Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/138/03. The contractual start date was in June 2015. The draft report began editorial review in February 2017 and was accepted for publication in August 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Antony Bayer was a member of the Health Technology Assessment (HTA) Dementia Themes Call Board from 2010 to 2011. Greta Rait is a member of the HTA Mental Health Methods Group and Panel. Jo Rycroft-Malone is the Director of the Health Services and Delivery Research (HSDR) programme and editor of the National Institute for Health Research HSDR journal. The authors declare no other financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Bunn et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2017 Queen’s Printer and Controller of HMSO
Chapter 1 Background
This chapter includes text from the protocol, which was published by Bunn et al. 1 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Introduction
Dementia and diabetes mellitus are common long-term conditions that may coexist in a large number of older people. 2,3 People with dementia may be less able to understand and manage their diabetes and may be at risk of complications such as hypoglycaemic episodes, cardiovascular conditions and amputations,4–6 which place a huge burden on health and social care economies. 7 Moreover, the impact of dementia and diabetes on patients and their families is considerable. There is a need to consider what kind of programmes or interventions are needed for the effective management of diabetes in people with dementia, including how interventions work, for whom and in what contexts; the barriers to, and facilitators of, the effective management of diabetes in people living with dementia; and how interventions might be tailored to this patient group.
Dementia and diabetes in older people
In the UK, there are an estimated 850,000 people living with dementia,8 the most common form being Alzheimer’s disease. 9 The number is forecast to exceed 2 million by 2050. 10 The prevalence of diabetes mellitus in the UK is also rising rapidly: since 1996, the number of people diagnosed with diabetes has more than doubled, from 1.4 million to almost 3.5 million. 11,12 The risk of both conditions increases with age. Dementia affects 1 in 20 people aged > 65 years and 1 in 5 people aged > 80 years. 13 In 2010, the prevalence of all types of diabetes was 0.4% in people aged 16–24 years, rising to 15% of people aged 70–84 years. 14 Owing to the high prevalence rates of both conditions in older people, they may inevitably coexist. A scoping review found data to suggest that rates of diabetes in people with dementia are between 13% and 20%. 2
There is increasing evidence that diabetes – in particular type 2 diabetes – is associated with an increased risk of cognitive impairment and dementia. Observational studies report that type 2 diabetes is associated with both of the major subtypes of dementia, with an approximate 2.5-fold increased risk of incident vascular dementia and a 1.5-fold increased risk of Alzheimer’s disease. 15,16 The association between vascular dementia and diabetes is not entirely surprising because diabetes is a risk factor for lacunar infarction and major stroke, and vascular disease is an essential aetiological factor of vascular dementia. 15 Although the underlying pathological pathways between type 2 diabetes and Alzheimer’s disease are not entirely clear, it is thought that metabolic factors may play a role,15 leading to Alzheimer’s disease being labelled by some as ‘type 3 diabetes’. 17
Dementia has been consistently and independently associated with an increased risk of hypoglycaemia,18,19 possibly because of an increased likelihood of errors in self-medication (e.g. with insulin or sulphonylurea treatments), irregular eating habits and an inability to recognise and treat hypoglyceamia. 20 Furthermore, there are a number of age-related changes that may affect diabetes treatment and management. Deficits in renal and liver functioning associated with increasing age can have an impact on medication effectiveness, making the older person either more or less sensitive to a drug’s potency. 21 In addition, older people may be more likely to demonstrate ‘hypoglycaemic unawareness’, whereby the central nervous system shows greater insensitivity to hypoglycaemic symptoms. 22 Hypoglycaemia is a serious cause of morbidity and mortality in frail older people23 and may increase the risk of robust individuals with diabetes becoming frail. 24 The relationship between dementia, frailty and hypoglycaemia is complex, and it has recently been reviewed. 25
Current guidance on the management of diabetes in people with dementia
Clinical guidance on the management of diabetes in older adults26–30 suggests that glycaemic targets should be individualised for older people and that care should be personalised to take into account factors such as age, dementia, frailty, comorbidities and polypharmacy. 31 However, despite this guidance, and although the harms of intensive treatment are likely to exceed the benefits for older people with complex or poor health status, a substantial proportion of older adults is potentially overtreated. 32 This may be because clinicians are not aware of existing guidelines, because they are uncertain about when and how to de-intensify diabetes medications and/or because they apply performance targets [such as the Quality and Outcomes Framework (QOF)] to the management of older people with diabetes rather than individualising care. 33
Self-management
The main approach to the management of long-term conditions such as diabetes revolves around self-management (SM) strategies that focus on the attitudes and self-efficacy of the patient. The successful SM of chronic conditions is based on the idea that the patient collaborates with health-care professionals (HCPs) in the management of the condition, allowing the patient to become knowledgeable about their condition, share in decision-making and receive educational support. 34 In relation to diabetes, self-care has been defined as ‘an evolutionary process of development of knowledge or awareness by learning to survive with the complex nature of the diabetes in a social context’,35 and consists of seven key behaviours: healthy eating, being physically active, monitoring blood sugar, complying with medications, good problem-solving skills, healthy coping skills and risk-reduction behaviours. 36
Although SM is well established as fundamental to the management of long-term conditions, there has been a paucity of literature about SM for people living with dementia. This may be due to the general belief of the ‘hopelessness’ of dementia promulgated by both professionals and lay people, coupled with limited research focusing on the daily needs and lives of those living with dementia. 37 Recently, these negative perceptions of people living with dementia have been challenged, and a more strength-based approach drawing on personhood has emerged. 38 There are, however, clearly differences between the skills needed to self-manage dementia and those required for diabetes, and people living with dementia are often reliant on others, usually family carers, to facilitate their access to services and support and to help them manage the condition. 39
Although there are differences in the physical and cognitive effects of the different types of dementias, all are usually progressive, involve increasing physical and mental deterioration, and lead to a person with dementia becoming increasingly dependent, all of which has an impact on their ability to understand and manage their diabetes. 2,4,6 Dementia has an impact on a person’s ability to undertake self-care management tasks such as managing medication, monitoring blood glucose and maintaining a healthy-eating regimen. 39–41 There are additional difficulties related to insulin management. Interviews conducted as part of a recent National Institute for Health Research (NIHR) study suggest that, as people living with dementia become unable to manage their own medication, they find injections distressing and painful. 39 The situation can be further complicated by the presence of behavioural and psychological symptoms which may have an impact on diabetes self-care regimens42 and lead to dementia becoming the focus of attention to the detriment of diabetes management. 43 Physical frailty or end-stage dementia compounds the complexity of diabetes management, with decisions needing to be made about whether to maintain strict treatment or consider admission into nursing home care. 44 Therefore, for people with dementia, SM needs to be conceptualised as a multidimensional, complex phenomenon affecting individuals, dyads and families, and interventions may need to target family carers. 41,45
Rationale for the research
As the population ages and the proportion of people with dementia and diabetes increases, the delivery of health and social care for this group becomes increasingly complex and challenging. 39 There is, however, currently no systematic approach to the management of dementia and diabetes,28 and many care pathways for diabetes do not take into account the needs of people with dementia. 46 Moreover, there is a gap in provision of services in mental health trusts for diabetes care and, similarly, a gap in acute hospital trusts for dementia care. 28 Recent guidance on the management of diabetes in people with dementia outlines a number of recommendations, including better case-finding of both conditions, better training for staff, adequate carer support, and care that is tailored to the needs of the individual. 28,31 However, currently there is little research evaluating interventions to improve the management of diabetes in people living with dementia; indeed, many diabetes-related studies exclude people with dementia or cognitive impairment.
Interventions designed to improve the management of diabetes in people with dementia are likely to be multicomponent, specific to different stages of the dementia trajectory, and dependent on the behaviours and choices of those delivering and receiving the care. They are also likely to be contingent on contextually situated decision-making. There is a need, therefore, to synthesise the different strands of research evidence in order to develop a theoretical understanding of the realities of working in and across complex, overlapping systems of care, and why and how different interventions may work. Realist synthesis is a systematic, theory-driven approach that aims to make explicit the mechanism(s) of how and why complex interventions work (or not) in particular settings or contexts. 47–49 Realist synthesis takes account of a broad and eclectic evidence base including the experiential and clinical knowledge that relates to the physiology and management of diabetes in older people, and specifically older people with dementia.
Aims and objectives
The overall aim was to identify the key features or mechanisms of programmes and approaches that aim to improve the management of diabetes in people with dementia, to understand how those mechanisms operate in different contexts to achieve particular outcomes for this population, to make explicit the barriers to and facilitators of implementation, and to identify areas needing further research.
We used an iterative four-stage approach that optimised the knowledge and networks of the research team. The synthesis was based on the stages set out by Pawson et al. 48,50 and follows the Realist and Meta-narrative Evidence Syntheses: Evolving Standards (RAMESES) publication standards. 47 The objectives were to:
-
identify how interventions, or elements of interventions, to manage diabetes in people with dementia are thought to work, on what range of outcomes (i.e. organisational, resource use, and patient care and safety) and for whom they work (or why they do not work) and in what contexts
-
identify the barriers to and facilitators of the acceptability, uptake and implementation of interventions designed to manage diabetes in people with dementia
-
establish what evidence there is on the feasibility and potential value of interventions to manage diabetes in people with dementia
-
establish what is known about the design of diabetes management technologies and identify the potential benefits of involving end-users (people with dementia and their carers) in their development.
Chapter 2 Methods
This chapter includes text from the protocol, which was published by Bunn et al. 1 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
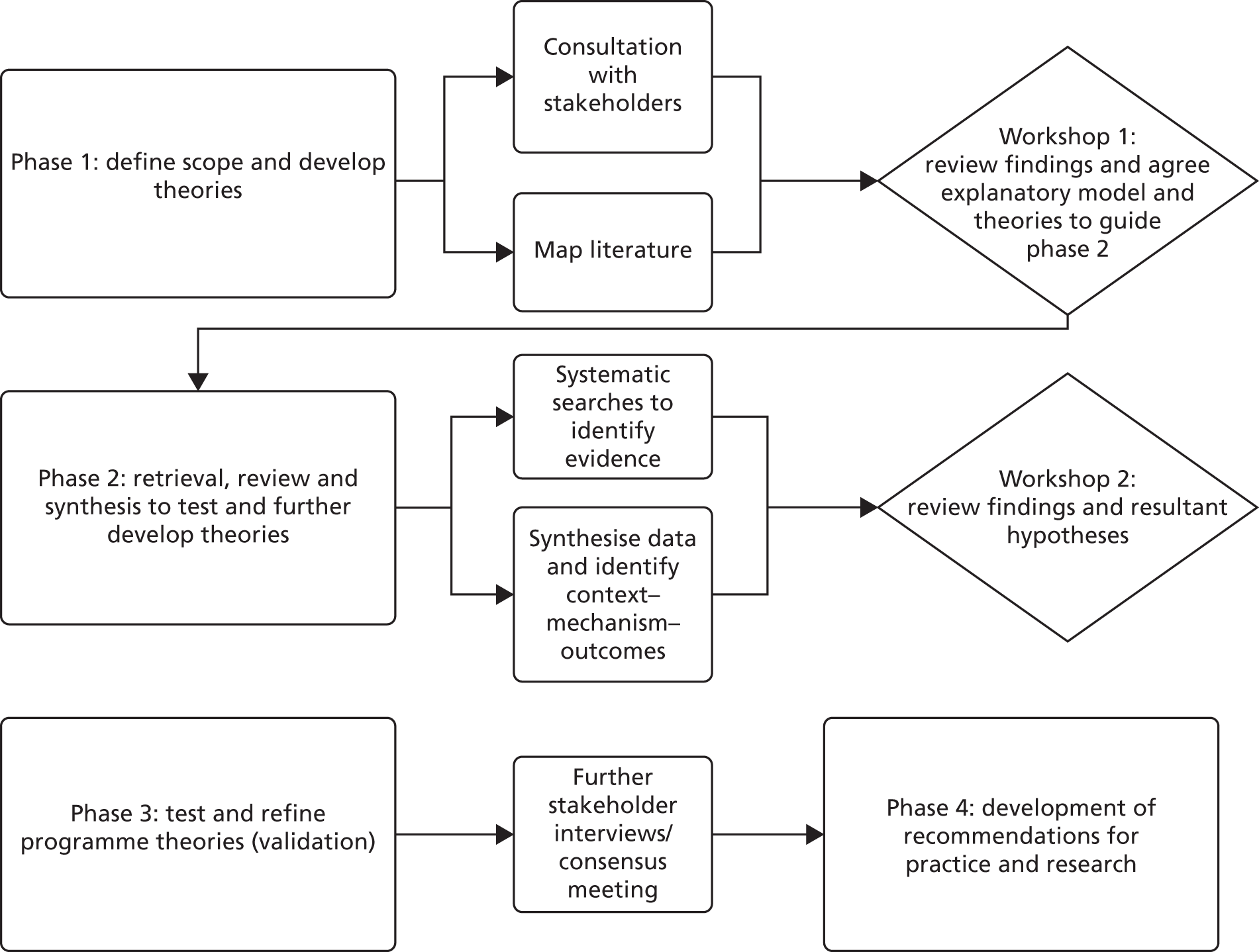
We used an iterative four-stage approach that optimised the knowledge and networks of the research team. The review was based on the stages for realist review set out by Pawson et al. 50 and follows the RAMESES publication standards for realist syntheses. 47 Figure 1 provides an overview of the study design.
FIGURE 1.
Summary of study design.
Rationale for using realist approach
The rationale for using a realist synthesis approach is that interventions for the management of diabetes in people living with dementia are likely to be multicomponent and will be dependent on the behaviours and choices of those delivering and receiving the care. Realist review is a theory-driven interpretive approach to evidence synthesis47,48,51 that assumes that there is more to reality than how we see it. There is an external reality or world that can be observed and measured, but how this reality is articulated and responded to is constantly being shaped by individuals’ perceptions and reasoning and/or dominant social and cultural mores. It is this constant interaction that creates particular responses that lead to observed outcomes. 52
Realist synthesis, therefore, endeavours to go beyond lists of barriers to and enablers of care, to unpack the ‘black box’ of how interventions might help people living with dementia manage their diabetes. The much-repeated statement used to explain the focus and purpose of realist synthesis is that it makes explicit ‘what works, for whom, why and in what circumstances’. It uses a theory-driven approach to articulate how particular contexts (C), including resources, have prompted certain mechanisms (M) or responses to lead to the observed outcomes (O). The iterative process of the review tests those theories that are thought to work (initial programme theory) against the observations reported in the evidence included in the syntheses. 53 The definitions of key realist terminology used in the review are provided in Box 1. The review process results in the emergence of ‘demi-regularities’ or patterns, which provide insight into how interventions work or not, and in what contexts. A realist synthesis enables us to take account of a broad evidence base, including experiential and clinical knowledge that relates to the physiology and management of diabetes in older people, and specifically older people living with dementia.
-
Context (C): the ‘backdrop’ conditions (which may change over time), for example provision of training in diabetes and/or dementia care delivery systems. Context can be broadly understood as any condition that triggers and/or modifies the behaviour of a mechanism.
-
Mechanism (M): a mechanism is the generative force triggered in particular contexts that leads to outcomes. Often denotes the reasoning (cognitive or emotional) of the various ‘actors’ (i.e. people living with dementia and diabetes, relatives and HCPs). Mechanisms are linked to, but are not the same as, a service’s strategies or interventions. Identifying the mechanisms goes beyond describing ‘what happened’ to theorising ‘why it happened, for whom and under what circumstances’.
-
Outcomes (O): the outcome is a result of the interaction between a mechanism and its triggering context. This may include greater engagement in SM behaviours or a reduction in adverse events.
-
Programme theory: those ideas about what needs to be changed or improved in how diabetes is managed for people living with dementia, what needs to be in place to achieve an improvement(s) and how programmes are believed to work. It specifies what is being investigated and the elements and scope of the review.
Changes in the review process
As recommended in the RAMESES publication standards,47 changes in the review process are documented in Table 1.
| Protocol | Revisions/changes | Agreed |
|---|---|---|
| The inclusion criteria stated that we would include people ‘resident in the community or a care home or other long-term setting’ | At the second project workshop (involving eight members of the RMT) it was decided that further refinement was needed of the inclusion and exclusion criteria. The RMT felt that the issues of managing diabetes for people living with dementia in care homes are different from those for people living in their own homes and that literature relating to care homes should be excluded | Research management team (April 2016) and supported by the PAG. The PAG agreed that the decision was appropriate because there were significant differences between the two environments |
| The protocol stated that in phase 3 we would review the hypotheses and supporting evidence through telephone interviews with up to 15 stakeholders | We conducted only seven individual interviews in phase 3. However, in addition, hypotheses (CMOs) and supporting evidence were presented and discussed at a consensus meeting involving 24 participants |
Phase 1: defining the scope of the realist review – concept mining and theory development
In phase 1, we were concerned with developing an initial programme theory/theories or hypotheses about why diabetes management programmes for people living with dementia work or do not work. This scoping or concept mining involved a variety of evidence sources, including the commissioning brief, policy/guidance and grey and published literature. In addition, we consulted with a range of content experts via interviews with stakeholders and discussions with the Research Management Team (RMT) and Project Advisory Group (PAG). The PAG included experts in the fields of diabetes, dementia, older people’s health and realist methods (see Appendix 1). The first RMT meeting included an open discussion in which the team were asked to draw on their expertise to articulate:
-
the dominant approaches and assumptions that informed current thinking about what supported the management of diabetes in people living with dementia
-
important outcomes.
Scoping interviews
To complement the expertise provided by the team, we interviewed 19 stakeholders (Table 2).
| Group | Rationale |
|---|---|
| 1. Clinicians with a special interest in the management of diabetes in older people | To understand organisational process and protocols and current ‘best practice’ for older people with diabetes. To be aware of factors which facilitate the implementation of guidelines |
| 2. Providers of care in primary and secondary care (e.g. diabetes specialist nurses, GPs and other clinicians) | To gain the perspectives of clinicians who are likely to be providing diabetes care for people living with dementia |
| 3. User representatives, including recipients of care and their family carers, and relevant diabetes or dementia charities | To give the closest possible approximation of the views of people living with dementia and diabetes |
| 4. Dementia specialists from primary, secondary and tertiary care and the voluntary sector (e.g. old-age psychiatrists, dementia specialist nurses and GPs with an interest in dementia) | To understand organisational process and protocols and current ‘best practice’ for caring for people living with dementia. To be aware of factors which facilitate the implementation of guidelines |
| 5. Academics and those involved in developing education and guidance for older people with diabetes | To ensure that we are up to date with the most relevant current research |
In the first instance, stakeholders were identified through the clinical and research networks of the RMT and PAG. A process of snowballing was used to identify additional participants.
Interview procedures
Interviews were conducted either face to face or via telephone by one of three researchers (FB, PRJ and BR). Participants were given a copy of the study information sheet, which provided contact details of the research team, and a consent form, which they were asked to read and sign. Interviews were conducted using an interview schedule and were audio-recorded and transcribed. Ethics approval was obtained from the University of Hertfordshire Health and Human Sciences Ethics Committee with delegated authority (CSK/SF/UH/00106). The interview schedules were designed to explore:
-
participants’ experiences of (a) working with people living with dementia and diabetes, (b) living with dementia and diabetes or (c) acting as an informal carer to someone with dementia and diabetes
-
current problems and challenges facing people and families who have to manage dementia and diabetes, and what needs to be in place to address the effects of dementia
-
what good diabetes care looks like for people living with dementia and what is needed to achieve it
-
changes required to improve the management of diabetes in people living with dementia.
First search and mapping of the literature
Literature for the scoping was initially drawn from a number of sources. This included searches recently undertaken for a scoping review for a NIHR study about dementia and comorbidity2 and for the development of clinical guidance on dementia and diabetes. 28 These were supplemented by a search of ProQuest Pro (2010–December 2015): this contains 13 databases, including British Nursing Index, PsycINFO and SocialSciences collection; Cumulative Index to Nursing and Allied Health Literature (CINAHL), MEDLINE, EBSCOhost, Web of Science, The Cochrane Library, Health Technology Assessment (HTA) database, National Institute for Health and Care Excellence guidelines and Google Scholar (Google Inc., Mountain View, CA, USA). Key words used in the searches included dementia OR Alzheimer’s disease OR vascular dementia OR mild cognitive impairment OR MCI OR frail elderly OR severe mental illness AND diabetes, T1DM OR T2DM AND self-management OR self-care OR chronic illness OR case-management OR assistive technology OR telemedicine/care OR family carer OR social support OR eating/meal times OR medicine management OR adherence OR exercise/leisure, OR health and social care professionals.
Records were originally categorised as diabetes biology/pathophysiology, candidate theories, case management, SM and technology. Portable Document Format (PDF) files of potentially relevant papers were stored in a private group on Mendeley (Elsevier, Amsterdam, the Netherlands) to which all members of the research team had access. Records were screened by two reviewers independently (from FB, PRJ, BR and DT) and decisions were recorded on a Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) spreadsheet. Disagreements were resolved by discussion.
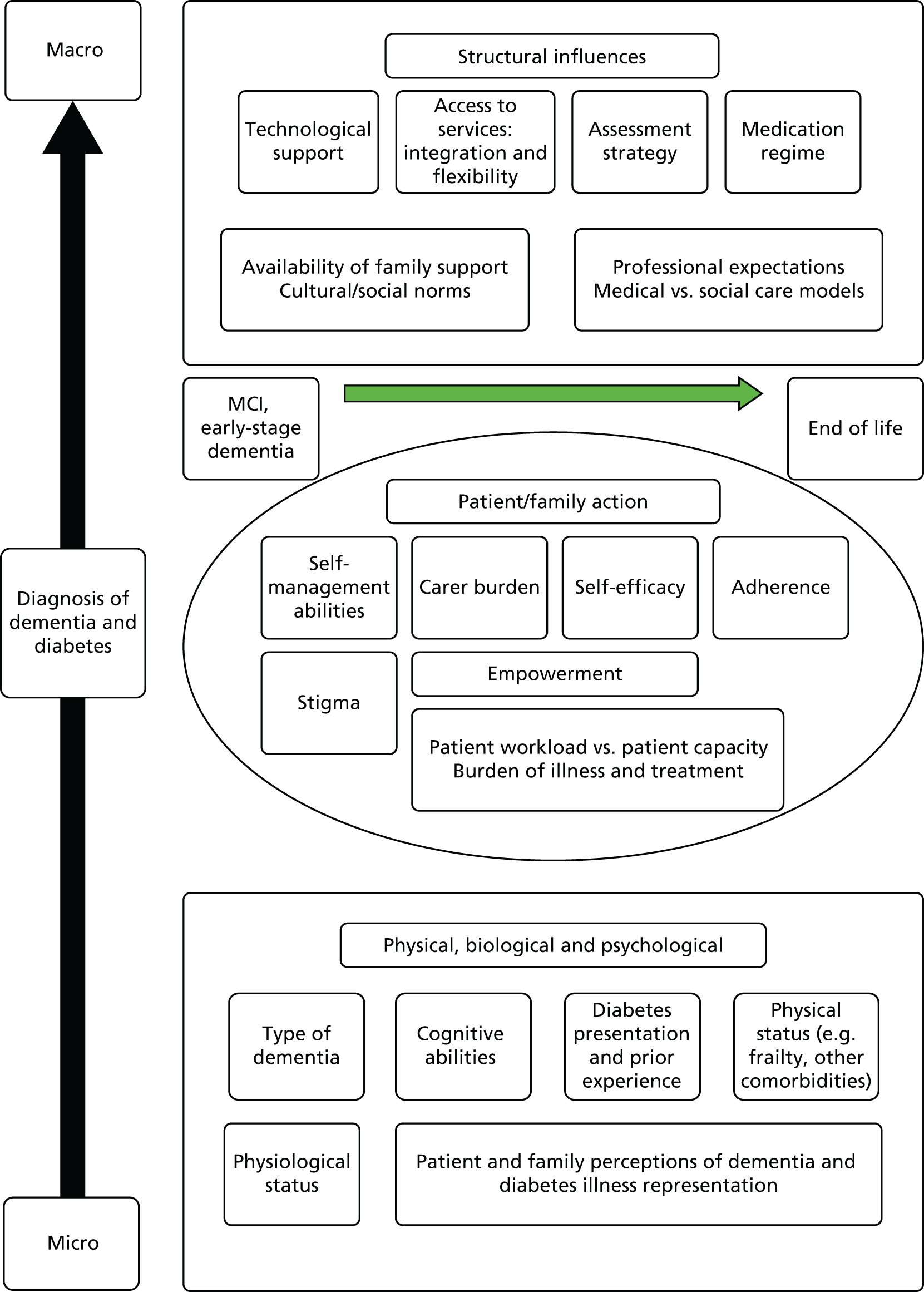
The initial scoping of the literature produced minimal research that investigated how diabetes is managed in people living with dementia, apart from the recognition that cognitive decline may be accelerated in people with diabetes. As an initial step to literature scoping, a framework was required that could link the management of diabetes (in older people) to the management of dementia (mild cognitive impairment to the end of life). Following the first project management meeting, a potential conceptual framework was identified based on the work of Glass and McAtee54 and extended by Greenhalgh et al. 55 This framework was used as a way of identifying key theories that could influence the management of dementia and diabetes care and linking the management of diabetes (in older people) and the management of dementia (mild cognitive impairment to the end of life). The framework highlights the complex influences that affect the management of diabetes in people living with dementia (Figure 2) and was used as a guide to ‘sketch the terrain’47 to be investigated and, through this process, to assist in refining the elements and scope for the review.
FIGURE 2.
Nested hierarchy of influences around diabetes management in people living with dementia. MCI, mild cognitive impairment.
From the literature and from listening to stakeholder transcripts, a series of explanatory accounts were built up that contained ‘if–then’ statements that helped to specify context and mechanism. ‘If–then’ statements are the identification of an intervention/activity linked to outcome(s), and they contain references to contexts and mechanisms (although these may not be very explicit at this stage) and/or barriers and enablers (which can be both mechanism and context). 56 The ‘if–then’ statements provided a helpful way of structuring our thinking. They also helped to focus the process of taking ideas and assumptions about how interventions work and testing them against the evidence we found. Initially, we generated 20 ‘if–then’ statements, which, after further discussion, were reduced to three (Box 2).
-
If interventions designed to promote SM use a comprehensive toolbox approach tailored to individual needs (including, for example, skill building, education, how to manage emotions, and coping mechanisms), then the person living with dementia and diabetes (and their family/carers) is (are) more likely to engage positively with managing their diabetes because they feel more in control (are more autonomous, confident, motivated, empowered), which results in (for the individual):
-
If models of care reflect a person-centred partnership approach (incorporating holism, and that are participatory, relational), and are balanced (with focus on diabetes management, therapeutic alliance), then relationships between the person living with dementia and diabetes (and family/carers) and HCPs are more likely to be effective because there is mutual trust and understanding and better communication between them, which results in:
-
If local specialist and primary services reflect a seamless approach that is responsive and accessible for people living with dementia and diabetes (including having the right systems, processes and people in place), then the person (and their family/carers) and the HCP will feel better supported and informed, which results in:
The ‘if–then’ statements were discussed with the PAG members, who made several suggestions that influenced our thinking and the development of our theory areas. For example, they suggested that we map what constitutes good diabetes care (e.g. that specified in current guidelines) against the barriers created by dementia. Members also felt that the review should not be too biomedically focused.
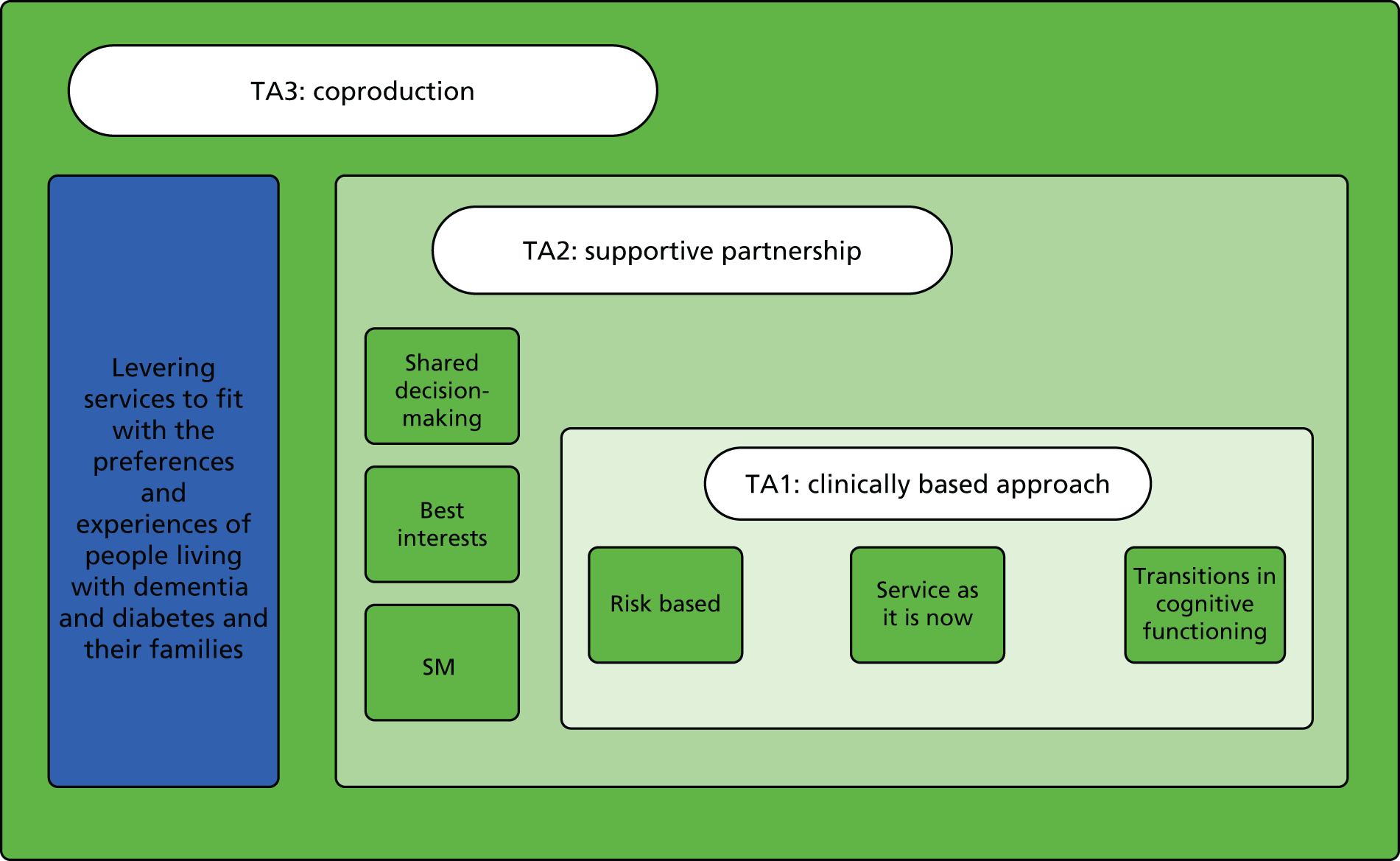
In response, we mapped ideas about ‘good’ diabetes care against the barriers for people living with dementia identified by the literature and the stakeholder interviews. We also identified potential interventions and emerging theory (Figure 3). This became theory area 1: clinically based approach. Theory area 1 was, however, felt to be rather biomedically focused, and additional theory areas around supportive partnerships (theory area 2) and coproduction (theory area 3) were developed to reflect other areas identified in the scoping. Figure 4 provides an overview of all of the initial theory areas.
FIGURE 3.
Good diabetes care mapped against potential barriers, interventions and emerging programme theory areas. PLWD, people living with dementia.
