Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/14/51. The contractual start date was in August 2009. The draft report began editorial review in December 2012 and was accepted for publication in June 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Professor Marion Walker is a member of the Global Stroke Community Advisory Panel (GSCAP), funded by Allergan. Hywel Williams is a member of the NIHR Journals Library Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Logan et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Introduction
In 2009 the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme funded a proposal to investigate the clinical effectiveness and cost-effectiveness of delivering a targeted outdoor mobility therapy provided within stroke services aimed at helping people get out of the house more often following a stroke. This report details the results of the Getting out of the House Study.
The effect of stroke on functioning, and rehabilitation
Stroke can have a devastating effect on people’s lives, with half of survivors being dependent on others 6 months later,1 one-third feeling socially isolated, one-quarter having abnormal moods, and half not getting out of their houses as much as they would like. 2 These are worldwide health-care issues but, with 130,000 people a year having a stroke in England and Wales,3 and stroke increasing with age, the number of people needing UK health and social care services is set to rise. The cost of stroke to the UK NHS is estimated to be over £2.5B per year3 in addition to the cost to social care services and to the stroke survivors. In the UK most people who have a stroke are admitted to hospital for acute care but are discharged on average 19.5 days later. 4 Treatment is then transferred to community-based teams and social care services.
Stroke unit care has been found to be the best way to organise inpatient care for stroke patients,5 allowing them to be transferred to evidenced-based Early Supported Stroke Discharge services6 when they go home. Once home, stroke rehabilitation interventions provided by occupational therapists have been shown to increase activity7 , 8 by helping people to regain independence in personal and domestic activities of daily living. Most stroke rehabilitation research has been conducted on people in the first year after their stroke. Although many consequences of stroke persist beyond a year there is little evidence for the benefit of interventions delivered after 1 year. 9 The early treatment and rehabilitation of stroke necessarily focuses on reducing neurological impairments but as time goes by after a stroke the activity and participation become more important. Also, the first goal of patients in hospital tends to be to get home, which requires a focus on self-care activities, but, once home, they set goals in terms of being able to optimise participation, and these tend to require an ability to get out of the house or to engage in social activities. However, these rehabilitation areas are rarely targeted.
Outdoor mobility after a stroke, and rehabilitation
Stroke can leave people with long-term and persistent impairments, leading to activity limitations and restriction in participation. These restrictions occur in addition to other impairments resulting from comorbidities, such as osteoarthritis or dementia, making recovery and evaluation of treatments difficult and complicated. In addition, stroke often leaves people with perceptual and cognitive impairments that can reduce performance of everyday activities, such as shopping, hobbies, cooking or using the bus. Lowering of mood, feelings of becoming a different person and lack of confidence are also common after stroke. 10 These hidden impairments often go untreated and, alongside the more obvious physical ones, contribute to activity limitations especially when people are discharged from health services. There is evidence that stroke patients may delay getting back to a normal life, even although they may have made a good physical recovery. 11 Hellstrom et al. 12 argued it is not until people go home after a stroke and attempt to try everyday activities that the real impact is realised. 12 In response to this, it is recommended that all stroke patients receive a 6-month review, with rehabilitation being available to enable people to maintain participation, reduce long-term misery and improve quality of life. These are priority areas identified in the Department of Health of England and Wales Stroke Strategy. 13
Limitations in outdoor mobility affect 42% of stroke patients,2 with 33% remaining permanently dependent on others long term. 3 Stroke patients can become housebound, leading to poor mental health, isolation and misery. 14 This can be devastating for patients and carers, and increase their reliance on home visits from medical staff, home care, social services and home adaptations. Stroke tends to affect older people, median age 77 years [interquartile range (IQR) 68–84 years],15 and, as outdoor mobility – including walking outside – declines with age, mobility disorders may also be due to impairments and social factors associated with ageing and comorbidity. People aged ≥ 80 years of age make half the number of outdoor journeys and travel less than one-quarter of the distance of those aged 50–54 years. 16 There is evidence that elderly people find it difficult to participate in transport services, owing to an inability to carry heavy loads and a fear of crime when outside at night. 14 People who are dependent on walking frames are, on average, getting out of the house less than twice each week. 17 Outdoor mobility is usually achieved by a combination of walking, cars and public transport, with very few people using specialist transport such as the provision of a taxi service for people with disabilities. 16 , 18
Specific outdoor mobility rehabilitation interventions exist for people with limited vision, learning disabilities and those who use wheelchairs19 but these are not routinely used for people who have had a stroke. The National Clinical Stroke Guidelines do not contain any evidence-based recommendations to guide therapists in how to treat outdoor mobility limitations. 20 However, the clinical guidelines recommend that stroke patients are encouraged to take physical exercise, as there is an increasing body of evidence that exercise is of benefit to stroke survivors. 21 In the UK the routine treatment for outdoor mobility problems is to provide information in a leaflet and verbally, delivered by a health-care professional. A Cochrane review22 concluded that the passive provision of leaflets is not effective and leaflets can be difficult to understand after a stroke. It is more likely that a multimodal rehabilitation intervention that includes exercises to decrease impairments (these can be physical and mental), education to provide information and behaviour change regimes, use of adaptive equipment and support from other people, both formal or informal sources, would be more effective at improving outdoor mobility. This study aimed to fill this evidence gap.
Justification for the current study
An outdoor mobility intervention for people with stroke was developed using qualitative interview findings, published literature, correspondence with the Department of Transport and expert opinion. 23 This intervention was evaluated in a single-centre randomised controlled study. 24 The study found clear benefits in outdoor mobility for people who received the intervention. The patients who received the intervention took twice as many journeys, many of these walking, as those in the control group, 4 and 10 months after recruitment into the study, and were significantly more likely to be satisfied with their outdoor mobility activity. Although the study was based on a real clinical need, recruited to target, had excellent follow-up and was published in a peer-reviewed journal, it had some limitations. The intervention was delivered by a single therapist, who was a stroke specialist, with experience in the delivery of community-based treatment. It was delivered in one UK city and no health economic evaluation was completed. Although this study has already influenced practice, alone it is insufficient to inform evidence-based guidelines.
Thus, the next step was to conduct a multicentre randomised controlled trial (RCT), using the lessons learned from the single-centre study. We completed a multicentre RCT to try and answer the following questions.
-
Whether:
-
the results of the pilot study were generalisable to other therapists and other sites.
-
the intervention could be implemented across a health system.
-
the intervention improves overall health and, if so, whether such an approach is conventionally cost-effective.
-
Structure of the Health Technology Assessment monograph
The monograph has been separated into methods and results for the randomised controlled study, including delivery of the intervention and clinical effectiveness, followed by methods and results for both the economic evaluation and the qualitative substudy. Finally, all results are discussed, followed by overall conclusions.
Appendices include the summary of the intervention manual and the statistical analysis plan.
Chapter 2 Randomised controlled study methods
This chapter describes the methods for the delivery and assessment of the intervention and collection of data to assess clinical effectiveness.
Summary of study design
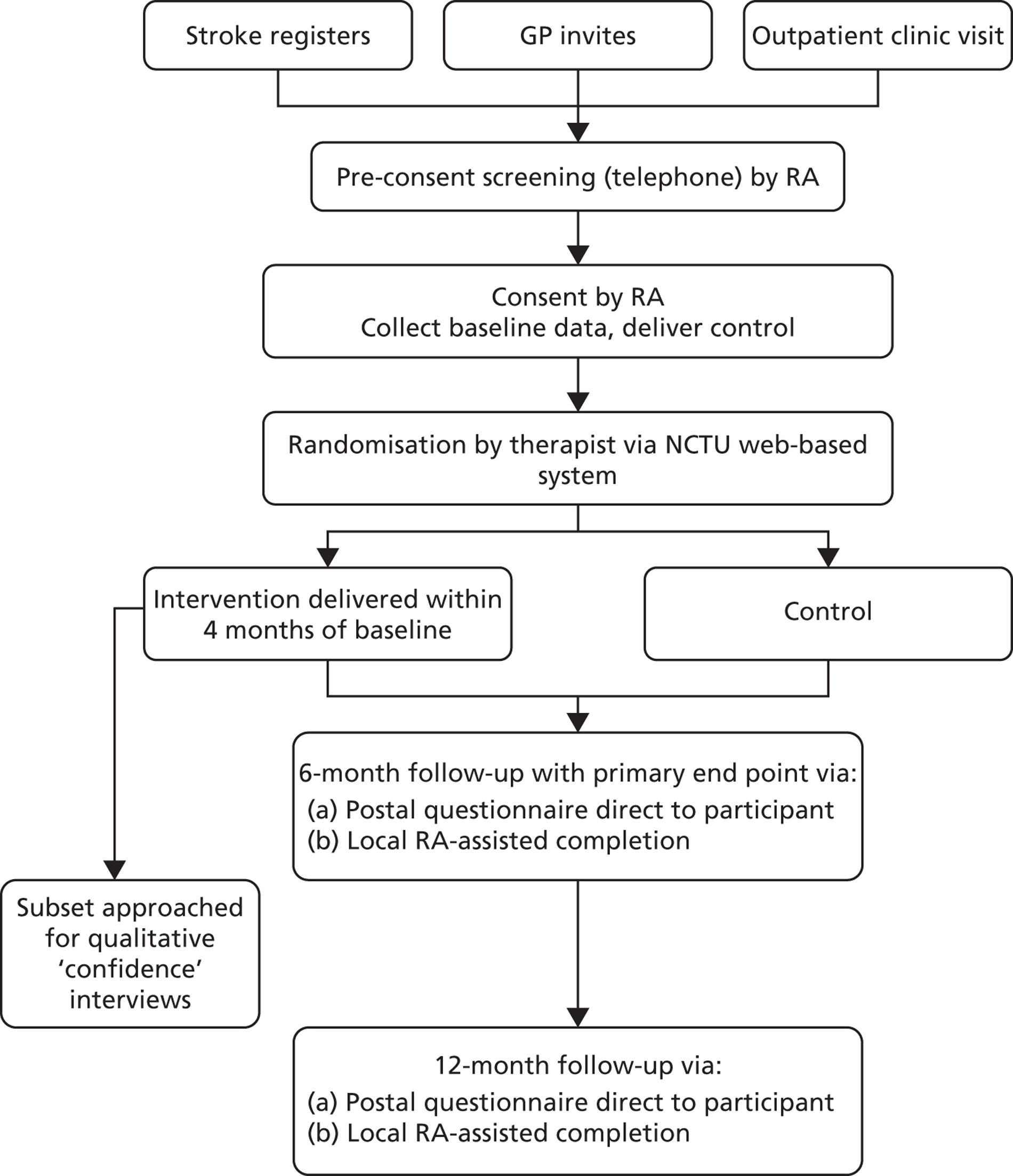
The Getting out of the House Study was a multicentre, parallel-group RCT comparing clinical effectiveness and cost-effectiveness for two groups, an intervention group and a control group. The methodology used was, as far as possible, a replication of that used in the single-centre study. 24 Figure 1 summarises the study design. Patients aged ≥ 18 years, who had experienced a stroke > 6 weeks previously were considered initially eligible for the study. The majority of patients were contacted by a patient invitation letter sent from either their general practitioner or from the local community rehabilitation or local hospital stroke service register. If interested, patients replied to their local research team, who contacted them and screened them by telephone call to further determine eligibility. Final confirmation of eligibility and subsequent consenting was carried out by the research assistant (RA) at the patient’s home. Following consent, a baseline assessment was completed and all participants received the control. They were provided with travel diaries in the form of 12 months of ‘travel calendar’ to record the number of journeys that they made and any falls that they had. All participants were then randomised by the local therapist, using the web-based randomisation system that was controlled by the Nottingham Clinical Trials Unit (NCTU), to either targeted outdoor mobility therapy (the intervention) or no further therapy contact (control). Those randomised to the intervention received up to 12 visits over a maximum of 4 months (from baseline). No further therapy contact was received after 4 months, allowing 2 months before the primary and secondary outcome data were collected. Outcomes were assessed at 6 and 12 months after recruitment by postal questionnaire and by monthly travel diaries. Local RAs blinded to treatment allocation were available to assist the participant with completion of questionnaires if needed. RAs recorded incidents where they were unblinded. A subset of participants at a single site were asked to participate in a nested qualitative study investigating confidence issues surrounding stroke.
FIGURE 1.
Study design flow chart. GP, general practice.
Primary objective
To test the clinical effectiveness and cost-effectiveness of treating people who have had a stroke with an outdoor mobility rehabilitation intervention in addition to routine care compared with routine care alone.
Secondary objectives
The secondary objectives were to measure whether the intervention was associated with:
-
improved:
-
mobility in and outside the house
-
patient well-being
-
participation in everyday activities
-
carer well-being
-
health-related quality of life.
-
Primary outcome measure
The primary outcome measure was the Social Function domain score of the Short Form questionnaire-36 items, version 2 (SF-36v2)25 measured at 6 months, as recorded in their questionnaire response. This is one of the eight domains of the SF-36v2 and was scored and transformed on to a scale of 0 (worst possible health state) to 100 (best possible health state).
Secondary outcome measures
The secondary outcome measures were:
-
functional activity in participants measured by the Nottingham Extended Activities of Daily Living (NEADL) scale26 , 27
-
mobility reported by participants in the completion of the Rivermead Mobility Index (RMI)28
-
the number of journeys as recorded by participants in the travel calendars
-
satisfaction with outdoor mobility (SWOM) as reported by participants in the completion of a yes/no question: ‘Do you get out of the house as much as you would like?’
-
psychological well-being as reported by participants in the completion of General Health Questionnaire 12 (GHQ-12). 29
-
carer psychological well-being as reported by participants in the completion of GHQ-1229
-
health-related quality of life as reported by participants in the completion of European Quality of Life-5 Dimensions (EQ-5D)30 and Short Form questionnaire-6 Dimensions (SF-6D)25 (based on a subset of questions from the SF-36 v231)
-
the resource use of health and social care, carer input and provision of equipment as reported by participant in the completion of the service use questionnaire and data collected relating to intervention visits
-
participant mortality (death data) as collected from NHS Information Centre (IC)/NHS Central Register.
Recruitment process
There were three methods of patient identification: searching general practice (GP) databases, searching stroke registers in secondary care hospitals, community hospitals or primary care community teams and approaching patients attending post-stroke outpatient clinics as they were discharged from rehabilitation. If the search team also had access to local stroke service rehabilitation records they cross referenced for any active stroke-related rehabilitation that may be ongoing.
All potential participants received a version of the participant information sheet (PIS) prior to the baseline visit and had sufficient time to consider the information. In addition, if required, an aphasic PIS and a summary PIS were available for participants and their carers.
Other recruitment strategies included placing adverts within local newspapers, local trust publications and relevant websites (e.g. the Stroke Association), establishing a study website, various articles within local trust publications and local press, visiting local stroke groups and widespread distribution of the study poster to relevant areas (e.g. GPs, rehabilitation teams, stroke groups, etc.).
Sample size
Our sample size calculations were based on the primary outcome measure, the Social Function domain of the SF-36v2 at 6-month follow-up. Although this was not used in the pilot study, a recent study had suggested a minimally clinically important difference (MCID) for the Social Function domain was 12.5 points. 32 The estimate of the standard deviation (SD) was taken from Brittle’s study33 and this closely matched the standard deviation obtained by assuming that the distribution of the nine possible integer-valued scores followed a roughly right-angled triangular distribution (i.e. that was positively skewed). This followed the general approach suggested by Deming34 for schematic distributions. Assuming a power of 90% and a two-sided significance level of 5%, we estimated that to detect a difference in mean SF-36 scores of 12.5 points, assuming a common SD of 28.2 points,33 a sample size of 135 patients per group was required. This calculation assumed an attrition rate of 20% over a 6-month period.
Clustering by delivery of treatment was allowed for by generating simulated data to mimic the multilevel structure of the proposed study, with site-specific variation between therapists. The likely existence of sharing of therapists by patients was addressed by a multiple membership model, and the variance of the treatment effect so found was compared with that of a regression model ignoring clustering (both models treating centre as a fixed effect). The data sets and analyses were performed in Stata v10 (StataCorp LP, College Station, TX, USA). The variance inflation observed was insensitive to variation in the simulated between-site variance and the average between-therapist variance, and a rounded value of 2.5 was selected as the multiplier for a sample size based on a naive analysis. After the allowance for clustering by delivery of treatment, a sample size of 338 participants per arm was required, with an overall sample size of 676, allowing for 20% lost to follow-up.
Revised sample size
Owing to an increase in the expected number of sites, the sample size was revised.
As more information had become available after the original sample size calculation was performed we developed an alternative method of calculating a sample size that was easier to replicate. This analytic approach was based on the same assumptions of the original sample size calculation and assumed that within each site there would potentially be four therapists delivering the intervention in the intervention group and one therapist delivering the control in both groups. In these calculations we assumed a therapist effect intraclass correlation coefficient (ICC) of 0.02 (Professor Stephen Walters, University of Sheffield, 30 October 2008, personal communication) and a site effect ICC of 0.04 [obtained from Aberdeen University’s database of ICCs (www.abdn.ac.uk/hsru/epp/iccs-web.xls)]. For comparison, based on seven sites this method gave a sample size of 746, allowing for 20% lost to follow-up. This method was adopted to provide a revised sample size.
As the number of sites had increase from the assumed 7 to 14 (at that time) we conducted further power calculations assuming a lower sample size and investigated the effect of varying the number of sites. This was based on detecting a difference in means of 12.5 (as in the original sample size calculation) and assuming a total sample size of 440 with the number of sites ranging from 7 (as originally planned) to 14. These calculations were based on the same assumptions made in the analytic sample size above (i.e. SD = 28.2 sites, two-sided significance level of 5%, ICC of 0.02 for therapist effect, and ICC of 0.04 for centre effect). These indicated that a sample size of 440 would have a power of 86% (assuming no attrition) and a power of 82% (assuming lost to follow-up rate of 20%) to detect a difference in means of 12.5 if there were seven sites and if there were 12 or more sites the corresponding power would be at least 90%. There were a total of 15 sites in this study so this met the latter calculations.
Sample size calculations can be performed only if a number of assumptions are made and although our calculations were based on the best estimates we could obtain at the time that there was some uncertainty about the true values of the ICCs used to adjust for clustering. Therefore, we decided to be cautious and aimed to recruit a sample size of 506 participants (440 + 15%) to ensure that we had recruited enough participants. This was agreed with the Trial Steering Committee (TSC).
Setting and locations
The study was coordinated by the NCTU and involved 15 sites from across England, Scotland and Wales. Initially seven sites were planned but during the course of recruitment an additional eight were added.
The sites were a mixture of settings, split in broad terms as follows.
Primary care community stroke rehabilitation service
-
NHS Nottingham City.
-
NHS Nottinghamshire County.
-
Gateshead Primary Care Trust (PCT).
-
NHS North Somerset.
-
Wolverhampton PCT.
-
Kent Community Health Trust.
-
Tower Hamlets PCT.
-
Norfolk Community Health and Care NHS Trust/NHS Norfolk.
-
NHS Bristol.
Secondary care community stroke rehabilitation service
-
NHS Lanarkshire.
-
NHS Grampian.
-
Cardiff and Vale University Health Board.
-
Cwm Taf Health Board.
Secondary care stroke service
-
United Lincolnshire Hospitals NHS Trust.
-
Southend Hospital NHS Foundation Trust.
Eligibility criteria
Patients were eligible for the study if they provided written informed consent and if they:
-
were aged ≥ 18 years
-
had experienced a stroke at least 6 weeks previously, and
-
wished to get out of the house more often.
Patients were not eligible for the study if they were:
-
not able to comply with the requirements of the protocol and therapy programme – in the opinion of the assessor
-
still in post-stroke intermediate care or active rehabilitation, or
-
previously enrolled in this study.
Patients who had suffered a transient ischaemic attack were not included.
Screening and baseline assessment
The majority of preconsent screening was undertaken over the telephone by the RA in response to a patient reply to a patient invitation, and involved a general discussion of the study background and participation requirements. In addition, key eligibility questions were asked in relation to wishing to get out of the house more often and establishing potential outdoor mobility goals related to the intervention manual. Following this process, if the potential participant remained eligible then a baseline visit would be arranged.
The baseline visit was conducted in the potential participant’s home. The visit consisted of four elements: first, confirming eligibility against inclusion and exclusion criteria; second, consenting (see Consent, below); third, baseline data collection; and, fourth, delivery of the control (see Control, below).
Only limited numbers of data were collected, including basic demographics, time since stroke and contact details.
Consent
Participant information sheets were available in several different formats, including full length, abbreviated leaflet style, summary and aphasic. In practice, a combination of all four of these were often used, as appropriate, for each individual participant. Only people who had the mental capacity to consent to participation, defined against the criteria in the English Mental Capacity Act,35 were eligible to take part. If there were communication problems then the participant’s carer or relative was involved in the process. In addition, owing to potential physical limitations with completion of the consent forms, it was acceptable for the participant to mark the consent form, accompanied by a witness signature to complete the consent process. If the physical challenges were more severe then a witness (usually a member of the participant’s family, carer or neighbour) could complete the consent form on the participant’s behalf.
During the baseline visit, carer information sheets and questionnaires were provided to all carers. They were allowed to complete them immediately or later, and the process of completing and returning the questionnaire was considered to be implied consent.
Control information
All participants in the study received the control information during the baseline visit by the RA. The control information was provision of verbal and written local mobility and transport information. This information was site specific. Each site collated a pack of local information and leaflets as guided by the research team. They were asked to include information about bus times, local community transport, taxi services, wheelchair services, disabled persons’ car badges, wheelchair borrowing schemes and mobility equipment. These individualised packs were given to the participant after their content had been discussed.
Randomisation
Following consent and completion of the baseline assessments and delivery of the control information, the participant’s details were passed to the local therapist. The therapist accessed the remote, secure web-based electronic case report form (eCRF) and randomisation system developed and maintained by the NCTU and entered basic demographic details. Once these details had been entered irrevocably into the system, the group to which the participant was randomly allocated was provided to the therapist. Randomisation was created using Stata/SE version 9 statistical software with a 1 : 1 allocation to intervention group or control. Participants and therapists were aware of the intervention allocation, whereas the RA responsible for assisting with collection of the outcome measures was kept blinded to the allocation throughout the study.
Randomisation was based on a computer-generated pseudorandom code using random permuted balanced blocks of randomly varying size, set up and maintained by the NCTU in accordance with its standard operating procedure and held on a secure server. The randomisation was stratified by age (< 65 years and ≥ 65 years) and site. Sixty-five years of age was chosen as a cut-off point because Mobility Allowance – a monetary allowance – was no longer available for people aged > 65 years and over. Access to the sequence was confined to the NCTU Informartion Technology (IT) Manager. The sequence of treatment allocations was concealed until all participants had been randomly assigned to a treatment group and recruitment, data collection and all other study-related assessments were complete.
If the participant was randomised to the intervention group then the therapist contacted the participant to arrange an initial goal-setting visit. If the participant was randomised to the control group the therapist would either, based on site staff preference, not contact the participant or contact the participant to explain their allocation. It was not recorded which approach was used for which participant.
Intervention
The development of the intervention followed the guidelines. 36 Qualitative interviews23 were used to identify ‘barriers and need’ theories. A group of senior and specialist therapists met to discuss the interviews and developed an intervention based on the results, their clinical skills and training. The intervention training manual that was used to train therapists in each site was developed using a systematic approach. Initially, a literature review was performed using the search strategy for the following terms ‘rehabilitation’, ‘stroke’, ‘occupational therapy’ and ‘outdoor mobility’, with the results reviewed by a panel of expert stroke clinicians from which a draft training manual was developed. This was then reviewed by a focus group of therapists (n = 15) who had either delivered the intervention in the pilot study or were clinical experts in stroke. The intervention training manual used for this study contained:
-
standardised assessments
-
case vignettes of treatment plans
-
goal planning and activity analysis guidelines
-
protocol for a first outing walking, using the bus and using a taxi
-
checklist of benefits and barriers to going outside
-
motivational and confidence-building strategies
-
examples of skills needed to catch a bus or train
-
examples of skills needed to be able to operate an outdoor mobility scooter.
The aim of the intervention was to increase outdoor mobility participation by alleviating physical difficulties, developing skills to maximise the individual’s potential and overcoming psychological barriers. The main component of the intervention was for participants to repeatedly practise outdoor mobility. This included buses, taxis, walking, voluntary drivers and mobility scooters until they felt confident to go alone or with a companion. The number of intervention sessions depended entirely on the participant. If they felt they did not require any further intervention, for whatever reason, then the intervention stopped. If they felt they required additional intervention for whatever reason, they could continue the intervention up to a maximum of 12 visits. We recorded the duration of each intervention visit, including the time taken to deliver the intervention, and travel to and from the participant’s home. A description of the intervention has been published37 and a training manual developed. A more detailed summary of the training manual can be found in Appendix 2 .
Training therapists to provide the intervention
Therapists in each site were trained by Pip Logan [chief investigator (CI) and sole therapist in the pilot study] to deliver the intervention using the intervention training manual and a standard presentation over the course of 2 hours. Following this face-to-face training, the majority of therapists attended an update session each year. On an ongoing basis they were able to e-mail other therapists on the study to discuss treatments and were able to contact the study CI with any queries relating to the intervention.
Each therapist was registered on the eCRF database and when the record of a particular intervention visit was added to the eCRF then a therapist, or therapists, had to be assigned to that particular visit, therefore providing an accurate record of the delivery of the intervention, which could be correlated against the training records.
Data collection
There were four methods of data collection: eCRF, questionnaire booklets, travel diaries and intervention records. The NCTU performed quality control checks on all aspects of data collected and entered onto the eCRF to ensure accuracy and completeness. Specifically, a 100% check of primary end points and a 10% check of all other data were completed.
Electronic case report form
Only the therapist [and principal investigator (PI)] at each site had access to the eCRF. The baseline demographic details, including the NHS number, were recorded and supplied to the NHS IC/NHS Central Register to allow mortality checks prior to follow-up. In addition, data for duration of visit, the occurrence of any adverse events and end of intervention details was also collected and directly entered on the eCRF, often transcribed from paper records (either study worksheets or the intervention records).
Questionnaire booklets
Baseline participant questionnaires were completed by participants, with assistance from the RA if needed, during the baseline screening assessment. Alternatively, the RA left the questionnaire for the participant to complete later and post direct to the NCTU. We asked participants to record at baseline and both the 6- and 12-month assessments, whether they completed the questionnaire themselves or required assistance. This was to indicate who physically completed the questionnaire and it was assumed that the participant answered the questionnaires regardless of who filled in the form. If the RA assisted with completion of the baseline questionnaire, they informed the NCTU that the participant would also require assistance at 6 and 12 months. The RA sent the baseline questionnaire booklets to the NCTU for entry onto the database. Baseline carer questionnaires were either completed during the baseline screening assessment, if the carer was available, or left with the participant for completion later.
Six- and 12-month participant and carer questionnaire data were collected either by post or with assistance of a blind-to-allocation RA. Following baseline assessment, each participant was assigned to the postal approach unless otherwise indicated by the RA. The NCTU tracked all questionnaires with the option to send a reminder letter or to telephone to assess the situation. If difficulties were encountered at any time during the study then participants were switched from the postal approach to the RA approach. These methods were found to be successful in the single-centre pilot study, with a 90% postal return rate of active participants (i.e. those who had not withdrawn, died or been lost to follow-up) with the further 10% collected by an independent assessor, the equivalent of the RA in this study.
Travel diary
Travel diaries were completed by all participants on a daily basis. This was in the form of a calendar with the participants entering the number of journeys made for each day. In addition, in order for the study team to assess safety, participants indicated whether they had experienced a fall on each day. The participant was provided with a 12- or 13-month calendar, depending on the date of the baseline visit. Training in the completion and return of the travel diaries was provided by the RA at baseline. A prepaid envelope was sent to participants at the end of each month for them to return the diaries. No reminder letters were sent.
Data from travel diaries were entered on to the database on a month-by-month basis but only if a journey was recorded (i.e. ≥ 1 journey for any given day). Months that contained only ‘0’ were also recorded. If the month was blank then no data were entered for that particular month.
Intervention records
As well as the intervention duration being recorded in the eCRF, therapists also completed a paper intervention record form detailing goals set, visit-by-visit clinical details plus a breakdown of therapy activities. These were collected at the end of the study and collated into a separate database. Therapists were trained to use the form as part of the intervention training. The type and duration of intervention within any given visit were recorded under the following headings: goal setting, mobility, confidence, adaptive equipment, information, other rehabilitation and referral on to other agencies. For each intervention visit the therapist recorded how many minutes they spent on each activity, they listed the agreed outdoor mobility goals and kept running records of each treatment session as they would in routine clinical care. The intervention records were kept in the NHS clinical setting until the end of the study when they were copied and collated by the central coordinating centre.
Treatment fidelity
To assess the fidelity of the intervention therapy provided, 20% of clinical records were monitored (10% of all participants). A RA who was independent of the site visited the site and checked the treatments being delivered against a predefined checklist by accompanying the therapist on a number of treatment visits and by monitoring the intervention records. The last question on the checklist (a yes/no question as to whether the assessor believed that treatment provided met the required standard) was used to indicate whether the intervention had been delivered as specified and, therefore, implied fidelity of treatment.
Adverse events/safety evaluation
The main risk identified from this study was the potential to increase the chance of a participant having a fall, as a result of increased mobility. For adverse event purposes we adopted the following definition: ‘A recordable adverse event is any fall that occurs during or following the first outdoor mobility intervention and for the remaining period of intervention in the study that specifically requires the assistance of a health-care professional’. If these criteria were met then an adverse event form was completed. We adopted the following approach to capture this information – the therapist would ask, during an intervention visit, the following questions:
-
‘Have you had any falls since my last visit?’
-
If yes, ‘How many?’
-
‘How many required health-care professional help?’
Therefore, adverse events were only recorded from the intervention group, as the control group did not receive any visits post baseline. No other adverse events were collected. The occurrence of a serious adverse event as a result of participation within this study was not expected and no serious adverse event data were collected.
In order to capture information from both groups for comparison and a further measure of safety, we adopted a second approach. Participants indicated a fall on the travel diary by marking or circling the preprinted ‘f’, denoting that ≥ 1 fall had occurred for that participant on that day. These would not be classed or recorded as adverse events. Also, a comparison was made of any untoward changes in the well-being score of all participants by comparing the baseline GHQ-12 score with the 6- and 12-month score.
In the event of a pregnancy occurring in a study participant then they were allowed to continue in the study regardless of which arm they had been randomised to.
Concealment of allocation during outcome assessments
Participants and therapists were aware of treatment allocation. However, the treatment allocation was concealed from the RA so that they were able to assist the participant with completion of the 6- and 12-month questionnaires, if necessary. In order to measure concealment of allocation we asked the RAs to complete a blinding assessment for each post-randomisation visit, indicating whether they were unblinded prior to or during the visit.
Participant retention and withdrawals
To try and reduce the bias in participant retention caused by people wishing to withdraw before the primary end point because they were not allocated to the intervention group, we allowed participants to stop completing the travel diaries and asked them whether they would be happy to remain in the study and complete the 6-month questionnaire.
No specific withdrawal criteria were defined for this study. If a participant left the study prematurely (i.e. prior to completion of the protocol), the primary reason for discontinuation was determined and recorded if at all possible. Withdrawn participants were not replaced. Participants were made aware (by the information sheet and consent form) that should they withdraw, the data collected prior to the withdrawal date would not be erased and would still be used in the final analysis.
For this study, and in reference to the CONSORT (Consolidated Standards of Reporting Trials) diagram (see Figure 3 ), the following definitions were applied in relation to the participants and the data:
Withdrawn consent The participant formally withdrew from the study and no further data were collected from that point. This was measured in relation to completion of 6- or 12-month follow-up and not the time of withdrawal, for example, if a participant withdrew after 7 months and did not complete their 6-month follow-up then they would be recorded as ‘withdrawn prior to 6-month follow-up’.
Lost to follow-up The study coordinating centre and/or local site staff were unable to contact the participant. This was recorded in relation to completion of 6- or 12-month follow-up as above.
