Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/16/01. The contractual start date was in August 2012. The draft report began editorial review in May 2013 and was accepted for publication in October 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Brazzelli et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Plain English summary
Gallstones are common, especially in women, but in many people they do not cause any symptoms.
About one in three people with gallstones develop symptoms. Symptoms usually include a severe pain in the upper right-hand side of the abdomen (known as ‘biliary colic’), and sometimes nausea and vomiting. Sometimes the pain is accompanied by inflammation of the gallbladder (cholecystitis).
Once gallstones start giving symptoms, painkillers, anti-inflammatory medicines and antibiotics are usually prescribed.
Surgery to remove the gallbladder, known as cholecystectomy, is the most common way to treat biliary pain or cholecystitis due to gallstones. About 70,000 cholecystectomies are performed every year in the UK, with significant costs for the NHS.
In the UK, surgery is commonly offered to people who present at secondary care with pain or cholecystitis due to gallstones. However, it is known that some patients do not have any more symptoms after the initial episode of pain and that surgery may not be necessary. This assessment has shown that some people with mild symptoms do not experience a recurrence or suffer complications for many years. A policy of ‘conservative treatment’ (painkillers/antibiotics and lifestyle advice) could, therefore, be appropriate in this group of people. Our results indicate that, for the NHS, surgery is more expensive than ‘conservative treatment’ but is still the most clinically effective treatment for gallstones. There are, however, great uncertainties in the data. There is a need for new clinical studies to address these uncertainties.
Chapter 1 Background
Description of health problem
Introduction
Gallstone disease (cholelithiasis) is one of the most common and costly gastrointestinal disorders in industrialised societies. 1–4 The prevalence of gallstones in these adult populations is approximately 10–15%. 1,5–9 Gallstones are more common in women and people over the age of 40 years. 10
Approximately 80% of people with cholelithiasis do not have symptoms,11,12 can remain asymptomatic for many years and do not require treatment. 13,14 About 20% of people experience pain and clinical complications. 1,5,6,15
Surgery remains the treatment of choice for symptomatic gallstones,16 even though early natural history studies showed that recurrent pain attacks may diminish in up to half of symptomatic people. 17 As many studies have concentrated on the best timing of performing surgery and on operative outcomes and complications, the question whether or not cholecystectomy is always required in people with mild, uncomplicated symptomatic gallstones has not been rigorously evaluated. There is evidence to show that it is feasible to conduct randomised controlled trials (RCTs) to compare the effects and safety of cholecystectomy with those of observation/conservative treatment. 18
The aim of the present assessment is to evaluate the clinical effectiveness and cost-effectiveness of cholecystectomy compared with observation/conservative management for preventing recurrent symptoms and complications in adults presenting initially with uncomplicated symptomatic gallstones for whom surgery is considered a treatment option.
Aetiology, pathophysiology and clinical presentation
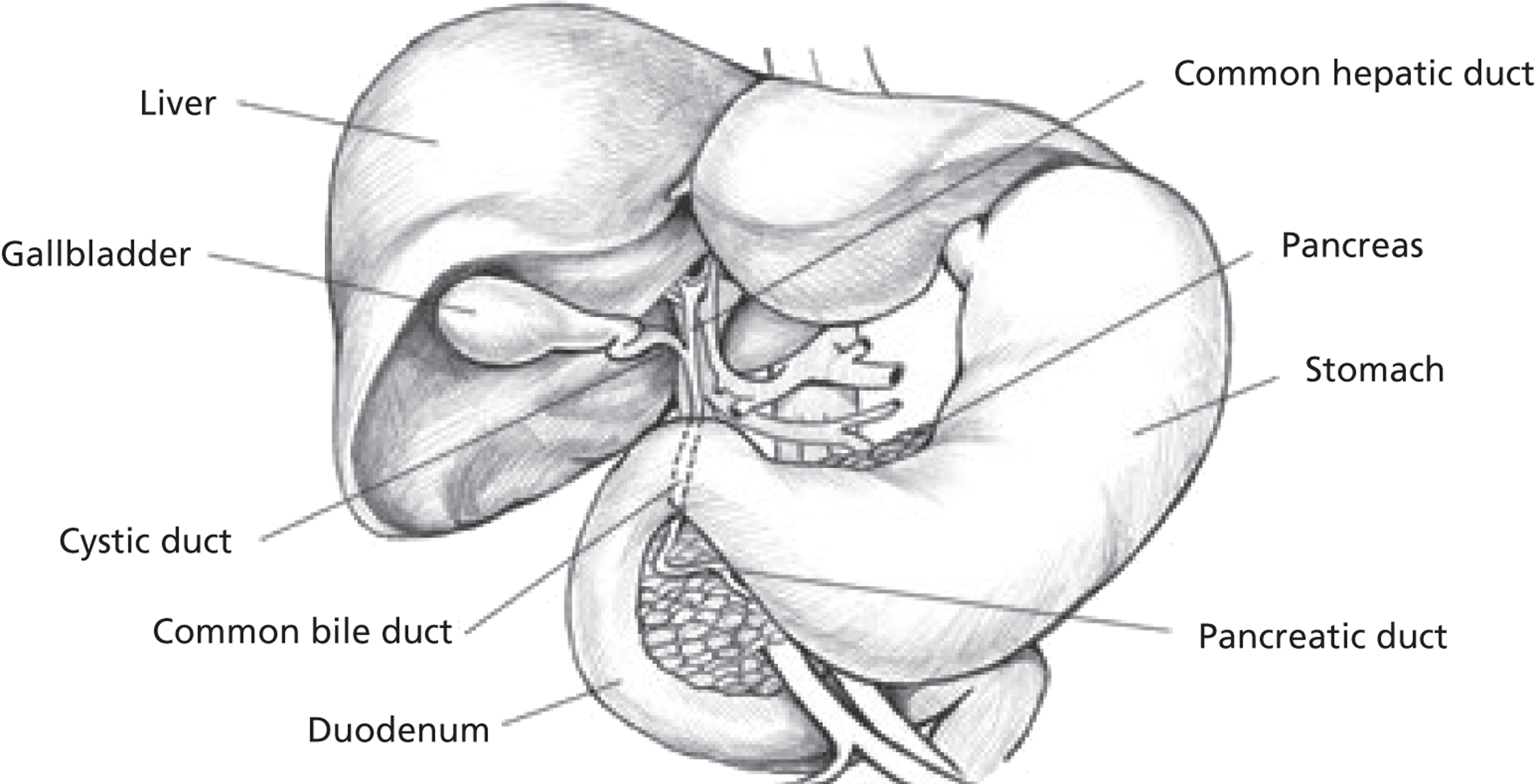
The gallbladder is a pear-shaped pouch which stores and concentrates bile entering the organ via the hepatic and cystic ducts (Figure 1). The concentrated bile is later ejected into the duodenum in response to food entering the intestines. The gallbladder is located beneath the liver and can hold between 30 and 50 ml of bile. Histologically, the gallbladder wall consists of serous, muscular and mucous layers. 19 Gallstones are solid accumulations of either cholesterol, mucin, calcium bilirubinate or protein20 and occur when the constituents come out of solution. 21 Most gallstones (> 80%) consist largely of cholesterol,7,22 whereas pigment stones consist of calcium bilirubinate and mucin glycoproteins. Both types of gallstone form in the gallbladder itself.
FIGURE 1.
Biliary system. Source: http://www.daviddarling.info/encyclopaedia/G/gall_bladder.html (last accessed May 2013). Reproduced with permission of David Darling.
Known risk factors for adults with gallstone disease include:8,23
-
increasing age
-
obesity
-
family history of gallstone disease
-
ethnicity
-
recent weight loss (for example, crash diets or weight-loss surgery)
-
digestive disorders (e.g. Crohn’s disease, irritable bowel syndrome)
-
cirrhosis
-
diabetes.
Women who:
-
take oral contraception
-
have high-dose oestrogen therapy or
-
are pregnant
are at higher risk (twice as likely as men) of developing gallstones. Some risk factors are modifiable, such as diet and obesity, while others are not (e.g. sex, age, concomitant diseases). 22
The most relevant symptom of gallstone disease is pain (i.e. biliary colic). Typically, biliary colic is defined as pain, moderate to severe, localised to the right upper quadrant or epigastrium lasting more than half an hour which occasionally can radiate to the right scapula. The pain may be accompanied by nausea and vomiting, may occur post-prandially and may wake the person at night. Although biliary pain is specific to gallstone disease, many people may present with other abdominal symptoms. 24 In some people, the symptoms are mild and consist of vague indigestion or dyspepsia. Dyspeptic symptoms (e.g. belching, bloating, heartburn), however, are not specific to gallstone disease. 5,7,25
Epidemiology and prognosis
Diseases of the gallbladder are widespread, affecting approximately 10–15% of the adult population. 26 Clinical surveys conducted in Europe, North and South America and Asia indicate that the prevalence rates for gallstone disease range from 5.9% to 25%27–37 and tend to increase with age. A clinical ultrasound survey conducted in the UK reported prevalence rates of 12% among men and 22% among women over 60 years of age. 35 A multicentre, population-based study conducted in Italy has reported a cumulative incidence of gallstone disease of 0.67% per year (0.66% in men and 0.81% in women). 38 In the developed world, gallstone disease is the most common gastrointestinal complaint for which people are admitted to hospital. 2,10,39 Hospital admissions increased in England by 30% in men and by 64% in women from 1989–90 to 1999–2000. 3
Natural history studies have shown low mortality from gallstone disease, with typically < 1% of people dying from gallbladder-related causes. 3,12,13,17 The natural course of gallstone disease is benign, with a relatively low progression from asymptomatic disease to symptomatic disease. In a recent population-based study, the overall frequency of symptom development in asymptomatic people was around 20% over a long follow-up period (mean 8.7 years). 12 An early natural history study (in which people were found to have gallstones through routine screening), showed that 10%, 15% and 18% of people assessed became symptomatic at 5, 10 and 15 years, respectively. 40
Overall, the annual risk of developing complications for people with asymptomatic gallstones is low, about 0.1–0.2%. In contrast, in people with symptomatic gallstone disease, the annual rates of developing complications have been reported to be higher, 1–3%. 41–43 The Italian Group for the Epidemiology and Prevention of Cholelithiasis study reported an annual incidence of complications of 0.3% for asymptomatic people and 0.7% for symptomatic people. 13 In particular, there is a proportion of people with uncomplicated gallstone disease who may experience only a few episodes of biliary pain without developing serious symptoms or complications for many years. 44,45 A recent multicentre study found that a considerable proportion of people with mild and severe symptoms (58% and 52%, respectively) did not experience subsequent episodes of biliary pain during the 10-year follow-up period and 10% suffered from gallstone complications, indicating a benign course of the disease even in those with initially severe symptoms. 12 Moreover, the severity of the disease does not seem to increase over time. 21,46
In the UK and in North America, the number of surgical procedures for gallstone disease increased steadily between the 1950s and 1990s, reflecting both the rise in prevalence of gallstone disease and the use of cholecystectomy as the treatment of choice. 3,47 Rates of surgical procedures stabilised in both countries towards the end of the twentieth century. 3,48
Impact of health problem
From a patient perspective, the defining symptom of gallstone disease is pain. 49,50 Commonly, general abdominal symptoms intensify over a period of time and become regular pain attacks, which require medical attention. Although the pain experienced may be described as ‘non-specific’,7,49,50 it tends to occur in a defined location and with characteristic patterns as described above. Best medical therapy includes the prescription of analgesics and, when necessary, antibiotics.
The most common complications associated with gallstones are acute cholecystitis, common bile duct (CBD) stones and acute pancreatitis. Acute cholecystitis is caused by a gallstone obstructing the cystic duct and results in unresolving upper right quadrant pain, nausea, vomiting, anorexia and fever. 51 CBD stones are found in up to 15% of people who undergo cholecystectomy. They may be asymptomatic or accompanied by biliary pain, jaundice, pancreatitis or cholangitis. 52 CBD stones can cause acute pancreatitis by obstructing the main pancreatic duct. 53 The typical symptoms of gallstone-related pancreatitis are epigastric abdominal pain, nausea and vomiting. 51 Pancreatitis is severe in over 20% of people with gallstone disease and can cause death in about one-third. 54
Even though removal of the gallbladder is considered the standard treatment for symptomatic gallstones, it does not guarantee eradication of symptoms. 55 Up to approximately 40% of people may continue to experience pain and abdominal symptoms after surgery. 56 In particular, marked biliary pain has been described in 4–9% of people after cholecystectomy, while persistent abdominal pain or non-specific pain persists in about 13–37% of people. 57–62 A recent systematic review of the literature found that up to one-third of people suffered continuing pain after cholecystectomy and up to 14% of people experienced de novo pain. 63 Some investigators have also reported a persistent pain similar to that experienced pre-operatively in about 20% of people with gallstones. In a prospective study conducted in Denmark, 21% of people experienced the same type of pain after surgery. 64 Similarly, in a RCT conducted in the UK, 19% of people complained of biliary pain 5 years after open cholecystectomy. 65 No difference has been observed between open and laparoscopic surgery in terms of persistent pain. 66,67 The term ‘post-cholecystectomy syndrome’ is an umbrella term that has been widely used to describe, though not accurately, the range of symptoms which occur after cholecystectomy. 68 The term ‘persistent post-cholecystectomy symptoms’ has been suggested as a more accurate description of these symptoms. 69 These symptoms include biliary and non-biliary abdominal pain, gastrointestinal disorders, dyspepsia, heartburn, nausea, vomiting, jaundice and cholangitis. Severe symptoms which occur early after surgery may represent complications of cholecystectomy, whereas those that manifest later (after months or years) are probably unrelated to cholecystectomy and explained by non-biliary causes. Non-biliary causes are more likely if the symptoms are similar to those experienced pre-operatively and no stones are found in the gallbladder. Recent research has suggested that, in some people, functional gastrointestinal disorders and not gallstone disease may be the cause of persistent post-surgery symptoms. 16 Nevertheless, there is no consistent pathophysiological explanation for persistent post-cholecystectomy symptoms, and in about 5% of people the reason for persistent abdominal pain remains unknown. 70
Current service provision
Management of disease
Management of gallstone disease may include pharmacological, non-pharmacological and surgical interventions. Surgery is the definitive way to treat gallstone disease. Laparoscopic cholecystectomy is currently preferred over open cholecystectomy for elective surgery in symptomatic gallstones. 5,71 In the UK, people with biliary pain are commonly put on a waiting list and undergo elective surgery several months after the original clinical diagnosis,72 although early and urgent surgery during the same admission is becoming more common.
People with symptomatic gallstones who are unfit or unwilling to undergo surgery are managed conservatively or may be offered alternative non-surgical treatments. In particular, conservative management is a feasible and relatively safe option for pregnant women and elderly people who are less likely to tolerate surgery. 73–75 The terms ‘observation’ and ‘conservative management’ are not clearly defined in the current literature and appear to be used interchangeably. In general, observation/conservative management in the context of gallstone disease involves the prescription of analgesics to relieve the biliary pain. Typical therapy includes narcotic analgesics (e.g. opiates) or non-steroidal anti-inflammatory drugs (e.g. ibuprofen) together with generic lifestyle advice. 15,55,76–78 Complicated biliary pain (i.e. inflammation of the gallbladder, namely acute cholecystitis) usually requires additional therapy with antibiotics. 15
Medical therapy for gallstone disease using bile acids, for example ursodeoxycholic acid, to dissolve stones (especially cholesterol stones) is presently restricted to a small, highly selected group of symptomatic people for whom a surgical intervention is not recommended and recurrence is likely to have particularly adverse consequences. 15 Novel experimental animal models and preliminary clinical findings indicate that future research could focus on the mechanisms of intestinal absorption of cholesterol, on hepatic cholesterol biosynthesis as well as on the role of gallstones genes. 15,79
Other non-pharmacological, non-surgical treatments include dissolution/fragmentation of gallstones using the cholesterol solvent methyl tert-butyl ether (MTBE) and extracorporeal shock wave lithotripsy (ESWL). These treatment options are very rarely used in clinical practice because of their potential side effects (MTBE), high recurrence rate in people with multiple stones (ESWL) and occurrence of transient biliary pain after successful stone fragmentation (ESWL). 5,80,81
The widespread use of laparoscopic cholecystectomy together with the use of imaging techniques for a more accurate diagnosis of symptomatic gallstone disease has contributed to the decline in non-surgical medical interventions such as oral bile acids and ESWL. 80–82
Current service cost
Even though the majority of gallstones are asymptomatic and do not require treatment, gallstone disease is still the most expensive of the digestive disorders in industrialised societies. 83 Considerable resources are involved in managing people with symptomatic gallstones. The bulk of the economic burden is mainly due to the costs associated with surgery for the removal of the gallbladder. 2,3
According to the NHS 2011/12 tariff for admitted patient care and outpatients procedures, for laparoscopic cholecystectomy, the day case tariff was £1689, whereas the elective spell tariff was £1370 and the non-elective spell tariff £3197. For open cholecystectomy, the combined day case and spell tariff was £2285, whereas the non-elective spell tariff was £4513. 84 Best practice tariffs were introduced in England in 2010–11 to incentivise cholecystectomy on a day-case basis, where clinically appropriate. 85
Variation in services and/or uncertainty about best practice
In the UK, people with biliary pain are usually put on a waiting list and undergo elective surgery several months after establishment of a clinical diagnosis of gallstone disease. The waiting time for elective cholecystectomy varies from hospital to hospital depending on the resources available, but generally lies between 4 and 12 months (although current clinical targets have led to a shortening of waiting lists). 86–89 There is a marked variation between UK health trusts in terms of rates for laparoscopic cholecystectomy. Approximately half of trusts perform < 5% of cholecystectomies as day cases, with many trusts performing no day procedures at all. 90 The variation is likely to be explained by the local resources available in each trust and the number of people with symptomatic gallstone disease being treated during the same emergency admission or electively after undergoing a period of conservative treatment.
Relevant national guidelines, including National Service Frameworks
There are no current published national guidelines on the management of gallstone disease.
Description of technologies under assessment
Summary of the surgical procedures under assessment
Laparoscopic cholecystectomy is the current standard surgical procedure for the management of gallstone disease. Since its development in the late 1980s,91 laparoscopic cholecystectomy has increasingly replaced open cholecystectomy, which remained the gold standard surgical treatment for over 100 years. 92 Laparoscopic cholecystectomy has been demonstrated to be a safe and cost-effective procedure with similar mortality and complications rates to those of open cholecystectomy, but significant shorter hospital stay, quicker recovery93 and lower total cost. 94
Open cholecystectomy
The first open cholecystectomy was performed in 1882 by Carl Langebuch. 95 After making an open incision, the peritoneum covering the triangle of Calot (the area bound by the inferior border of the liver, cystic duct and common hepatic duct) is dissected, permitting the cystic artery and duct to be identified then ligated and divided. The gallbladder can then be dissected from the liver bed (either from the infundibulum up or from the fundus down). The standard post-operative course is about 5 days as an inpatient, followed by 3–6 weeks’ convalescence,5 and remains significantly longer than laparoscopic cholecystectomy.
With the widespread use of laparoscopic procedures, there are now limited indications for initiating a cholecystectomy as an open procedure. Nowadays, the majority of open cholecystectomies consist of conversions from laparoscopic procedures. 96 Conversion to an open procedure may be required because of the presence of adhesions, difficulty in delineating the anatomy or a suspected complication. Conversion is needed more often in those with prior abdominal surgery, acute cholecystitis or severe/advanced diseases. 25,97 Conversion rates are typically < 10%, including emergency procedures. 71,98
Laparoscopic cholecystectomy
The majority of people with symptomatic gallstones are candidates for laparoscopic cholecystectomy unless they are unable to tolerate a general anaesthetic or have comorbidities that preclude surgery. 99
During laparoscopic cholecystectomy, the person is placed in the supine position on the operating table and anaesthetised. Access to the abdomen is gained by either a Veress needle, the open Hasson technique (direct trocar placement without prior pneumoperitoneum) or the optical view technique (in which the laparoscope is inside the trocar to allow the abdominal wall layers to be visualised as they are crossed). The surgeon inflates the abdominal cavity with carbon dioxide to create a working space. The camera is placed through the umbilical port and the abdominal cavity is inspected. Additional ports are inserted to allow the insertion of instruments. The gallbladder fundus is identified, grasped and retracted superiorly. With a second grasper, the gallbladder infundibulum is retracted laterally to expose and open Calot’s triangle. The cystic duct and the cystic artery are identified, clipped and divided. The gallbladder is then dissected from the liver bed and removed via the umbilical port100 or the epigastric port. Laparoscopic surgery requires meticulous and specific surgical skills. The procedure usually results in shorter hospital admission and recovery period than open cholecystectomy (1–2 days in hospital and 1–2 weeks of convalescence). 5
The National Institute of Health Consensus Conference statement concluded that laparoscopic cholecystectomy provides a safe and effective treatment for most people with symptomatic gallstones. 5
A Cochrane systematic review comparing open cholecystectomy with laparoscopic cholecystectomy for symptomatic gallstones93 demonstrated similar mortality and complications rates between the two procedures but shorter hospital stay, recovery period and lower total cost for laparoscopic surgery. 94
Single-incision laparoscopic cholecystectomy
Laparoscopic cholecystectomy typically results in three or four small scars caused by the port sites, which is a marked improvement over the scars left by open procedures. However, there have been recent efforts to further reduce the potential for scarring by the introduction of the so-called single-incision laparoscopic procedure. The laparoscope and instruments are both inserted using a single port in the umbilicus, a location in which a scar can effectively be camouflaged. 101 From a technical point of view, the single-incision laparoscopic procedure is very similar to standard laparoscopic cholecystectomy. 102
Several recent systematic reviews have compared single-incision and conventional laparoscopic cholecystectomy. Arezzo and colleagues103 concluded that the single-incision approach was not associated with more complications than the conventional laparoscopic approach. In addition, no mortality was reported and conversion rates to open cholecystectomy were similar for both procedures. Wang and colleagues104 found a higher conversion rate for single-incision cholecystectomy, but did not observe any difference in complication rates between single-incision and conventional laparoscopic surgery. Antoniou and colleagues105 observed higher complication rates for people over 45 years of age.
In a recent Cochrane systematic review comparing open cholecystectomy with small incision (< 8 cm) cholecystectomy for symptomatic gallstones,106 no differences were observed between the two surgical procedures with regard to complications and mortality rates. Hospital stay was, however, significantly shorter for small incision cholecystectomy.
Current usage in the National Health Service
At present, the definitive treatment choice for gallstone disease is surgery. Cholecystectomy is one of the most common surgical procedures performed in the UK. About 70,000 cholecystectomies were performed in England between 2011 and 2012. 107 The majority of these surgical procedures were performed after an elective admission and undertaken laparoscopically. 90 Laparoscopic cholecystectomy is technically more challenging to perform than the traditional open cholecystectomy and the success of the operation depends on the experience, specialisation and technical skills of the surgeon who performs the procedure. 5 In Scotland, the total cholecystectomy rate increased by 18.7% between 1989 and 1993. 108 Hospital Episode Statistics data for England for 2011–12 reported 65,926 admissions for excision of the gallbladder with a mean waiting time of 81 days. There were 6148 (9%) emergency admissions and 57,920 (88%) on the waiting list. 107
While waiting for surgery, people with symptomatic gallstones are treated conservatively. People who are unfit or unwilling to undergo surgery are managed by means of a ‘wait and see’ therapeutic strategy. Similarly, people with a single episode of biliary pain and no complications of gallstone disease may be potential candidates for conservative management, with a reasonable chance to remain symptom free.
Chapter 2 Definition of the decision problem
Decision problem
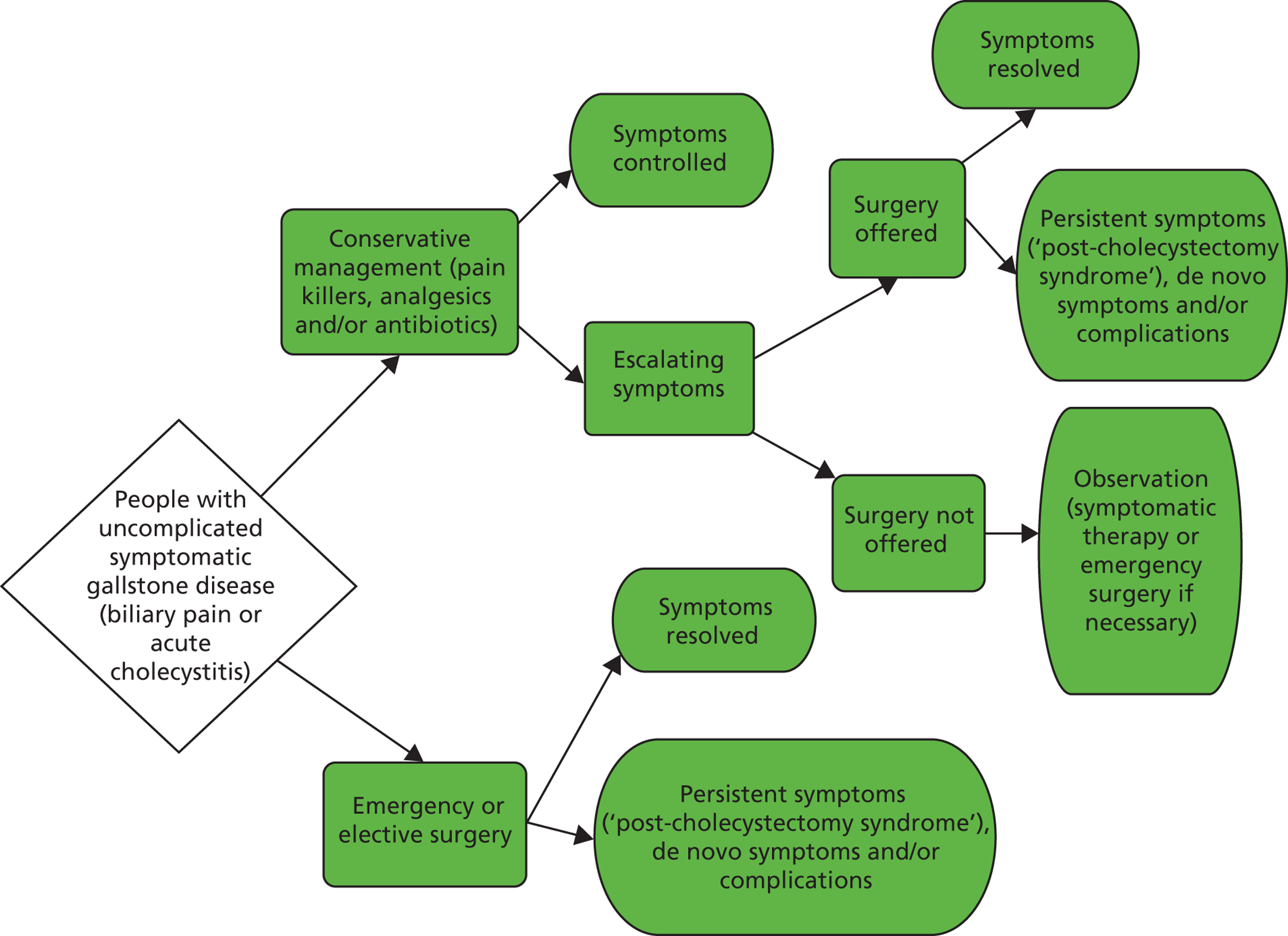
The purpose of this assessment was to evaluate the clinical effectiveness and the cost-effectiveness of cholecystectomy compared with observation/conservative management for preventing recurrent symptoms and complications in adults presenting with first episode of symptomatic gallstones in secondary care. The summary care pathway illustrating the decision problem addressed in this assessment is shown in Figure 2. The care pathway was developed using available published evidence, as well as the outcome of two advisory meetings with clinical experts convened for the purpose of this assessment. Although the pathway was primarily designed to guide the main phases of this assessment (e.g. gathering of existing evidence, development of an economic model), it is broadly consistent with previously published algorithms for the management of gallstone disease. 79 This chapter will consider the main components of the care pathway for gallstone disease. Specific information on the population, intervention, comparator and relevant outcomes considered for this assessment will be provided in Chapter 3 (Methods of the systematic review of clinical effectiveness).
FIGURE 2.
Flow chart of the clinical decision-making process for people with symptomatic uncomplicated gallstone disease.
Population
The population considered for this assessment is adults with symptomatic uncomplicated gallstone disease (biliary pain or acute cholecystitis) who are examined in a secondary care setting and considered suitable for cholecystectomy.
Clinical diagnosis of gallstone disease is usually confirmed by imaging and laboratory tests. Transabdominal ultrasonography is the standard imaging technique for the diagnosis of gallbladder stones. The technique is accurate, non-invasive and very widely available. 7,109,110
Intervention: cholecystectomy
Cholecystectomy is the standard treatment for symptomatic gallstone disease and its frequency is increasing worldwide. 50 Nowadays, nearly all cholecystectomies in the UK are performed using a minimally invasive laparoscopic approach (laparoscopic cholecystectomy). 5,111 Any cholecystectomies performed openly or laparoscopically (or variants thereof) are considered suitable for inclusion.
Comparator: observation/conservative management
The comparator intervention considered in this assessment is observation/conservative management. ‘Conservative management’ in the context of gallstone disease typically comprises the prescription of analgesics/anti-inflammatory drugs along with lifestyle advice. People with cholecystitis and signs of inflammation are usually prescribed antibiotics.
‘Delayed surgery’ is not considered a suitable comparator. The focus of recently published randomised trials and meta-analyses looking at early compared with delayed cholecystectomy (open and laparoscopic) has been on the optimal timing of surgical intervention as well as operative outcomes (in a patient population scheduled to receive surgery) rather than on the necessity of cholecystectomy in people with uncomplicated symptomatic gallstones presenting to secondary care.
Overall aims and objectives of assessment
The aim of this assessment was to evaluate the clinical effectiveness and cost-effectiveness of cholecystectomy compared with observation/conservative management for preventing recurrent symptoms and complications in adults presenting with symptomatic gallstones (biliary pain or cholecystitis) in secondary care for the first time.
The specific objectives of this assessment will help to facilitate decision-making on the most appropriate treatments for people suffering from uncomplicated symptomatic gallstone disease (biliary pain or cholecystitis) by:
-
conducting a systematic review of the evidence available on the clinical effectiveness of cholecystectomy compared with observation/conservative management
-
conducting a systematic review of the evidence available on the cost-effectiveness of cholecystectomy compared with observation/conservative management
-
developing an economic model to compare the cost-effectiveness cholecystectomy with that of observation/conservative management
-
determining which management options are most likely to be efficient for implementation into the UK NHS
-
identifying and prioritising future research.
Chapter 3 Methods of the systematic review of clinical effectiveness
The methods for this assessment were pre-specified in a protocol.
Identification of studies
Comprehensive literature searches were conducted to identify reports of studies on the clinical effectiveness and/or cost-effectiveness of cholecystectomy compared with non-surgical interventions for the management of people with symptomatic gallstone disease (biliary pain or acute cholecystitis). Highly sensitive search strategies were designed, including appropriate subject headings and free-text terms for the interventions under consideration and relevant study designs. No language restrictions were imposed on the literature searches. Searches were restricted to 1980 onwards, to mirror the use of surgical techniques currently available in clinical practice as well as the introduction of novel interventions for the management of symptomatic gallstones.
Databases searched were MEDLINE (1980 to 9 September 2012), MEDLINE In-Process & Other Non-Indexed Citations (10 September 2012), EMBASE (1980 to 10 September 2012), the Science Citation Index (1980 to 12 September 2012), Bioscience Information Service (BIOSIS; 1980 to 12 September 2012) and the Cochrane Central Register of Controlled Trials (date of inception to Issue 9, 2012). Reports of relevant evidence syntheses were also sought from the Cochrane Database of Systematic Reviews and the Database of Abstracts of Review of Effects.
