Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/62/02. The contractual start date was in December 2015. The draft report began editorial review in June 2016 and was accepted for publication in October 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Clive Ballard reports grants and personal fees from Lundbeck Ltd and ACADIA Pharmaceuticals Inc., and personal fees from Roche, Orion Pharma, GlaxoSmithKline, Otsuka Pharmaceutical, Heptares Therapeutics Ltd and Eli Lilly and Company outside the submitted work. Sube Banerjee reports grants and personal fees from AbbVie, personal fees and non-financial support from Eli Lilly and Company, and personal fees from Eleusis Pharmaceuticals Ltd, Daval International, Boehringer Ingelheim, Axovant Sciences, Lundbeck Ltd and Nutricia outside the submitted work. Sube Banerjee also reports being a member of the Health Technology Assessment (HTA) Mental, Psychological and Occupational Health Panel, and has been involved as the principal investigator in a series of National Institute for Health Research grants that has developed the Dementia Quality of Life measure (DEMQOL) system for the measurement of health-related quality of life in dementia, which is one of the candidate measures in this project. Alistair Burns reports being the editor for the International Journal of Geriatric Psychiatry and being the National Clinical Director for Dementia, NHS England, during the conduct of the study. Peter Garrard reports personal fees from Merck Sharp and Dohme Ltd outside the submitted work. Esme Moniz-Cook reports non-financial support from her contribution to the recent Joint Programme – Neurodegenerative Disease Research Outcome Measures, outside the submitted work. John T O’Brien reports personal fees from GE Healthcare, TauRx Pharmaceuticals, Cytox Ltd and Accera Inc, and grants and personal fees from Avid Radiopharmaceuticals Inc./Eli Lilly and Company outside the submitted work. James Pickett reports being a full-time employee of the Alzheimer’s Society. Rob Howard is a member of the HTA Commissioning Board. Sasha Shepperd is a member of the Health Services and Delivery Research Prioritisation Commissioning Panel.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Webster et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
It is estimated that 850,000 people in the UK are currently living with dementia (> 1% of the entire UK population),1 and one-third of people born in the UK in 2015 will develop dementia during their lifetime. 2 Dementia care currently costs in excess of £23B per annum,1 with costs expected to triple in the next 30 years as the number of older people increases. Dementia affects not only the person with the illness, but also their family and wider society, and with the current absence of a preventative treatment, the number of people with dementia is projected to reach > 1 million by 2020 and double again in the subsequent 20 years. 1 However, some recent population studies3,4 have suggested that the prevalence of dementia among those now reaching 65–75 years of age may be slightly lower and, therefore, the rate of increase, primarily driven by the ageing of the population, may not be as great as once thought. These new data increase the optimism about potential primary prevention of dementia and of finding a disease-modifying treatment5 that would either halt or delay underlying pathology.
There have been huge strides forward across dementia research, in particular with early diagnosis, information, advanced decision-making, psychological therapies, management of neuropsychiatric symptoms, strategies for family carers and cholinesterase inhibitors in Alzheimer’s disease (AD). There have also been positive changes in attitudes, including highlighting personhood and living well with dementia. There is, however, currently no cure or disease-modifying treatment for the common dementias. This may be partly because our research knowledge and funding of dementia lags behind that of other major diseases, such as cancer or heart disease. 6
Following the successful expansion of NHS Memory Services, the number of people diagnosed with dementia has increased dramatically in England. 7 Currently, dementia sufferers can be offered only symptomatic treatments, as well as access to social and psychological treatment, education, support and advice. 1,8 The NHS thus has a huge potential to use effective disease course-modifying treatments (which may be pharmacological or non-pharmacological, and aimed at dementia in general or individual subtypes) and provides a large and highly motivated group of affected patients and their families who would want to support research and developments in this area.
The National Institute for Health Research (NIHR) has identified this as an important area of research and, through the Efficacy and Mechanism Evaluation programme, is supporting two large drug-repurposing disease modification trials of AD. The first of these, the RADAR trial (Reducing pathology in Alzheimer’s Disease through Angiotensin TaRgeting),9 is a Phase II randomised controlled trial (RCT) evaluating the effect of losartan on brain tissue changes in AD, with magnetic resonance imaging (MRI) brain volume change over 12 months as a primary outcome measure. Second, the MADE study (Minocycline for Alzheimer’s Disease Trial),10 looking at the efficacy of minocycline in AD, measures change in cognition at 24 months with the Mini Mental State Examination (MMSE) and function with the Bristol Activities of Daily Living (BADL) scale. These trials were developed, funded and set up without any liaison between the trial teams. Indeed, both had been funded for almost 2 years before the chief investigators found out about each other’s trials and began to communicate about progress and difficulties. The completely different nature of the designs and choice of outcome measures for these trials, together with the lack of co-ordinated activity of the trial teams, illustrate starkly just how much more the UK dementia research community could do at this early stage to strategically develop a co-ordinated approach to developing research in an effective and cost-efficient way, particularly with the outcomes used across trials. Demonstrating efficacy in AD modification has so far defeated the resources and efforts of the global pharmaceutical industry, and it is unlikely that individual academic and NHS organisations will do any better if we cannot agree a unified approach that will allow us to co-ordinate resources and integrate findings.
Delivering high-quality research in dementia is fundamentally important to the NHS and, since May 2013, the NIHR has made good progress towards the target of recruiting 10% of dementia patients into clinical trials. Working in partnership with the Alzheimer’s Society (AS) and Alzheimer’s Research UK, the NIHR launched ‘Join Dementia Research’ to provide ‘ready’ cohorts of patients consenting to be approached about research. In addition, it has established ENRICH (Enabling Research in Care Homes), a network to support an increase of dementia research in care homes. In February 2015, the UK prime minister announced plans for a further £300M investment in dementia research. The UK has an experienced cadre of dementia triallists with a track record of designing and delivering studies of pharmacological, psychological, educational and other complex interventions, the results of which have often had an international impact on practice. The NIHR has intimated that prospective investigators’ testing of putative disease modifiers should very seriously consider the use of adaptive trial designs to improve the efficiency of trials.
Between 1998 and 2012, 101 new potential pharmacological treatments for AD entered trials internationally, but only four drugs have received regulatory approval, all of which were symptomatic treatments rather than disease modifying;11 these included cholinesterase inhibitors (donepezil, rivastigmine and galantamine). Memantine, the last new drug to receive regulatory approval, was approved more than 10 years ago. 12 Since then, many promising disease-modifying drugs have been proposed, but all have failed at the point of Phase III trials or at earlier stages of development. 13 As a consequence, the number of pharmaceutical companies with drug development programmes in neurodegeneration is shrinking. One suggested explanation for the failure of so many new drugs for AD has been that by the point of clinical presentation the burden of neurodegeneration may already be too great for disease-modifying interventions to have efficacy. Another is that the majority of new drugs target just one of the numerous pathological mechanisms (e.g. inflammation, hypoxia and oxidative stress, reduced energy metabolism) that are active and probably contribute to disease severity and progression. It is predicted that if a treatment that could slow the progression of mild to moderate dementia by 50% became available in 2020, this could reduce the numbers of people living with severe dementia from 14% to 2% by 2050. 14 At the G8 Dementia Summit held in London in December 2013, a commitment was made to find a disease-modifying treatment by 2025. 15
To improve the possibility of identifying a disease-modifying treatment, it is important that the outcomes measured in trials are appropriate, sensitive and clinically meaningful. 16–18 It is also essential that there is a harmonisation of the outcome measures being used across trials to combat the large variance of measures currently used. 19 In 2015, the NIHR commissioned a call for the development of a core set of outcomes to be used in future disease modification trials, particularly in mild to moderate dementia. Developing standardised outcome sets is now being recognised as important across medical research, so that there should be a commitment to measuring minimum sufficient sets of outcomes for every major medical condition. 20 An agreed core set of outcomes would improve the efficiency and effectiveness of trials, and enhance interpretation of data across disease-modifying trials. This applies to both drugs and non-pharmacological interventions so that the efficacy of, for example, exercise, diet changes and new drugs can be compared. It is also important to consider the acceptability of measurement packages and the burden that they put on patients and their families. The priorities of patients in disease-modifying trials may not be the same as those of researchers, and patients in focus groups appear to be less concerned about stigma and other negative effects of diagnoses but wish to unambiguously know their disease status and accept biological and possibly invasive tests. 21 These may include quality-of-life and related outcomes that people with dementia and their families report as being important to them, and can inform cost-effectiveness analysis, as well as more symptom-related scales, such as cognition. Finally, a standardised core outcome set would aid meta-analysis and thus enable the combination of small data sets to better inform practice. Therefore, there is an urgent need for a consensus from dementia researchers in the UK about the core outcomes that should be used in future disease modification trials in mild to moderate dementia.
This has led to the current project, which intends to produce a consensus within the dementia community about the core outcomes for disease modification trials. It is funded by the NIHR Health Technology Assessment programme, and brings together as co-applicants and collaborators, a large multidisciplinary team of experts who are co-authors of the report and the AS, which has led the public involvement of experts by experience arm of the study. The aims of the project are detailed in Chapter 2.
In workstream 1 we gathered possible relevant references that had been identified by other related systematic reviews. These came from co-applicant Louise Lafortune, who led a systematic review on non-pharmacological interventions for people living with dementia. We also searched the Cochrane Dementia and Cognitive Improvement Group study register (ALOIS), which is maintained by the Cochrane Dementia and Cognitive Improvement Group, represented by co-applicant Jenny McCleery. The use of additional references from workstream 1 is detailed in Chapter 3. In addition, we looked at other core sets being developed by members of the group, including an AS and European Union Joint Programme – Neurodegenerative Disease Research (JPND)-funded study about outcomes used in psychosocial interventions in dementia, led by co-applicant Gail Mountain, and a review of measures which are important to patients with dementia funded by the International Consortium for Health Outcomes Measurement Working Group (ICHOM) led by co-applicant Charlotte Roberts.
In workstream 2, we conducted a systematic review of the outcome measures that have been, and are currently, used in disease-modifying trials of dementia; to our knowledge this is the first systematic review of outcomes used in disease modification trials, the methods and results of which are detailed in Chapters 3 and 4.
We considered the frequency of outcome use and validation, and discussed these outcomes with people living with dementia and their carers. This patient and public involvement (PPI) consultation method and results are detailed in Chapters 5 and 6.
We then brought all of the systematic review information together, with each potential domain being presented by a champion, as well as presentation of the PPI focus groups results. This expert body debated the questions and came to conclusions. Summaries of these presentations and the conference discussion are detailed in Chapter 7. The discussions and conclusions are in Chapters 8 and 9.
Chapter 2 Research question and objectives
The research question we set out to answer was ‘what are the core clinical and patient-relevant health outcomes that should be used in all NIHR-funded trials of disease modification in mild to moderate dementia, and how should they be measured?’.
Objectives
-
To appraise existing research into outcome sets being developed for use in psychosocial interventions (funded by the AS and JPND) and around what is most important to patients (measured by the ICHOM), in the light of the goals of this study.
-
To update and add to the existing body of work by a systematic search of the outcomes used in pharmacological and non-pharmacological studies of disease modification.
-
To appraise the outcomes identified through this systematic search, either using the existing research as above or through the literature.
-
To synthesise the evidence to identify important, valid, reliable and acceptable outcome measures in mild to moderate dementia.
-
To ensure by consultation that these outcome measures are acceptable and relevant to patients, carers, clinicians and the research community, and that they would be practical to include in NIHR trials and other studies.
-
To produce updated, evidence-based recommendations on the best outcome measures for disease modification in mild to moderate dementia research and practice.
-
To validate these recommendations through a consensus conference.
-
To set out these results in a research paper and report for the Health Technology Assessment journal.
-
To enable the NIHR to specify an agreed set of core outcomes to be used for all funded trials of disease modification in mild to moderate dementia.
Core Outcome Measures in Effectiveness Trials
We registered the project with Core Outcome Measures in Effectiveness Trials (COMET), a database of planned, ongoing and completed core outcome sets. The project’s COMET record is accessible at www.comet-initiative.org/studies/details/819?result=true (accessed 7 April 2016).
Chapter 3 Systematic review methods
Protocol
We created the protocol for the systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria22 and registered it with PROSPERO [no. CRD42015027346; accessible at www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015027346 (accessed 7 April 2016)].
Search strategy
Database searches
As specified in the protocol, we searched Cochrane Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health Literature (CINAHL), EMBASE, MEDLINE, Latin American and Caribbean Health Sciences Literature (LILACS) and PsycINFO.
The search terms within the protocol were originally adapted from another systematic review being carried out by a member of the group. 23 These search terms, however, resulted in an unacceptably high number of irrelevant results, with > 85,000 references identified in a search of MEDLINE alone. Therefore, in consultation with the project steering group, we adapted the search terms to reduce irrelevant references being picked up. The full search strategy for MEDLINE (via OvidSP) is shown in Appendix 1, with the same strategy used for EMBASE and PsycINFO (via OvidSP), and modified for searches in CENTRAL (via The Cochrane Library), CINAHL (via EBSCOhost), and LILACS [via the Virtual Health Library (VHL) Regional Portal]. As we are interested in outcome measures available in English, we limited database searches to English language where possible (CINAHL, EMBASE, LILACS, MEDLINE and PsycINFO).
As part of workstream 1 we also searched ALOIS and added these references to the ones found in the other database searches. We used the advanced search for intervention studies, with a combination of search terms: (‘outcome’ OR outcome OR outcomes OR ‘instrument’ OR instrument OR instruments OR ‘measure’ OR measure OR measures) AND (intervention OR therapy OR therapeutic OR trial OR trials) AND (control OR controlled), selecting ‘Treatment dementia’ as the study aim, all study designs, <any> interventions, and <any> if included in Cochrane.
Additional databases
In order to ensure that we picked up all outcomes that are currently being used, we adapted the protocol to include searches of the International Standard Randomised Controlled Trial Number (ISRCTN) and ClinicalTrials.gov trial registries for ongoing disease modification trials in dementia. To search the ISRCTN database we selected the trial status as ‘ongoing’ and the condition as ‘dementia’. To search ClinicalTrials.gov we combined ‘dementia’ AND ‘(control OR controlled)’, and limited the search to ‘open studies’.
Hand-searches
We hand-searched the bibliographies of relevant systematic reviews that were found within the database searches. We then also searched additional references collected in workstream 1, from a systematic review of non-pharmacological interventions for dementia.
Search dates
Searches were conducted on 11 December 2015 (for ALOIS, CENTRAL, CINAHL, EMBASE, LILACS and PsycINFO), 22 January 2016 (for ISRCTN) and 29 January 2016 (for ClinicalTrials.gov). All of the searches were conducted from database inception, with no limit on the end date.
Study inclusion
Inclusion criteria
We defined a disease modification trial as one where the intervention aims to change the underlying pathology of the dementia disease. This is as opposed to trials that aim solely to treat the symptoms of dementia, but not affect the underlying illness.
We included trials if they met all of the following criteria:
-
The full text is written in English.
-
The trial is published in a peer-reviewed journal or is an ongoing trial.
-
At least some of the participants have clinically diagnosed mild or moderate dementia.
-
It is a trial that aimed to modify the dementia disease.
-
It is a RCT or clinical controlled trial (CCT) with:
-
the intervention directed at the person with dementia
-
the control or comparator arm comprising treatment as usual, no intervention, sham therapy, other therapy or placebo.
-
-
At least one quantitative outcome measure related to disease modification in mild or moderate dementia.
Exclusion criteria
We excluded studies where:
-
all participants had severe dementia (according to the study inclusion criteria, including a MMSE score of < 12 or equivalent)
-
the whole study was set in care homes, as the commission call specified a review of outcome measures that modify the disease of mild to moderate dementia and very few of whom would be resident in care homes
-
all participants had mild cognitive impairment (MCI)
-
the outcomes were only:
-
qualitative
-
related to carers
-
economic
-
related to drug levels.
-
Screening titles and abstracts, and full texts
We screened titles and abstracts found across the searches for relevance. Two reviewers (DG and LW) piloted this procedure by independently screening the first 20 titles and abstracts and then compared their decisions. There were no disagreements, confirming the reliability of the first screening.
Three raters (AGS, DG and LW) also piloted the screening of full texts for inclusion criteria. They screened the first 10 papers independently, comparing answers and discussing, and then repeating the process with the next 10 papers. The three raters agreed on 80% of the first 20 papers, with no decision to exclude a paper that was eventually included. The raters disagreed regarding whether or not four papers should be excluded (see Appendix 2). The disagreements were whether or not the intervention was aiming to modify the underlying pathology of the disease of dementia. We agreed to solve this by examining in detail how the intervention is described in the background section, as well as the aim of the intervention within the trial. We also agreed to discuss any trials where we were unsure if the aim was to modify the disease between the raters and Gill Livingston if necessary. If a trial seemed to fulfil the inclusion criteria but we needed extra information about it, we contacted the authors to ask for this.
Data extraction
We extracted data about trial location, trial type, dementia type and severity, how the dementia diagnosis was made, participants’ sex and age, description of the intervention (number of participants; n), description of the comparator group (n), outcomes related to disease modification (primary or secondary if reported) and when outcomes were measured. To assess the accuracy of extracted data, Derek Groskreutz and Lucy Webster independently extracted data from the same subsample of five trials and compared their answers. There were no differences between the raters’ extracted data. We used this exercise, and an additional five papers, to pilot the data extraction tool and ensure that all relevant data from trials were captured. After piloting the tool, we created a second data extraction tool, which included the time period from baseline when each outcome was measured for each study, as within trials different outcomes were measured at different time points.
Data synthesis
For each outcome measure we listed how frequently it was used (i.e. number of trials) with how many participants there were across the trials. We searched Google Scholar (Google Inc., Mountain View, CA, USA) to find a copy of each outcome measure in English, either the manual or a key paper relating to its development. We divided all outcomes into domains [specifically cognition, activities of daily living (ADLs), biological markers, neuropsychiatric symptoms, quality of life and global]. Initially we had not planned to consider global outcomes, but we added it as a separate outcome category as we found relevant measures.
Quality assessment
As our aim was to synthesise the outcomes used across trials, rather than to report results, we did not consider it necessary to assess the quality of studies.
Outcome validation
We conducted separate iterative searches on Google Scholar using the name of the measure and psychometric terms, and consulted within our expert group for each outcome measure to find information on the measures’ psychometric properties relevant to people living with dementia. Specifically, we sought information about:
-
if the measure is validated in people with mild to moderate dementia for the outcome in which it is used as a measure
-
if there are any relevant populations in which the measure is validated (e.g. mild to moderate dementia, ethnic groups, languages)
-
unit of measurement
-
sensitivity to change
-
minimal clinically important difference
-
reliability (inter-rater and test–retest)
-
acceptability
-
ceiling-and-floor effects
-
average time taken to complete
-
who fills in the questionnaire (i.e. researcher through patient, family carer, paid carer or observation, or self-complete)
-
any risks identified of use of the measure.
Chapter 4 Results of the systematic review
Details of included and excluded studies
Figure 1 shows a PRISMA flow diagram with the results of the searches. Altogether we found 22,918 original references from searches of databases and workstream 1 (ALOIS and another systematic review of non-pharmacological interventions for dementia). From the screening of all titles and abstracts, we excluded 22,021 references and sought the full texts of 897 abstracts. From the full texts, we excluded 748 papers; a list of excluded studies and reasons for exclusion is in Appendix 3. We included 149 reports of 125 trials.
FIGURE 1.
The PRISMA flow diagram.
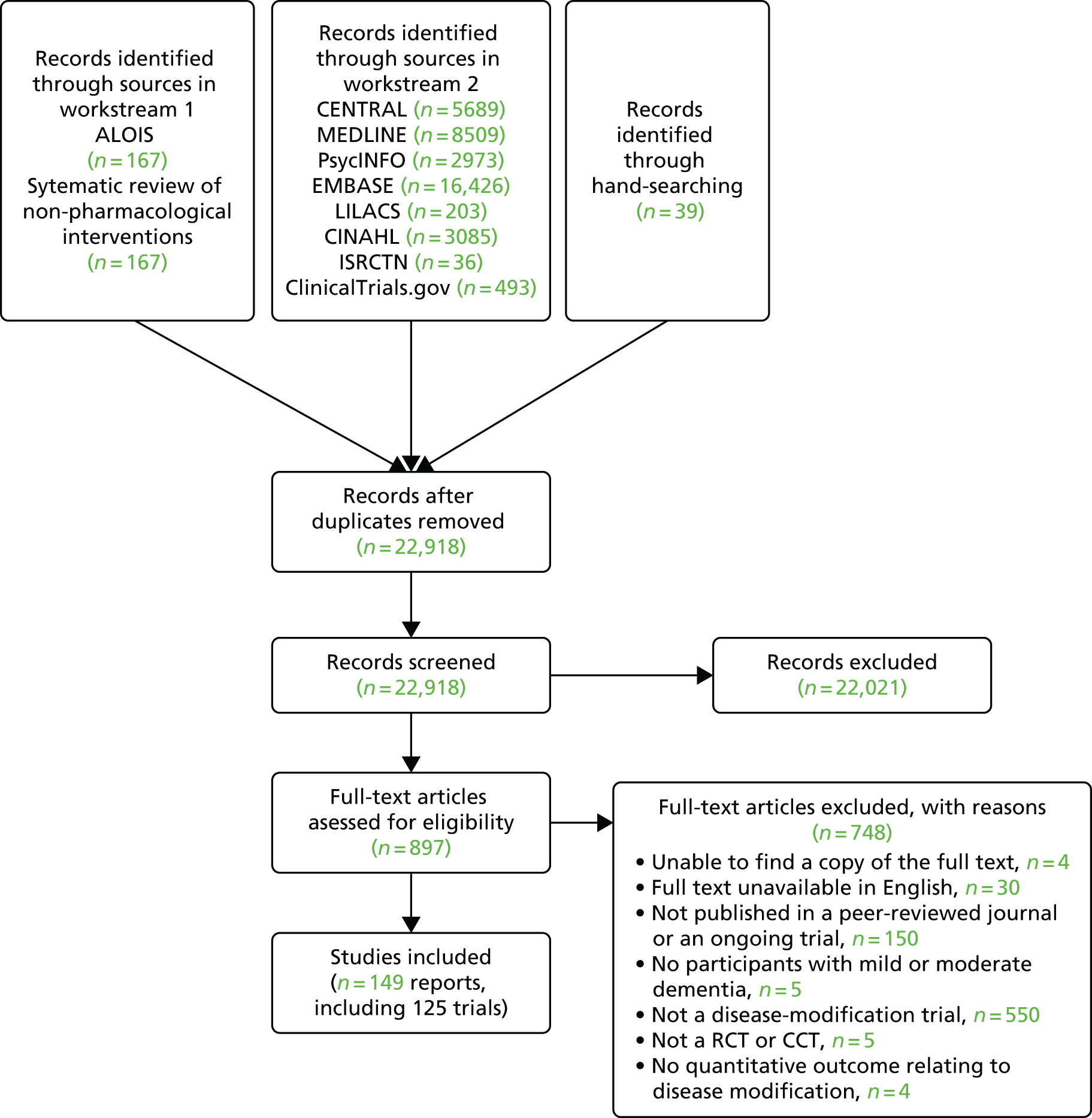
The 125 trials included 95 published trials, three published protocols and 27 ongoing trials, the characteristics of which are available, respectively, in Tables 1–3 in this chapter. One trial was a CCT, with the other 124 trials being RCTs. Trials were carried out in Australia (n = 2), Austria (n = 1), Canada (n = 1), China (n = 2), Denmark (n = 1), France (n = 2), Germany (n = 5), Iran (n = 2), Italy (n = 4), Japan (n = 1), Korea (n = 1), the Netherlands (n = 2), New Zealand (n = 1), Poland (n = 2), Russia (n = 1), Spain (n = 3), Sweden (n = 4), Taiwan (n = 1), the UK (n = 6) and the USA (n = 46); 37 were multicountry studies.
Across the trials, most participants had only AD; 16 studies included only patients with mild AD (seven of which specified early AD), six included patients with moderate AD, 84 involved patients with mild to moderate AD, two included patients with mild to severe AD, two studies included patients with moderate to severe AD and in one study all participants had AD of unspecified severity but were living at home. Eight trials also included participants with MCI, alongside mild (n = 5) or mild to moderate AD (n = 3). Three trials combined participants with AD and vascular dementia, two included patients with mild to moderate AD or vascular dementia, and one comprised patients with mild to moderate AD, with or without vascular dementia. Two trials included participants with vascular dementia only, one included patients with mild to moderately severe vascular dementia and one included patients with mild to moderate subcortical ischaemic vascular dementia. One trial included participants with mild to moderate primary degenerative dementia or vascular dementia.
Outcomes found in the review
An overview of the findings of the systematic review is available in Box 1.
Global: Alzheimer’s Disease Assessment Scale – Cognitive subscale (ADAS-Cog) (n = 92); Mattis Dementia Rating Scale (n = 1); MMSE (n = 83); Modified Telephone Interview for Cognitive Status (n = 1); and Vascular Dementia Assessment Scale Cognitive subscale (n = 1).
Batteries: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)’s Neuropsychological Test Battery (n = 2); Cogstate Alzheimer’s Battery (n = 6); Computerised Neuropsychological Test Battery (n = 1); Frontal Assessment Battery (n = 1); Mental Deterioration Battery (n = 1); Neuropsychological Test Battery (n = 7); Severe Impairment Battery (n = 1); Syndrome Short Test (n = 2); Wechsler Adult Intelligence Scale – Revised (n = 1); Wechsler Memory Scale (n = 5); and Western Aphasia Battery (n = 1).
Individual tests (either used in combination or to supplement the ADAS-Cog or MMSE): Buschke Selective Reminding Test (n = 3); Benton Visual Retention Test (n = 1); clock drawing test (n = 2); controlled oral word association test (n = 2); digit span test (n = 2); digit symbol (n = 3); dot counting n-back task (n = 1); fluency tests (n = 7); Mohs number cancellation test (n = 1); recall tasks (n = 3); Rey 15-Item Memory Test (n = 1); Stroop Colour Word Interference Test (n = 4); token test (n = 1); trail making test (n = 10); and word recognition (n = 1).
Techniques for biological markers (71 trials measured at least one biological marker; nine different techniques)Imaging: electroencephalography (n = 3); Doppler ultrasound (n = 1); MRI (n = 30); magnetic resonance spectroscopy (n = 1); positron emission tomography (n = 20); and single-photon emission computerised tomography (n = 1).
Fluid: blood tests (n = 35); cerebrospinal fluid analysis (n = 48); and urine analysis (n = 1).
Neuropsychiatric outcomes (58 trials measured at least one neuropsychiatric outcome; 16 different outcomes)Global: Alzheimer’s Disease Assessment Scale – Non-Cognitive subscale (n = 7); Behavioural Pathology in Alzheimer’s Disease Rating Scale (n = 1); Brief Psychiatric Rating Scale (n = 3); CERAD’s Behavioural Scale (n = 1); Dysfunctional Behavior Rating Instrument (n = 1); Neuropsychiatric Inventory (n = 38); Nurses’ Observation Scale for Geriatric Patients (n = 2); Plutchik Geriatric Rating Scale (n = 1); and Revised Memory and Behaviour Problems Checklist (n = 1).
Specific symptoms: Cohen-Mansfield Agitation Inventory (n = 1); Columbia Suicide Severity Rating Scale (n = 3); Cornell Scale for Depression in Dementia (n = 3); Geriatric Depression Scale (n = 10); Hamilton Depression Rating Scale (n = 5); Montgomery Depression Rating Scale (n = 2); and Zerssen Adjective Mood Scale (n = 2).
Quality-of-life outcomes (16 trials measured at least one quality-of-life outcome; three different measures)Dementia quality-of-life measure (n = 4); EuroQol-5 Dimensions Scale (n = 5); and Quality of Life in Alzheimer’s Disease Scale (n = 8).
Activities of daily living outcomes (68 trials measured at least one activities of daily living outcome; 12 different measures)Alzheimer’s Disease Co-operative Study – Activities of Daily Living Inventory (n = 35); Alzheimer’s Disease Functional Assessment and Change Scale (n = 2); BADL (n = 5); Dependence scale (n = 2); Disability Assessment For Dementia (n = 13); Functional Activities Questionnaire (n = 3); Interview for Deterioration in Daily Living Activities in Dementia (n = 2); Katz Index of Activities of Daily Living Scale (n = 4); Lawton Instrumental Activities of Daily Living Scale (n = 8); Nuremberg Gerontopsychological Rating Scale for Activities of Daily Living (n = 3); Physical Self-Maintenance Scale (n = 3); and Video Recorder Home Behavioural Assessment (n = 1).
Global outcomes (80 trials measured at least one global outcome; 10 different measures)Impression of change: Alzheimer’s Disease Cooperative Study – Clinical Global Impression of Change (n = 8); Clinical Global Impression’s Scale (n = 15); and Clinician’s Interview-Based Impression of Change plus Caregiver Input (n = 12).
Multiple domains: Blessed Dementia Rating Scale (n = 3); Dementia Severity Rating Scale (n = 3); Gottfries–Bråne–Steen Rating Scale for Dementia (n = 4); Sandoz Clinical Assessment-Geriatric Scale (n = 2); and Short Cambridge Mental Disorders of the Elderly Examination (n = 1).
Staging of dementia: Clinical Dementia Rating Scale (n = 48); and Global Deterioration Scale (n = 6).
We contacted three authors to request extra information regarding outcomes; two of whom provided this. 9,24 There were 81 different outcomes used across the trials; 72 questionnaire- or interview-based measures and nine biological techniques used to measure biomarkers. We categorised outcomes by the domain they measured. The domains were:
-
cognition
-
quality of life
-
ADLs
-
neuropsychiatric symptoms
-
global assessment
-
biological markers.
To help understand the findings of the review, we first provide an overview of the outcome measures used and their frequency of use in individual studies before describing the characteristics of these studies. We have divided the studies into published studies, ongoing trials and protocols.
Box 1 shows the outcome measures found. When outcome measures were categorised by domains, cognition was the largest domain in terms of the variety of instruments used, with 31 outcome measures used across the trials. Furthermore, cognition was the most widely used domain, being measured through at least one outcome measure in 117 of the 125 included trials. The domain included measures that look at cognition globally (n = 5), individual neuropsychological tests focusing on specific elements of cognition (n = 15) and then batteries of individual cognitive tests (n = 11). Of the included batteries, two are solely computerised (Cogstate Alzheimer’s Battery and Computerised Neuropsychological Test Battery).
The second most widely used outcomes were global measures, with 80 trials using at least one global outcome from a variety of 10 measures. The 10 measures included scales that look at clinical impressions of change (n = 3) that consider multiple domains (n = 5) and that stage dementia (n = 2). Seventy trials measured at least one biological marker, using one of nine biological techniques. The techniques can be further divided into imaging techniques [e.g. MRI (n = 6)] and fluid [e.g. blood tests (n = 3)]. Some of the biological markers measured via these techniques included the levels of amyloid-β peptide and microtubule-associated protein tau in blood and cerebrospinal fluid (CSF), changes in brain volume on MRI and changes in glucose metabolism and the density of amyloid-β peptide plaques on positron emission tomography (PET).
Activities of daily living were measured across 68 trials, with 12 different measures used. Neuropsychiatric symptoms were measured across 58 trials using 16 different measures. The neuropsychiatric outcomes included scales measuring symptoms globally (n = 9) and scales that focus on specific symptoms [e.g. depression or agitation (n = 7)]. Finally, quality-of-life outcomes were the least used across the trials; only 16 of the 124 trials measured this domain and they employed one of three measures.
Published trials
Characteristics of the included published trials are given in Table 1.
| Author and year | Trial location | Trial type | Dementia type and severity | Criteria for dementia diagnosis | Total number of participants | Description of | Participants | Outcomes related to disease modification | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (n) | Control/comparator group (n) | Sex (% of females) | Mean age (years) (range) | |||||||
| Aisen, 2000;25 Aisen et al., 200026 | USA | RCT | Mild to moderate AD | A MMSE score of 13–26 | 138 | 20 mg per day of prednisone (Deltasone, Pharmacia & Upjohn Inc., Kalamazoo, MI, USA) for 4 weeks, then 10 mg per day of prednisone for 1 year (69) | Placebo (69) | Prednisone, 49.3%; placebo, 50.7% | Prednisone, 73.4; placebo, 72.3 | Primary: ADAS-Cog Secondary: CDR (SB), BDRS, HAM-D and BPRS |
| Aisen et al., 200227 | USA | RCT | Mild to moderate AD | NINCDS-ADRDA | 40 | 200 mg per day of nimesulide (Mesulid, Helsinn Healthcare SA, Pazzallo, Switzerland) for 12 weeks (21) | Placebo (19) | Nimesulide, 38%; placebo, 47% | Nimesulide, 73; placebo, 74 | ADAS-Cog, MMSE, CDR (SB), BPRS, HAM-D and BDRS (ADL section) |
| Aisen et al., 200328 | USA | RCT | Mild to moderate AD | A MMSE score of 13–26 | 351 | 25 mg of rofecoxib (Vioxx, Merck Sharp & Dohme, Kenilworth, NJ, USA) per day (122) or 440 mg of naproxen per day (118) for 12 months | Placebo (111) | Placebo, 55.9%; naproxen, 48.3%; rofecoxib, 54.9% | Placebo, 73.8; naproxen, 74.1; rofecoxib, 73.7 | Primary: ADAS-Cog Secondary: CDR (SB), NPI, QOL-AD and ADCS-ADL |
| Aisen et al., 200629 | USA | RCT | Mild to moderate AD | DSM-430 and a MMSE score of 13–25 | 58 | 100 mg per day of 3-APS (15), 200 mg per day of 3-APS (16) or 300 mg per day of 3-APS (14) for 3 months | Placebo (13) | 100 mg, 33%; 200 mg, 75%; 300 mg, 50%; placebo, 46% | 75.1 | CSF analysis (Aβ40, Aβ42, and T-tau), ADAS-Cog, MMSE and CDR (SB) |
| Aisen et al., 200731 | USA | RCT | Mild to moderate AD | A MMSE score of 13–25 | 58 | 100 mg per day of tramiprosate (Alzhemed, BELLUS Health, Laval, QC, Canada), 200 mg per day of tramiprosate or 300 mg per day of tramiprosate for 3 months (n not specified) | Placebo (n not specified) | Not specified | Not specified | Primary: MMSE, ADAS-Cog and CDR (SB) Secondary: CSF analysis (biomarkers: only Aβ42 mentioned) |
| Aisen et al., 2008;32 National Institute on Ageing, and the General Clinical Research Centre Programme, 2008;33 Viswanathan, 200934 | USA | RCT | Mild to moderate AD | A MMSE score of 14–26 | 409 | 5 mg per day of folic acid, 1 mg per day of vitamin B12 and 25 mg per day of vitamin B6 for 18 months (240) | Placebo (169) | 56% | 76.3 (50+) | Primary: ADAS-Cog Secondary: MMSE, CDR (SB), ADCS-ADL, NPI, QOL-AD and blood tests (homocysteine levels) |
| Aisen et al., 2011;35 Gauthier et al., 2009;36 Saumier et al., 200937 | Canada and the USA | RCT | Mild to moderate AD | DSM-4,30 NINCDS-ADRDA and a MMSE score of 16–26 | 1052 | 200 mg per day of tramiprosate (352) or 300 mg per day of tramiprosate (347) for 78 weeks | Placebo (353) | 53% | 73.9 (48–94) | ADAS-Cog, CDR (SB), MMSE, CIBIC+, NPI, DAD, blood tests (Aβ), CSF analysis (tau and Aβ) and urine analysis (Aβ) In substudy: MRI (n = 312; volumetric) |
| Akhondzadeh et al., 201038 | Iran | RCT | Mild to moderate AD | DSM-4,30 NINCDS-ADRDA and a MMSE score of 15–26 | 54 | 30 mg per day of saffron for 22 weeks (27) | Donepezil 10mg per day (27) | Saffron, 48%; donepezil, 44% | Saffron, 72.7; donepezil 73.85 (55+) | MMSE, ADAS-Cog and CDR (SB) |
| Akhondzadeh et al., 201039 | Iran | RCT | Mild to moderate AD | DSM-430 and a MMSE score of 15–26 | 46 | 30 mg per day of saffron for 16 weeks (23) | Placebo (23) | Saffron, 43%; placebo, 48% | Saffron, 72.65; placebo, 73.13 (55+) | MMSE, ADAS-Cog and CDR (SB) |
| Alvarez et al., 200040 | Spain | RCT | Mild to moderate AD or vascular dementia | NINCDS-ADRDA, DSM-430 and a MMSE score of 14–26 | 45 | Polypodium leucotomos extract (Anapsos, A.S.A.C. Pharma, Alicante, Spain) 360 mg per day (15) or 720 mg per day (15) for 4 weeks | Placebo (15) | Not specified | 73.8 (56–89) | Primary: ADAS-Cog Secondary: EEG and Doppler ultrasound (blood flow haemodynamics) |
| Alvarez et al., 2006;41 Alvarez et al., 201142 | Spain | RCT | Mild to moderate AD | NINCDS-ADRDA, DSM-430 and a MMSE score of 14–25 | 279 | 10 ml per day of FPE 1070 (Cerebrolysin®, EVER Neuro Pharma GmBH, Unterach, Austria) (69), 30 ml per day of FPE 1070 (70), or 60 ml per day of FPE 1070 (71) 5 days per week for 4 weeks and on 2 days per week for next 8 weeks | Placebo (69) | 10 ml, 71.7%; 30 ml, 75.4%; 60 ml, 70.6%; placebo, 65.5% | 10 ml, 72.2; 30 ml, 73.4; 60 ml, 74.6; placebo, 73.9 | Primary: ADAS-Cog and CIBIC+ Secondary: MMSE, NPI, trail making test and DAD |
| Asthana et al., 199943 | USA | RCT | Mild to moderate AD | NINCDS-ADRDA | 12 | 0.05 mg per day of 17 β-oestradiol for 8 weeks (6) | Placebo (6) | 100% | β-oestradiol, 79.5 (66–89); placebo, 77.6 (70–86) | Buschke Selective Reminding Test, Wechsler Memory Scale, Stroop Colour Word Interference Test, trail making test, fluency test, token test, MMSE, BDRS (cognitive section), BPRS and blood tests (IGF-1 and IGFBP-3) |
| Babiloni et al., 2009;44 Pasqualetti et al., 200945 | Italy and the USA | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 16–25 | 132 | 800 mg per day of ibuprofen (and 20 mg per day of esomeprazole) for 12 months (66) | Placebo (66) | Placebo, 65%; ibuprofen, 61% | Placebo, 74.0; ibuprofen, 73.7 | Primary: ADAS-Cog Secondary: MMSE, Geriatric DS, Katz ADL, Lawton IADL, NPI, CDR (SB and global) and CIBIC+ |
| Bae et al., 200046 | Korea | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 10–24 | 53 | 30 ml per day of Cerebrolysin for 4 weeks (34) | Placebo (19) | Cerebrolysin, 68%; placebo, 63% | Cerebrolysin, 73.1; placebo, 69.0 | Primary: ADAS-Cog, CGI Secondary: MMSE, Geriatric DS, Katz ADL and Lawton IADL |
| Ban et al., 199047 | Italy | RCT | Mild to moderate primary degenerate dementia or vascular dementia | DSM-348 | 178 | 90 mg per day of nimodipine (Periplum, ITALFARMACO S.p.A., Milan, Italy) for 12 weeks (89) | Placebo (89) | Nimodipine, 55%; placebo, 61% | 75.4 (55–95) | CGI, HAM-D, MMSE, Global DS, SCAG, PGRS, Wechsler Memory Scale and blood tests [serum bilirubin, alkaline phosphatase, lactic dehydrogenase, electrolytes (Na, K, Cl), cholesterol, total CO2, SGOT, SGPT and BUN determinations] |
| Bayer et al., 2005;49 Holmes et al., 200850 | UK | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 14–26 | 80 | Immunisation of AN-1792 (Aβ1–42 peptide, 50 µg or 225 µg) using QS-21 adjuvant (50 µg or 100 µg) for 24 weeks (16 in each group) | QS-21, 50 (8); QS-21, 100 (8) | QS-21 50 µg, 25%; QS-21 100 µg, 25%; AN-1792 50 µg + QS-21 50 µg, 37.5%; AN-1792 50 µg + QS-21 100 µg, 43.8%; AN-1792 225 µg + QS-21 50 µg, 43.8%; AN-1792 225 µg + QS-21 100 µg, 31.3% | QS-21 50 µg, 70.3; QS-21 100 µg, 72.5; AN-1792 50 µg + QS-21 50 µg, 74.3; AN-1792 50 µg + QS-21 100 µg, 74.1; AN-1792 225 µg + QS-21 50 µg, 72.3; AN-1792 225 µg + QS-21 100 µg, 71.7 (under 85) | Primary: ADAS-Cog, MMSE, ADCS-CGIC and DAD |
| Bentham et al., 200851 | UK | RCT | Mild to moderate AD, with or without vascular dementia | DSM-4 | 310 | 75 mg per day of aspirin for 12 weeks (156) | Avoid aspirin (154) | Aspirin, 63%; non-aspirin, 62% | Aspirin, 51–90; non-aspirin, 46–90 | Primary: MMSE and Bristol ADL Secondary: NPI |
| Bilikiewicz, 200452 | Poland | RCT | Mild to moderate AD | DSM-4,30 NINCDS-ADRDA and a MMSE score of 10–24 | 105 | Colostrinin (Colostrinin, Biotech, ReGen Therapeutics Plc, London, UK) was 100 µg on alternate days for 3 weeks followed by 2-week drug-free cycle repeated three times for 15 weeks (53) | Placebo (52) | Not specified | 50+ | Primary: ADAS-Cog and CGI Secondary: Lawton IADL, MMSE, Global DS, Geriatric DS and ADAS-Noncog |
| Black et al., 201053 | USA | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 14–26 | 30 | 0.5 mg/kg of i.v. bapineuzumab (Janssen Alzheimer Immunotherapy, San Francisco, CA, USA) (6), 1.0 mg/kg of i.v. bapineuzumab (6) or 5.0 mg/kg of i.v. bapineuzumab (10) every 10 weeks for 52 weeks | Placebo (8) | Placebo, 87.5%; 0.5 mg, 50%; 1.5 mg, 16.67%; 5 mg, 30% | Placebo, 69.88; 0.5 mg, 74.67; 1.5 mg, 72.33; 5 mg, 74.70 (50–85) | Primary: MMSE Secondary: blood tests (Aβ1 and Aβ40) |
| Blennow et al., 2012;54 Rinne et al., 201055 | Finland and the UK | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 18−26 | 28 | 0.5 mg/kg of i.v. bapineuzumab (7), 1.0 mg/kg of i.v. bapineuzumab (7) or 2.0 mg/kg of i.v. bapineuzumab (6) every 13 weeks up to 78 weeks | Placebo (8) | Bapineuzumab groups, 42%; placebo, 57% | Bapineuzumab groups, 67.26; placebo, 70.00 (50–80) | Primary: ADAS-Cog, DAD, NTB, MMSE and PET (amyloid and glucose) Secondary: CDR (SB), NPI and MRI (volumetric) In substudy: CSF analysis (T-tau, P-tau and Aβ) |
| Blennow et al., 2012;54 Salloway et al., 200956 | USA | RCT | Mild to moderate AD | A MMSE score of 16–26 | 229 | Intravenous bapineuzumab in four ascending dose groups 0.15 mg/kg, 0.5 mg/kg, 1.0 mg/kg or 2.0 mg/kg every 13 weeks up to 78 weeks (124 between all 4 groups) | Placebo (110) | Bapineuzumab, 50.0%; placebo, 59.8% | 69.1 | Primary: ADAS-Cog and DAD Secondary: NTB, MMSE, CDR (SB) and MRI (volumetric) In substudy: CSF analysis (n = 35; T-tau, P-tau and Aβ42) |
| Bowen et al., 201557 | USA | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 12–24 | 108 | Leuprolide (Lupron, AbbVie, Maidenhead, UK) depot 11.25 mg (36) or 22.5 mg (36) every 12 weeks for 48 weeks | Placebo depot (36) | 100% | 11.25 mg, 78.75 (67–93); 22.5 mg, 78.25 (67–88); placebo, 76.97 (65–88) | Primary: ADAS-Cog and ADCS-CGIC Secondary: NPI, HAM-D and ADCS-ADL |
| Claxton et al., 201558 | USA | RCT | Amnestic MCI (n = 39) or mild to moderate AD (n = 21) | NINCDS-ADRDA and a MMSE score of > 15 | 60 | 20 IU per day insulin (20) or 40 IU per day insulin (20) for 21 days | Placebo (20) | Not specified | Not specified | Primary: recall tasks, Buschke Selective Reminding Test Secondary: DSRS, BVRT, dot counting n-back task, Stroop Colour Word Interference Test |
| Craft et al., 201259 | USA | RCT | Amnestic MCI (n = 64) or mild to moderate AD (n = 40) | NINCDS-ADRDA and a MMSE score of > 15 | 104 | 20 IU per day insulin (36) or 40 IU per day insulin (38) for 4 months | Placebo (30) | Placebo, 43.3%; 20 IU, 38.9%; 40 IU, 47.4% | Placebo, 74.9; 20 IU, 72.8; 40 IU, 69.9 | ADAS-Cog, ADCS-ADL and DSRS In substudy: PET (n = 40; metabolic rate of glucose) and CSF analysis (n = 23; Aβ42, Aβ40, tau protein and p181-tau) |
| Crapper McLachlan et al., 1991;60 Crapper McLachlan et al., 199361 | Canada and the USA | RCT | AD (not specified if mild or moderate but all living at home) | NINCDS-ADRDA | 48 | 250 mg of desferrioxamine intramuscularly daily, 5 days per week for 24 months (25) | Placebo (9) No treatment (14) | Desferrioxamine, 52%; placebo, 52% | 63.1 | Primary: Video Recorder Home Behavioural Assessment Secondary: Wechsler Adult Intelligence Scale – Revised, Wechsler Memory Scale and Western Aphasia Battery |
| Cucinotta et al., 199862 | Italy | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 15–23 | 142 | 40 mg per day of dihydroergocryptine mesylate for 1 year (70) | Placebo (72) | 70% | 74.2 (63–83) | Primary: GBS Scale Secondary: Mental Deterioration Battery |
| Dodel et al., 201363 | Germany and the USA | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 16–26 | 55 | 0.2 g/kg of i.v. immunoglobulin (12), 0.5 g/kg of i.v. immunoglobulin (15) or 0.8 g/kg of i.v. immunoglobulin (14) every 4 weeks until 20 or 22 weeks | Placebo (14) | Immunoglobulin, 37%; placebo, 64% | Immunoglobulin, 69.4; placebo, 72.0 | Primary: blood tests (Aβ1–40) Secondary: blood tests (Aβ1–42, and anti-Aβ autoantibodies), CSF analysis (Aβ1–40, Aβ1–42 and anti-Aβ autoantibodies, T-tau, and P-tau181), ADAS-Cog, CDR (SB), ADCS-ADL, MMSE, MRI (volumetric) and PET (glucose metabolism) |
| Doody et al., 200864 | Russia | RCT | Mild to moderate AD | DSM-4,30 NINCDS-ADRDA and a MMSE score of 10–24 | 183 | 40 mg per day of latrepirdine (Dimebon, Medivation, San Francisco, CA, USA) for 26 weeks (89) | Placebo (94) | Dimebon, 72%; placebo, 62% | Dimebon, 68.1; placebo, 68.4 | Primary: ADAS-Cog Secondary: MMSE, NPI, ADCS-ADL, CIBIC+ and ADCS-CGIC |
| Doody et al., 2013;65 Doody et al., 201566 | Argentina, Australia, Belgium, Canada, Chile, Denmark, Finland, France, Germany, India, Israel, Italy, Japan, Poland, South Africa, Spain, Sweden, the UK and the USA | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 16–26 | 1537 | 100 mg per day of semagacestat (Eli Lilly, Indianapolis, IN, USA) (507) or 140 mg per day of semagacestat (529) for 76 weeks | Placebo (501) | 53% | 73.2 | Primary: ADAS-Cog and ADCS-ADL Secondary: CDR (SB), NPI, MMSE, EQ-5D and blood tests (Aβ) In substudy: CSF analysis (n = 844; Aβ and tau), MRI (n = 208; volumetric) and PET (n = 108; Aβ) |
| Doody et al., 2014;67 Liu-Seifert et al., 201568 | France, Japan and the USA | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 16–26 | 1659 | 400 mg of i.v. solanezumab (Eli Lilly, Indianapolis, IN, USA) every 4 weeks for 18 months (cohort 1, 506; cohort 2, 521) | Placebo (cohort 1, 506; cohort 2, 519) | Cohort 1: placebo, 56.7%; solanezumab, 59.1% Cohort 2: placebo, 65.7%; solanezumab, 50.6% |
Cohort 1: placebo, 74.4; solanezumab, 75.0 Cohort 2: placebo, 72.5; solanezumab, 71.5 |
Primary: ADAS-Cog and ADCS-ADL Secondary: CDR (SB), NPI, MMSE, EQ-5D, blood tests (Aβ1–40 and Aβ1–42) and MRI (volumetric) In substudy: CSF analysis (n = 121; Aβ1–40, Aβ1–42 and tau) and PET (n = 266; amyloid) |
| Endres et al., 201469 | Germany | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 14–27 | 22 | 30 mg per day of acitretin (11) for 4 weeks | Placebo (11) | Placebo, 55%; acitretin, 82% | Placebo, 73; acitretin, 67 | CSF analysis (Aβ42, P-tau or t-tau), MMSE and NTB (CERAD) |
| Farlow et al., 201270 | USA | RCT | Mild to moderate AD | A MMSE score of 15–26 | 52 | 100 mg of solanezumab every 4 weeks (10), 100 mg weekly (11), 400 mg every 4 weeks (10) or 400 mg weekly (11) for 12 weeks | Placebo (10) | 53.8% | 71.2 (53–89) | CSF analysis (Aβ1–40 and Aβ1–42), blood tests (Aβ1–40 and Aβ1–42) and ADAS-Cog In substudy: PET (n = 24; amyloid) |
| Faux et al., 2010;71 Lannfelt et al., 200872 | Australia, Sweden, the UK and the USA | RCT | Mild to moderate AD | NINCDS-ADRDA, a MMSE score of 20–26 or an ADAS-Cog score of 10–25 | 78 | 50 mg of PBT2 per day (20) or 250 mg of PBT2 per day (29) for 12 weeks | Placebo (29) | Placebo, 52%; 50 mg, 45%; 250 mg, 52% | Placebo, 71.6 (60–83); 50 mg, 72.4 (58–83); 250 mg, 72.1 (58–83) | Blood tests (Aβ40, Aβ42, Zn2+, and Cu2+), CSF analysis (12 Aβ40, Aβ42, T-tau, P-tau, Zn2+ and Cu2+), NTB, ADAS-Cog and MMSE |
| Faxén-Irving et al., 2013;73 Freund-Levi et al., 200674 | Sweden | RCT | Mild to moderate AD | DSM-430 and a MMSE score of 15–30 | 174 | 1.7 g per day of DHA and 0.6 g per day of EPA for 6 months (89) | Placebo (85) | Intervention, 57%; placebo, 46% | Intervention, 72.6; placebo, 72.9 | Primary: MMSE and ADAS-Cog Secondary: CDR (SB and global) and blood tests (transthyretin) In substudy: CSF analysis (n = 35; transthyretin) |
| Ferrari et al., 199875 | Italy | RCT | Mild to moderate AD | ICD-1076 or DSM-4,48 NINCDS-ADRDA and a MMSE score of 10–23 | 213 | 10 mg per day of posatirelin (Poli Industria Chimica S.p.A., Rozzano, Italy) for 3 months (107) | Placebo (106) | 58% | 78.8 | Rey Memory test, GBS Scale, MMSE, HAM-D and Global DS |
| Fleisher et al., 200877 | USA | RCT | Mild to moderate AD | NINCDS-ADRDA | 51 | 100 mg per day of LY-450139 (22) or 140 mg per day of LY-450139 (14) for 14 weeks | Placebo (15) | Placebo, 33%; 100 mg, 64%; 140 mg, 43% | Placebo, 68.7; 100 mg, 70.8; 140 mg, 68.1 | Primary: blood tests (Aβ) and CSF analysis (Aβ) Secondary: ADAS-Cog and ADCS-ADL |
| Fleisher et al., 201178 | USA | RCT | Mild to moderate AD | A MMSE score of 12–20 | 89 | 10–12 mg/kg daily of divalproex sodium (Depakote, Abbott Laboratories, Chicago, IL, USA) for 24 months (43) | Placebo (46) | Placebo, 67%; divalproex sodium, 33% | Placebo, 76; divalproex sodium, 73 | Primary: MRI (volumetric) and NPI Secondary: ADAS-Cog, CDR (SB), MMSE, ADCS-ADL, ADCS-CGIC, CMAI and QOL-AD |
| Fox et al., 2005;79 Gilman et al., 2005;80 Hock et al., 2003;81 Koepsell et al., 2007;82 Orgogozo et al., 2003;83 Vellas et al., 200984 | France and USA | RCT | Mild to moderate AD | A MMSE score of 15–26 | 131 | Immunisation of 225 µg of AN-1792 plus 50 µg of adjuvant QS-21 at day 0 and months 1, 3, 6, 9 and 12 (59 responders) | Placebo (72) | Not specified | Intervention, 74.9; placebo, 73.7 | Primary: blood tests (Aβ), DAD, CDR (global), MMSE, ADAS-Cog and MRI (volumetric) Secondary: NTB |
| Galasko et al., 201285 | USA | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of ≥ 16 | 79 | 500 mg per day of vitamin C and 800 IU per day of vitamin E and 900 mg per day of α-lipoic acid (E/C/ALA) (28) OR 1200 mg per day of coenzyme Q (25) for 16 weeks | Placebo (26) | E/C/ALA, 46%; coenzyme Q, 44%; placebo, 48% | E/C/ALA, 73.6; coenzyme Q, 71.4; placebo, 73.2 | ADCS-ADL, MMSE and CSF analysis (tau, P-tau, Aβ42, F2-isoprostane) |
| Galasko et al., 201486 | USA | RCT | Mild to moderate AD | A MMSE score of 14–26 | 399 | 5 mg per day of PF-04494700 (132) or 20 mg of PF-04494700 per day (135) for 18 months | Placebo (132) | 5 mg, 53%; 20 mg, 61%; placebo, 57% | 5 mg 73.6; 20 mg 73.0; placebo, 72.2 | Primary: ADAS-Cog Secondary: CDR (SB), ADCS-ADL, NPI, MMSE, digit symbol substitution test, forward and backward digit span test, controlled oral word association test, Stroop Colour Word Interference Test, trail making test, DEMQOL and blood tests (Aβ1–40, Aβ1-x, Aβ1–42) In substudy: MRI (n = 186; volumetric) and CSF analysis (n = 52; Aβ1–40, Aβ1-x, Aβ1–42, tau and P-tau 181) |
| Gauthier et al., 201587 | Canada and the USA | RCT | Moderate AD | NINCDS-ADRDA and a MMSE score of 10–20 | 403 | 10 mg per day of ST101 (50), 60 mg per day of ST101 (51) and 120 mg per day of ST101 (51) for 12 weeks | Placebo (51) | Placebo, 41.2%; 10 mg, 50%; 60 mg, 60.8%; 120 mg, 52.9% | Placebo, 78.3; 10 mg, 74.4; 60 mg, 77.8; 120 mg, 75.7 | Primary: ADAS-Cog Secondary: ADCS-CGIC, ADCS-ADL, NPI and MMSE |
| Geldmacher et al., 201188 | USA | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 12–26 | 29 | 45 mg per day of pioglitazone (14) for 18 months | Placebo (15) | Pioglitazone, 64%; placebo, 60% | Pioglitazone, 74.9; placebo, 67.0 | Secondary: CDR (SB), ADAS-Cog, NPI, ADFACS, CIBIC+ and NOSGER |
| Gold et al., 201089 | Austria, Bulgaria, Chile, China, Croatia, Estonia, Germany, Greece, Hungary, Korea, Mexico, New Zealand, Pakistan, Peru, the Philippines, Puerto Rico, Russia, the UK and the USA | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 10–23 | 581 | 2 mg per day of extended-release rosiglitazone (166), 8 mg per day of extended-release rosiglitazone (165) for 24 weeks | Placebo (166) or 10 mg per day of donepezil (84) | 2 mg, 64%; 8 mg, 65%; placebo, 60%; donepezil, 63% | 2 mg, 71.7; 8 mg, 72.6; placebo, 72.5; donepezil, 72.9 | Primary: ADAS-Cog and CIBIC+ Secondary: NPI, DAD, MMSE, blood tests (glycated haemoglobin) and EQ-5D |
| Green et al., 2009;90 Myrexis, Inc., 201091 | USA | RCT | Mild to moderate AD | A MMSE score of 15–26 | 1684 | 800 mg per day of tarenflurbil (Flurizan, Myrexis, Inc., Salt Lake City, UT, USA) (42) or 1600 mg per day (820) for 18 months | Placebo (822) | 50.9% | 74.6 (55+) | Primary: ADAS-Cog and ADCS-ADL Secondary: CDR (SB), MMSE, NPI and QOL-AD |
| Grimaldi et al., 201492 | Italy | RCT | Mild to moderate AD | DSM-430 and a MMSE score of 20–26 | 42 | 66 µg per day of IFNβ1a for 28 weeks (23) | Placebo (19) | Placebo, 58%; IFNβ1a 65% | Placebo, 64.6; IFNβ1a, 63.0 | ADAS-Cog, Global DS, CIBIC+, MMSE, ADAS-Noncog, Lawton IADL, PSMS and Geriatric DS |
| Hampel et al., 200993 | Germany | RCT | Mild AD | DSM-4,30 NINCDS-ADRDA and a MMSE score of 21–26 | 71 | Lithium, various doses for 10 weeks (33) | Placebo (38) | 52.1% | 68.6 (50–84) | Primary: CSF analysis (P-tau) Secondary: CSF analysis (T-tau and Aβ42), blood tests (Aβ42), MMSE, NPI and ADAS-Cog |
| Hock et al., 2003;94 Hock et al., 200095 | Germany and Switzerland | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 12–26 | 40 | Talsaclidine (Boehringer Ingelheim, Rhein, Germany) various doses for 4 weeks (34) | Placebo (6) | Talsaclidine, 56%; placebo, 83% | Talsaclidine, 67.1; placebo, 69.7 | CSF analysis (Aβ42 and Aβ40) |
| Jhee et al., 200496 | Korea and the USA | RCT | Mild to moderate AD | NINCDS- ADRDA and a MMSE score of 10–24 | 20 | 100 mg per day of celecoxib (5), 400 mg per day of celecoxib (5) or 800 mg per day of celecoxib (5) for 28 days | Placebo (5) | Placebo, 20%; 100 mg, 20%; 400 mg, 40%; 800 mg, 20% | Placebo, 77.2; 100 mg, 68.6; 400 mg, 75.2; 800 mg, 69.7 | CSF analysis (PGE2, IL-6, Aβ1–42 and tau), blood tests (PGE2, IL-6, Aβ1–42 and tau), ADAS-Cog, MMSE and computerised NTB |
| de Jong et al., 200897 | The Netherlands | RCT | Mild to moderate AD | NINCDS- ADRDA and a MMSE score of 10–26 | 51 | 100 mg per day of indomethacin for 12 months (26) | Placebo (25) | Placebo, 76%; indomethacin, 54% | Placebo, 72.2; indomethacin, 72.7 | Primary: ADAS-Cog Secondary: MMSE, CIBIC+, ADAS-Noncog, NPI and IDDD |
| Kadir et al., 200898 | Sweden | RCT | Mild AD | NINCDS- ADRDA and a MMSE score of ≥ 21 | 20 | 30 mg per day of phenserine (QR Pharma, Inc., Berwyn, PA, USA) for 3 months (10) | Placebo (10) | 75% | 68 | PET (glucose and amyloid), CSF analysis (Aβ42, T-tau and P-tau, α- and β-secretase-cleaved amyloid precursor protein), blood tests (Aβ40 and Aβ42), MMSE, recall task, word recognition, digit symbol substitution test, trail making test and clock drawing task |
| Kessler et al., 200899,100 | Germany | RCT | Mild AD | NINCDS ADRDA and a MMSE score of < 25 | 68 | 51.62 mg per day of Cinnamomum verum (verum) extract containing 8 mg per day of copper orotate for 12 months (35) | Placebo (33) | Placebo, 55%; verum, 40% | Placebo, 69.4; verum, 69.6 | Primary: ADAS-Cog and MMSE |
| Landen et al., 2013101 | Australia, Canada, Sweden and the UK | RCT | Mild to moderate AD | DSM-4,30 NINCDS- ADRDA and a MMSE score of 16–26 | 37 | Ponezumab (Pfizer, Inc., New York City, NY, USA) – one infusion of 0.1 mg/kg (4), 0.3 mg/kg (4), 1 mg/kg (4), 3 mg/kg (6) or 10 mg/kg (8) | Placebo (11) | Ponezumab (altogether), 42.3%; placebo, 27.3% | Ponezumab (altogether), 70.0 (50–84); placebo, 71.8 (61–85) | ADAS-Cog, MMSE, Cogstate Alzheimer’s Battery and blood tests (Aβ1-x, Aβ1–40, and Aβ1–42) In substudy: CSF analysis (in 1- and 10-mg groups, n = 12; Aβ1-x, Aβ1–40, Aβ1–42, T-tau and P-tau) |
| Leszek et al., 1999102 | Poland | RCT | Mild to severe AD | NINCDS- ADRDA and DSM-348 | 46 | 100 mg every second day of Colostrinin for 3 weeks followed by a 2-week hiatus – 10 cycles (15) | 100 mg of selenium (15) or placebo (16) | Colostrinin, 80%; selenium, 80%; placebo, 62.5% | Colostrinin, 70.75 (45–83); selenium, 70.75 (50–82); placebo, 67.8 (59–76) | Primary: MMSE Secondary: additional psychosocial functioning of the AD patients provided by the patients’ caregivers – no information as to what measures |
| Li et al., 2015103 | China | CCT | Moderate AD | A MMSE score of 10–20 and an ADAS-Cog score of 29–40 | 24 | 0.9 g per day of Cistanches herb extract for 48 weeks (10) | No treatment (6) or 5 mg per day of donepezil (8) | Cistanches herb, 60%; no treatment, 50%; donepezil, 62.5% | Cistanches herb, 70.3; no treatment, 71.3; donepezil, 73.5 | MMSE, ADAS-Cog, MRI (volumetric), CSF analysis (protein, mRNA levels, T-tau, tumour necrosis factor alpha, and interleukin 1 beta) |
| Lovestone et al., 2015104 | Finland, France, Germany, Spain and the UK | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 14–26 | 307 | 500 mg per day (45) of tideglusib, 1000 mg per day of tideglusib (86) or 1000 mg of tideglusib every other day (91) for 26 weeks | Placebo (85) | Placebo, 55.3%; 500 mg, 64%; 1000 mg, 51.9%; 1000 mg every other day, 54.4% | Placebo, 70.8; 500 mg, 71.1; 1000 mg, 72.3; 1000 mg every other day 71.6 | Primary: ADAS-Cog Secondary: MMSE, fluency test, ADCS-ADL, EQ-5D, NPI and CGI In substudy: MRI (n = 86; cerebral atrophy) and CSF analysis (n = 21; P-tau and Aβ1–42) |
| Maher-Edwards et al., 2015105 | Bulgaria, Canada, Germany, Italy, Spain and Sweden | RCT | Mild AD | NINCDS-ADRDA and a MMSE score of 10–26 | 124 | 250 mg per day of rilapine (GlaxoSmithKline, London, UK) for 24 weeks (62) | Placebo (62) | Placebo, 54% rilapine, 47% | Placebo, 73.1; rilapine, 72.9 | Primary: Cogstate Alzheimer’s Battery and CSF analysis (Aβ1–42) Secondary: CSF analysis (Aβ1–40, T-tau, 181 P-tau, Lp-PLA2, neurofilament light chain and albumin quotient) and blood tests (Aβ1–40, Aβ1–42, and Lp-PLA2) |
| Marcusson et al., 1997106 | Belgium, Croatia, France, Germany, Sweden, the UK and Yugoslavia | RCT | Mild to moderate AD or vascular dementia | DSM-348 and a MMSE score of 15–25 | 261 | 300 mg of propentofylline (Hoechst Marion Roussel, Kansas City, MO, USA) three times daily for 12 months (130) | Placebo (131) | Not specified | Placebo, 72.9; propentofylline, 71.9 | Primary: GBS scale, CGI and SKT Secondary: digit symbol substitution test, MMSE, NAI-ADL and AMS |
| Molloy et al., 2013107 | Canada | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 14–26 | 406 | 200 mg per day of doxycycline + 300 mg per day of rifampin (101), 200 mg per day of doxycycline (102) or 300 mg per day of rifampin (101) for 12 months | Placebo (102) | Doxycycline and, 50.5%; doxycycline, 50%; rifampin, 48% | Doxycycline and rifampin, 79.2; doxycycline, 78.7; rifampin, 78.6 | Primary: ADAS-Cog and CDR Secondary: MMSE, Geriatric DS, CSDD, Lawton IADL and DBRI |
| Muresanu et al., 2002108 | Austria and Romania | RCT | Mild to moderate AD | NINCDS-ADRDA, DSM-430 and a MMSE score of 14–25 | 60 | 30 ml per day of Cerebrolysin for 5 days per week for 6 weeks (30) | Placebo (30) | Not specified | Not specified | DAD, ADAS-Cog and CGI |
| Muresanu et al., 2008109 | Romania and Spain | RCT | Mild to moderately severe vascular dementia | NINDS-AIREN and a MMSE score of 9–26 | 41 | 10 ml per day of Cerebrolysin (16) or 30 ml per day of Cerebrolysin (15) for 5 days per week for 4 weeks | Placebo (10) | 51% | 70.7 (51–88) | ADAS-Cog, MMSE and EEG |
| Nygaard et al., 2015110 | USA | RCT | Mild to moderate AD | A MMSE score of 16–26 | 24 | 50 mg per day of saracatinib, 100 mg per day of saracatinib or 125 mg per day of saracatinib for 4 weeks. Six in each of the three treatment groups | Placebo (6) | 61% | 73 | ADAS-Cog, ADCS-ADL, NPI, CDR (SB), MMSE, PET (glucose) and CSF analysis (Aβ40, Aβ42, Tau and P231-Tau) |
| Ostrowitzki et al., 2012111 | Denmark, Israel, the Netherlands, Sweden and the UK | RCT | Mild to moderate AD | NINCDS- ADRDA and a MMSE score of 16–26 | 18 | 60 mg of i.v. gantenerumab (F Hoffmann-La Roche AG, Basel Switzerland) (8) or 200 mg of i.v. gantenerumab (6) every 4 weeks for 3 months | Placebo (4) | Placebo, 75%; 60 mg, 25%; 200 mg, 50% | Placebo, 62.8; 60 mg, 70.9; 200 mg, 66.5 | Primary: ADAS-Cog, MMSE, a modified NTB (not specified) and DAD Secondary and in substudy: PET (n = 16; amyloid) |
| Quinn et al., 2010112 | USA | RCT | Mild to moderate AD | A MMSE score of 14–26 | 402 | 2 g per day of docosahexaenoic acid for 18 months (238) | Placebo (164) | 52.2% | 76 | Primary: ADAS-Cog and CDR (SB) Secondary: MMSE, ADCS-ADL, NPI and QOL-AD In substudy: MRI (n = 102; volumetric) |
| Regland et al., 2001113 | Sweden | RCT | Mild to moderate AD | NINCDS- ADRDA and a MMSE score of 10–24 | 20 | 20 mg per day of clioquinol (10) or 80 mg per day of clioquinol (10) for 21 days | 65% | 74.6 (61–83) | CSF analysis (Aβ42, tau and GAP43), MMSE, ADAS-Cog and GBS scale | |
| Reines et al., 2004114 | USA | RCT | Mild to moderate AD | NINCDS- ADRDA and a MMSE score of 14–26 | 692 | 25 mg per day of rofecoxib (Vioxx, Merck Sharp & Dohme) for 12 months (346) | Placebo (346) | Placebo, 52%; rofecoxib, 54% | Placebo, 75; rofecoxib, 76 | ADAS-Cog, CDR (global), MMSE, ADCS-ADL and CIBIC+ |
| Ringman et al., 2012115 | USA | RCT | Mild to moderate AD | NINCDS- ADRDA and a MMSE score of 17–29 | 36 | 2 g per day of curcumin C3 complex (12) or 4 g per day of curcumin C3 complex (12) for 24 weeks | Placebo (12) | 63% | 73.5 | ADAS-Cog, NPI, ADCS-ADL, MMSE and blood tests (Aβ1–40 and Aβ1–42), CSF analysis (Aβ1–42, T-tau, P-tau181 and isoprostanes) |
| Ritchie et al., 2003116 | Australia | RCT | Moderately severe AD | ADAS-Cog score of 20–45 and a MMSE score of 10–24 | 36 | Ascending doses of clioquinol up to 750 mg per day (18) | Placebo (18) | Clioquinol, 50.0%; placebo, 43.7% | 72.5 | ADAS-Cog and blood tests (Aβ, Zn and Cu) |
| Rüther et al., 2000;117 Rüther et al., 1994118 | Austria and Germany | RCT | Mild to moderate AD | DSM-348 | 120 | 30 ml per day of Cerebrolysin (for 5 days a week) for 4 weeks (60) | Placebo (60) | 66% | (55–85) | CGI, NAI-ADL, SCAG, trail making test and AMS |
| Ruether et al., 2001;119 Ruether et al., 2002120 | Austria and Germany | RCT | Mild to moderate AD | NINCDS-ADRDA, ICD-1076 and a MMSE score of 14–24 | 149 | 30 ml per day of Cerebrolysin for 5 days a week for 4 weeks (76) | Placebo (73) | Cerebrolysin, 64.9%; placebo, 51.4% | Cerebrolysin, 72.5; placebo, 73.5 | Primary: ADAS-Cog and CGI Secondary: SKT, MADR-S, NAI-ADL and ADAS-Noncog |
| Salloway et al., 2011121 | Canada and the USA | RCT | Moderate AD | A MMSE score of 16–26 | 353 | 500 mg per day of scyllo-inositol (89), 2000 mg per day of scyllo-inositol (89) or 4000 mg per day of scyllo-inositol (91) twice daily for 78 weeks | Placebo (84) | Placebo, 56.5%; 500 mg, 58.0%; 2000 mg, 53.9%; 4000 mg, 56.0% | Placebo, 73.4; 500 mg, 73.4; 2000 mg, 73.4; 4000 mg, 72.2 | Primary: NTB and ADCS-ADL Secondary: ADAS-Cog, CDR (SB), NPI and MRI (volumetric) In substudy: MRS (n not specified) and CSF analysis (n = 20; Aβx-40, Aβx-42, T-tau, P-tau) |
| Salloway et al., 2014122 | Austria, Canada, Germany and the USA | RCT | Mild to moderate AD | A MMSE score of 16–26 | 2451 | APOE ε4 carriers: 0.5 mg/kg of bapineuzumab every 13 weeks up to 78 weeks (673) Non-carriers: 0.5 mg/kg (337), 1.0 mg/kg (329) or 2.0 mg/kg (141 but discontinued and received 1.0 mg/kg) of bapineuzumab every 13 weeks up to 78 weeks |
Placebo (carriers, 448; non-carriers, 524) | Carriers: placebo, 56.0%; 0.5 mg/kg, 54.4% Non-carriers: placebo, 50.3%; 0.5 mg/kg, 52.5%; 1.0 mg/kg, 57.0% |
Carriers: placebo, 72.3; 0.5 mg/kg, 72.0 Non-carriers: placebo, 71.9; 0.5 mg/kg, 73.1; 1.0 mg/kg, 73.5 |
Primary: ADAS-Cog and DAD Secondary: NTB, CDR (SB), MMSE and Dependence Scale In substudy: PET (n = 154; amyloid) and CSF analysis (n = 390; P-tau181), MRI (n = 1149; volumetric) |
| Sano et al., 1996123 | USA | RCT | Moderate AD | NINDCS-ADRDA | 486 | Selegiline (4 mg per day) and atoc (1000 IU per day), placebo and atoc, selegiline and placebo for 2 years (n not specified) | Placebo (n not specified) | 64.9% | 73.3 | Primary: Bristol ADL and CDR (global) Secondary: ADAS-Cog, MMSE, Dependence Scale and CERAD’s Behavioural Rating Scale |
| Sano et al., 2011124 | USA | RCT | Mild to moderate AD | A MMSE score of 12–26 | 406 | Increasing dose of simvastatin up to 40 mg for 18 months (204) | Placebo (202) | Placebo, 59.9%; simvastatin, 58.8% | Placebo, 75.1; simvastatin, 74.0 | Primary: ADAS-Cog Secondary: ADCS-CGIC, MMSE, Dependence Scale, ADCS-ADL, NPI and QOL-AD |
| Scharf et al., 1999125 | Australia | RCT | Mild to moderate AD | DSM-430 and a MMSE score of 11–25 | 41 | 100 mg per day of diclofenac and 400 µg per day of misoprostol for 25 weeks (24) | Placebo (17) | Diclofenac/misoprostol group, 67%; placebo, 47% | Diclofenac/misoprostol group, 71.8; placebo, 73.9 | Primary: ADAS-Cog, Global DS and CGI Secondary: MMSE, ADAS-Noncog, Lawton IADL and PSMS |
| Schwam et al., 2014126 | Canada, Chile, Czech Republic and the USA | RCT | Mild to moderate AD | A MMSE score of 14–26 | 191 | 50 mg per day of PF-04447943 for 12 weeks (91) | Placebo (100) | PF-04447943, 64%; placebo, 64% | PF-0444794, 73.6; placebo, 73.5 | ADAS-Cog, NPI and CGI |
| del Ser et al., 2013127 | Germany | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 16–26 | 30 | Up to 1000 mg per day of tideglusib (Noscira SA, Madrid, Spain) (20) for 20 weeks | Placebo (10) | Tideglusib, 65%; placebo, 70% | Tideglusib, 73.1; placebo, 72.6 | Secondary: MMSE, ADAS-Cog, fluency test and CGI |
| Sevigny et al., 2008128 | USA | RCT | Mild to moderate AD | A MMSE score of 14–26 | 563 | 25 mg per day of ibutamoren for 12 months (282) | Placebo (281) | Ibutamoren, 56%; placebo, 59.8% | Ibutamoren, 75.9; placebo, 76.1 | Primary: CIBIC+ Secondary: ADAS-Cog, CDR (SB) and ADCS-ADL |
| Siemers et al., 2010129 | USA | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 14–26 | 19 | 0.5 mg/kg of solanezumab (Eli Lilly) (4), 1.5 mg/kg of solanezumab (4), 4.0 mg/kg of solanezumab (4) or 10.0 mg/kg of solanezumab (4) single dose | Placebo (3) | Placebo, 100%; 0.5 mg/kg, 25%; 1.5 mg/kg, 25%; 4 mg/kg, 50%; 10 mg/kg, 25% | Placebo, 70.3; 0.5 mg/kg, 61.0; 1.5 mg/kg, 71.5; 4 mg/kg, 67.5; 10 mg/kg, 75.3 | Blood tests (Aβ1–40 and Aβ1–42), CSF analysis (Aβ1–40 and Aβ1–42) and ADAS-Cog |
| Silverberg et al., 2002130 | USA | RCT | Mild to moderate AD | A MMSE score of 15–24 | 29 | Surgical shunt for low-flow CSF drainage (15) | No shunt (14) | 48% | 72.4 | Primary: MDRS and MMSE Secondary: CSF analysis (MAP-tau and Aβ1–42) |
| Silverberg et al., 2008131 | USA | RCT | Mild to moderate AD | A MMSE score of 15–24 | 164 | Low-flow ventriculoperitoneal shunt (COGNIshunt, Intergra LifeSciences, Plainsboro, NJ, USA) (110) | Sham (occluded) shunt (120) | Occluded, 56%; COGNIshunt, 61% | Occluded, 74.0; COGNIshunt, 74.5 | Primary: MDRS, Global DS Secondary: CSF analysis (Aβ1–42 and MAP-tau) |
| Simons et al., 2002132 | Germany | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 12–26 | 44 | 80 mg per day of simvastatin for 26 weeks (24) | Placebo (20) | Placebo, 47%; simvastatin, 63% | Placebo, 68.5; simvastatin, 68.0 | CSF analysis (AβA40, AβA42, lathosterol, cholesterol, and 24S-hydroxycholesterol), ADAS-Cog and MMSE |
| Soininen et al., 2007133 | Australia, Belgium, Finland, France, Germany, the Netherlands and the UK | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 12–26 | 425 | 400 mg per day of celecoxib for 52 weeks (285) | Placebo (140) | Placebo, 59%; celecoxib, 53% | Placebo, 73.3; celecoxib, 73.7 | Primary: ADAS-Cog and CIBIC+ Secondary: BEHAVE-AD, NOSGER and MMSE |
| Sparks et al., 2005134 | USA | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 12–28 | 63 | 80 mg per day of atorvastatin calcium for 12 months (32) | Placebo (31) | Placebo, 35.5%; atorvastatin calcium, 37.5% | Placebo, 78.9; atorvastatin calcium, 78.15 | Primary: ADAS-Cog and CGI Secondary: MMSE, Geriatric DS and ADCS-ADL |
| Sweetlove, 2012135 | New Zealand | RCT | Mild to moderate AD | A MMSE score of 12–24 | 1003 | 15 mg per day of latrepirdine or 60 mg per day of latrepirdine (n not specified) | Placebo (n not specified) | Males and females (n not specified) | (50+) | ADAS-Cog and ADCS-ADL |
| Tan and Pu, 2003136 | USA | RCT | Mild to moderate AD | NINCDS-ADRDA | 10 | 200 mg every 2 weeks of intramuscular testosterone enanthate (Delatestryl®, Endo Pharmaceuticals, Malvern, PA, USA) for 12 months (5) | Placebo (5) | 0% (all male) | 72.4 (68–80) | Primary: ADAS-Cog and MMSE Secondary: clock drawing test |
| Turner et al., 2015137 | USA | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 14–26 | 119 | Resveratrol (Aptuit Laurus, Inc., Hyderabad, India) escalating doses up to 2000 mg per day (64) | Placebo (55) | Resveratrol, 62.5%; placebo, 51% | Resveratrol, 69.8; placebo, 73 | Primary: blood tests (Aβ40 and Aβ42, and insulin and glucose) and CSF analysis (Aβ40, Aβ42, tau, and P-tau181), MRI (volumetric) Secondary: MMSE, ADAS-Cog, ADCS-ADL, CDR (SB) and NPI |
| Van Gool et al., 2001138 | The Netherlands | RCT | Mild AD | Minimal or mild severity scores according to the CAMDEX | 168 | A single dose of hydroxychloroquine (83; 400 mg in patients weighing ≥ 65 kg or 200 mg in those weighing < 65 kg) | Placebo (85) | Hydroxychloroquine, 54%; placebo, 60% | Hydroxychloroquine, 70.4; placebo, 70.7 | Primary: IDDD Secondary: ADAS-Cog and RMBCP |
| Vellas et al., 2011139 | France | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 12–24 | 159 | 40 mg per day of EHT 0202 (51) or 80 mg per day of EHT 0202 (55) for 3 months | Placebo (53) | 56% | 40 mg per day, 76.4; 80 mg per day, 76.7; placebo, 75.8 | Primary: ADAS-Cog, NTB, CDR (SB), NPI, ADCS-ADL, MMSE and CGI Secondary: blood tests (sAPPα) |
| Wang et al., 2013140 | China | RCT | Moderate to severe AD | NINCDS-ADRDA, DSM-430 and a MMSE score of 4–20 | 26 | 10 mg per day of memantine for 24 weeks (13) | Placebo (13) | Placebo, 54%; memantine, 54% | Placebo, 64.7; memantine, 65.7 (50–90) | Primary: Severe Impairment Battery, PET (glucose) and CSF analysis (T-tau, P-tau181, Aβ40 and Aβ42) Secondary: ADAS-Cog, MMSE and NPI |
| Watson et al., 2005141 | USA | RCT | Amnestic MCI (n = 9) or mild AD (n = 21) | NINCDS-ADRDA and a MMSE score of > 15 | 36 | 4 mg per day of rosiglitazone (Avandia, GlaxoSmithKline) for 6 months (24) | Placebo (12) | 30% | Rosiglitazone, 72.8; placebo, 73.3 | Blood tests (insulin and Aβ), Buschke Selective Reminding Test, recall task, Stroop Colour Word Interference Test, trail making test and fluency test |
| Wilcock et al., 2008142 | Canada and the UK | RCT | Mild to moderate AD | A MMSE score of 15–26 | 210 | 400 mg per day of tarenflurbil (69) or 800 mg per day of tarenflurbil (70) for 12 months | Placebo (71) | Placebo, 48%; 400 mg per day, 52%; 800 mg per day, 48% | Placebo, 74.4; 400 mg per day, 73.4; 800 mg per day, 75.8 | ADAS-Cog, ADCS-ADL and CDR (SB) |
| Winblad et al., 2001143 | Belgium, Germany, Italy, Sweden and the UK | RCT | Mild to moderate AD | MMSE score of 12–24 | 346 | 60 mg per day of nicergoline (Sermion, Pharmacia & Upjohn, Kalamazoo, MI, USA) for 6 months (177) | Placebo (169) | Nicergoline, 61%; placebo, 63.9% | 73.7 | Primary: ADAS-Cog, CGI and ADAS-Noncog Secondary: Lawton IADL and PSMS |
| Winblad et al., 2012144 | Sweden | RCT | Mild to moderate AD | DSM-430 and a MMSE score of 16–26 | 58 | Cohort 1: a 50-mg CAD106 injection at 0, 6 and 18 weeks (24) Cohort 2: a 150-mg CAD106 injection at 0, 2 and 6 weeks (22) |
Placebo (cohort 1, n = 7; cohort 2, n = 5) | Cohort 1: CAD106, 33%; placebo, 57% Cohort 2: CAD106, 59%; placebo, 60% |
Cohort 1: CAD106, 68.9; placebo, 70.6 Cohort 2: CAD106, 68.2; placebo, 67.0 |
Primary: CSF analysis (serum Aβ-antibody) Secondary: CSF analysis (amyloid biomarkers), blood tests (amyloid biomarkers), NTB (CERAD), MMSE, CDR (global), ADCS-ADL and MRI (volumetric) |
| Wischik, 2015145 | Singapore and the UK | RCT | Mild to moderate AD | DSM-4,30 NINCDS-ADRDA and a MMSE score of 10–26 | 321 | 69 mg per day of methylthioninium chloride (LMTX, TauRx Therapeutics Ltd, Singapore) (59), 138 mg per day of methylthioninium chloride (80) or 228 mg per day of methylthioninium chloride (90) for 24 weeks | Placebo (92) | 54% | 73.8 | Primary: ADAS-Cog Secondary: ADCS-CGIC, MMSE, CDR (SB), Bristol ADL, ADFACS, NPI and dementia ‘caseness’ short CAMDEX In substudy: SPECT (n = 135; for regional cerebral blood flow) |
| Wolkowitz et al., 2003146 | USA | RCT | Mild to severe AD | A MMSE score of > 8 | 58 | 100 mg per day of dehydroepiandrosterone (Neuroscience Pharma Inc., Montreal, QC, Canada) for 6 months (28) | Placebo (30) | 49% | Dehydroepiandrosterone, 75.5; placebo, 77.2 | ADAS-Cog, CIBIC+, MMSE, ADAS-Noncog and CSDD |
We included 95 trials published between 1990 and 2015. Most studies included all or some participants with AD (n = 94), and one study included only participants with vascular dementia. All were RCTs, except for one CCT. The trials included a total of 22,362 participants.
