Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/39/02. The contractual start date was in May 2008. The draft report began editorial review in January 2013 and was accepted for publication in April 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Stephen Allen has undertaken research in probiotics supported by Cultech Ltd, Baglan, Port Talbot, UK; the Children and Young People’s Research Network, Cardiff University, Wales; the Knowledge Exploitation Fund, Welsh Development Agency and the National Ankylosing Spondylitis Society, UK. He has also been an invited guest at the Yakult Probiotic Symposium in 2011 and received research funding from Yakult, UK, in 2010.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2013. This work was produced by Allen et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Antibiotic-associated diarrhoea (AAD), defined as diarrhoea in association with antibiotic treatment and without an alternative cause, occurs in 5–39% people within 12 weeks of exposure to antibiotics. 1,2 The predominant mechanism underlying AAD is disruption of the commensal gut flora. This impairs colonisation resistance and facilitates the emergence of a range of gut pathogens. 2,3 The major diarrhoeal pathogen associated with antibiotic treatment is Clostridium difficile, which accounts for 15–39% of AAD cases. 4 Altered commensal flora may also result in diarrhoea through changes in the metabolism of carbohydrates, short-chain fatty acids and bile acids, and some antibiotics cause diarrhoea through direct effects such as increased gut motility. 2,5
Clostridium difficile is an anaerobic bacterium that produces resistant spores that persist long term in the environment. Transmission is faecal–oral and 4–21% patients may acquire the infection during admission to hospital through contact with colonised patients, contaminated fomites and the hands of health-care staff. C. difficile diarrhoea (CDD) occurs in both endemic and outbreak scenarios. 6 Since 2003, an increase in the frequency and severity of CDD in North America and Europe has been attributable to the emergence of the hypervirulent 027 strain, which may produce higher amounts of toxin. 7–9
Antibiotic-associated diarrhoea occurs typically in older people admitted to hospital. 1,2 ADD complicates treatment with many antibiotic classes but especially broad-spectrum penicillins, cephalosporins, clindamycin and long-duration antibiotic treatments. 10 Additional risk factors include prolonged hospital stay, previous hospital admission, previous gastrointestinal surgery and use of a nasogastric tube (NGT). 3 In a retrospective study of European hospitals, risk factors for CDD included age ≥ 65 years, severe comorbidity and recent treatment with cephalosporins and aminopenillin-beta (β)-lactamase inhibitor combinations. 11 In a prospective study of people aged > 18 years admitted to Canadian hospitals, age, exposure to antibiotics, treatment with proton pump inhibitors (PPIs) and previous hospital admission within the last 2 months predicted CDD. 12
The severity of AAD varies greatly. Although usually a mild, self-limiting illness, it is associated with prolonged hospital stay and increased health-care costs. C. difficile infection may remain asymptomatic, but intestinal pathology results from secretion of toxins A and B causing increased mucosal fluid secretion and inflammation. Symptoms range from mild, self-limiting diarrhoea to severe diarrhoea complicated by pseudomembranous colitis, toxic megacolon and death. 6,8
Estimates of the financial burden of C. difficile infection (CDI) to the health-care service have varied between $2454 and $16,464 for every health-care-acquired case in the USA,13–15 £4107 in the UK16 and €7147 in Germany. 17 The annual cost of health care for CDD in the USA has been estimated to be $3B. 18,19 Nosocomial CDI increases hospital stay by between 7 and 26 days,16,17,20 and prolonged hospital admission was identified as the main cost driver in most studies. 13,15,16 Furthermore, an increase in length of hospital stay due to more severe disease in recent years has resulted in a rise of the incremental cost of CDI. 21
Treatment
Uncomplicated AAD usually responds to withdrawal of the offending antibiotics. CDD usually responds to treatment with metronidazole or vancomycin, but 20–25% patients go on to suffer from a recurrent form of the disease. Novel modes of treatment include probiotics, immunoglobulin infusion and faecal transplant from healthy donors. 3,6
Prevention of antibiotic-associated and Clostridium difficile diarrhoea
The frequency and severity of CDD in hospitals in the industrialised world have led to comprehensive strategies for prevention. These include decontamination of the environment, hand washing and isolation of patients with diarrhoea. 6 Antibiotic stewardship programmes have also been effective in reducing infection rates. 6,22,23
Probiotics
Probiotics are defined as live microbial organisms which, when administered in adequate numbers, are beneficial to health. 24 Although clinical trials are undertaken to determine whether or not a microbial preparation has a health benefit in a specific population, the term ‘probiotic’ is commonly used to refer to the preparation being evaluated and it is used in this sense here. Bacteria used as probiotics are among the organisms ‘generally regarded as safe’ by the Food and Agriculture Organization of the United Nations. 25 Live bacteria from several genera and the yeast Saccharomyces boulardii have been administered to vulnerable groups such as preterm infants and people with human immunodeficiency virus (HIV) infection without adverse effects. 26 Adverse events occurring in clinical trials evaluating the prevention of AAD have not been ascribed to probiotic intake. 26 Administration of lactic acid bacteria has been associated in rare cases with septicaemia in immunocompromised people and endocarditis in people with artificial heart valves. 27 Despite the apparent low risk of adverse events, careful assessment of safety in clinical trials is recommended. 26
Rationale
Specific probiotic strains have been identified in vitro and in vivo to possess several mechanisms that may prevent or ameliorate AAD and CDD through enhanced barrier function of the gut epithelium. 28 First, potential pathogens are killed or inhibited by the secretion of antimicrobial peptides and probiotics compete for attachment sites on intestinal mucus and epithelium. Acidification of the gut contents through the production of lactic acid also inhibits pathogen growth. Second, mucosal immunity is enhanced through increased secretory immunoglobulin A production and stimulation of antimicrobial peptide secretion by host cells. Finally, through direct effects on the epithelium, probiotics increase the secretion of mucus, enhance tight junction integrity and decrease epithelial cell apoptosis.
At the time that probiotic lactobacilli and bifidobacteria in antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in the elderly (PLACIDE) was developed, meta-analyses had suggested that probiotics may be effective in the prevention of AAD. McFarland pooled data from 25 randomised controlled trials (RCTs), which included a total of 2810 adults and children. 29 There was significant heterogeneity (χ2 = 82.5; p < 0.001) in a fixed-effects model. In random-effects analysis, the relative risk (RR) for AAD was 0.43 [95% confidence interval (CI) 0.31 to 0.58] in participants receiving a probiotic. This meta-analysis included all of the trials included in previous systematic reviews, which had broadly similar findings. 30–33 Although meta-analysis has provided some evidence that probiotics may be effective in the prevention of AAD, the marked differences between trials in the microorganisms evaluated (single strains and mixtures of bacteria, the yeast S. boulardii), administration regimens (mode of delivery, number of organisms, probiotics combined with prebiotics), patient populations (age and exposure to antibiotics) and period of follow-up for AAD probably underlie the statistical heterogeneity in the results and weaken the evidence for probiotic effectiveness.
Much less evidence from clinical trials was available for probiotics in the prevention of CDD. A pilot study in elderly hospitalised patients reported that 30 out of 138 (21.7%) patients developed diarrhoea and 5 out of 69 (7.2%) in the placebo group compared with 2 out of 69 (2.9%) who had received a combination of Lactobacillus acidophilus and Bifidobacterium bifidum tested positive for C. difficile toxin. Stool culture suggested that the main effect of the probiotic was neutralisation of toxin rather than prevention of colonisation. 34 Thomas et al. had assessed Lactobacillus rhamnosus GG for the prevention of AAD in 267 adults who were monitored for an average of 21 days, but the number of patients from whom stool samples had been collected and in which C. difficile was detected was too small to assess probiotic effect. 35 In randomised trials of 19336 and 180 adult patients,37 the occurrence of CDD was similar in those receiving S. boulardii and those receiving the placebo. Although there was trial evidence for probiotics in the treatment of established CDD or the prevention of recurrence,29 we are not aware of any other studies that had assessed probiotics in the prevention of CDD in adults.
We are not aware of any studies that have assessed the effect of probiotic on quality of life (QoL) in patients at risk of AAD.
Selection of the probiotic preparation
Although several mechanisms whereby probiotic organisms enhance gut barrier function have been identified,28 it remains unclear which of these are most relevant to the prevention or amelioration of AAD and to what extent these mechanisms are common to many different probiotic organisms or are strain specific. Therefore, the scientific evidence to inform the selection of a specific probiotic intervention for the prevention of AAD is limited. The meta-analyses included trials that had evaluated many different bacterial preparations and the yeast S. boulardii. 29 The bacterial interventions included single strains, mixtures of different organisms and mixtures of probiotics with prebiotics. Dosages (number of organisms) and modes of administration also varied markedly between studies. Factors associated with greater efficacy in preventing AAD in meta-analysis included the use of S. boulardii or L. rhamnosus GG, mixtures of probiotics and preparations with high numbers of organisms. 29 Efficacy was similar for bacterial preparations [five trials conducted on 384 adults and children; odds ratio (OR) 0.34; 95% CI 0.19 to 0.61] and yeast preparations (four trials conducted on 830 participants; OR 0.39; 95% CI 0.25 to 0.62). 31
In an attempt to maximise gut colonisation and, therefore, colonisation resistance, we used a multistrain preparation of Lactobacillus sp. and Bifidobacterium sp., bacterial species that had been evaluated extensively in clinical trials, with a high number of viable bacteria (total 6 × 1010 organisms per day). We intended to undertake a pragmatic trial to assess the clinical effectiveness and cost-effectiveness of the probiotic preparation in older people receiving antibiotics in secondary health-care settings representative of those in industrialised counties and with the causes of diarrhoea determined by routine laboratory practice.
Chapter 2 Methods
Trial design
Probiotic lactobacilli and bifidobacteria in antibiotic-associated diarrhoea and Clostridium difficile in the elderly was a prospective, multicentre, pragmatic, two-armed, double-blind, randomised, placebo-controlled trial with equal randomisation.
Approvals obtained
The Research Ethics Committee (REC) for Wales approved the study on 27 November 2008 (number 08/MRE09/18).
A clinical trial authorisation (CTA) was granted for the live bacterial preparation by the Medicines and Healthcare products Regulatory Agency (MHRA) on 6 October 2008 (CTA number AAD-CDD-001). The details of the REC for Wales, local RECs, competent authorities and research and development department approvals are provided in Appendix 1. The trial was assigned the International Standard Randomised Controlled Trial Number (ISRCTN) ISRCTN70017204 and EudraCT number 2007–002876–32.
Trial sites
Inpatients were recruited from medical and surgical wards of secondary care hospitals from two NHS regions: the Abertawe Bro Morgannwg University Health Board (ABMUHB) in South West Wales (Singleton, Morriston and Princess of Wales hospitals), and the County Durham and Darlington Foundation Trust (CDDFT; the University Hospital of North Durham and Darlington Memorial Hospital). Details of the trial sites are included in Appendix 2.
Participant inclusion criteria
People aged ≥ 65 years who were admitted to hospital and had been exposed to one or more antibiotics within the preceding 7 days, or were about to start antibiotic treatment, were eligible to join the study. Approval to invite the patient to participate in the study was secured from the patient’s consultant.
Participant exclusion criteria
People were excluded if they:
-
already had diarrhoea, which was defined as three or more watery or loose stools (Bristol Stool Form Scale types 5–7)38 in the preceding 24 hours
-
were sufficiently immunocompromised to require isolation and/or barrier nursing
-
had a severe illness requiring high-dependency or intensive care
-
had a prosthetic heart valve
-
had suffered from CDD in the previous 3 months
-
had inflammatory bowel disease that had required specific treatment in the previous 12 months
-
had suspected acute pancreatitis, which was defined as abdominal pain with serum amylase or lipase greater than three times the institutional upper limit of normal
-
had a known abnormality or disease of mesenteric vessels or coeliac axis that may compromise gut blood supply
-
had a jejunal tube in situ or were receiving jejunal feeds
-
had a previous adverse reaction to probiotics
-
were unwilling to discontinue their existing use of probiotics.
Investigational medicinal products
The active preparation consisted of a vegetarian capsule containing 6 × 1010 live bacteria as lyophilised powder comprising two strains of L. acidophilus [CUL60, National Collection of Industrial, Food and Marine Bacteria (NCIMB) 30157 and CUL21, NCIMB 30156; 3 × 1010 colony-forming units (cfu)] and two strains of Bifidobacterium (B. bifidum CUL20, NCIMB 30153 and Bifidobacterium lactis CUL34, NCIMB 30172; 3 × 1010 cfu). Obsidian Research Ltd, Port Talbot, UK, prepared the investigational medicinal products (IMPs) according to the randomisation schedule. The organisms were available commercially through BioCare® Ltd (Lakeside, Birmingham, UK) and Pharmax (Bellevue, WA, USA). The placebo capsules were identical to the probiotic capsules but contained inert maltodextrin powder. The dose was one capsule per day with food and, where possible, between antibiotic doses, for 21 days.
Each participant was provided with a bottle labelled with his or her unique study number and containing 21 capsules. The IMPs were given to participants on the day of recruitment and administration during the hospital stay was supervised by the research nurses in ABMUHB and by the ward nurses in CDDFT. To prevent cross-contamination, strict hygiene procedures were followed, for example where capsules were opened, and the contents were mixed with fluids for administration to participants with difficulty swallowing. Although the live bacteria had a shelf life of 2 months at room temperature, participants were instructed to keep the capsules in the refrigerator following discharge from the hospital and encouraged to complete the 21-day course.
In vitro antibiotic testing demonstrated that the lactobacilli and bifidobacteria were sensitive to broad-spectrum antibiotics. All four strains were sensitive to ceftriaxone, chloramphenicol, erythromycin, linezolid and tetracycline.
After recruitment of the first 50 participants, a research nurse perceived a slight difference in the colour of the powder contained in the probiotic and placebo capsules, which were transparent. Therefore, the IMPs were resupplied in opaque capsules according to the original random allocation sequence. No unmasking of participant allocation occurred.
To ensure the quality of the IMPs, unused capsules were collected from participants on an opportunistic basis (e.g. from participants who withdrew before completing the 21-day course). A laboratory independent of the research team performed quantitative bacterial culture. The findings were sent to the independent statistician to check against the randomisation sequence and for the total count of viable organisms.
Objectives
Primary objectives: to determine the clinical effectiveness and cost-effectiveness of a live preparation of two strains of lactobacilli and two strains of bifidobacteria in the prevention of AAD and CDD in people aged ≥ 65 years who were exposed to oral or intravenous antibiotics and who were representative of patients admitted to secondary care NHS facilities in the UK.
Secondary objectives: to assess the effect of the probiotic on the duration and severity of AAD, the acceptability of the probiotic preparation, serious adverse events (SAEs) of the probiotic preparation and its effect on QoL.
Outcomes
Primary outcomes: the occurrence of AAD within 8 weeks and CDD within 12 weeks of recruitment.
Secondary outcomes: the severity and duration of AAD; the severity and duration of CDD and incidence of recurrence within the follow-up period; the number of days with abdominal pain, bloating, flatus and nausea; the incidence of pseudomembranous colitis, the need for colectomy; well-being and QoL; duration of hospital stay; frequency of SAEs; the acceptability of the live bacterial preparation (to identify if the participants had any difficulty taking the bacterial preparation vs. the placebo); the viability of the bacterial preparation at point of administration and death.
Sample size
Based on a review of previous clinical trials, we estimated that AAD would occur in 20% and CDD in 4% of participants in the placebo arm. The detection of a 50% reduction in CDD to a frequency of 2% in the active arm with 80% power at the 5% significance level required 2478 subjects (1239 in each arm). This number of participants would provide a power of > 99% to detect a 50% reduction in the risk of AAD (to 10% frequency) and a power of 90% to detect a 25% risk reduction in AAD (to 15% frequency) in the active arm at the 5% significance level. We planned to recruit 2974 participants to allow for 10% drop-outs and 10% loss to follow-up due to deaths unrelated to diarrhoea.
Randomisation
The independent statistician prepared a random allocation sequence using blocks of variable sizes and stratified by hospital using SAS® PROC PLAN, version 9.1 (SAS Institute Inc., Cary, NC, USA). The sequence allocated participants in a 1 : 1 ratio to the two arms of the study.
Blinding
The allocation sequence was not available to any member of the research team until databases had been completed and locked. In view of the established safety record of lactobacilli and bifidobacteria27 and the sensitivity of the organisms used in this study to broad-spectrum antibiotics, there was no provision for the emergency unblinding of participants. Therefore, copies of the random allocation sequence were not held at the recruitment sites.
Recruitment
The research nurses received training in ‘Good Clinical Practice’ and were fully conversant with all aspects of the trial. Specific training was given in assessment of patient eligibility, recruitment and obtaining informed consent, the trial protocol, reporting of adverse events and collecting information. Presentations were made to clinical, nursing and pharmacy staff to ensure their familiarity with the purpose and conduct of the trial. Permission to approach patients and invite them to join the study was obtained from hospital consultants.
Research nurses visited wards daily to identify eligible patients and provide them with verbal and written information (see Appendix 3). Patients were revisited either later the same day or the next day after they had had the opportunity to discuss with relatives/carers and health-care personnel whether or not they wished to join the study. For people unable to provide consent, information was provided to their relatives/carers and assent sought (see Appendix 3). Although reasons for not joining the study were requested, patients and their relatives/carers were free to decline to participate without giving a reason. Patients in whom consent or assent was obtained were allocated the next unique study number in the random sequence for that site and the research nurses provided them with the corresponding trial preparation.
Baseline assessment
Information recorded at recruitment included basic demographic characteristics, use of cigarettes and alcohol, source of admission, principal diagnosis or reason for admission, comorbid illnesses, duration of hospital stay prior to recruitment, non-antibiotic drug treatment, indication for antibiotic treatment and antibiotics prescribed (see Appendix 4).
Participant follow-up
Participants were visited daily by the research staff during hospital stay. Changes to antibiotic treatment, gastrointestinal symptoms (including the presence of diarrhoea), compliance, difficulties taking the IMPs and occurrence of SAEs were recorded onto standard forms (see Appendix 4). The same information was requested weekly, after discharge from hospital, via a telephone questionnaire and continued for 8 weeks post recruitment. In addition, participants were encouraged to contact a named member of the research staff to report potential SAEs at any time during follow-up. Review of laboratory data regarding stool assays was continued until 12 weeks after recruitment.
Trial completion
Participants had completed the trial when they:
-
had completed follow-up
-
had withdrawn and declined collection of further follow-up data
-
were lost to follow-up
-
had died.
A chart showing participant flow through the study is included as Appendix 5.
Measurement of primary outcomes
Diarrhoea was defined as three or more loose stools (stool consistency 5–7 on the Bristol Stool Form Scale)38 in a 24-hour period. Diarrhoea was also diagnosed in participants with frequent stools that they described as loose but who were unable to describe stool consistency using the Bristol Stool Form Scale. The presence of diarrhoea according to these criteria was confirmed by the research nurses during admission with either the patient, their carers or a member of the medical staff. After discharge, this was confirmed during telephone interview.
Stool samples for analysis were collected only during episodes of diarrhoea, including diarrhoea that occurred after discharge from hospital. Stools were analysed for diarrhoeal pathogens according to routine NHS laboratory practice. Analyses included bacterial culture for Salmonella spp., Shigella spp., Campylobacter and Escherichia coli 0157 and wet film for ova, cysts and parasites. Detection of viruses depended on the clinical context (e.g. suspected norovirus outbreak). In ABMUHB, C. difficile toxins were detected by an in-house tissue culture assay with confirmation by enzyme immunoassay Premier™ Toxins A&B (Meridian Bioscience, Inc., Cincinnati, OH, USA). In CDDFT, toxins were detected by the VIDAS® C. difficile A&B (bioMérieux SA, Marcy l’Etoile, France). To improve the detection of CDD from June 2010, CDDFT also introduced detection of the C. difficile antigen glutamate dehydrogenase C. DIFF QUIK CHEK® (TECHLAB® Inc., Blacksburg, VA, USA) to be used in conjunction with the toxin assay. Further stools samples were requested if a cause of diarrhoea was not identified and especially if there was clinical suspicion of CDD. Stools positive for C. difficile toxin were cultured and the isolates sent to a central reference laboratory for ribotyping.
Antibiotic-associated diarrhoea was defined as occurring in association with antibiotic therapy but without diarrhoeal pathogens detected on routine laboratory analysis or an alternative explanation (e.g. laxative treatment). Among patients with AAD, CDD was diagnosed in those with stools testing positive for C. difficile toxins.
Measurement of secondary outcomes
Information regarding the severity and duration of AAD and CDD, number of stools per day and stool consistency, incidence of recurrence of CDD within the follow-up period, the number of days with abdominal pain, bloating, flatus and nausea, duration of hospital stay and acceptability of the live bacterial preparation was collected by the research nurses during follow-up (see Appendix 4). CDD was investigated and managed according to the current hospital practice and clinical and laboratory information from clinical records was recorded (see Appendix 4). Information regarding the occurrence of pseudomembranous colitis, the need for colectomy and death was also collected from the patient’s case records. The information from follow-up and patient case records was used to classify the severity of CDD according to UK national guidelines (see Appendix 6). 39 The severity of CDD was classified independently by two assessors, WH and SA, and differences resolved by discussion.
Quality of life was assessed by the generic Short Form questionnaire-12 items version 2 (SF-12 v2) administered by research nurses at baseline and at 4 and 8 weeks. SF-12 v2 QoL subscales (social function, role function–emotional, role function–physical, general health, mental health, bodily pain, vitality, physical functioning), physical component summary (PCS) and mental component summary (MCS) scores were calculated and quality assured according to the QualityMetric Incorporated guidance40 with imputation of missing scores using the SF-12 v2 Missing Data Estimator software where possible. 40 SF-12 v2 subdomain, PCS and MCS scores were allocated a value of 0 for the lowest/worse score and 100 for the highest/best score.
Serious adverse events were identified and reported according to standard guidelines. 41 Suspected unexpected serious adverse reactions were to be reported immediately to the independent safety monitor and, if appropriate, the ethics committee in accordance with local and national requirements, which were identified as:
-
any manifestation of infection (e.g. abscess, bacterial endocarditis, bacteraemia) in which lactobacilli or bifidobacteria were isolated from pathological specimens
-
the development of pancreatitis, which was defined as abdominal pain with serum amylase or lipase concentration equal to or greater than three times the institutional upper limit of normal
-
the development of multiorgan failure
-
the development of bowel ischaemia.
Additional data collected
Information was collected to identify subgroups of participants who may be at increased risk of AAD and CDD. This included potential risk factors at baseline, antibiotic treatment and duration of hospital stay.
Data management
All data were collected on standardised forms that were checked for missing values by the trial manager. Routine laboratory records were accessed to record results of stool analyses. Data were entered into Microsoft Access® (Microsoft Corporation, Redmond, WA, USA) and included range checks and double entry. Databases were compared using Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) to identify data entry errors.
Antibiotics were classified according to British National Formulary categories (see Appendix 7). 42 Indications for antibiotic treatment were classified according to the system organ class (SOC) terminology of the Medical Dictionary for Regulatory Activities (MedDRA). 43 Participants were allocated to each SOC for either suspected or proven infection of that organ or system. Antibiotic treatment for suspected infection where no system or organ was identified was classified as ‘suspected sepsis but site unclear’. SAEs were coded according to the most appropriate preferred terms (PTs) of the MedDRA. 43
After data cleaning, databases were locked and forwarded to the trial statistician for analysis. Initial analysis according to the randomisation sequence identified the two arms of the study as only ‘A’ or ‘B’. The identity of the two arms was only revealed after the Data Monitoring and Ethics Committee had reviewed these data and approved the analysis.
Statistical analysis
Demographic and baseline data were summarised by recruitment hospital and treatment group. Continuous variables were summarised using number of observations, median and interquartile range (IQR) and categorical variables by the number and percentage of events.
Analysis of primary and secondary end points was performed in a modified intention-to-treat (ITT) population that excluded a small number of subjects who withdrew shortly after randomisation and did not have follow-up data. The chi-squared test or Fisher’s exact test was used to compare proportions. Risk difference and RR together with the 95% CIs were calculated using a generalised linear model that included treatment as a single predictor. Similarly, CIs for the ORs of AAD and CDD were estimated from logistic regression models. Secondary continuous outcomes with no repeated measurements were summarised using number of observations, median and IQR. The t-test or Mann–Whitney method were used to compare continuous variables. Duration data were summarised by median and IQR and compared using the Mann–Whitney method. No transformation was used for any variables.
Analysis of primary end points was also performed by logistic regression model adjusting for the following prespecified baseline characteristics and potential risk factors for AAD that may be likely to affect the occurrence of the primary end point:
-
centre
-
age
-
sex
-
antibiotic class
-
duration of antibiotic treatment
-
antacid therapy (including PPI treatment)
-
NGT in situ
-
previous gastrointestinal surgery
-
recent previous hospital admission
-
duration of hospital stay.
We intended to include all 10 prespecified variables in the logistic model but some were not entered because their effects were inestimable. A per-protocol (PP) population excluded participants who did not receive any IMP doses or in whom compliance was unclear. Additional analyses also assessed probiotic effect on primary outcomes in participants who took all 21 doses, 14 or more doses, or seven or more doses of the IMP. Analysis methods for the PP population were similar to those described for the modified ITT population. All analyses were performed on both the modified ITT and PP populations.
SAS version 9.2 was used for data analyses.
Quality of life analysis
The main analysis compared the change from baseline in SF-12 v2 PCS and MCS composite scores at 4 weeks in the two study arms. We also compared SF-12 v2 PCS and MCS composite scores at 8 weeks and scores for individual SF-12 v2 subdomains (social function, role function–emotional, role function–physical, general health, mental health, bodily pain, vitality and physical functioning) at 4 and 8 weeks. Composite scores were compared using mixed model analysis using the SF-12 v2 score at baseline as a covariate, treatment, visit, interaction between the treatment and visit as fixed effects, and subject as a random effect. During the trial, some subjects dropped out, resulting in some incomplete observations. These incomplete observations were not computed but were assumed to be missing at random in the mixed model analysis. The treatment difference, together with the 95% CI at each visit, was derived from the mixed model.
Economic analysis
The cost-effectiveness evaluation was undertaken from the perspective of the UK NHS. Costs were assigned to the resources utilised by each participant. These consisted of the bacterial preparation, staff time involved in administering the preparation, treatment relating to potential adverse events, the assessment of cases of diarrhoea (stool collection and culture/toxin assay, endoscopy) and diarrhoea management costs (laundry, antibiotics, increased hospital stay and comorbidities). Unit costs (cost year 2011) were derived from published information44–46 and through discussions with relevant clinical and finance department staff. Missing data were addressed using the imputation-based method for QoL data and censored data relating to costs using the weighted cost method with known cost histories. 47 In view of the short timescale of the project, there was no discounting of the costs or benefits. Costs and benefits would have been discounted at the conventional rate of 3.5% if the time scale of the follow-up had exceeded 1 year.
Cost differences between the two arms of the study were determined and used in conjunction with differences in outcomes in undertaking a cost–consequences analysis, with cost per case of AAD averted as the primary outcome measure. We planned to undertake subgroup analyses to determine the relative cost-effectiveness of preventative strategies in different risk groups as indicated in the covariate analysis. In addition, a cost–utility analysis considered the differences in costs between the two study arms and differences in quality-adjusted life-years (QALYs) derived from European Quality of Life-5 Dimensions (EQ-5D) responses.
Resource use and costs
Health-care contacts
The number of health-care contacts, duration of initial hospital stay, days spent in care facilities and number and duration of readmissions were recorded routinely within the trial. If the discharge date was not known, the end of follow-up was assumed to be the discharge date. A weighted unit cost of £334.17 was applied for every day a participant spent in hospital (NHS reference costs 2011; extrapolated). 44 For the base-case analysis, all other health-care contacts were treated as general practitioner (GP) visits and published care home costs were applied on a daily rate basis. 45
Antibiotics
Antibiotic use was collected throughout the 8-week follow-up period and costed using published sources. 46 Staff time was assumed to be 5 minutes for the administration of oral antibiotics and 20 minutes for intravenous or intramuscular antibiotics. As 66% of doses during the study period were delivered orally and 34% intravenously, staff cost per dose was weighted to 10.1 minutes per dose and costed at £16.33 per dose. 45 Missing data on antibiotic dose were replaced by the most common value. If no data were available on number of doses per day or duration of antibiotic course, it was assumed that the patient was receiving a full course of the recorded antibiotic.
