Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/174/24. The contractual start date was in January 2018. The draft report began editorial review in April 2020 and was accepted for publication in July 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Fordham et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Cognitive–behavioural therapy (CBT) is an amalgam of interventions that emerged from cognitive and behavioural psychological models. It aims to improve patients’ quality of life by changing their maladaptive cognitions that maintain problematic symptoms. The basic principles of CBT are presented in Figure 1.
FIGURE 1.
Roth and Pilling’s1 generic therapeutic competencies: basic principles of CBT. © Crown copyright 2007. Contains public sector information licensed under the Open Government Licence v3.0.

Cognitive–behavioural therapy can be delivered in different formats. High-intensity CBT has been defined as formal CBT with a trained health professional, predominantly delivered face to face, in an individual or group format. 1 Low-intensity CBT focuses on self-help and can be delivered by health professionals with brief CBT training (non-psychologists) and via several platforms (internet, telephone, paper based or face to face). 1 The distinction can become less clear in some forms of CBT in which high-intensity therapy is combined with low-intensity self-help methods.
A previous overview of CBT reviews,2 conducted in 2012, identified 296 reviews. However, only 11 of these synthesised randomised controlled trial (RCT) evidence; therefore, the conclusions are subject to increased bias. 2 Since this overview,2 there have been many more trials and reviews. There has also been more guidance for conducting and reporting high-quality trials and reviews. 3–6 The existing CBT trial and review evidence base is large, yet the majority of expenditure on psychological treatment research remains focused on CBT effectiveness. 7 Some researchers have argued that CBT is in a ‘virtuous circle: money pours into research, evidence accumulates, more financial support is given to . . . [CBT] . . . and other forms of psychotherapy are excluded’8 (Peter Fonagy, University College London) (Copyright © The Economist Newspaper Limited 2014. Reproduced with permission). In providing a comprehensive cross-sectional map of the best-quality available evidence, we can provide an indication of where CBT has an evidence base to support its effectiveness and where future research resources would be best directed.
Cognitive–behavioural therapy interventions share an underlying process, common therapeutic style and employ similar techniques (e.g. guided discovery), yet the condition-, population- and context-specific protocols can look very different from one another. Because of the commonality of CBT interventions across conditions, it is plausible that CBT can produce a general effect across conditions. However, to date, to our knowledge, there is no overarching estimate regarding the consistency of CBT’s effect across different condition categories. We propose to generate such an effect by performing panoramic meta-analyses (PMAs) on the effect estimates generated in each condition.
The concept and methodology of systematically reviewing systematic reviews is established, and includes quality and reporting guidelines. 9,10 However, the existing reviews of systematic reviews are typically undertaken to compare multiple interventions in one condition. 11,12 For this overview, we are interested in examining one intervention (CBT) across multiple conditions. In this overview, the classification of a ‘condition’ was based on the World Health Organization’s (WHO’s) International Classification of Diseases, Eleventh Revision (ICD-11). 13 The WHO must consider cultural, religious and political differences that can influence condition categorisation. In using this internationally recognised tool, we developed research findings that are meaningful to a global audience. In looking across conditions, we examined the effect of CBT across all populations, with CBT compared with all types of comparator. Our primary outcome is health-related quality of life (HRQoL), and we include three secondary outcomes (depression, anxiety and pain) that would contribute to an individual’s quality-of-life rating.
We employ PMA, which is an emerging methodology to synthesise systematic reviews across conditions. 14,15 When methodological assumptions are met, it allows pooling of effect sizes across conditions. This produces an average effect across conditions, which, because of enlarged sample sizes, is estimated with more precision than within-condition pooled estimates.
Chapter 2 Aim and objectives
Aim
The overarching aim of the overview was to map the existing CBT systematic review evidence base and to examine if CBT produced an across-condition, general effect on HRQoL.
Objectives
To answer these research aims, the following steps were undertaken.
Step 1: data mapping
We identified all available systematic reviews of CBT and mapped these according to:
-
conditions (ICD-11 category, severity)
-
populations (age, sex, ethnicity, countries where the trials were conducted)
-
context (delivery format, care setting, intervention timing)
-
quality of the reviews.
The mapping exercise identified where there is/is not a high- or low-quality systematic review or meta-analysis of RCTs examining the effectiveness of CBT.
Step 2: panoramic meta-analysis
Reviews from step 1 that had sufficient quantitative data were entered into a PMA for the primary outcome of HRQoL and for the secondary outcomes of depression, anxiety and pain. Sensitivity analyses based on quality were performed.
If across-condition heterogeneity was not considerable, an across-condition general effect was generated for each outcome. Subgroup analyses based on age, CBT intensity, duration of follow-up and type of comparators were undertaken. We checked every within-condition and subgroup analysis to examine if there was evidence of inconsistency with the overall effect estimate.
In Chapter 6, we explore the extent to which the existing evidence base could be used to guide treatment, commissioning and research investment decisions. The aim of the patient and public input into the overview was to ensure that the overview produced work that remained rooted in the overall aim: to improve health for patients receiving CBT.
Chapter 3 Review methods
The methods for the mapping stage of the overview are presented first, followed by the methods for the PMA. The protocol was registered with PROSPERO, the international prospective register of systematic reviews (number CRD42017078690), and published open access. 16
Mapping
Inclusion and exclusion criteria for the mapping
Types of reviews
We included all reviews that reported RCTs if they met four of the five methodological criteria outlined by the Centre for Reviews and Dissemination (CRD) at the University of York, as part of the Database of Abstracts of Reviews of Effects (DARE). 17 DARE was consulted for its guidance in the application of the criteria:
-
Were inclusion/exclusion criteria reported?
-
Was the search adequate? (Databases stated, more than one database searched or one database plus checking references, hand-searching, contact with researchers, citation searching, internet searching.)
-
Were the included studies synthesised?
-
Was the quality of the included studies assessed?
-
Are sufficient details about the individual studies presented? (Details on the population/setting, intervention and a result for each included study.)
We included reviews of RCTs comparing CBT with an active or non-active comparator. Reviews containing randomised and non-randomised studies were included only if RCT data were summarised separately. We excluded reviews based on any other study designs (e.g. quasi-randomised, non-randomised).
Type of health condition
The ICD-11 classifies mental and physical diseases, disorders, injuries and other related health problems in a comprehensive and hierarchical fashion, and is used as a standard for both clinical and research purposes. 13 The term condition will be used throughout this report to represent diseases, disorders and injuries. Participants with any conditions recognised by the ICD-11 or its nominal categorisation, and of any severity, were included. Non-health-related problems, such as procrastination, were excluded.
For physical conditions, we categorised reviews under the primary ICD-11 codes. For mental conditions, we used the secondary level of ICD-11 codes listed underneath the primary code of 06 mental, behavioural or neurodevelopmental disorders. A review was categorised according to its primary aims. For example, if a review examined the effectiveness of CBT to improve quality of life for people living with diabetes, then 05: Endocrine diseases was the condition category. However, if a review examined the effectiveness of CBT for improving depression in people living with diabetes, then the review was classified as 6A60-80 mood disorders, with comorbid 05 endocrine diseases. Box 1 shows all of the primary and secondary codes that could be considered in grouping reviews together.
-
01 Certain infectious or parasitic diseases.
-
02 Neoplasms.
-
03 Diseases of the blood.
-
04 Diseases of the immune system.
-
05 Endocrine, nutritional or metabolic diseases.
-
07 Sleep–wake disorders.
-
08 Diseases of the nervous system.
-
09 Diseases of the visual system.
-
10 Diseases of the ear or mastoid process.
-
11 Diseases of the circulatory system.
-
12 Diseases of the respiratory system.
-
13 Diseases of the digestive system.
-
14 Diseases of the skin.
-
15 Diseases of the musculoskeletal system.
-
16 Diseases of the genitourinary system.
-
17 Conditions related to sexual health.
-
18 Pregnancy, childbirth or the puerperium.
-
19 Certain conditions originating in the perinatal period.
-
20 Developmental abnormalities.
-
21 Symptoms and signs not elsewhere classified.
-
6A00-06: neurodevelopmental disorders.
-
6A20-25: schizophrenia or other primary psychotic disorders.
-
6A40-41: catatonia.
-
6A60-80: mood disorders.
-
6B00-06: anxiety or fear-related disorders.
-
6B20-25: obsessive–compulsive disorders.
-
6B40-45: disorders specifically associated with stress.
-
6B60-66: dissociative disorders.
-
6B80-85: feeding or eating disorders.
-
6C00-01: elimination disorders.
-
6C20-21: disorders of bodily distress.
-
6C40-51: disorders due to substance use or addictive behaviours.
-
6C70-73: impulse control disorders.
-
6C90-91: disruptive behaviour or dissocial disorder.
-
6D10-11: personality disorders and related traits.
-
6D30-36: paraphilic disorders.
-
6D50-51: factitious disorders.
-
6D70-72: neurocognitive disorders.
-
6E20-21: mental or behavioural disorders associated with pregnancy, childbirth or the puerperium.
-
6E40: psychological or behavioural factors not elsewhere classified.
Types of participants
We included participants of any age [children/adolescents (aged < 18 years), adults (aged 18–65 years) and older adults (aged > 65 years)], either sex and any ethnicity.
Types of health-care setting
We included reviews of RCTs that were conducted in any setting [e.g. primary care, secondary care, school/university, institutional (residential care)] and in any country.
Types of delivery timing
We included reviews of RCTs in which CBT was delivered preventatively, as a standard responsive care or as a relapse prevention.
Types of interventions
We included only reviews that evaluated CBT. Interventions were accepted as CBT when authors explicitly stated so in the title, abstract or keywords, or when the review defined the intervention as including at least one cognitive and one behavioural element.
If a trial intervention combined CBT with another therapy and the other therapy was used as a comparator condition (e.g. CBT plus pharmacotherapy compared with pharmacotherapy), then we included these trials. If a trial combined CBT with another therapy and this was compared with another type of comparator [e.g. CBT plus pharmacotherapy compared with wait-list control (WLC)], then we excluded these reviews because we could not extract the isolated effects of CBT.
All modes of CBT delivery were included and categorised into high or low intensity, based on the definitions by Roth and Pilling. 1 High-intensity CBT was defined as face-to-face, individual or group therapy, delivered by a trained CBT therapist. Low-intensity was CBT delivered via media (internet, written, telephone), or was when face-to-face, individual or group CBT was administered by a non-CBT therapist (paraprofessional or layperson). If the review did not report the intensity of the intervention, it was assumed to be high-intensity CBT. We excluded all non-CBTs: cognitive therapy, behavioural therapy, third-wave CBT (e.g. acceptance and commitment therapy, mindfulness therapy), motivational interviewing, stress inoculation therapy, problem-solving therapy and stress management therapy.
Types of comparators
We included reviews that compared CBT with one of the following: (1) a non-CBT-based active comparator (e.g. other psychological therapy, pharmacotherapy), (2) a non-active comparator [e.g. placebo, WLC, treatment as usual (TAU), standard care, no treatment] or (3) a CBT-based active comparator of different intensity [e.g. face-to-face CBT (high intensity) compared with internet-based CBT (low intensity)]. We excluded reviews that compared variations of high-intensity (e.g. group CBT compared with individual CBT) or low-intensity CBT (internet CBT compared with bibliotherapy CBT).
Types of outcomes
We included reviews if they reported data on at least one of the following outcomes: HRQoL, anxiety, depression or a condition-specific outcome (e.g. pain).
Length of follow-up
We included reviews with post-treatment, short-term (< 12 months) or long-term (≥ 12 months) follow-up data. If both short- and long-term follow-up data were reported, the synthesis of only the longest follow-up time point was included.
Search methods for identification of systematic reviews
We followed the principles of the Cochrane Handbook for Systematic Reviews of Interventions3 and recommendations for conducting overviews of systematic reviews9 to identify systematic reviews for the overview.
Information sources
The DARE (up to March 2015), Cochrane Database of Systematic Reviews, MEDLINE (via Ovid), EMBASE (via Ovid), PsycINFO (via Ovid), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via EBSCOhost), Child Development & Adolescent Studies (via EBSCOhost) and OpenGrey databases were searched on 25–27 April 2018 to identify relevant systematic reviews published between 1992 and 2018. An updated search was run on all the above databases on 30 January 2019, covering the period from April 2018 to 30 January 2019, excluding DARE, which is no longer updated. Owing to the volume of material being processed and the time constraints associated with this process, the reference lists of included reviews were not hand-searched for additional reviews. We did not contact authors for additional information to confirm inclusion/exclusion.
Search strategy
Comprehensive search strategies for each of the eight databases were designed by a senior research information specialist (SK). Each search strategy was developed iteratively, and a sensitivity check was performed in each database for the ability of each strategy to retrieve 36 key known papers (where indexed) that had been identified a priori (see Appendix 1). The included search terms were identified from these reviews and their associated database indexing terms, and with input from the expert consultation group (ECG). The search strategies utilised a combination of free text and controlled vocabulary search terms covering variations of ‘CBT’ searched in the title, abstract or keyword fields, and were combined with validated study-type filters for ‘systematic review’. The Scottish Intercollegiate Guidelines Network systematic review search filters available on the InterTASC (Technology Appraisal Support Collaboration) Information Specialists’ Sub-Group website18 was used to search the MEDLINE, EMBASE and CINAHL databases. The McMaster University Health Information Research Unit systematic review filter19 was modified and used in the PsycINFO search. The full search strategies for all the databases can be found in Appendix 2.
Restrictions
The scoping work identified that the earliest published review of CBT is from 1992. 20 Therefore, we restricted our search to reviews published since 1992. The search was not restricted in terms of language, although we subsequently excluded non-English-language reviews (see Protocol revisions).
Data management
The database search results were exported into Endnote [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] for deduplication and then exported into Covidence (Melbourne, VIC, Australia), a Cochrane technology platform designed and recommended for systematic review management,3 and a final deduplication check was performed. The full texts of reviews shortlisted for full-text analysis were also uploaded to and screened in Covidence. Data extraction was performed using Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA).
Study selection
Two review authors (TS and BF) independently screened the titles and abstracts of all the references identified by the search strategy. The full texts of the selected reviews were obtained via online resources or through Bodleian Libraries. Reviews were screened for eligibility by two review authors (KE and TS), using the criteria stipulated in Inclusion and exclusion criteria for the mapping; disagreements were resolved by consensus or deliberation with a third reviewer (BF).
Data extraction
A bespoke data extraction form was developed. This form was piloted by two reviewers (BF and TS) using the sensitivity check papers recommended by the ECG (see Appendix 1).
We extracted the following information:
-
review identification details – author, date of publication, aim, number of included RCTs and number of participants, risk-of-bias tool used
-
participant details – primary condition (that which the intervention is primarily aiming to treat) and comorbid conditions, severity, age category (children and adolescents aged < 18 years, adults aged 18–65 years and older adults aged > 65 years), sex, ethnicity
-
setting – from where participants were recruited, treatment timing (e.g. preventative, early, standard, relapse prevention) and countries where the individual RCTs were conducted
-
intervention details – CBT intensity, and, if available, number, duration and frequency of sessions and intervention content description
-
comparator details – description of comparator interventions (active: CBT or non-CBT interventions; non-active: WLC, TAU, no treatment)
-
outcomes: what outcome was measured, follow-up period (short or long), the number of RCTs and number of participants summarised for this outcome, and whether or not a meta-analysis was conducted.
No numerical data were extracted at this stage. If a review had looked for one of our relevant outcomes but did not find any CBT RCTs, this was recorded. When available, we extracted information on patients’ perspectives of CBT, for example patient satisfaction ratings, levels of adherence, dropout rates and any reported adverse events. When available, we extracted information on patient satisfaction, acceptability, adverse events and economic evaluations. An example data extraction form can be found in Appendix 3.
Quality assessment of reviews
The methodological quality of all the included systematic reviews was independently assessed by two review authors (KE, TS or BC) using the A MeaSurement Tool to Assess systematic Reviews (AMSTAR)-2 checklist. 4 This checklist assesses the quality of the review design, analysis and reporting, but does not account for the risk of bias of the included RCTs. Because of the overview design (i.e. the review was conducted at the review level), it was outside the scope of this study to return to the RCT level to perform risk-of-bias assessments. Discrepancies between reviewers were adjudicated by another reviewer (BF). We used the online checklist21 (see Appendix 4) to complete the 16 items scored either as ‘yes’, ‘no’ or ‘partial yes.’ This automatically generated a review rating of ‘critically low’, ‘low’, ‘moderate’ or ‘high’ quality. We stratified the reviews based on their AMSTAR-2 score into higher-quality reviews (those rated ‘high’ or ‘moderate’ on the AMSTAR-2 checklist) and lower-quality reviews (those rated as ‘low’ or ‘critically low’) (Beverly Shea, University of Ottawa, 25 March 2019, personal communication).
We calculated the agreement on the overall quality rating between the two main reviewers (KE and TS) using weighted kappa (κw) (interpreted as < 0.20, poor; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, good; and 0.81–1.00, very good). 22
Independent, double data extraction was undertaken by two reviewers (KE, TS or BC). All data extraction forms and quality checklists were then cross-checked by a third reviewer (BF). All information from the data extraction sheets was entered into a review database, and graphic representation of quality was provided.
Visualisations mapping
The evidence from all the included systematic reviews was synthesised using the following types of charts, tables and maps.
Bubble chart
The evidence was grouped under the corresponding ICD-11 primary or secondary code. The volume of evidence, in terms of number of reviews, RCTs and participants, was then imported from Microsoft Excel into TIBCO Spotfire® (TIBCO, Software Inc., Palo Alto, CA, USA) software23 to produce a bubble chart. The axes of the bubble charts were very large, ranging from 0 to 45,000 participants. To help readability of the charts, we stratified reviews into those with < 1000 participants and those with ≥ 1000 participants.
Summary tables
The detailed description of each included review was represented in summary tables.
Gap maps
The condition and population and context characteristics extracted from the included reviews were populated in an Excel spreadsheet to identify any gaps in the evidence base, and were summarised.
Panoramic meta-analysis
Inclusion and exclusion criteria
From the reviews identified in the mapping stage, we selected the higher-quality reviews (rated ‘high’ or ‘moderate’ on AMSTAR-2) that contained quantitative data (either a single RCT or a meta-analytic effect estimate generated from pooling across multiple RCTs). We extracted these data for HRQoL, depression, anxiety and pain (the most commonly reported condition-specific outcome).
Reviews often contained multiple meta-analyses conducted on data from the same participants for a single outcome (e.g. CBT vs. active comparators, CBT vs. non-active comparators, symptom response, recovery, relapse, remission). To avoid double-counting studies, one meta-analysis per outcome per condition had to be chosen from each review. We used a predefined, step-by-step, hierarchy system in line with the review objectives. We included the meta-analysis (or single RCT) (1) with the longest follow-up time; (2) with the largest number of included RCTs; (3) that used measurement tools with the highest psychometric properties; (4) with the largest number of participants; (5) for which an active comparator was prioritised over non-active comparators; (6) for which continuous outcomes were prioritised over dichotomous outcomes; (7) for which, within dichotomous outcomes, the odds ratio (OR) was prioritised over the risk ratio (RR); (8) for which a random-effects meta-analysis was prioritised over fixed effects; and (9) for which self-report measures were prioritised over clinician-rated measures.
We then grouped the reviews that included quantitative data on each outcome (HRQoL, depression, anxiety and pain). Some of the reviews shared the same RCTs. To avoid double-counting evidence, we had to select one review to include in the PMA. We used a predefined selection process. 15 If two or more reviews shared the same RCT(s), we included the review (1) with the longest follow-up time, (2) with the highest AMSTAR-2 rating, (3) that was the most recently published, (4) with the largest number of RCTs or (5) with the largest number of participants.
Data extraction
We extracted the following data: number of participants in total and per group, number of participants who achieved the desired outcome in the case of dichotomous outcomes, effect sizes, confidence intervals (CIs), direction of effect, heterogeneity measures and type of meta-analysis. An example data extraction form can be found in Appendix 5.
Data management
Data from the data extraction sheets were entered into a master database (Excel) and exported into Stata® versions 13.1 and 16.0 (StataCorp LP, College Station, TX, USA). The PMAs were conducted by four reviewers (BC, HL, KE and TS).
Data analyses
Heterogeneity tests
We conducted a PMA per outcome measure. Review data were entered into an ICD-11 condition subgroup analysis and we tested the within-condition statistical heterogeneity across the reviews. In parallel, we tested the heterogeneity across the ICD-11 condition subgroup categories.
Assumptions for pooling across conditions
We developed three a priori conditions that must be met for us to pool the effect estimates across ICD-11 conditions categories:
-
Intervention homogeneity: the ECG and investigators (see Expert consultation group including patient and public involvement) agreed that, although investigators often use condition-, population- and context-specific protocols, the principles of CBT (see Figure 1) are the same across all conditions. This allows us to make a judgement of intervention homogeneity and, provided the other criteria are met, to pool estimates across conditions.
-
Design homogeneity: it is possible that meta-analytic estimates of effects would be moderated by differences between the review’s underlying design and methodologies. The review estimates would also be influenced by the quality of the included RCTs. However, a RCT-level quality assessment was beyond the scope of this overview. Therefore, we used the proxy of assuming that the highest-quality reviews would be more likely to be unbiased in their methods and would probably report from the best-available evidence. We minimised review design (but not RCT design) variation by including only reviews that adhered to the CRD review criteria and were graded as being of high or moderate quality on the AMSTAR-2 tool (higher-quality reviews); hence, we could claim design homogeneity. We ran a sensitivity analysis (which included higher- and lower-quality review data) to ascertain if the variation in review quality affected the homogeneity of effect estimates across conditions.
-
Statistical homogeneity: statistical heterogeneity was assessed using the I2 statistic; this is expressed as a percentage. A higher percentage is indicative of greater heterogeneity. I2 reflects the variation in effect estimates between reviews that is attributable to heterogeneity. 24 There is no guidance regarding acceptable heterogeneity for PMAs. We used the guidance for meta-analysis heterogeneity,25 which suggests that I2 of < 75% is acceptable for pooling across the categories.
Panoramic meta-analysis method
The PMA was undertaken using a two-step frequentist approach random-effects model using the ‘metan’ command in Stata (versions 13.1 and 16). The two-step analysis consists of performing a conventional meta-analysis of a series of meta-analyses. The first step is undertaken by the original reviewers in obtaining a pooled treatment effect based on their included trials. Many of these will have been estimated via a random-effects meta-analysis, but some will have been analysed using a fixed-effects approach. Nonetheless, we assume that within-review variability has been appropriately allowed for. In the second step, the pooled estimates (with CIs) from each of the systematic reviews are combined into an overall (over all reviews) pooled estimate. At this point, we use a random-effects approach using the DerSimonian–Laird26 approach. We obtained a pooled estimate from within condition and also across conditions, if the across-condition heterogeneity was < 75%. In the few cases where data required for the meta-analysis, such as standard deviations or CIs, were missing, we referred to the individual RCT paper to extract this information.
Primary analysis
The primary analysis was conducted on continuous, end-point data extracted from higher-quality reviews (AMSTAR-2 rating of ‘moderate’ or ‘high’ quality) if there were more than two systematic review per comparison. The primary outcome was HRQoL and the secondary outcomes were depression, anxiety and pain.
We analysed the standardised mean differences (SMDs). When reviews reported values as mean differences, we converted the pooled estimate into a SMD using the standard deviation reported. 27 We reported the 95% CIs and the prediction intervals. These offer a prediction of the distribution of SMDs from future reviews, perhaps in other conditions that have not been included in our overview.
Secondary analysis
Some reviews reported change scores only; we pooled these separately because of concerns that these may be biased as a result of regression to the mean. 28 We performed separate PMAs for RRs and ORs.
We grouped reviews that directly compared high- with low-intensity CBT, irrespective of the condition, and analysed this group separately.
Transforming the standardised mean difference into a mean difference
To make meaningful interpretations, the overall pooled estimate (i.e. the SMD) for each outcome was transformed into a mean difference. The SMD was multiplied by the standard deviation of the most commonly used outcome measure (e.g. Beck Depression Inventory for depression) for each outcome. 29 To find a suitable standard deviation for the measurement tool, we identified a higher-quality review, which included a low risk-of-bias RCT that had used the most common outcome measure. From that trial, we extracted the standard deviation of the outcome measure at baseline.
Publication bias
When ≥ 10 reviews were included in the meta-analysis, publication bias and small-study effects were tested for using Egger’s regression intercept30 and a visual assessment of funnel plot asymmetry. We used a conservative value of p < 0.1 at CIs of 95% to reflect asymmetry.
Subgroup analysis
Subgroup analyses were agreed a priori and were performed if four or more reviews were included in the meta-analysis across all conditions for each of the outcomes on the following: (1) CBT intensity (high/low intensity), (2) age (children and adolescents, adults, older adults), (3) duration of follow-up (short: < 12 months, long: > 12 months) and (4) comparator group (active, non-active). The subgroups were separated using the ‘by()’ command in Stata.
We ran interaction tests between the subgroups using an exploratory meta-regression. The meta-regression used the method of moments estimate of between-study variance and the ‘metareg’ command in Stata.
If we identified any reviews that directly compared high-intensity CBT with low-intensity CBT, we grouped these reviews together by outcomes (HRQoL, depression, anxiety and pain). If their estimates were homogeneous, then we pooled across the reviews as an example of direct comparison.
Sensitivity analysis
To test whether or not the quality of the reviews moderated the effect estimate and or heterogeneity, we ran a sensitivity analysis in which we included data from all reviews, irrespective of their AMSTAR-2 quality, for each outcome PMA. Then we compared the heterogeneity and pooled effect estimates between the sensitivity analyses and the primary analyses (which included only data from higher-quality reviews, i.e. those of ‘high’ and ‘moderate’ AMSTAR-2 quality).
We also conducted a sensitivity analysis in the HRQoL PMA for the two subscales of the Short Form questionnaire-12 items (SF-12)/Short Form questionnaire-36 items (SF-36) instruments. These instruments include a physical composite score and a mental composite score, but the tool does not pool them together. We prioritised the physical component scale (as recommended by the ECG; see Expert consultation group including patient and public involvement), then we re-ran the analyses using the mental component scale to determine if this changed the results.
Consistency of effect
We employed an ontological argument, which suggests that a lack of inconsistency across evidence suggests consistency of effect. 31 We examined the effect estimates from each condition subgroup (pooled effect across all reviews conducted within one condition), subgroup analyses (e.g. active/non-active comparator groups), sensitivity analyses (including the additional condition subgroup analyses) and secondary analyses (e.g. pooled effects across dichotomous outcomes). If any analyses produced a statistically significant effect in favour of the group (comparator or CBT) that was contrary to the overall pooled effect estimate, then the evidence for the general effect was inconsistent across the included conditions. If we did not identify any contrary evidence across any of the conditions or subgroups, then we declared that the overall effect was consistent across all included conditions.
Expert consultation group including patient and public involvement
We worked with a CBT ECG consisting of clinical academics (n = 6), research academics (n = 8) and patient representatives (n = 4). We met with this group directly on three occasions (January 2018, February 2019 and September 2019) and communicated via telephone/e-mail throughout the overview process. For each meeting, the group was sent a workbook of the work to date and a set of questions for the members to comment on. These were collected at the end of each meeting to ensure that all members’ contributions were collated and recognised. The group was not involved in any of the data extraction or quality assessment of the reviews. The ECG provided advice on methods and interpretation, but the final decisions were taken by the study investigators.
Expert consultation group meeting 1: January 2018
In the first meeting, we achieved consensus on the search strategy (terms and databases), the inclusion/exclusion criteria (population, intervention, comparison, outcomes, review design), data extraction form (data to extract) and analysis plan (avoid double-counting of RCTs, subgroup analyses).
Expert consultation group meeting 2: February 2019
The results of the data screening and extraction and the plan for the data synthesis were presented at this meeting. The ECG agreed the following actions:
-
Protocol amendment to include both pooled and single trial data in the PMAs
-
Protocol amendment to include behavioural outcomes as condition-specific outcomes.
-
The ECG did not reach consensus on how the generalisation framework should be used (see Chapter 6). Beth Fordham, Jeremy Howick and Karla Hemming were to continue work on how this could be conceptualised for sharing with the ECG at the next meeting.
Expert consultation group meeting 3: September 2019
The preliminary results were presented at the third meeting. The ECG were in agreement for the mapping and PMA processes. We agreed to prioritise higher-quality reviews over any-quality reviews. However, again, we did not reach agreement on the generalisation framework. The ECG agreed that there was intervention homogeneity, but could not agree that CBT always effects change through the same mechanisms. It agreed that CBT is implemented via the core principles (see Figure 1), but it felt that it was important to recognise that the mechanisms for change would be different for patients living with different conditions. The ECG suggested that a review of all the mechanistic evidence for CBT’s effectiveness was required in order to assume that there is a common mechanism of action.
The patient and public involvement (PPI) representatives guided our visual representation of the data and reflected that the real-life mechanisms of CBT will ‘feel’ very different for each individual receiving the treatment.
Protocol revisions
We intended to translate non-English-language reviews. However, the resource and time allocations were unprepared for the number and complexity of reviews that were found. On discussion with the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme board, we made a change to the protocol and excluded non-English-language reviews at the full-text screening stage.
In the protocol, we selected three general outcomes (HRQoL, depression and anxiety) and suggested collecting condition-specific outcomes, such as psychosis and physical/physiological outcomes. Subsequently, we chose to present the three general outcomes plus the most commonly reported other outcome, which was pain.
We had not envisaged the problem of a review reporting the mental and the physical component subscales of the SF-12 HRQoL tool separately. After consulting the ECG, we selected the physical subscale to be included in the primary analysis, and conducted a sensitivity analysis using the mental subscale data to examine if that affected the PMA estimates and heterogeneity.
Other specific changes included to:
-
include both single RCT data and pooled meta-analysis data in a PMA
-
include behavioural outcomes as condition-specific outcomes
-
prioritise higher-quality reviews over any-quality reviews.
All the above changes were approved by the NIHR HTA programme board.
In response to comments from reviewers of the draft HTA monograph, we have included prediction intervals to our primary panoramic meta-analyses.
Chapter 4 Results: mapping
Process of study selection
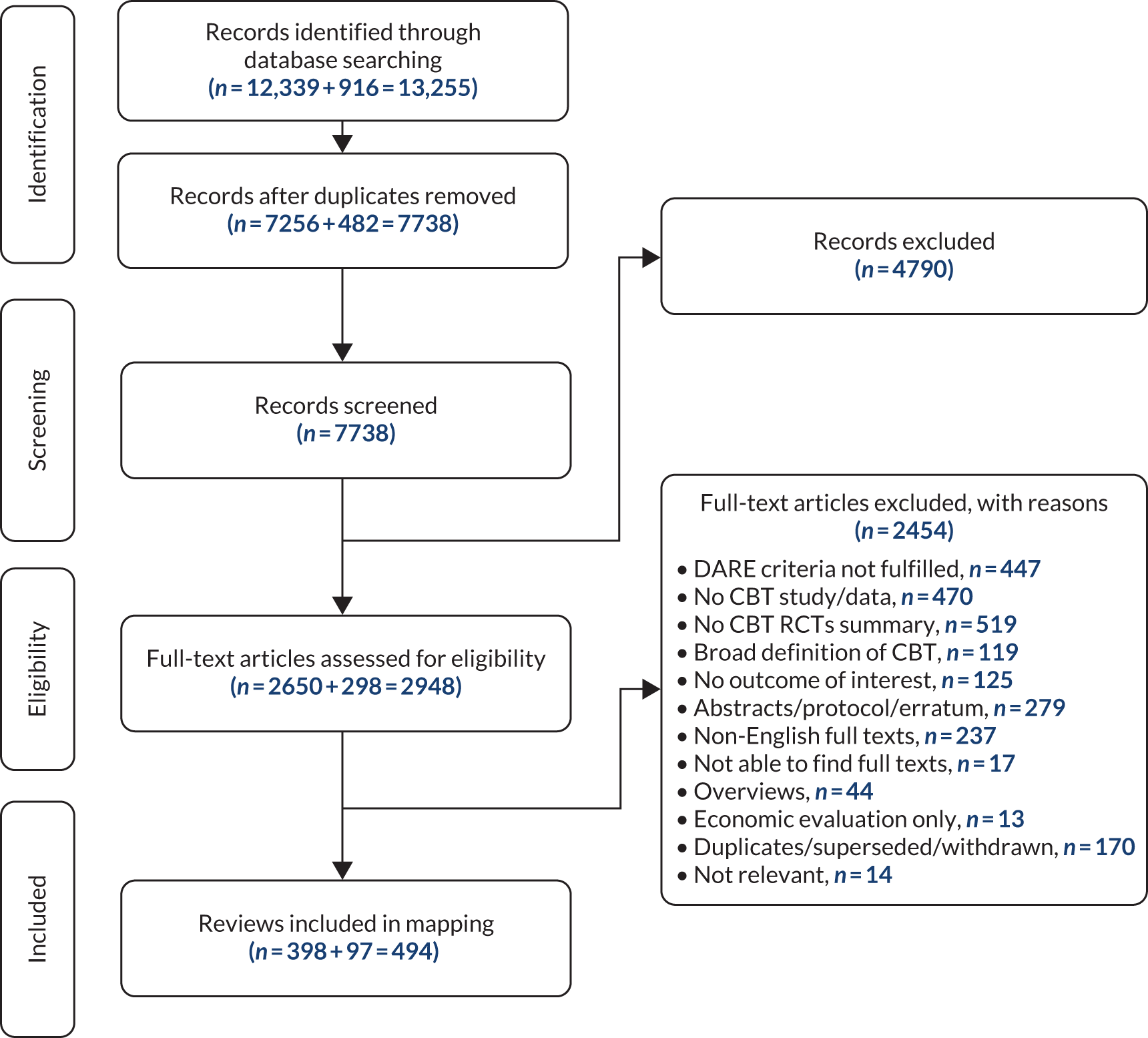
The initial search of eight databases in April 2018 retrieved 12,339 references, and the updated search in January 2019 retrieved 916 references. In total, 7738 titles and abstracts were screened after deduplication, from which 2948 reviews were selected for full-text analysis. On full-text analysis, 494 systematic reviews32–523 were selected for final inclusion. Data extraction for the mapping was done for all these reviews, the synthesis of which is presented in this chapter. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram describing each of these stages is presented in Figure 2.
FIGURE 2.
The PRISMA flow diagram describing review selection for mapping. Adapted from Fordham et al. 524 © The Author(s) 2021. Published by Cambridge University Press. This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted re-use, distribution and reproduction, provided the original article is properly cited.
Excluded studies
We excluded 2454 reviews at the full-text screening stage. Nearly half of these exclusions (1108/2454, 45%) were because the review did not provide a synthesis of CBT trials. Ten per cent (237/2454) of reviews were excluded because they were not available in English. References for the excluded reviews along with their reasons for exclusion are presented in Appendix 6.
Description of the included systematic reviews
We included 494 systematic reviews, which reported 2052 RCTs involving 221,128 participants. 32–523 The included reviews were synthesised in three main formats: summary tables, bubble charts and gap maps.
Summary tables
The summary tables provide comprehensive details of all the 494 included reviews, split into the ICD-11 codes (see Appendix 7, Tables 5–33).
Bubble charts
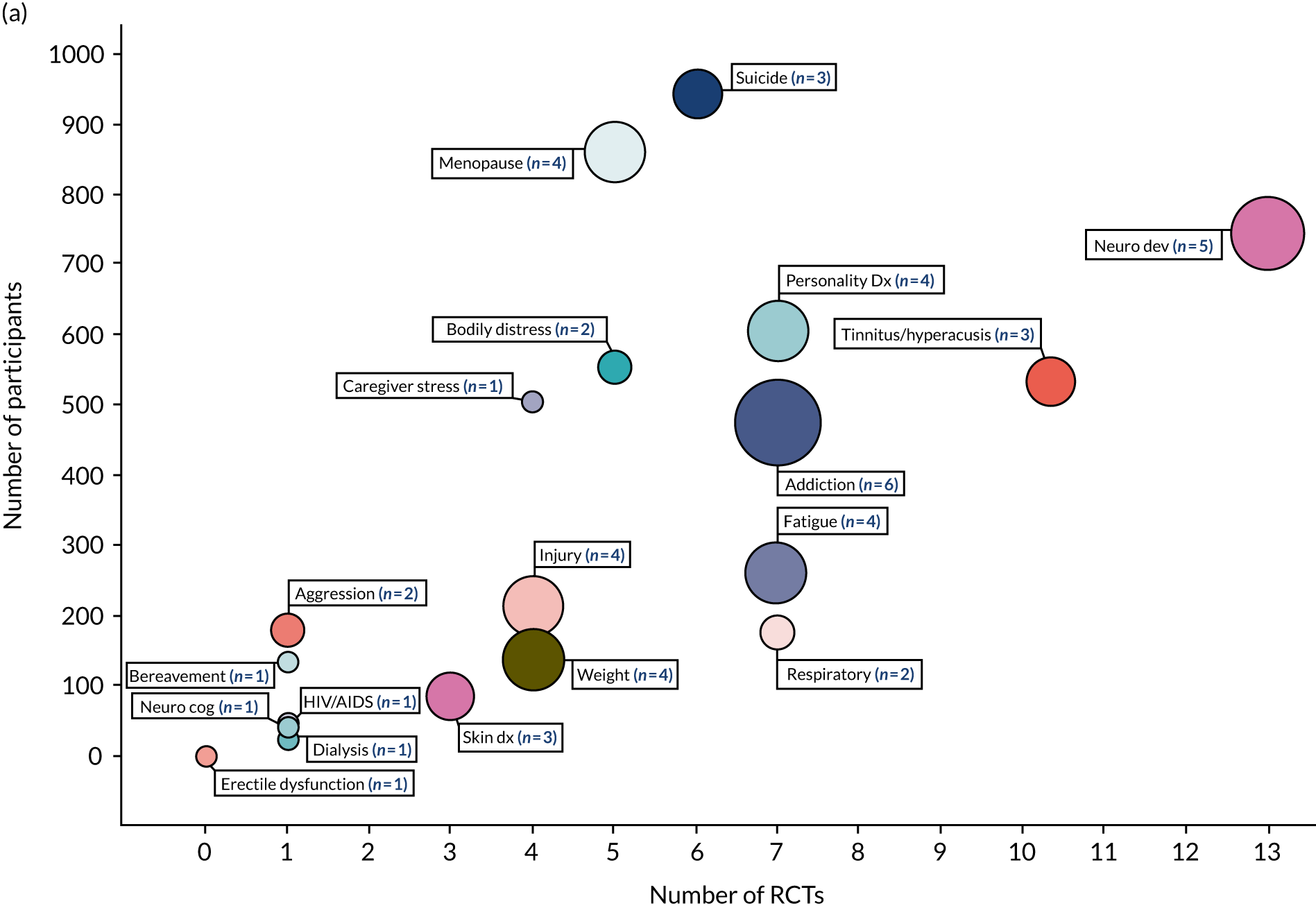
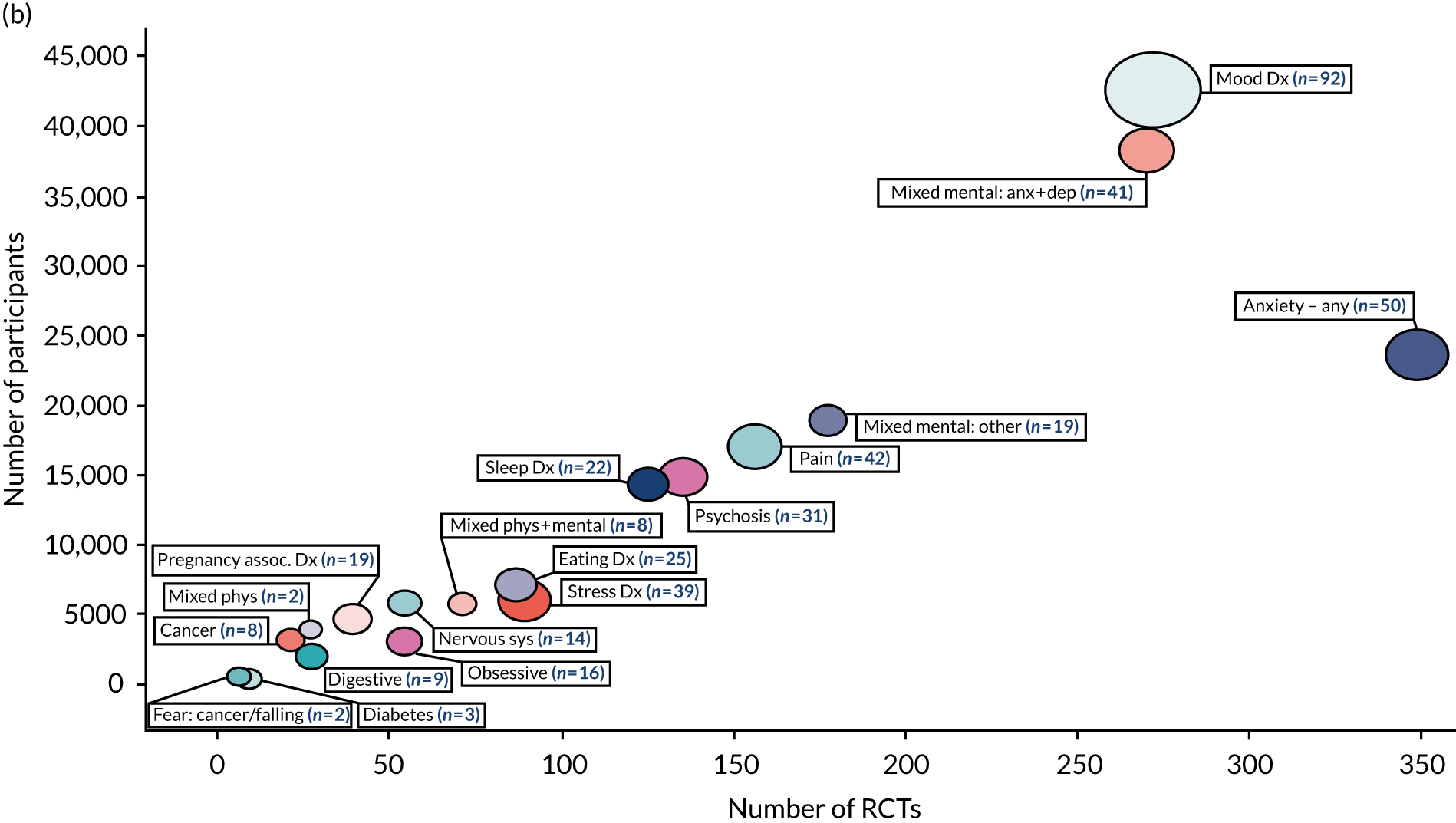
The 494 systematic reviews identified by the search examine the effectiveness of CBT on HRQoL, depression, anxiety or a condition-specific outcome in conditions represented in 14 out of 20 primary (physical) and 13 out of 20 secondary (mental) ICD-11 codes. This equates to 68% of all ICD-11 categories (27/40). ‘Mood disorders [6A60-80]’ were the most researched condition (92 reviews, 272 RCTs, 42,676 participants). The primary and secondary ICD-11 categories that are represented in the included reviews are listed in the unshaded rows presented in Box 2; those that are not represented (i.e. evidence gaps) are listed in the shaded rows in Box 2. The volume of reviews, trials and participants are represented in two bubble maps in Figure 3: (1) conditions that include < 1000 participants and (2) conditions that include > 1000 participants.
-
01 Certain infectious or parasitic diseases.
-
02 Neoplasms.
-
03 Diseases of the blood.
-
04 Diseases of the immune system.
-
05 Endocrine, nutritional or metabolic diseases.
-
07 Sleep–wake disorders.
-
08 Diseases of the nervous system.
-
09 Diseases of the visual system.
-
10 Diseases of the ear or mastoid process.
-
11 Diseases of the circulatory system.
-
12 Diseases of the respiratory system.
-
13 Diseases of the digestive system.
-
14 Diseases of the skin.
-
15 Diseases of the musculoskeletal system.
-
16 Diseases of the genitourinary system.
-
17 Conditions related to sexual health.
-
18 Pregnancy, childbirth or the puerperium.
-
19 Certain conditions originating in the perinatal period.
-
20 Developmental abnormalities.
-
21 Symptoms and signs NOS (MG30 pain, MG22 fatigue, MG43.6 excessive weight gain).
-
6A00-06: neurodevelopmental disorders.
-
6A20-25: schizophrenia or other primary psychotic disorders.
-
6A40-41: catatonia.
-
6A60-80: mood disorders.
-
6B00-06: anxiety or fear-related disorders.
-
6B20-25: obsessive–compulsive disorders.
-
6B40-45: disorders specifically associated with stress.
-
6B60-66: dissociative disorders.
-
6B80-85: feeding or eating disorders.
-
6C00-01: elimination disorders.
-
6C20-21: disorders of bodily distress.
-
6C40-51: disorders due to substance use or addictive behaviours.
-
6C70-73: impulse control disorders.
-
6C90-91: disruptive behaviour or dissocial disorder.
-
6D10-11: personality disorders and related traits.
-
6D30-36: paraphilic disorders.
-
6D50-51: factitious disorders.
-
6D70-72: neurocognitive disorders.
-
6E20-21: mental or behavioural disorders associated with pregnancy, childbirth or the puerperium.
-
6E40: psychological or behavioural factors NOS (MB23.0 aggressive behaviour).
NOS, not otherwise specified.
Shading indicates the ICD-11 categories that are not represented in the included reviews (i.e. evidence gaps).
FIGURE 3.
Bubble map representing the volume of systematic reviews, RCTs and participants included in the qualitative synthesis (n = 494 reviews). (a) Conditions with < 1000 participants in total; and (b) conditions with > 1000 participants in total. The size of a bubble and the number in brackets represents the number of reviews for each condition. AIDS, acquired immunodeficiency syndrome; anx, anxiety; assoc, associated; dep, depression; dx, disorder; HIV, human immunodeficiency virus; neuro cog, neurocognitive; neuro dev; neurodevelopmental; phys, physical; sys, system.
Gap maps
We produced a gap map that details the context and population characteristics of all the reviews conducted within each ICD-11 category (see Appendix 8, Tables 34–37). We have summarised the information in the following section.
Context characteristics of the included reviews
In Table 1, we present the number of reviews that included trials conducted in different contexts. One review could include some trials conducted in one context and also include trials conducted in another context. Therefore, that one review could represent two or more context characteristics. Consequently, the percentages presented across the rows will not always add up to 100% (n = 494 reviews). The shaded cells represent how many reviews did not report on this characteristic.
| Reviews | Reviews, n (%) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Who: severity | What: intensity | When: delivered | Where: participants recruited | Follow-up | ||||||||||||||||||
| Subclinical | Clinical | Chronic | Severe | NR | High | Low | NR | Preventative | Standard | Relapse prevention | NR | Community | GP primary | Outpatients | Inpatients | School/university | Institution | NR | Short term | Long term | NR | |
| Reviews included in mapping: N = 494 (2052 RCTs; 221,128 participants) | 16 (3) | 216 (44) | 19 (4) | 10 (2) | 247 (50) | 397 (80) | 139 (28) | 8 (2) | 29 (6) | 463 (94) | 7 (1) | 0 (0) | 92 (19) | 41 (8) | 114 (23) | 35 (7) | 36 (7) | 4 (1) | 283 (57) | 402 (81) | 130 (26) | 7 (1) |
Context characteristics well reported
Nearly all the included reviews (486/494, 98%) reported whether or not they examined high- or low-intensity CBT. The majority were conducted on high-intensity CBT, but low-intensity CBT trials were included in 28% (139/494) of reviews across 14 out of 40 (35%) ICD-11 categories. Nearly all reviews (487/494, 99%) reported when follow-up data were collected. One-third of reviews (130/494, 26%) included a long-term (> 12 months) follow-up time point.
Context characteristics poorly reported
Only half of the reviews (247/494, 50%) reported on the severity of participants’ symptoms. Of these, the majority described participants as having a clinical diagnosis (216/494, 44%), with no further description on the severity of the symptoms (i.e. chronic or severe). Only 3% (16/494) of reviews examined participants with subclinical symptoms.
Over half of the included reviews (283/494, 57%) did not report from which care setting they had recruited their samples. Of the reviews that did report this, the majority recruited their samples from outpatient settings (114/494, 23%).
Context characteristics rarely examined
All the included reviews reported when the intervention was delivered (494/494, 100%); only 7% (36/494) examined the use of CBT in a preventative context.
Population characteristics of the included reviews
In Table 2, we present the number of reviews that included trials with samples representing different characteristics. One review could include some trials conducted with one type of population (e.g. children and adolescents) and other trials conducted with another population (e.g. adults), or one trial that included children, adolescents and adults. Therefore, that one review could represent two (or more) sample characteristics. Consequently, the percentages presented across the rows will not always add up to 100% (n = 494 reviews). The shaded cells represent how many reviews did not report on this characteristic.
| Reviews | Reviews, n (%) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Sex | Ethnicity | Continent where the included RCTs were conducted | ||||||||||||||
| < 18 | 18–65 | > 65 | NR | < 50% female sample | > 50% female sample | Mixed | NR | < 25% non-white sample | 25–75% non-white sample | > 75% non-white sample | Mixed | NR | Europe, North America, Australasia | Asia | Africa, South America | NR | |
| Reviews included in mapping: N = 494 (2052 RCTs; 221,128 participants) | 108 (22) | 378 (77) | 30 (6) | 19 (4) | 44 (9) | 167 (34) | 65 (13) | 218 (44) | 10 (2) | 6 (1) | 9 (2) | 11 (2) | 458 (93) | 231 (47) | 37 (8) | 8 (2) | 218 (44) |
Population characteristics well reported
Most reviews reported the age of their samples (475/494, 96%); of these, only 6% (30/494 reviews, 81 RCTs and 6629 participants) were conducted with an older adult population.
Population characteristics poorly reported
Most reviews (458/494, 93%) did not report the ethnicity of the samples of their included trials. Of the 36 reviews that did report the ethnicity of their samples, we found an equal number of reviews that reported more white than non-white participants (10/494, 2%) and that reported more non-white than white participants (10/494, 2%).
Nearly half of the reviews (218/494, 44%) did not report on the sex of their trial samples or the country where their included trials were conducted. When reported, a higher number of reviews had a greater representation of female participants (167/494, 34%) in their trial samples, and most reviews included trials conducted in Europe, North America and Australasia (231/494, 47%).
The AMSTAR-2 review quality rating
Every review (n = 494) was assessed twice (by KE and TS) using the AMSTAR-2 checklist. The agreement between reviewers (KE and TS) in assessing the quality of reviews using the AMSTAR-2 checklist was good (327/494, 66%) (κw = 0.63, 95% CI 0.62 to 0.65). Figure 4 presents the proportion of reviews conducted over the preceding 20 years, classified into the four AMSTAR-2 quality categories.
FIGURE 4.
Publication year and AMSTAR-2 quality rating of the included reviews.
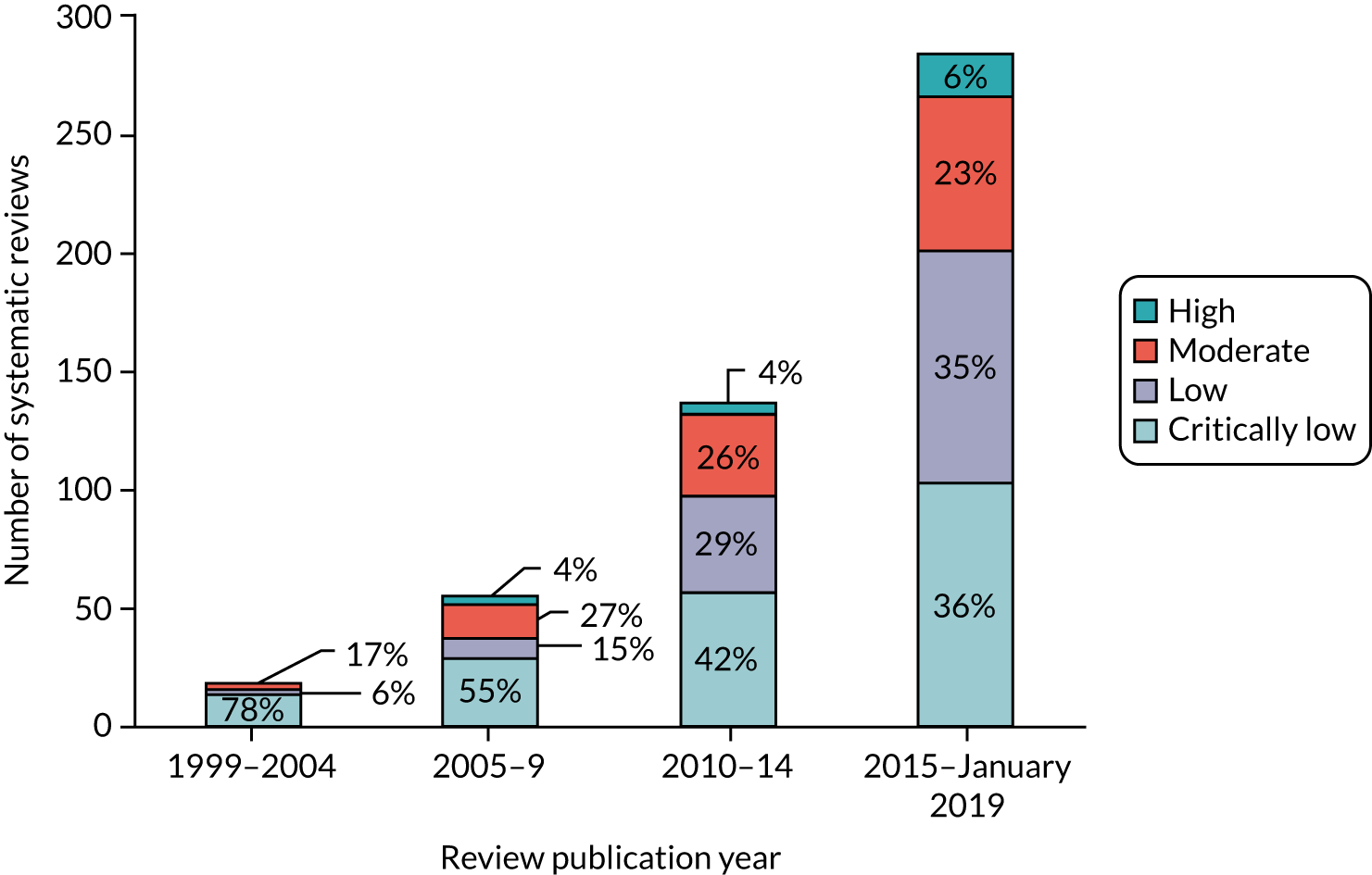
Over the previous 20 years, the quality of systematic reviews has improved; however, in the latest time epoch (2015–19), we still identified that 36% of the included reviews were of critically low quality and only 29% of reviews were classified as being of moderate or high quality.
Table 3 represents the item summaries from the AMSTAR-2 checklist. Of the ‘critical’ items on the checklist, 68% (336/494) of the reviews failed to register the protocol before commencement of the review (item 2), 76% (373/494) failed to provide the list of excluded studies along with the reasons for exclusion of each (item 7) and 50% (248/494) of reviews did not report an adequate search strategy.
| AMSTAR-2 items | Response, n (%) | ||
|---|---|---|---|
| Yes | Partial yes or 0 | No | |
| Components of PICO in research questions and inclusion criteria (item 1) | 266 (54) | – | 228 (46) |
| Protocol registered before commencement of the review (item 2)a | 153 (31) | 5 (1) | 336 (68) |
| Justification for selection of study design for inclusion (item 3) | 80 (16) | – | 414 (84) |
| Adequacy of the literature search (item 4)a | 67 (14) | 179 (36) | 248 (50) |
| Study selection performed in duplicate (item 5) | 325 (66) | – | 169 (34) |
| Data extraction performed in duplicate (item 6) | 322 (65) | – | 172 (35) |
| Justification for excluding individual studies (item 7)a | 108 (22) | 13 (3) | 373 (76) |
| Included studies reported in adequate detail (item 8) | 106 (21) | 350 (71) | 38 (8) |
| Risk of bias from individual studies being included in the review (item 9)a | 291 (59) | 27 (5) | 176 (36) |
| Reporting funding sources of included studies (item 10) | 48 (10) | – | 446 (90) |
| Appropriateness of meta-analytical methods (item 11)a | 292 (59) | 195 (39) | 7 (1) |
| Assessment of potential impact of risk of bias on results of the review (item 12) | 196 (40) | 186 (38) | 112 (23) |
| Consideration of risk of bias when interpreting the results of the review (item 13)a | 277 (56) | – | 217 (44) |
| Explanation/discussion of heterogeneity observed (item 14) | 299 (61) | – | 195 (39) |
| Assessment of presence and likely impact of publication bias (item 15)a | 209 (42) | 70 (14) | 215 (44) |
| Reporting conflicts of interest and funding (item 16) | 376 (76) | – | 118 (24) |
Patient perspective and safety data
Of the 494 reviews, 118 (24%) reviews reported data on dropout rates, adherence and satisfaction analyses. Twenty reviews32,53,56,68,78,103,133,153,165,198,219,234,244,251,266,367,376,402,464,469 searched for safety data, of which nine reviews included reports of adverse events occurring in the CBT groups. We have summarised all the patient perspective and safety data under the relevant conditions in Box 3.
Adelman et al. 34 reported that children, adolescents and adults with anxiety disorders are more likely to drop out of CBT than out of WLC groups (OR 1.76, 95% CI 1.27 to 2.44). Participants were more likely to drop out of internet-based CBT than out of face-to-face CBT (OR 1.36, 95% CI 0.79 to 2.33).
Three reviews of anxiety disorders in children and adolescents reported no difference in the risk of participants dropping out between CBT and WLC groups (OR 0.94, 95% CI 0.58 to 1.51),509 TAU groups (OR 1.01, 95% CI 0.31 to 3.3)227 or placebo-pill control groups (RR 0.53, 95% CI 0.30 to 0.95). 487
A review reported that more RCTs of CBT with adult participants than those with older adults report attrition levels below the 15% attrition threshold (67% vs. 40%). 252
Mood disordersThe dropout rates of adults and older adults from CBT groups ranged from 7% to 40%217,420 and were not significantly different between CBT and comparator groups (active or non-active). 59,145,280,501
Anxiety and/or moodDropout rates for CBT ranged from 6% to 50% across children/adolescent and adult populations. 101,459 A review45 found no difference in dropout rates between high- and low-intensity (internet-based CBT) interventions (OR 0.79, 95% CI 0.57 to 1.09).
Obsessive–compulsive disorders (hypochondriasis)A review of adults with hypochondriasis reported no difference between CBT and TAU/pharmacotherapy/placebo comparator in the likelihood for participants to drop out of their trial arm (OR 1.14, 95% CI 0.56 to 2.32). 450
Obsessive–compulsive disorders (body dysmorphic disorder)No difference was detected in the number of children/adolescents and adults who dropped out between CBT and WLC groups (RR 1.00, 95% CI 0.96 to 1.05), and the effects of CBT on depression outcomes were not altered if dropouts were treated as non-responders. 195
Eating disordersA review of adults with bulimia nervosa/binge-eating disorder reported no difference in dropout rates between CBT and active or non-active comparator groups [F(2,55) = 1.66; p = 0.20]. 179 This remained true in adults with binge-eating disorder when CBT was an adjunct to pharmacotherapy [i.e. fluoxetine alone (22%) or fluoxetine plus CBT (23%)]. 378
Stress disordersTwo reviews reported dropout rates of between zero and 30%355,422 from CBT in adult populations with post-traumatic stress disorder, and that these rates did not differ significantly between the CBT and other active comparator groups. 96
Bodily distress disordersThere were no significant differences in dropout rates between CBT and progressive muscle relaxation groups in adults with medically unexplained symptoms (SMD 0.98, 95% CI 0.83 to 1.15; n = 90). 469
AddictionOne review reported, without statistics, that fewer adults with psychostimulant abuse disorder dropped out of CBT groups than out of the TAU groups. 312
NeoplasmsTwo reviews report very similar dropout rates from CBT interventions: 15% and 22%. 65,238
Nervous system disorders (post-viral fatigue)Castell et al. 93 reported that 17% of adults with post-viral fatigue syndrome dropped out of the CBT groups. Participants were more likely to drop out of CBT than out of a no-treatment control (RR 1.71, 95% CI 1.29 to 2.27), but not when compared with other active comparators170 (RR 0.97, 95% CI 0.28 to 1.25), including exercise interventions266 (RR 0.59, 95% CI 0.28 to 1.25).
Conditions with symptoms of painThree reviews reported that a range of 0–22% of patients dropped out of CBT interventions for pain patients in both adult and children/adolescent populations. 32,285,375
Disorders of the ear (tinnitus)One review examined adults’ and older adults’ satisfaction with internet-based CBT and found that more participants dropped out of internet-based CBT than out of an online education programme. 213
Adherence MoodAcross the trials of adults with depression, the adherence rates ranged greatly, from 10% to 100%. 165,459 A review of CBT for depression in children and adolescents reported that only 50% completed the full CBT programme. 106 Adherence rates were reported to significantly predict treatment response (β = 0.90; p < 0.001). 240
Anxiety and moodAdherence (defined as between 75% and 100% adherence) rates from RCTs of children/adolescents (86%), adults and older adults (24–90%) across anxiety and depression ranged from 24% to 90%. 327,356,466
Eating disordersIn children and adolescents with bulimia nervosa or EDNOS, the adherence rates were similar between CBT and other psychotherapies (family therapy). 243 However, one review of bulimia nervosa in children, adolescents and adults estimated that 16% (95% CI 13% to 19%) of participants did not complete the CBT intervention. 438
Adherence rates were similar between high-intensity and low-intensity (i.e. internet-based) CBT. 353 However, the acceptability ratings were higher for high-intensity CBT than for internet-based CBT. 367
Conditions with symptoms of painOne review of predominantly male (92%) veterans with comorbid pain, depression and substance abuse reported completion rates of only 38% for CBT interventions. 58
InsomniaA review of internet-based CBT for insomnia reported that 78% of adult participants (range 67–100%) completed their treatment. 97 The review also reported that 71% of participants found internet-based CBT ‘mostly’ or ‘very’ effective.
Satisfaction MoodOne review49 suggests that older adults prefer psychological therapies, such as CBT, over pharmacotherapy because the side effects of pharmacotherapy become more problematic with increased comorbidity in older age. 49
There was no difference between children’s and adolescents’ reported levels of acceptability for CBT, compared with WLC or TAU comparator groups. 510
Anxiety and moodTwo reviews46,324 reported that 62–100% of adults were satisfied with internet-based CBT. One review324 found that participants reported more enjoyment when they were communicating with a therapist in face-to-face CBT.
Eating disordersThe acceptability ratings were higher for high-intensity CBT than for internet-based CBT. 367
Stress disordersOne review reported, in great depth, the acceptability of CBT for children and adolescents with post-traumatic stress disorder. 291 It is recognised to be acceptable for children and their caregivers, but there are concerns regarding the trauma exposure component. This is considered central to the effectiveness of CBT, but some evidence suggests that this element is linked to patient dropout rates. CBT is delivered most commonly in a clinic, but this review suggests that home delivery could be more acceptable. It also suggests that CBT delivered in a group setting is more acceptable than individual CBT in ethnically diverse, urban children in the USA. 291
PsychosisCognitive–behavioural therapy was reported to be less acceptable than TAU for schizophrenia patients in one review,66 whereas another review116 found no difference in acceptability. Therapists reported that it was harder to engage younger schizophrenic patients with the CBT intervention. 51
Disorders of the ear (tinnitus)Participants reported that the intervention was not engaging enough, and authors suggested that it might be too much of a commitment for those with low distress levels. 213
Adverse eventsOne or two patients per CBT group reported that their symptoms worsened because of participation in CBT in adults with post-viral fatigue syndrome,56,103,266 bodily distress (medically unexplained symptoms)469 and mixed mental conditions (mood disorder, anxiety and obsessive–compulsive disorders). 53 Adverse events were reported in reviews of other conditions, such as people with substance misuse conditions during withdrawal, schizophrenic patients and eating disorder patients, but there was no evidence to suggest that this was due to participating in CBT. 133,198,219,234
EDNOS, eating disorder not otherwise specified.
Overall, there does not seem to be a great difference in dropout rates between CBT and active or non-active comparator groups. However, it appears that more participants drop out of low-intensity internet-based CBT than out of face-to-face CBT, and patients reported greater satisfaction with the therapeutic relationship in face-to-face CBT. Older adults appeared to drop out more than younger adults, but they also reported preferring psychological therapies, such as CBT, over pharmacological therapies. There may be certain groups who do not find CBT acceptable; for example, one review of mainly male veterans reported very low completion rates. 58 Participants with common mental and physical conditions seemed generally satisfied with CBT, but schizophrenic patients seemed more likely to find CBT an unacceptable treatment option. In relation to adverse events, there is a lack of reporting on safety data from CBT reviews. However, the evidence we found does not suggest that participating in CBT could cause harm to participants.
Chapter 5 Results: panoramic meta-analysis
The map of the CBT review evidence base included 494 reviews. Of these, 171 reviews included data suitable for inclusion in the PMAs. The majority of the reviews reported in the mapping exercise, but excluded from the PMA, were not suitable because we could not extract the CBT RCT-specific data in isolation for any of the four outcomes (n = 279). This could be for any one of the following reasons:
-
The review may not have performed a meta-analysis or reported any quantitative data from single RCTs.
-
The review may have looked for RCTs reporting on the outcome but not identified any evidence.
-
We may have been unable to extract CBT RCT data in isolation, for example a review that presented a subgroup analysis of 10 CBT trials, but one of these trials was not a RCT. We could not isolate the purely CBT RCT evidence; therefore, the data were not included in the PMA. To have included these RCTs, we would have needed to return to the original RCT and perform a new meta-analysis including only the RCT data.
Of the 126 reviews eligible for the end-point PMA, 7132,37,39,46,50,59,63,68,89,102,117,126,134,143,149,165,167–169,175,188,193,197–199,205,206,211,219–221,227,231,234–236,246,249,251,259,261,267,275,286,291,299,315,317,340,343,347,357,369,371,373,397,401,405,409,432,445,446,448,450,454,464,469,480,484,507,518 were higher-quality reviews (i.e. ‘moderate’ or ‘high’ on AMSTAR-2); the primary analyses for each outcome were conducted using these 71 higher-quality reviews. The PRISMA flow diagram describing review selection for the PMAs from the mapping stage is presented in Figure 5.
FIGURE 5.
The PRISMA diagram from mapping to PMAs.
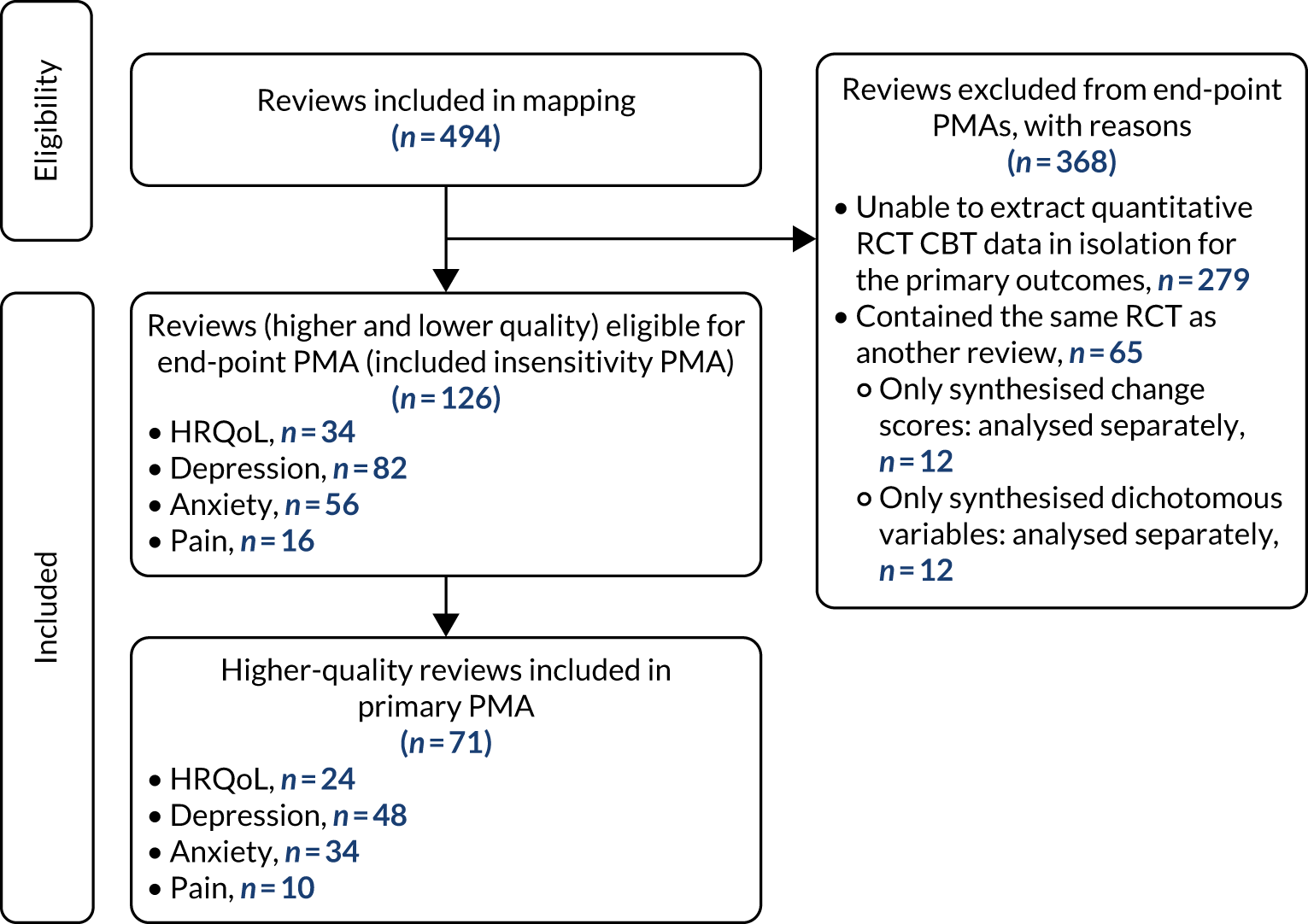
For reviews reporting data as change scores or dichotomous outcomes (RR, OR), separate PMAs for each outcome were undertaken; these are presented in Appendices 9–12.
Health-related quality of life
We identified 24 higher-quality systematic reviews32,39,63,165,188,193,219,220,231,235,236,275,286,299,317,343,347,371,409,445,446,464,469,518 that met the eligibility criteria to be included in the HRQoL PMA. One review343 included two meta-analyses for different disorders; hence, the number of comparisons is 25. These reviews included 49 RCTs, 4304 participants and represent 12 out of 40 ICD-11 categories (30%), as presented in Box 4. The white rows represent those ICD-11 codes that are represented in the primary analysis, the purple rows represent those conditions that are represented in the sensitivity analyses (see Sensitivity analysis) and the orange rows represent those ICD-11 codes that are not represented.
-
01 Certain infectious or parasitic diseases.
-
02 Neoplasms (in lower-quality reviews only).
-
03 Diseases of the blood.
-
04 Diseases of the immune system.
-
05 Endocrine, nutritional or metabolic diseases.
-
07 Sleep–wake disorders.
-
08 Diseases of the nervous system.
-
09 Diseases of the visual system.
-
10 Diseases of the ear or mastoid process.
-
11 Diseases of the circulatory system.
-
12 Diseases of the respiratory system.
-
13 Diseases of the digestive system (in lower-quality reviews only).
-
14 Diseases of the skin.
-
15 Diseases of the musculoskeletal system.
-
16 Diseases of the genitourinary system.
-
17 Conditions related to sexual health.
-
18 Pregnancy, childbirth or the puerperium.
-
19 Certain conditions originating in the perinatal period.
-
20 Developmental abnormalities.
-
21 Symptoms and signs NOS (MG30 pain, MG22 fatigue).
-
6A00-06: neurodevelopmental disorders.
-
6A20-25: schizophrenia or other primary psychotic disorders.
-
6A40-41: catatonia.
-
6A60-80: mood disorders.
-
6B00-06: anxiety or fear-related disorders.
-
6B20-25: obsessive–compulsive disorders.
-
6B40-45: disorders specifically associated with stress.
-
6B60-66: dissociative disorders.
-
6B80-85: feeding or eating disorders.
-
6C00-01: elimination disorders.
-
6C20-21: disorders of bodily distress.
-
6C40-51: disorders due to substance use or addictive behaviours.
-
6C70-73: impulse control disorders.
-
6C90-91: disruptive behaviour or dissocial disorder.
-
6D10-11: personality disorders and related traits.
-
6D30-36: paraphilic disorders.
-
6D50-51: factitious disorders.
-
6D70-72: neurocognitive disorders.
-
6E20-21: mental or behavioural disorders associated with pregnancy, childbirth or the puerperium.
-
6E40: psychological or behavioural factors NOS (MB23.0 aggressive behaviour).
NOS, not otherwise specified.
White rows represent ICD-11 codes represented in the primary analysis, purple shaded rows represent ICD-11 codes represented in the sensitivity analyses and orange shaded rows represent ICD-11 codes that are not represented in the HRQoL PMA.
The most commonly used measure of HRQoL was the Short Form questionnaire-36 items (SF-36) (n = 6). Other measurements included the Quality of Life Inventory (n = 5), the EuroQol-5 Dimensions (n = 2), the WHO Quality of Life-BREF (n = 1), the Global Assessment of Functioning (n = 1) and the Modular System for Quality of Life-54 (n = 1). The remaining reviews used population-specific (e.g. KIDSCREEN-27; KINDL-R; Comprehensive Quality of Life Scale, Intellectual Disability) or condition-specific [e.g. Fibromyalgia Impact Questionnaire, ADHD (attention deficit hyperactivity disorder) Impact Module-Adult™, Quality of Life in Alzheimer’s Disease, Diabetes Quality of Life for Youths] quality-of-life measurements.
Some reviews included trials with mixed characteristics, for example one review could include trials with adults and with older adults; in such a case, we would record that there was one review with adult data and one with older adult data. Consequently, the counts do not always add to 24 reviews. The mapping results demonstrate that the majority of these meta-analyses were focused on adults (n = 21), with only three reviews of children/adolescents and one review of older people. A higher number of reviews (12/24) had samples that included more female than male participants than reviews with samples of more male than female participants (6/24). Only three reviews reported the ethnicity of their samples: two reviews had samples with < 25% non-white participants and one included a sample with > 75% non-white participants.
The majority of reviews reported on the management of clinical conditions (16/24), through high-intensity CBT (17/24), delivered in outpatient settings (16/24), and with short-term follow-up (19/24). Seven reviews shared these three contexts but were conducted across different conditions. The majority of the included RCTs were from Europe, North America and Australasia (21/24).
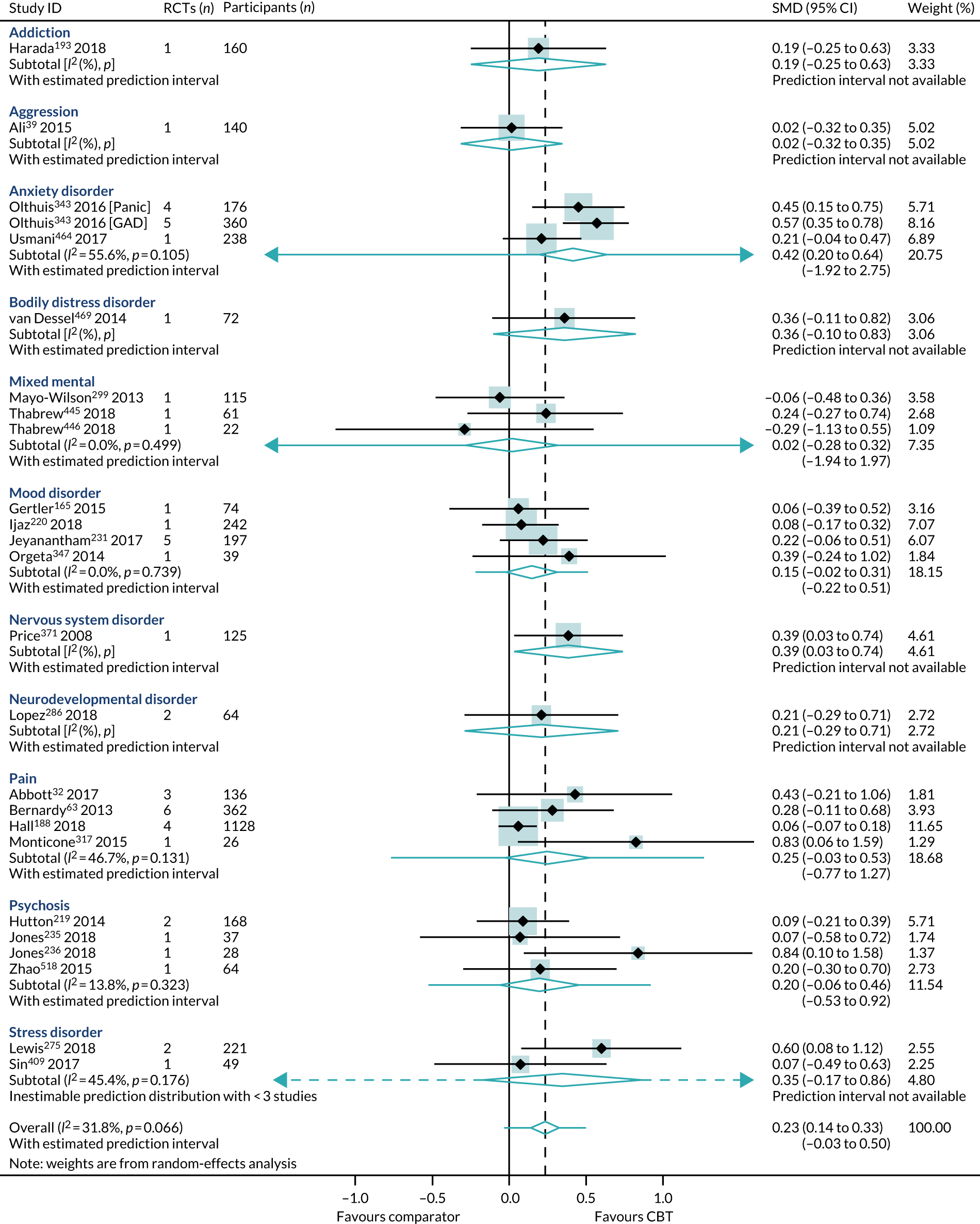
The number of reviews containing only one trial was 6 out of 25; for some conditions, the numbers in each trial were very small (Figure 6). Comparators were active (8/24), mixed (3/24) and non-active (13/24).
FIGURE 6.
Primary analysis of the primary outcome: HRQoL (end-point scores) from ‘high-quality’ reviews. GAD, generalised anxiety disorder. Adapted from Fordham et al. 524 © The Author(s) 2021. Published by Cambridge University Press. This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted re-use, distribution and reproduction, provided the original article is properly cited.
Primary analysis
Within-condition heterogeneity (I2) varied between 0% and 56%, and across-condition heterogeneity was 32%; hence, the criteria for PMA were met. The pooled across-condition SMD between control groups and CBT intervention groups gave a modest effect in favour of CBT on outcomes of HRQoL (SMD 0.23, 95% CI 0.14 to 0.33) (see Figure 6). Variation in effects was observed across conditions; for example, in aggression, the estimate mean effect was almost zero, although it was estimated with considerable uncertainty (SMD –0.02, 95% CI –0.28 to 0.32), whereas, in anxiety disorders, the estimated effect was positive and was estimated with much greater certainty (SMD 0.42, 95% CI 0.20 to 0.64). This heterogeneity is reflected in the resulting prediction intervals, which, indicated for the overall effect (within any given condition), were between –0.03 and 0.50, indicating, at worst (and with little support in the prediction interval), a small negative effect of CBT for some conditions and, at best, a large positive effect for other conditions.
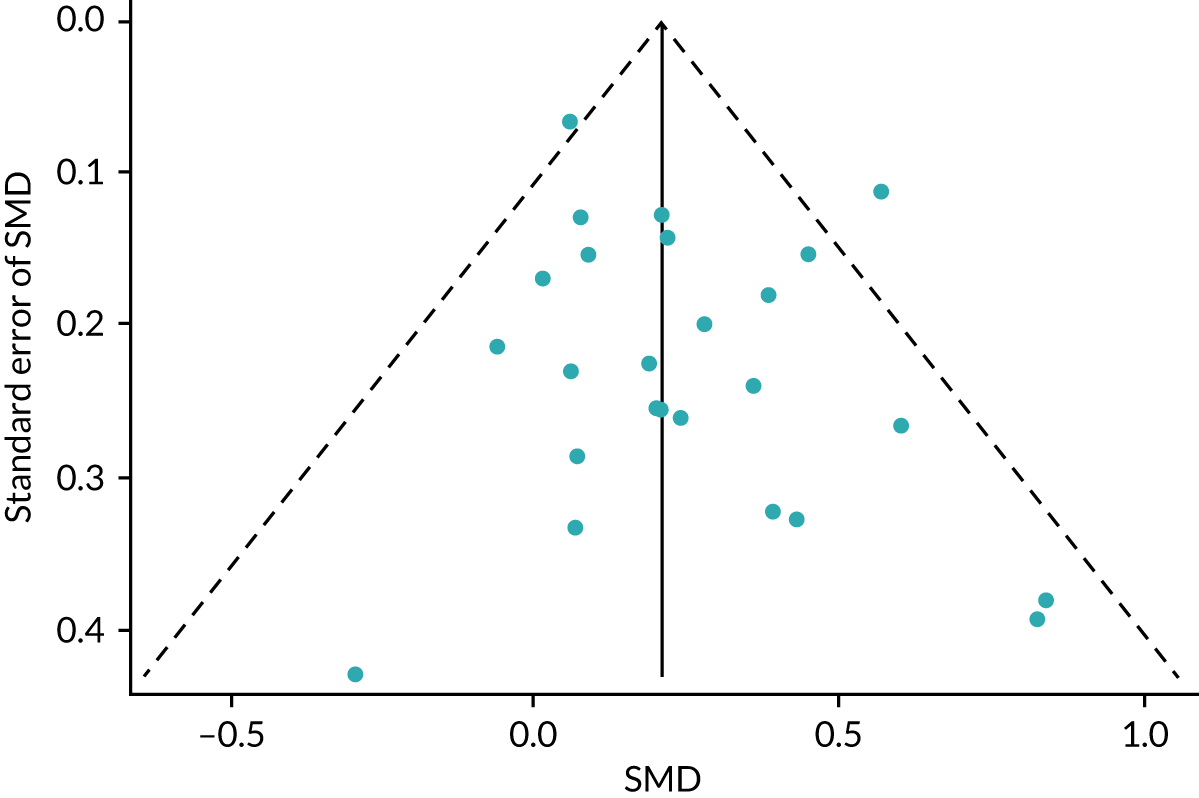
No publication bias was detected using funnel plots (Figure 7) and Egger’s test showed that there were no small-study effects (p = 0.18).
FIGURE 7.
The HRQoL funnel plot with pseudo-95% confidence limits (end-point data from high-quality reviews).
Mean difference in health-related quality of life
We identified a standard deviation (10.93 points) of the SF-36 physical composite score from a trial,525 deemed to have a low risk of bias, in a higher-quality review. 464 The SMD translated to an estimated mean difference on the SF-36 of 3 points (95% CI 2 to 4 points).
Subgroup analysis
The only interaction test that was statistically significant was between reviews of CBT compared with active comparators and reviews of CBT compared with non-active comparators. All other subgroup interaction tests were not statistically significant and are, therefore, consistent with the general effect of CBT on HRQoL outcomes.
Cognitive–behavioural therapy intensity
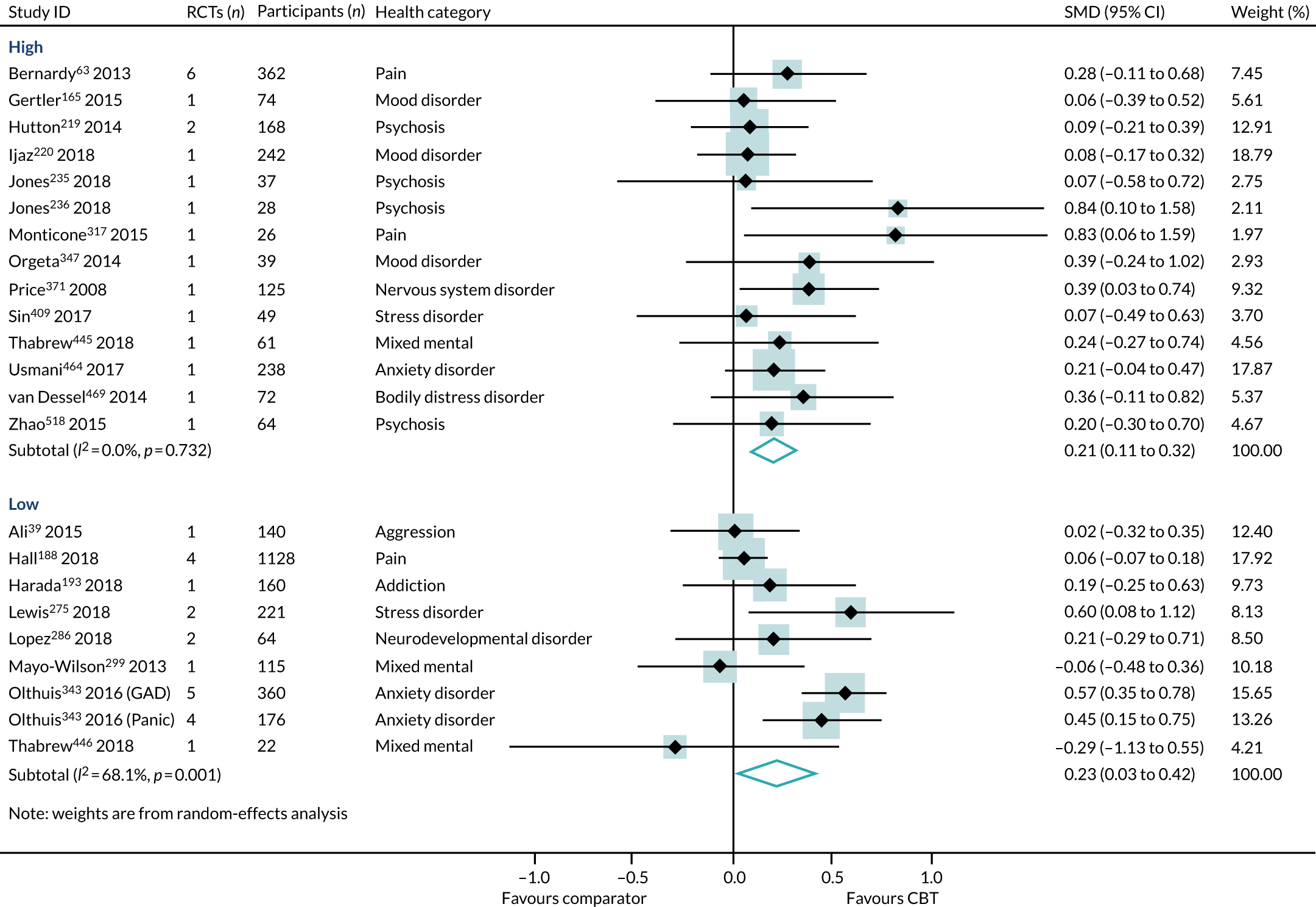
Overall, high- and low-intensity CBT reviews were distributed evenly across the different conditions and characteristics. High- and low-intensity CBT reviews both included populations diagnosed with 6B00-06 anxiety or fear-related disorders, 6A60-80 mood disorders, 6A00-06 neurodevelopmental disorders, 6B20-25 obsessive–compulsive disorders, 21 pain and 6B40-45 disorders specifically associated with stress. They included patients with chronic symptoms (6A60-80 mood disorders and 21 pain). The reviews included children, adolescents and adults, of both sexes, from all care settings in Europe, North America, Australasia and Asia. Reviews of both intensities included long-term follow-up data. Reviews of high-intensity, but not low-intensity, CBT included (1) populations diagnosed with 6C20-21 disorders of bodily distress, 08 diseases of the nervous system or 6A20-25 schizophrenia or other primary psychotic disorders; (2) older adults; and (3) CBT delivered in a preventative context. Reviews of low-intensity, but not high-intensity, CBT were conducted in populations diagnosed with 6C40-4H addiction and MB23 aggressive behaviour.
There was little difference between effect estimates in reviews of high-intensity and low-intensity CBT, although heterogeneity was substantially higher for low-intensity CBT (SMD 0.23, 95% CI 0.03 to 0.42; I2 = 68%) than for high-intensity CBT (SMD 0.21, 95% CI 0.11 to 0.32; I2 = 0%) (Figure 8). The interaction test between high- and low-intensity CBT reviews was not statistically significant (p = 0.99).
We identified three reviews318,343,521 (four RCTs, 243 participants; two reviews of lower quality and one review of higher quality) that directly compared high- with low-intensity CBT interventions on HRQoL outcomes in 6B00-06 anxiety or fear-related disorders and 6A60-80 mood disorders. One review provided separate data for both 6B01 panic and 6B04 social anxiety disorder populations, and so the PMA included four meta-analyses. In this subset of direct comparisons, there was no difference between high- and low-intensity CBT (SMD 0.15, 95% CI –0.10 to 0.40; I2 = 0%) (Figure 9).
FIGURE 9.
Health-related quality of life: high- vs. low-intensity CBT, direct comparison PMA. SAD, seasonal affective disorder.
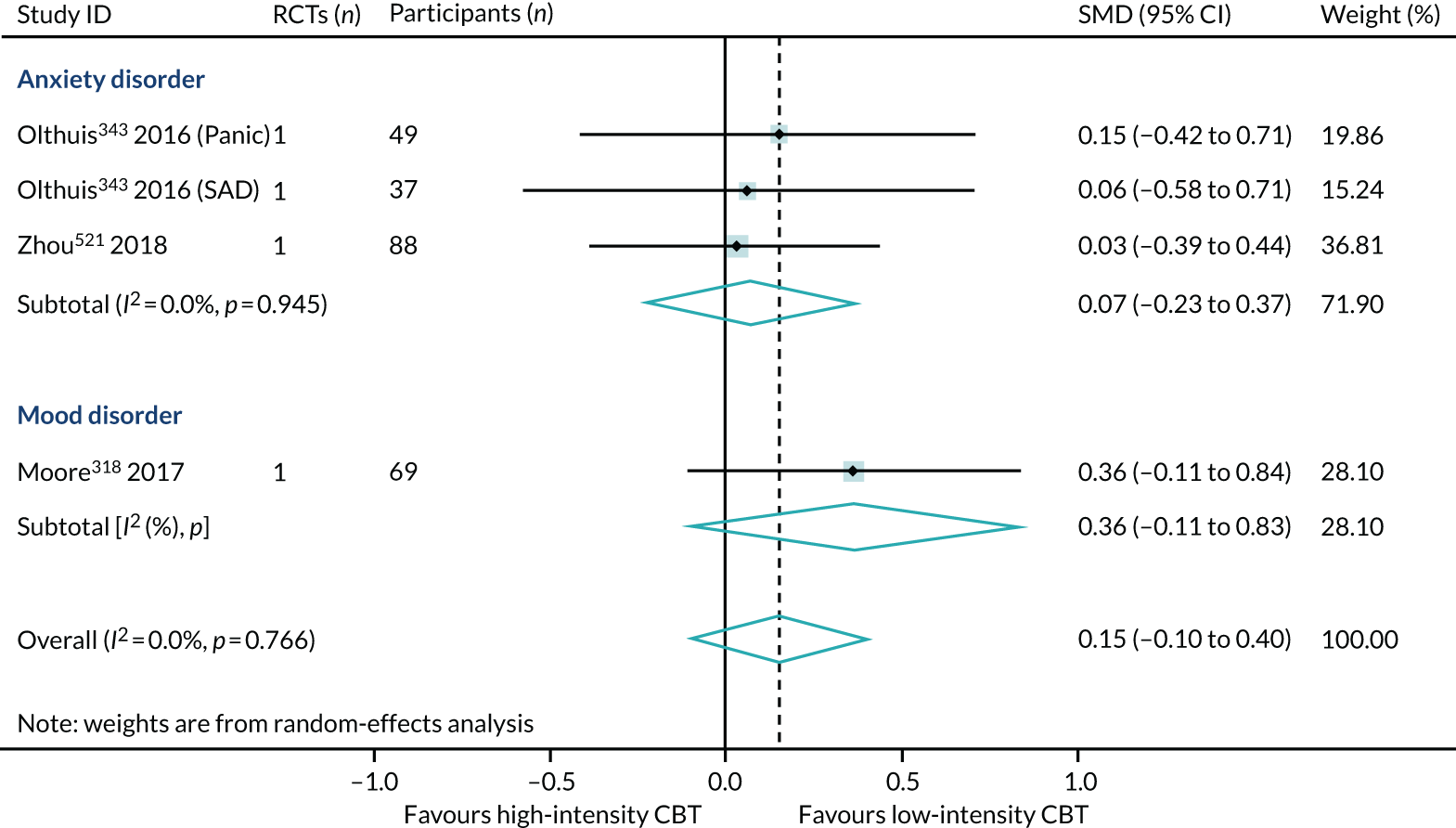
The direct evidence (see Figure 9) comparing high- with low-intensity CBT in 6B00-06: Anxiety and 6A60-80: Mood disorders supports our indirect evidence (see Figure 8) from subgroup analyses of high and low intensity. In summary, we have found no direct or indirect evidence that high- or low-intensity CBT produced different effect sizes.
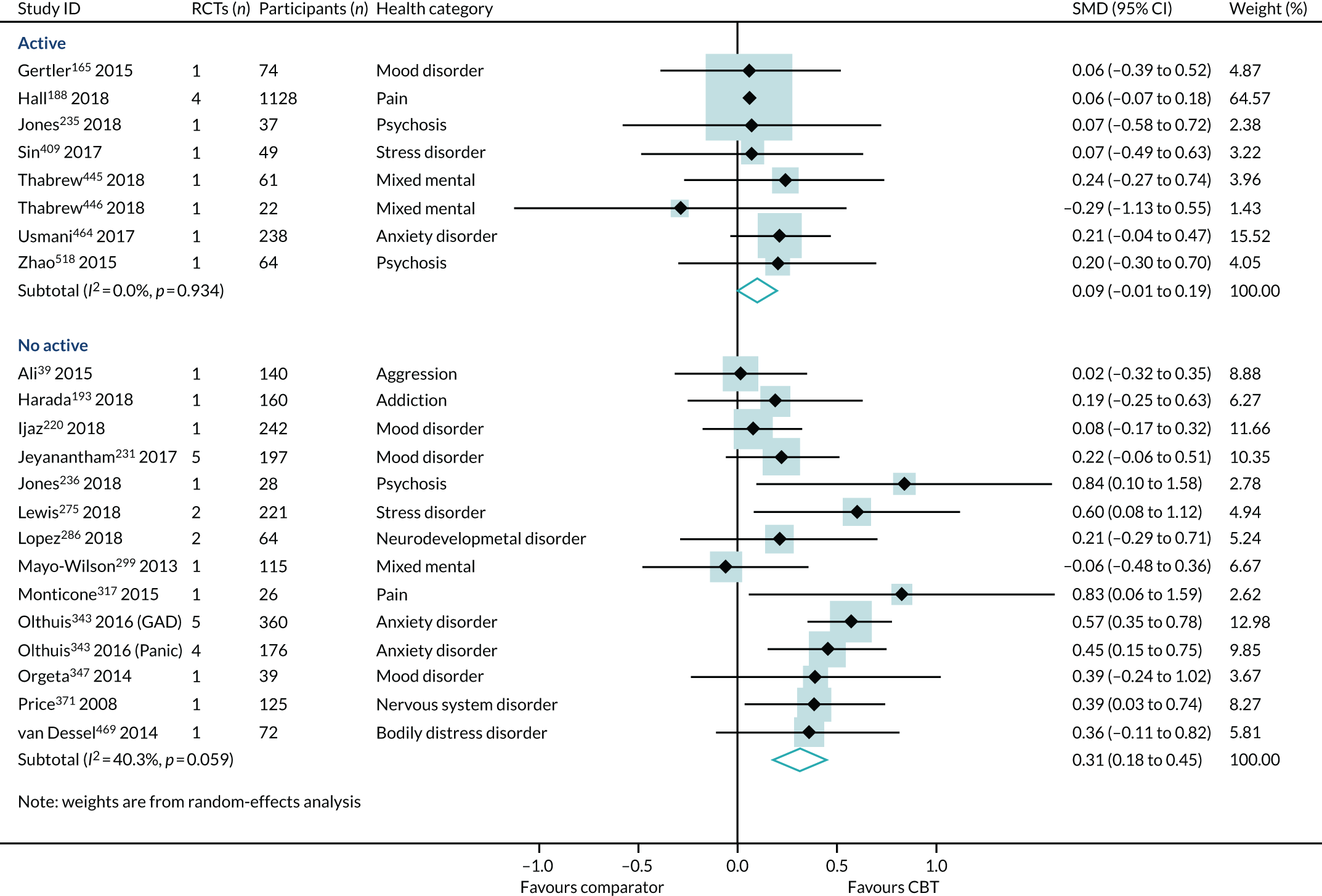
Type of comparators
The choice of comparator had a significant effect on the treatment estimates. Comparison to an active intervention was associated with a very small effect (SMD 0.09, 95% CI –0.01 to 0.19; I2 = 0%) (Figure 10). The active comparators tested in these reviews were education, exercise, pharmacotherapy, physiotherapy, psychotherapy/counselling and relaxation. Comparison with a non-active control was associated with a larger effect estimate (SMD 0.31, 95% CI 0.18 to 0.45; I2 = 40%). The interaction test was statistically significant (p = 0.04).
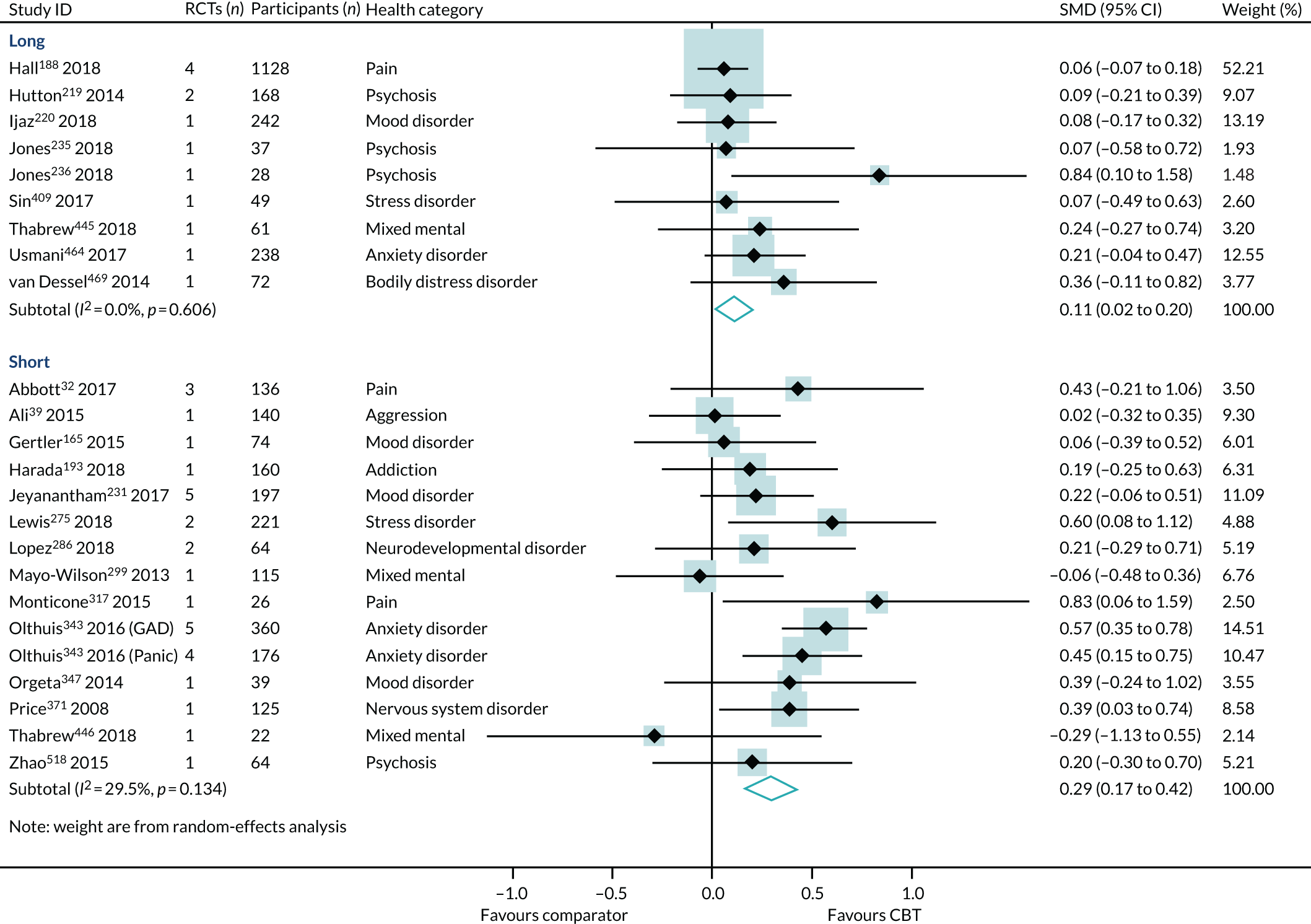
Duration of follow-up
Effect estimates were higher in reviews reporting short-term follow-up (SMD 0.29, 95% CI 0.17 to 0.42; I2 = 30%) than in reviews reporting long-term follow-up (SMD 0.11, 95% CI 0.02 to 0.20; I2 = 0%) (Figure 11). However, the interaction test did not find a statistically significant difference between the groups (p = 0.06).
FIGURE 11.
The HRQoL subgroup analysis (end-point data from high-quality reviews): duration of follow-up. Note that one review63 with combined short- and long-term follow-up is not included here. GAD, generalised anxiety disorder.
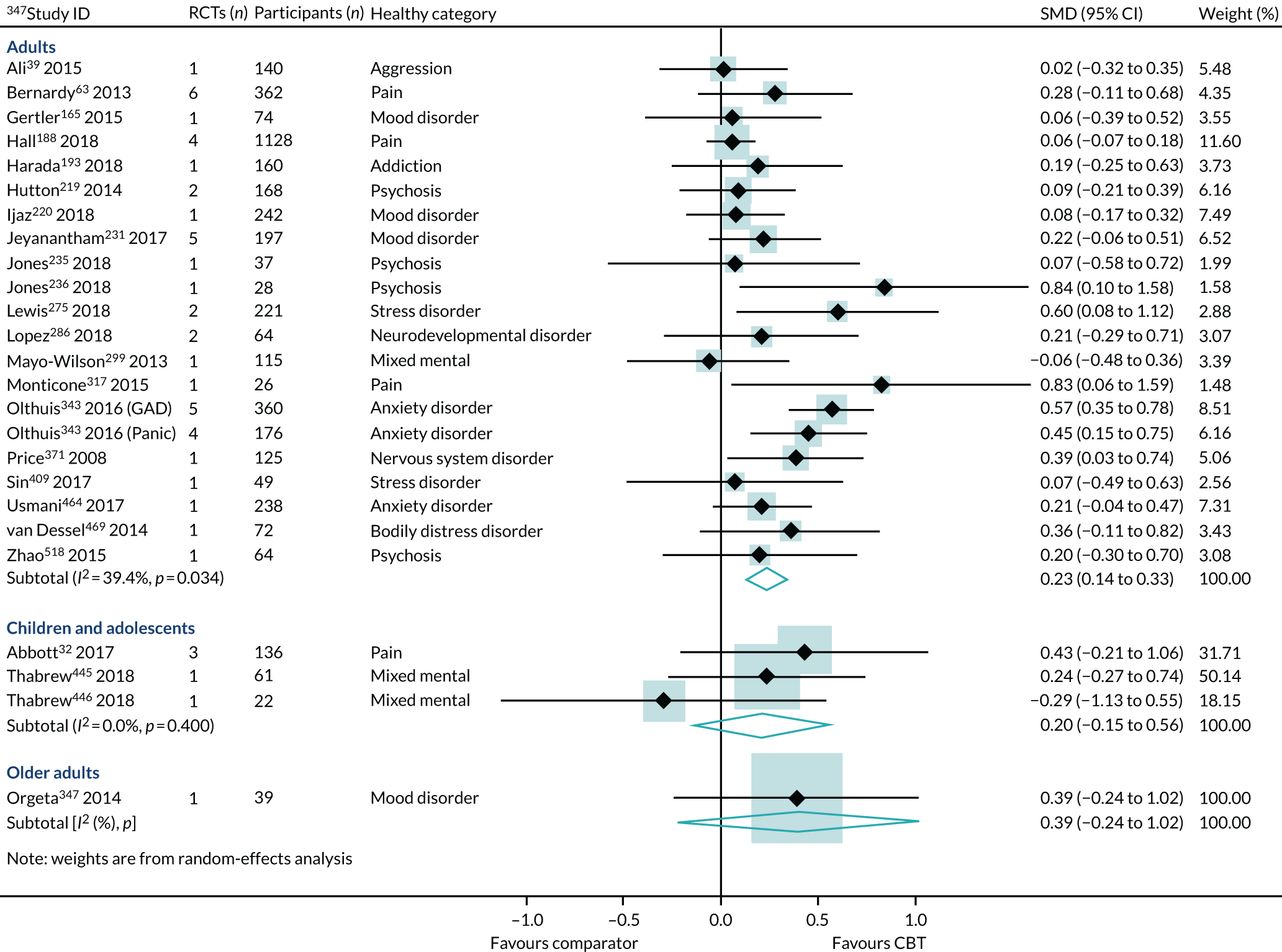
Age
Effect estimates were similar in reviews of children and adolescents (SMD 0.20, 95% CI –0.15 to 0.56; I2 = 0%) and adults (SMD 0.23, 95% CI 0.14 to 0.33; I2 = 39%) (Figure 12). However, the sample sizes were much smaller in the reviews of children and adolescents, and the consequent CIs crossed zero. The interaction test did not find a statistically significant difference between the children/adolescents and adult groups (p = 0.06). The effect size for older adults was larger (SMD 0.39, 95% CI –0.24 to 1.02), but was generated from one review, with one trial and only 39 participants, and, again, the 95% CIs crossed zero (see Figure 12).
FIGURE 12.
The HRQoL subgroup analysis (end-point data from high-quality reviews): age. GAD, generalised anxiety disorder.
Sensitivity analysis
The sensitivity analysis was conducted with an additional 10 reviews that had been rated as low or critically low on the AMSTAR-2. Therefore, the sensitivity analysis was conducted with 34 reviews (76 RCTs, 7466 participants). 32,39,63,82,165,188,193,219,220,231,235,236,270,275,276,279,286,299,317,329,343,347,356,371,409,413,445,446,464,467,469,513,518,521 Inclusion of lower-quality reviews increased the estimate of effect (SMD 0.28, 95% CI 0.17 to 0.38) and raised the levels of heterogeneity (I2 = 71%) (see Appendix 9, Figure 15). This analysis included reviews from more physical conditions: 13: Digestive system, 02: Neoplasms, 08: Headaches and epilepsy and 21: Symptoms such as tinnitus and fatigue (see Box 4). All of the additional within-condition group estimates were consistent with the general effect, that is, an absence of inconsistent effects.
We re-ran the PMA replacing the physical component scores with the mental component scores from the SF-12/SF-36 in the two reviews that presented both the physical and the mental component scores. 220,464 The replacement did not change the overall effect or heterogeneity rating for the HRQoL outcome (SMD 0.24, 95% CI 0.14 to 0.33; I2 = 38%) (see Appendix 9, Figure 16).
Health-related quality-of-life change scores and risk ratio data
Four reviews (four RCTs, 185 participants), two of higher and two of lower quality, presented HRQoL data as change scores. 158,246,406,523 These included reviews of 6B00-06 anxiety and 6A60-80 mood disorders, 13 digestive system, 21 pain and 12 respiratory system disorders. The overall pooling reported acceptable heterogeneity and a moderate effect in favour of CBT (SMD 0.58, 95% CI 0.15 to 1.00; I2 = 66%) (see Appendix 9, Figure 17).
One lower-quality review (two RCTs, 145 participants) presented HRQoL data as a RR. 170 This review identified a large effect (SMD 1.57, 95% CI 0.78 to 2.37; I2, not applicable) in favour of CBT (see Appendix 9, Figure 18).
Discussion
From the highest-quality reviews, we found that CBT produced consistent, positive effects on HRQoL across 10 different conditions. Effect estimates suggest a modest, long-term improvement, compared with no intervention. These effects became very small when CBT is compared with other active treatments, including education, exercise, pharmacotherapy, physiotherapy, psychotherapy/counselling and relaxation. We did not find a difference between the effect sizes of reviews conducted with low-intensity CBT or high-intensity CBT.
The effect estimates were generated by synthesising data from samples of children, adolescents and adults, of both sexes, mainly living in countries in Europe, North America and Australasia. There is a lack of higher-quality evidence of CBT’s effectiveness for older adults.
We do not know if CBT will be effective when delivered preventatively or when delivered to patients with severe or subclinical symptoms. We do not know if CBT is equally effective across different ethnic groups nor do we know its effect for people living in countries in Africa, Asia or South America.
Depression
We identified 48 higher-quality systematic reviews37,39,46,50,59,63,117,126,143,167–169,175,197–199,205,206,220,221,231,234,235,246,249,261,267,275,286,299,340,357,369,371,373,401,405,409,432,445,446,448,450,454,469,480,484,507 that met the eligibility criteria to be included in the primary depression PMA. One review included six meta-analyses for different disorders; hence, the number of comparisons is 53. These included 130 RCTs and 14,073 participants, and represent 16 out of 40 possible ICD-11 categories (40%). Box 5 includes the ICD-11 codes represented in the primary PMA (white cells) and those codes not represented (shaded cells).
-
01 Certain infectious or parasitic diseases.
-
02 Neoplasms.
-
03 Diseases of the blood.
-
04 Diseases of the immune system.
-
05 Endocrine, nutritional or metabolic diseases.
-
07 Sleep–wake disorders.
-
08 Diseases of the nervous system.
-
09 Diseases of the visual system.
-
10 Diseases of the ear or mastoid process.
-
11 Diseases of the circulatory system.
-
12 Diseases of the respiratory system.
-
13 Diseases of the digestive system.
-
14 Diseases of the skin.
-
15 Diseases of the musculoskeletal system.
-
16 Diseases of the genitourinary system.
-
17 Conditions related to sexual health.
-
18 Pregnancy, childbirth or the puerperium.
-
19 Certain conditions originating in the perinatal period.
-
20 Developmental abnormalities.
-
21 Symptoms and signs NOS (MG30 pain, MG22 fatigue).
-
6A00-06: neurodevelopmental disorders.
-
6A20-25: schizophrenia or other primary psychotic disorders.
-
6A40-41: catatonia.
-
6A60-80: mood disorders.
-
6B00-06: anxiety or fear-related disorders.
-
6B20-25: obsessive–compulsive disorders.
-
6B40-45: disorders specifically associated with stress.
-
6B60-66: dissociative disorders.
-
6B80-85: feeding or eating disorders.
-
6C00-01: elimination disorders.
-
6C20-21: disorders of bodily distress.
-
6C40-51: disorders due to substance use or addictive behaviours.
-
6C70-73: impulse control disorders.
-
6C90-91: disruptive behaviour or dissocial disorder.
-
6D10-11: personality disorders and related traits.
-
6D30-36: paraphilic disorders.
-
6D50-51: factitious disorders.
-
6D70-72: neurocognitive disorders.
-
6E20-21: mental or behavioural disorders associated with pregnancy, childbirth or the puerperium.
-
6E40: psychological or behavioural factors NOS (MB23.0 aggressive behaviour, QE01 caregiver stress).
NOS, not otherwise specified.
White rows represent ICD-11 codes represented in the primary analysis; purple shaded rows represent ICD-11 codes that are not represented in the depression PMA.
The most commonly used measure of depression was the Beck Depression Inventory (n = 22). Other measurements included the Hamilton Depression Rating Scale (n = 7), the Hospital Anxiety and Depression Scale (n = 4), the Patient Health Questionnaire-9 items (n = 2), the Montgomery–Åsberg Depression Rating Scale (n = 2), the Center for Epidemiologic Studies Depression Scale (n = 2), the Profile of Mood States (n = 1), the Depression Anxiety Stress Scale (n = 1) and the Hopkins Symptoms Checklist (n = 1). The remaining reviews used population-specific depression measures [Glasgow Depression Scale for People with a Learning Disability (n = 1), Children’s Depression Inventory-revised (n = 3)].
The majority of these meta-analyses were focused on adults (37/48), with seven reviews focusing on adolescents/children and one review focusing on older people. More reviews had samples that included more female than male participants (23/48) than samples with more male than female participants (9/48). Only seven reviews reported the ethnicity of their samples. Of these, four reviews had samples with < 25% non-white participants and one included a sample with > 75% non-white participants.
The majority of reviews reported on the management of clinical conditions (26/48), on interventions of high intensity (26/48), delivered in outpatient settings (27/48), and with short-term follow-up (39/48). The majority of included RCTs in the reviews were from Europe, North America and Australasia (33/48). Many of the reviews contained only one trial (54%, 26/48), and, for some conditions, such as personality disorders, the numbers in those trials were very small (see Appendix 10, Figure 19).
Primary analysis
Within-condition heterogeneity (I2) varied between 0% (6D10-11: Personality disorders) and 86.3% (6A60-80: Mood disorders), and across-condition heterogeneity was 81%. The across-condition heterogeneity was too high for us to pool across the ICD-11 category groups (see Appendix 10, Figure 19). Ten of the within-condition groups reported effects in favour of CBT with some certainty. However, aggression, eating disorders, mixed mental conditions, nervous system disorders and stress-related disorders report within-condition effects of close to zero.
The heterogeneity was too high to pool across ICD-11 categories in any of the subgroup or sensitivity analyses. There was no evidence of publication bias or of small-study effects (Egger’s test p = 0.87) (see Appendix 10, Figure 20).
Discussion
Depression was the most commonly reported outcome in the review evidence base. The variation between the effect estimates generated for the within-condition subgroups was too wide-ranging to pool across the ICD-11 condition groups. No further subgroup or sensitivity analyses were conducted to compare with the primary analysis.
Anxiety
We identified 34 higher-quality systematic reviews37,39,89,134,143,168,175,205,227,234–236,246,249,251,259,275,286,291,315,340,343,347,371,373,397,409,432,445,446,450,464,469,480 that met the eligibility criteria. Two reviews included meta-analyses for different disorders; hence, the number of comparisons is 36. These included 59 RCTs and 4673 participants, and represent 13 out of 40 possible ICD-11 categories (33%). Box 6 includes the ICD-11 codes represented in the primary PMA (white rows), those conditions represented in the sensitivity analysis only, namely lower-quality reviews (purple rows) and those codes not represented (orange rows).
-
01 Certain infectious or parasitic diseases.
-
02 Neoplasms.
-
03 Diseases of the blood.
-
04 Diseases of the immune system.
-
05 Endocrine, nutritional or metabolic diseases.
-
07 Sleep–wake disorders.
-
08 Diseases of the nervous system.
-
09 Diseases of the visual system.
-
10 Diseases of the ear or mastoid process.
-
11 Diseases of the circulatory system.
-
12 Diseases of the respiratory system.
-
13 Diseases of the digestive system.
-
14 Diseases of the skin.
-
15 Diseases of the musculoskeletal system.
-
16 Diseases of the genitourinary system.
-
17 Conditions related to sexual health.
-
18 Pregnancy, childbirth or the puerperium.
-
19 Certain conditions originating in the perinatal period.
-
20 Developmental abnormalities.
-
21 Symptoms and signs NOS (MG30 pain, MG22 fatigue).
-
6A00-06: neurodevelopmental disorders.
-
6A20-25: schizophrenia or other primary psychotic disorders.
-
6A40-41: catatonia.
-
6A60-80: mood disorders.
-
6B00-06: anxiety or fear-related disorders.
-
6B20-25: obsessive–compulsive disorders.
-
6B40-45: disorders specifically associated with stress.
-
6B60-66: dissociative disorders.
-
6B80-85: feeding or eating disorders.
-
6C00-01: elimination disorders.
-
6C20-21: disorders of bodily distress.
-
6C40-51: disorders due to substance use or addictive behaviours.
-
6C70-73: impulse control disorders.
-
6C90-91: disruptive behaviour or dissocial disorder.
-
6D10-11: personality disorders and related traits.
-
6D30-36: paraphilic disorders.
-
6D50-51: factitious disorders.
-
6D70-72: neurocognitive disorders.
-
6E20-21: mental or behavioural disorders associated with pregnancy, childbirth or the puerperium.
-
6E40: psychological or behavioural factors NOS (MB23.0 aggressive behaviour).
NOS, not otherwise specified.
White rows represent ICD-11 codes represented in the primary analysis, purple rows represent ICD-11 codes represented in the sensitivity analyses and orange rows represent ICD-11 codes that are not represented in the anxiety PMA.
The most commonly used measure of anxiety was the Beck Anxiety Inventory (BAI) (n = 9). Other measurements included the Hospital Anxiety and Depression Scale (n = 6), the State–Trait Anxiety Inventory (n = 6), the Hamilton Anxiety Rating Scale (n = 1), the Hopkins Symptom Checklist (n = 1) and the Profile of Mood States (n = 1). The remaining reviews used population-specific [Glasgow Anxiety Scale for People with an Intellectual Disability, Revised Children’s Manifest Anxiety Scale (n = 4)] or condition-specific [Generalised Anxiety Disorder-7 (n = 3), Dental Anxiety Scale, Cardiac Anxiety Questionnaire] measurements.
The majority of these meta-analyses focused on adults (23/34), with seven reviews of adolescents/children and two reviews of older people. More reviews had samples that included more female than male participants (14/34) than samples with more male than female participants (9/34). Only five reviews reported the ethnicity of their samples. Of these, four reviews had samples with > 75% white participants and none included a sample with < 25% white participants.
The majority of reviews reported on the management of clinical conditions (20/34), on interventions of high intensity (29/34), delivered in outpatient settings (22/34), and with a short-term follow-up (27/34). The majority of included RCTs were from Europe, North America and Australasia (22/34). Out of 34 reviews, 24 contained only one trial, and, in some cases, the numbers in each trial were very low (Figure 13 presents the data).
FIGURE 13.
Primary analysis of the secondary outcome: anxiety from ‘higher-quality’ reviews. OCD, obsessive–complusive disorder; PTSD, post-traumatic stress disorder. Adapted from Fordham et al. 524 © The Author(s) 2021. Published by Cambridge University Press. This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted re-use, distribution and reproduction, provided the original article is properly cited.
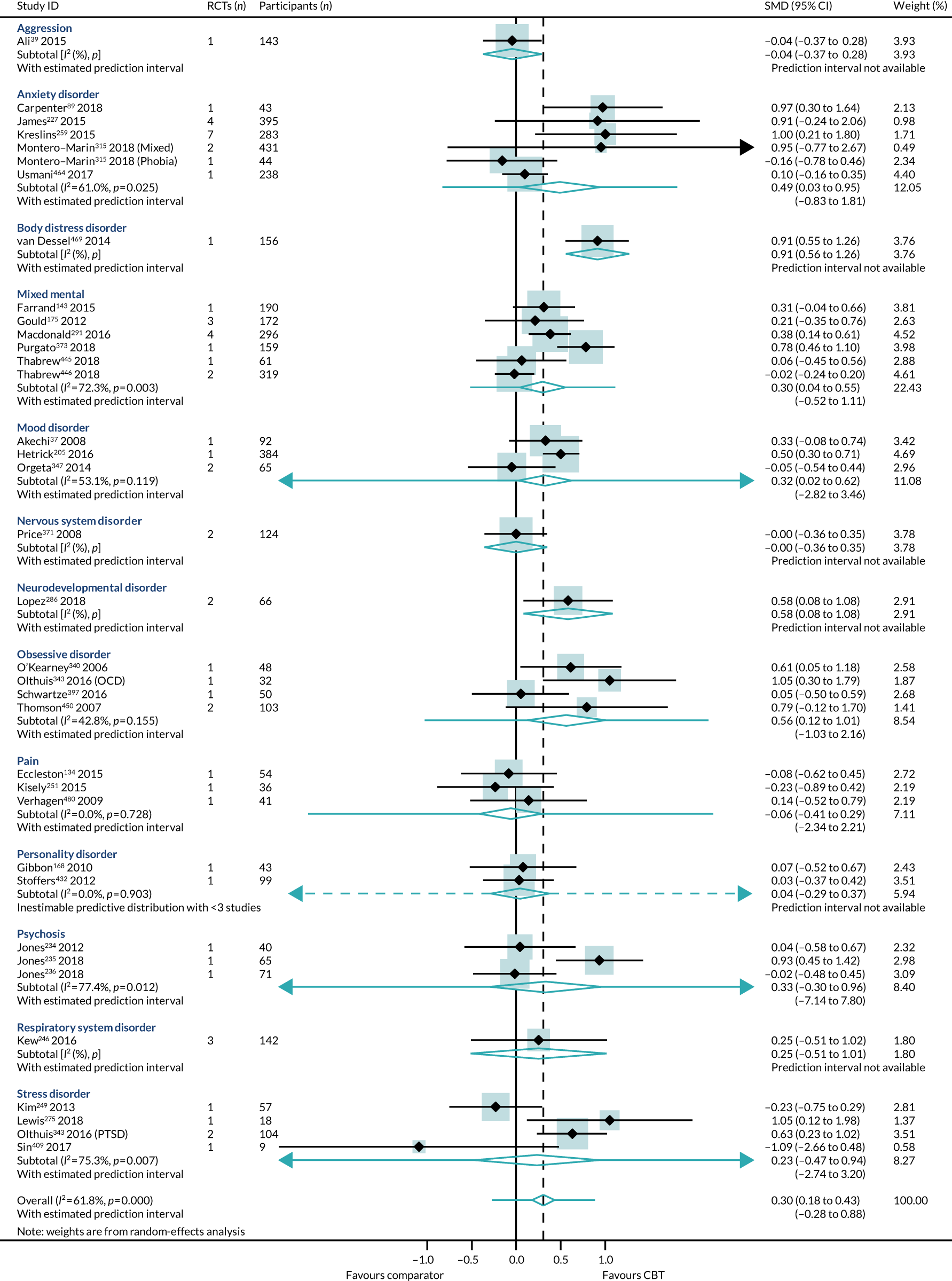
These analyses also included reviews with trials conducted in less common contexts and populations, for example patients with subclinical mood (6A60-80) conditions (n = 1), and CBT delivered in preventative contexts to mood disorder (6A60-80), psychosis (6A20-25) (n = 2) and inpatient psychosis patients (n = 2), and to older adults living with stress disorders (6B40-45), obsessive disorders (6B20-25), anxiety (6B00-06) and mood (6A60-80) disorders (n = 2). None of these specific reviews produced effect estimates that were inconsistent with the primary anxiety PMA.
Primary analysis
Within-condition heterogeneity varied between 0% (MG30 pain) and 75% (6B40-45 stress-related disorders) and across-condition heterogeneity was 62%. The pooled across-condition SMD gave a modest effect in favour of CBT on outcomes of anxiety (SMD 0.30, 95% CI 0.18 to 0.43) (see Figure 13). The prediction intervals for the overall effect were –0.28 to 0.88. No inconsistent effects were identified across the conditions.
Once again, variation in effects was observed across conditions. This heterogeneity is reflected in the resulting prediction interval, which, indicated for the overall effect (within any given condition), was between –0.28 to 0.88, indicating a possible small negative effect of CBT for some conditions and, at best, a large positive effect for other conditions.
There was no evidence of publication bias or of small-study effects (Egger’s test p = 0.70) (see Appendix 11, Figure 21).
Mean difference in anxiety
We transformed the across-condition SMD into a mean difference of the most commonly reported anxiety outcome, the BAI. 526 We identified a standard deviation (13.46 points) of the BAI from a low risk-of-bias trial525 in a higher-quality review. 464 The SMD translated to an estimated mean difference on the BAI of 4 points (95% CI 2 to 6 points).
Subgroup analysis
None of the interaction tests between the subgroups was significant and the evidence is consistent with the primary anxiety PMA.
Cognitive–behavioural therapy intensity
Reviews of low- and high-intensity CBT examined similar populations and conditions. The ICD-11 categories that were represented by high-intensity CBT only, and not low-intensity CBT, were 6A20-25 schizophrenia or other primary psychotic disorders, 6D10-11 personality disorders and related traits, 6A00-06 neurodevelopmental disorders and 12 diseases of the respiratory system. The populations who were sampled in reviews of high-intensity CBT but not in reviews of low-intensity CBT were older adults (06B40-45 disorders specifically associated with stress, 6B20-25 obsessive–compulsive disorders, 6B00-06 anxiety or fear-related disorders and 6A60-80 mood disorders) and subclinical populations (6A60-80 mood disorders). High-intensity, but not low-intensity, CBT reviews included trials delivered in preventative contexts (6A20-25 schizophrenia or other primary psychotic disorders and 6A60-80 mood disorders) and to inpatient samples (6A20-25 schizophrenia or other primary psychotic disorders).
The heterogeneity was too high to pool across the low-intensity CBT reviews (I2 = 78%). The low-intensity CBT reviews included trials examining CBT delivered via paraprofessionals (n = 3) or via the internet (n = 4). The heterogeneity was much lower in high-intensity CBT reviews (SMD 0.28, 95% CI 0.15 to 0.42; I2 = 54%). The interaction test between high- and low-intensity CBT reviews was not statistically significant (p = 0.62) (see Appendix 11, Figure 22).
We identified five reviews87,306,343,360,521 (11 RCTs, 503 participants) that directly compared high- with low-intensity CBT interventions on anxiety outcomes in 6B00-06: Anxiety, 6A60-80: Mood and pain [including tinnitus 21 Symptoms and signs not otherwise specified (MG30 pain)] conditions. In this subset of direct comparisons, there was no difference between high- and low-intensity CBT (SMD 0.03, 95% CI –0.14 to 0.21; I2 = 20%) (see Appendix 11, Figure 23). This direct evidence comparing high- with low-intensity CBT in anxiety and mood and pain conditions supports our indirect evidence (see Appendix 11, Figure 22) from the high- and low-intensity CBT subgroup analyses. We have found no direct or indirect evidence that high- or low-intensity CBT produce different effect sizes.
Type of comparators
The effect was larger and significant when CBT was compared with non-active comparators (SMD 0.37, 95% CI 0.19 to 0.55; I2 = 64%) and smaller and non-significant when compared with active comparators (SMD 0.19, 95% CI 0.00 to 0.37; I2 = 49%) (see Appendix 11, Figure 24). However, the interaction test between the two groups was not significant (p = 0.24).
Duration of follow-up
Effect estimates were higher in reviews reporting long-term follow-up (SMD 0.38, 95% CI 0.15 to 0.60; I2 = 66%) than in those reporting short-term follow-up (SMD 0.27, 95% CI 0.12 to 0.43; I2 = 59%) (see Appendix 11, Figure 25). However, the interaction test did not find a statistically significant difference between the groups (p = 0.48).
Age
Effect estimates were similar in reviews of children and adolescents (SMD 0.37, 95% CI 0.12 to 0.62; I2 = 67.1%) and adults (SMD 0.32, 95% CI 0.15 to 0.48; I2 = 63.6%). The estimates in the two reviews of older adults were much lower and the 95% CIs crossed zero (SMD 0.06, 95% CI –0.30 to 0.43; I2 = 0%) (see Appendix 11, Figure 26). The interaction test did not find a statistically significant difference between the three groups (p = 0.69).
Sensitivity analyses
We identified 56 reviews (117 RCTs, 11,409 participants)34,37,39,89,134,143,168,171,175,205,215,216,227,234–236,241,246,249,251,259,266,275,277,286,291,294,306,315,329,340,343,347,356,371,373,377,379,397,398,409,410,413,425,429,432,445,446,450,463,464,466,469,480,497,513 of any quality with data suitable for inclusion in the sensitivity anxiety PMA. Five reviews had separate valid data representing different conditions; therefore, the total number of comparisons in the all-quality anxiety PMA is 64. Inclusion of lower-quality reviews increased the heterogeneity across conditions (I2 = 76%) beyond our threshold for pooling across the conditions (see Appendix 11, Figure 27). All of the ICD-11 category within-condition effects were consistent with the primary analysis for anxiety outcomes.
Anxiety change scores/dichotomous outcomes
Four lower-quality reviews (four RCTs, 255 participants) reported anxiety outcome data as change scores. 56,105,336,457 These included reviews of 6B00-06 anxiety, 6A60-80 mood disorders and 08 diseases of the nervous system. The heterogeneity was too high (I2 = 88.1%) to pool across the reviews (see Appendix 11, Figure 28). One lower-quality review433 reported an OR (one RCT, 112 participants) and found a large effect in favour of CBT (SMD 1.01, 95% CI 0.96 to 1.06; I2, not applicable) (see Appendix 11, Figure 29). One lower-quality review332 (one RCT, 27 participants) reported a risk difference and presented a moderate effect in favour of CBT (SMD 0.36, 95% CI 0.01 to 0.71; I2, not applicable) (see Appendix 11, Figure 29). There were no data that were inconsistent with the primary analysis for anxiety outcomes from any of the change score or dichotomous data.
Discussion
The primary PMA reported that CBT produces a small, but meaningful, long-term improvement in anxiety symptoms. Results from the primary, subgroup, sensitivity subgroups, change scores and dichotomous data PMAs are all consistent with the primary PMA. Some individual reviews were conducted in less frequently researched contexts (e.g. trials conducted in Africa), under-represented populations (e.g. older adults) and less frequently researched delivery formats (e.g. preventative CBT). Every review generated effect estimates that were consistent with the overall general effect.
Cognitive–behavioural therapy was effective when it was delivered via high-intensity methods, but there was too much variation to conclude whether or not CBT was effective when it was delivered via low-intensity methods. However, there were no statistically significant differences between any of the subgroup tests.
The effect estimates were generated by synthesising data from samples of children, adolescents and adults, of both sexes, mainly living in countries in Europe, North America and Australasia. There is a lack of higher-quality evidence of CBT’s effectiveness for older adults.
We do not know if CBT will be effective when delivered preventatively or when delivered to patients with severe or subclinical symptoms. We do not know if CBT is effective across different ethnic groups nor do we know its effect for people living in countries in Africa, Asia or South America.
Pain
We identified 10 higher-quality systematic reviews32,68,102,134,149,188,211,251,317,446 that met the eligibility criteria. These included 22 RCTs (2581 participants) and represent 5 out of 40 possible ICD-11 categories (13%). Box 7 presents the ICD-11 codes represented in the primary PMA (white rows), those codes represented in the sensitivity analysis (i.e. lower-quality reviews) (purple rows) and those codes not represented (orange rows).
-
01 Certain infectious or parasitic diseases.
-
02 Neoplasms.
-
03 Diseases of the blood.
-
04 Diseases of the immune system.
-
05 Endocrine, nutritional or metabolic diseases.
-
07 Sleep–wake disorders.
-
08 Diseases of the nervous system (only lower-quality reviews).
-
09 Diseases of the visual system.
-
10 Diseases of the ear or mastoid process.
-
11 Diseases of the circulatory system.
-
12 Diseases of the respiratory system.
-
13 Diseases of the digestive system [DA01.11 oral mucositis (related to cancer treatments)].
-
14 Diseases of the skin.
-
15 Diseases of the musculoskeletal system (FA00–05 osteoarthritis).
-
16 Diseases of the genitourinary system.
-
17 Conditions related to sexual health.
-
18 Pregnancy, childbirth or the puerperium.
-
19 Certain conditions originating in the perinatal period.
-
20 Developmental abnormalities.
-
21 Symptoms and signs NOS (MG30 pain).
-
6A00-06: neurodevelopmental disorders.
-
6A20-25: schizophrenia or other primary psychotic disorders.
-
6A40-41: catatonia.
-
6A60-80: mood disorders.
-
6B00-06: anxiety or fear-related disorders.
-
6B20-25: obsessive–compulsive disorders.
-
6B40-45: disorders specifically associated with stress.
-
6B60-66: dissociative disorders.
-
6B80-85: feeding or eating disorders.
-
6C00-01: elimination disorders.
-
6C20-21: disorders of bodily distress.
-
6C40-51: disorders due to substance use or addictive behaviours.
-
6C70-73: impulse control disorders.
-
6C90-91: disruptive behaviour or dissocial disorder.
-
6D10-11: personality disorders and related traits.
-
6D30-36: paraphilic disorders.
-
6D50-51: factitious disorders.
-
6D70-72: neurocognitive disorders.
-
6E20-21: mental or behavioural disorders associated with pregnancy, childbirth or the puerperium.
-
6E40: psychological or behavioural factors NOS.
White rows represent ICD-11 codes represented in the primary analysis, purple shaded rows represent ICD-11 codes represented in the sensitivity analyses and orange shaded rows represent ICD-11 codes that are not represented in the pain PMA.
The most commonly used measure of pain was the 100-mm visual analogue scale (VAS) (n = 6). Other measurements included the numerical rating scale of pain intensity (n = 3), the Wong–Baker Faces Pain Rating Scale (n = 2), the modified von Korff scale (n = 1), the Chronic Pain Grade questionnaire (n = 1) and the McGill Pain Questionnaire (n = 1).
The majority of these meta-analyses were focused on adults (6/10),102,134,149,188,211,317 three reviews focused on adolescents/children32,68,446 and one review did not report the age of the samples. 251 All of the reviews included samples that were equally balanced between male and female participants. Only one review63 reported the ethnicity of its samples (> 75% white participants).
Two reviews specified examining CBT in patients with chronic symptoms of pain, anxiety and mood conditions. Half of the reviews examined high-intensity and half of the reviews examined low-intensity CBT. One review examined using CBT to prevent pain developing post orthodontic treatments, but all the others were using CBT in response to diagnosed problems. Two reviews observed the use of CBT for inpatients with pain conditions, whereas the remaining reviews examined CBT in outpatient/community settings. Three reviews included long-term follow-ups (abdominal pain, back pain, anxiety and mood disorders). Four reviews included only one trial, and five reviews included < 100 participants (Figure 14).
FIGURE 14.