A total of 79 different outcomes were used. The majority of trials (n = 94) used at least one cognitive outcome,25,27–29,31,33,37–41,43,45–47,49,51–55,57–59,61–64,66,68–71,73,75,77,78,81,85–90,92,93,96–99,101–116,118,119,121–146 of which there were 30 different measurement tools. The second most commonly measured domain was a global outcome, with nine different measures used across 64 trials. 25,27–29,31,33,37–39,41,45–47,49,52,54,55,57–59,62–64,66,68,73,75,78,81,86–90,92,97,104,106–108,110,112–114,118,119,121–128,131,133,134,137,139,142–146 Fifty trials included a neuropsychiatric outcome,25,27,28,33,37,41,43,45–47,51,52,55,57,64,66,68,75,78,86–93,97,104,106,107,110,112,115,118,119,121,123,125,126,130,131,133,134,137–140,143,145,146 with a variety of 16 measures used. ADLs were measured in 55 trials, using 12 measures. 27,28,33,37,41,45,46,49,51,52,54,55,57,59,61,63,64,66,68,77,78,81,85–90,92,97,104,106–108,110–112,114,115,118,119,121–125,128,134,135,137,138,142–145 Biological markers were measured in 51 trials using a variety of nine biological techniques. 29,31,33,37,40,43,47,53–55,59,63,66,68–71,73,77,78,81,85,86,89,93,95,96,98,101,103–105,109–113,115,116,121,122,129–132,137,139–141,144,145 Eleven of the published trials measured quality of life, using one of three outcomes. 28,33,66,68,78,86,89,90,104,112,124
Published protocols
The characteristics of the published protocols are described in Table 2.
| Author and year | Trial location | Trial type | Dementia type and severity | Criteria for dementia diagnosis | Participants | Description of | Outcomes related to disease modification | ||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Age range (years) | Intervention | Control/comparator group | ||||||
| Annweiler et al., 2011147 | France | RCT | Moderate AD | NINCDS-ADRDA, DSM-430 and a MMSE score of 10–20 | Males and females | ≥ 60 | 20 mg per day of memantine plus 3571 IU per day of vitamin D for 24 weeks | 20 mg per day of memantine plus placebo | Primary: ADAS-Cog Secondary: MMSE, Frontal Assessment Battery, trail making test, Katz ADL and Lawton IADL |
| Egefjord et al., 2012148 | Denmark | RCT | Mild to moderate AD | A MMSE score of 18–21 | Not specified | 50–80 | 1.8 mg per day of liraglutide for 6 months | Placebo | Primary: PET (glucose uptake and Aβ deposits) Secondary: MRI (perfusion) and Wechsler Memory Scale (Brief Cognitive Examination) |
| Lawlor et al., 2014149 | France, Germany, Greece, Holland, Hungary, Ireland, Italy, Sweden and the UK | RCT | Mild to moderate AD | NINCDS-ADRDA and a MMSE score of 12–27 | Males and females | ≥ 50 | 8 mg per day of nilvadipine for 78 weeks | Placebo | ADAS-Cog, CDR (SB) and DAD |
We included three protocols, published between 2011 and 2015. None of the results of the three trials have, as yet, been published. All three are randomised placebo-controlled trials, with participants with AD. All three are being conducted in Europe: one in Denmark,148 one in France147 and one multicountry study in France, Germany, Greece, the Netherlands, Hungary, Ireland, Italy, Sweden and the UK. 149
All three trials used at least one cognitive outcome; there were five different cognitive outcomes. ADLs were measured across two trials using a variety of three measures. One trial measured biological markers, via two biological techniques. Only one trial used a global outcome. None of the published protocols measured neuropsychiatric or quality-of-life outcomes.
Ongoing trials
The characteristics of the ongoing trials are available in Table 3.
| Trial register number | Trial location | Trial type | Dementia type and severity | Criteria for dementia diagnosis | Participants | Description of | Outcomes related to disease modification | ||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Age range (years) | Intervention | Control/comparator group | ||||||
| ISRCTN1610506410 | UK | RCT | Early AD | NIA-AA criteria and a MMSE score of > 23 | Males and females | 45–100 | 400 mg per day of minocycline or 200 mg per day of minocycline for 2 years | Placebo | MMSE and Bristol ADL |
| ISRCTN31208535150 | UK | RCT | Mild to moderate subcortical ischaemic vascular dementia | DSM-430 and a MMSE score of 15–26 | Males and females | ≥ 50 | 5 mg per day of amlodipine for 2 weeks then 10 mg per day of amlodipine for 50 weeks | Placebo | Primary: VADAS-Cog Secondary: MMSE, trail making test, TICS-M, CGI, MRI (quantitation of lacunar lesions and diffuse white matter lesions), EQ-5D, DEMQOL, DAD and NPI |
| ISRCTN89711766151 | UK | RCT | Early AD | Dubois criteria for early AD or NINCDS-ADRDA/NIA-AA, and a MMSE score of ≥ 22 | Males and females | 50–85 | 1.8 mg per day of i.v. liraglutide for 12 months | Placebo | PET (change in glucose metabolism) |
| ISRCTN936828789 | UK | RCT | Mild to moderate AD | A MMSE score of 15–28 or a MoCA score of 12–24 | Males and females | ≥ 55 | Losartan escalating doses to 100 mg per day for 12 months | Placebo | Primary: MRI (whole-brain atrophy) Secondary: MRI (white matter hyperintensity volume and cerebral blood flow), ADAS-Cog, DEMQOL, NPI and Bristol ADL |
| NCT0140991524 | USA | RCT | Mild to moderate AD | A MMSE score of 10–26 | Males and females | 55–85 | 250 µg/m2 of s.c. sargramostim (Leukine, Sanofi Genzyme, Cambridge, MA, USA) for 5 days per week for 3 weeks | Placebo | Secondary: MMSE, ADAS-Cog, CDR (global), trail making test and Mohs Number Cancellation Test |
| NCT01561053152 | Spain | RCT | Mild to moderate AD | A MMSE score of 18–26 | Males and females | 55–85 | Low-dose albumin and immunoglobulin, high-dose albumin and immunoglobulin, or low-dose albumin with no immunoglobulin | No intervention | Primary: ADAS-Cog and ADCS-ADL Secondary: MMSE, NTB (not specified), NPI, CDR (SB), ADCS-CGIC, CSDD, C-SSRS, QOL-AD, PET (glucose metabolism), CSF analysis (Aβ1–40 and Aβ1–42, T-tau and P-tau) and blood tests (Aβ1–40 and Aβ1–42) |
| NCT01767311153 | USA and Japan | RCT | MCI or mild AD | NIA-AA and a MMSE score of ≥ 22 | Males and females | 50–90 | BAN2401 2.5 mg/kg, 5 mg/kg or 10 mg/kg every 2 weeks; or 5 mg/kg or 10 mg/kg every 4 weeks, with placebo every 2 weeks | Primary: AD composite score [ADAS-Cog, MMSE, CDR (SB)] Secondary: MRI (volumetric) and PET (amyloid) |
|
| NCT01965756154 | USA | RCT | Early AD | A MMSE score of > 21 | Males and females | 55–80 | Metformin escalating doses to 4000 mg per day – then crossover with placebo | Placebo | Primary: ADAS-Cog Secondary: Cogstate Alzheimer’s Battery, DSRS and MRI (pCASL, MPRAGE and Flair) and CSF analysis |
| NCT01966666155 | USA | RCT | Mild to moderate AD | NIA-AA and a MMSE score of 14–26 | Males and females | 50–82 | 2 mg/m2 of TPI-287, 6.3 mg/m2 TPI-287 or 20 mg/m2 TPI-287 by i.v. infusion once every 3 weeks for 9 weeks | Placebo | Secondary: CSF analysis (biomarkers for AD, but not specified), MRI (changes in brain network functional and structural connectivity and perfusion), ADAS-Cog, MMSE, ADCS-ADL and Geriatric DS |
| NCT02036645156 | USA | RCT | Mild to moderate AD | Not specified | Males and females | 55–85 | Either i.v. or s.c. injection (single or multiple doses, 25–1800 mg) of MEDI1814 | Placebo | Primary: C-SSRS Secondary: blood tests (Aβ1–42), CSF analysis (Aβ1–42 and Aβ1–40) |
| NCT02051608157 | Argentina, Australia, Belgium, Bulgaria, Canada, Denmark, Finland, France, Germany, Guatemala, Hungary, Italy, Japan, Korea, the Netherlands, Portugal, Russia, Spain, Sweden, Switzerland, Turkey, the UK and USA | RCT | Mild AD | NINCDS-ADRDA | Males and females | 50–90 | Gantenerumab (F Hoffmann-La Roche AG) (dose not specified) s.c. every 4 weeks for 100 weeks | Placebo | Primary: ADAS-Cog and ADCS-ADL Secondary: CSF analysis (T-tau, P-tau and Aβ1–42), NPI, CDR (SB and global), MMSE and MRI (volumetric) In subsample: PET (for amyloid) |
| NCT02080364158 | USA | RCT | Mild AD | A MMSE score of 21–26 | Males and females | 50+ | 5 mg per day of Azeliragon (vTv Therapeutics Inc., High Point, NC, USA) for 18 months | Placebo | Primary: ADAS-Cog and CDR (SB) Secondary: MRI (volumetric), PET (glucose), NPI, MMSE, ADCS-ADL, controlled oral word association test, fluency test, DEMQOL and blood tests (Aβ) |
| NCT02245737159 | Argentina, Australia, Belgium, Canada, France, Germany, Hungary, Italy, Japan, Poland, Romania, Spain, South Korea, Sweden, the UK and USA | RCT | MCI or mild AD | NIA-AA and a MMSE score of 20–30 | Males and females | 55–85 | 20 mg per day of LY3314814 or 50 mg per day of LY3314814 for 104 weeks | Placebo | Primary: CDR (global) Secondary: ADAS-Cog, FAQ, ADCS-ADL and NPI In substudy: CSF analysis (Aβ1–42, Aβ1–40, T-tau and phosphorylated tau), PET (amyloid and glucose) and MRI (volumetric) |
| NCT02322021160 | USA | RCT | MCI or mild to moderate AD | NIA-AA | Males and females | 50–85 | Low, middle or high doses (not specified) of E2609 for 18 months | Placebo | Secondary: AD Composite Score [ADAS-Cog, MMSE and CDR (SB)] |
| NCT02353598161 | USA | RCT | Mild to moderate AD | NINCDS-ADRDA or DSM-5, and a MMSE score of 18–28 | Males and females | 50–90 | Dose level 1, 2 or 3 (not specified) of i.v. crenezumab (Genentech, South San Francisco, CA, USA) every 4 weeks until week 13 | Placebo | Primary: C-SSRS and MRI (amyloid) |
| NCT02386306162 | USA | RCT | Mild to moderate AD | NIA-AA and a MMSE score of 12–26 | Males and females | 55–85 | One of three different doses (not specified) of GC 021109 for 28 days | Placebo | Secondary: blood tests (IL-12, Aβ and tau) and CSF analysis (IL-12, Aβ and tau) |
| NCT02389413163 | Belgium, France, Germany, Finland, the Netherlands and Sweden | RCT | MCI or mild AD | A MMSE score of 21–30 | Males and females | 50–89 | PQ912 twice daily for 12 weeks | Placebo | Secondary: MMSE, fluency tests, Geriatric DS, Cogstate Alzheimer’s Battery, CSF analysis (QC activity, T-tau, P-tau, Aβ pattern and pro-inflammatory panel), MRI (brain functional connectivity) and EEG |
| NCT02406027164 | Belgium, France, Germany, the Netherlands, Spain and Sweden | RCT | Early AD | Not specified | Males and females | 50–85 | 10 mg per day of JNJ-54861911 or 25 mg per day of JNJ-54861911 for 52 weeks | Placebo | Secondary: CSF analysis [(Aβ1–37, Aβ1–38, Aβ1–40 and Aβ1–42), sAPP fragments (sAPP-alpha and sAPP-beta) and total] and blood tests [Aβ1–40 levels and sAPP fragments (sAPP-alpha and sAPP-beta)] |
| NCT02431468165 | USA | RCT | Moderately severe to severe AD | A MMSE score of 4–15 | Males and females | 55–85 | 10 µg of bryostatin 1 (Blanchette Rockefeller Neurosciences Institute, Rockville, MD, USA), 20 µg of bryostatin 1 or 40 µg of bryostatin 1 via i.v. for 45 minutes every other week | Placebo | Severe Impairment Battery |
| NCT02434718166 | Japan | RCT | Mild to moderate AD | Not specified | Males and females | 55–85 | Aducanumab (BIIB037) i.v. infusion in cohorts assigned to doses (single or multiple) up to 10 mg/kg | Placebo | MRI (for amyloid) |
| NCT02477800167 | Australia, Austria, Canada, Denmark, France, Germany, Hungary, Italy, Japan, Korea, Portugal, Spain, Taiwan, the UK and USA | RCT | Early AD | A MMSE score of 24–30 | Males and females | 55–85 | Aducanumab (BIIB037) (Biogen Idec Ltd, Maidenhead, UK) low or high dose via monthly i.v. infusion for 18 months | Placebo | Primary: CDR (SB) Secondary: MMSE and ADAS-Cog |
| NCT02484547168 | Belgium, Canada, France, Germany, Italy, Japan, the Netherlands, Poland, Spain, Sweden, Switzerland and the USA | RCT | Early AD | A MMSE score of 24–30 | Males and females | 50–85 | Aducanumab (BIIB037) low or high dose via monthly i.v. infusion for 18 months | Placebo | Primary: CDR (SB) Secondary: MMSE and ADAS-Cog |
| NCT02503501169 | USA | RCT | Amnestic MCI or mild AD | NINCDS-ARDRA and a MoCA score of 18–27 | Males and females | 50–90 | 40 IU per day of insulin glulisine for 6 months | Placebo | Primary: ADAS-Cog, FAQ, CDR (SB and global), CSF analysis (Aβ42, tau and P-tau) and PET (glucose) Secondary: digit span, trail making test, fluency test, Wechsler Memory Scale and Geriatric DS |
| NCT02547818170 | USA | RCT | Early AD | Score below the education-adjusted cut-off point on delayed paragraph recall (from the Wechsler Memory Scale) | Males and females | 55–79 | ALZT OP1a (cromoglicic acid) and ALZT OP1b (ibuprofen) together, or cromoglicic acid and placebo, or ibuprofen and placebo | Placebo | Primary: CDR (SB) |
| NCT02551809171 | Taiwan | RCT | Mild AD | A MMSE score of 20–26 | Males and females | 60+ | UB-311 either seven doses or five doses (with two placebo doses) | Placebo | Secondary: ADAS-Cog ADCS-ADL MMSE, CDR (SB) and NPI |
| NCT02579252172 | Austria | RCT | Mild AD | NIA-AA and a MMSE score of 20–26 | Males and females | 50–85 | AADvac1 (40 µg of axon peptide 108) for eight doses – six every 4 weeks, then two booster doses every 6 months | Placebo | Cogstate Alzheimer’s Battery, CDR (SB), PET (for glucose metabolism), MRI (volumetric) and CSF analysis (biomarkers not specified) |
| NCT02600130173 | USA | RCT | Mild to moderate AD | DSM-4, NINCDS-ADRDA, and a MMSE score of 18–24 | Males and females | 55–75 | Target dose of 20 million or 100 million longeveron mesenchymal stem cells via i.v. infusion | Placebo | ADAS-Cog, Cogstate Alzheimer’s Battery, MMSE, NPI, Geriatric DS, ADCS-ADL, QOL-AD, CSF analysis (inflammatory biomarkers, and tau, P-tau and Aβ), MRI (volumetric) and blood tests (IL-1, IL-6, TGF-β1, TNF-α, CRP, D-dimer, fibrinogen and ApoE) |
We included 27 ongoing trials registered on clinical trial databases as ongoing at the time of the search. 9,10,24,150–173 All are RCTs, including participants with vascular dementia (n = 1),150 only AD (n = 21),9,10,24,151,152,154–158,161,162,164–168,170–173 and AD or MCI (n = 5). 153,159,160,163,169 There was a total of 32 outcome measures used across the trials. Twenty of the trials measured cognition, using 1 of 12 outcome measures. 9,10,24,150,152–155,157–160,163,165,167–169,171–173 Similarly, 19 of the trials measured biological markers, using a variety of five biological techniques. 9,150–159,161–164,166,169,172,173 Thirteen trials measured the domain of neuropsychiatric symptoms, using four measures. 9,150,152,155–159,161,163,169,171,173 Quality of life was measured in five trials using three measures. 9,150,152,158,173 ADLs were measured across 11 trials, using four measures. 9,10,150,152,155,157–159,169,171,173 Fifteen trials measured a global outcome, using a variety of four measures. 24,150–154,157–160,167–172
Validation data
The validation data for the outcomes in each domain are available in Tables 4–9.
| Measure | Number of | Time taken (minutes) | Who completes | Relevant populations validated with | MCID | Floor-and-ceiling effects | Sensitivity to change | Acceptability | Reliability | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials | Participants | Inter-rater | Test–retest | ||||||||
| Global | |||||||||||
| Alzheimer’s Disease Assessment Scale – Cognitive subscale174 | 92 (75 published, two protocols and 15 ongoing) | 20,419 | 20–25 | Administered to PWD | Validated for mild to moderate dementia175 Validated in Chinese, Indian, Turkish, Brazilian, and Spanish176,177 |
MCID of 3 points may be relevant for early AD178 and was 4 points for the VISTA trial175 | No floor or ceiling effects reported across 20 studies176 Ceiling effects in mild dementia179 |
Sensitivity to change across 21 dementia treatment studies,176 but can have poor sensitivity to detect change in mild to moderate AD180,181 | No information found | Good across four studies174,182–184 | Good across seven studies,174,177,179,183–186 but low on some items179 |
| MMSE187 | 83 (68 published, one protocol and 14 ongoing) | 17,736 | 5–10 | Administered to PWD | Translated into 50 languages and validated in many, including Slovenian, Persian, Urdu, Greek and Spanish188–193 | MCID of 1.4 points in the DOMINO trial194 | Moderate ceiling effects and small floor effects195 | Sensitive to change in AD.196 May not be sensitive to change in early dementia and dementia with Lewy bodies/frontotemporal dementia197 | Described as acceptable to patients187 | Good187,198 | Good199–201 |
| Batteries | |||||||||||
| CERAD’s Neuropsychological Test Battery202 | Two (two published) | 80 | 30 | Interviewer administered with PWD | Validated for various types of dementia including AD and fronto-temporal203,204 Validated in French, Korean, Russian, and Cantonese205–208 |
No information found | No floor or ceiling effects209 | Sensitive to progression of AD202,210 | No information found | Good inter-rater reliability in PWD living in community211 | Good204 |
| Cogstate Alzheimer’s Battery212 | Six (two published and four ongoing) | 161 | 15–20 | Computer administered with PWD | Validated for use in dementia213 | No information found | No floor or ceiling effects214,215 | Sensitive to cognitive changes in dementia across three studies214–216 | No information found | No information found | Good in Alzheimer’s214,217 |
| Neuropsychological Test Battery218 | Seven (seven published) | 3429 | 70 for all components | Interviewer administered with PWD | Validated for mild to moderate dementia219 Validated for use in China, Taiwan, Singapore, Hong Kong and South Korea199 |
No information found | No floor or ceiling effects in total score, but floor effects on RAVLT delayed recall test in moderate dementia179 | Good ability to detect change in mild to moderate dementia179,199,219 | No information found | Good199 | Good in mild to moderate dementia179,218,219 |
| Measure | Number of | Time taken (minutes) | Who completes | Relevant populations validated with | MCID | Floor-and-ceiling effects | Sensitivity to change | Acceptability | Reliability | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials | Participants | Inter-rater | Test–retest | ||||||||
| Alzheimer’s Disease Assessment Scale – Non Cognitive Scale174 | Seven (all published) | 792 | 20–25 | Interview with PWD and caregiver | Validated for mild to moderate dementia220 | No information found | No floor or ceiling effects221 | Sensitivity to change in mild to moderate dementia185,222 | No information found | Good across three studies182,185,223 | Good185 |
| Behavioural Pathology in Alzheimer’s Disease224 | One (published) | 425 | 20 | Informant interview by clinician | Validated in French, Swedish, German, Dutch, Spanish, Chinese and Korean176 | No information found | No floor or ceiling effects225,226 | Sensitive to change in moderate to severe dementia227 | No information found | Good225,226,228 | No information found |
| Brief Psychiatric Rating Scale229 | Three (all published) | 190 | 20 | Rated by an observer | May be validated for AD230 | No information found | No information found | No information found | No information found | Good230 | No information found |
| CERAD’s Behavioural Scale231 | One (published) | 486 | 20–30 | Semistructured informant interview | Validated in French, Spanish, Arabic, Chinese and Japanese176,232 | No information found | No information found | Some evidence of sensitivity233 | No information found | Good231 | Good233 |
| Dysfunctional Behaviour Rating Instrument234 | One (published) | 406 | 20 | Informant rated | Validated for PWD living in the community234 | No information found | No information found | No information found | No information found | Good234 | Good235 |
| Neuropsychiatric Inventory236 | 38 (30 published and eight ongoing) | 11,756 | 10–20 | Informant interview | Validated across dementia severity236 Validated in Italian, Greek, Japanese, Korean, Mexican, Polish, Spanish and Dutch176,237 |
MCID of 8 points in the DOMINO trial194 | No floor or ceiling effects238 | Sensitive to change across dementia severities and types238–241 | No information found | Good236,237 | Good across three studies236,237,242 |
| Nurses’ Observation Scale for Geriatric Patients243 | Two (both published) | 454 | 3–5 | Nurses on wards normally rate with a caregiver | Validated for people with dementia in hospitals244 | No information found | No information found | Good sensitivity to change in two studies including PWD243,245 | 83% acceptability in mild to moderate dementia244 | Good244 | Good244 |
| Plutchik Geriatric Rating Scale246 | One (published) | 178 | 5–10 | Rated by an observer | Does not appear to be validated for use with people with dementia | No information found | No information found | No information found | No information found | No information found | No information found |
| Revised Memory and Behaviour Problems Checklist247 | One (published) | 168 | 10 | Informant questionnaire | Validated in Taiwanese and Spanish248,249 | No information found | No information found | May not be sensitive to detect progression of dementia in one study,250 but appears sensitive to changes in another study251 | No information found | No information found | Good248 |
| Measure | Number of | Time taken (minutes) | Who completes | Relevant populations validated with | MCID | Floor-and-ceiling effects | Sensitivity to change | Acceptability | Reliability | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials | Participants | Inter-rater | Test–retest | ||||||||
| DEMQOL252 | Four (one published and three ongoing) | 399 | 10–20 | PWD and/or informant | Valid for mild to moderate dementia living in the community or residential care252 Validated in Spanish, German and Russian253–255 |
No information found | No floor or ceiling effects252 | Sensitive to change in mild to moderate dementia256–258 | Good252 | Good inter-rater reliability of PWD and proxy versions for mild/moderate,252 thought PWD rate higher than proxy in one study259 and proxy rate higher than PWD in another study258 | Good across studies including PWD252,260 |
| EuroQol-5 Dimensions261 | Five (four published and one ongoing) | 4084 | 4–15 for PWD and 2 for proxy | PWD and/or informant | Validated for mild to moderate dementia living in the community or residential homes262 Available in 100 languages.263 Validated in French264 |
No information found | No floor or ceiling effects observed in one study.264 Substantial ceiling effect for patient ratings, but not proxy, in two studies265,266 | Not sufficiently sensitive to detect changes in the progression of dementia263 | High completion rate, but acceptability decreases with dementia severity263,264 | PWD provides significantly higher rating than proxy across four studies,263 but in one study PWD and proxy ratings are similar for mild to moderate dementia262 | Patients’ test–retest ratings unreliable for mild to moderate dementia and less reliable than carers ratings,263 but two studies264,265 report good test–retest reliability |
| Quality of Life in Alzheimer’s Disease267 | Eight (six published and two ongoing) | 3341 | 5 for informant version and 10–15 for PWD | PWD and/or informant | Validated for people living in the community or residential care268 Validated for use in MMSE scores of > 10269 Validated in English, French, Portuguese, Spanish, Japanese, Cantonese, Mandarin, Korean, Danish, Swedish, German and Greek176,269 |
One standard deviation270 | No floor or ceiling effects observed.271 Minimal floor-and-ceiling effects across eight studies28,272–278 | Sensitive to change in studies of mild to moderate dementia268 | Good271 | Mixed inter-rater reliability of PWD and informant responses across eight studies267,268,279–285 | Good267,268 |
| Measure | Number of | Time taken (minutes) | Who completes | Relevant populations validated with | MCID | Floor-and-ceiling effects | Sensitivity to change | Acceptability | Reliability | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials | Participants | Inter-rater | Test–retest | ||||||||
| Alzheimer’s Disease Co-operative Study – Activities of Daily Living Inventory286 | 34 (28 published and six ongoing) | 11,500 | 15–20 | Informant rated | Validated for person with mild to moderate dementia living in the community287 Validated for use in Spain, Sweden, Latvia, and Bosnia and Herzegovina176 |
No information found | No information found | Sensitive to change in mild to moderate dementia in three studies,288–290 but not in one291 | No information found | No information found | Good across four studies286,289,292,293 |
| Alzheimer’s Disease Functional Assessment and Change Scale286 | Two (two published) | 350 | 15–20 | Informant rated | Validated in Spanish294 | No information found | No information found | No information found | No information found | No information found | No information found |
| BADL295 | Five (three published and two ongoing) | 1117 | 15 | Informant rated | Validated for PWD living in community296 | MCID of 3.5 points in the DOMINO trial194 | No information found | Sensitive to change in dementia245,296 | Carers report it is easy to complete295 | Mixed across studies297 | Good295,297 |
| Dependence scale298 | Three (all published) | 3343 | 15–20 | Informant rated | Valid for use with PWD living in the community298 | No information found | Floor effect on cognition subscale299 | Sensitive to dementia progression,298 but may not pick up small changes in clinical trials300 | No information found | Good57 | Good298 |
| Disability Assessment For Dementia301 | 13 (11 published, one protocol and one ongoing) | 2914 | 15 | Informant rated | Validated across dementia severities living in the community.302 Validated in Korean, Chinese, Italian, Spanish, Persian, Portuguese and Turkish302–308 | No information found | No floor or ceiling effects301,309 | Good sensitivity to change in six studies,310–315 but one study316 found it was not sensitive to change in comparison to other measures | No information found | Good301 | Good301 |
| Functional Activities Questionnaire317 | Two (ongoing trials only) | N/A | 10 | Informant interview | Validated for mild dementia318 | No information found | No information found | No information found | No information found | Good317 | No information found |
| Interview for Deterioration in Daily Living Activities in Dementia319 | Two (published trials only) | 219 | 15 | Informant rated | Validated for mild dementia living at home320 Validated in Spanish and Dutch97,138,321 |
No information found | No floor or ceiling effects322 | Thought to be responsive to change299 | No information found | Good320 | Good299 |
| Katz Index of Activities of Daily Living Scale323 | Three (two published one protocol) | 185 | 10 | Informant rated | Validated as more of a clinical assessment than a measure of treatment effectiveness324 | No information found | No information found | Sensitive to dementia progression,325,326 but may not be sensitive to small changes327 | No information found | Good327,328 | Good327 |
| Lawton Instrumental Activities of Daily Living Scale329 | Eight (seven published and one protocol) | 1125 | 10 | Clinician/researcher rated | Validated in Asian older adults living in the community (some with dementia)330 | No information found | Ceiling effect reported331 | Sensitive to treatment effects in moderate to severe dementia332 | No information found | Good333 | Good333 |
| Nuremberg Gerontopsychological Rating Scale for Activities of Daily Living334 | Three (all published) | 530 | Not specified | Self-complete or informant questionnaire | Does not appear to be validated for use with people with dementia | No information found | No information found | May not be sensitive to change over time335 | No information found | Family carers rate more deficits than paid carers335 | No information found |
| Physical self-maintenance scale329 | Three (all published) | 429 | 5 | Self-complete or observer rated | Validated for PWD living in the community328 | No information found | Ceiling effect likely in PWD living in community299 | Sensitive to treatment effects in moderate to severe dementia332 | No information found | Good333 | Good333 |
| Video Recorder Home Behavioural Assessment60 | One (published) | 48 | Not specified | Researcher rated | Does not appear to be validated for use with people with dementia | No information found | No information found | May be sensitive to change60 | No information found | Good60 | Good60 |
| Measure | Number of | Time taken (minutes) | Who completes | Relevant populations validated with | MCID | Floor-and-ceiling effects | Sensitivity to change | Acceptability | Reliability | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials | Participants | Inter-rater | Test–retest | ||||||||
| Impression of change scales | |||||||||||
| Alzheimer’s Disease Cooperative Study – Clinical Global Impression of Change336 | Eight (seven published and one ongoing) | 1590 | 20 | PWD and informant | Validated for use in clinical and home settings337 | No information found | No information found | Sensitive to detect changes in mild to moderate dementia over 12 months336 | No information found | Good336 | Good336 |
| Clinical Global Impression’s Scale338 | 15 (14 published and one ongoing) | 2063 | 5 | Clinician rated by interview with PWD/informant | Does not appear to be validated for use with people with dementia | No information found | No information found | No information found | No information found | No information found | No information found |
| Clinician’s Interview-Based Impression of Change plus Caregiver Input339 | 12 (12 published) | 4087 | 10–40 | Clinician semistructured interview with PWD and informant | Validated in Japanese340 | No information found | No information found | Sensitive to change in dementia treatment studies336,341 | Good acceptability341 | Good342 | Good341 |
| Multiple domain scales | |||||||||||
| Short CAMDEX343 | One (published) | 321 | 30 | Clinician | Used to screen for and diagnose dementia343 | No information found | No information found | No information found | No information found | No information found | No information found |
| Blessed Dementia Rating Scale344 | Three (all published) | 190 | 15 | Informant rated | Validated in Taiwanese, Chinese, Korean and Czech345–348 | No information found | Floor-and-ceiling effects in two studies344,349 | Sensitive to progression of dementia350 | No information found | Good in two studies298,351 | Good in three studies350,352,353 |
| Dementia Severity Rating Scale354 | Three (two published and one ongoing) | 164 | 5 | Carer questionnaire | Validated for mild to severe dementia355 | No information found | No floor or ceiling effects354 | Sensitive to change across mild to severe dementia354–356 | No information found | Good inter-rater reliability of caregiver responses compared with clinician information354 | Good354,357 |
| Gottfries–Bråne–Steen rating Scale for Dementia358 | Four (all published) | 636 | 20–30 | Clinician interview with PWD and informant interview | Validated across dementia severities359 Translated into Czech, Danish, Italian, Japanese, Norwegian, Spanish and Swedish359 |
No information found | Can have a ceiling effect in mild dementia360 | Sensitive to change across dementia severities359 | No information found | Good across nine studies359 | No information found |
| Sandoz Clinical Assessment-Geriatric Scale361 | Two (two published) | 298 | 15–30 | Clinician observation | Validated in French and German176 | No information found | No information found | Sensitive to change across three studies361–363 | No information found | Good in two studies361,362 | Low across three studies352,362,364 |
| Staging of dementia scales | |||||||||||
| Clinical Dementia Rating scale365 | 48 (34 published, one protocol, and 13 ongoing) | 14,596 | 40 | PWD and carer | Valid for mild to severe dementia366 Valid in community and residential care settings176 Available in Chinese, Czech, Dutch, English, Finnish, French, German, Hebrew, Polish, Spanish, Swedish and Portuguese176,367,368 |
No information found | Minimal floor-and-ceiling effects across 11 studies369–379 Floor-and-ceiling effects in one study380 |
Sensitive to treatment effects across 12 studies369–379,381,382 | No information found | Good to very good across 12 studies365,380,383–392 | Good388 |
| Global Deterioration Scale | Six (six published) | 809 | 2 | Informant rated | Validated in community or residential care393 English version has been translated and validated in German and Korean176,394 |
No information found | No information found | Good sensitivity to change in two studies,395,396 but not in another study397 | No information found | Good across four studies230,398–400 | Good398 |
| Type of biological technique | Number of | Type of biological marker in trials | Accuracy | Sensitivity to change | Risks | |
|---|---|---|---|---|---|---|
| Trials | Participants | |||||
| MRI | 30 (16 published, one protocol, and 13 ongoing) | 4788 | Mostly serial structural MRI for volume (22 trials; 4788 participants) The other eight trials used MRI for:
|
Serial structural MRI: gives accurate measurements of hippocampal volume and correlates with neuronal numbers401 | Serial structural MRI:
|
|
We searched separately for validation information for each outcome measure, and also recorded how long each measure takes on average to complete and who completes it. Both the results of the review and the validation data were used at the consensus conference, which is discussed in Chapter 6.
Cognitive outcomes
As a result of the large number of cognitive outcomes, we shortlisted five cognitive outcomes for which we would search for validation data. These were the Alzheimer’s Disease Assessment Scale – Cognitive subscale (ADAS-Cog), MMSE, the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)’s Neuropsychological Battery, the Neuropsychological Battery and the Cogstate Alzheimer’s Battery.
We excluded the other cognitive outcomes for a number of reasons. They had only been used in one trial:
-
Mattis Dementia Rating Scale416 (one published trial; 149 participants)
-
Modified Telephone Interview for Cognitive Status417 (one ongoing trial)
-
Vascular Dementia Assessment Scale Cognitive Subscale418 (one ongoing trial)
-
Computerised Neuropsychological Test Battery419 (one published trial; 20 participants)
-
Frontal Assessment Battery420 (one protocol)
-
Mental Deterioration Battery421 (one published trial; 142 participants)
-
Wechsler Adult Intelligence Scale422 (one published trial; 48 participants).
They measure cognition in severe dementia:
-
Severe Impairment Battery423 (one published trial; 26 participants).
They are not available in the English language:
-
Syndrome Short Test424 (two published trials; 410 participants).
They refer to only one domain:
-
Wechsler Memory Scale425 (five trials; three published, one protocol and one ongoing; 238 participants)
-
Western Aphasia Battery426 (one published trial; 48 participants).
We also did not search for validation of the individual cognitive tests, as they are neuropsychological tests rather than cognitive scales, with the validation process of these tasks different as they are designed to do different things. 427 Furthermore, the individual tests focus on specific domains of cognition, and as dementia is an impairment of more than one cognitive domain a global scale is more appropriate. Most of the individual tests have only been used in a small number of trials and often in combination. These include:
-
Buschke Selective Reminding Test428 (three published trials; 108 participants)
-
Benton Visual Retention Test429 (one published trial; 60 participants)
-
clock drawing task430 (two published trials; 30 participants)
-
controlled oral word association test431 (two trials; one published and one ongoing; 399 participants)
-
digit span test432 (two trials; one published and one ongoing; 399 participants)
-
digit symbol substitution test422 (three published trials; 680 participants)
-
dot counting n-back task58 (one published trial; 60 participants)
-
fluency tests (seven trials; four published and three ongoing; 385 participants)
-
Mohs Number Cancellation Test433 (one ongoing trial)
-
recall tasks (three published trials; 116 participants)
-
Rey Memory Test434 (one published trial; 213 participants)
-
Stroop Colour Word Interference Test435 (four published trials; 507 participants)
-
token test436 (one published trial; 12 participants)
-
trail making test437 (10 trials; six published, one protocol and three ongoing; 866 participants)
-
word recognition (one published trial; 20 participants).
Neuropsychiatric outcomes
Similarly, for the neuropsychiatric outcomes we decided that we would not recommend measures of specific neuropsychiatric symptoms, such as agitation or depression; therefore, we did not search for validation data for the seven specific symptom measures included. These were:
-
Cohen-Mansfield Agitation Inventory438 (one trial, 89 participants)
-
Columbia Suicide Severity Rating Scale439 (three trials, all ongoing)
-
Cornell Scale for Depression in Dementia440 (three trials, 464 participants)
-
Geriatric Depression Scale441 (10 trials, 803 participants)
-
Hamilton Depression Rating Scale442 (five trials, 677 participants)
-
Montgomery Depression Rating Scale443 (two trials, 269 participants)
-
Zerssen Adjective Mood Scale444 (two trials, 381 participants).
Biological outcomes
For the validation of the biological outcomes, the two champions first used their expertise and own searches for validation information to make their recommendations. Afterwards we added to this with regard to serial structural MRI, as this is the only biological outcome we are recommending; therefore, MRI is the only biological outcome we have recorded validation information for in Table 9.
Chapter 5 Methods of patient and public involvement consultation
Purpose
The purpose of PPI within this project was to present people both directly and indirectly affected by dementia, including those with experience of research participation, with some of the findings from our systematic review to seek their views on which of the domains they considered core and their assessment of the acceptability of individual and packages of measures. We also wanted to know their thoughts about general matters around completing outcome measures, including the length of testing that was acceptable, who they thought should complete outcomes, opinions about invasive tests and travel distances to a research site.
Procedure
We planned to consult through face-to-face focus groups followed by e-mail consultation, with people recruited from AS’s Research Network volunteers. After meeting with the groups we sent a summary by e-mail to those who attended to check that the recommendations we intended to present at the consensus conference reflected what they thought had been said across the groups. We also asked participants if they had anything else to add.
After the consensus conference we conducted a second e-mail consultation with people from the AS Research Network, excluding those who had already attended the focus groups, to gain further feedback about the conclusions of the consensus conference.
Focus groups
We held three consultations, one in each of Cambridge, London and Sheffield, in February and March 2016. All were led by Lucy Webster and Anna Grinbergs-Saull from the AS. Champions within the group agreed to co-facilitate the focus groups. The AS had found that in previous focus groups including a clinician who uses these measures, and is therefore able to explain them, aids discussion, allowing participants to ask specific questions. The clinicians in each group were Gail Mountain in Sheffield, Gill Livingston in London and John O’Brien in Cambridge. Participants were e-mailed an information sheet and asked at the groups if they all consented to the session being recorded, with only Lucy Webster or the AS able to listen to the recording before it was destroyed (see Appendix 4). Participants who lived within travelling distance of each focus group were invited from the research network. For the Cambridge focus group, following recommendations made by a co-applicant, we also invited additional people from a local dementia and ageing research PPI group. Focus groups lasted 1–2 hours.
We focused on a different set of domains in each group, although there was an overlap between domains discussed at focus groups (Table 10). Discussions were audio-recorded and, to allow the participants to generate conclusions as easily as possible throughout the discussion, we summarised the conversations on flipchart paper. This allowed volunteers to see the notes that we were recording and refer to them when they felt it was necessary.
| Sheffield | London | Cambridge |
|---|---|---|
| ADLs, cognition | Quality of life, global, outcome sets | Biomarkers, neuropsychiatric, outcome sets |
First e-mail consultation
To gain a wider range of views, we ran an e-mail consultation with focus group participants on a summary report of the focus group discussions. This was to allow the volunteers to comment on measures that they had not discussed in their focus group and respond to the conclusions we drew (see Appendix 5).
Second e-mail consultation
We then ran a second e-mail consultation on a report including recommendations made at the consensus conference (see Appendix 6). This was sent to research network volunteers who had not participated in a focus group. This consultation included volunteers who had expressed an interest in focus groups but were unable to attend, and those living in different areas, including Wales, Northern Ireland and the Midlands.
Chapter 6 Results of patient and public involvement consultation
Demographic details of participants
Table 11 includes an overview of the experiences and geographical locations of all 18 participants who were involved in the PPI consultations.
| Background information | Number of participants |
|---|---|
| Care experience | |
| Person living with dementia | 4 |
| Current carer | 6 |
| Former carer | 7 |
| Ageing and research PPI | 1 |
| Dementia experience | |
| AD | 14 |
| Vascular | 2 |
| FTD | 1 |
| PCA | 1 |
| Research experience | |
| Participant | 6 |
| Carer of participant | 1 |
| Steering group | 7 |
| None | 7 |
| Geographical region | |
| London | 1 |
| East Anglia | 7 |
| North England | 8 |
| Northern Ireland | 1 |
| Wales | 1 |
Focus groups
The face-to-face focus groups involved 12 people overall: three people living with dementia, two current family carers, six former family carers and one PPI group member. Some had participated in research or supported a family member through participation; others had no trial experience. There was an even split between men and women. Participants gave their ages in bands as 45–54 years (n = 1), 55–64 years (n = 2), 65–74 years (n = 5) or ≥ 75 years (n = 4). Ten of the participants were white British and two were white other (one white South American and one not specified). Most participants were married or living with their partner (n = 7), four were widowed and one divorced. Participants had a range of occupational backgrounds with current or last employment given as architect, care assistant, civil servant, footwear manufacturer, ecotoxicologist, civil servant, information technology manager, marketing communications director, NHS team leader, NHS therapy manager, senior staff nurse and retiree.
First e-mail consultation
The first e-mail consultation was sent to the 12 people who participated in the focus groups, of which five responded.
Second e-mail consultation
The second consultation was sent to the wider AS research network, excluding focus group participants. We received six responses from people with experience of AD, including one person with dementia and five carers (three current carers and two former carers).
This report gives a summary of the views of those consulted on different outcome measures, their use and the design of a core outcome package. Where possible we have included direct quotes from volunteers, identifying only whether they were a person with dementia (PWD) or a carer. We have separated the recommendations by the most common themes that were suggested by participants during the focus groups.
What should be measured?
At the start of each focus group session people were asked ‘what should be measured?’. Responses were varied but gave broad support to each of the six domains covered. Suggestions not directly covered by the domains were:
-
side effects of pharmacological interventions
-
frequency of falls
-
sleep disturbance – thought to be a significant indicator of progression
-
carer outcomes – described by carers as a potential indicator of progression and likely to affect patient outcomes.
General recommendations
Throughout our discussions participants suggested potential improvements to the way in which outcome measures are delivered or packaged. Volunteers suggested several factors affecting the acceptability of outcome measures. We also received feedback on these suggestions during the e-mail consultation.
Questioning and terms used
Too many questions and fast delivery can cause anxiety in people with dementia, and intensive questioning can be perceived as ‘getting at you’ (carer).
The way in which questions were asked and the types of questions asked were also discussed. People with dementia stated that the questioning should be clear, as they may give completely different answers dependent on certain words. For example, the questions ‘On a good day? or ‘On a bad day?’ (PWD) would elicit different responses.
Across the questionnaires shown to people living with dementia, two people had a preference for positive questions that did not ‘focus on the negative stuff as much’ and rather the ‘things we can do’. However, another person living with dementia also noted ‘it’s more relevant what they cannot do than they can do, because there’s an awful lot more we can’t do than we can do’.
Several volunteers also strongly disagreed with the word ‘carer’, suggesting that this discounted those who did not identify as caring/cared for by a family member and those who lived alone. To conform to conventions within the literature, the word ‘carer’ is used here. However, we felt it was important to note the dislike for the term.
Time and travel requirements
The groups all thought that both time and travel could be a barrier to participation. Measures were deemed less appropriate if they required participants to travel to a specific centre each time. Long, single meetings were described as too tiring for people with dementia (most preferred a full day with several breaks). One and a half hours was described as the absolute maximum time for a single meeting or test without a break; however, volunteers suggested that researchers should be encouraged to aim for shorter periods. People with dementia particularly discussed reaching a stage where they cannot concentrate during longer sessions, but suggested that they may not be immediately aware that they had reached this point. Shorter testing would prevent this from happening:
Where it’s got to the stage that you’re not aware that you’re not giving the right answer, or what you would have given at the beginning.
PWD
Volunteers also stressed the importance of clearly explaining the time required, including any breaks and waiting periods. Carers suggested that waiting for a meeting can seem aimless and can therefore be particularly difficult to explain to the person they support.
Having to travel far is a barrier, as people with dementia are ‘going to have comorbidities . . . They’re going to have all the other things going on . . . so it makes it much more difficult’ (carer). Carers noted that being able to participate in research locally, rather than having to travel far, would encourage and help with participation.
Role of the ‘carer’ in completing outcomes
Discussing carer-assessed measures, volunteers highlighted the likely disparity between the answers given by people with dementia and carers. Although some thought that this was a problem, two volunteers also pointed out that a difference in responses might provide an additional source of data. The participants said that many people with dementia live alone. Although measures do not require co-residence, volunteers also pointed out that not all people with dementia will have a defined carer, family member or friend who could give an accurate assessment. However, all agreed the ‘importance of the carer in the decision-making’ around participating in research, as their time and availability play a part in the trial:
You can’t expect them to take days off work to come with you to an assessment . . . it’s difficult because then you’re relying on what I’m saying.
PWD
Engagement: communication and relevance
Many participants thought that clear communication about the reasons why particular measures were chosen would aid continued engagement in the trial. Participation requires commitment and time, and they felt that participants need to know what they are contributing and why. Although volunteers understood the need for standardisation to improve the usefulness of data, some people with dementia and carers did not like standardised questionnaires. It was suggested that standard sets of questions might not be relevant to different types of dementia. For example, a carer with frontotemporal dementia experience suggested that receiving questions about memory made the research seem ‘a waste of time’. Furthermore, some volunteers felt that standardised questions lack contextual detail about an individual’s background, and that results would be relative to each person’s experience of dementia and its symptoms.
Standardised interviews
Cognition
Overall, volunteers agreed that cognition should be core. However, volunteers felt that it should not be used in isolation as the only core measure and suggested that cognitive scores needed contextual, qualitative information. Carers, in particular, suggested that more weight should be placed on the interaction between cognition and ability rather than cognition alone:
It is more important to understand how a person’s cognitive impairments affect their activities of daily living and quality of life . . . than it is to rate their underlying cognitive skills.
Carer
Although they acknowledged its use as a standard cognitive measure, performed in a range of research and clinical contexts, several people did not like the MMSE. People with dementia suggested that it seemed irrelevant, as it gives a restricted account of dementia and carers described how they thought it would be difficult to have a cognitive measure that seemed relevant to all people with dementia:
It doesn’t seem useful.
PWD
It doesn’t have anything to do with what we have.
PWD
Cognitive testing, because we are so different and the way it progresses in the different forms of dementia makes it so complicated to try and produce some kind of standardised measure.
Carer
Volunteers, including both people with dementia and carers, felt that memory tests can be demoralising; carers particularly felt that it is distressing ‘watching someone fail a test’. People with dementia described the distress of seeing their score and performance worsen, and a tendency to try to prepare for tests to prevent this from happening. Some people preferred the ADAS-Cog to the MMSE, as it is more detailed in terms of the answers you can give.
Activities of daily living
Volunteers disagreed over the use of ADLs. Some focus group discussions concluded that it was not core; however, other people with dementia considered ADLs an accurate, practical measure of dementia. Volunteers consulted electronically also expressed some support for ADLs as a useful measure. Overall, volunteers preferred instrumental ADLs, rather than basic ADLs, as the activities seemed more relevant to daily life in mild to moderate dementia:
A lot of what’s down there now doesn’t apply to me.
PWD on the items of the Katz ADL scale that refers to basic ADLs
Some people with dementia also suggested that discussing reduced ability to perform tasks was less distressing than cognition:
Your body thinks it can do it, but your brain doesn’t allow it.
PWD
Some days you can do them and you are brilliant . . . other days you cannot do them.
PWD
The Katz instrument was not liked by some people with dementia because yes/no questions did not allow for gradations in ability. Volunteers suggested that questions should ask why someone cannot perform a task or what they do differently to complete it. For example, volunteers pointed out that they may still be able to do activities but in a different way to how they may have performed them before dementia, such as by wearing clothes that are easier to put on or cooking simpler meals:
Some task you can still do, just differently, just adapted.
PWD
People with dementia felt that this should be considered when questioning them about ADLs:
Say can you do them but differently than what you used to?