Intervention implementation
For the cost-effectiveness analysis, it was assumed that every patient would receive a course of 21 oral capsules containing the probiotic formulation at a retail cost of £10 and that capsules that were not taken by the participant would go to waste (as a high proportion of participants finished their course at home after hospital discharge). While the patient was in hospital, staff time of 5 minutes was allocated for administration of each dose. The number of days in hospital (and thus number of nurse contacts) was calculated individually for each patient according to his or her intervention start and hospital discharge dates. Nursing time was allocated even if the patient declined the intervention, as time would have been used for patient interaction and assessment of the situation. No staff time was allocated after the patient withdrew or died. Nursing time was costed at £8.08 per 5 minutes. 45 The cost of placebo formulation and staff cost for administration in the control group were excluded from the cost-effectiveness analysis as ‘routine use’ was considered.
Episodes of diarrhoea
Costs regarding health-care resource use, antibiotics and increase in length of hospital stay associated with diarrhoea were collected routinely during the trial. Other costs, such as diagnostics, clinical review, cleaning, laundry and isolation, were sourced from outside the trial setting. Resource use and costs of laboratory tests for C. difficile detection were obtained using a microcosting approach based on internal standard operating procedures and discussions with key laboratory staff and purchasing officers. Costs of other diagnostic tests (Salmonella, Shigella, Campylobacter, E. coli 0157 and norovirus) were taken from the literature. 48 Published reference costs44 were used to estimate the costs of diagnostic (endoscopy, abdominal computerised tomography and radiography) and therapeutic (colectomy) procedures, which were then weighted to reflect the probability of the event occurring in a population suffering from diarrhoea based on recent publications. 49,50 Costs of patient assessment, including for review of antibiotic treatment and nutritional requirements, and infection control measures were based on discussions with infection control nurses, clinicians and microbiologists. The medical team in the base case was assumed to consist of the treating clinician (costed as registrar), a gastroenterologist, a microbiologist, an infection control nurse, a ward nurse and a pharmacist. Staff time was estimated to be 45 minutes for the registrar and 15 minutes for the other professionals and unit costs were obtained from the literature. 45 Cleaning after patient discharge from the hospital or relocation after a diarrhoea episode was costed based on discussions with key members of infection control staff and includes domestic staff time, specialist cleaning agents (hypochlorite; TUFFIE 5®, Vernacare UK, Bolton, UK) and special cleaning equipment as well as laundry, steam cleaning and use of an autoclave. Costs were obtained from hospital human resource and purchasing departments, wholesalers, published literature51 and microcosting. All costs were allocated once per patient diarrhoea episode.
Daily costs of diarrhoeal disease included daily cleaning and bed and ward closures. Costing of daily cleaning included domestic staff time, specialist cleaning agents and special cleaning equipment. Costs were obtained from human resources and purchasing departments as well as wholesalers. A lost bed-day due to closures was costed at £334.17 by published reference costs44 and weighted for specialties (e.g. whether or not the bed is in intensive care) and activity. 44 Data on frequency of ward closures due to CDD and number of positive cases identified per year were obtained from discussions with key staff and Public Health Wales reports. 52 It was assumed that 1 in 115 cases resulted in an outbreak and subsequent ward closure. Based on an average ward size of 28 beds in Singleton and Morriston hospitals, ward closures could cost up to £9356.76 per day and occur in 0.87% of positive cases. Thus, a weighted cost of £81.40 was applied to each diarrhoea case per day to account for potential ward closures.
The cost per stool included disposables such as gloves and aprons, laundry and staff time for patient check-ups, spot cleaning and changing of beds. Costing of spot cleaning included nursing time, specialist cleaning agents and special cleaning equipment. Data on number of stools per day, duration of diarrhoea episode in days and whether or not the episode was managed in hospital were collected routinely as part of the trial. These data were used to calculate the cost of a diarrhoea episode individually for each patient by applying the one-off episode costs (microbiology, review, procedures, end cleaning) and adding daily and per stool costs according to duration and stool frequency. No cost was applied to participants whose diarrhoea was managed entirely at home. Episodes managed in care homes were treated as hospital episodes.
Cost-effectiveness analyses
Patient responses from EQ-5D questionnaires at baseline and after 4 and 8 weeks were translated into utility scores using a scoring algorithm. We planned to use health and cost outcomes to calculate the cost of probiotics per QALY gained in a cost–utility analysis, to obtain the cost per case of diarrhoea averted in a cost-effectiveness analysis and to present and compare outcomes in tabular form in a cost–consequences analysis. The results of cost-effectiveness analyses were expressed as incremental cost-effectiveness ratios (ICERs) and calculated by dividing the cost difference between the two alternatives being compared by the difference in the effect/benefit. In cost–utility analysis, the effect was expressed in QALYs. The cost per QALY was calculated by dividing the difference in costs by the difference in QALYs for each comparison.
Generally, the National Institute for Health and Care Excellence considers an intervention cost-effective if one of the following applies. 53
-
The intervention is less costly and more clinically effective than all other relevant alternatives. In this case, no ICER is calculated as the strategy in question dominates the alternatives.
-
The intervention has an ICER of < £20,000 per QALY compared with the next best alternative. This means that an investment of up to £20,000 in order to achieve an additional QALY is considered cost-effective.
Sensitivity analysis
Sensitivity analyses investigated the robustness of the results to changes in estimated costs and outcomes and probabilistic sensitivity analysis used bootstrap resampling to determine the probability that preventative strategies were within certain thresholds. We planned to assess the budgetary impact from a NHS perspective of adopting a policy of administering the bacterial preparation to prevent or ameliorate AAD and CDD in the target population.
During univariate sensitivity analysis, all ICERs were recalculated after changing the value of a range of parameters individually to estimate the robustness of the ICER (Table 1). Prolonged inpatient stay is the main cost driver when considering the cost of diarrhoea. 13,15,16 Other potentially important cost differences between the probiotic and placebo arms included staff time, which is naturally subject to variation, and diarrhoea-associated costs such as cleaning, laundry, microbiology, assessment, diagnostic and therapeutic procedures. As these costs were thought to make up a large proportion of the total health-care costs, the impact of changes to parameters contained within these costs was evaluated.
| Parameter | Minimum | Maximum | Change from base case |
|---|---|---|---|
| Costing of diarrhoea (£) | |||
| Microbiology ABMUHB | 30.17 | 109.88 | 50% discount on consumables; two samples tested per patient |
| Microbiology CDDFT | 27.51 | 90.56 | 50% discount on consumables; two samples tested per patient |
| Ward and bed closures | 0.00 | 497.61 | No cost effect; double the amount of wards closed per year |
| Clinical assessment and review | 103.01 | 259.67 | Reduced staff time, no gastroenterologist; increased staff time |
| Cleaning, laundry and disposables | 57.15 | 85.73 | ± 20% |
| Other costs (£) | |||
| Cost of one hospital inpatient day | 267.34 | 401.00 | ± 20% |
| Other parameters | |||
| Staff time/dose probiotics (minutes) | 2.5 | 10 | Half; double |
Probabilistic sensitivity analysis with changes to the values of all chosen parameters (usually within the 95% CI or a reasonable, defined range) calculated the probability that the intervention was cost-effective when all uncertainty associated with the individual parameters was considered. Results of the probabilistic sensitivity analysis were expressed as per cent probability that the intervention was cost-effective. Cost-effectiveness acceptability curves (CEACs) were generated to depict the probability of the intervention being cost-effective at different willingness-to-pay thresholds.
Chapter 3 Protocol changes
Inclusion and exclusion criteria
In practice, on assessment for eligibility, some patients were severely ill and not expected to survive for the intended period of follow-up; therefore, they were not approached regarding joining the study. Similarly, participants who were nil by mouth at initial assessment were not approached.
Follow-up
We had intended that diarrhoea outcomes would be assessed during antibiotic treatment and for 8 weeks after stopping antibiotics. However, prolonged follow-up for participants on long courses of antibiotics was not feasible. In practice, daily follow-up during hospital stay or weekly follow-up after discharge from hospital was continued to 8 weeks after recruitment. Review of laboratory data regarding stool assays was continued until 12 weeks after recruitment.
Assessment of quality of life
We considered modifying tools validated to measure QoL in treatment-induced diarrhoea in people with HIV54 and older patients with faecal incontinence. 55 However, we considered that completion of additional questionnaires would be too onerous for elderly inpatients and these were not pursued.
Chapter 4 Results
A total of 17,420 in-patients aged ≥ 65 years and who had been exposed to one or more antibiotics were assessed for eligibility (Figure 1). Exclusion criteria were present in 3202 (18.4%) patients, 9068 (52.1%) declined to participate, 2130 (12.2%) were too unwell to join the study and 39 (0.2%) were nil by mouth. We recruited 2981 (17.1%) patients, at randomisation 1493 (50.1%) were allocated to the probiotic and 1488 (49.9%) to the placebo arm.
FIGURE 1.
Trial profile.
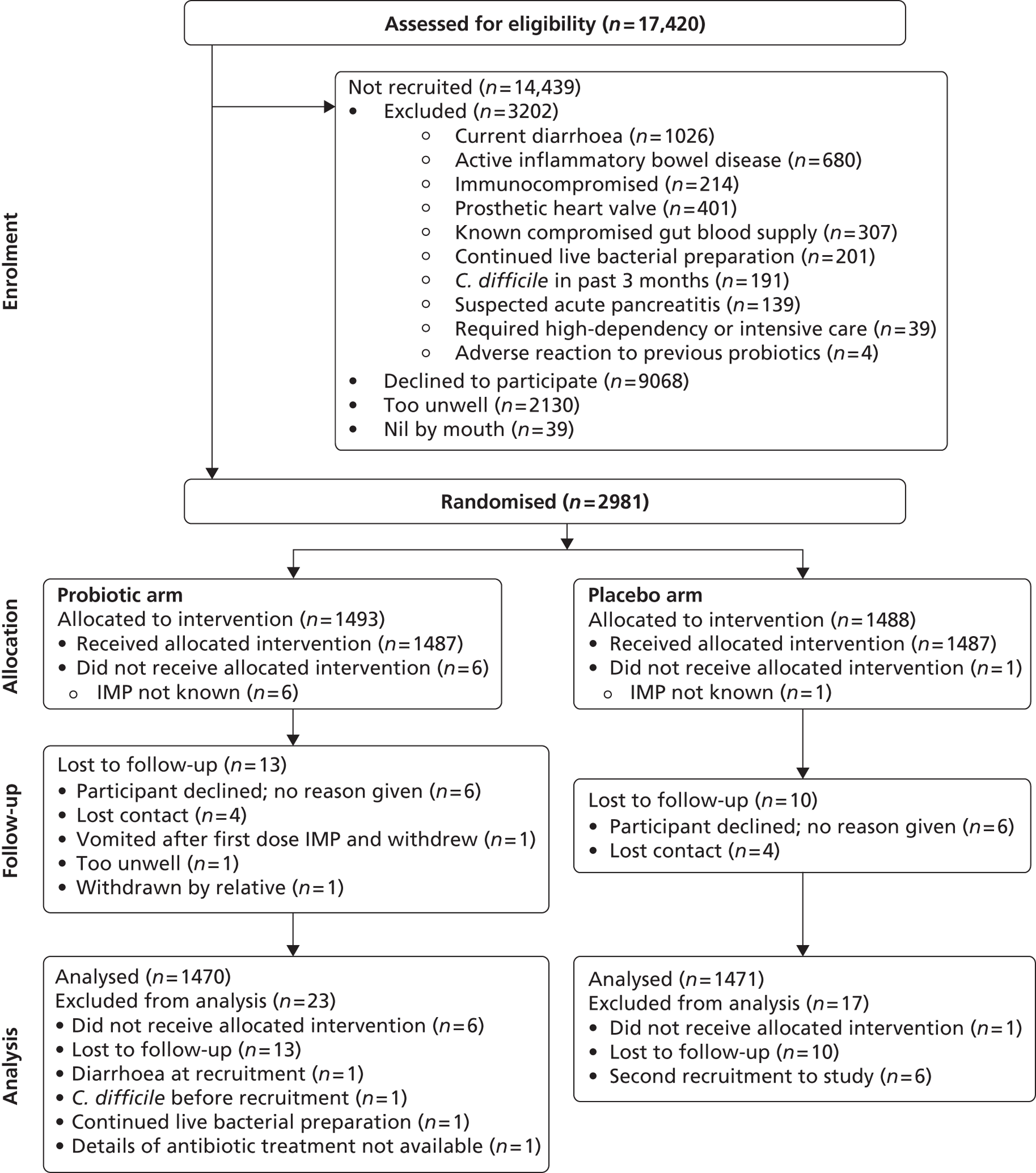
In total, 2941 (98.7%) were included in the analysis according to treatment allocated; 23 in the probiotic arm and 17 in the placebo arm were excluded. The identity of the IMP was unknown in seven participants (six allocated to the probiotic and one to the placebo arm) due to an error in IMP labelling at one hospital site. No outcome data were available in 23 patients who were lost to follow-up. In each arm of the study, these included six patients who declined further participation shortly after randomisation without giving a reason and contact was lost with four patients from each arm. Exclusion criteria were present at recruitment in three patients and the details of antibiotic treatment could not be determined in one patient in the probiotic arm. Six participants were recruited to the study for a second time and all were allocated to the placebo arm. Possible carry-over effects from their first involvement in the study could not be excluded and; therefore, data from their second involvement were excluded.
Participant characteristics according to intervention arm
Consent to participate in the trial was provided directly by 1398 patients (95.1%) in the probiotic arm and 1392 patients (94.6%) in the placebo arm. For patients unable to give consent themselves, assent was usually provided by a family member: daughter [in 24 cases (1.6%) in the probiotic arm and 34 cases (2.3%) in the placebo arm], wife [in 19 cases (1.3%) in the probiotic arm and 13 cases (0.9%) in the placebo arm], or son [in 15 cases (1.0%) in the probiotic arm and 18 cases (1.2%) in the placebo arm].
Baseline demographic and patient characteristics were similar in the two intervention arms except for a greater proportion of males than females in the probiotic arm and vice versa in the placebo arm (Table 2). The frailty of the study population is reflected in the median age of 77.1 years and common occurrence of comorbid illnesses: 54.6% of participants suffered from hypertension, 24.1% from chronic obstructive pulmonary disease (COPD) and 22.9% from diabetes. Participant age ranged from 65.0 to 107.5 years in the probiotic arm and from 65.0 to 104.4 years in the placebo arm. More participants were recruited during the winter than in the summer months. The majority of patients were admitted to hospital from home and approximately one-third had been admitted to hospital within the previous 8 weeks. Very few people of non-white ethnic origin were recruited. Cigarette smoking was uncommon, but approximately one in three participants drank alcohol. Recent consumption of foods containing live bacteria was uncommon among all participants and occurred with a similar frequency in both study arms.
| Characteristic | Probiotica (n = 1470) | Placeboa (n = 1471) | Total (n = 2941) |
|---|---|---|---|
| Median age in years (IQR) | 77.2 (70.8–83.6) | 77.0 (71.3–83.5) | 77.1 (71.0–83.5) |
| Male, n/N (%) | 777/1470 (52.9) | 679/1471 (46.2) | 1456/2941 (49.5) |
| Ethnicity, n/N (%) white | 1459/1461 (99.9) | 1461/1464 (99.8) | 2920/2925 (99.8) |
| Recruited during winter (October–March), n/N (%) | 845/1470 (57.5) | 845/1471 (57.4) | 1690/2941 (57.5) |
| Where admitted from | n = 1469 | n = 1468 | n = 2937 |
|
1345 (91.6) | 1334 (90.9) | 2679 (91.2) |
|
58 (3.9) | 67 (4.6) | 125 (4.3) |
|
37 (2.5) | 39 (2.7) | 76 (2.6) |
|
29 (2.0) | 28 (1.9) | 57 (1.9) |
| Hospital | |||
|
102 (6.9) | 101 (6.9) | 203 (6.9) |
|
742 (50.5) | 737 (50.1) | 1479 (50.3) |
|
94 (6.4) | 97 (6.6) | 191 (6.5) |
|
269 (18.3) | 278 (18.9) | 547 (18.6) |
|
263 (17.9) | 258 (17.5) | 521 (17.7) |
| Cigarette smoker, n/N (%) | 140/1470 (9.5) | 120/1471 (8.2) | 260/2941 (8.8) |
| Drinks alcohol, n/N (%) | 459/1470 (31.2) | 482/1471 (32.8) | 941/2941 (32.0) |
| Comorbid illnesses | |||
|
779/1455 (53.5) | 812/1457 (55.7) | 1591/2912 (54.6) |
|
350/1459 (24.0) | 354/1462 (24.2) | 704/2921 (24.1) |
|
357/1465 (24.4) | 314/1468 (21.4) | 671/2933 (22.9) |
|
237/1462 (16.2) | 232/1465 (15.8) | 469/2927 (16.0) |
|
127/1455 (8.7) | 139/1461 (9.5) | 266/2916 (9.1) |
|
61/1449 (4.2) | 80/1459 (5.5) | 141/2908 (4.8) |
|
28/1465 (1.9) | 26/1465 (1.8) | 54/2930 (1.8) |
|
978/1452 (67.4) | 1010/1458 (69.3) | 1988/2910 (68.3) |
| Previous gastrointestinal surgery, n/N (%) | 203/1448 (14.0) | 212/1449 (14.6) | 415/2897 (14.3) |
| NGT in situ, n/N (%) | 5/1469 (0.3) | 2/1464 (0.1) | 7/2933 (0.2) |
| Hospital admission in last 8 weeks, n/N (%) | 488/1470 (33.2) | 448/1471 (30.5) | 936/2941 (31.8) |
| Median number of hospital admissions in last 8 weeks (IQR) | 1465 0.0 (0.0–10) | 1467 0.0 (0.0–1.0) | 2932 0.0 (0.0–1.0) |
| Live bacteria consumed in last 7 days, n/N (%) | 72/1470 (4.9) | 45/1471 (3.1) | 117/2941 (4.0) |
Participant characteristics according to centre
Overall, 1873 (63.7%) inpatients were recruited in hospitals in ABMUHB (Singleton, Morriston and Princess of Wales) and 1068 (36.3%) in CDDFT (Durham and Darlington). In ABMUHB, recruitment began with a pilot study of 50 patients in Morriston Hospital on 1 December 2008 to evaluate the recruitment methods and data collection forms. Methods were found to be reliable and these patients were included in the final analysis. Recruitment continued until 28 February 2012 and a total of 1479 patients (50.3% of total) were recruited (see Table 2). Recruitment in Singleton Hospital began on 9 June 2009 but was terminated on 9 February 2011, after 203 (6.9%) patients had been recruited, because of falling numbers of eligible patients due to service reconfiguration. To maintain patient numbers, recruitment was undertaken at Princess of Wales Hospital from 5 May 2011 to 10 January 2012 and 191 (6.5%) patients were recruited. The start of recruitment was delayed in CDDFT for operational reasons. Darlington Memorial Hospital recruited 521 (17.7%) patients from 12 October 2009 to 27 February 2012 and University Hospital of North Durham recruited 547 (18.6%) patients from 17 November 2011 to 28 February 2012 (see Table 2).
Baseline participant characteristics were generally similar across the hospital sites (Table 3) with some exceptions. The greater proportion of males in the probiotic arm and females in the placebo arm occurred in all centres except for Singleton Hospital, where there were more females than males in the probiotic arm (data not shown). Participants recruited at Singleton Hospital were more likely to be female and tended to be older than participants from other hospitals. The period during which recruitment in each centre occurred was reflected in the lower proportion of patient recruitment during the winter months in Princess of Wales than in other hospitals. The frequency of COPD and hospital admission in the previous 8 weeks were both more common in hospitals in CDDFT than in ABMUHB.
| Variable | ABMUHBa | CDDFTa | |||
|---|---|---|---|---|---|
| Singleton | Morriston | Princess of Wales | Durham | Darlington | |
| Number participants recruited | n = 203 | n = 1479 | n = 191 | n = 547 | n = 521 |
| Median age in years (IQR) | 79.9 (74.1–86.3) | 76.8 (70.6–83.4) | 76.0 (70.4–82.7) | 77.7 (71.3–84.2) | 76.4 (70.8–82.1) |
| Male, n (%) | 85 (41.9) | 755 (51.0) | 93 (48.7) | 271 (49.5) | 252 (48.4) |
| Ethnicity, n/N (%) white | 203/203 (100.0) | 1467/1469 (99.9) | 188/188 (100.0) | 543/544 (99.8) | 519/521 (99.6) |
| Recruited during winter (October–March) n/N (%) | 130/203 (64.0) | 819/1479 (55.4) | 66/191 (34.6) | 368/547 (67.3) | 307/521 (58.9) |
| Where admitted from | |||||
|
184 (90.6) | 1312 (88.8) | 184 (96.3) | 506 (93.0) | 493 (94.6) |
|
14 (6.9) | 61 (4.1) | 3 (1.6) | 28 (5.1) | 19 (3.6) |
|
2 (1.0) | 64 (4.3) | 1 (0.5) | 4 (0.7) | 5 (1.0) |
|
3 (1.5) | 41 (2.8) | 3 (1.6) | 6 (1.1) | 4 (0.8) |
| Cigarette smoker, n/N (%) | 16/203 (7.9) | 122/1479 (8.2) | 18/191 (9.4) | 66/547 (12.1) | 38/521 (7.3) |
| Drinks alcohol, n/N (%) | 66/203 (32.5) | 487/1479 (32.9) | 43/191 (22.5) | 160/547 (29.3) | 185/521 (35.5) |
| Comorbid illnesses | |||||
|
88/198 (44.4) | 827/1467 (56.4) | 106/186 (57.0) | 261/545 (47.9) | 309/516 (59.9) |
|
54/201 (26.9) | 216/1468 (14.7) | 53/189 (28.0) | 216/544 (39.7) | 165/519 (31.8) |
|
48/202 (23.8) | 336/1477 (22.7) | 40/191 (20.9) | 138/543 (25.4) | 109/520 (21.0) |
|
43/202 (21.3) | 194/1473 (13.2) | 26/191 (13.6) | 96/542 (17.7) | 110/519 (21.2) |
|
20/202 (9.9) | 99/1467 (6.7) | 13/188 (6.9) | 68/540 (12.6) | 66/519 (12.7) |
|
17/202 (8.4) | 73/1458 (5.0) | 3/191 (1.6) | 32/539 (5.9) | 16/518 (3.1) |
|
3/202 (1.5) | 22/1475 (1.5) | 5/191 (2.6) | 13/542 (2.4) | 11/520 (2.1) |
|
91/199 (45.7) | 929/1470 (63.2) | 132/187 (70.6) | 420/542 (77.5) | 416/512 (81.3) |
| Previous gastrointestinal surgery, n/N (%) | 21/194 (10.8) | 201/1460 (13.8) | 18/188 (9.6) | 81/537 (15.1) | 94/518 (18.1) |
| NGT in situ, n/N (%) | 1/203 (0.5) | 1/1477 (0.1) | 1/191 (0.5) | 2/541 (0.4) | 2/521 (0.4) |
| Hospital admission in last 8 weeks, n/N (%) | 50/203 (24.6) | 311/1479 (21.0) | 30/191 (15.7) | 304/547 (55.6) | 241/521 (46.3) |
| Number of hospital admissions in last 8 weeks, n, median (IQR) | 201, 0.0 (0.0–0.0) | 1473, 0.0 (0.0–0.0) | 191, 0.0 (0.0–0.0) | 546, 1.0 (0.0–1.0) | 521, 0.0 (0.0–1.0) |
| Live bacteria consumed in last 7 days, n/N (%) | 7/203 (3.4) | 65/1479 (4.4) | 6/191 (3.1) | 12/547 (2.2) | 27/521 (5.2) |
Indications for initial antibiotic treatment
Indications for antibiotic treatment classified according to the MedDRA SOC43 were similar in the two study arms (Table 4). The most common indication was ‘respiratory, thoracic and mediastinal disorders’. Antibiotic treatment for suspected sepsis where the site was unclear was given to a small proportion of patients. About one in four patients in each arm of the study received antibiotics for prophylaxis rather than the treatment of infection and nearly all of these were for surgical and medical procedures.
| Indication for initial antibiotic treatment | Probiotic (n = 1470) (%) | Placebo (n = 1471) (%) | Total (n = 2941) (%) |
|---|---|---|---|
|
2 (0.1) | 0 (0.0) | 2 (0.1) |
|
5 (0.3) | 1 (0.1) | 6 (0.2) |
|
4 (0.3) | 1 (0.1) | 5 (0.2) |
|
0 (0.0) | 1 (0.1) | 1 (0.0) |
|
14 (1.0) | 8 (0.5) | 22 (0.7) |
|
32 (2.2) | 23 (1.6) | 55 (1.9) |
|
2 (0.1) | 3 (0.2) | 5 (0.2) |
|
67 (4.6) | 56 (3.8) | 123 (4.2) |
|
18 (1.2) | 17 (1.2) | 35 (1.2) |
|
3 (0.2) | 3 (0.2) | 6 (0.2) |
|
265 (18.0) | 278 (18.9) | 543 (18.5) |
|
2 (0.1) | 3 (0.2) | 5 (0.2) |
|
501 (34.1) | 525 (35.7) | 1026 (34.9) |
|
166 (11.3) | 147 (10.0) | 313 (10.6) |
|
338 (23.0) | 363 (24.7) | 701 (23.8) |
|
2 (0.1) | 3 (0.2) | 5 (0.2) |
| Suspected sepsis but site unclear | 49 (3.3) | 39 (2.7) | 88 (3.0) |
In keeping with differences in the frequency of COPD according to centre, a greater proportion of the patients in hospitals in CDDFT than ABMUHB were treated for ‘respiratory, thoracic and mediastinal disorders’ (Table 5).
| Indication for initial antibiotic treatment | ABMUHB | CDDFT | |||
|---|---|---|---|---|---|
| Singleton (n = 203) (%) | Morriston (n = 1479) (%) | Princess of Wales (n = 191) (%) | Durham (n = 547) (%) | Darlington (n = 521) (%) | |
| Blood and lymphatic system disorders | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) |
| Cardiovascular disorders | 0 (0.0) | 3 (0.2) | 1 (0.5) | 0 (0.0) | 2 (0.4) |
| Ear and labyrinth disorders | 0 (0.0) | 1 (0.1) | 1 (0.5) | 2 (0.4) | 1 (0.2) |
| Eye disorders | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 0 (0.0) |
| Gastrointestinal disorders | 0 (0.0) | 17 (1.1) | 1 (0.5) | 2 (0.4) | 2 (0.4) |
| Hepatobiliary disorders | 7 (3.4) | 24 (1.6) | 5 (2.6) | 13 (2.4) | 6 (1.2) |
| Infections and infestations | 1 (0.5) | 2 (0.1) | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| Injury, poisoning and procedural complications | 2 (1.0) | 100 (6.8) | 6 (3.1) | 5 (0.9) | 10 (1.9) |
| Musculoskeletal and connective tissue disorders | 0 (0.0) | 18 (1.2) | 2 (1.0) | 5 (0.9) | 10 (1.9) |
| Nervous system disorders | 0 (0.0) | 4 (0.3) | 0 (0.0) | 1 (0.2) | 1 (0.2) |
| Renal and urinary disorders | 56 (27.6) | 284 (19.2) | 37 (19.4) | 105 (19.2) | 61 (11.7) |
| Reproductive system and breast disorders | 1 (0.5) | 0 (0.0) | 1 (0.5) | 1 (0.2) | 2 (0.4) |
| Respiratory, thoracic and mediastinal disorders | 90 (44.3) | 331 (22.4) | 64 (33.5) | 313 (57.2) | 228 (43.8) |
| Skin and subcutaneous tissue disorders | 25 (12.3) | 162 (11.0) | 21 (11.0) | 52 (9.5) | 53 (10.2) |
| Surgical and medical procedures | 9 (4.4) | 488 (33.0) | 49 (25.7) | 28 (5.1) | 127 (24.4) |
| Vascular disorder | 0 (0.0) | 3 (0.2) | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| Suspected sepsis but site unclear | 12 (5.9) | 42 (2.8) | 1 (0.5) | 18 (3.3) | 15 (2.9) |
Antibiotic exposure
All of the participants were receiving one or more antibiotics when they started the IMPs. The date the participant began taking the antibiotics before recruitment was known in 1448 participants in the probiotic and 1443 in the placebo arm. The median (IQR) period of exposure to antibiotics before starting the IMP was 3.0 days (2.0–6.0 days) in both study arms (p = 0.38).