The World Health Organization International Clinical Trials Registry (date of inception to September 2012), Current Controlled Trials (date of inception to September 2012), Clinical Trials (date of inception to September 2012) and the National Institute for Health Research Portfolio (date of inception to September 2012) were also searched to identify potential relevant ongoing studies. Conference proceedings from 2011 and 2012 of key organisations such as the Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, the Association of Laparoscopic Surgeons of Great Britain and Ireland and the British Society of Gastroenterology were screened for further relevant reports. The reference lists of all included studies were perused to identify additional potentially relevant reports. Full details of the search strategy are reported in Appendix 1.
Inclusion and exclusion criteria
Types of studies
For assessing the clinical effectiveness of cholecystectomy compared with observation/conservative management in people with biliary pain or acute cholecystitis, the following types of study design were deemed suitable for inclusion:
-
RCTs which randomised people to either cholecystectomy (open or laparoscopic) or observation/conservative management, irrespective of study language, blinding and publication status
-
non-randomised comparative studies in which people received either cholecystectomy (open or laparoscopic) or observation/conservative management.
The following types of report were excluded:
-
reviews, editorials and opinions
-
case series and case reports
-
reports published in non-English languages for which a translation could not be organised.
Types of participants
Adults (18 years and older or ‘adults’ as defined by the triallists) with symptomatic gallstone disease (biliary pain or acute cholecystitis) being considered for cholecystectomy in a secondary care setting for the first time. Gallstone-related disease needed to be confirmed by ultrasonography. For the purpose of this assessment, ‘first episode of symptomatic gallstones’ was defined as the first instance in which the patient presented to secondary care attention (with the possibility of receiving surgical treatment) even though the clinical history documented previous clinical symptoms or pain attacks. This criterion was later relaxed to allow inclusion of studies in which up to 25% of participants had previously presented to secondary care for symptomatic gallstones. First episode was assumed unless studies explicitly described cases as recurrent. Eligibility for cholecystectomy, if not explicitly reported, was assumed in RCTs whereas non-randomised studies were assessed on an individual basis on this criterion. An accepted definition of ‘acute cholecystitis’ is based on a combination of relevant clinical symptoms (e.g. pain localised to the right upper quadrant of the abdomen; temperature exceeding 37.5 °C, leucocytosis greater than 10 × 109/l, increased C-reactive protein level) and ultrasonographic evidence of gallstones.
People with acute severe cholecystitis (e.g. obstruction of the cystic duct or neck of the gallbladder by gallstones) and/or cholangitis (inflammation, usually infection, of a bile duct) or pancreatitis were not considered suitable for inclusion, as they normally require urgent or emergency intervention. Similarly, people with symptomatic gallstones complicated by severe concomitant diseases, critically ill people, and/or people who were judged to be unfit or unsuitable for surgery (e.g. pregnant women) were not considered within the scope of this review.
Therefore, for those with acute cholecystitis, we included any study population presenting with acute, uncomplicated, cholecystitis.
Intervention
The intervention considered was surgical removal of the gallbladder (laparoscopic or open cholecystectomy).
Comparator interventions
The comparator interventions considered were observation (watchful waiting) and/or conservative treatment. Conservative treatment refers here to a course of analgesics/anti-inflammatory drugs accompanied by lifestyle advice. People with cholecystitis and signs of inflammation may also be prescribed antibiotics.
Types of outcomes
The following types of outcome measure were considered:
-
disease-related morbidity
-
recurrence of symptoms
-
complications (e.g. pancreatitis)
-
number of visits to primary care settings or hospital emergency department
-
analgesic requirements
-
need for surgical, endoscopic or radiological intervention
-
need for further medical intervention
-
mortality
-
-
surgery-related morbidity
-
bile duct injury
-
infection/bleeding
-
reoperation rate
-
diarrhoea
-
recurrent pain
-
mortality
-
-
patient-driven outcomes
-
generic and disease-specific quality of life (QoL; as defined by the studies’ authors)
-
-
cost of initial and any subsequent treatments.
Data extraction strategy
One reviewer (MC) screened all titles and, when available, abstracts of all citations identified by the search strategies. All potentially relevant reports were retrieved in full and assessed independently by two reviewers. Any disagreements were resolved by consensus or referred to a third party. A sample full-text screening form is presented in Appendix 2.
A data extraction form was designed specifically for the purpose of this assessment to collect data from included studies. Two independent reviewers (MC and MB) extracted details of study design, characteristics of participants, characteristics of interventions and outcome measures. Any disagreements were resolved by consensus. A sample data extraction form is presented in Appendix 3.
Critical appraisal strategy
Included RCTs were assessed by means of the Cochrane Collaboration’s risk of bias tool. 112 A sample form is presented in Appendix 4. Two reviewers (MC and MB) independently assessed the risk of bias within each included trial based on the following domains: sequence generation, allocation concealment, blinding, incomplete outcome data and selective outcome reporting. Individual outcomes were categorised as high risk of bias, low risk of bias or unclear risk of bias. Any disagreements between reviewers were resolved by consensus.
Methods of data synthesis
Results of each included study were tabulated for all outcomes with means reported for continuous outcomes and proportions for dichotomous outcomes. Where the same outcome was assessed by more than one included study, a quantitative synthesis of results was carried out using Review Manager (RevMan) computer program (version 5.2. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). Heterogeneity between studies was assessed by visual inspection of forest plots and from Mantel–Haenszel chi-squared and I2 tests. Meta-analyses using the Mantel–Haenszel method were carried out to estimate risk ratios pooled across studies, with corresponding 95% confidence intervals (CIs). Where there was unacceptable heterogeneity between studies, random-effects models were used, otherwise fixed-effects models were applied.
Chapter 4 Clinical effectiveness of cholecystectomy compared with observation/conservative management
Quantity of research available
Number and type of studies included
The primary literature searches identified 6779 potentially relevant citations. Seventy-seven reports were selected for full-text assessment. Seventy-one reports were subsequently excluded (see Appendix 5). Two RCTs published in six reports were included in this assessment. 46,55,77,78,113,114 No eligible non-randomised comparative studies were identified by the literature searches. A flow diagram of the screening process is outlined in Figure 3.
FIGURE 3.
Flow chart for the identification and selection of studies.
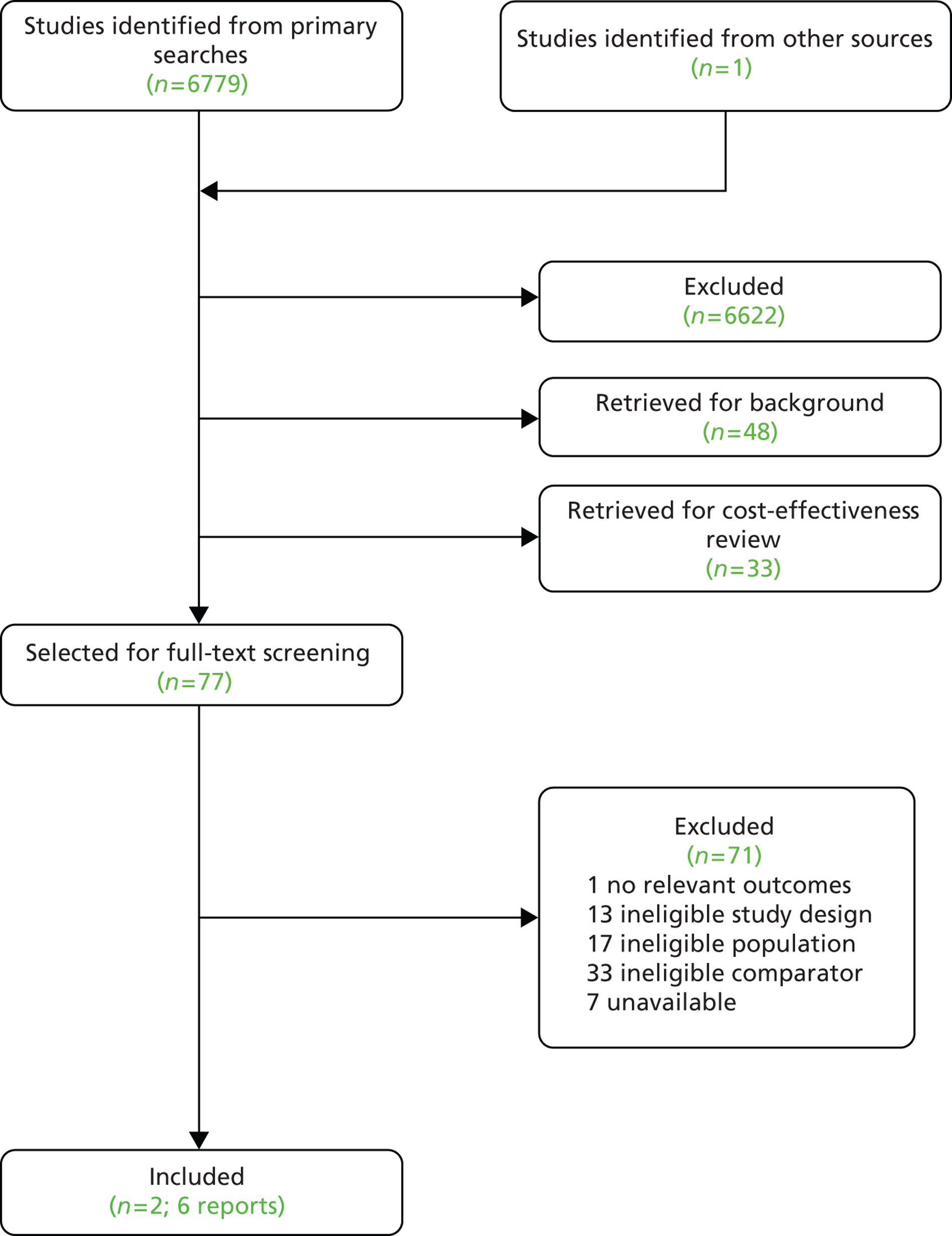
Appendix 5 provides the bibliographic details of the included and excluded studies.
Number and type of studies excluded
A list of the 71 full-text papers that were excluded along with the reasons for their exclusion is given in Appendix 5. These reports were excluded because they failed to meet one or more of the inclusion criteria in terms of the type of study, participants, intervention/comparator or outcomes reported.
Characteristics of the included studies
The search identified two randomised trials, Schmidt et al. 113 and Schmidt et al. ,46 published in full in six reports. 46,55,77,78,113,114 Participants were enrolled consecutively in both trials and data were collected prospectively. Both trials were conducted in Norway.
Table 1 presents the demographic information from the studies. The two trials included a total of 201 participants at enrolment. There were no dropouts during the study period and all 201 randomised participants were included in the statistical analyses. Participants’ diagnosis was uncomplicated symptomatic gallstones (biliary pain only) in Schmidt et al. 113 and acute cholecystitis in Schmidt et al. 46 In total, 149 women and 52 men were assessed. Of these, 72 women and 27 men were randomised to surgery, while 77 women and 25 men were randomised to observation. Median age was 50 years (range 20–79 years) in Schmidt et al. 113 and 58 years (range 27–77 years) in Schmidt et al. 46 Both trials enrolled some participants who had previously presented to secondary care for gallstone disease: 30 participants (22%) in Schmidt et al. 113 and 11 participants (17%) in Schmidt et al. 46 Both trials also reported the number of participants with concomitant diseases, specifically heart disease, diabetes and/or obstructive lung disease, in total 16 participants (12%) in Schmidt et al. 113 and 12 participants (19%) in Schmidt et al. 46 Baseline QoL, as assessed by the psychological general well being index (PGWB), Nottingham Health Profile (NHP) part II, pain score and Visual Analogue Pain Score (VAPS), were comparable across the studies. The mean follow-up was 14 years (range 13–16 years) for both trials. Similarly, the median follow-up was 14 years in Schmidt et al. 46
| Characteristics | Schmidt et al. 2011113 | Schmidt et al. 201146 | ||
|---|---|---|---|---|
| Surgery (n = 68) | Observation (n = 69) | Surgery (n = 31) | Observation (n = 33) | |
| Sex | ||||
| Men (%) | 13 (19) | 12 (17) | 14 (45) | 13 (39) |
| Women (%) | 55 (81) | 57 (83) | 17 (55) | 20 (61) |
| Age (years) | ||||
| Men (median, range) | 52 (27–74) | 60 (30–79) | 64 (41–77) | 64 (29–73) |
| Women (median, range) | 52 (20–77) | 48 (22–75) | 58 (27–77) | 47 (29–71) |
| Age overall (median, range) | 50 (20–79) | 58 (27–77) | ||
| Diagnosis | Uncomplicated symptomatic gallstones (biliary pain only) | Acute cholecystitis | ||
| Previous gallstones attacks (%) | 64 (94) | 63 (91) | NR | NR |
| Previous hospitalisation for gallstone disease (%) | 14 (21) | 16 (23) | 11 (17) | |
| Concomitant disease (heart disease/diabetes/obstructive lung disease) (%) | 16 (12) | 12 (19) | ||
| Baseline QoL | ||||
| PGWB score (higher score better) | 93.7 (n = 63) | 95.2 (n = 67) | 88.1 (n = 31) | 94.2 (n = 31) |
| NHP score (lower score better) | 2.0 (n = 50) | 1.8 (n = 53) | 2.2 (n = 28) | 2.0 (n = 29) |
| Pain score (lower score better) | 6.3 (n = 67) | 6.7 (n = 66) | 8.1 (n = 31) | 6.6 (n = 33) |
| VAPS (lower score better) | 47.2 (n = 67) | 48.1 (n = 65) | 57.7 (n = 31) | 57.1 (n = 31) |
Table 2 presents the key features of the two included studies.
| Study | Geographical location | Source of funding | No. of participants randomised | Diagnosis | Primary outcomes (patient level) | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|---|---|---|
| Schmidt et al. 2011113 (Vetrhus et al. 2002, Vetrhus et al. 2004)77,113 | Norway | Research Council of Norway; Research Committee of Rogaland Central Hospital; University of Bergen; Helga Semb’s Foundation Karla and Arne Oddmar’s Foundation Haraldsplass Deaconal Hospital and the Western Norway Regional Health Authority through the Centre for Clinical Research at Haukeland University Hospital |
Total number randomised: 137 Randomised to observation: 69 Randomised to surgery: 68 |
Uncomplicated symptomatic gallstones (biliary pain only) Symptomatic gallstone disease was defined as episodes of pain, commonly continuous, in the right subcostal or midline epigastric area, lasting more than 30 minutes with ultrasonographic signs of gallstones and no clinical or laboratory indication of other causes of the symptoms |
|
People with uncomplicated symptomatic gallstone disease | Age < 18 years or > 80 years Pregnancy Serious concomitant disease Suspected common bile duct stone People with infrequent and/or minimal pain needing only very occasional medication People with dyspeptic symptoms only |
| Schmidt et al. 201146 (Vetrhus et al. 2003, Vetrhus et al. 2005)55,78 | Norway | Research Council of Norway; Research Committee of Stavanger University Hospital; University of Bergen; Helga Semb’s Foundation Karla and Arne Oddmar’s Foundation Haraldsplass Deaconal Hospital and the Western Norway Regional Health Authority through the Centre for Clinical Research at Haukeland University Hospital |
Total number randomised: 64 Randomised to observation: 33 Randomised to surgery: 31 |
Acute cholecystitis was defined as acute abdominal pain, commonly in the right subcostal or midline epigastric area with a duration of > 8 hours and tenderness on clinical examination in the upper right quadrant. This was confirmed by the presence of gallbladder stones and inflammation signs on ultrasonography and by clinical biochemistry data |
|
People with AC | Age < 18 years or > 80 years; severe concomitant disease; suspected common bile duct stone; acalculous cholecystitis; localised peritonitis suggesting gallbladder perforation or gangrenous cholecystitis; people’s preferences; people who needed urgent treatment with surgery or percutaneous management |
Risk of bias of the included studies
The two published trials were assessed using The Cochrane Collaboration’s risk of bias tool. 112 Tables 3 and 4 and Figures 4 and 5 present the summary findings of this assessment. Both trials were judged to be at low risk for all domains assessed by the tool.
| Schmidt et al. 2011 | Judgement | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Low | Randomisation was by a computer program |
| Allocation concealment (selection bias) | Low | Brown, opaque, sealed envelopes were used to conceal the allocation |
| Blinding of participants and personnel (performance bias) | Low | Blinding was not an option, as the intervention being delivered would have been obvious to all concerned |
| Blinding of outcome assessment (detection bias) | Low | Blinding was not possible as outcomes were either participants’ self-reports or retrieved from medical records |
| Incomplete outcome data (attrition bias) | Low | Details of exclusions and attrition were fully reported. All participants were included in the analysis |
| Selective reporting (attrition bias) | Low | All stated outcomes were reported |
| Other sources of bias (reporting bias) | Low | No other potential sources of bias were identified |
| Schmidt et al. 2011 | Judgement | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Low | Randomisation was by a computer program |
| Allocation concealment (selection bias) | Low | Brown, opaque, sealed envelopes were used to conceal the allocation |
| Blinding of participants and personnel (performance bias) | Low | Blinding was not an option, as the intervention being delivered would have been obvious to all concerned |
| Blinding of outcome assessment (detection bias) | Low | Blinding was not possible as outcomes were either participants’ self-reports or retrieved from medical records |
| Incomplete outcome data (attrition bias) | Low | Details of exclusions and attrition were fully reported. All participants were included in the analysis |
| Selective reporting (attrition bias) | Low | All stated outcomes were reported |
| Other sources of bias (reporting bias) | Low | No other potential sources of bias were identified |
FIGURE 4.
Risk of bias graph for both included studies.
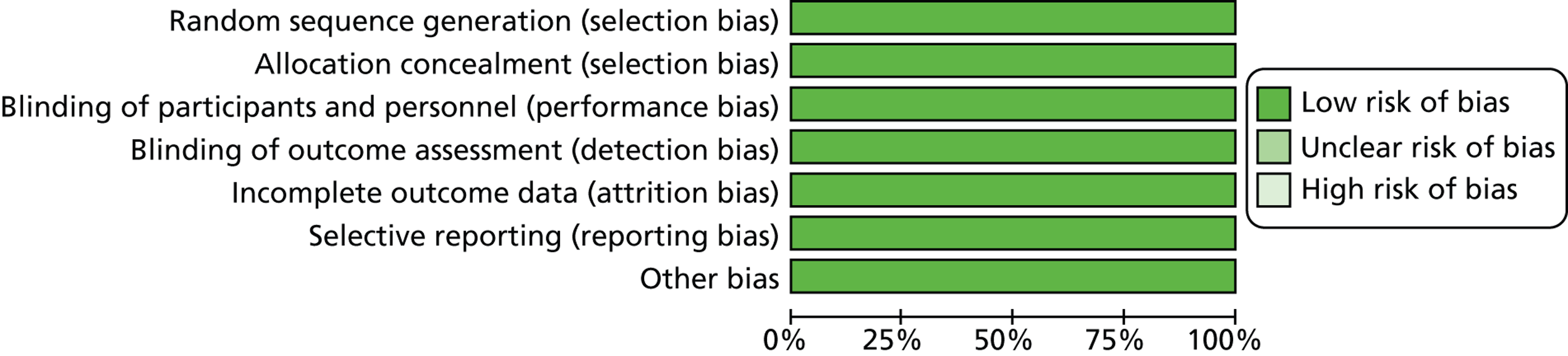
FIGURE 5.
Risk of bias summary figure.
Assessment of clinical effectiveness
A detailed description of the main clinical findings of Schmidt et al. 113 and Schmidt et al. 46 is given in Table 5. Meta-analyses of relevant clinical results were performed, when possible. Fixed-effects models were used only where there were acceptable levels of homogeneity between the studies (as identified by visual inspection of forest plots and from Mantel–Haenszel chi-squared and I2 tests), otherwise random-effects models were preferred.
| Outcomes and adverse events | Schmidt et al. 2011113 (n = 137) | Schmidt et al. 201146 (n = 64) | ||||||
|---|---|---|---|---|---|---|---|---|
| Observation (n = 69) | Surgery (n = 68) | Observation (n = 33) | Surgery (n = 31) | |||||
| 5 years | 14 years | 5 years | 14 years | 5 years | 14 years | 5 years | 14 years | |
| No. patients undergoing surgery (%) | 35 (51) | 35 (51) | 60 (88) | 60 (88) | 10 (30) | 11 (33) | 27 (87) | 27 (87) |
| Pain attacksa | NR | 23 (33) | NR | 8 (12) | NR | 3 (9) | NR | 4 (13) |
| Gallstone-related complicationsa (%) | 3 (4) | 3 (4) | 1 (1) | 1 (1) | 13 (39) | 11 (33) | 3 (10) | 1(3%) |
| Acute cholecystitis | 1 | 1 | 0 | 0 | 9 | 8 | 1 | 0 |
| CBD stones | 2 | 1 | 0 | 0 | 4b | 3 | 1c | 1 |
| Acute pancreatitis | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| Surgery-related complications (%) | 5 (7) | NR | 10 (15) | NR | 2 (6) | NR | 9 (29) | NR |
| Intra-abdominal infection/bile leakage | 3 | 2 | 1 | 1 | ||||
| Wound infection/dehiscence | 1 | 1 | 0 | 0 | ||||
| Bile duct injury | 0 | 0 | 0 | 1 | ||||
| Reoperation | 1 | 1 | 0 | 0 | ||||
| Minor complications | 0 | 6 | 1 | 7 | ||||
| Admission due to gallstone-related paina (%) | 12 (17) | NR | 2 (3) | NR | 4 (12) | NR | 3 (10) | NR |
| Mortality (%) | NRd | 8 (12) | NRd | 11 (16) | 0 (0) | 8 (24) | 4 (13) | 10 (32) |
| Further surgical intervention needed | NR | NR | NR | 4 (6) ERCP (CBD stones in one) |
NR | NR | NR | NR |
| Median time to surgery (months) from randomisation (range) | NR | 28 | NR | 3 (0–168) | NR | NR | 3.6 (0.5–12.8) | 4 (1–13) |
| QoL (mean values) – 6 months | ||||||||
| PGWB score (higher score better) | 99.9 (n = 59) | NR | 103.3 (n = 54) | NR | 110.2 (n = 33) | NR | 106.2 (n = 25) | NR |
| NHP score (lower score better) | 1.6 (n = 46) | 1.1 (n = 43) | 1.1 (n = 25) | 1.2 (n = 18) | ||||
| Pain score (lower score better) | 4.1 (n = 63) | 2.2 (n = 57) | 2.1 (n = 31) | 2.0 (n = 27) | ||||
| VAPS (lower score better) | 14.7 (n = 61) | 9.4 (n = 57) | 8.1 (n = 30) | 5.4 (n = 24) | ||||
| QoL (mean values) – 12 months | ||||||||
| PGWB score (higher score better) | 101.8 (n = 60) | NR | 102.3 (n = 57) | NR | 103.1 (n = 32) | NR | 103.2 (n = 25) | NR |
| NHP score (lower score better) | 1.6 (n = 46) | 1.0 (n = 43) | 0.9 (n = 22) | 1.2 (n = 18) | ||||
| Pain score (lower score better) | 3.7 (n = 61) | 2.3 (n = 58) | 3.1 (n = 32) | 2.4 (n = 25) | ||||
| VAPS (lower score better) | 15.7 (n = 60) | 7.0 (n = 55) | 15.1 (n = 31) | 9.9 (n = 24) | ||||
| QoL (mean values) – 60 months | ||||||||
| PGWB score (higher score better) | 104.1 (n = 55) | NR | 101.6 (n = 56) | NR | 112.0 (n = 30) | NR | 102.5 (n = 25) | NR |
| NHP score (lower score better) | 1.3 (n = 47) | 1.9 (n = 52) | 0.7 (n = 27) | 1.4 (n = 23) | ||||
| Pain score (lower score better) | 2.4 (n = 56) | 2.0 (n = 56) | 1.3 (n = 31) | 2.6 (n = 24) | ||||
| VAPS (lower score better) | 11.5 (n = 56) | 5.5 (n = 55) | 6.2 (n = 31) | 11.3 (n = 24) | ||||
Overview of outcomes included in meta-analyses
Number of participants undergoing surgery (14 years)
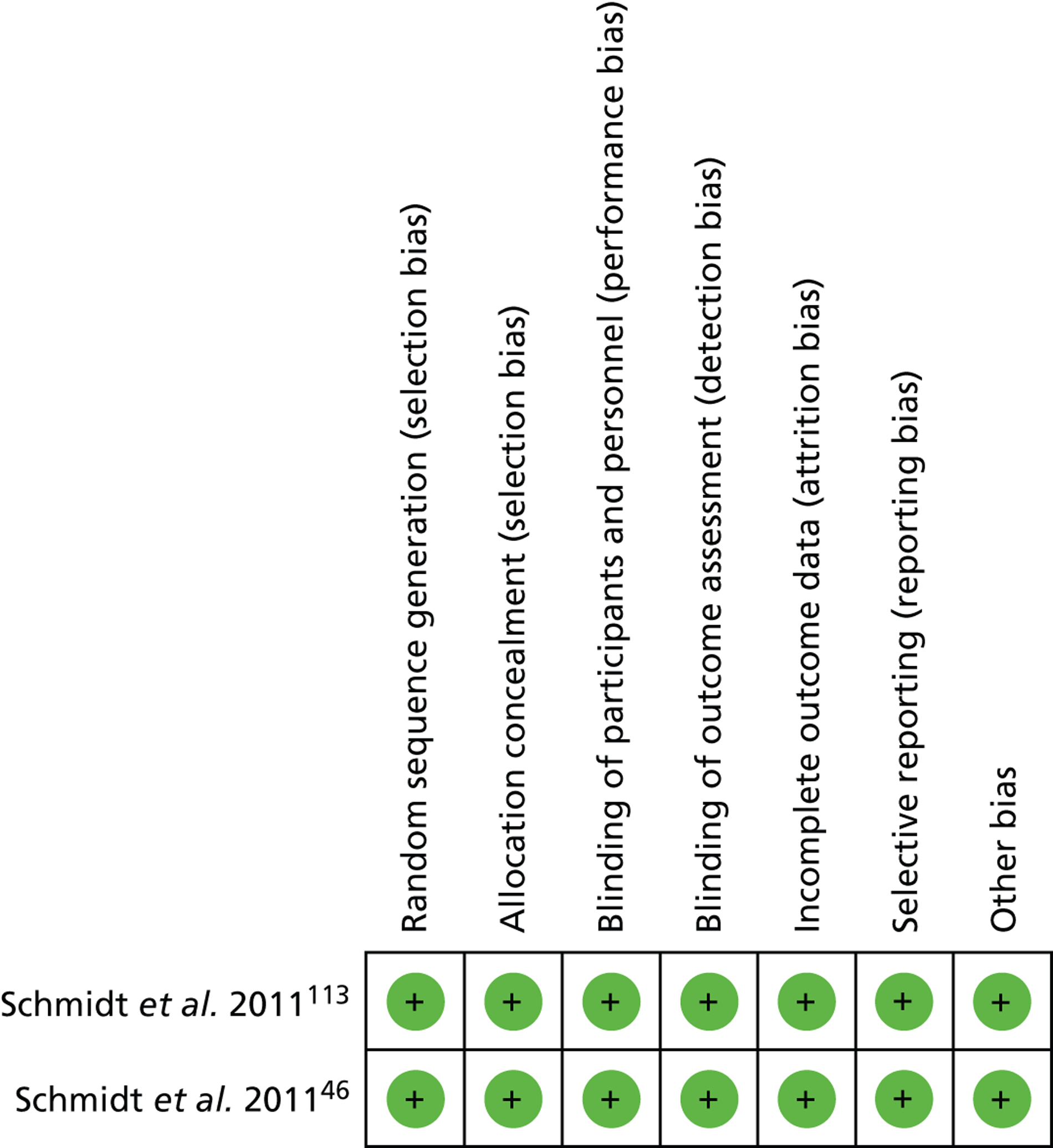
A total of 46 out of 102 (45%) participants randomised to observation and 87 out of 99 (88%) participants randomised to surgery underwent cholecystectomy (Figure 6). This was a statistically significant difference between the groups (risk ratio = 0.50; 95% CI 0.34 to 0.73; p = 0.0004).
FIGURE 6.
Forest plot of participants having surgery (14 years).
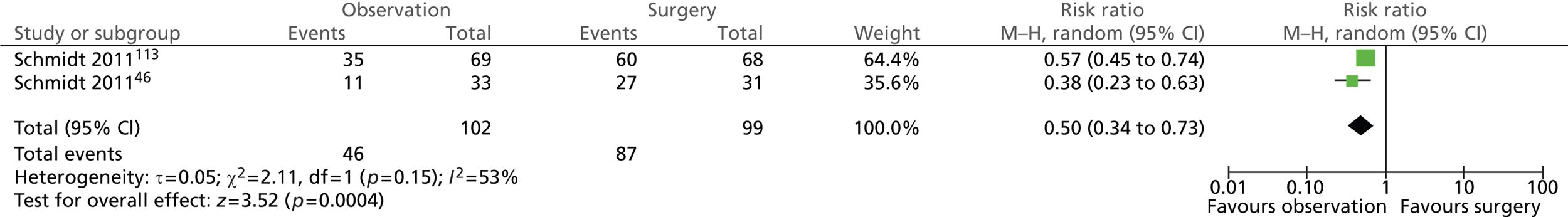
Pain attacks (14 years)
A total of 26 out of 102 (25%) participants randomised to observation and 12 out of 99 (12%) participants randomised to surgery experienced pain attacks (Figure 7). The difference between the groups was not statistically significant (risk ratio = 1.62; 95% CI 0.43 to 6.17; p = 0.48).
FIGURE 7.
Forest plot of pain attacks (14 years).
In general, pain attacks were reported more frequently in participants with uncomplicated symptomatic gallstone disease managed conservatively. One-third (33%) of participants with uncomplicated symptomatic gallstones (biliary pain only) randomised to observation experienced pain attacks after randomisation compared with only 9% of participants with acute cholecystitis. It is worth pointing out that a similar proportion of participants randomised to surgery experienced post-randomisation pain attacks across the groups (12%, Schmidt et al. ;113 13%, Schmidt et al. 46). Significantly more participants with uncomplicated symptomatic gallstones (biliary pain only) randomised to observation experienced pain attacks than those randomised to surgery (χ2 = 9.10; p = 0.0026), whereas no difference was observed between intervention groups within participants with an initial diagnosis of acute cholecystitis (χ2 = 0.24; p = 0.6253).
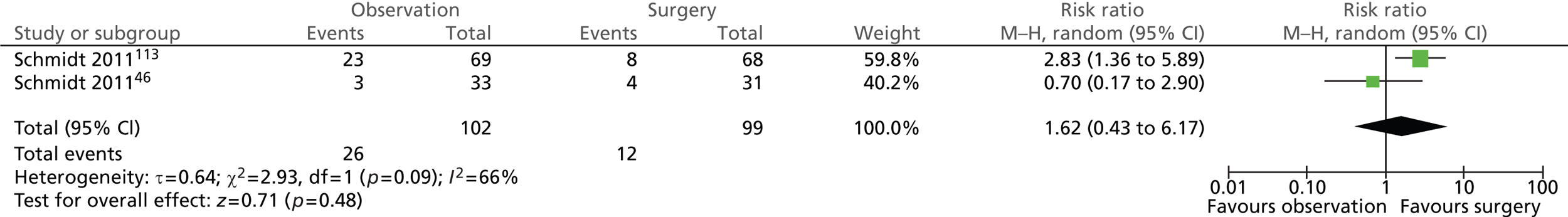
Gallstone-related complications: total (14 years)
Significantly more participants randomised to observation (14/102; 14%) than those randomised to surgery (2/99; 2%) experienced any of the specified gallstone-related complications (risk ratio = 6.69; 95% CI 1.57 to 28.51; p = 0.01) (Figure 8).
FIGURE 8.