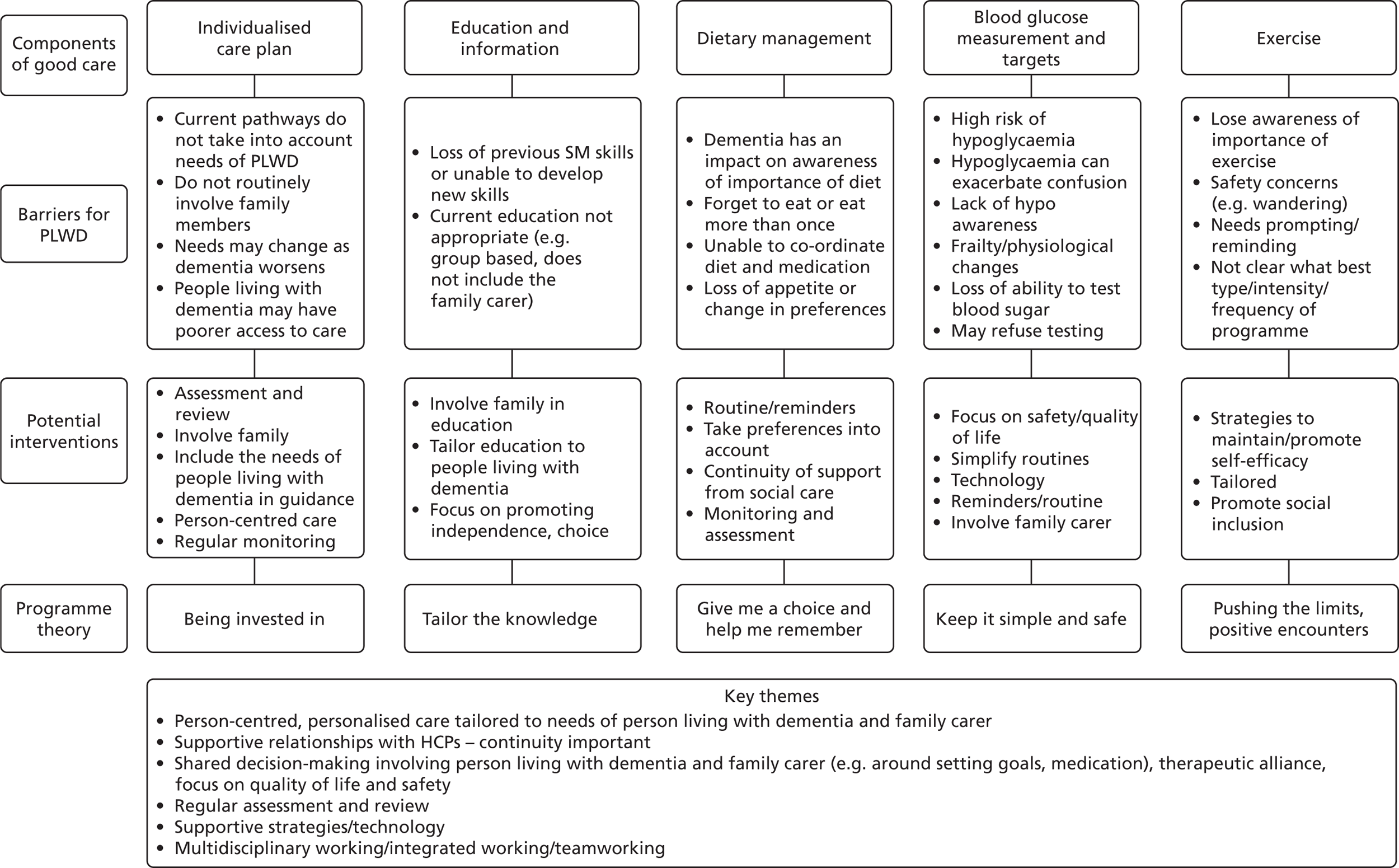
FIGURE 4.
Initial programme areas that emerged from phase 1. TA, theory area.
Phase 2: retrieval, review and synthesis
Searching processes
In phase 2 we undertook systematic searches of the evidence to test and develop the theories identified in phase 1. The main inclusion criteria were:
-
people with mild, moderate or advanced dementia [of any type, e.g. Alzheimer’s disease, vascular dementia, Lewy body dementia, Parkinson’s disease dementia, frontotemporal dementia (Pick’s disease) and alcohol-related dementia] and type 1 or type 2 diabetes, resident in the community
-
studies of any intervention designed to promote the management of diabetes in people living with dementia and the prevention of potential adverse effects associated with poorly managed diabetes, such as falls, blindness, vascular complications and renal failure
-
studies that provide evidence on barriers to, and facilitators of, the implementation and uptake of interventions designed to improve the physical health of people living with dementia (e.g. dementia-friendly initiatives, the impact of the cognitive vs. behavioural and psychological symptoms of dementia and the impact of the progression of dementia on family carers and service providers)
-
studies that offer opportunities for transferable learning, such as those that evaluate interventions for people living with dementia and other clinical conditions, or those that look at the way in which services are delivered and implemented for people living with dementia (e.g. interventions to improve access or continuity, tailor care to the needs of individuals with dementia or support family carers).
The purpose of the searches was not to identify an exhaustive set of studies but rather to be able to reach conceptual saturation in which sufficient evidence was identified to meet the aims of the review. 74 A diversity of evidence provides an opportunity for richer data mining and theory development. Therefore, we included studies of any design, including randomised controlled trials (RCTs), controlled studies, uncontrolled studies, interrupted time series studies, cost-effectiveness studies, process evaluations, surveys, and qualitative studies of participants’ views and experiences of interventions. We also included grey literature, policy documents and information about locally implemented programmes in the UK. As is usual with a realist review, the process of identifying relevant information and deciding what to include was iterative, involving tracking backwards and forwards between the literature and our review questions. 50 As such, the identification of relevant literature carried on during the course of the review, and some studies initially thought to be relevant were later excluded.
The search terms were devised in conjunction with an information scientist and chosen to reflect the theory areas identified in phase 1. The searches were split into three main categories:
-
A – theory areas + dementia AND diabetes
-
B – theory areas + dementia
-
C – theory areas + diabetes.
The main searches were category A, which included terms for the theory areas combined with both dementia and diabetes. However, because the scoping had identified little literature that covered both dementia and diabetes, we also searched for literature that focused on the theory areas and either dementia only (B searches) or diabetes only (C searches). As a result of discussions at the second project team workshop, an additional search was conducted (search D). This focused on tailored and individualised care for people with complex health needs (e.g. comorbidity and frailty). For full search terms, see Appendix 2.
For searches A, B and C, we searched the following electronic databases from 1990 to March 2016: MEDLINE (PubMed), CINAHL, Scopus, The Cochrane Library (including the Cochrane Database of Systematic Reviews), DARE (Database of Abstracts of Reviews of Effects), the HTA database, NHS EED (NHS Economic Evaluation Database), AgeInfo (Centre for Policy on Ageing – UK), Social Care Online, the NIHR portfolio database, NHS Evidence, Google (Google Inc., Mountain View, CA, USA) and Google Scholar. For search D, we searched PubMed, CINAHL, Scopus, Google Scholar and The Cochrane Library (1990–April 2016). An alert was set up in PubMed so that the team received weekly updates of new records identified by the search terms (March 2016–December 2016).
Previous dementia reviews undertaken by members of the project team have highlighted the importance of lateral searching for identifying studies for dementia-related reviews. 75 Therefore, in addition to the electronic database searches, we undertook lateral searches, which included checking reference lists, and citation searches using the ‘cited by’ option on Google and the ‘related articles’ option on PubMed.
Selection and appraisal of documents
Search results were downloaded into bibliographic software and, when possible, duplicates were deleted. Records from search A were split into two files and each file was screened independently by two reviewers (file 1 by PRJ and DT and file 2 by FB and BR). After each pair of reviewers had discussed the results of their screening, all four authors met to resolve any disagreements and amend the inclusion criteria as necessary. Records from the other searches (B and C) were screened by one reviewer, with 10% checked by a second. When studies appeared potentially relevant, PDF files were obtained, stored in Mendeley and screened by two reviewers. To enable us to keep track of the large number of records screened, and the changes in inclusion criteria, decisions made at different times were recorded in the Microsoft Excel spreadsheet created in phase 1.
Data extraction
A bespoke data extraction form was developed based on our three main theory areas. The form was piloted on six records by team members (FB, PRJ, BR and DT) and further refined as necessary. Once the final fields for data extraction were agreed, an electronic version was created in Microsoft Access® (Microsoft Corporation, Redmond, WA, USA). The data extraction form included fields relating to study aims, design and methods, the types of participants (e.g. dementia only, diabetes only, dementia and diabetes, other), outcomes, information relevant to the theory areas, and emerging context–mechanism–outcomes (CMOs) (see Appendix 3). Data were extracted by one reviewer, with 50% checked by a second. It should be noted that ‘data’ in a realist sense are not just restricted to the study results or outcomes measured. Therefore, author explanations and discussions can provide a rich source, or ‘nugget’, of ‘data’, and these were included in the data extraction form.
A test of whether or not to include an item in a realist review is to use ‘good enough and relevant enough’. 76 For a previous realist review,77 members of the research team created a set of constructs to ensure that the test of ‘good enough and relevant enough’ was transparent and clear to all team members. ‘Good enough’ was deconstructed as the quality of evidence expressed through fidelity, trustworthiness and value. ‘Relevant enough’ related to the contribution of the evidence to the theories and its potential contribution to the review. This set of constructs was added to the data extraction form in the form of a flow chart.
Analysis and synthesis processes
Realist reviews identify the task of synthesis as one of refining theory. Programmes operate through highly elaborate implementation processes. A realist review starts with a preliminary understanding of those processes, the initial programme theories (phase 1), and then seeks to refine them by extracting and evaluating the identified literature (phase 2).
The analytical task involved synthesising across the extracted information the relationships between mechanisms (e.g. underlying processes, structures and entities), contexts (e.g. conditions, types of setting, organisational configurations) and outcomes (i.e. intended and unintended consequences and impact). From the data fields in Microsoft Access, tables were constructed as the basis for further discussions about the emerging contingencies seen within and across the extracted data. These data were discussed with the wider RMT during a second half-day workshop. From these tables, we attempted to identify prominent recurrent patterns of contexts and outcomes (demiregularities) in the data and then sought to explain these through the means (mechanisms) by which they occurred. 78 This deliberative and iterative process enabled iteration from plausible hypotheses to the uncovering of potential CMO configurations.
The research team (FB, PRJ and BR) managed this extraction and synthesis process on a day-to-day basis, with regular consultation [via e-mail and telephone/SkypeTM (Microsoft Corporation, Redmond, WA, USA) conferencing]. Data synthesis involved individual reflection and team discussion that:
-
questioned the integrity of each theory
-
adjudicated between competing theories
-
considered the same theory in different settings
-
compared the stated theory with actual practice.
Phases 3 and 4: test and refine programme theories (validation) and develop actionable recommendations
To enhance the trustworthiness of the resultant hypotheses, and to develop a final review narrative to address what is necessary for the effective implementation of programmes to manage diabetes in people living with dementia, we reviewed the hypotheses and supporting evidence through consultation with the PAG and with stakeholders, some of whom had participated in the scoping. Stakeholder consultation was carried out by telephone interviews (n = 7) and by group discussions at a consensus conference involving 24 participants. Participants at the conference were purposively sampled to ensure that all of the key stakeholder groups in phase 1 were represented.
Consensus conference
The meeting began with a presentation from the research team in which the development of the hypotheses was outlined. This was followed by small group discussions about the proposed CMO configurations. Participants were split into three groups, with each including a mix of specialists in dementia and diabetes and at least one service user representative. Each group included two members of the research team, one to facilitate the group and one to take notes. At this stage there were six CMOs and each group was asked to focus on two. To encourage a quick generation of ideas, participants had just 10 minutes to write their recommendations on the ideas templates. The templates required participants to name what they thought the CMO might look like in practice (what needs to be in place), and what were the priorities and mediating factors. The facilitator for each group then asked participants to share their thoughts and these were recorded by the note-taker. Finally, the conference facilitator consolidated the recommendations by asking each of the groups to put forward their ideas to the larger group. Recommendations were discussed and recorded on a flip chart at the front of the room.
Following the consensus conference, members of the core project team (FB, PRJ and BR) met to review and discuss the outputs of the conference and amend the CMOs as appropriate. The revised CMOs were then checked against data from the literature and against transcripts of all the stakeholder interviews. The final CMOs and the supporting evidence are presented in Chapter 3.
Patient and public involvement
A well-established Public Involvement in Research Group at the University of Hertfordshire trains and provides support to public members and has a broad membership of service users and carers. Two members of this group (Dr Paul Millac and Mrs Diane Munday), both of whom have experience of caring for a family member with dementia and/or diabetes, were involved throughout the project. They were involved in defining the scope of the review (e.g. commenting on stakeholder interview transcripts), overseeing the project as members of the PAG and verifying findings as participants at the consensus meeting. As part of the realist review process, we also recruited additional patient and public involvement representatives for stakeholder interviews and the consensus conference. They were involved in defining the scope of the review and validation of the findings.
Chapter 3 Results
Description of studies
We included 89 papers. 25,28,30,37,39,42,43,45,57–73,79–142 These comprised 79 research papers and 10 guidelines or discussion pieces. Other papers cited in this chapter are for background information and did not undergo full data extraction. Twenty-two of the 79 research papers were reviews. Of those, 15 were systematic reviews,79,80,82,84,89,90,93,101,125–127,131,132,137,143 one was a realist review134 and six were non-systematic reviews. 25,37,45,66,73,105 The rest of the research papers (n = 57) related to primary research. Several studies were reported in more than one publication: the 57 primary research papers reported 51 studies. The main types of primary research were qualitative studies, RCTs or controlled studies. The rest were a mix of designs, including feasibility studies, surveys, before-and-after studies and observational studies. Ten papers25,28,39,42,59,64,66,83,123,142 focused on people living with dementia and diabetes; the rest were concerned with diabetes (n = 32), dementia (n = 31) or other groups, such as those with chronic illness or frailty. An overview of the selection process can be seen in Figure 5.
FIGURE 5.
Flow chart detailing study selection process. CI, cognitive impairment.
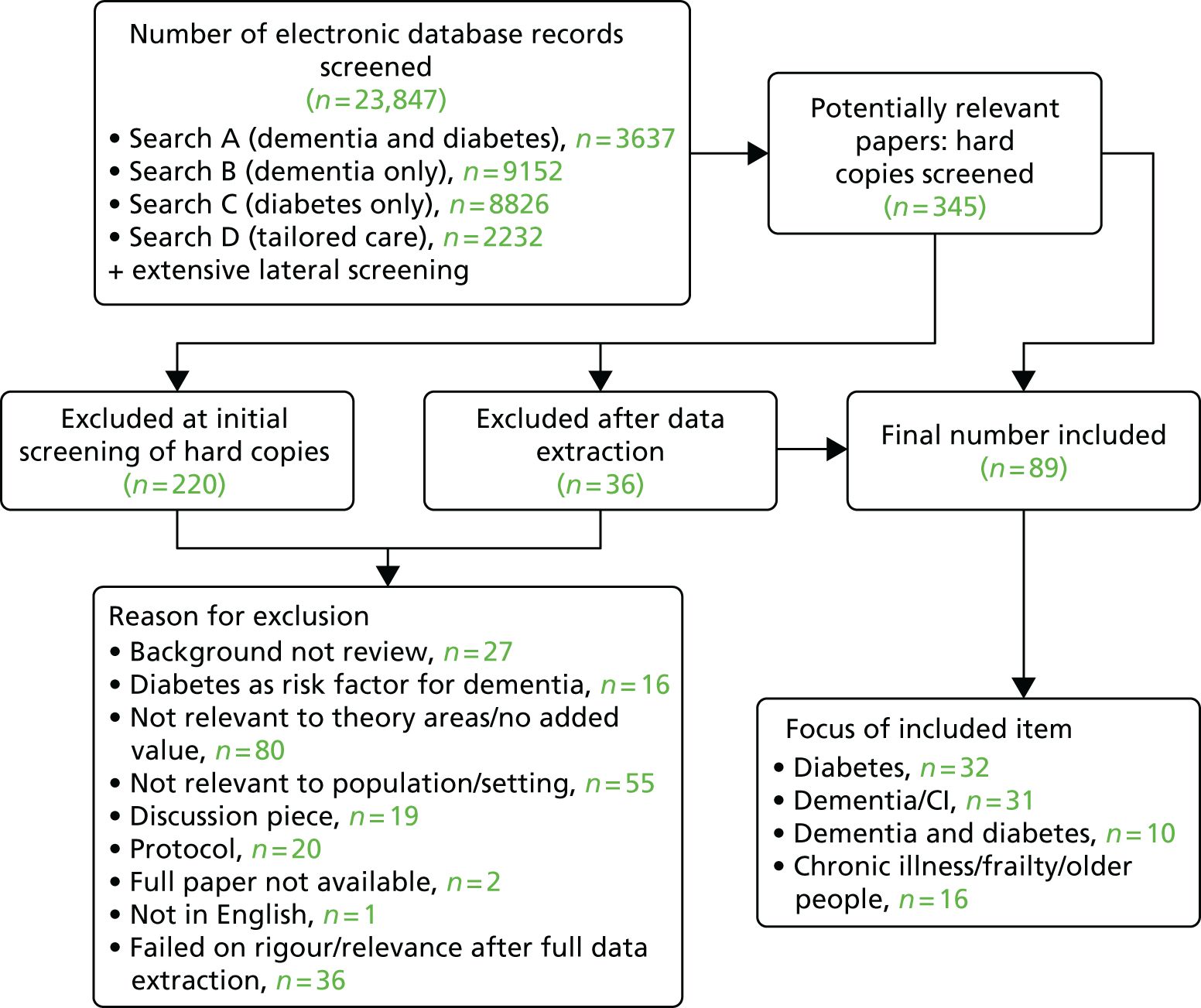
Of the 57 papers reporting primary research, the majority were from the UK (n = 2928,39,60,67,85–87,91,92,97,103–105,109–111,115,118–121,123,132,133,138–142), the USA (n = 1842,45,59,62–64,68,70,71,81,98–100,107,112,116,117,140) or Europe (n = 1058,61,72,95,96,106,113,114,122,124). As there was limited literature directly relevant to our target group, which we had anticipated, we included studies that offered opportunities for transferable learning, for example SM strategies for people living with dementia or diabetes care for older people. This evidence offered the opportunity to consider which challenges or issues were specific to people with dementia and diabetes and which were more general to other populations with diabetes (e.g. related to visual or sensory loss or other age-related problems). The types of papers we included, together with a summary of areas on which they focused and the sorts of outcomes they reported, can be seen in Table 3. Further details of individual studies are provided in Appendix 4. It is worth noting that much of the evidence on which we drew, particularly that relating to people with dementia and older people with diabetes, was detailing the absence or lack of care for these groups. Therefore, although there is literature that makes recommendations about what ought to be in place for these vulnerable groups, there is a lack of evidence that tests out these ideas.
| Focus | Included studies | Methodological approach | Types of outcomes |
|---|---|---|---|
Dementia AND diabetes (n = 10)
|
Abdelhafiz et al., 201625 |
|
|
| Brown et al., 201583 | |||
| Bunn et al., 201639 | |||
| Bunn et al., 2017142 | |||
| Camp et al., 201559 | |||
| Feil et al., 200942 | |||
| Feil et al., 201164 | |||
| Hackel, 201366 | |||
| Sachar, 2012123 | |||
| Sinclair et al., 201428 | |||
|
Dementia NOT diabetes (n = 31) Includes: |
Alsaeed et al., 201679 |
|
Patient outcomes include:
|
| Bahar-Fuchs et al., 201380 | |||
| Boots et al., 201482 | |||
| Boots et al., 201661 | |||
| Clare et al., 201385 | |||
| Clare et al., 201086 | |||
| Dhedi et al., 201487 | |||
| Dugmore et al., 201589 | |||
| Fleming and Sum, 201490 | |||
| Gibson et al., 201591 | |||
| Giebel et al., 201592 | |||
| Gillespie et al., 201293 | |||
| Goodwin et al., 2013138 | |||
| Graff et al., 200665 | |||
| Graff et al., 200896 | |||
| Graff et al., 200795 | Carer outcomes include:
|
||
| Iliffe et al., 200667 | |||
| Jekel et al., 2015101 | |||
| Knapp et al., 2015105 | |||
| Laakkonen et al., 2016106 | |||
| Lingler et al., 2016107 | |||
| Martin et al., 2013109 | |||
| Martin et al., 2015110 | |||
| Mountain, 200637 | |||
| Mountain and Craig, 2012115 | |||
| Quinn et al., 2015143 | |||
| Quinn et al., 2016136 | |||
| Schaller et al., 2016124 | |||
| Span et al., 2013127 | |||
| Suh et al., 2004128 | |||
| Toms et al., 2015132 | |||
|
Diabetes NOT dementia (n = 32) Participants include older adults, those with complex health needs (comorbidity, frailty, etc.), people with mental illness and adults with T2DM Includes: |
Aikens et al., 201563 |
|
|
| Bailey et al., 201681 | |||
| Baxter, 201457 | |||
| Beverly et al., 201470 | |||
| Branda et al., 201371 | |||
| Chrvala et al., 201684 | |||
| Care Quality Commission, 2016139 | |||
| Donald et al., 201388 | |||
| Goeman et al., 201694 | |||
| Heisler et al., 200398 | |||
| Hsu et al., 201699 | |||
| Huang et al., 2005100 | |||
| Jowsey et al., 201469 | |||
| Jowsey et al., 2016102 | |||
| Markle-Reid et al., 2016108 | |||
| Mathers et al., 2012111 | |||
| Mayberry et al., 201168 | |||
| Mayberry et al., 2016112 | |||
| McBain et al., 2016137 | |||
| McBain et al., 2016140 | |||
| Munshi et al., 2011117 | |||
| Munshi et al., 2013116 | |||
| Newton et al., 2016118 | |||
| Penn et al., 2015119 | |||
| Piette and Kerr, 200643 | |||
| Reinhardt Varming et al., 2015122 | |||
| Schulman-Green et al., 2016125 | |||
| Sherifali et al., 2015126 | |||
| IDF, 201330 | |||
| Sun et al., 2013129 | |||
| Tan et al., 2015130 | |||
| Taylor et al. 2016141 | |||
|
Other (e.g. people with chronic illness, frail older people, people with multimorbidity or LTCs) (n = 15) Includes: |
Anderson et al., 201573 |
|
|
| Bergdahl et al., 201372 | |||
| Davis et al., 201262 | |||
| De Vriendt et al., 201558 | |||
| Greenhalgh et al., 201397 | |||
| Kennedy et al., 2013103 | |||
| Kennedy et al., 2014104 | |||
| Kennedy et al., 201460 | |||
| Metzelthin et al., 2013;113 and Metzelthin et al., 2013114 | |||
| Procter et al., 2014120 | |||
| Ryan and Sawin, 200945 | |||
| Taylor et al., 2014131 | |||
| Wherton et al., 2012133 | |||
| Yardley et al., 2015134 |
Context–mechanism–outcome configurations
The theory development, refinement and testing process (see Chapter 2) led to the development of six CMO configurations (Table 4). Together, these explanations or hypotheses constitute a programme theory about ‘what works’ (or ‘what might work’) in the management of diabetes in people living with dementia. These CMO configurations were developed from evidence taken from interviews and the literature, and then were further tested in the literature and verified with stakeholders. The CMOs are not mutually exclusive and we would suggest that it is how the different elements of each interact that is important.
| Title | Context | Mechanism and outcome | Included evidence |
|---|---|---|---|
| 1: embedding positive attitudes towards people living with dementia | If health and social care delivery systems propagate and reinforce positive attitudes towards people living with dementia and diabetes and their families through tailored SM support . . . | . . . then this fosters a belief in staff that people living with dementia and diabetes have the potential to be involved in SM and the right to access diabetes-related services (even when the trajectory is one of deterioration), (M) prompting treatment confidence in people living with dementia and diabetes (M), which leads to engagement in SM practices by people living with dementia and diabetes, their family carers and HCPs (O) | 28,37,39,59,60,62,65,67,80,85,86,95,96,104–106,109,110,115,121,131,132,134,136,142 |
| 2: person-centred approaches to care planning | If delivery systems promote a person-centred and partnership approach to care, allowing HCPs to understand the individual needs and abilities of people living with dementia and diabetes and their families . . . | . . . then (1) HCPs feel confident that they are acting in the best interests of people living with dementia and diabetes and their families (M), and (2) this generates trust between HCPs and people living with dementia and diabetes and their families (M), leading to a better fit between care planning and patient and carer needs, and (potentially) a lessening of the burden of medicalisation experienced by people living with dementia and diabetes and their families (O) | 45,58,59,65,67,69,71–73,79,87,94,95,98,100,102,108,111,114,116,118,122,125–127,130,132,134,137,138 |
| 3: developing skills to provide tailored and flexible care | If HCPs are expected to develop skills that enhance the delivery of individualised and tailored care to people with dementia and diabetes (e.g. enablement rather than management, listening/communication/negotiation, shared decision-making) . . . | . . . then this legitimises the work creating the expectation in patients and in HCPs that diabetic care for people living with dementia is important (M), leading to the provision of more tailored diabetes care (O) and better engagement in SM by people living with dementia and diabetes and their family carers (O) | 25,60,67,69,71,79,88,89,98,103,104,111,113,114,116,117,119,122,123,129,134 |
| 4: regular contact | If HCPs maintain regular contact over time (e.g. face to face, by telephone or by e-mail) with the person living with dementia and diabetes and their family carer, monitoring and anticipating needs throughout the dementia trajectory . . . | . . . then HCPs feel more equipped to meet patients’ needs (M), and people living with dementia and diabetes and their families believe themselves to be supported (M) through the transition from functional independence to functional dependence (M), leading to improved diabetes management (O) | 28,43,59,61,62,64,66,79,83,84,87,116–119,124,128,130 |
| 5: family engagement | If family carers are routinely involved in care planning and information sharing, and are given the support they need to take on the tasks associated with managing diabetes in people living with dementia (e.g. medicine management, recognition of hypoglycaemia) . . . | . . . then family carers will feel supported and that their contribution is recognised and appreciated (M), leading to the development of effective SM strategies on the part of the family carer (O) | 39,42,63,64,69,72,79,82,95,107,108,115,124,130,142 |
| 6: usability of AT | As the dementia trajectory progresses, AT needs to be tailored and adapted to the needs and requirements of people living with dementia and diabetes and their families (includes social, environmental and cultural needs), with the focus on maintaining autonomy for the people living with dementia and diabetes . . . | . . . leading to people living with dementia and diabetes and their families gaining an understanding (awareness) of the usefulness of AT in their management of dementia and diabetes (M), leading to more effective and sustained use of AT to maintain autonomy and diabetes SM strategies (O) | 39,59,61,63,68,90,91,93,97,99,101,105,112,120,127,133,135 |
We now present each of the six CMOs in more detail. Further evidence supporting the CMOs can be seen in Appendices 5 and 6; this includes evidence from the papers (see Appendix 5) and interviews (see Appendix 6).
Context–mechanism–outcome 1: embedding positive attitudes towards people living with dementia
Programme theory
If health and social care delivery systems propagate and reinforce positive attitudes towards people living with dementia and diabetes and their families (C), through tailored SM support, then this fosters a belief in staff that people living with dementia and diabetes have the potential to be involved in SM and the right to access diabetes-related services (even when the trajectory is one of deterioration), (M) prompting treatment confidence in people living with dementia and diabetes (M), which leads to engagement in SM practices by people living with dementia and diabetes, their family carers and health-care professionals (HCPs) (O).
Stigma and barriers to care for people living with dementia
There is evidence that social stigma surrounds both dementia and diabetes not only in the general population but also among HCPs. 144 Stigma associated with dementia has an impact on the person living with dementia and may lead to them having poorer access to care than people with similar comorbidities but without dementia. 39 Stigma is also felt by the family carer, for example through increased social isolation. People with diabetes may also be stigmatised and blamed for their condition because of lifestyle choices and/or obesity. 144 Recent work by members of the team around community engagement and dementia-friendly health care has argued that without a foundation of awareness about what it is like to live with dementia, related initiatives will not succeed. A reliance, for example, on single initiatives such as dementia champions is insufficient. 145,146
People with dementia and comorbidity face many problems in accessing health care. This includes a lack of services designed around the needs of people living with dementia, poor communication between services, a lack of training on dementia care for health and social care staff and a reliance on others (such as family carers) to recognise a need for services and seek help. 39 A recent mixed-methods study, which included qualitative interviews and focus groups with clinicians, highlighted deficiencies in access to care and continuity of care for people with dementia and comorbidity. Access to care was affected by clinicians’ previous experiences and their attitudes towards risk. For example, there were contrasting opinions about the appropriateness of taking someone with dementia off insulin. 39,142 Stakeholders also highlighted the need to ensure that people living with dementia received equitable care:
. . . you shouldn’t be sort of swayed one way or the other, just because someone has dementia . . . I think certainly when they first start on their journey I think it’s really important that we do everything we can [of cross-disciplinary training to facilitate appropriate care].
Diab 1 (stakeholder interviews)
Involving people with dementia in self-management
We found 10 studies37,59,62,106,109,110,115,121,132,136 looking at SM interventions for people living with dementia or cognitive impairment, only one of which included people with dementia and diabetes. 59 This controlled study evaluated the impact of personalised education sessions on people with diabetes and cognitive impairment. 59 In this US-based study, certified diabetes educators delivered personalised education sessions over the internet to older adults with type 2 diabetes and cognitive impairment (mild cognitive impairment or early-stage dementia). They found a significant increase in self-efficacy but no difference in glycated haemoglobin (HbA1c) at 6-month follow-up. The authors say that the face-to-face nature of the contact (via Skype) was beneficial for establishing good rapport with participants. Mountain37 suggests that the lack of studies on SM for people living with dementia is a result of negative perceptions of the abilities of people with early dementia, which ‘leaves them on the periphery of any benefits that can be derived from the current focus of policies that support people with long term conditions’. 37
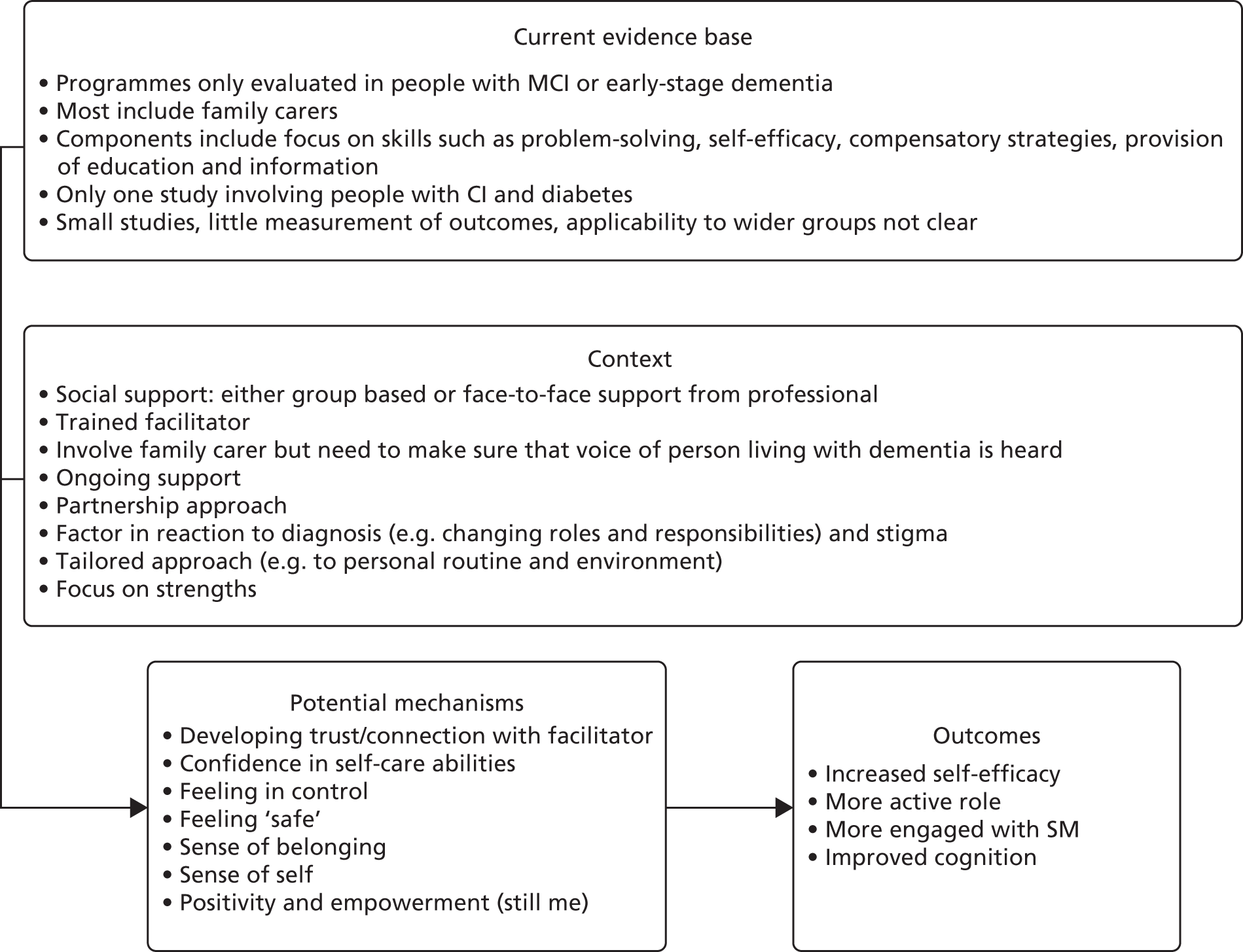
Studies on SM for people with dementia are mostly qualitative or small pilot or feasibility studies, and most do not report measurable health outcomes. They also tend to focus on couples or people who have a partner, which excludes many who might potentially benefit. 147 This literature does, however, provide valuable insights into what needs to be in place to support independence and appropriate outcomes in people living with dementia, much of which might form the basis for programmes for people with dementia and diabetes. Potentially important components of such programmes include the need to focus on the abilities and strengths of people with dementia rather than their disabilities,109,110 on emotions rather than problems,109 on building confidence62,121 and on respecting participant autonomy and enhancing empowerment. 106 Social interaction may also be important. 106,110,143 A small feasibility study121 suggested that SM interventions may foster independence and reciprocity, and promote social and clinical support. A larger RCT involving 136 people living with dementia–spouse dyads found that a group-based intervention to promote SM in people newly diagnosed with dementia led to improvements in health-related quality of life in spouses and in cognition in people living with dementia, although quality-of-life improvements in spouses were not maintained at longer-term (9-month) follow-up. 106 Further details of these studies can be seen in Appendix 7 and an overview of some key potential aspects of context, mechanism and outcomes can be seen in Figure 6.
