Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/24/02. The contractual start date was in November 2014. The draft report began editorial review in September 2019 and was accepted for publication in May 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. This report has been published following a shortened production process and, therefore, did not undergo the usual number of proof stages and opportunities for correction. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Batchelor et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Vitiligo is an acquired chronic skin condition that causes loss of skin pigmentation. This leads to milky white, well-demarcated, non-scaly patches on the affected skin and/or mucosal surfaces. The depigmentation seen in vitiligo is caused by destruction of pigment cells (melanocytes), although the precise cause of this is still unclear. Vitiligo is considered to be a multifactorial disease. 1–6 In the light of recent genome-wide studies, there is growing evidence that vitiligo has, at least in part, an autoimmune basis, and this is a target for future treatments, although these are still in development. 7
Vitiligo affects around 0.5–2% of the world’s population. Vitiligo can develop at any age but most commonly occurs between the ages of 10 and 30 years. 8–12 Although there is equal prevalence of vitiligo in adults and children of both sexes, females tend to seek treatment more often, possibly because of the greater social stigma experienced by women and girls with the condition. 10,13
Vitiligo may be segmental (affecting one specific area of skin) but is commonly non-segmental (affecting multiple, symmetrically-distributed areas). The most commonly affected sites are the face, neck and trunk. 14 The cosmetic disfigurement of this seemingly inconsequential skin disease has a major impact on quality of life. 15 It can be particularly distressing for people with darker skin types, especially if the vitiligo occurs on highly visible sites, such as the face and hands. 16 People with vitiligo can experience a number of psychological problems, such as depression and anxiety, which may lead to low self-esteem and social isolation. 15–18
Current clinical guidelines for the diagnosis and management of vitiligo recommend narrowband ultraviolet B light (NB-UVB), topical tacrolimus, topical corticosteroids (TCSs) and combination treatments. 19,20
Rationale for the HI-Light Vitiligo trial
Importance of the topic to patients and health-care practitioners
A James Lind Alliance Priority Setting Partnership identified priority topics for future vitiligo research, which were important to patients and health-care practitioners. 21 The Home Interventions and Light therapy for the treatment of vitiligo (HI-Light Vitiligo) trial has been designed to address two of the priority topics:
-
Which treatment is more effective for vitiligo: steroid creams/ointments or light therapy?
-
How effective is ultraviolet B light (UVB) therapy when combined with creams or ointments in treating vitiligo?
The Priority Setting Partnership also highlighted the importance of testing vitiligo treatments in children; so the HI-Light Vitiligo trial recruited both children and adults.
Existing evidence
A 2010 Cochrane systematic review looking at interventions for the treatment of vitiligo identified 57 trials covering 68 different treatment options. 22 The quality of the trials included in the review was generally poor, making it difficult to make firm recommendations. The use of NB-UVB light therapy was generally supported and the combination of light treatment with other active interventions appeared to be more effective than monotherapies. However, because of the heterogeneity of trial designs, optimal dosing and treatment regimen for NB-UVB could not be established. 23 In 2016, the Cochrane review was updated, and covered 96 trials, none of which provided evidence that was of sufficient quality to alter these overall conclusions.
When the HI-Light Vitiligo trial was first proposed in 2010, the only randomised controlled trial (RCT) that had been conducted to assess the use of hand-held NB-UVB devices for the treatment of vitiligo was the pilot study to the main HI-Light Vitiligo trial. 24 This demonstrated that the devices were safe and well tolerated when used to treat children and adults at home, and that people with vitiligo were keen to take part in a trial of home-based NB-UVB.
Following this pilot trial, other studies have suggested the efficacy of using hand-held NB-UVB devices for vitiligo, including in children, but the studies have been small or retrospective,25,26 making it difficult to draw firm conclusions.
Importance of assessing the use of hand-held narrowband UVB devices at home
In the UK, NB-UVB treatment is delivered almost exclusively in secondary care, requiring regular hospital visits. NB-UVB is usually reserved for people with widespread vitiligo, because most dermatology services are only equipped with large, full-body NB-UVB units. 19
There are various devices available for the administration of NB-UVB treatments at home, which avoids the need for hospital visits. Some dermatology departments in the UK now supply home NB-UVB units (large machines that look like portable sunbeds for treating large areas of skin) for use by patients with eczema and psoriasis. Early reports suggest that these are well tolerated and effective. 27–30
When treating vitiligo, the choice of the NB-UVB device is usually based on the extent and anatomical location of the vitiligo; limited areas of vitiligo can be treated with a small, hand-held NB-UVB devices. 31
There are several potential benefits of using hand-held NB-UVB devices for treating early, limited vitiligo:
-
reduction in attendance at hospital, and associated time and travel costs for patients
-
treating involved areas only, thus sparing uninvolved skin
-
when more extensive whole-body phototherapy is not indicated, NB-UVB treatment of vitiligo can still be used
-
low cost of the devices relative to expensive whole body units.
Should a hand-held device prove to be effective and safe for the treatment of vitiligo, this could be an important addition to the treatment options available to people with limited vitiligo in the early stages of the condition, or for those wishing to treat specific patches only.
Importance of treating early vitiligo
Clinical studies have suggested that treatment of vitiligo in its early stages is more likely to be beneficial than treatment of longstanding vitiligo. 25,32
For this reason, participants in the HI-Light Vitiligo trial were required to have at least one patch of vitiligo that had changed in the last 12 months (see Chapter 2, Participants, for further details).
Patient-reported outcome measures
A survey and systematic review of the outcome measures used in previous vitiligo trials suggested that patients and clinicians may have disparate views regarding which outcomes are most important in evaluating treatment response for vitiligo. 33
An international e-Delphi consensus exercise has established core outcome domains for future vitiligo trials. 34 Outcomes that should be measured in all future vitiligo trials include:
-
repigmentation
-
cosmetic acceptability of treatment response
-
maintenance of gained repigmentation
-
cessation of spread
-
quality of life
-
burden of treatment
-
safety.
The HI-Light Vitiligo trial will assess all of these core outcome domains. The core outcome domains include important patient-reported outcome measures (PROMs), including the cosmetic acceptability of treatment response. Prior to recruiting participants to the HI-Light Vitiligo trial, we developed a new patient-reported outcome measure to assess this domain: the Vitiligo Noticeability Scale (VNS). This instrument has been recommended for use within the core outcome set,35 and has been used as the primary outcome measure for the trial.
The VNS was co-produced with vitiligo patients, using surveys and focus group work to agree the construct of interest and to develop a preliminary version of the instrument. The VNS measures how ‘noticeable’ the patient thinks their vitiligo is after treatment, using a 5-point scale, with treatment success represented by response options 4 or 5 (‘a lot less noticeable’ or ‘no longer noticeable’).
National Institute for Health Research Health Technology Assessment funding call
In view of the limited evidence for home-based NB-UVB for vitiligo, the UK National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme issued a funding call and subsequently commissioned the HI-Light Vitiligo trial. To the best of our knowledge, the HI-Light Vitiligo trial is the first large-scale, multicentre, pragmatic RCT to evaluate the use of TCS and NB-UVB at home.
The trial includes a nested cost-effectiveness analysis and a mixed-methods process evaluation to explore the views of patients and health-care professionals on the trial treatments and the potential barriers to and facilitators of safe and effective use of the trial treatments within the NHS.
Chapter 2 Methods
The full trial protocol is available at www.journalslibrary.nihr.ac.uk/programmes/hta/122402/#/ (accessed 19 October 2020) and a summary protocol has been published. 36 The Consolidated Standards of Reporting Trials (CONSORT) guidelines have been followed for the analysis and reporting. Some parts of this text have been reproduced with permission from Haines et al. 36 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Trial objectives
-
To evaluate the comparative effectiveness and safety of home-based interventions for the management of active, limited vitiligo in adults and children. Comparing:
-
hand-held NB-UVB light with a potent TCS [mometasone furoate 0.1% ointment (Elocon®, Merck Sharp & Dohme Corp., Merck & Co., Inc., Whitehouse Station, NJ, USA)]
-
a combination of hand-held NB-UVB light plus a potent TCS with potent TCS alone.
-
-
To assess whether or not treatment response (if any) is maintained once the interventions are stopped.
-
To compare the cost-effectiveness of the interventions from an NHS and family perspective.
-
To understand the barriers to and facilitators of adoption of these interventions within the UK NHS.
Trial design
The HI-Light Vitiligo trial was a multicentre, three-arm, parallel-group, pragmatic, placebo-controlled RCT. The trial recruited adults (aged ≥ 16 years) and children (aged ≥ 5 years) with early or limited vitiligo (defined as a coverage of approximately ≤ 10% of the body surface area).
Trial treatments were administered at home by the participant, with or without assistance from a relative/carer. Participants were initially followed up in secondary care at 3 and 6 months post randomisation, and finally at 9 months post randomisation at which time the primary outcome was assessed. Long-term follow-up continued for a further 12 months, with online or postal questionnaires completed at 12, 15, 18 and 21 months post randomisation (Figure 1).
FIGURE 1.
The HI-Light Vitiligo trial flow chart. a, Topical corticosteroid (mometasone furoate 0.1% ointment). MED, minimum erythemal dose.
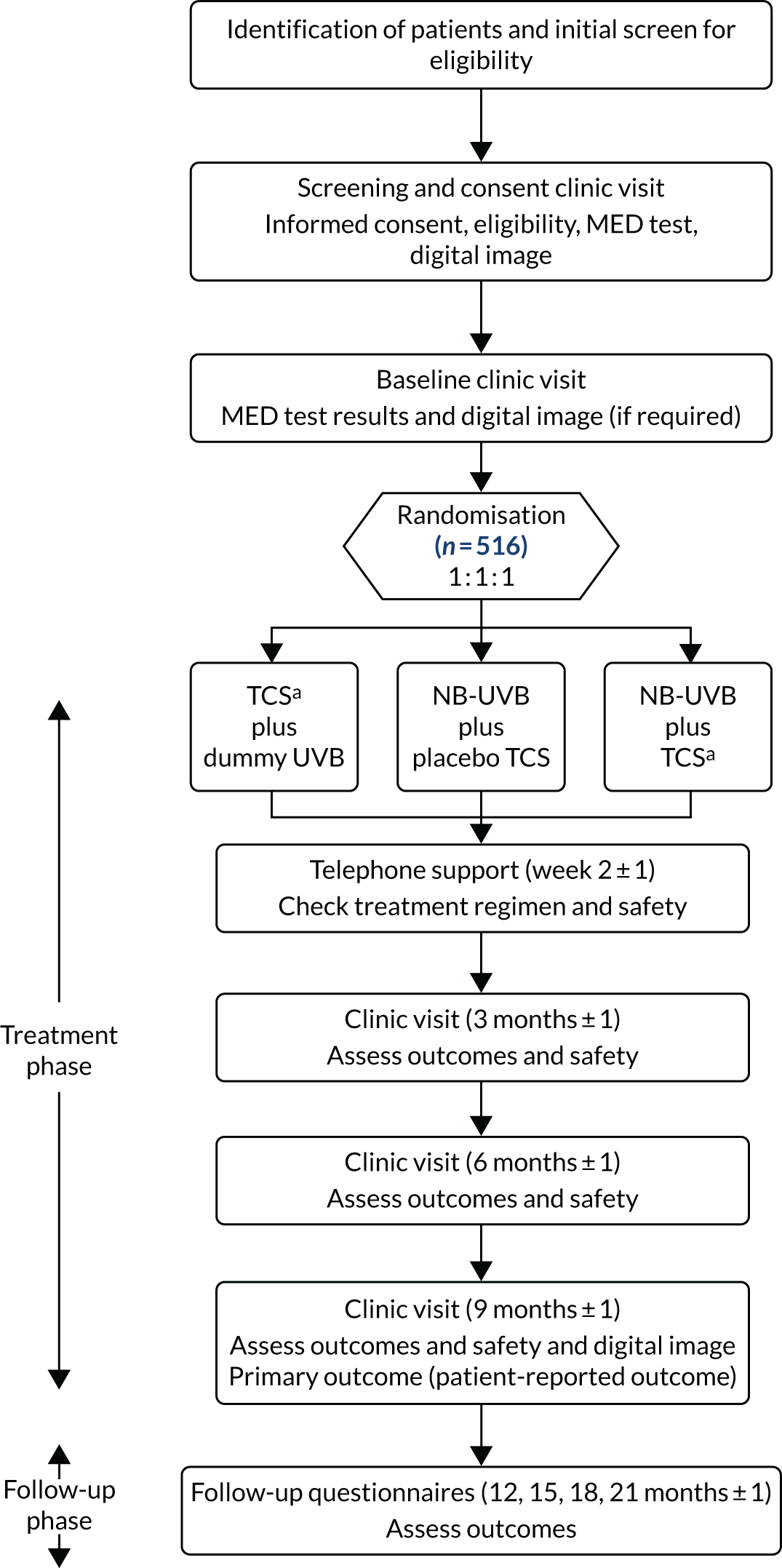
The HI-Light Vitiligo trial included a mixed-methods process evaluation and a health economic analysis. The study was approved by Health Research Authority East Midlands – Derby Ethics Committee (reference number 14/EM/1173) and by the local research and development department for each participating site prior to recruitment commencing. The trial was registered on Current Controlled Trials prior to start of recruitment (ISRCTN17160087). Subsequent changes to the protocol are summarised in Table 1.
| Protocol version | Date | Summary of changes |
|---|---|---|
| 2.0 | 11 March 2015 | Added details of the MRC START substudy |
| 3.0 | 30 September 2015 | Clarified inclusion and exclusion criteria; added more details about training participants to use trial treatments; procedures clarified for digital images outcome analyses; changes to AEs handling for erythema (grades 1 and 2 are not AE, but expected reactions); amendment of prespecified subgroup analysis to remove a comparison of active and inactive patches (as by definition all target patches will be active); addition of a subgroup analysis evaluating response of target patch by region of the body |
| 4.0 | 3 March 2017 | Added details of the nested process evaluation; updates to the safety handling section; introduction of an online automated blind-break procedure; change to sample size following sample size review by the DMC |
| 5.0 | 18 January 2018 | Amendment to reflect the fact that, owing to trial timelines, some participants would not receive the full 12-month follow-up but would receive quality-of-life questionnaires and study feedback questions; updates to statistical analyses section to reflect the statistical analysis plan; addition of output testing of NB-UVB devices after end of treatment phase |
Trial setting
Participants were identified when they attended secondary care dermatology clinics, or when they responded to mailshots sent out from general practices. Some participants self-referred in response to community advertising and trial publicity. A number of patient information sheets were used in the trial, depending on the age of the potential participant.
Recruitment took place at 16 UK sites, details of which are in the Acknowledgements.
Participants
Patients were considered for entry into the trial if the following criteria were met:
-
aged ≥ 5 years with a diagnosis of non-segmental vitiligo confirmed by a dermatologist
-
vitiligo limited to approximately ≤ 10% of body surface area, with at least one patch reported by the participant to have been active in the last 12 months
-
no other active therapy for vitiligo (or willing to stop current treatment; no washout period required)
-
able to administer the interventions safely at home
-
able and willing to give informed consent (or parental/guardian consent in the case of children).
In addition, patients were not entered into the trial if any of the following exclusions applied:
-
other types of vitiligo (e.g. segmental or universal vitiligo)
-
patients with vitiligo limited to areas of the body for which NB-UVB light treatment or potent TCS would be inappropriate (e.g. around the genitals)
-
history of skin cancer (ever)
-
history of radiotherapy use (ever)
-
photosensitivity (e.g. lupus, polymorphic light eruption, solar urticaria, chronic actinic dermatitis, actinic prurigo, porphyria or other photosensitivity disorders)
-
pregnant or breastfeeding women
-
current use of immunosuppressive or immune-modifying drugs (e.g. ciclosporin, azathioprine, mycophenolate mofetil, methotrexate)
-
allergy or contraindication to mometasone furoate or its components
-
current participation in another clinical trial or intervention study
-
marked evidence of Koebner phenomenon in the vitiligo (with the condition spreading extensively at the site of skin injury).
Informed consent
Written informed consent was obtained from each participant (or parent/carer in the case of children) prior to any trial procedures being carried out. Children provided assent as well if they wished to. Separate written consent was obtained for participation in the process evaluation, supported by a separate age-appropriate information sheet.
Randomisation and blinding
Randomisation was carried out via a secure web-based server created and maintained by Nottingham Clinical Trials Unit (NCTU). Randomisation was minimised by recruiting centre, body region of target patch (head and neck, hands and feet or rest of body) and age (5–15 years or ≥ 16 years).
Participants were randomised to one of three treatment groups in a ratio of 1 : 1 : 1 as follows:
-
TCS ointment plus dummy NB-UVB light (TCS only)
-
placebo (vehicle) ointment plus NB-UVB light (NB-UVB only)
-
TCS ointment plus NB-UVB light (combination treatment).
After completing training in the in use of the trial interventions, undergoing a minimum erythemal dose (MED) test and having photographs taken of the patches of vitiligo to be assessed in the trial, participants were randomised by staff at the recruiting hospital via a secure web-based server created and maintained by NCTU.
Participants, research nurses, principal investigators, members of the trial management group and data analysts were blinded to treatment allocation. The Senior Data Manager at NCTU (who created the randomisation schedule), medical physics staff (responsible for the testing of NB-UVB devices prior to distribution) and NCTU quality assurance staff (responsible for the blinding of NB-UVB devices) were all aware of the dummy/active nature of each device or ointment.
Although every effort was made to ensure that blinding of trial interventions was maintained, and that interventions were identical, there was a risk of blinding being compromised because of the nature of the treatments and their known side effect profile (in particular, erythema from NB-UVB treatment). Given this risk of unblinding, the following measures were taken to limit the impact that this had on trial results:
-
Information provided to participants emphasised that all participants received at least one active treatment for their vitiligo, reducing the risk of detection bias due to lack of treatment response.
-
Noticeability of vitiligo was assessed using the VNS by an independent panel of three people with vitiligo, all of whom were blinded, using images taken at baseline and after 9 months of treatment. These data are presented as a secondary outcome.
At the end of the treatment phase (9 months), participants and investigators were asked if they believed that they had become unblinded, and, if so, to what treatments they thought that they had been allocated. These data were used to support the interpretation of trial results.
Interventions
Topical therapy
Potent topical corticosteroid
Mometasone furoate 0.1% weight by weight (w/w) ointment (Elocon 0.1% ointment), a potent corticosteroid used once daily, has been recommended in the European Clinical Guidelines for the management of vitiligo. 20 To minimise the risk of adverse reactions, the guidelines recommend a discontinuous regimen involving periods of use followed by break periods. The possible adverse reactions to mometasone furoate 0.1%, as listed in the Summary of Product Characteristics,37 include infection, folliculitis, paraesthesia, burning sensation, contact dermatitis, skin hypopigmentation, hypertrichosis, skin striae, acneiform dermatitis, skin atrophy, pruritus, application site pain and visual disturbance. Participants were advised to stop use of the ointment if they noticed any side effects and to contact the local research team for review and advice on when to restart treatment.
Vehicle ointment
The vehicle ointment was white soft paraffin (an inert ointment) present in the base of mometasone furoate. The expected side effects from this treatment were minimal.
Treatment regimen
To reduce the risk of side effects, topical therapy was applied as a thin layer to the affected patches of skin on alternate weeks only (i.e. 1 week on, 1 week off), for a period of 9 months. To mitigate the risk of interaction between ointment and light therapy, participants were instructed to wait for at least 2 hours following light therapy before applying the ointment.
Light therapy
Narrowband UVB device
Several brands of NB-UVB units are Conformité Européenne (CE) marked for use in treating vitiligo and other skin conditions and are suitable for use at home. Dermfix 1000 MX units were used in the HI-Light Vitiligo trial, as guided by initial feasibility work. 24
Known adverse reactions to NB-UVB light therapy include erythema, blistering, burns, pruritus, perilesional hyperpigmentation, hypersensitivity reactions, cold sores and dry skin. Potential long-term risks include skin ageing and increased risk of skin cancer (although the latter is thought to be very low). 38,39 Side effects can be reduced by appropriate use of the device.
Dummy device
The dummy light therapy device was identical to the active device, with the exception of a specially designed spacer comb (identical to that found on the active device), which was used to block the transmission of NB-UVB light to the skin. The ‘spacer comb’ for the dummy devices was designed by the device manufacturer to be identical in appearance to the standard spacer comb in the normal devices. These dummy spacer combs filtered out UVB without changing the spectrum of visible light emitted by the device so that when the dummy devices were used, they would look and feel just like active devices.
Active and dummy devices were tracked using the manufacturer’s serial numbers. Experience from our pilot trial has shown that the use of a dummy device is acceptable to patients and is effective in blocking the NB-UVB radiation. 24
There are no known side effects of the dummy NB-UVB devices.
Quality control prior to distribution
All light therapy devices (both active and dummy) were tested for safety and ultraviolet output by the Medical Physics Department at Nottingham University Hospitals NHS Trust prior to distribution to participants (see Chapter 7, Device testing). Any device found to have an output that was ± 20% of the expected mean output, or a dummy device testing positive for any NB-UVB emission, was returned to the manufacturer. Any device that was damaged or ceased to function during the treatment phase was replaced with a new unit.
Treatment regimen
Although NB-UVB (ultraviolet radiation wavelengths of 311–312 nm) is now the most common form of light therapy used to treat skin conditions, many gaps remain in the knowledge about its use. In a 2016 paper, Madigan et al. 23 published a list of 12 key questions regarding the use of NB-UVB for generalised vitiligo. How each of these questions have been addressed in the context of the HI-Light Vitiligo trial is presented in Table 2.
| Question | Strategy tested in the HI-Light Vitiligo trial |
|---|---|
| 1. What is the optimal weekly frequency of NB-UVB treatment? | HI-Light Vitiligo trial: every other day (three or four times per week) |
| Rationale: this is the most commonly used treatment regimen in the UK | |
| 2. With regard to initial dosing, which strategy should ideally be employed? | HI-Light Vitiligo trial: all participants started on the same low dose, 0.05 J/cm2 |
| Rationale: MED test was carried out before treatment, but only to identify any undiagnosed cases of photosensitivity. Starting at a fixed low dose, to minimise the risk of symptomatic erythema, was felt to be safer for home delivery of NB-UVB | |
| 3. At subsequent treatments, what increments should be used for dose escalation in the absence of perceptible erythema? | HI-Light Vitiligo trial: 10% dosing increase after each treatment not followed by erythema |
| Rationale: this reflects typical clinical practice in UK phototherapy services | |
| 4. What is the maximum acceptable dose to be given in a single treatment? | HI-Light Vitiligo trial: maximum dose in the trial is 2.81 J/cm2 |
| Rationale: this reflects typical clinical practice in UK phototherapy services | |
| 5. What is the ideal practice for dose adjustment following symptomatic erythema? | HI-Light Vitiligo trial: patient self-adjustment for grades 1 and 2 erythema (according to flow chart in patient handbook) and investigator adjusted dosing for grades 3 and 4 |
| Rationale: the upwards and downwards dosing used in the trial reflects the clinical practice of most UK phototherapy services | |
| 6. How should the protocol be adjusted for missed doses? | HI-Light Vitiligo trial: varies in function of number of missed treatments. One or two missed: go back one step on treatment schedule; three missed: go back two steps on treatment schedule; four to six missed: 50% of last dose; more than six missed: restart treatment schedule from beginning |
| Rationale: this conservative approach ensured that participants who missed a lot of doses were not at risk of symptomatic erythema when they restarted treatment | |
| 7. How should a ‘course’ of NB-UVB therapy be defined? (i.e. At what interval should further exposure be reassessed?) | Not directly applicable within the scope of the trial |
| 8. What is the maximum number of exposures allowable for patients with vitiligo, given the potential risk of carcinogenesis with NB-UVB? | Not directly applicable within the scope of the trial |
| Participants in the trial only treated limited areas of skin and the total number of treatments was less than the current maximum recommended number of treatments | |
| 9. Should dosing strategies differ when treating children with vitiligo? | HI-Light Vitiligo trial: children were treated in the same way as adults. Parents were given the choice of what patches they were comfortable treating and could opt out of treating sensitive areas if they wished to do so |
| Rationale: the home-based treatment is more flexible than hospital-based full-body treatment, so it is possible for children to be treated in the same way as adults | |
| 10. Should shielding of sensitive structures (eyelids, areolas and genitals) be a universal requirement, or is it safe to expose these areas if affected by vitiligo? | HI-Light Vitiligo trial: the trial excluded treatment of vitiligo in the genital region. Other sensitive areas could be treated if they were affected by vitiligo, but would not otherwise be exposed to NB-UVB because of the localised nature of treatment using a hand-held device. If treating the eyes, patients were advised to seek assistance from someone else so that they could keep their eyes closed during treatment, thus reducing the risk of accidental exposure during treatment |
| 11. What is the most accurate definition of treatment unresponsiveness? | HI-Light Vitiligo trial: responsiveness to treatment was defined by patient report using the question ‘Compared with the start of the study, has there been a change in the vitiligo patch?’ |
| 12. How frequently should patients with vitiligo undergo surveillance following completion of a NB-UVB treatment protocol for both signs of relapse and adverse events? Is there a role for phototherapy in maintenance following repigmentation? | HI-Light Vitiligo trial: long-term treatment response was assessed 3-monthly for 1 year following completion of NB-UVB treatment. The trial was not designed to evaluate the use of intermittent treatment for maintenance of response. Long-term adverse events were not specifically collected in the trial |
| Rationale: patients are particularly interested in how long treatment response may last and this is now a core outcome domain for vitiligo clinical trials |
Prior to randomisation, all participants received a MED test to ensure eligibility for the trial. Results of the MED test were not used to determine starting dose of the light therapy, but instead to ensure that the participant did not have any undiagnosed photosensitivity disorder. All participants follow a predefined treatment schedule for the light treatment, with a starting dose of 0.05 J/cm2 (Table 3).
| Situation | What to do |
|---|---|
| No erythema or side effects after last treatment | Increase dose by one step for the next treatment |
| Erythema or overdose | |
| Grade 1 erythema after last treatment | Go back one step on treatment schedule for next treatment |
| Grade 2 erythema after last treatment | Skip next scheduled treatment. Go back one step on treatment schedule for following treatment |
| Grade 3 or 4 erythema after last treatment | Apply thick layer of trial ointment and contact local research team or local on-call dermatologist. Treatment to resume on advice of local research team only |
| Light overdose (used for ≥ 20% longer than intended treatment time) | Apply thick layer of trial ointment and seek medical attention (prescription for clobetasol propionate 0.05% twice per day for 2 or 3 days, as required). Treatment to resume on advice of local research team only |
| Missed treatments | |
| One or two missed treatments | At next session, go back one step on treatment schedule |
| Three missed treatments | At next session, go back two steps on treatment schedule |
| Four or more missed treatments | Contact local research team for advice on new starting dose |
| Side effects | |
| Itchy or dry skin | Apply moisturiser three or four times per day, but not within 2 hours before light treatment. Continue treatments as normal |
| Tan around edges | This is normal. Continue treatments as normal |
| Rash | Stop treatment immediately and seek medical advice. Treatment to resume on advice of local research team only |
| Cold sore | Stop light treatment until the cold sore has healed. Adjust next treatment time according to missed treatment advice |
Storage and distribution of trial treatments
Following quality control assessments (light devices) or qualified person release (ointment), blinded light devices and ointment tubes were dispatched to a central distribution centre (Mawdsleys, Doncaster, UK) for storage. On randomisation of a participant by the trial investigator/nurse, the distribution centre was notified of the container numbers of ointment and the device to be allocated to that participant via a web-based system. Trial treatments were then sent directly to the participant’s home following check and further qualified person release.
Training in use of interventions: ‘train the trainer’
As a part of the trial site initiation training, trial investigators/nurses were given in-depth training in the administering of trial interventions.
Before randomisation, all participants were trained by the site investigator/nurse in how to apply the ointment, including guidance on avoiding application to the eyelids (if < 1 cm away from the eyelid margin) and sensitive body sites, such as the genital area. In addition, participants received training in the correct use of the light therapy devices. Training also covered how to record treatment sessions using the trial handbook, how to follow the trial treatment schedule and how to manage adverse reactions. Participants were given either a digital versatile disc (DVD) or an electronic link allowing them to access a specifically designed training video at home, if they wished to revisit the training at any time. Written instructions were also included in the trial handbook. Any potential participant who was considered unable to follow the treatment regimen safely was excluded from the trial.
Participants received a telephone call from the research nurse 2 weeks post randomisation to check how they were getting on with the trial interventions and to confirm their understanding of treatment usage and completion of the treatment diaries. Additional training on the use of either treatment was provided to the participants at this time point (over the telephone or face to face), if deemed necessary.
Choice of vitiligo patch for treatment
During the baseline clinic appointment, participants were asked to select up to three patches of their vitiligo to be assessed as a part of the trial, one from each of three anatomical regions (head and neck, hands and feet, and rest of body), although they were permitted to treat as many patches as they liked throughout the treatment phase. As an aide-memoire for future appointments, investigators/nurses were encouraged to draw the patches chosen for assessment on ‘manikin drawings’ in the case report form (CRF) workbook. Of the three patches selected for assessment, participants chose one patch in which they would most like to see an improvement and this would be used as the target patch for the trial.
The target patch had to be one that the participant thought had been active in the past 12 months. Previous studies have suggested that patches that are hypomelanotic, with poorly defined borders, are more likely to be active patches and, therefore, are more responsive to treatment. 40 Patches were assessed at the point of randomisation using a Wood’s lamp and designated as hypomelanotic with poorly defined borders (or ‘hypomelanotic’ for short) or amelanotic with sharply defined borders (see Table 5).
Vitiligo is known to respond differently at different body sites, with the face and neck being more likely to respond to treatment than the hands and feet. 41 The training material provided to recruitment centres advised investigators/nurses to inform participants that patches on the hands and feet may be more difficult to treat and so they may wish to choose a target patch from one of the other body regions.
Adherence
Participants used a treatment diary as an aide-mémoire throughout the treatment phase of the trial. Participants were encouraged to record each treatment session (both for ointment and for light therapy) in the treatment diary, along with any additional comments (e.g. experienced adverse reactions). Treatment diaries were reviewed by investigators/nurses at clinic appointments at 3 and 6 months post randomisation to assess the participants’ understanding of the treatment regimen, to encourage adherence and to identify adverse events (AEs) and any potential additional training requirements.
Summary data obtained from the treatment diaries were used to assess adherence to the treatment regime.
Adherence will be expressed as a percentage, calculated by dividing the total number of treatment sessions reported by the participant by the total number of expected sessions from randomisation to the 9-month follow-up. The calculation will account for additional factors: (1) non-treatment session expected due to erythema, (2) discontinued treatment because of full repigmentation (adherence should be considered as 100% from the point where they achieved full repigmentation) and (3) discontinued treatment for any other reasons (adherence will be 0% from the point of reported discontinuation. Reported use up to this point will be used for calculation).
Concomitant medications
The risk of photosensitivity reaction from NB-UVB light in patients on medications is low and no change to existing medications was required at the onset of the trial. Participants were advised at the start of the trial that such reactions can sometimes occur and that they should contact a member of the research team if they developed a persistent rash during the treatment period. Any new medications that were started during the trial were documented on the CRF and also in the participant’s medical records at each visit (after 3, 6 and 9 months of treatment), and any medications known to cause photosensitivity were assessed alongside the reported adverse reactions as a part of the safety profile of the trial.
Because NB-UVB light is a form of radiation, participants were advised to avoid exposure to other forms of ultraviolet radiation during the treatment phase of the trial, including excessive exposure to sunlight.
Patients were eligible to take part in the trial only if they were not using, or were willing to stop using, active therapy for vitiligo. Participants were asked to refrain from using any active treatments for their vitiligo throughout the treatment and long-term follow-up phase to allow the duration of any treatment effect to be evaluated.
Treatment modifications following adverse events
Having been trained in recognising AEs, participants were instructed to record any AEs in their treatment diaries and to contact their recruiting centre if they experienced events of concern or a serious AE (whether or not they felt that it was related to trial treatment). For treatment-related side effects or drug-induced photosensitivity, the site research team provided telephone advice or arranged for a dermatology consultation, as necessary. If required, the research nurse or dermatologist suggested a treatment modification, including reduction or suspension (temporary or permanent) of either the TCS or the light therapy. An appointment was scheduled for a dermatologist to review the side effects if deemed necessary, in particular for reported episodes of skin thinning or for more severe episodes of erythema.
In the case of a medical emergency where an active treatment of the ointment or the device would need to be stopped, investigators and research nurses were advised to assume that both interventions were active. If knowledge of a participant’s allocation was necessary, the local investigator was able to access a 24-hour online blind-break system held by NCTU.
Outcomes
Primary outcome
The primary outcome was participant-reported treatment success at 9 months.
The primary outcome was assessed for each participant at 9 months (at the end of the treatment phase) at the target patch. Treatment success was defined as the participant reporting that their vitiligo was either ‘a lot less noticeable’ or ‘no longer noticeable’ in response to the question ‘Compared with the start of the study, how noticeable is the vitiligo now?’, using the previously validated VNS. 42
Secondary outcomes
-
VNS treatment success by blinded review of digital images at 9 months.
Assessed at 9 months at the target patch by three independent patient reviewers using digital images of the trial participants and using the same question for the primary outcome. Treatment success was derived from the score given by the majority of the three blinded reviewers.
-
Participant-reported treatment success by body region.
Assessed at 9 months, measured using the VNS and analysed by body region (A, B and C). Each participant assessed up to three patches from three different body regions, including the one chosen as the target patch. During the no-treatment follow-up phase, the same question was used at 12, 15, 18 and 21 months to assess long-term patient-reported noticeability for each body region.
-
Onset of treatment response.
Investigator-assessed onset of treatment response (including cessation of spread) for the target patch. To be assessed at 3, 6 and 9 months using the following question – ‘Compared with the start of the study, has there been a change in the vitiligo patch?’
-
Stayed the same (not worsened).
-
Improved.
-
Got worse.
A treatment response was considered to have occurred if the response given was ‘stayed the same’ or ‘improved’. Analyses for this secondary outcome used investigator-assessed responses because they were more likely to remain unblinded than the participants.
-
-
Maintenance of treatment response.
Participant-assessed maintenance of treatment response (including cessation of spread) for the target patch. This was assessed at 12, 15, 18 and 21 months to assess long-term patient-reported noticeability using the following question – ‘Compared to since you stopped using the study treatments, has there been a change in the vitiligo patch?’
-
Improved.
-
Stayed the same.
-
Got worse.
Loss of maintenance of treatment response was defined as ‘got worse’.
-
-
Percentage repigmentation at 9 months.
Percentage repigmentation was assessed at 9 months by a blinded independent dermatologist using digital images taken at baseline and at 9 months for the target patch. Investigator assessment of percentage repigmentation was also conducted at 3, 6 and 9 months.
-
Quality of life at the end of treatment (9 months) and at the end of follow-up (21 months). Assessments included:
-
Time burden of treatment: time per session for active light treatment and participant-reported treatment burden for TCS and light treatments during the treatment phase.
Safety outcomes
The safety end points are the number of adverse reactions during the treatment phase.
Participants were asked to record any AEs in their treatment diary and were also asked at the 3-, 6- and 9-month clinic visits about any AEs that they had experienced. Any AEs that were deemed related to trial treatments (adverse reactions) were reported in the CRF. Erythema (redness) of grade 1 or 2 was not considered an AE, as this is an expected treatment response from use of NB-UVB. All serious adverse events (SAEs) were reported directly to the trial co-ordinating centre and assessed for seriousness, expectedness and causality by the chief investigator, or delegated medical monitor. SAEs were recorded and reported to the Medicines Health Regulatory Authority and Research Ethics Committee as part of the annual reports.
Cost-effectiveness analysis
The within-trial economic evaluation estimates the incremental cost-effectiveness from an NHS perspective of:
-
NB-UVB light therapy (plus placebo ointment) compared with TCS (plus dummy light).
-
Combination of NB-UVB light therapy and TCS compared with TCS (plus dummy light).
The economic analysis uses individual participant-level data from the trial. The base-case analysis undertakes a cost-effectiveness analysis from an NHS perspective for all participants. Secondary analyses consider the cost–utility of the comparators of interest for those with EQ-5D-5L data available (participants aged ≥ 11 years) and separately for those with CHU-9D data available (participants aged 5 to ≤ 17 years). Full details of the methods can be found in Chapter 4, Methods.
Data collection
Trial data were entered into a web-based electronic case report form (eCRF) (MACRO 4.2.1 version 3800, Elsevier, London, UK). Staff at research sites had access to the data from their site only, with access controlled through person-specific login credentials. Access to the trial database and database maintenance was managed by NCTU.
To facilitate the data collection process, site staff members were provided with CRF workbooks that mirrored the data required for the eCRF. Investigators were asked to transcribe the data into the eCRF within 7 days of the data being collected, when possible.
Participants used a trial handbook, which included a detailed treatment diary, AE record, the use of any health-care resources and any prescribed medicines. Site staff reviewed these handbooks at the 3-, 6- and 9-month clinic visits and entered summary data into the eCRF.
The primary outcome was collected at the 9-month clinic visit. For those who did not attend this visit and who had not withdrawn from the trial, primary outcome data were obtained via telephone, post or text message, when possible.