Forest plot of total gallstone-related complications (14 years).
Details of participants with acute cholecystitis, CBD stones and acute pancreatitis are presented below.
Gallstone-related complications: acute cholecystitis (14 years)
Participants randomised to observation were more likely to suffer from acute cholecystitis as a complication (Figure 9). Nine of the 102 (9%) participants randomised to observation and none of the 99 (0%) participants randomised to surgery experienced acute cholecystitis as a complication. The difference between intervention groups was statistically significant (risk ratio = 9.55; 95% CI 1.25 to 73.27; p = 0.03).
FIGURE 9.
Forest plot of episodes of acute cholecystitis (14 years).
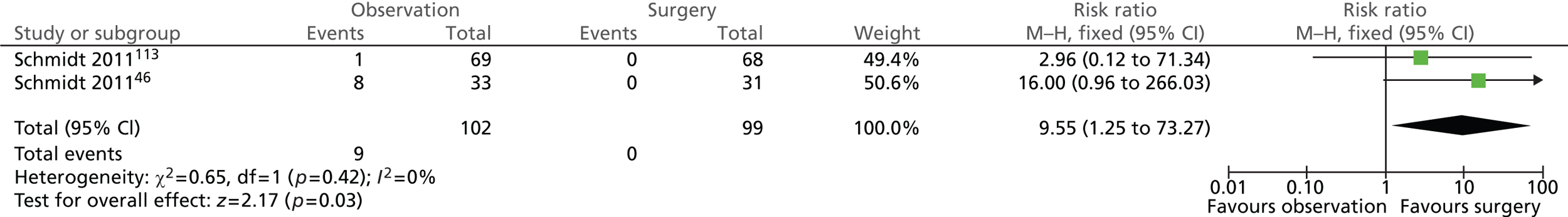
It is worth noting that, among those treated conservatively, new complicated events occurred more frequently in those in whom the initial diagnosis was acute cholecystitis than in those initially diagnosed as having uncomplicated symptomatic gallstones (biliary pain only).
Gallstone-related complications: common bile duct stones (14 years)
Few participants in both intervention groups suffered from CBD stones at the 14-year follow-up assessment (Figure 10). Four of the 102 (4%) participants randomised to observation and 1 of the 99 (1%) participants randomised to surgery experienced CBD stones. The difference between the groups was not statistically significant (risk ratio = 2.86; 95% CI 0.47 to 17.59; p = 0.26).
FIGURE 10.
Forest plot of episodes of CBD stones (14 years).
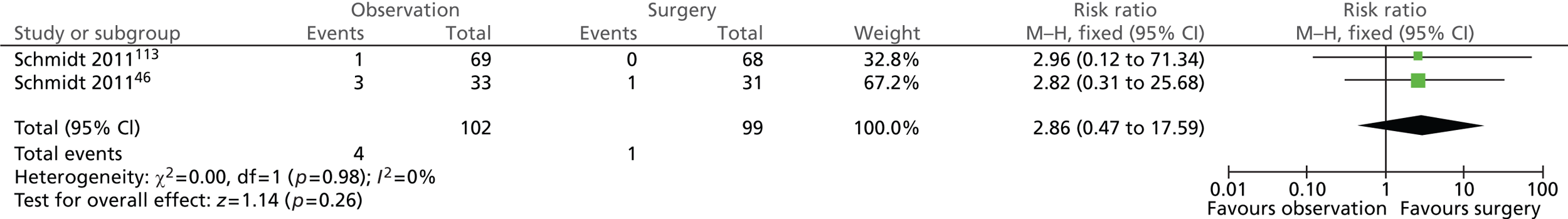
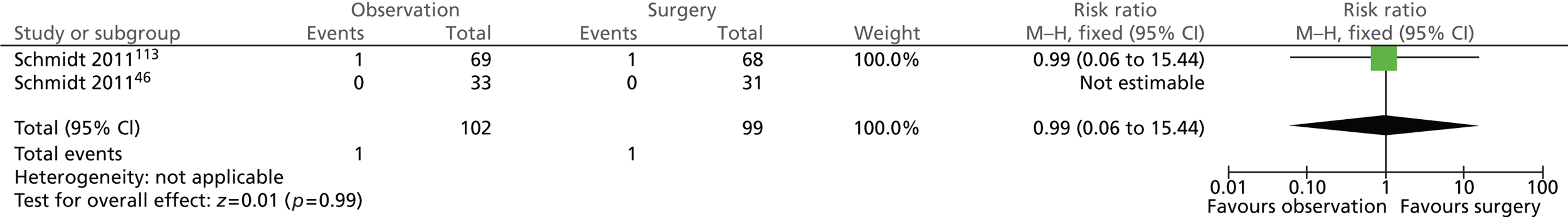
Gallstone-related complications: acute pancreatitis (14 years)
The risk of experiencing acute pancreatitis was the same in both intervention groups (Figure 11). One of the 102 (1%) participants randomised to observation and 1 of the 99 (1%) participants randomised to surgery experienced acute pancreatitis (risk ratio = 0.99; 95% CI 0.06 to 15.44; p = 0.99). Both events occurred in participants with uncomplicated symptomatic gallstones (biliary pain only) whereas participants with acute cholecystitis did not experience this complication.
FIGURE 11.
Forest plot of episodes of acute pancreatitis (14 years).
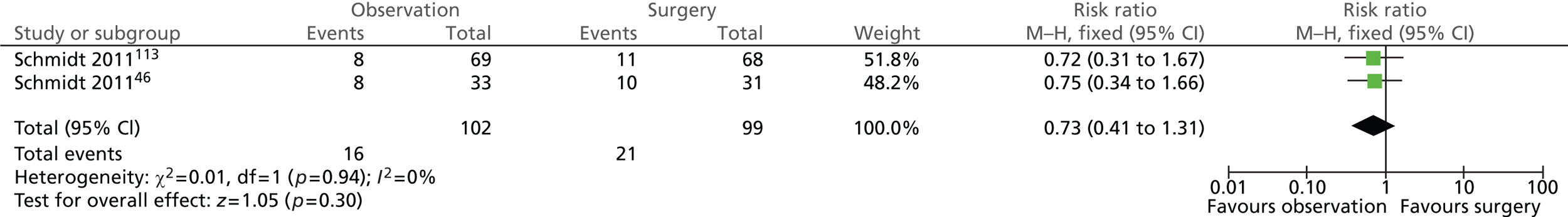
Surgery-related complications: total (5 years)
As expected, the risk of surgery-related complications was significantly lower in the observation group (Figure 12). In total, 7 out of 102 (7%) participants randomised to observation and 19 out of 99 (19%) participants randomised to surgery experienced surgery-related complications (risk ratio = 0.36; 95% CI 0.16 to 0.81; p = 0.01).
FIGURE 12.
Forest plot of total surgery-related complications (5 years).
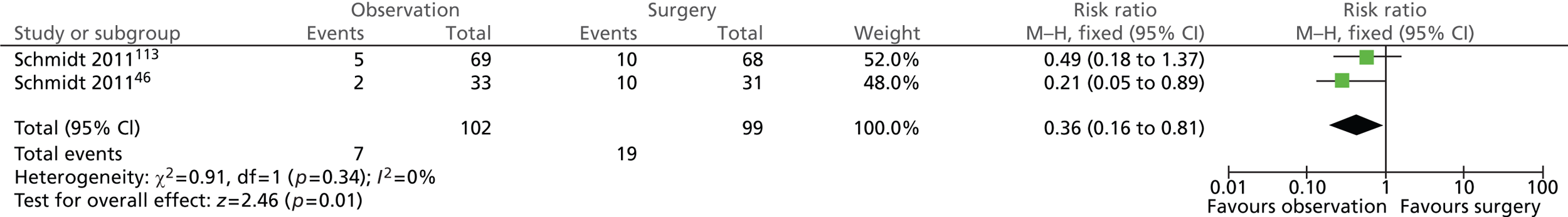
Further details of individual surgery-related complications are presented below.
Surgery-related complications: intra-abdominal infection/bile leakage (5 years)
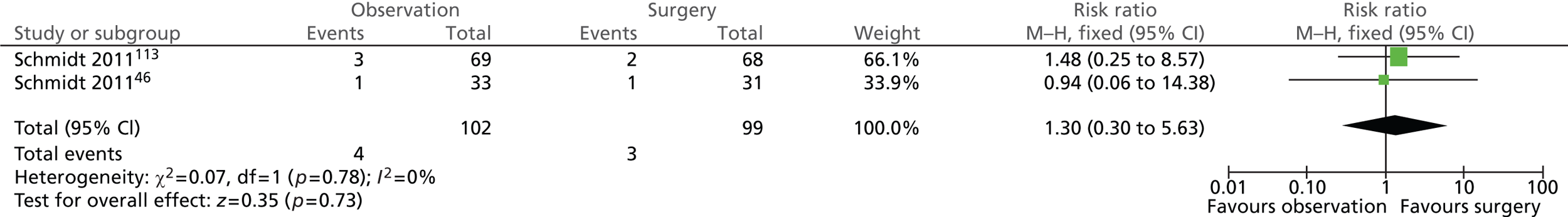
The risk of suffering from intra-abdominal infection/bile leakage was similar in both intervention groups (Figure 13). A total of 4 out of 102 (4%) participants randomised to observation and 3 out of 99 (3%) participants randomised to surgery experienced intra-abdominal infection or bile leakage (risk ratio = 1.30; 95% CI 0.30 to 5.63; p = 0.73). Five of the seven events were experienced by participants originally diagnosed with uncomplicated symptomatic gallstones (biliary pain only).
FIGURE 13.
Forest plot of intra-abdominal complications – infection/bile leakage (5 years).
Surgery-related complications: wound infection/dehiscence (5 years)
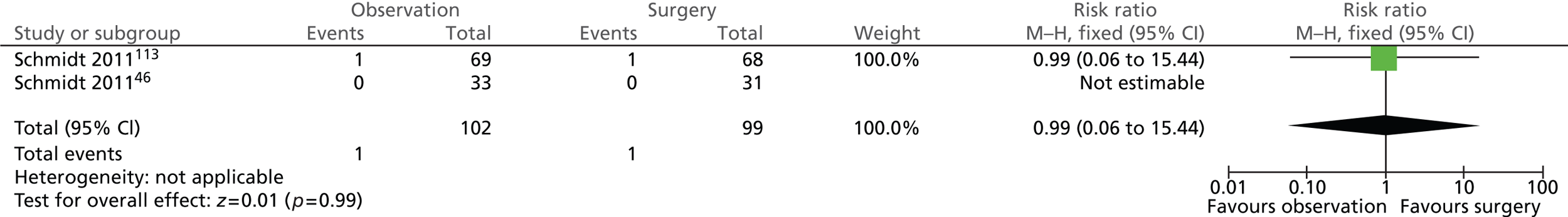
The number of participants who suffered from wound infection/dehiscence after surgery was the same in both intervention groups (Figure 14). One of the 102 (1%) participants randomised to observation and 1 of the 99 (1%) participants randomised to surgery experienced wound infection or dehiscence (risk ratio = 0.99; 95% CI 0.06 to 15.44; p = 0.99). Both events occurred in participants diagnosed with uncomplicated symptomatic gallstones (biliary pain only) whereas no participants diagnosed with acute cholecystitis experienced this complication.
FIGURE 14.
Forest plot of episodes of wound infection/dehiscence (5 years).
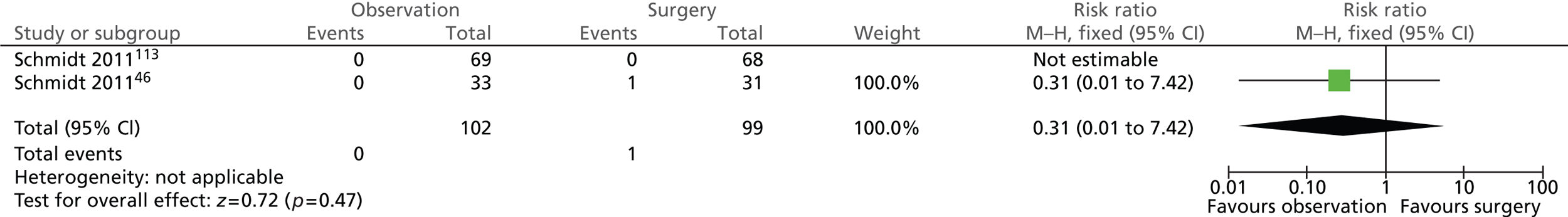
Surgery-related complications: bile duct injury
Figure 15 shows that only one participant randomised to surgery suffered from bile duct injury within 5 years (risk ratio = 0.31; 95% CI 0.01 to 7.42; p = 0.47).
FIGURE 15.
Forest plot of bile duct injury cases (5 years).
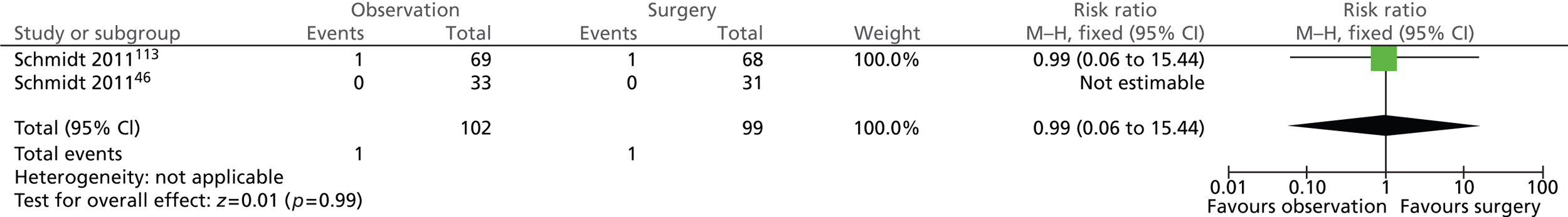
Surgery-related complications: reoperation (5 years)
The number of participants who required reoperation was the same in both intervention groups (Figure 16). One of the 102 (1%) participants randomised to observation and 1 of the 99 (1%) participants randomised to surgery underwent reoperation (risk ratio = 0.99; 95% CI 0.06 to 15.44; p = 0.99). Both participants who underwent reoperation were originally diagnosed with uncomplicated gallstones (biliary pain only).
FIGURE 16.
Forest plot of reoperation cases (5 years).
Surgery-related complications: minor complications (5 years)
Significantly fewer participants randomised to observation experienced minor complications (1/102; 1%) than participants randomised to surgery (13/99; 13%) (risk ratio = 0.11; 95% CI 0.02 to 0.56; p = 0.008) (Figure 17). A slightly larger proportion of participants originally diagnosed with acute cholecystitis experienced minor complications (8/64; 13%) than participants with uncomplicated symptomatic gallstones (biliary pain only) (6/137; 4%). The nature of minor complications was not described in the included trials.
FIGURE 17.
Forest plot of minor complications (5 years).
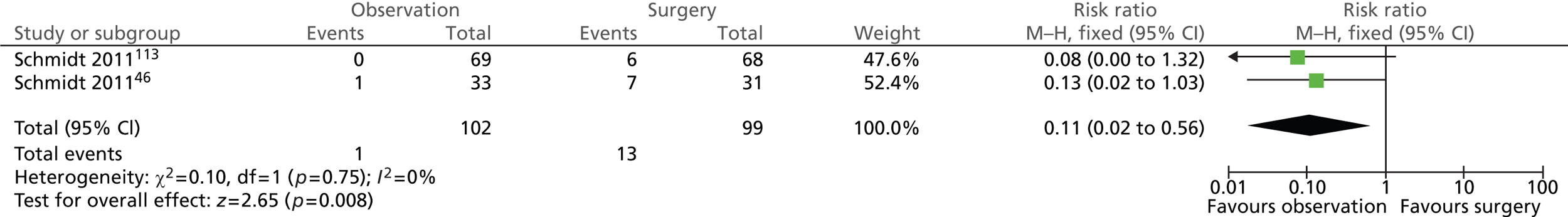
Admission due to gallstone-related pain (5 years)
A total of 16 out of 102 (16%) participants randomised to observation and 5 out of 99 (5%) participants randomised to surgery were admitted to hospital because of gallstone-related pain (Figure 18). There was no statistically significant difference between the intervention groups at the 5-year follow-up (risk ratio = 2.69; 95% CI 0.57 to 12.68; p = 0.21).
FIGURE 18.
Forest plot of number of admissions due to gallstone-related pain (5 years).
For both uncomplicated symptomatic gallstones (biliary pain only) and acute cholecystitis groups, more participants randomised to observation than to surgery were subsequently admitted to hospital for gallstone-related pain (12 vs. 2 people with biliary pain and 4 vs. 3 people with acute cholecystitis). It is worth noting that the difference between intervention groups was statistically significant for participants with uncomplicated symptomatic gallstones (biliary pain only) (χ2 = 7.79; p = 0.0052) but not for those with acute cholecystitis (χ2 = 0.10; p = 0.7542).
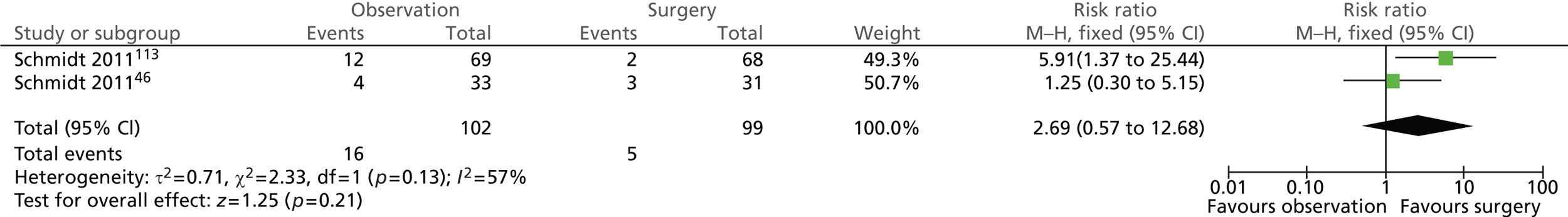
Mortality rate (14 years)
Schmidt et al. 113 reported that a total of 8 out of 137 participants with uncomplicated symptomatic gallstones (biliary pain only) had died by the 5-year follow-up, but they did not provide separate data for each intervention group. At the 14-year follow-up assessment, 8 out of 68 participants randomised to surgery and 11 out of 69 participants randomised to observation had died.
Schmidt et al. 46 reported that 4 out of 31 participants with acute cholecystitis who were randomised to surgery had died by the 5-year follow-up, whereas no deaths were observed among participants randomised to observation. By the 14-year follow-up, 8 out of 31 participants randomised to surgery and 10 out of 33 participants randomised to observation had died. None of the deaths was caused by gallstone disease.
A total of 16 out of 102 (16%) participants randomised to observation and 21 out of 99 (21%) participants randomised to surgery died (Figure 19). The difference between the groups was not statistically significant (risk ratio = 0.73; 95% CI 0.41 to 1.31; p = 0.30).
FIGURE 19.
Forest plot of number of deaths (14 years).
Relevant outcomes according to the treatment received by participants
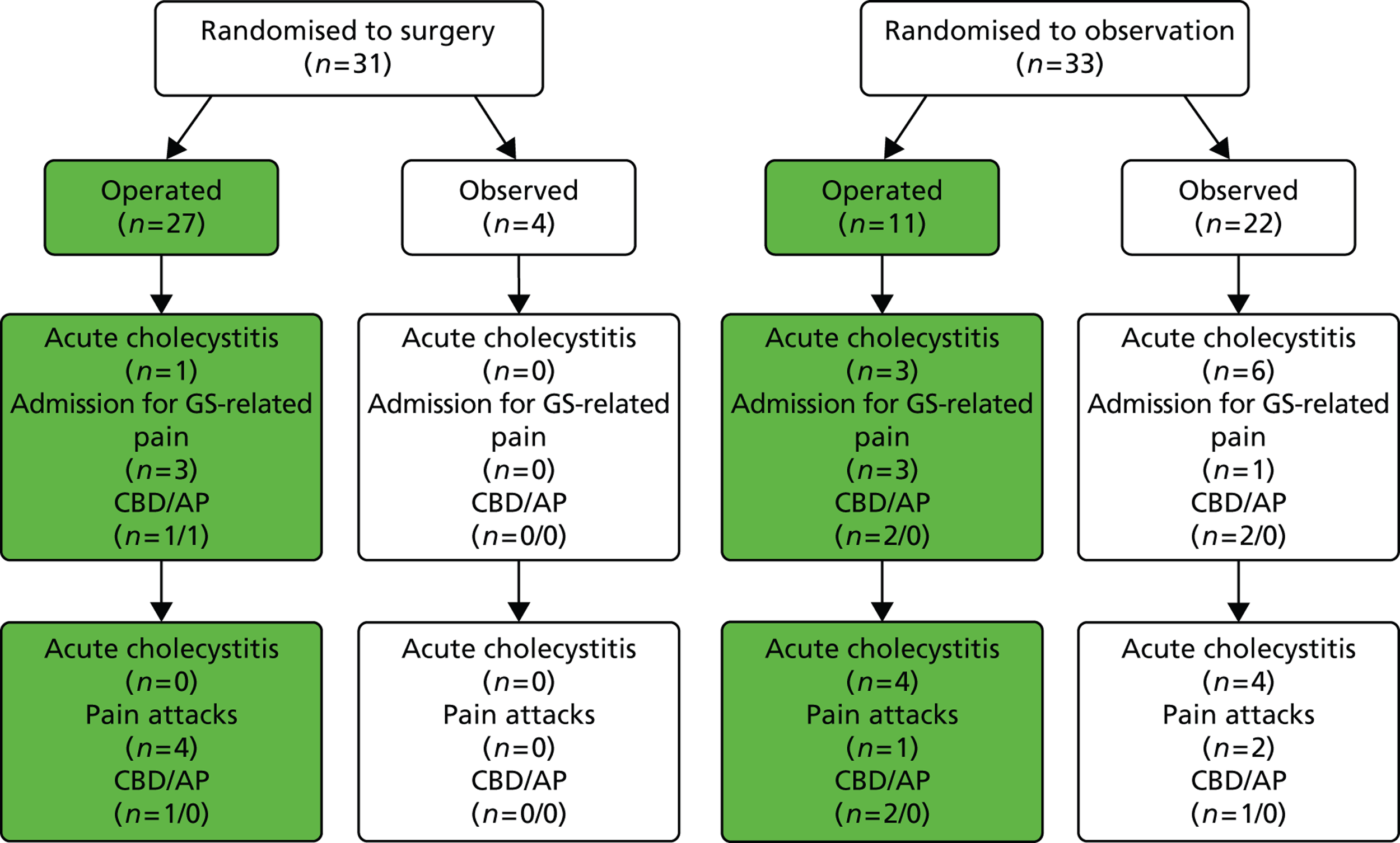
In both trials, a certain number of participants did not receive the treatment to which they were randomised but swapped over to receive the other intervention: a proportion of participants randomised to observation (46 people) underwent cholecystectomy and a proportion of participants randomised to receive surgery (12 people) opted for conservative management. The flow chart in Figure 20 illustrates the main outcomes according to the treatment that participants, in both trials, received. Figures 21 and 22 present this information for each trial (Schmidt et al. 113 and Schmidt et al. 46) separately. Note that in Figures 20 and 21 the numbers quoted for admission to hospital for gallstone-related pain for the observation group differ from those in Table 5 as a result of a discrepancy in table III of the Vetrhus et al. paper. 77 Moreover, the discrepancy between the 5 and 14 years’ findings is because outcomes at 5 years included events which occurred after randomisation but before surgery, whereas outcomes at 14 years did not include events that took place before surgery.
FIGURE 20.
Outcomes according to treatment received (both trials combined). AP, acute pancreatitis; GS, gallstone.
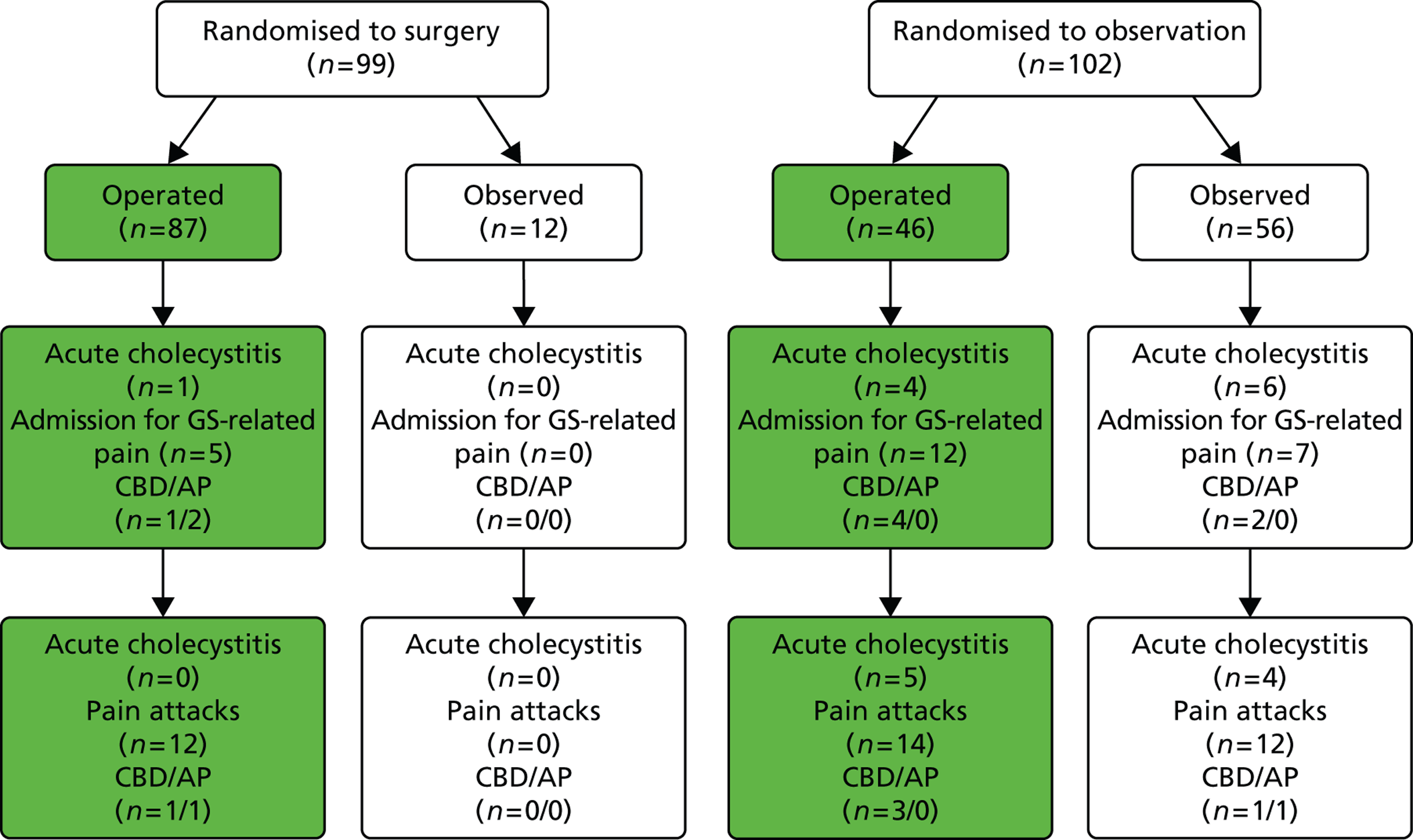
FIGURE 21.
Outcomes for the treatment received by participants with uncomplicated symptomatic gallstone disease only (Schmidt et al. 2011). 113 AP, acute pancreatitis; GS, gallstone.
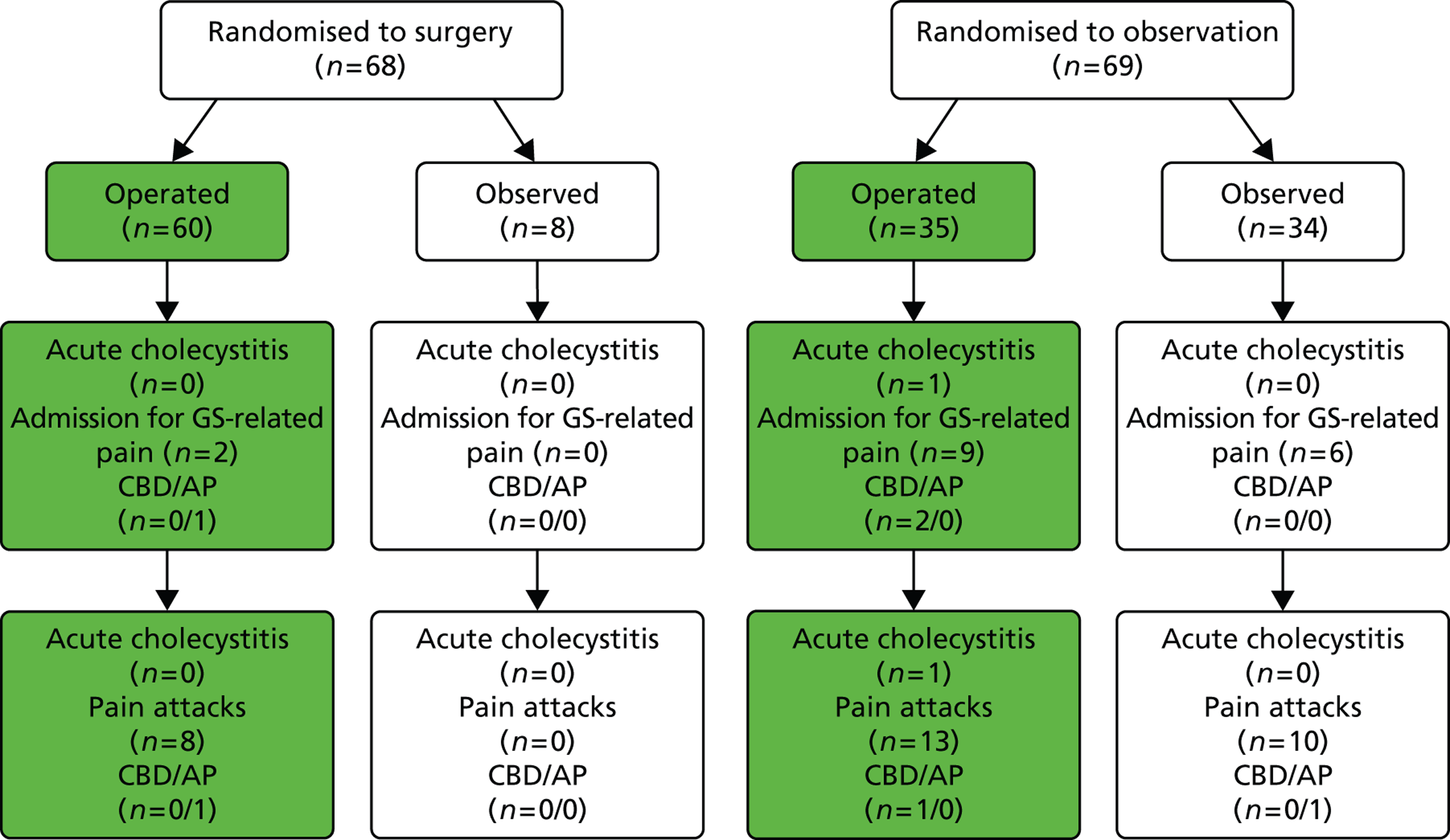
FIGURE 22.
Outcomes according to the treatment received by participants with acute cholecystitis only (Schmidt et al. 2011). 46 AP, acute pancreatitis; GS, gallstone.
Outcomes not included in meta-analyses
Further surgical intervention required
Schmidt et al. 113 reported that, at the 14-year follow-up, 4 out of 60 (7%) participants with uncomplicated symptomatic gallstones (biliary pain only) randomised to surgery who did undergo cholecystectomy subsequently required endoscopic retrograde cholangiopancreatography. Common bile duct stones were detected in one of these four participants.