No follow-up data The study coordinating centre and/or local site staff were in contact with the participant but follow-up data were not received; however, the participant was not formally withdrawn from the study. These participants at 6-month follow-up were classed as ‘no follow-up data’ on the CONSORT diagram and continued to participate in the study. These participants at 12 months’ follow-up were also classed as ‘no follow-up data’ on the CONSORT diagram.
Statistical methods
Full descriptions of the statistical methods of analysis are detailed in the statistical analysis plan (see Appendix 1 ).
Intervention and goals descriptive analysis A description of the components of the intervention was undertaken with the type of intervention therapy received by participants (e.g. mobility, goal setting, confidence, etc.) summarised in terms of number and percentage of participants who received the different types of therapy sessions and the mean, SD, median, IQR, and the minimum and maximum of the number of sessions for each type of therapy session. The goals set by each participant during the intervention sessions were recorded verbatim and this information was then sorted into categories of the goal types. These data were then analysed through calculating the number and percentage of participants who set each type of goal.
Comparison between treatment arms
All analyses were undertaken on an intention-to-treat basis, in that participants were analysed according to the group to which they were randomised, regardless of whether they received the intervention or not. Analyses were conducted on available case data. A sensitivity analysis, using multiple imputation (MI) to replace missing values, was performed for all outcome measures except travel journeys (see Sensitivity analysis adjusting for missing data section below for further information).
Basic data explorations using summary and graphical statistics (i.e. box plots, etc.) were conducted to check for outliers. If, after additional investigation, there was no evidence that these were errors then the analyses were repeated after Winsorising the data to assess the robustness of the results. Winsorising involves replacing extreme values (outliers) with a specified percentile of the data. 38 In this case, we replaced data values below the 5th percentile with the 5th percentile value and to replace data values above the 95th percentile with the 95th percentile value.
Descriptive analyses
Continuous data that were approximately normally distributed were summarised in terms of the mean, SD and number of observations. Skewed data were presented in terms of the median, lower and upper quartiles, minimum, maximum, and number of observations. Categorical data were summarised in terms of frequency counts and percentages.
Analysing primary and secondary outcomes
The mean social function score of the SF-36v2 was compared between treatment groups using a multiple membership form of mixed-effects multiple regression analysis, adjusting for site (as a random effect), age and baseline social function score as covariates, and therapist as a multiple membership random effect. A three-level hierarchical regression model was used with site at level 3, multiple membership effect of therapists at level 2, and participants at level 1. Regression coefficients and 95% credible intervals were presented. The robustness of these findings was assessed by repeating the analysis and including baseline variables that are likely to be associated with the outcome variable (namely gender and residential status) as covariates in the model.
Satisfaction with outdoor mobility was compared between treatment groups using a multiple membership form of a mixed-effects logistic regression model adjusting for site (as a random effect), baseline answer to the question and age as covariates, and therapist as a multiple membership random effect. Odds ratios and 95% credible intervals were presented.
Travel journeys were compared between treatment groups using a multiple membership form of a mixed-effects Poisson regression model, adjusting for site (as a random effect) and age as covariates, and therapist as a multiple membership random effect. Rate ratios and 95% credible intervals were presented. Travel journeys were presented as number of journeys per day. The analysis assumed that for a particular month for which journeys had been recorded then all other days were imputed with ‘0’. All months that were returned blank were not included in the analysis. When no travel diary was returned then that month was not included in the analysis.
All remaining secondary outcome variables were analysed using the same methods as for the primary outcome measure. We acknowledge the potential for type 1 errors associated with significance testing for multiple end points and, therefore, we consider the analyses of the secondary outcome measures to be partly exploratory in nature, and partly confirmatory of the findings for the primary outcome measure.
Sensitivity analysis adjusting for missing data
We assessed the effect of missing data on the overall conclusions of the study using MI. We used the Stata ‘ice’ command to generate 10 imputed data sets, and estimated the intervention effect in each imputed data set. We then used ‘Rubin’s Rules’ to combine these estimates into a pooled estimate of the intervention effect. 39
This process was conducted for all outcomes (except the travel journeys) for 6- and 12-month data. We considered the use of this process for the travel journeys to be inappropriate because, in addition to imputing the total number of journeys made, we would also have had to impute the number of days on which journeys were taken. Furthermore, there was no prior information available at baseline on the number of journeys made or the numbers of days on which the participants had made journeys that could be used to predict the data for 6 and 12 months.
Multiple membership random effect
In many studies, outcome measures are influenced by an intervention, such as a drug or a single person delivering an intervention, and, in these studies, assessing the effects of the drug or person on the outcome is clear. In our study, participants in the control group were treated by only one therapist but in the intervention group participants may have been treated by multiple therapists and some therapists may have delivered more therapy sessions to a specific individual than others. This means that the outcome obtained from a participant receiving treatment from different therapists may have been influenced by more than one person and, as some therapists may have delivered more therapy sessions to a participant than others (and the therapists may differ in their effectiveness), it is important to take account of this in the analysis. We used multiple membership random-effects models to deal with this situation and adjusted for the effect of the therapist by placing weights on the therapists delivering the intervention. We placed the greatest weight on the therapist who delivered the most therapy sessions for a particular participant. If two therapists delivered the same number of therapy sessions to a participant, equal weight was applied to each therapist, whereas if one therapist delivered one-third of the therapy sessions and another therapist delivered two-thirds of the therapy sessions (i.e. twice as many), the therapist who provided the most sessions was given the greatest weight (in this case, twice the weight of the other therapist). For participants treated by only one therapist, all weight was placed on that therapist, as only that therapist influenced the outcome. The weight applied to each therapist treating an individual therefore reflected the potential amount of influence that they may have had on the outcome with low weights indicating less influence on the outcome than higher weights.
Presentation of primary and secondary results
All main 6- and 12-month primary and secondary outcome data were presented descriptively by treatment group using means and SDs for continuous data or frequency counts and percentages for categorical data (unadjusted results), and then the results of the multivariable analysis – adjusting for therapy effect and site effect – were presented as differences in means for continuous data, odds ratios or rate ratios for binary and count data, respectively (adjusted results), with 95% credible intervals as a measure of significance. In addition, the ICCs indicating the size of the therapy effect and site effect were presented, apart from travel journey, for which these effects were not calculable. The interpretation of the results of the multivariable analysis of the 6- and 12-month primary and secondary outcome data are as follows:
-
Difference in means Where a score of ‘0’ indicates ‘no difference’ and hence if the 95% credible interval crossed the value ‘0’ then the result (difference in means between the two treatment groups) was not statistically significant. This approach was used for the primary outcome, NEADL, RMI and GHQ-12 outcome measures.
-
Odds ratio Where a score of ‘1’ indicates ‘no difference’ and hence if the 95% credible interval crossed the value ‘1’ then the result (the odds of getting out of the house as often as they would like in the intervention group relative to the control group) was not statistically significant. This approach was used only for the outcome SWOM.
-
Rate ratio Where a score of ‘1’ indicates ‘no difference’ and hence if the 95% credible interval crossed the value ‘1’ then the result was not considered to be statistically significant. This parameter was used for travel journeys.
Exploratory/other analyses
The following analyses were prespecified in the analysis plan:
-
NEADL by category Descriptive summaries (by treatment group) for the NEADL scale categories ‘Mobility’, ‘Kitchen’, ‘Domestic’ and ‘Leisure’.
-
Number of sessions by site Descriptive analysis of number of intervention visits by site compared with perceived successful completion of the intervention.
-
Number of travel journeys by intervention session Descriptive analysis comparing the number of travel journeys within the intervention group by participants who had received fewer than six sessions or six or more sessions. The pilot study found that a median of six sessions were effective, hence the pre-existing threshold of fewer than six or ≥ six or more sessions was used in this analysis.
-
Falls by age category and treatment allocation The total number (%) of ‘fall-days’ was described by treatment arm and age category (< 60 years of age and ≥ 60 years).
-
Satisfaction with outdoor mobility changes over time To explore the effect of the control information a descriptive comparison in the change of the SWOM scores from baseline to 6 months for each group.
-
Six-month follow-up by approach A descriptive comparison of collection of 6-month follow-up data from postal or RA approach.
Compliance in reference to CONSORT
Compliance in this study was effectively a measure of completeness of follow-up data. Baseline questionnaires were required to be completed before randomisation so that baseline CONSORT-presented numbers randomised. The CONSORT diagram presented compliance for this study as the number of returned questionnaires for the 6- and 12-month follow-ups, regardless of completeness of the questionnaire booklets.
For individual analyses of outcome measures, as the analysis relies on a comparison between follow-up data and the baseline data, the ‘n’ presented in the results section was a measure of the number of participants who completed the outcome measure at both baseline and follow-up. Data from participants who answered some, but not all, of the questions within a questionnaire based outcome measure were still included in the analysis; however, if the whole of the questionnaire was missing then their data were not used.
Study management and oversight
A number of committees were assembled to ensure the management and conduct of the study, and to uphold the safety and well-being of participants.
Trial Management Group
The Trial Management Group (TMG) oversaw the operational aspects of the study, which included the processes and procedures used and the day-to-day activities involved in study conduct.
Trial Steering and Data Committee
The Trial Steering and Data Committee (TSDC) had the overall responsibility for ensuring a scientifically sound study design, a well-executed study, and accurate reporting of the study results.
The data monitoring part of this committee evaluated the outcome and safety data in the context of the overall study and the currently existing information about the study. They considered the appropriate time frame for reviewing the data during the course of the study. The TSDC had access to grouped study data.
However, as part of the process of calculating a revised sample size, an ad hoc independent Data Monitoring Committee (DMC) was convened, which reviewed the suitability and legitimacy of the proposed revision.
Amendments to the study
Eligibility To simplify the screening process we removed ‘Able to comply with the requirements of the protocol’ as an inclusion criterion, and ‘Significant cognitive impairment which will impede ability to complete the assessments’ and ‘Diagnosis likely to interfere with rehabilitation or outcome assessments e.g. terminal illness’ as exclusion criteria. In their place we added a single exclusion ‘Not able to comply with the requirements of the protocol and therapy programme, in the opinion of the assessor/GP/investigator’. These amendments were made prior to the start of recruitment. We amended ‘At least 6 weeks but no longer than 5 years since stroke’ to ‘At least 6 weeks since stroke’ as an inclusion, as there was no valid reason for the 5-year limit and participant invitations asked participants whether the mobility issues were as a result of their stroke, so time frame was not relevant. Hence it was more inclusive to potential participants. This amendment was made after 4 months of recruitment.
Sample size Following consultation with the funders and the TSC, it was agreed that we would calculate a revised sample size, based on an increase in the number of sites. This revision is described in the ‘Revised sample size’ section earlier in the chapter. Independent statisticians from outside the study checked the validity of all calculations and assumptions. These amendments were made after 16 months of recruitment.
Summary of changes to recruitment material After initially using a standard PIS, in full and summary form, we introduced a supplementary PIS leaflet, in addition to rewording the PIS, consent form and patient invites. These were amended in order to make the aims of the study more transparent and patient friendly. Another significant change was the introduction of an aphasia-specific PIS consisting of images and key words. Though initially designed for aphasic patients it was used extensively, in conjunction with more standard formats, for other stroke patients. These amendments were made after 4 months of recruitment.
Qualitative study A study investigating confidence after stroke was incorporated into the existing study protocol with a view to interviewing existing study participants about issues relating to confidence and how it affected their recovery from stroke. This part of the study was only in Nottingham for 10 participants. The qualitative study is described in more detail in Chapter 5 . This amendment was made after 11 months of recruitment.
Recruitment timelines Initial recruitment timelines were 12 months; however, final recruitment timelines were extended to 22 months. This was managed through a combination of efficiencies within the overall project timelines and an extension to the overall project from 36 months to 40 months.
Chapter 3 Results: randomised controlled study
This chapter reports recruitment and retention of participants, and collection and analysis of study data specifically relating to the delivery and fidelity of the intervention, primary outcome measured at 6 months, and secondary outcomes measured at 6 and 12 months.
Study recruitment and follow-up
The study recruited 568 out of 506 (112.3%) of the required sample size from November 2009 to August 2011. Participants were recruited from 15 sites across England, Scotland and Wales, with an average recruitment of 38 participants (range from 5 to 99) per site, with nine sites recruiting > 30 participants. The participant involvement in the trial ended in August 2012, following completion of recruitment and follow-up. Figure 2 shows that, after an initial lag phase, steady recruitment was achieved with no noticeable seasonal variation.
FIGURE 2.
Recruitment rate.
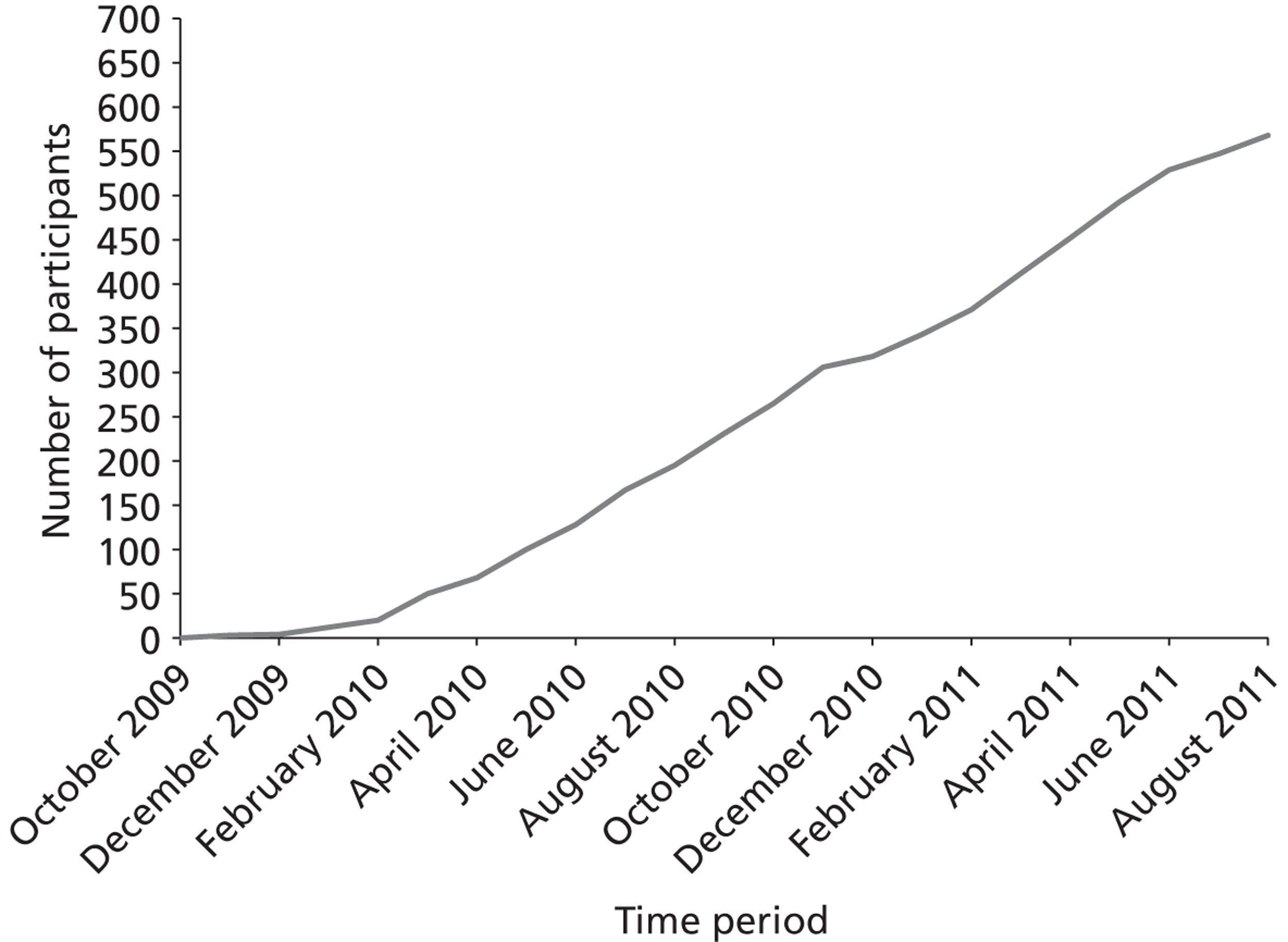
Table 1 provides a breakdown of screening figures collected from all sites, in terms of number of invitations sent, number of ‘yes’ replies, number of home screening visits, number randomised and the proportion of recruits per 100 invites. The different approaches were used to varying extents: GP-based approaches being responsible for recruiting 158 out of 568 (27.8%) of randomised participants at a rate of 2.5 per 100 invites, and stroke register-based approaches recruiting 410 out of 568 (72.2%) of randomised participants at a rate of 8.5 per 100 invites. The stroke register data includes participants recruited at outpatient clinics.
| Approach | Invites | ‘Yes’ replies (% of invites) | Screening visits (% of ‘yes’ replies) | Randomised (% of ‘yes’ replies) | Recruits/100 contacts |
|---|---|---|---|---|---|
| GP database | 6329 | 679 (10.7) | 261 (38.4) | 158 (23.3) | 2.5 |
| Stroke register | 4797 | 769 (16.0) | 591 (76.9) | 410 (53.3) | 8.5 |
| Overall | 11,126 | 1448 (13.0) | 852 (58.8) | 568 (39.2) | 5.1 |
Follow-up (CONSORT)
Figure 3 shows the CONSORT diagram for the study. The identification of participants was from 11,126 invites, either by letters of invitations or face-to-face approach. The exact breakdowns were not recorded; however, the majority were by letter of invitation. We had ‘yes’ replies from 1448 out of 11,126 (13.0%) approaches to participate in the study with 9678 out of 11,126 (87%) either not responding or providing negative replies. We did not record the number of ‘no’ replies. After initial pre-consent screening, usually by telephone, a further 596 out of 11,126 (5.4%) were considered ineligible. The main reason for ineligibility was that the potential participants felt they got out of the house as much as they wished already. Therefore, 852 out of 11,126 (7.7%) had a further screening visit in their own home from the RA. Overall, 568 out of 852 (66.7%) were found to be eligible to take part. Of the 11,126 letters sent, 568 people were included (5.1%). Of the 284 out of 11,126 (2.6%) who did not enter the study, only 1 out of 11,126 (0.01%) explicitly refused to participate (owing to their desire to be in the intervention group only).
FIGURE 3.
Final CONSORT diagram. a, Participant remained in the trial but no data were collected at that time point.
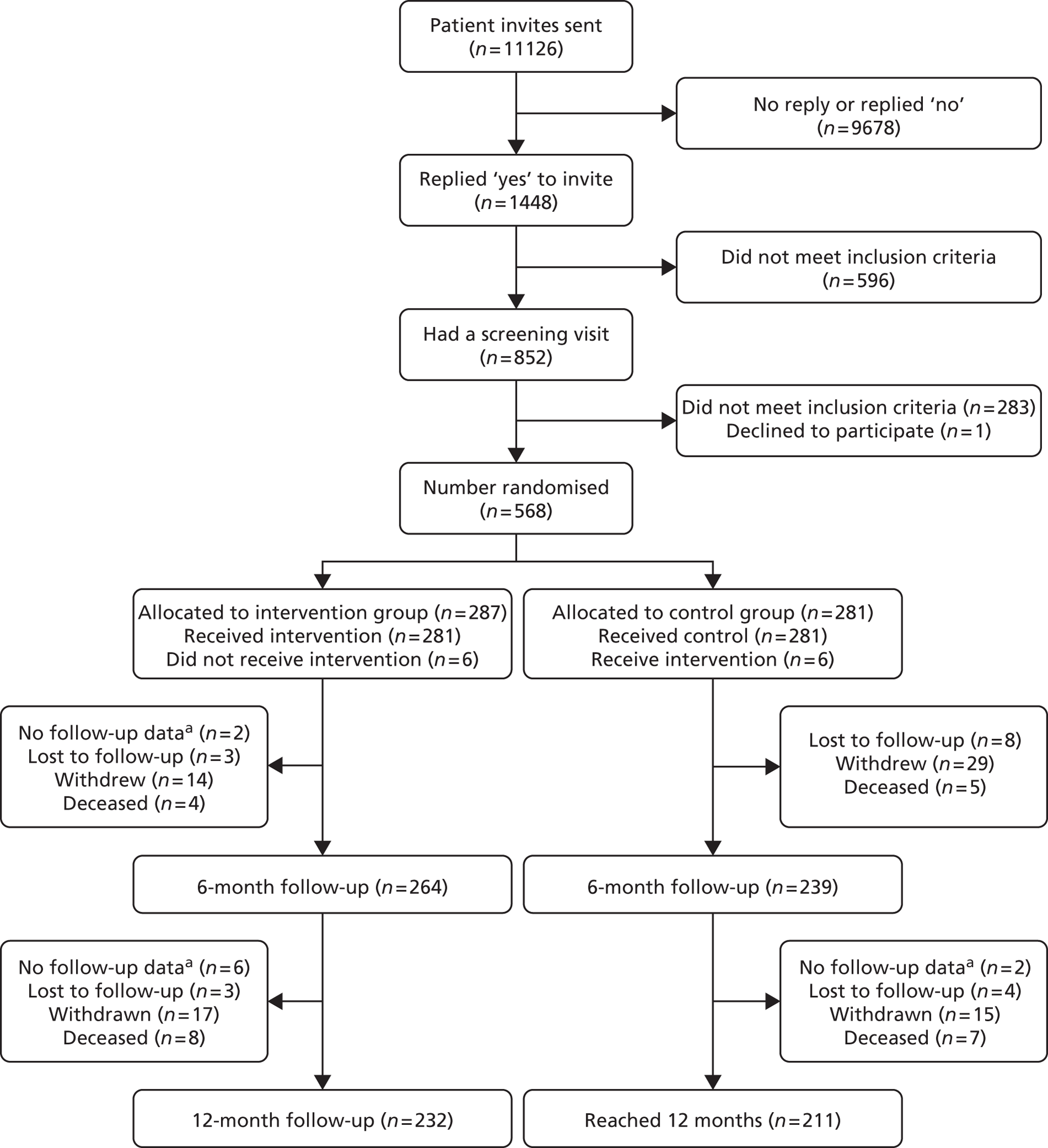
The baseline questionnaire was completed by 567 out of 568 (99.8%) participants, with the one outstanding being left with the participant and never being received by the study coordinating centre.
Following randomisation, 287 out of 568 (50.5%) participants were allocated to the intervention group and 281 out of 568 (49.5%) to the control group. In the control group, 1 out of 281 (0.4%) participants received two intervention visits in error. In the intervention group, 281 out of 287 (97.9%) of participants received at least one intervention visit. There was no evidence of crossover between the two groups.
Six-month follow-up was measured by the receipt of the 6-month questionnaire booklet by the research team. For the intervention group, 264 out of 287 (92.0%) reached 6-month follow-up. Of the 23 out of 287 (8.0%) who did not reach 6-month follow-up, 3 out of 287 (1.0%) were lost to follow-up, 14 out of 287 (4.9%) withdrew consent and 4 out of 287 (1.4%) died. A further 2 out of 287 (0.7%) did not complete the follow-up but remained in the study, with 266 participants remaining in the intervention group after 6-month follow-up. For the control group, 239 out of 281 (85.1%) reached 6-month follow-up. Of the 42 out of 281 (14.9%) who did not reach 6-month follow-up, 8 out of 281 (2.8%) were lost to follow-up, 29 out of 281 (10.3%) withdrew consent and 5 out of 281 (1.8%) died. Therefore, there were 239 participants remaining in the control group after 6-month follow-up.
Completion of 6- and 12-month follow-ups was defined as return of the fully/partially completed questionnaire booklet to the NCTU. It does not indicate the level of completeness. Six- and 12-month results for individual measures presented later in the chapter indicate the number of participants from which we have analysable data.
There was a differential follow-up rate between the two groups for the 6-month follow-up, with 92.0% collected for the intervention group and 85.1% collected for the control group. However, these were both less than the predefined 20% attrition rates.
Successful data collection at 12-month follow-up was measured by receipt of the 12-month questionnaire booklet. For the intervention group, 232 out of 287 (80.8%) reached 12-month follow-up. Of the 34 out of 287 (266 – 232 = 34; 11.8%) who did not reach 12-month follow-up, 3 out of 287 (1.0%) were lost to follow-up, 17 out of 287 (5.9%) withdrew consent and 8 out of 287 (2.8%) died. A further 6 out of 287 (2.1%) did not complete the follow-up but remained in the study. For the control group 211 out of 281 (75.1%) reached 12-month follow-up. Of the 28 out of 281 (239 – 211 = 28; 10.0%) who did not reach 12-month follow-up, 4 out of 281 (1.4%) were lost to follow-up, 15 out of 281 (5.3%) withdrew consent and 7 out of 281 (2.5%) died. A further 2 out of 281 (0.7%) did not complete the 12-month follow-up but remained in the study.
As with the 6-month follow-up, there were differential follow-up rates between the two groups for the 12-month follow-up. A total of 232 out of 287 (80.8%) of questionnaires were collected for the intervention group and 211 out of 281 (75.1%) collected for the control group. Although the control group follow-up rate was within the defined 20% attrition rate, the overall number of participants with 12-month follow-up data (n = 443) was within the threshold to ensure that the study had adequate power.
Table 2 summarises the results of who completed the questionnaire and indicates a consistent proportion of participants completed the questionnaires themselves, whereas at 6 and 12 months there were consistent completion rates for both carer and other (16% and approximately 37%, respectively). The ‘other’ completions at baseline were due to the presence of the RA.
| Questionnaire | Who completed the questionnaire | ||
|---|---|---|---|
| You (%) | Carer (%) | Other (%) | |
| Baseline (n = 567a) | 234 (41.3) | 32 (5.6) | 301 (53.1) |
| 6-month follow-up (n = 503) | 230 (45.7) | 81 (16.1) | 192 (38.2) |
| 12-month follow-up (n = 443) | 208 (47.0) | 74 (16.7) | 161 (36.4) |
Carer questionnaire follow-up
We received 192 carer questionnaires at baseline, with 100 out of 192 (52.1%) and 92 out of 192 (47.9%) from carers of participants in the intervention and control groups, respectively. We received 148 carer questionnaires at 6-month follow-up, with 84 out of 100 (84.8%) and 64 out of 92 (69.6%) from carers of participants in the intervention and control groups, respectively. We received 127 carer questionnaires at 12-month follow-up, with 71 out of 100 (71.0%) and 56 out of 92 (60.9%) from carers of participants in the intervention group and control, respectively.
Travel diary follow-up
Owing to the volume of travel diaries and the challenges of tracking their status, a decision was taken not to send reminders if the travel diary was not received. Overall, 70.6% of all expected travel diary months were received and assigned, regardless of whether they contained data, to 508 out of 568 (89.4%) of participants ( Table 3 ); 73.6% were received for the intervention group and 67.5% from the control group. Overall, 55.1% of participants returned diaries for the full 12 months.
| Travel diaries | Overall (expected n = 7363) | Control group (expected n = 3644) | Intervention group (expected n = 3719) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Total received | 5198 | 70.6 | 2462 | 67.5 | 2736 | 73.6 |
Baseline characteristics
Table 4 presents a summary of key baseline characteristics of each group. Baseline characteristics of the sample showed the majority of participants to be women, living with others, with a mean age of 71 years (SD 12.1 years). At baseline there was a slight gender imbalance, with a higher proportion of men in the control group than the intervention group (47% vs. 42.2%). Residential status was well balanced between groups with only a slightly higher proportion living alone in the control group (35.2% vs. 33.4%). The average time since stroke was 37 months (SD 43.8 months) for the control group and 43 months (SD 60.1 months) for the intervention group.
| Variable | Parameter | Allocation | ||
|---|---|---|---|---|
| Control | Intervention | |||
| Total | n (%) | 281 (49.5) | 287 (50.5) | |
| Age at inclusion (years) | Mean (SD) | 71.5 (12.1) | 71.7 (12.1) | |
| Median (IQR) | 75 (63–81) | 73 (64–81) | ||
| Min. to max. | 36–95 | 32–96 | ||
| Sex | Women | n (%) | 149 (53) | 166 (57.8) |
| Men | 132 (47) | 121 (42.2) | ||
| Ethnicity | White | n (%) | 263 (93.6) | 270 (94.1) |
| Black: Caribbean | 7 (2.5) | 4 (1.4) | ||
| Black: African | 2 (0.7) | 1 (0.3) | ||
| Black: other | 1 (0.4) | 3 (1) | ||
| Pakistani | 0 (0) | 0 (0) | ||
| Indian | 5 (1.8) | 4 (1.4) | ||
| Bangladeshi | 2 (.7) | 2 (0.7) | ||
| Chinese | 0 (0) | 0 (0) | ||
| Mixed | 0 (0) | 1 (0.3) | ||
| Not given | 1 (0.4) | 1 (0.3) | ||
| Other | 0 (0) | 1 (0.3) | ||
| Residential status | Lives alone | n (%) | 99 (35.2) | 96 (33.4) |
| Lives with others | 170 (60.5) | 179 (62.4) | ||
| Living in care home | 12 (4.3) | 12 (4.2) | ||
| Time since stroke (months) | Mean (SD) | 37 (43.8) | 43.2 (60.1) | |
| Median (IQR) | 21.3 (10.2–47.6) | 24.5 (12.8–44) | ||
| Min. to max. | 1.6–392.6 | 2–479.3 | ||
| Social functioning score | Mean (SD) | 50.1 (30.7) | 45.9 (30.3) | |
| Median (IQR) | 50 (25–75) | 37.5 (25–62.5) | ||
| Min. to max. | 0–100 | 0–100 | ||
| NEADL score | Mean (SD) | 10.1 (5.7) | 8.8 (5.2) | |
| Median (IQR) | 10 (5–14) | 9 (4–13) | ||
| Min. to max. | 0–22 | 0–22 | ||
| RMI score | Mean (SD) | 8.9 (4.1) | 8.1 (3.9) | |
| Median (IQR) | 10 (6–12) | 8 (5–11) | ||
| Min. to max. | 0–15 | 0–15 | ||
| SWOM | Yes | n (%) | 20 (7.1) | 18 (6.3) |
| No | 259 (92.2) | 268 (93.4) | ||
| General Health Questionnaire (participant) | Mean (SD) | 15.1 (6.8) | 14.9 (6.5) | |
| Median (IQR) | 13 (10–19) | 14 (10–19) | ||
| Min. to max. | 1–36 | 1–35 | ||
Baseline scores for mobility, activity measures, GHQ-12 and SWOM were similar in the two groups. However, for the primary outcome measure of quality of life (social functioning score), the control group had a higher mean (50.1 vs. 45.9) and median (50 vs. 37.5) than the intervention group.
Satisfaction with outdoor mobility scores indicated that overall 93.3% of participants were not currently satisfied with their level of outdoor mobility. The remaining participants who indicated SWOM (n = 38) were spread across both groups, in all sites except one, and had no other unusual characteristics.
It is important to clarify the relationship between the ‘SWOM’ (Do you get out of the house as much as you would like?) question as part of the baseline questionnaire and the eligibility criteria of ‘Wishing to get out of the house more often’. On 38 out of 568 (6.7%) occasions, 20 out of 281 (7.1%) in the control group and 18 out of 287 (6.3%) in the intervention group, the participant indicated that he or she met the eligibility criteria during the consent process but answered ‘Yes’ to the ‘SWOM’ question during completion of the baseline questionnaire. On further investigation it appeared that certain participants were viewing these questions within a different context. The eligibility question was related to aspirational view of getting out of the house (i.e. ‘Wishing to get out of the house more often’), whereas the SWOM question was related to day-by-day coping views of getting out of the house. Hence these participants were not classed as ineligible and were included in the analyses.
Delivery of the intervention
Number of therapists by site
There were 29 therapists taking part in this study, who delivered at least one treatment session. There was no restriction on how many therapists delivered the intervention at each site. This was mainly determined by how the service was structured, availability of staff to perform the research activity, and delivery of research and treatment costs to the relevant department. The therapists ranged from junior to senior (Agenda for Change bands 4–740). Three were physiotherapists, 17 were occupational therapists and nine were assistant practitioners. The number of therapists per site ranged from 1 to 4, with a median of two per site. A small proportion of visits were attended by two therapists (65/1939; 3.4%). These were mainly as a result of training junior staff and training as part of handover to replacement staff. Generally, only one therapist delivered the intervention while the second therapist observed as part of training.