After the treatment period (9 months), follow-up continued for a further 12 months, with participant-completed questionnaires at 12, 15, 18 and 21 months. These questionnaires were sent either by post with the data entered and returned on paper, or via e-mail using electronic questionnaires designed by staff at NCTU. Reminders were sent (via e-mail or post) if the questionnaire remained uncompleted after 2 weeks, and again after 3 weeks. Members of NCTU staff chased up outstanding questionnaires after 3 weeks by telephone.
Sample size
The choice of minimum clinically important difference between the groups was informed by a survey of the clinical membership of the UK Dermatology Clinical Trials Network (UK DCTN). Standard care was assumed to be TCS monotherapy and so ‘TCS plus dummy light therapy’ is the comparator group for all treatment comparisons. There are two comparisons of primary interest:
-
NB-UVB light therapy (plus placebo ointment) compared with TCS (plus dummy light).
-
Combination of NB-UVB light therapy and TCS compared with TCS (plus dummy light).
Assuming that 15% of participants who were allocated to receive TCS (plus dummy light therapy) would achieve treatment success as defined by the primary outcome, 372 participants were required to detect an absolute difference of 20%, with 2.5% two-sided alpha and 90% power. Allowing for 15% non-collection of primary outcome data, an original sample size of 440 participants was set.
As there were limited data available to inform the sample size calculation for the trial, the Data Monitoring Committee conducted a planned sample size review in December 2016. This review resulted in a recommendation to increase the sample size to 516 participants to maintain 90% power to detect a risk difference of 20% between the TCS arm and the other two arms. The Trial Steering Committee (TSC) and the funders approved this recommendation.
Statistical methods
Analyses were predefined in a statistical analysis plan, which was signed off prior to database lock. Points of clarification to the statistical analysis plan that were made after database lock are summarised in Appendix 2.
Primary outcome
The number and percentage of participants achieving ‘treatment success’ (defined as a response of either ‘a lot less noticeable’ or ‘no longer noticeable’ in response to the question ‘Compared with the start of the study, how noticeable is the vitiligo now?’) is reported for each treatment group at 9 months post randomisation.
The primary analysis was performed on the intention-to-treat (ITT) analysis set, where multiple imputation was used to account for missing primary outcome data at 9 months. Prior to primary analysis, baseline characteristics were summarised further by treatment arms and the availability of primary outcome at 9 months to check the missing at random assumption of multiple imputation.
Randomised groups were compared using a mixed-effects model for binary outcome adjusted by recruitment centre, body region of the target patch and age at randomisation (continuous). The primary effectiveness parameter comparing NB-UVB light with TCS alone, and NB-UVB light plus TCS with TCS alone, was the risk difference (risk ratio will also be included) in the percentage of participants achieving treatment success at 9 months along with 95% confidence interval (CI) and exact p-value. By default, risk differences are reported because these estimates are more clinically intuitive for binary outcomes. However, where models estimating risk difference do not converge, odds ratios will be reported instead of risk differences.
Sensitivity analyses were conducted to (1) adjust for any variables with imbalance at baseline, (2) repeat primary analysis based on participants whose primary outcome was available at 9 months and (3) investigate the effects of treatment adherence.
Planned subgroup analyses were (1) children versus adults, (2) body region of the target vitiligo patch, (3) hypomelanotic patch (an indicator of disease activity): definitely or maybe versus no and (4) ≥ 4 years duration of vitiligo versus < 4 years. These analyses were conducted by inclusion of appropriate interaction terms in the regression model and were considered as exploratory. An additional post hoc subgroup analysis explored the impact that skin type (types I–III vs. types IV–VI) had on the results.
Secondary outcomes
-
VNS treatment success by blinded review of digital images at 9 months.
Between-group comparisons were performed using a mixed-effect regression model for binary outcome, adjusting by recruitment centre, body region of target patch and age (continuous). The analysis was performed on a modified ITT set, where no imputation of missing data was required.
-
Participant-reported treatment success by body region (at 9 months).
VNS treatment success at 9 months for all assessed patches (up to three) was analysed using a multilevel mixed-effects model, accounting for potential correlation between treatment effects at different body regions in the same person. This analysis was conducted with multiple imputation of missing treatment success data. Patient-reported treatment success by body region at 3 and 6 months is presented descriptively.
-
Onset of treatment response (during treatment phase).
Summary data of all three categories (stayed the same, improved, got worse) is presented by treatment group and by timeline (3, 6 and 9 months). The cumulative percentage of participants who achieved a treatment response (i.e. stayed the same or improved) at target patch is presented. Analysis of treatment response at 9 months was analysed using a mixed-effect regression model for binary outcome, adjusting by recruitment centre, body region of target patch and age (continuous).
Participant-reported onset of treatment response is summarised as for investigator-assessed treatment response.
-
Maintenance of treatment response (during follow-up phase).
Maintenance of treatment response is presented separately for those who achieved and those who did not achieve treatment response at the end of the treatment phase. The cumulative percentage of participants with loss of maintenance of treatment response is presented by treatment arm. Data are reported for the target patch only.
-
Percentage repigmentation at 9 months (by blinded dermatologist and investigator).
Analysis of blinded dermatologist-assessed percentage repigmentation at 9 months was analysed using a mixed-effect regression model for binary outcome, adjusting by recruitment centre, body region of target patch and age (continuous). Where available, data from investigator assessments at 9 months were used for missing data based on blinded clinician assessment of digital images.
Treatment success based on investigator-assessed percentage repigmentation at 9 months is reported descriptively.
Assessments carried out by investigators at 3 and 6 and 9 months are presented descriptively.
-
Quality of life at the end of treatment (9 months) and at the end of follow-up (21 months).
Total scores for VitiQOL, Skindex-29, CHU-9D and EQ-5D questionnaires at 9 months and 21 months are summarised by treatment arm using appropriate summary statistics.
-
Time burden of treatment.
For active light therapy, the average time per treatment session was estimated using data collected at 3, 6 and 9 months. Time burden of TCS application was assumed to be minimal. The percentage of those who reported difficulties with the interventions are summarised, along with a description of the difficulties experienced.
Chapter 3 Results: clinical findings
Parts of this chapter have been reproduced with permission from Thomas et al. 48 This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Recruitment and participant characteristics
Recruitment took place between May 2016 and September 2017, and the database was closed for follow-up on 31 December 2018.
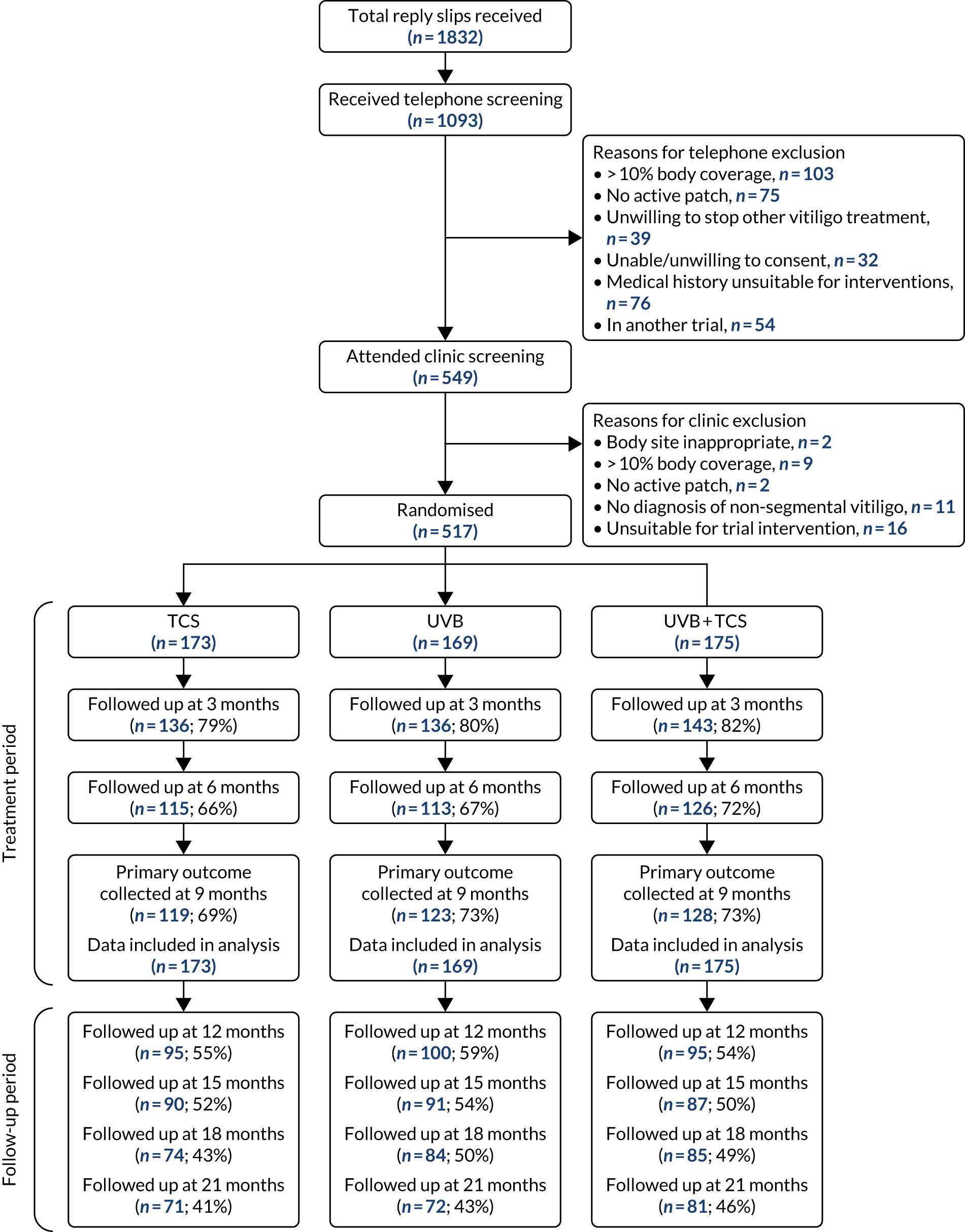
A total of 1832 reply slips were received, of which 1093 received telephone screening and 549 received clinic screening. A total of 517 participants (TCS only, n = 173; NB-UVB only, n = 169; and combination, n = 175) were randomised.
Primary outcome data at 9 months were available for 370 (72%) participants (Figure 2).
FIGURE 2.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. Note that reasons for non-collection of primary outcome at 9 months were participant was not assessed in clinic (n = 4), participant withdrew consent (n = 60), participant discontinued because of AE (n = 3), lost to follow-up (n = 75) and other (n = 5). These reasons were similarly distributed in each treatment arm. Of those participants who withdrew consent, 11 stated that this was because of lack of treatment response and 33 stated that this was because of the time burden. Of those participants who were lost to follow-up, one stated that this was because of lack of treatment response and two stated that it was because of the time burden.
Baseline characteristics and sources of recruitment are summarised in Table 4. Participants were recruited from primary care (118/517, 23%), secondary care (213/517, 41%) and through self-referral from community advertising (186/517, 36%).
| Characteristic | TCS (N = 173) | NB-UVB (N = 169) | Combination (N = 175) | Total (N = 517) |
|---|---|---|---|---|
| Age at randomisation (years) | ||||
| Mean (SD) | 38.6 (20.0) | 36.9 (18.9) | 37.0 (19.1) | 37.5 (19.3) |
| Age of adults at randomisation (years) | ||||
| Mean (SD) | 46.7 (15.2) | 44.7 (14.0) | 44.8 (14.2) | 45.4 (14.5) |
| n | 133 | 130 | 135 | 398 |
| Age of children at randomisation (years) | ||||
| Mean (SD) | 11.7 (3.7) | 10.8 (3.5) | 10.6 (3.3) | 11.1 (3.5) |
| n | 40 | 39 | 40 | 119 |
| Gender, n (%) | ||||
| Male | 75 (43) | 88 (52) | 105 (60) | 268 (52) |
| Ethnicity, n (%) | ||||
| White | 112 (65) | 114 (67) | 104 (59) | 330 (64) |
| Indian | 13 (8) | 13 (8) | 10 (6) | 36 (7) |
| Pakistani | 12 (7) | 15 (9) | 27 (15) | 54 (10) |
| Bangladeshi | 4 (2) | 4 (2) | 4 (2) | 12 (2) |
| Black | 5 (3) | 3 (2) | 7 (4) | 15 (2) |
| Chinese | 2 (1) | 1 (1) | 1 (1) | 4 (1) |
| Other Asian (non-Chinese) | 5 (3) | 6 (4) | 6 (3) | 17 (3) |
| Mixed ethnicity | 9 (5) | 6 (4) | 6 (3) | 21 (4) |
| Other | 10 (6) | 7 (4) | 9 (5) | 26 (5) |
| Missing | 1 (1) | 0 | 1 (1) | 2 (< 0.5) |
| Source of recruitment, n (%) | ||||
| Primary care | 35 (20) | 36 (21) | 47 (27) | 118 (23) |
| Secondary care | 74 (43) | 67 (40) | 72 (41) | 213 (41) |
| Self-referral | 64 (37) | 66 (39) | 56 (32) | 186 (36) |
| Skin photo type, n (%) | ||||
| Type I | 2 (1) | 2 (1) | 5 (3) | 9 (2) |
| Type II | 31 (18) | 32 (19) | 29 (17) | 92 (18) |
| Type III | 70 (40) | 66 (39) | 59 (34) | 195 (38) |
| Type IV | 29 (17) | 34 (20) | 33 (19) | 96 (19) |
| Type V | 35 (20) | 25 (15) | 44 (25) | 104 (20) |
| Type VI | 6 (3) | 10 (6) | 5 (3) | 21 (4) |
| Medical history, n (%) | ||||
| Type 1 diabetes | 5 (3) | 3 (2) | 4 (2) | 12 (2) |
| Hyperthyroidism | 4 (2) | 2 (1) | 6 (3) | 12 (2) |
| Hypothyroidism | 21 (12) | 18 (11) | 10 (6) | 49 (9) |
| Addison’s disease | 2 (1) | 0 | 3 (2) | 5 (1) |
| Pernicious anaemia | 5 (3) | 3 (2) | 6 (3) | 14 (3) |
| Alopecia areata | 3 (2) | 7 (4) | 3 (2) | 14 (3) |
| Duration of vitiligo (years) | ||||
| Mean (SD) | 11.5 (12.0) | 9.9 (11.1) | 11.3 (10.5) | 10.9 (11.2) |
| Median (25th, 75th centile) | 7 (3,6) | 5 (3,11) | 7 (4,15) | 7 (3,15) |
| Min., max. | 1, 60 | 1, 60 | 1, 45 | 1, 60 |
| Previous treatments used for vitiligo, n (%) | ||||
| Light therapy | 28 (16) | 26 (15) | 37 (21) | 91 (18) |
| Corticosteroid cream/ointment | 80 (46) | 75 (44) | 80 (46) | 235 (45) |
| Calcineurin inhibitor | 51 (29) | 39 (23) | 56 (32) | 146 (28) |
| Cosmetic camouflage | 45 (26) | 44 (26) | 40 (23) | 129 (25) |
| Other | 20 (12) | 15 (9) | 17 (10) | 52 (10) |
Baseline characteristics were well balanced between treatment groups. Almost one-quarter of the participants were children (119/517, 23%), and there was an equal balance of genders (268/517, 52% male). However, the majority were white (330/517, 64%). Participants of all skin types were enrolled, the most common being skin type III (195/517, 38%). Baseline characteristics for participants providing primary outcome data and those not providing primary outcome data are summarised in Table 5.
| Characteristic | TCS (N = 173) | NB-UVB (N = 169) | Combination (N = 175) | |||
|---|---|---|---|---|---|---|
| With primary outcome (n = 119) | Without primary outcome (n = 54) | With primary outcome (n = 123) | Without primary outcome (n = 46) | With primary outcome (n = 128) | Without primary outcome (n = 47) | |
| Age at randomisation (years) | ||||||
| Mean (SD) | 39.9 (21.2) | 35.8 (16.9) | 37.5 (20.2) | 35.2 (14.9) | 36.5 (200.2) | 38.3 (15.7) |
| Median (25th Q, 75th Q) | 43.6 (17.5, 58.7) | 32.4 (22.4, 49) | 39.5 (15.8, 52.8) | 34.4 (25.5, 43.6) | 36.4 (15.5, 51.2) | 39 (26.7, 46.5) |
| Min., max. | 6.1, 84.5 | 6.6, 65.1 | 5.2, 76.2 | 10, 68.7 | 5.4, 78.1 | 5.7, 72.7 |
| Age of adults at randomisation (years) | ||||||
| Mean (SD) | 49.8 (14.9) | 40.6 (14.2) | 47.1 (13.9) | 39.1 (12.8) | 46.6 (14.3) | 41.0 (13.4) |
| Median (25th Q, 75th Q) | 50.4 (38.9, 60.8) | 39.5 (28.6, 51.8) | 44.7 (38.1, 58.7) | 37.1 (29.6, 45.9) | 46.5 (35.9, 55.9) | 40.3 (30.8, 47.3) |
| Min., max. | 20, 84.5 | 20.9, 65.1 | 18.4, 76.2 | 18.1, 68.7 | 19.2, 78.1 | 18.4, 72.7 |
| Age of children at randomisation (years) | ||||||
| Mean (SD) | 11.7 (3.7) | 11.9 (3.7) | 10.2 (3.4) | 13.8 (2.0) | 10.9 (3.1) | 8.6 (4.9) |
| Median (25th Q, 75th Q) | 12 (7.9, 14.9) | 10.3 (10.2, 14.1) | 9.7 (7.3, 13.2) | 14.6 (12.3, 15) | 10.2 (8.9, 12.4) | 6.5 (5.9, 11.3) |
| Min., max. | 6.1, 17.8 | 6.6, 17.6 | 5.2, 16 | 10, 15.8 | 5.4, 17.8 | 5.7, 15.9 |
| Gender, n (%) | ||||||
| Male | 43 (36) | 32 (59) | 67 (54) | 21 (46) | 75 (59) | 30 (64) |
| Female | 76 (64) | 22 (41) | 56 (46) | 25 (54) | 53 (41) | 17 (36) |
| Ethnicity, n (%) | ||||||
| White | 74 (62) | 38 (70) | 85 (69) | 29 (63) | 77 (60) | 27 (57) |
| Indian | 11 (9) | 2 (4) | 8 (7) | 5 (11) | 9 (7) | 1 (2) |
| Pakistani | 9 (8) | 3 (6) | 10 (8) | 5 (11) | 21 (16) | 6 (13) |
| Bangladeshi | 3 (3) | 1 (2) | 3 (2) | 1 (2) | 3 (2) | 1 (2) |
| Black | 3 (3) | 2 (4) | 2 (2) | 1 (2) | 4 (4) | 3 (6) |
| Chinese | 1 (1) | 1 (2) | 1 (1) | 0 | 1 (1) | 0 |
| Other Asian (non-Chinese) | 4 (3) | 1 (2) | 4 (3) | 2 (4) | 4 (3) | 2 (4) |
| Mixed ethnicity | 7 (6) | 2 (4) | 4 (3) | 2 (4) | 3 (2) | 3 (6) |
| Other | 7 (6) | 3 (6) | 6 (5) | 1 (2) | 5 (4) | 4 (9) |
| Missing | 0 | 1 (2) | 0 | 0 | 1 (1) | 0 |
| Source of recruitment, n (%) | ||||||
| Primary care | 25 (21) | 10 (19) | 28 (23) | 8 (17) | 36 (28) | 11 (23) |
| Secondary care | 51 (43) | 23 (43) | 49 (40) | 18 (39) | 59 (46) | 13 (28) |
| Self-referral | 43 (36) | 21 (39) | 46 (37) | 20 (43) | 33 (26) | 23 (49) |
| Medical history, n (%) | ||||||
| Type 1 diabetes | 4 (3) | 1 (2) | 2 (2) | 1 (2) | 1 (1) | 3 (6) |
| Hyperthyroidism | 3 (3) | 1 (2) | 3 (3) | 0 | 3 (3) | 3 (6) |
| Hypothyroidism | 15 (13) | 6 (11) | 15 (12) | 3 (7) | 7 (5) | 3 (6) |
| Addison’s disease | 0 | 2 (4) | 0 | 0 | 2 (2) | 1 (2) |
| Pernicious anaemia | 3 (3) | 2 (4) | 2 (2) | 1 (2) | 2 (2) | 4 (9) |
| Alopecia areata | 1 (1) | 2 (4) | 5 (4) | 2 (4) | 2 (2) | 2 (4) |
| Skin photo type, n (%) | ||||||
| Type I | 1 (1) | 1 (2) | 2 (2) | 0 | 4 (3) | 1 (2) |
| Type II | 24 (20) | 7 (13) | 23 (19) | 9 (20) | 21 (16) | 8 (17) |
| Type III | 41 (34) | 29 (54) | 51 (41) | 15 (33) | 43 (34) | 16 (34) |
| Type IV | 23 (19) | 6 (11) | 21 (17) | 13 (28) | 22 (17) | 11 (23) |
| Type V | 28 (24) | 7 (13) | 20 (16) | 5 (11) | 37 (29) | 7 (15) |
| Type VI | 2 (2) | 4 (7) | 6 (5) | 4 (9) | 1 (1) | 4 (9) |
| Duration of vitiligo (years) | ||||||
| Mean (SD) | 11.8 (12.9) | 10.7 (9.9) | 9.7 (11.2) | 10.6 (10.9) | 10.8 (10.3) | 12.8 (10.9) |
| Median (25th Q, 75th Q) | 7 (3, 15.5) | 7 (3, 20) | 5 (2, 10) | 7.5 (4, 12.5) | 7 (3, 15) | 8 (5, 20) |
| Min., max. | 1, 60 | 1, 41 | 1, 60 | 1, 57 | 1, 45 | 1, 42 |
| Previous treatments used for vitiligo, n (%) | ||||||
| Light therapy | 18 (15) | 10 (19) | 18 (15) | 8 (17) | 29 (23) | 8 (17) |
| Corticosteroid cream/ointment | 55 (46) | 25 (46) | 54 (44) | 21 (46) | 62 (48) | 18 (38) |
| Calcineurin inhibitor | 41 (34) | 10 (19) | 27 (22) | 12 (26) | 46 (36) | 10 (21) |
| Cosmetic camouflage | 34 (29) | 11 (20) | 34 (28) | 10 (22) | 32 (25) | 8 (17) |
| Other | 17 (14) | 3 (6) | 10 (8) | 5 (11) | 10 (8) | 7 (15) |
The active target patches were located on the head and neck for 31% of participants (161/517), hands and feet for 32% of participants (164/517), and the rest of the body for 37% of participants (192/517). Not all participants chose to treat and assess three patches of vitiligo: 31% (162/517) chose one patch, 43% (224/517) chose two patches and 25% (131/517) chose three patches for assessment. Over half of the participants chose to treat patches in addition to the three being formally assessed in the trial, with 29% of participants (148/517) electing to treat six or more patches (Table 6).
| Patch characteristics | TCS (N = 173) | NB-UVB (N = 169) | Combination (N = 175) | Total (N = 517) |
|---|---|---|---|---|
| Target patch location, n (%) | ||||
| Head and neck | 53 (31) | 52 (31) | 56 (32) | 161 (31) |
| Hands and feet | 56 (32) | 53 (31) | 55 (31) | 164 (32) |
| Rest of the body | 64 (37) | 64 (38) | 64 (37) | 192 (37) |
| Total number of assessed patches included in study, n (%) | ||||
| 1 | 50 (29) | 50 (30) | 62 (35) | 162 (31) |
| 2 | 74 (43) | 77 (46) | 73 (42) | 224 (43) |
| 3 | 49 (28) | 42 (25) | 40 (23) | 131 (25) |
| Total number of patches the participant would like to treat, n (%) | ||||
| 1 | 13 (8) | 12 (7) | 14 (8) | 39 (8) |
| 2 or 3 | 61 (35) | 62 (37) | 67 (38) | 190 (37) |
| 4 or 5 | 52 (30) | 49 (29) | 39 (22) | 140 (27) |
| ≥ 6 | 47 (27) | 46 (27) | 35 (31) | 148 (29) |
| Activity of target patch, n (%) | ||||
| Hypomelanotic with poorly defined border | ||||
| Definitely | 52 (30) | 46 (27) | 52 (30) | 150 (29) |
| Maybe | 14 (8) | 20 (12) | 18 (10) | 52 (10) |
| No | 107 (62) | 103 (61) | 105 (60) | 315 (61) |
| Amelanotic with sharply defined border | ||||
| Definitely | 97 (56) | 101 (60) | 99 (57) | 297 (57) |
| Maybe | 10 (12) | 19 (11) | 19 (11) | 58 (11) |
| No | 56 (32) | 49 (29) | 56 (32) | 161 (31) |
Adherence to trial treatment and treatment burden
Adherence to treatment is reported in Table 7. The median percentage of NB-UVB treatment-days, as a percentage of expected days of treatment, was 81%, 77% and 74% for the TCS, NB-UVB and combination groups, respectively, and for the ointment was 79%, 83% and 77%, respectively. Just under half of the participants used the treatment for ≥ 75% of the expected number of occasions, which was used as an indicator of good adherence in the sensitivity analyses of the primary outcome accounting for treatment adherence. Just over one-quarter of participants in all groups discontinued one or both of the treatments before the end of the 9-month treatment phase.
| Treatment adherence | TCS (N = 173) | NB-UVB (N = 169) | Combination (N = 175) |
|---|---|---|---|
| Use of light treatment: reported number of treatment sessions as percentage of expected | |||
| Mean (SD) | 68 (31) | 68 (28) | 67 (27) |
| Median (IQR) | 81 (43–95) | 77 (51–90) | 74 (48–89) |
| Distribution of light adherence, n (%) | |||
| < 25% | 19 (11) | 16 (9) | 14 (8) |
| 25–49% | 21 (12) | 18 (11) | 26 (15) |
| 50–74% | 23 (13) | 31 (18) | 35 (20) |
| ≥ 75% | 82 (47) | 72 (43) | 74 (42) |
| Data not available | 28 (16) | 32 (19) | 26 (15) |
| Use of ointment treatment: reported number of treatment sessions as percentage of expected | |||
| Mean (SD) | 68 (29) | 73 (27) | 68 (28) |
| Median (IQR) | 79 (47–93) | 83 (57–95) | 77 (45–92) |
| Distribution of ointment adherence, n (%) | |||
| < 25% | 16 (9) | 12 (7) | 13 (7) |
| 25–49% | 22 (13) | 16 (9) | 28 (16) |
| 50–74% | 30 (17) | 27 (16) | 30 (17) |
| ≥ 75% | 74 (43) | 81 (48) | 77 (44) |
| Data not available | 31 (18) | 33 (20) | 27 (15) |
| Participant-reported average duration (minutes) per light treatment session, median (IQR), n | |||
| 3 months | 20 (10–30), 135 | 15 (10–30), 142 | |
| 6 months | 22.5 (12–42.5), 120 | 20 (15–35), 124 | |
| 9 months | 20 (13–40), 101 | 20 (12–30), 111 | |
| Burden of treatment, n/N (%) | |||
| NB-UVB burden reported | 36/142 (25) | 35/140 (25) | 32/149 (21) |
| TCS burden reported | 18/142 (13) | 14/140 (10) | 14/149 (9) |
| Any burden reported (from either treatment) | 42/142 (30) | 38/140 (27) | 36/149 (24) |
| Participants experienced difficulty using active light during the 9-month treatment period | |||
| Difficulties experienced,a n/N (%) | 76/140 (54) | 81/149 (54) | |
| Uncertainty of using light, n | 7 | 18 | |
| Treatment burden, n | 35 | 32 | |
| Side effect, n | 37 | 43 | |
| Other, n | 9 | 4 | |
| Participants experienced difficulty using active TCS treatment during the 9-month treatment period | |||
| Difficulties experienced,a n/N (%) | 35/142 (25) | 31/149 (21) | |
| Uncertainty of using TCS, n | 5 | 6 | |
| Treatment burden, n | 18 | 14 | |
| Side effect, n | 12 | 15 | |
| Other, n | 4 | 0 | |
| Participants discontinued NB-UVB | 50 (29) | 47 (28) | 43 (25) |
| Number discontinued within first 3 months, n (%) | 17 (10) | 22 (13) | 10 (6) |
| Reasons for NB-UVB discontinuation,a n (%) | |||
| All assessment patches repigmented, n | 1 | 1 | 3 |
| Time burden associated with treatment, n | 23 | 20 | 17 |
| Side effects, n | 4 | 9 | 4 |
| Lack of treatment response, n | 9 | 3 | 7 |
| Other, n | 13 | 14 | 12 |
| Participants discontinued TCS | 48 (28) | 41 (24) | 43 (25) |
| Number discontinued within first 3 months, n (%) | 17 (10) | 19 (11) | 10 (6) |
| Reasons for TCS discontinuation,a n (%) | |||
| All assessment patches repigmented, n | 1 | 1 | 3 |
| Time burden associated with treatment, n | 20 | 17 | 15 |
| Side effects, n | 3 | 1 | 5 |
| Lack of treatment response, n | 9 | 5 | 7 |
| Other, n | 15 | 17 | 13 |
For participants using active light devices the median time taken to administer the treatment was approximately 20 minutes, including time for set-up, administering the light, and documenting timings and side effects in the treatment diary. In addition to written and online video training, participants required just over 1 hour (mean 70 minutes) of face-to-face training with a trained health-care professional (usually a nurse) prior to using the treatment at home.
Difficulties in using the treatments are summarised in Table 7. Burden of treatment was identified as an issue by 42 out of 142 (30%) in the TCS group, 38 out of 140 (27%) in the NB-UVB group and 36 out of 149 (24%) in the combination group, although interpretation is difficult as all three groups used both treatments throughout (either active or dummy/placebo). Not surprisingly, NB-UVB treatment was more burdensome than treatment with TCS. The burden of treatment and side effects were the most commonly cited difficulties for both groups and were common reasons for discontinuation of treatment, along with lack of treatment response.
Blinding
At the 9-month clinic visit, investigators reported that they thought that they had become unblinded for 21% (31/145), 28% (43/153) and 27% (41/153) of participants in the TCS, NB-UVB and combination groups, respectively. Participants were more likely to report that they thought that they had become unblinded 39% (45/116), 55% (66/120) and 44% (55/125) for the TCS, NB-UVB and combination groups, respectively. Of the 115 investigators who thought that they had been unblinded, 78% (90/115) thought that it was due to either the presence or the absence of erythema.
Of those who indicated possible unblinding and were having NB-UVB, 83% (96/115) of investigators and 80% (132/166) of participants were correct. Of those who indicated possible unblinding and were using TCS, 32% (37/115) of investigators and 39% (64/166) of participants were correct (Table 8).
| Number of investigators | TCS | NB-UVB | Combination |
|---|---|---|---|
| 145 | 153 | 153 | |
| Number unblinded, n (%) | 31 (21) | 43 (28) | 41 (27) |
| Of those who indicated unblinding, n (%) | |||
| Investigator guess of light treatment received | |||
| Correct | 27 (87) | 35 (81) | 34 (83) |
| Incorrect | 4 (13) | 8 (19) | 7 (17) |
| Investigator guess of TCS treatment received | |||
| Correct | 21 (68) | 4 (9) | 12 (29) |
| Incorrect | 10 (32) | 39 (91) | 29 (71) |
| Number of participants | 116 | 120 | 125 |
| Number unblinded, n (%) | 45 (39) | 66 (55) | 55 (44) |
| Of those who indicated unblinding, n (%) | |||
| Participant guess of light treatment received | |||
| Correct | 25 (56) | 59 (89) | 48 (87) |
| Incorrect | 20 (44) | 7 (11) | 7 (13) |
| Participant guess of TCS treatment received | |||
| Correct | 23 (51) | 23 (35) | 18 (33) |
| Incorrect | 22 (49) | 43 (65) | 37 (67) |
Primary outcome
The percentage of participants who reported a treatment success (VNS) at 9 months was 17% (20/119) for the TCS-only group, 22% (27/123) for the NB-UVB-only group and 27% (34/128) for the combination group. For participants where the primary outcome was obtained, 96% (355/370) were obtained face to face at the 9-month clinic visit, 2% (9/370) via post, 1% (3/370) via telephone and 1% (3/370) via text message. The primary analysis was performed using multiple imputation. The adjusted risk difference was 5.2% (95% CI –4.4% to 14.9%; p = 0.29) for NB-UVB only compared with TCS only, and 10.9% (95% CI 1.1% to 20.9%; p = 0.03) for combination compared with TCS only (Table 9). The number needed to treat (NNT) for NB-UVB compared with TCS was 19 participants and for combination compared with TCS was 10 participants.
| Participant reported treatment success (VNS) | TCS (N = 173) | NB-UVB (N = 169) | Combination (N = 175) | Between-group comparisons (ITT) | |||
|---|---|---|---|---|---|---|---|
| NB-UVB vs. TCS | Combination vs. TCS | ||||||
| Adjusteda risk difference (95% CI) | Adjusted risk ratio (95% CI) | Adjusted risk difference (95% CI) | Adjusted risk ratio (95% CI) | ||||
| Patient response to VNS scale at 3 months, n (%) | |||||||
| More noticeable | 16 (12) | 26 (19) | 15 (10) | ||||
| As noticeable | 70 (52) | 57 (42) | 62 (43) | ||||
| Slightly less noticeable | 34 (25) | 34 (25) | 47 (33) | ||||
| A lot less noticeable | 13 (10) | 19 (14) | 17 (12) | ||||
| No longer noticeable | 2 (1) | 0 (0) | 2 (1) | ||||
| Patient response to VNS scale at 6 months, n (%) | |||||||
| More noticeable | 11 (10) | 23 (20) | 10 (8) | ||||
| As noticeable | 51 (44) | 37 (33) | 36 (29) | ||||
| Slightly less noticeable | 37 (32) | 33 (29) | 45 (36) | ||||
| A lot less noticeable | 14 (12) | 18 (16) | 28 (22) | ||||
| No longer noticeable | 2 (2) | 2 (2) | 7 (6) | ||||
| Participants with primary outcome data at 9 months | 119 (69) | 123 (73) | 128 (73) | ||||
| Patient response to VNS scale at 9 months, n (%) | |||||||
| More noticeable | 18 (15) | 27 (22) | 17 (13) | 5.2% (–4.4% to 14.9%) | 1.44 (0.77 to 2.70) | 10.9% (1.1% to 20.9%) | 1.93 (1.02 to 3.68) |
| As noticeable | 53 (45) | 33 (27) | 32 (25) | ||||
| Slightly less noticeable | 28 (24) | 36 (29) | 45 (35) | ||||
| A lot less noticeable | 15 (13) | 25 (20) | 27 (21) | ||||
| No longer noticeable | 5 (4) | 2 (2) | 7 (5) | ||||
| Patient-reported treatment successb using VNS scale at 9 months | 20 (17) | 27 (22) | 34 (27) | ||||
An additional 29.5% (109/370) of participants achieved a ‘partial treatment response’ (slightly less noticeable on the VNS): 24% (28/119) in the TCS group, 29% (36/123) in the NB-UVB group and 35% (45/128) in the combination group (see Table 9).
The percentage of participants with a treatment success at 3 and 6 months is shown in Table 9.
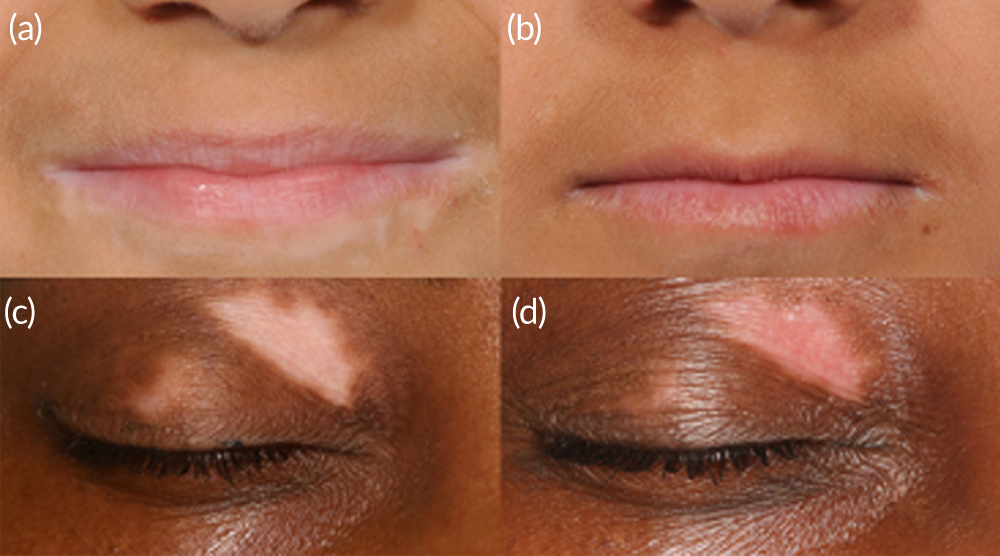
Images demonstrating examples of good and poor treatment responses are shown in Figure 3.
FIGURE 3.
Figure showing target lesions before (a, c) and after (b, d) treatment.
