Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/44/05. The contractual start date was in May 2008. The draft report began editorial review in January 2013 and was accepted for publication in June 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Christie et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction to CASCADE
Type 1 diabetes
Type 1 diabetes (T1D) is one of the most common chronic conditions of childhood and adolescence. Treatment is a taxing regimen of daily insulin therapy, blood glucose (BG) self-monitoring, controlling and calculating carbohydrate (CHO) intake and matching insulin to CHO, together with careful exercise management. Insulin replacement therapy easily controls hyperglycaemia and ketoacidosis, but the main treatment issue is supporting self-management by families and young people to maximise normality of BG and quality of life (QoL), preventing long-term complications.
Epidemiology of type 1 diabetes
There is a significant increase in the number of children and young people diagnosed with T1D and other variants of diabetes. The current prevalence of T1D in the UK is 1 per 700–1000 children, yielding a total population of over 29,000. 1 Peak age for diagnosis is between 10 and 14 years of age. 2
Diagnosis in the under-fives has risen relentlessly over the last 10–15 years. The reason for this is not clear. Changing incidence rates of childhood obesity have been suggested (‘The Accelerator Hypothesis’), with little to support this other than observational studies. 3 Young children have lower insulin needs and unpredictable food intake, and are poorly served by conventional multiple injection therapies. The UK National Institute for Health and Care Excellence (NICE) recommends insulin pump therapy at diagnosis for management of this age group to avoid persistent hyperglycaemia and inadvertent hypoglycaemia, which may be detrimental to the developing brain. 4
Type 1 diabetes is a major health burden for the individual and society. Early onset in children is associated with an increased risk of developing complications in their 30–40s, and the estimated cost of care and lost earnings in the USA has been estimated to escalate from teenage years to 60 years of age. T1D is also associated with a high mortality, both at diagnosis and especially in the critical period of transition to adult services.
Monitoring of glycaemic control
Avoiding hyperglycaemia and hypoglycaemia requires BG monitoring using two modalities. The first is regular self-monitoring of current BG, ideally six or more times a day. Greater frequency of BG self-monitoring is associated with better glycaemic control. Regular monitoring allows correction of high or low BG into the normal range (4–7 mmol/l), and aids accurate dosing of insulin for CHO intake with meals and snacks. Potentially painful finger-pricks needed for BG measurement are a significant disincentive to frequent testing for many and may impair QoL.
The second is assessment of long-term glycaemic exposure by measuring the glycosylated fraction of haemoglobin (HbA1c). This estimates the exposure of red cells to glucose in the bloodstream over a 8- to 12-week period. The recommended target for prevention of long-term complications is a HbA1c value of < 7.5% (< 58 mmol/mol). In the UK National Paediatric Diabetes Audit (NPDA), < 16% of children and adolescents reached this target. 5
Short-term complications
Diabetic ketoacidosis
In T1D, diabetic ketoacidosis (DKA) results from either insufficient insulin or lack of insulin efficacy (e.g. during intercurrent illness). DKA carries a significant mortality rate (0.2%), largely from cerebral oedema and hypokalaemia. DKA is often present at diagnosis. Approximately 25% of all newly diagnosed children are admitted in DKA at diagnosis (35% in those of < 5 years). 6,7
Hypoglycaemia
The most common short-term complication of T1D is low BG (hypoglycaemia). Hypoglycaemia is a result of an imbalance of insulin to both ingested CHO and the current BG level. Hypoglycaemia (BG level of < 4.0 mmol/l) initially causes symptoms associated with activation of the sympathetic nervous system (i.e. adrenaline release). Severe hypoglycaemia (BG level of < 2.5 mmol/l) results in insufficient glucose for neuronal activity (neuroglycopenia), resulting in impaired consciousness, bizarre behaviour, seizures, coma and death. Persistent or frequent recurrent hypoglycaemia may impact on short- and long-term neurocognitive functioning. 8
Consequences of long-term hyperglycaemia
Microvascular and macrovascular disease due to persistent exposure to excess glycaemia reduces life expectancy (on average by 23 years in people with T1D). 9 Microvascular complications may present within 5–10 years post diagnosis and are frequently seen in adolescence and early adulthood, and include significant visual loss, chronic renal failure and dialysis, and autonomic symptoms that include impaired peripheral sensation, pain, and gastrointestinal and genitourinary problems. The International Society for Paediatric and Adolescent Diabetes (ISPAD) recommend screening for retinopathy (by retinal review) and microalbuminuria (through urine albumin–creatinine ratio) at 11 years (with 2 years’ diabetes duration), at 9 years (with 5 years’ duration) and after 2 years’ duration in adolescence. 10
Macrovascular disease presents later in adult life with problems including heart attacks, strokes and lower limb problems including ulcers and gangrenous extremities requiring amputation. High glucose variability, i.e. rapid alternation of hyper-, normo- and hypoglycaemia, may independently increase risk of cardiovascular end points, although this remains controversial. 11
Suboptimal BG control through childhood and adolescence is a significant risk factor for complications in later adult life. 12 Key outcome studies [e.g. Diabetes Control and Complications Trial (DCCT)] have demonstrated improved diabetes control in childhood (or later improved control) can reduce the incidence and progression of microvascular complications. 13,14
The relationship between HbA1c and relative risk of developing eye, kidney and nerve problems is not linear. Small changes from very poor to reasonable control (e.g. 12–9%) reduces risk fivefold but does not reduce it to the population background. Only moving towards near-normal values of HbA1c will effectively reduce the risk in T1D to that of the general population.
Chronic hyperglycaemia leads to persistent glucose wasting in the urine, resulting in calorie insufficiency, poor or absent weight gain, and thus poor growth and pubertal delay. Optimising adherence to insulin therapy can reverse this.
Common comorbid conditions
The most common conditions comorbid with T1D are autoimmune conditions. These include thyroid disease (most commonly primary hypothyroidism), adrenal hypofunction (Addison’s disease) and coeliac disease, although other autoimmune conditions, such as cystic fibrosis, are described in association with diabetes.
Management of type 1 diabetes in children and adolescents
The goals of diabetes management are adequate glycaemic control while maintaining high QoL. Studies from the 1990s onwards have made it clear that tight glycaemic control is important to attain and maintain from the point of diagnosis. 14
The basic principle of insulin replacement therapy is to mimic the normal physiology of insulin secretion by:
-
Ensuring that there is always insulin around in the background throughout the 24-hour period. At night this is essential to switch off hepatic glucose production.
-
Deliver a bolus of insulin in a dose-dependent manner for whatever amount of CHO is consumed.
-
Bring any BG that is high or low back into target range.
-
Insulin can be delivered through multiple daily injections (MDIs) or through constant subcutaneous infusion of insulin. Intensive regimens are considered best practice and in many centres are commenced at diagnosis.
-
As regimens become more ‘physiological’, so too do requirements for adherence to achieve the best levels of glycaemic control.
Technological approaches
Advancing technologies such as the artificial pancreas are providing promising results in adults with diabetes. 15 Technological advances underpin increasingly sophisticated insulin delivery and self-monitoring devices. 15,16 Recent reviews report a wide range of current options. 17–22 Individual and group technology-based interventions include digital devices, video games, online chat rooms and social networks designed to engage, motivate, support and inform young people, although effect on glycaemic control remains modest. 23–30
The multidisciplinary team
Safe and effective diabetes care for children and young people requires a well-resourced multidisciplinary team (MDT) competent in integrating clinical, educational, dietetic, lifestyle, mental health and foot care aspects of diabetes. 31
Intensification of treatment supported by the MDT can produce dramatic improvements in HbA1c. Poor control is common when children are asked to take responsibility for self-care without sufficient cognitive and social maturity. 32 Involving families,33 supporting parental monitoring34 and facilitating shared responsibility for disease management contribute to good glycaemic control and adherence. The challenge is to find patient-centred models of care that can be implemented in clinics and create similar improvements in HbA1c level to those achieved in the DCCT, with subsequent reductions in the development of diabetes-related complications.
Psychosocial aspects of diabetes
Adolescence disrupts the precarious balance managing diet, activity and insulin. 35 There is an increased risk of depression, anxiety, disordered eating, disrupted body image, adverse effects on behaviour and increases in suicidal thoughts in this age group. Depression is associated with both poor glycaemic control and low regimen adherence. 36–44 Other outcomes include increased vigilance by parents and diabetes teams and family conflict. Given the significant number of young people who have unique needs as a consequence of this particular developmental stage, training in adolescent health and medicine is increasingly important.
Reactions to a diagnosis of diabetes
Initial reaction to a diagnosis of diabetes can be devastating. A loss of spontaneity and restrictions on activities create a sense that things will never be the same. 45 Adjustment improves with time after diagnosis. However, parents were clear that they never fully ‘accept’ the diagnosis. 46,47 Episodes of grief are described 7 years post diagnosis triggered by regimen changes, injections, hospitalisation, discussions about diabetes control and worry about complications, attending clinics and meeting new medical teams – reminding them that their child is different. 48
Parents worry over the long term, uncertainty about the future, medical procedures and finance. Loss of pay and costs of travelling to appointments creates additional stress. 45 For some, balancing a career and managing the complexities and demands of a child’s treatment regimen becomes untenable.
Adherence to the treatment regimen
Adherence is a common problem in adolescents in general. 49,50 Although adherence to different components of the regimen may be unrelated to each other,51 insulin omission is common in adolescent girls who are concerned with body weight issues. 52–56 Failure to monitor BG or adjust insulin is also common. 57 Demographic, psychosocial and health-care system factors all influence adherence. Poorer adherence is reported in children and adolescents with T1D from ethnic minority backgrounds, of low socioeconomic status (SES) and from single-parent families. 58 Adolescence is also a risk factor for low adherence and poor glycaemic control. This is related to the effects of diabetes on major developmental changes that occur in adolescence and include hormonal changes associated with puberty, resulting in decreased insulin sensitivity negatively affecting BG metabolism, leading to increases in BG levels. 59 Alongside these physical factors, self-management, increasing independence, emerging sexuality and increased stress from peer and academic pressures are all associated with deteriorating glycaemic control in adolescence. 60
Healthier family functioning just after diagnosis and greater family support are associated with better adherence over time. 61–63 Good family communication patterns,64 low family conflict65,66 and good problem-solving67 are also associated with better adherence. Frustration and guilt at failure to achieve optimal outcomes can be exacerbated by existing psychological issues within families.
Psychosocial variables
High self-esteem, appropriate health beliefs (e.g. perceived threat of diabetes low and perceived benefits to cost ratio high)68–70 and the ability to cope with the stress of negative life events (ranging from a new diagnosis to daily hassles) predict better adherence. 58,71 Peer support is positively related to adherence, particularly for dietary and exercise behaviours. 72 However, pressure to conform to social situations and be accepted by peers is related to decreased glucose monitoring. 49,73,74 Young people who see themselves as having little internal control over their health and who attribute negative events to external sources (i.e. out of their control) have lower adherence. 75
When young people identify other people’s reactions to their self-care as negative, they are also more likely to have adherence difficulties. 72,76,77 Maladaptive coping, such as risk behaviours or withdrawal from family or peer interaction, is associated with poor adherence. 78,79
Psychological and psychoeducational interventions
Person-centred therapies, motivational interviewing (MI), positive reinforcement behavioural contracts, negotiation of diabetes management goals and training in communication, coping and collaborative problem-solving skills are potential approaches to decreasing psychological distress and improving glycaemic control and QoL.
Managing the demands of a diabetes regimen
Stress management programmes using cognitive-restructuring and problem-solving strategies have limited impact. 80,81 In contrast, individual and group interventions focusing on peer group support, problem-solving and coping skills impact on short-term glycaemic control, showing positive effects on adherence including improved self-perception and increased knowledge about diabetes and decreases in diabetes-related conflict. 82,83 A problem-solving skills programme improved BG monitoring but failed to show improvements in problem-solving or HbA1c level. 84 In contrast, social problem-solving, social skills training, cognitive–behaviour techniques and conflict resolution skills for children transferring to intensive diabetes regimens reported improvement in glycaemic control and QoL, maintained at 1-year follow-up. 85–87 The groups did not affect adverse outcomes of hypoglycaemia, DKA or weight gain in boys but decreased the incidence of weight gain and hypoglycaemia in girls.
Diabetes summer camps offering peer interaction, sports and recreational activities alongside diabetes self-management training are highly evaluated; however, only show short-term changes in self-management and knowledge and fail to demonstrate lasting improvements in HbA1c level without ongoing support. 88,89
Focusing on psychological therapies
The effectiveness of psychological and psychoeducational interventions to improve adherence, glycaemic control, psychosocial functioning and QoL is debated, not only in T1D,90,91 but also in paediatric chronic illness in general. 92 Systematic reviews offer limited evidence that psychological interventions in children and young people with diabetes improve adherence and glycaemic control, with small to medium effects on physical and psychosocial outcomes. 91,93 Interventions that result in modest improvements focus on increasing knowledge/skills, addressing specific psychosocial issues and addressing self-management behaviours, but are often not sustained. Continuous support delivered by a diabetes MDT may be equally as important as short-term interventions. DeWit et al. 94,95 reported that asking young people about QoL during clinic visits improved QoL and satisfaction with care but had no effect on glycaemic control.
A recent meta-analysis of 15 randomised controlled trials (RCTs) of adherence interventions in 997 adolescents with T1D found a mean effect size of 0.11 (95% CI −0.01 to 0.23). 96 Modest improvements in glycaemic control were found in interventions that included emotional, social or family targets in addition to specific behavioural goals. 96 Interventions are most likely to be effective if they help the adolescent connect the many different aspects of diabetes management with all aspects of daily living. 97
Family interventions
Because of the complexity of diabetes management and the importance of family involvement and support, most interventions include a family component. In a review of family interventions, a positive effect was reported in 5 out of 19 studies, suggesting that family interventions may improve diabetes-related knowledge and glycaemic control. 98 A teamwork intervention increased family involvement and prevented an expected deterioration in glycaemic control. 98,99 However, the quality of family relationships may not be causally related to adherence, therefore decreasing family conflict and improving parent–child relationships alone may not result in improved adherence or glycaemic control.
Multisystemic therapy and an intensive home and community family intervention, incorporating developmentally appropriate negotiated responsibility,100 significantly improved adherence to BG testing, improved glycaemic control and decreased the number of inpatient admissions. 101,102
Behavioural family systems therapy (BFST) was developed for families of adolescents with clinically significant conduct-related problems. 103 The intervention includes cognitive restructuring of irrational beliefs and targets problematic family characteristics, family communication and problem-solving. Initial adaptations for children and families with diabetes addressed general developmental issues, such as managing curfews, chores and focused less on diabetes treatment-related treatment adherence or management. 104–106 BFST enhanced family communication, improved parent–adolescent relations, reduced behaviour problems and general and diabetes-related family conflict. 104–108 The effects on psychological adjustment depended on the adolescent’s age and gender but overall there was little effect on adjustment to diabetes or diabetic control. 104,105
A revised Behavioural Family Systems Therapy–Diabetes integrated empirically supported intervention strategies focusing on specific behaviours, as well as the social context of diabetes treatment-related behaviour including:
-
targeting at least two or more diabetes problems
-
behavioural contracting109
-
parental simulation of living with diabetes100
-
involving peers, siblings, and teachers, and running sessions in different locations.
Family conflict decreased as well as improvements in adherence. HbA1c level was significantly reduced, particularly among adolescents with poor metabolic control. Change in treatment adherence correlated significantly with change in HbA1c level at each follow-up. 105,106,111
These approaches show encouraging results; however, they are resource-intensive, requiring highly skilled practitioners who are able to travel into the family home. Although effective, this strategy is unlikely to be incorporated into UK clinical practice until shown to be clearly cost-effective.
Cognitive–behavioural therapy
Cognitive–behavioural therapy (CBT) can be delivered only by trained practitioners and usually requires 6–12 sessions. The client connects thoughts, feelings and behaviours. This requires a degree of engagement and participation that is often missing in adolescents. Although effective, standardised interventions for behaviour problems or depression in adolescents are available, no studies have targeted specific psychological disorders in adolescents with diabetes. A review found that the majority of studies used non-standardised approaches. 112
Motivational interviewing
Motivational interviewing is a person-centred therapy that works to resolve ambivalence about behaviour change. It creates a shared agenda, inviting the client to identify his/her goal (e.g. young people may say they want to talk about problems at school, whereas parents may want to talk about blood tests). The clinician negotiates how to explore these issues during the consultation. MI invites the client to be in charge of making changes, and looks for statements about intention to change and optimism about the future. The client identifies importance of change, confidence in their ability to change and when change will be a priority.
Motivational interviewing in paediatric populations has been increasingly explored. 113–115 Early work demonstrated the potential of MI to decrease harm-reduction behaviours in relation to alcohol and polysubstance use. 113,114 A review of nine RCTs in health-related domains, including diabetes, reported seven with positive findings on the effectiveness of MI. All RCTs that specifically addressed T1D related to the adolescent age group. 113,114,116 Two studies using MI alone117,118 found a significant reduction in HbA1c level, reduced fear of hypoglycaemia, and improved QoL and positive well-being. MI combined with other therapeutic approaches, such as dietary advice or CBT, has reported reductions in HbA1c level, a greater sense of control, improved perceptions of diabetes and improved self-esteem. 119–121
Attempts to incorporate MI into other formats, for example a six-session ‘personal trainer’ intervention delivered by non-clinical practitioners,122 have shown promising results, lowering HbA1c levels in older teenagers.
One response to the scarcity of mental health resources in the UK has been to ‘skill up’ staff and introduce components of successful interventions into routine consultations. MI122,123 and other brief therapy approaches have the potential to offer diabetes teams ways to communicate with young people and encourage greater self-management. 124,125 However, a recent study that attempted to deliver MI components as part of routine consultations proved challenging to implement and had no impact on either HbA1c level or psychosocial measures. 123–125
Solution-focused brief therapy
Solution-focused brief therapy (SFBT) relies on the clinician noticing what works successfully during conversations and doing more of this. 124 SFBT assumes the clients are the expert in their own situation and invites them to describe their preferred future and focus on what is already working. This creates opportunities to notice small changes and identify what makes this possible. 126 The clinician does not attempt to find the cause or ‘take the problem away’. The clinician and family work collaboratively to find already existing solutions to manage injections, finger-pricks or eating difficulties. They work together to stop diabetes getting in the way of family communication and find ways to manage sadness or anger. The family takes the lead in relation to ‘non-adherence’, focusing on what is working for them.
Solution-focused brief therapy is an effective approach for most, including those with severe and chronic problems. 127 The latest outcome evaluation research128 reviewed 109 SFBT studies including two meta-analyses and 19 RCTS. Nine RCTs found that SFBT has a greater effect than other approaches, for example CBT. Of the comparison studies, 34 out of 43 favoured SFBT. Solution-focused practice has been recommended within clinical practice guidelines. 120,129,130 SFBT combined with MI has been successfully integrated into diabetes clinical service,124 reducing HbA1c level in children and adolescents. 121
Solution-focused conversations create a different experience for families. Young people who have been blamed or criticised and described as non-adherent or manipulative ‘grow visibly taller in their chair’ given the opportunity to talk about their strengths and abilities and describe positive steps they are already taking to get their lives back on track.
This approach is increasingly relevant to the management of long-term chronic illness and models of empowerment. Nurses trained in SFBT showed positive changes in their practice and improved willingness to communicate with troubled people. 124,131 Changes to practice centred on the rejection of problem-orientated discourses and reduced feelings of inadequacy and emotional stress.
SFBT techniques may be relevant to nursing and a useful, cost-effective approach to the training of communication skills . . . provides a framework and easily understood tool-kit that are harmonious with nursing values.
Bowles, Mackintosh and Torn131
Case management/educational approaches
Other interventions have focused on case management and/or diabetes education. Enhanced case management increased the frequency of clinic visits, reduced hypoglycaemia and hospital admissions and improved glycaemic control in ‘high-risk’ youths. 132 Education and telephone case management has improved adherence and self-efficacy but with little influence on HbA1c level. 133,134 The NICE Health Technology Appraisal on patient education models (http://publications.nice.org.uk/guidance-on-the-use-of-patient-education-models-for-diabetes-ta60) and National Diabetes Support Team (NDST) identified a lack of nationally evaluated paediatric education programmes. 135 Very few, if any, paediatric clinics in the UK offer an evaluated structured education programme as part of routine clinical care. 4
In the last 5 years, a number of studies were initiated to tackle this issue. Programmes vary in methodology, style of intervention and number of patients recruited. Appendix 1 describes details of the most recent nine trials. All have failed to show positive change in glycaemic control. 136–138
The CASCADE study
The Child and Adolescent Structured Competencies Approach to Diabetes Education (CASCADE) intervention is a competency-driven, motivational, patient-centred structured intensive psychoeducational programme designed to improve diabetic control, self-management and QoL in children and adolescents. Intervention content and development are described in Chapter 2 . CASCADE is a complex intervention developed using the Medical Research Council (MRC) complex intervention evaluation framework. 139 We have already conducted the Phase I study [Modelling – defining components of the intervention and Phase II study (Exploratory Trial Phase)]. The present study is a multicentre cluster RCT with integral process and economic evaluation to investigate the effectiveness of CASCADE.
Research objectives
To:
-
assess the feasibility of the CASCADE intervention within a standard clinic setting for a diverse range of young people
-
investigate the effects on long-term glycaemic control of diabetes
-
evaluate the impact of the intervention on QoL using well-validated and reliable self-report and parental measures
-
investigate the impact on psychosocial functioning, including (1) emotional and behavioural adjustment of children and young people; (2) family functioning; and (3) self-management, decision-making and self-efficacy
-
investigate cost-effectiveness.
Conclusions
Type 1 diabetes in children and young people is increasing worldwide, with a particular increase in those of < 5 years. Effective glycaemic control requires a careful balancing act between insulin, food and physical activity. Intensive regimens are best practice, and in many centres these are commenced at diagnosis. Modern diabetes regimens can be oppressive for children, young people and families. Despite intensive regimens offering the best possible control, fewer than one in six children and young people achieve HbA1c values in the range identified as providing best future outcomes. One-third of all children with T1D in the UK are at significant risk for developing long-term complications. The challenge of how to address non-adherence is significant. Evidence to support one approach over another remains limited. Moderate evidence supports the effectiveness of psychological interventions in improving adherence. However, only 20% of children and adolescent services in the UK report adequate access to psychological services. 140
There is an urgent need for pragmatic, feasible and effective structured education programmes that are deliverable within clinics to targeted groups, which improve both glycaemic control and QoL. CASCADE was designed to respond to policy goals addressing strengths and weaknesses of other approaches. The aim was to refine and test the effectiveness of a pragmatic intervention delivered by trained educators. The intervention offers both structured education – to ensure that young people know what they need to know – and a delivery model designed to motivate self-management through empowerment techniques. The next chapter discusses the background to the development of structured education approaches and the CASCADE intervention.
Chapter 2 The development of the CASCADE intervention
Introduction
The CASCADE intervention is a manual-based structured education programme incorporating psychological approaches to increase engagement and enhance behaviour change in children, young people and families. The intervention has four modules led by a paediatric diabetes specialist nurse (PDSN) with a minimum of one additional member of the diabetes team.
This chapter outlines:
-
the background to structured education programmes
-
the CASCADE educational approach
-
the CASCADE underpinning philosophy
-
a summary of each CASCADE module
-
the educational theory and key components of the training workshops.
Structured education programmes
There are no effective structured education programmes for children and young people with diabetes in the UK. Patient-centred care and timely access to specialist education and support form the central pillars of the Diabetes National Service Framework,141 the NICE guidance for T1D4 and ‘Making Every Young Person with Diabetes Matter’. 142 The NICE Health Technology Appraisal on patient education models (http://publications.nice.org.uk/guidance-on-the-use-of-patient-education-models-for-diabetes-ta60) recommends that ‘structured patient education should be made available at the time of initial diagnosis and then as required on an ongoing basis, based on formal, regular assessment of need’.
At a recent structured education conference only 4 of 38 local structured educational programmes were paediatric. A number of programmes have recently been evaluated using different methodological approaches. Each differs in hours of education per patient, time period of the course, number of trained educators and number of patients taught per year (see Appendix 1 ).
International guidelines on how to establish, evaluate and improve diabetes education include the American Diabetes Association143 and ISPAD Consensus Guidelines. 144
The NDST guide to commissioning structured education identifies criteria by which to evaluate the effectiveness of patient education, including (1) quality assurance; (2) developmentally appropriate educational approaches; (3) a structured curriculum incorporating audit; (4) an underpinning philosophy; and (5) trained educators. 135 Although nationally evaluated structured education programmes exist for adults [Dose Adjustment For Normal Eating (DAFNE); Diabetes Education and Self Management for Ongoing and Newly Diagnosed (DESMOND); and Xpert],145–147 no UK paediatric clinics offer structured education that meets these standards. 135
The CASCADE intervention was developed in response to the Health Technology Assessment (HTA) review of psychoeducational interventions in childhood diabetes. 91 The intervention is based on Phase 1 pilot work and a non-randomised trial delivered by psychologists. 121 CASCADE was designed with robust quality assurance, developmentally appropriate educational approaches, a structured curriculum that incorporates an audit programme and most importantly an underpinning philosophy and trained educators. It uses approaches important in predicting success in improving long-term diabetic control as well as simply transferring knowledge.
Quality assurance
The programmes DAFNE and DESMOND have developed robust quality assurance processes;145,146 however, no standard template exists for paediatric and adolescent diabetes teams to assess patient knowledge and skills. The structure of quality assurance is crucial to ensure a rigorous process, with clear, written standards that can be monitored, regularly reviewed and updated. The quality assurance process include three main elements:
-
A defined programme with clear content, structure, curriculum and underlying philosophy. The training programme for educators should be included within the quality assurance process.
-
Quality assurance tools based on the programme structure, and a set of observable behaviours required to deliver the programme.
-
Internal and external processes to assess delivery and programme organisation.
Educational approach
Developmental level and perceived learning needs
Children of < 12 years use concrete thinking to combine, separate, order and transform objects and actions. An eight-year-old relies on parents to help test BG, supervise injections or manage hypoglycaemia at school or out with friends. Educational material and conversations about health care need to be simple, concrete and involve shared goals with the parent and child.
Adolescents start to develop a sense of identity, increasing their need for independence with a move from parental to peer influence. Another change is the beginning of complex thinking processes (formal logical operations), including abstract thinking (thinking about possibilities), reasoning from known principles (form own new ideas or questions), the ability to consider many points of view according to varying criteria (compare or debate ideas or opinions) and the ability to think about the process of thinking. Adolescence is a transitional time with expectations of increasing responsibility for independent diabetes self-management.
Age and developmental status are powerful contextual variables that influence diabetes self-management. An evolutionary concept analysis identifies three attributes of diabetes self-management in children and adolescents. 148 The first is process, which is proactive, flexible, and involves a shift of shared responsibility between the child and family, and collaboration with health-care providers (HCPs). The second is performance of simple to complex activities related to the adjustment of regimens, including self-adjustment of insulin. The third is where children and parents engage in this process to accomplish certain goals. Successful completion of process, performance and goals are developmentally influenced.
The CASCADE intervention was designed to be accessible for children and young people between 8 and 16 years. Four modules lasting approximately 120 minutes each are delivered to groups of three to four families with children and young people aged 8–11 years or 12–16 years over 4 months. Everyone in the group is included in all discussions, and encouraged to share ideas and thoughts and develop their own solutions to their goals by evaluating decisions made in the past and think about possibilities for the future.
Interventions that incorporate parents are generally associated with favourable outcomes. 145,146,149–151 Young people are invited to bring parents or significant family members to CASCADE unlike other current structured education courses. 137
Learning methods
Families work in the large group or individual family or peer groups (young people and parents). Delivery is non-didactic. Families are invited to discuss diabetes information from a position of their own knowledge and expertise. Their right to choose different behaviours is acknowledged. The literacy level for written material was age appropriate with additional visual and written information (handouts, charts, diagrams, flow charts). Children, young people and parents are invited to consider attitudes towards changing self-care behaviours and complete exercises that look at the pros and cons of changing behaviour and assess readiness to change. It is more important that the HCP ‘hear and understand what the child has to say, than the child hears and understands what the healthcare worker is telling them’. 152
The CASCADE structured curriculum
Modules were designed to develop confidence managing different aspects of diabetes, including how to adapt insulin dose, how to eat normally and how to manage exercise and illness. The curriculum conformed to the agreed core content for education programmes set out by the Diabetes Education network and Diabetes UK (DUK) (see Appendix 2 ).
Measuring change using a competencies framework
The aim of patient education is for people with diabetes (or their carers) to improve and put into practice knowledge and skills with confidence, enabling them to become experts at managing their (or their child’s) diabetes on a day-to-day basis. HCPs need to collaborate with education specialists to formulate appropriate and reliable evaluation methods that can be used in addition to measuring changes in HbA1c. 153
The CASCADE modules are informed by an eight-level competency system that assesses skills and knowledge designed by Children’s Hospital in Los Angeles ( Table 1 ). 154
| Competency | Level |
|---|---|
| Safety | 1 |
| Basics | 2 |
| CHO management | 3 |
| Correction doses | 4 |
| Daily changes | 5 |
| Base dose adjustment | 6 |
| Advanced management | 7 |
| Maximised control, basal and bolus therapy | 8 |
Families must demonstrate a minimum of competency level 5 to start on pump therapy. The assessment, completed in a clinical interview, in conjunction with insulin pump therapy showed significant and sustained reductions in HbA1c level over a 2-year period. 154
The manual
The manual provides a written curriculum. It includes Microsoft PowerPoint 2003, version 11 (Microsoft Corporation, Redmond, WA, USA) slides from each training session with reading lists and copies of key reading for each module.
Underpinning philosophy and delivery techniques
Psychological approaches used in CASCADE are MI and SFBT (see Chapter 1 ). Specific components integrated into each module to enhance engagement, develop confidence and motivation are described below.
Key communication skills
Key skills in motivational conversations are asking open-ended questions, affirming, reflective listening and summarising. Educators use open questions to elicit information and broaden and deepen conversations. Affirming positive actions and intentions of young people and families focuses on positive outcomes. Educators use reflective listening and simple summaries to show that they are listening.
Identifying skills abilities and strengths
Solution-focused brief therapy assumes that people possess the knowledge and resources they need to tackle their problems. 126 At the beginning of each module, educators invite descriptions of what has gone well, listening out for examples of skills, abilities and resources. Children, young people and families note positive change and identify what they are doing differently. The aim is for both educators and families to have ‘determined curiosity and relentless optimism’.
‘How come?’ and ‘What else?’
Describing examples of when they have been in charge of diabetes since the last session orientates people towards what is already working and acknowledges when their preferred future is happening. Educators ask families what they had noticed had worked (‘What else?’) and ask what made the positive changes possible (‘How come?’).
Focusing on the future
Families imagine themselves in the future having overcome difficulties or problems and describe this problem-free preferred future. Families think about what they will be doing differently, or more, or less of, and how this will have changed their relationships with friends and family. ‘Stepping into the future’ invites families to identify ‘their’ goal for change.
Scaling
Scaling helps families think about how close they currently are to their preferred future and break this into small and achievable steps. Scaling reinforces positive change and prevents young people from feeling stuck when change is difficult to notice. Asking how someone had moved a half or a whole point towards a goal reinforces a sense of personal agency having taken a step forward (or prevented themselves from slipping backwards).
Considering the pros and cons of behaviour change
Motivational interviewing assumes that young people and families are, to a greater or lesser extent, ambivalent about current self-management behaviours and the degree to which they put pre-existing knowledge into action. The majority of young people with high HbA1c levels will have had many conversations with diabetes consultants, nurses and dietitians, and possibly psychologists or counsellors. Uncertainty about implementing change can be expressed across emotional, practical and behavioural domains. In each module the young person and their parent/carer are invited to identify advantages and disadvantages of behaviours related to diabetes self-management (e.g. BG monitoring, using different insulin regimens, correcting for high or low BG) to help the educator understand the young person’s position and clarify the ‘pros and cons’ of change for the young person.
Establishing the importance and confidence of change
Importance and confidence are central to exploring the young person’s ambivalence to change. 156 Young people and parents scale (between 0 and 10) importance of change, confidence changing and how ready they feel they are to do things differently after completing a pros and cons exercise. The family identify what needs to be different for change to happen. If confidence is low the young person can think how to build confidence before making changes. If importance is low the group can think about what would need to happen for change to be more important.
Evocation not education: avoiding giving a choice or answer
The majority of young people with high HbA1c are aware of what they ‘need’ to do but are not willing, able or ready to put this knowledge into practice. Educators ask if it is ‘OK’ to discuss information about different topics and invite the group to talk about existing knowledge first. These ‘scaffolding’ questions help individuals discover further information for themselves, or ‘draw it out’. 157 Additional learning is a collaborative effort between individuals and trainers, reducing the sense of an expert imposing knowledge and moving towards a shared venture. This active rather than passive approach has been shown to be effective in eliciting behaviour change in other areas. 158
The CASCADE modules
The teaching plan includes session activities, objectives, time guides and resources, including key information essential for the educator, learning objective for the family and brief descriptions of each activity. Young people and parents completed homework tasks including a post-module quiz, designed to consolidate information, after each group. The educators encouraged the young person to complete the quiz with parents who could not attend, facilitating communication of key messages delivered during the module. The quiz and pre-prepared handouts could be sent to families if they missed a session.
Each module (apart from the first) starts with a review of the previous module creating an opportunity to highlight changes that have taken place and congratulate young people on successes. It also creates an opportunity to review the previous module for any family who missed the session.
Module 1: the relationship between food, insulin and blood glucose
Readings: (1) educator notes about CHO foods, BG and a healthy diet and (2) ISPAD guidelines on nutritional management in childhood and adolescent diabetes. 159 The introduction identifies strengths, resources and abilities. Focusing on the future, identify how things will be in the future if families get what they want from CASCADE. They scale how close they currently are to this. The session focuses on understanding food groups, particularly CHO. It talks about the role of insulin and different insulin regimens. Finally, families and young people consider the pros and cons of matching insulin to food to attain better glycaemic control.
Module 2: blood glucose testing
Readings: Implications of the DCCT13 and assessment and management of hypoglycaemia in children and adolescents. 160
After reviewing module 1, educators discuss the recommended target HbA1c. The group identify factors that cause BG to rise and fall and explore individual hypoglycaemia definitions, reviewing symptoms according to severity. Families discuss ways to treat hypoglycaemia and assess the pros and cons of BG testing.
Module 3: adjusting insulin – pros and cons
Reading: insulin analogues in diabetes care,161 using CHO counting162 and guidelines on assessment and monitoring of glycaemic control. 163
Following the review families discuss symptoms of hyperglycaemia and ketones. A brainstorming session considers when, how and who to contact for help with managing hyperglycaemia. The session focuses on managing high BG using temporary insulin changes and explains how to correctly calculate correction doses. The group explores the advantages of identifying trends in relation to permanent insulin dose changes and considers the advantages and disadvantages of CHO counting as a way of improving glycaemic control.
Module 4: living with diabetes
Reading: guidelines on exercise164 with a set of PowerPoint slides used in the workshop to explain the principles of exercise and management of insulin and CHOs.
Following the review, families identify the effect that low and high BG has on performance and concentration, and discuss effective strategies to bring BG into target range before starting exercise. Participants work in family groups to identify how different activities affect BG and discuss the timing of insulin injections in relation to exercise before considering the advantages of using CHO before, during and after exercise (to keep their BG stable during different exercise).
At the end of module 4, young people and families complete a ‘blueprint for success’. This marks the end of the sessions and acknowledges the steps into the future the young person has already made. It creates an opportunity to review the programme and strengthens long-term motivation to change by reviewing previous successful goals.
Trained educators
The Department of Health and DUK have both highlighted the importance of structured training for educators involved in delivering patient education. Members of UK paediatric and adolescent diabetes teams have a varying amount of training in relation to age-specific and developmental milestones for children and adolescents with no current consensus regarding uniformity of roles and qualifications for different members of teams delivering structured education.
Each diabetes team in the CASCADE intervention arm identified two primary site educators, one of whom had to be a PDSN. The 2006 Royal College of Nursing (RCN) document on specialist nursing services for children and young people with diabetes states that a PDSN based in hospital or community, working as a member of the team specialising in the management of childhood diabetes, should be able to provide:
. . . a source of specialist advice for children, young people and families on the nursing care and management of diabetes, including the provision of basic dietary advice and the management of acute complications.
. . . individual specialist teaching for children, young people and families, facilitating the development of self care skills and knowledge, at time of diagnosis and in planned, ongoing, age appropriate education, both individually and in groups.
Royal College of Nursing165
The second trainer could be any HCP within the team. These two trainers were expected to deliver the groups to families within their service. Teams were invited to bring along additional members to the training if they so wished.
Training and intervention
Pilot for intervention manual and training workshops
The intervention manual was piloted with a family known to the University College London Hospitals (UCLH) team. Content, delivery and resources were discussed to ensure that the principles and messages were clear and understandable. Training workshops were piloted with two UCLH PDSNs not involved in the intervention development, and five local PDSNs from centres not recruited to CASCADE. Feedback on the length of training (2 days), content and delivery was positive. Small changes were incorporated before training of the intervention sites began.
Training workshops
Key messages, based on the fundamental principles of CASCADE ran through all of the workshops:
-
Assume basic diabetic knowledge already provided at, and subsequent to, diagnosis.
-
Non-didactic educational principles and psychological models underpin behaviour change.
-
Not additional work for staff but working differently.
-
No right or wrong ways of saying things – just more or less helpful ways of talking.
-
Builds on families’ knowledge, skills and abilities, and uses their experience and expertise.
-
Assumes families have knowledge about how to manage diabetes and want to change their behaviours.
-
Use open questions; affirm and positively connote behaviours.
-
Reflect on what is heard and summarise to show good listening skills.
-
Explore the advantages and disadvantages of changing.
-
Focus on advantages of change and disadvantages of the status quo.
-
Encourage staff to be interested in the person rather than the problem.
-
Identify ways that people are already doing what they want to do by focusing on what is going well rather than what is not going well.
Training took place on two separate days with at least two intervention sites invited on each day. The following basic principles were emphasised:
-
Follow the manual as much as possible to maintain treatment fidelity.
-
Always have two people delivering each module.
-
Divide the different components in the module in whatever way works best for site educators.
-
Read key readings for each module to ensure knowledge of educational content.
Session 1 (morning of day 1)
The background to CASCADE was introduced with key delivery principles. These were to:
-
Deliver the intervention to all recruited families in each intervention clinic.
-
Offer four modules monthly to groups of three or four families with children in the same age group.
-
Offer groups if only one or two families turn up for a module. If only one family turns up, reschedule or complete the module on their own and then join another group for future modules.
-
If families miss a module they can continue with the next module and, where possible, be helped to catch up by discussing the module content with one of the site educators and/or being given the handouts from the module they missed.
The session introduced the basic principles of SFBT and MI and provided opportunities to try out the techniques embedded throughout the four modules.
Session 2 (afternoon of day 1)
This session introduced modules 1 and 2. Delivery and communication style was modelled by the UCLH trainers. Site educators participated in each exercise, giving answers or comments that they thought children might offer or the information they thought correct. The UCLH trainers worked together; one delivering the activities, while the second trainer commented on the process and performance, and invited discussion of content and delivery style. The trainers highlighted SFBT and MI techniques used in each activity.
Session 3 (morning of day 2)
Modules 3 and 4 were described using the same format as session 2.
Session 4 (afternoon of day 2): envisaging the future
Session 4 explored practicalities of running groups, organising sessions and how to engage and motivate participants to attend. After a brief slide presentation on groups, educators then worked in their clinic groups. They were asked to imagine themselves in 12 months’ time, having completed all of the CASCADE groups, and consider:
-
What made completing CASCADE possible?
-
What aspects of the process had gone well?
-
What had been possible challenges and barriers that they had overcome?
They were invited to identify their skills, abilities and knowledge that contributed to this ‘ideal outcome’ making the groups possible. As each ‘outcome’ was described, trainers asked questions to generate details about solutions, such as ‘How did you achieve this?’ ‘Which days worked best for you?’ The shared ideas from each clinic generated practical and achievable solutions to potential problems in advance to support group organisation (e.g. in one training session good attendance was ‘achieved’ by staff phoning families a week before the start as a reminder). Other ideas highlighted during this session included:
-
Enthusiasm for the project, successful recruitment and practicing sessions in front of peers for feedback.
-
Resources in place for delivering the programme; finding an appropriate venue for running sessions with staff and time to plan, organise and deliver sessions.
-
Good communication between educators, supportive management and other team members who would be receptive to the approaches and non-directive in communicating with young people.
-
Sessions delivered at the right pace, working as a team with families with good attendance and age groups that bonded well. Involving everyone in the group with good use of techniques and listening skills.
-
Positive feedback from young people and families. Families happy, more confident and accessing services appropriately.
-
All HbA1c levels of < 7.5%.
One-day refresher training
A 1-day refresher workshop was offered to all members of each intervention site when all workshops were finished. The morning session provided the training in the delivery process with a description of the four modules in the afternoon.
Ongoing support
Site educators could contact trainers at any time during the delivery of groups with queries or concern about timing of the groups, clarity in relation to aspects of the manual or what to do if a family did not turn up to training or composition of groups.
Chapter 3 Methods
Trial design
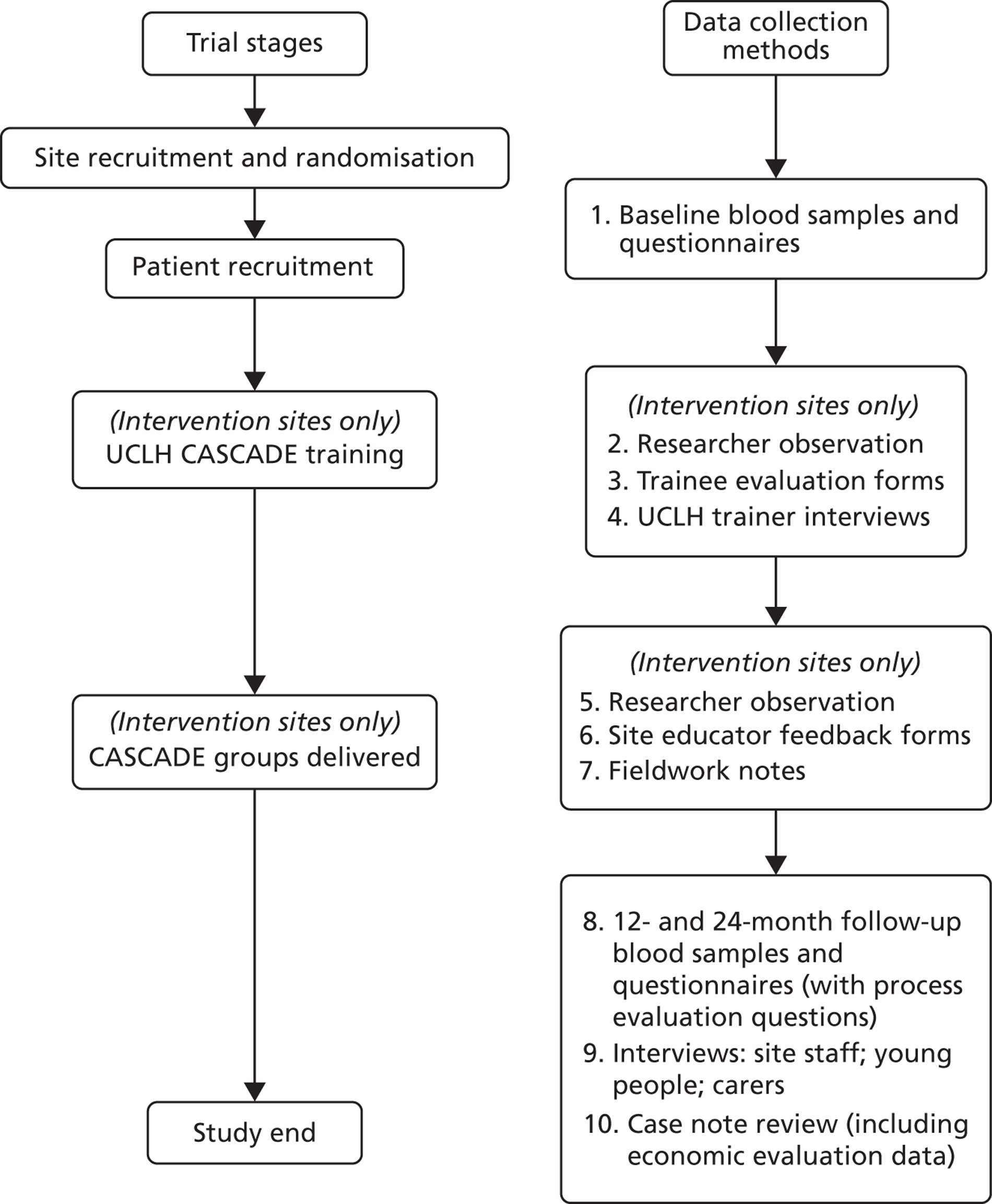
The study was a pragmatic, cluster RCT with paediatric/or adolescent diabetes clinic as the unit of randomisation. Integral process and economic evaluations were completed ( Figure 1 ). The protocol was published in BioMed Central trials. 166 A list of all substantial amendments made to the final protocol after trial commencement is shown in Appendix 3 .
FIGURE 1.
Data collection timing and methods.
Trial objectives
The primary trial objectives were to determine whether a structured, intensive educational programme could be provided within a standard clinic setting for a diverse range of young people with T1D and to assess whether the CASCADE intervention improved long-term clinical outcomes (HbA1c levels).
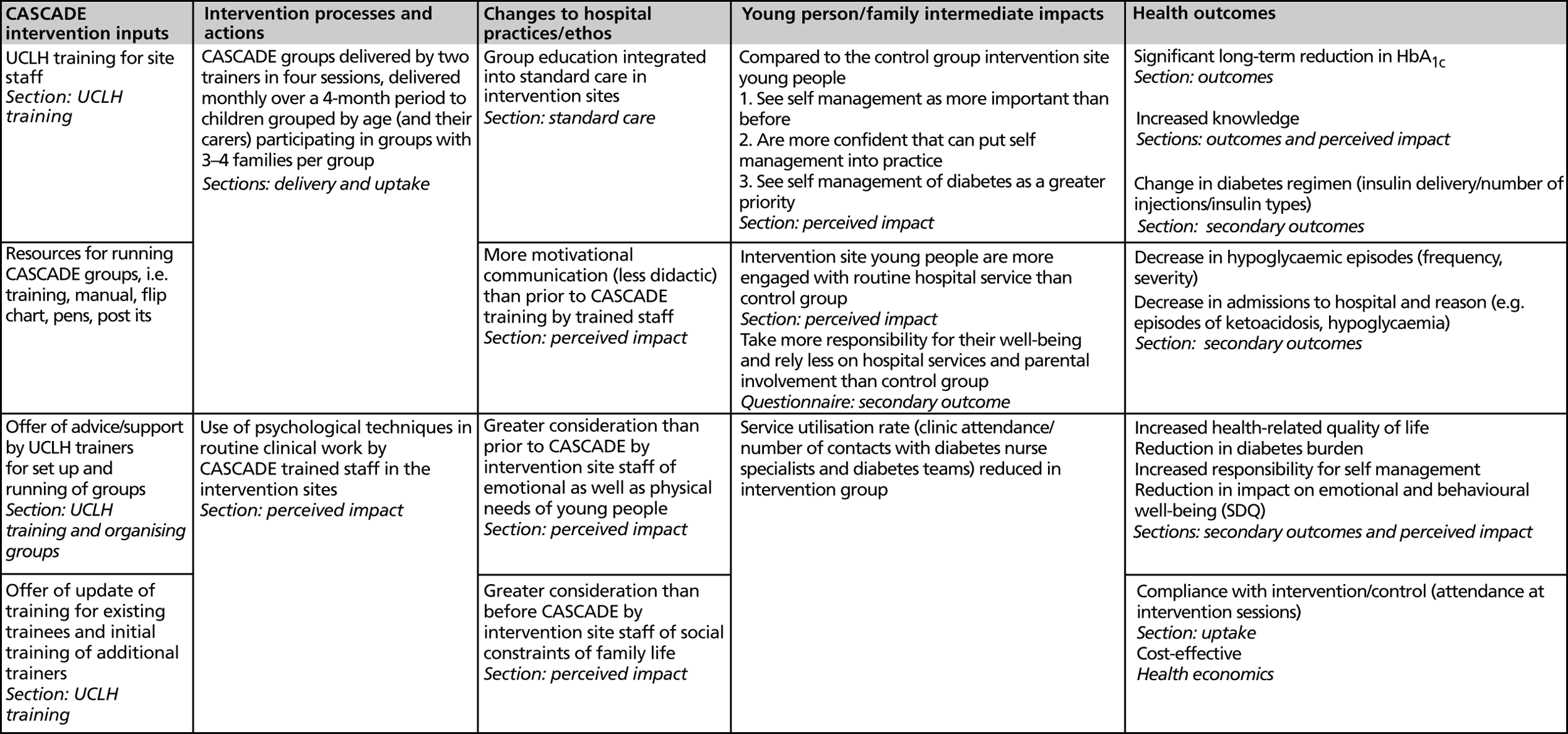
Secondary objectives included determining the impact of the intervention on other markers of diabetes control, psychological wellbeing and costs (see Outcomes, below). The logic model which informed evaluation of the intervention is shown in Figure 2 .