Number and duration of intervention sessions
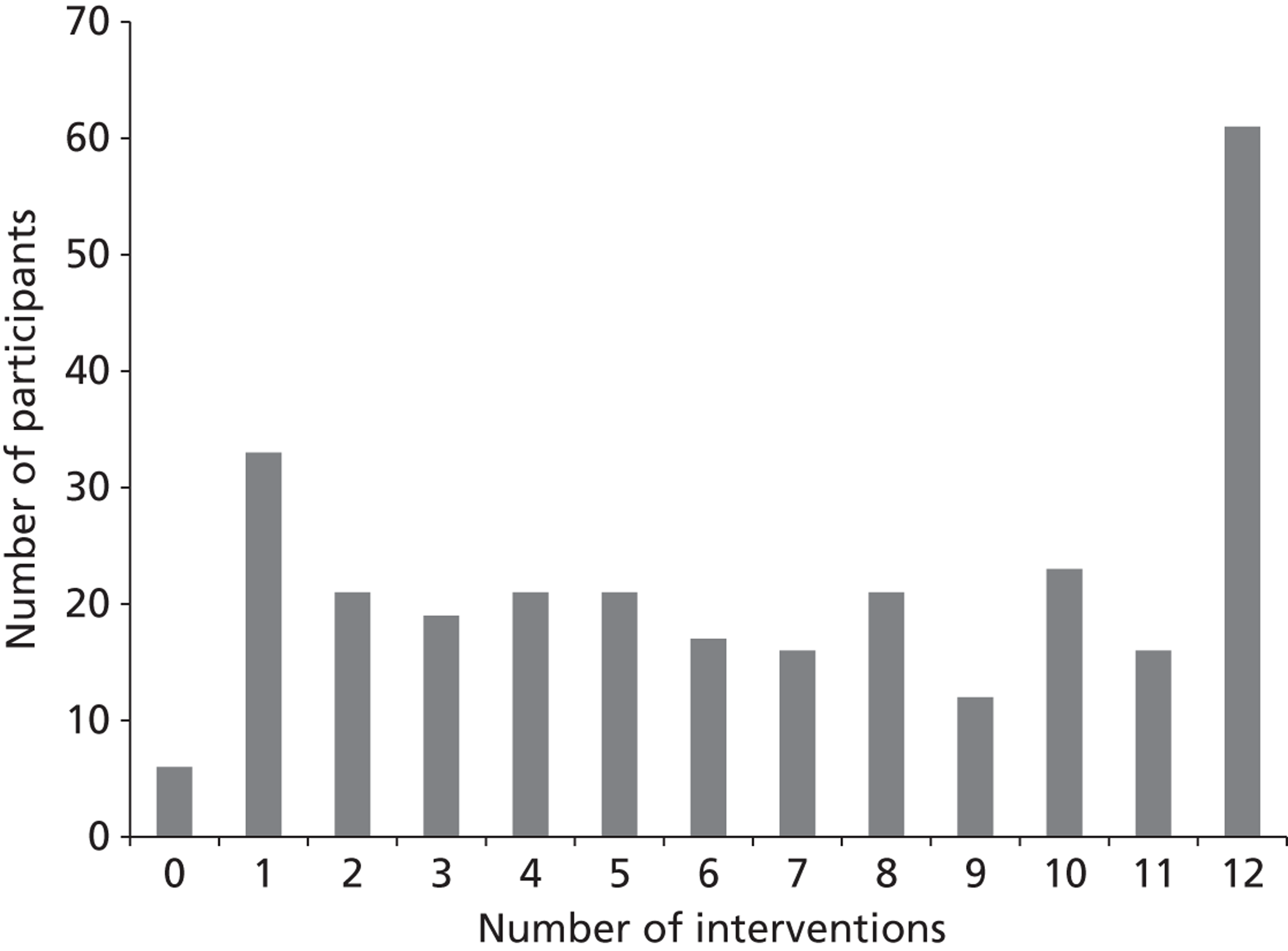
The intervention group received a median of seven intervention sessions (IQR 3–11 sessions), mean 6.80 sessions (SD 4.01 sessions). Figure 4 illustrates the distribution of intervention visits received by participants, ranging from zero intervention sessions (6/287, 2.1%) to the maximum 12 (61/287, 21.3%); 138 out of 287 (48.1%) received less than the median of seven visits, with 149 out of 287 (51.9%) receiving ≥ 7 visits. These were calculated from eCRF intervention visit data.
FIGURE 4.
Range of intervention visits.
Of the 287 intervention participants, paper intervention records completed by the therapists were returned for 269 out of 287 (93.7%) participants, 264 out of 287 (92.0%) of whom had received at least one treatment session. The median duration in total, in minutes, of intervention provided for these 264 participants was 369.5 minutes (IQR 170–691.5 minutes), mean 454.6 minutes (SD 352.20 minutes). These were calculated from intervention records data.
Description of set goals
Table 5 summarises the types of goals set for each participant. Of the 287 participants in the intervention group, information on goals set was recorded for 243. Participants were able to set more than one goal during the process. However, we have no direct measure, relating to individual goals set, to indicate the proportion of goals that were achieved. Instead, we have an overall measure of whether the intervention was delivered to the satisfaction of the therapist, detailed later in Table 11 . The most common goal was a long walk of > 100 m, set by 55.1% of participants, whereas increasing confidence was set for 36.2%. There was a wide range of goals set and the vast majority were considered appropriate for an intervention aimed at getting participants out of the house and were also considered attainable.
| Goal type | n participants | Percentage of 243 participants |
|---|---|---|
| Long walk > 100 m | 134 | 55.1 |
| Increase confidence | 88 | 36.2 |
| Short walk < 100 m | 83 | 34.2 |
| Othersa | 78 | 32.1 |
| Stamina | 56 | 23.1 |
| Access local shop | 54 | 22.2 |
| Bus | 50 | 20.6 |
| Attending social clubs/social activity | 46 | 18.9 |
| Training outdoor mobility equipment | 37 | 15.2 |
| Mobility scooter | 29 | 11.9 |
| Increase independence | 28 | 11.5 |
| Access town centre | 23 | 9.5 |
| Powered wheelchair | 16 | 6.6 |
| Crossing roads | 14 | 5.8 |
| Taxi | 12 | 4.9 |
| Car transfers | 12 | 4.9 |
| Increase journeys | 12 | 4.9 |
| Driving | 10 | 4.1 |
| Dial-a-Ride | 8 | 3.3 |
| Walk inside | 6 | 2.5 |
| Day centre | 6 | 2.5 |
| Shopmobility | 6 | 2.5 |
| Car | 1 | 0.4 |
Description of the key elements of the intervention
The average duration of an intervention visit, including travel to and from the participant’s home, was 96.6 minutes with a range of 10–390 minutes. A total of 1856 out of 1939 (95.7%) intervention visits were completed within the protocol guidelines of 4 months post baseline visit. The other 4.3% were outside the 4-month protocol guidance, although not all were recorded as protocol deviations by the therapists.
Table 6 summarises the proportion and type of intervention delivered to participants. Goal setting was delivered for 243 (92.1%) participants, with a median of two sessions provided (IQR 1–4 sessions). Mobility training was delivered the most often for a median of 5.5 visits for 222 participants (84.1%), and had the longest duration, with a median of 212.5 minutes (IQR 80–390 minutes) (data not reported in the table). Confidence building was used with 202 (76.5%) participants, with a median of four sessions provided (IQR 2–8 sessions). The least used treatment method was adaptive equipment training, with 63 (23.9%) participants receiving a median of one session (IQR 1–2 sessions). All treatment techniques listed on the record form were used at least once by each site.
| Intervention type | Participants, n (%)a | No. of sessions | ||
|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Min. to max. | ||
| Goal setting | 243 (92.1) | 3.32 (3.13) | 2 (1–4) | 1, 12 |
| Mobility | 222 (84.1) | 6.06 (3.89) | 5.5 (2–10) | 1, 12 |
| Information | 205 (77.7) | 3.89 (3.28) | 3 (1–10) | 1, 12 |
| Confidence | 202 (76.5) | 5.13 (3.53) | 4 (2–8) | 1, 12 |
| Other rehabilitation | 139 (52.7) | 3.55 (2.91) | 2 (1–5) | 1, 11 |
| Referral | 104 (39.4) | 2.04 (1.32) | 2 (1–3) | 1, 7 |
| Adaptive equipment | 63 (23.9) | 1.92 (1.24) | 1 (1–2) | 1, 6 |
Treatment fidelity
Of the 15 sites delivering the intervention, 14 were assessed for fidelity of treatment.
Treatment fidelity forms were completed for 59 out of 287 (20.6%) intervention participants. Fidelity of treatment assessments were not performed equally across sites, ranging from the intervention sessions for 10 out of 59 (17.0%) participants assessed at one site to 1 out of 59 (1.7%) participant at each of two sites, and no participants at one site.
Initially, the fidelity of treatment assessment was a combination of checking treatment records against a predefined checklist and also accompanying the treating therapists on visits. However, owing to the possibility that accompanying the therapists on visits might influence how a therapist undertook these sessions, it was decided that only reviewing the participants’ notes would be completed for the remainder. Of the 59 fidelity of treatment assessments, 12 (20.3%) were completed using the visit and the treatment notes and 47 out of 59 (79.7%) were completed by just assessing the treatment notes.
The final question asked the assessor to make a judgement as to whether the intervention had met the standard based on the checklist. They indicated that in 100% of the cases they believed it had.
Completion of intervention
At the end of the participant’s intervention period the therapist indicated in 193 out of 287 (67.3%) of participants that they, the therapists, felt that the participant had completed the intervention to the therapist’s satisfaction (i.e. a surrogate marker for achieving their set goals).
Contamination
There was one participant allocated to the control group who received two intervention visits in error. This was recorded as a protocol deviation and is reported later in the chapter. The nature of the study, with the intervention requiring a visit to the participant’s home, as well as the fact that all participants were not currently within the rehabilitation service, meant that participants in the control group were unlikely to have any contact with local site therapists and hence contamination was unlikely to occur. There is no evidence of any contamination within this study.
Numbers analysed
Figure 2 illustrates the flow of participants and their data; however, it does not provide the details on an outcome-by-outcome basis. A partially completed outcome at the particular follow-up was required for unadjusted measures. In order for any unadjusted outcome measure (apart from travel journeys) to be calculated, the particular measure must be at least partially completed at both baseline and at the particular follow-up. For travel journeys, the participant had to return at least one travel diary to be included in either unadjusted or adjusted analysis. Table 7 details the number of participants analysed for both adjusted and unadjusted outcome measure analysis.
| Outcome | Numbers analysed | |||
|---|---|---|---|---|
| 6 months | 12 months | |||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| SF-36 (SF domain) | 500 | 493 | 425 | 422 |
| NEADL | 494 | 493 | 438 | 437 |
| RMI | 499 | 497 | 442 | 440 |
| GHQ-12 | 495 | 493 | 436 | 434 |
| GHQ-12 (carer) | 148 | 145 | 127 | 125 |
| SWOM | 494 | 491 | 432 | 429 |
| Travel journeys | 504 | 504 | 504 | 504 |
Primary outcome
The primary outcome was the Social Function domain within the SF-36v2 relating to an improvement in participant’s quality of life. The predefined MCID was 12.5. 32
Table 8 summarises the primary outcome analysis. The variability of the social function score was similar in the two groups although the mean score was slightly higher in the intervention group (47.0) at 6 months compared with the control group (43.9). The adjusted difference in means between groups was 4.630 with a 95% credible interval of –0.549 to 9.848. This suggests a slightly higher social function score in the intervention group but this was not significant and it is less than the pre-defined MCID. The ICCs for the therapist (ICC = 0.0051) and site effects (ICC = 0.0099) are small, and less than we anticipated in our sample size calculations.
| Outcome | Unadjusted | Adjusted | ICC | |||
|---|---|---|---|---|---|---|
| Control, mean (SD) | Intervention, mean (SD) | Difference in means | 95% credible interval | Therapist | Site | |
| Social Function domain | 43.9 (29.8) (n = 239) | 47.0 (30.5) (n = 261) | 4.630 | –0.549 to 9.848 | 0.0051 | 0.0099 |
Sensitivity analyses of the primary outcome
Sensitivity analysis was conducted, as per the statistical analysis plan, to assess the effect of outliers and missing data. The results of the sensitivity analyses were consistent with those from the main analysis and did not result in any difference in the conclusions drawn.
Secondary outcomes at 6 months
Table 9 summarises the secondary outcome analysis at 6 months. The unadjusted mean scores in both groups were similar at 6 months for all secondary outcome measures. In the adjusted analyses there was a statistically significant difference observed between groups for travel journeys, with the intervention group being 42% more likely to make a journey than the control group [rate ratio 1.42, 95% confidence interval (95% CI) 1.14 to 1.67]. There were no other outcome differences between groups.
| Outcome | Unadjusted | Adjusted | ICC | |||
|---|---|---|---|---|---|---|
| Control mean (SD) | Intervention mean (SD) | Difference in means | 95% credible interval | Therapist | Site | |
| NEADL | 9.9 (5.7), (n = 233) | 9.0 (5.5), (n = 261) | 0.207 | –0.371 to 0.799 | 0.0052 | 0.0155 |
| RMI | 8.4 (4.2), (n = 236) | 7.8 (4.2), (n = 263) | 0.145 | –0.288 to 0.581 | 0.0111 | 0.0060 |
| GHQ-12 (participant) | 15.4 (6.6), (n = 234) | 14.2 (6.0), (n = 261) | –0.958 | –1.907 to 0.012 | 0.0028 | 0.0035 |
| GHQ-12 (carer) | 14.4 (6.4), (n = 64) | 13.6 (5.9), (n = 84) | –0.682 | –2.472 to 1.088 | 0.0176 | 0.0234 |
| SWOM | Yes = 52, no = 181, (n = 233) | Yes = 72, no = 189, (n = 261) | 1.37 | 0.89 to 2.22 | 0.0289 | 0.0123 |
| Travel journeys/day | 1.1 (1.2), (n = 241) | 1.0 (1.0), (n = 263) | 1.42 | 1.14 to 1.67 | – | – |
There was evidence of a therapist effect on the travel journeys taken but not for any of the other outcome measures. ICCs for therapist and centre effects were small, with ICCs for therapist effect being no greater than 0.0289 and ICCs for site effect being no greater than 0.0234. Therapy effect and site effect could not be calculated for travel journeys.
The other domains from the SF-36v2 were also analysed at 6 months and showed no significant difference between groups.
Outcome measures at 12 months
Table 10 summaries the secondary outcomes analysis at 12 months. The variability of the social function score in both groups at 12 months was similar to that observed at 6 months but, unlike the 6-month findings, the unadjusted mean social function score at 12 months was slightly less in the intervention group (45.5) compared with the control group (48.1) (see Table 10 ). The adjusted difference in means (–1.24) indicated that the intervention group scores were lower than the control, although the difference was not significant and no therapy or site effect was observed for this outcome measure.
| Outcome | Unadjusted | Adjusted | ICC | |||
|---|---|---|---|---|---|---|
| Control, mean (SD) | Intervention, Mean (SD) | Difference in means | 95% credible interval | Therapist | Site | |
| Social Function domain | 48.1 (28.7), (n = 207) | 45.5 (31.6), (n = 221) | –1.238 | –6.671 to 4.096 | 0.0025 | 0.0597 |
| NEADL | 9.9 (5.9), (n = 210) | 9.0 (5.8), (n = 228) | 0.487 | –0.216 to 1.168 | 0.0012 | 0.0171 |
| RMI | 8.4 (4.2), (n = 210) | 7.6 (4.4), (n = 232) | –0.044 | –0.530 to 0.432 | 0.0134 | 0.0057 |
| GHQ-12 (participant) | 14.3 (6.6), (n = 208) | 15.2 (6.6), (n = 228) | 1.069 | –0.045 to 2.150 | 0.0075 | 0.0043 |
| GHQ-12 (carer) | 13.7 (5.9), (n = 56) | 13.7 (5.3), (n = 71) | –0.399 | –2.338 to 1.543 | 0.0136 | 0.0066 |
| Outcome | Unadjusted | Adjusted | ICC | |||
| Control | Intervention | Odds ratio | 95% credible interval | Therapist | Site | |
| SWOM | Yes = 45, no = 160, (n = 205) | Yes = 56, no = 171, (n = 227) | 1.15 | 0.70 to 1.93 | 0.0315 | 0.0093 |
| Outcome | Unadjusted | Adjusted | ICC | |||
| Control, mean (SD) | Intervention, mean (SD) | Rate ratio | 95% credible interval | Therapist | Site | |
| Travel journeys/day | 1.1 (1.3) (n = 241) | 1.0 (1.1) (n = 263) | 1.76 | 1.36 to 1.95 | – | – |
The unadjusted mean scores in both groups were similar for all outcome measures. In the adjusted analysis there was a significant difference for travel journeys in the intervention group; rate ratio 1.76 journeys (95% CI 1.36 to 1.95 journeys). NEADL, RMI, SWOM and GHQ-12 (participant and carer) did not show any observable differences between groups. The ICCs for therapist effect were larger than those calculated for all secondary outcome measures at 6 months, with the exception of GHQ-12 (carer). The ICCs for site effect, however, were smaller at 12 months than at 6 months, apart from NEADL and participant-reported GHQ-12. There was a significant therapist effect for travel journeys at 12 months; however, we are unable to estimate the size of the effect because this is not calculable for this type of data (see Table 10 ).
Robustness of results
We assessed the robustness of the findings by repeating all analyses and adjusting for the baseline variables gender and residential status in addition to the other covariates already adjusted for in the models. The results of the analyses were consistent with those obtained from our original analysis. We were unable to conduct this analysis for the GHQ-12 carer outcome because the models would not converge.
Sensitivity analyses
The only potential outliers we found were for the GHQ-12 outcome measures for the participant and carers. After Winsorising these potential outliers we re-analysed the data but the findings were consistent with the original analysis. We also conducted a sensitivity analysis using MI to replace missing data but this did not change the findings of the study.
Exploratory/other analyses
Nottingham Extended Activities of Daily Living by category
No significant differences between groups were found for any of the four categories (mobility, kitchen, domestic and leisure) at 6 or 12 months.
Number of sessions by site
Table 11 details a site-by-site breakdown of the number of sessions delivered by therapists and the corresponding percentage of participants who completed the intervention to satisfaction. The median number of visits for this study was seven so this is a suitable point of comparison within the context of this study. Of the seven sites with a median of < 7 visits, only 3 out of 7 sessions (42.9%) had > 50% of participants who completed the intervention to satisfaction. Of the eight sites whose median was ≥ 7 visits, 8 out of 8 sessions (100.0%) had > 50% of participants who completed the intervention to satisfaction.
| Site | Percentage who completed intervention to satisfaction | No. of sessions | ||
|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Min. to max. | ||
| A | 18.4 | 3.93 (3.79) | 2 (1–6) | 1, 12 |
| B | 90.5 | 5.3 (4.16) | 4 (2–9) | 1, 12 |
| C | 40 | 5 (4.18) | 4 (1–10) | 1, 11 |
| D | 37.5 | 4.86 (2.67) | 5 (2–5) | 2, 10 |
| E | 66.7 | 6.67 (3.79) | 5 (4–11) | 4, 11 |
| F | 90.9 | 7.18 (3.84) | 6 (4–12) | 2, 12 |
| G | 50 | 6.25 (3.01) | 6.5 (4.5–8) | 1, 11 |
| H | 77.8 | 6.78 (3.14) | 7 (4–9) | 1, 12 |
| I | 70.6 | 7.41 (3.89) | 7 (5–11) | 1,12 |
| J | 77.4 | 7.03 (3.47) | 8 (4–10) | 1, 12 |
| K | 77.8 | 7.74 (3.24) | 8 (5–11) | 2, 12 |
| L | 80.0 | 8.7 (3.32) | 9.5 (6–12) | 2, 12 |
| M | 85.7 | 8.57 (3.63) | 10 (5.5–12) | 1, 12 |
| N | 100 | 8.71 (3.55) | 10 (5–12) | 3, 12 |
| O | 84.2 | 10.37 (3.37) | 12 (11–12) | 2, 12 |
The pilot study, however, showed that the intervention was effective with a median of six visits,24 so this is a suitable predefined point of comparison in terms of potential clinical effect. Of the five sites that had a median of < 6 visits, only 2 out of 5 sessions (40.0%) had > 50% of participants who completed the intervention to satisfaction. Of the 10 sites that had a median of ≥ 6 visits, 9 out of 10 sessions (90.0%) had > 50% of participants who completed the intervention to satisfaction. The results are exploratory but are suggestive that the more intervention visits a participant had the more likely they were to complete the intervention to satisfaction.
Number of travel journeys by intervention session
A further exploratory comparison of travel journeys within the intervention group, based on the number of sessions they received, was carried out ( Table 12 ). From the pilot study there was evidence that receiving six intervention visits would increase the number of journeys made, therefore it is considered to be a clinically important pre-existing threshold. The difference between travel journeys made by participants in the intervention group was summarised by < 6 and ≥ 6 or more intervention visits at both 6 and 12 months.
| Follow-up | Outcome measure | No. of intervention sessions | |
|---|---|---|---|
| < 6 (n = 109) | ≥ 6 (n = 156) | ||
| 6 months | No. of journeys | 16,141 | 25,979 |
| Mean (SD) | 148 (176) | 167 (169) | |
| Median (IQR) | 92 (36–199) | 111 (61–247) | |
| Min. to max. | 0–1088 | 0–1152 | |
| 12 months | No. of journeys | 31,679 | 49,214 |
| Mean (SD) | 291 (385) | 316 (321) | |
| Median (IQR) | 160 (54–381) | 208 (94–464) | |
| Min. to max. | 0–2195 | 0–2015 | |
These data show that participants who had six or more intervention visits were more likely to have a higher number of journeys at both 6 months (167 vs. 148) and 12 months (316 vs. 291). The results are exploratory but suggest that the more intervention visits a participant has the more likely they are to take more journeys beyond the completion of the intervention.
Falls by age category and allocation
All data described in this section were collected from travel diaries.
Table 13 presents falls data described by age, either < 60 years of age or ≥ 60 years. At randomisation, 104 participants (18.3%) were < 60 years of age, whereas 464 (81.7%) were ≥ 60 years. A total of 52 out of 104 (50%) of the participants aged < 60 years and 216 out of 464 (46.6%) of the participants aged ≥ 60 years had falls. In total, there were 1950 fall-days: 704 (36.1%) in the < 60 years age group and 1246 (63.9%) in the ≥ 60 years group. The median number of fall-days overall was 3 per year (IQR 1–6.5 fall-days). There appeared to be no difference between the median fall days of the two age groups.
| Fall-days | < 60 year | ≥ 60 years | Total |
|---|---|---|---|
| Participants, n (%) | 104 (18.3) | 464 (81.7) | 568 (100) |
| Participants with recorded falls, n (%) | 52 (50) | 216 (46.6) | 268 (47.2) |
| Total of fall days, n (%) | 704 (36.1) | 1246 (63.9) | 1950 (100) |
| Mean (SD) | 13.5 (22.2) | 5.8 (10.2) | 7.3 (13.7) |
| Median (IQR) | 3 (1–13) | 3 (1–5.5) | 3 (1–6.5) |
| Min. to max. | 1, 89 | 1, 79 | 1, 89 |
Table 14 presents falls data described by treatment allocation. The proportion of participants who had a fall was similar in each group: 133 out of 281 (47.4%) in the control group and 135 out of 287 (47%) in the intervention group.
| Fall-days | Allocation | Total | |
|---|---|---|---|
| Control | Intervention | ||
| Participants, n (%) | 281 (49.5) | 287 (50.5) | 568 (100) |
| Participants with recorded falls, n (%) | 133 (47.3) | 135 (47.0) | 268 (47.2) |
| Total of fall days, n (%) | 934 (47.90) | 1016 (52.10) | 1950 (100) |
| Mean (SD) | 7.0 (13.0) | 7.5 (14.5) | 7.3 (13.7) |
| Median (IQR) | 2 (1–7) | 3 (1–6) | 3 (1–6.5) |
| Min. to max. | 1, 80 | 1, 89 | 1, 89 |
Comparing changes in satisfaction with outdoor mobility over time
There was very strong evidence that the control group improved markedly. At baseline, 259 out of 281 (92.2%) participants were dissatisfied with outdoor mobility, but at the 6-month assessment this had reduced to 78% (160/205), a 15% reduction. The corresponding reduction in the intervention group was slightly greater (18%), with 268 out of 287 (93.4%) expressing dissatisfaction with outdoor mobility at baseline and 171 out of 227 (75.5%) expressing this at 6-month assessment. This suggests that the control (consisting of the baseline visits and completion of the travel diary) may have affected a change.
Six-month follow-up by follow-up approach
Table 15 details the questionnaire data collection from the different methods of approach. For 6-month questionnaires we received the questionnaire booklets for 503 out of 568 (88.6%) participants. The overall average difference in days from actual completion to expected due date was +4.7 days (range –33 to +133 days). Of those received, 280 out of 503 (55.7%) were via postal approach, 185 out of 503 (36.8%) were via the RA approach, with the remaining 38 out of 503 (7.6%) via the RA approach after switching from postal approach. RA assistance did not necessarily mean that the RA completed the questionnaire, just that assistance was provided; however, in the majority of cases the RA would ask the questions and complete on the participant’s behalf.
| Six months | Total received | Percentage of total |
|---|---|---|
| Total postal approach | 280 | 55.7 |
| Postal to RAa | 38 | 7.6 |
| Total RA approach | 185 | 36.8 |
| Total | 503 | 100 |
Adverse events
Adverse events records (i.e. a fall that required the assistance of a health-care professional) were collected from only participants in the intervention group over the course of delivering the intervention visits. There were 20 adverse events from 17 participants, with the majority of falls occurring at home. Two were recorded as ongoing, with the average mean duration of event being 5.6 days and median duration of 1 day (range 1–44). Overall, there was no effect on the delivery of the intervention and only one led to permanent discontinuation of the intervention.
Overall, 24 participants died, 12 in each group, indicating that the intervention group did not have an increased risk of death compared with what would be expected for this patient group. As part of the safety analysis there was also no significant difference in GHQ-12 score observed between the groups.
Protocol deviations
There were 68 protocol deviations recorded, with 37 out of 68 (54.4%) for visits not performed within the 4-month window (the majority of these were as a result of adverse weather conditions or temporary participant or therapist unavailability). However, from eCRF intervention data there were a total of 83 visits delivered outside the 4-month window, so there was an issue recording these as protocol deviations.
Twenty-five out of the 68 participants (36.8%) recorded other intervention therapy, which refers the intervention being delivered via non-protocol-defined methods (in all of these cases the intervention was delivered either by telephone or letter).
One out of 68 participants (1.5%) in the control group received the intervention (two visits); 1 out of 68 participants (1.5%) was in active rehabilitation at point of recruitment; and 1 out of 68 participants (1.5%) was an eligibility deviation, as the participant was unwell and not fit to receive treatment, whereas 1 out of 68 participants (1.5%) had a consent issue that was later resolved. Finally, 2 out of 68 participants (2.9%) had an intervention delay owing to unforeseen circumstances.
We considered these all to be minor deviations and that they were unlikely to have any effect on the outcome measure. As a result, no action was taken and all of these participants remained in the study and in the final analysis.
Concealment of allocation
There was a total of 223 RA-assisted participant visits at 6 months (see Table 15 ), for which the participant-reported primary outcome measure was collected. We received blinding assessments for 171 out of 223 (76.7%) [intervention group 88 out of 171 (51.5%); control group 83 out of 171 (48.5%)] RA-assisted participant visits at 6 months ( Table 16 ). The RA was unblinded for 87 out of 171 (50.9%) of those visits, with 13 out of 171 (7.6%) unblinded prior to the visit and 74 out of 171 (43.3%) unblinded during the visit. The RAs were unblinded more frequently for participants in the intervention group (48/87, 55.2%) than for those in the control group (39/87, 44.8%), although this is not indicative of any significant difference in unblinding rates between the two groups.
| Six months | Prior | During | Total |
|---|---|---|---|
| n visits | 171 | ||
| n unblinded | 13 | 74 | 87 |
| % n visits | 7.6 | 43.3 | 50.9 |
Summary
-
The study reached its recruitment targets and achieved strong retention rates at 6 months.
-
Differential follow-up occurred but not to an extent as to affect the power of the study. The attrition rates were greater within the first 6 months of follow-up.
-
The quality of life (social function) measure (primary outcome) showed no significant difference between groups at 6 months or at 12 months.
-
Six- and 12-month measures for functional mobility, SWOM, and participant and carer well-being showed no significant differences between groups.
-
The 6- and 12-month measure of travel journeys showed a significant difference in favour of the intervention group when the therapist and site effect was taken into consideration.
-
There was no evidence of a therapy or site effect in any of the outcome measures at either 6 or 12 months, apart from travel journeys at 6 and 12 months.
-
The intervention was delivered a median of seven times across the study as a whole, with 67.3% completed to the satisfaction of the participant; however, there was considerable variation amongst the sites for both these outcomes.
-
Exploratory data suggest that the more intervention visits a participant receives, the more likely they are to take more journeys beyond the completion of the intervention.
-
Exploratory data suggest that the more intervention visits a participant had, the more likely they were to complete the intervention to satisfaction.
-
The control group appears to have benefitted from inclusion in the study by becoming more satisfied with their outdoor mobility over time.
Chapter 4 Economic evaluation: methods and results
Health economic evaluation: methods used
This component of the study aimed to extend the evidence base by estimating for the first time the incremental cost-effectiveness of an outdoor mobility rehabilitation intervention, compared with control, from a health and personal social services perspective. In addition, carer time and certain patient-borne costs were also estimated. It was decided that medication costs would not be monitored, as it was considered that the intervention would not influence these. A within-trial analysis was conducted over a 12-month period, where an available case analyses approach41 was adopted. Thus, for each variable, we analysed all available data, which meant a different sample n was used for different variables. For example, the EQ-5D and SF-6D may have different response rates. Additionally, in the base-case analysis, it should be noted that when calculating levels of cost-effectiveness [see Cost-effectiveness, below, for a definition of incremental cost-effectiveness ratio (ICER)] we included only those participants who had both complete cost and effect data. As such, the number of participants for whom these data are available may well differ from the n for which separate cost and effect data are available.
Methods
Measuring costs
Overview
For each participant the total NHS and Personal Social Services (PSS) costs were estimated by summation of the intervention cost and other NHS and PSS costs. Carer input (coated in terms of lost productivity) and certain patient-borne costs were also estimated. Costs were estimated in UK sterling (£) at 2010–11 financial year levels.
Intervention
Training
Therapists were provided with training in order to deliver the intervention. Three types of training were provided: (1) group training in Nottingham; (2) individual training to a particular site therapist; and (3) within-site cascade training. The time inputs by all members of staff were estimated for each of these training methods, including preparation and travel time. The unit cost of NHS community therapy time, as estimated by Curtis et al. 42 was assumed to apply to all time inputs, where this was adjusted to be grade 7 for the individual and group trainer (compared with grade 5 for the cascade trainer and for those receiving the training and subsequently delivering the intervention). Travel distances were also estimated, and assigned a travel cost of 54p per mile. 42 Total training costs (the sum of staff and travel costs) were apportioned across all participants allocated to the intervention arm of the study.
Therapy contacts
Participants in both arms received a baseline visit; this was assumed to last 1 hour, including preparation, travel time and the writing up of notes, as well as the patient contact. Generally, this was provided by one therapist, although two therapists may undertake this (or subsequent visits) if there were issues relating to handover/safety, thus those who provided the intervention were asked to note which therapist(s) attended each visit (if this was not recorded it was assumed that one therapist provided the intervention). The associated travel costs for the baseline, and all subsequent therapy visits, were estimated in the following manner. After discussion with those who delivered the service, it was assumed that a travel time of 12 minutes would apply to each visit (this is in line with a previous assumption in relation to GP home visits42). Assuming an average speed of 29.9 miles per hour (mph) (the average free-flow vehicle speed in a 30-mph limit,43 this would equate to a total mileage of 5.98 miles per visit. Applying a travel cost of 54p per mile,42 this would equate to a travel cost of £3.23 per visit.