PWD
Additionally, people living with dementia suggested that they might avoid tasks because of a lack of confidence rather than an inability to complete them:
I can use the telephone, but I don’t have the confidence, I don’t like using the telephone.
PWD
Therefore, several volunteers felt that ADL measures lacked the nuance necessary to understand how, and if, an individual’s ability to perform daily tasks had been impaired.
Carers also raised the interesting point that some ADL questionnaires that they have had to complete were very similar to disability benefits assessments. The similarity was strong enough that they felt it could cause alarm and suspicion as to the use of trial data, and may affect the answers that participants give:
The link across into disability benefits . . . it’s suspicious.
Carer
Neuropsychiatric symptoms
Some participants said that behaviour is core because it is a significant aspect of dementia and seems more sensitive to illness than ADLs. However, some volunteers felt that behaviour should not be used in isolation, as it may be less applicable in mild to moderate stages and does not consider the reasons behind behaviour changes. Volunteers suggested that behavioural measures should be analysed alongside contextual information about the individual and their personality to give a more accurate insight into the behavioural changes caused by dementia:
It’s teasing out the brain damage from actual personality traits.
Carer
It’s about behavioural change over time.
Carer
In particular, some felt that behaviour should be measured alongside cognition and understanding, which could affect agitation and aggression. Volunteers suggested that, if behaviour were measured, it should, as it usually does, include sleep, agitation, walking (‘wandering’), violence/temperament, compulsive/repetitive behaviours and changes in diet and taste (i.e. preferences for certain foods). Some participants thought that measures should include an ability to hold a conversation or follow a television programme, which are usually not considered as behavioural.
Quality of life
Volunteers disagreed over the inclusion of quality-of-life measures as core. One volunteer considered these to be core, as they can give a summary of an individual’s experience of dementia. However, throughout the discussions and consultation there was some debate as to the sensitivity of quality-of-life measures. Some carers suggested that it would be difficult to assess another person’s quality of life and that these measures rely on accurate interpretation of an individual’s responses. Others thought that carer assessment would be necessary, as they may have a clearer insight into changes. It was suggested that comparing carer and patient responses would give the most accurate account of quality of life.
When one volunteer was shown all three of the measures, they described both the EuroQol-5 Dimensions (EQ-5D) and Quality of Life in Alzheimer’s Disease (QOL-AD) as lacking in detail. In particular, in reference to the EQ-5D, they said, ‘I don’t know how much you’d get out of it’ (carer). However, the Dementia Quality of Life measure (DEMQOL) was decided to be ‘reasonably easy to do and it’s going to give a lot of information’ (carer).
In particular, some felt that quality-of-life measures lack detail regarding the individual’s personality; for example, they may always have disliked social events. Therefore, in general, quality of life was considered important but not a core measure for all trials.
Global measures
Volunteers had differing opinions about global rating scales. Some approved of the breadth of the measure:
I like the global one . . . it’s all encompassing.
Carer
However, others suggested that global measures were superficial, depending too much on the individual’s experience on the day, and not meaningful:
You might be feeling particularly bad that day.
Carer
Is that really valuable . . . . Am I giving my time for something that’s meaningful? I’m not really convinced.
Carer
Those who did not like global scores suggested that a larger package of specific measures would give the holistic view of an individual with more detail.
Biological measures
Volunteers generally agreed that biomarkers should be core, viewing them as being the most reliable, objective measure and, therefore, least affected by environmental factors and day-to-day symptom variation. However, although these were thought to be more tangible and objective measures, there was also some uncertainty about their value and what type of data they actually provide.
For example, biomarkers were described as the more tangible measures:
Biological measures you’ve got something that everyone can agree on . . . you can compare like with like.
Carer
That said, there was some disagreement as to the use of certain measures, and volunteers also suggested that, although reliable, biomarkers should not be used in isolation and should be combined with measures such as cognition and behaviour.
Carers had a number of general questions about the type and quality of data biological measures could actually provide, including:
Do you get more information from a lumbar puncture than a scan?
Carer
Would you be looking for a reduction in amyloid or tau, or would you be looking for an arrest in the size of brain shrinkage?
Carer
How many people would you need for it to be significant?
Carer
Cerebrospinal fluid measures
Some volunteers particularly liked CSF measures, even though they were aware of possible side effects and general misconceptions about what the procedure involves (e.g. very large needles) that might discourage some from taking part. However, the volunteers felt that having to give CSF would not discourage most people from participating and that, although uncomfortable, it would be bearable. One volunteer described the person they care for as happy to undergo annual CSF as part of an ongoing trial:
A couple of hours and its done . . . it doesn’t put him off going the following year . . . he knows exactly what he’s let himself in for and he does it.
Indeed, for most, the main criticism was the need to take CSF measurements in a specific location. Those with experience of the measure suggested that travelling to and from the specified site was the greatest drawback. Some suggested that, if it were possible to take CSF in convenient locations, they would consent to two or three samples a year:
I think the practicalities of it would be the bit that concerns me . . . practicalities should be made easier for the patient.
Carer
That being said, the support was not unanimous, and one volunteer suggested that the two people she had cared for would not be able to cope with the procedure, and that ‘it might put people off taking part’ (carer).
Blood tests
The usefulness of blood tests in terms of showing disease modification was questioned by carers:
Do they produce something meaningful?
Carer
One volunteer who spoke against CSF measurements suggested that taking blood had been difficult for her family members (this was the reason that she doubted their tolerance for CSF procedures). However, overall, volunteers agreed that blood tests were unproblematic and that it would not be difficult to commit to frequent tests. The main barrier discussed was travel, with one group suggesting that, subject to location, ‘weekly blood tests would be very happily tolerated’ (carer).
Imaging
Most volunteers agreed that imaging could be core. However, their discussion focused on hopes and expectations that this would provide ‘sophisticated’, ‘sensitive’, ‘objective’ and ‘useful’ data:
Making sure the scans themselves are sophisticated enough to be able to give you what you’re looking for . . . are they sensitive enough to give the objective results you’re looking for?
Carer
Volunteers also suggested that many would consent to scans, as giving biological data can make the person with dementia feel that they are contributing useful information:
In going through that he is doing absolutely everything he can to further the research.
Carer
It’s a positive action.
Carer
It’s something physical happening . . . now logically you know it isn’t going to alter your dementia or do anything to it, but somehow it would make me feel better, that somebody somehow was actually doing something . . . although it has drawbacks obviously.
Carer
Practical issues around travel and the need for concentration were raised but, in general, 3- to 6-monthly scans were considered bearable. However, volunteers agreed that, although MRI is straightforward, it could be difficult for some people with dementia. Four people caring for someone with either vascular dementia or AD felt strongly that the person they cared for would be unable to lie still for 10 minutes and that doing so for 45–60 minutes would be impossible:
Vascular and Alzheimer’s when it gets to the middle . . . you can’t keep people still . . . they don’t understand what’s happening.
Carer
It bothers me about the scan . . . incredible noise being lashed down. I knew what was going on . . . but for people with dementia it could ruin them.
Carer
I’ve been in one and I can imagine her staying in there still . . . not for an hour.
Carer
I can’t imagine they’d tolerate an hour.
Carer
Participants did also note that ‘different people react in different ways’ (carer). Some had more positive experiences: one carer said that the person they care for ‘quite enjoys these’. Having music or a screen to watch was said to improve the experience, giving the individual something to focus on, and that could be done for most participants ‘If there is some flexibility’ (carer) around the MRI environment.
Carers agreed that PET scanners were less restrictive than MRI scanners, although the issue of having to keep still remains.
Overall, volunteers agreed with the suggestion made at the consensus conference regarding the use of MRI in a select number of participants who had consented to the procedure, rather than including MRI as a core component for every individual in the study design. Volunteers agreed that this could improve recruitment and retention, as the prospect of MRI can discourage participation. Allowing those who are anxious about MRI to opt out of that part of the study would enable them to join trials they would otherwise not consider.
Summary
During the focus groups, some volunteers thought that an outcome set should include biological tests, cognition and possibly behaviour. One volunteer suggested that a package could potentially include global measures. Most participants favoured biological measures as they perceived these to provide a more objective outcome. The volunteers who reviewed our report of the focus group discussions broadly supported this recommendation and the recommendations made in the consensus conference. Volunteers also suggested additional considerations for the design of a core package:
-
Burden of time: 1–1.5 hours maximum without a break.
-
Include both people with dementia and carers: carer assessments rely on positive relationships and an accurate understanding of an individual’s dementia. Consider how to involve those without a defined carer.
-
Prioritise explaining the reason for the test; clear idea of purpose may improve retention.
-
Efficiency: several tests within 1 day cuts further visits, weekend visits would remove the need for working carers to take time off.
-
Different types of dementia may need different core measures: some of those consulted felt that this was illustrated by the variation in volunteer opinions.
Volunteers suggested that the current research would present the opportunity to encourage dementia-friendly trial designs, with fewer individual measures and a lower burden on the person with dementia and their carer. This was despite consistently advocating for individualised and longer measures. One carer, when discussing a trial protocol involving a large number of exploratory measures, said:
There is a need to ensure that does not happen in dementia trials as the extra burden on patients cannot be justified.
The practicalities of research and carer and patient burden were in many ways a greater concern for those consulted here than the individual measures:
When you’re trying to recruit people, take as many of the barriers out of the way as possible.
Carer
The recommendations made by people with dementia and carers focus on the importance of reducing the personal impact of research participation, ensuring that the research methods chosen are relevant and acceptable to people affected by dementia, and the importance of trials enhancing a sense of achievement from participation:
That’s where I think research can come into its own on enhancing our situation by making us feel good that we’re taking part in something.
PWD
The experience of dementia is extremely isolating . . . being part of a dementia study certainly made me feel as though someone was remotely interested in what was going on, which was actually terribly important.
Carer
Chapter 7 Consensus conference
Purpose
The purpose of the consensus conference was to bring together the NIHR dementia research community to discuss which outcome domains should be core, any specific recommendations of outcomes within each domain and other issues that should be considered for potential participants completing the core set.
Preparations
We chose a central London venue for the conference; this was felt to be the easiest location for group members to travel to from around the UK and many of the participants were based in London.
We chose champions for each domain from within the group based on expertise. We split the biological marker domain into imaging markers and fluid-based markers with a champion for each, meaning that there were seven champions overall. The champions were:
-
ADLs: Gail Mountain
-
biological markers: fluid – Robert Perneczky
-
biological markers: imaging – John O’Brien
-
cognition: Rob Howard
-
global: Bob Woods
-
neuropsychiatric symptoms: Gill Livingston
-
quality of life: Sube Banerjee.
Each champion was sent the data from the systematic review about their domain, along with some validation information, and was asked to use both of these alongside their professional expertise and knowledge to evaluate if the domain was core and which measures they would recommend. Each champion was asked to prepare a short presentation (10–15 minutes) for the consensus conference around their recommendations and to write up their presentation in up to 1000 words.
Participants
The list of people who attended the conference is in Appendix 7.
We invited all participants who agreed to collaborate on the project within the protocol, and also additional people who had become involved during the project: a Master of Science student who had volunteered to work on the project (DG), the AS Research Engagement Officer who had led the day-to-day work on the PPI (AGS) and a representative from Alzheimer’s Research UK. A member of the original collaboration left academia (Mary Bond) and so put forward a colleague in her place (JTC).
Participants who attended had a range of academic and clinical expertise within dementia research, including:
-
AS research lead – James Pickett
-
applied psychosocial dementia research/occupational therapy – Gail Mountain (co-applicant)
-
dementia care – Frances Bunn and Claire Goodman
-
health service research – Sasha Shepperd
-
health outcome measurement – Sallie Lamb and Charlotte Roberts (co-applicant)
-
old age medicine – Roy Jones
-
old age psychiatry – Sube Banerjee, Chris Fox, Rob Howard (co-applicant), Gill Livingston (principal investigator), John T O’Brien and Robert Perneczky
-
psychology and dementia – Georgina Charlesworth, Esme Moniz-Cook and Bob Woods
-
public health and ageing – Louise Lafortune (co-applicant)
-
social care and social policy – David Challis, Katie Featherstone, Justine Schneider and Claire Surr
-
systematic reviews – Jo Thompson-Coon.
Conference proceedings
The conference began with a summary of the project, including the background, aims and workstreams of the project. Gill Livingston, who was chief investigator of the project, gave an overview of the purpose and workstreams of the project. Lucy Webster presented an overview of the systematic review, including the methods, numbers screened and searched, and the main findings from this in terms of outcomes. The main feedback from the focus groups was also presented by Anna Grinbergs-Saull. Gill Livingston and Rob Howard chaired the meeting and Gill Livingston and Lucy Webster took notes of the discussion.
Cognition
Champion: Rob Howard, Professor of Old Age Psychiatry, University College London
With the central feature of dementia being cognitive impairment, it is essential that a cognitive outcome would be a core measure in disease-modifying trials. By definition, at least two cognitive domains are disrupted in dementia and different dementias are characterised by disruption to different cognitive domains. Therefore, no single neuropsychological test will be sufficient as an outcome tool, hence the need to recommend a global cognitive scale or battery of tests that can be used across all dementia trials. We therefore did not consider cognitive scales that focused on only one cognitive domain. We shortlisted the five most commonly used scales and searched for validation data for these scales: ADAS-Cog, MMSE, CERAD’s Neuropsychological Battery, the Neuropsychological Battery and the Cogstate Alzheimer’s Battery.
For cognition, there are really only two serious contenders. The ADAS-Cog and the MMSE have overwhelmingly been the most widely used cognitive measures in disease-modifying trials. The ADAS-Cog has been used in 92 trials involving 20,419 participants, the MMSE in 83 trials involving 17,736 participants and both scales have been used in combination in 68 trials involving 15,949 participants. The MMSE has mostly been used with the ADAS-Cog or other cognitive tests; it has been the sole cognitive measure in only six of the included trials. ADAS-Cog, on the other hand, has solely been used in 20 of the trials.
Both scales are validated and demonstrated good sensitivity to change in the earlier cholinesterase inhibitor trials. The ADAS-Cog is scored out of 70 points, whereas the MMSE is scored out of 30 points. Minimum clinically important difference figures of 3.0 points for the ADAS-Cog and 1.4 points for the MMSE have been used. If trials can be designed to anticipate differences of this magnitude, either scale could be used.
Both scales are pencil and paper tests. The ADAS-Cog can only be administered by a trained tester and takes 45 minutes to complete. The MMSE can be administered by clinical staff with minimal extra training and takes 10 minutes to complete.
The MMSE has been criticised for being affected by age and education, for lacking sensitivity in differentiating between very early AD or MCI and normal ageing, for not including items sensitive to executive functioning and for being insensitive to disease progression in severe AD. However, in mild to moderate AD it has reasonable psychometric properties and performed well in the detection of small treatment effects in trials of cholinesterase inhibitors and memantine.
The ADAS-Cog was developed to detect incremental improvement or decline in cognitive functioning in clinical trials. It has been the gold standard cognitive assessment for use within AD trials conducted by the pharmaceutical industry. The larger number of items included certainly gives the impression of potential greater sensitivity to change than the MMSE, but superiority was not seen in the trials of symptomatic treatments. That is to say, in trials in which the ADAS-Cog showed a significant drug–placebo difference, the MMSE (if it was also used) would also detect this. Furthermore, because the ADAS-Cog is not used in clinical practice, clinicians do not have a ‘feel’ for what difference in score would constitute a meaningful change.
The ADAS-Cog has been criticised for not sufficiently assessing attention, planning, working memory and executive functioning, all of which are impaired at the earliest stages of AD. This has led to the addition of extra tests to the original ADAS-Cog, including delayed word recall, a maze and digit cancellation task, and a subjective assessment of concentration and distractibility.
Batteries of neuropsychological tests
In response to concerns that the ADAS-Cog and MMSE show low sensitivity to change in mild AD, with placebo-allocated patients typically showing a mean decline of as little as 1 ADAS-Cog point over 6 months in some trials, the Neuropsychological Test Battery (NTB) was developed to include measures of memory and executive function considered to be most affected at this stage of the disease. Through a combination of six validated neuropsychological tests, the NTB has been shown to have excellent psychometric properties across mild to moderate AD. The NTB has been used in seven trials involving 3429 participants. As a test of high sensitivity, but uncertain clinical significance, the NTB would seem most useful in early-phase trials in which detection of preliminary, proof of concept signal is sought. The battery can only be administered by trained raters.
Biological markers: imaging
Champion: John O’Brien, Professor of Old Age Psychiatry, University of Cambridge
Imaging biomarkers are used in clinical trials of disease-modifying therapies in AD for a number of reasons. First, they are used to clarify the clinical diagnosis through the application of research diagnostic criteria for AD. Second, they are used to stratify subjects for entry; for example, a trial using a therapy based on decreasing amyloid might stratify subjects by including only those who showed increased amyloid burden. This may be important, as previous failed studies of immunotherapy in AD have found that around one-third of subjects with supposed AD did not have any evidence of increased brain amyloid. 122 Third, imaging is used to ensure that inclusion criteria are met, for example the absence of multiple microbleeds on MRI for an immunotherapy trial. Fourth, MRI, in particular, is used as a safety outcome measure (examining for an increase in microbleeds and/or oedema). Fifth, imaging is used to demonstrate target engagement (e.g. showing that anti-amyloid therapy actually lowers brain amyloid). Finally, imaging markers are used as outcome measures in their own right, either because imaging changes are more directly related to pathology (e.g. amyloid or tau imaging) or because they have much greater reliability and sensitivity to change than clinical measures (e.g. serial structural MRI), therefore allowing studies to be done more quickly and with fewer subjects.
Imaging outcome measures used in 49 (30.4%) of the studies identified in the literature review included magnetic resonance spectroscopy (one study, 353 subjects), serial structural MRI (30 studies, 4788 subjects), PET (19 studies, 761 subjects), electroencephalography (EEG) (three studies, 96 subjects), perfusion single-photon emission computerised tomography (one study, 135 subjects) and Doppler ultrasound (one study, 45 subjects). PET can be divided into studies that used metabolic PET (13 studies) or amyloid PET (10 studies). Some studies have used multiple markers. Metabolic PET has been exclusively undertaken with fludeoxyglucose PET, while amyloid imaging most frequently uses Pittsburgh compound B (PiB) or florbetapir, although that may reflect the order in which amyloid imaging ligands became available, with PiB the best established, followed by florbetapir. There are two other amyloid imaging compounds that have been licensed for clinical use, flutemetamol and florbetaben. Tau imaging ligands are now available, with at least three (AV1451, PBB3 and THK) being the subject of ongoing validation studies. One ongoing trial was identified using AV1451 PET, but several more are planned. Magnetic resonance spectroscopy and Doppler blood flow measurement were each included in only one clinical trial. There is a strong suggestion that the inclusion of imaging biomarkers is increasing over time, as they were present in 25% of published studies but are included in 53% of currently ongoing studies.
Serial structural MRI has, therefore, been the most widely used imaging outcome measure, with scans repeated over a period ranging from 24 weeks to 2 years. Most studies have used 1.5-T magnetic resonance, although some a mixture of 1.5 T and 3 T. The outcome measures analysed most commonly are whole-brain volume change and/or change in hippocampal volume. Structural MRI has proved sensitive to change over time, but changes between placebo and treatment have not always been as expected. For example, in some of the early immunotherapy studies serial brain volume loss was actually higher in the treated (amyloid-lowering) groups,79 a finding explicable in terms of greater amyloid removal but not paralleling the accepted relationship between greater volumetric loss and worse disease progression.
Fludeoxyglucose PET has shown itself to be a sensitive marker of disease progression in studies over 6–18 months. In some studies, decreased glucose metabolism has been greater in placebo groups. 59,64,140 Amyloid imaging is becoming an almost essential requirement, both for target engagement and as an outcome measure, in clinical trials aimed at lowering brain amyloid. Its use was particularly important in the bapineuzumab studies,122 as clear differences in brain PiB retention could be demonstrated between placebo and actively treated groups, demonstrating to some extent target engagement, despite no effect on clinical outcome measures. This study was particularly interesting because whole-brain volume change was similar between groups, so not sensitive to the effect of amyloid lowering (although paralleling the lack of change in clinical measures). Perfusion single-photon emission computerised tomography, although a well-validated marker, has been used in only one Phase II study and is unlikely to be a useful biomarker for the future given the much greater sensitivity, wider availability and similar cost of fludeoxyglucose PET.
Electrophysiological and functional imaging markers have not been well studied in AD trials, despite the fact that they have potential for greater sensitivity to change over shorter temporal periods than structural, metabolic or amyloid imaging. Only three studies (of which two have completed) have used EEG, although, interestingly, both showed a change in EEG in the treated group. 40,109 There are too few data to draw any conclusions, but both EEG (and magnetoencephalography) and functional MRI merit further investigation and validation in future studies, especially those undertaken over short treatment periods (4–26 weeks).
Cerebrospinal fluid biomarkers can also capture information on tau and amyloid, and show a relationship to changes on imaging. Advantages of CSF include the ability to capture both amyloid and tau in a single measure, and the fact that it is relatively cheap to collect compared with imaging. Disadvantages include still considerable and largely unexplained variability between sites in results, the loss of any spatial (regional brain) information about pathology, a relatively indirect relationship to brain changes, the need for an invasive lumbar puncture and the inability to capture information provided by some imaging modalities (e.g. structural MRI).
In conclusion, around one-third of disease modification studies have used imaging biomarkers as outcome measures, with an increasing proportion over time. Serial structural MRI remains the most widely used and best-validated biomarker, and robustly demonstrates disease progression in untreated AD subjects. For example, it can be measured in a study to show that improvement in cognition is related to disease modification via a reduction in atrophy, rather than just a symptomatic change, and it does not require all participants to undergo MRI.
Metabolic PET has also been widely used and demonstrates sensitivity to change in untreated patients, and may be of particular interest for compounds that are purported to influence glucose or energy metabolism. More specific ligands, including those for amyloid and tau, are now available and are becoming increasingly validated as outcome markers. Longitudinal changes in amyloid PET, and most likely in tau PET (although this remains to be demonstrated), will be expected to be less sensitive to change over shorter periods than, for example, serial MRI. However, they will be essential to include in some studies of disease modification in AD, depending on the mechanism of action of the compound under study, to show target engagement.
Biological markers: cerebrospinal fluid
Cerebrospinal fluid and blood biomarkers
Champion: Robert Perneczky, Reader in Cognitive Impairment and Dementia, Imperial College London
Recently revised diagnostic guidelines for AD emphasise the use of biomarkers, heralding a paradigm shift towards a more biological definition of the disorder. Currently available biomarkers offer added diagnostic accuracy in certain situations, but their performance in terms of early diagnostic sensitivity and specificity does not fully live up to the desired standards. The hope for disease modification as well as technological advances in biomarker discovery fuel the search for biological indicators of the AD pathophysiological process, which can be used to identify neurodegeneration independently of its clinical manifestations. Ideally, such a biomarker, alone or in combination with other markers, would distinguish between individuals with and without AD pathology independently of the clinical symptomatology. Individuals with asymptomatic early AD would probably benefit most from interventions aiming to prevent further neural damage to maintain their independence, ability to work and fulfilment of social roles. Furthermore, pathophysiological markers may also offer the added benefit of directly assessing response to treatment options that target core processes of AD pathogenesis. The application of novel therapeutics with potentially significant side effects could thereby be restricted to patients with biological evidence of treatment response in line with the notion of personalised medicine. However, biomarker evidence of treatment efficacy should not replace clinical evidence of patient benefit.
Currently available AD biomarkers can generally be grouped into two categories. The first category comprises markers that indicate the type of pathology present, including CSF levels of amyloid-β (Aβ) 42, total tau (T-tau) and phosphorylated tau (P-tau)181 and PET tracers of fibrillar amyloid such as flutemetamol, florbetapir, florbetaben and PiB. The second category consists of markers that provide information on the topography of pathological changes, such as MRI and fludeoxyglucose PET. The diagnostic accuracy of the aforementioned biomarkers to distinguish between AD dementia and physiological ageing is high in selected patient cohorts, recruited at specialised centres and, therefore, enriched with relatively pure AD cases. However, the clinical usefulness of available biomarkers is limited when it comes to the identification of early or atypical AD cases, especially in unselected populations.
The sensitivity and specificity requirements set out in the consensus report of the Working Group on Molecular and Biochemical Markers of AD445 are rarely met when fluid biomarkers are compared with autopsy results. Little is known about the ability of biomarkers to identify AD pathophysiology in asymptomatic individuals. Studies in carriers of pathogenic mutations have shown that biomarkers can become abnormal many years before the onset of clinical symptoms. In addition, longitudinal observations in cognitively healthy older people have demonstrated that an AD-typical CSF biomarker profile is associated with greater cognitive decline. However, it is unclear how accurately current biomarkers can predict future dementia and the time of onset in individuals who have no symptoms. Furthermore, Aβ immunisation trials show that markers of AD pathophysiology show changes even if no clinical benefit is present, which limits their usefulness as study end points or surrogate measures (even though they may be useful to show target engagement).
Our systematic review confirms that the three established markers Aβ42, T-tau and P-tau181 are also the most widely used markers in AD dementia clinical trials. CSF levels were measured in 51 out of 125 (41%) studies, whereas 35 out of 125 (28%) studies reported blood levels. Even though AD fluid markers are useful for diagnosis to ensure that studies have AD cases with true AD pathology, and to measure target engagement, their usefulness as outcome measures is limited based on the available evidence. They are therefore not recommended as core outcomes for dementia clinical trials.
Neuropsychiatric symptoms
Champion: Gill Livingston, Professor of Psychiatry of Older People, University College London
Neuropsychiatric symptoms or behavioural and psychological symptoms of dementia are the symptoms of abnormal mood, disturbed behaviour, thinking or perception, which become more common as dementia progresses from mild to moderate so that each individual with dementia will almost inevitably develop neuropsychiatric symptoms at some point during the illness. 446
Although neuropsychiatric symptoms are almost universal if individuals are considered throughout the course of the disease, individuals with mild to moderate dementia occasionally have no neuropsychiatric symptoms and frequently have no clinically significant symptoms. For example, 94% of participants with dementia in a study designed to be representative of people with AD had one or more neuropsychiatric symptoms, but only 74% had clinically significant symptoms. 447 Similarly, a study of newly diagnosed patients with AD found that 78% had neuropsychiatric symptoms and 59% had clinically significant symptoms. 448 As people with mild to moderate dementia may not have any or clinically significant neuropsychiatric symptoms and, therefore, have no potential for these to improve, if the underlying disease is modified, this floor effect means that effective treatments for disease modification would require hugely increased samples size to show a significant effect on neuropsychiatric symptoms.
Neuropsychiatric symptoms are common and contribute significantly to decreased quality of life for patients with dementia, caregiver burden and care home admission. 449 These are therefore very important symptoms and we would like to encourage their measurements to understand what a disease-modifying treatment is doing in important domains. It is also important to recognise that neuropsychiatric symptoms can sometimes be influenced by social factors (e.g. care and relationships around a person). Therefore, if neuropsychiatric symptoms are being measured, studies would need to account for whether social or relational factors external to the intervention might be influencing this, possibly through measurement in control and intervention groups.
We will use the opportunity afforded by our systematic review of the literature in this field, PPI and consensus conference to make recommendations for which instrument to use.
Desirable characteristics of measures
-
We prespecified that potential measures must have some form of validation and reliability in the population to be tested. This particularly includes content validity for neuropsychiatric symptoms (i.e. not only reliability and concurrent validity but that the measure covers neuropsychiatric and only neuropsychiatric items rather than include, for example, ADLs or memory). In addition, these items should be neuropsychiatric symptoms found in dementia rather than, for example, symptoms of schizophrenia.
-
We measured how often they had been (or are being) used. Ideally, they would have been used often, as this is a measure of how an instrument is valued, how practical it is and how much it is likely to be used in practice. The frequency of use in disease-modifying trials to date is summarised in Table 12.
-
Neuropsychiatric symptoms can be considered in terms of severity or frequency. Some scales cover only one of these. Knowing the frequency without the severity or vice versa means that the scale is less useful in measuring change. This is because the symptoms may improve either by being of reduced frequency or severity, or one of these dimensions may improve while the other deteriorates. Both dimensions are important characteristics and are often used in a summary score in an instrument, and this is desirable.
-
The time taken should not be too long as the instrument would be part of a package ideally, but not essentially. In addition, the minimal clinically important difference should have been calculated and it should be translated into different languages.
| Name of measure | Number of | Time taken to complete measure (minutes) | |||
|---|---|---|---|---|---|
| Studies | Studies published | Studies ongoing | Participants | ||
| NPI | 38 | 30 | 8 | 11,756 | 10–20 |
| ADAS-Noncog | 7 | 7 | 0 | 792 | 20–25 |
| BPRS | 3 | 3 | 0 | 190 | 20 |
| Nurses’ Observation Scale for Geriatric Patients | 2 | 2 | 0 | 454 | 3–5 |
| CERAD’s Behavioural Rating Scale for Dementia | 1 | 1 | 0 | 486 | 20–30 |
| BEHAVE-AD | 1 | 1 | 0 | 425 | 20 |
| Dysfunctional Behaviour Rating Instrument | 1 | 1 | 0 | 406 | 20 |
| Plutchik Geriatric Rating Scale | 1 | 1 | 0 | 178 | 5–10 |
| Revised Memory and Behaviour Problems Checklist | 1 | 1 | 0 | 168 | 10 |
Our systematic review has identified nine measures that have been used to detail the range of neuropsychiatric symptoms in disease-modifying trials in dementia, detailed in Table 12. These are the Neuropsychiatric Inventory (NPI),236 Brief Psychiatric Rating Scale (BPRS),229 Alzheimer’s Disease Assessment Scale-Non Cognitive scale (ADAS-Noncog),174 CERAD’s Behavioural Rating Scale for Dementia,231 the Revised Memory and Behaviour Problems Checklist,247 Dysfunctional Behaviour Rating Instrument,234 Behavioural Pathology in Alzheimer’s Disease (BEHAVE-AD),450 Nurses’ Observation Scale for Geriatric Patients243 and Plutchik Geriatric Rating Scale. 246
Measure of individual neuropsychiatric symptoms
There are also some measures that have been used to consider individual neuropsychiatric symptoms (e.g. agitation451 and depression) but these are too specialist to recommend for all trials of disease modification as no specific symptom is common enough to measure individually, unless the object of the study is to change it. The NPI was the most commonly used measure and is being used most often currently. It is valid and reliable, and measures both frequency and severity. It has good reliability statistics and sensitivity to change. It takes slightly less time than CERAD and, possibly, BEHAVE-AD (10–20 minutes). The minimal clinically important difference has been calculated as 8 points. 452 It has also been translated into Italian, Greek, Japanese, Korean, Mexican, Polish, Spanish and Dutch. 176,237
The measures included that only assess the severity of symptoms are:
-
BEHAVE-AD – sensitivity to change in moderate and severe dementia, but unclear in mild dementia. It takes 20 minutes to complete, and there are 26 items on a four-point scale. There was no floor or ceiling effects. The questions ask about severity not frequency. It has been translated into French, Swedish, German, Dutch, Spanish, Chinese and Korean. 176
-
ADAS-Noncog – is commonly used (in terms of number of studies), but measures only the severity of symptoms. It is long, taking around 20–25 minutes to complete. It is sensitive to change in mild to moderate AD.
The measures included that only assess the frequency of symptoms are:
-
Nurses’ Observation Scale for Geriatric Patients – it only covers 3 days, which may be too short a period. It is commonly used (in terms of patient numbers), but measures only the frequency of symptoms. It is rapid to complete and has test–retest validity.
-
CERAD’s Behavioural Rating Scale for Dementia – is reliable and valid, and sensitive to change in mild to severe dementia. Takes 20–30 minutes to complete and is designed for mild to moderate dementia. It measures frequency but not severity, and has been translated into French and Spanish. 176
-
Dysfunctional Behaviour Rating Instrument – only measures frequency.
Content includes other domains:
-
BPRS – only measures severity and is designed for general psychiatric patients (e.g. includes blunted effect and concerns about physical illness).
-
Revised Memory and Behaviour Problems Checklist – incorporates memory problems in its total and, therefore, is not a pure test of behaviour. This may be why it is the least used.
-
Plutchik Geriatric Rating Scale – is validated in geriatric inpatients who are not necessarily those with mild to moderate dementia. It incorporates sensory impairment, social isolation and activities, and is therefore not a good measure of behaviour.
In summary, neuropsychiatric symptoms should not be core measures in dementia modification studies but, as they are very important, many studies may wish to measure them. We recommend the NPI for the reasons outlined above.
Activities of daily living
Champion: Gail Mountain, Professor of Health Services Research, University of Sheffield
The main rationale for applying ADL measures in disease-modifying studies is to assess changes in participant abilities to undertake functional activities following an intervention, with scores providing an estimation of deterioration or improvement. Such measures can include basic ADLs such as washing, dressing and toileting and complex or instrumental ADLs (IADL) such as cooking, shopping and money management. Newer measures are also taking into account other daily activities such as engagement with social and leisure activities.
The systematic review identified 12 measures that have been applied in disease-modifying studies:
-
Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory (ADCS-ADL) (34 studies: 28 studies published, six ongoing; 11,500 participants)
-
Alzheimer’s Disease Functional Activity and Change Scale (ADFACS) (two studies: two published; 350 participants)
-
BADL (five studies: three published, two ongoing; 1117 participants)
-
Dependence Scale (three studies: three published; 3343 participants)
-
Disability Assessment for Dementia (DAD) (12 studies: 10 published, one protocol and one ongoing; 2914 participants)
-
Functional Activities Questionnaire (two studies: two ongoing)
-
Interview for Deterioration in Daily Living Activities in Dementia (two studies: two published; 219 participants)
-
Katz ADL (four studies: three published and one protocol; 185 participants)
-
Lawton IADL (eight studies: seven published and one protocol; 1125 participants
-
Nuremberg ADL (three studies: three published; 530 participants)
-
Personal Self-Maintenance Scale (PSMS) (three studies: three published; 429 participants)
-
Video Recorder Home Behavioural Assessment (one published; 48 participants).
Initial considerations
-
The ADCS-ADL and ADFACS are both derived from the work of the Alzheimer’s Disease Cooperative Group and have the same primary reference. 286 The author has now confirmed that the ADFACS is a version of the ADCS-ADL developed by one of the team (Rachelle Doody, Baylor College of Medicine Medical Center), who has since participated in studies led by Douglas Galasko (University of California San Diego Medical School) using the ADCS-ADL.
-
Lawton IADLs and the PSMS are derived from the same authors and are frequently used in combination, thereby spanning both ADLs and IADLs.
-
The Video Recorder Home Behavioural Assessment has only been applied in a single study and, therefore, has been discounted.
This leaves nine measures for consideration. The following four measures have been used most frequently:
-
ADCS-ADL
-
DAD
-
Lawton/PSMS
-
BADL.
The Dependence Scale has been used in only three studies, but with a large number of participants.
Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale
There is a 19-item scale for basic ADLs and a 23-item IADL scale, which includes reading, leisure activities and basic chores. The measure has good test–retest reliability (assessed in four studies),286,289,292,293 is sensitive for use with people with mild to moderate dementia and sensitive to change in three out of four studies. 288–291 It has been translated and validated in several European languages.
Disability Assessment for Dementia
The 40-item measure was developed for use with people with different severities of AD living in the community. It can be applied in 15 minutes by a clinician interviewing the carer or by a trained observer rating performance. It determines whether or not the person with dementia needs help to initiate, organise, plan and perform in 10 ADL and IADL areas (including leisure activities). Content validity and reliability has been demonstrated independently331 and by the measure developers,301 and there are reportedly no floor or ceiling effects. 301,309
Lawton Instrumental Activities for Daily Living/Personal Self-Maintenance Scale
These two measures were originally developed for use with non-cognitively impaired older people and can be used separately or in combination. The PSMS contains six domains of basic ADL functions (toileting, bathing, feeding, dressing, mobility and physical ambulation), rated from 0 to 1. The Lawton ADL has eight IADL domains (ability to use the telephone, shopping, food preparation, housekeeping, laundry, transport, managing medications and finance) rated on a scale from 1 to 5. Both can be completed in a short (5- to 10-minute) interview with the person with dementia, as well as through a proxy. 331 There are questions regarding the extent of sensitivity with dementia. 331,453 It has also been noted that this ADL measure has a ceiling effect. 331
Bristol Activities of Daily Living scale
This 20-item questionnaire is used to measure abilities in both ADLs and IADLs by people in the early stages of dementia, using proxy report. 295 Face validity is determined by carer agreement regarding important items. BADL has good content and construct validity, but there are different views regarding test–retest reliability. 331,454
Dependence Scale
The Dependence Scale comprises 13 items, completed with an informant. It was designed to identify the level of required care. Items range from need for reminders or advice to undertake IADL/recreational activities to needs for assistance with basic ADLs and need for supervision. The final four items in the measure are concerned with needs for interventions to maintain basic function (catheterisation/tube feeding). The developer of the measure was able to demonstrate good psychometric properties and sensitivity to dementia progression, but this has not been replicated. 298 In addition, given the wide range of items within the measure, dependency at different stages of dementia is estimated by very few items.
The following measures are less frequently applied.
Interview for Deterioration in Daily Living Activities in Dementia
This is designed to assess both basic ADLs and IADLs in community living people with dementia, with both initiative to perform tasks and performance being assessed. The 33 items are assessed on a seven-point scale. 319 It was initially designed for use in a 15-minute carer interview, but there is now a self-complete version for the carer.
Functional Activities Questionnaire
This 10-item measure solely comprises IADL items, including travel and recreation, each being assessed on a six-point scale by an informant. It is valid for use only with people with mild dementia and has been used to distinguish between MCI and dementia.
Katz Activities of Daily Living
This measures independence in basic activities of older adults and is not dementia specific. Included items are bathing, feeding, dressing, toileting, transferring, continence and bathing, with rating being independence or dependence. The results from application of the Katz are highly correlated with MMSE scores,455 and it is sensitive to dementia progression.
Nuremberg Activities of Daily Living
The Nuremberg Activities of Daily Living is for use with people with severe dementia in a residential setting and, therefore, should not be considered for mild to moderate dementia.
Conclusions and recommendations
Only five of the most frequently applied instruments measure ADLs; the Dependence Scale includes items to measure dependency. All are for use with people with mild to moderate dementia apart from the PSMS and the Dependence Scale. All are dementia specific apart from the Lawton/PSMS measures.
The shortlist is:
-
DAD
-
Bristol ADL
-
Lawton ADL/PSMS
-
ADCS-ADL.
Although the ADL outcome is very important and is necessary for regulatory purposes and definitive trials, in some countries it is not core as it may change for other reasons than a change in dementia. However, we would encourage its inclusion and based on use and psychometrics the following measures are recommended:
-
DAD is the most often used for community living people and has an observation option
-
Lawton ADL/PSMS is the only measure that includes self-completion; however, this may not be reliable in terms of the answers given.
Quality of life
Champion: Sube Banerjee, Professor of Dementia, Brighton and Sussex Medical School
Health-related quality of life (HRQoL) is a multidimensional concept that reflects the individual’s subjective perception of the impact of a health condition on everyday life. 456 The science of developing instruments that can measure HRQoL has grown during the past 30 years. Such measures are increasingly seen as a useful complementary source of evidence of the value of impact throughout health care. They provide one approach to the measurement of overall impact and, importantly, they generate an appraisal of the subjective view of the individual affected. It has become clear that people with dementia have things that positively and negatively influence the quality of their lives, and that they can report these. 457–459
However, measuring HRQoL in people with dementia is difficult. In any type of dementia there are disorders of memory, attention, communication, insight, judgement and behaviour, all of which might impair the ability to complete measures of HRQoL. In addition, because of its progressive nature, what works at one stage of dementia may not work later on as the disease becomes more severe. All this means that instruments not developed for those with cognitive impairment will generally not work well in those with dementia. Thus, the generic instruments available to measure HRQoL across different disease states (e.g. the EQ-5D or Short Form Health Survey) do not work well in dementia. 252 For example, one study found that 48% of people with dementia self-reported being in full health using the EQ-5D questionnaire, with no problems on any dimension. 266 Disease-specific measures of HRQoL in dementia have therefore been developed. 267,460–462 The field is one that has developed rapidly, and there has been progressive improvement in methodology and measurement during the past 15 years.
There has been an underlying assumption in much of the literature until quite recently that HRQoL must necessarily decrease as dementia becomes more severe. From the outside, it might seem counterintuitive that HRQoL could do anything other than drop with increasing cognitive impairment and activity limitation. In this case there would be limited value in measuring multiple variables that all vary predictably together, rather than just measuring one (such as cognition). However, it now seems clear that activity limitation and cognition do not have a simple or linear relationship with HRQoL in dementia. 463–465 The data suggest that there is the possibility of good quality of life at all stages of dementia and that there are individuals with poor quality of life at all stages of the illness who may therefore benefit from interventions to improve quality of life in dementia. This does not mean that improving cognition would not cause an improvement in HRQoL. Woods et al. 272 looked in detail at data from a trial in which a psychological therapy had both a positive impact on HRQoL and a positive effect on cognition. In that study there were no cross-sectional associations between severity of dementia and HRQoL. This makes clear the limitations of using a profile approach, with HRQoL being more than the sum of the discrete measures used.
Depending on the specific questions being investigated, HRQoL can be a primary or secondary outcome in itself. The focus here is the evaluation of disease-modifying medication in mild to moderately severe AD, and it is unlikely that HRQoL would be used as a primary outcome measure in any Phase II or pivotal Phase III study. However, the measurement of HRQoL is an obligatory part of the economic evaluation of emerging health technologies or interventions. In cost–utility analyses, quality-adjusted life-years (QALYs) are used to measure the impact of an intervention on both quality and quantity of life. Quality of life is measured by using health-state values that are scored using preference information typically gained from a representative sample of the general population. Generic preference-based measures of health, such as the EQ-5D, discussed previously in this section, are widely used as a means of generating health-state values for use in the calculation of QALYs. Given the debate around the extent to which generic preference-based measures fully capture aspects of quality of life associated with some medical conditions, and that the validity of using generic preference-based measures in dementia is uncertain, there has been interest in developing preference-based measures from condition-specific measures to target medical conditions more effectively in terms of HRQoL. Recent developments of the DEMQOL system allow the use of a dementia-specific instrument to be used in cost-effectiveness analyses of interventions in dementia. 466–468
The systematic review identifies one generic measure of HRQoL, the EQ-5D, and two measures of disease-specific HRQoL that have been and are being used in evaluations of potentially disease-modifying compounds in AD. The information on their use is summarised in Table 13.
| Studies measure is used across | Instrument | ||
|---|---|---|---|
| EQ-5D | QOL-AD scale | DEMQOL | |
| Number of studies | 5 | 9 | 4 |
| Studies published | 4 | 7 | 1 |
| Studies ongoing | 1 | 2 | 3 |
| Number of participants | 4084 | 3341 | 399 |
An assessment of the relative merits of the two disease-specific systems in terms of their psychometrics is presented in Table 14. This enables a comparison of the two instruments with earlier instruments and shows that the current instruments are a significant advance on the earlier methodologies.
| Psychometric properties | Instrument | |||||
|---|---|---|---|---|---|---|
| Progressive Deterioration Scale | Pleasant Events Schedule – Alzheimer’s Disease | QOL-AD | DEMQOL | |||
| Patient | Proxy | Patient | Proxy | |||
| Conceptual model | 0 | + | ++ | 0 | +++ | +++ |
| Acceptability | 0 | 0 | ++ | ++ | ++ | ++ |
| Reliability | ||||||
| Internal consistency | 0 | 0 | +++ | +++ | +++ | +++ |
| Test–retest | +++ | 0 | +++ | ++ | +++ | +++ |
| Inter-rater reliability | 0 | 0 | NA | 0 | + | + |
| Validity | ||||||
| Content | + | 0 | +++ | +++ | +++ | +++ |
| Criterion related | 0 | 0 | 0 | 0 | ++ | ++ |
| Construct | ||||||
| Convergent validity | 0 | + | +++ | +++ | +++ | +++ |
| Discriminant validity | 0 | 0 | 0 | 0 | ++ | ++ |
| Known groups differences | + | 0 | +++ | 0 | +++ | +++ |
| Experimental intervention | 0 | 0 | 0 | 0 | ++ | ++ |
| Factor analysis | 0 | 0 | + | + | + | + |
| Responsiveness | 0 | 0 | + | + | + | + |
| Respondent burden | 0 | ++ | +++ | +++ | ++ | +++ |
| Cultural and language adaptations | 0 | 0 | ++ | ++ | ++ | ++ |
| Economic evaluations | 0 | 0 | 0 | 0 | +++ | +++ |
| Health state classification | 0 | 0 | 0 | 0 | +++ | +++ |
| Preference-based measures | 0 | 0 | 0 | 0 | +++ | +++ |
| Population values | 0 | 0 | 0 | 0 | +++ | +++ |
The main points from this analysis are as follows:
-
the QOL-AD is older and has been used more widely, particularly in the USA
-
the DEMQOL system development process was stronger in psychometric terms
-
the quality of development and subsequent data on DEMQOL-Proxy is symmetrical with that of the self-report versions, unlike the QOL-AD
-
there are questions about the validity of the framing of the questions in the proxy-rated QOL-AD, which are not present for DEMQOL-Proxy
-
the QOL-AD is less time-consuming for patients by 5–10 minutes
-
there are relatively few data on responsiveness published for either, although because of the DEMQOL’s longer form, development process and framing, it confers theoretical advantages in sensitivity to change
-
in economic evaluations DEMQOL has advantages because of the unique programme of work developing it as a preference-based measure with no additional burden to respondents or researchers.
In summary, there is a need for broad measures of overall impact of interventions in dementia but nonetheless, although important, it is not a core measure in disease modification. We are now at a stage in which HRQoL in dementia can be measured with instruments specifically designed to do so. It appears that cognition (or activity limitation) is not the same as HRQoL and is in fact a rather poor proxy for it. HRQoL should be measured as and for itself. It is complementary to the measurement of discrete specific outcomes, and a rational approach to measurement would ensure that both sorts of instrument were completed in evaluation of interventions in dementia. It is therefore not core but health decision-makers, such as the National Institute for Health and Care Excellence, place high value on such data when reimbursement decisions are being made. This is likely to include a generic quality-of-life measure, but a dementia-specific quality-of-life measure will be more likely to be valid. If one is used the current evidence suggests that it should be the DEMQOL.
Global
Champion: Bob Woods, Professor of Clinical Psychology of Older People, Bangor University
The development of global assessments of outcome in relation to disease-modifying treatments in the field of dementia may be seen as a rational response to the wide-ranging effects of the dementias. Diagnostic criteria have highlighted the presence of difficulties in multiple domains, including memory and other cognitive impairments, changes in day-to-day function and changes in behaviour. An outcome measure that is able to encompass the range of domains affected and, accordingly the range of potential targets for change, may have advantages over multiple measures, each evaluating a single domain. Specifically, if a treatment is an effective disease-modifying agent, but different aspects of the condition are improved in different individuals, for example through differences in disease progression or symptom expression, a global measure may reflect this through its combination of multiple domains. Global measures also have the potential to combine multiple perspectives, with input from caregivers and the person with dementia informing a global rating, again reflecting the reality of a condition that has effects beyond those reported by the person with the diagnosed condition.
Our literature search identified 10 global outcome measures in the included studies. These could be grouped into three distinct types: global rating scales, dementia staging instruments and clinician impression of change interviews. One of the staging instruments, the Clinical Dementia Rating scale (CDR) had been used in 48 studies. The Clinician Impression of Change interviews had been used in 35 studies altogether, with each of the five global rating scales being used in four or fewer studies.
The five global rating scales identified each included a number of domains, typically without a clear rationale for how each domain might contribute to any total global outcome score. Among the earliest of these scales was the Blessed Dementia Rating Scale, comprising a total of 22 items. These cover three domains, rated in an interview with an informant: everyday activities (eight items), ADLs – eating, dressing, toilet (three items) and personality changes (11 items). The scale does not specifically include cognitive items, but is often used with the Blessed Dementia Information, Memory and Concentration Test, a brief cognitive test. Completion of the Sandoz Clinical Assessment Geriatric Scale is based on an interview with the person with dementia and on observation. The 19 domains (which fall into five factors) include aspects of cognition (‘confusion’ and mental alertness), self-care ability, mood (e.g. anxiety, fatigue) and behaviour (e.g. hostility, ‘bothersome’, irritability, unsociability). The Gottfries–Bråne–Steen Rating Scale for Dementia is similarly completed from a semistructured interview with the person with dementia and observation, and comprises subscales measuring intellectual (12 items), emotional (three items), ADLs (primarily items of self-care) (six items), and behavioural and psychological symptoms of dementia (six items). The most recent of these global rating scales, the Dementia Severity Rating Scale (DSRS),354 is designed for completion by a carer, with 12 domains ranging from cognitive aspects such as memory, orientation, speech and language, and the ability to make decisions, through higher-level functional abilities such as social and community activity, home activities and responsibilities to basic functions (e.g. personal care and cleanliness, and control of urination and bowels). Rikkert et al. 469 comment from their systematic review of dementia staging scales that the reliability (inter-rater, intra-rater and internal consistency) of the DSRS and the Gottfries–Bråne–Steen scales are well established, but that evidence for their validity in staging severity of dementia has not been studied, although our review identified a report of sensitivity of change for each scale. The focus of the DSRS on ratings by a family carer, without external moderation, may introduce bias related to factors such as carer stress and depression.
Our review identified two staging scales for dementia severity that have been used as outcome measures: the CDR and the Global Deterioration Scale. The CDR is based on an interview with the person with dementia and caregivers, covering six domains: memory, orientation, judgement and problem-solving, community affairs, home and hobbies and personal care. An algorithm is used to combine the ratings of each domain into an overall stage categorisation, ranging from ‘no dementia’ to ‘severe dementia’, usually on a five-point scale (although extended versions are available). The algorithm does not weight different domains equally, although interestingly in many of the trials using the CDR, an unweighted ‘sum-of-boxes’ score is used, presumably to increase the range of scores. The Global Deterioration Scale has seven stages (three being ‘pre-dementia’) and is intended to be used with measures of cognition and functional ability. Rikkert et al. 469 comment on the strong support for the reliability of the CDR and for its discriminant validity (including with the sum-of-boxes scoring). Our review suggested that the CDR shows sensitivity to change, whereas the evidence for both reliability and sensitivity to change of the Global Deterioration Scale is more limited.
The third group of outcome measures has two additional features. First, they bring to bear the judgement of an experienced clinician in combining the different domains, balancing the significance and relevance of specific areas of strength and impairment in each case. There is no predetermined algorithm or a simple summation of scores across domain; the clinician simply rates their ‘impression’ of the person’s situation, taking into account all available sources of information. Second, at follow-up assessments, the clinician’s task is to rate how much change there has been since the baseline assessment, using detailed notes taken at that time to assist this judgement. The final outcome is then a score on a seven-point scale ranging from ‘marked worsening’ to ‘very much improved’. This overcomes the potential weakness of the staging instruments in that they would not be sensitive to change within what may in practice be quite a broad severity category, such as ‘mild dementia’, missing clinically significant change. The emphasis on change and being able to focus on changes of most importance to the individual are commendable features of these scales. It should be noted that they would only be suitable for studies that are double blind; in a trial of a non-pharmacological intervention where the participant is aware of the treatment being received, maintaining the blindness of the assessor would be impossible to guarantee.
Our review initially identified three scales of this type: the Clinical Global Impressions Scale, the Alzheimer’s Disease Cooperative Study – Clinical Global Impression of Change and the Clinician’s Interview-Based Impression of Change plus Caregiver Input (CIBIC+). However, for this purpose they may be viewed as a single entity. They are completed following a semistructured interview, with both the person with dementia and a caregiver. Four major categories are covered in the interview: (1) ‘general’ including relevant history (and at follow-up assessments changes since baseline), (2) cognitive function, (3) behaviour and (4) ADLs. Each category is divided into domains, with probe questions suggested for each domain. After completing the interview, the clinician is able to consult all available information, including cognitive tests such as the MMSE or ADAS-Cog carried out on that visit. Inter-rater reliability would be a key issue for these measures, which are so dependent on the clinician’s judgement, but there appears to be little evidence to support this,469 although there is better evidence of retest reliability. Sensitivity to change has been demonstrated in at least one study in our review.
Of the three types of approach to global assessment, both staging and impression of change outcome measures have merits, in combining different aspects through algorithms or clinical judgement, whereas the multiple domain rating scales do not offer advantages over carrying out a specific assessment of each of the included domains. The potential for the CIBIC+ to be sensitive to individual trajectories of change is attractive, in view of the diversity of profiles of function of people even with the same type of dementia. The CDR, which has been the most widely used staging scale, shows good reliability and has demonstrated sensitivity to change. Disease-modifying treatments should have a demonstrable influence on the rate of progress through the stages of dementia reflected by the CDR. However, it should be noted that, if the sum-of-boxes score is used, this places it in the same category as the multiple domain rating scales.
Discussion
Cognition
It was agreed that cognition is a core domain. Any recommended cognitive outcomes should show reliable detection of decline in cognition, which the ADAS-Cog and MMSE have both been shown to do. They are also the most commonly used and validated outcomes. The PPI consultation emphasised that patients dislike failing in tests of cognition and it was recommended that to improve the experience of cognitive tests in the future timed measures which estimate cognitive processing speed should be considered, although they would need validating, including looking for clinical significance and calculating the minimal clinically important difference. The ADAS-Cog is longer, but if more sensitive to change then it may need fewer participants than a trial powered on the MMSE. However, the ADAS-Cog has a problem when there are lots of missing data.
Recommendation
Overall, for cognition we recommend the MMSE or ADAS-Cog, as they are the best-available measures.
Imaging
Imaging is important; however, changes in imaging and cognition are not always highly correlated; between-group differences may show mechanism of change as well as disease modification.
Magnetic resonance imaging is the most sensitive imaging technique to change, and because of this may need fewer participants to still be fully powered, as well as to be able to see changes over 1 year. MRI changes, therefore, can be conducted in a subgroup of participants in disease modification trials. This means that, for example, it would be unnecessary to exclude from a study all patients who do not tolerate MRI, are unable to travel or who have a pacemaker. However, volumetric MRI does not always show expected changes in terms of atrophy (e.g. if amyloid is removed, then the volume may go down in treated patients), but does show changes, which is important.
Recommendation
The recommendation of the consensus conference was that serial structural MRI was the core biological marker that has utility to monitor disease progression. There is rapid innovation of biomarkers and this recommendation may change in the future.
Fluid makers
The four types of biological markers were discussed, as was how fluid biomarkers for AD are more advanced than other illnesses. However, CSF examination is expensive as people may need to go to hospital as a day case. Importantly, they are not helpful biomarkers for disease modification – a meta-analysis showed that changes in putative biomarkers of Aβ42 are not related to change in the MMSE470 – but they can be useful in aiding diagnosis.
Recommendation
It was agreed that currently CSF level does not have a role as an outcome measure for disease modification studies in dementia and, therefore, we do not recommend a fluid biomarker as part of the core set.
Neuropsychiatric symptoms
There was debate around whether or not neuropsychiatric symptoms should be a core domain. Not every individual with dementia, particularly in the early stages, will have neuropsychiatric symptoms, and if they do they may not be clinically significant, meaning that, if they are measured, no difference may be found. Problematic behavioural symptoms may also be measured in measures of global functioning or quality of life.
However, neuropsychiatric symptoms were considered to be core by many members of the group as symptoms increase with the progression of dementia severity. If a disease-modifying treatment was found, they would want behaviour to improve, or at least not decline. If cognition improved, but neuropsychiatric symptoms increased, potentially as a side effect of a pharmacological intervention, then this would be an important outcome to identify. The group judged this to be important but not core to every future study of disease modification as many people with mild dementia do not have any neuropsychiatric symptoms; if this was a core or primary measure it might lead to a false-negative finding.
Recommendation
We recommend that the NPI should be used to measure neuropsychiatric symptoms, as it includes frequency and severity of symptoms and has appropriate psychometric properties, but this is not a core outcome.
Activities of daily living
Function, in terms of ability to complete everyday activities, is often a driver of presentation. In the USA, ADLs is a possible measure in disease modification, whereas in Europe it has been mandatory. It should be measured by proxy, ideally as some people with dementia may underestimate any impairments, although not all will. Some scales try to differentiate between the performance and initiation of ADLs, and also may include that people with dementia may need prompting to complete ADLs.
Activity of daily living is being used less often in new disease modification trials as physical health confounds it and it may not demonstrate change over 1 year. Most of the included ADL scales have good psychometric properties, but sensitivity to change may be a problem and it may not measure what is relevant to mild to moderate dementia, and people from a range of backgrounds. Overall, it was therefore decided that it is not core.
Recommendation
Overall, we recommend the DAD as it is the best-available dementia-specific measure of ADLs.
Quality of life
Quality of life is different from other domains, as people with severe dementia can have good life quality and, thus, it is not a sensible primary outcome to measure disease progression. Nonetheless, it is a useful measure, as it could record any negative or positive impact of pharmacological interventions.
As the EQ-5D is not dementia specific, it may not necessarily give an accurate reflection. In addition, as the self-reporting of quality of life becomes worse over time, it is sensible to get a proxy report too so that measures can be compared over the whole population in a trial.
It was also discussed how quality of life is important in Phase III trials where economics need to be measured for cost by a dementia QALY. The conference did not have the required expertise to discuss or make recommendations about QALYs.
Recommendation
We recommend the DEMQOL, which measures quality of life from both the person with dementia and proxy perspectives, and from which QALYs can be calculated.
Global functioning
The group considered that global functioning could be an important measure as it encompasses several domains and could replace ADLs. Either a staging or impression-of-change outcome measure should be used, rather than a multidomain scale, with a staging instrument most favourably regarded.
Recommendation
We therefore recommend the CDR, as it has good psychometric properties. However, as the sum-of-boxes score of the CDR is similar to a multidomain scale, we recommend the global functioning score, which may be more appropriate.
Main findings
Although all domains are potentially relevant and important, cognition and biological outcomes are the only two domains that were decided to be core by consensus within the group (i.e. they should be measured in every trial of disease-modifying treatment in dementia). Overall, the group consensus was to recommend either the MMSE or ADAS-Cog for cognition. For biomarkers, serial structural MRI currently should be included as a core, but optional, outcome for individuals and used in a subset of participants. If and when future biological markers become available, it would be necessary to have a clear ability to measure progression if they were to be included.
There was debate about all other domains being core. Overall it was agreed that, although each domain is important, ADLs, neuropsychiatric, quality of life and global are not core domains that should be selected dependent on the type of study, and we have recommended the DAD, NPI, DEMQOL and CDR, respectively.
We also discussed the issue of informant-rated scales, and how the informant needs to have seen the person regularly in the weeks prior to completing the scale, so, therefore, the availability of information for each participant is required in study design.
After the conference
With the core recommendations from the conference, we conducted a second electronic consultation with the AS Research Network, the methods and results of which are detailed in Chapters 5 and 6. This additional consultation confirmed the main recommendations from the conference.
Chapter 8 Discussion
Findings
The main conclusions from the synthesis of the information at the consensus conference were that both cognition and biological outcome measures should be included in the core set of outcomes, although all domains are thought to be important. Most of the trials identified in the systematic review included only participants with AD, so our recommendations are mainly for AD.
Cognition is included as a core domain because it is the fundamental symptom of dementia, and there would be no purpose in modifying the underlying disease if this did not lead to cognition in an intervention group being better than in a control group over time. The biological outcome measures are core as they are the only way of being sure that the disease is being modified rather than the treatment being purely symptomatic.
The PPI conclusions are that the most important consideration for people with dementia and their carers are not what measures are used, but the overall burden of the study in terms of time, travel and inconvenience.
Recommendations of outcomes
Core domains
Within the cognitive domain, we recommend the use of either the ADAS-Cog or MMSE, as both have the best psychometrics of the included measures. Cognitive measures were described as often distressing, by both people with dementia and their families within the PPI consultation, as people are often aware that they are deteriorating, although as cognition is a core symptom of dementia it not possible to avoid measuring it. As one of the purposes of defining core measures is to make individual studies comparable and meta-analysable, it would be helpful for a future study to formulate an algorithm to be able to compare scores on both the ADAS-Cog and the MMSE and to consider developing algorithms specifically for individual subtypes of dementia. This has recently been accomplished for the ACE-3 (Addenbrooke’s Cognitive Examination – third edition)471 and the MMSE (Professor Gill Livingston, University College London, 4 April 2016, personal communication). This should be possible as a recently published study of the longitudinal cognitive decline of mild to moderate AD participants in placebo arms of 20 trials found the trajectories of the ADAS-Cog and MMSE to be similar. 472 We have longitudinal data from an earlier study in which 224 participants completed the ADAS-Cog and the MMSE and for whom, therefore, data are available. 473
For biological outcomes it was agreed that serial structural MRI currently offers the best indication in a biological outcome of disease progression, although it is not a perfect biomarker. As it would not require as large a number as a cognitive outcome for satisfactory power, we recommend MRI as a core outcome, but only as an optional part of the study. Therefore, people who want to be in the study but could not or would not undergo MRI could be included. Some people are unable to undergo MRI because they have a pacemaker or a fear of enclosed spaces or because it is too far for them to travel to the MRI scanner. Thus, not wanting to undergo MRI should not put people off participating.
To power a full-scale trial on MRI, an early paper recommends 200 participants per trial arm,474 and a more recent paper reduced this to 81 participants per trial arm. 475 Despite needing a reduced number to power a trial on MRI, the potential costs of both baseline and follow-up scans in a group of 162 participants are expensive. The commercial cost of MRI in research starts from £365.76, although the NHS tariff is around £110.
Non-core domains
The assumption of a recommendation of a core set of outcomes is that measures can be added to it, but none can be taken away. Although we do not judge the domains of ADLs, global, neuropsychiatric symptoms and quality of life to be core, they are still important outcomes and their need to be included as a chosen outcome depends on the specific focus of individual trials. We have made recommendations about which measures we would recommend within each of these domains. In addition, a decline in social engagement can be one of the most important outcomes in mild to moderate dementia. There are no current measures for this in the literature in dementia, but one has been developed (Gill Livingston, University College London, 10 May 2016, personal communication; Sommerlad et al. 476).
We judge ADLs to be an important domain, as functional impairment increases as dementia progresses, it is often one of the first areas where symptoms are noticed and many problems within families arise as people with dementia become more dependent but do not want to accept help. 477 We recommend that the measure used is a proxy measure as people with dementia can sometimes underestimate their functional impairment, although sometimes they can be more accurate than a proxy (Linda Clare, University of Exeter, 25 May 2016, personal communication; Martyr and Clare478). As described in Chapter 6, Standardised interviews, Activities of daily living, in view of the time taken, the acceptability and the psychometric properties, we recommend that the DAD, a dementia-specific proxy ADL measure, is the best instrument to use.
Global outcomes are also important as they encompass and summarise the range of functions affected by dementia, with a staging instrument thought to be better for disease modification trials than a multidomain or clinician impression scale. For global outcomes we recommend the CDR, a staging instrument specific to dementia, as it has the most satisfactory psychometric properties. In particular, we recommend the use of the global CDR score over the sum of boxes, as using the sum of boxes makes the CDR more of a multidomain scale rather than a staging one.
Neuropsychiatric symptoms are also an important domain, as most people with dementia will experience at least one symptom over the course of their dementia. However, although important, they do not change in line with disease progression; many neuropsychiatric symptoms are no worse or may improve as dementia progresses from moderate to severe and, thus, the use of these symptoms to measure disease progression might be misleading. They are distressing for people with dementia, their family and those around them, and often lead to care home admission. Within this category we are therefore recommending that triallists use the NPI, as this has acceptable psychometric properties in this domain and is the only measure included in ongoing disease modification trials.
The domain of quality of life is important, as if, for example, a pharmacological intervention has a positive impact on cognition, it is also possible that it would have a negative impact on quality of life, and then it would be important to record this. From the conference we recommend using a dementia-specific measure, the DEMQOL, as it has adequate psychometric properties and coverage of the domain. In addition, it can be collected from both the person with dementia and a proxy.
It was suggested that we should have different outcomes recommended for different types of dementia, particularly with cognitive tests, which are the most sensitive to the change within the specific cognitive domain that is key to a particular type of dementia. However, because the majority of studies include only participants with AD, it is not possible to make recommendations for the specific types of dementia. This is something to be considered in future trials of disease-modifying drugs.
It was also suggested that we should have different core outcomes for different phases of trials (e.g. we could recommend different measures within the domain for different phases of trials). However, the three phases of trials do not always translate to non-pharmacological intervention trials.
We did not include outcomes that related solely to economics, carers or drug levels. Although all three may be important outcomes in a disease modification trial, they are not necessarily core. Health economics is particularly relevant in Phase III trials, requiring data on costs and QALYs. We also did not include measures of side effects, which were mentioned across all three focus groups. These will always be measured as they are as relevant as measuring how effective a pharmacological intervention is.
Recommendations around completing outcomes
To ensure that side effects are measured accurately, an informant may also need to participate in the study. Having an informant for each participant would also be useful to ensure that, if the intervention is pharmacological, it is taken in adherence with the protocol, as medication management can be difficult for people with dementia, and often caregivers provide support. 479,480
Informants are also often involved in the completion of outcomes on behalf of the person with dementia they care for. There was discussion around who completes outcomes at both the PPI consultation and consensus conference and, although it is important that people with dementia should be enabled to complete measures, informal carers also need to be involved. This is due to the nature of dementia, including deterioration. Although this can mean that people with dementia who wish to participate in research are excluded because they do not have a defined carer, it is by definition the case that most people living with mild to moderate dementia have someone helping to care for them in some capacity, although not necessarily living with them.
