes and vaginal bleeding | fFN test ( n ) | ( n = 46) | Comparator ( n ) | ( n = 51) |
| Age in years (± SD)a | 27.4 (± 5.3) | Age in years (± SD) | 26.7 (± 5.8) | ||||
| Gravidity (IQR)b | 2.5 (2–3) | Gravidity in median (IQR) | 2 (1–3) | ||||
| Parity (IQR) | 1 (0–1) | Parity (IQR) | 1 (0–1) | ||||
| Multiple gestations (%) | 5 (11) | Singleton/twin gestation (%) | 3 (6) | ||||
| Gestational age at time of test (week) (IQR) | 30.4 (27.1–32.0) | Median gestational age at time of test (week) (IQR) | 30.6 (26.6–32.4) | ||||
| Previous PTB (%) | 12/46 (26) | Previous PTB (%) | 14/51 (27) | ||||
| Cervical dilation ≥ 3 cm | 4 (9%) | Cervical dilation ≥ 3 cm | 5 (10%) | ||||
| Grobman 200450 | 100 | EGA of 24–36 weeks, singleton pregnancy, primary complaint of uterine contractions, and more than six contractions/hour (by external tocodynometry) | Vaginal bleeding, non-intact amniotic membranes, ≥ 3 cm cervical dilation, or a VE or sexual intercourse within 24 hours; already received hospital observation, admission, or treatment for preterm contractions | fFN test ( n ) | ( n = 50) | Comparator ( n ) | ( n = 50) |
| Age in years (± SD)a | 29 ± (6) | Age in years (± SD)a | 29 ± (6) | ||||
| Nulliparity (n) | 28 (56%) | Nulliparity (n) | 20 (40%) | ||||
| White (n) | 23 (46%) | White (n) | 21 (42%) | ||||
| Previous PTB (%) | 4 (8) | Previous PTB (%) | 8 (16) | ||||
| Cervical dilation at admission (cm)a | 0.5 ± 0.7 | Cervical dilation at admission (cm)a | 0.5 ± 0.8 | ||||
| Dutta 201147 | 93 | Gestation between 24 + 0 and 34 + 6 weeks, and primary reason for presentation to hospital being uterine activity | Vaginal bleeding, membrane rupture, multiple pregnancies, history of recent intercourse, recent digital examination of the cervix in the last 24 hours, and cervical dilation of ≥ 3 cm, cervical cerclage, vaginal examination, use of lubricating jelly | fFN test ( n ) | ( n = 49) | Comparator ( n ) | ( n = 44) |
| Age in years (± SD)a | 26.66 (± 5.058) | Age in years (± SD) | 27.8714 (± 6.079) | ||||
| Ethnicity | 100% Caucasian | Ethnicity | 100% Caucasian | ||||
| Parity (± SD) | 1.0408 (± 1.2069) | Parity (± SD) | 0.9387 (± 1.162) | ||||
| Mean gestational age (± SD) | 30.82 (± 3.006) | Mean gestational age (± SD) | 30.7704 (± 2.7960) | ||||
| Notes: Pilot study | |||||||
| Plaut 200349 | 108 | Women with symptoms that suggested preterm labor between 24 weeks and 34 weeks 6 days | Cervical manipulation (intercourse, vaginal examination, or transvaginal ultrasonography scan) within the previous 24 hours, confirmed rupture of membranes, gross bleeding (more than bloody show), cervical dilation ≥ 3 cm, a cervical cerclage, or previous fFN testing within 2 weeks | fFN test ( n ) | ( n = 51) | Comparator ( n ) | ( n = 57) |
| Gestational age (weeks) mean ± SD | 29.9 ± 3.2 | Gestational age (weeks) mean ± SD | 30.4 ± 2.7 | ||||
| Twins (n) | 6 (11.8%) | Twins (n) | 6 (10.5%) | ||||
| Nulliparity (n) | 17 | Nulliparity (n) | 27 | ||||
| fFN positive (n) | 6 (11.8%) | fFN positive (n) | 4 (7%) | ||||
| Remark: discrepancy between table and text | Remark CT: discrepancy between table and text | ||||||
| Known risk factors (n) | 17 (33%) | Known risk factors (n) | 25 (44%) | ||||
| Osório 201051 | 66 (recruited, not sure randomised) | Women with gestational age between 24 weeks and 36 weeks 6 days which were seen at our hospital because of symptoms of preterm labor | NR | fFN test ( n ) | ( n = 33) | Comparator ( n ) | ( n = 33) |
| N = 66 not reported how many randomised to each arm but looking at the data it appears each arm had 33 participants | NR | NR | NR | ||||
| Note: Abstract reported that there was no difference between groups in demographic or obstetric characteristics | |||||||
Appendix 3 Risk of bias: Cochrane tool for risk of bias assessment
Study ID: Lowe 200448
| Bias | Support for judgement | Author’s judgement |
|---|---|---|
| Random sequence generation (selection bias) | Quote: ‘Randomisation was achieved through the use of computer generated table in blocks of 10’ | Low risk |
| Comment: The method of generation of random schedule was reported | ||
| Allocation concealment (selection bias) | Quote: ‘The results of randomisation were concealed through the use of opaque, sealed envelopes that were numbered sequentially’ | Low risk |
| Comment: The allocation was adequately concealed | ||
| Blinding of participants and personnel: all outcomes (performance bias) | Quote: ‘Physicians were not blinded to the results’ | Unclear risk |
| Comment: Given the design of this study the physicians cannot be blinded; however, the participants could have been blinded to treatment allocation. However, the information is not enough to pass a definitive judgement on adequate blinding of all personnel | ||
| Blinding of outcome assessment: all outcomes (detection bias) | Comment: Not stated | Unclear risk |
| Incomplete outcome data: all outcomes (dropouts/ITT) (attrition bias) | Quote: ‘Three women were assigned randomly to receive the fFN test but were discharged before it could be performed’ | Unclear risk |
| Comment: It was not clear if these were included in the ITT analysis | ||
| Selective reporting (reporting bias) | Comment: Based on paper only, protocol not obtained. All pre-specified outcomes were reported in the results | Low risk |
| Other bias | Comment: There was no difference between the baseline characteristics between the two groups. The study was funded by non-commercial organisation | Low risk |
Study ID: Grobman 200450
| Bias | Support for judgement | Author’s judgement |
|---|---|---|
| Random sequence generation (selection bias) | Quote: ‘Randomization was performed through the use of computer-generated random assignments’ | Low risk |
| Comment: The method of generation of random schedule was reported | ||
| Allocation concealment (selection bias) | Quote: ‘Patient assignments were placed in sequentially numbered opaque envelopes that were maintained at labor and delivery’ | Unclear risk |
| Comment: It was not clear from the text if these sequentially numbered opaque envelopes were sealed | ||
| Blinding of participants and personnel: all outcomes (performance bias) | Quote: ‘Laboratory personal [sic] who performed the fFN test were blinded to patients characteristics and outcomes’ | Unclear risk |
| Comment: Given the design of this study the physicians could not be blinded; however, the participants could have been blinded to treatment allocation. The laboratory personnel were reported to be blinded. The information is not enough to pass a definitive judgement on adequate blinding of all personnel | ||
| Blinding of outcome assessment: all outcomes (detection bias) | Comment: Not stated | Unclear risk |
| Incomplete outcome data: all outcomes (dropouts/ITT) (attrition bias) | Quote: ‘. . .the outcomes for this patient were analyzed along with other members of the group to which she was assigned randomly’ | Low risk |
| Comment: One patient was excluded post randomisation but was analysed using ITT analysis | ||
| Selective reporting (reporting bias) | Comment: Based on paper only, protocol not obtained. All pre-specified outcomes were reported in the results | Low risk |
| Other bias | Comment: There was no difference between the baseline characteristics between the two groups. The study was funded by commercial organisation | Unclear risk |
Study ID: Dutta 201147
| Bias | Support for judgement | Author’s judgement |
|---|---|---|
| Random sequence generation (selection bias) | Quote: ‘were randomized either to fFN testing or no fFN testing by admitting doctors using telephonic randomisation’ | Unclear risk |
| Comment: However, the method of generation of random schedule was not reported | ||
| Allocation concealment (selection bias) | Quote: ‘The telephonic randomisation was coordinated by the Health service research unit at Aberdeen’ | Low risk |
| Comment: The treatment was allocated by remote organisation using telephone | ||
| Blinding of participants and personnel: all outcomes (performance bias) | Quote: ‘The results of the fFN test of the patients in the active group were revealed to the clinician who performed the test. The clinician used this test in his/her decision making about how to manage the patients’ | Unclear risk |
| Comment: Given the design of this study the physicians could not be blinded; however, the participants could have been blinded to treatment allocation. It was not clear from the text if the participants were blinded to the treatment allocation | ||
| Blinding of outcome assessment: all outcomes (detection bias) | Comment: Not stated | Unclear risk |
| Incomplete outcome data: all outcomes (dropouts/ITT) (attrition bias) | Quote: ‘There are some missing values hence sample sizes are given in each case’ | High risk |
| ‘In three situations there was deviation from protocol and those patients were excluded from the study’ | ||
| Comment: ITT analysis was not done as all the randomised participants were not included in the final analysis | ||
| Selective reporting (reporting bias) | Comment: Based on paper only, protocol not obtained. It was not clear as the number of participants in each group vary for different outcomes | Unclear risk |
| Other bias | Comment: There was no difference between the baseline characteristics between the two groups. The study was funded by non-commercial organisation | Low risk |
Study ID: Plaut 200349
| Bias | Support for judgement | Author’s judgement |
|---|---|---|
| Random sequence generation (selection bias) | Quote: ‘Randomization of patients into two groups (result known to physician versus result not known to physician) was done in the laboratory by means of sequentially numbered opaque envelopes’ | Unclear risk |
| Comment: However, the method of generation of random schedule was not reported | ||
| Allocation concealment (selection bias) | Quote: ‘Randomization of patients into two groups (result known to physician versus result not known to physician) was done in the laboratory by means of sequentially numbered opaque envelopes’ | Unclear risk |
| Comment: The allocation was done using sequentially numbered opaque envelopes. However, it was not known if these envelopes were sealed | ||
| Blinding of participants and personnel: all outcomes (performance bias) | Quote: ‘Inside the envelopes were instructions to either notify the physician of the result or to notify the physician that the patient had been assigned randomly to the “not known” group’ | Unclear risk |
| Comment: Given the design of this study the physicians could not be blinded; however, the participants could have been blinded to treatment allocation. It was not clear from the text if the participants were blinded to the treatment allocation | ||
| Blinding of outcome assessment: all outcomes (detection bias) | Comment: Not stated | Unclear risk |
| Incomplete outcome data: all outcomes (dropouts/ITT) (attrition bias) | Comment: All the 108 swabs were reported in the results. However, the sample size calculated was much higher than the recruited because of which they could not assess the primary outcome. Hence, the information is not sufficient to adjudicate | Unclear risk |
| Selective reporting (reporting bias) | Quote: ‘Results are given for secondary outcomes of interests, with a focus on length of stay on hospital evaluation and treatment’ | High risk |
| Comment: Primary outcomes not assessed owing to low sample size, secondary outcomes reported selectively | ||
| Other bias | Quote: ‘Because of low enrolment rates, the study was terminated prematurely’ | High risk |
| Comment: This can cause early stopping bias. Also, this study was funded by a commercial organisation |
Study ID: Osório 201051
| Bias | Support for judgement | Author’s judgement |
|---|---|---|
| Random sequence generation (selection bias) | Quote: ‘Women were randomly assigned into two groups’ | Unclear risk |
| Comment: However, the method of generation of random schedule was not reported | ||
| Allocation concealment (selection bias) | Comment: Not stated | Unclear risk |
| Blinding of participants and personnel: all outcomes (performance bias) | Quote: ‘In group A, a rapid fFN test was performed and the results were known to the physicians’ | Unclear risk |
| Comment: Given the design of this study the physicians could not be blinded; however, the participants could have been blinded to treatment allocation. Only the abstract was available and it was not clear from the text if the participants were blinded to the treatment allocation | ||
| Blinding of outcome assessment: all outcomes (detection bias) | Comment: Not stated | Unclear risk |
| Incomplete outcome data: all outcomes (dropouts/ITT) (attrition bias) | Comment: Abstract available only. Information not sufficient | Unclear risk |
| Selective reporting (reporting bias) | Comment: Abstract available only. Information not sufficient | Unclear risk |