Median time to surgery (from randomisation)
Schmidt et al. 113 reported that the median time to surgery for participants with uncomplicated symptomatic gallstones (biliary pain only) was 3 months (range 0–68 months) for participants randomised to surgery and 28 months for participants randomised to observation (no range was given for the observation group). Schmidt et al. 46 stated that patients who were randomised to surgery, on average, underwent an operation 4 months (median) after randomisation (range 1–13 months). No details were provided for those patients randomised to observation who eventually underwent surgery.
Quality of life
We were not able to include QoL measures in the statistical analyses as measures of variability were not reported and were not available from the trial investigators. Quality-of-life measures were assessed only at 5 years. In the paragraphs below, scores for the observation group are reported first, followed by scores for the surgery group. None of the differences between groups was statistically significant.
Quality of life: Psychological General Well Being Index
In the Schmidt et al. trial113 both intervention groups reported PGWB scores lower than 105 at 6 months, which is the score normally expected in healthy control subjects (mean score of 99.9 for the observation group and 103.3 for the surgery group, respectively). 113 This indicates a slightly poorer level of general well-being than in healthy control subjects. In addition, the observation group reported lower scores than the surgery group. This difference was, however, not statistically significant. Notably, at this time point, 7 of the 35 participants in the observation group who crossed over to surgery had actually been operated on.
At 6 months, 46 participants with acute cholecystitis reported higher scores and, therefore, greater well-being than the average score of 105 expected in healthy control subjects (mean score of 110.2 for the observation group and 106.2 for the surgery group). In contrast to the Schmidt et al. trial,113 participants in the observation group reported higher (but not significantly higher) scores than participants who underwent surgery.
At 12 months, participants in both trials reported levels of well-being lower than those expected of healthy control subjects. In the Schmidt et al. trial,113 the mean PGWB scores were 101.8 for the observation group and 102.3 for the surgery group. Similarly, in the Schmidt et al. trial,46 the mean PGWB scores were 103.1 for the observation group and 103.2 for the surgery group. It is worth noting, however, that, at this time point, participants’ scores in both trials were similar.
In both trials, participants managed conservatively reported higher well-being scores at 60 months than those who underwent surgery. In the Schmidt et al. trial,113 the mean PGWB score was 104.1 for the observation group compared with 101.6 for the surgery group, whereas in the Schmidt et al. trial46 the mean PGWB score was 112.0 for the observation group and 102.5 for the surgery group. In Schmidt et al. participants randomised to observation reported levels of general well-being greater than scores which would be expected in healthy control subjects. 115
Quality of life: Nottingham Health Profile part II
At all time points, both trials reported NHP scores of < 2, indicating a limited number of specified domains (i.e. job, housework, social life, home life, sex life, interests/hobbies, holidays) affected by gallstone disease. In particular, the mean NPH scores for the observation group and the surgery group were, respectively, 1.6 and 1.1,113 and 1.1 and 1.246 at 6 months; 1.6 and 1.0113 and 0.9 and 1.246 at 12 months; and 1.3 and 1.9113 and 0.7 and 1.446 at 60 months. In both trials, scores did not vary significantly over time or between intervention groups.
Quality of life: pain score
Pain scores among participants with uncomplicated symptomatic gallstones (biliary pain only)113 showed a tendency to decrease between 6 and 60 months, with all scores being at the lower end of the possible range of 0–16. At 6 months, participants in the observation group had a mean score of 4.1 and participants in the surgery group a mean score of 2.2. At 12 months, the mean score was 3.7 and 2.3 for the observation group and the surgery group, respectively, whereas at 60 months the scores were 2.4 and 2.0, respectively. Pain scores were consistently higher for the observation group than for the surgery group (albeit the difference was not statistically significant).
Mean scores among participants with acute cholecystitis46 were also generally at the low end of the range (2.1 and 2.0 at 6 months, 3.1 and 2.4 at 12 months and 1.3 and 2.6 at 60 months for the observation group and surgery group, respectively). Scores for the surgery group increased between 6 and 60 months while scores for the observation group increased from 6–12 months and then decreased between 12 and 60 months. Furthermore, at 60 months, pain score for the surgery group was greater than that of the observation group (the only time point at which this occurred in either of the two trials).
Quality of life: Visual Analogue Pain Score
Schmidt et al. 113 reported mean VAPS at the lower end of the possible range (0–100) among participants with biliary pain only (14.7 and 9.4 at 6 months, 15.7 and 7.0 at 12 months and 11.5 and 5.5 at 60 months for the observation group and surgery group, respectively). In particular, scores in the observation group were consistently higher (but not significantly higher) than those in the surgery group. Pain scores tended to decrease in the surgery group between 6 and 60 months, whereas in the observation group scores increased between 6 and 12 months and subsequently decreased to pre-12-month levels at 60 months.
Mean VAPS in participants with acute cholecystitis allocated to surgery46 increased over time while in participants managed conservatively it varied over time, with a similar trend to that observed in the Schmidt et al. trial (8.1 and 5.4 at 6 months, 15.1 and 9.9 at 12 months and 6.2 and 11.3 at 60 months for the observation group and surgery group, respectively). 113
Summary
This assessment was based on the clinical data derived from two RCTs (published in six reports) with a total of 201 participants. Both trials were judged to be at low risk of bias. Table 6 presents a summary of the outcomes which were included in the meta-analyses. A summary of the clinical effect size for outcomes assessed at 5 years and for those assessed at 14 years is presented in Figures 23 and 24, respectively.
| Event | Risk ratio (95% CI) | Test for overall effect (p-value) |
|---|---|---|
| Undergoing surgery (14 years) | 0.50 (0.34 to 0.73) | 0.0004 |
| Pain attacks (14 years) | 1.62 (0.43 to 6.17) | 0.48 |
| Mortality (14 years) | 0.73 (0.41 to 1.31) | 0.30 |
| Gallstone-related complications (all 14 years) | ||
| Total | 6.69 (1.57 to 28.51) | 0.01 |
| Acute cholecystitis | 9.55 (1.25 to 73.27) | 0.03 |
| CBD stones | 2.86 (0.47 to 17.59) | 0.26 |
| Acute pancreatitis | 0.99 (0.06 to 15.44) | 0.99 |
| Surgery-related complications (all 5 years) | ||
| Total | 0.43 (0.21 to 0.91) | 0.03 |
| Intra-abdominal infection/bile leakage | 1.30 (0.30 to 5.63) | 0.73 |
| Wound infection/dehiscence | 0.99 (0.06 to 15.44) | 0.99 |
| Bile duct injury | 0.31 (0.01 to 7.42) | 0.47 |
| Reoperation | 0.99 (0.06 to 15.44) | 0.99 |
| Minor complications | 0.11 (0.02 to 0.56) | 0.008 |
| Admission due to gallstone-related pain (5 years) | 2.69 (0.57 to 12.68) | 0.21 |
FIGURE 23.
Summary of meta-analyses results (5-year data) [risk ratio (95% CI)].
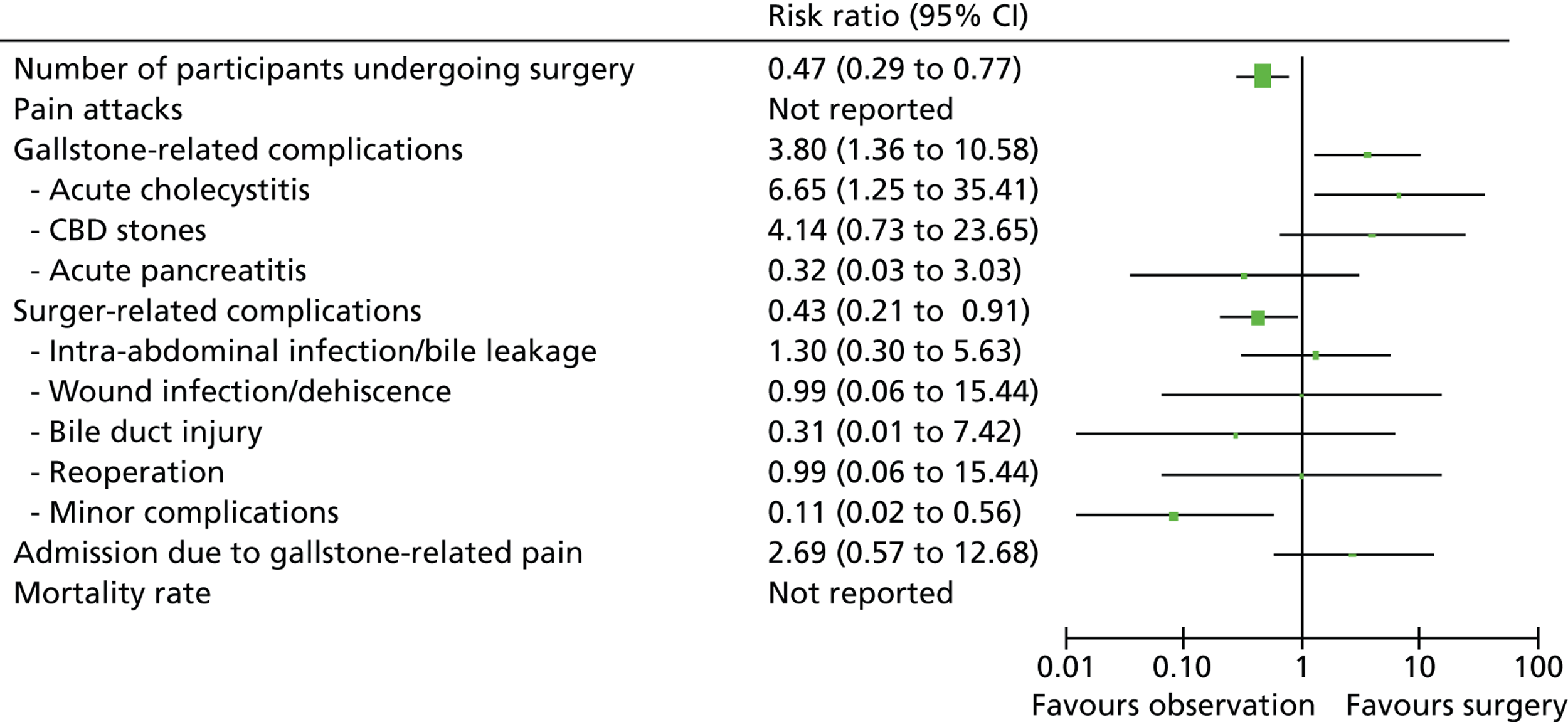
FIGURE 24.
Summary of meta-analyses results (14-year data) [risk ratio (95% CI)].
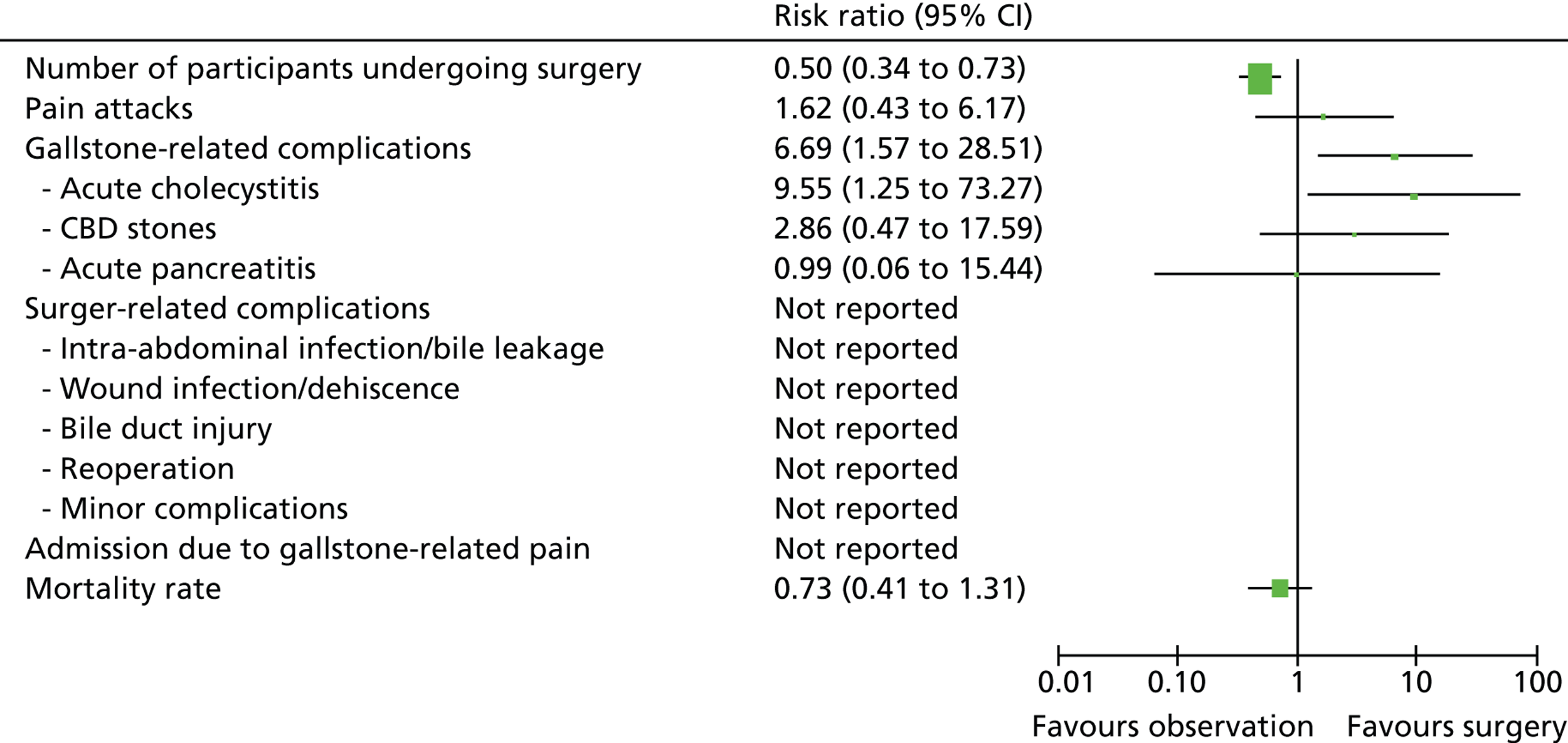
Participants randomised to surgery were significantly more likely to undergo surgery and had a greater risk of experiencing surgery-related complications as well as minor complications. Participants randomised to observation were at significantly greater risk of experiencing gallstone-related complications, in particular acute cholecystitis. Within participants with initial diagnosis of uncomplicated symptomatic gallstone (biliary pain only), those randomised to observation were more likely to experience pain attacks and be admitted to hospital for gallstone-related pain than those randomised to surgery. Mortality risk was greater (but not significantly greater) among participants randomised to surgery.
Chapter 5 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
A formal systematic review of economic evaluations studies was not attempted as initial scoping literature searches indicated that there were no existing economic evaluations comparing conservative management with surgery from the perspective of the UK NHS. Only two cost–utility papers based on the UK setting were identified, but they compared early with delayed laparoscopic cholecystectomy. 116,119 Therefore, this chapter focuses on presenting the methods and the results of a de novo economic model.
Economic modelling
Model development
A de novo economic model was developed to compare alternative treatment strategies for people with symptomatic gallstone disease (biliary pain or cholecystitis). According to the purpose of this assessment two strategies were considered:
-
conservative management
-
surgical management (cholecystectomy).
The structure of the model was informed by the findings of the trials included in the systematic review of clinical effectiveness, other existing evidence, as well as by the advice from health-care professionals within the research team for this assessment. The perspective adopted for the analysis is that of the UK NHS and the Personal Social Services.
The assumed care pathway of the model
The model described in this chapter is based on two clinically relevant care pathways for gallstone disease: a surgery care pathway and a conservative management care pathway (Figures 25 and 26). Both pathways were derived from the care pathway reported in Chapter 2 (Definition of the decision problem). As the current standard treatment for gallstone disease in the UK is cholecystectomy, the conservative management pathway portrays a plausible pathway that people with gallstones can follow.
FIGURE 25.
Surgery care pathway. Rectangles represent treatment options; ovals represent health states; arched arrows above the ovals represent health states in which people may return; and arrows indicate the possible directions in which individuals could move at the end of each cycle.
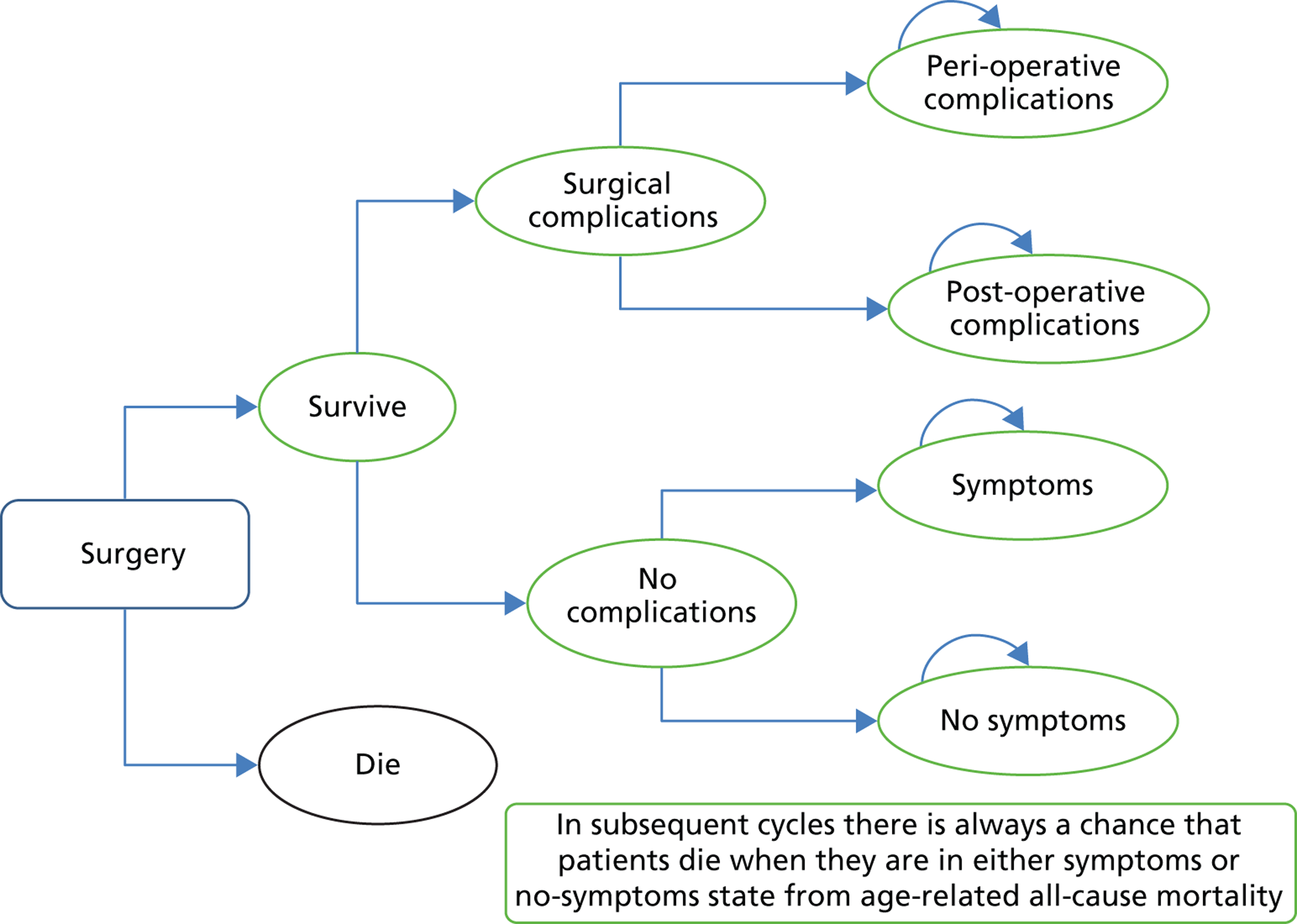
FIGURE 26.
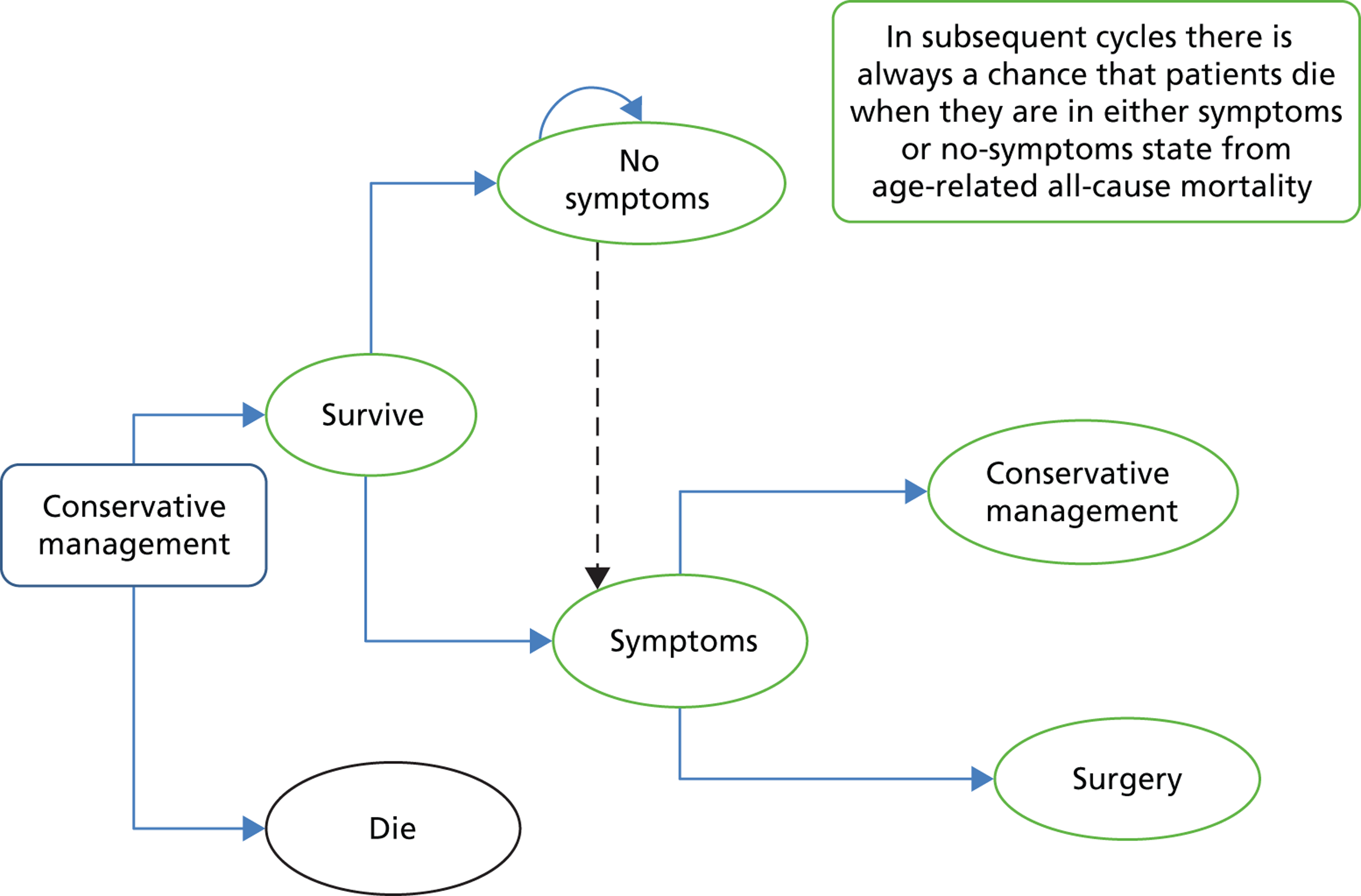
Conservative management care pathway. Rectangles represent treatment options; ovals represent health states; arched arrows above the ovals represent health states in which people may return; and arrows indicate the possible directions in which individuals could move at the end of each cycle.
The first care pathway (Figure 25) considers patients who are treated with surgery (mainly laparoscopic cholecystectomy). There are five health states that could arise from the surgical management: peri- and post-operative complications, no symptoms, post-surgical symptoms (such as pain that persists after surgery) and death. Patients who survive surgery can either have no complications or suffer from peri- or post-operative complications. These complications can either be managed without any further surgical intervention or require further surgery (e.g. surgery for bile duct injury). Those patients who experience no complications could end up with symptoms or they could have no symptoms. In subsequent cycles there is always a chance that patients die when they are in either a symptom or no symptom state from all-cause age-related mortality.
The second pathway (Figure 26) considers three health states for patients with gallstone disease who are managed conservatively: no symptoms, symptoms or death. Patients who are free from symptoms after conservative treatment have a chance of staying in this state (no symptoms) or they may continue to experience the recurrence of symptoms over time and, therefore, move into a symptoms state. Patients who continue to experience symptoms have a chance of continuing to be treated conservatively or they can be offered surgery. As in the surgery pathway, patients always have a chance of dying from all-cause age-related mortality. The key outcome of the conservative management (number of patients without symptoms) will be influenced by whether or not the treatment resolves the symptoms and, therefore, whether or not patients seek any further medical intervention, in particular surgery.
Model structure
A Markov model was developed to model the alternative management strategies (i.e. conservative management or surgery) that patients with symptomatic gallstone disease may be offered over time and to provide estimates of costs, clinical effectiveness [measured in quality-adjusted life-years (QALYs)] and cost-effectiveness (Figure 27). The model estimates the costs and consequences of both interventions and of any further events – such as the treatment for recurrent symptoms and/or complications. The model was developed using TreeAge Pro 2012 (TreeAge Software, Inc., Williamstown, MA, USA).
FIGURE 27.
Markov model structure.
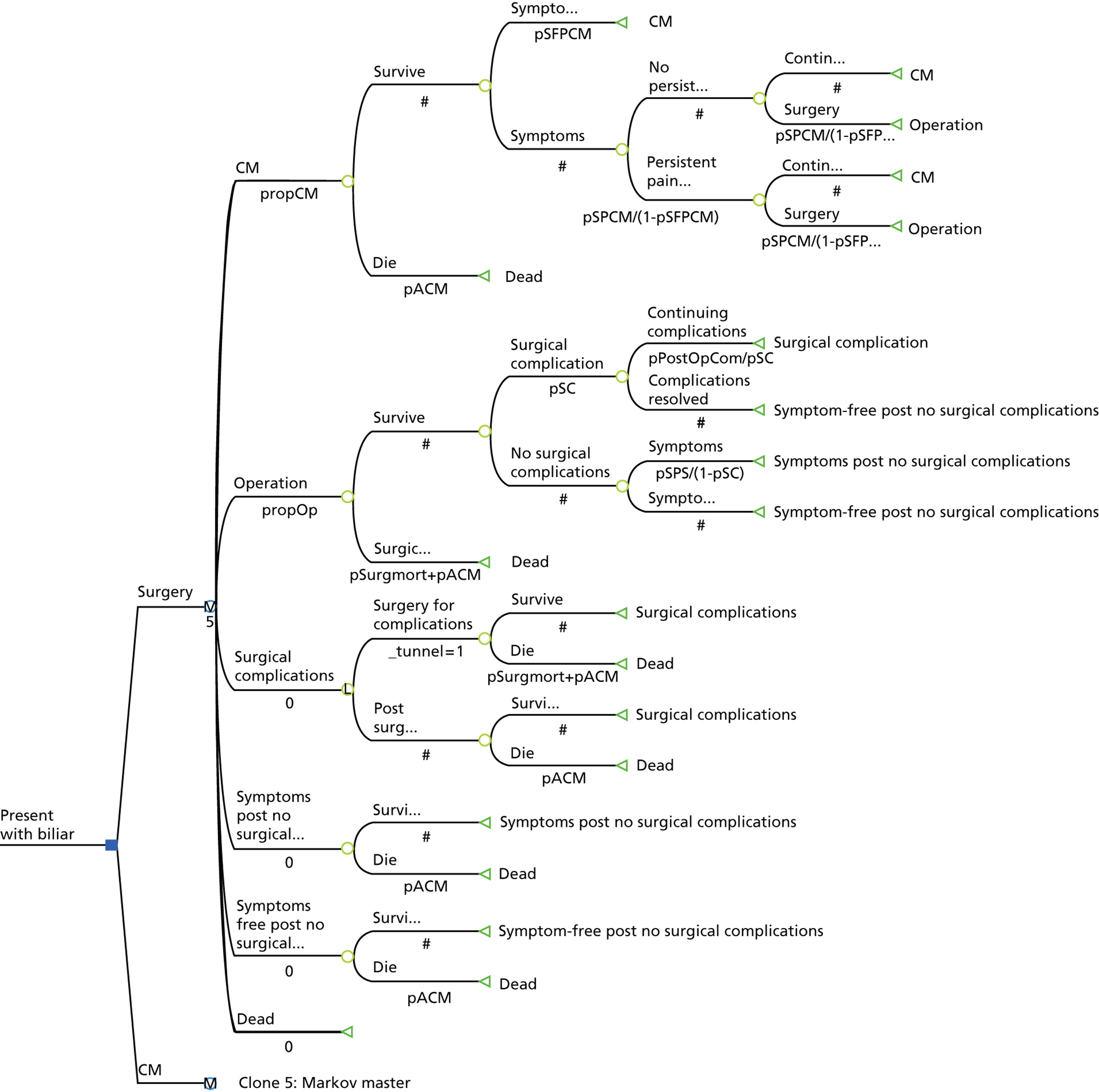
The model was based on a hypothetical cohort of patients presenting with symptomatic gallstone disease (biliary pain or cholecystitis). In the model, symptomatic patients receive an initial treatment of either conservative management or surgery (cholecystectomy) and then move into different health states, either surviving or dying. The assumption that over a given period of time a patient could die is always built into the model. Death takes into account that patients suffering from gallstone disease (i) are exposed to a very small risk of death when they undergo surgery and (ii) have a chance of dying from all other causes at any point in time (this chance is assumed to be equivalent to that of all-cause age-related mortality). 118 Death is referred to as an absorbing state (an absorbing state in a Markov model is a state that once entered cannot be exited again). Those patients who survive cholecystectomy end up in one of two health states: they can either have peri- or post-operative complications or they can have no complications for the remainder of the cycle. Those patients who have post-operative complications (e.g. bile duct injury, port incisional hernia) remain in the complications post-surgery state for the remainder of the cycle.
Similarly, those patients who have conservative management end up in one of two health states: their symptoms can either be resolved or they can persist continuously or intermittently over time. It is assumed that no further treatment is sought by those whose symptoms resolve. Those individuals who continue to experience symptoms can either undergo surgery or they can continue to be treated conservatively. In the model it is assumed that all individuals who continue to experience symptoms will present for surgery within 5 years, so by the end of 5 years all the people left under conservative management are symptom free.
The following are the key assumptions of the economic modelling exercise:
-
The time horizon of the model is 5 years. This time horizon was chosen as long-term follow-up data46 indicate that the majority of people with symptomatic gallstones undergo cholecystectomy during the first 5 years.
-
The cycle length (i.e. the time period by which transitions between health states are modelled) is 1 year.
-
In the base case, the typical person with symptomatic gallstone disease entering the model is a female of 51 years of age. This choice is justified by the average age at which people currently undergo surgery to remove their gallbladder and by the fact that women are more likely to suffer from gallstone disease than men. 84
The results of the model are presented in terms of incremental cost per QALY. The costs and benefits incurred beyond 1 year were discounted at 3.5% per year in accordance with the current National Institute for Health and Care Excellence (NICE) guidelines. 119
Data requirements and model inputs
Estimations of model probabilities
The model uses probabilities:
-
complications after surgery
-
resolution of complications
-
resolution of symptoms following surgery (symptomatic people can be cured from their symptoms – cholecystitis/biliary pain following surgery)
-
continuing to experience symptoms following initial conservative management;
-
transitioning to surgery following conservative management (assumed to be concentrated in those who continue to experience symptoms
-
surgical and all-cause mortality.