As we are recommending some measures that are informant based, either partly or fully, there also needs to be a way to standardise who the informant is that is completing outcomes. It is important to consider how pragmatic researchers can and should be in their identification of an informant (e.g. does this have to be a carer/can this be a neighbour/friend/health professional). An informant should also be the most reliable person available, in terms of both the information they give and their ability to commit to the full duration of the trial,481 as when an informant is changed between study visits, the answers on outcomes show greater variability than when the informant is the same. 482 Future research should also consider how to establish robust methods of using informant-supplied information for the completion of questionnaires, and also how to supplement responses from participants, as this could be very useful in terms of ensuring full data collection of all items and has the potential to expand the range of participants who can contribute to this research area.
As part of the project, we also considered the practicalities of participants completing the core set of outcomes, and how we can minimise any problems. We recommend that an outcome package at the very maximum should take no longer than 90 minutes without a break for a person with dementia, which was accepted by the people consulted within the PPI group. Researchers should aim for shorter time periods, particularly dependent on the person’s comorbid health conditions and how they are on the day. The people consulted at the focus groups also accepted that long assessments could take place over 1 day but be split into several time periods, with breaks and refreshments, if necessary.
They also want researchers to be clear about why assessments are being done as this would allow them to feel that their time is being well used. Despite giving informed or consultee consent at the beginning of the trial, people are required to be reminded of participation and what it involves at the time the assessments are happening. This is in line with process consent,483 which is the idea that consent runs throughout the whole research process, not just at the start of the trial when informant consent is taken, and is of particular importance in dementia research as a participant may lose capacity over the course of the trial period.
Patient and public involvement
A small but growing literature484–486 suggests that using qualitative methods to examine participant perspectives can provide triallists with important insights that can inform the design and implementation of clinical trials. A proactive approach to consider the design and development of clinical trials is needed at this early stage in the development of potentially disease course-modifying treatments that respond to the wider challenges in the design and implementation of clinical trials, and also to understand the needs, hopes and expectations of this patient population and their families.
The experiences and beliefs of our PPI group broadly reflect the wider literature. Reasons for refusals to participate appear to vary according to the type of trial and the severity of treatment,487 with key factors including inconvenience, difficulties with transport, too many clinic visits and time taken, as well as a distrust of medicine or the hospital and worries about side effects. 488,489 Much of the literature examining trial participation has identified altruism, trust in recruiting clinicians and an expectation of personal benefit as the main motivations for participation in trials. 490,491
However, altruism may be overstated as a motivation for participants, and a small but increasing number of studies using qualitative methods to examine the perspectives of clinical trial participants484–486 have concluded that patients participate in a clinical trial because they believe that they are receiving personalised care. One study486 found that, even when participants recalled the involvement of chance, most also held other coexisting (and sometimes contradictory) views about how and why they had been allocated to the treatment or intervention and believed that they would receive the best treatment for them. Similarly, a number of studies have identified that personal benefits, hope, access to the most effective treatment,492 and the enthusiasm and hopes of family and friends were often the driving force behind and key motivations for participation. 490 Such beliefs highlight the vulnerability of some groups and the risks that they are willing to take if there is a chance of survival,493 and this may be an important factor for trials involving potentially disease course-modifying treatments.
Future of biomarkers
Although outcomes, such as cognition, give an indication of clinical benefit, they do not necessarily demonstrate if an intervention has disease-modifying properties, whereas a biomarker could. 494 Change in current biomarkers of AD, in particular, does not always translate to changes in disease, and the improvement of current and development of new biomarkers are the key challenges in working towards a disease-modifying treatment, even more so with the development of adaptive trial designs. 19 Sensitive biomarkers could enable trials of potential disease-modifying interventions to be shorter, as they would be able to detect changes in disease progression; however, the development and validation of biomarkers takes time. 495 The development of biomarkers for dementia, particularly AD, is a hugely innovative field, meaning that it may be important to consider other biomarkers as core outcomes in the future if they are objective and reliable enough to show a potential disease-modifying effect. Ultimately, the choice of a biological marker depends, in a pharmacological study, on the compound being tested, and also the resources available.
When biomarkers are to be used in disease modification trials, it is also important to consider the impact this will have on participants’ willingness to take part in the research. From the PPI consultation, participants were generally enthusiastic about potentially invasive biological tests, but did also highlight how they may put off potential participants because of side effects, misconceptions and anxiety. They supported our recommendation that MRI should be optional rather than compulsory, and indicated that this would make them more willing to take part in a trial. As biomarkers develop, consideration will need to be given to reducing the side effects of the biological techniques, particularly CSF examination, as 30% of people in memory clinics report side effects, although very rarely are these serious or long-lasting problems. 496
Previous outcome recommendations
There have been previous recommendations related to the outcomes that should be used in disease modification trials. The current US Food and Drug Administration guidelines for all types of clinical trials for AD are that treatments must show efficacy on both a cognitive and a functional or global outcome measure. 497 For disease modification trials, in particular, the US Food and Drug Administration draft guidelines recommend that, as well as a benefit on clinical outcomes, a disease-modifying effect could be shown via a reliable biomarker, or by an adaptive trial design, such as a randomised start design, that can determine a long-lasting effect on disease course. Furthermore, the European Medicines Agency has recently published draft guidelines around medical trials of treatments for dementia. 498 These state that disease modification should be demonstrated by a slowing decline of clinical symptoms and also be linked to a change in validated biomarkers that reflect underlying disease pathology. These recommendations are in line with, but not as detailed as, our recommendations. Around a decade ago, a European task force reached consensus around a number of topics in dementia trials, including recommendations for the end points of disease modification trials. 499,500 They recommended measuring cognition, ADLs and global functioning as core, and within the domains, respectively, recommended the measures of the ADAS-Cog, ADCS-ADL and CDR sum of boxes.
Strengths and limitations
As part of the project, we have incorporated other research from members of our group around the development of core outcome sets for dementia from perspectives other than disease modification. The first is the recently published standard set of outcomes of what matters the most to people living with dementia, developed by ICHOM. 501 The second is funded by the JPND, and was a project to develop a set of core outcomes to be used in psychosocial intervention trials of dementia. 502,503 We also used references gathered for a project about non-pharmacological interventions for dementia. We conducted additional searches on top of those specified in the protocol, including for ongoing trials on two clinical trial registries.
We conducted both e-mail and face-to-face consultations with people living with dementia and carers, and gained a wide variety of views around completing outcomes from the 18 participants. Although the feedback from the PPI group was very useful, we conducted focus groups only in three locations, and the overall number of people consulted was small, even including the second e-mail consultation. In addition, all participants were part of the AS Research Network and/or a dementia PPI group, and were knowledgeable about dementia. Although this meant that they were able to give informed views, they did not cover the possible range of opinions, and only one-third of the participants had experience of participating in research. Furthermore, context makes a huge difference, and advocacy group members often have different ideas from the patient and carers attending memory clinics. The background of participants can also influence ideas, with the majority of those who participate in PPI being from middle-class backgrounds and of white ethnicity and thus not necessarily reflective of wider society,504 which is a potential limitation within our PPI sample. In addition, we did not conduct detailed, in-depth, open-ended interviews and, therefore, the information we gained was limited. Although there were three research team members present in each location, with differing backgrounds (so views were not filtered through a single researcher’s perspective), we did not do formal and independent thematic analyses. With more time and funding we would like to have further widened the consultation to include, for example, the clinical research nurses who often run dementia trials.
Many of the opinions arising from this consultation were contradictory. Thus, people suggested that they wanted more importance on interpreting scores within the context of an individual’s experiences and individualised instruments with more detail, but also wanted testing to be of shorter duration with fewer questions. Overall, it appeared that people were saying that they wanted to be recognised as a full person with an illness rather than to be summed up by declining scores on questionnaires. This is an important message for researchers, although most will think they do this already, as well as being the only acceptable way for health professionals to practise. Contextual information is of great importance in clinical practice and, although it should be considered in trials, it is of less relevance if participants have been randomised as those in each group have been matched for individual differences.
We included outcomes that are informant rated, which will limit people living with dementia taking part if they do not have an informant available. We also included only outcomes that have been or are currently being used in trials. Outcomes are not perfect and there may be better measures in the future. It has been suggested that one problem for disease modification trials is that outcomes need to be very sensitive in the early stages of dementia, when subtle and slow changes occur. 495 However, most people with dementia do deteriorate cognitively over the period of trials, and drugs which modify the disease need to be able to show a clinically important difference in cognitive deterioration, which would be seen in current measures. This may not be true of MCI, but this was not part of the study presented here.
This project was delivered in 6 months with a very limited budget, so we included only, as specified in the brief, mild to moderate dementia. We cannot make recommendations for disease modification trials involving other stages of dementia, such as severe, prodromal or mild cognitive impairment. Work has been completed around more appropriate measures to be used in other stages, such as those with dementia living in long-term care. 505 Similarly, we are unable to make recommendations for separate types of dementia, such as vascular or frontotemporal dementia, as most of the trials included only participants with AD. This is potentially a limitation of the outcome set for use in disease modification trials not focusing on participants with AD; although as AD is the most common type of dementia, the outcome set would reflect those most affected. If, and when, a disease modification treatment for AD is found, this could significantly decrease the burden of dementia on public health by up to 50%, although more work is needed to address the disease modification of the less prevalent types of dementia and reduce the burden of these. 506
We have also not had time or resources to consult with regulators and pharmaceutical industries; however, we have plans to do this in the future, possibly through collaboration with Alzheimer’s Research UK. Similarly, we did not consider economic measures as outcomes and did not include health economists on our team. This was because of the limitation in time and resources and the commissioning brief. Our recommendations did not consider the financial cost in detail although it was mentioned in the discussion. We did not attempt a cost analysis but are aware that MRI is expensive. If there are better biological measures in the future this recommendation will be superseded.
We included the highest standard of trials, RCTs and then CCTs, that compared the intervention with a control or another intervention. This means that we will have missed early-stage disease modification trials, in which the dosage and safety are the main focuses, rather than the efficacy from the comparison of interventions with each other or control groups. Despite including both ongoing and published trials, we may also have missed a number of trials that have been completed but not yet published in an academic journal article, whether a protocol or the study results. We did, however, conduct a large-scale systematic review, screening 22,918 references, in the hope of picking up the majority of relevant trials. Most of the trials included were conducted in English-speaking countries, with a large proportion conducted solely or partly in the USA. We included many trials from countries where the native language was not English as long as the trial was reported in English, and have reported whether or not validation of the measures has been carried out in other languages, but are unable to make definitive recommendations about measures in other languages. We excluded 30 full-text articles because they were not available in English.
Chapter 9 Conclusions
At present there are 81 different outcome measures used across disease modification trials, if we include published and ongoing trials. These include 72 questionnaire- or interview-based outcomes and nine techniques for measuring biological markers. This illustrates the importance and difficulty of the development of a consensus about a core set of the best available measures in allowing the comparison and combination of disease modification trials.
The main conclusions from the synthesis of all the information at the consensus conference were that cognition and biological markers are the only core outcome domains. For cognition we recommend the ADAS-Cog or MMSE, and for a biological marker we recommend using MRI as an outcome, which is optional for participants. This means that those who are unable or do not wish to undergo MRI can still participate. Other biological outcome markers would be appropriate depending on the proposed mechanism of action of the therapy, and selecting a universal set of biomarker outcome measures for all studies was not possible. We have also made outcome recommendations for the important, but non-core, domains of ADLs, global functioning, neuropsychiatric and quality of life, respectively, recommending the DAD, CDR, NPI and DEMQOL.
We have reached our conclusions and recommendations through discussion at the consensus conference based on the psychometric properties and suitability of the outcomes found within the systematic review, in discussion with expert researchers and clinicians, and informed by the voluntary sector and patients and families. We also considered the practicalities of the completion of the core outcome set, including timing, breaks, travel and if the person with dementia and/or an informant completes the measures.
As the recommended measures are currently the best available, we expect that additional or alternative outcome measures may supersede the current core set, particularly for biological markers where there is considerable current research. The principles on which we have made our choices are laid out here and they will not change.
Recommendations for future research
The recommendations throughout this report are in the most part for AD; therefore, there is a need to extend the development of core outcome sets for other dementias. As we are recommending the use of either the ADAS-Cog or MMSE, which are the most commonly used cognitive outcomes in the included disease modification trials, and we wish to be able to directly compare scores on each item, it would be useful to develop an algorithm to directly compare scores on both. Patients also feel that they are failing in tests of cognition as they notice their deterioration over time. It was suggested that timed measures, which estimate cognitive processing speed, should be further developed and validated against other measures, so that they can be considered in terms of clinical significance and minimal clinically important difference. These would mean that patients would be able to complete cognitive scales where they would not feel like they were failing.
We are also recommending measures that are partly or fully rated by informants, so it would also be useful to consider ways of making informant data more robust, including thinking about who the most appropriate informant is. We did not make any economic recommendations; however, future research could consider both QALYs and cost data.
Through PPI we gathered a wide variety of feedback about outcomes and trial participation; however, this was not a full qualitative study and only a small number of people participated, which highlights the need for further qualitative research. This could also include consultations with trial staff, such as clinical research nurses, and to see how those that administer outcomes can influence participants’ attitudes towards them.
Acknowledgements
We would like to thank the AS for providing support for the project through Anna Grinbergs-Saull’s time and for allowing access to the AS Research Network. We would also like to thank those who participated in the PPI.
Patient and public involvement
We consulted 18 people, either those living with dementia or family caregivers, to inform the recommendations of the core outcome set.
Contributions of authors
Lucy Webster, Anna Grinbergs-Saull and Gill Livingston drafted the report.
Lucy Webster, Derek Groskreutz and Anna Grinbergs-Saull screened papers, located references and extracted data.
Rob Howard, John T O’Brien, Gail Mountain, Sube Banerjee, Bob Woods, Robert Perneczky and Gill Livingston acted as champions for each of the domains in the review of outcomes.
Lucy Webster, Anna Grinbergs-Saull, John T O’Brien, Gail Mountain and Gill Livingston facilitated the PPI focus groups.
Gill Livingston is guarantor.
Derek Groskreutz, Rob Howard, John T O’Brien, Gail Mountain, Sube Banerjee, Bob Woods, Robert Perneczky, Louise Lafortune, Charlotte Roberts, Jenny McCleery, James Pickett, Frances Bunn, David Challis, Georgina Charlesworth, Katie Featherstone, Chris Fox, Claire Goodman, Roy Jones, Sallie Lamb, Esme Moniz-Cook, Justine Schneider, Sasha Shepperd, Claire Surr, Jo Thompson-Coon, Clive Ballard, Carol Brayne, Orlaith Burke, Alistair Burns, Linda Clare, Peter Garrard, Patrick Kehoe, Peter Passmore, Clive Holmes, Ian Maidment, Fliss Murtagh and Louise Robinson revised the paper critically for important intellectual content.
Data sharing statement
All data from the project are included in the report. Any further information can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Prince M, Knapp M, Guerchet M, Mccrone P, Prina M, Comas Herara A, et al. Dementia UK: Update. London: Alzheimer’s Society; 2014.
- Lewis F. Estimation of Future Cases of Dementia from those Born in 2015. London: Office of Health Economics Consulting Reports; 2015.
- Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 2013;382:1405-12. http://dx.doi.org/10.1016/S0140-6736(13)61570-6.
- Matthews FE, Stephan BC, Robinson L, Jagger C, Barnes LE, Arthur A, et al. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun 2016;7. http://dx.doi.org/10.1038/ncomms11398.
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014;13:788-94. http://dx.doi.org/10.1016/S1474-4422(14)70136-X.
- First WHO Ministerial Conference on Global Action Against Dementia: Meeting Report, WHO Headquarters, Geneva, Switzerland, 16–17 March 2015. Geneva: World Health Organization; 2015.
- Mukadam N, Livingston G, Rantell K, Rickman S. Diagnostic rates and treatment of dementia before and after launch of a national dementia policy: an observational study using English national databases. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-004119.
- Prince M, Bryce R, Ferri C. World Alzheimer Report 2011: The Benefits of Early Diagnosis and Intervention. London: Alzheimer’s Disease International; 2011.
- NIHR . ISRCTN93682878 Reducing Pathology in Alzheimer’s Disease Through Angiotensin Targeting 2015. www.isrctn.com/ISRCTN93682878 (accessed 22 January 2016).
- Medical Research Council/NIHR Efficacy and Mechanism Evaluation Programme . ISRCTN16105064 Minocycline in Alzheimer’s Disease 2013. www.isrctn.com/ISRCTN16105064 (accessed 22 January 2016).
- Researching Alzheimer’s Medicines: Setbacks and Stepping Stones. Washington, DC: Pharmaceutical Research and Manufacturers of America; 2012.
- Livingston G, Katona C. The place of memantine in the treatment of Alzheimer’s disease: a number needed to treat analysis. Int J Geriatr Psychiatry 2004;19:919-25. http://dx.doi.org/10.1002/gps.1166.
- Ghezzi L, Scarpini E, Galimberti D. Disease-modifying drugs in Alzheimer’s disease. Drug Des Devel Ther 2013;7:1471-8. http://dx.doi.org/10.2147/DDDT.S41431.
- Lewis F, Karlsberg Schaffer S, Sussex J. The Trajectory of Dementia in the UK – Making a Difference. London: Office of Health Economics Consulting Reports; 2014.
- G8 Dementia Summit Declaration. London: Department of Health; 2013.
- Zoda TE. New standards in Alzheimer’s disease trial design. Therapeutics J Clin Studies 2014;6:44-5.
- Rubinstein E, Duggan C, Van Landingham B, Thompson D, Warburton W. A Call to Action: The Global Response to Dementia Through Policy Innovation. Doha: World Innovation Summit for Health; 2015.
- Long R. Challenges to Finding Treatments for Dementia. London: Department of Health; 2015.
- Gauthier S, Albert M, Fox N, Goedert M, Kivipelto M, Mestre-Ferrandiz J, et al. Why has therapy development for dementia failed in the last two decades?. Alzheimers Dement 2016;12:60-4. http://dx.doi.org/10.1016/j.jalz.2015.12.003.
- Porter ME, Larsson S, Lee TH. Standardizing patient outcomes measurement. N Engl J Med 2016;374:504-6. http://dx.doi.org/10.1056/NEJMp1511701.
- Lawrence V, Pickett J, Ballard C, Murray J. Patient and carer views on participating in clinical trials for prodromal Alzheimer’s disease and mild cognitive impairment. Int J Geriatr Psychiatry 2014;29:22-31. http://dx.doi.org/10.1002/gps.3958.
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2535.
- Leeds Beckett University . ‘What Works’ in Dementia Education and Training? n.d. www.leedsbeckett.ac.uk/pages/what-works/ (accessed 11 April 2016).
- University of Colorado Denver . NCT01409915 Study of the Safety &Amp; Efficacy of Leukine® in the Treatment of Alzheimer’s Disease 2016. https://clinicaltrials.gov/ct2/show/NCT01409915 (accessed 29 January 2016).
- Aisen PS. Anti-inflammatory therapy for Alzheimer’s disease: implications of the prednisone trial. Acta Neurol Scand Suppl 2000;176:85-9. https://doi.org/10.1034/j.1600-0404.2000.00312.x.
- Aisen PS, Davis KL, Berg JD, Schafer K, Campbell K, Thomas RG, et al. A randomized controlled trial of prednisone in Alzheimer’s disease. Alzheimer’s Disease Cooperative Study. Neurology 2000;54:588-93. https://doi.org/10.1212/WNL.54.3.588.
- Aisen PS, Schmeidler J, Pasinetti GM. Randomized pilot study of nimesulide treatment in Alzheimer’s disease. Neurology 2002;58:1050-4. https://doi.org/10.1212/WNL.58.7.1050.
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 2003;289:2819-26. http://dx.doi.org/10.1001/jama.289.21.2819.
- Aisen PS, Saumier D, Briand R, Laurin J, Gervais F, Tremblay P, et al. A Phase II study targeting amyloid-beta with 3APS in mild to moderate Alzheimer disease. Neurology 2006;67:1757-63. https://doi.org/10.1212/01.wnl.0000244346.08950.64.
- Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994.
- Aisen PS, Gauthier S, Vellas B, Briand R, Saumier D, Laurin J, et al. Alzhemed: a potential treatment for Alzheimer’s disease. Curr Alzheimer Res 2007;4:473-8. https://doi.org/10.2174/156720507781788882.
- Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA 2008;300:1774-83. http://dx.doi.org/10.1001/jama.300.15.1774.
- National Institute of Aging, the General Clinical Research Center Programme . High-dose vitamin B does not slow cognitive decline in AD. Brown Uni Geriatr Psychopharmacol Update 2008;12.
- Viswanathan A. High-dose B vitamin supplementation as a disease-modifying therapy in Alzheimer disease. Arch Neurol 2009;66:520-2. http://dx.doi.org/10.1001/archneurol.2009.12.
- Aisen PS, Gauthier S, Ferris SH, Saumier D, Haine D, Garceau D, et al. Tramiprosate in mild to moderate Alzheimer’s disease – a randomized, double-blind, placebo-controlled, multi-centre study (the Alphase Study). Arch Med Sci 2011;7:102-11. http://dx.doi.org/10.5114/aoms.2011.20612.
- Gauthier S, Aisen PS, Ferris SH, Saumier D, Duong A, Haine D, et al. Effect of tramiprosate in patients with mild to moderate Alzheimer’s disease: exploratory analyses of the MRI sub-group of the Alphase study. J Nutr Health Aging 2009;13:550-7. https://doi.org/10.1007/s12603-009-0106-x.
- Saumier D, Duong A, Haine D, Garceau D, Sampalis J. Domain-specific cognitive effects of tramiprosate in patients with mild to moderate Alzheimer’s disease: ADAS-cog subscale results from the Alphase Study. J Nutr Health Aging 2009;13:808-12. https://doi.org/10.1007/s12603-009-0217-4.
- Akhondzadeh S, Shafiee Sabet M, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, et al. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild to moderate Alzheimer’s disease. Psychopharmacology 2010;207:637-43. http://dx.doi.org/10.1007/s00213-009-1706-1.
- Akhondzadeh S, Sabet MS, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, et al. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: a 16-week, randomized and placebo-controlled trial. J Clin Pharm Ther 2010;35:581-8. http://dx.doi.org/10.1111/j.1365-2710.2009.01133.x.
- Alvarez XA, Pichel V, Pérez P, Laredo M, Corzo D, Zas R, et al. Double-blind, randomized, placebo-controlled pilot study with Anapsos in senile dementia: effects on cognition, brain bioelectrical activity and cerebral hemodynamics. Methods Find Exp Clin Pharmacol 2000;22:585-94.
- Alvarez XA, Cacabelos R, Laredo M, Couceiro V, Sampedro C, Varela M, et al. A 24-week, double-blind, placebo-controlled study of three dosages of Cerebrolysin in patients with mild to moderate Alzheimer’s disease. Eur J Neurol 2006;13:43-54. https://doi.org/10.1111/j.1468-1331.2006.01222.x.
- Alvarez XA, Cacabelos R, Sampedro C, Aleixandre M, Linares C, Granizo E, et al. Efficacy and safety of Cerebrolysin in moderate to moderately severe Alzheimer’s disease: results of a randomized, double-blind, controlled trial investigating three dosages of Cerebrolysin. Eur J Neurol 2011;18:59-68. http://dx.doi.org/10.1111/j.1468-1331.2010.03092.x.
- Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen CP, et al. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology 1999;24:657-77. https://doi.org/10.1016/S0306-4530(99)00020-7.
- Babiloni C, Frisoni GB, Del Percio C, Zanetti O, Bonomini C, Cassetta E, et al. Ibuprofen treatment modifies cortical sources of EEG rhythms in mild Alzheimer’s disease. Clin Neurophysiol 2009;120:709-18. http://dx.doi.org/10.1016/j.clinph.2009.02.005.
- Pasqualetti P, Bonomini C, Dal Forno G, Paulon L, Sinforiani E, Marra C, et al. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer’s disease. Aging Clin Exp Res 2009;21:102-10. https://doi.org/10.1007/BF03325217.
- Bae CY, Cho CY, Cho K, Hoon Oh B, Choi KG, Lee HS, et al. A double-blind, placebo-controlled, multicenter study of Cerebrolysin for Alzheimer’s disease. J Am Geriatr Soc 2000;48:1566-71. https://doi.org/10.1111/j.1532-5415.2000.tb03865.x.
- Ban TA, Morey L, Aguglia E, Azzarelli O, Balsano F, Marigliano V, et al. Nimodipine in the treatment of old age dementias. Prog Neuropsychopharmacol Biol Psychiatry 1990;14:525-51. https://doi.org/10.1016/0278-5846(90)90005-2.
- Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1987.
- Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, et al. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology 2005;64:94-101. https://doi.org/10.1212/01.WNL.0000148604.77591.67.
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet 2008;372:216-23. http://dx.doi.org/10.1016/S0140-6736(08)61075-2.
- Bentham P, Gray R, Sellwood E, Hills R, Crome P, Raftery J. AD2000 Collaborative Group . Aspirin in Alzheimer’s disease (AD2000): a randomised open-label trial. Lancet Neurol 2008;7:41-9. https://doi.org/10.1016/S1474-4422(07)70293-4.
- Bilikiewicz A, Gaus W. Colostrinin (a naturally occurring, proline-rich, polypeptide mixture) in the treatment of Alzheimer’s disease. J Alzheimers Dis 2004;6:17-26.
- Black RS, Sperling RA, Safirstein B, Motter RN, Pallay A, Nichols A, et al. A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Dis Assoc Disord 2010;24:198-203. http://dx.doi.org/10.1097/WAD.0b013e3181c53b00.
- Blennow K, Zetterberg H, Rinne JO, Salloway S, Wei J, Black R, et al. Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol 2012;69:1002-10. https://doi.org/10.1001/archneurol.2012.90.
- Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol 2010;9:363-72. http://dx.doi.org/10.1016/S1474-4422(10)70043-0.
- Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 2009;73:2061-70. http://dx.doi.org/10.1212/WNL.0b013e3181c67808.
- Bowen RL, Perry G, Xiong C, Smith MA, Atwood CS. A clinical study of Lupron depot in the treatment of women with Alzheimer’s disease: preservation of cognitive function in patients taking an acetylcholinesterase inhibitor and treated with high dose Lupron over 48 weeks. J Alzheimers Dis 2015;44:549-60. http://dx.doi.org/10.3233/JAD-141626.
- Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, et al. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis 2015;44:897-906.
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 2012;69:29-38. http://dx.doi.org/10.1001/archneurol.2011.233.
- Crapper McLachlan DR, Dalton AJ, Kruck TP, Bell MY, Smith WL, Kalow W, et al. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 1991;337:1304-8. https://doi.org/10.1016/0140-6736(91)92978-B.
- McLachlan DR, Smith WL, Kruck TP. Desferrioxamine and Alzheimer’s disease: video home behavior assessment of clinical course and measures of brain aluminium. Ther Drug Monit 1993;15:602-7. https://doi.org/10.1097/00007691-199312000-00027.
- Cucinotta D, De Leo D, Frattola L, Trabucchi M, Albizatti MG, Beltramelli A, et al. Dihydroergocryptine as long-term treatment of Alzheimer type dementia: a multicenter two-year follow-up. Arch Gerontol Geriatr 1998;27:103-10. https://doi.org/10.1016/S0167-4943(98)80018-6.
- Dodel R, Rominger A, Bartenstein P, Barkhof F, Blennow K, Förster S, et al. Intravenous immunoglobulin for treatment of mild to moderate Alzheimer’s disease: a phase 2, randomised, double-blind, placebo-controlled, dose-finding trial. Lancet Neurol 2013;12:233-43. http://dx.doi.org/10.1016/S1474-4422(13)70014-0.
- Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, et al. Effect of Dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild to moderate Alzheimer’s disease: a randomised, double-blind, placebo-controlled study. Lancet 2008;372:207-15. http://dx.doi.org/10.1016/S0140-6736(08)61074-0.
- Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med 2013;369:341-50. http://dx.doi.org/10.1056/NEJMoa1210951.
- Doody RS, Raman R, Sperling RA, Seimers E, Sethuraman G, Mohs R, et al. Peripheral and central effects of γ-secretase inhibition by semagacestat in Alzheimer’s disease. Alzheimers Res Ther 2015;7. http://dx.doi.org/10.1186/s13195-015-0121-6.
- Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild to moderate Alzheimer’s disease. N Engl J Med 2014;370:311-21. http://dx.doi.org/10.1056/NEJMoa1312889.
- Liu-Seifert H, Siemers E, Holdridge KC, Andersen SW, Lipkovich I, Carlson C, et al. Delayed-start analysis: mild Alzheimer’s disease patients in solanezumab trials, 3.5 years. Alzheimers Dement Translat Res Clin Intervent 2015;1:111-21. https://doi.org/10.1016/j.trci.2015.06.006.
- Endres K, Fahrenholz F, Lotz J, Hiemke C, Teipel S, Lieb K, et al. Increased CSF APPs-α levels in patients with Alzheimer disease treated with acitretin. Neurology 2014;83:1930-5. http://dx.doi.org/10.1212/WNL.0000000000001017.
- Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, et al. Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimers Dement 2012;8:261-71. http://dx.doi.org/10.1016/j.jalz.2011.09.224.
- Faux NG, Ritchie CW, Gunn A, Rembach A, Tsatsanis A, Bedo J, et al. PBT2 rapidly improves cognition in Alzheimer’s Disease: additional phase II analyses. J Alzheimers Dis 2010;20:509-16. http://dx.doi.org/10.3233/JAD-2010-1390.
- Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol 2008;7:779-86. http://dx.doi.org/10.1016/S1474-4422(08)70167-4.
- Faxén-Irving G, Freund-Levi Y, Eriksdotter-Jönhagen M, Basun H, Hjorth E, Palmblad J, et al. Effects on transthyretin in plasma and cerebrospinal fluid by DHA-rich n-3 fatty acid supplementation in patients with Alzheimer’s disease: the OmegAD study. J Alzheimers Dis 2013;36:1-6. http://dx.doi.org/10.3233/JAD-121828.
- Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, Basun H, Faxén-Irving G, Garlind A, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol 2006;63:1402-8. http://dx.doi.org/10.1001/archneur.63.10.1402.
- Ferrari E, Cucinotta D, Albizatti M, Bartorelli L, Colombo N, Ferretti G, et al. Effectiveness and safety of posatirelin in the treatment of senile dementia: a multicenter, double-blind, placebo-controlled study. Arch Gerontol Geriatr 1998;6:163-74. https://doi.org/10.1016/S0167-4943(98)80024-1.
- The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992.
- Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, Dean RA, et al. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch Neurol 2008;65:1031-8. http://dx.doi.org/10.1001/archneur.65.8.1031.
- Fleisher AS, Truran D, Mai JT, Langbaum JB, Aisen PS, Cummings JL, et al. Chronic divalproex sodium use and brain atrophy in Alzheimer disease. Neurology 2011;77:1263-71. http://dx.doi.org/10.1212/WNL.0b013e318230a16c.
- Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, et al. AN1792(QS-21)-201 Study . Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 2005;64:1563-72. https://doi.org/10.1212/01.WNL.0000159743.08996.99.
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005;64:1553-62. https://doi.org/10.1212/01.WNL.0000159740.16984.3C.
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Müller-Tillmanns B, et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron 2003;38:547-54. https://doi.org/10.1016/S0896-6273(03)00294-0.
- Koepsell TD, Chi YY, Zhou XH, Lee WW, Ramos EM, Kukull WA. An alternative method for estimating efficacy of the AN1792 vaccine for Alzheimer disease. Neurology 2007;69:1868-72. https://doi.org/10.1212/01.wnl.0000278226.96003.f8.
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003;61:46-54. https://doi.org/10.1212/01.WNL.0000073623.84147.A8.
- Vellas B, Black R, Thal LJ, Fox NC, Daniels M, McLennan G, et al. Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr Alzheimer Res 2009;6:144-51. https://doi.org/10.2174/156720509787602852.
- Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, et al. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol 2012;69:836-41. https://doi.org/10.1001/archneurol.2012.85.
- Galasko D, Bell J, Mancuso JY, Kupiec JW, Sabbagh MN, van Dyck C, et al. Clinical trial of an inhibitor of RAGE-Aβ interactions in Alzheimer disease. Neurology 2014;82:1536-42. http://dx.doi.org/10.1212/WNL.0000000000000364.
- Gauthier S, Rountree S, Finn B, LaPlante B, Weber E, Oltersdorf T. Effects of the acetylcholine release agent ST101 with donepezil in Alzheimer’s disease: a randomized phase 2 study. J Alzheimers Dis 2015;48:473-81. http://dx.doi.org/10.3233/JAD-150414.
- Geldmacher DS, Fritsch T, McClendon MJ, Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch Neurol 2011;68:45-50. http://dx.doi.org/10.1001/archneurol.2010.229.
- Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, et al. Rosiglitazone monotherapy in mild to moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord 2010;30:131-46. http://dx.doi.org/10.1159/000318845.
- Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, et al. Tarenflurbil Phase 3 Study Group . Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA 2009;302:2557-64. http://dx.doi.org/10.1001/jama.2009.1866.
- Myrexis, Inc . No significant effect of tarenflurbil on cognition in early Alzheimer’s disease. Brown Uni Geriatr Psychopharmacol Update 2010;14:1-6.
- Grimaldi LM, Zappalà G, Iemolo F, Castellano AE, Ruggieri S, Bruno G, et al. A pilot study on the use of interferon beta-1a in early Alzheimer’s disease subjects. J Neuroinflammation 2014;11. http://dx.doi.org/10.1186/1742-2094-11-30.
- Hampel H, Ewers M, Bürger K, Annas P, Mörtberg A, Bogstedt A, et al. Lithium trial in Alzheimer’s disease: a randomized, single-blind, placebo-controlled, multicenter 10-week study. J Clin Psychiatry 2009;70:922-31. https://doi.org/10.4088/JCP.08m04606.
- Hock C, Maddalena A, Raschig A, Müller-Spahn F, Eschweiler G, Hager K, et al. Treatment with the selective muscarinic m1 agonist talsaclidine decreases cerebrospinal fluid levels of A beta 42 in patients with Alzheimer’s disease. Amyloid 2003;10:1-6. https://doi.org/10.3109/13506120308995249.
- Hock C, Maddalena A, Heuser I, Naber D, Oertel W, von der Kammer H, et al. Treatment with the selective muscarinic agonist talsaclidine decreases cerebrospinal fluid levels of total amyloid beta-peptide in patients with Alzheimer’s disease. Ann N Y Acad Sci 2000;920:285-91. https://doi.org/10.1111/j.1749-6632.2000.tb06937.x.
- Jhee SS, Frackiewicz EJ, Tolbert D, Sainati S, Karim A, Zolnouni PP, et al. A pharmacokinetic, pharmacodynamic, and safety study of celecoxib in subjects with probable Alzheimer’s disease. Clin Res Regul Aff 2004;21:49-66. http://dx.doi.org/10.1081/CRP-120030034.
- de Jong D, Jansen R, Hoefnagels W, Jellesma-Eggenkamp M, Verbeek M, Borm G, et al. No effect of one-year treatment with indomethacin on Alzheimer’s disease progression: a randomized controlled trial. PLOS ONE 2008;3. http://dx.doi.org/10.1371/journal.pone.0001475.
- Kadir A, Andreasen N, Almkvist O, Wall A, Forsberg A, Engler H, et al. Effect of phenserine treatment on brain functional activity and amyloid in Alzheimer’s disease. Ann Neurol 2008;63:621-31. http://dx.doi.org/10.1002/ana.21345.
- Kessler H, Pajonk FG, Bach D, Schneider-Axmann T, Falkai P, Herrmann W, et al. Effect of copper intake on CSF parameters in patients with mild Alzheimer’s disease: a pilot phase 2 clinical trial. J Neural Transm 2008;115:1651-9. http://dx.doi.org/10.1007/s00702-008-0136-2.
- Kessler H, Bayer TA, Bach D, Schneider-Axmann T, Supprian T, Herrmann W, et al. Intake of copper has no effect on cognition in patients with mild Alzheimer’s disease: a pilot phase 2 clinical trial. J Neural Transm 2008;115:1181-7. http://dx.doi.org/10.1007/s00702-008-0080-1.
- Landen JW, Zhao Q, Cohen S, Borrie M, Woodward M, Billing CB, et al. Safety and pharmacology of a single intravenous dose of ponezumab in subjects with mild to moderate Alzheimer disease: a phase I, randomized, placebo-controlled, double-blind, dose-escalation study. Clin Neuropharmacol 2013;36:14-23. https://doi.org/10.1097/WNF.0b013e31827db49b.
- Leszek J, Inglot AD, Janusz M, Lisowski J, Krukowska K, Georgiades JA. Colostrinin: a proline-rich polypeptide (PRP) complex isolated from ovine colostrum for treatment of Alzheimer’s disease. A double-blind, placebo-controlled study. Arch Immunol Ther Exp 1999;47:377-85.
- Li N, Wang J, Ma J, Gu Z, Jiang C, Yu L, et al. Neuroprotective Effects of cistanches herba therapy on patients with moderate Alzheimer’s Disease. Evid Based Complement Alternat Med 2015;2015. http://dx.doi.org/10.1155/2015/103985.
- Lovestone S, Boada M, Dubois B, Hüll M, Rinne JO, Huppertz HJ, et al. A phase II trial of tideglusib in Alzheimer’s disease. J Alzheimers Dis 2015;45:75-88. http://dx.doi.org/10.3233/JAD-141959.
- Maher-Edwards G, De’Ath J, Barnett C, Lavrov A, Lockhart A. A 24-week study to evaluate the effect of rilapladib on cognition and cerebrospinal fluid biomarkers of Alzheimer’s disease. Alzheimers Dement Translat Res Clin Intervent 2015;1:131-40. https://doi.org/10.1016/j.trci.2015.06.003.
- Marcusson J, Rother M, Kittner B, Rössner M, Smith RJ, Babic T, et al. A 12-month, randomized, placebo-controlled trial of propentofylline (HWA 285) in patients with dementia according to DSM III-R. The European Propentofylline Study Group. Dement Geriatr Cogn Disord 1997;8:320-8. https://doi.org/10.1159/000106650.
- Molloy DW, Standish TI, Zhou Q, Guyatt G. DARAD Study Group . A multicenter, blinded, randomized, factorial controlled trial of doxycycline and rifampin for treatment of Alzheimer’s disease: the DARAD trial. Int J Geriatr Psychiatry 2013;28:463-70. http://dx.doi.org/10.1002/gps.3846.
- Muresanu DF, Rainer M, Moessler H. Improved global function and activities of daily living in patients with AD: a placebo-controlled clinical study with the neurotrophic agent Cerebrolysin. J Neural Transm Suppl 2002;62:277-85. https://doi.org/10.1007/978-3-7091-6139-5_25.
- Muresanu DF, Alvarez XA, Moessler H, Buia M, Stan A, Pintea D, et al. A pilot study to evaluate the effects of Cerebrolysin on cognition and qEEG in vascular dementia: cognitive improvement correlates with qEEG acceleration. J Neurol Sci 2008;267:112-19. https://doi.org/10.1016/j.jns.2007.10.016.
- Nygaard HB, Wagner AF, Bowen GS, Good SP, MacAvoy MG, Strittmatter KA, et al. A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer’s disease. Alzheimers Res Ther 2015;7. http://dx.doi.org/10.1186/s13195-015-0119-0.
- Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol 2012;69:198-207. http://dx.doi.org/10.1001/archneurol.2011.1538.
- Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA 2010;304:1903-11. http://dx.doi.org/10.1001/jama.2010.1510.
- Regland B, Lehmann W, Abedini I, Blennow K, Jonsson M, Karlsson I, et al. Treatment of Alzheimer’s disease with clioquinol. Dement Geriatr Cogn Disord 2001;12:408-14. https://doi.org/10.1159/000051288.
- Reines SA, Block GA, Morris JC, Liu G, Nessly ML, Lines CR, et al. Rofecoxib: no effect on Alzheimer’s disease in a 1-year, randomized, blinded, controlled study. Neurology 2004;62:66-71. https://doi.org/10.1212/WNL.62.1.66.
- Ringman JM, Frautschy SA, Teng E, Begum AN, Bardens J, Beigi M, et al. Oral curcumin for Alzheimer’s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res Ther 2012;4. http://dx.doi.org/10.1186/alzrt146.
- Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, et al. Metal–protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol 2003;60:1685-91. http://dx.doi.org/10.1001/archneur.60.12.1685.
- Rüther E, Ritter R, Apecechea M, Freytag S, Gmeinbauer R, Windisch M. Sustained improvements in patients with dementia of Alzheimer’s type (DAT) 6 months after termination of Cerebrolysin therapy. J Neural Transm 2000;107:815-29. http://dx.doi.org/10.1007/s007020070061.
- Rüther E, Ritter R, Apecechea M, Freytag S, Windisch M. Efficacy of the peptidergic nootropic drug cerebrolysin in patients with senile dementia of the Alzheimer type (SDAT). Pharmacopsychiatry 1994;27:32-40. http://dx.doi.org/10.1055/s-2007-1014271.
- Ruether E, Husmann R, Kinzler E, Diabl E, Klingler D, Spatt J, et al. A 28-week, double-blind, placebo-controlled study with Cerebrolysin in patients with mild to moderate Alzheimer’s disease. Int Clin Psychopharmacol 2001;16:253-63. https://doi.org/10.1097/00004850-200109000-00002.
- Ruether E, Alvarez XA, Rainer M, Moessler H. Sustained improvement of cognition and global function in patients with moderately severe Alzheimer’s disease: a double-blind, placebo-controlled study with the neurotrophic agent Cerebrolysin. J Neural Transm Suppl 2002;62:265-75. https://doi.org/10.1007/978-3-7091-6139-5_24.
- Salloway S, Sperling R, Keren R, Porsteinsson AP, van Dyck CH, Tariot PN, et al. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology 2011;77:1253-62. http://dx.doi.org/10.1212/WNL.0b013e3182309fa5.
- Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild to moderate Alzheimer’s disease. N Engl J Med 2014;370:322-33. http://dx.doi.org/10.1056/NEJMoa1304839.
- Sano M, Ernesto C, Klauber MR, Schafer K, Woodbury P, Thomas R, et al. Rationale and design of a multicenter study of selegiline and alpha-tocopherol in the treatment of Alzheimer disease using novel clinical outcomes. Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1996;10:132-40. https://doi.org/10.1097/00002093-199601030-00004.
- Sano M, Bell KL, Galasko D, Galvin JE, Thomas RG, van Dyck CH, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology 2011;77:556-63. http://dx.doi.org/10.1212/WNL.0b013e318228bf11.
- Scharf S, Mander A, Ugoni A, Vajda F, Christophidis N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer’s disease. Neurology 1999;53:197-201. https://doi.org/10.1212/WNL.53.1.197.
- Schwam EM, Nicholas T, Chew R, Billing CB, Davidson W, Ambrose D, et al. A multicenter, double-blind, placebo-controlled trial of the PDE9A inhibitor, PF-04447943, in Alzheimer’s disease. Curr Alzheimer Res 2014;11:413-21. https://doi.org/10.2174/1567205011666140505100858.
- del Ser T, Steinwachs KC, Gertz HJ, Andrés MV, Gómez-Carrillo B, Medina M, et al. Treatment of Alzheimer’s disease with the GSK-3 inhibitor tideglusib: a pilot study. J Alzheimers Dis 2013;33:205-15. http://dx.doi.org/10.3233/JAD-2012-120805.
- Sevigny JJ, Ryan JM, van Dyck CH, Peng Y, Lines CR, Nessly ML. MK-677 Protocol 30 Study Group . Growth hormone secretagogue MK-677: no clinical effect on AD progression in a randomized trial. Neurology 2008;71:1702-8. http://dx.doi.org/10.1212/01.wnl.0000335163.88054.e7.
- Siemers ER, Friedrich S, Dean RA, Gonzales CR, Farlow MR, Paul SM, et al. Safety and changes in plasma and cerebrospinal fluid amyloid beta after a single administration of an amyloid beta monoclonal antibody in subjects with Alzheimer disease. Clin Neuropharmacol 2010;33:67-73. http://dx.doi.org/10.1097/WNF.0b013e3181cb577a.
- Silverberg GD, Levinthal E, Sullivan EV, Bloch DA, Chang SD, Leverenz J, et al. Assessment of low-flow CSF drainage as a treatment for AD: results of a randomized pilot study. Neurology 2002;59:1139-45. https://doi.org/10.1212/01.WNL.0000031794.42077.A1.
- Silverberg GD, Mayo M, Saul T, Fellmann J, Carvalho J, McGuire D. Continuous CSF drainage in AD: results of a double-blind, randomized, placebo-controlled study. Neurology 2008;71:202-9. http://dx.doi.org/10.1212/01.wnl.0000316197.04157.6f.
- Simons M, Schwärzler F, Lütjohann D, von Bergmann K, Beyreuther K, Dichgans J, et al. Treatment with simvastatin in normocholesterolemic patients with Alzheimer’s disease: a 26-week randomized, placebo-controlled, double-blind trial. Ann Neurol 2002;52:346-50. http://dx.doi.org/10.1002/ana.10292.
- Soininen H, West C, Robbins J, Niculescu L. Long-term efficacy and safety of celecoxib in Alzheimer’s disease. Dement Geriatr Cogn Disord 2007;23:8-21. https://doi.org/10.1159/000096588.
- Sparks DL, Sabbagh MN, Connor DJ, Lopez J, Launer LJ, Browne P, et al. Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol 2005;62:753-7. https://doi.org/10.1001/archneur.62.5.753.
- Sweetlove M. Phase III CONCERT trial of latrepirdine: negative results. Pharmaceut Med 2012;26:113-15. http://dx.doi.org/10.2165/11631260-000000000-00000.
- Tan RS, Pu SJ. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. Aging Male 2003;6:13-7. https://doi.org/10.1080/tam.6.1.13.17.
- Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015;85:1383-91. http://dx.doi.org/10.1212/WNL.0000000000002035.
- Van Gool WA, Weinstein HC, Scheltens P, Walstra GJ, Scheltens PK. Effect of hydroxychloroquine on progression of dementia in early Alzheimer’s disease: an 18-month randomised, double-blind, placebo-controlled study. Lancet 2001;358:455-60. https://doi.org/10.1016/S0140-6736(01)05623-9.
- Vellas B, Sol O, Snyder PJ, Ousset PJ, Haddad R, Maurin M, et al. EHT0202 in Alzheimer’s disease: a 3-month, randomized, placebo-controlled, double-blind study. Curr Alzheimer Res 2011;8:203-12. https://doi.org/10.2174/156720511795256053.
- Wang T, Huang Q, Reiman EM, Chen K, Li X, Li G, et al. Effects of memantine on clinical ratings, fluorodeoxyglucose positron emission tomography measurements, and cerebrospinal fluid assays in patients with moderate to severe Alzheimer dementia: a 24-week, randomized, clinical trial. J Clin Psychopharmacol 2013;33:636-42. http://dx.doi.org/10.1097/JCP.0b013e31829a876a.
- Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry 2005;13:950-8. https://doi.org/10.1176/appi.ajgp.13.11.950.
- Wilcock GK, Black SE, Hendrix SB, Zavitz KH, Swabb EA, Laughlin MA. Tarenflurbil Phase II Study investigators . Efficacy and safety of tarenflurbil in mild to moderate Alzheimer’s disease: a randomised phase II trial. Lancet Neurol 2008;7:483-93. http://dx.doi.org/10.1016/S1474-4422(08)70090-5.
- Winblad B, Bonura ML, Rossini BM, Battaglia A. Nicergoline in the treatment of mild to moderate Alzheimer’s disease: a European multicentre trial. Clin Drug Invest 2001;21:621-32. https://doi.org/10.2165/00044011-200121090-00004.
- Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T, et al. Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer’s disease: randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol 2012;11:597-604. http://dx.doi.org/10.1016/S1474-4422(12)70140-0.
- Wischik CM, Staff RT, Wischik DJ, Bentham P, Murray AD, Storey JM, et al. Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease. J Alzheimers Dis 2015;44:705-20. http://dx.doi.org/10.3233/JAD-142874.
- Wolkowitz OM, Kramer JH, Reus VI, Costa MM, Yaffe K, Walton P, et al. DHEA treatment of Alzheimer’s disease: a randomized, double-blind, placebo-controlled study. Neurology 2003;60:1071-6. https://doi.org/10.1212/01.WNL.0000052994.54660.58.
- Annweiler C, Fantino B, Parot-Schinkel E, Thiery S, Gautier J, Beauchet O. Alzheimer’s disease – input of vitamin D with mEmantine assay (AD-IDEA trial): study protocol for a randomized controlled trial. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-230.
- Egefjord L, Gejl M, Møller A, Brændgaard H, Gottrup H, Antropova O, et al. Effects of liraglutide on neurodegeneration, blood flow and cognition in Alzheimer’s disease – protocol for a controlled, randomized double-blinded trial. Dan Med J 2012;59.
- Lawlor B, Kennelly S, O’Dwyer S, Cregg F, Walsh C, Coen R, et al. NILVAD protocol: a European multicentre double-blind placebo-controlled trial of nilvadipine in mild to moderate Alzheimer’s disease. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-006364.
- British Heart Foundation . ISRCTN31208535 A Clinical Trial To Test Amlodipine As a New Treatment for Vascular Dementia 2016. www.isrctn.com/ISRCTN31208535 (accessed 22 January 2016).
- Novo Nordisk . ISRCTN89711766 Evaluating the Effects of the Novel GLP1 Analogue, Liraglutide, in Patients With Alzheimer’s Disease (ELAD Study) 2015. www.isrctn.com/ISRCTN89711766 (accessed 22 January 2016).
- Grifols Biologicals Inc . NCT01561053 A Study to Evaluate Albumin and Immunoglobulin in Alzheimer’s Disease (AMBAR) 2015. https://clinicaltrials.gov/ct2/show/NCT01561053 (accessed 29 January 2016).
- Eisai Inc . NCT01767311 A Study to Evaluate Safety, Tolerability, and Efficacy of BAN2401 in Subjects With Early Alzheimer’s Disease 2016. https://clinicaltrials.gov/ct2/show/NCT01767311 (accessed 29 January 2016).
- University of Pennsylvania . NCT01965756 Effect of Insulin Sensitizer Metformin on AD Biomarkers 2013. https://clinicaltrials.gov/ct2/show/NCT01965756 (accessed 29 January 2016).
- University of California, San Francisco . NCT01966666 A Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Preliminary Efficacy Study of TPI-287 in Alzheimer’s Disease 2016. https://clinicaltrials.gov/ct2/show/NCT01966666 (accessed 29 January 2016).
- AstraZeneca . NCT02036645 SAD MAD Study to Assess Safety, Tolerability, PK &Amp; PD of MEDI1814 in Subjects With Mild-Moderate Alzheimer’s Disease 2016. https://clinicaltrials.gov/ct2/show/NCT02036645 (accessed 29 January 2016).
- Hoffmann-La Roche . NCT02051608 A Study of Gantenerumab in Patients With Mild Alzheimer Disease 2016. https://clinicaltrials.gov/ct2/show/NCT02051608 (accessed 29 January 2016).
- vTv Therapeutics . NCT02080364 Evaluation of the Efficacy and Safety of Azeliragon (TTP488) in Patients With Mild Alzheimer’s Disease (STEADFAST) 2016. https://clinicaltrials.gov/ct2/show/NCT02080364 (accessed 29 January 2016).
- Eli Lilly and Company . NCT02245737 An Efficacy and Safety Study of LY3314814 in Early Alzheimer’s Disease (AMARANTH) 2016. https://clinicaltrials.gov/ct2/show/NCT02245737 (accessed 29 January 2016).
- Eisai Inc., Biogen . NCT02322021 Dose-Finding Study To Evaluate Safety, Tolerability, and Efficacy of E2609 in Subjects With Mild Cognitive Impairment Due to Alzheimer’s Disease (Prodromal Alzheimer’s Disease) and Mild to Moderate Dementia Due to Alzheimer’s Disease 2015. https://clinicaltrials.gov/ct2/show/NCT02322021 (accessed 29 January 2016).
- Genentech Inc . NCT02353598 A Study of Crenezumab in Participants With Mild to Moderate Alzheimer Disease 2016. https://clinicaltrials.gov/ct2/show/NCT02353598 (accessed 29 January 2016).
- GliaCure, Inc . NCT02386306 Study Evaluating Safety, Tolerability, and PK of Multiple Ascending Doses of GC021109 in Subjects With Mild to Moderate Alzheimer’s Disease 2016. https://clinicaltrials.gov/ct2/show/NCT02386306 (accessed 29 January 2016).
- Probiodrug A. Julius Clinical, VU University Medical Center . NCT02389413 Safety and Tolerability of PQ912 in Subjects With Early Alzheimer’s Disease (SAPHIR) 2016. https://clinicaltrials.gov/ct2/show/NCT02389413 (accessed 29 January 2016).
- Janssen Research & Development, LLC . NCT02406027 An Extension Study to Evaluate the Long-Term Safety and Tolerability of JNJ-54861911 in Participants in the Early Alzheimer’s Disease Spectrum 2016. https://clinicaltrials.gov/ct2/show/NCT02406027 (accessed 29 January 2016).
- Neurotrope Bioscience, Inc . NCT02431468 A Study Assessing Bryostatin in the Treatment of Moderately Severe to Severe Alzheimer’s Disease 2016. https://clinicaltrials.gov/ct2/show/NCT02431468 (accessed 29 January 2016).
- Biogen . NCT02434718 Single and Multiple Ascending Dose Study of BIIB037 in Japanese Participants With Alzheimer’s Disease (PROPEL) 2016. https://clinicaltrials.gov/ct2/show/NCT02434718 (accessed 29 January 2016).
- Biogen . NCT02477800 221AD301 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer’s Disease (ENGAGE) 2016. https://clinicaltrials.gov/ct2/show/NCT02477800 (accessed 29 January 2016).
- Biogen . NCT02484547 221AD302 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer’s Disease (EMERGE) 2016. https://clinicaltrials.gov/ct2/show/NCT02484547 (accessed 29 January 2016).
- HealthPartners Institute . NCT02503501 Intranasal Glulisine in Amnestic Mild Cognitive Impairment and Probable Mild Alzheimer’s Disease 2016. https://clinicaltrials.gov/ct2/show/NCT02503501 (accessed 29 January 2016).
- APCER Life Sciences . NCT02547818 Safety and Efficacy Study of ALZT-OP1 in Subjects With Evidence of Early Alzheimer’s Disease 2016. https://clinicaltrials.gov/ct2/show/NCT02547818 (accessed 29 January 2016).
- United Neuroscience Ltd . NCT02551809 Evaluate the Safety, Tolerability, Immunogenicity and Efficacy of UB-311 in Mild Alzheimer’s Disease (AD) Patients 2016. https://clinicaltrials.gov/ct2/show/NCT02551809 (accessed 29 January 2016).
- Axon Neuroscience SE . NCT02579252 24 Months Safety and Efficacy Study of AADvac1 in Patients With Mild Alzheimer’s Disease (ADAMANT) 2016. https://clinicaltrials.gov/ct2/show/NCT02579252 (accessed 29 January 2016).
- Longeveron LLC . NCT02600130 Allogeneic Human Mesenchymal Stem Cell Infusion Versus Placebo in Patients With Alzheimer’s Disease 2016. https://clinicaltrials.gov/ct2/show/NCT02600130 (accessed 29 January 2016).
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry 1984;141:1356-64. http://dx.doi.org/10.1176/ajp.141.11.1356.
- Rockwood K, Fay S, Gorman M. The ADAS-cog and clinically meaningful change in the VISTA clinical trial of galantamine for Alzheimer’s disease. Int J Geriatr Psychiatry 2010;25:191-20. http://dx.doi.org/10.1002/gps.2319.
- Sansoni J, Marosszeky N, Jeon Y, Chenoweth L, Hawthorne G, King MT, et al. Dementia Outcomes Measurement Suite (DOMS) Project: Final Report. Wollongong, NSW: Centre for Health Service Development, University of Wollongong; 2007.
- Peña-Casanova J, Aguilar M, Santacruz P, Bertran-Serra I, Hernández G, Sol JM, et al. Adaptation and normalization of the Alzheimer’s disease Assessment Scale for Spain (NORMACODEM) (II). Neurologia 1997;12:69-77.
- Schrag A, Schott JM. Alzheimer’s Disease Neuroimaging Initiative . What is the clinically relevant change on the ADAS-Cog?. J Neurol Neurosurg Psychiatr 2011;83:171-3. http://dx.doi.org/10.1136/jnnp-2011-300881.
- Karin A, Hannesdottir K, Jaeger J, Annas P, Segerdahl M, Karlsson P, et al. Psychometric evaluation of ADAS-Cog and NTB for measuring drug response. Acta Neurol Scand 2014;129:114-22. http://dx.doi.org/10.1111/ane.12153.
- Sevigny JJ, Peng Y, Liu L, Lines CR. Item analysis of ADAS-Cog: effect of baseline cognitive impairment in a clinical AD trial. Am J Alzheimers Dis Other Demen 2010;25:119-24. http://dx.doi.org/10.1177/1533317509350298.
- Grochowalski JH, Liu Y, Siedlecki KL. Examining the reliability of ADAS-Cog change scores. Aging Neuropsychol Cogn 2015:1-17.
- Mohs RC, Cohen L. Alzheimer’s Disease Assessment Scale (ADAS). Psychopharmacol Bull 1988;24:627-8.
- Chu LW, Chiu KC, Hui SL, Yu GK, Tsui WJ, Lee PW. The reliability and validity of the Alzheimer’s Disease Assessment Scale Cognitive Subscale (ADAS-Cog) among the elderly Chinese in Hong Kong. Ann Acad Med Singap 2000;29:474-85. https://doi.org/10.1016/s0197-4580(00)83371-0.
- Liu H, Lee Teng E, Chuang YY, Lin KN, Fuh JL, Wang PN. The Alzheimer’s Disease Assessment Scale: findings from a low education population. Dement Geriatr Cogn Disord 2002;13:21-6. https://doi.org/10.1159/000048629.
- Weyer G, Erzigkeit H, Kanowski S, Ihl R, Hadler D. Alzheimer’s Disease Assessment Scale: reliability and validity in a multicenter clinical trial. Int Psychogeriatr 1997;9:123-38. https://doi.org/10.1017/S1041610297004298.
- Kim YS, Nibbelink DW, Overall JE. Factor structure and reliability of the Alzheimer’s Disease Assessment Scale in a multicenter trial with linopirdine. J Geriatr Psychiatry Neurol 1994;7:74-83. https://doi.org/10.1177/089198879400700202.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. https://doi.org/10.1016/0022-3956(75)90026-6.
- Fountoulakis K, Tsolaki M, Chantzi H, Kazis A. Mini mental state examination (MMSE): a validation study in Greece. Am J Alzheimers Dis Other Dement 2000;15:342-5. https://doi.org/10.1177/153331750001500604.
- Beaman S, Beaman PE, Garcia-Peña C, Villa MA, Heres J, Córdova A, et al. Validation of a modified version of the Mini-Mental State Examination (MMSE) in Spanish. Aging Neuropsychol Cogn 2004;11:1-11. https://doi.org/10.1076/anec.11.1.1.29366.
- Ansari NN, Naghdi S, Hasson S, Valizadeh L, Jalaie S. Validation of a Mini-Mental State Examination (MMSE) for the Persian population: a pilot study. Appl Neuropsychol 2010;17:190-5. http://dx.doi.org/10.1080/09084282.2010.499773.
- Awan S, Shahbaz N, Akhtar SW, Ahmad A, Iqbal S, Ahmed S, et al. Validation Study of the Mini-Mental State Examination in Urdu Language for Pakistani Population. Open Neurol J 2015;9:53-8. http://dx.doi.org/10.2174/1874205X01509010053.
- Carnero-Pardo C. Should the mini-mental state examination be retired?. Neurologia 2014;29:473-81. http://dx.doi.org/10.1016/j.nrl.2013.07.003.
- Rakusa M, Granda G, Kogoj A, Mlakar J, Vodusek DB. Mini-Mental State Examination: standardization and validation for the elderly Slovenian population. Eur J Neurol 2006;13:141-5. https://doi.org/10.1111/j.1468-1331.2006.01185.x.
- Howard R, Phillips P, Johnson T, O’Brien J, Sheehan B, Lindesay J, et al. Determining the minimum clinically important differences for outcomes in the DOMINO trial. Int J Geriatr Psychiatry 2011;26:812-17. http://dx.doi.org/10.1002/gps.2607.
- Galasko DR, Gould RL, Abramson IS, Salmon DP. Measuring cognitive change in a cohort of patients with Alzheimer’s disease. Stat Med 2000;19:1421-32. https://doi.org/10.1002/(SICI)1097-0258(20000615/30)19:11/12<1421::AID-SIM434>3.0.CO;2-P.
- Salmon DP, Thal LJ, Butters N, Heindel WC. Longitudinal evaluation of dementia of the Alzheimer type: a comparison of 3 standardized mental status examinations. Neurology 1990;40:1225-30. https://doi.org/10.1212/WNL.40.8.1225.
- Velayudhan L, Ryu SH, Raczek M, Philpot M, Lindesay J, Critchfield M, et al. Review of brief cognitive tests for patients with suspected dementia. Int Psychogeriatr 2014;26:1247-62. http://dx.doi.org/10.1017/S1041610214000416.
- Schramm U, Berger G, Müller R, Kratzsch T, Peters J, Frölich L. Psychometric properties of Clock Drawing Test and MMSE or Short Performance Test (SKT) in dementia screening in a memory clinic population. Int J Geriatr Psychiatry 2002;17:254-60. https://doi.org/10.1002/gps.585.
- Shen JH, Shen Q, Yu H, Lai JS, Beaumont JL, Zhang Z, et al. Validation of an Alzheimer’s disease assessment battery in Asian participants with mild to moderate Alzheimer’s disease. Am J Neurodegener Dis 2014;3:158-69.
- Tombaugh TN. Test–retest reliable coefficients and 5-year change scores for the MMSE and 3MS. Arch Clin Neuropsychol 2005;20:485-503. https://doi.org/10.1016/j.acn.2004.11.004.
- Pangman VC, Sloan J, Guse L. An examination of psychometric properties of the mini-mental state examination and the standardized mini-mental state examination: implications for clinical practice. Appl Nurs Res 2000;13:209-13. https://doi.org/10.1053/apnr.2000.9231.
- Rossetti HC, Munro Cullum C, Hynan LS, Lacritz LH. The CERAD Neuropsychologic Battery Total Score and the progression of Alzheimer disease. Alzheimer Dis Assoc Disord 2010;24:138-42. http://dx.doi.org/10.1097/WAD.0b013e3181b76415.
- Haanpää RM, Suhonen NM, Hartikainen P, Koivisto AM, Moilanen V, Herukka SK, et al. The CERAD Neuropsychological Battery in patients with frontotemporal lobar degeneration. Dement Geriatr Cogn Dis Extra 2015;5:147-54. http://dx.doi.org/10.1159/000380815.
- Seo EH, Lee DY, Lee JH, Choo IH, Kim JW, Kim SG, et al. Total scores of the CERAD neuropsychological assessment battery: validation for mild cognitive impairment and dementia patients with diverse etiologies. Am J Geriatr Psychiatry 2010;18:801-9. https://doi.org/10.1097/JGP.0b013e3181cab764.
- Demers P, Robillard A, Laflèche G, Nash F, Heyman A, Fillenbaum G. Translation of clinical and neuropsychological instruments into French: the CERAD experience. Age Ageing 1994;23:449-51. https://doi.org/10.1093/ageing/23.6.449.
- Glezerman A, Drexler ML. The Russian Adaptation of the CERAD Battery (CERAD-RA). Arch Clin Neuropsychol 2001;16.
- Liu KP, Kuo MC, Tang KC, Chau AW, Ho IH, Kwok MP, et al. Effects of age, education and gender in the Consortium to Establish a Registry for the Alzheimer’s Disease (CERAD)-Neuropsychological Assessment Battery for Cantonese-speaking Chinese elders. Int Psychogeriatr 2011;23:1575-81. https://doi.org/10.1017/S1041610211001153.
- Lee JH, Lee KU, Lee DY, Kim KW, Jhoo JH, Kim JH, et al. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K): clinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci 2002;57:P47-53. https://doi.org/10.1093/geronb/57.1.P47.
- Paajanen T, Hänninen T, Aitken A, Hallikainen M, Westman E, Wahlund LO, et al. CERAD neuropsychological total scores reflect cortical thinning in prodromal Alzheimer’s disease. Dement Geriatr Cogn Disord Extra 2013;3:446-58. https://doi.org/10.1159/000356725.
- Hallikainen I, Hänninen T, Fraunberg M, Hongisto K, Välimäki T, Hiltunen A, et al. Progression of Alzheimer’s disease during a three-year follow-up using the CERAD-NB total score: Kuopio ALSOVA study. Int Psychogeriatr 2013;25:1335-44. http://dx.doi.org/10.1017/S1041610213000653.
- Trapp-Moen B, Tyrey M, Cook G, Heyman A, Fillenbaum GG. In-home assessment of dementia by nurses: experience using the CERAD evaluations. Consortium to Establish a Registry for Alzheimer’s Disease. Gerontologist 2001;41:406-9. https://doi.org/10.1093/geront/41.3.406.
- Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, et al. Validity of the Cogstate brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol 2009;24:165-78. https://doi.org/10.1093/arclin/acp010.
- Hammers D, Spurgeon E, Ryan K, Persad C, Barbas N, Heidebrink J, et al. Validity of a brief computerized cognitive screening test in dementia. J Geriatr Psychiatry Neurol 2012;25:89-9. http://dx.doi.org/10.1177/0891988712447894.