| Other bias | Comment: Abstract available only. Information not sufficient and sample size less | Unclear risk |
Appendix 4 Data extraction tables for test accuracy studies
| Authors | Year | Country | N | Study design | Inclusion | Exclusion | Testing gestation (week) | Description of test and threshold | Reference standard (days from testing or weeks’ gestation) |
|---|---|---|---|---|---|---|---|---|---|
| Asakura55 | 2009 | Japan | 108 | Cohort | Gestational age ranging from 22 to 33 weeks on admission, threatened PTD was diagnosed clinically when we observed at least one of the following symptoms: (1) regular uterine contractions at intervals of ≤ 10 minutes over a period of ≥ 2 hours, and (2) short cervix (< 20 mm) | Women with uterine anomaly and a clinical diagnosis of preterm rupture of the membrane, abruption placenta, placenta praevia, clinical evidence of chorio-amnionitis, gross cervical bleeding, and any contraindication for the use of tocolytic agents, or artificially induced PTD. Cases involving congenital fetal anomalies were also excluded | 22–33 | Swab from the posterior vaginal fornix for fFN sampling, as recommended by the manufacturer (Adeza Biochemical, Sunnyvale, CA, USA). Samples were immediately frozen and sent to a commercial laboratory (SRL, Tokyo, Japan) to assay concentrations of fFN using the Fibronectin collection kit with Biochemical TLiQ system rapid fFN automated analyser. An fFN result 50 ng/ml was considered positive | < 34, < 37 |
| Retrospective | With or without uterine contractions | ||||||||
| Rapid fFN testing | |||||||||
| Audibert64 | 2010 | Canada | 62 | Cohort | Women admitted to our tertiary care unit with a clinical diagnosis of preterm labour and intact membranes between 24 and 34 weeks were approached to participate in the study and were included after providing informed consent | Women were excluded if they had confirmed or suspected rupture of membranes, cervical dilation > 3 cm, cervical cerclage, vaginal bleeding, placental praevia, placental abruption, severe intrauterine growth restriction, pre-eclampsia, or medically indicated preterm delivery before 34 weeks | 24–34 | After digital examination 24 hours before entering the study each patient was first examined with vaginal speculum. A Dacron swab was rotated in posterior fornix of the vagina and sent to laboratory. The presence or absence of fFN was measured by a qualitative test and results were expressed as positive or negative | < 34, < 37 |
| Prospective | |||||||||
| Qualitative test | |||||||||
| Diaz54 | 2009 | Ecuador | 180 | Cohort | Any age attending hospital admission room, presenting singleton pregnancy first time, intact membranes, a gestational age 24–36 weeks, threatened PTB (painful regular contractions and cervical modifications) | Ruptured membranes, acute fetal distress, abnormal vaginal bleeding, in labour with ≥ 3 cm dilation, major fetal congenital malformation, multiple gestation, history of cervical cerclage or previous conisation. Patients with coitus and digital examination in other centre within 24 hours | 24–36 | Threshold: fFN test ≥ 50 ng/ml was positive when two lines appeared on dipstick. Cervicovaginal specimen taken from posterior vaginal fornix to perform the fFN dipstick test (Quick Check, Hologic, Bedford, MA, USA) | ≤ 21 days, ≤ 14 days, ≤ 7 days, < 35, < 37 |
| Prospective | |||||||||
| Test described as quick check dip stick | |||||||||
| Desjardins58 | 2008 | Canada | 361 | Cohort | Gestational age > 22 weeks and < 34 weeks, no rupture membranes, cervical dilation < 3 cm, no cervical cerclage, no vaginal examination or probe within 24 hours, no sexual intercourse within 24 hours, no lubricant gel used within 24 hours, and no presence of blood | NR | 22–34 | NR | < 7, < 14, < 35 |
| Retrospective | |||||||||
| Rapid fFN testing | |||||||||
| Groom60 | 2006 | New Zealand | 179 | Cohort | All women presenting with threatened preterm labour after 24 + 0 weeks’ gestation and prior to 34 + 0 weeks, with intact membranes and cervical dilation ≤ 3 cm. no use of lubricant prior to any other internal examination | NR | 22–34 | Samples are taken from the posterior fornix with a Dacron swab in accordance with the manufacturer’s guidelines (Adeza Biomedical, Sunnyvale, CA, USA). Samples are processed using the Adeza TLiIQ system Rapid fFN automated analyser. Threshold: fFN testing > 50 ng/ml was considered as positive | < 10 days, < 30, < 34, < 37 |
| Retrospective | |||||||||
| Rapid fFN testing | |||||||||
| Eroglu59 | 2007 | Turkey | 51 | Cohort | Women between 24 and 35 weeks of gestation with regular premature uterine contractions (> 10/hour). They also included asymptomatic women as controls but there are data for accuracy | Patients who had vaginal bleeding, cervical dilation of ≥ 3 cm, confirmed rupture of membranes, sexual intercourse within the past 24 hours, multiple pregnancy, uterine anomalies, congenital fetal abnormality, placenta praevia, abruptio placenta, intrauterine growth restriction, pre-eclampsia were excluded from the study | 24–35 | A sterile speculum was inserted into the vagina, a sterile Dacron swab (provided in the kit) was applied to the posterior fornix for 10–15 seconds in order to collect vaginal fluid specimens that were analysed for the presence of fFN using the rapid fFN assay (Adeza Fetal Fibronectin QuickCheck, Sunnyvale, CA, USA). ≥ 50 ng/ml | < 7 days, < 35 |
| Prospective | |||||||||
| Rapid fFN testing | |||||||||
| Lopez Farfan80 | 2011 | Mexico | 66 | Cohort | Pregnant women between 24 and 33.6 weeks of gestation based on reliable amenorrhoea, pregnancy of one fetus, and diagnosis of preterm labour | Confirmed rupture of membranes, cervical dilations ≥ 3 cm | 24–33.6 | fFN was searched in the cervicovaginal secretion with the rapid test, qualitative method for fFN Quick Check. The samples were collected from the posterior fornix with a sterile Dacron swab during 10 seconds, placed in a collection tube with buffer, and rotated for 45–60 seconds. The swab was then extracted from the tube and the reactive strip was introduced. Results from the strip were interpreted as (a) negative if one band appeared; or (b) positive if two bands appeared (≥ 50 mg/ml) | <37 weeks |
| Prospective | |||||||||
| Qualitative | |||||||||
| Rapid fFN | |||||||||
| Quick Check | |||||||||
| Henrich61 | 2010 | Germany | 125 | Cohort | Singleton pregnancies, regular uterine contractions, gestations between 23 and 33 (+6) weeks | NR | 23–33 | Samples were collected at the time speculum examination from the posterior cervical fornix. The probe was analysed using the rapid fetal fibronectin TLi system | < 35, <38 |
| Prospective | |||||||||
| Rapid fFN testing | |||||||||
| MacDonald62 | 2007 | Canada | 38 | Cohort | Women between 24 and 35 weeks EGA with symptoms of labour (abdominal pain, back pain, abdominal cramps, lower abdominal pelvic pressure) | NR | 24–35 | Rapid fetal fibronectin TLi System. ≥ 50 ng/ml | < 7 |
| Retrospective | |||||||||
| fFN assay not mentioned | |||||||||
| Singer66 | 2007 | USA | 516 | Cohort | Patients who presented to the Baystate Medical Centre obstetric triage area with complaints concerning preterm labour, such as contractions, pelvic pressure, and low back pain, and who met the following criteria were included in the study: fFN testing between 24.0 and 34.9 weeks of gestation, intact membranes, and cervical dilatation < 3 cm. If a patient presented to the triage area more than one time during their pregnancy for evaluation of preterm labour, only the first fFN test was included in our analysis | Patients were excluded if they had intercourse, a vaginal examination or a vaginal ultrasound within 24 hours of fFN testing. In addition, any patients with a cervical cerclage or those that required preterm delivery within 14 days of testing owing to maternal or fetal complications were excluded | 24–34.9 | A speculum examination was performed and a Dacron swab (E.I. du Pont de Nemours and Company, Inc., Wilmington, DE, USA) was placed in the posterior vaginal fornix, allowing it to absorb the vaginal secretions for 10 seconds. All samples were processed at Baystate Reference Laboratory within 24 hours using the rapid fFN TLi system qualitative method. Results as recommended by the manufacturer were reported by the laboratory as either positive (> 50 ng/ml) or negative (< 50 ng/ml). Laboratory personnel were not blinded to the clinical situation. Clinical information was obtained from the Baystate Medical Center laboratory and perinatal databases | < 34 |
| Retrospective | |||||||||
| Rapid fFN testing | |||||||||
| Skoll57 | 2006 | Canada | 160 | Cohort | Women between 24 and 34 weeks EGA with symptoms of preterm labour. No rupture of membrane, no moderate severe vaginal bleeding, no indication of preterm delivery, including non-reassuring fetal assessment, chorioamnionitis, several maternal hypertension, or fetal death | NR | 24–34 | Swabs were collected from the posterior vaginal fornix for fFN quantification. The specimens were kept at –4 °C and run in weekly batches. A level of ≥ 50 ng/ml is considered positive | < 7, < 34 |
| Prospective | |||||||||
| fFN quantification using TLiQ | |||||||||
| Swamy63 | 2005 | USA | 404 | Cohort | Women with symptoms of PTL, 22–34 weeks’ gestation, intact membrane, last digital cervical examination and sexual intercourse > 24 hours earlier and cervix < 2 cm dilated and >1 cm long | NR | 22–34 | All specimens were collected during sterile speculum examination prior to digital cervical examination. The fFN specimen collection kit contains a Dacron swab and a buffer-filled collection tube. The swab was used to obtain a sample of cervicovaginal secretions from posterior fornix. The swab was then immersed in buffer solution and sealed with collection tube. All samples were immediately transported to hospital laboratory and processed using rapid fFN assay. A positive test was defined as a fFN concentration > 50 ng/ml | < 7, < 34, < 37 |
| Prospective | |||||||||
| Rapid fFN testing | |||||||||
| Tsoi56 | 2006 | South Africa | 195 | Cohort | Women with singleton pregnancies presenting to labour ward with painful and regular uterine contractions at 24–36 weeks | Women in active labour, defined by the presence of cervical dilatation of ≥ 3 cm, and those of ruptured membranes, were excluded | 24–36 | A sterile speculum examination was performed, a specimen of cervicovaginal secretions was collected from posterior fornix or endocervix and qualitative detection of fFN was performed (Adeza Biomedical fFN testing). The test was performed at the bedside as described by manufacturer and a positive or negative result was recorded | < 7 |
| Prospective | |||||||||
| Bedside rapid fFN testing (qualitative) |
Appendix 5 QUADAS-2 completion guide for NIHR HTA fibronectin project
The version of QUADAS-2 used in this assessment included only the risk of bias components, as it was considered that the inclusion criteria matched the review question and that questions of applicability were, therefore, not relevant. The reference standard was the occurrence of preterm or term birth in all studies; we therefore considered that there were no issues of bias relating to the adequacy or application of the reference standard and the ‘reference standard’ domain of QUADAS-2 was omitted. Individual signalling questions not considered relevant to this review have also been omitted (e.g. those relating to the time between index test and reference standard because the reference standard was the occurrence of preterm or term birth).
Assessment of signalling questions and associated risk of bias due to signalling question
We considered each signalling question and included only those which we judged to be relevant to our review.
Domain 1: patient selection
Question 1: Was a consecutive or random sample of patients enrolled?
-
‘yes’ → low risk of bias
-
‘unclear’ → unclear risk of bias
-
‘no’ → high risk of bias.
Question 2: Did the study avoid inappropriate exclusions?
-
‘no’ for < 10% of patients or ‘yes’ → low risk of bias
-
‘unclear’ → unclear risk of bias
-
‘no’ for ≥ 10% of patients → high risk of bias.
Domain 2: index test
Did the study prespecify the threshold for a positive result?
-
‘yes’ → low risk of bias
-
‘unclear’ → unclear risk of bias
-
’no’ → high risk of bias.
Domain 3: flow and timing
Were all patients included in the analysis?
-
‘no’ but for < 10% of patients or ‘yes’ → low risk of bias
-
‘unclear’ → unclear risk of bias
-
‘no’ for ≥ 10% of patients → high risk of bias.
Assessment of the risk of bias per domain
-
If at least one of the signalling questions of a domain had an answer associated with a high risk of bias the domain would be judged to have a high risk of bias.
-
If the answer to any of the signalling questions was ‘unclear’ the risk of bias was also judged to be unclear.
-
The answer to all the signalling questions had to be ‘yes’ in order for the domain to be judged as having a low risk of bias.