During the period 7 days before, and 8 weeks following, recruitment, the most commonly used antibiotic class was the penicillins, with over half of all participants receiving a broad-spectrum penicillin. About one in four participants were exposed to a cephalosporin. Antibiotic exposure was similar in the two study arms (Table 6).
| Antibiotic (classes and individual drugs) | Probiotic (n = 1470) (%) | Placebo (n = 1471) (%) | Total (n = 2941) (%) |
|---|---|---|---|
| Penicillins | 1052 (71.6) | 1061 (72.1) | 2113 (71.8) |
| Benzylpenicillin | 115 (7.8) | 99 (6.7) | 214 (7.3) |
| Penicillinase-resistant penicillin – flucloxacillin | 322 (21.9) | 310 (21.1) | 632 (21.5) |
| Broad-spectrum penicillins | 822 (55.9) | 829 (56.4) | 1651 (56.1) |
|
310 (21.1) | 323 (22.0) | 633 (21.5) |
|
2 (0.1) | 1 (0.1) | 3 (0.1) |
|
612 (41.6) | 623 (42.4) | 1235 (42.1) |
| Anti-pseudomonas penicillins | 127 (8.6) | 118 (8.0) | 245 (8.3) |
|
3 (0.2) | 0 (0.0) | 3 (0.1) |
|
125 (8.5) | 118 (8.0) | 243 (8.3) |
| Cephalosporins | 359 (24.4) | 356 (24.2) | 715 (24.3) |
| First generation | 77 (5.2) | 74 (5.0) | 151 (5.1) |
|
77 (5.2) | 73 (5.0) | 150 (5.1) |
|
0 (0.0) | 1 (0.1) | 1 (0.0) |
| Second generation | 290 (19.7) | 304 (20.7) | 594 (20.2) |
|
27 (1.8) | 24 (1.6) | 51 (1.7) |
|
1 (0.1) | 0 (0.0) | 1 (0.0) |
|
284 (19.3) | 293 (19.9) | 577 (19.6) |
| Third generation | 11 (0.7) | 10 (0.7) | 21 (0.7) |
|
1 (0.1) | 2 (0.1) | 3 (0.1) |
|
7 (0.5) | 7 (0.5) | 14 (0.5) |
|
3 (0.2) | 2 (0.1) | 5 (0.2) |
| Other antibiotics | |||
| Carbapenems and other β-lactams | 33 (2.2) | 29 (2.0) | 62 (2.1) |
|
0 (0.0) | 1 (0.1) | 1 (0.0) |
|
2 (0.1) | 3 (0.2) | 5 (0.2) |
|
31 (2.1) | 26 (1.8) | 57 (1.9) |
| Tetracyclines | 211 (14.4) | 222 (15.1) | 433 (14.7) |
|
0 (0.0) | 2 (0.1) | 2 (0.1) |
|
199 (13.5) | 213 (14.5) | 412 (14.0) |
|
0 (0.0) | 1 (0.1) | 1 (0.0) |
|
10 (0.7) | 4 (0.3) | 14 (0.5) |
|
2 (0.1) | 4 (0.3) | 6 (0.2) |
| Aminoglycosides | 182 (12.4) | 196 (13.3) | 378 (12.9) |
|
182 (12.4) | 195 (13.3) | 377 (12.8) |
|
0 (0.0) | 1 (0.1) | 1 (0.0) |
| Macrolides | 249 (16.9) | 251 (17.1) | 500 (17.0) |
|
13 (0.9) | 11 (0.7) | 24 (0.8) |
|
203 (13.8) | 210 (14.3) | 413 (14.0) |
|
43 (2.9) | 41 (2.8) | 84 (2.9) |
| Clindamycin | 18 (1.2) | 14 (1.0) | 32 (1.1) |
| Sulphonamides and trimethoprim | 228 (15.5) | 242 (16.5) | 470 (16.0) |
|
0 (0.0) | 6 (0.4) | 6 (0.2) |
|
228 (15.5) | 236 (16.0) | 464 (15.8) |
| Metronidazole | 171 (11.6) | 142 (9.7) | 313 (10.6) |
| Quinolones | 185 (12.6) | 180 (12.2) | 365 (12.4) |
|
171 (11.6) | 157 (10.7) | 328 (11.2) |
|
14 (1.0) | 21 (1.4) | 35 (1.2) |
|
2 (0.1) | 5 (0.3) | 7 (0.2) |
|
0 (0.0) | 1 (0.1) | 1 (0.0) |
| Glycopeptides | 103 (7.0) | 75 (5.1) | 178 (6.1) |
|
82 (5.6) | 61 (4.1) | 143 (4.9) |
|
27 (1.8) | 20 (1.4) | 47 (1.6) |
| Anti-tuberculous antibiotics | 26 (1.8) | 20 (1.4) | 46 (1.6) |
|
1 (0.1) | 2 (0.1) | 3 (0.1) |
|
26 (1.8) | 20 (1.4) | 46 (1.6) |
|
1 (0.1) | 0 (0.0) | 1 (0.0) |
| Others | 38 (2.6) | 53 (3.6) | 91 (3.1) |
|
1 (0.1) | 0 (0.0) | 1 (0.0) |
|
3 (0.2) | 0 (0.0) | 3 (0.1) |
|
26 (1.8) | 45 (3.1) | 71 (2.4) |
|
9 (0.6) | 8 (0.5) | 17 (0.6) |
Antibiotic exposure varied according to centre (see Appendix 9, Table 31). In hospitals in CDDFT, exposure to broad-spectrum penicillins was greater than in hospitals in ABMUHB (67.8–70.6% vs. 47.3–57.1%, respectively), but exposure to cephalosporins was lower (1.9–3.3% vs. 13.6–29.1%, respectively), as was exposure to quinolones (6.9–7.5% vs. 8.4–21.2%, respectively).
Fewer than 1 in 10 participants received only a single dose of an antibiotic and most received antibiotics for ≥ 7, with one-third treated for at least 14 days (Tables 7 and 8). The majority of participants were exposed to antibiotics from two or more classes. Exposure to combination therapy and duration of antibiotic therapy was similar in the two study arms.
| Combination antibiotic therapya | Probiotic (n = 1470) | Placebo (n = 1471) | Total (n = 2941) |
|---|---|---|---|
| Number (%) participants who received an antibiotic from | |||
| One class only | 310 (21.1) | 310 (21.1) | 620 (21.1) |
| Two classes | 407 (27.7) | 397 (27.0) | 804 (27.3) |
| Three or more classes | 753 (51.2) | 764 (51.9) | 1517 (51.6) |
| Duration of antibiotic therapy | Probiotica (n = 1406) | Placeboa (n = 1398) | Total (n = 2804) |
|---|---|---|---|
| Number (%) participants who received | |||
| Single dose | 133 (9.5) | 123 (8.8) | 256 (9.1) |
| 1–6 days’ treatment | 389 (27.7) | 398 (28.5) | 787 (28.1) |
| 7–13 days’ treatment | 402 (28.6) | 426 (30.5) | 828 (29.5) |
| ≥ 14 days’ treatment | 482 (34.3) | 451 (32.3) | 933 (33.3) |
Non-antibiotic drug treatment
Use of drugs other than antibiotics was common, with many participants receiving antihypertensive therapy, aspirin, PPIs or angiotensin-converting enzyme (ACE) inhibitor therapy. Non-antibiotic drug treatment was similar in the two study arms (Table 9).
| Drugs | Probiotic,a n/N (%) | Placebo,a n/N (%) | Total, n/N (%) |
|---|---|---|---|
| Antacid therapies | |||
| PPI | 582/1459 (39.9) | 567/1460 (38.8) | 1149/2919 (39.4) |
| H2 blocker | 96/1449 (6.6) | 74/1454 (5.1) | 170/2903 (5.9) |
| Antacid | 30/1457 (2.1) | 34/1460 (2.3) | 64/2917 (2.2) |
| Other drugs | |||
| ACE inhibitor | 425/1449 (29.3) | 436/1453 (30.0) | 861/2902 (29.7) |
| Antihypertensive | 679/1450 (46.8) | 716/1453 (49.3) | 1395/2903 (48.1) |
| Aspirin | 597/1458 (40.9) | 589/1458 (40.4) | 1186/2916 (40.7) |
| Oral hypoglycaemic agent | 208/1460 (14.2) | 188/1460 (12.9) | 396/2920 (13.6) |
| Non-steroidal anti-inflammatory drug | 158/1450 (10.9) | 135/1454 (9.3) | 293/2904 (10.1) |
| Insulin | 96/1459 (6.6) | 78/1460 (5.3) | 174/2919 (6.0) |
| Feed containing probiotic | 8/1459 (0.5) | 9/1457 (0.6) | 17/2916 (0.6) |
Primary outcomes
Antibiotic-associated diarrhoea (including CDD) occurred with a similar frequency in the probiotic arm (159 participants, 10.8%) and placebo arm (153 participants, 10.4%; RR 1.04; 95% CI 0.84 to 1.28; p = 0.71; Table 10). This included 12 participants with frequent stools that they described as looser than normal but who were unable to describe stool consistency using the Bristol Stool Form Scale. 38
| Outcome | Probiotic n/N (%) | Placebo n/N (%) | RR (95% CI) [p-value] | OR (95% CI) [p-value] | Risk difference (95% CI) [p-value] |
|---|---|---|---|---|---|
| AADb | 159/1470 (10.8) | 153/1471 (10.4) | 1.04 (0.84 to 1.28) [0.72] | 1.04 (0.83 to 1.32) [0.72] | 0.42 (−1.81 to 2.64) [0.72] |
| CDD | 12/1470 (0.8) | 17/1471 (1.2) | 0.71 (0.34 to 1.47) [0.35] | 0.70 (0.34 to 1.48) [0.35] | −0.34 (−1.05 to 0.37) [0.35] |
Clostridium difficile diarrhoea was uncommon and occurred in 12 (0.8%) participants in the probiotic arm and 17 (1.2%) participants in the placebo arm (RR 0.71; 95% CI 0.34 to 1.47; p = 0.35; see Table 10). Based on this effect size and the low prevalence of CDD, the number needed to treat to prevent one case is 295. This would be reduced to 95 for an effect size at the lower limit of the 95% CI (a threefold reduction in CDD in the probiotic arm). The corresponding number needed to harm (the upper 95% CI) is 267.
Secondary outcomes
Clostridium difficile was isolated from stools in two participants with mild loose stools (not meeting the study criteria for diarrhoea) in the probiotic arm. One participant in each arm had an episode of CDD after an initial episode of AAD that was not associated with CDI; the participant allocated to the placebo arm required surgery for CDD and the participant allocated to the probiotic arm had gallstones and died during the episode of CDD. One patient with known carcinoma of the head of the pancreas with a biliary stent in situ died during an episode of CDD that occurred after withdrawal from the trial.
The adjusted treatment effect on occurrence of AAD from covariate analysis was similar to the unadjusted effect after controlling for nine prespecified covariates. Covariate analysis identified that the occurrence of AAD could be predicted by the duration of antibiotic treatment, antacid therapy and duration of hospital stay (Table 11).
| Variablea | Comparison | OR (95% CI) |
|---|---|---|
| Intervention arm | Probiotic vs. placebo | 1.00 (0.78 to 1.27) |
| Centre | Singleton vs. Darlington | 0.69 (0.38 to 1.25) |
| Morriston vs. Darlington | 0.84 (0.58 to 1.21) | |
| Princess of Wales vs. Darlington | 1.08 (0.62 to 1.88) | |
| Durham vs. Darlington | 0.93 (0.62 to 1.41) | |
| Age | ≤ 77 years vs. > 77 years | 1.05 (0.81 to 1.35) |
| Sex | Male vs. female | 1.07 (0.83 to 1.37) |
| Antibiotic class | Penicillins vs. other | 0.93 (0.70 to 1.22) |
| Cephalosporins vs. other | 1.41 (0.97 to 2.04) | |
| Duration of antibiotic therapy | ≤ 8 days vs. > 8 days | 0.48 (0.36 to 0.62) |
| Any antacid therapy | No vs. yes | 0.74 (0.58 to 0.95) |
| Previous gastrointestinal surgery | No vs. yes | 0.95 (0.67 to 1.33) |
| Recent previous hospital admission | No vs. yes | 1.05 (0.80 to 1.38) |
| Duration of hospital stay | < 7 days vs. ≥ 7 days | 0.74 (0.55 to 0.99) |
The frequency of AAD was similar in each centre: Morriston 162/1479 (11.0%), Singleton 20/203 (9.9%), Princess of Wales 21/191 (11.0%), Durham 56/547 (10.2%) and Darlington 53/521 (10.2%; p = 0.97). Subgroup analyses showed that the distribution of cases of AAD according to prespecified potential risk factors for AAD, including those identified as risk factors in covariate analysis, was similar in the two intervention arms and there was no evidence of a statistically significant interaction between prespecified potential risk factors for AAD and intervention arm (Table 12).
| Category | Probiotic,a n/N (%) | Placebo,a n/N (%) | RR (95% CI) | p-value for interaction |
|---|---|---|---|---|
| Centre | ||||
| Singleton | 11/102 (10.8) | 9/101 (8.9) | 1.21 (0.52 to 2.79) | 0.28 |
| Morriston | 81/742 (10.9) | 81/737 (11.0) | 0.99 (0.74 to 1.33) | |
| Princess of Wales | 15/94 (16.0) | 6/97 (6.2) | 2.58 (1.05 to 6.37) | |
| Durham | 26/269 (9.7) | 30/278 (10.8) | 0.90 (0.54 to 1.47) | |
| Darlington | 26/263 (9.9) | 27/258 (10.5) | 0.94 (0.57 to 1.57) | |
| Age | ||||
| ≤ 77 years | 86/730 (11.8) | 70/732 (9.6) | 1.23 (0.91 to 1.66) | 0.15 |
| > 77 years | 73/740 (9.9) | 83/739 (11.2) | 0.88 (0.65 to 1.18) | |
| Sex | ||||
| Male | 91/777 (11.7) | 68/679 (10.0) | 1.17 (0.87 to 1.57) | 0.25 |
| Female | 68/693 (9.8) | 85/792 (10.7) | 0.91 (0.68 to 1.24) | |
| Antibiotic class | ||||
| Penicillins | 78/761 (10.2) | 77/777 (9.9) | 1.03 (0.77 to 1.39) | 0.87 |
| Cephalosporins | 31/227 (13.7) | 26/220 (11.8) | 1.16 (0.71 to 1.88) | |
| Other | 50/482 (10.4) | 50/474 (10.5) | 0.98 (0.68 to 1.43) | |
| Duration of antibiotic treatment | ||||
| ≤ 8 days | 48/694 (6.9) | 52/709 (7.3) | 0.94 (0.65 to 1.38) | 0.66 |
| > 8 days | 107/712 (15.0) | 99/689 (14.4) | 1.05 (0.81 to 1.35) | |
| PPI treatment | ||||
| No | 86/877 (9.8) | 79/893 (8.8) | 1.11 (0.83 to 1.48) | 0.55 |
| Yes | 72/582 (12.4) | 72/567 (12.7) | 0.97 (0.72 to 1.32) | |
| Any antacid therapy (including PPI treatment) | ||||
| No | 77/802 (9.6) | 74/834 (8.9) | 1.08 (0.80 to 1.47) | 0.73 |
| Yes | 81/657 (12.3) | 77/627 (12.3) | 1.00 (0.75 to 1.34) | |
| NGT in situ | ||||
| No | 159/1464 (10.9) | 153/1462 (10.5) | 1.04 (0.84 to 1.28) | – |
| Yes | 0/5 (0.0) | 0/2 (0.0) | ||
| Previous gastrointestinal surgery | ||||
| No | 132/1245 (10.6) | 129/1237 (10.4) | 1.02 (0.81 to 1.28) | 0.71 |
| Yes | 25/203 (12.3) | 23/212 (10.8) | 1.14 (0.67 to 1.93) | |
| Recent previous hospital admission | ||||
| No | 107/982 (10.9) | 105/1023 (10.3) | 1.06 (0.82 to 1.37) | 0.85 |
| Yes | 52/483 (10.8) | 47/444 (10.6) | 1.02 (0.70 to 1.48) | |
| Duration of hospital stay | ||||
| < 7 days | 39/524 (7.4) | 40/498 (8.0) | 0.93 (0.61 to 1.42) | 0.54 |
| ≥ 7 days | 117/928 (12.6) | 111/949 (11.7) | 1.08 (0.85 to 1.37) | |
Most episodes of AAD (73.7%) occurred within 4 weeks of recruitment. On average, episodes of AAD lasted for 2 days with four stools in 24 hours and of consistency seven on the Bristol Stool Form Scale (Table 13). The most commonly associated symptoms were urgency, abdominal pain and nocturnal diarrhoea. The latter tended to occur more frequently in the placebo than the probiotic group (p = 0.051) and other characteristics of the diarrhoea episodes were similar in the two study arms (see Table 13). Most episodes of AAD were managed in hospital and stool samples were collected and tested for diarrhoeal pathogens in 58.6% of all cases. For many episodes of AAD, the short duration and occurrence after discharge from hospital complicated the collection of a stool specimen for testing for pathogens.
| Outcome | Probiotica | Placeboa | Total | p-value |
|---|---|---|---|---|
| Duration (days), n, median (IQR) | 135, 2.0 (1.0–4.0) | 125, 3.0 (1.0–6.0) | 260, 2.0 (1.0–5.0) | 0.11 |
| Number stools per 24 hours, n, median (IQR) | 158, 4.0 (3.0–5.0) | 152, 4.0 (3.0–5.0) | 310, 4.0 (3.0–5.0) | 0.69 |
| Stool consistency, n, median (IQR) | 152, 7.0 (6.0–7.0) | 145, 7.0 (6.0–7.0) | 297, 7.0 (6.0–7.0) | 0.85 |
| Nausea, n/N (%) | 35/154 (22.7) | 37/145 (25.5) | 72/299 (24.1) | 0.57 |
| Vomiting, n/N (%) | 20/155 (12.9) | 16/149 (10.7) | 36/304 (11.8) | 0.56 |
| Bloating, n/N (%) | 32/154 (20.8) | 31/146 (21.2) | 63/300 (21.0) | 0.92 |
| Flatus, n/N (%) | 41/153 (26.8) | 45/146 (30.8) | 86/299 (28.8) | 0.44 |
| Abdominal pain, n/N (%) | 55/154 (35.7) | 65/147 (44.2) | 120/301 (39.9) | 0.13 |
| Tenesmus, n/N (%) | 8/154 (5.2) | 7/145 (4.8) | 15/299 (5.0) | 0.88 |
| Fever, n/N (%) | 6/152 (3.9) | 4/143 (2.8) | 10/295 (3.4) | 0.59 |
| Faecal incontinence, n/N (%) | 27/151 (17.9) | 31/147 (21.1) | 58/298 (19.5) | 0.48 |
| Nocturnal diarrhoea, n/N (%) | 44/151 (29.1) | 59/148 (39.9) | 103/299 (34.4) | 0.051 |
| Urgency, n/N (%) | 78/151 (51.7) | 85/146 (58.2) | 163/297 (54.9) | 0.26 |
| Blood in stool, n/N (%) | 3/135 (2.2) | 3/134 (2.2) | 6/269 (2.2) | 0.99 |
| Mucus in stool, n/N (%) | 7/132 (5.3) | 12/131 (9.2) | 19/263 (7.2) | 0.23 |
| Managed in hospital, n/N (%) | 93/157 (59.2) | 75/146 (51.4) | 168/303 (55.4) | 0.17 |
| Stool sample tested, n/N (%) | 93/158 (58.9) | 88/151 (58.3) | 181/309 (58.6) | 0.92 |
As with AAD, the adjusted treatment effect for CDD was similar to the unadjusted estimate (Table 14). Covariate analysis showed that duration of antibiotic treatment was associated with CDD.
| Variablea | Comparison | OR (95% CI) |
|---|---|---|
| Intervention arm | Probiotic vs. placebo | 0.65 (0.29 to 1.47) |
| Centre | Singleton vs. Darlington | 0.52 (0.04 to 6.08) |
| Morriston vs. Darlington | 1.56 (0.34 to 7.20) | |
| Princess of Wales vs. Darlington | 0.00 (0.00 to incalculable) | |
| Durham vs. Darlington | 0.75 (0.10 to 5.46) | |
| Age | ≤ 77 vs. > 77 years | 0.95 (0.42 to 2.18) |
| Sex | Male vs. female | 1.12 (0.49 to 2.58) |
| Type of antibiotics | Penicillins vs. other | 0.43 (0.15 to 1.21) |
| Cephalosporins vs. other | 1.80 (0.69 to 4.67) | |
| Duration of antibiotic therapy | ≤ 8 days vs. > 8 days | 0.13 (0.03 to 0.56) |
| Any antacid therapy (including PPI treatment) | No vs. yes | 0.49 (0.21 to 1.12) |
| Previous gastrointestinal surgery | No vs. yes | 1.41 (0.41 to 4.88) |
| Recent previous hospital admission | No vs. yes | 0.97 (0.40 to 2.34) |
| Duration of hospital stay | < 7 days vs. ≥ 7 days | 0.00 (0.00 to incalculable) |
The frequency of CDD was similar in each centre: Morriston 21/1479 (1.4%), Singleton 2/203 (1.0%), Princess of Wales 0/191 (0.0%), Durham 3/547 (0.5%) and Darlington 3/521 (0.6%; p = 0.15). In subgroup analysis, there was a statistically significant interaction between intervention arm and age (p = 0.0015; Table 15). In patients aged > 77 years, the frequency of CDD was significantly lower in the probiotic arm than in the placebo arm. In contrast, the frequency of CDD was similar in the two intervention arms for patients aged ≤ 77 years. In addition, the interaction between treatment group and duration of antibiotic treatment was of borderline statistical significance (p = 0.054). There was no evidence of a significant interaction between the intervention arm and other prespecified potential risk factors for CDD (Table 15).
| Category | Probiotic,a n/N (%) | Placebo,a n/N (%) | RR (95% CI) | p-value for interaction |
|---|---|---|---|---|
| Centre | ||||
| Singleton | 1/102 (1.0) | 1/101 (1.0) | 0.99 (0.06 to 15.62) | 0.91 |
| Morriston | 8/742 (1.1) | 13/737 (1.8) | 0.61 (0.25 to 1.47) | |
| Princess of Wales | 0/94 (0.0) | 0/97 (0.0) | – | |
| Durham | 1/269 (0.4) | 2/278 (0.7) | 0.52 (0.05 to 5.67) | |
| Darlington | 2/263 (0.8) | 1/258 (0.4) | 1.96 (0.18 to 21.50) | |
| Age | ||||
| ≤ 77 years | 9/730 (1.2) | 3/732 (0.4) | 3.01 (0.82 to 11.07) | 0.0015 |
| > 77 years | 3/740 (0.4) | 14/739 (1.9) | 0.21 (0.06 to 0.74) | |
| Sex | ||||
| Male | 8/777 (1.0) | 7/679 (1.0) | 1.00 (0.36 to 2.74) | 0.31 |
| Female | 4/693 (0.6) | 10/792 (1.3) | 0.46 (0.14 to 1.45) | |
| Type of antibiotic | ||||
| Penicillins | 3/761 (0.4) | 6/777 (0.8) | 0.51 (0.13 to 2.03) | 0.86 |
| Cephalosporins | 4/227 (1.8) | 5/220 (2.3) | 0.78 (0.21 to 2.85) | |
| Other | 5/482 (1.0) | 6/474 (1.3) | 0.82 (0.25 to 2.67) | |
| Duration of antibiotic treatment | ||||
| ≤ 8 days | 2/694 (0.3) | 0/709 (0.0) | Incalculable | 0.054 |
| > 8 days | 10/712 (1.4) | 16/689 (2.3) | 0.60 (0.28 to 1.32) | |
| PPI treatment | ||||
| No | 6/877 (0.7) | 7/893 (0.8) | 0.87 (0.29 to 2.59) | 0.70 |
| Yes | 6/582 (1.0) | 9/567 (1.6) | 0.65 (0.23 to 1.81) | |
| Any antacid therapy (including PPI treatment) | ||||
| No | 5/802 (0.6) | 7/834 (0.8) | 0.74 (0.24 to 2.33) | 0.10 |
| Yes | 7/657 (1.1) | 9/627 (1.4) | 0.74 (0.28 to 1.98) | |
| NGT in situ | ||||
| No | 12/1464 (0.8) | 17/1462 (1.2) | 0.70 (0.34 to 1.47) | 1.00 |
| Yes | 0/5 (0.0) | 0/2 (0.0) | – | |
| Previous gastrointestinal surgery | ||||
| No | 10/1245 (0.8) | 15/1237 (1.2) | 0.66 (0.30 to 1.47) | 0.85 |
| Yes | 1/203 (0.5) | 2/212 (0.9) | 0.52 (0.05 to 5.71) | |
| Recent previous hospital admission | ||||
| No | 7/982 (0.7) | 13/1023 (1.3) | 0.56 (0.22 to 1.40) | 0.38 |
| Yes | 5/483 (1.0) | 4/444 (0.9) | 1.15 (0.31 to 4.25) | |
| Duration of hospital stay | ||||
| < 7 days | 0/524 (0.0) | 0/498 (0.0) | – | 1.00 |
| ≥ 7 days | 11/928 (1.2) | 16/949 (1.7) | 0.70 (0.33 to 1.51) | |
The timing of onset of CDD was similar to that of AAD, with 75.8% cases occurring within 4 weeks of recruitment. On average, the duration of CDD was 6.5 days and duration was similar in the two study arms (Table 16). Bloating was less common in the placebo arm than in the probiotic arm (risk difference 40.7%; 95% CI 7.4% to 74.0%) and median stool frequency tended to be lower in the placebo arm than in the probiotic arm (see Table 16). Otherwise, gastrointestinal symptoms, clinical findings and investigations and classification of severity were similar in the two study arms. During follow-up, no patient was identified as having peritonitis, ileus, toxic megacolon or life-threatening CDD or as having died from CDD. The majority of patients in both study arms were managed in hospital.
| Outcome | Probiotica | Placeboa | Total | p-value |
|---|---|---|---|---|
| Duration (days), n, median (IQR) | 11, 5.0 (3.0–8.0) | 11, 9.0 (6.0–13.0) | 22, 6.5 (3.0–12.0) | 0.16 |
| Number stools per 24 hours, n, median (IQR) | 12, 5.0 (3.0–6.0) | 17, 3.0 (3.0–4.0) | 29, 4.0 (3.0–5.0) | 0.057 |
| Stool consistency, n/N, median (IQR) | 12, 7.0 (6.0–7.0) | 17, 7.0 (6.0–7.0) | 29, 7.0 (6.0–7.0) | 0.62 |
| Nausea, n/N (%) | 3/12 (25.0) | 6/17 (35.3) | 9/29 (31.0) | 0.56 |
| Vomiting, n/N (%) | 1/12 (8.3) | 2/17 (11.8) | 3/29 (10.3) | 0.77 |
| Bloating, n/N (%) | 7/12 (58.3) | 3/17 (17.6) | 10/29 (34.5) | 0.023 |
| Flatus, n/N (%) | 7/12 (58.3) | 6/17 (35.3) | 13/29 (44.8) | 0.22 |
| Abdominal pain, n/N (%) | 8/12 (66.7) | 10/17 (58.8) | 18/29 (62.1) | 0.67 |
| Tenesmus, n/N (%) | 2/12 (16.7) | 1/17 (5.9) | 3/29 (10.3) | 0.35 |
| Fever, n/N (%) | 2/12 (16.7) | 1/17 (5.9) | 3/29 (10.3) | 0.35 |
| Faecal incontinence, n/N (%) | 3/12 (25.0) | 5/16 (31.3) | 8/28 (28.6) | 0.72 |
| Nocturnal diarrhoea, n/N (%) | 7/12 (58.3) | 11/17 (64.7) | 18/29 (62.1) | 0.73 |
| Urgency, n/N (%) | 8/12 (66.7) | 8/17 (47.1) | 16/29 (55.2) | 0.30 |
| Blood in stool, n/N (%) | 0/11 (0.0) | 0/16 (0.0) | 0/27 (0.0) | – |
| Mucus in stool, n/N (%) | 0/11 (0.0) | 4/15 (26.7) | 4/26 (15.4) | 0.063 |
| Managed in hospital, n/N (%) | 9/12 (75.0) | 14/17 (82.4) | 23/29 (79.3) | 0.63 |
| Findings on examination and clinical investigations | ||||
| Fever (temperature ≥ 38.5 °C), n/N (%) | 1/8 (12.5) | 0/11 (0.0) | 1/19 (5.3) | 0.23 |
| Abdominal distension, n/N (%) | 1/8 (12.5) | 4/11 (36.4) | 5/19 (26.3) | 0.24 |
| Abdominal tenderness, n/N (%) | 1/7 (14.3) | 3/11 (27.3) | 4/18 (22.2) | 0.52 |
| WCC (× 109/l), n, median (IQR) | 12, 8.5 (7.9–14.6) | 14, 11.7 (7.6–16.7) | 26, 9.6 (7.8–16.7) | 0.92 |
| Creatinine, n, median (IQR) | 9, 122 (64.0–207.0) | 13, 108 (58.0–133.0) | 22, 109 (58.0–155.0) | 0.43 |
| Sigmoidoscopy or colonoscopy performed, n/N (%) | 0/11 (0.0) | 1/15 (6.7) | 1/26 (3.8) | 0.38 |
| Severity classification,b n (%) | ||||
| 1 – mild | 7 (63.6) | 7 (50.0) | 14 (56.0) | 0.79 |
| 2 – moderate | 1 (9.1) | 2 (14.3) | 3 (12.0) | |
| 3 – severe | 3 (27.3) | 5 (35.7) | 8 (32.0) | |
Seven (0.5%) participants in the probiotic arm and 10 (0.7%) participants in the placebo arm had diarrhoea due to other causes (RR 0.70; 95% CI 0.27 to 1.84). In the probiotic arm, six had norovirus diarrhoea and one was diagnosed with non-specific colitis. In the placebo arm, six had norovirus diarrhoea, one had diarrhoea after taking laxatives, two patients attributed diarrhoea to drinking a large volume of fruit juice and one had melaena associated with abnormal clotting.