- Hammers D, Spurgeon E, Ryan K, Persad C, Heidebrink J, Barbas N, et al. Reliability of repeated cognitive assessment of dementia using a brief computerized battery. Am J Alzheimers Dis Other Demen 2011;26:326-33. http://dx.doi.org/10.1177/1533317511411907.
- Fredrickson J, Maruff P, Woodward M, Moore L, Fredrickson A, Sach J, et al. Evaluation of the usability of a brief computerized cognitive screening test in older people for epidemiological studies. Neuroepidemiology 2010;34:65-7. http://dx.doi.org/10.1159/000264823.
- Lim YY, Ellis KA, Harrington K, Ames D, Martins RN, Masters CL, et al. Use of the Cogstate Brief Battery in the assessment of Alzheimer’s disease related cognitive impairment in the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J Clin Exp Neuropsychol 2012;34:345-58. http://dx.doi.org/10.1080/13803395.2011.643227.
- Maruff P, Lim YY, Darby D, Ellis KA, Pietrzak RH, Snyder PJ, et al. Clinical utility of the cogstate brief battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer’s disease. BMC Psychol 2013;1. http://dx.doi.org/10.1186/2050-7283-1-30.
- Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol 2007;64:1323-9. https://doi.org/10.1001/archneur.64.9.1323.
- Harrison J, Rentz DM, McLaughlin T, Niecko T, Gregg KM, Black RS, et al. Cognition in MCI and Alzheimer’s disease: baseline data from a longitudinal study of the NTB. Clin Neuropsychol 2014;28:252-68. http://dx.doi.org/10.1080/13854046.2013.875595.
- Fernández M, Gobartt AL, Balañá M. COOPERA Study Group . Behavioural symptoms in patients with Alzheimer’s disease and their association with cognitive impairment. BMC Neurol 2010;10. http://dx.doi.org/10.1186/1471-2377-10-87.
- Cano SJ, Posner HB, Moline ML, Hurt SW, Swartz J, Hsu T, et al. The ADAS-cog in Alzheimer’s disease clinical trials: psychometric evaluation of the sum and its parts. J Neurol Neurosurg Psychiatr 2010;81:1363-8. http://dx.doi.org/10.1136/jnnp.2009.204008.
- Yesavage JA, Poulsen SL, Sheikh J, Tanke E. Rates of change of common measures of impairment in senile dementia of the Alzheimer’s type. Psychopharmacol Bull 1988;24:531-4.
- Standish TI, Molloy DW, Bédard M, Layne EC, Murray EA, Strang D. Improved reliability of the Standardized Alzheimer’s Disease Assessment Scale (SADAS) compared with the Alzheimer’s Disease Assessment Scale (ADAS). J Am Geriatr Soc 1996;44:712-16. https://doi.org/10.1111/j.1532-5415.1996.tb01838.x.
- Reisberg B, Borenstein J, Salob SP, Ferris SH, Franssen E, Georgotas A. Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment. J Clin Psychiatry 1987;48:9-15.
- Monteiro IM, Boksay I, Auer SR, Torossian C, Ferris SH, Reisberg B. Addition of a frequency-weighted score to the Behavioral Pathology in Alzheimer’s Disease Rating Scale: the BEHAVE-AD-FW: methodology and reliability. Eur Psychiatry 2001;16:5-24. https://doi.org/10.1016/S0924-9338(00)00524-1.
- Sclan S, Saillon A, Franssen E, Hugonot-Diener L, Saillon A, Reisberg B. The Behavior Pathology in Alzheimer’s Disease Rating Scale (BEHAVEAD): reliability and analysis of symptom category scores. Int J Geriatr Psychiatry 1996;11:819-30. http://dx.doi.org/10.1002/(SICI)1099-1166(199609)11:9%3C819::AID-GPS389%3E3.0.CO;2-S.
- Katz I, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Risperidone Study Group. J Clin Psychiatry 1999;60:107-15. https://doi.org/10.4088/JCP.v60n0207.
- Auer SR, Monteiro IM, Reisberg B. The Empirical Behavioral Pathology in Alzheimer’s Disease (E-BEHAVE-AD) Rating Scale. Int Psychogeriatr 1996;8:247-66. https://doi.org/10.1017/S1041610296002621.
- Overall J, Gorham D. The Brief Psychiatric Scale. Psychol Rep 1962;10:799-812. https://doi.org/10.2466/pr0.1962.10.3.799.
- Gottlieb GL, Gur RE, Gur RC. Reliability of psychiatric scales in patients with dementia of the Alzheimer type. Am J Psychiatry 1988;145:857-60. http://dx.doi.org/10.1176/ajp.145.7.857.
- Tariot P, Mack JL, Patterson MB, Edland SD, Weiner MF, Fillenbaum G, et al. The Behavior Rating Scale for Dementia of the Consortium to Establish a Registry for Alzheimer’s Disease. The Behavioral Pathology Committee of the Consortium to Establish a Registry for Alzheimer’s Disease. Am J Psychiatry 1995;152:1349-57. https://doi.org/10.1176/ajp.152.9.1349.
- Fillenbaum GG, van Belle G, Morris JC, Mohs RC, Mirra SS, Davis PC, et al. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): the first twenty years. Alzheimers Dement 2008;4:96-109. http://dx.doi.org/10.1016/j.jalz.2007.08.005.
- Patterson MB, Mack JL, Mackell JA, Thomas R, Tariot P, Weiner M, et al. A longitudinal study of behavioral pathology across five levels of dementia severity in Alzheimer’s disease: the CERAD Behavior Rating Scale for Dementia. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11:40-4. https://doi.org/10.1097/00002093-199700112-00006.
- Molloy DW, Mcllroy WE, Guyatt GH, Lever JA. Validity and reliability of the Dysfunctional Behaviour Rating Instrument. Acta Psychiatr Scand 1991;84:103-6. https://doi.org/10.1111/j.1600-0447.1991.tb01429.x.
- Molloy DW, Bédard M, Guyatt GH, Lever J. Dysfunctional Behavior Rating Instrument. Int Psychogeriatr 1996;8:333-41. https://doi.org/10.1017/S104161029700358X.
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308-14. https://doi.org/10.1212/WNL.44.12.2308.
- Frisoni GB, Rozzini L, Gozzetti A, Binetti G, Zanetti O, Bianchetti A, et al. Behavioral syndromes in Alzheimer’s disease: description and correlates. Dement Geriatr Cogn Disord 1999;10:130-8. https://doi.org/10.1159/000017113.
- Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology 1996;46:130-5. https://doi.org/10.1212/WNL.46.1.130.
- Hatoum H, Lin S-J, Arcona S, Thomas SK, Koumaris B, Mirski D. The use of the occupational disruptiveness scale of the neuropsychiatric inventory-nursing home version to measure the impact of rivistagmine on the disruptive behaviour of nursing home residents with Alzheimer’s disease. J Am Med Dir Assoc 2005;6:238-45. https://doi.org/10.1016/j.jamda.2005.04.003.
- Kaufer D, Cummings JL, Christine D. Differential neuropsychiatric symptom response in Tacrine in Alzhiemer’s disease: relationship to symptom severity. J Neuropsychiatry Clin Neurosci 1998;10. https://doi.org/10.1176/jnp.10.1.55.
- Cummings JL, Street J, Masterman D, Clark WS. Efficacy of olanzapine in the treatment of psychosis in dementia with Lewy bodies. Dement Geriatr Cogn Disord 2002;13:67-73. https://doi.org/10.1159/000048636.
- Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 1997;48:10-6. https://doi.org/10.1212/WNL.48.5_Suppl_6.10S.
- Spiegel R, Brunner C, Ermini-Fünfschilling D, Monsch A, Notter M, Puxty J, et al. A new behavioral assessment scale for geriatric out- and in-patients: the NOSGER (Nurses’ Observation Scale for Geriatric Patients). J Am Geriatr Soc 1991;39:339-47. https://doi.org/10.1111/j.1532-5415.1991.tb02897.x.
- Wahle M, Häller S, Spiegel R. Validation of the NOSGER (Nurses’ Observation Scale for Geriatric Patients): reliability and validity of a caregiver rating instrument. Int Psychogeriatr 1996;8:525-47. https://doi.org/10.1017/S1041610296002864.
- Tremmel L, Spiegel R. Clinical experience with the NOSGER (Nurses’ Observation Scale for Geriatric Patients): tentative normative data and sensitivity to change. Int J Geriatr Psychiatry 1993;8:311-17. https://doi.org/10.1002/gps.930080406.
- Plutchik R, Conte H, Lieberman M, Bakur M, Grossman J, Lehrman N. Reliability and validity of a scale for assessing the functioning of geriatric patients. J Am Geriatr Soc 1970;18:491-500. https://doi.org/10.1111/j.1532-5415.1970.tb01335.x.
- Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral problems in dementia: the revised memory and behavior problems checklist. Psychol Aging 1992;7:622-31. https://doi.org/10.1037/0882-7974.7.4.622.
- Fuh JL, Liu CY, Wang SJ, Wang HC, Liu HC. Revised memory and behavior problems checklist in Taiwanese patients with Alzheimer’s disease. Int Psychogeriatr 1999;11:181-9. https://doi.org/10.1017/S1041610299005736.
- Nogales-González C, Losada A, Romero-Moreno R. Confirmatory factor analysis of the Spanish version of the revised memory and behavior problems checklist. Int Psychogeriatr 2015;27:683-92. http://dx.doi.org/10.1017/S1041610214002476.
- Johnson MM, Wackerbarth SB, Schmitt FA. Revised memory and behavior problems checklist. Clin Gerontol 2001;22:87-108. https://doi.org/10.1300/J018v22n03_09.
- Weiner MF, Tractenberg R, Teri L, Logsdon R, Thomas RG, Gamst A, et al. Quantifying behavioral disturbance in Alzheimer’s disease patients. J Psychiatr Res 2000;34:163-7. https://doi.org/10.1016/S0022-3956(99)00042-4.
- Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9100.
- Lucas-Carrasco R, Lamping DL, Banerjee S, Rejas J, Smith SC, Gómez-Benito J. Validation of the Spanish version of the DEMQOL system. Int Psychogeriatr 2010;22:589-97. http://dx.doi.org/10.1017/S1041610210000207.
- Berwig M, Leicht H, Hartwig K, Gertz HJ. Self-rated quality of life in mild cognitive impairment and Alzheimer’s disease: the problem of affective distortion. GeroPsych 2011;24:45-51. http://dx.doi.org/10.1024/1662-9647/a000029.
- Gavrilova SI, Ferri CP, Mikhaylova N, Sokolova O, Banerjee S, Prince M. Helping carers to care – the 10/66 dementia research group’s randomized control trial of a caregiver intervention in Russia. Int J Geriatr Psychiatry 2009;24:347-54. http://dx.doi.org/10.1002/gps.2126.
- Shi GX, Liu CZ, Li QQ, Zhu H, Wang LP. Influence of acupuncture on cognitive function and markers of oxidative DNA damage in patients with vascular dementia. J Tradit Chin Med 2012;32:199-202. https://doi.org/10.1016/S0254-6272(13)60011-4.
- Orrell M, Aguirre E, Spector A, Hoare Z, Woods RT, Streater A, et al. Maintenance cognitive stimulation therapy for dementia: single-blind, multicentre, pragmatic randomised controlled trial. Br J Psychiatry 2014;204:454-61. http://dx.doi.org/10.1192/bjp.bp.113.137414.
- Low LF, Brodaty H, Goodenough B, Spitzer P, Bell JP, Fleming R, et al. The Sydney Multisite Intervention of LaughterBosses and ElderClowns (SMILE) study: cluster randomised trial of humour therapy in nursing homes. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-002072.
- Schulz R, Cook TB, Beach SR, Lingler JH, Martire LM, Monin JK, et al. Magnitude and causes of bias among family caregivers rating Alzheimer disease patients. Am J Geriatr Psychiatry 2013;21:14-25. http://dx.doi.org/10.1016/j.jagp.2012.10.002.
- Schulz R, Monin JK, Czaja SJ, Lingler JH, Beach SR, Martire LM, et al. Measuring the experience and perception of suffering. Gerontologist 2010;50:774-84. http://dx.doi.org/10.1093/geront/gnq033.
- EuroQol Group . EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Aguirre E, Kang S, Hoare Z, Edwards RT, Orrell M. How does the EQ-5D perform when measuring quality of life in dementia against two other dementia-specific outcome measures?. Qual Life Res 2016;25:45-9. http://dx.doi.org/10.1007/s11136-015-1065-9.
- Hounsome N, Orrell M, Edwards RT. EQ-5D as a quality of life measure in people with dementia and their carers: evidence and key issues. Value Health 2011;14:390-9. http://dx.doi.org/10.1016/j.jval.2010.08.002.
- Ankri J, Beaufils B, Novella JL, Morrone I, Guillemin F, Jolly D, et al. Use of the EQ-5D among patients suffering from dementia. J Clin Epidemiol 2003;56:1055-63. https://doi.org/10.1016/S0895-4356(03)00175-6.
- Naglie G, Tomlinson G, Tansey C, Irvine J, Ritvo P, Black SE, et al. Utility-based quality of life measures in Alzheimer’s disease. Qual Life Res 2006;15:631-43. http://dx.doi.org/10.1007/s11136-005-4364-8.
- Coucill W, Bryan S, Bentham P, Buckley A, Laight A. EQ-5D in patients with dementia: an investigation of inter-rater agreement. Med Care 2001;39:760-71. https://doi.org/10.1097/00005650-200108000-00003.
- Logsdon R, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer’s disease: patient and caregiver reports. J Ment Health Aging 1999;5:21-32.
- Thorgrimsen L, Selwood A, Spector A, Royan L, de Madariaga Lopez M, Woods RT, et al. Whose quality of life is it anyway? The validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Dis Assoc Disord 2003;17:201-8. https://doi.org/10.1097/00002093-200310000-00002.
- Bowling A, Rowe G, Adams S, Sands P, Samsi K, Crane M, et al. Quality of life in dementia: a systematically conducted narrative review of dementia-specific measurement scales. Aging Ment Health 2015;19:13-31. http://dx.doi.org/10.1080/13607863.2014.915923.
- Naglie G, Hogan DB, Krahn M, Black SE, Beattie BL, Patterson C, et al. Predictors of family caregiver ratings of patient quality of life in Alzheimer disease: cross-sectional results from the Canadian Alzheimer’s Disease Quality of Life Study. Am J Geriatr Psychiatry 2011;19:891-90. http://dx.doi.org/10.1097/JGP.0b013e3182006a7f.
- Wolak A, Novella JL, Drame M, Guillemin F, Di Pollina L, Ankri J, et al. Transcultural adaptation and psychometric validation of a French-language version of the QoL-AD. Aging Ment Health 2009;13:593-600. http://dx.doi.org/10.1080/13607860902774386.
- Woods B, Thorgrimsen L, Spector A, Royan L, Orrell M. Improved quality of life and cognitive stimulation therapy in dementia. Aging Ment Health 2006;10:219-26. https://doi.org/10.1080/13607860500431652.
- Selwood A, Thorgrimsen L, Orrell M. Quality of life in dementia – a one-year follow-up study. Int J Geriatr Psychiatry 2005;20:232-7. http://dx.doi.org/10.1002/gps.1271.
- Teri L, McCurry SM, Logsdon R, Gibbons LE. Training community consultants to help family members improve dementia care: a randomized controlled trial. Gerontologist 2005;45:802-11. https://doi.org/10.1093/geront/45.6.802.
- Sloane PD, Zimmerman S, Williams CS, Reed PS, Gill KS, Preisser JS. Evaluating the quality of life of long-term care residents with dementia. Gerontologist 2005;45:37-49. https://doi.org/10.1093/geront/45.suppl_1.37.
- Chapman SB, Weiner MF, Rackley A, Hynan LS, Zientz J. Effects of cognitive-communication stimulation for Alzheimer’s disease patients treated with donepezil. J Speech Lang Hear Res 2004;47:1149-63. https://doi.org/10.1044/1092-4388(2004/085).
- Orrell M, Spector A, Thorgrimsen L, Woods B. A pilot study examining the effectiveness of maintenance Cognitive Stimulation Therapy (MCST) for people with dementia. Int J Geriatr Psychiatry 2005;20:446-51. http://dx.doi.org/10.1002/gps.1304.
- Spector A, Thorgrimsen L, Woods B, Royan L, Davies S, Butterworth M, et al. Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: randomised controlled trial. Br J Psychiatry 2003;183:248-54. https://doi.org/10.1192/bjp.183.3.248.
- Edelman P, Fulton BR, Kuhn D. Comparison of dementia-specific quality of life measures in adult day centers. Home Health Care Serv Q 2004;23:25-42. http://dx.doi.org/10.1300/J027v23n01_02.
- Edelman P, Fulton BR, Kuhn D, Chang CH. A comparison of three methods of measuring dementia-specific quality of life: perspectives of residents, staff, and observers. Gerontologist 2005;45:27-36. https://doi.org/10.1093/geront/45.suppl_1.27.
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med 2002;64:510-19. https://doi.org/10.1097/00006842-200205000-00016.
- Fuh JL, Wang SJ. Assessing quality of life in Taiwanese patients with Alzheimer’s disease. Int J Geriatr Psychiatry 2006;21:103-7. http://dx.doi.org/10.1002/gps.1425.
- Spector A, Orrell M. Quality of life (QoL) in dementia: a comparison of the perceptions of people with dementia and care staff in residential homes. Alzheimer Dis Assoc Disord 2006;20:160-5. https://doi.org/10.1097/00002093-200607000-00007.
- Hoe J, Hancock G, Livingston G, Orrell M. Quality of life of people with dementia in residential care homes. Br J Psychiatry 2006;188:460-4. https://doi.org/10.1192/bjp.bp.104.007658.
- Shin IS, Carter M, Masterman D, Fairbanks L, Cummings JL. Neuropsychiatric symptoms and quality of life in Alzheimer disease. Am J Geriatr Psychiatry 2005;13:469-74. https://doi.org/10.1097/00019442-200506000-00005.
- Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11:33-9. https://doi.org/10.1097/00002093-199700112-00005.
- Galasko D, Bennett DA, Sano M, Marson D, Kaye J, Edland SD. ADCS Prevention Instrument Project: assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Dis Assoc Disord 2006;20:152-69. https://doi.org/10.1097/01.wad.0000213873.25053.2b.
- Bullock R, Bergman H, Touchon J, Gambina G, He Y, Nagel J, et al. Effect of age on response to rivastigmine or donepezil in patients with Alzheimer’s disease. Curr Med Res Opin 2006;22:483-94. http://dx.doi.org/10.1185/030079906X89685.
- Galasko D, Kershaw PR, Schneider L, Zhu Y, Tariot PN. Galantamine maintains ability to perform activities of daily living in patients with Alzheimer’s disease. J Am Geriatr Soc 2004;52:1070-6. http://dx.doi.org/10.1111/j.1532-5415.2004.52303.x.
- Brodaty H, Corey-Bloom J, Potocnik FC, Truyen L, Gold M, Damaraju CR. Galantamine prolonged-release formulation in the treatment of mild to moderate Alzheimer’s disease. Dement Geriatr Cogn Disord 2005;20:120-32. https://doi.org/10.1159/000086613.
- Peskind ER, Potkin SG, Pomara N, Ott BR, Graham SM, Olin JT, et al. Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomized, controlled trial. Am J Geriatr Psychiatry 2006;14:704-15. https://doi.org/10.1097/01.JGP.0000224350.82719.83.
- Schneider LS. Assessing outcomes in Alzheimer disease. Alzheimer Dis Assoc Disord 2001;15:8-18. https://doi.org/10.1097/00002093-200108001-00003.
- Burns A, Lawlor B, Craig S. Assessment Scales in Old Age Psychiatry. London: Taylor & Francis; 2004.
- Manero RM, Casals-Coll M, Sánchez-Benavides G, Rodríguez-de Los Reyes ON, Aguilar M, Badenes D, et al. Diagnostic validity of the Alzheimer’s Disease Functional Assessment and Change Scale in mild cognitive impairment and mild to moderate Alzheimer’s disease. Dement Geriatr Cogn Disord 2014;37:366-75. https://doi.org/10.1159/000350800.
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age Ageing 1996;25:113-20. https://doi.org/10.1093/ageing/25.2.113.
- Byrne LM, Wilson PM, Bucks RS, Hughes AO, Wilcock GK. The sensitivity to change over time of the Bristol Activities of Daily Living Scale in Alzheimer’s disease. Int J Geriatr Psychiatry 2000;15:656-61. https://doi.org/10.1002/1099-1166(200007)15:7<656::AID-GPS163>3.0.CO;2-Q.
- Bucks RS, Haworth J. Bristol Activities of Daily Living Scale: a critical evaluation. Expert Rev Neurother 2002;2:669-76. http://dx.doi.org/10.1586/14737175.2.5.669.
- Stern Y, Albert SM, Sano M, Richards M, Miller L, Folstein M, et al. Assessing patient dependence in Alzheimer’s disease. J Gerontol 1994;49:M216-22. https://doi.org/10.1093/geronj/49.5.M216.
- Demers L, Oremus M, Perrault A, Champoux N, Wolfson C. Review of outcome measurement instruments in Alzheimer’s disease drug trials: psychometric properties of functional and quality of life scales. J Geriatr Psychiatry Neurol 2000;13:170-80. https://doi.org/10.1177/089198870001300402.
- Bavazzano A, Magnolfi SU, Calvani D, Valente C, Boni F, Baldini A, et al. Functional evaluation of Alzheimer patients during clinical trials: a review. Arch Gerontol Geriatr 1998;26:27-32. https://doi.org/10.1016/S0167-4943(98)80005-8.
- Gélinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Ther 1999;53:471-81. https://doi.org/10.5014/ajot.53.5.471.
- Mok CC, Siu AM, Chan WC, Yeung KM, Pan PC, Li SW. Functional disabilities profile of Chinese elderly people with Alzheimer’s disease – a validation study on the Chinese version of the disability assessment for dementia. Dement Geriatr Cogn Disord 2005;20:112-19. https://doi.org/10.1159/000086612.
- Suh GH. Development of the Korean Version of Disability Assessment for Dementia Scale (DAD-K) to assess function in dementia. J Korean Geriatr Soc 2003;7:278-87.
- De Vreese LP, Caffarra P, Savarè R, Cerutti R, Franceschi M, Grossi E. Multicentre Study Group . Functional disability in early Alzheimer’s disease – a validation study of the Italian version of the disability assessment for dementia scale. Dement Geriatr Cogn Disord 2008;25:186-94. http://dx.doi.org/10.1159/000113415.
- Carthery-Goulart MT, Areza-Fegyveres R, Schultz RR, Okamoto I, Caramelli P, Bertolucci PH, et al. Cross-cultural adaptation of the Disability Assessment for Dementia (DAD). Arq Neuropsiquiatr 2007;65:916-19. https://doi.org/10.1590/S0004-282X2007000500038.
- Sánchez-Pérez A, López-Roig S, Pérez AP, Gómez PP, Pastor MÁ, Pomares MH. Validation study of the Spanish Version of the Disability Assessment for Dementia Scale. Medicine 2015;94. http://dx.doi.org/10.1097/MD.0000000000001925.
- Tozlu M, Cankurtaran M, Yavuz BB, Cankurtaran ES, Kutluer İ, Erkek BM. Functional disability in Alzheimer disease: a validation study of the Turkish version of the Disability Assessment for Dementia Scale. J Geriatr Psychiatry Neurol 2014;27:237-46. http://dx.doi.org/10.1177/0891988714532014.
- Zangbar HS, Mehraban AH, Akbarfahimi M, Rasanani FM. Validity and reliability of the Persian version of the Disability Assessment for Dementia Scale. Middle East J Rehabil Health 2016;3. http://dx.doi.org/10.17795/mejrh-35619.
- Feldman H, Sauter A, Donald A, Gélinas I, Gauthier S, Torfs K, et al. The Disability Assessment for Dementia Scale: a 12-month study of functional ability in mild to moderate severity Alzheimer disease. Alzheimer Dis Assoc Disord 2001;15:89-95. https://doi.org/10.1097/00002093-200104000-00008.
- Arrighi HM, Gélinas I, McLaughlin TP, Buchanan J, Gauthier S. Longitudinal changes in functional disability in Alzheimer’s disease patients. Int Psychogeriatr 2013;25:929-37. http://dx.doi.org/10.1017/S1041610212002360.
- Blesa R. Galantamine: therapeutic effects beyond cognition. Dement Geriatr Cogn Disord 2000;11:28-34. https://doi.org/10.1159/000051229.
- Blesa R, Davidson M, Kurz A, Reichman W, van Baelen B, Schwalen S. Galantamine provides sustained benefits in patients with ‘advanced moderate’ Alzheimer’s disease for at least 12 months. Dement Geriatr Cogn Disord 2003;15:79-87. http://dx.doi.org/67974.
- Farlow MR, Cyrus PA. Metrifonate therapy in Alzheimer’s disease: a pooled analysis of four randomized, double-blind, placebo-controlled trials. Dement Geriatr Cogn Disord 2000;11:202-11. https://doi.org/10.1159/000017238.
- Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: a 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology 2000;54:2261-8. https://doi.org/10.1212/WNL.54.12.2261.
- Gélinas I, Gauthier S, Cyrus PA. Metrifonate enhances the ability of Alzheimer’s disease patients to initiate, organize, and execute instrumental and basic activities of daily living. J Geriatr Psychiatry Neurol 2000;13:9-16. https://doi.org/10.1177/089198870001300102.
- Rockwood K, Fay S, Song X, MacKnight C, Gorman M. Video-Imaging Synthesis of Treating Alzheimer’s Disease (VISTA) Investigators . Attainment of treatment goals by people with Alzheimer’s disease receiving galantamine: a randomized controlled trial. CMAJ 2006;174:1099-105. https://doi.org/10.1503/cmaj.051432.
- Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol 1982;37:323-9. https://doi.org/10.1093/geronj/37.3.323.
- Teng E, Becker BW, Woo E, Knopman DS, Cummings JL, Lu PH. Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild Alzheimer disease. Alzheimer Dis Assoc Disord 2010;24:348-53. http://dx.doi.org/10.1097/WAD.0b013e3181e2fc84.
- Teunisse S, Derix MM. The interview for deterioration in daily living activities in dementia: agreement between primary and secondary caregivers. Int Psychogeriatr 1997;9:155-62. https://doi.org/10.1017/S1041610297004845.
- Teunisse S, Derix MM. Measurement of activities of daily living in patients with dementia living at home: development of a questionnaire. Tijdschr Gerontol Geriatr 1991;22:53-9.
- Böhm P, Peña-Casanova J, Aguilar M, Hernández G, Sol JM, Blesa R. Clinical validity and utility of the interview for deterioration of daily living in dementia for Spanish-speaking communities NORMACODEM Group. Int Psychogeriatr 1998;10:261-70. https://doi.org/10.1017/S1041610298005377.
- Voigt-Radloff S, Leonhart R, Schützwohl M, Jurjanz L, Reuster T, Gerner A, et al. Interview for Deterioration in Daily Living Activities in Dementia: construct and concurrent validity in patients with mild to moderate dementia. Int Psychogeriatr 2012;24:382-90. http://dx.doi.org/10.1017/S1041610211001785.
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of Adl: a standardized measure of biological and psychosocial function. JAMA 1963;185:914-19. https://doi.org/10.1001/jama.1963.03060120024016.
- Desai AK, Grossberg GT, Sheth DN. Activities of daily living in patients with dementia: clinical relevance, methods of assessment and effects of treatment. CNS Drugs 2004;18:853-75. https://doi.org/10.2165/00023210-200418130-00003.
- Londos E, Passant U, Brun A, Gustafson L. Clinical Lewy body dementia and the impact of vascular components. Int J Geriatr Psychiatry 2000;15:40-9. http://dx.doi.org/10.1002/(SICI)1099-1166(200001)15:1%3C40::AID-GPS74%3E3.0.CO;2-S.
- Agüero-Torres H, Fratiglioni L, Guo Z, Viitanen M, von Strauss E, Winblad B. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health 1998;88:1452-6. https://doi.org/10.2105/AJPH.88.10.1452.
- Katz S, Ford A, Moskowitz R, Jackson BA, Jaffe MW, Rush AJ, et al. Handbook of Psychiatric Measures. Washington, DC: American Psychiatric Association; 2000.
- Hokoishi K, Ikeda M, Maki N, Nomura M, Torikawa S, Fujimoto N, et al. Interrater reliability of the physical self-maintenance scale and instrumental activities of daily living scale in a variety of health professional representatives. Aging Ment Health 2001;5:38-40. https://doi.org/10.1080/13607860020020627.
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179-86. https://doi.org/10.1093/geront/9.3_Part_1.179.
- Ng TP, Niti M, Chiam PC, Kua EH. Physical and cognitive domains of the Instrumental Activities of Daily Living: validation in a multiethnic population of Asian older adults. J Gerontol A Biol Sci Med Sci 2006;61:726-35. https://doi.org/10.1093/gerona/61.7.726.
- Sikkes SA, de Lange-de Klerk ES, Pijnenburg YA, Scheltens P, Uitdehaag BM. A systematic review of Instrumental Activities of Daily Living scales in dementia: room for improvement. J Neurol Neurosurg Psychiatr 2009;80:7-12. http://dx.doi.org/10.1136/jnnp.2008.155838.
- Feldman H, Gauthier S, Hecker J, Vellas B, Emir B, Mastey V, et al. Donepezil MSAD Study Investigators Group . Efficacy of donepezil on maintenance of activities of daily living in patients with moderate to severe Alzheimer’s disease and the effect on caregiver burden. J Am Geriatr Soc 2003;51:737-44. https://doi.org/10.1046/j.1365-2389.2003.51260.x.
- Green CR, Mohs RC, Schmeidler J, Aryan M, Davis KL. Functional decline in Alzheimer’s disease: a longitudinal study. J Am Geriatr Soc 1993;41:654-61. https://doi.org/10.1111/j.1532-5415.1993.tb06740.x.
- Oswald W, Fleischmann UM. Nürnberger-Alters-Inventar (NAI)-Testmanual und-Textband. Göttingen: Hogrefe Ltd; 1995.
- Zank S, Frank S. Family and professional caregivers’ ratings of dementia symptoms and activities of daily living of day care patients: do differences change over time?. Aging Ment Health 2002;6:161-5. http://dx.doi.org/10.1080/13607860220126790.
- Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al. Validity and reliability of the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11:22-3. https://doi.org/10.1097/00002093-199700112-00004.
- Schneider LS, Clark CM, Doody R, Ferris SH, Morris JC, Raman R, et al. ADCS Prevention Instrument Project: ADCS-clinicians’ global impression of change scales (ADCS-CGIC), self-rated and study partner-rated versions. Alzheimer Dis Assoc Disord 2006;20:124-38. https://doi.org/10.1097/01.wad.0000213878.47924.44.
- Guy W. Clinical Global Impression Scale. ECDEU Assessment Manual for Psychopharmacology – Revised. Rockville, MD: National Institute of Mental Health; 1976.
- Schneider LS, Olin JT. Clinical global impressions in Alzheimer’s clinical trials. Int Psychogeriatr 1996;8:277-88. https://doi.org/10.1017/S1041610296002645.
- Homma A, Nakamura Y, Kobune S, Haraguchi H, Kodani N, Takami I, et al. Reliability study on the Japanese version of the Clinician’s Interview-Based Impression of Change. Dement Geriatr Cogn Disord 2006;21:97-103. https://doi.org/10.1159/000090296.
- Knopman DS, Knapp MJ, Gracon SI, Davis CS. The Clinician Interview-Based Impression (CIBI): a clinician’s global change rating scale in Alzheimer’s disease. Neurology 1994;44:2315-21. https://doi.org/10.1212/WNL.44.12.2315.
- Nakamura Y, Usui M, Nishikawa T, Takita M, Shigeta M, Imai Y, et al. CIBIC Plus-J Assessment using a videotaped method in Alzheimer’s disease patients. Dement Geriatr Cogn Dis Extra 2012;2:271-7. http://dx.doi.org/10.1159/000339953.
- Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, et al. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 1986;149:698-709. https://doi.org/10.1192/bjp.149.6.698.
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 1968;114:797-811. https://doi.org/10.1192/bjp.114.512.797.
- Lee DY, Yoon JC, Lee KU, Jhoo JH, Kim KW, Lee JH, et al. Reliability and validity of the Korean version of Short Blessed Test (SBT-K) as a dementia screening instrument. J Korean Neuropsychiatr Assoc 1999;38:1365-75.
- Lam LC, Chiu HF, Li SW, Chan WF, Chan CK, Wong M, et al. Screening for dementia: a preliminary study on the validity of the Chinese version of the Blessed-Roth Dementia Scale. Int Psychogeriatr 1997;9:39-46. https://doi.org/10.1017/S1041610297004183.
- Yang YH, Lai CL, Lin RT, Tai CT, Liu CK. Cut-off values of blessed dementia rating scale and its clinical application in elderly Taiwanese. Kaohsiung J Med Sci 2006;22:377-84. https://doi.org/10.1016/S1607-551X(09)70326-2.
- Vajdicková K, Kolibás E, Heretik A, Kosc M. Use of behavioral scales in the diagnosis of dementia in the aged. Ceska Slov Psychiatr 1995;91:7-14.
- Davis PB, Morris JC, Grant E. Brief screening tests versus clinical staging in senile dementia of the Alzheimer type. J Am Geriatr Soc 1990;38:129-35. https://doi.org/10.1111/j.1532-5415.1990.tb03473.x.
- Zillmer EA, Fowler PC, Gutnick HN, Becker E. Comparison of two cognitive bedside screening instruments in nursing home residents: a factor analytic study. J Gerontol 1990;45:69-74. https://doi.org/10.1093/geronj/45.2.P69.
- Landes AM, Sperry SD, Strauss ME. Prevalence of apathy, dysphoria, and depression in relation to dementia severity in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 2005;17:342-9. https://doi.org/10.1176/jnp.17.3.342.
- Villardita C, Lomeo C. Alzheimer’s disease: correlational analysis of three screening tests and three behavioral scales. Acta Neurol Scand 1992;86:603-8. https://doi.org/10.1111/j.1600-0404.1992.tb05496.x.
- Kawas C, Karagiozis H, Resau L, Corrada M, Brookmeyer R. Reliability of the Blessed Telephone Information-Memory-Concentration Test. J Geriatr Psychiatry Neurol 1995;8:238-42. https://doi.org/10.1177/089198879500800408.
- Clark CM, Ewbank DC. Performance of the dementia severity rating scale: a caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Dis Assoc Disord 1996;10:31-9. https://doi.org/10.1097/00002093-199603000-00006.
- Xie SX, Ewbank DC, Chittams J, Karlawish JH, Arnold SE, Clark CM. Rate of decline in Alzheimer disease measured by a Dementia Severity Rating Scale. Alzheimer Dis Assoc Disord 2009;23:268-74. http://dx.doi.org/10.1097/WAD.0b013e318194a324.
- Newberg A, Cotter A, Udeshi M, Alavi A, Clark C. A metabolic imaging severity rating scale for the assessment of cognitive impairment. Clin Nucl Med 2003;28:565-70. http://dx.doi.org/10.1097/01.RLU.0000073662.73501.BD.
- Karlawish JH, Casarett DJ, James BD, Tenhave T, Clark CM, Asch DA. Why would caregivers not want to treat their relative’s Alzheimer’s disease?. J Am Geriatr Soc 2003;51:1391-7. https://doi.org/10.1046/j.1532-5415.2003.51456.x.
- Gottfries CG, Bråne G, Gullberg B, Steen G. A new rating scale for dementia syndromes. Arch Gerontol Geriatr 1982;1:311-30. https://doi.org/10.1016/0167-4943(82)90031-0.
- Bråne G, Gottfries CG, Winblad B. The Gottfries–Bråne–Steen scale: validity, reliability and application in anti-dementia drug trials. Dement Geriatr Cogn Disord 2001;12:1-14. https://doi.org/10.1159/000051230.
- Robert P, Ferris S, Gauthier S, Ihl R, Winblad B, Tennigkeit F. Review of Alzheimer’s disease scales: is there a need for a new multi-domain scale for therapy evaluation in medical practice?. Alzheimers Res Ther 2010;2. http://dx.doi.org/10.1186/alzrt48.
- Shader RI, Harmatz JS, Salzman C. A new scale for clinical assessment in geriatric populations: Sandoz Clinical Assessment – Geriatric (SCAG). J Am Geriatr Soc 1974;22:107-13. https://doi.org/10.1111/j.1532-5415.1974.tb01521.x.
- Venn RD. The Sandoz Clinical Assessment-Geriatric (SCAG) scale. A general-purpose psychogeriatric rating scale. Gerontology 1983;29:185-98. https://doi.org/10.1159/000213113.
- Herrmann WM, Stephan K, Gaede K, Apeceche M. A multicenter randomized double-blind study on the efficacy and safety of nicergoline in patients with multi-infarct dementia. Dement Geriatr Cogn Disord 1997;8:9-17. https://doi.org/10.1159/000106595.
- Gräsel E. When home care ends – changes in the physical health of informal caregivers caring for dementia patients: a longitudinal study. J Am Geriatr Soc 2002;50:843-9. https://doi.org/10.1046/j.1532-5415.2002.50209.x.
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566-72. https://doi.org/10.1192/bjp.140.6.566.
- O’Bryant SE, Lacritz LH, Hall J, Waring SC, Chan W, Khodr ZG, et al. Validation of the new interpretive guidelines for the clinical dementia rating scale sum of boxes score in the national Alzheimer’s coordinating center database. Arch Neurol 2010;67:746-9. https://doi.org/10.1001/archneurol.2010.115.
- Montaño MB, Ramos LR. Validity of the Portuguese version of Clinical Dementia Rating. Rev Saude Publica 2005;39:912-17. https://doi.org/10.1590/S0034-89102005000600007.
- Chaves ML, Camozzato AL, Godinho C, Kochhann R, Schuh A, de Almeida VL, et al. Validity of the clinical dementia rating scale for the detection and staging of dementia in Brazilian patients. Alzheimer Dis Assoc Disord 2007;21:210-17. http://dx.doi.org/10.1097/WAD.0b013e31811ff2b4.
- Berg L, Miller JP, Baty J, Rubin EH, Morris JC, Figiel G. Mild senile dementia of the Alzheimer type 4. Evaluation of intervention. Ann Neurol 1992;31:242-9. http://dx.doi.org/10.1002/ana.410310303.
- Zemlan FP. Velnacrine for the treatment of Alzheimer’s disease: a double-blind, placebo-controlled trial. The Mentane Study Group. J Neural Transm 1996;103:1105-16. http://dx.doi.org/10.1007/BF01291795.
- Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med 1997;336:1216-22. http://dx.doi.org/10.1056/NEJM199704243361704.
- Jones RW, Soininen H, Hager K, Aarsland D, Passmore P, Murthy A, et al. A multinational, randomised, 12-week study comparing the effects of donepezil and galantamine in patients with mild to moderate Alzheimer’s disease. Int J Geriatr Psychiatry 2004;19:58-67. http://dx.doi.org/10.1002/gps.1038.
- Rockwood K. Size of the treatment effect on cognition of cholinesterase inhibition in Alzheimer’s disease. J Neurol Neurosurg Psychiatr 2004;75:677-85. https://doi.org/10.1136/jnnp.2003.029074.
- Cortes F, Gillette-Guyonnet S, Nourhashemi F, Andrieu S, Cantet C, Vellas B. REAL.FR Group . Recent data on the natural history of Alzheimer’s disease: results from the REAL.FR Study. J Nutr Health Aging 2005;9:86-93.
- McLendon BM, Doraiswamy PM. Defining meaningful change in Alzheimer’s disease trials: the donepezil experience. J Geriatr Psychiatry Neurol 1999;12:39-48. https://doi.org/10.1177/089198879901200108.
- Tariot PN, Cummings JL, Katz IR, Mintzer J, Perdomo CA, Schwam EM, et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of donepezil in patients with Alzheimer’s disease in the nursing home setting. J Am Geriatr Soc 2001;49:1590-9. https://doi.org/10.1111/j.1532-5415.2001.49266.x.
- Imbimbo BP, Troetel WM, Martelli P, Lucchelli F. A 6-month, double-blind, placebo-controlled trial of eptastigmine in Alzheimer’s disease. Dement Geriatr Cogn Disord 2000;11:17-24. https://doi.org/10.1159/000017208.
- Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology 1998;50:136-45. https://doi.org/10.1212/WNL.50.1.136.
- Burns A, Rossor M, Hecker J, Gauthier S, Petit H, Möller HJ, et al. The effects of donepezil in Alzheimer’s disease – results from a multinational trial. Dement Geriatr Cogn Disord 1999;10:237-44. https://doi.org/10.1159/000017126.
- Summers WK, DeBoynton V, Marsh GM, Majovski LV. Comparison of seven psychometric instruments used for evaluation of treatment effect in Alzheimer’s dementia. Neuroepidemiology 1990;9:193-207. https://doi.org/10.1159/000110773.
- Román GC, Wilkinson DG, Doody RS, Black SE, Salloway SP, Schindler RJ. Donepezil in vascular dementia: combined analysis of two large-scale clinical trials. Dement Geriatr Cogn Disord 2005;20:338-44. https://doi.org/10.1159/000088494.
- Riepe MW, Adler G, Ibach B, Weinkauf B, Gunay I, Tracik F. Adding memantine to rivastigmine therapy in patients with mild to moderate Alzheimer’s disease: results of a 12-week, open-label pilot study. Prim Care Companion J Clin Psychiatry 2006;8:258-63. https://doi.org/10.4088/PCC.v08n0501.
- Tractenberg RE, Schafer K, Morris JC. Interobserver disagreements on clinical dementia rating assessment: interpretation and implications for training. Alzheimer Dis Assoc Disord 2001;15:155-61. https://doi.org/10.1097/00002093-200107000-00007.
- Tractenberg RE, Weiner MF, Cummings JL, Patterson MB, Thal LJ. Independence of changes in behavior from cognition and function in community-dwelling persons with Alzheimer’s disease: a factor analytic approach. J Neuropsychiatry Clin Neurosci 2005;17:51-60. https://doi.org/10.1176/jnp.17.1.51.
- Waite L, Grayson D, Jorm AF, Creasey H, Cullen J, Bennett H, et al. Informant-based staging of dementia using the clinical dementia rating. Alzheimer Dis Assoc Disord 1999;13:34-7. https://doi.org/10.1097/00002093-199903000-00005.
- Rockwood K, Strang D, MacKnight C, Downer R, Morris JC. Interrater reliability of the Clinical Dementia Rating in a multicenter trial. J Am Geriatr Soc 2000;48:558-9. https://doi.org/10.1111/j.1532-5415.2000.tb05004.x.
- O’Connor DW, Blessed G, Cooper B, Jonker C, Morris JC, Presnell IB, et al. Cross-national interrater reliability of dementia diagnosis in the elderly and factors associated with disagreement. Neurology 1996;47:1194-9. https://doi.org/10.1212/WNL.47.5.1194.
- Marin DB, Flynn S, Mare M, Lantz M, Hsu MA, Laurans M, et al. Reliability and validity of a chronic care facility adaptation of the Clinical Dementia Rating scale. Int J Geriatr Psychiatry 2001;16:745-50. https://doi.org/10.1002/gps.385.
- Morris JC, Ernesto C, Schafer K, Coats M, Leon S, Sano M, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s Disease Cooperative Study experience. Neurology 1997;48:1508-10. https://doi.org/10.1212/WNL.48.6.1508.
- McCulla MM, Coats M, Van Fleet N, Duchek J, Grant E, Morris JC. Reliability of clinical nurse specialists in the staging of dementia. Arch Neurol 1989;46:1210-11. https://doi.org/10.1001/archneur.1989.00520470070029.
- Haroutunian V, Perl DP, Purohit DP, Marin D, Khan K, Lantz M, et al. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch Neurol 1998;55:1185-91. https://doi.org/10.1001/archneur.55.9.1185.
- Burke WJ, Miller JP, Rubin EH, Morris JC, Coben LA, Duchek J, et al. Reliability of the Washington University Clinical Dementia Rating. Arch Neurol 1988;45:31-2. https://doi.org/10.1001/archneur.1988.00520250037015.
- Reisberg B. Global measures: utility in defining and measuring treatment response in dementia. Int Psychogeriatr 2007;19:421-56. https://doi.org/10.1017/S1041610207005261.
- Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Jeong Y, et al. The validity of the Korean version of Global Deterioration Scale. J Korean Neurol Assoc 2002;20:612-17.
- Solomon PR, Adams FA, Groccia ME, DeVeaux R, Growdon JH, Pendlebury WW. Correlational analysis of five commonly used measures of mental status/functional abilities in patients with Alzheimer disease. Alzheimer Dis Assoc Disord 1999;13:147-50. https://doi.org/10.1097/00002093-199907000-00006.
- Solomon TM, deBros GB, Budson AE, Mirkovic N, Murphy CA, Solomon PR. Correlational analysis of 5 commonly used measures of cognitive functioning and mental status: an update. Am J Alzheimers Dis Other Demen 2014;29:718-22. http://dx.doi.org/10.1177/1533317514534761.
- Eisdorfer C, Cohen D, Paveza GJ, Ashford JW, Luchins DJ, Gorelick PB, et al. An empirical evaluation of the Global Deterioration Scale for Staging Alzheimer’s Disease. Am J Psychiatry 1992;149:190-4.
- Reisberg B, Franssen EH, Bobinski M, Auer S, Monteiro I, Boksay I, et al. Overview of methodologic issues for pharmacologic trials in mild, moderate, and severe Alzheimer’s disease. Int Psychogeriatr 1996;8:159-93. https://doi.org/10.1017/S1041610296002566.
- Auer SR, Reisberg B. Reliability of the Modified Ordinal Scales of Psychological Development: a cognitive assessment battery for severe dementia. Int Psychogeriatr 1996;8:225-31. https://doi.org/10.1017/S1041610296002608.
- Choi SH, Lee BH, Kim S, Hahm DS, Jeong JH, Yoon SJ, et al. Interchanging scores between clinical dementia rating scale and global deterioration scale. Alzheimer Dis Assoc Disord 2003;17:98-105. https://doi.org/10.1097/00002093-200304000-00008.
- Bobinski M, de Leon MJ, Wegiel J, Desanti S, Convit A, Saint Louis LA, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience 1999;95:721-5. https://doi.org/10.1016/S0306-4522(99)00476-5.
- Jack CR, Dickson DW, Parisi JE, Xu YC, Cha RH, O’Brien PC, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 2002;58:750-7. https://doi.org/10.1212/WNL.58.5.750.
- Apostolova LG, Zarow C, Biado K, Hurtz S, Boccardi M, Somme J, et al. Relationship between hippocampal atrophy and neuropathology markers: a 7T MRI validation study of the EADC-ADNI Harmonized Hippocampal Segmentation Protocol. Alzheimers Dement 2015;11:139-50. http://dx.doi.org/10.1016/j.jalz.2015.01.001.
- O’Brien JT, Paling S, Barber R, Williams ED, Ballard C, McKeith IG, et al. Progressive brain atrophy on serial MRI in dementia with Lewy bodies, AD, and vascular dementia. Neurology 2001;56:1386-8. https://doi.org/10.1212/WNL.56.10.1386.
- Jack CR, Shiung MM, Gunter JL, O’Brien PC, Weigand SD, Knopman DS, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 2004;62:591-600. https://doi.org/10.1212/01.WNL.0000110315.26026.EF.
- Fox NC, Scahill RI, Crum WR, Rossor MN. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology 1999;52:1687-9. https://doi.org/10.1212/WNL.52.8.1687.
- Henneman WJ, Sluimer JD, Barnes J, van der Flier WM, Sluimer IC, Fox NC, et al. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology 2009;72:999-1007. http://dx.doi.org/10.1212/01.wnl.0000344568.09360.31.
- Sluimer JD, Vrenken H, Blankenstein MA, Fox NC, Scheltens P, Barkhof F, et al. Whole-brain atrophy rate in Alzheimer disease: identifying fast progressors. Neurology 2008;70:1836-41. http://dx.doi.org/10.1212/01.wnl.0000311446.61861.e3.
- Sluimer JD, Bouwman FH, Vrenken H, Blankenstein MA, Barkhof F, van der Flier WM, et al. Whole-brain atrophy rate and CSF biomarker levels in MCI and AD: a longitudinal study. Neurobiol Aging 2010;31:758-64. http://dx.doi.org/10.1016/j.neurobiolaging.2008.06.016.
- Jack CR, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain 2009;132:1355-65. http://dx.doi.org/10.1093/brain/awp062.
- Archer HA, Edison P, Brooks DJ, Barnes J, Frost C, Yeatman T, et al. Amyloid load and cerebral atrophy in Alzheimer’s disease: an 11C-PIB positron emission tomography study. Ann Neurol 2006;60:145-7. http://dx.doi.org/10.1002/ana.20889.
- Marshall J, Martin T, Downie J, Malisza K. A comprehensive analysis of MRI research risks: in support of full disclosure. Can J Neurol Sci 2007;34:11-7. https://doi.org/10.1017/S0317167100005734.
- Shellock FG, Crues JV. MR procedures: biologic effects, safety, and patient care. Radiology 2004;232:635-52. http://dx.doi.org/10.1148/radiol.2323030830.
- Harris LM, Robinson J, Menzies RG. Evidence for fear of restriction and fear of suffocation as components of claustrophobia. Behav Res Ther 1999;37:155-9. https://doi.org/10.1016/S0005-7967(98)00110-7.
- Katz RC, Wilson L, Frazer N. Anxiety and its determinants in patients undergoing magnetic resonance imaging. J Behav Ther Exp Psychiatry 1994;25:131-4. https://doi.org/10.1016/0005-7916(94)90005-1.
- Mattis S, Karasu T, Bellack L, Karusu TB. Geriatric Psychiatry: A Handbook for Psychiatrics and Primary Care Physicians. New York, NY: Grune and Stratton; 1976.
- Welsh K, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using Telephone Screening of Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol 1993;6:103-10.
- Ferris SH. General measures of cognition. Int Psychogeriatr 2003;15:215-17. https://doi.org/10.1017/S1041610203009220.
- Veroff AE, Cutler NR, Sramek JJ, Prior PL, Mickelson W, Hartman JK. A new assessment tool for neuropsychopharmacologic research: the Computerized Neuropsychological Test Battery. J Geriatr Psychiatry Neurol 1991;4:211-17. https://doi.org/10.1177/089198879100400406.
- Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology 2000;55:1621-6. https://doi.org/10.1212/WNL.55.11.1621.
- Carlesimo GA, Caltagirone C, Gainotti G. The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol 1996;36:378-84. https://doi.org/10.1159/000117297.
- Wechsler D. The Measurement and Appraisal of Adult Intelligence. Baltimore, MD: Williams & Wilkins Company; 1939.
- Saxton J, McGoingle-Gibson K, Swihart A, Miller M, Boller F. Assessment of the severely impaired patient: description and validation of a new neuropsychological test battery. Psychol Assess 1990;2:298-303. https://doi.org/10.1037/1040-3590.2.3.298.
- Overall JE, Schaltenbrand R. The SKT neuropsychological test battery. J Geriatr Psychiatry Neurol 1992;5:220-7. https://doi.org/10.1177/002383099200500407.
- Wechsler D. Wechsler Memory Scale. San Antonio, TX: Psychological Corporation; 1945.
- Kertesz A, Poole E. The aphasia quotient: the taxonomic approach to measurement of aphasic disability. Can J Neurol Sci 1974;1:7-16.
- Lezak M. Neuropsychological Assessment. Oxford: Oxford University Press; 2004.
- Buschke H. Selective reminding for analysis of memory and learning. J Verb Learn Verb Beh 1973;12:543-50. https://doi.org/10.1016/S0022-5371(73)80034-9.
- Benton AL. A visual retention test for clinical use. Arch Neurol Psychiatry 1945;54:212-16. https://doi.org/10.1001/archneurpsyc.1945.02300090051008.
- Brodaty H, Moore CM. The clock drawing test for dementia of the Alzheimer’s type: a comparison of three scoring methods in a memory disorders clinic. Int J Geriatr Psychiatry 1997;12:619-27. https://doi.org/10.1002/(SICI)1099-1166(199706)12:6<619::AID-GPS554>3.0.CO;2-H.
- Benton A, Hamsher K, Sivan AB. Multilingual Aplasia Examination. Iowa City, IA: AJA Associates; 1983.
- Wechsler D. The Wechsler Intelligence Scale for Children. San Antonio, TX: The Psychological Corporation; 1949.
- Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11:13-21. https://doi.org/10.1097/00002093-199700112-00003.
- Rey A. The Clinical Examination in Psychology. Paris: University Press of France; 1964.
- Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol 1935;18:643-62. https://doi.org/10.1037/h0054651.
- De Renzi E, Vignolo LA. The token test: a sensitive test to detect receptive disturbances in aphasics. Brain 1962;85:665-78. https://doi.org/10.1093/brain/85.4.665.
- Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, AZ: Neuropsychology Press; 1985.
- Cohen-Mansfield J. Agitated behaviors in the elderly. II. Preliminary results in the cognitively deteriorated. J Am Geriatr Soc 1986;34:722-7. https://doi.org/10.1111/j.1532-5415.1986.tb04303.x.
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011;168:1266-77. http://dx.doi.org/10.1176/appi.ajp.2011.10111704.
- Alexopoulos G, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biol Psychiatry 1998;23:271-84. http://dx.doi.org/10.1016/0006-3223(88)90038-8.
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17:37-49. https://doi.org/10.1016/0022-3956(82)90033-4.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23. https://doi.org/10.1136/jnnp.23.1.56.
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382-9. https://doi.org/10.1192/bjp.134.4.382.
- von Zerssen D, Koeller DM, Rey ER. A scale for the objective evaluation of the state of subjective well-being as a method for longitudinal studies. Arzneimittelforschung 1970;20:915-18.
- National Institute on Aging Working Group . Consensus Report of the Working Group on: Molecular and Biochemical Markers of Alzheimer’s Disease. Neurobiol Aging 1998;2:109-16.
- Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h369.
- Ryu SH, Katona C, Rive B, Livingston G. Persistence of and changes in neuropsychiatric symptoms in Alzheimer disease over 6 months: the LASER-AD study. Am J Geriatr Psychiatry 2005;13:976-83. https://doi.org/10.1176/appi.ajgp.13.11.976.
- Spalletta G, Musicco M, Padovani A, Rozzini L, Perri R, Fadda L, et al. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. Am J Geriatr Psychiatry 2010;18:1026-35. https://doi.org/10.1097/JGP.0b013e3181d6b68d.
- Livingston G, Johnston K, Katona C, Paton J, Lyketsos CG. Old Age Task Force of the World Federation of Biological Psychiatry . Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021. https://doi.org/10.1176/appi.ajp.162.11.1996.
- Reisberg B, Monteiro I, Torossian C, Auer S, Shulman MB, Ghimire S, et al. The BEHAVE-AD assessment system: a perspective, a commentary on new findings, and a historical review. Dement Geriatr Cogn Disord 2014;38:89-146. http://dx.doi.org/10.1159/000357839.
- Cohen-Mansfield J, Billig N. Agitated behaviors in the elderly. I. A conceptual review. J Am Geriatr Soc 1986;34:711-21. https://doi.org/10.1111/j.1532-5415.1986.tb04302.x.
- Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 2012;366:893-90. http://dx.doi.org/10.1056/NEJMoa1106668.
- Kostka J, Borowiak E, Kostka T. Nutritional status and quality of life in different populations of older people in Poland. Eur J Clin Nutr 2014;68:1210-15. http://dx.doi.org/10.1038/ejcn.2014.172.
- Burns A, Lawlor B, Craig S. Rating scales in old age psychiatry. Br J Psychiatry 2002;180:161-7. http://dx.doi.org/10.1192/bjp.180.2.161.
- Giebel CM, Sutcliffe C, Challis D. Activities of daily living and quality of life across different stages of dementia: a UK study. Aging Ment Health 2015;19:63-71. http://dx.doi.org/10.1080/13607863.2014.915920.
- Bullinger M, Anderson R, Cella D, Aaronson N. Developing and evaluating cross-cultural instruments from minimum requirements to optimal models. Qual Life Res 1993;2:451-9. https://doi.org/10.1007/BF00422219.
- Cotrell V, Schulz R. The perspective of the patient with Alzheimer’s disease: a neglected dimension of dementia research. Gerontologist 1993;33:205-11. https://doi.org/10.1093/geront/33.2.205.
- Brod M, Stewart AL, Sands L. Conceptualisation of quality of life in dementia. J Ment Health Aging 1995;5:7-19.
- Kitwood T, Bredin K. Towards a theory of dementia care: personhood and well-being. Ageing Soc 1992;12:269-87. https://doi.org/10.1017/S0144686X0000502X.
- Brod M, Stewart AL, Sands L, Walton P. Conceptualization and measurement of quality of life in dementia: the dementia quality of life instrument (DQoL). Gerontologist 1999;39:25-3. https://doi.org/10.1093/geront/39.1.25.
- Black B, Rabins PV, Kasper JD. Alzheimer’s Disease Related Quality of Life (ADRQL) Users’ Manual. Baltimore, MD: Johns Hopkins School of Medicine; 2002.
- Smith S, Lamping D, Banerjee S, Harwood RH, Foley B, Smith P, et al. The development of a new measure of health related quality of life for people with dementia: DEMQOL. Psychol Med 2007;37:737-46. https://doi.org/10.1017/S0033291706009469.
- Banerjee S. Commentary on ‘Health economics and the value of therapy in Alzheimer’s disease’. Quality of life in dementia: development and use of a disease-specific measure of health-related quality of life in dementia. Alzheimers Dement 2007;3:166-71. http://dx.doi.org/10.1016/j.jalz.2007.04.384.
- Banerjee S, Samsi K, Petrie CD, Alvir J, Treglia M, Schwam EM, et al. What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and explanatory value of disease specific measures of health related quality of life in people with dementia. Int J Geriatr Psychiatry 2009;24:15-24. http://dx.doi.org/10.1002/gps.2090.
- Banerjee S, Smith SC, Lamping DL, Harwood RH, Foley B, Smith P, et al. Quality of life in dementia: more than just cognition. An analysis of associations with quality of life in dementia. J Neurol Neurosurg Psychiatr 2006;77:146-8. https://doi.org/10.1136/jnnp.2005.072983.
- Rowen D, Mulhern B, Banerjee S, van Hout B, Young TA, Knapp M, et al. Estimating preference-based single index measures for dementia using DEMQOL and DEMQOL-Proxy. Value Health 2012;15:346-56. http://dx.doi.org/10.1016/j.jval.2011.10.016.
- Mulhern B, Smith SC, Rowen D, Brazier JE, Knapp M, Lamping DL, et al. Improving the measurement of QALYs in dementia: developing patient- and carer-reported health state classification systems using Rasch analysis. Value Health 2012;15:323-33. http://dx.doi.org/10.1016/j.jval.2011.09.006.
- Mulhern B, Rowen D, Brazier J, Smith S, Romeo R, Tait R, et al. Development of DEMQOL-U and DEMQOL-PROXY-U: generation of preference-based indices from DEMQOL and DEMQOL-PROXY for use in economic evaluation. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17050.
- Rikkert MG, Tona KD, Janssen L, Burns A, Lobo A, Robert P, et al. Validity, reliability, and feasibility of clinical staging scales in dementia: a systematic review. Am J Alzheimers Dis Other Demen 2011;26:357-65. http://dx.doi.org/10.1177/1533317511418954.
- Zhou B, Teramukai S, Yoshimura K, Fukushima M. Validity of cerebrospinal fluid biomarkers as endpoints in early-phase clinical trials for Alzheimer’s disease. J Alzheimers Dis 2009;18:89-102. http://dx.doi.org/10.3233/JAD-2009-1124.
- Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR. Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord 2013;36:242-50. http://dx.doi.org/10.1159/000351671.
- Thomas RG, Albert M, Petersen RC, Aisen PS. Longitudinal decline in mild to moderate Alzheimer’s disease: analyses of placebo data from clinical trials. Alzheimers Dement 2016;12:598-603. http://dx.doi.org/10.1016/j.jalz.2016.01.002.
- Regan C, Katona C, Walker Z, Hooper J, Donovan J, Livingston G. Relationship of vascular risk to the progression of Alzheimer disease. Neurology 2006;67:1357-62. https://doi.org/10.1212/01.wnl.0000240129.46080.53.
- Fox NC, Cousens S, Scahill R, Harvey RJ, Rossor MN. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: power calculations and estimates of sample size to detect treatment effects. Arch Neurol 2000;57:339-44. https://doi.org/10.1001/archneur.57.3.339.
- Leung KK, Clarkson MJ, Bartlett JW, Clegg S, Jack CR, Weiner MW, et al. Robust atrophy rate measurement in Alzheimer’s disease using multi-site serial MRI: tissue-specific intensity normalization and parameter selection. Neuroimage 2010;50:516-23. http://dx.doi.org/10.1016/j.neuroimage.2009.12.059.
- Sommerlad A, Singleton D, Jones R, Banerjee S, Livingston G. Development of an instrument to assess social functioning in dementia: the Social Functioning in Dementia scale (SF-DEM). Alzheimers Dement 2017;7:88-9. https://doi.org/10.1016/j.dadm.2017.02.001.
- Livingston G, Leavey G, Manela M, Livingston D, Rait G, Sampson E, et al. Making decisions for people with dementia who lack capacity: qualitative study of family carers in UK. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4184.
- Martyr A, Clare L. Awareness of functional ability in people with early-stage dementia [published online ahead of print January 10 2017]. Int J Geriatr Psychiatry 2017. https://doi.org/10.1002/gps.4664.
- Gillespie R, Mullan J, Harrison L. Managing medications: the role of informal caregivers of older adults and people living with dementia. A review of the literature. J Clin Nurs 2014;23:3296-308. http://dx.doi.org/10.1111/jocn.12519.
- Poland F, Mapes S, Pinnock H, Katona C, Sorensen S, Fox C, et al. Perspectives of carers on medication management in dementia: lessons from collaboratively developing a research proposal. BMC Res Notes 2014;7. http://dx.doi.org/10.1186/1756-0500-7-463.
- Black BS, Taylor H, Rabins PV, Karlawish J. Researchers’ perspectives on the role of study partners in dementia research. Int Psychogeriatr 2014;26:1649-57. http://dx.doi.org/10.1017/S1041610214001203.
- Grill JD, Zhou Y, Karlawish J, Elashoff D. Frequency and impact of informant replacement in Alzheimer disease research. Alzheimer Dis Assoc Disord 2015;29:242-8. http://dx.doi.org/10.1097/WAD.0000000000000078.
- Dewing J. Participatory research: a method for process consent with persons who have dementia. Dementia 2007;6:11-25. https://doi.org/10.1177/1471301207075625.
- Appelbaum PS, Roth LH, Lidz CW, Benson P, Winslade W. False hopes and best data: consent to research and the therapeutic misconception. Hastings Cent Rep 1987;17:20-4. https://doi.org/10.2307/3562038.
- Snowdon C, Garcia J, Elbourne D. Making sense of randomization; responses of parents of critically ill babies to random allocation of treatment in a clinical trial. Soc Sci Med 1997;45:1337-55. https://doi.org/10.1016/S0277-9536(97)00063-4.
- Featherstone K, Donovan JL. ‘Why don’t they just tell me straight, why allocate it?’ The struggle to make sense of participating in a randomised controlled trial. Soc Sci Med 2002;55:709-19. https://doi.org/10.1016/S0277-9536(01)00197-6.
- Riordan F, Thomson AP. How to get patients’ consent to enter clinical trials. Reports of trials should state proportion of people who refuse to participate. BMJ 1996;312:185-6. https://doi.org/10.1136/bmj.312.7024.185c.
- Schwartz CE, Fox BH. Who says yes? Identifying selection biases in a psychosocial intervention study of multiple sclerosis. Soc Sci Med 1995;40:359-70. https://doi.org/10.1016/0277-9536(94)E0092-7.
- Bevan EG, Chee LC, McGhee SM, McInnes GT. Patients’ attitudes to participation in clinical trials. Br J Clin Pharmacol 1993;35:204-7. https://doi.org/10.1111/j.1365-2125.1993.tb05687.x.
- Charles C, Redko C, Whelan T, Gafni A, Reyno L. Doing nothing is no choice: lay constructions of treatment decision-making among women with early-stage breast cancer. Soc Sci Med 1978;20:71-95.
- Daugherty C, Ratain MJ, Grochowski E, Stocking C, Kodish E, Mick R, et al. Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol 1995;13:1062-72. http://dx.doi.org/10.1200/jco.1995.13.5.1062.
- Godskesen T, Hansson MG, Nygren P, Nordin K, Kihlbom U. Hope for a cure and altruism are the main motives behind participation in phase 3 clinical cancer trials. Eur J Cancer Care 2015;24:133-41. http://dx.doi.org/10.1111/ecc.12184.
- Cox K, Avis M. Psychosocial aspects of participation in early anticancer drug trials. Report of a pilot study. Cancer Nurs 1996;19:177-86. https://doi.org/10.1097/00002820-199606000-00004.
- Galimberti D, Scarpini E. Emerging amyloid disease modifying drugs for Alzheimer’s disease. Expert Opin Emerg Drugs 2016;21:5-6. http://dx.doi.org/10.1517/14728214.2016.1146678.
- Enhancing Translational Research and Clinical Development for Alzheimer’s Disease and other Dementias. Paris: Organisation for Economic Co-operation and Development; 2015.
- Duits F, Martinez-Lage P, Paquet C, Engelborghs S, Lleó A, Hausner L, et al. Performance and complications of lumbar puncture in memory clinics: results of the multicenter lumbar puncture feasibility study. Alzheimers Dement 2016;12:154-63. http://dx.doi.org/10.1016/j.jalz.2015.08.003.