FIGURE 2.
CASCADE logic model.
Ethics committee approvals
The trial was performed in accordance with the recommendations of guiding physicians in biomedical research involving human participants adopted by the 18th World Medical Assembly, Helsinki, Finland, 1964, amended at the 59th World Medical Association General Assembly, Seoul, October 2008. The study was approved by the University College London (UCL)/UCLH REC reference number 07/HO714/112 (see Appendix 4 ). Subsequent amendments to the original ethics approval are detailed below. Site-specific approval was granted at each site.
Outcomes
Primary outcome
The primary trial outcome was glycaemic control, assessed at the individual level using venous HbA1c value. Intravenous HbA1c samples for patients and questionnaires from patients and carers were collected at baseline. The original protocol stated outcomes would be collected 12 and 24 months post intervention. A substantial amendment to collect follow-up data 12 and 24 months after the date of baseline blood sample was approved and reported in protocol v6 301010 (HTA progress report 5). Where possible, data were collected within 3 months either side of the expected collection date.
Secondary outcomes
Secondary outcomes were outcomes directly and indirectly related to diabetes management, including hypoglycaemic episodes and hospital admissions, choice of diabetes regimen, knowledge and skills associated with diabetes management, responsibility for diabetes management, compliance with intervention and clinic utilisation. Information about psychological functioning in terms of emotional and behavioural adjustment and general and diabetes-specific QoL was measured using well-validated, reliable self-report and parental measures.
Questionnaire development
Specific versions of questionnaires were created for 8- to 12-year-olds, 13- to 16-year-olds and carers (questionnaires are available from the corresponding author). Those on pump therapy completed modified questionnaires reflecting differences in regimen. Demographic information and clinical data included years since diagnosis, insulin type dose, number of injections and time at current clinic. Carer outcomes included demographic information [age, gender, ethnic origin, socioeconomic status (SES)]. Questionnaires contained a number of validated instruments detailed below.
Psychosocial outcomes
Validated instruments appropriate for age were used wherever possible. Parental and self-report of health-related QoL was measured using the Pediatric Quality of Life Inventory (PedsQL). 167 The scale has good internal consistency, reliability and validity for both the generic and diabetes modules. The physical psychosocial health summary score produce an overall total score. The T1D module has four scales: Treatment I and II, Worry, and Communication, which combine to produce a total diabetes score.
The five-item ‘Impact Supplement’ of the Strengths and Difficulties Questionnaire (SDQ) (parent and child report versions) assessed the impact of identified emotional and behavioural difficulties on the young person’s life. 168
Young people were also asked to rate how happy they were with current weight and if they had skipped insulin using two self-report questions designed specifically for the study.
Diabetes outcomes directly related to patient management (parental report and case note review)
-
Diabetes regimen (insulin dose/number of injections/insulin types).
-
Hypoglycaemic episodes (frequency, severity) in last 6 months.
-
Admissions to hospital in last 6 months and reason (e.g. episodes of ketoacidosis, hypoglycaemia).
Diabetes outcomes indirectly related to patient management
-
Knowledge and skills associated with diabetes. 155
-
The Diabetes Family Responsibility Questionnaire (DFRQ)169 (parent and child report) reflects the sharing of diabetes-related responsibilities (such as deciding what to eat at meals and snacks, rotating injection sites and telling teachers about diabetes) within families. Parent and child versions of the questionnaire are scored separately. Higher responses (on both) indicate greater responsibility taken by the young person for their care. A dyadic score for the parent–child pair reflects agreement about who is taking responsibility. High dyadic scores when neither reports taking responsibility is linked to poor health outcomes.
-
The Diabetes Self-Efficacy Scale assesses confidence managing diabetes-related tasks. 170
Compliance with intervention/control
-
Attendance at intervention sessions.
-
Service utilisation rate.
-
Clinic attendance.
-
Contacts with PDSNs and diabetes teams.
Data were collected through process evaluation (PE) and case note review (CNR).
Service user perspectives
Service user perspectives were sought on the structure and content of all the questionnaires. Initial questionnaires were reviewed by a young woman with diabetes as part of the trial management group. One individual with research responsibilities for a diabetes charity and the mother of a girl with diabetes who was also research trained provided comments about the length, general tone and specific items on the questionnaire. Early data collection proved problematic as questionnaires were too long and were often only partially completed. After consulting a patient organisation and a carer researcher, the self-efficacy scale was removed and questions assessing knowledge and skills about self-management were removed from the 8- to 12-year-old child version.
Baseline data collection
Wherever possible, baseline data were collected at the point of consent. Otherwise it was collected at the next possible opportunity. A substantial amendment to allow researchers to collect follow-up questionnaire data by telephone was approved in June 2009 (progress report 3).
Primary outcome data collection
Arrangement for collection varied widely between and within sites. Research nurses or a member of the local clinical team trained in venesection took the sample in the paediatric outpatient clinic or occasionally at the patient’s home or GP surgery. Hospital phlebotomists also collected samples. Approximately 4 ml of blood was collected and stored in a tube prefilled with diluent and preservative. The samples were sent to a single UK laboratory (UCLH Special Biochemistry, London) for measurement of HbA1c concentrations. This ensured direct comparability of results from all clinics. Current legislation requires that non-infectious diagnostic specimens are transported in accordance with guidelines UN 3373 and need to conform to secure packing instructions P650 (The European Agreement concerning the International Carriage of Dangerous Goods by Road; Regulations concerning the International Carriage of Dangerous Goods by Rail; the International Air Transport Association). The research team provided the appropriate packaging. Samples were transported using a courier or more often delivered by hand by researchers or via the Royal Mail (identified as a biological substance, category B). HbA1c assays were carried out on a Tosoh G8 instrument (Tosoh Bioscience, Tessenderlo, Belgium) with International Federation of Clinical Chemistry aligned calibrators and results reported directly to the data manager following adjustment against the DCCT international standard.
When a sample was lost or spoiled in transit, the research team used their discretion with regard to requesting a repeat sample. A small number of young people were recruited in whom it was subsequently found they had variant haemoglobin, meaning that HbA1c levels could not be accurately measured. This issue came to light when the UCLH laboratory reported abnormal results for baseline blood samples. The clinic principal investigator (PI) was informed. These patients remained in the study and provided completed questionnaires but their HbA1c data were not used and no further study blood samples taken.
Secondary outcome data collection
For collection of secondary outcome data the researcher provided patients and carers with a copy of the appropriate questionnaire. Participants were encouraged to self-complete the questionnaires independently (i.e. parents and children were asked not to confer). The researcher provided help when required. If a young person and/or their carer was not able to complete the questionnaire in the clinic, either owing to insufficient time or the parent not being present, the researcher would go through the questionnaire with the young person/parent on the telephone. Patients recruited by site staff or research network nurses were contacted by telephone to complete the questionnaire.
The PI from each clinic ensured appropriate access to advice and psychological support should any participant express any concerns or worries with regard to taking part in research activities. In exceptional circumstances, for example disclosures of significant risk (to self or others), feedback to the family/carer and clinical team would be made by the research team. Participants were reminded that DUK provides an independent voice of support, through ‘Careline’, a dedicated helpline for all people with diabetes, friends, family, carers and HCPs.
Follow-up data collection
The process for collecting data at 12 and 24 months was the same as for baseline. A letter was sent to the young person and their parent/carer a few weeks prior to their clinic appointment, approximately 1 year after the baseline blood sample and 1 year later for the second-year follow-up. Families were asked to come to clinic early if possible to meet with the researcher and advised that a £10 high street gift voucher would be offered as a thank you for their participation. A substantial amendment to the protocol was submitted and approved (progress report 5, protocol v6) allowing the team to offer an additional £10 high street gift voucher at the second follow-up.
There was ongoing support from research nurses from networks that had capacity to continue to support the study at one or both follow-up time points. National Institute for Health Research (NIHR) flexibility and sustainability funding supported a part-time researcher from June 2010 to June 2011 to assist with follow-up data collection where research network nurses were not available.
During the first- and second-year follow-up, some patients transitioned to adult services. The risk of data loss was minimised by requesting that, wherever possible, clinics delayed transition. If this was not possible the research team wrote to the adult consultant to introduce the study and reassure him/her that the research team would take responsibility for overseeing data collection.
Trial Steering Committee and Data and Ethics Monitoring Committee
The NIHR-HTA appointed a Trial Steering Committee (TSC) and independent Data and Ethics Monitoring Committee (DMEC). The TSC met with the trial management group every 6 months. The DMEC reviewed, in strict confidence, data from the trial approximately halfway through the recruitment period. The Chair of the DMEC could also request additional meeting/analyses. In the light of these data and other evidence from relevant studies, the DMEC would inform the TSC if in their view:
-
There was proof that data indicated any part of the protocol under investigation was either clearly indicated or contra-indicated, either for all patients or a particular subgroup of patients, using the Peto and Haybittle stopping rule. 171,172
-
There were major ethical or safety concerns.
Details of committee membership are provided in Appendix 5 .
Centre recruitment and consent
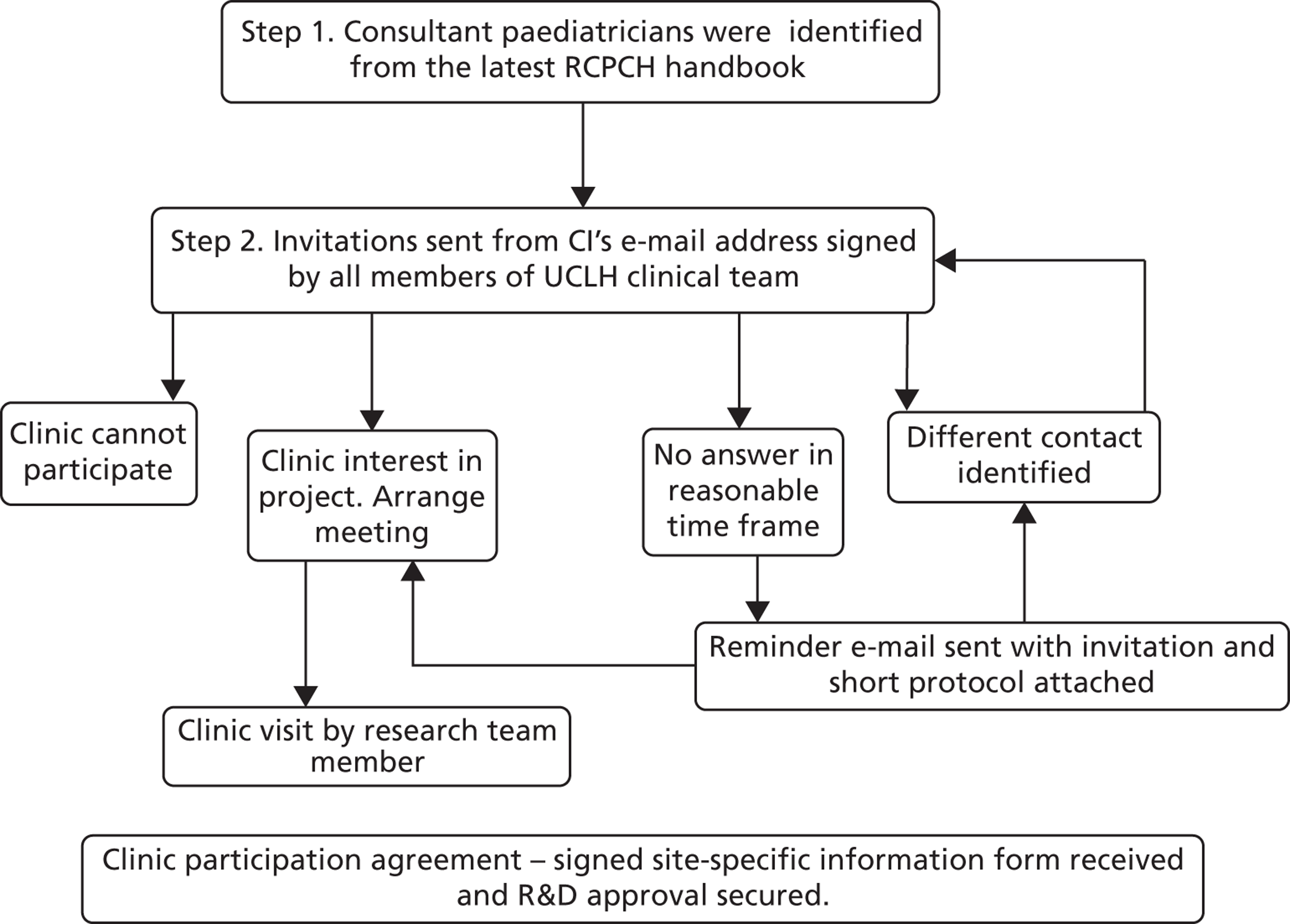
A database of potential diabetes clinics was created from a list of hospitals and consultant paediatricians identified from the Royal College of Paediatrics and Child Health (RCPCH) handbook. The Chief Investigator e-mailed the clinical lead for each potential clinic on behalf of the UCLH team to invite them to participate. Clinical teams in a few sites, hearing about the study, approached the study team to request inclusion. Clinical teams that expressed an interest were contacted by the research team to discuss the study in more detail and ensure that they met inclusion criteria. Consent to randomisation was obtained from the responsible clinician who agreed to act as PI ( Figure 3 ). Research and development (R&D) approval was then arranged with the participating acute trusts.
FIGURE 3.
Centre recruitment and consent process. R&D, research and development.
Eligibility criteria for clusters
-
NHS paediatric diabetes service – clinic had to be staffed by at least one paediatrician and paediatric nurse with an interest in diabetes.
-
Located within London, the south-east (SE) and the Midlands (maximum train journey time 2 hours).
-
Not running a group education programme at time of recruitment or during trial.
-
Not taken part in a similar paediatric diabetes trial within the last 12 months.
-
At least 40 eligible patients on current patient list.
Patient recruitment and consent
Prior to randomisation, clinics were asked to review patient lists to identify patients eligible to participate in the study. Guidance regarding patient eligibility was provided in writing, over the phone and sometimes face to face. The following criteria were used to determine eligibility.
Inclusion criteria for patients
-
Diagnosis of T1D with duration ≥ 12 months.
-
Aged 8–16 years.
-
Mean 12-month HbA1c value ≥ 8.5 mmol/l.
-
Under the care of a paediatric and/or adolescent diabetes clinic conducted by a specialist, or general paediatrician with an interest in diabetes.
Exclusion criteria for patients
-
Significant mental health problems unrelated to diabetes requiring specific mental health treatment.
-
Significant other chronic illness in addition to diabetes that may confound the results of the intervention. Patients with coeliac disease or hyperthyroidism were included.
-
Significant learning disability or insufficient command of English to enable full participation in the planned intervention. Young people with good command of English but whose parents have poor command of English would be eligible to attend by themselves if they wished as long as parents had given informed consent. Another relative who was one of the primary diabetes carers (e.g. sibling, aunt or uncle) who had good command of English could participate instead of the parents.
-
Participated in diabetes treatment trials in the 12 months prior to collection of baseline data.
Sample size
Based on NPDA figures, we calculated the average number of eligible patients would be 80–100 patients per site. A recruitment rate of 25% was predicted based on a previous study by the Chief Investigator. 121 The standard deviation (SD) of HbA1c in the target population is approximately 1.5%. The proposal was to recruit sufficient young people to allow the trial to detect a difference between groups of 0.5 SD, i.e. 0.75%, with 90% power at a significance level of 0.05 (two tailed), considered to represent a moderate size of effect. Power calculations were based upon an intracluster correlation coefficient (ICC) (the variability in outcome between clinics divided by the sum of the within-cluster and between-cluster variances) of 0.1. In the absence of reliable data on which to calculate an ICC for the clinic population, this was chosen to be compatible with databases of ICCs such as www.abdn.ac.uk/hsru/epp/cluster.shtml. With these assumptions, 13 clinics in each arm with an average of 20 young people in each would be required to detect a difference of half a SD (0.75) with > 90% statistical power at 5% significance. Given the possible loss to follow-up of approximately 10%, the target recruitment was inflated to 22 young people from each clinic. The original target sample size was therefore 572 children and young people recruited from 26 sites.
Early in the trial, the average number of eligible patients per clinic reported was 48 patients, with a range of 19–97 patients. This was considerably fewer than the 80–100 patients per clinic originally estimated.
Given the lower than expected number of eligible patients per clinic, the predicted recruitment rate of 25% would mean an average of only 11 patients per clinic was likely to be available (a total possible sample size of 286). Maintaining the original predicted effect size of 0.5 SD the reduced sample size would have power of only 80%.
A preliminary exploration of baseline data suggested that the ICC was 0.01, which was significantly lower than the original assumption of 0.1. Using this revised ICC and increasing the number of recruited clinics to 28, a minimum of 11 patients per clinic provided 87% power to detect the proposed effect size with 5% level of significance. This created a revised patient recruitment target of 308. The rationale for this was sent to the DMEC, TSC and NIHR-HTA and was approved on 21 December 2009.
In order to meet this overall target, the target recruitment rate per clinic was also raised from 25% to 33%. In clinics where eligible lists were particularly small, or patient recruitment began late, 33% was felt to be unrealistic and a minimum of seven recruits was set in these clinics. Where eligible patient numbers were felt to be particularly small, clinical teams were asked to review patient lists to identify any additional eligible patients who may have been inadvertently missed. Where requested, the research team visited clinical teams to help with this process.
Recruitment process
The research team provided electronic templates of letters and pre-printed information leaflets. Invitation packs were posted by clinics to the parent/guardian of all eligible patients and included:
-
an invitation letter (printed on trust headed paper) to parent/guardian
-
participant information leaflet for parent/guardian explaining the study
-
a separate non-sealed envelope containing an age appropriate invitation letter for the young person (printed on trust headed paper) and an age-appropriate information leaflet
-
separate invitation letters and participant information leaflets for 8- to 10-year-olds and 11- to 16-year-olds.
Administrative support to send out invitation packs was provided by the research team if requested by the site. The team liaised with site staff and travelled to outpatient clinics when eligible patients were attending routine appointments. Initial recruitment was carried out by two full-time members of the research team. Eligible children, young people and carers were approached in clinic and asked if they had received the invitation pack in the post. If not a duplicate pack was provided and patients were then approached at the next convenient opportunity. The trial was described as a comparison of different ways of helping young people to manage their diabetes. Participants were told that if they were in the intervention arm they would, in addition to standard care, be invited to a series of four 2-hour group sessions. If they were in the control arm they would continue with standard care only. It was made clear that all approaches had potential advantages and disadvantages. They were informed that the children and young people were required to give a venous blood sample and complete a questionnaire at three time points, and that the parent/carer would be required to complete a questionnaire at the same time points. Eligible patients that asked for more time to think about the study before consenting were followed up (by phone, letter or at subsequent clinic appointments).
Informed written consent or assent was obtained from both the young person and one parent/carer. The right of a child or parent to refuse participation without giving reasons was respected. The child/young person or their parent remained free to withdraw at any time from the study without giving reasons and without prejudicing further treatment. If a participant withdrew consent from further trial participation their data remained on file and was included in the final study analysis. If a participant withdrew consent for their data to be used, the data were destroyed immediately. 173,174
Additional strategies to support recruitment process
Five additional sessional workers were used to support recruitment on an ‘as required’ basis to increase capacity and maximize flexibility within the team.
Where possible clinics were asked to cluster eligible patients into specific clinics to minimise the number of trips researchers had to make. Reimbursement was offered to families for any expenses incurred through travel for research purposes.
The study was accepted as part of the NIHR portfolio and adopted by The Medicines for Children Research Network (MCRN) and the Diabetes Research Network (DRN). Research nurses from MCRN Trent and MCRN West Midlands Local Research Networks supported recruitment in clinics that fell within their geographical localities. Nurses connected to the London South East, North Central and East Research Network supported recruitment in a number of London clinics.
Protocol amendments were approved to allow local clinicians to recruit and take consent from young people and their families. For example, £100 was paid to the trust for each patient recruited. Baseline questionnaires were distributed and subsequent data collected over the telephone for patients recruited by local clinicians (progress report 1, 30 October 2008). Research nurses and site staff were trained in study procedures particularly with regard to minimising risk with respect to allocation. All patient recruitment took place on hospital sites with the exception of one site where a member of the clinical team recruited a patient at home. Ongoing support for staff assisting with the recruitment was provided by e-mail, phone and face-to-face contact. A DVD was made to support recruitment in clinics. A copy of the DVD was provided to each clinic and the file was also uploaded to YouTube (www.youtube.com/watch?v = XFgVKBYdCbU). A substantial amendment was submitted to allow the DVD to be used by site staff to support recruitment (progress report 2, 30 April 2009).
A regular newsletter was sent to clinics to maintain motivation to recruit.
Site randomisation
Clinics were randomly allocated to intervention or control. The randomisation schedule was drawn up at the London School of Hygiene and Tropical Medicine (LSHTM) and allocation concealed until after clinics had consented and a first participant recruited to minimise selection biases at entry of clusters to the trial. Randomisation was in a 1 : 1 ratio and minimised by factors likely to influence clinic mean HbA1c, such as age of target population of the clinic (paediatric or adolescent) and degree of clinic specialisation (district general hospital clinic or teaching hospital/tertiary clinic) and finally size of clinic to ensure balance in numbers of eligible children between the two arms of the trial. This information was provided by the site PI who completed a minimisation criteria form shortly after site recruitment. Allocation (i.e. whether the site was in the intervention or control arm) was conveyed to sites by the trial Chief Investigator. Staff were requested not to inform young people and families about randomisation status until recruitment had finished. Site staff then sent a letter informing them which arm the site had been randomised to (or told them in clinic). The research team sent the patients’ GP a letter informing them their patient was taking part in a trial and informing them which arm the hospital had been randomised to. The central laboratory assessing the primary outcome (HbA1c value, see below) remained blind to participant allocation.
Statistical analysis
The primary analysis was an intention-to-treat comparison of HbA1c values between the two groups of patients at 12 months and 24 months, using analysis of covariance with a random effect for clinic to allow for clustering at that level.
Psychological outcome measures were derived from baseline and follow-up questionnaires and analysed using analysis of covariance with a random effect for clinic to allow for clustering. As most of the psychological outcome measures were highly skewed, 95% confidence intervals (CIs) for these measures were estimated using 2000 bootstrap samples. For binary outcomes, logistic regression was used to estimate the effect of the intervention. Again, a random effect was included at the clinic level to allow for clustering and baseline values were adjusted for, where available.
Prespecified subgroup analyses based on age (paediatric/adolescent), gender, high/low (< 10.4 vs. ≥ 10.4 mmol/l) initial HbA1c levels and socioeconomic status (SES) (as measured by the multiple deprivation score) were carried out, and effect sizes and 95% CIs estimated from models that included an interaction term.
A per-protocol analysis of all primary and secondary outcomes was carried out with adherence to the protocol being defined as attending three or more of the four CASCADE sessions. The same statistical analysis techniques were used as for the intent-to-treat analysis.
Serious adverse event reporting procedures at site level
A member of the clinical team at each site was asked to take responsibility for overseeing the reporting of serious adverse events (SAEs) at that site.
This person was asked to ensure that all SAEs experienced by trial participants, from the time of consent to take part in the trial to the trial ending, were reported via a secure fax number to the research team within 24 hours of staff learning of the event. Assessment of seriousness, causality and expectedness relating to the CASCADE intervention of a reported event was carried out by a clinically trained member of the research team blind to allocation. The form was then signed by the study Chief Investigator and forwarded within 15 days to the Research Ethics Committee (REC).
All standard operating procedures (SOPs) are available on request.
Health-economic evaluation
The economic evaluation compares the CASCADE intervention with current NHS practice. It is assumed current diabetes education (dietary education, physical exercise and psychological support) is given in a non-standard, irregular way by health-care providers during clinic and home visits. Assessment of CASCADE cost-effectiveness with respect to current NHS practice focuses on the relative success controlling HbA1c level and the predicted impact on diabetic complications over time. Good BG control (HbA1c level of < 7.5%) is associated with a reduction in the risk of diabetic complications. 175
The cost-effectiveness model considered long-term cost and benefit implications of delaying onset of microvascular and macrovascular diabetic complications by comparing HbA1c levels in the intervention and control groups. A series of Markov chain Monte Carlo (MCMC) submodels simulated the progression of microvascular complications (kidney diseases, neuropathy – foot ulcer and risk of amputation, and retinopathy) and cardiovascular complication (infarction) were used to predict future treatment cost of patients receiving the CASCADE intervention or standard care. This is a similar approach to that adopted in the DAFNE study145,176–178 (DAFNE Study Group. 2002, unpublished). A detailed description of the methodology used in the economic analysis is provided in Chapter 7 .
Process evaluation
Aim
The PE was designed to assess the feasibility and acceptability of delivering CASCADE groups as part of standard care within an NHS setting. Should the CASCADE intervention fail to be effective, the PE was also designed to assess the extent to which theory or implementation was responsible.
Specific aims of the PE were to:
-
compare and contrast standard NHS paediatric/adolescent diabetes service across control and intervention sites
-
describe the UCLH training workshops for the CASCADE intervention and assess quality, fidelity and satisfaction
-
report on feasibility and acceptability for staff organising and delivering CASCADE groups in the sites
-
review feasibility and acceptability of the CASCADE intervention for families
-
describe parent/carer and young people’s perceptions of impact of participation in CASCADE on themselves and their families and views of staff regarding the impact on young people and on their own practice.
Design of the process evaluation
A mixed-methods approach to data collection was used. Data were collected from key stakeholder groups (UCLH trainers, site staff, young people and parents) at specific time points in the trial. Questionnaires, observation and CNR were used to collect qualitative and quantitative data. Qualitative data were collected through semistructured interviews, informal discussion following observation sessions and fieldwork notes. Participants were informed that all information would be confidential with only the research team able to access individual data. All data used would be anonymised. Permission to record interviews was sought. Interviews were transcribed verbatim. If permission to record was not granted then notes were taken by the interviewer during or immediately after the interview. All PE instruments used for data collection are available on request.
Process evaluation methods
University College London Hospitals training workshops
Observations
The aim of the researcher observation was to assess quality and fidelity across the workshops and describe delivery of training in terms of methodology, content, engagement and satisfaction (as perceived by the observer). Three each of workshops 1 and 2 were observed by researchers (between August 2009 and May 2010). A researcher observed the 1-day refresher workshop (June 2010) offered for members of the intervention clinics who were interested in learning more about the background to the intervention. Observers were introduced by the UCLH trainers at the start of the training sessions but observed from the back of the room as non-participants. Brief notes made during sessions were written up in more detail immediately afterwards. Details of number of attendees, professional background and role in CASCADE, and which hospital/clinic site they were from were also recorded.
Evaluation forms and questionnaires
A one-page anonymous evaluation form was completed by attendees immediately after each workshop session. Forms designed by the UCLH trainers captured immediate feedback on what participants most liked about the session, what they had not understood and what they would do differently as a result of being in the session. Trainers administered the forms and used the feedback to modify subsequent sessions if needed. For the purposes of the PE these data were used to support themes arising from other data sources.
After completing the second training workshop (and before the CASCADE groups started) a short questionnaire was sent to attendees. This collected further demographic data and evaluated the UCLH training workshops with the benefit of distance from the training.
Interviews with the University College London Hospitals trainers
A semistructured interview schedule was designed to gather views of the UCLH trainers on the process of intervention development and delivery of the training workshops. Perceptions of progress with group delivery in the sites were also captured. One interview was carried out soon after all training workshops had been conducted and before many sites started delivering groups. The second interview was conducted once the delivery in the sites was well under way.
CASCADE groups
Observation
Non-participant observation by a researcher of one complete group of four modules delivered by the trained site educators occurred in each of the 13 intervention clinics that ran any modules. The observation collected data on timings, setting, attendance, observers’ and staff views on what worked well and what worked less well, and recorded the extent to which the intervention was delivered as intended and noted problems that arose ‘in the real world’.
Groups in each site were purposively selected to ensure a mix of first, middle and last groups, based on the premise that trainers would gain confidence and expertise the more groups they ran.
A semistructured observation pro forma was used to enable a description of delivery of the intervention comparable across all sites, with unstructured observations to provide context and meaning to the observations. The pro forma was developed by the research team using the intervention manual and initial observations by researchers of sessions in four sites. Four researchers who had attended at least one UCLH training workshop carried out observations. The observer sat away from the group to be as unobtrusive as possible but near enough to be able to observe effectively. Observers were introduced to the group as a member of the research team by the site educators. Notes were used to complete the pro forma as soon as possible after the group had finished to maximise recall accuracy. Observers referred to the intervention manual throughout to be able to ‘score’ completion of each activity. Activity content in each module was rated on a scale of 1 (not completed) to 7 (completed). Observers also rated use of psychological techniques on a scale from 1 (not at all) to 5 (extensively) for each module. These two scales were designed to ensure high levels of validity and reliability. More intervals were used on the content than the technique scale because of an expectation that there would be more discernible difference with content than with technique. When developing the pro forma the research team discussed what each scale point meant to ensure a common view about amount of content and technique.
Site educator feedback
Site educators completed a short pro forma after every module to record information about young person and/or parent carer demographics, together with details of non-attendees. Staff rated the extent to which they felt they had delivered the required content on a scale of 1–7 and the extent to which they had used the key techniques on a scale of 1–5.
Interviews with site staff
Researchers interviewed one site educator from each intervention site and a key member of staff who had contact with young people in the control clinics (usually the lead nurse), as well as other key stakeholders working within the clinics. A purposive sampling approach ensured that those expected to have key information were included. Interviews were conducted either in person or over the telephone with the exception of the structured questions at the beginning, which could be e-mailed and completed in advance of the main interview. Interviews were conducted after all groups had been completed in the intervention sites. Control site interviews lasted about 30 minutes, and about 60 minutes for intervention sites. The schedule included structured and semistructured questions about service structure, current challenges regarding diabetes care, and current training/education. For the intervention sites semistructured questions were asked about the training and delivery of the CASCADE intervention.
Interviews with young people and parents
The main aim was to gather in-depth information on how young people and their families look after their diabetes, and the education they had been offered and/or received about diabetes. Control site interviews focused on standard practice, whereas in the intervention sites they included experience of the CASCADE modules.
The original protocol stated that these data would be collected from focus groups. Given the challenges experienced in the study with accessing young people at time points additional to routine clinic appointments, a decision was made to use individual interviews with young people and their carers instead. This minimised inconvenience for all stakeholders as interviews could be conducted in the clinic setting at the same time as follow-up data were collected or, alternatively, over the telephone. Interviews with up to four young people in each intervention site and two in each control site (plus parents) were planned. Saturation point (the point at which no new information was given) was reached after 30 intervention and 18 control site interviews were completed therefore target numbers were reduced to two and one per site respectively. To ensure a range of key characteristics within the sample, interviewees in each site were purposively selected using a sampling grid constructed for each site ( Box 1 ). The aim was to achieve diversity across as many characteristics as possible in each site and across the two arms of the trial.
Girl < 12 years.
Girl ≥ 12 years.
Boy < 12 years.
Boys ≥ 12 years.
At least one missed clinic appointment in previous year.
No missed clinic appointments in previous year.
The grids for the intervention sites had, in addition, attendance at:
-
all CASCADE sessions
-
some CASCADE sessions
-
no CASCADE sessions.
Individuals were invited to take part in an interview by a member of the research team, with parents/carers invited to take part once the young person consented. Young people were interviewed even if parents/carers declined.
Semistructured interview schedules used learning from data collected in the study and lasted approximately 20 minutes. They were piloted with a few young people and then further refined. All children and young people were asked about their experience and management of diabetes. Intervention site informants were also asked about their experience of CASCADE and any perceived impact. Those who had opted out of sessions were asked about their reasons for opting out. Carers were asked about views of their child’s management of diabetes, and, in the intervention sites, experience of CASCADE.
In control sites, interviews were completed at the time of first follow-up data collection. In intervention sites, interviews were carried out within 3 months of the young person completing groups. Most participants were invited to take part by letter that explained the main aim of the interview, and informed that they would receive a £10 voucher as a ‘thank you’ and compensation for their time.
Five researchers carried out most interviews face to face. Parent and young person interviews were designed to be conducted separately but a request to do the interview together was acceptable. Some were carried out over the phone when a face-to-face encounter was difficult to arrange. If a selected participant declined to be interviewed then a replacement participant was selected from the sampling grid.
Questions in secondary outcome measure questionnaires
A small number of PE questions were incorporated in the follow-up questionnaires for intervention families. This allowed data capture from the whole intervention sample (as opposed to just those interviewed). In the 12-month follow-up questionnaires the focus was on experience and perceived impact of the CASCADE groups. Those who had declined to take up the groups were asked to give reasons for this. In the 24-month follow-up questionnaires, questions were based on interview data from young people and carers focusing particularly on views of sustained perceived impact.
Field notes
Detailed field notes were kept during the 4 years of data collection.
Case note review
A CNR was conducted at each site around the time of the participants’ 24-month follow-up appointment. CNR for withdrawn patients was completed up to when they last provided data for the study. The same data were collected for participants at both control and intervention sites and entered on to a secure database with controlled access and no patient identifiable information. Data were used to describe standard care, specifically the level of nursing contacts in both control and intervention sites. Data were compared using Microsoft Excel version 2003, version 11 (Microsoft Corporation, Redmond, WA, USA).
Analysis
For large quantities of qualitative data analysis was supported by the use of NVivo software version 9 (QSR International, Southport, UK). Where the quantity was small, it was done by hand. Qualitative analysis of the interview transcripts identified key topics and issues that emerged from the data through familiarisation with transcripts. 179 Pertinent excerpts were coded and memos written to summarise and synthesise emerging themes. Researchers refined their analysis ensuring that themes were crosschecked with other data, first within and then between transcripts so that the validity of emerging explanations were tested and improved so themes were ordered within an analytical framework based on the data. To maximise the validity of the findings, a sample of the qualitative data were separately analysed and reviewed to agree a framework for full analysis of all data.
Quantitative data were analysed using an Excel spreadsheet and the Statistical Package for the Social Sciences (SPSS) version 19 software (SPSS Inc., Chicago, IL, USA) for statistical tests.
Composite fidelity delivery scores from individual observer and self-rated scores were created for each pro forma for content and for technique. This allowed the range and median score for content and technique to be calculated. A further composite variable was then calculated, which summed the content and technique scores for each site across all four modules. If any scores were missing for a module the total scores would be divided by the number of completed modules. This allowed comparison across sites and modules, as well as comparing the individual influence of content and techniques.
Analysis of the UCLH training workshops observation was carried out by a separate researcher reading through the notes made by the observers and identifying key themes and fidelity issues emerging from the data.
Chapter 4 Results
Clinic recruitment
Twenty-eight paediatric diabetes clinics in London, SE England and the Midlands were recruited between May 2008 and January 2010 with local research governance processes completed by March 2010 ( Figure 4 ). One clinic withdrew before randomisation owing to the departure of the PI. An additional clinic was recruited to replace it. A large number of clinics were ineligible because of involvement in three other large paediatric diabetes research studies [Development and Evaluation of a Psychosocial Intervention for Children and Teenagers Experiencing Diabetes (DEPICTED), Kids in Control of Food (KICk-OFF), and Family and Adolescent Communication and Teamwork Study (FACTS)]. 136,180,181 Concern about staff capacity was the most common reason for declining to participate.
FIGURE 4.
Cumulative (predicted vs. actual) clinic recruitment.
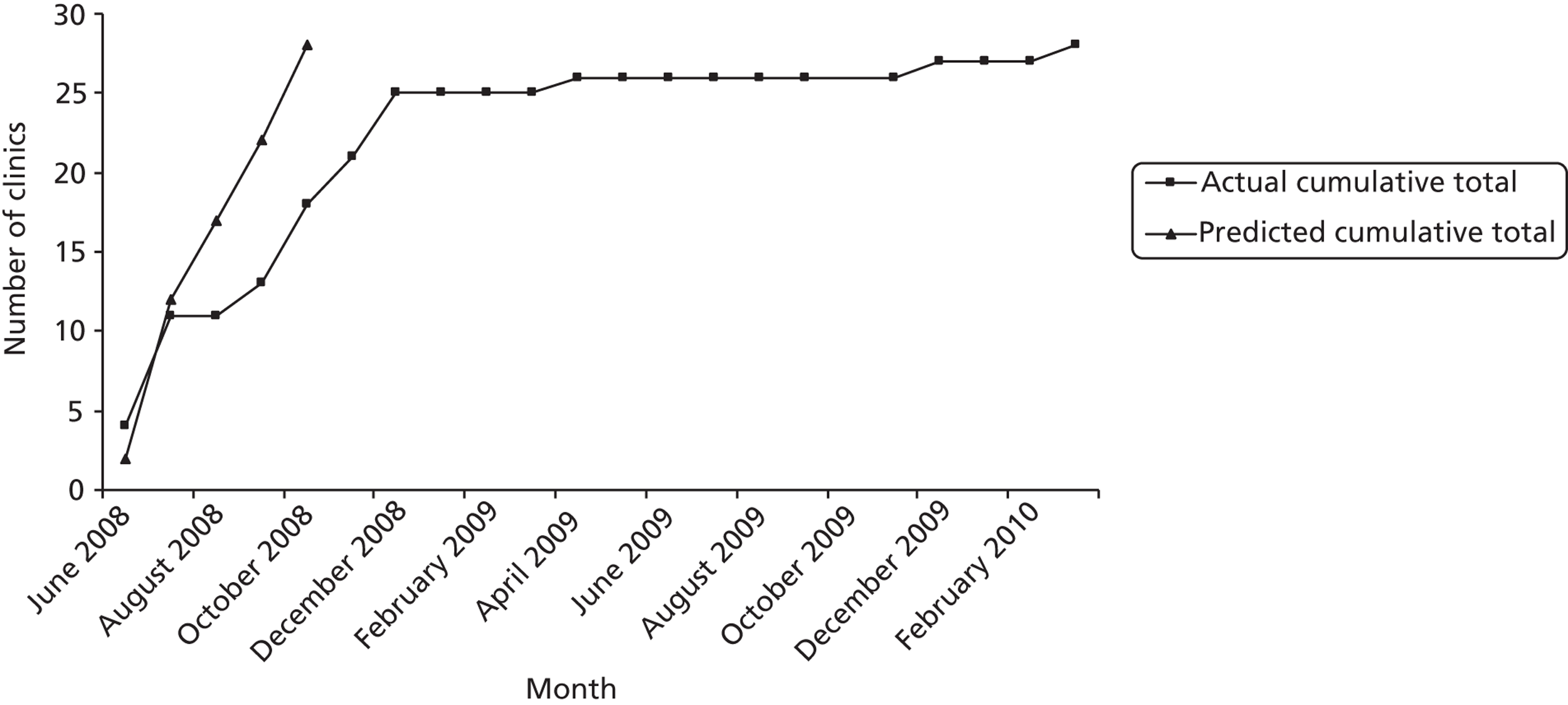
Characteristics of the clinics
Clinic-level characteristics clinics are shown in Table 2 . The two groups were similar at trial entry, with all clinics mixed with regards to age – 86% in each arm were general hospitals with similar numbers of eligible patients in each arm.
| Characteristic | Intervention (N = 14) | Control (N = 14) |
|---|---|---|
| Age profile of patients (years)a | ||
| Paediatric (0–12), n (%) | – | – |
| Adolescent (13–18), n (%) | – | – |
| Mixed, n (%) | 14 (100) | 14 (100) |
| Type of clinica | ||
| General hospital, n (%) | 12 (86) | 12 (86) |
| Specialist/tertiary, n (%) | 2 (14) | 2 (14) |
| No. of eligible patientsb | ||
| ≤ 32, n (%) | 6 (43) | 4 (28) |
| 32–50, n (%) | 5 (36) | 5 (36) |
| > 50, n (%) | 3 (21) | 5 (36) |
| Clinic size | ||
| No. attending clinic, mean (SD) | 122 (45.8) | 140 (51.3) |
| More than 50 young people registered, n (%) | 14 (100) | 14 (100) |
| More than 100 young people registered, n (%) | 11 (79) | 9 (64) |
Patient recruitment
Three hundred and sixty-five patients were recruited between February 2009 and September 2010. A total of 1340 eligible patients were identified and sent information about the study. Attempts were made to approach all eligible patients; however, some failed to attend multiple clinic appointments. Overall, 1177 were approached and invited to take part, representing a recruitment rate of 31% of those approached, 27% of eligible. Main reasons for declining to participate included lack of time and dislike of giving venous blood samples. Three patients were recruited with ineligible HbA1c levels and were therefore excluded from further analysis. The final recruitment figure was 362. Figure 5 shows the flow of clinics and young people through the trial.
FIGURE 5.
Consolidated Standards of Reporting Trials (CONSORT) diagram showing flow of clinics and young people through the trial. IQR, interquartile range. a, Includes one deceased; and b, only those with baseline measures as well as follow-up were included in the analysis.
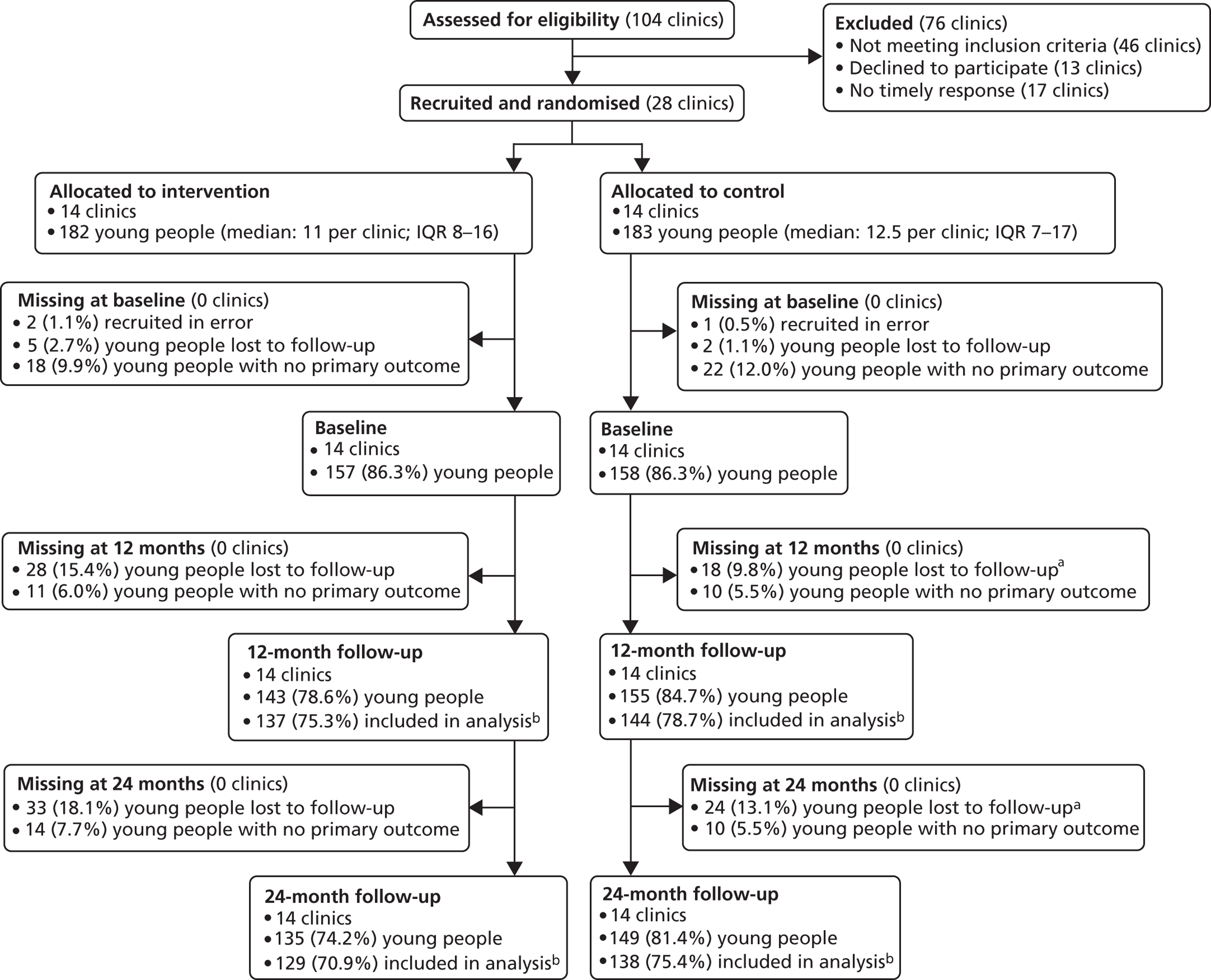
Baseline characteristics of the participants
Demographic information on 327 children and young people and 324 parents who completed the baseline questionnaire are shown in Tables 3 and 4 . Groups were similar at baseline, with a mean (child) age of 13 years (SD 2.1 years), and time since diagnosis of diabetes of 5.1 years in the intervention arm and 5.6 years in the control arm.
| Characteristic | Intervention (N = 159) | Control (N = 168) |
|---|---|---|
| Gender | ||
| Female, n (%) | 91 (57.2) | 90 (53.6) |
| Male, n (%) | 68 (42.8) | 78 (46.4) |
| Age (years), mean (SD) | 13.1 (2.1) | 13.2 (2.1) |
| 1 missing | 3 missing | |
| Ethnicity | ||
| White British, n (%) | 133 (83.7) | 129 (76.8) |
| White other, n (%) | 5 (3.1) | 5 (3.0) |
| Mixed, n (%) | 7 (4.4) | 4 (2.4) |
| Asian/Asian British, n (%) | 5 (3.1) | 14 (8.3) |
| Black/black British, n (%) | 5 (3.1) | 6 (3.6) |
| Chinese, n (%) | 0 | 0 |
| Other, n (%) | 4 (2.5) | 9 (5.4) |
| Time since diagnosis (years), mean (SD) | 5.7 (3.2) | 6.1 (3.3) |
| 27 missing | 32 missing | |
| Time since enrolled at participating clinic (years), mean (SD) | 5.1 (2.9) | 5.6 (3.2) |
| 32 missing | 32 missing | |
| Characteristic | Intervention (N = 159) | Control (N = 168) |
|---|---|---|
| Gender | ||
| Female, n (%) | 137 (87.8) | 151 (89.4) |
| Male, n (%) | 19 (12.2) | 17 (10.1) |
| 1 missing | ||
| Relationship to young person with diabetes | ||
| Mother, n (%) | 136 (87.2) | 153 (90.5) |
| Father, n (%) | 17 (10.9) | 13 (7.7) |
| Female guardian, n (%) | 0 | 1 (0.6) |
| Male guardian, n (%) | 0 | 0 |
| Other, n (%) | 2 (1.3) | 0 |
| 1 missing | 2 missing | |
| Partnership status | ||
| Married, n (%) | 89 (57.1) | 113 (66.9) |
| Cohabiting, n (%) | 18 (11.5) | 18 (10.7) |
| Single parent, n (%) | 45 (28.9) | 36 (21.3) |
| 4 missing | 2 missing | |
| Ethnicity | ||
| White British, n (%) | 135 (86.5) | 132 (78.1) |
| White other, n (%) | 7 (4.5) | 5 (3.0) |
| Mixed, n (%) | 0 | 2 (1.2) |
| Asian/Asian British, n (%) | 6 (3.9) | 15 (8.9) |
| Black/black British, n (%) | 4 (2.6) | 5 (3.0) |
| Chinese, n (%) | 1 (0.6) | 1 (0.6) |
| Other, n (%) | 3 (1.9) | 8 (4.7 |
| 1 missing | ||
| Tenure | 135 (86.5) | 132 (78.1) |
| Privately owned, n (%) | 106 (68.0) | 98 (58.0) |
| Council/rented, n (%) | 48 (30.8) | 68 (40.2) |
| 2 missing | 3 missing |
Table 5 shows the numbers providing data at each point of the analysis. Main reasons for loss to follow-up included an inability to contact participants at follow-up points (attempted directly and with assistance of clinic staff), and withdrawal (1) as moving away, (2) for reported reasons including needle phobia or pressure of GCSEs, and (3) for unexplained reasons. Some participants unavailable at follow-up 1, were re-engaged for data collection at follow-up 2. Table 5 shows some participants provided one type of follow-up data (blood sample or questionnaire) but not the other. Participants in the intervention arm who changed their mind about taking part in the CASCADE groups were informed that they were welcome to remain in the study despite choosing to opt out of the intervention.
| Type of follow-up | Intervention, n (%) | Control, n (%) | Total, n (%) |
|---|---|---|---|
| Eligible and randomised | 180 | 182 | 362 |
| Baseline | |||
| Both blood and questionnaires | 142 (78.9) | 146 (80.2) | 288 (79.6) |
| Blood only | 15 (8.3) | 12 (6.6) | 27 (7.5) |
| Questionnaires only | 17 (9.4) | 22 (12.1) | 39 (10.8) |
| Neither | 6 (3.3) | 2 (1.1) | 8 (2.2) |
| 12-month follow-up | |||
| Both blood and questionnaires | 138 (76.7) | 152 (83.5) | 290 (80.1) |
| Blood only | 5 (2.8) | 3 (1.6) | 8 (2.2) |
| Questionnaires only | 10 (5.6) | 7 (3.8) | 17 (4.7) |
| Neither | 27 (15.0) | 20 (11.0) | 47 (13.0) |
| 24-month follow-up | |||
| Both blood and questionnaires | 130 (72.2) | 141 (77.5) | 271 (74.9) |
| Blood only | 5 (2.8) | 8 (4.4) | 13 (3.6) |
| Questionnaires only | 14 (7.8) | 10 (5.5) | 24 (6.6) |
| Neither | 31 (17.2) | 23 (12.6) | 54 (14.9) |
Missing data
As there was no differential attrition by arm and no reason to believe the data were missing not at random, an intention-to-treat analysis based on randomised participants who provided primary outcome data was undertaken for all outcomes.