Those in the intervention arm received extra intervention visits, where the time associated with these visits (including travel to and from the participant’s home) was prospectively recorded by the therapist providing the intervention. It was additionally assumed that, for each visit, there would be 5 minutes’ preparation time and a further 10 minutes to write up associated patient records. Again for these visits the associated travel time was assumed to be 12 minutes, with a travel cost of £3.23 per visit. Thirty-minute supervisor meetings were also assumed to occur (one per site per month) for the duration of the study, where these were assumed to be 1 : 1 (therapy grade 7 and grade 5). Total supervisor meeting costs were equally apportioned across all visits. No other costs were included, as these were considered negligible (e.g. occasional bus trips with the participant, as part of the intervention).
Other NHS and Personal Social Services costs
Levels of resource use
The UK National Institute for Health and Care Excellence (NICE) recommends that costs can be calculated from the perspective of the NHS and PSS. 44 Accordingly, at both 6 and 12 months post randomisation, participants were asked to complete a resource-use questionnaire and return it by post. In this they were asked the number of times they had received different NHS and PSS services, any other care and certain patient costs that had been incurred. Specific questions with regard to health-care professional visits (and where they took place), hospital attendances and admissions, residential/nursing home admissions, home help (from community care assistant/someone who lives with them/other friends or family), Meals on Wheels and equipment purchased (to help with a health problem) were included.
Assumptions made in order to assign costs to items of resource use
Previously estimated unit costs42 , 45 were assigned to levels of resource use, where the following assumptions were made. Within Curtis et al. ,42 the length of time and/or cost associated with patient contacts (including home visit) is not stated for many health professionals, although the cost per hour of employment is generally available. Costs in terms of per hour of employment, per practice visit and per home visit are available, however, for the GP. Thus, the ratio of 1 hour of employment compared with (1) a practice visit and (2) a home visit can be calculated. This ratio was applied to the costs per hour of employment of other staff to estimate associated practice/hospital visit and home visit costs. Where the cost per hour of employment, for a particular health professional, was not reported within Curtis et al. 42 the average cost across the following health professionals was used: general practitioner, practice nurse, district nurse, dietitian, physiotherapist, occupational therapist, social worker, speech and language therapist, for the respective type of visit (GP, home or hospital). If the place of a health professional visit was not reported then it was assumed that the patient travelled to the health professional (patient travel costs were not estimated/included).
With regard to hospital admissions (in the past 6 months), if the length of a hospital admission was not reported the mean length of stay (per admission) for other respondents (who reported both the number of admissions and the accompanying length of stay) was applied to each admission that was reported but had no accompanying length of stay.
We also asked about the number of times a person had been admitted to both a residential home and a nursing home (in the past 6 months). We did not request that participants report the length of any associated stays in such care home we thereby made the assumption that each time a person reported they were admitted to a residential/nursing home they had stayed there for three of the preceding 6 months (the average length of stay in a care home has been estimated to be 801 days46 and we assumed that participants were on average admitted half-way through the 6-month period). If more than one admission was reported, it was assumed the participant had been in the home for the whole 6-month period in question.
Participants were asked whether they had attended a day-care centre in the past 6 months, and if they had, how many times per week they attended. The number of reported day centre attendances per week was assumed to apply to all weeks in the 6-month period in question.
Participants were asked to report the number of times (in the past week) they had home help or a visit from a community care assistant, and how long that person stayed. We requested that participants report the average time per visit. If the average time per visit was not reported, the average time per visit for other respondents (who reported both the number of times and the accompanying average time per visit) was applied to each visit that was reported but had no accompanying time per visit. Additionally, some of the responses were higher than what we considered to be a possible visit length. For example, one participant reported 28 visits and a visit length of 960 minutes (16 hours). This equates to more hours than there are in a week. We thereby assumed that this participant, and all others for whom the product of the number of visits and the associated time was greater than the number of hours in a week (a total of four participants at 6 months and five participants at 12 months), had misinterpreted the question and reported the total length of contact in the week, rather than the average per visit. Thus for these participants the reported visit length was assumed to be the total for the whole week. Again, it was assumed that the number of visits/hours reported for the week in question applied to all weeks in the past 6 months. In line with the therapist intervention, the associated travel time for each home help visit was assumed to be 12 minutes, with a travel cost of £3.23 per visit.
Participants were additionally asked how many times they had received help from someone they lived with, in the past week and the average associated length of time. They were also asked to report the level of such help from people they do not live with. In both these questions, we requested that people report the average length of time (per visit) for the help they received. If the average time per visit was not reported, the average time per visit for other respondents (who reported both the number of times and the accompanying average time per visit) was applied to each visit that was reported but had no accompanying time per visit. Also, for some participants, the product of the number of visits and the associated time was greater than the number of hours in a week (with regard to someone they lived with this occurred for a total of two participants at 6 months and two participants at 12 months, and for people they do not live with this occurred for one participant at 6 months). We thereby assumed that these participants had misinterpreted the question and reported the total length of contact in the week, rather than the average per visit. Thus for these participants the reported visit length was assumed to be the total for the whole week. Again it was also assumed that the number of visits/hours reported for the week in question was equivalent to the average per week across all weeks in the past 6 months. Within both these questions, participants were asked whether the person who provided the help had had to take time off work to provide this help. In order to provide an estimate of lost productivity, a cost was only applied to the total number of hours they were estimated to have received (both for people they live with, and do not live with) if they reported that the person that provided the care had to take time of work to provide such care. In this case, the average hourly earnings47 was applied to these times, consistent with the human capital approach. 48 However, as the costing of such care is sometimes considered controversial,49 these costs are also reported separately to the aforementioned NHS and PSS costs.
Participants were asked to report if they had Meals on Wheels and, if so, the number that they had received in the past week, and, if they paid for them, the amount they cost. Meals on Wheels that were not reported to be paid for by participants were assigned a previously estimated unit cost50 and classified as a PSS cost. The costs for those who reported that they paid for them themselves were classified as patient-borne costs. Again, it was assumed that the number of meals reported for the week in question was equivalent to the average per week across all weeks in the past 6 months.
Participants were also asked to report any equipment they had bought, or been given, to help with a health problem and, if so, to state the equipment, who paid for it (the participant/social services) and the cost of the item. Items that were not reported to be paid for by social services were classified as a patient-borne cost. When a cost was not stated, where possible, previously estimated unit costs were assigned to items (where these were taken from, e.g. Curtis et al. 42). When a unit cost for an item could not be identified, or the type of item was not reported, either the cost of what was considered to be a similar item or the average cost of all items for which a unit cost was identified was assigned to the item in question. This question did not specify the time frame over which it was interested in equipment purchases; consequently, even although all other questions specified the previous 6 months, it is possible that some of the reported items may have been purchased before the participant joined the study. The potential impact of this was reduced, however, as the equivalent annual cost of equipment purchases was calculated,48 for which the discount rate was assumed to be 3.5% per annum and the lifespan of the equipment was assumed to be 7 years.
Categorisation of costs
The above enabled a cost to be assigned to each of the resource-use questions. These were then categorised, as follows, where all costs relate to the 12-month post-intervention period: the costs associated with health professional or home help visits; visits to accident and emergency, walk-in centres, outpatients, day centres; and admissions to hospital, residential homes and nursing homes.
Meals on Wheels and equipment, for which the participant did not pay, were summed to estimate other NHS and PSS costs. Table 17 provides details of the resources monitored within each question. These were, in turn, added to the intervention costs to provide an estimate of total NHS and PSS costs (base-case analysis). The costs associated with help from people they live or do not live with were added together to provide an estimate of lost productivity. Similarly, the costs associated with Meals on Wheels and equipment, for which the participant did pay, provided an estimate of the costs borne by the patient. Finally, overall costs were estimated by the summation of intervention costs, other NHS and PSS costs, lost productivity and patient-borne costs.
| Resource item | Level of resource use | Associated unit cost (£) | Associated total cost (£) | Per-participant cost (£) |
|---|---|---|---|---|
| Provision of training | 35.75 hours of (central) trainer time | 44.74 per houra | 1599.59 | |
| Associated travel costs (800 miles) | 0.54 per milea | 432.00 | ||
| 14 hours of cascade training | 30.78 per houra | 430.92 | ||
| Receipt of training | 62 hours | 30.78 per houra | 1908.38 | |
| Associated travel costs (900 miles) | 0.54 per milea | 486.00 | ||
| Overall training cost | 4856.89 | 16.92 | ||
| Therapy contacts | Therapist time (mean = 6.76 visits)b,c | 30.78 per houra | 125,058.31 | |
| Associated travel costs (12 miles per visit) | 0.54 per milea | 6257.15 | ||
| Supervision meetings | 1 per month (over 18 months) in each of the 15 sites | 38.00 per meeting | 10,153.09 | |
| Overall intervention cost | 146,325.44 | 509.84 | ||
| Control visit | 1 visit, 1 hour in durationc | 34.78 | ||
| Incremental cost of the intervention | 475.07 |
Overall and incremental costs
For each of the aforementioned cost categories, the mean incremental cost of the intervention (over the 12-month follow-up period) was calculated by subtracting the mean cost for the control group from the mean cost for intervention group.
Measuring outcomes
To estimate the impact on health-related quality of life, participants were asked to complete the EQ-5D51 at baseline, 6 and 12 months post randomisation. The EQ-5D has five questions, through which the respondent is asked to report the level of problems they have (no problems, some/moderate problems, and severe/extreme problems) with regard to mobility, self-care, usual activities, pain and anxiety/depression. 30 The three-level version of the EQ-5D (EQ-5D-3L) was used. Responses to these five dimensions are converted into one of 243 different EQ-5D health-state descriptions, which range between no problems on all five dimensions (11111) and severe/extreme problems on all five dimensions (33333). A utility score (a scale where death = 0 and full health = 1) was assigned to each of these 243 health states using the York A1 tariff52 (associated EQ-5D scores range between –0.594 and 1.00). Completion of the EQ-5D enabled a cost–utility analysis to be undertaken, in which the benefits of different health-care treatments can be compared on a common utility scale. 53 The area-under-the-curve method53 was used to estimate the mean quality-adjusted life-year (QALY) gain/loss over the 12-month trial period for both groups. Within these QALY calculations, those who died within the study period were assigned a utility score of ‘0’ upon death.
In a similar way, responses to 11 of the questions on the SF-3654 were used to estimate a score on the SF-6D. 25 The SF-6D is composed of six dimensions (physical functioning, role limitations, social functioning, pain, mental health and vitality), which have between four and six levels. A non-parametric model (which uses Bayesian methods)55 was used to estimate SF-6D health-state utility values for each of 18,000 potential health states (associated SF-6D scores range between 0.203 and 1.00). QALY gains/losses were again calculated, as for the EQ-5D, and those who died were, again, assigned a score of ‘0’.
As the EQ-5D and SF-6D utility measures are based on both different health-state descriptions and use different valuation techniques, they could produce different utility scores for the same group of patients. This study therefore sought to explore the impact the choice of utility measure had on estimates of cost–utility, as further research has been argued to be necessary in this area. 56 It should be noted, however, that NICE currently recommends that the EQ-5D be used within the reference case analysis,39 and thus this constituted our main base-case analysis (see below).
Base-case analysis
Multiple regression57 was used to estimate the mean cost difference (incremental cost) and mean QALY difference (incremental effect) between the two treatment groups, where both the overall cost and the mean QALY gain/loss over the 12-month period were adjusted for baseline utility, age, sex and residential status (the last three variables were chosen as they were the only participant variables that were available for all participants who had complete cost and QALY data). The mean QALY difference was estimated for both the EQ-5D and SF-6D. In line with the clinical analysis, within the base-case analyses only participants with complete cost and QALY data were included.
Cost-effectiveness
After checking that dominance was not apparent (this would occur if one intervention were less costly and more effective than another),53 the incremental cost per QALY gain (ICER) associated with the intervention was calculated (mean incremental cost/mean incremental QALY gain). In line with NICE guidance44 we compared the ICER to a cost-effectiveness threshold (λ) of £20,000 per QALY.
Decision uncertainty
The bootstrap technique58 (with 5000 replications) was used to estimate the 95% CIs surrounding the incremental cost and incremental effect (where appropriate), the 95% CI was estimated using the percentile method. 59 As the ICER has the potential to be misinterpreted,60 we also estimated the incremental net benefit (INB) (and associated 95% CI) at a threshold of £20,000 per QALY. A negative INB would indicate that the intervention was not cost-effective at this threshold. The bootstrap samples were also used to estimate the cost-effectiveness acceptability curve (CEAC) for each group, where the CEAC depicts the probability that an intervention is cost-effective at different levels of λ. 61 The probability of the intervention being cost-effective was specifically estimated at the (λ) of £20,000 per QALY.
Sensitivity analysis
We assessed how robust conclusions were to the following changes:
-
Received six or more intervention visits:
-
On the assumption that those who received more visits might benefit more, here, only intervention participants who were included in the base-case analysis and had six or more visits were included in the analyses. The control group was the same as for the base case.
-
-
MI:
-
To impute missing data in this data set, we used regression methods to predict these values based on their relationship with other covariates (age, sex, residential status, cost and utility data). Imputation took place in 10 cycles, the estimates from which were then pooled and calculated using Rubin’s Rules. All MI was performed for incomplete cost and outcomes components at the patient level using the mi impute mvn procedure in Stata 11.
-
-
Winsorising:
-
As outlined previously, for the clinical data, we replaced data values below the 5th percentile with the 5th percentile value and to data values above the 95th percentile with the 95th percentile value. This was applied to the cost and QALY data for those individuals included in the base-case analysis. One reason for undertaking this analysis was the wide variation in some of the resource-use data that was reported, e.g. home help. This approach reduces the influence of extreme values and may partially test whether or not some of the assumptions in relation to these costs were correct.
-
Different cost perspective.
-
Results were re-estimated from an overall cost perspective.
Results
Costs
Training
Training costs were as follows (see Table 17 ). Total trainer time associated with group training (provided to eight sites at once) and individual training (to a further nine sites) was estimated to be 35.75 hours (including travel and preparation). Total trainer time for the cascade training (provided at seven sites) and time for receipt of all types of training (including travel) was estimated to be 14 and 62 hours, respectively. Trainer (group and individual) unit costs were estimated to be £45, compared with £31 per hour for cascade trainers and trainees. The total cost of all staff time associated with all aspects of training was thereby estimated to be £3938.89, with the addition of £918 for travel costs to sites (eight people attending the group training and nine site visits, with an average return trip of 100 miles), this gave a total training cost of £4856.89. Apportioned across all 287 participants in the intervention arm, this equates to £16.92 per participant.
Therapy contacts
All participants received a baseline visit. This was the only contact for those in the control arm and the associated mean total cost (staff time and travel costs) was estimated to be £34.78 per participant (see Table 17 ). (It should be noted that one participant in the control received two intervention visits – no associated intervention costs were assigned to this participant as this was provided in error.) The costs associated with therapist visits to those in the intervention arm are summarised in Table 17 . On average, participants in the intervention arm received a further 6.76 visits (range 0–12). The associated time for these visits was recorded for all but 1 of the 1939 visits, for which the mean time was 96.61 minutes (assuming a travel time of 12 minutes, this equates to an average contact time of 84.61 minutes). This mean value was assumed to apply to the visit where the time was not recorded. Each supervisor meeting was estimated to cost £37.87, one per month per site were estimated to occur across the 18-month period for which the intervention was provided. This equates to a total cost of £10,153.09 across all sites: £5.24 per visit undertaken. Visit, preparation and records’ write-up time were each costed at £31 per hour and, after adding the supervision cost (£5.24 per visit) and travel cost (£3.23 per visit), the mean cost of the intervention was estimated to be £492.92 per participant, where this increased to £509.84 after including the aforementioned training costs. The incremental intervention cost, compared with the baseline visit provided to the control arm (cost £34.78) was thereby estimated to be £475.07 per participant.
Other NHS and Personal Social Services costs
The 6-month resource-use questionnaire was returned by 259 out of 287 participants in the intervention arm and 235 out of 281 in the control arm; the numbers at 12 months were 230 and 209, respectively. However, not all returned questionnaires were fully completed and we accordingly note the response rate to each of the individual questions in Table 18 . (Note: A response was required at both 6 and 12 months in order for the 12-month cost to be estimated.) Mean levels of resource use, relating to each of the cost questions, are shown in Table 18 , in which it can be seen that participants frequently visit health professionals, outpatients and receive home help. The unit costs applied to the reported levels of resource use are summarised in Table 19 . Subsequently, costs were categorised into the following groups: other NHS and PSS costs, total NHS and PSS costs, lost productivity, and overall costs (see Table 18 for details of which questions contributed to each cost category). The mean cost (per participant) for the intervention and control groups, for each of these cost categories, are presented in Table 20 , in which it can be seen that, for each of these cost categories, the mean costs are estimated to be higher for the intervention group. It should be noted, however, that the number of participants for whom complete cost data are available falls when a broader perspective is taken. This can be explained largely by the fact that responses are required to a greater number of resource-use questions.
| Item | Respondents, n | Levels of resource use | Mean cost (£) | |||
|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |
| No. of health professional visitsa | 212 | 202 | 8.81 | 5.53 | 626.78 | 413.45 |
| No. of hospital admissionsa | 218 | 201 | 0.63 | 0.43 | 938.25 | 1078.05 |
| No. of A&E visitsa | 217 | 203 | 0.48 | 0.58 | 49.82 | 61.09 |
| No. of walk-in centre visitsa | 209 | 192 | 0.18 | 0.28 | 7.26 | 11.32 |
| No. of outpatient visitsa | 200 | 189 | 2.36 | 3.06 | 346.92 | 449.56 |
| No. of admissions to residential homesa | 208 | 186 | 0.22 | 0.16 | 652.31 | 451.58 |
| No. of admissions to nursing homesa | 207 | 187 | 0.05 | 0.09 | 451.55 | 649.79 |
| No. of day centre visitsa | 201 | 190 | 0.68 | 0.93 | 633.31 | 871.96 |
| No. of home help visitsa | 199 | 177 | 7.80 | 5.07 | 7097.08 | 6599.36 |
| Hours of help from someone they live withb | 188 | 177 | 6.68 | 6.50 | 412.28 | 398.83 |
| Percentage who took time off work | 202 | 193 | 4.00% | 3.10% | ||
| Hours of help from someone they do not live withb | 212 | 196 | 1.51 | 1.49 | 154.38 | 32.27 |
| Percentage who took time off work | 215 | 195 | 1.90% | 3.10% | ||
| Meals on Wheels | 208 | 193 | 0.09 | 0.12 | ||
| Cost when the participant did not paya | 5.25 | 14.55 | ||||
| Cost when the participant paidc | 5.38 | 14.55 | ||||
| Equipment | 208 | 194 | 84.10% | 77.80% | ||
| Cost when the participant did not paya | ||||||
| Cost when the participant paidc | 624.48 | 745.66 | ||||
| Item | Estimated unit cost (£) |
|---|---|
| GP visita | 30.00 |
| Practice nurse visita | 9.90 |
| District nurse visita | 13.20 |
| Dietitian visita | 9.30 |
| Physiotherapist visita | 9.60 |
| Occupational therapist visita | 9.60 |
| Social worker visita | 11.40 |
| Speech and language therapist visita | 9.30 |
| Cost per day in hospital (non-elective inpatient excess bed-day cost) | 246.0045 |
| A&E visit (non-admitted cost used) | 106.0042 |
| Walk-in centre visit (non-admitted cost used) | 41.0042 |
| Outpatient visit | 147.0042 |
| Admission to residential home (weekly cost) | 497.0042 |
| Admission to nursing home (weekly cost) | 719.0042 |
| Day centre (per visit cost) | 36.0042 |
| Home helpb (cost per hour of face-to-face contact) | 22.0042 |
| Help from someone they live with (who took time off work) (cost per hour) | 14.6562 |
| Meals on Wheels (where the participant did not pay) | 6.0042 |
| Equipment:c | |
| Grab rail | 94.0042 |
| Hoist | 934.0042 |
| Alarm system | 380.0042 |
| Stair lift | 2611.0042 |
| Concrete ramp | 646.0042 |
| Wheelchair | 402.0042 |
| Estimated variable | Intervention | Control | Difference |
|---|---|---|---|
| Intervention costs, £ (1) | 509.84 (n = 287), (95% CI 93.20 to 1051.71) | 34.78 (n = 281), (95% CI 34.23 to 34.23) | 475.07 (95% CI –58.97 to 1027.82) |
| Other NHS and PSS costs (2) | 11,707.93 (n = 162), (95% CI 256.80 to 55,886.08) | 9740.55 (n = 148), (95% CI 133 to 44,954.34) | 1967.38 (95% CI –35,921.68 to 48,398.27) |
| Total NHS and PSS costs (1 + 2) | 12,229.38 (n = 162), (95% CI 593.17 to 59,692.78) | 9775.19 (n = 148), (95% CI 163.83 to 44,988.56) | 2454.19 (95% CI –38,382.43 to 51,687.98) |
| Lost productivity (3) | 603.43 (n = 178), (95% CI 0.00 to 1333.15) | 291.45 (n = 165), (95% CI 0.00 to 761.80) | 311.98 (95% CI –761.80 to 1333.15) |
| Participant costs (4) | 273.26 (n = 203), (95% CI 0.00 to 1201.29) | 245.44 (n = 189), (95% CI 0.00 to 1109.98) | 27.82 (95% CI –1010.40 to 1123.98) |
| Overall cost (1 + 2 + 3 + 4)a | 14,008.45 (n = 143), (95% CI 597.61 to 70,841.91) | 9562.39 (n = 135), (95% CI 247.98 to 36,952.20) | 4446.06 (95% CI –30,413.18 to 58,969.86) |
| EQ-5D score: | |||
| Baseline | 0.408 (n = 281), (95% CI –0.182 to 0.848) | 0.420 (n = 280), (95% CI –0.124 to 0.850) | |
| 6 months | 0.398 (n = 255), (95% CI –0.181 to 0.848) | 0.436 (n = 229), (95% CI –0.181 to 0.883) | |
| 12 months | 0.384 (n = 223), (95% CI –0.182 to 0.850) | 0.447 (n = 204), (95% CI –0.164 to 1.000) | |
| Change (over 12-month period) | –0.022 (n = 218), (95% CI –0.570 to 0.435) | 0.022 (n = 203), (95% CI –0.531 to 0.651) | –0.044 (95% CI –0.814 to 0.691) |
| QALYb | 0.396 (n = 223), (95% CI –0.079 to 0.832) | 0.429 (n = 207), (95% CI –0.075 to 0.844) | –0.033 (95% CI –0.693 to 0.630) |
| SF-6D score: | |||
| Baseline | 0.517 (n = 271), (95% CI 0.320 to 0.665) | 0.530 (n = 267), (95% CI 0.376 to 0.668) | |
| 6 months | 0.510 (n = 239), (95% CI 0.340 to 0.651) | 0.514 (n = 226), (95% CI 0.317 to 0.660) | |
| 12 months | 0.507 (n = 209), (95% CI 0.317 to 0.649) | 0.521 (n = 198), (95% CI 0.320 to 0.687) | |
| Change (over 12-month period) | –0.007 (n = 198), (95% CI –0.182 to 0.161) | –0.011 (n = 190), (95% CI –0.196 to 0.170) | 0.004 (95% CI –0.241 to 0.255) |
| QALYc | 0.497 (n = 191), (95% CI 0.293 to 0.649) | 0.504 (n = 195), (95% CI 0.305 to 0.647) | –0.007 (95% CI –0.261 to 0.246) |
Overall and incremental costs
From the perspective of the NHS and PSS, the mean cost (per participant) was estimated to be approximately £2500 higher for the intervention group than the control group, and mean overall costs were estimated to be approximately £4500 higher. The confidence intervals in Table 20 do show, however, the large variations in relation to these figures. It can also be seen that the mean cost of the intervention is small in relation to other NHS and PSS costs incurred by this population group.
Outcomes
The mean baseline 6- and 12-month EQ-5D scores for both groups are shown in Table 20 . It can be seen that in the intervention arm, compared with baseline, the mean EQ-5D scores were lower by 0.022 at 12 months. Conversely, the mean EQ-5D score for the control group improved by 0.022 over the same period. Based on those who had complete EQ-5D data at baseline, 6 and 12 months, the mean QALY gain was 0.396 for the intervention group and 0.429 for the control group. The baseline score, however, was slightly higher for the control arm (we adjust for this in subsequent analyses). With regard to the SF-6D, both groups had slightly lower mean scores at the 12-month follow-up point than at baseline (see Table 20 ). The mean QALY gains were also similar in both groups.
Base-case analysis
For those who had both complete cost and QALY data (based on the EQ-5D), after adjusting for covariates, the mean incremental cost (total NHS and PSS cost) was estimated to be £3413.75 (95% CI –£448.43 to £7121.00), with an incremental QALY gain of –0.027 (95% CI –0.060 to 0.007) (see Table 21 for details of the numbers included in the analysis). An ICER was not calculated for this group, as the intervention was, on average, both more expensive and less effective. With regard to the SF-6D, the incremental cost was £2393.38 (95% CI –£2017.58 to £5999.37), with an incremental effect of –0.003 (95% CI –0.016 to 0.006). The intervention was thereby estimated to be dominated by the control group. The associated CEACs are shown in Figures 5 and 6 , for the EQ-5D and SF-6D, respectively. The probability that the intervention was cost-effective was < 20% at all cost-effectiveness thresholds.
| Analysis | Outcome measure | Incremental cost (£) | QALY gain | INB (£) |
|---|---|---|---|---|
| Base-case analysis (total NHS and PSS costs) | EQ-5D (n = 151; 139) | 3139.05a (95% CI –854.08 to 7086.78) | –0.040a (95% CI –0.094 to 0.013) | –3948.20 (6.5%),a (95% CI –8215.70 to 362.68) |
| 3413.75 (95% CI –448.43 to 7121.00) | –0.027 (95% CI –0.060 to 0.007) | –3944.90 (5.2%), (95% CI –7863.85 to 50.26) | ||
| SF-6D (n = 137; 139) | 1826.18a (95% CI –2259.47 to 5820.55) | –0.011a (95% CI –0.028 to 0.005) | –2053.78 (20.6%),a (95% CI –6139.58 to 2088.66) | |
| 2393.38 (95% CI –2017.58 to 5999.37) | –0.003 (95% CI –0.016 to 0.006) | –2237.37 (18.1%), (95% CI –6191.72 to 1936.07) | ||
| Received ≥ six intervention visits (total NHS and PSS costs) | EQ-5D (n = 91; 139) | 2663.38 (95% CI –1866.37 to 7415.26) | –0.036 (95% CI –0.074 to 0.003) | –6592.26 (4.8%), (95% CI –13268.98 to 104.40) |
| SF-6D (n = 85; 139) | 1937 (95% CI –2772.99 to 6637.36) | –0.008 (95% CI –0.021 to 0.004) | –2867.15 (22.9%), (95% CI –6844.37 to 2599.90) | |
| MI | EQ-5D (n = 287; 281) | –202.76 (95% CI –2651.37 to 2313.30) | –0.026 (95% CI –0.050 to –0.001) | –314.15 (40.7%), (95% CI –2867.61 to 2193.86) |
| SF-6D (n = 287; 281) | –454.62 (95% CI –2923.91 to 1939.63) | 0.001 (95% CI –0.010 to 0.013) | 483.76 (61.6%), (95% CI –1934.81 to 2963.58) | |
| Winsorising | EQ-5D (n = 151; 139) | 3088.47 (95% CI 6,35.90 to 5511.87) | –0.024 (95% CI –0.057 to 0.008) | –3570.48 (1.3%), (95% CI –6186.68 to –923.48) |
| SF-6D (n = 137; 139) | 2024.92 (95% CI –571.62 to 4622.48) | –0.005 (95% CI –0.014 to 0.005) | –2116.45 (9.1%), (95% CI –4748.48 to 499.28) | |
| Overall costs | EQ-5D (n = 133; 129) | 5144.81 (95% CI 823.17 to 9383.24) | –0.019 (95% CI –0.055 to 0.016) | –7273.84 (2.8%), (95% CI –13590.05 to –955.97) |
| SF-6D (n = 122; 126) | 3484.89 (95% CI –800.42 to 7663.75) | –0.003 (95% CI –0.014 to 0.009) | –3771.01 (8.8%), (95% CI –7663.75 to 800.42) |
FIGURE 5.
Cost-effectiveness acceptability curve for the intervention (green line) and control group (black line) (base case for EQ-5D data).
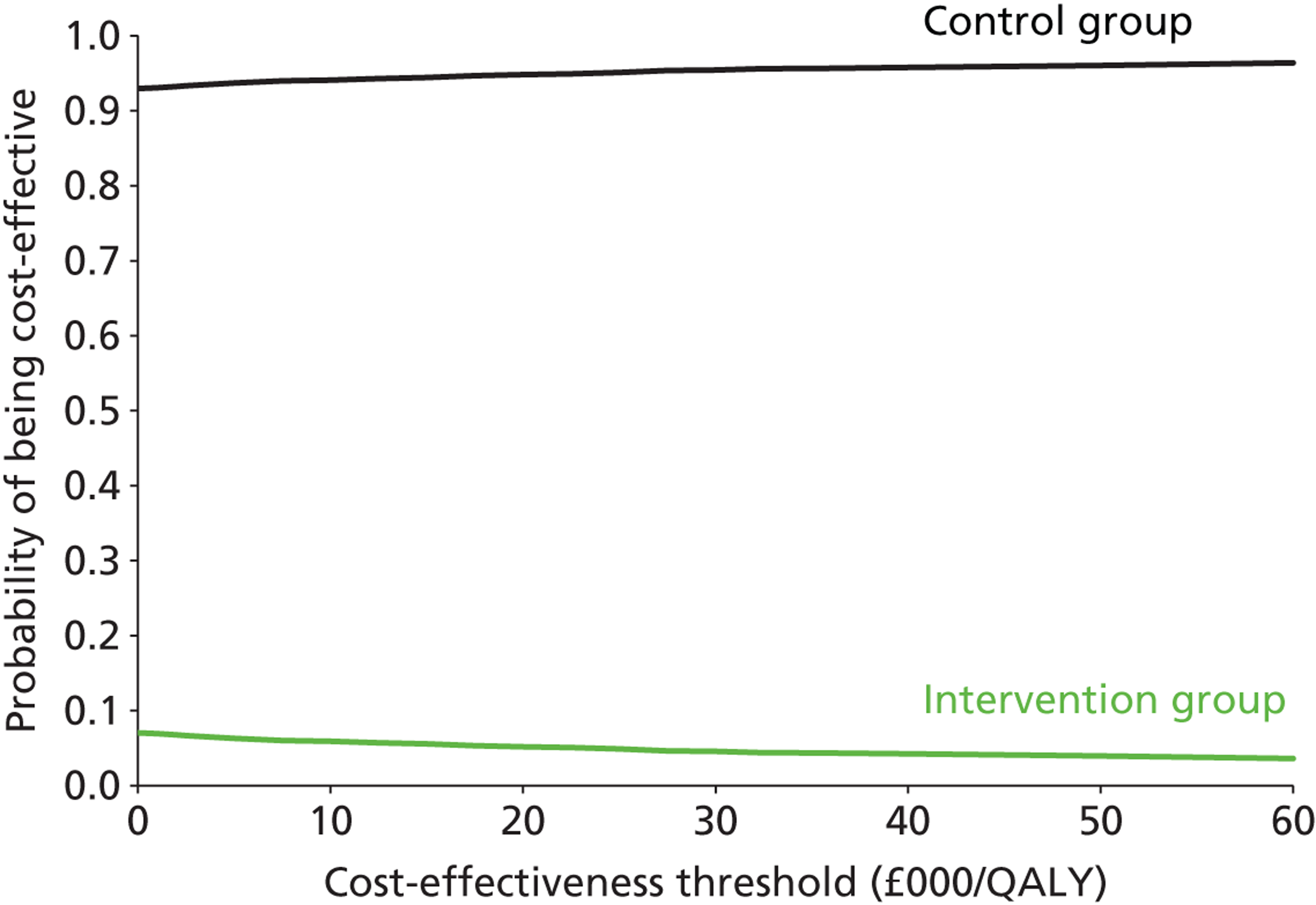
FIGURE 6.