Primary analysis of the secondary outcome: pain from ‘higher-quality’ reviews. Adapted from Fordham et al. 524 © The Author(s) 2021. Published by Cambridge University Press. This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted re-use, distribution and reproduction, provided the original article is properly cited.
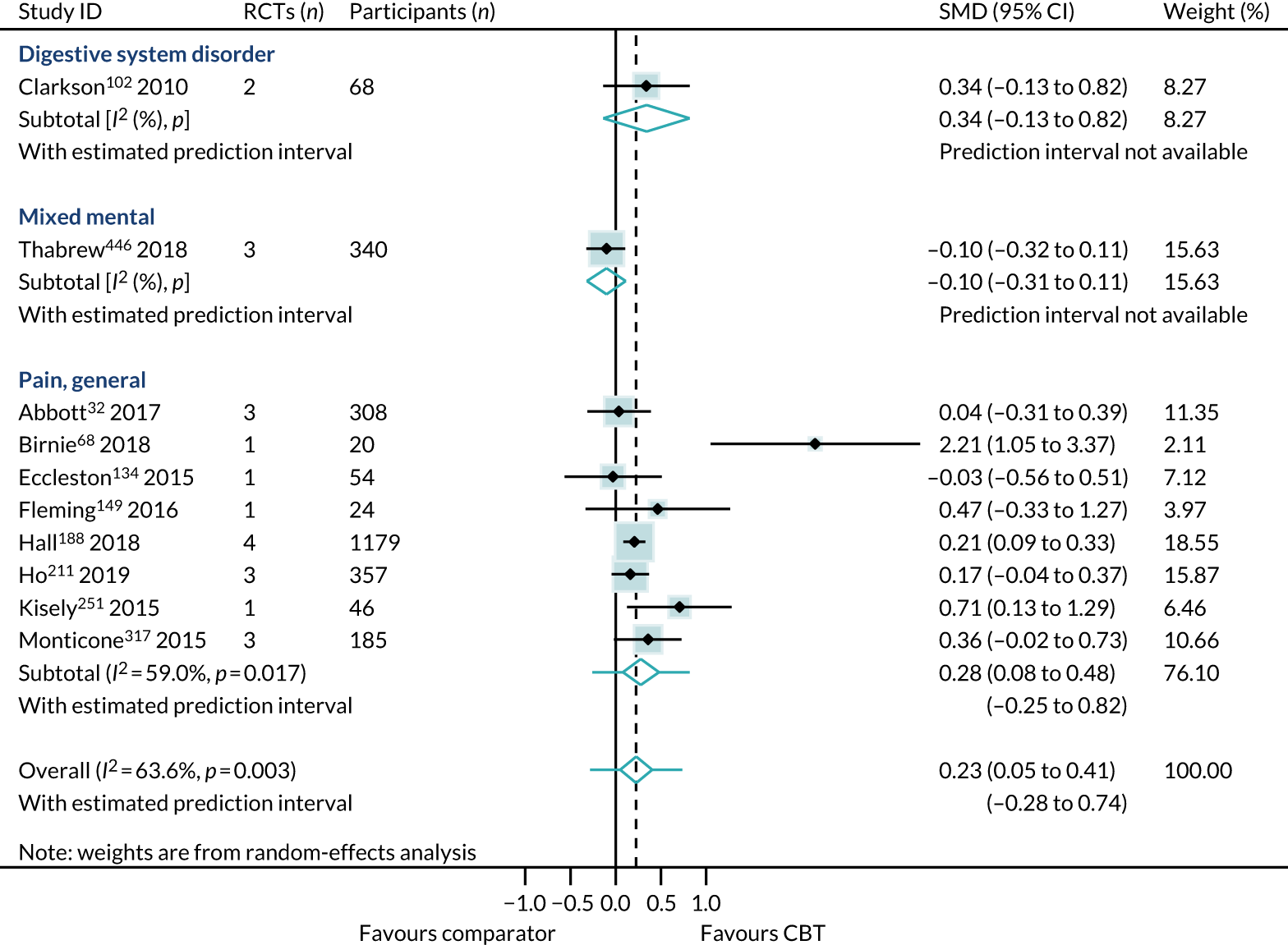
Primary analysis
The across-condition heterogeneity was 64% and the pooled across-condition SMD gave a modest effect in favour of CBT on outcomes of pain (SMD 0.23, 95% CI 0.05 to 0.41) (see Figure 14). The prediction intervals for the overall effect were –0.28 to 0.74.
There was no evidence of publication bias, nor of small-study effects (Eggers test p = 0.19) (see Appendix 12, Figure 30).
Mean difference in pain
We transformed the across-condition SMD into a mean difference of the most commonly reported pain outcome, the 100-mm VAS. We identified a standard deviation (27 mm) of the VAS from a trial527 with a low risk of bias in a higher-quality review. 32 The SMD translated to an estimated mean difference on the VAS of 6 mm (95% CI 1 to 11 mm).
Subgroup analysis
There were no statistically significant interaction effects between any subgroups; therefore, the evidence is consistent with the primary analysis.
Cognitive–behavioural intensity
High- and low-intensity CBT was examined in both children/adolescent and adult populations. The only review149 of preventative CBT delivered low-intensity treatment. There were no other differences in the populations or contexts tested with high- and low-intensity CBT.
The heterogeneity was too high to pool separately across the low-intensity CBT reviews (I2 = 84%). The low-intensity CBT reviews included trials examining CBT delivered by paraprofessionals (n = 4), through self-help tools (n = 6). The heterogeneity was much lower in high-intensity CBT reviews (SMD 0.19, 95% CI 0.01 to 0.37; I2 = 18%) (see Appendix 12, Figure 31). The interaction test between high- and low-intensity reviews was not statistically significant (p = 0.87).
No reviews directly compared the effectiveness of low-intensity compared with high-intensity CBT on pain outcomes. Therefore, no direct evidence is available to compare with our indirect evidence.
Type of comparators
The effect was larger when CBT was compared with non-active comparators (SMD 0.59, 95% CI 0.07 to 1.11; I2 = 69%) and was very small when compared with active comparators (SMD 0.14, 95% CI –0.11 to 0.38; I2 = 73%) (see Appendix 12, Figure 32). However, the difference between the two groups was not statistically significant when tested with the interaction test (p = 0.86).
Duration of follow-up
Effect estimates were higher in reviews reporting short-term follow-up (0.32, 95% CI 0.04 to 0.59; I2 = 70.5%) than in reviews reporting long-term follow-up (0.19, 95% CI 0.08 to 0.31; I2 = 0%) (see Appendix 12, Figure 33). However, the interaction test did not find a statistically significant difference between the groups (p = 0.62).
Age
The effect estimates extracted from reviews conducted in children and adolescent populations were too varied (I2 = 87%) to justify pooling across them. Conversely, there was 0% heterogeneity between the reviews in adult populations and the pooled effect was modest (SMD 0.21, 95% CI 0.12 to 0.31; I2 = 0%) (see Appendix 12, Figure 34). The interaction test between (1) children and adolescents and (2) adults did not find a significant difference between these groups (p = 0.68).
Sensitivity analysis
We identified 16 reviews (19 comparisons, 39 RCTs, 4592 participants)32,68,102,134,149,188,211,222,251,266,306,317,348,413,446,473 of any quality with data suitable for inclusion in the sensitivity pain PMA. This introduced 08: Nervous system disorders into the analysis. In the sensitivity analysis, all of the ICD-11 within-condition groups were consistent with the primary analysis for pain outcomes. Inclusion of lower-quality reviews marginally reduced the estimate of effect and heterogeneity (SMD 0.21, 95% CI 0.11 to 0.31; I2 = 51%), compared with the primary PMA (see Appendix 12, Figure 35).
Pain change scores/dichotomous data
The reviews that reported dichotomous data were consistent with the primary analysis. One lower-quality review, Palermo et al. ,354 presented pain outcome data as ORs. This review demonstrated a non-significant effect for CBT (SMD 7.99, 95% CI –2.72 to 18.70) (see Appendix 12, Figure 36). One lower-quality review, Bernardy et al. ,64 reported pain outcome data as risk differences and showed a non-significant effect for CBT (SMD 0.08, 95% CI –0.03 to 0.19) (see Appendix 12, Figure 36).
Discussion
From a smaller data set of the highest-quality reviews, we found that CBT produced consistent improvements in pain outcomes across six different conditions. Effect estimates suggest a modest long-term improvement in comparison with any other comparator intervention. We did not find a difference in the effect sizes between reviews conducted with low-intensity CBT and those conducted with high-intensity CBT.
The effect estimates were generated by synthesising data from samples of children, adolescents and adults, of both sexes, mainly living in countries in Europe, North America and Australasia. To our knowledge, there is no higher-quality evidence of CBT’s effectiveness for older adults.
The included reviews presented evidence to suggest that CBT can improve pain outcomes when CBT is delivered preventatively to patients with chronic or subclinical symptoms, but there is no evidence regarding severe symptoms. We do not know if CBT is equally effective across different ethnic groups, nor do we know its effect for people living in countries in Africa, Asia or South America.
Chapter 6 Generalisation
One of the aims of the project was to consider the extent to which the existing evidence base could be used to guide treatment, commissioning and research investment decisions.
This necessitated a layer of questions:
-
In the existing evidence base of systematic reviews, is there evidence of a general effect of CBT across different conditions (categorised by the ICD-11)?
Yes. Our PMA analyses (see Chapter 5) concluded that CBT does produce a general effect across conditions. We were able to meet the criteria of statistical, intervention and design homogeneity for our primary outcome of HRQoL, and two out of three secondary outcomes (anxiety and pain). We can feel confident in generalising the effect across these condition categories.
-
Can we assume that this effect is robust across all the specific conditions represented in each ICD-11 code?
In our methodology, we classified physical conditions at the primary ICD-11 code level and mental conditions at the secondary level. In each category, there are many subconditions. For some ICD-11 categories, such as musculoskeletal diseases (15), we have reviews representing three (arthropathies, spine conditions and osteopathies) of the five subcategories, whereas for others, such as respiratory disorders (12), we have reviews representing only one subcategory [lower respiratory tract diseases: asthma and chronic obstructive pulmonary disease (COPD)] out of six. We suggest that, as we found no evidence of inconsistent evidence, the effect of CBT will remain consistent across all conditions subsumed beneath each ICD-11 category. The consistency of effect mirrors and reassures the CBT field’s move towards transdiagnostic approaches. For example, literature suggests that a transdiagnostic manual is equally effective as condition-specific manuals for treating patients with all types of eating disorders (anorexia, bulimia, binge-eating and eating disorders not otherwise specified). 528 The benefit of using a transdiagnostic approach is that it is suitable for people with multiple conditions. Our PPI representatives suggested that this was a very important point, particularly because, with an ageing population, more and more people will live with multiple comorbidities.
-
Can we assume that this general effect is robust across conditions (ICD-11) that are represented by lower-quality reviews only?
Yes. We performed sensitivity analyses and found that the inclusion of lower-quality reviews increased the heterogeneity of review effect estimates, but did not alter the SMD. We can feel confident in generalising the effect across higher- and lower-quality systematic review evidence.
-
Can we assume that our general effect is robust across the populations and contexts?
Yes. We found no evidence of inconsistency in effect. We mapped the population and context details of the included reviews. We found no reviews, condition analyses or subgroup analyses that reported a statistically significant effect in favour of the comparator over CBT. Table 4 details the population, context and conditions of the reviews included in the primary HRQoL analyses. We identified each review from those populations or contexts that were under-represented and presented the individual review effect estimate. None of the individual or pooled meta-analyses produced an estimate that was inconsistent with the general effect of CBT on HRQoL.
| Generalisation parameter | Details from the included reviews |
|---|---|
| Condition | |
| Severity of symptoms | Most reviews examined the effect of CBT in patients who received a clinical diagnosis. Three reviews examined the effectiveness of CBT specifically for patients with chronic symptoms and found consistent effects: |
| Population | |
| Age | Reviews were conducted with children and adolescents (3/24) living with anxiety and/or mood445,446 and pain32 conditions and older adults with mood disorders (1/24)347 and adults (20/24) in nine different conditions (including anxiety, mood and pain conditions). The age subgroup analysis (see Chapter 5) did not identify a difference between children/adolescents and adults. The older adult review produced a consistent, but uncertain, effect (0.39, 95% CI –0.24 to 1.02)347 |
| Sex | The majority of the reviews were conducted with samples that were equally represented by male and female participants (21/24 reviews). One review of patients with neck pain had a female participant sample of > 75% and reported a consistent but uncertain effect (0.28, 95% CI –0.11 to 0.68).63 Two reviews reported samples with > 75% male participants. The review conducted in anxiety reported an uncertain but consistent effect (0.21, 95% CI –0.04 to 0.47).464 The effect was the same for the review conducted in bodily distress (0.36, 95% CI –0.11 to 0.82)469 |
| Ethnicity | Very few reviews reported the ethnicity of their samples. Of the five reviews that did, four included > 75% white participants. These all produced consistent evidence:
|
| Context | |
| Country | Five reviews included trials that were conducted in Asia. These were conducted in:All other trials included in the reviews were conducted in Europe, North America and Australasia |
| Health-care setting | Only five reviews recruited participants from inpatient settings. These were conducted in:All other review samples were recruited from community, primary and outpatient settings |
| Health-care timing | One review implemented CBT as a preventative intervention for patients with schizophrenia (0.09, 95% CI –0.21 to 0.39).219 The other reviews examined CBT delivered as a standard treatment |
-
Can we infer that this treatment effect might be observed across conditions that are not included in the current systematic review evidence base?
Our systematic approach meant that we classified reviews by the primary condition that the CBT was aiming to treat. For example, if a review examined the use of CBT to reduce symptoms of depression [i.e. a mood disorder (6A60-80)] in patients with COPD (12: respiratory disorder), then the review was classified as a review of the effectiveness of CBT for 6A60-80: Mood disorders with comorbid 12: Respiratory disorder (COPD). However, if CBT improves HRQoL in patients with mood disorders and comorbid COPD, then CBT is also improving quality of life for COPD patients. As quality of reviews did not affect the general effect, we generated a list of conditions for which CBT is effective from those reviews included in the sensitivity analyses. The following comorbid conditions are represented in the HRQoL, anxiety and pain outcome sensitivity analyses, but not represented in the primary list of conditions: intellectual disabilities (6A00-4), brain injury (6D70-2), dementia (6D70-2), migraines (08), epilepsy (08), circulatory diseases (11), COPD (12), irritable bowel syndrome (13), arthritis (15), tinnitus (21) and fatigue (21). Therefore, we conclude that CBT’s effect is consistent across 22 out of 40 ICD-11 codes (55%), as presented in Box 8.
-
01 Certain infectious or parasitic diseases (1C60-62: HIV).
-
02 Neoplasms.
-
03 Diseases of the blood.
-
04 Diseases of the immune system.
-
05 Endocrine, nutritional or metabolic diseases.
-
07 Sleep–wake disorders.
-
08 Diseases of the nervous system.
-
09 Diseases of the visual system.
-
10 Diseases of the ear or mastoid process.
-
11 Diseases of the circulatory system.
-
12 Diseases of the respiratory system.
-
13 Diseases of the digestive system.
-
14 Diseases of the skin.
-
15 Diseases of the musculoskeletal system.
-
16 Diseases of the genitourinary system (GA33 pregnancy loss).
-
17 Conditions related to sexual health.
-
18 Pregnancy, childbirth or the puerperium.
-
19 Certain conditions originating in the perinatal period.
-
20 Developmental abnormalities.
-
21 Symptoms and signs NOS (MG30 pain, MG22 fatigue, MC41 tinnitus).
-
6A00-06: neurodevelopmental disorders (6A02 autism, 6A00 intellectual disability and 6A05 ADHD).
-
6A20-25: schizophrenia or other primary psychotic disorders.
-
6A40-41: catatonia.
-
6A60-80: mood disorders.
-
6B00-06: anxiety or fear-related disorders.
-
6B20-25: obsessive–compulsive disorders.
-
6B40-45: disorders specifically associated with stress.
-
6B60-66: dissociative disorders.
-
6B80-85: feeding or eating disorders.
-
6C00-01: elimination disorders.
-
6C20-21: disorders of bodily distress.
-
6C40-51: disorders due to substance use or addictive behaviours.
-
6C70-73: impulse control disorders.
-
6C90-91: disruptive behaviour or dissocial disorder.
-
6D10-11: personality disorders and related traits.
-
6D30-36: paraphilic disorders.
-
6D50-51: factitious disorders.
-
6D70-72: neurocognitive disorders (6D80-86: dementia).
-
6E20-21: mental or behavioural disorders associated with pregnancy, childbirth or the puerperium.
-
6E40: psychological or behavioural factors NOS (MB23.0 aggressive behaviour).
HIV, human immunodeficiency virus; NOS, not otherwise specified.
Purple shaded rows indicate the ICD-11 categories that are not represented in any of the PMAs.
To generalise beyond the conditions and comorbidities represented in this overview is a challenging area for CBT, in which there are a diverse range of strongly held perspectives. We specified a priori that we would use a model of generalisation529,530 to guide our thinking, which necessitated that the evidence met three assumptions:
-
Statistical homogeneity in the systematic reviews being used to generate the estimate of effect. This was fulfilled.
-
Clinical homogeneity populations and contexts represented in the overview represent the contexts to which the generalisation is being made.
-
Shared mechanisms of action, which is the assumption that CBT effects change by changing the same or similar mediating variables across all conditions.
This would mean that, to assume an effect of CBT on HRQoL in a condition that has no systematic review evidence, there should be evidence of a general effect on HRQoL among similar patients, contexts and settings (i.e. there is no reason to believe that the treatment should work differently or be harmful). For the final assumption of shared mechanisms to be met, the highest level of evidence we would seek is that that from multiple mediation analyses.
As described, the ECG met on several occasions. We collectively agreed on the criteria for statistical homogeneity and the importance of consistency of effects, and that these were met in the PMA. For clinical homogeneity, we agreed the data extraction variables and framework, and were able to demonstrate where there is adequate evidence on context and where there is not adequate evidence (see Table 4). We demonstrated consistent effects for children, adolescents and adults, of both sexes, with clinical diagnoses, living in Europe, North America and Australasia, being treated in community, primary and secondary care settings with CBT being delivered in response to a diagnosis. However, there was less certainty for older adults, with subclinical, chronic or severe symptoms, living in Asia, Africa or South America, being treated in an inpatient setting or receiving CBT preventatively.
We did not search specifically for systematic reviews of mediation studies. At a basic level, the ECG and investigators were able to agree that the principles of CBT are the same regardless of the condition, namely that we intervene to modify thoughts and feelings, to influence behaviours and, ultimately, to improve health outcomes. The challenge is that the range of thoughts, feelings and behaviours is quite wide, and the ECG and investigators were unable to agree that CBT works through the same therapeutic mechanisms for every condition for which it has been used. The ECG recommended that future research should target identifying what the mechanisms of CBT are for each population. If these mechanisms could improve symptoms in other populations, then the evidence for effectiveness could be meaningfully generalised across the conditions. This will be a judgement call and there are likely to be situations in which details on mechanisms are available and similar to those included in reviews, and that the context and characteristics align to provide confidence in broader generalisation. The ECG suggested that a systematic review of mediation studies of CBT would be helpful in this respect, but this was outside the scope of this study.
Chapter 7 Discussion
Principal findings and their meaning
Cognitive–behavioural therapy has been evaluated (with systematic reviews) in most conditions (68%, 27/40 of ICD-11 categories). These reviews have summarised the RCT evidence of whether or not CBT improved outcomes in these conditions. The review estimates were similar enough between the different conditions for us to generate a general (as opposed to a condition-specific) effect estimate. We found that CBT produced a modest general benefit to HRQoL, anxiety and pain outcomes. The evidence was consistent across all 22 out of 40 (55%) conditions (and comorbidities), populations and contexts that have been tested.
The estimates for depression outcomes between conditions were too different; therefore, we could not produce a pooled general effect estimate. Although there were many more reviews in the depression PMA, the reviews used fewer different outcome measurements than in HRQoL, anxiety and pain PMAs. Therefore, it is unlikely that the high heterogeneity is due to the variation in the outcome measurements used. CBT has been shown to be very effective for people with clinical depression. 531,532 Our overview does not suggest that CBT is not effective for symptoms of depression, only that there was a great variation in how effective it was for changing depression symptoms across different conditions.
Cognitive–behavioural therapy was effective whether it was delivered in high- or low-intensity formats. This is not to imply that CBT can be delivered in high- or low-intensity formats interchangeably. The findings simply state that when low-intensity CBT has been tested in RCTs and synthesised into reviews, we found that it improved HRQoL, anxiety and pain outcomes. This adds strength to the argument that the mechanisms by which CBT is effective remain effective when delivered via high- or low-intensity formats.
Cognitive–behavioural therapy was effective in the short and long term. However, there was a paucity of reporting on the longer-term follow-ups (i.e. > 5 years post intervention); therefore, we have not captured the importance of relapse. We highlighted in the mapping exercise that there is a paucity of systematic review evidence regarding relapse prevention; this is an essential consideration to take into account when interpreting these findings.
When we pooled reviews that compared CBT with active interventions (e.g. pharmacotherapy, psychotherapy, exercise, education or relaxation), the effect estimates became very small. We found a significant interaction in the HRQoL analyses between those reviews that compared CBT with an active comparator and those that compared CBT with an inactive comparator. This could suggest that CBT and these other active interventions share mechanisms that improve HRQoL for patients.
We assume that CBT will help children, adolescents and adults, but we are uncertain as to how much it will help older adults, as there is less available evidence for this age population. We feel confident that CBT will be equally effective for male and female participants. The evidence base over-represents people who live in Europe, North America and Australasia, and poorly reports the ethnicity of the samples in the reviews. Consequently, we do not know if the effects will translate across people of different ethnicities in Europe, North America or Australasia or, to people who live in Asia, Africa or South America.
Strengths
When an individual systematic review pools evidence across many trials, the sample sizes can remain small. Small sample sizes mean that the effect estimate is less certain. One of the major strengths of this overview is that, by pooling data from many reviews across conditions, we become more certain of the effect estimates. Our HRQoL and anxiety outcome estimates include > 4000 participants, which guidance suggests indicates a certain effect. 533
To maintain the lowest risk of bias and the greatest design homogeneity, we conducted our primary analyses with the highest-quality (rated ‘moderate’ or ‘high’ on the AMSTAR-2 checklist) reviews. The most common criticism of all the reviews was that we, as readers, could not access a review protocol to check if the authors had performed what they had intended to perform and had not simply ‘cherry-picked’ results to present in the review publication. Another common problem was reviews not reporting the reasons why they excluded trials from their review. Without this information, we cannot check if there was any bias towards including some, but not other, trials. Our sensitivity analyses suggest that the higher-quality reviews report consistent findings (less heterogeneity), compared with poorer-quality reviews, but the quality of the reviews did not alter the effect estimates.
As the inclusion of the lower-quality reviews did not alter the effect estimates, we concluded that the general effect is consistent across conditions represented by higher- and lower-quality reviews. We also suggested that the effect could be generalised to comorbid conditions represented by these reviews. Consequently, the general effect can be generalised to over half (55%) of all conditions represented in the ICD-11.
Weaknesses
The main methodological weakness was due to our restriction in remaining at the review level, as opposed to including RCT-level extraction and analysis. The mapping exercise identified 494 reviews. Of these, 279 were not included in the PMA because we could not extract the purely CBT RCT evidence. For example, a review that synthesised 10 CBT RCTs with three non-RCTs would be excluded from the PMA unless the review had presented any of the purely RCT evidence in isolation (even if it was one single RCT, which we could include). Similarly, if a review included 30 CBT RCTs combined with six mindfulness-based cognitive therapy RCTs, then this would have been excluded unless the review also presented a separate CBT subgroup analysis. The only way we would have been able to include these RCTs would have been to return to the original RCTs, extract the data and perform a meta-analysis of those data for entry into the PMA. This was a conflicting decision. The evidence base was so large that we did not have the resources to perform RCT-level extraction or analysis, but this was at the expense of many RCTs being excluded from the PMAs.
Another consequence of remaining at the review level was the limitation of the quality assessments. A review of high quality may include RCTs judged to have a high risk of bias. Without performing additional RCT-level assessment and a separate analysis of RCTs with low risks of bias, we could not restrict the data to the best-quality RCT-level data.
This overview does not examine the health economics of the CBT evidence base, which is an essential element of commissioning and is the context of evidence-based medicine. We could not perform this analysis because it was beyond the scope of this current overview.
Our method for classifying reviews was to represent each review in one ICD-11 code. We classified the review by the primary condition the CBT was being used to treat. For example, a review of CBT for depression in COPD patients was classified as a review of CBT for depression with comorbid COPD. This meant that we could not reflect the multimorbidity represented in these reviews. A total of 158 out of 494 reviews included a comorbid condition such as alcohol abuse or dementia. Our methodology means that we have under-represented the number of different conditions for which CBT has been used to improve HRQoL and reduce symptoms of depression, anxiety and pain.
We have mapped the systematic review data across each condition and by the following groups: ‘who’ (populations with different clinical severity), ‘what’ (the CBT intensity format) and ‘when’ (delivered at what time, i.e. preventatively, in response to clinical diagnosis or as a relapse prevention). We were restricted to reporting and analysing the review-level data. Reviews often combined RCTs conducted across multiple subgroups. We did not perform RCT-level exploration of the subgroups, which limits the accuracy of our findings.
Our indirect (intensity subgroup analyses) and direct (high- compared with low-intensity CBT reviews) evidence suggested no difference in effectiveness between using high-intensity and using low-intensity CBT. However, although reviews of high-intensity CBT produced broadly similar estimates of CBT’s effectiveness, the estimates from low-intensity reviews varied widely. The large variation in the low-intensity CBT estimates may be due to our definition of low-intensity CBT. 1 We combined face-to-face delivery of CBT by paraprofessionals with self-help delivery of CBT (e.g. internet CBT). Future subgroup analyses could test if these two methods of delivery moderate the effectiveness of CBT.
When a review did not report how CBT was delivered in the included trials (i.e. high- or low-intensity CBT), we assumed that it was delivered face to face by a specialist (high intensity). We made this assumption because high-intensity CBT was the original and most common delivery method. When we developed our data extraction methods, we checked the trials in reviews that did not specify the CBT intensity. We found that these trials had tested high-intensity CBT. However, we did not check the included trials for every review included in our overview; therefore, this assumption may have led to us over-representing high-intensity CBT.
We made another assumption, whereby, if a review did not specify the time when the follow-up data were collected, we presumed that it was short term (< 12 months post intervention). We made this assumption because the majority of trials employed short-term follow-ups. However, as before, this assumption may be incorrect for some reviews.
Although most reviews estimated their effects with a random-effects meta-analysis, a few used a fixed-effects approach. We made an assumption that the within-review variability had been appropriately allowed for. If this assumption was incorrect, then our results might underestimate the amount of variation within conditions.
To make a meaningful interpretation of our effect estimates, we transformed them into mean differences using the standard deviation of the target outcome measure. If the value of this standard deviation is not a good approximate to the true standard deviation for this outcome, we might be underestimating or overestimating the effect size for each outcome considered.
We used techniques, such as using workbooks to record personal reflections, in the ECG meetings to ensure that each member could contribute equally. However, the debates often became polarised between academic discussions. Nevertheless, talking to PPI representatives more informally and during breaks generated rich feedback that helped the research group. On reflection, we should have included a formal forum at the end of each ECG meeting in which every member could summarise the day’s discussion and ask specific questions.
Implications
We have high-quality systematic review evidence, which demonstrates that, in comparison to no intervention, CBT improves HRQoL, anxiety and pain outcomes by a modest amount. This includes CBT that is delivered through low- and high-intensity formats. The benefit has been consistent in every condition, population and context in which it has been tested and synthesised into a systematic review. There are some conditions and contexts in which we are less certain about generalising the estimates from the PMA. These include conditions for which there is no similar condition already included in the review (e.g. vision impairments), or if CBT is being applied in contexts that we believe will vary substantially because of, among other things, cultural issues and health beliefs.
We have used a framework of broader generalisation that has considered pathophysiological rationale alongside the traditional quality indicators used in evidence-based medicine. 534,535 The early proponents of evidence-based medicine were more subtle in their approach, and demanded that values, circumstances, expertise and even pathophysiologic rationale be considered, especially when generalising evidence from clinical trials. 536,537 The most prominent evidence-based medicine rule of evidence is the GRADE system, which does not allow any role for pathophysiologic rationale at all, even though it does allow for recommendations to populations outside the trial (generalising). 5 We suggested that using the results of the PMA alongside knowledge of mechanistic actions of CBT in particular situations may enable the generalisation of this effective treatment (CBT) to a greater range of physical and mental conditions (and hence patients).
Chapter 8 Conclusion
Cognitive–behavioural therapy can help patients cope with the challenges of living with mental and physical conditions. We have found that it consistently improves quality of life and reduces anxiety and pain symptoms for people living with many different conditions across the 19 ICD-11 categories for which we have systematic review evidence. CBT has been tested in many different populations and contexts, and all these reviews report effects that are consistent with our general effects. High- and low-intensity CBT appear to be equally effective. The biggest area of uncertainty is around whether sociological constructs, such as ethnicity, religion, culture, country or language, could moderate the effectiveness of CBT or whether it will be equally effective across these constructs. We suggest that CBT will be effective for the conditions represented in the ICD-11 codes we have represented in the overview. However, we are unclear if the general effect can be applied to conditions that are not represented at all in the overview.
Chapter 9 Recommendations
Future research
The overview suggests a general benefit of CBT in physical and mental conditions. However, we also identified some unanswered questions. These are not presented in an order of priority:
-
We do not know what the mediating variables for CBT’s effectiveness are, nor do we know whether or not these are different across conditions. We recommend a review or an overview of CBT mechanisms across conditions.
-
We do not know the longer-term effects of CBT. Reviews of CBT trials that monitor their participants over ≥ 5 years, and that account for the relapse in patients, are needed to see if the effects remain across all conditions.
-
We found that CBT produced a modest benefit in HRQoL, anxiety and pain outcomes. Clinical research should focus on identifying how these effects from CBT can be magnified. For example –
-
Examining if the delivery of CBT can be modified to increase adherence and reduce dropouts (e.g. examining if the location of therapy delivery influences attendance rates).
-
Prioritising assessments of treatment fidelity and quality to check whether or not the therapists delivering CBT are adhering to the same core CBT principles. 1 A novel method to check the content of face-to-face CBT consultations is to use artificial intelligence to monitor if a therapist has used a core CBT technique.
-
-
We were not confident to recommend that CBT would work equally for older adults because of an absence of evidence. We are aware of new reviews and existing trials that suggest that CBT will be equally effective for older adults (aged > 65 years). 538 For example, a large, recent review found that the effects for psychotherapy (including CBT) on depression outcomes were equal between adults (aged 24–55 years), older adults (aged 55–75 years) and the oldest adults (aged > 75 years old). 538
-
We were unsure, owing to a lack of reporting on ethnicity of trial samples, whether or not CBT would be equally effective across ethnic groups. Another review of psychotherapy (including CBT) on depression outcomes between racial/ethnic groups concluded that race/ethnicity did not moderate the effectiveness of psychotherapy. However, authors highlight the problems of defining an ethnic group and encourage future research to include country of residence, language, religion and race in a definition of ethnicity. 539
-
Owing to an absence of evidence of trials conducted in Africa, Asia and South America, we were unsure if the general effects of CBT would apply to people living in those countries. The overview excluded reviews not published in English. Future research could prioritise reviews of CBT that include trials conducted in Africa, Asia or South America to see if the effects are the same as they have been in Europe, North America and Australasia.
Acknowledgements
Cognitive Behavioural Therapy – Overview Expert Consultation Group (CBT-O ECG)
-
Bethan Copsey, Medical Statistician, School of Medicine, University of Leeds.
-
Roshan das Nair, Professor of Clinical Psychology, School of Medicine, University of Nottingham.
-
Katherine Edwards, Research Assistant, Reviews and Implementation Group, University of Liverpool.
-
Douglas Findlay, Patient and Public Representative, who was an ECG member and reviewed the report.
-
Beth Fordham, Senior Psychology Fellow, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences (NDORMS), University of Oxford.
-
Daniel Freeman, Professor of Clinical Psychology, Department of Psychiatry, University of Oxford, who was a member of the ECG and reviewed the report.
-
Zara Hansen, CBT therapist specialising in pain conditions, NDORMS, University of Oxford.
-
Karla Hemming, Professor of Biostatistics, Institute of Applied Health Research Professor of Biostatistics, University of Birmingham.
-
Robert Howard, Professor of Psychiatry, Institute of Mental Health, University College London.
-
Jeremy Howick, Senior Fellow, Department of Philosophy, University of Oxford.
-
Sarah E Lamb, Mireille Gillings Professor of Health Innovation, College of Medicine and Health, University of Exeter.
-
Hopin Lee, Research Fellow, NDORMS, University of Oxford.
-
Richard Lilford, Professor and Director of National Institute for Health Research Applied Research Centre West Midlands, University of Birmingham, who was a member of the ECG.
-
Michael Lyle, Patient and Public Representative, who was a member of the ECG.
-
Paul Salkovskis, Professor of Clinical Psychology and Director for the Doctorate in Clinical Psychology, University of Oxford, who was a member of the ECG and reviewed the report.
-
Michael Sharpe, Professor of Psychological Medicine, Department of Psychiatry, University of Oxford.
-
Paul Stallard, Professor of Child and Family Mental Health, Department for Health, University of Bath.
-
Thavapriya Sugavanam, Research Fellow, NDORMS, University of Oxford.
Personal communications
Personal communication with Georgina MacKenzie, PROSPERO Project Manager/Research Project Support Officer, 2018 regarding operationalising the CRD guidelines.
Personal communication with Beverly Shea, developer of the AMSTAR-2 checklist, 2019, regarding the re-classification of the four AMSTAR-2 quality ratings into higher (‘high’ and ‘moderate’ AMSTAR-2 ratings) and lower quality (‘low’ and ‘critically low’ AMSTAR-2 ratings) reviews.
Contributions of authors
Dr Beth Fordham (https://orcid.org/0000-0001-5996-3563) (Senior Health Psychology Fellow) was the principal investigator and the grant holder.
Dr Thavapriya Sugavanam (https://orcid.org/0000-0002-3033-2028) (Post-doctoral Research Assistant) contributed to the protocol development, database selection, search term selection, reference management, title/abstract screening, full-text screening, data extraction, quality assessment, data analysis, interpretation and report writing.
Ms Katherine Edwards (https://orcid.org/0000-0002-1092-0092) (Research Associate) contributed to the full-text retrieval, reference management, title/abstract screening, full-text screening, data extraction, quality assessment, data analysis, interpretation and report writing.
Professor Karla Hemming (https://orcid.org/0000-0002-2226-6550) (Professor of Biostatistics) has expertise in PMA. She made a large contribution to the design, procedure, analysis, interpretation and report writing.
Dr Jeremy Howick (https://orcid.org/0000-0003-0280-7206) (Senior Fellow) has expertise in Epistemology Philosophy of Medicine. He developed the generalisation methodology and contributed to the report writing.
Dr Bethan Copsey (https://orcid.org/0000-0001-9783-6549) (Post-doctoral Research Fellow) designed and ran the PMA and contributed to the data extraction and interpretation.
Dr Hopin Lee (https://orcid.org/0000-0001-5692-0314) (Post-doctoral Research Fellow) contributed to the data analysis, interpretation and writing.
Ms Milla Kaidesoja (https://orcid.org/0000-0003-0372-8251) (HiLIFE Research Trainee) contributed to the data extraction, generalisation and interpretation.
Ms Shona Kirtley (https://orcid.org/0000-0002-7801-5777) (Senior Information Specialist) led the database search and keyword selection aspects of the overview and commented on the methods section of the final report.
Assistant Professor Sally Hopewell (https://orcid.org/0000-0002-6881-6984) (Associate Professor of Trials and Reviews) has methodology expertise and contributed to the design, procedure and interpretation.
Professor Roshan das Nair (https://orcid.org/0000-0001-8143-7893) (Professor of Clinical Psychology) contributed to the methodological design, interpretation and report writing. Professor das Nair has a special interest in cultural and minority representation.
Professor Robert Howard (https://orcid.org/0000-0002-3071-2338) (Professor of Psychiatry) was a member of the ECG and contributed to the report writing and dissemination.
Professor Paul Stallard (https://orcid.org/0000-0001-8046-0784) (Professor of Child and Family Mental Health) was a member of the ECG and contributed to the report writing and dissemination.
Ms Julia Hamer-Hunt (Patient and Public Representative) was an ECG member, reviewed the report and contributed to the plain English summary of the report.
Professor Zafra Cooper (https://orcid.org/0000-0001-7963-656X) (Professor of Psychiatry) was a member of the ECG, reviewed the report and contributed to the report writing.
Professor Sarah E Lamb (https://orcid.org/0000-0003-4349-7195) (Professor of Rehabilitation) was mentor to Dr Beth Fordham throughout the entire project. She contributed to every stage of this project from the design to the interpretation and writing.
Publication
Fordham B, Sugavanam T, Edwards K, Stallard P, Howard R, das Nair R, et al. The evidence for cognitive behavioural therapy in any condition, population or context: a meta-review of systematic reviews and panoramic meta-analysis. Psychol Med 2021;51:21–9.
Data-sharing statement
Requests for access to data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Roth AD, Pilling S. The Competencies Required to Deliver Effective Cognitive and Behavioural Therapy for People with Depression and with Anxiety Disorders. London: Department of Health and Social Care; 2007.
- Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit Ther Res 2012;36:427-40. https://doi.org/10.1007/s10608-012-9476-1.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 n.d. https://doi.org/10.1002/9781119536604 (accessed April 2020).
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358. https://doi.org/10.1136/bmj.j4008.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. https://doi.org/10.1136/bmj.39489.470347.AD.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred Reporting Items for Systematic reviews and Meta-Analyses: the PRISMA statement. BMJ 2009;339. https://doi.org/10.1136/bmj.b2535.
- Kirtley A. Preliminary Analysis of the UK Mental Health Research Funding Landscape: Psychological Treatments Research 2008–2013 2015. https://b.3cdn.net/joinmq/3b5f867c4e9564bdcd_24m6be88j.pdf (accessed April 2020).
- Fonagy P. Expanding the shrinks. The Economist 2014.
- Lorne AB, Andrew DO, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London: Cochrane; 2011.
- Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-15.
- O’Donnell A, Anderson P, Newbury-Birch D, Schulte B, Schmidt C, Reimer J, et al. The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews. Alcohol Alcohol 2014;49:66-78. https://doi.org/10.1093/alcalc/agt170.
- Ryan R, Santesso N, Lowe D, Hill S, Grimshaw J, Prictor M, et al. Interventions to improve safe and effective medicines use by consumers: an overview of systematic reviews. Cochrane Database Syst Rev 2014;4. https://doi.org/10.1002/14651858.CD007768.pub3.
- World Health Organization . ICD-11 for Mortality and Morbidity Statistics (ICD-11 MMS): 2018 Version n.d. https://icd.who.int/browse11/l-m/en (accessed 21 June 2018).
- Hemming K, Bowater RJ, Lilford RJ. Pooling systematic reviews of systematic reviews: a Bayesian panoramic meta-analysis. Stat Med 2012;31:201-16. https://doi.org/10.1002/sim.4372.
- Hemming K, Pinkney T, Futaba K, Pennant M, Morton DG, Lilford RJ. A systematic review of systematic reviews and panoramic meta-analysis: staples versus sutures for surgical procedures. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0075132.
- Fordham B, Sugavanam T, Hopewell S, Hemming K, Howick J, Kirtley S, et al. Effectiveness of cognitive-behavioural therapy: a protocol for an overview of systematic reviews and meta-analyses. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-025761.
- Centre for Reviews and Dissemination . Undertaking Systematic Reviews of Research on Effectiveness: CRD’s Guidance for Carrying Out or Commissioning Reviews (CRD Report 4) 2001.
- Information Specialists’ Sub-Group (ISSG) . Filters to Identify Systematic Reviews n.d. https://sites.google.com/a/york.ac.uk/issg-search-filters-resource/filters-to-identify-systematic-reviews (accessed 3 March 2017).
- Health Information Research Unit . Search Strategies for PsycINFO in Ovid Syntax n.d. https://hiru.mcmaster.ca/hiru/HIRU_Hedges_PsycINFO_Strategies.aspx (accessed 10 January 2018).
- Grossman PB, Hughes JN. Self-control interventions with internalizing disorders: a review and analysis. School Psychol Rev 1992;21:229-45. https://doi.org/10.1080/02796015.1992.12085609.
- AMSTAR . AMSTAR Checklist n.d. https://amstar.ca/Amstar_Checklist.php (accessed 24 September 2019).
- Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall; 1991.
- Tibco Spotfire Inc . TIBCO Spotfire Analytics n.d. www.tibco.com/products/tibco-spotfire (accessed 4 October 2019).
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. https://doi.org/10.1136/bmj.327.7414.557.
- Deeks JJ, Higgins JPT, Altman DG, Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0. London: Cochrane; 2019.
- DerSimonian R, Laird N. Meta-analysis in clincial trials. Control Clin Trials 1986;7:177-88. https://doi.org/10.1016/0197-2456(86)90046-2.
- Higgins JPT, Li T, Deeks JJ, Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0. London: Cochrane; 2019.
- Vickers AJ, Altman DG. Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ 2001;323:1123-4. https://doi.org/10.1136/bmj.323.7321.1123.
- Holger JS, Gunn EV, Julian PH, Nancy S, Jonathan JD, Paul G, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0. London: Cochrane; 2019.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. https://doi.org/10.1136/bmj.315.7109.629.
- Monk R. Ludwig Wittgenstein: The Duty of Genius. London: Vintage Books; 1990.
- Abbott RA, Martin AE, Newlove-Delgado TV, Bethel A, Thompson-Coon J, Whear R, et al. Psychosocial interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev 2017;1. https://doi.org/10.1002/14651858.CD010971.pub2.
- Ackermann RT, Williams JW. Rational treatment choices for non-major depressions in primary care: an evidence-based review. J Gen Intern Med 2002;17:293-301. https://doi.org/10.1046/j.1525-1497.2002.10350.x.
- Adelman CB, Panza KE, Bartley CA, Bontempo A, Bloch MH. A meta-analysis of computerized cognitive-behavioral therapy for the treatment of DSM-5 anxiety disorders. J Clin Psychiatry 2014;75:e695-704. https://doi.org/10.4088/JCP.13r08894.
- Adelufosi A, Edet B, Arikpo D, Aquaisua E, Meremikwu MM. Cognitive behavioral therapy for post-traumatic stress disorder, depression, or anxiety disorders in women and girls living with female genital mutilation: a systematic review. Int J Gynaecol Obstet 2017;136:56-9. https://doi.org/10.1002/ijgo.12043.
- Ahern E, Kinsella S, Semkovska M. Clinical efficacy and economic evaluation of online cognitive behavioral therapy for major depressive disorder: a systematic review and meta-analysis. Expert Rev Pharmacoecon Outcomes Res 2018;18:25-41. https://doi.org/10.1080/14737167.2018.1407245.
- Akechi T, Okuyama T, Onishi J, Morita T, Furukawa TA. Psychotherapy for depression among incurable cancer patients. Cochrane Database Syst Rev 2008;2. https://doi.org/10.1002/14651858.CD005537.pub2.
- Al Sayegh A, Sandford D, Carson AJ. Psychological approaches to treatment of postconcussion syndrome: a systematic review. J Neurol Neurosurg Psychiatry 2010;81:1128-34. https://doi.org/10.1136/jnnp.2008.170092.
- Ali A, Hall I, Blickwedel J, Hassiotis A. Behavioural and cognitive–behavioural interventions for outwardly-directed aggressive behaviour in people with intellectual disabilities. Cochrane Database Syst Rev 2015;4. https://doi.org/10.1002/14651858.CD003406.pub4.
- Álvarez-Jiménez M, Parker AG, Hetrick SE, McGorry PD, Gleeson JF. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull 2011;37:619-30. https://doi.org/10.1093/schbul/sbp129.
- Amato Nesbit S, Sharma R, Waldfogel JM, Zhang A, Bennett WL, Yeh HC, et al. Non-pharmacologic treatments for symptoms of diabetic peripheral neuropathy: a systematic review. Curr Med Res Opin 2019;35:15-2. https://doi.org/10.1080/03007995.2018.1497958.
- Amorim CSM, Espirito Santo AS, Sommer M, Marques AP. Effect of physical therapy in bruxism treatment: a systematic review. J Manipulative Physiol Ther 2018;41:389-404. https://doi.org/10.1016/j.jmpt.2017.10.014.
- Anagnostopoulou N, Kyriakopoulos M, Alba A. Psychological interventions in psychosis in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry 2019;28:735-46. https://doi.org/10.1007/s00787-018-1159-3.
- Andersen P, Toner P, Bland M, McMillan D. Effectiveness of transdiagnostic cognitive behaviour therapy for anxiety and depression in adults: a systematic review and meta-analysis. Behav Cogn Psychother 2016;44:673-90. https://doi.org/10.1017/S1352465816000229.
- Andersson G, Cuijpers P, Carlbring P, Riper H, Hedman E. Guided internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: a systematic review and meta-analysis. World Psychiatry 2014;13:288-95. https://doi.org/10.1002/wps.20151.
- Andrews G, Basu A, Cuijpers P, Craske M, McEvoy P, English C, et al. Computer therapy for the anxiety and depression disorders is effective, acceptable and practical health care: an updated meta-analysis. J Anxiety Disord 2018;55:70-8. https://doi.org/10.1016/j.janxdis.2018.01.001.
- Anie KA, Green J. Psychological therapies for sickle cell disease and pain. Cochrane Database Syst Rev 2015;5. https://doi.org/10.1002/14651858.CD001916.pub3.
- Antoniades J, Mazza D, Brijnath B. Efficacy of depression treatments for immigrant patients: results from a systematic review. BMC Psychiatry 2014;14. https://doi.org/10.1186/1471-244X-14-176.
- Apóstolo J, Queirós P, Rodrigues M, Castro I, Cardoso D. The effectiveness of nonpharmacological interventions in older adults with depressive disorders: a systematic review. JBI Database System Rev Implement Rep 2015;13:220-78. https://doi.org/10.11124/jbisrir-2015-1718.
- Arends I, Bruinvels DJ, Rebergen DS, Nieuwenhuijsen K, Madan I, Neumeyer-Gromen A, et al. Interventions to facilitate return to work in adults with adjustment disorders. Cochrane Database Syst Rev 2012;12. https://doi.org/10.1002/14651858.CD006389.pub2.
- Armando M, Pontillo M, Vicari S. Psychosocial interventions for very early and early-onset schizophrenia: a review of treatment efficacy. Curr Opin Psychiatry 2015;28:312-23. https://doi.org/10.1097/YCO.0000000000000165.
- Armento ME, Stanley MA, Marsh L, Kunik ME, York MK, Bush AL, et al. Cognitive behavioral therapy for depression and anxiety in Parkinson’s disease: a clinical review. J Parkinsons Dis 2012;2:135-51. https://doi.org/10.3233/JPD-2012-12080.
- Arnberg FK, Linton SJ, Hultcrantz M, Heintz E, Jonsson U. Internet-delivered psychological treatments for mood and anxiety disorders: a systematic review of their efficacy, safety, and cost-effectiveness. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0098118.
- Baardseth TP, Goldberg SB, Pace BT, Wislocki AP, Frost ND, Siddiqui JR, et al. Cognitive–behavioral therapy versus other therapies: redux. Clin Psychol Rev 2013;33:395-40. https://doi.org/10.1016/j.cpr.2013.01.004.
- Babowitch JD, Antshel KM. Adolescent treatment outcomes for comorbid depression and substance misuse: a systematic review and synthesis of the literature. J Affect Disord 2016;201:25-33. https://doi.org/10.1016/j.jad.2016.04.018.
- Bagnall A-M, Hempel S, Chambers D, Orton V, Forbes C. The Treatment and Management of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis in Adults and Children. York: University of York; 2007.
- Bandelow B, Seidler-Brandler U, Becker A, Wedekind D, Rüther E. Meta-analysis of randomized controlled comparisons of psychopharmacological and psychological treatments for anxiety disorders. World J Biol Psychiatry 2007;8:175-87. https://doi.org/10.1080/15622970601110273.
- Barrett K, Chang YP. Behavioral interventions targeting chronic pain, depression, and substance use disorder in primary care. J Nurs Scholarsh 2016;48:345-53. https://doi.org/10.1111/jnu.12213.
- Beltman MW, Voshaar RC, Speckens AE. Cognitive–behavioural therapy for depression in people with a somatic disease: meta-analysis of randomised controlled trials. Br J Psychiatry 2010;197:11-9. https://doi.org/10.1192/bjp.bp.109.064675.
- Bender JL, Radhakrishnan A, Diorio C, Englesakis M, Jadad AR. Can pain be managed through the internet? A systematic review of randomized controlled trials. Pain 2011;152:1740-50. https://doi.org/10.1016/j.pain.2011.02.012.
- Bennett S, Shafran R, Coughtrey A, Walker S, Heyman I. Psychological interventions for mental health disorders in children with chronic physical illness: a systematic review. Arch Dis Child 2015;100:308-16. https://doi.org/10.1136/archdischild-2014-307474.
- Bernard P, Romain AJ, Caudroit J, Chevance G, Carayol M, Gourlan M, et al. Cognitive behavior therapy combined with exercise for adults with chronic diseases: systematic review and meta-analysis. Health Psychol 2018;37:433-50. https://doi.org/10.1037/hea0000578.
- Bernardy K, Klose P, Busch AJ, Choy EH, Häuser W. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev 2013;9. https://doi.org/10.1002/14651858.CD009796.pub2.
- Bernardy K, Klose P, Welsch P, Häuser W. Efficacy, acceptability and safety of internet-delivered psychological therapies for fibromyalgia syndrome: a systematic review and meta-analysis of randomized controlled trials. Eur J Pain 2019;23:3-14. https://doi.org/10.1002/ejp.1284.
- Berry E, Davies M, Dempster M. Exploring the effectiveness of couples interventions for adults living with a chronic physical illness: a systematic review. Patient Educ Couns 2017;100:1287-303. https://doi.org/10.1016/j.pec.2017.02.015.
- Bighelli I, Salanti G, Huhn M, Schneider-Thoma J, Krause M, Reitmeir C, et al. Psychological interventions to reduce positive symptoms in schizophrenia: systematic review and network meta-analysis. World Psychiatry 2018;17:316-29. https://doi.org/10.1002/wps.20577.
- Bird V, Premkumar P, Kendall T, Whittington C, Mitchell J, Kuipers E. Early intervention services, cognitive–behavioural therapy and family intervention in early psychosis: systematic review. Br J Psychiatry 2010;197:350-6. https://doi.org/10.1192/bjp.bp.109.074526.
- Birnie KA, Noel M, Chambers CT, Uman LS, Parker JA. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev 2018;10. https://doi.org/10.1002/14651858.CD005179.pub4.
- Bisson JI. Post-traumatic stress disorder. BMJ 2015;351. https://doi.org/10.1136/bmj.h6161.
- Blainey S. Exploring Self Help Interventions Following Traumatic Experiences. Sheffield: University of Sheffield; 2012.
- Bledsoe SE, Grote NK. Treating depression during pregnancy and the postpartum: a preliminary meta-analysis. Res Soc Work Pract 2006;16:109-20. https://doi.org/10.1177/1049731505282202.
- Bohra MH, Espie CA. Is cognitive behavioural therapy for insomnia effective in treating insomnia and pain in individuals with chronic non-malignant pain?. Br J Pain 2013;7:138-51. https://doi.org/10.1177/2049463713489384.
- Boutin DL. Effectiveness of cognitive behavioral and supportive-expressive group therapy for women diagnosed with breast cancer: a review of the literature. J Specialists Group Work 2007;32:267-84. https://doi.org/10.1080/01933920701431594.
- Bower P, Rowland N, Hardy R. The clinical effectiveness of counselling in primary care: a systematic review and meta-analysis. Psychol Med 2003;33:203-15. https://doi.org/10.1017/s0033291702006979.
- Brasure M, Fuchs E, MacDonald R, Nelson VA, Koffel E, Olson CM, et al. Psychological and behavioral interventions for managing insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med 2016;165:113-24. https://doi.org/10.7326/M15-1782.
- Briani RV, Ferreira AS, Pazzinatto MF, Pappas E, De Oliveira Silva D, Azevedo FM. What interventions can improve quality of life or psychosocial factors of individuals with knee osteoarthritis? A systematic review with meta-analysis of primary outcomes from randomised controlled trials. Br J Sports Med 2018;52:1031-8. https://doi.org/10.1136/bjsports-2017-098099.
- Brooks SK, Dunn R, Amlôt R, Greenberg N, Rubin GJ. Training and post-disaster interventions for the psychological impacts on disaster-exposed employees: a systematic review [published online ahead of print February 15 2018]. J Ment Health 2018. https://doi.org/10.1080/09638237.2018.1437610.
- Brownley KA, Berkman ND, Peat CM, Lohr KN, Cullen KE, Bann CM, et al. Binge-eating disorder in adults: a systematic review and meta-analysis. Ann Intern Med 2016;165:409-20. https://doi.org/10.7326/M15-2455.
- Buckley LA, Maayan N, Soares-Weiser K, Adams CE. Supportive therapy for schizophrenia. Cochrane Database Syst Rev 2015;4. https://doi.org/10.1002/14651858.CD004716.pub4.
- Budhrani PH, Lengacher CA, Kip K, Tofthagen C, Jim H. An integrative review of subjective and objective measures of sleep disturbances in breast cancer survivors. Clin J Oncol Nurs 2015;19:185-91. https://doi.org/10.1188/15.CJON.185-191.
- Burgstaller JM, Jenni BF, Steurer J, Held U, Wertli MM. Treatment efficacy for non-cardiovascular chest pain: a systematic review and meta-analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0104722.
- Butler M, Urosevic S, Desai P, Sponheim SR, Popp J, Nelson VA, et al. Treatment for Bipolar Disorder in Adults: A Systematic Review. Rockville, MD: Agency for Healthcare Research and Quality; 2018.
- Caemmerer J, Correll CU, Maayan L. Acute and maintenance effects of non-pharmacologic interventions for antipsychotic associated weight gain and metabolic abnormalities: a meta-analytic comparison of randomized controlled trials. Schizophr Res 2012;140:159-68. https://doi.org/10.1016/j.schres.2012.03.017.
- Caldirola D, Alciati A, Riva A, Perna G. Are there advances in pharmacotherapy for panic disorder? A systematic review of the past five years. Expert Opin Pharmacother 2018;19:1357-68. https://doi.org/10.1080/14656566.2018.1504921.
- Cantor JB, Ashman T, Bushnik T, Cai X, Farrell-Carnahan L, Gumber S, et al. Systematic review of interventions for fatigue after traumatic brain injury: a NIDRR traumatic brain injury model systems study. J Head Trauma Rehabil 2014;29:490-7. https://doi.org/10.1097/HTR.0000000000000102.
- Cape J, Whittington C, Buszewicz M, Wallace P, Underwood L. Brief psychological therapies for anxiety and depression in primary care: meta-analysis and meta-regression. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-38.
- Carlbring P, Andersson G, Cuijpers P, Riper H, Hedman-Lagerlöf E. Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta-analysis. Cogn Behav Ther 2018;47:1-18. https://doi.org/10.1080/16506073.2017.1401115.
- Carlson P, Nicholson Perry K. Psychological interventions for psychogenic non-epileptic seizures: a meta-analysis. Seizure 2017;45:142-50. https://doi.org/10.1016/j.seizure.2016.12.007.
- Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety 2018;35:502-14. https://doi.org/10.1002/da.22728.
- Cartwright-Hatton S, Roberts C, Chitsabesan P, Fothergill C, Harrington R. Systematic review of the efficacy of cognitive behaviour therapies for childhood and adolescent anxiety disorders. Br J Clin Psychol 2010;43:421-36. https://doi.org/10.1348/0144665042388928.
- Cary CE, McMillen JC. The data behind the dissemination: a systematic review of trauma-focused cognitive behavioral therapy for use with children and youth. Child Youth Serv Rev 2012;34:748-57. https://doi.org/10.1016/j.childyouth.2012.01.003.
- Casacalenda N, Perry JC, Looper K. Remission in major depressive disorder: a comparison of pharmacotherapy, psychotherapy, and control conditions. Am J Psychiatry 2002;159:1354-60. https://doi.org/10.1176/appi.ajp.159.8.1354.
- Castell BD, Kazantzis N, Moss-Morris RE. Cognitive behavioral therapy and graded exercise for chronic fatigue syndrome: a meta-analysis. Clin Psychol Sci Prac 2011;18:311-24. https://doi.org/10.1111/j.1468-2850.2011.01262.x.
- Chan MF, Chua TL. The effectiveness of therapeutic interventions on quality of life for vitiligo patients: a systematic review. Int J Nurs Pract 2012;18:396-405. https://doi.org/10.1111/j.1440-172X.2012.02047.x.
- Chen D, Sun W, Liu N, Wang J, Zhao J, Zhang Y, et al. Fear of cancer recurrence: a systematic review of randomized, controlled trials. Oncol Nurs Forum 2018;45:703-12. https://doi.org/10.1188/18.ONF.703-712.
- Chen R, Gillespie A, Zhao Y, Xi Y, Ren Y, McLean L. The efficacy of eye movement desensitization and reprocessing in children and adults who have experienced complex childhood trauma: a systematic review of randomized controlled trials. Front Psychol 2018;9. https://doi.org/10.3389/fpsyg.2018.00534.
- Cheng SK, Dizon J. Computerised cognitive behavioural therapy for insomnia: a systematic review and meta-analysis. Psychother Psychosom 2012;81:206-16. https://doi.org/10.1159/000335379.
- Cheung JMY, Jarrin DC, Ballot O, Bharwani AA, Morin CM. A systematic review of cognitive behavioral therapy for insomnia implemented in primary care and community settings. Sleep Med Rev 2019;44:23-36. https://doi.org/10.1016/j.smrv.2018.11.001.
- Chibanda D, Cowan FM, Healy JL, Abas M, Lund C. Psychological interventions for common mental disorders for people living with HIV in low- and middle-income countries: systematic review. Trop Med Int Health 2015;20:830-9. https://doi.org/10.1111/tmi.12500.
- Chida Y, Steptoe A, Hirakawa N, Sudo N, Kubo C. The effects of psychological intervention on atopic dermatitis. A systematic review and meta-analysis. Int Arch Allergy Immunol 2007;144:1-9. https://doi.org/10.1159/000101940.
- Clarke AM, Kuosmanen T, Barry MM. A systematic review of online youth mental health promotion and prevention interventions. J Youth Adolesc 2015;44:90-113. https://doi.org/10.1007/s10964-014-0165-0.
- Clarkson JE, Worthington HV, Furness S, McCabe M, Khalid T, Meyer S. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2010;8. https://doi.org/10.1002/14651858.CD001973.pub4.
- Reid S, Chalder T, Cleare A, Hotopf M, Wessely S. Chronic fatigue syndrome. BMJ Clin Evid 2011;2011.
- Clevenger SS, Malhotra D, Dang J, Vanle B, IsHak WW. The role of selective serotonin reuptake inhibitors in preventing relapse of major depressive disorder. Ther Adv Psychopharmacol 2018;8:49-58. https://doi.org/10.1177/2045125317737264.
- Clond M. Emotional freedom techniques for anxiety: a systematic review with meta-analysis. J Nerv Ment Dis 2016;204:388-95. https://doi.org/10.1097/NMD.0000000000000483.
- Compton SN, Burns BJ, Helen LE, Robertson E. Review of the evidence base for treatment of childhood psychopathology: internalizing disorders. J Consult Clin Psychol 2002;70:1240-66. https://doi.org/10.1037//0022-006x.70.6.1240.
- Cong X, Perry M, Bernier KM, Young EE, Starkweather A. Effects of self-management interventions in patients with irritable bowel syndrome: systematic review. West J Nurs Res 2018;40:1698-720. https://doi.org/10.1177/0193945917727705.
- Coventry PA, Gellatly JL. Improving outcomes for COPD patients with mild-to-moderate anxiety and depression: a systematic review of cognitive behavioural therapy. Br J Health Psychol 2008;13:381-400. https://doi.org/10.1348/135910707X203723.
- Craig M, Howard L. Postnatal depression. BMJ Clin Evid 2009;2009.
- Cross H. Epilepsy: behavioural, psychological, and ketogenic diet treatments. BMJ Clin Evid 2015;2015.
- Cuijpers P, Cristea IA, Karyotaki E, Reijnders M, Hollon SD. Component studies of psychological treatments of adult depression: a systematic review and meta-analysis. Psychother Res 2019;29:15-29. https://doi.org/10.1080/10503307.2017.1395922.
- Cuijpers P, Hollon SD, van Straten A, Bockting C, Berking M, Andersson G. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002542.
- Cuijpers P, Munoz RF, Clarke GN, Lewinsohn PM. Psychoeducational treatment and prevention of depression: the ‘Coping with Depression’ course thirty years later. Clin Psychol Rev 2009;29:449-58. https://doi.org/10.1016/j.cpr.2009.04.005.
- Das A, Roy B, Schwarzer G, Silverman MG, Ziegler O, Bandyopadhyay D, et al. Comparison of treatment options for depression in heart failure: a network meta-analysis. J Psychiatr Res 2019;108:7-23. https://doi.org/10.1016/j.jpsychires.2018.10.007.
- Davidson KM, Tran CF. Impact of treatment intensity on suicidal behavior and depression in borderline personality disorder: a critical review. J Pers Disord 2014;28:181-97. https://doi.org/10.1521/pedi_2013_27_113.
- Davies C, Radua J, Cipriani A, Stahl D, Provenzani U, McGuire P, et al. Efficacy and acceptability of interventions for attenuated positive psychotic symptoms in individuals at clinical high risk of psychosis: a network meta-analysis. Front Psychiatry 2018;9. https://doi.org/10.3389/fpsyt.2018.00187.
- Davies EB, Morriss R, Glazebrook C. Computer-delivered and web-based interventions to improve depression, anxiety, and psychological well-being of university students: a systematic review and meta-analysis. J Med Internet Res 2014;16. https://doi.org/10.2196/jmir.3142.
- Davis R, de Souza MAM, Rigatti R, Heldt E. [Cognitive–behavioral therapy for anxiety disorders in children and adolescents: a systematic review of follow-up studies.]. J Bras Psiquiatr 2015;63:373-8. https://doi.org/10.1590/0047-2085000000047.
- De Crescenzo F, Perelli F, Armando M, Vicari S. Selective serotonin reuptake inhibitors (SSRIs) for post-partum depression (PPD): a systematic review of randomized clinical trials. J Affect Disord 2014;152–154:39-44. https://doi.org/10.1016/j.jad.2013.09.019.
- de Jong M, Schoorl M, Hoek HW. Enhanced cognitive behavioural therapy for patients with eating disorders: a systematic review. Curr Opin Psychiatry 2018;31:436-44. https://doi.org/10.1097/YCO.0000000000000452.
- de Koning MB, Bloemen OJ, van Amelsvoort TA, Becker HE, Nieman DH, van der Gaag M, et al. Early intervention in patients at ultra high risk of psychosis: benefits and risks. Acta Psychiatr Scand 2009;119:426-42. https://doi.org/10.1111/j.1600-0447.2009.01372.x.
- de Souza Moura AM, Lamego MK, Paes F, Ferreira Rocha NB, Simoes-Silva V, Rocha SA, et al. Effects of aerobic exercise on anxiety disorders: a systematic review. CNS Neurol Disord Drug Targets 2015;14:1184-93. https://doi.org/10.2174/1871527315666151111121259.
- Dekker RL. Cognitive behavioral therapy for depression in patients with heart failure: a critical review. Nurs Clin North Am 2008;43:155-70. https://doi.org/10.1016/j.cnur.2007.11.003.
- Demissie M, Hanlon C, Birhane R, Ng L, Medhin G, Fekadu A. Psychological interventions for bipolar disorder in low- and middle-income countries: systematic review. BJPsych Open 2018;4:375-84. https://doi.org/10.1192/bjo.2018.46.
- Dennis CL. Preventing postpartum depression part II: a critical review of nonbiological interventions. Can J Psychiatry 2004;49:526-38. https://doi.org/10.1177/070674370404900804.
- Dèttore D, Pozza A, Andersson G. Efficacy of technology-delivered cognitive behavioural therapy for OCD versus control conditions, and in comparison with therapist-administered CBT: meta-analysis of randomized controlled trials. Cogn Behav Ther 2015;44:190-211. https://doi.org/10.1080/16506073.2015.1005660.
- Devoe DJ, Farris MS, Townes P, Addington J. Attenuated psychotic symptom interventions in youth at risk of psychosis: a systematic review and meta-analysis. Early Interv Psychiatry 2019;13:3-17. https://doi.org/10.1111/eip.12677.
- Devoe DJ, Peterson A, Addington J. Negative symptom interventions in youth at risk of psychosis: a systematic review and network meta-analysis. Schizophr Bull 2018;44:807-23. https://doi.org/10.1093/schbul/sbx139.
- Macea DD, Gajos K, Daglia Calil YA, Fregni F. The efficacy of web-based cognitive behavioral interventions for chronic pain: a systematic review and meta-analysis. J Pain 2010;11:917-29. https://doi.org/10.1016/j.jpain.2010.06.005.
- Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M, Festinger D. A systematic review on the use of psychosocial interventions in conjunction with medications for the treatment of opioid addiction. J Addict Med 2016;10:93-103. https://doi.org/10.1097/ADM.0000000000000193.
- Ebert DD, Zarski AC, Christensen H, Stikkelbroek Y, Cuijpers P, Berking M, et al. Internet and computer-based cognitive behavioral therapy for anxiety and depression in youth: a meta-analysis of randomized controlled outcome trials. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0119895.
- Ebrahim S, Montoya L, Truong W, Hsu S, Kamal el Din M, Carrasco-Labra A, et al. Effectiveness of cognitive behavioral therapy for depression in patients receiving disability benefits: a systematic review and individual patient data meta-analysis. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0050202.
- Eccleston C, Fisher E, Thomas KH, Hearn L, Derry S, Stannard C, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev 2017;11. https://doi.org/10.1002/14651858.CD010323.pub3.
- Eccleston C, Hearn L, Williams AC. Psychological therapies for the management of chronic neuropathic pain in adults. Cochrane Database Syst Rev 2015;10. https://doi.org/10.1002/14651858.CD011259.pub2.
- Ehring T, Welboren R, Morina N, Wicherts JM, Freitag J, Emmelkamp PM. Meta-analysis of psychological treatments for posttraumatic stress disorder in adult survivors of childhood abuse. Clin Psychol Rev 2014;34:645-57. https://doi.org/10.1016/j.cpr.2014.10.004.
- Elliott S. Cognitive behavioural therapy and glycaemic control in diabetes mellitus. Practical Diabetes 2012;29:67-71. https://doi.org/10.1002/pdi.1661.
- Ernst E, Schmidt K, Baum M. Complementary/alternative therapies for the treatment of breast cancer. A systematic review of randomized clinical trials and a critique of current terminology. Breast J 2006;12:526-30. https://doi.org/10.1111/j.1524-4741.2006.00340.x.
- Escobar KM, Gorey KM. Cognitive behavioral interventions for depression among Hispanic people: promising meta-analytic evidence for deep cultural adaptations. Soc Work Ment Health 2018;16:743-55. https://doi.org/10.1080/15332985.2018.1476284.
- Ewing DL, Monsen JJ, Thompson EJ, Cartwright-Hatton S, Field A. A meta-analysis of transdiagnostic cognitive behavioural therapy in the treatment of child and young person anxiety disorders. Behav Cogn Psychother 2015;43:562-77. https://doi.org/10.1017/S1352465813001094.
- Fackrell K, Potgieter I, Shekhawat GS, Baguley DM, Sereda M, Hoare DJ. Clinical interventions for hyperacusis in adults: a scoping review to assess the current position and determine priorities for research. Biomed Res Int 2017;2017. https://doi.org/10.1155/2017/2723715.
- Farley AC, Hajek P, Lycett D, Aveyard P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev 2012;1. https://doi.org/10.1002/14651858.CD006219.pub3.
- Farrand P, Woodford J. Impact of support on the effectiveness of written cognitive behavioural self-help: a systematic review and meta-analysis of randomised controlled trials. Clin Psychol Rev 2013;33:182-95. https://doi.org/10.1016/j.cpr.2012.11.001.
- Farrand P, Woodford J. Effectiveness of cognitive behavioural self-help for the treatment of depression and anxiety in people with long-term physical health conditions: a systematic review and meta-analysis of randomised controlled trials. Ann Behav Med 2015;49:579-93. https://doi.org/10.1007/s12160-015-9689-0.
- Farrer L, Gulliver A, Chan JK, Batterham PJ, Reynolds J, Calear A, et al. Technology-based interventions for mental health in tertiary students: systematic review. J Med Internet Res 2013;15. https://doi.org/10.2196/jmir.2639.
- de Mello MF, de Jesus Mari J, Bacaltchuk J, Verdeli H, Neugebauer R. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. Eur Arch Psychiatry Clin Neurosci 2005;255:75-82. https://doi.org/10.1007/s00406-004-0542-x.
- Fernie BA, Kollmann J, Brown RG. Cognitive behavioural interventions for depression in chronic neurological conditions: a systematic review. J Psychosom Res 2015;78:411-19. https://doi.org/10.1016/j.jpsychores.2015.02.012.
- Field A, Cottrell D. Eye movement desensitization and reprocessing as a therapeutic intervention for traumatized children and adolescents: a systematic review of the evidence for family therapists. J Fam Ther 2011;33:374-88. https://doi.org/10.1111/j.1467-6427.2011.00548.x.
- Flament MF, Bissada H, Spettigue W. Evidence-based pharmacotherapy of eating disorders. Int J Neuropsychopharmacol 2012;15:189-207. https://doi.org/10.1017/S1461145711000381.
- Fleming PS, Strydom H, Katsaros C, MacDonald L, Curatolo M, Fudalej P, et al. Non-pharmacological interventions for alleviating pain during orthodontic treatment. Cochrane Database Syst Rev 2016;12. https://doi.org/10.1002/14651858.CD010263.pub2.
- Flodgren G, Rachas A, Farmer AJ, Inzitari M, Shepperd S. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2015;9. https://doi.org/10.1002/14651858.CD002098.pub2.
- Foa EB, Franklin ME, Moser J. Context in the clinic: how well do cognitive–behavioral therapies and medications work in combination?. Biol 2002;52:989-97. https://doi.org/10.1016/S0006-3223%2802%2901552-4.
- Ford AC, Lacy BE, Harris LA, Quigley EMM, Moayyedi P. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol 2019;114:21-39. https://doi.org/10.1038/s41395-018-0222-5.
- Forman-Hoffman V, McClure E, McKeeman J, Wood CT, Middleton JC, Skinner AC, et al. Screening for major depressive disorder in children and adolescents: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2016;164:342-9. https://doi.org/10.7326/M15-2259.
- Forman-Hoffman V, Middleton JC, Feltner C, Gaynes BN, Weber RP, Bann C, et al. Psychological and Pharmacological Treatments for Adults with Posttraumatic Stress Disorder: A Systematic Review Update. Rockville, MD: Agency for Healthcare Research and Quality; 2018.
- Forneris CA, Gartlehner G, Brownley KA, Gaynes BN, Sonis J, Coker-Schwimmer E, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med 2013;44:635-50. https://doi.org/10.1016/j.amepre.2013.02.013.
- Franco P, Gallardo AM, Urtubey X. Web-based interventions for depression in individuals with diabetes: review and discussion. JMIR Diabetes 2018;3. https://doi.org/10.2196/diabetes.9694.
- Frank JW, Bair MJ, Becker WC, Krebs EE, Liebschutz JM, Alford DP. Update in pain medicine for primary care providers: a narrative review, 2010–2012. Pain Med 2014;15:425-31. https://doi.org/10.1111/pme.12337.
- Fulton JJ, Newins AR, Porter LS, Ramos K. Psychotherapy targeting depression and anxiety for use in palliative care: a meta-analysis. J Palliat Med 2018;21:1024-37. https://doi.org/10.1089/jpm.2017.0576.
- Funderburk JS, Shepardson RL, Wray J, Acker J, Beehler GP, Possemato K, et al. Behavioral medicine interventions for adult primary care settings: a review. Fam Syst Health 2018;36:368-99. https://doi.org/10.1037/fsh0000333.
- Furuta M, Horsch A, Ng ESW, Bick D, Spain D, Sin J. Effectiveness of trauma-focused psychological therapies for treating post-traumatic stress disorder symptoms in women following childbirth: a systematic review and meta-analysis. Front Psychiatry 2018;9. https://doi.org/10.3389/fpsyt.2018.00591.
- van der Gaag M, Valmaggia LR, Smit F. The effects of individually tailored formulation-based cognitive behavioural therapy in auditory hallucinations and delusions: a meta-analysis. Schizophr Res 2014;156:30-7. https://doi.org/10.1016/j.schres.2014.03.016.
- Garcia-Escalera J, Chorot P, Valiente RM, Reales JM, Sandin B. Efficacy of transdiagnostic cognitive-behavioral therapy for anxiety and depression in adults, children and adolescents: a meta-analysis. Rev Psicopatol Psicol Clín 2016;21:147-75. https://doi.org/10.5944/rppc.vol.21.num.3.2016.17811.
- Gebara MA, Siripong N, DiNapoli EA, Maree RD, Germain A, Reynolds CF, et al. Effect of insomnia treatments on depression: a systematic review and meta-analysis. Depress Anxiety 2018;35:717-31. https://doi.org/10.1002/da.22776.
- George SI. What is the effectiveness of a biopsychosocial approach to individual physiotherapy care for chronic low back pain?. Internet J Allied Health Sci Pract 2008;6:1-10.
- Gertler P, Tate RL, Cameron ID. Non-pharmacological interventions for depression in adults and children with traumatic brain injury. Cochrane Database Syst Rev 2015;12. https://doi.org/10.1002/14651858.CD009871.pub2.
- Ghaderi A, Andersson G. Meta-analysis of CBT for bulimia nervosa: investigating the effects using DSM-III-R and DSM-IV criteria. Scandinavian J Behav Ther 1999;28:79-87. https://doi.org/10.1080/028457199440034.
- Ghaderi A, Odeberg J, Gustafsson S, Råstam M, Brolund A, Pettersson A, et al. Psychological, pharmacological, and combined treatments for binge eating disorder: a systematic review and meta-analysis. PeerJ 2018;6. https://doi.org/10.7717/peerj.5113.
- Gibbon S, Duggan C, Stoffers J, Huband N, Völlm BA, Ferriter M, et al. Psychological interventions for antisocial personality disorder. Cochrane Database Syst Rev 2010;6. https://doi.org/10.1002/14651858.CD007668.pub2.
- Gillies D, Maiocchi L, Bhandari AP, Taylor F, Gray C, O’Brien L. Psychological therapies for children and adolescents exposed to trauma. Cochrane Database Syst Rev 2016;10. https://doi.org/10.1002/14651858.CD012371.
- Gillings KL. The Role of Cognitive, Emotional and Behavioural Factors in Chronic Fatigue and Chronic Fatigue Syndrome Myalgic Encephalomyelitis: Content, Process and Therapeutic Outcome 2007.
- Giummarra MJ, Lennox A, Dali G, Costa B, Gabbe BJ. Early psychological interventions for posttraumatic stress, depression and anxiety after traumatic injury: a systematic review and meta-analysis. Clin Psychol Rev 2018;62:11-36. https://doi.org/10.1016/j.cpr.2018.05.001.
- Gómara-Toldrà N, Sliwinski M, Dijkers MP. Physical therapy after spinal cord injury: a systematic review of treatments focused on participation. J Spinal Cord Med 2014;37:371-9. https://doi.org/10.1179/2045772314Y.0000000194.
- Gonçalves R, Rodrigues H, Novaes F, Arbol J, Volchan E, Coutinho ES, et al. Listening to the heart: a meta-analysis of cognitive behavior therapy impact on the heart rate of patients with anxiety disorders. J Affect Disord 2015;172:231-40. https://doi.org/10.1016/j.jad.2014.09.058.
- Goode AP, Coeytaux RR, Maslow GR, Davis N, Hill S, Namdari B, et al. Nonpharmacologic treatments for attention-deficit/hyperactivity disorder: a systematic review. Pediatrics 2018;141. https://doi.org/10.1542/peds.2018-0094.
- Gould RL, Coulson MC, Howard RJ. Efficacy of cognitive behavioral therapy for anxiety disorders in older people: a meta-analysis and meta-regression of randomized controlled trials. J Am Geriatr Soc 2012;60:218-29. https://doi.org/10.1111/j.1532-5415.2011.03824.x.
- Greenwood J, McGregor A, Jones F, Mullane J, Hurley M. Rehabilitation following lumbar fusion surgery: a systematic review and meta-analysis. Spine 2016;41:E28-36. https://doi.org/10.1097/BRS.0000000000001132.
- Gregory VL. Cognitive-behavioral therapy for anxious symptoms in persons of African descent: a meta-analysis. J Soc Serv Res 2019;45:87-101. https://doi.org/10.1080/01488376.2018.1479344.
- Grenon R, Carlucci S, Brugnera A, Schwartze D, Hammond N, Ivanova I, et al. Psychotherapy for eating disorders: a meta-analysis of direct comparisons. Psychother Res 2019;29:833-45. https://doi.org/10.1080/10503307.2018.1489162.
- Grenon R, Schwartze D, Hammond N, Ivanova I, McQuaid N, Proulx G, et al. Group psychotherapy for eating disorders: a meta-analysis. Int J Eat Disord 2017;50:997-1013. https://doi.org/10.1002/eat.22744.
- Griffiths KM, Farrer L, Christensen H. The efficacy of internet interventions for depression and anxiety disorders: a review of randomised controlled trials. Med J Aust 2010;192:S4-11. https://doi.org/10.5694/j.1326-5377.2010.tb03685.x.
- Grist R, Cavanagh K. Computerised cognitive behavioural therapy for common mental health disorders, what works, for whom under what circumstances? A systematic review and meta-analysis. J Contemp Psychother 2013;43:243-51. https://doi.org/10.1007/s10879-013-9243-y.
- Grist R, Croker A, Denne M, Stallard P. Technology delivered interventions for depression and anxiety in children and adolescents: a systematic review and meta-analysis. Clin Child Fam Psychol Rev 2018;18. https://doi.org/10.1007/s10567-018-0271-8.
- Guest R, Tran Y, Gopinath B, Cameron ID, Craig A. Psychological distress following a motor vehicle crash: a systematic review of preventative interventions. Injury 2016;47:2415-23. https://doi.org/10.1016/j.injury.2016.09.006.
- Guidi J, Tomba E, Fava GA. The sequential integration of pharmacotherapy and psychotherapy in the treatment of major depressive disorder: a meta-analysis of the sequential model and a critical review of the literature. Am J Psychiatry 2016;173:128-37. https://doi.org/10.1176/appi.ajp.2015.15040476.
- Gupta A, Scott K, Dukewich M. Innovative technology using virtual reality in the treatment of pain: does it reduce pain via distraction, or is there more to it?. Pain Med 2018;19:151-9. https://doi.org/10.1093/pm/pnx109.
- Haibach JP, Beehler GP, Dollar KM, Finnell DS. Moving toward integrated behavioral intervention for treating multimorbidity among chronic pain, depression, and substance-use disorders in primary care. Med Care 2014;52:322-7. https://doi.org/10.1097/MLR.0000000000000098.
- Hajihasani A, Rouhani M, Salavati M, Hedayati R, Kahlaee AH. The influence of cognitive behavioral therapy on pain, quality of life, and depression in patients receiving physical therapy for chronic low back pain: a systematic review. PM&Amp;R 2018;25. https://doi.org/10.1016/j.pmrj.2018.09.029.
- Hall A, Richmond H, Copsey B, Hansen Z, Williamson E, Jones G, et al. Physiotherapist-delivered cognitive-behavioural interventions are effective for low back pain, but can they be replicated in clinical practice? A systematic review. Disabil Rehabil 2018;40:1-9. https://doi.org/10.1080/09638288.2016.1236155.
- Hall J, Kellett S, Berrios R, Bains MK, Scott S. Efficacy of cognitive behavioral therapy for generalized anxiety disorder in older adults: systematic review, meta-analysis, and meta-regression. Am J Geriatr Psychiatry 2016;24:1063-73. https://doi.org/10.1016/j.jagp.2016.06.006.
- Hall L, Skelton DA. Occupational therapy for caregivers of people with dementia: a review of the United Kingdom literature. Br J Occup Ther 2012;75:281-8. https://doi.org/10.4276/030802212X13383757345184.
- Hamers PCM, Festen DAM, Hermans H. Non-pharmacological interventions for adults with intellectual disabilities and depression: a systematic review. J Intellect Disabil Res 2018;62:684-700. https://doi.org/10.1111/jir.12502.
- Hanlon I, Hewitt C, Bell K, Phillips A, Mikocka-Walus A. Systematic review with meta-analysis: online psychological interventions for mental and physical health outcomes in gastrointestinal disorders including irritable bowel syndrome and inflammatory bowel disease. Aliment Pharmacol Ther 2018;48:244-59. https://doi.org/10.1111/apt.14840.
- Harada T, Tsutomi H, Mori R, Wilson DB. Cognitive-behavioural treatment for amphetamine-type stimulants (ATS)-use disorders. Cochrane Database Syst Rev 2018;12. https://doi.org/10.1002/14651858.CD011315.pub2.
- Harris P, Loveman E, Clegg A, Easton S, Berry N. Systematic review of cognitive behavioural therapy for the management of headaches and migraines in adults. Br J Pain 2015;9:213-24. https://doi.org/10.1177/2049463715578291.
- Harrison A, Fernández de la Cruz L, Enander J, Radua J, Mataix-Cols D. Cognitive-behavioral therapy for body dysmorphic disorder: a systematic review and meta-analysis of randomized controlled trials. Clin Psychol Rev 2016;48:43-51. https://doi.org/10.1016/j.cpr.2016.05.007.
- Hart SL, Hoyt MA, Diefenbach M, Anderson DR, Kilbourn KM, Craft LL, et al. Meta-analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. J Natl Cancer Inst 2012;104:990-1004. https://doi.org/10.1093/jnci/djs256.
- Hay PJ, Claudino AM, Touyz S, Abd Elbaky G. Individual psychological therapy in the outpatient treatment of adults with anorexia nervosa. Cochrane Database Syst Rev 2015;7. https://doi.org/10.1002/14651858.CD003909.pub2.
- Hay PP, Bacaltchuk J, Stefano S, Kashyap P. Psychological treatments for bulimia nervosa and binging. Cochrane Database Syst Rev 2009;4. https://doi.org/10.1002/14651858.CD000562.pub3.
- Bacaltchuk J, Hay P, Trefiglio R. Antidepressants versus psychological treatments and their combination for bulimia nervosa. Cochrane Database Syst Rev 2001;4.
- Hay PJ, Claudino AM. Bulimia nervosa. BMJ Clin Evid 2010;2010.
- Hay PJ, Touyz S, Sud R. Treatment for severe and enduring anorexia nervosa: a review. Aust N Z J Psychiatry 2012;46:1136-44. https://doi.org/10.1177/0004867412450469.
- van Hees ML, Rotter T, Ellermann T, Evers SM. The effectiveness of individual interpersonal psychotherapy as a treatment for major depressive disorder in adult outpatients: a systematic review. BMC Psychiatry 2013;13. https://doi.org/10.1186/1471-244X-13-22.
- Hendriks GJ, Oude Voshaar RC, Keijsers GP, Hoogduin CA, van Balkom AJ. Cognitive-behavioural therapy for late-life anxiety disorders: a systematic review and meta-analysis. Acta Psychiatr Scand 2008;117:403-11. https://doi.org/10.1111/j.1600-0447.2008.01190.x.
- Hennemann S, Farnsteiner S, Sander L. Internet- and mobile-based aftercare and relapse prevention in mental disorders: a systematic review and recommendations for future research. Internet Interv 2018;14:1-17. https://doi.org/10.1016/j.invent.2018.09.001.
- Hetrick SE, Cox GR, Witt KG, Bir JJ, Merry SN. Cognitive behavioural therapy (CBT), third-wave CBT and interpersonal therapy (IPT) based interventions for preventing depression in children and adolescents. Cochrane Database Syst Rev 2016;8. https://doi.org/10.1002/14651858.CD003380.pub4.
- Hetrick SE, Cox GR, Merry SN. Treatment-resistant depression in adolescents: is the addition of cognitive behavioral therapy of benefit?. Psychol Res Behav Manag 2011;4:97-112. https://doi.org/10.2147/PRBM.S13780.
- Hides L, Samet S, Lubman DI. Cognitive behaviour therapy (CBT) for the treatment of co-occurring depression and substance use: current evidence and directions for future research. Drug Alcohol Rev 2010;29:508-17. https://doi.org/10.1111/j.1465-3362.2010.00207.x.
- Hilbert A, Petroff D, Herpertz S, Pietrowsky R, Tuschen-Caffier B, Vocks S, et al. Meta-analysis of the efficacy of psychological and medical treatments for binge-eating disorder. J Consult Clin Psychol 2019;87:91-105. https://doi.org/10.1037/ccp0000358.
- Hines S, Ramis MA, Pike S, Chang AM. Effectiveness of psychosocial interventions for cognitive dysfunction in cancer patients who have received chemotherapy: a systematic review. JBI Libr Syst Rev 2011;9:2023-62. https://doi.org/10.11124/jbisrir-2011-99.
- Hjorth P, Davidsen AS, Kilian R, Skrubbeltrang C. A systematic review of controlled interventions to reduce overweight and obesity in people with schizophrenia. Acta Psychiatr Scand 2014;130:279-89. https://doi.org/10.1111/acps.12245.
- Ho KKN, Ferreira PH, Pinheiro MB, Aquino Silva D, Miller CB, Grunstein R, et al. Sleep interventions for osteoarthritis and spinal pain: a systematic review and meta-analysis of randomized controlled trials. Osteoarthr Cartil 2019;27:196-218. https://doi.org/10.1016/j.joca.2018.09.014.
- Ho MS, Lee CW. Cognitive behaviour therapy versus eye movement desensitization and reprocessing for post-traumatic disorder: is it all in the homework then?. Eur Rev Appl Psychol 2012;62:253-60. https://doi.org/10.1016/j.erap.2012.08.001.
- Hoare DJ, Kowalkowski VL, Kang S, Hall DA. Systematic review and meta-analyses of randomized controlled trials examining tinnitus management. Laryngoscope 2011;121:1555-64. https://doi.org/10.1002/lary.21825.
- Hobbs JD, Kushner MG, Lee SS, Reardon SM, Maurer EW. Meta-analysis of supplemental treatment for depressive and anxiety disorders in patients being treated for alcohol dependence. Am J Addict 2011;20:319-29. https://doi.org/10.1111/j.1521-0391.2011.00140.x.
- Huang J, Nigatu YT, Smail-Crevier R, Zhang X, Wang J. Interventions for common mental health problems among university and college students: a systematic review and meta-analysis of randomized controlled trials. J Psychiatr Res 2018;107:1-10. https://doi.org/10.1016/j.jpsychires.2018.09.018.
- Huang L, Zhao Y, Qiang C, Fan B. Is cognitive behavioral therapy a better choice for women with postnatal depression? A systematic review and meta-analysis. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0205243.
- Hundt NE, Barrera TL, Robinson A, Cully JA. A systematic review of cognitive behavioral therapy for depression in Veterans. Mil Med 2014;179:942-9. https://doi.org/10.7205/MILMED-D-14-00128.
- Huntley AL, Araya R, Salisbury C. Group psychological therapies for depression in the community: systematic review and meta-analysis. Br J Psychiatry 2012;200:184-90. https://doi.org/10.1192/bjp.bp.111.092049.
- Hutton P, Taylor PJ. Cognitive behavioural therapy for psychosis prevention: a systematic review and meta-analysis. Psychol Med 2014;44:449-68. https://doi.org/10.1017/S0033291713000354.
- Ijaz S, Davies P, Williams CJ, Kessler D, Lewis G, Wiles N. Psychological therapies for treatment-resistant depression in adults. Cochrane Database Syst Rev 2018;5. https://doi.org/10.1002/14651858.CD010558.pub2.
- Ipser JC, Sander C, Stein DJ. Pharmacotherapy and psychotherapy for body dysmorphic disorder. Cochrane Database Syst Rev 2009;1. https://doi.org/10.1002/14651858.CD005332.pub2.
- Iwasaki S, Shigeishi H, Akita T, Tanaka J, Sugiyama M. Efficacy of cognitive–behavioral therapy for patients with temporomandibular disorder pain: systematic review of previous reports. Int J Clin Exp Med 2018;11:500-9.
- Iyengar U, Snowden N, Asarnow JR, Moran P, Tranah T, Ougrin D. A further look at therapeutic interventions for suicide attempts and self-harm in adolescents: an updated systematic review of randomized controlled trials. Front Psychiatry 2018;9. https://doi.org/10.3389/fpsyt.2018.00583.
- Jackson CF, Makin SM, Baker GA. Neuropsychological and psychological interventions for people with newly diagnosed epilepsy. Cochrane Database Syst Rev 2015;7. https://doi.org/10.1002/14651858.CD011311.pub2.
- Jacob A, Moullec G, Lavoie KL, Laurin C, Cowan T, Tisshaw C, et al. Impact of cognitive–behavioral interventions on weight loss and psychological outcomes: a meta-analysis. Health Psychol 2018;37:417-32. https://doi.org/10.1037/hea0000576.
- Jacobsen P, Hodkinson K, Peters E, Chadwick P. A systematic scoping review of psychological therapies for psychosis within acute psychiatric in-patient settings. Br J Psychiatry 2018;213:490-7. https://doi.org/10.1192/bjp.2018.106.
- James AC, James G, Cowdrey FA, Soler A, Choke A. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev 2015;2. https://doi.org/10.1002/14651858.CD004690.pub4.
- Jayakody K, Gunadasa S, Hosker C. Exercise for anxiety disorders: systematic review. Br J Sports Med 2014;48:187-96. https://doi.org/10.1136/bjsports-2012-091287.
- Jennings C, Hewitt O. The use of cognitive behaviour therapy to treat depression in people with learning disabilities: a systematic review. Tizard Learning Disabil Rev 2015;20:54-6. https://doi.org/10.1108/TLDR-05-2014-0013.
- Jensen CM, Amdisen BL, Jørgensen KJ, Arnfred SM. Cognitive behavioural therapy for ADHD in adults: systematic review and meta-analyses. Atten Defic Hyperact Disord 2016;8:3-11. https://doi.org/10.1007/s12402-016-0188-3.
- Jeyanantham K, Kotecha D, Thanki D, Dekker R, Lane DA. Effects of cognitive behavioural therapy for depression in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev 2017;22:731-41. https://doi.org/10.1007/s10741-017-9640-5.
- Jiang Y, Shorey S, Seah B, Chan WX, Tam WWS, Wang W. The effectiveness of psychological interventions on self-care, psychological and health outcomes in patients with chronic heart failure – a systematic review and meta-analysis. Int J Nurs Stud 2018;78:16-25. https://doi.org/10.1016/j.ijnurstu.2017.08.006.
- Johnson JA, Rash JA, Campbell TS, Savard J, Gehrman PR, Perlis M, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev 2016;27:20-8. https://doi.org/10.1016/j.smrv.2015.07.001.
- Jones C, Hacker D, Cormac I, Meaden A, Irving CB. Cognitive behaviour therapy versus other psychosocial treatments for schizophrenia. Cochrane Database Syst Rev 2012;4. https://doi.org/10.1002/14651858.CD008712.pub2.
- Jones C, Hacker D, Meaden A, Cormac I, Irving CB, Xia J, et al. Cognitive behavioural therapy plus standard care versus standard care plus other psychosocial treatments for people with schizophrenia. Cochrane Database Syst Rev 2018;11. https://doi.org/10.1002/14651858.CD008712.pub3.
- Jones C, Hacker D, Xia J, Meaden A, Irving CB, Zhao S, et al. Cognitive behavioural therapy plus standard care versus standard care for people with schizophrenia. Cochrane Database Syst Rev 2018;12. https://doi.org/10.1002/14651858.CD007964.pub2.
- Julien D, O’Connor K, Aardema F. The inference-based approach to obsessive-compulsive disorder: a comprehensive review of its etiological model, treatment efficacy, and model of change. J Affect Disord 2016;202:187-96. https://doi.org/10.1016/j.jad.2016.05.060.
- Juvet LK, Elvsaas IKO, Leivseth G, Anker G, Bertheussen GF, Falkmer U, et al. Rehabilitation of breast cancer patients. Oslo: Knowledge Centre for the Health Services, The Norwegian Institute of Public Health; 2009.
- Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al. Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation. Health Technol Assess 2006;10. https://doi.org/10.3310/hta10330.
- Karyotaki E, Riper H, Twisk J, Hoogendoorn A, Kleiboer A, Mira A, et al. Efficacy of self-guided internet-based cognitive behavioral therapy in the treatment of depressive symptoms: a meta-analysis of individual participant data. JAMA Psychiatry 2017;74:351-9. https://doi.org/10.1001/jamapsychiatry.2017.0044.
- Kavanagh J, Oliver S, Caird J, Tucker H, Greaves A, Harden A, et al. Inequalities and the Mental Health of Young People: A Systematic Review of Secondary School-based Cognitive Behavioural Interventions. London: Institute of Education, Social Science Research Unit, EPPI-Centre, University of London; 2009.
- Kayrouz R, Dear BF, Kayrouz B, Karin E, Gandy M, Titov N. Meta-analysis of the efficacy and acceptability of cognitive-behavioural therapy for Arab adult populations experiencing anxiety, depression or post-traumatic stress disorder. Cogn Behav Ther 2018;47:412-30. https://doi.org/10.1080/16506073.2018.1445124.
- Keel PK, Haedt A. Evidence-based psychosocial treatments for eating problems and eating disorders. J Clin Child Adolesc Psychol 2008;37:39-61. https://doi.org/10.1080/15374410701817832.
- Kemper AR, Maslow GR, Hill S, Namdari B, Allen LaPointe NM, Goode AP, et al. Attention Deficit Hyperactivity Disorder: Diagnosis and Treatment in Children and Adolescents. Rockville, MD: Agency for Healthcare Research and Quality; 2018.
- Kennedy L, Xyrichis A. Cognitive behavioral therapy compared with non-specialized therapy for alleviating the effect of auditory hallucinations in people with reoccurring schizophrenia: a systematic review and meta-analysis. Community Ment Health J 2017;53:127-33. https://doi.org/10.1007/s10597-016-0030-6.
- Kew KM, Nashed M, Dulay V, Yorke J. Cognitive behavioural therapy (CBT) for adults and adolescents with asthma. Cochrane Database Syst Rev 2016;9. https://doi.org/10.1002/14651858.CD011818.pub2.
- Khan AM, Dar S, Ahmed R, Bachu R, Adnan M, Kotapati VP. Cognitive behavioral therapy versus eye movement desensitization and reprocessing in patients with post-traumatic stress disorder: systematic review and meta-analysis of randomized clinical trials. Cureus 2018;10. https://doi.org/10.7759/cureus.3250.
- Khera M, Goldstein I. Erectile dysfunction. BMJ Clin Evid 2011;2011.
- Kim YD, Heo I, Shin BC, Crawford C, Kang HW, Lim JH. Acupuncture for posttraumatic stress disorder: a systematic review of randomized controlled trials and prospective clinical trials. Evid Based Complement Alternat Med 2013;2013. https://doi.org/10.1155/2013/615857.
- Kiosses DN, Leon AC, Areán PA. Psychosocial interventions for late-life major depression: evidence-based treatments, predictors of treatment outcomes, and moderators of treatment effects. Psychiatric Clin N Am 2011;34:377-401. https://doi.org/10.1016/j.psc.2011.03.001.
- Kisely SR, Campbell LA, Yelland MJ, Paydar A. Psychological interventions for symptomatic management of non-specific chest pain in patients with normal coronary anatomy. Cochrane Database Syst Rev 2015;6. https://doi.org/10.1002/14651858.CD004101.pub5.
- Kishita N, Laidlaw K. Cognitive behaviour therapy for generalized anxiety disorder: is CBT equally efficacious in adults of working age and older adults?. Clin Psychol Rev 2017;52:124-36. https://doi.org/10.1016/j.cpr.2017.01.003.
- Klein JB, Jacobs RH, Reinecke MA. Cognitive-behavioral therapy for adolescent depression: a meta-analytic investigation of changes in effect-size estimates. J Am Acad Child Adolesc Psychiatry 2007;46:1403-13. https://doi.org/10.1097/chi.0b013e3180592aaa.
- Kluwe-Schiavon B, Sanvicente-Vieira B, Kristensen CH, Grassi-Oliveira R. Executive functions rehabilitation for schizophrenia: a critical systematic review. J Psychiatr Res 2013;47:91-104. https://doi.org/10.1016/j.jpsychires.2012.10.001.
- Koffel EA, Koffel JB, Gehrman PR. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev 2015;19:6-16. https://doi.org/10.1016/j.smrv.2014.05.001.
- Kolubinski DC, Frings D, Nikčević AV, Lawrence JA, Spada MM. A systematic review and meta-analysis of CBT interventions based on the Fennell model of low self-esteem. Psychiatry Res 2018;267:296-305. https://doi.org/10.1016/j.psychres.2018.06.025.
- Kowalik J, Weller J, Venter J, Drachman D. Cognitive behavioral therapy for the treatment of pediatric posttraumatic stress disorder: a review and meta-analysis. J Behav Ther Exp Psychiatry 2011;42:405-13. https://doi.org/10.1016/j.jbtep.2011.02.002.
- Koychev I, Okai D. Cognitive-behavioural therapy for non-motor symptoms of Parkinson’s disease: a clinical review. Evid Based Ment Health 2017;20:15-20. https://doi.org/10.1136/eb-2016-102574.
- Kreslins A, Robertson AE, Melville C. The effectiveness of psychosocial interventions for anxiety in children and adolescents with autism spectrum disorder: a systematic review and meta-analysis. Child Adolesc Psychiatry Ment Health 2015;9. https://doi.org/10.1186/s13034-015-0054-7.
- Kreuze LJ, Pijnenborg GHM, de Jonge YB, Nauta MH. Cognitive-behavior therapy for children and adolescents with anxiety disorders: a meta-analysis of secondary outcomes. J Anxiety Disord 2018;60:43-57. https://doi.org/10.1016/j.janxdis.2018.10.005.
- Krishna M, Lepping P, Jones S, Lane S. Systematic review and meta-analysis of group cognitive behavioural psychotherapy treatment for sub-clinical depression. Asian J Psychiatr 2015;16:7-16. https://doi.org/10.1016/j.ajp.2015.05.043.
- Kumar S, Malone D. Panic disorder. BMJ Clin Evid 2008;2008.
- Lai MH, Maniam T, Chan LF, Ravindran AV. Caught in the web: a review of web-based suicide prevention. J Med Internet Res 2014;16. https://doi.org/10.2196/jmir.2973.
- Lane DA, Millane TA, Lip GY. Psychological interventions for depression in adolescent and adult congenital heart disease. Cochrane Database Syst Rev 2013;10. https://doi.org/10.1002/14651858.CD004372.pub2.
- Larkin D, Lopez V, Aromataris E. Managing cancer-related fatigue in men with prostate cancer: a systematic review of non-pharmacological interventions. Int J Nurs Pract 2014;20:549-60. https://doi.org/10.1111/ijn.12211.
- Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev 2017;4. https://doi.org/10.1002/14651858.CD003200.pub7.
- Lau Y, Htun TP, Wong SN, Tam WSW, Klainin-Yobas P. Therapist-supported internet-based cognitive behavior therapy for stress, anxiety, and depressive symptoms among postpartum women: a systematic review and meta-analysis. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.6712.
- Lavender TJ, Ebert L, Jones D. An evaluation of perinatal mental health interventions: an integrative literature review. Women Birth 2016;29:399-406. https://doi.org/10.1016/j.wombi.2016.04.004.
- Lawrence R, Bradshaw T, Mairs H. Group cognitive behavioural therapy for schizophrenia: a systematic review of the literature. J Psychiatr Ment Health Nurs 2006;13:673-81. https://doi.org/10.1111/j.1365-2850.2006.01014.x.
- Laws KR, Darlington N, Kondel TK, McKenna PJ, Jauhar S. Cognitive behavioural therapy for schizophrenia – outcomes for functioning, distress and quality of life: a meta-analysis. BMC Psychol 2018;6. https://doi.org/10.1186/s40359-018-0243-2.
- Leavey K, Hawkins R. Is cognitive behavioural therapy effective in reducing suicidal ideation and behaviour when delivered face-to-face or via e-health? A systematic review and meta-analysis. Cogn Behav Ther 2017;46:353-74. https://doi.org/10.1080/16506073.2017.1332095.
- Leigh-Hunt N, Perry A. A systematic review of interventions for anxiety, depression, and PTSD in adult offenders. Int J Offender Ther Comp Criminol 2015;59:701-25. https://doi.org/10.1177/0306624X13519241.
- Leis JA, Mendelson T, Tandon SD, Perry DF. A systematic review of home-based interventions to prevent and treat postpartum depression. Arch Womens Ment Health 2009;12:3-13. https://doi.org/10.1007/s00737-008-0039-0.
- Lert CT, Fai CM. A systematic review of the effectiveness of therapeutic interventions on quality of life (QoL) for adult vitiligo patients. JBI Libr Syst Rev 2010;8:1169-201. https://doi.org/10.11124/jbisrir-2010-159.
- Lewis C, Roberts NP, Bethell A, Robertson L, Bisson JI. Internet-based cognitive and behavioural therapies for post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst Rev 2018;12. https://doi.org/10.1002/14651858.CD011710.pub2.
- Li C, Hou Z, Liu Y, Ji Y, Xie L. Cognitive-behavioural therapy in patients with inflammatory bowel diseases: a systematic review and meta-analysis. Int J Nurs Pract 2019;25. https://doi.org/10.1111/ijn.12699.
- Li C, Xu D, Hu M, Tan Y, Zhang P, Li G, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for patients with diabetes and depression. J Psychosom Res 2017;95:44-5. https://doi.org/10.1016/j.jpsychores.2017.02.006.
- Li JM, Zhang Y, Su WJ, Liu LL, Gong H, Peng W, et al. Cognitive behavioral therapy for treatment-resistant depression: a systematic review and meta-analysis. Psychiatry Res 2018;268:243-50. https://doi.org/10.1016/j.psychres.2018.07.020.
- Li L, Xiong L, Zhang S, Yu Q, Chen M. Cognitive-behavioral therapy for irritable bowel syndrome: a meta-analysis. J Psychosom Res 2014;77:1-12. https://doi.org/10.1016/j.jpsychores.2014.03.006.
- Linde K, Rücker G, Sigterman K, Jamil S, Meissner K, Schneider A, et al. Comparative effectiveness of psychological treatments for depressive disorders in primary care: network meta-analysis. BMC Fam Pract 2015;16. https://doi.org/10.1186/s12875-015-0314-x.
- Liu HX, Liang QJ, Xiao P, Jiao HX, Gao Y, Ahmetjiang A. The effectiveness of cognitive–behavioural therapy for temporomandibular disorders: a systematic review. J Oral Rehabil 2012;39:55-62. https://doi.org/10.1111/j.1365-2842.2011.02239.x.
- Liu TW, Ng GYF, Chung RCK, Ng SSM. Cognitive behavioural therapy for fear of falling and balance among older people: a systematic review and meta-analysis. Age Ageing 2018;47:520-7. https://doi.org/10.1093/ageing/afy010.
- Lockwood C, Page T, Conroy-Hiller T. Systematic review: effectiveness of individual therapy and group therapy in the treatment of schizophrenia. JBI Libr Syst Rev 2004;2:309-38. https://doi.org/10.11124/01938924-200402020-00001.
- LoGiudice JA, Massaro J. The impact of complementary therapies on psychosocial factors in women undergoing in vitro fertilization (IVF): a systematic literature review. Appl Nurs Res 2018;39:220-8. https://doi.org/10.1016/j.apnr.2017.11.025.
- Lonergan A. The effectiveness of cognitive behavioural therapy for pain in childhood and adolescence: a meta-analytic review. Ir J Psychol Med 2016;33:251-64. https://doi.org/10.1017/ipm.2015.59.
- Lopez PL, Torrente FM, Ciapponi A, Lischinsky AG, Cetkovich-Bakmas M, Rojas JI, et al. Cognitive–behavioural interventions for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev 2018;3. https://doi.org/10.1002/14651858.CD010840.pub2.
- Loucas CE, Fairburn CG, Whittington C, Pennant ME, Stockton S, Kendall T. E-therapy in the treatment and prevention of eating disorders: a systematic review and meta-analysis. Behav Res Ther 2014;63:122-31. https://doi.org/10.1016/j.brat.2014.09.011.
- Luckett T, Britton B, Clover K, Rankin NM. Evidence for interventions to improve psychological outcomes in people with head and neck cancer: a systematic review of the literature. Support Care Cancer 2011;19:871-81. https://doi.org/10.1007/s00520-011-1119-7.
- Ma D, Zhang Z, Zhang X, Li L. Comparative efficacy, acceptability, and safety of medicinal, cognitive-behavioral therapy, and placebo treatments for acute major depressive disorder in children and adolescents: a multiple-treatments meta-analysis. Curr Med Res Opin 2014;30:971-95. https://doi.org/10.1185/03007995.2013.860020.
- Mabey L, van Servellen G. Treatment of post-traumatic stress disorder in patients with severe mental illness: a review. Int J Ment Health Nurs 2014;23:42-50. https://doi.org/10.1111/inm.12007.
- Macdonald G, Livingstone N, Hanratty J, McCartan C, Cotmore R, Cary M, et al. The effectiveness, acceptability and cost-effectiveness of psychosocial interventions for maltreated children and adolescents: an evidence synthesis. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20690.
- Macedo T, Barbosa M, Rodrigues H, Coutinho EDSF, Figueira I, Ventura P. Does CBT have lasting effects in the treatment of PTSD after one year of follow-up? A systematic review of randomized controlled trials. Trends Psychiatry Psychother 2018;40:352-9. https://doi.org/10.1590/2237-6089-2017-0153.
- Maguire M, Marson AG, Ramaratnam S. Epilepsy (partial). BMJ Clin Evid 2011;2011.
- Maguire PN, Clark GI, Wootton BM. The efficacy of cognitive behavior therapy for the treatment of perinatal anxiety symptoms: a preliminary meta-analysis. J Anxiety Disord 2018;60:26-34. https://doi.org/10.1016/j.janxdis.2018.10.002.
- Markowitz JC, Lipsitz J, Milrod BL. Critical review of outcome research on interpersonal psychotherapy for anxiety disorders. Depress Anxiety 2014;31:316-25. https://doi.org/10.1002/da.22238.
- Markowitz SM, Gonzalez JS, Wilkinson JL, Safren SA. A review of treating depression in diabetes: emerging findings. Psychosomatics 2011;52:1-18. https://doi.org/10.1016/j.psym.2010.11.007.
- Martlew J, Pulman J, Marson AG. Psychological and behavioural treatments for adults with non-epileptic attack disorder. Cochrane Database Syst Rev 2014;2. https://doi.org/10.1002/14651858.CD006370.pub2.
- Matusiewicz AK, Hopwood CJ, Banducci AN, Lejuez CW. The effectiveness of cognitive behavioral therapy for personality disorders. Psychiatr Clin North Am 2010;33:657-85. https://doi.org/10.1016/j.psc.2010.04.007.
- Mayo-Wilson E, Montgomery P. Media-delivered cognitive behavioural therapy and behavioural therapy (self-help) for anxiety disorders in adults. Cochrane Database Syst Rev 2013;9. https://doi.org/10.1002/14651858.CD005330.pub4.
- McDaid C, Trowman R, Golder S, Hawton K, Sowden A. Systematic Review of the Effects of Interventions for People Bereaved by Suicide. York: Centre for Reviews and Dissemination, University of York; 2008.
- McDonagh MS, Matthews A, Phillipi C, Romm J, Peterson K, Thakurta S, et al. Depression drug treatment outcomes in pregnancy and the postpartum period: a systematic review and meta-analysis. Obstet Gynecol 2014;124:526-34. https://doi.org/10.1097/AOG.0000000000000410.
- McMahon SE, Smith TO, Raju K, Hing CB. A meta-analysis and systematic review of randomised controlled trials comparing cognitive behavioural therapy to conventional treatment of osteoarthritis. Curr Rheumatol Rev 2013;9:158-64. https://doi.org/10.2174/157339710903140130121431.
- de Medeiros Passarela C, Mendes DD, de Jesus Mari J. A systematic review to study the efficacy of cognitive behavioral therapy for sexually abused children and adolescents with posttraumatic stress disorder. Rev Psiquiatr Clin 2010;37:69-73.
- Mehl S, Werner D, Lincoln TM. Does cognitive behavior therapy for psychosis (CBTp) show a sustainable effect on delusions? A meta-analysis. Front Psychol 2015;6. https://doi.org/10.3389/fpsyg.2015.01450.
- Mehndiratta P, Sajatovic M. Treatments for patients with comorbid epilepsy and depression: a systematic literature review. Epilepsy Behav 2013;28:36-40. https://doi.org/10.1016/j.yebeh.2013.03.029.
- Mehta S, Peynenburg VA, Hadjistavropoulos HD. Internet-delivered cognitive behaviour therapy for chronic health conditions: a systematic review and meta-analysis. J Behav Med 2018;01. https://doi.org/10.1007/s10865-018-9984-x.
- Mendelson T, Cluxton-Keller F, Vullo GC, Tandon SD, Noazin S. NICU-based interventions to reduce maternal depressive and anxiety symptoms: a meta-analysis. Pediatrics 2017;139. https://doi.org/10.1542/peds.2016-1870.
- Mewton L, Andrews G. Cognitive behavioral therapy for suicidal behaviors: improving patient outcomes. Psychol Res Behav Manag 2016;9:21-9. https://doi.org/10.2147/PRBM.S84589.
- Michaelis R, Tang V, Goldstein LH, Reuber M, LaFrance WC, Lundgren T, et al. Psychological treatments for adults and children with epilepsy: evidence-based recommendations by the International League Against Epilepsy Psychology Task Force. Epilepsia 2018;59:1282-302. https://doi.org/10.1111/epi.14444.
- Michail M, Birchwood M, Tait L. Systematic review of cognitive–behavioural therapy for social anxiety disorder in psychosis. Brain Sci 2017;7. https://doi.org/10.3390/brainsci7050045.
- Miniati M, Callari A, Maglio A, Calugi S. Interpersonal psychotherapy for eating disorders: current perspectives. Psychol Res Behav Manag 2018;11:353-69. https://doi.org/10.2147/PRBM.S120584.
- Minozzi S, Saulle R, De Crescenzo F, Amato L. Psychosocial interventions for psychostimulant misuse. Cochrane Database Syst Rev 2016;9. https://doi.org/10.1002/14651858.CD011866.pub2.
- Mirosevic S, Jo B, Kraemer HC, Ershadi M, Neri E, Spiegel D. ‘Not just another meta-analysis’: sources of heterogeneity in psychosocial treatment effect on cancer survival. Cancer Med 2019;8:363-73. https://doi.org/10.1002/cam4.1895.
- Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract 2012;13. https://doi.org/10.1186/1471-2296-13-40.
- Montero-Marin J, Garcia-Campayo J, López-Montoyo A, Zabaleta-Del-Olmo E, Cuijpers P. Is cognitive-behavioural therapy more effective than relaxation therapy in the treatment of anxiety disorders? A meta-analysis. Psychol Med 2018;48:1427-36. https://doi.org/10.1017/S0033291717003099.
- Montgomery P, Dennis J. Cognitive behavioural interventions for sleep problems in adults aged 60+. Cochrane Database Syst Rev 2003;1. https://doi.org/10.1002/14651858.CD003161.
- Monticone M, Cedraschi C, Ambrosini E, Rocca B, Fiorentini R, Restelli M, et al. Cognitive–behavioural treatment for subacute and chronic neck pain. Cochrane Database Syst Rev 2015;5. https://doi.org/10.1002/14651858.CD010664.pub2.
- Moore LM, Carr A, Hartnett D. Does group CBT for depression do what it says on the tin? A systemic review and meta-analysis of group CBT for depressed adults (2000–2016). J Contemp Psychother 2017;47:141-52. https://doi.org/10.1007/s10879-016-9351-6.
- Moraes LJ, Miranda MB, Loures LF, Mainieri AG, Mármora CHC. A systematic review of psychoneuroimmunology-based interventions. Psychol Health Med 2018;23:635-52. https://doi.org/10.1080/13548506.2017.1417607.
- Moreno-Alcázar A, Treen D, Valiente-Gómez A, Sio-Eroles A, Pérez V, Amann BL, et al. Efficacy of eye movement desensitization and reprocessing in children and adolescent with post-traumatic stress disorder: a meta-analysis of randomized controlled trials. Front Psychol 2017;8. https://doi.org/10.3389/fpsyg.2017.01750.
- Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review. Sleep 1999;22:1134-56. https://doi.org/10.1093/sleep/22.8.1134.
- Mustafa M, Carson-Stevens A, Gillespie D, Edwards AG. Psychological interventions for women with metastatic breast cancer. Cochrane Database Syst Rev 2013;6. https://doi.org/10.1002/14651858.CD004253.pub4.
- Naeem F, Khoury B, Munshi T, Ayub M, Lecomte T, Kingdon D, et al. Brief cognitive behavioral therapy for psychosis (CBTp) for schizophrenia: literature review and meta-analysis. Int J Cognit Ther 2016;9:73-86. https://doi.org/10.1521/ijct_2016_09_04.
- Naidu VV, Giblin E, Burke KM, Madan I. Delivery of cognitive behavioural therapy to workers: a systematic review. Occup Med 2016;66:112-17. https://doi.org/10.1093/occmed/kqv141.
- Napa W, Tungpunkom P, Pothimas N. Effectiveness of family interventions on psychological distress and expressed emotion in family members of individuals diagnosed with first-episode psychosis: a systematic review. JBI Database System Rev Implement Rep 2017;15:1057-79. https://doi.org/10.11124/JBISRIR-2017-003361.
- Navarro-Bravo B, Parraga-Martinez I, Hidalgo JL-T, Andres-Pretel F, Rabanales-Sotos J. Group cognitive-behavioral therapy for insomnia: a meta-analysis. Anal Psicol 2015;31:8-18. https://doi.org/10.6018/analesps.31.1.168641.
- Newby JM, Twomey C, Yuan Li SS, Andrews G. Transdiagnostic computerised cognitive behavioural therapy for depression and anxiety: a systematic review and meta-analysis. J Affect Disord 2016;199:30-41. https://doi.org/10.1016/j.jad.2016.03.018.
- Nezu AM, Maguth Nezu C, Lombardo ER. Cognitive-behavior therapy for medically unexplained symptoms: a critical review of the treatment literature. Behav Ther 2001;32:537-83. https://doi.org/10.1016/S0005-7894(01)80035-6.
- Ng TK, Wong DFK. The efficacy of cognitive behavioral therapy for Chinese people: a meta-analysis. Aust N Z J Psychiatry 2018;52:620-37. https://doi.org/10.1177/0004867417741555.
- Niknejad B, Bolier R, Henderson CR, Delgado D, Kozlov E, Löckenhoff CE, et al. Association between psychological interventions and chronic pain outcomes in older adults: a systematic review and meta-analysis. JAMA Intern Med 2018;178:830-9. https://doi.org/10.1001/jamainternmed.2018.0756.
- Nillni YI, Mehralizade A, Mayer L, Milanovic S. Treatment of depression, anxiety, and trauma-related disorders during the perinatal period: a systematic review. Clin Psychol Rev 2018;66:136-48. https://doi.org/10.1016/j.cpr.2018.06.004.
- Noble AJ, Reilly J, Temple J, Fisher PL. Cognitive–behavioural therapy does not meaningfully reduce depression in most people with epilepsy: a systematic review of clinically reliable improvement. J Neurol Neurosurg Psychiatry 2018;89:1129-37. https://doi.org/10.1136/jnnp-2018-317997.
- Nocon A, Eberle-Sejari R, Unterhitzenberger J, Rosner R. The effectiveness of psychosocial interventions in war-traumatized refugee and internally displaced minors: systematic review and meta-analysis. Eur J Psychotraumatol 2017;8. https://doi.org/10.1080/20008198.2017.1388709.
- Noh D. Psychological interventions for runaway and homeless youth. J Nurs Scholarsh 2018;50:465-72. https://doi.org/10.1111/jnu.12402.
- Noone D, Stott J, Aguirre E, Llanfear K, Spector A. Meta-analysis of psychosocial interventions for people with dementia and anxiety or depression. Aging Ment Health 2019;23:1282-91. https://doi.org/10.1080/13607863.2018.1495177.
- Normann N, Morina N. The efficacy of metacognitive therapy: a systematic review and meta-analysis. Front Psychol 2018;9. https://doi.org/10.3389/fpsyg.2018.02211.
- Norris SC, Gleaves DH, Hutchinson AD. Treatment outcome research of enhanced cognitive behaviour therapy for eating disorders: a systematic review with narrative and meta-analytic synthesis. Eat Disord 2019;27:482-50. https://doi.org/10.1080/10640266.2018.1560240.
- O’Donnell ML, Metcalf O, Watson L, Phelps A, Varker T. A systematic review of psychological and pharmacological treatments for adjustment disorder in adults. J Trauma Stress 2018;31:321-31. https://doi.org/10.1002/jts.22295.
- Okajima I, Komada Y, Inoue Y. A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep Biol Rhythms 2011;9:24-3. https://doi.org/10.1111/j.1479-8425.2010.00481.x.
- O’Kearney RT, Anstey KJ, von Sanden C. Behavioural and cognitive behavioural therapy for obsessive compulsive disorder in children and adolescents. Cochrane Database Syst Rev 2006;4. https://doi.org/10.1002/14651858.CD004856.pub2.
- O’Keeffe M, Purtill H, Kennedy N, Conneely M, Hurley J, O’Sullivan P, et al. Comparative effectiveness of conservative interventions for nonspecific chronic spinal pain: physical, behavioral/psychologically informed, or combined? A systematic review and meta-analysis. J Pain 2016;17:755-74. https://doi.org/10.1016/j.jpain.2016.01.473.
- Oldham-Cooper R, Loades M. Disorder-specific versus generic cognitive–behavioral treatment of anxiety disorders in children and young people: a systematic narrative review of evidence for the effectiveness of disorder-specific CBT compared with the disorder-generic treatment, Coping Cat. J Child Adolesc Psychiatr Nurs 2017;30:6-17. https://doi.org/10.1111/jcap.12165.
- Olthuis JV, Watt MC, Bailey K, Hayden JA, Stewart SH. Therapist-supported internet cognitive behavioural therapy for anxiety disorders in adults. Cochrane Database Syst Rev 2016;3. https://doi.org/10.1002/14651858.CD011565.pub2.
- Opoka SM, Lincoln TM. The effect of cognitive behavioral interventions on depression and anxiety symptoms in patients with schizophrenia spectrum disorders: a systematic review. Psychiatr Clin North Am 2017;40:641-59. https://doi.org/10.1016/j.psc.2017.08.005.
- Opoka SM, Ludwig L, Lincoln TM. A systematic review of trials targeting depression and anxiety in patients with delusions: an emotion-focused perspective. Zeitschrift Fur Psychologie 2018;226:142-51. https://doi.org/10.1027/2151-2604/a000331.
- Opriş D, Pintea S, García-Palacios A, Botella C, Szamosközi Å, David D. Virtual reality exposure therapy in anxiety disorders: a quantitative meta-analysis. Depress Anxiety 2012;29:85-93. https://doi.org/10.1002/da.20910.
- Orgeta V, Qazi A, Spector AE, Orrell M. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database Syst Rev 2014;1. https://doi.org/10.1002/14651858.CD009125.pub2.
- Osborn RL, Demoncada AC, Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: meta-analyses. Int J Psychiatry Med 2006;36:13-34. https://doi.org/10.2190/EUFN-RV1K-Y3TR-FK0L.
- Østergaard KR. Treatment of selective mutism based on cognitive behavioural therapy, psychopharmacology and combination therapy – a systematic review. Nord J Psychiatry 2018;72:240-50. https://doi.org/10.1080/08039488.2018.1439530.
- Osugo M, Cooper SA. Interventions for adults with mild intellectual disabilities and mental ill-health: a systematic review. J Intellect Disabil Res 2016;60:615-22. https://doi.org/10.1111/jir.12285.
- O’Sullivan C, Bosqui T, Shannon C. Psychological interventions for children and young people affected by armed conflict or political violence: a systematic literature review. Intervention 2016;14:142-64. https://doi.org/10.1097/WTF.0000000000000110.
- Oud M, de Winter L, Vermeulen-Smit E, Bodden D, Nauta M, Stone L, et al. Effectiveness of CBT for children and adolescents with depression: a systematic review and meta-regression analysis. Eur Psychiatry 2019;57:33-45. https://doi.org/10.1016/j.eurpsy.2018.12.008.
- Palavras MA, Hay P, Filho CA, Claudino A. The efficacy of psychological therapies in reducing weight and binge eating in people with bulimia nervosa and binge eating disorder who are overweight or obese – a critical synthesis and meta-analyses. Nutrients 2017;9. https://doi.org/10.3390/nu9030299.
- Palermo TM, Eccleston C, Lewandowski AS, Williams AC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. Pain 2010;148:387-97. https://doi.org/10.1016/j.pain.2009.10.004.
- Palic S, Elklit A. Psychosocial treatment of posttraumatic stress disorder in adult refugees: a systematic review of prospective treatment outcome studies and a critique. J Affect Disord 2011;131:8-23. https://doi.org/10.1016/j.jad.2010.07.005.
- Păsărelu CR, Andersson G, Bergman Nordgren L, Dobrean A. Internet-delivered transdiagnostic and tailored cognitive behavioral therapy for anxiety and depression: a systematic review and meta-analysis of randomized controlled trials. Cogn Behav Ther 2017;46:1-28. https://doi.org/10.1080/16506073.2016.1231219.
- Patel N, Kellezi B, Williams AC. Psychological, social and welfare interventions for psychological health and well-being of torture survivors. Cochrane Database Syst Rev 2014;11. https://doi.org/10.1002/14651858.CD009317.pub2.
- Pateraki E, Morris PG. Effectiveness of cognitive behavioural therapy in reducing anxiety in adults and children with asthma: a systematic review. J Asthma 2018;55:532-54. https://doi.org/10.1080/02770903.2017.1350967.
- Peat CM, Berkman ND, Lohr KN, Brownley KA, Bann CM, Cullen K, et al. Comparative effectiveness of treatments for binge-eating disorder: systematic review and network meta-analysis. Eur Eat Disord Rev 2017;25:317-28. https://doi.org/10.1002/erv.2517.
- Pennant ME, Loucas CE, Whittington C, Creswell C, Fonagy P, Fuggle P, et al. Computerised therapies for anxiety and depression in children and young people: a systematic review and meta-analysis. Behav Res Ther 2015;67:1-18. https://doi.org/10.1016/j.brat.2015.01.009.
- Perna G, Caldirola D. Management of treatment-resistant panic disorder. Curr Treat Options Psychiatry 2017;4:371-86. https://doi.org/10.1007/s40501-017-0128-7.
- Perna G, Daccò S, Menotti R, Caldirola D. Antianxiety medications for the treatment of complex agoraphobia: pharmacological interventions for a behavioral condition. Neuropsychiatr Dis Treat 2011;7:621-37. https://doi.org/10.2147/NDT.S12979.
- Perveen T, Mahmood S, Gosadi I, Mehraj J, Sheikh SS. Long term effectiveness of cognitive behavior therapy for treatment of postpartum depression: a systematic review and meta-analysis. J Pioneer Med Sci 2013;3:198-204.
- Petricone-Westwood D, Jones G, Mutsaers B, Leclair CS, Tomei C, Trudel G, et al. A systematic review of interventions for health anxiety presentations across diverse chronic illnesses. Int J Behav Med 2018;29. https://doi.org/10.1007/s12529-018-9748-6.
- Pfeiffer PN, Heisler M, Piette JD, Rogers MA, Valenstein M. Efficacy of peer support interventions for depression: a meta-analysis. Gen Hosp Psychiatry 2011;33:29-36. https://doi.org/10.1016/j.genhosppsych.2010.10.002.
- Phelps AJ, Varker T, Metcalf O, Dell L. What are effective psychological interventions for veterans with sleep disturbances? A rapid evidence assessment. Mil Med 2017;182:e1541-e1550. https://doi.org/10.7205/MILMED-D-16-00010.
- Pittock A, Hodges L, Lawrie SM. The effectiveness of internet-delivered cognitive behavioural therapy for those with bulimic symptoms: a systematic review: a review of iCBT treatment for bulimic symptoms. BMC Res Notes 2018;11. https://doi.org/10.1186/s13104-018-3843-2.
- Podina IR, Mogoase C, David D, Szentagotai A, Dobrean A. A meta-analysis on the efficacy of technology mediated CBT for anxious children and adolescents. J Ration Emot Cogn Behav Ther 2016;34:31-50. https://doi.org/10.1007/s10942-015-0228-5.
- Polnay A, James VA, Hodges L, Murray GD, Munro C, Lawrie SM. Group therapy for people with bulimia nervosa: systematic review and meta-analysis. Psychol Med 2014;44:2241-54. https://doi.org/10.1017/S0033291713002791.
- Pompoli A, Furukawa TA, Imai H, Tajika A, Efthimiou O, Salanti G. Psychological therapies for panic disorder with or without agoraphobia in adults: a network meta-analysis. Cochrane Database Syst Rev 2016;4. https://doi.org/10.1002/14651858.CD011004.pub2.
- Price JR, Mitchell E, Tidy E, Hunot V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database Syst Rev 2008;3. https://doi.org/10.1002/14651858.CD001027.pub2.
- Provencher MD, Hawke LD, Thienot E. Psychotherapies for comorbid anxiety in bipolar spectrum disorders. J Affect Disord 2011;133:371-80. https://doi.org/10.1016/j.jad.2010.10.040.
- Purgato M, Gastaldon C, Papola D, van Ommeren M, Barbui C, Tol WA. Psychological therapies for the treatment of mental disorders in low- and middle-income countries affected by humanitarian crises. Cochrane Database Syst Rev 2018;7. https://doi.org/10.1002/14651858.CD011849.pub2.
- Raggi A, Grignani E, Leonardi M, Andrasik F, Sansone E, Grazzi L, et al. Behavioral approaches for primary headaches: recent advances. Headache 2018;58:913-25. https://doi.org/10.1111/head.13337.
- Ramond-Roquin A, Bouton C, Gobin-Tempereau AS, Airagnes G, Richard I, Roquelaure Y, et al. Interventions focusing on psychosocial risk factors for patients with non-chronic low back pain in primary care – a systematic review. Fam Pract 2014;31:379-88. https://doi.org/10.1093/fampra/cmu008.
- Randhawa K, Bohay R, Côté P, van der Velde G, Sutton D, Wong JJ, et al. The effectiveness of noninvasive interventions for temporomandibular disorders: a systematic review by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration. Clin J Pain 2016;32:260-78. https://doi.org/10.1097/AJP.0000000000000247.
- Rasing SPA, Creemers DHM, Janssens JMAM, Scholte RHJ. Depression and anxiety prevention based on cognitive behavioral therapy for at-risk adolescents: a meta-analytic review. Front Psychol 2017;8. https://doi.org/10.3389/fpsyg.2017.01066.
- Reas DL, Grilo CM. Review and meta-analysis of pharmacotherapy for binge-eating disorder. Obesity 2008;16:2024-38. https://doi.org/10.1038/oby.2008.333.
- Reavell J, Hopkinson M, Clarkesmith D, Lane DA. Effectiveness of cognitive behavioral therapy for depression and anxiety in patients with cardiovascular disease: a systematic review and meta-analysis. Psychosom Med 2018;80:742-53. https://doi.org/10.1097/PSY.0000000000000626.
- Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis 2001;189:278-87. https://doi.org/10.1097/00005053-200105000-00002.
- Reinholt N, Krogh J. Efficacy of transdiagnostic cognitive behaviour therapy for anxiety disorders: a systematic review and meta-analysis of published outcome studies. Cogn Behav Ther 2014;43:171-84. https://doi.org/10.1080/16506073.2014.897367.
- Richmond H, Hall AM, Copsey B, Hansen Z, Williamson E, Hoxey-Thomas N, et al. The effectiveness of cognitive behavioural treatment for non-specific low back pain: a systematic review and meta-analysis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0134192.
- Riper H, Andersson G, Hunter SB, Wit J, Berking M, Cuijpers P. Treatment of comorbid alcohol use disorders and depression with cognitive–behavioural therapy and motivational interviewing: a meta-analysis. Addiction 2014;109:394-406. https://doi.org/10.1111/add.12441.
- Roberts NP, Roberts PA, Jones N, Bisson JI. Psychological therapies for post-traumatic stress disorder and comorbid substance use disorder. Cochrane Database Syst Rev 2016;4. https://doi.org/10.1002/14651858.CD010204.pub2.
- Roberts NP, Kitchiner NJ, Kenardy J, Bisson JI. Systematic review and meta-analysis of multiple-session early interventions following traumatic events. Am J Psychiatry 2009;166:293-301. https://doi.org/10.1176/appi.ajp.2008.08040590.
- Robinson J, Hetrick SE, Martin C. Preventing suicide in young people: systematic review. Aust N Z J Psychiatry 2011;45:3-26. https://doi.org/10.3109/00048674.2010.511147.
- Rodenburg R, Benjamin A, de Roos C, Meijer AM, Stams GJ. Efficacy of EMDR in children: a meta-analysis. Clin Psychol Rev 2009;29:599-606. https://doi.org/10.1016/j.cpr.2009.06.008.
- Rodrigues H, Figueira I, Gonçalves R, Mendlowicz M, Macedo T, Ventura P. CBT for pharmacotherapy non-remitters – a systematic review of a next-step strategy. J Affect Disord 2011;129:219-28. https://doi.org/10.1016/j.jad.2010.08.025.
- Ruiz FJ. Acceptance and commitment therapy versus traditional cognitive behavioral therapy: a systematic review and meta-analysis of current empirical evidence. Int J Psychol Psychol Ther 2012;12:333-57.
- Russell C, Kyle SD, Wearden AJ. Do evidence based interventions for chronic fatigue syndrome improve sleep? A systematic review and narrative synthesis. Sleep Med Rev 2017;33:101-10. https://doi.org/10.1016/j.smrv.2016.05.001.
- Russell PL, Davis C. Twenty-five years of empirical research on treatment following sexual assault. Best Pract Ment Health 2007;3:21-37.
- Sakamoto JT, Burrell HA, Eucker SA. Are non-pharmacologic pain interventions effective at reducing pain in adult patients visiting the emergency department?. Ann Emerg Med 2016;68. https://doi.org/10.1016/j.annemergmed.2016.08.245.
- Sangsawang B, Wacharasin C, Sangsawang N. Interventions for the prevention of postpartum depression in adolescent mothers: a systematic review. Arch Women Ment Health 2018;16. https://doi.org/10.1007/s00737-018-0901-7.
- Sarin F, Wallin L, Widerlov B. Cognitive behavior therapy for schizophrenia: a meta-analytical review of randomized controlled trials. Nord J Psychiatry 2011;65:162-74. https://doi.org/10.3109/08039488.2011.577188.
- Schade A, Marquenie LA, Van Balkom AJLM, De Beurs E, Van Dyck R, Van Den Brink W. Do comorbid anxiety disorders in alcohol-dependent patients need specific treatment to prevent relapse?. Alcohol Alcohol 2003;38:255-62. https://doi.org/10.1093/alcalc/agg062.
- Schofield P, Reid D. The assessment and management of pain in older people: a systematic review of the literature. Int J Disabili Hum Dev 2006;5:9-15. https://doi.org/10.1515/IJDHD.2006.5.1.9.
- Schwartze D, Barkowski S, Burlingame GM, Strauss B, Rosendahl J. Efficacy of group psychotherapy for obsessive–compulsive disorder: a meta-analysis of randomized controlled trials. J Obsessive Compuls Relat Disord 2016;10:49-61. https://doi.org/10.1016/j.jocrd.2016.05.001.
- Schwartze D, Barkowski S, Strauss B, Burlingame GM, Barth J, Rosendahl J. Efficacy of group psychotherapy for panic disorder: meta-analysis of randomized, controlled trials. Group Dynam Theory Res Pract 2017;21:77-93. https://doi.org/10.1037/gdn0000064.
- Schwartze D, Barkowski S, Strauss B, Knaevelsrud C, Rosendahl J. Efficacy of group psychotherapy for posttraumatic stress disorder: systematic review and meta-analysis of randomized controlled trials. Psychother Res 2019;29:415-31. https://doi.org/10.1080/10503307.2017.1405168.
- Scope A, Leaviss J, Kaltenhaler E, Parry G, Sutcliffe P, Bradburn M, et al. Is group cognitive behaviour therapy for postnatal depression evidence-based practice? A systematic review. BMC Psychiatry 2013;13. https://doi.org/10.1186/1471-244X-13-321.
- Scott JL, Dawkins S, Quinn MG, Sanderson K, Elliott KE, Stirling C, et al. Caring for the carer: a systematic review of pure technology-based cognitive behavioral therapy (TB-CBT) interventions for dementia carers. Aging Ment Health 2016;20:793-80. https://doi.org/10.1080/13607863.2015.1040724.
- Sebastian B, Nelms J. The effectiveness of emotional freedom techniques in the treatment of posttraumatic stress disorder: a meta-analysis. Explore 2017;13:16-25. https://doi.org/10.1016/j.explore.2016.10.001.
- Seyffert M, Lagisetty P, Landgraf J, Chopra V, Pfeiffer PN, Conte ML, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0149139.
- Shamliyan TA, Kane RL, Taylor FR. Migraine in Adults: Preventive Pharmacologic Treatments. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- Sharp J, Holly D, Broomfield N. Computerized cognitive behaviour therapy for depression in people with a chronic physical illness. Br J Helath Psychol 2013;18:729-44. https://doi.org/10.1111/bjhp.12014.
- Shearer HM, Carroll LJ, Wong JJ, Côté P, Varatharajan S, Southerst D, et al. Are psychological interventions effective for the management of neck pain and whiplash-associated disorders? A systematic review by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration. Spine J 2016;16:1566-81. https://doi.org/10.1016/j.spinee.2015.08.011.