Estimates of probabilities of the events were derived from the results of the systematic review of clinical effectiveness reported in Chapter 6 as well as other UK sources. For probabilistic sensitivity analyses, appropriate probability distributions were assigned.
Complications during and after laparoscopic surgery
According to the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) the current rate of major bile duct injury in laparoscopic cholecystectomy has stabilised at 0.1–0.6%120–126 and series with no major bile duct injuries have been reported. 127 Although many believe that the rate of major bile duct injury in open cholecystectomy is lower than laparoscopic cholecystectomy, controversy remains. 125 Post-operative complications include bile leak, retained stone, wound infection, pneumonia and bile duct injury. The estimate used in the model for the probability of having peri-operative complications was 0.1 (10%), inclusive of those patients who had their laparoscopic cholecystectomy converted into open cholecystectomy. This value was based on advice from clinical experts and is a conservative assumption (in the sense that it is likely to be a lower rate and, based on SAGES data; therefore, our estimates of cost-effectiveness of surgery are less favourable than they would have been if SAGES data had been used). An estimate for the proportion of patients whose complications require further surgery was based on the information available to patients who undergo cholecystectomy in the NHS in England and expert opinion. 128 These values vary from 0.01 to 0.002 (1/100 to 1/500) and the estimate used in the base analysis was 0.01 (1/100).
Symptoms after surgery
Although cholecystectomy is the preferred treatment for symptomatic gallstones, the relationship between cholecystectomy and post-cholecystectomy symptoms is still unclear. 63 Individuals with gallstones have various abdominal symptoms that may be caused by gallstones or may be present as a separate condition but with common physiology. 50 The accompanying abdominal symptoms are referred to as functional gastrointestinal disorders. As both functional disorders and gallstones manifest with the same clinical symptoms (pain and discomfort) it is difficult to distinguish between the two conditions. Lack of clear distinction makes it difficult to treat the symptoms if they remain after the operation. Existing literature suggests that a proportion of people may still suffer from persistent or de novo symptoms after surgery. 63,129–131 Post-cholecystectomy syndrome occurs when abdominal symptoms persist after cholecystectomy. Reports suggest that about 4–40% of individuals who have their gallbladder removed may continue to experience symptoms after surgery. 57–62,132 The probability of having symptoms after laparoscopic cholecystectomy was informed by existing evidence and advice from clinical experts. The value used in the base-case analysis was 0.1 (10%).
Symptoms and surgery after conservative management
For people with gallstones treated conservatively, the model utilises the probability of individuals developing symptoms following initial treatment. The probability of experiencing recurrent symptoms while treated conservatively is based on the findings of the two RCTs reported in the systematic review of clinical effectiveness (Chapter 4). The estimate was derived from the number of patients in conservative management who switched to surgery or required further medical treatment during the 5-year follow-up period. 46 Forty-five out of 102 symptomatic people received further treatment, mainly surgery, over a 5-year period after randomisation to conservative management. The 5-year probability of presenting for surgery was converted into a fixed instantaneous rate and was reconverted into an annual probability using the following formula:133
where p is the probability, R is the rate, t is the time period of interest and ln is natural log.
The rate based on above data is therefore:
The 1-year probability of having symptoms is therefore:
The model was specified so that the proportion of patients remaining symptom free under conservative management increased over time (as those continuing to experience symptoms presented for surgery). As such, it was assumed that all those individuals remaining in conservative management at 5 years were symptom free.
Risk of death attached to surgery
This information was based on estimates of risk modelling for the probability of death after cholecystectomy using simulations for people that received elective surgery. 134 The estimate used in the base-case model was 0.00073 (Table 7).
| Definition | Value | Distribution | Source/notes |
|---|---|---|---|
| Probability surgical mortality | 0.00073 | Applied deterministically | Harrison 2012134 |
| Probability surgical complications | 0.10 | Applied deterministically | Clinical expert advice |
| Probability symptom free post-conservative management | 0.56 | Beta | Systematic review |
| Probability of getting surgery post-conservative management | 0.44 | Beta | 5-year probability reported in the systematic review converted to a 1-year probability within the model |
| Probability surgical complication needs further treatment | 0.01 | Applied deterministically | Literature and clinical advice |
| Probability of having symptoms after surgery | 0.10 | Applied deterministically | Clinical expert advice |
All-cause mortality rates in the UK
The probability that people with gallstone disease entering the model may die is based on the annual rates of all-cause age-specific mortality for both men and women (Table 8). 118
| Cycle | Men | Women |
|---|---|---|
| 0 | 0.003874 | 0.002496 |
| 1 | 0.004228 | 0.002831 |
| 2 | 0.004626 | 0.003019 |
| 3 | 0.005019 | 0.003503 |
| 4 | 0.005705 | 0.003615 |
Resource utilisation and cost estimation
Costs are presented in pounds sterling (£) in 2011–12 prices. Details of the sources used are summarised in Table 9. Data included the direct health service costs associated with each treatment strategy. The following resource use data were required to estimate costs incurred by the NHS: staff time, consumables, overheads and all the resources associated with any subsequent patient follow-up and management. Surgical procedures were mapped to their appropriate health-care resource group (HRG) and costed using NHS Reference Costs84 or the payment by results national tariff. The unit cost data for other resource utilisation was extracted from the literature or obtained from other relevant sources [e.g. Personal Social Service Research Unit].
| Resource | Value | Distribution | Source/notes |
|---|---|---|---|
| Cholecystectomy surgery | £2343 | Gamma | Elective inpatient HRG data, GA10Da laparoscopic cholecystectomy, 19 years and over, with length of stay 1 day or more, without CCs84 |
| Initial conservative management | £179 | Gamma | Consultant led: first attendance non-admitted face to face84 |
| Follow-up conservative management | £191 | Gamma | Based on one hospital outpatient visit (consultant led: follow-up attendance non-admitted face to face) and one GP visit £155 + £3684,135 |
| Operative surgical complications | £2877 | Gamma | Elective inpatient HRG data, GA10Fa open or laparoscopic cholecystectomy with CCs84 |
| Post-operative corrective surgery | £6123 | Gamma | GA03A&Ba to GA013 A&Ba average of hepatobiliary procedures categories 1–7 with and without CCs84 |
| Post-cholecystectomy syndrome | £191 | Gamma | Based on one hospital outpatient visit (consultant led: follow-up attendance non-admitted face to face) and one GP visit £155 + £3684,135 |
| Surgical complications follow-up | £532 | Gamma | Four follow-up outpatient consultant-led visits: follow-up attendance non-admitted face to face84 |
| Inpatient stayb | £949 | Gamma | Non-elective inpatient (short stay) HRG data. Average of all endoscopic or percutaneous, hepatobiliary or pancreatic procedures, with and without complications84 |
| GP visit | £36 | Gamma | Per surgery consultation lasting 11.7 minutes135 |
The cost of the surgery was based on the cost of laparoscopic cholecystectomy with the length of stay of 1 day without complications. Laparoscopic cholecystectomy was chosen as it is the most frequently performed surgical procedure for people presenting with cholecystitis or biliary pain. 84 There are two main dimensions of outcome after laparoscopic cholecystectomy: surgical complications or no complications. The cost of peri-operative complications was assumed to be the same for both surgical procedures, open and laparoscopic. Additional costs were considered for those people whose complications required further surgical interventions such as the treatment of bile duct injury. These additional costs were based on the average cost of all services relating to hepatobiliary procedures with and without complications. It was also assumed that these people had four follow-up visits to the hepatobiliary clinic every year. Costs were also included for people who experienced pain after cholecystectomy (post-cholecystectomy syndrome). People who had no surgical complications could either be completely cured or alternatively could have suffered from persistent symptoms (post-cholecystectomy syndrome). Based on clinical advice the cost of unresolved symptoms was assumed to involve one general practitioner and one outpatient visit per year.
The resources needed to provide conservative management included the number of visits to different health care providers, in particular the general practitioner (GP) and hospital outpatient department. In the base-case analysis it was assumed that all people presenting with biliary pain or cholecystitis had one hepatobiliary consultant clinic visit after the initial presentation (all people in the total cohort had one initial presentation at clinic). People whose symptoms were not resolved and continued with conservative management were assumed to have two additional visits within the first year of presentation to secondary care (one to the GP and a follow-up visit to the hepatobiliary clinic) and two similar visits each year in the following cycles. The remainder of people whose symptoms were not resolved were assumed to have had laparoscopic surgery and followed the surgery care pathway. Pain relief medications were not considered in the base-case analysis as these costs are either incurred by the people themselves (rest of the UK) or are free (Scotland).
Estimation of total cost of strategies
The total costs of the treatment strategies were determined by using recursive cost in the Markov model. As the model tree expands from the left to the right, the ‘cost’ variable is modified by adding new cost variables. In this way the value of ‘cost’ at each terminal node is unique to the path from the root node to that terminal node. Discounted costs are considered in the model to estimate the cost of each strategy by using the following formula:
Quality-of-life measures
Quality-of-life weights associated with the different outcomes of treatment and follow-up of people with symptomatic gallstones were identified in order to extend the economic evaluation into a cost–utility analysis. A focused search of the Cost-effectiveness Analysis Registry (Appendix 1) was performed to identify QoL data relevant to a UK setting. The search indicated that there were 15 studies reporting utility weights. None of these studies compared conservative management with cholecystectomy. There were six papers that compared outcomes of different interventions such as mini laparotomy, lithotripsy, open cholecystectomy with laparoscopic cholecystectomy116,117,136–139 and one paper compared the different methods of valuing health-related QoL techniques, i.e. visual analogue scale and the standard gamble in measuring people preferences for outcomes of gallstone disease. 140
Two of the studies117,139 that were conducted alongside RCTs used the European Quality of Life-5 Dimensions (EQ-5D). Although Nilsson and colleagues139 collected data at five different time points (pre-operatively, post-operatively, 1 week, 1 month and 1 year), they did not report preference-based weights or estimate a QALY. Macafee et al. 117 compared early with delayed cholecystectomy and reported utility scores of 0.85 (standard deviation 0.26) for the early group and 0.93 (standard deviation 0.13) for the conventional (delayed) group (QALYs estimated using the EQ-5D questionnaire were calculated 30–35 days after laparoscopic surgery). The final study138 estimated QALYs for different health states relating to two treatment options using a time trade-off method. The utility score for laparoscopic cholecystectomy was found to be similar to those of the Macafee et al. 117 study, 0.90 (95% CI 0.87 to 0.93). In the model, utility values were assigned to the types of potential different morbidities that could be associated with the procedures or health state represented in the model. Table 10 provides a summary of the estimates used in the model. Estimates of the utility for people who had cholecystectomy were based on the results of a RCT. 117 The utility estimate for people whose symptoms were resolved using laparoscopic cholecystectomy or conservative management was 0.93. The rest of the estimates were based on a study that used standard gamble to quantify how much a given type of morbidity would detract from their QoL. 140 The estimation of QALY values took into account the time people stayed in the state. For example, individuals who had further surgery were in a reduced state of health and were assigned a utility value of 0.64 for a month and 0.80 for the rest of the year. By applying utility weights to the modelled states and temporary events within states in the Markov model, QALYs gained over the 5-year follow-up period were tracked.
| Health state | Utility value | Distribution | Notes |
|---|---|---|---|
| Symptom-free post-laparoscopic cholecystectomy | 0.93 | Beta | Based on value of outcome for people who had conventional laparoscopic surgery117 |
| Symptom-free post-conservative management | 0.93 | Beta | Assumed to be the same as people who received laparoscopic surgery117 |
| Post-cholecystectomy syndrome | 0.79 | Beta | Based on standard gamble value of people having post-cholecystectomy syndrome as a chronic outcome140 |
| People with persistent symptoms | 0.71 | Beta | Based on Bass’s values for having biliary pain once a week each month for a lifetime140 |
| Symptoms not resolved in conservative management | 0.80 | Beta | Based on value of people experience biliary pain once a month for a life time140 |
| People undergoing corrective surgery | 0.64 | Beta | Based on standard gamble value for people needing surgery to repair a post-operative biliary stricture140 |
Assessment of cost-effectiveness
The base-case analysis was based on the costs and outcomes of a cohort of 51-year-old symptomatic women (the mean age of people receiving cholecystectomy in England and Wales). The outcome is the incremental cost per QALY. Data on the outcome are presented in two ways. First, the mean costs, QALYs and the incremental cost-effectiveness ratio (ICER), where appropriate, are calculated. These are ratios of the differences in costs and interventions divided by the differences in clinical effectiveness between the different strategies. These data reflect the rate of return in QALYs to the quantity of resources used (measured in monetary values). The second way in which the cost-effectiveness of the alternative care pathways is presented is in terms of cost-effectiveness acceptability curves (CEACs). CEACs which are derived from probabilistic sensitivity analysis are used to illustrate the uncertainty caused by the statistical variability in the parameters used in the model. CEACs illustrate the likelihood that a strategy is cost-effective at various threshold values for society’s willingness to pay for an additional QALY. The value society is willing to pay for a QALY is unclear, but in the UK NICE tends to recommend that an intervention is cost-effective when the incremental cost per QALY is less than £20,000–30,000. 119
Probabilistic and deterministic sensitivity analyses
Probabilistic and deterministic sensitivity analyses were applied to the model in order to assess the robustness of the results to realistic variations in the model parameters. The point estimates applied to costs, outcomes and probabilities of events in the model are all estimates of true population values. As such, distributions are assigned to these parameters to characterise the surrounding uncertainty due to sampling variation. Monte Carlo simulation was used to repeatedly sample from the distributions assigned to the parameters (Tables 7, 9 and 10) and reanalyse the model a large number of times.
Probabilistic sensitivity analyses of the base case were conducted by assuming a beta distribution for the probability of successful treatment outcome in the conservative management strategy (the 5-year probability of presenting for surgery being the complement of this). All cost parameters were assigned a gamma distribution as this distribution fits the skewed nature found in resource use and cost data. For health-care resource group inpatient unit cost data, the interquartile range was used to derive the standard error. For the normal distribution this is defined by the interval ± 0.67 standard errors either side of the mean. 133 An estimate of the cost standard error was derived by generating the difference between the interquartile ranges and dividing it by 2 then multiplying by 0.6, where 0.67 is the critical value which excludes 50% of the standard normal distribution. There was no distribution attached to all-cause mortality rate as the estimate was derived from the general population. The utility parameters were assigned a beta distribution to take into account the constraints in terms of their construction, which is infinity at the lower end (worst possible health) and 1.0 at the upper end (perfect health).
Sensitivity analyses varied the assumptions and/or parameter point estimates applied in the base-case analysis. One-way sensitivity analyses (varying the probabilities, costs and QALY weights one at a time) were performed.
The probability of people needing surgery after conservative management
The probability of the number of people needing surgery was varied by 25% as the results of the systematic review suggested that between 25% and 75% of people needed surgery after conservative management.
Probability of complications during surgery
Waiting lists are a common tool for managing access to elective surgery. 141 In the UK, the average reported time from referral by general practitioner to treatment for gallbladder problems varies greatly in different health trusts, from 5 to 18 weeks. There is little evidence on the health impact of delaying surgery for various conditions. According to cholecystectomy guidelines, conversion from laparoscopic to open cholecystectomy is halved (8% vs. 16%) when operating in the acute phase of the disease as opposed to allowing the acute phase to settle and being operated on later. The probability of surgical complications varies between 5% and 20%. 142
Cost of conservative management
There are no reliable data on the number of visits that people with symptoms of gallstone disease make to primary care providers before they are referred to a consultant, although it has been suggested that they may have multiple visits to their GP during the time they are awaiting treatment. The base-case analysis assumed that people with symptomatic gallstones had one primary care and one consultant visit. The number of visits was doubled to estimate the impact on cost-effectiveness. Another sensitivity analysis explored the inclusion of inpatient costs in the conservative management arm. According to the commissioner’s guide to cholecystectomy, it is not uncommon for a person to be readmitted as an emergency with a flare of symptoms three times during a prolonged period waiting for surgery. 90 A Canadian study on the risk of emergency while waiting for elective cholecystectomy reported that 6.7% of participants had emergency admissions. 141 Therefore, we estimated the cost of one, two and three additional inpatient stays for people who had persistent pain attacks while receiving conservative management. Further analysis was also performed assuming that all symptomatic people receiving conservative management had 1–3 non-elective episodes of short inpatient stay.
Utilities
As mentioned earlier, the data on utilities were based on published data and assumptions were made about the utility value of people who had received medical management. Although the RCTs included in the review of clinical effectiveness collected information on the number of pain attacks (within a pre-specified range) over a 5-year follow-up period, no data were provided on the duration of the pain attacks or on the number of pain attacks in each randomised group. Bass and colleagues140 estimated people preference values for selected outcomes by described frequency of pain or duration of outcome and their values ranged from 0.62 for having biliary pain once a day for a lifetime to 0.85 for having biliary pain once a week for 1 month. The CIs for these values spanned between 0.26 and 1.0 (full health).
Therefore, the utility of people that remained symptomatic in the conservative management arm was increased and decreased by 0.05 increments to explore the impact of this change on the results. Standard gamble utility weights for those who had open cholecystectomy with and without complications such as post-operative wound infections ranged from 0.57 to 0.83 for those with post-operative bile duct stone. Analysis was also performed to estimate the impact of increasing the utility of people who had continuing surgical complications from 0.64 to 0.80 (closer to the utility value of those patients who had open cholecystectomy 0.78).
Annual discount rate
The NICE guidelines recommend an annual discount rate of 3.5% for costs and outcomes and was applied in the base-case analysis. 119 A range of between 0% and 6% was considered in the sensitivity analyses.
Increasing follow-up to 10 years
Evidence from the long-term follow-up studies46,113 suggests that the number of people seeking further treatment reduces over time, so the base-case analysis considered the 5-year follow-up. A sensitivity analysis was performed to explore how the small variation in modelled health outcomes and ongoing management costs (at 5 years) influences cost-effectiveness over a longer time horizon (10 years).
Value of information analysis
The decisions related to the adoption or rejection of technologies should be based on expected cost-effectiveness given the current existing information. However, the decisions should not be based on little, or poor-quality evidence, as long as the decision to conduct further research to support adoption (or rejection) is made simultaneously. 132 Decisions to adopt or reject a technology based on existing information will be uncertain as there will always be a chance that the wrong decision could be made. Wrong decisions lead to opportunity costs due to forgone health benefits and resources. The expected cost of uncertainty is determined jointly by the probability that a decision based on existing information will be wrong and the consequences of a wrong decision. The estimates of the probability of error and opportunity costs of error can be used to calculate the expected cost of uncertainty or the expected loss surrounding the decisions. The expected cost of uncertainty can be interpreted as the expected value of perfect information (EVPI) and perfect information can be used to eliminate the possibility of making the wrong decision. We performed a value of information analysis to estimate the distribution of expected costs and expected QALYS of treatment of biliary pain/cholecystitis dependent on the uncertainty in the input parameters. The net monetary benefit (NMB) for the two strategies from each Monte Carlo iteration was computed to represent the possible values of NMB for all the possible realisations of the uncertain parameters. The maximum NMB among all the strategies was then calculated to determine the total EVPI per subject. The expected value of a decision undertaken with perfect information was derived by averaging the maximum net benefit with perfect information and the maximum net benefit with the current information. We then estimated the expected value for the entire population that could potentially benefit from more research (population EVPI). To calculate the population EVPI, we assumed the effective lifetime of the treatment to be 10 years. Benefits to future people were discounted at 3.5%. The annual patient population that could benefit from the results of a future study was based on the number of people undergoing a gallbladder excision per annum in England (Hospital Episode Statistics data84), multiplied by the time period of 10 years.
Results
Deterministic and probabilistic results
The cost-effectiveness analysis aggregates the average 5-year costs for the treatments and time spent in each of the various health states of the model. Table 11 shows the results for the base-case analysis for a hypothetical cohort of 51-year-old women with symptomatic gallstones. The table reports the performance of surgery compared with conservative management, which is less costly and less effective. On average, conservative management care pathway costs less but has lower benefits (QALYs) than the surgery pathway. Analysis performed using a cohort of 51-year-old men had lower QALYs (4.108 for conservative management and 4.21 for cholecystectomy) but the ICER was similar to that of the base-case analysis (ICER £12,178).
| Strategy | Costs (£) | Incremental cost | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CM | 1104 | 4.139 | |||
| Surgery | 2340 | 1236 | 4.232 | 0.094 | 13,205 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 60% | 51% | 46% | 44% | 42% |
| Surgery | 40% | 49% | 54% | 56% | 58% |
Probabilistic results
To account for the uncertainty surrounding ICER (for surgery vs. conservative management) resulting from the joint uncertainty surrounding all the model input parameters, probabilistic sensitivity analysis using Monte Carlo simulations was performed. The results of the probabilistic analysis are reported in the form of CEACs (Figure 28). The conservative management arm has a higher probability of being considered cost-effective for thresholds below £20,000 and the surgery arm has a higher probability of being considered cost-effective as the threshold increases beyond £20,000.
FIGURE 28.
Cost-effectiveness acceptability curve determined by society’s willingness to pay for an additional QALY. CM, conservative management.
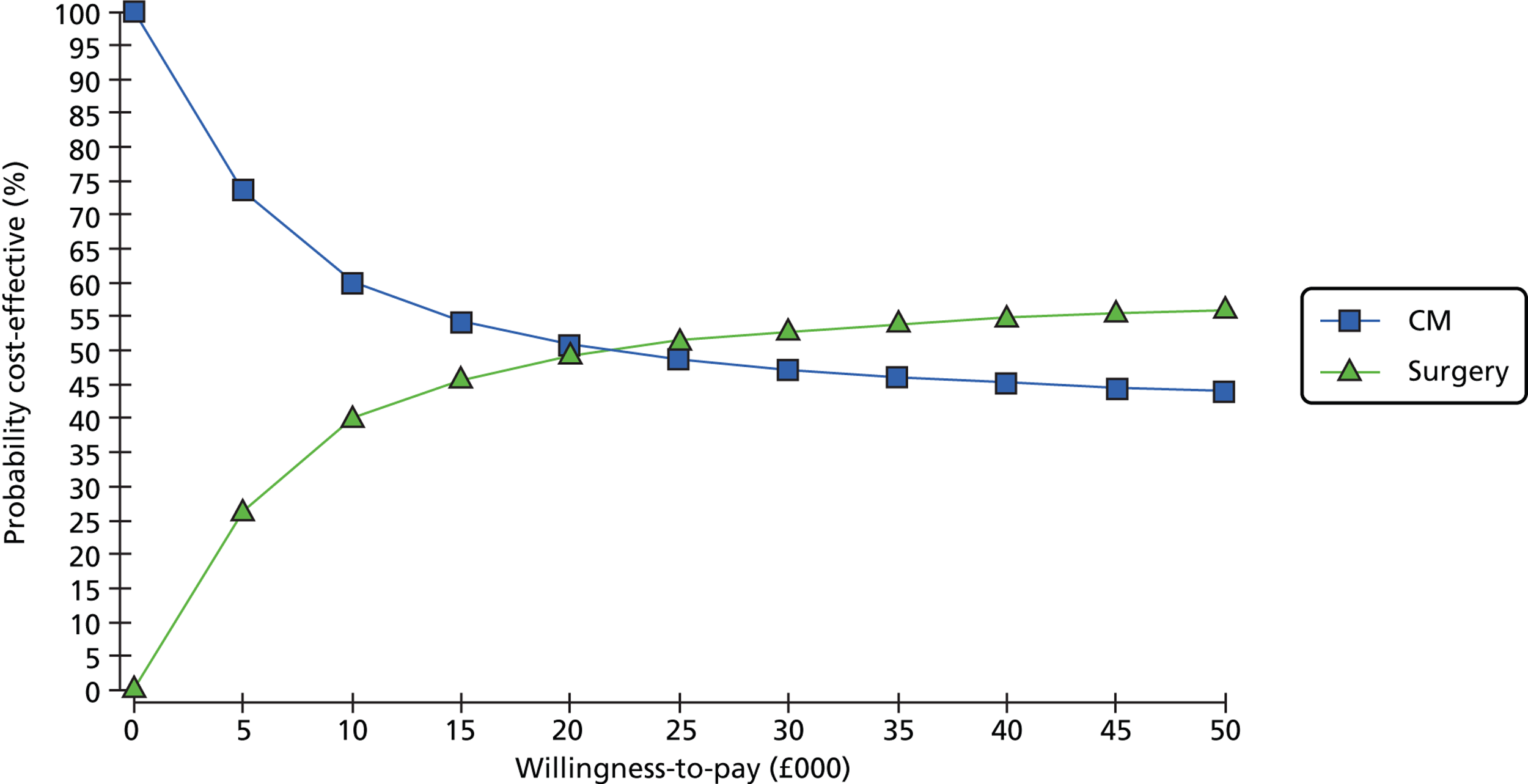
Sensitivity analyses
Probability of the number of people needing surgery
As anticipated, the value of the ICER doubles when we reduce the probability of people needing surgery after conservative management to 25%, as the costs of conservative management are reduced and its effectiveness increases (although the QALYs are still lower than those of the surgery strategy). This is equivalent to conservative management being targeted on a lower risk group (i.e. a group who have less frequent symptoms and better QoL than the average observed within the cohort).
On the other hand, the value of the ICER is reduced when the probability of people needing surgery after conservative management increases to 75%. The cost difference between the two strategies reduces but the difference between the QALYs increases, hence, making the surgery strategy more favourable (Table 12).
| Strategy | Costs (£) | Incremental cost (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| Probability of people needing surgery after conservative management reduced to 25% | |||||
| CM | 694 | – | 4.182 | – | – |
| Surgery | 2340 | 1646 | 4.222 | 0.049 | 33,542 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 75% | 61% | 56% | 51% | 48% |
| Surgery | 25% | 39% | 44% | 49% | 52% |
| Probability of people needing surgery after conservative management increased to 75% | |||||
| CM | 1757 | – | 4.095 | – | – |
| Surgery | 2340 | 583 | 4.231 | 0.136 | 4291 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 52% | 49% | 47% | 46% | 46% |
| Surgery | 48% | 51% | 53% | 54% | 54% |
Probability of complications during surgery
The results of varying the probability of complications between 0.05 and 0.20 produced similar results to those of the base case. The cost of conservative management was lower than surgery, but QALYs were also lower (Table 13).
| Strategy | Costs (£) | Incremental cost (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| Probability of surgical complications is 0.05 | |||||
| CM | 1140 | – | 4.139 | – | – |
| Surgery | 2427 | 1287 | 4.233 | 0.094 | 13,739 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 39% | 49% | 48% | 46% | 44% |
| Surgery | 61% | 51% | 52% | 54% | 56% |
| Probability of surgical complications is 0.20 | |||||
| CM | 1034 | – | 4.138 | – | – |
| Surgery | 2166 | 1133 | 4.231 | 0.093 | 12135 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 59% | 50% | 47% | 45% | 44% |
| Surgery | 41% | 50% | 53% | 55% | 56% |
Cost of conservative management
The ICER value reduced as the number of inpatient stays (under conservative management) increased (Table 14). When all the people that had symptoms in the conservative management strategy had an inpatient stay there was more than 50% chance of the surgery arm being considered to be cost-effective for the thresholds above £5000.
| Strategy | Costs (£) | Incremental cost (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| Assuming that the people with persistent pain had one inpatient stay | |||||
| CM | 1455 | – | 4.139 | – | – |
| Surgery | 2340 | 885 | 4.232 | 0.094 | 9455 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 54% | 48% | 45% | 43% | 43% |
| Surgery | 46% | 52% | 55% | 57% | 57% |
| Assuming that the people with persistent pain had two inpatient stays | |||||
| CM | 1807 | – | 4.139 | – | – |
| Surgery | 2340 | 533 | 4.232 | 0.094 | 5700 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 48% | 44% | 43% | 42% | 41% |
| Surgery | 52% | 56% | 57% | 58% | 59% |
| Assuming that the people with persistent pain had three inpatient stays | |||||
| CM | 2158 | – | 4.139 | – | – |
| Surgery | 2340 | 182 | 4.232 | 0.094 | 1950 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 43% | 41% | 40% | 40% | 40% |
| Surgery | 57% | 59% | 60% | 60% | 60% |
| Assuming that all the people with symptoms had one inpatient stay | |||||
| CM | 2223 | – | 4.139 | – | – |
| Surgery | 2340 | 117 | 4.232 | 0.094 | 1253 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 42% | 40% | 40% | 39% | 39% |
| Surgery | 58% | 60% | 60% | 61% | 61% |
Changes in the number of outpatient and GP visits had very little impact on the results.
Utilities
The ICER results were sensitive to the utility values applied (Table 15). When we assumed that the utility decrement for people who remain symptomatic under conservative management was greater than in the base case, the ICER reduced in favour of surgery (this is as expected, as there is more potential for health improvement if one’s utility value is lower). However, for values above the base case, the results favour conservative management as QALY gains with surgery decrease.
| Strategy | Costs (£) | Incremental cost (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| Assuming that the utility of those who remain symptomatic in conservative management is 0.60 | |||||
| CM | 1104 | – | 3.936 | – | – |
| Surgery | 2340 | 1236 | 4.232 | 0.296 | 4175 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 14% | 8% | 7% | 7% | 7% |
| Surgery | 68% | 92% | 93% | 93% | 93% |
| Assuming that the utility of those who remain symptomatic in conservative management is 0.65 | |||||
| CM | 1104 | – | 3.995 | – | – |
| Surgery | 2340 | 1236 | 4.232 | 0.237 | 5212 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 20% | 12% | 10% | 10% | 9% |
| Surgery | 80% | 88% | 90% | 90% | 91% |
| Assuming that the utility of those who remain symptomatic in conservative management is 0.70 | |||||
| CM | 1104 | – | 4.054 | – | – |
| Surgery | 2340 | 1236 | 4.232 | 0.178 | 6936 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 28% | 18% | 15% | 14% | 14% |
| Surgery | 72% | 82% | 85% | 86% | 86% |
| Assuming that the utility of those who remain symptomatic in conservative management is 0.75 | |||||
| CM | 1104 | – | 4.113 | – | – |
| Surgery | 2340 | 1236 | 4.232 | 0.119 | 10,365 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 40% | 26% | 22% | 21% | 20% |
| Surgery | 60% | 74% | 78% | 79% | 80% |
| Assuming that the utility of those who remain symptomatic in conservative management is 0.85 | |||||
| CM | 1104 | – | 4.231 | – | – |
| Surgery | 2340 | 1236 | 4.232 | 0.001 | 906528 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 79% | 55% | 47% | 44% | 42% |
| Surgery | 21% | 45% | 53% | 56% | 58% |
| Assuming that the utility of those who remain symptomatic in conservative management is 0.90 | |||||
| CM | 1104 | – | 4.29 | – | – |
| Surgery | 2340 | 1236 | 4.232 | –0.058 | Dominated |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 96% | 82% | 71% | 65% | 62% |
| Surgery | 4% | 18% | 29% | 35% | 38% |
| Increasing utility of those that had complications to 0.93 | |||||
| CM | 1104 | – | 4.138 | – | – |
| Surgery | 2340 | 1236 | 4.223 | 0.085 | 14549 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 61% | 53% | 49% | 47% | 46% |
| Surgery | 39% | 47% | 51% | 53% | 54% |
The analyses which we performed to estimate the impact of increasing the utility of people who had continuing surgical complications had a low impact on the overall results. On average, both surgery costs and benefits were higher, and led to a slightly lower ICER than that of the base-case analysis (£14,761).