- Guidance for Industry Alzheimer’s Disease: Developing Drugs for the Treatment of Early Stage Disease. Silver Spring, MD: Food and Drug Administration; 2013.
- Draft Guideline on the Clinical Investigation of Medicines for the Treatment of Alzheimer’s Disease and Other Dementias. London: European Medicines Agency; 2016.
- Vellas B, Andrieu S, Sampaio C, Wilcock G. Disease-modifying trials in Alzheimer’s disease: a European task force consensus. Lancet Neurol 2007;6:56-62.
- Vellas B, Andrieu S, Sampaio C, Coley N, Wilcock G. European Task Force Group . Endpoints for trials in Alzheimer’s disease: a European task force consensus. Lancet Neurol 2008;7:436-50. http://dx.doi.org/10.1016/S1474-4422(08)70087-5.
- ICHOM . Standard Set for Dementia 2016. www.ichom.org/medical-conditions/dementia/ (accessed 21 April 2016).
- Moniz-Cook E, Vernooij-Dassen M, Woods R, Verhey F, Chattat R, De Vugt M, et al. A European consensus on outcome measures for psychosocial intervention research in dementia care. Aging Ment Health 2008;12:14-29. http://dx.doi.org/10.1080/13607860801919850.
- Mountain G, Moniz-Cook ED, Øksnebjerg L. Dementia Outcome Measures: Charting New Territory. Brussels: JPND; 2015.
- Martin GP. ‘Ordinary people only’: knowledge, representativeness, and the publics of public participation in healthcare. Sociol Health Illn 2008;30:35-54. http://dx.doi.org/10.1111/j.1467-9566.2007.01027.x.
- Ellis-Smith C, Evans CJ, Bone AE, Henson LA, Dzingina M, Kane PM, et al. Measures to assess commonly experienced symptoms for people with dementia in long-term care settings: a systematic review. BMC Med 2016;14. http://dx.doi.org/10.1186/s12916-016-0582-x.
- Brookmeyer R, Kawas CH, Abdallah N, Paganini-Hill A, Kim RC, Corrada MM. Impact of interventions to reduce Alzheimer’s disease pathology on the prevalence of dementia in the oldest-old. Alzheimers Dement 2016;12:225-32. http://dx.doi.org/10.1016/j.jalz.2016.01.004.
- Adami M, Scarpini E, Bruno G, Zappala G, Richarz U, Gaudig M, et al. Cessation versus continuation of 12 months galantamine therapy in patients with Alzheimer’s disease: a randomised, double blind, placebo controlled withdrawal trial. Alzheimers Dement 2011;1. https://doi.org/10.1016/j.jalz.2011.05.2276.
Appendix 1 MEDLINE (via OvidSP) search strategy
Search strategy
-
exp Alzheimer Disease/
-
exp Dementia, Vascular/
-
exp ‘Pick Disease of the Brain’/
-
exp Dementia, Multi-Infarct/
-
exp Cognition Disorders/ or exp Dementia/
-
dement*.mp.
-
alzheimer*.mp.
-
(lewy* and dement*).af.
-
multiple infarcts.mp.
-
exp Supranuclear Palsy, Progressive/
-
(pick adj5 disease).mp.
-
‘Frontotemporal Dementia’.mp. or exp Frontotemporal Dementia/
-
(park* and dement*).af.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13
-
exp ‘Outcome Assessment (Health Care)’/
-
‘outcome’.mp.
-
‘instrument’.mp.
-
‘measure’.mp.
-
outcome*.mp.
-
instrument*.mp.
-
measure*.mp.
-
16 or 17 or 18 or 19 or 20 or 21
-
intervention.mp.
-
therap*.mp.
-
trial*.mp.
-
23 or 24 or 25
-
control*.mp.
-
22 or 26 or 27
-
limit 28 to english language
Appendix 2 Disagreements on exclusion of full texts
The raters disagreed on the inclusion of four full texts.
For the first paper,33 across the three raters there were two exclusions and one inclusion, with disagreement about the trial’s aim being symptomatic or disease modification. After looking at a second paper (within the database) that was referring to the same trial,32 it was much clearer that the aim of the trial was disease modification, so we agreed by consensus to include the first paper.
For the second paper, there was disagreement between raters – two raters excluded and one included – and we agreed to exclude, as it was a conference abstract. 507
The third and fourth paper referred to the same trial,25,26 and across the three raters there was one include, one undecided and one exclude. The raters could not reach a consensus, so we consulted with Gill Livingston who decided it should be included as the intervention was aiming to change neuronal dysfunction in AD.
Appendix 3 List of excluded studies and reasons for exclusion
| Reference | Reason for exclusion |
|---|---|
| Aarsland D, Ballard C, Walker Z, Bostrom F, Alves G, Kossakowski K, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol 2009;8:613–18 | Not a disease modification trial |
| AbbVie. NCT02573740 Safety, Tolerability and the Effects on Cerebrospinal Fluid Spectrin Breakdown Product-145 Levels of ABT-957 in Subjects With Mild Alzheimer’s Disease and Mild Cognitive Impairment. URL: https://clinicaltrials.gov/ct2/show/NCT02573740 (accessed 29 January 2016) | No quantitative outcome relating to disease modification |
| Adair JC, Knoefel JE, Morgan N. Controlled trial of N-acetylcysteine for patients with probable Alzheimer’s disease. Neurology 2001;57:1515–17 | Not a disease modification trial |
| Adami M, Scarpini E, Bruno G, Zappala G, Richarz U, Gaudig M, et al. Cessation versus continuation of 12 months galantamine therapy in patients with Alzheimer’s disease: a randomised, double blind, placebo controlled withdrawal trial. Alzheimers Dement 2011;1:S794 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Adamus WS, Leonard JP, Tröger W. Phase I clinical trials with WAL 2014, a new muscarinic agonist for the treatment of Alzheimer’s disease. Life Sci 1995;56:883–90 | No participants with mild or moderate dementia |
| Agid Y, Dubois B, Anand R, Gharabawi G. Efficacy and tolerability of rivastigmine in patients with dementia of the Alzheimer type. Curr Ther Res Clin Exp 1998;59:837–45 | Not a disease modification trial |
| Aguglia E, Onor ML, Saina M, Maso E. An open-label, comparative study of rivastigmine, donepezil and galantamine in a real-world setting. Curr Med Res Opin 2004;20:1747–52 | Not a disease modification trial |
| Aguiar P, Monteiro L, Feres A, Gomes I, Melo A. Rivastigmine transdermal patch and physical exercises for Alzheimer’s disease: a randomised clinical trial. Curr Alzheimer Res 2014;11:532–7 | Not a disease modification trial |
| Aguirre E, Spector A, Hoe J, Russell IT, Knapp M, Woods RT, et al. Maintenance Cognitive Stimulation Therapy (CST) for dementia: a single-blind, multi-centre, randomised controlled trial of Maintenance CST vs. CST for dementia. Trials 2010;11:46 | Not a disease modification trial |
| Ahlin A, Nyback H, Junthe T, Ohman G, Nordgren I. Tetrahydroaminoacridine in Alzheimer’s dementia: clinical and biochemical results of a double–blind crossover trial. Hum Psychopharmacol 1991;6:109–18 | Not a disease modification trial |
| Ahmed MA, Darwish ES, Khedr EM, El Serogy YM, Ali AM. Effects of low versus high frequencies of repetitive transcranial magnetic stimulation on cognitive function and cortical excitability in Alzheimer’s dementia. J Neurol 2012;259:83–92 | Not a disease modification trial |
| Akanuma K, Meguro K, Meguro M, Sasaki E, Chiba K, Ishii H, et al. Improved social interaction and increased anterior cingulate metabolism after group reminiscence with reality orientation approach for vascular dementia. Psychiatry Res 2011;192:183–7 | Not a disease modification trial |
| Akhondzadeh S, Noroozian M, Mohammadi M, Ohadinia S, Jamshidi AH, Khani M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: a double blind, randomised, placebo controlled trial. J Neurol Neurosurg Psychiatry 2003;74:863–6 | Not a disease modification trial |
| Akhondzadeh S, Noroozian M, Mohammadi M, Ohadinia S, Jamshidi AH, Khani M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: a double blind, randomised and placebo-controlled trial. J Clin Pharm Ther 2003;28:53–9 | Not a disease modification trial |
| Alva G, Grossberg GT, Schmitt FA, Meng X, Olin JT. Efficacy of rivastigmine transdermal patch on activities of daily living: item responder analyses. Int J Geriatr Psychiatry 2011;26:356–63 | Not a disease modification trial |
| Alva G, Isaacson R, Sadowsky C, Grossberg G, Meng X, Somogyi M. Efficacy of higher-dose 13.3 mg/24 h (15 cm2) rivastigmine patch on the Alzheimer’s Disease Assessment Scale-cognitive subscale: Domain and individual item analysis. Int J Geriatr Psychiatry 2014;29:920–7 | Not a disease modification trial |
| Alvarez XA, Cacabelos R, Sampedro C, Couceiro V, Aleixandre M, Vargas M, et al. Combination treatment in Alzheimer’s disease: results of a randomised, controlled trial with cerebrolysin and donepezil. Curr Alzheimer Res 2011;8:583–91 | Not a disease modification trial |
| Alvarez A, Iglesias O, Aleixandre M, Linares C, Figueroa J, Muresanu D, et al. Cerebrolysin and combination therapy enhance serum BDNF in AD patients. Alzheimer’s Dement 2014;10:774 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Amenta F, Carotenuto A, Fasanaro AM, Rea R, Traini E. The ASCOMALVA trial: association between the cholinesterase inhibitor donepezil and the cholinergic precursor choline alphoscerate in Alzheimer’s disease with cerebrovascular injury: interim results. J Neurol Sci 2012;322:96–101 | Not a disease modification trial |
| Amici S, Lanari A, Romani R, Antognelli C, Gallai V, Parnetti L. Cerebrospinal fluid acetylcholinesterase activity after long-term treatment with donepezil and rivastigmine. Mech Ageing Dev 2001;122:2057–62 | Not a disease modification trial |
| Amieva H, Dartigues JF. ETNA3, a clinical randomised study assessing three cognitive-oriented therapies in dementia: rationale and general design. Rev Neurol 2013;169:752–6 | Not a disease modification trial |
| Andersen F, Viitanen M, Halvorsen DS, Straume B, Wilsgaard T, Engstad TA. The effect of stimulation therapy and donepezil on cognitive function in Alzheimer’s disease. A community based RCT with a two-by-two factorial design. BMC Neurol 2012;12:59 | Not a disease modification trial |
| Andrade LP, Gobbi LT, Coelho FG, Christofoletti G, Costa JL, Stella F. Benefits of multimodal exercise intervention for postural control and frontal cognitive functions in individuals with Alzheimer’s disease: a controlled trial. J Am Geriatr Soc 2013;61:1919–26 | Not a disease modification trial |
| Annweiler C, Herrmann FR, Fantino B, Brugg B, Beauchet O. Effectiveness of the combination of memantine plus vitamin D on cognition in patients with Alzheimer disease: a pre–post pilot study. Cogn Behav Neurol 2012;25:121–7 | Not a RCT/CCT |
| Antonanzas F, Rive B, Badenas JM, Gomez-Lus S, Guilhaume C. Cost-effectiveness of memantine in community-based Alzheimer’s disease patients: an adaptation in Spain. Eur J Health Econ 2006;7:137–44 | Not a disease modification trial |
| Araki T, Wake R, Miyaoka T, Kawakami K, Nagahama M, Furuya M, et al. The effects of combine treatment of memantine and donepezil on Alzheimer’s disease patients and its relationship with cerebral blood flow in the prefrontal area. Int J Geriatr Psychiatry 2014;29:881–9 | Not a disease modification trial |
| Arcoverde C, Deslandes A, Moraes H, Almeida C, Araujo NB, Vasques PE, et al. Treadmill training as an augmentation treatment for Alzheimer’s disease: a pilot randomised controlled study. Arq Neuropsiquiatr 2014;72:190–6 | Not a disease modification trial |
| Arlt S, Muller-Thomsen T, Beisiegel U, Kontush A. Effect of one-year vitamin C- and E-supplementation on cerebrospinal fluid oxidation parameters and clinical course in Alzheimer’s disease. Neurochem Res 2012;37:2706–14 | Not a disease modification trial |
| Aronson S, Van Baelen B, Kavanagh S, Schwalen S. Optimal dosing of galantamine in patients with mild or moderate Alzheimer’s disease: post hoc analysis of a randomised, double-blind, placebo-controlled trial. Drugs Ageing 2009;26:231–9 | Not a disease modification trial |
| Arrigo A, Moglia A, Borsotti L. A double-blind, placebo-controlled, crossover trial with nicergoline in patients with senile dementia. Int J Clin Pharmacol Res 1982;2(Suppl. 1):33–41 | Not a disease modification trial |
| Ash E, Bregman N, Moore O, Korczyn A, Zangen A. Transcranial magnetic stimulation of deep brain regions in Alzheimer’s disease. Alzheimer’s Dement 2014;10:P450 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Ash EL, Vakhapova V, Bova I, Simon E, Korem M, Eldad M, et al. Transcranial magnetic stimulation of deep brain regions in Alzheimer’s disease: a pilot study. Ann Neurol 2012;72:S126 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Ashford JW, Adamson M, Beale T, La D, Hernandez B, Noda A, et al. MR spectroscopy for assessment of memantine treatment in mild to moderate Alzheimer dementia. J Alzheimers Dis 2011;26(Suppl. 3):331–6 | Not a disease modification trial |
| Asp E, Cloutier F, Fay S, Cook C, Robertson ML, Fisk J, et al. Verbal repetition in patients with Alzheimer’s disease who receive donepezil. Int J Geriatr Psychiatry 2006;21:426–31 | Not a disease modification trial |
| Asthana S, Baker LD, Craft S, Stanczyk FZ, Veith RC, Raskind MA, et al. High-dose estradiol improves cognition for women with AD: results of a randomised study. Neurology 2001;57:605–12 | Not a disease modification trial |
| Atri A, Hendrix S, Pejovic V, Graham S. Extended-release daily memantine provides increasing cumulative benefits across clinical domains over 24 weeks in patients with moderate to severe Alzheimer’s disease: an analysis of area under the curve. Neurology 2014;82:P1-006 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Atri A, Molinuevo JL, Lemming O, Wirth Y, Pulte I, Wilkinson D. Memantine in patients with Alzheimer’s disease receiving donepezil: new analyses of efficacy and safety for combination therapy. Alzheimers Res Ther 2013;5:6 | Not a disease modification trial |
| Atri A, Shaughnessy LW, Locascio JJ, Growdon JH. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Dis Assoc Disord 2008;22:209–21 | Not a disease modification trial |
| Auchus A, Brashear H, Salloway S, Korczyn A, De Deyn P, Gassmann-Mayer C. Galantamine treatment of vascular dementia: a randomised trial. Neurology 2007;69:448–58 | Not a disease modification trial |
| Avila R, Carvalho IA, Bottino CM, Miotto EC. Neuropsychological rehabilitation in mild and moderate Alzheimer’s disease patients. Behav Neurol 2007;18:225–33 | Not a disease modification trial |
| Bachynsky J, McCracken P, Lier D, Alloul K, Jacobs P. Propentofylline treatment for Alzheimer disease and vascular dementia: an economic evaluation based on functional abilities. Alzheimer Dis Assoc Disord 2000;14:102–11 | Not a disease modification trial |
| Bakchine S, Loft H. Memantine treatment in patients with mild to moderate Alzheimer’s disease: results of a randomised, double-blind, placebo-controlled 6-month study. J Alzheimers Dis 2007;11:471–9 | Not a disease modification trial |
| Ballard C, Sauter M, Scheltens P, He Y, Barkhof F, van Straaten ECW, et al. Efficacy, safety and tolerability of rivastigmine capsules in patients with probable vascular dementia: the VantagE study. Curr Med Res Opin 2008;24:2561–74 | Not a disease modification trial |
| Ban TA, Morey LC, Aguglia E, Batista R, Campanella G, Conti L, et al. Glycosaminoglycan polysulfate in the treatment of old age dementias. Prog Neuropsychopharmacol Biol Psychiatry 1991;15:323–42 | Not a disease modification trial |
| Barak Y, Levine J, Glasman A, Elizur A, Belmaker RH. Inositol treatment of Alzheimer’s disease: a double blind, cross-over placebo controlled trial. Prog Neuropsychopharmacol Biol Psychiatry 1996;20:729–36 | Not a disease modification trial |
| Barone P, Burn DJ, van Laar T, Hsu C, Poewe W, Lane RM. Rivastigmine versus placebo in hyperhomocysteinemic Parkinson’s disease dementia patients. Mov Disord 2008;23:1532–40 | Not a disease modification trial |
| Bars PL, Kieser M, Itil KZ. A 26-week analysis of a double-blind, placebo-controlled trial of the Ginkgo biloba extract EGb 761 in dementia. Dement Geriatr Cogn Disord 2000;11:230–7 | Not a disease modification trial |
| Batman MW. The effects of therapeutic aquatic exercise on patients with Alzheimer’s Disease (elderly). Diss Abstr Int B Sci Eng 1999;60:2933 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Battaglia A, Annon K, Pamparana F, De Paolis C, Bonura ML, Stekke W, et al. P-8–11 Nicergoline in the long term treatment of mild or moderate senile dementia. A multicenter double-blind, randomised, placebo-controlled trial. Eur Neuropsychopharmacol 1995;5:383 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Baum L, Lam CWK, Cheung SKK, Kwok T, Lui V, Tsoh J, et al. Six-month randomised, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol 2008;28:110–13 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Bayer AJ, Bokonjic R, Booya NH, Demarin V, Ersmark B, Fairbairn AF, et al. European pentoxifylline multi-infarct dementia study. Eur Neurol 1996;36:315–21 | Not a disease modification trial |
| Becker RE, Colliver JA, Markwell SJ, Moriearty PL, Unni LK, Vicari S. Double-blind, placebo-controlled study of metrifonate, an acetylcholinesterase inhibitor, for Alzheimer disease. Alzheimer Dis Assoc Disord 1996;10:124–31 | Not a disease modification trial |
| Becker RE, Colliver JA, Markwell SJ, Moriearty PL, Unni LK, Vicari S. Effects of metrifonate on cognitive decline in Alzheimer disease: a double-blind, placebo-controlled, 6-month study. Alzheimer Dis Assoc Disord 1998;12:54–7 | Not a disease modification trial |
| Belanoff JK, Jurik J, Schatzberg LD, DeBattista C, Schatzberg AF. Slowing the progression of cognitive decline in Alzheimer’s disease using mifepristone. J Mol Neurosci 2002;19:201–6 | Not a disease modification trial |
| Beller SA, Overall JE, Swann AC. Efficacy of oral physostigmine in primary degenerative dementia. A double-blind study of response to different dose level. Psychopharmacology 1985;87:147–51 | Not a disease modification trial |
| Bentham PW. A double-blind placebo-controlled trial of L-tryptophan to assess the degree of cognitive and behavioural improvement in patients with Alzheimer-type dementia and to compare differential response in clinical sub-groups. Int Clin Psychopharm 1990;5:261–72 | Not a disease modification trial |
| Bergamaschini LC, Scarpini E, Rossi E, Galimberti D, Case A, Lucca U, et al. [Randomised pilot study on the feasibility of Enoxaparin treatment in Alzheimer’s disease.] Neurodegener Dis 2011;8:1 | Full text unavailable in English |
| Bergamasco B, Scarzella L, La Commare P. Idebenone, a new drug in the treatment of cognitive impairment in patients with dementia of the Alzheimer type. Funct Neurol 1994;9:161–8 | Not a disease modification trial |
| Bergamasco B, Villardita C, Coppi R. Idebenone in the treatment of multi-infarct dementia: a randomised, double-blind, placebo controlled multicentre trial. Arch Gerontol Geriatr 1992;15:271–8 | Not a disease modification trial |
| Beversdorf DQ, Warner JL, Davis RA, Sharma UK, Nagaraja HN, Scharre DW. Donepezil in the treatment of dementia with Lewy bodies. Am J Geriatr Psychiatry 2004;12:542–3 | Not a disease modification trial |
| Bierer LM, Aisen PS, Davidson M, Ryan TM, Schmeidler J, Davis KL. A pilot study of clonidine plus physostigmine in Alzheimer’s disease. Dementia 1994;5:243–6 | Not a disease modification trial |
| Black RS, Barclay LL, Nolan KA, Thaler HT, Hardiman ST, Blass JP. Pentoxifylline in cerebrovascular dementia. J Am Geriatr Soc 1992;40:237–44 | Not a disease modification trial |
| Black S, Roman GC, Geldmacher DS, Salloway S, Hecker J, Burns A, et al. Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomised, placebo-controlled clinical trial. Stroke 2003;34:2323–30 | Not a disease modification trial |
| Blass JP, Cyrus PA, Bieber F, Gulanski B. Randomised, double-blind, placebo-controlled, multicenter study to evaluate the safety and tolerability of metrifonate in patients with probable Alzheimer disease. The Metrifonate Study Group. Alzheimer Dis Assoc Disord 2000;14:39–45 | Not a disease modification trial |
| Blesa R, Bullock R, He Y, Bergman H, Gambina G, Meyer J, et al. Effect of butyrylcholinesterase genotype on the response to rivastigmine or donepezil in younger patients with Alzheimer’s disease. Pharmacogenet Genomics 2006;16:771–4 | Not a disease modification trial |
| Blesa R, Davidson M, Kurz A, Reichman W, van Baelen B, Schwalen S. Galantamine provides sustained benefits in patients with ‘advanced moderate’ Alzheimer’s disease for at least 12 months. Dement Geriatr Cogn Disord 2003;15:79–87 | Not a disease modification trial |
| Boada-Rovira M. [Human albumin grifols 5% in plasmapheresis: a new therapy involving beta-amyloid mobilisation in Alzheimer’s disease.] Rev Neurol 2010;50(Suppl. 5):9–18 | Full text unavailable in English |
| Boada-Rovira M, Lopez O, Nunez L, Ortiz P, Anaya F, Hernandez I, et al. A phase II study to evaluate the efficacy and safety of plasma replacement with 5% albumin in beta-amyloid peptide clearance in cerebrospinal fluid, and its effects in patients with mild–moderate Alzheimer’s disease. Alzheimers Dement 2014;10:P274 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioural symptoms in Alzheimer disease. Arch Neurol 1997;54:465–73 | Not a disease modification trial |
| Bogaert P, Grinsven R, Tolson D, Wouters K, Engelborghs S, Mussele S. Effects of SolCos model-based individual reminiscence on older adults with mild to moderate dementia due to Alzheimer disease: a pilot study. J Am Med Dir Assoc 2013;14:528 | Not a disease modification trial |
| Bokde ALW, Karmann M, Teipel SJ, Born C, Lieb M, Reiser MF, et al. Decreased activation along the dorsal visual pathway after a 3-month treatment with galantamine in mild Alzheimer disease: a functional magnetic resonance imaging study. J Clin Psychopharmacol 2009;29:147–56 | Not a disease modification trial |
| Bottino CMC, Carvalho IAM, Alvarez AMM, Avila R, Zukauskas PR, Bustamante SEZ, et al. Cognitive rehabilitation combined with drug treatment in Alzheimer’s disease patients: a pilot study. Clin Rehabil 2005;19:861–9 | Not a disease modification trial |
| Boxer A, Knopman D, Kaufer D, Grossman M, Onyike C, Graf-Radford N, et al. A randomised, multicenter, placebo controlled trial of memantine for behavioural variant FTD and semantic variant PPA. Alzheimers Dement 2012;1:P405 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Boxer A, Knopman D, Kaufer D, Grossman M, Onyike C, Graf-Radford N, et al. A 26-week, multicenter, randomised, double blind, placebo controlled trial of memantine for behavioural variant FTD and semantic variant PPA. Dement Geriatr Cogn Disord 2012;34:47–8 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Boxer AL, Knopman DS, Kaufer DI, Grossman M, Onyike C, Graf-Radford N, et al. Memantine in patients with frontotemporal lobar degeneration: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol 2013;12:149–56 | Not a disease modification trial |
| Boxer A, Tartaglia M, Koestler M, Lasky A, Fine E, Heuer H, et al. A 12 week, randomised, double-blind, placebo-controlled pilot clinical trial of davunetide 15 mg intranasally twice daily for FTLD with predicted tau pathology (CBD,PNFA, PSP). Dement Geriatr Cogn Disord 2010;30:28–9 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Breeze RW, Cox S, Rodgers-Cox J. Changes in P-300 latency as a result of co-dergocrine mesylate therapy in patients with senile dementia. J Geriatr Psychiatry 1988;3:263–6 | Not a disease modification trial |
| Brem A-K, Atkinson NJ, Seligson EE, Pascual-Leone A. Differential pharmacological effects on brain reactivity and plasticity in Alzheimer’s disease. Front Psychiatry 2013;4:124 | Not a disease modification trial |
| Brem AK, Schilberg L, Freitas C, Atkinson N, Seligson E, Pascual-Leone A. Effects of cognitive training and rTMS in Alzheimer’s disease. Alzheimers Dement 2013;1:664 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Brem AK, Schilberg L, Freitas C, Atkinson N, Seligson E, Pascual-Leone A. Synergistic effects of rTMS and cognitive training in Alzheimer’s Disease. J Neurol Sci 2013;333:e343 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Brodaty H, Corey-Bloom J, Potocnik FC, Truyen L, Gold M, Damaraju CR. Galantamine prolonged-release formulation in the treatment of mild to moderate Alzheimer’s disease. Dement Geriatr Cogn Disord 2005;20:120–32 | Not a disease modification trial |
| Brooks JO 3rd, Yesavage JA, Carta A, Bravi D. Acetyl L-carnitine slows decline in younger patients with Alzheimer’s disease: a reanalysis of a double-blind, placebo-controlled study using the trilinear approach. Int Psychogeriatr 1998;10:193–203 | Not a disease modification trial |
| Brown D, Spanjers K, Atherton N, Lowe J, Stonehewer L, Bridle C, et al. Development of an exercise intervention to improve cognition in people with mild to moderate dementia: Dementia And Physical Activity (DAPA) Trial, registration ISRCTN32612072. Physiotherapy 2015;101:126–34 | Not a disease modification trial |
| Bullock R, Bergman H, Touchon J, Gambina G, He Y, Nagel J, et al. Effect of age on response to rivastigmine or donepezil in patients with Alzheimer’s disease. Curr Med Res Opin 2006;22:483–94 | Not a disease modification trial |
| Bullock R, Touchon J, Bergman H, Gambina G, He Y, Rapatz G, et al. Rivastigmine and donepezil treatment in moderate to moderately-severe Alzheimer’s disease over a 2-year period. Curr Med Res Opin 2005;21:1317–27 | Not a disease modification trial |
| Burgener SC, Yang Y, Gilbert R, Marsh-Yant S. The effects of a multimodal intervention on outcomes of persons with early-stage dementia. Am J Alzheimers Dis Other Demen 2008;23:382–94 | Not a disease modification trial |
| Burke D. Donepezil or memantine improved cognitive functioning in moderate-to-severe Alzheimer disease. ACP J Club 2012;156:1 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Burke WJ, Ranno AE, Roccaforte WH, Wengel SP, Bayer BL, Willcockson NK. L-deprenyl in the treatment of mild dementia of the Alzheimer type: preliminary results. J Am Geriatr Soc 1993;41:367–70 | Not a disease modification trial |
| Burke WJ, Roccaforte WH, Wengel SP, Bayer BL, Ranno AE, Willcockson NK. L-deprenyl in the treatment of mild dementia of the Alzheimer type: results of a 15-month trial. J Am Geriatr Soc 1993;41:1219–25 | Not a disease modification trial |
| Burn D, Emre M, McKeith I, De Deyn PP, Aarsland D, Hsu C, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Move Disord 2006;21:1899–907 | Not a disease modification trial |
| Burns A, Gauthier S, Perdomo C. Efficacy and safety of donepezil over 3 years: an open-label, multicentre study in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 2007;22:806–12 | Not a disease modification trial |
| Burns A, Rossor M, Hecker J, Gauthier S, Petit H, Moller HJ, et al. The effects of donepezil in Alzheimer’s disease – results from a multinational trial. Dement Geriatr Cogn Disord 1999;10:237–44 | Not a disease modification trial |
| Burns A, Spiegel R, Quarg P. Efficacy of rivastigmine in subjects with moderately severe Alzheimer’s disease. Int J Geriatr Psychiatry 2004;19:243–9 | Not a disease modification trial |
| Burns A GS, Perdomo C. Long-term use of donepezil may be safe and effective in elderly AD patients. Brown Uni Geriatr Psychopharmacol Update 2007;11:3–4 | Not a disease modification trial |
| Buschert VC, Friese U, Teipel SJ, Schneider P, Merensky W, Rujescu D, et al. Effects of a newly developed cognitive intervention in amnestic mild cognitive impairment and mild Alzheimer’s disease: a pilot study. J Alzheimers Dis 2011;25:679–94 | Not a disease modification trial |
| Butchart J, Brook L, Hopkins V, Teeling J, Puntener U, Culliford D, et al. Etanercept in Alzheimer disease: a randomised, placebo-controlled, double-blind, phase 2 trial. Neurology 2015;84:2161–8 | Not a disease modification trial |
| Camargo CH, Justus FF, Retzlaff G. The effectiveness of reality orientation in the treatment of Alzheimer’s disease. Am J Alzheimers Dis Other Dement 2015;30:527–32 | Not a disease modification trial |
| Canevelli M, Adali N, Kelaiditi E, Cantet C, Ousset PJ, Cesari M. Effects of Ginkgo biloba supplementation in Alzheimer’s disease patients receiving cholinesterase inhibitors: data from the ICTUS study. Phytomedicine 2014;21:888–92 | Not a disease modification trial |
| Caramelli P, Laks J, Palmini ALF, Nitrini R, Chaves MLF, Forlenza OV, et al. Effects of galantamine and galantamine combined with nimodipine on cognitive speed and quality of life in mixed dementia: a 24-week, randomised, placebo-controlled exploratory trial (the REMIX study). Arq Neuropsiquiatr 2014;72:411–17 | Not a disease modification trial |
| Carlson MC, Tschanz JT, Norton MC, Welsh-Bohmer K, Martin BK, Breitner JC. H2 histamine receptor blockade in the treatment of Alzheimer disease: a randomised, double-blind, placebo-controlled trial of nizatidine. Alzheimer Dis Assoc Disord 2002;16:24–30 | Not a disease modification trial |
| Ceccato E, Vigato G, Bonetto C, Bevilacqua A, Pizziolo P, Crociani S, et al. STAM protocol in dementia: a multicenter, single-blind, randomised, and controlled trial. Am J Alzheimers Dis Other Demen 2012;27:301–10 | Not a disease modification trial |
| Chan WC, Cheng ST, Shi L, Wang D, Lam LC-W. Would transcranial direct current stimulation (tDCS) enhance the effects of working memory training in older adults with mild neurocognitive disorder due to Alzheimer’s disease: study protocol for a randomised controlled trial. Trials 2015;16:1–7 | Not a disease modification trial |
| Chapman SB, Weiner MF, Rackley A, Hynan LS, Zientz J. Effects of cognitive-communication stimulation for Alzheimer’s disease patients treated with donepezil. J Speech Lang Hear R 2004;47:1149–63 | Not a disease modification trial |
| Chappell AS, Gonzales C, Williams J, Witte MM, Mohs RC, Sperling R. AMPA potentiator treatment of cognitive deficits in Alzheimer disease. Neurology 2007;68:1008–12 | Not a disease modification trial |
| Chatellier G, Lacomblez L. Tacrine (tetrahydroaminoacridine; THA) and lecithin in senile dementia of the Alzheimer type: A multicentre trial. BMJ 1990;300:495–9 | Not a disease modification trial |
| Chen J, Huang Y, Wang SX, Li QS, Liang YJ, Guo YN. [18FDG PET cerebral function imaging in 10 vascular dementia patients receiving needling at Baihui(DU20), Shuigou(DU26) and Shenmen(HT7).] J South Med Uni 2006;26:610–12 | Full text unavailable in English |
| Chen TS, Lang HC. Cost-effectiveness analysis of donepezil and rivastigmine for mild to moderate Alzheimer’s disease in Taiwan. Value Health 2013;16:A104 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Chen WW, Tian AL, Zhai L, Zhai XL, Zhang YL. [Effect of the xingding allied piracetam injection on improvement of cognitive function in patients with vascular dementia.] Chin J Clin Rehabil 2005;9:231–3 | Full text unavailable in English |
| Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, et al. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology 2005;64:2063–8 | Not a disease modification trial |
| Cheung SK. The effects of the music-with-movement intervention of the cognitive functions of people with moderate dementia. Diss Abstr Int B Sci Eng 2014;75 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Chiu C-C, Su K-P, Cheng T-C, Liu H-C, Chang C-J, Dewey ME, et al. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: a preliminary randomised double-blind placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:1538–44 | Not a disease modification trial |
| Choe YM, Kim KW, Jhoo JH, Ryu SH, Choo IH, Seo EH, et al. Multi-centre, randomised, placebo-controlled, double-blind clinical trial of escitalopram on the progression delaying effect in Alzheimer’s disease: Anmri study for atrophy delaying effect. Alzheimers Dement 2014;10:P302 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Choi SH, Park KW, Na DL, Han HJ, Kim EJ, Shim YS, et al. Tolerability and efficacy of memantine add-on therapy to rivastigmine transdermal patches in mild to moderate Alzheimer’s disease: a multicenter, randomised, open-label, parallel-group study. Curr Med Res Opin 2011;27:1375–83 | Not a disease modification trial |
| Chua KK, Wong A, Kwan P-L, Song JX, Chen LL, Chan A-T, et al. The efficacy and safety of the Chinese herbal medicine Di-Tan decoction for treating Alzheimer’s disease: protocol for a randomised controlled trial. Trials 2015;16:199 | Not a disease modification trial |
| Clare L, Linden DEJ, Woods RT, Whitaker R, Evans SJ, Parkinson CH, et al. Goal-oriented cognitive rehabilitation for people with early-stage Alzheimer disease: a single-blind randomised controlled trial of clinical efficacy. Am J Geriatr Psychiatry 2010;18:928–39 | Not a disease modification trial |
| Claus JJ, De Koning I, Van Harskamp F, Breteler MMB, Voet B, Gutzmann H, et al. Lisuride treatment of Alzheimer’s disease: a preliminary placebo-controlled clinical trial of safety and therapeutic efficacy. Clin Neuropharmacol 1998;21:190–5 | Not a disease modification trial |
| Claxton A, Baker L, Hanson A, Cholerton B, Trittschuh E, Morgan A, et al. Long-acting intranasal insulin detemir improves working memory for adults with mild cognitive impairment or early-stage Alzheimer’s dementia. Alzheimers Dement 2013;9(Suppl. 1):P657 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Coelho FG, Andrade LP, Pedroso RV, Santos-Galduroz RF, Gobbi S, Costa JL, et al. Multimodal exercise intervention improves frontal cognitive functions and gait in Alzheimer’s disease: a controlled trial. Geriatr Gerontol Int 2013;13:198–203 | Not a disease modification trial |
| Coker E. High-dose vitamin B supplements did not slow cognitive decline in patients with mild to moderate Alzheimer disease. Evid Based Nurs 2009;12:57 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Comelli M, Lucca U, Spagnoli A. Statistical analysis of the clinical trial of a therapy for Alzheimer’s disease. Univariate tests and logistic regression. Acta Neurologica 1990;12:222–30 | Not a disease modification trial |
| Concari L, Gardini S, Dieci F, Copelli S, Ferrari Pellegrini F, Ghetti C, et al. Cognitive and brain metabolism improvement after cognitive stimulation therapy Functional. Neurology 2013;28:21–2 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Connelly P. High dose vitamin B supplementation does not slow cognitive decline in mild to moderate Alzheimer’s disease. Evid Based Ment Health 2009;12:86 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Connelly PJ, Prentice NP, Cousland G, Bonham J. A randomised double-blind placebo-controlled trial of folic acid supplementation of cholinesterase inhibitors in Alzheimer’s disease. Int J Geriatr Psychiatry 2008;23:155–60 | Not a disease modification trial |
| Cook C, Fay S, Rockwood K. Decreased initiation of usual activities in people with mild to moderate Alzheimer’s disease: a descriptive analysis from the VISTA clinical trial. Int Psychogeriatr 2008;20:952–63 | Not a disease modification trial |
| Corey-Bloom J, Anand R, Veach J. A randomised trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriatr Psychopharmacol 1998;1:55–65 | Not a disease modification trial |
| Cornelli U. Treatment of Alzheimer’s disease with a cholinesterase inhibitor combined with antioxidants. Neurodegen Dis 2010;7:193–202 | Not a disease modification trial |
| Corona GI SG, Frattini P, Cucchi ML, Zerbi F, Tosca P, Savoldi F. Preliminary data on monoamine metabolic levels in cerebrospinal fluid and in urine during therapy in dementia. IRCS J Med Sci 1983;11:923–4 | Not a disease modification trial |
| Corona GL CM, Frattini P, Santagostino G, Schinelli S, Romani A, Pola A, et al. Clinical and biochemical responses to therapy in Alzheimer’s disease and multi-infarct dementia. Eur Arch Psychiatry Clin Neurosci 1989;239:79–86 | Not a disease modification trial |
| Corrigan FM VRA, Horrobin DF. Essential fatty acids in Alzheimer’s disease. Ann NY Acad Sci 1991;640:250–2 | Not a disease modification trial |
| Cotelli M, Calabria M, Manenti R, Rosini S, Zanetti O, Cappa SF, et al. Improved language performance in Alzheimer disease following brain stimulation. J Neurol Neurosurg Psychiatry 2011;82:794–7 | Not a disease modification trial |
| Courtney C, Farrell D, Grey R, Hills R, Lynch L, Sellwood E, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004;363:2105–15 | Not a disease modification trial |
| Cove J, Jacobi N, Donovan H, Orrell M, Stott J, Spector A. Effectiveness of weekly cognitive stimulation therapy for people with dementia and the additional impact of enhancing cognitive stimulation therapy with a carer training program. Clin Interv Ageing 2014;9:2143–50 | Not a disease modification trial |
| Craft S, Claxton A, Baker L, Cholerton B, Hanson A, Callaghan M, et al. Therapeutic effects of long-acting intranasal insulin detemir for Alzheimer’s dementia or mild cognitive impairment. Alzheimers Dement 2013;9(Suppl. 1):139–40 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Crook T, Petrie W, Wells C, Massari DC. Effects of phosphatidylserine in Alzheimer’s disease. Psychopharmacol Bull 1991;28:61–6 | Unable to find a copy of the full text |
| Crook T, Wilner E, Rothwell A, Winterling D, McEntee W. Noradrenergic intervention in Alzheimer’s disease. Psychopharmacol Bull 1991;28:67–70 | Unable to find a copy of the full text |
| Crumpacker DW. Retrospective evaluation of constructional praxis measurements among APOE4(–) subjects enrolled in the study of AC-1202 (Axona) in mild to moderate Alzheimer’s disease (AD). Am J Geriatr Psychiatry 2012;1:S129 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Cumbo E. Effects of levetiracetam, phenobarbital and lamotrigine on neuropsychological performance and moodin patients with Alzheimer’s disease and epilepsy. Epilepsia 2009;50:101 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Cummings J, Cho W, Ward M, Friesenhahn M, Brunstein F, Honigberg L, et al. A randomised, double-blind, placebo-controlled phase 2 study to evaluate the efficacy and safety of crenezumab in patients with mild to moderate Alzheimer’s disease. Alzheimers Dement 2014;10:P275 | Not a disease modification trial |
| Cummings J, Froelich L, Black SE, Bakchine S, Bellelli G, Molinuevo JL, et al. Randomised, double-blind, parallel-group, 48-week study for efficacy and safety of a higher-dose rivastigmine patch (15 vs. 10 cm2) in Alzheimer’s disease. Dement Geriatr Cogn Disord 2012;33:341–53 | Not a disease modification trial |
| Cummings JL, Cyrus PA, Bieber F, Mas J, Orazem J, Gulanski B. Metrifonate treatment of the cognitive deficits of Alzheimer’s disease. Neurology 1998;50:1214–21 | Not a disease modification trial |
| Cummings JL, Farlow MR, Meng X, Tekin S, Olin JT. Rivastigmine transdermal patch skin tolerability: results of a 1-year clinical trial in patients with mild to moderate Alzheimers disease. Clin Drug Investig 2010;30:41–9 | Not a disease modification trial |
| Cummings JL, Ferris SH, Farlow MR, Olin JT, Meng X. Effects of rivastigmine transdermal patch and capsule on aspects of clinical global impression of change in Alzheimer’s disease: a retrospective analysis. Dement Geriatr Cogn Disord 2010;29:406–12 | Not a disease modification trial |
| Cummings J, Grossberg G, Alva G, Caputo A, Downs P, Strohmaier C. High-dose 13.3 MG/24 h rivastigmine transdermal patch demonstrates efficacy on instrumental activities of daily living: individual item analysis. Alzheimers Dement 2012;8(Suppl. 1):P604 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Cummings J, Hendrix S, Miller M, Pejovic V, Graham S, Tocco M. Extended-release memantine (28 mg, once daily) and sustained behavioural improvement: post hoc responder analysis from a randomised trial in patients with moderate to severe Alzheimer’s disease. Neurology 2012;78:P04.197 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Cutler NR, Sramek JJ, Murphy MF, Nash RJ. Implications of the study population in the early evaluation of anticholinesterase inhibitors for Alzheimer’s disease. Ann Pharmacother 1992;26:1118–22 | Not a disease modification trial |
| D’Amico F, Rehill A, Knapp M, Aguirre E, Donovan H, Hoare Z, et al. Maintenance cognitive stimulation therapy: an economic evaluation within a randomised controlled trial. J Am Med Dir Assoc 2015;16:63–70 | Not a disease modification trial |
| Darreh-Shori T, Kadir A, Almkvist O, Grut M, Wall A, Blomquist G, et al. Inhibition of acetylcholinesterase in CSF versus brain assessed by 11C-PMP PET in AD patients treated with galantamine. Neurobiol Ageing 2008;29:168–84 | Not a disease modification trial |
| Darreh-Shori T ML, Pettersson T, Hugosson K, Hellstrom-Lindahl E, Andreasen N, Minthon L, et al. Changes in the activity and protein levels of CSF acetylcholinesterases in relation to cognitive function of patients with mild Alzheimer’s disease following chronic donepezil treatment. J Neural Transm 2006;113:1791–801 | Not a disease modification trial |
| Davidsson P, Blennow K, Andreasen N, Eriksson B, Minthon L, Hesse C. Differential increase in cerebrospinal fluid-acetylcholinesterase after treatment with acetylcholinesterase inhibitors in patients with Alzheimer’s disease. Neurosci Lett 2001;300:157–60 | Not a disease modification trial |
| Davis KL, Thal LJ, Gamzu ER, Davis CS, Woolson RF, Gracon SI, et al. A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer’s disease. The Tacrine Collaborative Study Group. N Engl J Med 1992;327:1253–9 | Not a disease modification trial |
| Davis RN, Massman PJ, Doody RS. Cognitive intervention in Alzheimer disease: a randomised placebo-controlled study. Alzheimer Dis Assoc Disord 2001;15:1–9 | Not a disease modification trial |
| de Waal H, Stam CJ, Lansbergen MM, Wieggers RL, Kamphuis PJ, Scheltens P, et al. The effect of Souvenaid on functional brain network organisation in patients with mild Alzheimer’s disease: a randomised controlled study. PLOS ONE 2014;27;9:e86558 | Not a disease modification trial |
| Dean R, Siemers E, Carlson C, Estergard W, Sundell K, Henley D, et al. Effects of solanezumab versus placebo administration on biomarkers in people with mild to moderate Alzheimer’s disease: results from two phase III clinical trials. Alzheimers Dement 2013;9(Suppl. 1):P283 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Demarin V, Podobnik SS, Storga-Tomic D, Kay G. Treatment of Alzheimer’s disease with stabilised oral nicotinamide adenine dinucleotide: a randomised, double-blind study. Drugs Exp Clin Res 2004;30:27–33 | Not a disease modification trial |
| Derouesne C, Renault B, Gueguen B, Van Der Linden M, Lacomblez L, Homeyer P, et al. Neuropsychophysiological evaluation of three doses of S 12024–2 in mild to moderate Alzheimer’s disease. Clin Drug Investig 1997;14:301–6 | Not a disease modification trial |
| Desire L, Marcade M, Peillon H, Drouin D, Sol O, Pando M. Clinical trials of EHT 0202, a neuroprotective and procognitive alpha-secretase stimulator for Alzheimer’s disease. Alzheimers Dement 2009;1:255–6 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Dichgans M, Markus HS, Salloway S, Verkkoniemi A, Moline M, Wang Q, et al. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. The Lancet Neurol 2008;7:310–18 | Not a disease modification trial |
| Di Lorenzo F. D2 agonist administration restores altered cortical plasticity in Alzheimer’s disease patients. Clin Neurophysiol 2014;125:S66 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Di Lorenzo F, Martorana A, Bonn S, Caltagirone C, Koch G. D2 agonist administration restores impaired LTP-like cortical plasticity in AD patients. Clin Neurophysiol 2013;124:e139–40 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Diehl-Schmid J, Hardlund J, Bentham P, Wischik CM. The first disease-modifying drug trial in frontotemporal dementia: initial experiences. Alzheimers Dement 2014;10:138 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Doggrell SA. Is memantine a breakthrough in the treatment of moderate-to-severe Alzheimer’s disease? Expert Opin Pharmacother 2003;4:1857–60 | Not a disease modification trial |
| Dong GS, Li X, Jiang QH, Yang HQ. [Effects of donepezil treatment on platelets and secretase activities in Alzheimer’s disease patients.] Zhonghua Yi Xue Za Zhi 2011;91:3341–5 | Full text unavailable in English |
| D’Onofrio G, Sancarlo D, Addante F, Ciccone F, Cascavilla L, Paris F, et al. A pilot randomised controlled trial evaluating an integrated treatment of rivastigmine transdermal patch and cognitive stimulation in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 2015;30:965–75 | Not a disease modification trial |
| Doody R, Galvin J, Farlow M, Shah R, Doraiswamy PM, Ferris S, et al. A new 26-week, double-blind, randomised, placebo-controlled, study of AC-1204 (caprylic triglyceride) in mild to moderate Alzheimer’s disease: presentation of study design. J Nutr Health Ageing 2012;16:868 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Doody RS, Geldmacher DS, Farlow MR, Sun Y, Moline M, Mackell J. Efficacy and safety of donepezil 23 mg versus donepezil 10 mg for moderate-to-severe Alzheimer’s disease: a subgroup analysis in patients already taking or not taking concomitant memantine. Dement Geriatr Cogn Disord 2012;33:164–73 | Not a disease modification trial |
| Doody RS, Geldmacher DS, Gordon B, Perdomo CA, Pratt RD. Open-label, multicenter, phase 3, extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol 2001;58:427–33 | Not a disease modification trial |
| Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, et al. Dimebon found safe and effective in mild to moderate Alzheimer’s disease. Brown Uni Geriatr Psychopharmacol Update 2008;12:1–6 | Not a disease modification trial |
| Doran MD. A Randomised 26 week Double-Blind Placebo Controlled Trial to Evaluate the Safety and Efficacy of Galantamine in the Treatment of Dementia Secondary to Cerebrovascular Disease. London: National Research Register; 2003 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Dorn M. Effect of Nimodipine on the Well-Being, Symptoms and Efficiency of Ambulatory Patients with Cerebrovascular Disorders. Stuttgart: Schattauer; 1985 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Dubois B, McKeith I, Orgogozo J-M, Collins O, Meulien D. A multicentre, randomised, double-blind, placebo-controlled study to evaluate the efficacy, tolerability and safety of two doses of metrifonate in patients with mild to moderate Alzheimer’s disease: The MALT Study. Int J Geriatr Psychiatry 1999;14:973–82 | Not a disease modification trial |
| Dubois B, Tolosa E, Katzenschlager R, Emre M, Lees AJ, Schumann G, et al. Donepezil in Parkinson’s disease dementia: a randomised, double-blind efficacy and safety study. Move Dis 2012;27:1230–8 | Not a disease modification trial |
| Dukoff R, Friz J, Lasser, Lev JPK, Sunderland T. A Comparison of Effects of Tacrine with Scopolamine Versus Tacrine with Placebo in Patients with Alzheimer’s Disease Conference Abstract. 11th Annual Meeting of the American Association for Geriatric Psychiatry San Diego, CA, USA, 8–11 March 1998 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Dysken M, Kuskowski M, Love S. Ondansetron in the treatment of cognitive decline in Alzheimer dementia. Am J Geriatr Psychiatry 2002;10:212–15 | Not a disease modification trial |
| Dysken MW, Guarino PD, Vertrees JE, Asthana S, Sano M, Llorente M, et al. Vitamin E and memantine in Alzheimer’s disease: clinical trial methods and baseline data. Alzheimers Dement 2014;10:36–44 | Not a disease modification trial |
| Dysken MW, Mendels J, LeWitt P, Reisberg B, Pomara N, Wood J, et al. Milacemide: a placebo-controlled study in senile dementia of the Alzheimer type. J Am Geriatr Soc 1992;40:503–6 | Not a disease modification trial |
| Dysken MW, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomised trial. JAMA 2014;311:33–44 | Not a disease modification trial |
| Eagger SA, Levy R, Sahakian BJ. Tacrine in Alzheimer’s disease. Acta Neurol Scand 1992;85:75–80 | Not a disease modification trial |
| Egan M, Yaari R, Liu L, Ryan M, Peng Y, Lines C, et al. Pilot randomised controlled study of a histamine receptor inverse agonist in the symptomatic treatment of Alzheimer’s disease. Alzheimers Dement 2011;1:S300 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Eisdorfer CJ, Wilkie FL. The effect of magnesium pemoline on cognition and behaviour. J Gerontol 1968;23:283–8 | Not a disease modification trial |
| Eliasova I, Anderkova L, Marecek R, Rektorova I. Non-invasive brain stimulation of the right inferior frontal gyrus may improve attention in early Alzheimer’s disease: a pilot study. J Neurol Sci 2014;346:318–22 | Not a disease modification trial |
| Emeriau JP, Lehert P, Mosnier M. Efficacy of naftidrofuryl in patients with vascular or mixed dementia: results of a multicenter, double-blind trial. Clin Ther 2000;22:834–44 | Not a disease modification trial |
| Emre M, Aarsland D, Albanese A, Byrne E, Deuschl G, De Deyn PP, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med 2004;351:2509–18 | Not a disease modification trial |
| Emre M, Poewe W, Deyn PP, Barone P, Kulisevsky J, Pourcher E, et al. Long-term safety of rivastigmine in Parkinson disease dementia: an open-label, randomised study. Clin Neuropharmacol 2014;37:9–16 | Not a disease modification trial |
| Emre M, Tsolaki M, Bonuccelli U, Destee A, Tolosa E, Kutzelnigg A, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:969–77 | Not a disease modification trial |
| Eriksdotter M, Vedin I, Falahati F, Freund-Levi Y, Hjorth E, Faxen-Irving G, et al. Plasma fatty acid profiles in relation to cognition and gender in Alzheimer’s Disease patients during oral omega-3 fatty acid supplementation: The OmegAD Study. J Alzheimers Dis 2015;48:805–12 | Not a disease modification trial |
| Erkinjuntti T, Gauthier S, Bullock R, Kurz A, Hammond G, Schwalen S, et al. Galantamine treatment in Alzheimer’s disease with cerebrovascular disease: responder analyses from a randomised, controlled trial (GAL-INT-6). J Psychopharmacol 2008;22:761–8 | Not a disease modification trial |
| Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet 2002;359:1283–90 | Not a disease modification trial |
| Erkinjuntti T, Kurz A, Small GW, Bullock R, Lilienfeld S, Damaraju CV, et al. An open-label extension trial of galantamine in patients with probable vascular dementia and mixed dementia. Clin Ther 2003;25:1765–82 | Not a disease modification trial |
| Erkinjuntti T, Skoog I, Lane R, Andrews C. Rivastigmine in patients with Alzheimer’s disease and concurrent hypertension. Int J Clin Prac 2002;56:791–6 | Not a disease modification trial |
| Etienne P, Dastoor D, Gauthier S, Ludwick R, Collier B. Alzheimer disease: lack of effect of lecithin treatment for 3 months. Neurology 1981;31:1552–4 | Not a disease modification trial |
| Fabbrini G, Martucci N, Battaglia A, Pamparana F, Annoni K. Nicergoline in the Treatment of Dementia: The Effects on Cerebral Blood Flow Measured by SPECT Conference Abstract. 8th European College of Neuropsychopharmacology Congress Venice, Italy, 30 September–4 October 1995 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Fakouhi TD, Jhee SS, Sramek JJ, Benes C, Schwartz P, Hantsburger G, et al. Evaluation of cycloserine in the treatment of Alzheimer’s disease. J Geriatr Psychiatry Neurol 1995;8:226–30 | Not a disease modification trial |
| Falsaperla A, Monici Preti PA, Oliani C. Selegiline versus oxiracetam in patients with Alzheimer-type dementia. Clin Ther 1990;12:376–84 | Not a disease modification trial |
| Farlow M, Gracon SI, Hershey LA, Lewis KW, Sadowsky CH, Dolan-Ureno J. A controlled trial of tacrine in Alzheimer’s disease. The Tacrine Study Group. JAMA 1992;268:2523–9 | Not a disease modification trial |
| Farlow MR. Rivastigmine three times daily improves cognition and response in Alzheimer’s disease. Evid Based Ment Health 2007;10:116 | Not a disease modification trial |
| Farlow MR, Alva G, Meng X, Olin JT. A 25-week, open-label trial investigating rivastigmine transdermal patches with concomitant memantine in mild to moderate Alzheimer’s disease: a post hoc analysis. Curr Med Res Opin 2010;26:263–9 | Not a disease modification trial |
| Farlow M, Anand R, Messina J Jr, Hartman R, Veach J. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol 2000;44:236–41 | Not a RCT/CCT |
| Farlow MR, Doraiswamy M, Meng X, Somogyi M. The effect of vascular risk factors on the efficacy of rivastigmine patch and capsule in Alzheimer’s disease. Am J Geriatr Psychiatry 2011;1:S120 | Not a disease modification trial |
| Farlow MR, Salloway S, Tariot PN, Yardley J, Moline ML, Wang Q, et al. Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer’s disease: a 24-week, randomised, double-blind study. Clin Ther 2010;32:1234–51 | Not a disease modification trial |
| Farokhnia M, Shafiee Sabet M, Iranpour N, Gougol A, Yekehtaz H, Alimardani R, et al. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer’s disease: a double-blind randomised clinical trial. Hum Psychopharmacol 2014;29:351–9 | Not a disease modification trial |
| Feldman H, Coric V, Sperling R, Greenberg S, Bronen R, Sorensen AG, et al. Cerebral microbleeds in a phase 2 clinical trial of mild to moderate Alzheimer’s disease with the gamma secretase inhibitor BMS-708163. Alzheimers Dement 2011;1:S375 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Feldman H, Gauthier S, Hecker J, VelIas B, Emir B, Mastey V, et al. Efficacy of donepezil on maintenance of activities of daily living in patients with moderate to severe Alzheimer’s disease and the effect on caregiver burden. J Am Geriatr Soc 2003;51:737–44 | Not a disease modification trial |
| Feldman H, Gauthier S, Hecker J, Vellas B, Hux M, Xu Y, et al. Economic evaluation of donepezil in moderate to severe Alzheimer disease. Neurology 2004;63:644–50 | Not a disease modification trial |
| Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E. A 24-week, randomised, double-blind study of donepezil in moderate to severe Alzheimer’s disease. Neurology 2001;57:613–20 | Not a disease modification trial |
| Feldman HH, Doody RS, Kivipelto M, Sparks DL, Waters DD, Jones RW, et al. Randomised controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology 2010;74:956–64 | Not a disease modification trial |
| Feldman HH, Lane R, Study G. Rivastigmine: a placebo controlled trial of twice daily and three times daily regimens in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2007;78:1056–63 | Not a disease modification trial |
| Feldman HH, Schmitt FA, Olin JT. Activities of daily living in moderate-to-severe Alzheimer disease: an analysis of the treatment effects of memantine in patients receiving stable donepezil treatment. Alzheimer Dis Assoc Disord 2006;20:263–8 | Not a disease modification trial |
| Ferris SH, Schmitt FA, Saxton J, Richardson S, MacKell J, Sun Y. Analysing the impact of 23 mg/day donepezil on language dysfunction in moderate to severe Alzheimer’s disease. Alzheimers Res Ther 2011;3:22 | Not a disease modification trial |
| Finger EC, MacKinley J, Blair M, Oliver LD, Jesso S, Tartaglia MC, et al. Oxytocin for frontotemporal dementia: a randomised dose-finding study of safety and tolerability. Neurology 2015;84:174–81 | Not a disease modification trial |
| Fischhof PK, Moslinger-Gehmayr R, Herrmann WM, Friedmann A, Russmann DL. Therapeutic efficacy of vincamine in dementia. Neuropsychobiology 1996;34:29–35 | Not a disease modification trial |
| Fischhof PK, Saletu B, Ruther E, Litschauer G, Moslinger-Gehmayr R, Herrmann WM. Therapeutic efficacy of pyritinol in patients with senile dementia of the Alzheimer type (SDAT) and multi-infarct dementia (MID). Neuropsychobiology 1992;26:65–70 | Not a disease modification trial |
| Fisman M. Double blind study of lecithin in patients with Alzheimer’s disease. Can J Psychiatry 1981;26:426–8 | Not a disease modification trial |
| Fleisher A, Tariot P, Truran D, Mai J, Aisen P, Cummings J, et al. Brain volume changes with divalproex sodium in Alzheimer’s disease. Neuropsychopharmacology 2010;35:S318–19 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Flicker C, Ferris SH, Kalkstein D, Serby M. A double-blind, placebo–controlled crossover study of ganglioside GM1 treatment for Alzheimer’s disease. Am J Geriatr Psychiatry 1994;151:126–9 | Not a disease modification trial |
| Foerster S, Buschert VC, Buchholz HG, Teipel SJ, Zach C, Hampel H, et al. Positive effects of a 6-month stage-specific cognitive intervention program on brain metabolism in subjects with amnestic mild cognitive impairment (AMCI) and mild Alzheimer’s disease (AD). Alzheimers Dement 2009;1:205–6 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Forbes D. Cognitive stimulation therapy improved cognition and quality of life in dementia. Evid Based Nurs 2004;7:54–5 | Not a disease modification trial |
| Forette F, Anand R, Gharabawi G. A phase II study in patients with Alzheimer’s disease to assess the preliminary efficacy and maximum tolerated dose of rivastigmine (Exelon). Eur J Neurol 1999;6:423–9 | Not a disease modification trial |
| Forette F, Gracon S, de Rotrou J, Hervy MP, Lechevalier B, Micas M, et al. A double-blind, placebo-controlled, enriched population study of tacrine in patients with Alzheimer’s disease. Eur J Neurol 1995;2:229–38 | Not a disease modification trial |
| Forster S, Buschert VC, Buchholz H-G, Teipel SJ, Friese U, Zach C, et al. Effects of a 6-month cognitive intervention program on brain metabolism in amnestic mild cognitive impairment and mild Alzheimer’s disease. J Alzheimers Dis 2011;25:695–706 | Not a disease modification trial |
| Forster S, Buschert VC, Buchholz HG, Teipel SJ, Rominger A, La Fougere C, et al. Attenuation of cerebral metabolic decline in patients with amnestic mild cognitive impairment (aMCI) or mild Alzheimers disease (AD) joining a six-month stage-specific cognitive intervention program. Nuklear Medizin 2010;49:A53 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Förster S, Buschert VC, Teipel SJ, Friese U, Buchholz HG, Drzezga A, et al. Effects of a 6-month cognitive intervention on brain metabolism in patients with amnestic MCI and mild Alzheimer’s disease. J Alzheimers Dis 2011;26(Suppl. 3):337–48 | Not a disease modification trial |
| Foroutanpour K. A Phase II, Single Centre, Randomised, Double-Blind, Placebo-Controlled, Parallel Group Trial to Evaluate the Safety and Efficacy of Three Different Dosages of Cerebrolysin in Patients with Probable Alzheimer’s Disease. PRA International Report prepared for EBEWE Pharma. Vienna: EBEWE Pharma; 2003 | Not published in a peer-reviewed journal article or is an ongoing trial |
| FORUM Pharmaceuticals Inc. NCT02149160 Study to Assess the Safety, Tolerability, and Pharmacodynamic (PD) Effects of FRM-0334 in Subjects With Prodromal to Moderate Frontotemporal Dementia With Granulin Mutation. URL: https://clinicaltrials.gov/ct2/show/NCT02149160 (accessed 29 January 2016) | No quantitative outcome relating to disease modification |
| Francois C, Sintonen H, Sulkava R, Rive B. Cost-effectiveness of memantine in moderately severe to severe Alzheimer’s disease: a Markov model in Finland. Clin Drug Investig 2004;24:373–84 | Not a disease modification trial |
| Freedman M, Rewilak D, Xerri T, Cohen S, Gordon A, Shandling M, et al. L-deprenyl in Alzheimer’s disease: cognitive and behavioural effects. Neurology 1998;50:660–8 | Not a disease modification trial |
| Freund-Levi Y, Vedin I, Hjorth E, Basun H, Faxen Irving G, Schultzberg M, et al. Effects of supplementation with omega-3 fatty acids on oxidative stress and inflammation in patients with Alzheimer’s disease: the OmegAD study. J Alzheimers Dis 2014;42:823–31 | Not a disease modification trial |
| Frolich L. High-dose rivastigmine patch: results from the optima study. Neurobiol Ageing 2012;33:S12 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Frolich L, Ashwood T, Nilsson J, Eckerwall G. Effects of AZD3480 on cognition in patients with mild to moderate Alzheimer’s disease: a phase IIb dose-finding study. J Alzheimers Dis 2011;24:363–74 | Not a disease modification trial |
| Gaitán A, Garolera M, Cerulla N, Chico G, Rodriguez-Querol M, Canela-Soler J. Efficacy of an adjunctive computer-based cognitive training program in amnestic mild cognitive impairment and Alzheimer’s disease: a single-blind, randomised clinical trial. Int J Geriatr Psychiatry 2013;28:91–9 | Not a disease modification trial |
| Galasko D, Kershaw PR, Schneider L, Zhu Y, Tariot PN. Galantamine maintains ability to perform activities of daily living in patients with Alzheimer’s disease. J Am Geriatr Soc 2004;52:1070–6 | Not a disease modification trial |
| Gang MG. Effect of the horticultural intervention program on cognitive function, emotion, communication and behaviour in the elderly with Alzheimer’s disease. Int Psychogeriatr 2013;25:S140 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Gault LM, Meier A, Florian H, Gauthier S, Lin Y, Tang Q, et al. A phase 2 trial of the efficacy and safety of the alpha7agonistabt-126 as an add-on treatment in mild to moderate Alzheimer’s dementia. Alzheimers Dement 2014;10:917–18 | Not a disease modification trial |
| Gauthier S. Dimebon improves cognitive function in people with mild to moderate Alzheimer’s disease. Evid Based Ment Health 2009;12:21 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Gauthier S, Bouchard R, Lamontagne A, Bailey P, Bergman H, Ratner J, et al. Tetrahydroaminoacridine–lecithin combination treatment in patients with intermediate-stage Alzheimer’s disease. Results of a Canadian double-blind, crossover, multicenter study. N Engl J Med 1990;322:1272–6 | Not a disease modification trial |
| Gavrilova SI, Kalyn Ia B, Kolykhalov IV, Roshchina IF, Selezneva ND. [Acetyl-L-carnitine (carnicetine) in the treatment of early stages of Alzheimer’s disease and vascular dementia.] Zh Nevrol Psikhiatr Im S S Korsakova 2011;111:16–22 | Full text unavailable in English |
| Gejl M, Egejord L, Moller A, Hansen SB, Vang K, Rodell A, et al. GLP-1 analogue liraglutide prevents decline of brain glucose metabolism in Alzheimer’s disease: randomised, placebo-controlled double-blinded clinical trial. Eur J Nucl Med Mol Imaging 2015;1:S132–3 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Gelmont D, Dyck-Jones J. Safety of intravenous immunoglobulin therapy in patients with probable Alzheimer’s disease: a randomised, placebo-controlled clinical study. J Allergy Clin Immunol 2015;135(Suppl.1):Ab97 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Giordano M, Dominguez LJ, Vitrano T, Curatolo M, Ferlisi A, Di Prima A, et al. Combination of intensive cognitive rehabilitation and donepezil therapy in Alzheimer’s disease (AD). Arch Gerontol Geriatr 2010;51:245–9 | Not a disease modification trial |
| Gleason CE, Fischer BL, Dowling NM, Setchell KDR, Atwood CS, Carlsson CM, et al. Cognitive effects of soy isoflavones in patients with Alzheimer’s disease. J Alzheimers Dis 2015;47:1009–19 | Not a disease modification trial |