- Shi Y, Zhao M, Chen S, Wang S, Li H, Ying J, et al. Effects of cognitive behavioral therapy on people living with HIV and depression: a systematic review and meta-analysis. Psychol Health Med 2019;24:578-94. https://doi.org/10.1080/13548506.2018.1549739.
- Silverman WK, Ortiz CD, Viswesvaran C, Burns BJ, Kolko DJ, Putnam FW, et al. Evidence-based psychosocial treatments for children and adolescents exposed to traumatic events. J Clin Child Adolesc Psychol 2008;37:156-83. https://doi.org/10.1080/15374410701818293.
- Sin J, Spain D, Furuta M, Murrells T, Norman I. Psychological interventions for post-traumatic stress disorder (PTSD) in people with severe mental illness. Cochrane Database Syst Rev 2017;1. https://doi.org/10.1002/14651858.CD011464.pub2.
- Singh K, Severn M. E-therapy Interventions for the Treatment of Anxiety: Clinical Evidence. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2018.
- Singh SK, Gorey KM. Relative effectiveness of mindfulness and cognitive behavioral interventions for anxiety disorders: meta-analytic review. Soc Work Ment Health 2018;16:238-51. https://doi.org/10.1080/15332985.2017.1373266.
- Skapinakis P, Caldwell DM, Hollingworth W, Bryden P, Fineberg NA, Salkovskis P, et al. Pharmacological and psychotherapeutic interventions for management of obsessive-compulsive disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatry 2016;3:730-9. https://doi.org/10.1016/S2215-0366(16)30069-4.
- Skelly AC, Chou R, Dettori JR, Turner JA, Friedly JL, Rundell SD, et al. Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review. Rockville, MD: Agency for Healthcare Research and Quality; 2018.
- Skelton M, Khokhar WA, Thacker SP. Treatments for delusional disorder. Cochrane Database Syst Rev 2015;5. https://doi.org/10.1002/14651858.CD009785.pub2.
- Sleight A. Coping with cancer-related cognitive dysfunction: a scoping review of the literature. Disabil Rehabil 2016;38:400-8. https://doi.org/10.3109/09638288.2015.1038364.
- Smallfield S, Molitor WL. Occupational therapy interventions addressing sleep for community-dwelling older adults: a systematic review. Am J Occup Ther 2018;72:7204190030p1-9. https://doi.org/10.5014/ajot.2018.031211.
- Smedslund G, Dalsbø TK, Steiro AK, Winsvold A, Clench-Aas J. Cognitive behavioural therapy for men who physically abuse their female partner. Cochrane Database Syst Rev 2007;3. https://doi.org/10.1002/14651858.CD006048.pub2.
- Smith SM, Sonego S, Ketcheson L, Larson JL. A review of the effectiveness of psychological interventions used for anxiety and depression in chronic obstructive pulmonary disease. BMJ Open Respir Res 2014;1. https://doi.org/10.1136/bmjresp-2014-000042.
- Snoek FJ, Skinner TC. Psychological counselling in problematic diabetes: does it help?. Diabet Med 2002;19:265-73. https://doi.org/10.1046/j.1464-5491.2002.00678.x.
- So M, Yamaguchi S, Hashimoto S, Sado M, Furukawa TA, McCrone P. Is computerised CBT really helpful for adult depression? A meta-analytic re-evaluation of CCBT for adult depression in terms of clinical implementation and methodological validity. BMC Psychiatry 2013;13. https://doi.org/10.1186/1471-244X-13-113.
- Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al. A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11390.
- Soo C, Tate R. Psychological treatment for anxiety in people with traumatic brain injury. Cochrane Database Syst Rev 2007;3. https://doi.org/10.1002/14651858.CD005239.pub2.
- Spain D, Sin J, Chalder T, Murphy D, Happe F. Cognitive behaviour therapy for adults with autism spectrum disorders and psychiatric co-morbidity: a review. Res Autism Spectr Disord 2015;9:151-62. https://doi.org/10.1016/j.rasd.2014.10.019.
- Spek V, Cuijpers P, Nyklícek I, Riper H, Keyzer J, Pop V. Internet-based cognitive behaviour therapy for symptoms of depression and anxiety: a meta-analysis. Psychol Med 2007;37:319-28. https://doi.org/10.1017/S0033291706008944.
- Spies G, Asmal L, Seedat S. Cognitive-behavioural interventions for mood and anxiety disorders in HIV: a systematic review. J Affect Disord 2013;150:171-80. https://doi.org/10.1016/j.jad.2013.04.018.
- Sprenger L, Gerhards F, Goldbeck L. Effects of psychological treatment on recurrent abdominal pain in children – a meta-analysis. Clin Psychol Rev 2011;31:1192-7. https://doi.org/10.1016/j.cpr.2011.07.010.
- Ssegonja R, Nystrand C, Feldman I, Sarkadi A, Langenskiöld S, Jonsson U. Indicated preventive interventions for depression in children and adolescents: a meta-analysis and meta-regression. Prev Med 2019;118:7-15. https://doi.org/10.1016/j.ypmed.2018.09.021.
- Starkstein SE, Brockman S. Management of depression in Parkinson’s disease: a systematic review. Mov Disord Clin Pract 2017;4:470-7. https://doi.org/10.1002/mdc3.12507.
- Stevens MWR, King DL, Dorstyn D, Delfabbro PH. Cognitive–behavioral therapy for internet gaming disorder: a systematic review and meta-analysis. Clin Psychol Psychother 2019;26:191-203. https://doi.org/10.1002/cpp.2341.
- Stevenson MD, Scope A, Sutcliffe PA, Booth A, Slade P, Parry G, et al. Group cognitive behavioural therapy for postnatal depression: a systematic review of clinical effectiveness, cost-effectiveness and value of information analyses. Health Technol Assess 2010;14. https://doi.org/10.3310/hta14440.
- Stock HR, Andrews J. A ten-year review of the efficacy of cognitive–behavioral treatment for obsessive–compulsive disorder in children and adolescents. Dev Disabil Bull 2004;32:173-90.
- Stoffers JM, Völlm BA, Rücker G, Timmer A, Huband N, Lieb K. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev 2012;8. https://doi.org/10.1002/14651858.CD005652.pub2.
- Stoll SVE, Crawley E, Richards V, Lal N, Brigden A, Loades ME. What treatments work for anxiety in children with chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME)? Systematic review. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-015481.
- Stratford HJ, Cooper MJ, Di Simplicio M, Blackwell SE, Holmes EA. Psychological therapy for anxiety in bipolar spectrum disorders: a systematic review. Clin Psychol Rev 2015;35:19-34. https://doi.org/10.1016/j.cpr.2014.11.002.
- Sukhodolsky DG, Bloch MH, Panza KE, Reichow B. Cognitive–behavioral therapy for anxiety in children with high-functioning autism: a meta-analysis. Pediatrics 2013;132:e1341-50. https://doi.org/10.1542/peds.2013-1193.
- Sullivan AL, Simonson GR. A systematic review of school-based social-emotional interventions for refugee and war-traumatized youth. Rev Educ Res 2016;86:503-30. https://doi.org/10.3102/0034654315609419.
- Sullivan KA, Blaine H, Kaye SA, Theadom A, Haden C, Smith SS. A systematic review of psychological interventions for sleep and fatigue after mild traumatic brain injury. J Neurotrauma 2018;35:195-209. https://doi.org/10.1089/neu.2016.4958.
- Svaldi J, Schmitz F, Baur J, Hartmann AS, Legenbauer T, Thaler C, et al. Efficacy of psychotherapies and pharmacotherapies for bulimia nervosa. Psychol Med 2019;49:898-910. https://doi.org/10.1017/S0033291718003525.
- Swan S, Keen N, Reynolds N, Onwumere J. Psychological interventions for post-traumatic stress symptoms in psychosis: a systematic review of outcomes. Front Psychol 2017;8. https://doi.org/10.3389/fpsyg.2017.00341.
- Tang WX, Zhang LF, Ai YQ, Li ZS. Efficacy of internet-delivered cognitive–behavioral therapy for the management of chronic pain in children and adolescents: a systematic review and meta-analysis. Medicine 2018;97. https://doi.org/10.1097/MD.0000000000012061.
- Tao WW, Tao XM, Song CL. Effects of non-pharmacological supportive care for hot flushes in breast cancer: a meta-analysis. Support Care Cancer 2017;25:2335-47. https://doi.org/10.1007/s00520-017-3691-y.
- Tay KW, Subramaniam P, Oei TP. Cognitive behavioural therapy can be effective in treating anxiety and depression in persons with dementia: a systematic review. Psychogeriatrics 2018;13. https://doi.org/10.1111/psyg.12391.
- Taylor DJ, Pruiksma KE. Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. Int Rev Psychiatry 2014;26:205-13. https://doi.org/10.3109/09540261.2014.902808.
- Taylor TL, Montgomery P. Can cognitive-behavioral therapy increase self-esteem among depressed adolescents? A systematic review. Child Youth Serv Rev 2007;29:823-39. https://doi.org/10.1016/j.childyouth.2007.01.010.
- Thabrew H, Stasiak K, Hetrick SE, Donkin L, Huss JH, Highlander A, et al. Psychological therapies for anxiety and depression in children and adolescents with long-term physical conditions. Cochrane Database Syst Rev 2018;12. https://doi.org/10.1002/14651858.CD012488.pub2.
- Thabrew H, Stasiak K, Hetrick SE, Wong S, Huss JH, Merry SN. E-Health interventions for anxiety and depression in children and adolescents with long-term physical conditions. Cochrane Database Syst Rev 2018;8. https://doi.org/10.1002/14651858.CD012489.pub2.
- Thakur ER, Shapiro J, Chan J, Lumley MA, Cully JA, Bradford A, et al. A systematic review of the effectiveness of psychological treatments for IBS in gastroenterology settings: promising but in need of further study. Dig Dis Sci 2018;63:2189-201. https://doi.org/10.1007/s10620-018-5095-3.
- Thomas PW, Thomas S, Hillier C, Galvin K, Baker R. Psychological interventions for multiple sclerosis. Cochrane Database Syst Rev 2006;1. https://doi.org/10.1002/14651858.CD004431.pub2.
- Thomas RE, Alves J, Vaska Mlis MM, Magalhaes R. Therapy and rehabilitation of mild brain injury/concussion: systematic review. Restor Neurol Neurosci 2017;35:643-66. https://doi.org/10.3233/RNN-170761.
- Thomson A, Page L. Psychotherapies for hypochondriasis. Cochrane Database Syst Rev 2007;4. https://doi.org/10.1002/14651858.CD006520.
- Toivonen KI, Zernicke K, Carlson LE. Web-based mindfulness interventions for people with physical health conditions: systematic review. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.7487.
- Torchalla I, Strehlau V. The evidence base for interventions targeting individuals with work-related PTSD: a systematic review and recommendations. Behav Modif 2018;42:273-30. https://doi.org/10.1177/0145445517725048.
- Torous J, Levin ME, Ahern DK, Oser ML. Cognitive behavioral mobile applications: clinical studies, marketplace overview, and research agenda. Cogn Behav Pract 2017;24:215-25. https://doi.org/10.1016/j.cbpra.2016.05.007.
- Townsend E, Walker DM, Sargeant S, Vostanis P, Hawton K, Stocker O, et al. Systematic review and meta-analysis of interventions relevant for young offenders with mood disorders, anxiety disorders, or self-harm. J Adolesc 2010;33:9-20. https://doi.org/10.1016/j.adolescence.2009.05.015.
- Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med 2015;163:191-204. https://doi.org/10.7326/M14-2841.
- Tremblay A, Sheeran L, Aranda SK. Psychoeducational interventions to alleviate hot flashes: a systematic review. Menopause 2008;15:193-202. https://doi.org/10.1097/gme.0b013e31805c08dc.
- Troeung L, Egan SJ, Gasson N. A meta-analysis of randomised placebo-controlled treatment trials for depression and anxiety in Parkinson’s disease. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0079510.
- Turner DT, van der Gaag M, Karyotaki E, Cuijpers P. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry 2014;171:523-38. https://doi.org/10.1176/appi.ajp.2013.13081159.
- Twomey C, O’Reilly G. Effectiveness of a freely available computerised cognitive behavioural therapy programme (MoodGYM) for depression: meta-analysis. Aust N Z J Psychiatry 2017;51:260-9. https://doi.org/10.1177/0004867416656258.
- Twomey C, O’Reilly G, Byrne M. Computerised cognitive behavioural therapy: helping Ireland log on. Ir J Psychol Med 2013;30:29-56. https://doi.org/10.1017/ipm.2012.5.
- Twomey C, O’Reilly G, Byrne M. Effectiveness of cognitive behavioural therapy for anxiety and depression in primary care: a meta-analysis. Fam Pract 2015;32:3-15. https://doi.org/10.1093/fampra/cmu060.
- Twomey C, O’Reilly G, Meyer B. Effectiveness of an individually-tailored computerised CBT programme (Deprexis) for depression: a meta-analysis. Psychiatry Res 2017;256:371-7. https://doi.org/10.1016/j.psychres.2017.06.081.
- Uchendu C, Blake H. Effectiveness of cognitive-behavioural therapy on glycaemic control and psychological outcomes in adults with diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Diabet Med 2017;34:328-39. https://doi.org/10.1111/dme.13195.
- Usmani ZA, Carson KV, Heslop K, Esterman AJ, De Soyza A, Smith BJ. Psychological therapies for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017;3. https://doi.org/10.1002/14651858.CD010673.pub2.
- Uwimana J, Louw Q. Effectiveness of palliative care including physiotherapy in HIV patients: a review of the literature. South Afr J Physiother 2007;63:41-50. https://doi.org/10.4102/sajp.v63i2.135.
- Välimäki M, Anttila K, Anttila M, Lahti M. Web-based interventions supporting adolescents and young people with depressive symptoms: systematic review and meta-analysis. JMIR Mhealth Uhealth 2017;5. https://doi.org/10.2196/mhealth.8624.
- van Beugen S, Ferwerda M, Hoeve D, Rovers MM, Spillekom-van Koulil S, van Middendorp H, et al. Internet-based cognitive behavioral therapy for patients with chronic somatic conditions: a meta-analytic review. J Med Internet Res 2014;16. https://doi.org/10.2196/jmir.2777.
- van der Maas M, Shi J, Elton-Marshall T, Hodgins DC, Sanchez S, Lobo DS, et al. Internet-based interventions for problem gambling: scoping review. JMIR Ment Health 2019;6. https://doi.org/10.2196/mental.9419.
- van Dessel N, den Boeft M, van der Wouden JC, Kleinstäuber M, Leone SS, Terluin B, et al. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev 2014;11. https://doi.org/10.1002/14651858.CD011142.pub2.
- van Driel CMG, Stuursma AS, Schroevers MJ, Mourits MJE, de Bock GH. Mindfulness, cognitive behavioural and behaviour-based therapy for natural and treatment-induced menopausal symptoms: a systematic review and meta-analysis. BJOG 2019;126:330-9. https://doi.org/10.1111/1471-0528.15153.
- van Ravesteyn LM, Lambregtse-van den Berg MP, Hoogendijk WJ, Kamperman AM. Interventions to treat mental disorders during pregnancy: a systematic review and multiple treatment meta-analysis. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0173397.
- Vandepitte S, Van Den Noortgate N, Putman K, Verhaeghe S, Faes K, Annemans L. Effectiveness of supporting informal caregivers of people with dementia: a systematic review of randomized and non-randomized controlled trials. J Alzheimers Dis 2016;52:929-65. https://doi.org/10.3233/JAD-151011.
- Veehof MM, Trompetter HR, Bohlmeijer ET, Schreurs KM. Acceptance- and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Cogn Behav Ther 2016;45:5-31. https://doi.org/10.1080/16506073.2015.1098724.
- Vélez Toral M, Godoy-Izquierdo D, Padial García A, Lara Moreno R, Mendoza Ladrón de Guevara N, Salamanca Ballesteros A, et al. Psychosocial interventions in perimenopausal and postmenopausal women: a systematic review of randomised and non-randomised trials and non-controlled studies. Maturitas 2014;77:93-110. https://doi.org/10.1016/j.maturitas.2013.10.020.
- Velleman S, Stallard P, Richardson T. A review and meta-analysis of computerized cognitive behaviour therapy for the treatment of pain in children and adolescents. Child Care Health Dev 2010;36:465-72. https://doi.org/10.1111/j.1365-2214.2010.01088.x.
- Velthorst E, Koeter M, van der Gaag M, Nieman DH, Fett AK, Smit F, et al. Adapted cognitive–behavioural therapy required for targeting negative symptoms in schizophrenia: meta-analysis and meta-regression. Psychol Med 2015;45:453-65. https://doi.org/10.1017/S0033291714001147.
- Venning A, Kettler L, Eliott J, Wilson A. The effectiveness of cognitive–behavioural therapy with hopeful elements to prevent the development of depression in young people: a systematic review. JBI Evid Implement 2009;7:15-33. https://doi.org/10.1111/j.1744-1609.2009.00122.x.
- Verberne DPJ, Spauwen PJJ, van Heugten CM. Psychological interventions for treating neuropsychiatric consequences of acquired brain injury: a systematic review. Neuropsychol Rehabil 2019;29:1509-42. https://doi.org/10.1080/09602011.2018.1433049.
- Verdeli H, Mufson L, Lee L, Keith JA. Review of evidence-based psychotherapies for pediatric mood and anxiety disorders. Curr Psychiatry Rev 2006;2:395-421. https://doi.org/10.2174/157340006778018102.
- Verhagen AP, Damen L, Berger MY, Passchier J, Koes BW. Behavioral treatments of chronic tension-type headache in adults: are they beneficial?. CNS Neurosci Ther 2009;15:183-205. https://doi.org/10.1111/j.1755-5949.2009.00077.x.
- Vidal-Estrada R, Bosch-Munso R, Nogueira-Morais M, Casas-Brugue M, Ramos-Quiroga JA. Psychological treatment of attention deficit hyperactivity disorder in adults: a systematic review. Actas Esp Psiquiatr 2012;40:147-54.
- Waddell C, Hua JM, Garland OM, Peters RD, McEwan K. Preventing mental disorders in children: a systematic review to inform policy-making. Can J Public Health 2007;98:166-73. https://doi.org/10.1007/BF03403706.
- Walklet E, Muse K, Meyrick J, Moss T. Do psychosocial interventions improve quality of life and wellbeing in adults with neuromuscular disorders? A systematic review and narrative synthesis. J Neuromuscul Dis 2016;3:347-62. https://doi.org/10.3233/JND-160155.
- Wang CW, Chan CL, Ho RT, Tsang HW, Chan CH, Ng SM. The effect of qigong on depressive and anxiety symptoms: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med 2013;2013. https://doi.org/10.1155/2013/716094.
- Wang MY, Tsai PS, Chou KR, Chen CM. A systematic review of the efficacy of non-pharmacological treatments for depression on glycaemic control in type 2 diabetics. J Clin Nurs 2008;17:2524-30. https://doi.org/10.1111/j.1365-2702.2008.02301.x.
- Wang SB, Wang YY, Zhang QE, Wu SL, Ng CH, Ungvari GS, et al. Cognitive behavioral therapy for post-stroke depression: a meta-analysis. J Affect Disord 2018;235:589-96. https://doi.org/10.1016/j.jad.2018.04.011.
- Wang Z, Whiteside SPH, Sim L, Farah W, Morrow AS, Alsawas M, et al. Comparative effectiveness and safety of cognitive behavioral therapy and pharmacotherapy for childhood anxiety disorders: a systematic review and meta-analysis. JAMA Pediatr 2017;171:1049-56. https://doi.org/10.1001/jamapediatrics.2017.3036.
- Wang ZD, Xia YF, Zhao Y, Chen LM. Cognitive behavioural therapy on improving the depression symptoms in patients with diabetes: a meta-analysis of randomized control trials. Biosci Rep 2017;37. https://doi.org/10.1042/BSR20160557.
- Ward EC. Examining differential treatment effects for depression in racial and ethnic minority women: a qualitative systematic review. J Natl Med Assoc 2007;99:265-74.
- Warwick H, Reardon T, Cooper P, Murayama K, Reynolds S, Wilson C, et al. Complete recovery from anxiety disorders following cognitive behavior therapy in children and adolescents: a meta-analysis. Clin Psychol Rev 2017;52:77-91. https://doi.org/10.1016/j.cpr.2016.12.002.
- Watson HJ, Joyce T, French E, Willan V, Kane RT, Tanner-Smith EE, et al. Prevention of eating disorders: a systematic review of randomized, controlled trials. Int J Eat Disord 2016;49:833-62. https://doi.org/10.1002/eat.22577.
- Watson HJ, Rees CS. Meta-analysis of randomized, controlled treatment trials for pediatric obsessive–compulsive disorder. J Child Psychol Psychiatry 2008;49:489-98. https://doi.org/10.1111/j.1469-7610.2007.01875.x.
- Watts SE, Turnell A, Kladnitski N, Newby JM, Andrews G. Treatment-as-usual (TAU) is anything but usual: a meta-analysis of CBT versus TAU for anxiety and depression. J Affect Disord 2015;175:152-67. https://doi.org/10.1016/j.jad.2014.12.025.
- Webb RT, Howard L, Abel KM. Antipsychotic drugs for non-affective psychosis during pregnancy and postpartum. Cochrane Database Syst Rev 2004;2. https://doi.org/10.1002/14651858.CD004411.pub2.
- Wendebourg MJ, Heesen C, Finlayson M, Meyer B, Pöttgen J, Köpke S. Patient education for people with multiple sclerosis-associated fatigue: a systematic review. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0173025.
- Werner-Seidler A, Johnston L, Christensen H. Digitally-delivered cognitive–behavioural therapy for youth insomnia: a systematic review. Internet Interv 2018;11:71-8. https://doi.org/10.1016/j.invent.2018.01.007.
- Wersebe H, Sijbrandij M, Cuijpers P. Psychological group-treatments of social anxiety disorder: a meta-analysis. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0079034.
- Weston L, Hodgekins J, Langdon PE. Effectiveness of cognitive behavioural therapy with people who have autistic spectrum disorders: a systematic review and meta-analysis. Clin Psychol Rev 2016;49:41-54. https://doi.org/10.1016/j.cpr.2016.08.001.
- Wide Boman U, Carlsson V, Westin M, Hakeberg M. Psychological treatment of dental anxiety among adults: a systematic review. Eur J Oral Sci 2013;121:225-34. https://doi.org/10.1111/eos.12032.
- Williams J, Hadjistavropoulos T, Sharpe D. A meta-analysis of psychological and pharmacological treatments for body dysmorphic disorder. Behav Res Ther 2006;44:99-111. https://doi.org/10.1016/j.brat.2004.12.006.
- Wilson KC, Mottram PG, Vassilas CA. Psychotherapeutic treatments for older depressed people. Cochrane Database Syst Rev 2008;1. https://doi.org/10.1002/14651858.CD004853.pub2.
- Woltz PC, Chapa DW, Friedmann E, Son H, Akintade B, Thomas SA. Effects of interventions on depression in heart failure: a systematic review. Heart Lung 2012;41:469-83. https://doi.org/10.1016/j.hrtlng.2012.06.002.
- Wood L, Byrne R, Varese F, Morrison AP. Psychosocial interventions for internalised stigma in people with a schizophrenia-spectrum diagnosis: a systematic narrative synthesis and meta-analysis. Schizophr Res 2016;176:291-303. https://doi.org/10.1016/j.schres.2016.05.001.
- Wootton BM. Remote cognitive-behavior therapy for obsessive–compulsive symptoms: a meta-analysis. Clin Psychol Rev 2016;43:103-13. https://doi.org/10.1016/j.cpr.2015.10.001.
- Wu JQ, Appleman ER, Salazar RD, Ong JC. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med 2015;175:1461-72. https://doi.org/10.1001/jamainternmed.2015.3006.
- Xiao F, Song X, Chen Q, Dai Y, Xu R, Qiu C, et al. Effectiveness of psychological interventions on depression in patients after breast cancer surgery: a meta-analysis of randomized controlled trials. Clin Breast Cancer 2017;17:171-9. https://doi.org/10.1016/j.clbc.2016.11.003.
- Xie CL, Wang XD, Chen J, Lin HZ, Chen YH, Pan JL, et al. A systematic review and meta-analysis of cognitive behavioral and psychodynamic therapy for depression in Parkinson’s disease patients. Neurol Sci 2015;36:833-43. https://doi.org/10.1007/s10072-015-2118-0.
- Xu GZ, Li YF, Wang MD, Cao DY. Complementary and alternative interventions for fatigue management after traumatic brain injury: a systematic review. Ther Adv Neurol Disord 2017;10:229-39. https://doi.org/10.1177/1756285616682675.
- Yang L, Zhou X, Pu J, Liu L, Cuijpers P, Zhang Y, et al. Efficacy and acceptability of psychological interventions for social anxiety disorder in children and adolescents: a meta-analysis of randomized controlled trials. Eur Child Adolesc Psychiatry 2018;28:79-8. https://doi.org/10.1007/s00787-018-1189-x.
- Yang L, Zhou X, Zhou C, Zhang Y, Pu J, Liu L, et al. Efficacy and acceptability of cognitive behavioral therapy for depression in children: a systematic review and meta-analysis. Acad Pediatr 2017;17:9-16. https://doi.org/10.1016/j.acap.2016.08.002.
- Yatham S, Sivathasan S, Yoon R, da Silva TL, Ravindran AV. Depression, anxiety, and post-traumatic stress disorder among youth in low and middle income countries: a review of prevalence and treatment interventions. Asian J Psychiatr 2018;38:78-91. https://doi.org/10.1016/j.ajp.2017.10.029.
- Ye BY, Jiang ZY, Li X, Cao B, Cao LP, Lin Y, et al. Effectiveness of cognitive behavioral therapy in treating bipolar disorder: an updated meta-analysis with randomized controlled trials. Psychiatry Clin Neurosci 2016;70:351-61. https://doi.org/10.1111/pcn.12399.
- Ye M, Du K, Zhou J, Zhou Q, Shou M, Hu B, et al. A meta-analysis of the efficacy of cognitive behavior therapy on quality of life and psychological health of breast cancer survivors and patients. Psycho-Oncology 2018;27:1695-703. https://doi.org/10.1002/pon.4687.
- Yorke J, Fleming S, Shuldham C, Rao H, Smith HE. Nonpharmacological interventions aimed at modifying health and behavioural outcomes for adults with asthma: a critical review. Clin Exp Allergy 2015;45:1750-64. https://doi.org/10.1111/cea.12511.
- Yoshinaga N, Nosaki A, Unozawa K, Hayashi Y, Shimizu E. A systematic review of cognitive behavioral therapy in nursing field in Japan. Asia-Pacific Psychiatry 2015;7.
- Zachariae R, Lyby MS, Ritterband LM, O’Toole MS. Efficacy of internet-delivered cognitive–behavioral therapy for insomnia – a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev 2016;30:1-10. https://doi.org/10.1016/j.smrv.2015.10.004.
- Zhang Z, Zhang L, Zhang G, Jin J, Zheng Z. The effect of CBT and its modifications for relapse prevention in major depressive disorder: a systematic review and meta-analysis. BMC Psychiatry 2018;18. https://doi.org/10.1186/s12888-018-1610-5.
- Zhao S, Sampson S, Xia J, Jayaram MB. Psychoeducation (brief) for people with serious mental illness. Cochrane Database Syst Rev 2015;4. https://doi.org/10.1002/14651858.CD010823.pub2.
- Zhou T, Li X, Pei Y, Gao J, Kong J. Internet-based cognitive behavioural therapy for subthreshold depression: a systematic review and meta-analysis. BMC Psychiatry 2016;16. https://doi.org/10.1186/s12888-016-1061-9.
- Zhou X, Michael KD, Liu Y, Del Giovane C, Qin B, Cohen D, et al. Systematic review of management for treatment-resistant depression in adolescents. BMC Psychiatry 2014;14. https://doi.org/10.1186/s12888-014-0340-6.
- Zhou X, Zhang Y, Furukawa TA, Cuijpers P, Pu J, Weisz JR, et al. Different types and acceptability of psychotherapies for acute anxiety disorders in children and adolescents: a network meta-analysis. JAMA Psychiatry 2018;31.
- Zhu Z, Zhang L, Jiang J, Li W, Cao X, Zhou Z, et al. Comparison of psychological placebo and waiting list control conditions in the assessment of cognitive behavioral therapy for the treatment of generalized anxiety disorder: a meta-analysis. Shanghai Arch Psychiatry 2014;26:319-31. https://doi.org/10.11919/j.issn.1002-0829.214173.
- Zijdenbos IL, de Wit NJ, van der Heijden GJ, Rubin G, Quartero AO. Psychological treatments for the management of irritable bowel syndrome. Cochrane Database Syst Rev 2009;1. https://doi.org/10.1002/14651858.CD006442.pub2.
- Fordham B, Sugavanam T, Edwards K, Stallard P, Howard R, das Nair R, et al. The evidence for cognitive behavioural therapy in any condition, population or context: a meta-review of systematic reviews and panoramic meta-analysis. Psychol Med 2021;51:21-9.
- Kunik ME, Veazey C, Cully JA, Souchek J, Graham DP, Hopko D, et al. COPD education and cognitive behavioral therapy group treatment for clinically significant symptoms of depression and anxiety in COPD patients: a randomized controlled trial. Psychol Med 2008;38:385-96. https://doi.org/10.1017/S0033291707001687.
- Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation; 1993.
- Palermo TM, Law EF, Fales J, Bromberg MH, Jessen-Fiddick T, Tai G. Internet-delivered cognitive–behavioral treatment for adolescents with chronic pain and their parents: a randomized controlled multicenter trial. Pain 2016;157:174-85. https://doi.org/10.1097/j.pain.0000000000000348.
- Fairburn CG, Cooper Z, Shafran R. Cognitive behaviour therapy for eating disorders: a ‘transdiagnostic’ theory and treatment. Behav Res Ther 2003;41:509-28. https://doi.org/10.1016/S0005-7967(02)00088-8.
- Howick J, Glasziou P, Aronson JK. Can understanding mechanisms solve the problem of extrapolating from study to target populations (the problem of ‘external validity’)?. J R Soc Med 2013;106:81-6. https://doi.org/10.1177/0141076813476498.
- Howick J, Glasziou P, Aronson JK. Problems with using mechanisms to solve the problem of extrapolation. Theor Med Bioeth 2013;34:275-91. https://doi.org/10.1007/s11017-013-9266-0.
- López-López JA, Davies SR, Caldwell DM, Churchill R, Peters TJ, Tallon D, et al. The process and delivery of CBT for depression in adults: a systematic review and network meta-analysis. Psychol Med 2019;49:1937-47. https://doi.org/10.1017/S003329171900120X.
- National Institute for Health and Care Excellence . Depression in Adults: Recognition and Management 2009.
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines. J Clin Epidemiol 2013;66:158-72. https://doi.org/10.1016/j.jclinepi.2012.01.012.
- Daly J. Evidence-based Medicine and the Search for a Science of Clinical Care. Berkeley, CA: University of California Press; 2005.
- Howick J. The Philosophy of Evidence-Based Medicine. Oxford: Wiley-Blackwell; 2011.
- Group . EBMW. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA 1992;268:2420-5. https://doi.org/10.1001/jama.1992.03490170092032.
- Howick J. Exploring the asymmetrical relationship between the power of finance bias and evidence. Perspect Biol Med 2019;62:159-87. https://doi.org/10.1353/pbm.2019.0009.
- Cuijpers P, Karyotaki E, Eckshtain D, Ng MY, Corteselli KA, Noma H, et al. Psychotherapy for depression across different age groups: a systematic review and meta-analysis. JAMA Psychiatry 2020;77:694-702. https://doi.org/10.1001/jamapsychiatry.2020.0164.
- Ünlü Ince B, Riper H, van ‘t Hof E, Cuijpers P. The effects of psychotherapy on depression among racial-ethnic minority groups: a metaregression analysis. Psychiatr Serv 2014;65:612-17. https://doi.org/10.1176/appi.ps.201300165.
- French P, Morrison AP. Early Detection and Cognitive Therapy for People at High Risk of Developing Psychosis: A Treatment Approach. Chichester: John Wiley & Sons Ltd; 2004.
- Shawyer F, Farhall J, Mackinnon A, Trauer T, Sims E, Ratcliff K, et al. A randomised controlled trial of acceptance-based cognitive behavioural therapy for command hallucinations in psychotic disorders. Behav Res Ther 2012;50:110-21. https://doi.org/10.1016/j.brat.2011.11.007.
- Beck AT. Cognitive Therapy of Depression. New York, NY; 1979.
- Kendall P, Choudary M, Hudson J, Webb A. The CAT Project Manual. Ardmore, PA: Workbook Publishing; 2002.
- Hay P, Trefiglio R. Antidepressants versus psychological treatments and their combination for bulimia nervosa. Cochrane Database Syst Rev 2001;2001. https://doi.org/10.1002/14651858.CD003385.
Appendix 1 Sensitivity check papers
Amick HR, Gartlehner G, Gaynes BN, Forneris C, Asher GN, Morgan LC, et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ 2015;351:h6019.
Beynon S, Soares-Weiser K, Woolacott N, Duffy S, Geddes JR. Psychosocial interventions for the prevention of relapse in bipolar disorder: systematic review of controlled trials. Br J Psychiatry 2008;192:5–11.
Carlbring P, Andersson G, Cuijpers P, Riper H, Hedman-Lagerlof E. Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta-analysis. Cogn Behav Ther 2018;47:1–18.
Cuijpers P, van Straten A, Andersson G. Internet-administered cognitive behavior therapy for health problems: a systematic review. J Behav Med 2008;31:169–77.
de Arellano MA, Lyman DR, Jobe-Shields L, George P, Dougherty RH, Daniels AS, et al. Trauma-focused cognitive–behavioral therapy for children and adolescents: assessing the evidence. Psychiatr Serv 2014;65:591–602.
Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 2008;165:179–87.
Farah WH, Alsawas M, Mainou M, Alahdab F, Farah MH, Ahmed AT, et al. Non-pharmacological treatment of depression: a systematic review and evidence map. Evid Based Med 2016;21:214–21.
Glombiewski JA, Sawyer AT, Gutermann J, Koenig K, Rief W, Hofmann SG. Psychological treatments for fibromyalgia: a meta-analysis. Pain 2010;151:280–95.
Hesser H, Weise C, Westin VZ, Andersson G. A systematic review and meta-analysis of randomized controlled trials of cognitive–behavioural therapy for tinnitus distress. Clin Psychol Rev 2011;31:545–53.
Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry 2008;69:621–32.
Hundt NE, Barrera TL, Robinson A, Cully JA. A systematic review of cognitive behavioral therapy for depression in veterans. Mil Med 2014;179:942–9.
James AC, James G, Cowdrey FA, Soler A, Choke A. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev 2013;6:CD004690.
Jones C, Hacker D, Cormac I, Meaden A, Irving CB. Cognitive behaviour therapy versus other psychosocial treatments for schizophrenia. Cochrane Database Syst Rev 2012;4:CD008712.
Kavanagh J, Oliver S, Lorenc T, Caird J, Tucker H, Harden A, et al. School-based cognitive–behavioural interventions: a systematic review of effects and inequalities. Health Sociol Rev 2009;18:61–78.
Kim JH. [A meta-analysis of effects of job stress management interventions (SMIs).] Taehan Kanho Hakhoe Chi 2007;37:529–39.
Leichsenring F, Leibing E. The effectiveness of psychodynamic therapy and cognitive behavior therapy in the treatment of personality disorders: a meta-analysis. Am J Psychiatry 2003;160:1223–32.
Okajima I, Komada Y, Inoue Y. A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep Biol Rhythm 2011;9:24–34.
Olthuis JV, Watt MC, Bailey K, Hayden JA, Stewart SH. Therapist-supported internet cognitive behavioural therapy for anxiety disorders in adults. Cochrane Database Syst Rev 2015;3:CD011565.
Orgeta V, Qazi A, Spector AE, Orrell M. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database Syst Rev 2014;1:CD009125.
O'Toole MS, Zachariae R, Renna ME, Mennin DS, Applebaum A. Cognitive behavioral therapies for informal caregivers of patients with cancer and cancer survivors: a systematic review and meta-analysis. Psycho-Oncology 2017;26:428–37.
Pallesen S, Mitsem M, Kvale G, Johnsen BH, Molde H. Outcome of psychological treatments of pathological gambling: a review and meta-analysis. Addiction 2005;100:1412–22.
Pearson FS, Lipton DS, Cleland CM, Yee DS. The effects of behavioral/cognitive-behavioral programs on recidivism. Crime Delinq 2002;48:476–96.
Peng XD, Huang CQ, Chen LJ, Lu ZC. Cognitive behavioural therapy and reminiscence techniques for the treatment of depression in the elderly: a systematic review. J Int Med Res 2009;37:975–82.
Pineros-Leano M, Liechty JM, Piedra LM. Latino immigrants, depressive symptoms, and cognitive behavioral therapy: a systematic review. J Affect Disord 2017;208:567–76.
Powers MB, Vedel E, Emmelkamp PM. Behavioral couples therapy (BCT) for alcohol and drug use disorders: a meta-analysis. Clin Psychol Rev 2008;28:952–62.
Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis 2001;189:278–87.
Richmond H, Hall AM, Copsey B, Hansen Z, Williamson E, Hoxey-Thomas N, et al. The effectiveness of cognitive behavioural treatment for non-specific low back pain: a systematic review and meta-analysis. PLOS One 2015;10:e0134192.
Santacruz I, Orgiles M, Rosa AI, Sanchez-Meca J, Mendez X, Olivares J. [Generalized anxiety, separation anxiety and school phobia: the predominance of cognitive–behavioural therapy.] Psicologia Conductual 2002;10:503–21.
Shinohara K, Honyashiki M, Imai H, Hunot V, Caldwell DM, Davies P, et al. Behavioural therapies versus other psychological therapies for depression. Cochrane Database Syst Rev 2013;10:CD008696.
Smith SM, Sonego S, Ketcheson L, Larson JL. A review of the effectiveness of psychological interventions used for anxiety and depression in chronic obstructive pulmonary disease. BMJ Open Respir Res 2014;1:e000042.
Sockol LE, Epperson CN, Barber JP. A meta-analysis of treatments for perinatal depression. Clin Psychol Rev 2011;31:839–49.
Soo S, Moayyedi P, Deeks J, Delaney B, Lewis M, Forman D. Psychological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev 2004;1:CD002301.
Taylor S, Asmundson GJG, Coons MJ. Current directions in the treatment of hypochondriasis. J Cogn Psychother 2005;19:285–304.
Thompson-Brenner HJ. Implications for the Treatment of Bulimia Nervosa: A Meta-analysis of Efficacy Trials and a Naturalistic Study of Treatment in the Community. PhD thesis. Ann Arbor, MI: University of Michigan; 2002.
van Dessel N, den Boeft M, van der Wouden JC, Kleinstauber M, Leone SS, Terluin B, et al. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev 2014;11:CD011142.
van Straten A, Geraedts A, Verdonck-de Leeuw I, Andersson G, Cuijpers P. Psychological treatment of depressive symptoms in patients with medical disorders: a meta-analysis. J Psychosom Res 2010;69:23–32.
Appendix 2 Detailed search strategies for review
MEDLINE
Database and platform
Ovid MEDLINE® Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®, 1946 to present.
Search filter
Scottish Intercollegiate Guidelines Network systematic review search filter for MEDLINE (via Ovid) [www.sign.ac.uk/search-filters.html (accessed February 2019)].
Date search was conducted
Original: 25 April 2018 (for publication years 1992 to present) (2967 references).
Updated: 30 January 2019 (for publication years 2018–19) (359 references).
Search strategy
-
(cognitive adj2 behavio?r adj3 (therap$ or theor$ or intervention$ or train$ or treatment$ or psychotherap$ or programme$ or program$ or method$ or approach$)).ti,ab,kw.
-
(cognitive adj2 behavio?ral adj3 (therap$ or theor$ or intervention$ or train$ or treatment$ or psychotherap$ or programme$ or program$ or method$ or approach$)).ti,ab,kw.
-
CBT.ti,ab,kw.
-
Cognitive Therapy/
-
or/1-4
-
Meta-Analysis as Topic/
-
meta analy$.tw.
-
metaanaly$.tw.
-
Meta-Analysis/
-
(systematic adj (review$1 or overview$1)).tw.
-
exp Review Literature as Topic/
-
or/6-11
-
cochrane.ab.
-
embase.ab.
-
(psychlit or psyclit).ab.
-
(psychinfo or psycinfo).ab.
-
(cinahl or cinhal).ab.
-
science citation index.ab.
-
bids.ab.
-
cancerlit.ab.
-
or/13-20
-
reference list$.ab.
-
bibliograph$.ab.
-
hand-search$.ab.
-
relevant journals.ab.
-
manual search$.ab.
-
or/22-26
-
selection criteria.ab.
-
data extraction.ab.
-
28 or 29
-
Review/
-
30 and 31
-
Comment/
-
Letter/
-
Editorial/
-
animal/
-
human/
-
36 and 37
-
36 not 38
-
or/33-35,39
-
12 or 21 or 27 or 32
-
41 not 40
-
5 and 42
-
limit 43 to yr = ‘1992-2018’.
EMBASE
Database and platform
Embase, 1974–2018, week 17 (via Ovid).
Search filter
The SIGN systematic review filter for EMBASE (via Ovid) [www.sign.ac.uk/search-filters.html (accessed February 2019)].
Date search was conducted
Original: 25 April 2018 (for publication years 1992 to present) (4862 references).
Update: 30 January 2019 (for publication years 2018–19) (192 references).
Search strategy
-
(cognitive adj2 behavio?r adj3 (therap$ or theor$ or intervention$ or train$ or treatment$ or psychotherap$ or programme$ or program$ or method$ or approach$)).ti,ab,kw.
-
(cognitive adj2 behavio?ral adj3 (therap$ or theor$ or intervention$ or train$ or treatment$ or psychotherap$ or programme$ or program$ or method$ or approach$)).ti,ab,kw.
-
CBT.ti,ab,kw.
-
Cognitive Therapy/
-
exp Cognitive Behavioral Therapy/
-
or/1-5
-
exp Meta Analysis/
-
((meta adj analy$) or metaanalys$).tw.
-
(systematic adj (review$1 or overview$1)).tw.
-
or/7-9
-
cancerlit.ab.
-
cochrane.ab.
-
embase.ab.
-
(psychlit or psyclit).ab.
-
(psychinfo or psycinfo).ab.
-
(cinahl or cinhal).ab.
-
science citation index.ab.
-
bids.ab.
-
or/11-18
-
reference list$.ab.
-
bibliograph$.ab.
-
hand-search$.ab.
-
relevant journals.ab.
-
manual search$.ab.
-
or/20-24
-
selection criteria.ab.
-
data extraction.ab.
-
26 or 27
-
review.pt.
-
28 and 29
-
Letter.pt.
-
Editorial.pt.
-
animal/
-
human/
-
33 and 34
-
33 not 35
-
or/31-32,36
-
10 or 19 or 25 or 30
-
38 not 37
-
6 and 39
-
limit 40 to yr = ‘1992-2018’.
Cumulative Index to Nursing and Allied Health Literature
Database and platform
CINAHL (via EBSCOhost).
Search filter
The SIGN systematic review filter for CINAHL (via EBSCOhost) [www.sign.ac.uk/search-filters.html (accessed February 2019)].
Date search was conducted
Original: 25 April 2018 (for publication years 1992 to present) (1062 references).
Update: 30 January 2019 (for publication years 2018–19) (174 references).
Search strategy
-
(TI (cognitive N2 behaviour N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*)) OR (AB (cognitive N2 behaviour N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*))
-
(TI (cognitive N2 behavior N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*)) OR (AB (cognitive N2 behavior N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*))
-
(TI (cognitive N2 behavioural N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*)) OR (AB (cognitive N2 behavioural N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*))
-
(TI cognitive N2 behavioral N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*)) OR (AB cognitive N2 behavioral N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*))
-
(TI ‘CBT’) OR (AB ‘CBT’)
-
(MH ‘Cognitive Therapy’) OR (SU ‘Cognitive Therapy’)
-
S1 OR S2 OR S3 OR S4 OR S5 OR S6
-
(MH Meta Analysis)
-
(TX ‘meta analys*’)
-
(TX ‘metaanaly*’)
-
(MH ‘Literature Review+’)
-
(TX systematic N1 (review or overview))
-
S8 OR S9 OR S10 OR S11 OR S12
-
(PT ‘Commentary’)
-
(PT ‘Letter’)
-
(PT ‘Editorial’)
-
(MH Animals)
-
S14 OR S15 OR S16 OR S17
-
S13 NOT S18
-
S7 AND S19
-
PY 1992-2018
-
S20 AND S21
PsycINFO
Database and platform:
PsycINFO, 1967 to April week 2 2018 (via Ovid).
Search filter
McMaster Hedges Maximises Specificity Systematic Review filter for PsycINFO (via Ovid) (modified) [https://hiru.mcmaster.ca/hiru/HIRU_Hedges_PsycINFO_Strategies.aspx#Reviews (accessed February 2019)].
Date search was conducted
Original: 27 April 2018 (for publication years 1992 to present) (2190 references).
Update: 30 January 2019 (for publication years 2018–19) (150 references).
Search strategy
-
(cognitive adj2 behavio?r adj3 (therap$ or theor$ or intervention$ or train$ or treatment$ or psychotherap$ or programme$ or program$ or method$ or approach$)).ti,ab.
-
(cognitive adj2 behavio?ral adj3 (therap$ or theor$ or intervention$ or train$ or treatment$ or psychotherap$ or programme$ or program$ or method$ or approach$)).ti,ab.
-
CBT.ti,ab.
-
Cognitive Behavior Therapy/
-
or/1-4
-
meta-analy$.tw.
-
systematic review.md.
-
meta analysis.md.
-
search:.tw.
-
or/6-9
-
5 and 10
-
limit 11 to yr = ‘1992-2018’
Notes
The McMaster Hedges Maximises Specificity Systematic Review filter for PsycINFO was modified in the following way:
-
Changed ‘meta-analysis.tw.’ to ‘meta-analy$.tw.’
-
Added ‘systematic review.md.’ and ‘meta analysis.md.’
This was to account for known missing papers with ‘meta-analyses’ or ‘meta-analytic’ in the abstract and for papers that do not use ‘search’ (e.g. where ‘retrieval’ or ‘databases surveyed’ is used). If the papers are systematic reviews or reviews, they should be assigned the methodology heading (‘.md.’).
Cochrane Database of Systematic Reviews
Database and platform
Cochrane Database of Systematic Reviews [via The Cochrane Library: http://cochranelibrary-wiley.com/cochranelibrary/search/ (accessed May 2020)].
Date search was conducted
Original: 26 April 2018 (for publication years 1992 to present) (176 references).
Update: 30 January 2019 (for publication years 2018–19) (20 references).
Search strategy
-
(cognitive next/2 behaviour* next/3 therap*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behaviour* next/3 theor*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behaviour* next/3 intervention*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behaviour* next/3 train*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behaviour* next/3 treatment*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behaviour* next/3 psychotherap*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behaviour* next/3 programme*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behaviour* next/3 program*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behaviour* next/3 method*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behaviour* next/3 approach*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behavior* next/3 therap*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behavior* next/3 theor*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behavior* next/3 intervention*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behavior* next/3 train*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behavior* next/3 treatment*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behavior* next/3 psychotherap*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behavior* next/3 programme*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behavior* next/3 program*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behavior* next/3 method*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
(cognitive next/2 behavior* next/3 approach*):ti,ab,kw in Cochrane Reviews (Reviews only)
-
‘CBT’:ti,ab,kw in Cochrane Reviews (Reviews only)
-
[mh ‘Cognitive Therapy’] in Cochrane Reviews (Reviews only)
-
#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22
-
#23 Publication Year from 1992 to 2018
Database of Abstracts of Reviews of Effects
Database and platform
Database of Abstracts of Reviews of Effect [via The Cochrane Library: https://cochranelibrary-wiley.com/cochranelibrary/search/ (accessed May 2020)].
Date search was conducted
Original: 26 April 2018 (for publication years 1992 to present) (610 references).
Update: no updated search on DARE as no longer updated.
Search strategy
-
cognitive next/2 behaviour* next/3 therap*:ti,ab,kw in Other Reviews
-
cognitive next/2 behaviour* next/3 theor*:ti,ab,kw in Other Reviews
-
cognitive next/2 behaviour* next/3 intervention*:ti,ab,kw in Other Reviews
-
cognitive next/2 behaviour* next/3 train*:ti,ab,kw in Other Reviews
-
cognitive next/2 behaviour* next/3 treatment*:ti,ab,kw in Other Reviews
-
cognitive next/2 behaviour* next/3 psychotherap*:ti,ab,kw in Other Reviews
-
cognitive next/2 behaviour* next/3 programme*:ti,ab,kw in Other Reviews
-
cognitive next/2 behaviour* next/3 program*:ti,ab,kw in Other Reviews
-
cognitive next/2 behaviour* next/3 method*:ti,ab,kw in Other Reviews
-
cognitive next/2 behaviour* next/3 approach*:ti,ab,kw in Other Reviews
-
cognitive next/2 behavior* next/3 therap*:ti,ab,kw in Other Reviews
-
cognitive next/2 behavior* next/3 theor*:ti,ab,kw in Other Reviews
-
cognitive next/2 behavior* next/3 intervention*:ti,ab,kw in Other Reviews
-
cognitive next/2 behavior* next/3 train*:ti,ab,kw in Other Reviews
-
cognitive next/2 behavior* next/3 treatment*:ti,ab,kw in Other Reviews
-
cognitive next/2 behavior* next/3 psychotherap*:ti,ab,kw in Other Reviews
-
cognitive next/2 behavior* next/3 programme*:ti,ab,kw in Other Reviews
-
cognitive next/2 behavior* next/3 program*:ti,ab,kw in Other Reviews
-
cognitive next/2 behavior* next/3 method*:ti,ab,kw in Other Reviews
-
cognitive next/2 behavior* next/3 approach*:ti,ab,kw in Other Reviews
-
‘CBT’:ti,ab,kw in Other Reviews
-
[mh ‘Cognitive Therapy’] in Other Reviews
-
#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22
-
#23 Publication Year from 1992 to 2018.
Child Development and Adolescent Studies
Database and platform
Child Development and Adolescent Studies (via EBSCOhost).
Date search was conducted
Original: 25 April 2018 (for publication years 1992 to present) (177 references).
Update: 30 January 2019 (for publication years 2018–19) (21 references).
Search strategy
-
(TI (cognitive N2 behaviour N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*)) OR (AB (cognitive N2 behaviour N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*)) OR (AS (cognitive N2 behaviour N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*))
-
(TI (cognitive N2 behavior N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*)) OR (AB (cognitive N2 behavior N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*)) OR (AS (cognitive N2 behavior N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*))
-
(TI (cognitive N2 behavioural N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*)) OR (AB (cognitive N2 behavioural N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*)) OR (AS (cognitive N2 behavioural N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*))
-
(TI cognitive N2 behavioral N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*)) OR (AB cognitive N2 behavioral N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*)) OR (AS cognitive N2 behavioral N3 (therap* or theor* or intervention* or train* or treatment* or psychotherap* or programme* or program* or method* or approach*))
-
(TI ‘CBT’) OR (AB ‘CBT’) OR (DE ‘CBT’)
-
(DE ‘Cognitive Therapy’) OR (SU ‘Cognitive Therapy’)
-
(DE ‘Cognitive-behavioral therapy’) OR (DE ‘Cognitive behavioral therapy’) OR (DE ‘Cognitive-behavioural therapy’) OR (DE ‘Cognitive behavioural therapy’) OR (DE ‘Cognitive-behavior therapy’) OR (DE ‘Cognitive behavior therapy’) OR (DE ‘Cognitive-behaviour therapy’) OR (DE ‘Cognitive behaviour therapy’)
-
(DE ‘BEHAVIOR therapy’) OR (SU ‘BEHAVIOR therapy’) OR (DE ‘BEHAVIOUR therapy’) OR (SU ‘BEHAVIOUR therapy’) OR (DE ‘BEHAVIORAL therapy’) OR (SU ‘BEHAVIORAL therapy’) OR (DE ‘BEHAVIOURAL therapy’) OR (SU ‘BEHAVIOURAL therapy’)
-
S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8
-
(DE ‘SYSTEMATIC reviews (Medical research)’) OR (DE ‘systematic review’) OR (SU ‘SYSTEMATIC reviews (Medical research)’) OR (DE ‘review’)
-
(DE ‘META-analysis’) OR (SU ‘META-analysis’)
-
(DE ‘Literature Review’)
-
(TX ‘metaanaly*’)
-
(TX ‘meta analy*’)
-
(TX systematic N3 (review or overview))
-
S10 OR S11 OR S12 OR S13 OR S14 OR S15
-
S9 AND S16
-
DT 1992-2018
-
S17 AND S18.
OpenGrey
Database and platform
OpenGrey [via www.opengrey.eu/ (accessed February 2019)].
Date search was conducted
Original: 26 April 2018 (295 references).
Update: 30 January 2019 (0 references).
Search strategy
-
‘cognitive behavioral’ OR ‘cognitive behavioural’ OR ‘cognitive behavior’ OR ‘cognitive behaviour’ OR ‘CBT’.
Appendix 3 Data extraction form for mapping
| Data extraction | Notes |
|---|---|
| Review ID (surname, year) | |
| Reviewer completing form | |
| Date completed | |
| Reference citation (first author, title, journal, volume, issue) | |
| Published in last 5 years (Y/N) | |
| Aim of the review | |
| Design of included studies [RCT n = [participants n = ]] | |
| Any risk-of-bias tool employed (Y/N) [Detail] | RoB not just quality assessment tool. Report the actual tool used in ‘details’ |
| Primary health problem | Report ICD-11 for every health problem reported |
| Secondary health problem | |
| Severity (mild, moderate, severe, NR) | Select one or many or ‘unclear’ |
| Age categories [number of RCTs] | Select one or many of children, adolescents, adults, older adults or ‘not reported’ or ‘unclear’ |
| Other characteristics reported: (1) gender, (2) ethnicity, (3) other specific/unique information | Report top-level information on (1) gender (2) ethnicity and (3) if available other information (but do not need to search) |
| Where recruited [number of RCTs] | Report as the review has reported: clinic, university, internet, etc |
| When delivered [number of RCTs] | Drop-down list [(1) preventative (2) preventative for relapse (3) early intervention (4) standard treatment (5) mixed (6) not reported (7) other (standard treatment is the norm, the others are if review specifies a target)] |
| Countries included [number of RCTs] | |
| CBT high/low/combined: description of CBT |
|
| CBT overall: number of sessions, duration and frequency | As much as is available in the review. Can synthesise ourselves but only at this top level |
| CBT content description 1 [number of RCTs/total RCTs] | Report high-intensity intervention first then low intensity |
| CBT description 2 [number of RCTs/total] | Complete for every type of CBT category the review includes |
| CBT description 3 [number of RCTs/total] | |
| CBT description 4 [number of RCTs/total] | |
| CBT description 5 [number of RCTs/total] | |
| Control description 1 [number of RCTs] | If review synthesises all non-active/active together, then extract as such; if reported as separate control groups, then we can extract as such and then later we will combine |
| Control description 2 [number of RCTs] | |
| Control description 3 [number of RCTs] | |
| Control description 4 [number of RCTs] | |
| Control description 5 [number of RCTs] | |
| Other details | Only most pertinent information if required |
| HRQoL category | Choose (1) category, (2) category but no data available, (3) not measured. If HRQoL emerges at individual RCT level, then we extract (but only for HRQoL) |
| How measured [name(s) of instruments/method] | Specific name of outcome |
| When measured [pegged time point?] | Short (majority < 12 months), long (majority ≥ 12 months) or ‘unclear’ [if the review reports where the follow-up time points are pegged to, i.e. post randomisation, post intervention, then report] |
| Number of RCTs [number of participants] | Number of RCTs [number of participants] |
| Meta-analysis [Y/N] | If no meta-analysis, please report the direction of results (i.e. in favour or not in favour of CBT) |
| Depression category | Choose (1) category, (2) category but no data available, (3) not measured |
| How measured [name(s) of instruments/method] | Specific name of outcome |
| When measured [pegged time point?] | Short (majority < 12 months), long (majority ≥ 12 months) or ‘unclear’ [if the review reports where the follow-up time points are pegged to, i.e. post randomisation, post intervention, then report] |
| Number of RCTs [number of participants] | Number of RCTs [number of participants] |
| Meta-analysis [Y/N] | If no meta-analysis, please report the direction of results (i.e. in favour or not in favour of CBT) |
| Anxiety category | Choose (1) category (2) category but no data available (3) not measured |
| How measured [name(s) of instruments/method] | Specific name of outcome |
| When measured [pegged time point?] | Short (majority < 12 months), long (majority ≥ 12 months) or ‘unclear’ [if the review reports where the follow-up time points are pegged to, i.e. post randomisation, post intervention, then report] |
| Number of RCTs [number of participants] | Number of RCTs [number of participants] |
| Meta-analysis [Y/N] | If no meta-analysis, please report the direction of results (i.e. in favour or not in favour of CBT) |
| Physical/physiological category | Choose (1) category, (2) category but no data available, (3) not measured |
| How measured [name(s) of instruments/method] | Specific name of outcome |
| When measured [pegged time point?] | Short (majority < 12 months), long (majority ≥ 12 months) or ‘unclear’ [if the review reports where the follow-up time points are pegged to, i.e. post randomisation, post intervention, then report] |
| Number of RCTs [number of participants] | Number of RCTs [number of participants] |
| Meta-analysis [Y/N] | If no meta-analysis, please report the direction of results (i.e. in favour or not in favour of CBT) |
| Psychosis category | Choose (1) category, (2) category but no data available, (3) not measured |
| How measured [name(s) of instruments/method] | Specific name of outcome |
| When measured [pegged time point?] | Short (majority < 12 months), long (majority ≥ 12 months) or ‘unclear’ [if the review reports where the follow-up time points are pegged to, i.e. post randomisation, post intervention, then report] |
| Number of RCTs [number of participants] | Number of RCTs [number of participants] |
| Meta-analysis [Y/N] | If no meta-analysis, please report the direction of results (i.e. in favour or not in favour of CBT) |
| All other outcomes reported in review | List format e.g. (1) PANSS psychosis |
| Overall | See AMSTAR-2 PDF21 |
| Mechanism data | Extraction of entire section ‘How the intervention might work’ (for Cochrane reviews), or similar (other reviews), and/or direct data: namely changes to beliefs such as self-efficacy or hypotheses for mechanisms such as presented in the discussions |
| Acceptability | |
| Satisfaction | |
| Adverse effects | |
| Economic analyses |
Appendix 4 The AMSTAR-2 checklist
Reproduced from Shea et al. 4 with permission. Copyright © 2017, BMJ Publishing Group Ltd. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Appendix 5 Data extraction form for the panoramic meta-analysis
| Meta-analysis details | |
|---|---|
| HRQoL | |
| GRADE or equivalent | |
| Intervention vs. control group | |
| Age group | |
| When measured | |
| Name of outcome instrument | Specific name of instrument on which meta-analysis was conducted |
| Number of RCTs | |
| Continuous SMD or MD [S/MD post or S/MD of change] (± favours intervention or not) | |
| Number of participants | |
| S/MD (effect size) and type [95% CI] | Hedges or Cohen (± favours intervention or not) |
| I 2 | |
| Fixed or random effects | |
| Reference | |
| Depression | |
| GRADE or equivalent | |
| Intervention vs. control group | |
| Age group | |
| Name of outcome instrument | |
| When measured | |
| Number of RCTs | |
| Binary OR or RR (± favours intervention or not) | |
| Number of participants | |
| Number of events | |
| OR/RR [95% CI] | |
| I 2 | |
| Fixed or random effects | |
| Continuous SMD or MD [S/MD post or S/MD of change] (± favours intervention or not) | |
| Number of participants | |
| S/MD (effect size) and type [95% CI] | |
| I 2 | |
| Fixed or random effects | |
| Reference | |
| Anxiety | |
| GRADE or equivalent | |
| Intervention vs. control group | |
| Age group | |
| Name of outcome instrument | |
| When measured | |
| Number of RCTs | |
| Binary OR or RR (± favours intervention or not) | |
| Number of participants | |
| Number of events | |
| OR/RR [95% CI] | |
| I 2 | |
| Fixed or random effects | |
| Continuous SMD or MD [S/MD post or S/MD of change] (± favours intervention or not) | |
| Number of participants | |
| S/MD (effect size) and type [95% CI] | |
| I 2 | |
| Fixed or random effects | |
| Reference | |
| Physical/physiological | |
| GRADE or equivalent | |
| Intervention vs. control group | |
| Age group | |
| When measured | |
| Name of outcome instrument | |
| Number of RCTs | |
| Binary OR or RR (± favours intervention or not) | |
| Number of participants | |
| Number of events | |
| OR/RR [95% CI] | |
| I 2 | |
| Fixed or random effects | |
| Continuous SMD or MD [S/MD post or S/MD of change] (± favours intervention or not) | |
| Number of participants | |
| S/MD (effect size) and type [95% CI] | |
| I 2 | |
| Fixed or random effects | |
| Reference | |
| Psychosis | |
| GRADE or equivalent | |
| Intervention vs. control group | |
| Age group | |
| Name of outcome instrument | |
| When measured | |
| Number of RCTs | |
| Binary OR or RR (± favours intervention or not) | |
| Number of participants | |
| Number of events | |
| OR/RR [95% CI] | |
| I 2 | |
| Fixed or random effects | |
| Continuous SMD or MD (± favours intervention or not) | |
| Number of participants | |
| S/MD (effect size) and type [95% CI] | |
| I 2 | |
| Fixed or random effects | |
| Reference | |
Appendix 6 References to studies excluded at the full-text screening stage, with reasons for exclusion
Studies excluded because no English full text or translation available (n = 237)
Abdel-Baki A, Nicole L. [Schizophrenia and cognitive–behavioural therapy (CBT).] Can J Psychiatry 2001;46:511–21.
Aghaie E, Abedi A, Paghale SJ. [Meta-analysis of the effectiveness of cognitive–behavior interventions in the reduction 3 of test anxiety in Iran.] Iran J Psychiatry Clin Psychol 2012;18:3–12.
Ahmadnia E, Haseli A, Karamat A. [Therapeutic interventions conducted on improving women’s sexual satisfaction and function during reproductive ages in Iran: a systematic review.] J Mazandaran Univ Med Sci 2017;27:146–62.
Ahonen S, Kivela SL. [Effects of cognitive and behavioral treatments on primary insomnia in old age.] Duodecim 2010;126:794–802.
Albert U, Barbaro F, Aguglia A, Maina G, Bogetto F. [Combined treatments in obsessive–compulsive disorder: current knowledge and future prospects.] Riv Psichiatr 2012;47:255–68.
Algar MJM, Garcia PB. [Approach to anxiety in patients diagnosed with cancer.] Psicooncologia 2016;13:227–48.
Almeida Lima Junior N, Lopes Paes DG, Belchior Pontes GC, Gomes Sancho A, da Silva Rosa JL, Dias Faria ÁC. Possíveis impactos do transtorno de ansiedade social no processo de envelhecimento. Fisioterap Bras 2018;19:577–81.
Almeida AM, Lotufo Neto F. [Cognitive–behavioral therapy in prevention of depression relapses and recurrences: a review.] Rev Bras Psiquiatr 2003;25:239–44.
Ambresin G, De Roten Y, Despland JN. [Psychotherapy of depression in primary care.] Schweiz Arch Neurol Psychiatr 2016;167:147–54.
Andanson J, Pourre F, Maffre T, Raynaud JP. [Social skills training groups for children and adolescents with Asperger syndrome: a review.] Arch Pediatr 2011;18:589–96.
Arteriole N. [Cognitive–Behavioral Therapy in Bipolar Disorder.] PhD thesis. Caen: Université de Caen Normandie & Université de Caen UFR de médecine; 2013.
Auclair V, Harvey PO, Lepage M. [Cognitive behavioral therapy and the treatment of ADHD in adults.] Sante Ment Que 2016;41:291–311.
Bacaltchuk J, Hay P. [Treatment of bulimia nervosa: a synthesis of evidence.] Rev Bras Psiquiatr 1999;21:184–7.
Banti S, Borri C, Montagnani MS, Cargioli C, Belli S, Cotugno B, et al. [Premenstrual disphoric disorder: an update.] J Psychopathol 2012;18:261–72.
Barreto EMDP, Elkis H. [Evidences from the efficacy of the cognitive behavior therapy on schizophrenia.] Rev Psiquiatr Clin 2007;34:204–7.
Baum E, Maisel P, Dorr C, Donner-Banzhoff N. [Update of the DEGAM-guideline fatigue: new developments and recommendations.] Z Allgemeinmed 2012;88:133–7.
Beelmann A, Schneider N. [The effects of psychotherapy with children and adolescents. A review and meta-analysis of German-language research.] Z klin Psychol Psychotherap 2003;32:129–43.
Belanger L, Vallieres A, Morin CM. [Insomnia and the increased use of sleeping pills among the elderly: problems and alternative therapy.] Can Fam Physician 2006;52:968–73.
Bellino S, Zizza M, Di Lorenzo R, Paradiso E, Falakfarsa R, Fulcheri M, et al. [Combined therapy of major depressive disorder: a critical review.] Italian J Psychopathol 2002;8:401–16.
Bellver Perez A, Moreno P. [Psychosocial risks and psychological intervention in the transplanted bone marrow patients.] Psicooncologia 2009;6:65–81.
Blanca-Gutierrez JJ, Jimenez-Diaz MDC, Escalera-Franco LF. [Effective interventions to reduce absenteeism among hospital nurses.] Gac Sanit 2013;27:545–51.
Block I, Loeber S. [Evidence-based psychotherapy of addictive disorders.] Nervenarzt 2018;89:283–9.
Bomba J. [Research on psychotherapy effectiveness in treatment of mental and behavioural disorders in children and adolescents.] Psychoterapia 2010:37–47.
Bornas X, Rodrigo T, Barcelo F, Toledo M. [New technologies in cognitive-behavioral therapy: a review.] Int J Clin Health Psychol 2002;2:533–41.
Boschi M, Santandrea S, Vanti C. [Efficacy of cognitive behavioural therapy in non-specific neck pain: a systematic review.] Sci Riabil 2010;12:5–15.
Bottlender M, Kohler J, Soyka M. [The effectiveness of psychosocial treatment approaches for alcohol dependence – a review.] Fortschr Neurol Psychiatr 2006;74:19–31.
Boudebesse C, Henry C. [Emotional hyper-reactivity and sleep disturbances in remitted patients with bipolar disorders.] Encephale 2012;38:S173–S8.
Brandao T, Mena Matos P. [Efficacy of psychological group interventions for women with breast cancer: a systematic review.] Rev Port Saude Publica 2015;33:98–106.
Brown RC. [Psychotherapeutic interventions for suicidal adolescents – a systematic review of the current literature.] Z Kind Jugendpsychiatr Psychotherap 2017;45:499–508.
Bruun Wyller V, Bjorneklett A, Brubakk O, Festvag L, Follestad I, Malt U, et al. [Diagnosis and Treatment of Chronic Fatigue Syndrome/Myalgic Encephalopathy (CFS/ME).] Report from Knowledge Centre No. 9. Oslo: National Knowledge Centre for Health Services at the Norwegian Institute of Public Health (NIPH); 2006.
Calzolari L, Fioravanti G. [A comparison between acceptance and commitment therapy and cognitive behavioural therapy: a review of literature.] Psicoter Cogn Comportamental 2016;22:103–17.
Caselli G, Manfredi C, Ruggiero GM, Sassaroli S. [Cognitive behavioural therapy for anxiety disorders: a review of efficacy studies.] Psicoter Cogn Comportamental 2016;22:81–101.
Castelein S, Knegtering H, Van Meijel B, Van Der Gaag M. [Dutch guideline on Schizophrenia 2012: basic care within the areas of psychosocial interventions and nursing care.] Tijdschr Psychiatr 2013;55:707–14.
Cavadas LF, Ribeiro L. [Management of adult secondary insomnia in primary health care.] Acta Med Port 2011;24:135–44.
Copanitsanou P, Sourtzi P. [The effect of educational interventions for the reduction of nursing staff’s occupational stress: systematic review.] Nosileftiki 2016;55:250–62.
Cuijpers P, Dekker J. [Psychological treatment of depression; a systematic review of meta-analyses.] Nederlands Tijdschr Geneesk 2005;149:1892–7.
Daga G, Quaranta M, Notaro G, Urani C, Amianto F, Fassino S. [Family therapy and eating disorders in young female patients: state of the art.] Ital J Psychopathol 2011;17:40–7.
Dahm KT, Landmark B, Kirkehei I, Reinar LM. [The Effects of School Health Services for Children and Young People’s Health and Growing Up Conditions.] Report from Knowledge Centre No. 17. Oslo: National Knowledge Centre for Health Services at the Norwegian Institute of Public Health (NIPH); 2010.
Dahm KT, Smedslund G, Havelsrud K, Hafstad E, Reinar LM. [Psychological Interventions for Children and Youth with Serious Somatic Illness in Primary Care.] Report from Knowledge Centre No.10. Oslo: National Knowledge Centre for Health Services at the Norwegian Institute of Public Health (NIPH); 2014.
de Almeida AM, Lotufo Neto F. [Cognitive–behavioral therapy in prevention of depression relapses and recurrences: a review.] Rev Bras Psiquiatr 2003;25:239–44.
de Carvalho MR, Nardi AE, Range B. [Comparison between cognitive, behavioral and cognitive–behavioral approaches in the treatment of panic disorder.] Rev Psiquiatr Clin 2008;35:66–73.
de Cerqueira ACR, Nardi AE. [Depression and multiple sclerosis: on overview.] Rev Bras Neurol 2011;47:11–6.
de Haan E, Huyser C, Boer F. [Obsessive–compulsive disorder in children and adolescents.] Tijdschr Psychiatr 2005;47:229–38.
de Haan L, Bakker JM. [Effectivity of individual psychotherapy in schizophrenia. A review of recent studies.] Tijdschr Psychiatr 2000;42:751–8.
Deffieux X, Billecocq S, Demoulin G, Rivain AL, Trichot C, Thubert T. [Pelvic floor rehabilitation for female urinary incontinence: mechanisms of action.] Progr Urol 2013;23:491–501.
Ding Y. [A review of the psychological characteristics and intervention of patients with malignant tumor before and atter treatment.] J Clin Rehabil Tissue Eng Res 2007;11:7951–4.
Dingemans AE, Bruna MJ, Van Furth EF. [Binge eating disorder. A review.] Tijdschr Psychiatr 2001;43:321–31.
Doerr JP, Hajak G, Riemann D. [Pharmacotherapy of primary insomnia – efficacy, effectiveness and safety of various therapeutic options.] Psycho Neuro 2008;34:86–93.
dos Reis E, Camargo Novelli MMP, Fernandes Guerra RL. Intervenções realizadas com grupos de cuidadores de idosos com síndrome demencial: revisão sistemática. Cad Bras Terap Ocup 2018;26:646–57.
Driessen E, Cuijpers P, Hollon SD, Van HL, Dekker JJM. [The efficacy of psychological treatments for depression: a review of recent research findings.] Tijdschr Psychiatr 2014;56:455–62.
Ducasse D, Denis H. [Pathological nighttime fears in children: clinical specificities and effective therapeutics.] Encephale 2015;41:323–31.
Duchesne M, Appolinario JC, Range BP, Freitas S, Papelbaum M, Coutinho W. [Evidence of cognitive–behavioral therapy in the treatment of obese patients with binge eating disorder.] Rev Psiquiatr Rio Grande Sul 2007;29:80–92.
박 수 인, 김 연 지, 오 의. 만성적인 신체질환을 가진 환자의 우울 감소를 위한 전화기반 인지행동치료의 효과: 메타분석. J Kor Acad Psychiatr Ment Health Nurs 2018;27:227–39.
김지현, 오복자. 수면장애가 있는 중장년 환자에게 적용한 비약물적 중재의 효과: 메타분석. Kor Adult Nurs 2016;28:13–29.
Elisha D, Karny N, Styr BB. [Psychotherapy – outcome studies and guidelines for evidence-based care policy in Israel.] Harefuah 2011;150:269–74, 302.
Espanol Armengol N, Mijan De La Torre A. [Eating disorders in obesity.] Rev Esp Obes 2006;4:317–27.
Fagiolo D, Berardelli I. [Use of cognitive–behavioural techniques in the treatment of headaches: a systematic review of the last 10-years’ literature.] Med Psicosomat 2007;52:117–24.
Faller H, Herschbach P. [Psychooncological interventions – how successful are they?] Nervenheilkunde 2011;30:133–7.
Fan RM, Yang L. [Progress in clinical treatment of insomnia.] Chin J Clin Rehabil 2006;10:149–51.
Fernandes PA, de Carvalho MR. [Neurobiological changes after cognitive–behavioral therapy of obsessive–compulsive disorder.] Psicologia: Teoria e Pesquisa 2016;32:1–9.
Fischer-Terworth C, Probst P, Glanzmann PG, Knorr CC. [Psychological interventions in dementia: an evaluative review.] Z Psychiatr Psycholog Psychotherap 2009;57:195–206.
Flores-Valdez IH, Leon-Santos MP, Vera-Hernandez E, del Rocio Hernandez Pozo M. [Psychological interventions on stress management and reduction for hypertensive patients: a review of their effectiveness.] Psychologia: Avances de la Disciplina 2013;7:25–44.
Fodor KE, Bitter I. [Psychological interventions following trauma to prevent posttraumatic stress disorder: a systematic review of the literature.] Orvosi Hetilap 2015;156:1321–4.
Foldes-Busque G, Marchand A, Landry P. [Early detection and treatment of panic disorder with or without agoraphobia.] Can Fam Physician 2007;53:1686–93.
Fond G, Franc N. [Treating specific childhood phobia in a single session? A systematic review of the literature.] Encephale 2013;39:109–14.
Fritsche G, Kroner-Herwig B, Kropp P, Niederberger U, Haag G. [Psychological therapy of migraine: systematic review.] Schmerz 2013;27:263–74.
Fu L, Wu MB, Hu Y. [Influence of cognitive behavioural therapy on depression, medication adherence and quality of life in people living with HIV/AIDS (PLHIV): a systematic review.] Chin J Evid Based Med 2014;14:734–42.
Gärtner-Tschacher N. [The effectiveness of combined active physiotherapy and cognitive, behavioural or cognitive–behavioural therapy approaches in patients with musculoskeletal pain.] Manuelle Therapie 2005;9:11–34.
Gai X-S, Lan G-R, Liu X-P. [A meta-analytic review on treatment effects of attention deficit/hyperactivity disorder children in China.] Acta Psychol Sin 2009;40:1190–6.
Gajdos P, Rigo A. [Irritable bowel syndrome: comorbid psychiatric disorders and psychological treatment options.] Orvosi Hetilap 2018;159:2115–21.
Galindo-Vazquez O, Perez-Barrientos H, Alvarado-Aguilar S, Rojas-Castillo E, Alvarez-Avitia MA, Aguilar-Ponce JL. [Cognitive behavioral therapy effects in cancer patients: a review.] Gaceta Mexicana de Oncologia 2013;12:108–15.
Garcia-Perez L, Valdivia-Salas S. [Acceptance and commitment therapy for social anxiety disorder: a systematic review.] Behav Psychol 2018;26:37–92.
Garcia-Torres F, Alos FJ, Perez-Duenas C. [Posttraumatic stress disorder in cancer survivors: a review of the psychological treatments available.] Psicooncologia 2015;12:293–301.
Garcia-Vera MP, Moreno N, Sanz J, Gutierrez S, Gesteira C, Zapardiel A, et al. [Efficacy and clinical utility (effectiveness) of treatments for adult victims of terrorist attacks: a systematic review.] Behavioral Psychology/Psicologia Conductual: Revista Internacional Clinica y de la Salud 2015;23:215–44.
Garcia-Vera MP, Sanz J. [Analysis of the situation of treatments for smoking cessation based on cognitive–behavioral therapy and nicotine patches.] Psicooncologia 2006;3:269–89.
Gellato C. [Eye Movement Desensitization and Reprocessing and Cognitive–Behavioral Therapy in the Treatment of Adult’s Post Traumatic Syndrome Disorder.] PhD thesis. Marseille: Université d’Aix-Marseille II; 2009.
Gomes JB, Matte BC, Vivan A, Viana A, Bortoncello CF, Salum GA, et al. [Cognitive behavioral therapy with family intervention for children and adolescents with obsessive compulsive disorder: a systematic review.] Rev Psiquiatr Rio Grande Sul 2011;33:121–7.
Gomez Puente JM, Martinez-Marcos M. [Overweight and obesity: effectiveness of interventions in adults.] Enfermeria Clin 2018;28:65–74.
Gonzalez Larrabe I, Torre Mollinedo F, Telletxea Benguria S, Arizaga Maguregi A. [Update in the multidisciplinary treatment of fibromyalgia.] Dolor 2008;23:194–206.
Groschwitz RC, Plener PL. [Psychotherapeutic interventions for non-suicidal self-injury.] Nervenheilkunde 2013;32:30–6.
Gunthner A, Batra A. [Prevention of burnout by stress management.] Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz 2012;55:183–9.
Guzman GAR, Lemus CAD, Garcia RR, Agraz FP. [Cognitive behavioral therapy for binge eating disorder: a review.] Psiquiatria 2005;21.
Haidl TK, Rosen M, Ruhrmann S, Klosterkotter J. [Social anxiety in individuals with clinical high-risk state for psychosis.] Fortschritte der Neurologie-Psychiatrie 2018;87:284–97.
Hauser W, Bernardy K. [Psychotherapeutic procedures for fibromyalgia syndrome.] Z Rheumatol 2015;74:584–90.
Hautzinger M, Meyer TD. [Psychotherapy for bipolar disorder: a systematic review of controlled studies.] Nervenarzt 2007;78:1248–60.
Hautzinger M, Wetzel H, Scheurich A, Mainz Univ, Saarland Univ, Rostock Univ, et al. [Serotonin 1A-agonist and Cognitive Behavior Therapy in Relapse Prevention of Alcoholics. Evaluation of Efficacy of Different Treatment Modalities and their Combination.] 2001.
Hautzinger M. [Behavioural therapy with affective and neurological disorders in the elderly.] Verhaltenstherapie & Verhaltensmedizin 2002;23:195–212.
Heckrath VC, Dohmen P. [On the empirical foundation of the highly significant superiority of the cognitive–behavior compared with the psychoanalytically oriented psychotherapies.] Z Psychosomat Med Psychoanal 1997;43:179–201.
Hendriks GJ, Keijsers GPJ, Kampman M, Verbraak MJPM, Broekman TG, Hoogduin CAL, et al. [Treatment of anxiety disorders in the elderly.] Tijdschrift voor Psychiatrie 2011;53:589–95.
Herr L, Mingebach T, Becker K, Christiansen H, Kamp-Becker I. [A systematic review of the effectiveness of parent-based interventions for children aged two to twelve years.] Kindheit Entwicklung: Z Klin Kinderpsychol 2015;24:6–19.
Hess M, Wirtz S, Allroggen M, Scheithauer H. [Intervention and therapy for perpetrators and victims of bullying: a systematic review.] Prax Kinderpsychol Kinderpsychiatr 2017;66:740–55.
Hilbert A, Brahler E. [Interpersonal psychotherapy for eating disorders: a systematic and practical review.] Verhaltenstherapie 2012;22:149–57.
Hirjak D, Bechdolf A, Thomann PA, Thiemann U, Wolf RC. [Prevention of psychosis.] Nervenheilkunde 2012;31:923–32.
Hua Y, Dai J. [Studies on occupational stress intervention in workplaces abroad: a systematic review.] Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2015;33:759–64.
Huang F-F, Li Z-J, Han H-Y, Xiong H-F, Ma Y. [Cognitive behavioral therapy combined with pharmacotherapy for obsessive compulsive disorder: a meta-analysis.] Chin Ment Health J 2013;27:643–9.
한수연, 황지혜, 김초희, 장혜영, 방경숙. 소아암 환자의 형제자매 중재에 관한 연구논문의 체계적 문헌고찰. Child Health Nurs Res 2017;23:394–404.
Isolan L, Pheula G, Manfro GG. [Treatment of social anxiety disorder in children and adolescents.] Rev Psiquiatr Clin 2007;34:125–32.
Jakle C, Basler HD. [Change of cognition in psychological pain therapy: a meta-analysis of the cognitive–behavioral mode.] Z klin Psychol Psychotherap 2000;29:127–39.
Jank R, Pieh C. [Efficacy and evidence base of group psychotherapy for depressive disorders.] Psychotherapie Forum 2016;21:62–71.
Jelonkiewicz I. [Psychotherapy of gambling – how to effectively treat gamblers? The effectivenes of applied therapeutic methods.] Psychoterapia 2014;2:31–41.
Juge C, Tubert-Jeannin S. [Effects of hypnosis in dental care.] Presse Medicale 2013;42:e114–e24.
Kamaradova D, Prasko J, Grambal A, Diveky T, Latalova K, Silhan P. [Pharmacoresistance in patients with panic disorder.] Psychiatrie 2012;16:150–6.
Kapfhammer H-P. [The relationship between depression, anxiety and heart disease – a psychosomatic challenge.] Psychiatr Danub 2011;23:412–25.
Kapusta ND, Fegert JM, Haring C, Plener PL. [Psychotherapeutic interventions for suicidal adolescents.] Psychotherapeut 2014;59:16–23.
Karjalainen P, Santalahti P, Sihvo S. [Are programs supporting parenthood skills effective in the prevention and reduction of conduct disorders and problems of childhood?] Duodecim 2016;132:967–74.
Kim JH. [A meta-analysis of effects of job stress management interventions (SMIs).] Taehan Kanho Hakhoe Chi 2007;37:529–39.
Kim Y, Park I, Park JS. [Meta-analysis of effects on adolescent smoking cessation programs in Korea.] Taehan Kanho Hakhoe Chi 2008;38:204–16.
Kirk I, Leiknes KA, Laru L, Hammerstrom KT, Bramness JG, Grawe RW, et al. [Dual Diagnoses – Severe Mental Illness and Substance Use Disorder. Part 2 – Effect of Psychosocial Interventions.] Report from Knowledge Centre No. 25. Oslo: National Knowledge Centre for Health Services at the Norwegian Institute of Public Health (NIPH); 2008.
Kirste U, Haugstad GK, Leganger S, Blomhoff S, Malt UF. [Chronic pelvic pain in women.] Tidsskr Nor Laegeforen 2002;122:1223–7.
Kollner V, Bernardy K, Greiner W, Krumbein L, Lucius H, Offenbacher M, et al. [Psychotherapy and psychological procedures for fibromyalgia syndrome: updated guidelines 2017 and overview of systematic review articles.] Schmerz 2017;31:266–73.
Kollner V, Hauser W, Klimczyk K, Kuhn-Becker H, Settan M, Weigl M, et al. [Psychotherapy for patients with fibromyalgia syndrome. Systematic review, meta-analysis and guideline.] Schmerz 2012;26:291–6.
Kornor H, Winje D, Ekeberg O, Johansen K, Weisaeth L, Ormstad SS, et al. [Psychosocial Interventions After Crises and Accidents.] Report from Knowledge Centre No. 14. Oslo: National Knowledge Centre for Health Services at the Norwegian Institute of Public Health (NIPH); 2007.
Kraft S, Schepker R, Goldbeck L, Fegert J. [Treatment of posttraumatic stress disorder in children and adolescents – a review of treatment outcome studies.] Nervenheilkunde: Z interdisziplin Fortbild 2006;25:709–16.
Krause K, Gurtler D, Bischof G, Rumpf H-J, Lucht M, John U, et al. [Computer-based interventions to reduce depressive symptoms – an overview of available and evidence-based programs.] Z Psychiatr Psycholog Psychother 2016;64:121–31.
Kreissl S, Burkle C, Ruffer U, Mehnert A, Borchmann P. [Cancer-related fatigue in Hodgkin’s lymphoma.] Onkologe 2018;24:329–34.
Kremberg E, Mitte K. [Cognitive–behavioral treatment of social phobia in children and adolescents: a review.] Z klin Psychol Psychother 2005;34:196–204.
Kroese MEA, de Vet HCW, Scholten RJP. [Review of research on the effectiveness of regular physical therapy for chronic benign pain.] Nederlands Tijdschr Fysiother 2002;112:42–9.
López RNA, Girona FG. [Cognitive–behavioral therapy in the treatment of generalized anxiety.] Metas de Enfermería 2011;14:70–3.
Laakmann M, Petermann U, Petermann F. [Parental participation in the context of anxiety treatment for children: a systematic review.] Kindheit und Entwicklung: Z Klin Kinderpsychol 2017;26:77–92.
Lagrue G, Le Foll B, Melihan-Cheinin P, Rostoker G, Ades J, de Beaurepaire R, et al. [Drug and non-drug treatment strategies to assist smoking cessation.] Therapie 2003;58:479–97.
Landuyt G, Dierckx B, De Nus PFA, Dieleman GC. [Treatment options for paediatric trichotillomania.] Tijdschr Psychiatr 2016;58:463–70.
Lang T, Helbig-Lang S, Petermann F. [Which interventions are crucial in CBT for panic disorder and agoraphobia? A systematic review.] Z Psychiatr Psychol Psychother 2009;57:161–75.
Larsson B. [Cognitive outcome of childhood depression using cognitive behavior therapy.] Lakartidningen 2002;99:1810–2, 5–9.
Larun L, Dalsbo TK, Hafstad E, Reinar LM. [Effects of Interventions for Prevention of Sick Leave and Disability for Health Personnel]. Report from Knowledge Centre No. 2. Oslo: Knowledge Centre for Health Services at the Norwegian Institute of Public Health (NIPH); 2014.
Lefio LA, Villarroel SR, Rebolledo C, Zamorano P, Rivas K. [Effective interventions in the problematic use of alcohol and other drugs.] Pan Am J Public Health 2013;34:257–66.
Leibetseder M, Laireiter AR, Vierhauser M, Hittenberger B. [Efficacy and effectiveness of psychological and psycho-pharmacological treatments in pathological gambling – a meta-analysis.] Sucht 2011;57:275–85.
Leichsenring F, Leibing E. [How effective are psychoanalytic-oriented therapy and behavioral therapy by personality disorders?] Forum der Psychoanalyse 2003;19:378–85.
Leite CEP, Vicentini HC, dos Santos Neves J, Torres AR. [Emetophobia: a critical review about an understudied disorder.] J Bras Psiquiatr 2011;60:123–30.
Levitan MN, Chagas MH, Crippa JA, Manfro GG, Hetem LA, Andrada NC, et al. [Guidelines of the Brazilian Medical Association for the treatment of social anxiety disorder.] Rev Bras Psiquiatr 2011;33:292–302.
Li JY, Ge LJ. [Epidemiologic characteristics and therapy of social anxiety.] J Clin Rehabil Tissue Eng Res 2007;11:10644–7.
Li J, Liu L, Li MQ, Zhang WW, Si Y. [Evidence-based evaluation of therapeutic measures for sleep disorders.] Chin J Contemp Neurol Neurosurg 2013;13:398–404.
Lin CW, Haas M, Maher CG, MacHado LA, Van Tulder MW, Joos S. [Cost-effectiveness of guideline-endorsed treatments for low back pain: a systematic review.] Deutsche Z Akupunktur 2011;54:26–7.
Lin W-C. [Cognitive behavioral treatment for insomnia on cancer patients: a systematic review.] Chin J Psychol;52:173–88.
Lincoln TM, Suttner C, Nestoriuc Y. [Effects of cognitive interventions for schizophrenia: a meta-analysis.] Psychologische Rundschau 2008;59:217–32.
Liu Q, Wang H, Wang W, Zhang F, Li J, Song WF, et al. [Interventions on preventing and treating mental health problems of involuntary migrants: a systematic review.] Chin J Evid Based Med 2009;9:929–37.
Liu Y-C, Lan Y-L, Liu C-J, Chou Y-J. [The 10-year meta-analysis of cognitive–behavioral group therapy for depressive symptoms.] Chin J Psychol 2008;50:383–402.
Loeber S, Dinter C, Mann K. [Psychotherapeutic treatment for addiction and comorbid depression.] Sucht 2011;57:373–81.
Lopes LO, Cachioni M. [Psychoeducational intervention for caregivers of elderly with dementia: a systematic review.] J Bras Psiquiatr 2012;61:252–61.
Mao Z-H, Zhao X-D. [Comprehensive analysis of articles on counseling and psychotherapy researches (2000–2009) in Chinese Mental Health Journal.] Chin Ment Health J 2011;25:254–8.
Marquez S, de la Vega R. [Exercise addiction: an emergent behavioral disorder.] Nutricion Hospitalaria 2015;31:2384–91.
Martin A, Gaab J. [Chronic fatigue syndrome. Evidenced-based psychotherapy for chronic medically unexplained fatigue.] Psychotherapeut 2011;56:231–8.
Martinez M, Miro E, Sanchez AI. [Global clinical benefits of cognitive behavioral therapy for insomnia and mindfulness-based therapy applied to fibromyalgia: systematic review and meta-analysis.] Behavioral Psychology/Psicologia Conductual: Revista Internacional Clinica y de la Salud 2016;24:459–80.
Martinez M, Sanchez AI, Martinez MP, Miro E. [Psychological treatment in patients with systemic lupus erythematosus: a systematic review.] Terapia Psicologica 2016;34:167–81.
Mate O, Somogyi K, Miklosi M. [Cognitive conceptualization of adult attention deficit hyperactivity disorder: a systematic review.] Psychiatr Hung 2015;30:68–77.
Mazoni CG, Fernandes S, Pierozan PS, Moreira T, Freese L, Ferigolo M, et al. [Efficacy of pharmacological and no-pharmacological treatments for smoking cessation.] Estud Psicol 2008;13:133–40.
Mehlum L, Dieserud G, Ekeberg O, Groholt B, Mellesdal L, Walby F, et al. [Effects of Interventions for Prevention of Sick Leave and Disability for Health Personnel]. Report from Knowledge Centre No. 2. Oslo: National Knowledge Centre for Health Services at the Norwegian Institute of Public Health (NIPH); 2006.
Meister R, Jansen A, Berger M, Baumeister H, Bschor T, Harfst T, et al. [Psychotherapy of depressive disorders. Procedures, evidence and perspectives.] Der Nervenarzt 2018;89:241–51.
Mendez Carrillo FX, Moreno PJ, Sanchez-Meca J, Olivares J, Espada JP. [Effectiveness of psychological treatment for child and adolescent depression: a qualitative review of two decades of research.] Psicologia Conductual 2000;8:487–510.
Mendez X, Rosa AI, Montoya M, Espada JP, Olivares J, Sanchez-Meca J. [The psychological treatment of childhood and adolescent depression: evidence or promise?] Psicologia Conductual 2002;10:563–80.
Meyer TD, Hautzinger M. [Cognitive behavioral therapy in addition to pharmacotherapy for manic depressive disorders. Empirical results.] Nervenarzt 2002;73:620–8.
Mikulska J, Brynska A. [Obsessive–compulsive disorder – methods of treatment and the factors influencing their efficacy.] Psychoterapia 2004:55–67.
Minelli A, Zambello F, Vaona A. [Effectiveness of cognitive-associated with behavioral therapy psychopharmacological depression. Literature review meta-analyses.] Riv Psichiatr 2011;46:18–23.
Mora Moscoso R, Guzman Ruiz M, Soriano Perez AM, De Alba-Moreno R. [Treatment of central neuropathic pain; future analgesic therapies: systematic review.] Revista de la Sociedad Espanola del Dolor 2014;21:270–80.
Moreno Gil PJ, Carrillo F, Sanchez Meca J. [Effectiveness of cognitive–behavioural treatment in social phobia: a meta-analytic review.] Psicothema 2000;12:346–52.
Mortan Sevi O, Tekinsav Sutcu S. [Cognitive–behavioral group treatment for schizophrenia and other psychotic disorders – a systematic review.] Turk Psikiyatri Dergisi 2012;23:206–18.
Muller A, De Zwaan M. [Compulsive buying: a review of the current literature.] Sucht 2008;54:271–9.
Muller H, Wiessmann T, Bechdolf A. [Interventions in people at risk of developing first episode psychosis: a survey of current randomised controlled studies.] Fortschritte der Neurologie 2012;80:570–9.
Mululo SCC, De Menezes GB, Fontenelle L, Versiani M. [Cognitive behavioral therapies, cognitive therapies and behavioral strategies for the treatment of social anxiety disorder.] Rev Psiquiatr Clin 2009;36:221–8.
Myrhaug HT, Strom V, Hafstad E, Kirkehei I, Reinar LM. [The Effect of Hydrotherapy for Persons with Musculoskeletal Disorders]. Report from Knowledge Centre No. 11. Oslo: National Knowledge Centre for Health Services at the Norwegian Institute of Public Health (NIPH); 2015.
Napierala M. [Cognitive–behavioural therapy in the treatment of bipolar affective disorders.] Neuropsychiatr Neuropsychol 2017;12:118–25.
Nardi B, Laurenzi S, Nicolo M, Bellantuono C. [Is the cognitive–behavioural therapy an effective strategy also in the prevention of post partum depression? A critical review.] Riv Psichiatr 2012;47:205–13.
Nauta KJ, Batelaan NM, Van Balkom AJLM. [Obsessive–compulsive disorder from a family perspective; implications for treatment and research.] Tijdschr Psychiatr 2012;54:439–48.
Navarro-Mateu F, Garriga-Puerto A, Sanchez-Sanchez JA. [Tree decision analysis of the therapeutic alternatives for panic disorders in primary care.] Atencion Primaria 2010;42:86–94.
Nedate K, Ichii M, Sekiguchi Y, Miyamae Y, et al. [Is cognitive behavior therapy effective? From the viewpoint of meta-analysis and individual differences.] Jpn J Counsel Sci 1995;28:87–103.
Nilges P. [Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain adults, excluding headache.] Schmerz 2001;15:69–70.
Oh PJ, Han SJ. [Meta-analysis of psychosocial interventions to reduce pain in patients with cancer.] J Korean Acad Nurs 2013;43:658–68.
Oliva VHS, Vianna A, Lotufo Neto F. [Cinematherapy as psychotherapeutic intervention: characteristics, applications and identification of cognitive–behavior techniques.] Rev Psiquiatr Clin 2010;37:138–44.
Olivares J, Rosa AI, Sanchez Meca J. [Meta-analysis of the effectiveness of coping skills in clinical and health problems in Spain.] Anuario de Psicologia 2000;31:43–61.
Park WJ, Park SJ, Hwang SD. [Effects of cognitive behavioral therapy on attention deficit hyperactivity disorder among school-aged children in Korea: a meta-analysis.] J Korean Acad Nurs 2015;45:169–82.
Pelissolo A. [Hypnosis for anxiety and phobic disorders: a review of clinical studies.] Presse Medicale 2016;45:284–90.
Pitschel-Walz G, Bauml J. [Efficacy of psychotherapy for patients with schizophrenia – results of meta-analyses.] Psychiatrische Praxis, Supplement 2007;34:S28–32.
Pok Ja O, Eun Ai L. [Cognitive behavioral therapy for psychological distress, self care and quality of life in patients with cancer: a meta-analysis]. Kor J Adult Nurs 2013;25:377–88.
Prados G, Miro E. [Fibromyalgia and sleep: a review.] Rev Neurol 2012;54:227–40.
Prasko JP, Ociskova M, Kamaradova D, Latalova K, Vrbova K, Sedlackova Z, et al. [Cognitive–behavioral therapy and dialectical–behavioral therapy in suicidal patients.] Psychiatrie 2014;18:8–17.
Prazeres AM, De Souza WF, Fontenelle LF. [Cognitive–behavior therapy for obsessive–compulsive disorder: a systematic review of the last decade.] Rev Bras Psiquiatr 2007;29:262–70.
Puschber B, Born A, Giesler A, Helm H, Becker T, Angermeyer MC. [Effects of interventions to improve compliance with antipsychotic medication in people suffering from schizophrenia results of recent reviews.] Psychiatrische Praxis 2005;32:62–7.
Puschner B, Vauth R, Jacobi F, Becker T. [Evidence basis of psychotherapy for schizophrenia patients in Germany.] Nervenarzt 2006;77:1301–2, 4.
Quintero MF, Finck C. [Effective psychological intervention for breast cancer patients in Latin America and Spain: a systematic review.] Psicooncologia 2018;15:49–64.
Raffin AL, Ferrao YA, De Souza FP, Cordioli AV. [Outcome predictor factors in the treatment of obsessive–compulsive disorder using behavior and cognitive–behavior therapies: a systematic review.] Rev Psiquiatr Rio Grande Sul 2008;30(Suppl. 1).
Rafihi-Ferreira RE, Soares MRZ. [Insomnia in patients with breast cancer.] Estudos de Psicologia 2012;29:597–607.
Ramos SAT, del Carmen Lara Munoz M. [Non-pharmacological interventions in primary insomnia: Controlled clinical trial findings (1998–2008).] Revista Colombiana de Psiquiatria 2011;40:310–35.
Reinecke H, Sorgatz H, German Society for the Study of P. [S3 guideline LONTS. Long-term administration of opioids for non-tumor pain.] Der Schmerz 2009;23:440–7.
Riehle M, Pillny M, Lincoln TM. [Are the negative symptoms of schizophrenia treatable at all? A systematic review on efficacy studies for targeted psychological interventions for negative symptoms.] Verhaltenstherapie 2017;27:199–208.
Rodrigues MGA, Krauss-Silva L, Martins ACM. [Meta-analysis of clinical trials on family intervention in schizophrenia.] Cadernos de Saude Publica 2008;24:2203–18.
Rojas E, Real T, Garcia-Silberman S, Medina-Mora ME. [Systematic review of addiction treatment in Mexico.] Salud Mental 2011;34:351–65.
Ruhmland M, Margraf J. [Efficacy of psychological treatments for generalized anxiety disorder and social phobia.] Verhaltenstherapie 2001;11:27–40.
Ruhmland M, Margraf J. [Efficacy of psychological treatments for panic and agoraphobia.] Verhaltenstherapie 2001;11:41–53.
Ruhmland M, Margraf J. [Efficacy of psychological treatments for specific phobia and obsessive compulsive disorder.] Verhaltenstherapie 2001;11:14–26.
Sánchez Ocón MT, Pérez Morente MÁ, Mingorance Ruiz MV, Pérez Robles MA, Muñoz de la Fuente JM. Revisión de las intervenciones farmacológicas y complementarias en el manejo del síndrome de fibromialgia. Enfermería Comunitaria 2013;9:1–9.
Santacruz I, Orgiles M, Rosa AI, Sanchez-Meca J, Mendez X, Olivares J. [Generalized anxiety, separation anxiety and school phobia: the predominance of cognitive–behavioural therapy.] Psicologia Conductual 2002;10:503–21.
Santandrea S, Boschi M, Vanti C. [Effectiveness of cognitive behavioural therapy in spinal pain: a systematic review.] Scienza Riabilitativa 2011;13:5–23.
Schmid G, Henningsen P, Dieterich M, Sattel H, Lahmann C. [Psychotherapy in dizziness-a systematic review.] PDP Psychodynamische Psychotherapie: Forum der tiefenpsychologisch fundierten Psychotherapie 2011;10:25–40.
Sikorski C, Luppa M, Kersting A, Konig HH, Riedel-Heller SG. [Computer-aided cognitive behavioral therapy for depression: a systematic review of the literature.] Psychiatrische Praxis 2011;38:61–8.
Siles J, Tarquinio C. [Psychosexual consequences and their treatments in the field of cancer: a systematic review of psychotherapeutics interventions.] Sexologies 2017;26:87–95.
Silva L, Morgado P. [Koro syndrome associated with obsessive–compulsive disorder: clinical case and brief review.] J Bras Psiquiatr 2018;67:135–9.
Slotema C, Blom J, Sommer I. [Treatment strategies for auditory verbal hallucinations.] Tijdschr Psychiatr 2014;56:247–55.
Smits CT, Van Der Gaag M. [Cognitive behavioural therapy for schizophrenia.] Tijdschr Psychiatr 2010;52:99–109.
Soulia V, Giannakopoulou M. [Non-invasive and non-pharmacological methods for the alleviation of neuropathic pain.] Nosileftiki 2011;50:147–62.
Soussana M, Sunyer B, Pry R, Baghdadli A. [Anxiety in children and adolescents with pervasive developmental disorder without mental retardation: review of literature.] Encephale 2012;38:16–24.
Spijker A, van Zaane J, Koenders M, Hoekstra R, Kupka R. [Bipolar disorder and alcohol use disorder: Practical recommendations for treatment, based on a literature review.] Tijdschr Psychiatr 2018;60:87–95.
Staccini L. [Psychological treatment of female sexual dysfunction: a critical review of the literature.] Riv Psichiatr 2015;50:265–73.
Stolarska-Werynska U, Biedron A, Kacinski M. [The links between neuropsychology and neurophysiology.] Przeglad Lekarski 2016;73:187–90.
Svec J, Svec P, Bencova V, Krcmery V. [Anxio-depressive syndrome – biopsychosocial model of supportive care.] Klin Onkol 2015;28:177–82.
SBU. [Treatment for Binge Eating Disorder. ] SBU report no 248. Stockholm: Swedish Agency for Health Technology Assessment and Assessment of Social Services; 2016.
Takano A, Miyamoto Y, Matsumoto T. [A review about new approaches using the internet and computer technology for people with drug use disorder.] Nihon Arukoru Yakubutsu Igakkai Zasshi 2015;50:19–34.
Tian L, Cao XY. [Effectiveness of cognitive behavioral therapy treating insomnia in patients with breast cancer: a systematic review.] Chin J Evid Based Med 2013;13:70–7.
Tomayo JM, Rosales-Barrera JI, Villasenor-Bayardon SJ, Rojas-Malpica C. [Literature review on management of treatment-resistant depression.] Salud Mental 2011;34:257–66.
Toorn SL, Ferdinand RF. [Anxiety disorders in children: which type of psychosocial treatment has proved effective.] Tijdschr Psychiatr 2004;46:167–77.
Turkcapar A, Turkcapar M. [Diagnosis and treatment of premenstrual syndrome and premenstrual dysphoric disorder: a review.] Klinik Psikiyatri Dergisi 2011;14:241–53.
Tutus D, Plener PL, Niemitz M. [Quality criteria of internet-based cognitive–behavioral interventions for children and adolescents and their parents – a systematic review.] Z Kinder Jugendpsychiatr Psychother 2018;48:1–17.
Valiune D. [Effectiveness of the cognitive behaviour therapy based interventions to children’s and adolescents’ anger and aggressive behaviour. Systematic analysis.] Int J Psychol Biopsychosoc Approach/Tarptautinis psichilogijos zurnalas: Biopsichosocialinis poziuris 2014;15:111–31.
van Alphen SP. [Prevalence, diagnosis and treatment of personality disorders in older adults.] Tijdschr gerontol geriatr 2010;41:79–86.
van den Berg B, Knoppert-Van der Klein E, van Zaane J. [Psychotherapeutic treatment options for bipolar disorders. A review of randomized controlled studies.] Tijdschr Psychiatr 2006;48:905–31.
van der Veen W, Renes J, Kupka R, Regeer E. [The effects of pharmacological and psychotherapeutic treatment of comorbid anxiety disorders in patients with bipolar disorder.] Tijdschr Psychiatr 2018;60:388–96.
Vancampfort D, Vanderlinden J, Pieters G, De Herdt A, Schueremans A, Adriaens A, et al. [The importance of movement-directed interventions in the multidisciplinary treatment of binge eating disorder: an overview.] Tijdschr Psychiatr 2012;54:719–30.
Vazquez FL. [Psychological and pharmacological smoking cessation approaches for smokers with depression disorders.] Clin Salud 2005;16:269–89.
Verhagen AP, Damen L, Berger MY, Lenssinck ML, Passchier J, Kroes BW. [Treatment of tension type headache: paracetamol and NSAIDs work: a systematic review.] Nederlands Tijdschr Geneeskunde 2010;154:A1924.
Verhagen A, Damen L, Bruijn J, Berger M, Passchier J, Koes B. [Effectiveness of interventions in children with migraine.] Huisarts en Wetenschap 2006;49:123–9.
Vetere G, Rodriguez Biglieri R. [Empirical validation of theoretical models and cognitive behavioral treatments for generalized anxiety disorder.] Vertex (Buenos Aires, Argentina) 2005;16:170–5.
Vist GE, Reinar LM, Straumann GH, Wisting L. [Treatment of Persons who Suffer from Both an Eating Disorder and Diabetes.] Report from Knowledge Centre No. 18. Oslo: National Knowledge Centre for Health Services at the Norwegian Institute of Public Health (NIPH); 2015.
Voderholzer U, Barton B. [Long-term effects of psychotherapy for non-chronic depressive disorder: a systematic review of studies in comparison with pharmacotherapy.] Verhaltenstherapie 2016;26:108–15.
Vogel-Mergaerts SML, Liessens D. [Panic disorder during pregnancy.] Tijdschr Psychiatr 2002;44:687–92.
von Hofe I, Latza U, Lonnfors S, Muckelbauer R. [Online health services for the prevention of stress-associated psychological impairments at the workplace.] Gesundheitswesen 2017;79:144–52.
Vulink NCC, Denys D. [Body dysmorphic disorder: an overview.] Tijdschr Psychiatr 2005;47:21–7.
Walter M, Dursteler KM, Petitjean SA, Wiesbeck GA, Euler S, Sollberger D, et al. [Psychosocial treatment of addictive disorders – an overview of psychotherapeutic options and their efficacy.] Fortschritte der Neurologie-Psychiatrie 2015;83:201–10.
Wang F, Wu HM, Huang CQ, Lun ZC, Dong BR. [Psychotherapy for depression in older patients: a systematic review.] Chin J Evid Based Med 2008;8:1079–85.
Willemse Y, Trijsburg R. [Cognitive behavior therapy and interpersonal psychotherapy. an analysis of factors leading to a successful treatment-outcome.] Tijdschr Psychiatr 2005;47:593–602.
Yablonsky PK, Sukhovskaya OA. [Smoking influence on the outcomes and complications of coronary bypass surgery.] Russ J Cardiol 2018;153:66–71.
Yang ZS, Chen JB. [Psychotheraphy for tumor patients.] Chin J Clin Rehabil 2005;9:77–9.
Ye YY, Jiang XJ, Liu J, Li XJ, Liu YZ, Lang Y, et al. [Efficacy of telephone-delivered cognitive behavioral therapy for insomnia: a meta-analysis.] Chin J Evid Based Med 2016;16:334–40.
Yu C, Liu XJ, Huang J, Zhou YT. [Effectiveness of psychological intervention on post-stoke depression: a systematic review.] Chin J Evid Based Med 2011;11:670–80.
Zabalegui Yarnoz A, Navarro Diez M, Cabrera Torres E, Fernandez-Puebla AG, Bardallo Porras D, Rodriguez Higueras E, et al. [Efficacy of interventions aimed at the main carers of dependent individuals aged more than 65 years old. A systematic review.] Rev Espanola Geriatr Gerontol 2008;43:157–66.
Zenner HP, Delb W, Kroner-Herwig B, Jager B, Peroz I, Hesse G, et al. [On the interdisciplinary S3 guidelines for the treatment of chronic idiopathic tinnitus.] HNO 2015;63:419–27.
Zhang YL, Meng B, Zhao GF. [Application of cognitive behavioral therapy in the rehabilitative treatment of mental diseases.] Chin J Clin Rehabil 2005;9:223–5.
Zimmermann T, Heinrichs N. [Psychosocial interventions for women with genital cancers.] Verhaltenstherapie & Verhaltensmedizin 2006;27:125–41.
Zonnenberg C, Niemantsverdriet M, Blom J, Slotema C. [Auditory verbal hallucinations in patients with borderline personality disorder.] Tijdschr Psychiatr 2016;58:122–9.
Studies excluded because Database of Abstracts of Reviews of Effects criteria not fulfilled (n = 447)
Aboujaoude E. Three decades of telemedicine in obsessive–compulsive disorder: a review across platforms. J Obsessive Compuls Relat Disord 2017;14:65–70.
Aboujaoude E, Salame W. Technology at the service of pediatric mental health: review and assessment. J Pediatr 2016;171:20–4. https://doi.org/10.1016/j.jpeds.2015.12.009
Abramowitz JS. Does cognitive–behavioral therapy cure obsessive–compulsive disorder? A meta-analytic evaluation of clinical significance. Behav Ther 1998;29:339–55.
Abramowitz JS, Franklin ME, Foa EB. Empirical status of cognitive–behavioral therapy for obsessive–compulsive disorder: a meta-analytic review. Romanian J Cogn Behav Psychother 2002;2:89–104.
Abramowitz JS, Whiteside SP, Deacon BJ. The effectiveness of treatment for pediatric obsessive–compulsive disorder: a meta-analysis. Behav Ther 2005;36:55–63.
Adams C, Wilson P, Bagnall A. Psychosocial interventions for schizophrenia. Qual Health Care 2000;9:251–6.
Adams N, Sim J. Rehabilitation approaches in fibromyalgia. Disabil Rehabil 2005;27:711–23.
Adams TG, Brady RE, Lohr JM, Jacobs W. A meta-analysis of CBT components for anxiety disorders. Cogn Behav Ther 2015;38:87–97.
Adili F, Larijani B, Haghighatpanah M. Diabetic patients: psychological aspects. Ann New York Acad Sci 2006;1084:329–49.
Agosti V, Nunes EV, O’Shea D. Do manualized psychosocial interventions help reduce relapse among alcohol-dependent adults treated with naltrexone or placebo? A meta-analysis. Am J Addict 2012;21:501–7. https://doi.org/10.1111/j.1521-0391.2012.00270.x
Albazaz R, Wong YT, Homer-Vanniasinkam S. Complex regional pain syndrome: a review. Ann Vasc Surg 2008;22:297–306.
Albert U, Aguglia A, Bramante S, Bogetto F, Maina G. Treatment-resistant obsessive–compulsive disorder (OCD): current knowledge and open questions. Clin Neuropsychiatry 2013;10:19–30.
Ale CM, McCarthy DM, Rothschild LM, Whiteside SP. Components of cognitive behavioral therapy related to outcome in childhood anxiety disorders. Clin Child Fam Psychol Rev 2015;18:240–51. https://doi.org/10.1007/s10567-015-0184-8
Allison DB, Faith MS. Hypnosis as an adjunct to cognitive–behavioral psychotherapy for obesity: a meta-analytic reappraisal. J Consult Clin Psychol 1996;64:513–16. https://doi.org/10.1037//0022-006x.64.3.513
Aman MM, Jason Yong R, Kaye AD, Urman RD. Evidence-based non-pharmacological therapies for fibromyalgia. Curr Pain Headache Rep 2018;22:33. https://doi.org/10.1007/s11916-018-0688-2
Andersson G, Hedman E. Effectiveness of guided internet-based cognitive behavior therapy in regular clinical settings. Verhaltenstherapie 2013;23:140–8.
Andersson G, Lyttkens L. A meta-analytic review of psychological treatments for tinnitus. Br J Audiol 1999;33:201–10. https://doi.org/10.3109/03005369909090101
Andersson G, Topooco N, Havik O, Nordgreen T. Internet-supported versus face-to-face cognitive behavior therapy for depression. Expert Rev Neurother 2016;16:55–60. https://doi.org/10.1586/14737175.2015.1125783
Andrews G, Corry J, Oakley-Browne M, Shepherd L. Australian and New Zealand clinical practice guidelines for the treatment of panic disorder and agoraphobia. Aust N Z J Psychiatry 2003;37:641–56.
Anthony MT, Farella M. Body dysmorphic disorder and orthodontics – an overview for clinicians. Aust Orthod J 2014;30:208–13.
Armenti NA, Babcock JC. Conjoint treatment for intimate partner violence: a systematic review and implications. Couple Fam Psychol Res Pract 2016;5:109-23.
Arnberg FK, Alaie I, Parling T, Jonsson U. Recent randomized controlled trials of psychological interventions in healthcare: a review of their quantity, scope, and characteristics. J Psychosom Res 2013;75:401–8. https://doi.org/10.1016/j.jpsychores.2013.08.019
Arumugham SS, Reddy JY. Augmentation strategies in obsessive–compulsive disorder. Expert Rev Neurother 2013;13:187–202. https://doi.org/10.1586/ern.12.160
Astin JA. Mind-body therapies for the management of pain. Clin J Pain 2004;20:27–32. https://doi.org/10.1097/00002508-200401000-00006
Bailey AP, Parker AG, Colautti LA, Hart LM, Liu P, Hetrick SE. Mapping the evidence for the prevention and treatment of eating disorders in young people. J Eat Disord 2014;2:5. https://doi.org/10.1186/2050-2974-2-5
Bain KT. Management of chronic insomnia in elderly persons. Am J Geriatr Pharmacother 2006;4:168–92.
Baker AL, Hides L, Lubman DI. Treatment of cannabis use among people with psychotic or depressive disorders: a systematic review. J Clin Psychiatry 2010;71:247–54. https://doi.org/10.4088/JCP.09r05119gry
Bakker A, van Balkom AJ, van Dyck R. Selective serotonin reuptake inhibitors in the treatment of panic disorder and agoraphobia. Int Clin Psychopharmacol 2000;15(Suppl. 2):25–30. https://doi.org/10.1097/00004850-200008002-00005
Ball K, Carver A, Downing K, Jackson M, O’Rourke K. Addressing the social determinants of inequities in physical activity and sedentary behaviours. Health Promot Int 2015;30(Suppl. 2):ii18–9. https://doi.org/10.1093/heapro/dav022
Barkham M, Moller NP, Pybis J. How should we evaluate research on counselling and the treatment of depression? A case study on how the National Institute for Health and Care Excellence’s draft 2018 guideline for depression considered what counts as best evidence. Couns Psychother Res 2017;17:253–68. https://doi.org/10.1002/capr.12141
Barrera M, Jr. Reaffirmation of behavioral approaches to depression treatment. Clin Psychol Sci Pract 2009;16:416–9.
Barron P, Hassiotis A, Banes J. Offenders with intellectual disability: the size of the problem and therapeutic outcomes. J Intellect Disabil Res 2002;46:454–63.
Barry TJ, Yeung SP, Lau JYF. Meta-analysis of the influence of age on symptom change following cognitive–behavioural treatment for anxiety disorders. J Adolesc 2018;68:232–41.
Bauer I, Wilansky-Traynor P, Rector NA. Cognitive–behavioral therapy for anxiety disorders with comorbid depression: a review. Int J Cogn Ther 2012;5:118–56.
Beelmann A, Lösel F. Child social skills training in developmental crime prevention: effects on antisocial behavior and social competence. Psicothema 2006;18:603–10.
Beintner I, Jacobi C, Taylor CB. Effects of an internet-based prevention programme for eating disorders in the USA and Germany – a meta-analytic review. Eur Eat Disord Rev 2012;20:1–8. https://doi.org/10.1002/erv.1130
Belli H, Belli S, Oktay MF, Ural C. Psychopathological dimensions of tinnitus and psychopharmacologic approaches in its treatment. Gen Hosp Psychiatry 2012;34:282–9. https://doi.org/10.1016/j.genhosppsych.2011.12.006
Bergfeld IO, Mantione M, Figee M, Schuurman PR, Lok A, Denys D. Treatment-resistant depression and suicidality. J Affect Disord 2018;235:362–7.
Best L, Stevens A. Cognitive Behavioural Therapy in the Treatment of Chronic Fatigue Syndrome. Southampton: Development and Evaluation Committee, Wessex Institute of Public Health Medicine; 1996.
Bhar SS, Beck AT. Treatment integrity of studies that compare short-term psychodynamic psychotherapy with cognitive-behavior therapy. Clin Psychol Sci Pract 2009;16:370–8.
Birmaher B, Ryan ND, Williamson DE, Brent DA. Childhood and adolescent depression: a review of the past 10 years. Part II. J Am Acad Child Adolesc Psychiatry 1996;35:1575–83.
Bisson JI. Post-traumatic stress disorder. BMJ 2007;14:789–93.
Blake CS, Hamrin V. Current approaches to the assessment and management of anger and aggression in youth: a review. J Child Adolesc Psychiatr Nurs 2007;20:209–21.
Blanco C, Bragdon LB, Schneier FR, Liebowitz MR. The evidence-based pharmacotherapy of social anxiety disorder. Int J Neuropsychopharmacol 2013;16:235–49.
Blanco C, Raza MS, Schneier FR, Liebowitz MR. The evidence-based pharmacological treatment of social anxiety disorder. Int J Neuropsychopharmacol 2003;6:427–42. https://doi.org/10.1017/S1461145703003791
Bloch MH, Storch EA. Assessment and management of treatment-refractory obsessive–compulsive disorder in children. J Am Acad Child Adolesc Psychiatry 2015;54:251–62. https://doi.org/10.1016/j.jaac.2015.01.011
Bluett EJ, Homan KJ, Morrison KL, Levin ME, Twohig MP. Acceptance and commitment therapy for anxiety and OCD spectrum disorders: an empirical review. J Anxiety Disord 2014;28:612–24. https://doi.org/10.1016/j.janxdis.2014.06.008
Boccia M, Piccardi L, Cordellieri P, Guariglia C, Giannini AM. EMDR therapy for PTSD after motor vehicle accidents: meta-analytic evidence for specific treatment. Front Hum Neurosci 2015;9:213. https://doi.org/10.3389/fnhum.2015.00213
Bolognesi F, Baldwin D, Ruini C. Psychological interventions in the treatment of generalized anxiety disorder: a structured review. J Psychopathol/Giornaledi Psicopatologia 2014;20:111–26.
Bosanac P, Castle D. How should we manage anxiety in patients with schizophrenia? Australas Psychiatry 2015;23:374–7.
Brewer M, Melnyk BM. Effective coping/mental health interventions for critically ill adolescents: an evidence review. Pediatr Nurs 2007;33:361–7, 373.
Bridle D, McCabe R, Priebe S. Incorporating psychotherapeutic methods in routine community treatment for patients with psychotic disorders. Psychos Psycholog Soc Integr Approaches 2013;5:154–65.
Broomfield NM, Laidlaw K, Hickabottom E, Murray MF, Pendrey R, Whittick JE, Gillespie DC. Post-stroke depression: the case for augmented, individually tailored cognitive behavioural therapy. Clin Psychol Psychother 2011;18:202–17. https://doi.org/10.1002/cpp.711
Brown WJ, Dewey D, Bunnell BE, Boyd SJ, Wilkerson AK, Mitchell MA, Bruce SE. A critical review of negative affect and the application of CBT for PTSD. Trauma Violence Abus 2018;19:176–94. https://doi.org/10.1177/1524838016650188
Buijs PCM, Bassett AS, Boot E. Non-pharmacological treatment of psychiatric disorders in individuals with 22q11.2 deletion syndrome; a systematic review. Am J Med Genet A 2018;176:1742–7. https://doi.org/10.1002/ajmg.a.38612
Burgener SC, Twigg P. Interventions for persons with irreversible dementia. Annu Rev Nurs Res 2002;20:89–124.
Burke BL, Dunn CW, Atkins DC, Phelps JS. The emerging evidence base for motivational interviewing: a meta-analytic and qualitative inquiry. J Cogn Psychother 2004;18:309–22.
Burton C. Beyond somatisation: a review of the understanding and treatment of medically unexplained physical symptoms (MUPS). Br J Gen Pract 2003;53:231–9.
Buse DC, Andrasik F. Behavioral medicine for migraine. Neurologic Clinics 2009;27:445–65.
Bussotti M, Sommaruga M. Anxiety and depression in patients with pulmonary hypertension: impact and management challenges. Vasc Health Risk Manag 2018;14:349–60. https://doi.org/10.2147/VHRM.S147173
Bustillo J, Lauriello J, Horan W, Keith S. The psychosocial treatment of schizophrenia: an update. Am J Psychiatry 2001;158:163–75. https://doi.org/10.1176/appi.ajp.158.2.163
Butts K. Innovative Strategies for Students with ADHD: How to Employ Best Practices Without Losing Student Interest. PhD thesis. Minneapolis, MN: Capella University; 2013.
Calati R, Courtet P, Lopez-Castroman J. Refining suicide prevention: a narrative review on advances in psychotherapeutic tools. Curr Psychiatry Rep 2018;20:14. https://doi.org/10.1007/s11920-018-0876-0
Canadian Agency for Drugs and Technologies in Health. Self-directed cognitive behavioural therapy for adults with diagnosis of depression: systematic review of clinical effectiveness, cost-effectiveness, and guidelines. CADTH Technology Overviews 2010;1:e0125.
Carpenter J, Gass MLS, Maki PM, Newton KM, Pinkerton JV, Taylor M, et al. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of the North American Menopause Society. Menopause 2015;22:1155–74.
Carr A. Depression in young people: description, assessment and evidence-based treatment. Dev Neurorehabil 2008;11:3–15.
Carroll KM. Relapse prevention as a psychosocial treatment: a review of controlled clinical trials. Exp Clin Psychopharmacol 1996;4:46–54.
Chambless DL, Gillis MM. Cognitive therapy of anxiety disorders. J Consult Clin Psychol 1993;61:248–60.
Chard KM. A Meta-analysis of Posttraumatic Stress Disorder Treatment Outcome Studies of Sexually Victimized Women. PhD thesis. Bloomington/Indianapolis, IN: Indiana University; 1995.
Charyton C, Elliott JO, Moore JL, Klatte ET. Is it time to consider cognitive behavioral therapy for persons with epilepsy? Clues from pathophysiology, treatment and functional neuroimaging. Expert Rev Neurother 2010;10:1911–27. https://doi.org/10.1586/ern.10.138
Chen E, Cole SW, Kato PM. A review of empirically supported psychosocial interventions for pain and adherence outcomes in sickle cell disease. J Pediatr Psychol 2004;29:197–209. https://doi.org/10.1093/jpepsy/jsh021
Chien WT, Leung SF, Yeung FK, Wong WK. Current approaches to treatments for schizophrenia spectrum disorders, part II: psychosocial interventions and patient-focused perspectives in psychiatric care. Neuropsychiatr Dis Treat 2013;9:1463–81. https://doi.org/10.2147/NDT.S49263
Christensen H, Batterham P, Calear A. Online interventions for anxiety disorders. Curr Opin Psychiatry 2014;27:7–13.
Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547–55.
Cochrane G. Role for a sense of self-worth in weight-loss treatments: helping patients develop self-efficacy. Can Fam Physician 2008;54:543–7.
Colom F, Lam D. Psychoeducation: improving outcomes in bipolar disorder. Eur Psychiatry 2005;20:359–64.
Colom F, Vieta E. A perspective on the use of psychoeducation, cognitive–behavioral therapy and interpersonal therapy for bipolar patients. Bipolar Disord 2004;6:480–6.
Compton SN, Kratochvil CJ, March JS. Pharmacotherapy for anxiety disorders in children and adolescents: an evidence-based medicine review. Pediatr Ann 2007;36:586–90, 592, 594–8. https://doi.org/10.3928/0090-4481-20070901-10
Comtois KA, Linehan MM. Psychosocial treatments of suicidal behaviors: a practice-friendly review. J Clin Psychol 2006;62:161–70. https://doi.org/10.1002/jclp.20220
Conn DK, Seitz DP. Advances in the treatment of psychiatric disorders in long-term care homes. Curr Opin Psychiatry 2010;23:516–21. https://doi.org/10.1097/YCO.0b013e32833efe56
Copeland J, Gates P, Pokorski I. A narrative review of psychological cannabis use treatments with and without pharmaceutical adjunct. Curr Pharm Des 2016;22:6397–408. https://doi.org/10.2174/1381612822666160831094811
Cordioli AV. [Cognitive-behavioral therapy in obsessive-compulsive disorder.] Braz J Psychiatry 2008;30(Suppl. 2):65–72.
Cottraux J. Nonpharmacological treatments for anxiety disorders. Dialogues Clin Neurosci 2002;4:305–19.
Crabb RM, Cavanagh K, Proudfoot J, Learmonth D, Rafie S, Weingardt KR. Is computerized cognitive–behavioural therapy a treatment option for depression in late-life? A systematic review. Br J Clin Psychol 2012;51:459–64. https://doi.org/10.1111/j.2044-8260.2012.02038.x
Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Prim Care Companion J Clin Psychiatry 2004;6:104–11.
Craig LA, Browne KD, Stringer I. Treatment and sexual offence recidivism. Trauma Violence Abus 2003;4:70–89.
Crider A, Glaros AG, Gevirtz RN. Efficacy of biofeedback-based treatments for temporomandibular disorders. Appl Psychophysiol Biofeedback 2005;30:333–45. https://doi.org/10.1007/s10484-005-8420-5
Crino RD. Psychological treatment of obsessive compulsive disorder: an update. Australas Psychiatry 2015;23:347–9. https://doi.org/10.1177/1039856215590030
Crowe M, Porter R. Inpatient treatment for mania: a review and rationale for adjunctive interventions. Aust N Z J Psychiatry 2014;48:716–21. https://doi.org/10.1177/0004867414540754
Cuijpers P. Are all psychotherapies equally effective in the treatment of adult depression? The lack of statistical power of comparative outcome studies. Evid Based Ment Health 2016;19:39–42. https://doi.org/10.1136/eb-2016-102341
Cuijpers P, Gentili C. Psychological treatments are as effective as pharmacotherapies in the treatment of adult depression: a summary from randomized clinical trials and neuroscience evidence. Res Psychother Psychopathol Process Outcome 2017;20:147–52.
Cunha LM. The Efficacy of Therapeutic Interventions for Adolescent Maltreatment Victims: A Meta-analysis. PhD thesis. San Diego, CA: Alliant International University; 2008.
Currier JM. Psychotherapeutic Interventions for Grief: A Comprehensive Review of Controlled Outcome Research. PhD thesis. Memphis, TN: University of Memphis; 2009.
Currier JM, Holland JM, Neimeyer RA. Do CBT-based interventions alleviate distress following bereavement? A review of the current evidence. Int J Cogn Ther 2010;3:77–93.
Cwikel J, Behar L, Rabson-Hare J. A comparison of a vote count and a meta-analysis review of intervention research with adult cancer patients. Res Soc Work Pract 2000;10:139–58.
Dautovich ND, McNamara J, Williams JM, Cross NJ, McCrae CS. Tackling sleeplessness: psychological treatment options for insomnia. Nat Sci Sleep 2010;2:23–37.
Davidson MA. ADHD in adults: a review of the literature. J Atten Disord 2008;11:628–41.
de Carvalho MR, Rozenthal M, Nardi AE. The fear circuitry in panic disorder and its modulation by cognitive–behaviour therapy interventions. World J Biol Psychiatry 2010;11:188–98.
De Silva S, Parker A, Purcell R, Callahan P, Liu P, Hetrick S. Mapping the evidence of prevention and intervention studies for suicidal and self-harming behaviors in young people. Crisis 2013;34:223–32. https://doi.org/10.1027/0227-5910/a000190
Deary V, Chalder T, Sharpe M. The cognitive behavioural model of medically unexplained symptoms: a theoretical and empirical review. Clin Psychol Rev 2007;27:781–97.
Decker SE, Kiluk BD, Frankforter T, Babuscio T, Nich C, Carroll KM. Just showing up is not enough: homework adherence and outcome in cognitive–behavioral therapy for cocaine dependence. J Consult Clin Psychol 2016;84:907–12.
Diseth TH, Christie HJ. Trauma-related dissociative (conversion) disorders in children and adolescents – an overview of assessment tools and treatment principles. Nord J Psychiatry 2005;59:278–92.
Amaral JM, Spadaro PT, Pereira VM, Silva AC, Nardi AE. The carbon dioxide challenge test in panic disorder: a systematic review of preclinical and clinical research. Braz J Psychiatry 2013;35:318–31. https://doi.org/10.1590/1516-4446-2012-1045
Domhardt M, Baumeister H. Psychotherapy of adjustment disorders: current state and future directions. World J Biol Psychiatry 2018;19:S21–S35. https://doi.org/10.1080/15622975.2018.1467041
Draper ML, Velligan DI, Tai S. Cognitive behavioral therapy for schizophrenia: a review of recent literature and meta-analyses. Minerva Psichiatrica 2010;51:85–94.
Dubicka B, Brent D. Combined therapy in adolescent depression. Int J Cogn Ther 2014;7:136–48.
Duff W, Haskey N, Potter G, Alcorn J, Hunter P, Fowler S. Non-pharmacologic therapies for inflammatory bowel disease: recommendations for self-care and physician guidance. Am J Gastroenterol 2017;112:S612–S3.
Duff W, Haskey N, Potter G, Alcorn J, Hunter P, Fowler S. Non-pharmacological therapies for inflammatory bowel disease: recommendations for self-care and physician guidance. World J Gastroenterol 2018;24:3055–70. https://doi.org/10.3748/wjg.v24.i28.3055
Dunn RL, Schwebel AI. Meta-analytic review of marital therapy outcome research. J Fam Psychol 1995;9:58–68.
Dutcher TD. A Meta-analytic Study of Marital Therapy Modalities and Clients’ Presenting Problems. PhD thesis. The Union Institute; 2000.
Dy SM, Apostol CC. Evidence-based approaches to other symptoms in advanced cancer. Cancer J 2010;16:507–13. https://doi.org/10.1097/PPO.0b013e3181f45877
Eckhardt CI, Murphy CM, Whitaker DJ, Sprunger J, Dykstra R, Woodard K. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abus 2013;4:196–231.
Eddy KT, Dutra L, Bradley R, Westen D. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive–compulsive disorder. Clin Psychol Rev 2004;24:1011–30.
Ehlers A, Clark D. Early psychological interventions for adult survivors of trauma: a review. Biol Psychiatry 2003;53:817–26.
Ehrenzeller MF, Mayer DK, Goldstein A. Smoking prevalence and management among cancer survivors. Oncol Nurs Forum 2018;45:55–68. https://doi.org/10.1188/18.ONF.55-68
Elliott R. The Effectiveness of Humanistic Therapies: A Meta-analysis. In Cain DJ, editor. Humanistic Psychotherapies: Handbook of Research and Practice. Washington, DC: American Psychological Association; 2002. pp. 57–81.
Elliott R. Person-centered/experiential psychotherapy for anxiety difficulties: theory, research and practice. Person-Centered Experiential Psychotherapies 2013;12:16–32.
Ellis P, Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for Depression. Australian and New Zealand clinical practice guidelines for the treatment of depression. Aust N Z J Psychiatry 2004;38:389–407. https://doi.org/10.1080/j.1440-1614.2004.01377.x
El-Mallakh P, Findlay J. Strategies to improve medication adherence in patients with schizophrenia: the role of support services. Neuropsychiatr Dis Treat 2015;11:1077–90. https://doi.org/10.2147/NDT.S56107
Fedoroff IC, Taylor S. Psychological and pharmacological treatments of social phobia: a meta-analysis. J Clin Psychopharmacol 2001;21:311–24. https://doi.org/10.1097/00004714-200106000-00011
Fernandez E, Salem D, Swift JK, Ramtahal N. Meta-analysis of dropout from cognitive behavioral therapy: magnitude, timing, and moderators. J Consult Clin Psychol 2015;83:1108–22. https://doi.org/10.1037/ccp0000044
Field T. Postnatal anxiety prevalence, predictors and effects on development: a narrative review. Infant Behav Dev 2018;51:24–32.
Fingeret MC, Teo I, Epner DE. Managing body image difficulties of adult cancer patients: lessons from available research. Cancer 2014;120:633–41. https://doi.org/10.1002/cncr.28469
Fisher EB, Thorpe CT, Devellis BM, Devellis RF. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educ 2007;33:1080–103.
Flannery V. Increasing breastfeeding rates: evidence-based strategies. Int J Childbirth Educ 2014;29:59–62.
Flückiger C, Del Re AC, Wampold BE, Symonds D, Horvath AO. How central is the alliance in psychotherapy? A multilevel longitudinal meta-analysis. J Couns Psychol 2012;59:10–17. https://doi.org/10.1037/a0025749
Foa EB. Psychosocial treatment of posttraumatic stress disorder. J Clin Psychiatry 2000;61(Suppl. 5):43–8.
Fontenelle LF, Nascimento AL, Mendlowicz MV, Shavitt RG, Versiani M. An update on the pharmacological treatment of obsessive–compulsive disorder. Expert Opin Pharmacother 2007;8:563–83. https://doi.org/10.1517/14656566.8.5.563
Foral P, Dewan N, Malesker M. Insomnia: a therapeutic review for pharmacists. Consult Pharm 2011;26:332–41. https://doi.org/10.4140/TCP.n.2011.332
Galante E, Gazzi L, Caffarra S. Psychological activities in neurorehabilitation: from research to clinical practice. G Ital Med Lav Ergon 2011;33(Suppl. 1):A19–28.
Gale CK, Millichamp J. Generalised anxiety disorder. BMJ Clin Evid 2007;2007:1002.
Ganasen KA, Ipser JC, Stein DJ. Augmentation of cognitive behavioral therapy with pharmacotherapy. Psychiatr Clin North Am 2010;33:687–99. https://doi.org/10.1016/j.psc.2010.04.008
Gasparini S, Beghi E, Ferlazzo E, Beghi M, Belcastro V, Biermann KP, et al. Management of psychogenic non-epileptic seizures: a multidisciplinary approach. Eur J Neurol 2019;26:205–e15. https://doi.org/10.1111/ene.13818
Gaudiano BA. Cognitive behavior therapies for psychotic disorders: current empirical status and future directions. Clin Psychol Sci Pract 2005;12:33–50.
Gaudin D, Krafcik BM, Mansour TR, Alnemari A. Considerations in spinal fusion surgery for chronic lumbar pain: psychosocial factors, rating scales, and perioperative patient education – a review of the literature. World Neurosurg 2017;98:21–7.
Gellatly J, Bower P, Hennessy S, Richards D, Gilbody S, Lovell K. What makes self-help interventions effective in the management of depressive symptoms? Meta-analysis and meta-regression. Psychol Med 2007;37:1217–28.
Garrido Genovés V, Anyela Morales L, Sánchez-Meca J. What works for serious juvenile offenders? A systematic review. Psicothema 2006;18:611–19.
Gewirtz A, Minen M. Adherence to behavioral therapy for migraine: knowledge to date, mechanisms for assessing adherence, and methods for improving adherence. Curr Pain Headache Rep 2019;23:3. https://doi.org/10.1007/s11916-019-0739-3
Ghafoori B. Effectiveness of Cognitive–Behavioral Therapy in Reducing Classroom Disruptive Behaviors: A Meta-analysis. (ERIC Document Reproduction Service No. ED457182). PhD thesis. Fresno, CA: The Educational Resources Information Center, California State University, Fresno; 2001.