Appendix 6 QUADAS-2 assessment for new accuracy studies
Asakura 200955
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Describe methods of patient selection: | |
| Retrospective study reviewed case notes for pregnant women admitted to our hospital due to threatened preterm delivery | |
| Was a consecutive or random sample of patients enrolled? | No |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | RISK: HIGH |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Describe the index test and how it was conducted and interpreted: | |
| Rapid fFN testing described and the threshold was mentioned to be > 50 ng/ml | |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
Domain 3: flow and timing
| A. Risk of bias | |
|---|---|
| Describe any patients who did not receive the index test(s) and/or reference standard (patients who were lost to follow-up) or who were excluded from the 2 × 2 table (refer to flow diagram): | |
| All patients were included in 2 × 2 data | |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
Audibert 201064
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Describe methods of patient selection: | |
| Prospective cohort. All women admitted in the tertiary care unit with symptoms of PTB were approached to participate in the study | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: LOW |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Describe the index test and how it was conducted and interpreted: | |
| The index test was well described but the threshold was not mentioned we had to obtain this information from the online kit manuals | |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
Domain 3: flow and timing
| A. Risk of bias | |
|---|---|
| Describe any patients who did not receive the index test(s) and/or reference standard (patients who were lost to follow-up) or who were excluded from the 2 × 2 table (refer to flow diagram): | |
| All 62 included in final analysis | |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
Diaz 200954
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Describe methods of patient selection: | |
| Pregnant women of any age attending hospital admission room. Prospective | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: LOW |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Describe the index test and how it was conducted and interpreted: | |
| A cervicovaginal specimen was taken from the posterior vaginal fornix to perform the fFN dipstick test (QuickCheck, Hologic, Bedford, MA). A positive fFN test (> 50 ng/ml) was considered when two distinct lines appeared on the dipstick | |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
Domain 3: flow and timing
| A. Risk of bias | |
|---|---|
| All patients included | |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
Desjardins 200858
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Describe methods of patient selection: | |
| Retrospective data | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | RISK: HIGH |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Describe the index test and how it was conducted and interpreted: | |
| The threshold was not mentioned we had to obtain this information form the online kit manuals | |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
Domain 3: flow and timing
| A. Risk of bias | |
|---|---|
| All included in the 2 × 2 tables | |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
Eroglu 200759
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Prospective cohort sequence not mentioned | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: UNCLEAR |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| They described the fashion in which index test was conducted but didn’t mention the threshold or in what way it was interpreted. The threshold was not mentioned we had to obtain this information form the online kit manuals | |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
Domain 3: flow and timing
| A. Risk of bias | |
|---|---|
| All included in the 2 × 2 tables | |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
Lopez Farfan 201180
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Describe methods of patient selection: | |
| Prospective cohort study of pregnant women 24–33.6 weeks’ gestation admitted due to diagnosis of preterm labor | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: LOW |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Describe the index test and how it was conducted and interpreted: | |
| The index test was well described, what a positive and negative test means, and the threshold mentioned (50 ng/ml) | |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
Domain 3: flow and timing
| A. Risk of bias | |
|---|---|
| Describe any patients who did not receive the index test(s) and/or reference standard (patients who were lost to follow-up) or who were excluded from the 2 × 2 table (refer to flow diagram): | |
| All patients were included in the 2 × 2 table | |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
Groom 200660
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Describe methods of patient selection: | |
| Retrospective data | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | RISK: HIGH |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Rapid fFN automated analyser. Threshold fFN test positive if ≥ 50 ng/ml | |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
Domain 3: flow and timing
| A. Risk of bias | |
|---|---|
| Management details were missing in a further 15 cases and results of these tests were included in analysis | |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
Henrich 201061
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Prospective cohort but did not mention the sequence in which patients were enrolled | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: UNCLEAR |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| The process of performing the test described but the threshold not reported | |
| If a threshold was used, was it prespecified? | No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: HIGH |
Domain 3: flow and timing
| A. Risk of bias | |
|---|---|
| All included in the 2 × 2 tables | |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
MacDonald 200762
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Describe methods of patient selection: | |
| Retrospective | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | RISK: HIGH |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Describe the index test and how it was conducted and interpreted: | |
| Test not described and threshold not specified. The threshold was not mentioned, we had to obtain this information form the online kit manuals | |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
Domain 3: flow and timing
| A. Risk of bias | |
|---|---|
| Describe any patients who did not receive the index test(s) and/or reference standard (patients who were lost to follow-up) or who were excluded from the 2 × 2 table (refer to flow diagram): | |
| All tested included in 2 × 2 data | |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
Singer 200766
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Describe methods of patient selection: | |
| Retrospective study, convenience sample | |
| Was a consecutive or random sample of patients enrolled? | No |
| Did the study avoid inappropriate exclusions? | No |
| Could the selection of patients have introduced bias? | RISK: HIGH |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Describe the index test and how it was conducted and interpreted: | |
| Index test and threshold described adequately | |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
Domain 3: flow and timing
| A. Risk of bias | |
|---|---|
| Describe any patients who did not receive the index test(s) and/or reference standard (patients who were lost to follow-up) or who were excluded from the 2 × 2 table (refer to flow diagram): | |
| All included in final analysis | |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
Skoll 200657
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Describe methods of patient selection: | |
| Prospective study. If the physician excluded the diagnosis of preterm labour on clinical assessment and vaginal examination and discharged patients home then they were not included in the final analysis. Hence, it was no clear if the patients were included serially | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: UNCLEAR |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Describe the index test and how it was conducted and interpreted: | |
| A level of ≥ 50 ng/ml was considered positive | |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
Domain 3: flow and timing
| A. Risk of bias | |
|---|---|
| 11 patients were loss to follow-up leaving behind 149 patient for final data analysis | |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: LOW |
Swamy 200563
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Describe methods of patient selection: | |
| Prospective cohort but unclear if the patients were recruited in series | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: UNCLEAR |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Describe the index test and how it was conducted and interpreted: | |
| Index test was described and positive test was defined to be > 50 ng/ml | |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
Domain 3: flow and timing
| A. Risk of bias | |
|---|---|
| Describe any patients who did not receive the index test(s) and/or reference standard (patients who were lost to follow-up) or who were excluded from the 2 × 2 table (refer to flow diagram): | |
| All patients were included in final analysis | |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
Tsoi 200656
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Describe methods of patient selection: | |
| Prospective cohort study. It included all women presenting to the labor ward with painful and regular uterine contraction | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: LOW |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| Describe the index test and how it was conducted and interpreted: | |
| Index test was described but the threshold was not defined. It was obtained from the manufacturer test kit manual. The threshold was not mentioned we had to obtain this information form the online kit manuals | |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW |
Domain 3: flow and timing
| A. Risk of bias | |
|---|---|
| Describe any patients who did not receive the index test(s) and/or reference standard (patients who were lost to follow-up) or who were excluded from the 2 × 2 table (refer to flow diagram): | |
| All included in the final analysis | |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: LOW |
Appendix 7 Summary of 2 × 2 data of accuracy studies from update and previous review
Reference standard: within 7–10 days of testing35
| Authors | Outcome | TP | FP | FN | TN | Sensitivity | Specificity | s/m | u/p | c/o |
|---|---|---|---|---|---|---|---|---|---|---|
| Bartnicki81 | 7 | 3 | 33 | 1 | 79 | 0.75 | 0.71 | m | p | o |
| Benattar82 | 7 | 8 | 11 | 1 | 104 | 0.89 | 0.90 | m | p | o |
| Closset83 | 7 | 5 | 11 | 1 | 44 | 0.83 | 0.80 | m | p | o |
| Desjardins58 | 7 | 6 | 23 | 4 | 328 | 0.60 | 0.93 | m | u | o |
| Diaz54 | 7 | 18 | 34 | 6 | 122 | 0.75 | 0.78 | s | u | c |
| Eroglu59 | 7 | 5 | 9 | 1 | 36 | 0.83 | 0.80 | s | u | o |
| Foxman84 | 7 | 6 | 25 | 1 | 107 | 0.86 | 0.81 | m | p | o |
| Giles85 | 7 | 11 | 34 | 5 | 100 | 0.69 | 0.75 | m | p | o |
| Gomez86 | 7 | 18 | 34 | 10 | 153 | 0.64 | 0.82 | m | p | o |
| Groom60 | 7 | 7 | 24 | 3 | 145 | 0.70 | 0.86 | m | u | o |
| Henrich61 | 7 | 5 | 17 | 0 | 59 | 1.00 | 0.78 | s | u | o |
| Iams87 | 7 | 13 | 32 | 1 | 146 | 0.93 | 0.82 | m | p | o |
| LaShay88 | 7 | 3 | 10 | 2 | 103 | 0.60 | 0.91 | s | p | c |
| Lopez89 | 7 | 8 | 12 | 1 | 64 | 0.89 | 0.84 | s | p | o |
| Lowe48 | 7 | 2 | 7 | 1 | 31 | 0.67 | 0.82 | s | p | o |
| Luzzi90 | 7 | 4 | 34 | 3 | 92 | 0.57 | 0.73 | m | p | c |
| MacDonald62 | 7 | 4 | 3 | 0 | 31 | 1.00 | 0.91 | m | u | o |
| Malak91 | 7 | 8 | 10 | 2 | 92 | 0.80 | 0.90 | s | p | o |
| McKenna92 | 7 | 5 | 13 | 1 | 35 | 0.83 | 0.73 | m | p | c |
| Peaceman93 | 7 | 19 | 123 | 2 | 581 | 0.90 | 0.83 | m | p | o |
| Sakai94 | 7 | 11 | 27 | 7 | 71 | 0.61 | 0.72 | s | p | o |
| Senden95 | 7 | 4 | 4 | 1 | 20 | 0.80 | 0.83 | s | p | c |
| Skoll57 | 7 | 12 | 20 | 3 | 114 | 0.80 | 0.85 | m | u | o |
| Sümer68 | 7 | 1 | 7 | 4 | 55 | 0.20 | 0.89 | s | u | o |
| Swamy63 | 7 | 14 | 31 | 7 | 352 | 0.67 | 0.92 | m | u | o |
| Tekesin96 | 7 | 9 | 37 | 2 | 122 | 0.82 | 0.77 | s | p | c |
| Tsoi56 | 7 | 18 | 67 | 1 | 109 | 0.95 | 0.62 | s | u | c |
Reference standard: < 34 weeks’ gestation35
| Authors | Outcome | TP | FP | FN | TN | Sensitivity | Specificity | s/m | u/p | c/o |
|---|---|---|---|---|---|---|---|---|---|---|
| Asakura55 | 34 | 10 | 11 | 6 | 81 | 0.63 | 0.88 | m | u | o |
| Audibert64 | 34 | 7 | 7 | 7 | 41 | 0.50 | 0.85 | m | u | c |
| Burrus97 | 34 | 23 | 6 | 3 | 5 | 0.88 | 0.45 | m | p | o |
| Chuileannain98 | 34 | 9 | 11 | 1 | 49 | 0.90 | 0.82 | s | p | o |
| Cox99 | 34 | 3 | 22 | 11 | 139 | 0.21 | 0.86 | s | p | o |
| Desjardins58 | 34 (35)a | 14 | 15 | 22 | 310 | 0.39 | 0.95 | m | u | o |
| Diaz 200954 | 34 (35)a | 12 | 40 | 0 | 128 | 1.00 | 0.76 | s | u | c |
| Driul65 | 34 | 11 | 31 | 4 | 36 | 0.73 | 0.54 | m | u | o |
| Eroglu59 | 34 (35)a | 7 | 7 | 3 | 34 | 0.70 | 0.83 | s | u | o |
| Goffeng100 | 34 | 7 | 7 | 4 | 45 | 0.64 | 0.87 | s | p | c |
| Groom60 | 34 | 13 | 18 | 1 | 147 | 0.93 | 0.89 | m | u | o |
| Henrich61 | 34 (35)a | 10 | 12 | 2 | 57 | 0.83 | 0.83 | s | u | o |
| Lopez89 | 34 | 11 | 9 | 4 | 61 | 0.73 | 0.87 | s | p | o |
| Musaad101 | 34 | 5 | 5 | 1 | 21 | 0.83 | 0.81 | m | p | c |
| Parker102 | 34 | 6 | 7 | 1 | 25 | 0.86 | 0.78 | s | p | o |
| Singer66 | 34 | 19 | 61 | 21 | 415 | 0.48 | 0.87 | m | u | o |
| Skoll57 | 34 | 17 | 15 | 10 | 107 | 0.63 | 0.88 | m | u | o |
| Swamy63 | 34 | 27 | 20 | 38 | 319 | 0.42 | 0.94 | m | u | o |
| Tekesin96 | 34 | 20 | 26 | 8 | 116 | 0.71 | 0.82 | s | p | c |
Reference standard: < 37 weeks’ gestation
| Authors | Outcome | TP | FP | FN | TN | Sensitivity | Specificity | s/m | u/p | c/o |
|---|---|---|---|---|---|---|---|---|---|---|
| Asakura55 | 37 | 14 | 7 | 26 | 61 | 0.35 | 0.90 | m | u | o |
| Audibert64 | 37 | 11 | 3 | 12 | 36 | 0.48 | 0.92 | m | u | c |
| Bartnicki81 | 37 | 27 | 7 | 13 | 65 | 0.68 | 0.90 | m | p | o |
| Benattar82 | 37 | 9 | 9 | 16 | 90 | 0.36 | 0.91 | m | p | o |
| Calda103 | 37 | 19 | 13 | 2 | 50 | 0.90 | 0.79 | m | p | o |
| Chuileannain98 | 37 | 13 | 7 | 1 | 49 | 0.93 | 0.88 | s | p | o |
| Closset83 | 37 | 12 | 4 | 11 | 34 | 0.52 | 0.89 | m | p | o |
| Diaz54 | 37 | 38 | 14 | 12 | 116 | 0.76 | 0.89 | s | u | c |
| Pieta-Dolinska104 | 37 | 28 | 8 | 10 | 69 | 0.74 | 0.90 | s | p | o |
| Driul65 | 37 | 25 | 17 | 14 | 26 | 0.64 | 0.61 | m | u | o |
| Farfan67 | 37 | 25 | 5 | 2 | 34 | 0.93 | 0.87 | m | u | o |
| Giles85 | 37 | 12 | 33 | 7 | 99 | 0.63 | 0.75 | m | p | o |