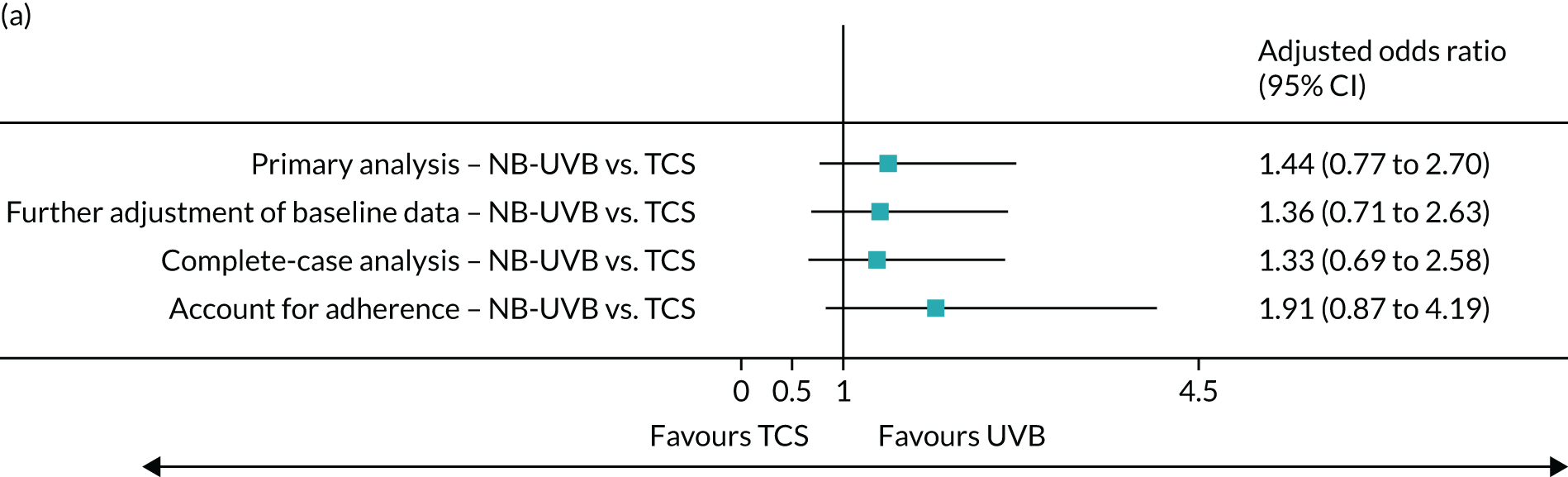
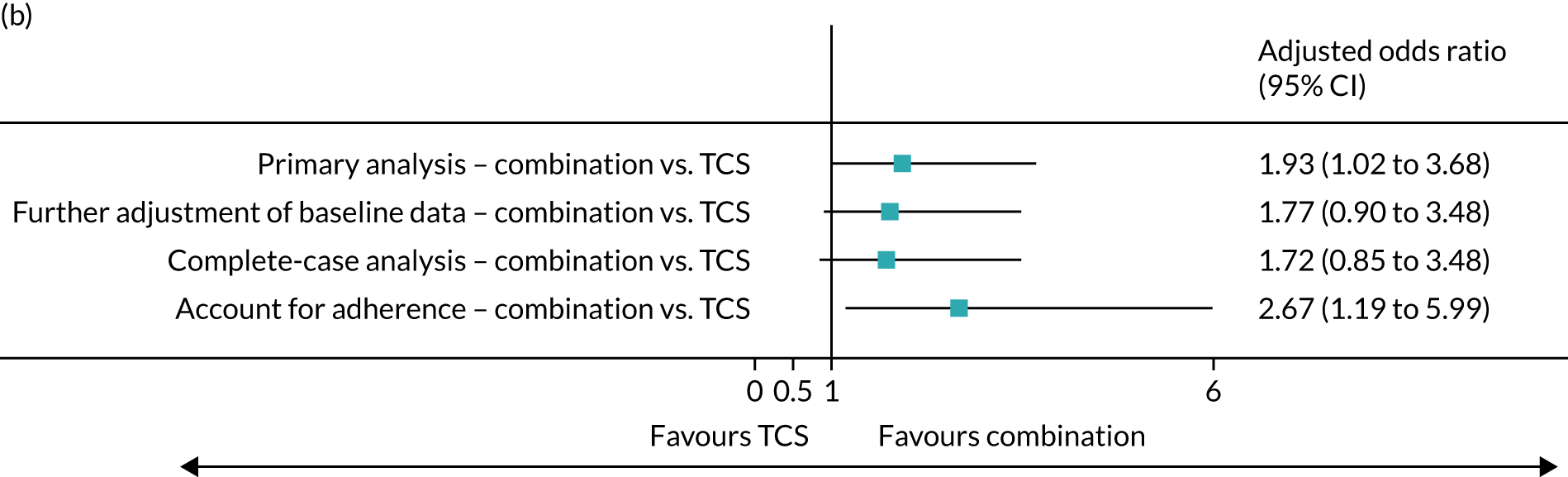
Sensitivity analyses were performed (1) with further adjustment of baseline data, (2) on participants only with primary outcome data at 9 months and (3) accounting for adherence to trial treatment. Results from sensitivity analyses were consistent with the primary analysis. Participants who adhered to treatment interventions by ≥ 75% of expected treatments were more likely to achieve a treatment success. The adjusted odds ratio was 1.91 (95% CI 0.87 to 4.19) for NB-UVB compared with TCS, and 2.67 (95% CI 1.19 to 5.99) for combination therapy compared with TCS (Figure 4).
FIGURE 4.
Sensitivity analyses of primary outcome. Note that further adjustment of baseline was for gender. Complete-case analysis was based on available data without imputation. Complier-average causal effect analyses were performed to account for the impact of treatment adherence.
Subgroup analyses of the primary outcome were performed according to (1) body region of the target patch (head and neck, hands and feet, or rest of the body), (2) age (adults, children), (3) hypomelanotic patch with poorly defined borders (definitely, maybe, no), (4) duration of vitiligo (< 4 years, ≥ 4 years) and (5) post hoc analysis by skin type (types I–III or types IV–VI).
No differences were found between the groups for any of the planned and post hoc subgroups, with the exception of body region of the target patch, where analyses based on patches on the rest of the body appeared to favour combination treatment compared with TCS (Table 10).
| Subgroup | VNS treatment success rate | Adjusteda odds ratio (95% CI) | |||
|---|---|---|---|---|---|
| TCS | NB-UVB | Combination | NB-UVB vs. TCSb | Combination vs. TCSb | |
| By body region of target patch, n (%) | |||||
| Head and neck (N = 161) | 10 (29) | 15 (42) | 11 (26) | 1.78 (0.70 to 4.52) | 1.15 (0.43 to 3.09) |
| Hands and feet (N = 164) | 2 (5) | 4 (12) | 4 (13) | 1.93 (0.35 to 10.78) | 2.56 (0.45 to 14.77) |
| Rest of body (N = 192) | 8 (17) | 8 (15) | 19 (36) | 1.01 (0.38 to 2.68) | 2.88 (1.06 to 7.80) |
| By age, n (%) | |||||
| Adults (N = 398) | 13 (15) | 20 (22) | 22 (24) | 1.64 (0.76 to 3.55) | 2.03 (0.93 to 4.43) |
| Children (N = 119) | 7 (23) | 7 (22) | 12 (33) | 1.03 (0.26 to 4.04) | 1.80 (0.60 to 5.37) |
| By hypomelanotic and poorly defined border, n (%) | |||||
| Definitely or maybe (N = 202) | 10 (22) | 11 (20) | 16 (30) | 1.05 (0.41 to 2.67) | 1.72 (0.64 to 4.66) |
| No (N = 315) | 10 (14) | 16 (23) | 18 (24) | 1.78 (0.83 to 3.82) | 2.08 (0.92 to 4.68) |
| By duration of vitiligo, n (%) | |||||
| ≥ 4 years (N = 348) | 11 (14) | 14 (21) | 18 (20) | 1.68 (0.73 to 3.82) | 1.72 (0.76 to 3.87) |
| < 4 years (N = 150) | 8 (22) | 10 (20) | 16 (47) | 0.99 (0.29 to 3.49) | 3.28 (0.91 to 11.92) |
| By skin type, n (%) | |||||
| Skin types I to III (N = 296) | 10 (15) | 14 (18) | 14 (21) | 1.18 (0.54 to 2.59) | 1.38 (0.64 to 2.96) |
| Skin types IV to VI (N = 221) | 10 (19) | 13 (28) | 20 (33) | 1.64 (0.57 to 4.78) | 2.56 (0.63 to 10.37) |
Secondary outcomes
Vitiligo Noticeability Scale treatment success from blinded patient and public involvement reviewers
Treatment success from blinded image assessment by patient reviewers were broadly consistent with the primary analysis but were more likely to suggest that there was a benefit from NB-UVB, with evidence of significant differences in treatment success for both the NB-UVB and the combination groups, compared with TCS (Table 11).
| Treatment phase | Treatment group, % (n/N) | Between-group comparison | |||||
|---|---|---|---|---|---|---|---|
| NB-UVB vs. TCS | Combination vs. TCS | ||||||
| TCS | NB-UVB | Combination | Adjusteda risk difference, % (95% CI) | Adjusteda risk ratio (95% CI) | Adjusteda risk difference, % (95% CI) | Adjusteda risk ratio (95% CI) | |
| Treatment success by blinded patient and public involvement assessors at 9 months (target patch) | 11 (12/112) | 20 (22/108) | 28 (32/116) | 9.7 (1.2 to 18.2) | 2.22 (1.14 to 4.31) | 16.3 (7.0 to 25.6) | 3.52 (1.80 to 6.89) |
Participant-reported Vitiligo Noticeability Scale treatment success by region of the body (including all assessed patches)
Patches on the hands and feet were less likely to respond to treatment than patches on other parts of the body, regardless of the treatments being used. However, the between-group comparisons given in Table 12 indicate that there was no evidence of a differential treatment effect according to the location of assessed patches (see Table 12 and Figure 5). Participant-reported VNS at 3 and 6 months by body region is summarised in Appendix 3.
| Treatment phase | Treatment group, % (n/N) | Between-group comparison | |||||
|---|---|---|---|---|---|---|---|
| TCS | NB-UVB | Combination | NB-UVB vs. TCS | Combination vs. TCS | |||
| Adjusteda odds ratiob for interactions (95% CI) | Adjusted odds ratio for interactions (95% CI) | ||||||
| Participant-reported treatment success at 9 months by body regions (maximum three patches per person) | |||||||
| Head and neck | 23 (14/61) | 32 (20/63) | 33 (23/69) | Hands and feet vs. head and neck | 0.89 (0.22 to 3.54) | Hands and feet vs. head and neck | 1.30 (0.31 to 5.52) |
| Hands and feet | 10 (8/83) | 11 (7/79) | 18 (13/74) | Rest of body vs. head and neck | 1.06 (0.30 to 3.74) | Rest of body vs. head and neck | 2.42 (0.67 to 8.76) |
| Rest of body | 15 (14/94) | 17 (16/92) | 34 (30/89) | ||||
FIGURE 5.
Treatment success at all assessed patches at 9 months.
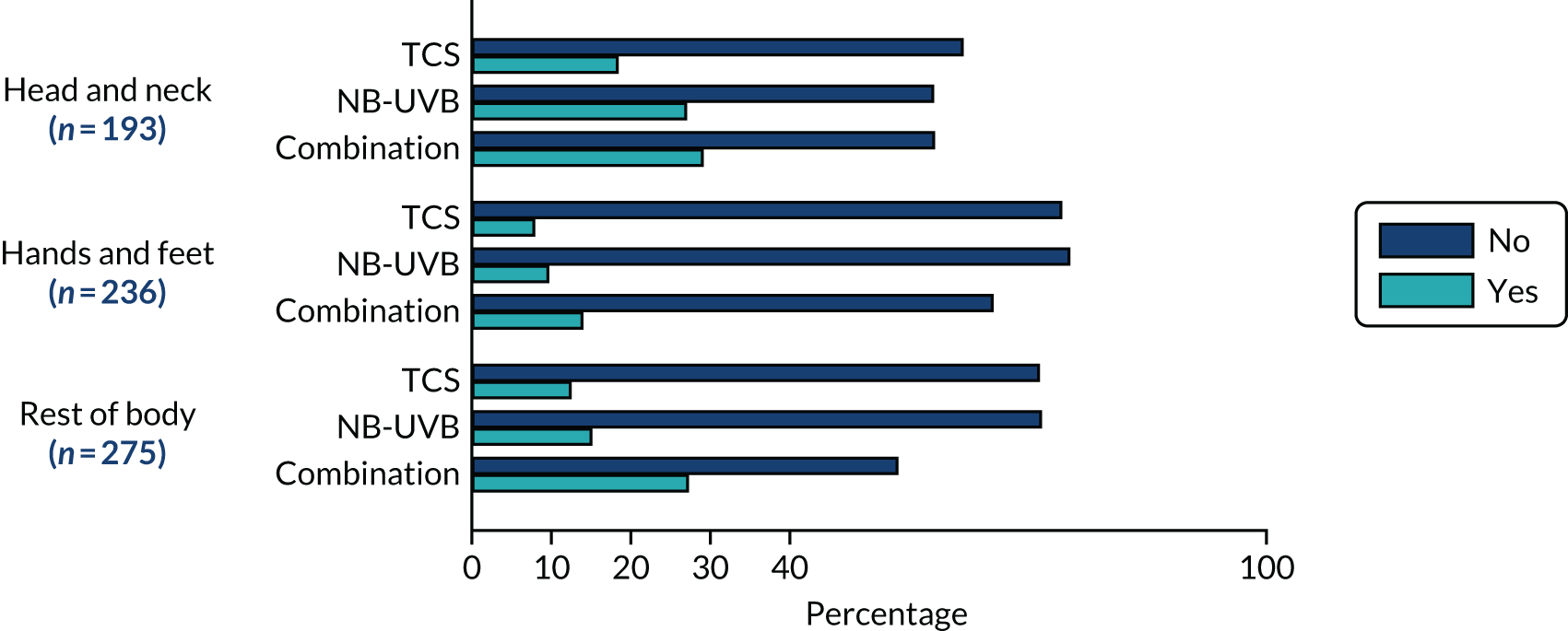
Overall, 94% of participants had achieved onset of treatment response by 3 months for all groups [defined as the active target patch having improved or stayed the same (i.e. not worsened) as assessed by investigators] (Figure 6): TCS (40% improved, 57% stayed the same), NB-UVB (61% improved, 35% stayed the same) and combination (60% improved, 38% stayed the same).
FIGURE 6.
Investigator assessed onset of treatment response.
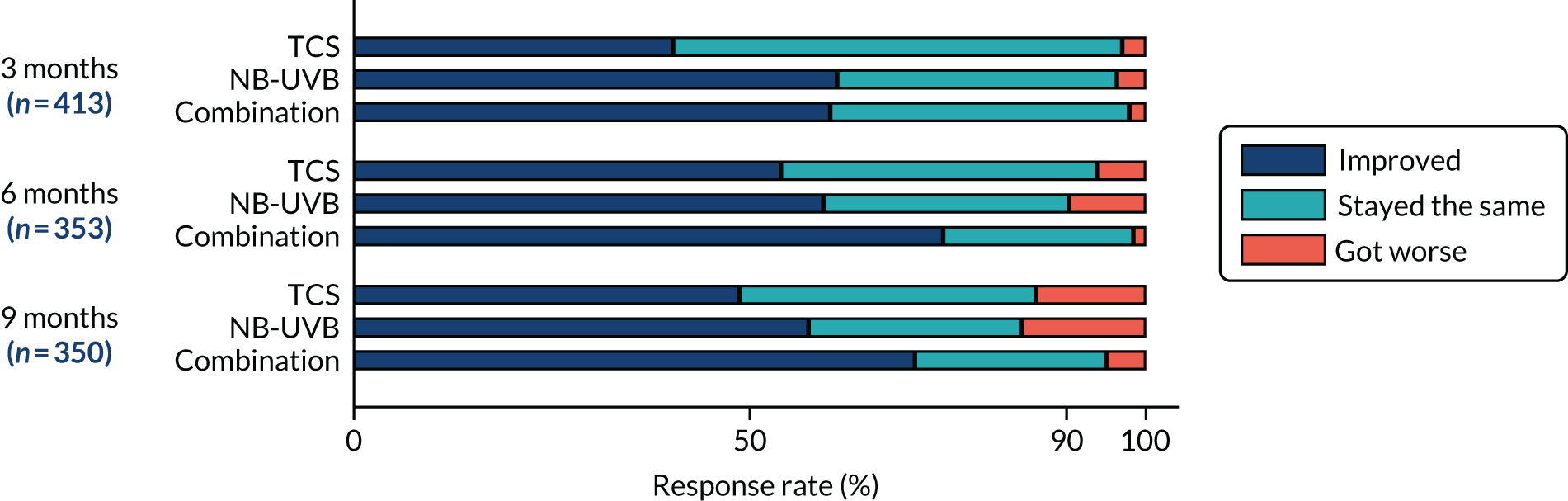
Participant-reported onset of treatment response is summarised in Appendix 4.
Treatment success: percentage repigmentation
Percentage repigmentation was assessed by a dermatologist using digital images taken at baseline and at 9 months. The results were supportive of the primary outcome, although the rates of treatment success were lower: 3% (4/115) for the TCS group, 8% (9/116) for the NB-UVB group and 15% (18/120) for the combination group. The adjusted odds ratio was 2.22 (95% CI 0.66 to 7.51) for NB-UVB compared with TCS, and was 4.62 (95% CI 1.50 to 14.24) for combination compared with TCS (Table 13). Review by blinded investigators during clinic visits were also supportive of the primary outcome (Table 13). Full details of repigmentation rates at all time points are summarised in Appendix 5.
| Treatment phase | Treatment group, % (n/N) | Between-group comparison | |||
|---|---|---|---|---|---|
| TCS | NB-UVB | Combination | NB-UVB vs. TCS | Combination vs. TCS | |
| Adjusteda odds ratiob (95% CI) | Adjusted odds ratio (95% CI) | ||||
| Percentage repigmentation – treatment success at 9 months assessed by blinded dermatologist (using digital images of target patch) | 3 (4/115) | 8 (9/116) | 15 (18/120) | 2.22 (0.66 to 7.51) | 4.62 (1.50 to 14.24) |
| Percentage repigmentation – treatment success assessed by investigators (target patch) at | |||||
| 3 months | 3 (4/134) | 4 (6/136) | 4 (6/143) | ||
| 6 months | 7 (8/115) | 5 (6/113) | 11 (14/125) | ||
| 9 months | 9 (10/134) | 10 (11/136) | 18 (21/143) | ||
Long-term follow-up (post intervention)
Long-term follow-up rates at 12, 15, 18 and 21 months were 56%, 52%, 47% and 43%, respectively, and so the results are presented descriptively.
By 21 months (12 months after stopping treatment), just over 40% (149/338) of participants reported that repigmentation had been lost (Table 14). These percentages were similar for those who achieved ‘treatment success’ at 9 months (Table 15).
| Long-term follow-up phase | Treatment group, % (n/N) | ||
|---|---|---|---|
| TCS | NB-UVB | Combination | |
| Loss of treatment response at target patch assessed by participant at | |||
| 12 months | 19 (18/95) | 23 (23/100) | 19 (18/95) |
| 15 months | 30 (31/105) | 35 (39/111) | 30 (32/107) |
| 18 months | 37 (39/107) | 40 (46/116) | 37 (41/112) |
| 21 months | 46 (50/108) | 43 (50/116) | 43 (49/114) |
| Long-term follow-up phase | Treatment group, % (n/N) | ||
|---|---|---|---|
| TCS (N = 173) | NB-UVB (N = 169) | Combination (N = 175) | |
| Loss of treatment response at target patch assessed by participant at | |||
| 12 months | 6 (1/17) | 13 (3/23) | 28 (7/25) |
| 15 months | 28 (5/18) | 36 (9/25) | 36 (10/28) |
| 18 months | 33 (6/33) | 38 (10/26) | 38 (11/29) |
| 21 months | 33 (6/18) | 38 (10/26) | 47 (14/30) |
Participant-reported Vitiligo Noticeability Scale throughout the trial (treatment and follow-up)
The VNS scores throughout the study treatment period (0 to 9 months) and follow-up period (12 to 21 months) are shown in Figure 7. The number included at each time point varies according to follow-up completion rates, but shows treatment success to be achieved by 6 months in the combination group and maintained for approximately 3 months before loss of gained pigmentation in the longer term.
FIGURE 7.
Percentage of participants reporting treatment success for target patch during the trial (VNS treatment success for target patch over time).
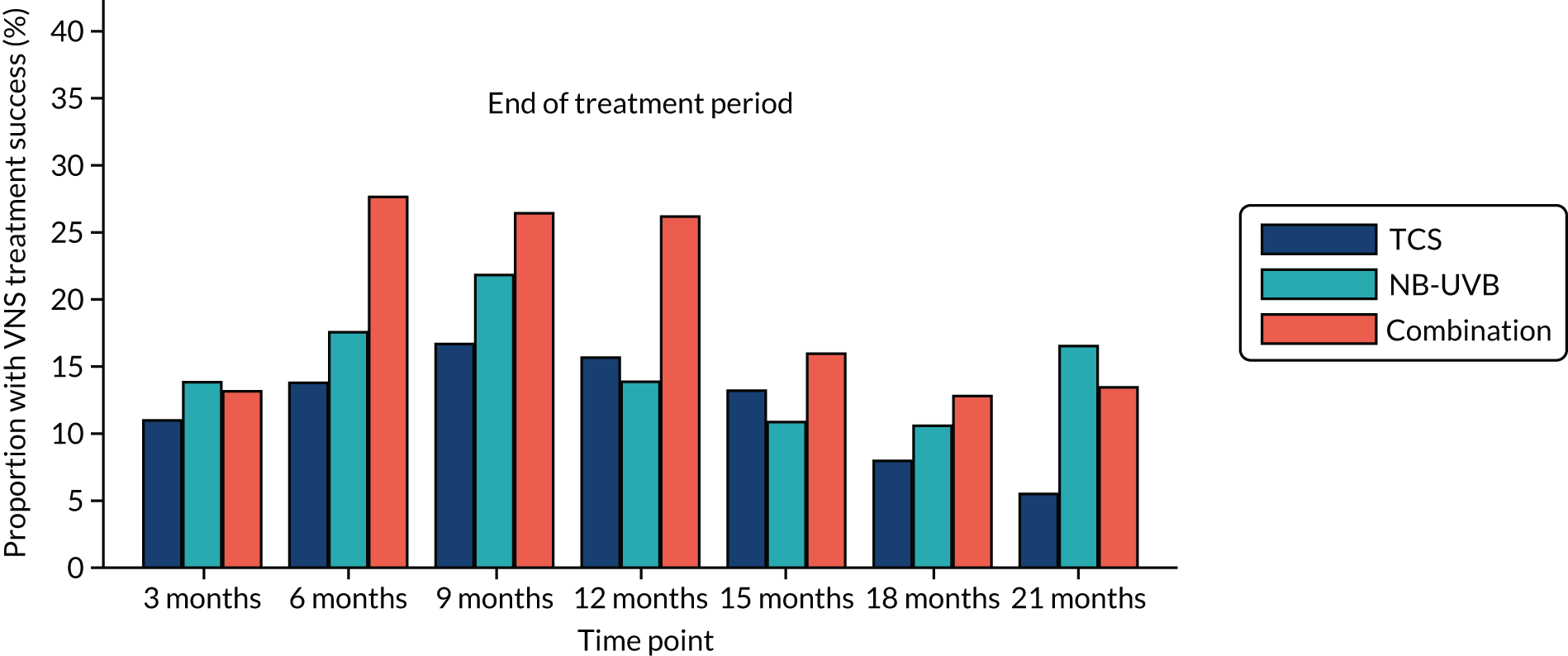
Quality of life
There was no difference between the groups in any of the generic or vitiligo-specific quality-of-life instruments at any time point (Table 16).
| Quality of life | TCS (N = 173) | NB-UVB (N = 169) | Combination (N = 175) | Between-group comparison | |
|---|---|---|---|---|---|
| NB-UVB vs. TCS, adjusted difference in means (95% CI) | Combination vs. TCS, adjusted difference in means (95% CI) | ||||
| At baseline | |||||
| VitiQOL score (adults) | |||||
| Mean (SD) | 34.7 (21.8) | 33.3 (23.8) | 35.6 (23.3) | ||
| n | 133 | 129 | 135 | ||
| Skindex-29 score (adults) | |||||
| Mean (SD) | 22.8 (15.7) | 21.4 (18.6) | 23.8 (18.7) | ||
| n | 132 | 130 | 133 | ||
| EQ-5D-5L utility score (all) | |||||
| Mean (SD) | 0.9 (0.1) | 0.9 (0.2) | 0.9 (0.2) | ||
| n | 151 | 140 | 147 | ||
| CHU-9D utility score (children) | |||||
| Mean (SD) | 1 (0.1) | 0.9 (0.1) | 0.9 (0.1) | ||
| n | 40 | 35 | 39 | ||
| At 9 months | |||||
| VitiQOL score (adults) | –5.5 (–11.8 to 0.8) | –2.0 (–8.3 to 4.4) | |||
| Mean (SD) | 32.7 (21.2) | 27.9 (22.0) | 31.7 (21.5) | ||
| n | 85 | 85 | 85 | ||
| Skindex-29 score (adults) | –2.2 (–6.8 to 2.4) | 0.3 (–4.3 to 4.9) | |||
| Mean (SD) | 19.2 (14.9) | 17.5 (16.6) | 20.3 (15.6) | ||
| n | 82 | 83 | 84 | ||
| EQ-5D-5L utility score (all) | 0.045 (0.003 to 0.087) | 0.031 (–0.010 to 0.073) | |||
| Mean (SD) | 0.9 (0.2) | 0.9 (0.1) | 0.9 (0.1) | ||
| n | 97 | 89 | 98 | ||
| CHU-9D utility score (children) | 0 (–0.028 to 0.027) | –0.023 (–0.048 to 0.002) | |||
| Mean (SD) | 1 (0.1) | 1 (0) | 0.9 (0.1) | ||
| n | 31 | 28 | 35 | ||
| At 21 months | |||||
| VitiQOL score (adults) | |||||
| Mean (SD) | 36.1 (21.1) | 31.1 (22.8) | 38.4 (23.6) | ||
| n | 56 | 57 | 63 | ||
| Skindex-29 score (adults) | |||||
| Mean (SD) | 22.5 (16.5) | 19.1 (16.6) | 25.9 (17.5) | ||
| n | 57 | 52 | 60 | ||
Adverse and serious adverse events
Safety
A total of 206 treatment-related AEs were reported by 124 (25%) participants, 33 AEs from 24 (14%) participants in the TCS group, 69 AEs from 48 (28%) participants in the NB-UVB group and 104 AEs from 52 (30%) participants in the combination group. A full listing of treatment-related AEs is provided in Appendix 6. There were five serious AEs reported from five participants, but none was related to trial interventions (Table 17). Details of grades 3 or 4 erythema and skin thinning are shown in Table 17. In general, fewer AEs were reported in children than in adults.
| Adverse events | Treatment group | ||
|---|---|---|---|
| TCS (N = 173) | NB-UVB (N = 169) | Combination (N = 175) | |
| Total number of participants who reported any related AEs, n (%) | 24 (14) | 48 (28) | 52 (30) |
| Total number of related AEs | 33 | 69 | 104 |
| AEs by severity, n | |||
| Mild | 30 | 32 | 58 |
| Moderate | 3 | 24 | 40 |
| Severe | 0 | 13 | 6 |
| AEs by outcome, n | |||
| Recovered | 20 | 53 | 92 |
| Resolved with sequelae | 3 | 6 | 3 |
| Ongoing | 7 | 5 | 6 |
| Unknown | 3 | 5 | 3 |
| Number of erythema events in adults, n (%) | 2 (2) | 22 (20) | 37 (26) |
| Grade 3 erythema | 0 | 8 | 33 |
| Grade 4 erythema | 2 | 14 | 4 |
| Number of erythema events in children, n (%) | 1 (1) | 7 (6) | 8 (7) |
| Grade 3 erythema | 1 | 6 | 8 |
| Grade 4 erythema | 0 | 1 | 0 |
| Erythema events by outcome, n | 3 | 29 | 45 |
| Recovered | 3 | 25 | 44 |
| Resolved with sequelae | 0 | 1 | 0 |
| Ongoing | 0 | 0 | 1 |
| Unknown | 0 | 3 | 0 |
| Number of skin thinninga event in adults, n (%) | 5 (5) | 2 (2) | 5 (5) |
| Number of skin thinninga events in children, n (%) | 1 (1) | 0 | 0 |
| Skin-thinning events by outcome, n | 6 | 2 | 5 |
| Recovered | 3 | 1 | 2 |
| Resolved with sequelae | 0 | 1 | 2 |
| Ongoing | 2 | 0 | 1 |
| Unknown | 1 | 0 | 0 |
There were five reported serious AEs (i.e. asthma, fracture, pancreatitis, pneumonia and syncope), but none was related to trial treatments.
Chapter 4 Health economic evaluation
Parts of this chapter have been reproduced with permission from Sach et al. 49 This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
A systematic review in 2018 showed that the economic evidence base for vitiligo treatment and care is virtually non-existent. 50 One of the two studies identified in this review estimated the annual direct cost of treating vitiligo in the USA to be US$15M for the price year 2004. 51 The other study demonstrated that 32.5% of people with vitiligo would be willing to make a one-off payment of €5000 for a cure (2006 price year),52 allowing an estimate of the maximum potential for benefit should a cure be found. These papers indicate the cost to both the person affected and the health-care system but do not provide evidence to inform resource allocation decisions. We did not identify any papers that undertook full economic evaluations of vitiligo treatments either alongside clinical trials or as economic modelling. McManus et al. 50 identified a need for full economic evaluations of currently prescribed vitiligo treatments. This chapter reports what we believe to be the first full economic evaluation of vitiligo treatment, of both a current standard treatment (TCS) and a new treatment (home-based NB-UVB light therapy) alone and in combination with TCS. In this chapter, the methods, results and discussion pertaining to the economic evaluation undertaken alongside the trial are reported.
Methods
The primary objective of the health economic evaluation was to estimate the within-trial cost-effectiveness of (1) active hand-held NB-UVB light compared with TCS only (standard care) and (2) active hand-held NB-UVB plus TCS compared with TCS only (standard care) in terms of cost per treatment success at the end of the 9-month treatment period for vitiligo, using individual-level data collected during the trial. These were deemed the appropriate economic questions as each compares with current standard care.
The secondary objective was to undertake two separate cost–utility analyses at the end of the trial intervention period (9 months) for those with (1) EQ-5D-5L utility values available (participants aged ≥ 11 years) and (2) CHU-9D utility values available (participants aged 5 to < 18 years). Including the EQ-5D-5L values of those aged 11–17 years described in (1) above deviates slightly from what was proposed in the protocol in recognition of the lower than expected response rates to the utility instruments, particularly at follow-up, which means that it makes more sense to use all the data available regardless of age.
The evaluation was undertaken in line with published guidelines for the economic evaluation of health-care interventions as appropriate. 53–57
The trial was conducted in the UK, which has a national health service (i.e. the NHS) providing publicly funded health care that is largely free of charge at the point of use. Therefore, the analysis was primarily undertaken from an NHS perspective, in keeping with the National Institute for Health and Care Excellence (NICE) reference case. 57 Out-of-pocket costs incurred by participants, and where applicable their parents/guardians, are presented separately reflecting a personal perspective.
The primary economic analysis compares the costs and outcomes over the 9-month intervention period from randomisation and, therefore, costs and benefits are not discounted.
Resource use and costs
Identification of resources
In keeping with the chosen perspective, the base case captured the intervention costs (including any side-effect costs) to the NHS and the participant’s wider use of the NHS (including health-care visits and prescriptions) as a result of vitiligo. Participants’ personal out-of-pocket expenses incurred as a result of their vitiligo were also captured in a separate analysis taking a broader perspective. The time spent by patients administering the interventions is presented descriptively in Chapter 3, Adherence to trial treatment and treatment burden, but participant time burden administering treatment was not costed.
Measurement of resource use data
Resource use for the intervention phase was collected at 3, 6 and 9 months, using information recorded by participants in daily diaries and in CRFs collected at follow-up visits. In the follow-up period, resource use was collected via online participant questionnaires at 12, 15, 18 and 21 months (or via paper copies if preferred).
Valuation of resource use data
The cost of the intervention was estimated at the individual level as follows.
Narrowband UVB device
In costing the intervention, the cost of the hand-held device was estimated using the manufacturer’s purchase price divided by an annuity factor (interest rate 3.5% for 5 years) to give an equivalent annual cost (EAC). EAC was divided by 12 months and multiplied by nine to give an equivalent cost of the 9-month time frame. The purchase price of personal protective equipment (e.g. goggles and glasses) were included at full cost as it is not believed that these would have the same durability as the device itself. We did not include in the analysis any costs for repairs or replacement devices required due to malfunction or damage, because if participants reported a faulty device during the trial, a replacement device was issued instead of repairing the existing device; in practice, repairs would be more likely. We do, however, report in Table 19 the mean number of NB-UVB devices used over the 9-month treatment period to show that malfunction of the devices was low. The price of the device was varied in sensitivity analyses and thus the uncertainty surrounding the cost of the device (including any replacement or repairs) that would change the conclusions of the study was explored. The number of devices received per participant over the course of the trial was recorded and reported descriptively to indicate the level of faults experienced in the trial.
Participants received training in how to use the device correctly, through practical demonstration, written instructions and a video. The time spent by investigators delivering this training was captured in the CRF.
As these devices are not currently routinely prescribed in the NHS, it is unclear how they would be rolled out if they were to be adopted. In the analysis we assume that the devices are given to patients by the dermatology department at hospital appointments and, once returned at 9 months, are given to a new patient.
Topical corticosteroid
Participants receiving the TCS intervention were supplied with two 90-g tubes of mometasone furoate 0.1% ointment (Elocon 0.1% ointment). The cost of the TCS was sourced from the Prescription Cost Analysis for 201758 and had the National Average Discount Percentage of 7.37% deducted59 and the professional pharmacist fee of £1.29 added, assuming that in practice a single tube would be prescribed at any one time.
When participants requested additional ointment, this was recorded and costed at the individual participant level.
Whichever intervention group participants were in, it was assumed that, in practice, all participants would see a dermatologist at 0, 3, 6 and 9 months and these were costed even though they will essentially cancel each other out between treatment arms.
Side effects requiring medical attention from either the NB-UVB device or TCS were recorded in the CRF. These unscheduled contacts were costed using published unit costs. 60
Unit costs
All resource use relevant to the NHS perspective, including wider NHS usage due to vitiligo, was valued using UK unit costs [in Great British pounds (GBP)] for the 2017 price year (the most recent price year available at the start of the analysis). Unit costs were identified from published sources, such as Unit Costs of Health and Social Care,61 Prescription Cost Analysis58 and NHS Reference Costs 2017. 60 A table of unit costs, together with their sources, is presented in Results.
Personal costs incurred by participants as out-of-pockets costs due to their vitiligo were valued using patient-reported estimates. These were not adjusted to reflect the year in which they were incurred because timing is likely to have had a negligible effect on price for the types of items reported [for instance, the majority of items were sun creams, emollients or camouflage products that are (or are similar to products) also available on prescription and the net ingredient cost (NIC) per item in the prescription cost analysis barely changed between 2016 and 2017 (from £8.34 in 2016 to £8.29 in 201757,62)].
Total costs
The cost of all reported resource use (relevant to an NHS perspective) was calculated for each participant. These figures were then summed for each participant, giving a total cost over the 9-month treatment period in the primary analysis. For each of the different intervention arms, a mean cost per participant was estimated.
Identification of outcome(s)
Vitiligo Noticeability Scale
The primary clinical outcome measure in the HI-Light Vitiligo trial is participant-reported treatment success, measured at 9 months, using the VNS. 42 Treatment success, a binary outcome, is defined by whether the participant responds that their target vitiligo patch is ‘a lot less noticeable’ or ‘no longer noticeable’ in response to the question: ‘Compared with the start of the study, how noticeable is the vitiligo now?’. To the best of our knowledge, no previous studies have compared the treatments being compared in this study, hence the use of single study-based estimates of effectiveness.
Quality of life
Quality-adjusted life-years (QALYs) were estimated in secondary analyses using the utility scores obtained from the EQ-5D-5L instrument for participants aged ≥ 11 years and the CHU-9D in the analysis focused on children aged < 18 years. 63,64 For participants aged 5–6 years, the CHU-9D was completed by parental proxy, but for all other ages these instruments were self-completed.
The decision to use the EQ-5D-5L was based on the EuroQoL EQ-5D-Y user guide (https://euroqol.org/publications/user-guides/; accessed November 2020) available at the time of study design, which stated that although the EQ-5D-Y ‘is generally recommended’ the adult version might be possible. We chose to use just the one version of the EQ-5D in the study for consistency and because the EQ-5D-Y does not currently have a UK valuation set. The CHU-9D was chosen over the EQ-5D-Y because a UK valuation set exists for it.
Neither generic utility instrument had been used in this disease area before. Therefore, their inclusion was somewhat experimental, seeking to start to build up some evidence for their potential for use in vitiligo.
Measurement of outcome(s)
Utility measurements were collected in person at clinic visits at baseline and 9 months and via online/postal questionnaire at 21 months.