FIGURE 6.
Summary of evidence around SM programmes for people living with dementia. CI, cognitive impairment; MCI, mild cognitive impairment.
Fostering confidence
The importance of confidence as a mechanism is underlined in a number of studies. For example, a systematic review130 of self-care interventions for older adults with diabetes (not dementia) found that interventions using concepts of self-efficacy, self-determination and proactive coping were effective in influencing diabetes self-care behaviours, leading to improved health outcomes. For example, proactive coping helped people to anticipate threats and act accordingly. In addition, work by Clare et al. 86 and Claire148 suggests that there is a link between independence, functional ability and self-care behaviour and feelings of confidence or self-efficacy in people living with dementia and their family carers. Clare et al. 86 argue that a loss of confidence can promote disability:
Negative influences can contribute to the development and maintenance of ‘excess’ disability – where the extent of functional disablement is greater than would be predicted by the degree of impairment: an example would be where an individual loses confidence.
Clare et al. 86
Therefore, interventions that promote confidence or self-efficacy may lead to increased engagement in SM activities. This ties in with literature on enablement, in which the focus is on what the person with dementia can do both cognitively and functionally to maintain their quality of life. 148 The process of enablement has been sponsored by the Department of Health in England in their 2010 guidance Nothing Ventured, Nothing Gained,149 which made it clear that HCPs need to be proportionate in their assessment of risk with people living with dementia and that a ‘safety first’ management perspective disempowers people living with dementia. Although we found no literature on enablement for people living with dementia and diabetes, there is some evidence that teaching goal-directed activities can improve the cognitive functioning of people living with dementia. 85,86 It has been suggested that the enablement philosophy can also be applied to identifying and treating accompanying physical illness and providing information and support to caregivers,148 although this has not been tested in people living with dementia and diabetes.
We included several studies that focused on the functional abilities of older people with multimorbidity. A case study138 of co-ordinated care for people with complex chronic conditions supported the idea that care co-ordination programmes should focus on supporting service users and carers to become more functional, independent and resilient. The authors suggested that this is preferable to a purely clinical focus on managing or treating medical symptoms. Two studies58,65 involved occupational therapists (OTs) delivering interventions aimed at improving the functional abilities of older people with multimorbidity. One, which found improvements in activities of daily living, suggested that an important aspect of the intervention was that OTs used motivational interviewing and goal-setting to understand patient priorities and to negotiate solutions in partnership. 58 In the other, a RCT of community OT for people living with dementia and their carers, the authors found that 10 sessions of OT led to improvements in mood, quality of life and health status at the 12-week follow-up. 65,95,96 The intervention involved the use of compensatory strategies to adapt activities of daily living to the disabilities of patients and adapt environments to their cognitive disabilities. Primary caregivers were trained to use effective supervision, problem-solving and coping strategies to sustain both their own and the patient’s autonomy and social participation. A potential mechanism was an increased sense of control.
Although these studies do not focus on SM for dementia and diabetes, and although they focused on people living with mild or early-stage dementia, they may provide transferable learning for the development of SM support for people living with dementia and diabetes, for example the focus on family relationships, maintenance of an active lifestyle, psychological well-being and techniques for coping with memory changes. Furthermore, they promote positive attitudes, with an emphasis on equality and collaboration between participants and facilitators. 106 Working with families is clearly key. However, studies highlight the need to ensure that the voice of the person living with dementia is heard and that their needs are balanced with those of the carer. 115 Qualitative studies on SM support for people living with dementia found that information provision may be aimed at carers, leaving people living with dementia feeling powerless,115 that carers may inadvertently take control away from people living with dementia109 and that people living with dementia can find support inappropriate or stifling. 132 As one stakeholder put it:
. . . an intervention should work at a level that people . . . particularly early stages of dementia . . . can be included . . . so it’s not decisions being made about them . . .
Dem 1
Further RCTs of SM support for people with diabetes and cognitive impairment are under way but these focus on intellectual disability150 and learning disability151 rather than dementia. Although the results are not available, the protocols do provide information about components of the intervention. For example, the intervention being evaluated by Walwyn et al. 151 involves establishing participants’ daily routines and lifestyles, identifying all supporters and helpers and their roles, setting realistic goals for change and monitoring progress against agreed-on goals, while the Taggart et al. 150 study involves evaluating an adapted version of DESMOND (The Diabetes and Self-Management for Ongoing and Newly Diagnosed patients with T2D programme). 152 The adapted version includes longer sessions, increased use of pictures in the educational information, greater involvement of carers, more repetition and interactive sessions, and a strong focus on celebration and fun,153 components that would appear appropriate for people living with dementia.
Context–mechanism–outcome 1 summary
Interventions for people with dementia and diabetes need to address the stigma that this group faces. There is evidence that people living with dementia have the potential to be involved in SM, although most research currently focuses on SM skills in people with mild or early-stage dementia, and there is little evidence relating to SM in people with dementia and a comorbid condition such as diabetes. However, the literature identified important components of interventions that are likely to be transferable to people with dementia and diabetes; for example, focusing on strengths and abilities, being emotion focused rather than problem focused, respecting autonomy and working to build confidence and empowerment. The involvement of family carers in programmes is key, but it is important to balance the needs of the people living with dementia with those of the carer to ensure that people living with dementia are not disempowered.
Context–mechanism–outcome 2: person-centred approaches to care planning
Programme theory
If delivery systems promote a person-centred and partnership approach to care, allowing HCPs to understand the individual needs and abilities of people living with dementia and diabetes and their families (C), then (1) HCPs feel confident that they are acting in the best interests of people living with dementia and diabetes and their families (M), and (2) this generates trust between HCPs and people living with dementia and diabetes and their families (M), leading to a better fit between care planning and patient and carer needs, and (potentially) a lessening of the burden of medicalisation experienced by people living with dementia and diabetes and their families (O).
Identifying patient and carer priorities
People living with dementia and diabetes have two chronic conditions with different trajectories. Dementia will generally ‘have a progressive or stepwise pattern of progression in illness severity, symptoms and disability over time, which will require continual adaptation to new issues or limitations’,154 whereas diabetes may have a more constant course with longer periods in which to adapt, but the trajectory of each is likely to have an impact on the other. Supporting people living with dementia and diabetes, and their families, with these ambiguous trajectories along the route from early-stage dementia to end-of-life care is a difficult clinical enterprise. Delivering appropriate and sustainable care for people living with dementia and diabetes requires a change from a curative, biomedical strategy to a more person-centred approach whereby patient priorities are at the forefront. 155 The Institute of Medicine defines patient-centred care as being ‘respectful of and responsive to individual patient preferences, needs, and values, and ensuring that patient values guide all clinical decisions’, and is a hallmark of high-quality care. 156
Maintaining independence and engagement with day-to-day activities was a clear priority for participants in all groups (e.g. people with dementia and diabetes, older people with diabetes, people with dementia). 59,70,100,113,114,132 For example, a qualitative study100 of older patients with type 2 diabetes found that their primary goal in diabetes SM was engagement with their day-to-day activities, rather than maintaining biomedical parameters. The authors concluded that HCPs have to prioritise each patient’s life experience when providing advice about diabetes management. This was supported by our stakeholder interviews, as seen in the following quotation from a general practitioner (GP):
But actually at this stage [referring to when people have complex health needs] people are interested in autonomy, mobility you know, retaining as much function and independence as they can, being a burden on their families you know, so all the normal things and they’re often much, much more important than a lot of the medical stuff.
Diab 12
Person-centred approach to self-management in people living with dementia and diabetes
A recognition of patient motivators and goals, and negotiation of a mutually agreed management plan, could improve adherence to SM regimens. 98,102,118,125,157 For example, in a qualitative study of older adults with diabetes (aged ≥ 65 years), participants reported a variety of motivators for maintaining SM once a routine had been established. HCPs needed to understand what these motivators were and work with patients towards their SM goals. Feedback from participants included the observation that HCPs did not listen to, or were dismissive of, their concerns and, as a result, this reduced the trust that they had in their HCP. 118
A Cochrane review137 looked at SM interventions for people with diabetes and severe mental illness. Searches, which were conducted in 2016, identified only one RCT that met the inclusion criteria. This was a small study (n = 64) involving adults with type 2 diabetes and schizophrenia or schizoaffective disorder (average age 54 years). The intervention, which involved a 24-week education programme, had no significant impact on glycaemic control, although there were small improvements in diabetes knowledge and self-efficacy. The authors suggested that the lack of impact could, in part, be explained by the lack of a person-centred approach within the programme.
We found only limited evidence that evaluated the impact of our identified context on glycaemic control or adverse events. Of the six included sources that focused on people with dementia and diabetes, only one59 evaluated an intervention. This was a controlled study evaluating the provision of personalised education sessions for diabetes and cognitive impairment. The study showed an increase in self-efficacy and a short-term effect on glycaemic control (HbA1c levels initially declined but had returned to baseline after 6 months). The authors suggested that this supported a need for long-term connection and maintenance programmes for this group.
Trusting relationships
A key element of achieving a person-centred or partnership approach to care is building a long-term trusting relationship with the people living with dementia and diabetes and their family69,70,72,73,79,102,125,142 that involves listening and valuing the patient’s subjective health experiences:
It’s allowing a two-way exchange of information, isn’t it, about how different conditions might affect things.
Res 1
The impact that a trusting relationship between HCPs and people with dementia can have on patient care is illustrated in a qualitative study87 exploring GPs’ perceptions of what is meant by a ‘timely’ diagnosis of dementia and how this differs from an early diagnosis. The timely diagnosis was seen as requiring a more patient-centred approach that recognises the issues that families go through in reconciling the stigma of diagnosis and consequences for the future. The authors argue that a ‘diagnosis’ of dementia was not a discrete act ‘. . . but a collective, cumulative, contingent process’. 87 The GPs in this study saw the diagnosis as a journey for both them and the patient and their family in reaching a nuanced and contingent resolution, leading to a co-constructed diagnosis. This study, like others,39 highlights how vital relationship continuity can be in creating trust and helping to secure appropriate and timely care for people living with dementia.
A feasibility study122 explored the use of patient-centred consultations for improving medication adherence and management among older people with diabetes. The intervention included the use of dialogue tools whereby the patient was asked to describe their day living with diabetes, to ‘. . . encourage participants to reflect, engage in dialogue, and verbalize their experiences’. 122 The findings showed that people living with type 2 diabetes felt listened to, which generated a trusting relationship with their HCP. Participants also reported feeling more capable of managing their diabetes, although, as this was a feasibility study, the authors did not measure the impact of the intervention on glycaemic control. HCPs reported that the process supported patient-centred partnership but the authors cautioned that the dialogue tools are, by themselves, not enough to ensure patient-centeredness; adequate communication skills are also required to prevent the process becoming a tick-box exercise.
We know that for people who have previously self-managed their diabetes (often for many years), the transition to needing SM support can be difficult and distressing. 39,142 Although we found some literature on enablement for people living with dementia (see Context–mechanism–outcome 1: embedding positive attitudes towards people living with dementia), we found no evidence about how (or whether or not) a patient’s previous diabetes knowledge or SM strategies are acknowledged or used by HCPs.
Shared decision-making
The management of people living with dementia and diabetes represents a complex process with difficult trade-offs to be made, so methods of involving patients and families in shared decision-making are important aspects of achieving person-centred care. In a multicentre cluster RCT of older people with type 2 diabetes (not dementia), Branda et al. 71 introduced a decision aid that encouraged shared decision-making between participants with diabetes and HCPs. There was no difference in glycaemic control between the decision-aid intervention group and the control group. However, the decision aids did facilitate patient-centred practice and co-construction of decision-making related to diabetes management. The participants also felt more engaged in their diabetes management despite the lack of clinical outcomes. Similarly, Mathers et al. ,111 in a RCT of a decision aid for patients with type 2 diabetes (adults aged between 42 and 87 years), found no statistical effect on glycaemic control (HbA1c decreased in both intervention and control groups), but decision-making by patients was more autonomous and promoted more realistic expectations and greater knowledge. The authors suggested that this can lead to better long-term SM practices.
Both of these studies involved the use of a decision aid in a single consultation between a clinician and a person with diabetes. In the Mathers et al. 111 study, the mean duration of the consultation was 15.31 minutes. People living with dementia and diabetes are likely to need interventions that involve more frequent contact and include repetition and reinforcement. Decision-making involving people living with dementia is likely to be complicated by issues of consent, concordance and the appropriateness of treatment for people with dementia.
Context–mechanism–outcome 2 summary
Self-management of diabetes in people with dementia is likely to be contingent on the development of a trusting relationship between the HCP and the person living with dementia and diabetes and their family, involving understanding and incorporating patient priorities and how these may change over time. This in turn facilitates a person-centred approach to care planning and diabetes management. There is currently little research that looks at person-centred approaches to diabetes management in people living with dementia, and further research is needed to develop interventions that support partnership working and incorporate the consideration of the risk-benefit balance for different treatment options.
Context–mechanism–outcome 3: developing skills to provide tailored and flexible care
Programme theory
If HCPs are expected to develop skills that enhance the delivery of individualised and tailored care to people with dementia and diabetes (e.g. enablement rather than management, listening/communication/negotiation, shared decision-making), then this legitimises the work and creates the expectation in patients and in HCPs that diabetic care for people living with dementia is important (M), leading to the provision of more tailored diabetes care (O) and better engagement with SM by people living with dementia and diabetes and their family carers (O).
Tailored and flexible
The importance of providing tailored care for older people with diabetes was emphasised in the literature. In a meta-analysis of SM programmes in older people living with diabetes, Sherifali et al. 126 concluded that the strategies most effective at reducing HbA1c levels were those that involved tailored interventions or psychological support. Tailored interventions consisted of patients receiving advice and support that met their particular needs and goals for diabetes SM and the psychological support emphasised coping strategies for depression and distress. The authors did not, however, unpick what it was about tailoring or psychological support that might have triggered these different responses. A RCT to assess barriers to SM experienced by older people with type 2 diabetes demonstrated the importance of assessing the specific self-care barriers faced by this cohort and then individualising diabetes management. 116 Individualisation was aided by a diabetes educator making telephone contact between clinic visits.
Current clinical guidance also highlights the need for individualised and flexible care for older people with dementia and diabetes. Clinical guidelines on diabetes recommend that target HbA1c levels be relaxed for older people who are frail or have comorbidities and/or dementia. 26,28,29,158 A summary of recommendations for glycaemic control in older people (including those with dementia) can be seen in Table 5.
| Guidance (author, year) | Population | Summary of recommendations: glycaemic control targets | Summary of recommendations: medications |
|---|---|---|---|
| Sinclair et al., 201428 | People with dementia | Aim to achieve fasting blood glucose 6–9 mmol/l (range HbA1c 53–64 mmol/mol; 7–8%) |
|
| IDF, 201330 | Older people but includes recommendations for people with dementia | A HbA1c target of up to 8.5%/70 mmol/mol may be appropriate |
|
| American Diabetes Association, 201529 | Older people but includes recommendations for those with complex health needs such as people with CI | A1C goal
|
|
| Sinclair et al., 201126 | For people whose hypoglycaemia risk is high and for whom symptom control and avoidance of metabolic decompensation is paramount (includes people with dementia), the target HbA1c range should be 7.6–8.5% | The physician should aim to establish a contract between himself/herself and the patient or principal carer in relation to treatment aims and goals of care, designed to optimise patient empowerment at all timesThe decision to offer treatment should be based on the likely benefit/risk ratio of the intervention for the individual concerned, but factors such as vulnerability to hypoglycaemia, ability to self-manage, the presence or absence of other pathologies, the cognitive status, and life expectancy must be considered |
For example, a best clinical practice statement28 by a multidisciplinary national expert working group recommends that, for people with dementia, clinicians should aim to achieve fasting blood glucose of 6–9 mmol/mol (range HbA1c 53–64 mmol/mol; 7–8%). Although guidance for general diabetic populations recommends levels of 6.5–7 mmol/mol, it also recommends that these targets be relaxed for those with complex health needs or those who are frail. 158 Despite this, evidence from observational studies suggests that many older people with diabetes are potentially overtreated. 25 This could be partly because these targets do not ‘fit’ with current performance measures159 (such as QOF targets in UK primary care), a factor suggested by a number of our stakeholders and illustrated by this quotation from a diabetes specialist:
. . . we encourage people to set agreed targets with the patient . . . that may well be . . . higher than the general population target which is a key message we get across to the GPs because they’re so driven by QOF.
Diab 9
Flexible diabetes care is not just about adjusting target HbA1c levels. A case study117 involving the use of continuous glucose monitoring to measure hypoglycaemia in older adults with diabetes found that simply relaxing HbA1c goals may not be adequate to protect frail older adults against hypoglycaemia. They found ‘an unexpectedly high frequency of hypoglycaemic episodes in older adults with poor glycaemic control’;117 this was true of those with type 2 diabetes as well as of those with type 1 diabetes. The authors concluded that there is a need for treatment regimens that better match patients’ self-care abilities, although they did not explore further what such regimens might look like. 117
Developing appropriate skills
Research suggests, however, that HCPs do not always have the skills needed to provide flexible individualised care for people with diabetes and/or dementia. 39 This was echoed by our stakeholders, as the following quotation illustrates:
I’ve seen very very few examples where it’s done well, any of this, any of this sort of self-management, shared decision-making, anything. It works well I think in a few types of situations where people feel they have the autonomy to act in the interests of the patient and are able to make decisions that might not necessarily follow the rules but they can see the patient as an individual and understands their circumstances, otherwise I think health professionals are possibly becoming themselves much more risk-averse and not wanting to suggest things that aren’t perceived as being healthy or might not be the right answer.
Res 1
One consequence of this is that care is not tailored appropriately. In a RCT116 involving older people with diabetes, the authors wrote that ‘the study team felt that many patients with multiple conditions were on complex regimens that were clearly beyond their coping abilities’. In a qualitative study with older adults with diabetes and other comorbidities (not dementia), participants reported that they felt overwhelmed when dealing with their diabetes and other conditions and that they felt that their HCPs did not understand the difficulties they experienced with balancing their comorbidities so that they could achieve a reasonable quality of life. 70 The following quotation from a GP highlights how ideas about assessing a patient’s abilities to cope with treatment plans are not yet part of routine care. The GP is referring to the ‘choosing wisely’ initiative from the American Board of Internal Medicine Foundation,160 which aims to promote conversations between clinicians and patients to prevent the use of unnecessary tests and procedures:
. . . choosing wisely American stuff you know, I think we’re all warming up to this agenda but I don’t think anyone’s quite cracked you know, it’s not mainstream yet.
Diab 12
In addition, Kennedy et al. 103 conducted a RCT of SM support for people with long-term conditions in primary care in the UK. The results of the RCT103 and accompanying process evaluation104 and qualitative study60 suggest that SM support did not fit with a biomedically focused ethos and, as a result, practices did not give it the priority needed to embed it in the day-to-day work of primary care. A qualitative study conducted in the USA also found that an overemphasis on biometrics and medicalisation by HCPs was a barrier to SM,100 and a study looking at SM pathways in primary care in the UK concluded that primary care is currently failing to support SM. 119 This is illustrated by the following stakeholder quotation:
. . . for the general population, self-management . . . is not working particularly effectively . . . translate that to a much more delicate and fragile group . . . who have other comorbidities and have dementia . . . then those types of responses are likely to be even less effective [of SM support strategies].
Diab 2
Several studies70,98,134 suggested that there is ‘goal divergence’ between patients, carers and HCPs, particularly when there is medical uncertainty, as is generally the case with the dynamic nature of living with multiple conditions. In a realist synthesis, Yardley et al. 134 suggested that, to reconcile discrepancies between the goals of patients, carers and HCPs, there is a need for less emphasis on a ‘diagnostic-cure model’. Patient-centred communication and collaboration between HCPs and patients/families appear to be key to achieving individualised care70,98,134 (see also Context–mechanism–outcome 2: person-centred approaches to care planning).
Context–mechanism–outcome 3 summary
We found evidence that, to be able to provide flexible and individualised care for people living with dementia and diabetes, HCPs need to prioritise communication, negotiation and partnership working. They need to be provided with appropriate training and support so that they have the confidence to focus more on quality of life and patient abilities and less on biometrics and clinical targets. However, currently the evidence to link our proposed context with glycaemic control or a reduction in adverse diabetes-related events is limited.
Context–mechanism–outcome 4: regular contact
Programme theory
If HCPs maintain regular contact over time (e.g. face to face, by telephone or by e-mail) with the person living with dementia and diabetes and their family carer, monitoring and anticipating needs throughout the dementia trajectory (C), then HCPs feel more equipped to meet patients’ needs (M), and people living with dementia and diabetes and their families believe themselves to be supported through the transition from functional independence to functional dependence (M), leading to improved diabetes management (O).
Anticipating needs
Regular contact between HCPs and the people living with dementia–carer dyad appears to be an important contextual factor for HCPs to anticipate transitions and help people living with dementia and diabetes and their family carers to manage changes in function and SM capabilities. 142 This is particularly important for people living with dementia for whom the dementia may progress in an uneven pattern of decline,92 and for whom the transition from autonomy to delegation or caregiver-led management may be particularly difficult. 39,79,118 Regular contact may have particular advantages for people living with dementia, as illustrated by this quotation from a stakeholder interview:
. . . if it’s set up on a regular basis, so the person knew, you know, like Tuesday afternoons when I speak to my diabetic nurse, that can be put in their diary.
Dem 4
Penn et al. 119 (looking at diabetes but not dementia) suggested that if SM support is offered at regular points throughout a person’s trajectory, rather than just focusing on the point of diagnosis, then HCPs are more likely to pick up problems with SM and be able to offer appropriate support. In a RCT116 focused on improving diabetes management in older people, the authors found that older adults were reluctant to make changes to their medication between clinic visits. However, regular telephone contact from a diabetes educator encouraged people to adjust their insulin dosage, leading to better glycaemic control. Regular contact is likely to be even more important for people with dementia, and guidance and commentary on the management of diabetes in people living with dementia recommends regular reassessment to identify additional care needs. 28,83 Older people with diabetes (including those with dementia) are particularly likely to be in need of support during vulnerable periods, such as after a period of hospitalisation. 116,142
Current care pathways, however, lack the capacity to consistently assess SM capabilities or provide SM support. 119 This is illustrated by the following quotation:
. . . since I’ve been in the care of the diabetic clinic everything else has gone out the window. When I was in the care of the specialist nurse at the GP’s, I would have a regular sort of every 6-month check on my feet . . . and the amount of protein in my urine, all those tests have now ceased, I’m now only looked at from a point of view of sugar levels.
Person with type 2 diabetes
What sort of contact and with whom?
The quotation above also illustrates another important point. It is not just about having regular contact but also about who that contact is with. The quotation suggests that care at the GP surgery, where the patient was known, was preferable to the less personalised (and less comprehensive) care received at the diabetic clinic. Clearly this is only one person’s experience; however, many of the studies we included39,59,62,64,66,116,130 support the link between regular contact with the same HCP, the development of a good relationship and improved SM practices. For example, a controlled study59 of diabetes SM education for people with cognitive impairment found that regular contact with a single diabetes educator led to the development of a rapport, which was important in helping participants to develop strategies to improve SM (e.g. remembering when to take their medication).
Of the studies we included, 38 described the development or evaluation of an intervention; two included people living with dementia and diabetes, 16 included people living with dementia (not diabetes), 14 included people with diabetes (not dementia) and six included other groups such as older people or those living with long-term conditions. This includes a diverse range of interventions, although many were aimed at improving SM. Interventions were delivered by a range of HCPs, most commonly GPs, nurses, OTs, psychologists and, in US studies, certified diabetes educators. Regardless of who was delivering the intervention, studies consistently highlighted the importance of the continuity and quality of the relationship. A number of studies looked at the development and delivery of interventions delivered remotely using technology (e.g. text messages). This is explored further in Context–mechanism–outcome 5: family engagement.
Although the quality of the relationship is important, people with dementia and diabetes need to be managed by practitioners with appropriate expertise. Stakeholders highlighted the particular challenges HCPs face when caring for people with dementia and diabetes, and the different skill sets that different specialists are likely to have. Dementia as a comorbidity may challenge a diabetes specialist, and a dementia specialist may lack appropriate diabetes knowledge. From our stakeholders we found examples of roles that involved someone with expertise in both mental and physical health, although this was the exception rather than the rule:
. . . I have a very good colleague . . . who is a specialist physical health-care nurse and a mental health nurse, and that is a, I’ve often thought of this person as a really interesting model for the future . . .
Dem 7
One paper described a programme that included embedding a psychiatrist within an integrated care pilot for people with diabetes in inner London. 123 The involvement of the psychiatrist raised awareness of patient mental health problems among other team members and meant that the team was able to identify if poor SM might be a result of mental health issues. The author said that clinicians became more engaged when they were able to ‘really think about the person in their entirety’. However, the paper provided no data on the impact of the intervention on patient outcomes.
Context–mechanism–outcome 4 summary
Continuity of care and regular contact are important for both people with dementia and those with diabetes, but it is likely to be even more critical for those with both dementia and diabetes. This is important so that professionals can recognise times of transition (e.g. worsening symptoms of dementia having an impact on diabetic control and increased risk of hypoglycaemia). At such times support may need to be ‘geared up’ but may be able to be ‘geared down’ once the transition has been successfully dealt with. Ensuring that all professionals have expertise in dementia and diabetes would be difficult, and collaborative practice is likely to be necessary for people with both conditions, particularly for more complex cases such as people who are insulin dependent or those with advanced dementia.
Context–mechanism–outcome 5: family engagement
Programme theory
If family carers are routinely involved in care planning and information sharing, and are given the support they need to take on the tasks associated with managing diabetes in people living with dementia (e.g. medicine management, recognition of hypoglycaemia), (C) then family carers will feel supported and that their contribution is recognised and appreciated (M), leading to the development of effective SM strategies on the part of the family carer (O).
There is a great deal of evidence that family members often provide significant SM support for people with long-term conditions such as diabetes,45,69 particularly when dementia has an impact on a person’s ability to undertake self-care-related tasks. 142 A mixed-methods study exploring the impact of dementia on access to non-dementia services found that family members were often proactive in facilitating continuity of care and negotiating access to services for their relatives with dementia and diabetes,39,142 and a review of medicine use in people living with dementia found that administering medicines was a huge component of being a caregiver of a person living with dementia. 79 Managing the needs of a family member with dementia and diabetes raises particular anxieties for carers because of the risk of hypoglycaemia and other adverse advents associated with diabetes. People with dementia are likely to forget to eat (or to eat too much), and ensuring that they eat appropriately and that this is co-ordinated with their medication is a source of great concern for carers, particularly if they live at a distance. 39 This is complicated by a lack of flexibility in service provision, such as district nurses being able to go in only at certain times of the day, or social services carers being unable to oversee medication administration for people with diabetes. 39,142
Family carers may also play an important part in supporting or motivating their relatives in their SM activities. A quasi-experimental study conducted in the USA (adults with diabetes but not dementia) found that involving a care partner may make a person with diabetes more likely to participate in an automated telephone SM system. The authors suggest that the mediating mechanisms were emotional support leading to improved ability to regulate one’s own behaviour, direct assistance with diabetes problem-solving provided by the care partner, and reinforcement of adherence. 63
Despite this, family carers often feel undervalued or excluded from decision-making, and they may be ill-prepared to take on responsibility for SM. 39,42,64,79 The situation is often further complicated by the fact that they may take on SM-related tasks only once there is a crisis or a failure to adhere to medication. 42 The need for appropriate support for carers is highlighted by studies that found that problematic medication management practices may persist despite the involvement of a family carer. 79,107 For example, a systematic review looking at challenges to optimal medicine use in people living with dementia found that family carers may adopt strategies that are not always safe and effective, and that it was difficult for them to make decisions about when to withhold or to give medicines. 79 A tailored problem-solving intervention to maximise medication management practices among caregivers of people with memory loss found no significant difference between the intervention and control groups, although both groups showed a significant reduction in the number of medication management problems. 107 The authors suggested that the lack of difference between the groups is because the caregivers in the control group participated in a face-to-face baseline assessment by a study nurse or social worker, which included questions about their knowledge of medications and approaches to managing them, medication reconciliation and medication-related resources.
Interventions aimed at supporting people with dementia to manage their diabetes should take into account the education and support needs of family carers as well as those of the person living with dementia,39,64,79 support that needs to include the issues arising from both conditions and the impact that dementia is likely to have on diabetes management. 39 We found no studies evaluating structured interventions to provide education and support to family carers of people with dementia and diabetes. Although stakeholders did talk about involving family members in education, they also recognised that this was not done in a structured or comprehensive way:
. . . I think we could probably do a lot more . . . supporting families and carers and to give them the confidence, I think they’re so worried, it can be so . . . frightening . . . to have both conditions . . .
Diab 13
. . . patients are educated one to one or through diabetes-structured education, again I’ve never heard of a patient education for carers and those with dementia to support them . . .
Diab 11
A number of studies linked support and education for carers to an improvement in SM. For example, qualitative studies looking at SM for people living with dementia115 and for people with diabetes (not dementia)69 argued that it is important to involve carers in the development of SM skills alongside the person they care for. This was also highlighted in the stakeholder interviews. For example:
We need to sort of normalise the situation where it is completely normal and expected that close family members will be involved in any decisions and there will be partnership.
Researcher: SM of long-term conditions
Context–mechanism–outcome 5 summary
Self-management for people with dementia and diabetes needs to be conceptualised as an activity that frequently involves not just the person with dementia but also their family members. Interventions need to take into account the needs and capabilities of family carers and the anxieties associated with managing medication and diet and preventing adverse events such as hypoglycaemic attacks. Including the family carers of people with dementia and diabetes should be the default option, and they should be included early, when the people living with dementia still have the capacity to make decisions and before SM breaks down.
Context–mechanism–outcome 6: usability of assistive technology
Programme theory
As the dementia trajectory progresses, assistive technology (AT) needs to be tailored and adapted to the needs and requirements of people living with dementia and diabetes and their families (includes social, environmental and cultural needs) with the focus on maintaining autonomy for the people living with dementia and diabetes (C), leading to people living with dementia and diabetes and their families gaining an understanding (awareness) of the usefulness of AT in their management of dementia (M) and to a more effective and sustained use of AT to maintain autonomy and diabetes SM strategies (O).