Baseline data
Values of the primary outcome at baseline are shown in Table 6 .
| Primary outcome | Intervention group | Control group | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| HbA1c (venepuncture) at baseline | 157 | 9.9 (1.5) | 158 | 10.0 (1.5) |
Baseline young people measures for secondary outcomes are shown in Table 7 , with baseline carer measures in Table 8 . The two groups appear well balanced at baseline both as reported by the young person and as reported by the carer.
| Secondary outcomes | Intervention group (N = 159) | Control group (N = 168) | ||||
|---|---|---|---|---|---|---|
| Missing | Mean (SD) | Median (IQR)/n (%) | Missing | Mean (SD) | Median (IQR)/n (%) | |
| PedsQL: generala | ||||||
| Physical health summary score | 46 | 87.6 (12.0) | 90.6 (84.4–96.9) | 15 | 87.4 (11.8) | 90.6 (81.3–96.9) |
| Psychosocial health summary score | 46 | 81.3 (13.5) | 83.9 (75.0–91.7) | 15 | 79.5 (13.8) | 81.7 (70.0–90.0) |
| Total score | 46 | 83.5 (12.1) | 87.0 (76.1–92.4) | 15 | 82.3 (11.7) | 84.8 (75.0–91.3) |
| PedsQL: diabetes module | ||||||
| Diabetes score | 1 | 63.3 (17.0) | 62.5 (50.0–77.3) | 1 | 62.1 (16.8) | 61.4 (50.0–72.7) |
| Treatment 1 score | 4 | 73.6 (20.1) | 75.0 (62.5–87.5) | 1 | 76.6 (20.5) | 81.3 (62.5–93.8) |
| Treatment 2 score | 2 | 82.5 (15.3) | 85.7 (75.0–92.9) | 1 | 83.7 (15.1) | 87.5 (71.4–96.4) |
| Worry score | 1 | 70.0 (25.2) | 75.0 (50.0–91.7) | 1 | 72.4 (23.2) | 75.0 (58.3–91.7) |
| Communication score | 1 | 70.5 (26.2) | 75.0 (50.0–91.7) | 1 | 77.5 (23.0) | 83.3 (66.7–100.0) |
| DFRQ | ||||||
| Family responsibility total score – young person | 24 | 34.8 (5.1) | 35.0 (31.0–39.0) | 20 | 35.5 (4.3) | 36.0 (32.5–39.0) |
| Family responsibility total score – young person (weightedb) | 2 | 34.9 (5.1) | 35.0 (31.0–38.3) | 1 | 35.3 (4.6) | 36.0 (32.0–39.0) |
| SDQ | ||||||
| Strengths and difficulties total average impact score | 3 | 0.38 (1.30) | 0.0 (0.0–0.0) | 5 | 0.31 (0.94) | 0.0 (0.0–0.0) |
| Normal | 3 | – | 139 (89.1) | 5 | – | 145 (89.0) |
| Borderline | – | 2 (1.3) | – | 3 (1.8) | ||
| Abnormal | – | 15 (9.6) | – | 15 (9.2) | ||
| Body weight and insulin | ||||||
| Happiness with body weight | 4 | 6.9 (2.7) | 8.0 (5.0–9.0) | 7 | 6.6 (2.8) | 7.0 (4.0–9.0) |
| No. of times skipped insulin in last month | 4 | – | 0 (0 to 2) | 6 | – | 0 (0 to 2) |
| Ever skipped insulin to lose weight | 0 | – | 8 (5.0) | 0 | – | 9 (5.4) |
| Baseline outcomes | Intervention group (N = 156) | Control group (N = 169) | ||||
|---|---|---|---|---|---|---|
| Missing | Mean (SD) | Median (IQR)/n (%) | Missing | Mean (SD) | Median (IQR)/n (%) | |
| Management | ||||||
| No. of severe hypoglycaemic episodes in last month | ||||||
| 1 | – | – | 12 (7.6) | – | – | 13 (7.8) |
| 2 | – | 2 (1.3) | – | 2 (1.2) | ||
| 3 | – | 1 (0.6) | – | 1 (0.6) | ||
| 12 | – | 1 (0.6) | – | 0 | ||
| No. of times admitted in last 6 months | ||||||
| 1 | – | – | 23 (14.7) | – | – | 16 (9.5) |
| 2 | – | 0 | – | 2 (1.2) | ||
| 3 | – | 1 (0.6) | – | 1 (0.6) | ||
| 10 | – | 1 (0.6) | – | 0 | ||
| No. of times attended diabetes clinic in last year | 7 | 3.7 (0.9) | 4.0 (3.5–4.0) | 13 | 3.9 (1.5) | 4.0 (3.5–4.0) |
| Visit hospital doctor most/every visit | 10 | – | 130 (89.0) | 9 | – | 142 (88.8) |
| Visit diabetes nurse most/every visit | 5 | – | 139 (92.1) | 4 | – | 158 (95.8) |
| Visit dietitian occasionally or more often | 16 | – | 129 (92.1) | 17 | – | 129 (84.9) |
| Visit psychologist occasionally or more often | 47 | – | 16 (14.7) | 46 | – | 19 (15.5) |
| PedsQL: general | ||||||
| Physical health summary score | 44 | 85.4 (14.2) | 89.1 (78.6–93.8) | 15 | 83.8 (17.6) | 87.5 (78.1–96.9) |
| Psychosocial health summary score | 44 | 74.6 (15.6) | 76.7 (63.8–86.7) | 15 | 71.9 (18.7) | 75.0 (61.7–85.0) |
| Total score | 44 | 78.4 (13.2) | 79.3 (70.1–89.1) | 15 | 76.0 (17.1) | 80.4 (68.5–88.0) |
| PedsQL: diabetes module | ||||||
| Diabetes score | 1 | 57.7 (15.8) | 56.8 (45.5–68.2) | 1 | 55.5 (17.3) | 54.5 (43.2–65.9) |
| Treatment 1 score | 1 | 60.9 (22.4) | 62.5 (43.8–75.0) | 1 | 61.6 (21.8) | 62.5 (50.0–75.0) |
| Treatment 2 score | 1 | 76.9 (16.3) | 78.6 (65.0–89.3) | 0 | 75.9 (18.2) | 75.0 (64.3–91.7) |
| Worry score | 1 | 64.8 (25.6) | 66.7 (50.0–83.3) | 1 | 65.5 (25.2) | 66.7 (50.0–83.3) |
| Communication score | 1 | 68.2 (28.0) | 66.7 (50.0–100.0) | 1 | 68.9 (29.6) | 75.0 (50.0–100.0) |
| DFRQ | ||||||
| Family responsibility total score – parent | 26 | 30.9 (4.6) | 31.0 (27.0–35.0) | 28 | 31.9 (5.0) | 32.0 (29.0–35.0) |
| Family responsibility total score – parent (weighteda) | 0 | 31.1 (4.7) | 31.0 (27.5–35.0) | 1 | 31.6 (5.4) | 31.8 (28.0–35.0) |
| Dyadic parent–child responsibility pattern | 44 | 0.82 (1.23) | 0 (0–1) | 40 | 0.72 (0.97) | 0 (0–1) |
| SDQ | ||||||
| Strengths and difficulties total average impact score | 2 | 0.57 (1.40) | 0.0 (0.0–0.0) | 1 | 0.79 (2.07) | 0.0 (0.0–0.0) |
| Normal | 2 | – | 126 (81.8) | 1 | – | 138 (82.1) |
| Borderline | – | 4 (2.6) | – | 5 (3.0) | ||
| Abnormal | – | 24 (15.6) | – | 25 (14.9) | ||
The DMEC confidentially reviewed unblinded interim analyses on three occasions and did not recommend stopping the trial early.
Primary outcomes
Results for the primary outcomes at 12 and 24 months are shown in Tables 9 and 10 .
| Primary outcome | Intervention group | Control group | Adjusted difference in meansa (95% CI) | ||
|---|---|---|---|---|---|
| n | Mean (SD)(mmol/l) | n | Mean (SD)(mmol/l) | ||
| HbA1c (venepuncture) at 12 months | 143 | 10.2 (2.0) | 155 | 10.1 (1.6) | 0.11 (–0.28 to 0.50), p = 0.584 |
| Change in HbA1c (venepuncture) at 12 months from baseline | 137 | 0.38 (1.34) | 144 | 0.28 (1.27) | |
| 6 missing | 11 missing | ||||
| Primary outcome | Intervention group | Control group | Adjusted difference in meansa (95% CI) | ||
|---|---|---|---|---|---|
| n | Mean (SD)(mmol/l) | n | Mean (SD)(mmol/l) | ||
| HbA1c (venepuncture) at 24 months | 135 | 10.1 (1.9) | 149 | 10.0 (1.7) | 0.03 (–0.36 to 0.41), p = 0.891 |
| Change in HbA1c (venepuncture) at 24 months from baseline | 129 | 0.10 (1.52) | 138 | 0.07 (1.53) | |
| 6 missing | 11 missing | ||||
The mean HbA1c value at 12 months was 10.2 mmol/l in the intervention group and 10.1 mmol/l in the control group [mean difference 0.11 (95% CI –0.28 to 0.50)]. The ICC for the primary outcome at 12 months was 0.134.
The mean HbA1c value at 24 months was 10.1 mmol/l in the intervention group and 10.0 mmol/l in the control group [mean difference 0.03 (95% CI –0.36 to 0.41)]. The ICC for the primary outcome at 24 months was 0.060. There was no impact of the intervention on the primary outcome.
Secondary outcomes
Secondary young person outcomes
The secondary outcomes as reported by the participants at 12 months and 24 months are shown in Tables 11 and 12 . They are similar between the two arms of the trial. At 24 months the intervention group has a higher DFRQ scores, which indicates that child/young person is taking more responsibility for self-management. There is also reduced self-reported happiness in body weight. However, it should be noted that with a 5% level of significance this number of significant findings is consistent with chance.
| Secondary outcomes | Intervention group (N = 148) | Control group (N = 159) | Adjusted effecta (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Missing | Mean (SD) | Median (IQR)/n (%) | Missing | Mean (SD) | Median (IQR)/n (%) | ||
| PedsQL: general | |||||||
| Physical health summary score | 1 | 87.9 (12.2) | 90.6 (84.4–96.9) | 1 | 86.6 (11.7) | 88.4 (78.1–96.9) | 0.34 (–2.51 to 2.62) |
| Psychosocial health summary score | 1 | 78.3 (13.6) | 81.7 (70.0–88.3) | 1 | 78.8 (13.7) | 81.7 (71.4–90.0) | –1.85 (–4.29 to 0.24) |
| Total score | 1 | 81.7 (12.0) | 83.7 (76.1–90.2) | 1 | 81.5 (11.7) | 82.6 (75.0–89.1) | –1.09 (–3.15 to 0.63) |
| PedsQL: diabetes module | |||||||
| Diabetes score | 0 | 62.1 (15.7) | 61.4 (51.1–72.7) | 2 | 60.8 (16.1) | 61.4 (50.0–70.5) | 0.62 (–2.35 to 3.04) |
| Treatment 1 score | 1 | 72.0 (20.6) | 75.0 (56.3–87.5) | 2 | 74.3 (22.1) | 81.3 (62.5–93.8) | –0.80 (–5.14 to 3.08) |
| Treatment 2 score | 0 | 82.5 (16.1) | 85.7 (75.0–92.9) | 2 | 83.2 (15.6) | 87.5 (75.0–96.4) | –1.90 (–4.99 to 1.97) |
| Worry score | 2 | 70.5 (24.7) | 75.0 (58.3–91.7) | 5 | 72.3 (24.4) | 75.0 (58.3–91.7) | –0.77 (–5.43 to 3.94) |
| Communication score | 1 | 71.6 (26.5) | 75.0 (58.3–91.7) | 1 | 75.5 (23.2) | 79.2 (58.3–91.7) | –1.34 (–6.31 to 4.01) |
| DFRQ | |||||||
| Family responsibility total score – young person | 13 | 36.0 (4.9) | 36.0 (32.0–39.0) | 15 | 36.3 (5.2) | 37.0 (32.5–40.0) | –0.12 (–1.04 to 0.75) |
| Family responsibility total score – young person (weightedb) | 1 | 36.0 (4.9) | 36.0 (32.9–39.0) | 2 | 36.2 (5.1) | 36.0 (32.6–40.0) | 0.01 (–0.85 to 0.81) |
| SDQ | |||||||
| Strengths and difficulties total average impact score | 0 | 0.47 (1.42) | 0.0 (0.0–0.0) | 1 | 0.25 (1.21) | 0.0 (0.0–0.0) | 0.09 (–0.21 to 0.43) |
| Normal, n (%) | 0 | – | 130 (87.8) | 1 | – | 148 (93.7) | – |
| Borderline, n (%) | – | 1 (0.7) | – | 3 (1.9) | |||
| Abnormal, n (%) | – | 17 (11.5) | – | 7 (4.4) | |||
| Body weight and insulin | |||||||
| Happiness with body weight | 7 | 6.5 (2.6) | 7.0 (4.0–9.0) | 12 | 6.5 (2.8) | 7.0 (4.0–9.0) | –0.24 (–0.65 to 0.29) |
| No. of times skipped insulin in last month | 2 | – | 0 (0–2) | 4 | – | 0 (0.3) | 0.82c (0.48 to 1.38) |
| Ever skipped insulin to lose weight, n (%) | 0 | – | 4 (2.7) | 0 | – | 11 (6.9) | 0.28 (0.07 to 1.13) |
| Secondary outcomes | Intervention group (N = 144) | Control group (N = 151) | Adjusted effecta (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Missing | Mean (SD) | Median (IQR)/N (%) | Missing | Mean (SD) | Median (IQR)/N (%) | ||
| PedsQL: general | |||||||
| Physical health summary score | 1 | 87.5 (11.2) | 90.6 (81.3–96.9) | 3 | 86.3 (12.7) | 87.5 (81.3–96.9) | 1.14 (–1.28 to 3.32) |
| Psychosocial health summary score | 1 | 78.3 (13.9) | 80.0 (68.3–88.3) | 3 | 79.7 (12.2) | 81.7 (71.7–88.3) | –1.17 (–3.69 to 1.45) |
| Total score | 1 | 81.5 (11.8) | 83.7 (75.0–90.2) | 3 | 82.0 (11.4) | 84.2 (75.0–90.2) | –0.33 (–2.53 to 1.97) |
| PedsQL: diabetes module | |||||||
| Diabetes score | 0 | 63.7 (15.6) | 63.6 (50.0–75.0) | 1 | 63.6 (15.2) | 63.6 (54.5–75.0) | –0.02 (–3.19 to 2.72) |
| Treatment 1 score | 0 | 72.9 (18.5) | 75.0 (62.5–87.5) | 1 | 75.2 (20.4) | 81.3 (62.5–93.8) | –1.05 (–4.52 to 2.32) |
| Treatment 2 score | 0 | 82.2 (14.6) | 85.7 (71.4–95.8) | 3 | 83.0 (15.5) | 85.7 (75.0–95.8) | –1.49 (–4.53 to 1.42) |
| Worry score | 0 | 70.1 (23.1) | 75.0 (58.3–87.5) | 2 | 72.5 (21.8) | 75.0 (58.3–91.7) | –0.32 (–4.64 to 4.52) |
| Communication score | 2 | 74.4 (23.3) | 83.3 (58.3–91.7) | 2 | 78.5 (21.8) | 83.3 (66.7–100.0) | –1.06 (–5.34 to 3.59) |
| DFRQ | |||||||
| Family responsibility total score – young person | 5 | 37.7 (4.8) | 38.0 (34.0–41.0) | 11 | 37.6 (5.0) | 37.0 (34.0–42.0) | 0.85 (0.03 to 1.61) |
| Family responsibility total score – young person (weightedb) | 0 | 37.8 (4.8) | 38.0 (34.0–41.0) | 1 | 37.5 (5.0) | 37.0 (34.0–42.0) | 0.71 (–0.07 to 1.44) |
| SDQ | |||||||
| Strengths and difficulties total average impact score | 2 | 0.28 (1.05) | 0.0 (0.0–0.0) | 0 | 0.27 (0.92) | 0.0 (0.0–0.0) | 0.04 (–0.14 to 0.21) |
| Normal, n (%) | 2 | – | 130 (91.6) | 0 | – | 135 (89.4) | – |
| Borderline, n (%) | – | 2 (1.4) | – | 4 (2.7) | |||
| Abnormal, n (%) | – | 10 (7.0) | – | 12 (8.0) | |||
| Body weight and insulin | |||||||
| Happiness with body weight | 5 | 6.3 (2.7) | 7.0 (4.0–9.0) | 8 | 6.6 (2.7) | 7.0 (5.0–9.0) | –0.56 (–1.03 to –0.06) |
| No. of times skipped insulin in last month | 2 | – | 0 (0–2) | 3 | – | 0 (0–2) | 1.30c (0.78 to 2.17) |
| Ever skipped insulin to lose weight, n (%) | 0 | – | 5 (3.5) | 0 | – | 7 (4.6) | 0.72 (0.22 to 2.33) |
Changes in knowledge and diabetes regimen were additional secondary outcomes. These data proved difficult to collect with accuracy. We present the results here ( Table 13 ), but these data should be interpreted with caution, as they are unadjusted for baseline and have a large number of missing participants. Young people showed no increase in knowledge at either follow-up.
| Knowledge and insulin injections | Intervention group (N = 148) | Control group (N = 159) | Effect (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Missing | Mean (SD) | Median (IQR)/n (%) | Missing | Mean (SD) | Median (IQR)/n (%) | ||
| Baseline | |||||||
| Knowledge (maximum possible = 7) | 57 | 4.1 (1.4) | 4.0 (3.0–5.0) | 60 | 3.9 (1.5) | 4.0 (3.0–5.0) | Not calculated |
| At 12 months | |||||||
| Knowledge (maximum possible = 7) | 38 | 4.1 (1.3) | 4.0 (3.0–5.0) | 47 | 4.1 (1.3) | 4.0 (3.0–5.0) | Not calculated |
| No. of injections per day | 39 | 4.0 (0.8) | 4.0 (4.0–4.0) | 62 | 3.9 (0.9) | 4.0 (4.0–4.0) | 0.08 (–0.22 to 0.34) |
| Average total dose of quick-acting insulin per day | 78 | 32.8 (15.9) | 30.0 (22.0–42.0) | 65 | 27.8 (16.6) | 25.5 (16.0–37.0) | 5.06 (1.04 to 9.93) |
| Average total dose of slow-acting insulin per day | 49 | 32.9 (16.3) | 30.0 (22.0–42.0) | 43 | 27.4 (16.0) | 26.5 (15.5–36.0) | 4.94 (0.61 to 9.16) |
| At 24 months | |||||||
| Knowledge (maximum possible = 7) | 20 | 4.3 (1.3) | 4.0 (4.0–5.0) | 24 | 4.3 (1.3) | 4.0 (3.5–5.0) | Not calculated |
| No. of injections per day | 53 | 3.9 (0.8) | 4.0 (4.0–4.0) | 50 | 4.1 (1.2) | 4.0 (4.0–4.0) | –0.14 (–0.43 to 0.15) |
| Average total dose of quick-acting insulin per day | 71 | 39.8 (21.2) | 34.0 (25.0–50.0) | 59 | 33.0 (22.3) | 30.0 (18.0–40.0) | 6.87 (1.26 to 12.57) |
| Average total dose of slow-acting insulin per day | 45 | 33.8 (19.3) | 29.0 (22.0–42.0) | 52 | 27.9 (13.8) | 27.5 (19.0–34.5) | 5.85 (1.58 to 9.73) |
Secondary parent/carer outcomes
Secondary outcomes reported by the parent/carer are shown in Tables 14 and 15 . They are similar between the two arms of the trial. At 12 months, the carer reports an increase in the family responsibility score, which indicates the child/young person is taking more responsibility for self-management of diabetes. As stated this significant finding is consistent with chance.
| Secondary outcome measures | Intervention group (N = 143) | Control group (N = 155) | Adjusted effecta (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Missing | Mean (SD) | Median (IQR)/n (%) | Missing | Mean (SD) | Median (IQR)/n (%) | ||
| Management | |||||||
| No. of severe hypoglycaemic episodes in last month, n (%) | |||||||
| 1 | – | – | 9 (6.3) | – | – | 11 (7.1) | 0.76b (0.35 to 1.67) |
| 2 | – | 2 (1.4) | – | 2 (1.3) | |||
| 3 | – | 1 (0.7) | – | 1 (0.7) | |||
| 4 | – | 0 | – | 1 (0.7) | |||
| 5 | – | 0 | – | 1 (0.7) | |||
| No. of times admitted in last 6 months, n (%) | |||||||
| 1 | – | – | 17 (11.9) | – | – | 14 (9.0) | 1.08b (0.54 to 2.14) |
| 2 | – | 2 (1.4) | – | 2 (1.3) | |||
| 6 | – | 0 | – | 1 (0.7) | |||
| 8 | – | 0 | – | 1 (0.7) | |||
| No. of times attended diabetes clinic in last year | 4 | 3.7 (1.0) | 4.0 (3.5–4.0) | 5 | 3.9 (0.9) | 4.0 (3.5–4.0) | 0.01 (–0.22 to 0.24) |
| Visit hospital doctor most/every visit, n (%) | 7 | – | 127 (93.4) | 14 | – | 127 (90.1) | 2.71 (0.81 to 9.05) |
| Visit diabetes nurse most/every visit, n (%) | 3 | – | 128 (91.4) | 5 | – | 143 (95.3) | 0.68 (0.22 to 2.05) |
| Visit dietitian occasionally or more often, n (%) | 11 | – | 121 (91.7) | 20 | – | 122 (90.37) | 1.64 (0.53 to 5.06) |
| Visit psychologist occasionally or more often, n (%) | 49 | – | 19 (20.2) | 46 | – | 12 (11.0) | 3.64 (0.56 to 23.63) |
| PedsQL: general | |||||||
| Physical health summary score | 0 | 86.7 (15.3) | 90.6 (81.3–96.9) | 0 | 81.9 (15.9) | 87.5 (71.9–93.8) | 2.24 (–1.34 to 5.10) |
| Psychosocial health summary score | 0 | 75.4 (17.1) | 78.3 (63.3–88.3) | 0 | 71.0 (17.6) | 71.7 (58.3–83.3) | 1.76 (–1.50 to 5.79) |
| Total score | 0 | 79.3 (14.7) | 82.6 (70.7–89.1) | 0 | 74.8 (15.7) | 77.2 (63.0–85.9) | 1.74 (–1.14 to 5.00) |
| PedsQL: diabetes module | |||||||
| Diabetes score | 2 | 59.1 (16.8) | 56.8 (50.0–70.5) | 1 | 55.7 (17.4) | 54.5 (40.9–65.9) | 1.13 (–1.72 to 4.02) |
| Treatment 1 score | 2 | 64.2 (21.2) | 62.5 (50.0–81.3) | 1 | 61.9 (22.3) | 62.5 (50.0–81.3) | 0.79 (–2.58 to 4.10) |
| Treatment 2 score | 0 | 77.6 (18.1) | 82.1 (64.3–92.9) | 1 | 73.3 (20.8) | 75.0 (62.5–89.3) | 2.37 (–0.40 to 5.37) |
| Worry score | 2 | 69.1 (24.7) | 75.0 (50.0–91.7) | 2 | 65.4 (25.0) | 66.7 (50.0–83.3) | 3.59 (–1.50 to 7.57) |
| Communication score | 0 | 71.5 (29.5) | 75.0 (50.0–100.0) | 3 | 68.5 (29.9) | 75.0 (50.0–100.0) | 2.30 (–2.44 to 8.64) |
| DFRQ | |||||||
| Family responsibility total score – parent | 10 | 32.9 (5.3) | 33.0 (29.0–36.0) | 12 | 32.4 (4.9) | 32.0 (29.0–36.0) | 0.74 (0.03 to 1.52) |
| Family responsibility total score – parent (weightedc) | 0 | 32.9 (5.3) | 33.0 (29.0–36.0) | 1 | 32.5 (5.1) | 32.5 (29.0–36.0) | 0.62 (–0.17 to 1.47) |
| Dyadic parent–child responsibility pattern | 28 | 0.58 (0.81) | 0 (0–1) | 30 | 0.69 (1.1) | 0 (0–1) | 0.00 (–0.22 to 0.20) |
| SDQ | |||||||
| Strengths and difficulties total average impact score | 2 | 0.59 (1.56) | 0.0 (0.0–0.0) | 1 | 0.60 (1.57) | 0.0 (0.0–0.0) | –0.03 (–0.31 to 0.27) |
| Normal, n (%) | 2 | – | 118 (83.7) | 1 | – | 127 (82.5) | – |
| Borderline, n (%) | – | 5 (3.6) | – | 5 (3.3) | |||
| Abnormal, n (%) | – | 18 (12.8) | – | 22 (14.3) | |||
| Secondary outcome measures | Intervention (N = 137) | Control (N = 140) | Adjusted effecta (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Missing | Mean (SD) | Median (IQR)/n (%) | Missing | Mean (SD) | Median (IQR)/n (%) | ||
| Management | |||||||
| No. of severe hypoglycaemic episodes in last month, n (%) | |||||||
| 1 | – | – | 6 (4.4) | – | – | 7 (5.0) | 0.92b (0.32 to 2.59) |
| 2 | – | 1 (0.7) | – | 3 (2.1) | |||
| 3 | – | 3 (2.2) | – | 1 (0.7) | |||
| No. of times admitted in last 6 months, n (%) | |||||||
| 1 | – | – | 11 (8.0) | – | – | 12 (8.6) | 0.95b (0.46 to 1.96) |
| 2 | 2 (1.5) | 3 (2.1) | |||||
| 3 | 2 (1.5) | 1 (0.7) | |||||
| 4 | – | 1 (0.7) | – | 0 (0.0) | |||
| 6 | – | 0 (0.0) | – | 1 (0.7) | |||
| No. of times attended diabetes clinic in last year | 11 | 3.5 (0.9) | 4.0 (3.0–4.0) | 11 | 3.9 (1.1) | 4.0 (3.5–4.0) | –0.18 (–0.41 to 0.06) |
| Visit hospital doctor most/every visit, n (%) | 8 | – | 121 (93.8) | 6 | – | 115 (85.8) | 3.09 (1.07 to 8.91) |
| Visit diabetes nurse most/every visit, n (%) | 5 | – | 125 (94.7) | 5 | – | 122 (90.4) | 2.70 (0.92 to 7.92) |
| Visit dietitian occasionally or more often, n (%) | 9 | – | 118 (92.2) | 16 | – | 106 (85.5) | 2.26 (0.86 to 5.93) |
| Visit psychologist occasionally or more often, n (%) | 31 | – | 27 (25.5) | 31 | – | 14 (12.8) | 3.85 (0.77 to 19.31) |
| PedsQL: general | |||||||
| Physical health summary score | 0 | 84.8 (15.7) | 87.5 (81.3–93.8) | 0 | 81.6 (16.4) | 87.5 (71.9–93.8) | 2.00 (–2.04 to 5.29) |
| Psychosocial health summary score | 2 | 74.0 (16.3) | 75.0 (63.3–88.3) | 0 | 71.8 (16.8) | 73.3 (61.7–84.2) | 0.03 (–3.08 to 3.66) |
| Total score | 1 | 77.8 (14.3) | 77.2 (69.6–90.2) | 0 | 75.2 (15.5) | 77.2 (66.3–87.0) | 0.58 (–2.70 to 3.46) |
| PedsQL: diabetes module | |||||||
| Diabetes score | 1 | 59.0 (16.4) | 56.8 (47.7–68.2) | 0 | 57.9 (18.6) | 56.8 (45.5–71.6) | –0.08 (–2.83 to 3.12) |
| Treatment 1 score | 1 | 65.2 (21.1) | 68.8 (50.0–81.3) | 0 | 63.5 (24.0) | 62.5 (50.0–81.3) | 2.20 (–2.31 to 6.88) |
| Treatment 2 score | 1 | 75.1 (17.9) | 78.6 (64.3–89.3) | 1 | 75.7 (18.8) | 78.6 (64.3–92.9) | –1.10 (–4.49 to 2.50) |
| Worry score | 2 | 68.2 (24.2) | 66.7 (50.0–83.3) | 0 | 68.5 (23.6) | 66.7 (50.0–89.6) | 0.66 (–3.75 to 5.14) |
| Communication score | 1 | 70.9 (28.4) | 75.0 (50.0–100.0) | 1 | 71.3 (30.4) | 83.3 (50.0–100.0) | –2.54 (–9.06 to 4.16) |
| DFRQ | |||||||
| Family responsibility total score – parent | 14 | 34.8 (4.8) | 35.0 (32.0–38.0) | 19 | 34.1 (5.5) | 34.0 (30.0–38.0) | 0.57 (–0.67 to 1.49) |
| Family responsibility total score – parent (weightedc) | 0 | 34.7 (4.8) | 35.0 (31.9–38.0) | 0 | 34.3 (5.4) | 34.0 (30.0–38.5) | 0.60 (–0.43 to 1.47) |
| Dyadic parent–child responsibility pattern | 23 | 0.69 (1.0) | 0 (0–1) | 43 | 0.73 (1.1) | 0 (0–1) | –0.03 (–0.26 to 0.23) |
| SDQ | |||||||
| Strengths and difficulties total average impact score | 4 | 0.62 (1.57) | 0.0 (0.0–0.0) | 1 | 0.73 (1.54) | 0.0 (0.0–0.0) | –0.09 (–0.41 to 0.26) |
| Normal, n (%) | 4 | – | 110 (82.7) | 1 | – | 107 (77.0) | – |
| Borderline, n (%) | – | 2 (1.5) | – | 6 (4.3) | |||
| Abnormal, n (%) | – | 21 (15.8) | – | 26 (18.7) | |||
Comparison of per-protocol and intention-to-treat analyses
A per-protocol analysis was completed using participants who had attended three or four modules. In general the results from the per-protocol analysis support the findings from the intention-to-treat analysis of no effect of the intervention on any of the primary or secondary outcomes. The per-protocol analysis tables are in Appendix 6 .
Subgroup analyses
A number of prespecified subgroup analyses were carried out. The results from these are given in Table 16 . The subgroup analysis suggested that those with higher baseline HbA1c levels experienced a small rise in HbA1c that was significant at 12 months but not at 24 months, suggesting that those with higher HbA1c levels responded poorly to the CASCADE intervention. Given the number of statistical tests completed this result is consistent with chance.
| Primary outcome | Follow-up | |||||
|---|---|---|---|---|---|---|
| 12 months | 24 months | |||||
| n | Adjusted difference in meansa (95% CI) | Interaction p-value | n | Adjusted difference in meansa (95% CI) | Interaction p-value | |
| Age group | ||||||
| ≤ 12 years | 129 | 0.17 (–0.34 to 0.68) | 0.639 | 118 | –0.20 (–0.75 to 0.36) | 0.268 |
| > 12 years | 149 | 0.02 (–0.45 to 0.50) | 146 | 0.20 (–0.30 to 0.71) | ||
| Sex | ||||||
| Males | 128 | 0.11 (–0.40 to 0.62) | 0.914 | 122 | 0.18 (–0.36 to 0.73) | 0.398 |
| Females | 153 | 0.08 (–0.40 to 0.56) | 145 | –0.12 (–0.62 to 0.39) | ||
| Baseline HbA1c level | ||||||
| < 10.4 mmol/l | 191 | –0.15 (–0.59 to 0.28) | 0.015 | 179 | –0.20 (–0.64 to 0.24) | 0.106 |
| ≥ 10.4 mmol/l | 90 | 0.63 (0.05 to 1.20) | 88 | 0.40 (–0.21 to 1.01) | ||
| SES (index of multiple deprivation) | ||||||
| Least deprived 80% | 204 | 0.09 (–0.32 to 0.50) | 0.201 | 189 | 0.11 (–0.34 to 0.57) | 0.895 |
| Most deprived 20% | 48 | 0.63 (–0.15 to 1.40) | 50 | 0.18 (–0.71 to 1.06) | ||
Serious adverse events
Serious adverse events were reported through clinics using a standardised reporting form following a SOP. Table 17 provides the number of SAEs reported by site staff.
| SAE | Intervention | Control |
|---|---|---|
| Severe hypoglycaemia | 0 | 2 |
| DKA (when pH < 7.1) | 2 | 6 |
| Death (as a result of DKA)a | 1 | |
| Less severe DKA (not meeting the criteria above) | 5 | 3 |
| Other (monitoring and advice) | 0 | 1 |
Despite procedures in place and review of these procedures during the trial, it was recognised that fewer than expected SAEs were being reported. Therefore, the CNR data were used to ascertain a clearer picture of the number of SAEs that occurred during the trial. Tables 18 and 19 show the number of SAEs identified through the CNR.
| Adverse event type | Intervention, n (N = 163) | Control, n (N = 180) | Overall, n (N = 343) |
|---|---|---|---|
| DKA | 58 | 67 | 125 |
| Hyperglycaemia with or without ketones | 29 | 19 | 48 |
| Hypoglycaemia | 8 | 7 | 15 |
| Severe hypoglycaemia | 2 | 4 | 6 |
| Uncontrolled diabetes/monitoring or insulin adjustment | 9 | 8 | 17 |
| Admission for general illness, for which T1D was a factor – includes gastric issues and pump problems | 19 | 27 | 46 |
| Accident or other problem (T1D not a complication) | 17 | 26 | 43 |
| Reason for admission not recorded | 3 | 29 | 32 |
| Total SAEs | 145 | 187 | 332 |
| No. of SAEs recorded | Intervention, n (%) | Control, n (%) | Overall, n (%) |
|---|---|---|---|
| 0 | 95 (58.3) | 106 (58.9) | 201 (58.6) |
| 1 | 34 (20.9) | 37 (20.6) | 71 (20.7) |
| 2 | 17 (10.4) | 14 (7.8) | 31(8.6) |
| 3 | 7 (4.3) | 5 (2.8) | 12 (3.5) |
| 4 | 2 (1.2) | 6 (3.3) | 8 (2.3) |
| 5 | 3 (1.8) | 4 (2.2) | 7 (2.0) |
| 6 | 3 (1.8) | 3 (1.7) | 6 (1.7) |
| 7 | 2 (1.2) | 1 (0.6) | 3 (0.8) |
| 8 | 0 (0.0) | 1 (0.6) | 1 (0.3) |
| 9 | 0 (0.0) | 1 (0.6) | 1 (0.3) |
| 12 | 0 (0.0) | 2 (1.1) | 2 (0.6) |
| Total no. of participants | 163 (100.0) | 180 (100.0) | 343 (100.0) |
Table 19 shows that there is no evidence of any difference in the number of patients experiencing SAEs between the two arms of the trial.
Chapter 5 Process evaluation results
This chapter reports on:
-
views of clinic staff participating in CASCADE
-
attendance, quality, fidelity and acceptability of the UCLH training workshops for the UCLH trainers and site educators
-
feasibility and acceptability for sites organising the intervention
-
feasibility and acceptability for sites delivering the intervention
-
feasibility and acceptability for families engaging with the intervention
-
parents’ and young people’s perceptions of impact of participation in CASCADE and views of staff regarding the impact on young people and their own practice.
Data sources and respondents
Sources of data used for these PE findings are listed below. Site codes given after each quote indicate if the respondent is from an intervention site (I) or a control site (C).
-
observation of the UCLH training
-
training workshop attendance data (provided by UCLH)
-
site educator CASCADE training evaluation forms for modules 1–4: 170 (99%) evaluation forms received immediately after 12 UCLH workshop sessions
-
site educator CASCADE training questionnaires: 27 (63%) received a few weeks after the UCLH training from 18 nurses, eight dietitians and one doctor
-
interviews with the two UCLH trainers
-
researcher observation of CASCADE modules delivery: 47 modules observed across 13 intervention sites (12 each of modules 1, 2 and 4 and 11 of module 3, between November 2009 and March 2011); observations were made in the first set of CASCADE groups run; in five sites they were in the second set and in three sites they were the third set; site educator views were obtained after each observed module
-
site educator pro formas completed following delivery of each module: 125 pro formas (94%) returned following completion of 131 modules
-
interviews with site staff: 30 staff interviewed (15 in each trial arm), after CASCADE groups delivered; 28 of the interviewees were nurses (of whom 14 were site educators)
-
interviews with young people and parents/carers: 53 young people (21 control/32 intervention) and 52 parents were interviewed; of the young people, 31 were female, 17 were 10–11 years old and 36 were 12–18 years old
-
young people and parent questionnaires at 12 and 24 months: PE questions were completed by 135 young people and 121 parents at 12 months and by 121 young people and 114 parents at 24 months.
Staff perspectives on taking part in the CASCADE trial
The decision to take part in the trial
Staff interviewed were mainly nurses. They had become involved in CASCADE mainly because of a consultant’s interest in being part of the study. About half of them remembered it being a collaborative team decision to take part:
Dr X said ‘I think we should do this, what do you think?’ And I remember two ladies coming down [presentation from the research team]. It sounded interesting, so we agreed to do it.
Nurse I11
For a significant minority, involvement was not something they had actively chosen, either because the current consultant, with little or no discussion, had instigated it or a previous consultant or other member of staff who was no longer part of the diabetes team had agreed to take part:
By that point I knew [my predecessor] wasn’t gonna come back but I felt that I couldn’t stop the process because it had gone too far and I’d sort of inherited it all and I just thought, ‘oh my goodness, how am I gonna cope with all of this?’
Nurse I4
Views on becoming involved in CASCADE
Becoming involved in CASCADE was an exciting prospect for many. They saw it as an opportunity to develop aspects of their service they wanted and would be expected to offer in the future.
I was quite keen because we’d had to stop giving the diet sessions because we hadn’t got . . . enough dietetic time and I just thought it would be great to have some structured education . . . and I thought it was something that we could be involved in and hopefully give something to benefit the kids.
Nurse I13
Many, however, including those who were generally enthusiastic about being involved, expressed concern they might not have the time or resources to do what would be required. Nurses were unsure whether consultants who had agreed to be part of the study were aware of the extent of the work involved.
I was a bit anxious at the time because I felt that the consultant didn’t truly understand who did what [in CASCADE] and I think she believed that everybody [from the central team] was gonna come in and deliver the sessions which wasn’t the thing so that made me a little bit anxious.
Nurse I1
Feelings about the outcome of the site randomisation process
Staff had varied reactions to which arm their site was randomised to. About half in the intervention arm were pleased. An equal number were anxious and concerned often owing to the delay that resulted from the extended patient recruitment process, which meant that the timing was no longer good for their site primarily because of lack of resources or staff shortages.
Horrified, well, because of the time delay and by the time we were starting it was just not a good time for me, so yes, I was horrified.
Nurse I1
Well, initially I think we were a little anxious because at the time we were having staff shortages and we think from a practical point of view it would have been better to have been in the other arm but it didn’t matter . . . we were excited to be in it anyway.
Nurse I5
Those randomised to the control arm were also evenly split between relief and disappointment at the zoutcome.
I was quite relieved because having to do the education [would have been] really difficult for me . . . actually carrying that out without any support [would have been] very difficult.
Nurse C2
We’d have liked to have been in the intervention arm partly because we were hoping that people would like to see what we could do to change our practice to improve the teenagers, particularly 12 and upwards [when they] start to get more independent and not wanting to comply.
C8
University College London Hospitals CASCADE training
Attendance at the training
Workshops 1 and 2 were run on six occasions each between 18 August 2009 and 21 May 2010 at UCLH. The UCLH trainers also travelled to deliver training to eight staff from two hospitals (in one site) over 2 days. The remaining workshops 1 and 2 were run between 1 and 7 weeks apart, with the majority ≤ 2 weeks apart. Each site sent the required minimum of two staff, with some sites sending more than two. A total of 43 staff attended. A few sites found it challenging to ‘free up’ staff to attend. Seven additional staff from intervention sites attended the 1-day refresher workshop. The numbers of participants at each workshop ranged from two to eleven. The number of clinics at workshops ranged from one to four ( Table 20 ).
| Workshop 1 | Workshop 2 | ||
|---|---|---|---|
| No. of individuals | No. of clinics | No. of individuals | No. of clinics |
| 5 | 3 | 7 | 3 |
| 7 | 3 | 9 | 4 |
| 11 | 4 | 2 | 1 |
| 8 | 3 | 7 | 2 |
| 5 | 2 | 6 | 3 |
| 6 | 3 | 6 | 3 |
The majority attending were PDSNs. Dietitians from 10 intervention sites attended, eight of whom were intending to be the second educator. One site, unable to release a second member of the diabetes team for training, sent a research nurse based at the site. One consultant attended both workshops. Three consultants attended one workshop or the 1-day refresher. Two nurses who attended the initial workshops also attended the 1-day workshop.
Fidelity across training workshops
Observation data demonstrated a high degree of fidelity across both workshops. The same trainers delivered all workshops and maintained a high degree of consistency in both content and style. The training approach was highly interactive with regular opportunities to clarify concerns in question and answer sessions.
Satisfaction and acceptability of training
Attendance
The majority of staff who completed the questionnaire (21/27) indicated they had been ‘extremely’ or ‘very’ keen to participate in the training. The remainder were ‘quite’ keen. Any reticence was generally the result of anxiety about lack of knowledge and experience, lack of control over the decision to take part, or lack of time to take on the delivery of the groups.
The UCLH trainers noted during workshop 1 that some attendees appeared to ‘not want to be there’. Approaches were used to change this resistance into a more relaxed position.
For some it was much more aggressive than that . . . ‘I haven’t got time for this, I shouldn’t be here’ . . . Anger actually, and so we had that conversation and then also started at the beginning . . . I guess the other side of that was how involved some of these people got, some of these people who came in clearly feeling a little bit bamboozled into it and not of their own choice, who actually went off really enthusiastic and positive and that was lovely.
UCLH trainer
Venue and timing
Ten workshops were run in London (at UCLH). Travelling to London was not generally a problem for intervention clinic staff, as long as they had enough notice. Two workshops were provided locally which had the advantage of reaching more members of the site team and made attendance easier.
Initially we thought we might have had to go down south and [a staff member] was going off and all those things . . . ‘oh, what have we done?’, but no, I mean [northern training location] was fine.
Nurse I10
Spreading the remaining workshops out (a few weeks apart) was acceptable to the majority, who said a 2-day block would have been more difficult to attend because of other commitments. Staff and trainers commented that 2 days of training felt the right amount.
A 45-minute lunch break was provided in every workshop. Breaks in the morning and afternoon depended on how well training was proceeding. When there were no additional breaks there was feedback that a break would have been an improvement in view of the high information load and opportunities to network.
Content and training style
Most staff thought the training was very good, motivating and comprehensive.
I thought it was excellent. I thought it was really good, we were quite motivated when we left, weren’t we . . . excited . . . I thought it was very well run.
Nurse I5
The primary focus of the workshops was to train site educators to use the specific techniques of the programme rather than teach specialist diabetes management. Site educators enjoyed learning about MI/SFBT techniques. Many found it refreshing to learn a communication style that was non-didactic, focused on positives and drew on the skills and knowledge of patients and families. They reported this approach might be useful in their practice.
I think it’s a really clever way of delivering your education. It’s very different to the way I’m used to. I tend to talk at people and teach that way . . . old fashioned teaching, and I liked seeing the different style how you actually get them to do the talking and draw it out of them.
Nurse I14
Not all trainees were positive about the approaches and their applicability in the ‘real world’. One trainee doubted the usability of the approach.
I can see that it would work for some patients, I don’t know it would work for all of them.
Nurse I4
For another trainee, the approaches were confusing until she put them into practice.
It didn’t make sense at all . . . when it was just talking about MI it kind of lost me a bit. I guess I’m not so much into psychology . . . but having said that, when you do try and put it into practice yourself it makes sense.
Nurse I13
Observation and evaluation form data showed variability in understanding by some site educators of some specialist diabetes management topics. These included food groups, matching food and insulin, CHO counting, insulin sensitivity ratios and exercise management.
I was shocked at the low level of knowledge for some of them to the point that some of it was ending up teaching them the content as opposed to teaching them the style of delivery. I hadn’t expected to be teaching content.
UCLH trainer
One secondary site educator commented
At the time I’d got very little diabetes knowledge so, for me, I was actually learning from it and I know that’s not really what it was about but a lot of it was that . . . I found it quite intimidating because of my lack of knowledge.
Nurse I3
The training modelled the approach to be used in module delivery. Witnessing examples of how to run modules in practice, including the integral group exercises, was considered to be a really useful training approach by the participants.
It was nice to see them actually do the session as they would anticipate us doing it so that was really helpful to see how . . . instead of just being talked about it and then left with it.
Nurse I6
The manual and resources
Site educators were positive about the manual and the resources. They found the manual easy to follow with useful prompts to promote wider discussion.
Preparedness to deliver CASCADE after training
The majority of the 27 participants who completed questionnaires rated their readiness to run sessions (on a scale of 1–10 with 10 being the highest) at 8 (range 6–9). Despite the majority saying that the 2 days’ training was adequate, there was concern at the scale of the task ahead considering the relative briefness of the training.
I remember thinking . . . gosh, they’ve thrown a lot of information at me and if and when we did ever get this running, I don’t know that I’m going to be able to remember all the things I’ve been taught.
Nurse I4
It was a lot in the few days. Teaching people theories and expecting them to suddenly change their behaviour I think is very difficult . . . cause some things are so set in your approach to leading things. I’ve taught student nurses before and to try and completely change how you do less talking [isn’t easy].
Nurse I1
The time gap between the training workshops and setting up the groups meant that some site educators said that they had lost focus and confidence.
I came away really buzzing inside to get going, but the downside for me was that we didn’t start then for probably another 3 months and I felt like I’d lost my confidence a bit.
Nurse I14
The 1-day refresher workshop was offered to all site educators to address this but only two site educators chose to attend. The UCLH trainers made it clear that they were available to give additional support to the site educators by phone or by e-mail.