Valuation of outcome(s)
In the cost–utility analysis, the responses received on the quality-of-life instruments was converted to utility scores using the EQ-5D-5L Crosswalk65 UK preference weights in the base-case analysis; this is in line with current recommendations. 66 The CHU-9D was valued using the UK value set. 63 Following this, the utility values were used to calculate the number of QALYs generated over the trial treatment period of 9 months and for sensitivity analyses over the treatment and follow-up period of 21 months, using both linear interpolation and area under the curve analysis with and without baseline adjustment. 67 Separate cost–utility analyses report the incremental cost per QALY based on the EQ-5D-5L responses (for participants aged ≥ 11 years) and the CHU-9D responses (for participants aged 5–17 years) from an NHS perspective. The impact of using different preference weights68,69 for the EQ-5D-5L was explored in sensitivity analyses.
Economic analysis
All analyses were conducted in Stata® MP (StataCorp LP, College Station, TX, USA) version 15. The economic base-case analysis was performed on the full analysis set, where, in line with that undertaken for the primary statistical analysis, multiple imputation was used to account for missing primary outcome data and cost data at 9 months. The final analysis was a within-trial analysis, taking a 9-month time horizon in the base-case analysis. As the time horizon being evaluated is 9 months in the base case, costs and benefits were not discounted.
The main base-case analysis was a cost-effectiveness analysis, meaning that decision-makers will need to make a value judgement about the acceptable value of the cost per treatment success. The cost-effectiveness analysis was chosen as the primary analysis because it enabled the whole sample to be analysed together, irrespective of the participant’s age. There was also some concern that available generic utility instruments may not be able to fully capture the health-related quality-of-life aspects of people living with vitiligo. Further details for this choice are reviewed in Discussion.
The secondary objective, to assess cost–utility analysis, combined the incremental mean costs and QALYs between comparator interventions. It used feasible range of values for decision-makers’ willingness to pay (ʎ), to obtain a distribution of net benefits for different levels of ʎ. In secondary analyses, the reported economic analysis used a cost-effectiveness threshold of £20,000 per QALY.
The mean [standard deviation (SD)] resource use per participant was estimated for each randomised group. The mean difference (95% CI) in mean resource use between arms (NB-UVB only compared with TCS only; and combination treatment compared with TCS only) is presented. The mean (SD) cost per participant is estimated for each randomised group. The mean difference (95% CI) in mean cost between arms (NB-UVB only compared with TCS only; and combination treatment compared with TCS only) is estimated unadjusted.
The primary outcome for the economic evaluation is cost per treatment success.
The secondary outcome for the economic evaluation is QALYs of participants over 9 months in the base case. The mean (SD) utility and mean (SD) QALYs per participant per randomised group is presented, and mean difference (95% CI) in utility and QALYs between arms (NB-UVB only compared with TCS only; and combination treatment compared with TCS only) is estimated unadjusted and adjusted.
Base-case analyses took into account missing data and are presented unadjusted and adjusted for age and target patch. The primary economic analysis, using the clinical outcome, used the imputation model and output of the primary clinical analysis presented in Chapter 3. Other analyses employed multiple imputation with chained equations using MI impute in Stata generating 60 (m = 60) data sets using predictive mean matching and separately by treatment allocation, the same approach as reported in Faria et al. 70 Costs were adjusted for age and location of target patch as were QALYs in addition to adjusting for baseline utility using seemingly unrelated regression. 71
Sampling uncertainty
Because costs and outcomes were skewed, non-parametric bootstrapping was used to determine the level of sampling uncertainty surrounding the mean incremental cost-effectiveness ratios by generating 10,000 estimates of incremental costs and benefits. These estimates were plotted on a cost-effectiveness plane. In addition, cost-effectiveness acceptability curves were produced, which show the probability that each intervention arm is cost-effective at different values of willingness to pay.
Subgroup analysis/analysis of heterogeneity
Other than doing separate pre-planned secondary analysis based on the different utility instruments used (EQ-5D-5L and CHU-9D), no subgroup analyses were undertaken.
Sensitivity analyses
Sensitivity analyses were undertaken to explore key uncertainties around important parameters in the economic evaluation.
-
The impact of missing data was explored by comparing base-case results using multiple imputation to a complete-case analysis.
-
Cost of the NB-UVB device: the cost-effectiveness of the interventions is likely to be significantly driven by the cost of the NB-UVB device. There is uncertainty about how the device would be prescribed and used, if it were found to be effective and adopted by the NHS. The base-case analysis annuitised the device cost assuming that the device would be used for a period of 5 years, but there is uncertainty surrounding this period of use and in practice the devices may not be returned by patients at the end of treatment. We estimate the device price at which a decision would switch from being cost-effective to cost-ineffective.
-
Wider cost perspective: as part of the trial, participants were asked about the costs (if any) incurred by themselves or their families in terms of out-of-pocket costs as a result of their vitiligo. These costs will be added to the base-case results to see if they would change the conclusions reached when considering NHS costs only.
-
Impact of treatment adherence: given any clinical effectiveness found and low adherence (defined as < 75% adherent), the economic analysis was repeated including only the adherent sample, where adherence was estimated as the total sessions used divided by the total expected sessions.
-
Longer-term analysis: if either comparison was found clinically effective at 9 months, then the cost-effectiveness and cost–utility analyses would be repeated at the 21-month follow-up point should the completion rate of follow-up data facilitate this. Although interventions will have stopped post 9 months, it might be useful to explore the longer-term cost-effectiveness of the comparators of interest beyond this point to see if value for money (if found at 9 months) is sustained. In any sensitivity analyses taking a 21-month time horizon, costs and benefits in months 13 to 21 would be discounted using the recommended rate of 3.5% for both costs and benefits. 57 It is expected that the majority of costs and benefits would be captured in this period, and, therefore, it is not considered necessary to develop a decision-analytic model.
This chapter has been written in line with Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting quality guidelines. Any deviations from the Health Economics Analysis Plan are described and justified in Results.
Results
Table 18 presents the unit costs (GBP, 2017), their source and any assumptions used throughout the economic analysis.
| Resource item | Unit cost (£) | Source (notes) |
|---|---|---|
| Intervention resources | ||
| Annuity factor | 4.515 based on r = 3.5% and n = 5 | Drummond et al.53 |
| Purchase price | 149.00 | Dermfix Ltd website (www.dermfix.uk; accessed November 2020) |
| Annuitised 9-month purchase pricea | 24.75 | (Purchase price divided by annuity factor to give EAC. EAC divided by 12 months and multiplied by nine) |
| Annuitised 9-month quality assurance (£17.83 multiplied by annuity factor) | 2.96 | Quality assurance: Medical Physics and Clinical Engineering, Nottingham University Hospitals NHS Trust (2018, personal communication) |
| Glasses (per set) | 15.00 | Dermfix Ltd website |
| Goggles (per set) | 7.00 | Dermfix Ltd website |
| TCS (per 90-g tube of mometasone furoate 0.1%) | 12.13 | NHS Digital Prescription Cost Analysis58 |
| Investigator face-to-face and telephone support (per minute, assumed band 7 £54 per hour) | 0.90 | PSSRU 201761 |
| Dermatologist face-to-face first appointment consultant led | 159.00 | NHS Schedule of Reference Costs 2017–2018 60 |
| Dermatologist face-to-face follow-up appointment consultant led | 129.00 | NHS Schedule of Reference Costs 2017–2018 60 |
| Dermatologist telephone appointment consultant led | 100.00 | NHS Schedule of Reference Costs 2017–2018 60 |
| Primary care resources (per visit) | ||
| GP | 37.00 | PSSRU 201761 |
| Practice nurse | 10.85 | PSSRU 201761 |
| Pharmacist (assumed to be a community pharmacist) | 11.11 | PSSRU 201761 |
| Hospital doctor | 53.33 | PSSRU 201761 |
| Hospital nurse | 15.00 | PSSRU 201761 |
| Therapist | 27.00 | PSSRU 201761 |
| Other (reported by participants) | Range from 15.00 to 86.00 | PSSRU 201761 and NHS Schedule of Reference Costs 2017–201860 |
| Other resources | ||
| Medication (various, NIC per item less NADP plus professional fee) | Range from 3.37 to 36.92 | Prescription Cost Analysis 2017 58 |
| Participant and family out-of-pocket costs | Various | Estimates reported by participants |
Intervention costs
The intervention costs consisted of the device plus consumables’ costs, drug costs, dermatologist appointments, training and unscheduled visit/telephone appointment costs. We did not include the costs of training the nurses to deliver the training session with participants or the costs of developing the video, as these were assumed to be sunk costs.
Glasses and goggles were given out for eye protection when using the NB-UVB device. These were costed for the actual number given out to participants (see Tables 20 and 22 for mean number used by group): some participants requested more than one set if their vitiligo patch was in a difficult-to-reach place or if they needed help (if the participant was a child).
Quality assurance
The process of setting up and checking the quality of devices before they were issued to patients was estimated using expert opinion from staff at the Nottingham University Hospitals medical physics department. The quality assurance process involved device in and device out processes. Before devices were issued to participants they were tested for electrical safety and output, spectral characterisation was carried out, and some data administration was involved. When devices were returned, they again had their output tested and some data administration was involved. Table 19 shows the time and cost for each aspect. Staff time was assumed to be a mid-point band 5 on Agenda for Change and the batch size was assumed to be 10 devices at once. Quality assurance costs were also multiplied by the annuity factor to gain the cost over the study period. In reality, quality assurance might be carried out more frequently than every 5 years or may be provided using a different service model (e.g. specialist vs. local sites undertaking the activity) that may affect cost but the impact of this assumption is tested in the sensitivity analysis section, where price is varied to see the impact on cost per treatment success.
| Process | Set-up time per batch (minutes) | Cost of set up per device (£) | Time per device (minutes) | Cost per device (£) | Total cost (£) |
|---|---|---|---|---|---|
| Device out | |||||
| Electrical safety testing | 10 | 0.52 | 5 | 2.58 | 3.10 |
| Output testing | 20 | 1.03 | 8 | 4.13 | 5.17 |
| Spectral characterisation | 30 | 1.55 | 10 | 5.17 | 6.72 |
| Data administration | 5 | 0.26 | 5 | 2.58 | 2.84 |
| Device in | |||||
| Output testing | 20 | 1.03 | 8 | 4.13 | 5.17 |
| Data administration | 5 | 2.58 | 2.58 | ||
It was assumed that devices would be given to patients at an appointment with the dermatologist. It was assumed they would have four visits with a dermatologist over the 9-month treatment period, whichever treatment group they were in. Those receiving the NB-UVB would also have had an appointment with a nurse in the dermatology department and a training session with the nurse. Tables 20 and 21 show that the training time had a mean of 73.08 minutes in the NB-UVB-only group and 69.17 minutes in the combination treatment group. In addition to routine visits to the dermatologist and nurse at set intervals, unscheduled contacts were also recorded. Such visits could either be face to face or over the telephone and occurred because of side effects or concerns over the use of treatments. The number of such contacts was small in all groups, although the combination treatment group had the most (see Tables 20 and 21).
| Resource | NB-UVB (N = 169) | TCS (N = 173) | Mean difference (95% CI) | ||
|---|---|---|---|---|---|
| Mean | SD (n) | Mean | SD (n) | ||
| Intervention | |||||
| NB-UVB interventiona | 1.08 | 0.30 (169) | 0.00 | 0.00 (173) | 1.083 (1.04 to 1.13) |
| Glasses | 1.41 | 0.58 (169) | 0.00 | 0.00 (173) | 1.41 (1.33 to 1.50) |
| Goggles | 0.46 | 0.60 (169) | 0.00 | 0.00 (173) | 0.46 (0.37 to 0.54) |
| TCS | 0.00 | 0.00 (169) | 2.15 | 0.55 (173) | –2.15 (–2.23 to –2.07) |
| Training time (minutes) | 73.08 | 40.47 (169) | 0.00 | 0.00 (173) | 73.08 (67.03 to 79.13) |
| Dermatologist time (clinic and telephone) | 4.00 | 0.00 (169) | 4.00 | 0.00 (173) | 0.00 (0.00 to 0.00) |
| Nurse time (clinic and telephone) | 2.00 | 0.00 (169) | 0.00 | 0.00 (173) | 2.00 (2.00 to 2.00) |
| Unscheduled clinic with nurse | 0.03 | 0.20 (169) | 0.01 | 0.11 (173) | 0.02 (–0.02 to 0.05) |
| Unscheduled telephone with nurse | 0.46 | 0.95 (169) | 0.39 | 0.87 (173) | 0.07 (–0.13 to 0.26) |
| Unscheduled clinic with dermatologist | 0.04 | 0.20 (169) | 0.02 | 0.13 (173) | 0.02 (–0.01 to 0.06) |
| Unscheduled telephone with dermatologist | 0.03 | 0.20 (169) | 0.02 | 0.17 (173) | 0.01 (–0.03 to 0.05) |
| Primary care and community | |||||
| Number | 0.17 | 0.64 (132) | 0.12 | 0.44 (136) | 0.06 (–0.07 to 0.19) |
| Secondary care | |||||
| Number | 0.20 | 0.61 (132) | 0.48 | 4.47 (136) | –0.28 (–1.05 to 0.49) |
| Other | |||||
| Medication | 0.08 | 0.35 (133) | 0.12 | 0.50 (138) | –0.04 (–0.14 to 0.06) |
| Out-of-pocket purchases | 0.28 | 0.88 (137) | 0.40 | 1.44 (141) | –0.12 (–0.40 to 0.16) |
| Resource | Combination (N = 175) | TCS (N = 173) | Mean difference (95% CI) | ||
|---|---|---|---|---|---|
| Mean | SD (n) | Mean | SD (n) | ||
| Intervention | |||||
| NB-UVB interventiona | 1.07 | 0.30 (175) | 0.00 | 0.00 (173) | 1.07 (1.03 to 1.12) |
| Glasses | 1.50 | 0.56 (175) | 0.00 | 0.00 (173) | 1.50 (1.41 to 1.58) |
| Goggles | 0.40 | 0.56 (175) | 0.00 | 0.00 (173) | 0.40 (0.32 to 0.48) |
| TCS | 2.12 | 0.49 (175) | 2.15 | 0.55 (173) | –0.03 (–0.14 to 0.08) |
| Training time (minutes) | 69.17 | 34.51 (175) | 0.00 | 0.00 (173) | 69.17 (64.01 to 74.33) |
| Dermatologist time (clinic and telephone) | 4.00 | 0.00 (175) | 4.00 | 0.00 (173) | 4.00 (4.00 to 4.00) |
| Nurse time (clinic and telephone) | 2.00 | 0.00 (175) | 0.00 | 0.00 (173) | 2.00 (2.00 to 2.00) |
| Unscheduled clinic with nurse | 0.13 | 0.51 (175) | 0.01 | 0.11 (173) | 0.12 (0.04 to 0.20) |
| Unscheduled telephone with nurse | 0.66 | 1.29 (175) | 0.39 | 0.87 (173) | 0.28 (0.04 to 0.51) |
| Unscheduled clinic with dermatologist | 0.10 | 0.43 (175) | 0.02 | 0.13 (173) | 0.09 (0.02 to 0.15) |
| Unscheduled telephone with dermatologist | 0.05 | 0.27 (175) | 0.02 | 0.17 (173) | 0.03 (–0.01 to 0.08) |
| Primary care and community | |||||
| Number | 0.12 | 0.55 (142) | 0.12 | 0.44 (136) | 0.002 (–0.12 to 0.12) |
| Secondary care | |||||
| Number | 0.20 | 0.63 (142) | 0.48 | 4.47 (136) | –0.28 (–1.03 to 0.46) |
| Other | |||||
| Medication | 0.09 | 0.34 (141) | 0.12 | 0.50 (138) | –0.03 (–0.13 to 0.07) |
| Out-of-pocket purchases | 0.31 | 1.27 (144) | 0.40 | 1.44 (141) | –0.09 (–0.41 to 0.23) |
Those participants receiving active TCS received two 90-g tubes of mometasone furoate 0.1% ointment at the outset of the study and any requests for further tubes were recorded and costed accordingly and similar amounts were requested in the TCS only and combination treatment groups (see Tables 20–22).
| Cost | NB-UVB (N = 169) | TCS (N = 173) | Mean difference (95% CI) | ||
|---|---|---|---|---|---|
| Mean | SD (n) | Mean | SD (n) | ||
| Intervention | |||||
| NB-UVB device | 24.75 | 0.00 (169) | 0.00 | 0.00 (173) | 24.75 (24.75 to 24.75) |
| Quality assurance for device | 2.96 | 0.00 (169) | 0.00 | 0.00 (173) | 2.96 (2.96 to 2.96) |
| Glasses | 21.21 | 8.74 (169) | 0.00 | 0.00 (173) | 21.21 (19.91 to 22.52) |
| Goggles | 3.19 | 4.18 (169) | 0.00 | 0.00 (173) | 3.19 (2.56 to 3.81) |
| TCS | 0.00 | 0.00 (169) | 26.08 | 6.67 (173) | –26.08 (–27.09 to –25.07) |
| Training time | 65.77 | 36.42 (169) | 0.00 | 0.00 (173) | 65.77 (60.32 to 71.22) |
| Dermatologist (clinic and telephone) | 546.00 | 0.00 (169) | 546.00 | 0.00 (173) | 0.00 (0.00 to 0.00) |
| Nurse (clinic and telephone) | 72.00 | 0.00 (169) | 0.00 | 0.00 (173) | 72.00 (72.00 to 72.00) |
| Unscheduled clinic with nurse | 0.53 | 3.64 (169) | 0.21 | 1.93 (173) | 0.32 (–0.29 to 0.94) |
| Unscheduled telephone with nurse | 8.34 | 17.53 (169) | 7.16 | 16.30 (173) | 1.19 (–2.41 to 4.79) |
| Unscheduled clinic with dermatologist | 5.34 | 25.78 (169) | 2.24 | 16.89 (173) | 3.11 (–1.52 to 7.73) |
| Unscheduled telephone with dermatologist | 2.96 | 20.20 (169) | 1.73 | 16.96 (173) | 1.22 (–2.74 to 5.19) |
| Total cost of intervention | 753.06 | 59.16 (169) | 583.42 | 29.59 (173) | 169.64 (159.73 to 179.56) |
| Primary care and community | |||||
| Cost | 5.90 | 22.20 (132) | 3.90 | 15.21 (136) | 2.00 (–2.56 to 6.57) |
| Secondary care | |||||
| Cost | 9.30 | 30.05 (132) | 11.05 | 77.14 (136) | –1.74 (–15.90 to 12.42) |
| Other | |||||
| Medication | 1.49 | 7.06 (133) | 2.48 | 10.52 (138) | –0.99 (–3.14 to 1.16) |
| Total mean cost per participant | 774.64 | 83.71 (131) | 599.98 | 96.18 (132) | 174.66 (152.75 to 196.56) |
| Out-of-pocket costs | 4.94 | 20.09 (137) | 14.44 | 96.78 (141) | –9.49 (–26.11 to 7.12) |
| Primary outcome | |||||
| VNS | 27 (21.95) | 20 (16.81) | 7 (5.14) | ||
Resource use, costs and primary clinical outcome
Use of resources for the intervention and wider health-care resource use related to vitiligo are shown in Tables 20 and 21 using available case data. These show that wider health-care resource use (primary care, secondary care and medicines) used for vitiligo but beyond those required for the intervention were not significantly different between groups. Patients with vitiligo can be seen to be low users of NHS health care, perhaps because there is a lack of treatments currently available for this condition, or because the trial was offering the best treatment for the condition and so they had little need for further care. Tables 22 and 23 display the mean resource use per participant by treatment group using available case data. It can be seen that the overall mean cost per participant in the NB-UVB-only group was £774.64 (SD £83.71) compared with £599.98 (SD £96.18) in the TCS-only group, giving an unadjusted mean difference in cost of £174.66 (95% CI £152.75 to £196.66). The combination treatment group had overall mean costs per participant of £813.38 (SD £111.39); compared with the TCS-only group this gave an unadjusted mean difference of £213.40 (95% CI £188.33 to £238.46) per participant. These figures suggest that the costs of the interventions are not offset by reductions in wider health-care resource use related to vitiligo, and that if the interventions are to be considered cost-effective, the additional cost of the interventions needs to be justified in terms of additional benefit attained.
| Cost | Combination (N = 175) | TCS (N = 173) | Mean difference (95% CI) | ||
|---|---|---|---|---|---|
| Mean | SD (n) | Mean | SD (n) | ||
| Intervention | |||||
| NB-UVB device | 24.75 | 0.00 (175) | 0.00 | 0.00 (173) | 24.75 (24.75 to 24.75) |
| Quality assurance for device | 2.96 | 0.00 (175) | 0.00 | 0.00 (173) | 2.96 (2.96 to 2.96) |
| Glasses | 22.46 | 8.34 (175) | 0.00 | 0.00 (173) | 22.46 (21.21 to 23.70) |
| Goggles | 2.80 | 3.90 (175) | 0.00 | 0.00 (173) | 2.80 (2.22 to 3.38) |
| TCS | 25.71 | 5.99 (175) | 26.08 | 6.67 (173) | –0.37 (–1.70 to 0.97) |
| Training time | 62.25 | 31.06 (175) | 0.00 | 0.00 (173) | 62.25 (57.61 to 66.90) |
| Dermatologist (clinic and telephone) | 546.00 | 0.00 (175) | 546.00 | 0.00 (173) | 546 (546.00 to 546.00) |
| Nurse (clinic and telephone) | 72.00 | 0.00 (175) | 0.00 | 0.00 (173) | 72.00 (72.00 to 72.00) |
| Unscheduled clinic with nurse | 2.41 | 9.53 (175) | 0.21 | 1.93 (173) | 2.20 (0.75 to 3.66) |
| Unscheduled telephone with nurse | 12.30 | 23.92 (175) | 7.16 | 16.30 (173) | 5.14 (0.82 to 9.46) |
| Unscheduled clinic with dermatologist | 13.27 | 55.45 (175) | 2.24 | 16.89 (173) | 11.03 (2.37 to 19.70) |
| Unscheduled telephone with dermatologist | 5.14 | 26.84 (175) | 1.73 | 16.96 (173) | 3.41 (–1.33 to 8.15) |
| Total cost of intervention | 792.06 | 94.61 (175) | 583.42 | 29.59 (173) | 208.64 (193.82 to 223.46) |
| Primary care and community | |||||
| Cost | 2.84 | 14.09 (142) | 3.90 | 15.21 (136) | –1.06 (–4.52 to 2.40) |
| Secondary care | |||||
| Cost | 8.52 | 26.87 (142) | 11.05 | 77.14 (136) | –2.53 (–16.05 to 11.00) |
| Other | |||||
| Medication | 1.20 | 6.09 (140) | 2.48 | 10.52 (138) | –1.28 (–3.30 to 0.75) |
| Total mean cost per participant | 813.38 | 111.39 (136) | 599.98 | 96.18 (132) | 213.40 (188.33 to 238.46) |
| Out-of-pocket costs | 6.62 | 28.45 (144) | 14.44 | 96.78 (141) | –7.81 (–24.37 to 8.75) |
| Primary outcome | |||||
| VNS, n successful (% successful) | 34 (26.56) | 20 (16.81) | 14 (9.75) | ||
Primary economic analysis
Cost-effectiveness analysis of narrowband UVB only compared with topical corticosteroid only
The unadjusted risk difference for NB-UVB compared to TCS was 3.64% (adjusted risk difference 5.20%), this equates to a NNT of 27 (19 adjusted); in other words, 27 (19) participants would need to be treated for one of them gain treatment success.
The incremental difference in cost was £174.65 (95% CI £152.75 to £96.55) unadjusted or £173.44 (95% CI £150.55 to £196.32) adjusted for age and body region of the target patch. The unadjusted incremental cost was £4801.92 (£3335.74 adjusted) per additional successful treatment. Figure 8 shows the probability that NB-UVB only is cost-effective at different possible levels of willingness to pay for an additional treatment success; probability increases as willingness to pay increases. It can be seen that there is a lot of uncertainty surrounding the decision of whether or not NB-UVB alone, compared with TCS alone, represents value for money as there is always at least 40% probability of making the wrong decision if choosing to fund NB-UVB alone below a threshold value of willingness to pay of £10,000 per additional treatment success.
FIGURE 8.
Cost-effectiveness acceptability curve for NB-UVB only vs. TCS only.
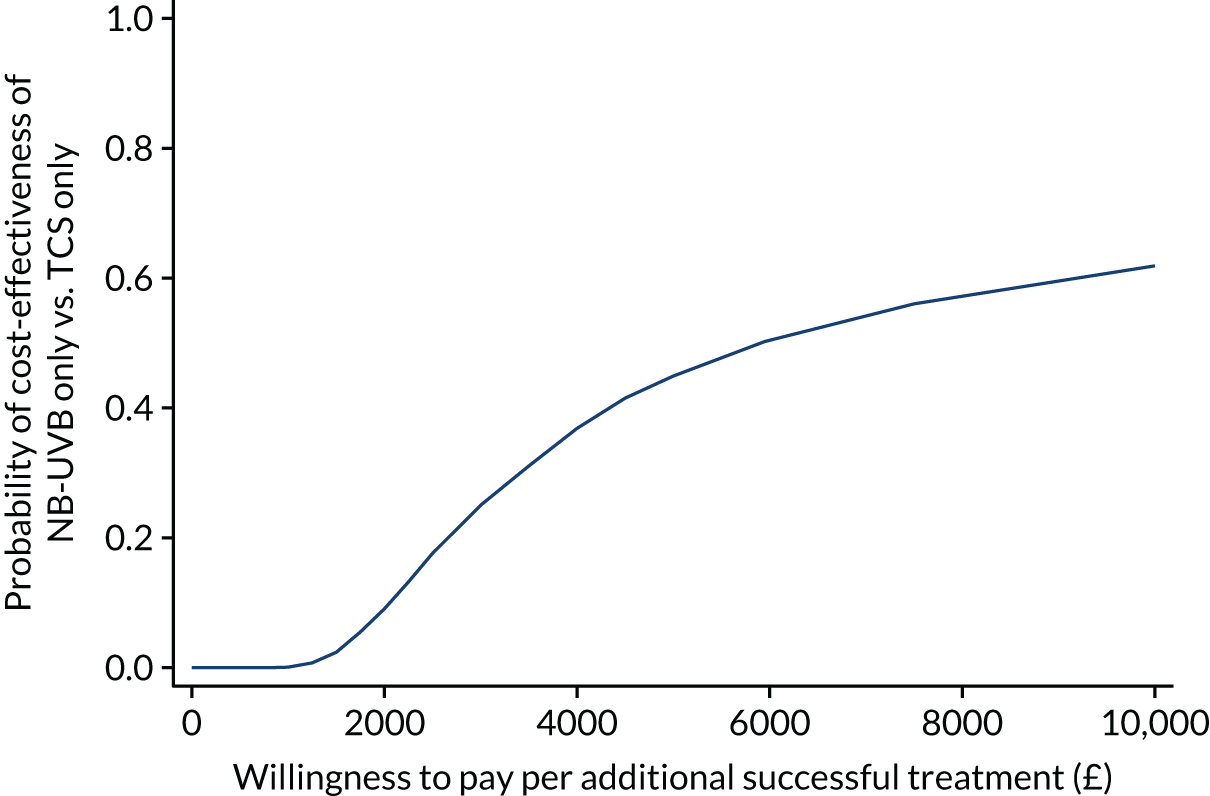
Cost-effectiveness analysis of combination treatment compared with topical corticosteroid only
The unadjusted risk difference for combination treatment compared to TCS was 9.16% (adjusted 10.94%). This equates to a NNT of 10 (9 adjusted) [i.e. 10 (9 adjusted) participants would need to be treated for one of them to gain a treatment success].
The incremental difference in cost was £213.40 (95% CI £190.02 to £236.78) unadjusted or £211.46 (95% CI £188.10 to £234.81) adjusted for age and location of the target patch. The unadjusted incremental cost was £2328.56 (£1932.35 adjusted) per additional successful treatment.
Figure 9 shows the probability that combination treatment is cost-effective at different possible levels of willingness to pay for an additional treatment success. It shows that combination treatment is likely to be cost-effective if the decision-maker is willing to pay > £3000 per additional treatment success. There is, however, currently no evidence to indicate how much a decision-maker would be willing to pay for an additional treatment success as defined in this study. Should the decision-maker’s willingness to pay per additional treatment success be low (i.e. < £2500) then it can be seen that uncertainty surrounding the decision to fund combination treatment is high.
FIGURE 9.
Cost-effectiveness acceptability curve for combination treatment vs. TCS only.
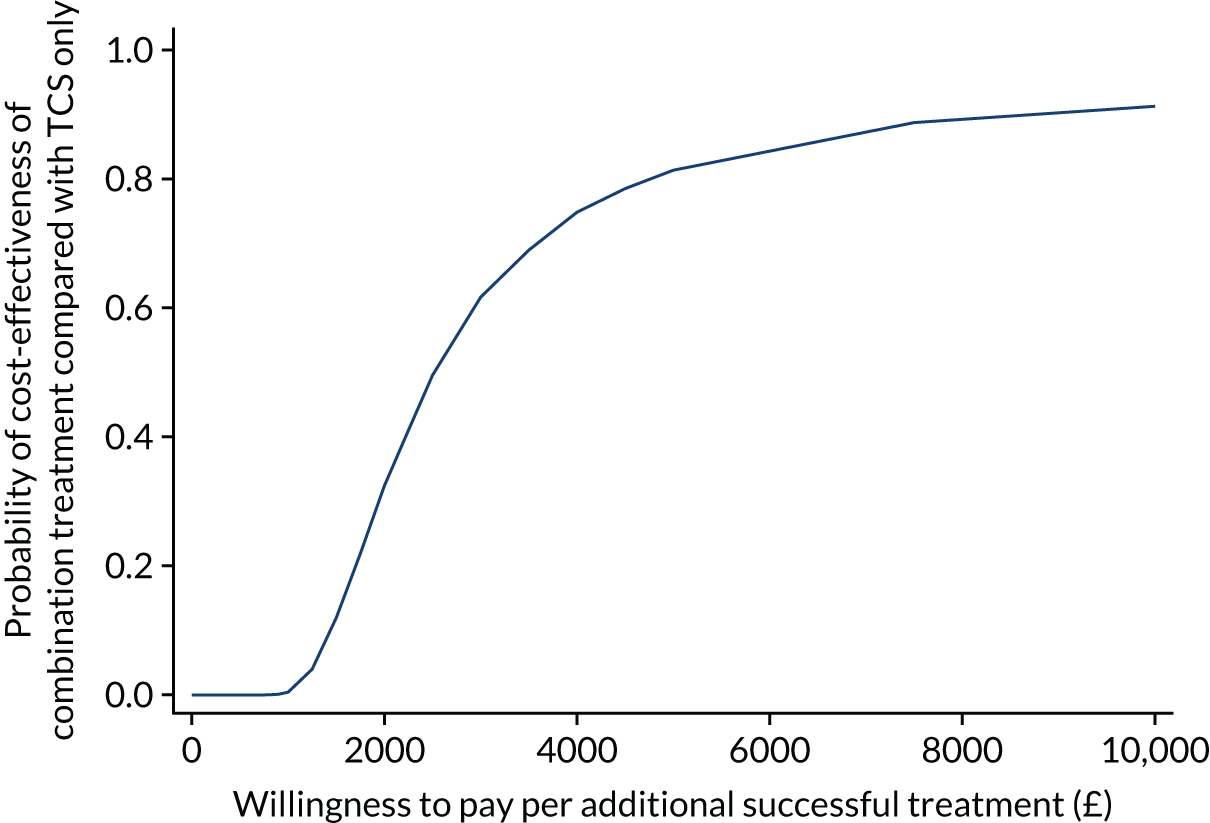
Sensitivity analysis
A number of sensitivity analyses were undertaken to explore key uncertainties around important parameters in the economic evaluation. The results of this are summarised in Table 24 with greater detail below for each analysis.
| Analysis | NB-UVB vs. TCS | Combination vs. TCS | ||||
|---|---|---|---|---|---|---|
| Incremental costs (£) | Incremental effect (risk difference), % | Incremental cost per treatment success (£) | Incremental costs (£) | Incremental effect (risk difference), % | Incremental cost per treatment success (£) | |
| Primary imputed | 173.44 | 5.20 | 3335.74 | 211.46 | 10.94 | 1932.35 |
| Complete case | 172.61 | 4.88 | 3535.40 | 212.59 | 9.96 | 2134.11 |
| Cost of device zero | 121.79 | 5.20 | 2342.35 | 158.54 | 10.94 | 1448.82 |
| Cost of device doubled | 225.02 | 5.20 | 4327.78 | 264.33 | 10.94 | 2415.55 |
| Wider cost perspective | 163.90 | 5.20 | 3152.30 | 200.95 | 10.94 | 1836.31 |
| Adherent patients only | 193.34 | 13.87 | 1393.98 | 230.83 | 20.06 | 1150.65 |
Complete-case analysis
The base case assumed data to be missing at random and undertook imputation to allow for this. 70 Table 24 presents the results for a complete-case analysis that includes participants with complete resource use and outcome data only to see if this changes the conclusions reached in the base-case analysis. In total, 348 participants had complete data on both cost and outcome (success of treatment): 113 in TCS only, 115 in NB-UVB only and 120 in combination treatment.
The cost of the narrowband UVB device
The cost-effectiveness of the interventions is likely to be driven significantly by the cost of the NB-UVB device. There is uncertainty about how the device would be prescribed and used in the NHS. If adopted as an effective treatment, patients may have to pay for the device themselves (with training, support and quality assurance paid for by the NHS), or the device might be adopted and provided free at point of use by the NHS for NHS patients. The base-case analysis annuitised the device cost, assuming that the device would be used for a period of 5 years, but there is uncertainty surrounding this period of use and, in practice, it may be that the devices are not returned by patients at the end of treatment.
We re-estimated the incremental cost per successful treatment assuming that patients paid for the device, quality assurance, glasses and goggles as one extreme and at the other we doubled the price of the device, quality assurance, goggles and glasses to provide an upper estimate.
As expected (see Table 23), reducing the cost of devices to zero reduced the incremental cost per treatment success, thereby lowering the amount that an NHS decision-maker would have to be willing to pay for this treatment to be implemented in the NHS. Conversely, doubling the cost of the device increased the incremental cost per treatment success and meant that the NHS would have to value a treatment success more highly than the base case to be willing to adopt the treatments.
Changes in the price of the device had less of an impact on the combination treatment versus TCS only comparison, due to the greater treatment success observed in the combination group. As noted in the primary base-case analysis, it is not clear how much a decision-maker would be willing to pay to achieve one more additional treatment success as measured on the VNS. Therefore, these figures just provide a range around the likely cost per treatment success.
Wider cost perspective
As part of the trial, participants were asked about the out-of-pocket costs (if any) incurred by themselves or their families as a result of their vitiligo. These costs were added to the base-case results (NHS perspective only) to see how they would have an impact on the incremental cost per treatment success. A total of 47 (11.1%) participants reported incurring out-of-pocket costs during the 9-month treatment period: 17 in the TCS-only group, 17 in the NB-UVB-only group and 13 in the combination group. The mean number of items and mean cost per participant by group can be seen in Tables 20–22. The type of items included (from most to least purchased) camouflage/makeup, sun cream and sun care, clothes/scarves, face creams/moisturisers/emollients, fake tan/tanning products, travel for appointments, private appointment including multivitamins, and herbal remedies.
Taking into account the participant out-of-pocket costs in relation to vitiligo reduced the incremental cost per treatment success, as these costs were higher in the standard care group (TCS only) (see Table 23 for results).
Impact of adherence
Because significant clinical effectiveness was found and a little under half of the participants used the treatment for > 75% of the expected duration, the primary economic analysis was repeated including the adherent sample only, where adherence was estimated as total sessions used divided by total expected sessions. In total, 227 participants adhered to treatments > 75% of the time; this sample was used as the adherent sample, minus three participants (one of whom had the primary outcome missing and two who had cost data missing).
The intervention was more cost-effective for patients who adhered to treatment, as they were the ones most likely to achieve a successful outcome (see Table 23 for estimates).
Longer-term analysis (12 to 21 months)
In the health economic analysis plan we intended to explore the longer-term cost-effectiveness of the comparators of interest beyond the 9-month treatment period (if either were found effective), to see if value for money was sustained. In the trial, only 30.4% of participants had complete data on NHS resource use in months 10–21, 44.5% of participants aged ≥ 11 years completed the EQ-5D-5L at 21 months and 43.3% of participants aged < 18 years at the beginning of the study had completed the CHU-9D at 21 months. Given the sparsity of data, we have not performed an economic evaluation over the longer-term follow-up as it would be too speculative. However, we report mean estimates of the participants’ (all ages, n = 517) wider NHS use over months 10 to 21 (the follow-up period) and utility at 21 months. Only 157 participants had complete resource use data for the whole 12-month follow-up period, which may have been for zero use, 64 had 9 months’ worth of data available, 56 had 6 months’ worth of data available, 59 had 3 months’ worth of data available and 181 had no resource use data recorded for the follow-up period. The mean quarterly NHS cost per participant over the 12-month follow-up period was £21.26 (SD £46.32) for combination treatment (n = 114), £25.89 (SD £52.82) for NB-UVB alone (n = 117) and £21.74 (SD £42.33) for TCS alone (n = 105). The mean prescription cost per participant over the 12-month follow-up period was £14.82 (SD £45.22) for combination treatment (n = 114), £13.78 (SD £45.63) for NB-UVB alone (n = 117) and £13.20 (SD £51.44) for TCS alone (n = 107). The mean out-of-pocket cost per participant over the 12-month follow-up period was £42.85 (SD £398.74) for combination treatment (n = 114), £3.62 (SD £16.93) for NB-UVB alone (n = 117) and £8.48 (SD £39.41) for TCS alone (n = 107).