Cost-effectiveness acceptability curve for the intervention (green line) and control group (black line) (base case for SF-6D data).
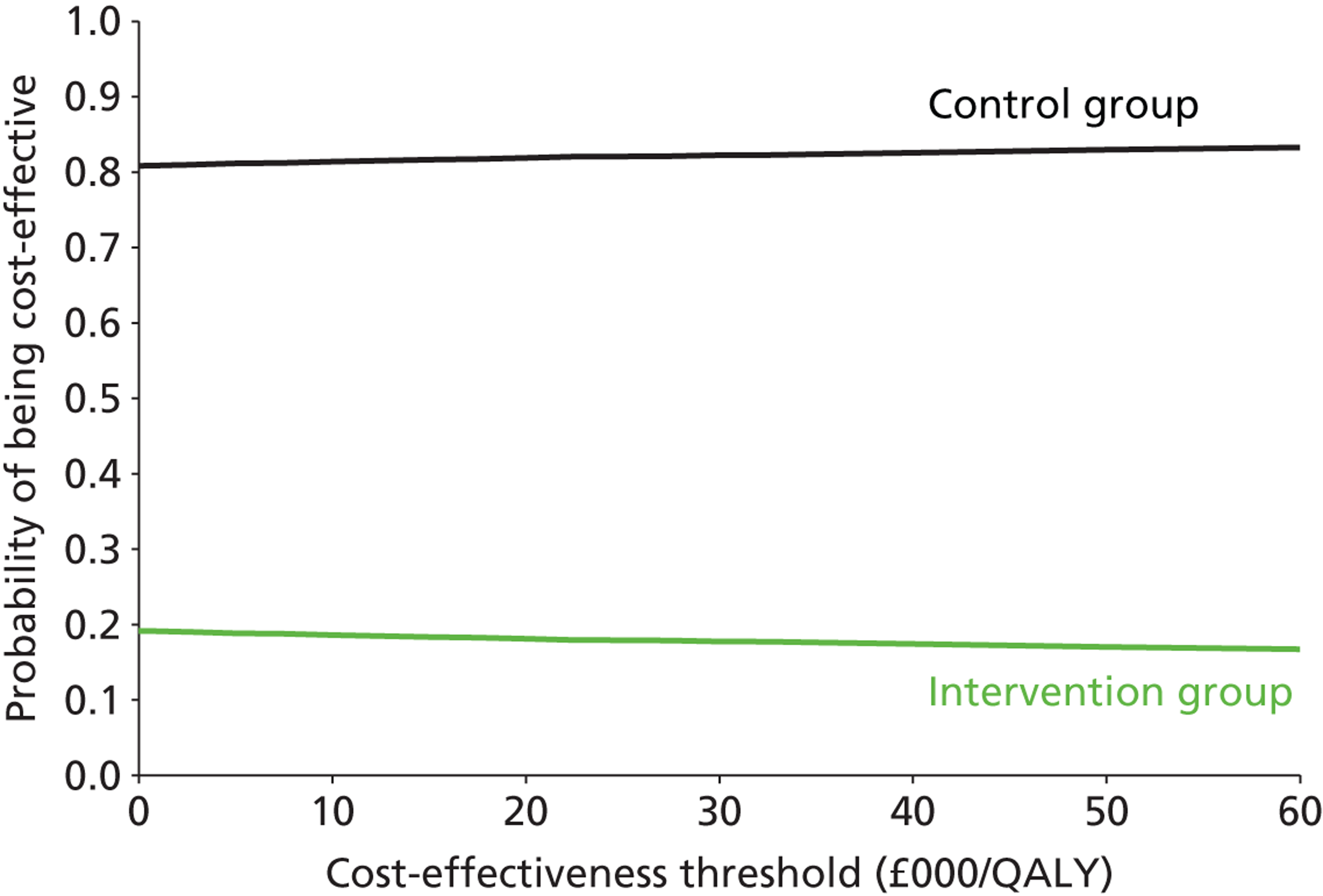
Sensitivity analysis The results of each of the sensitivity analyses are presented in Table 21 . Within all these analyses it can be seen that the 95% CI surrounding the INB is never wholly positive. Thus, in line with the base-case analysis, we are unable to conclude that the intervention is significantly (p < 0.05) more cost-effective at a λ of £20,000 per QALY. Indeed, the INB was more commonly negative and there was no suggestion that the intervention was more cost-effective for those who received six or more intervention visits.
One additional point to note is that although there is some consistency in these results (at a λ of £20,000 per QALY the 95% CI surrounding the INB is never wholly positive), there is some variation in the mean estimates. For example, in the base-case analysis (based on available data), compared with control, the intervention is estimated to be (on average) more costly and less effective. Conversely, when we look at the results based on MI, the intervention is estimated to be (on average) less costly and more effective. As such, at a λ of £20,000 per QALY, there is wide variation in the probability of the intervention being cost-effective (see Table 21 ).
Summary In the base (complete)-case analysis, the mean incremental cost of the intervention (total NHS and PSS costs) was estimated to be £3413.75 (95% CI –£448.43 to £7121.00), with an incremental QALY gain of –0.027 (95% CI –0.060 to 0.007), according to the EQ-5D. This suggests that the intervention was not cost-effective. The sensitivity analyses tended to support this conclusion as, at a cost-effectiveness threshold of £20,000 per QALY, the CIs around the mean INB were never wholly positive.
Chapter 5 Qualitative study: methods and results
Introduction
This chapter presents the aims and methods of the qualitative study, which explores the meaning of confidence after stroke, as described by the intervention participants. The principles of interpretive phenomenology analysis (IPA) were applied in both the collection and analysis phases of the study, resulting in broadening our understanding of the meaning of ‘confidence’ after having a stroke.
Aims
The aims of the qualitative study were to answer the following questions:
-
How does having a stroke affect self-confidence?
-
How do stroke survivors describe their experiences of regaining confidence after stroke?
-
What do stroke survivors identify as barriers to regaining confidence?
Sampling strategy and recruitment
All participants were selected from the ‘getting out of the house’ trial as IPA recommends that participants should be experts in the phenomenon being studied. ‘Lack of confidence’ was cited in the single centre24 as a reason for not getting out of the house, so it was felt that participants in the multicentre study were likely to have a perspective of individual confidence. To achieve maximum diversity a purposefully selected sample, ranging from no symptoms at all after stroke to severe disability, were recruited. The Modified Rankin Scale63 , 64 was used to identify this range, which was considered reflective of the stroke population. Ten potential participants were contacted by the qualitative researcher and invited to consent. All 10 participants agreed to be interviewed and a willingness to tell their stroke story was observed.
Participant sample characteristics
The sample was drawn from two trial sites for geographical convenience. Five men and five women were included in the study. One presented with no symptoms after stroke, three with slight disability, four with moderate disability, and two with moderately severe disability. Five lived alone, three lived with their spouses, and the remaining two lived with their spouses and children. Nine participants were of white British origin and one was of black Caribbean origin.
Data collection: interviewing approach
Semistructured, in-depth interviews that sought the perceptions of stroke survivors were the favoured method of data collection. It is advocated that in-depth interviews are the best ways to attain rich, comprehensive, first-person experience. 65 Interviews were conducted in participants’ own homes, which aimed to provide a safe and familiar context. A reflective journal to capture feelings and thoughts throughout the interview process was maintained.
An interview guide was developed for two pilot interviews, and improved for next eight interviews. This proved a useful tool for steering the interview. However, the first interview question elicited the majority of the data. ‘Tell me about your stroke, what happened when you had your stroke?’ The natural responses and flow of the interview as a result of this question, enabled participants to articulate meanings around losing confidence and regaining confidence, through their experiences, in their own words. Ten completed interviews were transcribed verbatim, using digital dictation transcription software. Transcripts reflected words, laughter, significant pauses and silences.
Data analysis
The framework for the data analysis was selected on the basis of the methodology. A six-stage process following IPA principles was chosen in favour of using a computer software package, such as, NVivo. IPA is interested in understanding the content of the data, rather than measuring frequency of words, or imposing a more tapered approach to the data. Therefore, principles advocated by an IPA expert65 were applied, and six-stage analysis process followed:
-
Reading and re-reading the data, making initial notes in margins.
-
Initial noting, taking one participant’s data at time, applying no rules and, therefore, making this stage as exploratory as possible.
-
Key data were then captured during the next analytical stage and the development of emergent themes progressed.
-
Stage four involved looking for patterns and connections across emergent themes and interpretation within these data was applied.
-
Stage five involved a repetition of stages 1–4 across all participants.
-
The final stage was a process that aimed to capture the essence of each individual story and also seek out differences and similarities within the data.
This process was audited by an experienced researcher in order to verify that all stages had been followed, adding to the trustworthiness of the data findings.
Presentation of the findings
The findings section follows with six key emergent themes that describe the phenomenon of confidence after a stroke. Although each participant provided a unique account, surprisingly, the emergent themes identified shared many concepts, despite difference in stroke impairments and personal context. The themes represent the essence of ‘confidence’ for the participants.
As there is an argument for the definition of confidence being different dependent upon who is asked,66 embedded in the interview data were a direct question about what confidence meant to participants in this study, conducted on the basis of providing some introductory data. Box 1 illustrates these findings:
Bob 12.16 ‘Going out and doing what you want to do’
Ryan 9.13 ‘Confidence to me, in relation to this, is doing something you want to do, when you want to do it.’
Mick 26.5 ‘It’s some way of going back to normal life. Being confident to leave the house, walking again, trying to talk erm . . .’
Ted 5.14 ‘Phewww God [quietly] [long pause] I’m thinking. Difficult one and I can’t really answer the question.’
June 22.17 ‘Confidence [long pause] . . . Can you say that again for me please?’
Alison 14.10 ‘I don’t know, I’ve never got it back completely. Scared to go out because I thought I was going to make a fool of myself . . .’
Leon 16.2 ‘I lose a lot since my strokes. Thing is, confidence is something I believe in, but you know, like my walking, for instance, how can I explain it? I walk inside here because the door is there. I hold on to there, right? But if I get outside, there’s nothing to hold on to . . .’
To three of the participants, confidence is about doing, choice and engaging in everyday activity. Two participants struggled with its definition in this direct context; however, were able to define the impact of confidence elsewhere in the data. A further two immediately describe losing their confidence and to these participants, confidence has a negative connotation and relates to fear and safety. Further analysis and interpretation is embedded within the themes that follow.
‘Robbed of life’
The notion that having a stroke questions who you are was articulated by many of the participants. Skill loss, decreased competency and lack of engagement in activities were described as contributing to a general feeling of being a lesser person, and uncertainty as to how competent one feels after having a stroke.
June illustrates a perceived link between activity and identity, associating what she does, to who she is:
Can’t walk far, can’t play badminton. I’m just a totally different person.
June 11.9
The data indicated that the participants’ sense of competence after stroke was challenged. Not feeling able to engage in previous, often familiar, skills or roles, such as ‘washing the pots or walking the dog [laughter]’ (Ryan 9.17) contributed to loss of confidence. However, embedded in the data, successfully regaining skills contributed to increased competence and self-beliefs, resulting in a regaining of confidence in their abilities.
Fear of having another stroke
Some element of fear was identified by all participants; however, the impact of fear on losing confidence and also regaining confidence varied between participants. The first fear that participants described was the fear of having another stroke:
. . . Every time I had a headache, I feel lightheaded, my leg hurts, there is always that question, erm, maybe [laughs], just kinda maybe.
Mick 13.2
. . . you never know what is going to happen tomorrow.
June 17.6
. . . I am going to have another stroke? – So that was on my mind.
Ted 12.1
. . . alert to any changes in my body at all, you know? Anything, because I think now, perhaps this is going to be the big one, you know.
Barbara 19.4
Participants described living with this fear and how it prevented them from participating in everyday activities. Mick experienced a second stroke, and articulates this period, as:
cementing the fear in my brain
Mick 12.14.
This second event had a huge impact on his recovery and confidence to leave the house, and resulted in a period of avoiding going out. For Mick, confidence has been about gradually overcoming this fear to enable him to regain confidence, to enable him to engage in what he chooses. He describes this process as gradual, and experiences ‘good and bad days’, suggesting confidence has a temporal component. Similar meanings evolved from June, indicating that fear is a factor that underpins her participation in going out of her home environment. Ted’s fear of having another stroke directly impacted on feeling anxious in crowds, and cites shopping centres as a place that held a particular anxiety. Avoidance was his coping strategy.
Fear of going out/social confidence
Fear of going out and being socially active after a stroke was a major concern voiced throughout the interviews by all of the 10 participants. Some were able to address their fears early on in their recovery, whereas others found it more difficult and, years after recovery, have not fully been able to achieve this, resulting in an avoidance of going out and a general sense of a diminished social lifestyle and social confidence.
I don’t know whether it was that I was self-conscious about the fact that I was struggling with my wheelchair, or struggling to walk, people looking at me or not, but . . .
Ryan 15.13
Eventually, as your confidence grows, your bubble starts to get bigger, erm, the garden, then the street, then the shops, eventually.
Mick 19.2
Participants also attributed positive encouragement from others as an enabler to going out and not avoiding activities that caused fear.
Although participants acknowledged huge fears about going out and social interactions with others, coupled with this fear was an immense desire to overcome these fears. For example, Ryan states ‘I had coping strategies pinned all over the wall’ (15.17). It is clear from the descriptions evolving from participants that confidence improves over time. Nevertheless, for some this is a slow process that may never be fully resolved. In summary, fear has a huge impact on daily activities and lives after stroke, often linked with low self-efficacy and avoidance of engagement in tasks, roles and events.
Team confidence/collective efficacy
The influence of significant others, as touched on within the previous theme, impact on levels of confidence. Although, not surprisingly, participants described positive influences as enablers to regaining confidence and conversely, negative influences act as barriers, the negatives and positives are not always overt in the context of participant’s daily lives and often require a period of reflection. Influence from a supportive friend, coupled with self-determination, one participant’s confidence levels increased to a point that enabled her to pursue ambitions she would not have considered prior to her stroke:
He [Friend] said ‘Let’s go to Cyprus.’ I said I’m not going to CYPRUS [increased tone: laughter] – you see I’ve never flown before, you see, so we flew to Cyprus.’ [laughter]
Freya 41.7
Conversely, another participant describes her family’s ‘help’ as being restrictive:
They don’t let me, well, I can’t go out of the back door without someone going with me.
Helen 11.13
Independently mobile and competent, this participant describes feelings of inadequate opportunities, to fulfil her potential. A message underpinning success for another participant, fundamental in gaining the confidence to mobilise outside after stroke, was a physiotherapy intervention:
. . . the best thing that happened to me, and the most erm, useful one to me, was being referred to a physiotherapist.
Barbara 11.1
Initially, unable to mobilise to the kerb without fear of falling, and apprehensive about physiotherapy intervention, she described factors such as humour, empathy, positive reinforcement and encouragement as the components that led to a positive treatment outcome. Keen to feedback her perceived success, once her treatment was completed, she describes telephoning the physiotherapist who delivered her treatment:
And I said to him, I can walk into town now, I can walk as far as Marks and Spencer’s, and he said ‘I can’t believe it, that’s wonderful.’ But I can, and I can now get as far as the shopping centre.
Barbara 24.19
Increased confidence after stroke appears to have a component that is influenced by the actions of others, in addition to the actions of self.
Role confidence
Participants perceived that ‘loss of roles’ had an impact on decreased confidence.
Loss of a driving role was considered hugely important to two participants and both these participants reported being more confident and active once they were able to resume their driving role. A gardening role, for another participant, despite a memory issue since stroke, still enables her to participate in an enjoyable role:
Couldn’t remember when they were supposed to flower, if they flowered and all those kind of things, but it didn’t bother me.
June 26.38
Confidence in meaningful roles appeared to make a difference to all participants. Increased confidence was evident when participants were motivated to engage in similar roles that were important pre-stroke or when they had found replacement roles that generated similar benefits.
‘It’s not I can’t, it’s I can’: skill mastery
Participants illustrated many examples of how relearning skills and becoming successful in mastering a new skill as being one of the biggest factors in regaining confidence. Some participants were slow to start to regain skills and gave examples of how they avoided activities that evoked fear and uncertainly. Others described being successful in a particular task or skill, which enabled them to believe they could increase their range of activities. This correlates closely to the self-efficacy theory. Participants who described themselves as confident pre-stroke tended to also describe a higher level of self-efficacy. This process is best described by participants:
Once I could get up and take a step, I knew I could do it, I know it seems daft.
Ryan 10.13
Gradually your confidence builds. The first time is always kinda nervous [pause] well it is for me now anyway.
Mick 31.4
Eventually, started cooking for myself, I thought, oh I can manage to do different things instead of using the microwave all the time.
Freya 30.2
Once participants began to succeed and achieve by repetitive practice, their motivation and confidence levels improved enabling them to move on to other activities. Practising skills was described as increasing competence and confidence; nonetheless, when practice did not improve skill, participants described becoming frustrated and found that their confidence decreased.
‘Inner strength’ and confidence
The final theme examines how the phenomenon of confidence is associated with other components of psychological distress. For three participants, episodes of depression were prevalent after stroke. Other participants identified periods of low mood, and most had some experience of anxiety. Low confidence was cited as underpinning their daily lives throughout these experiences. One participant stated being depressed ‘makes you feel a bit useless’ (Helen 23.7) and another describes similar sentiments: ‘Some days I feel helpless, alone’ (Mick 20.10). Interestingly, a participant describing herself as having very low self-esteem and a history of depression prior to her stroke, perceives she has a raised self-esteem and higher self-efficacy beliefs as a result of her stroke journey – ‘an inner strength almost that I didn’t realise I had’ (Freya 35.19). She illustrates this by telling us:
. . . the main barrier is within yourself, I think. The biggest one is within yourself, you think I can’t do that and you think about it and you think I’ll try. But you don’t try very hard because you think you can’t do it, you see? So you have to try a bit harder and then you realise you can do it, you know. So often the biggest barrier is within yourself.
Freya 50.11
Although focusing on doing and achieving, a process that increased self-efficacy beliefs was experienced by some participants; others clearly described it being a struggle to achieve the same:
I know in the back of my head there was something telling me you had to try and do these things, because, if you don’t June, you’ll just sit and vegetate, and life is too important for that.
June 23.17
As soon as I see a crowd of people I start to lose my balance. Go ‘hold me’ someone hold me [panic in tone of voice].
Alison 15.1
This latter quote suggests that success is about more than skill and ability. Alison was able to illustrate how negative thinking can often lead to an unsuccessful outcome.
Reinforcement from others suggests that another participant is not enough on its own: ‘Everybody kept saying “Oh, you are doing so well” but it wasn’t enough for me’ (Helen 8.2), suggesting that positive reinforcement does not compensate for intrinsic worth. The data signify that participants’ confidence levels have been affected by having a stroke to varying degrees. In seeking to explore the meaning of confidence after stroke, connections and interrelations between themes were identified, and similarities and differences in the data and between participants were disentangled, confirming that the phenomenon of confidence is multifaceted and complex.
Summary
-
Loss of confidence after stroke is a common experience.
-
The impact of stroke is truly realised only when a stroke survivor begins to establish routines, and continues with his/her life. Loss of former roles and a lack of competence when trying to engage in previously familiar tasks is prevalent in the early stages of stroke recovery, often linked to questioning self-identity.
-
Gradually regaining skills and re-establishing identity appeared to increase confidence over time.
-
Fear was identified in this study as a huge barrier to being confident to do the things participants wanted or needed to do.
-
Avoidance behaviours were evident and described in the data, lowering participant’s self-efficacy beliefs, further limiting the opportunity to become more competent and confident in activities and tasks.
-
Social confidence, fear of social interactions and stigma, was also embedded within the study’s findings. Gracey et al. 67 argued, if these issues fail to be resolved, a poor psychosocial outcome long term after stroke is realised.
-
Strategies that enable stroke survivors to resolve loss of confidence in social situations should be considered a necessary treatment component in stroke rehabilitation.
-
Confidence in ‘team’ and/or ‘partner’ was evident in the data. Descriptions of encouragement, patience and positive reinforcement are examples of factors that helped improve confidence and self-efficacy beliefs.
-
Prevention of opportunities for independence and choice of activities resulted in loss of confidence, control and disengagement during recovery.
-
A model to increase confidence in sport, which uses a multivaried framework encompassing self-confidence, role confidence, partner confidence, cohort confidence, team confidence, coach confidence and organisational confidence68 might be suitable for stroke patients when trying to improve outdoor mobility.
-
Highlighted in the literature is the concept that physical appearance often impacts on self-confidence; this was not prevalent in these study findings.
Chapter 6 Discussion
Key findings
Stroke patients can become housebound, miserable and in poorer health because they cannot participate in outdoor mobility. 14 This report presents the findings of a multicentre RCT to evaluate an outdoor mobility rehabilitation intervention for people with stroke. The intervention under evaluation was a complex mix of goal setting, practising outdoor mobility (which included walking outside), psychological support, and provision of information and self-monitoring of outdoor mobility through daily travel calendars. These calendars were used as an outcome measure. It was delivered by NHS therapists based in the primary care setting. The aim of the study was to replicate a single-centre study that found significant improvements in outdoor mobility participation when stroke patients were given the intervention by one highly skilled therapist. It aimed to evaluate whether the results were generalisable to NHS therapists in different UK locations. In addition, health-related quality of life and cost-effectiveness were measured.
The multicentre study replicated the methodology used in the single-site study. It reached its recruitment target on time. There was a high rate of follow-up at both the 6-month follow-up (to measure for an immediate effect of the intervention) and the 12-month follow-up (to assess whether it had a longer-lasting effect), and 70% of all travel diaries were returned. Stroke survivors and therapists were keen to take part in the study and although no formal qualitative study was completed to assess processes, it would appear from the proportion of patients who completed the travel diaries that getting out of the house is a major issue, even many years after stroke. A total of 568 participants from 15 UK-based sites were recruited over 18 months. The intervention was delivered, according to the protocol, to 287 participants, by 29 therapists, and was similar to that delivered in the single-centre study. Outcomes were collected by postal questionnaire and participants were supported, if needed, in their completion by RAs blind to allocation. The results were analysed using an intention-to-treat analysis, with the effect of different therapists at different sites being taken into consideration. A small qualitative study explored how a reduction in confidence might have affected people in the intervention group. The primary outcome measure used to calculate the sample size was health-related quality of life, as assessed by the Social Function domain of the SF-36v2. In addition, outcome measures used in the single-centre study – SWOM, travel journeys, activities of daily living ability and psychological well-being – were completed. A full economic evaluation was undertaken. This chapter will discuss the intervention, results, strengths and limitations of the study.
Intervention delivery
The intervention in this trial was developed over a number of years (2000–8), a treatment manual was produced and the CI taught therapists how to deliver it by a 2-hour training session. The intervention was a mixture of physical, psychological and planned preparation to achieve getting out of the house. Therapists were instructed to provide treatments as needed up to a maximum of 12 sessions over a 4-month period. They reviewed and planned their treatments following local protocols.
We consider that the intervention was delivered as per protocol to the majority of participants. We make this assertion because the intervention was delivered by occupational therapists and physiotherapists or by supervised rehabilitation assistants 100% of the time. These therapists had been trained to use the outdoor mobility manual by the study CI. It may have been that the manual was too prescriptive and did not allow therapists the possibility of providing more treatment sessions, but, owing to research methodology and financial restraints, therapists had to stop treatment after 12 sessions. The fidelity of treatment checking recorded that 100% of sample indicated that the therapists were delivering the correct treatment. We have to acknowledge that the fidelity of treatment checklist was not a standardised assessment owing to a lack of published measurements and that the last question which gave us this 100% rate had not undergone psychometric testing. The participants received a median of seven rehabilitation sessions with a duration of 3.5 hours. This is similar to that delivered in the single-centre study (six sessions, duration 4 hours). However, when the range of the intervention sessions was explored, it was found that approximately equal numbers (20 had one session, 20 had two sessions . . . , 20 had 11 sessions) but 60 participants received 12 sessions. It would appear that some patients may have needed > 12 sessions to achieve their goal. This could be the reason that nearly one-third did not complete their rehabilitation to the satisfaction of the therapist. Studies of upper limb rehabilitation suggest that > 3 hours per day for 30 days is needed to measure an improvement. 69
Although the number of intervention sessions was restricted to replicate what might be provided by the NHS, the content of the intervention had been developed to be person centred and therapists could be flexible in how it was delivered. Therapists who provided the intervention and participants provided informal feedback and said they felt it was an appropriate and clinically relevant treatment technique. The research team have received over 50 requests for the intervention manual and training over the last 3 years. This indicates a real need for an evidence-based outdoor mobility programme. A more recent study developed a person-centred rehabilitation intervention by asking 132 participants to prioritise their main goals. The goals stated were mainly related to active recreation, household and community management, mobility and socialisation, information on stroke and prevention of new strokes, outdoor mobility and transportation. 70
Effect of the intervention on health-related quality of life
The primary outcome measure of health-related quality of life found no significant differences between the control and intervention group at either the short- or long-term follow-up. The Social Function domain of the SF-36v2 was used to assess health-related quality of life in this population because the SF-36v2, as a whole, has been subjected to psychometric testing, used in numerous descriptive and intervention studies, and can be completed in < 10 minutes by self, proxy, interviewer or telephone. 71 This allows comparisons to be made with other health-care treatments. It includes a range of domains, such as loss of role, participation away from the home and social inclusion, which are areas that therapists hope to improve through the type of rehabilitation under evaluation. The Social Function domain of the SF-36v2 consists of two questions and was completed by most (500) participants out of the original sample (568 participants). The two questions were:
-
During the past 4 weeks, to what extent has your physical health or emotional problems interfered with your normal social activities with family, friends, neighbours or groups?
-
(Please tick one.) Not at all/Slightly/Moderately/Quite a bit/Extremely
-
-
During the past 4 weeks how much of the time have your physical health or emotional problems interfered with your social activities (like visiting friends, relatives, etc.)?
-
(Please tick one.) All of the time/Most of the time/Some of the time/A little of the time/None of the time
-
This measure was not used in the single-centre study and other trials of rehabilitation using this type of measure have found similar neutral findings. 72 – 74
Therapists and participants were concerned about the use of this measure, as they felt that the intervention aimed to improve outdoor mobility participation and the expectation that it would impact on social participation was overambitious, especially as the intervention was limited to 12 sessions over 4 months. In addition, they stated that participants wanted to undertake mobility activities for a number of reasons: getting to the doctors, dentists, shopping and work, which might not be classified as an improvement in quality of life as measured by these two questions. Improvement in outdoor mobility therefore might not have an impact on health-related quality of life as measured or it might take longer for the benefits to be realised. We conclude that the results were reliably collected and that the outdoor mobility intervention delivered in this study did not effectively improve stroke patient’s quality of life as measured by the Social Function domain of the SF-36v2. We suggest, however, that this is not a reason to stop outdoor mobility rehabilitation beginning offered to stroke patients. We believe that this targeted intervention has potential to increase outdoor mobility participation, which we consider an important part of activities of daily living. In addition, we have to consider whether the control group received a treatment that may have a role to play with patients for whom there have been many years since their stroke. We present our reasoning below.
Effect of the intervention on outdoor mobility participation
The main finding of the single-site study24 was that the intervention group were significantly more likely to participate in outdoor mobility. This positive outcome was considered an important clinical benefit. Stroke patients have complained about becoming housebound, isolated and miserable. This single-centre study demonstrated that, with a relatively short period of rehabilitation, patients could be taught how to increase their outdoor mobility. This included walking, using buses and electric pavement scooters. The outcome was assessed using two measures: SWOM participation and number of journeys. In the multicentre study, the intervention participants were no more satisfied with their outdoor mobility participation than the control participants at both 6 and 12 months but they were more likely to make journeys than those participants in the control group. However, this difference in number of journeys was apparent only when the data were adjusted to take the therapist effect into consideration. It appears that some therapists are able to produce these significant results, whereas others are not. We do not know whether it was the way the intervention was delivered, the characteristics of the participant or the skills of the therapist that produced this significant result. This outcome was measured using participant-reported journeys, which included outdoor walking, by travel diaries collected each month by post. Participants were taught how to use the calendars at the baseline visit by the RA and were encouraged to send them back using pre-paid envelopes. We feel that this was an objective measure as participants had to record each time they left the house and went somewhere. All participants completed the diaries and, as 70% of all travel diaries were returned, we feel this is a reliable outcome.
The results replicate those found in the single-site study, for which intervention participants took significantly more journeys at both 4 and 10 months. We need further research to explore the therapist and site effects in more detail. We consider that an increase in the number of outdoor journeys is an outcome that patients and health-care providers will feel is worthy of investment. It may be that an increase in outdoor mobility is helping people get to the doctors or health centre, they maybe going to work, or helping family with childcare. We know that people wish to get out of the house ‘just for the sake of it’ or ‘to get fresh air’ or to ‘enjoy just moving around’. 23 In addition, there is evidence from a systematic review of exercise after stroke, which found improvements in health-related quality of life in the short term. 75 It is possible that the outdoor mobility intervention provided in our study, which was mostly mobility training with the aim of walking > 100 m, was providing an exercise programme.
Satisfaction with outdoor mobility was measured using a single question: ‘Do you get out of the house as much as you would like?’ Although this measure was not designed as a patient-reported outcome measure (PROM) to assess health-related quality of life, it has many of the elements. It measures how people perceive the impact of a health intervention, is pertinent to the intervention being delivered and is easy to complete. Prior to this multicentre study, the measure had been subjected to some reliability testing76 and we have no reason to believe that the participants in the multicentre study found the question ambiguous. When we compared the groups we found no significant difference in outcome for this measure.
Effect of the control on outdoor mobility
There was very strong evidence that the control group improved markedly over time. At baseline, 259 out of 281 (92.2%) participants were dissatisfied with outdoor mobility but at the 6-month assessment this had reduced to 78% (160/205), a 15% reduction. The corresponding reduction in the intervention group was slightly greater (18%) with 268 out of 287 (93.4%) expressing dissatisfaction with outdoor mobility at baseline and 171 out of 227 (75.5%) expressing this at the 6-month assessment.
Participants in the control group must have been provided with a treatment that affected a change. It would be expected that people 3.5 years after stroke would remain stable over the next 6 months. This was a pragmatic trial and the therapists were told to deliver the baseline control as they would in the NHS. This was to replicate that given in the single-centre study. However, the combination of a face-to-face baseline visit presenting tailored (and participant focused) local transport and mobility information and use of daily travel diaries led to an effect in both the control and intervention groups. The baseline visit included a discussion about potential goals, which could have focused the participant’s thoughts towards improving outdoor mobility. We state previously that passive provision of leaflets and information is ineffective. However, we feel, with the motivated people who took part, that the control (targeted provision of leaflets and information) has acted in a different way. In addition, it is established that providing patients with daily diaries (e.g. food diaries) can lead to behavioural changes and an effect on the outcome being recorded. They provide a reference point for reflection on past and current behaviours, and they allow people to track changes over time and bring their behaviour to the front of their mind. In essence, they are a self-monitoring tool. Diaries have been used in behaviour modification programmes for a range of problems from weight loss and exercise programmes through to managing incontinence, tinnitus and migraines/headaches. Greenhalgh77 who investigated the feasibility of using diaries to measure the daily impact of multiple sclerosis on participants found that by completing a dairy participants became more aware of their symptoms, and this resulted in increased reporting. This impact may be further improved by the participants being aware that each month we would request the information they had recorded.