Organising the CASCADE groups
Numbers of groups
A total of 30 groups were run across 14 intervention sites. The modal number of completed groups was two, with a range of zero to six groups offered by clinics ( Table 21 ). Twelve sites delivered at least one complete group of four modules. One site only offered the first session and one site failed to offer any groups.
| No. of complete groups run | No. of sites |
|---|---|
| 0 | 2 |
| 1 | 2 |
| 2 | 5a |
| 3 | 4 |
| 6 | 1 |
Sites were not given a formal target number of groups but were aware that all recruited participants should be offered the opportunity to attend a group. A post hoc calculation suggested that 44 groups should have been run across the 14 intervention sites. Only three clinics completed the maximum number of groups possible ( Table 22 ).
| No. of groups | No. of sites |
|---|---|
| Completed ideal number of groups | 3 |
| One group fewer than ideal number completed | 8 |
| Two groups fewer than ideal number completed | 3 |
Size of groups
The aim was to deliver a group to three to four young people of a similar age. This was not achieved in most modules ( Table 23 ). The attendance ranged from one to five young people with a mode of two.
| Module | Total completed | No. of young people attending each module | ||
|---|---|---|---|---|
| Range | Mean | Mode | ||
| 1 | 38 | 1–5 | 2.6 | 2 |
| 2 | 32 | 1–4 | 2.4 | 2 |
| 3 | 33 | 1–4 | 2.3 | 2 |
| 4 | 30 | 1–4 | 2.2 | 3 |
Time between modules
The average interval between modules was slightly greater than 1 month ( Table 24 ) though the average length across all groups (from start to finish) was approximately 4 months. The main reason for delays was problems organising dates, as well as site educators being on sick leave or study leave.
| Intervals between modules: | Mean (days) | Range (days) |
|---|---|---|
| 1 and 2 | 38 | 7–98 |
| 2 and 3 | 43 | 14–111 |
| 3 and 4 | 43 | 14–91 |
| Total between 1 and 4 | 124 | 55–238 |
Time between training and delivery
As soon as site educators completed the UCLH training they were free to start preparing the groups. On average, sites ran the first CASCADE module 3 months after completing the UCLH training ( Table 25 ) with module 4 completed 7 months after training.
| Intervals | Mean (days) | Range (days) |
|---|---|---|
| Completing CASCADE training and running module 1 of first group | 101 (n = 13 sites) | 42–176 |
| Completing CASCADE training and running module 4 of first group | 213 (n = 12 sites) | 140–280 |
The training was very good, the only criticism is between training and then actually getting up and running was about 6 months which is . . . I think the training needs to be done a month before you go and launch your site.
Site educator I12
A key reason for delay setting up the first groups was delay in patient recruitment. Training began in August 2009; however, patient recruitment was not completed until September 2010. Earlier trained clinics often had to wait several months before they could start to organise and deliver groups. Many site educators cleared their diaries in anticipation of delivering groups; however, the delay meant that they were unable to sustain this with other commitments encroaching on the time required for the project. Where there were enough patients to make up a group, organising and delivering groups started before patient recruitment was completed. For sites with a low number of recruited patients this strategy prevented groups being organised by age.
Time between sets of groups
Where sites had sufficient participants groups were mostly run concurrently, organised by age, at different times to suit particular families. One site had a very long gap between running their first set of groups and their second owing to a site educator being on sick leave.
Days and time of CASCADE modules
Groups were run on a full range of weekdays. They were offered within school time (morning or afternoon), immediately after school (e.g. 1600), and evening (e.g. 1800). The most frequent time for running modules was the early afternoon ( Table 26 ). Saturdays and holidays did not appear to appeal to participants more than weekdays. One attempt at running a module on a Saturday ended when no participants arrived.
| Time of session | n (%) [N = 105a] |
|---|---|
| Morning | 20 (19) |
| Early afternoon | 42 (40) |
| Late afternoon | 26 (25) |
| Evening | 17 (16) |
Preparation for running groups
In the majority of sites a single site educator did the bulk of the preparatory work particularly when the second trainer had limited time allocated to paediatric diabetes (e.g. dietitians and mental health practitioners). Preparation involved deciding which participants should be grouped together, setting dates and times and booking rooms. This was felt to be particularly burdensome in sites where there was just one PDSN.
Initial decisions on how to group participants were considered straightforward, except in sites with small numbers of recruited patients. Where numbers were small, compromises were made about grouping by age. For sites with larger recruited numbers this compromise was usually made as they reached the last groups and the remaining pool of participants was small.
Setting dates and times was the most challenging aspect reported. The initial challenge was anticipating and/or determining time preferences for participants. Approaches used included contacting families to ask for individual timing preferences and offering a range of different days. Some staff were prepared to work outside normal working hours – including evenings and weekends; others were not.
The first one I think we held at about six in the evening having spoken to the families first and that was the preferred time for them and then we negotiated it as we went along. The first group was more flexible and were prepared to come in . . . let’s say less outside hours time. The second group was a nightmare because . . . there was no [flexibility]. ‘We want it at this time and I’m giving up this to get here’ and there was no concession at all that [my colleague] and I were giving up our [personal activities].
Nurse I11
Other sites made calculated guesses about the best times for holding the modules.
We did the sessions in the morning and I think the reason for that was really I didn’t feel they’d want to, or be able to, take on that after school when they’re tired.
Nurse I2
I think we did 6.30 to 8.30 [pm] for all our sessions, we thought it was a good choice because we didn’t want the young people to be missing school.
Nurse I8
The process of trying to reach an acceptable date was considered to be laborious and time-consuming. Site educators communicated via a combination of letter, telephone, and (occasionally) text. No site used e-mail or web-based diary systems to help organise dates. For most sites agreeing a date involved a series of negotiations to ensure that the greatest number of potential participants could attend.
We tried, we sent out an initial letter with a date like say, for example, the 8th of July as so those that didn’t respond I rang them again and said ‘We haven’t heard from you’ and they’d say ‘I’m on holiday that day’. So then I offered them an alternative date the following week and some of them were offered three or four opportunities to come before we abandoned them. So everybody was offered a selection of dates. It wasn’t through want of trying, it was a shame really.
Nurse I14
Group 2 was the problem. We probably offered something like August, October, November and December . . . really the people couldn’t make any of those dates.
Nurse I13
Following the setting of the date and time, most sites sent out a standard confirmation letter (provided by UCLH). Some sent a reminder letter (or made a reminder telephone call) prior to the date, which improved attendance. Teams that did not send a reminder expressed regret that they had not, thought it should not have been required or did so after early groups failed.
A common challenge was participants not attending agreed dates. When attendance was poor, site educators either cancelled or rescheduled to achieve better take-up or ran the session with one family who were then invited to join remaining modules of a different group. One of the sites that ran three complete groups offered seven module 1, five module 2, four module 3 and three module 4. Only one of the three groups ran with the same participants in (or at least invited to) all four sessions.
The majority of site educators began with great enthusiasm and goodwill trying to accommodate everyone but became more pragmatic about what was achievable over time. Additional administrative support would have made the task considerably easier for many sites, especially where site educators had little administrative experience and had to do this work in addition to normal work responsibilities. No site had any significant level of administrative support for organisation and running of groups.
Oh, it would have been very useful, to have somebody else running around making sure people could make certain dates . . . reminding them, cause one of the issues we had was that people claimed the letters had never gone out so I then started reminding everybody a week before.
Nurse I11
Staffing constraints
Staff turnover, absence and lack of flexibility around work hours also influenced the number of groups organised. In two sites a site educator left while groups were being delivered. One site found a replacement who had attended the refresher workshop and the groups recommenced relatively smoothly. The other site was unable to find a replacement and completed only one group (with the original educators), cancelling the second planned group. One site did not run any modules. The lead trainer went on extended sick leave and on return was deployed into a different role. In another site, educators ran module 1, twice in the morning, on a specific day of the week – inviting the same four families on each occasion. On the first date only one young person and parent turned up, on the second the previous attendee and parent returned along with one other participant and parent. Neither returned for module 2. No further groups were offered owing to the small number of recruited patients in this site.
The added burden of the research context
Problems setting up groups were exacerbated by CASCADE being a research project, not just an educational intervention. Staff felt pressured by additional trial-related tasks, such as organising research blood samples and liaison with research staff.
Restricting groups to a subset of recruited patients, instead of offering groups to the entire clinic list was perceived as making the organisation of the groups more challenging. This was particularly the case in sites with a small number of recruits and/or where a hospital served a large geographical area making travel arrangements complex. It also meant that natural groupings of patients (by age, or geographical area, etc.) were more difficult (and at times not possible). Site educators were aware that they would be observed delivering some of the modules which added to their concern about achieving reasonable group numbers.
Use of time
The theory behind use of time was explained in the training workshops:
How do we find the time to organise and run the sessions?
CASCADE is about organising time differently, not taking more time. For example, a home visit might take an hour and nothing changes afterwards. But, if you run a CASCADE session, you can say something to a group of children that you otherwise might have to say on an individual basis.
However, a great deal of frustration was expressed about the time required to organise CASCADE. In many sites complications around organising the modules took up any time that site educators had allocated to practice and preparation of the programme.
It suddenly hit me about how much commitment and organisation it was going to take and I do remember saying to [a colleague] ‘Gosh, I don’t know how we’re going to fit this in with all the other things that we’ve got!’.
Nurse I4
Staff perceptions were that so much time was spent on organisation it was unlikely to be outweighed by time saved.
I didn’t notice that it saved me any time because I was constantly chasing them [families] up to be there.
Nurse I1
The UCLH trainers were aware that organisation would be challenging and allocated time to discussing this in the training. The extent to which it was to emerge as a barrier had not been anticipated.
There have been no email, telephone requests back to [the trainers] . . . about the content of the manual, how to teach a particular element or uncertainty about ‘could you go through this again?’ I’ve had nothing. Nothing. The main one has been . . . ‘I can’t get people to come to this group, but also the admin organising side of it’.
UCLH trainer
Teamwork
Sites that were the most successful organising and running groups were similar. Two exemplary sites had dedicated MDTs who were flexible and willing to modify schedules in order to provide additional tailored care for the young people in their clinics. These sites had shown greater whole team dedication to the CASCADE project than some of the other sites, for example with greater consultant involvement.
Delivery of the CASCADE education sessions
Site educators
All four CASCADE modules should have been delivered by at least two trained site educators with the recommended level of diabetes knowledge. 165 Delivery in most sites was carried out by two trained educators (range 1–3). Staff delivering the modules were primarily PDSNs. In some sites the second (or third) site educator was a dietitian who shared the training equally at some sites, whereas in others covered only dietary topics. In one site an educator was a Child and Adolescent Mental Health Service (CAMHS) practitioner ( Table 27 ).The majority of site educators reported that they worked well together.
| No. of trained educators delivering modules | No. of sites | Specialist roles of educators |
|---|---|---|
| 1a | 1 | One PDSN |
| 2 | 8 | PDSN, paediatric nurse (one site) Two PDSNs (three sites) PDSN and dietitian (three sites)b PDSN and CAMHS practitioner (one site) |
| 3 | 3 | Two PDSNs and dietitian |
All sites had continuity with at least one trained site educator. Difficulties with second educators were experienced in a number of sites where they deviated from recommended CASCADE principles. In one site a dietitian who had not attended any of the CASCADE training assisted with the delivery of a module 4 session. In another site two members of staff who had only attended workshop 1 provided a support role in some modules. The rationale behind including untrained or partly trained staff was that site educators felt they did not have specific expertise that the untrained site educator had (e.g. dietetics or psychology). In a third site, two groups were delivered predominantly single-handed as the second site educator was sick. The site educator who ran these solo sessions said the lack of a second educator made delivering the modules very challenging.
Venues
Staff often had limited choice over venues. All groups, bar one, were held on hospital premises, usually in a seminar/training room. Most rooms had tables and chairs suitable for a teaching environment. The groups ran most smoothly when venues were comfortable, light and airy, and the set-up was conducive to interactive small group activities and discussion. One site held evening sessions in a pavilion in the community, creating a relaxed and informal environment away from the hospital setting.
Some sites managed with less suitable venues such as hospital wards and consulting rooms. One site held a module in an open waiting room area of children’s outpatients, with hospital staff passing through while the group was in progress. In another outpatient setting, there were no tables and the telephone in the background rang continuously. Distractions such as these disrupted module delivery.
Measuring fidelity of delivery
Activity completion was measured through site educator self-report and observer ratings. Self-report for each activity in all four modules had a median score of 7 (out of 7) for every activity (see Appendix 7 , Table 52 ). Observers scored activity completion lower than site educators, although still generally quite high. When observers rated an activity as being incomplete, the reasons given were mainly not using techniques enough or appropriately, rushing, missing a part of the activity or deviating from the protocol.
Time spent on activities
The manual recommends a guide time for each activity within each module. Modules were supposed to last between 90 and 110 minutes. Most lasted between 75 and 120 minutes (range 55–150 minutes).
Educators could be flexible with timing, depending on numbers in the group, group dynamics, family knowledge and interest in different topics. Although there was variation between sites, there were no activities that were either consistently truncated or that over ran excessively (see Appendix 7 , Table 53 ). A key factor affecting timing was the size of the group. Smaller groups took less time to complete an activity.
Very few observed modules incorporated a planned break. Observers noted modules flowed better when there was a scheduled short break to prevent children becoming restless and bored. Offering refreshments also appeared to help group mood and improved focus.
Content
CASCADE content was consistent with the recommended level of knowledge and skill of a PDSN. 165 However, there were areas of the curriculum that some site educators reported were beyond their expertise, for example use of CHO for exercise management. The educators spent less time than intended on these sections when delivering the modules. Educators were also more comfortable with activities where they felt families were engaged and/or knowledgeable.
The one thing that had a real impact on all of them was there was a chart that showed your HbA1c and the rise. The graph . . . and how when your HbA1c was 14, how much more at risk you were with your eyes and . . . they all sort of took a sharp intake of breath when they saw that and they’ve all said since, so it really makes you sort of think. We definitely would love to use that in the future.
Nurse I14
Site educators were less able to encourage discussion when there was initial hesitation from families or the family found the topic difficult to understand – e.g. matching food and insulin. When this happened some educators struggled to keep families engaged or interested.
The group introductions and reviews at the start of the modules were particularly challenging. The aim of introductions in module 1 was to help group members connect with each other, to increase engagement and encourage attendance. Despite the importance of this activity some site educators spent only a few minutes on it (see Appendix 7 , Table 53 ). This was particularly the case if they felt they knew the families well. Managing this activity was also difficult when groups were very small.
Module 1 . . . I thought the beginning of it where you’re trying to identify people’s strengths and weaknesses is a very difficult starting position.
I13
Educators also did not find it easy to facilitate reviews at the start of modules 2, 3 and 4. They struggled to ‘bring forth’ positive events and activities during the reviews specifically designed to build participants’ sense of knowledge, skills and abilities.
Reviewing the previous session, people couldn’t remember, it was too far away and I think some of that maybe because we’d have to re-schedule our sessions so it might have been longer than the month but still I do think a month is too long, you’ve forgotten . . . I couldn’t tell you what I did a month ago . . .
Nurse I13
Content: module 1
Module 1 was reported as the hardest module to deliver owing to ‘dry’ content. Others thought it was too long, especially for younger children. Activities that they reported most difficult to complete were (1) focusing on the future and (2) matching insulin to food. Many site educators found it difficult to deliver the ‘looking to future’ activity at the start of module 1 and thought it should be done later when people had warmed to the CASCADE process. They struggled to deliver specific activities when the content conflicted with their standard practice or knowledge of the topic, for example the testing of urine (vs. blood) for ketones. Variation in levels of knowledge about CHO counting also made this module particularly difficult for some site educators. The weakest activities reported by observers were (1) ‘identifying how insulin works’ and (2) ‘what is CHO?’.
The only thing that perhaps didn’t work from my point of view and [the other site educator]’s . . . It’s like a blood stream, how the sugar moves across into the cell, a lot of them didn’t really understand that . . . We had to spend longer on that . . . lots of blank looks.
Nurse I14
Content: module 2
Site educators reported few difficulties with activities in module 2 although observers found that (1) identifying hypoglycaemia and (2) the pros and cons of BG testing were areas of weakness.
It was pros and cons of BG testing, actually getting them to identify the category, extremely difficult and I don’t think they all got those words. They may have said them in the list, but it was really hard for them to actually come out with those words . . . detect, confirm, managing and adjusting. That was really hard to do that, to put that together.
Nurse I6
Content: module 3 and module 4
In modules 3 and 4 reviews of the previous session were rated as the hardest activity to complete. Observers rated the weakest activities in module 3 as (1) when to seek help and (2) permanent insulin changes. In module 4 the weakest were (1) review activity and (2) using CHO to keep BG stable.
Module 4 . . . It’s something we haven’t used before, the way they talk about the exercise and putting it into (blocks) . . . Found that very useful . . . I think had more children been able to come to that session, we would have had quite good results cause we’ve got a lot of kids that do a lot of sport and if they were able to receive that information it would have been good. And even those that maybe don’t have a high HbA1c that were recruited into CASCADE, if they could be delivered that information I think it would benefit a lot of people. I think that was probably the best session out of all of them cause that’s . . . something I don’t think we all cover in clinics very well. We tend to talk about hypos, talk about ketones, talk about illness . . . little bits here and there and I don’t think exercise is covered particularly very well at all.
Nurse I1
Use of techniques
Self-report from the site educators suggested that they felt they had used the SFBT and MI techniques ‘quite a bit’ on average, but less than had been hoped. ‘Asking open questions’ and ‘focusing on positive solutions’ were rated by site educators and observers as being used the most consistently (see Appendix 7 , Table 54 ). They were less confident in ‘avoiding giving choices’ and asking ‘how come?’ and ‘what else?’, which were designed to evoke responses from families. Observers rated these techniques as only ‘somewhat used’.
Overall completion of activities and use of techniques
An alternative way to calculate fidelity was derived to explore the degree to which individual site educators delivered the four modules as intended. From each observer activity score overall activity completion and technique fidelity scores were calculated. The scores only reflect observed modules and do not provide data about changes in fidelity over time for sites that ran more than one group.
The composite activity score was based on all 24 activities delivered in the modules (as listed in Table 28 ). With possible ratings of ‘1’ (not completed) to ‘7’ (completed) the highest possible composite score was 168. The range of composite activity scores achieved by the 12 sites that completed at least one full group of the four CASCADE modules was from 116 to 161 (or 69–96% of the possible maximum score). This indicates that there was a high level of activity completion in eight of the twelve sites.
| Average composite activity scoresa | No. of sitesb | Range of scores (% of possible 168 score) |
|---|---|---|
| > 144 (average ‘completed’ or ‘not quite completed’ across activities) | 3 | 147–161 (88–96) |
| 132–143 (average ‘not quite completed’ or ‘just more than partly completed’ across activities) | 5 | 134–140 (80–87) |
| 108–131 (average ‘partly completed’ or ‘just more than partly completed’ across activities | 4 | 116–129 (69–79) |
The techniques fidelity composite score was based on the six techniques used over four modules. With possible ratings of ‘1’ (not used at all) to ‘5’ (used extensively) the highest possible composite score was 120. Sites scored between 65 (54%) and 113 (94%). Half of the sites were able to use the techniques to an acceptable extent (> 70%) in delivery of the intervention ( Table 29 ).
| Average composite scoresa | No. of sitesb | Range of scores (% of possible 120 score) |
|---|---|---|
| Above 108 (use of techniques ‘extensively’ across all modules) | 1 | 108–113 (94) |
| 84–107 (use of techniques ‘quite a bit’ across all modules) | 5 | 88–108 (73–88) |
| 60–83 (use of techniques ‘somewhat’ across all modules) | 6 | 65–83 (54–69) |
There appeared to be relationship between technique fidelity and activity delivery fidelity scores – with sites having similar high, medium or low scores for both. However, there were a few anomalies – one of the sites that scored lowest in the activity completion rated high on use of techniques, whereas, in contrast, one of the most successful sites in terms of activity completion rated lower on the use of techniques. Both these sites took time to use the manual and plan the sessions.
Barriers and facilitators to delivery
Facilitators
Site educators liked the manual and found it easy to use when delivering the modules.
Sites with high delivery fidelity composite scores shared certain features. First, they were relatively well-resourced teams in terms of staffing. Site educators in these sites spent time preparing by completing the reading and familiarising themselves with the content and plan of activity before the modules were run, as emphasised during training. Site educators reported modules worked best when they read though the module in advance and agreed which educator would be delivering which activity. Modules that were most successful had two (or three) trained and enthusiastic educators who worked out their module plan in advance.
Successful sites also had site educators who had experience delivering groups, worked together for many years in the clinic and good knowledge of the participants. Highest fidelity ratings for individual modules were obtained where site educators were (1) knowledgeable about diabetes, (2) confident clear presenters and (3) persisted in encouraging reluctant participants to complete activities (e.g. breaking into small groups). These site educators were, in the main, very enthusiastic about CASCADE.
As site educators became more familiar with delivery and the group got to know each other there was improvement in the fidelity technique composite scores across the four modules. Early challenges were often overcome through increased confidence gained from running initial modules and increased familiarity with content and less need to check the manual during delivery. Site educators said they had started to use the approaches in every day clinical practice and felt better able to deal with silences and more able to facilitate discussion. Some said that when delivering a module they frequently referred to what a previous group had said. This worked well as a technique to help generate discussion in smaller quiet groups. Two sites started with relatively high scores in the first two modules dropped off in modules 3 and 4. These sites swapped site educators across the modules.
The modules ran well when site educators adapted to difficult circumstances (e.g. only one family attending, difficult family dynamics), ran the sessions at a good pace and finished on time.
Modules ran most smoothly when site educators had a strong grasp of the MI and SFBT techniques underpinning the CASCADE philosophy. Consistent and effective use of the techniques created a sense of inclusion and helped to engage less responsive participants. What worked well:
-
A friendly and non-judgemental manner that created a relaxed, informal atmosphere conducive to positive interaction within and between families.
-
Site educators working hard to ‘draw out’ quiet participants through asking open questions, encourage a thicker description of situations by asking ‘what else?’ and using affirmation and positive connotation.
-
Site educators actively listening to participants, explaining that there were no ‘right or wrong’ answers, as well as exploring issues and questions raised by asking the group for their views and ideas rather than ‘providing expert advice’.
An example of a well-run session is provided in Appendix 8 .
Barriers
The four sites rated as having the lowest composite scores had difficulty organising the modules, which took up much of the time and lowered their enthusiasm for delivering CASCADE. There was illness or disruption in the continuity of the educators with reported time conflicts and heavy workloads that meant the educators were often unprepared for the modules. When there was no preparation observers reported either less adherence to the manual or a stilted delivery needing to refer repeatedly to the manual. These groups were also held in problematic venues.
When there was a long gap between the UCLH training and starting the groups site educators said it was hard to remember what they had been taught and felt ‘very rusty’ delivering the modules.
Despite enthusiasm to use the approaches some found it a challenge to adopt the methods consistently and found it difficult to maintain the approach in larger or small groups or where there were difficult to manage group dynamics. Educators sometimes found it difficult to keep mixed age groups focused on activities and to engage the entire group at once. This resulted in boredom in some mixed aged groups. One site educator thought that the modules worked better with the adolescents because their knowledge was better.
Too far apart in age it doesn’t work, you use different methods for each age group even though the content is the same you do use different teaching methods for a 9-year-old than you do for a 14-year-old.
Nurse I5
Modules ran less smoothly when the site educator appeared confused, lacking in confidence or actively unhappy about participation in CASCADE. Some site educators fell back into didactic teaching, telling people what to do rather than using the approaches taught in CASCADE (e.g. asking open questions) to elicit knowledge. Reasons for reverting to telling and giving advice were because site educators wanted young people to understand, were trying to keep to time, or participants weren’t giving expected answers. One educator said she found it difficult asking questions and found it easier to ‘teach’ and tell people what to do, which was part of her nursing training. Some found it difficult to keep the young people focused when more challenging topics were introduced. Educators also found it challenging at times to work with parents and children and keep both of them focused on the task. Lively participants tended to dominate at the expense of quieter, less confident ones. Observers noticed that site educators often failed to engage quieter children whose contributions often went unnoticed. Many agreed that this had been a key challenge and did not have the confidence to use the approaches enough to address this. In one group a father was described as ‘very negative’, to which the site educators had found it difficult to respond.
Young people and families uptake and views on the intervention
Number of young people attending any CASCADE modules
Ninety-six of the 180 young people recruited to the intervention arm (53%) attended at least one module. Significantly more children (8–12 years) attended at least one module compared with teenagers (13–16 years) (64% vs. 44%, p < 0.01). Those who attended had significantly lower mean baseline HbA1c levels than those who did not attend (9.52 mmol/l vs. 10.33 mmol/l, p < 0.01). There were no significant differences in attendance by gender, length of time since diagnosis of diabetes, ethnicity or level of deprivation in the area in which they lived.
Non-attendance
Eighty-four young people failed to attend any module. There were three main categories for non-attendance at any CASCADE modules.
-
Seven (4%) dropped out of the study before the CASCADE workshops were offered across six sites.
-
Eleven (6%) were never offered modules to attend by their site either because the site did not run any modules or had stopped offering modules.
-
Sixty-six (37%) were offered the opportunity to attend the modules but opted not to do so.
-
All sites, bar the one that failed to offer any modules to anyone, had young people who chose to opt out.
There was no significant difference by intervention site when all three reasons for complete non-attendance were included. However, when the seven who withdrew from the study were removed, attendance compared with non-attendance was significantly different across the intervention sites (p < 0.04). Table 30 shows that only two sites kept non-attendance at less than one-quarter of the recruited young people from their site.
| Non-attendance (no. of modules attended by recruited young people)a (%) | No. of sites (n = 14) |
|---|---|
| 10–25 | 2 |
| 26–49 | 6 |
| 50–100 | 6 |
The two sites with the lowest non-attendance rate had the lowest mean baseline HbA1c levels for their recruited patients and completed the target number of groups for their site based on number of recruited families. The six sites with the highest number of non-attendance struggled with the organisation of groups, had high proportions of teens (vs. children) and relatively high baseline HbA1c means. There was a significant difference in mean baseline HbA1c level for the young people in the site with the highest level of non-attendance (75% non-attendance, mean 10.38 mmol/l) compared with the site with the lowest non-attendance (10% non-attendance, mean 8.75 mmol/l, p < 0.03).
Reasons for opting out of the CASCADE education sessions
Young people were asked at follow-up for reasons for non-attendance of CASCADE modules. For the 36 non-attenders that returned the questionnaire the most frequently cited reason was not wanting to miss school (n = 10). This was especially the case for teenagers, many of whom were preparing for GCSEs. Eight young people said they were not interested or did not think it would help. Other reasons are detailed in Appendix 7 , Table 55 .
I couldn’t be bothered. I just wanted to do the blood tests they asked me for.
Teenager I1
Parents provided very similar responses. Some explained work constraints hindered attendance, which made it more difficult for their child to as well. Only two parents suggested that they thought that CASCADE would not help their child.
[My daughter] does not understand diabetes. I hoped that she could attend to learn. I regret her not going.
Parent who had work conflicts I13
Site educators also commented on parents’ work being a barrier.
I think the child would have liked to have come but it’s more to do with one of parents, it was her dad. He was a very busy dad, making a lot of money I think, had a lot on his plate really.
Nurse I6
A few parents mentioned their child didn’t want to attend. One said her son was at a stage where he did not want to talk about his diabetes all the time. Some parents tried to persuade their children to go, others let this be their decision.
I tried to encourage my son to attend, so did the diabetes nurse. He just didn’t want to go. He said that if sessions had been during school time, he would definitely have attended so he could miss some school time!
Parent of teenager I14
When it came to the actual crunch, she goes ‘I don’t wanna do it’ and I was thinking ‘Okay, if you don’t wanna, don’t want to force you, don’t do it then’. So I wasn’t that bothered about it either.
Parent of child I12
Staff commonly cited lack of motivation when discussing the challenges of trying to encourage attendance by the most difficult to engage young people.
I would say the people that opted out I would have thought at the beginning wouldn’t have done it anyway so I was probably very surprised that they agreed to do the CASCADE study in the first place . . .
Nurse I6
I don’t think they were motivated enough, it’s just classic, isn’t it? That’s why they were invited in the first place, they were not motivated enough and they weren’t motivated enough to come.
Nurse I5
This view is consistent with the higher HbA1c levels among non-attenders.
Some staff also commented they thought attendance at CASCADE would be better for those who were closer to their diagnosis of diabetes, as these families tended to prioritise health needs over any other needs (including school work), thus facilitating uptake.
I think when you’re newly diagnosed, they tend to attend better, if you say you need to come to clinic because you’ve just been diagnosed, they’ll find the time to come that very first Monday whereas when they’ve been diagnosed 2 or 3 years, they’ll phone up and say . . . ‘I can’t get out of work’ or something, ‘can’t get out of school so I can’t come to clinic’. They’re a bit sort of more ‘do as they’re told’ for the first 12 months, they’re more likely to attend and perhaps take it on board, it gets them in the right frame of mind early.
Nurse I14
Attendance at modules
Attending the first module increased the likelihood of attending three or four modules. Of the 96 attendees, 57% attended the full intervention of four CASCADE modules ( Table 31 ) and 78 (82%) attended three or four modules. There were no significant differences by gender, age group, ethnicity, deprivation score, mean baseline HbA1c levels or length of time since diagnosis between those who attended one or two modules and those who attended three or four modules.
| Module(s) | n (%) [N = 96] |
|---|---|
| 1 | 11 (11) |
| 2 | 7 (7) |
| 3 | 24 (25) |
| 4 | 54 (57) |
Regular attendance did not significantly vary by site. Seventy-five per cent or more participants attended three or four sessions in 10 of the 12 sites that offered all the modules. The remaining two achieved 67% retention. Delivery fidelity by the site educators did not influence attendance.
The main reasons given by young people for missing modules were not wanting to miss school, illness, having no one to accompany them, snow and forgetting. Only three young people said that they had stopped attending because they were not interested or did not want to go. Parents’ responses were similar citing specific reasons why individual modules had to be missed, and disappointment that they were. A few parents suggested that attendance was a struggle. One parent commented:
We attended all the sessions but (my daughter) didn’t want to go but I insisted she went.
Parent of teenager I6
Staff agreed most participants seemed engaged and lack of attendance did not indicate a dislike of the programme.
I think it was personal issues really, one teenager had an exam on the last session, it was circumstantial rather than not wanting to come I think.
Nurse I6
Number of parents/others attending
The CASCADE intervention was designed for young people and family members to attend together. Young people were accompanied at 297 of the 327 modules (90%). The majority were accompanied by at least one parent (mostly mothers); however, other significant family members and one girlfriend also attended (see Appendix 7 , Table 56 ). In 50 modules (17%) more than one member of the young person’s family attended the session with them. One young man attended three modules on his own. Three modules were attended by family members but not by the young person.
Acceptability of CASCADE to young people and parents who attended
At 12 months, young people and parents who had attended modules were asked if they would recommend the CASCADE intervention to a friend in their situation. Overwhelmingly parents and young people said they would. Only one parent (out of 85) and two young people (out of 94) said that they would not make this recommendation.
I felt they were of little use to me as I already knew everything however this kind of session would be useful to someone who had just been diagnosed.
Teenager I9
I think they were very helpful and informative. The atmosphere at the sessions was very nice and friendly and I would definitely recommend it to friends.
Teenager I10
In general, responses were positive to specific questions about module content, group dynamics and the structure and length of sessions ( Table 32 ).
| Aspects of the group | Answered ‘quite a lot’ or ‘a great deal’: n/N (%) | |
|---|---|---|
| Young people | Parents/carers | |
| Group dynamic | ||
| Liked parents/young people being together in modules | 81/90 (90) | 81/84 (96) |
| Felt learnt something from other people in the group | 64/93 (69) | 60/85 (71) |
| Teaching style and length | ||
| Liked the way the trainers taught | 74/93 (81) | 81/86 (94) |
| Felt the sessions were too long | 22/94 (23) | 7/85 (8) |
| Content | ||
| Felt that some of the things covered were too complicated | 7/92 (8) | 8/85 (9) |
| Felt that some of the things covered they knew before | 48/91 (53) | 42/86 (49) |
Group dynamics
Most young people and parents liked being together in the same groups. In the questionnaires two-thirds said that they’d learned something from other people in their group with positive endorsement for group work from the interviews. Very small group sizes were criticised by parents and young people with mixed age groups described as problematic by some young people.
I quite liked the fact that there were other kids with the parents there, I think it takes the pressure off the individual child so for them to be with other kids that have the same problem and bringing it up there . . . their issues.
Parent of teenager I8
If more children and parents had turned up, then it might have been [easier to come]. It would be different; you needed more [participation]. Just one or two doesn’t make it beneficial, you need feedback from more, at least a good half dozen would’ve been good, I think.
Parent of child I5
[Being in a mixed age group] kind of restricted the things to talk about but if you make CASCADE each have a different age group, it would be better, bit more open.
Sort of sexual matters and drinking matters and sort of drugs and stuff like.
Teenager I11
Good [things about CASCADE] – meet other [people with] diabetes your age, with same probs and how they deal with it; Bad [things about CASCADE]- time consuming (coursework, homework . . .) and aimed at a younger age group.
Teenager I8
Teaching styles and length
Nearly all parents were positive about the length of sessions and methods used by trainers. In contrast nearly one-quarter of the young people were critical about the length of the sessions and said they would have preferred more sessions that were shorter.
I would have said . . . cause sometimes with like a concentration lapse, maybe [the sessions could be] a bit shorter . . . Yeah, cause like say if [the module] started at 3, they did then carry on till 5, all of us are just thinking about our stomachs.
Child I1
I thought it was OK, but I was a little bored, but my mum thought it was good.
Child I3
It was well planned . . . yeah, it was like humour as well . . . They gave us activities to do and then like we’d all go through it which helped like to get a good understanding . . . They stood out the front and did their bit and then they like handed it down to us, to let us like discuss our views on what like we thought about it.
Yeah . . . I think so, like we would all have different methods of like different things, so . . . yeah, it made the sessions better that we could all like discuss our views in the middle of the sessions.
Child I1
Most young people thought it was a good thing to know the site educator in advance of the modules. A few were critical of this, however, because they felt the nurse had preconceived ideas about them.
I think it makes it more personal [if you already know the site educator] . . . I think I would have felt less comfortable [if I hadn’t know them] cause I was meeting new people in the group and I would have had to have met new nurses as well, it could have been quite awkward cause we all knew [the site educators], it was all quite mutual.
Teenager I9
Content
About half the parents and young people thought the CASCADE content covered things they already knew, but many commented positively about having an opportunity to revisit this information.
Quite a lot was a reminder of what we might have known but it was still very helpful. I enjoyed it a lot
Teenager I6
I thought it was really useful – right balance between sciency and everyday terms and it was applicable.
Teenager I11
Well I didn’t enjoy it that much because they’re basically saying things like I already kind of knew and when I got diagnosed I got taught about all the things that they were doing.
Child I5
Perceived impact of CASCADE: young people, parents and staff
These data complement the quantitative data on the primary and secondary outcomes of the trial. They provide additional information in relation to the feasibility of using the selected trial outcomes to measure programme changes.
Perceived impact of CASCADE
Nearly all parents who completed follow-up questionnaires (95%) and/or took part in interviews perceived CASCADE to have had some positive impact. When parents reported no real changes, this was sometimes because they said they had always felt confident about managing the diabetes, and/or encouraging their son/daughter to participate in sports and other activities. During interviews, a few described some change since attending the groups but were unsure whether or not this should be attributed to CASCADE rather than other factors, such as increasing maturity or other life experiences of their child. One parent reported that although useful at the time, attendance at the sessions had no continuing impact. Another parent expressed disappointment that it hadn’t helped as she had hoped.
Like the parents, almost all young people identified some impact of the CASCADE sessions. Many indicated that changes had been both positive and sustained but often perceived as modest.
A little bit.
Cause like if it’s too high I give myself a few units and then I go and do like take the dog for a walk or something like that.
No.
I have, just like little bits? . . . Um, matching my food to insulin.
Teenager I1
Specific themes of how CASCADE was perceived to have had an impact are detailed below.
Improved knowledge and understanding
Ninety per cent of parents who attended the CASCADE sessions reported a greater understanding of the need for CHO counting with others reporting increased knowledge relating to insulin and CHO ( Table 33 ). In the interviews many parents reported increased knowledge as a result of CASCADE, either for specific topics or it had reinforced or supported their existing knowledge base.
| After attending some or all of the CASCADE diabetes education sessions, how much did your child/you: | Answered ‘quite a lot’/’a great deal’ | |
|---|---|---|
| Parent: n (%) [N = 90] | Young person: n (%) [N = 97] | |
| Knowledge | ||
| Understand better how insulin works | 66 (74)a | 70 (73)a |
| Understand better which foods contain CHO | 73 (81) | 68 (70) |
| See why counting the CHO in the food your child/you eat(s) can be helpful | 80 (90)a | 73 (75) |
| Intention to change | ||
| Want to stop your child’s/your glucose levels from going too low or high | 85 (94) | 85 (87.5)a |
| Want to test your child’s/your BG levels more often | 56 (43)a | 40 (42)b |
| Control | ||
| Feel more in charge of your child’s/your diabetes | 61 (69)a | 65 (68)b |
| Feel able to change your child’s/your insulin dose when they are exercising | 68 (77)b | 66 (69)a |
| Feel you are able to control your child’s/your BG levels better | 70 (78) | 64 (67)b |
| Access to care | ||
| Feel more able to ring/contact your diabetes nurse/GP/hospital if your child/you need(s) help | 72 (82)b | 52 (54)a |
| Family dynamic | ||
| Feel you had a better understanding of how diabetes affects your family | 67 (75)a | 69 (72)a |
At 12-month follow-up approximately three-quarters of young people identified increased knowledge and improved understanding in diabetes management as a positive outcome of attending CASCADE (see Table 33 ). CHO counting, matching insulin to food and exercise were most commonly cited as areas of increased knowledge and many young people gave examples of applying this knowledge to daily management.
I still eat but now I’m starting to inject the right amount.
[before CASCADE] I’d sometimes miss the injections.
Yeah, cause I understand a bit more.
Teenager I14
Many examples were given of how the young person had applied this knowledge and understanding to the management of their illness with subsequent outcomes.
Well I’ve learnt new things . . . yeah, I could do some things but it’s just like improved the way that I do it . . . I’d probably say like carb counting, and yeah, I used to have like a rough idea, now I know like things that are already in my head, like how much a piece of bread . . .
Child I1
Another young person was now able to make their own adjustments to match insulin to foods and explained the nurses had ‘shown her different ways’.
At 24-month follow-up 84% of parents who attended the modules continued to report that CASCADE had increased their knowledge ( Table 34 ).
| After attending some or all of the CASCADE diabetes education sessions, how much did your child/you: | Answered ‘quite a lot’/’a great deal’ | |
|---|---|---|
| Parents: n (%) [N = 80] | Young people: n (%) [N = 91] | |
| Knowledge | ||
| I/they learnt new things about diabetes | 67 (84) | 73 (80) |
| The sessions helped me understand things I already knew in relation to my child’s/my diabetes | 49 (61) | 51 (56) |
| Confidence | ||
| I felt more confident afterwards in regard to managing my child’s/my diabetes | 49 (61) | 55 (60) |
| I worry less now about my child’s/my diabetes | 23 (29) | 39 (43) |
| I am happier about how my child/I feel(s) about their/my diabetes now | 39 (49) | 27 (30) |
| Access to care | ||
| I liked spending time talking to the diabetes team without being in a rush | 48 (60) | 36 (40) |
| Family dynamic | ||
| It was helpful being with other parents and young people dealing with diabetes | 67 (84) | 41 (45) |
| Being with my child/parent at the sessions was helpful | 57 (71) | 53 (58) |
| I get on better with my child/parents with regard to their/my diabetes now | 29 (36) | 14 (15) |
| No impact | ||
| CASCADE did not help me/my child | 4 (5) | 8 (9) |
I found the sessions very informative, especially where growth hormones and ketones were concerned. I thought I was quite clued up on diabetes until I attended these sessions.
Parent I9, 24-month questionnaire
Greater confidence in managing diabetes and making decisions
At 12 months, over two-thirds of parents who attended any CASCADE module reported greater confidence in making decisions in the day-to-day management of their child’s condition; 78% felt that they were better able to control their child’s BG levels (see Table 33 ). Parents who attended the groups commonly reported anxiety and worry had decreased. Greater confidence was frequently attributed to an improved understanding of diabetes and its management, and a belief that their child’s diabetes was being managed more effectively. In a few instances parents identified a particular component of CASCADE which they had found helpful, for example a log book as a tool for assessing foods. Young people also reported greater confidence making decisions about insulin needs and application of this knowledge in day-to-day management of diabetes.
At 24 months, 61% of parents who had attended still reported more confidence managing their child’s diabetes. Over half said they were happier about how their child felt about their diabetes and just under one-third said they worried less now about their child’s diabetes as a result of CASCADE (see Table 34 ). Ambivalence in relation to putting knowledge into action was highlighted by nearly all the parents (93%) reported a greater desire to stop their child’s BG going too high or low; however, less than half (43%) said they wanted to test their child’s BG level more often.
Reported impacts on disease management and lifestyle
Improved management of diabetes was described by a number of interviewed parents. In one instance, a parent identified CASCADE as the reason for their daughter not having had an admission to hospital since the sessions. Improved BG levels were also reported in a number of cases.
Additionally some of the interviewed parents suggested practical and lifestyle impacts of attendance at CASCADE modules. Specific points made by parents included changes in shopping habits, closer reading of food labels, understanding of CHO counting, and managing sugars with exercise. In one instance, the parent reported that as a consequence of the participation in CASCADE, she was now able to leave the home for a day or two without worrying.
Changes observed in the young person by parents
Young people were described in interviews by parents as having greater awareness and understanding of their condition, and being more confident and comfortable with managing their condition. Examples given to illustrate this were changes to regimens and taking more responsibility.
Especially in school time, he is more responsible.
Parent of teenager I12
One parent described how her daughter, previously, apparently disinterested, was now motivated to manage her condition effectively and took responsibility for many tasks with which she had been previously less co-operative.
Social impacts were also reported. One parent observed that her daughter now felt able to discuss diabetes with friends and participate in social activities she had previously avoided. Another parent reported how her son had become more accepting of his condition and was more open with his friends.
Young people’s observations of self-change
Greater understanding of long-term complications of disease motivated one young person to change eating habits. This young person also reported
Keeping a lot more close eye on my BG . . . I think it made me realise . . . keep it under control now so I’ll be healthy when I’m older.
Teenager I3
Closer attention to, and/or more frequent testing of, BG levels was reported by many young people as well as the belief that they could make more appropriate adjustments particularly in relation to food and sport.
I suppose I’m taking it more seriously . . . I’m looking at more sort of how I should be eating and how like exercise and other things, how it all affects . . . you know . . . I change my units up and down based on what I’m eating and what I’m doing.
Teenager I11
Young people becoming more proactive in the management of their condition was common, for example one young person reported doing her own research:
[Regarding different foods] I noticed that they’re not good for me and I also got a list of things that were good and bad for me off the Internet.
Child I12
Changes of attitude and emotional factors
Sessions came along at the right time for this young person:
I had to finally accept and think that it was enough of actually going into hospital and getting into a lot of trouble.
Teenager I14
At 12 months young people felt more in control (68%). At 24 months they described a change in attitude, including ‘greater acceptance’ of the condition and the need for injections. Forty per cent reported that they were happier with their diabetes as a result of attending CASCADE and more confident (43%) and comfortable in the management of their condition (see Table 34 ).
[After CASCADE] I look after it much more than I used to. I feel good, just to know that I know.
Teenager, I5
I’ve been more happier . . . yeah, like around the house I’ve been more happier. Not so many strops . . . Cause my readings are better and we’ve been given a lot more information about the ketones and how to treat it . . . I found it really good . . . like useful the way they presented it.
Child, I13
In some cases young people also reported that they could take more responsibility:
I can do more things now, like by myself.
Child, I11
. . . if I’m in a low I don’t have to . . . nobody else has to worry about it that much cause I can handle it.
Teenager, I5
Partnership between parents and young people
At 12 months, three-quarters of parents said they had a better understanding of how diabetes affected their family following CASCADE. Participation had a positive influence on parents’ relationship with their son/daughter and their interaction managing diabetes.
Attending modules with their son/daughter was identified as beneficial by a number of parents. They described a shared understanding of the condition, which made communication at home easier. Parents also described how after sessions both parents and young people had a greater appreciation of each other’s perspectives and concerns. One parent also reported how they enjoyed spending this dedicated time, including the travel time and attending the sessions, with their son/daughter.
She’s not thinking ‘shut up’, she knows why I’m saying it, she understands . . . why I was saying it.
Parent of child I6
I think we’ve both been told the same . . . we’re both more on an even keel.
Parent of teenager I12
A number of parents reported they learnt more about their son/daughter by attending CASCADE with them. For example, they discovered that their son/daughter had greater knowledge and understanding of their diabetes than they previously thought, or gained more insight into how their son/daughter felt about having diabetes.
At 24 months, 71% of parents still reported it was helpful to have been at the sessions with their child and just over one-third said they get on better with their child regarding their diabetes now, as a result of CASCADE (see Table 34 ).
Young people also reported better co-operation with parents in managing diabetes, as a consequence of having a better understanding of the need for good management.
I just like, my relationship . . .
Like I can become less angry and stuff and like less agitated and stuff.
Teenager I14
. . . my mum used to say inject and I always did it like an hour later cause I was feeling it won’t affect me, but they showed me the graph and whenever mum told me to inject, I did it like seconds after.
Child I14
. . . she [mother] probably worries less because she knows I know a lot more about it.
Teenager, I12
One young person said his father now “paid more attention” and would engage in discussions to help achieve appropriate sugar levels.
Communication with diabetes team
Pre-existing relationships and accessibility were generally felt to be very good, therefore, the majority of parents and young people reported no change in the way they might contact or relate to clinic staff. However, at 12 months parents (82%) reported they were more able to contact health services in regards to their child’s diabetes after CASCADE. At 24 months, over half (60%) said that they liked spending time talking to the diabetes team without being in a rush. Reported changes were always in a positive direction, for example feeling more able to ask for help, or being more prepared to ask for explanations or to ‘challenge’ answers to questions. Positive changes for young people were that they found staff more approachable, were more confident raising issues for discussion and prepared to disclose their feelings:
I used to never tell the truth about my bloods because I was scared they would shout at me . . . But now . . . if I’m like high I’ll tell them . . . and they sort it out.
Child, I14
I wouldn’t say changed it [feelings about talking to nurses and other staff], but like I say, I have become more confident with knowing what I’m actually talking about.
Teenager, I11
Combined impact scores (12 months)
The scores for the nine factors in Table 33 (all except nursing contact) were combined to form a composite measure of the perceived impact of CASCADE at 12 months for parents and for young people. Scores could range between 0 and 27. Parents had a mean score of 19 with 85% rating CASCADE as having helped them ‘quite a lot’ or a ‘great deal’ ( Table 35 ). Young people who had a mean score of 17 with 78% rating CASCADE as having helped them ‘quite a lot’ or a ‘great deal’ with (see Table 35 ).
| Scores | Parents: n (%) | Young person: n (%) |
|---|---|---|
| Mean score 19 (IQR 16–23): N = 84 | Mean score 17 (IQR 14–21.5): N = 89 | |
| 0–4 (‘no impact’) | 2 (2) | 4 (5) |
| 5–13 (‘only a little’ impact) | 11 (13) | 16 (18) |
| 14–22 (‘quite a lot) | 47 (56) | 55 (62) |
| 23–27 (‘a great deal’) | 24 (29) | 14 (16) |
For the parents composite perceived impact score there were no significant differences by age or gender of children or by the number of sessions attended. Children reported a significantly higher impact than teenagers (mean 19.9 vs. 15.6, p < 0.01); however, there was no difference by gender or number of sessions attended.
Long-term impact on blood glucose control
Over half of parents reported that CASCADE had a long-term impact on motivating their child to keep trying to keep their BG well controlled. Just under half of young people reported some long-term impact on how hard they tried ( Table 36 ).
| Views of long-term impact on BG control | Parents: n (%) [N = 75] | Young people: n (%) [N = 86] |
|---|---|---|
| The sessions made my child/me want to try harder and he/she has/I have carried on trying hard | 42 (56) | 43 (50) |
| My child/I tried harder for a while after the sessions but has/I have not been able to keep this up | 24 (32) | 27 (31) |
| The sessions made no difference to how hard my child tries/I try | 9 (12) | 16 (19) |
Why CASCADE might have had less impact
A couple of parents said the information covered was known already. A few felt that group dynamics (differing characteristics of participants – e.g. ages, regimen, small group size) reduced the potential impact. Several parents acknowledged the difficulties of trying to change behaviour in young people.
I think being a ‘teenager’ has interfered with her understanding. She knows what she should do but doesn’t always do it. Hopefully this is something that she will grow out of and get back on track.
Site I2, 24-month questionnaire
Useful to have time to talk through issues surrounding management, but my child is at an age now where she will not always listen to advice.
Site I1, 24-month questionnaire
Two parents also suggested that although they liked CASCADE, the intervention did not seem enough on its own to overcome entrenched problems.
I will always worry about my child’s diabetes. My child is very angry about having diabetes and I don’t think the amount of lessons about diabetes will stop my child from asking ‘why me?’! . . . My child tries hard but feels disappointed when goes to clinic and the results aren’t what she expects. Then leaves feeling low and not wanting to do anything including blood readings.
Site I6, 24-month questionnaire
The most frequently mentioned reason for CASCADE failing to have an impact for young people was that they already knew the information covered. Some said they were bored or the sessions were too long or the content was boring. Young people also said that mixed groups with different ages and genders and varied regimens made the groups less helpful for them.