| Graham S, Hendrix S, Miller M, Pejovic V, Tocco M. Efficacy of high-dose, extended-release memantine (28 MG, once daily): post hoc responder analysis from a randomised trial in patients with moderate to severe Alzheimer’s disease. Alzheimers Dement 2011;1:S782 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Graham S, Tocco M, Hendrix S, Hofbauer RK, Perhach JL. Functional communication in patients with moderate Alzheimer’s disease treated with memantine. Eur Neuropsychopharmacol 2010;20:S557–8 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Graham SM, Hendrix S, Miller ML, Pejovic V, Tocco M. Extended-release memantine (28 mg, once daily) provides behavioural benefits across a wide range of disease severity in patients with moderate to severe Alzheimer’s disease: post hoc analysis from a randomised trial. Am J Geriatr Psychiatry 2013;1:S139 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Graham SM, Tocco M, Hendrix S, Hofbauer RK, Miller ML, Perhach JL. Memantine prevents worsening across multiple domains in a trial of patients with moderate Alzheimer’s disease. Eur Neuropsychopharmacol 2010;20:S557 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Green RC, Goldstein FC, Auchus AP, Presley R, Clark W, Van Tuyl L, et al. Treatment trial of oxiracetam in Alzheimer’s disease. Arch Neurol 1992;49:1135–6 | Not a disease modification trial |
| Greenberg SM, Tennis MK, Brown LB, Gomez-Isla T, Hayden DL, Schoenfeld DA, et al. Donepezil therapy in clinical practice: a randomised crossover study. Arch Neurol 2000;57:94–9 | Not a disease modification trial |
| Grossberg G, Alva G, Hendrix S, Hofbauer R, Pejovic V, Graham S. Efficacy and tolerability of memantine extended release added to stable donepezil regimen in individuals with moderate to severe Alzheimer’s disease: subset analysis of a randomised clinical trial. Neurology 2015;84:P7.101 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Grossberg G, Alva G, Hendrix S, Hofbauer R, Pejovic V, Graham SM. Efficacy and tolerability of memantine extended release added to stable donepezil regimen in individuals with moderate to severe Alzheimer’s disease: subset analysis of a randomised clinical trial. Alzheimers Dement 2014;10:P450 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Grossberg G, Cummings J, Frolich L, Bellelli G, Molinuevo JL, Krahnke T, et al. Efficacy of higher dose 13.3 mg/24 h rivastigmine patch on instrumental activities of daily living in patients with mild to moderate Alzheimer’s disease. AmJ Alzheimers Dis Other Dement 2013;28:583–91 | Not a disease modification trial |
| Grossberg G, Meng X, Olin JT. Impact of rivastigmine patch and capsules on activities of daily living in Alzheimer’s disease. Am J Alzheimers Dis Other Dement 2011;26:65–71 | Not a disease modification trial |
| Grossberg GT, Manes F, Allegri R, Robledo LMG, Gloger S, Xie L, et al. Extended-release memantine (28 mg, once daily) improves verbal fluency and behaviour in patients with moderate to severe Alzheimer’s disease: results of a multinational, double-blind, placebo-controlled trial. Am J Geriatr Psychiatry 2009;17:A78 | Not published in a peer reviewed journal article or an ongoing trial |
| Grossman H, Marzloff G, Luo X, LeRoith D, Sano M, Pasinetti G. NIC5–15 as a treatment for Alzheimer’s: safety, pharmacokinetics and clinical variables. Alzheimers Dement 2009;1:259 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Grove RA, Harrington CM, Mahler A, Beresford I, Maruff P, Lowy MT, et al. A randomised, double-blind, placebo-controlled, 16-week study of the H3 receptor antagonist, GSK239512 as a monotherapy in subjects with mild to moderate Alzheimer’s disease. Curr Alzheimer Res 2014;11:47–58 | Not a disease modification trial |
| Growdon JH, Corkin S, Huff FJ, Rosen TJ. Piracetam combined with lecithin in the treatment of Alzheimer’s disease. Neurobiol Ageing 1986;7:269–76 | Not a disease modification trial |
| Gu C, Shen T, An H, Yuan C, Zhou J, Ye Q, et al. Combined therapy of di-huang-yi-zhi with donepezil in patients with Parkinson’s disease dementia. Neurosci Lett 2015;606:13–17 | Not a disease modification trial |
| Guekht A, Doppler E, Moessler H, Gusev E. Cerebrolysin improves clinical outcome in moderate to moderately severe vascular dementia: results from a randomised, double-blind, placebo-controlled, multicenter trial. Eur J Neurol 2009;16(Suppl. 3):391 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Guekht AB, Moessler H, Novak PH, Gusev EI. Cerebrolysin in vascular dementia: improvement of clinical outcome in a randomised, double-blind, placebo-controlled multicenter trial. J Stroke Cerebrovasc Dis 2011;20:310–18 | Not a disease modification trial |
| Guimon J, Blanco J, Caso C. L-Dopa carbidopa treatment of senile dementia: a control study. Eur J Psychiat 1995;9:29–36 | Not a disease modification trial |
| Gustafson L. Physostigmine and tetrahydroaminoacridine treatment of Alzheimer’s disease. Acta Neurol Scand Suppl 1993;149:39–41 | Not a disease modification trial |
| Gustafson L, Risberg J, Johanson M, Fransson M, Maximilian VA. Effects of piracetam on regional cerebral blood flow and mental functions in patients with organic dementia. Psychopharmacology 1978;56:115–17 | Not a disease modification trial |
| Gustavsson A, Van Der Putt R, Jönsson L, McShane R. Economic evaluation of cholinesterase inhibitor therapy for dementia: comparison of Alzheimer’s disease and Dementia with Lewy bodies. Int J Geriatr Psychiatry 2009;24:1072–8 | Not a disease modification trial |
| Gutzmann H, Hadler D. Sustained efficacy and safety of idebenone in the treatment of Alzheimer’s disease: update on a 2-year double-blind multicentre study. J Neural Transm Supplementum 1998;54:301–10 | Not a disease modification trial |
| Haan J, Hörr R. [Delay in progression of dependency and need of care of dementia patients treated with Ginkgo special extract EGb 761.] Wien Med Wochenschr 2004;154:511–14 | Full text unavailable in English |
| Haase J, Halama P, Horr R. [Efficacy of short-term treatment with intravenously administered Ginkgo biloba special extract EGb 761 in Alzheimer type and vascular dementia.] Z Gerontol Geriatr 1996;29:302–9 | Full text unavailable in English |
| Haase J, Halama P, Hörr R. [Effectiveness of brief infusions with Ginkgo biloba special extract EGb 761 in dementia of the vascular and Alzheimer type.] Z Gerontol Geriatr 1996;29:302–9 | Full text unavailable in English |
| Hager K, Baseman AS, Han JH, Sano M, Richards HM. In a 2-year placebo-controlled randomised trial galantamine-treated patients had lower mortality rates and slower decline in cognition and activities of daily living. Neuropsychopharmacology 2012;38:S328 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Hager K, Baseman AS, Nye JS, Brashear H, Han J, Sano M, et al. Effects of galantamine in a 2-year, randomised, placebo-controlled study in Alzheimer’s disease. Neuropsychiat Dis Treat 2014;10:391–401 | Not a disease modification trial |
| Hagstadius S, Gustafson L, Risberg J. The effects of bromvincamine and vincamine on regional cerebral blood flow and mental functions in patients with multi-infarct dementia. Psychopharmacology 1984;83:321–6 | Not a disease modification trial |
| Haig G, Meier A, Pritchett Y, Hall C, Gault L, Lenz R. Evaluation of the efficacy and safety of the H3 antagonist ABT-288 in mild to moderate Alzheimer’s disease. Alzheimers Dement 2012;1:601–2 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Haig GM, Pritchett Y, Meier A, Othman AA, Hall C, Gault LM, et al. A randomised study of H3 antagonist ABT-288 in mild to moderate Alzheimer’s dementia. J Alzheimers Dis 2014;42:959–71 | Not a disease modification trial |
| Hancock G, Charlesworth G. Donepezil slows decline in daily life activities in people with moderate to severe Alzheimer’s disease and alleviates caregiver burden. Evid Based Ment Health 2004;7:20–1 | Not a disease modification trial |
| Hanney M, Prasher V, Williams N, Jones EL, Aarsland D, Corbett A, et al. Memantine for dementia in adults older than 40 years with Down’s syndrome (MEADOWS): a randomised, double-blind, placebo-controlled trial. Lancet 2012;379:528–36 | Not a disease modification trial |
| Hanyu H, Sato T, Kiuchi A, Sakurai H, Iwamoto T. Pioglitazone improved cognition in a pilot study on patients with Alzheimer’s disease and mild cognitive impairment with diabetes mellitus. J Am Geriatr Soc 2009;57:177–9 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Hara J, Shankle W. Long-term ivig treatment delays progression of Alzheimer’s and Lewy body disease. Alzheimers Dement 2011;1:S461 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Hashimoto M, Kazui H, Matsumoto K, Nakano Y, Yasuda M, Mori E. Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer’s disease? Am J Geriatr Psychiatry 2005;162:676–82 | Not a RCT/CCT |
| Hashimoto M, Yamashita K, Kato S, Tamai T, Matsumoto I, Tanabe Y, et al. Beneficial effects of dietary docosahexaenoic acid intervention on cognitive function in elderly people with very mild dementia in Japan. Alzheimers Dement 2011;1:610–11 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Hasselbalch SG, Hoffmann K, Frederiksen KS, Sobol NA, Beyer N, Vogel A, et al. A multicentre randomised clinical trial of physical exercise in Alzheimer’s disease (AD): Rationale and design of the ADEX study. Eur J Neurol 2012;19:95 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Hauer K, Schwenk M, Zieschang T, Essig M, Becker C, Oster P. Physical training improves motor performance in people with dementia: a randomised controlled trial. J Am Geriatr Soc 2012;60:8–15 | Not a disease modification trial |
| Heiss WD, Kessler J, Mielke R, Szelies B, Herholz K. Long-term effects of phosphatidylserine, pyritinol, and cognitive training in Alzheimer’s disease. A neuropsychological, EEG, and PET investigation. Dementia 1994;5:88–98 | Not a disease modification trial |
| Henderson ST, Barr LJ, Vogel JL, Garvin F. Evidence of an interaction between APOE and IDE in ketone body therapies in mild to moderate Alzheimer’s disease. J Neurol Sci 2009;285:S277 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomised, double-blind, placebo-controlled, multicenter trial. Nutri Metab 2009;6:31 | Not a disease modification trial |
| Henderson VW, Ala T, Sainani KL, Bernstein AL, Stephenson BS, Rosen AC, et al. Raloxifene for women with Alzheimer disease: a randomised controlled pilot trial. Neurology 2015;85:1937–44 | Not a disease modification trial |
| Herrmann WM, Stephan K. Moving from the question of efficacy to the question of therapeutic relevance: an exploratory reanalysis of a controlled clinical study of 130 inpatients with dementia syndrome taking piracetam. Int Psychogeriatr 1992;4:25–44 | Not a disease modification trial |
| Herrschaft H, Nacu A, Likhachev S, Sholomov I, Hoerr R, Schlaefke S. Ginkgo biloba extract EGb 761 in dementia with neuropsychiatric features: a randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J Psychiat Res 2012;46:716–23 | Not a disease modification trial |
| Heyman A, Schmechel D, Wilkinson W, Rogers H, Krishnan R, Holloway D, et al. Failure of long term high-dose lecithin to retard progression of early-onset Alzheimer’s disease. J Neural Transm Suppl 1987;24:279–86 | Not a disease modification trial |
| Hilt DC, Gawryl M, Koenig G, Dgetluck N, Harrison J, Moebius HJ, et al. Positive effects on cognition and clinical function in mild to moderate Alzheimer’s disease patients with a selective alpha-7 nicotinic partial agonists: interpretation of effects based on a PK/PD model. J Nutr Health Ageing 2012;16:819 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Ho RT, Cheung JK, Chan WC, Cheung IK, Lam LC. A 3-arm randomised controlled trial on the effects of dance movement intervention and exercises on elderly with early dementia. BMC Geriatr 2015;15:127 | Not a disease modification trial |
| Hofferberth B. The efficacy of EGb 761 in patients with senile dementia of the Alzheimer type, a double-blind, placebo-controlled study on different levels of investigation. Hum Psychopharm 1994;9:215–22 | Not a disease modification trial |
| Hoffmann K, Frederiksen KS, Sobol NA, Beyer N, Vogel A, Simonsen AH, et al. Preserving cognition, quality of life, physical health and functional ability in Alzheimer’s disease: the effect of physical exercise (ADEX trial): rationale and design. Neuroepidemiology 2013;41:198–207 | Not a disease modification trial |
| Homma A, Takeda M, Imai Y, Udaka F, Hasegawa K, Kameyama M, et al. Clinical efficacy and safety of donepezil on cognitive and global function in patients with Alzheimer’s disease: a 24-week, multicenter, double-blind, placebo-controlled study in Japan. Dement Geriatr Cogn Disord 2000;11:299–313 | Not a disease modification trial |
| Hooghiemstra A, Eggermont L, Flier W, Marum R, Campen J, Koppe P, et al. Feasibility of an RCT on exercise interventions in early-onset dementia: let’s move on! Alzheimers Dement 2013;9(Suppl. 1):P295 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Hooghiemstra AM, Eggermont LHP, Scheltens P, van der Flier WM, Bakker J, de Greef MHG, et al. Study protocol: EXERcise and Cognition In Sedentary adults with Early-ONset dementia (EXERCISE-ON). BMC Neurol 2012;12:75 | Not a disease modification trial |
| Hoogveldt B, Rive B, Severens J, Maman K, Guilhaume C. Cost-effectiveness analysis of memantine for moderate-to-severe Alzheimer’s disease in the Netherlands. Neuropsychiatr Dis Treat 2011;7:313–17 | Not a disease modification trial |
| Howard R. Donepezil or memantine improved cognitive functioning in moderate-to-severe Alzheimer disease. Ann Intern Med 2012;156:JC6–10 | Not a disease modification trial |
| Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 2012;366:893–903 | Not a disease modification trial |
| Huang WW, Zhang LH, Zhang H, Kong L. [Effects of hydrochloric donepezil in improving cognition and daily life ability in patients with Alzheimer disease.] Chin J Clin Rehabil 2004;8:5378–9 | Full text unavailable in English |
| Huang Y, Chen J, Htut WM, Lai X, Wik G. Acupuncture increases cerebral glucose metabolism in human vascular dementia. Int J Neurosci 2007;117:1029–37 | Not a disease modification trial |
| Huff FJ. Preliminary evaluation of besipirdine for the treatment of Alzheimer’s disease. Besipirdine Study Group. Ann NY Acad Sci 1996;777:410–14 | Not a disease modification trial |
| Huff FJ, Antuono P, Murphy M, Beyer J, Dobson C. Potential clinical use of an adrenergic/cholinergic agent (HP 128) in the treatment of Alzheimer’s disease. Ann NY Acad Sci 1991;640:263–7 | Not a disease modification trial |
| Hughes LE, Rittman T, Regenthal R, Robbins TW, Rowe JB. Improving response inhibition systems in frontotemporal dementia with citalopram. Brain 2015;138:1961–75 | Not a disease modification trial |
| Hwang HR, Choi SH, Yoon DH, Yoon BN, Suh YJ, Lee D, et al. The effect of cognitive training in patients with mild cognitive impairment and early Alzheimer’s disease: a preliminary study. J Clin Neurol 2012;8:190–7 | Not a disease modification trial |
| Ihl R, Bachinskaya N, Korczyn AD, Vakhapova V, Tribanek M, Hoerr R, et al. Efficacy and safety of a once-daily formulation of Ginkgo biloba extract EGb 761 in dementia with neuropsychiatric features: a randomised controlled trial. Int J Geriatr Psychiatry 2011;26:1186–94 | Not a disease modification trial |
| Ihl R, Tribanek M, Bachinskaya N. Baseline neuropsychiatric symptoms are effect modifiers in Ginkgo biloba extract (EGb 761®) treatment of dementia with neuropsychiatric features. Retrospective data analyses of a randomised controlled trial. J Neurol Sci 2010;299:184–7 | Not a disease modification trial |
| Ihl R, Tribanek M, Bachinskaya N. Efficacy and tolerability of a once daily formulation of Ginkgo biloba extract EGb 761® in Alzheimer’s disease and vascular dementia: results from a randomised controlled trial. Pharmacopsychiatry 2012;45:41–6 | Not a disease modification trial |
| Ikeda M, Mori E, Kosaka K, Iseki E, Hashimoto M, Matsukawa N, et al. Long-term safety and efficacy of donepezil in patients with dementia with Lewy bodies: Results from a 52-week, open-label, multicenter extension study. Dement Geriatr Cogn Disord 2013;36:229–41 | Not a disease modification trial |
| Imbimbo BP, Lucca U, Lucchelli F, Alberoni M, Thal L. A 25-week placebo-controlled study of eptastigmine in patients with Alzheimer disease. Alzheimer Dis Assoc Disord 1998;12:313–22 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Imbimbo BP, Lucchelli PE. Chronic low dose eptastigmine in Alzheimer patients: Relationship between acetylcholinesterase inhibition and cognitive effects. Neurobiol Ageing 1994;15(Suppl. 1):100 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Imbimbo BP, Martelli P, Troetel WM, Lucchelli F, Lucca U, Thal LJ. Efficacy and safety of eptastigmine for the treatment of patients with Alzheimer’s disease. Neurology 1999;52:700–8 | Not a disease modification trial |
| Imbimbo BP, Nicoli M, Martini C, Tomelleri GP, Martelli P, Ferrari GP, et al. Acetylcholinesterase assay may predict cognitive response of Alzheimer patients to eptastigmine treatment. Eur J Clin Pharmacol 1998;54:809–10 | Not a disease modification trial |
| Imbimbo BP, Troetel WM, Martelli P, Lucchelli F. A 6-month, double-blind, placebo-controlled trial of eptastigmine in Alzheimer’s disease. Dement Geriatr Cogn Disord 2000;11:17–24 | Not a disease modification trial |
| Imbimbo BP, Verdelli G, Martelli P, Marchesini D. Two-year treatment of Alzheimer’s disease with eptastigmine. Dement Geriatr Cogn Disord 1999;10:139–46 | Not a disease modification trial |
| Imbriano L, Podda L, Rendace L, Lucchese F, Campanelli A, D’Antonio F, et al. Long-lasting cognitive stimulation temporary improves cognitive impairment in patients with Alzheimer’s disease: the results from a 6-months follow-up controlled clinical study. Funct Neurol 2013;28:36–7 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Ishizaki J, Meguro K, Ohe K, Kimura E, Tsuchiya E, Ishii H, et al. Therapeutic psychosocial intervention for elderly subjects with very mild Alzheimer disease in a community: the tajiri project. Alzheimer Dis Assoc Disord 2002;16:261–9 | Not a disease modification trial |
| Isik AT, Bozoglu E. Acetylcholinesterase inhibition and insulin resistance in late onset Alzheimer’s disease. Int Psychogeriatr 2009;21:1127–33 | Not a disease modification trial |
| Ito T, Meguro K, Akanuma K, Ishii H, Mori E. A randomised controlled trial of the group reminiscence approach in patients with vascular dementia. Dement Geriatr Cogn Disord 2007;24:48–54 | Not a disease modification trial |
| Jack C, Slomkowski M, Gracon S, Hoover T, Felmlee J, Stewart K, et al. MRI as a biomarker of disease progression in a therapeutic trial of milameline for AD. Neurology 2003;60:253–60 | Not a disease modification trial |
| Jack CRSM, Gracon S, Hoover TM, Felmlee JP, Stewart K, Xu YC, et al. MRI as a Surrogate End Point for Disease Progression in a Therapeutic Trial of Milameline for Alzheimer’s Disease. Proceedings of the 8th International Conference on Alzheimer’s Disease and Related Disorders, Stockholm, Sweden, 20–25 July 2002 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Jacoby R. Rofecoxib or naproxen do not slow progression of mild to moderate Alzheimer’s disease. Evid Based Ment Health 2003;6:110 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Jann MW, Cyrus PA, Eisner LS, Margolin DI, Griffin T, Gulanski B. Efficacy and safety of a loading-dose regimen versus a no-loading-dose regimen of metrifonate in the symptomatic treatment of Alzheimer’s disease: a randomised, double-masked, placebo-controlled trial. Metrifonate Study Group. Clin Ther 1999;21:88–102 | Not a disease modification trial |
| Jelcic N, Agostini M, Meneghello F, Busse C, Parise S, Galano A, et al. Feasibility and efficacy of cognitive telerehabilitation in early Alzheimer’s disease: a pilot study. Clin Interv Ageing 2014;9:1605–11 | Not a disease modification trial |
| Jelcic N, Cagnin A, Meneghello F, Turolla A, Ermani M, Dam M. Effects of lexical-semantic treatment on memory in early Alzheimer disease: an observer-blinded randomised controlled trial. Neurorehabil Neural Repair 2012;26:949–56 | Not a disease modification trial |
| Jeong YH WR, Park CH, Suh YH. Therapeutic potentials of mefenamic acid for the treatment of Alzheimer’s disease. Neurobiol Ageing 2004;25:s589 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Jesso S, Diodati D, Morlog D, Pasternak S, Kertesz A, Finger E. A randomised, double-blind, placebo controlled, cross-over study of the effects of oxytocin in patients with frontotemporal dementia. Dement Geriatr Cogn Disord 2010;30:98 | Not a disease modification trial |
| Jhee SS, Fabbri L, Piccinno A, Monici P, Moran S, Zarotsky V, et al. First clinical evaluation of ganstigmine in patients with probable Alzheimer’s disease. Clin Neuropharmacol 2003;26:164–9 | Not a disease modification trial |
| Joffres C, Bucks RS, Haworth J, Wilcock GK, Rockwood K. Patterns of clinically detectable treatment effects with galantamine: a qualitative analysis. Dement Geriatr Cogn Disord 2003;15:26–33 | Not a disease modification trial |
| Johansson C, Ballard C, Hansson O, Palmqvist S, Minthon L, Aarsland D, et al. Efficacy of memantine in PDD and DLB: an extension study including washout and open-label treatment. Int J Geriatr Psychiatry 2011;26:206–13 | Not a RCT/CCT |
| Johannsen P, Salmon E, Hampel H, Xu Y, Richardson S, Qvitzau S, et al. Assessing therapeutic efficacy in a progressive disease: a study of donepezil in Alzheimer’s disease. CNS drugs 2006;20:311–25 | Not a disease modification trial |
| Johnsen K, Brynne N, Annas P, Hannesdottir K, Alexander R, Segerdahl M. The effects of AZD1446 (A neuronal nicotinic receptor agonist) on quantified electroencephalography (QEEG) in patients with mild to moderate Alzheimer’s disease. Quantitative measurements using a QEEG cholinergic index. J Nutr Health Ageing 2013;17:834 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Johnson KH. Donepezil minimally effective for patients with vascular dementia. J Fam Pract 2004;53:181–2 | Not a disease modification trial |
| Johnson NA, Rademaker A, Weintraub S, Gitelman D, Wienecke C, Mesulam M. Pilot trial of memantine in primary progressive aphasia. Alzheimer Dis Assoc Disord 2010;24:308 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Jolkkonen JT, Soininen HS, Riekkinen PJ. The effect of an ACTH4–9 analogue (Org2766) on some cerebrospinal fluid parameters in patients with Alzheimer’s disease. Life Sci 1985;37:585–90 | Not a disease modification trial |
| Jones RW, McCrone P, Guilhaume C. Cost-effectiveness of memantine in Alzheimer’s disease: an analysis based on a probabilistic Markov model from a UK perspective. Drugs Ageing 2004;21:607–20 | Not a disease modification trial |
| Jones RW, Soininen H, Hager K, Aarsland D, Passmore P, Murthy A, et al. A multinational, randomised, 12-week study comparing the effects of donepezil and galantamine in patients with mild to moderate Alzheimer’s disease. Int J Geriatr Psychiatry 2004;19:58–67 | Not a disease modification trial |
| Jorgenson S BA, Andersen J, Jensen HV, Olafsson K, Arup P, Moller SE. Fluvoxamine treatment of dementia: tryptophan levels. Biol Psychiatry 1993;34:587–8 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Jubert J, Navarra J, Canals R, Balaguer M. [Neuropsychologic evaluation of the action of oxovinca in the syndrome of diffuse deterioration of vascular origin.] Med Clín 1980;75:115–21 | Full text unavailable in English |
| Kadir A, Darreh-Shori T, Almkvist O, Wall A, Grut M, Strandberg B, et al. PET imaging of the in vivo brain acetylcholinesterase activity and nicotine binding in galantamine-treated patients with AD. Neurobiol Ageing 2008;29:1204–17 | Not a disease modification trial |
| Kalman J, Juhasz A, Rimanoczy A, Palotas A, Palotas M, Szabo Z, et al. Lack of influence of the apolipoprotein E genotype on the outcome of selegiline treatment in Alzheimer’s disease. Dement Geriatr Cogn Disord 2003;16:31–4 | Not a disease modification trial |
| Kamphuis P, Verhey F, Olde Rikkert M, Twisk J, Swinkels S, Scheltens P. Efficacy of a medical food on cognition in Alzheimer’s disease: results from secondary analyses of a randomised, controlled trial. J Nutr Health Ageing 2011;15:720–4 | Not a disease modification trial |
| Kanowski S, Hoerr R. Ginkgo biloba extract EGb 761 in dementia: intent-to-treat analyses of a 24-week, multi-centre, double-blind, placebo-controlled, randomised trial. Pharmacopsychiatry 2003;36:297–303 | Not a disease modification trial |
| Karaman Y, Erdogan F, Köseoglu E, Turan T, Ersoy AO. A 12-month study of the efficacy of rivastigmine in patients with advanced moderate Alzheimer’s disease. Dement Geriatr Cogn Disord 2005;19:51–6 | Not a disease modification trial |
| Keller C, Kadir A, Forsberg A, Porras O, Nordberg A. Long-term effects of galantamine treatment on brain functional activities as measured by PET in Alzheimer’s disease patients. J Alzheimers Dis 2011;24:109–23 | Not a disease modification trial |
| Kemp PM, Holmes C, Hoffmann S, Wilkinson S, Zivanovic M, Thom J, et al. A randomised placebo controlled study to assess the effects of cholinergic treatment on muscarinic receptors in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2003;74:1567–70 | Not a disease modification trial |
| Kennelly S, Abdullah L, Crawford F, Mullan M, Kenny RA, Lawlor B. APOE E4 genotype-specific short-term cognitive benefits of treatment with the antihypertensive nilvadipine in Alzheimer’s patients-an open-label trial. Ir J Med Sci 2011;180:S325 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Kennelly S, Abdullah L, Paris D, Parish J, Mathura V, Mullan M, et al. Demonstration of safety in Alzheimer’s patients for intervention with an anti-hypertensive drug nilvadipine: results from a 6-week open label study. Int J Geriatr Psychiatry 2011;26:1038–45 | Not a disease modification trial |
| Kertesz A, Morlog D, Light M, Blair M, Davidson W, Jesso S, et al. Galantamine in frontotemporal dementia and primary progressive aphasia. Dement Geriatr Cogn Disord 2008;25:178–85 | Not a disease modification trial |
| Kim SY, Choi SH, Rollema H, Schwam EM, McRae T, Dubrava S, et al. Phase II crossover trial of varenicline in mild to moderate Alzheimer’s disease. Dement Geriatr Cogn Disord 2014;37:232–45 | Not a disease modification trial |
| Koch HJ, Szecsey A. A randomised controlled trial of prednisone in Alzheimer’s disease. Neurology 2000;55:1067 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Knapp MJ, Gracon SI, Davis CS, Solomon PR, Pendlebury WW, Knopman DS. Efficacy and safety of high-dose tacrine: a 30-week evaluation. Alzheimer Dis Assoc Disord 1994;8(Suppl. 2):22–31 | Not a disease modification trial |
| Knapp MJ, Knopman DS, Solomon PR, Pendlebury WW, Davis CS, Gracon SI. A 30-week randomised controlled trial of high-dose tacrine in patients with Alzheimer’s disease. JAMA 1994;271:985–91 | Not a disease modification trial |
| Knopman D, Schneider L, Davis K, Talwalker S, Smith F, Hoover T, et al. Long-term tacrine (Cognex) treatment: effects on nursing home placement and mortality, Tacrine Study Group. Neurology 1996;47:166–77 | Not a disease modification trial |
| Knott V, Engeland C, Mohr E, Mahoney C, Ilivitsky V. Acute nicotine administration in Alzheimer’s disease: an exploratory EEG study. Neuropsychobiology 2000;41:210–20 | Not a disease modification trial |
| Knott V, Mohr E, Mahoney C, Engeland C, Ilivitsky V. Effects of acute nicotine administration on cognitive event-related potentials in tacrine-treated and non-treated patients with Alzheimer’s disease. Neuropsychobiology 2002;45:156–60 | Not a disease modification trial |
| Kolykhalov IV, Gavrilova SI, Kalyn Ia B, Selezneva ND, Fedorova Ia B. [Efficacy, safety and tolerability of a single dose of akatinol memantine in comparison to two-doses in patients with moderately expressed and moderately severe dementia in Alzheimer’s disease.] Zh Nevrol Psikhiatr Im S S Korsakova 2012;112:35–9 | Full text unavailable in English |
| Krishnan K, Charles H, Doraiswamy P, Mintzer J, Weisler R, Yu X, et al. Randomised, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer’s disease. Am J Geriatr Psychiatry 2003;160:2003–11 | Not a disease modification trial |
| Kruntoradova K, Mandelikova M, Mlcoch T, Dolezal T. Cost-effectiveness analysis of Ginkgo biloba extract (EGB761-tanakan) for the treatment of dementia in the Czech Republic. Value Health 2015;18:A756 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Kumar V, Anand R, Messina J, Hartman R, Veach J. An efficacy and safety analysis of Exelon in Alzheimer’s disease patients with concurrent vascular risk factors. Eur J Neurol 2000;7:159–69 | Not a disease modification trial |
| Kwak YS, Um SY, Son TG, Kim DJ. Effect of regular exercise on senile dementia patients. Int J Sports Med 2008;29:471–4 | Not a disease modification trial |
| Kwok T, Lee J, Law CB, Pan PC, Yung CY, Choi KC, et al. A randomised placebo controlled trial of homocysteine lowering to reduce cognitive decline in older demented people. Clinical Nutrition 2011;30:297–302 | Not a disease modification trial |
| Kwon JC, Kim EG, Kim JW, Kwon Oh D, Yoo BG, Yi HA, et al. A multicenter, open-label, 24-week follow-up study for efficacy on cognitive function of donepezil in Binswanger-type subcortical vascular dementia. Am J Alzheimers Dis Other Demen 2009;24:293–301 | Not a disease modification trial |
| Kyowa Hakko Kirin Pharma, Inc. NCT02127476 A Study of Single and Multiple Doses of KHK6640 in Subjects With Prodromal or Mild to Moderate Alzheimer’s Disease. URL: https://clinicaltrials.gov/ct2/show/NCT02127476 (accessed 29 January 2016) | No quantitative outcome relating to disease modification |
| Kyowa Hakko Kirin Pharma, Inc. NCT02377713 A Single Dose Study of KHK6640 in Japanese Patients With Alzheimer’s Disease. URL: https://clinicaltrials.gov/ct2/show/NCT02377713 (accessed 29 January 2016) | No quantitative outcome relating to disease modification |
| Lanier ER, Sturge G, McClernon D, Brown S, Halman M, Sacktor N, et al. HIV-1 reverse transcriptase sequence in plasma and cerebrospinal fluid of patients with AIDS dementia complex treated with abacavir. AIDS 2001;15:747–51 | Not a disease modification trial |
| Larsson V, Engedal K, Aarsland D, Wattmo C, Minthon L, Londos E. Quality of life and the effect of memantine in dementia with Lewy bodies and Parkinson’s disease dementia. Dement Geriatr Cogn Disord 2011;32:227–34 | Not a disease modification trial |
| Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Annals Neurol 2010;68:521–34 | Not a disease modification trial |
| Le Bars PL, Katz MM, Berman N, Itil TM, Freedman AM, Schatzberg AF. A placebo-controlled, double-blind, randomised trial of an extract of Ginkgo biloba for dementia. JAMA 1997;278:1327–32 | Not a disease modification trial |
| Leach L. Cognitive stimulation therapy improves cognition and quality of life in older people with dementia. Evid Based Ment Health 2004;7:19 | Not a disease modification trial |
| Lee DA, Ngo LY, Adamiak B, Gelmont D. A confirmatory phase 3 randomised, double-blind, placebo-controlled study of the safety and effectiveness of immune globulin intravenous (human), 10% solution (gammagard liquid/kiovig) for the treatment of mild to moderate Alzheimer’s disease. Alzheimers Dement 2011;1:S783–4 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Lee DY, Kim KW, Jhoo JH, Ryu SH, Choo H, Seo EH, et al. A multicenter, randomised, placebo-controlled, double-blind clinical trial of escitalopram on its atrophydelaying effect in Alzheimer’s disease. Alzheimers Dement 2012;8(Suppl. 1):603 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Lee GY, Yip CC, Yu EC, Man DW. Evaluation of a computer-assisted errorless learning-based memory training program for patients with early Alzheimer’s disease in Hong Kong: a pilot study. Clin Interv Ageing 2013;8:623–33 | Not a disease modification trial |
| Lefevre G, Sedek G, Jhee SS, Leibowitz MT, Huang HL, Enz A, et al. Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice-daily capsules in Alzheimer’s disease patients. Clin Pharmacol Ther 2008;83:106–14 | Not a disease modification trial |
| Lenz RA, Pritchett YL, Berry SM, Llano DA, Han S, Berry DA, et al. Adaptive, dose-finding Phase 2 trial evaluating the safety and efficacy of ABT-089 in mild to moderate Alzheimer disease. Alzheimer Dis Assoc Disord 2015;29:192–9 | Not a disease modification trial |
| Leroi I, Atkinson R, Overshott R. Memantine improves goal attainment and reduces caregiver burden in Parkinson’s disease with dementia. Int J Geriatr Psychiatry 2014;29:899–905 | Not a disease modification trial |
| Leroi I, Brandt J, Reich SG, Lyketsos CG, Grill S, Thompson R, et al. Randomised placebo-controlled trial of donepezil in cognitive impairment in Parkinson’s disease. Int J Geriatr Psychiatry 2004;19:1–8 | Not a disease modification trial |
| Leroi I, Overshott R, Byrne EJ, Daniel E, Burns A. Randomised controlled trial of memantine in dementia associated with Parkinson’s disease. Move Dis 2009;24:1217–21 | Not a disease modification trial |
| Levin OS, Batukaeva LA, Smolentseva IG, Amosova NA. [Efficacy and safety of memantine in dementia with Lewy bodies.] Zh Nevrol Psikhiatr Im S S Korsakova 2008;108:39–46 | Full text unavailable in English |
| Levin OS, Batukaeva LA, Smolentseva IG, Amosova NA. Efficacy and safety of memantine in Lewy body dementia. Neurosci Behav Physiol 2009;39:597–604 | Not a disease modification trial |
| Levy A, Brandeis R, Treves TA, Meshulam Y, Mawassi F, Feiler D, et al. Transdermal physostigmine in the treatment of Alzheimer’s disease. Alzheimer Dis Assoc Disord 1994;8:15–21 | Not a disease modification trial |
| Li CH, Liu CK, Yang YH, Chou MC, Chen CH, Lai CL. Adjunct effect of music therapy on cognition in Alzheimer’s disease in Taiwan: a pilot study. Neuropsychiatr Dis Treat 2015;11:291–6 | Not a disease modification trial |
| Liang E, Wagg J, Kurth M, Abushakra S. Effects of ELND005 (scyllo-inositol) on neuropsychiatric symptoms (NPS) in mild/moderate AD: correlations of ELND005 exposures to neuropsychiatric outcomes in a 78-week phase 2 study. J Nutr Health Ageing 2012;16:839 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Liang J, Li F, Wei C, Song H, Wu L, Tang Y, et al. Rationale and design of a multicenter, phase 2 clinical trial to investigate the efficacy of traditional Chinese medicine SaiLuoTong in vascular dementia. J Stroke Cerebrovasc Dis 2014;23:2626–34 | Not a disease modification trial |
| Liang P, Wang Z, Qian T, Li K. Acupuncture stimulation of Taichong (Liv3) and Hegu (LI4) modulates the default mode network activity in Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2014;29:739–48 | Not a disease modification trial |
| Likitjaroen Y, Meindl T, Friese U, Wagner M, Buerger K, Hampel H, et al. Longitudinal changes of fractional anisotropy in Alzheimer’s disease patients treated with galantamine: a 12-month randomised, placebo-controlled, double-blinded study. Eur Arch Psychiatry Clin Neurosci 2012;262:341–50 | Not a disease modification trial |
| Lin CH, Chen PK, Chang YC, Chuo LJ, Chen YS, Tsai GE, et al. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase Alzheimer disease: a randomised, double-blind, placebo-controlled trial. Biol Psychiatry 2014;75:678–85 | Not a disease modification trial |
| Lin CH, Chen PK, Chang YC, Chuo LJ, Chen YS, Tsai GE, et al. Enhancement of NMDA neurotransmission for the treatment of early-phase Alzheimer’s disease. J Neurochem 2014;130:34 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Litvan I, Phipps M, Pharr VL, Hallett M, Grafman J, Salazar A. Randomised placebo-controlled trial of donepezil in patients with progressive supranuclear palsy. Neurology 2001;57:467–73 | No participants with mild or moderate dementia |
| Liu X, Zhang J, Sun D, Fan Y, Zhou H, Fu B. Effects of fluoxetine on brain-derived neurotrophic factor serum concentration and cognition in patients with vascular dementia. Clin Interv Ageing 2014;9:411–18 | Not a disease modification trial |
| Liu-Seifert H, Andersen S, Holdridge K, Siemers E. Delayed-start analyses of solanezumab phase 3 studies in mild Alzheimer’s disease. Neurology 2015;84:P7.108 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Livingston GA, Sax KB, McClenahan Z, Blumenthal E, Foley K, Willison J, et al. Acetyl-l-carnitine in dementia. Int J Geriatr Psychiatry 1991;6:853–60 | Not a disease modification trial |
| Lloret A, Badía MC, Mora NJ, Pallardó FV, Alonso MD, Viña J. Vitamin E paradox in Alzheimer’s disease: it does not prevent loss of cognition and may even be detrimental. J Alzheimers Dis 2009;17:143–9 | Not a disease modification trial |
| Loeb MB, Molloy D, Smieja M, Standish T, Goldsmith CH, Mahony J, et al. A randomised, controlled trial of doxycycline and rifampin for patients with Alzheimer’s disease. J Am Geriatr Soc 2004;52:381–7 | Not a disease modification trial |
| Lojkowska W, Ryglewicz D, Jedrzejczak T, Minc S, Jakubowska T, Jarosz H, et al. The effect of cholinesterase inhibitors on the regional blood flow in patients with Alzheimer’s disease and vascular dementia. J Neurol Sci 2003;216:119–26 | Not a disease modification trial |
| Lopez-Pousa S, Turon-Estrada A, Garre-Olmo J, Pericot-Nierga I, Lozano-Gallego M, Vilalta-Franch M, et al. Differential efficacy of treatment with acetylcholinesterase inhibitors in patients with mild and moderate Alzheimer’s disease over a 6-month period. Dement Geriatr Cogn Disord 2005;19:189–95 | Not a disease modification trial |
| Lott IT, Doran E, Nguyen VQ, Tournay A, Head E, Gillen DL. Down syndrome and dementia: a randomised, controlled trial of antioxidant supplementation. Am J Med Genet A 2011;155A:1939–48 | Not a disease modification trial |
| Lu PH, Masterman DA, Mulnard R, Cotman C, Miller B, Yaffe K, et al. Preliminary results suggest testosterone therapy may benefit older patients with AD. Brown Uni Geriatr Psychopharmacol Update 2006;10:1–7 | Not a disease modification trial |
| Lucas P, Li D, Lobello K, Liu E, Brashear HR, Styren S. Intravenous bapineuzumab in mild to moderate Alzheimer’s disease: results from two double-blind, placebo-controlled phase 3 trials. J Nutr Health Ageing 2013;17:806–7 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Lucca U TM, Forloni G, Spagnoli A. Nonsteroidal antiinflammatory drug use in Alzheimer’s disease. Biol Psychiatry 1994;36:854–6 | Not a disease modification trial |
| Lyketsos CG, Reichman WE, Kershaw P, Zhu Y. Long-term outcomes of galantamine treatment in patients with Alzheimer disease. Am J Geriatr Psychiatry 2004;12:473–82 | Not a disease modification trial |
| Magdolna P, Anna J, Agnes F, Gergely D, Csilla FO, Tamas HL, et al. [Achetylcholinesterase (AchE) inhibition and serum lipokines in Alzheimer’s disease: Friend or foe?] Neuropsychopharmacol Hung 2012;14:19–27 | Full text unavailable in English |
| Maher-Edwards G, Watson C, Ascher J, Barnett C, Boswell D, Davies J, et al. Two randomised controlled trials of SB742457 in mild to moderate Alzheimer’s disease. Alzheimers Dement 2015;1:23–36 | Not a disease modification trial |
| Maher-Edwards G, Zvartau-Hind M, Hunter A, Gold M, Hopton G, Jacobs G, et al. Double-blind, controlled phase II study of a 5-HT6 receptor antagonist, SB-742457, in Alzheimer’s disease. Curr Alzheimer Res 2010;7:374–85 | Not a disease modification trial |
| Maina G, Fiori L, Torta R, Fagiani M, Ravizza L, Bonavita E, et al. Oxiracetam in the treatment of primary degenerative and multi-infarct dementia: a double-blind, placebo-controlled study. Neuropsychobiology 1989;21:141–5 | Not a disease modification trial |
| Maltby N, Broe GA, Creasey H, Jorm AF, Christensen H, Brooks WS. Efficacy of tacrine and lecithin in mild to moderate Alzheimer’s disease: double blind trial. BMJ 1994;308:879–83 | Not a disease modification trial |
| Marek GJ, Katz DA, Meier A, Greco NT, Zhang W, Liu W, et al. Efficacy and safety evaluation of HSD-1 inhibitor ABT-384 in Alzheimer’s disease. Alzheimers Dement 2014;10(Suppl.):364–73 | Not a disease modification trial |
| Marek G, Katz D, Meier A, Greco N, Zhang W, Liu W, et al. Evaluation of the efficacy and safety of the HSD-1 inhibitor ABT-384 in mild to moderate Alzheimer’s disease. Alzheimers Dement 2012;8(Suppl. 1):602 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Marin DB, Bierer LM, Lawlor BA, Ryan TM, Jacobson R, Schmeidler J, et al. L-deprenyl and physostigmine for the treatment of Alzheimer’s disease. Psychiatry Res 1995;58:181–9 | Not a disease modification trial |
| Marsh L, Biglan K, Williams JR. Randomised placebo-controlled trial of memantine for dementia in Parkinson’s disease. Parkinsonism Relat Disord 2009;15:S82 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Maruyama M, Tomita N, Iwasaki K, Ootsuki M, Matsui T, Nemoto M, et al. Benefits of combining donepezil plus traditional Japanese herbal medicine on cognition and brain perfusion in Alzheimer’s disease: a 12-week observer-blind, donepezil monotherapy controlled trial. J Am Geriatr Soc 2006;54:869–71 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Masterman D, Awipi T, Ashford E, Nave S, Yoo K, Vellas B, et al. A nicotinic-alpha-7 partial agonist as adjunctive therapy to stable donepezil. J Nutr Health Ageing 2012;16:838–9 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Matsuda O. Cognitive stimulation therapy for Alzheimer’s disease: the effect of cognitive stimulation therapy on the progression of mild Alzheimer’s disease in patients treated with donepezil. Int Psychogeriatr 2007;19:241–52 | Not a disease modification trial |
| Matsuda O, Shido E, Hashikai A, Shibuya H, Kouno M, Hara C, et al. Short-term effect of combined drug therapy and cognitive stimulation therapy on the cognitive function of Alzheimer’s disease. Psychogeriatrics 2010;10:167–72 | Not a disease modification trial |
| Maurer K, Ihl R, Dierks T, Frolich L. Clinical efficacy of Ginkgo biloba special extract EGb 761 in dementia of the Alzheimer type. J Psychiat Res 1997;31:645–55 | Not a disease modification trial |
| McCarney R, Fisher P, Iliffe S, Haselen R, Griffin M, Meulen J, et al. Ginkgo biloba for mild to moderate dementia in a community setting: a pragmatic, randomised, parallel-group, double-blind, placebo-controlled trial. Int J Geriatr Psychiatry 2008;23:1222–30 | Not a disease modification trial |
| McCarney R, Warner J, Iliffe S, Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol 2007;7:30 | Not a disease modification trial |
| McKeith I, Ser T, Spano P, Emre M, Wesnes K, Anand R, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000;356:2031–6 | Not a disease modification trial |
| McKeith IG. The clinical trial protocol of the Metrifonate in Alzheimer’s Trial (MALT). Dement Geriatr Cogn Disord 1998;9(Suppl. 2):2–7 | Not a disease modification trial |
| McShane RH. Memantine plus donepezil improves physical and mental health in people with Alzheimer’s disease. Evid Based Ment Health 2004;7:76 | Not a disease modification trial |
| Mellow AM, Sunderland T, Cohen RM, Lawlor BA, Hill JL, Newhouse PA, et al. Acute effects of high-dose thyrotropin releasing hormone infusions in Alzheimer’s disease. Psychopharmacology 1989;98:403–7 | Not a disease modification trial |
| Meyer JS, Chowdhury MH, Xu G, Li YS, Quach M. Donepezil treatment of vascular dementia. Ann NY Acad Sci 2002;977:482–6 | Not a disease modification trial |
| Meyer JS, Rogers RL, McClintic K, Mortel KF, Lotfi J. Randomised clinical trial of daily aspirin therapy in multi-infarct dementia. A pilot study. J Am Geriatr Soc 1989;37:549–55 | Not a disease modification trial |
| Minthon L, Edvinsson L, Gustafson L. Tacrine treatment modifies cerebrospinal fluid neuropeptide levels in Alzheimer’s disease. Dementia 1994;5:295–301 | Not a disease modification trial |
| Minthon L, Gustafson L, Dalfel G, Hagberg B, Nilsson K, Risberg J, et al. Oral tetrahydroaminoacridine treatment of Alzheimer’s disease evaluated clinically and by regional cerebral blood flow and EEG. Dementia 1993;4:32–42 | Not a disease modification trial |
| Mintzer JE, Kershaw P. The efficacy of galantamine in the treatment of Alzheimer’s disease: comparison of patients previously treated with acetylcholinesterase inhibitors to patients with no prior exposure. Int J Geriatr Psychiatry 2003;18:292–7 | Not a disease modification trial |
| Modrego PJ, Fayed N, Errea JM, Rios C, Pina MA, Sarasa M. Memantine versus donepezil in mild to moderate Alzheimer’s disease: a randomised trial with magnetic resonance spectroscopy. Eur J Neurol 2010;17:405–12 | Not a disease modification trial |
| Modrego PJ, Pina MA, Fayed N, Díaz M. Changes in metabolite ratios after treatment with rivastigmine in Alzheimer’s disease: a nonrandomised controlled trial with magnetic resonance spectroscopy. CNS Drugs 2006;20:867–77 | Not a disease modification trial |
| Moebius H, Loewen G, Dgetluck N, Hilt D. A randomised, double-blind, placebo-controlled, 24-week, phase 2b outcomes study of 3 different doses of encenicline or placebo in subjects with mild to moderate probable Alzheimer’s disease. Neurology 2015;84:P7.100 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Mohr E, Knott V, Sampson M, Wesnes K, Herting R, Mendis T. Cognitive and quantified electroencephalographic correlates of cycloserine treatment in Alzheimer’s disease. Clin Neuropharmacol 1995;18:28–38 | Not a disease modification trial |
| Mohr E, Nair NP, Sampson M, Murtha S, Belanger G, Pappas B, et al. Treatment of Alzheimer’s disease with sabeluzole: functional and structural correlates. Clin Neuropharmacol 1997;20:338–45 | Not a disease modification trial |
| Mohs RC, Doody R, Morris J, Ieni J, Rogers S, Perdomo C, et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology 2001;57:481–8 | Not a disease modification trial |
| Mohs RC, Shiovitz TM, Tariot PN, Porsteinsson AP, Baker KD, Feldman PD. Atomoxetine augmentation of cholinesterase inhibitor therapy in patients with Alzheimer disease: 6-month, randomised, double-blind, placebo-controlled, parallel-trial study. Am J Geriatr Psychiatry 2009;17:752–9 | Not a disease modification trial |
| Mok V, Wong A, Ho S, Leung T, Lam WWM, Wong KS. Rivastigmine in Chinese patients with subcortical vascular dementia. Neuropsychiatr Dis Treat 2007;3:943–8 | Not a disease modification trial |
| Molinuevo JL, Berthier ML, Rami L. Donepezil provides greater benefits in mild compared to moderate Alzheimer’s disease: implications for early diagnosis and treatment. Geriatric Arch Gerontol Geriatr 2011;52:18–22 | Not a disease modification trial |
| Moller HJ, Hampel H, Hegerl U, Schmitt W, Walter K. Double-blind, randomised, placebo-controlled clinical trial on the efficacy and tolerability of a physostigmine patch in patients with senile dementia of the Alzheimer type. Pharmacopsychiatry 1999;32:99–106 | Not a disease modification trial |
| Moller HJ, Maurer I, Saletu B. Placebo-controlled trial of the xanthine derivative propentofylline in dementia. Pharmacopsychiatry 1994;27:159–65 | Not a disease modification trial |
| Molloy DW, Standish TI, Almeida E, DiLoreto P, Lam-Au C, Guyatt GH. Doxycycline and rifampin for Alzheimer’s disease – The DARAD clinical trial. Eur Geriatr Med 2010;1:S3 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Moreno Moreno MDJ. Cognitive improvement in mild to moderate Alzheimer’s dementia after treatment with the acetylcholine precursor choline alfoscerate: a multicenter, double-blind, randomised, placebo-controlled trial. Clin Ther 2003;25:178–93 | Not a disease modification trial |
| Moretti DV. Alpha rhythm oscillations and MMSE scores are differently modified by transdermal or oral rivastigmine in patients with Alzheimer’s disease. Am J Neurodegener Dis 2014;3:72–83 | Not a disease modification trial |
| Moretti DV, Frisoni GB, Giuliano B, Zanetti O. Comparison of the effects of transdermal and oral rivastigmine on cognitive function and EEG markers in patients with Alzheimer’s disease. Front Ageing Neurosci 2014;6:179 | Not a disease modification trial |
| Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Rivastigmine in subcortical vascular dementia: an open 22-month study. J Neurol Sci 2002;203–204:141–6 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Rivastigmine in subcortical vascular dementia: a randomised, controlled, open 12-month study in 208 patients. Am J Alzheimers Dis Other Demen 2003;18:265–72 | Not a disease modification trial |
| Moretti R, Torre P, Antonello RM, Cazzato G, Griggio S, Ukmar M, et al. Rivastigmine superior to aspirin plus nimodipine in subcortical vascular dementia: an open, 16-month, comparative study. Int J Clin Pract 2004;58:346–53 | Not a disease modification trial |
| Moretti R, Torre P, Antonello RM, Cazzato G, Pizzolato G. Different responses to rivastigmine in subcortical vascular dementia and multi-infarct dementia. Am J Alzheimers Dis Other Demen 2008;23:167–76 | Not a disease modification trial |
| Morgan J, Sethi KD. Rivastigmine for dementia associated with Parkinson’s disease. Curr Neurol Neurosci Rep 2005;5:263–5 | Not a disease modification trial |
| Mori E, Ikeda M, Kosaka K. Donepezil for dementia with Lewy bodies: a randomised, placebo-controlled trial. Annals Neurol 2012;72:41–52 | Not a disease modification trial |
| Mori E, Ikeda M, Nagai R, Matsuo K, Nakagawa M, Kosaka K. Long-term donepezil use for dementia with Lewy bodies: results from an open-label extension of Phase III trial. Alzheimers Res Ther 2015;7:5 | Not a disease modification trial |
| Mori S, Mori E, Iseki E, Kosaka K. Efficacy and safety of donepezil in patients with dementia with Lewy bodies: preliminary findings from an open-label study. Psychiatry Clin Neurosci 2006;60:190–5 | Not a disease modification trial |
| Morillas-Ruiz JM, Rubio-Perez JM, Albaladejo MD, Zafrilla P, Parra S, Vidal-Guevara ML. Effect of an antioxidant drink on homocysteine levels in Alzheimer’s patients. J Neurol Sci 2010;299:175–8 | Not a disease modification trial |
| Morris JC, Cyrus PA, Orazem J, Mas J, Bieber F, Ruzicka BB, et al. Metrifonate benefits cognitive, behavioural, and global function in patients with Alzheimer’s disease. Neurology 1998;5:1222–30 | Not a disease modification trial |
| Moss DE, Berlanga P, Hagan MM, Sandoval H, Ishida C. Methanesulfonyl fluoride (MSF): a double-blind, placebo-controlled study of safety and efficacy in the treatment of senile dementia of the Alzheimer type. Alzheimer Dis Assoc Disord 1999;13:20–5 | Not a disease modification trial |
| Mossello E, Tonon E, Caleri V, Tilli S, Cantini C, Cavallini MC, et al. Effectiveness and safety of cholinesterase inhibitors in elderly subjects with Alzheimer’s disease: a ‘real world’ study. Arch Gerontol Geriatr 2004;38(Suppl.):297–307 | Not a disease modification trial |
| Mowla A, Mosavinasab M, Haghshenas H, Haghighi AB. Does serotonin augmentation have any effect on cognition and activities of daily living in Alzheimer’s dementia? A double-blind, placebo-controlled clinical trial. J Clin Psychopharmacol 2007;27:484–7 | Not a disease modification trial |
| Mulnard RA, Cotman CW, Kawas C, Dyck CH, Sano M, Doody R, et al. Oestrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomised controlled trial. JAMA 2000;283:1007–15 | Not a disease modification trial |
| Muniza R, Serraa CM, Reisberga B, Rojo JM, del Ser T, Casanova JP, et al. Cognitive-motor intervention in Alzheimer’s disease: long-term results from the Maria Wolff trial. J Alzheimers Dis 2015;45:295–304 | Not a disease modification trial |
| Muratorio A, Bonuccelli U, Nuti A, Battistini N, Passero S, Caruso V, et al. A neurotropic approach to the treatment of multi-infarct dementia using L-alpha-glycerylphosphorylchlorine. Curr Ther Res Clin Exp 1992;52:741–52 | Not a disease modification trial |
| Na HR, Kim S, Choi SH, Yang DW, Bae HJ, Kim JE, et al. Donepezil treatment in Alzheimer’s disease patients with and without cerebrovascular lesions: a preliminary report. Geriatr Gerontol Int 2011;11:90–7 | Not a disease modification trial |
| Naber D, Greenspan A, Schreiner A. Efficacy and safety of risperidone in the treatment of elderly patients suffering from organic brain disease (organic brain syndrome): results from a double-blind, randomised, placebo-controlled clinical trial. Psychopharmacology 2007;191:1027–9 | No participants with mild or moderate dementia |
| Nadeau SE, Malloy PF, Andrew ME. A crossover trial of bromocriptine in the treatment of vascular dementia. Ann Neurol 1988;24:270–2 | Not a disease modification trial |
| Nakamura Y, Strohmaier C, Tamura K, Kataoka N, Nakano M, Oda S, et al. A 24-week, randomised, controlled study to evaluate the tolerability, safety and efficacy of 2 different titration schemes of the rivastigmine patch in Japanese patients with mild to moderate Alzheimer’s disease. Dement Geriatr Cogn Disord Extra 2015;5:361–74 | Not a disease modification trial |
| Nakano S, Asada T, Matsuda H, Uno M, Takasaki M. Donepezil hydrochloride preserves regional cerebral blood flow in patients with Alzheimer’s disease. J Nucl Med 2001;42:1441–5 | Not a disease modification trial |
| Nakasujja N, Miyahara S, Evans S, Lee A, Musisi S, Katabira E, et al. Randomised trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology 2013;80:196–202 | Not a disease modification trial |
| Nakatsuka M, Nakamura K, Hamanosono R, Takahashi Y, Kasai M, Sato Y, et al. A cluster randomised controlled trial of nonpharmacological interventions for old-old subjects with a clinical dementia rating of 0.5: The Kurihara Project. Dement Geriatr Cogn DisordExtra 2015;5:221–32 | Not a disease modification trial |
| Nappi G, Bono G, Merlo P, Borromei A, Caltagirone C, Lomeo C, et al. Long-term nicergoline treatment of mild to moderate senile dementia. Results of a multicentre, double-blind, placebo-controlled study. Clin Drug Investig 1997;13:308–16 | Not a disease modification trial |
| Napryeyenko O, Borzenko I. Ginkgo biloba special extract in dementia with neuropsychiatric features. A randomised, placebo-controlled, double-blind clinical trial. Arzneimittelforschung 2007;57:4–11 | Not a disease modification trial |
| Napryeyenko O, Sonnik G, Tartakovsky I. Efficacy and tolerability of Ginkgo biloba extract EGb 761 by type of dementia: analyses of a randomised controlled trial. J Neurol Sci 2009;283:224–9 | Not a disease modification trial |
| Nasab NM, Bahrammi MA, Nikpour MR, Rahim F, Naghibis SN. Efficacy of rivastigmine in comparison to ginkgo for treating Alzheimer’s dementia. J Pak Med Assoc 2012;62:677–80 | Not a disease modification trial |
| Nascimento CMC, Teixeira CVL, Gobbi LTB, Gobbi S, Stella F. [A controlled clinical trial on the effects of exercise on neuropsychiatric disorders and instrumental activities in women with Alzheimer’s disease.] Braz J Phys Ther 2012;16:197–204 | Not a disease modification trial |
| Navia BA, Dafni U, Simpson D, Tucker T, Singer E, McArthur JC, et al. A phase I/II trial of nimodipine for HIV-related neurological complications. Neurology 1998;51:221–8 | Not a disease modification trial |
| Neumann PJ, Hermann RC, Kuntz KM, Araki SS, Duff SB, Leon J, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer’s disease. Neurology 1999;52:1138–45 | Not a disease modification trial |
| Nicholas T, Knebel W, Gastonguay MR, Bednar MM, Billing CB, Landen JW, et al. Preliminary population pharmacokinetic modelling of pf-04360365, a humanised anti-amyloid monoclonal antibody, in patients with mild to moderate Alzheimer’s disease. Alzheimers Dement 2009;1:253 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Nordberg A, Darreh-Shori T, Peskind E, Soininen H, Mousavi M, Eagle G, et al. Different cholinesterase inhibitor effects on CSF cholinesterases in Alzheimer patients. Curr Alzheimer Res 2009;6:4–14 | Not a disease modification trial |
| Novak G, Brashear HR, Di J, Werth J, Booth K, Margolin R, et al. Efficacy and safety of monthly subcutaneous bapineuzumab. Alzheimers Dement 2014;10:25 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Novak PH, Moessler H, Gusev EI, Guekht AB. Cerebrolysin in vascular dementia: a randomised, placebo controlled study. Alzheimers Dement 2009;1:249 | Not published in a peer-reviewed journal article or is an ongoing trial |
| O’Brien BJ, Goeree R, Hux M, Iskedjian M, Blackhouse G, Gagnon M, et al. Economic evaluation of donepezil for the treatment of Alzheimer’s disease in Canada. J Am Geriatr Soc 1999;47:570–8 | Not a disease modification trial |
| O’Caoimh R, Healy L, Gao Y, Svendrovski A, Kerins DM, Eustace J, et al. Effects of centrally acting angiotensin-converting enzyme inhibitors on functional decline in patients with Alzheimer’s disease. J Alzheimers Dis 2014;40:595–603 | Not a disease modification trial |
| Ohnishi T, Sakiyama Y, Okuri Y, Kimura Y, Sugiyama N, Saito T, et al. The prediction of response to galantamine treatment in patients with mild to moderate Alzheimer’s disease. Curr Alzheimer Res 2014;11:110–18 | Not a disease modification trial |
| Olazarán J, Muñiz R. Cognitive intervention in the initial stages of Alzheimer’s disease. Res Pract Alzheimers Dis 2006;11:376–80 | Not a disease modification trial |
| Olazarán J, Muñiz R, Reisberg B, Peña-Casanova J, Ser T, Cruz-Jentoft AJ, et al. Benefits of cognitive-motor intervention in MCI and mild to moderate Alzheimer disease. Neurology 2004;63:2348–53 | Not a disease modification trial |
| Onder G, Zanetti O, Giacobini E, Frisoni GB, Bartorelli L, Carbone G, et al. Reality orientation therapy combined with cholinesterase inhibitors in Alzheimer’s disease: randomised controlled trial. Br J Psychiatry 2005;187:450–5 | Not a disease modification trial |
| Orgeta V, Leung P, Yates L, Kang S, Hoare Z, Henderson C, et al. Individual cognitive stimulation therapy for dementia: a clinical effectiveness and cost-effectiveness pragmatic, multicentre, randomised controlled trial. Health Technol Assess 2015;19(64) | Not a disease modification trial |
| Orgogozo JM, Rigaud AS, Stöffler A, Möbius HJ, Forette F. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomised, placebo-controlled trial (MMM 300). Stroke 2002;33:1834–9 | Not a disease modification trial |
| Orrell M, Aguirre E, Spector A, Hoare Z, Woods RT, Streater A, et al. Maintenance cognitive stimulation therapy for dementia: single-blind, multicentre, pragmatic randomised controlled trial. Br J Psychiatry 2014;204:454–61 | Not a disease modification trial |
| Orrell M, Yates LA, Burns A, Russell I, Woods RT, Hoare Z, et al. Individual cognitive stimulation therapy for dementia (iCST): study protocol for a randomised controlled trial. Trials 2012;13:172 | Not a disease modification trial |
| Ortega L, Yassuda M, Nunes P, Aprahamian I, Santos F, Santos G, et al. The effects of a multiprofessional cognitive and functional rehabilitation program for patients with mild Alzheimer’s disease. Alzheimers Dement 2011;1:S660–1 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Othman A, Meier A, William Ritchie C, Florian H, Gault LM, Tang Q. Efficacy and safety of the ALPHA7 agonist ABT-126 as a monotherapy treatment in mild to moderate Alzheimer’s dementia: results of a phase 2b trial. Alzheimers Dement 2014;10:P137 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Ott BR, Blake LM, Kagan E, Resnick M. Open label, multicenter, 28-week extension study of the safety and tolerability of memantine in patients with mild to moderate Alzheimer’s disease. J Neurol 2007;254:351–8 | Not a disease modification trial |
| Paasschen J, Clare L, Yuen KS, Woods RT, Evans SJ, Parkinson CH, et al. Cognitive rehabilitation changes memory-related brain activity in people with Alzheimer disease. Neurorehabil Neural Repair 2013;27:448–59 | Not a disease modification trial |
| Panisset M, Gauthier S, Moessler H, Windisch M. Cerebrolysin in Alzheimer’s disease: a randomised, double-blind, placebo-controlled trial with a neurotrophic agent. J Neural Transm 2002;109:1089–104 | Not a disease modification trial |
| Pantoni L, Bianchi C, Beneke M, Inzitari D, Wallin A, Erkinjuntti T. The Scandinavian Multi-Infarct Dementia Trial: a double-blind, placebo-controlled trial on nimodipine in multi-infarct dementia. J Neurol Sci 2000;175:116–23 | Not a disease modification trial |
| Pantoni L, Rossi R, Inzitari D, Bianchi C, Beneke M, Erkinjuntti T, et al. Efficacy and safety of nimodipine in subcortical vascular dementia: a subgroup analysis of the Scandinavian Multi-Infarct Dementia Trial. J Neurol Sci 2000;175:124–34 | Not a disease modification trial |
| Pantoni L, Ser T, Soglian AG, Amigoni S, Spadari G, Binelli D, et al. Efficacy and safety of nimodipine in subcortical vascular dementia: a randomised placebo-controlled trial. Stroke 2005;36:619–24 | Not a disease modification trial |
| Parnetti L, Ambrosoli L, Abate G, Azzini C, Balestreri R, Bartorelli L, et al. Posatirelin for the treatment of late-onset Alzheimer’s disease: a double-blind multicentre study vs citicoline and ascorbic acid. Acta Neurol Scand 1995;92:135–40 | Not a disease modification trial |
| Parnetti L, Chiasserini D, Andreasson U, Ohlson M, Huls C, Zetterberg H, et al. Changes in CSF acetyl- and butyrylcholinesterase activity after long-term treatment with AChE inhibitors in Alzheimer’s disease. Acta Neurol Scand 2011;124:122–9 | Not a disease modification trial |
| Paskavitz JF, Gunstad JJ, Samuel JE. Clock drawing and frontal lobe behavioural effects of memantine in Alzheimer’s disease: a rater-blinded study. Am J Alzheimers Dis Other Dement 2006;21:454–9 | Not a disease modification trial |
| Pasinetti G, Rosenberg P. Repurposing anti-hypertensive drugs for Alzheimer’s disease. Alzheimers Dement 2012;1:707–P8 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Pasinetti GM, Rosenberg P. Repurposing cardiovascular drugs as Alzheimer’s disease modifying agents. J Nutr Health Ageing 2012;16:822 | Not published in a peer reviewed journal article or an ongoing trial |
| Patel KR. Biogen’s Aducanumab raises hope that Alzheimer’s can be treated at its source. Manag Care 2015;24:19 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Peng DT, Xu XH, Wang LN. [Efficiency and safety assessment of donepezil for treating mild and moderate Alzheimer disease.] Chin J Clin Rehabil 2005;9:170–2 | Full text unavailable in English |
| Perryman KM, Fitten LJ. Quantitative EEG during a double-blind trial of THA and lecithin in patients with Alzheimer’s disease. J Geriatr Psychiatry Neurol 1991;4:127–33 | Not a disease modification trial |
| Peskind ER, Potkin SG, Pomara N, Ott BR, Graham SM, Olin JT, et al. Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomised, controlled trial. Am J Geriatr Psychiatry 2006;14:704–15 | Not a disease modification trial |
| Petit H, Doody RS, Pratt RD. Donepezil Improves Cognition and Global Function in Alzheimer’s Disease: Results from us and Multinational Phase III Clinical Trials. 11th European College of Neuropsychopharmacology Congress Paris, France, 31 October–4 November 1998 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Pettegrew JW, Klunk WE, Panchalingam K, Kanfer JN, McClure RJ. Clinical and neurochemical effects of acetyl-L-carnitine in Alzheimer’s disease. Neurobiol Aging 1995;16:1–4 | Not a disease modification trial |