Annual discount rate for costs and benefits
Varying the discount rate for costs did not change the results substantially (Table 16). However, the ICERs appeared to vary more when the utility discount rate was changed. As expected, the ICER increased as the rates increased.
| Discount rate | Strategy | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| Sensitivity analysis varying the cost discount rate | ||||||
| 0% | CM | 1185 | – | 4.139 | – | – |
| Surgery | 2350 | 1165 | 4.232 | 0.094 | 12,454 | |
| 1% | CM | 1161 | – | 4.139 | – | – |
| Surgery | 2347 | 1186 | 4.232 | 0.094 | 12,678 | |
| 2% | CM | 1138 | – | 4.139 | – | – |
| Surgery | 2344 | 1207 | 4.232 | 0.094 | 12,894 | |
| 3% | CM | 1115 | – | 4.139 | – | – |
| Surgery | 2342 | 1226 | 4.232 | 0.094 | 13,103 | |
| 4% | CM | 1094 | – | 4.139 | – | – |
| Surgery | 2339 | 1245 | 4.232 | 0.094 | 13,306 | |
| 5% | CM | 1073 | – | 4.139 | – | – |
| Surgery | 2336 | 1264 | 4.232 | 0.094 | 13,502 | |
| 6% | CM | 1052 | – | 4.139 | – | – |
| Surgery | 2334 | 1281 | 4.232 | 0.094 | 13,691 | |
| Sensitivity analysis varying the benefit (QALYs) discount rate | ||||||
| 0% | CM | 1104 | – | 4.371 | – | – |
| Surgery | 2340 | 1236 | 4.522 | 0.15 | 8220 | |
| 1% | CM | 1104 | – | 4.301 | – | – |
| Surgery | 2340 | 1236 | 4.435 | 0.134 | 9230 | |
| 2% | CM | 1104 | – | 4.234 | – | – |
| Surgery | 2340 | 1236 | 4.352 | 0.118 | 10,505 | |
| 3% | CM | 1104 | – | 4.17 | – | – |
| Surgery | 2340 | 1236 | 4.271 | 0.102 | 12,168 | |
| 4% | CM | 1104 | – | 4.109 | – | – |
| Surgery | 2340 | 1236 | 4.194 | 0.086 | 14,430 | |
| 5% | CM | 1104 | – | 4.05 | – | – |
| Surgery | 2340 | 1236 | 4.12 | 0.07 | 17,689 | |
| 6% | CM | 1104 | – | 3.994 | – | – |
| Surgery | 2340 | 1236 | 4.049 | 0.054 | 22,797 | |
Increasing follow-up to 10 years
The results moved in the same directions as those of the base-case analysis as the probability of getting surgery in the conservative management strategy only increased by 0.01. The evidence from the systematic review of clinical effectiveness indicated that there was only one additional person that received surgery from the conservative management arm in the 14-year follow-up period bringing the total to 46 out of 102 (45%). There was quite a substantial drop in QALY gain over the longer follow-up period (Table 17). This was probably because, after 5 years, people on conservative management were no longer symptomatic.
| Strategy | Costs (£) | Incremental cost (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CM | 1275 | – | 7.645 | – | – |
| Surgery | 2436 | 1161 | 7.707 | 0.062 | 18,628 |
| Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (£) | |||||
| Threshold value | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 |
| CM | 62% | 54% | 51% | 49% | 48% |
| Surgery | 38% | 46% | 49% | 51% | 52% |
Value of information
The probabilistic analysis indicated that, for a threshold of £30,000, surgery had a 57% chance of being considered cost-effective. The expected saving from perfect information per person was £585.91 and the expected gain in effectiveness was 0.054 per person. The per person EVPI was £2209.65 and the resulting population EVPI was £1,206,730,653.
Chapter 6 Discussion
This assessment sought to answer the following research question posed by the UK National Institute for Health Research Health Technology Assessment programme: What is the clinical effectiveness and cost-effectiveness of cholecystectomy for preventing recurrent symptoms and complications from symptomatic gallstones or cholecystitis?
Summary of findings
This assessment, using the best available evidence and a health economic model, found that conservative management is on average less costly than surgery but also less effective for treating symptomatic gallstones. The results of the economic evaluation were most sensitive to the utility values used for people who were managed conservatively. The conservative management strategy dominated surgery (i.e. the conservative management was cheaper and more effective) when the utility value for persistent symptoms was assumed to be 0.90. However, when the utility value equalled 0.60, surgery was more effective, although more expensive, giving an ICER of £4175. With changes in the utility value of persistent symptoms from 0.60 to 0.90, the probability of the surgery strategy being considered cost-effective at a willingness-to-pay threshold of £20,000 changed from 92% to 18%. However, caution should be exercised owing to the uncertainty around some of the parameters used in the model. The findings of the clinical effectiveness review indicate that symptomatic people randomised to observation were at significantly greater risk of experiencing gallstone-related complications (in particular, acute cholecystitis), pain attacks and admissions to hospital for gallstone-related pain. Symptomatic people randomised to surgery were significantly more likely to undergo surgery and had a greater risk of experiencing surgery-related complications as well as minor complications. Forty-five per cent of people originally randomised to observation switched to surgery, whereas 12% of people originally randomised to surgery avoided the operation. It is interesting that nearly half of people randomised to observation were not operated on during the long-term follow-up period, demonstrating that there are a proportion of people who do not require surgery. When we considered outcomes according to the treatment people actually received, we noticed that people who did not undergo surgery had fewer episodes of pain attacks and fewer admissions to hospital as a result of pain than people who were treated with cholecystectomy.
Approximately 70,000 cholecystectomies are performed every year in the UK, the majority laparoscopically. However, surgery could be avoided in a proportion of people with symptoms but no complications, and this option appears to be safe in terms of subsequent events. The implications of this assessment in terms of planning the best care pathway for people suffering from gallstone disease are therefore crucial. Nevertheless, the uncertainty surrounding our findings and the paucity of the current evidence suggest a cautious interpretation.
Clinical effectiveness
The two included studies46,113 we identified in the current literature were good-quality RCTs, which randomised a total of 201 participants with symptomatic gallstone disease to either cholecystectomy or observation. Both trials reported relevant outcomes, which were combined in mathematical syntheses when appropriate. It is noteworthy that both trials were conducted in Norway and not in the UK. We do not know how well the health-care system in Norway mirrors the UK NHS.
Gallstone disease associated events
On the whole, significantly more people randomised to observation (14%) than to surgery (2%) experienced gallstone-related complications. New events that occurred in the observation group were acute cholecystitis, common bile duct stones and acute pancreatitis. In particular, further complications occurred more frequently (27%) in people with an initial diagnosis of acute cholecystitis46 than in those (1%) with uncomplicated symptomatic gallstones (biliary pain only). 113 Few people suffered from CBD stones during the 14-year follow-up with no significant difference between intervention groups (4% in the observation group vs. 1% in the surgery group). In particular, CBD stones occurred in only one person with uncomplicated symptomatic gallstones (biliary pain only) rather than acute cholecystitis during the 14-year follow-up period. Similarly, the proportion of people suffering from acute pancreatitis was the same in both intervention groups (1% observation group vs. 1% in the surgery group). These findings are in line with those of early natural history studies which report a cumulative rate of 2–6% for CBD stones or acute pancreatitis in participants observed for 5–10 years. 17,42,92 The overall risk of developing acute biliary complications in people with symptomatic gallstones has been estimated to be around 3% per annum during an observation period of 6 years. 143 In a recent population-based study,12 gallstone-related complications were observed in 7.5% and 22% of mild and severe symptomatic people, respectively. In short, in the included RCTs, the majority of people treated conservatively did not experience long-term complications (86%); CBD stones and episodes of acute pancreatitis were both rare.
Pain attacks
Overall, the proportion of people suffering from pain attacks was not significantly different between randomised groups (25% in the observation group vs. 12% in the surgery group). Pain attacks were observed more frequently in participants treated conservatively (26/102 people) than in those who underwent surgery (12/99 people) and in participants with uncomplicated symptomatic gallstones (biliary pain only – 23/69 people) rather than in those with an initial diagnosis of acute cholecystitis (3/33). The admissions to hospital as a result of pain were not significantly different between intervention groups. However, admissions were more frequently observed in people with uncomplicated symptomatic gallstones (biliary pain only – 12/69) rather than in those with acute cholecystitis (4/33). It is noteworthy that, when we considered people with gallstones according to the treatment they actually received and not according to randomisation, fewer episodes of pain were observed in people treated conservatively (12/68) than in those who were eventually operated on (26/133).
The review findings on pain attacks are broadly similar to those suggested in the clinical literature, i.e. cholecystectomy is not always successful in relieving symptomatic people from pain. Up to 40% of people may suffer from post-cholecystectomy symptoms. 56 Symptoms may persist or arise de novo after surgery. 63 Marked biliary pain has been described in 4–9% of people after cholecystectomy, while persistent abdominal pain or non-specific pain has been reported in about 13–37% of people. 57–62 Some investigators have also reported a persistent pain similar to that experienced pre-operatively in approximately 20% of people after cholecystectomy. 64,65
Surgery rate
The majority of people randomised to receive surgery (88%) and approximately half of the people randomised to observation (45%) eventually underwent cholecystectomy during the 14-year follow-up period. As few gallstone-related complications were experienced by symptomatic people randomised to observation, it is likely that pain was the reason why they underwent surgery. 114 Most of the surgical procedures were performed during the first 5 years and virtually no operations occurred after 5 years. These data are in accordance with previously published studies. McSherry and colleagues17 found that 44% of people with mild gallstone disease underwent surgery during a 5-year follow-up period. Similarly, Festi and colleagues12 reported that 17% and 41% of people with mild and severe symptoms, respectively, underwent surgery during a 10-year follow-up period, but the majority of the surgical procedures (68%) were performed during the first 4 years.
Not all participants in the observation group required cholecystectomy or experienced severe complications during the long-term follow-up. Interestingly, 55% of people randomised to observation did not require an operation during the 14-year follow-up period and 12% of people randomised to receive cholecystectomy did not undergo the scheduled operation, probably as a result of minimal, tolerable symptoms. These findings are in agreement with those of early natural history studies17,45 and with those of more recent observational and population-based studies. 12,56 Larsen and colleagues56 found that 45% of symptomatic people on watchful waiting were totally relieved of symptoms during a 1-year observation period. Similarly, Festi and colleagues12 observed that 58% of people with initially mild symptoms and 52% of those with more severe symptoms did not experience further episodes of pain during a follow-up period of 10 years.
Surgery associated events
The risk of surgery-related complications including abdominal infection, bile leakage, wound infection, dehiscence and bile duct injury was significantly higher in the cholecystectomy group. Nineteen per cent of participants originally randomised to surgery, compared with 7% participants randomised to observation (who were eventually operated on), experienced surgery-related complications. Complications of cholecystectomy have been well documented and the findings of our two RCTs46,113 are consistent with those of three recent Cochrane systematic reviews106,144,145 which indicate that the total number of people suffering complications after open, small-incision or laparoscopic cholecystectomy is high with no significant differences between the three surgical procedures. 106 In particular, the proportions of total complications after laparoscopic and small-incision cholecystectomy calculated from 13 clinical trials at low risk of bias (total 2337 participants) were 17% and 17.5%, respectively. 106
Mortality rate
There were no deaths caused by gallstone disease in the included trials. The all-cause mortality at 14 years was greater (but not significantly greater) among participants randomised to surgery. Even though a significant difference between intervention groups was not detected, it is noteworthy that the relative risk was 0.73 (95% CI 0.41 to 1.31) favouring the observation group. This finding suggests that people undergoing surgery had an increased probability of death compared with non-operated people. A clear explanation of this finding is difficult to determine. However, surgery in itself carries an intrinsic mortality risk, especially if there are pre-existing comorbidities.
Quality of life
We were not able to meta-analyse the scores of QoL scales. The trial investigators reported that, overall non-significant differences were observed between participants who underwent cholecystectomy and those treated conservatively, and no gallstone or surgery-related deaths occurred in the two randomised groups. Participants in both treatment groups experienced a decline over time in pain scores, and their QoL scores improved after randomisation. It is noteworthy that participants with more frequent and marked pain at randomisation were reported to have a higher probability of subsequent gallstone-related events. For the group with an initial diagnosis of acute cholecystitis, the trial investigators analysed the results according to the treatment participants actually received and unexpectedly did not find any difference between observed and operated participants. It is likely, however, that the sample size of the two groups (64 participants in total) was too small to detect statistically significant differences.
Early compared with delayed surgery
We identified only two RCTs in the current literature, which compared observation/conservative management with cholecystectomy. Several RCTs have been conducted on the effects and safety of early compared with delayed surgery. Although these studies do not focus specifically on people with uncomplicated symptomatic gallstone disease or with a first episode of biliary pain presenting to secondary care, and the delayed period is relatively short (a few weeks), it is worth considering the outcomes of people allocated to ‘delayed surgery’ in terms of complications and need for emergency surgery in light of our review results. In particular, two Cochrane systematic reviews have been recently published on the effects of early laparoscopic cholecystectomy compared with delayed cholecystectomy in people with either acute cholecystitis or biliary pain. 72,146
The Cochrane systematic review on early laparoscopic cholecystectomy (less than 7 days from symptom onset) compared with delayed cholecystectomy (more than 6 weeks after initial admission) in people with an initial diagnosis of acute cholecystitis146 included five trials with a total of 451 participants. No statistically significant differences between the two groups were detected for any of the outcomes considered, including complication rate (bile duct injury odds ratio 0.6; 95% CI 0.15 to 2.70) and conversion to open cholecystectomy (odds ratio 0.85; 95% CI 0.53 to 1.34). Length of follow-up was not reported. However, 18% of the people randomised to the delayed group had non-resolution or recurrence of symptoms before their planned operation and, therefore, required emergency laparoscopic cholecystectomy. The proportion of these emergency procedures converted to open cholecystectomy was 45%.
The same authors conducted a systematic review to evaluate the benefits and harms of early compared with delayed laparoscopic cholecystectomy in participants with biliary pain due to gallstone disease. 72 They identified only one trial with a total of 75 participants in the literature. 87 Morbidity, and in particular severe morbidity (e.g. acute pancreatitis, bile duct injury, gallbladder perforation), was significantly lower in the early group (0%, 0/35) than in the delayed group (15%, 6/40). Overall, during the delayed period (mean 4.2 months) 35% of people in the delayed group (14/40) required 18 hospital admissions for symptoms related to gallstone disease (hospital admissions for people in the early group were not reported). Hospital stay was significantly shorter in the early group than in the delayed group (weighted mean difference −1.25 days; 95% CI −2.05 to −0.45). The trial was at high risk of bias (unclear allocation concealment, lack of blinding, no sample size calculation and no intention-to-treat analysis) hence its results can only be interpreted tentatively.
Factors influencing the decision to undertake a cholecystectomy
From a clinical point of view, the review findings suggest that it would be crucial to identify which characteristics of gallstone disease could predict the evolution of symptoms. In particular, it would be very important to be able to single out people with uncomplicated symptomatic gallstones who could be treated conservatively. Unfortunately, at present no definitive factors have been identified. Even the presence of pain in the upper right abdominal quadrant may not consistently be related to gallbladder stones147 and, overall, the symptoms caused by gallstone disease are still not completely understood, rendering it difficult to identify people who could benefit from surgery. Furthermore, people vary considerably in the way they report their symptoms depending on their individual pain threshold as well as their preference for having or avoiding surgery. At present, in many cases, surgery is offered independently from the clinical symptoms of people presenting with gallstone disease and without considering the potential benign course of the disease partly due to the wide diffusion of laparoscopic cholecystectomy. According to the World Gastroenterology Organisation Practice Guideline148 on gallstone disease, when a group of nine surgeons were asked to evaluate 252 people who underwent cholecystectomy, they agreed that surgery was the appropriate option in only 52% of cases and failed to find an agreement on 44% of cases. There is now an indication that removal of the gallbladder may also potentially lead to a slightly increased risk of colon cancer in women in the long term. 149,150
Summary
In our assessment, approximately half of the people in the observation group were eventually operated on. Almost no surgical operations were performed after the first 5 years of follow-up. Participants who underwent cholecystectomy experienced more surgery-related complications and showed a slight, non-significant, increase in mortality rate (all-cause mortality) than those who were treated conservatively. Conversely, participants in the observation groups had more episodes of cholecystitis, but the incidence of other gallstone-related complications (e.g. common bile duct stones, acute pancreatitis) was low. Approximately half of the people in the observation group did not required surgery at long-term indicating that there is probably a subgroup of people with symptomatic gallstone disease who could be safely treated conservatively.
Summary of findings from the economic model
The economic model was developed using evidence synthesis from three main sources: a systematic review and meta-analysis of clinical effectiveness, advice from clinical experts and a prior health state valuation survey. The economic model compared two treatment strategies: conservative management and surgery. The model compared cumulative costs and QALYs for a cohort of 51-year-old women for a 5-year time horizon. This time was chosen as the available data indicated that the results did not markedly change if a longer-term horizon was adopted. On average, the surgery strategy cost £1236 more than the conservative management strategy but was, on average, more effective, and generated 0.094 additional QALYs. The incremental cost per QALY was £13,205. The incremental cost-effectiveness result indicated that the conservative management strategy had a 51% chance of being considered cost-effective at society’s willingness-to-pay threshold of £20,000. The probability of cost-effectiveness was not sensitive to changes in willingness to pay, when the threshold increased to £30,000, the conservative management strategy had a 46% chance of being considered cost-effective.
The result of the analysis using a male cohort aged 51 years was similar to that of the base-case analysis. On average, surgery cost more but was more clinically effective, even though the effectiveness for both arms was slightly lower (conservative management 4.108 and cholecystectomy 4.21) leading to an ICER of £12,718. This slight reduction in QALYs was the result of the higher mortality of the male cohort.
The results were sensitive to the probability of the number of people needing surgery in the conservative management strategy. The systematic review indicated that there was uncertainty around the estimate derived in the meta-analysis. On average, the cost of the conservative management strategy reduced to £694 when the probability was reduced to 25% leading to an ICER of £33,542 per QALY for the surgical strategy. The probability that the conservative management strategy would be considered cost-effective was between 56% and 61% (for willingness to pay for QALY values of £20,000–30,000, respectively). However, the ICER reduced to £4291 when the probability of surgery among those initially managed conservatively was increased to 75%. The probability that the surgery strategy would be considered cost-effective at the recommended threshold was between 51% and 53%.
There was no evidence to indicate that the risk of surgical intervention increased over time for patients that required surgery after conservative management. Therefore, these patients were assumed to follow a similar pathway to those who went straight into surgery. However, the model considered that patients who had surgery after conservative management had a lower utility prior to surgery – to take into account the fact that they remained symptomatic.
Evidence suggests that the number of complications from laparoscopic cholecystectomy has reduced over the years as the procedure has become widely used. The sensitivity analyses results around the probability of surgical complications were similar to those of the base-case analysis (£13,739 when probability of complications = 0.05, and £12,135 when probability of complications = 0.2), suggesting that the model is robust to the changes in the probability of surgical complications.
The results were more sensitive to changes in the costs of conservative management. As there are no data to support the average number of visits people may have to the GP and the hospital, various exploratory analyses were performed to assess the impact of additional inpatient stays for people in the conservative management strategy. As anticipated, as the number of inpatient stays (each year) for the group with persistent pain increased, the cost of the conservative management strategy increased, thereby reducing the cost difference between the two strategies. This led to a reduction of the ICER from £13,205 (no inpatient stay) to £1253 (three non-elective inpatient short stays).
The results were most sensitive to the utility value that was used for people who were managed conservatively. Based on a previous valuation survey, in the base-case analysis it was assumed that the utility was 0.71 among those with persistent symptoms and 0.80 for those with no persistent pain. As appropriate information on the number of pain attacks and their impact on QoL was not available from the trial data, these values could have been over- or underestimated, and were therefore scrutinised in sensitivity analysis. The ICERs varied from £4175 (when utility value equalled 0.60 for persistent symptoms), to conservative management strategy dominating surgery when the utility value for persistent symptoms was assumed to be 0.9. The probability of conservative management strategy being considered cost-effective for society’s willingness-to-pay thresholds of £20,000–30,000 ranged from 8% to 82%. With changes in the utility value of persistent symptoms from 0.60 to 0.90, the probability of the surgery strategy being considered cost-effective at willingness-to-pay threshold of £20,000 changed from 92% to 18%. The results were not sensitive to changes in the utility of complications associated with surgery. The results were not markedly sensitive to changes in the discount rates applied to both the costs and the benefits.
Strengths and limitations of the assessment
The methods used to conduct this assessment were detailed and thorough. In particular, we performed comprehensive literature searches of the major electronic databases and we contacted experts in the field to identify all existing relevant evidence. We reviewed all potential eligible studies for inclusion and we did not restrict study selection to English-language publications. We assessed the methodological quality of included studies using the best recommended risk of bias tool. We developed specific data extraction forms on pre-specified outcome parameters and two reviewers independently extracted data from included studies. Despite all these efforts there is still the possibility that some relevant evidence remains buried in non-indexed journals or hidden as a result of non-publication.
We identified only two RCTs from the same investigators (published in six reports) in the current literature and no non-randomised comparative studies. Both trials, even though at low risk of bias, were of small sample size and it likely that they were underpowered to detect some important treatment differences between the interventions. Moreover, as a consequence of the small number of participants, few events were available for some of the analyses with wide confidence intervals around the estimates of treatment effect. It is also worth noting that even though statistical heterogeneity was not identified in the performed meta-analyses, the small sample sizes may have contributed to a lack of power to detect statistical heterogeneity.
The two trials did not include post-cholecystectomy symptoms within their outcome measurements. As cholecystectomy is supposed to completely cure gallstone symptoms, an important outcome to consider would have been the number of people who still experience pain post surgery. Similarly, little information was available on the type of ‘conservative’ management – in terms of regimen of medication and GP visits – for participants in the observation group. Even though ‘episodes of pain’ were recorded in both intervention groups in both trials, the severity of these episodes was not reported and uncertainty remains as to how these episodes were subsequently treated. Both trials reported the number of admissions to hospital as a result of pain but without providing details of what exactly ‘admission to hospital’ entailed. We were able to contact one of the trials investigators who explained that in Norway, where the trials were conducted, participants who were admitted to hospital as a result of pain are commonly offered further imaging and laboratory tests to confirm the nature of the pain before opting for the best medical or surgical treatment. This is somewhat different from what happens in the UK where further admissions to hospital as a result of pain are normally treated by surgery, unless contraindicated.
Another limitation of this assessment is that both trials were conducted outside the UK and it is likely that their findings cannot be easily generalised to a UK clinical setting. Conservative management is not a policy that is (or has been) used in the NHS. As such, there was an inherent difficulty in extrapolating the review findings to the NHS setting. We addressed this difficulty by consulting with various clinical experts in the field. However, until prospective data are collected, the exact structure of a conservative management strategy in the NHS, in the UK, is unknown.
A major challenge in analysing and presenting the results of the included trials was the number of participants in the observation group who crossed over to receive surgery and similarly, although less numerous, the number of participants in the surgery group who refused to undergo the operation. We analysed the outcomes using an intention-to-treat approach (i.e. according to randomisation), which allows a proper comparison between treatment groups. However, when the number of participants who did not adhere to the intervention they were originally allocated to is so high, the overall validity of the results becomes questionable and their interpretation problematic. For this reason we decided to also use an efficacy approach. We present the main results of the two trials according to the treatment participants actually received, regardless of randomisation (Figures 20–22 in Chapter 4). As this approach has the advantage of comparing the specific effects of surgery compared with those of pure observation, it does, however, violate randomisation and potentially introduce biases.
Modelling and estimation of cost-effectiveness were challenging because of the lack of data on many of the parameters required in the model. As indicated in Chapter 5, considerable effort was made to identify relevant data and sensitivity analyses were used to explore the impact of uncertainties. There were very few data on the conservative management strategy, as it is not practised in the UK and the modelling parameters were based on the best available data. Therefore, the findings of this study should be treated cautiously.
The effectiveness of treatment was measured in QALYs which were derived from different sources: a study published in the UK and a study from the USA that used standard gamble to estimate utility preferences for different health states. As there were no data relating to the QoL of people that do not receive surgery, it was assumed that the utility of people who did not progress to surgery in the conservative management strategy was similar to those that were cured by surgery. This decision was based on the clinical judgement that people who do not receive surgery are unlikely to be experiencing persistent symptoms and are likely to be ‘doing well’ under the conservative management approach.
Another assumption made in the model was that all people in the surgery strategy received surgery. Although there is evidence to suggest that a minority of people do not take up surgery when it is offered, this assumption was considered to reflect the current practice in the UK, as surgery is the recommended treatment for people presenting with uncomplicated symptomatic gallstones or cholecystitis.
People’s preferences regarding the utility or disutility associated with the delivery of conservative management relative to surgical treatment (e.g. treatment time, pre-operative anxiety, post-operative pain) were not explicitly taken into account. People may have preferences over these attributes. Those who considered the disutility of surgery to outweigh the symptoms they experience would likely not agree to the procedure. As such, this model applies only to people who are considering surgery as a potential treatment option. It may be that additional incorporation of these factors among this group would change the QALY estimates to some degree. Further research to elicit preferences for the process and outcomes of treatment may be useful to explore the impact of these preferences.
The economic model focused on costs to the NHS. However, there are no data on the number of pain relief medications that people may procure to take care of their symptoms. There is a need for a prospective follow-up of people who do not have surgery to have further insight regarding their health-seeking behaviour, particularly the number of times they visit their GP and other health-care providers for the management of uncomplicated symptomatic gallstone disease (biliary pain or cholecystitis).
Chapter 7 Conclusions
Implications for health care
Cholecystectomy, and in particular laparoscopic cholecystectomy, is still the standard treatment for symptomatic gallstone disease (biliary pain or cholecystitis). The main clinical indication for performing cholecystectomy is persistent or excruciating pain. The results of this assessment, although associated with some uncertainty, suggest that uncomplicated symptomatic gallstones may have a more benign natural course than previously assumed and it is likely that a proportion of patients may not require surgery in the long term and this seems to be safe in terms of subsequent events.
Cholecystectomy is more costly to the NHS because of the use of resources associated with surgery and the costs related to the treatment of post-surgery complications. Our modelling does show, however, that conservative treatment/observation fails to become a cost-effective option when a high proportion of people develop complications and require emergency surgery. A policy of surgery for all, as opposed to a policy of conservative management followed by surgery for people whose symptoms persist, is likely to be more costly but more effective, even though the difference between the two policies is modest. Owing to the current dearth of evidence for some of the relevant health states for gallstone disease and, consequently, the uncertainty around some utility values used in the model, the findings of our economic evaluation require a cautious interpretation.
At present, our information suggests that in many centres in the UK, cholecystectomy is the default option for all people with symptomatic gallstone disease with no attempts to identify people who might benefit from a conservative approach. Approximately 70,000 surgical procedures are performed every year in the UK and, in many hospitals, people are put on a waiting list and operated electively. However, conservative management may be a valid option in people presenting with uncomplicated symptoms (biliary pain or cholecystitis) depending on their age and sex, as well as on the clinical presentation of the disease and the evolution of symptoms over time. In addition, as uncomplicated symptoms are usually not urgent, it may be reasonable to take into consideration a non-surgical option first, alongside people’s personal preference and convenience. The recognition of symptoms related to gallstones remains, however, a main challenge in the management of the disease.
Suggested research priorities
The main gap in the current evidence is the dearth of randomised trials comparing cholecystectomy with observation/conservative management in uncomplicated, symptomatic gallstone disease, especially in the UK setting. For this assessment we identified only two RCTs,46,113 conducted in Norway, and we are not aware of any ongoing studies on this topic.
A large, good-quality, clinical trial needs to be undertaken to compare the effects and safety of conservative management with cholecystectomy in people presenting with uncomplicated symptomatic gallstones (biliary pain only) or cholecystitis. Ideally, such a trial would be multicentre, with long-term follow-up, would include a pre-specified assessment of people’s symptoms, relevant outcome measures, such as post-cholecystectomy symptoms and QoL measurements, and a full economic evaluation.
Areas in which further research would be important are the following:
-
research based on well-designed clinical trials to compare the effects and safety of observation/conservative management with cholecystectomy in people presenting with uncomplicated symptomatic gallstones (biliary pain) or cholecystitis to secondary care
-
research based on large, long-term, prospective cohorts or population studies to evaluate the natural history of symptomatic gallstone disease (including uncomplicated cases) in terms of frequency, characteristics of disease presentation; evolution of symptoms over time and clinical outcomes
-
research to elicit factors that may predict the evolution of symptoms in people with symptomatic gallstone disease
-
research on people’s personal preferences in terms of treatment options and outcomes
-
research to identify resource use for people undergoing cholecystectomy (pre-operative assessment, surgical admissions, post-operative management) and for those receiving conservative management, in order to develop more robust cost estimates for the UK.
Acknowledgements
We are grateful to Lara Kemp for her secretarial support; Kieran Rothnie for contributing to the assessment of full-text papers; Graham Scotland for contributing to the checking of the economic model and for his comments on the draft of the cost-effectiveness chapter; Jonathan Cook for providing statistical advice; John Leeds (Consultant Gastroenterologist, Aberdeen Royal Infirmary) and Yuen Soon (Consultant Surgeon, Royal Surrey County Hospital) for providing clinical guidance as members of the advisory group for this assessment; Heather Peace for providing valuable consumer insight and advice through her participation as member of the advisory group for this assessment; and Karl Søndenaa, University of Bergen, Norway, for providing additional information and assisting with queries relating to the two included trials.
This report was commissioned by the National Institute for Health Research Health Technology Assessment programme as project number 12/16/01. The Health Services Research Unit and Health Economics Research Unit are core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorates. The views and opinions expressed are those of the authors and do not necessarily reflect those of the funders.
Contribution of authors
Miriam Brazzelli (Senior Research Fellow) oversaw and co-ordinated all aspects of the assessment and wrote the original protocol.
Moira Cruickshank (Research Fellow) led the day-to-day running of the assessment and reviewed the evidence on the clinical effectiveness of the interventions.
Mary Kilonzo (Research Fellow) developed the care pathway for gallstone disease with clinical advice from Irfan Ahmed and conducted the economic evaluation with supervision from Paul McNamee (Professor of Health Economics).
Irfan Ahmed (Consultant Surgeon) and Alison Avenell (Clinical Professor of Health Services Research) provided expert advice on the clinical aspects and management of the disease.
Fiona Stewart (Information Specialist) developed and ran the search strategies with supervision from Cynthia Fraser (Senior Information Officer) and was responsible for obtaining full-text papers and for compiling the reference list of the report.
Andrew Elders provided statistical advice.
Craig Ramsay (Health Care Assessment Programme Director) jointly co-ordinated the assessment, led and co-ordinated the expert advisory group participation, and commented on the draft version of the report.
The authors of the draft version report were as follows: scientific summary, Miriam Brazzelli; background, Miriam Brazzelli and Moira Cruickshank; definition of the decision problem, Miriam Brazzelli; methods, Moira Cruickshank, Fiona Stewart, Andrew Elders and Miriam Brazzelli; clinical effectiveness, Moira Cruickshank, Andrew Elders and Miriam Brazzelli; cost-effectiveness, MaryKilonzo and Paul McNamee; and discussion, Miriam Brazzelli;Mary Kilonzo; Conclusions: Miriam Brazzelli.
All authors commented on the draft of the report and approved its final version.
Publication
Brazzelli M, Cruickshank M, Kilonzo M, Ahmed I, Stewart F, McNamee P, et al. Systematic review of the clinical and cost effectiveness of cholecystectomy versus observation/conservative management for uncomplicated symptomatic gallstones or cholecystitis. Surg Endosc 2014: in press. doi: 10.1007/s00464-014-3712-6
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- American College of Physicians . Guidelines for the treatment of gallstones. Ann Intern Med 1993;119:620-2.
- Aerts R, Penninckx F. The burden of gallstone disease in Europe. Aliment Pharmacol Ther Suppl 2003;18:49-53. http://dx.doi.org/10.1046/j.0953-0673.2003.01721.x.