The mean utility (EQ-5D-5L) per participant aged ≥ 11 years at 21 months was 0.856 (SD 0.230) for combination treatment (n = 73), 0.865 (SD 0.231) for NB-UVB alone (n = 61) and 0.833 (SD 0.274) for TCS alone (n = 69). The mean utility (CHU-9D) per participant (aged < 18 years at the outset of the study) at 21 months was 0.938 (SD 0.054) for combination treatment (n = 20), 0.941 (SD 0.056) for NB-UVB alone (n = 16) and 0.937 (SD 0.118) for TCS alone (n = 16).
Secondary economic analysis
Cost–utility analysis for those aged ≥ 11 years
Of the 517 participants in the trial, 456 (88%) participants were aged 11 years, 155 were randomised to TCS only, 148 were randomised to NB-UVB only and 153 were randomised to combination treatment.
The cost–utility analysis was planned as a secondary analysis due to the fact that, to our knowledge, no prior study had utilised the EQ-5D-5L, or CHU-9D for children, in patients with vitiligo. There were some concerns that such generic quality-of-life instruments might not be appropriate for this condition, as much of the effect may be visual or psychological rather than on physical quality of life. Such concerns seem to have been borne out in the study, Table 25 shows the domains on the EQ-5D-5L selected by participants at baseline. In total, 55% of participants reported having no problems on any of the five domains of the EQ-5D-5L at baseline, suggesting that over half of the sample started the study in perfect health as defined by this instrument. This is a large ceiling effect that was also observed at subsequent follow-up (Table 25). No floor effect was observed at any time point.
| Levels | Mobility, n (%) | Self-care, n (%) | Usual activities, n (%) | Pain/discomfort, n (%) | Anxiety/depression, n (%) | Percentage of participants in health state 11111 (55555) |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| 1 (no problems) | 416 (91.8) | 436 (96.3) | 394 (87.0) | 376 (83.0) | 292 (64.5) | 55.0% |
| 2 | 22 (4.9) | 8 (1.8) | 41 (9.1) | 44 (9.7) | 108 (23.8) | |
| 3 | 9 (2.0) | 4 (0.9) | 7 (1.6) | 22 (4.9) | 40 (8.8) | |
| 4 | 4 (0.9) | 1 (0.2) | 6 (1.3) | 7 (1.6) | 5 (1.1) | |
| 5 (unable to/extreme) | 0 (0) | 1 (0.2) | 2 (0.4) | 1 (0.2) | 6 (1.3) | (0%) |
| Blank | 2 (0.4) | 3 (0.7) | 3 (0.7) | 3 (0.7) | 2 (0.4) | |
| 9 months | ||||||
| 1 | 271 (90.0) | 290 (96.4) | 271 (90.3) | 249 (82.7) | 215 (71.4) | 59.8% |
| 2 | 19 (6.3) | 6 (2.0) | 16 (5.3) | 29 (9.6) | 55 (18.3) | |
| 3 | 9 (3.0) | 3 (0.7) | 9 (3.0) | 18 (6.0) | 22 (7.3) | |
| 4 | 1 (0.3) | 1 (0.3) | 3 (1.0) | 4 (1.3) | 5 (1.6) | |
| 5 | 0 (0) | 0 (0) | 1 (0.3) | 1 (0.3) | 4 (1.3) | (0%) |
| Blank | 1 (0.2) | 1 (0.3) | 1 (0.3) | 0 (0) | 0 (0) | |
| Final assessment (19 or 21 months) | ||||||
| 1 | 131 (64.5) | 180 (88.7) | 192 (94.6) | 173 (85.2) | 153 (75.4) | 50.3% |
| 2 | 50 (24.6) | 14 (6.9) | 2 (1.0) | 21 (10.3) | 36 (17.7) | |
| 3 | 19 (9.4) | 3 (1.5) | 0 (0.0) | 3 (1.5) | 9 (4.4) | |
| 4 | 1 (0.5) | 1 (0.5) | 1 (0.5) | 1 (0.5) | 2 (1.0) | |
| 5 | 0 (0.0) | 1 (0.5) | 1 (0.5) | 1 (0.5) | 0 (0.0) | (0%) |
| Blank | 2 (1.0) | 4 | 7 | 4 | 3 (1.5) | |
Cost–utility analysis for participants aged ≥ 11 years for narrowband UVB only compared with topical corticosteroid only
The unadjusted mean cost per participant in the NB-UVB-only treatment group (n = 131) was £774.64 (SD £83.71, 95% CI £760.17 to £789.11) compared to £599.99 (SD £96.18, 95% CI £583.43 to £616.55) for the TCS-only group (n = 132) giving an unadjusted mean incremental cost per participant of £174.65 (95% CI £152.75 to £196.55). The imputed, adjusted and bootstrapped mean incremental cost per participant was £169.58 (95% CI £165.50 to £173.65) more for the NB-UVB-only treatment group than the TCS-only group.
The imputed, adjusted and bootstrapped mean incremental QALYs gained were 0.0204 (95% CI 0.0180 to 0.0229) in favour of the NB-UVB only compared with TCS only (Table 26). The adjusted incremental cost per QALY was £8293.88.
| Secondary outcomes | NB-UVB (N = 148) | TCS (N = 155) | Mean difference (95% CI) | Combination (N = 153) | TCS (N = 155) | Mean difference (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD (n) | Mean | SD (n) | Mean | SD (n) | Mean | SD (n) | |||
| van Hout et al.65 utility value set known as the ‘crosswalk’ | ||||||||||
| EQ-5D-5L at baseline | 0.8920 | 0.1866 (140) | 0.9172 | 0.1145 (151) | –0.0252 (–0.0607 to 0.0102) | 0.8906 | 0.1719 (147) | 0.9172 | 0.1145 (151) | –0.0266 (–0.0599 to 0.0066) |
| EQ-5D-5L at 9 months | 0.9287 | 0.1422 (89) | 0.8843 | 0.1666 (97) | 0.0444 (–0.0006 to 0.0894) | 0.9182 | 0.1325 (98) | 0.8843 | 0.1666 (97) | 0.0339 (–0.0086 to 0.0764) |
| QALYs at 9 months | 0.6871 | 0.0913 (89) | 0.6721 | 0.0983 (97) | 0.0150 (–0.0125 to 0.0425) | 0.6843 | 0.0993 (96) | 0.6721 | 0.0983 (97) | 0.0122 (–0.0159 to 0.0402) |
Cost–utility analysis for participants aged ≥ 11 years for combination treatment compared with topical corticosteroid only
The unadjusted mean cost per participant in the combination treatment group (n = 136) was £813.38 (SD £111.39, 95% CI £794.49 to £832.27) compared with £599.99 (SD £96.18, 95% CI £583.43 to £616.55) for the TCS-only group (n = 132) giving an unadjusted mean incremental cost per participant of £213.40 (95% CI £188.33 to £238.46). The imputed, adjusted and bootstrapped mean incremental cost per participant was £203.93 (95% CI £199.39 to £208.47) more for the combination treatment group than the TCS-only group.
The imputed, adjusted and bootstrapped mean incremental QALYs gained were 0.0145 (95% CI 0.0123 to 0.0167) in favour of the combination treatment compared with TCS only (see Table 26). The adjusted incremental cost per QALY was £14,081.
Alternative utility value sets were tested to see if they had any impact on the results but because the size of QALY gains were so small in this study (see Appendix 7) the choice of value set did not affect the results presented above in the cost–utility analysis.
Cost–utility analysis for participants aged < 18 years
A total of 119 participants were aged < 18 years, 40 received TCS only, 39 NB-UVB only and 40 the combination treatment. Complete cost and outcome data were available for only 91 (75.8%) of these participants. The results presented here are based on a complete-case analysis only, as an imputed, adjusted analysis was not possible due to the small sample size. Table 27 shows the utility, as measured on the CHU-9D at baseline and 9 months, and QALYs for the 9-month treatment period. The incremental QALYs were non-significantly different from zero.
| Secondary outcomes | NB-UVB (N = 39) | TCS (N = 40) | Mean difference (95% CI) | Combination (N = 40) | TCS (N = 40) | Mean difference (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD (n) | Mean | SD (n) | Mean | SD (n) | Mean | SD (n) | |||
| CHU-9D at baseline | 0.9450 | 0.0635 (35) | 0.9506 | 0.0528 (40) | –0.0056 (–0.0324 to 0.0212) | 0.9326 | 0.0605 (39) | 0.9506 | 0.0528 (40) | –0.0180 (–0.043 to 0.0074) |
| CHU-9D at 9 months | 0.9538 | 0.0416 (28) | 0.9513 | 0.0523 (31) | 0.0025 (–0.0223 to 0.0273) | 0.9318 | 0.0590 (35) | 0.9513 | 0.0523 (31) | –0.0195 (–0.0471 to 0.0080) |
| QALYs at 9 months | 0.7154 | 0.0312 (28) | 0.7135 | 0.0392 (31) | 0.0019 (–0.0167 to 0.0205) | 0.6988 | 0.0443 (35) | 0.7135 | 0.0392 (31) | –0.0147 (–0.0353 to 0.0060) |
The ceiling effect on this instrument was better than the EQ-5D-5L but still high; 30% of participants had no problems according to any of the nine dimensions on the CHU-9D (Table 28). The domains of worry, tiredness and sleeping were those in which problems were reported most often.
| Level | Worry, n (%) | Sad, n (%) | Pain, n (%) | Tired, n (%) | Annoyed, n (%) | Schoolwork, n (%) | Sleep, n (%) | Routine, n (%) | Activities, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Baseline | |||||||||
| 1 (no problems) | 91 (75.8) | 109 (90.8) | 104 (86.7) | 67 (55.8) | 104 (86.7) | 97 (80.8) | 90 (75.0) | 110 (91.7) | 106 (88.3) |
| 2 | 20 (16.7) | 7 (5.8) | 13 (10.8) | 30 (25.0) | 11 (9.2) | 20 (16.7) | 20 (16.7) | 6 (5.0) | 6 (5.0) |
| 3 | 5 (4.2) | 2 (1.7) | 2 (1.7) | 10 | 2 (1.7) | 0 (0.0) | 6 (5.0) | 3 (2.5) | 3 (2.5) |
| 4 | 1 (0.8) | 0 (0.0) | 0 (0.0) | 6 (5.0) | 2 (1.7) | 0 (0.0) | 2 (1.7) | 0 (0.0) | 1 (0.8) |
| 5 (very problematic) | 2 (1.7) | 1 (0.8) | 0 (0.0) | 5 (4.2) | 0 (0.0) | 1 (0.8) | 1 (0.8) | 0 (0.0) | 1 (0.8) |
| Blank | 1 (0.8) | 1 (0.8) | 1 (0.8) | 2 (1.7) | 1 (0.8) | 2 (1.7) | 1 (0.8) | 1 (0.8) | 3 (2.5) |
| Proportion of participants in health state 111111111 (555555555) | 30.0% (0.0%) | ||||||||
| 9 months | |||||||||
| 1 (no problems) | 83 (88.3) | 88 (93.6) | 84 (89.4) | 42 (44.7) | 86 (91.5) | 81 (86.2) | 66 (70.2) | 82 (87.2) | 83 (88.3) |
| 2 | 8 (8.5) | 4 (4.3) | 9 (9.6) | 25 (26.6) | 5 (5.3) | 10 (10.6) | 17 (18.1) | 7 (7.4) | 3 (3.2) |
| 3 | 0 (0.0) | 1 (1.1) | 0 (0.0) | 19 | 2 (2.1) | 0 (0.0) | 8 (8.5) | 2 (2.1) | 3 (3.2) |
| 4 | 1 (1.1) | 0 (0.0) | 0 (0.0) | 4 (4.3) | 0 (0.0) | 2 (2.1) | 1 (1.1) | 2 (2.1) | 2 (2.1) |
| 5 (very problematic) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 3 (3.2) | 0 (0.0) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 2 (2.1) |
| Blank | 1 (1.1) | 1 (1.1) | 1 (1.1) | 1 (1.1) | 1 (1.1) | 1 (1.1) | 1 (1.1) | 1 (1.1) | 1 (1.1) |
| Proportion of participants in health state 111111111 (555555555) | 29.8% (0.0%) | ||||||||
| 21 months | |||||||||
| 1 (no problems) | 48 (92.3) | 47 (90.4) | 47 (90.4) | 26 (50.0) | 32 (61.5) | 44 (84.6) | 41 (78.8) | 49 (94.2) | 46 (88.5) |
| 2 | 2 (3.8) | 3 (5.8) | 4 (7.7) | 18 (34.6) | 8 (15.4) | 6 (11.5) | 7 (13.5) | 2 (3.8) | 4 (7.7) |
| 3 | 1 (1.9) | 1 (1.9) | 0 | 4 (7.7) | 1 (1.9) | 2 (3.8) | 4 (7.7) | 1 (1.9) | 1 (1.9) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.9) |
| 5 (very problematic) | 1 (1.9) | 1 (1.9) | 1 (1.9) | 2 (3.8) | 2 (3.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Blank | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Proportion of participants in health state 111111111 (555555555) | 36.5% (0.0%) | ||||||||
Narrowband UVB compared with topical corticosteroid: cost–utility analysis for participants aged < 18 years (Table 27)
For those participants with complete cost and utility data, the unadjusted mean cost per participant in the NB-UVB-only treatment group (n = 28) was £818.47 (SD £91.20, 95% CI £787.16 to £849.81) compared with £597.51 (SD £49.31, 95% CI £579.43 to £615.60) for the TCS-only group (n = 31). The unadjusted mean incremental cost per participant was £171.50 (95% CI £137.35 to £205.65). The unadjusted incremental cost per QALY was £92,381.98. This figure is significantly higher than accepted threshold values and thus would not be considered cost-effective.
Combination treatment compared with topical corticosteroids: cost–utility analysis for participants aged < 18 years
For those participants with complete cost and utility data, the unadjusted mean cost per participant in the combination treatment group (n = 35) was £818.47 (SD £91.20, 95% CI £787.16 to £849.81) compared with £597.51 (SD £49.31, 95% CI £579.43 to £615.60) for the TCS-only group (n = 31). The unadjusted mean incremental cost per participant of £220.97 (95% CI £184.23 to £257.69). As mean cost was higher for the combination treatment group and QALYs less (albeit by a very small amount), it is possible to say that for this group of participants standard care (TCS only) dominates (i.e. it is both cheaper and more effective than combination treatment). However, one should note the small sample sizes.
Discussion
This chapter has presented the results for, to our knowledge, the first full economic evaluation of treatments for vitiligo, and uses standard care of TCS as the comparator. The additional cost of the combination treatment was not offset by NHS cost savings but did result in significant treatment success over the 9-month treatment period that could be gained if decision-makers were willing to pay more than the unadjusted incremental cost of £2328.56 (£1932.35 adjusted) per additional successful treatment (as defined in this study by ‘a lot less noticeable’ or ‘no longer noticeable’ on the VNS). NB-UVB alone was less costly than combination treatment but also less effective such that the incremental cost per successful treatment was higher than for combination treatment, suggesting that the NHS would get better value for money from combination treatment than light therapy alone.
This study also suggests that patients with vitiligo do not use NHS services for their condition very much. This may be because the range of treatments available is limited and the condition is often viewed by some as being cosmetic, making people with vitiligo feel that there is not much help available.
We undertook the cost-effectiveness analysis as the primary analysis because it enabled us to analyse all participants together, irrespective of age. We also had a prior belief that available generic utility instruments may not be able to fully capture the health-related quality-of-life aspects people living with vitiligo experience. This seems to have been borne out with a high ceiling effect on the EQ-5D-5L, where 55% of participants were in the health state 11111 (perfect health) at the beginning of the study. Although there was less of a ceiling effect on the CHU-9D, with 30% of participants in the best possible health state, in nearly one-third of children there was no capacity to measure any gain using these instruments. Those with less than perfect health reported problems in terms of anxiety/depression, pain and discomfort, and with usual activities. Undertaking the cost–utility analysis gave slightly contradictory results to the clinical and cost-effectiveness results, in that NB-UVB only appeared more cost-effective than combination treatment for those aged ≥ 11 years, whereas neither treatment arm appeared cost-effective for those aged < 18 years using the CHU-9D (although this probably reflects the small sample size for this age group). The cost–utility results are not that useful, and probably reflect a lot of uncertainty around the QALYs gained as the gain between groups was effectively very close to zero in both comparisons. Therefore, more weight should be attached to the clinical effectiveness results and further work to explore the measurement properties of the EQ-5D-5L and CHU-9D in this patient group is warranted, given the high ceiling effect observed in this study. A number of sensitivity analyses were undertaken and these suggest that perspective, cost of the NB-UVB light device and method of dealing with missing data do not change the conclusions reached. Repeating the analysis including only adherent participants does not change the overall conclusions either, although in this case the results do suggest that if it were possible to predict which individuals were likely to be adherent to treatment, the cost per treatment success for this group would be lower than the base case as they have a higher probability of success.
New treatments such as Janus kinase inhibitors are currently being developed as a novel treatment for vitiligo, especially for those with more extensive skin involvement. Although these treatments show some initial signs of promise, they are likely to be very costly when they become available within health-care systems. The relatively lower costs of the interventions assessed in this trial may therefore be advantageous when resources are limited, and the trial has yielded useful cost-effectiveness data that can be used for comparison with these novel treatments.
Conclusion
The combination treatment has a lower incremental cost per successful treatment than NB-UVB only but whether or not this is considered cost-effective will depend on the judgement of health-care decision-makers regarding how much they are willing to pay to achieve a successful treatment. The fact that vitiligo has few treatment options available, and the likely high cost of newer treatments being developed, may be important to consider in this regard.
Chapter 5 Process evaluation
Introduction
The clinical effectiveness (or otherwise) of home-based interventions for vitiligo is only one factor in determining whether or not such interventions will eventually be implemented across the NHS. This chapter considers the health economic impact of home-based provision; here we explore the process of intervention delivery and the experience of managing treatment at home. Understanding how treatments are experienced, and unpicking the opinions of those involved in providing them (in the trial and potentially in future clinical services), will help us to navigate and interpret the HI-Light Vitiligo trial’s clinical and economic findings. It will also help us to generate better informed recommendations for future clinical practice; recommendations that are supported by the subjective experience and preferences of those affected by vitiligo.
This focus has pertinence for home-based light treatment as neither dermatology nor primary care services currently routinely prescribe hand-held (home-based) NB-UVB for any dermatological condition. New services would need to be commissioned and designed. Should such provision be initiated it would necessarily involve the provision of relatively expensive equipment for long-term domestic use. Although TCSs are already being prescribed for vitiligo, potent TCSs are prescribed mainly in secondary care, whereas, in primary care, lower-potency TCSs tend to be used, and for shorter time periods. Clinicians and patients recognise that both NB-UVB and potent TCSs may have potentially harmful side effects. Moreover, the complexity of treatments delivered in combination, side-effect monitoring and routine dose adjustment might all influence how well home-based treatment is accepted and integrated in personal and domestic circumstances. How this complexity is managed in the HI-Light Vitiligo trial may also inform the nature and scope of future clinical supervision and the support that is made available for future home-based treatment.
At the outset, a programme theory (Box 1) was described that outlines how home-based treatment for vitiligo might work in ideal circumstances. Although informed by prior development work and a pilot trial,24 the programme theory, by its very nature, includes a number of speculative or idealistic assumptions. Home-based treatment for vitiligo is viable when clinicians and commissioners consider vitiligo to be a condition that warrants treatment and when patients decide that they wish to receive treatment for their vitiligo. Clinicians and commissioners need to understand their role in the pathway to making these treatments available to patients and patients need to be happy to receive this treatment (for both treatment options). It is important that patients should not be overburdened or confused by using both treatments concurrently and patients should be able to access support from the medical professionals as required. Previous research might suggest that achieving all these criteria can be challenging. 24
Aims and objectives
The aim of this process evaluation is to generate insight from a range of stakeholders that will support the interpretation of the HI-Light Vitiligo trial’s clinical and cost-effectiveness data, and inform the generation of recommendations for future clinical practice.
Specific objectives are to:
-
contextualise clinical and cost-effectiveness data with subjective reports of the experience of home-based therapy
-
consider whether or not stakeholders (patients, clinicians and commissioners) view home-based treatment for vitiligo to be acceptable and feasible
-
identify difficulties with the delivery and management of home-based treatment for vitiligo
-
consider implementation issues associated with the future delivery of home-based treatment for vitiligo.
The process evaluation will use qualitative and quantitative data to test the programme theory and inform recommendations for the future delivery of home-based treatment for vitiligo.
Methods
Study design
This is a mixed-methods process evaluation incorporating stakeholder interviews, interviews with NHS commissioners, an online survey of those who delivered home-based treatment in the trial and focus groups with those who delivered the trial.
Ethics approval was obtained for the process evaluation on 10 April 2017 (ethics reference 14/EM/1173, SA04) from the National Research Ethics Service (NRES) Committee East Midlands – Derby.
Participants
-
Trial participants were recruited from the main HI-Light Vitiligo trial.
Sampling was purposive, focused initially on age, treatment allocation, recruiting site and treatment success (based on the primary outcome). Other factors, such as treatment adherence, early stopping of treatment, ethnicity/skin type, gender, extent of vitiligo, number of patches being treated, and whether or not the participant experienced problems with treatment, guided later-stage recruitment of interviewees.
Participants were approached at the 9-month time point to minimise the impact that this had on treatment adherence.
-
Commissioners were identified via online directories of Clinical Commissioning Groups and via personal contact with members of the study team.
-
On completion of the trial, site investigators (principal investigators and research nurses) at all recruiting centres were invited to take part in an online survey and/or a focus group to review the delivery of NB-UVB.
-
To avoid having any impact on recruitment to the trial, all activities with recruiting site staff were conducted after recruitment had finished.
(This document describes the ideal situation for a person with vitiligo who is seeking treatment within the NHS.)
Initial consultation in primary careA patient visits their GP because they are concerned about pale patches on their skin. The GP correctly diagnoses vitiligo. The GP is aware that vitiligo is treatable and is knowledgeable about all possible management options and recognises the importance of early treatment. After a discussion about the physical and psychological impact of their vitiligo and the possible management options, the patient decides they wish to receive treatment for their vitiligo. The GP is supportive and offers to prescribe a potent topical corticosteroid and manage within primary care if appropriate, and/or offers referral to a dermatologist. The GP also refers the patient for other relevant services such as camouflage and psychological support as required and provides advice on sun protection.
Topical corticosteroidsThe GP or dermatologist prescribes TCS on an intermittent regimen to avoid side effects and the patient is happy to receive this treatment. A health-care professional fully educates the patient on the use of TCS (including information on frequency of application, amount to be used, and sites to avoid e.g. the genital area) and prescribes the TCS for as long as is required to achieve the desired outcome. The patient feels empowered to use the TCS, is aware that the treatments are slow acting but is prepared to stick to the recommended duration and frequency of application. The patient is willing and able to return for regular follow-up visits for monitoring of side effects and efficacy. The patient experiences no side effects from the TCS. If after 3 months of TCS there is no beneficial effect, the treatment is stopped. If there is a beneficial effect, the TCS is continued for up to 1 year, with regular follow-up. The TCS is stopped once the vitiligo is completely cleared.
Hand-held narrowband UVB light therapyThe dermatologist prescribes hand-held NB-UVB. The patient is happy to receive this treatment and is able to commit sufficient time to use the device. A phototherapy service is available and home phototherapy is supported. A medical professional with a full understanding of how to use hand-held NB-UVB for vitiligo fully educates the patient on the use of hand-held NB-UVB (including treatment regimen). The patient feels empowered to use the hand-held NB-UVB device. The patient is then given a hand-held NB-UVB device that has been checked for output and safety to take home. The patient is aware that the treatments are slow acting but is prepared to stick to the recommended duration and frequency of application despite it taking a significant amount of time each day and is able to treat all patches of vitiligo without experiencing any problems with the regimen or the device. The patient is willing and able to access support from the medical professionals as required. The patient is willing and able to return for regular follow-up visits for monitoring of side effects and efficacy. If after 3 months of NB-UVB there is no beneficial effect, the treatment will be stopped. If there is a beneficial effect, the NB-UVB is continued for up to 1 year, with regular follow up. The NB-UVB is stopped once the vitiligo is completely cleared.
Combination treatment of topical corticosteroids and hand-held narrowband (NB) UVB light therapyCombination treatment of TCS and hand-held NB-UVB is considered to be the most appropriate treatment option for this patient so a dermatologist prescribes these as above. The patient is not overburdened or confused by using both treatments concurrently.
Service provision/clinician perspectiveClinicians are able to diagnose vitiligo and recognise the importance of early treatment. Clinicians and commissioners consider vitiligo to be a condition that warrants treatment. They are willing to offer hand-held NB-UVB and/or TCS therapy for vitiligo to all suitable patients. Both clinicians and commissioners understand their role in the pathway to making these treatments available to patients. Clinicians have the knowledge, skills and resources required to prescribe these treatments, train patients in using them, and to ensure that the hand-held NB-UVB devices are correctly maintained. Support services are available including medical physics, phototherapy and medical photography.
GP, general practitioner.
Data collection
-
Semistructured interviews were carried out with those trial participants who consented to this element of the study.
Interviews lasted between 30 and 60 minutes; they were conducted by telephone or video call. All data were recorded using digital audio-recording equipment and interviews were transcribed in full by a professional transcription service.
-
Interviews focused on experiences of participating in the trial, perceptions of treatment and views on whether or not treatments should be available to more people with vitiligo. Topic guides were used to structure the interviews and participants were encouraged to focus on (or introduce) any topic that they felt was important.
Participants were offered a £20 gift voucher to compensate for their time if they participated in an interview.
Participants were asked to sign an online consent form before the interview; however, if it was not possible to obtain consent in this way, verbal consent was obtained prior to the interview commencing (in line with the ethics approval).
Interviews with commissioners were similarly guided by a semistructured topic guide. Interviews with commissioners were shorter in duration, approximately 20–30 minutes. They were also delivered via telephone or video call and recorded using digital recording equipment. Data were transcribed in full.
-
An online survey (see Appendix 8) was delivered to all recruiting centre staff via the SurveyMonkey® (Palo Alto, CA, USA) online survey software. Questions considered the challenge of delivering NB-UVB and sought insight and recommendations about the nature and form of any future implementation of NB-UVB in the treatment of vitiligo. The survey was live between 25 March and 24 April 2019.
Following the online survey, site investigators were invited to a 1-day ‘results’ meeting where progress in the HI-Light Vitiligo trial was presented (29 May 2019). At this meeting, participants took part in short focus group discussions (60 minutes) as well as whole group discussion of the trial findings. Discussions were guided by a semistructured topic guide (see www.journalslibrary.nihr.ac.uk/programmes/hta/122402#/; accessed November 2020) with each focus group facilitated by an experienced facilitator. All discussion at this meeting was audio recorded and transcribed in full.
Data analysis
-
Data were anonymised and handled using the NVivo software package (QSR International, Warrington, UK) for qualitative data analysis (version 12). Transcripts were coded following the conventions of framework analysis72,73 using a framework initially derived from the underpinning programme theory and set out in three broad matrices: experience of treatment, need for treatment and future implementation. The coding framework was developed and amended as the data suggested new insight and topics. Coding and thematic development were checked by multiple members of the team to ensure valid and relevant interpretation.
-
Data were anonymised and handled using the NVivo software package for qualitative data analysis. Data were charted to the analytic framework described above, although into separate matrices so as to distinguish commissioner data. Coding and thematic development were checked by multiple members of the team to ensure valid and relevant interpretation.
-
Descriptive statistics were generated for the online survey responses.
Free-text responses (in the online survey) and focus group discussions were anonymised and handled using the NVivo software package for qualitative data analysis.
Again, data were charted to matrices as described above; site investigator data were charted to dedicated matrices to ensure that these data could be considered independently. Coding and thematic development were checked by multiple members of the team to ensure valid and relevant interpretation.
Themes across matrices for (1), (2) and (3) were compared, contrasted and synthesised in order to address study objectives.
Results
Data overview
-
In total, 25 interviews with trial participants (or parents) were conducted between 13 July 2017 and 20 July 2018 (Table 29).
Twelve out of the 16 recruiting sites were represented in the sample.
-
Nine commissioner interviews, involving 10 individuals, were conducted between 5 June 2017 and 10 October 2017.
Participants included strategic and operational roles in the commissioning process, and represented a geographic spread across England. Most participants were medically trained, and included general practitioners (GPs) with a special interest in dermatology.
-
Twenty-four recruiting site staff completed the online survey: seven doctors, 16 nurses and one other.
Ten of these had prior experience of phototherapy services, for others the HI-Light Vitiligo trial had been their introduction to this treatment. To support anonymity we did not collect data about which site they represented.
Thirteen site staff participated in the focus groups representing 10 recruiting sites. Eleven nurses were split into two groups; two doctors formed the final focus group.
Thematic analysis
Thematic analysis is organised around three questions:
-
Can home-based treatment for vitiligo be adequately managed by HI-Light Vitiligo trial participants, and supported by those clinical teams involved in the trial?
-
Do stakeholders feel that home-based treatment should be made available as part of routine NHS provision?
-
Do stakeholders feel that home-based treatment for vitiligo could be integrated within the current NHS organisation and pathways?
Is home-based narrowband UVB treatment and topical corticosteroid for vitiligo manageable for participants?
All bar two of the (health-care professional) survey respondents agreed that home-based treatments are ‘easy’ for participants; discussion group data (with site investigators) reinforced this, with health-care professionals reporting that the phototherapy device is (superficially) simple to operate and most trial participants seemed to understand the instructions offered about light therapy and TCS ointment. Trial participants offered a similar assessment indicating that they generally understood how to use the individual treatments; training and support offered by the research nurses and demonstration video had helped in this.
Some practical difficulties with individual treatments were described (by both site investigators and trial participants) but nothing of a magnitude to prevent participants effectively managing their treatments. Practical difficulties for light therapy were timers that failed, guard teeth that broke off, difficulties reaching parts of the body and difficulties using (a flat device) on curved parts of the body. Practical difficulties for the use of TCS ointment were an unpleasant smell, a greasy feeling and poor absorption. The time commitment required for light therapy was a common cause for comment:
It felt like an awful amount of time, I am pretty busy and to eventually be spending in excess of three-quarters of an hour per 2 days just felt like an inordinate amount of time.
Adult participant 3
Treating multiple patches could leave participants feeling overburdened:
I started with more than that because I was quite positive, I was doing different parts of my body like six or seven or something . . . Then I just did three, the three patches they were interested in so I was just treating them, no more.
Adult participant 4
Time seemed to be more of an issue for parents treating a young child (i.e. ‘keeping them still!’), or if there were other children in the household that required attention. Participants described linking treatment to ‘treat time’ for children (e.g. watching television); others described building routines that facilitated their time commitment:
Yes it was always around 6 o’clock after my tea and after I’d washed up and whatever you know, then I didn’t have time to just sit and do it then.
Adult participant 12
Yeah, so I used to sort of do each week in advance so that I knew what I’d got to do the next week and how long each treatment was going to be, and I’d put my notes next to it so I knew I’d done it, so no, I was quite comfortable with that.
Parent of child participant 3
Although time-consuming, home-based phototherapy was considered less disruptive than regular hospital visits for phototherapy, and the potential for treatments at home was important to the majority of trial participants.
Despite a generally positive assessment of each treatment, health-care professionals and some participants flagged the complexity of treatments in combination. Site investigators reported that trial participants who they thought had a good understanding of treatments made errors with TCS and phototherapy dose, and suggested that some individuals had disregarded instructions and used the ointment/device excessively on multiple body sites. One trial participant admitted as much:
I just ramped it up pretty much straight away back to what it was, but again no redness whatsoever which only really served to confirm it’s a dummy.
Adult participant 11
Some trial participants said that they found the combination treatment protocol complicated, particularly early on, and expressed caution when initially using the treatments:
Yeah I found it confusing for the first few weeks, it was like 1 week on 1 week off [for TCS], and every other day for the light and stuff.
Parent of child participant 5
Some trial participants acknowledged that they had made mistakes with treatment:
I was completely knackered and was [. . .] at the end of the day, had done the light treatment. So, I sat and did my chest which was one the areas being treated and part of, one of my, part of my left hand which is the other bit of the treatment and then started to do the second bit on the left hand and fell asleep so I ended up burning myself.
Adult participant 3
Stepping-up or down NB-UVB dose (as part of the treatment protocol or in response to erythema) was recognised to confuse and cause difficulties, with site investigators concerned that some participants never appeared to fully understand the process of incremental dose change. Trial participants indicated that their treatment diary was essential in guiding them:
Yes, without that it would be nowhere, without the form that you fill in with boxes I mean and writing down the time you would be absolutely nowhere, there’s no chance in a million that you would actually keep to anything like the protocol.
Adult participant 3
A further area of complexity identified by trial participants was in assessing whether or not treatment was making any difference and the importance of the photographs in determining any change:
Well I thought [things had improved] but when we got the photographs, you know we got the photographs on the computer; it didn’t seem to be any different to be honest.
Adult participant 12
Only when I went back and saw the difference in the photographs, so as I was treating it I couldn’t really see any difference, when you saw the photographs versus my face in the mirror actually, there was a difference.
Adult participant 5
Seasonal variation in skin tone might add to this complexity:
. . . because usually I do find I do get quite tanned and therefore, between the summer and winter there is a contrast, so without looking at the photographs I couldn’t tell whether actually it was making any difference or not.
Adult participant 5
Trial participants described nurses as having an important role in supporting them in assessing whether or not treatment was leading to improvement; nurses were also considered important in supporting the management of erythema (especially when it occurred for the first time). Overall, participants viewed nurse support positively; they responded quickly, were helpful and friendly, they offered reassurance, and generally ensured that participants were doing the right thing.
In contrast, participants were sceptical of the potential for GP-led support:
In all honesty if I rang my GP up and I had an issue, they’re useless anyway, you have to wait God knows how long to get an appointment and whatever else.
Parent of child participant 6
Despite the acknowledged complexity of the combination treatment, trial participants reported that they felt able to adhere to treatment regimen without any fundamental difficulty. Where they had not adhered to protocol this would more often be with light therapy and participants would point to legitimate (practical) reasons for this, such as other health conditions or holidays:
. . . like I say it was literally just when we went away on holiday, I just, I probably wouldn’t have done it if I did take it to be honest when I’m on holiday.
Adult participant 9
Others had not adhered to the light treatment protocol when they recognised no benefit from treatment, many associated this with receiving a dummy device:
I think I only really found it onerous because I was just convinced it was a dummy, and I just felt as if I was, about 20 minutes I was really just wasting basically because I thought this was not going to be any good at all.
Adult participant 11
As soon as I realised that it wasn’t even tanning my skin I just, it was really hard to continue because it was really time-consuming.
Adolescent participant 2
These comments suggest the importance of expectations in shaping how treatments are managed; some individuals ceased treatment because expected improvement had not occurred. That noted, it should be made explicit that most trial participants demonstrated generally realistic expectations, often borne out of previous treatment experience:
I mean it’s not like sunburn or a suntan is it? Where the skin sort of changes overnight practically, if you’ve got something happening in the cell structure maybe that takes a much longer time because you’re waiting for the cells to regenerate.
Adult participant 10
. . . 6 months I would have expected to have seen something.
Adult participant 2
So, it’s just I didn’t have great expectations, possibly because of previous treatments and stuff.
Adult participant 13
Expectations were, however, often tempered by an intuitive, emotional response to the offer of a new treatment:
I was hoping for them to shrink or bring some of the pigmentation, like away, get her back to her normal colour.
Parent of child participant 5
Actually, I was very pessimistic about the whole thing but I was, I didn’t really think that I was going to get any benefit.
Adult participant 13
Should home-based treatment be made more widely available?
Although potentially complex (and confusing for some), there was a general sense that this type of combination treatment should be made available to more vitiligo patients; 18 out of 24 health-care professional survey respondents agreed or strongly agreed with this. Focus groups with site investigators supported this by highlighting a clinical population that have few treatment options, and for whom the impact of vitiligo can be very distressing:
We have always said that it is the best of a bad bunch of treatments, and it probably still is. There is no fantastic treatment out there for vitiligo, there doesn’t seem to be, and the trial doesn’t show that it’s fantastic. It’s shown that for patients it’s worthwhile doing because the quality of life is impaired for a lot of patients. They are pinning hopes on it.
Site investigator 9 – research nurse
This is reflected in those reasons offered by trial participants for taking part in the HI-Light Vitiligo trial. Some hoped that participation would bring them access to new treatments for themselves or their children, some subsequently hoped for complete remission, whereas others hoped that their disease would stop spreading. For a minority of participants there was a sense of having ‘nothing to lose’:
. . . had hoped it would totally recover the 9 months or earlier you know, the sort of blemishes would disappear.
Adult participant 5
I decided to take part because why not, it would be working on my skin or not but I just decided to take part to see what happened.
Adult participant 4
I don’t know, probably half and half of me was hoping that yes, something would work and it would help her, but if it didn’t then we wasn’t really going to lose anything.
Parent of child participant 7
Others hoped that their involvement in the HI-Light Vitiligo trial would benefit the broader vitiligo population by contributing to the development of a new treatment pathway. Given these assessments, it is understandable that site investigators recognised the importance of new treatments for their patient population, even suggesting that (irrespective of the clinical impact) new treatments offer vitiligo patients hope and the potential to engage with their condition.