Staff perceptions of impact
At the point of delivering sessions staff were generally positive about the potential for impact and the level of involvement of families who attended. Several site educators noted some children were opening up more in the sessions and were committed to attending. These staff were generally positive about the impact that the activities were having on the knowledge and engagement of young people who attended.
I think it’s made them more aware of how important it is looking at the future and complications.
Nurse I14
Staff were aware of young people who they believed as a consequence of CASCADE tested their BG more often, had better clinic attendance, were more confident and relaxed, which, in a few cases, had improved relationships with staff and might have better HbA1c levels.
I have seen some changes and at least a better understanding in all of the others. One of the young men from the first session . . . is a little less frightened of controlling his diabetes and used to be very reticent to put doses up cause he would be frightened of hypos and things like that, so it’s improved that. As to HbA1cs and things, I haven’t seen a huge difference so far . . . I would say a slight improvement in parents and child relationships and slightly better base knowledge but I’ll wait and see whether it’s implemented fully.
Nurse I11
One of the people who came to . . . all four sessions, they’ve turned theirself around and I think if it’s only helped one out of 8 . . . then it’s done its job. Yes, they’ve changed and the parents have commented how much it’s changed them.
Nurse I12
In follow-up interviews, often several months after all groups had been delivered, site educators expressed disappointment that the intervention did not seem to be having a more measurable impact, although they did feel that some young people had benefited.
A variety of reasons were given by staff for this potential lack of impact including entrenched behaviour, lack of engagement, individual personality, lack of motivation to change and insufficient parental involvement.
Some people aren’t comfortable in a group setting, you will always get that, some will do fine and engage and get things out of it, others will always sit back and not engage.
Nurse I5
I’ve spoken to a parent of one of the young ladies who was saying that she’s very good at interacting in the session and she’s very knowledgeable and all that involved . . . given information willingly but then the mum has said to me that behind the scenes that they’re still very much at loggerheads at times over the diabetes and she doesn’t feel necessarily that behind the scenes much has changed with that individual.
Nurse I8
Well, it’s only going to benefit the ones that actually want to make a change I think really or want to consider making a change let’s say but there’s obviously those who just wouldn’t be interested regardless of what you offered, so . . . yeah, it’s going to benefit some more than others.
Nurse I3
No comments suggesting a negative impact of attending the groups CASCADE were received.
Having taken part, many staff valued the experience of participating in the intervention arm of the trial and felt that the CASCADE approach was a very positive one. Some suggested they would like to carry on running CASCADE groups but were concerned about the resources required in terms of staff time and capacity. Some staff having being trained in CASCADE reported an impact on their own practice beyond the education groups themselves. In particular, they were, and expected to continue, using MI techniques, for example employing them in consultations with young people.
Biggest thing for me was the MI and that way of doing things and I consistently pick myself up in clinic when I find myself slipping into the whole ‘you should do this, you should do that’. Well, I like to think I use it all the time.
Nurse I11
There were examples where staff explained that they had actively chosen not to continue with such techniques.
We’d met with mum 2 weeks ago and said we’ll try the motivational approach with [your daughter] and see if we can get her to reach her own outcomes . . . goals, and she said ‘Oh no, don’t try that with [my daughter], she knows what you’re up to and she doesn’t like it, just talk to her straight’. So we were very straight with her recently and her HbA1cs improved by us using the old-fashioned method. Well, we weren’t too harsh on her but we did have to say ‘ if you don’t follow the rules to keep yourself safe we’ll have to consider you stop using the pump’ and she actually liked that.
Nurse I5
Conclusions
The mixed-methods PE demonstrated that the CASCADE intervention had promise but faced some challenges in implementation.
It proved possible to train site educators in every site and the intervention was delivered with relatively high levels of fidelity to some families in most of the sites. Young people and parents who attended and staff who delivered it were enthusiastic about CASCADE’s relevance and members of all stakeholder groups perceived that it had some impact.
The PE illuminated key contextual factors that affected implementation. Not all the site educators had the level of expertise expected. Setting up groups proved frustrating and time consuming and used site educator time that might otherwise have been devoted to practising delivery of the intervention groups. Approximately half of the families that were offered the groups attended; take-up was particularly low for those young people with the highest HbA1c levels. Fewer groups than expected were run, and those that did were smaller in size and more mixed in terms of ages of participants than considered ideal. Delivering the intervention to these non-ideal groups was sometimes very challenging.
The PE findings suggested that some aspects of the CASCADE model contributed to the failure of the intervention to achieve expected outcomes. In particular, the expectation that the site educators could organise and deliver the intervention with high fidelity but minimal ongoing support. The theory that young people with the worst glycaemic control would voluntarily attend a group intervention outside of normal clinic appointments also was shown to be problematic as was the expectation that CASCADE would not add significantly to the workload of site educators.
These implementation and model factors were compounded by trial factors, including the small numbers of recruits in some sites, an extended time lapse between training and delivery caused by delays in trial patient recruitment, and workload related to supporting data collection. We would recommend that the site educators carry out some practice runs delivering modules before actual delivery in the trial and be assessed for fidelity of delivery and constructive feedback in any future such interventions.
Chapter 6 Standard care in NHS clinics
Introduction
The recommended core paediatric diabetes team should include a consultant paediatrician (or diabetologist) with specialist expertise in diabetes; a PDSN; a registered dietitian and clinical psychologist (or mental health professional). 4 The most recent survey in the UK showed that staffing had improved, with 98% of consultants having a special interest in diabetes; a PDSN working solely in diabetes in 88% of services; 93% having a paediatric dietitian in clinic; and 21% having a psychologist as an integrated member of the team. 182,183 The NPDA collected data on 23,676 children and young people aged < 25 years diagnosed with diabetes. 5 Fewer than 16% achieved the recommended target glycaemic control of < 7.5%.
Although the aim of any diabetes team is to provide expert practical guidance and skill training, until recently there has been no national curriculum for either HCP training or a nationally agreed curriculum. 184 The SWEET project (better control in paediatric and adolescent diabetes in the EU: working to create centres of reference) identifies different levels of knowledge expected from each core HCP. 185
This chapter describes the type of diabetes care that children, young people and families currently receive and explores the context in which CASCADE was delivered within NHS clinics. It describes clinical teams and patient numbers, outpatient services, education at time of diagnosis and any ongoing structured education including group work. Key challenges to delivery are also detailed.
Data sources
-
Staff interviews after the CASCADE groups were completed.
-
Parent/carer and young people’s interviews.
-
Detailed fieldwork notes.
-
Data collected for the minimisation criteria.
-
CNR.
Patient numbers and staffing
Patient numbers
The number of registered patients per clinic ranged from 78 to 220 (control) and 90 to 280 (intervention) at the start of the study, increasing slightly across both arms during the study.
Consultants
The majority of sites had one paediatric consultant. Six had two consultants and one had three. In 27 sites the ratio of consultants (including staff grade) to patients ranged between 1 : 80 and 1 : 120. In one site (intervention) it was 1 : 170. In one intervention site the consultant was on long-term sick leave for the majority of the period from patient recruitment to completion of delivery of the intervention.
Nursing
Approximately half of the 28 sites had one PDSN, one (control) had four, with the remainder having two or three ( Table 37 ). Nurse to patient ratio ranged from 1 : 51 to 1 : 170, with seven having a ratio of ≥ 1 : 100. The RCN recommends a ratio of 1 : 70. 165 The majority worked part time. At the start of the trial there were 29 nurses in the control clinics [22.4 whole-time equivalents (WTE)] and 24 in the intervention clinics (17.9 WTE). In control sites there was little change in nursing time over the course of the study. Two intervention sites had a 100% increase in nursing WTE. All control clinic nurses and over 90% of the intervention clinic nurses described themselves as being employed as PDSNs, with the majority having at least one nurse who had worked at the site, in their current role, for over 5 years.
| Baseline levels | Intervention sites (n = 14) | Control sites (n = 14) |
|---|---|---|
| No. of paediatric diabetes consultants | ||
| 1 | 11 | 10 |
| 2 | 2 | 4 |
| 3 | 1 | 0 |
| No. of PDSNs in clinic | ||
| 1 | 6 | 4 |
| 2 | 6 | 6 |
| 3 | 2 | 3 |
| 4 | 0 | 1 |
Dietetic support
In control sites, 13 out of 14 received approximately 0.1 WTE dietetic support compared with 0.2 WTE in intervention sites. The dietitian was not always a paediatric dietitian. The majority of dietetic time was used to cover routine paediatric diabetes outpatient clinics and newly diagnosed patients.
Psychological support
Very few sites had any integrated psychology provision in the early stages of the study. Lack of psychological support was a major concern for many staff. In general, referrals go to local CAMHSs. This was almost unanimously considered to be very unsatisfactory with long waiting lists and the threshold for accepting young people often too high for most referrals from the diabetic team.
Unless it’s a suicide attempt it’s difficult to get anyone to see children.
Site educator I12
Two intervention sites appointed a psychologist during CASCADE, at which point patients began to see a dietitian and psychologist at every clinic. Two other intervention sites referred patients to the UCLH diabetes team to access psychology services.
Team working
In most sites all members of the MDT were in an acute setting; however, there were a number of sites where the PDSN was based in the children’s community nursing team – working alongside generic community nurse colleagues.
We’ve no longer got a Band 7 [Nurse] so we’ve been incorporated into the Children’s Community Nursing team. It does mean that we don’t have anybody leading diabetes and keeping us as one team which we’re meant to be. A lot of things that are challenges to us at the moment are due to the fact that we don’t have a Band 7 because the Children’s Community Nursing team leader who is currently our team leader has no concept how diabetes as a specialism works really and doesn’t really understand how we work.
Nurse/site educator I3
Provision of 24-hour service
Twenty-four-hour advice was an area described as inadequate and needed improving. For the majority sites patients were directed to the on-call registrar out of hours, except for newly diagnosed patients or new pump starts for whom 24-hour contact with the PDSN for a short period was the norm.
Diabetes team . . . no, we don’t . . . we used to and it’s something that I do believe in but again due to resources, you need more people who are able to give the right advice available for a 24/7 service and that’s what we haven’t got but we will always . . . we give out a contact sheet to all our families that says . . . ring this number at this time, this number at this time . . . and that’s essentially just Monday to Friday 9 to 5 . . . outside of hours we tell them to ring the ward.
Nurse C7
The PDSNs offered training to ward staff – but this was either not taken up or the ward staff did not have time.
[Nurse A] and I are on 9 to 5 and then any other times after that or weekends it will be done by the children’s ward, from hopefully a designated nurse which is what we want on the ward which we’re working towards.
No . . . and they speak to doctors and the doctors are not always that well informed.
Site educator/nurse I12
Outpatient services
Paediatric diabetes clinics
Most sites ran a consultant-led clinic at least once a week. At least two-thirds of control sites ran additional regular nurse-led clinics. Fewer intervention sites ran nurse-led clinics. Nurse-led clinics aimed to be more flexible and informal than consultant-led clinics and were primarily a key source of support for patients with persistently poor control and their parents/carers. In most sites nurses also attended consultant-led clinics. This meant attending 8–10 clinics per month in addition to home visits, school visits and telephone contact. Sites without nurse-led clinics recognised the need for them and had plans to start running them. Some nurse-led clinics were held in community venues.
Frequency of nursing contacts from 2008 to 2012 was obtained from a detailed CNR. The mean length of time data were collected was 49.7 months (range 44–54 months). There was huge variability in how contact information was recorded. Nurse contacts included home visits, nurse-led clinics, telephone calls, school visits and ward visits excluding routine clinic visits with the consultant. Data were obtained for 164 control and 162 intervention patients. The average number of nurse contacts per patient per year in control sites was 5.08 (range 1–121) compared with 7.12 (range 1–168) in intervention sites.
Clinic ‘did not attend’ rates
Clinic staff were asked to estimate the average number of young people who ‘did not attend’ (DNA) clinic appointments. The rate reported was 5–42% for controls (with most around 15%) and 5–20% for intervention clinics (with most around 10%).
All clinics reported higher DNA rates for adolescents and young adults (20–50%). One nurse had offered nurse-led clinics to encourage clinic attendance, which had no impact on attendance. One nurse said it was ‘a regular few’ who usually did not turn up. In one clinic, a nurse felt the low rate of 5% was because clinics were scheduled in the afternoon so young people did not miss too much school. Some staff mentioned working towards a policy on how to deal with DNAs as part of the preparation for the Best Practice Tariff (www.diabetes.nhs.uk/networks/paediatric_network/best_practice_tariff_for_paediatric_diabetes/).
Annual review clinics
All clinics carried out annual reviews – the number of clinics reporting what was offered (at the end of the intervention period) is listed in Table 38 .
| Clinical assessments | Intervention: n = 14 | Control: n = 14 | Notes |
|---|---|---|---|
| Height and weight | 12 | 12 | Some sites measured these at all clinics |
| HbA1c | 13 | 11 | Some sites measured this at all clinics. Some asked for a
blood sample in advance of the annual review, so results
could be discussed at clinic Nearly all sites collected venous samples for the annual review and all collected capillary samples at 3 monthly clinic appointments |
| Urine samples (> 12 years) (checking for microalbuminuria) | 13 | 13 | Patients often forget to bring a sample. ‘We try desperately, they’re so averse to peeing some of the older ones’ (C6) |
| Thyroid check (> 5 years) | 14 | 11 | |
| Eye examinations with a digital camera | 6 | 0 | Inconsistent and often done independently by retinal screening service via GP referral or less often by the GP. Some sites will check patients had been to the opticians for retinal screening and if not refer to the consultant ophthalmologist. Some examinations were done but not with a digital camera. Many felt eye examinations apply only to young people aged > 12 years, as it is unlikely they will have complications before that. An exception to this might be if they had been diagnosed for some years then staff might think about it earlier |
| Coeliac screen | 11 | 9 | NICE guidelines originally said to complete on diagnosis and every 3 years thereafter, unless young people were displaying symptoms. Some staff said consultants screening at every annual review or at least every 3 or 5 years |
| Foot examination | 11 | 10 | Some done at clinic; some sites do not do foot examinations even at annual review ‘No . . . we don’t, if there were problems with feet . . . refer to podiatry’ – others just said ‘probably not’ or ‘not always’ |
| Injection site checks | 14 | 13 | Most done at every clinic as well |
| Blood pressure | 12 | 13 | Some at clinic for those aged > 12 years but might be sooner if patients diagnosed > 5 years |
| Lipids | 3 | 0 | One clinic measures lipids, liver function, urea and
electrolytes One site measures cholesterol in those aged > 12 years |
| Food intake review | Undertaken in a small number of clinics but, owing to limited dietetic support, often completed by the PDSN | ||
| Psychological review | A number of sites said that team available for discussion of parents’ and young people’s worries and realistic targets following annual review or on an ongoing basis | ||
Pump therapy
At the start of CASCADE one intervention and three control sites had no patients on pumps. Seven intervention and four control sites had fewer than 5% of patients on pump therapy. The remaining four intervention and six control sites had 6–20% of their case load on pumps. Most clinics saw a small increase in the number of patients on pumps over the time of the study. Data were not available for three clinics.
Transition to adult services
In general young people were transitioned between 15 and 19 years of age. Nurses managed the transition process in all clinics. Young people transferred to the adult diabetic service in the same trust in 27 clinics. One discharged patients back to the GP, with a request to refer to adult services. Flexibility in transition and preparing young people beforehand was common; however, there was no clear transition policy for the majority of clinics. Individual factors that drove transition included personality of the young person, cognitive maturity, the need to ease pressure on overcrowded paediatric clinic, fitting in with examinations and negotiating with young people on an ad hoc basis. Some young people remained in paediatric clinics even although it was felt appropriate for them to move. Reasons included:
-
no pump services locally
-
a young person was autistic so was kept in the paediatric clinic
-
no spare capacity in the young adult clinic to accommodate them
-
insufficient clinic appointments/dates available.
The care pathway from the paediatric to adult diabetic clinic occurred via interim clinics with a number of stages:
-
Handover clinic/transition clinic
-
Joint adolescent clinic
-
Young adult clinic
-
Adult clinic.
Staff and young people noted the stark differences between paediatric and adult service.
They have to work harder and I do warn them . . . you know, for the last couple of years that they’re sort of with me, I do warn them that once you move to adult services, there will not be somebody ringing you up saying ‘is everything alright?’, there will not be someone popping round the house to make sure everything’s okay, it will be down to you to ring and say ‘I need some help’.
Site educator/nurse I4
They’re a lot nicer . . . the children’s service . . . They’re just nicer, they seem like they have time for you, it’s not always about facts and figures, it’s more about how you’re feeling and what you’re doing . . . Yeah, I suppose I’ve grown up with them, like they all know me and I’ve grown up with them for 15 years, so . . . you know, it’s nice. I think the adult one is just very rushed, it’s like . . . right, you’re not dying, fine okay.
YP male I11
Diabetes education
Education at diagnosis
All sites provided initial diabetes education at diagnosis on the ward. PDSNs were primarily responsible for education with contributions from other members of the team, including consultants and dietitians and ward staff.
Well, I do [the initial education] myself . . . obviously the parents are seen by the consultant within the 24 hours and he tells them a little bit, and then I have a package that I work through . . . that’s all we do really before they get home but they do see a dietitian as well for a brief description.
Nurse C6
Consultants had a minimal role in education beyond a brief description of the diagnosis. One intervention site offered an appointment with an Emotional Wellbeing Practitioner based in the primary care service, whereas another referred to a psychologist at diagnosis to assist families with adjustment and problem-solving.
Initial education at diagnosis usually followed a checklist. It was generally regarded as important to deliver information in manageable ‘chunks’ so as not to overwhelm families who were dealing with the initial shock and implications of the new diagnosis. The checklist covered the ‘basics’ about diabetes including a general overview, managing BG levels, living with diabetes at home and MDI. One clinic uses a DVD from DUK for families, as well as books and leaflets.
It’s quite intensive really . . . how to do an injection, how to prepare it . . . We like them to have a decent understanding of hypoglycaemia because that’s the biggest emergency . . . high blood sugar levels and DKA . . . We call those the initial survival skills.
Nurse C7
Dietitians covered topics related to food and CHO. Only one control site introduced CHO counting from the beginning.
We try and do that right from the start so they’ve got a good idea of adjusting their own doses because it’s much harder to change once they’re doing something different.
Nurse C1
Nurses kept in close contact with families after their return home, giving them further information, supporting them through home visits and telephone contact before their first clinic visit. Families were encouraged to telephone nurses with queries or concerns. Some patients had their first hospital appointment 1 week after diagnosis, but for others first appointments could be up to 6 weeks later.
Contact between nurses and families with a newly diagnosed child varied depending on the perceived individual needs, as well as available resources. Many nurses offered several home visits to deliver more information about managing diabetes. Other nurses offered telephone support, often daily, in the first week. Following that, telephone contact was on an as-needed basis for ‘trouble-shooting’.
It’s very much on an individual basis because I say to the families, ‘I’m here to support you’, and some families will need daily visits for maybe the first 4 or 5 days . . . I’m very much guided by what the families want cause some need more support than others.
Site educator/nurse I4
Usually go and do education on the ward, follow them up with a home visit and then do an evening session for their family and friends and then do as much education (as required) for their swimming teachers, scouts, brownies, community education . . . I usually follow them up once a week initially and then once a month until they feel confident but I phone them every day and we talk about what the day is like and then put into place anything we need to for the following 24 hours.
Nurse C2
Nurses also visited schools to deliver training to staff often within a week after diagnosis and before the child returned to school. This could involve several visits to implement a health-care plan and to train staff, including first aiders and school nurses. One nurse mentioned training ‘whole school employees’ including receptionists. This could be more intensive for primary school children who were more dependent on staff.
I go into the schools when they’re newly diagnosed if they want me to educate the staff and (help with) health care plans. If they change to different teachers, then I’ll go in and see the new teacher to educate them. I’ll go into whole schools like the training days if they let me and just educate all the staff for that and like if they change to Basal Bolus or a pump, then again I’d go in and help support the staff.
Nurse C3
A minority of sites were less involved with schools. In one intervention site, the nurse acted in an advisory capacity but considered the care plan to be a contract between school nurses and parents. A nurse at one control site said they were having difficulty implementing comprehensive care plans because schools were reluctant to take responsibility for administering injections and blood tests.
Most staff rated education provided for newly diagnosed families as ‘sufficient’, ‘good’ or ‘very good’. The majority of staff had developed education programmes over time using teaching methods that they felt worked best for them and their patients. This was rarely formally evaluated. In contrast one site was infuriated by the impact of lack of dietetic time.
What are the key challenges for your service at the moment, would you say?
What do you have in the way of that?
Is that a change?
How do you get round it?
So is that a better service?
Ongoing diabetes education
The majority of ongoing education was delivered by PDSNs with some input from dietitians and consultants. Dietitians were particularly involved in teaching CHO counting. When nurses felt that children and families had grasped ‘the basics’ further information would be discussed depending on individual need. Education was delivered on an informal basis in consultant-led and nurse-led clinics, during home and school visits. Clinic appointments were time limited and were regarded by some as not the best venue for delivering education, which tended to be unstructured and ad hoc.
I would say if you’re attending a clinic appointment we do try and fit in education around that but it’s very, very limited.
Nurse C4
I think we cover everything, it’s pretty comprehensive, there’s no time limit to do it, some do it all in 6 months, some do it in 6 weeks. We do an awful lot of . . . we do training for members of the family as well.
Nurse C6
Most clinic staff went into schools on an annual basis, or on request, to update schools. Although parents were the first port of call for schools in emergencies, PDSNs had ongoing contact with schools by telephone, fax and e-mail and through school visits. A nurse at a control site was frequently called to visit schools that did not have school nurses. In one intervention site staff went into schools four times at the beginning of a school year to train staff in giving injections, to explain regimens, to offer advice on school trips and managing hypoglycaemic attacks and to make sure they had adequate supplies. In another intervention site the nurse had also taught other school children about diabetes.
I’ll go in and do education sessions to the other children so that they know what little J’s having to go through and if there’s issues of teasing or bullying I’ve been into schools as well to talk about insulin and . . . it’s not fun.
Nurse/site educator I13
Two sites offered training for health-care and education professionals. One site had organised a group session for up to 40 teachers; however, schools were reluctant to pay the £5 the clinic was charging to cover costs.
Group education for patients
Many sites had run groups at some point. These tended to be occasional and ad hoc rather than a formal or regular programme. A few sites had run more formal programmes with a planned curriculum that generally focused on CHO counting. One intervention site nurse and dietitian ran a series of 3-hour sessions for a group of 4–6 people on CHO counting over a period of 3 years prior to CASCADE.
Limited resources were seen as a barrier to the development of group work. Although some site staff reported positive experiences with good attendance and good feedback from attendees, others had found them difficult to organise with poor attendance.
[Organising groups has been] a bit ad hoc. It’s always taken ages; different languages (spoken by families) and times that people can make it are so different.
Nurse C9
One nurse said she had run groups in the past but felt they did not work as well as one-to-one training where individual needs, abilities and stages could be catered for.
About half of the control sites ran some kind of education groups during the trial, which were described as informal, loosely structured and ‘ad hoc’. A few had attempted to use a theoretical base and a curriculum.
Examples of groups offered in control sites during the trial are listed below.
-
One PDSN organised educational and social events, affiliated to DUK, for children and families to meet and gain support in living with diabetes. Age-banded events were held including a meal at a restaurant for the teenagers, a party for the younger ones and an activity day for 7- to 17-year-olds. A parents workshop was facilitated by an experienced counsellor. A Healthy Eating family workshop was facilitated by a dietitian. The PDSN also organised a ‘boys-only night’ for boys aged 11 years and their Dad, brother or best friend (male). A few adults with diabetes share their experiences of life with diabetes – from leaving home to getting a job and what happens when things go wrong. Boys with diabetes who attended got a £10 voucher.
-
A group has been run by a dietitian for the past five years on CHO and insulin adjustment based on DAFNE. 145
-
‘If it’s about CHO counting, you know, the grandparents are struggling, then we get the scales out and the food and start weighing so they understand and it’s much easier with practical sessions’ (Nurse C2).
-
A series of 10 education sessions over 12 months (in 2010) was offered through a quarterly newsletter. Topics included ‘What is diabetes and what does it mean for me?’; CHO counting; exercise and travel; sick day management; pumps; insulin dependent devices; sex, drugs and alcohol; and transition to adult services. Attendance at sessions was poor.
Almost all group sessions were run by nurses and dietitians with minimal involvement of consultants at three control sites. Two control sites mentioned the involvement of employees from companies that provide pumps in CHO counting training.
We also have a rep that comes from his company . . . helps with the carb counting so that’s why he’s been involved and he’s offered financial help as well.
Nurse C13
Education groups were primarily run on hospital premises. The majority took place outside school hours in the late afternoon, evening or during the school holidays, and occasionally during weekends.
Certainly every main holiday and often every half term as well, it depends how many youngsters have expressed an interest and obviously the availability of the dietitian as well but certainly every Easter, summer, Christmas holiday we would do one session and often we would do one in between at half term.
Nurse C8
In the majority of cases, attendance was a patient’s choice. Sessions were open to those who ‘expressed an interest’ or who wanted to come along to a particular session. Families were invited to group education on the basis of their ‘stage’ in the clinical pathway.
We have an ongoing list so that’s decided as we get to know the patients and their families and we know what the next stage is for them or if they’re going to be going on to an insulin pump that’s part of pump (pathway).
Nurse C10
Groups varied by site according to type of education offered and whether it was targeted to a specific group or the entire caseload. PDSNs were aware that mixed age groups did not work well so organised groups on the basis of age or gender with age regarded as more important than gender. Parents were involved in some groups.
Rather than doing a group of 12, it’s probably better to do a group of 6 so we’re looking at splitting that one but when we actually came to it we discovered the older teenagers weren’t particularly impressed, so we’ve actually had to split the very young from moderately young.
Nurse C8
A lot of stuff around sport and exercise . . . I would say one of the best attended ones because we’ve had an outside speaker and she’s excellent and we’ve had two or three sessions on that. I would say we’ve had 60 people there at those sessions. Yeah, it makes it worthwhile for her coming as well
Nurse C4
Group attendance varied from two to three individuals up to groups of 60. The percentage of invited people who attended was generally quite low and was often difficult to predict.
Challenges to the service
A lack of administrative support was a key challenge.
We have the consultants’ secretaries who are very good and will do the odd one but the majority . . . yeah, the clerical stuff is down to us . . . you know, clinic letter, filing and all that sort of thing.
Site educator/nurse I2
Staffing levels were also considered a challenge by many although a few felt the level of staffing was acceptable.
Not enough manpower . . . there’s too many patients126 and only me . . . and I feel we can’t meet the needs of our patients really, there’s only so many pump (starts) we can do and we’re jiggling clinics all the time to try and fit all our patients in so very over-stretched.
Site educator/nurse I7
From a nursing point view we feel very fortunate . . . yeah, it’s probably not quite within the (RCN) guidance of one nurse to 70 cause we’ve got about 120 but I think compared typically it’s not far off, it’s not too bad.
Nurse C11
Views about continuing professional development varied.
All study leave’s been cancelled . . . for the moment, they’ve cancelled it from January to the end of March and then we were meant to get a message at the end of March to let us know whether they’ve lifted the ban and at the moment we haven’t been given the all-clear.
Nurse C8
We feel we have enough training which is lovely . . . Yeah, nice to be able to say that we attend conferences when we feel we need to. We, we do it as a team. And everybody in diabetes [has had] pump training, that’s all been offered to us, so we’re very fortunate . . .
Nurse C11
Increases in referrals and referring to CAMHS were seen by some as a challenge:
Referrals are going up. It’s a little bit of a challenge because we have had . . . I think it’s nearly 40 children since November, obviously new children take priority over everything and they take longer.
Site educator/nurse I5
I think the CAMHS . . . the referring into CAMHS, I think that’s something I would . . . you know, we can’t always guarantee that the child will be seen . . . yeah, that’s an area really.
Site educator/nurse I2
In contrast, some felt referrals were not a problem:
We haven’t had many new ones to be fair, until very recently we’ve had these three but even that that’s absolutely fine . . . no problem with that, if we need to refer them on like I said to CAMHS for example, got the psychologist, she comes to all the clinics, she sees everybody anyway and then follows them up outside of that so we’re quite fortunate that we’ve got that service and the dietitian attached to the clinic . . .
Site educator/nurse I3
Other key challenges mentioned by staff at control sites included providing education, the demand for insulin pumps, teaching requests, social problems, standardisation of services and management issues. Answers from intervention site staff covered issues such as providing managing transition, preparation for best practice tariff and management and divisional changes.
Chapter 7 Cost-effectiveness analysis
In this chapter the cost-effectiveness of CASCADE compared with current NHS practice is assessed. Given findings from the PE a scenario analysis is undertaken of a hypothetical Enhanced CASCADE intervention.
Methods
The economic evaluation compares CASCADE with current NHS practice. Education on diet, insulin dosage, regimen and exercise are delivered in a non-standard and irregular way during diabetes clinics (see Chapter 6 ). To assess the cost-effectiveness of CASCADE with respect to current NHS practice, the analysis focuses on the relative success in controlling HbA1c level and predicted impact on diabetic complications over time. Good control (HbA1c value of < 7.5%) is associated with a reduction in the risk of diabetic complications. 175
The cost-effectiveness model considered long-term costs and benefit implications of delaying the onset of microvascular and macrovascular complications of diabetes by comparing HbA1c levels in the intervention and control groups. A series of MCMC submodels simulated the progression of microvascular complications (kidney diseases, neuropathy – foot ulcer and risk of amputation, and retinopathy) and cardiovascular complication (infarction). These predicted future treatment cost of patients receiving the CASCADE intervention or routine NHS care. This is a similar approach to that adopted in the DAFNE study. 145,176–178,186
The proportion of children starting in each health state was based on baseline data from the CASCADE study and the literature ( Table 39 ).
| Complications | Probabilities (%) | Source |
|---|---|---|
| High HbA1c value (> 7.5%) | 81 | CASCADE |
| Uncomplicated kidney disease | 0.92 | |
| ESKD | 0a | |
| Retinopathy | 2.75 | |
| Uncomplicated foot ulcer | 0.61 | |
| Foot amputation | 0a | |
| Myocardial infarction | 0a |
The model simulates the progression of diabetes through a number of mutually exclusive health states. At the end of each cycle, the person can move from one stage to another. The same model is used for both current NHS practice and CASCADE. The only elements allowed to vary between arms are the extent to which patients achieve improved metabolic control. Transition probabilities are assumed to depend on HbA1c levels. For instance, consider the MCMC assessment for kidney diseases ( Figure 6 ). Those without kidney disease continue without developing kidney disease, die, develop uncomplicated kidney disease or end-stage kidney disease (ESKD) in the next time period. Those with diabetic complications could continue with complications, return to the without-complications state or die in the next period. For each model, health states and transition probabilities were determined, based on study data and from the literature.
FIGURE 6.
A MCMC for kidney disease. incr rwd, incremental reward; init rwd, initial reward; term rwd, final reward.
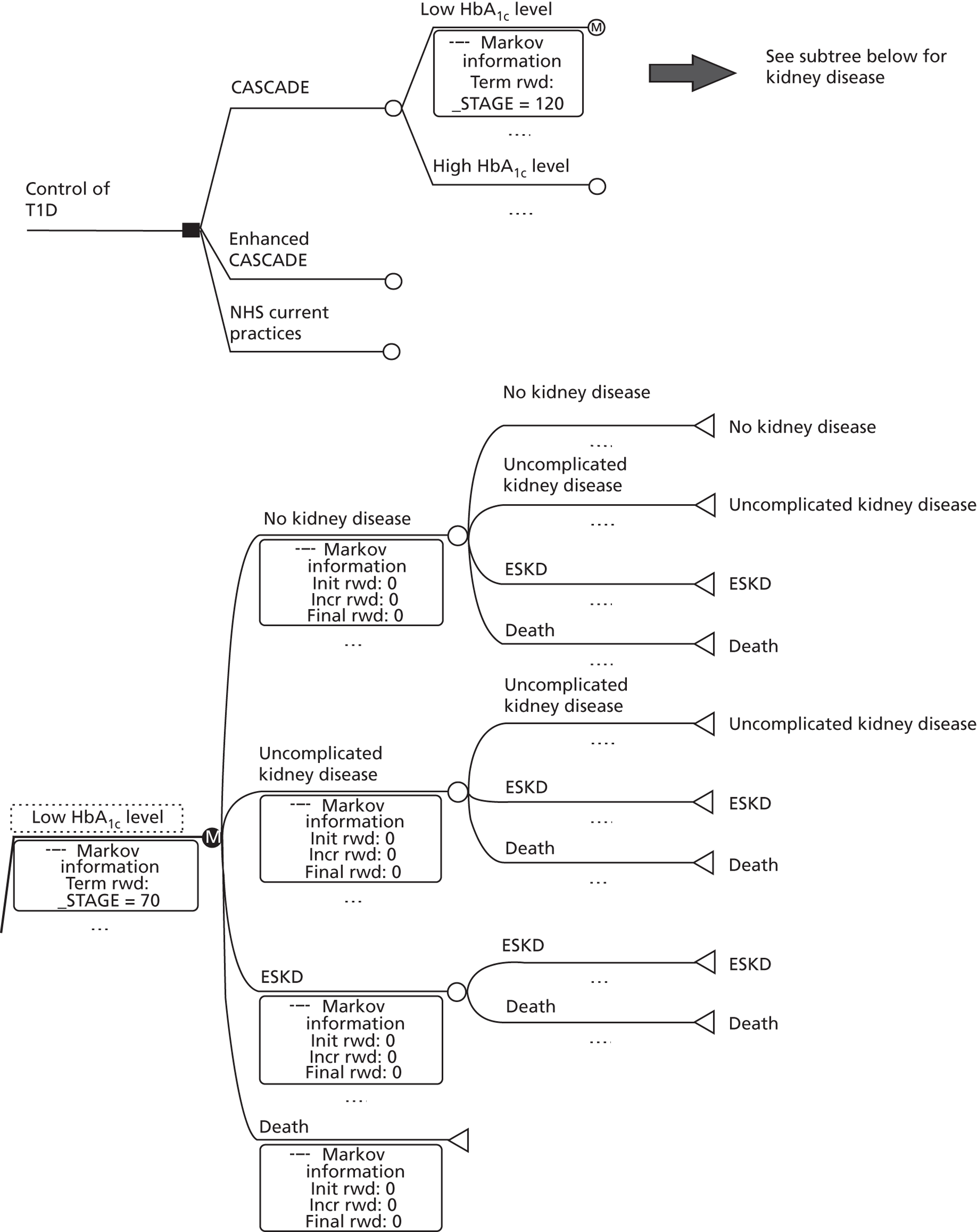
The transition probabilities for diabetes complications did not consider whether patients move to a more or less severe stage of disease (for instance, from a kidney disease to a cardiovascular infarction) in a given year. This was because these probabilities depend on a series of parameters that were either incomplete or unavailable in our database. The sub-MCMC models use HbA1c level (low or high) to predict if patients will or will not develop diabetes complications. The present MCMC submodels contain different health states depending on diabetic complications. The lifetime costs associated with each complication were estimated and incorporated in the model. Table 40 shows the transition probabilities owing to intensive HbA1c control between health states for different diabetes complications. Mortality rates were assigned to all the diabetes outcomes.
| Transitions | Low HbA1c (< 7.5%) | High HbA1c (> 7.5%) | Source | |
|---|---|---|---|---|
| Kidney disease | ||||
| No kidney disease | No kidney disease | 0.989945 | 0.989428 | |
| Uncomplicated kidney disease | 0.009090 | 0.009200 | CASCADE | |
| ESKD | 0.000965 | 0.001372 | Roze et al. 2005;187 Burke 2011188 | |
| Death | 0 | 0 | Assumption | |
| Uncomplicated kidney disease | Uncomplicated kidney disease | 0.906235 | 0.905828 | |
| ESKD | 0.000965 | 0.001372 | Assumed same as no kidney disease to ESKD | |
| Death | 0.0928 | 0.0928 | Assumed same as ESKD to death minus 20% | |
| ESKD | ESKD | 0.884 | 0.884 | |
| Death | 0.116 | 0.116 | Diabetes UK 2010186 | |
| Retinopathy | ||||
| No retinopathy | No retinopathy | 0.9028275 | 0.901671 | |
| Retinopathy | 0.0043725 | 0.005529 | Roze et al. 2005;187 Burke 2011188 | |
| Death | 0.0928 | 0.0928 | Assumption | |
| Retinopathy | Retinopathy | 0.884 | 0.884 | |
| Death | 0.116 | 0.116 | DUK 2010186 | |
| Foot ulcer | ||||
| No foot ulcer | No foot ulcer | 0.9874482 | 0.891589 | |
| Uncomplicated foot ulcer | 0.0015a | 0.097a | Tennvall et al. 2001189 | |
| Foot amputation | 0.0040518 | 0.004411 | Roze et al. 2005;187 Burke 2011188 | |
| Death | 0.007b | 0.007b | Tennvall et al. 2001189 | |
| Uncomplicated foot ulcer | Uncomplicated foot ulcer | 0.896 | 0.896 | |
| Foot amputation | 0.097 | 0.097 | Tennvall et al. 2001189 | |
| Death | 0.007b | 0.007b | Tennvall et al. 2001189 | |
| Foot amputation | Foot amputation | 0.884 | 0.884 | |
| Death | 0.116 | 0.116 | DUK, 2010186 | |
| Myocardial infarction | ||||
| No MI | No MI | 0.995578 | 0.995001 | |
| MI | 0.004422 | 0.004999 | Roze et al., 2005;187 Burke, 2011188 | |
| Death | 0 | 0 | Assumption | |
| MI | MI | 0.884 | 0.884 | |
| Death | 0.116 | 0.116 | DUK, 2010186 | |
Markov chain Monte Carlo for diabetes complications are presented in Appendix 9 (see Figures 9 – 11 ). The DCCT study showed the risk reduction owing to intensive HbA1c control for kidney disease is 50%, for nerve disease 60%, for eye disease 76% and for any cardiovascular disease event 42%. 190,191 CASCADE was modelled as a one-off intervention (for any particular child), through which any improvement in HbA1c level control achieved is assumed to be sustained. The model is run with an annual cycle for 70 years. An individual is either in the health state with or without diabetic complications until they exit the model (at death), and the next patient selected from the hypothetical sample. This process is repeated for a sample of 10,000 patients of ≥ 13 years. At the end of the simulation, the time spent in each of the treatments and health states, and the time spent alive are calculated in terms of lifetime costs and lifetime health benefits comparing current NHS practice to CASCADE.
The analysis was undertaken from the perspective of the NHS and thus only considered directly incurred NHS costs. Future costs were discounted at 3%. The model captures the direct costs of CASCADE and the direct NHS costs in the treatment of diabetes (inpatient treatment and diabetes complications). Direct costs for CASCADE included start-up costs for planning, reviewing and producing the educational material and training workshop costs. Recurrent costs associated with module delivery included (1) planning, administration and setting-up each session; (2) supporting the site educators during module delivery; and (3) staff time to deliver sessions. Start-up and recurrent costs were collected by the CASCADE team. As start-up costs are not incurred every time CASCADE is offered at a particular site it is assumed (arbitrarily) that groups are offered on average twice a year for 3 years.
Costs for CASCADE were estimated using standard methods where the mean use of resource was multiplied by the unit cost of that resource to produce the estimated direct mean cost incurred by the intervention. 192 The resources involved in the development and delivery of CASCADE are shown in Table 41 .
| Activity | CASCADE (actual resource use) |
|---|---|
| Start-up | Type of resource (quantity; time in days) |
| Developing, reviewing and producing the educational material | Nurse (1; 49) Psychologist (1; 24) Assistant psychologist (1, 48) Dietitian (1; 5) Stationery and equipment |
| Training the site educators | Nurse (1; 12) Psychologist (1; 12) Assistant psychologist (1, 14) Clinic staff being trained (43;a 86) Stationery, venue and equipment |
| Recurrent (per clinic) | Type of resource (quantity; time in hours – per clinic) |
| Planning activities | Nurse (2; 1) |
| Administrative tasks | Nurse (2; 2) |
| Setting up the education sessions | Nurse (2; 1) |
| Delivering education sessions | Nurse (varied; varied) Stationery, transport, materials, venues and equipment |
| Supporting the site educators during delivery of the modules | Nurse (1; 6.5) Psychologist (1; 6.5) Nurse for support (1; 7) 1-day refresher workshop |
The salaries of the UCLH team responsible for developing the educational material, running workshops and supporting site educators were PDSN (Band 7), clinical psychologist (Band 8d), assistant psychologist (Band 4) and dietitian (Band 6). Consultants and registrars were assumed to be paid average salaries for their scales (http://careers.bmj.com/careers/static/advice-salary-scales.html). The same salary points were used to cost the Enhanced CASCADE scenario. The site PDSNs and dietitians were allocated at Band 6. Salaries were downloaded from the NHS pay rates website: www.nhscareers.nhs.uk/details/default.aspx?id = 766 (accessed 30 May 2012).
An additional 20% was added to all salary costs to allow for pension costs and National Insurance contributions incurred by the NHS. A unit cost per day was estimated by dividing the annual cost by 220. Direct costs incurred by the NHS for the treatment of diabetes were also estimated using standard methodology. 192 Frequency of use of NHS resources by patients enrolled in intervention and control groups were collected by the CASCADE team. NHS costs were related hospital admissions to manage the diabetes ( Table 42 ). The costs of diabetes complications (kidney disease, neuropathy – foot ulcer and the risk of amputation, retinopathies and cardiovascular infarction) are based on 2010/2011 NHS reference costs figures, as the estimates for the 2011/2012 period are not yet available. 193
| Cost item | Unit cost (£) |
|---|---|
| Hospital admission | 2485 |
| General renal disorder (kidney diseases) | 2688 |
| ESKD (haemodialysis and transplant)a | 18,560 |
| Uncomplicated foot ulcer (without major cardiovascular problems) | 2236 |
| Foot amputation (without major cardiovascular problems) | 8726 |
| Retinopathy (eye diseases) | 1187 |
| Cardiovascular infarction | 1410 |
A one-off treatment cost is included for patients experiencing \eskd, cardiovascular infarction and foot amputation. No costs associated with dialysis and cardiovascular complications have been included. Costs for hospital admissions to treat general glycaemic disorders were entered for all health states. The average hospital length of stay was assumed as 1.4 days for no complications and retinopathy, 3.2 days for uncomplicated kidney diseases and uncomplicated foot ulcer, 5.4 days for myocardial infarction, 23 days for ESKD and 23.7 days for foot amputation. 20
Health gains from improved HbA1c level are estimated by predicting the quality-adjusted life-years (QALYs) experienced by CASCADE patients and comparing them to those predicted for current practice patients. These QALYs were estimated by combining the years spent in each health state predicted by the model with health-state values from the literature. 194 These health-state values are not directly related to educational programmes to control T1D, but rather to the use of insulin and are presented in Table 43 . QALYs were discounted at 1.5% since for this patient group they potentially arise over a long time horizon. 195
| Health state | Utility | Reference |
|---|---|---|
| No kidney disease | 0.814 | Clarke et al., 2002;196 Cummins et al., 2010194 |
| Uncomplicated kidney disease | 0.720 | Assumed to be average of values for ESKD and no kidney disease; Clarke et al., 2002;196 Cummins et al., 2010194 |
| ESKD | 0.626 | Assumed as an average of values for haemodialysis (0.490) and kidney transplant (0.762); Tengs et al., 2000;197 Cummins et al., 2010194 |
| No retinopathy | 0.814 | Clarke et al., 2002;196 Cummins et al., 2010194 |
| Retinopathy | 0.774 | Assumed as an average of values for macular oedema (0.794), severe vision loss/blindness (0.734) and cataract (0.794); AIHW, 2003;198 Brown et al., 2004;199 Cummins et al., 2010194 |
| No foot ulcer | 0.814 | Clarke et al., 2002;196 Cummins et al., 2010194 |
| Uncomplicated foot ulcer | 0.549 | Assumed as an average of values for foot amputation and no foot ulcer; Clarke et al., 2002;196 Cummins et al., 2010194 |
| Foot amputation | 0.285 | Assumed as an average of utilities values for amputation, year of event (–0.109) and amputation, 2 years after the event (0.680); Clarke et al., 2002;196 Cummins et al., 2010194 |
| No MI | 0.814 | Clarke et al., 2002;196 Cummins et al., 2010194 |
| MI | 0.710 | Assumed as an average of values for recent MI (0.685) and remote MI (0.736); Clarke et al., 2002196 |
| Death | 0 | Assumption |
A probabilistic sensitivity analysis was performed using Monte Carlo simulation whereby input parameters were varied simultaneously over specified ranges to capture uncertainty in the model. The simulation drew values for each input parameter and calculated expected cost and effectiveness for each arm of the model by repeating the simulation process 10,000 times to give a distribution of expected cost and clinical effectiveness values. Gamma distributions were specified for the cost parameters and beta distributions were specified for the probabilities, utilities and risk reduction parameters. The distributions assumed for these parameters are reported in Appendix 10 . In addition a one-way sensitivity analysis was conducted to identify the variables that had the largest impact on the model results. For this analysis all parameters were varied by ± 20%. The parameters that had the largest impact on the model are presented in a tornado diagram (a bar chart where cost categories are ordered so the largest impact is presented on the top of the chart followed by the next largest impact categories).
Results
The costs incurred in the development and delivery of CASCADE are reported in Table 44 . As can be seen, the bulk of costs is recurrent and will be incurred each time the education programme is offered.
| Activity | CASCADE |
|---|---|
| Start-up | |
| Developing, reviewing and producing the educational material | 4485 |
| Training the site educators | 3963 |
| Total start-up cost | 8448 (21%) |
| Recurrent | |
| Planning activities | 2378 |
| Administrative tasks | 4755 |
| Setting-up the education sessions | 2378 |
| Delivering education sessions | 12,308 |
| Supporting the site educators during delivery of the modules | 5334 |
| Other resources | 5291 |
| Total recurrent cost | 32,444 (79%) |
| TOTAL COST | 40,892 (100%) |
| COST PER SITE | 3145 |
| COST PER CHILD | 422 |
Lifetime costs and QALYs predicted under current NHS practice and with CASCADE are reported in Table 45 . Current NHS practice dominates CASCADE in that it achieves the same number of QALYs at a lower cost. This follows directly from the failure of CASCADE patients to demonstrate improved metabolic control.
| Type of practice | Cost (discounted) | QALY (discounted) | Incremental cost | Incremental QALY | ICER |
|---|---|---|---|---|---|
| Current NHS practice | 247,551 | 14.4293 | |||
| CASCADE | 247,973 | 14.4293 | 422 | 0 | Dominated |
Scenario analysis
The PE identified a lack of (1) support setting up groups, (2) knowledge and (3) difficulty maintaining the SFBT/MI behaviour change approaches by some of the site educators. There was, however, highly positive feedback from parents and young people who attended the groups. They reported changes in behaviours that were not detected in either the primary or secondary outcome measures. As a consequence of these delivery issues, an Enhanced CASCADE scenario was modelled, which addressed the reported difficulties with delivery and uptake. To estimate the costs of the enhanced scenario, the clinical team identified areas that could be improved using a different combination of resources and/or the inclusion/exclusion of a specific resource. Delivery to a different group of patients and earlier in time after diagnosis would have no cost implications for the model. The Enhanced CASCADE involves more intensive training and support for those delivering the intervention and increasing the proportion of site educators who are psychologists ( Table 46 ). The amount of training was modelled on the current DAFNE training course shown to be widely acceptable to clinical teams (www.dafne.uk.com/downloads/04_fact_sheet_four.pdf).
| Training component | No. of hours | Notes | |
|---|---|---|---|
| CASCADE | Enhanced CASCADE | ||
| Staff required | PDSN plus any other team member | PDSN plus team psychologist | The use of a psychologist will improve the delivery of the behaviour change techniques |
| Other team members | None required | 15 hours training (2-day workshop) for consultant/clinic lead and other team members | Similar to DAFNE training so team can support delivery of programme |
| Orientation | 0.5 | 1 | |
| Background reading | Not compulsory | 5 | Educational knowledge will be assessed |
| Educators observe CASCADE modules 1–4 delivered by trainer | 0 | 8 | Could be live or video |
| Workshops | 15 | 30 | |
| Preparation (training provided for first group run) | 0 | 10 | Educators required to keep portfolio of preparation activity that is reviewed by trainers |
| Educators first group observed by peer reviewer | 0 | 8 | Peer reviewer will be PDSN or psychologist that has successfully completed training |
| Follow-up workshop | 0.5 | 8 | To reinforce skills and sort any issues |
| Total | 16 | 85 | |
The anticipated resource use is reported in Table 47 (in comparison with Table 41 ) and estimated cost in Table 48 . As can be seen by comparing Tables 45 and 48 , for a fourfold increase in training there is only a doubling of the estimated cost per child.
| Activity | Enhanced CASCADE (hypothetical resource use) |
|---|---|
| Start-up | Type of resource (quantity; days) |
| Developing, reviewing and producing the educational material | Nurse (1; 49) Psychologist (1; 24) Assistant psychologist (1, 48) Dietitian (1; 5) Stationery and equipment |
| Training the site educators | Nurse (1; 43) Psychologist (1; 43) Assistant psychologist (1, 30) Nurses being trained (13;104) Psychologists being trained (13; 104) Nurse to observe the training (1; 4.67) Psychologist to observe the training (1; 4.67) Stationery, venue and equipment |
| Recurrent | Type of resource (quantity; hours per clinic) |
| Planning activities | Nurse (2; 5) |
| Administrative tasks | Nurse (2; 2) |
| Setting up education sessions | Nurse (2; 1) |
| Delivering education sessions | Nurse (1; 8) Psychologist (1; 8) Stationery, transport materials, venues and equipment |
| Supporting site educators during module delivery (UCLH staff) | Nurse (1; 1) Psychologist (1; 1) |
| Activity | Enhanced CASCADE |
|---|---|
| Start-up | |
| Developing, reviewing and producing educational material | 4485 |
| Training site educators | 12,097 |
| Total start-up cost | 16,582 (14%) |
| Recurrent | |
| Planning activities | 11,888 |
| Administrative tasks | 30,304 |
| Setting up education sessions | 2378 |
| Delivering education sessions | 43,602 |
| Supporting site educators during module delivery | 7619 |
| Other resources | 9260 |
| Total recurrent cost | 105,052 (86%) |
| TOTAL COST | 121,634 (100%) |
| COST PER SITE | 9356 |
| COST PER CHILD | 1254 |
The cost-effectiveness of the Enhanced CASCADE scenario was explored by assuming that risk reductions in the prevalence of complications would be similar to those observed in the DCCT study at 12 months. 190,191 These reductions in complications are arbitrary and illustrative as there is no evidence as to what an Enhanced CASCADE intervention could achieve.