| Goffeng100 | 37 | 10 | 4 | 18 | 31 | 0.36 | 0.89 | s | p | c |
| Grandi105 | 37 | 4 | 9 | 4 | 9 | 0.50 | 0.50 | s | p | c |
| Groom60 | 37 | 18 | 13 | 17 | 131 | 0.51 | 0.91 | m | u | o |
| Henrich61 | 38 | 17 | 5 | 12 | 47 | 0.59 | 0.90 | s | u | o |
| Hincz106 | 37 | 10 | 5 | 4 | 63 | 0.71 | 0.93 | m | p | c |
| Iams87 | 37 | 27 | 18 | 35 | 112 | 0.44 | 0.86 | m | p | o |
| Inglis107 | 37 | 7 | 2 | 9 | 20 | 0.44 | 0.91 | s | p | o |
| Irion108 | 37 | 15 | 11 | 7 | 31 | 0.68 | 0.74 | m | p | o |
| Langer109 | 37 | 10 | 8 | 8 | 35 | 0.56 | 0.81 | m | p | o |
| LaShay88 | 37 | 10 | 8 | 24 | 76 | 0.29 | 0.90 | s | p | c |
| Lockwood13 | 37 | 49 | 10 | 11 | 47 | 0.82 | 0.82 | m | p | o |
| Lopez89 | 37 | 17 | 3 | 31 | 34 | 0.35 | 0.92 | s | p | o |
| Lowe48 | 37 | 3 | 6 | 6 | 26 | 0.33 | 0.81 | s | p | o |
| Malak91 | 37 | 17 | 5 | 10 | 109 | 0.63 | 0.96 | s | p | o |
| Mansouri110 | 37 | 13 | 12 | 12 | 53 | 0.52 | 0.82 | m | p | o |
| Morrison111 | 37 | 9 | 5 | 1 | 13 | 0.90 | 0.72 | s | p | c |
| Musaad101 | 37 | 5 | 3 | 5 | 15 | 0.50 | 0.83 | m | p | c |
| Peaceman93 | 37 | 61 | 81 | 78 | 505 | 0.44 | 0.86 | m | p | o |
| Rizzo112 | 37 | 40 | 12 | 9 | 45 | 0.82 | 0.79 | s | p | o |
| Rozenberg113 | 37 | 14 | 17 | 6 | 39 | 0.70 | 0.70 | s | p | o |
| Sakai94 | 37 | 26 | 12 | 36 | 42 | 0.42 | 0.78 | m | p | o |
| Stevens114 | 37 | 32 | 20 | 37 | 86 | 0.46 | 0.81 | m | p | o |
| Swamy63 | 37 | 30 | 17 | 90 | 267 | 0.25 | 0.94 | m | u | o |
| Tekesin96 | 37 | 31 | 15 | 4 | 120 | 0.89 | 0.89 | s | p | c |
| Gomez-Bravo Topete115 | 37 | 24 | 4 | 10 | 36 | 0.71 | 0.90 | m | p | o |
| Vercoustre116 | 37 | 12 | 21 | 1 | 44 | 0.92 | 0.68 | s | p | o |
| Vetr117 | 37 | 5 | 11 | 4 | 26 | 0.56 | 0.70 | m | p | o |
Appendix 8 Summary and quality checklist of cost-effectiveness studies
Summary of cost-effectiveness studies
| Study details | Mozurkewich et al.69 | Tsourapas et al.71 |
|---|---|---|
| Time horizon | Until the time of hospital discharge or death of the neonate | Not available |
| Objective | To compare nine different treatment strategies for the management of women presented with threatened preterm labour | To investigate the potential cost-effectiveness of alternative ‘test-and-treat’ strategies in the prevention of spontaneous PTB |
| Source of effectiveness information/testing accuracy data | Based on literature review | Based on meta-analysis of results of systematic literature review |
| Comparators |
|
The study compares all possible combinations of test (fibronectin, phIGFBP, CRP, absence of fetal breathing, and previous history of PTB) and treatment (atosiban, indomethacin, calcium channel blockers, magnesium sulphate, terbutaline, prophylactic antibiotics) options as below:
|
| Reference standard | NA | NA |
| Unit costs118–121 | Statistical data of University of Michigan Hospital as well as the literature (1999 prices in Canadian dollars) | Based on literature reviews and the Birmingham Women’s Hospital, Birmingham, UK |
| Measure of benefit | Neonatal deaths avoided/1000 births | Proportion of women avoiding threatened preterm labour or PTB |
| RDS avoided/100 births | ||
| Study type | Cost-effectiveness analysis | Cost-effectiveness analysis |
| Model assumptions |
|
As there were no data on the improvement in neonatal outcomes it was assumed that the delaying of the preterm labour or preterm delivery was beneficial |
| Perspective | Third-party payer perspective | Hospital |
| Discount rate | NA | NA |
| Uncertainty around cost-effectiveness ratio expressed | No | No |
| Sensitivity analysis | Sensitivity analysis was performed on all variables by varying them on plausible ranges | A deterministic and probabilistic sensitivity was reported based on different levels of willingness to pay |
| Outcome (cost and LYS/QALYs) per comparator | Expressed as mean costs per women:
|
Expressed as mean costs per women:
|
| Summary of incremental analysis | Expressed as RDS avoided:
|
Expressed as incremental costs per PTB/labour avoided:
|
Cost-effectiveness study quality checklist
| Item | Mozurkewich et al.69 | Tsourapas et al.71 |
|---|---|---|
| Study design | ||
| The research question is stated | ✓ | ✓ |
| The economic importance of the research question is stated | ✓ | ✓ |
| The viewpoint(s) of the analysis are clearly stated and justified | ✓ | ✓ |
| The rationale for choosing alternative programmes or interventions compared is stated | ✓ | ✓ |
| The alternatives being compared are clearly described | ✓ | ✓ |
| The form of economic evaluation used is stated | ✓ | ✓ |
| The choice of form of economic evaluation is justified in relation to the questions addressed | ✓ | ✓ |
| Data collection | ||
| The source(s) of effectiveness estimates used are stated | ✓ | ✓ |
| Details of the design and results of effectiveness study are given (if based on a single study) | ✓ | ✓ |
| Details of the methods of synthesis or meta-analysis of estimates are given (if based on a synthesis of a number of effectiveness studies) | ✗ | ✗ |
| The primary outcome measure(s) for the economic evaluation are clearly stated | ✓ | ✓ |
| Methods to value benefits are stated | ✓ | ✓ |
| Details of the subjects from whom valuations were obtained were given | ✓ | ✓ |
| Productivity changes (if included) are reported separately | NA | NA |
| The relevance of productivity changes to the study question is discussed | ✓ | ✗ |
| Quantities of resource use are reported separately from their unit costs | ✓ | ✓ |
| Methods for the estimation of quantities and unit costs are described | ✓ | ✓ |
| Currency and price data are recorded | ✓ | ✓ |
| Details of currency of price adjustments for inflation or currency conversion are given | ✓ | ✓ |
| Details of any model used are given | NA | NA |
| The choice of model used and the key parameters on which it is based are justified | ✓ | ✓ |
| Analysis and interpretation of results | ||
| Time horizon of costs and benefits is stated | ✓ | ✓ |
| The discount rate(s) is stated | ✗ | ✗ |
| The choice of discount rate(s) is justified | ✗ | ✗ |
| An explanation is given if costs and benefits are not discounted | ✗ | ✗ |
| Details of statistical tests and CIs are given for stochastic data | ✓ | ✓ |
| The approach to sensitivity analysis is given | ✓ | ✓ |
| The choice of variables for sensitivity analysis is justified | ✓ | ✗ |
| The ranges over which the variables are varied are justified | ✓ | ✗ |
| Relevant alternatives are compared | ✓ | ✓ |
| Incremental analysis is reported | ✓ | ✓ |
| Major outcomes are presented in a disaggregated as well as aggregated form | ✓ | ✓ |
| The answer to the study question is given | ✓ | ✓ |
| Conclusions follow from the data reported | ✓ | ✓ |
| Conclusions are accompanied by the appropriate caveats | ✓ | ✓ |
Appendix 9 Excluded studies list along with rationale
The following is a list of studies excluded at the full paper screening stage of the review, along with the primary reason for their exclusion. For simplicity, studies were assigned a single reason for exclusion; however, many studies failed more than one inclusion criteria.
Effectiveness studies
The reasons for study exclusion are coded as follows:
Study design: The study is not a RCT of if the randomisation was done post testing.
Intervention: If the intervention was not rapid fFN testing. The studies were also excluded if the intervention group had fFN testing with a combination of any other test(s) to detect PTB and no separate data were reported for fFN testing. Also, if the fFN testing was done in both arms and the results were available for both groups. Studies with ELISA fFN testing were excluded.
Population: The studies with asymptomatic women for PTB were excluded.
Outcomes: Studies that did not report the outcomes of interest or if the data were not sufficient to extract the outcomes of interest.
References and reasons
-
Adeniji AO, Olayemi O, Odukogbe AA, Oladokun A, Adeniji OI, Egbewale BE, et al. Cervico-vaginal foetal fibronectin: a predictor of cervical response at pre-induction cervical ripening. West Afr J Med 2005;24:334–7. (Intervention)
-
Akers A, Jarzembowski JA, Johnson CT, Lieberman RW, Dalton VK. Examining the relationship between positive mid-gestational fetal fibronectin assays and histological evidence of acute placental inflammation. J Perinat Med 2007;35:36–42. (Study design)
-
Andersen HF. Use of fetal fibronectin in women at risk for preterm delivery. Clin Obstet Gynecol 2000;43:746–58. (Study design)
-
Andrews WW, Goldenberg RL, National Institute of Child Health, Human Development Maternal-Fetal Medicine Units Network. What we have learned from an antibiotic trial in fetal fibronectin positive women. Semin Perinatol 2003;27:231–8. (Population)
-
Burwick RM, Zork NM, Lee GT, Ross MG, Kjos SL. Cervilenz assessment of cervical length compared to fetal fibronectin in the prediction of preterm delivery in women with threatened preterm labor. J Matern Fetal Neonatal Med 2011;24:127–31. (Intervention)
-
Conde-Agudelo A, Romero R. Fetal fibronectin as a predictor of spontaneous preterm delivery in multiple gestations: a systematic review and meta-analysis. Am J Obstet Gynecol 2009;201:S196. (Study design)
-
Crane J. The use of fetal fibronectin in predicting successful labor induction: a systematic review. Am J Obstet Gynecol 2005;193:S40. (Outcomes)
-
Elliott JP, Miller HS, Coleman S, Rhea D, Abril D, Hallbauer K, et al. A randomized multicenter study to determine the efficacy of activity restriction for preterm labor management in patients testing negative for fetal fibronectin. J Perinatol 2005;25:626–30. (Intervention)
-
Goldenberg RL, Andrews WW, Hoffman I, Fawzi W, Valentine M, Young A. Fetal fibronectin and adverse infant outcomes in a predominantly human immunodeficiency virus-infected African population: a Randomized controlled trial. Obstet Gynecol 2007;110:936. (Study design)
-
Goldenberg RL, Andrews WW, Hoffman I, Fawzi W, Valentine M, Young A, et al. Fetal fibronectin and adverse infant outcomes in a predominantly human immunodeficiency virus-infected African population: a randomized controlled trial. Obstet Gynecol 2007;109(2 Part 1):392–401. (Intervention)
-
Gomez R, Romero R, Medina L, Nien JK, Chaiworapongsa T, Carstens M, et al. Cervicovaginal fibronectin improves the prediction of preterm delivery based on sonographic cervical length in patients with preterm uterine contractions and intact membranes. Am J Obstet Gynecol 2005;192:350–9. (Study design)
-
Gomez-Bravo Topete E, Castillo-Lechuga C, Villegas-Su A, Briones-Garduno JC. [Predictive value of fetal fibronectin for preterm labor.] Cir Cir 2004;72:491–4. (Study design)
-
Hayes, Inc. Fetal fibronectin test in women with symptoms of preterm labor. Lansdale, PA: Hayes, Inc.; 2006. (Study design)
-
Imai M, Tani A, Saito M, Saito K, Amano K, Nisijima M. Significance of fetal fibronectin and cytokine measurement in the cervicovaginal secretions of women at term in predicting term labor and post-term pregnancy. Eur J Obstet Gynecol Reprod Biol 2001;97:53–8. (Study design)
-
Institute for Clinical Systems Improvement (ICSI). Fetal fibronectin for the prediction of preterm labor. Bloomington, IN: ICSI; 2000. (Study design)
-
Institute of Health Economics (IHE). Using fetal fibronectin to diagnose pre-term labour. Edmonton, AB: IHE; 2008. p. 79. (Study design)
-
Institute of Health Economics (IHE). Actim Partus Test BS TLi System as rapid response diagnostic tests. Edmonton, AB: IHE; 2008. (Study design)
-
Keeler SM, Roman AS, Coletta JM, Kiefer DG, Feuerman M, Rust OA. Fetal fibronectin testing in patients with short cervix in the midtrimester: can it identify optimal candidates for ultrasound-indicated cerclage? Am J Obstet Gynecol 2009;200:158.e1–6. (Study design)
-
Koenn ME. Fetal fibronectin. Clin Lab Sci 2002;15:96–8. (Study design)
-
Kurtzman J, Chandiramani M, Briley A, Poston L, Das A, Shennan A. Quantitative fetal fibronectin screening in asymptomatic high-risk patients and the spectrum of risk for recurrent preterm delivery. Am J Obstet Gynecol 2009;200:263.e1–6. (Population)
-
Lopez RL, Francis JA, Garite TJ, Dubyak JM. Fetal fibronectin detection as a predictor of preterm birth in actual clinical practice. Am J Obstet Gynecol 2000;182:1103–6. (Study design)
-
Mateus J, Pereira L, Baxter J, Berghella V, Tolosa J. Effectiveness of fetal fibronectin testing compared with digital cervical assessment of women with preterm contractions. Am J Perinatol 2007;24:381–5. (Study design)
-
Mercorio F, Mercorio A, Votino C, Di Spiezio Sardo A, Barba GV, et al. Fetal fibronectin as predictor of successful induction of mid-trimester abortion. Acta Obstet Gynecol Scand 2005;84:390–4. (Study design)
-
Mozurkewich EL, Naglie G, Krahn MD, Hayashi RH. Predicting preterm birth: a cost-effectiveness analysis. Am J Obstet Gynecol 2000;182:1589–97. (Study design)
-
Ness A, Visintine J, Ricci E, Berghella V. Does knowledge of cervical length and fetal fibronectin affect management of women with threatened preterm labor? A randomized trial. Am J Obstet Gynecol 2007;197:426.e1–7. (Intervention)
-
Ness A, Visintine J, Ricci E, Boyle K, Berghella V. Use of fetal fibronectin and transvaginal ultrasound cervical length to triage women with suspected preterm labor: a randomized trial. Am J Obstet Gynecol 2006;195:S67. (Intervention)
-
Nguyen TCQ. The cost-effectiveness of fetal fibronectin testing in suspected preterm labor: a randomized trial. Obstet Gynecol 2002;99(Suppl. 4):97S. (Outcomes)
-
Smith V, Devane D, Begley CM, Clarke M, Higgins S. A systematic review and quality assessment of systematic reviews of fetal fibronectin and transvaginal length for predicting preterm birth. Eur J Obstet Gynecol Reprod Biol 2007;133:134–42. (Study design)
-
Tsourapas A, Roberts TE, Barton PM, Honest H, Forbes C, Hyde CJ, et al. An economic evaluation of alternative test-intervention strategies to prevent spontaneous pre-term birth in singleton pregnancies. Acta Obstet Gynecol Scand 2009;88:1319–30. (Population)
-
Vis JY, Wilms FF, Oudijk MA, Porath MM, Scheepers HCJ, Bloemenkamp KWM, et al. Cost-effectiveness of fibronectin testing in a triage in women with threatened preterm labor: alleviation of pregnancy outcome by suspending tocolysis in early labor (APOSTEL-I trial). BMC Pregnancy Childbirth 2009;9(38). (Intervention)
Accuracy studies
The reasons for study exclusion are coded as follows:
Study design: We excluded all non-DTA studies published since completion of the previous HTA report by Honest et al. 10
Index test: Studies were excluded if the used quantitative ELISA fFN testing to predict PTB or any other biomarkers. The studies were also excluded if the intervention group had fFN testing with a combination of any other test(s) to detect PTB and no separate data were reported for fFN testing.
Population: The studies with asymptomatic women for PTB were excluded.
Outcomes: The study did not report any of the outcomes specified in Chapter 3, Inclusion criteria, OR, for diagnostic test accuracy studies, insufficient data were reported to allow the construction of 2 × 2 contingency tables (numbers of TP, FN, FP, and TN test results).