Overall, 2927/2940 (99.6%) participants took at least one dose of the IMP, with a similar proportion in the probiotic (1462/1469, 99.5%) and placebo arms (1465/1471, 99.6%; p = 0.78; compliance unknown for one participant in the probiotic arm). The median number of days that participants were observed or reported taking the IMP in the first 3 weeks was similar in the probiotic [n = 1469, 21 days (IQR 14–21 days)] and placebo arms [n = 1471, 21 days (IQR 14–21 days); p = 0.55; Figure 2]. The full 21-day course was completed by 52.5% of participants. Overall, 1076/2934 (36.7%) participants reported that they disliked taking the IMP, and this proportion was similar in the probiotic (529/1466, 36.1%) and placebo arms (547/1468, 37.3%; p = 0.51). Taking account of compliance in covariate analysis did not materially alter the risk of AAD (OR 1.02; 95% CI 0.80 to 1.30) or CDD (OR 0.66; 95% CI 0.30 to 1.47).
FIGURE 2.
Total number of days participants took the IMPs according to intervention arm.
Unused IMPs were collected opportunistically at three time points during the study, from participants who had withdrawn or died, for assessing correct identity according to active versus placebo and number of viable organisms in the probiotic preparation. Thirty-four probiotic capsules were tested and all contained ≥ 1.62 × 1010 viable bacteria. All of the 33 placebo capsules tested were sterile.
During the first 3 weeks while participants were taking the IMPs, the duration of hospital stay was similar in the probiotic [n = 1469, median 6 days (IQR 2–13 days)] and placebo arms [n = 1470, median 6 days (IQR 2–13 days); p = 0.65]. The most commonly reported gastrointestinal symptoms were nausea (14.9%), abdominal pain (13.4%) and diarrhoea (any loose stools reported by the participants; 12.3%; Table 17). The frequency of gastrointestinal symptoms was similar in the two study arms with the exception of flatus, which was marginally less common in the placebo than the probiotic arm (risk difference 2.3%; 95% CI 0.0% to 4.6%). Futhermore, although very few participants had a NGT in situ, this was significantly more common in the probiotic than placebo arm. With these two exceptions, the duration that symptoms were present was also similar in the two study arms (p > 0.17 for all comparisons).
| Variable | Probiotic,a n/N (%) | Placebo,a n/N (%) | Total, n/N (%) | p-value |
|---|---|---|---|---|
| Diarrhoea | 189/1460 (12.9) | 172/1464 (11.7) | 361/2924 (12.3) | 0.33 |
| Nocturnal diarrhoea | 55/1459 (3.8) | 51/1464 (3.5) | 106/2923 (3.6) | 0.68 |
| Faecal incontinence | 46/1460 (3.2) | 53/1463 (3.6) | 99/2923 (3.4) | 0.48 |
| Tenesmus | 22/1458 (1.5) | 22/1464 (1.5) | 44/2922 (1.5) | 0.99 |
| Abdominal pain | 200/1458 (13.7) | 193/1464 (13.2) | 393/2922 (13.4) | 0.67 |
| Nausea | 228/1458 (15.6) | 207/1462 (14.2) | 435/2920 (14.9) | 0.26 |
| Vomiting | 124/1459 (8.5) | 110/1463 (7.5) | 234/2922 (8.0) | 0.33 |
| Bloating | 155/1457 (10.6) | 143/1464 (9.8) | 298/2921 (10.2) | 0.44 |
| Flatus | 183/1459 (12.5) | 149/1462 (10.2) | 332/2921 (11.4) | 0.045 |
| NGT in situ | 8/1460 (0.5) | 1/1463 (0.1) | 9/2923 (0.3) | 0.019 |
| Other morbidity | 442/1462 (30.2) | 463/1468 (31.5) | 905/2930 (30.9) | 0.44 |
| Sought consultation for new health problem | 238/1469 (16.2) | 257/1471 (17.5) | 495/2940 (16.8) | 0.36 |
There were no statistically significant differences in either the frequency or duration of gastrointestinal symptoms or other morbidity according to study arm during weeks 4–8 of the study (data not shown). Overall, average duration of hospital stay was known in 2899 participants and was similar in the probiotic [n = 1452, median 4 days (IQR 1–11 days)] and placebo arms [n = 1447, median 4 days (IQR 1–11 days); p = 0.87; Figure 3].
FIGURE 3.
Duration of hospital stay according to intervention arm.
Eighteen participants were excluded from PP analysis. Seven patients in the probiotic and six in the placebo arm declined to take any of the IMPs. Investigation of the IMP labelling error that occurred at one centre resulted in the IMPs being withdrawn before completion of the 21-day course in one participant in the probiotic arm and four in the placebo arm. Among these participants excluded from PP analysis, none developed CDD, but one allocated to the probiotic arm developed AAD. Analysis of primary outcomes in the PP population did not materially alter the assessment of the efficacy of the intervention (data not shown). In addition, the risk of developing AAD or CDD was as similar among those participants who took all 21 IMP doses, 14 or more doses or seven or more doses as it was in all participants (data not shown).
Serious adverse events
Serious adverse events were common in the study population with 578 (19.7%) participants experiencing one or more SAE (Table 18). The most common MedDRA SOC classifications for SAEs were respiratory, thoracic and mediastinal disorders, gastrointestinal disorders, and cardiac disorders.
| SOC | Probiotic (n = 1470) (%) | Placebo (n = 1471) (%) | Total (n = 2941) (%) |
|---|---|---|---|
| Blood and lymphatic system disorders | 7 (0.5) | 5 (0.3) | 12 (0.4) |
| Cardiac disorders | 42 (2.9) | 28 (1.9) | 70 (2.4) |
| Ear and labyrinth disorders | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Eye disorders | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Gastrointestinal disorders | 44 (3.0) | 35 (2.4) | 79 (2.7) |
| General disorders and administration site conditions | 14 (1.0) | 9 (0.6) | 23 (0.8) |
| Immune system disorders | 0 (0.0) | 4 (0.3) | 4 (0.1) |
| Infections and infestations | 20 (1.4) | 23 (1.6) | 43 (1.5) |
| Injury, poisoning and procedural complications | 20 (1.4) | 21 (1.4) | 41 (1.4) |
| Investigations | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Metabolism and nutrition disorders | 3 (0.2) | 9 (0.6) | 12 (0.4) |
| Musculoskeletal and connective tissue disorders | 4 (0.3) | 4 (0.3) | 8 (0.3) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 11 (0.7) | 11 (0.7) | 22 (0.7) |
| Nervous system disorders | 13 (0.9) | 15 (1.0) | 28 (1.0) |
| Psychiatric disorders | 3 (0.2) | 0 (0.0) | 3 (0.1) |
| Renal and urinary disorders | 22 (1.5) | 25 (1.7) | 47 (1.6) |
| Respiratory, thoracic and mediastinal disorders | 83 (5.6) | 87 (5.9) | 170 (5.8) |
| Skin and subcutaneous tissue disorders | 13 (0.9) | 4 (0.3) | 17 (0.6) |
| Social circumstances | 4 (0.3) | 1 (0.1) | 5 (0.2) |
| Surgical and medical procedures | 9 (0.6) | 11 (0.7) | 20 (0.7) |
| Vascular disorder | 8 (0.5) | 7 (0.5) | 15 (0.5) |
| Unclassifiedc | 5 (0.3) | 4 (0.3) | 9 (0.3) |
| Any SAE | 294 (20.0) | 284 (19.3) | 578 (19.7) |
Serious adverse events classified as gastrointestinal disorders occurred in 79 (2.7%) participants with a similar frequency in both study arms (see Table 18). With SAEs classified according to MedDRA PTs43 (see Appendix 9, Table 32), gastrointestinal haemorrhage occurred in 15 participants in the probiotic arm (specified as upper gastrointestinal haemorrhage in four participants and lower in five participants) and 11 participants in the placebo arm (specified as upper gastrointestinal haemorrhage in one participant and lower in seven participants). Peptic ulcer occurred in four participants in the probiotic arm (specified as duodenal ulcer in one participant and perforated peptic ulcer in two participants) and one participant in the placebo arm experienced a perforated duodenal ulcer. Abdominal pain occurred in four participants in the probiotic arm and three in the placebo arm. Gastroenteritis occurred in three participants in the probiotic arm and two in the placebo arm. Constipation occurred in one participant in the probiotic arm and two in the placebo arm. Peritonitis, volvulus and dysentery each occurred in one participant in the probiotic arm and appendix abscess, colostomy performed, diarrhoea, liver abscess and pancreatitis each occurred in one participant in the placebo arm. There was no occurrence of intestinal ischaemia.
The frequency of SAEs that were, or may have been, due to bacterial infection occurred with similar frequency in each arm (see Appendix 9, Table 32). Pneumonia occurred in 52 participants in the probiotic arm (specified as caused by Pseudomonas sp. in two of these participants) and 53 in the placebo arm (specified as caused by Pseudomonas sp. in three of these participants). Abscesses occurred in one participant in the probiotic arm (specifically, a groin abscess) and four participants in the placebo arm (specifically groin, mediastinal, liver and psoas abscesses). Urinary tract infection occurred in 15 participants in the probiotic arm and 12 in the placebo arm, wound infection or cellulitis occurred in 16 participants in the probiotic arm and nine in the placebo arm, infected implant site occurred in two participants in the probiotic arm and one in the placebo arm and infected haematoma occurred in one participant in the probiotic arm. Sepsis occurred in 10 participants in the probiotic and 12 in the placebo arm and organ failure in one participant in each study arm.
The most frequent SAEs classified according to MedDRA PTs43 were pneumonia (3.3%), obstructive pulmonary disorder (1.6%) and falls (1.1%). Overall, 143 (4.9%) participants experienced a SAE that resulted in death, 10 (0.3%) SAEs that were considered to be life-threatening, 447 (15.2%) SAEs that prolonged hospitalisation, four (0.1%) SAEs that resulted in persistent or significant disability or incapacity and 11 (0.4%) experienced other SAEs that were considered to be significant medical events (see Appendix 9, Table 32). The proportion of patients in each SAE severity category, the frequency of individual SAEs within each category and the proportion of participants experiencing one or more SAE were similar in the two study arms. (see Appendix 9, Table 32).
Following the occurrence of a SAE, the patient’s clinical team withdrew the IMP from 14 (0.5%) participants and discontinued them temporarily in 90 (3.1%) participants, with a similar proportion in each study arm (see Appendix 9, Table 33). A common reason for discontinuing the IMPs was to reduce the number of medications for the patient rather than any concern regarding the safety of the bacterial organisms.
Quality of life analysis
European Quality of Life-5 Dimensions
There was a tendency for the EQ-5D visual analogue scale (VAS) and index values to increase over time from baseline to 4 weeks and then 8 weeks, indicating an improvement in health status over time within each of the study arms. Median scores were similar in the two study arms (Table 19).
| Visit | Probiotic,a n, median (IQR) | Placebo,a n, median (IQR) | Total, n, median (IQR) |
|---|---|---|---|
| VAS | |||
| Baseline | 1432, 50.0 (40.0–70.0) | 1435, 50.0 (40.0–70.0) | 2867, 50.0 (40.0–70.0) |
| 4 weeks | 1160, 60.0 (50.0–75.0) | 1197, 60.0 (50.0–75.0) | 2357, 60.0 (50.0–75.0) |
| 8 weeks | 1133, 60.0 (50.0–78.0) | 1151, 65.0 (50.0–80.0) | 2284, 61.0 (50.0–80.0) |
| Index values | |||
| Baseline | 1457, 0.62 (0.22–0.74) | 1461, 0.62 (0.24–0.74) | 2918, 0.62 (0.22–0.74) |
| 4 weeks | 1180, 0.69 (0.52–0.81) | 1219, 0.69 (0.52–0.81) | 2399, 0.69 (0.52–0.81) |
| 8 weeks | 1153, 0.71 (0.59–0.81) | 1178, 0.69 (0.59–0.82) | 2331, 0.69 (0.59–0.81) |
The change from baseline EQ-5D VAS to that at 4 weeks was similar in both study arms but at 8 weeks there was a statistically significant difference between the two arms. However, this was a change of less than 2 points on the 100-point scale and, therefore, is unlikely to represent a clinically important change in health status (Table 20).
| Comparison | Difference (95% CI) | p-value |
|---|---|---|
| VAS | ||
| Probiotic vs. placebo at 4 weeks | −0.44 (−1.98 to 1.11) | 0.58 |
| Probiotic vs. placebo at 8 weeks | −1.76 (−3.32 to −0.19) | 0.028 |
| Index values | ||
| Probiotic vs. placebo at 4 weeks | 0.00 (−0.02 to 0.03) | 0.74 |
| Probiotic vs. placebo at 8 weeks | 0.01 (−0.01 to 0.03) | 0.45 |
Generic Short Form questionnaire-12 items version 2
As with the EQ-5D, there was a tendency for SF-12 v2, MCS, PCS and subdomain scores, with the exception of vitality, to increase over time, also indicating that the level of health increased in both study arms (Table 21).
| Subdomains and component summaries | Probiotic,a n, median (IQR) | Placebo,a n, median (IQR) | Total, n, median (IQR) |
|---|---|---|---|
| Physical function | |||
| Baseline | 1458, 25.0 (0.0–50.0) | 1461, 25.0 (0.0–50.0) | 2919, 25.0 (0.0–50.0) |
| 4 weeks | 1179, 25.0 (0.0–50.0) | 1214, 25.0 (0.0–50.0) | 2393, 25.0 (0.0–50.0) |
| 8 weeks | 1150, 50.0 (0.0–50.0) | 1176, 50.0 (0.0–50.0) | 2326, 50.0 (0.0–50.0) |
| Role physical | |||
| Baseline | 1458, 37.5 (25.0–62.5) | 1461, 37.5 (25.0–62.5) | 2919, 37.5 (25.0–62.5) |
| 4 weeks | 1179, 50.0 (25.0–75.0) | 1214, 37.5 (25.0–75.0) | 2393, 42.5 (25.0–75.0) |
| 8 weeks | 1150, 50.0 (25.0–75.0) | 1176, 50.0 (25.0–75.0) | 2326, 50.0 (25.0–75.0) |
| Bodily pain | |||
| Baseline | 1458, 50.0 (25.0–100.0) | 1461, 50.0 (25.0–100.0) | 2919, 50.0 (25.0–100.0) |
| 4 weeks | 1179, 75.0 (50.0–100.0) | 1214, 75.0 (50.0–100.0) | 2393, 75.0 (50.0–100.0) |
| 8 weeks | 1150, 75.0 (50.0–100.0) | 1176, 75.0 (50.0–100.0) | 2326, 75.0 (50.0–100.0) |
| General health | |||
| Baseline | 1458, 25.0 (25.0–60.0) | 1461, 25.0 (25.0–60.0) | 2919, 25.0 (25.0–60.0) |
| 4 weeks | 1179, 60.0 (60.0–85.0) | 1214, 60.0 (60.0–85.0) | 2393, 60.0 (60.0–85.0) |
| 8 weeks | 1150, 60.0 (25.0–60.0) | 1176, 60.0 (25.0–67.5) | 2326, 60.0 (25.0–60.0) |
| Vitality | |||
| Baseline | 1458, 25.0 (0.0 to 50.0) | 1461, 25.0 (0.0 to 50.0) | 2919, 25.0 (0.0 to 50.0) |
| 4 weeks | 1179, 25.0 (0.0 to 50.0) | 1214, 25.0 (0.0 to 50.0) | 2393, 25.0 (0.0 to 50.0) |
| 8 weeks | 1150, 25.0 (25.0 to 50.0) | 1176, 25.0 (25.0 to 50.0) | 2326, 25.0 (25.0 to 50.0) |
| Social function | |||
| Baseline | 1458, 50.0 (25.0 to 75.0) | 1461, 50.0 (0.0 to 100.0) | 2919, 50.0 (0.0 to 100.0) |
| 4 weeks | 1179, 50.0 (25.0 to 75.0) | 1214, 50.0 (25.0 to 75.0) | 2393, 50.0 (25.0 to 75.0) |
| 8 weeks | 1150, 75.0 (25.0 to 100.0) | 1176, 75.0 (25.0 to 100.0) | 2326, 75.0 (25.0 to 100.0) |
| Role emotional | |||
| Baseline | 1458, 70.5 (37.5 to 100.0) | 1461, 62.5 (37.5 to 100.0) | 2919, 62.5 (37.5 to 100.0) |
| 4 weeks | 1179, 75.0 (50.0 to 100.0) | 1214, 75.0 (50.0 to 100.0) | 2393, 75.0 (50.0 to 100.0) |
| 8 weeks | 1150, 75.0 (50.0 to 100.0) | 1176, 75.0 (50.0 to 100.0) | 2326, 75.0 (50.0 to 100.0) |
| Mental health | |||
| Baseline | 1458, 62.5 (50.0 to 87.5) | 1461, 62.5 (50.0 to 87.5) | 2919, 62.5 (50.0 to 87.5) |
| 4 weeks | 1179, 75.0 (50.0 to 87.5) | 1214, 75.0 (50.0 to 87.5) | 2393, 75.0 (50.0 to 87.5) |
| 8 weeks | 1150, 75.0 (60.0 to 87.5) | 1176, 75.0 (50.0 to 87.5) | 2326, 75.0 (55.9 to 87.5) |
| MCS | |||
| Baseline | 1458, 33.3 (26.2 to 41.9) | 1461, 33.32 (25.8 to 41.9) | 2919, 33.32 (25.9 to 41.9) |
| 4 weeks | 1179, 37.50 (30.9 to 43.8) | 1214, 37.63 (30.5 to 43.6) | 2393, 37.51 (30.7 to 43.7) |
| 8 weeks | 1150, 38.12 (28.3 to 45.4) | 1176, 38.35 (29.3 to 45.5) | 2326, 38.25 (28.9 to 45.4) |
| PCS score | |||
| Baseline | 1458, 45.8 (36.0 to 54.4) | 1461, 45.4 (36.2 to 54.4) | 2919, 45.5 (36.2 to 54.4) |
| 4 weeks | 1179, 48.0 (38.2 to 55.3) | 1214, 47.94 (37.9 to 55.4) | 2393, 47.98 (38.1 to 55.4) |
| 8 weeks | 1150, 51.0 (41.0 to 57.0) | 1176, 50.64 (40.2 to 56.8) | 2326, 50.86 (40.6 to 56.8) |
Analysis of changes from baseline in SF-12 v2 summary and subdomain scores at 4 weeks and 8 weeks by treatment allocated showed no statistically significant differences between the two study arms (Table 22).
| Comparison | Difference (95% CI) | p-value |
|---|---|---|
| Physical function | ||
| Probiotic vs. placebo at 4 weeks | 0.61 (−1.95 to 3.17) | 0.64 |
| Probiotic vs. placebo at 8 weeks | −0.28 (−2.87 to 2.31) | 0.83 |
| Role physical | ||
| Probiotic vs. placebo at 4 weeks | 1.44 (−1.04 to 3.92) | 0.26 |
| Probiotic vs. placebo at 8 weeks | 0.91 (−1.61 to 3.42) | 0.48 |
| Bodily pain | ||
| Probiotic vs. placebo at 4 weeks | 0.63 (−1.52 to 2.77) | 0.57 |
| Probiotic vs. placebo at 8 weeks | 0.49 (−1.68 to 2.66) | 0.66 |
| General health | ||
| Probiotic vs. placebo at 4 weeks | −0.88 (−3.05 to 1.30) | 0.43 |
| Probiotic vs. placebo at 8 weeks | −1.29 (−3.50 to 0.92) | 0.25 |
| Vitality | ||
| Probiotic vs. placebo at 4 weeks | −1.26 (−3.21 to 0.68) | 0.20 |
| Probiotic vs. placebo at 8 weeks | −1.49 (−3.46 to 0.48) | 0.14 |
| Social function | ||
| Probiotic vs. placebo at 4 weeks | 0.19 (−2.58 to 2.97) | 0.89 |
| Probiotic vs. placebo at 8 weeks | −0.55 (−3.36 to 2.26) | 0.70 |
| Role emotional | ||
| Probiotic vs. placebo at 4 weeks | 0.88 (−1.54 to 3.29) | 0.48 |
| Probiotic vs. placebo at 8 weeks | 1.77 (−0.68 to 4.22) | 0.16 |
| Mental health | ||
| Probiotic vs. placebo at 4 weeks | 0.55 (−1.16 to 2.26) | 0.53 |
| Probiotic vs. placebo at 8 weeks | 1.33 (−0.40 to 3.06) | 0.13 |
| MCS | ||
| Probiotic vs. placebo at 4 weeks | 0.29 (−0.63 to 1.20) | 0.54 |
| Probiotic vs. placebo at 8 weeks | −0.51 (−1.44 to 0.41) | 0.28 |
| PCS score | ||
| Probiotic vs. placebo at 4 weeks | 0.13 (−0.76 to 1.03) | 0.77 |
| Probiotic vs. placebo at 8 weeks | 0.51 (−0.39 to 1.41) | 0.27 |
Economic analysis
Resource use and costs
Health-care contacts
Average duration of initial hospital stay was 0.03 days longer in the probiotic arm than in the placebo arm (Table 23). Overall, during the 8-week follow-up period, 18.3% patients were readmitted to hospital with a similar frequency in each study arm; however, patients in the probiotic arm remained in hospital for 0.62 days less than those in the placebo arm during each readmission. In the probiotic arm, 38.4% of patients reported other health-care contacts for a new problem compared with 40.9% in the placebo arm (costed as GP visits) and spent an additional 0.11 days, on average, in care facilities. None of these differences was statistically significant.
| Health-care contact | Probiotic (n = 1470) | Placebo (n = 1471) | Total (n = 2941) |
|---|---|---|---|
| Mean duration of initial hospital stay in days (95% CI) | 17.38 (16.36 to 18.39) | 17.35 (16.31 to 18.39) | 17.36 (16.63 to 18.09) |
| Number of readmissions (%) | 260 (17.7) | 279 (19.0) | 539 (18.3) |
| Mean duration of readmission inpatient stay in days (95% CI) | 10.99 (9.78 to 12.19) | 11.61 (10.38 to 12.84) | 11.31 (10.45 to 12.17) |
| Mean number of other health-care contacts per patient | 0.69 | 0.73 | 0.71 |
| Mean number of days in care home per patient (95% CI) | 3.73 (3.17 to 4.28) | 3.62 (3.08 to 4.15) | 3.67 (3.29 to 4.06) |
The mean cost of health-care contacts per patient was similar in the two trial arms (Table 24).
| Health-care contact | Probiotic (95% CI) | Placebo (95% CI) | p-value |
|---|---|---|---|
| Mean cost of initial hospital stay (£) | 5806.60 (5467.94 to 6145.27) | 5797.66 (5450.63 to 6144.68) | 0.97 |
| Mean cost of readmissions (£) | 3672.01 (3268.90 to 4075.12) | 3879.50 (3468.30 to 4290.70) | 0.48 |
| Mean cost of other health-care contacts (£) | 64.47 (57.50 to 71.44) | 63.85 (59.45 to 68.25) | 0.88 |
| Mean cost of care home (£) | 2680.96 (2464.09 to 2897.82) | 2505.79 (2298.39 to 2713.18) | 0.25 |
Antibiotics
The mean cost of antibiotics was £105.38 in the probiotic arm and £90.94 in the placebo arm. Staff costs for administration of antibiotics was £759.71 in the probiotic and £738.34 in the placebo arm. Overall antibiotics cost per patient was £35.80 less in the placebo arm than in the probiotic arm, but the difference was not statistically significant (Table 25).
| Health event | Probiotic (95% CI) | Placebo (95% CI) | p-value |
|---|---|---|---|
| Mean cost of antibiotics including staff time (£) | 865.09 (816.65 to 913.54) | 829.29 (780.38 to 878.19) | 0.31 |
| Mean cost of probiotic including staff time (£) | 73.02 (70.20 to 75.83) | – | – |
| Episode of diarrhoea (all causes included) (£) | 1817.20 (1519.88 to 2114.52) | 2219.83 (1725.61 to 2714.06) | 0.17 |
| Mean cost of an episode of AAD (£) | 1742.15 (1438.39 to 2045.92) | 2220.38 (1696.52 to 2744.23) | 0.12 |
| Mean total cost (£) | 8020.11 (7622.31 to 8417.90) | 8011.37 (7600.53 to 8422.22) | 0.98 |
Intervention implementation
The mean nursing time required to administer the probiotic course was 39 minutes at a cost of £63.02. Including the retail cost of the formulation and accounting for duration of hospital stay, the mean implementation cost of the probiotic was £73.02 (range £10.00–179.68; Table 25). No adverse events requiring additional health-care contacts were observed.
Episodes of diarrhoea
A summary of costs associated with gastroenteritis while patients are in hospital and collected outside the trial can be found in Table 26. When all causes of diarrhoea were included but the costs of antibiotics, other health-care contacts and increased duration of hospital stay were excluded, an episode of diarrhoea cost £402.63 more in the placebo arm than in the probiotic arm. When only AAD was considered, the differential cost was £478.23 more in the placebo arm than in the probiotic arm (see Table 25). However, these differences were not statistically significant.
| Cost component | Base case (£) | Range (£) |
|---|---|---|
| Per patient episode | ||
| Microbiology at ABMUHB hospitals | 54.94 | 30.17–109.88 |
| Microbiology at CDDFT hospitals | 45.28 | 27.51–90.56 |
| Diagnostic and therapeutic procedures | 69.65 | 49.25–101.25 |
| Clinical assessment and review | 194.76 | 103.01–259.67 |
| End cleaning | 20.76 | 16.62–24.91 |
| Per day | ||
| Bed and ward closures | 415.57 | 0–497.61 |
| Daily cleaning | 9.54 | 7.63–11.45 |
| Per stool | ||
| Disposables and staff time | 8.38 | 6.70–10.06 |
| Spot cleaning and changing | 32.85 | 26.28–39.42 |
Independent of study arm, the mean duration of hospital stay was 22.31 days for patients with AAD versus 16.73 days for non-diarrhoea patients. This difference of 5.58 days (95% CI 2.78 to 8.39 days) accrued, on average, £4531.36 (95% CI £3439.80 to £5622.92) more health-care costs (p = 0.01). In addition to increased length of hospital stay, the main cost drivers for this difference were additional costs of £1976.66 (95% CI £1677.24 to £2276.09) attributed to assessment and management of diarrhoea episodes including microbiology, staff time, diagnostics, cleaning, laundry and infection control measures.