Gil PJ, Carrillo F, Meca JS. Effectiveness of cognitive–behavioural treatment in social phobia: a meta-analytic review. Psychol Spain 2001;5:17–25.
Giles TR, Prial EM, Neims DM. Evaluating psychotherapies: a comparison of effectiveness. Int J Ment Health 1993;22:43–65.
Goldenberg DL, Burckhardt C, Crofford L, Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA 2004;292:2388–95.
Gomez-de-Regil L, Alvarez-Nemegyei J. Open access scientific evidence of cognitive behavioral therapy for patients with fibromyalgia. Actualidadesen Psicologia 2016;30:91–102.
Gontard A, Niemczyk J, Wagner C, Equit M. Voiding postponement in children – a systematic review. Eur Child Adolesc Psychiatry 2016;25:809–20.
Gonzales AH, Bergstrom L. Adolescent Non-Suicidal Self-Injury (NSSI) Interventions. J Child Adolesc Psychiatr Nurs 2013;26:124–30.
Goodwin PJ. Psychosocial support for women with advanced breast cancer. Breast Cancer Res Treatment 2003;81:S103–S10.
Gould MS, Greenberg T, Velting DM, Shaffer D. Youth suicide risk and preventive interventions: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 2003;42:386–405.
Gould R A, Johnson MW. Comparative Effectiveness of Cognitive–Behavioral Treatment and Pharmacotherapy for Social Phobia: Meta-analytic Outcome. In Hofmann SG, DiBartolo RM, editors. From Social Anxiety to Social Phobia: Multiple Perspectives. Needham Heights, MA: Allyn and Bacon; 2001. pp. 379–90.
Graham J. Cognitive behavioural therapy for occupational trauma: a systematic literature review exploring the effects of occupational trauma and the existing CBT support pathways and interventions for staff working within mental healthcare including allied professions. Cogn Behav Ther 2012;5:24–45.
Grambal A, Prasko J, Ociskova M, Slepecky M, Kotianova A, Sedlackova Z, et al. Borderline personality disorder and unmet needs. Neuro Endocrinol Lett 2017;38:275–89.
Grant JE, Odlaug BL, Donahue C. Gambling. In Hofmann SG, editor. The Wiley Handbook of Cognitive Behavioural Therapy. Chichester: Wiley-Blackwell; 2014.
Grech E. Psychological interventions for psychosis: a critical review of the current evidence. Int J Ment Health 2004;1:1–9.
Gregory VL. Cognitive-behavioral therapy for depression in bipolar disorder: a meta-analysis. J Evid Based Soc Work 2010;7:269–79. https://doi.org/10.1080/15433710903176088
Gremeaux V, Coudeyre E. [The internet and the therapeutic education of patients: a systematic review of the literature.] Ann Phys Rehabil Med 2010;53:669–92.
Grossman LS, Martis B, Fichtner CG. Are sex offenders treatable? A research overview. Psychiatr Serv 1999;50:349–61. https://doi.org/10.1176/ps.50.3.349
Guay DR. Drug treatment of paraphilic and nonparaphilic sexual disorders. Clin Ther 2009;31:1–31. https://doi.org/10.1016/j.clinthera.2009.01.009
Guidi J, Tomba E, Cosci F, Park SK, Fava GA. The role of staging in planning psychotherapeutic interventions in depression. J Clin Psychiatry 2017;78:456–63. https://doi.org/10.4088/JCP.16r10736
Gutiérrez M, Sánchez M, Trujillo A, Sánchez L. Cognitive-behavioral therapy for chronic psychosis. Actas Esp Psiquiatr 2009;37:106–14.
Hallberg SC, Lisboa CS, de Souza DB, Mester A, Braga AZ, Strey AM, da Silva CS. Systematic review of research investigating psychotherapy and information and communication technologies. Trends Psychiatry Psychother 2015;37:118–25. https://doi.org/10.1590/2237-6089-2014-0055
Halvorsen JG, Metz ME. Sexual dysfunction, part II: diagnosis, management, and prognosis. J Am Board Fam Pract 1992;5:177–92.
Ham P, Waters DB, Oliver MN. Treatment of panic disorder. Am Fam Physician 2005;71:733–810.
Hammond A. Rehabilitation in rheumatoid arthritis: a critical review. Musculoskeletal Care 2004;2:135–51. https://doi.org/10.1002/msc.66
Harrington R, Whittaker J, Shoebridge P. Psychological treatment of depression in children and adolescents. A review of treatment research. Br J Psychiatry 1998;173:291–8.
Häuser W, Arnold B, Eich W, Felde E, Flügge C, Henningsen P, et al. Management of fibromyalgia syndrome – an interdisciplinary evidence-based guideline. Ger Med Sci 2008;6:Doc14.
Hayee B, Forgacs I. Psychological approach to managing irritable bowel syndrome. BMJ 2007;334:1105–9.
Heimberg RG. Current status of psychotherapeutic interventions for social phobia. J Clin Psychiatry 2001;62(Suppl. 1):36–42.
Heimberg RG. Cognitive–behavioral therapy for social anxiety disorder: current status and future directions. Biol Psychiatry 2002;51:101–8.
Herpertz S, Hagenah U, Vocks S, von Wietersheim J, Cuntz U, Zeeck A, German Society of Psychosomatic Medicine and Psychotherapy. The diagnosis and treatment of eating disorders. Dtsch Arztebl Int 2011;108:678–85. https://doi.org/10.3238/arztebl.2011.0678
Hetrick SE, Cox GR, Fisher CA, Bhar SS, Rice SM, Davey CG, Parker AG. Back to basics: could behavioural therapy be a good treatment option for youth depression? A critical review. Early Interv Psychiatry 2015;9:93–9. https://doi.org/10.1111/eip.12142
Hewitt J, Coffey M. Therapeutic working relationships with people with schizophrenia: literature review. J Adv Nurs 2005;52:561–70.
Hitsman B, Papandonatos GD, McChargue DE, DeMott A, Herrera MJ, Spring B, et al. Past major depression and smoking cessation outcome: a systematic review and meta-analysis update. Addiction 2013;108:294–306. https://doi.org/10.1111/add.12009
Ho BP, Stephenson J, Carter M. Cognitive–behavioral approaches for children with autism spectrum disorder: a trend analysis. Res Autism Spectr Disord 2018;45:27–41.
Hoch E, Bonnetn U, Thomasius R, Ganzer F, Havemann-Reinecke U, Preuss UW. Risks associated with the non-medicinal use of cannabis. Dtsch Arztebl Int 2015;112:271–8. https://doi.org/10.3238/arztebl.2015.0271
Hollon SD, Jarrett RB, Nierenberg AA, Thase ME, Trivedi M, Rush AJ. Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment? J Clin Psychiatry 2005;66:455–68. https://doi.org/10.4088/jcp.v66n0408
Howell D, Oliver TK, Keller-Olaman S, Davidson J, Garland S, Samuels C, et al. A Pan-Canadian practice guideline: prevention, screening, assessment, and treatment of sleep disturbances in adults with cancer. Support Care Cancer 2013;21:2695–706. https://doi.org/10.1007/s00520-013-1823-6
Hruschak V, Cochran G, Wasan AD. Psychosocial interventions for chronic pain and comorbid prescription opioid use disorders: a narrative review of the literature. J Opioid Manag 2018;14:345–58.
Hutchinson AD, Wilson C. Improving nutrition and physical activity in the workplace: a meta-analysis of intervention studies. Health Promot Int 2012;27:238–49. https://doi.org/10.1093/heapro/dar035
Huynh ME, Vandvik IH, Diseth TH. Hypnotherapy in child psychiatry: the state of the art. Clin Child Psychol Psychiatry 2008;13:377–93. https://doi.org/10.1177/1359104508090601
Hwang MS, Yeagley KL, Petosa R. A meta-analysis of adolescent psychosocial smoking prevention programs published between 1978 and 1997 in the United States. Health Educ Behav 2004;31:702–19.
Janicke DM, Finnev JW. Empirically supported treatments in pediatric psychology: recurrent abdominal pain. J Pediatr Psychol 1999;24:115–27. https://doi.org/10.1093/jpepsy/24.2.115
Jarrell JF, Vilos GA, Allaire C, Burgess S, Fortin C, Gerwin R, et al. No. 164-consensus guidelines for the management of chronic pelvic pain. J Obstet Gynaecol Can 2018;40:e747–e787.
Jassogne C, Zdanowicz N. Management of adult patients with anorexia nervosa: a literature review. Psychiatria Danubina 2018;30:533–6.
Jayasekara R, Procter N, Harrison J, Skelton K, Hampel S, Draper R, Deuter K. Cognitive behavioural therapy for older adults with depression: a review. J Ment Health 2015;24:168–71. https://doi.org/10.3109/09638237.2014.971143
Jeffries FW, Davis P. What is the role of eye movements in eye movement desensitization and reprocessing (EMDR) for post-traumatic stress disorder (PTSD)? A review. Behav Cogn Psychother 2013;41:290–300. https://doi.org/10.1017/S1352465812000793
Jhanjee S. Evidence based psychosocial interventions in substance use. Indian J Psychol Med 2014;36:112–18. https://doi.org/10.4103/0253-7176.130960
Julius RJ, Novitsky MA, Dubin WR. Medication adherence: a review of the literature and implications for clinical practice. J Psychiatr Pract 2009;15:34–44. https://doi.org/10.1097/01.pra.0000344917.43780.77
Kaplan MS, Krueger RB. Cognitive-behavioral treatment of the paraphilias. Israel J Psychiatry Relat Sci 2012;49:291–6.
Kaptein AA, van Dijk S, Broadbent E, Falzon L, Thong M, Dekker FW. Behavioural research in patients with end-stage renal disease: a review and research agenda. Patient Educ Couns 2010;81:23–9. https://doi.org/10.1016/j.pec.2009.10.031
Kazantzis N, Deane FP, Ronan KR. Homework assignments in cognitive and behavioral therapy: a meta-analysis. Clin Psychol Sci Pract 2000;7:189-202.
Killgore WD. Academic and research interest in several approaches to psychotherapy: a computerized search of literature in the past 16 years. Psychol Reports 2000;87:717–20.
Kirsch I. Hypnotic enhancement of cognitive–behavioral weight loss treatments – another meta-reanalysis. J Consult Clin Psychol 1996;64:517–19. https://doi.org/10.1037//0022-006x.64.3.517
Kirsch I, Montgomery G, Sapirstein G. Hypnosis as an adjunct to cognitive–behavioral psychotherapy: a meta-analysis. J Consult Clin Psychol 1995;63:214–20. https://doi.org/10.1037//0022-006x.63.2.214
Knouse LE, Teller J, Brooks MA. Meta-analysis of cognitive–behavioral treatments for adult ADHD. J Consult Clin Psychol 2017;85:737–50. https://doi.org/10.1037/ccp0000216
Koder DA, Brodaty H, Anstey KJ. Cognitive therapy for depression in the elderly. Int J Geriatr Psychiatry 1996;11:97–107.
Korczak D, Wastian M, Schneider M. Therapy of the burnout syndrome. GMS Health Technol Assess 2012;8:Doc05.
Kovacs M, Lopez-Duran N. Prodromal symptoms and atypical affectivity as predictors of major depression in juveniles: implications for prevention. J Child Psychol Psychiatry 2010;51:472–96. https://doi.org/10.1111/j.1469-7610.2010.02230.x
Kroon Van Diest AM, Powers SW. Cognitive behavioral therapy for pediatric headache and migraine: why to prescribe and what new research is critical for advancing integrated biobehavioral care. Headache 2019;59:289–97. https://doi.org/10.1111/head.13438
Krueger SJ, Glass CR. Integrative psychotherapy for children and adolescents: a practice-oriented literature review. J Psychother Integr 2013;23:331–44.
Kunkle B, Bae S, Singh KP, Roy D. Increased risk of childhood brain tumors among children whose parents had farm-related pesticide exposures during pregnancy. JP J Biostat 2014;11:89–101.
Kwekkeboom KL. Cancer symptom cluster management. Semin Oncol Nurs 2016;32:373–82.
Lam S, Macina LO. Therapy update for insomnia in the elderly. Consult Pharm 2017;32:610–22. https://doi.org/10.4140/TCP.n.2017.610
Lamarche LJ, De Koninck J. Sleep disturbance in adults with posttraumatic stress disorder: a review. J Clin Psychiatry 2007;68:1257–70. https://doi.org/10.4088/jcp.v68n0813
Lampe L, Coulston CM, Berk L. Psychological management of unipolar depression. Acta Psychiatr Scand Suppl 2013;443:24–37. https://doi.org/10.1111/acps.12123
Lampe LA. Social anxiety disorder: recent developments in psychological approaches to conceptualization and treatment. Aust N Z JPsychiatry 2009;43:887–98.
Landenberger NA, Lipsey MW. The positive effects of cognitive–behavioral programs for offenders: a meta-analysis of factors associated with effective treatment. J Exp Criminol 2005;1:451–76.
Lazar SG, Offenkrantz W. Psychotherapy in the Treatment of Posttraumatic Stress Disorder. In Lazar SG, editor. Psychotherapy Is Worth It: A Comprehensive Review of Its Cost-Effectiveness. Washington, DC: American Psychiatric Publishing, Inc; 2010. pp. 87–102.
Le Grange D, Schmidt U. The treatment of adolescents with bulimia nervosa. J Ment Health 2005;14:587–97.
Le HN, Boyd RC. Prevention of major depression: early detection and early intervention in the general population. Clin Neuropsychiatry 2006;3:6–22.
Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012;4:147–54. https://doi.org/10.1177/1941738111433673
Leichsenring F, Hiller W, Weissberg M, Leibing E. Cognitive–behavioral therapy and psychodynamic psychotherapy: techniques, efficacy, and indications. Am J Psychother 2006;60:233–59. https://doi.org/10.1176/appi.psychotherapy.2006.60.3.233
Leichsenring F, Leibing E. Psychodynamic psychotherapy: a systematic review of techniques, indications and empirical evidence. Psychol Psychother Theory Res Pract 2007;80:217–28.
Lejoyeux M, Weinstein A. Compulsive buying. Am J Drug Alcohol Abus 2010;36:248–53.
Lener MS, Iosifescu DV. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: a review of the literature. Ann N Y Acad Sci 2015;1344:50–65. https://doi.org/10.1111/nyas.12759
Lenz A, Haktanir A, Callender K. Meta-analysis of trauma-focused therapies for treating the symptoms of posttraumatic stress disorder. J Couns Dev 2017;95:339–53.
Lett HS, Davidson J, Blumenthal JA. Nonpharmacologic treatments for depression in patients with coronary heart disease. Psychosom Med 2005;67(Suppl. 1):58–62.
Leung KS, Cottler LB. Treatment of pathological gambling. Curr Opin Psychiatry 2009;22:69–74.
Levenstein S. The Evidence for Treatments for Somatoform Disorders: A View from the Trenches. In Gresser M, editor. Somatic Presentations of Mental Disorders: Refining the Research Agenda for DSM-V. Arlington, VA: American Psychological Association; 2009. pp. 165–9.
Linden W, Chambers L. Clinical effectiveness of non-drug treatment for hypertension: a meta- analysis. Ann Behav Med 1994;16:35–45.
Linton SJ, Bergbom S. Understanding the link between depression and pain. Scand J Pain 2011;2:47–54. https://doi.org/10.1016/j.sjpain.2011.01.005
Liu P, Parker AG, Hetrick SE, Callahan P, de Silva S, Purcell R. An evidence map of interventions across premorbid, ultra-high risk and first episode phases of psychosis. Schizophr Res 2010;123:37–44. https://doi.org/10.1016/j.schres.2010.05.004
Ljotsson B, Hedman E, Mattsson S, Andersson E. The effects of cognitive–behavioral therapy for depression are not falling: a re-analysis of Johnsen and Friborg (2015). Psychol Bull 2017;143:321–5.
Lock J. An update on evidence-based psychosocial treatments for eating disorders in children and adolescents. J Clin Child Adolesc Psychol 2015;44:707–21.
Loerinc AG, Meuret AE, Twohig MP, Rosenfield D, Bluett EJ, Craske MG. Response rates for CBT for anxiety disorders: need for standardized criteria. Clin Psychol Rev 2015;42:72–82. https://doi.org/10.1016/j.cpr.2015.08.004
Losel F, Beelmann A. Effects of child skills training in preventing antisocial behavior: a systematic review of randomized evaluations. Ann Am Acad Political Soc Sci 2003;587:84–109.
Lourenço Leite P, Pereira VM, Nardi AE, Silva AC. Psychotherapy for compulsive buying disorder: a systematic review. Psychiatry Res 2014;219:411–19. https://doi.org/10.1016/j.psychres.2014.05.037
Luby JL. Treatment of anxiety and depression in the preschool period. J Am Acad Child Adolesc Psychiatry 2013;52:346–58.
Luhmann D, Stoll S, Burkhardt-Hammer T, Raspe H. Prevention of relapsing backache. GMS Health Technol Assess 2006;2:Doc12.
Lundgren JD, Danoff-Burg S, Anderson DA. Cognitive–behavioral therapy for bulimia nervosa: an empirical analysis of clinical significance. Int J Eat Disord 2004;35:262–74. https://doi.org/10.1002/eat.10254
Luskin FM, Newell KA, Griffith M, Holmes M, Telles S, DiNucci E, et al. A review of mind/body therapies in the treatment of musculoskeletal disorders with implications for the elderly. Altern Ther Health Med 2000;6:46–56.
Luskin FM, Newell KA, Griffith M, Holmes M, Telles S, Marvasti FF, et al. A review of mind–body therapies in the treatment of cardiovascular disease. Part 1: implications for the elderly. Altern Ther Health Med 1998;4:46–61.
Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complicat 2005;19:113–22.
Lustyk MK, Gerrish WG, Shaver S, Keys SL. Cognitive–behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health 2009;12:85–96. https://doi.org/10.1007/s00737-009-0052-y
MacLeod S, Musich S, Kraemer S, Wicker E. Practical non-pharmacological intervention approaches for sleep problems among older adults. Geriatr Nurs 2018;39:506–12.
Mai F. Somatization disorder: a practical review. Can J Psychiatry 2004;49:652–62.
Maia AC, Braga AA, Soares-Filho G, Pereira V, Nardi AE, Silva AC. Efficacy of cognitive behavioral therapy in reducing psychiatric symptoms in patients with implantable cardioverter defibrillator: an integrative review. Braz J Med Biol Res 2014;47:265–72. https://doi.org/10.1590/1414-431X20133418
Makris UE, Abrams RC, Gurland B, Reid MC. Management of persistent pain in the older patient: a clinical review. JAMA 2014;312:825–36. https://doi.org/10.1001/jama.2014.9405
Mallick S. Palliative care in Parkinson’s disease: role of cognitive behavior therapy. Indian J Palliat Care 2009;15:51–6. https://doi.org/10.4103/0973-1075.53512
Manfro GG, Heldt E, Cordioli AV, Otto MW. Cognitive–behavioral therapy in panic disorder. Rev Bras Psiquiatr 2008;30:S81–S7.
Maquet D, Demoulin C, Croisier JL, Crielaard JM. Benefits of physical training in fibromyalgia and related syndromes. Ann Readapt Med Phys 2007;50:363–8, 356–62.
Marazziti D, Consoli G. Treatment strategies for obsessive–compulsive disorder. Expert Opin Pharmacother 2010;11:331–43. https://doi.org/10.1517/14656560903446948
Marcotte D. Treating depression in adolescence: a review of the effectiveness of cognitive–behavioral treatments. J Youth Adolesc 1997;26:273–83.
Marcus DK, O’Connell D, Norris AL, Sawaqdeh A. Is the Dodo bird endangered in the 21st century? A meta-analysis of treatment comparison studies. Clin Psychol Rev 2014;34:519–30. https://doi.org/10.1016/j.cpr.2014.08.001
Maricutoiu LP, Sava FA, Butta O. The effectiveness of controlled interventions on employees’ burnout: a meta-analysis. J Occup Organ Psychol 2016;89:1–27.
Marsch LA, Hegel MT, Greene MA. Leveraging digital technology to intervene on personality processes to promote healthy aging. Personal Disord 2019;10:33–45. https://doi.org/10.1037/per0000275
Martin GW, Rehm J. The effectiveness of psychosocial modalities in the treatment of alcohol problems in adults: a review of the evidence. Can J Psychiatry 2012;57:350–8. https://doi.org/10.1177/070674371205700604
Math SB, Janardhan Reddy YC. Issues in the pharmacological treatment of obsessive–compulsive disorder. Int J Clin Pract 2007;61:1188–97.
Maxwell N, Scourfield J, Featherstone B, Holland S, Tolman R. Engaging fathers in child welfare services: a narrative review of recent research evidence. Child Fam Soc Work 2012;17:160–9.
McClellan JM, Werry JS. Evidence-based treatments in child and adolescent psychiatry: an inventory. J Am Acad Child Adolesc Psychiatry 2003;42:1388–400.
McCormack M, Tierney K, Brennan D, Lawlor E, Clarke M. Lack of insight in psychosis: theoretical concepts and clinical aspects. Behav Cogn Psychother 2014;42:327–38. https://doi.org/10.1017/S1352465813000155
McCracken LM, Thompson M. Psychological advances in chronic pain: a concise selective review of research from 2010. Curr Opin Support Palliat Care 2011;5:122–6. https://doi.org/10.1097/SPC.0b013e328345a3ff
McCracken LM, Turk DC. Behavioral and cognitive–behavioral treatment for chronic pain: outcome, predictors of outcome, and treatment process. Spine 2002;27:2564–73. https://doi.org/10.1097/00007632-200211150-00033
McCrady BS, Owens MD, Borders AZ, Brovko JM. Psychosocial approaches to alcohol use disorders since 1940: a review. J Stud Alcohol Drugs Suppl 2014;75(Suppl. 17):68–78.
McGuire AW, Ahearn E, Doering LV. Psychological distress and cardiovascular disease. J Clin Outcomes Manag 2015;22:421–32.
McHugh RK, Hearon BA, Otto MW. Cognitive behavioral therapy for substance use disorders. Psychiatr Clin North Am 2010;33:511–25. https://doi.org/10.1016/j.psc.2010.04.012
McIntyre RS, Alsuwaidan M, Goldstein BI, Taylor VH, Schaffer A, Beaulieu S, Kemp DE, Canadian Network for Mood and Anxiety Treatments (CANMAT) Task Force. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid metabolic disorders. Ann Clin Psychiatry 2012;24:69–81.
McMain S, Pos AE. Advances in psychotherapy of personality disorders: a research update. Curr Psychiatry Rep 2007;9:46–52. https://doi.org/10.1007/s11920-007-0009-7
McManus F, Shafran R, Cooper Z. What does a ‘transdiagnostic’ approach have to offer the treatment of anxiety disorders? Br J Clin Psychol 2010;49:491–505.
McPherson S, Evans C, Richardson P. The NICE depression guidelines and the recovery model: is there an evidence base for IAPT? J Ment Health 2009;18:405–14.
Mehler-Wex C, Kölch M. Depression in children and adolescents. Dtsch Arztebl Int 2008;105:149–55. https://doi.org/10.3238/arztebl.2008.0149
Meléndez JC, McCrank E. Anxiety-related reactions associated with magnetic resonance imaging examinations. JAMA 1993;270:745–7. https://doi.org/10.1001/jama.1993.03510060091039
Menon V, Rajan TM, Kuppili PP, Sarkar S. Cognitive behavior therapy for medically unexplained symptoms: a systematic review and meta-analysis of published controlled trials. Indian J Psychol Med 2017;39:399–406. https://doi.org/10.4103/IJPSYM.IJPSYM_17_17
Middaugh SJ, Pawlick K. Biofeedback and behavioral treatment of persistent pain in the older adult: a review and a study. Appl Psychophysiol Biofeedback 2002;27:185–202. https://doi.org/10.1023/a:1016208128254
Milin R, Walker S, Chow J. Major depressive disorder in adolescence: a brief review of the recent treatment literature. Can J Psychiatry 2003;48:600–6. https://doi.org/10.1177/070674370304800906
Miller LJ, Mittenberg W. Brief cognitive behavioral interventions in mild traumatic brain injury. Appl Neuropsychol 1998;5:172–83. https://doi.org/10.1207/s15324826an0504_2
Mitchell JE, Raymond NC. Cognitive–Behavioral Therapy in Treatment of Bulimia Nervosa. In Halmi K, editor. Psychobiology and Treatment of Anorexia Nervosa and Bulimia Nervosa. Arlington, VA: American Psychiatric Association; 1992. pp. 307–27.
Mitchell JE, Roerig J, Steffen K. An Update on Treatment Strategies for Bulimia Nervosa. In Stein D, Latzer Y, editors. Treatment and Recovery of Eating Disorders. Hauppauge, NY: Nova Science Publishers; 2012. pp. 27–39.
Mitte K. Meta-analysis of cognitive–behavioral treatments for generalized anxiety disorder: a comparison with pharmacotherapy. Psychol Bull 2005;131:785–95.
Mitte K. A meta-analysis of the efficacy of psycho- and pharmacotherapy in panic disorder with and without agoraphobia. J Affect Disord 2005;88:27–45.
Miziou S, Tsitsipa E, Moysidou S, Karavelas V, Dimelis D, Polyzoidou V, Fountoulakis KN. Psychosocial treatment and interventions for bipolar disorder: a systematic review. Ann Gen Psychiatry 2015;14:19. https://doi.org/10.1186/s12991-015-0057-z
Mobini S, Grant A. Clinical implications of attentional bias in anxiety disorders: an integrative literature review. Psychotherapy 2007;44:450–62. https://doi.org/10.1037/0033-3204.44.4.450
Molinuevo B, Batista-Miranda JE. Under the tip of the iceberg: psychological factors in incontinence. Neurourol Urodyn 2012;31:669–71. https://doi.org/10.1002/nau.21216
Mulcahy RA, Blue RS, Vardiman JL, Castleberry TL, Vanderploeg JM. Screening and mitigation of layperson anxiety in aerospace environments. Aerosp Med Hum Perform 2016;87:882–9. https://doi.org/10.3357/AMHP.4536.2016
Muller A, Mitchell JE, De Zwaan M. Compulsive buying. Am J Addict 2015;24:132–7.
Musiat P, Tarrier N. Collateral outcomes in e-mental health: a systematic review of the evidence for added benefits of computerized cognitive behavior therapy interventions for mental health. Psychol Med 2014;44:3137–50. https://doi.org/10.1017/S0033291714000245
Mychailyszyn MP. School-based Interventions for Anxious and Depressed Youth: A Meta-analysis of Outcomes. PhD thesis. Philadelphia, PA: Temple University; 2012.
Mychailyszyn MP, Brodman DM, Read KL, Kendall PC. Cognitive–behavioral school-based interventions for anxious and depressed youth: a meta-analysis of outcomes. Clin Psychol Sci Pract 2012;19:129–53.
Nalajala N, Walls K, Hili E. Insomnia in chronic lower back pain: non-pharmacological physiotherapy interventions. Int J TherRehabil 2013;20:510–6.
Nardi B, Francesconi G, Catena-Dell’Osso M, Bellantuono C. Adolescent depression: clinical features and therapeutic strategies. Eur Rev Med Pharmacol Sci 2013;17:1546–51.
Nardi B, Massei M, Arimatea E, Moltedo-Perfetti A. Effectiveness of group CBT in treating adolescents with depression symptoms: a critical review. Int J Adolesc Med Health 2016;29. https://doi.org/10.1515/ijamh-2015-0080
Neuderth S, Jabs B, Schmidtke A. Strategies for reducing test anxiety and optimizing exam preparation in German university students: a prevention-oriented pilot project of the University of Würzburg. J Neural Transm 2009;116:785–90. https://doi.org/10.1007/s00702-008-0123-7
Nicholas MK, Molloy AR, Brooker C. Using opioids with persisting noncancer pain: a biopsychosocial perspective. Clin J Pain 2006;22:137–46.
Nicholl C, Thompson A. The psychological treatment of post traumatic stress disorder (PTSD) in adult refugees: a review of the current state of psychological therapies. J Ment Health 2004;13:351–62.
Nordin M, Balagué F, Cedraschi C. Nonspecific lower-back pain: surgical versus nonsurgical treatment. Clin Orthop Relat Res 2006;443:156–67. https://doi.org/10.1097/01.blo.0000198721.75976.d9
Norman RM, Townsend LA. Cognitive–behavioural therapy for psychosis: a status report. Can J Psychiatry 1999;44:245–52. https://doi.org/10.1177/070674379904400304
Norris AL, Marcus DK. Cognition in health anxiety and hypochondriasis: recent advances. Curr Psychiatry Rev 2014;10:44–9.
Norton PJ, Price EC. A meta-analytic review of adult cognitive–behavioral treatment outcome across the anxiety disorders. J Nerv Ment Dis 2007;195:521–31. https://doi.org/10.1097/01.nmd.0000253843.70149.9a
O’Connor K. Research in young people at ultra-high risk for psychosis: a review of the current evidence. Ir J Psychol Med 2013;30:77–89. https://doi.org/10.1017/ipm.2012.9
Oei TP, Free ML. Do cognitive behaviour therapies validate cognitive models of mood disorders? A review of the empirical evidence. Int J Psychol 1995;30:145–80.
Olatunji BO, Cisler JM, Deacon BJ. Efficacy of cognitive behavioral therapy for anxiety disorders: a review of meta-analytic findings. Psychiatr Clin North Am 2010;33:557–77. https://doi.org/10.1016/j.psc.2010.04.002
Olatunji BO, Cisler JM, Tolin DF. A meta-analysis of the influence of comorbidity on treatment outcome in the anxiety disorders. Clin Psychol Rev 2010;30:642–54. https://doi.org/10.1016/j.cpr.2010.04.008
Olivares JM, Sermon J, Hemels M, Schreiner A. Definitions and drivers of relapse in patients with schizophrenia: a systematic literature review. Ann Gen Psychiatry 2013;12:32. https://doi.org/10.1186/1744-859X-12-32
O’Neal P, Jackson A, McDermott F. A review of the efficacy and effectiveness of cognitive–behaviour therapy and short-term psychodynamic therapy in the treatment of major depression: implications for mental health social work practice. Aust Soc Work 2014;67:197–213.
O’Reilly R, Wilkes L, Luck L, Jackson D. The efficacy of family support and family preservation services on reducing child abuse and neglect: what the literature reveals. J Child Health Care 2010;14:82–94. https://doi.org/10.1177/1367493509347114
Ost LG. Cognitive behavior therapy for anxiety disorders: 40 years of progress. Nord J Psychiatry 2008;62(Suppl. 47):5–10. https://doi.org/10.1080/08039480802315590
Otte C. Cognitive behavioral therapy in anxiety disorders: current state of the evidence. Dialogues Clin Neurosci 2011;13:413–21.
Parikh SV, Kusumakar V, Haslam DR, Matte R, Sharma V, Yatham LN. Psychosocial interventions as an adjunct to pharmacotherapy in bipolar disorder. Can J Psychiatry 1997;42(Suppl. 2):74S–8S.
Parikh SV, Quilty LC, Ravitz P, Rosenbluth M, Pavlova B, Grigoriadis S, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 2. Psychological treatments. Can J Psychiatry 2016;61:524–39. https://doi.org/10.1177/0706743716659418
Parikh SV, Segal ZV, Grigoriadis S, Ravindran AV, Kennedy SH, Lam RW, Patten SB, Canadian Network for Mood and Anxiety Treatments (CANMAT). Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. II. Psychotherapy alone or in combination with antidepressant medication. J Affect Disord 2009;117(Suppl. 1):15–25. https://doi.org/10.1016/j.jad.2009.06.042
Parker G, Fletcher K. Treating depression with the evidence-based psychotherapies: a critique of the evidence. Acta Psychiatr Scand 2007;115:352–9.
Parker G, Parker I, Brotchie H, Stuart S. Interpersonal psychotherapy for depression? The need to define its ecological niche. J Affect Disord 2006;95:1–11.
Parker G, Roy K, Eyers K. Cognitive behavior therapy for depression? Choose horses for courses. Am J Psychiatry 2003;160:825–34. https://doi.org/10.1176/appi.ajp.160.5.825
Parker GB, Crawford J, Hadzi-Pavlovic D. Quantified superiority of cognitive behaviour therapy to antidepressant drugs: a challenge to an earlier meta-analysis. Acta Psychiatr Scand 2008;118:91–7. https://doi.org/10.1111/j.1600-0447.2008.01196.x
Patel N, C de C Williams A, Kellezi B. Reviewing outcomes of psychological interventions with torture survivors: conceptual, methodological and ethical issues. Torture 2016;26:2–16.
Patterson E, Wan YW, Sidani S. Nonpharmacological nursing interventions for the management of patient fatigue: a literature review. J Clin Nurs 2013;22:2668–78. https://doi.org/10.1111/jocn.12211
Pattison S, Harris B. Counselling children and young people: a review of the evidence for its effectiveness. Counsel Psychother Res 2006;6:233–7.
Pavan C, Simonato P, Marini M, Mazzoleni F, Pavan L, Vindigni V. Psychopathologic aspects of body dysmorphic disorder: a literature review. Aesthetic Plast Surg 2008;32:473–84. https://doi.org/10.1007/s00266-008-9113-2
Pearsall DF. Psychotherapy outcome research in child psychiatric disorders. Can J Psychiatry 1997;42:595–601.
Pearson FS, Lipton DS, Cleland CM, Yee DS. The effects of behavioral/cognitive–behavioral programs on recidivism. Crime Delinq 2002;48:476–96.
Penckofer S, Doyle T, Byrn M, Lustman PJ. State of the science: depression and type 2 diabetes. West J Nurs Res 2014;36:1158–82. https://doi.org/10.1177/0193945914524491
Peng XD, Huang CQ, Chen LJ, Lu ZC. Cognitive behavioural therapy and reminiscence techniques for the treatment of depression in the elderly: a systematic review. J Int Med Res 2009;37:975–82. https://doi.org/10.1177/147323000903700401
Perry JC, Banon E, Ianni F. Effectiveness of psychotherapy for personality disorders. Am J Psychiatry 1999;156:1312–21. https://doi.org/10.1176/ajp.156.9.1312
Petrocelli JV. Effectiveness of group cognitive–behavioral therapy for general symptomatology: a meta-analysis. J Specialists Group Work 2002;27:92–115.
Pfammatter M, Junghan UM, Brenner HD. Efficacy of psychological therapy in schizophrenia: conclusions from meta-analyses. Schizophr Bull 2006;32(Suppl. 1):64–80.
Pheng TL, Hassan S. A review of psychotherapy as add-on treatment to pharmacotherapy for bipolar disorder. Res J Med Sci 2014;8:99–108.
Pinquart M, Sörensen S. How effective are psychotherapeutic and other psychosocial interventions with older adults? A meta-analysis. J Ment Health Aging 2001;7:207–43.
Pinquart M, Sörensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects? Int Psychogeriatr 2006;18:577–95.
Pinto TF, Da Silva FGC, De Bruin VMS, De Bruin PFC. Night eating syndrome: how to treat it? Rev Assoc Med Bras 2016;62:701–7.
Piotrowski C. Chronic pain in the elderly: mapping the mental health literature. J Instr Psychol 2014;41:16–8.
Podea D, Suciu R, Suciu C, Ardelean M. An update on the cognitive behavior therapy of obsessive compulsive disorder in adults. J Cogn Behav Psychother 2009;9:221–33.
Poiraudeau S, Rannou F, Revel M. Functional restoration programs for low back pain: a systematic review. Ann Readapt Med Phys 2007;50:425–9, 419–24.
Polackwich AS, Shoskes DA. Chronic prostatitis/chronic pelvic pain syndrome: a review of evaluation and therapy. Prostate Cancer Prostatic Dis 2016;19:132–8. https://doi.org/10.1038/pcan.2016.8
Polaschek DL, Collie RM. Rehabilitating serious violent adult offenders: an empirical and theoretical stocktake. Psychol Crime Law 2004;10:321–34.
Ponniah K, Hollon SD. Empirically supported psychological interventions for social phobia in adults: a qualitative review of randomized controlled trials. Psychol Med 2008;38:3–14.
Portzky G, van Heeringen K. Deliberate self-harm in adolescents. Curr Opin Psychiatry 2007;20:337–42.
Pospos S, Young IT, Downs N, Iglewicz A, Depp C, Chen JY, et al. Web-based tools and mobile applications to mitigate burnout, depression, and suicidality among healthcare students and professionals: a systematic review. Acad Psychiatry 2018;42:109–20. https://doi.org/10.1007/s40596-017-0868-0
Post MW, van Leeuwen CM. Psychosocial issues in spinal cord injury: a review. Spinal Cord 2012;50:382–9. https://doi.org/10.1038/sc.2011.182
Poynter BA, Hunter JJ, Coverdale JH, Kempinsky CA. Hard to swallow: a systematic review of deliberate foreign body ingestion. Gen Hosp Psychiatry 2011;33:518–24. https://doi.org/10.1016/j.genhosppsych.2011.06.011
Prajapati AR. Pharmacology vs. psychotherapy in the management of depression and anxiety disorders. Int J Psychother 2014;18:50–61.
Pratchett LC, Daly K, Bierer LM, Yehuda R. New approaches to combining pharmacotherapy and psychotherapy for posttraumatic stress disorder. Expert Opin Pharmacother 2011;12:2339–54. https://doi.org/10.1517/14656566.2011.604030
Price A, Hotopf M. The treatment of depression in patients with advanced cancer undergoing palliative care. Curr Opin Support Palliat Care 2009;3:61–6. https://doi.org/10.1097/SPC.0b013e328325d17a
Prout SM, Prout H. A meta-analysis of school-based studies of counseling and psychotherapy: an update. J School Psychol 1998;36:121–36.
Pull CB. Combined pharmacotherapy and cognitive–behavioural therapy for anxiety disorders. Curr Opin Psychiatry 2007;20:30–5. https://doi.org/10.1097/YCO.0b013e3280115e52
Pull CB. Current empirical status of acceptance and commitment therapy. Curr Opin Psychiatry 2009;22:55–60. https://doi.org/10.1097/YCO.0b013e32831a6e9d
Pull CB, Damsa C. Pharmacotherapy of panic disorder. Neuropsychiatr Dis Treat 2008;4:779–95.
Putnam FW. Ten-year research update review: child sexual abuse. J Am Acad Child Adolesc Psychiatry 2003;42:269–78.
RachBeisel J, Scott J, Dixon L. Co-occurring severe mental illness and substance use disorders: a review of recent research. Psychiatr Serv 1999;50:1427–34. https://doi.org/10.1176/ps.50.11.1427
Radziwon CD, Lackner JM. Cognitive behavioral therapy for IBS: how useful, how often, and how does it work? Curr Gastroenterol Rep 2017;19:49.
Ramacciotti CE, Coli E, Marazziti D, Segura-García C, Brambilla F, Piccinni A, Dell’osso L. Therapeutic options for binge eating disorder. Eat Weight Disord 2013;18:3–9. https://doi.org/10.1007/s40519-013-0003-5
Rathod S, Hansen L, Kingdon D. Insight in Psychosis and Cognitive Behaviour Therapy. In Abelian M, editor. Focus on Psychotherapy Research. Hauppauge, NY: Nova Science Publishers; 2005. pp. 51–67.
Rathod S, Kingdon D, Weiden P, Turkington D. Cognitive–behavioral therapy for medication-resistant schizophrenia: a review. J Psychiatr Pract 2008;14:22–33. https://doi.org/10.1097/01.pra.0000308492.93003.db
Reinecke MA, Ryan NE, DuBois DL. Cognitive–behavioral therapy of depression and depressive symptoms during adolescence: a review and meta-analysis. J Am Acad Child Adolesc Psychiatry 1998;37:26–34.
Richmond J, Berman BM, Docherty JP, Goldstein LB, Kaplan G, Keil JE, et al. Integration of behavioral and relaxation approaches into the treatment of chronic pain and insomnia. JAMA 1996;276:313–8.
Ridings LE, Moreland AD, Petty KH. Implementing trauma-focused CBT for children of veterans in the VA: providing comprehensive services to veterans and their families. Psychol Serv 2018;30:30.
Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev 2009;13:205–14. https://doi.org/10.1016/j.smrv.2008.06.001
Rimbaut S, Van Gutte C, Van Brabander L, Vanden Bossche L. Chronic fatigue syndrome – an update. Acta Clin Belg 2016;71:273–80.
Rimes KA, Chalder T. Treatments for chronic fatigue syndrome. Occup Med 2005;55:32–9.
Ritz T, Meuret AE, Trueba AF, Fritzsche A, von Leupoldt A. Psychosocial factors and behavioral medicine interventions in asthma. J Consult Clin Psychol 2013;81:231–50. https://doi.org/10.1037/a0030187
Rodrigo C, Rajapakse S, Jayananda G. The ‘antisocial’ person: an insight in to biology, classification and current evidence on treatment. Ann Gen Psychiatry 2010;9:31. https://doi.org/10.1186/1744-859X-9-31
Roepke AM, Seligman ME. Depression and prospection. Br J Clin Psychol 2016;55:23–48.
Roffman JL, Marci CD, Glick DM, Dougherty DD, Rauch SL. Neuroimaging and the functional neuroanatomy of psychotherapy. Psychol Med 2005;35:1385–98.
Rogers-Nicastro J. A Meta-analytic Review of Play Therapy Outcomes and the Role of Age: Implications for School Psychologists. PhD thesis. New York, NY: St John’s University; 2006.
Ronconi JM, Shiner B, Watts BV. Inclusion and exclusion criteria in randomized controlled trials of psychotherapy for PTSD. J Psychiatr Pract 2014;20:25–37. https://doi.org/10.1097/01.pra.0000442936.23457.5b
Roshanaei-Moghaddam B, Pauly MC, Atkins DC, Baldwin SA, Stein MB, Roy-Byrne P. Relative effects of CBT and pharmacotherapy in depression versus anxiety: is medication somewhat better for depression, and CBT somewhat better for anxiety? Depress Anxiety 2011;28:560–7.
Rostain AL. Attention-deficit/hyperactivity disorder in adults: evidence-based recommendations for management. Postgrad Med 2008;120:27–38. https://doi.org/10.3810/pgm.2008.09.1905
Roth AD, Pilling S, Turner J. Therapist training and supervision in clinical trials: implications for clinical practice. Behav Cogn Psychother 2010;38:291–302. https://doi.org/10.1017/S1352465810000068
Rotheram-Fuller E, MacMullen L. Cognitive–behavioral therapy for children with autism spectrum disorders. Psychol School 2011;48:263–71.
Rowa K, Antony MM. Psychological treatments for social phobia. Can J Psychiatry 2005;50:308–16.
Rozental A, Magnusson K, Boettcher J, Andersson G, Carlbring P. For better or worse: an individual patient data meta-analysis of deterioration among participants receiving internet-based cognitive behavior therapy. J Consult Clin Psychol 2017;85:160–77. https://doi.org/10.1037/ccp0000158
Rubin A, Miao Y. Within-group effect size benchmarks for cognitive--behavioral therapy in the treatment of adult depression. Soc Work Res 2017;41:135-44.
Rubinchik SM, Kablinger AS, Gardner JS. Medications for panic disorder and generalized anxiety disorder during pregnancy. Prim Care Companion J Clin Psychiatry 2005;7:100–5.
Rustad JK, Stern TA, Hebert KA, Musselman DL. Diagnosis and treatment of depression in patients with congestive heart failure: a review of the literature. Prim Care Companion CNS Disord 2013;15:PCC.13r01511. https://doi.org/10.4088/PCC.13r01511
Rutherford L, Couturier J. A review of psychotherapeutic interventions for children and adolescents with eating disorders. J Can Acad Child Adolesc Psychiatry 2007;16:153–7.
Saini M. A meta-analysis of the psychological treatment of anger: developing guidelines for evidence-based practice. J Am Acad Psychiatry Law 2009;37:473–88.
Sakinofsky I. The aftermath of suicide: managing survivors’ bereavement. Can J Psychiatry 2007;52(Suppl. 6):129–36.
Salmoiraghi A, Sambhi R. Early termination of cognitive–behavioural interventions: literature review. Psychiatrist 2010;34:529–32.
Sampaio FM, Sequeira CA, Lluch Canut MT. Nursing psychotherapeutic interventions: a review of clinical studies. J Clin Nurs 2015;24:2096–105. https://doi.org/10.1111/jocn.12808
Samson JE, Tanner-Smith EE. Single-session alcohol interventions for heavy drinking college students: a systematic review and meta-analysis. J Stud Alcohol Drugs 2015;76:530–43.
Schaffer A, McIntosh D, Goldstein BI, Rector NA, McLntyre RS, Beaulieu S, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid anxiety disorders. Ann Clin Psychiatry 2012;24:6–22.
Schoenwald SK, Garland AF. A review of treatment adherence measurement methods. Psychol Assess 2013;25:146–56. https://doi.org/10.1037/a0029715
Schwalbe C, Gearing R. The moderating effect of adherence-promoting interventions with clients on evidence-based practices for children and adolescents with mental health problems. Am J Orthopsychiatry 2012;82:146–55. https://doi.org/10.1111/j.1939-0025.2011.01133.x
Scogin F, Welsh D, Hanson A, Stump J, Coates A. Evidence-based psychotherapies for depression in older adults. Clin Psychol Sci Pract 2005;12:222–37.
Segal ZV, Whitney DK, Lam RW, CANMAT Depression Work G. Clinical guidelines for the treatment of depressive disorders. III. Psychotherapy. Can J Psychiatry 2001;46(Suppl. 1):29S–37S.
Sharma L. Clinical. Osteoarthr Cartil 2015;23:A24.
Shen Y, Huang JY, Li J, Liu CF. excessive daytime sleepiness in Parkinson’s disease: clinical implications and management. Chin Med J 2018;131:974–81. https://doi.org/10.4103/0366-6999.229889
Shen YH, Nahas R. Complementary and alternative medicine for treatment of irritable bowel syndrome. Can Fam Physician 2009;55:143–8.
Siegel P, Tencza M, Apodaca B, Poole JL. Effectiveness of occupational therapy interventions for adults with rheumatoid arthritis: a systematic review. Am J Occup Ther 2017;71:7101180050p.
Silva JAMD, Siegmund G, Bredemeier J. Crisis interventions in online psychological counseling Intervencoes em crise nos atendimentos psicologicos online. Trend Psychiatry Psychother 2015;37:171–82.
Sinclair D, Adams CE. Treatment resistant schizophrenia: a comprehensive survey of randomised controlled trials. BMC Psychiatry 2014;14:253. https://doi.org/10.1186/s12888-014-0253-4
Skeem JL, Steadman HJ, Manchak SM. Applicability of the risk–need–responsivity model to persons with mental illness involved in the criminal justice system. Psychiatr Serv 2015;66:916–22. https://doi.org/10.1176/appi.ps.201400448
Skriner LC, Chu BC, Kaplan M, Bodden DHM, Bögels SM, Kendall PC, et al. Trajectories and predictors of response in youth anxiety CBT: integrative data analysis. J Consult Clin Psychol 2019;87:198–211. https://doi.org/10.1037/ccp0000367
Slater J, Townend M. cognitive behaviour therapy for psychosis in high secure services: an exploratory hermeneutic review of the international literature. Behav Cogn Psychother 2016;44:652–72.
Slobodin O, de Jong JTVM. Mental health interventions for traumatized asylum seekers and refugees: what do we know about their efficacy? Int J Soc Psychiatry 2015;61:17–26.
Spattini L, Rioli G, Longo F, Ferrari S, Galeazzi GM. An update on current clinical management of eating disorders. Minerva Psichiatrica 2017;58:54–69.
Speisman BB, Storch EA, Abramowitz JS. Postpartum obsessive–compulsive disorder. J Obstet Gynecol Neonatal Nurs 2011;40:680–90.
Spielmans GI, Pasek LF, McFall JP. What are the active ingredients in cognitive and behavioral psychotherapy for anxious and depressed children? A meta-analytic review. Clin Psychol Rev 2007;27:642–54.
Stacciarini JM, O’Keeffe M, Mathews M. Group therapy as treatment for depressed Latino women: a review of the literature. Issues Ment Health Nurs 2007;28:473–88.
Staub C. Concept of diverse sleep treatments in physiotherapy. Eur J Physiother 2018;21:177–84.
Stewart RE, Chambless DL. Cognitive–behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol 2009;77:595–606. https://doi.org/10.1037/a0016032
Stuart S, Bowers WA. Cognitive therapy with inpatients: review and meta-analysis. J Cogn Psychother 1995;9:85–92.
Sturmey P. Behavioral activation is an evidence-based treatment for depression. Behav Modif 2009;33:818–29. https://doi.org/10.1177/0145445509350094
Sturmey P. Treatment of psychopathology in people with intellectual and other disabilities. Can J Psychiatry 2012;57:593–600. https://doi.org/10.1177/070674371205701003
Sukhodolsky DG. Cognitive–Behavioral Treatment Programs for Anger-related Problems in Children and Adolescents: A Meta-analytic Study. PhD thesis. Hempstead, NY: Hofstra University; 1998.
Sukhodolsky DG, Kassinove H, Gorman BS. Cognitive–behavioral therapy for anger in children and adolescents: a meta-analysis. Aggress Violent Behav 2004;9:247–69.
Sun M, Rith-Najarian LR, Williamson TJ, Chorpita BF. Treatment features associated with youth cognitive behavioral therapy follow-up effects for internalizing disorders: a meta-analysis. J Clin Child Adolesc Psychol 2018;00:1–15.
Sussman S, Sun P, Dent CW. A meta-analysis of teen cigarette smoking cessation. Health Psychol 2006;25:549–57.
Sveinsdottir V, Eriksen HR, Reme SE. Assessing the role of cognitive behavioral therapy in the management of chronic nonspecific back pain. J Pain Res 2012;5:371–80. https://doi.org/10.2147/JPR.S25330
Syka A. Depression in pregnancy and ways of dealing. Int J Caring Sci 2015;8:231–7.
Szentagotai A, David D. The efficacy of cognitive–behavioral therapy in bipolar disorder: a quantitative meta-analysis. J Clin Psychiatry 2010;71:66–72. https://doi.org/10.4088/JCP.08r04559yel
Taddio A, Appleton M, Bortolussi B, Chambers C, Dubey V, Halperin S, et al. Reducing the pain of childhood immunization – an evidence-based clinical practice guideline. Can J Infect Dis Med Microbiol 2010;21:179.
Tavares H, Lobo DS, Fuentes D, Black DW. [Compulsive buying disorder: a review and a case vignette.] Braz J Psychiatry 2008;30(Suppl. 1):16–23.
Tavares H, Zilberman ML, El-Guebaly N. Are there cognitive and behavioural approaches specific to the treatment of pathological gambling? Can J Psychiatry 2003;48:22–7.
Taylor S. Meta-analysis of cognitive–behavioral treatments for social phobia. J Behav Ther Exp Psychiatry 1996;27:1–9.
Taylor S, Asmundson GJG, Coons MJ. Current directions in the treatment of hypochondriasis. J Cogn Psychother 2005;19:285–304.
Thapar A, Collishaw S, Potter R, Thapar AK. Managing and preventing depression in adolescents. BMJ 2010;340:c209. https://doi.org/10.1136/bmj.c209
Thorpe CT, Fahey LE, Johnson H, Deshpande M, Thorpe JM, Fisher EB. Facilitating healthy coping in patients with diabetes: a systematic review. Diabetes Educ 2013;39:33–52. https://doi.org/10.1177/0145721712464400
Tiffin PA, Welsh P. Practitioner review: schizophrenia spectrum disorders and the at-risk mental state for psychosis in children and adolescents – evidence-based management approaches. J Child Psychol Psychiatry 2013;54:1155–75. https://doi.org/10.1111/jcpp.12136
Tolin DF. Beating a dead dodo bird: looking at signal vs. noise in cognitive–behavioral therapy for anxiety disorders. Clin Psychol Sci Pract 2014;21:351–62.
Tsiros MD, Sinn N, Coates AM, Howe PR, Buckley JD. Treatment of adolescent overweight and obesity. Eur J Pediatr 2008;167:9–16. https://doi.org/10.1007/s00431-007-0575-z
Turchiano TP. A Meta-analysis of Behavioral and Cognitive Therapies for Children and Adolescents with Attention Deficit Hyperactivity and/or Impulsivity Disorders. PhD thesis. Hempstead, NY: Hofstra University; 1999.
Turner JA. Educational and behavioral interventions for back pain in primary care. Spine 1996;21:2851–7. https://doi.org/10.1097/00007632-199612150-00010
Twohig MP, Levin ME. Acceptance and commitment therapy as a treatment for anxiety and depression: a review. Psychiatr Clin North Am 2017;40:751–70.
Ulloa-Flores RE, de la Pena-Olvera F, Nogaies-lmoca I. The multimodal treatment for children and adolescents with depression. Salud Ment 2011;34:403–7.
Usher AM, Stewart LA. Effectiveness of correctional programs with ethnically diverse offenders: a meta-analytic study. Int J Offender Ther Comp Criminol 2014;58:209–30. https://doi.org/10.1177/0306624X12469507
van der Feltz-Cornelis CM, Hoedeman R, Keuter EJ, Swinkels JA. Presentation of the multidisciplinary guideline medically unexplained physical symptoms (MUPS) and somatoform disorder in the Netherlands: disease management according to risk profiles. J Psychosom Res 2012;72:168–9. https://doi.org/10.1016/j.jpsychores.2011.11.007
van der Klink JJ, Blonk RW, Schene AH, van Dijk FJ. The benefits of interventions for work-related stress. Am J Public Health 2001;91:270–6.
van Emmerik AA, Reijntjes A, Kamphuis JH. Writing therapy for posttraumatic stress: a meta-analysis. Psychother Psychosom 2013;82:82–8. https://doi.org/10.1159/000343131
van Ingen DJ, Freiheit SR, Vye CS. From the lab to the clinic: effectiveness of cognitive–behavioral treatments for anxiety disorders. Prof Psychol Res Pract 2009;40:69–74.
Vanderploeg RD, Belanger HG, Curtiss G, Bowles AO, Cooper DB. Reconceptualizing rehabilitation of individuals with chronic symptoms following mild traumatic brain injury. Rehabil Psychol 2019;64:1–12. https://doi.org/10.1037/rep0000255
Vasa RA. Assessment and management of anxiety in youth with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2016;55:S98.
Vieta E, Colom F. Psychological interventions in bipolar disorder: from wishful thinking to an evidence-based approach. Acta Psychiatr Scand Suppl 2004;422:34–8. https://doi.org/10.1111/j.1600-0447.2004.00411.x
Vieta E, Pacchiarotti I, Scott J, Sánchez-Moreno J, Di Marzo S, Colom F. Evidence-based research on the efficacy of psychologic interventions in bipolar disorders: a critical review. Curr Psychiatry Rep 2005;7:449–55. https://doi.org/10.1007/s11920-005-0066-8
Vigil TM, Orellana AF, Garcia RR, Correa MD. Cognitive behavioral therapy and negative symptoms in schizophrenia. Salud Ment 2015;38:371–7.
Vitoula K, Venneri A, Varrassi G, Paladini A, Sykioti P, Adewusi J, Zis P. Behavioral therapy approaches for the management of low back pain: an up-to-date systematic review. Pain Ther 2018;7:1–12. https://doi.org/10.1007/s40122-018-0099-4
Vittengl JR, Jarrett RB, Weitz E, Hollon SD, Twisk J, Cristea I, et al. Divergent outcomes in cognitive–behavioral therapy and pharmacotherapy for adult depression. Am J Psychiatry 2016;173:481–90. https://doi.org/10.1176/appi.ajp.2015.15040492
Volavka J. Violence in schizophrenia and bipolar disorder. Psychiatr Danub 2013;25:24–33.
Wagner JJ. A Meta-analysis/Literature Review Comparing the Effectiveness of SSRI Antidepressants, Cognitive Behavioral Therapy, and Placebo for the Treatment of Depression. PhD thesis. San Juan: Carlos Albizu University; 2005.
Waldron HB, Kaminer Y. On the learning curve: the emerging evidence supporting cognitive–behavioral therapies for adolescent substance abuse. Addiction 2004;99(Suppl. 2):93–105.
Walitza S, Melfsen S, Jans T, Zellmann H, Wewetzer C, Warnke A. Obsessive–compulsive disorder in children and adolescents. Dtsch Arztebl Int 2011;108:173–9. https://doi.org/10.3238/arztebl.2011.0173
Walther L, Gantner A, Heinz A, Majić T. Evidence-based treatment options in cannabis dependency. Dtsch Arztebl Int 2016;113:653–9. https://doi.org/10.3238/arztebl.2016.0653
Wang X, Huang S, Qi HB. Comparative efficacy and acceptability of seven augmentation agents for treatment-resistant depression: a multiple-treatments meta-analysis. South Afr J Psychiatry 2014;20:71–6.
Wellen DG. A Meta-analysis of Single-subject Studies of Therapies for Children and Adolescents with Aggression. PhD thesis. New York, NY: St John’s University; 1998.
Wellington J. Noninvasive and alternative management of chronic low back pain (efficacy and outcomes). Neuromodulation 2014;17(Suppl. 2):24–30. https://doi.org/10.1111/ner.12078
Whitbread J, McGown A. The treatment of bulimia nervosa: what is effective? A meta-analysis. Indian J Clin Psychol 1994;21:32–44.
Wiers RW, Boffo M, Field M. What’s in a trial? On the importance of distinguishing between experimental lab studies and randomized controlled trials: the case of cognitive bias modification and alcohol use disorders. J Stud Alcohol Drugs 2018;79:333–43.
Wijeratne C, Sachdev P. Treatment-resistant depression: critique of current approaches. Aust N Z J Psychiatry 2008;42:751–62. https://doi.org/10.1080/00048670802277206
Wild D, von Maltzahn R, Brohan E, Christensen T, Clauson P, Gonder-Frederick L. A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns 2007;68:10–15.
Wilks CR, Zieve GG, Lessing HK. Are trials of computerized therapy generalizable? A multidimensional meta-analysis. Telemed J E Health 2016;22:450–7.
Williams CR. Long-term Effectiveness of CBT for Anxiety Disorders: A Meta-analytic Review. PhD thesis. West Hartford, CT: University of Hartford; 2016.
Williams NH. Optimising the psychological benefits of osteopathy. Int J Osteopath Med 2007;10:36–41.
Wilson IR. Management of chronic pain through pain management programmes. Br Med Bull 2017;124:55–64. https://doi.org/10.1093/bmb/ldx032
Wurz A, Sungur MZ. Combining cognitive behavioural therapy and pharmacotherapy in the treatment of anxiety disorders: true gains or false hopes? Klin Psikofarmakol Bulteni 2009;19:436–47.
Zaider TI, Heimberg RG. Non-pharmacologic treatments for social anxiety disorder. Acta Psychiatr Scand Suppl 2003;417:72–84.
Zaretsky AE, Rizvi S, Parikh SV. How well do psychosocial interventions work in bipolar disorder? Can J Psychiatry 2007;52:14–21.
Zenner HP, Delb W, Kröner-Herwig B, Jäger B, Peroz I, Hesse G, et al. A multidisciplinary systematic review of the treatment for chronic idiopathic tinnitus. Eur Arch Otorhinolaryngol 2017;274:2079–91. https://doi.org/10.1007/s00405-016-4401-y
Zinzow HM, Jeffirs SM. Driving aggression and anxiety: intersections, assessment, and interventions. J Clin Psychol 2018;74:43–82. https://doi.org/10.1002/jclp.22494
Studies excluded because cognitive–behavioural therapy only data could not be extracted (n = 470)
Aguilera M. Post-surgery support and the long-term success of bariatric surgery. Pract Nurs 2014;25:455–9.
Alanazi MH, Parent EC, Dennett E. Effect of stabilization exercise on back pain, disability and quality of life in adult with scoliosis: a systematic review. Eur J Phys Rehabil Med 2017;16:16.
Albert U, Marazziti D, Di Salvo G, Solia F, Rosso G, Maina G. A systematic review of evidence-based treatment strategies for obsessive–compulsive disorder resistant to first-line pharmacotherapy. Curr Med Chem 2017;22:22.
Alcântara-Silva TR, Freitas-Junior R, Freitas NM, Machado GD. Fatigue related to radiotherapy for breast and/or gynaecological cancer: a systematic review. J Clin Nurs 2013;22:2679–86. https://doi.org/10.1111/jocn.12236
Alessi C, Vitiello MV. Insomnia (primary) in older people: non-drug treatments. BMJ Clin Evid 2015;2015:2302.
Alosaimi FD, Baker B. Clinical review of treatment options for major depressive disorder in patients with coronary heart disease. Saudi Med J 2012;33:1159–68.
Altena AM, Brilleslijper-Kater SN, Wolf JL. Effective interventions for homeless youth: a systematic review. Am J Prev Med 2010;38:637–45. https://doi.org/10.1016/j.amepre.2010.02.017
Álvarez-Jiménez M, Alcazar-Corcoles MA, González-Blanch C, Bendall S, McGorry PD, Gleeson JF. Online, social media and mobile technologies for psychosis treatment: a systematic review on novel user-led interventions. Schizophr Res 2014;156:96–106. https://doi.org/10.1016/j.schres.2014.03.021
Anderson RU, Wise D, Nathanson BH. Chronic prostatitis and/or chronic pelvic pain as a psychoneuromuscular disorder – a meta-analysis. Urology 2018;120:23–9.
Ángel García D, Martínez Nicolás I, Saturno Hernández PJ. [Clinical approach to fibromyalgia: synthesis of evidence-based recommendations, a systematic review.] Reumatol Clin 2016;12:65–71. https://doi.org/10.1016/j.reuma.2015.06.001
Arias E, Arce R, Vilarino M. Batterer intervention programmes: a meta-analytic review of effectiveness. Psychosoc Interv 2013;22:153–60.
Aricò D, Raggi A, Ferri R. Cognitive behavioral therapy for insomnia in breast cancer survivors: a review of the literature. Front Psychol 2016;7:1162. https://doi.org/10.3389/fpsyg.2016.01162
Armelius BA, Andreassen TH. Cognitive–behavioral treatment for antisocial behavior in youth in residential treatment. Cochrane Database Syst Rev 2007;4:CD005650. https://doi.org/10.1002/14651858.CD005650.pub2
A-Tjak JG, Davis ML, Morina N, Powers MB, Smits JA, Emmelkamp PM. A meta-analysis of the efficacy of acceptance and commitment therapy for clinically relevant mental and physical health problems. Psychother Psychosom 2015;84:30–6. https://doi.org/10.1159/000365764
Auty KM, Cope A, Liebling A. Psychoeducational programs for reducing prison violence: a systematic review. Aggress Violent Behav 2017;33:126–43.
Badawy SM, Cronin RM, Hankins J, Crosby L, DeBaun M, Thompson AA, Shah N. Patient-centered eHealth interventions for children, adolescents, and adults with sickle cell disease: systematic review. J Med Internet Res 2018;20:e10940. https://doi.org/10.2196/10940
Baez S, Hoch MC, Hoch JM. Evaluation of cognitive behavioral interventions and psychoeducation implemented by rehabilitation specialists to treat fear-avoidance beliefs in patients with low back pain: a systematic review. Arch Phys Med Rehabil 2017;14:14.
Baez S, Hoch MC, Hoch JM. Evaluation of cognitive behavioral interventions and psychoeducation implemented by rehabilitation specialists to treat fear-avoidance beliefs in patients with low back pain: a systematic review. Arch Phys Med Rehabil 2018;99:2287–98.
Bahrami HR, Hamedi S, Salari R, Noras M. Herbal medicines for the management of irritable bowel syndrome: a systematic review. Electron Physician 2016;8:2719–25. https://doi.org/10.19082/2719
Baird E, Williams ACC, Hearn L, Amris K. Interventions for treating persistent pain in survivors of torture. Cochrane Database Syst Rev 2017;8:CD012051. https://doi.org/10.1002/14651858.CD012051.pub2
Baker AL, Hiles SA, Thornton LK, Hides L, Lubman DI. A systematic review of psychological interventions for excessive alcohol consumption among people with psychotic disorders Acta Psychiatri Scand 2012;126:243–55.
Baker GA, Brooks JL, Goodfellow L, Bodde N, Aldenkamp A. Treatments for non-epileptic attack disorder. Cochrane Database Syst Rev 2007;2:CD006370.
Baliousis M, Rennoldson M, Snowden JA. Psychological interventions for distress in adults undergoing haematopoietic stem cell transplantation: a systematic review with meta-analysis. Psycho-Oncology 2016;25:400–11. https://doi.org/10.1002/pon.3925
Barlow J, Bennett C, Midgley N, Larkin SK, Wei Y. Parent-infant psychotherapy for improving parental and infant mental health. Cochrane Database Syst Rev 2015;1:CD010534. https://doi.org/10.1002/14651858.CD010534.pub2
Barnason S, Zimmerman L, Young L. An integrative review of interventions promoting self-care of patients with heart failure. J Clin Nurs 2012;21:448–75. https://doi.org/10.1111/j.1365-2702.2011.03907.x
Barnes TN, Smith SW, Miller MD. School-based cognitive–behavioral interventions in the treatment of aggression in the United States: a meta-analysis. Aggress Violent Behav 2014;19:311–21.
Barrett S, Begg S, O’Halloran P, Kingsley M. Integrated motivational interviewing and cognitive behaviour therapy for lifestyle mediators of overweight and obesity in community-dwelling adults: a systematic review and meta-analyses. BMC Public Health 2018;18:1160. https://doi.org/10.1186/s12889-018-6062-9
Beumont P, Hay P, Beumont D, Birmingham L, Derham H, Jordan A, et al. Australian and New Zealand clinical practice guidelines for the treatment of anorexia nervosa. Aust N Z J Psychiatry 2004;38:659–70. https://doi.org/10.1080/j.1440-1614.2004.01449.x
Belleville G, Cousineau H, Levrier K, St-Pierre-Delorme ME. Meta-analytic review of the impact of cognitive–behavior therapy for insomnia on concomitant anxiety Clin Psychol Rev 2011;31:638–52.
Bennett C, Barlow J, Huband N, Smailagic N, Roloff V. Group-based parenting programs for improving parenting and psychosocial functioning: a systematic review. J Soc Soc Work Res 2013;4:300–32.
Beynon S, Soares-Weiser K, Woolacott N, Duffy S, Geddes JR. Psychosocial interventions for the prevention of relapse in bipolar disorder: systematic review of controlled trials. Br J Psychiatry 2008;192:5–11. https://doi.org/10.1192/bjp.bp.107.037887
Binford MC, Kahana SY, Altice FL. A systematic review of antiretroviral adherence interventions for HIV-infected people who use drugs. Curr HIV/AIDS Rep 2012;9:287–312. https://doi.org/10.1007/s11904-012-0134-8
Binks CA, Fenton M, McCarthy L, Lee T, Adams CE, Duggan C. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev 2006;1:CD005652. https://doi.org/10.1002/14651858.CD005652
Bjornstad GJ, Montgomery P. Family therapy for attention-deficit disorder or attention-deficit/hyperactivity disorder in children and adolescents. Cochrane Database Syst Rev 2005;18:CD005042.
Blanchet C, Mathieu MÈ, St-Laurent A, Fecteau S, St-Amour N, Drapeau V. A systematic review of physical activity interventions in individuals with binge eating disorders. Curr Obes Rep 2018;7:76–88. https://doi.org/10.1007/s13679-018-0295-x
Bodryzlova Y, Audet JS, Bergeron K, O’Connor K. Group cognitive–behavioural therapy for hoarding disorder: systematic review and meta-analysis. Health Soc Care Community 2018;23:23.
Boniface S, Malet-Lambert I, Coleman R, Deluca P, Donoghue K, Drummond C, Khadjesari Z. The effect of brief interventions for alcohol among people with comorbid mental health conditions: a systematic review of randomized trials and narrative synthesis. Alcohol Alcohol 2018;53:282–93. https://doi.org/10.1093/alcalc/agx111
Borsay C. Anger management interventions for adults with learning disabilities living in the community: a review of recent (2000–2010) evidence. Br J Learn Disabil 2013;41:38–44.
Bougea A, Darviri C, Alexopoulos EC. A systematic review of randomized controlled interventions for parents’ distress in pediatric leukemia. ISRN Oncol 2011;2011:959247. https://doi.org/10.5402/2011/959247
Bowker E, Dorstyn D. Hypnotherapy for disability-related pain: a meta-analysis. J Health Psychol 2016;21:526–39. https://doi.org/10.1177/1359105314530452
Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry 2005;162:214–27.
Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, Paisley S. Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation. Health Technol Assess 2006;10(35). https://doi.org/10.3310/hta10350
Brettle A, Hill A, Jenkins P. Counselling in primary care: a systematic review of the evidence. Counsel Psychother Res 2008;8:207–14.
Brewer M, Melnyk BM. Effective coping/mental health interventions for critically ill adolescents: an evidence review. Pediatr Nurs 2007;33:361–7, 373.
Brok EC, Lok P, Oosterbaan DB, Schene AH, Tendolkar I, van Eijndhoven PF. Infant-related intrusive thoughts of harm in the postpartum period: a critical review. J Clin Psychiatry 2017;78:e913–e923. https://doi.org/10.4088/JCP.16r11083
Baker GA, Brooks JL, Goodfellow L, Bodde N, Aldenkamp A. Treatments for non-epileptic attack disorder. Cochrane Database Syst Rev 2007;1:CD006370. https://doi.org/10.1002/14651858.CD006370
Brown JL, Vanable PA. Cognitive-behavioral stress management interventions for persons living with HIV: a review and critique of the literature. Ann Behav Med 2008;35:26–40. https://doi.org/10.1007/s12160-007-9010-y
Brox JI, Storheim K, Grotle M, Tveito TH, Indahl A, Eriksen HR. Systematic review of back schools, brief education, and fear-avoidance training for chronic low back pain. Spine J 2008;8:948–58.
Brunner E, De Herdt A, Minguet P, Baldew SS, Probst M. Can cognitive behavioural therapy based strategies be integrated into physiotherapy for the prevention of chronic low back pain? A systematic review. Disabil Rehabil 2013;35:1–10. https://doi.org/10.3109/09638288.2012.683848
Buchanan JA, Zakrzewska JM. Burning mouth syndrome. BMJ Clin Evid 2010;2010:1301.
Busse JW, Montori VM, Krasnik C, Patelis-Siotis I, Guyatt GH. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychother Psychosom 2009;78:6–15. https://doi.org/10.1159/000162296
Butler R, Radhakrishnan R. Dementia. BMJ Clin Evid 2012;2012:1001.
Cameron LC. Treatment of Bulimia Nervosa: A Meta-analysis. PhD thesis. Oxford, MS: The University of Mississippi; 1999.
Cape J, Barker C, Buszewicz M, Pistrang N. General practitioner psychological management of common emotional problems (I): Definitions and literature review. Br J Gen Pract 2000;50:313–18.
Carson KV, Brinn MP, Peters M, Veale A, Esterman AJ, Smith BJ. Interventions for smoking cessation in Indigenous populations. Cochrane Database Syst Rev 2012;1:CD009046. https://doi.org/10.1002/14651858.CD009046.pub2
Carville SF, Arendt-Nielsen L, Arendt-Nielsen S, Bliddal H, Blotman F, Branco JC, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis 2008;67:536–41.
Casement MD, Swanson LM. A meta-analysis of imagery rehearsal for post-trauma nightmares: effects on nightmare frequency, sleep quality, and posttraumatic stress. Clin Psychol Rev 2012;32:566–74. https://doi.org/10.1016/j.cpr.2012.06.002
Castelnuovo G, Giusti EM, Manzoni GM, Saviola D, Gatti A, Gabrielli S, et al. Psychological treatments and psychotherapies in the neurorehabilitation of pain: evidences and recommendations from the Italian Consensus Conference on Pain in Neurorehabilitation. Front Psychol 2016;7:115. https://doi.org/10.3389/fpsyg.2016.00115
Castillo-Bueno MD, Moreno-Pina JP, Martínez-Puente MV, Artiles-Suárez MM, Company-Sancho MC, García-Andrés MC, et al. Effectiveness of nursing intervention for adult patients experiencing chronic pain: a systematic review. JBI Libr Syst Rev 2010;8:1112–68.
Cattelani R, Zettin M, Zoccolotti P. Rehabilitation treatments for adults with behavioral and psychosocial disorders following acquired brain injury: a systematic review. Neuropsychol Rev 2010;20:52–85. https://doi.org/10.1007/s11065-009-9125-y
Chalfoun C, Karelis AD, Stip E, Abdel-Baki A. Running for your life: a review of physical activity and cardiovascular disease risk reduction in individuals with schizophrenia. J Sports Sci 2016;34:1500–15. https://doi.org/10.1080/02640414.2015.1119875
Challet-Bouju G, Bruneau M, Victorri-Vigneau C, Grall-Bronnec M, IGNACE Group. Cognitive remediation interventions for gambling disorder: a systematic review. Front Psychol 2017;8:1961. https://doi.org/10.3389/fpsyg.2017.01961
Chambers SK, Pinnock C, Lepore SJ, Hughes S, O’Connell DL. A systematic review of psychosocial interventions for men with prostate cancer and their partners. Patient Educ Couns 2011;85:e75–88. https://doi.org/10.1016/j.pec.2011.01.027
Chan E, Fogler JM, Hammerness PG. Treatment of attention-deficit/hyperactivity disorder in adolescents: a systematic review. JAMA 2016;315:1997–2008. https://doi.org/10.1001/jama.2016.5453
Chatterton ML, Stockings E, Berk M, Barendregt JJ, Carter R, Mihalopoulos C. Psychosocial therapies for the adjunctive treatment of bipolar disorder in adults: network meta-analysis. Br J Psychiatry 2017;210:333–41. https://doi.org/10.1192/bjp.bp.116.195321
Chen I, Opiyo N, Tavender E, Mortazhejri S, Rader T, Petkovic J, et al. Non-clinical interventions for reducing unnecessary caesarean section. Cochrane Database Syst Rev 2018;9:CD005528. https://doi.org/10.1002/14651858.CD005528.pub3
Cheney G. Psychology in Schools: Understanding and Intervening within Vulnerable Populations. PhD thesis. Kingston upon Hull: University of Hull; 2011.
Chew HSJ, Cheng HY, Chair SY. The suitability of motivational interviewing versus cognitive behavioural interventions on improving self-care in patients with heart failure: a literature review and discussion paper. Appl Nurs Res 2019;45:17–22.
Chi NC, Demiris G, Lewis FM, Walker AJ, Langer SL. Behavioral and educational interventions to support family caregivers in end-of-life care: a systematic review. Am J Hosp Palliat Care 2016;33:894–908.
Clare L, Teale JC, Toms G, Kudlicka A, Evans I, Abrahams S, et al. Cognitive rehabilitation, self-management, psychotherapeutic and caregiver support interventions in progressive neurodegenerative conditions: a scoping review. Neuro Rehabilitation 2018;43:443–71. https://doi.org/10.3233/NRE-172353
Clough BA, March S, Chan RJ, Casey LM, Phillips R, Ireland MJ. Psychosocial interventions for managing occupational stress and burnout among medical doctors: a systematic review. Syst Rev 2017;6:144. https://doi.org/10.1186/s13643-017-0526-3
Clucas C, Sibley E, Harding R, Liu L, Catalan J, Sherr L. A systematic review of interventions for anxiety in people with HIV. Psychol Health Med 2011;16:528–47. https://doi.org/10.1080/13548506.2011.579989
Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2012;9:CD010078. https://doi.org/10.1002/14651858.CD010078
Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2015;12:CD010078. https://doi.org/10.1002/14651858.CD010078.pub2
Collatz A, Johnston SC, Staines DR, Marshall-Gradisnik SM. A systematic review of drug therapies for chronic fatigue syndrome/myalgic encephalomyelitis. Clin Ther 2016;38:1263–71.e9.
Conway A, Schadewaldt V, Clark R, Ski CF, Thompson DR, Kynoch K, et al. The effectiveness of non-pharmacologic interventions in improving psychological outcomes for heart transplant recipients: a systematic review. JBI Database Syst Rev Implement Rep 2013;11:112–32.
Cooney P, Tunney C, O’Reilly G. A systematic review of the evidence regarding cognitive therapy skills that assist cognitive behavioural therapy in adults who have an intellectual disability. J Appl Res Intellect Disabil 2018;31:23–42. https://doi.org/10.1111/jar.12365
Couch R, Jetha M, Dryden DM, Hooten N, Liang Y, Durec T, et al. Diabetes education for children with type 1 diabetes mellitus and their families. Evid Rep Technol Assess 2008;166:1–144.
Coughtrey A, Millington A, Bennett S, Christie D, Hough R, Su MT, et al. The effectiveness of psychosocial interventions for psychological outcomes in pediatric oncology: a systematic review. J Pain Symptom Manage 2018;55:1004–17.
Covin R, Ouimet AJ, Seeds PM, Dozois DJ. A meta-analysis of CBT for pathological worry among clients with GAD. J Anxiety Disord 2008;22:108–16.
Cramer H, Lauche R, Paul A, Langhorst J, Kümmel S, Dobos GJ. Hypnosis in breast cancer care: a systematic review of randomized controlled trials. Integr Cancer Ther 2015;14:5–15. https://doi.org/10.1177/1534735414550035
Cuijpers P, Andersson G, Donker T, van Straten A. Psychological treatment of depression: results of a series of meta-analyses. Nord J Psychiatry 2011;65:354–64. https://doi.org/10.3109/08039488.2011.596570
Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson KS. A meta-analysis of cognitive–behavioural therapy for adult depression, alone and in comparison with other treatments. Can J Psychiatry 2013;58:376–85. https://doi.org/10.1177/070674371305800702
Cuijpers P, Cristea IA, Weitz E, Gentili C, Berking M. The effects of cognitive and behavioural therapies for anxiety disorders on depression: a meta-analysis. Psychol Med 2016;46:3451–62.
Cuijpers P, Donker T, Weissman MM, Ravitz P, Cristea IA. Interpersonal psychotherapy for mental health problems: a comprehensive meta-analysis. Am J Psychiatry 2016;173:680–7. https://doi.org/10.1176/appi.ajp.2015.15091141
Cuijpers P, Weitz E, Lamers F, Penninx BW, Twisk J, DeRubeis RJ, et al. Melancholic and atypical depression as predictor and moderator of outcome in cognitive behavior therapy and pharmacotherapy for adult depression. Depress Anxiety 2017;34:246–56. https://doi.org/10.1002/da.22580
Cullen KL, Irvin E, Collie A, Clay F, Gensby U, Jennings PA, et al. Effectiveness of workplace interventions in return-to-work for musculoskeletal, pain-related and mental health conditions: an update of the evidence and messages for practitioners. J Occup Rehabil 2018;28:1–15. https://doi.org/10.1007/s10926-016-9690-x
Cusack K, Jonas DE, Forneris CA, Wines C, Sonis J, Middleton JC, et al. Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin Psychol Rev 2016;43:128–41. https://doi.org/10.1016/j.cpr.2015.10.003
Cusimano MD, Nastis S, Zuccaro L. Effectiveness of interventions to reduce aggression and injuries among ice hockey players: a systematic review. CMAJ 2013;185:E57–69. https://doi.org/10.1503/cmaj.112017
Cwikel JG, Behar LC. Social work with adult cancer patients: a vote-count review of intervention research. Soc Work Health Care 1999;29:39–67. https://doi.org/10.1300/J010v29n02_03
Daigle MS, Pouliot L, Chagnon F, Greenfield B, Mishara B. Suicide attempts: prevention of repetition. Can J Psychiatry 2011;56:621–9.
Dainty AD. Irritable bowel syndrome: psychological comorbidities and cognitive behavioural therapy. A review of the literature. Gastrointest Nurs 2012;10:44–50.
Damen L, Bruijn J, Koes BW, Berger MY, Passchier J, Verhagen AP. Prophylactic treatment of migraine in children. Part 1. A systematic review of non-pharmacological trials. Cephalalgia 2006;26:373–83.
Damgaard P, Bartels EM, Ris I, Christensen R, Juul-Kristensen B. Evidence of physiotherapy interventions for patients with chronic neck pain: a systematic review of randomised controlled trials. ISRN Pain 2013;2013:567175. https://doi.org/10.1155/2013/567175
David D, Cotet C, Matu S, Mogoase C, Stefan S. 50 years of rational-emotive and cognitive–behavioral therapy: a systematic review and meta-analysis. J Clin Psychol 2018;74:304–18. https://doi.org/10.1002/jclp.22514
Davis AM, MacKay C. Osteoarthritis year in review: outcome of rehabilitation. Osteoarthr Cartil 2013;21:1414–24. https://doi.org/10.1016/j.joca.2013.08.013
Davis ML, Powers MB, Handelsman P, Medina JL, Zvolensky M, Smits JA. Behavioral therapies for treatment-seeking cannabis users: a meta-analysis of randomized controlled trials. Eval Health Prof 2015;38:94–114. https://doi.org/10.1177/0163278714529970
D’Egidio V, Sestili C, Mancino M, Sciarra I, Cocchiara R, Backhaus I, et al. Counseling interventions delivered in women with breast cancer to improve health-related quality of life: a systematic review. Qual Life Res 2017;26:2573–92. https://doi.org/10.1007/s11136-017-1613-6
Devine EC. Meta-analysis of the effect of psychoeducational interventions on pain in adults with cancer. Oncol Nurs Forum 2003;30:75–89. https://doi.org/10.1188/03.ONF.75-89
Dirmaier J, Steinmann M, Krattenmacher T, Watzke B, Barghaan D, Koch U, Schulz H. Non-pharmacological treatment of depressive disorders: a review of evidence-based treatment options. Rev Recent Clin Trials 2012;7:141–9.
Dixon S, Dantas JA. Best practice for community-based management of postnatal depression in developing countries: a systematic review. Health Care Women Int 2017;38:118–43. https://doi.org/10.1080/07399332.2016.1255213
Dölemeyer R, Tietjen A, Kersting A, Wagner B. Internet-based interventions for eating disorders in adults: a systematic review. BMC Psychiatry 2013;13:207. https://doi.org/10.1186/1471-244X-13-207
Dolle K, Schulte-Körne G. The treatment of depressive disorders in children and adolescents. Dtsch Arztebl Int 2013;110:854–60. https://doi.org/10.3238/arztebl.2013.0854
Dorstyn D, Mathias J, Denson L. Efficacy of cognitive behavior therapy for the management of psychological outcomes following spinal cord injury: a meta-analysis. J Health Psychol 2011;16:374–91. https://doi.org/10.1177/1359105310379063
Dossa NI, Hatem M. Cognitive-behavioral therapy versus other PTSD psychotherapies as treatment for women victims of war-related violence: a systematic review. Scientific World J 2012;2012:181847. https://doi.org/10.1100/2012/181847
Dubicka B, Elvins R, Roberts C, Chick G, Wilkinson P, Goodyer IM. Combined treatment with cognitive–behavioural therapy in adolescent depression: meta-analysis. Br J Psychiatry 2010;197:433–40. https://doi.org/10.1192/bjp.bp.109.075853
Duncan LR, Pearson ES, Maddison R. Smoking prevention in children and adolescents: a systematic review of individualized interventions. Patient Educ Couns 2018;101:375–88.
DuPaul GJ, Eckert TL, Vilardo B. The effects of school-based interventions for attention deficit hyperactivity disorder: a meta-analysis 1996–2010. School Psychol Rev 2012;41:387–412.
Eilender P, Ketchen B, Maremmani I, Saenger M, Fareed A. Treatment approaches for patients with opioid use disorder and chronic noncancer pain: a literature review. Addict Disord Treat 2016;15:85–98.
Eisenberg DM, Delbanco TL, Berkey CS, Kaptchuk TJ, Kupelnick B, Kuhl J, et al. Cognitive behavioral techniques for hypertension: are they effective? Ann Intern Med 1993;118:964–72.
Elderton AJ. Posttraumatic Growth in Survivors of Interpersonal Violence in Additional. PhD thesis. Oxford: University of Oxford; 2013.
El-Serag HB, Olden K, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther 2002;16:1171–85.
Enck P, Junne F, Klosterhalfen S, Zipfel S, Martens U. Therapy options in irritable bowel syndrome. Eur J Gastroenterol Hepatol 2010;22:1402–11.
Engels GI, Vermey M. Efficacy of nonmedical treatments of depression in elders: a quantitative analysis. J Clin Geropsychol 1997;3:17–35.
Everitt H, Baldwin DS, Stuart B, Lipinska G, Mayers A, Malizia AL, et al. Antidepressants for insomnia in adults. Cochrane Database Syst Rev 2018;14:CD010753.
Fann JR, Hart T, Schomer KG. Treatment for depression after traumatic brain injury: a systematic review. J Neurotrauma 2009;26:2383–402. https://doi.org/10.1089/neu.2009.1091
Farooq S, Sherin A. Interventions for psychotic symptoms concomitant with epilepsy. Cochrane Database Syst Rev 2008;4:CD006118. https://doi.org/10.1002/14651858.CD006118.pub2
Farooq S, Sherin A. Interventions for psychotic symptoms concomitant with epilepsy. Cochrane Database Syst Rev 2015;12:CD006118. https://doi.org/10.1002/14651858.CD006118.pub3
Faulkner G, Cohn T, Remington G. Interventions to reduce weight gain in schizophrenia. Cochrane Database Syst Rev 2007;1:CD005148. https://doi.org/10.1002/14651858.CD005148.pub2
Fernandez E, Malvaso C, Day A, Guharajan D. 21st century cognitive behavioural therapy for anger: a systematic review of research design, methodology and outcome. Behav Cogn Psychother 2018;46:385–404. https://doi.org/10.1017/S1352465818000048
Feske U, Chambless DL. Cognitive behavioral versus exposure only treatment for social phobia: a meta-analysis. Behav Ther 1995;26:695–720.
Field T. Postnatal anxiety prevalence, predictors and effects on development: a narrative review. Infant Behav Dev 2018;51:24–32.
Fontenelle LF, Coutinho ES, Lins-Martins NM, Fitzgerald PB, Fujiwara H, Yücel M. Electroconvulsive therapy for obsessive-compulsive disorder: a systematic review. J Clin Psychiatry 2015;76:949–57. https://doi.org/10.4088/JCP.14r09129
Forbes D, Creamer M, Phelps A, Bryant R, McFarlane A, Devilly GJ, et al. Australian guidelines for the treatment of adults with acute stress disorder and post-traumatic stress disorder. Aust N Z J Psychiatry 2007;41:637–48.
Forman-Hoffman VL, Zolotor AJ, McKeeman JL, Blanco R, Knauer SR, Lloyd SW, et al. Comparative effectiveness of interventions for children exposed to nonrelational traumatic events. Pediatrics 2013;131:526–39. https://doi.org/10.1542/peds.2012-3846
Foster ER, Bedekar M, Tickle-Degnen L. Systematic review of the effectiveness of occupational therapy-related interventions for people with Parkinson’s disease. Am J Occup Ther 2014;68:39–49. https://doi.org/10.5014/ajot.2014.008706
Frazer CJ, Christensen H, Griffiths KM. Effectiveness of treatments for depression in older people. Med J Aust 2005;182:627–32.
Fréchette-Simard C, Plante I, Bluteau J. Strategies included in cognitive behavioral therapy programs to treat internalized disorders: a systematic review. Cogn Behav Ther 2018;47:263–85. https://doi.org/10.1080/16506073.2017.1388275
Freire RC, Zugliani MM, Garcia RF, Nardi AE. Treatment-resistant panic disorder: a systematic review. Expert Opin Pharmacother 2016;17:159–68. https://doi.org/10.1517/14656566.2016.1109628
Freudenstein U, Jagger C, Arthur A, Donner-Banzhoff N. Treatments for late life depression in primary care – a systematic review. Fam Pract 2001;18:321–7. https://doi.org/10.1093/fampra/18.3.321
Fricton JR, Ouyang W, Nixdorf DR, Schiffman EL, Velly AM, Look JO. Critical appraisal of methods used in randomized controlled trials of treatments for temporomandibular disorders. J Orofac Pain 2010;24:139–51.
Friedman MA, Detweiler-Bedell JB, Leventhal HE, Horne R, Keitner GI, Miller IW. Combined psychotherapy and pharmacotherapy for the treatment of major depressive disorder. Clin Psychol Sci Pract 2004;11:47–68.
Fu F, Zhao H, Tong F, Chi I. A systematic review of psychosocial interventions to cancer caregivers. Front Psychol 2017;8:834. https://doi.org/10.3389/fpsyg.2017.00834
Fuller TE, Haider HF, Kikidis D, Lapira A, Mazurek B, Norena A, et al. Different teams, same conclusions? A systematic review of existing clinical guidelines for the assessment and treatment of tinnitus in adults. Front Psychol 2017;8:206. https://doi.org/10.3389/fpsyg.2017.00206
Furlong M, McGilloway S, Bywater T, Hutchings J, Smith SM, Donnelly M. Behavioural and cognitive–behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years. Cochrane Database Syst Rev 2012;2:CD008225. https://doi.org/10.1002/14651858.CD008225.pub2
Furlong M, McGilloway S, Bywater T, Hutchings J, Smith SM, Donnelly M. Cochrane review: behavioural and cognitive–behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years (Review). Evid Based Child Health 2013;8:318–692. https://doi.org/10.1002/ebch.1905
Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract 2016;22(Suppl. 3):1–203. https://doi.org/10.4158/EP161365.GL
Gerson S, Belin TR, Kaufman A, Mintz J, Jarvik L. Pharmacological and psychological treatments for depressed older patients: a meta-analysis and overview of recent findings. Harv Rev Psychiatry 1999;7:1–28.
Ghadiri-Sani M, Silver N. Headache (chronic tension-type). Clin Evid 2016;05:05.
Gibson E, Sabo MT. Can pain catastrophizing be changed in surgical patients? A scoping review. Can J Surg 2018;61:311–18. https://doi.org/10.1503/cjs.015417
Gilchrist G, Swan D, Widyaratna K, Marquez-Arrico JE, Hughes E, Mdege ND, et al. A systematic review and meta-analysis of psychosocial interventions to reduce drug and sexual blood borne virus risk behaviours among people who inject drugs. AIDS Behav 2017;21:1791–811. https://doi.org/10.1007/s10461-017-1755-0
Gilpin HR, Keyes A, Stahl DR, Greig R, McCracken LM. Predictors of treatment outcome in contextual cognitive and behavioral therapies for chronic pain: a systematic review. J Pain 2017;18:1153–64.
Goldbeck L, Fidika A, Herle M, Quittner AL. Psychological interventions for individuals with cystic fibrosis and their families. Cochrane Database Syst Rev 2014;6:CD003148. https://doi.org/10.1002/14651858.CD003148.pub3
González S, Artal J, Gómez E, Caballero P, Mayoral J, Moreno T, et al. Early intervention in bipolar disorder: the Jano program at Hospital Universitario Marqués de Valdecilla. Actas Esp Psiquiatr 2012;40:51–6.
Gordon J, King NJ, Gullone E, Muris P, Ollendick TH. Treatment of children’s nighttime fears: the need for a modern randomised controlled trial. Clin Psychol Rev 2007;27:98–113.
Gould RA, Safren SA, Washington DON, Otto MW. A Meta-Analytic Review of Cognitive–Behavioral Treatments. In Meimberg RG, Turk CL, Mennin DS, editors. Generalized Anxiety Disorder: Advances in Research and Practice. New York, NY: Guilford Publications, Inc; 2004. pp. 248–64.
Gould RL, Coulson MC, Brown RG, Goldstein LH, Al-Chalabi A, Howard RJ. Psychotherapy and pharmacotherapy interventions to reduce distress or improve well-being in people with amyotrophic lateral sclerosis: a systematic review. Amyotroph Lateral Scler Frontotemporal Degener 2015;16:293–302. https://doi.org/10.3109/21678421.2015.1062515
Gouzoulis-Mayfrank E, Hartel-Petri R, Hamdorf W, Havemann-Reinecke U, Muhlig S, Wodarz N. Methamphetamine-related disorders. Dtsch Arztebl Int 2017;114:455–61.
Grewal R, Spielmann PM, Jones SE, Hussain SS. Clinical efficacy of tinnitus retraining therapy and cognitive behavioural therapy in the treatment of subjective tinnitus: a systematic review. J Laryngol Otol 2014;128:1028–33. https://doi.org/10.1017/S0022215114002849
Gu W, Storch EA, Zhao Q, Xu T, Wang Z. Effects of D-cycloserine augmentation on cognitive behavioral therapy in patients with obsessive–compulsive disorder: a systematic review and meta-analysis. J Obsess Compuls Relat Disord 2017;13:24–9.
Hackett KL, Gotts ZM, Ellis J, Deary V, Rapley T, Ng WF, et al. An investigation into the prevalence of sleep disturbances in primary Sjögren’s syndrome: a systematic review of the literature. Rheumatology 2017;56:570–80. https://doi.org/10.1093/rheumatology/kew443
Haines T, Gross A, Burnie SJ, Goldsmith CH, Perry L. Patient education for neck pain with or without radiculopathy. Cochrane Database Syst Rev 2009;1:CD005106. https://doi.org/10.1002/14651858.CD005106.pub3
Haines T, Gross A, Goldsmith CH, Perry L. Patient education for neck pain with or without radiculopathy. Cochrane Database Syst Rev 2008;4:CD005106. https://doi.org/10.1002/14651858.CD005106.pub2
Haines T, Gross AR, Burnie S, Goldsmith CH, Perry L, Graham N, Cervical Overview Group (COG). A Cochrane review of patient education for neck pain. Spine J 2009;9:859–71. https://doi.org/10.1016/j.spinee.2009.04.019
Hall GC. Sexual offender recidivism revisited: a meta-analysis of recent treatment studies. J Consult Clin Psychol 1995;63:802–9. https://doi.org/10.1037/0022-006x.63.5.802
Hartnett D, Carr A, Hamilton E, O’Reilly G. The effectiveness of functional family therapy for adolescent behavioral and substance misuse problems: a meta-analysis. Fam Process 2017;56:607–19. https://doi.org/10.1111/famp.12256
Häuser W, Thieme K, Turk DC. Guidelines on the management of fibromyalgia syndrome – a systematic review. Eur J Pain 2010;14:5–10. https://doi.org/10.1016/j.ejpain.2009.01.006
Heaton LJ. Behavioral interventions may reduce dental anxiety and increase acceptance of dental treatment in dentally fearful adults. J Evid Based Dent Pract 2013;13:160–2. https://doi.org/10.1016/j.jebdp.2013.10.008
Hegedüs A, Kozel B. Does adherence therapy improve medication adherence among patients with schizophrenia? A systematic review. Int J Ment Health Nurs 2014;23:490–7. https://doi.org/10.1111/inm.12089
Hersch J, Juraskova I, Price M, Mullan B. Psychosocial interventions and quality of life in gynaecological cancer patients: a systematic review. Psychooncology 2009;18:795–810. https://doi.org/10.1002/pon.1443
Hesse M. Achieving abstinence by treating depression in the presence of substance-use disorders. Addict Behav 2004;29:1137–41. https://doi.org/10.1016/j.addbeh.2004.03.006
Hetrick SE, Purcell R, Garner B, Parslow R. Combined pharmacotherapy and psychological therapies for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2010;7:CD007316. https://doi.org/10.1002/14651858.CD007316.pub2
Hilfiker R, Meichtry A, Eicher M, Nilsson BL, Knols RH, Verra ML, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med 2017;13:13.
Hill A, Brettle A. The effectiveness of counselling with older people: results of a systematic review. Counsel Psychother Res 2005;5:265–72.
Himelhoch S, Medoff DR, Oyeniyi G. Efficacy of group psychotherapy to reduce depressive symptoms among HIV-infected individuals: a systematic review and meta-analysis. AIDS Patient Care STDS 2007;21:732–9. https://doi.org/10.1089/apc.2007.0012
Hirschtritt ME, Bloch MH, Mathews CA. Obsessive–compulsive disorder: advances in diagnosis and treatment. JAMA 2017;317:1358–67.
Hjorthoj C, Fohlmann A, Nordentoft M. Reprint of ‘Treatment of cannabis use disorders in people with schizophrenia spectrum disorders – a systematic review’. Addict Behav 2009;34:846–51.
Hjorthoj CR, Baker A, Fohlmann A, Nordentoft M. Intervention efficacy in trials targeting cannabis use disorders in patients with comorbid psychosis systematic review and meta-analysis. Curr Pharm Des 2014;20:2205–11.
Ho BP, Carter M, Stephenson J. Anger management using a cognitive-behavioural approach for children with special education needs: a literature review and meta-analysis. Int J Disabil Dev Educ 2010;57:245–65.
Ho ECM, Siu AMH. Occupational therapy practice in sleep management: a review of conceptual models and research evidence. Occup Ther Int 2018;2018:8637498. https://doi.org/10.1155/2018/8637498
Hoch E, Preuss UW, Ferri M, Simon R. Digital interventions for problematic cannabis users in non-clinical settings: findings from a systematic review and meta-analysis. Eur Addict Res 2016;22:233–42. https://doi.org/10.1159/000445716
Hoffman BM, Papas RK, Chatkoff DK, Kerns RD. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol 2007;26:1–9.
Hofmann SG, Curtiss J, Carpenter JK, Kind S. Effect of treatments for depression on quality of life: a meta-analysis. Cogn Behav Ther 2017;46:265–86. https://doi.org/10.1080/16506073.2017.1304445
Hofmann SG, Sawyer AT, Korte KJ, Smits JA. Is it beneficial to add pharmacotherapy to cognitive–behavioral therapy when treating anxiety disorders? A meta-analytic review. Int J Cogn Ther 2009;2:160–75.
Hofmann SG, Wu JQ, Boettcher H. Effect of cognitive–behavioral therapy for anxiety disorders on quality of life: a meta-analysis. J Consult Clin Psychol 2014;82:375–91. https://doi.org/10.1037/a0035491
Hoogsteder LM, Stams GJJM, Figge MA, Changoe K, van Horn JE, Hendriks J, et al. A meta-analysis of the effectiveness of individually oriented Cognitive Behavioral Treatment (CBT) for severe aggressive behavior in adolescents. J Forensic Psychiatry Psychol 2015;26:22–37.
Howard MC. Science Fiction Meets Scientific Inquiry: A Task–Technology Fit/Computer-based Training Framework and a Meta-analysis of Virtual Reality Applications for Intervention, Training, and Therapy Purposes. PhD thesis. University Park, PA: Pennsylvania State University; 2018.
Hubbard JB. Psychotherapy Outcome for Eating Disorders: A Meta-analysis. PhD thesis. Provo, UT: Brigham Young University; 2014.
Huguet A, McGrath PJ, Stinson J, Tougas ME, Doucette S. Efficacy of psychological treatment for headaches: an overview of systematic reviews and analysis of potential modifiers of treatment efficacy. Clin J Pain 2014;30:353–69. https://doi.org/10.1097/AJP.0b013e318298dd8b
Hui PS, Yi SM, Kuswanto CN, Kang S. Evidence-based options for treatment-resistant adult bipolar disorder patients. Ann Acad Med Singap 2012;1:S230.
Hunot V, Moore TH, Caldwell DM, Furukawa TA, Davies P, Jones H, et al. ‘Third wave’ cognitive and behavioural therapies versus other psychological therapies for depression. Cochrane Database Syst Rev 2013;10:CD008704. https://doi.org/10.1002/14651858.CD008704.pub2
Hunt GE, Siegfried N, Morley K, Sitharthan T, Cleary M. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database Syst Rev 2013;10:CD001088. https://doi.org/10.1002/14651858.CD001088.pub3
In-Albon T, Schneider S. Psychotherapy of childhood anxiety disorders: a meta-analysis. Psychother Psychosom 2007;76:15–24.
Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol 2006;25:3–14.
IsHak WW, Bolton MA, Bensoussan JC, Dous GV, Nguyen TT, Powell-Hicks AL, et al. Quality of life in body dysmorphic disorder. CNS Spectr 2012;17:167–75. https://doi.org/10.1017/S1092852912000624
IsHak WW, Wen RY, Naghdechi L, Vanle B, Dang J, Knosp M, et al. Pain and depression: a systematic review. Harv Rev Psychiatry 2018;26:352–63.
Itlescas SR, Sanchez-Meca J, Genoves VG. Treatment of offenders and recidivism: assessment of the effectiveness of programmes applied in Europe. Psychol Spain 2001;5:47–62.
Jackson CF, Makin SM, Marson AG, Kerr M. Non-pharmacological interventions for people with epilepsy and intellectual disabilities. Cochrane Database Syst Rev 2015;9:CD005502. https://doi.org/10.1002/14651858.CD005502.pub3
Jarrell JF, Vilos GA, Allaire C, Burgess S, Fortin C, Gerwin R, et al. Consensus guidelines for the management of chronic pelvic pain. J Obstet Gynaecol Can 2005;27:869–910.
Järvinen M, Stolt M, Honkala E, Leino-Kilpi H, Pöllänen M. Behavioural interventions that have the potential to improve self-care in adults with periodontitis: a systematic review. Acta Odontol Scand 2018;76:612–20. https://doi.org/10.1080/00016357.2018.1490964
Jaworska-Burzynska L, Kanaffa-Kilijanska U, Przysiezna E, Szczepanska-Gieracha J. The role of therapy in reducing the risk of job burnout – a systematic review of literature. Arch Psychiatry Psychother 2016;18:43–52.
Jenkinson E, Williamson H, Byron-Daniel J, Moss TP. Systematic review: psychosocial interventions for children and young people with visible differences resulting from appearance altering conditions, injury, or treatment effects. J Pediatr Psychol 2015;40:1017–33. https://doi.org/10.1093/jpepsy/jsv048
Jewell LM, Wormith J. Variables associated with attrition from domestic violence treatment programs targeting male batterers: a meta-analysis. Crim Justice Behav 2010;37:1086–113.
Jones B, Williams ACDC. CBT to reduce healthcare use for medically unexplained symptoms: systematic review and meta-analysis. Br JGen Pract 2019;28:28.
Jones C, Hacker D, Cormac I, Meaden A, Irving CB. Cognitive behavior therapy versus other psychosocial treatments for schizophrenia. Schizophr Bull 2012;38:908–10. https://doi.org/10.1093/schbul/sbs090
Jong E, Oudhoff LA, Epskamp C, Wagener MN, van Duijn M, Fischer S, van Gorp EC. Predictors and treatment strategies of HIV-related fatigue in the combined antiretroviral therapy era. AIDS 2010;24:1387–405. https://doi.org/10.1097/QAD.0b013e328339d004
Jorm AF, Christensen H, Griffiths KM, Rodgers B. Effectiveness of complementary and self-help treatments for depression. Med J Aust 2002;176:S84–96.
Kaptein AA, Scharloo M, Fischer MJ, Snoei L, Cameron LD, Sont JK, et al. Illness perceptions and COPD: an emerging field for COPD patient management. J Asthma 2008;45:625–9. https://doi.org/10.1080/02770900802127048
Kelly KP, Kirschenbaum DS. Immersion treatment of childhood and adolescent obesity: the first review of a promising intervention. Obes Rev 2011;12:37–49. https://doi.org/10.1111/j.1467-789X.2009.00710.x
Kennedy CA, Amick BC, Dennerlein JT, Brewer S, Catli S, Williams R, et al. Systematic review of the role of occupational health and safety interventions in the prevention of upper extremity musculoskeletal symptoms, signs, disorders, injuries, claims and lost time. J Occup Rehabil 2010;20:127–62. https://doi.org/10.1007/s10926-009-9211-2
Kent P, Kjaer P. The efficacy of targeted interventions for modifiable psychosocial risk factors of persistent nonspecific low back pain – a systematic review. Man Ther 2012;17:385–401. https://doi.org/10.1016/j.math.2012.02.008
Kenworthy T, Adams CE, Bilby C, Brooks-Gordon B, Fenton M. Psychological interventions for those who have sexually offended or are at risk of offending. Cochrane Database Syst Rev 2004;3:CD004858. https://doi.org/10.1002/14651858.CD004858
Kim HS, Kim EJ. Effects of relaxation therapy on anxiety disorders: a systematic review and meta-analysis. Arch Psychiatr Nurs 2018;32:278–84.
King DL, Delfabbro PH, Wu AMS, Doh YY, Kuss DJ, Pallesen S, et al. Treatment of internet gaming disorder: an international systematic review and CONSORT evaluation. Clin Psychol Rev 2017;54:123–33.
Klinsophon T, Thaveeratitham P, Sitthipornvorakul E, Janwantanakul P. Effect of exercise type on smoking cessation: a meta-analysis of randomized controlled trials. BMC Res Notes 2017;10:442. https://doi.org/10.1186/s13104-017-2762-y
Knight L, Mukumbang FC, Schatz E. Behavioral and cognitive interventions to improve treatment adherence and access to HIV care among older adults in sub-Saharan Africa: an updated systematic review. Syst Rev 2018;7:114. https://doi.org/10.1186/s13643-018-0759-9
Koehler JA, Losel F, Akoensi TD, Humphreys DK. A systematic review and meta-analysis on the effects of young offender treatment programs in Europe. J Exp Criminol 2013;9:19–43.
Krishnan A, Silver N. Headache (chronic tension-type). BMJ Clin Evid 2009;2009:1205.
Kroll C, Doebler P, Nuesch S. Meta-analytic evidence of the effectiveness of stress management at work. Eur J Work Organ Psychol 2017;26:677–93.
Kuder SJ, Accardo A. What works for college students with autism spectrum disorder. J Autism Dev Disord 2018;48:722–31. https://doi.org/10.1007/s10803-017-3434-4
Kuerbis A, Sacco P. A review of existing treatments for substance abuse among the elderly and recommendations for future directions. Subst Abuse 2013;7:13–37. https://doi.org/10.4137/SART.S7865
Kuin N, Masthoff E, Kramer M, Scherder E. The role of risky decision-making in aggression: a systematic review. Aggress Violent Behav 2015;25:159–72.
Kwan I, Onwude JL. Premenstrual syndrome. BMJ Clin Evid 2007;2007:0806.
Kwekkeboom KL, Bratzke LC. A systematic review of relaxation, meditation, and guided imagery strategies for symptom management in heart failure. J Cardiovasc Nurs 2016;31:457–68. https://doi.org/10.1097/JCN.0000000000000274
Kyle SD, Aquino MR, Miller CB, Henry AL, Crawford MR, Espie CA, Spielman AJ. Towards standardisation and improved understanding of sleep restriction therapy for insomnia disorder: a systematic examination of CBT-I trial content. Sleep Med Rev 2015;23:83–8. https://doi.org/10.1016/j.smrv.2015.02.003
Lancee J, Spoormaker VI, Krakow B, van den Bout J. A systematic review of cognitive–behavioral treatment for nightmares: toward a well-established treatment. J Clin Sleep Med 2008;4:475–80.
Lapp LK, Agbokou C, Peretti CS, Ferreri F. Management of post traumatic stress disorder after childbirth: a review. J Psychosom Obstet Gynaecol 2010;31:113–22. https://doi.org/10.3109/0167482X.2010.503330
Lee J, Kim Y, Kim YL. Non-pharmacological therapies for sleep disturbances in people with Parkinson’s disease: a systematic review. J Adv Nurs 2018;27:27.
Leichsenring F, Leibing E. The effectiveness of psychodynamic therapy and cognitive behavior therapy in the treatment of personality disorders: a meta-analysis. Am J Psychiatry 2003;160:1223–32. https://doi.org/10.1176/appi.ajp.160.7.1223
Lemos IL, De Abreu CN, Sougey EB. Internet and video game addictions: a cognitive behavioral approach. Rev Psiquiatr Clin 2014;41:82–8.
Lewandowski LM, Gebing TA, Anthony JL, O’Brien WH. Meta-analysis of cognitive–behavioral treatment studies for bulimia. Clin Psychol Rev 1997;17:703–18.
Lilliengren P, Johansson R, Lindqvist K, Mechler J, Andersson G. Efficacy of experiential dynamic therapy for psychiatric conditions: a meta-analysis of randomized controlled trials. Psychotherapy 2016;53:90–104. https://doi.org/10.1037/pst0000024
Lockwood C, Page T, NursCert H, Conroy-Hiller T. Effectiveness of individual therapy and group therapy in the treatment of schizophrenia. JBI Libr Syst Rev 2004;2:1–44.
Hoon LS, Chi Sally CW, Hong-Gu H. Effect of psychosocial interventions on outcomes of patients with colorectal cancer: a review of the literature. Eur J Oncol Nurs 2013;17:883–91. https://doi.org/10.1016/j.ejon.2013.05.001
Lin MY, Liu MF, Hsu LF, Tsai PS. Effects of self-management on chronic kidney disease: a meta-analysis. Int J Nurs Stud 2017;74:128–37.
Linardon J, Fitzsimmons-Craft EE, Brennan L, Barillaro M, Wilfley DE. Dropout from interpersonal psychotherapy for mental health disorders: a systematic review and meta-analysis. Psychother Res 2019;29:870–81. https://doi.org/10.1080/10503307.2018.1497215
Linde K, Treml J, Steinig J, Nagl M, Kersting A. Grief interventions for people bereaved by suicide: a systematic review. PLOS ONE 2017;12:e0179496. https://doi.org/10.1371/journal.pone.0179496
Lindhiem O, Higa J, Trentacosta CJ, Herschell AD, Kolko DJ. Skill acquisition and utilization during evidence-based psychosocial treatments for childhood disruptive behavior problems: a review and meta-analysis. Clin Child Fam Psychol Rev 2014;17:41–66. https://doi.org/10.1007/s10567-013-0136-0
Liu C, Liao M, Smith DC. An empirical review of internet addiction outcome studies in China. Res Soc Work Pract 2012;22:282–92.
Lockwood C, Page T, Conroy-Hiller T. Comparing the effectiveness of cognitive behaviour therapy using individual or group therapy in the treatment of depression. JBI Libr Syst Rev 2004;2:1–33.
Lorenzo-Luaces L, Johns E, Keefe JR. The generalizability of randomized controlled trials of self-guided internet-based cognitive behavioral therapy for depressive symptoms: systematic review and meta-regression analysis. J Med Internet Res 2018;20:e10113. https://doi.org/10.2196/10113
Losel F, Schmucker M. The effectiveness of treatment for sexual offenders: a comprehensive meta-analysis. J Exp Criminol 2005;1:117–46.
Lyman DR, Kurtz MM, Farkas M, George P, Dougherty RH, Daniels AS, et al. Skill building: assessing the evidence. Psychiatr Serv 2014;65:727–38. https://doi.org/10.1176/appi.ps.201300251
Lynch D, Laws KR, McKenna PJ. Cognitive behavioural therapy for major psychiatric disorder: does it really work? A meta-analytical review of well-controlled trials. Psychol Med 2010;40:9–24. https://doi.org/10.1017/S003329170900590X
Mackin RS, Areán PA. Evidence-based psychotherapeutic interventions for geriatric depression. Psychiatr Clin North Am 2005;28:805–20, vii–viii.
Makdissi M, Schneider KJ, Feddermann-Demont N, Guskiewicz KM, Hinds S, Leddy JJ, et al. Approach to investigation and treatment of persistent symptoms following sport-related concussion: a systematic review. Br J Sports Med 2017;51:958–68. https://doi.org/10.1136/bjsports-2016-097470
Manassis K, Lee TC, Bennett K, Zhao XY, Mendlowitz S, Duda S, et al. Types of parental involvement in CBT with anxious youth: a preliminary meta-analysis. J Consult Clin Psychol 2014;82:1163–72. https://doi.org/10.1037/a0036969
Manfredini D, Ahlberg J, Winocur E, Lobbezoo F. Management of sleep bruxism in adults: a qualitative systematic literature review. J Oral Rehabil 2015;42:862–74. https://doi.org/10.1111/joor.12322
Marc I, Toureche N, Ernst E, Hodnett ED, Blanchet C, Dodin S, Njoya MM. Mind-body interventions during pregnancy for preventing or treating women’s anxiety. Cochrane Database Syst Rev 2011;7:CD007559. https://doi.org/10.1002/14651858.CD007559.pub2
Mariano TY, Urman RD, Hutchison CA, Jamison RN, Edwards RR. Cognitive Behavioral Therapy (CBT) for subacute low back pain: a systematic review. Curr Pain Headache Rep 2018;22:15. https://doi.org/10.1007/s11916-018-0669-5
Marker I, Norton PJ. The efficacy of incorporating motivational interviewing to cognitive behavior therapy for anxiety disorders: a review and meta-analysis. Clin Psychol Rev 2018;62:1–10.
Marques DR, Gomes AA, Clemente V, dos Santos JM, Caetano G, Castelo-Branco M. Neurobiological correlates of psychological treatments for insomnia: a review. Eur Psychol 2016;21:195–205.
Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev 2006;18:CD004718.
Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev 2011;15:CD004718.
Martlew J, Baker GA, Goodfellow L, Bodde N, Aldenkamp A. Behavioural treatments for non-epileptic attack disorder. Cochrane Database Syst Rev 2009;4:CD006370.
Mas-Exposito L, Amador-Campos JA, Gomez-Benito J, Lalucat-Jo L. Review of psychotherapeutic interventions for people with schizophrenia. Anu Psicol 2013;43:101–16.
Masterton KJ, Tariman JD. Effective transitional therapy for adolescent and young adult patients with cancer: an integrative literature review. Clin J Oncol Nurs 2016;20:391–6. https://doi.org/10.1188/16.CJON.391-396
Mataix-Cols D, Fernández de la Cruz L, Monzani B, Rosenfield D, Andersson E, Pérez-Vigil A, et al. D-Cycloserine augmentation of exposure-based cognitive behavior therapy for anxiety, obsessive-compulsive, and posttraumatic stress disorders: a systematic review and meta-analysis of individual participant data. JAMA Psychiatry 2017;74:501–10. https://doi.org/10.1001/jamapsychiatry.2016.3955
Matthys F, Stes S, van den Brink W, Joostens P, Mobius D, Tremmery S, et al. Guideline for screening, diagnosis and treatment of ADHD in adults with substance use disorders. Int J Ment Health Addict 2014;12:629–47.
McCart MR, Priester PE, Davies WH, Azen R. Differential effectiveness of behavioral parent-training and cognitive–behavioral therapy for antisocial youth: a meta-analysis. J Abnorm Child Psychol 2006;34:527–43. https://doi.org/10.1007/s10802-006-9031-1
McCart MR, Sheidow AJ. Evidence-based psychosocial treatments for adolescents with disruptive behavior. J Clin Child Adolesc Psychol 2016;45:529–63.
McClintock SM, Brandon AR, Husain MM, Jarrett RB. A systematic review of the combined use of electroconvulsive therapy and psychotherapy for depression. J ECT 2011;27:236–43. https://doi.org/10.1097/YCT.0b013e3181faaeca
McDonald P, Colwell B, Backinger CL, Husten C, Maule CO. Better practices for youth tobacco cessation: evidence of review panel. Am J Health Behav 2003;27(Suppl. 2):144–58. https://doi.org/10.5993/ajhb.27.1.s2.5
McGuire JF, Orr SP, Essoe JK, McCracken JT, Storch EA, Piacentini J. Extinction learning in childhood anxiety disorders, obsessive compulsive disorder and post-traumatic stress disorder: implications for treatment. Expert Rev Neurother 2016;16:1155–74. https://doi.org/10.1080/14737175.2016.1199276
McGuire JF, Piacentini J, Lewin AB, Brennan EA, Murphy TK, Storch EA. A meta-analysis of cognitive behavior therapy and medication for child obsessive-compulsive disorder: moderators of treatment efficacy, response, and remission. Depress Anxiety 2015;32:580–93. https://doi.org/10.1002/da.22389
McKenzie PS. Chronic Low Back Pain and Insomnia: Understanding the Experience and Attributions Made by Out-patients about Sleeplessness, Pain and their Interaction. PhD thesis. Edinburgh: University of Edinburgh; 2012.
McLaughlin B, Ryder D, Taylor MF. Effectiveness of interventions for grandparent caregivers: a systematic review. Marriage Fam Rev 2017;53:509–31.
McMurran M, Theodosi E. Is treatment non-completion associated with increased reconviction over no treatment? Psychol Crime Law 2007;13:333–43.
Mehta S, Orenczuk S, Hansen KT, Aubut JA, Hitzig SL, Legassic M, Teasell RW, Spinal Cord Injury Rehabilitation Evidence Research Team. An evidence-based review of the effectiveness of cognitive behavioral therapy for psychosocial issues post-spinal cord injury. Rehabil Psychol 2011;56:15–25. https://doi.org/10.1037/a0022743
Melnik T, Hawton K, McGuire H. Interventions for vaginismus. Cochrane Database Syst Rev 2012;12:CD001760.
Menzies RE, Zuccala M, Sharpe L, Dar-Nimrod I. The effects of psychosocial interventions on death anxiety: a meta-analysis and systematic review of randomised controlled trials. J Anxiety Disord 2018;59:64–73.
Meyer C, Denis CM, Berquin AD. Secondary prevention of chronic musculoskeletal pain: a systematic review of clinical trials. Ann Phys Rehabil Med 2018;61:323–38.
Miller BJ. A review of second-generation antipsychotic discontinuation in first-episode psychosis. J Psychiatr Pract 2008;14:289–300. https://doi.org/10.1097/01.pra.0000336756.65308.83
Milling LS, Gover MC, Moriarty CL. The effectiveness of hypnosis as an intervention for obesity: a meta-analytic review. Psychol Conscious Theory Res Pract 2018;5:29–45.
Mirza SK, Deyo RA. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine 2007;32:816–23. https://doi.org/10.1097/01.brs.0000259225.37454.38
Mogul A, Irby MB, Skelton JA. A systematic review of pediatric obesity and family communication through the lens of addiction literature. Child Obes 2014;10:197–206. https://doi.org/10.1089/chi.2013.0157
Molenaar NM, Kamperman AM, Boyce P, Bergink V. Guidelines on treatment of perinatal depression with antidepressants: an international review. Aust N Z J Psychiatry 2018;52:320–7. https://doi.org/10.1177/0004867418762057
Momani TG, Berry DL. integrative therapeutic approaches for the management and control of nausea in children undergoing cancer treatment: a systematic review of literature. J Pediatr Oncol Nurs 2017;34:173–84. https://doi.org/10.1177/1043454216688638
Montgomery P, Lilly J. Insomnia in the elderly. BMJ Clin Evid 2007;2007:2302.
Monticone M, Ambrosini E, Cedraschi C, Rocca B, Fiorentini R, Restelli M, et al. Cognitive–behavioral treatment for subacute and chronic neck pain: a Cochrane review. Spine 2015;40:1495–504. https://doi.org/10.1097/BRS.0000000000001052
Moore KE, Hacker RL, Oberleitner L, McKee SA. Reentry interventions that address substance use: a systematic review. Psychol Serv 2018;11:11.
Moore M, Carr A. Depression and Grief. In Carr A, editor. What Works with Children and Adolescents? A Critical Review of Psychological Interventions with Children, Aolescents and their Families. Florence, KY/London: Taylor & Frances/Routledge; 2000. pp. 203–32.
Moore THM, King AJL, Evans M, Sharp D, Persad R, Huntley AL. Supportive care for men with prostate cancer: why are the trials not working? A systematic review and recommendations for future trials. Cancer Med 2015;4:1240–51.
Moreno Gil PJ, Mendez Carrillo FX, Sanchez Meca J. Effectiveness of cognitive–behavioural treatment in social phobia: a meta-analytic review. Psychol Spain 2001;5:17–25.
Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1–13. https://doi.org/10.1016/s0304-3959(98)00255-3
Morrell CJ, Sutcliffe P, Booth A, Stevens J, Scope A, Stevenson M, et al. A systematic review, evidence synthesis and meta-analysis of quantitative and qualitative studies evaluating the clinical effectiveness, the cost-effectiveness, safety and acceptability of interventions to prevent postnatal depression. Health Technol Assess 2016;20(37). https://doi.org/10.3310/hta20370
Morris L, Stander J, Ebrahim W, Eksteen S, Meaden OA, Ras A, Wessels A. Effect of exercise versus cognitive behavioural therapy or no intervention on anxiety, depression, fitness and quality of life in adults with previous methamphetamine dependency: a systematic review. Addict Sci Clin Pract 2018;13:4. https://doi.org/10.1186/s13722-018-0106-4
Mpofu E, Athanasou JA, Rafe C, Belshaw SH. Cognitive–behavioral therapy efficacy for reducing recidivism rates of moderate- and high-risk sexual offenders: a scoping systematic literature review. Int J Offender Ther Comp Criminol 2018;62:170–86. https://doi.org/10.1177/0306624X16644501
Munn Z, Jordan Z. The effectiveness of interventions to reduce anxiety, claustrophobia, sedation and non-completion rates of patients undergoing high technology medical imaging. JBI Libr Syst Rev 2012;10:1122–85.
Munn Z, Jordan Z. Interventions to reduce anxiety, distress and the need for sedation in adult patients undergoing magnetic resonance imaging: a systematic review. Int J Evid Based Healthc 2013;11:265–74. https://doi.org/10.1111/1744-1609.12045
Munn Z, Jordan Z. Interventions to reduce anxiety, distress, and the need for sedation in pediatric patients undergoing magnetic resonance imaging: a systematic review. J Radiol Nurs 2013;32:87–96.
Murawski B, Wade L, Plotnikoff RC, Lubans DR, Duncan MJ. A systematic review and meta-analysis of cognitive and behavioral interventions to improve sleep health in adults without sleep disorders. Sleep Med Rev 2018;40:160–9.
Murray M, Murray L, Donnelly M. Systematic review of interventions to improve the psychological well-being of general practitioners. BMC Fam Pract 2016;17:36. https://doi.org/10.1186/s12875-016-0431-1
Nagy D, Szamoskozi S. Efficacy of cognitive behavioral interventions on complicated grief in adults: a quantitative meta-analysis. Erdelyi Pszichologiai Szemle 2013;14:39–54.
Nelson MK. Meta-analysis: Hypnotherapy/Cognitive–Behavioral Therapy and its Efficacy on Depression Compared to Pharmacotherapy. PhD thesis. San Diego, CA: Alliant International University; 2002.
Nicoll M, Beail N, Saxon D. Cognitive behavioural treatment for anger in adults with intellectual disabilities: a systematic review and meta-analysis. J Appl Res Intellect Disabil 2013;26:47–62. https://doi.org/10.1111/jar.12013
Nielson WR, Weir R. Biopsychosocial approaches to the treatment of chronic pain. Clin J Pain 2001;17(Suppl. 4):114–27. https://doi.org/10.1097/00002508-200112001-00020
Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014;11:CD000011. https://doi.org/10.1002/14651858.CD000011.pub4
Nieuwsma JA, Williams JW, Namdari N, Washam JB, Raitz G, Blumenthal JA, et al. Diagnostic accuracy of screening tests and treatment for post-acute coronary syndrome depression: a systematic review. Ann Intern Med 2017;167:725–35. https://doi.org/10.7326/M17-1811
Nolan M, Carr A. Attention Deficit Hyperactivity Disorder. In Carr A, editor. What Works with Children and Adolescents? A Critical Review of Psychological Interventions with Children, Adolescents and their Families. Florence, KY/London: Taylor & Frances/Routledge; 2000. pp. 65–101.
Norman A, Moss TP. Psychosocial interventions for adults with visible differences: a systematic review. Peer J 2015;3:e870. https://doi.org/10.7717/peerj.870
Norton C, Czuber-Dochan W, Artom M, Sweeney L, Hart A. Systematic review: interventions for abdominal pain management in inflammatory bowel disease. Aliment Pharmacol Ther 2017;46:115–25. https://doi.org/10.1111/apt.14108
Nowak I, Sabariego C, Świtaj P, Anczewska M. Disability and recovery in schizophrenia: a systematic review of cognitive behavioral therapy interventions. BMC Psychiatry 2016;16:228. https://doi.org/10.1186/s12888-016-0912-8
Nyenhuis N, Golm D, Kröner-Herwig B. A systematic review and meta-analysis on the efficacy of self-help interventions in tinnitus. Cogn Behav Ther 2013;42:159–69. https://doi.org/10.1080/16506073.2013.803496
Ochentel O, Humphrey C, Pfeifer K. Efficacy of exercise therapy in persons with burnout. A systematic review and meta-analysis. J Sports Sci Med 2018;17:475–84.
O’Connor M, Halkett GK. A systematic review of interventions to reduce psychological distress in pediatric patients receiving radiation therapy. Patient Educ Counsel 2018;29:29.
Odeen M, Magnussen LH, Maeland S, Larun L, Eriksen HR, Tveito TH. Systematic review of active workplace interventions to reduce sickness absence. Occup Med 2013;63:7–16. https://doi.org/10.1093/occmed/kqs198
Oei TP, Dingle G. The effectiveness of group cognitive behaviour therapy for unipolar depressive disorders. J Affect Disord 2008;107:5–21.
Okoniewski W, Lu KD, Forno E. Weight loss for children and adults with obesity and asthma. A systematic review of randomized controlled trials. Ann Am Thorac Soc 2019;16:613–25. https://doi.org/10.1513/AnnalsATS.201810-651SR
Okumura Y, Ichikura K. Efficacy and acceptability of group cognitive behavioral therapy for depression: a systematic review and meta-analysis. J Affect Disord 2014;164:155–64. https://doi.org/10.1016/j.jad.2014.04.023
Olatunji BO, Davis ML, Powers MB, Smits JA. Cognitive–behavioral therapy for obsessive–compulsive disorder: a meta-analysis of treatment outcome and moderators. J Psychiatr Res 2013;47:33–41. https://doi.org/10.1016/j.jpsychires.2012.08.020
Oliver K, Cronan TA, Walen HR. A review of multidisciplinary interventions for fibromyalgia patients: where do we go from here? J Musculoskelet Pain 2001;9:63–80.
Olsson KL, Cooper RL, Nugent WR, Reid RC. Addressing negative affect in substance use relapse prevention. J Hum Behav Soc Environ 2016;26:2–14.
Öst LG, Havnen A, Hansen B, Kvale G. Cognitive behavioral treatments of obsessive–compulsive disorder. A systematic review and meta-analysis of studies published 1993–2014. Clin Psychol Rev 2015;40:156–69. https://doi.org/10.1016/j.cpr.2015.06.003
Othman A, Blunden S. Psychological interventions for parents of children who have cancer: a meta-analytic review. Curr Pediati Rev 2009;5:118–27.
O’Toole MS, Zachariae R, Renna ME, Mennin DS, Applebaum A. Cognitive behavioral therapies for informal caregivers of patients with cancer and cancer survivors: a systematic review and meta-analysis. Psycho-Oncology 2017;26:428–37. https://doi.org/10.1002/pon.4144
Ougrin D. Efficacy of exposure versus cognitive therapy in anxiety disorders: systematic review and meta-analysis. BMC Psychiatry 2011;11:200. https://doi.org/10.1186/1471-244X-11-200
Oustric P, Gibbons C, Beaulieu K, Blundell J, Finlayson G. Changes in food reward during weight management interventions – a systematic review. Obes Rev 2018;19:1642–58. https://doi.org/10.1111/obr.12754
Oyebode JR, Parveen S. Psychosocial interventions for people with dementia: an overview and commentary on recent developments. Dementia 2016;04:04.
Ozabaci N. Cognitive behavioural therapy for violent behaviour in children and adolescents: a meta-analysis. Child Youth Serv Rev 2011;33:1989–93.
Paintain E, Cassidy S. First-line therapy for post-traumatic stress disorder: a systematic review of cognitive behavioural therapy and psychodynamic approaches. Couns Psychother Res 2018;18:237–50. https://doi.org/10.1002/capr.12174
Parahoo K, McDonough S, McCaughan E, Noyes J, Semple C, Halstead EJ, et al. Psychosocial interventions for men with prostate cancer. Cochrane Database Syst Rev 2013;12:CD008529. https://doi.org/10.1002/14651858.CD008529.pub3
Parahoo K, McDonough S, McCaughan E, Noyes J, Semple C, Halstead EJ, et al. Psychosocial interventions for men with prostate cancer: a Cochrane systematic review. BJU Int 2015;116:174–83. https://doi.org/10.1111/bju.12989
Parcesepe AM, Martin SL, Pollock MD, Garcia-Moreno C. The effectiveness of mental health interventions for adult female survivors of sexual assault: a systematic review. Aggress Violent Behav 2015;25:15–25.
Paul CL, Carey ML, Sanson-Fisher RW, Houlcroft LE, Turon HE. The impact of web-based approaches on psychosocial health in chronic physical and mental health conditions. Health Educ Res 2013;28:450–71. https://doi.org/10.1093/her/cyt053
Paulik G. The role of social schema in the experience of auditory hallucinations: a systematic review and a proposal for the inclusion of social schema in a cognitive behavioural model of voice hearing. Clin Psychol Psychother 2012;19:459–72. https://doi.org/10.1002/cpp.768
Payne KT, Marcus DK. The efficacy of group psychotherapy for older adult clients: a meta-analysis. Group Dyn Theory Res Pract 2008;12:268–78.
Pearson FS, Lipton DS. A meta-analytic review of the effectiveness of corrections-based treatments for drug abuse. Prison J 1999;79:384–410.
Pengel HM, Maher CG, Refshauge KM. Systematic review of conservative interventions for subacute low back pain. Clin Rehabil 2002;16:811–20. https://doi.org/10.1191/0269215502cr562oa
Perkes SJ, Bowman J, Penkala S. Psychological therapies for the management of co-morbid depression following a spinal cord injury: a systematic review. J Health Psychol 2014;19:1597–612. https://doi.org/10.1177/1359105313496445
Pham H, Torres H, Sharma P. Mental health implications in bladder cancer patients: a review. Urol Oncol 2019;37:97–107.
Phianmongkhol Y, Thongubon K, Woottiluk P. Effectiveness of cognitive behavioral therapy techniques for control of pain in lung cancer patients: an integrated review. Asian Pac J Cancer Prev 2015;16:6033–8. https://doi.org/10.7314/apjcp.2015.16.14.6033
Phillips AS. A Meta-analysis of Treatments for Pediatric Obsessive–Compulsive Disorder. PhD thesis. Manhattan, KS: Kansas State University; 2004.
Pinquart M, Duberstein PR, Lyness JM. Effects of psychotherapy and other behavioral interventions on clinically depressed older adults: a meta-analysis. Aging Ment Health 2007;11:645–57.
Pomaki G, Franche RL, Murray E, Khushrushahi N, Lampinen TM. Workplace-based work disability prevention interventions for workers with common mental health conditions: a review of the literature. J Occup Rehabil 2012;22:182–95. https://doi.org/10.1007/s10926-011-9338-9
Poole JL, Siegel P. Effectiveness of occupational therapy interventions for adults with fibromyalgia: a systematic review. Am J Occup Ther 2017;71:7101180040p1–7101180040p10. https://doi.org/10.5014/ajot.2017.023192
Posadzki P, Choi J, Lee MS, Ernst E. Yoga for addictions: a systematic review of randomised clinical trials. Focus Altern Complement Ther 2014;19:1–8.
Preti A, Cella M. Randomized-controlled trials in people at ultra high risk of psychosis: a review of treatment effectiveness. Schizophr Res 2010;123:30–6. https://doi.org/10.1016/j.schres.2010.07.026
Qaseem A, Barry MJ, Kansagara D, Clinical Guidelines Committee of the American College of Physicians. Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2016;164:350–9. https://doi.org/10.7326/M15-2570
Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD, Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2016;165:125–33. https://doi.org/10.7326/M15-2175
Qaseem A, Wilt TJ, McLean RM, Forciea MA, Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2017;166:514–30. https://doi.org/10.7326/M16-2367
Quinn J, Kolla NJ. From clozapine to cognitive remediation: a review of biological and psychosocial treatments for violence in schizophrenia. Can J Psychiatry 2017;62:94–101.
Raine R, Haines A, Sensky T, Hutchings A, Larkin K, Black N. Systematic review of mental health interventions for patients with common somatic symptoms: can research evidence from secondary care be extrapolated to primary care? BMJ 2002;325:1082–5.
Rakofsky JJ, Dunlop BW. Treating nonspecific anxiety and anxiety disorders in patients with bipolar disorder: a review. J Clin Psychiatry 2011;72:81–90. https://doi.org/10.4088/JCP.09r05815gre
Ranasinghe I, Sin J. A systematic review of evidence-based treatment for individuals with treatment-resistant schizophrenia and a suboptimal response to clozapine monotherapy. Psychos Psychol Soc Integr Approaches 2014;6:253–65.
Reavley N, Jorm AF. Prevention and early intervention to improve mental health in higher education students: a review. Early Interv Psychiatry 2010;4:132–42. https://doi.org/10.1111/j.1751-7893.2010.00167.x
Reddy LA, De Thomas CA, Newman E, Chun V. School-based prevention and intervention programs for children with emotional disturbance: a review of treatment components and methodology. Psychol Schools 2009;46:132–53.
Redondo S, Sanchez-Meca J, Garrido V. The influence of treatment programmes on the recidivism of juvenile and adult offenders: an European meta-analytic review. Psychol Crime Law 1999;5:251–78.
Rees G, Ponczek E, Hassell J, Keeffe JE, Lamoureux EL. Psychological outcomes following interventions for people with low vision: a systematic review. Expert Rev Ophthalmol 2010;5:385–403.
Renton T, Tang H, Ennis N, Cusimano MD, Bhalerao S, Schweizer TA, Topolovec-Vranic J. Web-based intervention programs for depression: a scoping review and evaluation. J Med Internet Res 2014;16:e209. https://doi.org/10.2196/jmir.3147
Richards MC, Ford JJ, Slater SL, Hahne AJ, Surkitt LD, Davidson M, McMeeken JM. The effectiveness of physiotherapy functional restoration for post-acute low back pain: a systematic review. Man Ther 2013;18:4–25. https://doi.org/10.1016/j.math.2012.06.005
Richardson A, McNoe B, Derrett S, Harcombe H. Interventions to prevent and reduce the impact of musculoskeletal injuries among nurses: a systematic review. Int J Nurs Stud 2018;82:58–67.
Richardson J, Smith JE, McCall G, Richardson A, Pilkington K, Kirsch I. Hypnosis for nausea and vomiting in cancer chemotherapy: a systematic review of the research evidence. Eur J Cancer Care 2007;16:402–12.
Richardson KM, Rothstein HR. Effects of occupational stress management intervention programs: a meta-analysis. J Occup Health Psychol 2008;13:69–93. https://doi.org/10.1037/1076-8998.13.1.69
Rihn JA, Radcliff K, Norvell DC, Eastlack R, Phillips FM, Berland D, et al. Comparative effectiveness of treatments for chronic low back pain: a multiple treatment comparison analysis. Clin Spine Surg 2017;30:204–25. https://doi.org/10.1097/BSD.0000000000000410
Ritvo PG, Irvine MJ, Lindsay EA, Kraetschmer N, Blair N, Shnek ZM. A critical review of research related to family physician-assisted smoking cessation interventions. Cancer Prev Control 1997;1:289–303.