Reference and reasons
-
fFN testing not always so accurate. Contemp Ob Gyn 2009;54:18. (Population)
-
Abenhaim HA, Morin L, Benjamin A. Does availability of fetal fibronectin testing in the management of threatened preterm labour affect the utilization of hospital resources? J Obstet Gynaecol Can 2005;27:689–94. (Study design)
-
Akers A, Jarzembowski JA, Johnson CT, Lieberman RW, Dalton VK. Examining the relationship between positive mid-gestational fetal fibronectin assays and histological evidence of acute placental inflammation. J Perinat Med 2007;35:36–42. (Outcomes)
-
Ayala-Mendez JA, Kurtzman J, Rosales-Ortiz S, Martinez-Alvarez O, Das A, Jimenez-Solis G. Fetal fibronectin status markedly modifies the risk of preterm delivery in low risk patients with symptomatic preterm labor and cervical shortening. Am J Obstet Gynecol 2008;199:S235. (Outcomes)
-
Bahado-Singh RO, Argoti P, Wilson L, Kruger M, Sorokin Y. Simultaneous evaluation of epidemiologic, psychosocial, biochemical and sonographic markers for prediction of spontaneous preterm birth. Paper presented at 57th Annual Scientific Meeting of the Society for Gynecologic Investigation, Orlando, FL, 24–27 March 2010. Reprod Sci 2010;17(Suppl. 1):277A. (Intervention)
-
Berghella V. MFM consult. When to use fetal fibronectin. Contemp Ob Gyn 2009;54:26. (Study design)
-
Bittar RE, Zugaib M. [Risk predictors for preterm birth.] Rev 2009;31:203–9. (Study design)
-
Chandiramani M, Di Renzo GC, Gottschalk E, Helmer H, Henrich W, Hoesli I, et al. Fetal fibronectin as a predictor of spontaneous preterm birth: a European perspective. J Matern Fetal Neonatal Med 2011;24:330–6. (Study design)
-
Chandiramani M, Shennan AH. Cervical insufficiency: prediction, diagnosis and prevention. Obstet Gynaecol 2008;10:99–106. (Intervention)
-
Facchinetti F, Paganelli S, Venturini P, Dante G, Palama L. [Biochemical mediators of the cervical modifications in the preterm birth.] G Ital Ostet Ginecol 2006;28:11–15. (Intervention)
-
Fox N, Saltzman D, Klauser C, Peress D, Gutierrez C, Rebarber A. Prediction of spontaneous preterm birth and very preterm birth in twin pregnancies using serial fetal fibronectin and cervical length. Am J Obstet Gynecol 2008;199:S25. (Population)
-
Franco R, Shaw K, Williams C, Hickok D. Risk of preterm delivery with a positive fetal fibronectin test result and a normal cervical length. Obstet Gynecol 2005;105:114S. (Outcomes)
-
Fuchs I, Dudenhausen JW. [Use of foetal fibronectin for prediction of a premature birth.] Gynakol Prax 2008;32:646–8. (Outcomes)
-
Ghidini A, Poggi SH, Korker V. Performance of vaginal fetal fibronectin as predictor of preterm delivery in a community hospital. J Soc Gynecol Investig 2006;13:338A. (Outcomes)
-
Giles W. Fetal fibronectin use in the management of threatened preterm labour. O & G Magazine 2006;8:39. (Outcomes)
-
Gottschalk EM, Wenzel S, Salomon NS, Dudenhausen JW, Henrich W. Importance of cervical length measurement and fetal fibronectin (fFN) to the prediction of lower premature birth rate. Geburtshilfe Frauenheilkunde 2008;68:S25. (Outcomes)
-
Hee L. Likelihood ratios for the prediction of preterm delivery with biomarkers. Acta Obstet Gynecol Scand 2011;90:1189–99. (Intervention)
-
Herbst A, Nilsson C. Diagnosis of early preterm labour. BJOG 2006;113(Suppl. 3):60–7. (Intervention)
-
Holmgren C, Lacoursiere DY, Esplin MS. Clinical predictors of a false negative fetal fibronectin (FFN). Am J Obstet Gynecol 2007;197:S204. (Outcomes)
-
Incerti M, Ghidini A, Korker V, Pezzullo JC. Performance of cervicovaginal fetal fibronectin in a community hospital setting. Arch Gynecol Obstet 2007;275:347–51. (Outcomes)
-
Jenkins SM, Kurtzman JT, Osann K. Dynamic cervical change: is real-time sonographic cervical shortening predictive of preterm delivery in patients with symptoms of preterm labor? Ultrasound Obstet Gynecol 2006;27:373–6. (Intervention)
-
Johnson CT, Dalton VK, Akers AY, Jarzembrowski JA, Lieberman RW, Johnson TRB. Fetal fibronectin assay results and placental histopathology in multiple gestation pregnancies. Obstet Gynecol 2006;107:80S. (Intervention)
-
Kang JH, Lee SE, Park CW, Jun JK, Romero R, Yoon BH. Cervical fetal fibronectin: an index of intra-amniotic inflammation, histologic chorioamnionitis and impending preterm delivery in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2007;197:S47. (Outcomes)
-
Khan KS. Systematic reviews of diagnostic tests: a guide to methods and application. Best Pract Res Clin Obstet Gynaecol 2005;19:37–46. (Study design)
-
Knee A, Belisle E, Markenson G, Malshe A, Plevyak M, Bsat F, et al. Do pregnancies complicated by preterm births with a negative fetal fibronectin screen have different characteristics compared to those that have positive fibronectin results? Paper presented at 31st Annual Meeting of the Society for Maternal-Fetal Medicine: The Pregnancy Meeting, San Francisco, CA, 7–12 Feb 2011. Am J Obstet Gynecol 2011;204(Suppl. 1):S190. (Population)
-
Kosec V, Herman R. [Diagnosis of preterm birth.] Gynaecol Perinatol Suppl 2008;17:S38–41. (Outcomes)
-
Kuin RA, Vis JY, Mol BW. Fetal fibronectin as a short-term predictor of preterm birth in symptomatic patients. Obstet Gynecol 2010;115:186–7. (Study design)
-
Leitich H. Controversies in diagnosis of preterm labour. BJOG 2005;112(Suppl. 1):61–3. (Outcomes)
-
Markenson G. Predicting preterm labor. Female Patient 2008;33:19–20. (Study design)
-
Menon R, Torloni MR, Voltolini C, Torricelli M, Merialdi M, Betran AP, et al. Biomarkers of spontaneous preterm birth: an overview of the literature in the last four decades. Reprod Sci 2011;18:1046–70. (Intervention)
-
Muller A, McCullough M, Obican S, Norton H, Carter J, Gonzalez-Quintero VH. ‘False’ positive fetal fibronectin and rates of adverse obstetrical outcomes. Am J Obstet Gynecol 2006;195:S233. (Intervention)
-
Myint H, Singh V, Roberts NJ. Introducing fetal fibronectin testing to a district general hospital in women with symptoms of preterm labour. Paper presented at Perinatal Medicine conference; 15–17 Jun 2011; Harrogate, UK. Arch Dis Child Fetal Neonatal Ed 2011;96:Fa83. (Intervention)
-
O’Sullivan M. Fetal fibronectin as a tool to reduce preterm labour admissions. Aust Midwifery News 2006;6:14–15. (Study design)
-
Passuello V, Puhl AG, Seufert R, Fischl F, Kobl H. Fetal fibronectin: is (still actual) a marker of premature birth? Geburtshilfe Frauenheilkunde 2008;68:S50. (Intervention)
-
Pizzo A, Ardita FV, Oteri F, Caruso C, La Spada R, Accardo FM. [Fibronectin and preterm labour.] Gaz Med Ital Archiv Sci Med 2006;165:175–9. (Outcomes)
-
Ponting J, Tomlin M. Diagnosis of preterm labour: introducing the fetal fibronectin test. Brit J Midwifery 2011;19:24–31. (Study design)
-
Pucillo K, Munneke S. Fetal fibronectin as predictor of preterm delivery in women with symptoms of preterm labor: women with negative FFN test results are unlikely to deliver in the following 2 weeks; observation is still warranted, but expensive interventions may be unnecessary. Evid Based Pract 2008;11:11–12. (Outcomes)
-
Ramirez Pineda M, Duenas Diez JL, Sala Turrens J, Polo Padillo J, Bedoya Bergua C. [Analysis of two strategies for the management of threatened preterm labor.] Prog Obstet Ginecol 2010;53:261–6. (Study design)
-
Riboni F, Vitulo A, Dell’avanzo M, Plebani M, Battagliarin G, Paternoster D. Biochemical markers predicting pre-term delivery in symptomatic patients: phosphorylated insulin-like growth factor binding protein-1 and fetal fibronectin. Arch Gynecol Obstet 2011;284:1325–9. (Intervention)
-
Rouse DJ. Improved management in threatened preterm labor with rapid fetal fibronectin testing: commentary. Obstet Gynecol Surv 2006;61:688–9. (Study design)
-
Sanchez-Ramos L, Zamora J, Kaunitz AM. Fetal fibronectin as a short-term predictor of preterm birth in symptomatic patients reply. Obstet Gynecol 2010;115:187. (Study design)
-
Schmitz T, Maillard F, Bessard-Bacquaert S, Kayem G, Fulla Y, Cabrol D, et al. Selective use of fetal fibronectin detection after cervical length measurement to predict spontaneous preterm delivery in women with preterm labor. Am J Obstet Gynecol 2006;194:138–43. (Intervention)
-
Tanir HM, Sener T, Yildiz Z. Cervicovaginal fetal fibronectin (FFN) for prediction of preterm delivery in symptomatic cases: a prospective study. Clin Exp Obstet Gynecol 2008;35:61–4. (Outcomes)
-
Tekesin I, Eberhart LHJ, Schaefer V, Wallwiener D, Schmidt S. Evaluation and validation of a new risk score (CLEOPATRA score) to predict the probability of premature delivery for patients with threatened preterm labor. Ultrasound Obstet Gynecol 2005;26:699–706. (Outcomes)
-
Tekesin I, Marek S, Hellmeyer L, Reitz D, Schmidt S. Assessment of rapid fetal fibronectin in predicting preterm delivery. Obstet Gynecol 2005;105:280–4. (Study design)
-
Ting H-S, Chin P-S, Yeo GSH, Kwek K. Comparison of bedside test kits for prediction of preterm delivery: phosphorylated insulin-like growth factor binding protein-1 (pIGFBP-1) test and fetal fibronectin test. Ann Acad Med Singapore 2007;36:399–402. (Outcomes)
-
Vintzileos AM. Predicting preterm births in high-risk patients. Female Patient 2005;30:10–13. (Study design)
-
Wilms FF, van Stralen G, Porath MM, Papatsonis DNM, Oei SGG, Mol B-W, et al. [Predicating imminent preterm labour based on a determination of foetal fibronectin in a vaginal smear.] Ned Tijdschr Geneeskd 2009;153:B398. (Intervention)
-
Yoneda S, Sakai M, Sasaki Y, Shiozaki A, Hidaka T, Saito S. Interleukin-8 and glucose in amniotic fluid, fetal fibronectin in vaginal secretions and preterm labor index based on clinical variables are optimal predictive markers for preterm delivery in patients with intact membranes. J Obstet Gynaecol Res 2007;33:38–44. (Intervention)
Appendix 10 Protocol
DIAGNOSTIC ASSESSMENT REPORT COMMISSIONED BY THE NIHR HTA PROGRAMME
Title of project
The cost effectiveness of fetal fibronectin (fFN) testing in suspected premature labour.
Name of External Assessment Group (EAG) and project lead
Kleijnen Systematic Reviews Ltd. Assessment Group.
Project lead:
Marie Westwood
Kleijnen Systematic Reviews Ltd
Unit 6, Escrick Business Park
Riccall Road, Escrick
York YO19 6FD
Tel: 01904 727983
Email: marie@systematic-reviews.com
1. Plain English Summary:
The World Health Organization (WHO) defines a premature birth as an infant born before 37 completed weeks of gestation. 1 The incidence of spontaneous preterm birth is 7–12% of pregnancies before 37 weeks’ gestation and about 4% of pregnancies before the completion of 34 weeks’ gestation. 2–5 One in 13 live births in England and Wales are preterm. 6 The incidence of preterm births before 37 weeks’ gestation is reported to be greater in multiple pregnancies (61.9%) as compared to singleton pregnancies (11.1%). 4 In the majority of developed countries, preterm birth is one of the major causes of neonatal mortality and severe morbidities1. Preterm births account for about 60 to 80% of the neonatal mortality and about 75% of severe morbidities. 7,8 These severe morbidities can cause significant psychological, sociological and financial burdens on the parents or the carers. 9
The recent developments in perinatal health care have not significantly reduced the incidence of spontaneous preterm labour. 4 The Cochrane review by Crowley10 reported the effectiveness of antenatal steroids in significantly reducing the rate of neonatal mortality and morbidities in symptomatic women. However, to maximise the effectiveness of antenatal steroid therapy, it is important to diagnose preterm labor in early stages after the appearance of signs and symptoms.
The use of fetal fibronectin (fFN) testing is proposed to diagnose preterm labor in the women displaying symptoms. fFN can be detected in cervicovaginal secretions in early pregnancy and before birth. fFN is released into the cervix or vagina because of the mechanical damage caused to the fetal membrane before the onset of birth. However, in the normal course of pregnancy it is unusual to detect fFN between 22 to 37 weeks’ gestational ages. 11 Hence, the detection of elevated levels of fFN in cervicovaginal secretion between 22 to 37 weeks’ gestation can be considered an indicator of preterm labour in symptomatic women. 12
The purpose of this project is to assess the cost-effectiveness of adding fFN to conventional management, compared with conventional management alone, in women who are symptomatic for preterm birth. The conventional methods of managing pre-term labour in symptomatic women include hospitalisation for longer periods, antenatal steroid therapy and occasional in-utero transfer. 13 However, only about 20% of admissions for suspected preterm labor will actually progress and deliver the baby prematurely. The remaining 80% of admissions have normal delivery after 37 weeks’ gestation; this means that there are many unnecessary and costly inpatient admissions and treatments for suspected preterm labor. 14 It is hoped that the addition of fFN testing to the diagnostic work-up of women with suspected pre-term labour will help to identify those 20% of women who require active management, and thus avoid unnecessary interventions, hospitalizations and associated costs.