Total health-care cost
Total health-care cost was £8.74 greater in the probiotic arm than in the placebo arm (see Table 25). According to our analysis, the main cost drivers that make up a high proportion of the total health-care costs were the duration of the initial hospital stay and readmissions, staff time for antibiotic and probiotic administration and diarrhoea-associated costs (including microbiology, clinical review and assessment, diagnostic and therapeutic procedures, disposables, cleaning, laundry and infection control procedures).
Utility and quality-adjusted life-years
Mean EQ-5D index values at the baseline were 0.51 for both the probiotic and placebo arms, and this value increased over time. At 4 weeks, scores for both trial arms were 0.60, and at 8 weeks this had further increased to 0.64 in the probiotic and 0.63 in the placebo arm. The slightly better 8-week follow-up outcome for the patients who were administered the probiotic (average utility difference of 0.01) was not statistically significant. Extrapolated to 1 year, the total QALY gain in the probiotic group was 0.0004 as no further QALY gain was to be expected after 8 weeks and any further changes in QoL would probably be due to general recovery (Table 27).
| Outcome | Difference: probiotic minus placebo | p-value |
|---|---|---|
| Incremental utility – 8 weeks (95% CI) | 0.01 (−0.01 to 0.03) | 0.45 |
| Incremental QALY – 1 year (95% CI) | 0.0004 (−0.0006 to 0.0014) | – |
| Incremental total health-care cost (95% CI) | £8.74 (−£4.32 to £21.78) | 0.98 |
| ICER (1 year) | £22,701 per QALY | |
| Probability cost-effective at £20,000 | 0.48 | |
| Probability cost-effective at £30,000 | 0.54 | |
| Probiotic implementation cost (95% CI) | £73.02 (£70.20 to £75.83) | |
| ICER (1 year) | £189,662 per QALY | |
| Probability cost-effective at £20,000 | < 0.01 | |
| Probability cost-effective at £30,000 | 0.02 | |
Cost-effectiveness and uncertainty
Base-case analysis showed only a small total health-care cost difference between the probiotic and placebo arms (see Table 25). This was mainly due to the relatively small implementation cost of the probiotic and the marginal cost savings for diarrhoea episodes in the probiotic arm. The cost difference resulted in an ICER of £22,701 per QALY at 1 year with a probability of the intervention being cost-effective at a £20,000 willingness-to-pay threshold of 48%. The CEAC depicts the probability of the intervention being cost-effective at different willingness-to-pay thresholds. (Figure 4).
FIGURE 4.
Cost-effectiveness acceptability curve, base case analysis: total health-care cost.
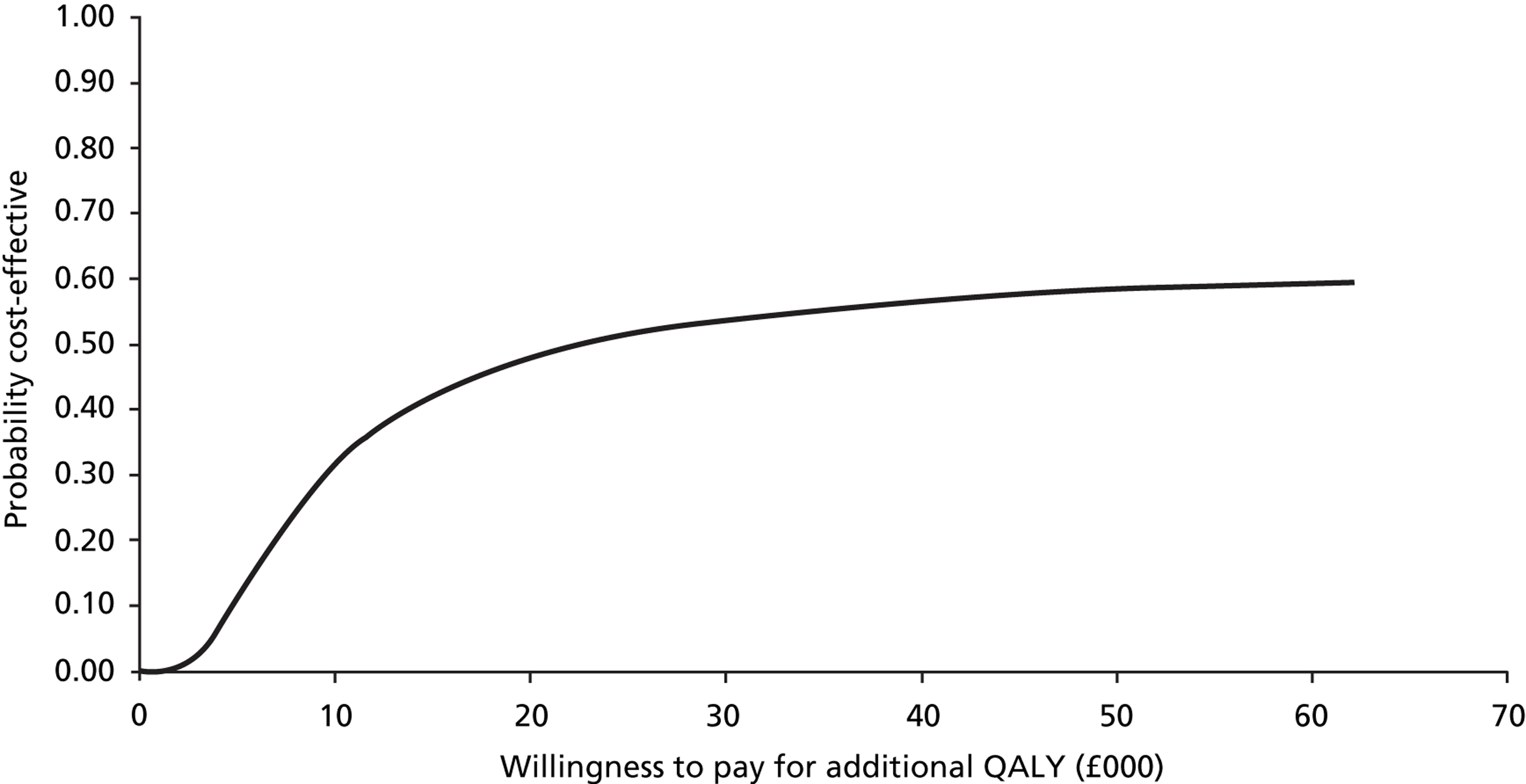
If the implementation costs of the probiotics only are taken into account, without consideration of any downstream effects, the ICER increases to £189,662 per QALY at 1 year with a probability of cost-effectiveness at £30,000 of 2% (Figure 5). Thus, based on a £30,000 willingness-to-pay threshold and implementation costs, probiotics are not cost-effective.
FIGURE 5.
Cost-effectiveness acceptability curve, base case analysis: probiotics implementation cost.
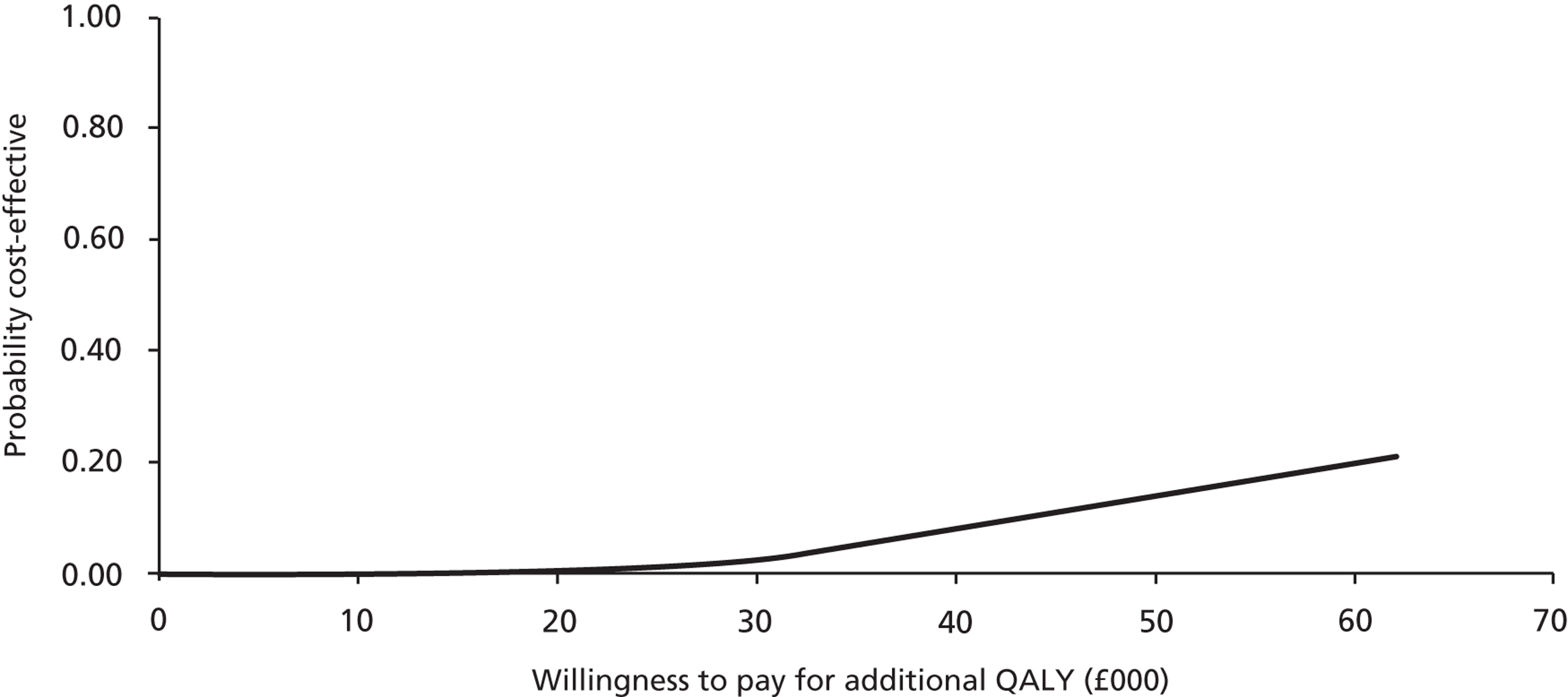
Results of the cost–consequences analysis are reported in Table 28. As overall differences in costs and clinical outcomes between the two arms were small, the clinical effectiveness and cost-effectiveness of probiotics in the prevention of AAD in this study can be considered limited. Even though probiotics appeared cost-effective in the cost–utility analysis based on total health-care costs, no significant budgetary impact can be anticipated. This is due to the small differences in total cost between the probiotic and placebo arms and the lack of statistical significance in the primary outcomes. Subgroup analysis was not undertaken, as the covariate analysis did not identify any specific population that clearly benefited from receiving the probiotic. Cost per case of diarrhoea averted was not analysed as the study did not demonstrate a difference in diarrhoea frequency between the two groups.
| Impact variable | Probiotic | Placebo | Difference | p-value |
|---|---|---|---|---|
| Costs impact | ||||
| Implementation cost per patient (£) | 73.02 (70.20 to 75.83) | 0.00 (0.00 to 0.00) | 73.02 (70.20 to 75.83) | 0.01 |
| Total health-care cost per patient (£) | 8020.11 (7622.31 to 8417.90) | 8011.37 (7600.53 to 8422.22) | 8.74 (−4.32 to 21.78) | 0.98 |
| Cost of AAD episode (£) | 1742.15 (1438.39 to 2045.92) | 2220.38 (1696.52 to 2744.23) | −478.23 (−1192.34 to 235.89) | 0.12 |
| QoL impact | ||||
| EQ-5D scores at baseline | 0.51 (SD 0.341) | 0.51 (SD 0.332) | 0.00 | – |
| EQ-5D changes baseline – 4 weeks | 0.08 (SD 0.343) | 0.08 (SD 0.334) | 0.00 (−0.03 to 0.03) | 0.74 |
| EQ-5D changes baseline – 8 weeks | 0.12 (SD 0.348) | 0.11 (SD 0.352) | 0.01 (−0.01 to 0.03) | 0.45 |
| Health impact | ||||
| Number of AAD cases per arm | 159/1470 (10.8%) | 153/1471 (10.4%) | 6 (0.4%) | 0.72 |
| Number of CDD cases per arm | 12/1470 (0.8%) | 17/1471 (1.2%) | −5 (−0.4%) | 0.35 |
Sensitivity analysis
Changes in the parameters included in the microcosting of a diarrhoea episode and changes in the cost of an inpatient day (the average cost per day amounted by a patient while in hospital) did not result in significant changes to the difference in overall cost between the probiotic and the placebo arms (Table 29). Furthermore, a decrease or increase in staff time for probiotic administration did not significantly change the cost-effectiveness results (Table 30). Considering probiotic implementation costs only, a reduction in staff time by 50% resulted in an ICER of £107,818 per QALY and a probability of cost-effectiveness at £30,000 of 16%, whereas doubling of staff time increased the ICER to £353,402 per QALY and was associated with a probability of cost-effectiveness at £30,000 of < 1%. The CEACs for these results can be found in Figures 6 and 7.
| Parameter changed | Mean health-care cost per patient | |||
|---|---|---|---|---|
| Probiotic (95% CI) | Placebo (95% CI) | Difference | p-value | |
| Costing of diarrhoea (£) | ||||
| All diarrhoea costs lower value | 7871.70 (7483.57 to 8259.83) | 7843.83 (7445.22 to 8242.44) | 27.87 | 0.92 |
| All diarrhoea costs upper value | 8095.37 (7694.56 to 8496.19) | 8111.94 (7691.43 to 8532.46) | −16.57 | 0.96 |
| Other costs (£) | ||||
| Hospital inpatient day lower value | 6716.00 (6389.38 to 7042.61) | 6699.56 (6359.39 to 7039.73) | 16.14 | 0.95 |
| Hospital inpatient day upper value | 9298.27 (8833.97 to 9762.58) | 9312.79 (8831.04 to 9794.54) | −14.52 | 0.97 |
| Probiotic implementation cost (£) | Cost per patient (95% CI) | ICER | Probability cost-effective |
|---|---|---|---|
| Mean staff time per dose lower value | 41.51 (40.11 to 42.92) | 107,818 per QALY | 0.046 |
| Mean staff time per dose upper value | 136.06 (130.44 to 141.68) | 353,402 per QALY | 0.00 |
FIGURE 6.
Cost-effectiveness acceptability curve, sensitivity analysis: probiotic implementation cost when staff cost halved.
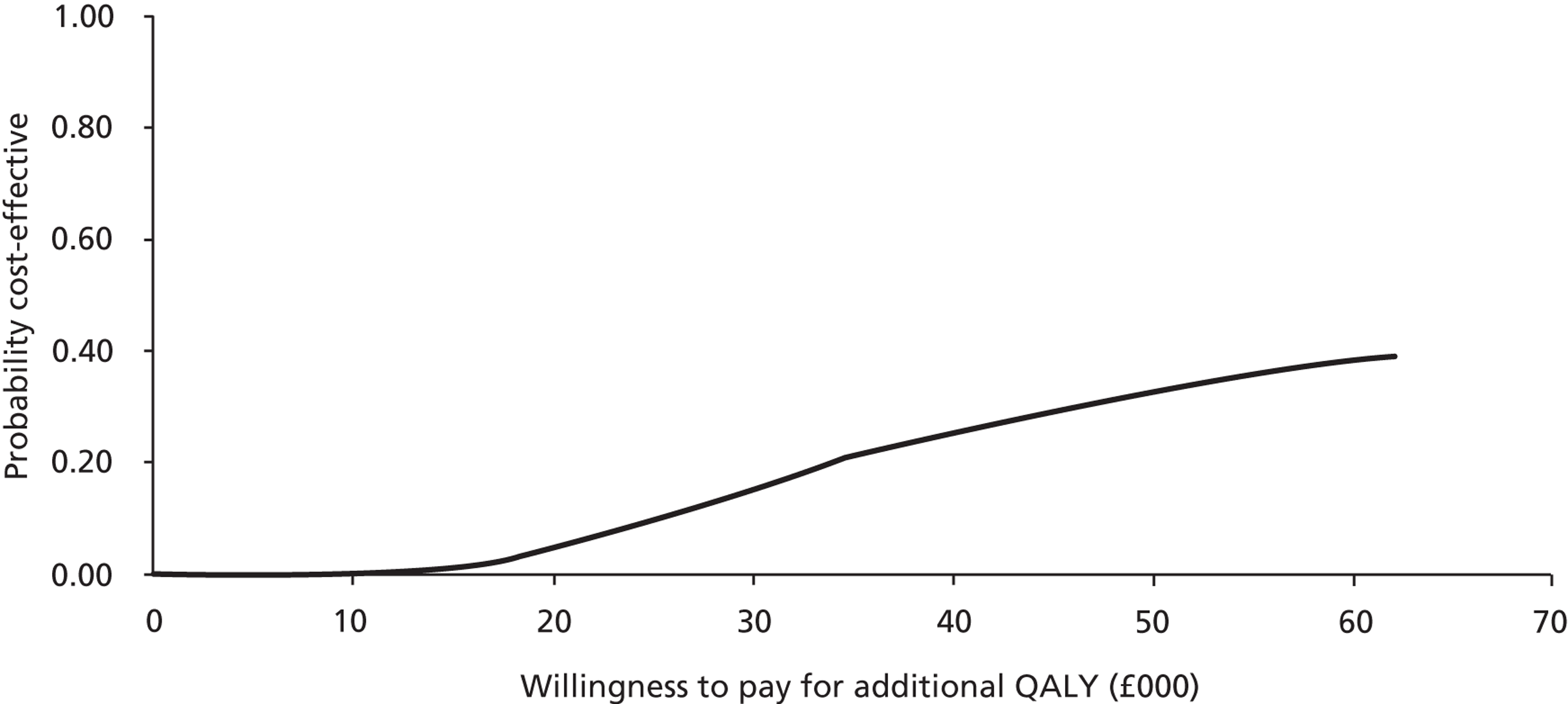
FIGURE 7.
Cost-effectiveness acceptability curve, sensitivity analysis: probiotic implementation cost when staff cost doubled.
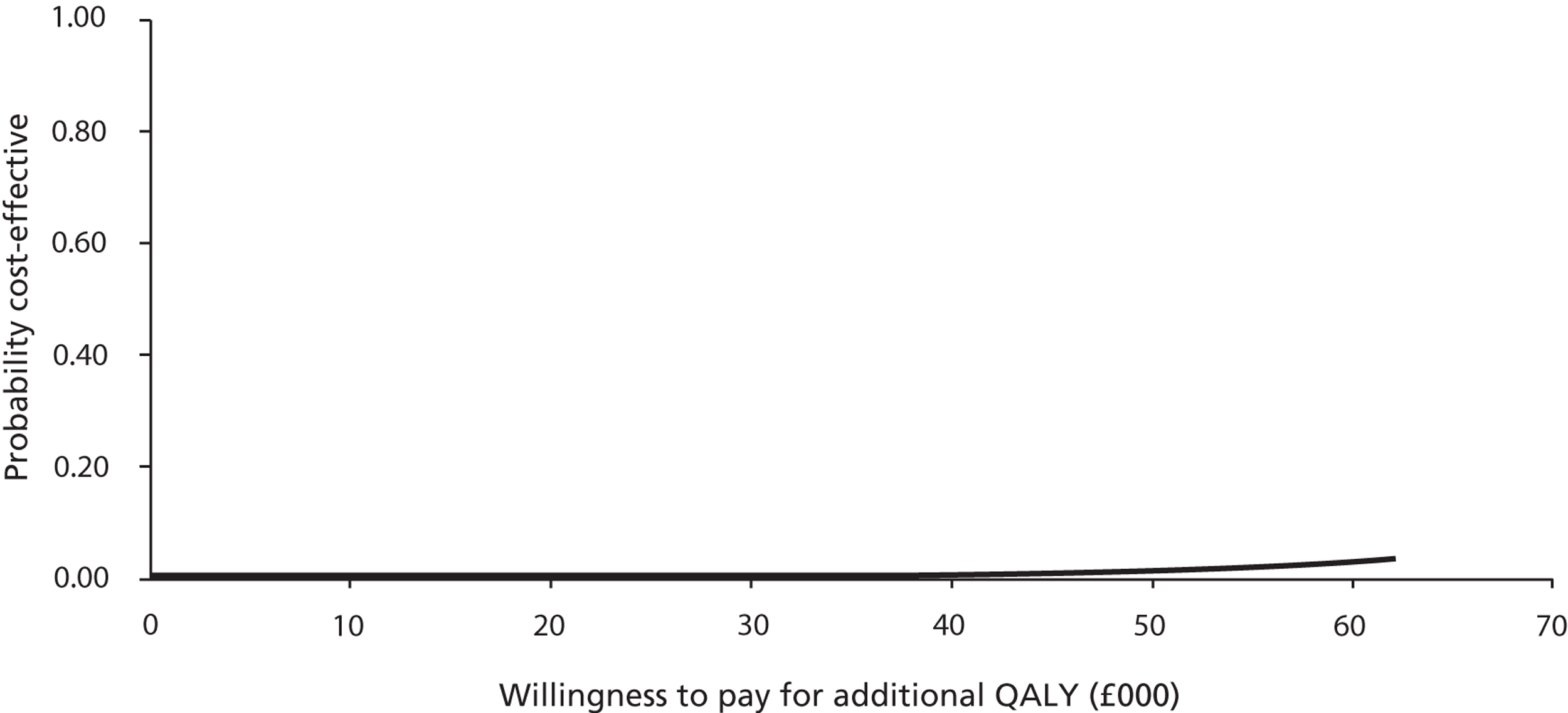
Summary of cost-effectiveness results
-
Cost and duration of hospital stays, cost of diarrhoea, cost of antibiotics and total health-care cost per patient were very similar between the probiotic and the placebo arms. No statistically significant cost differences were found between the two study arms.
-
Incremental total health-care cost of participants who suffered from AAD was £4531.36 (95% CI £3439.80 to £5622.92), which was significantly higher than for non-diarrhoea patients, independent of study arm. This was mainly due to increased length of hospital stay and additional diarrhoea-associated costs.
-
Duration of hospital stay (initial stay and readmissions), staff time for antibiotic administration and diarrhoea-associated costs were identified as main components of the total health-care costs across both study arms.
-
Between baseline and 8 weeks, mean QoL increase was 0.01 QALY higher in the probiotic than in the placebo arm; however, this difference was not statistically significant.
-
The ICER associated with probiotic use at 1 year was estimated at £22,701 per QALY gained when total health-care costs were considered and £189,662 per QALY gained considering probiotic implementation costs only. However, the similarity in total cost, number of diarrhoea cases and patient QoL between the two trial arms limits the relevance of the ICERs.
-
One-way sensitivity analyses did not show any significant effect on difference in total health-care costs between the trial arms and the overall conclusion of the cost-effectiveness assessment.
Chapter 5 Discussion
Here we report the results of a RCT that evaluated the clinical effectiveness and cost-effectiveness of a high-dose, multistrain bacterial preparation in the prevention or amelioration of AAD and CDD. PLACIDE was designed and undertaken in response to an increased frequency and severity of CDD in hospitals in industrialised countries8,9 and a meta-analysis29 that highlighted the need for more high-quality evidence regarding the role of probiotics. We aimed to undertake a pragmatic trial of older people receiving antibiotics in secondary health-care settings representative of those in industrialised countries and with the causes of diarrhoea determined by routine laboratory practice. This section summarises the key findings, compares our results with published studies, considers the strengths and limitations of PLACIDE and discusses the clinical and research implications of our findings.
Key findings
In an analysis according to treatment allocated, we did not find adequate evidence that a preparation of four strains of live lactobacilli and bifidobacteria was clinically effective in preventing or ameliorating AAD in 2941 inpatients aged between 65.0 and 107.5 years. Overall, AAD occurred in 312 (10.6%) participants, 159 (10.8%) participants in the probiotic arm and 153 (10.4%) participants in the placebo arm (RR 1.04; 95% CI 0.84 to 1.28; p = 0.72). CDD was an uncommon cause of AAD, occurring in 29 (1.0%) participants. CDD occurred less frequently in the probiotic (12 participants; 0.82%) arm than in the placebo arm (17 participants; 1.2%). However, this difference was not statistically significant (RR 0.71; 95% CI 0.34 to 1.47; p = 0.35). Analysis adjusting for potential risk factors did not significantly change the findings [OR for AAD 1.0 (95% CI 0.78 to 1.27); OR for CDD 0.65 (95% CI 0.29 to 1.47)]. Secondary outcome measures including the severity of diarrhoea, frequency and duration of gastrointestinal symptoms, duration of hospital stay, and QoL were also generally similar in the two study arms. Although an episode of AAD increased total health-care costs by £4531.36 (95% CI £3439.80 to £5622.92) independent of study arm, this was not reflected in a difference in total health-care cost between the probiotic and placebo arms. Cost-effectiveness analysis based on probiotic implementation costs showed that the probiotic was not cost-effective with an ICER of £189,662 per QALY. This result was robust to changes in the key parameters.
Comparison with other studies/reviews
Antibiotic-associated diarrhoea
Probiotic efficacy in our study (RR 1.04; 95% CI 0.84 to 1.28) is in marked contrast with meta-analyses that reported findings in adults (≥ 18 years of age),29,56–59 as well as a recent review of 63 randomised trials (11,811 participants of all ages and mostly outpatients), in which the pooled estimate showed a statistically significant effect of probiotics (random-effects model; RR 0.58; 95% CI 0.50 to 0.68). 60 We are not aware of further trial findings published since this recent review.
Although meta-analyses have reported similar estimates of probiotic efficacy, authors have emphasised caution in the interpretation of pooled results because of marked clinical heterogeneity between studies. In the recent review, marked heterogeneity in the pooled result (I2 = 54%) was not explained by subgroup analyses according to differences between trials in the probiotic preparation used, antibiotic treatment and patient characteristics. 60 Review authors have also highlighted deficiencies in the evidence base, especially the limited number of well-conducted trials and variations in the quality control of probiotic preparations. 61 Despite the addition of many recent studies, the authors of the latest review commented that research methods and reporting were generally poor, most studies were underpowered, probiotic strains were not clearly identified, only half of the studies defined the diarrhoea outcome and the follow-up period was limited or not specified. 60 A recent Cochrane review of probiotics in the prevention of AAD in children also highlighted the low quality of clinical trial evidence. 62
Causes of AAD may vary according to age and other patient characteristics. For example, the effects of antibiotics may vary according to the characteristics of the pretreatment enteric flora. The composition of the enteric flora varies between individuals,63 to a greater degree in the elderly than in younger people,64 and is influenced by chronic disease, frailty, diet, residence and care setting. 64 Most clinical trials recruited people of all ages. Although age was not significantly associated with probiotic efficacy in metaregression analysis in the recent review, interestingly, in three trials that recruited only patients aged > 65 years, there was no clear beneficial effect of probiotics (pooled RR 0.81; 95% CI 0.40 to 1.63). 60 Age and other host factors may modulate probiotic effects and need to be considered when evaluating the effect of interventions. Recommendations that probiotics should be used routinely for the prevention of AAD65 seem premature and more evidence is needed to inform the selection of a specific probiotic preparation for a well-defined population group. 60
Clostridium difficile diarrhoea
Probiotic efficacy in our study (RR 0.71; 95% CI 0.34 to 1.47) is consistent with pooled estimates from meta-analyses,60,66 including that of a recent meta-analysis of 20 trials including 3818 children and adults (random-effects model; RR 0.34; 95% CI 0.24 to 0.49). 67 Although this meta-analysis included trials that evaluated many different probiotics, including the yeast S. boulardii, and research methods and reporting were poor in many studies, there was consistency in results across studies and probiotics remained clinically effective in worse case assumptions for missing outcome data.
Interestingly, our findings suggested that the probiotic may have been clinically effective in preventing CDD in the older patients (aged > 77 years). However, we are not aware of similar findings in other reviews or randomised trials. Given the large number of analyses in our study, the apparent difference in probiotic effect according to age may have occurred by chance.
Strengths and weaknesses of probiotic lactobacilli and bifidobacteria in antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in the elderly
Research setting and population evaluated
We aimed to maximise the generalisability of our findings by limiting the participant exclusion criteria to conditions associated with diarrhoea and potential risk factors for probiotic adverse effects. 26,68,69 However, this still resulted in the exclusion of about one in five patients assessed for eligibility, and a further 1 in 10 were considered too unwell to join the study. In addition, > 50% of patients receiving antibiotics declined to participate. Reluctance to take additional medications was commonly stated as a reason for declining to join the trial. Among those recruited, use of numerous medications was common, and over one in three stated that they disliked taking the IMP as an additional medication. These practical difficulties need to be considered when developing novel interventions for the older population at risk of AAD.