- Kang JY, Ellis C, Majeed A, Hoare J, Tinto A, Williamsons RCN, et al. Gallstones – an increasing problem: a study of hospital admissions in England between 1989/1990 and 1999/2000. Aliment Pharmacol Ther 2003;17:561-9. http://dx.doi.org/10.1046/j.1365-2036.2003.01439.x.
- Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, et al. The burden of selected digestive diseases in the United States. Gastroenterology 2002;122:1500-11. http://dx.doi.org/10.1053/gast.2002.32978.
- National Institutes of Health . Gallstones and Laparoscopic Cholecystectomy, NIH Consensus Statement. Consensus Development Conference Statement, September 14–16 1992. http://consensus.nih.gov/1992/1992gallstoneslaparoscopy090html.htm (accessed May 2013).
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. Br J Surg 2004;91:734-8. http://dx.doi.org/10.1002/bjs.4547.
- Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet 2006;368:230-9. http://dx.doi.org/10.1016/S0140-6736(06)69044-2.
- Shaffer EA. Epidemiology of gallbladder stone disease. Clin Gastroenterol 2006;20:981-96. http://dx.doi.org/10.1016/j.bpg.2006.05.004.
- Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterol Clin North Am 2010;39:157-69. http://dx.doi.org/10.1016/j.gtc.2010.02.003.
- Bateson MC. Gallstones and cholecystectomy in modern Britain. Postgrad Med J 2000;76:700-3. http://dx.doi.org/10.1136/pmj.76.901.700.
- Attili AF, Carulli N, Roda E, Barbara B, Capocaccia L, Menotti A, et al. Epidemiology of gallstone disease in Italy: prevalence data of the Multicenter Italian Study on Cholelithiasis (M.I.COL.). Am J Epidemiol 1995;141:158-65.
- Festi D, Reggiani MLB, Attili AF, Loria P, Pazzi P, Scaioli E, et al. Natural history of gallstone disease: Expectant management or active treatment? Results from a population-based cohort study. J Gastroenterol Hepatol 2010;25:719-24. http://dx.doi.org/10.1111/j.1440-1746.2009.06146.x.
- Attili AF, De Santis A, Capri R, Repice AM, Maselli S, Capocaccia L, et al. The natural history of gallstones: the GREPCO experience. Hepatology 1995;21:656-60. http://dx.doi.org/10.1002/hep.1840210309.
- Schmidt M, Hausken T, Glambek I, Schleer C, Eide GE, Søndenaa K. A 24-year controlled follow-up of patients with silent gallstones showed no long-term risk of symptoms or adverse events leading to cholecystectomy. Scand J Gastroenterol 2011;46:949-54. http://dx.doi.org/10.3109/00365521.2011.571710.
- Portincasa P, Di Ciaula A, Wang HH, Moschetta A, Wang DQH. Medicinal treatments of cholesterol gallstones: old, current and new perspectives. Curr Med Chem 2009;16:1531-42. http://dx.doi.org/10.2174/092986709787909631.
- Schmidt M, Søndenaa K, Dumot JA, Rosenblatt S, Hausken T, Ramnefjell M, et al. Post-cholecystectomy symptoms were caused by persistence of a functional gastrointestinal disorder. World J Gastroenterol 2012;18:1365-72. http://dx.doi.org/10.3748/wjg.v18.i12.1365.
- McSherry CK, Ferstenberg H, Calhoun WF, Lahman E, Virshup M. The natural history of diagnosed gallstone disease in symptomatic and asymptomatic patients. Ann Surg 1985;202:59-63. http://dx.doi.org/10.1097/00000658-198507000-00009.
- Søndenaa K, Nesvik I, Solhaug JH, Soreide O. Randomization to surgery or observation in patients with symptomatic gallbladder stone disease: the problem of evidence-based medicine in clinical practice. Scand J Gastroenterol 1997;32:611-16. http://dx.doi.org/10.3109/00365529709025108.
- Patton KD, Thibodeau GA. Anatomy and Physiology. Missouri: Mosby Elsevier; 2012.
- Thudichum JLW. A Treatise on Gallstones their Chemistry, Pathology and Treatment. London: John Churchill & Sons; 1983.
- Friedman GD. Natural-history of asymptomatic and symptomatic gallstones. Am J Surg 1993;165:399-404. http://dx.doi.org/10.1016/S0002-9610(05)80930-4.
- Acalovschi M. Cholesterol gallstones: from epidemiology to prevention. Postgrad Med J 2001;77:221-9. http://dx.doi.org/10.1136/pmj.77.906.221.
- National Digestive Diseases Information Clearinghouse (NDDIC) . Gallstones 2010. www.digestive.niddk.nih.gov/ddiseases/pubs/gallstones/index.aspx (accessed May 2013).
- Berger MY, van der Velden JJ, Lijmer JG, de KH, Prins A, Bohnen AM. Abdominal symptoms: do they predict gallstones? A systematic review. Scand J Gastroenterol 2000;35:70-6.
- Society for Surgery of the Alimentary Tract patient care guidelines. Treatment of gallstone and gallbladder disease . J Gastrointest Surg 2007;11:1222-4.
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 2012;6:172-87. http://dx.doi.org/10.5009/gnl.2012.6.2.172.
- Barbara L, Sama C, Labate AMM. A population study on the prevalence of gallstone disease: the Sirmione study. Hepatology 1987;7:913-17. http://dx.doi.org/10.1002/hep.1840070520.
- Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 1999;117:632-9. http://dx.doi.org/10.1016/S0016-5085(99)70456-7.
- Everhart JE, Yeh F, Lee ET, Hill MC, Fabsitz R, Howard BV, et al. Prevalence of gallbladder disease in American Indian populations: Findings from the Strong Heart Study. Hepatology 2002;35:1507-12. http://dx.doi.org/10.1053/jhep.2002.33336.
- Glambek I, Kvaale G, Arnesjo B, Soreide O. Prevalence of gallstones in a Norwegian population. Scand J Gastroenterol 1987;22:1089-94. http://dx.doi.org/10.3109/00365528708991963.
- Jorgensen T. Prevalence of gallstones in a Danish population. Am J Epidemiol 1987;126:912-21.
- Loria P, Dilengite A, Bozzoli M, Carubbi F, Messora R, Sassatelli R, et al. Prevalence rates of gallstone disease in Italy. Eur J Epidemiol 1994;10:143-50. http://dx.doi.org/10.1007/BF01730363.
- Miquel JF, Covarrubias C, Villaroel L, Mingrone G, Greco AV, Puglielli L, et al. Genetic epidemiology of cholesterol cholelithiasis among Chilean Hispanics, Amerindians, and Maoris. Gastroenterology 1998;115:937-46. http://dx.doi.org/10.1016/S0016-5085(98)70266-5.
- Nomura H, Kashiwagi S, Hayashi J, Kajiyama W, Ikematsu H, Noguchi A, et al. Prevalence of gallstone disease in a general population of Okinawa, Japan. Am J Epidemiol 1988;128:598-605.
- Heaton KW, Braddon FEM, Mountford RA, Hughes AO, Emmett PM. Symptomatic and silent gall stones in the community. Gut 1991;32:316-20. http://dx.doi.org/10.1136/gut.32.3.316.
- Khuroo MS, Mahajan R, Zargar SA, Javid G, Sapru S. Prevalence of biliary tract disease in India: a sonographic study in adult population in Kashmir. Gut 1989;30:201-5. http://dx.doi.org/10.1136/gut.30.2.201.
- Williams JG, Roberts SE, Ali MF, Cheung WY, Cohen DR, Demery G, et al. Gastroenterology services in the UK. The burden of disease, and the organisation and delivery of services for gastrointestinal and liver disorders: a review of the evidence. Gut 2007;56:1-113. http://dx.doi.org/10.1136/gut.2006.117598.
- Festi D, Dormi A, Capodicasa S, Staniscia T, Attili AF, Loria P, et al. Incidence of gallstone disease in Italy: results from a multicenter, population-based Italian study (the MICOL project). World J Gastroenterol 2008;14:5282-9. http://dx.doi.org/10.3748/wjg.14.5282.
- Holland C, Heaton KW. Increasing frequency of gall bladder operations in the Bristol clinical area. Br Med J 1972;3:672-5. http://dx.doi.org/10.1136/bmj.3.5828.672.
- Gracie WA, Ransohoff DF. The natural history of silent gallstones. The innocent gallstone is not a myth. N Engl J Med 1982;307:798-800. http://dx.doi.org/10.1056/NEJM198209233071305.
- Beckingham IJ. ABC of diseases of liver, pancreas, and biliary system: gallstone disease. BMJ 2001;322:91-4. http://dx.doi.org/10.1136/bmj.322.7278.91.
- Friedman GD, Raviola CA, Fireman B. Prognosis of gallstones with mild or no symptoms: 25 years of follow-up in a health maintenance organization. J Clin Epidemiol 1989;42. http://dx.doi.org/10.1016/0895-4356(89)90086-3.
- Thistle JL, Cleary PA, Lachin JM, Tyor MP, Hersh T. The natural history of cholelithiasis: the National Cooperative Gallstone Study. Ann Int Med 1984;101:171-5. http://dx.doi.org/10.7326/0003-4819-101-2-171.
- Newman HF, Northup JD, Rosenblum M, Abrams H. Complications of cholelithiasis. Am J Gastroenterol 1968;50:476-96.
- Ransohoff DF, Gracie WA. Treatment of gallstones. Ann Int Med 1993;119:606-19. http://dx.doi.org/10.7326/0003-4819-119-7_Part_1-199310010-00010.
- Schmidt M, Søndenaa K, Vetrhus M, Berhane T, Eide GE. Long-term follow-up of a randomized controlled trial of observation versus surgery for acute cholecystitis: non-operative management is an option in some patients. Scand J Gastroenterol 2011;46:1257-62. http://dx.doi.org/10.3109/00365521.2011.598548.
- Nenner RP, Imperato PJ, Rosenberg C, Ronberg E. Increased cholecystectomy rates among Medicare patients after the introduction of laparoscopic cholecystectomy. J Community Health 1994;19:409-15. http://dx.doi.org/10.1007/BF02260323.
- Zacks SL, Sandler RS, Rutledge R, Brown J. A population-based cohort study comparing laparoscopic cholecystectomy and open cholecystectomy. Am J Gastroenterol 2002;97:334-40. http://dx.doi.org/10.1016/S0002-9270(01)04028-X.
- Berhane T, Vetrhus M, Hausken T, Olafsson S, Søndenaa K. Pain attacks in non-complicated and complicated gallstone disease have a characteristic pattern and are accompanied by dyspepsia in most patients: the results of a prospective study. Scand J Gastroenterol 2006;41:93-101. http://dx.doi.org/10.1080/00365520510023990.
- Schmidt M, Dumot JA, Soreide O, Søndenaa K. Diagnosis and management of gallbladder calculus disease. Scand J Gastroenterol 2012;47:1257-65. http://dx.doi.org/10.3109/00365521.2012.704934.
- Duncan CB, Riall TS. Evidence-based current surgical practice: calculous gallbladder disease. J Gastrointest Surg 2012;16:2011-25. http://dx.doi.org/10.1007/s11605-012-2024-1.
- Shojaiefard A, Esmaeilzadeh M, Ghafouri A, Mehrabi A. Various techniques for the surgical treatment of common bile duct stones: a meta review. Gastroenterol Res Pract 2009. http://dx.doi.org/10.1155/2009/840208.
- Johnston DE, Kaplan MM. Medical progress: pathogenesis and treatment of gallstones. N Engl J Med 1993;328:412-21.
- Whitcomb DC. Acute pancreatitis. N Engl J Med 2006;354:2142-50. http://dx.doi.org/10.1056/NEJMcp054958.
- Vetrhus M, Soreide O, Eide GE, Nesvik I, Søndenaa K. Quality of life and pain in patients with acute cholecystitis. Results of a randomized clinical trial. Scand J Surg 2005;94:34-9.
- Larsen TK, Qvist N. The influence of gallbladder function on the symptomatology in gallstone patients, and the outcome after cholecystectomy or expectancy. Dig Dis Sci 2007;52:760-3. http://dx.doi.org/10.1007/s10620-006-9498-1.
- Bates T, Ebbs SR, Harrison M, A’Hern RP. Influence of cholecystectomy on symptoms. Br J Surg 1991;78:964-7. http://dx.doi.org/10.1002/bjs.1800780823.
- Borly L, Anderson IB, Bardram L, Christensen E, Sehested A, Kehlet H, et al. Preoperative prediction model of outcome after cholecystectomy for symptomatic gallstones. Scand J Gastroenterol 1999;34:1144-52. http://dx.doi.org/10.1080/003655299750024968.
- Gui GP, Cheruvu CV, West N, Sivaniah K, Fiennes AG. Is cholecystectomy effective treatment for symptomatic gallstones? Clinical outcome after long-term follow-up. Ann R Coll Surg Engl 1998;80:25-32.
- Middelfart HV, Kristensen JU, Laursen CN, Qvist N, Hojgaard L, Funch-Jensen P, et al. Pain and dyspepsia after elective and acute cholecystectomy. Scand J Gastroenterol 1998;33:10-4.
- Ure BM, Troidl H, Spangenberger W, Lefering R, Dietrich A, Eypasch EP, et al. Long-term results after laparoscopic cholecystectomy. Br J Surg 1995;82:267-70. http://dx.doi.org/10.1002/bjs.1800820243.
- Vetrhus M, Berhane T, Soreide O, Søndenaa K. Pain persists in many patients five years after removal of the gallbladder: observations from two randomized controlled trials of symptomatic, noncomplicated gallstone disease and acute cholecystitis. J Gastrointest Surg 2005;9:826-31. http://dx.doi.org/10.1016/j.gassur.2005.01.291.
- Lamberts MP, Lugtenberg M, Rovers MM, Roukema AJ, Drenth JPH, Westert GP, et al. Persistent and de novo symptoms after cholecystectomy: a systematic review of cholecystectomy effectiveness. Surg Endosc 2013;27:709-18. http://dx.doi.org/10.1007/s00464-012-2516-9.
- Jorgensen T, Stubbe Teglbjerg J, Wille-Jorgensen P, Bille T, Thorvaldsen P. Persisting pain after cholecystectomy. A prospective investigation. Scand J Gastroenterol 1991;26:124-8. http://dx.doi.org/10.3109/00365529108996493.
- Ahmed R, Freeman JV, Ross B, Kohler B, Nicholl JP, Johnson AG. Long term response to gallstone treatment – problems and surprises. Eur J Surg 2000;166:447-54.
- Peterli R, Schuppisser JP, Herzog U, Ackermann C, Tondelli PE. Prevalence of postcholecystectomy symptoms: long-term outcome after open versus laparoscopic cholecystectomy. World J Surg 2000;24:1232-5.
- McMahon AJ, Ross S, Baxter JN, Russell IT, Anderson JR, Morran CG, et al. Symptomatic outcome 1 year after laparoscopic and minilaparotomy cholecystectomy: a randomized trial. Br J Surg 1995;82:1378-82. http://dx.doi.org/10.1002/bjs.1800821028.
- Girometti R, Brondani G, Cereser L, Como G, Del Pin M, Bazzocchi M, et al. Post-cholecystectomy syndrome: spectrum of biliary findings at magnetic resonance cholangiopancreatography. Br J Radiol 2010;83:351-61. http://dx.doi.org/10.1259/bjr/99865290.
- Luman W, Adams WH, Nixon SN, Mcintyre IM, Hamer-Hodges D, Wilson G, et al. Incidence of persistent symptoms after laparoscopic cholecystectomy: a prospective study. Gut 1996;39:863-6. http://dx.doi.org/10.1136/gut.39.6.863.
- Bisgaard T, Rosenberg J, Kehlet H. From acute to chronic pain after laparoscopic cholecystectomy: A prospective follow-up analysis. Scand J Gastroenterol 2005;40:1358-64. http://dx.doi.org/10.1080/00365520510023675.
- Livingston EH, Rege RV. A nationwide study of conversion from laparoscopic to open cholecystectomy. Am J Surg 2004;188:205-11. http://dx.doi.org/10.1016/j.amjsurg.2004.06.013.
- Gurusamy KS, Koti R, Fusai G, Davidson BR. Early versus delayed laparoscopic cholecystectomy for uncomplicated biliary colic. Cochrane Database Syst Rev 2008;4. http://dx.doi.org/10.1002/14651858.CD007196.pub3.
- Al-Akeely MH. Management of complicated gallstone disease during pregnancy. Saudi J Gastroenterol 2003;9:135-8.
- Date RS, Kaushal M, Ramesh A. A review of the management of gallstone disease and its complications in pregnancy. Am J Surg 2008;196:599-608. http://dx.doi.org/10.1016/j.amjsurg.2008.01.015.
- McGillicuddy EA, Schuster KM, Barre K, Suarez L, Hall MR, Kaml GJ, et al. Non-operative management of acute cholecystitis in the elderly. Br J Surg 2012;99:1254-61. http://dx.doi.org/10.1002/bjs.8836.
- Daradkeh S, Sumrein I, Daoud P, Zaidin K, Abu-Khalaf M. Management of gallbladder stones during pregnancy: conservative treatment or laparoscopic cholecystectomy?. Hepato-gastroenterology 1999;46:3074-6.
- Vetrhus M, Soreide O, Solhaug JH, Nesvik I, Søndenaa K. Symptomatic, non-complicated gallbladder stone disease. Operation or observation? A randomized clinical study. Scand J Gastroenterol 2002;37:834-9.
- Vetrhus M, Soreide O, Nesvik I, Søndenaa K. Acute cholecystitis: delayed surgery or observation. A randomized clinical trial. Scand J Gastroenterol 2003;38:985-90.
- Di Ciaula A, Wang DQH, Wang HH, Bonfrate L, Portincasa P. Targets for current pharmacologic therapy in cholesterol gallstone disease. Gastroenterol Clin North Am 2010;39:245-64. http://dx.doi.org/10.1016/j.gtc.2010.02.005.
- Sackmann M, Niller H, Klueppelberg U, Von Ritter C, Pauletzki J, Holl J, et al. Gallstone recurrence after shock-wave therapy. Gastroenterology 1994;106:225-30.
- Villanova N, Bazzoli F, Taroni F, Frabboni R, Mazzella G, Festi D, et al. Gallstone recurrence after successful oral bile acid treatment: A 12-year follow-up study and evaluation of long-term postdissolution treatment. Gastroenterology 1989;97:726-31.
- Carrilho-Ribeiro L, Pinto-Correia A, Velosa J, Carneiro de Moura M. Long-term gallbladder stone recurrence and risk factors after successful lithotripsy. Eur J Gastroenterol Hepatol 2000;12:209-15. http://dx.doi.org/10.1097/00042737-200012020-00013.
- Kim WR, Brown J, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology 2002;36:227-42. http://dx.doi.org/10.1053/jhep.2002.34734.
- UK Department of Health . NHS Reference Costs 2011–12 2012. www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012 (accessed May 2013).
- UK Department of Health . 2011–12 Tarriff Information Spreadsheet 2011. www.gov.uk/government/uploads/system/uploads/attachment_data/file/151912/dh_125398.xls.xls (accessed May 2012).
- Lawrentschuk N, Hewitt PM, Pritchard MG. Elective laparoscopic cholecystectomy: implications of prolonged waiting times for surgery. ANZ J Surg 2003;73:890-3. http://dx.doi.org/10.1046/j.1445-2197.2003.02826.x.
- Salman B, Yuksel O, Irkorucu O, Akyurek N, Tezcaner T, Dogan I, et al. Urgent laparoscopic cholecystectomy is the best management for biliary colic. Dig Surg 2005;22:95-9. http://dx.doi.org/10.1159/000085300.
- Somasekar K, Shankar PJ, Foster ME, Lewis MH. Costs of waiting for gall bladder surgery. Postgrad Med J 2002;78:668-9. http://dx.doi.org/10.1136/pmj.78.925.668.
- Rutledge D, Jones D, Rege R. Consequences of delay in surgical treatment of biliary disease. Am J Surg 2000;180:466-9. http://dx.doi.org/10.1016/S0002-9610(00)00520-1.
- A Guide for Commissioners. Coventry: NHS Institution for Innovation and Improvement; 2006.
- Chamberlain RS, Sakpal SV. A Comprehensive review of single-incision laparoscopic surgery (SILS) and natural orifice transluminal endoscopic surgery (NOTES) techniques for cholecystectomy. J Gastrointest Surg 2009;13:1733-40. http://dx.doi.org/10.1007/s11605-009-0902-y.
- McSherry CK. Cholecystectomy: the gold standard. Am J Surg 1989;158:174-8. http://dx.doi.org/10.1016/0002-9610(89)90246-8.
- Keus F, de Jonge T, Gooszen HG, Buskens E, van Laarhoven CJHM. Cost-minimization analysis in a blind randomized trial on small-incision versus laparoscopic cholecystectomy from a societal perspective: sick leave outweighs efforts in hospital savings. Trials 2009;10. http://dx.doi.org/10.1186/1745-6215-10-80.
- Charlo Dupont T, Fernandez Martin M, Tejido Sanchez C. A cost analysis of laparoscopic cholecystectomy compared with the open technic. Rev Esp Enferm Dig 1995;87:449-52.
- Langebuch C. Ein fall von extirpation der gallenblase wegen chronischer cholelithiasis. Berl Klinisch Wochenschr 1882;48:725-7.
- Visser BC, Parks RW, Garden OJ. Open cholecystectomy in the laparoendoscopic era. Am J Surg 2008;195:108-14. http://dx.doi.org/10.1016/j.amjsurg.2007.04.008.
- Tang B, Cuschieri A. Conversions during laparoscopic cholecystectomy: risk factors and effects on patient outcome. J Gastrointest Surg 2006;10:1081-91. http://dx.doi.org/10.1016/j.gassur.2005.12.001.
- Ballal M, David G, Willmott S, Corless DJ, Deakin M, Slavin JP. Conversion after laparoscopic cholecystectomy in England. Surg Endosc 2009;23:2338-44. http://dx.doi.org/10.1007/s00464-009-0338-1.
- Overby DW, Apelgren KN, Richardson W, Fanelli R. SAGES guidelines for the clinical application of laparoscopic biliary tract surgery. Surg Endosc 2010;24:2368-86. http://dx.doi.org/10.1007/s00464-010-1268-7.
- Saad S, Strassel V, Sauerland S. Randomized clinical trial of single-port, minilaparoscopic and conventional laparoscopic cholecystectomy. Br J Surg 2013;100:339-48. http://dx.doi.org/10.1002/bjs.9003.
- Stegenga H, Patrick H, Campbell B. NICE news in the annals: Recent NICE Guidance of interest to surgeons. Ann R Coll Surg Engl 2010;92:254-6. http://dx.doi.org/10.1308/003588410X12664192075215.
- Interventional Procedure Overview of Single-Incision Laparoscopic Cholecystectomy. London: NICE; 2009.
- Arezzo A, Scozzari G, Famiglietti F, Passera R, Morino M. Is single-incision laparoscopic cholecystectomy safe? Results of a systematic review and meta-analysis. Surg Endosc 2013;27:2293-304. http://dx.doi.org/10.1007/s00464-012-2763-9.
- Wang D, Wang Y, Ji ZL. Laparoendoscopic single-site cholecystectomy versus conventional laparoscopic cholecystectomy: a systematic review of randomized controlled trials. ANZ J Surg 2012;82:303-10. http://dx.doi.org/10.1111/j.1445-2197.2012.06044.x.
- Antoniou SA, Pointner R, Granderath FA. Single-incision laparoscopic cholecystectomy: a systematic review. Surg Endosc 2011;25:367-77. http://dx.doi.org/10.1007/s00464-010-1217-5.
- Keus F, de Jong J, Gooszen HG, Laarhoven CJHM. Small-incision versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev 2006;4. http://dx.doi.org/10.1002/14651858.CD004788.pub2.
- Health & Social Care Information Centre . Main Procedures and Interventions: 4 Character 2011–12 2011. http://www.hscic.gov.uk/catalogue/PUB08288/hosp-epis-stat-admi-main-ops-4cha-11-12-tab.xls (accessed May 2013).
- Lam CM, Murray FE, Cuschieri A. Increased cholecystectomy rate after the introduction of laparoscopic cholecystectomy in Scotland. Gut 1996;38:282-4. http://dx.doi.org/10.1136/gut.38.2.282.
- Leopold GR, Amberg J, Gosink BB, Mittelstaedt C. Gray scale ultrasonic cholecystography: a comparison with conventional radiographic techniques. Radiology 1976;121:445-8.
- Portincasa P, Di Ciaula A, Baldassarre G, Palmieri V, Gentile A, Cimmino A, et al. Gallbladder motel function in gallstone patients: sonographic and in vitro studies on the role of gallstones; smooth muscle function and gallbladder wall inflammation. J Hepatol 1994;21:430-40.
- Fullarton GM, Bell G. Prospective audit of the introduction of laparoscopic cholecystectomy in the west of Scotland. Gut 1994;35:1121-6. http://dx.doi.org/10.1136/gut.35.8.1121.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 2011. www.cochrane-handbook.org (accessed April 2013).
- Schmidt M, Søndenaa K, Vetrhus M, Berhane T, Eide GE. A randomized controlled study of uncomplicated gallstone disease with a 14-year follow-up showed that operation was the preferred treatment. Dig Surg 2011;28:270-6. http://dx.doi.org/10.1159/000329464.
- Vetrhus M, Soreide O, Eide GE, Solhaug JH, Nesvik I, Søndenaa K. Pain and quality of life in patients with symptomatic, non-complicated gallbladder stones: results of a randomized controlled trial. Scand J Gastroenterol 2004;39:270-6. http://dx.doi.org/10.1080/00365520310008502.
- Dupuy HJ, Wenger NK, Mattson ME, Furburg CD, Elinson J. Assessment if Quality of Life in Clinical Trials of Cardiovascular Therapies. New York, NY: Le Jacq Publications; 1984.
- Wilson E, Gurusamy K, Gluud C, Davidson BR. Cost-utility and value-of-information analysis of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg 2010;97:210-19. http://dx.doi.org/10.1002/bjs.6872.
- Macafee DA, Humes DJ, Bouliotis G, Beckingham IJ, Whynes DK, Lobo DN. Prospective randomized trial using cost-utility analysis of early versus delayed laparoscopic cholecystectomy for acute gallbladder disease. Br J Surg 2009;96:1031-40.
- Interim Life Tables 2013. www.gad.gov.uk/Demography%20Data/Life%20Tables/index.html (accessed May 2013).
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Debru E, Dawson A, Leibman S, Richardson M, Glen L, Hollinshead J, et al. Does routine intraoperative cholangiography prevent bile duct transection?. Surg Endosc 2005;19:589-93. http://dx.doi.org/10.1007/s00464-004-8711-6.
- Diamantis T, Tsigris C, Kiriakopoulos A, Papalambros E, Bramis J, Michail P, et al. Bile duct injuries associated with laparoscopic and open cholecystectomy: an 11-year experience in one institute. Surg Today 2005;35:841-5. http://dx.doi.org/10.1007/s00595-005-3038-z.
- Karvonen J, Gullichsen R, Laine S, Salminen P, Gronroos JM. Bile duct injuries during laparoscopic cholecystectomy: primary and long-term results from a single institution. Surg Endosc 2007;21:1069-73. http://dx.doi.org/10.1007/s00464-007-9316-7.
- Nuzzo G, Giuliante F, Giovannini I, Ardito F, D’Acapito F, Vellone M, et al. Bile duct injury during laparoscopic cholecystectomy: results of an Italian national survey on 56 591 cholecystectomies. Arch Surg 2005;140:986-92. http://dx.doi.org/10.1001/archsurg.140.10.986.
- Singh K, Ohri A. Anatomic landmarks: their usefulness in safe laparoscopic cholecystectomy. Surg Endosc 2006;20:1754-8. http://dx.doi.org/10.1007/s00464-005-0528-4.
- Tantia O, Jain M, Khanna S, Sen B. Iatrogenic biliary injury: 13,305 cholecystectomies experienced by a single surgical team over more than 13 years. Surg Endosc 2008;22:1077-86. http://dx.doi.org/10.1007/s00464-007-9740-8.
- Waage A, Nilsson M. Iatrogenic bile duct injury: a population-based study of 12 776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg 2006;141:1207-13.
- Avgerinos C, Kelgiorgi D, Touloumis Z, Baltatzi L, Dervenis C. One thousand laparoscopic cholecystectomies in a single surgical unit using the ‘critical view of safety’ technique. J Gastrointest Surg 2009;13:498-503.
- NHS Choices . Gallbladder 2012. www.nhs.uk/conditions/Laparoscopiccholecystectomy/Pages/Introduction.aspx (accessed May 2013).
- Finan KR, Leeth RR, Whitley BM, Klapow JC, Hawn MT. Improvement in gastrointestinal symptoms and quality of life after cholecystectomy. Am J Surg 2006;192:196-202. http://dx.doi.org/10.1016/j.amjsurg.2006.01.020.
- Ros E, Zambon D. Postcholecystectomy symptoms. A prospective study of gall stone patients before and two years after surgery. Gut 1987;28:1500-4. http://dx.doi.org/10.1136/gut.28.11.1500.
- Weinert CR, Arnett D, Jacobs D, Kane RL. Relationship between persistence of abdominal symptoms and successful outcome after cholecystectomy. Arch Int Med 2000;160:989-95. http://dx.doi.org/10.1001/archinte.160.7.989.
- Glasgow RE, Mulvihill SJ, Feldman M. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Philadelphia, PA: Saunders; 2010.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Harrison EM, O’Neill S, Meurs TS, Wong PL, Duxbury M, Paterson-Brown S, et al. Hospital volume and patient outcomes after cholecystectomy in Scotland: retrospective, national population based study. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e3330.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit; 2012.
- Bass EB, Steinberg EP, Pitt HA, Saba GP, Lillemoe KD, Kafonek DR, et al. Cost-effectiveness of extracorporeal shock-wave lithotripsy versus cholecystectomy for symptomatic gallstones. Gastroenterology 1991;101:189-99.
- Bass EB, Pitt HA, Lillemoe KD. Cost-effectiveness of laparoscopic cholecystectomy versus open cholecystectomy. Am J Surg 1993;165:466-71. http://dx.doi.org/10.1016/S0002-9610(05)80942-0.
- Cook J, Richardson J, Street A. A cost utility analysis of treatment options for gallstone disease: methodological issues and results. Health Econ 1994;3. http://dx.doi.org/10.1002/hec.4730030305.
- Nilsson E, Ros A, Rahmqvist M, Backman K, Carlsson P. Cholecystectomy: Costs and health-related quality of life: A comparison of two techniques. Int J Qual Health Care 2004;16:473-82. http://dx.doi.org/10.1093/intqhc/mzh077.
- Bass EB, Steinberg EP, Pitt HA, Griffiths RI, Lillemoe KD, Saba GP, et al. Comparison of the rating scale and the standard gamble in measuring patient preferences for outcomes of gallstone disease. Med Decis Making 1994;14:307-14. http://dx.doi.org/10.1177/0272989X9401400401.
- Sobolev B, Mercer D, Brown P, FitzGerald M, Jalink D, Shaw R. Risk of emergency admission while awaiting elective cholecystectomy. CMAJ 2003;169:662-5.
- NHS Choices . Guide to Waiting Times 2012. www.nhs.uk/choiceinthenhs/rightsandpledges/waitingtimes/pages/guide%20to%20waiting%20times.aspx (accessed May 2013).
- Persson GE. Expectant management of patients with gallbladder stones diagnosed at planned investigation: a prospective 5- to 7-year follow-up study of 153 patients. Scand J Gastroenterol 1996;31:191-9. http://dx.doi.org/10.3109/00365529609031985.
- Keus F, De Jong JAF, Gooszen HG, van Laarhoven CJHM. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev 2006;4. http://dx.doi.org/10.1002/14651858.CD006231.