The definition of AT used for this CMO is ‘any product or service designed to enable independence for disabled and older people’. 161 This broad definition has the merit of including both high- and low-technology devices, ranging from objects that may not be considered ‘technological’, such as notice boards and calendar clocks, to devices that are clearly technology based, such as GPS (Global Positioning System) locators, blood-glucose monitors, reminders for medical management, and the internet. 135 Gibson et al. 91 further subdivided AT into telecare and telemedicine. Telecare involves remotely monitoring people in their own homes and communicating with them at a distance via telephone and the internet, while telemedicine is technology-supported medical or nursing tasks that assess biometrics sent from the patient and instructions returned to the patient from their HCP. The use of AT in health and social care is seen as an important element in enabling older people to live independently, and is part of the Department of Health’s Prime Minister’s Challenge on Dementia. 162 Despite this, a review of the use of AT for people living with dementia suggested that the technology industry has limited awareness of the needs of people living with dementia and their carers, and needs to view the ‘dementia market’ as an attractive option. 105
We included 17 papers39,59,61,63,68,90,91,93,97,99,101,105,112,120,127,133,135 (five of which were reviews) in this section. Two39,59 relate to people with dementia and diabetes, seven61,90,91,93,101,105,127 relate to the use of AT by people with dementia, four97,120,133,135 concern the use of AT by older people and four63,68,99,112 involve people with diabetes (not dementia). There is clearly a large body of literature relating to the use of AT by people with diabetes (in particular telehealth and telecare), but most of this was beyond the remit of this review. We found only one study59 that looked at the use of AT to help manage diabetes in people with cognitive impairment, but there is a lot of evidence on the use of technology by people living with dementia. 91,97,120,135 More details of the included studies can be seen in Table 6.
| Study (author, year) | Study type | Condition | Focus | ||
|---|---|---|---|---|---|
| Dementia | Diabetes | Other | |||
| Aikens et al., 201563 | Before-and-after study | ✗ | Investigating potential benefits to medication adherence of integrating a patient-selected support person into an automated diabetes telemonitoring and SM programme | ||
| Boots et al., 201661 | Qualitative | ✗ | Development and evaluation of web-based SM program for family caregivers of people with early-stage dementia | ||
| Bunn et al., 201639 | Mixed methods | ✗ | ✗ | People living with dementia and carer experiences of service delivery, but refers to use of AT | |
| Camp et al., 201559 | Controlled study | ✗ | ✗ | Evaluating a distance-based education intervention for people with both diabetes and CI | |
| Fleming and Sum, 201490 | SR | ✗ | Assessing the empirical support for the use of AT in the care of people living with dementia | ||
| Gibson et al., 201591 | Qualitative | ✗ | Exploring the everyday use of AT by people with dementia and their families | ||
| Gillespie et al., 201293 | SR | ✗ | Examining the relationship between AT for cognition and cognitive function | ||
| Greenhalgh et al., 201397 (links to Procter et al.120) | Qualitative | ✗ (older people) | Defining quality in telehealth and telecare, with the aim of improving the proportion of patients who receive appropriate, acceptable and workable technologies and services | ||
| Hsu et al., 201699 | RCT | ✗ | Cloud-based diabetes management programme for insulin initiation and titration | ||
| Jekel et al., 2015101 | SR | ✗ (CI) | Focuses on performance of patients with MCI in specific IADL (sub)domains but refers to problems with use of technology | ||
| Knapp et al., 2015105 | Rapid review | ✗ | Cost–benefit analysis to consider the hypothesis that accelerated investment in technology could, over a series of different time frames, deliver savings on the overall cost of care | ||
| Mayberry et al., 201168 | Mixed methods | ✗ | Exploring the role of patient health literacy, numeracy and computer literacy on the usage of health IT | ||
| Mayberry et al., 2016112 | Qualitative | ✗ | Developing and testing a telephone coaching system to improve self-care for people with T2DM from low-SES groups | ||
| Procter et al., 2014120 | Qualitative | ✗ (older people) | Exploring the experiences of older people living with assisted-living technologies and care services | ||
| Span et al., 2013127 | SR | ✗ | Gaining insight into the involvement of people with dementia in developing supportive IT applications | ||
| Wherton et al., 2012133 | Qualitative | ✗ (older people) | Understanding the assisted living needs of older people in domestic settings and methods to support their involvement in the coproduction of assisted living technologies | ||
Assistive technology to maintain autonomy
For people living with dementia and diabetes, being able to remain in their own home and community is very important to their quality of life. AT that can include the monitoring of biometrics, provide reminders for medicine management and use sensors and alarms to track movement is seen as one way of maintaining autonomy:135
. . . there’s electronic dosette boxes . . . linked to telecare, so if the person doesn’t take the medication, telecare will come through the intercom and say, ‘Mr so-and-so, you need to take your tablets’, and then if they don’t . . . [it] locks anyway so they can’t overdose.
Dem 2
Simple technology, such as dosette boxes, can also be used to maintain independence, as this stakeholder suggests:
I was familiar with the sort of dosette box, if that’s what you’re talking about, which is a great idea and does really help people.
Dem 1
However, such strategies become less successful as the dementia progresses,142 and even people with mild cognitive impairment are likely to have problems with the everyday use of technology, such as telephone and television, that limit their independence and autonomy. 101 These problems are also likely to apply to technology for the management of diabetes, as this stakeholder points out:
But, I mean some of the insulin pens are really fiddly as well, like they’ve got really tiny numbers and you have to dial it up and all that kind of thing, I don’t know how well they’re adapted for people with visual problems or cognitive problems.
Dem 6
A systematic review90 of AT for people living with dementia (n = 41 studies) found that there is currently little evidence to suggest that AT has an impact on the independence, safety or security of people living with dementia. There was some evidence to support the use of AT to facilitate communication and provide access to support and information for carers. The authors say that the research is characterised by the poor performance of the technology, small samples and high drop-out rates, and that some AT (e.g. that to improve safety and security) may not be acceptable to users. Furthermore, they suggest that ‘there is quite a short span of time during which the person with dementia is able to use the technology’. 90
Tailoring assistive technology to the needs of people living with dementia
In the UK, AT for people living with dementia is provided by a variety of sources, including health and social care services and the private sector and AT that has been adapted by users for their own particular needs. Qualitative research suggests that the bulk of provision involves the direct purchase of ‘off-the-shelf’ technology, which, if not specific to user needs, is adapted by family carers. 91,97 A review of AT for people living with dementia found that family carers were most likely to use technology that is not specific to caring or to dementia, such as tablets, baby monitors, smart phones and light sensors. 105 Health and social care services appear to provide inadequate support because they have limited provision of appropriate technology and/or they do not have the requisite knowledge base. 91,118 Moreover, provision may be variable:
In one local authority, we went to one recently, a dementia kind of carers group and sat with them and showed them a list from somewhere else and some of the devices on that weren’t on theirs, you know? It’s not equitable . . .
Res 2
A number of commentators argue that it is important to involve people living with dementia in the development of supportive technology. A systematic review127 examining the involvement of people living with dementia in developing information technology (IT) applications found that people living with dementia wanted to, and could, contribute to IT design. The authors argued that the involvement of people living with dementia and their family carers can help to ensure the development of appropriate AT that is ‘fit for purpose’ and that it can also enhance feelings of empowerment in people living with dementia. 127 However, even when included in the design of AT, people living with dementia are likely to need ongoing support. In an ethnographic study133 looking at methods to support the involvement of older people in the coproduction of AT, participants suggested that they would need ongoing support and assistance once the technology had been provided and that family members would need to be involved throughout the provision process. Coproduction involves the sharing of power between professionals and members of the public, working together in equal partnership. 163
Support in the use of assistive technology
One of the ways in which AT needs to be tailored to people living with dementia and diabetes is to ensure that interventions include appropriate support, for example from a family carer or a health or social care professional:
. . . technologies are great but you need to think is it the right thing for the patient . . . is there enough support around it to implement it and respond to it . . .
Res 2
The role of support in the use of AT, most often from family members, was a common theme. 68,90,91,112,120,133,142 For example, a mixed-methods study68 on older people with diabetes and a qualitative study91 on people living with dementia highlight the importance of family carer assistance in the use of AT. Family carers were found to invest a substantial amount of effort in embedding AT to help their relatives to maintain independence and reduce potential risks, which in turn minimises carer anxiety. 91 Family support was also thought to increase motivation to use, and participation in the use of, automated SM support. 63 However, a feasibility study of diabetes self-care support suggested that family ties are complex and family involvement in SM is not always helpful. 112
Technology in itself is unlikely to solve the problem of independent living for older people,97 particularly those living with dementia. 105 Knapp et al. 105 write that ‘successful interventions need to recognise the high value that many PLWD [people living with dementia] and their carers place on face-to-face service contacts . . . technologies that seek to reduce these contacts are unlikely to be acceptable or used (or, if they are, could exacerbate problems associated with social isolation and loneliness)’. AT, such as telehealth or telecare, appears to be most effective when technology augmented or involved face-to-face contact. 59,90 For example, a controlled study59 evaluated the impact of a tailored educational intervention, delivered via Skype on an iPad (Apple Inc., Cupertino, CA, USA), for older people with both type 2 diabetes and mild cognitive impairment. There was an initial decrease in HbA1C levels post intervention but this was not sustained at 6 months. The authors account for this through the fact that contact with the HCPs ended, and that their input had been instrumental in sustaining participant adherence. Participants in this study had few problems using their iPads and wanted to continue using them after the trial to maintain social networking, retrieve information and communicate via e-mail. The authors suggest that using such AT with trained volunteers over the longer term may enable people living with dementia and diabetes to receive effective and efficient medication management. However, it should be noted that participants were paid to take part in the study and were given the iPads (neither of which is likely to be possible outside a research setting).
The importance of contact with a HCP was supported by a qualitative study61 exploring the use of a SM programme for carers of people living with dementia; participants preferred blended care (face-to-face and online modules) as they valued personal contact with a professional. As one participant said, ‘people experience emotions, whilst a computer is just an object’. 61 The assumption was that personal contact could increase motivation and adherence, although the mechanism for achieving this was not identified in the paper. This is supported by an evaluation of web-based appointments for people with diabetes in Newham, East London. Older people were less likely to have broadband at home and only 11% of those aged 70–79 years agreed to participate in Skype consultations, compared with 82% of those aged < 50 years. 164 In addition, a RCT of a cloud-based diabetes management programme aimed at improving SM (not dementia) suggested that connectivity with a coach helped people to feel less anxious and more motivated. 99
Context–mechanism–outcome 6 summary
Evidence suggests that, in order to make AT usable for people living with dementia and diabetes and their families, AT needs to be focused on the needs of the user (e.g. maintaining autonomy), involve people living with dementia and their carers in its development and include family carers in installation and training. Telehealth and telecare should be designed to involve (as the default option) care partners such as other members in the family network. Appropriate support in the form of face-to-face contact appears to be an important contextual factor that may lead to improved motivation and adherence. Service providers need to recognise the high value that people living with dementia and their family carers place on face-to-face contacts.
Conclusions
The CMO configurations outlined in this chapter require changes in individual or organisational behaviour or understanding, and in many cases both. For example, CMO 3 – which focuses on skills development – requires the development of skills at an individual level but also organisational changes which legitimise the importance of those skills and allows the time for them to be acquired and practised. The outcomes we specified in the protocol for this synthesis included a number of clinical outcomes, such as the prevention of hypoglycaemia, the management of cardiovascular risk factors and the identification and management of long-term complications such as neuropathy. 1 However, the outcomes that emerged from the evidence available are primarily experiential rather than clinical, focusing on the need to trigger mechanisms such as trust, confidence and empowerment. A general metamechanism that emerges is that there is some form of synergy between an intervention strategy, disease progression and social and environmental factors (in particular the involvement of family members). A flexible service model for people with dementia and diabetes would enable this synergy in a way that would lead to the improved management of diabetes in people living with dementia. These ideas are explored further in Chapter 4.
Chapter 4 Discussion
The overall aims of this study were to identify key features or mechanisms of programmes and approaches that aim to improve the management of diabetes in people with dementia, to understand how those mechanisms operate in different contexts to achieve particular outcomes for this population and to identify the areas needing further research. We used an iterative four-stage approach that optimised the knowledge and networks of the research team and that was guided by the RAMESES criteria for realist review. 47 In this chapter we start by giving an overview of the findings and their implications; we then go on to discuss the limitations of the study, and finish by outlining our suggestions for practice and future research.
Summary of findings
We included 89 papers, 10 of which focused directly on our target group of people living with dementia and diabetes. The majority of the evidence related to people with dementia or diabetes or other long-term conditions and was included because of the opportunities it provided for transferable learning. Our review has resulted in an explanatory account of how interventions might work to improve the management of diabetes in people living with dementia. We have generated six CMO configurations that explain the importance of:
-
embedding positive attitudes towards people living with dementia
-
person-centred approaches to care planning
-
developing skills to provide tailored and flexible care
-
regular contact
-
family engagement
-
usability of AT.
These CMO configurations are summarised in Figure 7 and are grounded in evidence from the literature and stakeholder perspectives. Although designed to be specific to people with dementia, the configurations are also likely to be transferable to other groups who experience problems with diabetes management, for example older people with complex health and social care needs.
FIGURE 7.
Summary of CMO configurations. PLWD, people living with dementia.
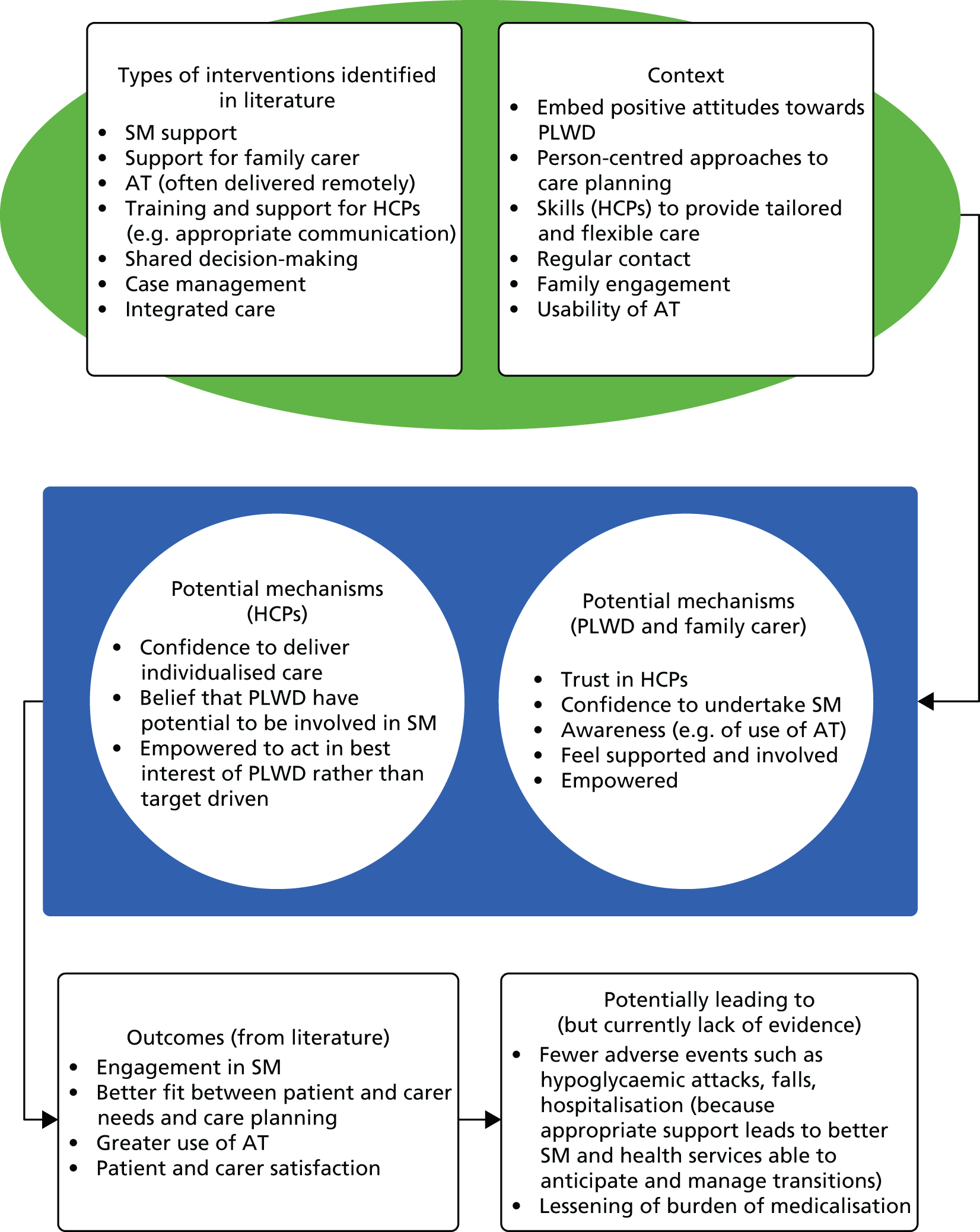
Each CMO configuration involves a contingent change in individual or organisational behaviour or understanding, and the programmes draw on a range of theoretical traditions and perspectives, some of which come through in the analysis more strongly than others. Some key mid-range theories that relate to the CMO configurations identified in the synthesis are summarised in Table 7. These mid-range, more abstract theoretical perspectives provide a further resource that can be drawn on by people designing and delivering interventions to manage diabetes in people living with dementia.
| CMO configuration | Illustrative (mid-range) theories | Explanation |
|---|---|---|
| Embedding positive attitudes towards people living with dementia | Enablement148 | Enablement theory emphasis what the person living with dementia can do rather than what they cannot. In the early stages of dementia the focus may be on functionality and personal choice (e.g. involvement in SM) and then, as the dementia progresses, the focus shifts towards maintaining dignity148 |
| Person-centred approaches to care planning | Agency and personhood38 | A person living with dementia should continue to be recognised and respected as an individual and, when applicable, as one member of a trusting relationship or relationships.38 Supportive person-centred caregiving from HCPs who know the individual’s biography and focus on their assets rather than their losses will lead to resources being used to support people living with dementia in managing their diabetes |
| Developing skills to provide tailored and flexible care | RCC165 | To practise enablement with people living with dementia and diabetes, HCPs need to acquire skills related to RCC.165 RCC is the process by which the HCP achieves patient-centred care through the relationships that they build over time with the patient, family and other professional colleagues.166 RCC theory has three aspects:
|
| Regular contact | Continuity of care | This refers to how contact, co-ordination of care, information and shared decision-making is achieved and sustained over time between patients and practitioners. Continuity of care is particularly important for those with complex health needs, such as people with dementia and diabetes167 Continuity has moved to a partnership paradigm in which continuity of care is recognised to be constructed by patients, families and professionals, all of whom have a part to play in its accomplishment168,169 |
| Family engagement | Therapeutic quadrangle154,170 | The therapeutic quadrangle considers not just the interconnectedness of the patient, main family carer and HCPs in a ‘triangle of care’ model, but also the type of chronic disease(s) that the patient suffers from. Within this model, the interaction between the HCPs, patient and family will vary according to the level of ‘technological’ care required at any given time through the disease trajectory. In people living with dementia and diabetes, there are two conditions that have different characteristics, necessitating a flexible approach to the management of care between family carer and HCP, taking into account the preferences of the person living with dementia and diabetes. The HCP needs to be alert to the progression of dementia with its impact on diabetes and vice versa. In addition, the HCP needs to consider how treatment regimens might increase the burden of care on patient and family, leading to the unintended consequence of people disengaging from SM practices because they are too difficult or because they feel overwhelmed |
| Usability of AT | Coproduction97 | Coproduction, and ‘ageing in place’, positions the person living with dementia at the centre of the design process for health technologies. It is a social process involving family and HCPs and the customisation of technology for individual use97 |
| Activity theory171 | Activity theory is a framework for understanding human–computer interaction.171 Consciousness is located in everyday actions and embedded in social interactions between people and artefacts. These may be physical, such as a smart phone, or phenomena, such as human language. Understanding the way in which people living with dementia and diabetes, carers, HCPs and artefacts interact in everyday activity provides a basis for achieving useable AT.172 As a consequence, people living with dementia are enabled to use AT through a ‘scaffolding’ process as others (carers and HCPs) adjust their help and support to match the abilities of the people living with dementia |
Explanatory framework
An overarching metamechanism emerges from the data. This relates to the convergence between an intervention strategy, disease progression and social and environmental factors. Although this framework (Figure 8) takes into account the trajectory of both dementia and diabetes, the dementia trajectory dominates. This is because the progressive nature of most dementia means that people living with dementia, and their family members, have to adapt continually to new issues or limitations,75,154 which may include adaptations in the way in which diabetes is managed. Environmental factors include physical, cultural and economic elements, the most important of which is the availability of support from informal carers, particularly family members. 173
FIGURE 8.
Diagrammatic representation of explanatory framework.
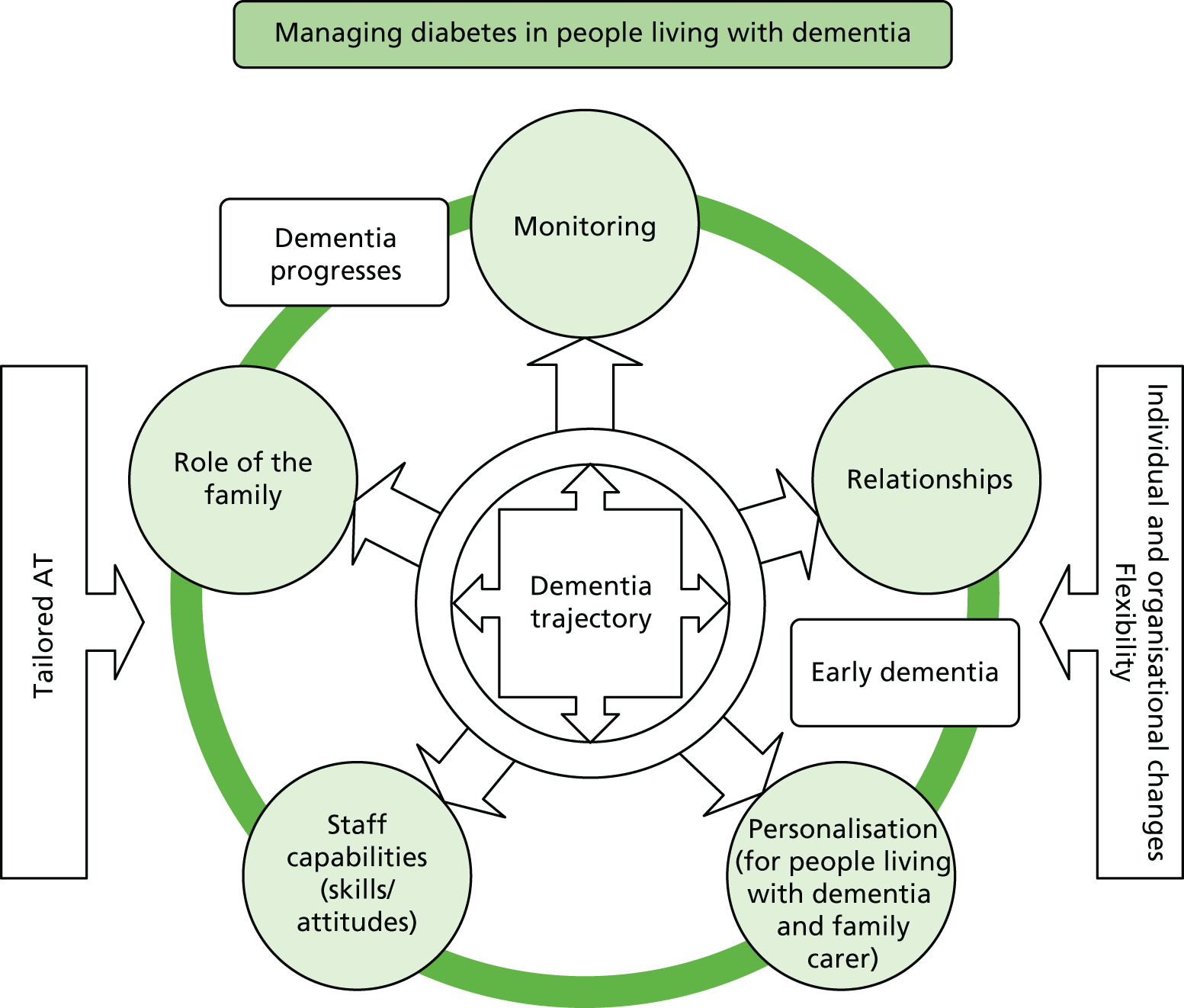
The framework in Figure 8 represents a ‘dial’, whereby the arrows turn in relation to the dementia trajectory. In the early stages, when people living with dementia and diabetes can still retain some functionality to make decisions about their diabetes management, ‘personalisation’ and ‘relationship-building’ are key components of care that involve the person living with dementia and diabetes, their family carer and their HCPs. This requires a reorientation of staff capabilities towards a more patient-centred care perspective, prioritising people living with dementia and diabetes and family perspectives over biomedical directives. As the dementia trajectory moves towards increased cognitive difficulties, and independent functioning becomes more problematic for the person living with dementia and diabetes, the dial moves towards greater ‘monitoring’ by the HCP and family carer as risks of poorer memory or behaviours have an impact on diabetes SM. This risk-management perspective may increasingly use technology as a way of maintaining diabetes SM. The relationship and personalisation perspectives are still essential but are adapted through the trajectory, requiring HCPs to anticipate the needs of people living with dementia and diabetes and their family carers by ‘gearing up’ interventions during periods of cognitive decline and, when functionality has stabilised, ‘gearing-down’ support, thus maintaining a personalised management approach to the uneven cognitive decline.
Context
The CMOs highlight a number of contextual factors that are likely to impact on the success, or otherwise, of interventions or programmes to manage diabetes in people with dementia. These would include the following.
-
The broader organisational strategy and goals: to what extent do systems legitimise and prioritise SM support and person-centred approaches for people with complex needs? Do current pathways meet the needs of people with dementia and diabetes? Evidence from this review suggests that significant changes are necessary at an organisational level.
-
The specific requirements of the workforce: ordered so that they can provide individualised care to people with dementia and diabetes. This may relate to individual development needs (e.g. necessary knowledge of dementia and diabetes, appropriate communication skills) or to organisational or team issues (e.g. appropriate skill mix, interprofessional working).
-
Service organisation and delivery: the extent to which services are organised to engage with and support family carers, provide continuity of care and anticipate changing needs (e.g. through regular contact/monitoring).
Mechanisms
A realist approach argues that exposing the resources and reasoning within mechanisms and their relationship to the context of their implementation is key to the evaluation of a complex programme of change. 174 Our review suggests that the design and delivery of programmes or services for people living with dementia and diabetes will have a greater chance of ‘working’ (i.e. reducing feelings of powerlessness, increasing independence and, potentially, improving glycaemic control) by paying attention to those activities, or contexts, that engender the following mechanisms.
-
Assets: stigma and negative assumptions about the capabilities of people living with dementia may adversely affect their access to appropriate care. Systems need to foster the expectation in individuals (patients and professionals) that people with dementia have the right to receive diabetes-related services and that they (and their family carers) should be involved in the management of their condition.
-
Inclusion/engagement: family carers need to feel valued and supported. Support may come through one-to-one contact with professionals or from peers via group-based activities. Systems need routinely to promote the involvement of family carers (e.g. information-sharing with family carers is the default option).
-
Confidence of HCPs: HCPs need to feel confident that they are acting in the best interests of the people living with dementia and diabetes and that they are equipped to meet the patient’s needs. For example, to change treatment goals or simplify medication regimens, they need to feel that it is the ‘right’ thing to do. They also need to feel confident that the system supports them to deliver personalised care.
-
Confidence/empowerment of patients and carers: people living with dementia and diabetes and their family carers need to feel confident in the treatment/care they are receiving and in their own abilities to undertake SM-related tasks. Resources to create confidence in maintaining SM practices in the early stages of the dementia trajectory might include diabetes-related education, programmes to teach compensatory strategies to cope with memory changes, continuity of contact with specialist staff (in both dementia and diabetes) and enablement-focused activities.
-
Trust: the evidence suggests that relationship continuity175 (e.g. a continuous therapeutic relationship with one or more HCPs over time) is an important resource for triggering trust and the related mechanism of confidence. Face-to-face contact is important in facilitating trust and encouraging the person to participate in their care.
-
Resonance: programmes and initiatives need to be cognitively, emotionally and physically relevant and meaningful to people living with dementia and their family carers. For example, AT for people living with dementia needs to focus on promoting independence and care plans need to ‘fit’ with the needs and priorities of patients and their family carers.
Mechanisms are dynamic and may be interacting with each other;176 for example, in our programme theory, trust is likely to operate in parallel to confidence. In addition, what is seen as a mechanism or outcome in one CMO, such as a belief that people living with dementia have the right to diabetes care, may, in turn, become a context in a subsequent CMO. 177
Implications of the findings
Engaging with family carers
The need to engage with, and support, family carers of people living with dementia and diabetes is an overarching theme throughout the CMOs. This review highlights the way in which the emotional support and practical assistance provided by families is key in the management of long-term conditions and of particular importance for those with complex needs such as dementia and diabetes. Despite this, carers often feel undervalued or ill-prepared to take on caring responsibilities. A survey of carers of older people with diabetes found that 40% of family carers had never received any information about diabetes from professionals. 41 Many of the support needs of family carers identified in this review are not specific to people caring for a family member with dementia and diabetes; they could equally apply to those caring for people with dementia and other comorbidities. However, there are clearly some concerns that are particularly pertinent to family members of people living with dementia and diabetes. Although many carers may find medication management stressful, this is often exacerbated for family carers of people with dementia and diabetes owing to their concerns about the prevention of adverse events associated with either hypoglycaemia or hyperglycaemia. 39,64,142
To generate carers’ confidence in their ability to undertake diabetes-related care, we argue that they are likely to need education combined with ongoing support from a specialist in diabetes about timing of medication, diet, how to recognise the common signs and symptoms of hypoglycaemia and how to distinguish between symptoms of dementia and diabetes. 28 They may also have needs related to the dementia, such as how to manage behaviours that challenge. Support needs to be individualised and adapted over time to reflect the changing needs of the person living with dementia and diabetes–carer dyad, and to create opportunities to review priorities.
How best to support carers of people living with dementia and diabetes is unclear. Caregivers often report high level of satisfaction with interventions178 but the evidence of effectiveness for most interventions is weak. 157 The lack of effectiveness may be due, in part, to methodological problems with the outcomes used in these studies. Although depressive symptoms are one of the most widely used outcomes in caregiver intervention studies, not all carers will have depressive symptoms. 179 Zarit and Femia179 argue that caregiving should not be viewed as if it were a psychiatric disorder like major depression. In addition, they argue that there is heterogeneity in caregivers’ experiences of a relative’s dementia and not all will react to stressors and resources in the same way. Interventions need to be adaptive or tailored,180 but for interventions to be tailored appropriately we need to better understand what caregivers want. 181
Minimally disruptive medicine
The complex needs of people with dementia and diabetes require new ways of looking at how we organise and deliver care for this patient group. Minimally disruptive medicine is focused on achieving patient goals for life and health while imposing the smallest possible burden on patients’ lives. 182,183 It is an approach that recognises that people living with multiple chronic conditions are likely to be overwhelmed by the work involved in being a patient183 and suggests that there is a need to move away from targets to practices that foster trust and care. For someone with dementia and diabetes, rather than intensifying treatment in the face of poor outcomes, it may be that clinicians needs to focus on ‘can you really do what I’m asking you to do?’. 184 Minimally disruptive medicine for someone with dementia and diabetes might involve changing or simplifying medication regimens, for example by reducing the total number of tablets prescribed and/or giving tablets once per day if possible. 28 Fostering trust, for example through a continuous therapeutic relationship between a professional and the patient–carer dyad, is likely to make this easier. 185
People living with dementia without family carers or support
There is very little evidence on which to base any recommendations for practice for people living with dementia and diabetes who live alone and have very limited networks of support from family or friends. The focus on the role of the carer highlighted in this work is clearly very significant, not just because of what it indicates about the support needs of family carers but also because of the implications for service provision for people who do not have family support. Research suggests that people with dementia who live alone are at an increased risk of having unmet social, environmental, psychological and medical needs. 39,186 Our CMO on person-centred approaches to care planning identified the generation of trust between the HCPs and the people living with dementia as necessary to improve care planning and SM. Building trusting relationships with HCPs may be particularly important for those who live alone as they do not have a family member to facilitate access and continuity of care39 and are likely to be more dependent on HCPs to perform this role. 187 However, a qualitative study188 reported that people living with dementia who lived alone found it difficult to trust others and admit to their mistakes or challenges, because they feared being placed in long-term care.