Despite the relatively low conversion rate among patients who were assessed for their eligibility to participate in the trial (17.1%), we included patients from a range of medical and surgical wards in five hospitals in two NHS regions and, as far as we are aware, recruited a greater number of participants than other similar trials. 60,67 Therefore, we consider that our findings are generalisable to older patients managed in secondary care facilities in settings similar to the UK.
Overall, participant characteristics, including common comorbid illnesses and potential risk factors for AAD were similar at baseline, indicating that randomisation had been successful. The imbalance in sex between the two arms is likely to have arisen by chance and would not appear to have influenced the evaluation of probiotic efficacy; sex was not a risk factor for AAD and there was no evidence of an interaction between sex and probiotic effect. The higher frequency of COPD in the CDDFT hospitals probably reflects exposure to mining dust and asbestos in the north-east of England related to the ship building and mining industries in the second half of the 20th century.
Treatment with PPIs has been identified as an important risk factor for CDD,12,70 which led to recommendations for hospital antacid policies to restrict their use. 71 Antacid and PPI use were common among our participants (39.4%); both AAD and CDD were more frequent among patients receiving antacid medications, although this finding was statistically significant only for AAD. However, we found no evidence of an interaction between antacid treatment and probiotic effect and, therefore, no indication that probiotics should be targeted to this specific patient population.
Bacterial preparation
Several bacterial strains share antipathogen mechanisms such as the production of lactic acid;72 however, other mechanisms that may prevent AAD may be strain specific. Probiotic mixtures appear to better maintain a beneficial gut microbiota than single strains, although this may be due to a greater number of organisms rather than strains. 73 Evidence for a range of health outcomes, mainly from animal studies, also suggests that multispecies mixtures may be more effective than either multistrain or monostrain probiotics. 74 In meta-analysis, subgroup analyses suggested that the use of S. boulardii, L. rhamnosus GG, probiotic mixtures and a high number of organisms were associated with greater efficacy in preventing AAD. 29 These findings informed the selection of the probiotic preparation used in our study, which consisted of a high number (6 × 1010) of four strains of bacteria from two species, Lactobacillus and Bifidobacterium, that are most commonly evaluated in trials of the prevention of AAD. 60
In meta-analysis of CDD, subgroup analysis did not show significant changes in probiotic effect according to probiotic strain or dose. However, there was a trend towards increased clinical effectiveness of mixtures compared with single probiotic species. 67 Although not a commensal organism of the human gastrointestinal tract, the yeast S. boulardii may also be clinically effective in preventing AAD in adults [10 trials, 1866 adults, pooled RR of 0.47 (95% CI. 0.35 to 0.63) with homogeneity between trials in random-effects analysis]. 75
Several authors have highlighted deficiencies in previous studies in the identification of probiotic strains and confirmation of the viability of organisms following storage and at the point of delivery. The organisms in this study are deposited in an established repository (the NCIMB, UK). Confirmation of viability, and also identity according to the random allocation sequence, was undertaken in capsules collected from the point of use.
Overall, compliance with the IMPs was good, with over half of the participants reporting completion of the full 21-day course. The similarity in compliance in the two study arms and the lack of interaction between compliance and probiotic effect in covariate analysis suggests that the absence of probiotic effect was not due to poor compliance.
Frequency of Clostridium difficile diarrhoea
A major limitation of our study was the uncommon occurrence of CDD, and this prevented a reliable assessment of the efficacy of the probiotic. This occurred despite recruiting patients from general medicine, elderly care and nephrology wards that are known to have the highest CDD rates. 52 In line with Department of Health recommendations,39 all of the hospitals in this study actively implemented measures to prevent and control CDI during the period of the trial. These included antibiotic stewardship (especially reduced cephalosporin and broad-spectrum antibiotic use),22,23 enhanced cleaning, use of chlorine-based cleaning agents, enhanced hand hygiene and improved recognition and isolation of patients with C. difficile.
The low frequency is in keeping with the marked fall in CDD in hospitals in the UK during the period of recruitment to this study. Mandatory reporting for patients aged > 65 years admitted to hospitals in Wales with CDD identified 2744 cases from July 2008 to June 2009 and 1295 from April 2011 to March 2012 (a fall of 52.8%). 52 In ABMUHB, the annual number of cases fell from 344 to 229 (a fall of 33.4%) during the equivalent time periods. In patients aged > 65 years admitted by NHS Trusts in England, the total number of CDD cases fell from 20,191 in April 2009 to March 2010 to 13,836 in April 2011 to March 2012 (a fall of 31.5%). 76 The number of reported cases in CDDFT was 228 and 123, respectively (a fall of 46.1%).
Stool samples were not available to test for C. difficile toxins in 41.4% of the participants with diarrhoea in our study. As a result, some cases of CDD will not have been identified, and this weakens our estimate of probiotic efficacy. In practice, collection of stool samples was often difficult due to diarrhoea episodes of short duration (median duration of 2 days) and many occurring after hospital discharge. Where reported, the frequency of missing assessment for CDD has generally been lower in smaller trials (5–45%)66 than in our pragmatic study. In view of the low sensitivity of enzyme-linked immunosorbent assay-based toxin assays in the diagnosis of CDD,77–79 we undertook additional laboratory analyses in stored stool samples from participants with diarrhoea to try to increase the detection rate. These results will be reported in a separate publication.
Although CDI has been associated with single doses of cephalosporins,80 the limited duration of exposure to antibiotics in some patients may have also reduced CDD frequency in our study. Although most participants in our study were exposed to antibiotics from two or more classes and 12.4% were exposed to fluoroquinolones, 9.1% received only a single antibiotic dose and a further 28.1% received treatment for ≤ 6 days. A recent retrospective review of patients aged ≥ 18 years identified the number of antibiotics prescribed, increasing cumulative dose, duration of treatment and exposure to fluoroquinolones as increasing the risk of CDD. 81 We also found that longer duration of exposure (≥ 8 days) was associated with an increased frequency of both AAD and CDD. However, the practical value of longer exposure to antibiotics as a risk factor for CDD may be limited as duration of treatment is often unpredictable when commencing antibiotic therapy. Importantly, to prevent a single case of CDD, the number of patients needed to treat with any novel preventative strategy will increase as other measures reduce the frequency of CDD. This needs to be considered in the clinical management of individual patients and also in the design of trials to assess probiotic effectiveness for this indication.
Most antibiotic classes have been associated with AAD with increased risk with broad-spectrum antibiotics such as aminopenicillins, coamoxiclav (amoxicillin and clavulanic acid), cephalosporins and clindamycin. 82 Despite differences in antibiotic prescribing, rates of AAD and CDD were similar across the centres. Antibiotic exposure was similar in the two study arms and exposure to the two main antibiotic classes, penicillins and cephalosporins, did not modulate probiotic effect. We plan to undertake more detailed analysis to identify specific antibiotic treatment regimens that may increase the risk of AAD.
Serious adverse events
The daily follow-up during hospital stay and weekly telephone calls after discharge provided opportunities to identify SAEs. SAEs were expected to occur commonly in this vulnerable population and one or more SAE occurred in about one in five participants. Based on mortality data for the 3 months after hospital admission for patients aged ≥ 65 years in England over the period 2004–7, we had expected an overall mortality rate of 9.1% (Professor David Ford, Swansea University, March 2009, personal communication). The lower mortality rate in our study (4.9%) probably results from the exclusion of severely unwell patients. The frequency and severity of all SAEs, and those that resulted in the temporary or permanent withdrawal of the IMPs, were broadly similar in the two study arms. No SAE was attributed to the participant’s involvement in the study. The probiotic used in this study was not associated with adverse events in a previous study of 52 adults with irritable bowel syndrome. 83 Our findings are consistent with recent systematic reviews of probiotic safety in humans,84 including a recent meta-analysis of 208 RCTs where mainly single strains or mixtures of Lactobacillus sp. and Bifidobacterium sp. administered to medium-risk and critically ill patients were not associated with increased adverse events, including gastrointestinal disorders and infections. 26
Quality of life
The application of SF-12 v2, a generic tool to evaluate health-related quality of life (HRQL), to specific gastrointestinal disorders is unclear. Erminia et al. were unable to detect differences in SF-12 v2 scores between patients with and without lactose intolerance. 85 A systematic review that included four studies of people with irritable bowel syndrome reported that despite improvements in symptoms in the probiotic group compared with controls, little improvement was seen in HRQL. 86 However, Koloski et al. 87 reported poor HRQL in patients with functional gastrointestinal disorders compared with healthy controls. We found that, overall, both SF-12 v2 and EQ-5D scores improved during follow-up, which was consistent with improved HRQL as patients responded to treatment. However, administration of the probiotic did not result in a significant improvement in HRQL. We are not aware of any studies that have examined the HRQL effects of probiotics on diarrhoea.
Health economic analysis
In our study, the probiotic was not effective in preventing AAD and the total health-care costs were very similar in both arms, indicating that the probiotic had virtually no budgetary impact. Furthermore, based on a non-significant difference in QoL as assessed by EQ-5D index values with very similar data ranges in both arms, the cost–utility analysis revealed an ICER of £189,662 per QALY gained, consistent with an absence of cost-effectiveness of probiotics in regards to patient QoL. The main limitation of the cost-effectiveness analysis was the relatively low number of diarrhoea cases (especially CDD), which limited the potential to detect cost differences between the study arms.
Although the need for rigorous economic evaluation of preventative therapies for AAD has been recognised,88,89 to our knowledge no formal cost-effectiveness evaluations of probiotics have been undertaken in appropriately powered RCTs. In contrast with our findings, Kamdeu Fansi et al. 90 undertook a cost–consequences analysis based on a decision tree model with a short-term horizon (3 weeks follow-up) and concluded that substantial cost savings could be achieved by the routine use of probiotics due to a significant reduction in AAD incidence and total health-care cost per patient. Their model was based on a single trial of 225 participants divided into three trial arms (two different Lactobacillus formulations compared with no prophylactic intervention), patient QoL was not considered and costs included in the total cost were restricted to those of microbiological testing, the probiotic preparation, antibiotics and hospital stay (which was based on data from previously published studies and assumptions). In a pilot study, probiotics were cost-effective based on the relatively low implementation cost and a lower frequency of AAD in the placebo arm. 91 However, the sample size was small (n = 23) and no formal cost-effectiveness analysis was undertaken. Therefore, both of these cost-effectiveness estimates have severe limitations.
In our study, AAD resulted in an average increased duration of hospital stay of 5.58 days and an average incremental total cost of £4531.36. These findings correspond well with those of previous studies, in which CDD resulted in increased length of hospital stay of 3–21 days13,16,17,20,92,93 and was associated with additional total health-care costs of between £3101 and £6195 converted to pounds sterling, correct in 2011. 13,14,16,17 In our study, no SAEs that required additional health-care resources were attributed to the probiotics, which is in line with reports from the published literature. 94,95
Despite only moderate evidence of clinical effectiveness, some authorities have recommended that the use of probiotics is justified for the prevention of AAD96 and CDD. 67 The modest effect of probiotics has also led to the recommendation that they should be considered a supplement rather than a replacement for conventional therapy. 97 In our study, the similar diarrhoea and QoL outcomes in the two study arms and lack of cost-effectiveness of the probiotic preparation lead us to recommend that further research is needed before a specific probiotic preparation can be recommended for a specific population group.
Clinical implications
-
The high-dose, multistrain preparation of lactobacilli and bifidobacteria evaluated in our study is unlikely to benefit unselected older inpatients exposed to antibiotics.
-
Clinical judgement regarding the benefits and risks of novel interventions to prevent AAD needs to take account of the impact of other preventative measures, such as antibiotic stewardship, on disease frequency.
-
The administration of additional medications to vulnerable older people, many of whom are already taking multiple medications, may not be well tolerated in practice.
-
The clinical effectiveness of our preparation in preventing CDD was unclear. However, even if it is effective, the falling prevalence of CDD needs more patients to take the probiotic to prevent a single case.
-
The probiotic preparation was not associated with SAEs in our study. However, surveillance for potentially uncommon adverse events is required in future studies.
Research implications
-
A better understanding of the multiple potential mechanisms underlying AAD and CDD, how these may vary in specific populations and the strain-specific effects of probiotics is needed before further clinical trials of specific probiotic preparations are undertaken. Further research to identify populations at increased risk of AAD and CDD is needed to facilitate the future evaluation of probiotic interventions.
-
The design of studies to evaluate the efficacy of alternative probiotics in the prevention of CDD needs to consider the effect of other measures that have reduced the frequency of CDI in some health-care institutions.
-
Further research into the effect of probiotics on patient QoL will be necessary to better determine patient benefit and cost-effectiveness.
Acknowledgements
We would like to thank the participants for taking part in the trial, the clinicians who gave permission for their patients to be approached to partake in the study and the ward nurses for helping with collection of stool samples. We would also like to thanks the members of the Trial Steering Committee and Data Monitoring and Ethics Committee for overseeing the trial, and Dr Barney Hawthorne, the independent safety monitor.
We would specifically like to thank the research team in ABMUHB: Claire Fagan, study co-ordinator and assisted with training and monitoring at CDDFT and ongoing training of staff at Swansea; Scott Davies, data manager who set up and managed the databases ably assisted by data entry clerks Tara Ghuman and Matthew Hanney; and substantive research nurses Christine Jones, Natalie Blytt Jordans, Gillian Scott, Tina Morgan, Claire Stafford and Rebeccah Thomas. In CDDFT: Jill Deane, project supervisor; Fiona Bezzina, Jean Dent, Lynn Dixon, Lisa Godwin, Rachel Hayman, Gil Horner, Graham Naylor, Lyndsey Taylor, Glynis Rose and Claire Shaw, research nurses; and Geraldine Brown, data entry.
We would also like to thank:
-
Mrs Mel Storey and Dr Alan Watkins, West Wales Organisation for Rigorous Trials in Health, Professor Ian Russell and Professor John Williams for valuable comments on the manuscript.
-
Eugene Rees, Dr Khalid El-Bouri, Katie Davies, Rhian Bourne, Public Health Wales Microbiology Swansea, Singleton Hospital, Swansea.
-
Dr Yan Yiannakou, Research and Development Director, CDDFT, for his support during of the local ethics approval process and for securing funding from the National Institute for Health Research (NIHR) Comprehensive Local Research Network.
-
The County Durham and Tees Valley, NIHR Comprehensive Local Research Network for a grant to provide additional funding to support authors CB, AD, HB and AF.
Contributions of authors
Professor Stephen J Allen wrote the original protocol, was the chief investigator, oversaw the study, wrote the initial draft of the main report and contributed to the final report.
Ms Kathie Wareham was a co-applicant and refined the protocol, was the trial manager and contributed to the final report.
Dr Duolao Wang was a co-applicant and refined the protocol, wrote the statistical analysis plan and undertook the statistical analysis and contributed to the final report.
Mrs Caroline Bradley was a co-applicant and refined the protocol and contributed to the final report.
Dr Bernadette Sewell designed the economic analysis, wrote the initial draft of the economics analysis and contributed to the final report.
Ms Hayley Hutchings wrote the initial draft of the QoL analysis and contributed to the final report.
Dr Wyn Harris was a co-applicant and refined the protocol and contributed to the final report.
Dr Anjan Dhar was a co-applicant and refined the protocol and contributed to the final report.
Dr Helga Brown was a co-applicant and refined the protocol and contributed to the final report.
Dr Alwyn Foden was a co-applicant and refined the protocol and contributed to the final report.
Professor Mike B Gravenor was a co-applicant and refined the protocol, contributed data analysis and interpretation of the statistics and contributed to the final report.
Professor Dietrich Mack was a co-applicant and refined the protocol and contributed to the final report.
Professor Ceri J Phillips was a co-applicant and refined the protocol, designed the economic analysis and contributed to the final report.
Trial Steering Committee members
Professor Stephen Bain (Chairperson), Chairperson in Medicine (Diabetes), College of Medicine, Swansea University; Diabetes Lead Clinician, ABMUHB.
Dr John Sloss, Consultant Microbiologist, Darlington Memorial Hospital, Hollyhurst Road, Darlington, County Durham.
Mr Graham Tanner, Royal British Legion welfare case worker.
Data monitoring and Ethics Committee members
Professor John Williams (Chairperson), Professor of Health Services Research, College of Medicine, Swansea University and Consultant Gastroenterologist, ABMUHB.
Dr Duolao Wang; Senior Lecturer in Medical Statistics, Department of Medical Statistics, London School of Hygiene and Tropical Medicine, London, UK. Independent Statistician from study start to January 2012.
Professor Kerenza Hood, Director, South East Wales Trials Unit, Institute of Translation, Innovation, Methodology and Engagement, Cardiff University School of Medicine, Neuadd Meirionnydd, Heath Park, Cardiff, UK. Independent Statistician from January 2012.
Dr John Brazier, Anaerobe Reference Laboratory, Cardiff, UK, retired.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis 1998;16:292-307. http://dx.doi.org/10.1159/000016879.
- Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 2002;346:334-9.
- McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol 2008;3:563-78. http://dx.doi.org/10.2217/17460913.3.5.563.
- Viswanathan VK, Mallozzi MJ, Vedantam G. Clostridium difficile infection: an overview of the disease and its pathogenesis, epidemiology and interventions. Gut Microbes 2010;1:234-42.
- Antunes LC, Han J, Ferreira RB, Lolic P, Borchers CH, Finlay BB. Effect of antibiotic treatment on the intestinal metabolome. Antimicrob Agents Chemother 2011;55:1494-503. http://dx.doi.org/10.1128/AAC.01664-10.
- Badger VO, Ledeboer NA, Graham MB, Edmiston CE. Clostridium difficile: epidemiology, pathogenesis, management, and prevention of a recalcitrant healthcare-associated pathogen. JPEN J Parenter Enteral Nutr 2012;36:645-62. http://dx.doi.org/10.1177/0148607112446703.
- Cartman ST, Heap JT, Kuehne SA, Cockayne A, Minton NP. The emergence of ‘hypervirulence’ in Clostridium difficile. Int J Med Microbiol 2010;300:387-95. http://dx.doi.org/10.1016/j.ijmm.2010.04.008.
- Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med 2006;145:758-64. http://dx.doi.org/10.7326/0003-4819-145-10-200611210-00008.
- Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 2005;366:1079-84. http://dx.doi.org/10.1016/S0140-6736(05)67420-X.
- Wistrom J, Norrby SR, Myhre EB, Eriksson S, Granstrom G, Lagergren L, et al. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: a prospective study. J Antimicrob Chemother 2001;47:43-50. http://dx.doi.org/10.1093/jac/47.1.43.
- Bauer MP, Notermans DW, van Benthem BH, Brazier JS, Wilcox MH, Rupnik M, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet 2011;377:63-7. http://dx.doi.org/10.1016/S0140-6736(10)61266-4.
- Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med 2011;365:1693-703.
- Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 2002;34:346-53. http://dx.doi.org/10.1086/338260.
- Dubberke ER, Reske KA, Olsen MA, McDonald LC, Fraser VJ. Short- and long-term attributable costs of Clostridium difficile-associated disease in nonsurgical inpatients. Clin Infect Dis 2008;46:497-504. http://dx.doi.org/10.1086/526530.
- McGlone SM, Bailey RR, Zimmer SM, Popovich MJ, Tian Y, Ufberg P, et al. The economic burden of Clostridium difficile. Clin Microbiol Infect 2012;18:282-9. http://dx.doi.org/10.1111/j.1469-0691.2011.03571.x.
- Wilcox MH, Cunniffe JG, Trundle C, Redpath C. Financial burden of hospital-acquired Clostridium difficile infection. J Hosp Infect 1996;34:23-30. http://dx.doi.org/10.1016/S0195-6701(96)90122-X.
- Vonberg RP, Reichardt C, Behnke M, Schwab F, Zindler S, Gastmeier P. Costs of nosocomial Clostridium difficile-associated diarrhoea. J Hosp Infect 2008;70:15-20. http://dx.doi.org/10.1016/j.jhin.2008.05.004.
- Dubberke ER, Wertheimer AI. Review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol 2009;30:57-66.
- O’Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol 2007;28:1219-27.
- Forster AJ, Taljaard M, Oake N, Wilson K, Roth V, van Walraven C. The effect of hospital-acquired infection with Clostridium difficile on length of stay in hospital. CMAJ 2012;184:37-42. http://dx.doi.org/10.1503/cmaj.110543.
- Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of Clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol 2008;29:823-8.
- Valiquette L, Cossette B, Garant MP, Diab H, Pepin J. Impact of a reduction in the use of high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease caused by the hypervirulent NAP1/027 strain. Clin Infect Dis 2007;45:S112-21. http://dx.doi.org/10.1086/519258.
- Fowler S, Webber A, Cooper BS, Phimister A, Price K, Carter Y, et al. Successful use of feedback to improve antibiotic prescribing and reduce Clostridium difficile infection: a controlled interrupted time series. J Antimicrob Chemother 2007;59:990-5. http://dx.doi.org/10.1093/jac/dkm014.
- Joint FAO/WHO Expert Consultation . Health and Nutritional Properties of Probiotics in Food Including Powder Milk With Live Lactic Acid Bacteria 2001. ftp://ftp.fao.org/es/esn/food/probio_report_en.pdf (accessed 10 April 2013).
- Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food . Guidelines for the Evaluation of Probiotics in Food 2002. ftp://ftp.fao.org/es/esn/food/wgreport2.pdf (accessed 16 July 2013).
- Hempel S, Newberry S, Ruelaz A, Wang Z, Miles JN, Suttorp MJ, et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep Technol Assess 2011;200:1-645.
- Hammerman C, Bin-Nun A, Kaplan M. Safety of probiotics: comparison of two popular strains. BMJ 2006;333:1006-8. http://dx.doi.org/10.1136/bmj.39010.630799.BE.
- Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol 2010;298:G807-19. http://dx.doi.org/10.1152/ajpgi.00243.2009.
- McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol 2006;101:812-22. http://dx.doi.org/10.1111/j.1572-0241.2006.00465.x.
- Cremonini F, Di Caro S, Nista EC, Bartolozzi F, Capelli G, Gasbarrini G, et al. Meta-analysis: the effect of probiotic administration on antibiotic-associated diarrhoea. Aliment Pharmacol Ther 2002;16:1461-7. http://dx.doi.org/10.1046/j.1365-2036.2002.01318.x.
- D’Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. BMJ 2002;324. http://dx.doi.org/10.1136/bmj.324.7350.1361.
- Sazawal S, Hiremath G, Dhingra U, Malik P, Deb S, Black RE. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis 2006;6:374-82. http://dx.doi.org/10.1016/S1473-3099(06)70495-9.
- Hawrelak JA, Whitten DL, Myers SP. Is Lactobacillus rhamnosus GG effective in preventing the onset of antibiotic-associated diarrhoea: a systematic review. Digestion 2005;72:51-6. http://dx.doi.org/10.1159/000087637.
- Plummer S, Weaver MA, Harris JC, Dee P, Hunter J. Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int Microbiol 2004;7:59-62.
- Thomas MR, Litin SC, Osmon DR, Corr AP, Weaver AL, Lohse CM. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: a randomized, placebo-controlled trial. Mayo Clin Proc 2001;76:883-9. http://dx.doi.org/10.4065/76.9.883.
- McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Moyer KA, Melcher SA, et al. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol 1995;90:439-48.
- Surawicz CM, Elmer GW, Speelman P, McFarland LV, Chinn J, van Belle G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology 1989;96:981-8.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920-4. http://dx.doi.org/10.3109/00365529709011203.
- London, UK: Department of Health; 2008.
- Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. User’s Manual for the SF-12v2® Health Survey (with a Supplement Documenting SF-12v2® Health Survey). Lincoln, RI: QualityMetric Incorporated; 2002.
- International Conference on Harmonisation . International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Guidelines for Good Clinical Practice 1996. www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html (accessed 10 April 2013).
- British National Formulary n.d. www.bnf.org/bnf/index.htm (accessed September 2012).
- MedDRA . MedDRA Term Selection: Points To Consider 2011. ww.ich.org/fileadmin/Public_Web_Site/ICH_Products/MedDRA/MedDRA_Documents/MedDRA_Term_Selection/Release_4.2_based_on_14.1/TermSelection_PTC_R4.2_ October2011.pdf (accessed 10 April 2013).
- Department of Health . NHS Reference Costs 2011 2012. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_131140 (accessed December 2012).
- Curtis L. Unit Costs Of Health and Social Care 2011. Canterbury: PSSRU: University of Kent; 2011.
- Joint Formulary Committee . British National Formulary (online) 2012. www.medicinescomplete.com (accessed January 2013).
- Young TA. Estimating mean total costs in the presence of censoring: a comparative assessment of methods. Pharmacoeconomics 2005;23:1229-42. http://dx.doi.org/10.2165/00019053-200523120-00007.
- Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al. A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food. Health Technol Assess 2007;11:1-216.
- Burkart NE, Kwaan MR, Shepela C, Madoff RD, Wang Y, Rothenberger DA, et al. Indications and relative utility of lower endoscopy in the management of Clostridium difficile infection. Gastroenterol Res Pract 2011;2011. http://dx.doi.org/10.1155/2011/626582.
- Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P. Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg 2012;99:1501-13.
- Doan L, Forrest H, Fakis A, Craig J, Claxton L, Khare M. Clinical and cost effectiveness of eight disinfection methods for terminal disinfection of hospital isolation rooms contaminated with Clostridium difficile 027. J Hosp Infect 2012;82:114-21. http://dx.doi.org/10.1016/j.jhin.2012.06.014.
- Public Health Wales . All Wales Commentaries: Clostridium Difficile Reports 2012. http://www.wales.nhs.uk/sites3/page.cfm?orgid=379&pid=18490 (accessed 25 June 2013).
- Guide to the Methods of Technology Appraisal. 2008.
- Thielman NM, Rust PF, Guerrant RL. Criterion-related validity of a diarrhea questionnaire in HIV-infected patients. Dig Dis Sci 2002;47:1421-6.
- Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. Fecal incontinence quality of life scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum 2000;43:9-16. http://dx.doi.org/10.1007/BF02237236.
- Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther 2012;35:1355-69. http://dx.doi.org/10.1111/j.1365-2036.2012.05104.x.
- Ritchie ML, Romanuk TN. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS One 2012;7. http://dx.doi.org/10.1371/journal.pone.0034938.
- Avadhani A, Miley H. Probiotics for prevention of antibiotic-associated diarrhea and Clostridium difficile-associated disease in hospitalized adults – a meta-analysis. J Am Acad Nurse Pract 2011;23:269-74. http://dx.doi.org/10.1111/j.1745-7599.2011.00617.x.
- Kale-Pradhan PB, Jassal HK, Wilhelm SM. Role of Lactobacillus in the prevention of antibiotic-associated diarrhea: a meta-analysis. Pharmacotherapy 2010;30:119-26.
- Hempel S, Newberry SJ, Maher AR, Wang Z, Miles JN, Shanman R, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA 2012;307:1959-69.
- McFarland LV. Evidence-based review of probiotics for antibiotic-associated diarrhea and Clostridium difficile infections. Anaerobe 2009;15:274-80. http://dx.doi.org/10.1016/j.anaerobe.2009.09.002.
- Johnston BC, Goldenberg JZ, Vandvik PO, Sun X, Guyatt GH. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev 2011;11. http://dx.doi.org/10.1002/14651858.CD004827.
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature 2011;473:174-80. http://dx.doi.org/10.1038/nature10187.
- Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA 2011;108:4586-91. http://dx.doi.org/10.1073/pnas.1000097107.
- Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488:178-84. http://dx.doi.org/10.1038/nature11319.
- Dendukuri N, Costa V, McGregor M, Brophy JM. Probiotic therapy for the prevention and treatment of Clostridium difficile-associated diarrhea: a systematic review. CMAJ 2005;173:167-70. http://dx.doi.org/10.1503/cmaj.050350.
- Johnston BC, Ma SS, Goldenberg JZ, Thorlund K, Vandvik PO, Loeb M, et al. Probiotics for the Prevention of Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Ann Intern Med 2012;18:878-88. http://dx.doi.org/10.7326/0003-4819-157-12-201212180-00563.
- Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008;371:651-9. http://dx.doi.org/10.1016/S0140-6736(08)60207-X.
- Sand J, Nordback I. Probiotics in severe acute pancreatitis. Lancet 2008;371:634-5. http://dx.doi.org/10.1016/S0140-6736(08)60284-6.
- Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol 2012;107:1001-10.
- Thachil J. Overprescribing PPIs: time for a hospital antacid policy on Clostridium difficile. BMJ 2008;336.
- Kolling GL, Wu M, Warren CA, Durmaz E, Klaenhammer TR, Guerrant RL. Lactic acid production by Streptococcus thermophilus alters Clostridium difficile infection and in vitro Toxin A production. Gut Microbes 2012;3:523-9. http://dx.doi.org/10.4161/gmic.21757.
- Chapman CM, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains?. Eur J Nutr 2011;50:1-17. http://dx.doi.org/10.1007/s00394-010-0166-z.
- Timmerman HM, Koning CJ, Mulder L, Rombouts FM, Beynen AC. Monostrain, multistrain and multispecies probiotics – a comparison of functionality and efficacy. Int J Food Microbiol 2004;96:219-33. http://dx.doi.org/10.1016/j.ijfoodmicro.2004.05.012.
- McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol 2010;16:2202-22.
- Health Protection Agency . Results from the Mandatory Clostridium Difficile Reporting Scheme. Acute Trust Counts and Rates by Quarter and Financial Year- April 2007 Onwards 2012. www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/ClostridiumDifficile/EpidemiologicalData/MandatorySurveillance/cdiffMandatoryReportingScheme/ (accessed 25 June 2013).
- Eastwood K, Else P, Charlett A, Wilcox M. Comparison of nine commercially available Clostridium difficile toxin detection assays, a real-time PCR assay for C. difficile tcdB, and a glutamate dehydrogenase detection assay to cytotoxin testing and cytotoxigenic culture methods. J Clin Microbiol 2009;47:3211-17. http://dx.doi.org/10.1128/JCM.01082-09.
- Novak-Weekley SM, Marlowe EM, Miller JM, Cumpio J, Nomura JH, Vance PH, et al. Clostridium difficile testing in the clinical laboratory by use of multiple testing algorithms. J Clin Microbiol 2010;48:889-93. http://dx.doi.org/10.1128/JCM.01801-09.
- Chapin KC, Dickenson RA, Wu F, Andrea SB. Comparison of five assays for detection of Clostridium difficile toxin. J Mol Diagn 2011;13:395-400. http://dx.doi.org/10.1016/j.jmoldx.2011.03.004.
- Privitera G, Scarpellini P, Ortisi G, Nicastro G, Nicolin R, de Lalla F. Prospective study of Clostridium difficile intestinal colonization and disease following single-dose antibiotic prophylaxis in surgery. Antimicrob Agents Chemother 1991;35:208-10. http://dx.doi.org/10.1128/AAC.35.1.208.
- Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis 2011;53:42-8. http://dx.doi.org/10.1093/cid/cir301.
- Lo Vecchio A, Zacur GM. Clostridium difficile infection: an update on epidemiology, risk factors, and therapeutic options. Curr Opin Gastroenterol 2012;28:1-9. http://dx.doi.org/10.1097/MOG.0b013e32834bc9a9.
- Williams EA, Stimpson J, Wang D, Plummer S, Garaiova I, Barker ME, et al. Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment Pharmacol Ther 2009;29:97-103. http://dx.doi.org/10.1111/j.1365-2036.2008.03848.x.
- Whelan K, Myers CE. Safety of probiotics in patients receiving nutritional support: a systematic review of case reports, randomized controlled trials, and nonrandomized trials. Am J Clin Nutr 2010;91:687-703. http://dx.doi.org/10.3945/ajcn.2009.28759.
- Erminia R, Ilaria B, Tiziana M, Silvia P, Antonio N, Pierpaolo D, et al. HRQoL questionnaire evaluation in lactose intolerant patients with adverse reactions to foods. Intern Emerg Med 2013;8:493-6. http://dx.doi.org/10.1007/s11739-011-0630-7.
- Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol 2009;9. http://dx.doi.org/10.1186/1471-230X-9-15.
- Koloski NA, Talley NJ, Boyce PM. The impact of functional gastrointestinal disorders on quality of life. Am J Gastroenterol 2000;95:67-71. http://dx.doi.org/10.1016/S0002-9270(99)00794-7.
- Stone PW. Economic burden of healthcare-associated infections: an American perspective. Expert Rev Pharmacoecon Outcomes Res 2009;9:417-22. http://dx.doi.org/10.1586/erp.09.53.
- Hickson M. Probiotics in the prevention of antibiotic-associated diarrhoea and Clostridium difficile infection. Therap Adv Gastroenterol 2011;4:185-97. http://dx.doi.org/10.1177/1756283X11399115.
- Kamdeu Fansi AA, Guertin JR, LeLorier J. Savings from the use of a probiotic formula in the prophylaxis of antibiotic-associated diarrhea. J Med Econ 2012;15:53-60. http://dx.doi.org/10.3111/13696998.2011.629015.
- Cimperman L, Bayless G, Best K, Diligente A, Mordarski B, Oster M, et al. A randomized, double-blind, placebo-controlled pilot study of Lactobacillus reuteri ATCC 55730 for the prevention of antibiotic-associated diarrhea in hospitalized adults. J Clin Gastroenterol 2011;45:785-9. http://dx.doi.org/10.1097/MCG.0b013e3182166a42.
- MacGowan AP, Feeney R, Brown I, McCulloch SY, Reeves DS, Lovering AM. Health care resource utilization and antimicrobial use in elderly patients with community-acquired lower respiratory tract infection who develop Clostridium difficile-associated diarrhoea. J Antimicrob Chemother 1997;39:537-41. http://dx.doi.org/10.1093/jac/39.4.537.
- Al-Eidan FA, McElnay JC, Scott MG, Kearney MP. Clostridium difficile-associated diarrhoea in hospitalised patients. J Clin Pharm Ther 2000;25:101-9. http://dx.doi.org/10.1046/j.1365-2710.2000.00266.x.
- Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol 2010;105:1636-41. http://dx.doi.org/10.1038/ajg.2010.11.
- Song HJ, Kim JY, Jung SA, Kim SE, Park HS, Jeong Y, et al. Effect of probiotic Lactobacillus (Lacidofil(R) cap) for the prevention of antibiotic-associated diarrhea: a prospective, randomized, double-blind, multicenter study. J Korean Med Sci 2010;25:1784-91.
- Cremonini F, Videlock EJ. Probiotics are associated with a decreased risk of antibiotic-associated diarrhoea. Evid Based Med 2013;18:71-2. http://dx.doi.org/10.1136/eb-2012-100863.
- Ciorba MA. A gastroenterologist’s guide to probiotics. Clin Gastroenterol Hepatol 2012;10:960-8. http://dx.doi.org/10.1016/j.cgh.2012.03.024.
Appendix 1 Regulatory approvals
Competent authority approval, MHRA. Approval granted on 8 October 2008.
Medical REC for Wales, Sixth Floor Churchill House, 17 Churchill Way, Cardiff, CF10 2TW. Approval granted on 27 November 2008.
Singleton, Morriston and Princess of Wales hospitals; Research and Development Department, ABMUHB (change of name from Abertawe Bro Morgannwg NHS Trust on 1 October 2009), Swansea, SA6 6NL. Approval granted on 11 September 2008.
County Durham and Tees Valley 2 REC. Approval granted on 17 June 2008.
Research and Development Department, Darlington Memorial Hospital, Hollyhurst Road, Darlington, DL3 6HX. Approval granted on 5 December 2008.
Appendix 2 Details of wards involved in the recruitment of patients by centre
| Specialty | Morriston Hospital (n = 23) | Singleton Hospital (n = 8) | Princess of Wales Hospital (n = 13) | Darlington Memorial Hospital (n = 12) | University Hospital of North Durham (n = 11) |
|---|---|---|---|---|---|
| Medical specialties | |||||
| General medicine | ✓ | ✓ × 2 | – | – | ✓ |
| Medical admissions | ✓ | – | ✓ | ✓ | ✓ |
| Respiratory/general medicine | ✓ | – | ✓ | ✓ | ✓ |
| Elderly care/general medicine | – | ✓ | ✓ × 2 | ✓ | ✓ |
| Stroke/general medicine | – | ✓ | – | ✓ | ✓ |
| Elderly care/stroke medicine | ✓ | – | ✓ | – | ✓ |
| Cardiology/general medicine | ✓ | ✓ | ✓ | ✓ | ✓ |
| Cardiology | ✓ | – | – | – | – |
| Haematology/general medicine | – | – | – | ✓ | ✓ |
| Hepatology/gastroenterology/general medicine | – | – | – | ✓ | – |
| Gastroenterology/general medicine | ✓ | – | ✓ | – | – |
| Renal medicine | ✓ | – | – | – | – |
| Neurology/general medicine | ✓ | – | – | – | – |
| Total number of medical wards | 9 | 5 | 7 | 7 | 8 |
| Surgical specialties | |||||
| Surgical admissions | ✓ | – | – | – | – |
| Male genitourinary | ✓ | – | – | – | ✓ |
| Female genitourinary and gynaecology | ✓ | ✓ | ✓ | – | ✓ |
| Orthopaedic | ✓ × 3 | – | ✓ × 2 | ✓ × 2 | ✓ |
| General surgery | ✓ | ✓ × 2 | ✓ × 2 | ✓ × 2 | – |
| Colorectal surgery | ✓ | – | – | – | – |
| Ear, nose and throat/general surgery | – | – | ✓ | ✓ | – |
| Neurosurgery | ✓ | – | – | – | – |
| Burns | ✓ | – | – | – | – |
| Burns and plastic surgery | ✓ × 2 | – | – | – | – |
| Maxillary facial surgery/plastic surgery | ✓ | – | – | – | – |
| Cardiac surgery | ✓ | – | – | – | – |
| Total number of surgical wards | 14 | 3 | 6 | 5 | 3 |
Appendix 3 Patient information sheet, consent form, advocate information sheet and advocate assent form
Patient information sheet
The PLACIDE study
Probiotic Lactic acid bacteria and Antibiotic-associated and C. difficile diarrhoea in the Elderly
Patient information sheet
Full title of the study: A multicentre, randomised, placebo controlled trial of lactic acid bacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in patients aged 65 years and over admitted to hospital and receiving antibiotics.
Part 1
You are invited to participate in a clinical study. Before agreeing to participate in this study it is important that you read and understand why the research is being done and what will happen during it. Please take time to read this information carefully. Take time to ask as many questions as you want. The study personnel will explain any word or information you do not clearly understand. You may to talk to others about the study if you wish.
Part 1 tells you the purpose of the research and explains what will happen to you if take part.
Part 2 gives you more information about the conduct of the study if you are still interested after reading Part 1.
1. What is the purpose of the study?
We want to find out whether or not giving a probiotic food supplement prevents the diarrhoea that often affects people taking antibiotics. Probiotics are the safe, live, ‘friendly’ bacteria that are found in live yoghurts that you can buy in supermarkets.
Many people admitted to hospital require treatment with antibiotics. Antibiotics change the ‘healthy’ bacteria that live in the gut and this results in diarrhoea in about 1 in 5 people. Diarrhoea is distressing for patients and may also delay recovery from illness and prolong the hospital admission.
Occasionally, antibiotics result in an overgrowth of a potentially dangerous bacterium called ‘C. difficile’. This is also known as ‘C. diff’ and is talked about a lot in the press. This can cause a severe and life-threatening diarrhoeal illness that may require additional medical or surgical treatment. The clinicians working in the hospitals involved in this study perceive C. difficile diarrhoea as a very important problem. This underlies our interest to search for new ways to prevent this problem.
2. Why have I been chosen?
You have been approached because you fulfil the entry criteria for the study. These criteria are:
-
aged 65 years or more and have been admitted to hospital
-
already taking antibiotics or are about to start antibiotic treatment
-
do not have diarrhoea at the moment
-
have not had an adverse reaction to a probiotic food supplement in the past
-
do not have an impaired immune system, an artificial heart valve or active inflammatory bowel disease.
The study is being conducted in Wales and in Durham and we hope to recruit around 2500 patients.
3. Do I have to take part?
It is up to you to decide whether or not to take part. If you do, you will be given this information sheet to keep and be asked to sign a consent form. You are still free to withdraw consent from the study at any time and without giving a reason. A decision to withdraw at any time, or a decision not to take part, will not affect the standard of care you receive.
4. What will happen to me if I take part?
The doctors will treat the illness as usual, including treatment with antibiotics. If you develop diarrhoea, the nurses and doctors will take stool samples to investigate the cause and treat it in the usual way.
We will ask you to take a small amount of powder by mouth once daily for 21 days. You will have an equal chance of receiving either the probiotic food supplement (the active intervention) or an inactive substance (the placebo). We will also ask you to answer questions about any symptoms you may have (diarrhoea, abdominal pain, bloating, flatus, nausea) and also general questions that assess the impact of your illness on your quality of life.
We will keep in-touch with patients to check for these symptoms for 8 weeks after you have finished the course of antibiotics. Our research nurses will visit you in hospital or telephone or visit you at home if discharged from hospital.
5. Expenses and payments?
Joining the trial will not result in any expenses for you. None of the participants will receive any payment.
6. What do I have to do?
Once you have consented to join the trial, you will be asked to give information to our research nurses about any symptoms you have, including diarrhoea, and also answer some questions that assess how your quality of life is affected. You should take the trial intervention as directed together with other medications as prescribed by the doctor. Please note that it is important that the participant avoids taking any probiotic preparations (other than the trial intervention) or live yogurts during the study. The research nurse can give you more guidance on what products to avoid if needed. Please inform the study nurse if any of your medications change during the study period.
7. What is the drug that is being tested?
We are testing a probiotic food supplement and this is not a drug. The supplement consists of lactobacilli and bifidobacteria that are just like the bacteria that live in the bowels of healthy people. The bacteria are Lactobacillus acidophilus (2 strains: CUL60 and CUL21), Bifidobacterium bifidum (CUL20) and Bifidobacterium lactis (CUL34).
People in the placebo group will receive an inactive, inert powder called maltodextrin.
8. What are the alternative procedures or treatments?
There are no other ways for people to protect themselves against antibiotic-associated and C. difficile diarrhoea. The hospitals are trying their best to reduce these problems for all patients by improving hygiene and also being careful with using antibiotics.
9. What are the side effects of treatment?
Probiotics are very safe and we do not anticipate any side effects. There are a small number of case reports where probiotics may themselves have caused infections in people with markedly impaired immune systems or an artificial heart valve. We will not include such people in our study.
10. What are the other possible disadvantages and risks of taking part?
We are not aware of any disadvantages or risks of joining our study.
11. What are the possible benefits of taking part?
All patients in the study, including those in the placebo group, will benefit from regular follow-up for diarrhoea and other symptoms. This may improve the recognition of problems and overall care for all participants.
Participants who receive the active intervention may have a reduced risk of developing antibiotic-associated and C. difficile diarrhoea or may develop milder disease. If the food supplement proves to be successful against C. difficile diarrhoea, fewer cases will reduce the risk of other patients acquiring this infection.
12. What happens when the research stops?
Patients will leave the trial at the end of the 8 weeks follow-up. If you require any further treatment in respect of diarrhoea this will be under the care of the GP or hospital doctor.
We plan to publicise the findings of the study widely in the medical literature and local press. Depending on the results, the NHS may recommend probiotic food supplements as part of routine care for older people receiving antibiotics in hospitals.
13. What if there is a problem?
We do not expect any problems related to the study because probiotics are very safe. If you, or your doctor feels that the trial intervention is causing you to have an adverse effect then it will be stopped and any necessary treatment instigated.
14. Will my taking part in the study be kept confidential?
Yes. The information about you will only be known to members of the research team. All information about you will be held using a unique research number so that you cannot be identified in any results, publications or publicity related to the study.
15. Contact details?
Please note the name and phone number of your research nurse. You can contact this person at any time should you have any questions about the study.
If you (your representative) have any questions as to your rights as a research subject you may contact:
If you have further questions about this study or your participation, or if during your participation you experience a study related injury, or any side effect should occur between the nurse visits/telephone calls, please feel free to contact the nurse for further information and or for action to be taken.
This completes Part 1 of the Information Sheet
If the information in Part 1 has interested you and you are considering participation, please continue to read the additional information in Part 2 before making any decision.
Part 2
16. What if relevant new information becomes available?
Sometimes during the course of a study, new information becomes available about the intervention being studied. If this happens, the doctor will tell you about it and discuss whether or not you wish to continue in the study. If you wish to withdraw from the study, the study doctor will make arrangements for your continued care. If you decide to continue in the study, you may be asked to sign an updated consent form containing the new information. Both the researchers and an independent committee of experts will be looking-out for any new information. If the study is stopped for any reason you will be told why and your continuing care will be arranged.
17. What will happen if I don’t want to carry on with the study?
You are free to withdraw consent from the study without giving a reason at any time. This will not affect the medical care that you receive in any way.
18. What if there is a problem?
Complaints: If you have a concern about any aspect of this study, you should ask to speak with the researchers who will do their best to answer your questions. If you remain unhappy and wish to complain formally, you can do this through the NHS complaints procedure (or private institution). Details can be obtained from the hospital.
Harm: If you are harmed, due to participating in the study and or this is due to someone’s negligence then you may have grounds for a legal action for compensation against the Trust but you may have to pay your legal costs. The normal National Health Service complaints mechanisms will still be available to you.
19. Will my taking part in the study be kept confidential?
Yes. The information about you will only be known to members of the research team. No individuals will be identified in any results, publications or publicity related to the study.
We will ask your permission to inform your General Practitioner about your participation in the study.
20. What will happen to any samples given?
If you develop diarrhoea, your doctors or nurses will collect a stool sample in the usual way. This will be tested in the NHS laboratories to identify the cause of the diarrhoea. For quality control purposes, further testing to identify the strain of bacteria will be done in some of the stools samples that test positive for C. difficile.
21. Will any genetic tests be done?
Genetic tests may be done on the bacteria in stool samples but not on any of the participants. The genetic results will be coded so that the patient is not identifiable.
22. What will happen to the results of the research study?
We will inform the NHS Research and Development Department and publish the findings of the study in the medical literature and widely in the local press. Depending on the results, the NHS may recommend probiotic food supplements as part of routine care for older people receiving antibiotics in hospitals. You will not be identified in any reports or publications.
23. Who is organising and funding this research?
The project is organised and carried-out by the research teams in Swansea University and NHS Trust and County Durham and Darlington Foundation Trust. The NHS Research and Development Health Technology Association are providing funding.
24. Who has reviewed the study?
The NHS Research and Development Health Technology Association has reviewed the study. It has also been given a favourable ethical opinion for conduct in the NHS by the South Wales Ethics Committee. The Medicines and Health Regulatory Authority have approved the probiotic food supplement.
Thank you for taking the time to read this patient information sheet.
Patient consent form
Patient advocate information sheet
The PLACIDE study
Probiotic Lactic acid bacteria and Antibiotic-associated and C. difficilediarrhoea in the Elderly
Patient representatives information sheet
Note: for persons being asked to consider inclusion of a patient who is unable them self to give informed consent (e.g. relative/friend/spouse) it is important that you consider all aspects of the study on their behalf and act in their best interest in respect of inclusion in the study.
Full title of the study: A multicentre, randomised, placebo controlled trial of lactic acid bacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in patients aged 65 years and over admitted to hospital and receiving antibiotics.
Part 1
The patient you represent is invited to participate in a clinical study. Before agreeing to them participate in this study it is important that you read and understand why the research is being done and what will happen during it. Please take time to read this information carefully. Take time to ask as many questions as you want. The study personnel will explain any word or information you do not clearly understand. You may to talk to others about the study if you wish.
Part 1 tells you the purpose of the research and explains what will happen to the patient you represent if they take part.
Part 2 gives you more information about the conduct of the study if you are still interested after reading Part 1.
1. What is the purpose of the study?
We want to find out whether or not giving a probiotic food supplement prevents the diarrhoea that often affects people taking antibiotics. Probiotics are the safe, live, ‘friendly’ bacteria that are found in live yoghurts that you can buy in supermarkets.
Many people admitted to hospital require treatment with antibiotics. Antibiotics change the ‘healthy’ bacteria that live in the gut and this results in diarrhoea in about 1 in 5 people. Diarrhoea is distressing for patients and may also delay recovery from illness and prolong the hospital admission.
Occasionally, antibiotics result in an overgrowth of a potentially dangerous bacterium called ‘C. difficile’. This is also known as ‘C. diff’ and is talked about a lot in the press. This can cause a severe and life-threatening diarrhoeal illness that may require additional medical or surgical treatment. The clinicians working in the hospitals involved in this study perceive C. difficile diarrhoea as a very important problem. This underlies our interest to search for new ways to prevent this problem.
2. Why has the patient I represent been chosen?
The patient has been approached because he/she fulfils the entry criteria for the study. These criteria are:
-
aged 65 years or more and have been admitted to hospital
-
already taking antibiotics or are about to start antibiotic treatment
-
do not have diarrhoea at the moment
-
have not had an adverse reaction to a probiotic food supplement in the past
-
do not have an impaired immune system, an artificial heart valve or active inflammatory bowel disease.
The study is being conducted in Wales and in Durham and we hope to recruit around 2,500 patients.
3. Does the patient have to take part?
It is up to you to decide whether or not the patient you represent can participate. If you do, you will be given this information sheet to keep and be asked to sign an assent form giving consent on behalf of the patient. As the patient’s representative you are still free to withdraw assent from the study at any time and without giving a reason. A decision to withdraw at any time, or a decision not to take part, will not affect the standard of care the patient receives.
4. What will happen to the patient I represent if they take part?
The doctors will treat the illness as usual, including treatment with antibiotics. If the patient represent develops diarrhoea, the nurses and doctors will take stool samples to investigate the cause and treat it in the usual way.
We will ask the patient to take a small amount of powder by mouth once daily for 21 days. He/she will have an equal chance of receiving either the probiotic food supplement (the active intervention) or an inactive substance (the placebo). We will also ask you or the patient to answer questions about any symptoms they may have (diarrhoea, abdominal pain, bloating, flatus, nausea) and also general questions that assess the impact of their illness on their quality of life.
We will keep in-touch with patients to check for these symptoms for 8 weeks after the patient has finished the course of antibiotics. Our research nurses will visit the patient in hospital or telephone or visit you him/her at home if discharged from hospital.
5. Expenses and payments?
Joining the trial will not result in any expenses for the patient. None of the participants will receive any payment.
6. What do I have to do?
Once you have given assented on behalf of the patient to join the trial, we will ask the patient or you on his/her behalf to give information to our research nurses about any symptoms the patient has, including diarrhoea, and also answer some questions that assess how their quality of life is affected. The patient should take the trial intervention as directed together with other medications as prescribed by the doctor. Please note that it is important that the participant avoids taking any probiotic preparations (other than the trial intervention) or live yogurts during the study. The research nurse can give you more guidance on what products to avoid if needed. Please inform the study nurse if any of the patient’s medications change during the study period. While in hospital the research nurse will be able to confirm all medicines that are prescribed.
7. What is the drug that is being tested?
We are testing a probiotic food supplement and this is not a drug. The supplement consists of lactobacilli and bifidobacteria, which are just like the bacteria that live in the bowels of healthy people. The bacteria are Lactobacillus acidophilus (2 strains: CUL60 and CUL21), Bifidobacterium bifidum (CUL20) and Bifidobacterium lactis (CUL34).
People in the placebo group will receive an inactive, inert powder called maltodextrin.
8. What are the alternative procedures or treatments?
There are no other ways for people to protect themselves against antibiotic-associated and C. difficile diarrhoea. The hospitals are trying their best to reduce these problems for all patients by improving hygiene and also being careful with using antibiotics.
9. What are the side effects of treatment?
Probiotics are very safe and we do not anticipate any side effects. There are a small number of case reports where probiotics may themselves have caused infections in people with markedly impaired immune systems or an artificial heart valve. We will not include such people in our study.
10. What are the other possible disadvantages and risks of taking part?
We are not aware of any disadvantages or risks of joining our study.
11. What are the possible benefits of taking part?
All patients in the study, including those in the placebo group, will benefit from regular follow-up for diarrhoea and other symptoms. This may improve the recognition of problems and overall care for all participants.
Participants who receive the active intervention may have a reduced risk of developing antibiotic-associated and C. difficile diarrhoea or may develop milder disease. If the food supplement proves to be successful against C. difficile diarrhoea, fewer cases will reduce the risk of other patients acquiring this infection.
12. What happens when the research stops?
Patients will leave the trial at the end of the 8 weeks follow-up. If the patient you represent requires any further treatment in respect of diarrhoea this will be under the care of the GP or hospital doctor.
We plan to publicise the findings of the study widely in the medical literature and local press. Depending on the results, the NHS may recommend probiotic food supplements as part of routine care for older people receiving antibiotics in hospitals.
13. What if there is a problem?
We do not expect any problems related to the study because probiotics are very safe. If the patient you represent, or your doctor feels that the trial intervention is causing you to have an adverse effect then it will be stopped and any necessary treatment instigated.
14. Will my taking part in the study be kept confidential?
Yes. The information about the patient you represent will only be known to members of the research team. All information about him/her will be held using a unique research number so that they cannot be identified in any results, publications or publicity related to the study.
15. Contact details?
Please note the name and phone number of the patient’s research nurse. You can contact this person at any time should you have any questions about the study.
If you have any questions as to the rights of a research subject you may contact:
If you have further questions about this study or the patient’s participation, or if during you’re their participation they experience a study related injury, or any side effect should occur between the nurse visits/telephone calls, please feel free to contact the nurse for further information and or for action to be taken.
This completes Part 1 of the Information Sheet
If the information in Part 1 has interested you and you are considering participation, please continue to read the additional information in Part 2 before making any decision.
Part 2
16. What if relevant new information becomes available?
Sometimes during the course of a study, new information becomes available about the intervention being studied. If this happens, the doctor will tell you about it and discuss whether or not you wish the patient to continue in the study. If you wish them to be withdrawn from the study, the study doctor will make arrangements for their continued care. If you allow them to continue in the study, you may be asked to sign an updated assent form containing the new information. Both the researchers and an independent committee of experts will be looking-out for any new information. If the study is stopped for any reason you will be told why and the patient’s continuing care will be arranged.
17. What will happen if I/the patient you represent don’t/doesn’t want to carry on with the study?
You are free to withdraw assent for the study without giving a reason at any time. This will not affect the medical care that the patient receives in any way.
18. What if there is a problem?
Complaints: If you have a concern about any aspect of this study, you should ask to speak with the researchers who will do their best to answer your questions. If you remain unhappy and wish to complain formally, you can do this through the NHS complaints procedure (or private institution). Details can be obtained from the hospital.
Harm: If the patient you represent is harmed, due to participating in the study and or this is due to someone’s negligence then you may have grounds for a legal action for compensation against the Trust but you may have to pay your legal costs. The normal National Health Service complaints mechanisms will still be available to you.
19. Will my taking part in the study be kept confidential?
Yes. The information about the patient will only be known to members of the research team. No individuals will be identified in any results, publications or publicity related to the study.
We will ask your permission to inform the patient’s General Practitioner about (their) participation in the study.
20. What will happen to any samples given?
If the patient develops diarrhoea, the doctors or nurses will collect a stool sample in the usual way. This will be tested in the NHS laboratories to identify the cause of the diarrhoea. For quality control purposes, further testing to identify the strain of bacteria will be done in some of the stools samples that test positive for C. difficile.
21. Will any genetic tests be done?
Genetic tests may be done on the bacteria in stool samples but not on any of the participants. The genetic results will be coded so that the patient is not identifiable.
22. What will happen to the results of the research study?
We will inform the NHS Research and Development Department and publish the findings of the study in the medical literature and widely in the local press. Depending on the results, the NHS may recommend probiotic food supplements as part of routine care for older people receiving antibiotics in hospitals. The patient you represent will not be identified in any reports or publications.
23. Who is organising and funding this research?
The project is organised and carried-out by the research teams in Swansea University and NHS Trust and County Durham and Darlington Foundation Trust. The NHS Research and Development Health Technology Association are providing funding.
24. Who has reviewed the study?
The NHS Research and Development Health Technology Association has reviewed the study. It has also been given a favourable ethical opinion for conduct in the NHS by the South Wales Ethics Committee. The Medicines and Health Regulatory Authority have approved the probiotic food supplement.
Thank you for taking the time to read this patient information sheet.