4. Decision problem
4.1 Aims & objectives:
Aim:
The aim of this project is to assess the impact of early diagnosis of pre-term labour, using fetal fibronectin testing, on NHS resources and to propose possible changes in maternal management.
Objectives:
-
To assess the effectiveness and accuracy of the fFN test (commercial rapid test kit) in diagnosing spontaneous pre-term labour in symptomatic women.
-
To assess, from an NHS perspective, the cost-effectiveness of the use of Fibronectin (rapid fFN testing) to diagnose spontaneous pre-term labor in symptomatic women in comparison to no testing (current care).
4.2 Intervention:
Fetal fibronectin is an extracellular matrix glycoprotein produced by amniocytes and by cytotrophoblast. 1 It is thought to be present mainly in the choriodecidual interface, which is a union between maternal and fetal tissues. 15 Normally, fFN is present in the cervicovaginal secretions of pregnant women until 22 weeks’ gestation. However, the level of fFN in cervicovaginal secretions drops after 22 weeks’ gestation (< 50 ng/mL). If the pregnancy is not normal, the level of fFN found in a cervicovaginal swab may be high (≥ 50 ng/mL) at or after 22 weeks’ gestation; elevated levels of fFN may indicate early onset of labour. 1
The fFN test can be used to assess the risk of preterm birth, within 7 to 14 days of testing, in symptomatic women. The fetal fibronectin test is available in two formats: a quantitative solid-phase enzyme-linked immunosorbent assay (ELISA) or a qualitative membrane immunosorbent assay (Rapid fFN for the TLi™ System, which recently changed to FullTerm™). 16–18 Rapid fFN testing is a more practical approach for diagnosing preterm labour as it gives the results instantly (30 min) unlike the ELISA assay which delivers the results 4 to 48 hours after sample collection. 17 However, there is limited clinical evidence on the use of rapid fFN to detect preterm labour as majority of evidence is based on ELISA.
Rapid fFN testing is a lateral flow, solid-phase immunosorbent assay designed to perform a qualitative detection (positive/negative) of fFN in cervicovaginal specimens collected in the Adeza Biomedical Collection Kit. 17 The cervicovaginal specimen (vaginal swab) is mixed with a liquid buffer in a collection tube and a portion of this sample is pipetted to the lateral flow, rapid fFN cassette in the TLi™ IQ Analyser. 17 The assay takes about approximately 30 min to process the sample and deliver the results. The TLi™ automatically prints and displays positive or negative results along with patient details (an fFN level of ≥ 50 ng/mL is positive result and an fFN level of < 50 ng/mL is negative result). 17
The intervention considered in this review is rapid fFN testing in addition to usual care.
Population:
The data from England and Wales suggest that the estimated number of spontaneous preterm births before 37 weeks’ gestation was 76,000 in 2004. 6 The majority of neonatal deaths occur in the infants born before 34 weeks’ gestation; surviving babies tend to suffer from serious morbidities such as bronchopulmonary dysplasia, respiratory distress syndrome (RDS), necrotizing enterocolitis, intraventricular haemorrhage (IVH), retrolental fibroplasia, sepsis, long term cognitive difficulties etc. 1,15 Also, some of the premature infants who are classified as normal with respect to their development, or who have mild abnormalities, can have multiple health problems later in life. 19 Preterm births not only affect the infant and family but also increases NHS resource use (longer hospital stays, or use of neonatal intensive care services). 20
The actual pathogenesis of preterm labour is unknown but there are several risk factors which are believed to be predictive of preterm birth (e.g. ethnicity, smoking, young/old maternal age, multiple pregnancy, stress, infection, low socioeconomic status and history of previous preterm birth). 21,22 Multiple pregnancies are more likely to be at risk of preterm labour than singleton pregnancies. In developed countries the incidence of multiple pregnancies has increased in last 20–30 years mainly because of advanced reproductive techniques such as drugs used to induce ovulation and in vitro fertilisation. 20 Most studies on fFN testing exclude women with multiple pregnancies because of the associated complications. However, in this review both singleton and multiple pregnancies will be considered.
This assessment will consider the population of women with singleton or multiple pregnancies displaying symptoms of labour before completing the 37 weeks gestational period (preterm labour). The clinical signs and symptoms that indicate onset of preterm labour are uterine contractions, low abdominal pain, dull backache, pelvic pressure, change in volume or consistency of vaginal discharge, and menstrual-like or intestinal cramping. 23–25 Also, an important sign of preterm labour is cervical effacement (80%) and dilation (< 3 cm).
Comparator (usual care):
Currently, the diagnosis of preterm labour is based mainly on signs and symptoms, clinical history and physical examination of the patient. Physical examination of the cervix indicating dilation of ≥ 3 cm and at least 80% effacement is indicative of preterm labour within 24 hours to 7 days. 17 If a woman is diagnosed with preterm labour by a physical examination then she can be treated to postpone her delivery by administering tocolytic agents. However, in some cases it is not possible to postpone the delivery and preparations have to be made for a preterm delivery. Clinicians need to take a number of key decisions before preparing for a preterm delivery, e.g. use of maternal intramuscular corticosteroid injection to facilitate the development of lungs and to avoid respiratory distress syndrome. 9 Antenatal corticosteroids are most effective in the infants who have been delivered after 2–7 days after the administration of the drugs. 10 Also, it is important to check for the availability of neonatal intensive care unit space before in utero transfers. The arrangements for in utero transfers may take some time due to geographical constrains or long waiting periods. 26 Thus, considering the time required for the corticosteroid drugs to show maximum effectiveness (2–7 days) as well as the time required for making in utero transfer arrangements it is very important for the clinicians to have advance timely knowledge of preterm birth in symptomatic women.
Where physical examination does not confirm the diagnosis of preterm labour, symptomatic women have to be hospitalised under observation for longer periods to assess if the symptoms are subsiding or increasing. 21,24,25 During this period of hospitalisation, complete bed rest is suggested and clinicians may administer tocolytic drugs or antibiotics as required. The main concern for clinical diagnosis based on symptoms is that it is very unreliable, and leads to over diagnosis of preterm labour. 27 The overdiagnosis of preterm labour incurs unnecessary hospitalisation, unnecessary interventions and wastage of resources; there is, therefore, a need for improved diagnostic testing.
Current evidence:
A number of systematic reviews have previously evaluated the effectiveness of the fFN testing. Honest et al. 9 conducted a HTA review on screening to prevent spontaneous preterm birth in symptomatic and asymptomatic women. They evaluated several screening interventions which can be used to predict and prevent spontaneous preterm birth, including the fFN test. However, the conclusions of this review did not focus on the cost effectiveness of fFN testing. A recent systematic review, exclusively evaluating the accuracy of fFN testing to predict the preterm birth in women with multiple pregnancies, concluded that fFN testing can be most accurate in predicting the spontaneous preterm birth within 7 days of testing (pooled sensitivity, specificity, and positive and negative likelihood ratios of 85%, 78%, 3.9, and 0.20, respectively) in women with twin pregnancies. 24 Similarly, an earlier review by Honest et al. 15 evaluated the accuracy of fFN testing in predicting spontaneous preterm labour and concluded that fFN testing is most accurate in predicting spontaneous preterm birth with 7–10 days of testing among the symptomatic women. However, this review evaluated only the quantitative solid-phase ELISA test. A systematic review by Ramos,28 evaluated the effectiveness of fFN testing and, in contrast to the studies detailed above, concluded that fFN has limited accuracy in predicting preterm birth within 7 days of sampling in symptomatic pregnant women.
Two previous systematic reviews have assessed rapid fFN testing for predicting preterm labour in symptomatic women. The first study was carried out in Australia by the Medical Service Advisory Committee29 which determined the test to be safe but it did not determine the effectiveness for symptomatic labour. The second study was carried out by the Institute of Health Economics in Canada. The study concludes by supporting the previous findings that the rapid fFN test can be used to diagnose preterm labour in symptomatic women based on its higher negative predictive values. 17
Given the current evidence base and clinical imperative for rapid information, evaluate rigorous, up-to-date evaluation of the cost effectiveness of rapid fFN testing to predict the preterm labour in the symptomatic women is needed. Some countries (Australia and Canada) have already assessed rapid fFN testing with respect to their healthcare settings. However, to date, no similar assessment has been carried out for the UK setting; the current assessment will evaluate the cost effectiveness of fetal fibronectin testing in suspected premature labour in the UK.
5. Methods of assessing clinical effectiveness
5.1 Inclusion and Exclusion criteria:
Population:
Studies including pregnant women with singleton or twin gestations who have signs and symptoms of pre-term labour (e.g. uterine contractions, dull backache, pelvic pressure, change in volume or consistency of vaginal discharge, and menstrual-like or intestinal cramping) before 37 weeks’ gestation.
Setting:
Secondary care.
Intervention:
Studies assessing swab testing for fetal fibronectin using a commercial rapid test kit done before 37 weeks’ gestation + usual care, for the diagnosis of pre-term labour. Studies using rapid fetal fibronectin test in participants after 37 weeks’ gestation or studies assessing fetal fibronectin for detecting any other risks than preterm birth will be excluded from this review.
Comparator (clinical effectiveness studies only):
Usual care, without fibronectin, testing for managing pre-term birth.
Reference Standard (accuracy studies only):
Spontaneous preterm births before 37 weeks’ gestation.
Outcomes:
-
Incidence of spontaneous pre-term birth before 37 weeks’ gestation, before 34 weeks’ gestation, or within 24 hours, 48 hours, or 7–10 days of testing (time required for corticosteroids to exert beneficial effects and the potential for in utero transfer and tocolytic administration). – primary outcome measure.
-
Changes in maternal management
-
Admission to hospital
-
Use of corticosteroids
-
Changes in frequency of monitoring
-
Changes from usual care.
-
-
Outcomes in the new born, morbidity, mortality.
-
Outcomes of maternal health.
-
Diagnostic accuracy of the test.
-
Cost-effectiveness.
Study Design:
Step 1: Randomised and non-randomised trials where participants are assigned to the intervention group or comparator group, and which report patient-relevant outcomes (changes to maternal management, maternal health outcomes, new born morbidity and mortality) and/or incidence of pre-term birth (before 37 weeks).
Step 2: If insufficient evidence for the clinical effectiveness testing is identified, diagnostic cohort studies will be included in order to assess test accuracy.
Included test accuracy studies will be required to report sufficient data to construct 2 × 2 contingency tables, i.e. numbers of true positive, false negative, false positive, and true negative test results.
The following study/publication types will be excluded:
-
Studies with < 10 participants.
-
Pre-clinical and animal.
-
Reviews, editorials, and opinion pieces.
-
Case reports and diagnostic case-control studies.
5.2 Search strategy
Search strategies will be based on target condition and intervention, as recommended in the Centre for Reviews and Dissemination (CRD) guidance for undertaking reviews in health care and the Cochrane Handbook for Diagnostic Test Accuracy Reviews. 30–32
Additional supplementary searches, for data to populate economic models, will be carried out as necessary. Searches for studies for cost and quality of life will also be included, see Section 6 for further detail.
The following databases will be searched for relevant studies from 2000 to the present:
-
MEDLINE (OvidSP)
-
MEDLINE In-Process Citations and Daily Update (OvidSP)
-
EMBASE (OvidSP)
-
Cochrane Database of Systematic Reviews (CDSR) (Internet)
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Internet)
-
Database of Abstracts of Reviews of Effects (DARE) (CRD website)
-
Health Technology Assessment Database (HTA) (CRD website)
-
NHS Economic Evaluation Database (NHSEED) (CRD website)
-
Science Citation Index (SCI) (Web of Science)
-
Maternity and Infant Care
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO)
Completed and on-going trials will be identified by searches of the following resources (2000–2010):
-
ClinicalTrials.gov (http://www.clinicaltrials.gov/)
-
Current Controlled Trials (http://www.controlled-trials.com/)
-
International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/)
-
EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/)
Key conference proceedings will be screened for the last five years. These may include Society for Maternal-Fetal Medicine, Blair Bell Research Society, European Association of Perinatal Medicine.
Identified references will be downloaded in Endnote X4 software for further assessment and handling.
The bibliographies of retrieved articles and relevant systematic reviews will be checked for additional studies.
Search strategies will be developed specifically for each database and the keywords will be adapted according to the configuration of each database.
No restrictions on language or publication status will be applied. Limits will be applied to remove animal studies. Searches will take into account generic and other product names for the intervention. Examples of the search strategies to be used are presented in Appendix 1.
5.3 Data extraction strategy
Two reviewers will independently screen titles and abstracts of all reports identified by searches and discrepancies will be discussed. Full copies of all studies deemed potentially relevant, after discussion, will be obtained and two reviewers will independently assess these for inclusion; any disagreements will be resolved by consensus or discussion with a third reviewer.
Data relating to study details, participants, intervention and comparator tests, gold standard (test accuracy studies only), and outcome measures will be extracted by one reviewer, using a piloted, standard data extraction form. A second reviewer will check data extraction and any disagreements will be resolved by consensus or discussion with a third reviewer.
5.4. Quality assessment strategy
The methodological quality of included studies will be assessed using standard tools. 32 The QUADAS tool,33 is recommended for assessing the methodological quality of test accuracy studies,30,32 but a revised version of QUADAS (QUADAS-2) is soon to be published (submitted for publication May 2011). QUADAS-2 will more closely resemble the approach and structure of the Cochrane risk of bias tool. The QUADAS-2 tool will be used in this assessment, with the permission of the QUADAS steering group, of which one of the reviewers is a member.
The results of the quality assessment will be used for descriptive purposes to provide an evaluation of the overall quality of the included studies and to provide a transparent method of recommendation for design of any future studies. In addition, if enough data are available from the included studies, quality components will be included as covariates in SROC models, to investigate their possible association with test performance. Based on the findings of the quality assessment, recommendations will be made for the conduct of future studies.
5.5. Methods of analysis/synthesis
The results of initial scoping searches suggest that trial data are likely to be sparse or non-existent. This section therefore focuses on the synthesis of data from test accuracy studies.