Tailoring and person-centred care
Running through our programme theory are ideas about person-centred care, tailoring and individualised care. These are identified as resources necessary to trigger mechanisms such as trust, empowerment and a belief that SM is achievable and worthwhile. These ideas are not new189 and have been applied to both those with dementia38 and those with diabetes. 190 Guidance for both conditions recommends person-centred care. 30,191,192 What this synthesis does is begin to identify what some of the key components of person-centred care or tailoring would be and what changes this might entail in individual practice and organisational approaches. For example, the instigation of individualised (possibly simplified) diabetes regimens requires that the person living with dementia and diabetes sees an appropriate specialist(s) or that generalists such as GPs and practice nurses have appropriate knowledge about how to tailor diabetes care. It also relies on HCPs knowing that such actions are legitimised, for example through less focus on biomedical targets and time allocation to establish an understanding of the individual’s story and priorities. This is, inevitably, linked to continuity and having a practitioner who both understands the dementia trajectory and can respond as care needs alter. 39
Collaboration and communication
Poor communication and collaboration between different specialties have been identified as significant barriers to continuity of, and access to, care for people with dementia and comorbidities such as diabetes. 39,142,193 This also emerged very strongly from our stakeholder interviews. Many of our stakeholders talked about the problems caused by poor communication between disciplines, an inability to share records across different sectors and specialties, and a lack of understanding of each other’s roles and responsibilities. We considered developing this theme as one of our CMOs. However, although this issue was clearly of great importance, we felt that it was generic rather than specific to dementia and diabetes and, as such, that it fell outside the remit of this review. Nevertheless, future initiatives for people with dementia and diabetes will need to consider how to improve communication and collaboration between individual health and care professionals and between organisations; perhaps through the co-location of different specialties. This will include the need for better documentation and communication of decisions around treatment burden and risk (e.g. decisions to deprescribe).
Care pathways
One of the questions that arose throughout the review was the extent to which ‘good care’ for people with dementia and diabetes required specific diabetes interventions and how much related more generally to interventions for the management of older people with complex health and social care needs. A UK study194 found that, on average, people living with dementia had 4.6 chronic illnesses in addition to their dementia. Therefore, people with dementia and diabetes are likely to have other conditions, such as hypertension or arthritis,195 that may further complicate management and create clinical uncertainty.
A Cochrane review looked at managing multimorbidity in primary and community care settings. 196 The review, which includes 18 studies, found some evidence to suggest that interventions targeted at specific risk factor management (e.g. the management of vascular risk factors and depression in people with comorbid vascular disease and depression) or focused on areas where people have difficulties, such as with functional ability or medicines management, were effective. Initiatives also need to be integrated into health-care systems. 196 Our review would add to this by suggesting that building confidence in medicines management and promoting functional ability in people living with dementia and their carers is reliant on multiple contexts that allow clinicians to base assessment on familiarity with the patient’s (and carer’s) story, priorities and needs. Longer consultations are needed for patients with the ‘cumulative complexity’ of multiple chronic conditions and for those less able to articulate their priorities and needs,197 both of which apply to people with dementia and diabetes.
Strengths and limitations
One of the main limitations of this synthesis was the lack of evidence relating to the management of diabetes in people living with dementia. The qualitative and observational evidence detailed some of the individual and organisational challenges involved in managing diabetes in people living with dementia, but there was a paucity of discussion about the underlying assumptions of the research or interventions tested for this group. However, in realist methodology the unit of analysis is the programme theory, or underpinning mechanism of action, rather than the intervention;50 as such, we were able to draw on a wider literature that provided opportunities for transferable learning. This enabled us to develop a theory-driven explanation, in the form of six CMO configurations that make up a programme theory, to inform the care of people living with dementia and diabetes. The programme theory that we have developed can be used to guide future initiatives and interventions.
The outcomes in our CMOs are largely experiential rather than clinical. This reflects the evidence available. Outcomes such as increased engagement in SM are potential surrogates for better clinical management of diabetes, but this is not proven. We included a number of clinical outcomes such as the prevention of hypoglycaemia, the management of cardiovascular risk factors and the identification and management of long-term complications such as neuropathy. Although these outcomes may be important, the literature suggests that key goals for this group are maintaining independence and creating treatment regimens that ‘fit’ with the needs and abilities of people living with dementia and their family carers. However, literature in this area is scarce, and further work is needed to identify what it is that people living with dementia and diabetes and their family carers want from interventions. 180
Much of the evidence we included related to either people living with dementia or people with diabetes, rather than people with both conditions. Inevitably, the aims, focuses and outcomes of these two sets of studies are very different. Moreover, because we drew on this larger literature, there were many more potentially relevant sources of information than we could possibly cover. However, the nature of realist synthesis means that there is not a finite set of relevant papers that can be found. Rather, the reviewer takes a more purposive approach to sampling,50 with the aim of reaching conceptual saturation rather than identifying an exhaustive set of studies. 74 In this review, conceptual saturation was reached in relation to findings about the need to address stigma, personalise care, increase patient and practitioner trust and confidence, and to identify what supports independence. Owing to the limited nature of the evidence, we felt that including further studies would not add anything new to the programme theory.
A realist review takes a particular position on how the quality of evidence is judged. 50,198 The traditional hierarchy of evidence is rejected in favour of an approach that prioritises the way in which studies contribute to the development of the programme theory. For example, ‘do the inferences made in a study gel with those from other studies?’. 198 A realist review is concerned with theoretical depth and transferability rather than with developing statements or recommendations that have statistical certainty about questions of effectiveness or cost-effectiveness. 50 In line with this approach, we assessed evidence on how it contributed to our theory development. We did not undertake formal quality assessment, but in our data extraction process we included questions that allowed us to assess the relevance and rigour of the evidence. This included an appraisal of whether or not the evidence linked to the theory areas, whether or not it provided valuable information, if it could be relied on and if it contributed to the review.
The use of a realist approach allowed us to develop a plausible programme theory about what works in the management of diabetes in people with dementia. However, the lack of directly relevant evidence means that the extent to which our CMOs could be tested using empirical studies was limited. In addition, in developing our CMOs we found it much easier to describe the context or resources that were needed than either the mechanisms or the outcomes. Most studies did not report the outcomes specified in our protocol and few were explicit about what they thought the mechanisms were that explained their study outcomes. However, the plausibility of our programme theory was refined through extensive consultation with experts in dementia and diabetes and with service user representatives. This included one-to-one interviews, a consensus meeting at the end of the study, workshops with the project team and consultation with the experts on our advisory committee.
Conclusions
Dementia and diabetes mellitus are common long-term conditions that coexist in a large number of older people. 2,3 The cognitive and physical consequences of dementia have an impact on the ability of people living with dementia to manage their diabetes and puts them at risk of complications such as hypoglycaemic episodes, cardiovascular conditions and amputations,4–6 which place a huge burden on health and social care economies. 7 Moreover, the impact of dementia and diabetes on patients and their families is considerable.
The priority for HCPs is how to accommodate the challenges of living with dementia as a long-term condition with the minimum requirements of good diabetic control, recognising that perceptions of ‘good’ are situation specific, differ for people living with dementia and diabetes and for family carers and will change over time. The dearth of literature on people living with dementia and diabetes and, as importantly, their supporters and family carers is mirrored in the wider literature. This review suggests that there is a need for further work to establish a shared understanding of what needs to be in place to engage effectively with people living with dementia including those with diabetes and their supporters to establish how ‘good support’ is operationalised and measured.
The role of family carers in managing the health-care conditions of people living with dementia, and their contribution in facilitating continuity of, and access to, care, are indisputable. 39 It is important, therefore, that HCPs conceptualise the provision of care for people with dementia and a comorbidity as a complex phenomenon that affects not just individuals but also dyads and families. 45 The challenges of being a carer have been exhaustively documented; legislation and staff training to ensure that carers are recognised and supported has been in place for some time. What is not so well understood is how to involve and support family carers at different stages of the trajectory for people with dementia and diabetes. 39
Although both hyperglycaemia and hypoglycaemia can have adverse effects for people living with dementia and diabetes, the prevention of hypoglycaemia seems to be particularly important. 25 Despite this, evidence suggests that older people with diabetes are often overtreated and given inappropriate medications. 25 Moreover, the ability of people living with dementia and diabetes and their family carers to cope with medication regimens is not taken into account. This review suggests that there is a need to prioritise quality of life, independence and patient and carer priorities over a more biomedical, target-driven approach. Much of the research included in this review, particularly that specific to people living with dementia and diabetes, identifies deficiencies and problems with current systems. Although we have highlighted the need for personalised care, continuity and family-centred approaches, there is much evidence to suggest this is not currently happening. Future research on the management of diabetes in older people with complex health needs, including those with dementia, needs to look at how organisational structures and workforce development can be better aligned to the needs of people living with dementia and diabetes.
Implications for practice
The following implications for practice have emerged from the review.
-
The evidence suggests that SM for people with dementia and diabetes needs to be conceptualised as an activity that frequently involves not just the person with dementia but also family members. Therefore, SM should include the identification of family carers, appropriate training in carer engagement for staff, and protocols regarding confidentiality and information sharing.
-
Self-management support needs to be seen by HCPs as a legitimate activity, and pathways should be adapted to enable the regular assessment of SM capabilities and to provide appropriate SM support for people living with dementia and diabetes and their family carers.
-
Family carers are likely to require diabetes-specific education and advice (e.g. about the appropriate timing of medication and access to food, how to recognise the common signs and symptoms of hypoglycaemia and how to distinguish between symptoms of dementia and those of diabetes).
-
Staff caring for people with diabetes need appropriate training on dementia and the impact that this might have on the management of diabetes. This applies to staff at all levels, including those more senior.
-
HCPs caring for people living with dementia and diabetes need education in enablement approaches to SM.
-
HCPs caring for people with dementia and diabetes need regularly to assess a patient’s ability to self-manage and to identify when they, or their family carer, may need additional support.
-
HCPs caring for people with dementia and diabetes may need training or guidance on how to incorporate ideas about deprescribing and minimally disruptive medicine (e.g. the management of uncertainty).
-
There is a need for better integration of physical and mental health-care systems, that is, old-age psychiatry teams and geriatric teams working together and community-based geriatric and frailty teams having specialist mental health staff as an integral part of the team.
-
People with dementia and diabetes who live alone, or who do not have family support, may be particularly disadvantaged and may need additional help and monitoring from HCPs and care staff.
-
People living with dementia and diabetes are likely to benefit from longer appointments in both primary and secondary care, and booking systems should allow for this.
-
People living with dementia and diabetes, particularly those who live alone, are likely to need regular (preferably face-to-face) contact with HCPs who are familiar with their needs and problems.
Suggestions for future research
A number of potential areas for future research were identified by the review. These are listed in order of priority and include the following.
-
What is the impact of SM interventions for people with dementia and diabetes that involve family carers?
-
What interventions can be used to improve medication management in people with dementia and diabetes and their family carers; for example, what is the impact of pharmacist-led interventions?
-
What sort of care pathway is most appropriate and effective? For example, a specific dementia and diabetes pathway or a pathway for older adults with complex needs (vulnerability pathway)?
-
What sort of support do family caregivers of people with dementia and diabetes want and how can interventions be designed to reflect this?
-
How can professionals caring for people with dementia and diabetes be helped to recognise when a person is no longer able to self-manage, or when there is a need to ‘gear-up’ or ‘gear-down’ support?
-
What are important outcomes and goals for people with dementia and diabetes and their family carers?
-
How can AT support SM for people with dementia and diabetes and how do their needs change as the dementia trajectory progresses?
-
What impact does the stage/extent of cognitive and physical impairment have on the uptake and outcomes of interventions?
-
Are interventions that take an assets-based approach to the care of people with dementia and diabetes (e.g. promoting confidence, empowerment and independence) more effective?
Acknowledgements
We would like to acknowledge the contribution and support of the PAG: Professor Steve Iliffe (chairperson), Mr Tim Beanland, Dr Simon Conroy, Professor Angus Forbes, Dr Charles Fox, Professor Yoon Loke, Dr Ana Manzano-Santaella, Dr Paul Millac, Mrs Diane Munday and Ms Debbie Stanisstreet.
We would like to thank Mrs Tina Gibbons for assistance with proofreading the report.
Contributions of authors
Professor Frances Bunn (Professor in Health and Complex Conditions) was the principal investigator, led the study, was involved in all aspects of the review process and is the lead author of this report.
Professor Claire Goodman (Professor in Health Services Research) was a coapplicant on the grant, was involved in study design, was involved in research team meetings and workshops, gave feedback between meetings and participated in the synthesis process and the preparation of the final report.
Mr Peter Reece Jones (Lecturer) took day-to-day responsibility for project management and for the review process, was involved in all aspects of the review process and participated in the preparation of the final report.
Ms Bridget Russell (Research Assistant) assisted with project management, was involved in all aspects of the review process and participated in the preparation of the final report.
Dr Daksha Trivedi (Senior Research Fellow in Evidence Based Practice) was a coapplicant on the grant, was involved in review processes, attended research team meetings and workshops, and participated in the preparation of the final report.
Professor Alan Sinclair (Director, Foundation for Diabetes Research in Older People) was a coapplicant on the grant, was involved in study design, attended workshops and meetings, provided clinical expertise and participated in the preparation of the final report.
Professor Antony Bayer (Professor of Geriatric Medicine) was a coapplicant on the grant, was involved in study design, attended workshops and meetings, provided clinical expertise and participated in the preparation of the final report.
Dr Greta Rait (Reader in Primary Care) was a coapplicant on the grant, was involved in study design, attended workshops and meetings, provided clinical expertise and participated in the preparation of the final report.
Professor Jo Rycroft-Malone (Pro-Vice Chancellor, Research & Impact, Bangor University) was a coapplicant on the grant, was involved in study design, provided methodological expertise and participated in the preparation of the final report.
Professor Chris Burton (Professor of Rehabilitation and Nursing Research) was a coapplicant on the grant, was involved in study design, provided methodological expertise, and participated in the synthesis process and preparation of the final report.
This work is supported by the NIHR HTA programme (project reference 13/138/03).
Publications
Bunn F, Goodman C, Malone JR, Jones PR, Burton C, Rait G, et al. Managing diabetes in people with dementia: protocol for a realist review. Syst Rev 2016;5:5.
Bunn F, Goodman C, Jones PR, Russell B, Trivedi D, Sinclair A, et al. What works for whom in the management of diabetes in people living with dementia: a realist review. BMC Med 2017;15:141.
Data sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Bunn F, Goodman C, Malone JR, Jones PR, Burton C, Rait G, et al. Managing diabetes in people with dementia: protocol for a realist review. Syst Rev 2016;5. https://doi.org/10.1186/s13643-015-0182-4.
- Bunn F, Burn AM, Goodman C, Rait G, Norton S, Robinson L, et al. Comorbidity and dementia: a scoping review of the literature. BMC Med 2014;12. https://doi.org/10.1186/s12916-014-0192-4.
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64-7. https://doi.org/10.1016/S1474-4422(05)70284-2.
- Feil DG, Zhu CW, Sultzer DL. The relationship between cognitive impairment and diabetes self-management in a population-based community sample of older adults with type 2 diabetes. J Behav Med 2012;35:190-9. https://doi.org/10.1007/s10865-011-9344-6.
- Hewitt J, Smeeth L, Chaturvedi N, Bulpitt CJ, Fletcher AE. Self management and patient understanding of diabetes in the older person. Diabet Med 2011;28:117-22. https://doi.org/10.1111/j.1464-5491.2010.03142.x.
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 2000;50:203-12. https://doi.org/10.1016/S0168-8227(00)00195-9.
- The Hidden Impact of Diabetes in Social Care. Bedfordshire: Institute of Diabetes for Older People, Novo Nordisk; 2013.
- Dowrick A, Southern A. Alzheimer’s Society – Dementia 2014: Opportunity for Change 2014. www.alzheimers.org.uk/downloads/download/1484/dementia_2014_opportunity_for_change (accessed 13 July 2016).
- Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Herrara M, et al. Dementia UK: Update Second Edition. London: Alzheimer’s Society; 2014.
- Fraser L, Karlsbery Schaffer S, Sussex J, O’Neil P, Cockcroft L. The Trajectory of Dementia in the UK – Making a Difference 2014. www.ohe.org/publications/trajectory-dementia-uk-making-difference (accessed 22 November 2016).
- Diabetes UK . Facts and Stats 2015. www.diabetes.org.uk/About_us/What-we-say/Statistics/ (accessed 13 July 2016).
- Quality and Outcomes Framework Achievement Data. Leeds: NHS Digital; 2013.
- World Alzheimer Report 2010. The Global Economic Impact of Dementia. London: Alzheimer’s Disease International; 2010.
- NHS Digital . National Diabetes Audit Executive Summary 2009–2010 2011. papers2://publication/uuid/779E10F3-176B-4C37-9C69-032FD2E17128%5Cnhttp://www.hscic.gov.uk/catalogue/PUB02573/nati-diab-audi-09-10-exec-summ.pdf (accessed 13 July 2016).
- Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 2012;42:484-91. https://doi.org/10.1111/j.1445-5994.2012.02758.x.
- Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLOS ONE 2009;4. https://doi.org/10.1371/journal.pone.0004144.
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease – is this type 3 diabetes?. J Alzheimers Dis 2005;7:63-80. https://doi.org/10.3233/JAD-2005-7107.
- Bloomfield HE, Greer N, Newman D, MacDonald R, Carlyle M, Fitzgerald P, et al. Predictors and Consequences of Severe Hypoglycemia in Adults with Diabetes – A Systematic Review of the Evidence. Washington, DC: Department of Veterans Affairs; 2012.
- Punthakee Z, Miller ME, Launer LJ, Williamson JD, Lazar RM, Cukierman-Yaffee T, et al. Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care 2012;35:787-93. https://doi.org/10.2337/dc11-1855.
- Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in type 2 diabetes. Diabet Med 2008;25:245-54. https://doi.org/10.1111/j.1464-5491.2007.02341.x.
- Gates BJ, Walker KM. Physiological changes in older adults and their effect on diabetes treatment. Diabetes Spectr 2014;27:20-8. https://doi.org/10.2337/diaspect.27.1.20.
- Bremer JP, Jauch-Chara K, Hallschmid M, Schmid S, Schultes B. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care 2009;32:1513-17. https://doi.org/10.2337/dc09-0114.
- Laubscher T, Regier L, Bareham J. Diabetes in the frail elderly: individualization of glycemic management. Can Fam Physician 2012;58:543-6.
- Abdelhafiz AH, Rodríguez-Mañas L, Morley JE, Sinclair AJ. Hypoglycemia in older people - a less well recognized risk factor for frailty. Aging Dis 2015;6:156-67. https://doi.org/10.14336/AD.2014.0330.
- Abdelhafiz AH, McNicholas E, Sinclair AJ. Hypoglycemia, frailty and dementia in older people with diabetes: reciprocal relations and clinical implications. J Diabetes Complications 2016;30:1548-54. https://doi.org/10.1016/j.jdiacomp.2016.07.027.
- Sinclair AJ, Paolisso G, Castro M, Bourdel-Marchasson I, Gadsby R, Rodriguez Manas L. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab 2011;37:27-38. https://doi.org/10.1016/S1262-3636(11)70962-4.
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577-96. https://doi.org/10.1007/s00125-012-2534-0.
- Sinclair AJ, Hillson R, Bayer AJ. National Expert Working Group. Diabetes and dementia in older people: a best clinical practice statement by a multidisciplinary national expert working group. Diabet Med 2014;31:1024-31. https://doi.org/10.1111/dme.12467.
- American Diabetes Association . Older adults. Diabetes Care 2015;38:67-9. https://doi.org/10.2337/dc15-S013.
- Managing Older People with Type 2 Diabetes: Global Guideline. Brussels: IDF; 2013.
- Morley JE, Sinclair A. Individualising treatment for older people with diabetes. Lancet 2013;382:378-80. https://doi.org/10.1016/S0140-6736(13)61055-7.
- Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356-62. https://doi.org/10.1001/jamainternmed.2014.7345.
- Genere N, Sargis RM, Masi CM, Nathan AG, Quinn MT, Huang ES, et al. Physician perspectives on de-intensifying diabetes medications. Medicine (Baltimore) 2016;95. https://doi.org/10.1097/MD.0000000000005388.
- Ciechanowski P, Katon WJ. The interpersonal experience of health care through the eyes of patients with diabetes. Soc Sci Med 2006;63:3067-79. https://doi.org/10.1016/j.socscimed.2006.08.002.
- Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. J Diabetes Metab Disord 2013;12. https://doi.org/10.1186/2251-6581-12-14.
- Suhl E, Bonsignore P. Diabetes self-management education for older adults: general principles and practical application. Diabetes Spectr 2006;19:234-40. https://doi.org/10.2337/diaspect.19.4.234.
- Mountain G. Self-management for people with early dementia: an exploration of concepts and supporting evidence. Dementia 2006;5:429-46. https://doi.org/10.1177/1471301206067117.
- Kitwood T. Dementia Reconsidered: The Person Comes First. Buckingham: Open University Press; 1997.
- Bunn F, Burn A, Goodman C, Robinson L, Rait G, Norton S, et al. Comorbidity and dementia: a mixed method study on improving health care for people with dementia (CoDem). Health Serv Deliv Res 2016;4.
- Tomlin A, Sinclair A. The influence of cognition on self-management of type 2 diabetes in older people. Psychol Res Behav Manag 2016;9:7-20. https://doi.org/10.2147/PRBM.S36238.
- Sinclair AJ, Armes DG, Randhawa G, Bayer AJ. Caring for older adults with diabetes mellitus: characteristics of carers and their prime roles and responsibilities. Diabet Med 2010;27:1055-9. https://doi.org/10.1111/j.1464-5491.2010.03066.x.
- Feil DG, Pearman A, Victor T, Harwood D, Weinreb J, Kahle K, et al. The role of cognitive impairment and caregiver support in diabetes management of older outpatients. Int J Psychiatry Med 2009;39:199-214. https://doi.org/10.2190/PM.39.2.h.
- Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care 2006;29:725-31. https://doi.org/10.2337/diacare.29.03.06.dc05-2078.
- End of Life Care for People with Dementia: Commissioning Guide. London: National Institute for Health and Care Excellence; 2010.
- Ryan P, Sawin KJ. The individual and family self-management theory: background and perspectives on context, process, and outcomes. Nurs Outlook 2009;57:217-25.e6. https://doi.org/10.1016/j.outlook.2008.10.004.
- Diabetes Overview NICE Pathways. London: National Institute for Health and Care Excellence; 2014.
- Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. RAMESES publication standards: realist syntheses. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-21.
- Pawson R, Greenhalgh T, Harvey G, Walshe KR. Realist Synthesis: An Introduction. Manchester: University of Manchester; 2004.
- Rycroft-Malone J, McCormack B, Hutchinson AM, DeCorby K, Bucknall TK, Kent B, et al. Realist synthesis: illustrating the method for implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-33.
- Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review – a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10:21-34. https://doi.org/10.1258/1355819054308530.
- Pawson R. Evidence-based Policy: A Realist Perspective. London: Sage Publications; 2006.
- Pawson R, Greenhalgh J, Brennan C, Glidewell L. Do reviews of healthcare interventions teach us how to improve healthcare systems? The depth ontology of demand management. Soc Sci Med 2014;114:129-37. https://doi.org/10.1016/j.socscimed.2014.05.032.
- Shepperd S, Lewin S, Straus S, Clarke M, Eccles MP, Fitzpatrick R, et al. Can we systematically review studies that evaluate complex interventions?. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000086.
- Glass TA, McAtee MJ. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc Sci Med 2006;62:1650-71. https://doi.org/10.1016/j.socscimed.2005.08.044.
- Greenhalgh T, Clinch M, Afsar N, Choudhury Y, Sudra R, Campbell-Richards D, et al. Socio-cultural influences on the behaviour of South Asian women with diabetes in pregnancy: qualitative study using a multi-level theoretical approach. BMC Med 2015;13:1-15. https://doi.org/10.1186/s12916-015-0360-1.
- Pearson M, Brand SL, Quinn C, Shaw J, Maguire M, Michie S, et al. Using realist review to inform intervention development: methodological illustration and conceptual platform for collaborative care in offender mental health. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0321-2.
- Baxter MA. The new NHS and diabetes care. Br J Diabetes Vasc Dis 2014;14:87-94. https://doi.org/10.15277/bjdvd.2014.026.
- De Vriendt P, Peersman W, Florus A, Verbeke M, Van de Velde D. Improving health related quality of life and independence in community dwelling frail older adults through a client-centred and activity-oriented program. A pragmatic randomized controlled trial. J Nutr Heal Aging 2015;20:35-40. https://doi.org/10.1007/s12603-016-0673-6.
- Camp CJ, Fox K, Skrajner MJ, Antenucci V, Haberman J. Creating effective self-management for older adults with Type 2 Diabetes and cognitive impairment. Adv Aging Res 2015;4:33-41. https://doi.org/10.4236/aar.2015.42005.
- Kennedy A, Rogers A, Bowen R, Lee V, Blakeman T, Gardner C, et al. Implementing, embedding and integrating self-management support tools for people with long-term conditions in primary care nursing: a qualitative study. Int J Nurs Stud 2014;51:1103-13. https://doi.org/10.1016/j.ijnurstu.2013.11.008.
- Boots LM, de Vugt ME, Withagen HE, Kempen GI, Verhey FR. Development and initial evaluation of the web-based self-management program ‘Partner in Balance’ for family caregivers of people with early stage Dementia: an exploratory mixed-methods study. JMIR Res Protoc 2016;5. https://doi.org/10.2196/resprot.5142.
- Davis KK, Mintzer M, Dennison Himmelfarb CR, Hayat MJ, Rotman S, Allen J. Targeted intervention improves knowledge but not self-care or readmissions in heart failure patients with mild cognitive impairment. Eur J Heart Fail 2012;14:1041-9. https://doi.org/10.1093/eurjhf/hfs096.
- Aikens JE, Trivedi R, Aron DC, Piette JD. Integrating support persons into diabetes telemonitoring to improve self-management and medication adherence. J Gen Intern Med 2015;30:319-26. https://doi.org/10.1007/s11606-014-3101-9.
- Feil DG, Lukman R, Simon B, Walston A, Vickrey B. Impact of dementia on caring for patients’ diabetes. Aging Ment Health 2011;15:894-903. https://doi.org/10.1080/13607863.2011.569485.
- Graff MJL, Vernooij-Dassen MJM, Thijssen M, Dekker J, Hoefnagels WHL, Rikkert MGMO. Community based occupational therapy for patients with dementia and their care givers: randomised controlled trial. BMJ 2006;333. https://doi.org/10.1136/bmj.39001.688843.BE.
- Hackel JM. ‘Patient-centered care’ for complex patients with type 2 diabetes mellitus-analysis of two cases. Clin Med Insights Endocrinol Diabetes 2013;6:47-61. https://doi.org/10.4137/CMED.S12231.
- Iliffe S, Wilcock J, Haworth D. Delivering psychosocial interventions for people with dementia in primary care: jobs or skills?. Dementia 2006;5:327-38. https://doi.org/10.1177/1471301206067107.
- Mayberry LS, Kripalani S, Rothman RL, Osborn CY. Bridging the digital divide in diabetes: family support and implications for health literacy. Diabetes Technol Ther 2011;13:1005-12. https://doi.org/10.1089/dia.2011.0055.
- Jowsey T, Pearce-Brown C, Douglas KA, Yen L. What motivates Australian health service users with chronic illness to engage in self-management behaviour?. Health Expect 2014;17:267-77. https://doi.org/10.1111/j.1369-7625.2011.00744.x.
- Beverly EA, Wray LA, Chiu CJ, LaCoe CL. Older adults’ perceived challenges with health care providers treating their type 2 diabetes and comorbid conditions. Clin Diabetes 2014;32:12-7. https://doi.org/10.2337/diaclin.32.1.12.
- Branda ME, LeBlanc A, Shah ND, Tiedje K, Ruud K, Van Houten H, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res 2013;13. https://doi.org/10.1186/1472-6963-13-301.
- Bergdahl E, Benzein E, Ternestedt BM, Elmberger E, Andershed B. Co-creating possibilities for patients in palliative care to reach vital goals – a multiple case study of home-care nursing encounters. Nurs Inq 2013;20:341-51. https://doi.org/10.1111/nin.12022.
- Anderson RA, Bailey DE, Wu B, Corazzini K, McConnell ES, Thygeson NM, et al. Adaptive leadership framework for chronic illness: framing a research agenda for transforming care delivery. ANS Adv Nurs Sci 2015;38:83-95. https://doi.org/10.1097/ANS.0000000000000063.
- Ford JA, Wong G, Jones AP, Steel N. Access to primary care for socioeconomically disadvantaged older people in rural areas: a realist review. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010652.
- Bunn F, Goodman C, Sworn K, Rait G, Brayne C, Robinson L, et al. Psychosocial factors that shape patient and carer experiences of dementia diagnosis and treatment: a systematic review of qualitative studies. PLOS Med 2012;9. https://doi.org/10.1371/journal.pmed.1001331.
- Pawson R. Evidence-based policy: the promise of ’realist synthesis’. Evaluation 2002;8:340-58. https://doi.org/10.1177/135638902401462448.
- Rycroft-Malone J, Burton C, Hall B, McCormack B, Nutley S, Seddon D, et al. Improving skills and care standards in the support workforce for older people: a realist review. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005356.
- Wong G, Pawson R, Owen L. Policy guidance on threats to legislative interventions in public health: a realist synthesis. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-222.
- Alsaeed D, Jamieson E, Orlu Gul M, Smith FJ. Challenges to optimal medicines use in people living with dementia and their caregivers: a literature review. Int J Pharm 2016;512:396-404. https://doi.org/10.1016/j.ijpharm.2015.12.050.
- Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer‘s disease and vascular dementia. Cochrane Database Syst Rev 2013;6. https://doi.org/10.1002/14651858.CD003260.pub2.
- Bailey RA, Pfeifer M, Shillington AC, Harshaw Q, Funnell MM, VanWingen J, et al. Effect of a patient decision aid (PDA) for type 2 diabetes on knowledge, decisional self-efficacy, and decisional conflict. BMC Health Serv Res 2016;16. https://doi.org/10.1186/s12913-016-1262-4.
- Boots LM, de Vugt ME, van Knippenberg RJ, Kempen GI, Verhey FR. A systematic review of Internet-based supportive interventions for caregivers of patients with dementia. Int J Geriatr Psychiatry 2014;29:331-44. https://doi.org/10.1002/gps.4016.
- Brown J, Carson A, Waugh A, Park D. Managing diabetes in people with dementia. Nurs Times 2015;111:16-9.
- Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns 2016;99:926-43. https://doi.org/10.1016/j.pec.2015.11.003.
- Clare L, Bayer A, Burns A, Corbett A, Jones R, Knapp M, et al. Goal-oriented cognitive rehabilitation in early-stage dementia: study protocol for a multi-centre single-blind randomised controlled trial (GREAT). Trials 2013;14. https://doi.org/10.1186/1745-6215-14-152.
- Clare L, Linden DE, Woods RT, Whitaker R, Evans SJ, Parkinson CH, et al. Goal-oriented cognitive rehabilitation for people with early-stage Alzheimer disease: a single-blind randomized controlled trial of clinical efficacy. Am J Geriatr Psychiatry 2010;18:928-39. https://doi.org/10.1097/JGP.0b013e3181d5792a.
- Dhedhi SA, Swinglehurst D, Russell J. ‘Timely’ diagnosis of dementia: what does it mean? A narrative analysis of GPs’ accounts. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004439.
- Donald M, Dower J, Coll JR, Baker P, Mukandi B, Doi SA. Mental health issues decrease diabetes-specific quality of life independent of glycaemic control and complications: findings from Australia’s living with diabetes cohort study. Heal Qual Life Outcomes 2013;11. https://doi.org/10.1186/1477-7525-11-170.