The lifetime costs and QALYs predicted under current NHS practice and for the Enhanced CASCADE intervention are reported in Table 49 . Current NHS practice is therefore assumed to be less costly and less effective (in terms of QALYs) compared with Enhanced CASCADE. Enhanced CASCADE is estimated to have a cost per QALY gained of £15,920 compared with current NHS practice. This is less than the cost-effectiveness threshold of £20,000 to £30,000 currently specified by NICE.
| Type of practice | Cost (discounted) | QALY (discounted) | Incremental cost (£) | Incremental QALY | ICER |
|---|---|---|---|---|---|
| Current NHS practice | 247,551 | 14.4293 | |||
| Enhanced CASCADE | 252,053 | 14.7121 | 4502 | 0.2828 | 15,920 |
The 95% credible ranges for the costs and QALYs based on 10,000 simulations are reported in Appendix 11 . Figure 7 illustrates the cost-effectiveness acceptability curves that indicate the probability that an intervention is cost-effective for a range of willingness-to-pay thresholds. The probability that Enhanced CASCADE is cost-effective compared with CASCADE and current NHS practice increases as the threshold increases. For a threshold between £20,000 and £30,000 per QALY, the Enhanced CASCADE scenario would have a probability of 42–45% of being cost-effective. However, the difference between the Enhanced CASCADE scenario and the CASCADE scenario is small for the £20,000–30,000 threshold.
FIGURE 7.
Cost-effectiveness acceptability curves for CASCADE, Enhanced CASCADE and current NHS practice.
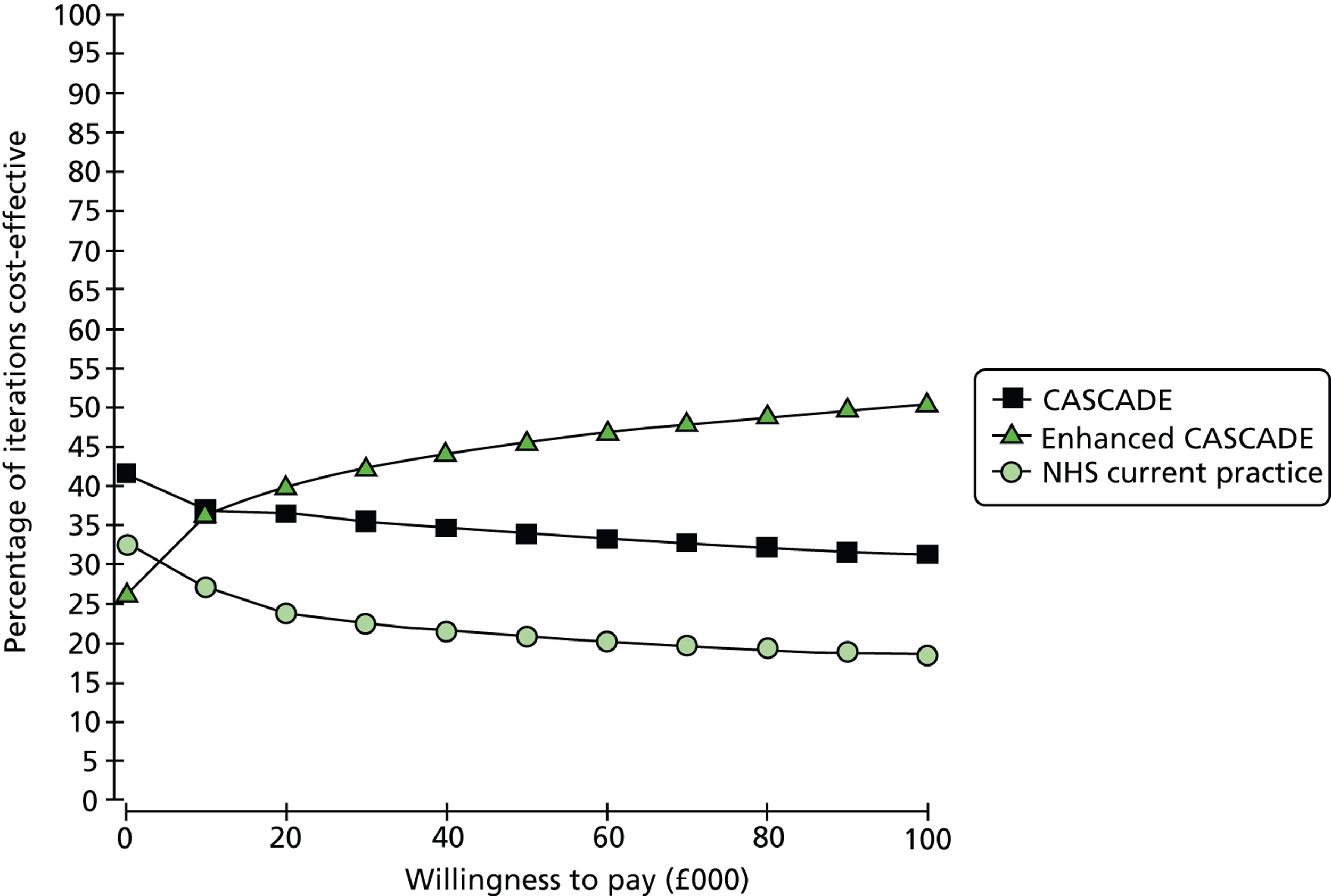
The univariate sensitivity analysis results are shown in the tornado diagram ( Figure 8 ). The diagram reports the possible net monetary benefit of Enhanced CASCADE compared with current NHS practice for the 10 variables that have the largest impact on the model results. Net monetary benefit is calculated by taking the difference in clinical effectiveness and multiplying by society’s willingness to pay less the difference in costs. A positive net monetary benefit indicates a scenario where Enhanced CASCADE may be cost-effective relative to current NHS practice, whereas a negative net monetary benefit indicates that current NHS practice may be cost-effective compared with Enhanced CASCADE. The variables that had the largest impact on the model results were the cost of hospitalisation, the utility of diabetes with no complications, the utility of uncomplicated foot ulcer, and the transition probability from uncomplicated foot ulcer to uncomplicated foot ulcer.
FIGURE 8.
Tornado diagram showing the 10 variables with the largest impact. pHTFAFA, probability of remaining in the foot amputated state; cTreatment, refers to the cost of the treatment for diabetes, in this case, the cost of hospitalisation; uNocomplications, utility for no complications of diabetes; uUfootulcer, utility for uncomplicated foot ulcer; pHTUFUHb, probability of remaining in the uncomplicated foot ulcer state; pHTRetinoRetino, probability of remaining in the retinopathy state; pLowHbA1c, probability of low HbA1c level; pHTUFUFA, transition probability from uncomplicated foot ulcer to foot amputation; uFootamputation, utility for foot amputation; uUkidnedisease, utility for uncomplicated kidney disease.
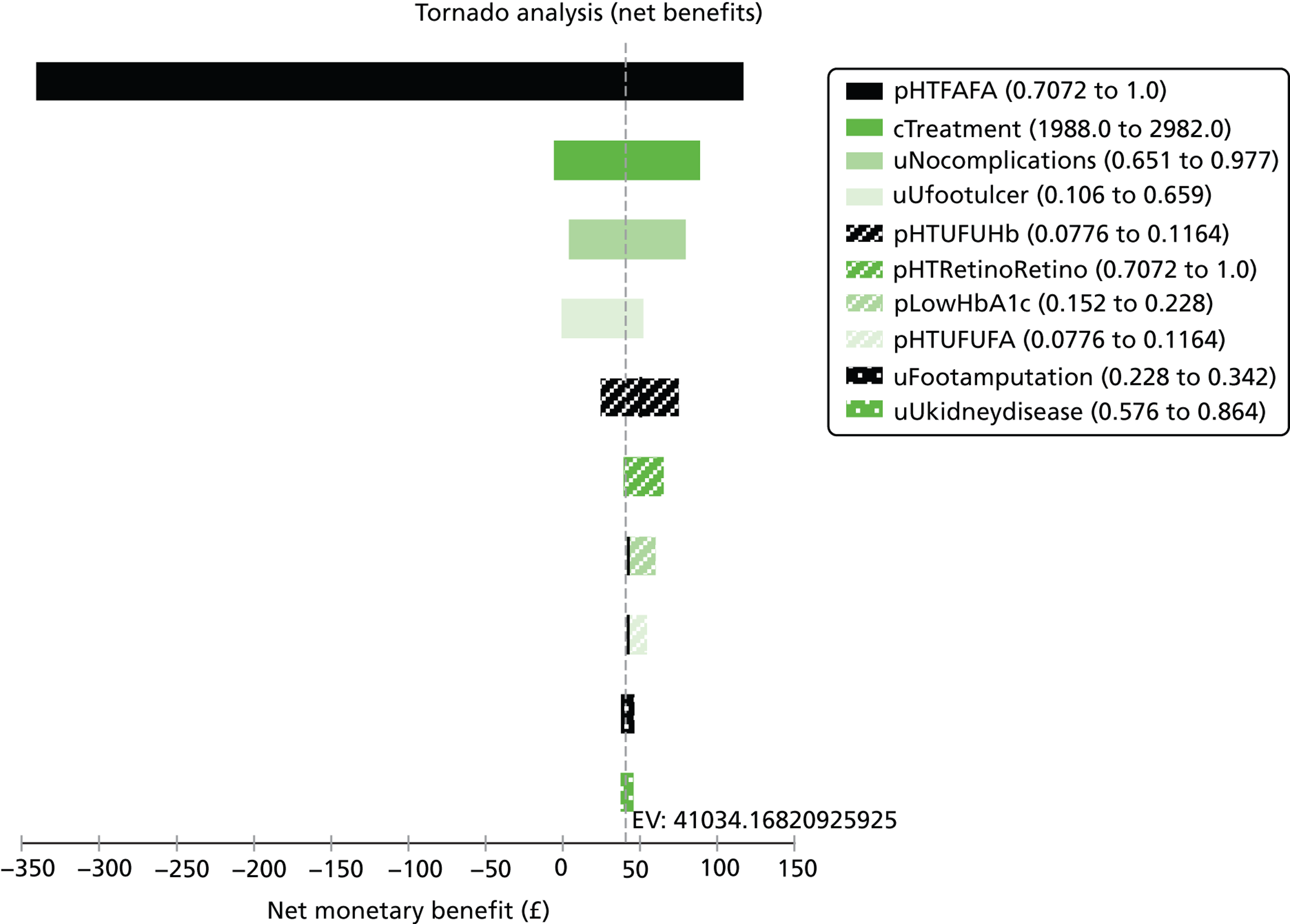
Discussion
A number of fundamental challenges are faced when estimating the cost-effectiveness of an educational intervention such as CASCADE. The most significant is that the costs of delivering the intervention are largely incurred in the near future, whereas many consequences for patient health and utilisation of health-care resources (should the intervention be effective and improved metabolic control be sustained) are experienced in the more distant future, some more than 30 years hence. Thus, although many costs can be captured as part of the trial, most of the consequences in terms of health effects and future treatment costs must be modelled rather than observed. The reduction in risk of diabetic complications owing to improved glycaemic control is at the heart of the assessment of the cost-effectiveness of CASCADE.
These future consequences were modelled by specifying a relationship between the likelihood of diabetic complications and relatively short-run changes in HbA1c. In the modelling it was assumed that any change in the successful management of diabetes taking place as a consequence of CASCADE would be adequately captured in the HbA1c level. Because of limited follow-up it is not possible to be sure that changes in HbA1c level will be sustained in the longer term. Moreover, the model assumes that the significant change in HbA1c is from ≥ 7.5% to < 7.5%, and that the relationship between HbA1c level and annual risk of different complications can be specified. The DCCT trial, conducted from 1983 to 1993 in the USA and Canada, involved 1441 volunteers, ages 13–39 years, with T1D and showed that keeping HbA1c level as close to 7.5% as possible slows the onset and progression of eye, kidney and nerve damage caused by diabetes. 200 However, neither the DAFNE nor DCCT studies, nor even the UKPDS study for T2D,201,202 provide evidence that good metabolic control reduces chronic complications. In other words, no definitive assessment shows a causal relationship between specific levels of glycaemic exposure and the risk of complications, as confounding is possible from a number of sources (DAFNE Study Group. Clinical and cost effectiveness for education people with type 1 diabetes mellitus in diabetes self-management. 2002, unpublished).
Data show that those of Afro-Caribbean, African and South Asian ethnicity are more likely to develop diabetes, as well as the complications associated with the disease, than other ethnic groups. 186 These differences have not been explored because the CASCADE intervention and control groups were, respectively, 91% and 81% white British or other white people.
The CASCADE intervention and NHS current practice might have also been compared in terms of daily insulin dose; however, as recognised by the DAFNE UK study, and as shown in our data, the number of insulin injections varies widely across both study arms and many of the data on insulin dose were incomplete in our database145,183 (also DAFNE Study Group. Clinical and cost effectiveness for education people with type 1 diabetes mellitus in diabetes self-management. 2002, unpublished).
The cost of treating future diabetic complications and the consequence of these for the patient’s health state must necessarily be based on literature rather than on trial data. Although the costs of delivering the intervention are firmly rooted in data from the trial, even here assumptions must be made. In particular, if CASCADE were to be introduced into routine clinical practice only some of the costs would be incurred each time the programme was delivered, whereas other costs with respect to training educators would not. Thus assumptions must be made regarding on how many occasions educators will deliver the programme.
Despite the lack of impact of CASCADE as delivered on HbA1c level, the considerable support from the PE for the intervention introduced the question of whether an Enhanced CASCADE compared with current practice might be cost-effective. Although a reasonable estimate of the costs of an Enhanced CASCADE can be made, any estimate of cost-effectiveness is highly speculative given there is no evidence regarding the likely success of an Enhanced CASCADE programme. As is highlighted by the probabilistic sensitivity analysis, there is great uncertainty regarding the cost-effectiveness of the different interventions.
Chapter 8 Discussion
In the following discussion, we highlight the strengths and weaknesses of the study and discuss the generalisability of the findings. Interpretation of the study results will be considered, taking into account the study hypothesis. Considerations for future delivery of clinical services and research are addressed.
Key findings
Training PDSNs and other members of diabetes teams to deliver CASCADE structured education groups using behaviour change methods was not shown to impact beneficially on glycaemic control as measured by HbA1c levels.
Both parents and young people reported an increase in young people’s responsibility for diabetes self-management, although young people’s self-reported happiness with body weight decreased. At 12 months’ follow-up, 87.5% of children in the intervention arm were on ≥ 4 injections per day compared with 80% in the control arm. At both 12 and 24 months’ follow-up, there was a tendency for young people in the intervention group to use higher doses of slow- and quick-acting insulin. This is potentially consistent with those receiving the intervention being more likely to take more insulin to correct high BG readings.
The majority of parents who attended the intervention groups described improved knowledge and understanding of diabetes. They also described positive impacts on lifestyle, for example shopping habits, managing BG and exercise, as well as greater confidence managing diabetes, motivation and decision-making in their son/daughter and improved family relationships. Positive impacts reported by children and teenagers included improved understanding of diabetes, greater motivation in managing diabetes and increased confidence and changes in attitude, for example in discussing their diabetes and improved relationships with parents and peers. Twenty-four months after the intervention, nearly half of the young people who had attended CASCADE reported that the sessions had made them want to try harder to control BG levels and that they had carried on trying. Interviews with young people suggested less perceived impact by teenagers than younger children.
The CASCADE study shows that a high-quality, complex, pragmatic trial of structured education can be successfully conducted alongside standard care in NHS diabetes clinics with a number of provisos discussed below. Despite challenging circumstances we were able to recruit teams and patients from clinical services reflecting a wide range of philosophies and research experience throughout London, south-east England and the Midlands. The pragmatic components of a NICE-compliant structured education programme can be successfully delivered following a relatively brief 2-day training workshop, whereas paediatric health-care professionals benefit from training in behaviour change skills.
The workshop component of the training represented 12.5% of the overall costs of the intervention. CASCADE was not found to be cost-effective. However, in the economic model cost-effectiveness could be achieved only by improving metabolic control, and no value is being assigned to increased competence, or any changes in behaviour, if they do not translate into better metabolic control.
Strengths and limitations
Design strengths
A randomised cluster design was used to good effect in this study. An appropriate number of clinics and additional participants were recruited to allow for dropout without compromising statistical power. We achieved a sample size that allowed adequate power to test our hypotheses. All centres recruited and randomised into the study completed the structural and training elements of the study.
Delivery by a large number of practitioners minimised the influence of individual practitioners’ skills and personality. Secondary outcomes used included several shown to perform well in measuring QoL in children with diabetes in a range of contexts. 203 Assessment of practitioner fidelity used extensive observation of a large sample of workshops delivered at different time points, as well as self-assessment by practitioners after delivery of every group. 204
Data loss
Many young people had issues with providing the blood samples, which was a major barrier to both recruitment and subsequent collection of HbA1c data after consent. Considerable negotiation was often required as to how and when the sample would be collected. Clinics often struggled to find an appropriate HCP to collect the blood samples. Primary (HbA1c) and secondary (questionnaire) measures were sometimes collected on separate occasions at both follow-up points. Trying to organise telephone questionnaires, when they could not be completed in the clinic, was a key contributor to data loss.
Internal validity
Measurement bias was minimised by the use of a central laboratory for all HbA1c assays. The two trial arms were well balanced at baseline. One limitation of pragmatic cluster trials is possible bias due to trial arm allocation knowledge. Allocation was concealed until after clinics had consented and a first participant recruited to minimise selection biases at entry of clusters to the trial. Checks for bias revealed no obvious imbalances in demographic data. It is unlikely that allocation knowledge bias affected recruitment rates as there was no difference between trial arms in number of eligible patients or final recruitment rates.
Intervention development
A strength of the CASCADE trial was incorporation of recommendations from previous systematic reviews of psychoeducational interventions in childhood diabetes. 91 The intervention had a firm theoretical basis that drew significantly on principles of MI and SFBT previously shown to have potential in childhood diabetes117,121 and incorporated key components of structured education programmes following NICE guidelines. 4
Educational content
The study highlighted different levels of knowledge in practitioners for aspects of key diabetes content. Problematic topics were related to food groups, matching food and insulin, CHO counting and insulin sensitivity ratios. A small number of secondary site educators did not have a thorough grounding in diabetes knowledge. In interviews only half of the parents and young people said they had been familiar with the content of the groups even though the majority of the content should have been covered in the average 5 years since diagnosis. This confirms a lack of consistent and effective structured education currently being offered as part of standard care by the majority of diabetes teams.
Lay and professional stakeholder perspectives
The CASCADE trial involved the views of both lay and professional stakeholders at many levels. A parent of a young child, a young person and two adults with diabetes were members of the initial study design team and reviewed the structure and content of the length, general tone and specific items on the questionnaire. Two adults with diabetes were members of the study management team. Comments on the content and tone of questionnaires by the parent and a staff member with research responsibilities for a diabetes charity raise questions about how to involve lay perspectives in designing data collection instruments. In particular, the content and language of some tools were better suited to the cultural norms for health and illness in their country of origin (USA) than the UK context of this trial. This challenge is heightened where changes to research instruments have implications for their validation or copyright status.
A parent and young person from the UCLH diabetes service reviewed the content and delivery of the intervention, and an independent group of PDSNs reviewed and trialled the training workshop content and delivery. The extensive integral PE can be used to inform interpretation of the results and ensure that parents and young people can make a valid contribution to future research programme, such as CASCADE, to ensure that further developments are likely to be feasible and practical in an NHS paediatric diabetes context.
Serious adverse effects
The CNR highlighted the difficulties involved in attempting to set up a formal reporting system in this context. The data from the CNR showed that the actual number of SAEs was in line with expected levels. Overall number of DKA episodes found in the CNR matches data from the recent UK NPDA and Hospital Episode Statistics data, which is 200–400 per 1000 for 0- to 20-year-olds, and highest in teenagers. There was also a lower number of cases of hypoglycaemia reported than might be expected, reflecting the fact that we chose a population with higher HbA1c levels. However, the CNR highlighted that the clinical teams involved were very poor at reporting SAEs.
Feasibility and training exposure
Training to deliver CASCADE
Overall the training programme was positively evaluated although motivation to attend the workshops varied, often depending on how site educators had become involved. Training (from the same two trainers) took place over 2 days, primarily at UCLH. The majority of site educators regarded 2 days of training as feasible and acceptable, although some felt there was too much to take in. All site educators received substantially the same or very similar training, with some variation depending on group dynamics and types of questions and discussion. Site educators generally thought that the training was very good and were initially excited and motivated.
Running groups
Despite the post-training enthusiasm of the site educators, only 30 out of a possible 44 groups were run (68%). One site did not manage to run a full set of sessions and one site failed to run any groups owing to lack of staff.
Experience of setting up and running groups by site educators was often minimal. Most sites found organising the groups very difficult and time-consuming, with little use of electronic media to facilitate this process. Some found it very difficult and time-consuming to agree dates and times with families. Practical issues, such as finding appropriate rooms to house the groups and identifying time for nursing staff to deliver the group, were also barriers to delivery. Some thought that more time could have been spent on this in the training, despite 25% of the training being focused on getting clinics to think about how to get the groups up and running as well as practical issues like arranging rooms. Site educators suggested that more administrative support would have been helpful. Key barriers to running groups included lack of administrative support for organisation of groups, low use electronic media for communication with patients, competing priorities for potential participants and factors specific to being part of a research study.
The research context
Highly variable turnaround times obtaining necessary governance approvals at sites led to significant delays in patient recruitment, which had an impact on clinics’ ability to organise groups soon after completion of training. Site educators felt that momentum was lost because of the time lag between training and the setting up of the CASCADE groups.
The Research Passport system (www.nihr.ac.uk/systems/Pages/systems_research_passports.aspx) and Integrated Research Application System were introduced as the CASCADE study started. This meant that R&D processes were very slow and in some cases stopped for protracted periods while staff drew up new local policies and procedures. (e.g. one trust refused the research team access for 6 months). A strength of CASCADE was the quality of the study processes, capacity and continuity between collection of all data by the research team. However, there were significant delays obtaining honorary contracts/letters of access for research staff. All R&D departments requested additional supporting documentation, resulting in long delays, and Criminal Record Bureau checks taking up to 6 months. A pragmatic decision was taken to arrange contracts for selected research staff to work in selected clinics rather than across all sites as originally intended. Although this ultimately sped up the recruitment process it complicated the management of fieldwork. The negative impact of negotiating these regulatory procedures on recruitment has been reported by others. 205
Where possible the research team liaised closely with secretarial, clerical and path laboratory staff to check clinic dates, demographic details, etc. as noted in the extensive acknowledgement list; however, staff reported feeling pressured by additional trial-related tasks (such as organising for research blood samples to be taken and liaison with research staff over clinic dates, etc.).
Restricting the groups to a subset of patients who were recruited to the trial, instead of being able to offer the groups to the entire clinic list was perceived as making the organisation of the groups more challenging. This was particularly the case in sites where small numbers of recruits and/or where a hospital served a large geographical area making travel arrangements complex. It also meant that natural groupings of patients (by age, or geographical area, etc.) were more difficult (and at times not possible).
Age-appropriate groups
The educators found it difficult to keep the young people focused when more challenging topics were introduced. This may have been because the language used by the educator was not age appropriate and was particularly challenging in groups with mixed ages. The UCLH training team emphasised the need to have similar age groups. However, for pragmatic reasons compromises were sometimes made in terms of the CASCADE model for optimum group composition. Rather than grouping by age, groups were often mixed. Educators found it difficult to keep mixed age groups focused on activities and to engage the entire group at once. Additionally, the age mix of the groups was felt to be problematic by some young people.
Delivery
The intervention appears to be deliverable within the context of routine care without major impacts on service structure with some provisos. Although at least two practitioners from each study site attended both workshops, there was a failure to use trained educators in a few sites in the delivery of some groups, which compromised the integrity of the programme. Two sites that swapped site educators across the modules started with higher fidelity scores in the first two modules, which tailed off in modules 3 and 4.
Fidelity of delivery of activities was generally high across all sites. Site educators delivered activities as described in the manual and used aspects of the approaches demonstrated in the training sessions. However, less time than was recommended was often spent on some activities. Some of the key exercises that would have had the greatest impact on HbA1c level were the ones on which less time was spent. Key factors that influenced effective delivery included having an appropriate venue and having planned breaks. In addition, advance preparation for sessions by both site educators and skilled group management using the psychological approaches with confidence made the groups run smoothly.
Although fidelity of CASCADE techniques was good, it was not as high as desired. Site educators were aware that they needed to use CASCADE approaches more often (e.g. evoking knowledge by asking ‘What else?’) but found it difficult to do in larger or small groups or where there were group dynamics they found difficult to manage. Educators found it challenging at times to work with parents and children and keep both of them focused on the task and would have benefited from more training in how to manage the group when there was a dominant member of the group, usually a parent. Some site educators found it difficult to move away from didactic teaching and struggled with ‘avoiding giving a choice or giving people the answer’ and asking ‘how come?’. They said it was ‘impossible’ to keep asking ‘what ifs’ if only one family attended a session. The frequency with which groups would comprise single families had not been anticipated in the training, hence this was not prepared for. The training workshops seemed to have ‘deskilled’ the PDSNs, as in normal practice they are more used to working with single families than groups. As PDSNs reported lacking confidence delivering the CASCADE groups to a single family this would have to be addressed in future training.
Generalisability
The trial was conducted in NHS paediatric diabetes clinics spread throughout London, SE England and the Midlands. Eligible list size varied but was well balanced across trial arms although the number of eligible patients reported by clinics was surprisingly low. Exclusion criteria were kept to a minimum and the heterogeneity of the paediatric diabetes population is well reflected by trial participants.
Uptake
Staff thought that many of the young people who were eligible for CASCADE were the least motivated in terms of their diabetes, and that these remained the least motivated to attend groups, even if recruited. Of the 180 families recruited, 66 (37%) failed to attend any of the groups offered, but were happy to provide baseline and follow-up data. The majority of reasons for opting out of groups were a desire not to miss school (young people) and not missing work (parents). Older teenagers and young people with higher baseline HbA1c levels were significantly less likely to attend than those with lower baseline HbA1c levels. The mean HbA1c value of the patients at baseline was higher than the population average as reported by the NPDA. This was because the study selected young people with a higher HbA1c level. The data suggest that the intervention does not appeal to young people with baseline HbA1c levels of ≥ 10.4 mmol/l and an alternative, more personalised and/or intensive, intervention may be required for this subgroup of patients.
Staffing problems and difficulties organising groups prevented 14 of the expected 44 groups being offered. The most suitable timing for when groups are best offered and which specific age groups should be targeted remain unclear. Some staff felt that CASCADE would have been more effective if offered during first 12 months after diagnosis when young people and families are more likely to take on information and are more in ‘the right frame of mind’ (site educator, I14). Children (8–12 years) were significantly more likely to attend CASCADE than teenagers (13–16 years), which would suggest that parents found it easier to ‘bring’ younger children to the session. In addition, reported perceived impact was significantly higher for younger children than teenagers.
We contend that the generalisability of these results is high for diabetes patients in the UK aged 8–16 years and their carers with participation of a large number of diverse clinics, serving diverse populations and suggest it is feasible to set up and run structured education with adequate support. A low dropout rate and good attendance for the subgroup that attended the intervention, as well as reported impact by this group, strengthens the findings of the study; however, it suggests that uptake and effectiveness might be improved if offered to younger individuals with lower HbA1c levels.
Although the start-up cost associated with the intervention could be spread over more patients were CASCADE to become routine clinical practice, about four-fifths of the costs of the intervention are closely related to the number of patients. Thus, the costs will differ by number of patients and will be lower for larger clinics. However, these differences will not be large.
Pragmatic RCTs tend to demonstrate high external validity and assess effectiveness rather than efficacy. The intervention has been evaluated in ‘real-life’ and representative settings, with all the challenges that this presents, ultimately providing invaluable information on barriers and opportunities regarding future similar interventions.
Changing the clinic culture
In hindsight it might have been helpful to have trained the whole clinical team in the CASCADE philosophy to create a clinic environment that supported the delivery of the structured education groups. It is possible that children, young people and families attending groups that effectively encouraged behaviour change may have then been faced by didactic appointments with other clinicians. This approach can create resistance and may have cancelled out a positive effect of the groups, particularly in older teenagers with higher HbA1c.
Sites that remained most faithful to the delivery of activities and used the techniques most effectively generally came from relatively well-resourced teams. They were able to take time to prepare before the modules were run and had suitable venues available. The site educators were enthusiastic about CASCADE, had experience in delivering groups, had worked together in the clinic for some years and had good knowledge of the participants.
The failure of the study to demonstrate an effect on HbA1c level is disappointing, given the influence of MI and SFBT on its design and previous evidence that behaviour change approaches can have beneficial effects on glycaemic control. Positive results have been reported in studies that used trained psychologists with more extensive training and skills in MI and SFBT. The interventions had a much greater emphasis on the psychological components without needing to teach new educational content. 117,121
Many site educators made real attempts to adhere to the approach, but there were instances when they found this difficult or unachievable. It is possible that the approach was not delivered consistently. Site educators were less able to deliver content that conflicted with their standard practice or reported knowledge of a topic, for example testing urine for ketones. Less adherence to the manual was reported in exercises involving CHO (e.g. CHO counting and use of CHO to keep BG stable and exercise management), as well as identifying how insulin works, matching insulin to food and making permanent insulin changes, and identifying hypoglycaemia. Encouraging all of these behaviours is critical to good diabetes management.
In addition, some educators struggled to complete aspects of the delivery that are key to the theory of change. Limited time was spent on the introduction (module 1) and reviews (modules 2, 3 and 4) designed to ‘bring forth’ positive events and activities to build participants’ sense of knowledge, skills and abilities. These activities were identified as challenging. Some site educators also misunderstood the intention of the ‘looking to the future’ activity and struggled to deliver it effectively. Key behaviour change techniques that address ambivalence (e.g. the pros and cons of BG testing and when to seek help) were also not delivered consistently. Ambivalence and discrepancy between knowledge and action is a key target for approaches delivering MI effectively. The importance of using these key techniques to address ambivalence towards behaviours is highlighted by data that showed that, although nearly all parents (93%) reported a greater desire to stop their child’s BG going too high or low, less than half (43%) reported wanting to test their child’s BG level more often.
The PE showed that some nurses lacked basic knowledge, some did not adopt the CASCADE approach and some struggled to adhere to the approach consistently. However, it is unclear whether failure to deliver the content and approach with adequate fidelity or organisational issues and the capacity to set up groups are the critical components for the failure to demonstrate effectiveness in improving glycaemic control.
Change in patient and family behaviour
Just over half (53%) of young people in the intervention sites attended one or more CASCADE education sessions, with the majority attending three or four modules. However, the per-protocol analysis showed no effect of the intervention in those who attended. School and parental work commitments, holidays and forgetting were the most common reasons to miss a group. Young people and parents liked being in the sessions together. Staff also found it helpful to include parents (albeit finding it challenging at times). The majority of participants considered sessions to be the right length with appropriate content level. Nearly all would recommend CASCADE to other families. Parents and young people who attended at least one CASCADE group reported positive changes. Some nurses reported significant changes in specific challenging individual patients but this was not confined to sites where delivery was rated highly.
Glycaemic control is crucial to the short- and long-term well-being of patients with T1D. The primary trial outcome established in the research commissioning brief was levels of HbA1c. Effective glycaemic control is consequent upon several contributing factors. It is therefore important to also focus attention on the secondary outcomes, given their potential position as potential mediators or moderators; however, the small number of significant secondary outcomes consistent with change described by parents were in line with what would have been expected by chance.
Impact on standard care
The challenge of delivering standard NHS care identified by staff interviewed at the end of the 4-year study period showed some differences in control and intervention sites. Control sites identified their main challenges under the theme of ‘providing education’. This included; how to provide education, the demand for insulin pumps, requests for more teaching, help managing social problems, the need for standardisation of services and management issues. Several control sites expressed disappointment that they were not offered the training for the CASCADE groups at the end of the trial, even although they were aware that the intervention was not effective in changing glycaemic control.
Intervention sites identified key issues that fell under a theme of ‘teaching requests’ related to topics that included managing transition, preparation for best practice tariff and management and divisional changes. Six of the 12 intervention sites that offered groups continued to use the CASCADE material as part of their clinic structured education programme (before the trial results were reported).
Changes for future delivery
Site educators want (and need) more supervision and support to ensure that the key techniques and activities are completed according to the intention of the CASCADE philosophy. The pragmatic nature of this intervention did not allow us to specifically assess whether or not these techniques would have been more effective if combined with other efforts to intensify diabetes management. It is also possible that failure to address the entire clinic culture (as DEPICTED tried to) may have had better overall impact, although the DEPICTED trial similarly did not have a significant effect on HbA1c level.
Interpretation of results
The training and programme delivered in the CASCADE trial was not effective in changing glycaemic control over a 1- and 2-year follow-up period post intervention.
This disappointing outcome may be due to one or more of the following influences:
-
The level of support for the intervention was inconsistent across teams. The impact may be greater if the whole diabetes team value the importance of the approach. This would require practitioners to adopt a holistic approach to behaviour change in routine consultations with families alongside structured education sessions.
-
Support for delivery would require involvement by commissioners and trust management to facilitate the process of integrating structured education into routine clinic systems.
-
The level of diabetes specific education of site educators was inconsistent and, therefore, insufficient to improve patient knowledge
-
The level of training provided did not provide the skills required to facilitate behaviour change in the patient.
-
The level of support for site educators was insufficient for them to achieve the sustained fidelity required.
-
The timing of delivery was not optimal. Staff have suggested that they would have found it more useful for young people diagnosed more recently.
-
Mixed age groups did not work well for staff, as some of the older age group reported that they held back asking for specific issues to be addressed in deference to the younger age group being present (issues such as drugs, sex and alcohol consumption).
-
It would have been better to have focused the intervention only on the younger age group.
-
The intervention should have been a universal one rather than a targeted one. Focusing on a higher HbA1c group made it more difficult to recruit a population who, by definition, were less likely to attend groups and are also more resistant to change.
-
On the basis of the results, the training as defined in the CASCADE trial cannot be recommended to produce clinically significant changes in children with diabetes, although the PE suggests it was beneficial for some children and young people with questions regarding timing, readiness to change in some groups, and the need to value small steps in the right direction.
-
There were high levels of initial exposure to the training with good levels of attendance of workshops and high levels of enthusiasm to participate. There were problems with the timing of training delivery and group delivery which resulted in a loss of skill and confidence.
Recommendations and reflections
On the basis of the results, the training as defined in the CASCADE trial cannot be recommended to produce clinically significant changes in children with diabetes. However, HbA1c levels – the primary trial outcome – is dependent upon several intervening steps. The intention of the session delivery style is to invite young people to think about change as much as actively change behaviours. Increase in insulin in the intervention group may suggest small changes in attitude that have not yet impacted on HbA1c level. Changes in knowledge, attitude and family relationships were reported in the PE. There was some evidence of an impact on patients’ and carers’ appraisal of their own self-management, although, as two of many secondary outcomes, these may be significant by chance. It is possible therefore that we did not choose the best secondary outcome measures in this trial, or that instruments used chosen were not sensitive enough to identify reported change. Changes between groups in insulin dosage at 12 and 24 months are similar at both time points. However, without baseline data this is difficult to interpret and there is no suggestion of a continuing effect.
The MRC guidance on complex interventions recommends that the results are interpreted within two domains. In CASCADE the first was the effort of the study to change the behaviour of practitioners through provision of training workshops. The second was subsequent efforts to change the behaviour of patients (children and parents) through development of the structured education programme.
The research team assumed a level of basic knowledge missing in some cases and variable in many. It is suggested that educational knowledge must be assessed and addressed in any training workshop prior to attempting to change the behaviour of practitioners. Currently the Nursing and Midwifery Council (NMC) sets standards of education, training, conduct and performance so nurses can deliver high-quality health care consistently throughout their careers. The need to develop and implement a standardised national competency framework for diabetes specialist nurses is being addressed by SWEET. 185 NHS England now oversees and shapes the development of the health and care workforce and promotes high-quality education and training responsive to the changing needs of patients and local communities.
It has been suggested that more extensive training with attendant allocated resources to address organisational, administrative and clinical support would have been beneficial in delivery of the CASCADE groups. Despite the majority of site educators saying 2 days’ training was adequate, there was some concern expressed at a later date at the scale of the task ahead considering the relative briefness of the training.
Because of unfamiliarity with the psychological approaches, some felt that confidence using the new style of teaching and techniques would have increased if there had been a middle step between being told how to do it and actually doing it with families. Research has shown that nurses can find it difficult to adjust to using a non-didactic approach compared with how they have been trained. 131
Limited training, such as the half-day described in DEPICTED,123 or 2-day courses, such as provided in the CASCADE study, may not be enough. Programmes need to provide ongoing supervision and skill development. Having more of the clinical team attend the training could help change the team culture with the added benefit of reducing the risk of staff turnover or absence, which was a major problem. Providing training locally may also improve attendance.
A more extended training is used by the successful DAFNE programme. 145 The ‘ideal’ training programme (based on DAFNE) would include (1) extended training with more role play and assessment of educational knowledge; (2) more support and training in preparation for organising and running groups; (3) site educators having an opportunity to observe a peer trainer deliver sessions to patients; (4) peer trainers observing site educators delivering their first groups; and (5) increased support and supervision throughout the delivery of the first group.
An enhanced intervention involving more intensive training and support for those delivering the intervention and increasing the proportion of educators who are psychologists was costed. Current NHS practice is less costly and less effective (in terms of QALYs) than this Enhanced CASCADE were it to be effective. Enhanced CASCADE is estimated to have a cost per QALY gained of £28,100 compared with current NHS practice. This lies within the cost-effectiveness threshold range of £20,000–30,000 currently specified by NICE.
Change in practice behaviour
There are mixed results in the use of the techniques in group delivery. The PE sheds doubt about the ability and willingness staff who attended training (and those who did not) wanting to use the approach or deliver the groups. There was therefore a limited embrace of the shift in style, attitude and skills. The unavoidable delay before groups were delivered that impacted on skill and confidence may be avoidable if sessions were delivered as part of usual service.
Conclusions
Determining the effective elements of complex interventions is a significant challenge.
Members of paediatric diabetes services trained to deliver the CASCADE structured education package using behaviour change techniques did not improve glycaemic control in patients compared with control subjects 1 and 2 years after the intervention.
The training workshops for practitioners were well evaluated; however, more intensive training was needed. Maintaining skills following training was a particular challenge.
The cost of the intervention was £683 per patient but it was not cost-effective because it did not improve metabolic control. Although a reasonable estimate of the costs of an Enhanced CASCADE can be made, any estimate of cost-effectiveness is highly speculative given that there is no evidence regarding the likely success of an Enhanced CASCADE programme. Investment of significantly greater resources in training, ongoing supervision of the techniques and administrative support is required. As is highlighted by the probabilistic sensitivity analysis, there is great uncertainty regarding the cost-effectiveness of the different interventions.
Practitioners can be trained to use a range of MI and SFBT techniques in the delivery of structured education, although some aspects were more difficult to maintain than others were. Limited use and adoption of specific activities and/or techniques by site educators suggested a lack of adequate education.
Skill levels in the use of behaviour change techniques were insufficient to impact on glycaemic control, unlike the outcomes of trials using intensive MI.
There was a failure to effect changes in the whole team approach owing to training only part of the clinical team. Greater involvement by consultants could have made them (1) more likely to use the techniques themselves when working with patients; (2) more engaged with the project and thus more sympathetic for site educators need for time to prepare sessions; and (3) have a better understanding of the problems with organising groups/uptake.
A complex, pragmatic intervention is deliverable and the integral PE has provided extensive data to identify necessary improvements in training and programme content. Key factors that influenced effective delivery included preparation in advance of delivering modules; confidence using the psychological approaches, having at least two site educators, site educators who could manage groups effectively and team having adequate resources to be able to offer comfortable, quiet venues with planned breaks. Factors that made delivery more challenging included delays between training and delivery of modules, small numbers or mixed age groups, not preparing or practising sessions in advance and a lack of experience or confidence in site educators.
Key messages that emerged from the PE that are important for the development of future psychosocial interventions include:
-
creating a sense of ownership in whole teams adopting the approach
-
creating solutions to the practical logistical challenges to organising the groups and making them accessible and attractive to those with the poorest glycaemic management
-
ensuring resources are available to provide clerical support to set up and organise the sending of invitations, making appropriate appointments, communicating with young people/parents/carers and reminding them of appointments, changing and rescheduling appointments, room hire and organising refreshments
-
offering better training in group working and management of group dynamics; clinicians are often poor at involving children in group conversations or managing ‘difficult individuals;206,207 children resent being ‘left out’ of discussions about their medical care208
-
a collaborative approach to who should be invited to the intervention in order to address the needs of different patient subgroups
-
training on how to engage families and young people in the process and recognition of the difficulties integrating diabetes into everyday life and embedding CASCADE philosophy in standard clinical education.
Implications for clinical services
Structured education packages, which are NICE compliant, are urgently needed, as well as effective communication skills training. It is essential that regulatory bodies emphasise the need for effective communication underlying high-quality clinical care for children with diabetes. 4,209 These skills need to be learned early on in professionals careers and/or as they enter the field of paediatric diabetes. Evidence for the effectiveness in changing difficult behaviour is provided in MI and SFBT research. 210
Consideration needs to be given to the practical organisation of structured education and alternative approaches alongside clinical appointments. Our experience suggests that additional voluntary appointments will not be taken up by those who are in the greatest need and are the most difficult to engage.
Resource implications for delivery of an effective structured education programme
The failure of CASCADE to impact on metabolic control suggests that alternative educational approaches should be pursued. Additional training is required to standardise both the educational level of PDSNs and behaviour change techniques and communication sills. Teams must ensure that they are adherent to evidence-based international guidelines in preference to preferred local delivery. Future training would demand that education was a key component of training to ensure adequate knowledge levels as well as providing training in communication and behaviour change skills with support in reinforcing and maintaining skills as suggested in the Enhanced CASCADE model.
Future developments
There is a demand for acceptable, deliverable and effective structured education in paediatric diabetes. The CASCADE study has provided an evidence base from which to develop future programmes. As highlighted by previous studies,123 a key consideration will be where responsibility lies for provision and regulation of such programmes (e.g. with NHS trusts or professional bodies, such as the Royal Colleges, the NMC or General Medical Council).
Additional support for practising delivery and reduced time lag between training and delivery were identified as key improvements. Some trainees thought they already used the skills taught. Many thought that professionally they should be practising in this manner. Clinicians often complain that the pressure of time in clinic demands a directive approach. However, they are also aware that this is not satisfactory, which is an important message and challenge for future researchers. 180,211
The challenge is to help practitioners see behaviour change and communication skills as a way of working differently rather than as tasks that need to be imposed on top of what is already done. Non-threatening ways to evaluate and feedback to clinicians on consultation skills are required ensuring that parents and children also participate in this process. The RCPCH is currently evaluating a patient-reported experience measure in all paediatric diabetes clinics in England and Wales. It is hoped this will enable the 29,000 children and young people living with diabetes to let clinicians know their thoughts about their clinic visits. 212
Some practitioners demonstrated particular determination in trying to apply and retain skills and it is essential to explore how to maintain skills in routine clinical practice in a cost-effective manner. Ongoing training of nurses (who provide the majority of day-to-day care in diabetes services) should be invested in with adequate administrative support provided to deliver the latest evidence-based service to their patients.
Implications for future research
There is widespread evidence for effectiveness of psychosocial interventions in diabetes. 97 The particular utility of MI/SFBT in diabetes has been highlighted although it is not effective in every setting. 213 Process research points to the central value of using reflective listening to evoke change talk, which, in turn, predicts behaviour change. 214 Researchers in paediatric diabetes might respond to this by following the approaches described by Channon et al. and/or Christie. 123
A better understanding of the cost-effectiveness of an educational intervention for this patient group will require data on the extent to which any resulting improvements in metabolic control are sustained in the future, and on the relationship between improvements in metabolic control and changes in the number and timing of diabetic complications.
The dilemma is whether we should be using counsellors or psychologists to deliver MI-linked evidence-based therapy with selected patients at a more individual level or persevering in our attempts to train different team members to deliver group interventions to the required standard. Perhaps the answer is that we need both.
The CASCADE groups were based on a programme delivered at UCLH by a psychologist and senior PDSN with training in MI in the context of a whole team practising the CASCADE philosophy. The creation of a deliverable manual and training programme to enable the trial to take place therefore by definition required a degree of dilution of the approach. There is a need to address how to help teams work effectively as part of the delivery of structured education programmes. The lack of consistent educational knowledge by practitioners must be addressed.
Pragmatic RCTs tend to demonstrate high external validity and assess effectiveness rather than efficacy. The intervention has been evaluated in ‘real-life’ and representative settings, with all of the challenges that this presents. Thus, it provides invaluable information on barriers and opportunities regarding future, similar interventions. A low dropout rate and good attendance for the subgroup that attended the intervention, as well as reported impact by this group, strengthens the findings of the study but, however, suggests there might be improved uptake if offered to young people with lower HbA1c level. Understanding why 37% chose not to attend is another important area.
Although acquiring mastery and maintaining skills is a focus of training, it is important to ensure that teams ‘sign up’ to the approach and are given adequate supervision to ensure that they continue to use the approach when things get difficult. Testing whether this approach can be more successful with a robust ongoing supervisory element should be a target of further research.
Finally, follow-up of HbA1c levels within the current trial cohort at 4 years may help to address whether reported changes in psychosocial outcomes and self-management behaviours have resolved into subsequent differences in glycaemic control.
Acknowledgements
Contributions of authors
All of the following named authors contributed to the development of the research question and study design, study implementation (including membership of study management group), analysis and/or interpretation of data and submission of the final report.
Contributions to individual study outputs/particular study contributions are noted below.
Dr Deborah Christie (Consultant Clinical Psychologist) Chief Investigator, lead applicant, the study guarantor. Led the development of research question and study design, study implication and report submission, and codeveloped the intervention, provided training in intervention delivery.
Rebecca Thompson (Consultant Nurse) Co-applicant. Codeveloped the intervention, provided training in intervention delivery.
Mary Sawtell (Research Officer) Project manager from September 2010 until the end of the study. Involved in analysis and writing-up of the PE.
Dr Elizabeth Allen (Statistician) Co-applicant. Completed the quantitative analysis.
Professor John Cairns (Professor of Health Economics) Co-applicant. Contributed to initial study design and conducted health-economic analysis.
Professor Felicity Smith (Professor of Pharmacy Practice) Co-applicant. Contributed to initial study design, in particular approaches to, and instruments for, the PE. She was involved in discussions throughout the project on the progress of the PE and assisted with the analysis.