For test accuracy data, absolute numbers of true positive, false negative, false positive and true negative test results, as well as sensitivity and specificity values, with 95% confidence intervals will be presented for each study and patient group reported. Where appropriate, and where sufficient accuracy data are available, summary receiver operating characteristic (SROC) curves will be calculated to summarise test accuracy data. SROC modelling will use the bivariate approach. 30,34,35 Potential sources of heterogeneity will be investigated by extending SROC models to include study level covariates, (e.g. participant age, ethnicity, smoking status, concomitant infection, previous history of pre-term birth, risk of bias criteria); the bivariate approach to modelling allows investigation of the effects of covariates on sensitivity and specificity separately.
Where meta-analysis is considered unsuitable for some or all of the data identified (e.g. due to the heterogeneity and/or small numbers of studies), we will employ a narrative synthesis. Typically, this will involve the use of text and tables to summarise data. These will allow the reader to consider any outcomes in the light of differences in study designs and potential sources of bias for each of the studies being reviewed. Studies will be organised by clinical application (singleton, multiple pregnancies), relevant patient sub-groups, and the outcomes assessed. Where data are insufficient to support meta-analyses, the following graphical representations will be presented: plots in ROC space (without summary curves) and/or paired forest plots of sensitivity and specificity for test accuracy data; forest plots for any trial data.
A detailed commentary on the major methodological problems or biases that affected the studies will also be included, together with a description of how this may have affected the individual study results. Recommendations for further research will be made based on any gaps in the evidence or methodological flaws.
6. Methods of assessing cost-effectiveness
The economic component of the project, assessing the value of the use of Fibronectin (rapid fFN testing) to diagnose spontaneous pre-term labor in symptomatic women will consist of two parts. First a review of the economic literature will be performed. Secondly, a de novo cost-effectiveness model will be built and run. We consider the design and use of a de novo model (or adaptation of any other suitable model that might be identified in the literature) essential since the cost-effectiveness model that was described in the HTA-report by Honest et al. was based on estimating the incremental cost per preterm birth avoided or cost per perinatal death avoided. This analysis did not distinguish between preterm birth at < 34 weeks’ gestation and between 34 to 37 weeks’ gestation, which has impact on costs. In the de novo model we intend to differentiate between < 34 and 34–37 weeks of gestation Also, the analysis by Honest et al did not take into account the long-term effects on costs, life expectancy and quality of life of the child resulting from the use of fFN testing. Dependent on time and budgetary constraints, we may also adopt a longer time horizon, use outcome measures such as life expectancy and QALYs, and explore the possible impact of the use of Fibronectin on the life expectancy of the mother.
6.1 Identifying and reviewing published cost-effectiveness studies
The objective of the review of economic evaluations of the diagnosis of preterm labor is to summarize methods and findings of existing peer reviewed studies.
Exploration of the literature regarding published economic evaluations will be performed in the databases listed in the systematic review part of this protocol. In addition, specific health economic databases will be searched (e.g. NHSEED (NHS Economic Evaluation Database), PEDE (Paediatric Economic Database Evaluation), and HEED (Health Economic Evaluation Database); an example search strategy is included in Appendix 1. Searches will focus on original papers that report on cost, cost-accuracy, cost-effectiveness or cost-utility analyses studying diagnostics of preterm labor. For our assessment only full economic evaluations, i.e. those that explicitly compare different decision options will be selected. Clinical trials as well as modelling studies and cohort studies will be considered relevant within the frame of our project. The intention of this component of the project is not to perform a systematic review, but to use the studies identified to support the development of an economic model that will aim to answer the research questions of this project.
The results and the methodological quality of the studies selected will be summarised. Assessment of methodological quality will follow the criteria for economic evaluations in health care as described in the NICE methodological guidance. 32 Data extraction will focus on technologies compared, indicated population, main results in terms of costs and consequences of the alternatives compared, and the incremental cost-effectiveness, but also on methods of modelling used (if applicable), analytical methods and robustness of the study findings.
6.2 Evaluation of costs, quality of life and cost-effectiveness
The model will evaluate the cost-effectiveness of rapid fFN testing in symptomatic women in addition to a diagnosis based on clinical signs and symptoms (uterine contractions, low abdominal pain, dull backache, pelvic pressure, change in volume or consistency of vaginal discharge, and menstrual-like or intestinal cramping). The focus of the evaluation of fFN testing in this population will be in assessing the accuracy of testing. Identifying a woman to be at high risk for preterm labor, either based on clinical signs and symptoms only or based on fFN-testing, will lead to preventive actions. In the model the following actions will be included: hospitalisation under observation for longer periods (including complete bed rest, and possibly administration of tocolytic drugs or antibiotics) to assess if the symptoms are subsiding or increasing. If preterm birth is unavoidable preparation is required, e.g. by administration of maternal intramuscular corticosteroid injection. In the current situation a false positive identification of high risk of preterm labor will lead to inefficient care (avoidable hospitalization and treatment). A false negative judgement based on testing might lead to preventable preterm delivery and preventable maternal and paediatric morbidity and mortality.
The perspective will be that of the NHS and the timeframe used will initially consider time to delivery (short term analysis) and may also consider a lifetime time horizon (long time analysis). Short-term costs will include the costs of fFN-testing, perinatal hospitalization cost of mother and child, and costs of delivery. For this purpose a distinction will be made between the situation of delivery after 37 weeks’ gestation, between 34 and 37 weeks’ gestation and earlier than 34 weeks’ gestation. Short-term consequences will be expressed as probability of preterm delivery (< 37 weeks’ gestation) and very early preterm delivery (< 34 weeks’ gestation) and consequently the cost-effectiveness will be expressed as the cost per case of preterm delivery avoided (both < 34 and < 37 weeks’ gestation). Besides this, perinatal death will be assessed in this short term analysis and expressed as the cost per perinatal death avoided. If undertaken, long-term cost-effectiveness will assess the costs per life year and the cost per QALY. In this analysis life expectancy and QALY will be based on the general population expectancy, according to the three subgroups specified based on duration of gestation (< 34 weeks; 34–37 weeks, and > 37 weeks). Lifetime health care costs (non-perinatal costs) may be considered if sufficient data are available for analysis. Data for the cost analyses will be drawn from routine NHS sources (e.g. NHS reference costs, Personal Social Services Research Unit (PSSRU), British National Formulary (BNF)), and discussions with individual hospitals where necessary.
Besides the impact of preterm diagnosis on the child a possible impact on the mortality of the mother will be assessed in a separate calculation.
Any assumption used in the models and any parameter value will be based primarily on literature and supplemented by clinical expert opinion as appropriate. Extensive one-way sensitivity analyses will be performed, besides a comprehensive probabilistic sensitivity analysis. If assessed, longer-term costs and consequences will be discounted using the UK discount rates of 3.5% of both costs and effects. Decision uncertainty regarding the alternatives will be reflected using cost-effectiveness planes and cost-effectiveness acceptability curves. Value of information analysis will be performed for those model parameters for which empirical distributions can be defined.
The following major assumptions will be basis for the cost-effectiveness calculations:
-
A possible impact on preterm delivery will impact on the perinatal mortality, life expectancy, and quality of life of the child. The possible short-term impact of preterm birth on the morbidity of the mother and the long-term impact on the parents are considered to be beyond the scope of this economic model.
-
The analysis will be based on a closed cohort population. Variability within the population is not part of the analyses. If the review on efficacy of fibronectin reveals heterogeneity within the population, this will be dealt with using subgroup analyses in the model.
-
Life expectancy and quality of life of a newborn that is born > 37 weeks’ gestation is considered to be equal to the general population.
-
In the analysis no distinctions will be made between singletons and twins.
-
Preterm birth before 34 weeks’ gestation will have impact on perinatal mortality and morbidity of the child. Besides this, preterm birth will have impact on life expectancy and quality of life of the child.
-
Elective preterm deliveries are considered beyond the scope of this model.
A preliminary version of the decision analytic model is shown in the figure 1 below. Validation and possibly adaptation of the structure of this model will depend on the findings from the literature review and consultation with clinical experts. In addition, the existence/availability of any other electronic models that reflect the cost-effectiveness of diagnostic and treatment pathways for these patients, and are representative of current care within the NHS, will be determined.
FIGURE 1.
Decision analytic model.
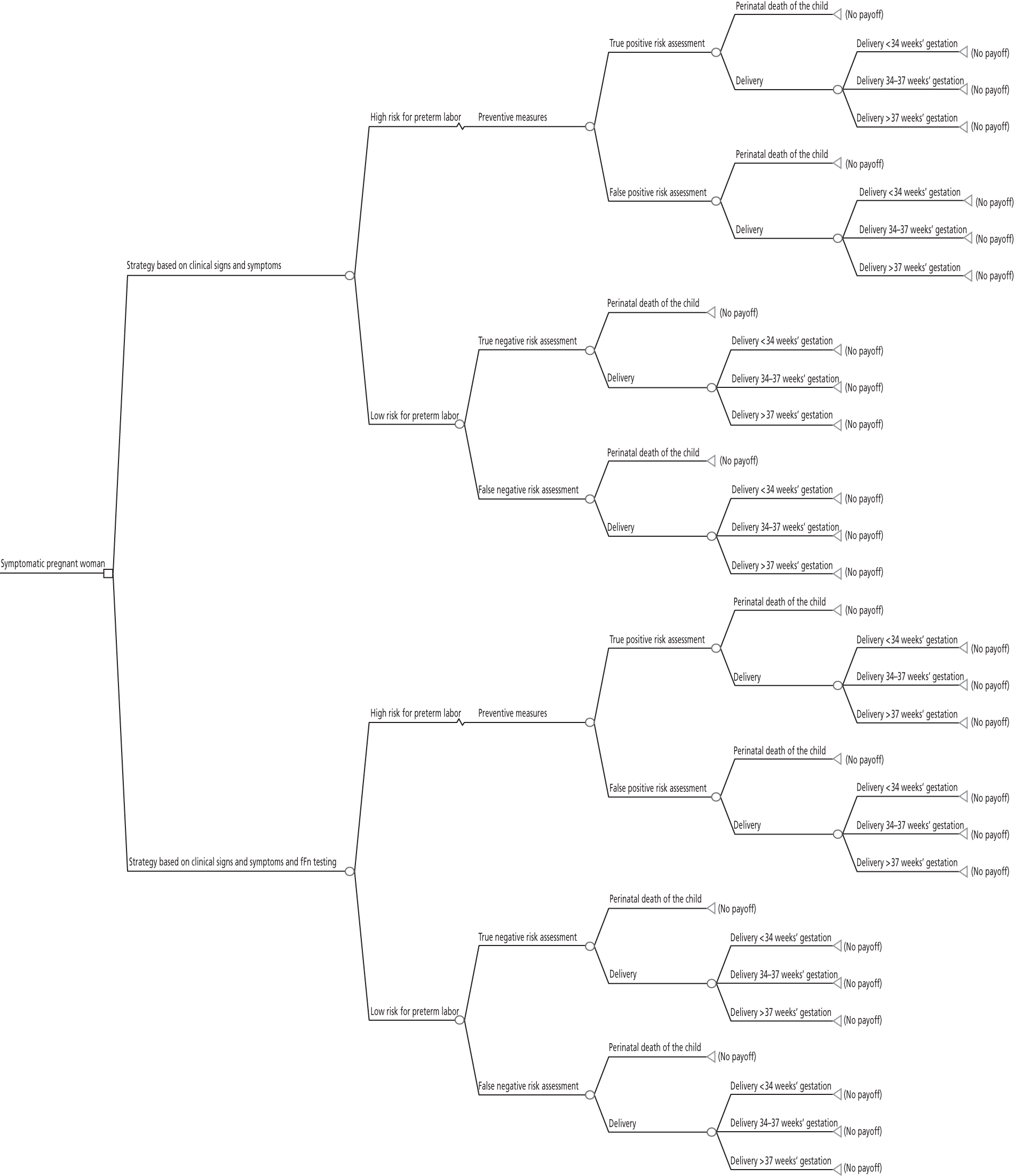
References:
- Berghella V, Hayes E, Visintine J, Baxter JK. Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database Systematic Review 2008. URL: 10.1002/14651858.CD006843.pub2.
- Morrison JJ, Rennie JM. Clinical, scientific and ethical aspects of fetal and neonatal care at extremely preterm periods of gestation. BJOG 1997;104:1341-50.
- Byrne B, Morrison JJ. Preterm birth. Clinical Evidence 2004;11:1903-22.
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2003. National Vital Statistics Reports 2005;54:1-116.
- McPheeters ML, Miller WC, Hartmann KE, Savitz DA, Kaufman JS, Garrett JM, et al. The epidemiology of threatened preterm labor: a prospective cohort study. Am J Obstet Gynecol 2005;192:1325-9.
- Office for National Statistics (ONS) . Live Births in 2004, Selected Background Data (final), England and Wales [Internet] 2004. URL: http://www.statistics.gov.uk/statbase/Product.asp?vlnk=14010 (accessed 13.06.11).
- Kramer MS, Demissie K, Yang H, Platt RW, Sauve R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. JAMA 2000;248:843-9.
- Mathews TJ, Menacker F, MacDorman MF. Infant mortality statistics from the 2002 period linked birth/infant death data set. National Vital Statistics Reports 2004;53:1-30.
- Honest H, Forbes CA, Duree KH, Norman G, Duffy SB, Tsourapas A, et al. Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess 2009;13.
- Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database Syst Rev 2006. URL: 10.1002/14651858.CD000065.pub2.
- Ascarelli MH, Morrison JC. Use of fetal fibronectin in clinical practice. Obstet Gynecol Surv 1997;52:S1-12.
- Lockwood CJ, Senyei AE, Dische MR, Casal D, Shah KD, Thung SN, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med 1991;325:669-74.
- Treatment of women presenting in preterm labour. BTHU; 2008.
- Morgan MA, Goldenberg RL, Schulkin J. Obstetrician-gynecologists' knowledge of preterm birth frequency and risk factors. J Matern Fetal Neonatal Med 2007;20:895-901.
- Honest H, Bachmann LM, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervicovaginal fetal fibronectin test in predicting risk of spontaneous preterm birth: systematic review. BMJ 2002;325.
- Andersen HF. Use of fetal fibronectin in women at risk for preterm delivery. Clin Obstet Gynecol 2000;43:746-58.