As this desire for new treatments might suggest, commissioners confirmed that treatment pathways for vitiligo are often lacking, suggesting that dermatology, let alone vitiligo, is unlikely to be a priority in commissioning discussions. They also indicated that some commissioners perceive vitiligo to be a ‘cosmetic’ condition, which adds a further barrier to it being considered a priority area. Variation in cosmetic impact (and associated concerns) was also manifest in comments made by trial participants, which suggests that willingness to pursue (complex) treatment might vary according to site or visibility of vitiligo:
. . . if I had, say if it was like more in a cosmetic important place I’m pretty sure I would be prepared to have a go at it long term, when saying long term, I mean over a period of years or whatever is required.
Adult participant 3
I never felt that for a chap of my age, I mean I’m 74, it’s a bit irrelevant because they’re worse when you’ve got a bit of a sun tan obviously, but it’s not like being perhaps a lady who cosmetically can look a bit odd.
Adult participant 7
These comments illustrate and support the assessment of site investigators that home-based therapy might not be appropriate for all vitiligo patients. In the online survey, 12 site investigators indicated that home-based therapy would be appropriate for most people, 11 indicated that it would be appropriate for some, but none indicated that it would be appropriate for all people with vitiligo. Considerations for providing home-based treatment might be (1) clinical, (2) practical, or related to (3) personal circumstances:
-
Comments from consultant dermatologists (as part of the survey and site investigator discussions) speculated that combination treatment might be particularly beneficial for new patients as an early intervention.
Trial findings point to variation in outcome according to body site of vitiligo patch (see Chapter 3).
Target patches were chosen by trial participants, and had to have been active in the preceding 12 months, as reported by participants. Discussions with site investigators suggested that some participants may not have been able to judge this very well, with some investigators feeling that potential participants may have exaggerated the activity of their vitiligo to obtain access to the treatments offered in the trial.
-
The practical challenge of managing combination treatment (long-term use, dose fluctuation, potential side effects, etc.) was recognised by both participants and site investigators. Participants suggested that not all people are sufficiently organised for these treatments; they thought that individuals need to plan ahead, be committed to the treatments and to be willing to incorporate them into their routine:Adult participant 1
If you’re an organised type of person it becomes part of second nature after a while.
In some cases, hospital-based light therapy was considered more appropriate:Adult participant 4
Yes, to go to the cabinet and spend 5, 10 minutes and that is all because you will have treated all your body.
The duration of home-based treatment was also a factor to be considered when considering individuals:Parent of child participant 2Adult participant 3
I think doing it for any longer [than 9 months] probably would have been a bit challenging probably, because it became, it did start to become a bit more of a hassle to do it so regularly.
Towards the end, I mean I really did find, it just felt like I was spending a lot of time.
Health-care professionals felt that mental health issues, other health complaints, or significant caring responsibilities (e.g. multiple children) might all challenge an individual’s ability to maintain a complex treatment regimen over a long period of time.
-
Beyond practical challenges, there was concern from health-care professionals that an individual’s level of understanding about vitiligo and their expectations of treatment might also be important
-
Those with potential difficulties adhering to a long-term treatment programme may be unlikely to benefit from home-based treatment and those with unrealistic expectations might find it difficult to adhere. Trial participants recognised that some individuals might exaggerate, or provide inaccurate information about patches, to gain access to a new treatment.
Establishing which individuals might benefit from home-based treatment was considered difficult by health-care providers; fully sharing information about effectiveness, treatment burden and treatment duration may help support shared decision-making. Some site investigators even suggested that some kind of test, formal or informal, of whether or not a patient understands the treatment regimen might also be appropriate.
Could home-based treatment be made more widely available outside the trial?
A small number of site investigators indicated that they were already reusing devices from the trial and incorporating them into clinical practice for vitiligo. Indeed, some sites in the UK (e.g. Ninewells Hospital, Dundee) are already offering such devices for use at home, often to treat other skin conditions such as scalp psoriasis.
Other site investigators pointed to the importance of situating new pathways within existing phototherapy provision for other skin conditions, with appropriate support from medical physics to monitor device output. However, they recognised that this would not be possible in all locations and some suggested that home-based treatment should be managed regionally by specialist centres.
These perspectives mirror commissioners’ concerns that any new treatment would need to sit within (or at least not disrupt) existing service pathways. In contrast to site investigators, commissioners were, however, less aware of a need for new treatment pathways and perceived no explicit demand from patients, clinicians or health-care providers:
I’m not getting any complaints for example about the services that we provide. Like GPs aren’t coming to me saying, we’re not happy with this. As far as our GPs are concerned, they’re getting a good service because their patients aren’t complaining to them. It’s not coming up on our monitoring in terms of performance.
MC: commissioner
Should a new treatment pathway be considered, commissioners stressed that clinical and cost-effectiveness would be key to any decision, they warned that significant changes to services would need to be supported by considerable evidence of clinical or cost improvement:
I think the only issue would be time . . . a change in service is something that can be time-consuming. So the benefit has to be significant. So we’re making an assessment how significant the change is.
JB: commissioner
Investigators recognised that commissioners might be reluctant to commission new services and speculated on different mechanisms for the provision of phototherapy devices.
All investigators recognised that these devices can be easily purchased online and some reported that participants had indicated that they might buy one independently of NHS support. Similarly, several participants described considering purchasing a device, although for some the thought of ‘going it alone’ deterred them:
I think they’re about £100 aren’t they? They’re not fantastically expensive but I didn’t then think I might go and buy one of those, largely because I wasn’t sure how I would use it you know, it’s very secure and comforting isn’t it to have that kind of regime and do this, that and the other every day, and then you think ‘right OK so I know where I’m up to’ and so on, so to suddenly be cut loose from that would be a little bit more you know, anxiety provoking, when you know that it’s potentially dangerous.
Adult participant 6
Investigators recognised that the publication of (positive) trial findings might accelerate this type of independent use among a patient population desperate for treatment options. Most investigators were apprehensive about this, in the online survey only 2 out of 24 felt that NHS involvement was not important and 13 felt that this was essential or very important. Concerns for safety led some investigators to suggest that devices should be automatic (i.e. patients cannot adjust) or managed by a dermatology nurse. Some patients also suggested that devices could have safety features, such as automatic cut-outs, to prevent overuse. Investigators also suggested that patient monitoring should be frequent and sooner than 3 months (as in the trial). Some participants recognised the value of an earlier monitoring visit, whereas others felt that this was not necessary:
. . . personally think it needs an interim visit, if only to compare the photograph, because I do think that you forget what it was like and you do think ‘oh it’s not making any difference’, but then when you see the photograph and you see the shape changing.
Parent of child participant 3
The potential for some form of ‘mixed economy’, where patients lease or purchase a phototherapy device within an NHS service, was considered by site investigators to be the most likely way that effective provision could be offered. However, this is not without its difficulties. Both trial participants and site investigators were concerned about unequal access for those that cannot afford to purchase or lease a device. Some health-care professionals suggested that ‘purchasing health care’ may lead to unreasonable expectations and/or incorrect use (‘if I’m paying for it will work!’). In addition, the failure to return leased devices might make a service economically not viable (127 light therapy devices were not returned during this trial).
Discussion
It is perhaps unsurprising that data generated from multiple sources (with contrasting clinical and patient perspectives) produces a complex, and at times contradictory, set of insights:
-
treatments that are ‘easy’, but are complex in combination
-
treatments that should be made available to more vitiligo patients, but where selection of patients might be essential
-
treatment that may be purchased independently by patients, but that would need significant monitoring and support from the NHS.
Through much of the process evaluation data (especially in participant and site investigator insights), there is a marked divergence between the recognised potential for home-based treatment and concerns for harm associated with its inappropriate or unsupervised use.
Trial fidelity
The process evaluation offers some insight to support the interpretation of the clinical effectiveness and cost-effectiveness data.
Participant interviews demonstrated that adherence to the treatments was not hampered by a lack of knowledge about how to use the treatments, nor by a lack of support when using them. Intermittent non-adherence to treatment was acknowledged (and is perhaps unsurprising given the treatment burden associated with light therapy) but this was most often a pragmatic response to life circumstances and events. Trial adherence data (see Table 7) show that around two-thirds of participants used the treatments as specified in the treatment protocol while they were still using them.
All of this suggests that trial procedures were adequate in supporting normal practice, and that clinical findings were neither inflated nor diminished by unrealistic or disastrous levels of treatment adherence.
Where trial procedures were, however, less successful was in blinding participants to treatment allocation. The trial data demonstrates that a relatively high percentage of participants stopped treatment early, with only around half using treatments for at least three-quarters of the expected duration (see Table 7). Although some participants stopped treatment because they had achieved complete remission, more commonly participants indicated that a lack of effect led them to cease treatment with some suggesting that they knew that their light device was a dummy. A lack of any redness in the skin or other indications such as warmth meant that around half correctly guessed that they had the dummy device (89% of those using active light guessed correctly).
With regard to trial outcomes, participants were largely able to judge the primary outcome (VNS). The use of photographs was crucial in this, owing to the duration between visits, and/or because of difficulties in establishing whether or not minor changes had taken place. It is perhaps worth noting that participants indicated that seasonal variation in the noticeability of their vitiligo (i.e. it was more noticeable in summer) may have had an impact on their assessments. The consistency of other trial outcomes, however, suggests that this was not a major issue.
Population in need
Site investigators recognised a clinical population with few treatment options, commissioners acknowledged that vitiligo is not a priority area, and trial participants expressed a desire to try new treatments that might work where normal clinical practice had failed. Health economic assessment suggests that many individuals with vitiligo manage with little or no input from the NHS. The culmination of these insights demonstrate that there is an unmet need for effective treatments for vitiligo.
Participants were willing to go to great lengths to accommodate the time-consuming and complex treatment regimen; many were willing to continue treatment in the absence of any effect. Expectations were realistic, participants hoped for partial improvement or halting the spread of vitiligo (rather than complete repigmentation) and few expected immediate results. These characteristics may suggest that the levels of treatment success observed in the trial, and ability of the interventions to stop the spread of vitiligo, offer sufficient potential for individuals with vitiligo to be willing to try (and persist with) home-based treatment in the future.
Assuming that vitiligo does, however, have at least some degree of psychological impact in many people with the condition, it seems important that future recipients of vitiligo treatments should be fully informed about the likely success rate of home-based treatment, to ensure realistic expectations of treatment.
Easy to do but complex to use
Site investigators and trial participants recognised that treatments were relatively straightforward to use, with appropriate instruction and support.
However, economic assessment points to the cost-effectiveness of combination treatment (more so than treatments in isolation); a complex treatment protocol coupled with a considerable time burden (both each day and over a period of months) creates potential for incorrect use that can result in either increased side effects or reduced effectiveness. Site investigators were particularly concerned about the potential for participants to harm themselves. Treatment diary planners and site staff were considered essential by trial participants in helping them to navigate this complexity. It is notable that some participants had considered purchasing a light therapy device, but had decided against this because of a lack of ongoing NHS support. Site investigators stressed the importance of ongoing support and monitoring of patients in any future clinical service.
The clinical data indicate a need for some form of intermittent maintenance therapy, as effectiveness diminished once treatments had been stopped. Many trial participants were relieved to stop light therapy after 9 months, suggesting that maintenance therapy with TCS is more likely to be a preferred approach over maintenance light therapy.
Treatments may not be suitable for all
Treatment burden means that home-based therapy is less appropriate for those wishing to treat a high number of patches. Lifestyle and personal circumstances may make adhering to a complex treatment regimen over an extended period of time difficult for some. It is also pertinent to remember that not everyone with vitiligo wants or is seeking treatment.
Site investigators stressed the difficulty of predicting which participants were most likely to benefit from home-based treatment. Fully discussing the advantages and disadvantages, treatment burden and time scale of home-based treatments are essential in helping people with vitiligo reach informed decisions about treatment.
Integrating within the NHS
Site investigators were positive about the trial results (‘any improvement is worthwhile’ was a common sentiment) and participants were keen to see effective treatments for vitiligo become available. However, it is unclear whether or not sufficient improvement is manifest here to convince commissioners of the value of home-based phototherapy for the management of vitiligo. Home phototherapy services that support treatment of a broad range of skin conditions, as seen in existing specialist phototherapy units offering home phototherapy (e.g. Ninewells Hospital in Dundee), are likely to be more attractive.
Both site investigators and trial participants recognised that light therapy devices might be privately purchased. Some site investigators were concerned about this, and some trial participants indicated interest in privately purchasing, but at the same time being concerned about a lack of clinical support if they did so. It is pertinent to stress that training, treatment diaries, technical support and staff support were all considered essential by site investigators (and many trial participants) to the success and safety of home-based treatment. Medical physics is required to ensure that devices are appropriately calibrated and to ensure that bulb output is consistent; phototherapy services are required to support a complex treatment regimen, monitor effects and support/temper patient expectations.
The culmination of these two strands points to the potential for some form of mixed economy provision where light therapy devices are leased or privately purchased (independently or via the NHS) with treatment defined, supported and monitored by NHS services. The number of devices (127) not returned after the end of the treatment period in this trial might make NHS leasing of devices a less appealing prospect to commissioners.
Site investigators recognised that not all settings are well placed to provide medical physics support and commissioners suggested that new pathways are more attractive if they sit within existing provision (rather than requiring infrastructure development). This might point to a hub and spoke model of regional delivery whereby specialist sites with existing medical physics expertise could provide access to home phototherapy devices across a number of NHS trusts, but clinical provision including training and monitoring of side effects could be delivered locally.
It is perhaps appropriate to conclude by recognising that the challenge of any future home-based treatment for vitiligo will be navigating the needs of patients and their enthusiasm for new treatment alongside the concerns of health-care professionals about the potential side effects associated with light therapy and long-term TCS use.
Study strengths and limitations
This process evaluation synthesises data from a range of relevant stakeholders to provide insight into the delivery and experience of home-based treatment for vitiligo. It complements the clinical and health economic data summarised elsewhere in this report. The subjective experiences that are reported here provide an important context to support interpretation of the clinical findings and provide situated detail to inform future service development and delivery.
The evaluation is comprehensive in its coverage, in that those exposed to home-based treatment, those that delivered it, and those that might commission it in the future were all consulted. Insight may have been enhanced further with the inclusion of more teenagers in the process evaluation, but other than this it is positive that the views of adults, young people and parents of children with vitiligo were all incorporated into the data collection. It is also positive that among those trial participants who engaged with the evaluation some had positive a experience of treatment, others less so.
As with all research of this kind we acknowledge that participants were to some extent self-selecting, and it may be that those with particularly positive or strong views about home-based treatment were more likely to consent to involvement in the process evaluation. Site investigators could potentially have a vested interest in the future commissioning of home-based treatment for vitiligo and so may be inclined to give more positive views.
As with all qualitative data, there is some degree of interpretation in our analysis of the interview and discussion group data. Although we have tried to ensure some rigour in this process (multiple coders/group discussion about interpretations) there is always potential for us to misunderstand or misinterpret what we were told.
Chapter 6 Patient and public involvement
Background
The involvement of key stakeholders, such as patients and their carers, representatives from patient support groups, and health-care professionals, is important when identifying clinical research priorities and when developing and designing clinical trials. This helps to ensure that the resulting research evidence is useful and relevant to clinical care, is delivered efficiently and that recruitment targets are achieved with minimum unwarranted burden on participants.
Many of the current treatments for vitiligo have been assessed through clinical trials, but variation in the design of these studies and a lack of standardised outcome measures makes it difficult to compare the effectiveness of these treatments. 74 Systematic reviews have also shown that there is wide variation in the choice of outcome measures used in vitiligo trials. 33
In addition to the lack of standardised outcome measures and limited use of PROMs in vitiligo trials, until now there has been very limited stakeholder involvement in identifying the most important areas for future vitiligo research, or in designing new vitiligo trials.
We sought to address these issues when we started to develop the HI-Light Vitiligo trial, and here we report how we did so.
Aims
To evaluate the impact that stakeholder involvement had in the design, delivery and dissemination of the HI-Light Vitiligo trial.
Methods
This work is reported using the Guidance for Reporting Involvement of Patients and the Public (GRIPP2) guidelines. 75 It outlines the breadth of stakeholder activities that have contributed to the delivery of a multicentre RCT from 2009 to present, and the impact that this involvement has on the design, delivery and dissemination of the HI-Light Vitiligo trial. For the purpose of this report, we use the term ‘stakeholder’ to include people with vitiligo and their carers, representatives from organisations representing people with vitiligo (e.g. patient support groups), health-care professionals who treat people with vitiligo and health-care commissioners.
Data were collected and logged throughout the trial using the eight core principles of the Public Involvement Impact Assessment Framework (PiAFF) identified by Telford et al. ,76 as outlined in Table 30.
| Core principles | Inclusion in the HI-Light Vitiligo trial |
|---|---|
| Principle 1: the roles of the stakeholder are agreed between the researchers and the stakeholders involved in the research | The role of the stakeholder representatives was documented in the funding application, protocol and final report |
| Principle 2: researchers budget appropriately for the costs of the stakeholder involvement in research | Stakeholder costs were included in the trial budget. Costs associated with stakeholder work throughout the trial (e.g. travel expenses, time commitments) were reimbursed |
| Principle 3: researchers respect the differing skills, knowledge and experience of stakeholders | Different stakeholders (people with vitiligo and their carers, representatives from organisations representing people with vitiligo, e.g. patient support groups and health-care professionals) were involved in various aspects of the trial. Requests for involvement were tailored to each individual stakeholder group’s skills, knowledge, experience and stage of the trial |
| Principle 4: stakeholders are offered training and personal support to enable them to be involved in research | Patient partners invited to join the Centre of Evidence Based Dermatology’s Patient Panel, which provides regular sharing of information, an annual face-to-face training day and opportunities to attend relevant national training events and conferences |
| Principle 5: researchers ensure that they have the necessary skills to involve stakeholders in the research process | Researchers involved in the HI-Light Vitiligo trial are experienced in the involvement of stakeholders in research and embedded within institutions that value the central role of patients and the public as partners in research |
| Principle 6: stakeholders are involved in decisions about how participants are both recruited and kept informed about the progress of the research | Stakeholders were involved in the design and development of trial recruitment procedures and documentation. Through The Vitiligo Society in particular, stakeholders were pivotal in the communication of trial developments to both participants and the wider vitiligo community and in giving advice about recruitment |
| Principle 7: stakeholder involvement is described in research reports | Stakeholder contribution and analysis of its impact included in the final report and written up as a separate paper |
| Principle 8: research findings are available to stakeholders, in formats and in language that they can easily understand | Stakeholders were invited to the HI-Light Vitiligo trial results meeting to discuss the findings of the research from a stakeholder perspective. Lay summaries of trial findings were developed with input from stakeholders |
Details of stakeholder involvement and evidence of impact were collected on dedicated logs throughout the development and duration of the trial.
Results
Contextual factors relating to stakeholder involvement
Funding body
The HI-Light Vitiligo trial was funded by the NIHR HTA programme. This funding body is dedicated to the involvement of the public in the delivery of research, rather than through participation in clinical trials alone. The NIHR defines public involvement in research as ‘research being carried out “with” or “by” members of the public rather than “to”, “about” or “for” them’ (INVOLVE copyright © all rights reserved 2020; reproduced with permission). 77
Working in collaboration with NIHR ensured committed funds for the involvement of the public throughout the delivery of the HI-Light Vitiligo trial, from the identification of the research question to the dissemination of trial results.
Research group
Both the Centre for Evidence Based Dermatology (CEBD) and NCTU have extensive experience in the involvement of the public in the delivery of clinical trials. The CEBD were able to utilise existing networks, including a well-established patient panel whose members receive training and support through face-to-face workshops, newsletters and attendance at relevant training courses/conferences.
Sponsor organisation
The University of Nottingham places strategic importance on the involvement of patients and the public in both teaching and research activities. A public engagement lead is employed to support each faculty of the university, including the Faculty of Medicine and Health Sciences, to support researchers in developing patient and public involvement (PPI) initiatives to engage effectively with PPI partners throughout project delivery.
Patient support groups
The research team had strong pre-existing links with UK-based charity The Vitiligo Society.
Stakeholders involved in the HI-Light Vitiligo trial
Stakeholders (including people with vitiligo and health-care professionals) were involved across all areas of trial design and delivery. They were involved in prioritising the initial research question; completing surveys to inform trial design; developing and testing the primary outcome; assisting in trial conduct, recruitment and oversight; and contributed to the analysis and interpretation of the trial results. The number and types of stakeholder involved at each stage in the trial life cycle are shown in Figure 10.
FIGURE 10.
Stakeholder contribution to the design, development and execution of the HI-Light Vitiligo trial.
Stages of research and opportunities for stakeholder impact
The impact that stakeholder contribution had in the development and delivery of the HI-Light Vitiligo trial is summarised in Table 31.
| Stage of the research | Methods used in the HI-Light Vitiligo trial | Measures of impact |
|---|---|---|
| Establishing the research question | ||
| In 2009, members of the HI-Light Vitiligo trial team led the Vitiligo PSP in collaboration with the James Lind Alliance and The Vitiligo Society21 | The PSP identified the top priorities for future research as defined by people with vitiligo and health-care professionals, while also highlighting the importance of assessing the suitability of vitiligo treatments for children. The NIHR prioritised a commissioned research call to address two of the top 10 research priority topics:
|
|
| In total, 302 people with vitiligo, 142 health-care professionals and 17 people from other sources contributed to the survey | ||
| Development of primary outcome measure | A systematic review was conducted to assess which outcomes are measured most frequently in vitiligo trials.33 In parallel, a survey conducted between January and March 2009 asked people with vitiligo or their carers (n = 165) to suggest which outcomes should be used in future clinical trials. These two processes laid the foundation for work to identify a core set of outcomes measures that should be captured in future vitiligo trials | Emphasised the inconsistencies across the reporting of outcome measures in vitiligo trials and the need for standardised outcome measures |
| Helped to establish the most important outcome measures in vitiligo research, including cosmetic acceptability of treatment response | ||
| Survey work among people with vitiligo showed that the outcome domains that are important to people with vitiligo included ‘cosmetically acceptable repigmentation’ and ‘normal looking skin’33 | ||
| Three online discussion groups involving people with vitiligo (n = 12) were held with an overall aim to further narrow down (1) the most important concepts when measuring treatment success and (2) potential wording for questions to assess treatment success. As with the earlier PSP, participants for the focus groups were identified via the CEBD mailing list and through The Vitiligo Society | Further work determined the most appropriate way of assessing cosmetic acceptability of treatment response contributed to the development of the VNS42 | |
| Validation of primary outcome measure | Work was carried out to validate the primary outcome measure (VNS) through the scoring of baseline and after treatment images by health-care professionals (n = 33) and people with vitiligo (n = 101) | This work showed that (1) the VNS has good construct validity, acceptability and interpretability, supporting its inclusion as a patient-reported measure of the cosmetic acceptability of treatment response in vitiligo trials, (2) the VNS is a better and more consistent indicator of global treatment success than percentage repigmentation, (3) VNS scores of 4 or 5 can be interpreted as representing treatment success and (4) further validation of the VNS is required42 |
| Research design and delivery | ||
| Trial oversight | An experienced patient researcher (MW) was a co-applicant on the grant application for the HI-Light Vitiligo trial, acting also as a representative of The Vitiligo Society. Another patient representative of The Vitiligo Society (MS) acted as a lay member of the TSC | The presence of patient representatives on trial oversight committees was invaluable throughout the design and delivery of the trial, helping to ensure that patients remained at the forefront of trial objectives |
| A patient researcher (MW) joined the trial team as a co-applicant and was a regular participant in Trial Development Group meetings during the funding application process | ||
| Trial documentation | Five patients with experience of vitiligo advised on the content and ease of use of patient information sheets and treatment diaries | Feedback was incorporated into all aspects of patient-facing trial documentation to ensure that it was both meaningful and informative to vitiligo patients |
| Data collection | The patient researcher (MW) advised and commented on the development of the CRF, suggesting wording changes and amendments | Feedback from MW led to the following changes to the CRF:
|
| Nine patient representatives were involved in the review and testing of the online follow-up questionnaires for the trial | ||
| Recruitment and engagement activities | The patient researcher (MW) and a young person with vitiligo and her mother assisted with the recording of a video to aid recruitment, and a video demonstration of how to use the light treatment (Figures 11a and 11b). These videos were used for training purposes. Site staff were encouraged to play the videos to participants at their baseline clinic appointment as a part of their intervention training. Participants were then given either a DVD of the training videos to take home, or a link that they could use to watch the videos online | Participants reported feeling confident in their ability to use the treatments appropriately |
| Interviews carried out with trial participants as a part of the trial process evaluation suggested that, alongside the participant handbook, the videos were a useful tool to assist with the treatment regime | ||
| The patient researcher and trial chief investigators gave presentations at The Vitiligo Society conferences to raise awareness of the trial. They also contributed pieces to The Vitiligo Society newsletter on an ongoing basis, to update society members about the progress of the trial and to help with trial recruitment | ||
| Supporting analysis | ||
| Providing blinded outcome assessment from the perspective of people with vitiligo | Blinded assessment of baseline and post-treatment digital images were undertaken by three lay assessors (people with vitiligo) and one clinical assessor | Data provided by these blinded assessors were used to inform interpretation of the trial results |
| Process evaluation | ||
| A subset of the HI-Light Vitiligo trial participants and their carers were interviewed once they had completed the trial. Questions explored the patients’ experiences in using the treatments and possible barriers to and facilitators of their use | Results of the process evaluation informed interpretation of the trial results and implementation planning for uptake of the treatments within the NHS | |
| Interpretation | ||
| Four people with vitiligo attended the internal trial results reveal meeting | Having a strong patient presence at the internal results meeting ensured that interpretation of the results was appropriate and helped inform discussions around the clinical relevance of the observed treatment effects | |
| Patient partners were involved in producing and reviewing lay summaries of the trial findings, participant newsletters and social media communications | Patient representatives also provided guidance on the delivery of trial results to the vitiligo community | |
| Summaries of results were provided in accessible formats for multiple stakeholder groups | ||
FIGURE 11.
Stills from the HI-Light Vitiligo trial training videos. Figures reproduced with permission (2019, personal communication). (a) A patient researcher and (b) a young person with vitiligo and her mother demonstrate the use of the NB-UVB hand-held light device.

From stakeholder to participant
During the delivery of the HI-Light Vitiligo trial, the patient researcher (MW) expressed an interest in being involved as a trial participant. In such instances, care should be taken to ensure that there is a clear distinction between being a member of the trial team (in this case a patient representative) and trial participation to protect the blinding and to preserve the equipoise of the research team. Following discussions between the research team and the patient researcher, it was decided that the prior role as a member of the research team should cease for the duration of her involvement as a trial participant. However, our patient researcher was able to provide valuable input at the trial results meeting, where she assisted with interpretation of the results and ongoing dissemination activities.
Reflections from our stakeholders
I feel very privileged to have been involved with the HI-Light project from its inception. I was impressed by the way the trial was organised and conducted which involved patients in many aspects of trial design. I took part in videos, including the [NB-]UVB training video and other forms of publicity to encourage participation in the trial. My observation that small improvements in percentage repigmentation was not meaningful for patients led to the decision to develop a new scale for use as the primary outcome measure in the trial. I also commented on the wording of all patient-related study materials and online questionnaires to make them easier for patients to complete and I helped to develop the VNS scale.
Patient researcher and member of the Trial Management Group
As I have vitiligo, and have experienced the difficulties in getting access to treatment. The trial seemed to provide a bit of hope for those wanting to treat their patches. If people have an opportunity to do this kind of voluntary work, I really recommend taking it. It’s been really interesting and I hope the lay volunteers have made a positive contribution to the trial overall.
Blinded image assessor
Being a vitiligo sufferer myself, I felt honoured to be asked to be part of this trial by looking at before and after treatment photos. It is exciting to see the research that is still being done and how the ways of treatment are still being explored. I would also be happy to take part in any future trials and participate in any further research.
Blinded image assessor
As someone with vitiligo it is always pleasing to be asked to help in research. I have a PhD [doctor of philosophy] in pain control based on research I did in the NHS many years ago so I know how important it is to have people willing to give their support to research projects in a committed and consistent manner – I was more than happy to help. I would hope that if the results are in favour of a treatment effect then this treatment can then be offered to more people – but it may only work for some and not at all for others so we’d need to try it and see on a case-by-case basis. I look forward to learning more about this and thank you for allowing me to participate as an image reviewer.
Blinded image assessor
I was delighted to be approached and take part in this trial. I agreed to take part because (a) it felt like a professionally run trial and (b) my role as a trustee of a related charity.
I felt the organisation of the trial, the regular communications and reading materials were excellent. I felt part of the team and I recognised that extra effort that was made to include me in conversations/debates even though I was not a medical expert. As a senior manager in business I was able to draw parallels with the formal board meetings and governance I have encountered in my day-to-day work. This felt like a serious undertaking.
I felt a little energy and momentum has been lost recently but that is also a recognition of the very high standards encountered at the beginning.
Going forwards I would like to have sight of any publications relating to the research and would be delighted to support any further research relating to vitiligo.
Patient member of the TSC
When [name of child] and I were approached to take part in the HI-Light trial, it gave us a great opportunity to be involved in research that was being undertaken in order to help those who were living with vitiligo. Both [name of child] and I were happy to contribute to this trial as we believed that any outcome would be one step nearer to finding a cure for vitiligo or at least being able to manage the symptoms of living with vitiligo.
I believe that during the trial the journey was made easier from the training tape that we were allowed to follow but also from the support that we were given during this time from those involved in the trial.
Mother of child participant
Maintaining communication
A trustee of The Vitiligo Society, who has vitiligo, actively contributed as a member of the TSC. This, along with the involvement of the patient researcher on the Trial Management Group, provided invaluable patient perspectives on key trial decisions. It also helped to maintain a connection between the trial management team and the community of people with vitiligo. The chief investigator and patient researcher attended meetings organised by The Vitiligo Society to keep the vitiligo community aware of trial progress and encourage participation in the trial.
Discussion
This report documents the diverse involvement of stakeholders in the development, delivery and dissemination of a large multicentre RCT. The importance of PPI and wider stakeholder involvement in all aspects of research delivery were recognised by the trial funder, trial sponsor and research team. This shared passion helped to facilitate successful stakeholder involvement throughout the life cycle of the HI-Light Vitiligo trial.
We have demonstrated significant impact from stakeholder involvement, particularly in prioritising the research question, defining the primary outcome and informing the trial design. Involving a panel of people with vitiligo in the blinded assessment of the digital images was innovative and provided reassurance that the primary outcome was not influenced by accidental unmasking of the trial participants to their treatment allocation. Involvement of a range of key stakeholders during discussion of the trial results was key to understanding the clinical relevance of the findings, which demonstrated a statistically significant, but relatively small, treatment effect.
We used existing partnerships between people with vitiligo and the research team to facilitate meaningful stakeholder contribution across all aspects of the trial, with almost 800 individuals contributing overall. Given the large number of children involved in the trial, it is possible that a greater impact could have been made by involving young people and parents of children with vitiligo.
There was some criticism from stakeholders that communication towards the later stages of the trial was less evident and this probably reflects the long time delay between the end of treatment at 9 months and the end of long-term follow-up after 21 months. For trials such as this with long-term follow-up, special efforts could helpfully be made to ensure that participants and stakeholders understand the reason for apparent inactivity and delays in hearing about the study results.
Conclusion
The NIHR-funded HI-Light Vitiligo trial had a strong stakeholder contribution in all aspects of trial design and delivery. With invaluable input from patients, patient carers and health-care professionals, we were able to deliver the largest multicentre vitiligo trial to our knowledge to date, and have successfully developed a patient-reported outcome and used it to assess a patient-led intervention. Our working relationship with the vitiligo patient community has proven to be mutually beneficial and one that we hope continues to grow.
Chapter 7 Device testing
Parts of this chapter have been reproduced with permission from Rogers et al. 78 This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
Usually NB-UVB treatment is carried out in a hospital setting, although in some countries there are well-developed systems for allowing NB-UVB treatment to be carried out at home. 79 For vitiligo, although treatment is usually administered in a hospital setting, there is increasing interest in hand-held devices that can be used at home to deliver NB-UVB to localised areas of vitiligo. 24,80,81 However, there are a number of evidence and knowledge gaps in the optimum use of localised NB-UVB treatment for vitiligo. 23,24 In particular, there is little evidence regarding the consistency of dosing delivered by localised NB-UVB units, the quality assurance measures that should be followed in their use, and whether or not there are any significant safety issues, especially when people with vitiligo use the units at home.
In preparation for the main trial, a pilot study was carried out that identified potential dosimetry issues that may arise during the use of hand-held NB-UVB units, which could have possible implications for maintaining adequate control of participant NB-UVB exposure. The following potential dosimetry issues were identified:
-
The absolute device output was lower than the manufacturer’s specification, which has implications for defining the treatment protocol exposure times.
-
There was variation in device output, which could make it difficult to evaluate treatment effects.
-
There was a change in tube output during use, with implications for defining the treatment protocol exposure times.
-
Short-term, early-life change in device output were observed, which suggests that a pre-burn of bulbs prior to delivery to a participant might be necessary.
These issues have the potential to be critically important in the context of home-based treatment, without the usual degree of control over treatment that would be achieved in a hospital-based phototherapy unit. Furthermore, we wanted to minimise potential variance in trial outcomes caused by NB-UVB dosimetry issues. Therefore, it was clear that we needed to conduct a thorough analysis of device output prior to their use in the main trial.
The aim of the work reported here was to ensure that the hand-held NB-UVB devices used in the HI-Light Vitiligo trial delivered a consistent and safe dose for all trial participants (addressing the issues identified above), so that any variance in trial outcomes attributable to variance in the output of the NB-UVB devices would be kept to an absolute minimum. We planned to achieve this aim using the following objectives:
-
establish whether or not the device output was consistent with the outputs as specified by the manufacturer and quantify the variation in device output across all devices used
-
quantify the likely drop in output over time and establish whether or not a pre-burn period was necessary prior to distribution of devices to trial participants
-
provide quality control checks on all trial devices prior to distribution to ensure that all issued devices had outputs within a predetermined range
-
develop a dosing schedule for use in the trial that ensured patient safety while delivering a clinically useful dose of NB-UVB.
Materials and methods
Use of narrowband UVB devices in the HI-Light Vitiligo trial
Before commencing trial recruitment, we undertook a photometric characterisation set of measurements as described in Study 1: photometric characterisation of the narrowband UVB devices prior to their use in the trial to achieve objectives 1, 2 and 4.
The HI-Light Vitiligo trial recruited 517 participants (children aged ≥ 5 years and adults) who were randomised into one of three parallel groups. In total, 425 live devices and 175 placebo devices were tested. This was more than the number of participants because of some tested devices not being suitable for participants, and anticipation of some devices requiring replacement during treatment. Participants (and their carers) were recruited at 16 secondary care sites around the UK and trained in how to use the NB-UVB device by watching a training video, and receipt of a written manual during a thorough face-to-face training with a research nurse. If a participant felt that the NB-UVB device was not working properly, they reported this to the co-ordinating clinical trials unit and a replacement unit was sent out from the central trial pharmacy. Faulty devices were returned by the participant and replaced. The faulty devices were sent back to the manufacturer.
Once the trial started recruitment, we performed the tests described in Study 2: quality assurance of devices prior to distribution to trial participants prior to issuing the devices, to achieve objective 3. All study tests were performed by a single team of scientists and technologists experienced in ultraviolet measurements.
Devices and test equipment
The hand-held NB-UVB device used in the HI-Light Vitiligo trial was the Dermfix 1000MX unit (Androv Medical, Leatherhead, UK). This unit is provided with a suggested dosing schedule, to be used after consultation with a supervising medical professional. We sought to develop a simplified dosing schedule that could be used in the trial. Initially the manufacturer supplied 10 Dermfix 1000MX units and two fluorescent tubes for characterisation [LightTech LTC 9W/G23 (LightTech Lamp Technology Ltd., Dunakeszi, Hungary) and Philips PL-S 9W/01/2P tubes (Koninklijke Philips N.V., Amsterdam, The Netherlands)]. Although there were small differences in spectral emissions (differing relative intensities at equivalent wavelengths) and a reduced output from the LightTech tube, the cost differential between the tubes was felt to outweigh these emission differences and so for all further characterisation and trial utilisation LightTech tubes were used in the hand-held devices. This is the standard configuration for this unit.
A Bentham DMc150 spectroradiometer (Bentham Instruments, Reading, UK), comprising a radiometer and double monochromator, was used to verify spectral outputs and an ILT 1700 radiometer (International Light Technologies, Peabody, MA, USA) was used for instantaneous irradiance measurements and integrated dose measurements. The spectroradiometer’s double monochromator and radiometer were calibrated against a mercury lamp with traceable spectral emissions. The ILT 1700 radiometer was field calibrated against the spectroradiometer.
To ensure consistency of output measurements, a jig to hold the Bentham and ILT sensors was designed and built by the Clinical Engineering Department at Nottingham University Hospitals NHS Trust (Figures 12 and 13). This ensured that the sensors were positioned over the centre of the lamp at the comb tip to consistently simulate desired clinical use.
FIGURE 12.
Elevation drawing of the measurement jig.
FIGURE 13.
Photographs showing three differing views of the test jig.