- Dugmore O, Orrell M, Spector A. Qualitative studies of psychosocial interventions for dementia: a systematic review. Aging Ment Health 2015;19:955-67. https://doi.org/10.1080/13607863.2015.1011079.
- Fleming R, Sum S. Empirical studies on the effectiveness of assistive technology in the care of people with dementia: a systematic review. J Assist Technol 2014;8:14-3. https://doi.org/10.1108/JAT-09-2012-0021.
- Gibson G, Dickinson C, Brittain K, Robinson L. The everyday use of assistive technology by people with dementia and their family carers: a qualitative study. BMC Geriatr 2015;15. https://doi.org/10.1186/s12877-015-0091-3.
- Giebel CM, Sutcliffe C, Challis D. Activities of daily living and quality of life across different stages of dementia: a UK study. Aging Ment Health 2015;19:63-71. https://doi.org/10.1080/13607863.2014.915920.
- Gillespie A, Best C, O’Neill B. Cognitive function and assistive technology for cognition: a systematic review. J Int Neuropsychol Soc 2012;18:1-19. https://doi.org/10.1017/S1355617711001548.
- Goeman D, Conway S, Norman R, Morley J, Weerasuriya R, Osborne RH, et al. Optimising health literacy and access of service provision to community dwelling older people with diabetes receiving home nursing support. J Diabetes Res 2016. https://doi.org/10.1155/2016/2483263.
- Graff MJL, Vernooij-Dassen MJM, Thijssen M, Dekker J, Hoefnagels WHL, OldeRikkert MGM. Effects of community occupational therapy on quality of life, mood, and health status in dementia patients and their caregivers: a randomized controlled trial. J Gerontol Med Sci 2007;62A:1002-9. https://doi.org/10.1093/gerona/62.9.1002.
- Graff MJ, Adang EM, Vernooij-Dassen MJ, Dekker J, Jönsson L, Thijssen M, et al. Community occupational therapy for older patients with dementia and their care givers: cost effectiveness study. BMJ 2008;336:134-8. https://doi.org/10.1136/bmj.39408.481898.BE.
- Greenhalgh T, Wherton J, Sugarhood P, Hinder S, Procter R, Stones R. What matters to older people with assisted living needs? A phenomenological analysis of the use and non-use of telehealth and telecare. Soc Sci Med 2013;93:86-94. https://doi.org/10.1016/j.socscimed.2013.05.036.
- Heisler M, Vijan S, Anderson RM, Ubel PA, Bernstein SJ, Hofer TP. When do patients and their physicians agree on diabetes treatment goals and strategies, and what difference does it make?. J Gen Intern Med 2003;18:893-902. https://doi.org/10.1046/j.1525-1497.2003.21132.x.
- Hsu WC, Hei K, Lau K, Ghiloni S, Le H, Gilroy S, et al. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare. Diabetes Technol Ther 2016;18:1-9. https://doi.org/10.1089/dia.2015.0160.
- Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc 2005;53:306-11. https://doi.org/10.1111/j.1532-5415.2005.53119.x.
- Jekel K, Damian M, Wattmo C, Hausner L, Bullock R, Connelly PJ, et al. Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review. Alzheimers Res Ther 2015;7. https://doi.org/10.1186/s13195-015-0099-0.
- Jowsey T, Dennis S, Yen L, Mofizul Islam M, Parkinson A, Dawda P. Time to manage: patient strategies for coping with an absence of care coordination and continuity. Sociol Health Illn 2016;38:854-73. https://doi.org/10.1111/1467-9566.12404.
- Kennedy A, Bower P, Reeves D, Blakeman T, Bowen R, Chew-Graham C, et al. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ 2013;346. https://doi.org/10.1136/bmj.f2882.
- Kennedy A, Rogers A, Chew-Graham C, Blakeman T, Bowen R, Gardner C, et al. Implementation of a self-management support approach (WISE) across a health system: a process evaluation explaining what did and did not work for organisations, clinicians and patients. Implement Sci 2014;9. https://doi.org/10.1186/s13012-014-0129-5.
- Knapp M, Barlow J, Comas-Herrera A, Damant J, Freddolino P, Hamblin K, et al. The Case for Investment in Technology to Manage the Global Costs of Dementia. Policy Innovation Research Unit; 2015.
- Laakkonen ML, Kautiainen H, Hölttä E, Savikko N, Tilvis RS, Strandberg TE, et al. Effects of self-management groups for people with dementia and their spouses – randomized controlled trial. J Am Geriatr Soc 2016;64:752-60. https://doi.org/10.1111/jgs.14055.
- Lingler JH, Sereika SM, Amspaugh CM, Arida JA, Happ ME, Houze MP, et al. An intervention to maximize medication management by caregivers of persons with memory loss: intervention overview and two-month outcomes. Geriatr Nurs 2016;37:186-91. https://doi.org/10.1016/j.gerinurse.2015.12.002.
- Markle-Reid M, Ploeg J, Fisher K, Reimer H, Kaasalainen S, Gafni A, et al. The Aging, Community and Health Research Unit – Community Partnership Program for older adults with type 2 diabetes and multiple chronic conditions: a feasibility study. Pilot Feasibility Stud 2016;2. https://doi.org/10.1186/s40814-016-0063-1.
- Martin F, Turner A, Wallace LM, Choudhry K, Bradbury N. Perceived barriers to self-management for people with dementia in the early stages. Dementia 2013;12:481-93. https://doi.org/10.1177/1471301211434677.
- Martin F, Turner A, Wallace LM, Stanley D, Jesuthasan J, Bradbury N. Qualitative evaluation of a self-management intervention for people in the early stage of dementia. Dementia 2015;14:418-35. https://doi.org/10.1177/1471301213498387.
- Mathers N, Ng CJ, Campbell MJ, Colwell B, Brown I, Bradley A. Clinical effectiveness of a patient decision aid to improve decision quality and glycaemic control in people with diabetes making treatment choices: a cluster randomised controlled trial (PANDAs) in general practice. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2012-001469.
- Mayberry LS, Berg CA, Harper KJ, Osborn CY. The design, usability, and feasibility of a family-focused diabetes self-care support mhealth intervention for diverse, low-income adults with type 2 diabetes. J Diabetes Res 2016;2016. https://doi.org/10.1155/2016/7586385.
- Metzelthin SF, Daniels R, van Rossum E, Cox K, Habets H, de Witte LP, et al. A nurse-led interdisciplinary primary care approach to prevent disability among community-dwelling frail older people: a large-scale process evaluation. Int J Nurs Stud 2013;50:1184-96. https://doi.org/10.1016/j.ijnurstu.2012.12.016.
- Metzelthin SF, van Rossum E, de Witte LP, Ambergen AW, Hobma SO, Sipers W, et al. Effectiveness of interdisciplinary primary care approach to reduce disability in community dwelling frail older people: cluster randomised controlled trial. BMJ 2013;347. https://doi.org/10.1136/bmj.f5264.
- Mountain GA, Craig CL. What should be in a self-management programme for people with early dementia?. Aging Ment Health 2012;16:576-83. https://doi.org/10.1080/13607863.2011.651430.
- Munshi MN, Segal AR, Suhl E, Ryan C, Sternthal A, Giusti J, et al. Assessment of barriers to improve diabetes management in older adults: a randomized controlled study. Diabetes Care 2013;36:543-9. https://doi.org/10.2337/dc12-1303.
- Munshi MN, Segal AR, Suhl E, Staum E, Desrochers L, Sternthal A, et al. Frequent hypoglycemia among elderly patients with poor glycemic control. Arch Intern Med 2011;171:362-4. https://doi.org/10.1001/archinternmed.2010.539.
- Newton L, Dickinson C, Gibson G, Brittain K, Robinson L. Exploring the views of GPs, people with dementia and their carers on assistive technology: a qualitative study. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011132.
- Penn ML, Kennedy AP, Vassilev II, Chew-Graham CA, Protheroe J, Rogers A, et al. Modelling self-management pathways for people with diabetes in primary care. BMC Fam Pract 2015;16. https://doi.org/10.1186/s12875-015-0325-7.
- Procter R, Greenhalgh T, Wherton J, Sugarhood P, Rouncefield M, Hinder S. The day-to-day co-production of ageing in place. Comput Support Coop Work 2014;23:245-67. https://doi.org/10.1007/s10606-014-9202-5.
- Quinn C, Toms G, Jones C, Brand A, Edwards RT, Sanders F, et al. A pilot randomized controlled trial of a self-management group intervention for people with early-stage dementia (the SMART study). Int Psychogeriatr 2016;28:787-800. https://doi.org/10.1017/S1041610215002094.
- Reinhardt Varming A, Møller Hansen U, Andrésdóttir G, Reventlov Husted R, Willaing I. Empowerment, motivation, and medical adherence (EMMA): the feasibility of a program for patient-centered consultations to support medication adherence and blood glucose control in adults with type 2 diabetes. Patient Prefer Adherence 2015;9:1243-53.
- Sachar A. How important is mental health involvement in integrated diabetes care? The Inner North West London experience. London J Prim Care 2012;5:63-7. https://doi.org/10.1080/17571472.2013.11493377.
- Schaller S, Marinova-Schmidt V, Setzer M, Kondylakis H, Griebel L, Sedlmayr M, et al. Usefulness of a tailored ehealth service for informal caregivers and professionals in the dementia treatment and care setting: the eHealthMonitor Dementia Portal. JMIR Res Protoc 2016;5. https://doi.org/10.2196/resprot.4354.
- Schulman-Green D, Jaser SS, Park C, Whittemore R. A metasynthesis of factors affecting self-management of chronic illness. J Adv Nurs 2016;72:1469-89. https://doi.org/10.1111/jan.12902.
- Sherifali D, Bai JW, Kenny M, Warren R, Ali MU. Diabetes self-management programmes in older adults: a systematic review and meta-analysis. Diabet Med 2015;32:1404-14. https://doi.org/10.1111/dme.12780.
- Span M, Hettinga M, Vernooij-Dassen M, Eefsting J, Smits C. Involving people with dementia in the development of supportive IT applications: a systematic review. Ageing Res Rev 2013;12:535-51. https://doi.org/10.1016/j.arr.2013.01.002.
- Suh GH, Ju YS, Yeon BK, Shah A. A longitudinal study of Alzheimer’s disease: rates of cognitive and functional decline. Int J Geriatr Psychiatry 2004;19:817-24. https://doi.org/10.1002/gps.1168.
- Sun X, Guyatt GH. Interventions to enhance self management support. BMJ 2013;346. https://doi.org/10.1136/bmj.f3949.
- Tan CC, Cheng KK, Wang W. Self-care management programme for older adults with diabetes: an integrative literature review. Int J Nurs Pract 2015;21:115-24. https://doi.org/10.1111/ijn.12388.
- Taylor SJC, Pinnock H, Epiphaniou E, Pearce G, Parke HL, Schwappach A, et al. A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions: PRISMS – Practical systematic Review of Self-Management Support for long-term conditions. Health Sev Deliv Res 2014;2.
- Toms GR, Quinn C, Anderson DE, Clare L. Help yourself: perspectives on self-management from people with dementia and their caregivers. Qual Health Res 2015;25:87-98. https://doi.org/10.1177/1049732314549604.
- Wherton J, Sugarhood P, Procter R, Rouncefield M, Dewsbury G, Hinder S, et al. Designing assisted living technologies ‘in the wild’: preliminary experiences with cultural probe methodology. BMC Med Res Methodol 2012;12. https://doi.org/10.1186/1471-2288-12-188.
- Yardley S, Cottrell E, Rees E, Protheroe J. Modelling successful primary care for multimorbidity: a realist synthesis of successes and failures in concurrent learning and healthcare delivery. BMC Fam Pract 2015;16. https://doi.org/10.1186/s12875-015-0234-9.
- Greenhalgh T, Procter R, Wherton J, Sugarhood P, Hinder S, Rouncefield M. What is quality in assisted living technology? The ARCHIE framework for effective telehealth and telecare services. BMC Med 2015;13. https://doi.org/10.1186/s12916-015-0279-6.
- Quinn C, Toms G, Anderson D, Clare L. A review of self-management interventions for people with dementia and mild cognitive impairment. J Appl Gerontol 2016;35:1154-88. https://doi.org/10.1177/0733464814566852.
- McBain H, Mulligan K, Haddad M, Flood C, Jones J, Simpson A. Self management interventions for type 2 diabetes in adult people with severe mental illness. Cochrane Database Syst Rev 2016;4. https://doi.org/10.1002/14651858.CD011361.pub2.
- Goodwin N, Sonola L, Thiel V, Kodner D. Co-ordinated Care for People with Complex Chronic Conditions: Key Lessons and Markers for Success. London: The King’s Fund; 2013.
- My Diabetes, My Care. Newcastle upon Tyne: Care Quality Commission; 2016.
- McBain H, Mulligan K, Lamontagne-Godwin F, Jones J, Haddad M, Flood C, et al. Implementation of recommended type 2 diabetes care for people with severe mental illness – a qualitative exploration with healthcare professionals. BMC Psychiatry 2016;16. https://doi.org/10.1186/s12888-016-0942-2.
- Taylor B, Bircher J, Wilson C, Barr S. RCGP National Diabetes Audit Quality Improvement Programme Report. London: Royal College of General Practitioners; 2016.
- Bunn F, Burn AM, Robinson L, Poole M, Rait G, Brayne C, et al. Healthcare organisation and delivery for people with dementia and comorbidity: a qualitative study exploring the views of patients, carers and professionals. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013067.
- Quinn C, Toms G, Anderson D, Clare L. Self-management group interventions for people with MCI or dementia: a systematic review. Alzheimer’s Dement J Alzheimer’s Assoc 2015;10. https://doi.org/10.1016/j.jalz.2014.05.1474.
- Browne JL, Ventura A, Mosely K, Speight J. ‘I call it the blame and shame disease’: a qualitative study about perceptions of social stigma surrounding type 2 diabetes. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003384.
- Handley M, Bunn F, Goodman C. Interventions that support the creation of dementia friendly environments in health care: protocol for a realist review. Syst Rev 2015;4. https://doi.org/10.1186/s13643-015-0168-2.
- Goodman C, Buswell M, Russell B, Bunn F, Mayrhofer A. Community Engagement Evidence Synthesis: A Draft Report for Alzheimer’s Society. Hatfield: Centre for Research in Primary and Community Care, University of Hertfordshire; 2016.
- Mountain G. Self-management programme for people with dementia and their spouses demonstrates some benefits, but the model has limitations. Evid Based Nurs 2017;20:26-7. https://doi.org/10.1136/eb-2016-102408.
- Clare L. An Enablement Approach to Dementia Care and Prevention n.d.
- Nothing Ventured, Nothing Gained: Risk Guidance for People with Dementia. London: Department of Health; 2010.
- Taggart L, Coates V, Clarke M, Bunting B, Davies M, Carey M, et al. A study protocol for a pilot randomised trial of a structured education programme for the self-management of type 2 diabetes for adults with intellectual disabilities. Trials 2015;16.
- Walwyn RE, Russell AM, Bryant LD, Farrin AJ, Wright-Hughes AM, Graham EH, et al. Supported self-management for adults with type 2 diabetes and a learning disability (OK-Diabetes): study protocol for a randomised controlled feasibility trial. Trials 2015;16. http://dx.doi.org/10.1186/s13063-015-0832-9.
- Davies MJ, Heller S, Skinner TC, Campbell MJ, Carey ME, Cradock S, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336:491-5. https://doi.org/10.1136/bmj.39474.922025.BE.
- Taggart L, Truesdale-Kennedy M, Scott J. Working with people with intellectual and developmental disabilities who have diabetes. J Diabetes Nurs 2015;19:190-4.
- Rolland JS. Chronic illness and the life cycle: a conceptual framework. Fam Process 1987;26:203-21. https://doi.org/10.1111/j.1545-5300.1987.00203.x.
- Eaton S, Roberts S, Turner B. Delivering person centred care in long term conditions. BMJ 2015;350. https://doi.org/10.1136/bmj.h181.
- Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC, WA: National Academies Press; 2001.
- Bunn F, Goodman C, Pinkney E, Drennan VM. Specialist nursing and community support for the carers of people with dementia living at home: an evidence synthesis. Health Soc Care Community 2016;24:48-67. https://doi.org/10.1111/hsc.12189.
- Managing Blood Glucose in Adults with Type 2 Diabetes. Manchester: National Institute for Health and Care Excellence; 2016.
- Caverly TJ, Fagerlin A, Zikmund-Fisher BJ, Kirsh S, Kullgren JT, Prenovost K, et al. Appropriate prescribing for patients with diabetes at high risk for hypoglycemia: National Survey of Veterans Affairs Health Care Professionals. JAMA Intern Med 2015;175:1994-6. https://doi.org/10.1001/jamainternmed.2015.5950.
- American Board of Internal Medicine Foundation . Choosing Wisely n.d. www.choosingwisely.org/about-us/ (accessed 9 December 2016).
- Down K, Mitchell M, Wardle D. Assistive Technology: Standards for Service Provision. London: Foundation for Assistive Technology; 2006.
- Prime Minister’s Challenge on Dementia: Delivering Major Improvements in Dementia Care and Research by 2015. London: Department of Health; 2012.
- Dineen R. Co-Producing Prudent Healthcare: Putting People in the Picture n.d. www.prudenthealthcare.org.uk/coproduction/ (accessed 26 January 2017).
- Vijayaraghavan S, O’Shea T, Campbell-Richards D, Sudra R, Morris J, Byrne E, et al. DAWN: Diabetes Appointments via Webcam in Newham. Br J Diabetes 2015;15:123-6. https://doi.org/10.15277/bjdvd.2015.032.
- Tresolini C. Health Professions Education and Relationship-Centered Care. San Francisco, CA: Pew Health Professions Commission; 1994.
- Suchman A, Sluyter D, Williamson P. Leading Change in Healthcare. London: Radcliffe Publishing; 2011.
- Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R. Continuity of care: a multidisciplinary review. BMJ 2003;327:1219-21. https://doi.org/10.1136/bmj.327.7425.1219.
- Parker G, Corden A, Heaton J. Synthesis and Conceptual Analysis of the SDO Programme’s Research on Continuity of Care. Southampton: NIHR Service Delivery and Organisation programme; 2009.
- Heaton J, Corden A, Parker G. ‘Continuity of care’: a critical interpretive synthesis of how the concept was elaborated by a national research programme. Int J Integr Care 2012;12. https://doi.org/10.5334/ijic.794.
- Rolland J. In sickness and in health: the impact of illness on couples’ relationships. J Marital Fam Ther 1994;20:327-47. https://doi.org/10.1111/j.1752-0606.1994.tb00125.x.
- Nardi BA. Context and Consciousness: Activity Theory and Human-Computer Interaction. Cambridge, MA: MIT Press; 1996.
- Torsi S, Nasr N, Wright PC, Mawson SJ, Mountain GA. User-Centered Design for Supporting the Self-Management of Chronic Illnesses: An Interdisciplinary Approach n.d.
- International Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization; 2001.
- Dalkin SM, Greenhalgh J, Jones D, Cunningham B, Lhussier M. What’s in a mechanism? Development of a key concept in realist evaluation. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0237-x.
- Freeman GK, Woloshynowych M, Baker R, Boulton M, Guthrie B, Car J, et al. Continuity of Care 2006: What Have We Learned Since 2000 and What Are Policy Imperatives Now?. National Co-ordinating Centre for NHS Service Delivery and Organisation R & D; 2007.
- Lacouture A, Breton E, Guichard A, Ridde V. The concept of mechanism from a realist approach: a scoping review to facilitate its operationalization in public health program evaluation. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0345-7.
- Jagosh J, Pluye P, Wong G, Cargo M, Salsberg J, Bush PL, et al. Critical reflections on realist review: insights from customizing the methodology to the needs of participatory research assessment. Res Synth Methods 2014;5:131-41. https://doi.org/10.1002/jrsm.1099.
- Schoenmaker N, Van Gool WA. The age gap between patients in clinical studies and in the general population: a pitfall for dementia research. Lancet Neurol 2004;3:627-30. https://doi.org/10.1016/S1474-4422(04)00884-1.
- Zarit SH, Femia EE. A future for family care and dementia intervention research? Challenges and strategies. Aging Ment Health 2008;12:5-13. https://doi.org/10.1080/13607860701616317.
- Zarit SH, Femia EE, Kim K, Whitlatch CJ. The structure of risk factors and outcomes for family caregivers: implications for assessment and treatment. Aging Ment Health 2010;14:220-31. https://doi.org/10.1080/13607860903167861.
- Zarit SH. Past is prologue: how to advance caregiver interventions [published online ahead of print 16 May 2017]. Aging Ment Health 2017. https://doi.org/10.1080/13607863.2017.1328482.
- May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ 2009;339. https://doi.org/10.1136/bmj.b2803.
- Leppin A, Montori V, Gionfriddo M. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare 2015;3:50-63. https://doi.org/10.3390/healthcare3010050.
- Mair FS, May CR. Thinking about the burden of treatment. BMJ 2014;349. https://doi.org/10.1136/bmj.g6680.
- Anderson K, Foster M, Freeman C, Luetsch K, Scott I. Negotiating ‘unmeasurable harm and benefit’. Qual Health Res 2017;27:1936-47. https://doi.org/10.1177/1049732316687732.
- Miranda-Castillo C, Woods B, Orrell M. People with dementia living alone: what are their needs and what kind of support are they receiving?. Int Psychogeriatr 2010;22:607-17. https://doi.org/10.1017/S104161021000013X.
- de Witt L, Ploeg J. Caring for older people living alone with dementia: healthcare professionals’ experiences. Dementia 2016;15:221-38. https://doi.org/10.1177/1471301214523280.
- de Witt L, Ploeg J, Black M. Living alone with dementia: an interpretive phenomenological study with older women. J Adv Nurs 2010;66:1698-707. https://doi.org/10.1111/j.1365-2648.2010.05295.x.
- Stewart M. Towards a global definition of patient centred care. The patient should be the judge of patient centred care. BMJ 2001;322:444-5. https://doi.org/10.1136/bmj.322.7284.444.
- Skovlund SE, Peyrot M. DAWN International Advisory Panel: lifestyle and behavior: the Diabetes Attitudes, Wishes and Needs (DAWN) program: a new approach to improving outcomes of diabetes care. Diabetes Spectr 2005;18:136-42. https://doi.org/10.2337/diaspect.18.3.136.
- Type 1 Diabetes in Adults: Diagnosis and Management. Manchester: National Institute for Health and Care Excellence; 2015.
- Dementia: Supporting People with Dementia and their Carers in Health and Social Care. London: National Institute for Health and Care Excellence; 2006.
- Sinnott C, McHugh S, Browne J, Bradley C. GPs’ perspectives on the management of patients with multimorbidity: systematic review and synthesis of qualitative research. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003610.
- Guthrie B, Payne K, Alderson P, McMurdo ME, Mercer SW. Adapting clinical guidelines to take account of multimorbidity. BMJ 2012;345. https://doi.org/10.1136/bmj.e6341.
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43. https://doi.org/10.1016/S0140-6736(12)60240-2.
- Smith SM, Wallace E, O’Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2016;3. https://doi.org/10.1002/14651858.CD006560.pub3.
- Roland M, Paddison C. Better management of patients with multimorbidity. BMJ 2013;346:21-2. https://doi.org/10.1136/bmj.f2510.
- Pawson R. Assessing the Quality of Evidence in Evidence-based Policy: Why, How and When? Working Paper No 1. Economic and Social Research Council; 2003.
- Beverly EA, Wray LA, Chiu CJ, Weinger K. Perceived challenges and priorities in co-morbidity management of older patients with type 2 diabetes. Diabet Med 2011;28:781-4. https://doi.org/10.1111/j.1464-5491.2011.03282.x.
- Heifetz R, Grashow A, Linsky M. The Practice of Adaptive Leadership: Tools and Tactics for Changing your Organization and the World. Boston, MA: Harvard Business Review Press; 2009.
Appendix 1 Project Advisory Group
| Names | Role |
|---|---|
| Professor Steve Iliffe (chairperson) | Professor of Primary Care for Older People, University College London |
| Mr Tim Beanland | Knowledge Manager, Alzheimer’s Society |
| Dr Simon Conroy | Head of Geriatric Medicine, University of Leicester Hospitals |
| Professor Angus Forbes | Chairperson of Clinical Diabetes Nursing, King’s College London |
| Dr Charles Fox | Consultant Physician with Expertise in Diabetes |
| Professor Yoon Loke | Professor of Medicine and Pharmacology, Norwich Medical School (interest in the harms of hypoglycaemia) |
| Dr Ana Manzano-Santaella | Lecturer in Health and Social Policy, University of Leeds (expertise in evaluation of complex health-care interventions using realist approaches) |
| Dr Paul Millac | Member of University of Hertfordshire Patient and Public Involvement Group |
| Ms Diane Munday | Member of University of Hertfordshire Patient and Public Involvement Group |
| Ms Debbie Stanisstreet | Lead Diabetes Specialist Nurse |
Appendix 2 Search strategy
Search strategy
Search terms were devised by Frances Bunn and Peter Reece Jones and then discussed with other team members and with an information scientist (Beth Hall). The search terms were chosen to reflect our three main theory areas.
The searches were split into three categories: A, dementia and diabetes; B, dementia only; and C, diabetes only. Each category was then divided into three to reflect each of the programme theories. For example, A1 is the category of dementia and diabetes plus theory area 1 (clinically based approach).
The main searches are A1, A2 and A3, which include terms for both dementia and diabetes. However, because there was little that covered both, we also looked at literature that focused on either dementia only (B1, B2 and B3) or diabetes only (C1, C2 and C3). An overview of the search strategy can be seen in Table 8. This is followed by details of the full search terms for PubMed. The terms were adapted as appropriate for other databases.
| Focus of search | Theory area 1: clinically based approach | Theory area 2: collaborative partnerships | Theory area 3: coproduction |
|---|---|---|---|
| Dementia and diabetes | A1 | A2 | A3 |
| Theory area 3 produced very few hits, so all searches (A, B and C) were combined | |||
| Dementia only | B1 | B2 | B3 |
| Diabetes only | C1 | C2 | C3 |
| Large area so was agreed could use clinical guidelines (e.g. recent Sinclair guidelines26,28,30) as these provide up-to-date evidence |
PubMed: first searches were run on 8 March 2016 (all searches were limited to 1990 onwards to coincide with some of the early papers from Alan Sinclair).
#1 ((((((“diabetes”[Title/Abstract]) OR “insulin”[Title/Abstract]) OR “hypoglycaemia”[Title/Abstract]) OR “hyperglycaemia”[Title/Abstract]) OR “glycaemic control”[Title/Abstract]) OR “glycemic control”[Title/Abstract]) OR “hba1c”[Title/Abstract]) OR (diabetes[Title/Abstract] OR insulin[Title/Abstract] OR (glycaemic control)[Title/Abstract] OR (glycemic control)[Title/Abstract] OR hypoglycaem*[Title/Abstract] OR hypoglycemi*[Title/Abstract] OR hyperglycaem*[Title/Abstract] OR hyperglycem*[Title/Abstract] OR Hba1C[Title/Abstract] – includes both MeSH and free text
#2 (“dementia”[Title/Abstract]) OR “alzheimer”[Title/Abstract]) OR “alzheimers”[Title/Abstract]) OR “mild cognitive impairment”[Title/Abstract]) OR “cognitive impairment”[Title/Abstract]) OR (dement*[Title/Abstract] OR alzheimer*[Title/Abstract] OR MCI[Title/Abstract] OR cognitive impairment[Title/Abstract])
#3 = #1 AND #2
#4 (((manage*[Title/Abstract] OR treat*[Title/Abstract] OR intervention*[Title/Abstract] OR programme*[Title/Abstract] OR program*[Title/Abstract] OR controlled[Title/Abstract] OR randomized[Title/Abstract] OR randomised[Title/Abstract] OR interview*[Title/Abstract] OR qualitative[Title/Abstract] OR trial[Title/Abstract])) OR “randomised controlled trial”) OR “intervention study”
#5 = #3 AND #4
Not
((((“cross sectional study”) OR “epidemiological studies”) OR “case control”) OR “cohort study”) OR “cross sectional studies”
N = 2868
A2#1 ((((((“diabetes”[Title/Abstract]) OR “insulin”[Title/Abstract]) OR “hypoglycaemia”[Title/Abstract]) OR “hyperglycaemia”[Title/Abstract]) OR “glycaemic control”[Title/Abstract]) OR “glycemic control”[Title/Abstract]) OR “hba1c”[Title/Abstract]) OR (diabetes[Title/Abstract] OR insulin[Title/Abstract] OR (glycaemic control)[Title/Abstract] OR (glycemic control)[Title/Abstract] OR hypoglycaem*[Title/Abstract] OR hypoglycemi*[Title/Abstract] OR hyperglycaem*[Title/Abstract] OR hyperglycem*[Title/Abstract] OR Hba1C[Title/Abstract] – includes both MeSH and free text
#2 (“dementia”[Title/Abstract]) OR “alzheimer”[Title/Abstract]) OR “alzheimers”[Title/Abstract]) OR “mild cognitive impairment”[Title/Abstract]) OR “cognitive impairment”[Title/Abstract]) OR (dement*[Title/Abstract] OR alzheimer*[Title/Abstract] OR MCI[Title/Abstract] OR cognitive impairment[Title/Abstract])
#3 = #1 AND #2
#4 (((“self care”[Title/Abstract]) OR “self management”[Title/Abstract]) OR “self medication”[Title/Abstract]) OR “self administration”[Title/Abstract]) OR “minimally disruptive medicine”[Title/Abstract]) OR “adherence”[Title/Abstract]) OR “shared decision making”[Title/Abstract]) OR “patient preference”[Title/Abstract]) OR “patient participation”[Title/Abstract]) OR “patient involvement”[Title/Abstract]) OR “patient centred care”[Title/Abstract]) OR “personalised care”[Title/Abstract]) OR “individualised care”[Title/Abstract]) OR (partnership[Title/Abstract] OR collaboration[Title/Abstract]) Filters: Publication date from 1990/01/01
#5 = #3 AND #4
N = 113
A3#1 ((((((“diabetes”[Title/Abstract]) OR “insulin”[Title/Abstract]) OR “hypoglycaemia”[Title/Abstract]) OR “hyperglycaemia”[Title/Abstract]) OR “glycaemic control”[Title/Abstract]) OR “glycemic control”[Title/Abstract]) OR “hba1c”[Title/Abstract]) OR (diabetes[Title/Abstract] OR insulin[Title/Abstract] OR (glycaemic control)[Title/Abstract] OR (glycemic control)[Title/Abstract] OR hypoglycaem*[Title/Abstract] OR hypoglycemi*[Title/Abstract] OR hyperglycaem*[Title/Abstract] OR hyperglycem*[Title/Abstract] OR Hba1C[Title/Abstract] – includes both MeSH and free text
#2 (“dementia”[Title/Abstract]) OR “alzheimer”[Title/Abstract]) OR “alzheimers”[Title/Abstract]) OR “mild cognitive impairment”[Title/Abstract]) OR “cognitive impairment”[Title/Abstract]) OR (dement*[Title/Abstract] OR alzheimer*[Title/Abstract] OR MCI[Title/Abstract] OR cognitive impairment[Title/Abstract])
#3 = #1 AND #2
#4 (((((“co production”) OR “co design”) OR “codesign”) OR “coproduction”) OR “co creation”) OR (co-produc* OR coproduc* OR co-design* OR codesign* OR co-creat* OR cocreat* OR co-commission* OR cocommission) Filters: Publication date from 1990/01/01 (MeSH and free text)
#5 = #3 AND #4 = 0
#1 ((((“dementia”[Title/Abstract]) OR “alzheimer”[Title/Abstract]) OR “alzheimers”[Title/Abstract]) OR “mild cognitive impairment”[Title/Abstract]) OR “cognitive impairment”[Title/Abstract]) OR (dement*[Title/Abstract] OR alzheimer*[Title/Abstract] OR MCI[Title/Abstract] OR cognitive impairment[Title/Abstract])
#2 (diet[Title/Abstract] OR exercise[Title/Abstract] OR blood glucose[Title/Abstract] OR blood pressure[Title/Abstract] OR medication[Title/Abstract] OR adherence[Title/Abstract] OR self management[Title/Abstract]) Filters: Publication date from 1990/01/01
#3 = #1 AND #2
#4 (((manage*[Title/Abstract] OR treat*[Title/Abstract] OR intervention*[Title/Abstract] OR programme*[Title/Abstract] OR program*[Title/Abstract] OR controlled[Title/Abstract] OR randomized[Title/Abstract] OR randomised[Title/Abstract] OR interview*[Title/Abstract] OR qualitative[Title/Abstract] OR trial[Title/Abstract])) OR “randomised controlled trial”) OR “intervention study”
#5 = #3 AND #4
#6 Not
((((“cross sectional study”) OR “epidemiological studies”) OR “case control”) OR “cohort study”) OR “cross sectional studies”
N = 4767
B2#1 ((((“dementia”[Title/Abstract]) OR “alzheimer”[Title/Abstract]) OR “alzheimers”[Title/Abstract]) OR “mild cognitive impairment”[Title/Abstract]) OR “cognitive impairment”[Title/Abstract]) OR (dement*[Title/Abstract] OR alzheimer*[Title/Abstract] OR MCI[Title/Abstract] OR cognitive impairment[Title/Abstract])
#2 (self care[Title/Abstract] OR self management[Title/Abstract] OR self medication[Title/Abstract] OR self administration[Title/Abstract] OR minimally disruptive medicine[Title/Abstract] OR adherence[Title/Abstract] OR shared decision making[Title/Abstract] OR patient preference[Title/Abstract] OR patient participation[Title/Abstract] OR patient involvement[Title/Abstract] OR patient centred care[Title/Abstract] OR patient Centered care[Title/Abstract] OR personalised care[Title/Abstract] OR individualised care[Title/Abstract] OR individualized care[Title/Abstract] OR personalized care[Title/Abstract]) Filters: Publication date from 1990/01/01
#3 = #1 AND #2
N = 1257
B3#1 ((((“dementia”[Title/Abstract]) OR “alzheimer”[Title/Abstract]) OR “alzheimers”[Title/Abstract]) OR “mild cognitive impairment”[Title/Abstract]) OR “cognitive impairment”[Title/Abstract]) OR (dement*[Title/Abstract] OR alzheimer*[Title/Abstract] OR MCI[Title/Abstract] OR cognitive impairment[Title/Abstract])
#2 ((((“co production”) OR “co design”) OR “codesign”) OR “coproduction”) OR “co creation”) OR (co-produc* OR coproduc* OR co-design* OR codesign* OR co-creat* OR cocreat* OR co-commission* OR cocommission) Filters: Publication date from 1990/01/01
#3 = #1 AND #2
N = 20
Use clinical guidelines.