Dr Elizabeth Jamieson (Research Officer). Conducted all processes involved in outcome and PE data collection. Carried out analysis of the PE data.
Dr Katrina Hargreaves (Research Officer). Conducted all processes involved in outcome and PE data collection. Carried out analysis of the PE data.
Anne Ingold (Research Officer). Conducted all processes involved in outcome and PE data collection. Carried out analysis of the PE data.
Lucy Brooks (Data Manager/Trial Manager). Managed the LSHTM database and collected CNR data.
Meg Wiggins (Senior Research Officer). Involved in design of the study materials and the obtaining of ethics and research and development approvals. Involved in analysis and writing-up of the PE.
Professor Sandy Oliver (Professor of Pubic Policy) Co-applicant. Contributed to initial study design. Designed materials to invite informed consent and elicited patient views on questionnaire design.
Dr Rebecca Jones (Medical Statistician). Conducted quantitative data analysis (from September 2012).
Professor Diana Elbourne (Professor of Healthcare Evaluation) Co-applicant. Contributed to initial study design.
Dr Andreia Santos (Lecturer, Health Economics). Conducted analysis for health economics chapter (from September 2012).
Professor Ian Chi Kei Wong (Professor, Department of Pharmacology and Pharmacy) Co-applicant. Contributed to initial study design.
Simon O’Neill (Diabetes UK, Director of Health Intelligence) Co- applicant. Contributed to initial study design and provided patient and professional policy perspective.
Dr Vicki Strange (Senior Research Officer) Co-applicant. Contributed to initial study design. Primary responsibility for design of the integral PE. Project manager until August 2010.
Professor Peter Hindmarsh (Professor of Paediatric Endocrinology) Co-applicant. Contributed to initial study design. Specific support for clinic recruitment.
Francesca Annan (Paediatric Diabetes Dietician) Co-applicant. Contributed to development of intervention.
Professor Russell Viner (Professor of Adolescent Health) Co-applicant. Contributed to initial study design and gave specific advice in relation to support for recruitment.
The report authors gratefully acknowledge the substantial contribution of the following in the conduct and delivery of the CASCADE study.
Administrative, methodological and technical support
Ben Weiner, Hasina Khatun, Susie Colville, Izzy Girling, Kate Davenport, Christina Bull (Assistant Psychologists, UCLH), Dr Meena Khatwa, Veena Methoo, Claire Planner, Carol Ann Vigurs [Research Officers, Social Sciences Research Unit (SSRU/SOP)], Christine Delaney (Transcriber, SSRU). Dr Shazia Basaria, Rosie Davies (Sessional Researchers, SSRU), Mike Bennett, Joe Strachan (Database Support, LSHTM), Korotimi Diallo, Elly Morris, Paulo Ramos (Extra Data Processing, LSHTM) and Joanna Sturgess (General Trial Support, LSHTM).
Members of the Trial Steering Committee
Professor David Dunger (Chair), Professor Jacqueline Barnes and Professor Tim Cole.
Members of the Data Monitoring and Ethics Committee
Professor Chris Kelnar (Chair), Professor Darren Ashcroft, Professor Robert Coe and Dr Chris Patterson.
Participating clinical centres and research networks
The local principal investigators, members of each clinical team and local UK Clinical Research Network research staff and all R&D departments participating in the 28 trial centres.
Dr Naeem Ahmad (Consultant Paediatrician), Diane Cluley (PDSN) and Marie Sparrow (Clinic Nurse) at Alexandra Hospital (Redditch); Beverley Anderson (PDSN), Dr Mark Cohen (Consultant in Endocrinology and Diabetes), Anuja Gamegedura (Phlebotomist), Julie Greenwood (Receptionist), Dr Vaseem Hakeem (Consultant Paediatrician), Cher Johnson (Liaison Nurse, Child Psychiatry) and Sarah Winter (Secretary) at Barnet & Chase Farm Hospital.
Julia Brown (PDSN), Sheena Hawkes (Paediatric Phlebotomist), Clare Keech (Endocrine Nurse), Dr Mira Leigh (Consultant Paediatrician), Julia Turner (Family Care Assistant) and Marielle Vallette (Paediatric Dietitian) at Bedford Hospital.
Emily Benson (Clinical Research Facilitator), Indy Birak (Clinical Research Nurse/West Midlands MCRN) and Dr Sam Heyton (West Midlands MCRN) at Birmingham Children’s Hospital.
Nicola Getlevog (PDSN), Dr Swati Karandikar (Consultant Paediatrician), Elaine Keegan (PDSN), Jodie Owen (Dietitian) and Dr Stephen Rose (Consultant Paediatrician) at Birmingham Heartlands Hospital.
Dr Alok Gupta (Consultant Paediatrician), Dr Mohamed Ismail (Consultant, Adolescent and Adult Diabetes Services), Jackie Simister (Paediatric Specialist Nurse) and Craig Ticehurst (Paediatric Specialist Nurse) at Darent Valley Hospital.
Susan Beames (Research Nurse Paediatrics), Kate Dembenski (PDSN), Jill Gethin (PDSN) and Dr Susan Matthai (Consultant Paediatrician) at Gloucestershire Royal Hospital.
Bev Simmons (Children’s Diabetes Nurse) at Grantham Hospital.
Lorraine Hodsdon (Clinical Research Manager) at Great Ormond St Hospital.
Tracey Fallows (PDSN) and Jo Hankey (PDSN) at Hanford Health Centre.
Dr Joanne Baker (Consultant Paediatrician), Jennifer Brunsdon (PDSN), Carol Marsh (Receptionist), Sheena McIntosh (Dietitian) and June Tjerkstra (Secretary) at Kent and Canterbury Hospital.
Rebekah Ford (Dietitian), Sarah Hodgkinson (Senior Paediatric Staff Nurse), Kate Johnson (Paediatric Link Nurse), Lynsey Judd (Paediatric Research Nurse), Helen Marsh (PDSN), Maria Meyrick (Emotional Health Practitioner) and Dr Ursula Ngwu (Consultant Paediatrician) at Kings Mill Hospital.
Emily Cavell Clarke (Paediatric Diabetes Liaison Nurse), Barbara Clarke (Phlebotomist), Jane Gwynne (Clinical Nurse Specialist Paediatric Diabetes), Dr Vinayak Pai (Consultant Paediatrician), Sue Thomas (Dietitian) and Cindy Wood (Health Care Assistant) at Kingston Hospital.
Katy McMillan (Dietitian), Caroline Pearce (Health Care Support Worker), Amanda Roper (Children’s Research Office), Dr Dougie Thomas (Consultant Paediatrician) and Helen Warhurst (Children’s Diabetes Nurse) at Lincoln County Hospital; Gemma Barry and Adrian Green (Children’s Research Nurses) at London and South East MCRN.
Sultana Begum (Assistant Paediatric Secretary), Sarah Edwards (Dietitian), Carol Hynes (Paediatric Nurse), Karen McClurg (Nursery Nurse), Denise Morrison (PDSN), Julie Murphy (Secretary), Dr Nisha Nathwani (Consultant Paediatrician), Meeta Patel (PDSN), Dr Tariq Rehman (Consultant Endocrinologist), Annie Roxas (Paediatric Nurse), Angela Stafford (Secretary) and Hayley Tack (Secretary) at Luton and Dunstable Hospital.
Jenny Endean (PDSN), Ann Hinton (Lead Phlebotomist) and Dr Kala Pathy (Consultant Paediatrician) at Maidstone Hospital.
Carol Boorman (Secretary), Louise La Plage (PDSN), Dr Asankha Ranasinghe (Consultant Paediatrician), Nicola Richards (PDSN), Dr Paul Williams (Consultant Paediatrician) and Melissa Wooley (Secretary) at Medway Maritime Hospital.
Sue Appleby (Receptionist), Dr James Bursell (Consultant Paediatrician), Jackie Higgins (Lead PDSN) and Gill Tatum (Phlebotomy) at Milton Keynes Hospital.
Dr Anjum Bahadur (Paediatric Registrar), Jenny Hurley (PDSN), Dr Abdul Moodambail (Consultant Paediatrician) and Vimmy Venugopal (Paediatric Registrar) at Newham University Hospital.
Marie Phipps (Clinical Research Facilitator) at The North Staffordshire Medical Institute.
Lucy Gilmour (Research nurse) at North West London Diabetes Research Network; Anna Frost (MCRN) at Nottingham Integrated Clinical Research Centre.
Penny Erskine (East MCRN, Regional Lead South Yorkshire & Trent) at Nottingham University Hospitals NHS Trust.
Kirsty Henderson (Secretary) at Paula Carr Centre, William Harvey Hospital.
Dr Zafar Ahmed (Consultant Paediatrician), Emma Boole (PDSN), Katie Butler (Paediatric Diabetes Nurse), Sarah Davis (PDSN), Lezanne Niehaus (Dietitian) and Anne Prestt (Lead Nurse for Diabetes) at Pilgrim Hospital.
Anne Kelly (Community nurse), Dr Awais Khan (Consultant Paediatrician), Lesley Simpson (PDSN) and Katie Ward (MCRN) at Queen Elizabeth Hospital.
Dr Jacob Eyers (Consultant Paediatrician), Jan Kalra (PDSN), Sarah Putney (PDSN), Queen Mary’s Hospital.
Dr Mansoor Ahmed (Consultant Paediatrician), Stephanie Boswell (Clinical Research Sister), Jane Humphries (PDSN), Jane Maiden (Clinical Research Sister), Clare Mewies (Clinical Trials Co-ordinator), Jane Reynolds (Research Nurse) and Dr Jacob Samuel (Consultant Paediatrician) at Queen’s Hospital (Burton).
Dr Kausik Banerjee (Consultant Paediatrician), Diane Harvey-Coggans (Paediatric Specialist Nurse), Emily Quek (Dietitian) and Dr Judit Szollar (Consultant Paediatrician) at Queen’s Hospital (Romford).
Katie Burns (Community Paediatric Nurse), Caroline Byrne (Community Nurse), Dr Victoria Dublon (Consultant Paediatrician) and Anthony Geraets (Administrator) at Royal Free Hospital.
Dr Jeremy Allgrove (Consultant Paediatrician), Freya Brown (PDSN), Sharanjit Cheema (PDSN) and Claire Wyatt (PDSN) at The Royal London Hospital; Dr Atanu Dutta (Consultant Paediatrician), Debbie Hammond (PDSN), Michelle Parker (Paediatric Diabetes Nurse) and Belinda Sparkes (Senior Staff Nurse) at Stoke Mandeville Hospital.
Pamela Coward (Research Nurse) and Annette Williams (Senior Research Nurse) at Thames Valley Research Network; Dr Gill Rumsby (Consultant Biochemist) at Special Biochemistry, University College London Hospital.
Jacqui Daglish (Senior Clinical Research Facilitator) and Fiona Thompson (Clinical Research Facilitator) at University Hospitals Coventry and Warwickshire NHS Trust.
Janet Hagan (Clinic Manager), Dr Parakkal Rafeeq (Consultant Paediatrician) and Dr Tricia Smith (Consultant) at University Hospital of North Staffordshire.
Sharon Baker (Secretary), Ruth Bayram (PDSN), Cathy Butler (Outpatient Sister), Sharon Buckingham (Secretary), Kay Chandler (Receptionist), Dr Thomas Galiford (Consultant in General Internal Medicine, Endocrinology and Diabetes), Dr Lola Harold-Sodipo (Staff Grade Consultant), Dr Chantal Kong (Consultant in General Internal Medicine, Endocrinology and Diabetes), Flavia Marcus (PDSN), Diane McLaughlin (Directory of Services Manager), Abbie Mitchell (PDSN), Dr Heather Mitchell (Consultant Paediatrician), Heather Norris (Receptionist), Dr Arla Ogilvie (Consultant in General Internal Medicine, Endocrinology and Diabetes), Sue Perkins (Administrator), Liz Searle (Secretary), Nadene Short (Phlebotomy Manager), Barbara Sherlock (Secretary) and Chris Tominey (Supervisor) at West Hertfordshire Hospitals NHS Trust
Jane Edwards (PDSN), Lee Potiphar (DRN) and Dr Jayanti Rangasami (Consultant Paediatrician) at West Middlesex University Hospital.
Jan Dent (Sister in Charge Children’s Clinic), Dr Zilla Huma (Consultant Paediatrician), Helen Timms (Paediatric Specialist Nurse) and Diana Yardley (Advanced Nurse Practitioner in Paediatric Diabetes) at Wexham Park Hospital.
Dr Paramita Cifeeli (Consultant Paediatrician), Lynne Ellis (PDSN), Mabeh Fang (Paediatric Research Nurse), Teresa O’Donoghue (Clinic Receptionist), Jackie Pratchett-Pearce (Deputy Supervisor Phlebotomy Department) and Dr Justine Rweyemanu (Consultant) at Whipps Cross University Hospital.
Esther Harrison (PDSN), Terry Martin (Senior Programme Research Nurse CLRN), Sarah Phillip (Sister of Children’s Outpatients) and Dr John Scanlon (Consultant Paediatrician) at Worcestershire Royal Hospital.
Kausar Hassan (PDSN) and Dr Michelle Russell-Taylor (Consultant Paediatrician) at Wycombe Hospital.
User representative
We particularly acknowledge the dedicated input of our patient representative Ms Cassandra Solomon (co-applicant) in particular during the development phase of the study. Katy Sutcliffe (mother of a nine-year-old with diabetes, researcher and doctoral student) and Rachel Connor (Research Relations Manager, Juvenile Diabetes Research Foundation) both commented on the questionnaires from the perspective of service users.
Institutional support
We acknowledge, with thanks, the trial funders, the UK National Institute for Health Research Health Technology Assessment (NIHR HTA) programme.
We thank all those not otherwise mentioned above who have contributed to the CASCADE study.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Growing Up With Diabetes: Children and Young People with Diabetes in ENGLAND. London: RCPCH; 2009.
- EURODIAB ACE. Study Group. Variation and trends in incidence of childhood diabetes in Europe. Lancet 2000;355:873-6.
- Viner RM, Hindmarsh PC, Taylor B, Cole TJ. Childhood body mass index (BMI), breastfeeding and risk of Type 1 diabetes: findings from a longitudinal national birth cohort. Diabet Med 2008;25:1056-61. http://dx.doi.org/10.1111%2Fj.1464-5491.2008.02525.x.
- Diagnosis and Management of Type 1 Diabetes in Children, Young People and Adults. London; 2004.
- National Paediatric Diabetes Audit 2010–2011. London: RCPCH; 2012.
- Pinkey JH, Bingley PJ, Sawtell PA, Dunger DB, Gale EA. Presentation and progress of childhood diabetes mellitus: a prospective population-based study. The Bart’s-Oxford Study Group. Diabetologia 1994;37:70-4.
- Ali K, Wilson IV, Edge JA, Bingley PJ. Diabetic Ketoacidosis at diagnosis has not declined in children over the last 20 years: data from the Bart’s-Oxford Study, 2009, SD18. Diabet Med 2009;26.
- Griffin A, Christie D, Christie D, Martin C. Psychosocial Aspects of Diabetes: Children, Adolescents and Their Families. London: Radcliffe Publishing; 2012.
- Bryden KS, Dunger DB, Mayou RA, Peveler RC, Neil HA. Poor prognosis of young adults with type 1 diabetes: a longitudinal study. Diabet Care 2003;26:1052-7. http://dx.doi.org/10.2337%2Fdiacare.26.4.1052.
- Donaghue KC, Chiarelli F, Trotta D, Allgrove J, Dahl-Jorgensen K. Microvascular and macrovascular complications associated with diabetes in children and adolescents. Pediatr Diabet 2009;10:195-203. http://dx.doi.org/10.1111%2Fj.1399-5448.2009.00576.x.
- Monnier L, Colette C, Owens D. The glycaemic triumvirate and diabetic complications: is the whole greater than the sum of its component parts?. Diabet Res Clin Pract 2012;95:303-11. http://dx.doi.org/10.1016%2Fj.diabres.2011.10.014.
- Diabetes Control and Complications Trial (DCCT) Group . The effect of intensive diabetes treatment on the development and progression of long-term complications in insulin dependent diabetes mellitus. The Diabetes Control and Complications Trial. N Engl J Med 1993;329:977-86.
- Diabetes Control and Complications Trial (DCCT) Group . The effect of intensive diabetes therapy on the development and progression of neuropathy. The Diabetes Control and Complications Trial Research Group. Ann Intern Med 1995;122:561-8.
- White N, Cleary P, Dahms W, Goldstein D, Malone J, Tamborlane W. Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Pediatr 2001;139:804-12.
- Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010;375:743-51. http://dx.doi.org/10.1016%2FS0140-6736%2809%2961998-X.
- Kumareswaran K, Elleri D, Allen JM, Harris J, Xing D, Kollman C, et al. Meta-analysis of overnight closed-loop randomized studies in children and adults with type 1 diabetes: the Cambridge cohort. J Diabet Sci Technol 2011;5:1352-62.
- Shalitin S, Peter CH. Diabetes technology and treatments in the paediatric age group. Int J Clin Pract Suppl 2011;170:76-82. http://dx.doi.org/10.1111%2Fj.1742-1241.2010.02582.x.
- Sussman A, Taylor EJ, Patel M, Ward J, Alva S, Lawrence A, et al. Performance of a glucose meter with a built-in automated bolus calculator versus manual bolus calculation in insulin-using subjects. J Diabet Sci Technol 2012;6:339-44.
- Slover RH. Continuous glucose monitoring in children and adolescents. Curr Diab Rep 2012;12:510-16. http://dx.doi.org/10.1007%2Fs11892-012-0303-6.
- Hirose M, Beverly EA, Weinger K. Quality of Life and Technology: Impact on Children and Families With Diabetes. Curr Diab Rep 2012;12:711-20. http://dx.doi.org/10.1007%2Fs11892-012-0313-4.
- Mauras N, Fox L, Englert K, Beck RW. Continuous glucose monitoring in type 1 diabetes. Endocrine 43:41-50. http://dx.doi.org/10.1007%2Fs12020-012-9765-1.
- Harris MA, Hood KK, Mulvaney SA. Pumpers, skypers, surfers and texters: technology to improve the management of diabetes in teenagers. Diabet Obes Metab 2012;14:967-72. http://dx.doi.org/10.1111%2Fj.1463-1326.2012.01599.x.
- Cafazzo JA, Casselman M, Hamming N, Katzman DK, Palmert MR. Design of a mHealth app for the self-management of adolescent type 1 diabetes: a pilot study. J Med Internet Res 2012;14. http://dx.doi.org/10.2196%2Fjmir.2058.
- Froisland DH, Arsand E, Skarderud F. Improving diabetes care for young people with type 1 diabetes through visual learning on mobile phones: mixed-methods study. J Med Internet Res 2012;14. http://dx.doi.org/10.2196%2Fjmir.2155.
- Mulvaney SA, Rothman RL, Wallston KA, Lybarger C, Dietrich MS. An internet-based program to improve self-management in adolescents with type 1 diabetes. Diabet Care 2010;33:602-4. http://dx.doi.org/10.2337%2Fdc09-1881.
- Mulvaney SA, Rothman RL, Osborn CY, Lybarger C, Dietrich MS, Wallston KA. Self-management problem solving for adolescents with type 1 diabetes: intervention processes associated with an Internet program. Patient Educ Couns 2011;85:140-2. http://dx.doi.org/10.1016%2Fj.pec.2010.09.018.
- Mulvaney SA, Rothman RL, Dietrich MS, Wallston KA, Grove E, Elasy TA, et al. Using mobile phones to measure adolescent diabetes adherence. Health Psychol 2012;31:43-50. http://dx.doi.org/10.1037%2Fa0025543.
- Kumar VS, Wentzell KJ, Mikkelsen T, Pentland A, Laffel LM. The DAILY (Daily Automated Intensive Log for Youth) trial: a wireless, portable system to improve adherence and glycemic control in youth with diabetes. Diabet Technol Ther 2004;6:445-53. http://dx.doi.org/10.1089%2F1520915041705893.
- Hanauer DA, Wentzell K, Tovar A, Zeuhlke J, Kumar V, Laffel LM. Parent and youth assessments of a handheld wireless device to enhance diabetes mellitus management. Arch Pediatr Adolesc Med 2006;160. http://dx.doi.org/10.1001%2Farchpedi.160.3.321-a.
- Brown SJ, Lieberman DA, Germeny BA, Fan YC, Wilson DM, Pasta DJ. Educational video game for juvenile diabetes: results of a controlled trial. Med Inform (London) 1997;22:77-89. http://dx.doi.org/10.3109%2F14639239709089835.
- Williamson S. The best model of care for children and young people with diabetes. J R Coll Physicians Edinburgh 2010;40:25-32. http://dx.doi.org/10.4997%2FJRCPE.2010.S04.
- Wysocki T, Taylor A, Hough BS, Linscheid TR, Yeates KO, Naglieri JA. Deviation from developmentally appropriate self-care autonomy. Association with diabetes outcomes. Diabet Care 1996;19:119-25. http://dx.doi.org/10.2337%2Fdiacare.19.2.119.
- Helgeson VS, Reynolds KA, Siminerio L, Escobar O, Becker D. Parent and adolescent distribution of responsibility for diabetes self-care: links to health outcomes. J Pediatr Psychol 2008;33:497-508. http://dx.doi.org/10.1093%2Fjpepsy%2Fjsm081.
- Ellis DA, Yopp J, Templin T, Naar-King S, Frey MA, Cunningham PB, et al. Family mediators and moderators of treatment outcomes among youths with poorly controlled type 1 diabetes: results from a randomized controlled trial. J Pediatr Psychol 2007;32:194-205. http://dx.doi.org/10.1093%2Fjpepsy%2Fjsj116.
- Christie D, Viner R. Adolescent development. BMJ 2005;330:301-4. http://dx.doi.org/10.1136%2Fbmj.330.7486.301.
- Grey M, Cameron ME, Thurber FW. Coping and adaptation in children with diabetes. Nurs Res 1991;40:144-9. http://dx.doi.org/10.1097%2F00006199-199105000-00004.
- Grey M, Davidson M, Boland EA, Tamborlane WV. Clinical and psychosocial factors associated with achievement of treatment goals in adolescents with diabetes mellitus. J Adolesc Health 2001;28:377-85. http://dx.doi.org/10.1016%2FS1054-139X%2800%2900211-1.
- Grey M, Whittemore R, Tamborlane W. Depression in type 1 diabetes in children: natural history and correlates. J Psychosom Res 2002;53:907-11.
- Grylli V, Hafferl-Gattermayer A, Wagner G, Schober E, Karwautz A. Eating disorders and eating problems among adolescents with type 1 diabetes: exploring relationships with temperament and character. J Pediatr Psychol 2005;3:197-206. http://dx.doi.org/10.1093%2Fjpepsy%2Fjsi007.
- Helgeson VS, Siminerio L, Escobar O, Becker D. Predictors of metabolic control among adolescents with diabetes: a 4-year longitudinal study. J Pediatr Psychol 2009;34:254-70. http://dx.doi.org/10.1093%2Fjpepsy%2Fjsn079.
- Herzer M, Hood KK. Anxiety symptoms in adolescents with type 1 diabetes: association with blood glucose monitoring and glycemic control. J Pediatr Psychol 2010;35:415-25. http://dx.doi.org/10.1093%2Fjpepsy%2Fjsp063.
- McGrady A, Brennan J, Lynch D. Effects of wellness programs in family medicine. Appl Psychophysiol Biofeedback 2009;34:121-6. http://dx.doi.org/10.1007%2Fs10484-009-9084-3.
- Hislop AL, Fegan PG, Schlaeppi MJ, Duck M, Yeap BB. Prevalence and associations of psychological distress in young adults with Type 1 diabetes. Diabet Med 2008;25:91-6. http://dx.doi.org/10.1111%2Fj.1464-5491.2007.02310.x.
- Reynolds KA, Helgeson VS. Children with diabetes compared to peers: depressed? Distressed? A meta-analytic review. Ann Behav Med 2011;42:29-41. http://dx.doi.org/10.1007%2Fs12160-011-9262-4.
- Gannoni AF, Shute RH. Parental and child perspectives on adaptation to childhood chronic illness: a qualitative study. Clin Child Psychol Psychiatr 2010;15:39-53. http://dx.doi.org/10.1177%2F1359104509338432.
- Lowes L, Lyne P. A normal lifestyle: parental stress and coping in childhood diabetes. Br J Nurs 1999;8:133-9.
- Lowes L, Lyne P. Chronic sorrow in parents of children with newly diagnosed diabetes: a review of the literature and discussion of the implications for nursing practice. J Adv Nurs 2000;32:41-8. http://dx.doi.org/10.1046%2Fj.1365-2648.2000.01418.x.
- Bowes S, Lowes L, Warner J, Gregory JW. Chronic sorrow in parents of children with type 1 diabetes. J Adv Nurs 2009;65:992-1000. http://dx.doi.org/10.1111%2Fj.1365-2648.2009.04963.x.
- Delamater AM, Kurtz SM, White NH, Santiago JV. Effects of social demand on reports of self-monitored blood glucose in adolescents with Type 1 diabetes mellitus. J Appl Soc Psychol 1988;18:491-502. http://dx.doi.org/10.1111%2Fj.1559-1816.1988.tb00031.x.
- Schmidt LE, Klover RV, Arfken CL, Delamater AM, Hobson D. Compliance with dietary prescriptions in children and adolescents with insulin-dependent diabetes mellitus. J Am Diet Assoc 1992;92:567-70.
- Kurtz SM. Adherence to diabetes regimens: empirical status and clinical applications. Diabet Educ 1990;16:50-9. http://dx.doi.org/10.1177%2F014572179001600112.
- Peveler RC, Bryden KS, Neil HAW, Fairburn CG, Mayou RA, Dunger DB, et al. The relationship of disordered eating habits and attitudes to clinical outcomes in young females with type 1 diabetes. Diabet Care 2005;28:84-8. http://dx.doi.org/10.2337%2Fdiacare.28.1.84.
- Bryden KS, Neil A, Mayou RA, Peveler RC, Fairburn CG, Dunger DB. Eating habits, body weight, and insulin misuse. A longitudinal study of teenagers and young adults with type 1 diabetes. Diabet Care 1999;22:56-60. http://dx.doi.org/10.2337%2Fdiacare.22.12.1956.
- Neumark-Sztainer D, Story M, Toporoff E, Cassuto N, Resnick MD, Blum RW. Psychosocial predictors of binge eating and purging behaviours among adolescents with and without diabetes mellitus. J Adolesc Health 1996;19:289-96. http://dx.doi.org/10.1016%2FS1054-139X%2896%2900082-1.
- Schwartz SA, Weissberg-Benchell J, Perlmuter LC. Personal control and disordered eating in female adolescents with type 1 diabetes. Diabet Care 2002;25:1987-91. http://dx.doi.org/10.2337%2Fdiacare.25.11.1987.
- Weissberg-Benchell J, Glasgow AM, Tynan WD, Wirtz P, Turek J, Ward J. Adolescent diabetes management and mismanagement. Diabet Care 1995;18:77-82. http://dx.doi.org/10.2337%2Fdiacare.18.1.77.
- Delamater AM, Davis SG, Bubb J, Santiago JV, Smith JA, White NH. Self-monitoring of blood glucose by adolescents with diabetes: technical skills and utilization of data. Diabet Educ 1989;15:56-61. http://dx.doi.org/10.1177%2F014572178901500115.
- Jacobson AM, Hauser ST, Willett JB, Wolfsdorf JI, Dvorak R, Herman L, et al. Psychological adjustment to IDDM: 10-year follow-up of an onset cohort of child and adolescent patients. Diabet Care 1997;20:811-18. http://dx.doi.org/10.2337%2Fdiacare.20.5.811.
- Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med 1986;315:215-19.
- La Greca AM, Mackey ER, Roberts MC, Steele RG. Handbook of Pediatric Psychology. New York, NY: The Guilford Press; 2009.
- Hauser ST, Jacobson AM, Lavori P, Wolfsdorf JI, Herskowitz RD, Milley JE, et al. Adherence among children and adolescents with insulin-dependent diabetes mellitus over a four-year longitudinal follow-up: II. Immediate and long-term linkages with the family milieu. J Pediatr Psychol 1990;15:527-42. http://dx.doi.org/10.1093%2Fjpepsy%2F15.4.527.
- Ellis DA, Podolski CL, Frey M, Naar-King S, Wang B, Moltz K. The role of parental monitoring in adolescent health outcomes: impact on regimen adherence in youth with type 1 diabetes. J Pediatr Psychol 2007;32:907-17. http://dx.doi.org/10.1093%2Fjpepsy%2Fjsm009.
- Skinner TC, John M, Hampson SE. Social support and personal models of diabetes as predictors of self-care and well-being: a longitudinal study of adolescents with diabetes. J Pediatr Psychol 2000;25:257-67. http://dx.doi.org/10.1093%2Fjpepsy%2F25.4.257.
- Bobrow ES, Avruskin TW, Siller J. Mother–daughter interaction and adherence to diabetes regimens. Diabet Care 1985;8:146-51. http://dx.doi.org/10.2337%2Fdiacare.8.2.146.
- Lewandowski A, Drotar D. The relationship between parent-reported social support and adherence to medical treatment in families of adolescents with type 1 diabetes. J Pediatr Psychol 2007;32:427-36. http://dx.doi.org/10.1093%2Fjpepsy%2Fjsl037.
- Miller-Johnson S, Emery RE, Marvin RS, Clarke W, Lovinger R, Martin M. Parent–child relationships and the management of insulin-dependent diabetes mellitus. J Consult Clin Psychol 1994;62:603-10.
- Wysocki T, Iannotti R, Weissberg-Benchell J, Laffel L, Hood K, Anderson B, et al. Diabetes problem solving by youths with type 1 diabetes and their caregivers: measurement, validation, and longitudinal associations with glycemic control. J Pediatr Psychol 2008;33:875-84. http://dx.doi.org/10.1093%2Fjpepsy%2Fjsn024.
- Brownlee-Duffeck M, Peterson L, Simonds JF, Goldstein D, Kilo C, Hoette S. The role of health beliefs in the regimen adherence and metabolic control of adolescents and adults with diabetes mellitus. J Consult Clin Psychol 1987;55:139-44. http://dx.doi.org/10.1037%2F%2F0022-006X.55.2.139.
- Bond G, Aiken L, Somerville S. The health belief model and adolescents with insulin-dependent diabetes mellitus. Health Psychol 2012;11:190-6. http://dx.doi.org/10.1037%2F%2F0278-6133.11.3.190.
- Littlefield CH, Craven JL, Rodin GM, Daneman D, Murray MA, Rydall AC. Relationship of self-efficacy and binging to adherence to diabetes regimen among adolescents. Diabet Care 1992;15:90-4. http://dx.doi.org/10.2337%2Fdiacare.15.1.90.
- Jacobson AM, Hauser ST, Lavori P, Wolfsdorf JI, Herskowitz RD, Milley JE, et al. Adherence among children and adolescents with insulin-dependent diabetes mellitus over a four-year longitudinal follow-up: I. The influence of patient coping and adjustment. J Pediatr Psychol 1990;15:511-26.
- La Greca AM, Bearman KJ. The diabetes social support questionnaire-family version: evaluating adolescents’ diabetes-specific support from family members. J Pediatr Psychol 2002;27:665-79.
- Helgeson VS, Snyder PR, Escobar O, Siminerio L, Becker D. Comparison of adolescents with and without diabetes on indices of psychosocial functioning for three years. J Pediatr Psychol 2007;32:794-806. http://dx.doi.org/10.1093%2Fjpepsy%2Fjsm020.
- Thomas AM, Peterson L, Goldstein D. Problem solving and diabetes regimen adherence by children and adolescents with IDDM in social pressure situations: A reflection of normal development. J Pediatr Psychol 1997;22:541-61. http://dx.doi.org/10.1093%2Fjpepsy%2F22.4.541.
- Murphy L, Thompson RJ, Morris MA. Adherence behavior among adolescents with type 1 insulin dependent diabetes: the role of cognitive processes. J Pediatr Psychol 1997;22:811-25.
- Hains AA, Berlin KS, Davies WH, Smothers MK, Sato AF, Alemzadeh R. Attributions of adolescents with type 1 diabetes related to performing diabetes care around friends and peers: the moderating role of friend support. J Pediatr Psychol 2007;32:561-70. http://dx.doi.org/10.1093%2Fjpepsy%2Fjsl040.
- Hains AA, Berlin KS, Davies WH, Parton EA, Alemzadeh R. Attributions of adolescents with type 1 diabetes in social situations: relationship with expected adherence, diabetes stress, and metabolic control. Diabet Care 2006;29:818-22. http://dx.doi.org/10.2337%2Fdiacare.29.04.06.dc05-1828.
- Hanson CL, Cigrang JA, Harris MA, Carle DL, Relyea G, Burghen GA. Coping styles in youths with insulin-dependent diabetes mellitus. J Consult Clin Psychol 1989;57:644-51. http://dx.doi.org/10.1037%2F%2F0022-006X.57.5.644.
- Hanson CL, Henggeler SW, Harris MA, Burghen GA, Moore M. Family system variables and the health status of adolescents with insulin-dependent diabetes mellitus. Health Psychol 1989;8:239-53. http://dx.doi.org/10.1037%2F%2F0278-6133.8.2.239.
- Hains AA, Davies WH, Parton E, Totka J, Moroso-Camarata J. A stress management intervention for adolescents with type 1 diabetes. Diabet Educ 2000;26:417-24.
- Boardway RH, Delamater AM, Tomakowsky J, Gutai JP. Stress management training for adolescents with diabetes. J Pediatr Psychol 1993;18:29-45. http://dx.doi.org/10.1093%2Fjpepsy%2F18.1.29.
- Anderson BJ, Wolf FM, Burkhart MT, Cornell RG, Bacon GE. Effects of peer-group intervention on metabolic control of adolescents with IDDM. Randomized outpatient study. Diabet Care 1989;12:179-83. http://dx.doi.org/10.2337%2Fdiacare.12.3.179.
- Greco P, Pendley JS, McDonell K, Reeves G. A peer group intervention for adolescents with type 1 diabetes and their best friends. J Pediatr Psychol 2001;26:485-90. http://dx.doi.org/10.1093%2Fjpepsy%2F26.8.485.
- Cook S, Herold K, Edidin DV, Briars R. Increasing problem solving in adolescents with type 1 diabetes: the choices diabetes program. Diabet Educ 2002;28:115-24. http://dx.doi.org/10.1177%2F014572170202800113.
- Grey M, Boland EA, Davidson M, Yu C, Tamborlane WV. Coping skills training for youths with diabetes on intensive therapy. Appl Nurs Res 1999;12:3-12. http://dx.doi.org/10.1016%2FS0897-1897%2899%2980123-2.
- Grey M, Boland EA, Davidson M, Li J, Tamborlane WV. Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. J Pediatr 2000;137:107-13. http://dx.doi.org/10.1067%2Fmpd.2000.106568.
- Grey M, Berry D. Coping skills training and problem solving in diabetes. Curr Diabet Rep 2004;4:126-31. http://dx.doi.org/10.1007%2Fs11892-004-0068-7.
- Karaguzel G, Bircan I, Erisir S, Bundak R. Metabolic control and educational status in children with type 1 diabetes: effects of a summer camp and intensive insulin treatment. Acta Diabetol 2005;42:156-61. http://dx.doi.org/10.1007%2Fs00592-005-0196-9.
- Misuraca A, Di GM, Lioniello M, Duval M, Aloi G. Summer camps for diabetic children: an experience in Campania, Italy. Diabet Res Clin Pract 1996;32:91-6. http://dx.doi.org/10.1016%2F0168-8227%2896%2901219-3.
- Delamater AM, Jacobson AM, Anderson B, Cox D, Fisher L, Lustman P, et al. Psychosocial therapies in diabetes: report of the Psychosocial Therapies Working Group. Diabet Care 2001;24:1286-92. http://dx.doi.org/10.2337%2Fdiacare.24.7.1286.
- Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al. Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review. Health Technol Assess 2001;5.
- Rapoff MA. Adherence to Pediatric Medical Regimens. New York, NY: Kluwer Academic; 1999.
- Murphy HR, Rayman G, Skinner TC. Psycho-educational interventions for children and young people with type 1 diabetes. Diabet Med 2006;23:935-43. http://dx.doi.org/10.1111%2Fj.1464-5491.2006.01816.x.
- DeWit M, Delemarre-van de Waal HA, Bokma JA, Haasnoot K, Houdijk MC, Gemke RJ, et al. Follow-up results on monitoring and discussing health-related quality of life in adolescent diabetes care: benefits do not sustain in routine practice. Pediatr Diabet 2010;11:175-81.
- DeWit M, Delemarre-van de Waal HA, Bokma JA, Haasnoot K, Houdijk MC, Gemke RJ, et al. Monitoring and discussing health-related quality of life in adolescents with type 1 diabetes improve psychosocial well-being: a randomized controlled trial. Diabet Care 2008;31:1521-6.
- Hood KK, Rohan JM, Peterson CM, Drotar D. Interventions with adherence-promoting components in pediatric type 1 diabetes: meta-analysis of their impact on glycemic control. Diabet Care 2010;33:1658-64. http://dx.doi.org/10.2337%2Fdc09-2268.
- Delamater AM. Psychological care of children and adolescents with diabetes. Pediatr Diabet 2009;10:175-84. http://dx.doi.org/10.1111%2Fj.1399-5448.2009.00580.x.
- Armour TA, Norris SL, Jack L, Zhang X, Fisher L. The effectiveness of family interventions in people with diabetes mellitus: a systematic review. Diabet Med 2005;22:1295-305. http://dx.doi.org/10.1111%2Fj.1464-5491.2005.01618.x.
- Laffel LM, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. J Pediatr 2003;142:409-16. http://dx.doi.org/10.1067%2Fmpd.2003.138.
- Satin W, La Greca AM, Zigo MA, Skyler JS. Diabetes in adolescence: effects of multifamily group intervention and parent simulation of diabetes. J Pediatr Psychol 1989;14:259-75. http://dx.doi.org/10.1093%2Fjpepsy%2F14.2.259.
- Ellis DA, Frey MA, Naar-King S, Templin T, Cunningham P, Cakan N. Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in chronic poor metabolic control: a randomized controlled trial. Diabet Care 2005;28:1604-10. http://dx.doi.org/10.2337%2Fdiacare.28.7.1604.
- Ellis DA, Templin T, Naar-King S, Frey MA, Cunningham PB, Podolski CL, et al. Multisystemic therapy for adolescents with poorly controlled type I diabetes: stability of treatment effects in a randomized controlled trial. J Consult Clin Psychol 2007;75:168-74. http://dx.doi.org/10.1037%2F0022-006X.75.1.168.
- Robin A, Foster S. Negotiating Parent–Adolescent Conflict: A Behavioural Family Systems Approach. New York, NY: Guilford Press; 1989.
- Wysocki T, Greco P, Harris MA, Bubb J, White NH. Behavior therapy for families of adolescents with diabetes: maintenance of treatment effects. Diabet Care 2001;24:441-6. http://dx.doi.org/10.2337%2Fdiacare.24.3.441.
- Wysocki T, Harris MA, Greco P, Bubb J, Danda CE, Harvey LM, et al. Randomized, controlled trial of behavior therapy for families of adolescents with insulin-dependent diabetes mellitus. J Pediatr Psychol 2000;25:23-3. http://dx.doi.org/10.1093%2Fjpepsy%2F25.1.23.
- Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie S, Taylor A, et al. Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. J Pediatr Psychol 2006;31:928-38. http://dx.doi.org/10.1093%2Fjpepsy%2Fjsj098.
- Harris MA, Freeman KA, Beers M. Family therapy for adolescents with poorly controlled diabetes: initial test of clinical significance. J Pediatr Psychol 2009;34:1097-107. http://dx.doi.org/10.1093%2Fjpepsy%2Fjsp009.
- Harris MA, Harris BS, Mertlich D. Brief report: In-home family therapy for adolescents with poorly controlled diabetes: failure to maintain benefits at 6-month follow-up. J Pediatr Psychol 2005;30:683-8. http://dx.doi.org/10.1093%2Fjpepsy%2Fjsi055.
- Wysocki T, Green L, Huxtable K. Blood glucose monitoring by diabetic adolescents: compliance and metabolic control. Health Psychol 1989;8:267-84. http://dx.doi.org/10.1037%2F%2F0278-6133.8.3.267.
- Delamater AM, Bubb J, Davis SG, Smith JA, Schmidt L, White NH, et al. Randomized prospective study of self-management training with newly diagnosed diabetic children. Diabet Care 1990;13:492-8. http://dx.doi.org/10.2337%2Fdiacare.13.5.492.
- Wysocki T, Nansel TR, Holmbeck GN, Chen R, Laffel L, Anderson BJ, et al. Collaborative involvement of primary and secondary caregivers: associations with youths’ diabetes outcomes. J Pediatr Psychol 2009;34:869-81.
- Northam EA, Todd S, Cameron FJ. Interventions to promote optimal health outcomes in children with Type 1 diabetes: are they effective?. Diabet Med 2006;23:113-21. http://dx.doi.org/10.1111%2Fj.1464-5491.2005.01678.x.
- Suarez M, Mullins S. Motivational interviewing and pediatric health behavior interventions. J Dev Behav Pediatr 2008;29:417-28. http://dx.doi.org/10.1097%2FDBP.0b013e31818888b4.
- Erickson SJ, Gerstle M, Feldstein SW. Brief interventions and motivational interviewing with children, adolescents, and their parents in pediatric health care settings: a review. Arch Pediatr Adolesc Med 2005;159:1173-80. http://dx.doi.org/10.1001%2Farchpedi.159.12.1173.
- Channon S, Huws-Thomas MV, Rollnick S, Gregory JW. The potential of motivational interviewing. Diabet Med 2005;22.
- Winkley K, Ismail K, Landau S, Eisler I. Psychological interventions to improve glycaemic control in patients with type 1 diabetes: systematic review and meta-analysis of randomised controlled trials. BMJ 2006;333. http://dx.doi.org/10.1136%2Fbmj.38874.652569.55.
- Channon SJ, Huws-Thomas MV, Rollnick S, Hood K, Cannings-John RL, Rogers C, et al. A multicenter randomized controlled trial of motivational interviewing in teenagers with diabetes. Diabet Care 2007;30:1390-5. http://dx.doi.org/10.2337%2Fdc06-2260.
- Channon S, Smith VJ, Gregory JW. A pilot study of motivational interviewing in adolescents with diabetes. Arch Dis Child 2003;88:680-3. http://dx.doi.org/10.1136%2Fadc.88.8.680.
- Knight KM, Bundy C, Morris R, Higgs JF, Jameson RA, Unsworth P, et al. The effects of group motivational interviewing and externalizing conversations for adolescents with Type 1 diabetes. Psychol Health Med 2003;8:149-57. http://dx.doi.org/10.1080%2F1354850031000087528.
- Viner R, Christie D, Taylor V, Hey S. Motivational/solution-focused intervention improves HbA1c in adolescents with Type 1 diabetes: a pilot study. Diabet Med 2003;20:739-42. http://dx.doi.org/10.1046%2Fj.1464-5491.2003.00995.x.
- Nansel TR, Iannotti RJ, Simons-Morton BG, Cox C, Plotnick LP, Clark LM, et al. Diabetes personal trainer outcomes: short-term and 1-year outcomes of a diabetes personal trainer intervention among youth with type 1 diabetes. Diabet Care 2007;30:2471-7. http://dx.doi.org/10.2337%2Fdc06-2621.
- Robling M, McNamara R, Bennert K, Butler CC, Channon S, Cohen D, et al. The effect of the Talking Diabetes consulting skills intervention on glycaemic control and quality of life in children with type 1 diabetes: cluster randomised controlled trial (DEPICTED study). BMJ 2012;344. http://dx.doi.org/10.1136%2Fbmj.e2359.
- Christie D. Dancing with diabetes: brief therapy conversations with children, young people and families living with diabetes. Eur Diabet Nurs 2008;5:1-5. http://dx.doi.org/10.1002%2Fedn.99.
- Davis ED, Vander Meer JM, Yarborough PC, Roth SB. Using solution-focused therapy strategies in empowerment-based education. Diabetes Educ 1999;25:249-57. http://dx.doi.org/10.1177%2F014572179902500210.
- George E, Iveson C, Ratner H. Problem to Solution: Brief Therapy with Individuals and Families. London: BT Press; 1990.
- McKeel AJ, Miller SD, Hubble MA, Duncan BL. Handbook of Solution-Focused Brief Therapy. San Francisco, CA: Jossey Bass; 1996.
- MacDonald A. Solution Focused Brief Therapy Evaluation List 2012. www.solutionsdoc.co.uk/sft.html (accessed November 2012).
- Cardiac Rehabilitation: A National Clinical Guideline. Edinburgh: SIGN; 2002.
- Guidance on Cancer Services: Improving Supportive and Palliative Care for Adults with Cancer. The Manual. London: NICE; 2004.
- Prevention and Lifestyle Behaviour Change: A Competence Framework. London: NHS; 2011.
- Bowles N, Mackintosh C, Torn A. Nurses’ communication skills: an evaluation of the impact of solution-focused communication training. J Adv Nurs 2001;36:347-54.
- Svoren BM, Butler D, Levine BS, Anderson BJ, Laffel LM. Reducing acute adverse outcomes in youths with type 1 diabetes: a randomized, controlled trial. Pediatrics 2003;112:914-22. http://dx.doi.org/10.1542%2Fpeds.112.4.914.
- Howe CJ, Jawad AF, Tuttle AK, Moser JT, Preis C, Buzby M, et al. Education and telephone case management for children with type 1 diabetes: a randomized controlled trial. J Pediatr Nurs 2005;20:83-95. http://dx.doi.org/10.1016%2Fj.pedn.2004.12.010.
- Howells L, Wilson AC, Skinner TC, Newton R, Morris AD, Greene SA. A randomized control trial of the effect of negotiated telephone support on glycaemic control in young people with Type 1 diabetes. Diabet Med 2002;19:643-8. http://dx.doi.org/10.1046%2Fj.1464-5491.2002.00791.x.
- Building the Capacity to Deliver Structured Education for All People with Diabetes: A Briefing Document for SHA Diabetes Leads and PCT Commissioning. London: NHS; 2008.
- Murphy HR, Wadham C, Rayman G, Skinner TC. Approaches to integrating paediatric diabetes care and structured education: experiences from the Families, Adolescents, and Children’s Teamwork Study (FACTS). Diabet Med 2007;24:1261-8. http://dx.doi.org/10.1111%2Fj.1464-5491.2007.02229.x.
- Waller H, Eiser C, Knowles J, Rogers N, Wharmby S, Heller S, et al. Pilot study of a novel educational programme for 11–16 year olds with type 1 diabetes mellitus: the KICk-OFF course. Arch Dis Child 2008;93:927-31.
- Chaney D, Coates V, Shevlin M. Running a complex educational intervention for adolescents with type 1 diabetes: lessons learnt. J Diabet Nurs 2010;14:370-9.
- A Framework for Development and Evaluation of RCTs for Complex Interventions to Improve Health. London: MRC; 2000.
- Royal College of Paediatrics and Child Health (RCPCH) . National Paediatric Diabetes Audit for 2010–2011 2012. www.rcpch.ac.uk/npda.
- National Service Framework for Diabetes; Standards. London: Department of Health; 2001.
- Making Every Young Person with Diabetes Matter. London: Department of Health; 2007.
- Funnell MM, Brown TL, Childs BP, Haas LB, Hosey GM, Jensen B, et al. National standards for diabetes self-management education. Diabet Care 2012;35:101-8. http://dx.doi.org/10.2337%2Fdc12-s101.
- Swift PG. Diabetes education in children and adolescents. Pediatr Diabet 2009;10:51-7. http://dx.doi.org/10.1111%2Fj.1399-5448.2009.00570.x.
- DAFNE Study Group . Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325.
- Davies MJ, Heller S, Skinner TC, Campbell MJ, Carey ME, Cradock S, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336:491-5. http://dx.doi.org/10.1136%2Fbmj.39474.922025.BE.
- Deakin TA, Cade JE, Williamst R, Greenwood DC. Structured patient education: the diabetes X-PERT programme makes a difference. Diabet Med 2006;23:944-54. http://dx.doi.org/10.1111%2Fj.1464-5491.2006.01906.x.
- Schilling LS, Grey M, Knafl KA. The concept of self-management of type 1 diabetes in children and adolescents: an evolutionary concept analysis. J Adv Nurs 2002;37:87-99. http://dx.doi.org/10.1046%2Fj.1365-2648.2002.02061.x.