Effect of the outcome on functional ability and psychological distress
The intervention did not lead to greater levels of activity or reduce participant or carer psychological distress. The assessments used to collect the outcomes were well known and standardised measures used in many stroke studies. 78 – 81 We have to conclude that the intervention, as delivered, does not impact on these areas. This result is in line with previous studies, which has found that a targeted rehabilitation intervention delivered over approximately six sessions can improve the specific domain being addressed but does not affect other domains. 82
Was the intervention cost-effective?
In the base-case analysis the mean incremental cost (total NHS and PSS cost) was estimated to be £3413.75 (95% CI –£448.43 to £7121.00) with an incremental QALY gain of –0.027 (95% CI –0.060 to 0.007). This would suggest that the intervention was dominated compared with the control. The sensitivity analyses were broadly in line with the results of the base case.
Effect of the intervention on falls
Falls occurred in all age groups of stroke patients, with a median of three falls per year compared with one in four people from an aged-matched, non-stroke population falling each year. 83 It may be that we need to explore specific falls-prevention interventions for people with stroke. There were no differences in falls between the intervention group and the control group, providing evidence that the intervention did not cause people to fall over more often.
What did we learn about confidence after stroke?
Strongly emerging themes indicated that barriers to high levels of confidence included loss of former self, negative reinforcement from others including health professionals, loss of confidence in roles and fear of everyday activities. Confidence levels were found to change over time, with participants having ‘good and bad days’, but, generally, the participants spoke of improved confidence as time since stroke increased. The factors that helped stroke patients regain their confidence were positive reinforcement from others, successful skill mastery and self-defined positive changes in roles. The study demonstrated that stroke survivors in this study were experts of the confidence phenomenon by identifying a variety of factors that have both enabled them to achieve and succeed, resulting in increased confidence levels, and also identifying barriers that prevent them from regaining confidence. These findings suggest there is a need for therapists to understand the impact that low confidence might have on outdoor mobility and to aim to provide interventions to overcome this barrier.
Adverse events
We had a small number of adverse events [20 from 17 (17/287; 5.9%) participants] within the intervention group during the delivery of the intervention. There was no evidence to suggest that the intervention led to an increase in falls that required the assistance of a health-care professional.
Methodological issues
Comparison with other studies
This Getting out of the House Study evaluated a complex, but targeted, outdoor mobility intervention for community-based people who had experienced a stroke many years previously. Owing to the number of unique methodological features there is only one study to which we can compare the results. This was the single-centre study on which this multicentre study was based. 24 We aimed to replicate the recruitment and intervention delivery but a major difference between the studies was the time from stroke. In the single-centre study, participants were recruited 10–11 months after stroke, whereas in the multicentre study they were recruited 37–40 months after stroke. By completing a pragmatic multicentre trial and recruiting participants many years after stroke, we may have found a population more adapted to their situation and less likely to respond to the intervention. This could have contributed to the neutral effects found in the multicentre study compared with the positive effects found in the single-centre study. This highlights the difficulty of completing a pragmatic study over an explanatory study where inclusion criteria are more rigid. It would appear by comparing the two studies that the population most likely to benefit from the outdoor mobility training are those 1 year after their stroke. The other major difference between the studies is that in the single centre only one therapist delivered the intervention, whereas in the multicentre 29 therapists provided the intervention. The results from both studies provide some evidence that outdoor mobility rehabilitation is more effective when provided by specific therapists. However, we are not sure what characteristics these therapists need to have to elicit the benefit. Again, owing to the multicentre study being pragmatic and using routine NHS therapists, we consider that the results are more likely to be those seen if the intervention was provided to all stroke patients.
Generalisability of findings
The intervention delivered in this study was developed through university academics working with NHS and international partners. It was found to be feasible for delivery in the NHS by both occupational therapists and physiotherapists in all parts of the UK and suitable for patients, both men and women, many years post stroke. The age of the population was similar to the stroke population generally, so the intervention could be delivered to routine patients. The results cannot be generalised to patients early after stroke, who are most likely receiving Early Supported Discharge services or Community Rehabilitation. The evidence from the single-centre study is most applicable to this population and provides evidence that outdoor mobility participation can be increased by receiving a targeted intervention.
Strengths and limitations
The main strengths of the study are that it was conducted and reported following the CONSORT recommendations. 84 The Nottingham CTU managed the data collection, keeping the group allocation locked until the analysis had been completed. The study was overseen by an independent TSDC who provided rigorous governance checks. The patient and carer outcomes were collected by post to reduce bias and three statisticians independently checked the analysis. This study was designed to be as pragmatic as possible by recruiting participants from a variety of settings and using NHS staff to deliver the intervention in 15 different sites across Scotland, Wales and England. The participants were randomised using a secure and independent service and the baseline characteristics of the two groups are balanced, providing evidence that the randomisation process worked. Key areas of strengths and weakness are discussed below.
Economic evaluation
The study was strengthened by having the economic data collection and evaluation being nested within the trial. The analysis was predefined and completed by a research team at a separate university. A weakness was that a number of assumptions were required in order to estimate some of the cost variables, for example it was unclear (for a few participants) whether they were reporting average times per carer/home help visit or total times for the week. That said, the results are broadly consistent, for example for the different cost perspectives, so it does not seem that this has any great effect on the results of the cost-effectiveness analysis.
Qualitative study
The inclusion of a small qualitative interview study that explored confidence levels in participants who received the intervention provided some indications that confident people with stroke are very keen to get out of their houses and are often restricted by a reduction in confidence as much as a physical impairments. A major limitation of this study was that a full qualitative study to explore processes was not undertaken. This may have reinforced the main findings, provided valuable implementation information and let us understand how the therapist effect was influencing the travel journeys. In future studies of this kind it is recommended that participants, staff, commissioners and managers are interviewed.
Choice of outcome measures
The study was strengthened by measuring outcomes at the functional and social participation level, using standardised patient-reported outcomes but weakened by not having available measures that have been used in people late after stroke. The main outcome measure has been discussed above. The rest of the measures have been used successfully with people in other stroke rehabilitation trials8 up to a year after stroke. There are very few outcome measures designed for changes in people with long-term neurological conditions. The participants would most likely have a number of comorbidities that may have affected their outcomes, such as arthritis. Therapists who provided the intervention were concerned that the clinical changes they observed were not measured. They felt that a goal attainment scale might have been a better primary outcome measure as it would have reflected the diverse needs of the patients. Some were aiming to walk 20 m and some were aiming to use the bus.
Participant identification and randomisation
Originally, we calculated that seven sites would be needed to reach the sample size over the period of the study. It was obvious that recruitment was slower than anticipated and the sites were doubled, and then the recruitment period was lengthened by 4 months. This improved the generalisability, as the sites were more representative of different locations – rural, cities, suburban – and participants were recruited in all seasons. Invitations from patients identified from stroke registers led to a higher proportion of randomised participants than identification from the GP database, with anecdotal evidence of involvement of the stroke survivor’s original rehabilitation team leading to enhanced response rates. This also indicated that more use of stroke rehabilitation services in stroke research would be beneficial for participant identification and recruitment purposes, as well as these services acting as hosts to future research. Whether there is a correlation between participant perception of stroke care from the sender of the invitation and their willingness to participate is unclear and deserves further investigation. GP approach yielded a poor response rate; however, this is not unusual among other research studies from a range of disciplines.
The majority of participants who were not randomised did not meet eligibility criteria, with a small number wishing to enter the study only if they could receive the intervention. Overall, there was a high level of acceptance and enthusiasm for the study and the intervention in particular. Whether the acceptance and enthusiasm was due to existing services not meeting the participants needs and the opportunity to receive any sort of therapy was appealing is not clear, but again deserves further investigation. Anecdotal evidence from participants showed that they were very appreciative of receiving the intervention and found it of great benefit; however, conversely, there were high levels of dissatisfaction and frustration from participants in the control group, indicating that maybe there is a strong desire from participants to receive ongoing therapy to address ongoing issues with their recovery from stroke. The eligibility criteria was very broad, with an aim to include as many potential people as possible, so people living in care homes, including those in wheelchairs who needed hoisting to transfer. Although this makes the findings very generalisable it often caused concerns to the treating therapists, as they felt that 12 sessions was not enough to achieve the goals in severely impaired patients.
An additional concern from the sites, but again with no real evidence, was that delivery of the intervention to the quality expected by NHS staff was sometimes difficult despite the great deal of enthusiasm and support from research networks to recruit participants to the study.
Collection of follow-up data
The dual approach of collection of follow-up questionnaire by either postal approach or RA approach proved very effective and logistically was easily managed. Response rates for travel diaries were surprisingly strong considering that only a single postal attempt per month was implemented. These diaries were the source of a vast number of data and, potentially, for future trials, could be adapted to collect additional information, for example social activity and resource use (GP appointments, carers).
Issues of unblinding
Although there was a high level of unblinding either prior to or during 6-month visits by the RA, it was tempered by the fact that the outcomes were still participant reported and the RAs were not assessing a subjective outcome measure for either primary or secondary outcomes. However, there was evidence from personal communication with RAs that they clarified certain questions that participants (in the opinion of the RA) had a tendency to misunderstand or misinterpret. Any impact of this bias will be minimal in the overall interpretation of the data, as this incidence is likely to be equally distributed across both treatment groups and occurred independently of participant allocation or RA unblinding status.
There was a clear risk of unblinding when collecting outcome data in a complex intervention in stroke patients via RA-assisted questionnaires, so there is a need for prevention methods and alternative approaches to be evaluated, while balancing that with the potential bias to outcome data from unblinding.
Differential follow-up
A common issue with studies that have a participant-perceived differential benefit, as apparent as in the Getting out of the House Study, is that a proportion of participants will be inclined to feel that continued participation in the control group is of no overall benefit. This benefit is related to the participant themselves, whereas others feel that it is of no benefit to study data overall. Although the former may be true, of course, at the outset of the study there was only preliminary evidence that the intervention was effective and the latter is definitely not the case. Despite efforts to maintain engagement with participants wishing to withdraw, of which the majority were in the control group, there was still differential follow-up between the two groups. However, this does not affect the overall study results, as we have 6-month follow-up data from both groups within predefined attrition rates.
Attention control
One of the biggest limitations of this study was the input given to the control participants. The exploratory data imply that control-group participants were given a treatment that caused an improvement, which was more than that expected from routine care. This is a methodological weakness and one that is difficult to overcome in rehabilitation research. We considered that the information given to all participants was similar to that provided routinely but collaborating therapists disagreed. They would have preferred in retrospect for control participants to receive nothing, as they believe this is what routine care is at present for people late after stroke. The addition of the monthly travel diaries seems to have changed the behaviour of participants whom therapists would have expected to be stable. As with other rehabilitation research projects it is impossible to complete a double-blind trial and control participants may have been eager to use the travel information and the monthly diaries to change their lives.
Therapist and site effect
A strength of the analysis undertaken in this study was that the statistical analysis plan was predefined and locked prior to the start of the analysis (see Appendix 1 ).
The two patient groups were compared using an adjusted modelling method, which took into consideration the fact that many therapists were providing the intervention and that some patients would be treated by a number of different therapists. This technique has been recommended for studies of this kind,85 , 86 as there is the potential for clustering of outcomes (owing to which site a patient is at and which therapist treated them). However, this adjusted modelling method has not been readily used in rehabilitation studies and is therefore a point of discussion.
Before the adjusted modelling method could be used, a weighting for each therapist providing the intervention needed to be calculated using an approach that considered the number of therapists treating each patient and the number of times that each patient was treated. This weighting was then applied to the outcome results while the groups were compared using the adjusted method. The adjusted results and the unadjusted results can be seen in Tables 8–10 . Only one of the outcomes was affected by the therapist and site adjustments, and that was the number of journeys made. The result went from a neutral one to a significant positive one. A concern, though, is that the descriptive data in the 6- and 12-month tables suggests that the control group are perhaps slightly more likely to do more journeys. Another concern for the number of journeys outcome is that it was not possible to calculate the sizes of the obviously influential site and therapy effects (owing to the modelling process used). This meant that it was therefore impossible to determine which of these effects had the most weight on the adjusted result. However, even if it was possible to calculate the site and therapy effects for the number of journeys outcome, as was done for the other outcomes of this study, it would be very difficult to determine how these effects specifically altered the outcome. For instance, it would be hard to discover if any of the individual sites or therapists had a strong (or weak) influence on an outcome.
These results have been checked by three medical statisticians. This therapist effect can be explained by understanding that the intervention is a combination of the therapist and the techniques prescribed in the manual. It is very reliant on the skills of the therapist and the willingness of the participant. This type of analysis and understanding of the therapist effect needs further research.
Chapter 7 Conclusions
What the study found
This study provides robust evidence that getting out of the house is a real and substantive limitation among stroke survivors even after many years. It demonstrates that rehabilitation interventions that aim to improve outdoor mobility are appropriate for delivery by NHS therapists and, therefore, the results provide definitive and generalisable answers to clinically important questions.
The intervention has the potential to increase outdoor mobility participation in stroke patients as measured by journeys and SWOM but it has to be delivered in a specified way. In the single-centre study that found similar results, the patients were treated by the same therapist who was experienced and skilled in stroke care. This multicentre study has highlighted the issues of implementing an intervention that was developed for a single-centre study across the NHS.
The intervention had no measureable effect on quality of life (social function), activities of daily living or mood so we cannot recommend that this intervention is used to improve limitations in these areas.
Unfortunately, a major limitation of this study is that control participants who were expected to remain stable over the period of the study improved in their SWOM over the first 6 months. We suggest that the information and verbal advice plus monthly travel diaries may have acted as a treatment that may have affected the results of the primary analysis. The intervention was more costly than the control and did not improve quality of life compared with control.
Implications for clinical practice
This study replicated the results found in the single-centre study. An outdoor mobility intervention can increase outdoor mobility participation but this change does not appear to have a significant effect on quality of life (social function) compared with a control. So is this increase in journeys a clinically relevant finding that NHS commissioners will be paying for?
To implement this intervention into clinical care, it would appear from the single-centre study that the intervention needs to be provided by one experienced and stroke-trained therapist, who would provide all of the intervention sessions therefore becoming an expert in outdoor mobility.
To implement the control, outdoor mobility information and verbal advice needs to be personalised and provided face to face by an expert in community services. This needs to be supported by a self-reporting process that allows patients to let the therapists know how they are progressing.
Recommendations for research
-
Further research is needed to find new and innovative techniques and interventions that might help stroke patients get out of their houses as much as they would like. The results of our multicentre study indicated that we should be looking at the control, where we targeted the motivated participants, gave people decent information, and provided a structured behavioural change programme that included charting the behaviour changes.
-
Research is needed to explore alternative outcome measures that are clinically relevant but that can be used to compare health-care techniques. In this multicentre study we used a health-related quality of life (social function) measure but the intervention was targeted at outdoor mobility participation. Stroke patient’s value increased outdoor mobility in its own right. Further qualitative research is needed to explore how increased journeys may impact on quality of life.
-
We recommend that research is completed to explore the divergence in results between single-site and multisite studies. This successfully completed multicentre trial followed from a positive single-centre study. However, the results were different. We considered that we had replicated the methodology used in the single-centre study as far as possible when completing a pragmatic study, but we realise that this was a complex intervention delivered in a complex way to complex patients.
-
Research is needed to develop stroke-specific falls prevention interventions and psychological interventions to improve confidence. In this study falls occurred in all age groups of stroke patients and more often than in an age-matched group without stroke. Fear of falling and reduced confidence have been shown to reduce outdoor mobility.
-
Data from this study need to be shared with other research groups to allow discussion and comparison of multiple modelling analysis.
Acknowledgements
Many thanks to all of the PIs and local network clinical researchers and coordinators. Without their help this study would have not been possible. The study was managed by a TMG (Mat Leighton, Sarah Armstrong, Pip Logan, Tony Avery, Hywel Williams, Marion Walker, Simon Leach, Kathleen O’Neil, John Gladman, Tracey Sach, Shirley Smith, Ossie Newell, James Scott, Kathryn Brown) and a TSDC (Tom Robinson, Norma Fenton, Cath Sackley, Nadina Lincoln, Helen McCloughry, Angela Shone, Sarah Armstrong, Pip Logan, Mat Leighton). We would especially like to thank Dr Annie McCluskey from the University of Sydney, Sydney, Australia, for helping to develop the intervention manual.
A trial manager (Mat Leighton, NCTU) was in charge of the day-to-day running of the trial, a trial administrator (Pat Morris, NCTU) was responsible for sending and receiving questionnaires, both supported with dedicated time from the CI (Pip Logan). Mrs Gail Arnold helped with trial administration and organisation of the therapist events. The trial sponsor is the University of Nottingham. Full ethical committee and R&D director approval were granted for each site (09/H0403/55).
The 15 sites were:
-
NHS Nottingham City: PI, Pip Logan; RAs Jane Horne and Amy Moody; therapists, Jane Horne, Lorraine Lancaster and Janet Darby.
-
NHS Nottinghamshire County: PI, Pip Logan; RAs Janet Darby and Amy Moody; therapists, Jane Horne, Lorraine Lancaster and Janet Darby.
-
NHS Lincolnshire/United Lincolnshire Hospitals NHS Trust: PI, Simon Leach, RA Debbie McRobbie; therapist, Allison Read.
-
Gateshead PCT/Gateshead Health NHS Foundation Trust: PI Dave Barer; RAs, Ellie Morrell, Linda Walker and Maria Bokhari; therapists, Kathleen O’Neil and Charlotte Callinan.
-
NHS Lanarkshire: PI, Claire Ritchie; RAs, Derek Esson, Stephen Kirk and Elaine Feely; therapist, Melanie Campbell.
-
NHS North Somerset: PI, Ailie Turton; RA, Sarah Dunn; therapist, Louise Biffin.
-
Wolverhampton City PCT: PI and RA, Jane Bisiker; therapists, Michelle Corr and Helen Jones.
-
NHS Norfolk/Norfolk Community Health and Care NHS Trust: PI, Ingrid Watmough; RA Sue Allen; therapists, Charmaine Chandler and Jo Scrivens.
-
East Kent Community Services: PI, David Smithard; RAs Linda Cowie and Laura Brockway; therapist, Brian Macnally.
-
Tower Hamlets Primary Care Trust: PI, Tess Baird; RAs, Stephanie Pohlman, Claire Pentecost and Selina Gann; therapists, Emma Belton and Suong Nguyen.
-
NHS South East Essex/Southend University Hospital NHS Foundation Trust: PI, Dr Paul Guyler; RAs, Sharon Tysoe and Ajithra Praveen; therapist, Janusz Mendyka.
-
NHS Grampian: PI, Therese Jackson; RAs, Emma Wilson and Christine Dallas; therapist, Mae Ong.
-
Cwm Taf Local Health Board: PI, Janet Ivey; RAs Matthew Williams and Claire Nott; therapists, Julie Thomas and Karu Kodi.
-
Cardiff and Vale University Health Board: PI, Maggie Webster; RAs, Matthew Williams and Claire Nott; therapist, Rachel Smyth.
-
NHS Bristol: PI, Ailie Turton; RA, Sarah Dunn; therapist, Jo Corr.
Contributions of authors
Philippa A Logan conceived the study, was the grant holder and CI for the study, and wrote this summary and the original protocol.
Mat P Leighton, based in the NCTU, was responsible for day-to-day running of the trial and data collection/management, and was responsible for most revisions of the protocol, incorporating suggestions and comments from Philippa A Logan, Marion F Walker, John RF Gladman, Tony J Avery, Hywel C Williams, Ossie Newell, Sarah Armstrong, Tracey H Sach, Kathleen O’Neil and Garry R Barton.
Marion F Walker, John RF Gladman, Tony J Avery, Hywel C Williams, Ossie Newell, Sarah Armstrong, Tracey H Sach, Kathleen O’Neil, Simon Leach, David Barer, Jane Horne, Janet Darby, Garry R Barton and Nadina B Lincoln have contributed to the writing of this summary and helped to write the original full protocol.
Hywel C Williams, when director of the NCTU, helped to design the study and secure funding.
In addition, Sarah Armstrong was the study statistician and has particular responsibility for the analysis. Samir Mehta and Lisa J Woodhouse were study statisticians and analysed the data.
In addition, Tracey H Sach and Garry R Barton were the study health economists and were responsible for economic analysis.
All authors have read and approved the final manuscript.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publication
Logan P, Leighton M, Walker M, Armstrong S, Gladman J, Sach T, et al. A multi-centre randomized controlled trial of rehabilitation aimed at improving outdoor mobility for people after stroke: study protocol for a randomized controlled trial. Trials 2012;13:86.
References
- Feigin VL, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2003;2:43-5. http://dx.doi.org/10.1016/S1474-4422(03)00266-7.
- Logan P, Gladman J, Radford K. Use of transport by stroke patients. Br J Occup Ther 2001;64:261-4.
- Reducing Brain Damage: Faster Access to Better Stroke Care. London; 2005.
- May C. The hard work of being ill. Chronic Illn 2006;2:161-2. http://dx.doi.org/10.1179/174592006X129464.
- Langhorne P, Legg L. Evidence behind stroke rehabilitation. J Neurol Neurosurg Psychiatry 2003;74:iv18-iv21.
- Early Supported Discharge Trialists . Services for reducing duration of hospital care for acute stroke patients. Cochrane Database Syst Rev 2005;2.
- Legg L, Langhorne P, Andersen HE, Corr S, Drummond A, Duncan P, et al. Rehabilitation therapy services for stroke patients living at home: systematic review of randomised trials. Lancet 2004;363:352-6.
- Walker MF, Leonardi-Bee J, Bath P, Langhorne P, Dewey M, Corr S, et al. An individual patient meta-analysis of randomised controlled trials of community occupational therapy for stroke patients. Stroke 2004;35:2226-32.
- Aziz N, Leonardi-Bee J, Phillips M, Gladman J, Legg L, Walker M. Therapy-based rehabilitation services for patients living at home more than one year after stroke. Cochrane Database Syst Rev 2008;2. http://dx.doi.org/10.1002/14651858.CD005952.
- Hackett ML, Anderson CS. Frequency, management, and predictors of abnormal mood after stroke: the Auckland Regional Community Stroke (ARCOS) study, 2002 to 2003. Stroke 2006;37:2123-8. http://dx.doi.org/10.1161/01.STR.0000231387.58943.1f.
- Parker CJ, Gladman JR, Drummond AE. The role of leisure in stroke rehabilitation. Disabil Rehabil 1997;19:1-5. http://dx.doi.org/10.3109/09638289709166438.
- Hellstrom K, Nilsson L, Fugl Meyer AR. Relationship of confidence in task performance with balance and motor function after stroke. Physiother Theory Pract 2001;17:55-6. http://dx.doi.org/10.1080/095939801750334149.
- National Stroke Strategy. London; 2007.
- Gilhooly M, Hamilton K, O’Neil M, Gow J, Webster J, Pike F. Transport and Ageing: Extending Quality of Life for Older People via Public and Private Transport. Sheffield: ESRC Growing Older Programme; 2003.
- Robertson K, Logan P, Ward M, Pollard J, Gordon A, Williams W, et al. Thinking falls-taking action: a falls prevention tool for care homes. Br J Community Nurs 2012;17:206-9.
- Older People: Their Transport Needs and Requirements. London: Department of Transport, Publications; 2000.
- Oxley P, Alexander J. Disability and Mobility in London. London: Transport Research Laboratory; 1994.
- Rabbitt P, Carmicheal A, Jones S, Holland C. When and Why Older Drivers Give Up Driving. Cheadle: AA Foundation for Road Safety Research; 1996.
- Virgili G, Rubin G. Orientation and mobility training for adults with low vision. Cochrane Database Syst Rev 2003;4. http://dx.doi.org/10.1002/14651858.CD003925.
- National Clinical Guideline for Stroke. London: Royal College of Physicians; 2012.
- Brazzelli M, Saunders DH, Greig CA, Mead GE. Physical fitness training for stroke patients. Cochrane Database Syst Rev 2011;11. http://dx.doi.org/10.1002/14651858.CD003316.pub4.
- Forster A, Brown L, Smith J, House A, Knapp P, Wright JJ, et al. Information provision for stroke patients and their caregivers. Cochrane Database Syst Rev 2012;11. http://dx.doi.10.1002/14651858.CD001919.pub3.
- Logan P, Dyas J, Gladman J. Using an interview study of transport use by people who have had a stroke to inform rehabilitation. Clin Rehabil 2004;18:703-8. http://dx.doi.org/10.1191/0269215504cr742oa.
- Logan PA, Gladman JRF, Avery AJ, Walker MF, Groom L, Dyas J. Randomised controlled trial of an occupational therapy intervention to increase outdoor mobility after stroke. BMJ 2004;329:1372-5. http://dx.doi.org/10.1136/bmj.38264.679560.8F.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. http://dx.doi.org/10.1016/S0167-6296(01)00130-8.
- Nouri F, Lincoln N. An extended activities of daily living scale for stroke patients. Clin Rehabil 1987;1:301-5. http://dx.doi.org/10.1177/026921558700100409.
- Gladman JRF, Lincoln NB, Adams SA. Use of the Extended ADL Scale with stroke patients. Age Ageing 1993;22:419-24. http://dx.doi.org/10.1093/ageing/22.6.419.
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. Int Disabil Stud 1991;13:50-4. http://dx.doi.org/10.3109/03790799109166684.
- Goldberg DP, Williams P. A Users Guide to the General Health Questionnaire. Windsor: NFER Nelson; 1992.
- Brooks R. EuroQol EQ-5D: EuroQol: the current state of play. Health Policy 1996;37:53-72.
- Brazier J, Walters SJ, Nicholl JP, Kohler B. Using the SF-36 and the Euroqol on an elderly population. Qual Life Res 1996;5:195-204. http://dx.doi.org/10.1007/BF00434741.
- Wyrwich K, Bullinger M, Aaronson N, Hays R, Patrick D, Symonds T, et al. Estimating clinically significant differences in quality of life outcomes. Qual Life Res 2005;14:285-95. http://dx.doi.org/10.1007/s11136-004-0705-2.
- Brittle N, Brown M, Mant J, McManus R, Riddoch J, Sackley C. Short term effects on mobility, activities of daily living and health-related quality of life of a Conductive Education programme for adults with multiple sclerosis, Parkinson’s disease and stroke. Clin Rehabil 2008;22:329-37. http://dx.doi.org/10.1177/0269215507082334.
- Deming WE. Sample Design in Business Research. New York, NY: Wiley & Sons; 1960.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- A Framework for Development and Evaluation of RCTs for complex Interventions to Improve Health. London: MRC; 2000.
- Logan P, Walker M, Gladman J. Description of an occupational therapy intervention aimed at improving outdoor mobility. Br J Occup Ther 2006;69:2-6.
- Salkind NJ. Encyclopedia of Research Design. London: SAGE; 2010.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley and Sons; 1987.
- The NHS Knowledge and Skills Framework (NHS KSF) and the Development Review Process. London: DH; 2004.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing . . . presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. http://dx.doi.org/10.1002/hec.766.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: PSSRU; 2011.
- Free Flow Vehicle Speeds on Built-up Roads in Great Britain, Annual from 2006 to 2011. London: Department for Transport; 2011.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- NHS Reference Costs 2010–11. London: DH; 2011.
- Forder J, Fernandez J-L. Length of Stay in Care Homes. Canterbury: Bupa Care Services; 2011.
- Statistical Bulletin . 2010 Annual Survey of Hours and Earnings 2010. www.ons.gov.uk/ons/rel/ashe/annual-survey-of-hours-and-earnings/2010-revised-results/index.html (accessed 21 May 2013).
- Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; 2005.
- Sach TH, Whynes DK. Measuring indirect costs: is there a problem?. Appl Health Econ Health Policy 2003;2:135-9.
- Curtis L. Unit Costs of Health and Social Care 2009. Canterbury: PSSRU; 2009.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Sach T, Barton GR, Jenkinson C, Doherty M, Avery A, Muir KR. Comparing cost-utility estimates: does the choice of EQ-5D or SF-6D matter?. Med Care 2009;47:889-94. http://dx.doi.org/10.1097/MLR.0b013e3181a39428.
- Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection. Med Care 1992;30:473-83. http://dx.doi.org/10.1097/00005650-199206000-00002.
- Kharroubi SA, Brazier JE, Roberts J, O’Hagan A. Modelling SF-6D health state preference data using a nonparametric Bayesian method. J Health Econ 2007;26:597-612. http://dx.doi.org/10.1016/j.jhealeco.2006.09.002.
- Grieve R, Grishchenko M, Cairns J. SF-6D versus EQ-5D: reasons for differences in utility scores and impact on reported cost-utility. Eur J Health Econ 2009;10:13-5. http://dx.doi.org/10.1007/s10198-008-0097-2.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002;23:377-401.
- Heyse JF, Cook JR, Carides GW. Statistical Considerations in Analysing Health Care Resource Utilization and Cost Data. Oxford: Oxford University Press; 2001.
- Stinnett AA, Mullahy J. The negative side of cost-effectiveness analysis. JAMA 1997;277:1931-2.
- Barton GR, Briggs AH, Fenwick EA. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health 2008;11:886-97. http://dx.doi.org/10.1111/j.1524-4733.2008.00358.x.
- Statistical Bulletin: 2010 Annual Survey of Hours and Earnings. Newport: ONS; 2010.
- Rankin J. Cerebral vascular accidents in patients over the age of 60. Scott Med J 1957;2:200-15.
- Van Swieten JC, Koubstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. http://dx.doi.org/10.1161/01.STR.19.5.604.
- Smith J, Flowers P, Larkin M. Interpretative Phenomenological Analysis: Theory, Method and Research. London: Sage; 2009.
- Henschen K, Newton M. Building Confidence in Sport. Milton, QLD: John Wiley & Sons; 2004.
- Gracey F, Palmer S, Malley D, Keohane C, Cooper J, Prince L, et al. Cases illustrating a ‘Y-shaped’ model of identity and participation change processes in holistic rehabilitation of brain injury. Brain Impair 2008;9:205-35.
- Vealey R, Brewer BW. Confidence in Sport. Chichester: Wiley Blackwell; 2009.
- Han C, Wang Q, Meng PP, Qi MZ. Effects of intensity of arm training on hemiplegic upper extremity motor recovery in stroke patients: a randomized controlled trial. Clin Rehabil 2013;27:75-81. http://dx.doi.org/10.1177/0269215512447223.
- Lund A, Michelet M, Kjeken I, Wyller TB, Sveen U. Development of a person-centred lifestyle intervention for older adults following a stroke or transient ischaemic attack. Scand J Occup Ther 2012;19:140-9. http://dx.doi.org/10.3109/11038128.2011.603353.
- Geyh S, Cieza A, Kollerits B, Grimby G. Content comparison of health related quality of life measures used in stroke based on the international classification of functioning, disability and health (ICF): a systematic review. Qual Life Res 2007;16:833-51. http://dx.doi.org/10.1007/s11136-007-9174-8.
- Forster A, Young J, Nixon J, Kalra L, Smithard D, Patel A, et al. A cluster randomized controlled trial of a structured training programme for caregivers of inpatients after stroke (TRACS). Int J Stroke 2012;7:94-9. http://dx.doi.org/10.1111/j.1747-4949.2011.00722.x.
- Tilling K, Coshall C, McKevitt C, Daneski K, Wolfe C. A family support organiser for stroke patients and their carers: a randomised controlled trial. Cerebrovasc Dis 2005;20:85-91. http://dx.doi.org/10.1159/000086511.
- Ellis G, Mant J, Langhorne P, Dennis M, Winner S. Stroke liaison workers for stroke patients and carers: an individual patient data meta-analysis. Cochrane Database Syst Rev 2010;5. http://dx.doi.org/10.1002/14651858.CD005066.pub2.
- Chen M, Rimmer J. Effects of exercise on quality of life in stroke survivors: a meta-analysis. Stroke 2011;42:832-7. http://dx.doi.org/10.1161/STROKEAHA.110.607747.
- Logan PA. Outdoor Mobility After Stroke. Nottingham: University of Nottingham; 2004.
- Greenhalgh J. An assessment of the feasibility and utility of the MS Symptom and Impact Diary (MSSID). Qual Life Res 2005;14:1363-74. http://dx.doi.org/10.1007/s11136-004-5389-0.