Roberts NP, Kitchiner NJ, Kenardy J, Bisson JI. Early psychological interventions to treat acute traumatic stress symptoms. Cochrane Database Syst Rev 2010;3:CD007944. https://doi.org/10.1002/14651858.CD007944.pub2
Robinson PD, Oberoi S, Tomlinson D, Duong N, Davis H, Cataudella D, et al. Management of fatigue in children and adolescents with cancer and in paediatric recipients of haemopoietic stem-cell transplants: a clinical practice guideline. Lancet Child Adolesc Health 2018;2:371–8.
Rodrigues H, Figueira I, Lopes A, Gonçalves R, Mendlowicz MV, Coutinho ES, Ventura P. Does D-cycloserine enhance exposure therapy for anxiety disorders in humans? A meta-analysis. PLOS ONE 2014;9:e93519. https://doi.org/10.1371/journal.pone.0093519
Rogers D. Which educational interventions improve healthcare professionals’ resilience? Med Teach 2016;38:1236–41.
Rometsch-Ogioun El Sount C, Windthorst P, Denkinger J, Ziser K, Nikendei C, Kindermann D, et al. Chronic pain in refugees with posttraumatic stress disorder (PTSD): a systematic review on patients’ characteristics and specific interventions. J Psychosom Res 2018;30:30.
Roozen HG, de Waart R, van der Windt DA, van den Brink W, de Jong CA, Kerkhof AJ. A systematic review of the effectiveness of naltrexone in the maintenance treatment of opioid and alcohol dependence. Eur Neuropsychopharmacol 2006;16:311–23.
Rosa-Alcázar AI, Sánchez-Meca J, Rosa-Alcázar Á, Iniesta-Sepúlveda M, Olivares-Rodríguez J, Parada-Navas JL. Psychological treatment of obsessive–compulsive disorder in children and adolescents: a meta-analysis. Span J Psychol 2015;18:E20. https://doi.org/10.1017/sjp.2015.22
Rosendal M, Blankenstein AH, Morriss R, Fink P, Sharpe M, Burton C. Enhanced care by generalists for functional somatic symptoms and disorders in primary care. Cochrane Database Syst Rev 2013;10:CD008142. https://doi.org/10.1002/14651858.CD008142.pub2
Ross SD, Estok RP, Frame D, Stone LR, Ludensky V, Levine CB. Disability and chronic fatigue syndrome: a focus on function. Arch Intern Med 2004;164:1098–107. https://doi.org/10.1001/archinte.164.10.1098
Ruiz-Perez I, Murphy M, Pastor-Moreno G, Rojas-García A, Rodríguez-Barranco M. The effectiveness of HIV prevention interventions in socioeconomically disadvantaged ethnic minority women: a systematic review and meta-analysis. Am J Public Health 2017;107:e13–e21. https://doi.org/10.2105/AJPH.2017.304067
Ruotsalainen JH, Verbeek JH, Mariné A, Serra C. Preventing occupational stress in healthcare workers. Cochrane Database Syst Rev 2014;11:CD002892. https://doi.org/10.1002/14651858.CD002892.pub3
Ruotsalainen JH, Verbeek JH, Mariné A, Serra C. Preventing occupational stress in healthcare workers. Cochrane Database Syst Rev 2015;4:CD002892. https://doi.org/10.1002/14651858.CD002892.pub5
Sadowski L, Casteel C. Intimate partner violence towards women. BMJ Clin Evid 2010;2010:1013.
Salathé CR, Melloh M, Crawford R, Scherrer S, Boos N, Elfering A. Treatment efficacy, clinical utility, and cost-effectiveness of multidisciplinary biopsychosocial rehabilitation treatments for persistent low back pain: a systematic review. Global Spine J 2018;8:872–86. https://doi.org/10.1177/2192568218765483
Salt S, Mulvaney CA, Preston NJ. Drug therapy for symptoms associated with anxiety in adult palliative care patients. Cochrane Database Syst Rev 2017;5:CD004596. https://doi.org/10.1002/14651858.CD004596.pub3
Sánchez-Meca J, Rosa-Alcázar AI, Iniesta-Sepúlveda M, Rosa-Alcázar A. Differential efficacy of cognitive–behavioral therapy and pharmacological treatments for pediatric obsessive–compulsive disorder: a meta-analysis. J Anxiety Disord 2014;28:31–44. https://doi.org/10.1016/j.janxdis.2013.10.007
Sánchez-Meca J, Rosa-Alcazar AI, Lopez-Soler C. The psychological treatment of sexual abuse in children and adolescents: a meta-analysis. Int J Clin Health Psychol 2011;11:67–93.
van der Sande R, Buskens E, Allart E, van der Graaf Y, van Engeland H. Psychosocial intervention following suicide attempt: a systematic review of treatment interventions. Acta Psychiatr Scand 1997;96:43–50. https://doi.org/10.1111/j.1600-0447.1997.tb09903.x
Sankar A, Melin A, Lorenzetti V, Horton P, Costafreda SG, Fu CHY. A systematic review and meta-analysis of the neural correlates of psychological therapies in major depression. Psychiatry Res Neuroimaging 2018;279:31–9.
Satapathy S, Kaushal T, Bakhshi S, Chadda RK. Non-pharmacological interventions for pediatric cancer patients: a comparative review and emerging needs in India. Indian Pediatr 2018;55:225–32.
Savage J, Waddell A. Tinnitus. BMJ Clin Evid 2014;2014:0506.
Scaini S, Belotti R, Ogliari A, Battaglia M. A comprehensive meta-analysis of cognitive–behavioral interventions for social anxiety disorder in children and adolescents. J Anxiety Disord 2016;42:105–12. https://doi.org/10.1016/j.janxdis.2016.05.008
Scanlan JN. Interventions to reduce the use of seclusion and restraint in inpatient psychiatric settings: what we know so far a review of the literature. Int J Soc Psychiatry 2010;56:412–23. https://doi.org/10.1177/0020764009106630
Schaafsma F, Schonstein E, Ojajärvi A, Verbeek J. Physical conditioning programs for improving work outcomes among workers with back pain. Scand J Work Environ Health 2011;37:1–5.
Schaefert R, Hausteiner-Wiehle C, Häuser W, Ronel J, Herrmann M, Henningsen P. Non-specific, functional, and somatoform bodily complaints. Dtsch Arztebl Int 2012;109:803–13. https://doi.org/10.3238/arztebl.2012.0803
Scheer SJ, Watanabe TK, Radack KL. Randomized controlled trials in industrial low back pain. Part 3. Subacute/chronic pain interventions. Arch Phys Med Rehabil 1997;78:414–23.
Schirmbeck F, Zink M. Cognitive behavioural therapy for obsessive–compulsive symptoms in schizophrenia. Cogn Behav Ther 2013;6:E7.
Schleider JL, Weisz JR. Little treatments, promising effects? Meta-analysis of single-session interventions for youth psychiatric problems. J Am Acad Child Adolesc Psychiatry 2017;56:107–15.
Schmucker M, Lösel F. Does sexual offender treatment work? A systematic review of outcome evaluations. Psicothema 2008;20:10–19.
Schmucker M, Losel F. The effects of sexual offender treatment on recidivism: an international meta-analysis of sound quality evaluations. J Exp Criminol 2015;11:597–630.
Schneider M, Vernon H, Ko G, Lawson G, Perera J. Chiropractic management of fibromyalgia syndrome: a systematic review of the literature. J Manipulative Physiol Ther 2009;32:25–40. https://doi.org/10.1016/j.jmpt.2008.08.012
Schneider RL, Arch JJ, Wolitzky-Taylor KB. The state of personalized treatment for anxiety disorders: a systematic review of treatment moderators. Clin Psychol Rev 2015;38:39–54. https://doi.org/10.1016/j.cpr.2015.02.004
Schonstein E, Kenny D, Keating J, Koes B, Herbert RD. Physical conditioning programs for workers with back and neck pain: a cochrane systematic review. Spine 2003;28:E391–5. https://doi.org/10.1097/01.BRS.0000092482.76386.97
Schonstein E, Kenny DT, Keating J, Koes BW. Work conditioning, work hardening and functional restoration for workers with back and neck pain. Cochrane Database Syst Rev 2003;1:CD001822. https://doi.org/10.1002/14651858.CD001822
Schroeck JL, Ford J, Conway EL, Kurtzhalts KE, Gee ME, Vollmer KA, Mergenhagen KA. Review of safety and efficacy of sleep medicines in older adults. Clin Ther 2016;38:2340–72.
Schwartz C, Schlegl S, Kuelz AK, Voderholzer U. Treatment-seeking in OCD community cases and psychological treatment actually provided to treatment-seeking patients: a systematic review. J Obsess Compuls Relat Disord 2013;2:448–56.
Seda G, Sanchez-Ortuno MM, Welsh CH, Halbower AC, Edinger JD. Comparative meta-analysis of prazosin and imagery rehearsal therapy for nightmare frequency, sleep quality, and posttraumatic stress. J Clin Sleep Med 2015;11:11–22. https://doi.org/10.5664/jcsm.4354
Seferiadis A, Rosenfeld M, Gunnarsson R. A review of treatment interventions in whiplash-associated disorders. Eur Spine J 2004;13:387–97. https://doi.org/10.1007/s00586-004-0709-1
Segool NK, Carlson JS. Efficacy of cognitive-behavioral and pharmacological treatments for children with social anxiety. Depress Anxiety 2008;25:620–31. https://doi.org/10.1002/da.20410
Segredou I, Xenitidis K, Panagiotopoulou M, Bochtsou V, Antoniadou O, Livaditis M. Group psychosocial interventions for adults with schizophrenia and bipolar illness: the evidence base in the light of publications between 1986 and 2006. Int J Soc Psychiatry 2012;58:229–38. https://doi.org/10.1177/0020764010390429
Sepede G, Sarchione F, Matarazzo I, Di Giannantonio M, Salerno RM. Premenstrual dysphoric disorder without comorbid psychiatric conditions: a systematic review of therapeutic options. Clin Neuropharmacol 2016;39:241–61. https://doi.org/10.1097/WNF.0000000000000173
Sereda M, Xia J, El Refaie A, Hall DA, Hoare DJ. Sound therapy (using amplification devices and/or sound generators) for tinnitus. Cochrane Database Syst Rev 2018;12:CD013094. https://doi.org/10.1002/14651858.CD013094.pub2
Sesel AL, Sharpe L, Naismith SL. Efficacy of psychosocial interventions for people with multiple sclerosis: a meta-analysis of specific treatment effects. Psychother Psychosom 2018;87:105–11. https://doi.org/10.1159/000486806
Sharma E, Thennarasu K, Reddy YC. Long-term outcome of obsessive–compulsive disorder in adults: a meta-analysis. J Clin Psychiatry 2014;75:1019–27. https://doi.org/10.4088/JCP.13r08849
Shen J, Rouse J, Godbole M, Wells HL, Boppana S, Schwebel DC. Systematic review: interventions to educate children about dog safety and prevent pediatric dog-bite injuries: a meta-analytic review. J Pediatr Psychol 2017;42:779–91. https://doi.org/10.1093/jpepsy/jsv164
Shergis JL, Ni X, Jackson ML, Zhang AL, Guo X, Li Y, et al. A systematic review of acupuncture for sleep quality in people with insomnia. Complement Ther Med 2016;26:11–20. https://doi.org/10.1016/j.ctim.2016.02.007
Sherr L, Clucas C, Harding R, Sibley E, Catalan J. HIV and depression – a systematic review of interventions. Psychol Health Med 2011;16:493–527. https://doi.org/10.1080/13548506.2011.579990
Shingler E, Robles LA, Perry R, Penfold C, Ness AR, Thomas S, et al. Systematic review evaluating randomized controlled trials of smoking and alcohol cessation interventions in people with head and neck cancer and oral dysplasia. Head Neck 2018;40:1845–53. https://doi.org/10.1002/hed.25138
Sim J, Adams N. Systematic review of randomized controlled trials of nonpharmacological interventions for fibromyalgia. Clin J Pain 2002;18:324–36. https://doi.org/10.1097/00002508-200209000-00008
Simon SS, Cordás TA, Bottino CM. Cognitive behavioral therapies in older adults with depression and cognitive deficits: a systematic review. Int J Geriatr Psychiatry 2015;30:223–33. https://doi.org/10.1002/gps.4239
Singer GH, Ethridge BL, Aldana SI. Primary and secondary effects of parenting and stress management interventions for parents of children with developmental disabilities: a meta-analysis. Ment Retard Dev Disabil Res Rev 2007;13:357–69. https://doi.org/10.1002/mrdd.20175
Singh N, Reece J. Psychotherapy, pharmacotherapy, and their combination for adolescents with major depressive disorder: a meta-analysis. Aust Educ Dev Psychol 2014;31:47–65.
Sivaraman B, Nye E, Bowes L. School-based anti-bullying interventions for adolescents in low- and middle-income countries: a systematic review. Aggress Violent Behav 2018;45:154–62.
Slotema CW, Blom JD, Niemantsverdriet MBA, Sommer IEC. Auditory verbal hallucinations in borderline personality disorder and the efficacy of antipsychotics: a systematic review. Front Psychiatry 2018;9:347. https://doi.org/10.3389/fpsyt.2018.00347
Smith CA, Armour M, Lee MS, Wang LQ, Hay PJ. Acupuncture for depression. Cochrane Database Syst Rev 2018;3:CD004046.
Smith CA, Hay PP. Acupuncture for depression. Cochrane Database Syst Rev 2005;2:CD004046.
Smith CA, Hay PP, MacPherson H. Acupuncture for depression. Cochrane Database Syst Rev 2010;1:CD004046.
Smith DP, Dunn KI, Harvey PW, Battersby MW, Pols RG. Assessing randomised clinical trials of cognitive and exposure therapies for gambling disorders: a systematic review. Behav Change 2013;30:139–58.
Smits JA, Hofmann SG. A meta-analytic review of the effects of psychotherapy control conditions for anxiety disorders. Psychol Med 2009;39:229–39. https://doi.org/10.1017/S0033291708003498
Soomro GM. Obsessive compulsive disorder. BMJ Clin Evid 2012;2012:1004.
Spahn JM, Reeves RS, Keim KS, Laquatra I, Kellogg M, Jortberg B, Clark NA. State of the evidence regarding behavior change theories and strategies in nutrition counseling to facilitate health and food behavior change. J Am Diet Assoc 2010;110:879–91. https://doi.org/10.1016/j.jada.2010.03.021
Spence JD, Barnett PA, Linden W, Ramsden V, Taenzer P. Recommendations on stress management. CMAJ 1999;160:S46–S50.
Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut 2007;56:1770–98.
Spoelstra SL, Schueller M, Hilton M, Ridenour K. Interventions combining motivational interviewing and cognitive behaviour to promote medication adherence: a literature review. J Clin Nurs 2015;24:1163–73. https://doi.org/10.1111/jocn.12738
Springer KS, Levy HC, Tolin DF. Remission in CBT for adult anxiety disorders: a meta-analysis. Clin Psychol Rev 2018;61:1–8.
Stanton A, Grimshaw G. Tobacco cessation interventions for young people. Cochrane Database Syst Rev 2013;8:CD003289. https://doi.org/10.1002/14651858.CD003289.pub5
Steel JL, Bress K, Popichak L, Evans JS, Savkova A, Biala M, et al. A systematic review of randomized controlled trials testing the efficacy of psychosocial interventions for gastrointestinal cancers. J Gastrointest Cancer 2014;45:181–9. https://doi.org/10.1007/s12029-014-9605-z
Steinert C, Hofmann M, Leichsenring F, Kruse J. What do we know today about the prospective long-term course of social anxiety disorder? A systematic literature review. J Anxiety Disord 2013;27:692–702. https://doi.org/10.1016/j.janxdis.2013.08.002
Sullivan PJ. A Meta-analysis of the Effectiveness and Efficiency of Dcycloserine-augmented Exposure Therapy with Treatment Resistant Pediatric OCD Patients. PhD thesis. Palo Alto, CA: Palo Alto University; 2018.
Sumathipala A. What is the evidence for the efficacy of treatments for somatoform disorders? A critical review of previous intervention studies. Psychosom Med 2007;69:889–900.
Tan L, Wang MJ, Modini M, Joyce S, Mykletun A, Christensen H, Harvey SB. Preventing the development of depression at work: a systematic review and meta-analysis of universal interventions in the workplace. BMC Med 2014;12:74. https://doi.org/10.1186/1741-7015-12-74
Tarrier N, Taylor K, Gooding P. Cognitive–behavioral interventions to reduce suicide behavior: a systematic review and meta-analysis. Behav Modif 2008;32:77–108.
Taylor TL, Killaspy H, Wright C, Turton P, White S, Kallert TW, et al. A systematic review of the international published literature relating to quality of institutional care for people with longer term mental health problems. BMC Psychiatry 2009;9:55. https://doi.org/10.1186/1471-244X-9-55
Thabane L, Chu R, Cuddy K, Douketis J. What is the quality of reporting in weight loss intervention studies? A systematic review of randomized controlled trials. Int J Obes 2007;31:1554–9.
Thombs BD, de Jonge P, Coyne JC, Whooley MA, Frasure-Smith N, Mitchell AJ, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA 2008;300:2161–71. https://doi.org/10.1001/jama.2008.667
Tobon JI, Ouimet AJ, Dozois DJA. Attentional bias in anxiety disorders following cognitive behavioral treatment. J Cogn Psychother 2011;25:114–29.
Tolin DF. Is cognitive-behavioral therapy more effective than other therapies? A meta-analytic review. Clin Psychol Rev 2010;30:710–20. https://doi.org/10.1016/j.cpr.2010.05.003
Tolin DF. Can cognitive behavioral therapy for anxiety and depression be improved with pharmacotherapy? A meta-analysis. Psychiatr Clin North Am 2017;40:715–38.
Tolin DF, Frost RO, Steketee G, Muroff J. Cognitive behavioral therapy for hoarding disorder: a meta-analysis. Depress Anxiety 2015;32:158–66. https://doi.org/10.1002/da.22327
Tumur I, Kaltenthaler E, Ferriter M, Beverley C, Parry G. Computerised cognitive behaviour therapy for obsessive–compulsive disorder: a systematic review. Psychother Psychosom 2007;76:196–202.
Turner BJ, Austin SB, Chapman AL. Treating nonsuicidal self-injury: a systematic review of psychological and pharmacological interventions. Can J Psychiatry 2014;59:576–85.
Turner W, Macdonald GM, Dennis JA. Cognitive–behavioural training interventions for assisting foster carers in the management of difficult behaviour. Cochrane Database Syst Rev 2007;1:CD003760. https://doi.org/10.1002/14651858.CD003760.pub3
van Balkom AJ, Bakker A, Spinhoven P, Blaauw BM, Smeenk S, Ruesink B. A meta-analysis of the treatment of panic disorder with or without agoraphobia: a comparison of psychopharmacological, cognitive-behavioral, and combination treatments. J Nerv Ment Dis 1997;185:510–16. https://doi.org/10.1097/00005053-199708000-00006
Van Cauwenbergh D, De Kooning M, Ickmans K, Nijs J. How to exercise people with chronic fatigue syndrome: evidence-based practice guidelines. Eur J Clin Invest 2012;42:1136–44. https://doi.org/10.1111/j.1365-2362.2012.02701.x
van den Heuvel JF, Groenhof TK, Veerbeek JH, van Solinge WW, Lely AT, Franx A, Bekker MN. eHealth as the next-generation perinatal care: an overview of the literature [published online ahead of print 06 05 2018]. J Med Internet Res 2018. https://doi.org/10.2196/jmir.9262
van der Sande R, Buskens E, Allart E, van der Graaf Y, van Engeland H. Psychosocial intervention following suicide attempt: a systematic review of treatment interventions. Acta Psychiatr Scand 1997;96:43–50. https://doi.org/10.1111/j.1600-0447.1997.tb09903.x
van der Straten AL, Denys D, van Wingen GA. Impact of treatment on resting cerebral blood flow and metabolism in obsessive compulsive disorder: a meta-analysis. Sci Rep 2017;7:17464. https://doi.org/10.1038/s41598-017-17593-7
Vancampfort D, Vanderlinden J, De Hert M, Adámkova M, Skjaerven LH, Catalán-Matamoros D, et al. A systematic review on physical therapy interventions for patients with binge eating disorder. Disabil Rehabil 2013;35:2191–6. https://doi.org/10.3109/09638288.2013.771707
Vandborg SK, Hartmann TB, Bennedsen BE, Pedersen AD, Eskildsen A, Videbech PB, Thomsen PH. Do cognitive functions in obsessive–compulsive disorder change after treatment? A systematic review and a double case report. Nord J Psychiatry 2012;66:60–7. https://doi.org/10.3109/08039488.2011.626869
Vaughn MG, Howard MO. Adolescent substance abuse treatment: a synthesis of controlled evaluations. Res Soc Work Pract 2004;14:325–35.
Veale D, Naismith I, Miles S, Gledhill LJ, Stewart G, Hodsoll J. Outcomes for residential or inpatient intensive treatment of obsessive–compulsive disorder: a systematic review and meta-analysis. J Obsess Compuls Relat Disord 2016;8:38–49.
Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain 2011;152:533–42. https://doi.org/10.1016/j.pain.2010.11.002
Verheul R, Herbrink M. The efficacy of various modalities of psychotherapy for personality disorders: a systematic review of the evidence and clinical recommendations. Int Rev Psychiatry 2007;19:25–38.
Verhey R, Chibanda D, Brakarsh J, Seedat S. Psychological interventions for post-traumatic stress disorder in people living with HIV in resource poor settings: a systematic review. Trop Med Int Health 2016;21:1198–208. https://doi.org/10.1111/tmi.12756
Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive-behavioral therapy’s effects. J Consult Clin Psychol 2007;75:475–88.
Vocks S, Tuschen-Caffier B, Pietrowsky R, Rustenbach SJ, Kersting A, Herpertz S. Meta-analysis of the effectiveness of psychological and pharmacological treatments for binge eating disorder. Int J Eat Disord 2010;43:205–17. https://doi.org/10.1002/eat.20696
Voshaar RC, Couvée JE, van Balkom AJ, Mulder PG, Zitman FG. Strategies for discontinuing long-term benzodiazepine use: meta-analysis. Br J Psychiatry 2006;189:213–20.
Wade AG. Use of the internet to assist in the treatment of depression and anxiety: a systematic review. Prim Care Companion J Clin Psychiatry 2010;12:PCC.09r00876. https://doi.org/10.4088/PCC.09r00876blu
Wagner KD, Unger JB, Bluthenthal RN, Andreeva VA, Pentz MA. Cognitive behavioral theories used to explain injection risk behavior among injection drug users: a review and suggestions for the integration of cognitive and environmental models. Health Educ Behav 2010;37:504–32. https://doi.org/10.1177/1090198109357319
Walker DF, McGovern SK, Poey EL, Otis KE. Treatment effectiveness for male adolescent sexual offenders: a meta-analysis and review. J Child Sex Abus 2004;13:281–93. https://doi.org/10.1300/j070v13n03_14
Wariki WM, Ota E, Mori R, Koyanagi A, Hori N, Shibuya K. Behavioral interventions to reduce the transmission of HIV infection among sex workers and their clients in low- and middle-income countries. Cochrane Database Syst Rev 2012;2:CD005272. https://doi.org/10.1002/14651858.CD005272.pub3
Warner JP, Butler R, Gupta S. Dementia. BMJ Clin Evid 2010;2010:1001.
Watanabe N, Churchill R, Furukawa TA. Combined psychotherapy plus benzodiazepines for panic disorder. Cochrane Database Syst Rev 2009;1:CD005335. https://doi.org/10.1002/14651858.CD005335.pub2
Wegner I, Hall DA, Smit AL, McFerran D, Stegeman I. Betahistine for tinnitus. Cochrane Database Syst Rev 2018;12:CD013093.
Weisz JR, Kuppens S, Ng MY, Eckshtain D, Ugueto AM, Vaughn-Coaxum R, et al. What five decades of research tells us about the effects of youth psychological therapy: a multilevel meta-analysis and implications for science and practice. Am Psychol 2017;72:79–117. https://doi.org/10.1037/a0040360
Werneke U, Taylor D, Sanders TA, Wessely S. Behavioural management of antipsychotic-induced weight gain: a review. Acta Psychiatr Scand 2003;108:252–9.
Wheeler S, Acord-Vira A, Davis D. Effectiveness of interventions to improve occupational performance for people with psychosocial, behavioral, and emotional impairments after brain injury: a systematic review. Am J Occup Ther 2016;70:7003180060p1–9. https://doi.org/10.5014/ajot.115.020677
Williams M, Viscusi JA. Hoarding disorder and a systematic review of treatment with cognitive behavioral therapy. Cogn Behav Ther 2016;45:93–110. https://doi.org/10.1080/16506073.2015.1133697
Wilson DB, Gottfredson DC, Najaka SS. School-based prevention of problem behaviors: a meta-analysis. J Quant Criminol 2001;17:247.
Winkley K, Ismail K, Landau S, Eisler I. Psychological interventions to improve glycaemic control in patients with type 1 diabetes: systematic review and meta-analysis of randomised controlled trials. BMJ 2006;333:65.
Winter D, Bradshaw S, Bunn F, Wellsted D. A systematic review of the literature on counselling and psychotherapy for the prevention of suicide: 1. Quantitative outcome and process studies. Counsel Psychother Res 2013;13:164–83.
Witt K, de Moraes DP, Salisbury TT, Arensman E, Gunnell D, Hazell P, et al. Treatment as usual (TAU) as a control condition in trials of cognitive behavioural-based psychotherapy for self-harm: impact of content and quality on outcomes in a systematic review. J Affect Disord 2018;235:434–47.
Woodruff SC. The Effects of Mindfulness and Acceptance-based Interventions and Cognitive–Behavioral Interventions on Positive and Negative Affect: A Meta-analysis. PhD thesis. Washington, DC: The Catholic University of America; 2015.
Wozney L, Huguet A, Bennett K, Radomski AD, Hartling L, Dyson M, et al. How do eHealth programs for adolescents with depression work? A realist review of persuasive system design components in internet-based psychological therapies. J Med Internet Res 2017;19:e266. https://doi.org/10.2196/jmir.7573
Yorke J, Fleming SL, Shuldham CM. Psychological interventions for adults with asthma. Cochrane Database Syst Rev 2006;1:CD002982. https://doi.org/10.1002/14651858.CD002982.pub3
Young SC. A Failure to Self-regulate? A Research Synthesis of the Cognitive–Behavioral Literature Targeting the Improvement of Self-regulation among School-age Males. PhD thesis. Virginia Beach, VA: Regent University; 2015.
Yusufov M, Nicoloro-SantaBarbara J, Grey NE, Moyer A, Lobel M. Meta-analytic evaluation of stress reduction interventions for undergraduate and graduate students. Int J Stress Manag 2019;26:132–45.
Zakrzewska J, Buchanan JA. Burning mouth syndrome. BMJ Clin Evid 2016;2016:1301.
Zakrzewska JM, Forssell H, Glenny AM. Interventions for the treatment of burning mouth syndrome. Cochrane Database Syst Rev 2005;1:CD002779. https://doi.org/10.1002/14651858.CD002779.pub2
Zalta AK. A meta-analysis of anxiety symptom prevention with cognitive–behavioral interventions. J Anxiety Disord 2011;25:749–60.
Zech N, Hansen E, Bernardy K, Häuser W. Efficacy, acceptability and safety of guided imagery/hypnosis in fibromyalgia – a systematic review and meta-analysis of randomized controlled trials. Eur J Pain 2017;21:217–27. https://doi.org/10.1002/ejp.933
Zeppegno P, Gattoni E, Mastrangelo M, Gramaglia C, Sarchiapone M. Psychosocial suicide prevention interventions in the elderly: a mini-review of the literature. Front Psychol 2018;9:2713. https://doi.org/10.3389/fpsyg.2018.02713
Zhang H, Zhang Y, Yang L, Yuan S, Zhou X, Pu J, et al. Efficacy and acceptability of psychotherapy for anxious young children: a meta-analysis of randomized controlled trials. J Nerv Ment Dis 2017;205:931–41. https://doi.org/10.1097/NMD.0000000000000749
Studies excluded because no cognitive–behavioural therapy randomised controlled trial summary (n = 520)
Aafjes-van Doorn K, Barber JP. Systematic review of in-session affect experience in cognitive behavioral therapy for depression. Cogn Ther Res 2017;41:807–28.
Aderka IM, Nickerson A, Bøe HJ, Hofmann SG. Sudden gains during psychological treatments of anxiety and depression: a meta-analysis. J Consult Clin Psychol 2012;80:93–101. https://doi.org/10.1037/a0026455
Aggarwal VR, Tickle M, Javidi H, Peters S. Reviewing the evidence: can cognitive behavioral therapy improve outcomes for patients with chronic orofacial pain? J Orofac Pain 2010;24:163–71.
Alam-mehrjerdi Z, Daneshmand R, Samiei M, Samadi R, Abdollahi M, Dolan K. Women-only drug treatment services and needs in Iran: the first review of current literature. Daru 2016;24:3. https://doi.org/10.1186/s40199-016-0141-1
Aldi GA, Bertoli G, Ferraro F, Pezzuto A, Cosci F. Effectiveness of pharmacological or psychological interventions for smoking cessation in smokers with major depression or depressive symptoms: a systematic review of the literature. Subst Abus 2018;39:289–306. https://doi.org/10.1080/08897077.2018.1439802
Alexander H. Coping with Sickle Cell Disease using Cognitive Behavior Therapy. PhD thesis. Minneapolis, MN: Walden University; 2018.
Allen S, Dalton WT. Treatment of eating disorders in primary care: a systematic review. J Health Psychol 2011;16:1165–76. https://doi.org/10.1177/1359105311402244
Altayar O, Sharma V, Prokop LJ, Sood A, Murad MH. Psychological therapies in patients with irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Gastroenterol Res Pract 2015;2015:549308. https://doi.org/10.1155/2015/549308
Andersson G, Rozental A, Shafran R, Carlbring P. Long-term effects of internet-supported cognitive behaviour therapy. Expert Rev Neurother 2018;18:21–8. https://doi.org/10.1080/14737175.2018.1400381
Andreasson S, Ojehagen A. Psychosocial Treatment for Alcohol Dependence. In Berglund M, Thelander S, Johnsson E, editors. Treating Alcohol and Drug Abuse: An Evidence Based Review. Weinheim: Wiley-VCH Veriag GmbH and Co KGaA; 2003. pp. 43–188.
Apolinario-Hagen J. Internet-delivered psychological treatment options for panic disorder: a review on their efficacy and acceptability. Psychiatry Investig 2018;20:20.
Arroyo K, Lundahl B, Butters R, Vanderloo M, Wood DS. Short-term interventions for survivors of intimate partner violence: a systematic review and meta-analysis. Trauma Violence Abuse 2017;18:155–71. https://doi.org/10.1177/1524838015602736
Astin JA, Beckner W, Soeken K, Hochberg MC, Berman B. Psychological interventions for rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum 2002;47:291–302. https://doi.org/10.1002/art.10416
Aydın A. [Parental involvement in cognitive–behavioral therapy for children with anxiety disorders.] Turk Psikiyatri Derg 2014;25:181–9.
Ayers CR, Sorrell JT, Thorp SR, Wetherell JL. Evidence-based psychological treatments for late-life anxiety. Psychol Aging 2007;22:8–17.
Baandrup L, Østrup Rasmussen J, Klokker L, Austin S, Bjørnshave T, Fuglsang Bliksted V, et al. Treatment of adult patients with schizophrenia and complex mental health needs – a national clinical guideline. Nord J Psychiatry 2016;70:231–40. https://doi.org/10.3109/08039488.2015.1074285
Baker AL, Thornton LK, Hiles S, Hides L, Lubman DI. Psychological interventions for alcohol misuse among people with co-occurring depression or anxiety disorders: a systematic review. J Affect Disord 2012;139:217–29. https://doi.org/10.1016/j.jad.2011.08.004
Ballesio A, Aquino MRJV, Feige B, Johann AF, Kyle SD, Spiegelhalder K, et al. The effectiveness of behavioural and cognitive behavioural therapies for insomnia on depressive and fatigue symptoms: a systematic review and network meta-analysis. Sleep Med Rev 2018;37:114–29.
Bandelow B, Lichte T, Rudolf S, Wiltink J, Beutel ME. The diagnosis of and treatment recommendations for anxiety disorders. Dtsch Arztebl Int 2014;111:473–80. https://doi.org/10.3238/arztebl.2014.0473
Bandelow B, Reitt M, Röver C, Michaelis S, Görlich Y, Wedekind D. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol 2015;30:183–92. https://doi.org/10.1097/YIC.0000000000000078
Bandelow B, Sagebiel A, Belz M, Görlich Y, Michaelis S, Wedekind D. Enduring effects of psychological treatments for anxiety disorders: meta-analysis of follow-up studies. Br J Psychiatry 2018;212:333–8. https://doi.org/10.1192/bjp.2018.49
Baraniak A, Sheffield D. The efficacy of psychologically based interventions to improve anxiety, depression and quality of life in COPD: a systematic review and meta-analysis. Patient Educ Couns 2011;83:29–36. https://doi.org/10.1016/j.pec.2010.04.010
Barkowski S, Schwartze D, Strauss B, Burlingame GM, Barth J, Rosendahl J. Efficacy of group psychotherapy for social anxiety disorder: a meta-analysis of randomized-controlled trials. J Anxiety Disord 2016;39:44–64.
Barlow J, Bergman H, Kornør H, Wei Y, Bennett C. Group-based parent training programmes for improving emotional and behavioural adjustment in young children. Cochrane Database Syst Rev 2016;8:CD003680. https://doi.org/10.1002/14651858.CD003680.pub3
Barlow J, Smailagic N, Huband N, Roloff V, Bennett C. Group-based parent training programmes for improving parental psychosocial health. Cochrane Database Syst Rev 2012;6:CD002020. https://doi.org/10.1002/14651858.CD002020.pub3
Barlow JH, Ellard DR, Hainsworth JM, Jones FR, Fisher A. A review of self-management interventions for panic disorders, phobias and obsessive–compulsive disorders. Acta Psychiatr Scand 2005;111:272–85.
Barrett PM, Farrell L, Pina AA, Peris TS, Piacentini J. Evidence-based psychosocial treatments for child and adolescent obsessive–compulsive disorder. J Clin Child Adolesc Psychol 2008;37:131–55. https://doi.org/10.1080/15374410701817956
Barth J, Munder T, Gerger H, Nüesch E, Trelle S, Znoj H, et al. Comparative efficacy of seven psychotherapeutic interventions for patients with depression: a network meta-analysis. PLOS Med 2013;10:e1001454. https://doi.org/10.1371/journal.pmed.1001454
Beck R, Fernandez E. Cognitive–behavioral therapy in the treatment of anger: a meta-analysis. Cogn Ther Res 1998;22:63–74.
Belleville G, Cousineau H, Levrier K, St-Pierre-Delorme ME, Marchand A. The impact of cognitive–behavior therapy for anxiety disorders on concomitant sleep disturbances: a meta-analysis. J Anxiety Disord 2010;24:379–86.
Bennett DS, Gibbons TA. Efficacy of child cognitive–behavioral interventions for antisocial behavior: a meta-analysis. Child Fam Behav Ther 2000;22:1–15.
Berkman ND, Bulik CM, Brownley KA, Lohr KN, Sedway JA, Rooks A, et al. Management of eating disorders. Evid Rep Technol Assess 2006;135:1–166.
Bernardy K, Klose P, Welsch P, Häuser W. Efficacy, acceptability and safety of cognitive behavioural therapies in fibromyalgia syndrome – a systematic review and meta-analysis of randomized controlled trials. Eur J Pain 2018;22:242–60. https://doi.org/10.1002/ejp.1121
Berner M, Günzler C. Efficacy of psychosocial interventions in men and women with sexual dysfunctions – a systematic review of controlled clinical trials: part 1 – the efficacy of psychosocial interventions for male sexual dysfunction. J Sex Med 2012;9:3089–107. https://doi.org/10.1111/j.1743-6109.2012.02970.x
Berryhill MB, Halli-Tierney A, Culmer N, Williams N, Betancourt A, King M, et al. Videoconferencing psychological therapy and anxiety: a systematic review. Fam Pract 2018;04:04.
Bethel NJ. Self-applied Interventions for Social Anxiety. PhD thesis. Sheffield: University of Sheffield; 2010.
Beumont P, Hay P, Beumont D, Birmingham L, Derham H, Jordan A, et al. Australian and New Zealand clinical practice guidelines for the treatment of anorexia nervosa. Aust N Z J Psychiatry 2004;38:659–70. https://doi.org/10.1080/j.1440-1614.2004.01449.x
Bhatia U, Nadkarni A, Murthy P, Rao R, Crome I. Recent advances in treatment for older people with substance use problems: an updated systematic and narrative review. Eur Geriatr Med 2015;6:580–6.
Biajar A, Mollayeva T, Sokoloff S, Colantonio A. Assistive technology to enable sleep function in patients with acquired brain injury: issues and opportunities. Br J Occup Ther 2017;80:225–49.
Binnie J, Blainey S. The use of cognitive behavioural therapy for adults with autism spectrum disorders: a review of the evidence. Ment Health Rev J 2013;18:93–104.
Birur B, Moore NC, Davis LL. An evidence-based review of early intervention and prevention of posttraumatic stress disorder. Community Ment Health J 2017;53:183–201. https://doi.org/10.1007/s10597-016-0047-x
Blake MJ, Sheeber LB, Youssef GJ, Raniti MB, Allen NB. Systematic review and meta-analysis of adolescent cognitive–behavioral sleep interventions. Clin Child Fam Psychol Rev 2017;20:227–49. https://doi.org/10.1007/s10567-017-0234-5
Bodde NM, Brooks JL, Baker GA, Boon PA, Hendriksen JG, Mulder OG, Aldenkamp AP. Psychogenic non-epileptic seizures – definition, etiology, treatment and prognostic issues: a critical review. Seizure 2009;18:543–53. https://doi.org/10.1016/j.seizure.2009.06.006
Bogdanov S, Naismith S, Lah S. Sleep outcomes following sleep-hygiene-related interventions for individuals with traumatic brain injury: a systematic review. Brain Inj 2017;31:422–33. https://doi.org/10.1080/02699052.2017.1282042
Bomasang-Layno E, Fadlon I, Murray AN, Himelhoch S. Antidepressive treatments for Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 2015;21:833–42. https://doi.org/10.1016/j.parkreldis.2015.04.018
Bomasang-Layno E, Fadlon I, Murray AN, Himelhoch S. Antidepressive treatments for Parkinson’s disease: a systematic review and meta-analysis. Am J Geriatr Psychiatry 2016;24(3 Suppl. 1):S102–S3.
Bouman TK. Psychological treatments for hypochondriasis: a narrative review. Curr Psychiatry Rev 2014;10:58–69.
Bourke L, Boorjian SA, Briganti A, Klotz L, Mucci L, Resnick MJ, et al. Survivorship and improving quality of life in men with prostate cancer. Eur Urol 2015;68:374–83. https://doi.org/10.1016/j.eururo.2015.04.023
Braun SR, Gregor B, Tran US. Comparing bona fide psychotherapies of depression in adults with two meta-analytical approaches. PLOS ONE 2013;8:e68135. https://doi.org/10.1371/journal.pone.0068135
Brendel KE. A Systematic Review and Meta-analysis of the Effectiveness of Child–Parent Interventions for Children and Adolescents with Anxiety Disorders. Dissertation Abstracts International Section A: Humanities and Social Sciences. 2012;72:2965.
Brent M, Lobato D, LeLeiko N. Psychological treatments for pediatric functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr 2009;48:13–21. https://doi.org/10.1097/MPG.0b013e3181761516
Britton EP. A Meta-analysis of Group Cognitive Behavior Therapy for Children and Adolescents with Social Phobia. PhD thesis. Berkeley, CA: The Wright Institute; 2007.
Brooks AT, Wallen GR. Sleep disturbances in individuals with alcohol-related disorders: a review of cognitive–behavioral therapy for insomnia (CBT-I) and associated non-pharmacological therapies. Subst Abuse 2014;8:55–62. https://doi.org/10.4137/SART.S18446
Brown RC, Witt A, Fegert JM, Keller F, Rassenhofer M, Plener PL. Psychosocial interventions for children and adolescents after man-made and natural disasters: a meta-analysis and systematic review. Psychol Med 2017;47:1893–905. https://doi.org/10.1017/S0033291717000496
Browne C, Smith IC. Psychological interventions for anger and aggression in people with intellectual disabilities in forensic services. Aggress Violent Behav 2018;39:1–14.
Brownley KA, Berkman ND, Sedway JA, Lohr KN, Bulik CM. Binge eating disorder treatment: a systematic review of randomized controlled trials. Int J Eat Disord 2007;40:337–48. https://doi.org/10.1002/eat.20370
Brunwasser SM, Gillham JE, Kim ES. A meta-analytic review of the Penn Resiliency Program’s effect on depressive symptoms. J Consult Clin Psychol 2009;77:1042–54. https://doi.org/10.1037/a0017671
Buhrman M, Gordh T, Andersson G. Internet interventions for chronic pain including headache: a systematic review. Internet Interv 2016;4:17–34. https://doi.org/10.1016/j.invent.2015.12.001
Bulik CM, Berkman ND, Brownley KA, Sedway JA, Lohr KN. Anorexia nervosa treatment: a systematic review of randomized controlled trials. Int J Eat Disord 2007;40:310–20. https://doi.org/10.1002/eat.20367
Burghardt S, Koranyi S, Magnucki G, Strauss B, Rosendahl J. Non-pharmacological interventions for reducing mental distress in patients undergoing dental procedures: systematic review and meta-analysis. J Dent 2018;69:22–31.
Burin AB, Osorio FL. Interventions for music performance anxiety: results from a systematic literature review. Rev Psiquiatr Clin 2016;43:116–31.
Cairns R, Hotopf M. A systematic review describing the prognosis of chronic fatigue syndrome. Occup Med 2005;55:20–31.
Calati R, Pedrini L, Alighieri S, Alvarez MI, Desideri L, Durante D, et al. Is cognitive behavioural therapy an effective complement to antidepressants in adolescents? A meta-analysis. Acta Neuropsychiatr 2011;23:263–71. https://doi.org/10.1111/j.1601-5215.2011.00595.x
Calear AL, Christensen H. Systematic review of school-based prevention and early intervention programs for depression. J Adolesc 2010;33:429–38. https://doi.org/10.1016/j.adolescence.2009.07.004
Calear AL, Christensen H. Review of internet-based prevention and treatment programs for anxiety and depression in children and adolescents. Med J Aust 2010;192:S12–4.
Cameron SK, Rodgers J, Dagnan D. The relationship between the therapeutic alliance and clinical outcomes in cognitive behaviour therapy for adults with depression: a meta-analytic review. Clin Psychol Psychother 2018;25:446–56. https://doi.org/10.1002/cpp.2180
Campbell CL, Campbell LC. A systematic review of cognitive behavioral interventions in advanced cancer. Patient Educ Couns 2012;89:15–24. https://doi.org/10.1016/j.pec.2012.06.019
Carnevale TD. Universal adolescent depression prevention programs: a review. J Sch Nurs 2013;29:181–95. https://doi.org/10.1177/1059840512469231
Carolan S, Harris PR, Cavanagh K. Improving employee well-being and effectiveness: systematic review and meta-analysis of web-based psychological interventions delivered in the workplace. J Med Internet Res 2017;19:e271. https://doi.org/10.2196/jmir.7583
Carrico AW, Zepf R, Meanley S, Batchelder A, Stall R. Critical review: when the party is over: a systematic review of behavioral interventions for substance-using men who have sex with men. J Acquir Immune Defic Syndr 2016;73:299–306.
Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Bärnighausen T. Interventions to improve adherence to antiretroviral therapy: a rapid systematic review. AIDS 2014;28(Suppl. 2):187–204. https://doi.org/10.1097/QAD.0000000000000252
Chambers CT, Taddio A, Uman LS, McMurtry CM, HELPinKIDS Team. Psychological interventions for reducing pain and distress during routine childhood immunizations: a systematic review. Clin Ther 2009;31(Suppl. 2):77–103. https://doi.org/10.1016/j.clinthera.2009.07.023
Chambers D, Bagnall AM, Hempel S, Forbes C. Interventions for the treatment, management and rehabilitation of patients with chronic fatigue syndrome/myalgic encephalomyelitis: an updated systematic review. J R Soc Med 2006;99:506–20.
Chambers SK, Hyde MK, Smith DP, Hughes S, Yuill S, Egger S, et al. New challenges in psycho-oncology research III: a systematic review of psychological interventions for prostate cancer survivors and their partners: clinical and research implications. Psycho-Oncology 2017;26:873–913. https://doi.org/10.1002/pon.4431
Chan EK-H. Efficacy of Cognitive–Behavioral, Pharmacological, and Combined Treatments of Depression: A Meta-analysis. PhD thesis. Calgary, AB: University of Calgary; 2006.
Chandler ML. Psychotherapy for adult attention deficit/hyperactivity disorder: a comparison with cognitive behaviour therapy. J Psychiatr Ment Health Nurs 2013;20:814–20. https://doi.org/10.1111/jpm.12023
Chapman A, Liu S, Merkouris S, Enticott JC, Yang H, Browning CJ, Thomas SA. Psychological interventions for the management of glycemic and psychological outcomes of type 2 diabetes mellitus in China: a systematic review and meta-analyses of randomized controlled trials. Front Public Health 2015;3:252. https://doi.org/10.3389/fpubh.2015.00252
Chatters R, Cooper K, Day E, Knight M, Lagundoye O, Wong R, et al. Psychological and psychosocial interventions for cannabis cessation in adults: a systematic review. Addict Res Theory 2016;24:93–110.
Chen L, Zhang G, Hu M, Liang X. Eye movement desensitization and reprocessing versus cognitive–behavioral therapy for adult posttraumatic stress disorder: systematic review and meta-analysis. J Nerv Ment Dis 2015;203:443–51. https://doi.org/10.1097/NMD.0000000000000306
Chiang KJ, Tsai JC, Liu D, Lin CH, Chiu HL, Chou KR. Efficacy of cognitive–behavioral therapy in patients with bipolar disorder: a meta-analysis of randomized controlled trials. PLOS ONE 2017;12:e0176849. https://doi.org/10.1371/journal.pone.0176849
Chien CH, Liu KL, Chien HT, Liu HE. The effects of psychosocial strategies on anxiety and depression of patients diagnosed with prostate cancer: a systematic review. Int J Nurs Stud 2014;51:28–38. https://doi.org/10.1016/j.ijnurstu.2012.12.019
Christensen H, Pallister E, Smale S, Hickie IB, Calear AL. Community-based prevention programs for anxiety and depression in youth: a systematic review J Prim Prev 2010;31:139–70.
Chu BC, Harrison TL. Disorder-specific effects of CBT for anxious and depressed youth: a meta-analysis of candidate mediators of change. Clin Child Fam Psychol Rev 2007;10:352–72. https://doi.org/10.1007/s10567-007-0028-2
Chun J, Shim H, Kim S. A meta-analysis of treatment interventions for internet addiction among Korean adolescents. Cyberpsychol Behav Soc Netw 2017;20:225–31. https://doi.org/10.1089/cyber.2016.0188
Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, Wessely S. A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression. Health Technol Assess 2001;5(35). https://doi.org/10.3310/hta5350
Ciketic S, Hayatbakhsh MR, Doran CM, Najman JM, McKetin R. A review of psychological and pharmacological treatment options for methamphetamine dependence. J Subst Use 2012;17:363–83.
Cima RF, Andersson G, Schmidt CJ, Henry JA. Cognitive–behavioral treatments for tinnitus: a review of the literature. J Am Acad Audiol 2014;25:29–61. https://doi.org/10.3766/jaaa.25.1.4
Clarke K, Mayo-Wilson E, Kenny J, Pilling S. Can non-pharmacological interventions prevent relapse in adults who have recovered from depression? A systematic review and meta-analysis of randomised controlled trials. Clin Psychol Rev 2015;39:58–70. https://doi.org/10.1016/j.cpr.2015.04.002
Cleary M, Hunt GE, Matheson S, Walter G. Psychosocial treatments for people with co-occurring severe mental illness and substance misuse: systematic review. J Adv Nurs 2009;65:238–58. https://doi.org/10.1111/j.1365-2648.2008.04879.x
Clery P, Stahl D, Ismail K, Treasure J, Kan C. Systematic review and meta-analysis of the efficacy of interventions for people with type 1 diabetes mellitus and disordered eating. Diabet Med 2017;34:1667–75. https://doi.org/10.1111/dme.13509
Cobb B, Sample PL, Alwell M, Johns NR. Cognitive–behavioral interventions, dropout, and youth with disabilities: a systematic review. Remedial Spec Educ 2006;27:259–75.
Cobeanu O, David D. Alleviation of side effects and distress in breast cancer patients by cognitive–behavioral interventions: a systematic review and meta-analysis. J Clin Psychol Med Settings 2018;25:335–55. https://doi.org/10.1007/s10880-017-9526-7
Cohan SL, Chavira DA, Stein MB. Practitioner review: psychosocial interventions for children with selective mutism: a critical evaluation of the literature from 1990–2005. J Child Psychol Psychiatry 2006;47:1085–97.
Cohen G, Harvey J. The use of psychological interventions for adult male sex offenders with a learning disability: a systematic review. J Sex Aggress 2016;22:206–23.
Collado A, Lim AC, MacPherson L. A systematic review of depression psychotherapies among Latinos. Clin Psychol Rev 2016;45:193–209. https://doi.org/10.1016/j.cpr.2016.04.001
Comer JS, Hong N, Poznanski B, Silva K, Wilson M. Evidence base update on the treatment of early childhood anxiety and related problems. J Clin Child Adolesc Psychol 2019;48:1–15. https://doi.org/10.1080/15374416.2018.1534208
Compton SN, March JS, Brent D, Albano AM, Weersing R, Curry J. Cognitive–behavioral psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry 2004;43:930–59.
Conejo-Cerón S, Moreno-Peral P, Rodríguez-Morejón A, Motrico E, Navas-Campaña D, Rigabert A, et al. Effectiveness of psychological and educational interventions to prevent depression in primary care: a systematic review and meta-analysis. Ann Fam Med 2017;15:262–71. https://doi.org/10.1370/afm.2031
Cong X, Perry M, Bernier KM, Young EE, Starkweather A. Effects of self-management interventions in patients with irritable bowel syndrome: systematic review. West J Nurs Res 2018;40:1698–720. https://doi.org/10.1177/0193945917727705
Cooper C, Balamurali TB, Selwood A, Livingston G. A systematic review of intervention studies about anxiety in caregivers of people with dementia. Int J Geriatr Psychiatry 2007;22:181–8. https://doi.org/10.1002/gps.1656
Cooper K, Chatters R, Kaltenthaler E, Wong R. Psychological and psychosocial interventions for cannabis cessation in adults: a systematic review short report. Health Technol Assess 2015;19(56). https://doi.org/10.3310/hta19560
Coss-Adame E, Erdogan A, Rao SS. Treatment of esophageal (noncardiac) chest pain: an expert review. Clin Gastroenterol Hepatol 2014;12:1224–45. https://doi.org/10.1016/j.cgh.2013.08.036
Cossu G, Cantone E, Pintus M, Cadoni M, Pisano A, Otten R, et al. Integrating children with psychiatric disorders in the classroom: a systematic review. Clin Pract Epidemiol Ment Health 2015;11:41–57.
Costa MB, Melnik T. Effectiveness of psychosocial interventions in eating disorders: an overview of Cochrane systematic reviews. Einstein 2016;14:235–77. https://doi.org/10.1590/S1679-45082016RW3120
Coull G, Morris PG. The clinical effectiveness of CBT-based guided self-help interventions for anxiety and depressive disorders: a systematic review. Psychol Med 2011;41:2239–52. https://doi.org/10.1017/S0033291711000900
Cowlishaw S, Merkouris S, Dowling N, Anderson C, Jackson A, Thomas S. Psychological therapies for pathological and problem gambling. Cochrane Database Syst Rev 2012;11:CD008937. https://doi.org/10.1002/14651858.CD008937.pub2
Crawford C, Wallerstedt DB, Khorsan R, Clausen SS, Jonas WB, Walter JA. A systematic review of biopsychosocial training programs for the self-management of emotional stress: potential applications for the military. Evid Based Complement Alternat Med 2013;2013:747694. https://doi.org/10.1155/2013/747694
Creswell C, Cartwright-Hatton S. Family treatment of child anxiety: outcomes, limitations and future directions. Clin Child Fam Psychol Rev 2007;10:232–52. https://doi.org/10.1007/s10567-007-0019-3
Crumlish N, O’Rourke K. A systematic review of treatments for post-traumatic stress disorder among refugees and asylum-seekers. J Nerv Ment Dis 2010;198:237–51. https://doi.org/10.1097/NMD.0b013e3181d61258
Cruz CG. Integrative Review and Development of Algorithm for the Treatment of Insomnia in the Geriatric Population. PhD thesis. Las Cruces, NM: New Mexico State University; 2016.
Cuijpers P. A psychoeducational approach to the treatment of depression: a meta-analysis of Lewinsohn’s ‘Coping With Depression’ course. Behav Ther 1998;29:521–33.
Cuijpers P. Psychological outreach programmes for the depressed elderly: a meta-analysis of effects and dropout. Int J Geriatr Psychiatry 1998;13:41–8.
Cuijpers P, Ebert DD, Acarturk C, Andersson G, Cristea IA. Personalized psychotherapy for adult depression: a meta-analytic review. Behav Ther 2016;47:966–80.
Cuijpers P, Gentili C, Banos RM, Garcia-Campayo J, Botella C, Cristea IA. Relative effects of cognitive and behavioral therapies on generalized anxiety disorder, social anxiety disorder and panic disorder: a meta-analysis. J Anxiety Disord 2016;43:79–89.
Cuijpers P, Karyotaki E, Pot AM, Park M, Reynolds CF. Managing depression in older age: psychological interventions. Maturitas 2014;79:160–9. https://doi.org/10.1016/j.maturitas.2014.05.027
Cuijpers P, van Straten A, Andersson G, van Oppen P. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. J Consult Clin Psychol 2008;76:909–22. https://doi.org/10.1037/a0013075
Cuijpers P, van Straten A, Smit F. Psychological treatment of late-life depression: a meta-analysis of randomized controlled trials. Int J Geriatr Psychiatry 2006;21:1139–49. https://doi.org/10.1002/gps.1620
Cullen KL, Irvin E, Collie A, Clay F, Gensby U, Jennings PA, et al. Effectiveness of workplace interventions in return-to-work for musculoskeletal, pain-related and mental health conditions: an update of the evidence and messages for practitioners. J Occup Rehabil 2018;28:1–15. https://doi.org/10.1007/s10926-016-9690-x
Cunningham JEA, Shapiro CM. Cognitive behavioural therapy for insomnia (CBT-I) to treat depression: a systematic review. J Psychosom Res 2018;106:1–12.
da Costa RT, Sardinha A, Nardi AE. Virtual reality exposure in the treatment of fear of flying. Aviat Space Environ Med 2008;79:899–903.
da Silva Roggi PM, da Gama MFN, Neves FS, Garcia F. Update on treatment of craving in patients with addiction using cognitive behavioral therapy. Clin Neuropsychiatry 2015;12:118–27.
Dagnan D, Jackson I, Eastlake L. A systematic review of cognitive behavioural therapy for anxiety in adults with intellectual disabilities. J Intellect Disabil Res 2018;62:974–91. https://doi.org/10.1111/jir.12548
Dale HL, Adair PM, Humphris GM. Systematic review of post-treatment psychosocial and behaviour change interventions for men with cancer. Psycho-Oncology 2010;19:227–37. https://doi.org/10.1002/pon.1598
David AR, Szamoskozi S. A meta-analytical study on the effects of cognitive behavioral techniques for reducing distress in organizations. J Cogn Behav Psychother 2011;11:221–36.
David D, Cotet C, Matu S, Mogoase C, Stefan S. 50 years of rational-emotive and cognitive–behavioral therapy: a systematic review and meta-analysis. J Clin Psychol 2018;74:304–18. https://doi.org/10.1002/jclp.22514
Davies C, Cipriani A, Ioannidis JPA, Radua J, Stahl D, Provenzani U, et al. Lack of evidence to favor specific preventive interventions in psychosis: a network meta-analysis. World Psychiatry 2018;17:196–209. https://doi.org/10.1002/wps.20526
de Arellano MA, Lyman DR, Jobe-Shields L, George P, Dougherty RH, Daniels AS, et al. Trauma-focused cognitive–behavioral therapy for children and adolescents: assessing the evidence. Psychiatr Serv 2014;65:591–602. https://doi.org/10.1176/appi.ps.201300255
de Bitencourt Machado D, Braga Laskoski P, Trelles Severo C, Margareth Bassols A, Sfoggia A, Kowacs C, et al. A psychodynamic perspective on a systematic review of online psychotherapy for adults. Br J Psychother 2016;32:79–108.
de Swart JJW, Van den Broek H, Stams GJJM, Asscher JJ, Van der Laan PH, Holsbrink-Engels GA, et al. The effectiveness of institutional youth care over the past three decades: a meta-analysis. Child Youth Serv Rev 2012;34:1818–24.
Deady M, Choi I, Calvo RA, Glozier N, Christensen H, Harvey SB. eHealth interventions for the prevention of depression and anxiety in the general population: a systematic review and meta-analysis. BMC Psychiatry 2017;17:310. https://doi.org/10.1186/s12888-017-1473-1
Deas G, Kelly C, Hadjinicolaou AV, Holt C, Agius M, Zaman R. An update on: meta-analysis of medical and non-medical treatments of the prodromal phase of psychotic illness in at risk mental states. Psychiatr Danub 2016;28:31–8.
De-Bacco C, Marzola E, Fassino S, Abbate-Daga G. Psychodynamic psychotherapies for feeding and eating disorders. Minerva Psichiatrica 2017;58:162–80.
Deenadayalan Y, Perraton L, Machotka Z, Kumar S. Day therapy programs for adolescents with mental health problems: a systematic review. Internet J Allied Health Sci Pract 2010;8:1–14.
Denis C, Lavie E, Fatséas M, Auriacombe M. Psychotherapeutic interventions for cannabis abuse and/or dependence in outpatient settings. Cochrane Database Syst Rev 2006;3:CD005336. https://doi.org/10.1002/14651858.CD005336.pub2
Dennis CL. Treatment of postpartum depression, part 2: a critical review of nonbiological interventions. J Clin Psychiatry 2004;65:1252–65. https://doi.org/10.4088/jcp.v65n0915
Devoe DJ, Peterson A, Addington J. Negative symptom interventions in youth at risk of psychosis: a systematic review and network meta-analysis. Schizophr Bull 2017;24:24.
Di Giulio G. Therapist, Client Factors, and Efficacy in Cognitive Behavioural Therapy: A Meta-analytic Exploration of Factors that Contribute to Positive Outcome. PhD thesis. Ottawa, ON: University of Ottawa; 2007.
Dickerson SS, Connors LM, Fayad A, Dean GE. Sleep–wake disturbances in cancer patients: narrative review of literature focusing on improving quality of life outcomes. Nat Sci Sleep 2014;6:85–100. https://doi.org/10.2147/NSS.S34846
Doki S, Sasahara S, Matsuzaki I. Psychological approach of occupational health service to sick leave due to mental problems: a systematic review and meta-analysis. Int Arch Occup Environ Health 2015;88:659–67. https://doi.org/10.1007/s00420-014-0996-8
Dopp AR, Borduin CM, Brown CE. Evidence-based treatments for juvenile sexual offenders: review and recommendations. J Aggress Confl Peace Res 2015;7:223–36.
Dorsey S, McLaughlin KA, Kerns SEU, Harrison JP, Lambert HK, Briggs EC, et al. Evidence base update for psychosocial treatments for children and adolescents exposed to traumatic events. J Clin Child Adolesc Psychol 2017;46:303–30. https://doi.org/10.1080/15374416.2016.1220309
Dowell KA, Ogles BM. The effects of parent participation on child psychotherapy outcome: a meta-analytic review. J Clin Child Adolesc Psychol 2010;39:151–62. https://doi.org/10.1080/15374410903532585
Dowling NA, Merkouris SS, Lorains FK. Interventions for comorbid problem gambling and psychiatric disorders: advancing a developing field of research. Addict Behav 2016;58:21–30. https://doi.org/10.1016/j.addbeh.2016.02.012
Dray J, Bowman J, Campbell E, Freund M, Wolfenden L, Hodder RK, et al. Systematic review of universal resilience-focused interventions targeting child and adolescent mental health in the school setting. J Am Acad Child Adolesc Psychiatry 2017;56:813–24.
Duncan EA, Nicol MM, Ager A, Dalgleish L. A systematic review of structured group interventions with mentally disordered offenders. Crim Behav Ment Health 2006;16:217–41. https://doi.org/10.1002/cbm.631
Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 2008;165:179–87. https://doi.org/10.1176/appi.ajp.2007.06111851
Easthall C, Song F, Bhattacharya D. A meta-analysis of cognitive-based behaviour change techniques as interventions to improve medication adherence. BMJ Open 2013;3:e002749. https://doi.org/10.1136/bmjopen-2013-002749
Eccleston C, Fisher E, Craig L, Duggan GB, Rosser BA, Keogh E. Psychological therapies (internet-delivered) for the management of chronic pain in adults. Cochrane Database Syst Rev 2014;2:CD010152. https://doi.org/10.1002/14651858.CD010152.pub2
Eccleston C, Morley S, Williams A, Yorke L, Mastroyannopoulou K. Systematic review of randomised controlled trials of psychological therapy for chronic pain in children and adolescents, with a subset meta-analysis of pain relief. Pain 2002;99:157–65.
Edwards AR. Psychotherapy and Pharmacotherapy for Social Anxiety Disorder: A Comprehensive Meta-analysis. PhD thesis. Philadelphia, PA: Temple University; 2011.
Ekers D. Behavioural Activation for Depression: A Systematic Review and Controlled Clinical Trial. PhD thesis. York: University of York; 2011.
Ekers D, Richards D, Gilbody S. A meta-analysis of randomized trials of behavioural treatment of depression. Psychol Med 2008;38:611–23.
Escobar KM, Gorey KM. Cognitive–behavioral interventions for anxiety disorders: rapid review suggestion of larger effects among Hispanic than non-Hispanic white people. J Soc Serv Res 2018;44:132–40.
Faizi F, Tavallaee A, Rahimi A, Saburi A, Saghafinia M. Quality assessment of randomized control trials applied psychotherapy for chronic pains in iran: a systematic review of domestic trials. Iran Red Crescent Med J 2014;16:e15312. https://doi.org/10.5812/ircmj.15312
Farhood Z, Ong AA, Discolo CM. PANDAS: a systematic review of treatment options. Int J Pediatr Otorhinolaryngol 2016;89:149–53.
Farronato NS, Dürsteler-Macfarland KM, Wiesbeck GA, Petitjean SA. A systematic review comparing cognitive–behavioral therapy and contingency management for cocaine dependence. J Addict Dis 2013;32:274–87. https://doi.org/10.1080/10550887.2013.824328
Farver-Vestergaard I, Jacobsen D, Zachariae R. Efficacy of psychosocial interventions on psychological and physical health outcomes in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Psychother Psychosom 2015;84:37–50. https://doi.org/10.1159/000367635
Ferriter M, Kaltenthaler E, Parry G, Beverley C. Computerised cognitive behaviour therapy for phobias and panic disorder: a systematic review. J Public Ment Health 2008;7:15–22.
Feuerstein M, Burrell LM, Miller VI, Lincoln A, Huang GD, Berger R. Clinical management of carpal tunnel syndrome: a 12-year review of outcomes. Am J Ind Med 1999;35:232–45.
Firth J, Torous J, Nicholas J, Carney R, Pratap A, Rosenbaum S, Sarris J. The efficacy of smartphone-based mental health interventions for depressive symptoms: a meta-analysis of randomized controlled trials. World Psychiatry 2017;16:287–98. https://doi.org/10.1002/wps.20472
Fisher PL. The Efficacy of Psychological Treatments for Generalised Anxiety Disorder? In Davey GCL, Wells A, editors. Worry and its Psychological Disorders: Theory, Assessment and Treatment. Hoboken, NJ: Wiley Publishing; 2006. pp. 359–77.
Flik CE, Bakker L, Laan W, van Rood YR, Smout AJ, de Wit NJ. Systematic review: the placebo effect of psychological interventions in the treatment of irritable bowel syndrome. World J Gastroenterol 2017;23:2223–33. https://doi.org/10.3748/wjg.v23.i12.2223
Ford AC, Vandvik PO. Irritable bowel syndrome. BMJ Clin Evid 2010;2010:0410.
Ford AC, Vandvik PO. Irritable bowel syndrome. BMJ Clin Evid 2012;2012:0410.
Forte AL, Hill M, Pazder R, Feudtner C. Bereavement care interventions: a systematic review. BMC Palliat Care 2004;3:3. https://doi.org/10.1186/1472-684X-3-3
Fossum S, Handegard BH, Adolfsen F, Vis SA, Wynn R. A meta-analysis of long-term outpatient treatment effects for children and adolescents with conduct problems. J Child Fam Stud 2016;25:15–29.
Fréchette-Simard C, Plante I, Bluteau J. Strategies included in cognitive behavioral therapy programs to treat internalized disorders: a systematic review. Cogn Behav Ther 2018;47:263–85. https://doi.org/10.1080/16506073.2017.1388275
Frederiksen Y, Farver-Vestergaard I, Skovgård NG, Ingerslev HJ, Zachariae R. Efficacy of psychosocial interventions for psychological and pregnancy outcomes in infertile women and men: a systematic review and meta-analysis. BMJ Open 2015;5:e006592. https://doi.org/10.1136/bmjopen-2014-006592
Fredette C, El-Baalbaki G, Palardy V, Rizkallah E, Guay S. Social support and cognitive–behavioral therapy for posttraumatic stress disorder: a systematic review. Traumatology 2016;22:131–44.
Freeman JB, Choate-Summers ML, Moore PS, Garcia AM, Sapyta JJ, Leonard HL, Franklin ME. Cognitive behavioral treatment for young children with obsessive–compulsive disorder. Biol Psychiatry 2007;61:337–43.
French LRM, Turner KM, Dawson S, Moran P. Psychological treatment of depression and anxiety in patients with co-morbid personality disorder: a scoping study of trial evidence. Personal Ment Health 2017;11:101–17. https://doi.org/10.1002/pmh.1372
Friedrich A, Schlarb AA. Let’s talk about sleep: a systematic review of psychological interventions to improve sleep in college students. J Sleep Res 2018;27:4–22. https://doi.org/10.1111/jsr.12568
Fristad MA, MacPherson HA. Evidence-based psychosocial treatments for child and adolescent bipolar spectrum disorders. J Clin Child Adolesc Psychol 2014;43:339–55. https://doi.org/10.1080/15374416.2013.822309
Furukawa TA, Watanabe N, Churchill R. Combined psychotherapy plus antidepressants for panic disorder with or without agoraphobia. Cochrane Database Syst Rev 2007;1:CD004364. https://doi.org/10.1002/14651858.CD004364.pub2
Galdston MR, John RM. Mind over gut: psychosocial management of pediatric functional abdominal pain. J Pediatr Health Care 2016;30:535–45.
Gale CK, Millichamp J. Generalised anxiety disorder. BMJ Clin Evid 2011;2011:1002.
Gallagher-Thompson D, Coon DW. Evidence-based psychological treatments for distress in family caregivers of older adults. Psychol Aging 2007;22:37–51.
Galvao-de Almeida A, Araujo Filho GM, Berberian Ade A, Trezsniak C, Nery-Fernandes F, Araujo Neto CA, et al. The impacts of cognitive-behavioral therapy on the treatment of phobic disorders measured by functional neuroimaging techniques: a systematic review. Braz J Psychiatry 2013;35:279–83. https://doi.org/10.1590/1516-4446-2012-0922
Gandy M, Sharpe L, Perry KN. Cognitive behavior therapy for depression in people with epilepsy: a systematic review. Epilepsia 2013;54:1725–34. https://doi.org/10.1111/epi.12345
Garland SN, Johnson JA, Savard J, Gehrman P, Perlis M, Carlson L, Campbell T. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat 2014;10:1113–24. https://doi.org/10.2147/NDT.S47790
Gartlehner G, Forneris CA, Brownley KA, Gaynes BN, Sonis J, Coker-Schwimmer E, et al. Interventions for the Prevention of Posttraumatic Stress Disorder (PTSD) in Adults After Exposure to Psychological Trauma. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
Gaudiano BA. Is symptomatic improvement in clinical trials of cognitive–behavioral therapy for psychosis clinically significant? J Psychiatric Pract 2006;12:11–23.
Gaudiano BA, Weinstock LM, Miller IW. Improving treatment adherence in bipolar disorder: a review of current psychosocial treatment efficacy and recommendations for future treatment development. Behav Modif 2008;32:267–301. https://doi.org/10.1177/0145445507309023
Gaynor D, Cock H, Agrawal N. Psychological treatments for functional non-epileptic attacks: a systematic review. Acta Neuropsychiatr 2009;21:158–68. https://doi.org/10.1111/j.1601-5215.2009.00376.x
Gearing RE, Schwalbe CS, Lee R, Hoagwood KE. The effectiveness of booster sessions in CBT treatment for child and adolescent mood and anxiety disorders. Depress Anxiety 2013;30:800–8. https://doi.org/10.1002/da.22118
Geiger-Brown JM, Rogers VE, Liu W, Ludeman EM, Downton KD, Diaz-Abad M. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev 2015;23:54–67. https://doi.org/10.1016/j.smrv.2014.11.007
George N, Abdallah J, Maradey-Romero C, Gerson L, Fass R. Review article: the current treatment of non-cardiac chest pain. Aliment Pharmacol Ther 2016;43:213–39. https://doi.org/10.1111/apt.13458
Gibby BA, Casline EP, Ginsburg GS. Long-term outcomes of youth treated for an anxiety disorder: a critical review. Clin Child Fam Psychol Rev 2017;20:201–25. https://doi.org/10.1007/s10567-017-0222-9
Gillen PA, Sinclair M, Kernohan WG, Begley CM, Luyben AG. Interventions for prevention of bullying in the workplace. Cochrane Database Syst Rev 2017;1:CD009778. https://doi.org/10.1002/14651858.CD009778.pub2
Giroux I, Goulet A, Mercier J, Jacques C, Bouchard S. Online and mobile interventions for problem gambling, alcohol, and drugs: a systematic review. Front Psychol 2017;8:954. https://doi.org/10.3389/fpsyg.2017.00954
Glenn CR, Franklin JC, Nock MK. Evidence-based psychosocial treatments for self-injurious thoughts and behaviors in youth. J Clin Child Adolesc Psychol 2015;44:1–29.
Glombiewski JA, Sawyer AT, Gutermann J, Koenig K, Rief W, Hofmann SG. Psychological treatments for fibromyalgia: a meta-analysis. Pain 2010;151:280–95. https://doi.org/10.1016/j.pain.2010.06.011
Goldberg SB, Buck B, Raphaely S, Fortney JC. Measuring psychiatric symptoms remotely: a systematic review of remote measurement-based care. Curr Psychiatry Rep 2018;20:81. https://doi.org/10.1007/s11920-018-0958-z
Gomes HS, Viana KA, Batista AC, Costa LR, Hosey MT, Newton T. Cognitive behaviour therapy for anxious paediatric dental patients: a systematic review [published online ahead of print July 08 2018]. Int J Paediatr Dent 2018. https://doi.org/10.1111/ipd.12405
Goncalves R, Lages AC, Rodrigues H, Pedrozo AL, Coutinho ESF, Neylan T, et al. Potential biomarkers of cognitive behavior-therapy for post-traumatic stress disorder: a systematic review. Rev Psiquiatr Clin 2011;38:155–60.
Gonçalves R, Pedrozo AL, Coutinho ES, Figueira I, Ventura P. Efficacy of virtual reality exposure therapy in the treatment of PTSD: a systematic review. PLOS ONE 2012;7:e48469. https://doi.org/10.1371/journal.pone.0048469
Gonzalez-Pinto A, Gonzalez C, Enjuto S, Fernandez de Corres B, Lopez P, Palomo J, et al. Psychoeducation and cognitive–behavioral therapy in bipolar disorder: an update. Acta Psychiatr Scand 2004;109:83–90.
Gooding P, Tarrier N. A systematic review and meta-analysis of cognitive–behavioural interventions to reduce problem gambling: hedging our bets? Behav Res Ther 2009;47:592–607.
Goodyer IM, Wilkinson PO. Practitioner review: therapeutics of unipolar major depressions in adolescents. J Child Psychol Psychiatry Allied Discip 2018;25:25.
Gordon D, Heimberg RG, Tellez M, Ismail AI. A critical review of approaches to the treatment of dental anxiety in adults. J Anxiety Disord 2013;27:365–78. https://doi.org/10.1016/j.janxdis.2013.04.002
Gorin SS, Badr H, Jacobsen PB, Janke EA, Jim HS, Krebs P. Meta-analysis of behavioral interventions to reduce cancer pain. J Clin Oncol 2011;29:S1.
Goslin RE, Gray RN, McCrory DC, Penzien D, Rains J, Hasselblad V. Behavioural and Physical Treatments for Migraine Headache. Rockville, MD: Agency for Health Care Policy and Research; 1999.
Gottdiener WH. The Benefits of Individual Psychotherapy for Schizophrenic Patients: A Meta-analytic Review of the Psychotherapy Outcome Literature. PhD thesis. New York City, NY: New School for Social Research; 2000.
Gottdiener WH, Haslam N. The benefits of individual psychotherapy for people diagnosed with schizophrenia: a meta-analytic review. Ethical Hum Sci Serv 2002;4:163–87.
Gould RA, Buckminster S, Pollack MH, Otto MW, Yap L. Cognitive–behavioral and pharmacological treatment for social phobia: a meta-analysis. Clin Psychol Sci Pract 1997;4:291–306.
Gould RA, Otto MW, Pollack MH. A meta-analysis of treatment outcome for panic disorder. Clin Psychol Rev 1995;15:819–44.
Gould RA, Otto MW, Pollack MH, Yap L. Cognitive behavioral and pharmacological treatment of generalized anxiety disorder: a preliminary meta-analysis. Behav Ther 1997;28:285–305.
Goulding L, Furze G, Birks Y. Randomized controlled trials of interventions to change maladaptive illness beliefs in people with coronary heart disease: systematic review J Adv Nurs 2010;66:946–61.
Gregory B, Peters L. Changes in the self during cognitive behavioural therapy for social anxiety disorder: a systematic review. Clin Psychol Rev 2017;52:1–18.
Gregory Jr VL. Cognitive–behavioral therapy for comorbid bipolar and substance use disorders: a systematic review of controlled trials. Ment Health Subst Use Dual Diagn 2011;4:302–13.
Gregory VL, Jr. Cognitive–behavioral therapy for bipolar disorder: implications for clinical social workers. J Soc Serv Res 2010;36:460–9.
Gregory VL, Jr. Cognitive–behavioral therapy for depressive symptoms in persons of African descent: a meta-analysis. J Soc Serv Res 2016;42:113–29.
Groff SE. Is enhanced cognitive behavioral therapy an effective intervention in eating disorders? A review. J Evid Inf Soc Work 2015;12:272–88.
Guggisberg KW. Methodological Review and Meta-analysis of Treatments for Child and Adolescent Obsessive–Compulsive Disorder. PhD thesis. Salt Lake City, UT: The University of Utah; 2005.
Günzler C, Berner MM. Efficacy of psychosocial interventions in men and women with sexual dysfunctions – a systematic review of controlled clinical trials: part 2 – the efficacy of psychosocial interventions for female sexual dysfunction. J Sex Med 2012;9:3108–25. https://doi.org/10.1111/j.1743-6109.2012.02965.x
Gutermann J, Schreiber F, Matulis S, Schwartzkopff L, Deppe J, Steil R. Psychological treatments for symptoms of posttraumatic stress disorder in children, adolescents, and young adults: a meta-analysis. Clin Child Fam Psychol Rev 2016;19:77–93. https://doi.org/10.1007/s10567-016-0202-5
Hans E, Hiller W. Effectiveness of and dropout from outpatient cognitive behavioral therapy for adult unipolar depression: a meta-analysis of nonrandomized effectiveness studies. J Consult Clin Psychol 2013;81:75–88. https://doi.org/10.1037/a0031080
Hans E, Hiller W. A meta-analysis of nonrandomized effectiveness studies on outpatient cognitive behavioral therapy for adult anxiety disorders. Clin Psychol Rev 2013;33:954–64. https://doi.org/10.1016/j.cpr.2013.07.003
Hanson RK, Gordon A, Harris AJ, Marques JK, Murphy W, Quinsey VL, Seto MC. First report of the collaborative outcome data project on the effectiveness of psychological treatment for sex offenders. Sex Abuse 2002;14:169–94. https://doi.org/10.1177/107906320201400207
Harding R, Liu L, Catalan J, Sherr L. What is the evidence for effectiveness of interventions to enhance coping among people living with HIV disease? A systematic review. Psychol Health Med 2011;16:564–87. https://doi.org/10.1080/13548506.2011.580352
Hassiotis A, Hall I. Behavioural and cognitive-behavioural interventions for outwardly-directed aggressive behaviour in people with learning disabilities. Cochrane Database Syst Rev 2004;4:CD003406. https://doi.org/10.1002/14651858.CD003406.pub2
Hassiotis AA, Hall I. Behavioural and cognitive-behavioural interventions for outwardly-directed aggressive behaviour in people with learning disabilities. Cochrane Database Syst Rev 2008;3:CD003406. https://doi.org/10.1002/14651858.CD003406.pub3
Hawley DJ. Psycho-educational interventions in the treatment of arthritis. Baillieres Clin Rheumatol 1995;9:803–23.
Hay P. A systematic review of evidence for psychological treatments in eating disorders: 2005–2012. Int J Eat Disord 2013;46:462–9. https://doi.org/10.1002/eat.22103
Hazell CM, Hayward M, Cavanagh K, Strauss C. A systematic review and meta-analysis of low intensity CBT for psychosis. Clin Psychol Rev 2016;45:183–92. https://doi.org/10.1016/j.cpr.2016.03.004
Hazell P. Depression in children and adolescents. BMJ Clin Evid 2009;2009:1008.
Hedman E, Ljótsson B, Lindefors N. Cognitive behavior therapy via the internet: a systematic review of applications, clinical efficacy and cost-effectiveness. Expert Rev Pharmacoecon Outcomes Res 2012;12:745–64. https://doi.org/10.1586/erp.12.67
Hellenbach M, Brown M, Karatzias T, Robinson R. Psychological interventions for women with intellectual disabilities and forensic care needs: a systematic review of the literature. J Intellect Disabil Res 2015;59:319–31. https://doi.org/10.1111/jir.12133
Hembree EA, Foa EB, Dorfan NM, Street GP, Kowalski J, Tu X. Do patients drop out prematurely from exposure therapy for PTSD? J Trauma Stress 2003;16:555–62.
Henwood KS, Chou S, Browne KD. A systematic review and meta-analysis on the effectiveness of CBT informed anger management. Aggress Violent Behav 2015;25:280–92.
Herbert V, Kyle SD, Pratt D. Does cognitive behavioural therapy for insomnia improve cognitive performance? A systematic review and narrative synthesis. Sleep Med Rev 2018;39:37–51.
Hernandez JP, Macgowan MJ. Psychosocial interventions for women with HIV/AIDS: a critical review. Res Soc Work Pract 2015;25:103–16.
Hershcovici T, Achem SR, Jha LK, Fass R. Systematic review: the treatment of noncardiac chest pain. Aliment Pharmacol Ther 2012;35:5–14. https://doi.org/10.1111/j.1365-2036.2011.04904.x
Hetzel-Riggin MD, Brausch AM, Montgomery BS. A meta-analytic investigation of therapy modality outcomes for sexually abused children and adolescents: an exploratory study. Child Abuse Negl 2007;31:125–41.
Hilfiker R, Meichtry A, Eicher M, Nilsson Balfe L, Knols RH, Verra ML, Taeymans J. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med 2018;52:651–8. https://doi.org/10.1136/bjsports-2016-096422
Hill A, Brettle A. Counselling Older People: A Systematic Review. Lutterworth: British Association for Counselling and Psychotherapy; 2005.
Hind D, Cotter J, Thake A, Bradburn M, Cooper C, Isaac C, House A. Cognitive behavioural therapy for the treatment of depression in people with multiple sclerosis: a systematic review and meta-analysis. BMC Psychiatry 2014;14:5. https://doi.org/10.1186/1471-244X-14-5
Hogue A, Henderson CE, Ozechowski TJ, Robbins MS. Evidence base on outpatient behavioral treatments for adolescent substance use: updates and recommendations 2007–2013. J Clin Child Adolesc Psychol 2014;43:695–720. https://doi.org/10.1080/15374416.2014.915550
Høifødt RS, Strøm C, Kolstrup N, Eisemann M, Waterloo K. Effectiveness of cognitive behavioural therapy in primary health care: a review. Fam Pract 2011;28:489–504. https://doi.org/10.1093/fampra/cmr017
Hollis C, Falconer CJ, Martin JL, Whittington C, Stockton S, Glazebrook C, Davies EB. Annual research review: digital health interventions for children and young people with mental health problems – a systematic and meta-review. J Child Psychol Psychiatry 2017;58:474–503. https://doi.org/10.1111/jcpp.12663
Hollon SD, Ponniah K. A review of empirically supported psychological therapies for mood disorders in adults. Depress Anxiety 2010;27:891–932. https://doi.org/10.1002/da.20741
Holvast F, Massoudi B, Oude Voshaar RC, Verhaak PFM. Non-pharmacological treatment for depressed older patients in primary care: a systematic review and meta-analysis. PLOS ONE 2017;12:e0184666. https://doi.org/10.1371/journal.pone.0184666
Hoon LS, Chi Sally CW, Hong-Gu H. Effect of psychosocial interventions on outcomes of patients with colorectal cancer: a review of the literature. Eur J Oncol Nurs 2013;17:883–91. https://doi.org/10.1016/j.ejon.2013.05.001
Howell D, Oliver TK, Keller-Olaman S, Davidson JR, Garland S, Samuels C, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol 2014;25:791–800.
Huertas-Ceballos A, Logan S, Bennett C, Macarthur C. Psychosocial interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database Syst Rev 2008;1:CD003014. https://doi.org/10.1002/14651858.CD003014.pub2
Hulgaard D, Dehlholm-Lambertsen G, Rask CU. Family-based interventions for children and adolescents with functional somatic symptoms: a systematic review. J Fam Ther 2019;41:4–28.
Inouye J, Braginsky N, Kataoka-Yahiro M. Randomized clinical trials of self-management with Asian/Pacific Islanders. Clin Nurs Res 2011;20:366–403. https://doi.org/10.1177/1054773811417708
Ipser JC, Singh L, Stein DJ. Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin Neurosci 2013;67:311–22. https://doi.org/10.1111/pcn.12055
Iruthayarajah J, Alibrahim F, Mehta S, Janzen S, McIntyre A, Teasell R. Cognitive behavioural therapy for aggression among individuals with moderate to severe acquired brain injury: a systematic review and meta-analysis. Brain Inj 2018;32:1443–9. https://doi.org/10.1080/02699052.2018.1496481
Ishak WW, Bagot K, Thomas S, Magakian N, Bedwani D, Larson D, et al. Quality of life in patients suffering from insomnia. Innov Clin Neurosci 2012;9:13–26.
Ismail A, Moore C, Alshishani N, Yaseen K, Alshehri MA. Cognitive behavioural therapy and pain coping skills training for osteoarthritis knee pain management: a systematic review. J Phys Ther Sci 2017;29:2228–35. https://doi.org/10.1589/jpts.29.2228
Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 2004;363:1589–97. https://doi.org/10.1016/S0140-6736(04)16202-8
Itza F, Zarza D, Gómez-Sancha F, Salinas J, Bautrant E. [Update on the diagnosis and treatment of vulvodynia.] Actas Urol Esp 2012;36:431–8. https://doi.org/10.1016/j.acuro.2011.11.004
Ivarsson T, Skarphedinsson G, Kornør H, Axelsdottir B, Biedilæ S, Heyman I, et al. The place of and evidence for serotonin reuptake inhibitors (SRIs) for obsessive compulsive disorder (OCD) in children and adolescents: views based on a systematic review and meta-analysis. Psychiatry Res 2015;227:93–103. https://doi.org/10.1016/j.psychres.2015.01.015
Jarry JL, Ip K. The effectiveness of stand-alone cognitive-behavioural therapy for body image: a meta-analysis. Body Image 2005;2:317–31.
Jibb LA, Nathan PC, Stevens BJ, Seto E, Cafazzo JA, Stephens N, et al. Psychological and physical interventions for the management of cancer-related pain in pediatric and young adult patients: an integrative review. Oncol Nurs Forum 2015;42:E339–57. https://doi.org/10.1188/15.ONF.E339-E357
Johnsen TJ, Friborg O. The effects of cognitive behavioral therapy as an anti-depressive treatment is falling: a meta-analysis. Psychol Bull 2015;141:747–68. https://doi.org/10.1037/bul0000015
Johnsen TJ, Thimm JC. A meta-analysis of group cognitive–behavioral therapy as an antidepressive treatment: are we getting better? Can Psychol 2018;59:15–30.
Jones HC, McKenzie-McHarg K, Horsch A. Standard care practices and psychosocial interventions aimed at reducing parental distress following stillbirth: a systematic narrative review. J Reprod Infant Psychol 2015;33:448–65.
Jonsson U, Bertilsson G, Allard P, Gyllensvärd H, Söderlund A, Tham A, Andersson G. Psychological treatment of depression in people aged 65 years and over: a systematic review of efficacy, safety, and cost-effectiveness. PLOS ONE 2016;11:e0160859. https://doi.org/10.1371/journal.pone.0160859
Kaltenthaler E, Parry G, Beverley C. Computerized cognitive behaviour therapy: a systematic review. Behav Cogn Psychother 2004;32:31–55.
Kaltenthaler E, Parry G, Beverley C, Ferriter M. Computerised cognitive–behavioural therapy for depression: systematic review. Br J Psychiatry 2008;193:181–4. https://doi.org/10.1192/bjp.bp.106.025981
Kampman O, Lehtinen K. Compliance in psychoses. Acta Psychiatr Scand 1999;100:167–75.
Kanapathy J, Bogle V. The effectiveness of cognitive behavioural therapy for depressed patients with diabetes: a systematic review. J Health Psychol 2019;24:137–49. https://doi.org/10.1177/1359105317713360
Kang HS, Kim HK, Park SM, Kim JH. Online-based interventions for sexual health among individuals with cancer: a systematic review. BMC Health Serv Res 2018;18:167. https://doi.org/10.1186/s12913-018-2972-6
Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: a systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull 2008;134:700–41. https://doi.org/10.1037/a0012825
Kani AS, Shinn AK, Lewandowski KE, Öngür D. Converging effects of diverse treatment modalities on frontal cortex in schizophrenia: a review of longitudinal functional magnetic resonance imaging studies. J Psychiatr Res 2017;84:256–76.
Kanters S, Park JJ, Chan K, Socias ME, Ford N, Forrest JI, et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. Lancet HIV 2017;4:e31–e40.
Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat 2011;7:167–81. https://doi.org/10.2147/NDT.S10389
Kazlauskas E, Zelviene P, Lorenz L, Quero S, Maercker A. A scoping review of ICD-11 adjustment disorder research. Eur J Psychotraumatol 2017;8:1421819. https://doi.org/10.1080/20008198.2017.1421819
Kennedy CE, Fonner VA, Armstrong KA, O’Reilly KR, Sweat MD. Increasing HIV serostatus disclosure in low and middle-income countries: a systematic review of intervention evaluations. AIDS 2015;29(Suppl. 1):7–23. https://doi.org/10.1097/QAD.0000000000000671
Kenny DT. A systematic review of treatments for music performance anxiety. Anxiety Stress Coping Int J 2005;18:183–208.
Khoury B, Lecomte T, Fortin G, Masse M, Therien P, Bouchard V, et al. Mindfulness-based therapy: a comprehensive meta-analysis. Clin Psychol Rev 2013;33:763–71. https://doi.org/10.1016/j.cpr.2013.05.005
Kleinstäuber M, Witthöft M, Hiller W. Efficacy of short-term psychotherapy for multiple medically unexplained physical symptoms: a meta-analysis. Clin Psychol Rev 2011;31:146–60. https://doi.org/10.1016/j.cpr.2010.09.001
Kleinstäuber M, Witthöft M, Hiller W. Cognitive–behavioral and pharmacological interventions for premenstrual syndrome or premenstrual dysphoric disorder: a meta-analysis. J Clin Psychol Med Settings 2012;19:308–19. https://doi.org/10.1007/s10880-012-9299-y
Kliem S, Kröger C. Prevention of chronic PTSD with early cognitive behavioral therapy. A meta-analysis using mixed-effects modeling. Behav Res Ther 2013;51:753–61. https://doi.org/10.1016/j.brat.2013.08.005
Kneebone II, Dunmore E. Psychological management of post-stroke depression. Br J Clin Psychol 2000;39:53–65.
Knight SJ, Scheinberg A, Harvey AR. Interventions in pediatric chronic fatigue syndrome/myalgic encephalomyelitis: a systematic review. J Adolesc Health 2013;53:154–65. https://doi.org/10.1016/j.jadohealth.2013.03.009
Knowles SR, Monshat K, Castle DJ. The efficacy and methodological challenges of psychotherapy for adults with inflammatory bowel disease: a review. Inflamm Bowel Dis 2013;19:2704–15. https://doi.org/10.1097/MIB.0b013e318296ae5a
Kornør H, Winje D, Ekeberg Ø, Weisaeth L, Kirkehei I, Johansen K, Steiro A. Early trauma-focused cognitive–behavioural therapy to prevent chronic post-traumatic stress disorder and related symptoms: a systematic review and meta-analysis. BMC Psychiatry 2008;8:81. https://doi.org/10.1186/1471-244X-8-81
Korotana LM, Dobson KS, Pusch D, Josephson T. A review of primary care interventions to improve health outcomes in adult survivors of adverse childhood experiences. Clin Psychol Rev 2016;46:59–90. https://doi.org/10.1016/j.cpr.2016.04.007
Kozasa EH, Hachul H, Monson C, Pinto L, Garcia MC, Mello LE, Tufik S. Mind–body interventions for the treatment of insomnia: a review. Braz J Psychiatry 2010;32:437–43.
Krishna M, Honagodu A, Rajendra R, Sundarachar R, Lane S, Lepping P. A systematic review and meta-analysis of group psychotherapy for sub-clinical depression in older adults. Int J Geriatr Psychiatry 2013;28:881–8. https://doi.org/10.1002/gps.3905
Krishna M, Jauhari A, Lepping P, Turner J, Crossley D, Krishnamoorthy A. Is group psychotherapy effective in older adults with depression? A systematic review. Int J Geriatr Psychiatry 2011;26:331–40. https://doi.org/10.1002/gps.2546
Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med 2007;69:881–8.
Kroenke K, Swindle R. Cognitive-behavioral therapy for somatization and symptom syndromes: a critical review of controlled clinical trials. Psychother Psychosom 2000;69:205–15.
Kuester A, Niemeyer H, Knaevelsrud C. Internet-based interventions for posttraumatic stress: a meta-analysis of randomized controlled trials. Clin Psychol Rev 2016;43:1–16. https://doi.org/10.1016/j.cpr.2015.11.004
Kwekkeboom KL, Cherwin CH, Lee JW, Wanta B. Mind–body treatments for the pain–fatigue–sleep disturbance symptom cluster in persons with cancer. J Pain Symptom Manage 2010;39:126–38. https://doi.org/10.1016/j.jpainsymman.2009.05.022
Labelle R, Pouliot L, Janelle A. A systematic review and meta-analysis of cognitive behavioural treatments for suicidal and self-harm behaviours in adolescents. Can Psychol 2015;56:368–78.
Lami MJ, Martínez MP, Sánchez AI. Systematic review of psychological treatment in fibromyalgia. Curr Pain Headache Rep 2013;17:345. https://doi.org/10.1007/s11916-013-0345-8
Lang R, Regester A, Lauderdale S, Ashbaugh K, Haring A. Treatment of anxiety in autism spectrum disorders using cognitive behaviour therapy: a systematic review. Dev Neurorehabil 2010;13:53–63. https://doi.org/10.3109/17518420903236288
Lavenberg JG. Effects of School-based Cognitive–Behavioral Anger Interventions: A Meta-analysis. Philadelphia, PA: University of Pennsylvania; 2007.
Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev 2008;27:309–17. https://doi.org/10.1080/09595230801919494
Leenarts LE, Diehle J, Doreleijers TA, Jansma EP, Lindauer RJ. Evidence-based treatments for children with trauma-related psychopathology as a result of childhood maltreatment: a systematic review. Eur Child Adolesc Psychiatry 2013;22:269–83. https://doi.org/10.1007/s00787-012-0367-5
Leichsenring F. Comparative effects of short-term psychodynamic psychotherapy and cognitive–behavioral therapy in depression: a meta-analytic approach. Clin Psychol Rev 2001;21:401–19.
Leland NE, Marcione N, Niemiec SLS, Kelkar K, Fogelberg D. What is occupational therapy’s role in addressing sleep problems among older adults? Occup Particip Health 2014;34:141–9.
Lenz A, Hollenbaugh K. Meta-analysis of trauma-focused cognitive behavioral therapy for treating PTSD and co-occurring depression among children and adolescents. Counsel Outcome Res Eval 2015;6:18–32.
Letourneau NL, Dennis CL, Cosic N, Linder J. The effect of perinatal depression treatment for mothers on parenting and child development: a systematic review. Depress Anxiety 2017;34:928–66. https://doi.org/10.1002/da.22687
Lewey JH, Smith CL, Burcham B, Saunders NL, Elfallal D, O’Toole SK. Comparing the effectiveness of EMDR and TF-CBT for children and adolescents: a meta-analysis. J Child Adolesc Trauma 2018;11:457–72. https://doi.org/10.1007/s40653-018-0212-1
Lim C, Sim K, Renjan V, Sam HF, Quah SL. Adapted cognitive–behavioral therapy for religious individuals with mental disorder: a systematic review. Asian J Psychiatr 2014;9:3–12. https://doi.org/10.1016/j.ajp.2013.12.011
Linardon J. Meta-analysis of the effects of cognitive–behavioral therapy on the core eating disorder maintaining mechanisms: implications for mechanisms of therapeutic change. Cogn Behav Ther 2018;47:107–25. https://doi.org/10.1080/16506073.2018.1427785
Linardon J. Rates of abstinence following psychological or behavioral treatments for binge-eating disorder: meta-analysis. Int J Eat Disord 2018;51:785–97. https://doi.org/10.1002/eat.22897
Linardon J, Brennan L. The effects of cognitive–behavioral therapy for eating disorders on quality of life: a meta-analysis. Int J Eat Disord 2017;50:715–30. https://doi.org/10.1002/eat.22719
Linardon J, Fairburn CG, Fitzsimmons-Craft EE, Wilfley DE, Brennan L. The empirical status of the third-wave behaviour therapies for the treatment of eating disorders: a systematic review. Clin Psychol Rev 2017;58:125–40.
Linardon J, Wade T, de la Piedad Garcia X, Brennan L. Psychotherapy for bulimia nervosa on symptoms of depression: a meta-analysis of randomized controlled trials. Int J Eat Disord 2017;50:1124–36. https://doi.org/10.1002/eat.22763
Linardon J, Wade TD. How many individuals achieve symptom abstinence following psychological treatments for bulimia nervosa? A meta-analytic review. Int J Eat Disord 2018;51:287–94. https://doi.org/10.1002/eat.22838
Linardon J, Wade TD, de la Piedad Garcia X, Brennan L. The efficacy of cognitive–behavioral therapy for eating disorders: a systematic review and meta-analysis. J Consult Clin Psychol 2017;85:1080–94. https://doi.org/10.1037/ccp0000245
Lincoln TM, Peters E. A systematic review and discussion of symptom specific cognitive behavioural approaches to delusions and hallucinations. Schizophr Res 2019;203:66–79.
Liu J, Nie J, Wang Y. Effects of group counseling programs, cognitive behavioral therapy, and sports intervention on internet addiction in East Asia: a systematic review and meta-analysis. Int J Environ Res Public Health 2017;14:E1470.
Liu ZQ, Zeng X, Duan CY. Neuropsychological rehabilitation and psychotherapy of adult traumatic brain injury patients with depression: a systematic review and meta-analysis. J Neurosurg Sci 2018;62:24–35. https://doi.org/10.23736/S0390-5616.17.03953-4
Loades ME, Sheils EA, Crawley E. Treatment for paediatric chronic fatigue syndrome or myalgic encephalomyelitis (CFS/ME) and comorbid depression: a systematic review. BMJ Open 2016;6:e012271. https://doi.org/10.1136/bmjopen-2016-012271
Lohnberg JA. A review of outcome studies on cognitive–behavioral therapy for reducing fear-avoidance beliefs among individuals with chronic pain. J Clin Psychol Med Setting 2007;14:113–22.
Lopes AP, Macedo TF, Coutinho ES, Figueira I, Ventura PR. Systematic review of the efficacy of cognitive–behavior therapy related treatments for victims of natural disasters: a worldwide problem. PLOS ONE 2014;9:e109013. https://doi.org/10.1371/journal.pone.0109013
López-Pinar C, Martínez-Sanchís S, Carbonell-Vayá E, Fenollar-Cortés J, Sánchez-Meca J. Long-term efficacy of psychosocial treatments for adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Front Psychol 2018;9:638. https://doi.org/10.3389/fpsyg.2018.00638
Lopresti AL. Cognitive behaviour therapy and inflammation: a systematic review of its relationship and the potential implications for the treatment of depression. Aust N Z J Psychiatry 2017;51:565–82. https://doi.org/10.1177/0004867417701996
Lovell K, Bee P. Optimising treatment resources for OCD: a review of the evidence base for technology-enhanced delivery. J Ment Health 2011;20:525–42. https://doi.org/10.3109/09638237.2011.608745
Luk BH, Loke AY. A review of supportive interventions targeting individuals or couples undergoing infertility treatment: directions for the development of interventions. J Sex Marital Ther 2016;42:515–33. https://doi.org/10.1080/0092623X.2015.1074133
Ma ZR, Shi LJ, Deng MH. Efficacy of cognitive behavioral therapy in children and adolescents with insomnia: a systematic review and meta-analysis. Braz J Med Biol Res 2018;51:e7070.
Magill M. Cognitive–Behavioral Treatment With Adult Substance Users: A Meta-analysis. Dissertation Abstracts International Section A: Humanities and Social Sciences. 2008;68:4479.
Magill M, Ray LA. Cognitive–behavioral treatment with adult alcohol and illicit drug users: a meta-analysis of randomized controlled trials. J Stud Alcohol Drugs 2009;70:516–27.
Malejko K, Abler B, Plener PL, Straub J. Neural correlates of psychotherapeutic treatment of post-traumatic stress disorder: a systematic literature review. Front Psychiatry 2017;8:85. https://doi.org/10.3389/fpsyt.2017.00085
Malouff JM, Thorsteinsson EB, Rooke SE, Bhullar N, Schutte NS. Efficacy of cognitive behavioral therapy for chronic fatigue syndrome: a meta-analysis. Clin Psychol Rev 2008;28:736–45.
Marchesi C, Ossola P, Amerio A, Daniel BD, Tonna M, De Panfilis C. Clinical management of perinatal anxiety disorders: a systematic review. J Affect Disord 2016;190:543–50.
Marotta PL. A systematic review of behavioral health interventions for sex offenders with intellectual disabilities. Sex Abuse 2017;29:148–85. https://doi.org/10.1177/1079063215569546
Martorella G, Boitor M, Berube M, Fredericks S, Le May S, Gélinas C. Tailored web-based interventions for pain: systematic review and meta-analysis. J Med Internet Res 2017;19:e385. https://doi.org/10.2196/jmir.8826
Mason L, Peters E, Kumari V. Functional connectivity predictors and mechanisms of cognitive behavioural therapies: a systematic review with recommendations. Aust N Z J Psychiatry 2016;50:311–21. https://doi.org/10.1177/0004867415624970
Matcham F, Rayner L, Hutton J, Monk A, Steel C, Hotopf M. Self-help interventions for symptoms of depression, anxiety and psychological distress in patients with physical illnesses: a systematic review and meta-analysis. Clin Psychol Rev 2014;34:141–57. https://doi.org/10.1016/j.cpr.2014.01.005
Matthews E, Carter P, Page M, Dean G, Berger A. Sleep–wake disturbance: a systematic review of evidence-based interventions for management in patients with cancer. Clin J Oncol Nurs 2018;22:37–52. https://doi.org/10.1188/18.CJON.37-52
Matthews H, Grunfeld EA, Turner A. The efficacy of interventions to improve psychosocial outcomes following surgical treatment for breast cancer: a systematic review and meta-analysis. Psychooncology 2017;26:593–607. https://doi.org/10.1002/pon.4199
Mayo-Wilson E, Dias S, Mavranezouli I, Kew K, Clark DM, Ades AE, Pilling S. Psychological and pharmacological interventions for social anxiety disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatry 2014;1:368–76. https://doi.org/10.1016/S2215-0366(14)70329-3
McCombie A, Gearry R, Andrews J, Mikocka-Walus A, Mulder R. Computerised cognitive behavioural therapy for psychological distress in patients with physical illnesses: a systematic review. J Clin Psychol Med Settings 2015;22:20–44. https://doi.org/10.1007/s10880-015-9420-0
McCurry SM, Logsdon RG, Teri L, Vitiello MV. Evidence-based psychological treatments for insomnia in older adults. Psychol Aging 2007;22:18–27.
McDonagh MS, Dana T, Selph S, Devine EB, Cantor A, Bougatsos C, et al. Treatments for Schizophrenia in Adults: A Systematic Review. Rockville, MD: Agency for Healthcare Research and Quality; 2017.
McNaughton JL. Brief interventions for depression in primary care: a systematic review. Can Fam Physician 2009;55:789–96.
Mellentin AI, Skøt L, Nielsen B, Schippers GM, Nielsen AS, Stenager E, Juhl C. Cue exposure therapy for the treatment of alcohol use disorders: a meta-analytic review. Clin Psychol Rev 2017;57:195–207.
Mendes DD, Mello MF, Ventura P, Passarela Cde M, Mari Jde J. A systematic review on the effectiveness of cognitive behavioral therapy for posttraumatic stress disorder. Int J Psychiatry Med 2008;38:241–59. https://doi.org/10.2190/PM.38.3.b
Merlin JS, Bulls HW, Vucovich LA, Edelman EJ, Starrels JL. Pharmacologic and non-pharmacologic treatments for chronic pain in individuals with HIV: a systematic review. AIDS Care 2016;28:1506–15.
Mewton L, Smith J, Rossouw P, Andrews G. Current perspectives on internet-delivered cognitive behavioral therapy for adults with anxiety and related disorders. Psychol Res Behav Manag 2014;7:37–46. https://doi.org/10.2147/PRBM.S40879
Miklowitz DJ, Scott J. Psychosocial treatments for bipolar disorder: cost-effectiveness, mediating mechanisms, and future directions. Bipolar Disord 2009;11(Suppl. 2):110–22. https://doi.org/10.1111/j.1399-5618.2009.00715.x
Miles LA, Cooper RL, Nugent WR, Ellis RA. Sexual addiction: a literature review of treatment interventions. J Hum Behav Soc Environ 2016;26:89–99.
Miller-Graff LE, Campion K. Interventions for posttraumatic stress with children exposed to violence: factors associated with treatment success. J Clin Psychol 2016;72:226–48. https://doi.org/10.1002/jclp.22238
Minen MT, Torous J, Raynowska J, Piazza A, Grudzen C, Powers S, et al. Electronic behavioral interventions for headache: a systematic review. J Headache Pain 2016;17:51. https://doi.org/10.1186/s10194-016-0608-y
Mitchell K, Carr A. Anorexia and Bulimia. In Carr A, editor. What Works with Children, Adolescents and Adults? A Critical Review of Psychological Interventions with Children, Adolescents and their Families. Florence, KY: Taylor & Frances/Routledge; 2000. pp. 233–57.
Montgomery L, Robinson C, Seaman EL, Haeny AM. A scoping review and meta-analysis of psychosocial and pharmacological treatments for cannabis and tobacco use among African Americans. Psychol Addict Behav 2017;31:922–43. https://doi.org/10.1037/adb0000326
Moore M, Carr A. Anxiety Disorders. In Carr A, editor. What Works with Children, Adolescents and Adults? A Critical Review of Psychological Interventions with Children, Adolescents and their Families. Florence, KY: Taylor & Frances/Routledge; 2000. pp. 178–202.
Moore TH, Kapur N, Hawton K, Richards A, Metcalfe C, Gunnell D. Interventions to reduce the impact of unemployment and economic hardship on mental health in the general population: a systematic review. Psychol Med 2017;47:1062–84. https://doi.org/10.1017/S0033291716002944
Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998–2004). Sleep 2006;29:1398–414. https://doi.org/10.1093/sleep/29.11.1398
Morrison AP. Cognitive behaviour therapy for first episode psychosis: good for nothing or fit for purpose? Psychos Psychol Soc Integr Approach 2009;1:103–12.
Mu PF, Chen YC, Cheng SC. The effectiveness of non-pharmacological pain management in relieving chronic pain for children and adolescents. JBI Libr Syst Rev 2009;7:1489–543.
Mueller C, Wesenberg S, Nestmann F, Stubbs B, Bebbington P, Raymont V. Interventions to enhance coping after traumatic brain injury: a systematic review. Int J The Rehabil 2018;25:107–19.
Muller I, Yardley L. Telephone-delivered cognitive behavioural therapy: a systematic review and meta-analysis. J Telemed Telecare 2011;17:177–84. https://doi.org/10.1258/jtt.2010.100709
Murphy E, Carr A. Paediatric Pain Problems. In Carr A, editor. What Works with Children and Adolescents? A Critical Review of Psychological Interventions with Children, Adolescents and their Families. Florence, KY: Taylor & Frances/Routledge; 2000. pp. 258–79.
Nadiga DN, Hensley PL, Uhlenhuth EH. Review of the long-term effectiveness of cognitive behavioral therapy compared to medications in panic disorder. Depress Anxiety 2003;17:58–64. https://doi.org/10.1002/da.10084
Necrason E. Cognitive Behavioral Interventions for Pediatric Procedure-related Pain: A Comprehensive Methodological Review. PhD thesis. West Hartford, CT: University of Hartford; 2016.
Neil AL, Christensen H. Australian school-based prevention and early intervention programs for anxiety and depession: a systematic review. Med J Aust 2007;186:305–8.
Neil AL, Christensen H. Efficacy and effectiveness of school-based prevention and early intervention programs for anxiety. Clin Psychol Rev 2009;29:208–15. https://doi.org/10.1016/j.cpr.2009.01.002
Nevo GA, Manassis K. Outcomes for treated anxious children: a critical review of long-term-follow-up studies. Depress Anxiety 2009;26:650–60. https://doi.org/10.1002/da.20584
Newby JM, McKinnon A, Kuyken W, Gilbody S, Dalgleish T. Systematic review and meta-analysis of transdiagnostic psychological treatments for anxiety and depressive disorders in adulthood. Clin Psychol Rev 2015;40:91–110. https://doi.org/10.1016/j.cpr.2015.06.002
Nicholls JL, Azam MA, Burns LC, Englesakis M, Sutherland AM, Weinrib AZ, et al. Psychological treatments for the management of postsurgical pain: a systematic review of randomized controlled trials. Patient Relat Outcome Meas 2018;9:49–64. https://doi.org/10.2147/PROM.S121251
Nieuwsma JA, Trivedi RB, McDuffie J, Kronish I, Benjamin D, Williams JW. Brief psychotherapy for depression: a systematic review and meta-analysis. Int J Psychiatry Med 2012;43:129–51.
Nigatu YT, Huang J, Rao S, Gillis K, Merali Z, Wang J. Indicated prevention interventions in the workplace for depressive symptoms: a systematic review and meta-analysis. Am J Prev Med 2019;56:e23–e33.
Normann N, van Emmerik AA, Morina N. The efficacy of metacognitive therapy for anxiety and depression: a meta-analytic review. Depress Anxiety 2014;31:402–11. https://doi.org/10.1002/da.22273
Norton AR, Abbott MJ, Norberg MM, Hunt C. A systematic review of mindfulness and acceptance-based treatments for social anxiety disorder. J Clin Psychol 2015;71:283–301. https://doi.org/10.1002/jclp.22144
O’Brien AP, McNeil KA, Fletcher R, Conrad A, Wilson AJ, Jones D, Chan SW. New fathers’ perinatal depression and anxiety-treatment options: an integrative review. Am J Mens Health 2017;11:863–76. https://doi.org/10.1177/1557988316669047
O’Connor E, Rossom RC, Henninger M, Groom HC, Burda BU. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016;315:388–406. https://doi.org/10.1001/jama.2015.18948
O’Connor E, Rossom RC, Henninger M, Groom HC, Burda BU, Henderson JT, et al. Screening for Depression in Adults: An Updated Systematic Evidence Review for the U.S. Preventative Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
Oei TPS, Llamas M, Devilly GJ. The efficacy and cognitive processes of cognitive behaviour therapy in the treatment of panic disorder with agoraphobia. Behav Cogn Psychother 1999;27:63–88.
Oing T, Prescott J. Implementations of virtual reality for anxiety-related disorders: systematic review. JMIR Serious Games 2018;6:e10965. https://doi.org/10.2196/10965
Okuzawa N, Kline E, Fuertes J, Negi S, Reeves G, Himelhoch S, Schiffman J. Psychotherapy for adolescents and young adults at high risk for psychosis: a systematic review. Early Interv Psychiatry 2014;8:307–22. https://doi.org/10.1111/eip.12129
Öst LG, Ollendick TH. Brief, intensive and concentrated cognitive behavioral treatments for anxiety disorders in children: a systematic review and meta-analysis. Behav Res Ther 2017;97:134–45.
Öst LG, Riise EN, Wergeland GJ, Hansen B, Kvale G. Cognitive behavioral and pharmacological treatments of OCD in children: a systematic review and meta-analysis. J Anxiety Disord 2016;43:58–69.
Ostadhashemi L, Khankeh HR, Eghlima M, Arshi M, Nafei A, Asangari B, et al. Family-oriented psychosocial intervention in children with cancer: a systematic review. J Kermanshah Univ Med Sci 2016;20:43–50.
Pajak R, Lackner J, Kamboj SK. A systematic review of minimal-contact psychological treatments for symptom management in irritable bowel syndrome. J Psychosom Res 2013;75:103–12. https://doi.org/10.1016/j.jpsychores.2013.05.007
Patel MX, Baker D, Nosarti C. Injection phobia: a systematic review of psychological treatments. Behav Cogn Psychother 2005;33:343–9.
Pearl SB, Norton PJ. Transdiagnostic versus diagnosis specific cognitive behavioural therapies for anxiety: a meta-analysis. J Anxiety Disord 2017;46:11–24.
Pedersen SS, van den Broek KC, Sears SF. Psychological intervention following implantation of an implantable defibrillator: a review and future recommendations. Pacing Clin Electrophysiol 2007;30:1546–54.
Penn DL, Waldheter EJ, Perkins DO, Mueser KT, Lieberman JA. Psychosocial treatment for first-episode psychosis: a research update. Am J Psychiatry 2005;162:2220–32.
Petry NM, Ginley MK, Rash CJ. A systematic review of treatments for problem gambling. Psychol Addict Behav 2017;31:951–61. https://doi.org/10.1037/adb0000290
Phillipou A, Rossell SL, Wilding HE, Castle DJ. Randomised controlled trials of psychological pharmacological treatments for body dysmorphic disorder: a systematic review. Psychiatry Res 2016;245:179–85.
Pichora-Fuller MK, Santaguida P, Hammill A, Oremus M, Westerberg B, Ali U, et al. Evaluation and Treatment of Tinnitus: Comparative Effectiveness. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
Pilling S, Bebbington P, Kuipers E, Garety P, Geddes J, Orbach G, Morgan C. Psychological treatments in schizophrenia: I. Meta-analysis of family intervention and cognitive behaviour therapy. Psychol Med 2002;32:763–82. https://doi.org/10.1017/s0033291702005895
Pineros-Leano M, Liechty JM, Piedra LM. Latino immigrants, depressive symptoms, and cognitive behavioral therapy: a systematic review. J Affect Disord 2017;208:567–76.
Pittock A, Mair E. Are psychotherapies effective in the treatment of anorexia nervosa? A systematic review. J Indian Assoc Child Adolesc Ment Health 2010;6:55–71.
Polizzi DM, MacKenzie DL, Hickman LJ. What works in adult sex offender treatment? A review of prison- and non-prison-based treatment programs. Int J Offender Ther Comp Criminol 1999;43:357–74.
Ponniah K, Hollon SD. Empirically supported psychological treatments for adult acute stress disorder and posttraumatic stress disorder: a review. Depress Anxiety 2009;26:1086–109. https://doi.org/10.1002/da.20635
Ponniah K, Magiati I, Hollon SD. An update on the efficacy of psychological therapies in the treatment of obsessive–compulsive disorder in adults. J Obsessive Compuls Relat Disord 2013;2:207–18. https://doi.org/10.1016/j.jocrd.2013.02.005
Pontillo M, De Crescenzo F, Vicari S, Pucciarini ML, Averna R, Santonastaso O, Armando M. Cognitive behavioural therapy for auditory hallucinations in schizophrenia: a review. World J Psychiatry 2016;6:372–80. https://doi.org/10.5498/wjp.v6.i3.372
Post KE, Flanagan J. Web based survivorship interventions for women with breast cancer: an integrative review. Eur J Oncol Nurs 2016;25:90–9.
Prazeres AM, Nascimento AL, Fontenelle LF. Cognitive–behavioral therapy for body dysmorphic disorder: a review of its efficacy. Neuropsychiatr Dis Treat 2013;9:307–16. https://doi.org/10.2147/NDT.S41074
Priemer M, Talbot F. CBT guided self-help compares favourably to gold standard therapist-administered CBT and shows unique benefits over traditional treatment. Behav Change 2013;30:227–40.
Probyn K, Bowers H, Mistry D, Caldwell F, Underwood M, Patel S, et al. Non-pharmacological self-management for people living with migraine or tension-type headache: a systematic review including analysis of intervention components. BMJ Open 2017;7:e016670. https://doi.org/10.1136/bmjopen-2017-016670
Querstret D, Cropley M. Assessing treatments used to reduce rumination and/or worry: a systematic review. Clin Psychol Rev 2013;33:996–1009. https://doi.org/10.1016/j.cpr.2013.08.004
Quinn A, Mowbray O. Effective treatments for older adult baby boomers with alcohol-use disorders: a literature review. J Soc Work Pract Addict 2018;18:389–410.
Radu M, Moldovan R, Pintea S, Băban A, Dumitrascu D. Predictors of outcome in cognitive and behavioural interventions for irritable bowel syndrome. A meta-analysis. J Gastrointestin Liver Dis 2018;27:257–63. https://doi.org/10.15403/jgld.2014.1121.273.bab
Ramchandani P, Jones DP. Treating psychological symptoms in sexually abused children: from research findings to service provision. Br J Psychiatry 2003;183:484–90.
Rampling J, Furtado V, Winsper C, Marwaha S, Lucca G, Livanou M, Singh SP. Non-pharmacological interventions for reducing aggression and violence in serious mental illness: a systematic review and narrative synthesis. Eur Psychiatry 2016;34:17–28.
Rathbone AL, Clarry L, Prescott J. Assessing the efficacy of mobile health apps using the basic principles of cognitive behavioral therapy: systematic review. J Med Internet Res 2017;19:e399. https://doi.org/10.2196/jmir.8598
Read H, Roush S, Downing D. Early intervention in mental health for adolescents and young adults: a systematic review. Am J Occup Ther 2018;72:7205190040p1–7205190040p8. https://doi.org/10.5014/ajot.2018.033118
Rees-Jones A. Examining the Utility of Assessment Tools and Group Intervention Programmes for Mentally Disordered Offenders. PhD thesis. Birmingham: University of Birmingham; 2011.
Regan B, Varanelli L. Adjustment, depression, and anxiety in mild cognitive impairment and early dementia: a systematic review of psychological intervention studies. Int Psychogeriatr 2013;25:1963–84. https://doi.org/10.1017/S104161021300152X
Regehr C, Glancy D, Pitts A. Interventions to reduce stress in university students: a review and meta-analysis. J Affect Disord 2013;148:1–11. https://doi.org/10.1016/j.jad.2012.11.026
Reger MA, Gahm GA. A meta-analysis of the effects of internet- and computer-based cognitive–behavioral treatments for anxiety. J Clin Psychol 2009;65:53–75. https://doi.org/10.1002/jclp.20536
Rice SM, Goodall J, Hetrick SE, Parker AG, Gilbertson T, Amminger GP, et al. Online and social networking interventions for the treatment of depression in young people: a systematic review. J Med Internet Res 2014;16:e206. https://doi.org/10.2196/jmir.3304
Richardson T, Stallard P, Velleman S. Computerised cognitive behavioural therapy for the prevention and treatment of depression and anxiety in children and adolescents: a systematic review. Clin Child Fam Psychol Rev 2010;13:275–90. https://doi.org/10.1007/s10567-010-0069-9
Richter K, Acker J, Adam S, Niklewski G. Prevention of fatigue and insomnia in shift workers – a review of non-pharmacological measures. EPMA J 2016;7:16. https://doi.org/10.1186/s13167-016-0064-4
Riemann D, Workshop Participants. Does effective management of sleep disorders reduce depressive symptoms and the risk of depression? Drugs 2009;69:43–64.
Rith-Najarian LR, Mesri B, Park AL, Sun M, Chavira DA, Chorpita BF. Durability of cognitive behavioral therapy effects for youth and adolescents with anxiety, depression, or traumatic stress: a meta-analysis on long-term follow-ups. Behav Ther 2019;50:225–40.
Rodgers M, Asaria M, Walker S, McMillan D, Lucock M, Harden M, et al. The clinical effectiveness and cost-effectiveness of low-intensity psychological interventions for the secondary prevention of relapse after depression: a systematic review. Health Technol Assess 2012;16(28). https://doi.org/10.3310/hta16280
Rolfsnes ES, Idsoe T. School-based intervention programs for PTSD symptoms: a review and meta-analysis. J Trauma Stress 2011;24:155–65. https://doi.org/10.1002/jts.20622
Romanelli RJ, Wu FM, Gamba R, Mojtabai R, Segal JB. Behavioral therapy and serotonin reuptake inhibitor pharmacotherapy in the treatment of obsessive–compulsive disorder: a systematic review and meta-analysis of head-to-head randomized controlled trials. Depress Anxiety 2014;31:641–52. https://doi.org/10.1002/da.22232
Romero-Martinez A, Hidalgo-Moreno G, Moya-Albiol L. Neuropsychological consequences of chronic stress: the case of informal caregivers. Aging Ment Health 2018;18:1–13.
Rooksby M, Elouafkaoui P, Humphris G, Clarkson J, Freeman R. Internet-assisted delivery of cognitive behavioural therapy (CBT) for childhood anxiety: systematic review and meta-analysis. J Anxiety Disord 2015;29:83–92. https://doi.org/10.1016/j.janxdis.2014.11.006
Rossy LA, Buckelew SP, Dorr N, Hagglund KJ, Thayer JF, McIntosh MJ, et al. A meta-analysis of fibromyalgia treatment interventions. Ann Behav Med 1999;21:180–91. https://doi.org/10.1007/BF02908299
Rubin GJ, Das Munshi J, Wessely S. A systematic review of treatments for electromagnetic hypersensitivity. Psychother Psychosom 2006;75:12–18.
Rutten JM, Korterink JJ, Venmans LM, Benninga MA, Tabbers MM. Nonpharmacologic treatment of functional abdominal pain disorders: a systematic review. Pediatrics 2015;135:522–35. https://doi.org/10.1542/peds.2014-2123
Sajid S, Kotwal AA, Dale W. Interventions to improve decision making and reduce racial and ethnic disparities in the management of prostate cancer: a systematic review. J Gen Intern Med 2012;27:1068–78. https://doi.org/10.1007/s11606-012-2086-5
Salcedo S, Gold AK, Sheikh S, Marcus PH, Nierenberg AA, Deckersbach T, Sylvia LG. Empirically supported psychosocial interventions for bipolar disorder: current state of the research. J Affect Disord 2016;201:203–14. https://doi.org/10.1016/j.jad.2016.05.018
Salmoirago-Blotcher E, Ockene IS. Methodological limitations of psychosocial interventions in patients with an implantable cardioverter-defibrillator (ICD): a systematic review. BMC Cardiovasc Disord 2009;9:56. https://doi.org/10.1186/1471-2261-9-56
Salomonsson S, Hedman-Lagerlöf E, Öst LG. Sickness absence: a systematic review and meta-analysis of psychological treatments for individuals on sick leave due to common mental disorders. Psychol Med 2018;48:1954–65. https://doi.org/10.1017/S0033291718000065
Sawyer MC, Nunez DE. Cognitive–behavioral therapy for anxious children: from evidence to practice. Worldviews Evid Based Nurs 2014;11:65–71. https://doi.org/10.1111/wvn.12024
Schmid G, Henningsen P, Dieterich M, Sattel H, Lahmann C, Schmid G, et al. Psychotherapy in dizziness: a systematic review. J Neurol Neurosurg Psychiatry 2011;82:601–6.
Scott RW, Mughelli K, Deas D. An overview of controlled studies of anxiety disorders treatment in children and adolescents. J Natl Med Assoc 2005;97:13–24.
Seidler GH, Wagner FE. Comparing the efficacy of EMDR and trauma-focused cognitive–behavioral therapy in the treatment of PTSD: a meta-analytic study. Psychol Med 2006;36:1515–22.
Shapiro JR, Berkman ND, Brownley KA, Sedway JA, Lohr KN, Bulik CM. Bulimia nervosa treatment: a systematic review of randomized controlled trials. Int J Eat Disord 2007;40:321–36. https://doi.org/10.1002/eat.20372
Shepherd C, Beail N. A systematic review of the effectiveness of psychoanalysis, psychoanalytic and psychodynamic psychotherapy with adults with intellectual and developmental disabilities: progress and challenges. Psychoanal Psychother 2017;31:94–117.
Shin JC, Kim J, Grigsby-Toussaint D. Mobile phone interventions for sleep disorders and sleep quality: systematic review. JMIR M health Uhealth 2017;5:e131. https://doi.org/10.2196/mhealth.7244
Sigra S, Hesselmark E, Bejerot S. Treatment of PANDAS and PANS: a systematic review. Neurosci Biobehav Rev 2018;86:51–65.
Sikorski C, Lakhanpaul M, Costello A, Heys M. A systematic review: ‘Can postnatal women’s groups improve health outcomes for women and children in high-income countries?’. Arch Dis Child 2014;1:A200.
Silverman WK, Pina AA. Psychosocial Treatments for Phobic and Anxiety Disorders in Youth. In Steele RG, Elkin TD, Roberts MC, editors. Handbook of Evidence-Based Therapies for Children and Adolescents. New York, NY: Springer Science + Business Media; 2008. pp. 65–82.
Silverman WK, Pina AA, Viswesvaran C. Evidence-based psychosocial treatments for phobic and anxiety disorders in children and adolescents. J Clin Child Adolesc Psychol 2008;37:105–30. https://doi.org/10.1080/15374410701817907
Simblett S, Birch J, Matcham F, Yaguez L, Morris R. A systematic review and meta-analysis of e-mental health interventions to treat symptoms of posttraumatic stress. JMIR Ment Health 2017;4:e14. https://doi.org/10.2196/mental.5558
Simning A, Simons KV. Treatment of depression in nursing home residents without significant cognitive impairment: a systematic review. Int Psychogeriatr 2017;29:209–26. https://doi.org/10.1017/S1041610216001733
Sinclair S, Ob Sutherland R, Henderson S, O’Callaghan V, Dalton T, Jefford M, et al. The impact of fear of cancer recurrence on wellness. Asia-Pac J Clin Oncol 2013;3:84–5.
Sinha JW, Rosenberg LB. A critical review of trauma interventions and religion among youth exposed to community violence. J Soc Serv Res 2013;39:436–4.
Skapinakis P, Caldwell D, Hollingworth W, Bryden P, Fineberg N, Salkovskis P, et al. A systematic review of the clinical effectiveness and cost-effectiveness of pharmacological and psychological interventions for the management of obsessive–compulsive disorder in children/adolescents and adults. Health Technol Assess 2016;20(43). https://doi.org/10.3310/hta20430
Skarphedinsson G, Hanssen-Bauer K, Kornør H, Heiervang ER, Landrø NI, Axelsdottir B, et al. Standard individual cognitive behaviour therapy for paediatric obsessive–compulsive disorder: a systematic review of effect estimates across comparisons. Nord J Psychiatry 2015;69:81–92. https://doi.org/10.3109/08039488.2014.941395
Smith TE, Weston CA, Lieberman JA. Schizophrenia (maintenance treatment). BMJ Clin Evid 2009;2009:1007.
Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affect Disord 2015;177:7–21. https://doi.org/10.1016/j.jad.2015.01.052
Sockol LE, Epperson CN, Barber JP. A meta-analysis of treatments for perinatal depression. Clin Psychol Rev 2011;31:839–49. https://doi.org/10.1016/j.cpr.2011.03.009
Song F, Huttunen-Lenz M, Holland R. Effectiveness of complex psycho-educational interventions for smoking relapse prevention: an exploratory meta-analysis. J Public Health 2010;32:350–9. https://doi.org/10.1093/pubmed/fdp109
St Amand A, Bard DE, Silovsky JF. Meta-analysis of treatment for child sexual behavior problems: practice elements and outcomes. Child Maltreat 2008;13:145–66. https://doi.org/10.1177/1077559508315353
Stafford MR, Mayo-Wilson E, Loucas CE, James A, Hollis C, Birchwood M, Kendall T. Efficacy and safety of pharmacological and psychological interventions for the treatment of psychosis and schizophrenia in children, adolescents and young adults: a systematic review and meta-analysis. PLOS ONE 2015;10:e0117166. https://doi.org/10.1371/journal.pone.0117166
Stapleton JL, Hillhouse J, Levonyan-Radloff K, Manne SL. Review of interventions to reduce ultraviolet tanning: need for treatments targeting excessive tanning, an emerging addictive behavior. Psychol Addict Behav 2017;31:962–78. https://doi.org/10.1037/adb0000289
Stefanopoulou E, Grunfeld EA. Mind-body interventions for vasomotor symptoms in healthy menopausal women and breast cancer survivors. A systematic review. J Psychosom Obstet Gynaecol 2017;38:210–25. https://doi.org/10.1080/0167482X.2016.1235147
Steinert C, Munder T, Rabung S, Hoyer J, Leichsenring F. Psychodynamic therapy: as efficacious as other empirically supported treatments? A meta-analysis testing equivalence of outcomes. Am J Psychiatry 2017;174:943–53. https://doi.org/10.1176/appi.ajp.2017.17010057
Stevanovic D, Tadic I, Knez R. Are antidepressants effective in quality of life improvement among children and adolescents? A systematic review. CNS Spectr 2014;19:134–41. https://doi.org/10.1017/S1092852913000576
Stratton E, Lampit A, Choi I, Calvo RA, Harvey SB, Glozier N. Effectiveness of eHealth interventions for reducing mental health conditions in employees: a systematic review and meta-analysis. PLOS ONE 2017;12:e0189904. https://doi.org/10.1371/journal.pone.0189904
Subnis UB, Starkweather AR, McCain NL, Brown RF. Psychosocial therapies for patients with cancer: a current review of interventions using psychoneuroimmunology-based outcome measures. Integr Cancer Ther 2014;13:85–104. https://doi.org/10.1177/1534735413503548
Sylvia LG, Tilley CA, Lund HG, Sachs GS. Psychosocial interventions: empirically-derived treatments for bipolar disorder. Curr Psychiatry Rev 2008;4:108–13.
Sztein DM, Koransky CE, Fegan L, Himelhoch S. Efficacy of cognitive behavioural therapy delivered over the internet for depressive symptoms: a systematic review and meta-analysis. J Telemed Telecare 2018;24:527–39. https://doi.org/10.1177/1357633X17717402
Tatrow K, Montgomery GH. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. J Behav Med 2006;29:17–27. https://doi.org/10.1007/s10865-005-9036-1
Tavares LR, Barbosa MR. Efficacy of group psychotherapy for geriatric depression: a systematic review. Arch Gerontol Geriatr 2018;78:71–80.
Teng EJ, Hiatt EL, McClair V, Kunik ME, Frueh B, Stanley MA. Efficacy of posttraumatic stress disorder treatment for comorbid panic disorder: a critical review and future directions for treatment research. Clin Psychol Sci Pract 2013;20:268–84.
Thoma NC, McKay D, Gerber AJ, Milrod BL, Edwards AR, Kocsis JH. A quality-based review of randomized controlled trials of cognitive-behavioral therapy for depression: an assessment and metaregression. Am J Psychiatry 2012;169:22–30. https://doi.org/10.1176/appi.ajp.2011.11030433
Thomas WJ, Hauson AO, Lambert JE, Stern MJ, Gamboa JM, Allen KE, et al. A meta-analysis of the effectiveness of cognitive–behavioural therapies for late-life depression. Can J Counsel Psychother 2018;52:78–117.
Thomas WJF. Effectiveness of Cognitive–Behavioral Therapies for Late-life Depression: Research Synthesis and Meta-analysis. PhD thesis. San Diego, CA: Alliant International University; 2015.
Thompson C, Fernández de la Cruz L, Mataix-Cols D, Onwumere J. A systematic review and quality assessment of psychological, pharmacological, and family-based interventions for hoarding disorder. Asian J Psychiatr 2017;27:53–66.
Thompson DM, Hall DA, Walker DM, Hoare DJ. Psychological therapy for people with tinnitus: a scoping review of treatment components. Ear Hear 2017;38:149–58. https://doi.org/10.1097/AUD.0000000000000363
Thompson-Brenner HJ. Implications for the Treatment of Bulimia Nervosa: A Meta-analysis of Efficacy Trials and a Naturalistic Study of Treatment in the Community. PhD thesis. Ann Arbor, MI: University of Michigan; 2003.
Thorp SR, Ayers CR, Nuevo R, Stoddard JA, Sorrell JT, Wetherell JL. Meta-analysis comparing different behavioral treatments for late-life anxiety. Am J Geriatr Psychiatry 2009;17:105–15. https://doi.org/10.1097/JGP.0b013e31818b3f7e
Tol WA, Stavrou V, Greene MC, Mergenthaler C, van Ommeren M, García Moreno C. Sexual and gender-based violence in areas of armed conflict: a systematic review of mental health and psychosocial support interventions. Confl Health 2013;7:16. https://doi.org/10.1186/1752-1505-7-16
Trautmann E, Lackschewitz H, Kröner-Herwig B. Psychological treatment of recurrent headache in children and adolescents – a meta-analysis. Cephalalgia 2006;26:1411–26.
Tribe RH, Sendt KV, Tracy DK. A systematic review of psychosocial interventions for adult refugees and asylum seekers. J Ment Health 2017;12:1–15.
Trinidade A, Goebel JA. Persistent postural–perceptual dizziness – a systematic review of the literature for the balance specialist. Otol Neurotol 2018;39:1291–303. https://doi.org/10.1097/MAO.0000000000002010
Tullar JM, Brewer S, Amick BC, Irvin E, Mahood Q, Pompeii LA, et al. Occupational safety and health interventions to reduce musculoskeletal symptoms in the health care sector. J Occup Rehabil 2010;20:199–219. https://doi.org/10.1007/s10926-010-9231-y
Tundo A, Necci R. Cognitive–behavioural therapy for obsessive–compulsive disorder co-occurring with psychosis: systematic review of evidence. World J Psychiatry 2016;6:449–55. https://doi.org/10.5498/wjp.v6.i4.449
Turner DT, McGlanaghy E, Cuijpers P, van der Gaag M, Karyotaki E, MacBeth A. A meta-analysis of social skills training and related interventions for psychosis. Schizophr Bull 2018;44:475–91. https://doi.org/10.1093/schbul/sbx146
Uman LS, Birnie KA, Noel M, Parker JA, Chambers CT, McGrath PJ, Kisely SR. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev 2013;10:CD005179. https://doi.org/10.1002/14651858.CD005179.pub3
Ung D, Selles R, Small BJ, Storch EA. A systematic review and meta-analysis of cognitive–behavioral therapy for anxiety in youth with high-functioning autism spectrum disorders. Child Psychiatry Hum Dev 2015;46:533–47. https://doi.org/10.1007/s10578-014-0494-y
Unwin G, Tsimopoulou I, Kroese BS, Azmi S. Effectiveness of cognitive behavioural therapy (CBT) programmes for anxiety or depression in adults with intellectual disabilities: a review of the literature. Res Dev Disabil 2016;51–52:60–75. https://doi.org/10.1016/j.ridd.2015.12.010
Vallarino M, Henry C, Etain B, Gehue LJ, Macneil C, Scott EM, et al. An evidence map of psychosocial interventions for the earliest stages of bipolar disorder. Lancet Psychiatry 2015;2:548–63. https://doi.org/10.1016/S2215-0366(15)00156-X
Vally Z, Maggott C. Evaluating the outcome of cultural adaptations of cognitive–behavioural therapy for adult depression: a meta-analysis of treatment studies in developing countries. Int J Adv Counsel 2015;37:293–304.