- Gowers SG, Jones JC, Kiana S, North CD, Price DA. Family functioning: a correlate of diabetic control?. J Child Psychol Psychiatr 1995;36:993-1001. http://dx.doi.org/10.1111%2Fj.1469-7610.1995.tb01345.x.
- Anderson BJ, Brackett J, Ho J, Laffel LM. An office-based intervention to maintain parent-adolescent teamwork in diabetes management. Impact on parent involvement, family conflict, and subsequent glycemic control. Diabet Care 1999;22:713-21. http://dx.doi.org/10.2337%2Fdiacare.22.5.713.
- Gage H, Hampson S, Skinner TC, Hart J, Storey L, Foxcroft D, et al. Educational and psychosocial programmes for adolescents with diabetes: approaches, outcomes and cost-effectiveness. Patient Educ Couns 2004;53:333-46. http://dx.doi.org/10.1016%2Fj.pec.2003.06.003.
- Koopman HM, Baars RM, Chaplin J, Zwinderman KH. Illness through the eyes of the child: the development of children’s understanding of the causes of illness. Patient Educ Couns 2004;55:363-70. http://dx.doi.org/10.1016%2Fj.pec.2004.02.020.
- Assal JP, Muhlhauser I, Pernet A, Gfeller R, Jorgens V, Berger M. Patient education as the basis for diabetes care in clinical practice and research. Diabetologia 1985;28:602-13. http://dx.doi.org/10.1007%2FBF00281995.
- Kaufman FR, Halvorson M, Carpenter S, Devoe D, Pitukcheewanont P. View 2: Insulin pump therapy in young children with diabetes. Diabet Spectr 2001;14:84-9. http://dx.doi.org/10.2337%2Fdiaspect.14.2.84.
- Kaufman FR, Austin J, Lloyd J, Halvorson M, Carpenter S, Pitukcheewanont P. Characteristics of glycemic control in young children with type 1 diabetes. Pediatr Diabet 2002;3:179-83. http://dx.doi.org/10.1034%2Fj.1399-5448.2002.30402.x.
- Rollnick S, Miller WR, Butler C. Motivational Interviewing in Healthcare: Helping Patients Change Behaviour. London: Guilford Press; 2008.
- Welle DL, Clatts MC. Scaffolded interviewing with lesbian, gay, bisexual, transgender, queer, and questioning youth: a developmental approach to HIV education and prevention. J Assoc Nurses AIDS Care 2007;18:5-14. http://dx.doi.org/10.1016%2Fj.jana.2007.01.004.
- Albarracin D, Gillette JC, Earl AN, Glasman LR, Durantini MR, Ho MH. A test of major assumptions about behavior change: a comprehensive look at the effects of passive and active HIV-prevention interventions since the beginning of the epidemic. Psychol Bull 2005;131:856-97. http://dx.doi.org/10.1037%2F0033-2909.131.6.856.
- Smart C, Aslander-van VE, Waldron S. Nutritional management in children and adolescents with diabetes. Pediatr Diabet 2009;10:100-17. http://dx.doi.org/10.1111%2Fj.1399-5448.2009.00572.x.
- Clarke W, Jones T, Rewers A, Dunger D, Klingensmith GJ. Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabet 2009;10:134-45. http://dx.doi.org/10.1111%2Fj.1399-5448.2009.00583.x.
- Thompson R, Christie D, Hindmarsh PC. The role for insulin analogues in diabetes care. Curr Paediatr 2006;16:117-22. http://dx.doi.org/10.1016%2Fj.cupe.2005.12.011.
- Gillespie SJ, Kulkarni KD, Daly AE. Using carbohydrate counting in diabetes clinical practice. J Am Diet Assoc 1998;98:897-905. http://dx.doi.org/10.1016%2FS0002-8223%2898%2900206-5.
- Rewers M, Pihoker C, Donaghue K, Hanas R, Swift P, Klingensmith GJ. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabet 2009;10:71-8. http://dx.doi.org/10.1111%2Fj.1399-5448.2009.00582.x.
- Robertson K, Adolfsson P, Scheiner G, Hanas R, Riddell MC. Exercise in children and adolescents with diabetes. Pediatr Diabet 2009;10:154-68. http://dx.doi.org/10.1111%2Fj.1399-5448.2009.00567.x.
- Specialist Nursing Services for Children and Young People with Diabetes. London: RCN; 2006.
- Christie D, Strange V, Allen E, Oliver S, Wong IC, Smith F, et al. Maximising engagement, motivation and long term change in a Structured Intensive Education Programme in Diabetes for children, young people and their families: Child and Adolescent Structured Competencies Approach to Diabetes Education (CASCADE). BMC Pediatr 2009;9. http://dx.doi.org/10.1186%2F1471-2431-9-57.
- Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabet Care 2003;26:631-7.
- Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol 1997;38:581-6. http://dx.doi.org/10.1111%2Fj.1469-7610.1997.tb01545.x.
- Anderson BJ, Auslander WF, Jung KC, Miller JP, Santiago JV. Assessing family sharing of diabetes responsibilities. J Pediatr Psychol 1990;15:477-92. http://dx.doi.org/10.1093%2Fjpepsy%2F15.4.477.
- Grossman HY, Brink S, Hauser ST. Self-efficacy in adolescent girls and boys with insulin-dependent diabetes mellitus. Diabet Care 1987;10:324-9. http://dx.doi.org/10.2337%2Fdiacare.10.3.324.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J 1976;34:585-612. http://dx.doi.org/10.1038%2Fbjc.1976.220.
- Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol 1971;44:793-7. http://dx.doi.org/10.1259%2F0007-1285-44-526-793.
- Weijer C, Grimshaw JM, Eccles MP, McRae AD, White A, Brehaut JC, et al. The Ottawa Statement on the Ethical Design and Conduct of Cluster Randomized Trials. PLoS Med 2012;9. http://dx.doi.org/10.1371%2Fjournal.pmed.1001346.
- Campbell MK, Piaggio G, Elbourne DR, Altman DG. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345. http://dx.doi.org/10.1136%2Fbmj.e5661.
- Diabetes UK . Guide to HbA1c 2012. what-is-hba1c.html (accessed November 2012).
- Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N. The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation. Health Technol Assess 2003;7.
- Shearer A, Bagust A, Sanderson D, Heller S, Roberts S. Cost-effectiveness of flexible intensive insulin management to enable dietary freedom in people with Type 1 diabetes in the UK. Diabet Med 2004;21:460-7. http://dx.doi.org/10.1111%2Fj.1464-5491.2004.01183.x.
- Owen C, Woodward S. Effectiveness of dose adjustment for normal eating (DAFNE). Br J Nurs 2012;21:224-32.
- Lofland J, Lofland H. Analyzing Social Settings: A Guide to Qualitative Observation and Analysis. Belmont CA: Wadsworth; 1995.
- Gregory J, Robling M, Bennert K, Channon S, Cohen D, Crowne E, et al. Development and evaluation by a cluster randomised trial of a psychosocial intervention in children and teenagers experiencing diabetes: the DEPICTED study. Health Technol Assess 2011;15.
- Knowles J, Price K, Fox M. ‘KICk-OFF’: A Structured Education Programme for Children With Diabetes Based on the Adult DAFNE Course 2012. www.kick-off.org.uk/team.php (accessed 1 May 2012).
- Gosden C, Edge JA, Holt RI, James J, Turner B, Winocour P, et al. The fifth UK paediatric diabetes services survey: meeting guidelines and recommendations?. Arch Dis Child 2010;95:837-40. http://dx.doi.org/10.1136%2Fadc.2009.176925.
- James J, Gosden C, Winocour P, Walton C, Nagi D, Turner B, et al. Diabetes specialist nurses and role evolvement: a survey by Diabetes UK and ABCD of specialist diabetes services 2007. Diabet Med 2009;26:560-5. http://dx.doi.org/10.1111%2Fj.1464-5491.2009.02716.x.
- Pihoker C, Forsander G, Wolfsdorf J, Klingensmith GJ. The delivery of ambulatory diabetes care to children and adolescents with diabetes. Pediatr Diabetes 2009;10:58-70. http://dx.doi.org/10.1111%2Fj.1399-5448.2009.00585.x.
- Special Issue: SWEET – Better control in pediatric and adolescent diabetes: working to create centres of reference . Pediatr Diabetes 2013;13:1-75.
- Diabetes in the UK 2010: Key Statistics on Diabetes. London: Diabetes UK; 2010.
- Roze S, Valentine WJ, Zakrzewska KE, Palmer AJ. Health-economic comparison of continuous subcutaneous insulin infusion with multiple daily injection for the treatment of Type 1 diabetes in the UK. Diabet Med 2005;22:1239-45. http://dx.doi.org/10.1111%2Fj.1464-5491.2005.01576.x.
- Burke M. Cost Impact of Continuous Subcutaneous Insulin Infusion (CSII) for the Management of Patients with Type 1 Diabetes in England. York: University of York NHS Technology Adoption Centre Health Economics Consortium; 2011.
- Tennvall GR, Apelqvist J, Eneroth M. Costs of deep foot infections in patients with diabetes mellitus. Pharmacoeconomics 2000;18:225-38. http://dx.doi.org/10.2165%2F00019053-200018030-00003.
- Diabetes Control and Complications Trial Research Group (DCCT) . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86.
- Diabetes Control and Complications Trial Research Group (DCCT) . Lifetime Benefits and Costs of Intensive Therapy as Practiced in the Diabetes Control and Complications Trial. JAMA 1996;276:1409-15.
- Akobundu E, Ju J, Blatt L, Mullins CD. Cost-of-illness studies: a review of current methods. Pharmacoeconomics 2006;24:869-90. http://dx.doi.org/10.2165%2F00019053-200624090-00005.
- NHS Reference Costs 2010–11. London: Department of Health; 2012.
- Cummins E, Royle P, Snaith A, Greene A, Robertson L, McIntyre L, et al. Clinical effectiveness and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes: systematic review and economic evaluation. Health Technol Assess 2010;14.
- Discounting of Health Benefits in Special Circumstances. London: NICE; 2011.
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340-9. http://dx.doi.org/10.1177%2F027298902400448902.
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000;38:583-637. http://dx.doi.org/10.1097%2F00005650-200006000-00004.
- The Burden of Disease and Injury in Australia. Canberra: AIHW; 2003.
- Brown GC, Brown MM, Sharma S, Brown H, Smithen L, Leeser DB, et al. Value-based medicine and ophthalmology: an appraisal of cost-utility analyses. Trans Am Ophthalmol Soc 2004;102:177-85.
- The Diabetes Control and Complications Trial Research Group . Effects of Intensive Diabetes Therapy on Neuropsychological Function in Adults in the Diabetes Control and Complications Trial. Ann Intern Med 1996;124:379-88.
- UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53.
- UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854-65.
- DeWit M, Delemarre-van de Waal HA, Pouwer F, Gemke RJ, Snoek FJ. Monitoring health related quality of life in adolescents with diabetes: a review of measures. Arch Dis Child 2007;92:434-9.
- Moyers TB, Martin T, Catley D, Harris KJ, Ahluwalia JS. Assessing the integrity of motivational interventions: reliability of the Motivational Interviewing Skills Code. Behav Cogn Psychother 2003;31:177-84. http://dx.doi.org/10.1017%2FS1352465803002054.
- A New Pathway for Regulation and Governance of Health Research. London: Academy for Medical Sciences; 2011.
- Pantell RH, Stewart TJ, Dias JK, Wells P, Ross AW. Physician communication with children and parents. Pediatrics 1982;70:396-402.
- Stewart TJ, Pantell RH, Dias JK, Wells PA, Ross AW. Children as patients: a communications process study in family practice. J Fam Pract 1981;13:827-35.
- Lewis C, Knopf D, Chastain-Lorber K, Ablin A, Zoger S, Matthay K, et al. Patient, parent, and physician perspectives on pediatric oncology rounds. J Pediatr 1988;112:378-84. http://dx.doi.org/10.1016%2FS0022-3476%2888%2980316-0.
- Edge JA, Swift PG, Anderson W, Turner B. Diabetes services in the UK: fourth national survey; are we meeting NSF standards and NICE guidelines?. Arch Dis Child 2005;90:1005-9. http://dx.doi.org/10.1136%2Fadc.2005.071613.
- Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract 2005;55:305-12.
- Lowes L, Robling MR, Bennert K, Crawley C, Hambly H, Hawthorne K, et al. Involving lay and professional stakeholders in the development of a research intervention for the DEPICTED study. Health Expect 2011;14:250-60. http://dx.doi.org/10.1111%2Fj.1369-7625.2010.00625.x.
- Christie D. Developing the National Paediatric Diabetes Audit Patient Reported Experience Measure. Diabet Care Child Young People 2012;1:47-8.
- Welch G, Rose G, Ernst D. Motivational Interviewing and diabetes: what is it, how is it used and does it work. Diabet Spectr 2012;19:5-11. http://dx.doi.org/10.2337%2Fdiaspect.19.1.5.
- Moyers TB, Martin T, Christopher PJ, Houck JM, Tonigan JS, Amrhein PC. Client language as a mediator of motivational interviewing efficacy: where is the evidence?. Alcohol Clin Exp Res 2007;31:40-7. http://dx.doi.org/10.1111%2Fj.1530-0277.2007.00492.x.
Appendix 1 Tables describing different paediatric education programmes
| Name | Type of trial | Participants recruited | Programme duration | Module themes (general) | No. of educators | Size of groups | Patients taught per year | Hours of education | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| FACTS, Murphy et al. 2007141 |
Multicentre, RCT | 305 adolescents recruited (mean 13.1 ± 1.9 years) from 10 centres (not clustered) | 6 × 1.5 hours Education over 6 months |
CHO counting and insulin dose adjustment, parental responsibility and communication | Members of the existing diabetes MDT (dietitian, paediatric nurse specialist, physician) | 4–6 families (parents and adolescents) | ? | 9 hours, over 6 months | HbA1c (3-monthly), episodes of hypoglycaemia/DKA,
Diabetes Quality of Life Youth scale, Health Behaviour in School
Children, Problem Areas in Diabetes Scale and
DFRQ Baseline and 12 months (6 months post FACTS) |
| KICk-OFF | Pilot data (Waller et al., 2008142) Currently trialling multicentre, cluster RCT |
48 young people aged 11–16 years (mean 13.6 years) from three centres | Five consecutive days Six groups completed |
Monday: what is diabetes, food groups, insulin–CHO
relationship Tuesday: BG levels, hypoglycaemia, CHO counting Wednesday: Insulin dose adjustments, doses and illness, diabetes + long-term health Thursday: Healthy eating, weight, sweets, alcohol, exercise Friday: (friends can attend) friends/school awareness of diabetes and support, puberty |
Three educators per group (two PDSNs and one dietitian) | Eight young people per group (stratified by age and sex) | 48 participants from 3 centres over 5 months | 5 weekdays | Mean HbA1c value, body mass index, hypoglycaemia
episodes, Diabetes Family Conflict Scale, DFRQ, Diabetes
Treatment Satisfaction Questionnaire (DTSQ), PedsQL,
Self-Efficacy for Diabetes Baseline, 3 months, 6 months |
| CHO, Insulin Collaborative Education Programme (CHOICE) | Multicentre, RCT (protocol data) | 140 adolescents (13–19 years) from six sites in Northern Ireland | Four evening sessions over 4 weeks | CHO content in food/drink, relationship between CHO and insulin, BG and exercise, adjusting insulin, illness, alcohol and drug use | Two: one research assistant leading and one dietitian | 12 hours over 4 consecutive weeks | HbA1c, hypoglycaemia episodes, Diabetes quality of life questionnaire, Diabetes empowerment scale, Diabetes Care Profile | ||
| DEPICTED | Multicentre, cluster RCT | Clinical teams from 26 paediatric diabetes clinics (and 693 young people from those clinics) | ‘Talking Diabetes’ training package for clinic staff | 1.5 hours e-learning module plus two afternoon seminars | HbA1c values of young people in clinics during trial
and in year after staff have received training Health Care Climate, PedsQL, Diabetes Continuity of Care, Problem Areas in Diabetes Scale, Patient Enablement, hypoglycaemic episodes in parents and young people |
||||
| Evidence into Practice: Evaluating a child-centred intervention for diabetes medicine management (EPIC) | Multicentre, RCT (protocol data) | Up to 10 sites will recruit a target of 252 participants (6–18 years); 168 in intervention, 84 in control | Intervention delivered as part of usual clinic appointment | Young people receive individually tailored, age-appropriate information pack at clinic visits | Information pack to be delivered by nurse/clinician as part of clinic visit | Diabetes PedsQL at 6 months. HbA1c value at each routine clinic visit, EQ-5D and generic PedsQL, episodes of DKA | |||
| Diabetes Information Given Because you are Young (DIGBY) | Clinic education programme at Addenbrooke’s Hospital | Newly diagnosed young people | How diabetes affects the body, how to manage diabetes and how to adjust insulin doses according to diet and activity | ||||||
| Managing Insulin and Carbohydrate for Kids and Young people with Success (MICK&YS) | Pilot study | Young people (11–17 years), MDI regimen | 2 × 3.5-hour sessions 1 week apart | Healthy eating and CHO counting, insulin dose adjustment; pairs also shared a meal together | Can be delivered by member of MDT, e.g. dietitian, diabetes specialist nurse, paediatrician | Parent–child pairs | 7 hours over 2 weeks |
Qualitative evaluation (QoL and satisfaction outcomes) | |
| Flexible Adjustment of Basal Boluses (FABB) | Clinic education programme at Leicester Royal | 146 families so far | 2 × 2.5-hour sessions | Training to enable young people/parents to dose adjust insulin as part of a basal bolus regimen | Four trained educators; courses led by nurse and dietitian | Six children and six family members | 5 hours over 2 weeks | HbA1c post programme – currently being audited | |
| STeP | Clinic education programme at Leeds Teaching Hospitals NHS Trust | Children starting on insulin pump therapy | 6–12 hours either consecutive days or within
4-day period |
Appendix 2 Curriculum for Education programmes
Type 1 Education network: agreed learning methods and core content for programmes
| Method |
| Goal setting |
| Time for patients own agenda |
| Group interaction |
| Problem-solving |
| Experiential learning |
| Patient feedback and reflecting |
| Content |
| CHO counting |
| Insulin dose adjustment |
| How insulin works |
| Hypoglycaemia |
| How to manage exercise |
| Lifestyle issues |
| Complications |
| Other self-management skills |
American Diabetes Association National standards recommended curriculum for Diabetes Self-Management Education
| Describing the diabetes disease process and treatment options |
| Incorporating nutritional management into lifestyle |
| Incorporating physical activity into lifestyle |
| Using medication(s) safely and for maximum therapeutic effectiveness |
| Monitoring BG and other parameters and interpreting and using the results for self-management decision-making |
| Preventing, detecting and treating acute complications |
| Preventing detecting and treating chronic complications |
| Developing personal strategies to address psychosocial issues and concerns |
| Developing personal strategies to promote health and behaviour change |
Appendix 3 Record of reported changes in HTA project protocol
Changes to protocol reported in 6-monthly progress reports.
| Change to protocol | Progress report | Date | Approved by |
|---|---|---|---|
| Recruitment and consent taken by clinical staff with appropriate expertise in addition to research staff | 1 | 30 October 2008 | Simon Bevan |
| Use of a DVD to assist with recruitment | 2 | 30 April 2009 | |
| Timing of primary and secondary outcomes | 2 | 30 April 2009 | |
| Questionnaires to be carried out over the telephone where required | 3 | 14 December 2009 | |
| Revision of recruitment target (non-substantial amendment) | 3 | 14 December 2009 | |
| Participants to be offered £10 reward for completing follow-up | 5 | 2 November 2010 |
Appendix 4 PDF file of letter of favourable intent from ethics
Appendix 5 Trial Steering Committee and Data Monitoring and Ethics Committee membership details
Trial Steering Committee members
Professor David Dunger, Professor of Paediatrics
Department of Paediatrics
University of Cambridge
Box 116, Level 8
Addenbrooke’s Hospital
Hills Road
Cambridge
CB2 0QQ
Professor Jacqueline Barnes, Professor of Psychology
Department of Psychological Sciences
Institute for the Study of Children, Families and Social Issues
Birkbeck
Malet Street
London
WC1E 7HX
Professor Tim Cole, Professor of Medical Statistics
Centre for Paediatric Epidemiology and Biostatistics
University College Institute of Child Health
30 Guilford Street
London
WC1N 1EH
Data Monitoring and Ethics Committee members
Professor Christopher JH Kelnar, Honorary Professor of Paediatric Endocrinology
University of Edinburgh
9 Easter Belmont Road
Edinburgh
EH12 6EX
Professor Darren Ashcroft, Professor of Pharmacoepidemiology
University of Manchester
School of Pharmacy and Pharmaceutical Sciences
1st Floor, Stopford Building
Oxford Road
Manchester
M13 9PT
Professor Robert Coe, Professor of Education
Director of the Centre for Evaluation and Monitoring (CEM)
Durham University
School of Education
Leazes Road
Durham
DH1 1TA
Dr Chris Patterson, Reader in Medical Statistics
Centre for Public Health
Queen’s University Belfast
Room 3.014, ICS Block B,
Royal Victoria Hospital
Grosvenor Road
Belfast
BT12 6BA
Appendix 6 Per-protocol analyses tables for young people and parent/carers
| Outcomes | 12-month follow-up | 24-month follow-up | ||
|---|---|---|---|---|
| Intention-to-treat analysis: effect (95% CI) | Per-protocol analysis: effect (95% CI) | Intention-to-treat analysis: effect (95% CI) | Per-protocol analysis: effect (95% CI) | |
| HbA1c | ||||
| HbA1c value from venepuncture (mmol/l) | 0.11 (–0.28 to 0.50), p = 0.584 | 0.02 (–0.43 to 0.46), p = 0.944 | 0.03 (–0.36 to 0.41), p = 0.891 | 0.00 (–0.42 to 0.42), p = 0.994 |
| PedsQL: general | ||||
| Physical health summary score | 0.34 (–2.51 to 2.62) | 2.09 (–1.60 to 4.88) | 1.14 (–1.28 to 3.32) | 1.43 (–1.62 to 4.69) |
| Psychosocial health summary score | –1.85 (–4.29 to 0.24) | 0.33 (–3.04 to 3.52) | –1.17 (–3.69 to 1.45) | 0.15 (–2.63 to 3.38) |
| Total score | –1.09 (–3.15 to 0.63) | 0.87 (–1.90 to 3.49) | –0.33 (–2.53 to 1.97) | 0.53 (–2.04 to 3.76) |
| PedsQL: diabetes module | ||||
| Diabetes score | 0.62 (–2.35 to 3.04) | 1.77 (–1.80 to 4.97) | –0.02 (–3.19 to 2.72) | –0.25 (–3.18 to 2.94) |
| Treatment 1 score | –0.80 (–5.14 to 3.08) | 0.60 (–5.00 to 5.82) | –1.05 (–4.52 to 2.32) | –2.10 (–7.09 to 2.80) |
| Treatment 2 score | –1.90 (–4.99 to 1.97) | 0.17 (–2.59 to 3.66) | –1.49 (–4.53 to 1.42) | –1.48 (–5.05 to 2.22) |
| Worry score | –0.77 (–5.43 to 3.94) | 0.21 (–5.64 to 6.06) | –0.32 (–4.64 to 4.52) | 0.39 (–4.17 to 5.55) |
| Communication score | –1.34 (–6.31 to 4.01) | 1.54 (–3.84 to 7.70) | –1.06 (–5.34 to 3.59) | –0.78 (–6.22 to 5.78) |
| DFRQ | ||||
| Family responsibility total score – young person | –0.12 (–1.04 to 0.75) | –0.32 (–1.37 to 0.63) | 0.85 (0.03 to 1.61) | 0.71 (–0.54 to 1.80) |
| Family responsibility total score – young person (weighted) | 0.01 (–0.85 to 0.81) | –0.20 (–1.18 to 0.72) | 0.71 (–0.07 to 1.44) | 0.54 (–0.64 to 1.59) |
| Strengths and difficulties questionnaire | ||||
| Strengths and difficulties total average impact score | 0.09 (–0.21 to 0.43) | –0.06 (–0.41 to 0.32) | 0.04 (–0.14 to 0.21) | –0.18 (–0.32 to –0.03) |
| Body weight | ||||
| Happiness with body weight | –0.24 (–0.65 to 0.29) | –0.15 (–0.70 to 0.71) | –0.56 (–1.03 to –0.06) | –0.37 (–0.95 to 0.25) |
| Outcomes | 12-month follow-up | 24-month follow-up | ||
|---|---|---|---|---|
| Intention-to-treat analysis: effect (95% CI) | Per-protocol analysis: effect (95% CI) | Intention-to-treat analysis: effect (95% CI) | Per-protocol analysis: effect (95% CI) | |
| Management | ||||
| No. of times attended diabetes clinic in last year | 0.01 (–0.22 to 0.24) | 0.03 (–0.21 to 0.33) | –0.18 (–0.41 to 0.06) | –0.13 (–0.42 to 0.18) |
| PedsQL: general | ||||
| Physical health summary score | 2.24 (–1.34 to 5.10) | 1.29 (–4.18 to 6.09) | 2.00 (–2.04 to 5.29) | 1.92 (–2.78 to 5.92) |
| Psychosocial health summary score | 1.76 (–1.50 to 5.79) | 2.26 (–1.57 to 6.58) | 0.03 (–3.08 to 3.66) | 2.49 (–1.19 to 6.22) |
| Total score | 1.74 (–1.14 to 5.00) | 1.62 (–2.26 to 5.91) | 0.58 (–2.70 to 3.46) | 2.13 (–1.47 to 5.20) |
| PedsQL: diabetes module | ||||
| Diabetes score | 1.13 (–1.72 to 4.02) | 0.82 (–2.74 to 4.09) | –0.08 (–2.83 to 3.12) | 0.54 (–2.56 to 3.86) |
| Treatment 1 score | 0.79 (–2.58 to 4.10) | 0.28 (–4.81 to 4.16) | 2.20 (–2.31 to 6.88) | 3.08 (–1.55 to 8.21) |
| Treatment 2 score | 2.37 (–0.40 to 5.37) | 2.44 (–0.66 to 5.59) | –1.10 (–4.49 to 2.50) | –0.31 (–4.25 to 3.51) |
| Worry score | 3.59 (–1.50 to 7.57) | 4.12 (–1.85 to 8.80) | 0.66 (–3.75 to 5.14) | 1.54 (–2.83 to 6.07) |
| Communication score | 2.30 (–2.44 to 8.64) | 6.76 (–1.77 to 15.34) | –2.54 (–9.06 to 4.16) | 1.79 (–5.55 to 9.06) |
| DFRQ | ||||
| Family responsibility total score – parent | 0.74 (0.03 to 1.52) | 0.56 (–0.35 to 1.46) | 0.57 (–0.67 to 1.49) | 0.28 (–0.90 to 1.33) |
| Family responsibility total score – parent (weighted) | 0.62 (–0.17 to 1.47) | 0.57 (–0.46 to 1.57) | 0.60 (–0.43 to 1.47) | 0.15 (–0.81 to 1.16) |
| Strengths and difficulties questionnaire | ||||
| Strengths and difficulties total average impact score | –0.03 (–0.31 to 0.27) | –0.06 (–0.41 to 0.29) | –0.09 (–0.41 to 0.26) | –0.25 (–0.69 to 0.18) |
Appendix 7 Process evaluation tables
| Module contents | Site educator self-ratings:a median scores (range) | Research observer rating:b median scores (range) | |||
|---|---|---|---|---|---|
| n = 35 modules | n = 10 modules | ||||
| Module 1 | |||||
| 1 | Introductions | 7 | (1–7) | 6 | (4–7) |
| 2a and 2b | Identifying strengths and abilities and looking at the
future Focusing on the future and scaling |
7 | (4–7) | 6 | (4–7) |
| 3a/3b | ‘All food is good!’ and ‘What is a healthy diet?’ | 7 | (5–7) 1 missing | 6 | (5–7) |
| 4 | What is CHO? | 7 | (5–7) | 5 | (3–7) |
| 5 | How insulin works | 7 | (5–7) 1 missing | 5.5 | (4–7) |
| 6a and 6b | How insulin works in somebody without diabetes How your insulin injections work | 7 | (5–7) 1 missing | 5 | (4–7) |
| 7 | Matching insulin to food (ambivalence task) | 7 | (4–7) | 6 | (5–7) |
| n = 30 modules | n = 10 modules | ||||
| Module 2 | |||||
| 1 | Review of previous session | 7 | (4–7) | 6 | (4–7) |
| 2 | HbA1c | 7 | (5–7) | 6 | (4–7) |
| 3 | Factors that influence insulin requirements | 7 | (5–7) | 6 | (5–7) |
| 4 | Identifying hypoglycaemia | 7 | (6–7) | 6 | (4–7) |
| 5 | Treating hypoglycaemia | 7 | (5–7) | 6 | (5–7) |
| 6 | Pros and cons of BG testing | 7 | (5–7) | 5 | (4–7) |
| n = 33 modules | n = 10 modules | ||||
| Module 3 | |||||
| 1 | Review of previous session | 7 | (5–7) 2 missing | 6 | (4–7) |
| 2 and 2b | Identifying hyperglycaemia Identifying hyperglycaemia + ketones |
7 | (5–7) 1 missing | 6 | (4–7) |
| 3 | When to get help: Treat at home vs. contact a health-care professional | 7 | (5–7) 1 missing | 5 | (5–7) |
| 4a and 4b | Managing high BG levels – temporary insulin changes, calculating a correction dose | 7 | (3–7) | 5.5 | (3–7) |
| 5 | Managing high BG levels – permanent insulin changes | 7 | (5–7) | 5 | (4–7) |
| 6a and 6b | CHO counting: What is it? CHO counting: Addressing ambivalence |
7 | (4–7) 1 missing | 6 | (4–7) |
| n = 28 modules | n = 10 modules | ||||
| Module 4 | |||||
| 1 | Review of previous session | 7 | (4–7) 1 missing | 5.5 | (4–7) |
| 2a, 2b and 2c | Low BG levels before activity High BG levels before activity BG levels before activity |
7 | (5–7) | 6 | (4–7) |
| 3a and 3b | Activities that affect the BG level | 7 | (4–7) | 6 | (4–7) |
| 4 | Timing of insulin injections in relation to activity | 7 | (4–7) | 6 | (4–7) |
| 5 | Using CHO to keep your BG stable | 7 | (4–7) | 5.5 | (2–7) |
| Module contents | Suggested minutes | Mean minutes spent | Range | |
|---|---|---|---|---|
| Module 1 (nine observed groups) | ||||
| Activity | ||||
| 1 | Introductions | 10 | 8 | 3–15 |
| 2a and 2b | Focusing on the future and scaling Identifying strengths and abilities and looking at the future |
15 | 18 | 10–30 |
| 3a and 3b | What is a healthy diet? All food is good! |
10 | 21 | 13–35 |
| 4 | CHO | 15 | 11 | 3–17 |
| 5 | How insulin works | 20 | 10 | 3–20 |
| 6a and 6b | How insulin works in somebody without diabetes How your insulin injections work |
20 | 17 | 10–25 |
| 7 | Matching insulin to food (ambivalence task) | 10 | 12 | 5–23 |
| Module 2 (11 observed groups) | ||||
| 1 | Review of previous session | 15 | 11 | 3–20 |
| 2 | HbA1c | 5 | 5 | 3–7 |
| 3 | Factors that influence insulin requirements | 15 | 17 | 13–22 |
| 4 | Identifying hypoglycaemia | 20 | 16 | 4–29 |
| 5 | Treating hypoglycaemia | 20 | 18 | 9–32 |
| 6 | Pros and cons of BG testing | 30 | 26 | 15–37 |
| Module 3 (nine observed groups) | ||||
| 1 | Review of previous session | 10 | 10 | 4–20 |
| 2a and 2b | Identifying hyperglycaemia Identifying hyperglycaemia + ketones |
20 | 18 | 10–33 |
| 3 | When to get help: treat at home vs. contact a health care professional | 15 | 18 | 10–30 |
| 4a and 4b | Managing high BG levels – temporary insulin
changes Calculating a correction dose |
30 | 28 | 15–52 |
| 5 | Managing high BG levels – permanent insulin changes | 10 | 5 | 3–8 |
| 6a and 6b | CHO counting – What is it? CHO counting – addressing ambivalence |
15 | 22 | 7- 33 |
| Module 4 (11 observed groups) | ||||
| 1 | Review of previous session | 10 | 6 | 3–13 |
| 2a and 2b | Low BG levels before activity High BG levels before activity |
10 | 20 | 11–28 |
| 3a and 3b | BG levels before activity Activities that affect the BG level |
40 | 29 | 15–41 |
| 4 | Timing of insulin injections in relation to activity | 10 | 11 | 2–33 |
| 5 | Using CHO to keep your BG stable | 15 | 10 | 5–19 |
| Scores | Module 1 | Module 2 | Module 3 | Module 4 |
|---|---|---|---|---|
| Median (range) | Median (range) | Median (range) | Median (range) | |
| n = 35 | n = 30 | n = 33 | n = 28 | |
| Site educators’ self-scoring | ||||
| Asking open questions | 4 (4–5) | 4 (4–5) | 4 (3–5) | 4 (4–5) |
| Focusing on positive solutions | 4 (3–5) | 4 (3–5) | 4 (3–5) | 4 (3–5) |
| Avoiding giving a choice or answer | 4a (2–5) | 4 (2–5) | 4 (2–5) | 4 (3–5) |
| Encouraging families and children to identify previous successes | 4 (2–5) | 4 (3–5) | 4 (3–5) | 4 (3–5) |
| Asking ‘how come?’ | 4 (1–5) | 4 (3–5) | 4 (3–5) | 4 (3–5) a |
| Asking ‘what else?’ | 4b (3–5) | 4 (3–5) | 4 (3–5) | 4 (3–5) |
| n = 10 | n = 11 | n = 10 | n = 12 | |
| Observer scoresc | ||||
| Asking open questions | 4 (3–4) | 4 (3–5) | 4 (3–5) | 4 3–5) |
| Focusing on positive solutions | 4 (2–5) a | 4 (2–5) | 4 (2–5) | 4 (3–5) |
| Avoiding giving a choice or the answer | 3 (2–4) | 4 (3–5) | 3 (2–4) | 4 (2–5) |
| Encouraging families and children to identify previous successes | 4 (2–5) | 3 (2–5) | 4 (2–5) | 4 (2–5) |
| Asking ‘how come?’ | 3 (2–4) | 3 (2–5) | 3 (2–4) | 3 (2–4) |
| Asking ‘what else?’ | 4 (3–5) | 4 (3–5) | 4 (2–5) | 3.5 (2–5) |
| Reasons (multiple allowed) | No. (N = 36) |
|---|---|
| Didn’t want to/couldn’t miss school | 10 |
| Wasn’t told when the modules were | 6 |
| Ill on the day | 5 |
| Wasn’t interested/didn’t want to go | 5 |
| Clashed with other out of school activities/work | 4 |
| No one to attend with them/parent work | 4 |
| Didn’t think they would help | 3 |
| Couldn’t get to the place where they were being organised | 2 |
| Forgot to attend | 2 |
| Was away/on holiday | 2 |
| Felt too shy/scared to attend group sessions | 1 |
| Accompanied by: | No. of modules attended (N = 297) |
|---|---|
| Mother | 203 (68%) |
| Father | 34 (11%) |
| Mother and father (or stepfather) | 41 (14%) |
| Parent(s) and sibling | 7 (2%) |
| Sibling | 4 (1%) |
| Other family member (grandmother, aunt) | 3 (1%) |
| Mother and grandmother | 1 (0%) |
| Girlfriend | 1 (0%) |
| No child, just family members (2 × mother; 1 × father and grandmother) | 3 (1%) |
Appendix 8 Case example of exemplary CASCADE session
The team of site educators were well prepared and organised – they had done ‘dummy runs’ of each module beforehand. They worked well together and each had a role. The site educators were authoritative, positive and caring. They appeared confident and relaxed. The team were very respectful of the families. They thanked them for their thoughts/contributions. They listened well and used MI techniques consistently and appropriately.
A site educator explained activities clearly so patients and carers knew what was required of them. They reiterated key points at different stages of the activity. There was consistent use of the flipchart so key points and outcomes of discussion were recorded and visible to everyone.
Site educators were inclusive in discussions; they asked for feedback from everyone without ‘picking on’ individual people. They went into discussions in depth, facilitating this in a way that seemed to invite families to contribute quite openly. They asked people to think about the possibility of doing things differently in managing their diabetes. They asked them if they might be willing to try to do things differently without telling them what they should do and got feedback from families to their open questions.
Site educators gave families concrete examples to illustrate points made so they could use the information in their everyday life. More time was spent on some sections of module 4 than was noted in other sites, for example they spent over 30 minutes on the subject of the timing of insulin injections in relation to exercise. This was because they explained the topic in depth, as it had particularly relevance to some of the group attendees.
Ideas, questions and experiences raised by families were followed through by site educators either in the group as a whole or on a one-to-one basis so nothing was left uncertain or unexplored.
Appendix 9 Markov chain Monte Carlo submodels for retinopathy, foot ulcer and myocardial infarction
FIGURE 9.
Markov chain Monte Carlo submodel for retinopathy. incr rwd, incremental reward; init rwd, initial reward; term rwd, final reward.
FIGURE 10.
Markov chain Monte Carlo submodel for foot ulcer. incr rwd, incremental reward; init rwd, initial reward; term rwd, final reward.
FIGURE 11.
Markov chain Monte Carlo submodel for infarction. incr rwd, incremental reward; init rwd, initial reward; term rwd, final reward.
Appendix 10 Statistical distribution of parameters used in the model
| Description of distributions | Type of distributiona | Parameters |
|---|---|---|
| Cost ESKD | γ β |
α: (185602)/(23202), λ: 18,560/(23202) |
| Cost enhanced CASCADE | α: (1253.962)/(156.742), λ: 1253.96/(156.742) | |
| Cost uncomplicated foot ulcer | α: (22362)/(279.502), λ: 2236/(279.502) | |
| Cost treatment (hospitalisations) | α: (24852)/(310.622), λ: 2485/(310.622) | |
| Cost myocardial infarction | α: (14102)/(176.252), λ: 1410/(176.252) | |
| Cost CASCADE | α: (421.572)/(52.702), λ: 421.57/(52.702) | |
| Cost uncomplicated kidney disease | α: (26882)/(3362), λ: 2688/(3362) | |
| Cost foot amputation | α: (87262)/(1090.752), λ: 8726/(1090.752) | |
| Cost retinopathy | α: (11872)/(148.372), λ: 1187/(148.372) | |
| Initial probability for no complications | Subtype: 2, α: 0.2724, β: 0.01218 | |
| Initial probability for retinopathy | Subtype: 2, α: 15.5325, β: 549.2857 | |
| Initial probability for uncomplicated foot ulcer | Subtype: 2, α: 15.8963, β: 2590.055 | |
| Initial probability for uncomplicated kidney disease | Subtype: 2, α: 15.8436, β: 1706.287 | |
| Probability of remaining in the no complication stage in the low HbA1c level | Subtype: 2, α: 0.81534, β: 0.00895 | |
| Probability of remaining in the no complication stage in the high HbA1c level | Subtype: 2, α: 1.082687, β: 0.15116 | |
| Probability of moving from no complications to uncomplicated kidney disease in the low HbA1c level | Subtype: 2, α: 15.92274, β: 3487.829 | |
| Probability of moving from no complications to uncomplicated kidney disease in the high HbA1c level | Subtype: 2, α: 0.600924, β: 0.062473 | |
| Probability of moving from no complication to ESKD in the low HbA1c level | Subtype: 2, α: 15.9918, β: 33127.63 | |
| Probability of moving from no complication to ESKD in the high HbA1c level | Subtype: 2, α: 15.9836, β: 16547.33 | |
| Probability of moving from no complication to retinopathy in the low HbA1c level | Subtype: 2, α: 15.98216, β: 15213.83 | |
| Probability of moving from no complication to retinopathy in the high HbA1c level | Subtype: 2, α: 15.92567, β: 3626.308 | |
| Probability of moving from no complication to uncomplicated foot ulcer in the low HbA1c level | Subtype: 2, α: 15.9898, β: 26633.68 | |
| Probability of moving from no complication to uncomplicated foot ulcer in the high HbA1c level | Subtype: 2, α: 15.9745, β: 10633.69 | |
| Probability of moving from no complication to foot amputation in the low HbA1c level | Subtype: 2, α: 15.97245, β: 9839.183 | |
| Probability of moving from no complication to foot amputation in the high HbA1c level | Subtype: 2, α: 15.92501, β: 3594.37 | |
| Probability of moving from no complication to myocardial infraction in the low HbA1c level | Subtype: 2, α: 15.9564, β: 6205.444 | |
| Probability of moving from no complication to myocardial infraction in the high HbA1c level | Subtype: 2, α: 15.91502, β: 3167.725 | |
| Probability of remaining in the uncomplicated kidney disease in the low HbA1c level | Subtype: 2, α: 0.203, β: 0.00999 | |
| Probability of remaining in the uncomplicated kidney disease in the high HbA1c level | Subtype: 2, α: 0.18876, β: 0.00946 | |
| Probability of moving from the uncomplicated kidney disease to ESKD in the low HbA1c level | Subtype: 2, α: 15.9918, β: 33127.63 | |
| Probability of moving from the uncomplicated kidney disease to ESKD in the high HbA1c level | Subtype: 2, α: 15.97668, β: 11628.83 | |
| Probability of moving from uncomplicated kidney disease to death in the low HbA1c level | Subtype: 2, α: 15.2112, β: 312.6164 | |
| Probability of moving from uncomplicated kidney disease to death in the high HbA1c level | Subtype: 2, α: 14.4224, β: 140.9914 | |
| Probability of remaining in the ESKD state in the low HbA1c level | Subtype: 2, α: 8.486, β: 10.7131 | |
| Probability of remaining in the ESKD state in the high HbA1c level | Subtype: 2, α: 14.028, β: 106.903 | |
| Probability of moving from ESKD to death in the low HbA1c level | Subtype: 2, α: 6.514, β: 5.160 | |
| Probability of moving from ESKD to death in the high HbA1c level | Subtype: 2, α: 14.028, β: 106.903 | |
| Probability of remaing in the retinopathy stage in the low HbA1c level | Subtype: 2, α: 12.39328, β: 46.0215 | |
| Probability of remaining in the retinopathy stage in the high HbA1c level | Subtype: 2, α: 14.028, β: 106.903 | |
| Probability of moving from retinopathy to death in the low HbA1c level | Subtype: 2, α: 2.60572, β: 0.701972 | |
| Probability of moving from retinopathy to death in the high HbA1c level | Subtype: 2, α: 14.028, β: 106.903 | |
| Probability of remaining in the uncomplicated foot ulcer stage in the low HbA1c level | Subtype: 2, α: 0.2928, β: 0.01271 | |
| Probability of remaining in the uncomplicated foot ulcer stage in the high HbA1c level | Subtype: 2, α: 0.768, β: 0.089143 | |
| Probability of moving from uncomplicated foot ulcer to foot amputation in the low HbA1c level | Subtype: 2, α: 15.3404, β: 380.0307 | |
| Probability of moving from uncomplicated foot ulcer to foot amputation in the high HbA1c level | Subtype: 2, α: 14.351, β: 133.5975 | |
| Probability of moving from uncomplicated foot ulcer to death in the low HbA1c level | Subtype: 2, α: 15.9524, β: 5681.333 | |
| Probability of moving from uncomplicated foot ulcer to death in the high HbA1c level | Subtype: 2, α: 15.881, β: 2252.833 | |
| Probability of remaining in the foot amputation stage in the low HbA1c level | Subtype: 2, α: 9.9888, β: 18.26007 | |
| Probability of remaining in the foot amputation stage in the high HbA1c level | Subtype: 2, α: 14.028, β: 106.903 | |
| Probability of moving from foot amputation to death in the low HbA1c level | Subtype: 2, α: 5.0112, β: 2.741275 | |
| Probability of moving from foot amputation to death in the high HbA1c level | Subtype: 2, α: 14.028, β: 106.903 | |
| Probability of remaining in the myocardial infraction stage in the low HbA1c level | Subtype: 2, α: 7.28376, β: 6.922356 | |
| Probability of remaining in the myocardial infraction stage in the low HbA1c level | Subtype: 2, α: 0.972, β: 0.127548 | |
| Probability of moving from the myocardial infraction stage to death in the low HbA1c level | Subtype: 2, α: 7.71624, β: 8.119091 | |
| Probability of moving from the myocardial infraction stage to death in the high HbA1c level | Subtype: 2, α: 14.028, β: 106.903 | |
| Probability of utility for no complication | Subtype: 2, α: 2.162, β: 0.49402 | |
| Probability of utility for uncomplicated kidney disease | Subtype: 2, α: 3.76, β: 1.46222 | |
| Probability of utility for uncomplicated foot ulcer | Subtype: 2, α: 6.667, β: 5.476898 | |
| Probability of utility for retinopathy | Subtype: 2, α: 2.842, β: 0.829835 | |
| Probability of utility for foot amputation | Subtype: 2, α: 11.155, β: 27.98535 | |
| Probability of utility for myocardial infarction | Subtype: 2, α: 3.93, β: 1.605211 | |
| Probability of utility for ESKD | Subtype: 2, α: 5.358, β: 3.201105 | |
| Probability of low HbA1c level | Subtype: 2, α: 12.77, β: 54.44053 | |
| Probability of high HbA1c level | Subtype: 2, α: 2.23, β: 0.523086 |
Appendix 11 Expected costs and effectiveness for the control of diabetes type 1 interventions with 95% credible ranges based on the results from the 10,000 Monte Carlo simulations
| Practices | Cost, £ (range) | QALY (range) |
|---|---|---|
| Current NHS practice | 247,551 (209,080–289,642) | 14.4293 (13.48–15.59) |
| Enhanced CASCADE | 252,053 (211,590–290,023) | 14.7121 (13.78–15.80) |
| CASCADE | 247,972 (205,852–293,964) | 14.4293(13.48–15.59) |
Appendix 12 Follow-up questionnaire (parent of young person > 12 years)
List of abbreviations
- BFST
- Behavioural Family Systems Therapy
- BG
- blood glucose
- CAMHS
- Child and Adolescent Mental Health Service
- CASCADE
- Child and Adolescent Structured Competencies Approach to Diabetes Education
- CBT
- cognitive–behavioural therapy
- CHO
- carbohydrate
- CI
- confidence interval
- CNR
- case note review
- DAFNE
- Dose Adjustment for Normal Eating
- DCCT
- Diabetes Control and Complications Trial
- DEPICTED
- Development and Evaluation of a Psychosocial intervention for Children and Teenagers Experiencing Diabetes
- DESMOND
- Diabetes Education and Self Management for Ongoing and Newly Diagnosed
- DFRQ
- Diabetes Family Responsibility Questionnaire
- DKA
- diabetic ketoacidosis
- DMEC
- Data and Ethics Monitoring Committee
- DNA
- did not attend
- DRN
- Diabetes Research Network
- DUK
- Diabetes UK
- DVD
- digital versatile disk
- ESKD
- end-stage kidney disease
- FACTS
- Family and Adolescent Communication and Teamwork Study
- HbA1c
- glycosylated fraction of haemoglobin
- HCP
- health-care providers
- HTA
- Health Technology Assessment
- ICC
- intracluster correlation coefficient
- ISPAD
- International Society for Paediatric and Adolescent Diabetes
- KICk-OFF
- Kids in Control of Food
- LSHTM
- London School of Hygiene and Tropical Medicine
- MCMC
- Markov chain Monte Carlo
- MCRN
- Medicines of Children Research Network
- MDI
- multiple daily injection
- MDT
- multidisciplinary team
- MI
- motivational interviewing
- MRC
- Medical Research Council
- NDST
- National Diabetes Support Team
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute of Health Research
- NMC
- Nursing and Midwifery Council
- NPDA
- National Paediatric Diabetes Audit
- PDSN
- paediatric diabetes specialist nurse
- PE
- process evaluation
- PedsQL
- Paediatric Quality of Life Inventory
- PI
- principal investigator
- PREM
- patient-reported experience measure
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- R&D
- research and development
- RCN
- Royal College of Nursing
- RCPCH
- Royal College of Paediatrics and Child Health
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- SDQ
- Strengths and Difficulties Questionnaire
- SE
- south-east
- SES
- socioeconomic status
- SFBT
- solution-focused brief therapy
- SOP
- standard operating procedure
- SSRU
- Social Sciences Research Unit
- SWEET
- better control in paediatric and adolescent diabetes in the EU: working to create centres of reference
- T1D
- type 1 diabetes
- TSC
- Trial Steering Committee
- UCL
- University College London
- UCLH
- University College London Hospitals
- WTE
- whole-time equivalent