Patient advocate assent form
Appendix 4 Data collection forms
Case report form
Daily follow-up log
Weekly follow-up log
Additional antibiotics form
Severe adverse events form
Diarrhoea sheet
Classification of severity of Clostridium difficile diarrhoea
Classification of severity of Clostridium difficile diarrhoea (PDF download)
Appendix 5 Participant flow chart
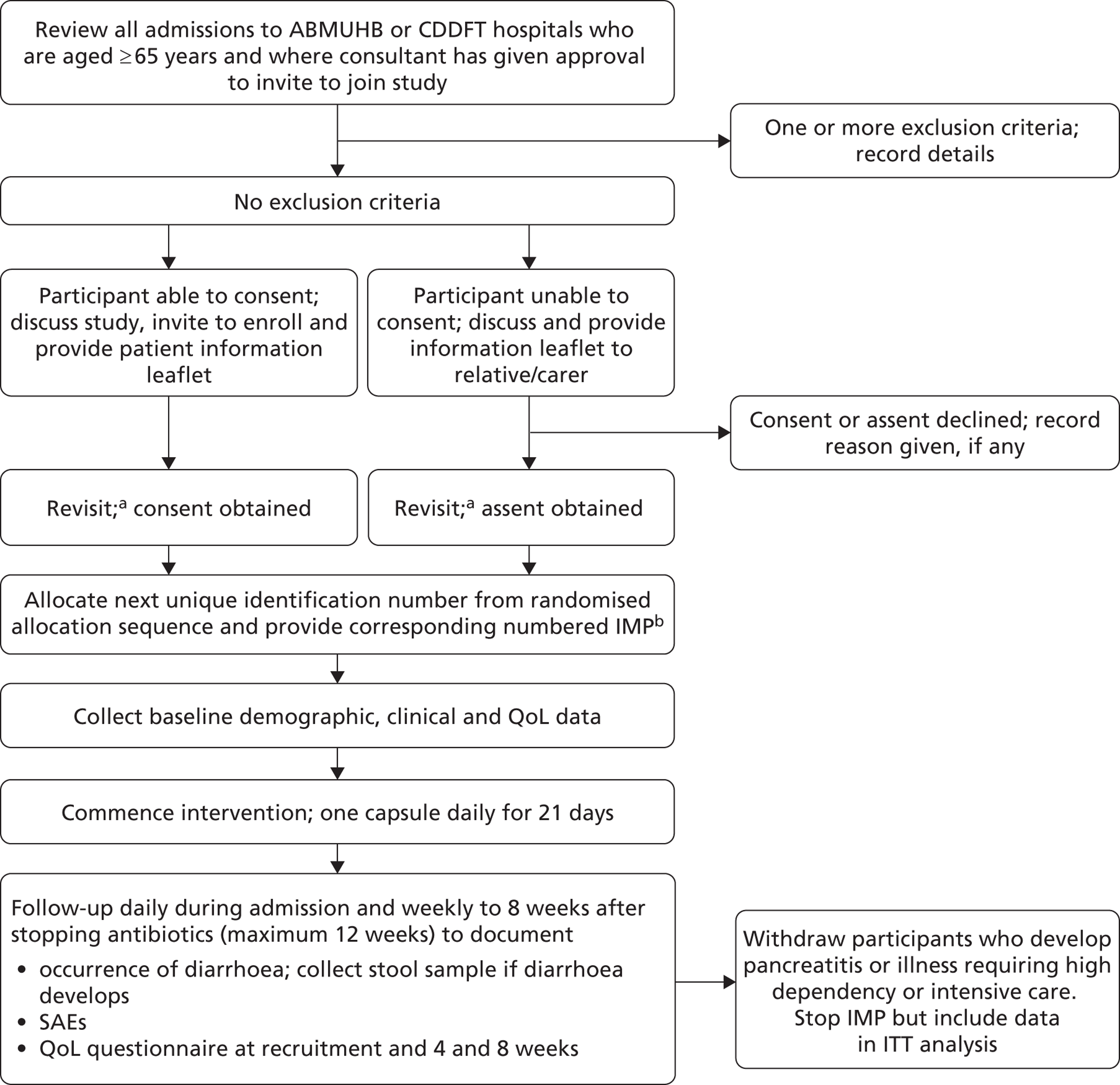
Notes
(a) The patient or next of kin is approached for consent in the afternoon if verbal and written information about the trial is provided in the morning, or the following day if provided in the afternoon; and (b) either 21 capsules of probiotic or placebo.
Appendix 6 Criteria for severity of Clostridium difficile infection
Based on information from case records, the severity of episodes of C. difficile diarrhoea was classified according to the following guidelines:39
Mild
Moderate
-
WCC raised but < 15 × 109/l and
-
stool frequency three to five per day. 39
Severe
-
WCC raised but > 15 × 109/l or
-
an acute rising serum creatinine (> 50% increase above baseline) or
-
temperature > 38.5 °C or
-
evidence of severe colitis (based on clinical examination or imaging). 39
Life-threatening
-
hypotension or
-
partial or complete ileus or
-
toxic megacolon or
-
radiological evidence of severe disease. 39
Appendix 7 Classification of antibiotics (according to British National Formulary 2012)
| Antibiotic class | Drug names |
|---|---|
| Penicillins | |
|
Benzylpenicillin |
|
Flucloxacillin |
|
Amoxicillin, ampicillin, co-amoxiclav |
|
Piperacillin, piperacillin plus tazobactam, ticaricillin |
| Cephalosporins | |
|
Cefalexin, cefradine, cefadroxil |
|
Cefuroxime, cefaclor, cefixime |
|
Cefotaxime, ceftazidime, ceftriaxone |
| Carbapenems and other β-lactams | Ertapenem, imipenem, meropenem, aztreonam |
| Tetracyclines | Tetracycline, demeclocycline, doxycycline, lymecycline, minocylcine, oxytetracycline |
| Aminoglycosides | Gentamicin, amikacin, tobramycin, neomycin |
| Macrolides | Erythromycin, azithromycin, clarithromycin |
| Clindamycin | Clindamycin |
| Sulphonamides and trimethoprim | Co-trimoxazole, trimethoprim |
| Metronidazole | Metronidazole |
| Quinolones | Nalidixic acid, ciprofloxacin, norfloxacin, ofloxacin, levofloxacin, moxifloxacin |
| Glycopeptides | Vancomycin, teicoplanin |
| TB drugs | Ethambutol, rifampicin, streptomycin |
| Others | Chloramphenicol, daptomycin, sodium fusidate, linezolid, nitrofurantoin |
Appendix 8 Protocol
Appendix 9 Information regarding exposure to antibiotics according to centre and severe adverse events in the two intervention arms classified according to MedDRA preferred term and actions taken regarding the trial interventions
| Antibiotic (classes and drugs) | ABMUHB | CDDFT | |||
|---|---|---|---|---|---|
| Singleton, n (%) | Morriston, n (%) | Princess of Wales, n (%) | Durham, n (%) | Darlington, n (%) | |
| Penicillins | 138 (68.0) | 968 (65.4) | 147 (77.0) | 436 (79.7) | 424 (81.4) |
| Benzylpenicillin | 18 (8.9) | 130 (8.8) | 23 (12.0) | 20 (3.7) | 23 (4.4) |
| Penicillinase-resistant penicillin – flucloxacillin | 29 (14.3) | 372 (25.2) | 68 (35.6) | 83 (15.2) | 80 (15.4) |
| Broad-spectrum penicillins | 116 (57.1) | 700 (47.3) | 96 (50.3) | 371 (67.8) | 368 (70.6) |
|
32 (15.8) | 224 (15.1) | 43 (22.5) | 209 (38.2) | 125 (24.0) |
|
0 (0.0) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 2 (0.4) |
|
97 (47.8) | 559 (37.8) | 68 (35.6) | 227 (41.5) | 284 (54.5) |
| Antipseudomonas penicillins | 18 (8.9) | 126 (8.5) | 24 (12.6) | 52 (9.5) | 25 (4.8) |
|
0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.4) | 1 (0.2) |
|
18 (8.9) | 126 (8.5) | 24 (12.6) | 51 (9.3) | 24 (4.6) |
| Cephalosporins | 59 (29.1) | 602 (40.7) | 26 (13.6) | 18 (3.3) | 10 (1.9) |
| First generation | 10 (4.9) | 113 (7.6) | 6 (3.1) | 15 (2.7) | 7 (1.3) |
|
10 (4.9) | 112 (7.6) | 6 (3.1) | 15 (2.7) | 7 (1.3) |
|
0 (0.0) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Second generation | 52 (25.6) | 517 (35.0) | 22 (11.5) | 3 (0.5) | 0 (0.0) |
|
13 (6.4) | 38 (2.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
0 (0.0) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
45 (22.2) | 507 (34.3) | 22 (11.5) | 3 (0.5) | 0 (0.0) |
| Third generation | 2 (1.0) | 16 (1.1) | 0 (0.0) | 0 (0.0) | 3 (0.6) |
|
1 (0.5) | 2 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
0 (0.0) | 11 (0.7) | 0 (0.0) | 0 (0.0) | 3 (0.6) |
|
1 (0.5) | 4 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other antibiotics | 153 (75.4) | 1036 (70.0) | 138 (72.3) | 332 (60.7) | 295 (56.6) |
| Carbapenems and other beta-lactams | 8 (3.9) | 43 (2.9) | 4 (2.1) | 6 (1.1) | 1 (0.2) |
| Ertapenem | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 0 (0.0) |
| Imipenem | 0 (0.0) | 5 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Meropenem | 8 (3.9) | 39 (2.6) | 4 (2.1) | 5 (0.9) | 1 (0.2) |
| Tetracyclines | 36 (17.7) | 235 (15.9) | 26 (13.6) | 74 (13.5) | 62 (11.9) |
| Demeclocycline | 0 (0.0) | 2 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Doxycycline | 36 (17.7) | 226 (15.3) | 26 (13.6) | 69 (12.6) | 55 (10.6) |
| Lymecycline | 0 (0.0) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Oxytetracycline | 0 (0.0) | 4 (0.3) | 0 (0.0) | 6 (1.1) | 4 (0.8) |
| Tetracycline | 0 (0.0) | 2 (0.1) | 0 (0.0) | 1 (0.2) | 3 (0.6) |
| Aminoglycosides | 7 (3.4) | 271 (18.3) | 28 (14.7) | 16 (2.9) | 56 (10.7) |
| Gentamicin | 6 (3.0) | 271 (18.3) | 28 (14.7) | 16 (2.9) | 56 (10.7) |
| Tobramycin | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Macrolides | 45 (22.2) | 144 (9.7) | 34 (17.8) | 152 (27.8) | 125 (24.0) |
| Azithromycin | 0 (0.0) | 2 (0.1) | 4 (2.1) | 9 (1.6) | 9 (1.7) |
| Clarithromycin | 39 (19.2) | 98 (6.6) | 26 (13.6) | 137 (25.0) | 113 (21.7) |
| Erythromycin | 9 (4.4) | 52 (3.5) | 5 (2.6) | 12 (2.2) | 6 (1.2) |
| Clindamycin | 2 (1.0) | 16 (1.1) | 1 (0.5) | 2 (0.4) | 11 (2.1) |
| Sulphonamides and trimethoprim | 40 (19.7) | 267 (18.1) | 30 (15.7) | 88 (16.1) | 45 (8.6) |
| Co-trimoxazole | 0 (0.0) | 5 (0.3) | 0 (0.0) | 1 (0.2) | 0 (0.0) |
| Trimethoprim | 40 (19.7) | 262 (17.7) | 30 (15.7) | 87 (15.9) | 45 (8.6) |
| Metronidazole | 22 (10.8) | 217 (14.7) | 21 (11.0) | 30 (5.5) | 23 (4.4) |
| Quinolones | 43 (21.2) | 229 (15.5) | 16 (8.4) | 41 (7.5) | 36 (6.9) |
| Ciprofloxacin | 38 (18.7) | 224 (15.1) | 15 (7.9) | 31 (5.7) | 20 (3.8) |
| Levofloxacin | 5 (2.5) | 7 (0.5) | 1 (0.5) | 11 (2.0) | 11 (2.1) |
| Moxifloxacin | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.4) | 5 (1.0) |
| Norfloxacin | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Glycopeptides | 6 (3.0) | 133 (9.0) | 9 (4.7) | 11 (2.0) | 19 (3.6) |
| Teicoplanin | 4 (2.0) | 102 (6.9) | 8 (4.2) | 11 (2.0) | 18 (3.5) |
| Vancomycin | 2 (1.0) | 43 (2.9) | 1 (0.5) | 0 (0.0) | 1 (0.2) |
| TB drugs | 1 (0.5) | 31 (2.1) | 3 (1.6) | 6 (1.1) | 5 (1.0) |
| Ethambutol | 0 (0.0) | 0 (0.0) | 2 (1.0) | 1 (0.2) | 0 (0.0) |
| Rifampicin | 1 (0.5) | 31 (2.1) | 3 (1.6) | 6 (1.1) | 5 (1.0) |
| Streptomycin | 0 (0.0) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Others | 5 (2.5) | 56 (3.8) | 8 (4.2) | 17 (3.1) | 5 (1.0) |
| Daptomycin | 0 (0.0) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Linezolid | 0 (0.0) | 2 (0.1) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| Nitrofurantoin | 5 (2.5) | 41 (2.8) | 4 (2.1) | 17 (3.1) | 4 (0.8) |
| Sodium fusidate | 0 (0.0) | 12 (0.8) | 4 (2.1) | 0 (0.0) | 1 (0.2) |
| PT | Probiotic (N = 1470), n (%)b | Placebo (N = 1471), n (%)b | Total (N = 2941), n (%)b |
|---|---|---|---|
| SAE resulted in death | 79 (5.4) | 64 (4.4) | 143 (4.9) |
| Pneumonia | 15 (1.0) | 12 (0.8) | 27 (0.9) |
| General physical health deterioration | 10 (0.7) | 6 (0.4) | 16 (0.5) |
| Obstructive airways disorder | 9 (0.6) | 7 (0.5) | 16 (0.5) |
| Lung neoplasm malignant | 3 (0.2) | 5 (0.3) | 8 (0.3) |
| Cardiac failure | 4 (0.3) | 3 (0.2) | 7 (0.2) |
| Cerebrovascular accident | 4 (0.3) | 3 (0.2) | 7 (0.2) |
| Metastatic neoplasm | 2 (0.1) | 3 (0.2) | 5 (0.2) |
| Sepsis | 3 (0.2) | 2 (0.1) | 5 (0.2) |
| Cardiac arrest | 2 (0.1) | 2 (0.1) | 4 (0.1) |
| Pleural effusion | 2 (0.1) | 1 (0.1) | 3 (0.1) |
| Cardiac failure congestive | 2 (0.1) | 1 (0.1) | 3 (0.1) |
| Elderly | 2 (0.1) | 1 (0.1) | 3 (0.1) |
| Myocardial infarction | 1 (0.1) | 2 (0.1) | 3 (0.1) |
| Pulmonary embolism | 1 (0.1) | 2 (0.1) | 3 (0.1) |
| Renal failure chronic | 2 (0.1) | 1 (0.1) | 3 (0.1) |
| Respiratory failure | 2 (0.1) | 1 (0.1) | 3 (0.1) |
| Urinary tract infection | 0 (0.0) | 3 (0.2) | 3 (0.1) |
| Gastrointestinal haemorrhage | 2 (0.1) | 0 (0.0) | 2 (0.1) |
| Peptic ulcer perforation | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Renal failure | 0 (0.0) | 2 (0.1) | 2 (0.1) |
| Aortic aneurysm rupture | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Bacterial sepsis | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Bladder cancer | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Bladder neoplasm | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Bronchiectasis | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Cardiac death | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Chronic renal failure | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Creutzfeldt–Jakob disease | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Dementia | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Diverticulitis | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Duodenal ulcer perforation | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Hiatus hernia, obstructive | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Hyponatraemia | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Implant site infection | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Ischaemic heart disease | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Left ventricular failure | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Lower gastrointestinal haemorrhage | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Lung infection – pseudomonal | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Pleural neoplasm | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Pulmonary fibrosis | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Pulmonary oedema | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Insufficient details to classify | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| SAE was life-threatening | 6 (0.4) | 5 (0.3) | 11 (0.4) |
| Cardiac arrest | 1 (0.1) | 2 (0.1) | 3 (0.1) |
| Acute renal failure | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Gastric cancer | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Lung neoplasm malignant | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Metastatic neoplasm | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Pulmonary oedema | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Sepsis | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Small intestinal obstruction | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Subdural haemorrhage | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| SAE prolonged or required hospitalisation | 222 (15.1) | 223 (15.2) | 445 (15.1) |
| Pneumonia | 35 (2.4) | 38 (2.6) | 73 (2.5) |
| Obstructive airways disorder | 17 (1.2) | 15 (1.0) | 32 (1.1) |
| Fall | 18 (1.2) | 13 (0.9) | 31 (1.1) |
| Urinary tract infection | 14 (1.0) | 9 (0.6) | 23 (0.8) |
| Cerebrovascular accident | 6 (0.4) | 10 (0.7) | 16 (0.5) |
| Wound infection | 5 (0.3) | 7 (0.5) | 12 (0.4) |
| Cellulitis | 10 (0.7) | 2 (0.1) | 12 (0.4) |
| Lower gastrointestinal haemorrhage | 3 (0.2) | 7 (0.5) | 10 (0.3) |
| Sepsis | 4 (0.3) | 6 (0.4) | 10 (0.3) |
| Cardiac failure congestive | 6 (0.4) | 3 (0.2) | 9 (0.3) |
| Abdominal pain | 4 (0.3) | 3 (0.2) | 7 (0.2) |
| Anaemia | 5 (0.3) | 3 (0.2) | 8 (0.3) |
| Myocardial infarction | 7 (0.5) | 1 (0.1) | 8 (0.3) |
| Angina pectoris | 4 (0.3) | 3 (0.2) | 7 (0.2) |
| Cardiac failure | 3 (0.2) | 4 (0.3) | 7 (0.2) |
| Dyspnoea | 4 (0.3) | 3 (0.2) | 7 (0.2) |
| Gastrointestinal haemorrhage | 4 (0.3) | 2 (0.1) | 6 (0.2) |
| Pleural effusion | 1 (0.1) | 4 (0.3) | 5 (0.2) |
| Viral infection | 3 (0.2) | 3 (0.2) | 6 (0.2) |
| Bacterial sepsis | 2 (0.1) | 3 (0.2) | 5 (0.2) |
| Chest pain | 4 (0.3) | 1 (0.1) | 5 (0.2) |
| Cholecystitis | 2 (0.1) | 2 (0.1) | 4 (0.1) |
| Cholelithiasis | 4 (0.3) | 1 (0.1) | 5 (0.2) |
| Gastroenteritis | 3 (0.2) | 2 (0.1) | 5 (0.2) |
| Joint dislocation reduction | 2 (0.1) | 2 (0.1) | 4 (0.1) |
| Lung infection – pseudomonal | 1 (0.1) | 3 (0.2) | 4 (0.1) |
| Urinary retention | 1 (0.1) | 3 (0.2) | 4 (0.1) |
| Diverticulitis | 4 (0.3) | 0 (0.0) | 4 (0.1) |
| Drug hypersensitivity | 0 (0.0) | 4 (0.3) | 4 (0.1) |
| Haemorrhagic diathesis | 1 (0.1) | 2 (0.1) | 3 (0.1) |
| Pulmonary oedema | 2 (0.1) | 2 (0.1) | 4 (0.1) |
| Constipation | 1 (0.1) | 2 (0.1) | 3 (0.1) |
| General physical health deterioration | 2 (0.1) | 1 (0.1) | 3 (0.1) |
| Pulmonary embolism | 1 (0.1) | 2 (0.1) | 3 (0.1) |
| Renal failure | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Upper gastrointestinal haemorrhage | 3 (0.2) | 0 (0.0) | 3 (0.1) |
| Acute coronary syndrome | 0 (0.0) | 2 (0.1) | 2 (0.1) |
| Arrhythmia | 2 (0.1) | 0 (0.0) | 2 (0.1) |
| Bladder catheter management | 0 (0.0) | 2 (0.1) | 2 (0.1) |
| Convulsion | 2 (0.1) | 0 (0.0) | 2 (0.1) |
| Decubitus ulcer | 0 (0.0) | 2 (0.1) | 2 (0.1) |
| Deep-vein thrombosis postoperative | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Dehydration | 0 (0.0) | 2 (0.1) | 2 (0.1) |
| Haematoma | 2 (0.1) | 0 (0.0) | 2 (0.1) |
| Haematuria | 0 (0.0) | 2 (0.1) | 2 (0.1) |
| Hepatic cirrhosis | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Hypercalcaemia | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Ischaemic heart disease | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Metastatic neoplasm | 2 (0.1) | 0 (0.0) | 2 (0.1) |
| Oesophageal neoplasm | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Organ failure | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Pulmonary fibrosis | 0 (0.0) | 2 (0.1) | 2 (0.1) |
| Pyelonephritis | 0 (0.0) | 2 (0.1) | 2 (0.1) |
| Social problem | 2 (0.1) | 0 (0.0) | 2 (0.1) |
| Transient ischaemic attack | 2 (0.1) | 0 (0.0) | 2 (0.1) |
| Upper respiratory tract infection | 0 (0.0) | 2 (0.1) | 2 (0.1) |
| Wound dehiscence | 0 (0.0) | 2 (0.1) | 2 (0.1) |
| Adverse drug reaction | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Agitation | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Anorectal varices haemorrhage | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Aortic surgery | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Appendiceal abscess | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Arterial thrombosis limb | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Arthritis infective | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Atrial fibrillation | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Bile duct T-tube removal | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Bile duct stent removal | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Bone trimming | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Brain neoplasm | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Bronchial fistula repair | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Bronchospasm | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Cardiac pacemaker replacement | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Cardiac pacemaker revision | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Cholecystectomy | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Colostomy | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Cystoscopy | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Deep-vein thrombosis | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Dementia | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Diabetic foot infection | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Dialysis related complication | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Diarrhoea | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Duodenal ulcer haemorrhage | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Eating disorder | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Eczema | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Foreign-body aspiration | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Gallbladder empyema | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Gangrene | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Gastroenteritis norovirus | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Gastrointestinal examination | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Groin abscess | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Haematoma infection | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Heart valve replacement | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Herpes zoster ophthalmic | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Hip fracture | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Hyperkalaemia | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Hypertension | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Hypocalcaemia | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Hypoglycaemia | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Hypokalaemia | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Hyponatraemia | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Implant site infection | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Infected bites | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| International normalised ratio abnormal | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Intestinal polyp | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Ischaemic limb pain | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Joint injection | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Joint surgery | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Leg amputation | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Limb crushing injury | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Liver abscess | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Lung neoplasm malignant | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Malnutrition | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Mediastinal abscess | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Mouth haemorrhage | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Multiple myeloma | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Muscle swelling | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Musculoskeletal chest pain | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Myalgia | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Nephrectomy | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Nephrolithiasis | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Non-Hodgkin’s lymphoma | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Oesophagogastroscopy | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Oral discharge | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Otitis externa | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Pain in extremity | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Pancreatitis | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Peptic ulcer haemorrhage | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Peptic ulcer perforation | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Peritonitis | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Pneumonia aspiration | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Pneumothorax | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Renal failure chronic | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Shunt infection | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Skin graft infection | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Skin ulcer | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Supraventricular tachycardia | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Syncope | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Trigeminal neuralgia | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Umbilical hernia, obstructive | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Urethral stent insertion | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Ventricular tachycardia | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Viral upper respiratory tract infection | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Volvulus | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Wound complication | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Wound haematoma | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Wound infection | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Insufficient details to classify | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| SAE resulted in persistent or significant disability or incapacity | 2 (0.1) | 2 (0.1) | 4 (0.1) |
| Cerebrovascular accident | 1 (0.1) | 2 (0.1) | 3 (0.1) |
| Urinary tract infection | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Other significant medical event | 6 (0.4) | 5 (0.3) | 11 (0.4) |
| Upper gastrointestinal haemorrhage | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Cardiac failure | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Cholelithiasis | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Dysentery | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Haemorrhoids | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Incision site haematoma | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Joint dislocation reduction | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Lower gastrointestinal haemorrhage | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Psoas abscess | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Renal failure | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| PT | Probiotic (N = 1470), n (%)b | Placebo (N = 1471), n (%)b | Total (N = 2941), n (%)b |
|---|---|---|---|
| IMP withdrawn | 8 (0.5) | 6 (0.4) | 14 (0.5) |
| Lower gastrointestinal haemorrhage | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Pneumonia | 2 (0.1) | 0 (0.0) | 2 (0.1) |
| Acute renal failure | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Cerebrovascular accident | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Cholelithiasis | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Drug hypersensitivity | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Duodenal ulcer perforation | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Haemorrhagic diathesis | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Metastatic neoplasm | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Peptic ulcer perforation | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Sepsis | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Upper gastrointestinal haemorrhage | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| IMP withdrawn temporarily | 44 (3.0) | 46 (3.1) | 90 (3.1) |
| Pneumonia | 4 (0.3) | 5 (0.3) | 9 (0.3) |
| Urinary tract infection | 3 (0.2) | 3 (0.2) | 6 (0.2) |
| Cerebrovascular accident | 3 (0.2) | 2 (0.1) | 5 (0.2) |
| Obstructive airways disorder | 3 (0.2) | 2 (0.1) | 5 (0.2) |
| Angina pectoris | 2 (0.1) | 2 (0.1) | 4 (0.1) |
| Fall | 1 (0.1) | 3 (0.2) | 4 (0.1) |
| Wound infection | 1 (0.1) | 3 (0.2) | 4 (0.1) |
| Cholecystitis | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Myocardial infarction | 3 (0.2) | 0 (0.0) | 3 (0.1) |
| Viral infection | 3 (0.2) | 0 (0.0) | 3 (0.1) |
| Abdominal pain | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Bacterial sepsis | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Cardiac arrest | 0 (0.0) | 2 (0.1) | 2 (0.1) |
| Cellulitis | 2 (0.1) | 0 (0.0) | 2 (0.1) |
| Gastrointestinal haemorrhage | 2 (0.1) | 0 (0.0) | 2 (0.1) |
| Haematoma | 2 (0.1) | 0 (0.0) | 2 (0.1) |
| Joint dislocation reduction | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Lung infection – pseudomonal | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Appendiceal abscess | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Bronchospasm | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Cardiac pacemaker revision | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Chest pain | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Cholelithiasis | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Deep-vein thrombosis postoperative | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Diabetic foot infection | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Diverticulitis | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Dysentery | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Gastroenteritis | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Hepatic cirrhosis | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Incision site haematoma | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| International normalised ratio abnormal | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Ischaemic limb pain | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Joint injection | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Leg amputation | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Lower gastrointestinal haemorrhage | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Nephrectomy | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Pain in extremity | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Pneumonia aspiration | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Psoas abscess | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Pulmonary embolism | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Pulmonary oedema | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Sepsis | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Shunt infection | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Skin graft infection | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Small intestinal obstruction | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Upper gastrointestinal haemorrhage | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Upper respiratory tract infection | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Viral upper respiratory tract infection | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Wound dehiscence | 0 (0.0) | 1 (0.1) | 1 (0.0) |
| Wound haematoma | 0 (0.0) | 1 (0.1) | 1 (0.0) |
List of abbreviations
- AAD
- antibiotic-associated diarrhoea
- ABMUHB
- Abertawe Bro Morgannwg University Health Board
- ACE
- angiotensin-converting enzyme
- CDD
- Clostridium difficile diarrhoea
- CDDFT
- County Durham and Darlington Foundation Trust
- CDI
- Clostridium difficile infection
- CEAC
- cost-effectiveness acceptability curve
- cfu
- colony-forming unit
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CTA
- clinical trial authorisation
- EQ-5D
- European Quality of Life-5 Dimensions
- GP
- general practitioner
- HIV
- human immunodeficiency virus
- HRQL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IMP
- investigational medicinal product
- IQR
- interquartile range
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ITT
- intention to treat
- MCS
- mental component summary
- MedDRA
- Medical Dictionary for Regulatory Activities
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NCIMB
- National Collection of Industrial, Food and Marine Bacteria
- NGT
- nasogastric tube
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PCS
- physical component summary
- PLACIDE
- probiotic lactobacilli and bifidobacteria in antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in the elderly
- PP
- per protocol
- PPI
- proton pump inhibitor
- PT
- preferred term
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- relative risk
- SAE
- serious adverse event
- SF-12 v2
- generic Short Form questionnaire-12 items version 2
- SOC
- system organ class
- VAS
- visual analogue scale
- WCC
- white blood cell count