Van der Oord S, Prins PJ, Oosterlaan J, Emmelkamp PM. Efficacy of methylphenidate, psychosocial treatments and their combination in school-aged children with ADHD: a meta-analysis. Clin Psychol Rev 2008;28:783–800.
van Hout MS, Wekking EM, Berg IJ, Deelman BG. Psychological treatment of patients with chronic toxic encephalopathy: lessons from studies of chronic fatigue and whiplash. Psychother Psychosom 2003;72:235–44. https://doi.org/10.1159/000071894
van Koulil S, Effting M, Kraaimaat FW, van Lankveld W, van Helmond T, Cats H, et al. Cognitive–behavioural therapies and exercise programmes for patients with fibromyalgia: state of the art and future directions. Ann Rheum Dis 2007;66:571–81.
van Straten A, Geraedts A, Verdonck-de Leeuw I, Andersson G, Cuijpers P. Psychological treatment of depressive symptoms in patients with medical disorders: a meta-analysis. J Psychosom Res 2010;69:23–32. https://doi.org/10.1016/j.jpsychores.2010.01.019
van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, Lancee J. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev 2018;38:3–16.
van Vilsteren M, van Oostrom SH, de Vet HC, Franche RL, Boot CR, Anema JR. Workplace interventions to prevent work disability in workers on sick leave. Cochrane Database Syst Rev 2015;10:CD006955. https://doi.org/10.1002/14651858.CD006955.pub3
van Zoonen K, Buntrock C, Ebert DD, Smit F, Reynolds CF, Beekman AT, Cuijpers P. Preventing the onset of major depressive disorder: a meta-analytic review of psychological interventions. Int J Epidemiol 2014;43:318–29. https://doi.org/10.1093/ije/dyt175
Vasa RA, Carroll LM, Nozzolillo AA, Mahajan R, Mazurek MO, Bennett AE, et al. A systematic review of treatments for anxiety in youth with autism spectrum disorders. J Autism Dev Disord 2014;44:3215–29. https://doi.org/10.1007/s10803-014-2184-9
Verdejo-Garcia A, Alcazar-Corcoles MA, Albein-Urios N. Neuropsychological interventions for decision-making in addiction: a systematic review. NeuropsycholRev 2018;26:26.
Vereenooghe L, Langdon PE. Psychological therapies for people with intellectual disabilities: a systematic review and meta-analysis. Res Dev Disabil 2013;34:4085–102. https://doi.org/10.1016/j.ridd.2013.08.030
Visser E, Gosens T, Den Oudsten BL, De Vries J. The course, prediction, and treatment of acute and posttraumatic stress in trauma patients: a systematic review. J Trauma Acute Care Surg 2017;82:1158–83. https://doi.org/10.1097/TA.0000000000001447
von der Embse N, Barterian J, Segool N. Test anxiety interventions for children and adolescents: a systematic review of treatment studies from 2000–2010. Psychol Schools 2013;50:57–71.
Wadephul F, Jones C, Jomeen J. The impact of antenatal psychological group interventions on psychological well-being: a systematic review of the qualitative and quantitative evidence. Healthcare 2016;4:E32. https://doi.org/10.3390/healthcare4020032
Walczak M, Ollendick T, Ryan S, Esbjørn BH. Does comorbidity predict poorer treatment outcome in pediatric anxiety disorders? An updated 10-year review. Clin Psychol Rev 2018;60:45–61.
Waldron B, Casserly LM, O’Sullivan C. Cognitive behavioural therapy for depression and anxiety in adults with acquired brain injury: what works for whom? Neuropsychol Rehabil 2013;23:64–101. https://doi.org/10.1080/09602011.2012.724196
Waldron HB, Turner CW. Evidence-based psychosocial treatments for adolescent substance abuse. J Clin Child Adolesc Psychol 2008;37:238–61. https://doi.org/10.1080/15374410701820133
Wan Mohd Yunus WMA, Musiat P, Brown JSL. Systematic review of universal and targeted workplace interventions for depression. Occup Environ Med 2018;75:66–75. https://doi.org/10.1136/oemed-2017-104532
Wang L, Chang Y, Kennedy SA, Hong PJ, Chow N, Couban RJ, et al. Perioperative psychotherapy for persistent post-surgical pain and physical impairment: a meta-analysis of randomised trials. Br J Anaesth 2018;120:1304–14.
Wang MY, Wang SY, Tsai PS. Cognitive behavioural therapy for primary insomnia: a systematic review. J Adv Nurs 2005;50:553–64.
Watson HJ, Bulik CM. Update on the treatment of anorexia nervosa: review of clinical trials, practice guidelines and emerging interventions. Psychol Med 2013;43:2477–500. https://doi.org/10.1017/S0033291712002620
Weisz JR, McCarty CA, Valeri SM. Effects of psychotherapy for depression in children and adolescents: a meta-analysis. Psychol Bull 2006;132:132–49.
Weitlauf AS, McPheeters ML, Peters B, Sathe N, Travis R, Aiello R, et al. Therapies for Children with Autism Spectrum Disorder: Behavioural Interventions Update. Rockville, MD: Agency for Healthcare Research and Quality; 2014.
Werner-Seidler A, Johnston L, Christensen H. Digitally-delivered cognitive–behavioural therapy for youth insomnia: a systematic review. Internet Interv 2018;11:71–8. https://doi.org/10.1016/j.invent.2018.01.007
Weydert JA, Ball TM, Davis MF. Systematic review of treatments for recurrent abdominal pain. Pediatrics 2003;111:e1–11. https://doi.org/10.1542/peds.111.1.e1
Whiting P, Bagnall AM, Sowden AJ, Cornell JE, Mulrow CD, Ramírez G. Interventions for the treatment and management of chronic fatigue syndrome: a systematic review. JAMA 2001;286:1360–8.
Williams S, Dale J. The effectiveness of treatment for depression/depressive symptoms in adults with cancer: a systematic review. Br J Cancer 2006;94:372–90.
Wilson DB, Bouffard LA, Mackenzie DL. A quantitative review of structured, group-oriented, cognitive–behavioral programs for offenders. Crim Justice Behav 2005;32:172–204.
Wolpert M, Dalzell K, Ullman R, Garland L, Cortina M, Hayes D, et al. Strategies not accompanied by a mental health professional to address anxiety and depression in children and young people: a scoping review of range and a systematic review of effectiveness. Lancet Psychiatry 2019;6:46–60.
Wu Y, Lang Z, Zhang H. Efficacy of cognitive–behavioral therapy in pediatric obsessive–compulsive disorder: a meta-analysis. Med Sci Monit 2016;22:1646–53.
Yang B, Xu J, Xue Q, Wei T, Xu J, Ye C, et al. Non-pharmacological interventions for improving sleep quality in patients on dialysis: systematic review and meta-analysis. Sleep Med Rev 2015;23:68–82. https://doi.org/10.1016/j.smrv.2014.11.005
Yang S, Sajatovic M, Walter BL. Psychosocial interventions for depression and anxiety in Parkinson’s disease. J Geriatr Psychiatry Neurol 2012;25:113–21. https://doi.org/10.1177/0891988712445096
Yatham S, Sivathasan S, Yoon R, Da Silva T, Ravindran AV, Levitt SE. Depression and anxiety disorders in child and adolescent populations in low and middle income countries: a review. Ann Glob Health 2017;83:87.
Ye YY, Chen NK, Chen J, Liu J, Lin L, Liu YZ, et al. Internet-based cognitive–behavioural therapy for insomnia (ICBT-i): a meta-analysis of randomised controlled trials. BMJ Open 2016;6:e010707. https://doi.org/10.1136/bmjopen-2015-010707
Ye YY, Zhang YF, Chen J, Liu J, Li XJ, Liu YZ, et al. Internet-based cognitive behavioral therapy for insomnia (ICBT-i) improves comorbid anxiety and depression-a meta-analysis of randomized controlled trials. PLOS ONE 2015;10:e0142258. https://doi.org/10.1371/journal.pone.0142258
Yeh ML, Chung YC, Hsu MYF, Hsu CC. Quantifying psychological distress among cancer patients in interventions and scales: a systematic review topical collection on cancer pain. Curr Pain Headache Rep 2014;18:399.
Ying L, Wu LH, Loke AY. The effects of psychosocial interventions on the mental health, pregnancy rates, and marital function of infertile couples undergoing in vitro fertilization: a systematic review. J Assist Reprod Genet 2016;33:689–701. https://doi.org/10.1007/s10815-016-0690-8
Yohannes AM, Caton S. Management of depression in older people with osteoarthritis: a systematic review. Aging Ment Health 2010;14:637–51. https://doi.org/10.1080/13607860903483094
Yohannes AM, Junkes-Cunha M, Smith J, Vestbo J. Management of dyspnea and anxiety in chronic obstructive pulmonary disease: a critical review. J Am Med Dir Assoc 2017;18:1096.e1–1096.e17.
Yoon IA, Slade K, Fazel S. Outcomes of psychological therapies for prisoners with mental health problems: a systematic review and meta-analysis. J Consult Clin Psychol 2017;85:783–802. https://doi.org/10.1037/ccp0000214
Zantvoord JB, Diehle J, Lindauer RJ. Using neurobiological measures to predict and assess treatment outcome of psychotherapy in posttraumatic stress disorder: systematic review. Psychother Psychosom 2013;82:142–51. https://doi.org/10.1159/000343258
Zhou SG, Hou YF, Liu D, Zhang XY. Effect of cognitive behavioral therapy versus interpersonal psychotherapy in patients with major depressive disorder: a meta-analysis of randomized controlled trials. Chin Med J 2017;130:2844–51. https://doi.org/10.4103/0366-6999.219149
Zhou X, Hetrick SE, Cuijpers P, Qin B, Barth J, Whittington CJ, et al. Comparative efficacy and acceptability of psychotherapies for depression in children and adolescents: a systematic review and network meta-analysis. World Psychiatry 2015;14:207–22. https://doi.org/10.1002/wps.20217
Zimmermann G, Favrod J, Trieu VH, Pomini V. The effect of cognitive behavioral treatment on the positive symptoms of schizophrenia spectrum disorders: a meta-analysis. Schizophr Res 2005;77:1–9.
Zimmermann P, Muhlig S, Sonntag D, Buhringer G, Wittchen HU. Review on psychotherapeutic interventions for cannabis disorders. Sucht 2004;50:334–42.
Studies excluded because of broad definition of cognitive–behavioural therapy (n = 119)
Álvarez-Jiménez M, Hetrick SE, González-Blanch C, Gleeson JF, McGorry PD. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry 2008;193:101–7. https://doi.org/10.1192/bjp.bp.107.042853
Amick HR, Gartlehner G, Gaynes BN, Forneris C, Asher GN, Morgan LC, et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ 2015;351:h6019. https://doi.org/10.1136/bmj.h6019
Andersson G, Nordgren LB, Buhrman M, Carlbring P. Psychological treatments for depression delivered via the internet and supported by a clinician: an update. Rev Psicopatol Psicol Clin 2014;19:217–25.
Arnberg A, Ost LG. CBT for children with depressive symptoms: a meta-analysis. Cogn Behav Ther 2014;43:275–88. https://doi.org/10.1080/16506073.2014.947316
Barlow J, Smailagic N, Huband N, Roloff V, Bennett C. Group-based parent training programmes for improving parental psychosocial health. Cochrane Database Syst Rev 2014;5:CD002020. https://doi.org/10.1002/14651858.CD002020.pub4
Barrera TL, Mott JM, Hofstein RF, Teng EJ. A meta-analytic review of exposure in group cognitive behavioral therapy for posttraumatic stress disorder. Clin Psychol Rev 2013;33:24–32. https://doi.org/10.1016/j.cpr.2012.09.005
Battagliese G, Caccetta M, Luppino OI, Baglioni C, Cardi V, Mancini F, Buonanno C. Cognitive–behavioral therapy for externalizing disorders: a meta-analysis of treatment effectiveness. Behav Res Ther 2015;75:60–71. https://doi.org/10.1016/j.brat.2015.10.008
Bennett K, Manassis K, Walter SD, Cheung A, Wilansky-Traynor P, Diaz-Granados N, et al. Cognitive behavioral therapy age effects in child and adolescent anxiety: an individual patient data metaanalysis. Depress Anxiety 2013;30:829–41. https://doi.org/10.1002/da.22099
Bighelli I, Huhn M, Schneider-Thoma J, Krause M, Reitmeir C, Wallis S, et al. Response rates in patients with schizophrenia and positive symptoms receiving cognitive behavioural therapy: a systematic review and single-group meta-analysis. BMC Psychiatry 2018;18:380. https://doi.org/10.1186/s12888-018-1964-8
Bisson JI, Roberts NP, Andrew M, Cooper R, Lewis C. Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst Rev 2013;12:CD003388. https://doi.org/10.1002/14651858.CD003388.pub4
Burns AM, Erickson DH, Brenner CA. Cognitive–behavioral therapy for medication-resistant psychosis: a meta-analytic review. Psychiatr Serv 2014;65:874–80. https://doi.org/10.1176/appi.ps.201300213
Buscemi N, Vandermeer B, Friesen C, Bialy L, Tubman M, Ospina M, et al. Manifestations and management of chronic insomnia in adults. Evid Rep Technol Assess 2005;125:1–10.
Chung KF, Lee CT, Yeung WF, Chan MS, Chung EW, Lin WL. Sleep hygiene education as a treatment of insomnia: a systematic review and meta-analysis. Fam Pract 2018;35:365–75. https://doi.org/10.1093/fampra/cmx122
Chung KF, Lee CT, Yeung WF, Chan MS, Chung EW, Lin WL. Sleep hygiene education as a treatment of insomnia: a systematic review and meta-analysis. Fam Pract 2017;29:29.
Cooper K, Gregory JD, Walker I, Lambe S, Salkovskis PM. Cognitive behaviour therapy for health anxiety: a systematic review and meta-analysis. Behav Cogn Psychother 2017;45:110–23. https://doi.org/10.1017/S1352465816000527
Crepaz N, Passin WF, Herbst JH, Rama SM, Malow RM, Purcell DW, Wolitski RJ, HIV/AIDS Prevention Research Synthesis Team. Meta-analysis of cognitive–behavioral interventions on HIV-positive persons’ mental health and immune functioning. Health Psychol 2008;27:4–14. https://doi.org/10.1037/0278-6133.27.1.4
Cristea IA, Stefan S, Karyotaki E, David D, Hollon SD, Cuijpers P. The effects of cognitive behavioral therapy are not systematically falling: a revision of Johnsen and Friborg (2015). Psychol Bull 2017;143:326–40.
Cuijpers P, Cristea IA, Karyotaki E, Reijnders M, Huibers MJ. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta-analytic update of the evidence. World Psychiatry 2016;15:245–58. https://doi.org/10.1002/wps.20346
Cuijpers P, Sijbrandij M, Koole S, Huibers M, Berking M, Andersson G. Psychological treatment of generalized anxiety disorder: a meta-analysis. Clin Psychol Rev 2014;34:130–40. https://doi.org/10.1016/j.cpr.2014.01.002
Cuijpers P, van Straten A, Andersson G. Internet-administered cognitive behavior therapy for health problems: a systematic review. J Behav Med 2008;31:169–77. https://doi.org/10.1007/s10865-007-9144-1
Cuijpers P, Straten A, Warmerdam L. Are individual and group treatments equally effective in the treatment of depression in adults: a meta-analysis. Eur J Psychiatry 2008;22:38–51.
Cuijpers P, van Straten A, Warmerdam L, Andersson G. Psychotherapy versus the combination of psychotherapy and pharmacotherapy in the treatment of depression: a meta-analysis. Depress Anxiety 2009;26:279–88. https://doi.org/10.1002/da.20519
Cuijpers P, van Straten A, Smit F, Mihalopoulos C, Beekman A. Preventing the onset of depressive disorders: a meta-analytic review of psychological interventions. Am J Psychiatry 2008;165:1272–80. https://doi.org/10.1176/appi.ajp.2008.07091422
De Los Reyes A. Identifying Evidence-based Interventions Using the Range of Possible Changes Model: A Meta-analytic Illustration. PhD thesis. New Haven, CT: Yale University; 2008.
Dickens C, Cherrington A, Adeyemi I, Roughley K, Bower P, Garrett C, et al. Characteristics of psychological interventions that improve depression in people with coronary heart disease: a systematic review and meta-regression. Psychosom Med 2013;75:211–21. https://doi.org/10.1097/PSY.0b013e31827ac009
Diehle J, Schmitt K, Daams JG, Boer F, Lindauer RJ. Effects of psychotherapy on trauma-related cognitions in posttraumatic stress disorder: a meta-analysis. J Trauma Stress 2014;27:257–64. https://doi.org/10.1002/jts.21924
Dissanayake RK, Bertouch JV. Psychosocial interventions as adjunct therapy for patients with rheumatoid arthritis: a systematic review. Int J Rheum Dis 2010;13:324–34. https://doi.org/10.1111/j.1756-185X.2010.01563.x
Dorrepaal E, Thomaes K, Hoogendoorn AW, Veltman DJ, Draijer N, van Balkom AJ. Evidence-based treatment for adult women with child abuse-related Complex PTSD: a quantitative review. Eur J Psychotraumatol 2014;5:23613. https://doi.org/10.3402/ejpt.v5.23613
Eccleston C, Palermo TM, Fisher E, Law E. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst Rev 2012;8:CD009660. https://doi.org/10.1002/14651858.CD009660.pub2
Feng CY, Chu H, Chen CH, Chang YS, Chen TH, Chou YH, et al. The effect of cognitive behavioral group therapy for depression: a meta-analysis 2000-2010. Worldviews Evid Based Nurs 2012;9:2–17.
Fentz HN, Arendt M, O’Toole MS, Hoffart A, Hougaard E. The mediational role of panic self-efficacy in cognitive behavioral therapy for panic disorder: a systematic review and meta-analysis. Behav Res Ther 2014;60:23–33. https://doi.org/10.1016/j.brat.2014.06.003
Filges T, Jorgensen A-MK. Cognitive–behavioral therapies for young people in outpatient treatment for nonopioid drug use. Res Soc Work Pract 2018;28:363–85.
Flint J, Cuijpers P, Horder J, Koole S, Munafo M. Is there an excess of significant findings in published studies of psychotherapy for depression? Psychol Med 2015;45:439–46.
Fors EA, Bertheussen GF, Thune I, Juvet LK, Elvsaas IK, Oldervoll L, et al. Psychosocial interventions as part of breast cancer rehabilitation programs? Results from a systematic review. Psycho-Oncology 2011;20:909–18. https://doi.org/10.1002/pon.1844
Forti-Buratti MA, Saikia R, Wilkinson EL, Ramchandani PG. Psychological treatments for depression in pre-adolescent children (12 years and younger): systematic review and meta-analysis of randomised controlled trials. Eur Child Adolesc Psychiatry 2016;25:1045–54. https://doi.org/10.1007/s00787-016-0834-5
Furukawa TA, Noma H, Caldwell DM, Honyashiki M, Shinohara K, Imai H, et al. Waiting list may be a nocebo condition in psychotherapy trials: a contribution from network meta-analysis. Acta Psychiatr Scand 2014;130:181–92. https://doi.org/10.1111/acps.12275
Furukawa TA, Watanabe N, Omori IM, Churchill R. Can pill placebo augment cognitive–behavior therapy for panic disorder? BMC Psychiatry 2007;7:73.
Furukawa TA, Weitz ES, Tanaka S, Hollon SD, Hofmann SG, Andersson G, et al. Initial severity of depression and efficacy of cognitive–behavioural therapy: individual-participant data meta-analysis of pill-placebo-controlled trials. Br J Psychiatry 2017;210:190–6. https://doi.org/10.1192/bjp.bp.116.187773
Galsworthy-Francis L, Allan S. Cognitive Behavioural Therapy for anorexia nervosa: a systematic review. Clin Psychol Rev 2014;34:54–72. https://doi.org/10.1016/j.cpr.2013.11.001
Garg S, Garg D, Turin TC, Chowdhury MF. Web-based interventions for chronic back pain: a systematic review. J Med Internet Res 2016;18:e139. https://doi.org/10.2196/jmir.4932
Gartlehner G, Gaynes BN, Amick HR, Asher G, Morgan LC, Coker-Schwimmer E, et al. Nonpharmacological Versus Pharmacological Treatments for Adult Patients with Major Depressive Disorder. Comparative Effectiveness Review No. 161. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
Ghahramanlou M. Cognitive Behavioral Treatment Efficacy for Anxiety Disorders: A Meta-analytic Review. PhD thesis. New Jersey: Fairleigh Dickinson University; 2003.
Gøtzsche PC, Gøtzsche PK. Cognitive behavioural therapy halves the risk of repeated suicide attempts: systematic review. J R Soc Med 2017;110:404–10. https://doi.org/10.1177/0141076817731904
Gould RL, Coulson MC, Howard RJ. Cognitive behavioral therapy for depression in older people: a meta-analysis and meta-regression of randomized controlled trials. J Am Geriatr Soc 2012;60:1817–30. https://doi.org/10.1111/j.1532-5415.2012.04166.x
Gracie DJ, Irvine AJ, Sood R, Mikocka-Walus A, Hamlin PJ, Ford AC. Effect of psychological therapy on disease activity, psychological comorbidity, and quality of life in inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2:189–99.
Haby MM, Donnelly M, Corry J, Vos T. Cognitive behavioural therapy for depression, panic disorder and generalized anxiety disorder: a meta-regression of factors that may predict outcome. Aust N Z J Psychiatry 2006;40:9–19.
Harrington R, Whittaker J, Shoebridge P, Campbell F. Systematic review of efficacy of cognitive behaviour therapies in childhood and adolescent depressive disorder. BMJ 1998;316:1559–63.
Hawton K, Witt KG, Taylor Salisbury TL, Arensman E, Gunnell D, Hazell P, et al. Psychosocial interventions for self-harm in adults. Cochrane Database Syst Rev 2016;5:CD012189. https://doi.org/10.1002/14651858.CD012189
Hesser H, Weise C, Westin VZ, Andersson G. A systematic review and meta-analysis of randomized controlled trials of cognitive-behavioral therapy for tinnitus distress. Clin Psychol Rev 2011;31:545–53. https://doi.org/10.1016/j.cpr.2010.12.006
Ho FY, Chan CS, Tang KN. Cognitive–behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: a meta-analysis of randomized controlled trials. Clin Psychol Rev 2016;43:90–102. https://doi.org/10.1016/j.cpr.2015.09.005
Ho FY, Chung KF, Yeung WF, Ng TH, Kwan KS, Yung KP, Cheng SK. Self-help cognitive–behavioral therapy for insomnia: a meta-analysis of randomized controlled trials. Sleep Med Rev 2015;19:17–28. https://doi.org/10.1016/j.smrv.2014.06.010
Hockenhull JC, Whittington R, Leitner M, Barr W, McGuire J, Cherry MG, et al. A systematic review of prevention and intervention strategies for populations at high risk of engaging in violent behaviour: update 2002–8. Health Technol Assess 2012;16(3). https://doi.org/10.3310/hta16030
Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry 2008;69:621–32.
Honyashiki M, Furukawa TA, Noma H, Tanaka S, Chen P, Ichikawa K, et al. Specificity of CBT for depression: a contribution from multiple treatments meta-analyses. Cogn Ther Res 2014;38:249–60.
Hopkinson MD, Reavell J, Lane DA, Mallikarjun P. Cognitive behavioral therapy for depression, anxiety, and stress in caregivers of dementia patients: a systematic review and meta-analysis. Gerontologist 2018;24:24.
Hunot V, Churchill R, Silva de Lima M, Teixeira V. Psychological therapies for generalised anxiety disorder. Cochrane Database Syst Rev 2007;1:CD001848. https://doi.org/10.1002/14651858.CD001848.pub4
Iniesta-Sepúlveda M, Rosa-Alcázar AI, Sánchez-Meca J, Parada-Navas JL, Rosa-Alcázar Á. Cognitive–behavioral high parental involvement treatments for pediatric obsessive–compulsive disorder: a meta-analysis. J Anxiety Disord 2017;49:53–64.
Ishikawa SI, Okajima I, Matsuoka H, Sakano Y. Cognitive behavioural therapy for anxiety disorders in children and adolescents: a meta-analysis. Child Adolesc Ment Health 2007;12:164–72. https://doi.org/10.1111/j.1475-3588.2006.00433.x
Jassim GA, Whitford DL, Hickey A, Carter B. Psychological interventions for women with non-metastatic breast cancer. Cochrane Database Syst Rev 2015;5:CD008729. https://doi.org/10.1002/14651858.CD008729.pub2
Jauhar S, McKenna PJ, Radua J, Fung E, Salvador R, Laws KR. Cognitive–behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. Br J Psychiatry 2014;204:20–9. https://doi.org/10.1192/bjp.bp.112.116285
Jonas DE, Cusack K, Forneris CA, Wilkins TM, Sonis J, Middleton JC, et al. Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder (PTSD). Rockville, MD: Agency for Healthcare Research and Quality; 2013.
Jónsson H, Hougaard E. Group cognitive behavioural therapy for obsessive–compulsive disorder: a systematic review and meta-analysis. Acta Psychiatr Scand 2009;119:98–106. https://doi.org/10.1111/j.1600-0447.2008.01270.x
Kaddour L, Kishita N, Schaller A. A meta-analysis of low-intensity cognitive behavioral therapy-based interventions for dementia caregivers [published online ahead of print November 12 2018]. Int Psychogeriatr 2018. https://doi.org/10.1017/S1041610218001436
Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J. A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety. Health Technol Assess 2002;6(22). https://doi.org/10.3310/hta6220
Kampmann IL, Emmelkamp PM, Morina N. Meta-analysis of technology-assisted interventions for social anxiety disorder. J Anxiety Disord 2016;42:71–84. https://doi.org/10.1016/j.janxdis.2016.06.007
Keles S, Idsoe T. A meta-analysis of group cognitive behavioral therapy (CBT) interventions for adolescents with depression. J Adolesc 2018;67:129–39.
Kishita N, Hammond L, Dietrich CM, Mioshi E. Which interventions work for dementia family carers?: an updated systematic review of randomized controlled trials of carer interventions. Int Psychogeriatr 2018;30:1679–96. https://doi.org/10.1017/S1041610218000947
Kwon OY, Ahn HS, Kim HJ, Park KW. Effectiveness of cognitive behavioral therapy for caregivers of people with dementia: a systematic review and meta-analysis. J Clin Neurol 2017;13:394–404. https://doi.org/10.3988/jcn.2017.13.4.394
Laird KT, Tanner-Smith EE, Russell AC, Hollon SD, Walker LS. Comparative efficacy of psychological therapies for improving mental health and daily functioning in irritable bowel syndrome: a systematic review and meta-analysis. Clin Psychol Rev 2017;51:142–52.
Le LK, Barendregt JJ, Hay P, Mihalopoulos C. Prevention of eating disorders: a systematic review and meta-analysis. Clin Psychol Rev 2017;53:46–58.
Lee M, Ryoo JH, Chung M, Anderson JG, Rose K, Williams IC. Effective interventions for depressive symptoms among caregivers of people with dementia: a systematic review and meta-analysis [published online ahead of print January 12 2019]. Dementia 2019. https://doi.org/10.1177/1471301218822640
Liu J, Gill NS, Teodorczuk A, Li ZJ, Sun J. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord 2019;245:98–112.
Lo K, Waterland J, Todd P, Gupta T, Bearman M, Hassed C, Keating JL. Group interventions to promote mental health in health professional education: a systematic review and meta-analysis of randomised controlled trials. Adv Health Sci Educ Theory Pract 2018;23:413–47. https://doi.org/10.1007/s10459-017-9770-5
Lutgens D, Gariepy G, Malla A. Psychological and psychosocial interventions for negative symptoms in psychosis: systematic review and meta-analysis. Br J Psychiatry 2017;210:324–32. https://doi.org/10.1192/bjp.bp.116.197103
Maag JW, Swearer SM, Toland MD. Cognitive–Behavioral Interventions for Depression in Children and Adolescents: Meta-analysis, Promising Programs, and Implications for School Personnel. York: Centre for Reviews and Dissemination, University of York; 2009.
Maddock JE. Statistical Power and Effect Size in the Field of Health Psychology. PhD thesis. Kingston, RI: University of Rhode Island; 2000.
Martinez Devesa P, Waddell A, Perera R, Theodoulou M. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev 2007;1:CD005233.
Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev 2010;8:CD005233.
Mello PG, Silva GR, Donat JC, Kristensen CH. An update on the efficacy of cognitive–behavioral therapy, cognitive therapy, and exposure therapy for posttraumatic stress disorder. Int J Psychiatry Med 2013;46:339–57. https://doi.org/10.2190/PM.46.4.b
Menon V, Rajan TM, Kuppili PP, Sarkar S. Cognitive behavior therapy for medically unexplained symptoms: a systematic review and meta-analysis of published controlled trials. Indian J Psychol Med 2017;39:399–406. https://doi.org/10.4103/IJPSYM.IJPSYM_17_17
Montero-Marin J, Garcia-Campayo J, López-Montoyo A, Zabaleta-Del-Olmo E, Cuijpers P. Is cognitive–behavioural therapy more effective than relaxation therapy in the treatment of anxiety disorders? A meta-analysis. Psychol Med 2018;48:1427–36. https://doi.org/10.1017/S0033291717003099
Morina N, Koerssen R, Pollet TV. Interventions for children and adolescents with posttraumatic stress disorder: a meta-analysis of comparative outcome studies. Clin Psychol Rev 2016;47:41–54. https://doi.org/10.1016/j.cpr.2016.05.006
Muresan V, Montgomery GH, David D. Emotional outcomes and mechanisms of change in online cognitive–behavioral interventions: a quantitative meta-analysis of clinical controlled studies. J Technol HumServ 2012;30:1–13.
Nardi B, Laurenzi S, Di Nicolò M, Bellantuono C. Is the cognitive–behavioral therapy an effective intervention to prevent the postnatal depression? A critical review. Int J Psychiatry Med 2012;43:211–25. https://doi.org/10.2190/PM.43.3.b
Nenova M, Morris L, Paul L, Li Y, Applebaum A, DuHamel K. Psychosocial interventions with cognitive-behavioral components for the treatment of cancer-related traumatic stress symptoms: a review of randomized controlled trials. J Cogn Psychother 2013;27:258–84. https://doi.org/10.1891/0889-8391.27.3.258
Newton-Howes G, Wood R. Cognitive behavioural therapy and the psychopathology of schizophrenia: systematic review and meta-analysis. Psychol Psychother 2013;86:127–38. https://doi.org/10.1111/j.2044-8341.2011.02048.x
Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta-analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache 2017;57:349–62. https://doi.org/10.1111/head.13016
Nosè M, Ballette F, Bighelli I, Turrini G, Purgato M, Tol W, et al. Psychosocial interventions for post-traumatic stress disorder in refugees and asylum seekers resettled in high-income countries: systematic review and meta-analysis. PLOS ONE 2017;12:e0171030. https://doi.org/10.1371/journal.pone.0171030
Nüesch E, Häuser W, Bernardy K, Barth J, Jüni P. Comparative efficacy of pharmacological and non-pharmacological interventions in fibromyalgia syndrome: network meta-analysis. Ann Rheum Dis 2013;72:955–62. https://doi.org/10.1136/annrheumdis-2011-201249
Okajima I, Inoue Y. Efficacy of cognitive behavioral therapy for comorbid insomnia: a meta-analysis. Sleep Biol Rhythm 2018;16:21–35.
Olatunji BO, Kauffman BY, Meltzer S, Davis ML, Smits JA, Powers MB. Cognitive–behavioral therapy for hypochondriasis/health anxiety: a meta-analysis of treatment outcome and moderators. Behav Res Ther 2014;58:65–74. https://doi.org/10.1016/j.brat.2014.05.002
Perrot S, Russell IJ. More ubiquitous effects from non-pharmacologic than from pharmacologic treatments for fibromyalgia syndrome: a meta-analysis examining six core symptoms. Eur J Pain 2014;18:1067–80. https://doi.org/10.1002/ejp.564
Philipp R, Kriston L, Lanio J, Kuhne F, Harter M, Moritz S, et al. Effectiveness of metacognitive interventions for mental disorders in adults – a systematic review and meta-analysis (METACOG). Clin Psychol Psychother 2018;19:19.
Phyo AZZ, Demaneuf T, De Livera AM, Jelinek GA, Brown CR, Marck CH, et al. The efficacy of psychological interventions for managing fatigue in people with multiple sclerosis: a systematic review and meta-analysis. Front Neurol 2018;9:149. https://doi.org/10.3389/fneur.2018.00149
Pijnenborg GH, van Donkersgoed RJ, David AS, Aleman A. Changes in insight during treatment for psychotic disorders: a meta-analysis. Schizophr Res 2013;144:109–17. https://doi.org/10.1016/j.schres.2012.11.018
Pozza A, Andersson G, Antonelli P, Dèttore D. Computer-delivered cognitive–behavioural treatments for obsessive compulsive disorder: preliminary meta-analysis of randomized and non-randomized effectiveness trials. Cogn Behav Ther 2014;7:e16.
Pozza A, Dèttore D. Drop-out and efficacy of group versus individual cognitive behavioural therapy: what works best for obsessive–compulsive disorder? A systematic review and meta-analysis of direct comparisons. Psychiatry Res 2017;258:24–36.
Reynolds S, Wilson C, Austin J, Hooper L. Effects of psychotherapy for anxiety in children and adolescents: a meta-analytic review. Clin Psychol Rev 2012;32:251–62. https://doi.org/10.1016/j.cpr.2012.01.005
Saddichha S, Al-Desouki M, Lamia A, Linden IA, Krausz M. Online interventions for depression and anxiety – a systematic review. Health Psychol Behav Med 2014;2:841–81. https://doi.org/10.1080/21642850.2014.945934
Santoft F, Axelsson E, Öst LG, Hedman-Lagerlöf M, Fust J, Hedman-Lagerlöf E. Cognitive behaviour therapy for depression in primary care: systematic review and meta-analysis. Psychol Med 2019;49:1266–74. https://doi.org/10.1017/S0033291718004208
Shinohara K, Honyashiki M, Imai H, Hunot V, Caldwell DM, Davies P, et al. Behavioural therapies versus other psychological therapies for depression. Cochrane Database Syst Rev 2013;10:CD008696. https://doi.org/10.1002/14651858.CD008696.pub2
Sijbrandij M, Kunovski I, Cuijpers P. Effectiveness of internet-delivered cognitive behavioral therapy for posttraumatic stress disorder: a systematic review and meta-analysis. Depress Anxiety 2016;33:783–91. https://doi.org/10.1002/da.22533
Smeets KC, Leeijen AA, van der Molen MJ, Scheepers FE, Buitelaar JK, Rommelse NN. Treatment moderators of cognitive behavior therapy to reduce aggressive behavior: a meta-analysis. Eur Child Adolesc Psychiatry 2015;24:255–64. https://doi.org/10.1007/s00787-014-0592-1
Spielmans GI, Benish SG, Marin C, Bowman WM, Menster M, Wheeler AJ. Specificity of psychological treatments for bulimia nervosa and binge eating disorder? A meta-analysis of direct comparisons. Clin Psychol Rev 2013;33:460–9. https://doi.org/10.1016/j.cpr.2013.01.008
Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ 2013;346:f185. https://doi.org/10.1136/bmj.f185
Stephens S, Ford E, Paudyal P, Smith H. Effectiveness of psychological interventions for postnatal depression in primary care: a meta-analysis. Ann Fam Med 2016;14:463–72. https://doi.org/10.1370/afm.1967
Tirado-Muñoz J, Gilchrist G, Farré M, Hegarty K, Torrens M. The efficacy of cognitive behavioural therapy and advocacy interventions for women who have experienced intimate partner violence: a systematic review and meta-analysis. Ann Med 2014;46:567–86. https://doi.org/10.3109/07853890.2014.941918
van Bronswijk S, Moopen N, Beijers L, Ruhe HG, Peeters F. Effectiveness of psychotherapy for treatment-resistant depression: a meta-analysis and meta-regression. Psychol Med 2019;49:366–79. https://doi.org/10.1017/S003329171800199X
van den Akker LE, Beckerman H, Collette EH, Eijssen IC, Dekker J, de Groot V. Effectiveness of cognitive behavioral therapy for the treatment of fatigue in patients with multiple sclerosis: a systematic review and meta-analysis. J Psychosom Res 2016;90:33–42.
van Der Gaag M, Smit F, Bechdolf A, French P, Linszen DH, Yung AR, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res 2013;149:56–62.
van der Heijden I, Abrahams N, Sinclair D. Psychosocial group interventions to improve psychological well-being in adults living with HIV. Cochrane Database Syst Rev 2017;3:CD010806. https://doi.org/10.1002/14651858.CD010806.pub2
Vugts MAP, Joosen MCW, van der Geer JE, Zedlitz AMEE, Vrijhoef HJM. The effectiveness of various computer-based interventions for patients with chronic pain or functional somatic syndromes: a systematic review and meta-analysis. PLOS ONE 2018;13:e0196467. https://doi.org/10.1371/journal.pone.0196467
Weitz ES, Hollon SD, Twisk J, van Straten A, Huibers MJ, David D, et al. Baseline depression severity as moderator of depression outcomes between cognitive behavioral therapy vs pharmacotherapy: an individual patient data meta-analysis. JAMA Psychiatry 2015;72:1102–9. https://doi.org/10.1001/jamapsychiatry.2015.1516
Wells MJ, Owen JJ, McCray LW, Bishop LB, Eells TD, Brown GK, et al. Computer-assisted cognitive–behavior therapy for depression in primary care: systematic review and meta-analysis. Prim Care Companion CNS Disord 2018;20:17r02196. https://doi.org/10.4088/PCC.17r02196
Wethington HR, Hahn RA, Fuqua-Whitley DS, Sipe TA, Crosby AE, Johnson RL, et al. The effectiveness of interventions to reduce psychological harm from traumatic events among children and adolescents: a systematic review. Am J Prev Med 2008;35:287–313. https://doi.org/10.1016/j.amepre.2008.06.024
Whittal ML, Agras WS, Gould RA. Bulimia nervosa: a meta-analysis of psychosocial and pharmacological treatments. Behav Ther 1999;30:117–35.
Williams ACC, Fisher E, Hearn L, Eccleston C. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2020;8:CD007407. https://doi.org/10.1002/14651858.CD007407.pub4
Young Z, Moghaddam N, Tickle A. The efficacy of cognitive behavioral therapy for adults with ADHD: a systematic review and meta-analysis of randomized controlled trials. J Attention Disord 2016;22:22.
Zhang M, Huang L, Feng Z, Shao L, Chen L. Effects of cognitive behavioral therapy on quality of life and stress for breast cancer survivors: a meta-analysis. Minerva Med 2017;108:84–93. https://doi.org/10.23736/S0026-4806.16.04528-6
Studies excluded because outcomes not relevant (n = 125)
Abreu Costa M, D’Alo de Oliveira GS, Tatton-Ramos T, Manfro GG, Salum GA. Anxiety and stress-related disorders and mindfulness-based interventions: a systematic review and multilevel meta-analysis and meta-regression of multiple outcomes. Mindfulness 2018;10:996–1005.
Alammehrjerdi Z, Ezard N, Dolan K. Methamphetamine dependence in methadone treatment services in Iran: the first literature review of a new health concern. Asian J Psychiatr 2018;31:49–55.
Anderson N, Heywood-Everett S, Siddiqi N, Wright J, Meredith J, McMillan D. Faith-adapted psychological therapies for depression and anxiety: systematic review and meta-analysis. J Affect Disord 2015;176:183–96. https://doi.org/10.1016/j.jad.2015.01.019
Applebaum AJ, Breitbart W. Care for the cancer caregiver: a systematic review. Palliat Support Care 2013;11:231–52. https://doi.org/10.1017/S1478951512000594
Ashman L, Duggan L. Interventions for learning disabled sex offenders. Cochrane Database Syst Rev 2008;1:CD003682. https://doi.org/10.1002/14651858.CD003682.pub2
Babcock JC, Green CE, Robie C. Does batterers’ treatment work? A meta-analytic review of domestic violence treatment. Clin Psychol Rev 2004;23:1023–53.
Benbow AA, Anderson PL. A meta-analytic examination of attrition in virtual reality exposure therapy for anxiety disorders. J Anxiety Disord 2019;61:18–26.
Bernecker SL, Coyne AE, Constantino MJ, Ravitz P. For whom does interpersonal psychotherapy work? A systematic review. Clin Psychol Rev 2017;56:82–93.
Bhui K, Aslam RW, Palinski A, McCabe R, Johnson MR, Weich S, et al. Interventions designed to improve therapeutic communications between black and minority ethnic people and professionals working in psychiatric services: a systematic review of the evidence for their effectiveness. Health Technol Assess 2015;19(31). https://doi.org/10.3310/hta19310
Brendel KE, Maynard BR. Child–parent interventions for childhood anxiety disorders: a systematic review and meta-analysis. Res Soc Work Pract 2014;24:287–95.
Brooks SJ, Stein DJ. A systematic review of the neural bases of psychotherapy for anxiety and related disorders. Dialogues Clin Neurosci 2015;17:261–79.
Clarke C, Skokauskas N. CBT for adolescent pathological gambling – lessons from adult research. Ir J Psychol Med 2009;26:140–6. https://doi.org/10.1017/S0790966700000458
Clements KM, Hydery T, Tesell MA, Greenwood BC, Angelini MC. A systematic review of community-based interventions to improve oral chronic disease medication regimen adherence among individuals with substance use disorder. Drug Alcohol Depend 2018;188:141–52.
Cole RL. A systematic review of cognitive–behavioural interventions for adolescents with anger-related difficulties. Educ Child Psychol 2008;25:27–47.
Cristea IA, Huibers MJ, David D, Hollon SD, Andersson G, Cuijpers P. The effects of cognitive behavior therapy for adult depression on dysfunctional thinking: a meta-analysis. Clin Psychol Rev 2015;42:62–71. https://doi.org/10.1016/j.cpr.2015.08.003
Cuijpers P, Smit F, Bohlmeijer E, Hollon SD, Andersson G. Efficacy of cognitive–behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry 2010;196:173–8. https://doi.org/10.1192/bjp.bp.109.066001
Cuijpers P, Weitz E, Twisk J, Kuehner C, Cristea I, David D, et al. Gender as predictor and moderator of outcome in cognitive behavior therapy and pharmacotherapy for adult depression: an ‘individual patient data’ meta-analysis. Depress Anxiety 2014;31:941–51.
da Silva JA, Siegmund G, Bredemeier J. Crisis interventions in online psychological counseling. Trends Psychiatry Psychother 2015;37:171–82. https://doi.org/10.1590/2237-6089-2014-0026
Darker CD, Sweeney BP, Barry JM, Farrell MF, Donnelly-Swift E. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev 2015;5:CD009652. https://doi.org/10.1002/14651858.CD009652.pub2
De Crescenzo F, Ciabattini M, D’Alò GL, De Giorgi R, Del Giovane C, Cassar C, et al. Comparative efficacy and acceptability of psychosocial interventions for individuals with cocaine and amphetamine addiction: a systematic review and network meta-analysis. PLOS Med 2018;15:e1002715. https://doi.org/10.1371/journal.pmed.1002715
De Giorgi R, Cassar C, Loreto D’alò G, Ciabattini M, Minozzi S, Economou A, et al. Psychosocial interventions in stimulant use disorders: a systematic review and qualitative synthesis of randomized controlled trials. Riv Psichiatr 2018;53:233–55. https://doi.org/10.1708/3000.30003
Dennis JA, Khan O, Ferriter M, Huband N, Powney MJ, Duggan C. Psychological interventions for adults who have sexually offended or are at risk of offending. Cochrane Database Syst Rev 2012;12:CD007507. https://doi.org/10.1002/14651858.CD007507.pub2
Depont F, Berenbaum F, Filippi J, Le Maitre M, Nataf H, Paul C, et al. Interventions to improve adherence in patients with immune-mediated inflammatory disorders: a systematic review. PLOS ONE 2015;10:e0145076. https://doi.org/10.1371/journal.pone.0145076
Dou C, Rebane J, Bardal S. Interventions to improve benzodiazepine tapering success in the elderly: a systematic review. Aging Ment Health 2019;23:411–16. https://doi.org/10.1080/13607863.2017.1423030
Dumont M, Thériault J, Briand C, Dumais A, Potvin S. Psychosocial approaches for individuals with schizophrenia in correctional and forensic psychiatric settings: a rapid review. J Forensic Pract 2018;20:152–66.
Ebrahim S. Psychotherapy for depression in claimants receiving wage replacement benefits: review of the evidence. J Insur Med 2014;44:53–7.
Eskildsen A, Hougaard E, Rosenberg NK. Pre-treatment patient variables as predictors of drop-out and treatment outcome in cognitive behavioural therapy for social phobia: a systematic review. Nord J Psychiatry 2010;64:94–105. https://doi.org/10.3109/08039480903426929
Evans K, Spiby H, Morrell JC. Non-pharmacological interventions to reduce the symptoms of mild to moderate anxiety in pregnant women. A systematic review and narrative synthesis of women’s views on the acceptability of and satisfaction with interventions. Arch Womens Ment Health 2020;23:11–28.
Fisher H, Gardner FE, Montgomery P. Cognitive-behavioural interventions for preventing youth gang involvement for children and young people (7–16). Cochrane Database Syst Rev 2008;2:CD007008. https://doi.org/10.1002/14651858.CD007008.pub2
Fjermestad KW, Mowatt Haugland BS, Heiervang E, Ost LG. Relationship factors and outcome in child anxiety treatment studies. Clin Child Psychol Psychiatry 2009;14:195–214. https://doi.org/10.1177/1359104508100885
Franklin G, Carson AJ, Welch KA. Cognitive behavioural therapy for depression: systematic review of imaging studies. Acta Neuropsychiatr 2016;28:61–74. https://doi.org/10.1017/neu.2015.41
Gallagher M, McLeod HJ, McMillan TM. A systematic review of recommended modifications of CBT for people with cognitive impairments following brain injury. Neuropsychol Rehabil 2019;29:1–21. https://doi.org/10.1080/09602011.2016.1258367
Gilchrist G, Munoz JT, Easton CJ. Should we reconsider anger management when addressing physical intimate partner violence perpetration by alcohol abusing males? A systematic review. Aggress Violent Behav 2015;25:124–32.
Gilinsky A, Swanson V, Power K. Interventions delivered during antenatal care to reduce alcohol consumption during pregnancy: a systematic review. Addict Res Theory 2011;19:235–50.
Ginsburg GS, Kingery JN, Drake KL, Grados MA. Predictors of treatment response in pediatric obsessive–compulsive disorder. J Am Acad Child Adolesc Psychiatry 2008;47:868–78.
Gray H, Howe T. Physiotherapists’ assessment and management of psychosocial factors (Yellow and Blue Flags) in individuals with back pain. Phys Ther Rev 2013;18:379–.94
Hay P, Chinn D, Forbes D, Madden S, Newton R, Sugenor L, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of eating disorders. Aust N Z J Psychiatry 2014;48:977–1008. https://doi.org/10.1177/0004867414555814
Heber E, Ebert DD, Lehr D, Cuijpers P, Berking M, Nobis S, Riper H. The benefit of web- and computer-based interventions for stress: a systematic review and meta-analysis. J Med Internet Res 2017;19:e32. https://doi.org/10.2196/jmir.5774
Henry C, Ghaemi SN. Insight in psychosis: a systematic review of treatment interventions. Psychopathology 2004;37:194–9. https://doi.org/10.1159/000080131
Heron-Speirs HA, Harvey ST, Baken DM. Moderators of psycho-oncology therapy effectiveness: meta-analysis of therapy characteristics. J Psychosoc Oncol 2013;31:617–41. https://doi.org/10.1080/07347332.2013.835022
Hesser H, Weise C, Rief W, Andersson G. The effect of waiting: a meta-analysis of wait-list control groups in trials for tinnitus distress. J Psychosom Res 2011;70:378–84. https://doi.org/10.1016/j.jpsychores.2010.12.006
Ho BP, Stephenson J, Carter M. Cognitive–behavioural approach for children with autism spectrum disorder: a literature review. J Intellect Dev Disabil 2015;40:213–29.
Ho M, Jensen ME, Burrows T, Neve M, Garnett SP, Baur L, et al. Best practice dietetic management of overweight and obese children and adolescents: a 2010 update of a systematic review. JBI Database of Syst Rev Implement Rep 2013;11:190–293.
Hodgkinson B, Evans D, O’Donnell A, Walsh K. Comparing the Effectiveness of Individual Therapy and Group Therapy in the Treatment of Depression: Systematic Review. Systematic Review 3. Adelaide, SA: Joanna Briggs Institute for Evidence Based Nursing and Midwifery; 1999.
Hogue A, Henderson CE, Becker SJ, Knight DK. Evidence base on outpatient behavioral treatments for adolescent substance use, 2014–2017: outcomes, treatment delivery, and promising horizons. J Clin Child Adolesc Psychol 2018;47:499–526. https://doi.org/10.1080/15374416.2018.1466307
Honagodu AR, Krishna M, Sundarachar R, Lepping P. Group psychotherapies for depression in persons with HIV: a systematic review. Indian J Psychiatry 2013;55:323–30. https://doi.org/10.4103/0019-5545.120541
Huguet A, Rao S, McGrath PJ, Wozney L, Wheaton M, Conrod J, Rozario S. A systematic review of cognitive behavioral therapy and behavioral activation apps for depression. PLOS ONE 2016;11:e0154248. https://doi.org/10.1371/journal.pone.0154248
Hundt NE, Mignogna J, Underhill C, Cully JA. The relationship between use of CBT skills and depression treatment outcome: a theoretical and methodological review of the literature. Behav Ther 2013;44:12–26.
Ince P, Haddock G, Tai S. A systematic review of the implementation of recommended psychological interventions for schizophrenia: rates, barriers, and improvement strategies. Psychol Psychother 2016;89:324–50. https://doi.org/10.1111/papt.12084
Jonsson H, Kristensen M, Arendt M. Intensive cognitive behavioural therapy for obsessive–compulsive disorder: a systematic review and meta-analysis. J Obsess Compuls Relat Disord 2015;6:83–96.
Joyce S, Shand F, Tighe J, Laurent SJ, Bryant RA, Harvey SB. Road to resilience: a systematic review and meta-analysis of resilience training programmes and interventions. BMJ Open 2018;8:e017858. https://doi.org/10.1136/bmjopen-2017-017858
Kaltenthaler E, Sutcliffe P, Parry G, Beverley C, Rees A, Ferriter M. The acceptability to patients of computerized cognitive behaviour therapy for depression: a systematic review. Psychol Med 2008;38:1521–30. https://doi.org/10.1017/S0033291707002607
Karyotaki E, Kemmeren L, Riper H, Twisk J, Hoogendoorn A, Kleiboer A, et al. Is self-guided internet-based cognitive behavioural therapy (iCBT) harmful? An individual participant data meta-analysis. Psychol Med 2018;48:2456–66. https://doi.org/10.1017/S0033291718000648
Kazantzis N, Whittington C, Zelencich L, Kyrios M, Norton PJ, Hofmann SG. Quantity and quality of homework compliance: a meta-analysis of relations with outcome in cognitive behavior therapy. Behav Ther 2016;47:755–72.
Khan A, Tansel A, White DL, Kayani WT, Bano S, Lindsay J, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review. Clin Gastroenterol Hepatol 2016;14:191–202.e1-4. https://doi.org/10.1016/j.cgh.2015.07.047
Klimas J, Tobin H, Field CA, O’Gorman CS, Glynn LG, Keenan E, et al. Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users. Cochrane Database Syst Rev 2014;12:CD009269. https://doi.org/10.1002/14651858.CD009269.pub3
Koffel E, Bramoweth AD, Ulmer CS. Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): a narrative review. J Gen Intern Med 2018;33:955–62. https://doi.org/10.1007/s11606-018-4390-1
Kregel J, Coppieters I, DePauw R, Malfliet A, Danneels L, Nijs J, et al. Does conservative treatment change the brain in patients with chronic musculoskeletal pain? A Systematic Review. Pain Physician 2017;20:139–54.
Kurtz MM. Neurocognition as a predictor of response to evidence-based psychosocial interventions in schizophrenia: what is the state of the evidence? Clin Psychol Rev 2011;31:663–72.
Lam LT, Lam MK. eHealth intervention for problematic internet use (PIU). Curr Psychiatry Rep 2016;18:107. https://doi.org/10.1007/s11920-016-0747-5
Lavielle M, Puyraimond-Zemmour D, Romand X, Gossec L, Senbel E, Pouplin S, et al. Methods to improve medication adherence in patients with chronic inflammatory rheumatic diseases: a systematic literature review. RMD Open 2018;4:e000684. https://doi.org/10.1136/rmdopen-2018-000684
Law EF, Fisher E, Fales J, Noel M, Eccleston C. Systematic review and meta-analysis of parent and family-based interventions for children and adolescents with chronic medical conditions. J Pediatr Psychol 2014;39:866–86. https://doi.org/10.1093/jpepsy/jsu032
Lee EB, An W, Levin ME, Twohig MP. An initial meta-analysis of acceptance and commitment therapy for treating substance use disorders. Drug Alcohol Depend 2015;155:1–7. https://doi.org/10.1016/j.drugalcdep.2015.08.004
Linardon J, Hindle A, Brennan L. Dropout from cognitive-behavioral therapy for eating disorders: a meta-analysis of randomized, controlled trials. Int J Eat Disord 2018;51:381–91. https://doi.org/10.1002/eat.22850
Linardon J, de la Piedad Garcia X, Brennan L. Predictors, moderators, and mediators of treatment outcome following manualised cognitive–behavioural therapy for eating disorders: a systematic review. Eur Eat Disord Rev 2017;25:3–12. https://doi.org/10.1002/erv.2492
Lundkvist-Houndoumadi I, Hougaard E, Thastum M. Pre-treatment child and family characteristics as predictors of outcome in cognitive behavioural therapy for youth anxiety disorders. Nord J Psychiatry 2014;68:524–35. https://doi.org/10.3109/08039488.2014.903295
Markel C. Child-focused psychosocial interventions for childrenand adolescents with attention-deficit hyperactivity disorder (ADHD): a systematic review and meta-analysis. PhD thesis. Toronto, ON: University of Toronto; 2018.
Martire LM. The ‘relative’ efficacy of involving family in psychosocial interventions for chronic illness: are there added benefits to patients and family members? Fam Syst Health 2005;23:312–28.
Maseroli E, Scavello I, Rastrelli G, Limoncin E, Cipriani S, Corona G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med 2018;15:1752–64.
Matteson ML, Russell C. Interventions to improve hemodialysis adherence: a systematic review of randomized-controlled trials. Hemodial Int 2010;14:370–82. https://doi.org/10.1111/j.1542-4758.2010.00462.x
Matthews EE, Arnedt JT, McCarthy MS, Cuddihy LJ, Aloia MS. Adherence to cognitive behavioral therapy for insomnia: a systematic review. Sleep Med Rev 2013;17:453–64. https://doi.org/10.1016/j.smrv.2013.01.001
Mbuagbaw L, Sivaramalingam B, Navarro T, Hobson N, Keepanasseril A, Wilczynski NJ, Haynes RB, Patient Adherence Review (PAR) Team. Interventions for enhancing adherence to antiretroviral therapy (ART): a systematic review of high quality studies. AIDS Patient Care STDS 2015;29:248–66. https://doi.org/10.1089/apc.2014.0308
McLean SM, Burton M, Bradley L, Littlewood C. Interventions for enhancing adherence with physiotherapy: a systematic review. Man Ther 2010;15:514–21. https://doi.org/10.1016/j.math.2010.05.012
Melendez-Torres GJ, Bonell C. Systematic review of cognitive behavioural interventions for HIV risk reduction in substance-using men who have sex with men. Int J STD AIDS 2014;25:627–35. https://doi.org/10.1177/0956462413515638
Montgomery EC, Kunik ME, Wilson N, Stanley MA, Weiss B. Can paraprofessionals deliver cognitive–behavioral therapy to treat anxiety and depressive symptoms? Bull Menninger Clin 2010;74:45–62.
Mukhtar F, Oei TP. A review on assessment and treatment for depression in malaysia. Depress Res Treat 2011;2011:123642. https://doi.org/10.1155/2011/123642
Naeem F, Farooq S, Kingdon D. Cognitive behavioural therapy (brief versus standard duration) for schizophrenia. Cochrane Database Syst Rev 2015;10:CD010646. https://doi.org/10.1002/14651858.CD010646.pub3
Nascimento SS, Oliveira LR, DeSantana JM. Correlations between brain changes and pain management after cognitive and meditative therapies: a systematic review of neuroimaging studies. Complement Ther Med 2018;39:137–45.
Nesset MB, Lara-Cabrera ML, Dalsbø TK, Pedersen SA, Bjørngaard JH, Palmstierna T. Cognitive behavioural group therapy for male perpetrators of intimate partner violence: a systematic review. BMC Psychiatry 2019;19:11. https://doi.org/10.1186/s12888-019-2010-1
Nieuwenhuijsen K, Faber B, Verbeek JH, Neumeyer-Gromen A, Hees HL, Verhoeven AC, et al. Interventions to improve return to work in depressed people. Cochrane Database Syst Rev 2014;12:CD006237. https://doi.org/10.1002/14651858.CD006237.pub3
O’Keeffe J, Conway R, McGuire B. A systematic review examining factors predicting favourable outcome in cognitive behavioural interventions for psychosis. Schizophr Res 2017;183:22–30.
Ougrin D, Tranah T, Stahl D, Moran P, Asarnow JR. Therapeutic interventions for suicide attempts and self-harm in adolescents: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 2015;54:97–107.e2. https://doi.org/10.1016/j.jaac.2014.10.009
Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, Paisley S. Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation. Health Technol Assess 2006;10(35). https://doi.org/10.3310/hta10350
Perkins DO. Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry 2002;63:1121–8. https://doi.org/10.4088/jcp.v63n1206
Petry NM, Armentano C. Prevalence, assessment, and treatment of pathological gambling: a review. Psychiatr Serv 1999;50:1021–7. https://doi.org/10.1176/ps.50.8.1021
Pihlaja S, Stenberg JH, Joutsenniemi K, Mehik H, Ritola V, Joffe G. Therapeutic alliance in guided internet therapy programs for depression and anxiety disorders – a systematic review. Internet Interv 2018;11:1–10. https://doi.org/10.1016/j.invent.2017.11.005
Pompoli A, Furukawa TA, Efthimiou O, Imai H, Tajika A, Salanti G. Dismantling cognitive–behaviour therapy for panic disorder: a systematic review and component network meta-analysis. Psychol Med 2018;48:1945–53. https://doi.org/10.1017/S0033291717003919
Porter E, Chambless DL. A systematic review of predictors and moderators of improvement in cognitive–behavioral therapy for panic disorder and agoraphobia. Clin Psychol Rev 2015;42:179–92. https://doi.org/10.1016/j.cpr.2015.09.004
Porto PR, Oliveira L, Mari J, Volchan E, Figueira I, Ventura P. Does cognitive behavioral therapy change the brain? A systematic review of neuroimaging in anxiety disorders. J Neuropsychiatry Clin Neurosci 2009;21:114–25. https://doi.org/10.1176/appi.neuropsych.21.2.114
Potier F, Degryse JM, de Saint-Hubert M. Impact of caregiving for older people and pro-inflammatory biomarkers among caregivers: a systematic review. Aging Clin Exp Res 2018;30:119–32. https://doi.org/10.1007/s40520-017-0765-0
Powers MB, Vedel E, Emmelkamp PM. Behavioral couples therapy (BCT) for alcohol and drug use disorders: a meta-analysis. Clin Psychol Rev 2008;28:952–62. https://doi.org/10.1016/j.cpr.2008.02.002
Rapley HA, Loades ME. A systematic review exploring therapist competence, adherence, and therapy outcomes in individual CBT for children and young people. Psychother Res 2019;29:1010–19. https://doi.org/10.1080/10503307.2018.1464681
Rathbun AM, Reed GW, Harrold LR. The temporal relationship between depression and rheumatoid arthritis disease activity, treatment persistence and response: a systematic review. Rheumatology 2013;52:1785–94. https://doi.org/10.1093/rheumatology/kes356
Rhodes RE, Fiala B. Building motivation and sustainability into the prescription and recommendations for physical activity and exercise therapy: the evidence. Physiother Theory Pract 2009;25:424–41.
Riblet NBV, Shiner B, Young-Xu Y, Watts BV. Strategies to prevent death by suicide: meta-analysis of randomised controlled trials. Br J Psychiatry 2017;210:396–402. https://doi.org/10.1192/bjp.bp.116.187799
Rohden AI, Benchaya MC, Camargo RS, Moreira TC, Barros HMT, Ferigolo M. Dropout prevalence and associated factors in randomized clinical trials of adolescents treated for depression: systematic review and meta-analysis. Clin Ther 2017;39:971–92.e4.
Room J, Hannink E, Dawes H, Barker K. What interventions are used to improve exercise adherence in older people and what behavioural techniques are they based on? A systematic review. BMJ Open 2017;7:e019221. https://doi.org/10.1136/bmjopen-2017-019221
Rudge S, Feigenbaum JD, Fonagy P. Mechanisms of change in dialectical behaviour therapy and cognitive behaviour therapy for borderline personality disorder: a critical review of the literature. J Ment Health 2020;29:92–102.
Rueda S, Park-Wyllie LY, Bayoumi AM, Tynan AM, Antoniou TA, Rourke SB, Glazier RH. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev 2006;3:CD001442. https://doi.org/10.1002/14651858.CD001442.pub2
Sajatovic M, Davies M, Hrouda DR. Enhancement of treatment adherence among patients with bipolar disorder. Psychiatr Serv 2004;55:264–9. https://doi.org/10.1176/appi.ps.55.3.264
Schaafsma F, Schonstein E, Whelan KM, Ulvestad E, Kenny DT, Verbeek JH. Physical conditioning programs for improving work outcomes in workers with back pain. Cochrane Database Syst Rev 2010;1:CD001822. https://doi.org/10.1002/14651858.CD001822.pub2
Schaafsma FG, Whelan K, van der Beek AJ, van der Es-Lambeek LC, Ojajärvi A, Verbeek JH. Physical conditioning as part of a return to work strategy to reduce sickness absence for workers with back pain. Cochrane Database Syst Rev 2013;8:CD001822. https://doi.org/10.1002/14651858.CD001822.pub3
Schütze R, Rees C, Smith A, Slater H, Campbell JM, O’Sullivan P. How can we best reduce pain catastrophizing in adults with chronic noncancer pain? A systematic review and meta-analysis. J Pain 2018;19:233–56.
Simon W. Follow-up psychotherapy outcome of patients with dependent, avoidant and obsessive–compulsive personality disorders: a meta-analytic review. Int J Psychiatry Clin Pract 2009;13:153–65. https://doi.org/10.1080/13651500802570972
Smits JA, Berry AC, Tart CD, Powers MB. The efficacy of cognitive–behavioral interventions for reducing anxiety sensitivity: a meta-analytic review. Behav Res Ther 2008;46:1047–54. https://doi.org/10.1016/j.brat.2008.06.010
Smits JA, Julian K, Rosenfield D, Powers MB. Threat reappraisal as a mediator of symptom change in cognitive–behavioral treatment of anxiety disorders: a systematic review. J Consult Clin Psychol 2012;80:624–35. https://doi.org/10.1037/a0028957
Spinhoven P, Klein N, Kennis M, Cramer AOJ, Siegle G, Cuijpers P, et al. The effects of cognitive–behavior therapy for depression on repetitive negative thinking: a meta-analysis. Behav Res Ther 2018;106:71–85.
Storebø OJ, Skoog M, Damm D, Thomsen PH, Simonsen E, Gluud C. Social skills training for attention deficit hyperactivity disorder (ADHD) in children aged 5 to 18 years. Cochrane Database Syst Rev 2011;12:CD008223. https://doi.org/10.1002/14651858.CD008223.pub2
Stott J, Charlesworth G, Scior K. Measures of readiness for cognitive behavioural therapy in people with intellectual disability: a systematic review. Res Dev Disabil 2017;60:37–51.
Surley L, Dagnan D. A review of the frequency and nature of adaptations to cognitive behavioural therapy for adults with intellectual disabilities. J Appl Res Intellect Disabil 2019;32:219–37. https://doi.org/10.1111/jar.12534
Thulin U, Svirsky L, Serlachius E, Andersson G, Ost LG. The effect of parent involvement in the treatment of anxiety disorders in children: a meta-analysis. Cogn Behav Ther 2014;43:185–200. https://doi.org/10.1080/16506073.2014.923928
Thurgood SL, McNeill A, Clark-Carter D, Brose LS. A systematic review of smoking cessation interventions for adults in substance abuse treatment or recovery. Nicotine Tob Res 2016;18:993–1001. https://doi.org/10.1093/ntr/ntv127
Turner C, O’Gorman B, Nair A, O’Kearney R. Moderators and predictors of response to cognitive behaviour therapy for pediatric obsessive-compulsive disorder: a systematic review. Psychiatry Res 2018;261:50–60.
Vallury KD, Jones M, Oosterbroek C. Computerized cognitive behavior therapy for anxiety and depression in rural areas: a systematic review. J Med Internet Res 2015;17:e139. https://doi.org/10.2196/jmir.4145
van Ballegooijen W, Cuijpers P, van Straten A, Karyotaki E, Andersson G, Smit JH, Riper H. Adherence to internet-based and face-to-face cognitive behavioural therapy for depression: a meta-analysis. PLOS ONE 2014;9:e100674. https://doi.org/10.1371/journal.pone.0100674
van der Put CE, Assink M, Gubbels J, Boekhout van Solinge NF. Identifying effective components of child maltreatment interventions: a meta-analysis. Clin Child Fam Psychol Rev 2018;21:171–202. https://doi.org/10.1007/s10567-017-0250-5
Vázquez Rivera S, Gómez Magariños S, González-Blanch C. [Effects on the brain of effective psychological treatments for anxiety disorders: a systematic review.] Actas Esp Psiquiatr 2010;38:239–48.
Vigerland S, Lenhard F, Bonnert M, Lalouni M, Hedman E, Ahlen J, et al. Internet-delivered cognitive behavior therapy for children and adolescents: a systematic review and meta-analysis. Clin Psychol Rev 2016;50:1–10.
Vujanovic AA, Farris SG, Bartlett BA, Lyons RC, Haller M, Colvonen PJ, Norman SB. Anxiety sensitivity in the association between posttraumatic stress and substance use disorders: a systematic review. Clin Psychol Rev 2018;62:37–55.
Waller R, Gilbody S. Barriers to the uptake of computerized cognitive behavioural therapy: a systematic review of the quantitative and qualitative evidence. Psychol Med 2009;39:705–12. https://doi.org/10.1017/S0033291708004224
Williamson JPA. Assessing the Suitability of Cognitive–Behavioural Therapy for Specialised Client Populations and Clinical Practice Reports. PhD thesis. Birmingham; University of Birmingham; 2011.
Windgassen S, Moss-Morris R, Chilcot J, Sibelli A, Goldsmith K, Chalder T. The journey between brain and gut: a systematic review of psychological mechanisms of treatment effect in irritable bowel syndrome. Br J Health Psychol 2017;22:701–36. https://doi.org/10.1111/bjhp.12250
Windsor LC, Jemal A, Alessi EJ. Cognitive behavioral therapy: a meta-analysis of race and substance use outcomes. Cultur Divers Ethnic Minor Psychol 2015;21:300–13. https://doi.org/10.1037/a0037929
Wray TB, Grin B, Dorfman L, Glynn TR, Kahler CW, Marshall BD, et al. Systematic review of interventions to reduce problematic alcohol use in men who have sex with men. Drug Alcohol Rev 2016;35:148–57. https://doi.org/10.1111/dar.12271
Xu Z, Huang F, Kösters M, Rüsch N. Challenging mental health related stigma in China: systematic review and meta-analysis. II. Interventions among people with mental illness. Psychiatry Res 2017;255:457–64.
Studies excluded because economic evaluation only (n = 13)
Bereza BG, Machado M, Einarson TR. Systematic review and quality assessment of economic evaluations and quality-of-life studies related to generalized anxiety disorder. Clin Ther 2009;31:1279–308. https://doi.org/10.1016/j.clinthera.2009.06.004
Bosmans JE, van Schaik DJF, de Bruijne MC, van Hout HPJ, van Marwijk HWJ, van Tulder MW, et al. Are psychological treatments for depression in primary care cost-effective? J Ment Health Policy Econ 2008;11:3–15.
Dieng M, Cust AE, Kasparian NA, Mann GJ, Morton RL. Economic evaluations of psychosocial interventions in cancer: a systematic review. Psychooncology 2016;25:1380–92. https://doi.org/10.1002/pon.4075
Gajic-Veljanoski O, Sanyal C, McMartin K, Xie X, Walter M, Higgins C, et al. Economic evaluations of commonly used structured psychotherapies for major depressive disorder and generalized anxiety disorder: a systematic review. Can Psychol 2018;59:301–14.
Heuzenroeder L, Donnelly M, Haby MM, Mihalopoulos C, Rossell R, Carter R, et al. Cost-effectiveness of psychological and pharmacological interventions for generalized anxiety disorder and panic disorder. Aust N Z J Psychiatry 2004;38:602–12. https://doi.org/10.1080/j.1440-1614.2004.01423.x
Karyotaki E, Tordrup D, Buntrock C, Bertollini R, Cuijpers P. Economic evidence for the clinical management of major depressive disorder: a systematic review and quality appraisal of economic evaluations alongside randomised controlled trials. Epidemiol Psychiatr Sci 2017;26:501–16. https://doi.org/10.1017/S2045796016000421
Konnopka A, Schaefert R, Heinrich S, Kaufmann C, Luppa M, Herzog W, König HH. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom 2012;81:265–75. https://doi.org/10.1159/000337349
Lin CW, Haas M, Maher CG, Machado LA, van Tulder MW. Cost-effectiveness of guideline-endorsed treatments for low back pain: a systematic review. Eur Spine J 2011;20:1024–38. https://doi.org/10.1007/s00586-010-1676-3
Mavranezouli I, Mayo-Wilson E, Dias S, Kew K, Clark DM, Ades AE, Pilling S. The cost effectiveness of psychological and pharmacological interventions for social anxiety disorder: a model-based economic analysis. PLOS ONE 2015;10:e0140704. https://doi.org/10.1371/journal.pone.0140704
Ophuis RH, Lokkerbol J, Heemskerk SC, van Balkom AJ, Hiligsmann M, Evers SM. Cost-effectiveness of interventions for treating anxiety disorders: a systematic review. J Affect Disord 2017;210:1–13.
Sanyal C, Stolee P, Juzwishin D, Husereau D. Economic evaluations of eHealth technologies: a systematic review. PLOS ONE 2018;13:e0198112. https://doi.org/10.1371/journal.pone.0198112
Stevens M, Roberts H, Shiell A. Research review: economic evidence for interventions in children’s social care: revisiting the What Works for Children project. Child Fam Soc Work 2010;15:145–54.
van der Velde G, Yu H, Paulden M, Côté P, Varatharajan S, Shearer HM, et al. Which interventions are cost-effective for the management of whiplash-associated and neck pain-associated disorders? A systematic review of the health economic literature by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration. Spine J 2016;16:1582–97.
Studies excluded because abstracts/protocol/erratum (n = 278)
Adamus C, Pfammatter M. The effects of subjective recovery-oriented psychological interventions on schizophrenic disorders-a systematic review. Eur Arch Psychiatry Clin Neurosci 2017;267(Suppl. 1):S53.
Addington J, Devoe D. Meta-analytic review of treatment options for CHR youth. Early Interv Psychiatry 2018;12(Suppl. 1):10.
Aljudaibi S, Duane B. Non-pharmacological pain relief during orthodontic treatment. Evid Based Dent 2018;19:48–9. https://doi.org/10.1038/sj.ebd.6401305
Alwani S. Promoting mothers’ psychological health after fetal loss maternal mortality and morbidity is alarming and need extensive interventions. BJOG 2016;123(Suppl. 2):110.
Anagnostopoulou N, Kyriakopoulos M, Alba A. Psychological interventions in early onset psychosis: a systematic review. Eur Psychiatry 2018;48(Suppl. 1):S193.
Andrews L. Systematic review summary – psychosocial interventions to improve quality of life and emotional well-being for recently diagnosed cancer patients. Singap Nurs J 2013;40:47–9.
Angerer P, Li J. Evaluation of short-term and long-term effects of work stress interventions on CVD risk factors: focusing on the individual level. Eur J Prevent Cardiol 2017;24:13.
Anonymous. Useful treatments for fibromyalgia syndrome. J Fam Pract 2005;54:105.
Anonymous. Cognitive behavioral therapy helps ease tinnitus discomforts. Harvard Womens Health Watch 2007;14:2.
Anonymous. Computerised cognitive-behavioural therapy for depression: systematic review. Br J Psychiatry 2008;193:181–4. [Erratum published in Br J Psychiatry 2008;193:346.]
Anonymous. Cognitive behavioral therapy and psychoeducation reduce relapse in bipolar disorder. J Natl Med Assoc 2008;100:1108–9.
Anonymous. Early treatment for PTSD. J Natl Med Assoc 2009;101:742.
Anonymous. Effect of cognitive–behavioral therapy for anxiety disorders on quality of life: a meta analysis. J Consult Clin Psychol 2014;82:375–91. [Erratum published in J Consult Clin Psychol 2014;82:1228.]
Anonymous. ‘The effects of cognitive behavioral therapy as an anti-depressive treatment is falling: a meta-analysis’: correction to Johnsen and Friborg (2015). Psychol Bull 2016;142:290.
Anonymous. Depression severity does not moderate differences between medication and CBT. Brown University Psychopharmacology Update 2016;27:4.
Anonymous. CBT results overstated. Ther Today 2016;27:5.
Anonymous. ‘Meta-analysis of cognitive–behavioral treatments for adult ADHD’: correction to Knouse, Teller, and Brooks (2017). J Consult Clin Psychol 2017;85:882.
Anonymous. Meta-analysis of cognitive–behavioral treatments for adult ADHD. J Consult Clin Psychol 2017;85:737–750 [Erratum published in J Consult Clin Psychol 2017;85:882.]
Anonymous. ‘Effect of cognitive–behavioral therapy for anxiety disorders on quality of life: a meta-analysis’: correction to Hofmann, Wu, and Boettcher (2014). J Consult Clin Psychol 2014;82:1228.
Arts M, Petrykiv S, Fennema J, De Jonge L. The role of psychiatry in the approach of neurocardiogenic syncope. Eur Psychiatry 2018;48(Suppl. 1):S218–S9.
Aydin C, Tibbo P, Ursuliak Z. Psychosocial interventions to reduce cannabis use in the early psychosis population. Early Interv Psychiatry 2012;6(Suppl. 1):115.
Baandrup L, Rasmussen JO, Klokker L, Austin S, Bjornshave T, Bliksted VF, et al. ‘Treatment of adult patients with schizophrenia and complex mental health needs – a national clinical guideline’: corrigendum. Nord J Psychiatry 2017;71:163.
Baglioni C, Hertenstein E, Bostanova Z, Rucker G, Riemann D, Feige B. A systematic review and network meta-analysis of complementary and alternative interventions for insomnia. J Sleep Res 2018;27(Suppl. 1):172.
Ballesio A, Aquino M, Feige B, Johann A, Kyle SD, Spiegelhalder K, et al. The impact of cognitive behavioral therapy for insomnia on fatigue symptoms: a systematic examination of randomized controlled trials. Sleep 2016;39(Suppl.):A211.
Ballesio A, Aquino MRJ, Feige B, Johann A, Kyle SD, Spiegelhalder K, et al. Network meta-analysis on the effectiveness of cognitive behavioral therapies for insomnia on daytime depression and fatigue. J Sleep Res 2016;25(Suppl. 1):90–1.
Barbato A. Effective strategies for health information, self-help and psychoeducation in bipolar disorder. Eur Psychiatry 2011;26(Suppl. 1):2214.
Barber B, Zhang H, Mitchell N, O’Connell DA, Harris JR, Seikaly H. Optimal interventions for depression after diagnosis of head and neck cancer: a systematic review. Otolaryngol Head Neck Surg 2014;151(Suppl. 1):P174.
Barrett D. Systematic review summary – media-delivered cognitive behavioural therapy and behavioural therapy (self-help) for anxiety disorders in adults. Singap Nurs J 2015;42:39–40.
Basu A, Andrews G. Bandelow revisited: a conventional analysis of a meta-analysis of anxiety disorder treatments. Aust N Z J Psychiatry 2018;52(Suppl. 1):128.
Basu A, Andrews G, Cuijpers P, Craske M, McEvoy P, English C, et al. Computer therapy for the anxiety and depression disorders is effective, acceptable and practical healthcare: an updated meta-analysis. Aust N Z J Psychiatry 2017;51(Suppl. 1):117.
Bei B, Wiley JF, Trinder J, Member R. Beyond the mean: a systematic review on the correlates of daily sleep variability. Sleep 2015;1:A89.
Belleville G, Cousineau H, Levrier K, St-Pierre-Delorme ME. Does CBT-I decrease concomitant anxiety? A meta-analytic review. Sleep Med 2011;12(Suppl. 1):S61.
Benz F, Knoop T, Ballesio A, Bacaro V, Johann A, Rucker G, et al. The efficacy of cognitive and behavior therapies for insomnia on daytime symptoms: a systematic review and network meta-analysis. J Sleep Res 2018;27(Suppl. 1):289.
Bertolin-Guillen JM, Bertolin-Colilla M. P.2.a.024 Effectiveness of mindfulness-based therapies as an alternative or adjuvant of antidepressants in the treatment of depression. Eur Neuropsychopharmacol 2011;21(Suppl. 3):S367–S8.
Bhattacharya R, Kelley G, Bhattacharjee S. Long-term follow-up effects of computerized or internet-based cognitive behavioral therapy for depression and anxiety: a metaanalysis. Value Health 2012;15:A82.
Bighelli I, Leucht S. Psychological interventions for positive symptoms in schizophrenia: a network meta-analysis. Schizophr Bull 2018;44(Suppl. 1):S305.
Blodgett JC, Maisel NC, Fuh IL, Wilbourne PL, Finney JW. How effective is continuing care for substance use disorders? A metaanalytic review. Alcohol Clin Exp Res 2012;36(Suppl. 1):156A.
Bos T. The efficacy of CBT-I on insomnia and depressive symptoms in comorbid adult patients: a systematic review and meta-analysis. Sleep 2018;41(Suppl. 1):A166–A7.
Bougea A, Spantideas N, Despoti A, Belegri S, Kleisarhakis M, Chrousos G. Proposed practical recommendations of stress management for headaches in children and adolescents. Eur J Neurol 2018;25(Suppl. 2):160.
Boyle JT, Muench A, Gencarelli A, Khader W, Perlis ML. How does intensive sleep retraining (ISR) compare to CBT-I? Sleep 2018;41(Suppl. 1):A155–A6.
Bradley R, Greene J, Russ E, Dutra L, Westen D. ‘A multidimensional meta-analysis of psychotherapy for PTSD’: correction. Am J Psychiatry 2006;163:330.
British Association for Counselling and Psychotherapy. CBT in schools: a new systematic review. Ther Today 2009;20:52.
Brotherton H, Hallmark C, Rogers H. The impact of stress management on immune response in breast cancer patients: a systematic review and meta-analysis of lymphocyte outcomes shortly after intervention. Psychooncology 2014;23(Suppl. 3):124.
Brown RF, Subnis U, Starkweather A, McCain N. The effect of psychosocial interventions for patients with cancer on psychoneuroimmunologic outcomes: a systematic review. Asia Pac J Clin Oncol 2012;8(Suppl. 3):145.
Bryden PA, Caldwell DM, Welton N, Churchill R, Baxter H, Lewis G, Skapinakis P. Network meta-analysis of the relative efficacy of pharmacological and psychological interventions in adults with obsessive compulsive disorder. Value Health 2014;17:A454–5. https://doi.org/10.1016/j.jval.2014.08.1241
Bucur M, Whale R. Systematic review of randomised interventions for patients at high risk of developing psychosis: 2012 update. Early Interv Psychiatry 2012;6(Suppl. 1):123.
Carpenter JS. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 NAMS position statement. Menopause 2016;23:1365.
Carson J. Mindful pain management. J Altern Complement Med 2016;22:A71.
Carson K, Jayasinghe H, Ali A, Singh K, Peters M, Esterman A, et al. TO 012. Culturally-tailored interventions for smoking cessation in indigenous populations: a Cochrane systematic review and meta-analysis. Respirology 2015;20(Suppl. 2):17.
Carson KV, Brinn MP, Peters M, Veale A, Esterman AJ, Smith BJ. Interventions for tobacco use cessation in indigenous populations: a Cochrane meta-analysis. Am J Respir Crit Care Med 2014;189:A1086.
Castelein S, Knegtering H. Treatment of negative symptoms: which psychosocial interventions are effective? Schizophr Bull 2011;37(Suppl. 1):261.
Cattelani R, Zettin M, Zoccolotti P. Rehabilitation treatments for adults with behavioral and psychosocial disorders following acquired brain injury: a systematic review. Neuropsychol Rev 2010;20:52–85. [Erratum published in Neuropsychol Rev 2011;21:224.]
Cheung JMY, Jarrin DC, Ballot O, Bharwani A, Morin CM. A systematic review of adaptive cognitive behavioral therapy for insomnia implemented in primary care settings. Sleep Med 2017;40(Suppl. 1):e59.
Chow KM, H Chan CW, Chan JC. Effects of psychoeducational interventions on sexual functioning, quality of life and psychological outcomes in patients with gynaecological cancer: a systematic review. JBI Libr Syst Rev 2012;10:4077–164. https://doi.org/10.11124/jbisrir-2012-406
Chung P, Khan F. Traumatic brain injury (TBI) diagnosis and treatment: a systematic review and update. Brain Injury 2014;28(5–6):744–5.
Chvatalova B. A comparison of the effectiveness of various psychotherapeutic approaches to first-episode psychosis in terms of recovery: a systematic review. Early Interv Psychiatry 2018;12(Suppl. 1):137.
Coffey BJ. 39.3 Update on pharmacological treatments in tics and tourette’s disorder. J Am Acad Child Adolesc Psychiatry 2016;55:S61.
Costa A, Melina F, Sansalone A, Iannacchero R. P004. Evidence based psychological treatments in pain management: a review of controlled and randomized trials about chronic headache, neuropathic pain and fibromyalgia. J Headache Pain 2015;16(Suppl. 1):A151.
Creasey N, Benger J, Wright I, Lyttle M. 0589 Non-pharmacological interventions to reduce psychological sequalae of mild traumatic brain injury in adults and children: a systematic review. Brain Injury 2016;30:709–10.
Creasey NJ, Lyttle M. Psychological interventions for patients of all ages with mild traumatic brain injury: a systematic review. Arch Dis Child 2016;101(Suppl. 1):A138–9.
Creed F, Rizzo M. W07-04-UK NICE guidelines for depression in people with a chronic physical illness. Eur Psychiatry 2011;26(Suppl. 1):2212.
Cullen K, Irvin E, Van Eerd D, Saunders R. Preventing work disability in workers with depression; a systematic review. Occup Environ Med 2018;75(Suppl. 2):A589.
Dahdah M, Driver S, Shafi S, Callender L, Collinsworth A, Brown R, et al. Development of evidence-based treatment guidelines for TBI rehabilitation care: the cognitive–behavioral treatment ARM. Arch Phys Med Rehabil 2016;97:e100–1.
Dancet E, Vermeulen N, Boivin J, Gameiro S. The effectiveness of psychosocial interventions that can be delivered by all staff members: a systematic review. Hum Reprod 2015;30(Suppl. 1):i61.
De Groot V, Beckerman H. Rehabilitation to treat MS-related fatigue: the TREFAMS research programme. Mult Scler 2016;22(Suppl. 3):40–1.
De Rooij A, Roorda L, Otten R, Dekker J, Steultjens M. Predictors of multidisciplinary rehabilitation outcome in fibromyalgia: a systematic review. Physiotherapy 2011;97(Suppl. 1):eS269–70.
De Vera MA, Galo J. FRI0212 What are the effects of interventions targetting medication adherence in rheumatic diseases: a systematic review. Ann Rheum Dis 2014;73(Suppl. 2):459.
Desai P, Urosevic S, Butler M. Psychosocial and other non-drug treatments for bipolar disorder in adults: a systematic review. Value Health 2018;21(Suppl. 1):S180–1.
Devoe D, Farris M, Addington J. Symptoms of depression and anxiety in youth at risk for psychosis: a systematic review and metaanalysis. Early Interv Psychiatry 2018;12(Suppl. 1):174.
Devoe D, Farris MS, Townes P, Addington J. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analysis. Early Interv Psychiatry 2018;12(Suppl. 1):173.
Devoe D, Farris MS, Townes P, Addington J. Interventions and social functioning in youth at risk of psychosis: a systematic review and meta-analysis. Early Interv Psychiatry 2018;12(Suppl. 1):172.
Dickens C, Cherrington A, Garrett C, Bower P, Bundy C, Gask L, et al. Characteristics of psychosocial interventions that improve depression in people with coronary heart disease: a systematic review with meta-regression. J Psychosom Res 2010;68:619.
Dickinson C, Whittingham K, Sheffield J, Wotherspoon J, Boyd R. Efficacy of interventions to improve psychological adjustment for parents who have an infant diagnosed with neurodevelopmental disability: a systematic review. Devel Med Child Neurol 2018;60(Suppl. 1):20.
Dieng M, Cust AE, Kasparian NA, Mann GJ, Morton RL. Economic evaluation of psychosocial interventions in cancer: a systematic review. Asia Pac J Clin Oncol 2015;11(Suppl. 4):95.
Doyle C, Foster N, Jordan J. Psychological interventions for long-term conditions: a review of approaches, content and outcomes. Physiother Res Int 2008;13:138.
Dragioti E, Dimoliatis I, Evangelou V. 1810 – An empirical assessment of psychotherapy allegiance in randomised controlled trials. Eur Psychiatry 2013;28(Suppl. 1):1.
Ebrahim S, Montoya L, Truong W, Hsu S, Kamal el Din M, et al. Correction: effectiveness of cognitive behavioral therapy for depression in patients receiving disability benefits: a systematic review and individual patient data meta-analysis. PLOS ONE 2013;8(6).
Elstner S. Psychotherapy for ADHD in people with IDD. Eur Psychiatry 2016;33(Suppl. 1):S475–S6.
Erdogan A, Coss-Adame E, Rao SSC. Systematic review of treatments for esophageal (noncardiac) chest pain. Neurogastroenterol Motil 2013;25(Suppl. 1):19.
Evans J, Jack R, Harrison A. The Scottish Intercollegiate Guideline Network (SIGN) guideline on rehabilitation of cognitive and mood disorders after brain injury. Brain Impair 2012;13:144.
Eyrenci A, Ayalp GC. A meta-analysis of group psychotherapy studies for early stage breast cancer patients. Psychooncology 2014;23(Suppl. 3):195.
Fagundes SBR, Fagundes DJL, Molen YF, Prado LBF, Carvalho JEC, Carvalho LBC, et al. What’s new in primary insomnia treatment? Sleep Med 2009;10(Suppl. 2):S17.
Falsaperla R, Saporito MAN, Di Stefano V, Pavone P. A31 Pandas: tip of the iceberg. Riv Ital Pediatr 2017;43(Suppl. 2):15.
Fangtham M, Nash JL, Hyon S, Bannuru RR, Wang C. Non-pharmacological treatment on fatigue, depression, disease activity, and quality of life of systemic lupus erythematosus: a systematic review [abstract]. Arthr Rheumatol 2017;69(Suppl. 10).
Farris M, Devoe D, Addington J. Attrition rates in treatment trials: a systematic review and meta-analysis of clinical high-risk for psychosis interventions. Early Interv Psychiatry 2018;12(Suppl. 1):182.
Ferreira PH, Ho KK, Pinheiro MB, Aquino Silva D, Miller C, Grunstein R, et al. Sleep interventions for osteoarthritis and spinal pain: a systematic review of randomized control trials. Osteoarthr Cartil 2018;26(Suppl. 1):S243.
Fishpool K, Jones B, Hewlett S, Ndosi M. 277 Online interventions for addressing psychological distress in people with rheumatoid arthritis and other long-term conditions: a systematic review. Rheumatology 2018;57(Suppl. 3).
Fishpool K, Jones B, Hewlett S, Ndosi M. THUR0730-HPR A systematic review of online interventions for addressing psychological distress in rheumatoid arthritis and other long-term conditions. Ann Rheum Dis 2018;77(Suppl. 2):1792.
Forti Buratti M, Ramchandani P, Saikia R, Wilkinson E, Mehta N. Systematic review of psychological treatments for depression in children below 13 years old. Eur Child Adolesc Psychiatry 2015;24(Suppl. 1):S167.
Fredrikson M, Faria V, Agren T, Engman J, Furmark T. Meta-analytical evidence for segregating and integrating brain activation to symptom provocation in social anxiety disorder, specific phobia and post traumatic stress disorder. Biol Psychiatry 2011;69:S71–2.
French LA, Nikolic-Novakovic L. P01-327 New dimensionsin assessing and treating traumatic stress: a meta-analysis of historical and recent contributions to the field. Eur Psychiatry 2010;25(Suppl. 1):540.
Garbutt J. Predictors of response to naltrexone in alcohol dependence: a systematic review and integration of the world literature. J Addict Med 2013;7:E7–8.
Garg S, Garg D, Chowdhury F, Barron G, Turin TC. Web-based intervention for chronic back pain. Can Fam Physician 2016;62(Suppl. 1):S34.
Garg SK, Wadhwa V, Anugwom CM, Gupta N, George J, Sanaka MR, et al. Mo1655 Effectiveness of pharmacological and non-pharmacological therapies for irritable bowel syndrome: a systematic review and Bayesian network meta-analysis. Gastroenterology 2016;(Suppl. 1):S–744.
Gaudin D, Krafcik B, Mansour T, Alnemari AA. P160 – considerations in spinal fusion surgery for chronic lumbar pain: psychosocial factors, rating scales, and perioperative patient education: a review of the literature. Glob Spine J 2017;7:268S–9S.
Gertler P, Tate RL, Cameron ID. A systematic review of non-pharmacological treatments for depression after TBI. Brain Impair 2011;(Suppl. 1):70.
Glozier N, Tofler G, Colquhoun D, Bunker S, Clarke D, D LH, et al. The National Heart Foundation of Australia consensus statement on depression, work stress and coronary heart disease (CHD). Aust N Z J Psychiatry 2013;47(S1):21.
Goodman JH. Group treatment of postpartum depression: a systematic review. Arch Womens Ment Health 2011;14:S68–9.
Goyal D, Parikh T, Fitzgerald J, Pruett J. 5.50 Assessment and treatment of irritability in children and adolescents: a review of literature for evidence-based recommendations. J Am Acad Child Adolesc Psychiatry 2018;57:S242–3.
Grigoriadis S, Kennedy S, Robinson G, VonderPorten E, Mamisashvili L, Peer M. A systematic review of treatments for depression in perimenopausal and postmenopausal women. J Womens Health 2017;26:A22.
Gritzner S, Antick J, Michael P, Cavanaugh R. Cognitive behavior therapy for fibromyalgia: a meta analysis. J Pain 2012;13:S97.
Gudmundsdottir E. Support interventions for parents of children with cancer: a review of effectiveness reducing parental distress. Pediatr Blood Cancer 2011;57:760.
Hackett KL. Sleep disturbances in primary sjogren’s syndrome: evidence from the literature, patient sleep diaries and a qualitative focus group study. Ann Rheum Dis 2018;77(Suppl. 2):45.
Hamrick N, Dickinson T. Spiritually-based interventions: meta-analysis of impact on psychological well-being and comparison with cognitive interventions. Psychooncology 2010;19(Suppl. 1):S13.
Han Shi Jocelyn Chew HSJ, Chair SY, Cheng HY. Comparing the effectiveness of cognitive behavioral interventions and motivational interviewing on improving self-care behaviors. Eur J Heart Fail 2018;20(Suppl. 1):72.
Harsh R, Kundi PS, Ezeh D. A systematic review of the effectiveness of psychological interventions for adult sex-offenders in prisons, community clinics and forensic hospitals. Eur Neuropsychopharmacol 2015;25:S653–4.
Hauser W. 141. Managing fibromyalgia: what works and what doesn’t. Rheumatol 2014;53(Suppl. 1):i9.
Hauser W, Kopp I. 0P0070 Update of the german evidence-based guideline on the management of fibromyalgia syndrome. Ann Rheum Dis 2012;71(Suppl. 3):77.
Hauser W, Nuesch E, Juni P. 0P0192 Comparative efficacy of pharmacological and non-pharmacological interventions in fibromyalgia. Ann Rheum Dis 2012;71(Suppl. 3):119–20.
Hauser W, Thieme K, Denis T. 150 A comparison of the US–American and German guideline with eular recommendations for the management of fibromyalgia. Eur J Pain 2009;13(Suppl. 1):S52.
Hay P, Galletly C, Carter G, Andrews G, Chinn D, Forbes D, et al. RANZCP clinical practice guidelines for eating disorders. Aust N Z J Psychiatry 2015;49(Suppl. 1):30–1.
Hazell P. Interventions for deliberate self-harm in adolescents. Aust N Z J Psychiatry 2017;51(Suppl. 1):82.
Hegerl U. Self-management in affective disorders: how to use both patient generated data and internet and CBT-based programmes in routine care. Eur Psychiatry 2018;48(Suppl. 1):S42.
Heidari E, Kamal KM, Giannetti V, Covvey JR. The impact of antidepressant medications on clinical outcomes of patients with type 2 diabetes: a systematic review. Value Health 2018;21(Suppl. 1):S70.
Henwood KS, Chou S, Browne KD. ‘A systematic review and meta-analysis on the effectiveness of CBT informed anger management’: corrigendum. Aggress Violent Behav 2016;27:121.
Heron H, Baken D, Harvey S. Meta-analysis of moderators of psycho-oncology therapy effectiveness: ‘It’s the sick who need a doctor’. Psychooncology 2009;18(Suppl. 2):S107.
Hershberger A, Um M, Cyders M. The role of the UPPS-p impulsive personality traits in cognitive behavioral therapy based substance use treatment: a meta-analysis. Alcohol Clin Exp Res 2017;41(Suppl. 1):229A.
Hetrick SE, Purcell R, Garner B, Parslow R. Combined pharmacotherapy and psychological therapies for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2010;7:CD007316. https://doi.org/10.1002/14651858.CD007316.pub2
Hewlett S. SP0147 Using cognitive–behavioural therapy to help patients self-manage their fatigue. Ann Rheum Dis 2013;72(Suppl. 3):A34.
Hochard KH, Burger K, Hulbert-Williams NJ. Comprehensive systematic review of insomnia treatments for people being treated for curable cancer. Psychooncology 2016;25(Suppl. 1):15.
Hofmann SG, Asnaani A, Vonk IJJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cogn Ther Res 2012;36:427–440. [Erratum published in Cogn Ther Res 2014;38:368.]
Horton MS, Pugh M, Lawrence VA. Nonpharmacological therapy for insomnia in elders: a systematic review. J Am Geriatr Soc 2010;58:S65.
Howard LM. Partner violence in pregnancy and postpartum. Arch Womens Ment Health 2013;16:S5.
Hulbert-Williams N, Flynn S, Heaton-Brown L, Scanlon K. Interventions to improve the well-being of breast cancer survivors at the end of active treatment: a systematic review of the literature. Psychooncology 2013;22(Suppl. 3):329–30.
Hunt JIE. Treatments for OCD in pediatric patients . . . obsessive–compulsive disorder. Brown University Child & Adolescent Psychopharmacology Update 2008;10:4–5.
Huntley A, Moore T, King A, Evans M, Persad R, Sharp D. Supportive care interventions for men with prostate cancer: a systematic review. Support Care Cancer 2015;23:S369.
Indrielle T, Keay S. P0196 Does psychotherapy improve success rates of in vitro fertilisation? systematic review. Int J Gynecol Obstet 2015;5:E371.
Isaac D. Clinical benefits of exercise and psychological interventions in patients with cancer-related fatigue. J Clin Outcomes Manag 2017;24:200–2.
Jayasekara R. Cognitive behavioral therapy for men who physically abuse their female partner. J Adv Nurs 2008;64:129–30.
Jomaa I, Saini B, Miller C. Is cognitive behavioural therapy for the treatment of insomnia harmful? A systematic review of the literature. J Sleep Res 2017;26(Suppl. 1):37–8.
Junghan UM, Pfammatter M. What are the therapeutic ingredients of cognitive behavior therapy for psychosis? A systematic review. Eur Arch Psychiatry Clin Neurosci 2011;261:S36.
Kangas M, Bovbjerg DH, Montgomery GH. ‘Cancer-related fatigue: a systematic and meta-analytic review of non-pharmacological therapies for cancer patients’: correction to Kangas, Bovbjerg, and Montgomery (2008). Psychol Bull 2009;135:172.
Kaplan SG. Motivational interviewing in the treatment of pediatric obesity: research and promise. Psychosom Med 2011;73:A7.
Kazantzis N. Translating science into practice, collaborative empiricism and engagement in homework assignments in cognitive behavior therapy. Bull Clin Psychopharmacol 2011;21(Suppl. 2):S41.
Khan A, Tansel A, White D, Blais P, Lindsay J, El-Serag HB, et al. Mo1248 Psychosocial interventions to achieve abstinence in patients with chronic liver disease and alcohol use disorders: a systematic review. Gastroenterology 2015;148:S–649.
Kingdon D. FC19-03 – advances in cognitive behaviour therapy for psychosis. Eur Psychiatry 2011;26(Suppl. 1):1917.
Kitsumban V, Thapinta D, Picheansathian W. The effectiveness of cognitive–behavioural therapy on depression in the elderly. JBI Libr Syst Rev 2012;10(Suppl. 14):1–9. https://doi.org/10.11124/jbisrir-2012-269
Koehler M, Hoppe S, Peplinski D, Richter D, Frommer J, Flechtner HH, et al. Psycho-oncologic interventions for parents of cancer patients: systematic review. Oncol Res Treat 2015;38(Suppl. 5):172.
Kohli IS, Kataria A, Singla S, Kaushik P, Jindal R, Aggarwal A. Effect of cognitive behavioural therapy in multiple sclerosis fatigue: a systematic review of randomised controlled trials. Value Health 2012;15:A545–6.
Konnopka A, Schaefert R, Heinrich S, Leicht H, Kaufmann C, Luppa M, et al. The economic burden of somatization syndromes: a systematic review of cost-of-illness studies and cost-effectiveness analyses. J Ment Health Policy Econ 2011;1:S16–S7.
Kuehner C. S11-04 Psychological treatments and prevention in bipolar disorder – recent developments. Eur Psychiatry 2009;24(Suppl. 1):S61.
Kuehner C. S11-03 – evidence-based psychotherapy for chronic depression. Eur Psychiatry 2010;25(Suppl. 1):41.
Kuo MH, Brown CA, Phillips L, Berry R, Tan M. Non-pharmacological sleep interventions for youth with chronic health conditions: a systematic review. Sleep 2012;35(Suppl. 1):A307–8.
Laird K, Tanner-Smith E, Russell A, Hollon S, Walker L. Comparative efficacy of psychological therapies for improving mental health and functioning in irritable bowel syndrome: a systematic review and meta-analysis. J Altern Complement Med 2016;22:A93.
Landa-Ramirez E, Rivero-Rosas A, Cardenas-Lopez G, Greer JA, Sanchez-Roman S, Field A. Cognitive–behavioral therapy for depression and anxiety in advanced cancer: a literature review. Psycho-Oncology 2013;22(Suppl. 3):226.
Landmark B, Reinar LM, Brurberg KG, Hammerstrom KA, Almas E, Aars H, et al. Effects of sexological therapy for sex offenders. A systematic review. J Sex Med 2013;10(Suppl. 5):315.
Larkin D, Lopez V, Aromataris E. Interventions for managing cancer-related fatigue in men treated for prostate cancer: a systematic review. Support Care Cancer 2013;21:S101–2.
Lars Jerden L, Kiessling A, Wetterqvist A, Hambreus K, Perk J. Establishment of national guidelines in Sweden for lifestyle modification in healthcare. Eur J Prevent Cardiol 2013;20(Suppl. 1):S65.
Laska I, Swieca J, Meaklim H, Kelly D, Cunnington D. Psychologically-based treatment strategies for restless legs syndrome: a review. J Sleep Res 2017;26(Suppl. 1):38.
Lee M, Patel T, Lee L. Nonpharmacologic outpatient interventions for benzodiazepine discontinuation in elderly persons. Can Fam Physician 2015;61(Suppl. 1):S55.
Lee V, Balucani C, DeLuca J, Lederman YS, Arnedo V, Lushbough CA, et al. Abstract 3684: Post-stroke fatigue: a systematic evidence-based critique. Stroke 2012;43(Suppl. 1):A3684.
Lehman A, Yohannes S, MacDonald C. Moving from patient-centred to family-centred care? A systematic review of psycho-educational programs for people and partners affected by arthritis. Reumatol Clin Supl 2011;7:167.
Leverich AE, Acke S, Verbrugghe M, Schmickler MN, De Brouwer C. Efficiency of vocational rehabilitation programs for workers with schizophrenia: a systematic literature review according to the PRISMA guidelines. Occup Environ Med 2018;75(Suppl. 2):A612–13.
Li L, Xiong L, Zhang S, Yu Q, Chen M. Cognitive-behavioral therapy for irritable bowel syndrome: a meta-analysis. J Psychosom Res 2014;77:1–12. https://doi.org/10.1016/j.jpsychores.2014.03.006
Lim C, Sam HF, Renjan V, Quah SL. A systematic review of adapted cognitive–behavioural therapy for religious individuals with severe mental illness. Ann Acad Med Singap 2011;40(Suppl.):S62.
Lipsey MW. Variability across treatments and outcomes: meta-analysis of the effects of adolescent substance abuse treatment. Alcohol Clin Exp Res 2012;36(Suppl. 1):330A.
Liu P, Parker A, Hetrick S, Purcell R. Evidence mapping for early psychotic disorders in young people. Schizophr Res 2010;117:438–9.
Lock J. 3.3 Evidence-based psychosocial treatments for eating disorders in children and adolescents. J Am Acad Child Adolesc Psychiatry 2016;55:S88–9.
Loucas C, Pennant M, Whittington C, Naqvi S, Sealey C, Stockton S, et al. G130 E-therapies for mental health problems in children and young people: a systematic review and focus group investigation. Arch Dis Child 2014;99:A58.
Lynch MJ, George TP. Therapeutic mechanisms underlying the effects of Alcoholics Anonymous: results of a systematic literature review. Am J Addict 2013;22:306.
MacMillan H, Jack SM, MacGregor J, Wathen N. 22.1 Identifying and responding to intimate partner violence exposure among children and adolescents in pediatric settings. J Am Acad Child Adolesc Psychiatry 2017;56:S32.
Marian A, Dumitrascu DL. Predictors of response to cognitive–behavioral therapy in patients with irritable bowel syndrome. J Gastrointest LiverDis 2017;26(Suppl. 1):59.
Matthews EE, McCarthy MS. Adherence to Cognitive Behavioral Therapy for Insomnia. Paper presented at the Western Institute of Nursing Annual Communicating Nursing Research Conference, Seattle, WA, April 2014.
Matthews H, Grunfeld B, Turner A. The efficacy of psychosocial interventions among women following surgical treatment for breast cancer: a systematic review and meta-analysis. Eur J Cancer 2016;57(Suppl. 2):S151.
Mayo-Wilson E. Internet-based cognitive behaviour therapy for symptoms of depression and anxiety: a meta-analysis. Psychol Med 2007;37:1211. https://doi.org/10.1017/s0033291707000608
McDaid D, Park A, Parsonage M. Making the economic case for tackling somatoform disorders. Psychiatr Prax 2011;38(Suppl. 1):S13_4_EC.
McKay GD, Berkowitz Sturgis EK, Grandner MA, Gehrman P, Perlis ML. 0646 Response and remission definitions for CBT-I: a quantitative review. Sleep 2012;35(Suppl. 1):A219.
British Journal of Hospital Medicine. Early cognitive behavioural therapy reduces risk of psychosis, finds meta-analysis. Br J Hosp Med 2013;74:1750–8460.
Mehta S, Orenczuk S, Teasell R. Poster 47. Evidence based management of depression following spinal cord injury: a meta-analysis. Arch Phys Med Rehabil 2011;92:1706–7.
Mei Ling L, Creedy DK, Moon Fai C. Effectiveness of psychological-based interventions to enhance coping of nursing students: a systematic review. JBI Database Syst Rev Implement Rep 2010;8:S1–24.
Menezes A. 50 – psychosocial interventions for common mental disorders in primary health care. J Psychosom Res 2013;74:554.
Messer SC. 1148. Differential effectiveness of psychological and pharmacotherapy interventions for ptsd: a meta-analytic review. Am J Epidemiol 2011;173(Suppl.):S287.
Michura D, Bonn J. O012. The effectiveness of group cognitive behavioural therapy in the management of chronic low back pain. Physiotherapy 2017;103(Suppl. 1):e8–e9.
Mikocka-Walus A. 51 – Psychosomatic medicine and inflammatory bowel disease: a new integrated model of care. J Psychosom Res 2013;74:554.
Mikocka-Walus A. 245. Cognitive-behavioural therapy in inflammatory bowel disease: what’s the evidence? Psychother Psychosom 2013;82(Suppl. 1):67.
Mikocka-Walus AA, Turnbull DA, Holtmann G, Andrews JM. Sa1031. Coping with the unmet needs of gastroenterology and hepatology outpatients: a systematic approach towards an integrated model of care in South Australia. Gastroenterology 2011;58:S–208.
Minen M, Jinich S, Vallespir EG. Behavioral therapies and mind body interventions for post traumatic headache and post-concussive symptoms: a systematic review. Headache 2018;58(Suppl. 2):201.
Morell GFC. Insufficient evidence to support or reject effect of conservative TMD therapies on otologic signs and symptoms. Evid Based Dent 2018;19:26–7. https://doi.org/10.1038/sj.ebd.6401293
Moritz S. A Reply to ‘Effectiveness of an individually-tailored computerised CBT programme (Deprexis) for depression: a meta-analysis’ by Twomey and colleagues [Psychiatry Res. 256 (2017) 371–377.] Psychiatry Res 2018;263:282.
Moss-Morris R, Castell BD, Kazantzis N. Cognitive behavioral therapy and graded exercise for chronic fatigue syndrome: a comparative meta-analysis including moderators of effects. Psychosom Med 2012;74:A89.
Munn Z, Yifan X, Moola S, McArthur A. Children’s views about obesity, body size, shape and weight; inequalities and the mental health of young people: a systematic review of secondary school-based cognitive behavioural interventions; intervention strategies that support self-care activities: an integrative study across disease/impairment groupings; advocacy interventions to reduce or eliminate violence and promote the physical and psychosocial well-being of women who experience intimate partner abuse. J Adv Nurs 2011;67:954–60.
Natale P, Palmer S, Ruospo M, Saglimbene V, Hegbrant J, Strippoli G. FO025. Interventions to improve sleep quality in people with chronic kidney disease: a Cochrane systematic review. Nephrol Dial Transplant 2018;33(Suppl. 1):i28.
Nickel F, Kolominsky-Rabas PL. Health economic evidence on non-pharmacological interventions for persons with dementia: a systematic review. Value Health 2017;20:A713–14.
Nikandrou D. What psychological interventions have been found to reduce overall distress in cancer patients? A systematic review. Psychooncology 2016;25(Suppl. 1):20.
Niknejad B, Bolier R, Delgado D, Henderson C, Reid M. The efficacy of cognitive–behavioral interventions for chronic pain in older adults: a meta-analysis. J Am Geriatr Soc 2017;65(Suppl. 1):S144–5.
Noble A, Reilly J, Temple J, Fisher P, Snape D. Cognitive–behavioural therapy does not meaningfully reduce depression in most people with epilepsy: a systematic review with a reliable change analysis. Epilepsia 2018;59(Suppl. 3):S242–3.
Noble F, Marshman Z. The effectiveness of cognitive behavioural therapy in the reduction of dental anxiety in children. Evid Based Dent 2018;19:104. https://doi.org/10.1038/sj.ebd.6401339
Nordentoft M, Austin S, Jeppesen P, Melau M, Thorup A. Poster #244. Crucial elements in treatment of first-episode psychosis patient, psychosocial aspects. Schizophr Res 2012;136(Suppl. 1):S369.
Nordentoft M, Melau M. A90. Crucial elements in treatment of first-episode psychosis patient, psychosocial aspects. Early Interv Psychiatry 2014;8(Suppl. 1):86.
Norman L, Taylor S, Liu Y, Radua J, Abelson J, Angstadt M, et al. S20. Error-processing in OCD: a meta-analysis of fMRI studies and investigation of changes following CBT. Biol Psychiatry 2018;83:S354.
Norton C, Czuber-Dochan W, Artom M, Sweeney L, Hart A. P555 Systematic review of interventions for chronic abdominal pain management in inflammatory bowel disease. J Crohns Colitis 2017;11(Suppl. 1):S363.
O’Toole MS, Zachariae RS, Renna ME, Mennin D, Applebaum A. Cognitive behavioral therapies for informal caregivers of patients with cancer and cancer survivors: a systematic review and meta-analysis. Psychosom Med 2016;78:A99–100.
Olthuis JV, Watt MC, Bailey K, Hayden JA, Stewart SH. Therapist-supported internet cognitive behavioural therapy for anxiety disorders in adults. Cochrane Database Syst Rev 2015;3:CD011565. https://doi.org/10.1002/14651858.CD011565
Omlin X, Ballesio A, Aquino MRJ, Espie CA, Kyle SD. The effect of cognitive behavioural therapy for insomnia on quality of life outcomes: a meta-analysis of randomised controlled trials. J Sleep Res 2018;27(Suppl. 1):174–5.
Panciroli G, Marchi M, Mattei G, Galeazzi GM, Ferrari S. Efficacy of music therapy interventions in the treatment of mood disorders: a systematic review. Eur Psychiatry 2018;48(Suppl. 1):S357.
Penades R. Integrating cognitive remediation and other interventions to improve social functioning. Eur Arch Psychiatry Clin Neurosci 2009;259(Suppl. 1):S41–2.
Perez PV, De Azua SR, Martinez M, Ron S, Oliveros RG, Asua J, et al. P.3.a.006. Psychological treatment in the first psychotic episode. Eur Neuropsychopharmacol 2009;19(Suppl. 3):S483–4.
Pfammatter M. The empirical status of CBT for psychosis: controlled efficacy, indication and therapeutic factors. A systematic review of metaanalytic findings. Eur Psychiatry 2011;26(Suppl. 1):1475.
Phianmongkhol Y, Thongubon K, Woottiluk P. Cognitive behavioral therapy techniques for pain in lung cancer patients: a systematic review. JBI Libr Syst Rev 2011;9:1–11.
Pijnenborg M, Van Donkersgoed R, David A, Aleman A. Treatment of insight in psychosis: a meta-analysis. Schizophr Res 2012;136(Suppl. 1):S76–S7.
Poiraudeau S, Palazzo C. Therapeutic advances in chronic low back pain management: a systematic review of the litterature over the 5 past years. Ann Phys Rehabil Med 2012;55(Suppl. 1):e284–e5.
Police R, Zhao Y, Russell M, Foster T. A systematic review of the burden, epidemiology, costs and treatment of chronic low back pain. J Pain 2009;10:S5.
Pompoli A, Furukawa TA, Imai H, Tajika A, Efthimiou O, Salanti G. Psychological therapies for panic disorder with or without agoraphobia in adults: a network meta-analysis. Cochrane Database Syst Rev 2016;4:CD011004.
Ponnuthurai SA, Brown J. Meta-analysis of the outcome of rcts of preventative or early intervention universal group psychological therapies in under 18s. J Am Acad Child Adolesc Psychiatry 2018;57:S189.
Pozza A, Andersson G, Dèttore D. Therapist-guided internet-based cognitive–behavioural therapy for adult obsessive–compulsive disorder: a meta-analysis. Eur Psychiatry 2016;33(Suppl.):S276–7.
Puyraimond-Zemmour D, Romand X, Lavielle M, Molto A, Gaudin P, Soubrier M, et al. Adherence to disease-modifying drugs in chronic inflammatory rheumatic diseases: several questionnaires, diverse patient characteristics and some efficacious interventions – a systematic literature review. Ann Rheum Dis 2018;77(Suppl. 2):1194.
Raciti AE. Effectiveness of interventions for parasomnias among soldiers who have post traumatic stress disorder. Sleep 2016;39(Suppl.):A282–A3.
Ramsenthaler C, Siegert RJ, Weatherall M, Bausewein C, Koffman J, Higginson IJ, et al. Cognitive behavioural therapy (CBT) for panic and anxiety in chronic obstructive pulmonary disease – a systematic review. Palliat Med 2012;26:405–6.
Rebbeck T, Stewart M, Cameron I, Stewart J. Treatment of chronic whiplash: a systematic review and clinical guidelines. Physiotherapy 2011;97(Suppl. 1):eS1038–9.
Rezaie L, Rassafiani. Is cognitive behavior therapy (CBT) an alternative treatment for pharmacotherapy for children with obsessive compulsive disorders? (A critically appraised topic). Iran J Psychiatry 2012;1:133.
Rice S, Goodall J, Hetrick S, Parker A, Gilbertson T, Amminger P, et al. Online and social networking interventions for the treatment of depression in young people: literature review and new horizons. Early Interv Psychiatry 2014;8(Suppl. 1):74.
Riehle M, Pillny M, Buggisch S, Lincoln T. The efficacy of psychosocial treatments for patients with present negative symptoms: a meta-analysis. Eur Arch Psychiatry Clin Neurosci 2017;267(Suppl. 1):S52.
Robbins R, Underwood PE, Jackson C, Chen M, Kuriakose S, Jean-Louis G, et al. A systematic review of worksite interventions and their impact on employee sleep. Sleep 2018;41(Suppl. 1):A219.
Rogers VE, Diaz-Abad M, Liu W, Geiger-Brown J. Meta-analysis of CBT-I for the treatment of comorbid insomnia. Sleep 2015;38(Suppl. 1):A228.
Rolls C, Prior Y. 285 Non-pharmacological interventions for people with fibromyalgia: a systematic review. Rheumatology 2018;57(Suppl. 3).
Rueda S, Rzeznikiewiz D, Park-Wyllie L, Bayoumi A, Tynan AM, Antoniou T, et al. A systematic review of the effectiveness of sociobehavioral interventions to improve adherence to combination antiretroviral therapy in HIV. J Int Assoc Physician AIDS Care 2010;9:58.
Sahota P, Wordley J, Woodward J. Effective behavioural components in child and adolescent weight management programmes. Obes Rev 2011;12(Suppl. 1):57–8.
Sajid SS, Kotwal A, Dale W. Interventions to reduce health disparities in prostate cancer: a systematic literature review. J Am Geriatr Soc 2011;59:S94.
Sanchez Ortuno M, Seda G, Welsh C, Halbower A, Edinger J. Comparative meta-analysis of prazosin and imagery rehearsal therapy for nightmares, sleep disturbance and post-traumatic stress. Sleep Med 2013;14(Suppl. 1):e48.
Sanchez-Meca J, Rosa-Alcazar AI, Lopez-Soler C. The psychological treatment of sexual abuse in children and adolescents: a meta-analysis. Int J Clin Health Psychol 2011;11:67–93. [Erratum published in Int J Clin Health Psychol 2011;11.]
Sanjuan J, Liano V, Balaguer E, Aguilar EJ. Neuroimaging techniques and response to psychological intervention in psychotic patients: a systematic review. Schizophr Bull 2011;37(Suppl. 1):151–2.
Seda G, Sanchez-Ortuno MM, Welsh CH, Ann HC, Edinger JD. Comparative meta-analysis of prazosin and imagery rehearsal therapy for nightmares, sleep disturbance and post-traumatic stress. Sleep 2014;37(Suppl. 1):A276.
Sedlar N, Lainscak M, Farkas J. Psychological interventions to improve psychological outcomes in patients with heart failure: a systematic literature review. Eur J Heart Fail 2018;20(Suppl. 1):586.
Seppala A, Miettunen J, Hirvonen N, Isohanni M, Moilanen J, Koponen H, et al. What do we know about treatment-resistant schizophrenia? A systematic review. Eur Psychiatry 2016;33(Suppl.):S586.
Seppala J, Seppala A, Isohanni M, Miettunen J, Jaaskelainen E. Treatment-resistant and difficult-to treat schizophrenia as a challenge for clinical practices. Data from Finnish samples: Northern Finland birth cohort 1966 and Perfect-project. Eur Arch Psychiatry Clin Neurosci 2017;267(Suppl. 1):S16–17.
Sesel AL, Sharpe L, Naismith S. The efficacy of psychosocial interventions for people with multiple sclerosis: a meta-analysis of specific treatment effects. Mult Scler J 2017;23:NP1.
Shi Q, Xiang T, Liangren L, Zhenhua L, Lu Y, Qiang W. 946. Psychosocial interventions to improve the quality of life for men with prostate cancer: a Bayesian network meta-analysis of 31 randomised controlled trails. Eur Urol Suppl 2017;16:e1648.
Silva M, Figueiredo AR, Fornelos A, Macedo P, Nunes S. Psychotherapeutic interventions in Tinnitus. Eur Psychiatry 2016;33(Suppl.):S561.
Singh N, Dornadula I, Ramezani A, Sheth S, De Mesa C. Psychological preparation for neuromodulation: a systematic review. Neuromodulation 2018;21:e72.
Sjogren T, Avikainen L, Calleeuw N, Forsblom K, Keranen K, Lautamaki L, et al. Motor control exercise for non-specific low back pain: a systematic review and meta-analysis of randomized controlled trials. Physiotherapy 2015;101(Suppl. 1):eS1405.
Sleight A. Rehabilitation interventions and coping techniques for chemotherapy-related cognitive dysfunction. Arch Phys Med Rehabil 2015;96:e115–e6.
Sleight A, Clark F. Chemotherapy-related cognitive dysfunction and the role of rehabilitation: a scoping review of the literature. Support Care Cancer 2015;23:S310–S1.
Smeets KC, Rommelse NNJ, Scheepers FE, Buitelaar JK. Responder and non-responder profiles of a cognitive behavior therapy to reduce aggression in adolescents. Eur Child Adolesc Psychiatry 2015;24:S50–S1.
Smith K. Network meta-analysis: a useful tool for comparing different psychological treatments for panic disorder?: Commentary on . . . Cochrane Corner. BJPsych Adv 2018;24:3–8.
Spiegel D, Krizanec S, Kraemer H, Jo B, Ershadi M, Neri E, et al. Meta-analysis of psychosocial treatment effects on cancer survival and sources of heterogeneity. Neuropsychopharmacology 2017;43(Suppl. 1):S331.
Ssegonja R, Nystrand C, Feldman I, Sarkadi A, Langenskiold S, Jonsson U. Indicated preventive interventions for depression in children and adolescents: a meta-analysis and meta-regression. Value Health 2018;21(Suppl. 1):S181.
Staugaard CF, Uhre V, Lonfeldt N, Pretzmann L, Vangkilde S, Plessen K, et al. Cognitive behavioral therapy for obsessive compulsive disorder in children and adolescents – preliminary results from a systematic review. Early Interv Psychiatry 2018;12(Suppl. 1):166.
Strauss N, Rabin J, Temel B, Philpotts L, Ostroff J, Park ER, et al. Smoking cessation interventions for Black and Hispanic cancer patients: a systematic review of tobacco trials. Psycho-Oncology 2017;26(Suppl. 1):87.
Stuhldreher N, Konnopka A, Wild B, Herzog W, Zipfel S, Lowe B, et al. Cost-of-illness studies and cost-effectiveness analyses in eating disorders: a systematic review. J Ment Health Policy Econ 2013;1:S33.
Sutherland R, O’Callaghan V, Henderson S, Nelson A, Jefford M, Zorbas H. Recommendations for the identification and management of fear of cancer recurrence in adult cancer survivors. Psychooncology 2014;23(Suppl. 3):109–10.
Swanson S, Dolce A, Marsh K, Summers J, Sheldon LK. Interventions to prevent and treat anxiety: a review of the evidence and implications for practice. Oncol Nurs Forum 2008;35:525.
Szeverenyi C, Kekecs Z, Elkins G, Csernatony Z, Varga K, Johnson A. Psychosocial interventions as adjuncts to orthopedic surgery: a systematic review and meta-analysis. Glob Adv Health Med 2018;7:258–9.
Teo SY, Loy FL. A physiotherapy clinical pathway for the management of chronic neck pain. Ann Acad Med Singap 2015;44(Suppl.):S304.
Thombs BD. Screening for depression in cardiac patients. J Psychosom Res 2010;68:670.
Tirado-Munoz J, Gilchrist G, Torrens M. Intimate partner violence among drug dependent women: state of the art. Heroin Addict Relat Clin Probl 2016;18(3 Suppl. 1):25–6.
Tolin DF. ‘Beating a dead dodo bird: Looking at signal vs. noise in cognitive–behavioral therapy for anxiety disorders’: corrigendum. Clin Psychol Sci Pract 2015;22:315–6.
Towbin K, DeLonga K. 21.1 Integrated treatment of refractory depression in youth: a review of medication, psychological treatments, and barriers to optimal care. J Am Acad Child Adolesc Psychiatry 2018;57:S31.
Trauer J, Qian M, Doyle J, Rajaratnum S, Cunnington D. 180 Efficacy of cognitive behavioural therapy for insomnia: a systematic review and meta-analysis. Sleep Biol Rhythm 2014;12(Suppl.):58.
Troy D, Arora J, Siefferman J. (370) Addressing attention deficits in the treatment of chronic pain: a systematic review. J Pain 2014;15:S68.
van Ameringen M, Patterson B. T92. The use of augmentation strategies in treatment resistant anxiety disorders: a systematic review and meta-analysis. Neuropsychopharmacology 2014;39:S350–1.
van Berkel DM, Van Den Heuvel S, Van Ravensberg D. Effects of specialized psychosomatic physical therapy in patients with stress related health problems: a systematic literature review. Physiotherapy 2011;97(Suppl. 1):eS1277–8.
van der Gaag M, Ising H. Latest developments of psychological psychosis prevention strategies in the Netherlands. Early Interv Psychiatry 2018;12(Suppl. 1):76.
van der Gaag M, Smit F, Bechdolf A, French P, Linszen DH, Yung A, et al. Preventing a first episode of psychosis: a meta-analysis. Schizophr Res 2014;153(Suppl. 1):S42.
van der Gaag M, Smit F, Bechdolf A, French P, Linszen DH, Yung AR, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials. Schizophr Bull 2013;39(Suppl. 1):S356.
van der Gaag M, Velthorst E, Smit F, Meyer C, Koeter M, Fett AK, et al. The battle for ameliorating negative symptoms has a restart. Early Interv Psychiatry 2014;8(Suppl. 1):23.
van der Gaag M, Velthorst E, Smit F, Meyer C, Koeter M, Fett AK, et al. Psychotherapy in people with negative symptoms. Eur Arch Psychiatry Clin Neurosci 2015;265(Suppl.):S40.
van Straten A, Van Der Zweerde T, Morin C, Lancee J. Cognitive and behavioural therapies in the treatment of insomnia: a systematic metaanalysis of all the literature. J Sleep Res 2018;27(Suppl. 1):167.
Vandepitte S, Van Den Noortgate N, Putman K, Verhaeghe S, Faes K, Annemans L. Effectiveness of supporting informal caregivers of people with dementia: a systematic review. Value Health 2015;18(7):A407–8.
Vasa RA. 8.5 Assessment and management of anxiety in youth with ASD. J Am Acad Child Adolesc Psychiatry 2018;57:S134–5.
Verberne D, Spauwen P, Van Heugten C. Neuropsychological interventions for treating neuropsychiatric consequences of acquired brain injury: a systematic review. Brain Injury 2017;31(6–7):797.
Vitinius F, Bassou S, Albus C. Psychotherapeutic and psychiatric interventions after bonemarrow/stemcell transplantation – a systematic review. J Psychosom Res 2011;70:621–2.
Vitinius F, Imlau C, Albus C. Psychosocial strain and therapeutic approaches of hereditary neurological diseases: a systematic review. J Psychosom Res 2013;74:560.
Vitinius F, Piontek K, Albus C. Psychosocial interventions to improve quality of life, depression and anxiety in lung transplant patients – a systematic review of randomized controlled trials. J Psychosom Res 2011;70:620.
Vollm B, Gibbon S, Khalifa N, Duggan C, Stoffers J, Huband N, et al. S08-01-Cochrane reviews of pharmacological and psychological interventions for antisocial personality disorder (ASPD). Eur Psychiatry 2010;25(Suppl. 1):90.
Walling AD. Which treatments are effective for reducing adolescent alcohol abuse? Am Fam Physician 2010;82:532–4.
Wang C, Bayes S. Effectiveness of acupuncture as an add-on treatment for women with postnatal depression: a systematic review. Women Birth 2018;31(Suppl. 1):S27.
Wang Z. Errors in Meta-analysis of trial comparing effectiveness and safety of cognitive behavioral therapy with pharmacotherapy for childhood anxiety disorders. JAMA Pediatr 2018;172:983–4. https://doi.org/10.1001/jamapediatrics.2018.2951
Wearden AJ, Russell C, Emsley R, Fairclough G, Kyle SD. Sleep and fatigue in chronic fatigue syndrome. Psychosom Med 2016;78:A24.
Webb M. A review of web-based applications used to support self-management of non-specific chronic low back pain. Br J Pain 2017;11(Suppl. 2):51–2.
Wendebourg MJ, Heesen C, Finlayson M, Meyer B, Pottgen J, Kopke S. Patient education for people with multiple sclerosis associated fatigue: a systematic review. Mult Scler 2016;22(Suppl. 3):700.
Whale R, Bucur M. Systematic review of randomised interventions for preonset phase psychosis. Early Interv Psychiatry 2010;4(Suppl. 1):116.
White D, Luther L. Efficacy of cognitive behavior therapy in early psychosis. Schizophr Bull 2017;43(Suppl. 1):S209.
Wiener J, Mehta S, Iruthayarajah J, Janssen S, Teasell R. The effectiveness of cognitive behavioural therapy for the management of post-stroke depressive symptoms. Arch Phys Med Rehabil 2017;98:e137.
Williams A, Eccleston C, Morley S. 1012 Systematic review and meta-analysis of psychological treatments for persistent pain in adults, excluding headache. Eur J Pain 2009;13(Suppl. 1):S284.
Williams A, Morley S. Systematic review and meta-analysis of psychological treatments for persistent pain in adults, excluding headache. J Pain 2009;10:S62.
Yoshinaga N, Nosaki A, Unozawa K, Hayashi Y, Shimizu E. A systematic review of cognitive behavioral therapy in nursing field in Japan. Asia Pac Psychiatry 2015;7(Suppl. 1):26.
Zafar Usmani A, Ni Cheng J, Smith BJ, Carson KV. A meta-analysis (Cochrane review) of pharmacological and psychological interventions for anxiety and depression in COPD. Respirology 2010;15(Suppl. 1):A19.
Zangi HA. The evidence for patient education in inflammatory arthritis. Ann Rheum Dis 2014;73(Suppl. 2):55–6.
Studies excluded because duplicates/superceded/withdrawn (n = 170)
Abbott RA, Martin AE, Newlove-Delgado TV, Bethel A, Whear RS, Thompson Coon J, Logan S. Recurrent abdominal pain in children: summary evidence from 3 systematic reviews of treatment effectiveness. J Pediatr Gastroenterol Nutr 2018;67:23–33. https://doi.org/10.1097/MPG.0000000000001922
Aggarwal VR, Lovell K, Peters S, Javidi H, Joughin A, Goldthorpe J. Psychosocial interventions for the management of chronic orofacial pain. Cochrane Database Syst Rev 2011;11:CD008456. https://doi.org/10.1002/14651858.CD008456.pub2
Akechi T, Okuyama T, Onishi J, Morita T, Furukawa TA. WITHDRAWN: Psychotherapy for depression among incurable cancer patients. Cochrane Database Syst Rev 2018;11:CD005537. https://doi.org/10.1002/14651858.CD005537.pub3
Alessi C, Vitiello MV. Insomnia (primary) in older people. BMJ Clin Evid 2011;2011:2302.
Andrews G, Cuijpers P, Craske MG, McEvoy P, Titov N. Computer therapy for the anxiety and depressive disorders is effective, acceptable and practical health care: a meta-analysis. PLOS ONE 2010;5:e13196. https://doi.org/10.1371/journal.pone.0013196
Anie KA, Green J. Psychological therapies for sickle cell disease and pain. Cochrane Database Syst Rev 2002;2:CD001916.
Anie KA, Green J. Psychological therapies for sickle cell disease and pain. Cochrane Database Syst Rev 2012;2:CD001916. https://doi.org/10.1002/14651858.CD001916.pub2
Apóstolo J, Bobrowicz-Campos E, Rodrigues M, Castro I, Cardoso D. The effectiveness of non-pharmacological interventions in older adults with depressive disorders: a systematic review. Int J Nurs Stud 2016;58:59–70.
Arroyo K, Lundahl B, Butters R, Vanderloo M, Wood DS. Short-term interventions for survivors of intimate partner violence: a systematic review and meta-analysis. Trauma Violence Abuse 2015;2.
Ashman L, Duggan L. Interventions for learning disabled sex offenders. Cochrane Database Syst Rev 2002;2:CD003682.
Auclair V, Harvey P-O, Lepage M. La thérapie cognitive-comportementale dans le traitement du TDAH chez l’adulte. Sante Mental Quebec 2016;41:291–311.
Bandelow B, Seidler-Brandler U, Becker A, Wedekind D, Rüther E. Meta-analysis of randomized controlled comparisons of psychopharmacological and psychological treatments for anxiety disorders. World J Biol Psychiatry 2007;8:175–87.
Beelmann A, Losel F. El entrenamiento en habilidades sociales en la prevencion temprana de la delincuencia: los efectos en la conducta antisocial y la competencia social. Psicothema 2006;18:603–10.
Belleville G, Cousineau H, Levrier K, St-Pierre-Delorme MÈ. Meta-analytic review of the impact of cognitive–behavior therapy for insomnia on concomitant anxiety. Clin Psychol Rev 2011;31:638–52. https://doi.org/10.1016/j.cpr.2011.02.004
Beltman MW, Oude Voshaar RC, Speckens AE. Cognitive–behavioural therapy for depression in people with a somatic disease: meta-analysis of randomised controlled trials Br J Psychiatry 2010;197:11–9.
Bender JL, Radhakrishnan A, Diorio C, Englesakis M, Jadad AR. Can pain be managed through the internet? A systematic review of randomized controlled trials. Pain 2011;152:1740–50. https://doi.org/10.1016/j.pain.2011.02.012
Bernardy K, Füber N, Köllner V, Häuser W. Efficacy of cognitive–behavioral therapies in fibromyalgia syndrome – a systematic review and metaanalysis of randomized controlled trials. J Rheumatol 2010;37:1991–2005. https://doi.org/10.3899/jrheum.100104
van Beugen S, Ferwerda M, Hoeve D, Rovers MM, Spillekom-van Koulil S, van Middendorp H, Evers AW. Internet-based cognitive behavioral therapy for patients with chronic somatic conditions: a meta-analytic review. J Med Internet Res 2014;16:e88. https://doi.org/10.2196/jmir.2777
Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2005;2:CD003388. https://doi.org/10.1002/14651858.CD003388.pub2
Bisson J, Andrew M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2007;3:CD003388. https://doi.org/10.1002/14651858.CD003388.pub3
Bisson JI, Ehlers A, Matthews R, Pilling S, Richards D, Turner S. Psychological treatments for chronic post-traumatic stress disorder. Systematic review and meta-analysis. Br J Psychiatry 2007;190:97–104.
Brasure M, MacDonald R, Fuchs E, Olson CM, Carlyle M, Diem S, et al. Management of Insomnia Disorder. Comparative Effectiveness Review No. 159. Report No: 15(16)-EHC027-EF. Rockville, MD: Agency for Healthcare Research and Quality (US); 2015.
Brennan L, Murphy KD, Shaw KA, McKenzie JE. WITHDRAWN: Psychological interventions for overweight or obesity. Cochrane Database Syst Rev 2014;5:CD003818. https://doi.org/10.1002/14651858.CD003818.pub3
Buchanan J, Zakrzewska J. Burning mouth syndrome. BMJ Clin Evid 2008;14:1301.
Buckley LA, Pettit T, Adams CE. Supportive therapy for schizophrenia. Cochrane Database Syst Rev 2007;18:CD004716.
Casacalenda N, Perry JC, Looper K. Remission in major depressive disorder: a comparison of pharmacotherapy, psychotherapy, and control conditions. Am J Psychiatry 2002;159:1354–60. https://doi.org/10.1176/appi.ajp.159.8.1354
Chang CW, Mu PF, Jou ST, Wong TT, Chen YC. The effectiveness of non-pharmacological interventions on fatigue in children and adolescents with cancer: a systematic review. JBI Libr Syst Rev 2012;10:574–614. https://doi.org/10.11124/jbisrir-2012-60
Chi NC, Demiris G, Lewis FM, Walker AJ, Langer SL. Behavioral and educational interventions to support family caregivers in end-of-life care: a systematic review. Am J Hosp Palliat Care 2016;33:894–908.
Cleary M, Hunt G, Matheson S, Siegfried N, Walter G. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database Syst Rev 2008;1:CD001088. https://doi.org/10.1002/14651858.CD001088.pub2
Cormac I, Jones C, Campbell C. Cognitive behaviour therapy for schizophrenia. Cochrane Database Syst Rev 2002;1:CD000524.
Covin R, Ouimet AJ, Seeds PM, Dozois DJ. A meta-analysis of CBT for pathological worry among clients with GAD. J Anxiety Disord 2008;22:108–16.
Cuijpers P, van Straten A, Andersson G. Internet-administered cognitive behavior therapy for health problems: a systematic review. J Behav Med 2008;31:169–77. https://doi.org/10.1007/s10865-007-9144-1
Cuijpers P, van Straten A, Smit F. Psychological treatment of late-life depression: a meta-analysis of randomized controlled trials. Int J Geriatr Psychiatry 2006;21:1139–49. https://doi.org/10.1002/gps.1620
Cuijpers P, van Straten A, Warmerdam L, Andersson G. Psychotherapy versus the combination of psychotherapy and pharmacotherapy in the treatment of depression: a meta-analysis. Depress Anxiety 2009;26:279–88. https://doi.org/10.1002/da.20519
de Arellano MA, Lyman DR, Jobe-Shields L, George P, Dougherty RH, Daniels AS, et al. Trauma-focused cognitive–behavioral therapy for children and adolescents: assessing the evidence. Psychiatr Serv 2014;65:591–602. https://doi.org/10.1176/appi.ps.201300255
Driessen E, Cuijpers P, Hollon SD, Van HL, Dekker JJ. [The efficacy of psychological treatments for depression: a review of recent research findings.] Tijdschr Psychiatr 2014;56:455–62.