C2#1 ((((((“diabetes”[Title/Abstract]) OR “insulin”[Title/Abstract]) OR “hypoglycaemia”[Title/Abstract]) OR “hyperglycaemia”[Title/Abstract]) OR “glycaemic control”[Title/Abstract]) OR “glycemic control”[Title/Abstract]) OR “hba1c”[Title/Abstract]) OR (diabetes[Title/Abstract] OR insulin[Title/Abstract] OR (glycaemic control)[Title/Abstract] OR (glycemic control)[Title/Abstract] OR hypoglycaem*[Title/Abstract] OR hypoglycemi*[Title/Abstract] OR hyperglycaem*[Title/Abstract] OR hyperglycem*[Title/Abstract] OR Hba1C[Title/Abstract] – includes both MeSH and free text
#2
(((“frailty”) OR “older”) OR “elderly”) OR “geriatric”) OR “elder”) OR “aged” Filters:Publication date from 1990/01/01 – all MeSH
#3 (self care[Title/Abstract] OR self management[Title/Abstract] OR self medication[Title/Abstract] OR self administration[Title/Abstract] OR minimally disruptive medicine[Title/Abstract] OR adherence[Title/Abstract] OR shared decision making[Title/Abstract] OR patient preference[Title/Abstract] OR patient participation[Title/Abstract] OR patient involvement[Title/Abstract] OR patient centred care[Title/Abstract] OR patient Centered care[Title/Abstract] OR personalised care[Title/Abstract] OR individualised care[Title/Abstract] OR individualized care[Title/Abstract] OR personalized care[Title/Abstract]) Filters: Publication date from 1990/01/01
#4 = #1 AND #2 AND #3
N = 4670
C3#1 ((((((“diabetes”[Title/Abstract]) OR “insulin”[Title/Abstract]) OR “hypoglycaemia”[Title/Abstract]) OR “hyperglycaemia”[Title/Abstract]) OR “glycaemic control”[Title/Abstract]) OR “glycemic control”[Title/Abstract]) OR “hba1c”[Title/Abstract]) OR (diabetes[Title/Abstract] OR insulin[Title/Abstract] OR (glycaemic control)[Title/Abstract] OR (glycemic control)[Title/Abstract] OR hypoglycaem*[Title/Abstract] OR hypoglycemi*[Title/Abstract] OR hyperglycaem*[Title/Abstract] OR hyperglycem*[Title/Abstract] OR Hba1C[Title/Abstract] – includes both MeSH and free text
#2 ((((“co production”) OR “co design”) OR “codesign”) OR “coproduction”) OR “co creation”) OR (co-produc* OR coproduc* OR co-design* OR codesign* OR co-creat* OR cocreat* OR co-commission* OR cocommission) Filters: Publication date from 1990/01/01
#3 = #1 AND #2
N = 57
Updated searches around tailored care, individualised care and multimorbidity
A new search was run on 27 April 2016 as a result of discussions at the project workshop. The suggestion was that we should be looking at care for people with comorbidity.
PubMed
#1 Tailored care OR tailoring OR individualised care OR individualized care OR personalised care OR personalized care or needs based care (all MESH)
#2 (tailored[Title/Abstract] OR tailor*[Title/Abstract] OR individualised[Title/Abstract] OR individualized[Title/Abstract] OR personalised[Title/Abstract] OR personalized[Title/Abstract] OR “needs based”[Title/Abstract])
#3 #1 OR #2
#4 multimorbidity OR multimorbid Or comorbidity OR comorbid OR frailty (all MESH)
#5 #3 AND #4
#6 (manage*[Title/Abstract] OR treat*[Title/Abstract] OR intervention*[Title/Abstract] OR programme*[Title/Abstract] OR program*[Title/Abstract] OR controlled[Title/Abstract] OR randomized[Title/Abstract] OR randomised[Title/Abstract] OR trial[Title/Abstract])) OR “randomised controlled trial”) OR “intervention study”)
#7 #5 AND #6
N = 162
Appendix 3 Data extraction form
Appendix 4 Table of included studies
| Study (author, year) | Country | Research type | Aim/purpose | Description of intervention | Participants | Type of participants |
|---|---|---|---|---|---|---|
| Abdelhafiz et al., 201625 | NA | Review | To explore the relationship between hypoglycaemia, frailty and dementia and the implications for clinical practice | NA | Dementia AND diabetes | Older people with frailty diabetes and/or dementia |
| Aikens et al., 201563 | USA | Before-and-after study | To investigate the potential benefits of integrating a patient-selected support person into an automated diabetes telemonitoring and SM program | Automated telemonitoring service for diabetes that used interactive voice response telemonitoring in which patients respond to prerecorded queries using their telephone keypad. System then provided tailored SM messages and notified clinical team about problems that might require additional follow-up | Diabetes NOT dementia | People with T2DM who are non-adherent to medication. Majority of care partners were adult children |
| Alsaeed et al., 201679 | NA | Literature review | To investigate the facilitators of optimal medicines use from the perspectives of people living with dementia and their caregivers | NA | Dementia NOT diabetes | People living with dementia and/or care partners |
| Anderson et al., 201573 | USA | Opinion/discussion | To shift focus from symptoms to symptoms and the challenges that they pose for patients/families | NA | Other | Relates to people with chronic illness |
| Bahar-Fuchs et al., 201380 | NA | SR | To evaluate the effectiveness and impact of cognitive training and/or rehabilitation for people with mild Alzheimer’s disease or vascular dementia in relation to important cognitive and non-cognitive outcomes for the person and their primary caregiver in the short, medium and long term | Cognitive rehabilitation or training for people with dementia (interventions that directly or indirectly target cognitive functioning as opposed to those that focus on behavioural, emotional or physical functions) | Dementia NOT diabetes | People with Alzheimer’s disease or vascular dementia |
| Bailey et al., 201681 | USA | RCT | To determine the impact of a PDA for decisions about antihyperglycaemic medications on key elements of shared decision-making | The interactive diabetes decision aid for T2DM: presented on computer, designed to help people understand T2DM, and the full range of treatment options | Diabetes NOT dementia | English-speaking participants with T2DM receiving metformin with persistent hyperglycaemia who were recommended to consider medication intensification |
| Baxter, 201457 | UK | Opinion/discussion | To improve standards of diabetes care | NA | Diabetes NOT dementia | Article relates to people with diabetes |
| Bergdahl et al., 201372 | Sweden | Qualitative | To explore planned home care nursing encounters in palliative care | NA | Other | Older people with cancer and comorbidities, family carers and nurses |
| Beverly et al., 201470 (same study as Beverly 2011199) | USA | Qualitative | To explore how older adults manage and cope with T2DM and self-care and other chronic conditions | NA | Diabetes NOT dementia | Older adults diagnosed with T2DM and at least one other chronic health condition |
| Boots et al., 201482 | NA | SR | To provide an overview of the evidence for the effectiveness, feasibility and quality of internet interventions for informal caregivers of people with dementia | A variety of internet interventions for carers of people living with dementia are reviewed (e.g. website or website and support, workshop sessions, online training) | Dementia NOT diabetes | Informal carers of people living with dementia |
| Boots et al., 201661 | Netherlands | Qualitative | To (1) develop an online SM programme for early-stage dementia carers to increase self-efficacy and goal attainment, and (2) evaluate the programme’s feasibility and report preliminary data on effectiveness | Face-to-face meeting with an appointed ‘coach’ (always the same HCP) followed by completion of the online tool to ‘skill up’ informal carers of people living with dementia and improve their coping strategies; during this stage the coach is available to help with any issues and a final meeting with the coach occurs after completion | Dementia NOT diabetes | Spousal carers of people living with mild to moderate dementia |
| Branda et al., 201371 | USA | RCT | To evaluate the impact of patient decision aids vs. usual care on decision making measures, metabolic control and medication adherence | Diabetes medication choice decision aid vs. statin choice decision aid. Clinicians received minimal training | Diabetes NOT dementia | HCPs: physicians, nurses, nurse practitioners and physician assistants who cared for patients with T2DM. Patients: adults with T2DM of a duration > 1 year and a reason, identified by a clinician, to consider changing their antihyperglycaemic or lipid-lowering regimens |
| Brown et al., 201583 | UK | Opinion/discussion | To inform nursing staff about current practice for managing diabetes for people living with dementia | NA | Dementia AND diabetes | Relates to people living with dementia and diabetes, their carers and family |
| Bunn et al., 201639 | UK | Mixed methods | To explore the impact of dementia on access to non-dementia services and identify ways of improving the integration of services for this population | NA | Dementia AND diabetes | Older people living with dementia and comorbid conditions, including diabetes, stroke and vision impairment |
| Camp et al., 201559 | USA | Non-randomised controlled study | To determine whether or not a distance-based education intervention would result in positive health outcomes for people with both diabetes and CI | PRIDE: providing resources for independence through diabetes education. Certified diabetic educators were linked with older adults with diabetes and CI. Provided personalised education sessions over the internet. Initial focus was on medication adherence and an additional goal selected by participant–certified diabetes educator dyad (e.g. diet, blood glucose testing). A subset of participants also received a cognitive intervention called spaced retrieval | Dementia AND diabetes | Older adults with T2DM and CI (MCI or early-stage dementia) living independently in their homes |
| Care Quality Commission, 2016139 | UK | Thematic review | Improve understanding of people’s experiences of diabetes care across England, and consider how well different care services work together | Diabetes care in England | Diabetes NOT dementia | Adults aged 18–65 years with type 1 and type 2 diabetes. Particular focus on people from black and minority ethnic populations |
| Chrvala et al., 201684 | NA | SR | To assess the effect of diabetes SM education and support methods, providers, duration and contact time on glycaemic control in adults with T2DM | Diabetes SM education interventions including elements and activities intended to improve participants’ knowledge, skills, and ability to perform SM activities that had the potential to improve glycaemic control | Diabetes NOT dementia | Studies involving people aged > 18 years with any HbA1C level and all intervals of diabetes duration with a comorbid health condition. Sample to include > 50% of participants with T2DM |
| Clare et al., 201086 | UK | RCT | To provide evidence of the clinical efficacy of cognitive rehabilitation in early-stage Alzheimer’s disease | Eight weekly 1-hour individual sessions of cognitive rehabilitation, personalised to address individually relevant goals; also techniques for learning new information, practice maintaining attention and concentration, and techniques for stress management. Two control groups: one treatment as usual and the other with eight weekly 1-hour sessions of relaxation | Dementia NOT diabetes | People with dementia and informal carers |
| Clare et al., 201385 | UK | Study protocol | To establish whether or not cognitive rehabilitation is a clinically effective and cost-effective intervention for people with early-stage dementia and their carers | Ten sessions of cognitive rehabilitation over 3 months followed by four maintenance sessions over 6 months | Dementia NOT diabetes | People with early-stage dementia |
| Davis et al., 201262 | USA | RCT | To test the effect of a targeted intervention on self-care, heart failure knowledge and 30-day readmissions in people with MCI | Intervention focused on environmental manipulations (by simplifying tasks and providing external cues or prompts to initiate action) and training in compensatory strategies for working with impairments in memory | Other | People hospitalised for exacerbation of heart failure who screened positive for MCI |
| De Vriendt et al., 201558 | Belgium | RCT | To investigate the effectiveness of a client-centred, activity-orientated intervention on ADL for frail older people | Client-centred goal-setting: OT trained to clarify older persons’ prioritised goals in self-care, leisure activities, etc. | Other | Community-dwelling frail older adults |
| Dhedi et al., 201487 | UK | Qualitative | To explore GPs’ perspectives on the meaning of ‘timeliness’ in dementia diagnosis | NA | Dementia NOT diabetes | Practising GPs in an academic department of primary care and public health |
| Donald et al., 201388 | Australia/New Zealand | Observational | To assess the impact of complications and comorbidities on diabetes-specific quality of life in a large population-based cohort of type 2 diabetic patients | NA | Diabetes NOT dementia | Adults with diabetes |
| Dugmore et al., 201589 | NA | SR | To explore[W]hat existing qualitative studies reveal about the implementation, effects and processes of psychosocial interventions for dementia | Any non-pharmacological, clinical intervention for people with dementia | Dementia NOT diabetes | Six out of 16 studies on people living with dementia, 5/16 studies on people living with dementia and family/professional carers, and 5/16 studies on professional carers only |
| Feil et al., 200942 | USA | Observational | To examine the role of CI and caregiver support in diabetes care adherence and glycaemic control | NA | Dementia AND diabetes | Older adults with T2DM and CI |
| Feil et al., 201164 | USA | Qualitative | To explore caregivers’ challenges and quality-of-life issues managing diabetes in patients with dementia | NA | Dementia AND diabetes | Family carers, but no details of their relationship with people living with dementia |
| Fleming and Sum, 201490 | NA | SR | To assess the empirical support for the use of AT in the care of people with dementia as an intervention to improve independence, safety, communication, well-being and carer support | Any intervention utilising AT and focused on the care of people with dementia aged > 50 years | Dementia NOT diabetes | People with dementia |
| Gibson et al., 201591 | UK | Qualitative | To explore the everyday use of AT by people with dementia and their families | NA | Dementia NOT diabetes | Total of 39 participants: 13 people with dementia and 26 carers |
| Giebel et al., 201592 | UK | Observational | To investigate which activities are impaired at each stage of dementia and to what extent this is associated with variations in quality of life across the different stages | NA | Dementia NOT diabetes | People with dementia aged ≥ 65 years and their carers |
| Gillespie et al., 201293 | NA | SR | To examine the relationship between AT for cognition and cognitive function | AT for cognition is defined as ‘any technology which compensates for cognitive deficit during task performance’. One of the categories is around reminders and time management | Dementia NOT diabetes | People of all ages with CI of any aetiology, including acquired brain injury, neurodevelopment disorder, psychiatric disorder, dementia and/or intellectual disability |
| Goeman et al., 201694 | Australia/New Zealand | Other: mixed methods | To improve the approach to diabetes education and give better SM support to clients |
Training nurses to identify learning styles and deliver prescribed information in an appropriate way and confirm the patient’s understanding Education of people with diabetes by the specially trained nurses to improve health literacy and understanding of diabetes and so improve SM |
Diabetes NOT dementia | People aged > 50 years, with diabetes |
| Goodwin et al., 2013138 | UK | Case study | To examine key lessons and markers for success in the ‘how’ of care co-ordination that might be transferable to different contexts and settings | Care co-ordination, covering a variety of conditions including palliative care and advanced dementia service. The dementia intervention is about supporting carers to provide palliative care for people with advanced dementia | Dementia NOT diabetes | People with advanced stage dementia and their family carers |
| Graff et al., 200665 (linked to Graff 200896) | Netherlands | RCT | To determine the effectiveness of community based occupational therapy on daily functioning of patients with dementia and the sense of competence of their caregivers | Ten sessions of occupational therapy over 5 weeks, including cognitive and behavioural interventions, to train patients in the use of aids to compensate for cognitive decline and caregivers in coping behaviours and supervision | Dementia NOT diabetes | People with dementia and their informal carers |
| Graff et al., 200896 (linked to Graff et al., 200665) | Netherlands | RCT | To assess the cost-effectiveness of community-based occupational therapy, compared with usual care, in older patients with dementia and their care givers from a societal viewpoint | Ten sessions of occupational therapy over five weeks, including cognitive and behavioural interventions, to train patients in the use of aids to compensate for cognitive decline and care givers in coping behaviours and supervision | Dementia NOT diabetes | People with dementia and their informal carers |
| Graff et al., 200795 | Netherlands | RCT | To investigate the effects of community occupational therapy on quality of life, mood and health status in dementia patients and their caregivers | Occupational therapists trained in collaborative assessment (80 hours) and familiar with dementia patients provided 10 × 1-hour sessions to people living with dementia and carers. The first four sessions were assessment and goal-setting through collaborative techniques. The remaining six sessions implemented compensatory strategies to adapt patients’ ADL; caregiver supervision skills were also monitored | Dementia NOT diabetes | People with dementia and family carers |
| Greenhalgh et al., 201397 (part of Athene study and related to Procter et al., 2014120) | UK | Qualitative | To define quality in telehealth and telecare with the aim of improving the proportion of patients who receive appropriate, acceptable and workable technologies and services | NA | Other | People aged ≥ 60 years with multimorbidity |
| AT/telecare service providers | ||||||
| AT designers | ||||||
| Hackel, 201366 | USA | Opinion/discussion | . . . to apply and compare aspects of person centred care and recent consensus guidelines to two cases of older adults with poorly controlled diabetes in the context of relatively similar multimorbidity | NA | Dementia AND diabetes | Older adults with T2DM and complex multimorbidities |
| Heisler et al., 200398 | USA | Survey | To assess the extent to which patients with T2DM agree with their primary care providers on diabetes treatment goals and strategies, the factors that predict agreement, and whether or not greater agreement is associated with better patient SM of diabetes | NA | Diabetes NOT dementia | Adults with diabetes |
| Hsu et al., 201699 | USA | RCT | To help patients develop improved self-efficacy and more accurate management models for diabetes | Training in the use of a software interface for glucose monitors and a cloud-based system to track HbA1c level in order to self-administer insulin more accurately | Diabetes NOT dementia | Independent adults with T2DM |
| Huang et al., 2005100 | USA | Qualitative | To explore. . . self-reported healthcare goals, factors influencing these goals, and self-care practices of older patients with diabetes mellitus | NA | Diabetes NOT dementia | Older people with T2DM |
| Iliffe et al., 200667 | UK | Qualitative | If the job categories cannot expand as fast as is needed, the tasks of dementia care will have to be redistributed, suggesting that skills will have to be shared and transferred between different disciplines. The question for service commissioners and providers is: how can smarter working be achieved? This article attempts to answer this question . . . | NA | Dementia NOT diabetes | HCPs |
| Institute of Diabetes in Older People, 201330 | UK (and International) | Guidance | International group of diabetes experts considered the key issues that require attention in supporting the highest quality of diabetes care for older people | NA | Dementia AND diabetes | Older people with diabetes, including those with dementia |
| Jekel et al., 2015101 | NA | SR | To summarise the research results regarding the performance of patients with MCI in specific IADL (sub)domains compared with persons who are cognitively normal and/or patients with dementia | NA | Dementia NOT diabetes | People with MCI |
| Jowsey, 201469 | Australia/New Zealand | Qualitative | To describe motivation towards or away from SM in diverse group of older Australians with T2DM, COPD and CHF | NA | Diabetes NOT dementia | Older people with T2DM, COPD or CHF |
| Jowsey et al., 2016102 | Australia/New Zealand | Qualitative | To explore how patients with chronic illness (COPD, CHF or T2DM) manage their condition in the absence of health organisation continuity of care | NA | Diabetes NOT dementia | People with T2DM, COPD or CHF |
| Kennedy et al., 2013103 | UK | RCT | To determine the effectiveness of an intervention to enhance SM support for patients with chronic conditions in UK primary care | Practice-level training in a whole-systems approach to SM support. Practices were trained to use a range of resources: a tool to assess the support needs of patients, guidebooks on SM, and a web-based directory of local SM resources. Training facilitators were employed by the health management organisation | Other | People with LTCs |
| Kennedy et al., 2014104 | UK | Survey and interviews | To identify influences affecting the implementation of an intervention of a SM support approach (WISE) at patient, clinical and organisational levels | SM support intervention (this is a process evaluation of the intervention; see Kennedy et al.103) | Other | Organisational stakeholders, practice staff and trial participants |
| Kennedy et al., 201460 | UK | Qualitative | To evaluate the implementation and embedding of SM support in a UK primary care setting | SM support intervention (this is a qualitative study linked to the intervention; see Kennedy et al.103) | Other | Practice nurses |
| Knapp et al., 2015105 | UK | Rapid review | To undertake a cost–benefit analysis to consider the hypothesis that accelerated investment in technology could, over a series of different time frames, deliver savings on the overall cost of care (for people with dementia) | IT | Dementia NOT diabetes | Academics, managers and telecare representatives |
| Laakkonen et al., 2016106 | Finland | RCT | To investigate the effects of SM group rehabilitation for people with dementia and their spouses on their health-related quality of life | SM group intervention in which SM capabilities such as problem-solving, self-efficacy and mastery were built gradually | Dementia NOT diabetes | People with a recent diagnosis of dementia and their spouses |
| Lingler et al., 2016107 | USA | RCT | To develop and examine the efficacy of a tailored problem-solving intervention on informal caregivers’ management of medications for community-dwelling persons with memory loss | The intervention was delivered by either a nurse or a social worker and included two or three home visits, 2 weeks apart, followed by two or three telephone sessions, 7–10 days apart. Sessions always included discussion of issues that the caregiver was experiencing. Carers received a copy of the intervention ‘manual’ for reference between sessions and after the trial. The intervention addressed seven basic aspects of the caregiver’s role in managing medications (e.g. ‘preventing errors’ and ‘contingency planning’) | Dementia NOT diabetes | People with memory loss and their informal carers |
| Markle-Reid et al., 2016108 | Canada | Before-and-after study | To investigate the feasibility of a RCT to examine the effectiveness of the ACHRU Partnership Program, an interprofessional nurse-led programme for community-living older adults with T2DM and other multimorbidities | Home visits by a nurse and a dietitian from the diabetes education centre, monthly group sessions for participants, monthly nurse-led case conferences for team members and nurse-led care co-ordination | Diabetes NOT dementia | Older people with T2DM and at least two comorbid conditions |
| Martin et al., 2013109 | UK | Qualitative | To explore barriers to SM among people living with dementia | NA | Dementia NOT diabetes | People with dementia, carers, service providers |
| Martin et al., 2015110 | UK | Qualitative | To evaluate the experiences of attending a novel SM programme and initial process evaluation. The programme was designed with and for people with dementia | Six sessions, each lasting 2.5 hours, held weekly, and covering, practical, emotional, physical and physiological and lifestyle aspects of managing the issues encountered by people in the early stages of living with dementia | Dementia NOT diabetes | People in the early stages of dementia |
| Mathers et al., 2012111 | UK | RCT | To test the PANDA decision aid, which facilitates decision-making between people with T2DM and clinicians, when the patient is taking at least two oral glucose-lowering drugs at maximum tolerated dose and has a high HbA1c level, and for whom the introduction of insulin is being considered | Brief training of clinicians and use of PDA with patients in a single consultation. The development of the intervention was based on the UK MRC framework for the development and evaluation of complex interventions | Diabetes NOT dementia | People with T2DM aged ≥ 21 years |
| Mayberry et al., 201168 | USA | Mixed methods, focus groups and survey | To explore the role of patient health literacy, numeracy and computer literacy on usage of a different patient web portal and other forms of health IT | NA | Diabetes NOT dementia | Adults with T2DM |
| Mayberry et al., 2016112 | USA | Qualitative | To develop and test a telephone coaching system to improve self-care for people with T2DM from low-SES groups | Two weeks of tele-health coaching, a discussion to set personal goals and follow-up individualised text messaging to encourage participants to achieve the goals | Diabetes NOT dementia | People with T2DM and family members, from low-SES backgrounds |
| McBain et al., 2016137 | NA | SR | To assess the effects of diabetes SM interventions specifically tailored for people with T2DM and severe mental illness | Diabetes SM interventions designed for people with mental health problems | Diabetes NOT dementia | Adults with T2DM and severe mental illness |
| McBain et al., 2016140 | UK | Qualitative | Explore the barriers and facilitators HCPs experience when managing T2DM in people with severe mental illness | NA | Diabetes NOT dementia | Adults with T2DM and severe mental illness |
| Metzelthin et al., 2013113 (linked to Metzelthin et al., 2013114) | Netherlands | Process evaluation | To examine the extent to which the interdisciplinary care approach is implemented as planned and gain insight into HCPs’ and frail older people’s experiences regarding the benefits, burden, stimulating factors and barriers | Nurse-led interdisciplinary care approach (care management approach). Called Prevention of Care, it involves screening for frailty, care planning and a ‘flexible toolbox of interventions’ (e.g. enhancing meaningful activities, stimulating health). A nurse acts as case manager. The intervention is based on the ‘5 As’ behaviour change model (Assess, Advise, Agree, Assist, Arrange) | Other | Frail older people |
| Metzelthin et al., 2013114(linked to Metzelthin et al., 2013113) | Netherlands | RCT | To evaluate whether an interdisciplinary primary care approach for community-dwelling frail older people is more effective than usual care in reducing disability and preventing (further) functional decline | Nurse-led interdisciplinary care approach (care management approach). Called Prevention of Care, it involves screening for frailty, care planning and a ‘flexible toolbox of interventions’ (e.g. enhancing meaningful activities, stimulating health). A nurse acts as case manager. The intervention is based on the ‘5 As’ behaviour change model | Other | Frail older people |
| Mountain, 200637 | NA | Scoping review | To describe the concept of SM and how it is being promoted and consider how people with early dementia might be enabled to SM | NA | Dementia NOT diabetes | People with early dementia |
| Mountain and Craig 2012115 | UK | Qualitative | To identify priority topics for a potential SM programme and to explore the relevance of the identified topics with a consultation group of people living with dementia and their carers to inform the creation of a draft SM programme | NA | Dementia NOT diabetes | People with early dementia |
| Munshi et al., 2011117 | US | Case study | To evaluate hypoglycaemia in older people with HbA1c levels of ≥ 8% with continuous glucose monitoring | NA | Diabetes NOT dementia | People aged ≥ 69 years with HbA1c values of ≥ 8% |
| Munshi et al., 2013116 | US | RCT | To evaluate whether or not assessment of barriers to self-care and strategies to cope with these barriers in older adults with diabetes is superior to usual care with attention control | Geriatric diabetes team (a geriatric diabetologist, a diabetes educator and a nutritionist) identified strategies to help patients cope with barriers. Strategies were designed to optimise patients’ self-care, leading to better treatment adherence. Patients were given individualised strategies either in person or by telephone | Diabetes NOT dementia | Community-dwelling adults aged ≥ 69 years with T2DM |
| Newton et al., 2016118 | UK | Qualitative | To examine the motives that people living with T2DM have for SM and the methods they use to assess their success | NA | Diabetes NOT dementia | People with T2DM |
| Penn et al., 2015119 | UK | Case study | To explore and illuminate the processes and points where people struggle to find SM support | NA | Diabetes NOT dementia | Staff from primary care general practices in the UK |
| Piette and Kerr, 200643 | NA | Opinion/discussion | To examine the effectiveness of diabetes care when diabetes mellitus is one of several long-term health problems and to identify pathways for improvement | NA | Diabetes NOT dementia | People with diabetes and comorbidities |
| Procter et al., 2014120 (part of Athene study and related to Greenhalgh et al., 201397) | UK | Qualitative | To explore the experiences of older people who use assisted living technologies and care services | NA | Other | Older adults |
| Quinn et al., 2016121 | UK | RCT | To explore the feasibility of SM intervention for people with early-stage dementia compared with treatment as usual | Each session began with time for social interaction, then a discussion. Each session focused one of the following: information about dementia, enjoying favourite activities, staying well, managing memory difficulties, coping skills, maintaining relationships and planning for the future. Sessions ended with a 5-minute mindfulness exercise. Caregivers attended the first and last sessions and could join each session at the end for a summary of what had been discussed | Dementia NOT diabetes | People with dementia or MCI |
| Quinn et al., 2016136 | International | Review | Identify group-based psychosocial interventions for people with dementia or MCI that incorporate significant elements of SM | Group-based psychosocial interventions | Dementia NOT diabetes | People with dementia or MCI |
| Reinhardt Varming et al., 2015122 | Denmark | Feasibility study | To explore the feasibility of a research-based programme for patient-centred consultations to improve medical adherence and blood glucose control in patients with T2DM | Patient-centred empowerment, motivation and medical adherence consultation programme. Intervention developed by the action learning process. Three one-to-one consultations with the same HCP (nurse or physician) to ensure continuity. The main focus of the programme is to explore and resolve challenges patients may have with implementing prescribed medication and in obtaining good glycaemic control | Diabetes NOT dementia | People with T2DM aged 49–85 years |
| Ryan and Sawin, 200945 | USA | Qualitative | To clarify the concept of SM, comment on the divergence of research, theoretical and conceptual thinking, present the individual and family SM theory and identify opportunities for future study of SM | The study evaluated multiple SM interventions aimed at improving various health outcomes | Other | People with LTCs |
| Sachar, 2012123 | UK | Opinion/discussion | Aim not stated: describes the service | Integrated diabetes care (Inner North West London Integrated Care Pilot). Integrates diabetes care and mental health care | Dementia AND diabetes | People with diabetes and mental health problems that could involve CI or dementia |
| Schaller et al., 2016124 | Germany | Before-and-after study | To assess the usefulness and impact of the European eHealth Monitor project Dementia Portal service in the dementia care setting from two user perspectives: informal caregivers and professionals | A web portal for informal caregivers and professionals was tested for a 12-week period | Dementia NOT diabetes | Family carers of people living with dementia and community HCPs in Germany |
| Schulman-Green et al., 2016125 | NA | SR | To identify factors that are facilitators and barriers to SM by adults with chronic illness | NA | Diabetes NOT dementia | Of 53 included studies, 28 reported on diabetes, and a remaining 20 reported on cardiovascular disease |
| Sinclair et al., 201428 | UK | Guideline | To provide guidance for the care of people with dementia and diabetes | NA | Dementia AND diabetes | People with dementia and diabetes |
| Span et al., 2013127 | NA | SR | To gain insight into the involvement of people with dementia in developing supportive IT applications | Computer applications involving people with dementia | Dementia AND diabetes | People with dementia |
| Suh et al., 2004128 | Other | Observational | To measure rates of decline in cognition and function in patients with Alzheimer’s disease and to investigate their accelerating risk factors in Republic of Korea | NA | Dementia NOT diabetes | People with a diagnosis of Alzheimer’s disease |