- Using fetal fibronectin to diagnose pre-term labour. Edmonton, AB: IHE; 2008.
- Ramsey PS, Andrews WW. Biochemical predictors of preterm labor: fetal fibronectin and salivary estriol. Clin Perinatol 2003;30:701-33.
- Kollee LA. Twenty-five week limit for viability of the foetus is ethically correct. Ned Tijdschr Geneeskd 2005;149.
- Tucker J, McGuire W. Epidemiology of preterm birth. BMJ 2004;329:675-8.
- Haram K, Mortensen JH, Wollen AL. Preterm delivery: an overview. Acta Obstet Gynecol Scand 2003;82:687-704.
- Logghe H, Walker JJ. Towards improved neonatal outcome: future strategies. Semin Fetal Neonatal Med 2004;9:491-8.
- Koenn ME. Fetal fibronectin. Clin Lab Sci 2002;15:96-8.
- Iams JD. Prediction and early detection of preterm labor. Obstet Gynecol 2003;101:402-12.
- Hole JW, Tressler TB. Management of preterm labor. J Am Osteopath Assoc 2001;101:S14-8.
- Lee SK, McMillan DD, Ohlsson A, Boulton J, Lee DS, Ting S, et al. The benefit of preterm birth at tertiary care centers is related to gestational age. Am J Obstet Gynecol 2003;188:617-22.
- Terzidou V, Bennett PR. Preterm labour. Curr Opin Obstet Gynecol 2002;14:105-13.
- Sanchez-Ramos L, Delke I, Zamora J, Kaunitz AM. Fetal Fibronectin as a Short-Term Predictor of Preterm Birth in Symptomatic Patients. Obstet Gynecol 2009;114.
- Fetal fibronectin test for predicting preterm labour: MSAC application 1103. Assessment report [internet]. Camberra: Commonwealth Department of Health and Ageing; 2006.
- Systematic Reviews: CRD's guidance for undertaking reviews in health care [Internet]. York: University of York; 2009.
- Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. J Clin Epidemiol 2010;64:602-7.
- Diagnostics Assessment Programme: interim methods statement (version 2) [Internet]. London: National Institute for Health and Clinical Excellence; 2010.
- Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3.
- Reitsma JB, Glas AS, Rutjes AWS, Scholten RJPM, Bossuyt PMM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90.
- Harbord RM, Deek JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51.
Appendix: 1
Clinical effectiveness search for SRs and RCTs
Medline (OvidSP): 2000-2011/6/wk1
Searched: 10.06.11
-
fibronectins/ (18793)
-
(86088-83-7 or fibronectin$).af. (30593)
-
(fFN or tli system$).ti,ab,ot. (126)
-
(tli adj iq).ti,ab,ot. (1)
-
or/1-4 (30620)
-
exp Obstetric Labor, Premature/ (14510)
-
((Pre term or preterm or premature or early or immature) adj5 (labo?r or birth$ or childbirth$ or deliver$ or partu$ or ruptur$)).ti,ab,ot,hw. (39734)
-
(PROM or PROM or PTB).ti,ab,ot. (3046)
-
((Short$ or reduced or multiple) adj4 gestation$).ti,ab,ot. (3052)
-
or/6-9 (44101)
-
5 and 10 (404)
-
randomized controlled trial.pt. (308386)
-
controlled clinical trial.pt. (82578)
-
randomized.ab. (214849)
-
placebo.ab. (125246)
-
drug therapy.fs. (1456618)
-
randomly.ab. (155580)
-
trial.ab. (221813)
-
groups.ab. (1034167)
-
meta-analysis.mp,pt. or review.pt. or search:.tw. (1741286)
-
or/12-20 (4106833)
-
exp animals/ not humans.sh. (3598690)
-
21 not 22 (3569496)
-
23 and 11 (173)
-
limit 24 to yr="2000 -Current" (116)
SR filter:
Montori VM, Wilczynski NL, Morgan D, Haynes RB. Optimal search strategies for retrieving systematic reviews from MEDLINE: analytical survey (top strategy minimising the difference between sensitivity and specificity). BMJ 2005;330(7482):68.
RCT filter:
Lefebvre C, Manheimer E, Glanville J. Chapter 6: searching for studies. Box 6.4.c: Cochrane Highly sensitive search strategy for identifying randomized controlled trials in Medline: Sensitivity-maximizing version (2008 version); OVID format. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org
Economic evaluations search
Medline (OvidSP): 1948-2011/6/wk1
Searched: 13.06.11
-
fibronectins/ (18793)
-
(86088-83-7 or fibronectin$).af. (30593)
-
(fFN or tli system$).ti,ab,ot. (126)
-
(tli adj iq).ti,ab,ot. (1)
-
or/1-4 (30620)
-
exp Obstetric Labor Complications/ (43593)
-
(labo?r or birth$ or childbirth$ or deliver$ or partu$ or ruptur$).ti,ab,ot,hw. (711882)
-
(PROM or PROM or PTB).ti,ab,ot. (3046)
-
((Short$ or reduced or multiple) adj4 gestation$).ti,ab,ot. (3052)
-
or/6-9 (720210)
-
5 and 10 (1116)
-
economics/ (26052)
-
exp "costs and cost analysis"/ (156819)
-
economics, dental/ (1829)
-
exp "economics, hospital"/ (17190)
-
economics, medical/ (8404)
-
economics, nursing/ (3847)
-
economics, pharmaceutical/ (2236)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (335890)
-
(expenditure$ not energy).ti,ab. (14210)
-
(value adj1 money).ti,ab. (20)
-
budget$.ti,ab. (14415)
-
or/12-22 (448370)
-
((energy or oxygen) adj cost).ti,ab. (2292)
-
(metabolic adj cost).ti,ab. (594)
-
((energy or oxygen) adj expenditure).ti,ab. (13122)
-
or/24-26 (15392)
-
23 not 27 (444870)
-
letter.pt. (716157)
-
editorial.pt. (276466)
-
historical article.pt. (275084)
-
or/29-31 (1254908)
-
28 not 32 (420635)
-
Animals/ (4763447)
-
Humans/ (11766611)
-
34 not (34 and 35) (3517445)
-
33 not 36 (396750)
-
37 and 11 (37)
Costs filter:
Centre for Reviews and Dissemination. NHS EED Economics Filter: Medline (Ovid) monthly search [Internet]. York: Centre for Reviews and Dissemination; 2010 [cited 28.9.10]. Available from: http://www.crd.york.ac.uk/crdweb/html/helpdoc.htm#MEDLINE_NHSEED
Appendix 11 Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | p. i |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | pp. xiii–xvii |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | Chapter 1, pp. 1–4 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | Chapter 2, p. 5 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g. Web address), and, if available, provide registration information including registration number | PROSPERO |
| CRD42011001468 (URL: www.crd.york.ac.uk/prospero/) | |||
| NICE (URL: http://guidance.nice.org.uk/DT/6) | |||
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language, publication status) used as criteria for eligibility, giving rationale | Chapter 3, Inclusion criteria, pp. 7–8 |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | Chapter 3, Search strategy, pp. 8–9 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Appendix 1 |
| Study selection | 9 | State the process for selecting studies (i.e. screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | Chapter 3, Inclusion screening and data extraction, p. 9 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | Chapter 3, Inclusion screening and data extraction, p. 9 |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made | Chapter 3, Inclusion screening and data extraction, p. 9 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | Chapter 3, Quality assessment, pp. 9–10 |
| Summary measures | 13 | State the principal summary measures (e.g. RR, difference in means) | Chapter 3, Methods of analysis/synthesis, pp. 10–11 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g. I2) for each meta-analysis | Chapter 3, Methods of analysis/synthesis, pp. 10–11 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g. publication bias, selective reporting within studies) | NA |
| Additional analyses | 16 | Describe methods of additional analyses (e.g. sensitivity or subgroup analyses, metaregression), if done, indicating which were pre-specified | Chapter 3, Methods of analysis/synthesis, pp. 10–11 |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | Chapter 3, Results, pp. 11–12, Figure 1, p. 12, and Appendix 9 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g. study size, PICOS, follow-up period) and provide the citations | Chapter 1, Table 1, pp. 13–14, and Appendices 2 and 4 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | Chapter 3, Clinical effectiveness, Figures 2 and 3, pp. 15–16, Appendix 3 |
| Chapter 3, Test accuracy, Table 6, pp. 20–22, Appendix 7 | |||
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group; (b) effect estimates and confidence intervals, ideally with a forest plot | Chapter 3, Clinical effectiveness, Tables 2–5, pp. 16, 18, 20, 22 |
| Chapter 3, Test accuracy, Tables 7, 9 and 11, pp. 25, 27, 29 | |||
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | Chapter 3, Clinical effectiveness Figure 4–8, pp. 17, 19, 21, 23 |
| Chapter 3, Test accuracy, Figures 9–11 and Tables 8, 10 and 12, pp. 26, 28, 30 | |||
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see item 15) | NA |
| Additional analysis | 23 | Give results of additional analyses, if done [e.g. sensitivity or subgroup analyses, metaregression (see item 16)] | Chapter 3, Test accuracy, Tables 8, 10 and 12. pp. 26, 28, 30 |
| Discussion | |||
| Summary of evidence | 24 | Summarise the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g. health-care providers, users, and policy makers) | Chapter 5, Statement of principal findings, pp. 41–3 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g. risk of bias), and at review-level (e.g. incomplete retrieval of identified research, reporting bias) | Chapter 5, Strengths, limitations and uncertainties of the assessment, pp. 43–5 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | Chapter 6, pp. 47–8 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g. supply of data); role of funders for the systematic review | p. vi |
Glossary
- Bronchopulmonary dysplasia
- A condition, characterised by inflammation and scarring of the lungs, which arises from prolonged mechanical ventilation in premature infants, and can further compromise oxygenation of the blood.
- Cost-effectiveness analysis
- An economic analysis that converts effects into health terms and describes the costs for additional health gain.
- Decision modelling
- A mathematical construct that allows the comparison of the relationship between costs and outcomes of alternative health-care interventions.
- Diagnostic odds ratio
- An overall measure of diagnostic accuracy, calculated as the odds of positivity among persons with disease divided by the odds of positivity among persons without disease. When a test provides no diagnostic evidence the diagnostic odds ratio is 1.0.
- False-negative
- Incorrect negative test result – number of diseased persons with a negative test result.
- False-positive
- Incorrect positive test result – number of non-diseased persons with a positive test result.
- Gestational age
- The age of an embryo or fetus or a newborn infant.
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Index test
- The test whose performance is being evaluated.
- Intraventricular haemorrhage
- Bleeding into the brain's ventricular system, which is thought to result from changes in perfusion of the delicate cellular structures that are present in the growing brain, increased by the immaturity of the cerebral circulatory system, and is especially vulnerable to hypoxic ischemic encephalopathy. The lack of blood flow results in cell death and subsequent breakdown of the blood vessel walls, leading to bleeding.
- Markov model
- An analytical method particularly suited to modelling repeated events, or the progression of a chronic disease over time.
- Meta-analysis
- Statistical techniques used to combine the results of two or more studies and obtain a combined estimate of effect.
- Metaregression
- A statistical technique used to explore the relationship between study characteristics and study results.
- Necrotising enterocolitis
- A condition, seen in premature infants, in which portions of the bowel undergo necrosis (tissue death). Initial symptoms include feeding intolerance, increased gastric residuals, abdominal distension and bloody stools. Symptoms may progress rapidly to abdominal discoloration with intestinal perforation and peritonitis and systemic hypotension requiring intensive medical support.
- Opportunity costs
- The cost of forgone outcomes that could have been achieved through alternative investments.
- Preterm birth
- An infant born before 37 completed weeks of gestation.
- Preterm labour
- Labour that occurs earlier in pregnancy than normal, either before the fetus has reached a weight of 2000–2500 g or before the 37th or 38th week of gestation.
- Publication bias
- Bias arising from the preferential publication of studies with statistically significant results.
- Quality of life
- An individual's emotional, social and physical well-being and their ability to perform the ordinary tasks of living.
- Quality-adjusted life-year
- A measure of health gain, used in economic evaluations, in which survival duration is weighted or adjusted by the patient's quality of life during the survival period.
- Receiver operating characteristic curve
- A graph which illustrates the trade-offs between sensitivity and specificity which result from varying the diagnostic threshold.
- Reference standard
- The best currently available diagnostic test(s), against which the index test is compared.
- Respiratory distress syndrome
- A syndrome in premature infants caused by developmental insufficiency of surfactant production and structural immaturity in the lungs.
- Retrolental fibroplasias or retinopathy of prematurity
- An eye disease that affects premature infants and is thought to be caused by disorganised growth of retinal blood vessels, which may result in scarring and retinal detachment. The condition can be mild and may resolve spontaneously, but it may lead to blindness in serious cases.
- Sensitivity
- Proportion of people with the target disorder who have a positive test result.
- Specificity
- Proportion of people without the target disorder who have a negative test result.
- True-negative
- Correct negative test result – number of non-diseased persons with a negative test result.
- True-positive
- Correct positive test result – number of diseased persons with a positive test result.
List of abbreviations
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CRP
- C-reactive protein
- DARE
- Database of Abstracts of Reviews of Effects
- DOR
- diagnostic odds ratio
- DTA
- diagnostic test accuracy
- ELISA
- enzyme-linked immunosorbent assay
- fFN
- fetal fibronectin
- FN
- false-negative
- FP
- false-positive
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- i.v.
- intravenous
- LR–
- likelihood ratio for negative test result
- LR+
- likelihood ratio for positive test result
- NHS EED
- NHS Economic Evaluation Database
- NICU
- neonatal intensive care unit
- NIH
- National Institutes of Health
- NR
- not reported
- phIGFBP
- highly phosphorylated insulin-like growth factor binding proteins
- PTB
- preterm birth
- RCT
- randomised controlled trial
- RDS
- respiratory distress syndrome
- ROC
- receiver operating characteristic
- RR
- risk ratio
- SCI
- Science Citation Index
- SROC
- summary receiver operating characteristic
- TN
- true-negative
- TP
- true-positive
- WHO
- World Health Organization