- Keus F, de Jong JA, Gooszen HG, van Laarhoven CJ. Laparoscopic versus small-incision cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev 2006;4. http://dx.doi.org/10.1002/14651858.CD006229.
- Gurusamy KS, Davidson C, Gluud C, Davidson BR. Early versus delayed laparoscopic cholecystectomy for people with acute cholecystitis. Cochrane Database Syst Rev 2006;4. http://dx.doi.org/10.1002/14651858.CD005440.pub3.
- Berger MY, Olde H, van d, Bohnen AM. Is biliary pain exclusively related to gallbladder stones? A controlled prospective study. Br J Gen Pract 2004;54:574-9.
- Johnson AG, Fried M, Tytgat GNJ, Krabshuis JH. WGO Practice Guideline – Asymptomatic Gallstone Disease. Milwaukee, WI: World Gastroenterology Organisation; 2006.
- Goldacre MJ, Abisgold JD, Seagroatt V, Yeates D. Cancer after cholecystectomy: record-linkage cohort study. Br J Cancer 2005;92:1307-9. http://dx.doi.org/10.1038/sj.bjc.6602392.
- Shao T, Yang YX. Cholecystectomy and the risk of colorectal cancer. Am J Gastroenterol 2005;100:1813-20. http://dx.doi.org/10.1111/j.1572-0241.2005.41610.x.
Appendix 1 Search strategies
Clinical effectiveness
Databases
MEDLINE (1946 to week 37, 2012)
MEDLINE In-Process & Other Non-Indexed Citations (10 September 2012)
EMBASE (1974 to 10 September 2012)
Ovid multifile search URL: http://shibboleth.ovid.com/
Search terms
-
cholecystitis/
-
cholecystitis, acute/
-
cholecystolithiasis/
-
gallstones/
-
cholelithiasis/
-
biliary colic/
-
(gall?bladder adj3 (empyema or inflam$)).tw.
-
(biliary colic or gall?stone$ or cholecystitis or cholecystolithiasis).tw.
-
((pain or biliary symptom$) adj5 (cholecystitis or cholecystolithiasis or gall?bladder)).tw.
-
or/1-9
-
exp Cholecystectomy/
-
cholecystectom$.tw.
-
((excis$ or remov$) adj4 gall?bladder).tw.
-
((surgery or surgical) adj5 (cholecystitis or cholecystolithiasis or gall?bladder)).tw.
-
or/11-14
-
exp clinical trial/
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomi?ed.ab.
-
randomly.ab.
-
trial.ab.
-
placebo.ab.
-
drug therapy.fs.
-
groups.ab.
-
comparative study/ use prmz
-
(prospective$ or retrospective$).tw.
-
(compare$ or compara$).ti,ab.
-
or/16-27
-
10 and 15 and 28
-
(review or editorial or case report$ or letter).pt.
-
exp animals/ not humans/
-
29 not (30 or 31)
-
limit 32 to yr="1980 -Current"
-
limit 33 to yr="2000-Current"
-
limit 33 to yr="1980-1999"
-
remove duplicates from 34
-
remove duplicates from 35
-
36 or 37
The Cochrane Library (date of inception to September 2012)
URL: www.thecochranelibrary.com
Search terms
-
MeSH descriptor Cholecystitis, Acute, this term only
-
MeSH descriptor Cholecystitis, this term only
-
MeSH descriptor Cholecystolithiasis, this term only
-
MeSH descriptor Gallstones, this term only
-
MeSH descriptor Cholelithiasis, this term only
-
(gall*bladder) near/3 (empyema or inflam*)
-
biliary colic or gall*stone* or cholecystitis or cholecystolithiasis
-
(pain or biliary symptom*) near/5 (cholecystitis or cholecystolithiasis or gall*bladder)
-
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8)
-
MeSH descriptor Cholecystectomy explode all trees
-
cholecystectom*
-
(excis* or remov*) near/4 (gall*bladder)
-
(surgery or surgical) near/5 (cholecystitis or cholecystolithiasis or gall*bladder)
-
(#10 OR #11 OR #12 OR #13)
-
(#9 AND #14), from 1980 to 2012
Database of Abstract of Reviews of Effects – Health Technology Assessment – NHS Economic Evaluation Database (date of inception to September 2012)
Centre for Reviews and Dissemination URL: www.crd.york.ac.uk
Search terms
-
Medical subject heading (MeSH) DESCRIPTOR Cholecystitis
-
MeSH DESCRIPTOR Cholecystitis, Acute
-
MeSH DESCRIPTOR Cholecystolithiasis EXPLODE 2
-
MeSH DESCRIPTOR Cholelithiasis
-
MeSH DESCRIPTOR Gallstones
-
(gallbladder or gall bladder) adj3 (empyema or inflam*)
-
biliary colic or gallstone* or gall stone* or cholecystitis or cholecystolithiasis
-
(pain or biliary symptom*) adj5 (cholecystitis or cholecystolithiasis or gallbladder or gall bladder)
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
-
MeSH DESCRIPTOR Cholecystectomy EXPLODE 1
-
cholecystectom*
-
(excis* or remov*) adj4 (gallbladder or gall bladder)
-
(surgery or surgical) adj5 (cholecystitis or cholecystolithiasis or gallbladder or gall bladder)
-
#10 OR #11 OR #12 OR #13
-
#9 and #14
Science Citation Index (1970 to 12 September 2012)
Conference Proceedings Citation Index – Science (1990 to 12 September 2012)
URL: www.isiknowledge.com
Search terms
-
TS =(cholecystitis or cholecystolithiasis or gallstones or cholelithiasis or biliary colic)
-
TS=((gall-bladder or gall*bladder or gallbladder) near/3 (empyema or inflam*))
-
TS=((gall-bladder or gall*bladder or gallbladder or cholecystitis or cholecystolithiasis) near/3 (pain or symptom*))
-
#3 OR #2 OR #1
-
TS=cholecystectom*
-
TS=((excis* or remov*) near/4 (gall-bladder or gall*bladder or gallbladder))
-
TS=((surgery or surgical) near/5 (cholecystitis or cholecystolithiasis or gall-bladder or gall*bladder or gallbladder))
-
#7 OR #6 OR #5
-
#8 AND #4
-
TS=(trial* or random* or comparison or compare or comparative or prospective or retrospective)
-
#10 AND #9
-
#11[excluding] Document Types=( LETTER OR EDITORIAL MATERIAL OR NOTE)
-
Timespan=1980-01-01 - 2012-09-12
Bioscience Information Service (1956 to 10 September 2011)
URL: www.isiknowledge.com
Search terms
-
TS =(cholecystitis or cholecystolithiasis or gallstones or cholelithiasis or biliary colic)
-
TS=((gall-bladder or gall*bladder or gallbladder) near/3 (empyema or inflam*))
-
TS=((gall-bladder or gall*bladder or gallbladder or cholecystitis or cholecystolithiasis) near/3 (pain or symptom*))
-
#3 OR #2 OR #1
-
TS=cholecystectom*
-
TS=((excis* or remov*) near/4 (gall-bladder or gall*bladder or gallbladder))
-
TS=((surgery or surgical) near/5 (cholecystitis or cholecystolithiasis or gall-bladder or gall*bladder or gallbladder))
-
#7 OR #6 OR #5
-
#8 AND #4
-
TS=(trial* or random* or comparison or compare or comparative or prospective or retrospective)
-
#10 AND #9
-
#11[excluding] Document Types=( LETTER)
-
Timespan=1980-2012
Trials
Clinical Trials (date of inception to September 2012)
URL: http://clinicaltrials.gov/
Search terms
cholecystectomy AND (cholecystitis OR gallstones OR cholelithiasis OR biliary colic)
gallbladder removal AND (cholecystitis OR cholelithiasis)
World Health Organization Clinical Trials Registry (date of inception to September 2012)
URL: http://apps.who.int/trialsearch/
Search terms
(cholecystitis or gallstones) AND cholecystectomy
Current Controlled Trials (date of inception to September 2012)
National Institute for Health Research Portfolio (date of inception to September 2012)
Conference proceedings from:
Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland.
Association of Laparoscopic Surgeons of Great Britain and Ireland.
British Society of Gastroenterology.
Cost-effectiveness
Databases
MEDLINE (1946 to 1 October 2012)
MEDLINE In-Process (8 October 2012)
EMBASE (1974 to 8 October 2012)
Ovid multifile search URL: http://shibboleth.ovid.com/
Search terms
-
cholecystitis/
-
cholecystitis, acute/
-
cholecystolithiasis/
-
gallstones/
-
cholelithiasis/
-
(gall?bladder adj3 (empyema or inflam$)).tw.
-
(biliary colic or gall?stone$ or cholecystitis or cholecystolithiasis).tw.
-
((pain or biliary symptom$) adj5 (cholecystitis or cholecystolithiasis or gall?bladder)).tw.
-
or/1-8
-
exp *Cholecystectomy/
-
cholecystectom$.ti.
-
((excis$ or remov$) adj2 gall?bladder).tw.
-
((surgery or surgical) adj3 (cholecystitis or cholecystolithiasis or gall?bladder)).tw.
-
or/10-13
-
exp "costs and cost analysis"/
-
economics/
-
exp economics,hospital/
-
exp economics,medical/
-
economics,pharmaceutical/
-
exp budgets/
-
exp models, economic/
-
exp decision theory/
-
ec.fs.
-
monte carlo method/
-
markov chains/
-
exp health status indicators/
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimis$)).ab.
-
economic$ model$.tw.
-
(economic$ or pharmacoeconomic$ or pharmaco-economic$).tw.
-
(price$ or pricing).tw.
-
(financial or finance or finances or financed).tw.
-
((value adj2 money) or monetary).tw.
-
markov$.tw.
-
monte carlo.tw.
-
(decision$ adj2 (tree? or analy$ or model$)).tw.
-
(standard adj1 gamble).tw.
-
trade off.tw.
-
or/15-36
-
9 and 14 and 39
-
exp animals/ not humans/
-
40 not 41
-
remove duplicates from 42
-
limit 43 to yr="1980 -Current"
Science Citation Index (1970 to 10 October 2012)
Conference Proceedings Citation Index – Science (1990 to 10 October 2012)
URL: www.isiknowledge.com
Search terms
-
TS =(cholecystitis or cholecystolithiasis or gallstones or cholelithiasis or biliary colic)
-
TS=((gall-bladder or gall*bladder or gallbladder) near/3 (empyema or inflam*))
-
TS=((gall-bladder or gall*bladder or gallbladder or cholecystitis or cholecystolithiasis) near/3 (pain or symptom*))
-
#3 OR #2 OR #1
-
TS=cholecystectom*
-
TS=((excis* or remov*) near/4 (gall-bladder or gall*bladder or gallbladder))
-
TS=((surgery or surgical) near/5 (cholecystitis or cholecystolithiasis or gall-bladder or gall*bladder or gallbladder))
-
#7 OR #6 OR #5
-
#8 AND #4
-
TS=(economic* or cost* or price* or pricing* or financ* or markov* or monte carlo)
-
TS=(decision near/3 (tree* OR analy* OR model*))
-
#11 OR #10
-
#12 AND #9
-
Refined by: [excluding] Document Types=( LETTER OR EDITORIAL MATERIAL)
-
Timespan=1980-01-01 - 2012-10-10
NHS Economic Evaluation Database (date of inception to October 2012)
Centre for Reviews and Dissemination URL: www.crd.york.ac.uk
Search terms
-
MeSH DESCRIPTOR Cholecystitis
-
MeSH DESCRIPTOR Cholecystitis, Acute
-
MeSH DESCRIPTOR Cholecystolithiasis EXPLODE 2
-
MeSH DESCRIPTOR Cholelithiasis
-
MeSH DESCRIPTOR Gallstones
-
(gallbladder or gall bladder) adj3 (empyema or inflam*)
-
biliary colic or gallstone* or gall stone* or cholecystitis or cholecystolithiasis
-
(pain or biliary symptom*) adj5 (cholecystitis or cholecystolithiasis or gallbladder or gall bladder)
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
-
MeSH DESCRIPTOR Cholecystectomy EXPLODE 1
-
cholecystectom*
-
(excis* or remov*) adj4 (gallbladder or gall bladder)
-
(surgery or surgical) adj5 (cholecystitis or cholecystolithiasis or gallbladder or gall bladder)
-
#10 OR #11 OR #12 OR #13
-
#9 and #14
-
* IN NHSEED
-
#9 AND #16
-
#14 AND #16
-
#17 OR #18
CEA Registry (date of inception to October 2012)
URL: https://research.tufts-nemc.org/cear4/SearchingtheCEARegistry/SearchtheCEARegistry.aspx
IDEAS (RePEc) (date of inception to October 2012)
Quality of life
Databases
MEDLINE (1946 to week 50 2012)
MEDLINE In-Process (10 December 2012)
EMBASE (1974 to 10 December 2012)
Ovid multifile search URL: http://shibboleth.ovid.com/
Search terms
-
Quality of life/
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
(sf36 or sf 36 or short form 36 or shortform 36).tw.
-
(sf6 or sf 6 or short form 6 or shortform 6).tw.
-
(sf12 or sf 12 or short form 12 or shortform 12).tw.
-
(sf16 or sf 16 or short form 16 or shortform 16).tw.
-
(sf20 or sf 20 or short form 20 or shortform 20).tw.
-
or/1,2-7
-
*cholecystitis/
-
*cholecystitis, acute/
-
*cholecystolithiasis/
-
*biliary colic/
-
(gall?bladder adj3 (empyema or inflam$)).tw.
-
(biliary colic or gall?stone$ or cholecystitis or cholecystolithiasis).tw.
-
((pain or biliary symptom$) adj5 (cholecystitis or cholecystolithiasis or gall?bladder)).tw.
-
or/9-15
-
8 and 16
-
(review or editorial or case report$ or letter or conference abstract).pt.
-
exp animals/ not humans/
-
17 not (18 or 19)
-
cholecystitis/
-
cholecystitis, acute/
-
cholecystolithiasis/
-
gallstones/
-
cholelithiasis/
-
(gall?bladder adj3 (empyema or inflam$)).tw.
-
(biliary colic or gall?stone$ or cholecystitis or cholecystolithiasis).tw.
-
((pain or biliary symptom$) adj5 (cholecystitis or cholecystolithiasis or gall?bladder)).tw.
-
or/21-28
-
exp *Cholecystectomy/
-
cholecystectom$.ti.
-
((excis$ or remov$) adj2 gall?bladder).tw.
-
((surgery or surgical) adj3 (cholecystitis or cholecystolithiasis or gall?bladder)).tw.
-
or/30-33
-
exp "costs and cost analysis"/
-
economics/
-
exp economics,hospital/
-
exp economics,medical/
-
economics,pharmaceutical/
-
exp budgets/
-
exp models, economic/
-
exp decision theory/
-
ec.fs.
-
monte carlo method/
-
markov chains/
-
exp health status indicators/
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimis$)).ab.
-
economic$ model$.tw.
-
(economic$ or pharmacoeconomic$ or pharmaco-economic$).tw.
-
(price$ or pricing).tw.
-
(financial or finance or finances or financed).tw.
-
((value adj2 money) or monetary).tw.
-
markov$.tw.
-
monte carlo.tw.
-
(decision$ adj2 (tree? or analy$ or model$)).tw.
-
(standard adj1 gamble).tw.
-
trade off.tw.
-
or/35-56
-
29 and 34 and 59
-
exp animals/ not humans/
-
60 not 61
-
remove duplicates from 62
-
limit 63 to yr="1980 -Current"
-
20 not 64
-
limit 65 to yr="1980 -Current"
Appendix 2 Full-text screening form
Appendix 3 Data extraction form
Appendix 4 Risk of bias form
Appendix 5 List of included and excluded studies
Included studies
Schmidt et al. 2011113
Schmidt M, Søndenaa K, Vetrhus M, Berhane T, Eide GE. A randomized controlled study of uncomplicated gallstone disease with a 14-year follow up showed that operation was the preferred treatment. Dig Surg 2011;28:270–6.
Secondary publications:
Vetrhus M, Soreide O, Solhaug JH, Nesvik I, Søndenaa K. Symptomatic, non-complicated gallbladder stone disease. Operation or observation? A randomized clinical study. Scand J Gastroenterol 2002;37:834–839.
Vetrhus M, Soreide O, Eide GE, Solhaug JH, Nesvik I, Søndenaa K. Pain and quality of life in patients with symptomatic, non-complicated gallbladder stones: Results of a randomized controlled trial. Scand J Gastroeneterol 2004; 39:270–6.
Schmidt et al. 201146
Schmidt M, Søndenaa K, Vetrhus M, Berhane T, Eide GE. Long-term follow-up of a randomized controlled trial of observation versus surgery for acute cholecystitis: Non-operative management is an option in some patients. Scand J Gastroenterol 2011;46:1251–6.
Secondary publications
Vetrhus M, Soreide O, Nesvik I, Søndenaa K. Acute cholecystitis: delayed surgery or observation. A randomized clinical trial. Scand J Gastroenterol 2003;38:985–90.
Vetrhus M, Soreide O, Eide GE, Nesvik I, Søndenaa K. Quality of life and pain in patients with acute cholecystitis. Results of a randomized clinical trial. Scand J Surg 2005;94:34–9.
Excluded studies
Ineligible comparator
Ahmad I. Cholecystectomy in acute cholecystitis. J Pak Med Assoc 1992;42:112–15.
Ajao OG, Al-Saigh AA, Malatani T, Ladipo JK. Timing of surgery in acute cholecystitis. East Afr Med J 1991;68:490–5.
Al-Khuwaitir S. Early versus delayed surgery for acute cholecystitis. J Pak Med Assoc 1984;34:210–13.
Banz VM, Gsponert T, Candinas D, Guller U. Population-based analysis of 4126 patients with acute cholecystitis: Defining the optimal time-point for laparoscopic cholecystectomy. Surg Endosc 2011;25:S2.
Cameron IC, Chadwick C, Phillips J, Johnson AG. Acute cholecystitis – room for improvement? Ann R Coll Surg Engl 2002;84:10–13.
Chang TC, Lin MT, Wu MH, Wang MY, Lee PH. Evaluation of early versus delayed laparoscopic cholecystectomy in the treatment of acute cholecystitis. Hepato-Gastroenterology 2009;56:26–8.
Eitan A, Toledano C, Rivlin E, Linn S, Barzilai A. Early vs. delayed cholecystectomy for acute cholecystitis. Harefuah 1991;120:319–68.
Fowkes FG, Gunn AA. The management of acute cholecystitis and its hospital cost. Br J Surg 1980;67:613–17.
Gomez-Ferrer F, Ortega J, Cortes J, Ruiz J, Arnal C. Early or delayed operations in acute cholecystitis. Rev Esp Enferm Apar Dig 1988;73:259–63.
Isoda N, Ido K, Kawamoto C, Suzuki T, Nagamine N, Ono K, et al. Laparoscopic cholecystectomy in gallstone patients with acute cholecystitis. J Gastroenterol 1999;34:372–5.
Jaeger G, Rothenbuhler JM, Famos M, Tondelli P. When should cholecystectomy in acute cholecystitis be planned? Schweiz Med Wochenschr 1983;113:552–4.
Jarvinen HJ, Hastbacka J. Early cholecystectomy for acute cholecystitis: a prospective randomized study. Ann Surg 1980;191:501–5.
Johansson M, Thune A, Lundell L. A prospective randomised trial comparing early versus delayed laparoscopic cholecystectomy in the treatment of acute cholecystitis. Gastroenterology 2002;123:24.
Johansson M, Thune A, Blomqvist A, Nelvin L, Lundell L. Management of acute cholecystitis in the laparoscopic era: results of a prospective, randomized clinical trial. J Gastrointest Surg 2003;7:642–5.
Johansson M, Thune A, Blomqvist A, Nelvin L, Lundell L. Impact of choice of therapeutic strategy for acute cholecystitis on patient’s health-related quality of life. Results of a randomized, controlled clinical trial. Dig Surg 2004;21:359–62.
Kontopodis N, Spiridakis K, Panagiotakis G, Grigoraki M, Kokkinakis T, Rokadakis L. Acute cholecystitis: conservative treatment and delayed cholecystectomy. Chirurgia 2011;24:121–4.
Kozelj M, Flis V, Potrc S, Horvat M, Mursak M. Comparison between delayed and early cholecystectomy in acute cholecystitis. J Hepatol 1996;25:209.
Lai PB, Kwong KH, Leung KL, Kwok SP, Chan AC, Chung SC, et al. Randomized trial of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg 1998;85:764–7.
Lawrentschuk N, Hewitt PM, Pritchard MG. Elective laparoscopic cholecystectomy: implications of prolonged waiting times for surgery. ANZ J Surg 2003;73:890–3.
Lin A, Stiven P, Bagshaw P, Connor S. Cholecystectomy following acute presentation to a major New Zealand metropolitan hospital: change to the timing of surgery is needed. N Z Med J 2006;119:U2104.
Mare LD, Saadi A, Roulin D, Demartines N, Halkic N. Delayed versus early laparoscopic cholecystectomy for acute cholecystitis: A prospective randomized study. HPB 2012;14:130.
Maroske D, Röher HD. Early but not delayed cholecystectomy in acute cholecystitis. Dig Surg 1984;1:98.
Navarra S, Melita P, Calbo L, Barbuscia M, Palmeri R, Lorenzini C. Acute cholecystitis. Immediate or postponed treatment? Minerva Chir 1983;38:1391–6.
Norby S, Herlin P, Holmin T. Early or delayed cholecystectomy in acute cholecystitis? A clinical trial. Br J Surg 1983;70:163–5.
Papi C, Catarci M, D’Ambrosio L, Gili L, Koch M, Grassi GB, et al. Timing of cholecystectomy for acute calculous cholecystitis: a meta-analysis. Am J Gastroenterol 2004;99:147–55.
Paran H, Zissin R, Rosenberg E, Griton I, Kots E, Gutman M. Prospective evaluation of patients with acute cholecystitis treated with percutaneous cholecystostomy and interval laparoscopic cholecystectomy. Int J Surg 2006;4:101–5.
Sanchez Bueno F, Parrilla P, Ayllon JG, Lujan JA, Robles R, Ramirez P, et al. Timing of operation in acute cholecystitis: Prospective study of 375 cases divided into three series – early, delayed and late surgery. Dig Surg 1993;10:72–7.
Schaefer D, Barth H, Thon K, Jostarndt L, Maroske D. Early or delayed operation in patients with acute cholecystitis. Results of a prospective randomized controlled clinical trial. Chir Forum Exp Klin Forsch 1980;149–53.
Serralta AS, Bueno JL, Planells MR, Rodero DR. Prospective evaluation of emergency versus delayed laparoscopic cholecystectomy for early cholecystitis. Surg Laparosc Endosc Percutan Tech 2003;13:71–5.
Siddiqui T, MacDonald A, Chong PS, Jenkins JT. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis: a meta-analysis of randomized clinical trials. Am J Surg 2008;195:40–7.
van der Linden W, Edlund G. Early versus delayed cholecystectomy: the effect of a change in management. Br J Surg 1981;68:753–7.
Williamson WA, Simpson CJ, Mclatchie G, Gillespie C, McArdle CS. Prospective randomized trial of early vs. delayed surgery for acute biliary tract disease. Gut 1984;25:A1140–1.
Zargar-Shoshtari K, Short H, Poole GH, Hill AG. Acute laparoscopic cholecystectomy: preferred treatment for acute biliary disease. ANZ J Surg 2008;78:771–4.
Ineligible study design
Al-Saleh MS, Almuhiza I, Alharthy M. Early cholecystectomy for acute cholecystitis. Saudi Med J 1995;16:227–30.
Contini S, Corradi D, Busi N, Alessandri L, Pezzarossa A, Scarpignato C. Can gangrenous cholecystitis be prevented?: a plea against a ‘wait and see’ attitude. J Clin Gastroenterol 2004;38:710–16.
D’Ambrosio R, Leone L, Peppas C, Grasso A, Di M, D’Ambrosio E, et al. Acute cholecystitis: timing of surgery. Minerva Chir 1995;50:39–46.
Festi D, Reggiani ML, Attili AF, Loria P, Pazzi P, Scaioli E, et al. Natural history of gallstone disease: Expectant management or active treatment? Results from a population-based cohort study. J Gastroenterol Hepatol 2010;25:719–24.
Gambiez L. How should biliary lithiasis be managed? Gastroenterol Clin Biol 2001;25:S128–39.
Ghani AA, Jan WA, Ul H. Acute cholecystitis: Immediate versus interval cholecystectomy. J Postgrad Med Inst 2005;19:192–5.
Goldfinger SE. By the way, doctor... Last month I had a night of severe abdominal pain. My doctor ordered an ultrasound test, and it showed gallstones. She is recommending surgery to remove my gallbladder, but I’d rather not have an operation because of this one episode. Is it dangerous to hold off on surgery? Is there any diet to follow or medicine I can take to prevent another attack? I am 53 years old and healthy. Harv Health Lett 1999;24:3.
Jorgensen T. Treatment of patients with uncomplicated gallbladder stones? Scand J Gastroenterol 2002;37:745.
Kubo G, Mann H, Nicko HJ. Treatment of acute cholecystitis by comparing conservative therapy, interval operation and early operation. Zentralbl Chir 1985;110:1054–65.
Malik A, I, Tou S, Nelson RL. Early laparoscopy versus observation for patients with acute abdominal pain. Cochrane Database Syst Rev 2009;1:CD008035.
Petakovic G, Korica M, Gavrilovic S. Acute cholecystitis – early or delayed cholecystectomy. Med Pregl 2002;55:135–9.
Ranalli M, Testi W, Genovese A, Bing C, Tumbiolo S, Andolfi E, et al. Early vs conservative treatment of acute cholecystitis. Personal experience and review of the literature. Minerva Chir 2004;59:547–53.
Sackmann M. Conservative treatment of gallstones: pro. Dtsch Med Wochenschr 2000;125:1051–2.
Ineligible population
Arthur JD, Edwards PR, Chagla LS. Management of gallstone disease in the elderly. Ann R Coll Surg Engl 2003;85:91–6.
Boerma D, Rauws EA, Keulemans YC, Janssen IM, Bolwerk CJ, Timmer R, et al. Wait-and-see policy or laparoscopic cholecystectomy after endoscopic sphincterotomy for bile-duct stones: a randomised trial. Lancet 2002;360:761–5.
Boerma D, Rauws EAJ, Keulemans YCA, Janssen IMC, Bolwerk CJM, Timmer R, et al. More recurrent complaints with a wait-and-see approach than with laparoscopic cholecystectomy after endoscopic sphincterotomy for common bile duct stones; randomised clinical trial. Ned Tijdschr Geneeskd 2002;146:1786–92.
Calero Garcia P, Ruiz Tovar J, Sanjuanbenito Dehesa A, Calero Amaro A, Diez Tabernilla M, Latorre Fragua R, et al. Acute cholecystitis: is it still justified to delay surgery? Cir Esp 2010;88:92–6.
Chiedozi LC, Al H, Salem MM, Al M, Okpere EE. Management of symptomatic cholelithiasis in pregnancy. Ann Saudi Med 2001;21:38–41.
Daradkeh S, Sumrein I, Daoud F, Zaidin K, Abu-Khalaf M. Management of gallbladder stones during pregnancy: conservative treatment or laparoscopic cholecystectomy? Hepato-Gastroenterology 1999;46:3074–6.
Elamin Ali M, Al-Shehri MY, Abu-Eshy S, Cheema MA, Mustafa Z, Sadek A. Is surgical intervention in acute cholecystitis in pregnancy justified? J Obstet Gynaecol 1997;17:435–8.
Gonzalez Gonzalez JJ, Sanz Alvarez L, Grana Lopez JL, Bermejo Abajo G, Navarrete Guijosa F, Martinez Rodriguez E. Gallstone disease in patients over the age of 80. Surgery or long-term medical treatment? Rev Esp Enferm Dig 1997;89:196–205.
Irvin TT, Arnstein PM. Management of symptomatic gallstones in the elderly. Br J Surg 1988;75:1163–5.
Keulemans YC, Rauws EA, Huibregtse K, Gouma DJ. Current management of the gallbladder after endoscopic sphincterotomy for common bile duct stones. Gastrointest Endosc 1997;46:514–19.
Kuy S, Roman SA, Desai R, Sosa JA. Outcomes following cholecystectomy in pregnant and nonpregnant women. Surgery 2009;146:358–66.
Lau J, Leow CK, Fung TMK, Suen BY, Yu LM, Lai PBS, et al. Five year follow-up of a randomized comparison of laparoscopic cholecystectomy or gallbladder in situ after endoscopic sphincterotomy and clearance of bile duct stones in patients older than 60. Gastroenterology 2005;128(Suppl. 2):A79.
Lu EJ, Curet MJ, El-Sayed YY, Kirkwood KS. Medical versus surgical management of biliary tract disease in pregnancy. Am J Surg 2004;188:755–9.
Plaisier PW, Nijs HG, van der Hul RL, den Toom R, Terpstra OT. 3-year results of ‘gallstone outpatient clinic’ in an academic hospital. Ned Tijdschr Geneeskd 1992;136:978–82.
Sanchez-Beorlegui J, Lamata-Hernandez F, Lagunas-Lostao E, Monsalve-Laguna E, Peñalva-Segura P. Choice of therapeutic approach for acute cholecystitis in the elderly. Rev Gastroenterol Mex 2010;75:149–57.
Swisher SG, Schmit PJ, Hunt KK, Hiyama DT, Bennion RS, Swisher EM, et al. Biliary disease during pregnancy. Am J Surg 1994;168:576–81.
Yamashita Y, Takada T, Hirata K. A survey of the timing and approach to the surgical management of patients with acute cholecystitis in Japanese hospitals. J Hepatobiliary Pancreat 2006;13:409–15.
No relevant outcomes
Søndenaa K, Nesvik I, Solhaug JH, Soreide O. Randomization to surgery or observation in patients with symptomatic gallbladder stone disease. The problem of evidence-based medicine in clinical practice. Scand J Gastroenterol 1997;32:611–16.
Unavailable
Borodach VA, Borodach AV. Surgical treatment of cholelithiasis in elderly and aged patients. Khirurgiia 2002;11:38–41.
Dimov R, Strangev G, Kandilarov K, Marinov V, Argirov D, Velkov B, et al. Prospective trial of early and delayed laparoscopic cholecistectomy in patients presented with acute cholecystitis. Bulg Med 2005;13:6–9.
Fisher DA. Watchful waiting after endoscopic removal of common bile duct stones: cheaper and better? Am J Gastroenterol 2006;101:753–4.
Grinev MV. Time of the operations in acute cholecystitis. Vestn Khir Im I I Grek 1988;140:22–6.
Hernandez Estrada AI, Aquirre Osete X, Pedraza Gonzalez LA. Laparoscopic cholecystectomy in pregnancy. Five years experience in the Spanish Hospital of Mexico and literature review. Ginecol Obstet Mex 2011;79:200–5.
Khan SSA. Early versus delayed cholecystectomy for acute cholecystitis, a prospective randomized study. Pak J Gastroenterol 2002;16:30–4.
Lopez EV, Florez JB, Fernandez CH, Valens PE, Caperochipi JA. Acute cholecystitis 2. Comparative study of the surgical treatment – early, deferred, or elective. Cir Esp 1982;36:244–51.
List of abbreviations
- CBD
- common bile duct
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- EQ-5D
- European Quality of Life-5 Dimensions
- ESWL
- extracorporeal shock wave lithotripsy
- EVPI
- expected value of perfect information
- GP
- general practitioner
- HRG
- health-care resource group
- ICER
- incremental cost-effectiveness ratio
- MeSH
- medical subject heading
- MTBE
- methyl tert-butyl ether
- NHP
- Nottingham Health Profile part II
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- PGWB
- Psychological General Well Being index
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAGES
- Society of American Gastrointestinal and Endoscopic Surgeons
- VAPS
- Visual Analogue Pain Score