- Walker MF, Sunderland A, Fletcher-Smith J, Drummond A, Logan P, Edmans JA, et al. The DRESS trial: a feasibility randomized controlled trial of a neuropsychological approach to dressing therapy for stroke inpatients. Clin Rehabil 2012;26:675-85. http://dx.doi.org/10.1177/0269215511431089.
- Legg L, Drummond A, Leonardi-Bee J, Gladman JR, Corr S, Donkervoort M, et al. Occupational therapy for patients with problems in personal activities of daily living after stroke: systematic review of randomised trials. BMJ 2007;335. http://dx.doi.org/10.1136/bmj.39343.466863.55.
- Corr S, Phillips CJ, Walker M. Evaluation of a pilot service designed to provide support following stroke: a randomized cross-over design study. Clin Rehabil 2004;18:69-75. http://dx.doi.org/10.1191/0269215504cr703oa.
- Gilbertson L, Langhorne P, Walker A, Allen A, Murray G. Domicillary occupational therapy for stroke patients discharged from hospital: a randomised controlled trial. BMJ 2000;320. http://dx.doi.org/10.1136/bmj.320.7235.603.
- Legg L, Drummond A, Langhorne P. Outpatient Service Trialists: occupational therapy for patients with problems in activities of daily living after stroke. Cochrane Database Syst Rev 2006;4.
- Sayer AA, Syddall HE, Martin HJ, Dennison EM, Anderson FH, Cooper C. Falls, sarcopenia, and growth in early Life: Findings from the Hertfordshire Cohort Study. Am J Epidemiol 2006;164:665-71. http://dx.doi.org/10.1093/aje/kwj255.
- Zwarenstein M, Treweek S, Gagnier J, Altman D, Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a2390.
- Lee KJ, Thompson SG. Clustering by health professional in individually randomised trials. BMJ 2005;330:140-2. http://dx.doi.org/10.1136/bmj.330.7483.142.
- Walters SJ. Therapist effects in randomised controlled trials: what to do about them. J Clin Nurs 2010;19:1102-12. http://dx.doi.org/10.1111/j.1365-2702.2009.03067.x.
Appendix 1 Statistical analysis plan
TOMAS
Getting out of the House Study
Statistical analysis plan
| The following people have reviewed the Final Statistical Analysis Plan and are in agreement with the contents | |||
| Name | Role | Signature | Date |
| Author | |||
| Statistical Reviewer | |||
| Chief investigator | |||
Abbreviations
| Abbreviation | Description |
|---|---|
| AE | Adverse Event |
| CI | Chief investigator |
| CRF | Case Report Form |
| DMC | Data Monitoring Committee |
| EOT | End of trial |
| GCP | Good Clinical Practice |
| ICF | Informed consent form |
| NHS | National Health Service |
| NHS IC | NHS Information Centre |
| ONS | Office of National Statistics |
| OT | Occupational Therapist |
| PI | Principle Investigator at a local centre |
| PIS | Participant information sheet |
| RA | Research assistant |
| REC | Research Ethics Committee |
| R&D | Research & Development department |
| SAE | Serious Adverse Event |
| TCC | Trial Coordinating Centre |
| TMG | Trial Management Group |
| TSC | Trial Steering Committee |
1. INTRODUCTION AND PURPOSE
This document details the rules proposed and the presentation that will be followed, as closely as possible, when analysing and reporting the main trial results.
The purpose of the plan is to:
-
Ensure that the analysis is appropriate for the aims of the trial, reflects good statistical practice in general, and minimises bias by preventing inappropriate post hoc analyses.
-
Explain in detail how the data will be handled and analysed to enable others to perform the actual analysis in the event of sickness or other absence.
-
Protect the project by helping it keep to timelines and within scope.
Additional exploratory or auxiliary analyses of data not specified in the protocol are permitted but fall outside the scope of this analysis plan (although such analyses would be expected to follow Good Statistical Practice).
The analysis strategy will be made available if required by journal editors or referees when the main papers are submitted for publication. Additional analyses suggested by reviewers or editors will, if considered appropriate, be performed in accordance with the Statistical Analysis Plan, but if reported the source of such a post hoc analysis will be declared.
Amendments to the statistical analysis plan will be described and justified in the final report of the trial.
2. SYNOPSIS OF STUDY DESIGN AND PROCEDURES
2.1. Trial objectives and aims
The purpose of this trial is to confirm or refute the clinical effectiveness and cost effectiveness of treating outdoor mobility limitations after stroke with a novel targeted rehabilitation therapy intervention versus standard clinical practice.
2.1.1. Primary objective
-
The health care objective is to improve the quality of people’s lives after a stroke by enabling them to get out of the house more often and when they wish.
-
The research objective is to test the effectiveness and cost effectiveness of treating people who have had a stroke with a new outdoor mobility rehabilitation intervention.
2.1.2. Secondary objectives
The secondary objectives are to measure whether this new therapy intervention is associated with:
-
improved mobility in and outside the house
-
improved patient mood
-
improved participation in everyday activities
-
improved carer mood
-
improved health-related quality of life.
2.2. Trial design and configuration
This is a multicentre parallel group randomised controlled trial. The trial consists of an initial baseline visit to provide information and explain the study, take informed consent, provide the standard clinical stroke rehabilitation treatment, completion of set of questionnaires and provide a travel diary with instructions. All participants will have to complete travel diaries every month and questionnaires at baseline, 6 and 12 months.
2.3. Trial centres
Approximately 15 Primary Care Trusts throughout the UK.
2.4. Eligibility criteria
2.4.1. Inclusion criteria
-
Age 18 years or over.
-
At least six weeks since stroke.
-
Wishing to get out of the house more often.
-
The participant must give informed consent before completing any study-related procedure, which means any assessment or evaluation that would not have formed part of their normal care.
2.4.2. Exclusion criteria
-
Not able to comply with the requirements of the protocol and therapy programme, in the opinion of the assessor.
-
Still in post-stroke intermediate care or active rehabilitation.
-
Previous enrolment in this study.
2.5. Description of interventions
A novel rehabilitation technique (intervention) group will be compared with a usual care (control) group.
Control group
Participants will receive what is considered clinically to be routine intervention for outdoor mobility limitations. That is verbal, advice and provision of leaflets provided during the baseline assessment visit.
Intervention group
This group will receive the same treatment as the control group but in addition, participants in this group will receive up to 12 rehabilitation outdoor mobility sessions of about an hour each over 4 months. The main component of the intervention is that therapists go repeatedly with patients to try outdoor mobility, including, buses, taxis, walking, voluntary drivers and mobility scooters until they feel confident to go alone or with a companion.
The number of intervention depends entirely on the participant. If they feel they do not require any further interventions, for whatever reason, then the interventions can stop. If they feel they require additional intervention, for whatever reason, they can continue the intervention up to a maximum of 12 visits.
2.6. Randomisation procedures
The randomisation will be based on a computer generated pseudo-random code using random permuted balanced blocks of randomly varying size, stratified by age (< 65 years, ≥ 65 years) and centre. All participants will be given the control group standard information intervention prior to randomisation in order to minimise performances and reduce contamination.
Participants will be randomly allocated (1 : 1 ratio) to either intervention or to the control group.
2.7. Sample size and justification
Our sample size calculations are based on the primary outcome measure, the Social Function domain of the Short Form 36 version 2 (SF-36v2) at six month follow-up. Although this was not used in the original study a recent study has suggested a minimally clinically important difference (MCID) for the social function domain is 12.5 points (Wyrwich, Tierney et al. 2005). The estimate of the standard deviation is taken from Brittle’s study (Brittle, Brown et al. 2008) and this closely matches the standard deviation obtained by assuming the distribution of the nine possible integer-valued scores follows a roughly right-angled triangular distribution (i.e. that is positively skewed). This follows the general approach suggested by Deeming for schematic distributions. Assuming a power of 90% and a two-sided significance level of 5%, we estimate that to detect a difference in mean SF-36 scores of 12.5 points assuming a common standard deviation of 28.2 (Brittle et al. 2008) a sample size of 135 patients per arm is required. This calculation assumes an attrition rate of 20% over 6 month period.
Clustering by delivery of treatment was allowed for by generating simulated data to mimic the multilevel structure of the proposed trial, with centre-specific variation between therapists. The likely existence of sharing of therapists by patients was addressed by a multiple membership model, and the variance of the treatment effect so found was compared with that of a regression model ignoring clustering (both models treating centre as a fixed effect). The data sets and analyses were performed in Stata v10. The variance inflation observed was insensitive to variation in the simulated between-centre variance and the average between-therapist variance, and a rounded value of 2.5 was selected as the multiplier for a sample size based on a naive analysis. After the allowance for clustering by delivery of treatment a sample size of 338 participants per arm is required.
A further sample size was also calculated using an alternative and easier to replicate method. This analytic approach was based on the same assumptions of the original sample size calculation and assumed that there would be 7 centres and within each centre there would potentially be 4 therapists delivering the intervention in the intervention group and one therapist delivering the control intervention in both groups (verbal advice and provision of leaflets at baseline visit prior to randomisation). In these calculations we assumed a therapist effect ICC of 0.02 (obtained from personal communication) and a centre effect ICC of 0.04 [obtained from Aberdeen University’s database of ICCs (http://www.abdn.ac.uk/hsru/epp/iccs-web.xls)]. This gave a revised total sample size (with no allowance for attrition) of 596 participants. If an attrition rate of 20% is assumed a total sample size of 746 would be required.
Owing to an increase in the number of sites, the sample size was revised. Power calculations to detect a difference in means of 12.5 (as in the original sample size calculation) and assuming a total sample size of 440 with the number of centres ranging from 7 (as originally planned) to 14 were then performed. These calculations were based on the same assumptions made in the analytic sample size above (i.e. SD = 28.2, two-sided significance level of 5%, ICC of 0.02 for therapist effect and ICC of 0.04 for centre effect). These indicated that a sample size of 440 would have a power of 86% (assuming no attrition) and a power of 82% (assuming attrition rate of 20%) to detect a difference in means of 12.5 if there were 7 centres and if there were 12 or more centres the corresponding power would be at least 90% if an attrition rate of 20% was assumed. There are a total of 15 centres in this trial so this meets the latter calculations.
Sample size calculations can only be performed if a number of assumptions are made and although our calculations are based on the best estimates we could obtain there is some uncertainty about the true values of the ICCs used to adjust for clustering. We therefore intended to be cautious and aimed to recruit a sample size of 506 (440 + 15%) to ensure we have recruited enough participants.
2.8. Blinding and breaking of blind
The research assistants (RA) who will help the participants complete the travel diaries and questionnaires, will not be aware of which group the participant has been randomised to. Following any visits, at 6 and 12 months, to assist with questionnaire completion the RA will complete a blinding questionnaire to assess if the blind had been broken.
Neither the participants nor the therapist are blinded to the intervention. The RA and the outcome assessors are blinded to the allocation of the treatment group and there is no foreseeable situation whereby they would need to know the treatment allocation of a particular participant. As a result there will be no procedures in place for breaking the randomisation code.
2.9. Trial committees
A number of committees will be assembled to ensure the proper management and conduct of the trial, and to uphold the safety and well-being of participants.
Trial Management Group (TMG): The TMG will oversee the operational aspects of the trial, which include the processes and procedures employed, and the day-to-day activities involved in study conduct.
Trial Steering and Data Committee (TSDC): The TSDC has the overall responsibility for ensuring a scientifically sound study design, a well-executed trial, and accurate reporting of the study results.
The Data Monitoring part of this committee will evaluate the outcome and safety data in the context of the overall trial and the currently existing information about the study. The DM will consider the appropriate timeframe for reviewing the data during the course of the study. The TSDC will have access to all trial data.
2.10. Outcome measures
2.10.1. Primary outcome
-
The primary outcome is the mean social function domain score of the SF-36v2 questionnaire measured at 6 months. The final social function domain score is then transformed into a 0–100 scale (see section 3.2).
2.10.2. Secondary outcomes
-
Functional ability (Nottingham Extended Activities Daily Living scale)
-
A 22-item questionnaire developed to detect how people who have suffered a stroke can carry out complex everyday tasks such as outdoor mobility and shopping. Each of the 22 item has the following categories ‘0 = not at all’, ‘0 = with help’, ‘1 = on your own with difficulty’ and ‘1 = on your own easily’. The final score is a score that ranges from 0–22 and higher values indicate greater independence.
-
-
Mobility (Rivermead Mobility Index)
-
This is a 15 item questionnaire used as a measure of disability related to bodily mobility. It demonstrates the patient’s ability to move his/her own body. All 15 items are ‘yes/no’ answers with values of 1 = yes and 0 = no. These are summed together to give a final score that ranges from 0 to 15 with higher values indicating greater mobility.
-
-
The number of journeys (travel diaries)
-
The travel diaries are in the form of a calendar and the participants are given 12 months worth of travel diary. The participants indicate the number of journeys (any journeys out of the house) they took for each particular day of the month for 12 months.
-
-
Satisfaction with outdoor mobility assessed using one yes/no question ‘Do you get out of the house as much as you would like?’
-
This question is included in the baseline, 6 months and 12 months questionnaires and asks the participant ‘Do you get out of the house as much as you would like’ with a binary answer of either yes/no.
-
-
Participant Mood (General Health Questionnaire – 12)
-
Carer psychological distress (General Health Questionnaire – 12)
-
The GHQ-12 is a 12 item questionnaire and it is administered in exactly the same way for participant and carer. Each of the 12 items are scored on a 0–3 scale. The final score is calculated by summing together the scores for these questions, giving a score that ranges from 0 to 36 with lower scores indicating a better outcome (mood/psychological distress).
2.11. Interim analysis
No formal interim analysis is planned.
3. GENERAL ANALYSIS CONSIDERATIONS
3.1. Analysis populations
Safety set: All randomised participants who receive at least 1 intervention.
Full analysis set: All randomised participants for whom the 6 month assessment of primary endpoint is available.
3.2. Derived variables
1. Social Functioning score (SF-score) transformed to 0–100 scale
The final SF-score is the addition of the 2 items (questions 6 and 10) from the SF36 questionnaire and the range for this is from 0–10.
Question 10 of the SF36 (focusing on how much of the participants time their physical health or emotional problems has interfered with their social activities) has the following categories; ‘1 = all the time’, ‘2 = most of the time’, ‘3 = some of the time’, ‘4 = a little of the time’ and ‘5 = none of the time’.
Question 6 of the SF36 (focusing on the extent to which the participants physical health or emotional problems has interfered with their normal social activities with family, friends, neighbours or groups) has the following categories; ‘1 = not at all’, ‘2 = slightly’, ‘3 = moderately’, ‘4 = quite a bit’ and ‘5 = extremely’. However, the scoring of this question was reversed to ‘1 = extremely’, ‘2 = quite a bit’, ‘3 = moderately’, ‘4 = slightly’ and ‘5 = not at all’ to ensure that higher values indicate better scores.
The final score is therefore the score of the questions 6 and 10 summed together and it ranges from 2 to 10, where high scores indicate better social functioning. However we need to transform this into a 0–100 scale and is calculated as shown below.
Lowest possible SFscore = 2, Possible SFscore range = 8
2. Nottingham Extended Activities of Daily Living Scale (NEADL) score
The total NEADL score is the addition of 22 items questionnaire with each item having the category ‘0 = not at all’, ‘0 = with help’, ‘1 = on your own with difficulty’ and ‘1 = on your own easily’. Hence, the range for the total NEADL score will be 0–22.
3. Rivermead Mobility Index (RMI) score
The total RMI score is the addition of the 15 items of the RMI questionnaire. Each item is either ‘1 = yes’ or ‘0 = no’ and so the range will be 0–15.
4. General Health Questionnaire – 12 (GHQ-12) score
The GHQ-12 is a 12 item questionnaire with each item is scored on a 0–3 scale.
Usually the categories for questions 2, 5, 6, 9, 10 and 11 are reversed before scoring, since the GHQ-12 questionnaire is composed of positively phrased and negatively phrased statements about mood states. However in our questionnaire the categories for questions for questions 2, 5, 6, 9, 10 and 11 were placed in reverse order so there is no need to reverse the scoring for these questions.
This questionnaire is completed by both the participant and the carer and so there will be a GHQ-12 final score for both participant and carer. The range of this final score will be 0–36.
5. Calculation of age
Age is calculated as age at inclusion → (consent date – date of birth)/365.25
3.3. Procedures for missing data
A complete case analysis will be conducted for the primary and secondary outcome measures. The replacement of missing data with imputed data (either by single or multiple imputation, as appropriate) will then be performed as a sensitivity analysis.
3.4. Levels of significance
The tests will be two-tailed and a result will be declared ‘statistically significant’ if p < 0.05 for all outcomes. Results will be presented with 95% confidence intervals.
3.5. Study centre effects
Centre will be handled as a random effect.
3.6. Outliers
Basic data explorations using summary and graphical statistics (i.e. box plots, etc.) will be conducted to check for any outliers. If after additional investigation there is no evidence that these are errors then the analyses will be repeated after winsoring the data to assess the robustness of the results.
Winsoring involves replacing extreme values (outliers) with a specified percentile of the data. In this case, data values below the 5th percentile will be replaced with the 5th percentile value and data values above the 95th percentile will be replaced with the 95th percentile value.
4. DESCRIPTION OF SUBJECT CHARACTERISTICS
4.1. Disposition
A flow of patients through the trial will be summarised in a CONSORT diagram that will include the eligibility, reasons for exclusion, numbers randomised to the two treatment groups, losses to follow-up and the numbers analysed.
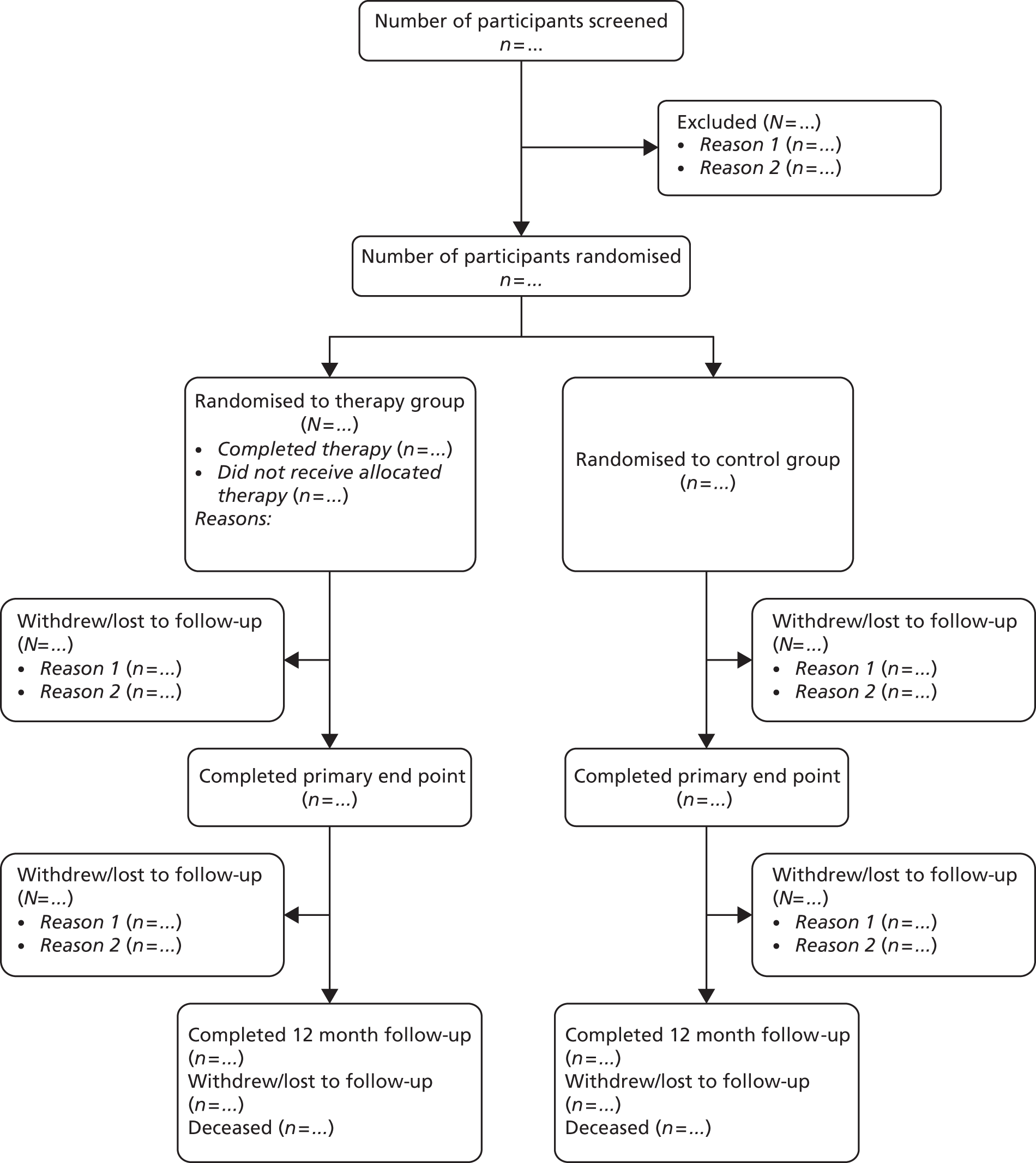
4.2. Baseline characteristics
Participants in the two arms will be described separately with respect to age, sex, ethnicity and time since last stroke (see Table 1 ).
Continuous data will be summarised in terms of the mean, standard deviation, median, lower and upper quartiles, minimum, maximum and number of observations. Categorical data will be summarised in terms of frequency counts and percentages.
5. ASSESSMENT OF STUDY QUALITY
5.1. Eligibility checks
The number of participants meeting the eligibility criteria will be tabulated (see Table 2 ).
5.2. Randomisation checks
The randomisation sequence as generated was evaluated using a multiple logistic model with treatment arm as response and block, sequence in block and stratum as explanatory variables. The AIC was used to confirm that the best fitting (minimum AIC) model was the null one, with no explanatory variables.
5.3. Data validation
The data validation checks will be conducted in-accordance to the CTU standard operating procedures. Final analyses will be conducted once the data validation programs developed within the CTU have checked for and cleaned the original data sets and saved them as new ‘Stata.dta’ data sets so that the original data sets are not tampered.
5.4. Withdrawals
The number and percentage of withdrawals will be summarised by treatment arm (see Table 3 ).
5.5. Compliance
Compliance in this trial will focus on the completion and returning of all diaries and questionnaires. Baseline questionnaires will be required to be completed before randomisation and so in practice this means 12 monthly travel diaries and 2 sets of assessment questionnaires, one each at 6 months and 12 months.
-
Compliance of diary calendars:
-
The number (%) of diary calendars returned at each month will be summarised (see Table 4 ).
-
-
Compliance of questionnaires returned and completed:
5.6. Extent of study intervention
The total number of therapy sessions each participant received will be summarised for the treatment group only (see Table 6 ).
Also the number of therapists providing the intervention in each centre will be presented (see Table 7 ). Furthermore, the minimum and maximum number of therapists treating the participants will be provided.
5.7. Protocol deviations
A listing of all participants with a protocol deviation will be provided (see Table 8 ).
5.8. Specify & justify changes made to the planned statistical analyses
-
Centre will be handled as a random effect rather than fixed effect (as mentioned in the protocol) due to the increase in the number of centres, we feel that adjusting for centre as a fixed effect will take up a lot of degrees of freedom.
6. ANALYSIS OF EFFICACY
6.1. Mis-randomised patients
Patients will be analysed as randomised, in order to follow the ITT principle.
6.2. Summary of primary and secondary outcomes
Baseline summaries of primary and secondary outcomes are described in the baseline characteristics table (see Table 1 ).
6.3. Primary analysis
The mean Social Function score (SFscore) will be compared between treatment arms using a multiple membership form of mixed effects multiple regression analysis adjusting for centre (as a random effect), age and baseline SFscore as covariates and therapist as a multiple membership random effect (87).
The table below gives examples of the multiple membership effect of therapist:
| Therapist 1 | Therapist 2 | Therapist 3 | |
|---|---|---|---|
| Patient 1 | × (8) | × (2) | × (2) |
| Patient 2 | × (12) | ||
| Patient 3 | × (6) | × (2) |
Accounting for the therapists
The effect of therapists will be accounted for by assigning weights. Based on the table above, patient 1 attended all the 12 sessions and was treated by therapist 1 eight times, therapist 2 two times and therapist 3 also two times.
Hence the total number of sessions attended by patient 1 was 12 and the weight that will be attributed to each therapist will be:
-
therapist 1 is 8/12 = 0.667
-
therapist 2 is 2/12 = 0.167
-
therapist 3 is 2/12 = 0.167.
Patient 2 also attended all 12 sessions but this patient was only treated by therapist 1 throughout the study and so therapist 1 will be assigned all the weight, i.e. 1.
Hence each therapist will be given a weight depending on the number of therapy sessions he/she conducted for a particular participant. ( Note : The therapist effect at baseline will be ignored since we are only interested on the therapist effect during the intervention period.)
A 3-level multiple membership model will be conducted using MLwiN once the data has been sorted and converted into the appropriate format for MLwiN. Centre will be the level 3 variable, with therapists as a multiple membership form level 2 and participants as level 1.
The robustness of these findings will be assessed by repeating the analysis and including baseline variables gender and residential status (see Table 9 and Appendix 12.2.A for code). Deviance will be used to compare the performance of the models. Regression coefficients and 95% confidence intervals will be presented for the model with the lowest Deviance.
6.4. Secondary analyses
The secondary outcome measures are collected at 6 months and 12 months. Data for the 6 months and 12 months will be analysed separately. The time point of primary interest is at 6 months.
-
Nottingham Extended Activities of Daily Living Scale (Functional ability)
-
The range of the NEADL score will be 0–66, with higher scores means greater independence.
-
The mean NEADL score will be compared between treatment arms using the same approach as for the primary outcome measure, namely a multiple membership form of mixed effects multiple regression analysis adjusting for centre (as a random effect), age and baseline score as covariates and therapist as a multiple membership random effect (see Table 10 ). The robustness of these findings will be assessed by repeating the analysis and including baseline variables gender and residential status.
-
Deviance will be used to compare the performance of the models. Regression coefficients and 95% confidence intervals will be presented for the model with the lowest Deviance.
-
-
Rivermead Mobility Index (Mobility)
-
The range of the RMI score will be 0–15 with higher scores indicating greater mobility.
-
The mean RMI score will be compared between treatment arms using the same method of analysis used for the primary outcome measure (see Table 11 ).
-
The robustness of these findings will be assessed by repeating the analysis and including baseline variables gender and residential status. Deviance will be used to compare the performance of the models. Regression coefficients and 95% confidence intervals will be presented for the model with the lowest Deviance.
-
-
Travel Diary (number of journeys)
-
Participants are required to complete 12 months worth of travel diary. They are asked to record how many journeys (any journey out of the house) they made on each particular day of the month for the period of 12 months.
-
The total number of journeys will be compared between treatment arms using a multiple membership form of mixed effects Poisson regression model adjusting for centre (as a random effect), age as a covariate and therapist as a multiple membership random effect (see Table 12 ). The robustness of these findings will be assessed by repeating the analysis and including baseline variables gender and residential status.
-
Deviance will be used to compare the performance of the models. Regression coefficients and 95% confidence intervals will be presented for the model with the lowest Deviance.
-
-
Yes or no question: ‘Do you get out of the house as much as you would like?’ (Satisfaction with outdoor mobility)
-
This question asking the participant ‘Do you get out of the house as much as you would like’ is part of the baseline, 6 months and 12 months questionnaires. The response is a binary variable coded as either 1 = yes or 0 = no.
-
The proportion of participants saying they get out of the house as often as they would like will be compared between treatment arms using a multiple membership form of mixed effects logistic regression model adjusting for centre (as a random effect) and age as covariates and therapist as a multiple membership random effect (see Table 13 ).
-
The robustness of these findings will be assessed by repeating the analysis and including baseline variables gender and residential status. Deviance will be used to compare the performance of the models. Odds ratios and 95% confidence intervals will be presented for the model with the lowest Deviance.
-
-
General Health Questionnaire (GHQ-12) (Mood)
-
GHQ-12 (Carer psychological distress)
-
The GHQ-12 score is a score that ranges from 12 to 48 with lower scores indicating better mood.
-
The mean final GHQ-12 score for the participant and for the carer will be compared (using separate models) between treatment arms using the same method of analysis used for the primary outcome measure (see Tables 14 and 15 ).
-
The robustness of the findings for the GHQ-12 score (assessed by repeating these analyses and including baseline characteristics for gender and residential status) will only be assessed for the participant. Deviance will be used to compare the performance of the models. Regression coefficients and 95% confidence intervals will be presented for the model with the lowest Deviance.
-
-
Participant mortality (death data) will be collected from NHS IC/NHS Central Register
-
The number and percentage of participants who died during the period of follow-up will be reported by treatment group.
-
-
EQ-5D and SF-6D (subset of SF-36 v2) – Resource use of health and social care and provision of equipment
-
This outcome will be analysed by the health economist (see Health Economics section).
-
6.5. Sensitivity analysis
Sensitivity analyses will be conducted for all outcome measures accounting for any outliers and missing data.
6.6. Exploratory/other analysis
Additional analyses include descriptive summaries (by treatment group) for the NEADL scale categories (see below):
-
mobility – questions 1–6
-
kitchen – questions 7–11
-
domestic – questions 12–16
Basic descriptive summaries (by treatment arm) for the total number of journeys made will also be conducted (see Table 19 ).
A description of the components of the intervention will be presented. The type of intervention received by each participant and the number of sessions the participants received for each type of intervention will be summarised in terms of n (%)s, Mean (SD), Median (IQR) and Min;Max (see Table 20 ).
The type of goals to be achieved by each participant is recorded as a list. This information will be categorised into standard categories and the number of participants assigned each type of goals will be presented as n (%) (see Table 21 ).
6.7. Assessment of blinding
All participants fill out the questionnaires either by post or require the help of a research assistant (RA). Of those that require the help of a RA, the number and percentage [n (%)] of the RAs who became unblinded will be presented by treatment group (see Table 22 ).
7. HEALTH ECONOMIC DATA ANALYSIS
This analysis will be conducted separately by a Health Economist. This evaluation aims to estimate the incremental cost effectiveness of an outdoor mobility rehabilitation intervention, compared with usual care, from a health and personal social services perspective. In addition the patient and carer perspective will be examined separately.
Any censored data will be adjusted for, using appropriate published techniques. If non-dominance occurs, an incremental cost effectiveness ratio will be produced. The confidence region around the ratio will be estimated using appropriate statistical techniques such as the non-parametric bootstrap method. This stochastic analysis will enable a cost effectiveness acceptability curve to be produced illustrating the uncertainty surrounding the optimal decision.
Probabilistic sensitivity analysis will be undertaken to test the robustness of the results. Estimates of the incremental cost, incremental cost effectiveness and the uncertainty around these estimates will enable us to discover whether ‘The getting out of the house intervention’ offers value for money.
8. QUALITATIVE DATA ANALYSIS
Data from the semi-structured interviews to assess the participants’ confidence after their stroke will be analysed by a qualitative researcher. Data will be analysed using Interpretive Phenomenology Analysis (IPA) principle using a six stage framework (89). The aim is to identify enablers and barriers to regaining confidence from the patient perspective.
9. ANALYSIS OF EFFICACY
Fidelity of treatment will be assessed by reviewing the notes of therapists against a predefined checklist to determine whether the intervention was being delivered according to the protocol. Furthermore, a selection of therapy visits will be observed and scored against a predefined checklist.
10. ANALYSIS OF SAFETY
10.1. Adverse events
All participants who experience any adverse events will be listed by treatment group (see Table 23 ).
10.2. Falls data
Each participant was asked to record in their diaries whether they had experienced a fall on a given day of the month.
The total number (%) of falls day, i.e. number of days when the participants recorded they experienced at least one fall, will be described by treatment group and by age group (see Tables 24–25 ).
11. FINAL REPORT TABLES AND FIGURES
COMMENT
Specify layout, for example by presenting dummy tables. The following rules may be adopted when creating the summary tables:
Number of decimal places (DP):
-
for minimum and maximum the number of DPs will be the same as the raw data
-
for mean, median and SD the number of DPs will be one more than the raw data
-
percentages – Round up to the nearest whole number
-
p-values – 3 decimal points will be presented
-
no more than 4 significant numbers will be used.