Study 1: photometric characterisation of the narrowband UVB devices prior to their use in the trial
To achieve objectives 1, 2 and 4, we developed the following protocol to test the photometric characteristics (spectral output, actual irradiance, consistency of irradiance during warm-up, longer-term stability of the irradiance-time curve) of the device with the LightTech tubes:
-
Measure the spectral output for 10 tubes and compare with the manufacturer’s specification using the Bentham monochromator.
This test was to ensure that the tube emission spectrum was both as expected and consistent across tubes (objective 1).
-
Measure the irradiance at 2 minutes following ‘switch on’ (to compare with manufacturer’s specification) and integrated dose at various time points (from 15 to 210 seconds in 15-second intervals) to calculate average dose rate as a function of time for each LightTech tube, then calculate the mean values and variance across all LightTech tubes.
We carried out these tests both to ensure that the tubes met the stated manufacturer’s output at 2 minutes and also to investigate the irradiance changes during tube warm-up when the irradiance initially rises fairly rapidly, before dropping off more slowly. These tests evaluated whether or not the tube outputs were repeatable, thus allowing more confidence in any set treatment protocol (objectives 1, 2 and 4).
-
Measure irradiance to simulate various treatment regimen simulations (2 × 30% total, 2 × 65% total, 2 × 100% total) following the skin type VI treatment protocol (longest treatment times) for three lesions, simulating actual usage.
We carried out these tests to investigate whether the irradiance-time curve shape remained constant irrespective of usage (objectives 1, 2 and 4).
-
Measure integrated dose for 50% total, 2 × 50% total, 100% total in single exposures. The 100% single exposure was conducted on two LightTech tubes.
We carried out this test to investigate if ‘fractionation’ of total exposure changed tube characteristics (objectives 2 and 4).
Tests a and b were designed to test the absolute characteristics of the tubes and to assess the variability of performance. This helped us to develop cut-off tolerances for irradiance and integrated exposure when testing trial devices prior to issue to participants, excluding devices whose characteristics fell outside these tolerances.
Tests c and d were designed to test the fall-off in irradiance and integrated dose, to ascertain if any pre-burn was required and to inform the trial treatment protocols. It also gave an insight into whether or not tests carried out on used devices following participant use could be used to determine treatment protocol adherence by the trial participants.
Study 2: quality assurance of devices prior to distribution to trial participants
Study 2 addressed objective 3, ensuring consistency of performance for devices issued in the trial. For reasons of efficiency, devices were tested in batches of approximately 15–25 prior to release to participants. The tests undertaken included:
-
a spectral irradiance test to look for any gross fault or set-up error in the supplied device (such as an incorrect tube fitted)
-
peak irradiance (3 minutes after start-up for all devices) to check if the device irradiance was within 20% of our validated irradiance values.
Owing to the large variability in tube irradiance at the manufacturer’s specified time of 2 minutes post start-up, we measured irradiance at 3 minutes post start-up, as we discovered that this was a more stable measure of individual tube performance (see Results).
Results
Study 1: characterisation of devices
Ten tubes from the same manufacturing batch were tested for spectral irradiance. The results of these measurements are shown in Figure 14. The results show almost exact coincidence of the spectral irradiance for all 10 tubes. They also show good coincidence with the manufacturer’s specified spectral irradiance. The main irradiance was at 313 nm, with subsidiary peaks at 365, 405 and 435 nm. There was a very small ultraviolet A light (UVA) component (365 nm).
FIGURE 14.
Spectral irradiance for the first batch of 10 tubes (spectral irradiance for LightTech tubes).
Table 32 shows the irradiance results from the sample of 10 tubes. These results are to be compared with the manufacturer’s specification (‘typical irradiance at 120 seconds’) at the tube’s mid-point at the comb tip of 7 mW per cm2. These results showed that most tubes would fall within our acceptance criteria of an output ± 20% from the value used to inform the clinical treatment protocol.
| Mean irradiance (mW per cm2) | Minimum irradiance (mW per cm2) | Maximum irradiance (mW per cm2) | SD (mW per cm2) | Manufacturer’s specified irradiance (mW per cm2) |
|---|---|---|---|---|
| 4.02 | 3.58 | 4.50 | 0.26 | 7 |
The mean device output was only 53% of the manufacturer’s specified output. For clinical protocol derivation, a mean tube irradiance of 4 mW per cm2 was used.
The results of simulating various participant protocols on the irradiance are shown in Table 33.
| Treatment time (% of total fractions from the start) | Average % drop (from mean start maximum value) |
|---|---|
| 30 | 23 (n = 5) |
| 65 | 31 (n = 4) |
| 100 | 37 (n = 2) |
The table shows the average irradiance and percentage drop from the initial maximum average irradiance at various time points during the treatment protocol for skin type VI for three lesions (the maximum number of lesions to be assessed in the trial). It allows for the repeated turning on and off of the device on different days and, therefore, includes multiple warm-up times, as would be the case for a real participant treatment regimen.
Study 2: results of devices prior to issue to participants
Although the spectral irradiance test in study 2 was designed to ensure that no devices with gross set-up errors (e.g. incorrect tube wavelength) were issued to participants, it also allowed us to track changes in tube manufacturing. The peak irradiance wavelength was noted to change from 313 to 314 nm after about one-fifth of the batch processing. This slight change in the wavelength of the peak irradiance was not due to any calibration drift of our monochromator and was probably due to changes in the phosphor composition of the fluorescent tubes. This change did not produce any significant change in the amount of UVA radiation.
Twenty-one batches were tested and 9 out of 21 (43%) batches had devices where one or more devices showed an irradiance outside the range 3.22–4.82 mW per cm2 (mean value ± 20%). A total of 54 live devices were rejected out of a total tested of 425 (13%). One batch (24 live devices) was tested where all devices lay outside the required range. After confirming that this was not due to faulty bulbs, combs or voltage supply, it was assumed that a power supply fault was responsible and the devices were returned to the manufacturer. The batch sizes ranged from 15 to 25 units.
Discussion
The results of the characterisation tests on the LightTech tubes clearly showed a real output much lower than in supplier information. This difference (a factor of approximately 0.6) would have required an increase in treatment time of 75% to allow for the drop in output performance of the real-world tubes. These results show the importance of thoroughly characterising NB-UVB devices prior to use to ensure adequate treatment. Although the much lower output of the units could have potentially led to a reduction in efficacy, we accounted for the lower output by adjusting the treatment schedule used in the trial. Based on our measurements, there is a need for cost-effective, higher irradiance tubes that can be used in such devices.
The tube irradiance as a function of time (Figure 15) was broadly the same for all tubes tested. For all tubes there was a brief (within the first 2 minutes) increase in output, followed by a decrease in output characterised by a reducing gradient with a plateau reached after about 50% of the treatment time. To pre-burn the tubes to enable a constant output would therefore have required a pre-burn time of many hours. This could have been done in the setting of a research study but would be costly and potentially impractical when using the devices in clinical practice. One alternative approach would be to adjust the treatment schedule in the early stages of treatment, making allowances for the gradual loss of output. However, this would have been complicated to calculate and would not have allowed us to use a simple treatment schedule with fixed increments between treatments. We also felt that asking participants to recalculate doses themselves would add further complexity to the treatment, which may have reduced adherence. Moreover, any gradual loss of output in the early life of a unit will simply require that it is used for slightly longer periods with each subsequent use to achieve the expected mild degree of skin erythema and subsequent therapeutic response, and this would be achieved by simply moving on to the next step of the dosing schedule. Therefore, as the trial was pragmatic, reflective of real life clinical practice, we decided not to pre-burn the tubes but instead to use them from new and to ask participants to follow the planned treatment schedule that included a 2-minute ‘stabilisation’ period for the device prior to commencing treatment.
FIGURE 15.
Tube irradiance (normalised to the maximum irradiance) as a function of time.
The fact that one in eight devices were rejected because of their output lying outside the ± 20% cut-off point shows the importance of testing the devices prior to use. This quality control reduced the variance in treatment exposure among trial participants attributable to device output. It also demonstrates the need to check the output of devices before they are used in clinical practice as part of quality assurance, as is current practice for clinic-based treatment units.
Furthermore, as these devices may be purchased by members of the public, the output variation from specification, and output variation between tubes shows the need for clinical supervision, backed up by robust quality assurance, during their use. Given these results, we would recommend any member of the public purchasing such a device directly from a supplier seek specialist dermatological advice before use.
The device tests described in this report require expensive ultraviolet test equipment and scientific and technical expertise to interpret the results. These staff and equipment are not available at all hospitals and so may support the development of specialist centres of expertise supporting many dermatology services (a hub and spoke model).
We hope that our findings regarding the dosimetry and performance of hand-held NB-UVB units will help to inform the design of community-based phototherapy services in the future. There are, however, some additional considerations regarding the external validity of the work. In the trial, each device was used by only one participant. In a clinical service each device is likely to be used by several patients in succession, so the clinical service would have to decide whether to reissue the unit with the same bulb or to fit a new bulb prior to the unit being reissued. For the tubes used in this study the manufacturer states a useful tube life of 400 hours, which is far longer than the integrated treatment time for three lesions on a participant with skin type VI for 9 months, so a single tube could potentially be used for multiple patients, although protocols would have to be developed to ensure that devices were fit for purpose when reissued.
As the study team required detailed technical specifications of the devices and tubes, a close working relationship with the supplier was essential in order to access such information; we received good support from the manufacturer in this respect. This would also be an essential requirement in the future when setting up a home-based phototherapy service using the devices.
Chapter 8 Discussion and conclusion
Main findings
The HI-Light Vitiligo trial was a large, pragmatic RCT of home interventions (potent TCS and NB-UVB light therapy) for people with active and limited vitiligo. The combination of hand-held NB-UVB plus potent TCS for 9 months was found to be superior to potent TCS used on their own, was well tolerated and was potentially cost-effective (£2329 unadjusted or adjusted £1932 per additional treatment success, although there is currently no evidence to indicate how much a decision-maker would be willing to pay for an additional treatment success). NB-UVB used as monotherapy was not superior to potent TCS and had a higher incremental cost per additional successful treatment than combination treatment compared with TCS alone.
Blinded evaluation of treatment success as assessed by a panel of three people with vitiligo supported the primary outcome, although treatment effects were larger and both NB-UVB and combination treatment were significantly better than TCS alone. It is unclear why blinded observers would value the treatments more than the participants in the trial, but it is possible that the trial participants balanced the observed treatment effects against the burden of adhering to treatment over 9 months.
Results for investigator-assessed percentage repigmentation, using digital images of the vitiligo, were also consistent with the participant-reported primary outcome (VNS). Percentage repigmentation is the most commonly used outcome in vitiligo trials,33 and so these results provide a useful context for comparison with other studies.
Quality of life was high for all groups at baseline and no differences were observed between groups following treatment.
Both NB-UVB and potent TCS were well tolerated. Erythema (grade 3 or 4) was a relatively common side effect, but these episodes were limited to the small areas being treated and were managed effectively. The incidence of clinical skin thinning was rare despite the relatively long-term intermittent use of potent TCS, including on the face.
Sensitivity analyses were supportive of the main findings and participants who adhered to the treatment regimen by ≥ 75% were more likely to achieve treatment success. There was no difference between the treatment groups according to age (adults vs. children) or duration of vitiligo (≥ 4 vs. < 4 years).
In line with clinical experience, vitiligo patches on the hands and feet responded less well to treatment; this was true for whatever interventions were being used.
At 3 months, > 90% of participants in all three groups showed onset of treatment response at the target patch, suggesting that all were effective in stopping the spread of vitiligo. However, onset of treatment response was defined as ‘stopped spreading’ (i.e. ‘stayed the same’ or ‘improved’), which could have resulted in an overestimation of treatment effect if potential participants over-reported recent changes to the target patch to gain access to treatment.
Interpretation of results for ‘maintenance of treatment response’ were limited by low follow-up rates at 12 to 21 months. Nevertheless, the results suggest that treatment response may be lost quite rapidly once interventions are stopped and that maintenance therapy may be required to retain the pigmentation gained during treatment.
Process evaluation findings suggested that patients and health-care professionals were positive about the role of combination treatment in the management of vitiligo.
Despite being time-consuming and (potentially) complex, both participants and health-care professionals indicated that, with appropriate support, combination treatment could be managed at home. Appropriate training and ongoing monitoring, particularly in the early stages of treatment, are essential, especially given concerns about potential side effects.
People with vitiligo were perceived to have few treatment options, thus supporting the broader use of combination treatment in the NHS, with some caveats about which patients might benefit most. Those with a lifestyle that is incompatible with regular time-consuming treatments, unrealistic expectations of treatment, or poor levels of adherence to prior treatments may be poor candidates for combination treatment.
Both health-care professionals and commissioners recognised that the need for a developed infrastructure (including nursing support and medical physics provision) may be a barrier to broader NHS provision. Regional clinics may be a possible solution, as might some form of mixed economy approach, where patients purchase light therapy devices alongside NHS support and training.
Relevance to the wider literature
These results show that combination treatment with NB-UVB and potent TCS is more effective than a single intervention (in this case, TCS). This is consistent with previous research, which has shown that combination treatments are generally more effective than monotherapies in treating vitiligo, although overall response rates, both in our study and in previous research, are generally modest. 22,25,26
Although there have not been any studies assessing the same interventions as those used in this study, the response rates are comparable with other studies. A meta-analysis of studies assessing phototherapy for vitiligo,82 including 29 prospective studies of NB-UVB, reported a ‘marked response’ (> 75% repigmentation) in around 19% of participants after 6 months of NB-UVB monotherapy. This is similar to the rates of treatment success in our study, measured using the VNS (18% for NB-UVB only and 28% for combination at 6 months), although we observed lower success rates based on ≥ 75% repigmentation (5% for NB-UVB only and 11% for combination at 6 months). The same meta-analysis reported better response rates for vitiligo on the head and neck, which is consistent with our study. 82
No other studies have compared the specific combination of NB-UVB and mometasone furoate with mometasone alone, so direct comparison is difficult. One study comparing the combination of NB-UVB and clobetasol propionate (a more potent TCS) with NB-UVB alone83 was identified in the Cochrane systematic review of interventions for vitiligo. 22 This study suggested that combination treatment may be more effective than NB-UVB monotherapy, but the study was small and so lacked power to demonstrate any statistically significant difference between the intervention groups; the relative risk ratio for achieving > 75% repigmentation was 1.38 (95% CI 0.71 to 2.68). 83
No significant safety issues have been identified in previous small studies of home-based hand-held phototherapy devices for vitiligo, used instead of hospital NB-UVB therapy,25,26 and this is confirmed by the findings of our study. Long-term NB-UVB treatment (mean number of treatments = 211) in a study of patients with darker skin types conferred no increase in skin cancer risk, suggesting that NB-UVB can safely be continued for longer periods of time than in our study, although most patients in that study were skin types IV–VI. 84 Large cohort studies of patients having long-term treatment with NB-UVB have also shown no significant increased risk in skin cancer risk from this treatment. 38,39
Although combining NB-UVB and calcineurin inhibitors (e.g. tacrolimus) was discouraged in the past, because of concerns over a possible increased risk of skin cancer, a number of studies assessing this combination of treatment have been published over the last few years. A systematic review by Arora et al. 85 identified three studies comparing a combination of NB-UVB and tacrolimus with NB-UVB monotherapy. Meta-analysis of two of these studies showed combination treatment to be more effective than NB-UVB monotherapy in achieving > 75% repigmentation, although only just (risk ratio 1.34, 95% CI 1.05 to 1.71). It is possible that further studies comparing these interventions will provide sufficient data to make the confidence estimates stronger, but this remains to be seen.
Strengths and limitations
This was a large, pragmatic trial that was designed and managed in collaboration with an accredited clinical trials unit. Using a patient-reported primary outcome meant that treatment success reflected the views of people with vitiligo and was supported by blinded outcome assessment using digital images for both VNS and percentage repigmentation; both of which have been recommended for inclusion in vitiligo clinical trials by people with vitiligo. 35
As found in other vitiligo trials,22 retention throughout the trial was challenging. Just over 70% of participants provided primary outcome data at 9 months, and < 50% provided data by 21 months. This limited interpretation of some of the results, especially during the long-term follow-up phase.
Because loss to follow-up was higher than originally anticipated, the trial lacked power to provide a high level of precision around the point estimates.
Adherence to treatment regimens was quite low (see Table 6). This was probably due to the time burden of treatments, particularly the active or dummy NB-UVB devices. This is a limitation of the study but this was a pragmatic trial and treatments were delivered by the participant and/or their carers at home (with nursing support). It is possible that participants adhered more to trial interventions as a result of being in a trial, and this may have led to an overestimate of treatment effects. However, we think that, overall, the level of use of the treatments is reflective of how they would be used in real life.
We used a single, standardised treatment schedule, which we asked all participants to follow. This started at a very low dose and then built up to higher doses in small increments. This will have meant that for participants with darker skin types, the first few doses will have been lower than those used in conventional hospital-based phototherapy, where starting doses are determined by measuring the MED prior to starting treatment. However, participants would all move up the schedule to longer treatment times and over the course of treatment (up to 9 months) these smaller initial doses are not likely to have had a significant impact on the total NB-UVB dose received by those participants using active devices.
Participants were encouraged to choose a target patch that was genuinely the one in which they most wanted to see a difference. If they had two patches on different parts of the body that they were equally keen to see an improvement in, and if one of the patches was on their hands or feet, they were advised that the response may not be as good on the hands and feet, but they could still choose for the hand/foot patch to be their target patch. Many participants still chose a hand/foot patch as their target patch, but it is possible that some participants may have decided to change the target patch to one on the head and neck or rest of the body. This could have introduced bias into the study findings. However, this is similar to the situation in clinical practice, where a patient may be advised that treatment of vitiligo in certain anatomical locations may be less effective and that it may make more sense to concentrate on treating areas of vitiligo that are more likely to respond to treatment.
Generalisability
This trial has good external validity as it was a large, pragmatic trial with few exclusions, although all participants were required to have active vitiligo that affected < 10% of their body surface area. People with more extensive vitiligo are unlikely to find these interventions helpful as the treatments would become overly burdensome.
The trial included both children and adults and treated different body sites. Planned subgroup analyses explored the impact of these characteristics but found no evidence of differential treatment response by age or body site, other than the overall poorer response rates on the hands and feet. People with all skin types and ethnicities were included in the trial as this reflected the types of patients typically presenting for vitiligo treatment within the UK NHS. We did not exclude participants with lighter skin types (types I and II), as vitiligo can cause considerable distress in such people as well as those with darker skin types. 16,86 A post hoc analysis by skin type found no differential treatment response in people with paler skin types (types I–III) or those with darker skin types (types IV–VI), although we would emphasise that this was an exploratory, post hoc analysis and the study was not specifically powered for this analysis.
The trial was designed to reflect normal clinical practice as far as possible. Hand-held NB-UVB devices such as those tested in this study are not widely available within the UK NHS at present, although a few sites do offer treatment with similar devices and they can also be purchased online and used at the user’s own risk. In the trial, nurses in secondary care dermatology departments delivered training on use of the treatments, and participants were reviewed every 3 months during clinic visits. Additional support was provided by telephone as required, if participants had queries about use of the interventions or experienced side effects.
The process evaluation conducted alongside this study identified the importance of the support provided to participants, to enable them to use the treatments safely. Participants and investigators agreed that the complexity of the treatments meant that support and close monitoring were essential. Some participants had considered purchasing a light therapy device, but had decided against this because of a lack of the necessary support infrastructure. If the treatments were introduced into the NHS, the cost of providing this support infrastructure would need to be taken into account and health-care decision-makers would have to decide how much they are willing to pay to achieve a successful treatment. The relative lack of other treatment options, and the likely high cost of newer drug treatments currently being developed, would be important to consider when making such decisions.
Conclusions
Implications for health care
The HI-Light Vitiligo trial demonstrates that combination treatment with NB-UVB and potent TCS is superior to potent TCS alone, although the benefits are likely to be modest. Combination treatment was safe, well tolerated and was potentially cost-effective for people with limited vitiligo, but there is uncertainty over how much a decision-maker would be willing to pay to achieve an additional treatment success.
Patients starting vitiligo treatments should be made aware of the considerable time commitment required, and the likely duration of treatment over many months. Clinical review at 3 months appears to be an appropriate time point at which to judge whether or not further treatment is likely to be beneficial.
Our study confirmed that vitiligo on the hands and feet responds less well to treatment, so treating these anatomical areas may be difficult to justify when resources are limited.
Hand-held, home NB-UVB therapy appears to be a useful treatment option for people with vitiligo and provides considerable advantages over hospital NB-UVB therapy (which requires hospital visits two or three times per week). Home NB-UVB requires training and support from health-care professionals with experience of delivering phototherapy services and is time intensive for patients.
Use of mometasone furoate 0.1% ointment (a potent corticosteroid) as first-line treatment for vitiligo is supported, as it achieved treatment success in one in six individuals and was effective in stopping the spread of active vitiligo patches. Stopping the spread of vitiligo is an important treatment outcome to people with the condition. 21,33 These trial results suggest that potent TCS is safe in both adults and children when used 1 week on, 1 week off for 9 months.
Treatment effects were lost once interventions were stopped, suggesting that maintenance therapy is likely to be needed to prevent further loss of pigment.
Compared with potent TCS, combination treatment had a lower incremental cost-effectiveness ratio than NB-UVB monotherapy (meaning that an additional treatment success can be attained for a lower cost), although the mechanism for widespread implementation of a home-based NB-UVB service for skin disorders within the NHS has yet to be established.
Qualitative findings from our mixed-methods process evaluation study suggested that people with vitiligo and health-care professionals who treat them would value the provision of home NB-UVB as a useful treatment option for the management of vitiligo, despite the relatively modest treatment effects. Both trial participants and health-care professionals suggested that some form of ‘mixed economy’ may be the most effective way of providing home-based light therapy. This could potentially involve patients leasing or purchasing a phototherapy device, and the NHS providing the necessary training, quality assurance and support for patients. This would reduce the likely cost to the NHS (see Chapter 4, Sensitivity analysis) but would have equity implications in that treatment would only be accessible to those patients able to afford it.
These findings need to be disseminated to a wide audience. People seeking treatment for vitiligo are unlikely to receive any treatment if they do not receive appropriate advice from health-care professionals. In the UK, people with vitiligo are likely to consult a GP initially, and research among members of The Vitiligo Society suggest people view their GP as their primary source of information, although GPs appear to have low awareness of vitiligo. 87 The NICE Clinical Knowledge Summaries guideline suggests that people seeking treatment for vitiligo may be prescribed TCSs and/or referred to dermatology. 88 However, anecdotally, such management does not always seem to be followed. The safety data from this trial suggest that GPs can be reassured that adverse effects are rare if potent TCSs are used long-term once daily on alternate weeks (i.e. 1 week on, 1 week off).
Implications for research
Participants in the HI-Light Vitiligo trial reported relatively high quality-of-life scores at baseline using both generic and vitiligo-specific quality-of-life instruments. Despite having good quality of life, all participants were keen to access vitiligo treatments and were wiling to use them over many months, suggesting that something other than quality of life was motivating treatment choices. It is not clear whether this was because the trial focused on people with limited vitiligo (which had a limited impact on their quality of life), or the quality-of-life instruments themselves were insufficiently sensitive to detect the impact of vitiligo, particularly in relation to the psychological impact of the condition.
Because home-based phototherapy services for the management of skin disorders are currently available in only a small number of specialist centres, further research is required to establish the best ways of implementing a home-based light therapy service across the UK. This might usefully involve a hub and spoke model whereby specialist medical physics units perform the testing and maintenance of devices for a number of departments.
We used participant-reported treatment success as the primary outcome, based on the noticeability of the vitiligo (VNS), to ensure that vitiligo treatments were judged against criteria that are meaningful to people with vitiligo. Further work is required to establish the validity, responsiveness and interpretability of the VNS. In particular, it would be helpful to establish how patients value a ‘partial treatment’ response as measured by the VNS.
The HI-Light Vitiligo trial was designed to address two of the questions prioritised by health-care professionals and people with vitiligo in the James Lind Alliance Vitiligo Priority Setting Partnership. 21 Many of the top 10 priorities remain unanswered (Box 2).
-
How effective are systemic immunosuppressants in treating vitiligo?
-
How much do psychological interventions help people with vitiligo?
-
Which treatment is more effective for vitiligo: light therapy or calcineurin inhibitors (e.g. tacrolimus, pimecrolimus)?
-
How effective is UVB light therapy when combined with creams or ointments in treating vitiligo?
-
What role might gene therapy play in the treatment of vitiligo?
-
How effective are hormones or hormone-related substances that stimulate pigment cells (MSH analogues, afamelanotide) in treating vitiligo?
-
Which treatment is more effective for vitiligo: calcineurin inhibitors or steroid creams/ointments?
-
Which treatment is more effective for vitiligo: steroid creams/ointments or light therapy?
-
How effective is the addition of psychological interventions to patients using cosmetic camouflage for improving their quality of life?
-
How effective is pseudocatalase cream (combined with brief exposure to UVB light) in treating vitiligo?
In addition, two treatment uncertainties were suggested as ‘ones to watch’, as these interventions were still in an early investigative stage:
-
How effective is piperine (black pepper) cream in treating vitiligo?
-
What role might stem cell therapy play in treating vitiligo?
Future research priorities that have emerged from the HI-Light Vitiligo trial include the need for:
-
development and testing of new vitiligo treatments with a greater response and longer-lasting effects
-
investigation of treatments suitable for people with widespread vitiligo
-
research into different strategies to maintain treatment response once treatments are stopped
-
further development and validation of outcome instruments to be included in the vitiligo core outcome set, to facilitate combining of trial results in meta-analyses.
Acknowledgements
We would like to thank all 517 people with vitiligo who participated in the trial.
The NB-UVB light devices used in the trial were provided by Dermfix Ltd (Großaitingen, Germany, and Chalfont St Giles, UK). The authors would like to thank Dermfix for their support, specifically in providing the devices at a reduced cost, ensuring timely support for problem resolution and also for providing detailed technical information.
The trial was sponsored by the University of Nottingham, was co-ordinated from the NCTU and was supported by the NIHR Clinical Research Network and the UK DCTN. The UK DCTN is grateful to the British Association of Dermatologists and the University of Nottingham for financial support of the network.
The authors would also like to thank the entire trial team for their support in this work.
Contributors to the HI-Light Vitiligo trial:
-
Independent TSC – Khaled Ezzedine (Department of Dermatology, Paris-Est Créteil Val-de-Marne University), Chris Edwards (Consultant Medical Physicist, Department of Dermatology, St Woolos Hospital, Newport), Marco Singh (patient representative and Trustee and Treasurer, The Vitiligo Society) and Ophelia Dadzie (Consultant Dermatologist, Hillingdon Hospital, Uxbridge).
-
Data Monitoring Committee – Robert Dawe (Consultant Dermatologist, Dermatology Department, Ninewells Hospital, Dundee), Michelle Collinson (Senior Medical Statistician, University of Leeds), Robert Sarkany (Consultant Dermatologist, St John’s Institute of Dermatology, London) and Bonnie Cundill (Principal Statistician, Complex Interventions Division Clinical Trials Research Unit, Leeds).
-
NCTU – Daniel Simpkins and Chris Rumsey (management of the trial database systems); Jessica Haywood, Sarah Walker, Mathew Foster and Stella Tarr (data management); Alexandra Erven and Isobel Hawley (blinded control of light devices); Gill Bumphrey and Paula Blance (clinical trials pharmacy); Jenny Smithson and Hayley Gregory (monitoring activities); Melanie Boulter, Sharon Ellender and Hayley Foster (quality assurance).
-
University of Nottingham – Dr Natasha Rogers for help with study set-up, dissemination materials and editorial support.
-
Nottingham University Hospitals NHS Trust – Janine Crutchley and Mariusz Grocki (medical physics – testing of light devices).
-
Blinded image assessors – Dev Shah (Consultant Dermatologist, Amersham Hospital), Emma Rush (patient representative), Emily Haskell (patient representative) and Ramon Pediani (patient representative).
-
Members of CEBD’s patient panel and people who responded to our online survey to inform the trial design.
-
Contributors at recruiting centres:
-
Cannock Chase Hospital and New Cross Hospital, The Royal Wolverhampton NHS Trust (Walter Machado, Lyndsay Bibb, Jolante Frankiewicz, Sharon Kempson, Krys Castro-Foskett, Lynette Wheeler, Sue Price, Nor Adzura Mohd Azam, Oluwadamilola Jagun, Julia Brockley, Julie Edwards, Sarah Johnson, Joanne Logan, Emma Sharman, Joannna Butler, Rebecca Pate, Katherine Jones and Tammy Smith).
-
St Luke’s Hospital, Bradford Teaching Hospitals NHS Foundation Trust (Annette Essex, Jenny Ott, Susanne Hatfield, Carol Fleming, Jonathan France, Anthony Dock, Sarah Walton and Paul Creasey).
-
University Hospital Wales, Cardiff and Vale University Health Board (Beverley Gambles, Gladys Makuta and Geraldine Haebich).
-
The Norfolk and Norwich University Hospital, Norfolk and Norwich University Hospitals NHS Foundation Trust (David Tomlinson, Rebecca Phillips, Alison Wiltshire, Aza Yaakub, Urvi Popli, Puran Gurung, Karen Banks Dunnell, Cathy Haughton and Jocelyn Keshet-Price).
-
Solihull Hospital, Heart of England NHS Foundation Trust (Athanasia Filippa, Ruth Jocelyn, Samantha Stafford, Maxine Lovell, Angela Burden, Aaron Wernham, Alexis Cuell, Georgina Fremlin, Helen Goodyear, Indy Atwal, Catherine Cotter, Gabriella Petrof and James Carr).
-
West Glasgow Ambulatory Care Hospital, NHS Greater Glasgow and Clyde (Gabrielle Becher, Anne Thorat, Fiona Cunningham and Cristina MacNeil).
-
Whipps Cross Hospital and The Royal London Hospital, Barts Health NHS Trust (Ceri Guarnieri, Jason Thomson, Satwinderjit Shinhmar, Bibi Badal, Jennifer Ross, Vanessa Van-de-Velde, Claire Marshall, Daniel Lieberman, Khatija Sarkar, Adebanke Aboaba, Nadira Qureshi, Maria-Angeliki Gikini, Waffa Girshab, Zeeshaan-ul Hasan, Jonathan Kentley, Sarah Hogan, Darren Hawkins, Denise Andrews, Ausra Zdanaviciene, Chipo Chitsenga, Syed Arafath, Jason Thomson and Beebee Meeajun).
-
Birmingham Children’s Hospital, Birmingham Children’s Hospital NHS Foundation Trust [Gemma Baker, Jason Pitt, Lucy Cooper, Karen Doherty, Gemma Grovenor, Dharshini Sathish Kumar, Needa Mohamed, Katie Heffernan, Charlotte Cottrill (nee Lilley) and Melanie Rooney].
-
York Hospital, York Teaching Hospital NHS Foundation Trust (Jill Green, Sian Sturdy, Sarah Russell-Sharpe, Helen Marson and David Thompson).
-
Royal Derby Hospital and the London Road Community Hospital, Derby Teaching Hospitals NHS Foundation Trust (Sally-Ann Bell, Vanessa Unsworth, Melody MacGregor, Mariana Bernado, Padma Mohandas, Andrew Martin, Sarah Murfin, William Stone and Michelle Scott).
-
Queens Medical Centre, Nottingham University Hospitals NHS Trust (Jo Llewellyn, Rebecca Phillips and Li-lin Hong).
-
Chelsea and Westminster Hospital, Chelsea and Westminster Hospital NHS Foundation Trust (Miriam Fitzgerald, Charlotte Edwards, Steven Guichard-Kenny, Chris Priest, Amanda Parry, Kribashnie Nundlall, Muna Dahir Hassan, Sarah Riaz, Catherine Sheehan, Rhian Bull, Carina Bautista, Jaime Carungcong, Nicole Kerry, Sathya Visvendra, Mini Thankachen and Elna Cifra).
-
University Hospital of North Durham; County Durham and Darlington NHS Foundation Trust (Anne Thomson, Parastoo Babakinejad and Maneesha Vatve).
-
The James Cook University Hospital, South Tees Hospitals NHS Foundation Trust (Lisa Fisher, Debbie Banks, Alison Williamson, Louise Palmer, Jessica Hollingsworth, Jackie Dodds, Alycon Walker and Lynsey Searle).
-
Birmingham City Hospital, Sandwell and West Birmingham Hospitals NHS Trust (Christine Baker, Raakhee Ramesh, Anne Rutland, Rohima Khatun, Saibal Sanyal, Sara Mirhadi, Saima Naqvi, Imogen Hughes, Laura Croxton, Sophie Musson, Erin Lynskey, Cat Shuttleworth, Niharika Bansal, Almar Hermosilla, Samia Hussain, Sue Ann Chan and Nadia Shamim).
-
Contributions of authors
Jonathan M Batchelor (https://orcid.org/0000-0002-7728-2021) (Consultant Dermatologist, Chief Investigator) contributed to the conception and design of the study, trial oversight, interpretation of the data, and drafted and critically reviewed the report.
Kim S Thomas (https://orcid.org/0000-0001-7785-7465) (Professor of Applied Dermatology Research, lead applicant) contributed to the conception and design of the study, trial oversight, interpretation of the data, and drafted and critically reviewed the report.
Perways Akram (https://orcid.org/0000-0002-2510-0345) (Technical Services Manager, testing and calibration of NB-UVB devices) contributed to the design of the study, data collection and analysis, interpretation of the data, and drafted and critically reviewed the report.
Jaskiran Azad (https://orcid.org/0000-0002-4139-5980) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
Anthony Bewley (https://orcid.org/0000-0003-1195-0290) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
Joanne R Chalmers (https://orcid.org/0000-0002-2281-7367) (Senior Research Fellow, lead for process evaluation study) contributed to the conception and design of the study, data collection and analysis, interpretation of the data, and drafted and critically reviewed the report.
Seau Tak Cheung (https://orcid.org/0000-0001-7773-5177) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
Lelia Duley (https://orcid.org/0000-0001-6721-5178) (Former Director of NCTU) contributed to the conception and design of the study and critically reviewed the report.
Viktoria Eleftheriadou (https://orcid.org/0000-0001-5776-1466) (Dermatology Specialist Registrar) contributed to the conception and design of the study, contributed to data collection and critically reviewed the report.
Robert Ellis (https://orcid.org/0000-0001-6305-2121) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
Adam Ferguson (https://orcid.org/0000-0002-7793-0850) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
Jonathan MR Goulding (https://orcid.org/0000-0003-1310-9972) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
Rachel H Haines (https://orcid.org/0000-0001-7924-0602) (Senior Trial Manager) contributed to the conception and design of the study, trial oversight, interpretation of the data, and critically reviewed the report.
Hamdi Hamad (https://orcid.org/0000-0002-5215-3792) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
John R Ingram (https://orcid.org/0000-0002-5257-1142) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
Bisola Laguda (https://orcid.org/0000-0002-7551-2663) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
Paul Leighton (https://orcid.org/0000-0001-5208-0274) (Associate Professor of Applied Health Services Research, qualitative research lead for process evaluation) contributed to the conception and design of the study, data collection and analysis, interpretation of the data, and drafted and critically reviewed the report.
Nick Levell (https://orcid.org/0000-0003-3393-8305) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
Areti Makrygeorgou (https://orcid.org/0000-0002-1589-7469) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
Garry D Meakin (https://orcid.org/0000-0001-8593-3421) (Trial Manager) contributed to the design and delivery of the study, trial oversight, interpretation of the data, and drafted and critically reviewed the report.
Adam Millington (https://orcid.org/0000-0001-9601-1924) (Specialist Pharmacist, Trial Pharmacist) contributed to the design and delivery of the study, development and packaging of the investigational medicinal product, and critically reviewed the report.
Malobi Ogboli (https://orcid.org/0000-0002-8861-6998) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
Amirtha Rajasekaran (https://orcid.org/0000-0001-6700-0708) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
Jane C Ravenscroft (https://orcid.org/0000-0002-1609-7084) (Consultant Dermatologist, Principal Investigator) contributed to the conception and design of the study, trial oversight, data collection, interpretation of the data, and critically reviewed the report.
Andrew Rogers (https://orcid.org/0000-0003-4734-9528) (Lead Interventional Medical Physics Expert, testing and calibration of NB-UVB devices) contributed to the conception and design of the study, trial oversight, data collection, interpretation of the data, and drafted and critically reviewed the report.
Tracey H Sach (https://orcid.org/0000-0002-8098-9220) (Professor of Health Economics, Senior Health Economist) contributed to the conception and design of the study, trial oversight, analysis and interpretation of the data, and drafted and critically reviewed the report.
Miriam Santer (https://orcid.org/0000-0001-7264-5260) (Associate Professor in Primary Care Research, GP) contributed to the conception and design of the study, trial oversight, interpretation of the data, and critically reviewed the report.
Julia Stainforth (https://orcid.org/0000-0002-2403-2264) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
Wei Tan (https://orcid.org/0000-0001-8037-240X) (Trial Statistician) contributed to the conception and design of the study, trial oversight, interpretation of the data, and drafted and critically reviewed the report.
Shyamal Wahie (https://orcid.org/0000-0003-2798-8429) (Consultant Dermatologist, Principal Investigator) responsible for oversight at recruiting centre and critically reviewed the report
Jennifer White (https://orcid.org/0000-0003-1945-7644) (Trial Co-ordinator) contributed to delivery of the study, trial oversight, interpretation of the data, and critically reviewed the report.