Eccleston C, Williams AC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2009;2:CD007407. https://doi.org/10.1002/14651858.CD007407.pub2
Edwards AG, Hailey S, Maxwell M. Psychological interventions for women with metastatic breast cancer. Cochrane Database Syst Rev 2004;2:CD004253. https://doi.org/10.1002/14651858.CD004253.pub2
Edwards AG, Hulbert-Williams N, Neal RD. Psychological interventions for women with metastatic breast cancer. Cochrane Database Syst Rev 2008;3:CD004253. https://doi.org/10.1002/14651858.CD004253.pub3
Elderton AJ. Posttraumatic Growth in Survivors of Interpersonal Violence in Adulthood. PhD thesis. Oxford: University of Oxford; 2013.
Ewing DL, Monsen JJ, Thompson EJ, Cartwright-Hatton S, Field A. A meta-analysis of transdiagnostic cognitive behavioural therapy in the treatment of child and young person anxiety disorders. Behav Cogn Psychother 2015;43:562–77. https://doi.org/10.1017/S1352465813001094
Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol 2014;109:1350–65. https://doi.org/10.1038/ajg.2014.148
Furlong M, McGilloway S, Bywater T, Hutchings J, Smith SM, Donnelly M. Behavioural and cognitive–behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years. Cochrane Database Syst Rev 2012;2:CD008225.
Galsworthy-Francis L. The Development and Exploration of the Experiences of Humiliation Scale (EHS) in an Eating Disordered Population. Leicester: University of Leicester; 2012.
Gandy M, Sharpe L, Perry KN. Cognitive behavior therapy for depression in people with epilepsy: a systematic review. Epilepsia 2013;54:1725–34. https://doi.org/10.1111/epi.12345
Gartlehner G, Gaynes BN, Amick HR, Asher GN, Morgan LC, Coker-Schwimmer E, et al. Comparative benefits and harms of antidepressant, psychological, complementary, and exercise treatments for major depression: an evidence report for a clinical practice guideline from the American College of Physicians. Ann Intern Med 2016;164:331–41. https://doi.org/10.7326/M15-1813
Gómez Puente JM, Martínez-Marcos M. Sobrepeso y obesidad: eficacia de las intervenciones en adultos. Enferm Clin 2018;28:65–74.
Goschwitz R, Plener P. Psychotherapeutic interventions for non-suicidal self-injury. Nervenheilkd Z Interdiszip Fortbild 2013;32:30–6.
Gregory VL. Cognitive–behavioral therapy for comorbid bipolar and substance use disorders: a systematic review of controlled trials. Ment Health Subst Use 2011;4:302–13.
Haniffa M, Lasserson TJ, Smith I. Interventions to improve compliance with continuous positive airway pressure for obstructive sleep apnoea. Cochrane Database Syst Rev 2004;4:CD003531. https://doi.org/10.1002/14651858.CD003531.pub2
Hay PJ, Bacaltchuk J. Psychotherapy for bulimia nervosa and binging. Cochrane Database Syst Rev 2000;2:CD000562.
Hay PJ, Bacaltchuk J. Psychotherapy for bulimia nervosa and binging. Cochrane Database Syst Rev 2001;3:CD000562.
Hay PJ, Bacaltchuk J. Psychotherapy for bulimia nervosa and binging. Cochrane Database Syst Rev 2003;1:CD000562. https://doi.org/10.1002/14651858.CD000562
Hay PJ, Bacaltchuk J. Bulimia nervosa. BMJ Clin Evid 2008;2008:1009.
Hay PJ, Bacaltchuk J, Stefano S. Psychotherapy for bulimia nervosa and binging. Cochrane Database Syst Rev 2004;3:CD000562. https://doi.org/10.1002/14651858.CD000562.pub2
Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2008;2:CD000011. https://doi.org/10.1002/14651858.CD000011.pub3
Hazell P. Depression in children and adolescents. BMJ Clin Evid 2011;2011:1008.
Hetrick SE, Cox GR, Merry SN. Treatment-resistant depression in adolescents: is the addition of cognitive behavioral therapy of benefit? Psychol Res Behav Manag 2011;4:97–112.
Hetrick SE, Cox GR, Merry SN. Where to go from here? An exploratory meta-analysis of the most promising approaches to depression prevention programs for children and adolescents. Int J Environ Res Public Health 2015;12:4758–95. https://doi.org/10.3390/ijerph120504758
Hjorthøj C, Fohlmann A, Nordentoft M. Treatment of cannabis use disorders in people with schizophrenia spectrum disorders – a systematic review. Addict Behav 2009;34:520–5. https://doi.org/10.1016/j.addbeh.2009.02.001
Ho BPV, Carter M, Stephenson J. Anger management using a cognitive–behavioural approach for children with special education needs: a literature review and meta-analysis. Int J Disab Dev Educ 2010;57:245–65.
Hofmann SG, Sawyer AT, Korte KJ, Smits JA. Is it beneficial to add pharmacotherapy to cognitive–behavioral therapy when treating anxiety disorders? A meta-analytic review. Int J Cogn Ther 2009;2:160–75.
Hofmann SG, Smits JA. Cognitive–behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry 2008;69:621–32.
Huang FF, Li ZJ, Han HY, Xiong HF, Ma Y, et al. Cognitive behavioral therapy combined with pharmacotherapy for obsessive compulsive disorder: a meta-analysis. Chin Ment Health J 2013;27:643–9.
Huertas-Ceballos AA, Logan S, Bennett C, Macarthur C. WITHDRAWN: Psychosocial interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database Syst Rev 2014;2:CD003014. https://doi.org/10.1002/14651858.CD003014.pub3
Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol 2006;25:3–14.
James A, Soler A, Weatherall R. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev 2005;4:CD004690. https://doi.org/10.1002/14651858.CD004690.pub2
James AC, James G, Cowdrey FA, Soler A, Choke A. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev 2013;6:CD004690. https://doi.org/10.1002/14651858.CD004690.pub3
Jones C, Cormac I, Mota J, Campbell C. Cognitive behaviour therapy for schizophrenia. Cochrane Database Syst Rev 2000;2:CD000524.
Jones C, Cormac I, Silveira da Mota Neto JI, Campbell C. Cognitive behaviour therapy for schizophrenia. Cochrane Database Syst Rev 2004;4:CD000524.
Jones C, Hacker D, Meaden A, Cormac I, Irving CB. WITHDRAWN: Cognitive behaviour therapy versus other psychosocial treatments for schizophrenia. Cochrane Database Syst Rev 2011;4:CD000524. https://doi.org/10.1002/14651858.CD000524.pub3
Jones C, Hacker D, Cormac I, Meaden A, Irving CB. Cognitive behaviour therapy versus other psychosocial treatments for schizophrenia. Cochrane Database Syst Rev 2012;4:CD008712. https://doi.org/10.1002/14651858.CD008712.pub2
Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J. A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety. Health Technol Assess 2002;6(22). https://doi.org/10.3310/hta6220
Kavanagh J, Oliver S, Lorenc T, Caird J, Tucker H, Harden A, et al. School-based cognitive–behavioural interventions: a systematic review of effects and inequalities. Health Sociol Rev 2009;18:61–78.
Kenworthy T, Adams CE, Bilby C, Brooks-Gordon B, Fenton M. WITHDRAWN: Psychological interventions for those who have sexually offended or are at risk of offending. Cochrane Database Syst Rev 2008;4:CD004858. https://doi.org/10.1002/14651858.CD004858.pub2
Kisely S, Campbell LA, Skerritt P. Psychological interventions for symptomatic management of non-specific chest pain in patients with normal coronary anatomy. Cochrane Database Syst Rev 2005;1:CD004101. https://doi.org/10.1002/14651858.CD004101.pub2
Kisely SR, Campbell LA, Skerritt P, Yelland MJ. Psychological interventions for symptomatic management of non-specific chest pain in patients with normal coronary anatomy. Cochrane Database Syst Rev 2010;1:CD004101. https://doi.org/10.1002/14651858.CD004101.pub3
Kisely SR, Campbell LA, Yelland MJ, Paydar A. Psychological interventions for symptomatic management of non-specific chest pain in patients with normal coronary anatomy. Cochrane Database Syst Rev 2012;6:CD004101. https://doi.org/10.1002/14651858.CD004101.pub4
Klimas J, Field CA, Cullen W, O’Gorman CS, Glynn LG, Keenan E, et al. Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users. Cochrane Database Syst Rev 2012;11:CD009269. https://doi.org/10.1002/14651858.CD009269.pub2
Klimas J, Field CA, Cullen W, O’Gorman CS, Glynn LG, Keenan E, et al. Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users: Cochrane Review. Syst Rev 2013;2:3. https://doi.org/10.1186/2046-4053-2-3
Köllner V, Häuser W, Klimczyk K, Kühn-Becker H, Settan M, Weigl M, Bernardy K, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften. [Psychotherapy for patients with fibromyalgia syndrome. Systematic review, meta-analysis and guideline.] Schmerz 2012;26:291–6. https://doi.org/10.1007/s00482-012-1179-8
Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med 2007;69:881–8.
Larkin D, Lopez V, Aromataris E. Non-pharmacological interventions for cancer-related fatigue in men treated for prostate cancer: a systematic review. JBI Libr Syst Rev 2012;10:3764–811.
Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev 2015;2:CD003200. https://doi.org/10.1002/14651858.CD003200.pub3
Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev 2016;12:CD003200.
Lewandowski LM, Gebing TA, Anthony JL, O’Brien WH. Meta-analysis of cognitive–behavioral treatment studies for bulimia. Clin Psychol Rev 1997;17:703–18.
Linde K, Sigterman K, Kriston L, Rücker G, Jamil S, Meissner K, Schneider A. Effectiveness of psychological treatments for depressive disorders in primary care: systematic review and meta-analysis. Ann Fam Med 2015;13:56–68. https://doi.org/10.1370/afm.1719
Lip GY, Lane DA, Millane TA, Tayebjee MH. Psychological interventions for depression in adolescent and adult congenital heart disease. Cochrane Database Syst Rev 2003;3:CD004394. https://doi.org/10.1002/14651858.CD004394
Liu HX, Liang QJ, Xiao P, Jiao HX, Gao Y, Ahmetjiang A. The effectiveness of cognitive–behavioural therapy for temporomandibular disorders: a systematic review. J Oral Rehabil 2012;39:55–62. https://doi.org/10.1111/j.1365-2842.2011.02239.x
Lockwood C, Page T, NursCert H, Conroy-Hiller T. Effectiveness of individual therapy and group therapy in the treatment of schizophrenia. JBI Libr Syst Rev 2004;2:1–44.
Macdonald G, Higgins JP, Ramchandani P, Valentine JC, Bronger LP, Klein P, et al. Cognitive–behavioural interventions for children who have been sexually abused. Cochrane Database Syst Rev 2012;5:CD001930. https://doi.org/10.1002/14651858.CD001930.pub3
Macdonald GM, Higgins JP, Ramchandani P. Cognitive–behavioural interventions for children who have been sexually abused. Cochrane Database Syst Rev 2006;4:CD001930. https://doi.org/10.1002/14651858.CD001930.pub2
Macea DD, Gajos K, Daglia Calil YA, Fregni F. The efficacy of Web-based cognitive behavioral interventions for chronic pain: a systematic review and meta-analysis. J Pain 2010;11:917–29. https://doi.org/10.1016/j.jpain.2010.06.005
Malouff JM, Thorsteinsson EB, Rooke SE, Bhullar N, Schutte NS. Efficacy of cognitive behavioral therapy for chronic fatigue syndrome: a meta-analysis. Clin Psychol Rev 2008;28:736–45.
Marshall M, Lockwood A. Early intervention for psychosis. Cochrane Database Syst Rev 2004;2:CD004718.
Marson AG, Maguire M, Ramaratnam S. Epilepsy. BMJ Clin Evid 2009;2009:1201.
Matthews EE, Arnedt JT, McCarthy MS, Cuddihy LJ, Aloia MS. Adherence to cognitive behavioral therapy for insomnia: a systematic review. Sleep Med Rev 2013;17:453–64. https://doi.org/10.1016/j.smrv.2013.01.001
Mayo-Wilson E. Media-delivered Behavioural and Cognitive Behavioural Therapy for Anxiety: A Systematic Review of Effectiveness and an Exploratory Study of Consumer Preferences. PhD thesis. Oxford: Oxford University; 2011.
Mayo-Wilson E, Montgomery P. Media-delivered cognitive behavioural therapy and behavioural therapy (self-help) for anxiety disorders in adults. Cochrane Database Syst Rev 2013;9:CD005330. https://doi.org/10.1002/14651858.CD005330.pub4
McDaid C, Trowman R, Golder S, Hawton K, Sowden A. Interventions for people bereaved through suicide: systematic review. Br J Psychiatry 2008;193:438–43. https://doi.org/10.1192/bjp.bp.107.040824
Meyer T, Hautzinger M. Cognitive behavioral therapy in addition to pharmacotherapy for manic depressive disorders: empirical results. Der Nervenarzt 2002;73:620–8.
Montgomery P, Dennis J. Cognitive behavioural interventions for sleep problems in adults aged 60+. Cochrane Database Syst Rev 2002;2:CD003161.
Montgomery P, Dennis J. A systematic review of non-pharmacological therapies for sleep problems in later life. Sleep Med Rev 2004;8:47–62. https://doi.org/10.1016/S1087-0792(03)00026-1
Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review. Sleep 1999;22:1134–56. https://doi.org/10.1093/sleep/22.8.1134
Ng TK, Wong DFK. The efficacy of cognitive behavioral therapy for Chinese people: a meta-analysis. Aust N Z J Psychiatry 2018;52:620–37. https://doi.org/10.1177/0004867417741555
Oakley-Browne MA, Adams P, Mobberley PM. Interventions for pathological gambling. Cochrane Database Syst Rev 2000;2:CD001521.
Oakley-Browne MA, Adams P, Mobberley PM. WITHDRAWN: Interventions for pathological gambling. Cochrane Database Syst Rev 2007;1:CD001521. https://doi.org/10.1002/14651858.CD001521.pub2
Oei TP, Dingle G. The effectiveness of group cognitive behaviour therapy for unipolar depressive disorders. J Affect Disord 2008;107:5–21.
O’Kearney R. Benefits of cognitive–behavioural therapy for children and youth with obsessive–compulsive disorder: re-examination of the evidence. Aust N Z J Psychiatry 2007;41:199–212.
Orgeta V, Qazi A, Spector A, Orrell M. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry 2015;207:293–8. https://doi.org/10.1192/bjp.bp.114.148130
Parsons AC, Shraim M, Inglis J, Aveyard P, Hajek P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev 2009;1:CD006219. https://doi.org/10.1002/14651858.CD006219.pub2
Passarela CDM, Mendes DD, De Jesus Mari J. A systematic review to study the efficacy of cognitive behavioral therapy for sexually abused children and adolescents with posttraumatic stress disorder. Rev Psiquiatr Clin 2010;37:63–73.
Perveen T, Mahmood S, Gosadi I, Mehraj J, Sheikh SS. Long term effectiveness of cognitive behavior therapy for treatment of postpartum depression: a systematic review and meta-analysis. J Pak Med Stud 2013;3:198–204.
Pignone M, Gaynes BN, Rushton JL, Mulrow CD, Orleans CT, Whitener BL, et al. Screening for depression. Systematic Evidence Reviews, No. 6. Rockville, MD: Agency for Healthcare Research and Quality; 2002.
Pinquart M, Sörensen S. How effective are psychotherapeutic and other psychosocial interventions with older adults? J Ment Health Aging 2001;7:207–43.
Ponniah K, Magiati I, Hollon SD. An update on the efficacy of psychological therapies in the treatment of obsessive–compulsive disorder in adults. J Obsessive Compuls Relat Disord 2013;2:207–18. https://doi.org/10.1016/j.jocrd.2013.02.005
Pratt HD. Psychotherapy in the age of pharmacology. Int J Child Adolesc Health 2010;3:137–42.
Pratt HD. Psychotherapy in the Age of Pharmacology. In Merrick J, editor. Child and Adolescent Health Yearbook. New York, NY: Noval Science Publishers, Inc.; 2012. pp. 149–56.
Price JR, Couper J. Cognitive behaviour therapy for adults with chronic fatigue syndrome. Cochrane Database Syst Rev 2000;2:CD001027.
Ramaratnam S, Baker GA, Goldstein L. Psychological treatments for epilepsy. Cochrane Database Syst Rev 2001;4:CD002029.
Ramaratnam S, Baker GA, Goldstein L. Psychological treatments for epilepsy. Cochrane Database Syst Rev 2003;4:CD002029.
Ramaratnam S, Baker GA, Goldstein LH. Psychological treatments for epilepsy. Cochrane Database Syst Rev 2005;19:CD002029.
Ramaratnam S, Baker GA, Goldstein LH. Psychological treatments for epilepsy. Cochrane Database Syst Rev 2008;16:CD002029.
Ramaratnam S, Baker GA, Goldstein LH. Psychological treatments for epilepsy. Cochrane Database Syst Rev 2016;25:CD002029.
Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis 2012;200:832–9. https://doi.org/10.1097/NMD.0b013e31826dd9af
Reid SF, Chalder T, Cleare A, Hotopf M, Wessely S. Chronic fatigue syndrome. BMJ Clin Evid 2008;2008:1101.
Rimer J, Dwan K, Lawlor DA, Greig CA, McMurdo M, Morley W, et al. Exercise for depression. Cochrane Database Syst Rev 2012;7:CD004366.
Roberts NP, Roberts PA, Jones N, Bisson JI. Psychological interventions for post-traumatic stress disorder and comorbid substance use disorder: a systematic review and meta-analysis. Clin Psychol Rev 2015;38:25–38. https://doi.org/10.1016/j.cpr.2015.02.007
Schirmbeck F, Zink M. Cognitive behavioural therapy for obsessive-compulsive symptoms in schizophrenia. Cogn Behav Ther 2013;6:1–13.
Schmid G, Henningsen P, Dieterich M, Sattel H, Lahmann C. Psychotherapy in dizziness: a systematic review. J Neurol Neurosurg Psychiatry 2011;82:601–6.
Segal ZV, Whitney DK, Lam RW. Psychotherapy. Can J Psychiatry 2001;46:29S–37S.
Shaw K, O’Rourke P, Del Mar C, Kenardy J. Psychological interventions for overweight or obesity. Cochrane Database Syst Rev 2005;2:CD003818. https://doi.org/10.1002/14651858.CD003818.pub2
Silver N. Headache (chronic tension-type). BMJ Clin Evid 2007;2007:1205.
Sin J, Spain D. Psychological interventions for trauma in individuals who have psychosis: a systematic review and meta-analysis. Psychos Psychol Soc Integr Approach 2017;9:67–81.
Smedslund G, Dalsbø TK, Steiro AK, Winsvold A, Clench-Aas J. Cognitive behavioural therapy for men who physically abuse their female partner. Cochrane Database Syst Rev 2007;3:CD006048. https://doi.org/10.1002/14651858.CD006048.pub2
Soo S, Forman D, Delaney BC, Moayyedi P. A systematic review of psychological therapies for nonulcer dyspepsia. Am J Gastroenterol 2004;99:1817–22. https://doi.org/10.1111/j.1572-0241.2004.30086.x
Soo S, Moayyedi P, Deeks J, Delaney B, Lewis M, Forman D. Psychological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev 2001;4:CD002301.
Soo S, Moayyedi P, Deeks J, Delaney B, Lewis M, Forman D. Psychological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev 2004;1:CD002301. https://doi.org/10.1002/14651858.CD002301.pub2
Soo S, Moayyedi P, Deeks J, Delaney B, Lewis M, Forman D. Psychological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev 2005;2:CD002301. https://doi.org/10.1002/14651858.CD002301.pub4
Soo S, Moayyedi P, Deeks JJ, Delaney B, Lewis M, Forman D. WITHDRAWN: Psychological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev 2011;2:CD002301. https://doi.org/10.1002/14651858.CD002301.pub5
Soo S, Moayyedi P, Deeks Jonathan J, Delaney B, Lewis M, Forman D. Psychological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev 2011;16:CD002301.
Soomro GM. Obsessive compulsive disorder. BMJ Clin Evid 2007;2007:1004.
Spek V, Cuijpers P, Nyklícek I, Riper H, Keyzer J, Pop V. Internet-based cognitive behaviour therapy for symptoms of depression and anxiety: a meta-analysis. Psychol Med 2007;37:319–28.
Spence JD, Barnett PA, Linden W, Ramsden V, Taenzer P. Lifestyle modifications to prevent and control hypertension. 7. Recommendations on stress management. Canadian Hypertension Society, Canadian Coalition for High Blood Pressure Prevention and Control, Laboratory Centre for Disease Control at Health Canada, Heart and Stroke Foundation of Canada. CMAJ 1999;160(Suppl. 9):46–50.
Stratford HJ. Anxiety and Bipolar Spectrum Disorders: Psychological Treatments and Mental Imagery. PhD thesis. Oxford: University of Oxford; 2013.
Sumathipala A. What is the evidence for the efficacy of treatments for somatoform disorders? A critical review of previous intervention studies. Psychosom Med 2007;69:889–900.
Summerbell CD, Ashton V, Campbell KJ, Edmunds L, Kelly S, Waters E. Interventions for treating obesity in children. Cochrane Database Syst Rev 2003;3:CD001872. https://doi.org/10.1002/14651858.CD001872
Sztein DM, Koransky CE, Fegan L, Himelhoch S. Efficacy of cognitive behavioural therapy delivered over the internet for depressive symptoms: a systematic review and meta-analysis. J Telemed Telecare 2018;24:527–39. https://doi.org/10.1177/1357633X17717402
Liu TW, Ng GYF, Chung RCK, Ng SSM. Cognitive behavioural therapy for fear of falling and balance among older people: a systematic review and meta-analysis. Age Ageing 2018;47:520–7. https://doi.org/10.1093/ageing/afy010
Tatrow K, Montgomery GH. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. J Behav Med 2006;29:17–27. https://doi.org/10.1007/s10865-005-9036-1
Thakur ER, Shapiro J, Chan J, Lumley MA, Cully JA, Bradford A, El-Serag HB. A systematic review of the effectiveness of psychological treatments for IBS in gastroenterology settings: promising but in need of further study. Dig Dis Sci 2018;63:2189–201. https://doi.org/10.1007/s10620-018-5095-3
Torchalla I, Strehlau V. The evidence base for interventions targeting individuals with work-related PTSD: a systematic review and recommendations. Behav Modif 2018;42:273–303. https://doi.org/10.1177/0145445517725048
Turner DT, van der Gaag M, Karyotaki E, Cuijpers P. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry 2014;171:523–38. https://doi.org/10.1176/appi.ajp.2013.13081159
Turner W, Macdonald GM, Dennis JA. Cognitive–behavioural training interventions for assisting foster carers in the management of difficult behaviour. Cochrane Database Syst Rev 2005;2:CD003760. https://doi.org/10.1002/14651858.CD003760.pub2
Turner W, Macdonald GM, Dennis JA. Cognitive–behavioural training interventions for assisting foster carers in the management of difficult behaviour. Cochrane Database Syst Rev 2007;1:CD003760. https://doi.org/10.1002/14651858.CD003760.pub3
Uman LS. Psychological interventions for needle-related procedural pain and distress in children and adolescents: A systematic review and quality analysis. PhD thesis. Halifax, NS: Dalhousie University; 2010.
Uman LS, Chambers CT, McGrath PJ, Kisely S. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev 2006;4:CD005179. https://doi.org/10.1002/14651858.CD005179.pub2
Uman LS, Chambers CT, McGrath PJ, Kisely S. A systematic review of randomized controlled trials examining psychological interventions for needle-related procedural pain and distress in children and adolescents: an abbreviated cochrane review. J Pediatr Psychol 2008;33:842–54. https://doi.org/10.1093/jpepsy/jsn031
van Balkom AJ, Bakker A, Spinhoven P, Blaauw BM, Smeenk S, Ruesink B. A meta-analysis of the treatment of panic disorder with or without agoraphobia: a comparison of psychopharmacological, cognitive-behavioral, and combination treatments. J Nerv Ment Dis 1997;185:510–16. https://doi.org/10.1097/00005053-199708000-00006
Veronese A, Hunot V, Cipriani A, Churchill R, Barbui C. Psychological therapies versus pharmacotherapy for obsessive compulsive disorder. Cochrane Database Syst Rev 2008;3:CD007319.
Vulink N, Denys D. Body dysmorphic disorder: an overview. Tijdschriftvoor Psychiatrie 2005;47:21–7.
Waddell C, Hua JM, Garland OM, Peters RD, McEwan K. Preventing mental disorders in children. Can J Public Health 2007;98:166–73.
Warren Z, Veenstra-VanderWeele J, Stone W, Bruzek JL, Nahmias AS, Foss-Feig JH, et al. Therapies for Children with Autism Spectrum Disorders. Comparative Effectiveness Review No. 26. Report No: 11-EHC029-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
Windmill J, Fisher E, Eccleston C, Derry S, Stannard C, Knaggs R, Moore RA. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev 2013;9:CD010323. https://doi.org/10.1002/14651858.CD010323.pub2
Yorke J, Fleming SL, Shuldham C. Psychological interventions for adults with asthma: a systematic review. Respir Med 2007;101:1–14.
Zakrzewska JM, Forssell H, Glenny AM. Interventions for the treatment of burning mouth syndrome: a systematic review. J Orofac Pain 2003;17:293–300.
Zakrzewska JM, Glenny AM, Forssell H. Interventions for the treatment of burning mouth syndrome. Cochrane Database Syst Rev 2001;3:CD002779.
Studies excluded as no cognitive–behavioural therapy in title/abstract/keywords or not relevant to the overview (n = 14)
Alharbi F, el-Guebaly N. Disulfiram: the survivor medication. Addict Disord Treat 2012;11:212–23.
Allen LA, Escobar JI, Lehrer PM, Gara MA, Woolfolk RL. Psychosocial treatments for multiple unexplained physical symptoms: a review of the literature. Psychosom Med 2002;64:939–50. https://doi.org/10.1097/01.psy.0000024231.11538.8f
Anderson EM, Lambert MJ. Short-term dynamically oriented psychotherapy: a review and meta-analysis. Clin Psychol Rev 1995;15:503–14.
Anonymous. Exam 2: efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review. Clin Gastroenterol Hepatol 2016;14:e20.
Berglund M. A better widget? Three lessons for improving addiction treatment from a meta-analytical study. Addiction 2005;100:742–50.
Bloom K, Tam JA. Walk-in services for child and family mental health. J Syst Ther 2015;34:61–77.
Brown M, O’Neill N, van Woerden H, Eslambolchilar P, Jones M, John A. Gamification and adherence to web-based mental health interventions: a systematic review. JMIR Ment Health 2016;3:e39. https://doi.org/10.2196/mental.5710
Chakraborty K, Basu D, Vijaya Kumar KG. Internet addiction: consensus, controversies, and the way ahead. East Asian Arch Psychiatry 2010;20:123–32.
Clark GI, Rock AJ. Processes contributing to the maintenance of flying phobia: a narrative review. Front Psychol 2016;7:754. https://doi.org/10.3389/fpsyg.2016.00754
Daker-White G, Rogers A. What is the potential for social networks and support to enhance future telehealth interventions for people with a diagnosis of schizophrenia: a critical interpretive synthesis. BMC Psychiatry 2013;13:279. https://doi.org/10.1186/1471-244X-13-279
Edwards CJ, Cella M, Tarrier N, Wykes T. Investigating the empirical support for therapeutic targets proposed by the temporal experience of pleasure model in schizophrenia: a systematic review. Schizophr Res 2015;168:120–44. https://doi.org/10.1016/j.schres.2015.08.013
Folmer RL, Theodoroff SM, Martin WH, Shi Y. Experimental, controversial, and futuristic treatments for chronic tinnitus. J Am Acad Audiol 2014;25:106–25. https://doi.org/10.3766/jaaa.25.1.7
Marks R, Allegrante JP. Effectiveness of psychoeducational interventions in osteoarthritis. Crit Rev Phys Rehabil Med 2002;14:173–95.
Rozental A, Bennett S, Forsström D, Ebert DD, Shafran R, Andersson G, Carlbring P. Targeting procrastination using psychological treatments: a systematic review and meta-analysis. Front Psychol 2018;9:1588. https://doi.org/10.3389/fpsyg.2018.01588
Studies excluded as not able to obtain full text (n = 17)
Anders M, Christiansen H. Unaccompanied refugee minors: a systematic review of psychological interventions. Kindh Entwickl Z Klin Kinderpsychol 2016;25:216–30.
Åslund L, Arnberg F, Kanstrup M, Lekander M. Cognitive and behavioral interventions to improve sleep in school-age children and adolescents: a systematic review and meta-analysis. J Clin Sleep Med 2018;14:1937–47. https://doi.org/10.5664/jcsm.7498
Cardi V, Treasure J. Treatments in eating disorders: towards future directions. Minerva Psichiatrica 2010;51:191–206.
Chang CW, Mu PF, Jou ST, Wong TT, Chen YC. The effectiveness of non-pharmacological interventions on fatigue in children and adolescents with cancer: a systematic review. JBI Libr Syst Rev 2012;10:574–614. https://doi.org/10.11124/jbisrir-2012-60
Corbière M, Shen J. A systematic review of psychological return-to-work interventions for people with mental health problems and/or physical injuries. Can J Community Ment Health 2006;25:261–88.
de Wit N, Rubin G, Jones RH. Irritable bowel syndrome. BMJ Clin Evid 2007;2007:0410.
DiMauro J. Exposure therapy for posttraumatic stress disorder: a meta-analysis. Mil Psychol 2014;26:120–30.
Gherman A, David D. Are psychological interventions effective in diabetes care? A quantitative meta-analysis. Erdelyi Pszichologiai Szemle 2011;12:155–69.
Gratton S. Evaluation of a Computer-based CBT Package for Exam Anxiety. PhD thesis. Guildford: University of Surrey; 2007.
Heaton K. Men with Intellectual Disabilities who Display Sexually Abusive Behaviour. PhD thesis. Lancaster: Lancaster University; 2010.
Hedgpeth NL. Systematic Review of Psychosocial Interventions for Anxiety in Adult Cancer Patients. PhD thesis. DNPc; 2012.
Kinsella PJ. Paranoid Ideation after Traumatic Brain Injury: An Exploration of Related Factors. PhD thesis. Bangor: Bangor University; 2012.
Larkin D, Lopez V, Aromataris E. Non-pharmacological interventions for cancer-related fatigue in men treated for prostate cancer: a systematic review. JBI Libr Syst Rev 2012;10:3764–811.
Liu Z. The Effectiveness of Cognitive Behavioural Interventions for the Psychological Morbidity of Dementia Family Caregivers: A Systematic Review and Meta-analysis. PhD thesis. Hong Kong: The Chinese University of Hong Kong; 2017.
Nicoll M. Anger in Offenders with Intellectual Disabilities. PhD thesis. Sheffield: University of Sheffield; 2011.
Paskins RT, Brady AM, Schultz JC. Applied interventions in college settings to assist adult students with autism spectrum disorder: a systematic review of the literature. J Appl Rehabil Counsel 2018;49:39–45.
Pratt HD. Psychotherapy in the Age of Pharmacology. In Greydanus DE, Calles JL Jr, Patel DR, Nazeer A, Merrick J, editors. Clincial Aspects of Psychopharmacology in Childhood and Adolescence. 2nd edn. Hauppauge, NY: Nova Science Publishers; 2017. pp. 83–91.
Studies excluded because overviews of systematic reviews (n = 44)
Aguiluz J, Álvarez M, Pimentel E, Abarca C, Moore P. How to face a patient with benzodiazepine dependence in primary health care? Strategies for withdrawal. Medwave 2018;18:e7159. https://doi.org/10.5867/medwave.2018.01.7159
Astin JA, Shapiro SL, Eisenberg DM, Forys KL. Mind–body medicine: state of the science, implications for practice. J Am Board Fam Pract 2003;16:131–47.
Barlow JH, Ellard DR. Psycho-educational interventions for children with chronic disease, parents and siblings: an overview of the research evidence base. Child Care Health Dev 2004;30:637–45.
Bennett K, Manassis K, Duda S, Bagnell A, Bernstein GA, Garland EJ, et al. Treating child and adolescent anxiety effectively: overview of systematic reviews. Clin Psychol Rev 2016;50:80–94.
Bhui KS, Dinos S, Stansfeld SA, White PD. A synthesis of the evidence for managing stress at work: a review of the reviews reporting on anxiety, depression, and absenteeism. J Environ Public Health 2012;2012:515874. https://doi.org/10.1155/2012/515874
Bidonde J, Menseses J. The Effect of Interventions for Children who have Experienced Violence in Close Relationships: An Overview of Reviews. Oslo: The Norwegian Institute of Public Health; 2017.
Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive–behavioral therapy: a review of meta-analyses. Clin Psychol Rev 2006;26:17–31.
Buysse DJ. Insomnia. JAMA 2013;309:706–16.
Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med 2007;147:492–45. [Erratum published in Ann Intern Med 2008 Feb 5;148(3):247].
Cislak A, Safron M, Pratt M, Gaspar T, Luszczynska A. Family-related predictors of body weight and weight-related behaviours among children and adolescents: a systematic umbrella review. Child Care Health Dev 2012;38:321–31. https://doi.org/10.1111/j.1365-2214.2011.01285.x
Cox G, Hetrick S. Psychosocial interventions for self-harm, suicidal ideation and suicide attempt in children and young people: What? How? Who? And Where? Evid Based Ment Health 2017;20:35–40.
Crowe K, McKay D. Efficacy of cognitive–behavioral therapy for childhood anxiety and depression. J Anxiety Disord 2017;49:76–87.
Das JK, Salam RA, Lassi ZS, Khan MN, Mahmood W, Patel V, Bhutta ZA. Interventions for adolescent mental health: an overview of systematic reviews. J Adolesc Health 2016;59:S49–S60.
Dragioti E, Dimoliatis I, Fountoulakis KN, Evangelou E. A systematic appraisal of allegiance effect in randomized controlled trials of psychotherapy. Ann Gen Psychiatry 2015;14:25. https://doi.org/10.1186/s12991-015-0063-1
Dragioti E, Karathanos V, Gerdle B, Evangelou E. Does psychotherapy work? An umbrella review of meta-analyses of randomized controlled trials. Acta Psychiatr Scand 2017;136:236–46. https://doi.org/10.1111/acps.12713
Driot D, Bismuth M, Maurel A, Soulie-Albouy J, Birebent J, Oustric S, et al. Management of first depression or generalized anxiety disorder episode in adults in primary care: a systematic meta review. La Presse Medicale 2017;46:1124–38.
Dufour S, Chamberland C. The effectiveness of selected interventions for previous maltreatment: enhancing the well-being of children who live at home. Child Fam Soc Work 2004;9:39–56.
Duncan M, Moschopoulou E, Herrington E, Deane J, Roylance R, Jones L, et al. Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open 2017;7:e015860. https://doi.org/10.1136/bmjopen-2017-015860
Eccleston C, Morley SJ, Williams AC. Psychological approaches to chronic pain management: evidence and challenges. Br J Anaesth 2013;111:59–63. https://doi.org/10.1093/bja/aet207
Enns J, Holmqvist M, Wener P, Halas G, Rothney J, Schultz A, et al. Mapping interventions that promote mental health in the general population: a scoping review of reviews. Prev Med 2016;87:70–80.
Farah WH, Alsawas M, Mainou M, Alahdab F, Farah MH, Ahmed AT, et al. Non-pharmacological treatment of depression: a systematic review and evidence map. Evid Based Med 2016;21:214–21. https://doi.org/10.1136/ebmed-2016-110522
Fishbain DA. Non-surgical chronic pain treatment outcome: a review. Int Rev Psychiatry 2000;12:170–80.
Foroushani PS, Schneider J, Assareh N. Meta-review of the effectiveness of computerised CBT in treating depression. BMC Psychiatry 2011;11:131. https://doi.org/10.1186/1471-244X-11-131
Gartlehner G, Wagner G, Matyas N, Titscher V, Greimel J, Lux L, et al. Pharmacological and non-pharmacological treatments for major depressive disorder: review of systematic reviews. BMJ Open 2017;7:e014912. https://doi.org/10.1136/bmjopen-2016-014912
Gustafsson C, Öjehagen A, Hansson L, Sandlund M, Nyström M, Glad J, et al. Effects of psychosocial interventions for people with intellectual disabilities and mental health problems: a survey of systematic reviews. Res Soc Work Pract 2009;19:281–90.
Health Quality Ontario. Psychotherapy for major depressive disorder and generalized anxiety disorder: a health technology assessment. Ont Health Technol Assess Ser 2017;17:1–167.
Health Quality Ontario. Cognitive behavioural therapy for psychosis: a health technology assessment. Ont Health Technol Assess Ser 2018;18:1–141.
Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit Ther Res 2012;36:427–40. https://doi.org/10.1007/s10608-012-9476-1
Jacobs S, Hassell K, Johnson S. The effectiveness of organisational stress management and prevention strategies: what can community pharmacy learn from existing evidence? Int J Pharm Pract 2013;21:78–9.
Joyce S, Modini M, Christensen H, Mykletun A, Bryant R, Mitchell PB, Harvey SB. Workplace interventions for common mental disorders: a systematic meta-review. Psychol Med 2016;46:683–97. https://doi.org/10.1017/S0033291715002408
Khan F, Amatya B. Rehabilitation in multiple sclerosis: a systematic review of systematic reviews. Arch Phys Med Rehabil 2017;98:353–67.
Lee AH, DiGiuseppe R. Anger and aggression treatments: a review of meta-analyses. Curr Opin Psychol 2018;19:65–74.
Louw Q, Morris L, Sklaar J. Evidence of physiotherapeutic interventions for acute LBP patients. South Afr J Physiother 2007;63:7–14.
Miller P, Soundy A. The pharmacological and non-pharmacological interventions for the management of fatigue related multiple sclerosis. J Neurol Sci 2017;381:41–54.
Minelli A, Vaona A. Effectiveness of cognitive behavioral therapy in the treatment of fibromyalgia syndrome: a meta-analytic literature review. Reumatismo 2012;64:151–7. https://doi.org/10.4081/reumatismo.2012.151
Morin L, Franck N. Rehabilitation interventions to promote recovery from schizophrenia: a systematic review. Front Psychiatry 2017;8:100. https://doi.org/10.3389/fpsyt.2017.00100
Munthe-Kaas HM, Johansen S, Blaasvaer N, Hammerstrom KT, Nilsen W. The Effect of Psychosocial Interventions for Prevention and Treating Depression and Anxiety among At-risk Children and Adolescents (Report 22). Oslo: The Norwegian Institute of Public Health; 2014.
Palmer T. Programmatic and Nonprogrammatic Aspects of Successful Intervention. In Harland AT, editor. Choosing Correctional Options That Work: Defining the Demand and Evaluating the Supply. Thousand Oaks, CA: Sage Publications, Inc.; 1996. pp. 131–82.
Paulus FW, Ohmann S, Popow C. Practitioner Review: school-based interventions in child mental health. J Child Psychol Psychiatry 2016;57:1337–59. https://doi.org/10.1111/jcpp.12584
Peters LW, Kok G, Ten Dam GT, Buijs GJ, Paulussen TG. Effective elements of school health promotion across behavioral domains: a systematic review of reviews. BMC Public Health 2009;9:182. https://doi.org/10.1186/1471-2458-9-182
Prothero L, Barley E, Galloway J, Georgopoulou S, Sturt J. The evidence base for psychological interventions for rheumatoid arthritis: a systematic review of reviews. Int J Nurs Stud 2018;82:20–9.
Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res 2017;26:675–700. https://doi.org/10.1111/jsr.12594
Stamou G, García-Palacios A, Botella C. Cognitive–behavioural therapy and interpersonal psychotherapy for the treatment of post-natal depression: a narrative review. BMC Psychol 2018;6:28. https://doi.org/10.1186/s40359-018-0240-5
Turrini G, Purgato M, Ballette F, Nosè M, Ostuzzi G, Barbui C. Common mental disorders in asylum seekers and refugees: umbrella review of prevalence and intervention studies. Int J Ment Health Syst 2017;11:51. https://doi.org/10.1186/s13033-017-0156-0
Appendix 7 Summary tables of included systematic reviews grouped according to the conditions they targeted as per the ICD-11
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of patients) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Uwimana and Louw465 2007 | Human immunodeficiency virus | NA | Critically low | 1 (NR) | Adults | NR | Group CBT | NA | Peer support counselling | NA | Short | ✓ | ✓ | ✓ | ✗ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Berry et al.65 2017 | Neoplasms | NA | Low | 1 (118) | Adults | The Netherlands | Face-to-face CBT (couples therapy) | NA | NA | TAU | Short | ✗ | ✓ | ✗ | ≤ |
| Boutin73 2007 | Malignant neoplasms of breast | NA | Critically low | 2 (213) | Adults | NR | Individual CBT | NA | NR | NR | Short–long | ✗ | ✓ | ✗ | ✗ |
| Ernst et al.137 2006 | Malignant neoplasms of breast | NA | Critically low | 1 (92) | NR | NR | Therapist-led group CBT + standard oncological care | NA | NA | TAU | Long | ✗ | ✗ | ✗ | ✓ |
| Hines et al.209 2011 | Malignant neoplasms | Cognitive dysfunction | Moderate | 1 (40) | Adults | USA | Individual CBT+ telephone booster | NA | NA | WLC | Short | ✓ | ✗ | ✗ | ✗ |
| Juvet et al.238 2009 | Malignant neoplasms of breast | NA | Moderate | 1 (114) | Adults | Israel | Group CBT | NA | Relaxation + guided support | TAU | Short | ≤ | ≤ | ≤ | ✓ |
| Mirosevicet al.313 2019 | Malignant neoplasms | NA | Critically low | 4 (625) | Adults | NR | Group CBT | NA | NA | TAU | Long | ✗ | ✗ | ✗ | ✓ |
| Mustafa et al.322 2013 | Malignant neoplasms of breast | NA | Moderate | 1 (63) | Adults | Australia | Individual CBT | NA | NA | TAU | Short–long | ≤ | ✓ | ✓ | ✓ |
| Ye et al.513 2018 | Malignant neoplasms of breast | NA | Low | 10 (1939) | Adults | USA, China, Ireland, France, the Netherlands, Australia | Individual CBT | NA | Behavioural placebo, relaxation, drug | WLC, TAU | Short | ✓ | ✓ | ✓ | ✓ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Amato Nesbit et al.41 2019 | Diabetic peripheral neuropathy | NA | Critically low | 1 (19) | Adults | NR | Individual CBT | NA | NA | Sham | Short | ✗ | ✗ | ✗ | ✓ |
| Elliot136 2012 | Diabetes mellitus | Depression | Critically low | 2 (158) | Adults | The Netherlands, USA | Group CBT, individual CBT | NA | Education, blood glucose awareness training | NA | Short–long | ✗ | ✓ | ✗ | ✓ |
| Uchendu and Blake463 2017 | Diabetes mellitus | Depression, anxiety | Low | 6 (NR) | Adults | The Netherlands, USA, Sweden | Group CBT, individual CBT | Telephone CBT | NA | TAU, NR | Long | ✓ | ✓ | ✓ | ✓ |
Reviews in this ICD-11 primary classification 06 mental, behavioural or neurodevelopmental problems are presented at the secondary level of classification.
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of patients) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Goode et al.174 2018 | ADHD | NA | Critically low | 1 (119) | Children, adolescents | NR | NR | NA | NA | TAU | Short | ✗ | ≤ | ≤ | ✓ |
| Jensen et al.230 2016 | ADHD | Anxiety, depression | Low | 2 (85) | Adults | NR | Manualised group CBT or individual CBT | NA | NA | TAU | Short | ≤ | ✓ | ✓ | ✓ |
| Kemper et al.244 2018 | ADHD | NA | High | 1 (119) | Children, adolescents | NR | NR | NA | NA | TAU | Short | ✗ | ✗ | ✗ | ✓ |
| Lopez et al.286 2018 | ADHD | NA | High | 6 (277) | Adults | Sweden, Finland, Spain, Iceland, USA | Individual CBT | Internet-based CBT | Relaxation, education, cognitive training, drug | WLC | Short | ✓ | ✓ | ✓ | ✓ |
| Vidal-Estrada et al.481 2012 | ADHD | NA | Critically low | 3 (146) | Adults | NR | Individual CBT + medication | NA | Medication | NA | Short | ✗ | ✓ | ✓ | ✓ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of patients) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Álvarez-Jiménez et al.40 2011 | Psychosis | NA | Moderate | 1 (309) | Adults | NR | Manualised individual CBT | NA | Supportive counselling therapy | TAU | Long | ✗ | ✗ | ✗ | ✓ |
| Anagnostopoulou et al.43 2018 | Psychosis | NA | Critically low | 1 (30) | Adolescents | UK | Individual CBT | NA | Family therapy | TAU | Short | ✗ | ✗ | ✗ | ✓ |
| Armando et al.51 2015 | Psychosis | NA | Critically low | 1 (309) | Adolescents, adults | NR | NR | NA | Supportive counselling + TAU | TAU | Long | ✗ | ✗ | ✗ | ✓ |
| Bighelli et al.66 2018 | Psychosis | NA | Moderate | 2 (323) | Adults | UK, Norway | Individual CBT | NA | NA | WLC | Short | ′ | ′ | ✗ | ✓ |
| Bird et al.67 2010 | Early-onset psychosis | NA | Moderate | 3 (529) | Adults | NR | Group CBT, individual CBT | NA | NA | TAU | Long | ✗ | ✗ | ✗ | ✓ |
| Buckley et al.79 2015 | Psychosis | NA | High | 5 (349) | Adults | USA, UK, Italy | Individual CBT | NA | Supportive therapy | NA | Short | ✗ | ✓ | ✗ | ✓ |
| Davies et al.116 2018 | Psychosis | NA | Moderate | 6 (712) | Adolescents, adults | Canada, UK, Germany, Australia | Individual CBT (French and Morrison540 protocol) | NA | Drug | Needs-based intervention | Long | ✗ | ✗ | ✗ | ✓ |
| de Koning et al.121 2009 | Attenuated psychosis syndrome | NA | Critically low | 1 (128) | Adults | Germany | Comprehensive individual CBT | NA | Supportive counselling | NA | Long | ✗ | ✗ | ✗ | ✓ |
| Devoe et al.128 2018 | Attenuated psychosis syndrome | NA | Moderate | 3 (236) | Young adults | Canada, the Netherlands, Australia | Individual CBT + TAU, individual CBT + placebo | NA | Supportive therapy, supportive therapy + placebo drug | TAU + monitoring | Short | ✗ | ✗ | ✗ | ✓ |
| Devoe et al.127 2019 | Psychosis | NA | Low | 6 (843) | Young adults | Canada, the Netherlands, UK, Australia, New Zealand | Individual CBT, individual CBT +TAU, individual CBT + placebo, individual CBT + monitoring | NA | Supportive therapy, supportive therapy + placebo | TAU + monitoring | Long | ✗ | ✗ | ✗ | ✓ |
| Gaag et al.161 2014 | Psychosis | NA | Moderate | 9 (768) | Adults | UK, the Netherlands, Canada, Germany, Norway | Individually tailored case formulation + culturally adapted CBT | NA | Supportive counselling, social activity treatment | Attention placebo, TAU | Short | ✗ | ✗ | ✗ | ✓ |
| Hutton and Taylor219 2014 | Psychosis | NA | Moderate | 3 (324) | Adults | Canada, Australia, the Netherlands | Individual CBT | NA | Supportive therapy | Monitoring | Long | ✓ | ✓ | ✓ | ✓ |
| Jacobsen et al.226 2018 | Psychosis | NA | Low | 1 (90) | Adults | UK | NR | NA | NA | TAU | Long | ✗ | ′ | ′ | ✓ |
| Jones et al.234 2012 | Psychosis | NA | Moderate | 5 (378) | Adults | NR | Group CBT, individual CBT | NA | Supportive psychotherapy, family intervention, supportive counselling, enhanced supportive therapy, befriending, TAU | TAU | Long | ✓ | ✓ | ✓ | ✓ |
| Jones et al.235 2018 | Psychosis | NA | High | 14 (1561) | Adults | Scotland, Germany, UK, the Netherlands, Canada, USA, China | Group CBT, individual CBT | NA | Psychoeducation, family intervention, supportive psychotherapy, health education, group social skills training, supportive counselling, social activities therapy, befriending | NA | Short–long | ✓ | ✓ | ✓ | ✓ |
| Jones et al.236 2018 | Psychosis | NA | High | 12 (1827) | Adults | UK, Pakistan, China | Individual CBT | NA | NA | TAU | Long | ✓ | ✓ | ✓ | ✓ |
| Kennedy and Xyrichis245 2017 | Psychosis | NA | Low | 2 (105) | Adults | USA, Australia | Group CBT, TORCH541 individual CBT | NA | Supportive therapy, befriending | NA | Short | ✗ | ✗ | ✗ | ✓ |
| Kluwe-Schiavon et al.254 2013 | Psychosis | NA | Critically low | 1 (40) | Adults | NR | Individual CBT | NA | Cognitive remediation therapy | TAU | Short | ≤ | ✗ | ✗ | ✓ |
| Lawrence et al.269 2006 | Psychosis | NA | Critically low | 1 (88) | Adults | Australia, UK, Germany | Group CBT | NA | Psychoeducation | TAU, NR | Long | ✗ | ✓ | ✓ | ✓ |
| Laws et al.270 2018 | Psychosis | NA | Critically low | 9 (626) | Adults | NR | Group CBT, individual CBT | NA | Psychoeducation, befriending, drug | WLC, TAU | Short | ✓ | ✗ | ✗ | ✓ |
| Lockwood et al.283 2004 | Psychosis | Social anxiety | Critically low | 4 (825) | Adults | UK, NR | Group CBT, individual CBT | NA | Supportive counselling | WLC, TAU | Short–long | ✓ | ✓ | ✓ | ✓ |
| Mehl et al.304 2015 | Delusional disorder | NA | Critically low | 5 (842) | Adults | NR | Individual CBT for psychosis | NA | Supportive therapy, social activity therapy, family intervention | TAU | Short | ✗ | ✗ | ✗ | ✓ |
| Naeem et al.323 2016 | Psychosis | NA | Critically low | 7 (1128) | Adults | NR | Individual CBT for psychosis, group CBT for psychosis, culturally adapted individual CBT for psychosis | NA | Supportive counselling, befriending | TAU | Short–long | ′ | ✓ | ′ | ✓ |
| Rector and Beck380 2001 | Psychosis | NA | Critically low | 6 (341) | Adults | NR | NR | NA | Problem-solving, supportive therapy, informal support, befriending | TAU | Short | ✗ | ✓ | ✗ | ✓ |
| Sarin et al.394 2011 | Psychosis | NA | Moderate | 5 (623) | Adults | NR | Group CBT, individual CBT | NA | Other psychotherapy (family intervention, supportive therapy) | TAU | Short, NR | ✗ | ✓ | ✓ | ′ |
| Skelton et al.414 2015 | Delusional disorder | NA | Moderate | 1 (17) | Adults | NR | Individual CBT | NA | NA | Attention placebo | Short | ≤ | ✓ | ✓ | ✓ |
| Turner et al.458 2014 | Psychosis | NA | Low | 8 (497) | NR | NR | Group CBT, individual CBT | NA | Supportive counselling | NA | Short | ✗ | ✗ | ✗ | ✓ |
| van der Gaag et al.161 2014 | Psychosis | NA | Low | 9 (768) | Adults | UK, Norway, Germany, the Netherlands, Canada | Individual CBT | NA | Supportive counselling, social activity treatment | TAU, attention placebo | Short | ✗ | ✗ | ✗ | ✓ |
| Velthorst et al.476 2015 | Psychosis | NA | Critically low | 2 (238) | Adults | USA, Germany | Group CBT, individual CBT | NA | Cognitive remediation | TAU | Short | ✗ | ✗ | ✗ | ✓ |
| Wood et al.503 2016 | Psychosis | NA | Low | 1 (29) | Adults | UK | Individual CBT | NA | NA | TAU | Short | ✗ | ✓ | ✗ | ✓ |
| Zhao et al.518 2015 | Psychosis | NA | High | 1 (41) | Adults | Germany | Group CBT | NA | Group psychological programme | NA | Short | ✓ | ≤ | ≤ | ✓ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of patients) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Ackermann and Williams33 2002 | Premenstrual dysphoric disorder | NA | Critically low | 1 (49) | NR | NR | Individual CBT | NA | NA | Placebo | NR | ✗ | ✓ | ✗ | ✗ |
| Ahern et al.36 2018 | Depressive disorder | NA | Critically low | 24 (2379) | Adults | Sweden, Australia, USA, Norway, UK, the Netherlands, Spain | NA | Internet-based CBT + therapist, internet-based CBT + administrative support | Individual CBT | WLC, TAU, attention control | Short | ✗ | ✓ | ✗ | ✗ |
| Akechi et al.37 2008 | Depressive disorder | Metastatic breast cancer | High | 1 (92) | Adults | NR | Group CBT | NA | NA | TAU | NR | ≤ | ✓ | ✓ | ✗ |
| Antoniades et al.48 2014 | Depressive disorder | NA | Low | 1 (63) | Adults | NR | NA | Culturally adapted internet-based CBT | NA | WLC | Short | ✗ | ✓ | ✗ | ✗ |
| Apóstolo et al.49 2015 | Depressive disorder | NA | Moderate | 1 (92) | Older adults | UK | Individual CBT + TAU (modified for older people) | NA | Talking control group | TAU | Short | ✓ | ✓ | ✗ | ✗ |
| Babowitch and Antshel55 2016 | Depressive disorder | Substance abuse | Critically low | 1 (40) | Adolescents | NR | Individual CBT, family CBT | NA | NA | TAU | Long | ✗ | ✓ | ✗ | ✗ |
| Beltman et al.59 2010 | Depressive disorder | Cancer, HIV, MS, RA, vascular disease, COPD, renal failure | Moderate | 6 (725) | Adults | NR | Group CBT, individual CBT | NA | Other psychotherapy | WLC, TAU | Short | ✗ | ✓ | ✗ | ✗ |
| Bennett et al.61 2015 | Subthreshold depression | Epilepsy, IBD | Low | 2 (71) | Children, adolescents | Serbia and Montenegro, USA | Individual CBT | NA | NA | TAU (counselling, depression information leaflet) | Short–long | ✓ | ✓ | ✗ | ✓ |
| Briani et al.76 2018 | Depressive disorder | Pain (knee osteoarthritis) | Low | 1 (69) | Adults | NR | NA | Internet-based CBT | NA | TAU | Short | ≤ | ✓ | ✗ | ✗ |
| Butler et al.82 2018 | Bipolar or related disorder | NA | Low | 4 (405) | Adults | UK, USA, Germany, Canada | Individual CBT, individual CBT for insomnia | NA | Psychoeducation, supportive therapy | TAU | Short–long | ✓ | ✓ | ✗ | ✓ |
| Casacalenda et al.92 2002 | Depressive disorder | NA | Critically low | 1 (239) | Adults | NR | Individual CBT | NA | Imipramine (drug), interpersonal therapy | Placebo | Short | ✗ | ✓ | ✗ | ✗ |
| Clevenger et al.104 2018 | Depressive disorder | NA | Critically low | 1 (40) | Children, adolescents, adults | NR | Individual CBT + SSRI | NA | SSRI | NA | Long | ✗ | ✓ | ✗ | ✗ |
| Compton et al.106 2002 | Depressive disorder | NA | Critically low | 1 (57) | Children, adolescents | NR | Individual CBT | NA | Non-focused supportive intervention | NA | Long | ✗ | ✓ | ✗ | ✗ |
| Cuijpers et al.113 2009 | Subthreshold depression | Subthreshold depression | Critically low | 10 (1159) | Adolescent, adults | NR | Group CBT (coping with depression) | Guided self-help CBT (coping with depression) | Internet-based CBT | WLC, TAU | Short | ✗ | ✓ | ✗ | ✗ |
| Cuijpers et al.112 2013 | Depressive disorder | NA | Low | 9 (506) | Adults | USA, UK, Romania | Acute phase individual CBT + 3 or 4 booster (Beck542 protocol) | NA | Drug | NA | Short–long | ✗ | ✓ | ✗ | ✗ |
| Cuijpers et al.111 2017 | Depressive disorder | Diabetes | Low | 3 (200) | Adults | NR | Individual CBT | NA | Cognitive therapy, behavioural activation | NA | NR | ✗ | ✓ | ✗ | ✗ |
| Das et al.114 2019 | Depressive disorder | Heart failure | Critically low | 1 (60) | Adults | USA | NR | NA | Exercise | TAU | Short | ✗ | ✓ | ✗ | ✗ |
| de Mello et al.145 2005 | Depressive disorder | NA | Critically low | 3 (204) | Adults | NR | Individual CBT | NA | Interpersonal therapy | NA | Short | ✗ | ✓ | ✗ | ✗ |
| Dekker123 2008 | Depressive disorder | Stroke, diabetes (type 2) | Critically low | 2 (174) | NR | NR | Individual tailored CBT | NA | Diabetes education | Attention placebo, no intervention | Short | ✗ | ✓ | ✗ | ✗ |
| Demissie et al.124 2018 | Bipolar or related disorder | NA | Low | 2 (91) | Adults | Brazil | Group CBT | NA | NA | TAU | Short–long | ✓ | ✓ | ✓ | ✓ |
| Ebrahim et al.132 2012 | Depressive disorder | NA | Low | 8 (1400) | Adults | USA, Canada, Pakistan, Iran, the Netherlands | Mostly individualised face-to-face CBT | Internet-based CBT, CBT (delivered by female health workers) | Drug | No intervention, TAU | NR | ✗ | ✓ | ✗ | ✗ |
| Escobar and Gorey138 2018 | Depressive disorder | NA | Low | 5 (485) | Adults | USA | Group CBT, individual CBT | Telephone CBT | NA | TAU | Short | ✗ | ✓ | ✗ | ✗ |
| Fernie et al.146 2015 | Depressive disorder | Epilepsy | Low | 1 (59) | Adults | NR | Individual CBT | NA | NA | WLC | NR | ✗ | ✓ | ✗ | ✗ |
| Forman-Hoffman et al.153 2016 | Depressive disorder | NA | Low | 1 (123) | Children, adolescents | USA, NR | Individual CBT | NA | Drug, CBT + drug | Placebo | Short | ✓ | ✓ | ✗ | ✗ |
| Franco et al.156 2018 | Depressive disorder | Diabetes | Critically low | 2 (345) | Adults | NR | NA | Internet-based CBT | NA | WLC, TAU | Short | ✓ | ′ | ✓ | ✓ |
| Gertler et al.165 2015 | Depressive disorder | Brain injury | High | 2 (177) | Adults | USA | Individual CBT | NA | Supportive psychotherapy | WLC | Short | ✓ | ✓ | ✗ | ✗ |
| Griffiths et al.180 2010 | Depressive disorder | NA | Low | 5 (2166) | Adults | USA | NA | Internet-based CBT | NA | WLC, attention control, passive control | Short–long | ✗ | ✓ | ≤ | ✗ |
| Guidi et al.184 2016 | Depressive disorder | NA | Moderate | 1 (40) | Adults | NR | Individual CBT | NA | NA | TAU | Long | ✗ | ✓ | ✗ | ✗ |
| Haibach et al.186 2014 | Depressive disorder | Substance abuse | Critically low | 1 (66) | Adults | USA | Integrated individual CBT | NA | 12-step facilitation | NA | Long | ✗ | ✓ | ✗ | ✓ |
| Hall and Skelton190 2012 | Depressive disorder | Caregivers (dementia) | Critically low | 1 (42) | Adults | UK | CBT family intervention for caregivers | NA | NR | NR | Short | ✗ | ✓ | ✗ | ✗ |
| Hamers et al.191 2018 | Depressive disorder | Intellectual disabilities | Low | 1 (32) | Adults | NR | Manualised individual CBT | NA | NA | TAU | Short | ✗ | ✓ | ✗ | ✗ |
| Hart et al.196 2012 | Depressive disorder | Cancer (mixed) | Low | 1 (78) | Adults | USA | NA | Group CBT (delivered by social worker) | Crisis consultation and individual therapy | NA | Short | ✗ | ✓ | ✗ | ✗ |
| van Hees et al.202 2013 | Depressive disorder | NA | Low | 3 (333) | Adults | NR | Individual CBT | NA | Interpersonal therapy | NA | Short | ✗ | ✓ | ✗ | ✗ |
| Hennemann et al.204 2018 | Depressive disorder | NA | Low | 1 (84) | Adults | Sweden | NA | Internet-based CBT | NA | TAU | Long | ✓ | ✓ | ✓ | ✗ |
| Hetrick et al.205 2016 | Depressive disorder | NA | Moderate | 4 (1600) | Children, adolescents | Australia, New Zealand, USA | Manualised group CBT | Telephone CBT | NA | No intervention, TAU, attention placebo | Long | ✗ | ✓ | ✓ | ✗ |
| Hetrick et al.206 2011 | Depressive disorder | NA | Moderate | 2 (536) | Children, adolescents | UK, NR | Individual CBT + SSRI | NA | SSRI | NA | Short | ✗ | ✓ | ✗ | ✗ |
| Hides et al.207 2010 | Depressive disorder | Substance abuse | Critically low | 1 (66) | Adults | NR | NA | Group CBT | Group 12-step facilitation | NA | Short | ✗ | ✓ | ✗ | ✓ |
| Hundt et al.217 2014 | Depressive disorder | Substance use disorder | Critically low | 3 (357) | Older adults | NR | Group CBT | Telephone CBT | 12-step facilitation | TAU | Short | ✗ | ✓ | ✗ | ✗ |
| Huntley et al.218 2012 | Depressive disorder | NA | Moderate | 3 (113) | Adults | UK | Group CBT + usual care | NA | NA | TAU | Short | ✗ | ✓ | ✗ | ✗ |
| Ijaz et al.220 2018 | Treatment resistant depressive disorder | Treatment resistant | Moderate | 1 (242) | Adults | UK | Individual CBT | NA | NA | TAU | Long | ✓ | ✓ | ✗ | ✗ |
| Jackson et al.224 2015 | Depressive disorder | Epilepsy | Moderate | 1 (30) | Adolescents | Serbia | Individual CBT | NA | NA | TAU | Short | ✓ | ✓ | ≤ | ✗ |
| Jeyanantham et al.231 2017 | Depressive disorder | Heart failure | Moderate | 5 (204) | Adults | USA | Face-to-face individual CBT ± telephone sessions (delivered by a therapist) | Face-to-face individual CBT (delivered by nurse) + telephone sessions | Exercise, CBT + exercise | TAU | Short | ✓ | ✓ | ✗ | ✓ |
| Jiang et al.232 2018 | Depressive disorder | Heart failure | Low | 5 (383) | Adults | Philippines, USA | Face-to-face group CBT and individual CBT (delivered by a therapist) ± telephone call | Face-to-face CBT (delivered by nurse) ± telephone call | NR | NR | Short | ✓ | ✓ | ′ | ✓ |
| Karyotaki et al.240 2017 | Depressive disorder | NA | Moderate | 13 (3876) | Adults | Switzerland, Australia, the Netherlands, UK, Germany, Spain | NA | Self-guided internet-based CBT | NA | TAU, WLC, attention placebo, no intervention | Short | ✗ | ✓ | ✗ | ✗ |
| Kavanagh et al.241 2009 | Depressive disorder | NA | Critically low | 2 (1153) | Adolescents | USA, Australia | Coping with depression | Coping with depression (delivered by teachers) | NA | No intervention | Short | ✗ | ′ | ✓ | ✗ |
| Kiosses et al.250 2011 | Depressive disorder | NA | Critically low | 1 (100) | Older adults | NR | NR | NA | Drug | NA | NR | ≤ | ✓ | ≤ | ≤ |
| Klein et al.253 2007 | Depressive disorder | NA | Critically low | 1 (88) | Adolescents | NR | Group CBT | NA | NA | TAU | Long | ✗ | ✓ | ✗ | ✗ |
| Krishna et al.261 2015 | Depressive disorder | NA | Moderate | 1 (96) | Adults | The Netherlands | Group CBT | NA | NA | TAU | Long | ✗ | ✓ | ✗ | ✗ |
| Lane et al.264 2013 | Depressive disorder | Congenital heart disease | Moderate | 0 (0) | Adolescents, adults | NR | NA | NA | NA | NA | NA | ≤ | ≤ | ✗ | ≤ |
| Li et al.277 2017 | Depressive disorder | Diabetes | Low | 3 (336) | Adults | NR | Group CBT/individual CBT (diabetes specific and generic) | NA | Diabetes education | TAU, WLC | Short | ′ | ✓ | ✓ | ′ |
| Li et al.278 2018 | Depressive disorder | NA | Low | 2 (468) | Adults | UK, Japan | Individual CBT | NA | NA | TAU | Long | ✗ | ✓ | ✗ | ✗ |
| Linde et al.280 2015 | Depressive disorder | NA | Moderate | 7 (1273) | Adults | NR | Individual CBT | Telephone CBT, internet-based CBT | Drug, counselling | TAU | Short | ✗ | ✓ | ✗ | ✗ |
| Luckett et al.288 2011 | Depressive disorder | Cancer (head and neck) | Moderate | 1 (184) | Adults | USA | NA | Individual CBT (delivered by nurse) | NA | TAU | Short | ≤ | ✓ | ≤ | ✗ |
| Ma et al.289 2014 | Depressive disorder | NA | Low | 1 (208) | Children, adolescents | UK | Individual CBT | NA | Fluoxetine (drug) | NA | Short | ✗ | ✓ | ✗ | ✗ |
| Markowitz et al.296 2011 | Depressive disorder | Diabetes | Critically low | 1 (51) | Adults | NR | Individual CBT | NA | Education | NA | Short | ✗ | ✓ | ✗ | ✓ |
| Mewton and Andrews308 2016 | Depressive disorder | Including suicide ideation | Critically low | 2 (208) | Adults | NR | NR | Internet-based CBT | High-intensity CBT, interperson therapy, drug + clinical management | Placebo + clinical management | Short | ✗ | ✓ | ✗ | ✗ |
| Michaelis et al.309 2018 | Depressive disorder | Epilepsy | Critically low | 1 (NR) | Children | NR | Individual CBT | NR | NR | NR | Short | ≤ | ✓ | ≤ | ≤ |
| Moore et al.318 2017 | Depressive disorder | NA | Low | 5 (631) | Adults | Taiwan, England, Australia, Hong Kong, Sweden | Group CBT | NA | Guided internet-based CBT | WLC, TAU | Short–long | ✓ | ✓ | ✗ | ✗ |
| Napa et al.325 2017 | Depressive disorder | Caregivers (psychosis) | Critically low | 1 (21) | Adults | NR | Family CBT relapse prevention | NA | NA | TAU | Long | ✗ | ✓ | ✗ | ✗ |
| Noh334 2018 | Depressive disorder | Homeless youth | Critically low | 1 (27) | Adolescents, young adults | Republic of Korea | Group CBT | NA | NA | TAU | Short | ✗ | ✓ | ✗ | ✗ |
| Opoka and Lincoln344 2017 | Depressive disorder | Psychosis | Critically low | 1 (30) | Adults | NR | Individual CBT | NA | NA | TAU | Short | ✗ | ✓ | ′ | ✓ |
| Orgeta et al.347 2014 | Depressive disorder | Dementia | Moderate | 2 (82) | Older adults | UK, USA | Individual CBT | NA | NA | TAU | Short | ✓ | ′ | ✓ | ✓ |
| Osugo and Cooper350 2016 | Depressive disorder | Intellectual disabilities | Critically low | 1 (32) | Adults | NR | Individual CBT | NA | NA | TAU | Short | ′ | ′ | ✓ | ✗ |
| Oud et al.352 2019 | Subthreshold depression | Subclinical depression or clinical depressive disorder | Low | 4 (976) | Children, adolescents | USA, the Netherlands | Group CBT, individual CBT | Mixed CBT (group CBT/bibliotherapy CBT/online CBT) | NA | Placebo, online monitoring | Short | ✓ | ✓ | ✗ | ✗ |
| Pfeiffer et al.365 2011 | Depressive disorder | NA | Low | 7 (488) | Adults | NR | Group CBT | NA | Peer support interventions | NA | Short | ✗ | ✓ | ✗ | ✗ |
| Riper et al.383 2013 | Depressive disorder | Alcohol misuse | Low | 1 (164) | Adults | USA | Individual CBT for depression + TAU | NA | NA | TAU + placebo | Short | ✗ | ✓ | ✓ | ✓ |
| Robinson et al.386 2011 | Depressive disorder | Including suicide ideation | Low | 1 (90) | Adolescents, young adults | NR | Individual CBT | NA | NA | TAU | Short | ✗ | ✓ | ✓ | ✓ |
| Sharp et al.405 2013 | Depressive disorder | Diabetes | Moderate | 1 (167) | Adults | The Netherlands | NA | Computerised CBT (adapted from coping with depression) | NA | WLC | Short | ≤ | ✓ | ✓ | ✓ |
| Shi et al.407 2018 | Depressive disorder | HIV | Low | 4 (319) | NR | USA | Individual CBT | NA | Education on HIV medication adherence | TAU | Short–long | ✗ | ✓ | ✓ | ✓ |
| So et al.420 2013 | Depressive disorder | NA | Moderate | 5 (976) | Adults | Sweden, the Netherlands, Germany | NA | Internet-based CBT (unguided, guided) | NA | WLC, TAU | Short | ′ | ✓ | ✗ | ✗ |
| Soares-Weiser et al.421 2007 | Bipolar or related disorder | NA | Moderate | 1 (253) | Adults | UK | Individual CBT | NA | NA | TAU | Long | ✗ | ✗ | ✗ | ✓ |
| Ssegonja et al.427 2019 | Subthreshold depression | Subsyndromal | Low | 7 (3931) | Children, adolescents | Australia, USA, UK, the Netherlands | Group CBT (‘Friends’, ‘PRP’, ‘the resourceful adolescent’, ‘Friends for life’) | Group CBT (delivered by health-care professionals, teachers and psychologists) | Bibliotherapy, supportive expressive therapy | TAU, WLC, brochure, attention control | Long | ✗ | ✓ | ✗ | ✗ |
| Starkstein and Brockman428 2017 | Depressive disorder | Parkinson’s disease | Critically low | 1 (80) | Adults | NR | Individual CBT | NA | NA | Clinical monitoring | Short | ✗ | ✓ | ✗ | ✗ |
| Taylor and Montgomery444 2007 | Depressive disorder | NA | Low | 1 (30) | Adolescents | NR | Group CBT | NA | Relaxation | WLC | Short | ✗ | ✓ | ✗ | ✗ |
| Thomas et al.448 2006 | Depressive disorder | MS | Moderate | 3 (106) | Adults | NR | Group CBT, individual CBT | Telephone CBT | Drug | TAU, WLC | Short | ≤ | ✓ | ✗ | ✓ |
| Townsend et al.454 2010 | Depressive disorder | NA | Moderate | 1 (93) | Children, adolescents | USA | Group adolescent coping with depression | NA | Life skills tutoring | NA | Long | ✗ | ✓ | ≤ | ✗ |
| Twomey et al.462 2017 (Deprexis) | Depressive disorder | NA | Moderate | 8 (2402) | Adults | USA, Germany, Switzerland | NA | Computerised CBT (Deprexis) | NA | WLC, TAU | Short | ✗ | ✓ | ′ | ✗ |
| Vandepitte et al.472 2016 | Anxiety and depression | Caregivers (dementia) | Low | 2 (292) | Adults | Spain | Group CBT | NA | NA | TAU | Short | ✓ | ✓ | ✗ | ✗ |
| Venning et al.477 2009 | Depressive disorder | NA | Critically low | 5 (2195) | Children, adolescents | USA, New Zealand, Australia | Group CBT [‘PEP’, ‘PRP’, ‘Problem-solving for Life’, ‘RAP-kiwi’, ‘the resourceful adolescent’) | NA | NA | TAU, NR | Long | ✗ | ✓ | ✓ | ✗ |
| Wang et al.485 2008 | Depressive disorder | Diabetes | Critically low | 1 (42) | Adults | NR | Individual CBT | NA | Diabetes self-management education | NA | Short | ✗ | ✓ | ✗ | ✓ |
| Wang et al.488 2017 | Depressive disorder | Diabetes | Low | 2 (368) | Adults | The Netherlands, Germany | NR | NA | Diabetes education | TAU | Long | ✗ | ✓ | ✗ | ✗ |
| Wang et al.486 2018 | Depressive disorder | Post stroke | Critically low | 22 (1911) | Adults | UK, China | Individual CBT ± drug | NA | Drug | Placebo, placebo or drug, attention control, TAU | Short | ✗ | ✓ | ′ | ✗ |
| Ward489 2007 | Depressive disorder | Racial and ethnic minority women | Critically low | 1 (267) | Adults | USA | NR | NA | Drug | TAU | Short | ✓ | ✓ | ✗ | ✗ |
| Wilson et al.501 2008 | Depressive disorder | NA | Moderate | 2 (148) | Older adults | USA | Individual CBT | NA | Focused visual imagery, education, psychodynamic therapy | NA | Short | ✓ | ✓ | ✗ | ✗ |
| Woltz et al.502 2012 | Depressive disorder | Heart failure | Critically low | 1 (74) | Adults | USA | Individual CBT | NA | Walking programme | Attention control | Short | ✗ | ✓ | ✗ | ✗ |
| Xiao et al.506 2017 | Depressive disorder | Cancer (breast) | Low | 1 (60) | Adults | USA | Individual CBT | NA | NA | No intervention | Short | ✗ | ✓ | ✗ | ✗ |
| Xie et al.507 2015 | Depressive disorder | Parkinson’s disease | Moderate | 6 (381) | Adults | UK, USA, China | Individual CBT + drug/monitoring, mixed individual CBT + telephone sessions | Bibliotherapy (self-help) | Drug, telephone support | Monitoring, no intervention | Short | ✗ | ✓ | ✗ | ✓ |
| Yang et al.510 2017 | Depressive disorder | NA | Low | 3 (93) | Children | NR | Group CBT | NA | NA | WLC, no intervention, placebo | Short | ✗ | ✓ | ✗ | ✗ |
| Ye et al.512 2016 | Bipolar or related disorder | NA | Low | 1 (76) | Adults | USA, Germany | Individual CBT | NA | Education, support therapy | NA | Short | ✗ | ✓ | ✗ | ✓ |
| Zhang et al.517 2018 | Depressive disorder | NA | Critically low | 3 (164) | Adults | NR | Individual CBT | Internet-based CBT | NA | Placebo | Long | ✗ | ✓ | ✗ | ✗ |
| Zhou et al.520 2014 | Depressive disorder | NA | Low | 1 (334) | Adolescents | NR | Individual CBT + drug | NA | Drug | NA | Short | ✗ | ✓ | ✗ | ✗ |
| Zhou et al.519 2016 | Depressive disorder | NA | Low | 3 (1066) | Adults | NR | NA | Internet-based CBT | NA | Attention control, WLC | Short–long | ✓ | ✓ | ✓ | ✗ |
The reviews in Table 11 are labelled using the tertiary-level ICD-11 labels:
-
6B00 Generalised anxiety disorder
-
6B01 Panic disorder
-
6B02 Agoraphobia
-
6B03 Specific phobia
-
6B04 Social anxiety disorder
-
6B05 Separation anxiety disorder
-
6B06 Selective mutism.
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Adelman et al.34 2014 | GAD, SAD, panic, phobia | NA | Low | 34 (2029) | Children, adults | NR | NA | Computerised CBT | High-intensity CBT | WLC | Short | ✗ | ✗ | ✓ | ✗ |
| Baardseth et al.54 2013 | Anxiety | NA | Critically low | 2 (123) | Adults | NR | NR | NA | Short-term dynamic psychotherapy, emotion-focused therapy | NR | NA | ✗ | ✗ | ✓ | ✓ |
| Bandelow et al.57 2007 | GAD, SAD, panic | NA | Critically low | 13 (NR) | NR | NR | NR | NA | Drug | NA | Short | ✗ | ✗ | ✓ | ✗ |
| Caldirola et al.84 2018 | Panic | NA | Critically low | 1 (150) | Adults | The Netherlands | Individual CBT, individual CBT + SSRI | NA | SSRI | NA | Long | ✗ | ✗ | ✓ | ✗ |
| Carpenter et al.89 2018 | GAD, panic | NA | Moderate | 1 (43) | Adults | NR | Individual CBT | NA | NA | Placebo (non-directive) | Short | ✗ | ✗ | ✓ | ✗ |
| Cartwright-Hatton et al.90 2004 | Anxiety | NA | Critically low | 10 (636) | Children, adolescents | USA, Australia | Group CBT, individual CBT | NA | NA | WLC | Short | ✗ | ✗ | ✓ | ✗ |
| Clond105 2016 | Anxiety | NA | Critically low | 1 (42) | Children, adolescents | USA | A brief form of individual CBT based on ‘Coping Cat’ and the C.A.T. Project543 | NA | EFT | WLC | Short | ✗ | ✗ | ✓ | ✗ |
| Davis et al.118 2015 | GAD, phobia, separation | NA | Critically low | 1 (51) | Children, adolescents | NR | Group CBT | NA | NA | WLC | Long | ✗ | ′ | ✓ | ✗ |
| de Souza Moura et al.122 2015 | Panic | NA | Critically low | 1 (36) | Adults | NR | Individual CBT | NA | Physical exercise | NA | Long | ✗ | ✗ | ✓ | ✗ |
| Ewing et al.139 2015 | Anxiety | NA | Moderate | 20 (2099) | Children | NR | Trans-diagnostic individual CBT or group CBT | NA | NA | WLC | NC | ✗ | ✗ | ✓ | ✗ |
| Farrer et al.144 2013 | SAD, exam anxiety | NA | Low | 3 (300) | Adults | Australia, Spain, UK | NA | Internet-based CBT | Education | WLC | Short | ✗ | ′ | ✓ | ✗ |
| Foa et al.151 2002 | GAD, panic | NA | Critically low | 2 (425) | Adults | NR | Individual CBT | NA | Drug | Placebo | Short | ✗ | ✗ | ✓ | ✗ |
| Hall et al.189 2016 | GAD | NA | Moderate | 5 (242) | Older adults | NR | Group CBT, individual CBT | NA | Information only, enhanced usual care, supportive psychotherapy, drug, acceptance and commitment therapy | NA | Short | ✗ | ✗ | ✓ | ✓ |
| Hendriks et al.203 2008 | GAD, panic, SAD, agoraphobia | NA | Low | 7 (396) | Older adults | NR | Group CBT, individual CBT | NA | Supportive psychotherapy | WLC, TAU, discussion group | Short | ✗ | ✓ | ✓ | ✗ |
| James et al.227 2015 | Anxiety | NA | Moderate | 10 (707) | Children, adolescents | NR | Group CBT, school-based group CBT, clinic-based group CBT, modular CBT, individual CBT, ‘Facing your Fears’ programme, manualised group CBT | NA | Group CBT with parental advice, group support and attention, family-based CBT, family-based education support, social recreation programme, CBT without cognitive restructuring (IAFS) | TAU, WLC | Long | ✗ | ✗ | ✓ | ✗ |
| Jayakody et al.228 2014 | Anxiety | NA | Low | 0 (0) | Adults | NR | NA | NA | NA | NA | NA | ≤ | ✗ | ≤ | ✗ |
| Kishita and Laidlaw252 2017 | GAD | NA | Critically low | 7 (323) | Older adults | NR | Group CBT, individual CBT | NA | Supportive therapy, telephone calls, discussion groups | WLC | Short | ✗ | ✗ | ✓ | ✗ |
| Kreslins et al.259 2015 | Anxiety | Autistic spectrum disorder | Moderate | 7 (283) | Children, adolescents | NR | Group CBT, individual CBT, mixed individual CBT + group CBT | NA | NA | WLC, TAU | Short | ✗ | ✗ | ✓ | ✗ |
| Kreuze et al.260 2018 | Mixed anxiety, SAD | NA | Low | 13 (1073) | Children, adolescents | USA, Australia, Germany, UK | Group CBT, individual CBT | Audio CBT, partially guided CBT, computerised CBT | ‘Test-busters’, computer-assisted education support and attention, education support | WLC, TAU, placebo | Short | ✗ | ′ | ′ | ✓ |
| Kumar and Malone262 2008 | Panic | Alcohol dependence | Critically low | 2 (122) | Adults | NR | Group CBT, individual CBT | NA | Exposure, alcohol treatment programme, internet self-help | NA | Long | ≤ | ✓ | ✓ | ✓ |
| Markowitz et al.295 2014 | Panic | NA | Critically low | 1 (91) | Adults | The Netherlands | Individual CBT | NA | Interpersonal therapy | NA | Short | ✗ | ✗ | ✓ | ✓ |
| Michail et al.310 2017 | SAD | Psychosis | Moderate | 1 (16) | Adults | Australia | Group CBT | NA | NA | WLC | Short | ✓ | ✓ | ✓ | ✓ |
| Oldham-Cooper and Loades342 2017 | Anxiety | NA | Low | 4 (257) | Children, adolescents | Australia, USA | Manualised individual CBT ‘Coping Cat’ | NA | NA | WLC | Short | ✗ | ✗ | ✓ | ✗ |
| Opriş et al.346 2012 | Phobia (flying) | NA | Critically low | 1 (45) | Adults | NR | NR | NA | Behavioural therapy + virtual reality exposure | NA | Short | ✗ | ✗ | ✓ | ✓ |
| Østergaard349 2018 | Selective mutism | NA | Critically low | 1 (21) | Children | NR | Individual CBT (integrated behaviour therapy for selective mutism) | NA | NA | WLC | Short–long | ✗ | ✗ | ✓ | ′ |
| Pateraki and Morris358 2018 | Anxiety | Asthma | Moderate | 1 (94) | Children, adolescents, adults | UK | Individual CBT | NA | NA | TAU | Short | ✗ | ✗ | ✓ | ✗ |
| Perna and Caldirola361 2017 | Panic (treatment resistant) | NA | Critically low | 1 (58) | Adults | NR | Individual CBT | NA | SSRI | NA | Short | ✗ | ✗ | ✓ | ✓ |
| Perna et al.362 2011 | Agoraphobia with panic | NA | Critically low | 1 (49) | Older adults | NR | NR | NA | Drugs | WLC | Short | ✗ | ✗ | ✓ | ✓ |
| Podina et al.368 2016 | Anxiety | NA | Critically low | 8 (404) | Children, adolescents | NR | NA | Internet-based CBT | High-intensity CBT | WLC | Short | ✗ | ✗ | ✓ | ✗ |
| Pompoli et al.370 2016 | Panic with or without agoraphobia | NA | Moderate | 18 (1090) | Adults | NR | Group CBT, individual CBT | NA | NA | WLC | Short | ✗ | ✗ | ✓ | ✓ |
| Provencher et al.372 2011 | Anxiety | Bipolar | Critically low | 1 (108) | NR | NR | Individual CBT for PTSD | NA | NA | TAU | Short | ✗ | ≤ | ✓ | ✓ |
| Reinholt and Krogh381 2014 | Anxiety | NA | Critically low | 5 (1240) | Adults | NR | Trans-diagnostic group CBT and individual CBT | NA | NA | TAU, WLC | Short | ✗ | ✗ | ✓ | ✗ |
| Schade et al.395 2003 | Anxiety | Substance abuse (alcohol) | Critically low | 2 (206) | Adults | NR | Group CBT, individual CBT | NA | Substance abuse treatment | NA | Short | ✗ | ✗ | ✓ | ✓ |
| Schwartze et al.398 2017 | Panic | NA | Low | 1 (12) | Adults | NR | Group CBT | NA | Drug | NA | Short | ✗ | ′ | ✓ | ✓ |
| Singh and Gorey411 2018 | SAD | NA | Critically low | 1 (26) | Adults | NR | Individual CBT | NA | Mindfulness-based cognitive therapy | NA | Short | ✗ | ✗ | ✓ | ✗ |
| Singh and Servern410 2018 | Anxiety | NA | Critically low | 3 (326) | Adults, older adults | Romania, Sweden, Switzerland, Austria, Germany | NA | Internet-based CBT guided group, unguided and guided individual CBT | NA | WLC | Short | ✓ | ✓ | ✓ | ✗ |
| Stoll et al.433 2017 | Anxiety | Chronic fatigue syndrome/ myalgic encephalomyelitis | Low | 1 (112) | Children, adolescents | The Netherlands | NA | Internet-based CBT | NA | TAU | Short–long | ✗ | ✗ | ✓ | ✓ |
| Stratford et al.434 2015 | Anxiety | Bipolar | Critically low | 2 (149) | Adults | USA, Brazil | Group CBT and individual CBT for borderline personality disorder and PTSD | NA | NA | TAU | Short | ✗ | ✗ | ✓ | ✗ |
| Sukhodolsky et al.435 2013 | Anxiety | Autism | Moderate | 5 (224) | Children, adolescents | NR | Group CBT, individual CBT | NA | Social recreation | WLC, TAU | Short | ✗ | ✗ | ✓ | ✗ |
| Torous et al.453 2017 | SAD | NA | Critically low | 1 (52) | Adults | NR | NA | CBT app | Interpersonal therapy app | NA | Short | ✗ | ≤ | ✓ | ✗ |
| Usmani et al.464 2017 | Anxiety | COPD | High | 1 (238) | Adults | USA | Group CBT | NA | Education | NA | Long | ✓ | ✗ | ✓ | ✓ |
| Verberne et al.478 2018 | Anxiety | Acquired brain injury | Low | 1 (12) | Adults | NR | Individual CBT | NA | NA | WLC | Short | ✗ | ✗ | ✓ | ✗ |
| Waddell et al.482 2007 | Anxiety | NA | Critically low | 1 (594) | Children, adolescents | Australia | NA | ‘Friends’ child group CBT (delivered by teachers) | NR | NR | Long | ✗ | ✓ | ✓ | ✗ |
| Wang et al.487 2017 | Anxiety | NA | Low | 20 (1811) | Children, adolescents | Australia, USA, Spain, Mexico, Ireland | Group CBT, individual CBT, mixed group CBT + individual CBT | NA | Education, non-specific counselling | Placebo, no intervention, TAU, group attention support | Short | ✗ | ✗ | ✓ | ✗ |
| Warwick et al.490 2017 | Anxiety | Autistic spectrum disorders | Critically low | 19 (1085) | Children, adolescents | NR | Group CBT, individual CBT | NA | NR | WLC | Short | ✗ | ✗ | ✓ | ✗ |
| Wersebe et al.497 2013 | SAD | NA | Low | 8 (403) | Adults | USA, China, Europe, Australia, NR | Group CBT, intensive group CBT | NA | NA | Placebo, WLC, TAU | Short | ✗ | ✗ | ✓ | ✓ |
| Wide Boman et al.499 2013 | Phobia (dental) | NA | Low | 1 (41) | Adults | USA | NR | NA | Behavioural therapy | WLC, positive dental experience | Long | ≤ | ✗ | ✓ | ✗ |
| Yang et al.509 2018 | SAD | NA | Moderate | 14 (1000) | Children, adolescents | NR | Group CBT, individual CBT | Internet-based CBT | Not used in analysis | WLC, placebo, no intervention | Short | ′ | ′ | ✓ | ✗ |
| Zhou et al.521 2018 | GAD, SAD, panic, separation, selective mutism | NA | Low | 78 (5035) | Children, adolescents | USA, Australia, Spain, UK, Iran, Norway, the Netherlands, China, Denmark, Canada, Switzerland, Germany, Sweden, Turkey | Group CBT ± parental involvement, individual CBT ± parental involvement, parent CBT, mixed individual CBT + group CBT | Internet-based CBT, bibliotherapy CBT | Bibliotherapy CBT, group behavioural therapy, individual behavioural therapy + parental involvement | No intervention, WLC, TAU, placebo | Short | ✓ | ✗ | ✓ | ✗ |
| Zhu et al.522 2014 | GAD | NA | Moderate | 4 (199) | Adults | NR | Group CBT, individual CBT | NA | Non-directive therapy, minimal contact control, supportive psychotherapy, discussion groups | NA | NR | ✗ | ✗ | ✓ | ✗ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Dèttore et al.126 2015 | OCD | NA | Moderate | 5 (420) | Children, adolescents, adults | Sweden, UK, USA, Australia, UK and USA mixed | NA | Internet-based CBT, behavioural therapy steps, web-camera family-based CBT | Online non-directive therapy, progressive relaxation, bibliotherapy | WLC | Short | ✗ | ✓ | ✓ | ✓ |
| Funderburk et al.159 2018 | Hypochondriasis | NA | Critically low | 2 (104) | Adults | NR | Individual CBT, enhanced individual CBT, individual CBT + physiotherapy | NA | Physiotherapy/exercise | WLC | Short | ✗ | ✗ | ✓ | ✓ |
| Grist and Cavanagh181 2013 | OCD | NA | Moderate | 1 (204) | Adults | NR | NA | Computerised CBT | E-mail therapy | NA | Short | ✗ | ′ | ✓ | ✓ |
| Harrison et al.195 2016 | Body dysmorphic disorder | NA | Moderate | 5 (225) | Children, adolescents, adults | NR | Individual CBT | Internet-based CBT | Anxiety management, psychoeducation + telephone calls, supportive therapy | WLC | Short | ✗ | ✓ | ✗ | ✗ |
| Ipser et al.221 2009 | Body dysmorphic disorder | NA | High | 2 (73) | Adults | NR | Individual CBT | NA | NA | WLC, no intervention | Short | ≤ | ✓ | ✓ | ✓ |
| Julien et al.237 2016 | OCD | NA | Critically low | 2 (134) | NR | NR | Individual CBT | NA | Inference-based therapy, exposure and response prevention therapy | NA | Short | ✓ | ′ | ✓ | ✓ |
| O’Kearney et al.340 2006 | OCD | NA | Moderate | 6 (351) | Children, adolescents | Australia, USA, Brazil, the Netherlands, UK | Individual CBT, group CBT, family CBT | NA | Medication | Placebo, WLC, NR | Short | ≤ | ✓ | ✓ | ✓ |
| Petricone-Westwood et al.364 2018 | Hypochondriasis | Diabetes, cardiac, cancer | Critically low | 3 (NR) | Adults | NR | NR | Internet-based CBT | Motivational enhancement therapy | Online discussion, NR | Short | ✗ | ✗ | ✓ | ✗ |
| Schwartze et al.397 2016 | OCD | NA | Moderate | 3 (253) | Adults | NR | Group CBT | NA | Drug | NA | Short–long | ✗ | ✓ | ✓ | ✓ |
| Skapinakis et al.412 2016 | OCD | Depression | Moderate | 9 (231) | Adults | NR | Individual CBT | NA | Drug | WLC, placebo | Short | ✗ | ✗ | ✓ | ✓ |
| Stock and Andrews431 2004 | OCD | NA | Critically low | 1 (112) | Children, adolescents | NR | Individual CBT | NA | Drug | Placebo | Short | ✗ | ✗ | ✓ | ✓ |
| Thomson and Page450 2007 | Hypochondriasis | NA | Moderate | 3 (294) | Adults | UK, the Netherlands, USA | Individual CBT | NA | NA | WLC, TAU, placebo | Short–long | ✗ | ✓ | ✓ | ✓ |
| Watson and Rees492 2008 | OCD | Paediatric OCD | Critically low | 4 (189) | Children, adolescents | UK, Australia, USA | Individual CBT, group CBT, mixed individual CBT + group CBT | NA | NA | WLC, placebo | Short | ✗ | ✗ | ✓ | ✓ |
| Weston et al.498 2016 | OCD | Autism spectrum disorder | Low | 1 (46) | Adolescents, adults | UK | Individual CBT | NA | Anxiety management | NA | Short | ✗ | ′ | ✓ | ✓ |
| Williams et al.500 2006 | Body dysmorphic disorder | NA | Critically low | 1 (19) | Adults | NR | Individual CBT | NA | NA | WLC | Short | ✗ | ✓ | ✗ | ′ |
| Wootton504 2016 | OCD | NA | Critically low | 6 (462) | Children, adolescents, adults | Sweden, UK, USA, Norway, Australia, multiple countries | NA | Remote CBT (internet-based CBT, computerised CBT, video conferencing CBT, bibliotherapy CBT, telephone CBT) | Face-to-face CBT, relaxation | WLC, attention control | Short–long | ✗ | ✗ | ✓ | ✓ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of patients) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Arends et al.50 2012 | Adjustment disorder | NA | Moderate | 2 (204) | Adults | Netherlands | Group CBT, individual CBT | NA | NA | TAU, no intervention | Long | ≤ | ✓ | ✗ | ✗ |
| Bisson69 2010 | PTSD | Physical injury | Critically low | 3 (358) | Adults | NR | Individual CBT | NA | Person-centred therapy | TAU, no intervention | Short–long | ✗ | ✓ | ✓ | ✓ |
| Blainey70 2012 | PTSD | Birth trauma | Critically low | 1 (56) | Adults | NR | Individual CBT | NA | NA | TAU | NR | ✗ | ✗ | ✓ | ✓ |
| Brooks et al.77 2018 | PTSD | NA | Critically low | 1 (21) | Adults | USA | Individual CBT (modified for disaster workers) | NA | NA | TAU | Short | ✗ | ✓ | ✓ | ✓ |
| Cary and McMillen91 2012 | PTSD | NA | Critically low | 3 (333) | Children, adolescents | NR | Trauma-focused individual CBT | NA | NA | WLC, standard community care, non-directive supportive care | Long | ✗ | ✓ | ✓ | ✓ |
| Chen et al.96 2018 | PTSD | Sexual abuse | Low | 1 (14) | Children | Syria | Individual CBT | NA | EMDR | NA | Short | ✗ | ≤ | ≤ | ✓ |
| Ehring et al.135 2014 | PTSD | NA | Low | 1 (74) | Adults | NR | Trauma-focused individual CBT | NA | Present-centred therapy | WLC | Short | ✗ | ✓ | ✓ | ✓ |
| Field and Cottrell147 2011 | PTSD | Sexual abuse | Critically low | 1 (14) | Children | Iran | Individual CBT | NA | EMDR | NA | Short | ✗ | ✗ | ✓ | ✓ |
| Forman-Hoffman et al.154 2018 | PTSD | Combat assault | Low | 9 (809) | Adults | NR | Individual CBT ‘Seeking safety’ | NA | Exposure therapy, imaginal exposure, relaxation | WLC,TAU | Short | ′ | ✓ | ✓ | ✗ |
| Forneris et al.155 2013 | PTSD | Motor vehicle, industrial, non-sexual assault | Low | 3 (105) | Adults | Australia | Brief individual CBT | NA | Supportive counselling | NA | Short | ✗ | ✓ | ✓ | ✓ |
| Furuta et al.160 2018 | PTSD | Traumatic birth | Low | 2 (161) | Adults | Sweden, USA | Trauma-focused individual CBT | Internet-based CBT + TAU | NA | WLC + TAU, attention control + TAU | Short | ✗ | ✗ | ✓ | ✓ |
| Gillies et al.169 2016 | PTSD | Exposed to traumatic events | Moderate | 5 (319) | Children, adolescents | USA, Netherlands, Iran | Trauma-focused individual CBT | NA | Play therapy, EMDR | TAU | Short | ≤ | ✓ | ✓ | ✓ |
| Guest et al.183 2016 | PTSD | Post vehicle accident | Low | 2 (95) | Adults | China, Germany | Individual CBT | NA | Self-help book CBT | WLC | Short | ✗ | ✓ | ✓ | ✓ |
| Ho and Lee212 2012 | PTSD | NA | Critically low | 8 (227) | Adults | NR | Trauma-focused individual CBT | NA | EMDR | NA | Short | ✗ | ✓ | ✓ | ✓ |
| Khan et al.247 2018 | PTSD | Traumatic events | Critically low | 1 (23) | Adults | NR | NR | NA | EMDR | NA | Short | ✗ | ✓ | ′ | ′ |
| Kim et al.249 2013 | PTSD | NA | Moderate | 1 (55) | NR | USA | Individual CBT | NA | NA | WLC | NR | ✗ | ✓ | ✓ | ✓ |
| Kowalik et al.257 2011 | PTSD | Sexual abuse | Critically low | 4 (427) | Children | NR | Individual CBT or parent–child CBT, sexual specific/trauma-focused/adapted for sexually abused pre-school children (CBT-SAP) | NA | Non-directive support therapy, child-centred play therapy | NA | Short | ✗ | ✗ | ✓ | ✓ |
| Leigh-Hunt and Perry272 2015 | PTSD | Offenders | Low | 1 (44) | Adults | USA | Group CBT | NA | NA | TAU | Short | ≤ | ≤ | ✓ | ✓ |
| Lewis et al.275 2018 | PTSD | NA | High | 6 (437) | Adults | USA, Sweden, Iraq, UK | NA | Internet-based CBT | Other internet-based psychological therapy | WLC, TAU | Short | ✓ | ✓ | ✓ | ✓ |
| Mabey and van Servellen290 2014 | PTSD | Severe mental illness (depression, bipolar, schizophrenia) | Critically low | 1 (108) | Adults | USA | Individual CBT | NA | NA | TAU | Short | ≤ | ✓ | ✓ | ✓ |
| Macedo et al.292 2018 | PTSD | NA | Moderate | 1 (156) | Adults | NR | Individual CBT | NA | Brief treatment programme (breathing + psychoeducation) | NA | Long | ✗ | ✗ | ✓ | ✗ |
| Moreno-Alcázar et al.320 2017 | PTSD | NA | Moderate | 3 (119) | Children, adolescents | NR | Individual CBT | NA | EMDR | NA | Short | ✗ | ✓ | ✓ | ✓ |
| Nocon et al.333 2017 | PTSD | War displacement | Critically low | 1 (399) | Children | Sri Lanka | Individual CBT | NA | NA | WLC | Short | ✗ | ✓ | ✓ | ✓ |
| O’Donnell et al.338 2018 | Adjustment disorder | NA | Critically low | 1 (54) | Adults | NR | NA | Self-help bibliotherapy | NA | WLC | Short | ✗ | ✓ | ✓ | ✗ |
| Palic and Elklit355 2011 | PTSD | Refugees | Critically low | 5 (106) | Adults | Vietnam, Cambodia, mixed southern Asian nationality | Culturally sensitive individual CBT for South East Asians, individual CBT | NA | Psychoactive medication, exposure therapy | WLC | Short | ✓ | ✓ | ✓ | ✓ |
| de Medeiros Passarela et al.303 2010 | PTSD | Sexual abuse | Low | 2 (136) | Children, adolescents | USA, Australia | Family/parent CBT | NA | NA | TAU | Short | ✗ | ✗ | ✓ | ✓ |
| Patel et al.357 2014 | PTSD | Torture survivors | High | 1 (16) | Adults | Sweden | Individual CBT | NA | Behavioural exposure | NA | Short | ✓ | ✓ | ✓ | ✓ |
| Roberts et al.385 2009 | PTSD | NA | Moderate | 3 (248) | Adults | NR | Trauma-focused individual CBT | NA | Supportive counselling | WLC | Long | ✗ | ✗ | ✓ | ✓ |
| Roberts et al.384 2016 | PTSD | Substance misuse | Moderate | 1 (353) | Adults | USA | Group CBT or individual CBT: ‘seeking safety’, integrated CBT | NA | Group women’s health education | NA | Short–long | ✗ | ✗ | ✓ | ✓ |
| Rodenburg et al.387 2009 | PTSD | NA | Critically low | 2 (52) | Children, adolescents | NR | NR | NR | EMDR | NA | Short | ✗ | ✗ | ✓ | ✓ |
| Russell and Davis391 2007 | PTSD | Sexual assault | Critically low | 1 (10) | Adults | NR | NR | NA | NA | WLC | Short | ✗ | ✓ | ✗ | ✓ |
| Schwartze et al.399 2017 (PTSD) | PTSD | NA | Moderate | 1 (24) | Adults | NR | Culturally adapted group CBT | NA | Applied muscle relaxation + TAU | NA | Short | ✗ | ′ | ✓ | ✓ |
| Sebastian and Nelms402 2017 | PTSD | Sexual violence | Critically low | 1 (50) | NR | Democratic republic of Congo | NR | NA | EFT | NA | Short | ✗ | ≤ | ✓ | ✓ |
| Silverman et al.408 2008 | PTSD | Sexual abuse, traumatic event | Critically low | 3 (164) | Children, adolescents | Iran, NR | Trauma-focused group CBT, trauma-focused individual CBT | NA | EMDR | WLC | Short | ✗ | ✓ | ✓ | ✓ |
| Sin et al.409 2017 | PTSD | Psychosis | Moderate | 3 (145) | Adults | USA, UK | Trauma-focused individual CBT | NA | Brief psychoeducation | TAU | Short–long | ✓ | ✓ | ✓ | ✓ |
| Soo and Tate422 2007 | Acute stress disorder | Traumatic brain injury | Moderate | 1 (24) | Adults | UK | Individual CBT | NA | Supportive counselling | NA | Short | ✗ | ✗ | ✓ | ✗ |
| Sullivan and Simonson436 2016 | PTSD | Refugees | Critically low | 1 (31) | Children, adolescents | USA (refugees/immigrants from Burundi, Congo, Kenya, Liberia, Rwanda, Somalia, Tanzania, Myanmar, Nepal, Russia, Bosnia, Afghanistan, Iraq, Turkey and Uzbekistan) | Trauma-focused individual CBT | NA | Child-centred play therapy | NA | Short | ✗ | ✗ | ✓ | ✗ |
| Swan et al.439 2017 | PTSD | Psychosis | Critically low | 1 (108) | Adults | USA | Individual CBT | NA | NA | TAU | Short | ✗ | ✓ | ✓ | ≤ |
| Torchalla and Strehlau452 2018 | PTSD | Workplace trauma | Critically low | 1 (31) | Adults | USA | Trauma-focused individual CBT | NA | NA | No intervention | Short | ✗ | ✗ | ✓ | ✗ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of patients) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Brownley et al.78 2016 | BED | NA | Low | 7 (699) | Adults | USA | Therapist-led individual CBT, partially therapist-led individual CBT | Guided or pure self help | Interpersonal therapy | WLC | Short | ✗ | ✓ | ✗ | ✓ |
| de Jong et al.120 2018 | Bulimia nervosa, BED, EDNOS | NA | Low | 1 (130) | Adults | UK | NR | NA | Interpersonal therapy | NA | Long | ✗ | ✗ | ✗ | ✓ |
| Flament et al.148 2012 | BED and anorexia nervosa | NA | Critically low | 1 (108) | Adults | Italy | Individual CBT | NA | Drug | NA | Long | ✗ | ✗ | ✗ | ✓ |
| Flodgren et al.150 2015 | BED | NA | High | 1 (128) | Adults | USA | NA | Video-teleconference CBT | Face-to-face CBT | NA | Short | ✓ | ✓ | ✗ | ✓ |
| Ghaderi and Andersson166 1999 | Mixed | NA | Critically low | 5 (334) | NR | NR | NR | NA | Drug, supportive–expressive therapy, interpersonal therapy | WLC | Short | ✗ | ✗ | ✗ | ✓ |
| Ghaderi et al.167 2018 | Bulimia nervosa | NA | Moderate | 9 (949) | Adults | USA, Switzerland, the Netherlands | Individual CBT, group CBT, mixed individual CBT + group CBT | Self-help, guided self-help | Interpersonal therapy, behavioural weight loss | WLC, placebo | Short–long | ≤ | ✓ | ✗ | ✓ |
| Grenon et al.179 2017 | BED | NA | Moderate | 4 (328) | Adults | USA, Canada | Group CBT | NA | Behavioural therapy, interpersonal therapy, group psychodynamic interpersonal therapy | NA | Short | ✗ | ′ | ✗ | ✓ |
| Grenon et al.178 2018 | Bulimia nervosa, BED | NA | Critically low | 1 (42) | Adults | USA | Group CBT | NA | Behavioural therapy | NA | Short | ✗ | ′ | ✗ | ✓ |
| Hay and Claudino200 2010 | Mixed | NA | Critically low | 3 (220) | Adults | NR | Individual CBT for bulimia nervosa | NA | Guided self-help CBT | WLC | Short | ≤ | ✓ | ✗ | ✓ |
| Hay et al.201 2012 | Bulimia nervosa | NA | Low | 1 (33) | Adults | NR | Individual CBT | NA | Individual nutritional counselling | NA | Long | ✗ | ✗ | ✗ | ✓ |
| Hay et al.199 2001 | Anorexia nervosa | NA | Moderate | 4 (141) | Adults | USA, Canada | Individual CBT | NA | Drug | NA | Short | ✗ | ✓ | ✗ | ✓ |
| Hay et al.198 2009 | Bulimia nervosa | NA | Moderate | 17 (1367) | Adults | USA, Canada, UK, Australia | Group CBT or individual CBT for bulimia, mixed group CBT + individual CBT | NA | Self-monitoring, CBT/exposure and response prevention, interpersonal therapy, behavioural therapy, short-term focal psychotherapy, psychoeducation, supportive–expressive therapy, hypo behavioural therapy, prescriptive therapy non-directive counselling and ‘focused evocative unfolding’ | WLC, no intervention | Short | ✗ | ✓ | ✗ | ✓ |
| Hay et al.197 2015 | Anorexia nervosa | NA | Moderate | 2 (56) | Adults | New Zealand, Germany | Individual CBT | NA | Interpersonal therapy, SSCM, FPDT | Optimised TAU | Long | ✗ | ✓ | ✗ | ✓ |
| Hilbert et al.208 2019 | BED | NA | Low | 5 (494) | Adults | NR | Group CBT, individual CBT | NA | Weight loss treatment, drug | NA | Short | ✗ | ′ | ✗ | ✓ |
| Keel and Haedt243 2008 | Bulimia nervosa, EDNOS | NA | Critically low | 1 (85) | Adolescents | NR | NA | CBT guided self-care | Family therapy | NA | Long | ✗ | ✗ | ✗ | ✓ |
| Loucas et al.287 2014 | Anorexia nervosa, bulimia nervosa, BED, EDNOS | NA | Low | 4 (474) | Adults | NR | NA | Internet-based CBT, CD-ROM CBT | High-intensity face-to-face CBT | WLC, TAU | Short–long | ✗ | ✗ | ✗ | ✓ |
| Miniati et al.311 2018 | Anorexia nervosa | NA | Low | 1 (56) | Adults | NR | Individual CBT | NA | Interpersonal therapy | Non-specific supportive clinical management | Short | ✗ | ✗ | ✗ | ✓ |
| Norris et al.337 2019 | Mixed | NA | Critically low | 5 (249) | Adults | Australia, UK, USA, Germany | Enhanced individual CBT | NA | NR | NR | Short | ✗ | ✗ | ✗ | ✓ |
| Palavras et al.353 2017 | BED | NA | Low | 3 (261) | Adults | USA, Switzerland | Group CBT, individual CBT | Guided self-help | Behavioural weight loss therapy, interpersonal therapy | NA | Long | ✗ | ✗ | ✗ | ✓ |
| Peat et al.359 2017 | BED | NA | Critically low | 2 (170) | Adults | NR | Group CBT | NA | Behavioural weight loss therapy | NA | Long | ✗ | ✓ | ✗ | ✓ |
| Pittock et al.367 2018 | Bulimia nervosa | NA | Low | 1 (196) | Adults | USA | NA | Internet-based CBT | Face-to-face CBT | NA | Long | ✗ | ✗ | ✗ | ✓ |
| Polnay et al.369 2014 | Bulimia nervosa | NA | Moderate | 3 (133) | Adults | USA, Norway | Group CBT (delivered by doctoral qualified psychologists) | NA | NA | WLC | Short | ✗ | ✓ | ✗ | ✓ |
| Reas and Grilo378 2008 | BED | NA | Critically low | 1 (108) | Adults | USA | Individual CBT | NA | Fluoxetine | Placebo | Short | ✗ | ✗ | ✗ | ✓ |
| Svaldi et al.438 2018 | Bulimia nervosa | NA | Moderate | 4 (295) | Adolescents, adults | NR | Individual CBT | NA | (Active arms not included in analysis) | WLC | Short | ✗ | ✓ | ✗ | ✓ |
| Watson et al.491 2016 | Mixed | NA | Low | 1 (76) | Adults | USA | NA | Internet-based CBT | Psychoeducation | NA | Short | ✗ | ✗ | ✗ | ✓ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of patients) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Nezu et al.328 2001 | Bodily distress or experience disorder | NA | Critically low | 1 (50) | Adults | NR | Group CBT | NA | NA | WLC | Short | ✗ | ✓ | ✗ | ✓ |
| van Dessel et al.469 2014 | Bodily distress or experience disorder | NA | High | 4 (504) | Adults | USA, Spain, Germany, the Netherlands | Group CBT, individual CBT | NA | Progressive muscle relaxation | TAU | Short–long | ✓ | ✓ | ✓ | ✓ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of patients) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Dugosh et al.130 2016 | Opioid addiction | NA | Critically low | 1 (60) | NR | NR | Individual CBT + methadone maintenance treatment | NA | Methadone maintenance treatment | NA | Long | ✓ | ✗ | ✗ | ✗ |
| Eccleston et al.133 2017 | Opioid misuse | Chronic back/neck pain | Moderate | 1 (42) | Adults | NR | Group CBT, individual CBT for the prevention of opioid misuse | NA | NA | TAU | Short | ✗ | ✓ | ✓ | ✓ |
| Harada et al.193 2018 | Due to use of stimulants | Amphetamine-type stimulants | High | 1 (160) | Adults | Australia | NA | Internet-based CBT | NA | WLC | Short | ✓ | ✗ | ✗ | ✗ |
| Minozzi et al.312 2016 | Due to use of hallucinogens | Psychostimulant | High | 0 (0) | Adults | NA | NA | NA | NA | NA | NA | ✗ | ≤ | ✗ | ≤ |
| Stevens et al.429 2018 | Internet gaming disorder | NA | Critically low | 3 (148) | Adolescents, NR | China, Republic of Korea, NR | Group CBT, group CBT + medication | NA | Medication only, physical exercise | No intervention | Short | ✗ | ✓ | ✓ | ✗ |
| van der Maas et al.468 2019 | Gambling disorder | NA | Critically low | 1 (66) | Adults | Sweden | NA | Internet-based CBT and e-mail and discussion groups | NA | WLC | Long | ✗ | ✓ | ✓ | ✗ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of patients) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Davidson and Tran115 2014 | Personality disorder | NA | Critically low | 1 (106) | Adults | NR | Individual CBT for personality disorder | NA | NA | TAU | Long | ✗ | ✓ | ✗ | ✗ |
| Gibbon et al.168 2010 | Personality disorder | Cocaine dependence | Moderate | 1 (43) | Adults | UK | Individual CBT | NA | NA | TAU | Short | ≤ | ✓ | ✓ | ✗ |
| Matusiewicz et al.298 2010 | Personality disorder | NA | Critically low | 4 (348) | Adults | NR | Individual CBT | NA | Brief dynamic therapy, brief relational therapy | WLC, TAU | Short | ✗ | ✓ | ✓ | ✓ |
| Stoffers et al.432 2012 | Personality disorder | NA | Moderate | 1 (106) | Adults | UK | Individual CBT | NA | NA | TAU | Short | ✗ | ✓ | ✓ | ✓ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of patients) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Sleight415 2016 | Mild neurocognitive disorder | Cancer (breast) | Critically low | 1 (41) | Adults | USA | Individual CBT intervention ‘Memory and Attention Adaption Training’ | NA | NA | WLC | Short | ✓ | ✗ | ✗ | ✗ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of patients) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Bledsoe and Grote71 2006 | Depressive disorder | Pregnancy and postpartum depression | Critically low | 3 (172) | Adults | UK, France | Home-based individual CBT | NA | NA | TAU, placebo, NR | Short | ✗ | ✓ | ✗ | ✗ |
| Craig and Howard109 2009 | Postnatal depression | NA | Critically low | 5 (502) | Adults | NR | Individual CBT, individual CBT + antidepressants, group CBT | Individual CBT (delivered by early-childhood nurses), group CBT (delivered by health visitors) | Non-directive counselling, psychodynamic therapy, antidepressants | TAU | Short–long | ≤ | ✓ | ✗ | ✗ |
| De Crescenzo119 2014 | Postnatal depression | NA | Moderate | 1 (35) | Adults | NR | Individual CBT + paroxetine | NA | Paroxetine only | NA | Short | ✗ | ✓ | ✗ | ✗ |
| Dennis125 2004 | Postnatal depression | NA | Critically low | 1 (176) | Adults | Finland | Individual CBT with between-session telephone support | NA | NA | TAU | Short | ✗ | ✓ | ✗ | ✗ |
| Huang216 2018 | Postnatal depression | NA | Low | 6 (510) | Adults | Canada, Australia, France, Iran | Individual CBT, group CBT | Internet-based CBT | NA | TAU | Short | ′ | ✓ | ✓ | ✗ |
| Lau267 2017 | Postnatal depression | Pregnancy loss, PTSD, depression | Moderate | 6 (530) | Adults | Canada, USA, Australia, Germany, Sweden | NA | Internet-based CBT with therapist support | NA | TAU, WLC | Short | ✗ | ✓ | ✓ | ✗ |
| Lavender268 2016 | Antenatal depression | NA | Critically low | 1 (55) | Adults | USA | Modified individual CBT | NA | NR | NR | Short | ✗ | ✓ | ≤ | ✗ |
| Leis273 2009 | Postnatal depression | NA | Critically low | 1 (37) | Adults | Australia | NA | CBT (delivered by early-childhood nurses) | Support sessions | NA | Short | ✗ | ✓ | ✗ | ✗ |
| LoGiudice284 2018 | Undergoing IVF | NA | Critically low | 1 (31) | Adults | Iran | Group CBT | NA | NR | NR | Short | ≤ | ✓ | ✓ | ✗ |
| Maguire294 2018 | Postnatal depression | NA | Critically low | 2 (172) | Adults | Germany, Australia | Group CBT, individual CBT | NA | NA | TAU | Short | ✗ | ✗ | ✓ | ✗ |
| McDonagh301 2014 | Postnatal depression | NA | Low | 1 (35) | Adults | Canada | NR | NA | Paroxetine only | NA | Short | ✗ | ✓ | ✗ | ✗ |
| Mendelson307 2017 | Perinatal anxiety, depression | NA | Low | 1 (39) | Adults | USA | Individual CBT | NA | NA | TAU | Short | ✗ | ✓ | ≤ | ✗ |
| Nillni331 2018 | Perinatal anxiety, depression | NA | Critically low | 1 (105) | Adults | NR | Individual CBT | NA | NA | TAU + parent mentor programme | Short | ✗ | ✓ | ✓ | ✗ |
| Perveen363 2013 | Postnatal depression | NA | Critically low | 5 (607) | Adults | UK, USA, Australia | Individual CBT (delivered by therapists) | CBT (delivered by health visitor or research staff) | NA | TAU | Short | ✗ | ✓ | ✓ | ✗ |
| Sangsawang393 2018 | Postnatal depression | NA | Critically low | 1 (47) | Adolescents | USA | NA | CBT adapted for Apache adolescents, ‘Living in Harmony’ programme | Educational support | NA | Short | ✗ | ✓ | ≤ | ≤ |
| Scope400 2013 | Postnatal depression | NA | Low | 1 (192) | Adults | Australia | Group CBT | NA | Group + individual counselling | TAU | Short | ✗ | ✓ | ✗ | ✗ |
| Stevenson430 2010 | Postnatal depression | NA | Moderate | 1 (192) | Adults | Australia | Group CBT | NA | Group counselling | NA | Short | ✗ | ✓ | ✗ | ✗ |
| van Ravesteyn471 2017 | Antenatal depression | NA | Low | 1 (1300) | Adults | NR | Group CBT, individual CBT | NA | Supportive counselling, psychoeducation, health-care visits | TAU, booklet | Short | ✗ | ✓ | ✗ | ✗ |
| Webb494 2004 | Perinatal psychosis | Perinatal period | Moderate | 0 (0) | Adults | NR | NA | NA | NA | NA | NA | ✗ | ✗ | ≤ | ✗ |
| ICD-11 primary/secondary | Condition description | Study | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of patients) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition-specific | ||||||||
|
|
Adelufosi35 2017 | Female genital mutilation | Critically low | 0 (0) | Children, adults | NR | NA | NA | NA | NA | NA | ✗ | ≤ | ≤ | ≤ |
|
|
Andersen44 2016 | NA | Moderate | 5 (435) | Adults | Australia, USA | Transdiagnostic individual or group CBT | Transdiagnostic internet-based CBT + e-mail/telephone | NA | WLC | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Andrews46 2018 | NA | Moderate | 53 (8279) | Adults | NR | NA | Internet-based CBT | High-intensity face-to-face CBT, bibliotherapy CBT | WLC ± discussion group, WLC ± attention placebo, TAU, information, self-monitoring | Short–long | ✗ | ✓ | ✓ | ✓ |
|
|
Armento52 2012 | Parkinson’s disease | Critically low | 2 (174) | Older adults | NR | Individual CBT, 1 session of individual CBT + telephone sessions | NA | Supportive therapy | Clinical monitoring | Short | ✓ | ✓ | ✓ | ✓ |
|
|
Arnberg53 2014 | NA | Low | 21 (1855) | Children, adolescents, adults | Australia, Sweden, Switzerland | NA | Internet-based CBT | High-intensity individual CBT, online supportive therapy | WLC, online forum | NR | ✗ | ✓ | ✓ | ✓ |
|
|
Bower74 2003 | NA | Low | 1 (NR) | Adults | NR | NR | NA | Non-directive counselling | NA | Long | ✗ | ✓ | ≤ | ✗ |
|
|
Cape86 2010 | NA | Critically low | 11 (709) | Adults | Australia, UK | Individual CBT | NA | NA | TAU | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Chibanda99 2015 | HIV | Low | 1 (13) | Adults | China | Group CBT | NA | NA | WLC | Short | ✗ | ✓ | ≤ | ✗ |
|
|
Clarke101 2015 | NA | Low | 1 (1477) | Adolescents | Australia | NA | Internet-based CBT | NA | WLC | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Coventry108 2008 | COPD | Low | 1 (48) | Adults | USA | Individual CBT | NA | Education | NA | NR | ✗ | ✓ | ✓ | ✗ |
|
|
Davies117 2014 | NA | Moderate | 4 (198) | Adults | Australia, Canada, Spain | NA | Internet-based CBT | Other psychotherapy | WLC, no intervention | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Ebert131 2015 | NA | Low | 11 (778) | Children, adolescents | Australia, the Netherlands, New Zealand, Sweden | NA | Internet-based CBT | NA | WLC, placebo | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Farrand143 2015 | Myocardial infarction, multiple sclerosis | Moderate | 2 (235) | Adults | NR | NA | Internet-based CBT | NA | TAU, attention control | Long | ✗ | ✓ | ✓ | ✓ |
|
|
Fulton158 2018 | Breast cancer | Critically low | 1 (13) | Adults | NR | Group CBT | NA | Supportive therapy | NA | Short | ✓ | ′ | ′ | ✗ |
|
|
Garcia-Escalera162 2016 | NA | Low | 14 (1337) | Children, adults | Australia, UK, USA | Transdiagnostic individual or group CBT | Transdiagnostic internet-based CBT | NA | WLC, discussion group | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Giummarra171 2018 | Traumatic injury | Low | 3 (366) | Adults | Australia, the Netherlands | Individual CBT, modular CBT | Internet-based CBT | Supportive counselling | TAU | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Gonçalves173 2015 | NA | Low | 3 (67) | Adults | NR | Individual CBT | NA | NA | WLC | Short | ✗ | ✗ | ✓ | ✓ |
|
|
Gould175 2012 | NA | Moderate | 3 (172) | Older adults | NR | Individual CBT | NA | Drug, supportive therapy | TAU | Long | ✗ | ✓ | ✓ | ✗ |
|
|
Gregory177 2018 | NA | Critically low | 2 (53) | Adults | USA | Individual CBT, group CBT | NA | NR | NR | Short | ✗ | ✗ | ✓ | ✗ |
|
|
Grist182 2018 | NA | Low | 3 (408) | Children, adolescents | The Netherlands, Sweden | NA | Internet-based CBT | High-intensity, face-to-face CBT | WLC | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Hobbs214 2011 | Alcohol dependence | Critically low | 2 (131) | Adults | NR | NR | NA | NR | NR | Short | ✗ | ✓ | ✓ | ✓ |
|
|
Huang215 2018a | NA | Critically low | 2 (79) | Adults | Australia, USA | Group CBT | Internet-based CBT | NA | Placebo, no intervention | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Jennings229 2015 | Learning disability | Critically low | 1 (32) | Adults | UK | Individual CBT | NA | NA | TAU | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Kaltenthaler239 2006 | NA | Low | 1 (274) | Adults | UK, USA | NA | Internet-based CBT | NA | TAU, information | Short | ≤ | ✓ | ✓ | ≤ |
|
|
Kayrouz242 2018 | NA | Moderate | 1 (159) | Adults | Iraq | NA | Internet-based CBT | NA | WLC | Short | ✗ | ✓ | ✓ | ✓ |
|
|
Mac-donald291 2016 | Childhood abuse | Moderate | 6 (658) | Children, adolescents | Australia, Norway, USA | Individual CBT, trauma-focused individual CBT, sexual-abuse individual CBT, child individual CBT | NA | Supportive therapy, child-centred therapy | WLC, TAU | Short | ≤ | ✓ | ✓ | ✓ |
|
|
Mayo-Wilson299 2013 | NA | High | 22 (2001) | Adults | Australia, Canada, the Netherlands, Spain, Sweden, UK, USA | NA | Internet-based CBT, telephone CBT, bibliotherapy CBT, mixed CBT (bibliotherapy + internet-based CBT) | High-intensity CBT | WLC, TAU | Short | ✓ | ✓ | ✓ | ✓ |
|
|
Mehndiratta305 2013 | Epilepsy | Critically low | 1 (37) | Older adults | Australia | Group CBT | NA | NA | TAU | Short | ✗ | ✓ | ≤ | ✓ |
|
|
Montero-Marin315 2018 | NA | Moderate | 4 (495) | Adults | Spain, USA, Norway | Group CBT, individual CBT | NA | Progressive relaxation, applied relaxation | NA | Short–long | ′ | ✓ | ✓ | ✓ |
|
|
Naidu324 2016 | NA | Low | 5 (390) | Adults | Sweden, Australia | NA | Internet-based CBT | High-intensity face-to-face individual or group CBT | NA | Short | ✗ | ≤ | ✓ | ✗ |
|
|
Newby327 2016 | NA | Low | 14 (1606) | Adults, older adults | Sweden, Canada, Australia, UK, Switzerland, Germany, Austria | NA | Transdiagnostic internet-based CBT | Relaxation | WLC, TAU, discussion, forum | Short | ✓ | ✓ | ✓ | ✗ |
|
|
Ng329 2018 | NA | Critically low | 1 (NR) | Adults, older adults | China | Group CBT | NA | NA | WLC, no intervention | Short | ✓ | ≤ | ✓ | ✗ |
|
|
Noble332 2018 | Epilepsy | Critically low | 1 (59) | Adults | Australia | Individual CBT | NA | NA | WLC | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Noone335 2018 | Dementia | Low | 2 (82) | Older adults | UK, USA | Individual CBT | NR | NA | TAU | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Normann336 2018 | NA | Critically low | 2 (122) | Adults | NR | Individual CBT | NA | Metacognitive therapy | NA | Short–long | ✗ | ✓ | ✓ | ✗ |
|
|
Olthuis343 2016 | NA | Moderate | 28 (4998) | Adults | Australia, Austria, Germany, the Netherlands, Sweden, Switzerland | NA | Internet-based CBT | High-intensity face-to-face CBT | WLC, attention control, information, discussion | Short–long | ✓ | ✗ | ✓ | ✓ |
|
|
Opoka344 2018 | Psychosis | Critically low | 1 (150) | Adults | NR | Individual CBT | NA | NA | TAU | Short | ✗ | ✗ | ✗ | ✓ |
|
|
O’Sullivan351 2016 | War-affected children and young people | Critically low | 2 (102) | Children, adolescents | Democratic Republic of the Congo | Group trauma-focused CBT | NA | NA | WLC | ✗ | ′ | ✓ | ✓ | |
|
|
Păsărelu356 2017 | NA | Low | 4 (283) | Adults | Sweden, Australia | NA | Transdiagnostic internet-based CBT | NA | WLC | Long | ✓ | ✓ | ✓ | ✗ |
|
|
Pennant360 2015 | NA | Low | 15 (2615) | Children, adolescents, young adults | NR | NA | Computerised CBT | High-intensity face-to-face CBT, attention bias modification, computer control | WLC, TAU, no intervention | Short | ✗ | ✓ | ✓ | ✓ |
|
|
Purgato373 2018 | NA | Moderate | 1 (313) | Children, adolescents, adults | Thailand, China, Iraq, Democratic Republic of the Congo | Trauma-focused individual CBT | Web-based CBT, group CBT (delivered by school staff and paraprofessionals) | NA | TAU, no intervention, general support, WLC | Short | ✓ | ✓ | ✓ | ✓ |
|
|
Rasing377 2017 | NA | Critically low | 16 (4422) | Adolescents | Australia, USA, the Netherlands, Canada | Group CBT | NA | Activity | Information | Long | ✗ | ✓ | ✓ | ✗ |
|
|
Reavell379 2018 | Cardiovascular disease | Low | 1 (38) | Adults | Australia | Individual CBT | NA | NA | TAU | Long | ′ | ✓ | ✓ | ≤ |
|
|
Rodrigues388 2011 | NA | Critically low | 5 (132) | Adults | NR | Individual CBT | NA | Drug | WLC | Short | ✗ | ✗ | ✓ | ✓ |
|
|
Smith418 2014 | COPD | Low | 4 (378) | Adults | Norway, Australia, USA | Group CBT, individual CBT | NA | COPD education | TAU | Short | ′ | ✓ | ✓ | ✗ |
|
|
Snoek419 2002 | Diabetes | Critically low | 8 (85) | Adults | NR | Group CBT, individual CBT | NA | Non-prescriptive therapy | No intervention | Short | ✗ | ✓ | ✓ | ✓ |
|
|
Spain423 2015 | Autism spectrum disorder | Critically low | 1 (37) | Adults | UK | Individual CBT for OCD | NA | Anxiety management | NA | Short | ✗ | ✓ | ✓ | ✓ |
|
|
Spek424 2007 | NA | Low | 10 (1465) | Adults | NR | NA | Internet-based CBT | NA | WLC, TAU, placebo, self-monitoring, information, online discussion group | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Spies425 2013 | HIV/AIDS | Critically low | 3 (250) | Adults | Hong Kong, USA | Group CBT, individual CBT | NA | Supportive psychotherapy, peer counselling, intervention control | No intervention | NR | ✓ | ✓ | ✓ | ✗ |
|
|
Tay442 2018 | Dementia | Critically low | 1 (50) | Older adults | NR | Individual CBT (for mild to moderate dementia patients) | NA | NA | TAU | Short | ✗ | ✓ | ′ | ✗ |
|
|
Thabrew445 2018 | Long-term physical health problems | High | 8 (770) | Children, adolescents | USA, UK, India, Australia, Serbia, Montenegro | Group CBT, individual CBT, home-based individual CBT, clinic-based group CBT, transdiagnostic CBT | NA | Education, supportive psychotherapy, supportive non-directive therapy, non-directive behavioural counselling | WLC, TAU, information | Short–long | ✓ | ✓ | ✓ | ✓ |
|
|
Thabrew446 2018b | Long-term physical health problems | High | 4 (469) | Children, adolescents | USA, Germany | NA | Internet-based CBT or Web-MAP | Specialised headache treatment, education, applied relaxation | WLC | Short–long | ✓ | ✓ | ✓ | ✓ |
|
|
Troeung457 2013 | Parkinson’s disease | Critically low | 1 (80) | Older adults | USA | Individual CBT | NA | NA | Clinical monitoring | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Twomey461 2015 | NA | Low | 25 (3716) | Adults | NR | Individual CBT, group CBT | Computerised CBT, guided CBT, telephone CBT | NA | WLC, no intervention, TAU | Short | ✗ | ✓ | ✓ | ✓ |
|
|
Twomey459 2017 (MoodGYM) | NA | Critically low | 11 (4570) | Adults | Australia, Ireland, UK, Norway | NA | Internet-based CBT ‘MoodGYM’ | Telephone support or website | TAU, no intervention | Short | ✗ | ✓ | ≤ | ✗ |
|
|
Valimaki466 2017 | NA | Low | 1 (187) | Adolescents | New Zealand | NA | Computerised CBT ‘SPARX’ | NA | TAU | Short | ✗ | ′ | ✓ | ✗ |
|
|
Verdeli479 2006 | NA | Critically low | 3 (118) | Adolescents | NR | Group CBT, individual CBT, school-based CBT | NA | Interpersonal therapy | No intervention, WLC, attention support | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Wang484 2013 | NA | Moderate | 2 (115) | Adults | Hong Kong | Group CBT, individual CBT | NA | Dejian mind–body intervention | WLC | Short | ✗ | ✓ | ≤ | ✗ |
|
|
Watts493 2015 | NA | Critically low | 48 (6926) | Adults | NR | Individual CBT (delivered by therapists, psychologists) | CBT (delivered by nurses, social workers, behavioural health specialists) | NA | TAU | Short | ✗ | ✓ | ✓ | ✗ |
|
|
Yatham511 2018 | NA | Critically low | 2 (615) | Children, adolescents | Palestine | Individual CBT ‘teaching recovery techniques’, individual CBT adapted ‘teaching recovery techniques’ | NA | NA | WLC | Short | ✗ | ′ | ✓ | ✗ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Bohra 201372 | Insomnia | Pain | Critically low | 1 (51) | Older adults | NR | Group CBT for insomnia | NA | Stress management and wellness | NA | Short | ✗ | ✗ | ✗ | ✓ |
| Brasure 201675 | Insomnia | NA | Low | 11 (1182) | Adults | USA, UK, Europe | Group CBT for insomnia, individual CBT for insomnia | Self-help CBT for insomnia, internet-based CBT for insomnia | Sleep hygiene, self-help | Internet sham, placebo (drug), WLC, TAU | Short | ✗ | ✗ | ✗ | ✓ |
| Budhrani 201580 | Insomnia | Breast cancer | Critically low | 1 (72) | Adults | NR | Individual CBT | NA | NR | NR | Short | ✗ | ✗ | ✗ | ✓ |
| Cheng 201297 | Insomnia | NA | Low | 4 (300) | Adults | USA, Canada, Sweden | NA | Computerised CBT for insomnia | NA | WLC, self-monitoring | Short | ✗ | ✗ | ✗ | ✓ |
| Cheung 201898 | Insomnia | NA | Critically low | 1 (139) | Adults | UK | NA | Group CBT (delivered by health visitors) | NA | Sleep monitoring | Long | ✗ | ✗ | ✗ | ✓ |
| Gebara 2018163 | Insomnia | Depression | Critically low | 3 (124) | Adults | USA, Sweden | Group CBT, individual CBT | NA | Drug, relaxation | WLC | Short | ✗ | ✓ | ✗ | ✓ |
| Johnson 2016233 | Insomnia | Cancer | Critically low | 5 (621) | Adults | NR | Individual CBT for insomnia, group CBT for insomnia | Video-based CBT for insomnia | Behavioural placebo, mindfulness-based cancer recovery | WLC, TAU | Short | ✗ | ✗ | ✗ | ✓ |
| Koffel 2015255 | Insomnia | NA | Low | 6 (408) | Adults | NR | Group CBT | NA | Sleep/pain education | WLC, TAU, diary-keeping | Short | ✗ | ✓ | ✗ | ✓ |
| Mitchell 2012314 | Insomnia | NA | Critically low | 3 (201) | Adults, older adults | Norway, Canada, China | Group CBT, individual CBT | NA | Drug | NA | Short–long | ✓ | ✓ | ✓ | ✓ |
| Montgomery 2003316 | Insomnia | NA | Moderate | 2 (102) | Older adults | USA | Group CBT, individual CBT | NA | NA | WLC, placebo | Short | ≤ | ✗ | ✗ | ✓ |
| Morin 1999321 | Insomnia | NA | Critically low | 1 (24) | Adults | NR | Multicomponent group CBT | NA | NA | WLC | Long | ✗ | ✗ | ✗ | ✓ |
| Navarro-Bravo 2015326 | Insomnia | NA | Critically low | 5 (456) | Adults | NR | Individual CBT | NA | Stress management and wellness, sleep hygiene education | WLC, TAU | Short | ✗ | ✗ | ✗ | ✓ |
| Okajima 2011339 | Insomnia | NA | Critically low | 4 (226) | Adults | NR | Group CBT, individual CBT | NA | Drug | WLC, placebo, TAU | Long | ✗ | ′ | ✗ | ✓ |
| Phelps 2017366 | Insomnia | NA | Critically low | 1 (81) | Adults | USA | NR | NA | Sleep hygiene | NA | Short | ✗ | ✗ | ✗ | ✓ |
| Seyffert 2016403 | Insomnia | NA | Low | 11 (1886) | Adults | Canada, Hong Kong, UK, Sweden, the Netherlands, Germany, USA | NA | Internet-based CBT | Group CBT, telehealth CBT, internet-based CBT without support, internet control, imagery relief therapy | WLC | Short–long | ✗ | ✓ | ✗ | ✓ |
| Smallfield 2018416 | Insomnia | NA | Critically low | 1 (60) | Older adults | NR | NA | Telephone CBT (delivered using workbook) | NA | Information | Short | ✗ | ✗ | ✗ | ✓ |
| Taylor 2014443 | Insomnia | Depression, PTSD, alcohol abuse | Critically low | 4 (132) | Adults | NR | Individual CBT | Self-help CBT with telephone support | NA | Placebo, WLC, NR | Short | ✗ | ✓ | ✓ | ✓ |
| Trauer 2015455 | Insomnia | NA | Moderate | 5 (240) | Adults | Canada, USA, China, Australia | Group CBT for insomnia, individual CBT for insomnia | NA | Sleep hygiene education | WLC, placebo, tablets | Short–long | ✗ | ✗ | ✗ | ✓ |
| Twomey 2013460 | Insomnia | NA | Critically low | 33 (5768) | Adults | Australia, UK, Spain, USA, Switzerland, Canada, Germany | NA | Computerised CBT | High-intensity face-to-face CBT, psychoeducation, computer-guided self-relaxation | WLC, TAU, NR | Short | ✗ | ✓ | ✓ | ✓ |
| Werner-Seidler 2018496 | Insomnia | NA | Low | 2 (173) | Adolescents, young adults | The Netherlands, UK | NA | eCBT for insomnia | NA | WLC | Short | ≤ | ✓ | ✓ | ✓ |
| Wu 2015505 | Insomnia | Medical/psychiatric conditions | Low | 10 (609) | Adults | NR | Individual CBT for insomnia, individual CBT for insomnia + drug | NA | Sleep hygiene, escitalopram plus quasi desensitisation, mindfulness-based stress reduction, obstructive sleep apnoea surgery, wellness education, audiotape relaxation training | WLC, TAU | Short | ✗ | ′ | ′ | ✓ |
| Zachariae 2016516 | Insomnia | NA | Low | 11 (1460) | Adults | NR | NA | Internet-based CBT for insomnia | NR | WLC | Short | ✗ | ✗ | ✗ | ✓ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Bagnell 200756 | Post-viral fatigue syndrome | NA | Low | 4 (459) | Children, adults | NR | Individual CBT | NA | Relaxation, guided support group | TAU, WLC, no intervention | Long | ✓ | ✓ | ✓ | ✓ |
| Carlson 201788 | Psychogenic non-epileptic seizures | NA | Low | 1 (34) | Adults | USA | Individual CBT | NA | NA | TAU | Short | ✗ | ✗ | ✗ | ✓ |
| Castell 201193 | Post-viral fatigue syndrome | NA | Critically low | 16 (1457) | Adults | NR | Individual CBT, group CBT | NA | Education/support, relaxation, supportive listening, adaptive pacing, counselling | WLC, placebo, injection, TAU, medical care | Short | ✓ | ′ | ′ | ′ |
| Cleare 2015103 | Post-viral fatigue syndrome | NA | Critically low | 9 (1552) | Children, adolescents, adults | NR | Group CBT, individual CBT | Internet-based CBT | Guided support, relaxation, education and support, psychoeducation, specialist medical care | No intervention, TAU | Long | ✓ | ✓ | ✓ | ✓ |
| Cross 2015110 | Epilepsy or seizures | NA | Critically low | 2 (60) | Adolescents, adults | NR | Group CBT, individual CBT | NA | Supportive counselling | No intervention, TAU | Short | ✓ | ✓ | ✗ | ✓ |
| Gillings 2007170 | Post-viral fatigue syndrome | NA | Low | 4 (NR) | Adults | NR | NR | NA | Relaxation and guided support | No intervention | Short–long | ✓ | ✓ | ✓ | ✓ |
| Koychev 2017258 | Parkinson’s disease | NA | Critically low | 1 (45) | Adults | NR | Individual CBT | NA | NA | WLC | Short | ′ | ✓ | ✓ | ✓ |
| Larun 2017266 | Post-viral fatigue syndrome | NA | Low | 2 (331) | Adults | UK, USA | Individual CBT | NA | Anaerobic activity therapy, cognitive therapy, relaxation, specialist medical care, adaptive pacing therapy, graded exercise therapy | NA | Long | ′ | ✓ | ✓ | ✓ |
| Maguire 2011293 | Epilepsy or seizures | NA | Critically low | 2 (60) | Adolescents, adults | NR | NR | NA | Supportive counselling | TAU, no intervention | Short | ✓ | ✓ | ✗ | ✓ |
| Martlew 2014297 | Epilepsy or seizures | NA | Moderate | 1 (66) | Adults | UK | Individual CBT | NA | NA | TAU | Short | ≤ | ✓ | ✓ | ✓ |
| Price 2008371 | Post-viral fatigue syndrome | NA | Moderate | 8 (1050) | Adults | UK, USA, the Netherlands, Australia | Group CBT, individual CBT | NA | Education, relaxation, psychodynamic, exercise, cognitive therapy, counselling, guided support group | WLC, TAU, no intervention | Short | ✓ | ✓ | ✓ | ✓ |
| Reid 2011103 | Post-viral fatigue syndrome | NA | Critically low | 1 (69) | Children, adolescents | NR | Individual CBT | NA | NA | No intervention | Short | ≤ | ≤ | ≤ | ✓ |
| Russell 2017390 | Post-viral fatigue syndrome | NA | Critically low | 1 (640) | Adults | NR | Individual CBT | NA | NA | TAU | Long | ✗ | ✗ | ✗ | ✓ |
| Walklet 2016483 | Motor neuron diseases or related disorders | NA | Low | 2 (60) | Adults | NR | Individual CBT | Na | NA | TAU | Short | ✓ | ✗ | ✗ | ✗ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Fackrell 2017140 | Hyperacusis | NA | Critically low | 1 (60) | Adults | NR | Individual CBT | NA | NA | WLC | Long | ✓ | ✓ | ✓ | ✓ |
| Hoare 2011213 | Tinnitus | NA | Low | 8 (410) | Adults, older adults | UK | Therapist-delivered individual CBT | Internet-based CBT | Internet-based education, education, minimal contact education relaxation, tinnitus retraining therapy | WLC | Short | ✗ | ✓ | ✓ | ✓ |
| Toivonen 2017451 | Tinnitus | NA | Moderate | 1 (64) | Adults | Sweden | NA | Internet-based CBT | Asynchronous web-based acceptance and commitment therapy | Online discussion forum | Short–long | ✓ | ✓ | ✓ | ✓ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of patients) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Kew 2016246 | Asthma | NA | High | 6 (134) | Adults | UK, India, Belgium, Canada, Italy | Individual CBT | NA | NA | WLC, no intervention TAU, self-management | Short | ✓ | ✓ | ✓ | ✓ |
| Yorke 2015514 | Asthma | NA | Low | 1 (40) | Adults | NR | Individual CBT | NA | Self-management intervention | NA | Short | ✓ | ≤ | ′ | ✓ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Amorim 201842 | Dentofacial parafunctional disorders | NA | Low | 1 (57) | Adults | Germany | NA | CBT (delivered by health-care professional) | Occlusal splint | NA | Short | ✗ | ✓ | ≤ | ✓ |
| Clarkson 2010102 | Oral mucositis | Cancer | Low | 2 (68) | Adults | USA | Individual CBT | NA | Hypnosis, relaxation and imagery | Therapist contact, TAU | Short | ≤ | ✗ | ✗ | ✓ |
| Cong 2018107 | Irritable bowel syndrome | NA | Critically low | 2 (149) | Adults | UK, Sweden | NA | Internet-based CBT, self-management CBT | NA | WLC, TAU | Short | ✓ | ✓ | ≤ | ✓ |
| Ford 2019152 | Irritable bowel syndrome | NA | Critically low | 5 (307) | Adults | USA, New Zealand, Sweden | NA | Internet-based CBT, self-administered/minimal contact CBT | NA | NR | Short | ✗ | ✗ | ✗ | ✓ |
| Hanlon 2018192 | Irritable bowel disorder/syndrome | NA | Moderate | 7 (770) | Adolescents, adults | Sweden, UK, USA, Australia | NA | Internet-based CBT | Stress management, drug | TAU, placebo | Short–long | ✓ | ✓ | ✓ | ✓ |
| Li 2014279 | Irritable bowel syndrome | NA | Low | 4 (NR) | Adults | Australia, USA, the Netherlands, UK | Individual CBT (delivered by psychologists) | Internet-based CBT (delivered by nurses) | Relaxation, drug | TAU, WLC | Short | ✓ | ′ | ′ | ✓ |
| Li 2018276 | Irritable bowel syndrome | NA | Low | 1 (199) | Adults | New Zealand | NA | Internet-based CBT | NA | TAU | Short | ✓ | ✗ | ≤ | ✓ |
| Thakur 2018447 | Irritable bowel disorder | NA | Critically low | 1 (32) | Adults | Iran | Group CBT | NA | NA | WLC | Short | ✗ | ✓ | ≤ | ≤ |
| Zijdenbos 2009523 | Irritable bowel syndrome | NA | Critically low | 4 (497) | Adults | NR | Group CBT, individual CBT | NA | NA | TAU, placebo, education | Short–long | ✓ | ✗ | ✗ | ✓ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Chan 201294 | Diseases of the skin | NA | Critically low | 1 (16) | Adults | UK | Group CBT (delivered by psychologist) | NA | NA | TAU | Short | ✓ | ✗ | ✗ | ✗ |
| Chida 2007100 | Atopic eczema | NA | Critically low | 1 (55) | Adults | Germany | Individual CBT | NA | Dermatological education | NA | Long | ≤ | ✓ | ✓ | ✓ |
| Lert 2010274 | Vitiligo | NA | Low | 1 (16) | Adults | UK | Group CBT | NA | NA | TAU | Short | ✓ | ✗ | ✗ | ✗ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Tao 2017441 | Menopausal hot flushes | Breast Cancer | Critically low | 2 (518) | Adults | UK, the Netherlands | Individual CBT | NA | Physical exercise | TAU, WLC | Short | ✗ | ✗ | ✗ | ✓ |
| Tremblay 2008456 | Menopausal hot flushes | NA | Low | 1 (29) | Adults | NR | Group CBT | NA | NA | WLC | Short | ✓ | ✗ | ✗ | ✓ |
| van Driel 2018470 | Menopause | Breast cancer | Low | 1 (173) | Adults | NR | Group CBT | NA | NA | WLC | Short | ✗ | ✗ | ✗ | ✓ |
| Vélez Toral 2014474 | Menopause | NA | Critically low | 1 (140) | Adults | NR | Individual CBT | NA | Self-help CBT | WLC | Short | ✓ | ✓ | ≤ | ✓ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Khera 2011248 | Male erectile dysfunction | NA | Critically low | 0 (0) | Adults | NA | NA | NA | NA | NA | NA | ≤ | ✗ | ✗ | ≤ |
Reviews falling under ICD-11 primary code 21 (Table 29) have been subdivided into those with mental conditions and those with physical conditions. The reviews are listed under each of their respective categories.