| Sun and Guyatt, 2013129 | NA | Opinion/discussion | NA | NA | Diabetes NOT dementia | People with LTCs including diabetes |
| Tan et al., 2015130 | NA | SR | To evaluate the effectiveness of diabetes self-care interventions for older adults with diabetes and identify the factors influencing self-care behaviours | Diabetes self-care interventions for older adults | Diabetes NOT dementia | Older adults with diabetes |
| Taylor et al., 2014131 | NA | SR | To evaluate the evidence on SM support for people with one or more LTCs in order to inform commissioners and health-care providers about what works, for whom and in what contexts | SM support interventions | Other | People with LTCs |
| Taylor et al., 2016141 | UK | Evaluation | To evaluate the pilot of an evidence-based care quality improvement intervention for diabetes in primary care | Quality improvement programme for diabetes in primary care | Diabetes NOT dementia | General practices in the UK |
| Toms et al., 2015132 | UK | Qualitative | To explore:. . . the views of people with dementia and family caregivers on the use of self-management in dementia | NA | Dementia NOT diabetes | People with dementia and carers |
| Wherton et al., 2012133 | UK | Qualitative | To discuss the challenges of understanding the assisted living needs of older people in domestic settings and methods to support their involvement in the coproduction of assisted living technologies | NA | Other | People with LTCs including heart disease, stroke, COPD, diabetes, Alzheimer’s disease, falls, visual impairment and osteoarthritis |
| Yardley et al., 2015134 | Other | Realist synthesis | To investigate how primary health-care delivery and professional experiential learning interact to generate outcomes valued by patients with multimorbidity and HCPs | NA | Other | People with multimorbidity |
Appendix 5 Evidence supporting context–mechanism–outcome configurations from the literature
| Study (type) | Condition | Evidence | ||
|---|---|---|---|---|
| Dementia | Diabetes | Other | ||
| Stigma and barriers | ||||
| Bunn et al., 201639 (mixed methods) | ✗ | ✗ |
|
|
| Iliffe et al., 200667 (qualitative) | ✗ |
|
||
| Kennedy et al., 201460 (RCT) | ✗ | ✗ |
|
|
| Knapp et al., 2015105 (review) | ✗ |
|
||
| Metzelthin et al., 2013113 and Metzelthin et al., 2013114 (RCT and process evaluation) | ✗ |
|
||
| Sinclair et al., 201428 (best practice statement) | ✗ | ✗ |
|
|
| Yardley et al., 2015134 (realist synthesis) | ✗ (LTC) |
|
||
| Reinforce positive attitudes (e.g. focus on abilities rather than disabilities) | ||||
| Bahar-Fuchs et al., 201380 (SR) | ✗ |
|
||
| Camp et al., 201559 (non-randomised controlled) | ✗ (CI) | ✗ |
|
|
| Clare et al., 201385 (protocol for RCT) | ✗ | Negative influences can contribute to the development and maintenance of ‘excess’ disability – where the extent of functional disablement is greater than would be predicted by the degree of impairment [e.g. through loss of confidence]
|
||
| Davis et al., 201262 | ✗ (CI) | ✗ |
|
|
| Graff et al., 200665 (RCT) (links to Graff et al., 200795 and Graff et al., 200896) | ✗ |
|
||
| Laakkonen et al., 2016106 (RCT) | ✗ |
|
||
| Martin et al., 2013109 (qualitative) | ✗ |
|
||
| Martin et al., 2015110 (qualitative) | ✗ |
|
||
| Mountain 200637 (review) | ✗ | Neglect of the potential of self-management can be attributed to the gap that exists between the commonly held interpretations of self-management and the prevailing understandings of the abilities of people with early dementia
|
||
| Mountain and Craig, 2012115 (qualitative) | ✗ |
|
||
| Quinn et al., 2016121 (feasibility study) | ✗ |
|
||
| Taylor et al., 2016141 (SR) | ✗ |
|
||
| Toms et al., 2015132 (qualitative) | ✗ |
|
||
| Study (type) | Condition | Evidence | ||
|---|---|---|---|---|
| Dementia | Diabetes | Other | ||
| Identifying patient and carer priorities | ||||
| Alsaeed et al., 201679 (SR) | ✗ |
|
||
| Anderson et al., 201573 (review) | ✗ |
|
||
| Bergdahl et al., 201372 (case study) | ✗ |
|
||
| Branda et al., 201371 (RCT) | ✗ |
|
||
| Jowsey et al., 201469 (qualitative) |
|
|||
| Jowsey et al., 2016102 (qualitative) |
|
|||
| Dhedi et al., 201487 (qualitative) | ✗ |
|
||
| Heisler et al., 200398 (survey) | ✗ |
|
||
| Mathers et al., 2012111 (RCT) | ✗ |
|
||
| Newton et al., 2016118 (qualitative) | ✗ |
|
||
| Schulman-Green et al., 2016125 (SR) |
|
|||
| Person-centred approaches | ||||
| Anderson et al., 201573 (review) | ✗ |
|
||
| Bailey et al., 201681 (RCT) | ✗ |
|
||
| Bergdahl et al., 201372 (case study) | ✗ |
|
||
| Huang et al., 2005100 (qualitative) | ✗ |
|
||
| De Vriendt et al., 201558 (RCT) | ✗ |
|
||
| Goeman et al., 201694 (uncontrolled before-and-after study) | ✗ |
|
||
| Heisler et al., 200398 (survey) | ✗ |
|
||
| Markle-Reid et al., 2016108 (feasibility study) |
|
|||
| McBain et al., 2016137 (SR) | ✗ | ✗ |
|
|
| Munshi et al., 2011117 (case study) |
|
|||
| Munshi et al., 2013116 (RCT) | ✗ |
|
||
| Reinhardt Varming et al., 2015122 (feasibility study) | ✗ |
|
||
| Ryan and Swain, 200945 (description of development of model) | ✗ | ✗ |
|
|
| Schulman-Green et al., 2016125 (SR) | ✗ |
|
||
| Sherifali et al., 2015126 (SR) | ✗ |
|
||
| Span et al., 2013127 (SR) | ✗ | ✗ |
|
|
| Yardley et al., 2015134 (realist synthesis) | ✗ (LTC) |
|
||
| Functional abilities | ||||
| Camp et al., 201559 (controlled study) | ✗ (CI) | ✗ |
|
|
| De Vriendt et al., 201558 (qualitative) |
|
|||
| Goodwin et al., 2013138 (case study) | ✗ |
|
||
| Graff et al., 2006,65 Graff et al., 200795 (RCT) | ✗ |
|
||
| Huang et al., 2005100 (qualitative) | ✗ |
|
||
| Iliffe et al., 200667 (qualitative) | ✗ |
|
||
| Metzelthin et al., 2013113 | ✗ |
|
||
| Tan et al., 2015130 (SR) | ✗ |
|
||
| Toms et al., 2014132 (qualitative) | ✗ |
|
||
| Yardley et al., 2015134 (realist synthesis) |
|
|||
| Study (type) | Condition | Evidence | ||
|---|---|---|---|---|
| Dementia | Diabetes | Other | ||
| Appropriate skills (e.g. enablement, listening, communication, negotiation) | ||||
| Branda et al., 201371 (RCT) | ✗ |
|
||
| Iliffe et al., 200667 (qualitative) | ✗ |
|
||
| Kennedy et al., 2013103 (qualitative) | ✗ | ✗ |
|
|
| Kennedy et al., 2014104 and Kennedy et al., 201460 (RCT) | ✗ | ✗ |
|
|
| Mathers et al., 2012111 (RCT) | ✗ |
|
||
| Metzelthin et al., 2013113 and Metzelthin et al., 2013114 (RCT and process evaluation) | ✗ |
|
||
| Penn et al., 2015119 (modelling pathways) | ✗ |
|
||
| Reinhardt Varming et al., 2015122 (feasibility study) | ✗ |
|
||
| Sachar, 2012123 (opinion/discussion) | ✗ | ✗ |
|
|
| Sun and Guyatt, 2013129 (editorial on Kennedy trial) | ✗ | One might seriously question doctors’ awareness of the importance of patient SM of chronic conditions
|
||
| Tailored and flexible | ||||
| Abdelhafiz et al., 201625 (review) | ✗ |
|
||
| Alsaeed et al., 201679 (SR, qualitative) |
|
|||
| Donald et al., 201388 (observational) |
|
|||
| Dugmore et al., 201589 (SR) | ✗ |
|
||
| Heisler et al., 200398 (survey) | ✗ |
|
||
| Jowsey et al., 201469 | ✗ |
|
||
| Munshi et al., 2011117 (case study using continuous glucose monitoring) | ✗ |
|
||
| Munshi et al., 2013116 (RCT) | ✗ |
|
||
| Taylor et al., 2016141 (case study) | ✗ |
|
||
| Yardley et al., 2015134 (realist synthesis) |
|
|||
| Study (type) | Condition | Evidence | ||
|---|---|---|---|---|
| Dementia | Diabetes | Other | ||
| Communication and regular contact (allows one to anticipate needs) | ||||
| Alsaeed et al., 201679 (SR) | ✗ |
|
||
| Boots et al., 201661 (mixed methods) |
|
|||
| Brown et al., 201583 (opinion/discussion) | ✗ | ✗ |
|
|
| Bunn et al., 2017142 (qualitative) (and Bunn et al., 201639) | ✗ | ✗ |
|
|
| Camp et al., 201559 (controlled study) | ✗ | ✗ |
|
|
| Chrvala et al., 201684 (SR) | ✗ |
|
||
| Davis et al., 201262 (RCT) | ✗ (MCI) | ✗ (heart failure) |
|
|
| Dhedi et al., 201487 (qualitative) | ✗ |
|
||
| Feil et al., 201164 (qualitative) | ✗ | ✗ |
|
|
| Giebel et al., 201592 (qualitative) | ✗ |
|
||
| Hackel, 201366 (case report) | ✗ |
|
||
| Munshi et al., 2013116 (RCT) | ✗ | ✗ |
|
|
| Newton et al., 2016118 (qualitative) | ✗ |
|
||
| Penn et al., 2015119 (case study) | ✗ |
|
||
| Piette and Kerr, 200643 (opinion/discussion) | ✗ |
|
||
| Sinclair et al., 201428 (guidelines) | ✗ | ✗ |
|
|
| Suh et al., 2004128 (observational) | ✗ |
|
||
| Tan et al., 2015130 (review) |
|
|||
| Study (type) | Condition | Evidence | ||
|---|---|---|---|---|
| Dementia | Diabetes | Other | ||
| Engaging with families | ||||
| Aikens et al., 201563 (before-and-after study) | ✗ |
|
||
| Alsaeed et al., 201679 (review) | ✗ |
|
||
| Bergdahl et al., 201372 (qualitative case studies) |
|
|||
| Boots et al., 201482 (SR) | ✗ |
|
||
| Boots et al., 201661 (mixed methods) |
|
|||
| Bunn et al., 201639 (mixed methods) | ✗ | ✗ |
|
|
| Bunn et al., 2017142 (qualitative) (related to Bunn et al., 201639) | ✗ | ✗ |
|
|
| Graff et al., 200665 (RCT) | ✗ |
|
||
| Graff et al., 200795 (links to Graff et al., 200665) (RCT) | ✗ |
|
||
| Feil et al., 201142 (qualitative) | ✗ | ✗ |
|
|
| Lingler et al., 2016107 (RCT) | ✗ |
|
||
| Markle-Reid et al., 2016108 (feasibility study) | ✗ | ✗ (older people) |
|
|
| Mountain and Craig, 2012115 (qualitative) | ✗ |
|
||
| Feil et al., 200942 (cross-sectional) | ✗ | ✗ |
|
|
| Jowsey et al., 201469 (qualitative) | ✗ |
|
||
| Schaller et al., 2016124 (before-and-after study) | ✗ |
|
||
| Tan et al., 2015130 (review) |
|
|||
| Study (type) | Condition | Evidence | ||
|---|---|---|---|---|
| Dementia | Diabetes | Other | ||
| Tailored and adapted | ||||
| Aikens et al., 201563 (before-and-after study) | ✗ |
|
||
| Boots et al., 201661 (qualitative) | ✗ |
|
||
| Camp et al., 201559 (controlled study) | ✗ | ✗ |
|
|
| Fleming and Sum, 201490 (SR) | ✗ |
|
||
| Gibson et al., 201591 (SR) | ✗ |
|
||
| Gillespie et al., 201293 (SR) | ✗ |
|
||
| Greenhalgh et al., 201397 (qualitative) | ✗ |
|
||
| Hsu et al., 201699 (RCT) | ✗ |
|
||
| Jekel et al., 2015101 (SR) | ✗ (CI) |
|
||
| Knapp et al., 2015105 (review, economic evaluation) | ✗ |
|
||
| Mayberry et al., 201168 (mixed methods) | ✗ |
|
||
| Mayberry et al., 2016112 (qualitative) | ✗ |
|
||
| Procter et al., 2014120 (qualitative) | ✗ |
|
||
| Span et al., 2013127 (SR) | ✗ |
|
||
| Wherton et al., 2012133 (qualitative) | ✗ |
|
||
Appendix 6 Evidence supporting context–mechanism–outcomes from the stakeholder interviews
| CMO name | Context | Mechanism and outcome | Interview endorsements |
|---|---|---|---|
| 1: embedding positive attitudes towards people living with dementia | If health and social care delivery systems propagate and reinforce positive attitudes towards people living with dementia and diabetes and their families through tailored SM support . . . | . . . then this fosters a belief in staff that people living with dementia and diabetes have the potential to be involved in SM and the right to access diabetes-related services (even when the trajectory is one of deterioration) (M), prompting treatment confidence in people living with dementia and diabetes (M), which leads to engagement in SM practices by people living with dementia and diabetes, their family carers and HCPs (O) | Dem (n = 46) |
| Diab (n = 28) | |||
| Res (n = 3) | |||
| Total (N = 77) | |||
| . . . people with dementia . . . will go to all sorts of different wards, so I guess it’s just that we need to continue with that work to help people [staff] to understand, you know, how we can support people with dementia . . .Dem 1. . . we still have this thing where if you’ve got dementia, and everything you display is because of your dementiaDem 2. . . not just the education of the staff that go in, but that’s engaging with the heads of councils I guess really, who have the money, who are responsible for the funding of home carersDem 2. . . part of the CQC guidelines are that, in terms of dementia, the guidelines are part of the training . . . but we [nurses] get . . . a 45-minute slot . . . health-care assistants, because it’s part of this new certificate . . . they get 5 hours, so that’s a bit better. So how do you incorporate all the NICE guidelines into that?Dem 2So people with dementia aren’t even allowed to make cups of tea. I think we’ve got better at that, you know, care homes now don’t have locks on kitchens, and you know, there’s tea and coffee available for people, but we are still I think quite risk averseDem 2a. . . she missed four memory clinic appointments and they phoned me and said they were discharging her for non-compliance . . .Dem 4I’ve not come across many in the diabetic team who can really get their heads around what’s happening with the person and that, you know, the person isn’t deliberately non-compliant . . .Dem 6. . . the Getting To Know Me project here in [XXXX] we trained over 600, or 700, frontline practitioners about dementia, just a general aspects of dementia, what it is, how to communicate, what to look for, what people might be saying when they maybe can’t tell you through words . . .Dem 7. . . all of our diabetes specialist nurses have subspeciality . . . I’m hoping that the two DSNs that are now the dementia champions will become exactly that . . .Diab 1I think the main points are getting policy changed around targets and making it OK for people that are frail elderly with dementia to have a higher HbA1c target . . .Diab 9. . . one of my profound frustrations is that you can have people talking about multiple long-term conditions and they’re excluding mental health diagnoses, and yet we know that you know, 30% to 40% of people with diabetes will have anxiety and depression you know, and often early unrecognised memory issues . . .Diab 12a | |||
| CMO name | Context | Mechanism and outcome | Interview endorsements |
|---|---|---|---|
| 2: person-centred approaches to care planning | If delivery systems promote a person-centred and partnership approach to care, allowing HCPs to understand the individual needs and abilities of people living with dementia and diabetes and their families . . . | . . . then (1) HCPs feel confident that they are acting in the best interests of people living with dementia and diabetes and their families (M) and (2) this generates trust between HCPs and people living with dementia and diabetes and their families (M), leading to a better fit between care planning and patient and carer needs and (potentially) a lessening of the burden of medicalisation experienced by people living with dementia and diabetes and their families (O) | Dem (n = 44) |
| Diab (n = 80) | |||
| Res (n = 11) | |||
| Total (N = 135) | |||
| . . . what’s in their best interests and does it really matter if we don’t have perfectly tight diabetic control if they’re enjoying what they’re eatingDem 6I would want people with dementia to be able to eat anything that they want, no diabetes diet, normal foodDiab 6Where the client is fully able to contribute [to a care plan], that usually goes very well because we can then discuss the likes and dislikes, their routines, how they manage their diabetes themselves . . .Dem 4. . . I’m not an expert on dementia, but for me it seems logical . . . that you try and keep it as normal as possible for them, it’s what they’re familiar with isn’t it, you know, so even if it was a case of for example their wife bringing in the blood glucose meter that they use at home . . .Diab 1I inject twice a day and I try to eat to match the insulin . . . It was the only thing I was offered so, you know, at that point and I thought well I can manage that . . .Diab 3a. . . in some places where perhaps they’re doing very good personalised care for that patient, they’ll bring the carers in and they’ll be part of that personalised care process, because they’ll recognise the importance of that carer with somebody with dementiaDiab 4b. . . they’re now working much more towards outcomes . . . so that gives you flexibility to do different things with different people . . .Diab 4. . . since I’ve been in the care of the diabetic clinic everything else has gone out the window. When I was in the care of the specialist nurse at the GP’s, I would have a regular sort of every 6-month check on my feet, you know, yeah, sensitivity in my feet, my kidney and the amount of protein in my urine, all those tests have now ceased, I’m now only looked at from a point of view of sugar levelsDiab 8a,c. . . how could I help this person? What’s their network of support and is that going to be enough? Who would I need to get involved? So you’re really breaking it down like thatDiab 9I think different targets for certain groups of people you know, and quality of life targets rather than all about number crunching . . .Diab 13. . . educate the GPs that the targets might not need to be so strict for these people [with dementia and diabetes]Diab 13I think health professionals are possibly becoming themselves much more risk averse and not wanting to suggest things that aren’t perceived as being healthy or might not be the right answerRes 1. . . you go and get your monthly prescription . . . and you have to do it for numerous medications that are often out of sync and then pharmacy doesn’t have things in stock, and it’s just, you know, a huge burden of work involved, which is invisible to care providersRes 3 | |||
| CMO name | Context | Mechanism and outcome | Interview endorsements |
|---|---|---|---|
| 3: developing skills to provide tailored and flexible care | If HCPs are expected to develop skills that enhance the delivery of individualised and tailored care to people with dementia and diabetes (e.g. enablement rather than management, listening/communication/negotiation, shared decision-making) . . . | . . . then this legitimises the work, creating the expectation in patients and HCPs that diabetic care for people living with dementia is important (M), leading to the provision of more tailored diabetes care (O) and better engagement in SM by people living with dementia and diabetes and family carers (O) | Dem (n = 46) |
| Diab (n = 44) | |||
| Res (n = 6) | |||
| Total (N = 96) | |||
| . . . we’ve had very little training about the management of diabetes, so, and I’m imagining that a lot of my, you know, my ideas about diabetes management are quite old-fashioned and if I’m trying to educate other people then that’s not a great position . . .Dem 1I don’t think we’re supporting people with diabetes [and dementia] as well as we could, because of this training issue and where responsibility lies . . .Dem 4. . . we don’t tend to think of dementia as a kind of life-limiting condition which we need to perhaps change that and to think a little bit more . . .Dem 1You tailor around maximising the possibility the person will be able to access that treatment . . .Dem 5a. . . if somebody’s not doing something at all and you can get them doing something a little bit, it’s really noticeable what a difference that makes to their cognition . . .Dem 5. . . you can conceptualise it as tailoring, but I suppose in the way that I see it in my mind is there’s probably a checklist of about 10 or 15 things that we think about, and you sort of run through them and you think what’s most relevant to that personDem 5. . . in the memory clinic we try to do shared decision-making and thinking about what people wanted . . . we’d talk to them about various options for management of their dementia . . . I’m not sure I even said to people . . . let’s think about how we manage your other health problems, like I’m just thinking now, that’s probably something that we didn’t address . . .Dem 6. . . it’s about having . . . different levels of intervention or help or support or enablement that are actually needed, given the scenarios in which people find themselves . . .Dem 7. . . there’s national guidance, isn’t there, in terms of what the target HbA1c is per se, but as I say when we’re actually seeing individuals we certainly do tailor that to their needs, to their comorbidities, to the situation at the time and for their safety ultimatelyDiab 1NICE has released five guidelines . . . the one on type 2 diabetes . . . does suggest goals for glucose, but it does say very clearly that these may need to be modified in people on an individual basis in people who are older who have got significant comorbiditiesDiab 6. . . I don’t know if they have QOF indicators for dementia, but there are QOF indicators for diabetes, and often they can see that as a tick box . . . health-care professionals will just look at what’s happening with the diabetes now, instead of thinking . . . what could happen in 3 or 4 years?Diab 4. . . people shouldn’t be running high . . . let’s say 15 or 12 . . . but much more important is avoiding hypoglycaemia which affects them in all sorts of ways, it makes their dementia worse, it makes them more likely to fall . . .Diab 5. . . the diabetes needs expert attention but it needs expert attention to simplify the regime . . .Diab 6b | |||
| CMO name | Context | Mechanism and outcome | Interview endorsements |
|---|---|---|---|
| 4: regular contact | If HCPs maintain regular contact over time (e.g. face to face, by telephone or by e-mail) with the person living with dementia and diabetes and their family, monitoring and anticipating needs throughout the dementia trajectory . . . | . . . then HCPs feel more equipped to meet patients’ needs (M), and people living with dementia and diabetes and their family believe themselves to be supported (M) through the transition from functional independence to functional dependence (M), leading to improved diabetes management (O) | Dem (n = 6) |
| Diab (n = 6) | |||
| Res (n = 1) | |||
| Total (N = 13) | |||
| . . . it’s a kind of a whole-system approach really, so you know, it’s having the district nurses on board . . .Dem 2. . . once a year for a formal annual review as it were and whenever I’ve needed it, if there’s been any problems I’ve always been able to be seen and there’s a very good nurse who’s the diabetes person . . .Diab 3a,b. . . if you have one health-care person who you know is almost like your keyworker, your key contact, you build up a relationship, which is very important . . .Diab 4. . . our local GPs are pretty well bogged down and you can’t get an appointment with them just to see a doctor in less than a month . . .Diab 8. . . the ideal thing would be to have a mobile team of nurses who would visit people in their homes very frequently to keep an eye on things and maybe to educate the carers if there are carers . . .Diab 8a,b,c | |||
| CMO name | Context | Mechanism and outcome | Interview endorsements |
|---|---|---|---|
| 5: family engagement | If family carers are routinely involved in care planning and information sharing, and are given the support they need to take on the tasks associated with managing diabetes in people living with dementia (e.g. medicines management, recognition of hypoglycaemia) . . . | . . . then family carers will feel supported and that their contribution is recognised and appreciated (M), leading to the development of effective SM strategies on the part of the family carer (O) | Dem (n = 23) |
| Diab (n = 33) | |||
| Res (n = 4) | |||
| Total (N = 60) | |||
| I wouldn’t say most of the patients I look after are managing their diabetes really themselves, it’s managed by those around them . . .Dem 1. . . more communication between families and people like diabetic nurses . . . a diabetic nurse . . . is going to want to manage diabetes . . . the relative’s going to have to manage the dementia . . .Dem 2It’s very difficult to learn new things, I understand as in this case, [he’s] also got Parkinson’s and Lewy body, so she’s at a breaking point . . .Dem 4. . . you’ve got someone with diabetes who used to perfectly manage their diabetes well themselves, suddenly can’t and now the wife has taken it over, she’s chosen to continue to support him . . . it must be really hard for her . . . she’s gone from being a partner to a carer . . .Diab 1Big responsibility [taking on the management of diabetes], yeah, and I think, yeah, we shouldn’t take that for granted, yeah, it could be a bit daunting for a relativeDiab 7. . . you need to think about the whole care network and that’s where you start thinking about how much can we ask carers to undertake, who have never been in a caring role . . .Diab 10. . . carers are almost always very conscious of the need to help that person to be as independent as possible . . .Dem 5. . . I’ve had this conversation with carers saying, ‘Yes, he has always throughout his life made a decision to drink, but now the decision’s different because now when he drinks, he punches walls, wanders off, makes himself very unsafe,’ and so actually the nature of the decision has changedDem 5a. . . it’s common for families to disagree. Particularly around whether somebody should be allowed to live at home in a risky situation rather than go to a care homeDem 5. . . it’s really hard then [for families] working with that person because they don’t think they’ve got dementia or any memory problems, they may even still be off driving, you’ve got all those issues to deal with as well . . .Dem 6b,c. . . for the family, how lovely would it be for them to just speak to one person who knew about diabetes and the dementia . . .Diab 1c,d. . . dementia is a terminal illness . . . and that relatives very often don’t know that . . .Diab 6. . . as a carer, you are faced with an uphill struggle to actually understand the coherence of the care offered to your loved one with dementia and diabetesDiab 10. . . it’s relationships and it’s negotiating that and making sure that it’s in place and for health professionals to recognise when that’s really fragile and what can be done to just sort of sure things up a bitRes 1a,c | |||
| CMO name | Context | Mechanism and outcome | Interview endorsements |
|---|---|---|---|
| 6: usability of AT | As the dementia trajectory progresses, AT needs to be tailored and adapted to the needs and requirements of the person living with dementia and diabetes and their family (includes social, environmental and cultural needs), with the focus on maintaining autonomy for the person living with dementia and diabetes . . . | . . . leading to the person living with dementia and diabetes and their family gaining an understanding (awareness) of the usefulness of AT in their management of dementia and diabetes (M), leading to a more effective and sustained use of AT to maintain autonomy and diabetes SM strategies (O) | Dem (n = 6) |
| Diab (n = 15) | |||
| Res (n = 13) | |||
| Total (N = 34) | |||
| . . . I was familiar with the sort of dosette box . . . which is a great idea and does really help people, you know, to manage their medications . . .Dem 1. . . there’s electronic dosette boxes that can be linked to telecare, so if the person doesn’t take the medication, telecare will come through the intercom . . . and then if they don’t take them it clicks on and locks anyway so they can’t overdoseDem 2. . . you can get clocks that actually tell you. . .exactly which 7 o’clock of the day it is, so it will tell you it’s 7 o’clock in the evening rather than the morning. . .Dem 2. . . some of the insulin pens are really fiddly as well, like they’ve got really tiny numbers and you have to dial it up and all that kind of thing, I don’t know how well they’re adapted for people with visual problems or cognitive problemsDem 6Timesulin device . . . they can use that lid instead of the normal lid and then when the person takes the lid off, it times when their next inject, when they last took the lid off . . .Diab 9Luckily meters do keep a record of what the blood sugar levels were so you don’t have to write them downDiab 8. . . as health-care professionals, we need to be taught . . . what are the best resources out there that we can use as, if you like, a menu of options that we’ve got with these patients, that we can say well you could try using this alert system, you know, or you can try using this app . . .Diab 9a,bWith socialising there’s technologies that can make things simpler like telephones that can have direct dials with pictures that they could access medical attention if they needed it or their carerRes 2Well, there’s lots of technologies that prompt reminders for anything and you can actually set them upRes 2. . . the pendant alarms . . . now are kind of more standard and that people know that when they press the button that someone’s speaking at the end . . . there’s something about whether they keep the pendant on . . .Res 2I think technologies are great but you need to think is it the right thing for the patient, what they want, is there enough support around it to implement it and respond to it and just to make sure that it’s all OK?Res 2. . . what we found is quite a lot of the carers liked these things because . . . it meant they [carers] would have a sleep, the alarm wouldn’t go off unless they [person with dementia] got out of bedRes 2 | |||
Appendix 7 Table of included studies on self-management for people living with dementia
| Study ID | Aim/focus | Details of intervention and context | Mechanisms | Outcomes |
|---|---|---|---|---|
| Camp et al., 201559 | Determine whether or not a distance-based education intervention would result in positive health outcomes for persons with both diabetes and CI (n = 40) |
|
Develop a connection with CDE: trust (long-term connections important) Participants feel that contacts are positive |
HbA1c: initially declined after treatment but returned to baseline levels after 6 months Self-efficacy: significant increase, which was maintained across follow-ups (increased in both groups) |
| Davis et al., 201262 | Test the effect of targeted intervention on self-care, heart failure knowledge and 30-day readmissions in people with MCI |
|
Teaching in hospital environment could not be translated into self-care management when the person returned home. Self-care behaviours may improve, not as a function of increased knowledge, but more as a function of confidence | Improved knowledge but had no impact on readmission rates or self-care |
| Laakkonen et al., 2016106 | Investigate the effects of SM group rehabilitation for people living with dementia and their spouses on their health-related quality of life (136 couples recruited: intervention group, n = 67; usual care group, n = 69) |
|
Suggest that elements of the programme that encouraged participants’ sense of control, empowerment and self-efficacy may have improved health-related quality of life Development of trust between participants |
Better health-related quality of life in carers at 3 months but not at 9 months Cognition of people living with dementia in SM group improved No increase in health and social service costs |
| Martin et al., 2013109 | To explore barriers to SM among people living with dementia |
|
Barriers lead to people living with dementia feeling disempowered | If people living with dementia are disempowered, then they are more likely to be disengaged from SM |
| Martin et al., 2015110 | Qualitative evaluation of a SM intervention for people in the early stage of dementia (n = 6 participants) |
|
Positivity and empowerment (‘still me’) Group environment and ability to share experience of living with dementia led to participants ‘feeling safe’ Feeling of belonging |
Potentially enables people to reject a passive role |
| Mountain, 200637 | Considers concept of SM for people living with dementia |
|
Maintaining a sense of self Stimulate strengths and personal abilities |
|
| Mountain and Craig, 2012115 | Identify priority topics for a potential SM programme and to explore the relevance of the identified topics with a consultation group of people living with dementia and their carers |
|
Information aimed at carers may increase feelings of powerlessness or helplessness in people living with dementia Maintaining a sense of self |
Not explored in this paper |
| Quinn et al., 2016121 | A pilot RCT of a SM group intervention for people with early-stage dementia (the SMART study) (n = 24) |
|
Findings from qualitative interviews suggest that increased self-efficacy may be related to increased confidence and the widening of social support opportunities | Small effect on self-efficacy in intervention group |
| Quinn et al., 2016136 | To identify group-based psychosocial interventions for people living with dementia or MCI that incorporate significant elements of SM. Included 15 studies (12 with people living with dementia and three with people with MCI) |
|
Reduce feeling of helplessness Assist people to feel confidence in their abilities to perform certain behaviours successfully Development of reciprocal relationships (people living with dementia as provider as well as receiver of information and support) |
Many of the included studies did not report any measurable outcomes or reported patient feedback only The findings from these studies suggest that interventions for people with MCI could improve memory functioning, memory strategy use, knowledge and acceptance. Interventions for people with dementia could improve quality of life and depression |
| Toms et al., 2015132 | Explored the views of people living with dementia and family caregivers on the use of SM in dementia |
|
Mental activity (C), keeps the neural pathways active (M), which maintains cognitive abilities for as long as possible |
Glossary
- Context
- The condition that influences the success or failure of an intervention or programme.
- Mechanism
- The generative force that leads to outcomes; that which influences the reasoning and behaviours of people.
- Mid-range theory
- Delimited in its area of application, the intermediate between a working hypothesis for testing and an all-inclusive grand theory.
- Outcome
- A pattern resulting from the interplay between context and mechanism.
- Programme theory
- Practical and specific to each programme or intervention, it specifies the components of a programme (or intervention) intended to mitigate or resolve the problem and the expected outcomes.
- Realist synthesis
- The process of evidence review that follows the realist approach (also known as realist review).
List of abbreviations
- AT
- assistive technology
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CMO
- context–mechanism–outcome
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HCP
- health-care professional
- HTA
- Health Technology Assessment
- IT
- information technology
- NIHR
- National Institute for Health Research
- OT
- occupational therapist
- PAG
- Project Advisory Group
- Portable Document Format
- QOF
- Quality and Outcomes Framework
- RAMESES
- Realist and Meta-narrative Evidence Syntheses: Evolving Standards
- RCT
- randomised controlled trial
- RMT
- Research Management Team
- SM
- self-management