Maxine E Whitton (https://orcid.org/0000-0001-9902-7809) (Patient Partner) contributed to the conception and design of the study, trial oversight, interpretation of the data, and critically reviewed the report.
Hywel C Williams (https://orcid.org/0000-0002-5646-3093) (Consultant Dermatologist) contributed to the conception and design of the study, interpretation of the data, and critically reviewed the report.
Andrew Wright (https://orcid.org/0000-0001-5154-1227) (Consultant Dermatologist, Principal Investigator) was responsible for oversight at the recruiting centre and critically reviewed the report.
Alan A Montgomery (https://orcid.org/0000-0003-0450-1606) (Director of NCTU, Senior Trial Statistician) contributed to the conception and design of the study, trial oversight, interpretation of the data, and drafted and critically reviewed the report.
Publications
Tour SK, Thomas KS, Walker DM, Leighton P, Yong ASW, Batchelor JM. Survey and online discussion groups to develop a patient-rated outcome measure on acceptability of treatment response in vitiligo. BMC Dermatol 2014;14:10.
Batchelor JM, Tan W, Tour S, Yong A, Montgomery AA, Thomas KS. Validation of the Vitiligo Noticeability Scale: a patient-reported outcome measure of vitiligo treatment success. Br J Dermatol 2016;174:386–94.
Haines RH, Thomas KS, Montgomery AA, Ravenscroft JC, Akram P, Chalmers JR, et al. Home interventions and light therapy for the treatment of vitiligo (HI-Light Vitiligo Trial): study protocol for a randomised controlled trial. BMJ Open 2018;8:e018649.
Sach TH, Thomas KS, Batchelor JM, Akram P, Chalmers JR, Haines RH, et al. An economic evaluation of the randomised controlled trial of topical corticosteroid and home-based narrowband UVB for active and limited vitiligo (The HI-Light Trial) [published online ahead of print September 12 2020]. Br J Dermatol 2020.
Thomas KS, Batchelor JM, Akram P, Chalmers JR, Haines RH, Meakin GD, et al. Randomised controlled trial of topical corticosteroid and home-based narrowband UVB for active and limited vitiligo – results of the HI-Light Vitiligo trial [published online ahead of print October 2 2020]. Br J Dermatol 2020.
Rogers A, Akram P, Batchelor JM, Crutchley J, Grocki M, Haines RH, et al. Quality assurance and characterisation of NB-UVB devices for use at home – lessons from the Hi-Light Vitiligo trial [published online ahead of print October 27 2020]. BJD 2020.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Dell’anna ML, Picardo M. A review and a new hypothesis for non-immunological pathogenetic mechanisms in vitiligo. Pigment Cell Res 2006;19:406-11. https://doi.org/10.1111/j.1600-0749.2006.00333.x.
- Denman CJ, McCracken J, Hariharan V, Klarquist J, Oyarbide-Valencia K, Guevara-Patiño JA, et al. HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J Invest Dermatol 2008;128:2041-8. https://doi.org/10.1038/jid.2008.45.
- Le Poole IC, Das PK, van den Wijngaard RM, Bos JD, Westerhof W. Review of the etiopathomechanism of vitiligo: a convergence theory. Exp Dermatol 1993;2:145-53. https://doi.org/10.1111/j.1600-0625.1993.tb00023.x.
- Le Poole IC, Wankowicz-Kalinska A, van den Wijngaard RM, Nickoloff BJ, Das PK. Autoimmune aspects of depigmentation in vitiligo. J Investig Dermatol Symp Proc 2004;9:68-72. https://doi.org/10.1111/j.1087-0024.2004.00825.x.
- Schallreuter KU, Bahadoran P, Picardo M, Slominski A, Elassiuty YE, Kemp EH, et al. Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else?. Exp Dermatol 2008;17:139-40. https://doi.org/10.1111/j.1600-0625.2007.00666_1.x.
- Taïeb A. Intrinsic and extrinsic pathomechanisms in vitiligo. Pigment Cell Res 2000;13:41-7. https://doi.org/10.1034/j.1600-0749.13.s8.9.x.
- Spritz RA. The genetics of generalized vitiligo: autoimmune pathways and an inverse relationship with malignant melanoma. Genome Med 2010;2. https://doi.org/10.1186/gm199.
- Howitz J, Brodthagen H, Schwartz M, Thomsen K. Prevalence of vitiligo. Epidemiological survey on the Isle of Bornholm, Denmark. Arch Dermatol 1977;113:47-52. https://doi.org/10.1001/archderm.113.1.47.
- Sehgal VN, Srivastava G. Vitiligo: compendium of clinico-epidemiological features. Indian J Dermatol Venereol Leprol 2007;73:149-56. https://doi.org/10.4103/0378-6323.32708.
- Singh M, Singh G, Kanwar AJ, Belhaj MS. Clinical pattern of vitiligo in Libya. Int J Dermatol 1985;24:233-5. https://doi.org/10.1111/j.1365-4362.1985.tb05445.x.
- Das SK, Majumder PP, Chakraborty R, Majumdar TK, Haldar B. Studies on vitiligo. I. Epidemiological profile in Calcutta, India. Genet Epidemiol 1985;2:71-8. https://doi.org/10.1002/gepi.1370020107.
- Zhang Y, Cai Y, Shi M, Jiang S, Cui S, Wu Y, et al. The prevalence of vitiligo: a meta-analysis. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0163806.
- Fitzpatrick TB. Hypomelanosis. South Med J 1964;57:995-1005. https://doi.org/10.1097/00007611-196409000-00001.
- Hann SK, Park YK, Chun WH. Clinical features of vitiligo. Clin Dermatol 1997;15:891-7. https://doi.org/10.1016/S0738-081X(97)00130-2.
- Porter J, Beuf AH, Nordlund JJ, Lerner AB. Psychological reaction to chronic skin disorders: a study of patients with vitiligo. Gen Hosp Psychiatry 1979;1:73-7. https://doi.org/10.1016/0163-8343(79)90081-1.
- Ezzedine K, Grimes PE, Meurant JM, Seneschal J, Léauté-Labrèze C, Ballanger F, et al. Living with vitiligo: results from a national survey indicate differences between skin phototypes. Br J Dermatol 2015;173:607-9. https://doi.org/10.1111/bjd.13839.
- Lai YC, Yew YW, Kennedy C, Schwartz RA. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol 2017;177:708-18. https://doi.org/10.1111/bjd.15199.
- Ezzedine K, Sheth V, Rodrigues M, Eleftheriadou V, Harris JE, Hamzavi IH, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol 2015;73:883-5. https://doi.org/10.1016/j.jaad.2015.07.039.
- Gawkrodger DJ, Ormerod AD, Shaw L, Mauri-Sole I, Whitton ME, Watts MJ, et al. Guideline for the diagnosis and management of vitiligo. Br J Dermatol 2008;159:1051-76. https://doi.org/10.1111/j.1365-2133.2008.08881.x.
- Taieb A, Alomar A, Böhm M, Dell’anna ML, De Pase A, Eleftheriadou V, et al. Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol 2013;168:5-19. https://doi.org/10.1111/j.1365-2133.2012.11197.x.
- Eleftheriadou V, Whitton ME, Gawkrodger DJ, Batchelor J, Corne J, Lamb B, et al. Future research into the treatment of vitiligo: where should our priorities lie? Results of the vitiligo priority setting partnership. Br J Dermatol 2011;164:530-6. https://doi.org/10.1111/j.1365-2133.2010.10160.x.
- Whitton ME, Pinart M, Batchelor J, Lushey C, Leonardi-Bee J, Gonzalez U. Interventions for vitiligo. Cochrane Database Syst Rev 2010;1. https://doi.org/10.1002/14651858.CD003263.pub4.
- Madigan LM, Al-Jamal M, Hamzavi I. Exploring the gaps in the evidence-based application of narrowband UVB for the treatment of vitiligo. Photodermatol Photoimmunol Photomed 2016;32:66-80. https://doi.org/10.1111/phpp.12228.
- Eleftheriadou V, Thomas K, Ravenscroft J, Whitton M, Batchelor J, Williams H. Feasibility, double-blind, randomised, placebo-controlled, multi-centre trial of hand-held NB-UVB phototherapy for the treatment of vitiligo at home (HI-Light trial: Home Intervention of Light therapy). Trials 2014;15. https://doi.org/10.1186/1745-6215-15-51.
- Dong DK, Pan ZY, Zhang J, Lu XF, Jin C, Tao SQ, et al. Efficacy and safety of targeted high-intensity medium-band (304-312 nm) ultraviolet B light in pediatric vitiligo. Pediatr Dermatol 2017;34:266-70. https://doi.org/10.1111/pde.13098.
- Tien Guan ST, Theng C, Chang A. Randomized, parallel group trial comparing home-based phototherapy with institution-based 308 excimer lamp for the treatment of focal vitiligo vulgaris. J Am Acad Dermatol 2015;72:733-5. https://doi.org/10.1016/j.jaad.2014.12.026.
- Cameron H, Yule S, Dawe RS, Ibbotson SH, Moseley H, Ferguson J. Review of an established UK home phototherapy service 1998–2011: improving access to a cost-effective treatment for chronic skin disease. Public Health 2014;128:317-24. https://doi.org/10.1016/j.puhe.2014.01.011.
- Nolan BV, Yentzer BA, Feldman SR. A review of home phototherapy for psoriasis. Dermatol Online J 2010;16.
- Wind BS, Kroon MW, Beek JF, van der Veen JP, Nieuweboer-Krobotová L, Meesters AA, et al. Home vs. outpatient narrowband ultraviolet B therapy for the treatment of nonsegmental vitiligo: a retrospective questionnaire study. Br J Dermatol 2010;162:1142-4. https://doi.org/10.1111/j.1365-2133.2010.09678.x.
- Yule S, Sanyal S, Ibbotson S, Moseley H, Dawe RS. Self-administration of hospital-based narrowband ultraviolet B (TL-01) phototherapy: a feasibility study in an outpatient setting. Br J Dermatol 2013;169:464-8. https://doi.org/10.1111/bjd.12312.
- Ibbotson SH, Bilsland D, Cox NH, Dawe RS, Diffey B, Edwards C, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group Workshop Report. Br J Dermatol 2004;151:283-97. https://doi.org/10.1111/j.1365-2133.2004.06128.x.
- Hallaji Z, Ghiasi M, Eisazadeh A, Damavandi MR. Evaluation of the effect of disease duration in generalized vitiligo on its clinical response to narrowband ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed 2012;28:115-19. https://doi.org/10.1111/j.1600-0781.2012.00648.x.
- Eleftheriadou V, Thomas KS, Whitton ME, Batchelor JM, Ravenscroft JC. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol 2012;167:804-14. https://doi.org/10.1111/j.1365-2133.2012.11056.x.
- Eleftheriadou V, Thomas K, van Geel N, Hamzavi I, Lim H, Suzuki T, et al. Developing core outcome set for vitiligo clinical trials: international e-Delphi consensus. Pigment Cell Melanoma Res 2015;28:363-9. https://doi.org/10.1111/pcmr.12354.
- Eleftheriadou V, Hamzavi I, Pandya AG, Grimes P, Harris JE, Huggins RH, et al. International Initiative for Outcomes (INFO) for vitiligo: workshops with patients with vitiligo on repigmentation. Br J Dermatol 2019;180:574-9. https://doi.org/10.1111/bjd.17013.
- Haines RH, Thomas KS, Montgomery AA, Ravenscroft JC, Akram P, Chalmers JR, et al. Home interventions and light therapy for the treatment of vitiligo (HI-Light Vitiligo Trial): study protocol for a randomised controlled trial. BMJ Open 2018;8.
- Electronic Medicines Compendium . Elocon Ointment: Summary of Product Characteristics n.d. www.medicines.org.uk/emc/product/79/smpc (accessed 7 January 2020).
- Hearn RM, Kerr AC, Rahim KF, Ferguson J, Dawe RS. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol 2008;159:931-5. https://doi.org/10.1111/j.1365-2133.2008.08776.x.
- Lin TL, Wu CY, Chang YT, Juan CK, Chen CC, Yu SH, et al. Risk of skin cancer in psoriasis patients receiving long-term narrowband ultraviolet phototherapy: results from a Taiwanese population-based cohort study. Photodermatol Photoimmunol Photomed 2019;35:164-71. https://doi.org/10.1111/phpp.12443.
- Benzekri L, Gauthier Y, Hamada S, Hassam B. Clinical features and histological findings are potential indicators of activity in lesions of common vitiligo. Br J Dermatol 2013;168:265-71. https://doi.org/10.1111/bjd.12034.
- Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D. Rook’s Textbook of Dermatology. Oxford: Wiley–Blackwell; 2016.
- Batchelor JM, Tan W, Tour S, Yong A, Montgomery AA, Thomas KS. Validation of the Vitiligo Noticeability Scale: a patient-reported outcome measure of vitiligo treatment success. Br J Dermatol 2016;174:386-94. https://doi.org/10.1111/bjd.14208.
- Lilly E, Lu PD, Borovicka JH, Victorson D, Kwasny MJ, West DP, et al. Development and validation of a vitiligo-specific quality-of-life instrument (VitiQoL). J Am Acad Dermatol 2013;69:e11-8. https://doi.org/10.1016/j.jaad.2012.01.038.
- Fernandez-Peñas P, Jones-Caballero M, Espallardo O, García-Díez A. Comparison of Skindex-29, Dermatology Life Quality Index, Psoriasis Disability Index and Medical Outcome Study Short Form 36 in patients with mild to severe psoriasis. Br J Dermatol 2012;166:884-7. https://doi.org/10.1111/j.1365-2133.2012.10806.x.
- Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717-27. https://doi.org/10.1007/s11136-012-0322-4.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Ratcliffe J, Stevens K, Flynn T, Brazier J, Sawyer MG. Whose values in health? An empirical comparison of the application of adolescent and adult values for the CHU-9D and AQOL-6D in the Australian adolescent general population. Value Health 2012;15:730-6. https://doi.org/10.1016/j.jval.2012.04.005.
- Thomas KS, Batchelor JM, Akram P, Chalmers JR, Haines RH, Meakin GD, et al. Randomised controlled trial of topical corticosteroid and home-based narrowband UVB for active and limited vitiligo – results of the HI-Light Vitiligo trial [published online ahead of print October 2 2020]. Br J Dermatol 2020. https://doi.org/10.1111/bjd.19592.
- Sach TH, Thomas KS, Batchelor JM, Akram P, Chalmers JR, Haines RH, et al. An economic evaluation of the randomised controlled trial of topical corticosteroid and home-based narrowband UVB for active and limited vitiligo (The HI-Light Trial) [published online ahead of print September 12 2020]. Br J Dermatol 2020.
- McManus E, Sach T, Levell NJ. Are vitiligo treatments cost-effective? A systematic review. Br J Dermatol 2018;178:e57-e58. https://doi.org/10.1111/bjd.15881.
- Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol 2006;55:490-50. https://doi.org/10.1016/j.jaad.2006.05.048.
- Radtke MA, Schäfer I, Gajur A, Langenbruch A, Augustin M. Willingness-to-pay and quality of life in patients with vitiligo. Br J Dermatol 2009;161:134-9. https://doi.org/10.1111/j.1365-2133.2009.09091.x.
- Drummond M SM, Claxton K, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Cost Eff Resour Alloc 2013;11. https://doi.org/10.1186/1478-7547-11-6.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II – An ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- Glick HA DJ, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials (Handbooks in Health Economic Evaluation). Oxford University Press; 2014.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 7 January 2020).
- NHS Digital . Prescription Cost Analysis 2017. https://data.gov.uk/dataset/prescription-cost-analysis-england (accessed 7 January 2020).
- NHS Business Services Authority . Financial Forecasting n.d. www.nhsbsa.nhs.uk/prescription-data/understanding-our-data/financial-forecasting (accessed 3 October 2020).
- NHS improvement . NHS Schedule of Reference Costs 2017–2018 n.d. https://improvement.nhs.uk/resources/reference-costs/ (accessed 7 January 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- NHS Digital . Prescription Cost Analysis 2018. https://data.gov.uk/dataset/prescription-cost-analysis-england (accessed 3 Febuary 2020).
- Stevens K. Valuation of the Child Health Utility 9D Index. PharmacoEconomics 2012;30:729-47. https://doi.org/10.2165/11599120-000000000-00000.
- Stevens KJ, Brazier JE, McKenna SP, Doward LC, Cork MJ. The development of a preference-based measure of health in children with atopic dermatitis. Br J Dermatol 2005;153:372-7. https://doi.org/10.1111/j.1365-2133.2005.06736.x.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- National Institute for Health and Care Excellence (NICE) . Position Statement on the Use of the EQ-5D-5L Valuation Set 2018. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 7 January 2020).
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Devlin N, Shah K, Feng Y, B. M, van Hout B. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England. London: Office of Health Economics; 2016.
- Devlin N, Shah K, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Ritchie J, Spencer L, Bryman A, Burgess R. Analyzing Qualitative Data. London: Taylor & Francis; 1994.
- Tour SK, Thomas KS, Walker DM, Leighton P, Yong AS, Batchelor JM. Survey and online discussion groups to develop a patient-rated outcome measure on acceptability of treatment response in vitiligo. BMC Dermatol 2014;14. https://doi.org/10.1186/1471-5945-14-10.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. Res Involv Engagem 2017;3. https://doi.org/10.1186/s40900-017-0062-2.
- Telford R, Boote JD, Cooper CL. What does it mean to involve consumers successfully in NHS research? A consensus study. Health Expect 2004;7:209-20. https://doi.org/10.1111/j.1369-7625.2004.00278.x.
- INVOLVE . What Is Public Involvement in Research? n.d. www.invo.org.uk/find-out-more/what-is-public-involvement-in-research-2/ (accessed 7 January 2020).
- Rogers A, Akram P, Batchelor JM, Crutchley J, Grocki M, Haines RH, et al. Quality assurance and characterisation of NB-UVB devices for use at home – lessons from the Hi-Light Vitiligo trial [published online ahead of print October 27 2020]. BJD 2020.
- Koek MB, Buskens E, van Weelden H, Steegmans PH, Bruijnzeel-Koomen CA, Sigurdsson V. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ 2009;338. https://doi.org/10.1136/bmj.b1542.
- Mysore V. Targeted phototherapy. Indian J Dermatol Venereol Leprol 2009;75:119-25. https://doi.org/10.4103/0378-6323.48655.
- Eleftheriadou V, Ezzedine K. Portable home phototherapy for vitiligo. Clin Dermatol 2016;34:603-6. https://doi.org/10.1016/j.clindermatol.2016.05.010.
- Bae JM, Jung HM, Hong BY, Lee JH, Choi WJ, Lee JH, et al. Phototherapy for vitiligo: a systematic review and meta-analysis. JAMA Dermatol 2017;153:666-74. https://doi.org/10.1001/jamadermatol.2017.0002.
- Lim-Ong M, Leveriza R, Ong B. M. F. Comparison between narrow-band UVB with topical corticosteroid and narrow-band UVB with placebo in the treatment of vitiligo: a randomized controlled trial. J Phillipine Dermatol Soc 2005;14:17-22.
- Momen S, Sarkany R. Are very high cumulative doses of narrowband UVB safe in vitiligo?. Photodermatol Photoimmunol Photomed 2017;33:220-1. https://doi.org/10.1111/phpp.12306.
- Arora CJ, Rafiq M, Shumack S, Gupta M. The efficacy and safety of tacrolimus as mono- and adjunctive therapy for vitiligo: a systematic review of randomised clinical trials. Australas J Dermatol 2020;61:e1-e9. https://doi.org/10.1111/ajd.13096.
- Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: a survey of members of The Vitiligo Society. Clin Exp Dermatol 2010;35:736-9. https://doi.org/10.1111/j.1365-2230.2009.03765.x.
- Teasdale E, Muller I, Abdullah Sani A, Thomas KS, Stuart B, Santer M. Views and experiences of seeking information and help for vitiligo: a qualitative study of written accounts. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-018652.
- National Institute for Health and Care Excellence (NICE) . Clinical Knowledge Summaries (CKS) Vitiligo – Scenario: Management of Vitiligo 2016. https://cks.nice.org.uk/vitiligo#!scenarioRecommendation:2 (accessed 7 January 2020).
Appendix 1 Full list of authors
Jonathan M Batchelor,1† Kim S Thomas,1*† Perways Akram,2 Jaskiran Azad,3 Anthony Bewley,4 Joanne R Chalmers,1 Seau Tak Cheung,5 Lelia Duley,6 Viktoria Eleftheriadou,7 Robert Ellis,8,9 Adam Ferguson,10 Jonathan MR Goulding,11 Rachel H Haines,6 Hamdi Hamad,12 John R Ingram,13 Bisola Laguda,14 Paul Leighton,1 Nick Levell,15 Areti Makrygeorgou,16 Garry D Meakin,6 Adam Millington,17 Malobi Ogboli,18 Amirtha Rajasekaran,19 Jane C Ravenscroft,20 Andrew Rogers,2 Tracey H Sach,21 Miriam Santer,5 Julia Stainforth,22 Wei Tan,6 Shyamal Wahie,23 Jennifer White,6 Maxine E Whitton,1 Hywel C Williams,1 Andrew Wright24 and Alan A Montgomery6
Appendix 2 Addendum to HI-Light Vitiligo statistical analysis plan final version 1.0 dated 15 October 2018
Changes from protocol v5.0
Additional points of clarification are outlined below. These amendments provide additional information on how the outcomes were reported and analysed, but do not substantially change the outcomes as defined prior to database lock.
| Protocol | Statistical analysis plan | Justification |
|---|---|---|
| Digital image assessment of the target patch at 9 months by independent assessors is described as a secondary analysis of the primary outcome | The digital image assessment of the target patch at 9 months by independent assessors will be reported as an additional secondary outcome | More appropriate as a secondary outcome as based on new data from a different source to the primary outcome |
| Maintenance of treatment response will be reported for each of the three body regions | Maintenance of treatment response will be reported for the target patch only | Because of lower than expected follow-up rates, there are insufficient data to present the maintenance of treatment response at 12, 15, 18 and 21 months for each body region |
| Patient-reported treatment success by body region will be assessed at 3, 6 and 9 months | Patient-reported treatment success by body region will be assessed at 9 months only. Treatment success at 3 and 6 months will be presented descriptively | Minimise risk of type I errors from multiple hypothesis testing |
| Percentage repigmentation will be assessed at 3, 6 and 9 months | Percentage repigmentation will be assessed using digital image assessment by a blinded clinical assessor at 9 months. Where available, data from nurse assessments at 9 months will be used for missing blinded assessor data. Assessments carried out by nurses at 3 and 6 months will be presented descriptively | Minimise risk of type I errors from multiple hypothesis testing |
| Participant-reported treatment burden will be presented at 3, 6 and 9 months based on average duration and number of treatment sessions and adherence with the treatment schedule. To be presented for light therapy and topical corticosteroid therapy separately | Treatment burden will focus on the burden of light therapy and will be presented alongside adherence data as a process measure | Calculating the duration of light treatment based on placebo devices is not appropriate as the dosing schedule would always increase as no erythema will have been experienced during the dosing schedule, and so treatment times are likely to be longer |
| Average session duration for those who received an active light device will be reported at 3, 6 and 9 months and the proportion of participants who reported difficulties with treatment (including time burden) with be presented over 9 months | Data regarding the duration of treatment sessions were not collected for TCS treatment as the time required to apply ointment is minimal | |
| For TCS, average time per session will not be reported as the time required for this was felt to be minimal. However, treatment burden for those receiving active TCS will be presented for those who reported experiencing difficulties with treatment (including time burden) presented over 9 months |
Appendix 3 Participant-reported Vitiligo Noticeability Scale treatment success at all assessed patches at 3 and 6 months
| Treatment success | TCS, n/N (%) | NB-UVB, n/N (%) | Combination, n/N (%) |
|---|---|---|---|
| Head and neck | |||
| At 3 months | 13/73 (18) | 15/71 (21) | 19/73 (26) |
| At 6 months | 15/62 (24) | 15/57 (26) | 29/68 (43) |
| Hands and feet | |||
| At 3 months | 7/94 (7) | 10/89 (11) | 2/87 (2) |
| At 6 months | 10/81 (12) | 11/73 (15) | 10/74 (14) |
| Rest of body | |||
| At 3 months | 7/106 (7) | 8/99 (8) | 11/104 (11) |
| At 6 months | 11/91 (12) | 13/86 (15) | 25/91 (27) |
Appendix 4 Participant-assessed onset of treatment response
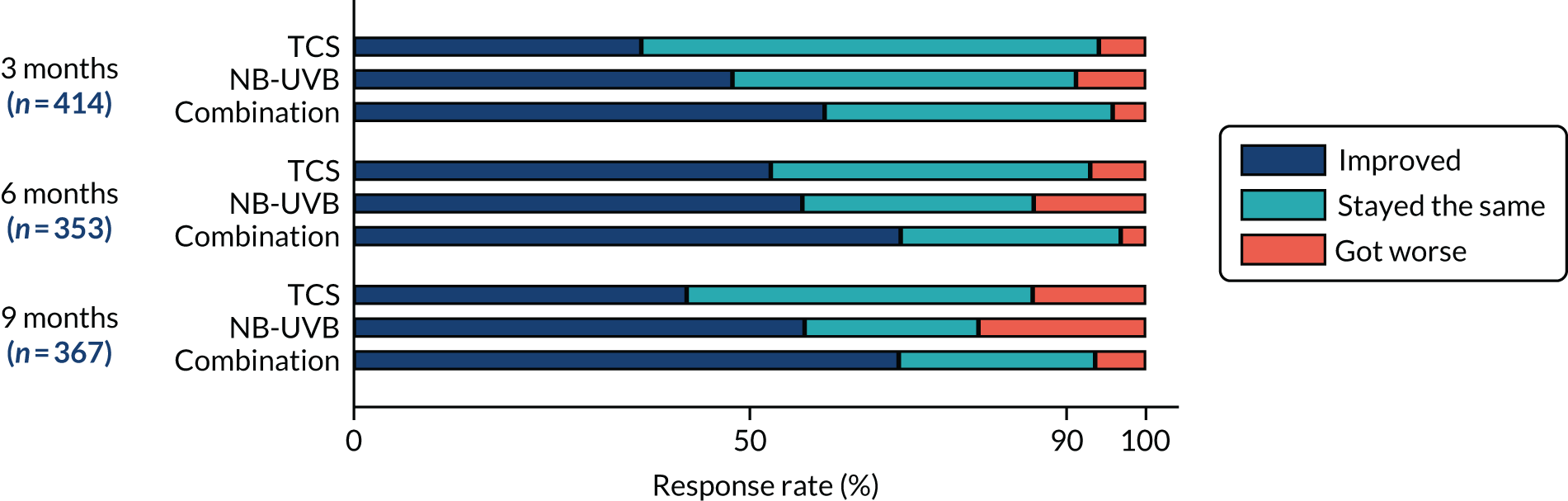
Appendix 5 Target patch percentage repigmentation assessed by nurse at 3, 6 and 9 months
| Repigmentation of target patch | TCS, n (%) | NB-UVB, n (%) | Combination, n (%) |
|---|---|---|---|
| At 3 months | |||
| 0–24% | 119 (89) | 110 (81) | 111 (78) |
| 25–49% | 7 (5) | 13 (10) | 15 (10) |
| 50–74% | 4 (3) | 7 (5) | 11 (8) |
| 75–100% | 4 (3) | 6 (4) | 6 (4) |
| At 6 months | |||
| 0–24% | 91 (79) | 73 (65) | 75 (60) |
| 25–49% | 6 (5) | 24 (21) | 17 (14) |
| 50–74% | 10 (9) | 10 (9) | 19 (15) |
| 75–100% | 8 (7) | 6 (5) | 14 (11) |
| At 9 months | |||
| 0–24% | 83 (72) | 72 (63) | 66 (55) |
| 25–49% | 16 (14) | 14 (12) | 18 (15) |
| 50–74% | 6 (5) | 18 (16) | 14 (12) |
| 75–100% | 10 (9) | 11 (10) | 21 (18) |
Appendix 6 Summary of related adverse events by preferred term name in MedDRA coding
| Adverse event | TCS (n = 33) | NB-UVB (n = 69) | Combination (n = 104) | Total (n = 206) |
|---|---|---|---|---|
| Acne | 0 | 1 | 2 | 3 |
| Application site pruritus | 0 | 2 | 0 | 2 |
| Blister | 0 | 4 | 2 | 6 |
| Contusion | 1 | 0 | 2 | 3 |
| Dry skin | 3 | 0 | 5 | 8 |
| Erythema | 3 | 29 | 45 | 77 |
| Folliculitis | 0 | 0 | 1 | 1 |
| Haemangioma | 0 | 1 | 0 | 1 |
| Hair growth abnormal | 5 | 2 | 4 | 11 |
| Herpes virus infection | 0 | 2 | 0 | 2 |
| Herpes zoster infection | 0 | 0 | 1 | 1 |
| Koebner phenomenon | 0 | 1 | 0 | 1 |
| Lip dry | 0 | 0 | 1 | 1 |
| Lip pain | 0 | 0 | 1 | 1 |
| Melanocytic naevus | 0 | 1 | 0 | 1 |
| Miliaria | 0 | 0 | 2 | 2 |
| Night sweats | 0 | 1 | 0 | 1 |
| Oral discomfort | 0 | 0 | 1 | 1 |
| Oral herpes | 1 | 4 | 6 | 11 |
| Pain in extremity | 0 | 0 | 1 | 1 |
| Pain in jaw | 1 | 0 | 0 | 1 |
| Pain of skin | 1 | 0 | 0 | 1 |
| Paraesthesia | 0 | 0 | 1 | 1 |
| Polymorphic light eruption | 0 | 1 | 0 | 1 |
| Pruritus | 3 | 7 | 10 | 20 |
| Pustular psoriasis | 0 | 0 | 1 | 1 |
| Rash | 6 | 3 | 4 | 13 |
| Rash pruritic | 1 | 2 | 6 | 9 |
| Rhinalgia | 1 | 0 | 0 | 1 |
| Skin atrophy | 5 | 1 | 1 | 7 |
| Skin depigmentation | 1 | 0 | 0 | 1 |
| Skin exfoliation | 0 | 5 | 0 | 5 |
| Skin hyperpigmentation | 0 | 0 | 2 | 2 |
| Skin papilloma | 0 | 0 | 1 | 1 |
| Skin striae | 1 | 0 | 0 | 1 |
| Spider vein | 0 | 1 | 3 | 4 |
| Telangiectasia | 0 | 0 | 1 | 1 |
| Vitiligo | 0 | 1 | 0 | 1 |
Appendix 7 Utility and quality-adjusted life-years for participants aged ≥ 11 years (available case data, secondary cost–utility analysis)
| Utility value | NB-UVB (N = 148) | TCS (N = 155) | Mean difference (95% CI) | Combination (N = 153) | TCS (N = 155) | Mean difference (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD (n) | Mean | SD (n) | Mean | SD (n) | Mean | SD (n) | |||
| Devlin et al.68 utility value | ||||||||||
| Secondary outcomes | ||||||||||
| EQ-5D-5L at baseline | 0.9300 | 0.1346 (139) | 0.9456 | 0.0805 (151) | –0.0156 (–0.0410 to 0.0098) | 0.9247 | 0.1381 (147) | 0.9456 | 0.0805 (151) | –0.0209 (–0.0466 to 0.0048) |
| EQ-5D-5L at 9 months | 0.9527 | 0.1108 (89) | 0.9231 | 0.1240 (97) | 0.0295 (–0.0046 to 0.0637) | 0.9446 | 0.1057 (97) | 0.9231 | 0.1240 (97) | 0.0215 (–0.0111 to 0.0540) |
| QALYs at 9 months | 0.7082 | 0.0699 (89) | 0.6989 | 0.0694 (97) | 0.0093 (–0.0109 to 0.0295) | 0.7064 | 0.0757 (96) | 0.6989 | 0.0694 (97) | 0.0075 (–0.0131 to 0.0282) |
| Devlin et al.69 utility value set | ||||||||||
| Secondary outcomes | ||||||||||
| EQ-5D-5L at baseline | 0.9299 | 0.1374 (139) | 0.9461 | 0.0800 (151) | –0.0162 (–0.0420 to 0.0095) | 0.9250 | 0.1399 (147) | 0.9461 | 0.0800 (151) | –0.0211 (–0.0470 to 0.0048) |
| EQ-5D-5L at 9 months | 0.9537 | 0.1101 (89) | 0.9239 | 0.1245 (97) | 0.0298 (–0.0044 to 0.0639) | 0.9448 | 0.1059 (98) | 0.9239 | 0.1245 (97) | –0.0209 (–0.0118 to 0.0535) |
| QALYs at 9 months | 0.7086 | 0.0696 (89) | 0.6996 | 0.0690 (97) | 0.0090 (–0.0110 to 0.0291) | 0.7068 | 0.0754 (96) | 0.6996 | 0.0690 (97) | 0.0073 (–0.0132 to 0.0278) |
Appendix 8 Survey of recruiting centre staff
Introductory page
In anticipation of the HI-Light Vitiligo trial results day we would like to ask you some questions about your experience of providing hand-held phototherapy to the HI-Light Vitiligo trial participants.
We are particularly interested to hear about any insight that you would like to share with those that might be thinking about providing a similar therapy to their patients.
Context to these questions
Existing evidence points to the benefits of phototherapy (in combination with other treatments) in the management of vitiligo; existing evidence points to the potential for home-based phototherapy using hand-held devices.
Some consideration of the clinical aspects of this (dosing, etc.) is manifest in the literature, but little has been said about service organisation and how best to delivery this type of therapy.
The NHS is a distinct context for delivering this type of service.
About you
Are you a doctor/a nurse/a specialist dermatology nurse/other?
Prior to the HI-Light Vitiligo trial had you been involved in any form of phototherapy service? Yes/No.
What was your role in the HI-Light Vitiligo trial?
Are you already aware of the HI-Light Vitiligo trial results? Yes/No.
Question 1
Do you agree that home-based phototherapy should be made more widely available for vitiligo patients?
Strongly agree/agree/neutral/disagree/strongly disagree.
We appreciate that the HI-Light Vitiligo trial results will ultimately inform this decision, but at this point we would welcome your intuitive response.
Can you explain your response? What is it about home-based phototherapy (and your experiences as part of the HI-Light Vitiligo trial) that encourages, or discourages, you about its use?
Free-text response box . . .
Question 2
Do you think that home-based phototherapy is appropriate for all vitiligo patients?
All patients/most patients/some patients/few patients/no patients.
Could you explain your answer? What factors might influence whether a patient is appropriate for home-based phototherapy?
We would be interested to hear if you think that there are types of vitiligo presentation which are more, or less, appropriate for home-based phototherapy.
We would also be interested to hear if you think that lifestyle/personality/personal circumstance are important in this decision.
Free-text response box . . .
Question 3
Do you agree that delivering a home-based phototherapy service is feasible in the NHS?
Strongly agree/agree/neutral/disagree/strongly disagree.
We appreciate that ultimately this is a decision that commissioners will make, but we would invite your comment about the practical challenges that this might involve.
What were the difficulties and challenges that you found in delivering the HI-Light Vitiligo trial? Do you have any suggestions that would make a home-based phototherapy service easier to deliver or manage?
Free-text response box . . .
Question 4
Do you think that participants (and their families) found hand-held phototherapy easy to do at home?
Very easy/easy/neutral/difficult/very difficult.
We would be interested to hear about any difficulties or challenges that participants experienced with the hand-held phototherapy (and/or steroid cream).
We would be interested to hear about any strategies or techniques that participants used to manage, and about the nature of support that you offered them in this.
Free-text response box . . .
Question 5
How important do you think it is for any hand-held phototherapy devices to be provided and maintained by an NHS provider?
Can you explain why you think this?
Do you have any thoughts about patients purchasing their own hand-held phototherapy unit? Via the NHS? Via a commercial provider?
Free-text response box . . .
Question 6
Do you have any other comments, or recommendations, that would help others to establish and run a home-based phototherapy service for vitiligo?
Do you have any top-tips that you would like to share?
Free-text response box . . .
List of abbreviations
- AE
- adverse event
- CEBD
- Centre for Evidence Based Dermatology
- CHU-9D
- Child Health Utility 9D
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- DVD
- digital versatile disc
- EAC
- equivalent annual cost
- eCRF
- electronic case report form
- EQ-5D-5L
- EuroQol 5-Dimensions, five-level version
- GBP
- Great British pounds
- GP
- general practitioner
- HI-Light Vitiligo
- Home Interventions and Light therapy for the treatment of vitiligo
- HTA
- Health Technology Assessment
- ITT
- intention to treat
- MED
- minimum erythemal dose
- NB-UVB
- narrowband ultraviolet B light
- NCTU
- Nottingham Clinical Trials Unit
- NIC
- net ingredient cost
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NNT
- number needed to treat
- PPI
- patient and public involvement
- PROM
- patient-reported outcome measure
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- TCS
- topical corticosteroid
- TSC
- Trial Steering Committee
- UK DCTN
- UK Dermatology Clinical Trials Network
- UVA
- ultraviolet A light
- UVB
- ultraviolet B light
- VitiQOL
- Vitiligo-Specific Health-Related Quality-of-Life Instrument
- VNS
- Vitiligo Noticeability Scale