Data presentation:
-
Treatment group will be in columns with the visits in rows.
-
Column headers in mixed case, with ‘(n = nn)’ below treatments to denote the denominator.
-
Decimal places will be aligned.
-
n (%) as a separate column rather than included in brackets for each element of the table.
-
Categories (i.e. in column 1) in sentence case, in the order on the CRF.
-
Ordering of statistics N, Missing, Mean, SD, CV%, Minimum, Median and Maximum. Geometric Means inserted between Mean and SD for pharmacokinetics.
11.1. Subject characteristics and background summaries
| Variable | Parameter | Treatment arm | ||
|---|---|---|---|---|
| A | B | |||
| Total | N | |||
| Age at inclusion | Mean (SD) | |||
| Median (IQR) | ||||
| Minimum, maximum | ||||
| Sex | Female | n (%) | ||
| Male | ||||
| Ethnicity | White | n (%) | ||
| Black – Caribbean | ||||
| Black – African | ||||
| Black – Other | ||||
| Pakistani | ||||
| Indian | ||||
| Bangladeshi | ||||
| Chinese | ||||
| Mixed | ||||
| Not given | ||||
| Other | ||||
| Residential status | Lives alone | n (%) | ||
| Lives with others | ||||
| Living in care home | ||||
| Time since stroke | Mean (SD) | |||
| Median (IQR) | ||||
| Minimum, maximum | ||||
| Social functioning score | Mean (SD) | |||
| Median (IQR) | ||||
| Minimum, maximum | ||||
| NEADL1 score | Mean (SD) | |||
| Median (IQR) | ||||
| Minimum, maximum | ||||
| Rivermead Mobility Index score | Mean (SD) | |||
| Median (IQR) | ||||
| Minimum, maximum | ||||
| Satisfaction with outdoor mobility | Yes | n (%) | ||
| No | ||||
| General Health Questionnaire (Participant) | Mean (SD) | |||
| Median (IQR) | ||||
| Minimum, maximum | ||||
11.2. Study quality summaries
| Inclusion criteria | Yes | No |
|---|---|---|
| Is the participant aged 18 years or older? | ||
| At least 6 weeks but no longer than 5 years since stroke? | ||
| Wishing to get out of the house more often? | ||
| Has the participant given written informed consent? | ||
| Exclusion criteria | Yes | No |
| Not able to comply with the requirements of the protocol and therapy programme, in the opinion of the assessor? | ||
| Still in post-stroke intermediate care or active rehabilitation? | ||
| Previous enrolment in this study? |
| Withdrawals | A (n = . . .) | B (n = . . .) | Total (n = . . .) | ||||
|---|---|---|---|---|---|---|---|
| 1. Participant withdrew from the study | |||||||
| 2. Participant lost to follow-up | |||||||
| 3. Participant experienced an adverse event | |||||||
| 4. Investigator withdrew participant | |||||||
| 5. Other (specify) | |||||||
| Compliance of the diary calendars | Total overall (N = . . .) | Total excluding any withdrawals (N = . . .) |
|---|---|---|
| n (%) returned no calendars | ||
| n (%) of participants who returned 1 month of travel calendar | ||
| n (%) of participants who returned 2 months of travel calendar | ||
| n (%) of participants who returned 3 months of travel calendar | ||
| n (%) of participants who returned 4 months of travel calendar | ||
| n (%) of participants who returned 5 months of travel calendar | ||
| n (%) of participants who returned 6 months of travel calendar | ||
| n (%) of participants who returned 7 months of travel calendar | ||
| n (%) of participants who returned 8 months of travel calendar | ||
| n (%) of participants who returned 9 months of travel calendar | ||
| n (%) of participants who returned 10 months of travel calendar | ||
| n (%) of participants who returned 11 months of travel calendar | ||
| n (%) of participants who returned 12 months of travel calendar |
| Compliance of the questionnaires | Total (N = . . .) |
|---|---|
| n (%) of the 6 months questionnaires returned | |
| n (%) of the participants who completed the SF361 questionnaire in full | |
| n (%) of the participants who completed NEADL2 questionnaire in full | |
| n (%) of the participants who completed RMI3 questionnaire in full | |
| n (%) who answered the SWOM4 question | |
| n (%) of the participants who completed the GHQ-125 questionnaire in full | |
| n (%) of the 12 months questionnaires returned | |
| n (%) of the participants who completed the SF361 questionnaire in full | |
| n (%) of the participants who completed NEADL2 questionnaire in full | |
| n (%) of the participants who completed RMI3 questionnaire in full | |
| n (%) who answered the SWOM4 question | |
| n (%) of the participants who completed the GHQ-125 questionnaire in full |
| Extent of study intervention | Total (N = . . .) |
|---|---|
|
| Centre | No. of therapists providing the intervention |
|---|---|
| Nottingham City PCT | |
| Nottinghamshire County Teaching PCT | |
| Lincolnshire PCT | |
| Gateshead PCT | |
| NHS Lanarkshire | |
| North Somerset PCT | |
| Wolverhampton PCT | |
| East Kent Community Services | |
| Tower Hamlets | |
| Southend | |
| Aberdeen | |
| Norwich | |
| Cwm Taf | |
| Cardiff & Vale | |
| Bristol PCT | |
| (Min; Max) No. of therapists seen by the participants |
| Patient number | Treatment arm | Description of deviation | Date of deviation |
|---|---|---|---|
11.3. Primary outcome results at 6 months
| Social Functioning Score | Mean | Standard error | p-value | 95% credible interval |
|---|---|---|---|---|
| Treatment A (baseline) | – | – | – | – |
| Treatment B | XXX | XXX | XXX | XXX to XXX |
| Age | XXX | XXX | XXX | XXX to XXX |
| Constant | XXX | XXX | XXX | XXX to XXX |
| Random-effects parameters | Mean | Standard error | 95% credible interval | |
| Level 3: Centre | XXX | XXX | XXX to XXX | |
| Level 2: Therapist | XXX | XXX | XXX to XXX | |
| Level 1: Participants | XXX | XXX | XXX to XXX | |
11.4. Secondary outcomes results at 6 months
| Burn-in period = xxxx | No. of observation = xxx | |||
|---|---|---|---|---|
| Chain = xxxx | Estimation algorithm = MCMC | |||
| Thinning = xxxx | ||||
| Deviance = xxxx | ||||
| Bayesian deviance = xxxx | ||||
| NEADL score | Mean | Standard error | p-value | 95% credible interval |
| Treatment A (baseline) | – | – | – | – |
| Treatment B | XXX | XXX | XXX | XXX to XXX |
| Age | XXX | XXX | XXX | XXX to XXX |
| Constant | XXX | XXX | XXX | XXX to XXX |
| Random-effects parameters | Mean | Standard error | 95% credible interval | |
| Level 3: Centre | XXX | XXX | XXX to XXX | |
| Level 2: Therapist | XXX | XXX | XXX to XXX | |
| Level 1: Participants | XXX | XXX | XXX to XXX | |
| Burn-in period = xxxx | No. of observation = xxx | |||
|---|---|---|---|---|
| Chain = xxxx | Estimation algorithm = MCMC | |||
| Thinning = xxxx | ||||
| Deviance = xxxx | ||||
| Bayesian deviance = xxxx | ||||
| RMI score | Mean | Standard error | p-value | 95% credible interval |
| Treatment A (baseline) | – | – | – | – |
| Treatment B | XXX | XXX | XXX | XXX to XXX |
| Age | XXX | XXX | XXX | XXX to XXX |
| Constant | XXX | XXX | XXX | XXX to XXX |
| Random-effects parameters | Mean | Standard error | 95% credible interval | |
| Level 3: Centre | XXX | XXX | XXX to XXX | |
| Level 2: Therapist | XXX | XXX | XXX to XXX | |
| Level 1: Participants | XXX | XXX | XXX to XXX | |
| Burn-in period = xxxx | No. of observation = xxx | |||
|---|---|---|---|---|
| Chain = xxxx | Estimation algorithm = MCMC | |||
| Thinning = xxxx | ||||
| Deviance = xxxx | ||||
| Bayesian deviance = xxxx | ||||
| Travel journeys | Mean | Standard error | p-value | 95% credible interval |
| Treatment B | – | – | – | – |
| XXX | XXX | XXX | XXX to XXX | |
| Age | XXX | XXX | XXX | XXX to XXX |
| Constant | XXX | XXX | XXX | XXX to XXX |
| Random-effects parameters | Mean | Standard error | 95% credible interval | |
| Level 3: Centre | XXX | XXX | XXX to XXX | |
| Level 2: Therapist | XXX | XXX | XXX to XXX | |
| Level 1: Participants | XXX | XXX | XXX to XXX | |
| Burn-in period = xxxx | No. of observation = xxx | |||
|---|---|---|---|---|
| Chain = xxxx | Estimation algorithm = MCMC | |||
| Thinning = xxxx | ||||
| Deviance = xxxx | ||||
| Bayesian deviance = xxxx | ||||
| Satisfaction with outdoor mobility (yes/no) | Coef. | Standard error | p-value | 95% credible interval |
| Treatment B | – | – | – | – |
| XXX | XXX | XXX | XXX to XXX | |
| Age | XXX | XXX | XXX | XXX to XXX |
| Constant | XXX | XXX | XXX | XXX to XXX |
| Random-effects parameters | Mean | Standard error | 95% credible interval | |
| Level 3: Centre | XXX | XXX | XXX to XXX | |
| Level 2: Therapist | XXX | XXX | XXX to XXX | |
| Level 1: Participants | XXX | XXX | XXX to XXX | |
| Burn-in period = xxxx | No. of observation = xxx | |||
|---|---|---|---|---|
| Chain = xxxx | Estimation algorithm = MCMC | |||
| Thinning = xxxx | ||||
| Deviance = xxxx | ||||
| Bayesian deviance = xxxx | ||||
| GHQ-12 participant score | Mean | Standard error | p-value | 95% credible interval |
| Treatment A (baseline) | – | – | – | – |
| Treatment B | XXX | XXX | XXX | XXX to XXX |
| Age | XXX | XXX | XXX | XXX to XXX |
| Constant | XXX | XXX | XXX | XXX to XXX |
| Random-effects parameters | Mean | Standard error | 95% credible interval | |
| Level 3: Centre | XXX | XXX | XXX to XXX | |
| Level 2: Therapist | XXX | XXX | XXX to XXX | |
| Level 1: Participants | XXX | XXX | XXX to XXX | |
| Burn-in period = xxxx | No. of observation = xxx | |||
|---|---|---|---|---|
| Chain = xxxx | Estimation algorithm = MCMC | |||
| Thinning = xxxx | ||||
| Deviance = xxxx | ||||
| Bayesian deviance = xxxx | ||||
| GHQ-12 carer score | Mean | Standard error | p-value | 95% credible interval |
| Treatment A (baseline) | – | – | – | – |
| Treatment B | XXX | XXX | XXX | XXX to XXX |
| Age | XXX | XXX | XXX | XXX to XXX |
| Constant | XXX | XXX | XXX | XXX to XXX |
| Random-effects parameters | Mean | Standard error | 95% credible interval | |
| Level 3: Centre | XXX | XXX | XXX to XXX | |
| Level 2: Therapist | XXX | XXX | XXX to XXX | |
| Level 1: Participants | XXX | XXX | XXX to XXX | |
11.5. Primary endpoint at 12 months
This is the same analysis as 6 months, repeated for data at 12 months.
11.6. Secondary endpoints at 12 months
This is the same analysis as 6 months, repeated for data at 12 months.
11.7. Exploratory/other analysis results
| Category | Treatment arm | |
|---|---|---|
| A | B | |
| Mobility | ||
| n | ||
| Mean (SD) | ||
| Median (IQR) | ||
| Min. to max. | ||
| Kitchen | ||
| n | ||
| Mean (SD) | ||
| Median (IQR) | ||
| Min. to max. | ||
| Domestic | ||
| n | ||
| Mean (SD) | ||
| Median (IQR) | ||
| Min. to max. | ||
| Leisure | ||
| n | ||
| Mean (SD) | ||
| Median (IQR) | ||
| Min. to max | ||
| Category | Treatment arm | |
|---|---|---|
| A | B | |
| Mobility | ||
| n | ||
| Mean (SD) | ||
| Median (IQR) | ||
| Min. to max. | ||
| Kitchen | ||
| n | ||
| Mean (SD) | ||
| Median (IQR) | ||
| Min. to max. | ||
| Domestic | ||
| n | ||
| Mean (SD) | ||
| Median (IQR) | ||
| Min. to max. | ||
| Leisure | ||
| n | ||
| Mean (SD) | ||
| Median (IQR) | ||
| Min. to max. | ||
| Category | Treatment arm | |
|---|---|---|
| A | B | |
| Mobility | ||
| n | ||
| Mean (SD) | ||
| Median (IQR) | ||
| Min. to max | ||
| Kitchen | ||
| n | ||
| Mean (SD) | ||
| Median (IQR) | ||
| Min. to max. | ||
| Domestic | ||
| n | ||
| Mean (SD) | ||
| Median (IQR) | ||
| Min. to max. | ||
| Leisure | ||
| n | ||
| Mean (SD) | ||
| Median (IQR) | ||
| Min. to max. | ||
| Travel journeys | Treatment arm | |
|---|---|---|
| A | B | |
| Total journeys | ||
| Mean (SD) | ||
| Median (IQR) | ||
| Min. to max. | ||
| Intervention type | n (%) Participants | No. of sessions | ||
|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Min – Max | ||
| Mobility | ||||
| Confidence | ||||
| Information | ||||
| Goal Setting | ||||
| Referral | ||||
| Other Rehab | ||||
| Adaptive Equipment | ||||
| Goal type* | n (%) Participants |
|---|---|
| Goal 1 | |
| Goal 2 | |
| . . . | |
| Goal N |
| Unblinding | Treatment arm | Total | |
|---|---|---|---|
| A | B | ||
| n (%) of RAs unblinded | |||
11.8. Safety results
| Ref-no | Record no | AE name | AE start date | AE ongoing | AE end date | Action taken |
|---|---|---|---|---|---|---|
| Fall days | Treatment arm | Total | |
|---|---|---|---|
| A | B | ||
| Total n (%) of fall days | |||
| Mean (SD) | |||
| Median (IQR) | |||
| Min. to max. | |||
| Fall days | Age | |
|---|---|---|
| Under 60 | 60 plus | |
| Total n (%) of fall days | ||
| Mean (SD) | ||
| Median (IQR) | ||
| Min. to max. | ||
12. APPENDICES
12.1. Stata code for derived variables
1. Social Functioning score into 0–100 scale
*Transformation into 0–100 scale (Higher score are better)
2. Age
*Generate the age variable
3. Nottingham Extended Activities of Daily Living Scale (NEADL) score
*Recode to range from 0 to 1
*Generate the total NEADL score
4. Rivermead Mobility Index (RMI) score
*Generate the total NEADL score
5. General Health Questionnaire-12 (GHQ-12) score
*Recode to range from 0 to 3
*Generate the total GHQ-12 score
6. Number of journeys made by each participant
*Now count how many days are recorded for each participant
*Now use the collapse command to get a summary for each participant
12.2. Stata code for final analyses
9.2.A. Primary analysis code and instructions
*Status.dta
*Generate age
*Merge with sf36.dta
*Only need baseline and 6 months data
*Only keep the relevant variables
/*Transform SFscore into 0–100 scale
SFscore is calculated from 2 items (6 and 10) of the SF36 questionnaire and both range from 1–5 hence the final range for SFscore is between 2–10.
lowest possible score = 2
range = 10 – 2 = 8
Transformation = (sfscore – 2)/8
*Transformation into 0–100 scale (Higher score are better)*/
*Reshape
*Now save this file
*Now sort the visits.dta
*Only keep relevant variables
/*The variable ‘therid2’ is a recording of another therapist that was present at some interventions stages. Pip Logan (CI) said that the 1st therapist delivered the treatment and that the second therapist only supported. Hence we only account for 1st therapist.
/*The following will replace all recordings of therapists at baseline to be ‘.’ as we are only interested in the therapist effect for the therapy sessions and they are not done at baseline.
Also this recording of therapist at baseline is inconsistent as there are recording of therapist for some but not for all at baseline. */
*Need to replace all missing values and this is necessary to do else data set will not assign weights in MLwiN
/*Now reshape to wide format
Note: in doing this we will use the refno as the ‘stub’ and this will therefore be deleted and will need to be re-created later */
*Need to re-generate the original patno
*This will give the names and values as we want it
*Drop any unnecessary variables
*The following therapist names were not involved in delivering the intervention → Therapist2–15, 17–23
*This will count how many session each participant had with each therapist
*Generate weights
/*This will count the total therapists support involved per-participant
EG: If say refno=1001 had 12 total interventions of which 6 were done by therapist 1 and for other 6 interventions, therapist 2 was involved. The participant had the max 12 sessions, i.e. 6 from therapist 1 and 6 from therapist 2.
Hence, the weights will be: Therapist 1 = 6/12 = 0.5 and Therapist 2 = 6/12 = 0.5
The weight for the other therapists will be ‘0’ for this participant.
*/
*Assigning weights based on the example shown above
Important decision
Now that we have established how many sessions each participant did and how many sessions each therapist delivered, we need to change the no of sessions by each therapist to be replaced by the therapist number (see example below).
refno therapist1 therapist2 w_therapist1 w_therapist2 Total
1001 6 6 6/12 = 0.5 6/12 = 0.5 12
-
So initially we will have the number of interventions each therapist did of the total for the participant.
-
So in this example we can see that therapist1 did 10 out 12 interventions.
-
Hence the weight for therapist1 is 10/12.
-
Now that we have calculated the weights, we need to rename the therapist columns by their appropriate number.
-
So for this example we need to change the therapist1 column to be ‘1’ instead of ‘10’ and similarly for all therapists.
-
Note we leave the weights as they are.
-
So for the example above we need to change it so it looks like this (see below).
refno therapist1 therapist2 w_therapist1 w_therapist2 Total
1001 1 2 6/12 = 0.5 6/12 = 0.5 12
This needs to be done for all the therapists columns and the code below will do this.
*Save data set
Instructions in MLwiN
-
Open the saved file in MLwiN
-
Select model and equations and the equations window should appear
-
In the equations window at the bottom, select notation and unselect the multiple subscript option, and click done
-
Now click on the ‘Y’ in the equations window and select the SFscore from the list
-
Select the 3 levels from the N-Levels options
-
Then select level 3 to be siteid, level 2 to be therapist1 and level 1 to be refno
-
Now select ‘B0’ from the equations window and choose it to be the constant vector of 1’s and select this to be random at level 3, level 2 and level 1.
-
Now click on ‘Add term’ option at the bottom of the equations window and select the covariates described for the primary analysis (Note: none of these should be selected as a random effect)
-
Once all the terms are added, make sure that the estimation procedure is on the option ‘IGLS’ and click on start
-
Then change the estimation procedure to MCMC and run the model once more
-
Once the model is run using MCMC, click on Model/MCMC/Classification from the menu bar
-
Select the option for multiple classification Level 2 option and in the option for number of columns enter the number of columns there are for the therapists
-
Then click on the drop down menu for Weight start column and click on the variable from where the weight for the first therapist is
-
Then click on start and run the model for the final time and the results will be the final results to be used for the analysis
All other secondary analyses will be done in the same manner as the primary endpoint.
13. References
- Gladman JRF, Lincoln NB, Adams SA. Use of the Extended ADL Scale with Stroke Patients. Age Ageing 1993;22:419-24.
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Motorbility Index: a further development of the Rivermead Motor Assessment. Int Disabil Stud 1991;13:50-4.
- Goldberg DP, Williams P. A Users Guide to the General Health Questionnaire. Windsor: NFER-NELSON; 1992.
- Browne WJ. MCMC estimation in MLwiN. 2011.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. New York: Oxford University Press, USA; 2007.
- Smith JA, Flowers P, Larkin M. Interpretative Phenomenological Analysis: Theory, Method and Research. London: Sage; 2009.
Appendix 2 Summary of intervention training manual
Introduction
An Outdoor Mobility Rehabilitation Training Manual was produced for therapists to provide a guide on an outdoor mobility intervention to be offered to stroke patients. As this was a large multicentre trial, this manual served as an important guide to ensure that therapists followed a similar treatment programme across sites. The intervention outlined in the manual was based on knowledge gained during an earlier single-centre study. In this study the intervention was found to have made a massive difference to people’s lives by helping them out of the house more often. The manual is split into different sections and each section is summarised below.
Background
Background information on the prevalence of stroke, and the effect of strokes upon the lives of people with stroke, is provided in this section. Research data are provided to support the figures presented. The section moves on to provide the rationale behind providing an outdoor mobility training intervention for people with stroke. Research is cited to support the need for such an intervention study.
It is outlined in this section that the multicentre trial, to which the manual relates, follows on from a single-centre study that found clear benefits with the intervention. However, the generalisability of this study was limited as the intervention was delivered by a single therapist, in one city, and there was no health-economic evaluation. It is argued that a multicentre trial is now needed to determine whether the intervention is effective and cost-effective before it can be recommended for wider adoption across the NHS.
Models and approaches to rehabilitation
In everyday practice, therapists use a combination of physical and psychological models and approaches when devising treatment programmes for stroke patients. These models and approaches provide a theoretical base for the intervention. Therapists will often use a combination of models and approaches when deciding on a treatment programme. Although there have been papers published describing the models there is very little research to evaluate or compare the models. There is no strong evidence to suggest that one model or approach is superior to another. The manual therefore lists and describes models and approaches that therapists may choose to use in their treatment sessions. These may be used together with the practice of outdoor mobility activities. The models described include the Compensatory or Functional model, the Model of Human Occupation, the Cognitive Frame of Reference, and the Adaptive Skill model. The neurodevelopmental approach is also described, along with the main techniques used by therapists, including the Bobath, Rood, Conductive Education, Proprioceptive Neuromuscular Facilitation and Sensory Integration techniques. It is stressed in the manual that this is not a comprehensive list and other models and approaches can be adopted.
Benefits and barriers to going outside
This section looks at why people wish to get out of their home and the barriers preventing this. It includes two checklists. The first checklist provides a list of possible destinations. This serves as an aid to prompt patients to identify outings of personal interest to them. The second checklist provides a list of possible factors that may hinder trips out of the home for the patient. These issues can then be discussed and addressed by the therapist and patient.
Assessing for limitations
This section provides a screening form to be completed with the patient to help the therapist and patient to plan the outdoor intervention. The form includes 11 screening questions on transport options and outdoor mobility. The form is completed during the treatment planning stage of the intervention. The form can also be used at intervals during therapy if progress seems slow.
Building a useful address and information resource
This section provides a template for the therapist/other team members/patient to insert useful contact details of local transport and community services. Examples include the Blue Badge Scheme for car parking, Dial-a-Ride, voluntary car schemes, Shopmobility and the local Disability Living Centre contact numbers.
Goal planning and activity analysis
The importance of goal setting is emphasised in this section. Several activities are broken down into smaller more detailed goals as examples of possible outdoor goals that could be completed with patients. The activities include walking outside, driving a car, using the bus and train, using Dial-a-Ride, the voluntary driver scheme and electric pavement scooters.
Pre-outing checklist
In this section a checklist is provided to help the therapist and patient to identify any potential risks, hazards or challenges that might arise during community outings. This checklist is completed prior to the first outing. It ensures that factors are considered which will help to make the trip both safe and more successful. The checklist includes mobility, upper limb function, cognitive and perceptual function, vision and continence. Each area is broken down into skills and abilities that need consideration.
It is recommended in the manual that the therapist observes performance around the home before venturing outside.
Protocol for the first outing
This section prompts the therapist to assess and plan ahead for all contingencies prior to the first outing. Examples of early outings recommended in the manual are walking to the garden gate, walking to the street corner, walking to the closest bus stop or a walk around the block. A checklist is provided of considerations and contingencies for this first outing, along with consideration of activities that can be practised first in the home environment.
Protocol for outdoor walking
In the single-site outdoor mobility study, walking outdoors was found to be an important goal for patients. Of the 78 main goals recorded, 22% focused on walking outdoors. In the protocol it is recommended that this activity starts with simple and small goals, such as walking to the garden gate, and progresses once skills are consolidated to encompass more demanding situations. The protocol stipulates setting practice targets between sessions aimed at increasing confidence and fitness, and includes activities such as daily trips to the street corner with a family member.
The protocol for walking is broken down into three levels. The first level incorporates walking over a short distance in a quiet environment, requiring minimal attention and physical demands. The second level progresses to incorporate more moderate demands involving road crossings, kerbs and gradients. The final level requires multitasking activities, such as carrying a bag, handling money while standing, and walking in a busy location.
Protocol for using the bus
In the single-site outdoor mobility study, catching the bus was the second most popular goal. Of the 78 main goals recorded, 17% focused on taking a bus. It is recognised in this protocol that not all therapists themselves may be familiar with using public transport. To this end guidance is provided on the steps and skills to consider for this activity, including recognising and hailing the correct bus, managing money and ticketing, boarding and disembarking the bus, and recognising the correct destination.
Once again the protocol is broken down into three levels. The first level involves home practice of reading timetables, handling money, and preparing contingency plans. The second level moves on to supervised outings. Maximum prompting and close supervision is provided at this level. The final level progresses to just standby or distant supervision.
Protocol for using the train
As with the bus protocol it is recognised in this section that not all therapists will be familiar with using trains. Equally people with stroke may not be familiar with this mode of transport, particularly if they drove before their stroke. To this end guidance is provided on the steps and skills to consider for this activity, including reading the indicator board, purchasing a ticket, accessing the platform, identifying the correct train, negotiating crowds, and boarding and leaving the train.
Similar to the bus protocol the train protocol is broken down into three levels. The first level involves home practice of reading timetables, managing money and preparing contingency plans. The second level progresses to supervised outings with close supervision and maximum prompting. The final level involves just standby or distant supervision.
Protocol for using outdoor pavement scooters
In the single-site outdoor mobility study, being able to use a (personally owned) motorised scooter was a primary goal for six people with stroke, although using a scooter on a temporary loan for shopping around town (part of the Shopmobility service in England) was a primary goal for another six people with stroke.
The protocol for using an outdoor pavement scooter is broken down in the manual into two levels. The first level involves a trial off road. This enables practice operating the scooter controls and familiarisation with charging the scooter battery. The second level progresses to a trial on the road. Practice progresses from quiet to busy roads, crossing at traffic lights, negotiating kerbs, slopes, gradients, obstacles and pedestrians, and parking the scooter outside shops and home beside the battery-charging location.
Once again, the protocol includes a checklist of the skills and competencies needed to safely drive an outdoor pavement scooter.
Each level of the protocol for outdoor walking, using the bus, using the train and using outdoor pavement scooters is clearly stipulated so that therapists across sites follow similar treatment programmes for each activity. Family members are encouraged to assist in the practice of skills and, importantly, the protocols advocate the setting of practice targets between sessions to assist in the consolidation of skills.
Intervention record form
This section provides the therapist intervention paperwork for the study, which includes:
-
A form requiring the therapist to break down the components of each intervention into minutes. The therapist records the time spent on mobility task training, other rehabilitation training, adaptive equipment, referral to other agencies, information provision, confidence and motivation, and goal-setting activities.
-
A form to record the patient’s goals and their relevant medical history.
-
A form to record the running records of each session completed.
Case vignette of treatment plans
A case vignette taken from rehabilitation notes is provided as a guide for therapists in this section when planning treatment sessions. This vignette outlines a clinical history of a person with stroke. Information is provided on their ability and skills around personal and domestic activities of daily living, their transfer ability and their level of mobility. The vignette incorporates their social situation and outlines their local environment, including access to their home property. The vignette moves on to focus on the person’s experience of using different modes of transport, and then highlights the goals set with this person. This is followed by a detailed breakdown of the six treatment sessions completed.
Confidence and motivation
As self-confidence to complete a task and the motivation to persevere are such important aspects of the outdoor mobility intervention, this section of the manual is dedicated to explaining the significance of these two attributes.
Self-confidence in relation to the study is about having the confidence to complete outdoor mobility. This not only relates to confidence in self, but also to having confidence and trust in others, such as the therapist. This is particularly important when therapists are encouraging people with stroke to try new activities or to push themselves harder.
Motivation is the activation or energisation of goal-orientated behaviour. It requires that those enrolled on the study have some desire to undertake outdoor mobility. Individuals need to be able to identify meaningful reasons why they wish to attain goals in order to have the motivation to succeed.
Sample letter for requesting help with purchasing equipment
In this section a sample letter is inserted to provide therapists with guidance when seeking to apply for funding for outdoor mobility equipment for their patients. In the UK, motorised scooters are not generally available via government schemes. Individuals therefore have to purchase their own scooter, and may seek financial assistance from philanthropic agencies, local church groups or service clubs. In these situations therapists may wish to support their applications by writing a letter, as per the sample provided.
References
This section of the training manual provides the full references that have been quoted in the main body of the manual.
PowerPoint slides
The PowerPoint (2007) slides (Microsoft Corporation, Redmond, WA, USA) presented during the therapist training days are provided in this section. The training was provided by Dr Pip Logan, CI; Dr Annie McCluskey, external expert; Professor Marion Walker, Professor of Stroke Rehabilitation; and Dr Matt Leighton, Trial Manager. The training day provided information on the following:
-
An overview of the multicentre outdoor mobility rehabilitation study.
-
Roles and responsibilities of those working on the study, and lines of communication and help.
-
Information on the earlier single-site outdoor mobility study.
-
The mobility outcome measures being used in the outdoor mobility rehabilitation study.
-
Timings of baseline visits, intervention visits and follow-up visits.
-
The outdoor mobility rehabilitation study documentation.
-
Goal setting.
-
The role of the Stroke Research Network.
-
The role of the Collaboration in Leadership in Applied Health Research and Care (CLAHRC).
-
Information on CLAHRC trials being completed.
-
Information on an Australian study that audited the amount of outdoor mobility training being incorporated into therapy intervention programmes. The barriers behind including outdoor mobility intervention were studied, training on outdoor mobility was provided, and a repeat audit of outdoor mobility intervention was completed.
Published papers
The following published papers are included in the final section of the manual:
Logan PA, Gladman JRF, Radford KA. The use of transport by stroke patients. Br J Occup Ther 2001;64:261–4.
Logan PA, Gladman JRF, Avery A, Walker MF, Dyas J, Groom L. Randomised controlled trial of an occupational therapy intervention to increase outdoor mobility after stroke. BMJ 2004;329:1372–5.
Logan PA, Walker MF, Gladman JRF. Description of an occupational therapy intervention aimed at improving outdoor mobility. Br J Occup Ther 2006;69:1, 2–6.
List of abbreviations
- 95% CI
- 95% confidence interval
- CEAC
- cost-effectiveness acceptability curve
- CI
- chief investigator
- CONSORT
- Consolidated Standards of Reporting Trials
- DMC
- Data Monitoring Committee
- eCRF
- electronic case report form
- EQ-5D
- European Quality of Life-5 Dimensions
- GHQ-12
- General Health Questionnaire-12 items
- GP
- general practice
- HTA
- Health Technology Assessment
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- INB
- incremental net benefit
- IPA
- interpretive phenomenology analysis
- IQR
- interquartile range
- MCID
- minimally clinically important difference
- MI
- multiple imputation
- mph
- miles per hour
- NCTU
- Nottingham Clinical Trials Unit
- NEADL
- Nottingham Extended Activities of Daily Living
- NHS-IC
- NHS Information Centre
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute of Health Research
- PCT
- primary care trust
- PI
- principal investigator
- PIS
- patient information sheet
- PROM
- patient-reported outcome measure
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RA
- research assistant
- RCT
- randomised controlled trial
- RMI
- Rivermead Mobility Index
- SD
- standard deviation
- SF-36v2
- Short Form questionnaire-36 items, version 2
- SF-6D
- Short Form questionnaire-6 Dimensions
- SWOM
- satisfaction with outdoor mobility
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- TSDC
- Trial Steering and Data Committee