| Pfizer. NCT00912288 A Phase 3, Multi-Centre, Randomised, Double-Blind, Placebo-Controlled 26-Week Trial to Evaluate the Efficacy and Safety of Dimebon in Patients with Moderate-to-Severe Alzheimer’s Disease. URL: http://clinicaltrials.gov/show/NCT00912288 (accessed 29 January 2016) | Not published in a peer-reviewed journal article or is an ongoing trial |
| Phillips MA, Childs CE, Calder PC, Rogers PJ. No effect of omega-3 fatty acid supplementation on cognition and mood in individuals with cognitive impairment and probable Alzheimer’s disease: a randomised controlled trial. Int J Mol Sci 2015;16:24600–13 | Not a disease modification trial |
| Pirttilä T, Wilcock G, Truyen L, Damaraju CV. Long-term efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicenter trial. Eur J Neurol 2004;11:734–41 | Not a disease modification trial |
| Pitkälä KH, Pöysti MM, Laakkonen M-L, Tilvis RS, Savikko N, Kautiainen H, et al. Effects of the Finnish Alzheimer Disease Exercise Trial (FINALEX): a randomised controlled trial. JAMA Intern Med 2013;173:894–901 | Not a disease modification trial |
| Pitkala K, Raivio M, Laakkonen ML, Tilvis R, Kautiainen H, Strandberg T. Effects of intensive exercise intervention on Alzheimer’s patients – a randomised, controlled trial. Eur Geriatr Med 2011;2:S59 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Pitkala KH, Raivio MM, Laakkonen ML, Tilvis RS, Savikko N, Kautiainen H, et al. Effectiveness and costs of intensive exercise intervention on Alzheimer’s patients – a randomised, controlled trial. Eur Geriatr Med 2013;4:S8 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Poewe W, Wolters E, Emre M, Onofrj M, Hsu C, Tekin S, et al. Long-term benefits of rivastigmine in dementia associated with Parkinson’s disease: an active treatment extension study. Move Dis 2006;21:456–61 | Not a disease modification trial |
| Pomara N, Block R, Abraham J. Combined cholinergic precursor treatment and dihydroergotoxine mesylate in Alzheimer’s disease. IRCS J Med Sci 1983;11:1048–9 | Not a disease modification trial |
| Pomara N, Ott BR, Peskind E, Resnick E. Memantine treatment of cognitive symptoms in mild to moderate Alzheimer disease: secondary analyses from a placebo-controlled randomised trail. Alzheimer Dis Assoc Disord 2007;21:60–4 | Not a disease modification trial |
| Poole Hoffmann V, Case M, Hake AM. Effects of treatment with solanezumab in patients with Alzheimer’s disease who receive current standard of care. J Nutr Health Ageing 2013;17:847–8 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Poon P, Hui E, Dai D, Kwok T, Woo J. Cognitive intervention for community-dwelling older persons with memory problems: telemedicine versus face-to-face treatment. Int J Geriatr Psychiatry 2005;20:285–6 | Not a disease modification trial |
| Porsteinsson AP, Grossberg GT, Mintzer J, Olin JT. Memantine treatment in patients with mild to moderate Alzheimer’s disease already receiving a cholinesterase inhibitor: a randomised, double-blind, placebo-controlled trial. Curr Alzheimer Res 2008;5:83–9 | Not a disease modification trial |
| Potkin SG, Alva G, Gunay I, Koumaras B, Chen M, Mirski D. A pilot study evaluating the efficacy and safety of rivastigmine in patients with mixed dementia. Drugs Ageing 2006;23:241–9 | Not a disease modification trial |
| Prasher VP, Huxley A, Haque MS. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Down syndrome and Alzheimer’s disease – pilot study. Int J Geriatr Psychiatry 2002;17:270–8 | Not a disease modification trial |
| Pratt RD. Patient populations in clinical studies of donepezil in vascular dementia. Int Psychogeriatr 2003;15(Suppl. 1):195–200 | Not a disease modification trial |
| Prentice N, Van Beck M, Dougall NJ, Moffoot APR, O’Carroll RE, Goodwin GM, et al. A double-blind, placebo-controlled study of tacrine in patients with Alzheimer’s disease using SPET. J Psychopharmacol 1996;10:175–81 | Not a disease modification trial |
| Pressman P, Gottfried JA. Journal Club: a randomised, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology 2012;79:33–6 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Rabey J, Dobronevsky E, Marton RG, Aichenbau S, Khaigrech M. Improved cognitive function following treatment of Alzheimer’s patients with repetitive transcranial magnetic stimulation (rTMS) interlaced with cognitive learning treatment. Alzheimers Dement 2011;1:S694–5 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Rafii MS, Walsh S, Little JT, Behan K, Reynolds B, Ward C, et al. A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology 2011;76:1389–94 | Not a disease modification trial |
| Rankinen T. NCT00083811 A Phase 3, Multi-Centre, Randomised, Double-Blind Placebo-Controlled Study to Evaluate the Safety and Tolerability of Dimebon (PF-01913539) for up to 26-Weeks in Patients with Mild to Moderate Alzheimer’s Disease. URL: http://clinicaltrials.gov/ct2/show/NCT0083811 (accessed 29 January 2016) | Not published in a peer-reviewed journal article or is an ongoing trial |
| Randolph C, Roberts JW, Tierney MC, Bravi D, Mouradian MM, Chase TN. D-cycloserine treatment of Alzheimer disease. Alzheimer Dis Assoc Disord 1994;8:198–205 | Not a disease modification trial |
| Raskind M, Liang E, Sperling R, Boxer A, Ross J, Brody M, et al. Pharmacokinetics and pharmacodynamics of bapineuzumab following multiple intravenous infusions in patients with mild to moderate Alzheimer’s disease. Alzheimers Dement 2009;1:415–16 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Raskind MA, Peskind ER, Truyen L, Kershaw P, Damaraju CV. The cognitive benefits of galantamine are sustained for at least 36 months: a long-term extension trial. Arch Neurol 2004;61:252–6 | Not a disease modification trial |
| Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: a 6-month randomised, placebo-controlled trial with a 6-month extension. Neurology 2000;54:2261–8 | Not a disease modification trial |
| Ravina B, Putt M, Siderowf A, Farrar JT, Gillespie M, Crawley A, et al. Donepezil for dementia in Parkinson’s disease: a randomised, double blind, placebo controlled, crossover study. J Neurol Neurosurg Psychiatry 2005;76:934–9 | Not a disease modification trial |
| Rebok GW, Ball K, Guey LT, Jones RN, Kim H-Y, King JW, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc 2014;62:16–24 | Not a disease modification trial |
| Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol 2006;63:49–54 | Not a disease modification trial |
| Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003;348:1333–41 | Not a disease modification trial |
| Reisberg B, Kenowsky S, Boksay I, Golomb J, Heller S, Ghimire S, et al. Memantine and comprehensive, individualised, person-centred management (CI-PCM) of Alzheimer’s disease: a randomised controlled trial. Alzheimers Dement 2013;1:295–6 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Riekkinen M, Laakso MP, Jakala P, Riekkinen P Jr. Clonidine impairs sustained attention and memory in Alzheimer’s disease. Neuroscience 1999;92:975–82 | Not a disease modification trial |
| Riekkinen P, Kuikka J, Soininen H, Helkala EL, Hallikainen M, Riekkinen P. Tetrahydroaminoacridine modulates technetium-99 m labelled ethylene dicysteinate retention in Alzheimer’s disease measured with single photon emission computed tomography imaging. Neurosci Lett 1995;195:53–6 | Not a disease modification trial |
| Riekkinen P, Riekkinen M. THA improves word priming and clonidine enhances fluency and working memory in Alzheimer’s disease. Neuropsychopharmacology 1999;20:357–64 | Not a disease modification trial |
| Riekse RG, Li G, Petrie EC, Leverenz JB, Vavrek D, Vuletic S, et al. Effect of statins on Alzheimer’s disease biomarkers in cerebrospinal fluid. J Alzheimers Dis 2006;10:399–406 | No participants with mild or moderate dementia |
| Rigaud AS, André G, Vellas B, Touchon J, Pere JJ, Loria-Kanza Y. [Oestro-progestagen treatment combined with rivastigmine in menopausal women suffering from Alzheimer’s disease. The results of a 28-weeks controlled study.] Presse Med 2003;32:1649–54 | Full text unavailable in English |
| Rijpma A, Meulenbroek O, Van Hees AM, Sijben JW, Scheltens P, Olde Rikkert MG. Effects of a medical food on plasma micronutrient and fatty acid levels in mild to moderate Alzheimer’s disease. Clin Nutr 2014;33:S193 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Rijpma A, Meulenbroek O, van Hees AM, Sijben JW, Vellas B, Shah RC, et al. Effects of Souvenaid on plasma micronutrient levels and fatty acid profiles in mild and mild to moderate Alzheimer’s disease. Alzheimers Res Ther 2015;7:51 | Not a disease modification trial |
| Risner ME, Saunders AM, Altman JFB, Ormandy GC, Craft S, Foley IM, et al. Efficacy of rosiglitazone in a genetically defined population with mild to moderate Alzheimer’s disease. Pharmacogenomics J 2006;6:246–54 | Not a disease modification trial |
| Rive B, Vercelletto M, Damier FD, Cochran J, Francois C. Memantine enhances autonomy in moderate to severe Alzheimer’s disease. Int J Geriatr Psychiatry 2004;19:458–64 | Not a disease modification trial |
| Rockwood K, Dai D, Mitnitski A. Patterns of decline and evidence of subgroups in patients with Alzheimer’s disease taking galantamine for up to 48 months. Int J Geriatr Psychiatry 2008;23:207–14 | Not a disease modification trial |
| Rockwood K, Fay S, Gorman M. The ADAS-Cog and clinically meaningful change in the VISTA clinical trial of galantamine for Alzheimer’s disease. Int J Geriatr Psychiatry 2010;25:191–201 | Not a disease modification trial |
| Rockwood K, Fay S, Song X, MacKnight C, Gorman M. Video-imaging synthesis of treating Alzheimer’s disease I attainment of treatment goals by people with Alzheimer’s disease receiving galantamine: a randomised controlled trial. CMAJ 2006;174:1099–105 | Not a disease modification trial |
| Rockwood K, Mintzer J, Truyen L, Wessel T, Wilkinson D. Effects of a flexible galantamine dose in Alzheimer’s disease: a randomised, controlled trial. J Neurol Neurosurg Psychiatry 2001;71:589–95 | Not a disease modification trial |
| Rodriguez-Sanchez E, Criado-Gutierrez JM, Mora-Simon S, Muriel-Diaz MP, Gomez-Marcos MA, Recio-Rodriguez JI, et al. Physical activity program for patients with dementia and their relative caregivers: randomised clinical trial in primary health care (AFISDEMyF study). BMC Neurol 2014;14:63 | Not a disease modification trial |
| Rogers J, Kirby LC, Hempelman SR, Berry DL, McGeer PL, Kaszniak AW, et al. Clinical trial of indomethacin in Alzheimer’s disease. Neurology 1993;43:1609 | Not a disease modification trial |
| Rogers S, Perdomo C, Friedhoff L. Clinical Benefits are Maintained During Long-term Treatment of Alzheimer’s Disease with the Acetylcholinesterase Inhibitor, E2020. 8th European College of Neuropsychopharmacology Congress Venice, Italy, 30 September–4 October 1995 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Arch Intern Med 1998;158:1021–31 | Not a disease modification trial |
| Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology 1998;50:136–45 | Not a disease modification trial |
| Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT, Ieni J, et al. Donepezil improved cognitive and global function in mild to moderate Alzheimer disease. Evid Based Med 1998;3:155 | Not a disease modification trial |
| Rogers SL, Friedhoff LT. Donepezil Improves Cognition in Patients with Mild to Moderate AD: Results of ADAS-Cog Analysis in a 30-Week Phase III Study. 10th European College of Neuropsychopharmacology Congress Vienna, Austria, 13–17 September 1997 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Rogers SL, Mohs RC, Friedhoff LT. Donepezil (E2020) Improves Cognition and Function in Patients with Mild to Moderately Severe Alzheimer’s Disease Results from Phase. 150th Annual Meeting of the American Psychiatric Association San Diego, CA, 17–22 May 1997 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Román GC, Salloway S, Black SE, Royall DR, Decarli C, Weiner MW, et al. Randomised, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke 2010;41:1213–21 | Not a disease modification trial |
| Román GC, Wilkinson DG, Doody RS, Black SE, Salloway SP, Schindler RJ. Donepezil in vascular dementia: combined analysis of two large-scale clinical trials. Dement Geriatr Cogn Disord 2005;20:338–44 | Not a disease modification trial |
| Rosenbloom MH, Barclay TR, Pyle M, Owens BL, Cagan AB, Anderson CP, et al. A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild-moderate Alzheimer’s disease. CNS Drugs 2014;28:1185–9 | Not a disease modification trial |
| Rosengarten B, Paulsen S, Molnar S, Kaschel R, Gallhofer B, Kaps M. Acetylcholine esterase inhibitor donepezil improves dynamic cerebrovascular regulation in Alzheimer patients. J Neurol 2006;253:58–64 | Not a disease modification trial |
| Roshchina IF, Kolykhalov IV, Selezneva ND, Zharikov GA, Gerasimov NP, Gavrilova SI. [The influence of Cerebrolysin on the efficiency of subsequent therapy with amiridine++ in Alzheimer’s disease patients (neuropsychological investigation).] Zh Nevrol Psikhiatr Im S S Korsakova 1999;99:43–6 | Full text unavailable in English |
| Rosler M, Anand R, Cincin-Sain A, Gauthier S, Agid Y, Dal-Bianco P, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ 1999;318:633–40 | Not a disease modification trial |
| Sabbagh M, Cummings J, Christensen D, Doody R, Farlow M, Liu L, et al. Evaluating the cognitive effects of donepezil 23 mg/d in moderate and severe Alzheimer’s disease: analysis of effects of baseline features on treatment response. BMC Geriatr 2013;13:56 | Not a disease modification trial |
| Sacktor N, Kieburtz K, Schifitto G, McDermott M, Bourgeois K, Palumbo D, et al. A randomised, double-blind, placebo-controlled trial of deprenyl and thioctic acid in human immunodeficiency virus-associated cognitive impairment. Neurology 1998;50:645–51 | No participants with mild or moderate dementia |
| Sadowsky CH, Grossberg GT, Somogyi M, Meng X. Predictors of sustained response to rivastigmine in patients with Alzheimer’s disease: a retrospective analysis. Prim Care Companion J Clin Psychiatry 2011;13:PCC.10m01101 | Not a disease modification trial |
| Sahakian BJ, Coull JT. Tetrahydroaminoacridine (THA) in Alzheimer’s disease: an assessment of attentional and mnemonic function using CANTAB. Acta Neurol Scand Suppl 1993;149:29–35 | Not a disease modification trial |
| Sahakian BJ, Coull JT. Nicotine and tetrahydroaminoacradine: evidence for improved attention in patients with dementia of the Alzheimer type. Drug Develop Res 1994;31:80–8 | Not a disease modification trial |
| Sainati SM ID, Talwalker S, Geis GS. Results of a Double-Blind, Randomised, Placebo-Controlled Study of Celecoxib in the Treatment of Progression of Alzheimer’s Disease. Proceedings of the 6th International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy, Stockholm, Sweden, 5–8 April 2000 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Saletu B, Anderer P, Fischhof PK, Lorenz H, Barousch R, Böhmer F. EEG mapping and psychopharmacological studies with denbufylline in SDAT and MID. Biol Psychiatry 1992;32:668–81 | Not a disease modification trial |
| Saletu B, Hochmayer I, Grunberger J, Bohmer F, Paroubek J, Wicke L, et al. [Therapy of multi-infarct-dementia with nicergoline: double-blind, clinical, psychometric and EEG-imaging-investigation with two different drug administration schedules.] Wien Med Wochenschr 1987;137:513–24 | Full text unavailable in English |
| Salloway S, Sperline R, Gregg K, Black R, Grundman M. Cognitive and functional outcomes from a phase II trial of bapineuzumab in mid to moderate Alzheimer’s disease. Neurology 2009;72(Suppl. 3):A271 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Salloway S, Sperling R, Gregg K, Yu P, Joshi A, Lu M, et al. Incidence and clinical progression of placebo-treated amyloidnegative subjects with mild to moderate Alzheimer’s disease (AD): results from the phase III PET substudies of bapineuzumab and solanezumab. Alzheimers Dement 2013;9(Suppl. 1):888–9 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Salloway S, Sperling R, Honig L, Porsteinsson A, Sabbagh M, Liu E, et al. A randomised, double-blind, placebo-controlled clinical trial of intravenous bapineuzumab in patients with Alzheimer’s disease who are apolipoprotein E 4 non-carriers. Eur J Neurol 2012;19:70 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Salotti P, De Sanctis B, Clementi A, Fernandez Ferreira M, De Silvestris T. Evaluation of the efficacy of a cognitive rehabilitation treatment on a group of Alzheimer’s patients with moderate cognitive impairment: a pilot study. Aging Clin Exp Res 2013;25:403–9 | Not a disease modification trial |
| Salva A, Andrieu S, Fernandez E, Schiffrin E, Moulin J, Decarli B, et al. Health and nutrition promotion program for patients with dementia (NutriAlz): cluster randomised trial. J Nutr Health Aging 2011;15:822–30 | Not a disease modification trial |
| Salva A, Andrieu S, Fernandez E, Schiffrin EJ, Moulin J, Decarli B, et al. Health and nutritional promotion program for patients with dementia (NutriAlz Study): design and baseline data. J Nutr Health Aging 2009;13:529–37 | Not a disease modification trial |
| Samorajski T, Vroulis GA, Smith RC. Piracetam plus lecithin trials in senile dementia of the Alzheimer type. Ann NY Acad Sci 1985;444:478–81 | Not a disease modification trial |
| Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med 1997;336:1216–22 | Not a disease modification trial |
| Sanofi, ICD CSD. NCT00104013 A Randomised, Multicenter, Double-Blind, Placebo-Controlled, 18-Month Study of the Efficacy of SR57746A in Patients with Mild to Moderate Dementia of the Alzheimer. URL: https://clinicaltrials.gov/ct2/show/NCT00104013 (accessed 29 January 2016) | Not published in a peer-reviewed journal article or is an ongoing trial |
| Sanofi, ICD CSD. NCT00103649 18-month Study of the Efficacy of Xaliproden (SR57746A) in Patients with Mild to Moderate Dementia of the Alzheimer. URL: https://clinicaltrials.gov/ct2/show/NCT00103649 (accessed 29 January 2016) | Not published in a peer-reviewed journal article or is an ongoing trial |
| Santens P, Ventura M. Donepezil in the treatment of mild to moderate Alzheimer’s disease: report of a Belgian multicenter study. Acta Neurol Belg 2003;103:159–63 | Not a disease modification trial |
| Saxton J, Hofbauer RK, Woodward M, Gilchrist NL, Potocnik F, Hsu HA, et al. Memantine and functional communication in Alzheimer’s disease: results of a 12-week, international, randomised clinical trial. J Alzheimers Dis 2012;28:109–18 | Not a disease modification trial |
| Schecker M, Pirnay-Dummer P, Schmidtke K, Hentrich-Hesse T, Borchardt D. Cognitive interventions in mild Alzheimer’s disease: a therapy-evaluation study on the interaction of medication and cognitive treatment. Dement Geriatr Cogn Disord Extra 2013;3:301–11 | Not a disease modification trial |
| Scheltens NME, Van Berckel BNM, Boellaard R, Barkhof F, Van Der Flier WM, Kamphuis PJGH, et al. A Dutch 24-week randomised controlled study exploring the effect of a nutritional intervention on brain glucose metabolism in early Alzheimer’s disease (NL-ENIGMA); rationale and design. Eur Geriatr Med 2014;5:91 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Scheltens P, Kamphuis PJGH, Verhey FRJ, Olde Rikkert MGM, Wurtman RJ, Wilkinson D, et al. Efficacy of a medical food in mild Alzheimer’s disease: a randomised, controlled trial. Alzheimers Dement 2010;6:1–10.e1 | Not a disease modification trial |
| Scheltens P, Sperling R, Salloway S, Fox N. Bapineuzumab IV phase 3 results. J Nutr Health Aging 2012;16:797 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Scheltens P, Stam C, Shah R, Bennett D, Wieggers R, Hartmann T, et al. Medical nutrition in disease management of Alzheimer’s patients. Clin Nutr 2013;32:35 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Scheltens P, Verhey FRJ, Rikkert MGMO, Kamphuis PJGH, Wilkinson D, Kurz A. The efficacy of a medical food (Souvenaid) in Alzheimer’s disease: results from the first trial and design of future trials. Alzheimers Dement 2009;1:258–9 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Scherder E, Knol D, van Tol MJ, van Someren E, Deijen JB, Swaab D, et al. Effects of high-frequency cranial electrostimulation on the rest-activity rhythm and salivary cortisol in Alzheimer’s disease: a pilot study. Dement Geriatr Cogn Disord 2006;22:267–72 | Not a disease modification trial |
| Scherder EJ, Tol MJ, Swaab DF. High-frequency cranial electrostimulation (CES) in patients with probable Alzheimer’s disease. Am J Phys Med Rehabil 2006;85:614–18 | Not a disease modification trial |
| Schiffczyk C, Romero B, Jonas C, Muller F, Riepe MW. Efficacy of multimodal intervention for people with Alzheimer’s disease. Alzheimers Dement 2013;1:654 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Schifitto G, Navia BA, Yiannoutsos CT, Marra CM, Chang L, Ernst T, et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS 2007;21:1877–86 | Not a disease modification trial |
| Schifitto G, Zhang J, Evans SR, Sacktor N, Simpson D, Millar LL, et al. A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology 2007;69:1314–21 | Not a disease modification trial |
| Schmidt R, Ropele S, Pendl B, Ofner P, Enzinger C, Schmidt H, et al. Longitudinal multimodal imaging in mild to moderate Alzheimer disease: a pilot study with memantine. J Neurol Neurosurg Psychiatry 2008;79:1312–17 | Not a disease modification trial |
| Schmitt F, Farlow M, Olin J. Effects of rivastigmine on executive function in Parkinson’s disease dementia: results from a 24-week placebo-controlled clinical trial. Ann Neurol 2009;66:48 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Schmitt FA, Aarsland D, Bronnick KS, Meng X, Tekin S, Olin JT. Evaluating rivastigmine in mild to moderate Parkinson’s disease dementia using ADAS-Cog items. Am J Alzheimers Dis Other Demen 2010;25:407–13 | Not a disease modification trial |
| Schmitt FA, Aarsland D, Bronnick KS, Olin JT, Meng X. Evaluating cognitive effects of oral rivastigmine using subscales and items of the ADAS-Cog in patients with mild to moderate Parkinson’s disease dementia. Am J Geriatr Psychiatry 2010;1:79 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Schmitt FA, Farlow MR, Meng X, Tekin S, Olin JT. Efficacy of rivastigmine on executive function in patients with Parkinson’s disease dementia. CNS Neurosci Ther 2010;16:330–6 | Not a disease modification trial |
| Schmitt FA, Saxton J, Ferris SH, Mackell J, Sun Y. Evaluation of an 8-item Severe Impairment Battery (SIB-8) vs. the full SIB in moderate to severe Alzheimer’s disease patients participating in a donepezil study. J Clin Pract 2013;67:1050–6 | Not a disease modification trial |
| Schneider L, Porsteinsson A, Farlow M, Shimakura A, Nakagawa M, Iwakami N. The neuroprotective and neurotrophic agent T-817MA for Alzheimer’s disease: randomised, double-blind, placebo controlled proof-of-concept trial outcomes. Alzheimers Dement 2013;1:530–1 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Schneider LS, DeKosky ST, Farlow MR, Tariot PN, Hoerr R, Kieser M. A randomised, double-blind, placebo-controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer’s type. Curr Alzheimer Res 2005;2:541–51 | Not a disease modification trial |
| Schneider LS, Farlow M. Combined tacrine and oestrogen replacement therapy in patients with Alzheimer’s disease. Ann NY Acad Sci 1997;826:317–22 | Not a disease modification trial |
| Schneider LS, Farlow MR, Henderson VW, Pogoda JM. Effects of oestrogen replacement therapy on response to tacrine in patients with Alzheimer’s disease. Neurology 1996;46:1580–4 | Not a disease modification trial |
| Schneider LS, Farlow MR, Pogoda JM. Potential role for oestrogen replacement in the treatment of Alzheimer’s dementia. Am J Med 1997;103:46–50 | Not a disease modification trial |
| Schwam E, Evans R, Nicholas T, Chew R, Davidson W, Ambrose D, et al. PF-04447943: A phase II controlled clinical trial of a selective pde9a inhibitor in Alzheimer’s disease. Alzheimers Dement 2011;1:695 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Seltzer B, Zolnouni P, Nunez M, Goldman R, Kumar D, Ieni J, et al. Efficacy of donepezil in early-stage Alzheimer disease: a randomised placebo-controlled trial. Arch Neurol 2004;61:1852–6 | Not a disease modification trial |
| Seltzer B, Zolnouni P, Nunez M, Goldman R, Noble Y, Kumar D, et al. Donepezil Treatment Improves Cognitive Performance in Patients with Very Mild Alzheimer’s Disease. European Neuropsychopharmacology; 15th International Congress of the European College of Neuropsychopharmacology, Barcelona, Spain, 5–9 October, 2002 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Ser T, Lovestone S, Boada-Rovira M, Dubois B, Hull M, Rinne J, et al. A phase II randomised, double-blind, parallel group, 26-week study of GSK-3 inhibitor tideglusib in Alzheimer’s disease (argo trial). Alzheimers Dement 2013;1:689–90 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Ser TD, Lovestone S, Rovira MB, Dubois B, Hull M, Rinne J, et al. Argo trial. Alzheimers Dement 2012;1:455–6 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Shah RC, Matthews DC, Andrews RD, Capuano AW, Fleischman DA, VanderLugt JT, et al. An evaluation of MSDC-0160, a prototype mTOT modulating insulin sensitiser, in patients with mild Alzheimer’s disease. Curr Alzheimer Res 2014;11:564–73 | Not a disease modification trial |
| Shankle WR, Hara J. Longitudinal measure of IVIG treatment effect in patients with Alzheimer’s and Lewy body disease. Alzheimers Dement 2009;1:430 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Shatenstein B, Kergoat M, Reid I, Chicoine ME. Dietary intervention in older adults with early-stage Alzheimer dementia: early lessons learned. J Nutr Health Ageing 2008;12:461–9 | Not a disease modification trial |
| Sheehan B, Phillips P, Juszczak E, Adams J, Baldwin A, Ballard C, et al. DOMINO-AD protocol: donepezil and memantine in moderate to severe Alzheimer’s disease – a multicentre RCT. Trials 2009;10:57 | Not a disease modification trial |
| Shifu X, Heqin Y, Peifen Y, Luning W, Jianjun J, Xin M, et al. Efficacy of FPF 1070 (Cerebrolysin) in patients with Alzheimer’s disease. A multicentre, randomised, double-blind, placebo-controlled trial. Clin Drug Investig 2000;19:43–53 | Not a disease modification trial |
| Shikiar R, Shakespeare A, Sagnier PP, Wilkinson D, McKeith I, Dartigues JF, et al. The impact of metrifonate therapy on caregivers of patients with Alzheimer’s disease: results from the MALT clinical trial. Metrifonate in Alzheimer’s Disease Trial. J Am Geriatr Soc 2000;48:268–74 | Not a disease modification trial |
| Shimizu S, Kanetaka H, Hirose D, Sakurai H, Hanyu H. Differential effects of acetylcholinesterase inhibitors on clinical responses and cerebral blood flow changes in patients with Alzheimer’s disease: a 12-month, randomised, and open-label trial. Dement Geriatr Cogn Disord Extra 2015;5:135–46 | Not a disease modification trial |
| Shinto L, Quinn J, Montine T, Dodge HH, Woodward W, Baldauf-Wagner S, et al. A randomised placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. J Alzheimers Dis 2014;38:111–20 | Not a disease modification trial |
| Shrotriya RC, Cutler NR, Sramek JJ, Veroff AE, Hironaka DY. Efficacy and safety of BMY 21,502 in Alzheimer disease. Ann Pharmacother 1996;30:1376–80 | Not a disease modification trial |
| Sidtis JJ, Gatsonis C, Price RW, Singer EJ, Collier AC, Richman DD, et al. Zidovudine treatment of the AIDS dementia complex: results of a placebo-controlled trial. Ann Neurol 1993;33:343–9 | Not a disease modification trial |
| Siemers E, Henley D, Sundell K, Sethuraman G, Dean R, Wrobleski K, et al. Evaluating semagacestat, a gammasecretase inhibitor, in a phase iii trial. Alzheimers Dement 2011;1:484–5 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Silva HA, Pathmeswaran A, Gunatilake SB. Efficacy of rivastigmine on activities of daily living in Sri Lankan patients with Alzheimer disease and on improving caregiver burden: a prospective study. Ceylon Med J 2005;50:106–9 | Not a disease modification trial |
| Sinforiani E, Iannuccelli M, Mauri M, Costa A, Merlo P, Bono G, Nappi G. Neuropsychological changes in demented patients treated with acetyl-L-carnitine. Int J Clin Pharmacol Res 1989;10:69–74 | Unable to find a copy of the full text |
| Small G, Erkinjuntti T, Kurz A, Lilienfeld S. Galantamine in the treatment of cognitive decline in patients with vascular dementia or Alzheimer’s disease with cerebrovascular disease. CNS Drugs 2003;17:905–14 | Not a disease modification trial |
| Spagnoli A, Lucca U, Menasce G, Bandera L, Cizza G, Forloni G, et al. Long-term acetyl-L-carnitine treatment in Alzheimer’s disease. Neurology 1991;41:1726–32 | Not a disease modification trial |
| Spector A, Orrell M, Woods B. Cognitive Stimulation Therapy (CST): effects on different areas of cognitive function for people with dementia. Int J Geriatr Psychiatry 2010;25:1253–8 | Not a disease modification trial |
| Spector A, Thorgrimsen L, Woods B, Royan L, Davies S, Butterworth M, et al. Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia. Br J Psychiatry 2003;183:248–54 | Not a disease modification trial |
| Spector A, Woods B, Orrell M. Cognitive stimulation for the treatment of Alzheimer’s disease. Expert Rev Neurother 2008;8:751–7 | Not a disease modification trial |
| Spilich GJ, Wannenmacher W, Duarte A, Buendia R, Gomez JT, Ramirez S, et al. Efficacy of pyritinol versus hydergine upon cognitive performance in patients with senile dementia of the Alzheimer’s type: a double-blind multi-centre trial. Alzheimers Res 1996;2:79–84 | Not a disease modification trial |
| Sramek JJ, Viereck C, Huff FJ, Wardle T, Hourani J, Stewart JA, et al. A ‘bridging’ (safety/tolerance) study of besipirdine hydrochloride in patients with Alzheimer’s disease. Life Sci 1995;57:1241–8 | Not a disease modification trial |
| Standridge JB. Donepezil did not reduce the rate of institutionalisation or disability in people with mild to moderate Alzheimer’s disease. Evid Based Ment Health 2004;7:112 | Not a disease modification trial |
| Steele LS, Glazier RH. Is donepezil effective for treating Alzheimer’s disease? Can Fam Physician1999;45:917–19 | Not a disease modification trial |
| Stein MS, Scherer SC, Ladd KS, Harrison LC. A randomised controlled trial high-dose vitamin D2 followed by intranasal insulin in Alzheimer’s disease. J Alzheimers Dis 2011;26:477–84 | Not a disease modification trial |
| Stein AM, Vital TM, Coelho FGM, Andrade LP, Pereira JR, Garuffi M, et al. Aerobic exercise training effect on cognitive functions, neuropsychiatric disorders, functionality, quality of life and lipid profile in elderly with Alzheimer’s disease. Eur Geriatr Med 2014;5:105–6 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Stern Y, Sano M, Mayeux R Long-term administration of oral physostigmine in Alzheimer’s disease. Neurology 1988;38:1837–41 | Not a disease modification trial |
| Storey P HL, Duke L, Callaway R, Marson D. Does chronic oral physostigmine alter the course of Alzheimer’s disease? Neurobiol Ageing 1992;13(Suppl. 1):126 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Stott DJ, MacIntosh G, Lowe GDO, Rumley A, McMahon AD, Langhorne P, et al. Randomised controlled trial of homocysteine-lowering vitamin treatment in elderly patients with vascular disease. Am J Clin Nutr 2005;82:1320–6 | Not a disease modification trial |
| Streater A, Spector A, Aguirre E, Hoe J, Hoare Z, Woods R, et al. Maintenance cognitive stimulation therapy (CST) in practice: study protocol for a randomised controlled trial. Trials 2012;13:91 | Not a disease modification trial |
| Stubendorff K, Larsson V, Ballard C, Minthon L, Aarsland D, Londos E. Treatment effect of memantine on survival in dementia with Lewy bodies and Parkinson’s disease with dementia: a prospective study. BMJ Open 2014;4:e005158 | Not a RCT/CCT |
| Suh GH, Jung HY, Lee CU, Choi S, Korean Galantamine Study G. Economic and clinical benefits of galantamine in the treatment of mild to moderate Alzheimer’s disease in a Korean population: a 52-week prospective study. J Korean Med Sci 2008;23:10–17 | Not a disease modification trial |
| Suh GH, Jung HY, Lee CU, Lee SK, Lee NJ, Kim JH. [Effect of galantamine on caregiver time and activities of daily living in mild to moderate Alzheimer’s disease: a 1-year prospective study.] J Korean Geriatr Soc 2007;11:74–82 | Full text unavailable in English |
| Suh GH, Jung HY, Lee CU, Oh BH, Lee SK, Lee N, et al. Effect of the apolipoprotein E epsilon4 allele on the efficacy and tolerability of galantamine in the treatment of Alzheimer’s disease. Dement Geriatr Cogn Disord 2006;21:33–9 | Not a disease modification trial |
| Suh GH, Yeon Jung H, Uk Lee C, Hoon Oh B, Nam Bae J, Jung HY, et al. A prospective, double-blind, community-controlled comparison of three doses of galantamine in the treatment of mild to moderate Alzheimer’s disease in a Korean population. Clinical Therapeutics 2004;26:1608–18 | Not a disease modification trial |
| Sun Y, Lu C-J, Chien K-L, Chen S-T, Chen R-C. Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer’s disease: a 26-week, randomised, double-blind, placebo-controlled study in Taiwanese patients. Clin Ther 2007;29:2204–14 | Not a disease modification trial |
| Suzuki T, Futami S, Igari Y, Matsumura N, Watanabe K, Nakano H, et al. A Chinese herbal medicine, choto-san, improves cognitive function and activities of daily living of patients with dementia: a double-blind, randomised, placebo-controlled study. J Am Geriatr Soc 2005;53:2238–40 | Not a disease modification trial |
| Tadaka E, Kanagawa K. A randomised controlled trial of a group care program for community-dwelling elderly people with dementia. Jpn J Nurs Sci 2004;1:19–25 | Not a disease modification trial |
| Tadaka E, Kanagawa K. Effects of reminiscence group in elderly people with Alzheimer disease and vascular dementia in a community setting. Geriatr Gerontol Int 2007;7:167–73 | Not a disease modification trial |
| Tajadini H, Saifadini R, Choopani R, Mehrabani M, Kamalinejad M, Haghdoost AA. Herbal medicine Davaie Loban in mild to moderate Alzheimer’s disease: a 12-week randomised double-blind placebo-controlled clinical trial. Complement Ther Med 2015;23:767–72 | Not a disease modification trial |
| Takahashi T, Matsushita H. Long-term effects of music therapy on elderly with moderate/severe dementia. J Music Ther 2006;43:317–33 | Not a disease modification trial |
| Tariot P, Sabbagh M, Flitman S, Reyes P, Taber I, Seely L. A safety, tolerability and pharmacokinetic study of Dimebon in patients with Alzheimer’s disease already receiving donepezil. Alzheimers Dement 2009;5:251 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Tariot P, Salloway S, Yardley J, Mackell J, Moline M. Long-term safety and tolerability of donepezil 23 mg in patients with moderate to severe Alzheimer’s disease. BMC Res Notes 2012;5:283 | Not a disease modification trial |
| Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomised controlled trial. JAMA 2004;291:317–24 | Not a disease modification trial |
| Tariot PN, Goldstein B, Podgorski CA, Cox C, Frambes N. Short-term administration of selegiline for mild to moderate dementia of the Alzheimer’s type. Am J Geriatr Psychiatry 1998;6:145–54 | Not a disease modification trial |
| Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C. A 5-month, randomised, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology 2000;54:2269–76 | Not a disease modification trial |
| Tarraga L, Boada M, Modinos G, Espinosa A, Diego S, Morera A, et al. A randomised pilot study to assess the efficacy of an interactive, multimedia tool of cognitive stimulation in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2006;77:1116–21 | Not a disease modification trial |
| Teipel SJ, Drzezga A, Bartenstein P, Moller HJ, Schwaiger M, Hampel H. Effects of donepezil on cortical metabolic response to activation during 18FDG-PET in Alzheimer’s disease: a double-blind cross-over trial. Psychopharmacology 2006;187:86–94 | Not a disease modification trial |
| Thai LJ, Carta A, Clarke WR, Ferris SH, Friedland RP, Petersen RC, et al. A 1-year multicenter placebo-controlled study of acetyl-L-carnitine in patients with Alzheimer’s disease. Neurology 1996;47:705–11 | Not a disease modification trial |
| Thal L, Grundman M, Berg J, Ernstrom K, Margolin R, Pfeiffer E, et al. Idebenone treatment fails to slow cognitive decline in Alzheimer’s disease. Neurology 2003;61:1498–502 | Not a disease modification trial |
| Thal LJ, Calvani M, Amato A, Carta A. A 1-year controlled trial of acetyl-l-carnitine in early-onset AD. Neurology 2000;55:805–10 | Not a disease modification trial |
| Thal LJ, Ferguson JM, Mintzer J, Raskin A, Targum SD. A 24-week randomised trial of controlled-release physostigmine in patients with Alzheimer’s disease. Neurology 1999;52:1146–52 | Not a disease modification trial |
| Thal LJ, Forrest M, Loft H, Mengel H. Lu 25–109, a muscarinic agonist, fails to improve cognition in Alzheimer’s disease. Lu25–109 Study Group. Neurology 2000;54:421–6 | Not a disease modification trial |
| Thal LJ, Fuld PA, Masur DM, Sharpless NS. Oral physostigmine and lecithin improve memory in Alzheimer disease. Ann Neurol 1983;13:491–6 | Not a disease modification trial |
| Thal LJ, Masur DM, Sharpless NS. Acute and chronic effects of oral physostigmine and lecithin in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 1986;10:627–36 | Not a disease modification trial |
| Thal LJ, Schwartz G, Sano M, Weiner M, Knopman D, Harrell L, et al. A multicenter double-blind study of controlled-release physostigmine for the treatment of symptoms secondary to Alzheimer’s disease. Physostigmine Study Group. Neurology 1996;47:1389–95 | Not a disease modification trial |
| Thomas AJ, Burn DJ, Rowan EN, Littlewood E, Newby J, Cousins D, et al. A comparison of the efficacy of donepezil in Parkinson’s disease with dementia and dementia with Lewy bodies. Int J Geriatr Psychiatry 2005;20:938–44 | Not a disease modification trial |
| Thompson ITL, Filley CM, Mitchell WD, Culig KM, LoVerde M, Byyny RL. Lack of efficacy of hydergine in patients with Alzheimer’s disease. N Engl J Med 1990;323:445–8 | Not a disease modification trial |
| Tian J, Shi J, Miao Y, Wei M. Efficacy and safety of an herbal therapy in patients with early stage of Alzheimer’s disease: a 24-week randomised phase III trial. Alzheimers Dement 2011;1:790 | Not a disease modification trial |
| Tian J, Shi J, Wei M, Qin R, Chen Y, Wang Y. Efficacy and safety of FFDS tablets in people with mild to moderate vascular dementia: a 24-week randomised, double-blind, placebo, parallel-controlled trial. Alzheimers Dement 2013;1:670–1 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Tinklenberg JR, Kraemer HC, Yaffe K, Ross L, Sheikh J, Ashford JW, et al. Donepezil treatment and Alzheimer disease: can the results of randomised clinical trials be applied to Alzheimer disease patients in clinical practice? Am J Geriatr Psychiatry 2007;15:953–60 | Not a disease modification trial |
| Tocco M, Hendrix S, Miller M, Pejovic V, Graham S. Effects of extended-release memantine (28 MG/DAY) on activities of daily living in patients with moderate to severe Alzheimer’s disease: post hoc factor analysis of a randomised trial. Alzheimers Dement 2011;1:790–1 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Tocco M, Hendrix S, Miller M, Pejovic V, Graham S. Effects of extended-release memantine (28 MG/day) on cognitive domains in patients with moderate to severe Alzheimer’s disease: post hoc analysis of a randomised trial. Alzheimers Dement 2011;1:784–5 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Tocco M, Hendrix S, Miller M, Pejovic V, Graham S. Clinical benefits of extended-release memantine (28 mg, once daily) as a function of disease severity in people with moderate to severe Alzheimer’s disease: post hoc analysis from a randomised trial. Alzheimers Dement 2013;1:655 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Tocco M, Hendrix S, Miller ML, Pejovic V, Graham SM. Effects of extended-release memantine (28 mg, once daily) on language and communication abilities in patients with moderate to severe Alzheimer’s disease. Ann Neurol 2012;72:S52–3 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Tollefson GD. Short-term effects of the calcium channel blocker nimodipine (Bay-e-9736) in the management of primary degenerative dementia. Biol Psychiatry 1990;27:1133–42 | Not a disease modification trial |
| Trollor JN SP, Haindl W, Brodaty H, Wen W, Walker BM. Combined cerebral blood flow effects of a cholinergic agonist (milameline) and a verbal recognition task in early Alzheimer’s disease. Psychiatry Clin Neurosci 2006;60:616–25 | Not a disease modification trial |
| Tsai GE, Falk WE, Gunther J. A preliminary study of D-cycloserine treatment in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 1998;10:224–6 | Not a disease modification trial |
| Tzimopoulou S, Cunningham VJ, Nichols TE, Searle G, Bird NP, Mistry P, et al. A multi-centre randomised proof-of-concept clinical trial applying [18F]FDG-PET for evaluation of metabolic therapy with rosiglitazone XR in mild to moderate Alzheimer’s disease. J Alzheimers Dis 2010;22:1241–56 | Not a disease modification trial |
| Van de Winckel A, Fey H, De Weerdt W, Dom R. Cognitive and behavioural effects of music-based exercises in patients with dementia. Clin Rehabil 2004;18:253–60 | Not a disease modification trial |
| van Dongen M, van Rossum E, Kessels A, Sielhorst H, Knipschild P. Ginkgo for elderly people with dementia and age-associated memory impairment: a randomised clinical trial. J Clin Epidemiol 2003;56:367–76 | Not a disease modification trial |
| Van Dyck CH, Lin CH, Robinson R, Cellar J, Smith EO, Nelson JC, et al. The acetylcholine releaser linopirdine increases parietal regional cerebral blood flow in Alzheimer’s disease. Psychopharmacology 1997;132:217–26 | Not a disease modification trial |
| van Dyck CH, Newhouse P, Falk WE, Mattes JA. Extended-release physostigmine in Alzheimer disease: a multicenter, double-blind, 12-week study with dose enrichment. Physostigmine Study Group. Arch Gen Psychiatry 2000;57:157–64 | Not a disease modification trial |
| van Dyck CH, Tariot PN, Meyers B, Resnick E. A 24-week randomised, controlled trial of memantine in patient with moderate-to-severe Alzheimer disease. Alzheimer Dis Assoc Disord 2007;21:136–43 | Not a disease modification trial |
| Venneri A, Shanks MF, Staff RT, Pestell SJ, Forbes KE, Gemmell HG, et al. Cerebral blood flow and cognitive responses to rivastigmine treatment in Alzheimer’s disease. Neuroreport 2002;13:83–7 | Not a disease modification trial |
| Vereschagin NV NY, Lebedeva NV, Suslina ZA, Solovyev OI, Piradov MA, et al. [Mild forms of multiinfarct dementia – effectiveness of Cerebrolysin.] Sov Meditsina 1991;11:6–8 | Full text unavailable in English |
| Veroff AE, Bodick NC, Offen WW, Sramek JJ, Cutler NR. Efficacy of xanomeline in Alzheimer disease: cognitive improvement measured using the Computerised Neuropsychological Test Battery (CNTB). Alzheimer Dis Assoc Disord 1998;12:304–12 | Not a disease modification trial |
| Villardita C, Grioli S, Lomeo C, Cattaneo C, Parini J. Clinical studies with oxiracetam in patients with dementia of Alzheimer type and multi-infarct dementia of mild to moderate degree. Neuropsychobiology 1992;25:24–8 | Not a disease modification trial |
| Villardita C, Parini J, Grioli S, Quattropani M, Lomeo C, Scapagnini U. Clinical and neuropsychological study with oxiracetam versus placebo in patients with mild to moderate dementia. J Neural Transm Suppl 1987;24:293–8 | Not a disease modification trial |
| Villemagne VL, Rowe CC, Barnham KJ, Cherny R, Woodward M, Pejoska S, et al. A 52-week pilot study targeting abeta with PBT2: neuroimaging results. Neurodegen Dis 2015;15:308 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Viola LF, Nunes PV, Yassuda MS, Aprahamian I, Santos FS, Santos GD, et al. Effects of a multidisciplinary cognitive rehabilitation program for patients with mild Alzheimer’s disease. Clinics 2011;66:1395–400 | Not a disease modification trial |
| Vreugdenhil A, Cannell J, Davies A, Razay G. A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: a randomised controlled trial. Scand J Caring Sci 2012;26:12–19 | Not a disease modification trial |
| Wade AG, Farmer M, Harari G, Fund N, Laudon M, Nir T, et al. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomised, placebo-controlled, multicenter trial. Clin Interv Ageing 2014;9:947–61 | Not a disease modification trial |
| Waldemar G, Hoffmann K, Sobol N, Frederiksen K, Beyer N, Vogel A, et al. Effect of moderate-to-high intensity endurance exercise in elderly community-dwelling persons with mild-moderate Alzheimer’s disease. Eur J Neurol 2015;22:95 | Not published in a peer-reviewed journal article or an ongoing trial |
| Walzl M, Walzl B, Kleinert G, Schied G, Lechner H. [Heparin-induced extracorporeal LDL precipitation (HELP). A new therapeutic possibility in cerebral multi-infarct dementia.] Der Nervenarzt 1993;64:648–52 | Full text unavailable in English |
| Wang P, Yang J, Liu G, Chen H, Yang F. [Effects of moxibustion at head-points on levels of somatostatin and arginine vasopressin from cerebrospinal fluid in patients with vascular dementia: a randomised controlled trial.] Chin J Integr Med 2010;8:636–40 | Full text unavailable in English |
| Wang PN, Liao SQ, Liu RS, Liu CY, Chao HT, Lu SR, et al. Effects of oestrogen on cognition, mood, and cerebral blood flow in AD: a controlled study. Neurology 2000;54:2061–6 | Not a disease modification trial |
| Warner J. Donepezil is more effective than galantamine for mild to moderate Alzheimer’s disease. Evid Based Ment Health 2004;7:77 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Watkins PB, Zimmerman HJ, Knapp MJ, Gracon SI, Lewis KW. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994;271:992–8 | Not a disease modification trial |
| Wattmo C, Wallin AK, Londos E, Minthon L. Predictors of long-term cognitive outcome in Alzheimer’s disease. Alzheimers Res Ther 2011;3:23 | Not a disease modification trial |
| Wei MQ, Tian JZ, Shi J, Ma FY, Miao YC, Wang YY. Effects of Chinese medicine for promoting blood circulation and removing blood stasis in treating patients with mild to moderate vascular dementia: a randomised, double-blind and parallel-controlled trial. Chin J Integr Med 2012;10:1240–6 | Not a disease modification trial |
| Weiner MF, Bonte FJ, Tintner R, Ford N, Svetlik D, Riall T. ACE inhibitor lacks acute effect on cognition or brain blood flow in Alzheimer’s disease. Drug Dev Res 1992;26:467–71 | Not a disease modification trial |
| Weinstein HC, Teunisse S, van Gool WA. Tetrahydroaminoacridine and lecithin in the treatment of Alzheimer’s disease. Effect on cognition, functioning in daily life, behavioural disturbances and burden experienced by the carers. J Neurol 1991;238:34–8 | Not a disease modification trial |
| Wesnes KA, Aarsland D, Ballard C, Londos E. Improvements to attention and verbal episodic memory with memantine in Parkinson’s disease dementia and dementia with Lewy bodies. J Nutr Health Aging 2013;17:781–2 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Wesnes K, Aarsland D, Ballard C, Londos E. Memantine improves attention and verbal episodic memory in Parkinson’s disease dementia and dementia with Lewy bodies: a double-blind, placebo-controlled multicentre trial. Alzheimers Dement 2013;1:890 | Not a disease modification trial |
| Wesnes KA, McKeith IG, Ferrara R, Emre M, Ser T, Spano PF, et al. Effects of rivastigmine on cognitive function in dementia with Lewy bodies: a randomised placebo-controlled international study using the cognitive drug research computerised assessment system. Dement Geriatr Cogn Disord 2002;13:183–92 | Not a disease modification trial |
| Weyer G, Babej-Dölle RM, Hadler D, Hofmann S, Herrmann WM. A controlled study of 2 doses of idebenone in the treatment of Alzheimer’s disease. Neuropsychobiology 1997;36:73–82 | Not a disease modification trial |
| Weyer G, Erzigkeit H, Hadler D, Kubicki S. Efficacy and safety of idebenone in the long-term treatment of Alzheimer’s disease: a double-blind, placebo controlled multicentre study. Hum Psychopharmacol 1996;11:53–65 | Not a disease modification trial |
| Weyer G, Eul A, Milde K, Wierich W, Herrmann WM. Cyclandelate in the treatment of patients with mild to moderate primary degenerative dementia of the Alzheimer type or vascular dementia: experience from a placebo controlled multi-centre study. Pharmacopsychiatry 2000;33:89–97 | Not a disease modification trial |
| White HK, Levin ED. Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer’s disease. Psychopharmacology 1999;143:158–65 | Not a disease modification trial |
| Wieggers RL, Kamphuis P, Stam C, Shah R, Bennett D, Hartmann T, et al. An overview of the medical food Souvenaid clinical trial program. Alzheimers Dement 2013;9(Suppl. 1):669 | Not a disease modification trial |
| Wilcock G, Howe I, Coles H, Lilienfeld S, Truyen L, Zhu Y, et al. A long-term comparison of galantamine and donepezil in the treatment of Alzheimer’s disease. Drugs Aging 2003;20:777–89 | Not a disease modification trial |
| Wilcock G, Möbius HJ, Stöffler A. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500). Int Clin Psychopharm 2002;17:297–305 | Not a disease modification trial |
| Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. BMJ 2000;321:1445–9 | Not a disease modification trial |
| Wilcock GK, Surmon DJ, Scott M, Boyle M, Mulligan K, Neubauer KA, et al. An evaluation of the efficacy and safety of tetrahydroaminoacridine (THA) without lecithin in the treatment of Alzheimer’s disease. Age Ageing 1993;22:316–24 | Not a disease modification trial |
| Wilkinson D, Colding-Jorgensen E, Windfeld K. A clinical phase II study of LU AE58054 added to stable donepezil treatment in patients with moderate Alzheimer’s disease. Alzheimers Dement 2013;9(Suppl. 1):P529 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Wilkinson D, Doody R, Helme R, Taubman K, Mintzer J, Kertesz A, et al. Donepezil in vascular dementia: a randomised, placebo-controlled study. Neurology 2003;61:479–86 | Not a disease modification trial |
| Wilkinson D, Fox NC, Barkhof F, Phul R, Lemming O, Scheltens P. Memantine and brain atrophy in Alzheimer’s disease: a 1-year randomised controlled trial. J Alzheimers Dis 2012;29:459–69 | Not a disease modification trial |
| Wilkinson D, Murray J. Galantamine: a randomised, double-blind, dose comparison in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 2001;16:852–7 | Not a disease modification trial |
| Wilkinson D, Róman G, Salloway S, Hecker J, Boundy K, Kumar D, et al. The long-term efficacy and tolerability of donepezil in patients with vascular dementia. Int J Geriatr Psychiatry 2010;25:305–13 | Not a disease modification trial |
| Wilkinson DG, Passmore AP, Bullock R, Hopker SW, Smith R, Potocnik FC, et al. A multinational, randomised, 12-week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer’s disease. Int J Clin Pract 2002;56:441–6 | Not a disease modification trial |
| Willan AR, Goeree R, Pullenayegum EM, McBurney C, Blackhouse G. Economic evaluation of rivastigmine in patients with Parkinson’s disease dementia. Pharmacoeconomics 2006;24:93–106 | Not a disease modification trial |
| Wimo A, Gaudig M, Schauble B, Jedenius E. The economic impact of galantamine vs placebo: an analysis based on functional capacity in a Swedish cohort study. J Med Econ 2012;15:1019–24 | Not a disease modification trial |
| Wimo A, Winblad B, Shah SN, Chin W, Zhang R, McRae T. Impact of donepezil treatment for Alzheimer’s disease on caregiver time. Curr Med Res Opin 2004;20:1221–5 | Not a disease modification trial |
| Wimo A, Winblad B, Soininen H, Verhey F, Waldemar G, Wetterholm AL, et al. An economic evaluation of donepezil in mild to moderate Alzheimer’s disease: results of a 1-year, double-blind, randomised trial. Dement Geriatr Cogn Disord 2003;15:44–54 | Not a disease modification trial |
| Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, et al. A 1-year, randomised, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 2001;57:489–95 | Not a disease modification trial |
| Winblad B, Giacobini E, Frölich L, Friedhoff LT, Bruinsma G, Becker RE, et al. Phenserine efficacy in Alzheimer’s disease. J Alzheimers Dis 2010;22:1201–8 | Not a disease modification trial |
| Winblad B, Grossberg G, Frolich L, Farlow M, Zechner S, Nagel J, et al. A 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer disease. Neurology 2007;69(Suppl. 1):14–22 | Not a disease modification trial |
| Winblad B, Wimo A, Engedal K, Soininen H, Verhey F, Waldemar G, et al. 3-year study of donepezil therapy in Alzheimer’s disease: effects of early and continuous therapy. Dement Geriatr Cogn Disord 2006; 21:353–63 | Not a disease modification trial |
| Wirth Y, Rive B. Memantine enhances autonomy in moderate to severe Alzheimer’s disease patients already receiving donepezil. Eur J Neurol 2012;19:474 | Not a disease modification trial |
| Wolters E, Riekkinen P, Lowenthal A, Van der Plaats J, Zwart J, Sennef C. DGAVP (Org 5667) in early Alzheimer’s disease patients: an international double-blind, placebo-controlled, multicenter trial. Neurology 1990;40:1099–101 | Not a disease modification trial |
| Wong WJ, Liu HC, Fuh JL, Wang SJ, Hsu LC, Wang PN, et al. A double-blind, placebo-controlled study of tacrine in Chinese patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 1999;10:289–94 | Not a disease modification trial |
| Woods B, Thorgrimsen L, Spector A, Royan L, Orrell M. Improved quality of life and cognitive stimulation therapy in dementia. Ageing Ment Health 2006;10:219–26 | Not a disease modification trial |
| Wouters CJ, Dautzenberg L, Thissen A, Dautzenberg PL. [Oral galantamine versus rivastigmine transdermal patch: a descriptive study at a memory clinic in The Netherlands.] Tijdschr Gerontol Geriatr 2010;41:146–50 | Full text unavailable in English |
| Xiao S, Wang T, Huang Q, Chen K, Reiman E. Changes of biological markers and brain PET imaging and clinical effects of memantine for patients with moderate to severe Alzheimer’s disease: a 24 week double-blind, randomised, placebo-controlled study. J Nutr Health Aging 2012;16:855 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Xiao S, Yan H, Yao P. The efficacy of Cerebrolysin in patients with vascular dementia: results of a Chinese multicentre, randomised, double-blind, placebo-controlled trial. The Cerebrolysin Study Group. Hong Kong J Psychiatry 1999;9:13–9 | Unable to find a copy of the full text |
| Xu SS, Gao ZX, Weng Z, Du ZM, Xu WA, Yang JS, et al. Efficacy of tablet huperzine-A on memory, cognition, and behaviour in Alzheimer’s disease. Zhongguo Yao Li Xue Bao 1995;16:391–5 | Not a disease modification trial |
| Xue SW, Ding JM, Zhong P, Liang K, An HY, Bo Y. Impacts of huperzine A on the level of Fas, Apo2.7 and Bcl-2 on the platelet membrane and the cognitive function in patients with Alzheimer disease. Chin J Clin Rehabil 2005;9:188–9 | Full text unavailable in English |
| Yamanaka K, Kawano Y, Noguchi D, Nakaaki S, Watanabe N, Amano T, et al. Effects of cognitive stimulation therapy Japanese version (CST-J) for people with dementia: a single-blind, controlled clinical trial. Aging Ment Health 2013;17:579–86 | Not a disease modification trial |
| Yan YX, Liang LZ, Zhou ZL. Clinical study of combined treatment with compound reinhartdt and sea cumber capsule and donepezil for vascular dementia. Zhongguo Zhong Xi Yi Jie He Za Zhi 2007;27:887–90 | Full text unavailable in English |
| Yancheva S, Ihl R, Nikolova G, Panayotov P, Schlaefke S, Hoerr R, et al. Ginkgo biloba extract EGb 761(R), donepezil or both combined in the treatment of Alzheimer’s disease with neuropsychiatric features: a randomised, double-blind, exploratory trial. Aging Ment Health 2009;13:183–90 | Not a disease modification trial |
| Yoo HB, Choi JS, Yoon EJ, Lee HY, Kim YK, Lee H, et al. Efficacy of cilostazol augmentation treatment in Alzheimer’s disease with white matter lesion by FDG PET. Int Psychogeriatr 2013;25:S58 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Yoon BK, Kim DK, Kang Y, Kim JW, Shin MH, Na DL. Hormone replacement therapy in postmenopausal women with Alzheimer’s disease: a randomised, prospective study. Fertil Steril 2003;79:274–80 | Not a disease modification trial |
| Yu F, Bronas U, Nelson N, Dysken M, Jack C, Konety S, et al. Aerobic exercise in Alzheimer’s disease: The FIT-AD trial. Alzheimers Dement 2014;10:851–2 | Not a disease modification trial |
| Yu F, Bronas UG, Konety S, Nelson NW, Dysken M, Jack C Jr, et al. Effects of aerobic exercise on cognition and hippocampal volume in Alzheimer’s disease: study protocol of a randomised controlled trial (The FIT-AD trial). Trials 2014;15:394 | Not a disease modification trial |
| Yu J, Zhang X, Liu C, Meng Y, Han J. Effect of acupuncture treatment on vascular dementia. Neurol Res 2006;28:97–103 | Not a disease modification trial |
| Yu L, Lin SM, Zhou RQ, Tang WJ, Huang PX, Dong Y, et al. Chinese herbal medicine for patients with mild to moderate Alzheimer disease based on syndrome differentiation: a randomised controlled trial. Chin J Integr Med 2012;10:766–76 | Full text unavailable in English |
| Zamfirescu A, Capisizu A, Slavila M, Capisizu AA, Romila A. Physical exercise training in older adults diagnosed with mild to moderate dementia. J Nutr Health Aging 2012;16:861–2 | Not published in a peer-reviewed journal article or is an ongoing trial |
| Zanetti O, Frisoni GB, De Leo D, Dello Buono M, Bianchetti A, Trabucchi M. Reality orientation therapy in Alzheimer disease: useful or not? A controlled study. Alzheimer Dis Assoc Disord 1995;9:132–8 | Not a disease modification trial |
| Zemlan FP, Keys M, Richter RW, Strub RL. Double-blind placebo-controlled study of velnacrine in Alzheimer’s disease. Life Sci 1996;58:1823–32 | Not a disease modification trial |
| Zhang FR. Observation of Chinese herb kangnaoling in improving cognitive and daily living ability of patients with vascular dementia. Chin J Clin Rehabil 2006;10:161–3 | Full text unavailable in English |
| Zhang MZ, Dong ZX, Mao YJ, Du F. Effect of yizhi capsule on the intelligence of patient with multi-infarct dementia. Chin J Clin Rehabil 2005;9:155–7 | Full text unavailable in English |
| Zhang Y, Lin C, Zhang L, Cui Y, Gu Y, Guo J, et al. Cognitive improvement during treatment for mild Alzheimer’s disease with a Chinese herbal formula: a randomised controlled trial. PLOS ONE 2015;10:e0130353 | Not a disease modification trial |
| Zhang YX, Luo G, Guo ZJ, Cui RY, Wang LQ, Zhou CL. Quantitative evaluation of the interventional effect of oestrogen on Alzheimer disease. Chin J Clin Rehabil 2006;10:37–9 | Full text unavailable in English |
| Zhang YY, Yang LQ, Guo LM. Effect of phosphatidylserine on memory in patients and rats with Alzheimer’s disease. Genet Mol Res 2015;14:9325–33 | Not a disease modification trial |
| Zhou BR XZ, Kuang YF, Deng YH, Liu ZF. Effectiveness of polydrug therapy for senile dementia. Chin J Clin Rehabil 2004;8:1214 | Full text unavailable in English |
| Zhu M, Xiao S, Li G, Li X, Tang M, Yang S, et al. Effectiveness and safety of generic memantine hydrochloride manufactured in China in the treatment of moderate to severe Alzheimer’s disease: a multicenter, double-blind randomised controlled trial. Shanghai Jingshen Yixue 2013;25:244–53 | Not a disease modification trial |
| Zieschang T, Schwenk M, Oster P, Hauer K. Long-term effect of a standardised motor training on cognition in patients with dementia: results of a RCT. Eur Geriatr Med 2013;4:S211 | Not published in a peer-reviewed journal article or is an ongoing trial |
Appendix 4 Information sheet for focus group participants
Appendix 5 First e-mail consultation
Appendix 6 Second e-mail consultation
Appendix 7 List of consensus conference attendees
Twenty-seven attendees:
-
Sube Banerjee
-
Frances Bunn
-
David Challis
-
Georgina Charlesworth
-
Alison Evans
-
Katie Featherstone
-
Chris Fox
-
Claire Goodman
-
Anna Grinbergs-Saull
-
Derek Groskreutz
-
Rob Howard
-
Roy Jones
-
Louise Lafortune
-
Sallie Lamb
-
Gill Livingston
-
Esme Moniz-Cook
-
Gail Mountain
-
John O’Brien
-
Robert Perneckzy
-
James Pickett
-
Charlotte Roberts
-
Justine Schneider
-
Sasha Shepperd
-
Clare Surr
-
Jo Thompson-Coon
-
Lucy Webster
-
Bob Woods.
List of abbreviations
- AD
- Alzheimer’s disease
- ADAS-Cog
- Alzheimer’s Disease Assessment Scale – Cognitive subscale
- ADAS-Noncog
- Alzheimer’s Disease Assessment Scale-Non Cognitive scale
- ADCS-ADL
- Alzheimer’s Disease Co-operative Study – Activities of Daily Living Inventory
- ADFACS
- Alzheimer’s Disease Functional Activity and Change Scale
- ADL
- activity of daily living
- ALOIS
- Cochrane Dementia and Cognitive Improvement Group study register
- AS
- Alzheimer’s Society
- BADL
- Bristol Activities of Daily Living
- BEHAVE-AD
- Behavioural Pathology in Alzheimer’s Disease
- BPRS
- Brief Psychiatric Rating Scale
- CCT
- clinical controlled trial
- CDR
- Clinical Dementia Rating scale
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CERAD
- Consortium to Establish a Registry for Alzheimer’s Disease
- CIBIC+
- Clinician’s Interview-Based Impression of Change plus Caregiver Input
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- COMET
- Core Outcome Measures in Effectiveness Trials
- CSF
- cerebrospinal fluid
- DAD
- Disability Assessment For Dementia
- DEMQOL
- Dementia Quality of Life measure
- DSRS
- Dementia Severity Rating Scale
- EEG
- electroencephalography
- EQ-5D
- EuroQol-5 Dimensions
- HRQoL
- health-related quality of life
- IADL
- instrumental activities of daily living
- ICHOM
- International Consortium for Health Outcomes Measurement Working Group
- ISRCTN
- International Standard Randomised Controlled Trial Number
- JPND
- European Union Joint Programme – Neurodegenerative Disease Research
- LILACS
- Latin American and Caribbean Health Sciences Literature
- MCI
- mild cognitive impairment
- MMSE
- Mini Mental State Examination
- MRI
- magnetic resonance imaging
- NIHR
- National Institute for Health Research
- NPI
- Neuropsychiatric Inventory
- NTB
- Neuropsychological Test Battery
- P-tau
- phosphorylated tau
- P-tau181
- phosphorylated tau 181
- PET
- positron emission tomography
- PiB
- Pittsburgh compound B
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSMS
- Personal Self-Maintenance Scale
- PWD
- person with dementia
- QALY
- quality-adjusted life-year
- QOL-AD
- Quality of Life in Alzheimer’s Disease
- RCT
- randomised controlled trial
- T-tau
- total tau