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Mental conditions | |||||||||||||||
| Ali 201539 | Aggression | Intellectual disabilities | High | 1 (179) | Adults | UK | NA | Manualised group CBT anger management therapy (delivered by lay therapist) | NA | WLC, TAU | Short | ✓ | ✓ | ✓ | ✓ |
| Smedslund 2007417 | Aggression | NA | Moderate | 0 (0) | Adults | NA | NA | NA | NA | NA | NA | ✗ | ≤ | ≤ | ≤ |
| Chen 201895 | Fear of cancer | Cancer | Critically low | 1 (88) | NR | NR | Blended individual CBT + additional electronic consultations | NA | NR | NR | Short | ✓ | ✓ | ✓ | ✗ |
| Liu 2018282 | Fear of falling | NA | Moderate | 5 (1103) | Older adults | NR | NA | Group or individual CBT (delivered by nurses) | Tai Chi | TAU | Short | ✗ | ✗ | ✗ | ✓ |
| Iyengar 2018223 | Suicidal behaviour | NA | Critically low | 1 (40) | Adolescents | NR | Integrated individual CBT and group CBT | NA | NA | TAU | Long | ✗ | ✓ | ✗ | ✗ |
| Lai 2014263 | Suicidal behaviour | NA | Critically low | 2 (351) | Adults | NR | NA | Internet-based CBT | Information website | TAU | Short | ✗ | ✓ | ✗ | ✗ |
| Leavey 2017271 | Suicidal behaviour | NA | Low | 3 (551) | Adults | USA, the Netherlands, Australia | NA | Internet-based CBT | Information website | TAU | Short | ✗ | ✓ | ✗ | ✗ |
| Scott 2016401 | Stress | Caregiver (dementia) | Moderate | 4 (505) | Adults | NR | NA | Technology-based CBT | NA | WLC | Short | ✓ | ✓ | ✓ | ✗ |
| Physical conditions | |||||||||||||||
| Abbott 201732 | Chronic pain | NA | High | 5 (369) | Children, adolescents | Germany, USA, Australia, the Netherlands | Individual CBT | CD-ROM CBT | Education, medical dietary advice | WLC | Short–long | ✓ | ✓ | ✓ | ✗ |
| Anie 201547 | Chronic pain | Sickle cell disease | Moderate | 1 (53) | Adolescents | USA | Family home-based group CBT | NA | Disease education | NA | Long | ≤ | ≤ | ✗ | ✓ |
| Bender 201160 | Chronic pain | NA | Moderate | 4 (299) | Children, adolescents, adults | USA, Germany, Canada | NA | Family internet-based CBT + online support, internet-based CBT + e-mail/telephone support | Internet psychoeducation with support | WLC | Short | ≤ | ✓ | ✓ | ✓ |
| Bernardy 201363 | Chronic pain | NA | High | 13 (840) | Children, adolescents, adults | Europe, USA | Group CBT, individual CBT, mixed group CBT + individual CBT | NA | Exercise, FMS education and information | WLC, TAU, attention control | Short | ✓ | ✓ | ✗ | ✓ |
| Bernardy 201964 | Chronic pain | NA | Low | 3 (212) | Adults | Spain, USA, Canada | NA | Guided or unguided internet-based CBT | High-intensity CBT | WLC, TAU | Short | ✓ | ✓ | ✗ | ✓ |
| Birnie 201868 | Chronic pain | NA | Moderate | 1 (20) | Children, adolescents | Greece | NA | CBT (delivered by non-psychologists) | Hypnosis | TAU | Short | ✗ | ✗ | ✗ | ✓ |
| Burgstaller 201481 | Chronic pain | NA | Moderate | 4 (191) | Adults | USA, Norway, UK, the Netherlands | Individual CBT | NA | NA | TAU | Short–long | ✗ | ✓ | ✓ | ′ |
| Caemmerer 201283 | Excessive weight gain | Schizophrenia and schizoaffective, bipolar, schizotypal, depression and personality disorders | Critically low | 1 (61) | Adults | NR | Individual CBT | NA | NR | NR | Short | ✗ | ✗ | ✗ | ✓ |
| Cantor 201485 | Fatigue | Fatigue | Critically low | 1 (12) | Adults | NR | Individual CBT for social anxiety | NA | NA | WLC | NR | ✗ | ✗ | ✓ | ✓ |
| Eccleston 2015134 | Chronic pain | NA | Moderate | 1 (54) | Adults | The Netherlands | Individual CBT | NA | NA | WLC | Short | ✓ | ✗ | ✓ | ✓ |
| Farley 2012141 | Excessive weight gain | Smoking | Moderate | 1 (44) | Adults | USA | Weight gain individual CBT | NA | NA | TAU, standard cessation counselling + placebo | Long | ✗ | ✗ | ✗ | ✓ |
| Fleming 2016149 | Chronic pain | NA | High | 1 (54) | Adults | China | NA | Self-help listening to 10 minutes of CBT | Brain wave music therapy | No intervention | Short | ≤ | ✗ | ✗ | ✓ |
| Frank 2014157 | Chronic pain | NA | Critically low | 1 (701) | Adults | UK | NA | Group CBT + advice (delivered by physiotherapist) | Advice | NA | Long | ✓ | ✗ | ✗ | ✓ |
| George 2008164 | Chronic pain | NA | Moderate | 1 (223) | Adults | NR | Individual CBT + physiotherapy | NA | Physiotherapy | WLC | NC | ✗ | ✗ | ✗ | ✓ |
| Greenwood 2016176 | Chronic pain | NA | Moderate | 1 (130) | Adults | NR | Individual CBT + supervised exercise (TAU) | NA | NA | Supervised exercise (TAU) | Long | ✓ | ✗ | ✗ | ✓ |
| Gupta 2018185 | Chronic pain | NA | Critically low | 1 (61) | Adults | NR | Group CBT using virtual reality | NA | NA | TAU (medication) | Short | ✓ | ✓ | ✗ | ✗ |
| Hajihasani 2018187 | Chronic pain | NA | Low | 8 (404) | Adults | NR | Individual CBT + physical therapy | NA | Physical therapy | NA | NR | ✓ | ✓ | ✗ | ✓ |
| Hall 2018188 | Chronic pain | NA | Moderate | 4 (1128) | Adults | UK | NA | Individual CBT, group CBT, mixed individual + group CBT (all physiotherapist led) | Spinal stability, physiotherapy, advice, advice + exercises | NA | Long | ✓ | ✗ | ✗ | ✓ |
| Harris 2015194 | Chronic pain | NA | Low | 5 (305) | Adults, older adults | Germany, Australia, USA, Canada | Individual CBT + relaxation, group CBT + relaxation | NA | Relaxation, biofeedback, self-management CBT | WLC | Short–long | ✗ | ✗ | ✗ | ✓ |
| Hjorth 2014210 | Excessive weight gain | Schizophrenia | Critically low | 1 (15) | Adults | NR | Group CBT | NA | NA | TAU | Short | ✗ | ✗ | ✗ | ✓ |
| Ho 2019211 | Chronic pain | NA | Moderate | 4 (457) | Adults | USA, UK, Canada | Individual CBT or group CBT for pain and/or insomnia | NA | Education, behavioural desensitisation (placebo) | Symptom monitoring, WLC | Short | ≤ | ✓ | ✓ | ✓ |
| Iwasaki 2018222 | Chronic pain | NA | Critically low | 2 (256) | Adults | USA | Intensive individual CBT, brief individual CBT | N | NA | TAU | Long | ✗ | ✗ | ✗ | ✓ |
| Jacob 2018225 | Excessive weight gain | NA | Low | 1 (18) | Adults | Portugal | Individual CBT | NA | NA | WLC | Short | ✗ | ′ | ✓ | ′ |
| Kisely 2015251 | Chronic pain | NA | Moderate | 8 (422) | NR | NR | Group CBT, individual CBT | Brief CBT (delivered by nurses) | Paroxetine only | TAU, WLC, placebo, assessment | Short | ′ | ′ | ✓ | ✓ |
| Larkin 2014265 | Fatigue | Prostate cancer | Critically low | 2 (85) | Adults | The Netherlands, Scotland | Individual CBT (delivered by therapists) | CBT (delivered by nurses) | Brief nursing intervention | TAU | Short | ✗ | ✗ | ✗ | ✓ |
| Liu 2012281 | Chronic pain | NA | Low | 2 (275) | Adults | NR | NR | NA | NA | TAU, attention control | Long | ✗ | ✓ | ✗ | ✓ |
| Lonergan 2016285 | Chronic pain | NA | Critically low | 6 (501) | Children, adolescents | Australia, the Netherlands, Germany, USA | Family CBT | NA | Education, education + drug | TAU, WLC | Short–long | ✗ | ✗ | ✗ | ✓ |
| Macea 2010129 | Chronic pain | NA | Critically low | 1 (51) | Adults | Sweden | NA | Internet-based CBT | NA | WLC | Short | ✗ | ✗ | ✗ | ✓ |
| McMahon 2013302 | Chronic pain | NA | Moderate | 1 (29) | Adults | NR | Individual CBT | NA | Education | NA | Short | ✓ | ✓ | ✓ | ✓ |
| Mehta 2018306 | Chronic pain | NA | Low | 22 (3014) | Adults | USA, Sweden, Australia, Spain, Germany, Canada, the Netherlands | NA | Internet-based CBT | High-intensity face-to-face CBT, positive psychology therapy, internet-based acceptance and commitment therapy | WLC, TAU, attention control, discussion groups, information | Short | ✗ | ✓ | ✓ | ✓ |
| Monticone 2015317 | Chronic pain | NA | Moderate | 4 (226) | Adults | NR | Individual CBT (delivered by psychologist) | CBT ([delivered by health-care professional) | Physiotherapy or medication | WLC | Short | ✓ | ≤ | ≤ | ✓ |
| Niknejad 2018330 | Chronic pain | NA | Critically low | 2 (370) | Older adults | NR | Group CBT or individual CBT for pain | NA | Education | Symptom monitoring | Short | ✗ | ′ | ′ | ✓ |
| O’Keeffe 2016341 | Chronic pain | NA | Low | 1 (80) | Adults | NR | NR | NA | Neck exercises | NA | Long | ✗ | ✗ | ✗ | ✓ |
| Palermo 2010354 | Chronic pain | NA | Low | 2 (68) | Children, adolescents | NR | NA | Internet-based CBT and CD-ROM CBT | NA | WLC | Short | ≤ | ✗ | ✗ | ✓ |
| Raggi 2018374 | Chronic pain | NA | Critically low | 4 (391) | Children, adolescents, adults | NR | NR | NA | Education, education + drug | NA | Short | ≤ | ✓ | ✗ | ✓ |
| Ramond-Roquin 2014375 | Chronic pain | NA | Moderate | 3 (348) | Adults | USA, Sweden | NR | NA | Education | TAU, NR | Long | ✓ | ✗ | ✗ | ✓ |
| Randhawa 2016376 | Chronic pain | NA | Low | 1 (117) | Adults | USA | Individual CBT | NA | NA | TAU | Long | ≤ | ✓ | ≤ | ✓ |
| Richmond 2015382 | Chronic pain | NA | Moderate | 14 (2706) | Adults | NR | Individual or group CBT (delivered by psychologists) | Individual CBT or goup CBT (delivered by physiotherapist or other health-care professionals) | NA | TAU, WLC | Long | ✓ | ✗ | ✗ | ✓ |
| Sakamoto 2016392 | Chronic pain | NA | Moderate | 2 (127) | Adults | USA, UK | NR | NA | NA | No intervention | Short | ✓ | ✗ | ✓ | ′ |
| Schofield 2006396 | Chronic pain | NA | Critically low | 1 (28) | Older adults | Canada | Individual CBT | NR | Attention support | NA | Short | ✗ | ✗ | ✗ | ✓ |
| Shamliyan 2013404 | Chronic pain | NA | Low | 1 (158) | Adults | Germany | Individual CBT minimal contact programme | NA | NA | Brochures | NR | ≤ | ✓ | ✓ | ✓ |
| Shearer 2016406 | Chronic pain | NA | Low | 1 (80) | Adults | Italy | NA | CBT (delivered by physiotherapist) | Multimodal physiotherapy | NA | Long | ✓ | ✗ | ✗ | ✓ |
| Skelly 2018413 | Chronic pain | NA | Low | 9 (1708) | Adults | Spain, USA, UK, Finland, NR | Group CBT, individual CBT, mixed group + individual CBT | Group CBT, individual CBT, mixed group + individual CBT (delivered by health-care professionals) | Drug, exercise | TAU, attention control, active management | Short–long | ✓ | ✓ | ✓ | ✓ |
| Sprenger 2011426 | Chronic pain | NA | Critically low | 5 (225) | Children, adolescents | NR | Individual CBT, family CBT | Internet-based CBT | NR | NR | Short | ✗ | ✗ | ✗ | ✓ |
| Tang 2018440 | Chronic pain | NA | Low | 3 (404) | Children, adolescents | USA | NA | Internet-based CBT, family internet-based CBT | Internet-based education, specialised headache treatment programme | WLC | Short | ✗ | ✓ | ✓ | ✓ |
| Veehof 2016473 | Chronic pain | NA | Low | 2 (190) | Adults | NR | Group CBT | NA | Acceptance and commitment therapy, mindfulness-based stress reduction | NA | Short | ≤ | ✓ | ≤ | ✓ |
| Velleman 2010475 | Chronic pain | NA | Critically low | 4 (179) | Children, adolescents | NR | NA | Computerised CBT | Computerised education programme | WLC | Short | ✗ | ✗ | ✗ | ✓ |
| Verhagen 2009480 | Chronic pain | NA | Moderate | 2 (68) | Adults | NR | Individual CBT | NA | Drug, relaxation | NA | Short | ✗ | ✓ | ✓ | ✓ |
| Wendebourg 2017495 | Fatigue | Multiple sclerosis | Low | 3 (152) | Adults | UK, New Zealand, USA | Face-to-face individual CBT, face-to-face individual CBT + telephone contact | Internet-based CBT + telephone contact | Group intervention (not CBT), relaxation | No intervention | Short | ′ | ′ | ✗ | ✓ |
| Xu 2017508 | Fatigue | Traumatic brain injury | Low | 1 (12) | Adults | Australia | Individual CBT | NA | NA | WLC | Short | ✗ | ✗ | ✗ | ✓ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Al Sayegh 201038 | Injuries to the head | Head injury | Low | 1 (16) | Adults | NR | Individual CBT adapted to account for difficulties with attention, concentration, fatigue and memory, single session individual CBT + manual | NA | NA | WLC, TAU | Short | ≤ | ✓ | ✓ | ✓ |
| Gómara-Toldrà 2014172 | Spinal cord injury | NA | Low | 1 (61) | Adults | The Netherlands | Multidisciplinary individual CBT for coping with neuropathic pain | NA | NA | WLC | Short | ✓ | ✗ | ✗ | ✓ |
| Sullivan 2018437 | Intracranial injury | NA | Low | 1 (58) | Adults | USA | Single session individual CBT with therapist | NA | NA | TAU | Short | ✗ | ✗ | ✗ | ✓ |
| Thomas 2017449 | Intracranial injury | Depression | Moderate | 1 (77) | Adults | USA | Individual CBT | NA | Supportive psychotherapy | NA | Short | ✓ | ′ | ′ | ✗ |
Reviews falling under primary ICD-11 code 24 (Table 31) have been subdivided into those with mental conditions and those with physical conditions. The reviews are listed under each of their respective categories.
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Mental conditions | |||||||||||||||
| McDaid 2008300 | Bereavement | NA | Moderate | 1 (134) | Adolescents, adults | The Netherlands | Family CBT | NA | NA | TAU | Long | ✗ | ✓ | ✗ | ✗ |
| Physical conditions | |||||||||||||||
| Moraes 2018319 | Care involving peritoneal dialysis | NA | Critically low | 1 (24) | NR | NR | Individual CBT | NA | NR | NR | Short | ✗ | ≤ | ≤ | ✓ |
| ICD-11 primary/secondary | Condition description | Study | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | ||||||||
|
|
Andersson 201445 | NA | Low | 11 (934) | Adults | Australia, the Netherlands, Spain, Sweden, Switzerland, USA | NA | Internet-based CBT | High-intensity CBT | NA | Short | ✗ | ✓ | ✓ | ✓ |
|
|
Barrett 201658 | Substance abuse | Critically low | 1 (66) | Adults | USA | Integrated individual CBT for substance abuse disorder, depression and pain | NA | Twelve-step facilitation | NA | Long | ✗ | ✓ | ✗ | ✗ |
|
|
Carlbring 201887 | NA | Low | 16 (914) | Adults | Australia, the Netherlands, Spain, Sweden, Germany, USA | NA | Internet-based CBT | High-intensity CBT | NA | Short | ✗ | ′ | ✓ | ✓ |
|
|
Farrand 2013142 | NA | Low | 35 (2977) | Adults | NR | NA | Guided self-help CBT, minimal contact CBT, self-administered CBT | NA | WLC TAU Attention control | Short | ✗ | ✓ | ✓ | ✓ |
|
|
Kolubinski 2018256 | Psychosis, bipolar, cognitive deficit, brain injury, learning disability, autism | Critically low | 2 (562) | Adults | UK | Group CBT | NA | NA | WLC | Short | ✗ | ✓ | ✗ | ✗ |
|
|
Osborn 2006348 | Cancer survivors | Critically low | 1 (34) | Adults | NR | Individual CBT | NA | NR | NR | Short | ✗ | ✓ | ✓ | ✓ |
|
|
Ruiz 2012389 | NA | Critically low | 3 (258) | Adults | NR | Group CBT, individual CBT | NA | Acceptance and commitment therapy | NA | Short–long | ✓ | ✓ | ✓ | ✓ |
|
|
Yoshinaga 2015515 | NA | Critically low | 2 (68) | NR | NR | Group CBT, individual CBT | NA | Group occupational therapy | TAU | Short | ✓ | ✓ | ✓ | ✓ |
| Study | Condition description | Comorbid/specific symptoms | AMSTAR-2 quality rating | Number of RCTs (number of participants) | Age | Country of RCTs | CBT intervention group | Comparator group | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High intensity (description) | Low intensity (description) | Active (description) | Non-active (description) | Follow-up time | HRQoL | Depression | Anxiety | Condition specific | |||||||
| Bernard 201862 | COPD, heart failure, cancer, chronic pain | NA | Critically low | 8 (NR) | Adults | Brazil, the Netherlands, Pakistan, USA, international | CBT + exercise therapy (some group) | NA | Exercise therapy | NA | Short | ✗ | ✓ | ✓ | ✓ |
| van Beugen 2014467 | Tinnitus, diabetes, chronic pain, cancer, headache, epilepsy, fatigue, functional gastrointestinal | NA | Low | 19 (3974) | Adults | NR | NA | Internet-based CBT | NA | TAU, WLC | Short | ✓ | ✓ | ✓ | ✓ |
Appendix 8 Gap maps of included systematic reviews grouped according to the condition each targeted, as per the ICD-11
| Secondary ICD-11 (number of reviews, RCTs, participants) | Who: severity | What: intensity | When: delivered | Where: participants recruited | Follow-up | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subclinical | Clinical | Chronic | Severe | Not reported | High | Low | Not reported | Preventative | Standard | Relapse prevention | Not reported | Community | GP primary | Outpatients | Inpatients | School/university | Institution | Not reported | Short | Long | Not reported | |
| 6B00-06: Anxiety or fear-related disorders [agoraphobia with panic disorder, GAD, panic, phobia, SAD, selective mutism, mixed anxiety disorders (GAD, panic, phobia, SAD, separation, selective mutism)], n = 50 reviews (346 RCTs; 24,078 participants) | 0 | 30 | 1 | 1 | 20 | 42 | 10 | 1 | 2 | 48 | 0 | 0 | 5 | 2 | 8 | 4 | 6 | 0 | 35 | 41 | 10 | 1 |
| 6C40-51: Disorders due to substance use or addictive behaviours (gambling, gaming, hallucinogens, stimulants, substance use), n = 6 reviews (7 RCTs; 476 participants) | 0 | 1 | 0 | 0 | 5 | 3 | 2 | 1 | 0 | 6 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 2 | 3 | 2 | 1 |
| 6C20-21: Disorders of bodily distress or bodily experience (somatoform/MUS], n = 2 reviews (5 RCTs; 554 participants) | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 1 | 0 |
| 6B40-45: Disorders specifically associated with stress (acute stress disorders, adjustment disorders, post-traumatic stress disorder), n = 39 reviews (89 RCTs; 6110 participants) | 4 | 17 | 0 | 1 | 18 | 37 | 3 | 0 | 3 | 36 | 0 | 0 | 11 | 2 | 6 | 1 | 6 | 1 | 22 | 35 | 7 | 0 |
| 6B80-85: Feeding or eating disorders [AN, BED, BN, mixed (AN, BN, BED, EDNOS)], n = 25 reviews (87 RCTs; 7132 participants) | 2 | 13 | 1 | 1 | 10 | 20 | 8 | 0 | 1 | 23 | 1 | 0 | 8 | 3 | 8 | 1 | 1 | 0 | 13 | 17 | 10 | 0 |
| 6B80-85: Mental disorders associated with pregnancy, childbirth or the puerperium (during IVF, antenatal depression, perinatal anxiety and depression, postnatal depression), n = 19 reviews (39 RCTs; 4737 participants) | 0 | 7 | 0 | 0 | 12 | 15 | 6 | 1 | 3 | 16 | 0 | 0 | 4 | 0 | 0 | 2 | 0 | 0 | 13 | 18 | 1 | 1 |
| 6A60-80: Mood disorders (bipolar or related disorder, depressive disorders, premenstrual dysphoric disorder, subthreshold depression, treatment-resistant depression), n = 92 reviews (272 RCTs; 42,676 participants) | 9 | 43 | 4 | 3 | 39 | 76 | 29 | 2 | 8 | 84 | 4 | 0 | 14 | 11 | 19 | 6 | 7 | 2 | 48 | 72 | 26 | 1 |
| 6D70-72: Neurocognitive disorders, n = 1 review (1 RCT; 41 participants) | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| 6A00-06: Neurodevelopmental disorders (attention deficit hyperactive disorder), n = 5 reviews (13 RCTs; 746 participants) | 0 | 3 | 0 | 0 | 2 | 5 | 1 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 5 | 0 | 0 |
| 6B20-25: Obsessive–compulsive or related disorders (body dysmorphic disorder, hypochondriasis, OCD), n = 16 reviews (54 RCTs; 3117 participants) | 0 | 9 | 0 | 1 | 7 | 13 | 5 | 0 | 0 | 16 | 0 | 0 | 1 | 4 | 2 | 0 | 1 | 0 | 12 | 16 | 3 | 0 |
| 6D10-11: Personality disorders and related traits, n = 4 reviews (7 RCTs; 603 participants) | 0 | 2 | 0 | 0 | 2 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 3 | 1 | 0 |
| 6A20-25: Schizophrenia or other primary psychotic disorders (attenuated psychosis syndrome, delusional disorder, early-onset psychosis, psychosis), n = 31 reviews (135 RCTs; 14,924 participants) | 0 | 14 | 0 | 1 | 16 | 31 | 0 | 0 | 6 | 24 | 1 | 0 | 3 | 1 | 10 | 8 | 0 | 0 | 17 | 20 | 14 | 0 |
| Mixed mental (anxiety, addictive, bodily distress, depression, eating disorder, obsessive, stress), n = 60 reviews (447 RCTs; 57,213 participants) | 1 | 31 | 1 | 1 | 28 | 40 | 26 | 1 | 2 | 58 | 0 | 0 | 16 | 8 | 18 | 3 | 11 | 0 | 26 | 53 | 13 | 1 |
| Primary and secondary ICD-11 (number of reviews, RCTs, participants) | Who: severity | What: intensity | When: delivered | Where: participants recruited | Follow-up | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subclinical | Clinical | Chronic | Severe | Not reported | High | Low | Not reported | Preventative | Standard | Relapse prevention | Not reported | Community | GP primary | Outpatients | Inpatients | School/university | Institution | Not reported | Short | Long | Not reported | |
| 21. Symptoms, signs or clinical findings, not elsewhere classified: mental, behavioural symptoms (aggression, caregivers stress, fear of cancer, fear of falling, suicidal behaviour), n = 8 reviews (17 RCTs; 2817 participants) | 0 | 2 | 0 | 1 | 6 | 2 | 5 | 1 | 1 | 7 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 6 | 6 | 1 | 1 |
| 24. Factors influencing health: associated with absence, loss or death of others (bereavement through suicide), n = 1 review (1 RCT; 134 participants) | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Primary ICD-11 (number of reviews, RCTs, participants) | Who: severity | What: intensity | When: delivered | Where: participants recruited | Follow-up | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subclinical | Clinical | Chronic | Severe | Not reported | High | Low | Not reported | Preventative | Standard | Relapse prevention | Not reported | Community | GP primary | Outpatients | Inpatients | School/university | Institution | Not reported | Short | Long | Not reported | |
| 01 Certain infectious or parasitic diseases (HIV/AIDS), n = 1 review (1 RCT; 46 participants) | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| 02 Neoplasms (chemotherapy, malignant breast), n = 8 reviews (21 RCTs; 3204 participants) | 0 | 6 | 0 | 0 | 2 | 8 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 7 | 6 | 4 | 0 |
| 05 Endocrine, nutritional or metabolic diseases (diabetes mellitus, diabetic neuropathy), n = 3 reviews (8 RCTs; 1061 participants) | 0 | 1 | 0 | 0 | 2 | 3 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 2 | 2 | 0 |
| 07 Sleep–wake disorders (insomnia disorders), n = 22 reviews (125 RCTs; 14,315 participants) | 0 | 8 | 2 | 0 | 12 | 15 | 10 | 0 | 0 | 22 | 0 | 0 | 6 | 1 | 2 | 0 | 1 | 0 | 15 | 19 | 6 | 0 |
| 08 Diseases of the nervous system (epilepsy or seizure, neuromuscular disorders, non-epileptic seizures, Parkinson’s disease, post-viral fatigue syndrome), n = 14 reviews (54 RCTs; 5883 participants) | 0 | 5 | 0 | 0 | 9 | 14 | 1 | 0 | 0 | 14 | 0 | 0 | 1 | 1 | 4 | 2 | 0 | 0 | 8 | 10 | 5 | 0 |
| 10 Diseases of the ear or mastoid process (hyperacusis, tinnitus), n = 3 reviews (9 RCTs; 534 participants) | 0 | 0 | 0 | 0 | 3 | 2 | 2 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 1 | 0 |
| 12 Diseases of the respiratory system (asthma), n = 2 reviews (7 RCTs; 174 participants] | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 |
| 13 Diseases of the digestive system (dentofacial parafunctional disorders, irritable bowel disorder/syndrome, oral mucositis), n = 9 reviews (27 RCTs; 2079 participants) | 0 | 2 | 0 | 0 | 7 | 4 | 6 | 0 | 0 | 9 | 0 | 0 | 1 | 2 | 4 | 0 | 0 | 0 | 5 | 9 | 2 | 0 |
| 14 Diseases of the skin (atopic eczema, vitiligo), n = 3 reviews (3 RCTs; 87 participants) | 0 | 1 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 |
| 16 Diseases of the genitourinary system (menopausal hot flushes, menopause), n = 4 reviews (5 RCTs; 860 participants) | 0 | 0 | 0 | 0 | 4 | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4 | 0 | 0 |
| 17 Conditions related to sexual health (male erectile dysfunction), n = 1 review (0 RCTs; 0 participants) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 21 General symptoms, signs or clinical findings: fatigue, n = 4 reviews (7 RCTs; 261 participants) | 0 | 0 | 0 | 0 | 4 | 4 | 2 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 4 | 0 | 0 |
| 21 General symptoms, signs or clinical findings: pain (back pain, chronic non-cancer pain, chronic pain, headaches and migraines, fibromyalgia, leukaemia, lumbar fusion surgery, neck pain, non-specific chest pain, spinal pain and injury, OA, orthodontic treatment, RA, RAP, sickle cell disease pain, tempro mandibular disorder), n = 42 reviews (156 reviews; 17,105 participants) | 0 | 11 | 9 | 0 | 22 | 30 | 18 | 0 | 2 | 39 | 1 | 0 | 10 | 4 | 15 | 3 | 3 | 1 | 22 | 31 | 16 | 0 |
| 21 General symptoms, signs or clinical findings: weight gain, n = 4 reviews (4 RCTs; 138 participants) | 0 | 2 | 0 | 0 | 2 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 1 | 0 |
| 22 Injury, poisoning or certain other consequences of external causes (intracranial injury, spinal cord injury), n = 4 reviews (4 RCTs; 212 participants) | 0 | 2 | 0 | 1 | 1 | 4 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 0 | 2 | 3 | 0 | 0 | 1 | 4 | 0 | 0 |
| 24 Factors influencing health status (peritoneal dialysis), n = 1 review (1 RCT; 24 participants) | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Mixed physical conditions (COPD, cancer, chronic pain, diabetes, epilepsy, headache, heart failure, fatigue, functional gastrointestinal, tinnitus), n = 2 reviews (27 RCTs; 3974 participants) | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Primary physical/secondary mental ICD-11 category (number of reviews, RCTs, participants) | Who: severity | What: intensity | When: delivered | Where: participants recruited | Follow-up | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subclinical | Clinical | Chronic | Severe | Not reported | High | Low | Not reported | Preventative | Standard | Relapse prevention | Not reported | Community | GP primary | Outpatients | Inpatients | School/university | Institution | Not reported | Short | Long | Not reported | |
| Physical: brain injury, conditions related to sexual health, chronic pain, diseases of the ear or mastoid process, sleep–wake disorders; mental: anxiety, autism, bipolar, bodily distress, depression, eating disorders, learning disability, psychosis, stress disorders, n = 8 (71 RCTs; 5813 participants) | 0 | 2 | 0 | 0 | 6 | 5 | 3 | 0 | 0 | 8 | 0 | 0 | 3 | 0 | 4 | 1 | 0 | 0 | 3 | 7 | 2 | 0 |
Appendix 9 Health-related quality of life
Sensitivity analysis: review quality
The sensitivity analysis was conducted with an additional 10 reviews that had been rated as being of low or critically low quality on the AMSTAR-2. Therefore, the sensitivity analysis was conducted with 34 reviews (76 RCTs, 7466 participants). 32,39,63,82,165,188,193,219,220,231,235,236,270,275,276,279,286,299,317,329,343,347,356,371,409,413,445,446,464,467,469,513,518,521 Inclusion of lower-quality reviews increased the estimate of effect (SMD 0.28, 95% CI 0.17 to 0.38) and produced higher levels of heterogeneity (I2 = 71%) (Figure 15).
FIGURE 15.
Sensitivity analysis for quality in HRQoL outcomes. GAD, generalised anxiety disorder; OA, osteoarthritis.
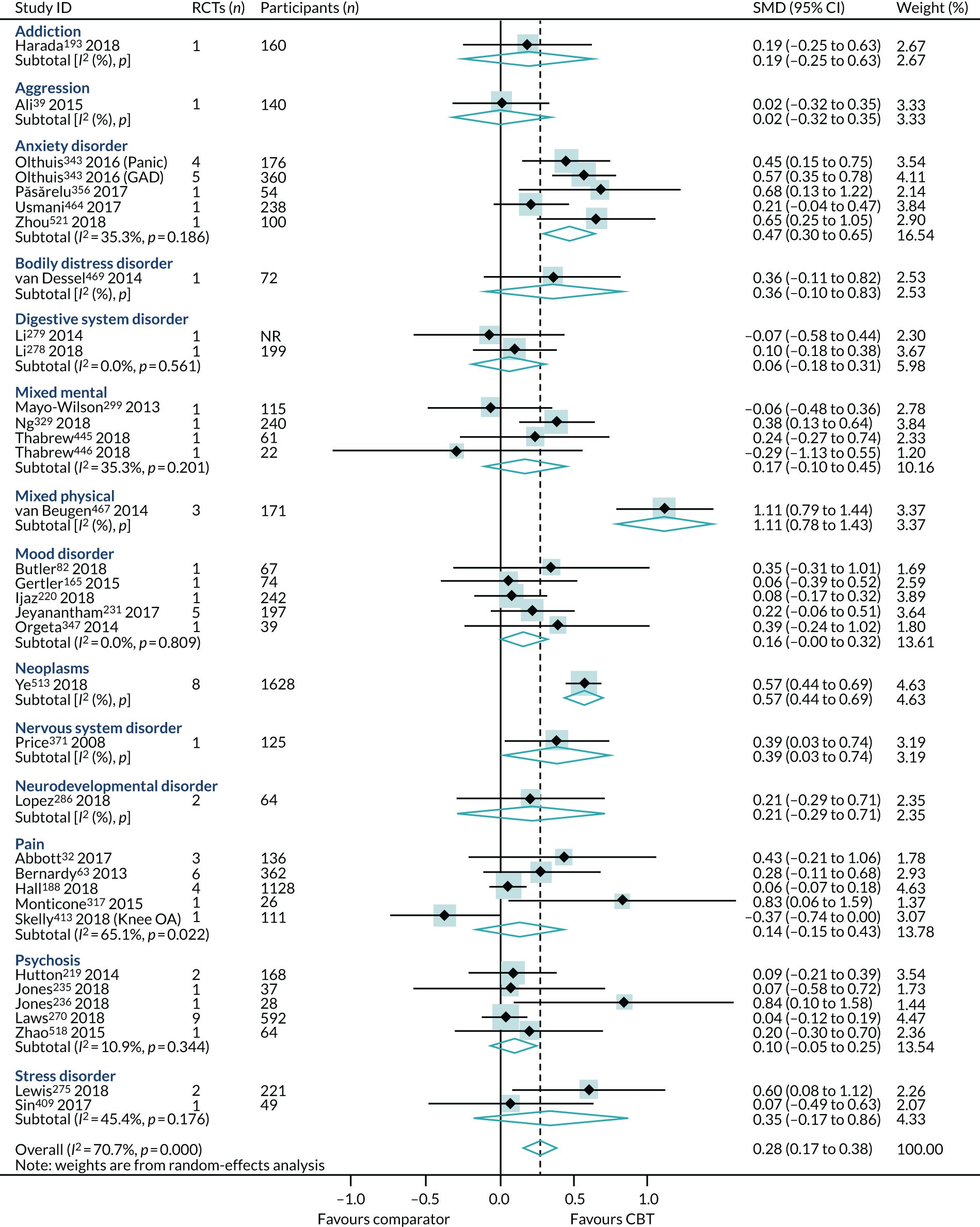
Sensitivity analysis: prioritisation of mental subscales
We re-ran the PMA, replacing the physical component scores with the mental component scores from the SF-12/SF-36 in the two reviews that presented both the physical and mental component scores. 220,464 The replacement did not change the overall effect or heterogeneity rating for the HRQoL outcome (SMD 0.24, 95% CI 0.14 to 0.33; I2 = 38%) (Figure 16).
FIGURE 16.
The HRQoL sensitivity analysis: prioritisation of mental subscales.
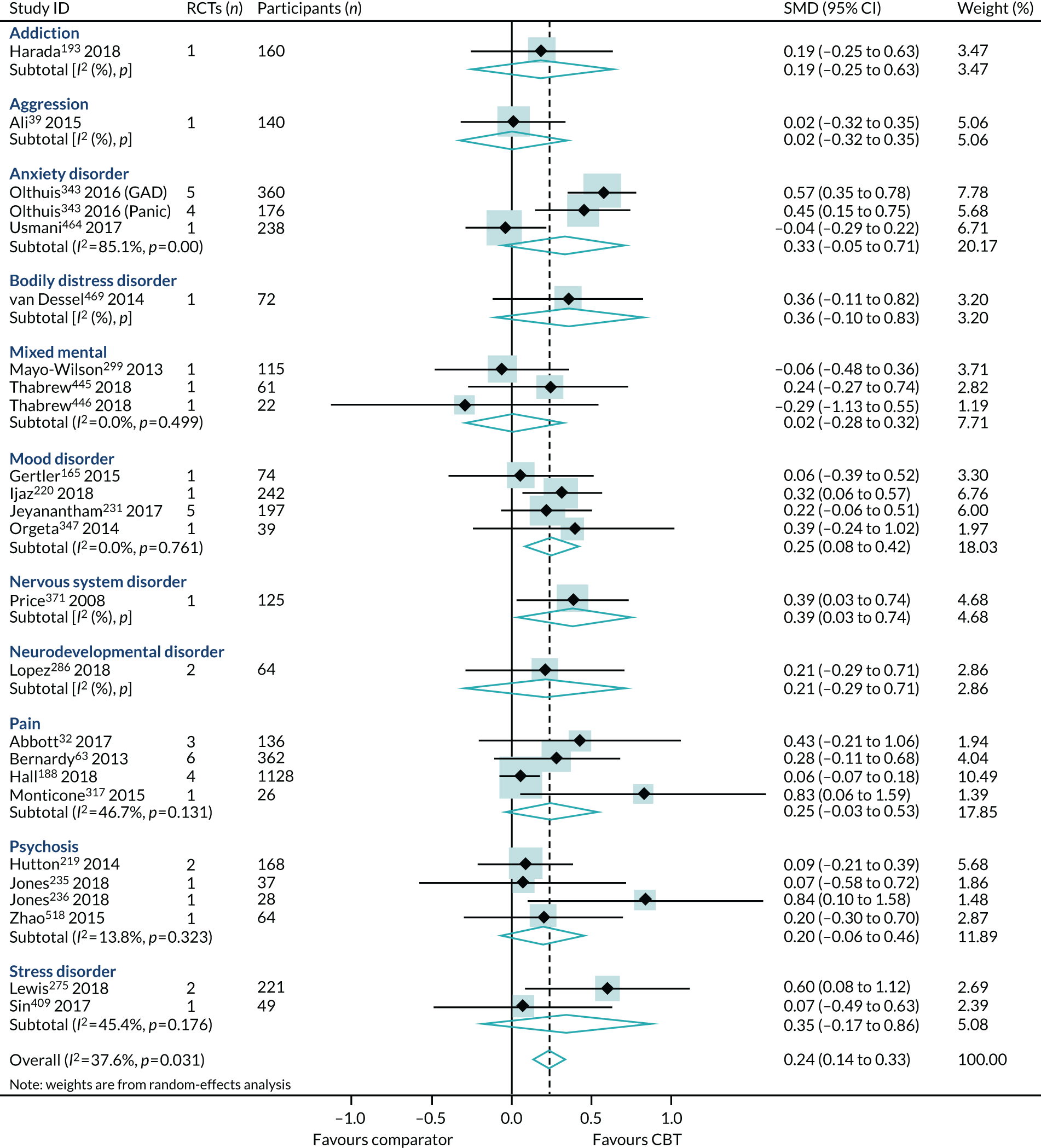
Synthesis of reviews reporting change scores
Data were presented as change scores for the HRQoL outcome in four reviews (four RCTs, 185 participants). 158,246,406,523 Each review represented a different condition: digestive system diseases (irritable bowel syndrome), mixed mental problems (anxiety and depression), pain (neck and whiplash) and respiratory system diseases (asthma). Pooled results showed a moderate effect in favour of CBT (SMD 0.58, 95% CI 0.15 to 1.00; I2 = 66%) (Figure 17).
FIGURE 17.
The HRQoL outcome (change score) synthesis across all conditions.
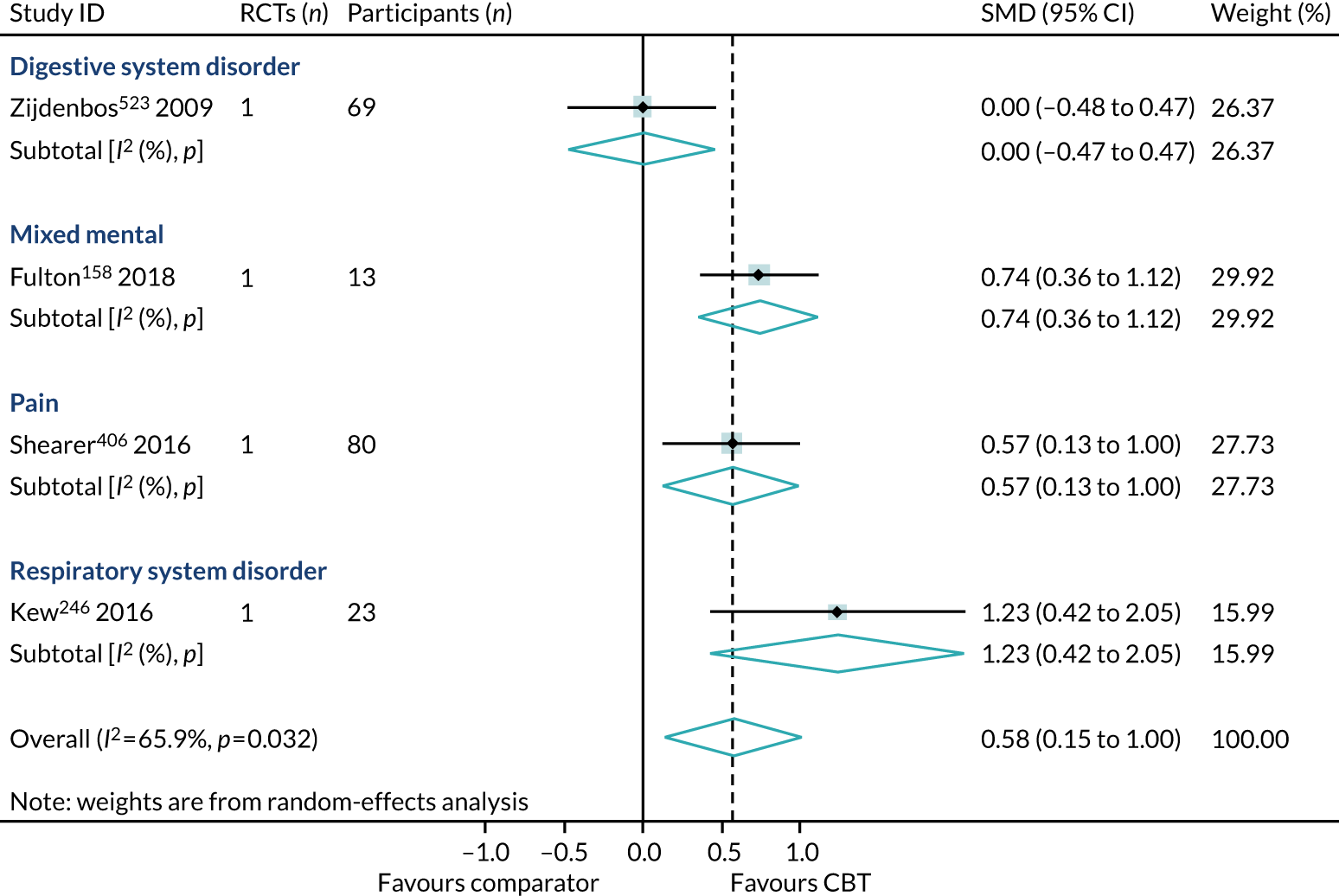
Synthesis of reviews reporting dichotomous outcomes
Only one review (two RCTs, 145 participants) reported HRQoL outcome results as RRs. 170 This review in nervous system disorder (post-viral fatigue syndrome) identified a large effect for CBT (SMD 1.57, 95% CI 0.78 to 2.37) (Figure 18).
FIGURE 18.
The HRQoL outcome (RR) synthesis across all conditions.
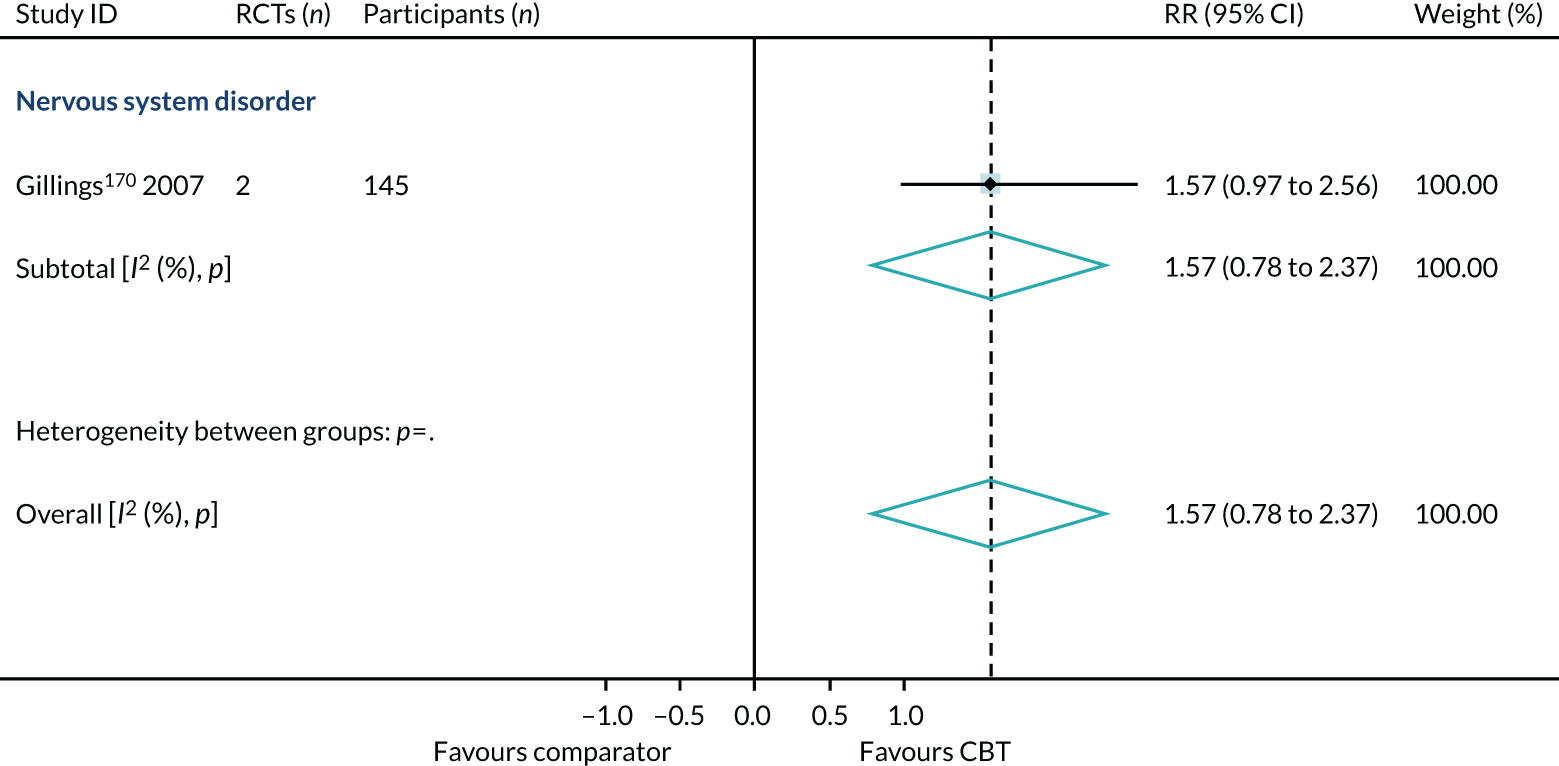
Appendix 10 Depression
Primary analysis
Within-condition heterogeneity varied between 0% (6D10-11: Personality disorders) and 86.3% (6A60-80: Mood disorders) and across-condition heterogeneity was 81%. The across-condition heterogeneity was too high for us to pool across the ICD-11 category subgroups (Figure 19).
FIGURE 19.
Primary analysis of the secondary outcome: depression from ‘higher-quality’ reviews.
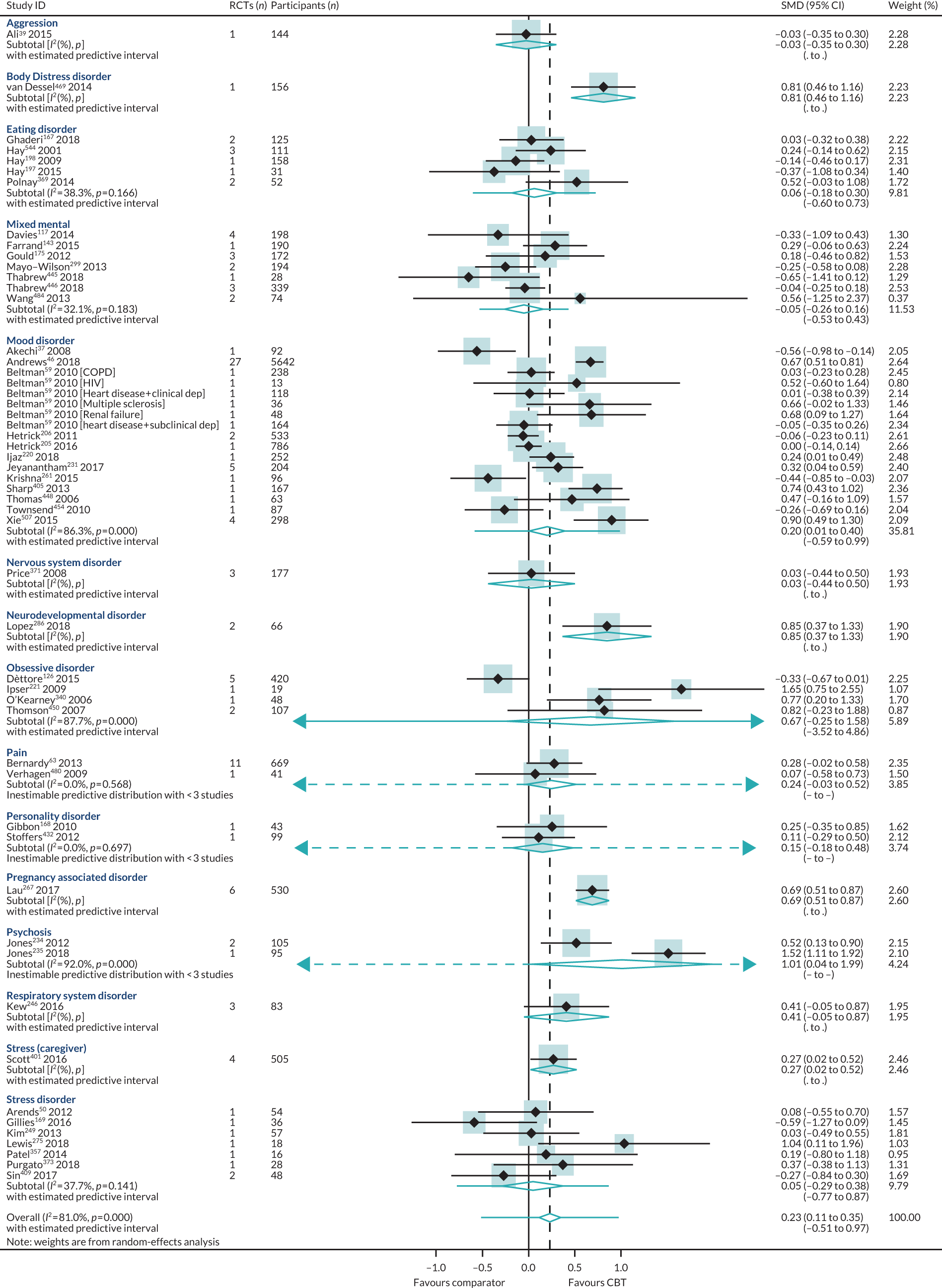
Publication bias
No publication bias was detected using funnel plots (Figure 20) and Egger’s test showed no small-study effects (p = 0.87).
FIGURE 20.
Depression funnel plot (end-point data from high-quality reviews).
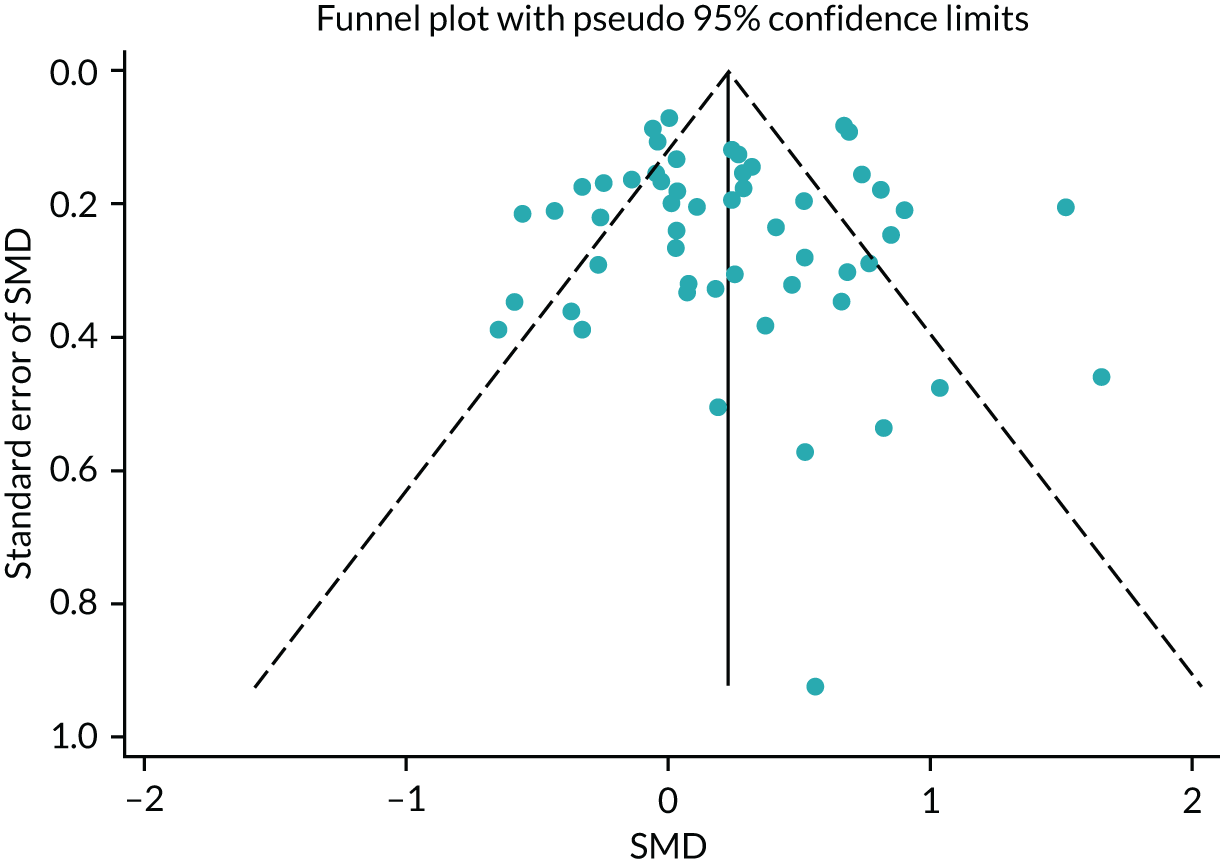
Appendix 11 Anxiety
Publication bias
There was no evidence of publication bias, nor of small-study effects (Egger’s test p = 0.70) (Figure 21).
FIGURE 21.
Anxiety funnel plot (end-point data from high-quality reviews).
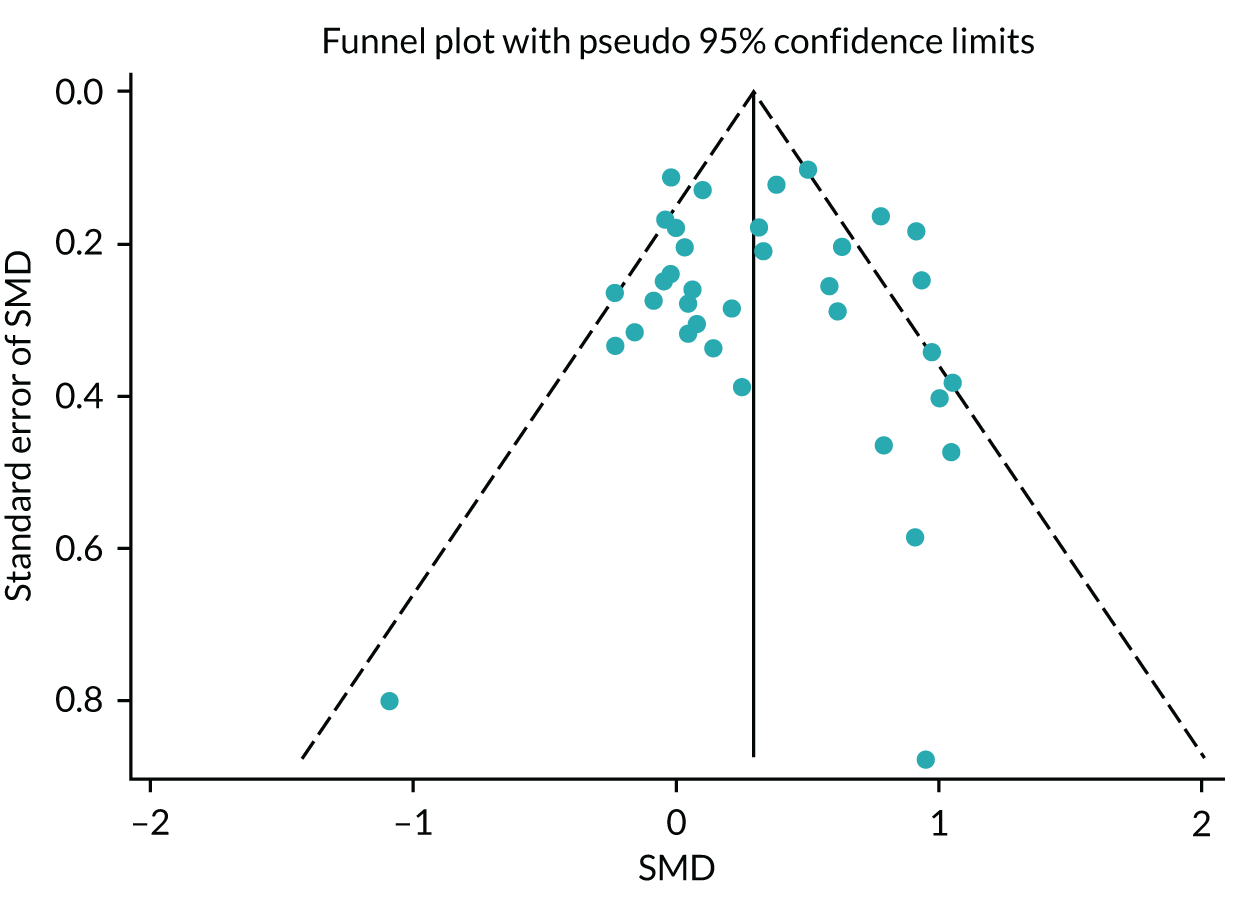
Subgroup analysis
Cognitive–behavioural therapy intensity
The pooling of 28 meta-analyses collected from reviews of high-intensity CBT (face to face with a highly trained CBT therapist) found a modest effect in favour of CBT (SMD 0.28, 95% CI 0.15 to 0.42; I2 = 54.3%) (Figure 22).
FIGURE 22.
Anxiety subgroup analysis (end-point data from high-quality reviews): CBT intensity. OCD, obsessive–compulsive disorder; PTSD, post-traumatic stress disorder.
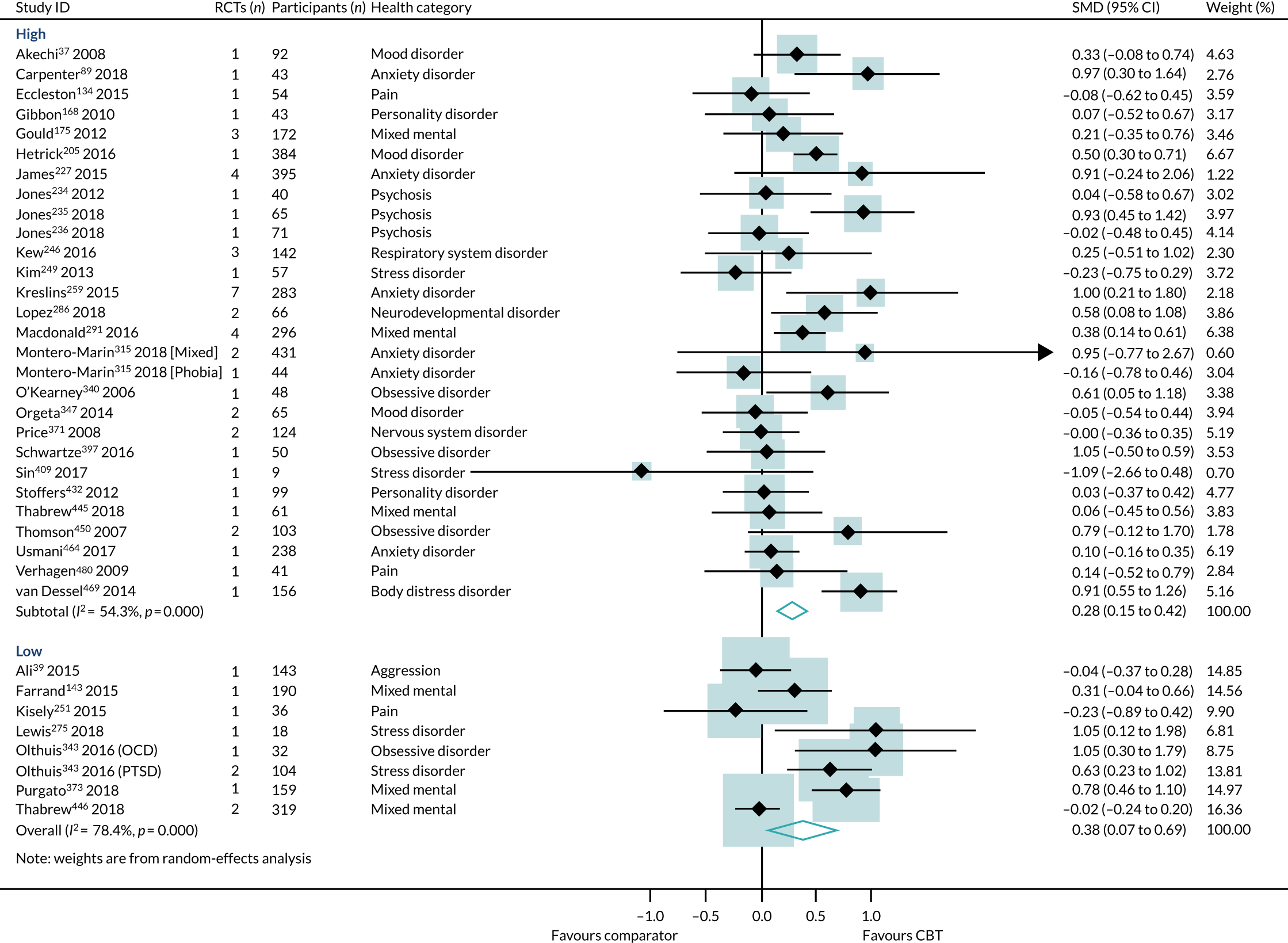
We identified five reviews87,306,343,360,521 (11 RCTs, 503 participants) that directly compared high- with low-intensity CBT interventions on anxiety outcomes in 6B00-06: Anxiety, 6A60-80: Mood and pain [tinnitus 21 Symptoms and signs not otherwise specified (MG30 pain)] conditions. In this subset of direct comparisons, there was no difference between high- and low-intensity CBT (SMD 0.03, 95% CI –0.14 to 0.21; I2 = 20%) (Figure 23).
FIGURE 23.
Anxiety: high- vs. low-intensity CBT direct comparison PMA. SAD, seasonal affective disorder.
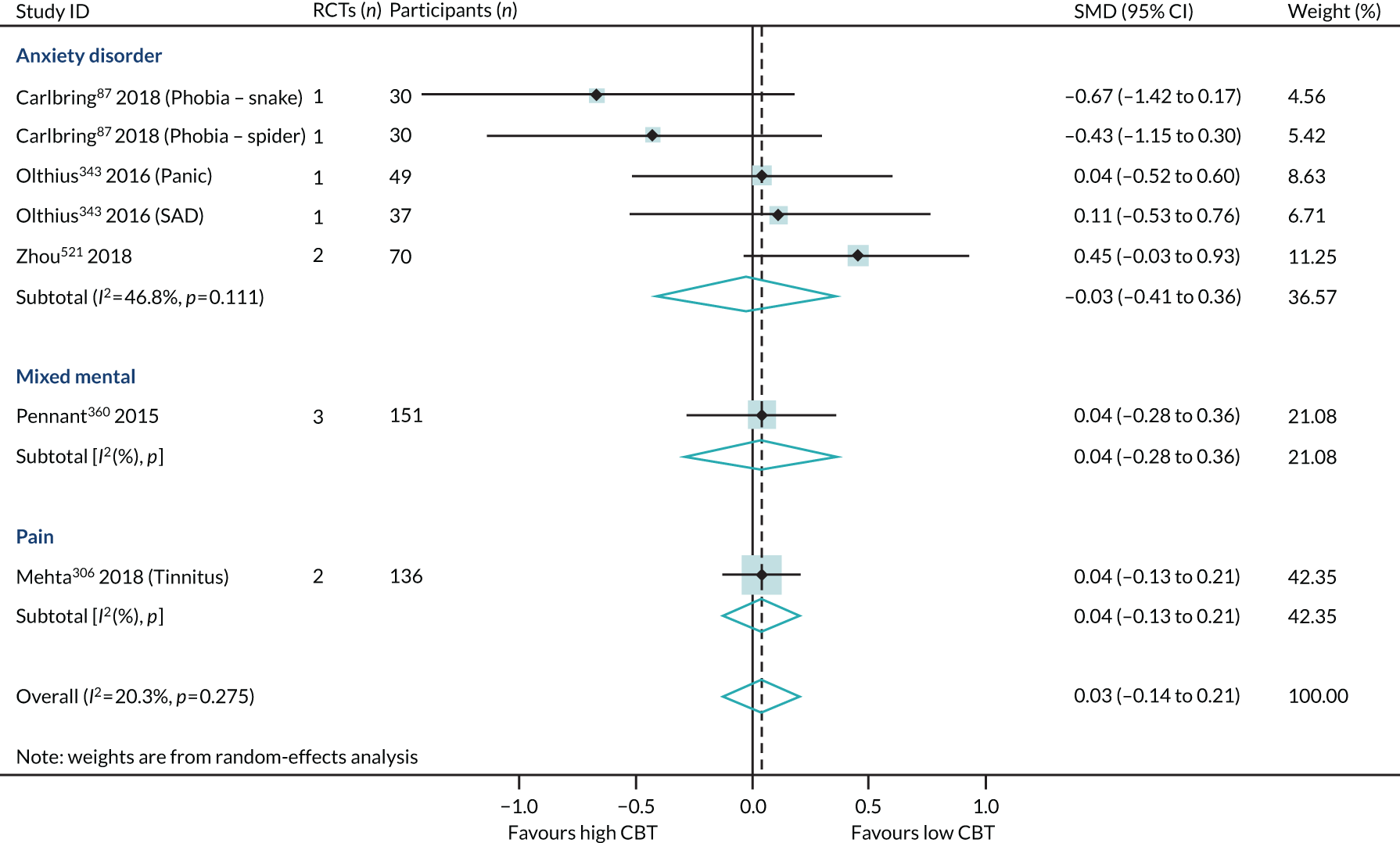
Type of comparators
The pooling of 14 meta-analyses collected from reviews of CBT compared with an active comparator (i.e. another type of therapy) found a non-significant effect of CBT (SMD 0.19, 95% CI –0.00 to 0.37; I2 = 48.6%). The pooling of 20 meta-analyses collected from reviews of CBT compared with a non-active comparator (e.g. WLC) found a modest effect in favour of CBT (SMD 0.37, 95% CI 0.19 to 0.55; I2 = 64.3%) (Figure 24).
FIGURE 24.
Anxiety subgroup analysis (end-point data from high-quality reviews): type of comparators. Note that two reviews that included both active and non-active comparators are not included here. OCD, obsessive–compulsive disorder; PTSD, post-traumatic stress disorder.
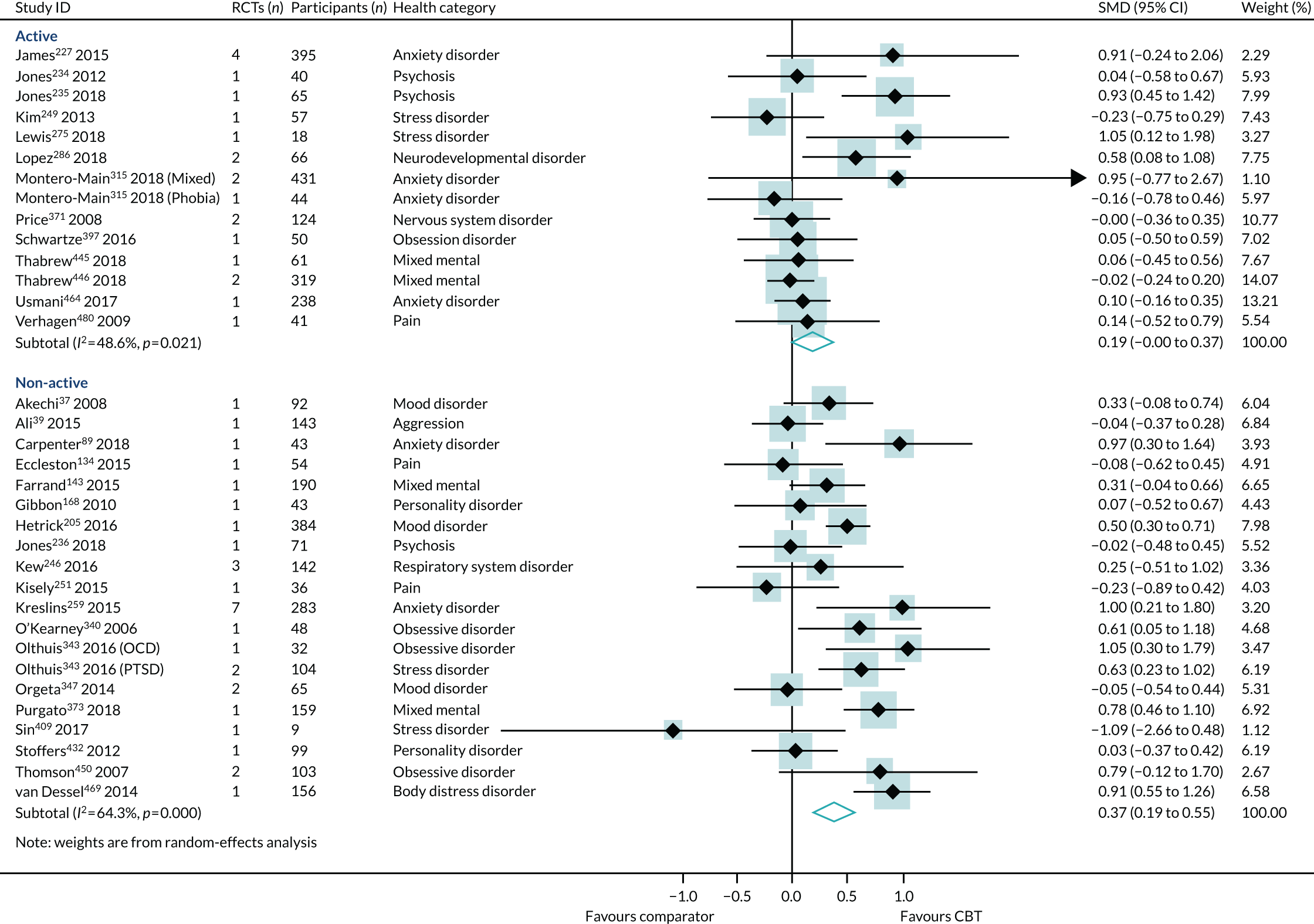
Duration of follow-up
The pooling of 10 meta-analyses collected from reviews that collected long-term follow-up data (> 12 months post intervention) found a modest effect of CBT (SMD 0.38, 95% CI 0.15 to 0.60; I2 = 65.9%). The pooling of 26 meta-analyses collected from reviews that collected only short-term follow-up data (< 12 months) found a similar effect in favour of CBT (SMD 0.27, 95% CI 0.12 to 0.43; I2 = 59.4%) (Figure 25).
FIGURE 25.
Anxiety subgroup analysis (end-point data from high-quality reviews): duration of follow-up. OCD, obsessive–compulsive disorder; PTSD, post-traumatic stress disorder.
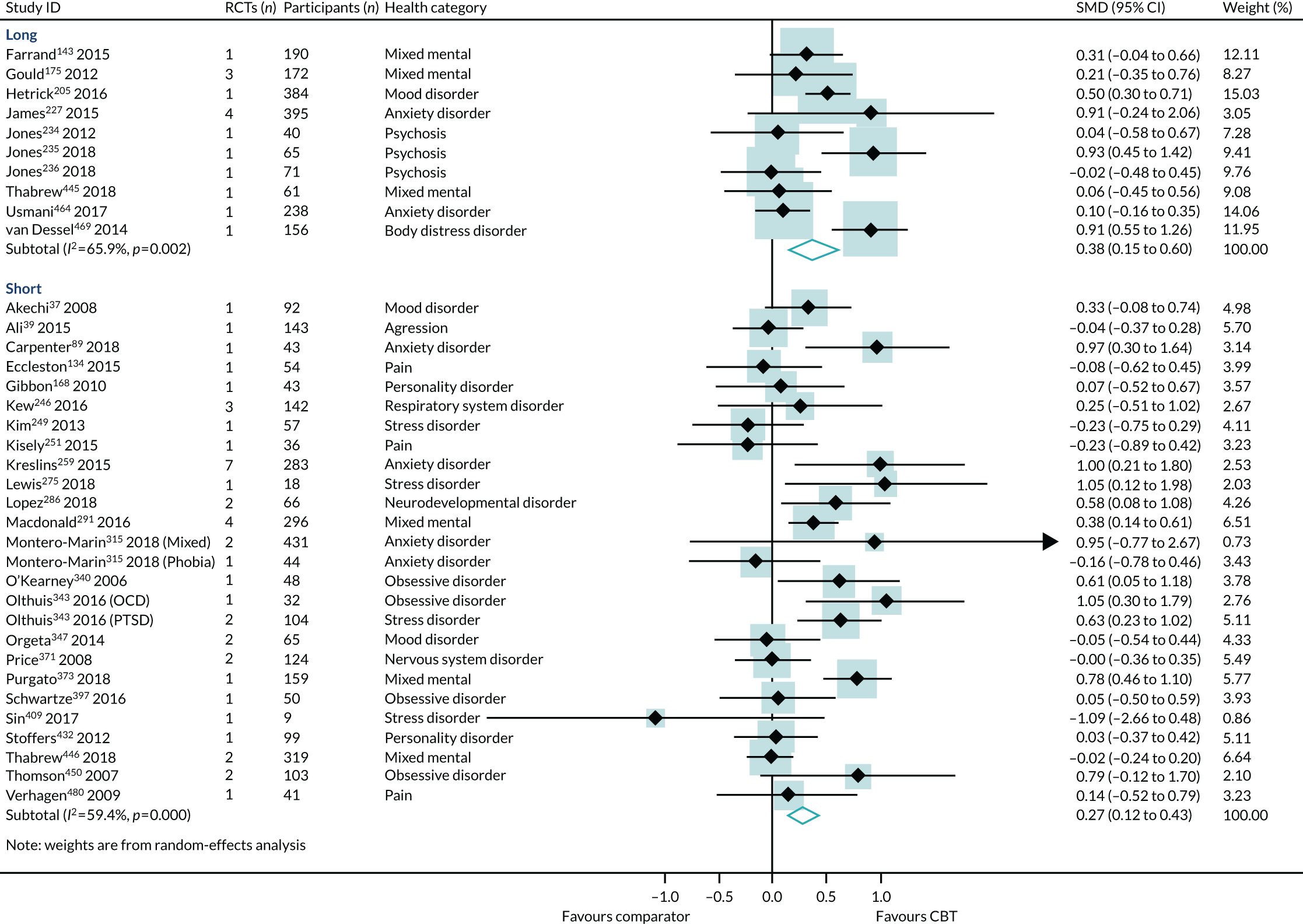
Age
The pooling of seven meta-analyses collected from child and adolescent populations (aged < 18 years) found an effect in favour of CBT (SMD 0.37, 95% CI 0.12 to 0.62; I2 = 67%). The pooling of 26 meta-analyses collected from adult populations (aged 18–65 years) found a significant effect in favour of CBT (SMD 0.32, 95% CI 0.15 to 0.48; I2 = 64%). The pooling of two meta-analyses collected from older adult populations (aged > 65 years) found a non-significant effect of CBT on anxiety outcomes (SMD 0.06, 95% CI –0.30 to 0.43; I2 = 0%) (Figure 26).
FIGURE 26.
Anxiety subgroup analysis (end-point data from high-quality reviews): age. Note that one review that did not report the age group is not included here. OCD, obsessive–compulsive disorder; PTSD, post-traumatic stress disorder.
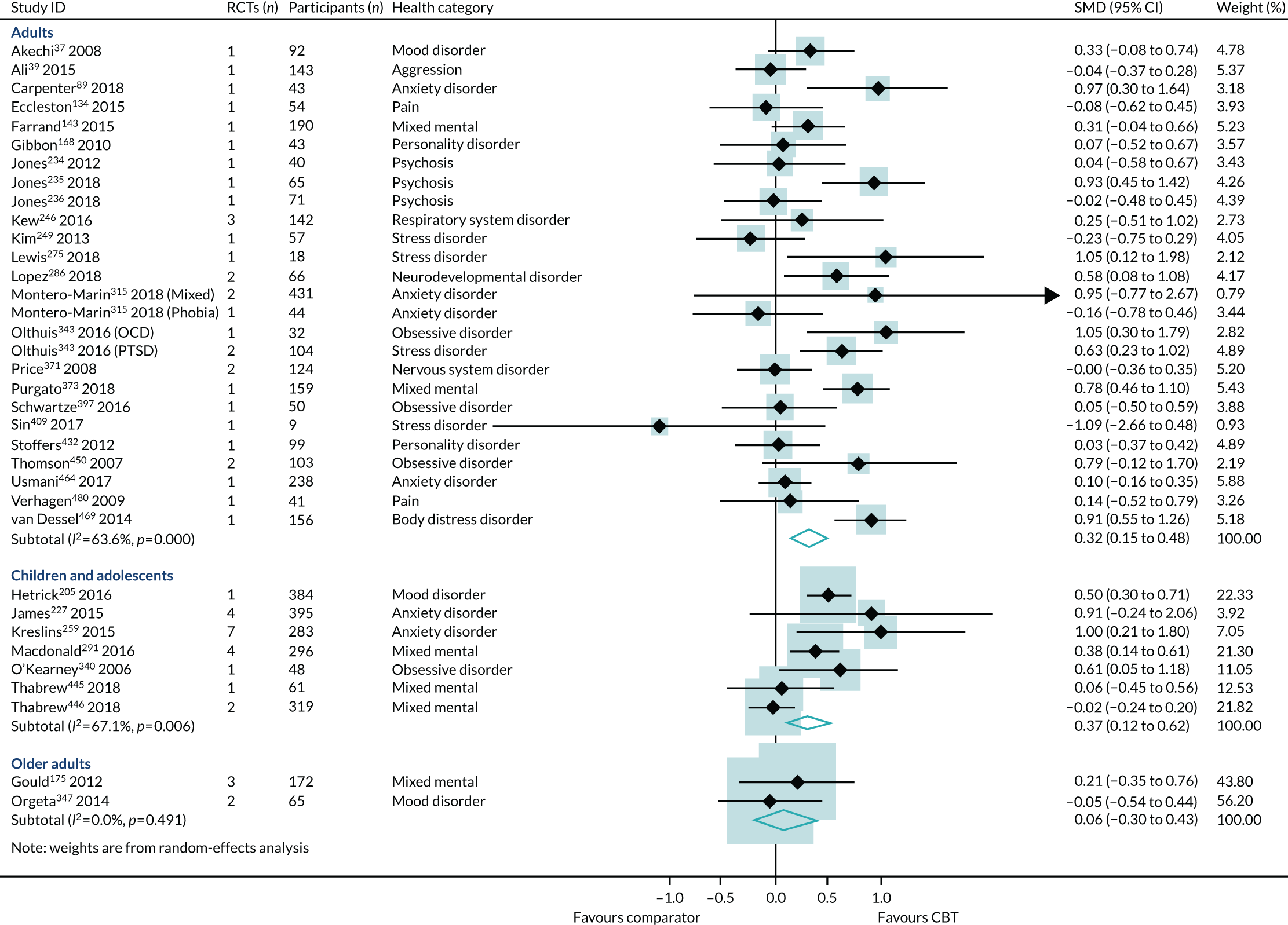
Sensitivity analysis
As the heterogeneity across the conditions’ subgroups was too large, we did not pool effects across conditions (Figure 27).
FIGURE 27.
Anxiety sensitivity analysis (end-point data from all-quality reviews). OCD, obsessive–compulsive disorder; PTSD, post-traumatic stress disorder; SAD, seasonal affective disorder.
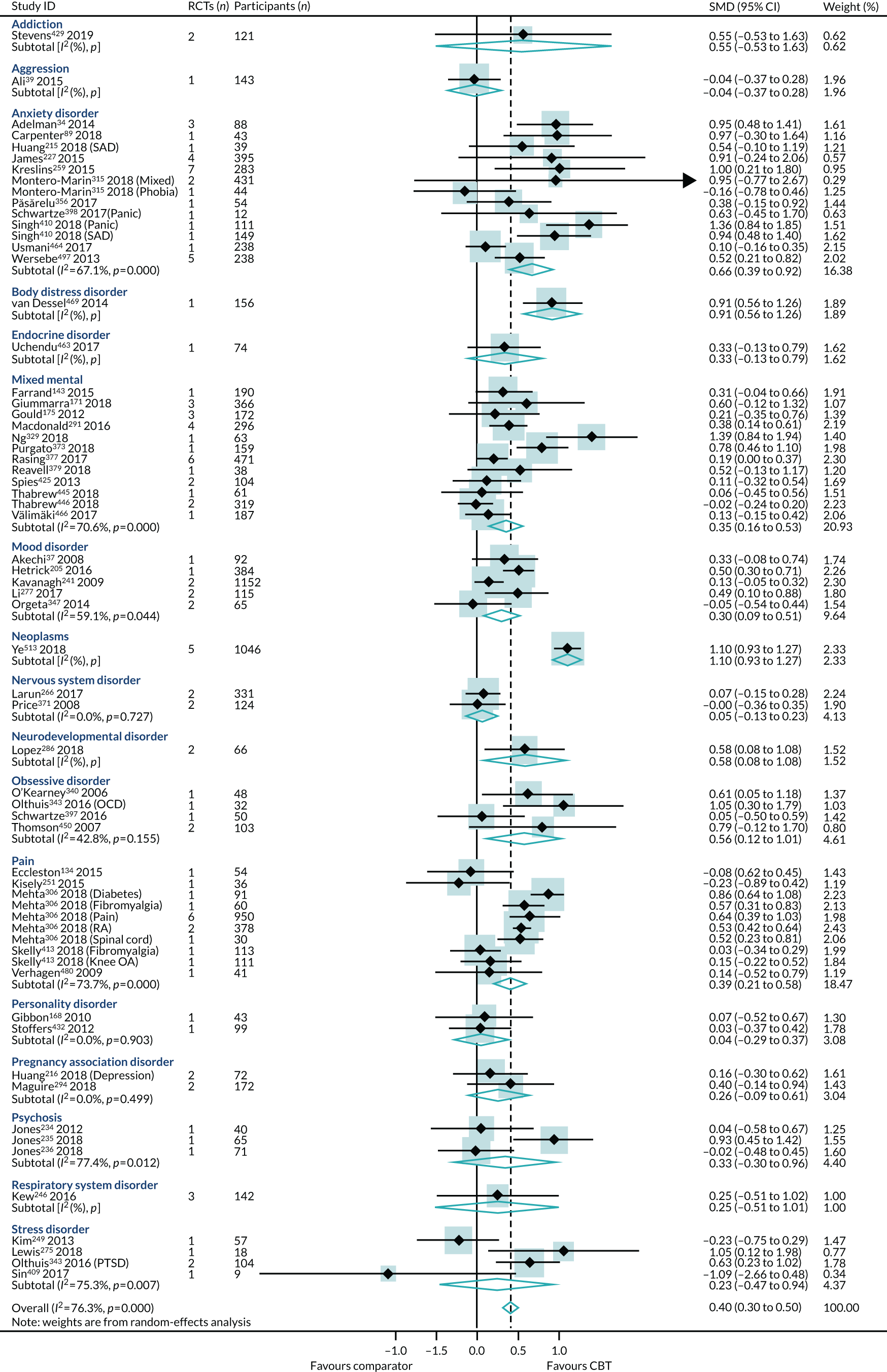
Synthesis of reviews reporting change scores
Four lower-quality reviews (four RCTs, 255 participants) reported anxiety outcome data as change scores. 56,105,336,457 These included reviews of 6B00-06: Anxiety, 6A60-80: Mood and 08 Diseases of the nervous system. The heterogeneity was too high (I2 = 88%) to pool across reviews (Figure 28).
FIGURE 28.
Anxiety outcome synthesis (change scores) across all conditions.
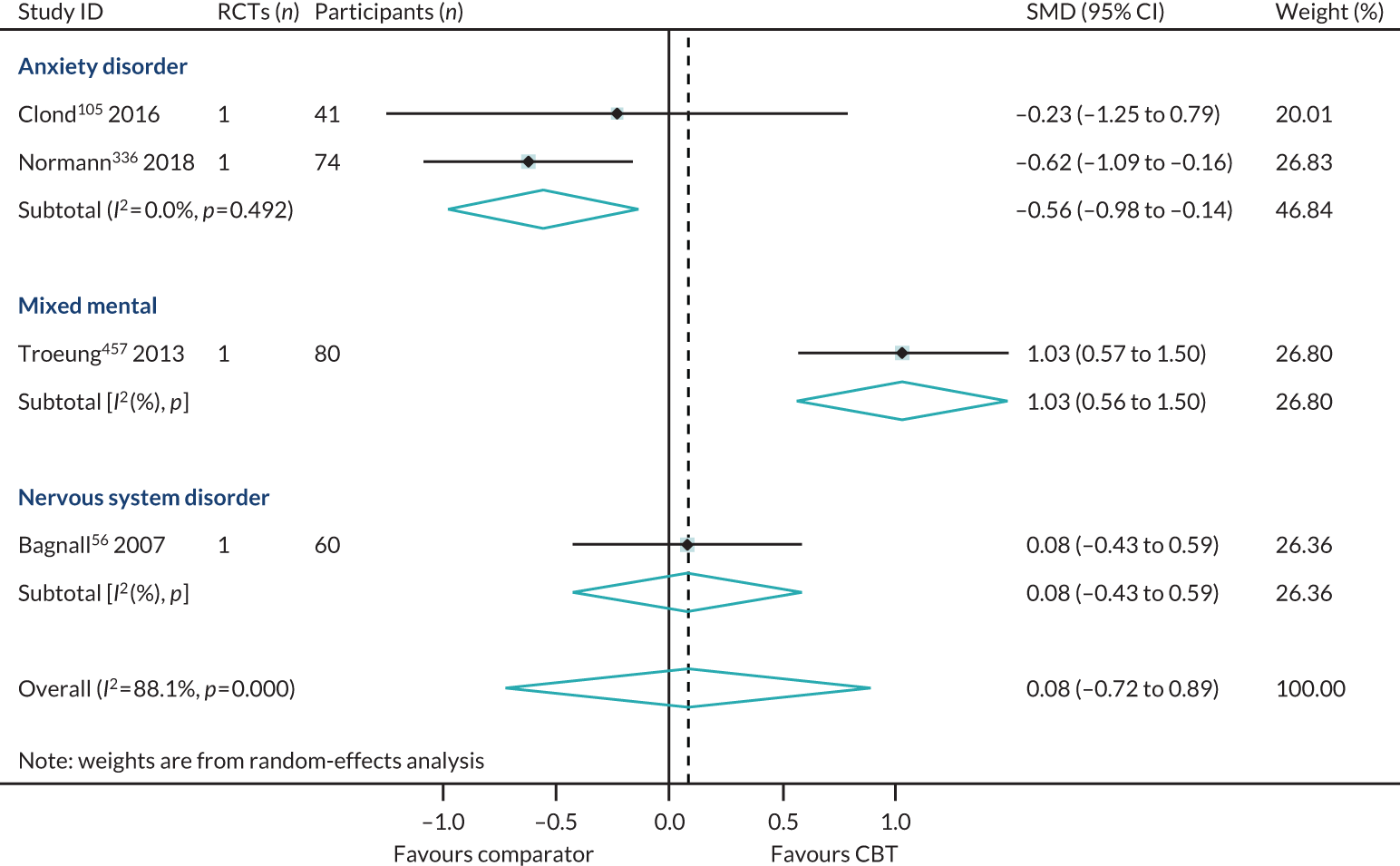
Synthesis of reviews reporting dichotomous outcomes
The lower-quality review of Noble et al. 332 (one RCT, 27 participants), classified under mixed mental conditions (anxiety and depression), reported anxiety data as risk differences and showed an effect in favour of CBT (SMD 0.36, 95% CI 0.01 to 0.71). The lower-quality review of Stoll et al. 433 (one RCT, 112 participants), targeting anxiety disorders, presented anxiety outcome data as ORs and reported an effect in favour of CBT (SMD 1.01, 95% CI 0.96 to 1.06) (Figure 29).
FIGURE 29.
Anxiety outcome synthesis across all conditions: (a) risk difference; and (b) OR.
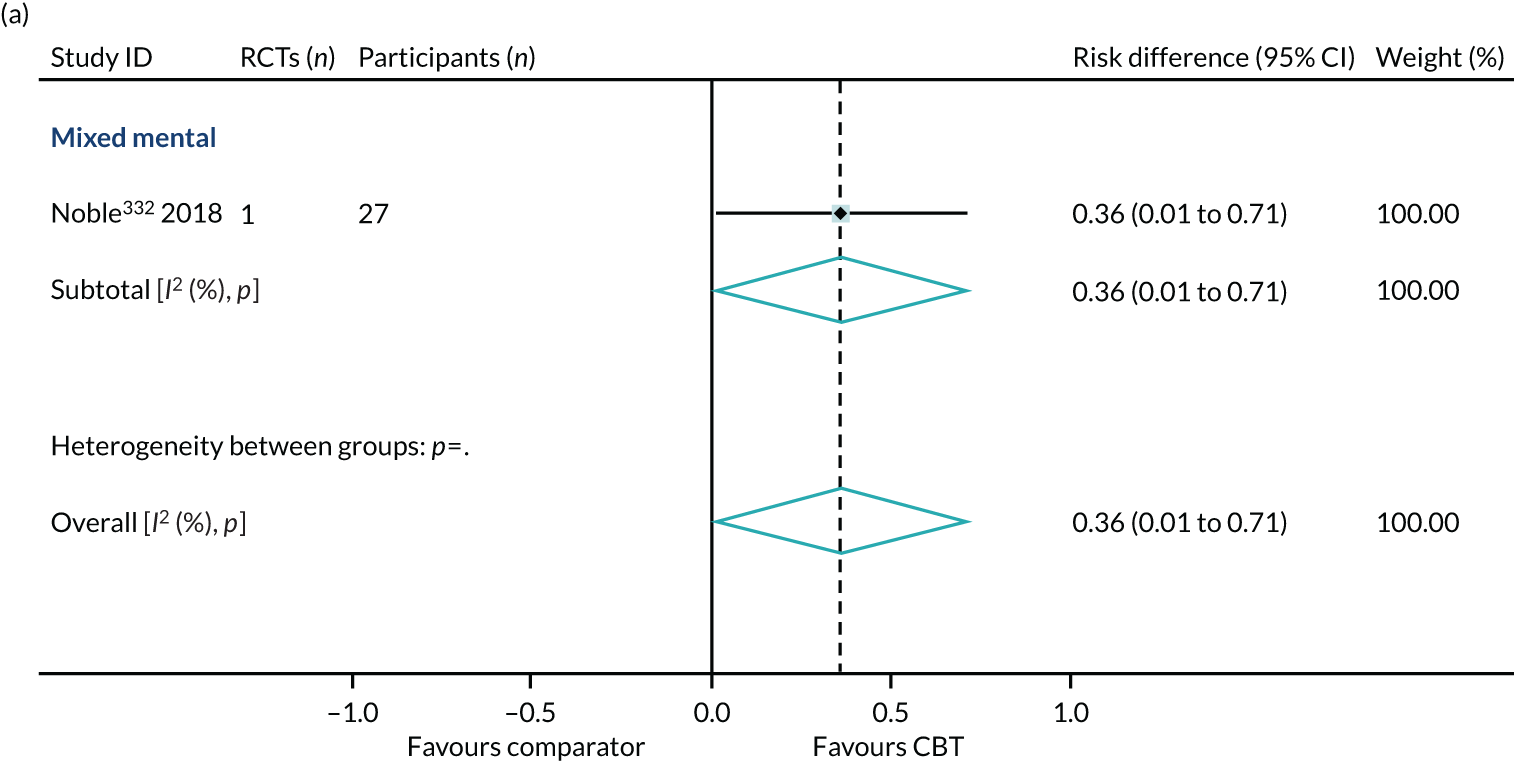
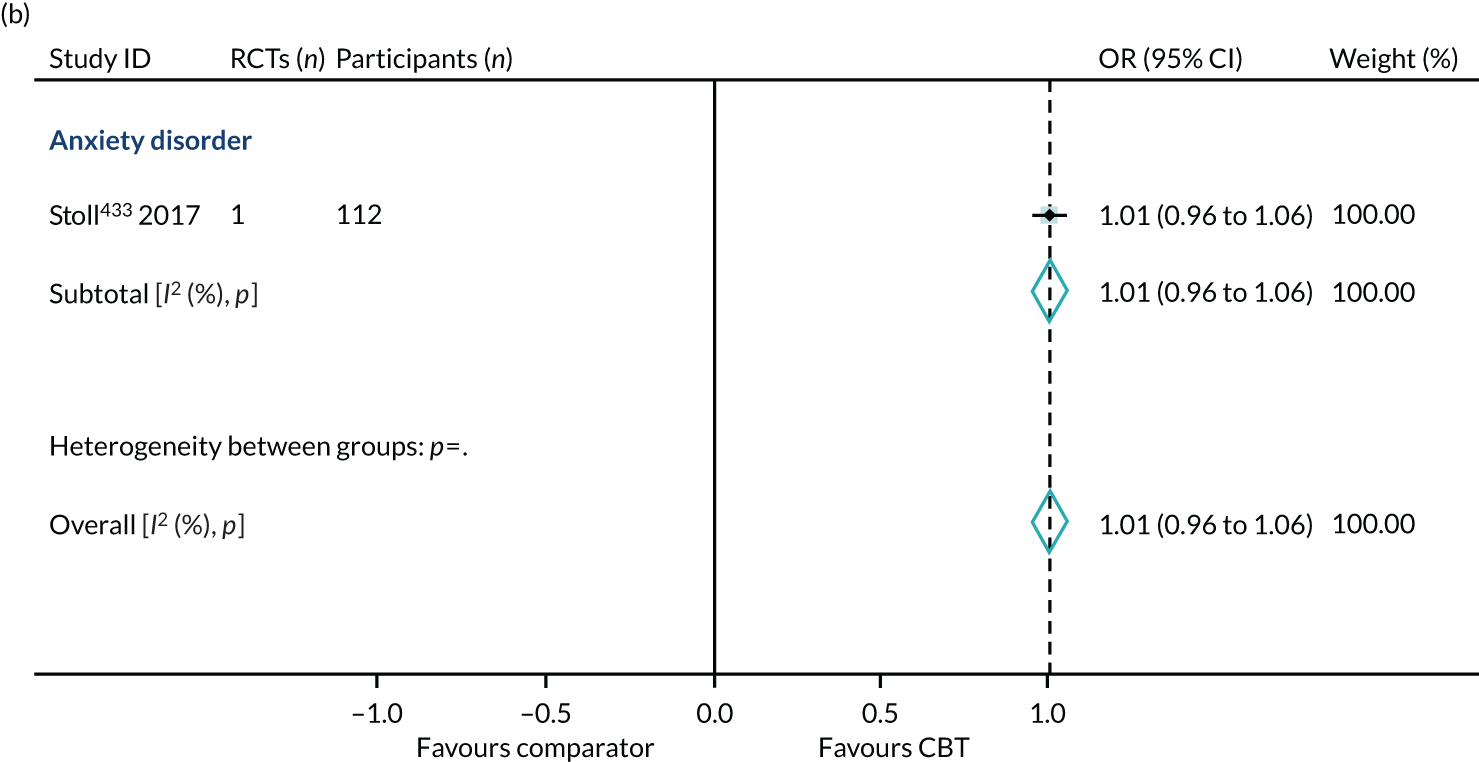
Appendix 12 Pain
Publication bias
No publication bias was detected using funnel plots (Figure 30), and Egger’s test showed no small-study effects (p = 0.19).
FIGURE 30.
Pain funnel plot (end-point data from high-quality reviews).
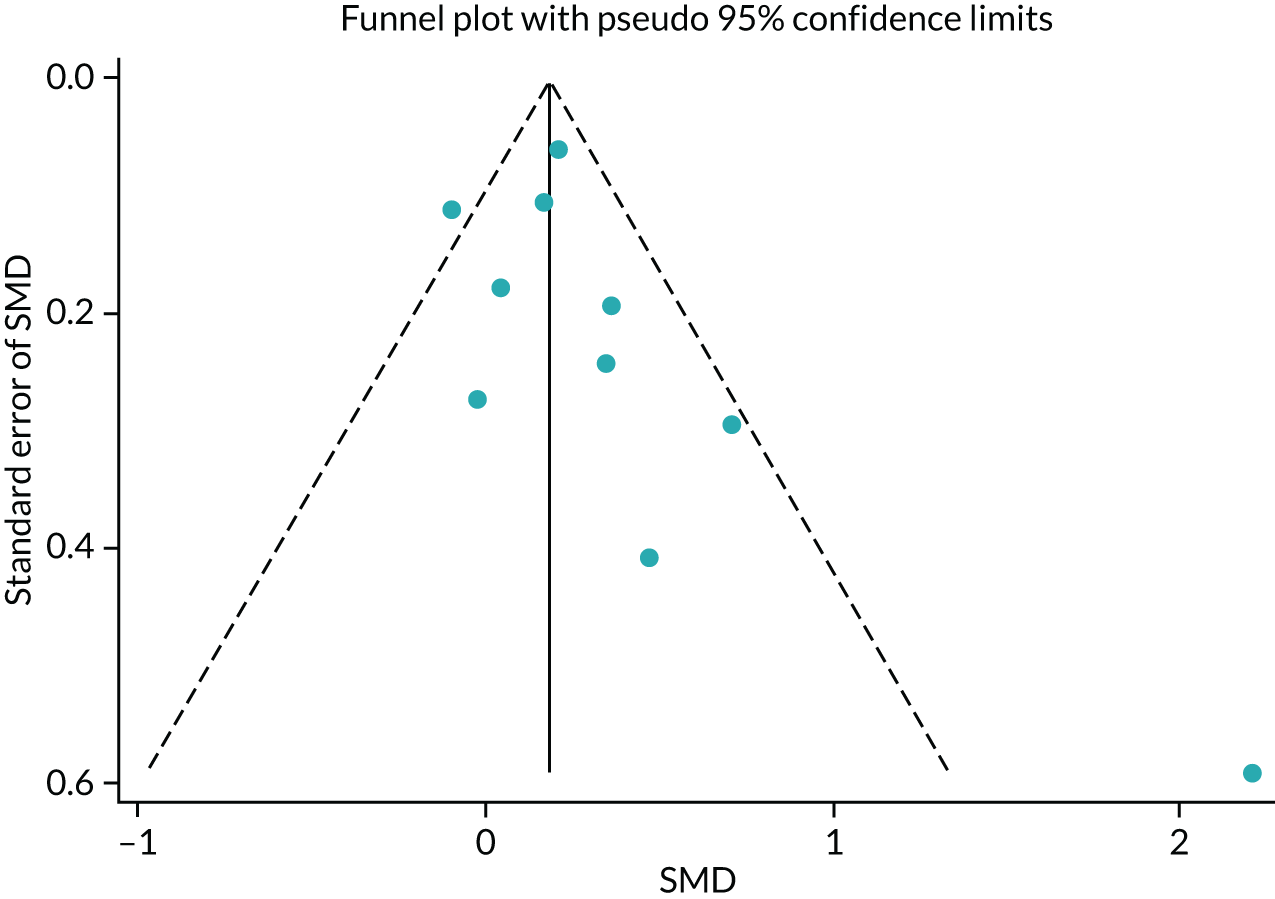
Subgroup analysis
Cognitive–behavioural therapy intensity
The SMD generated across the high-intensity reviews was in favour of CBT (SMD 0.19, 95% CI 0.01 to 0.37; I2 = 18%) (Figure 31).
FIGURE 31.
Pain subgroup analysis (end-point data from high-quality reviews): CBT intensity. Note that one review with combined high- and low-intensity CBT is not included here.
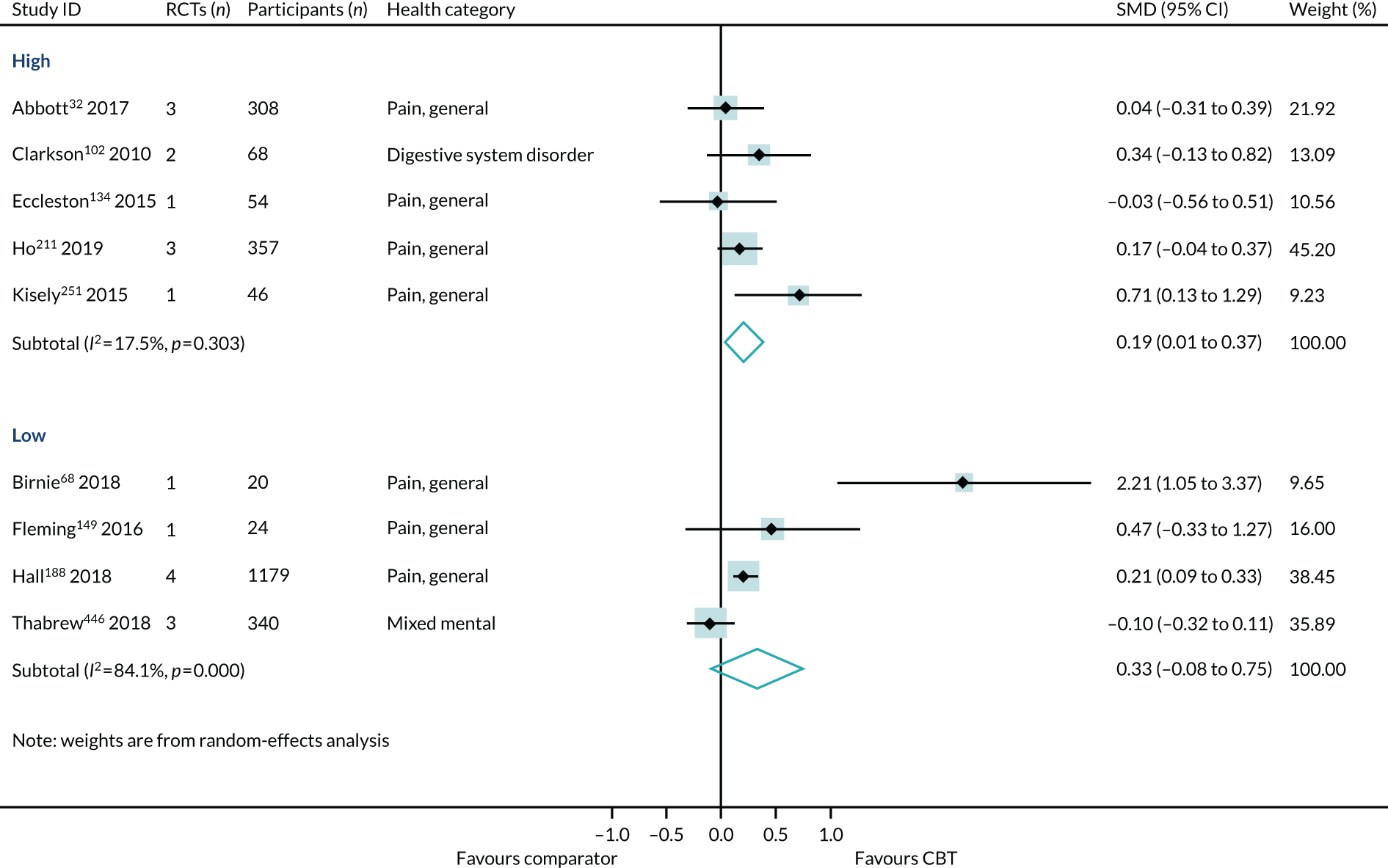
Type of comparators
We found an effect in favour of CBT across reviews in the non-active comparator group (SMD 0.59, 95% CI 0.07 to 1.11; I2 = 69%), but the effect was much smaller across reviews in the active comparator subgroup (SMD 0.14, 95% CI –0.11 to 0.38; I2 = 73%) (Figure 32).
FIGURE 32.
Pain subgroup analysis (end-point data from high-quality reviews): type of comparators. Note that two reviews with mixed active and non-active comparators are not included here.
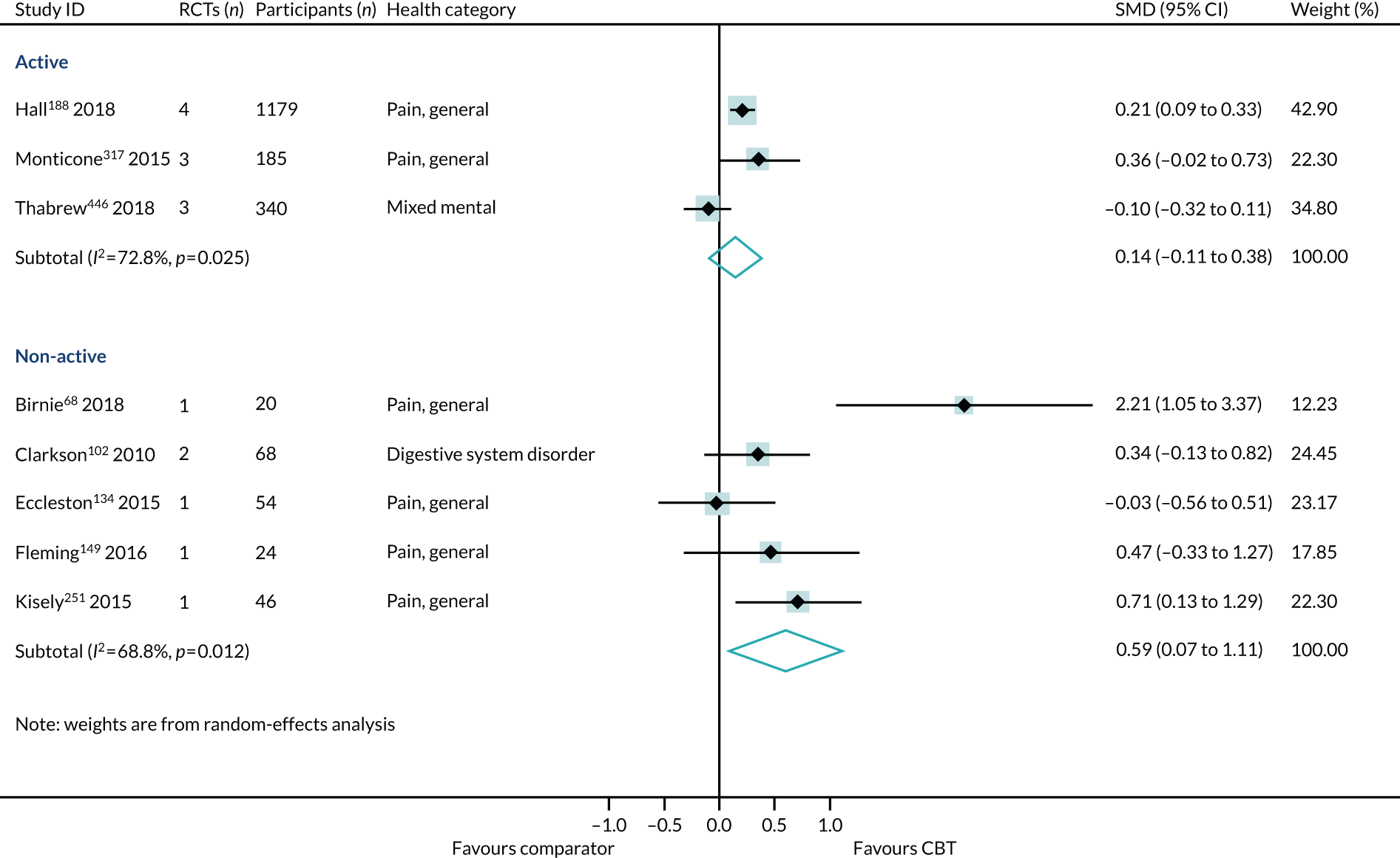
Duration of follow-up
Both subgroups produced effects in favour of CBT. The effect was larger in the short-term follow-up group (SMD 0.32, 95% CI 0.04 to 0.59; I2 = 71%) than in the long-term follow-up group (SMD 0.19, 95% CI 0.08 to 0.31; I2 = 0%) (Figure 33).
FIGURE 33.
Pain subgroup analysis (end-point data from high-quality reviews): duration of follow-up.
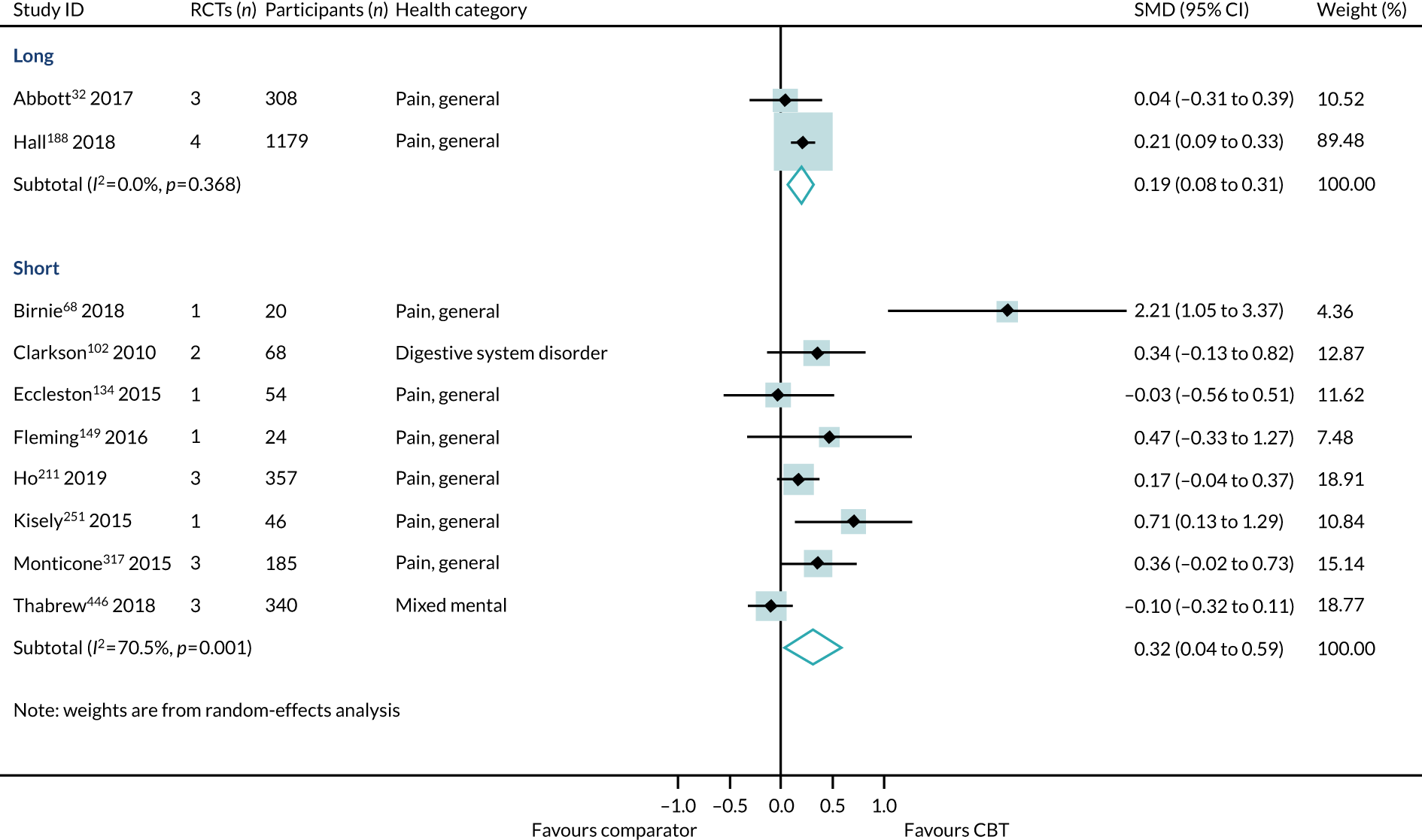
Age
The standardised mean effect across the reviews conducted in adults was in favour of CBT (SMD 0.21, 95% CI 0.12 to 0.31; I2 = 0%) (Figure 34).
FIGURE 34.
Pain subgroup analysis (end-point data from high-quality reviews): age. Note that one review that did not report the age group is not included here.
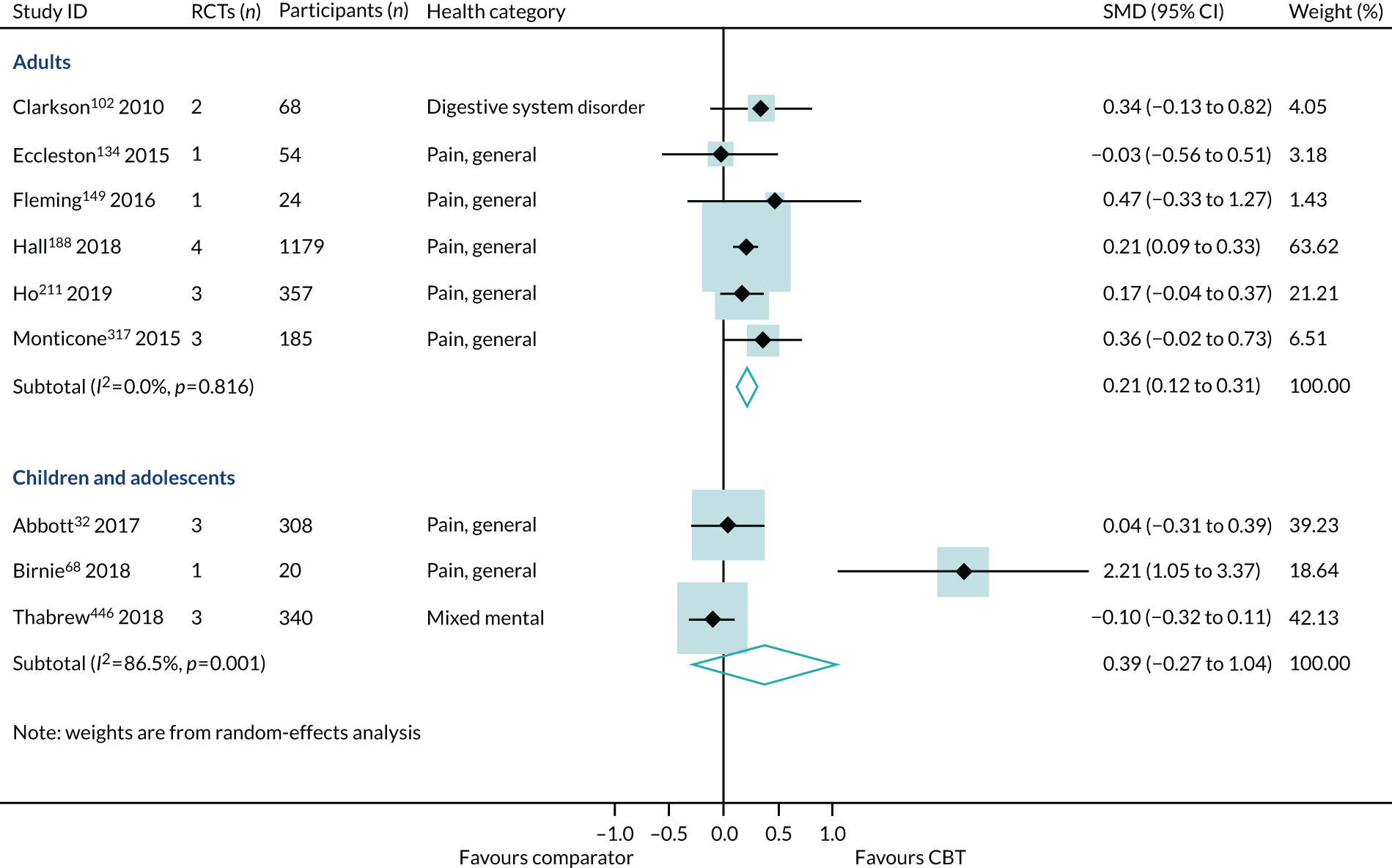
Sensitivity analysis
The across-condition pooled effect was in favour of CBT (SMD 0.21, 95% CI 0.11 to 0.31; I2 = 51%) for reducing pain intensity (Figure 35).
FIGURE 35.
Pain sensitivity analysis (end-point data from all-quality reviews).
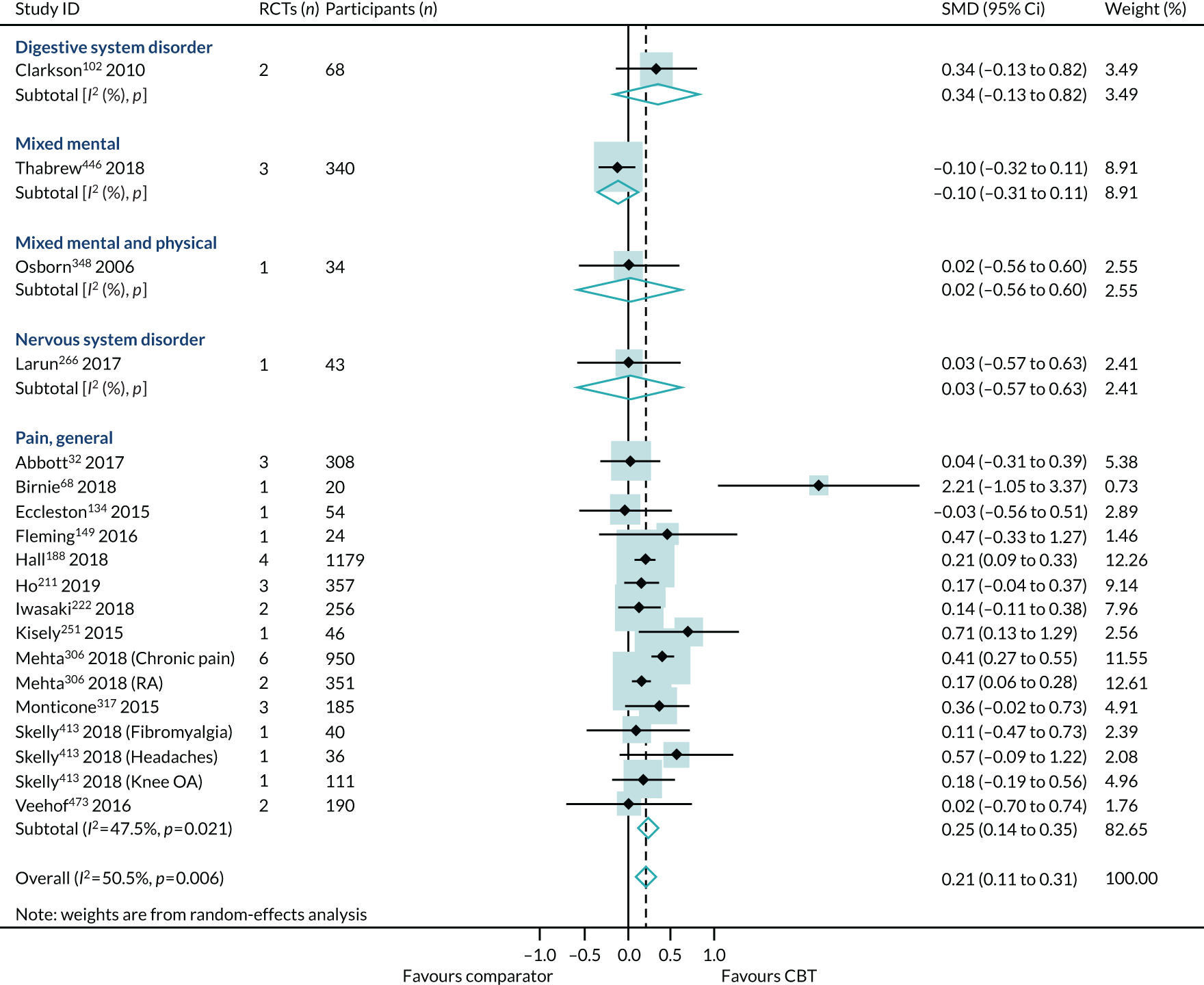
Synthesis of reviews reporting dichotomous outcomes
The review of Bernardy et al. 64 (one RCT, 118 participants) reported pain outcome data as risk difference and showed a non-significant effect for CBT (SMD 0.08, 95% CI –0.03 to 0.19) (Figure 36a). One review, Palermo et al. 354 (two RCTs, 68 participants), presented pain outcome data as ORs. This review demonstrated a non-significant effect for CBT (SMD 7.99, 95% CI –2.72 to 18.70) (Figure 36b).
FIGURE 36.
Pain outcome synthesis across all conditions: (a) risk difference; and (b) OR. Axes labels amended for space.
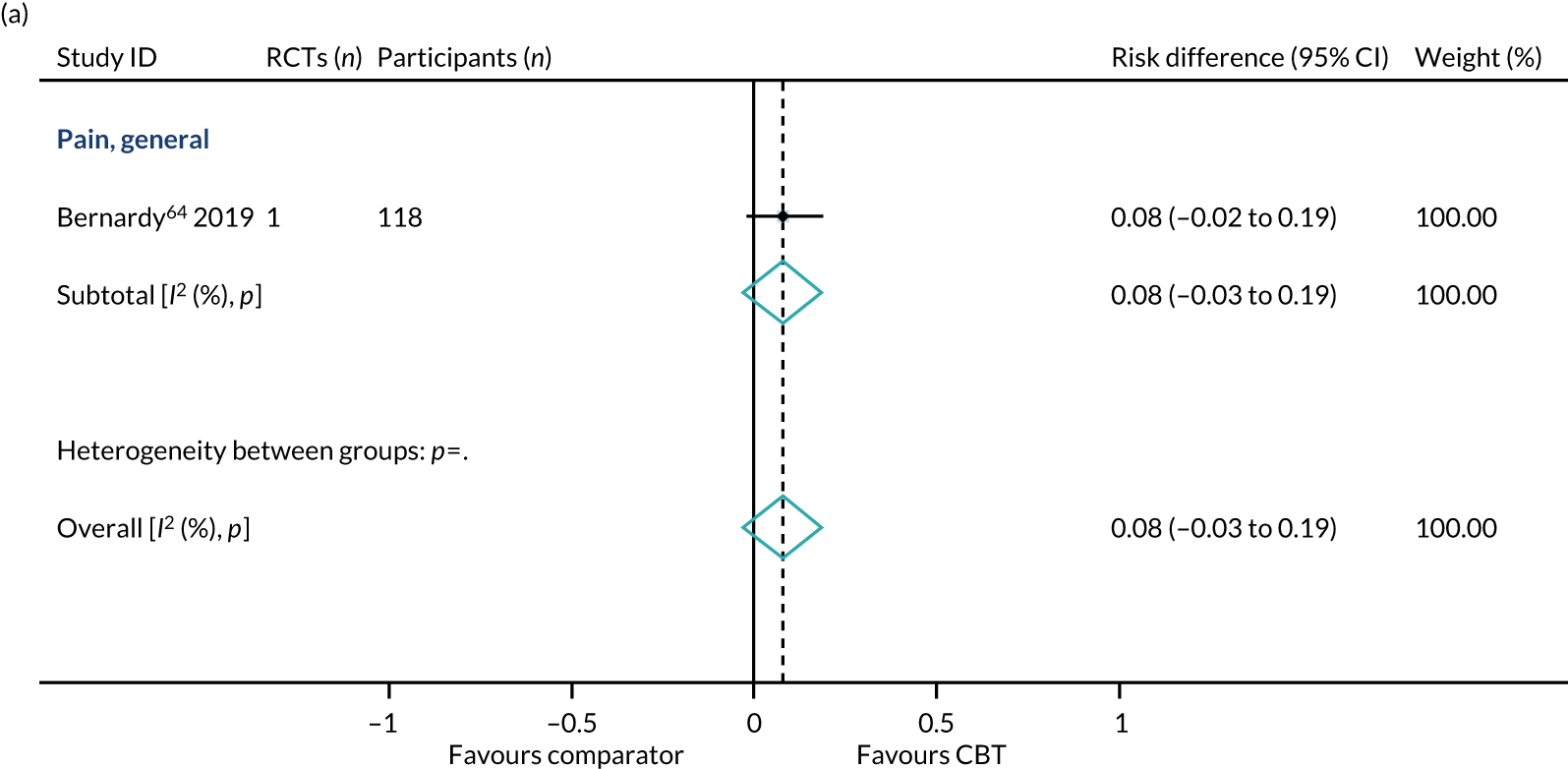
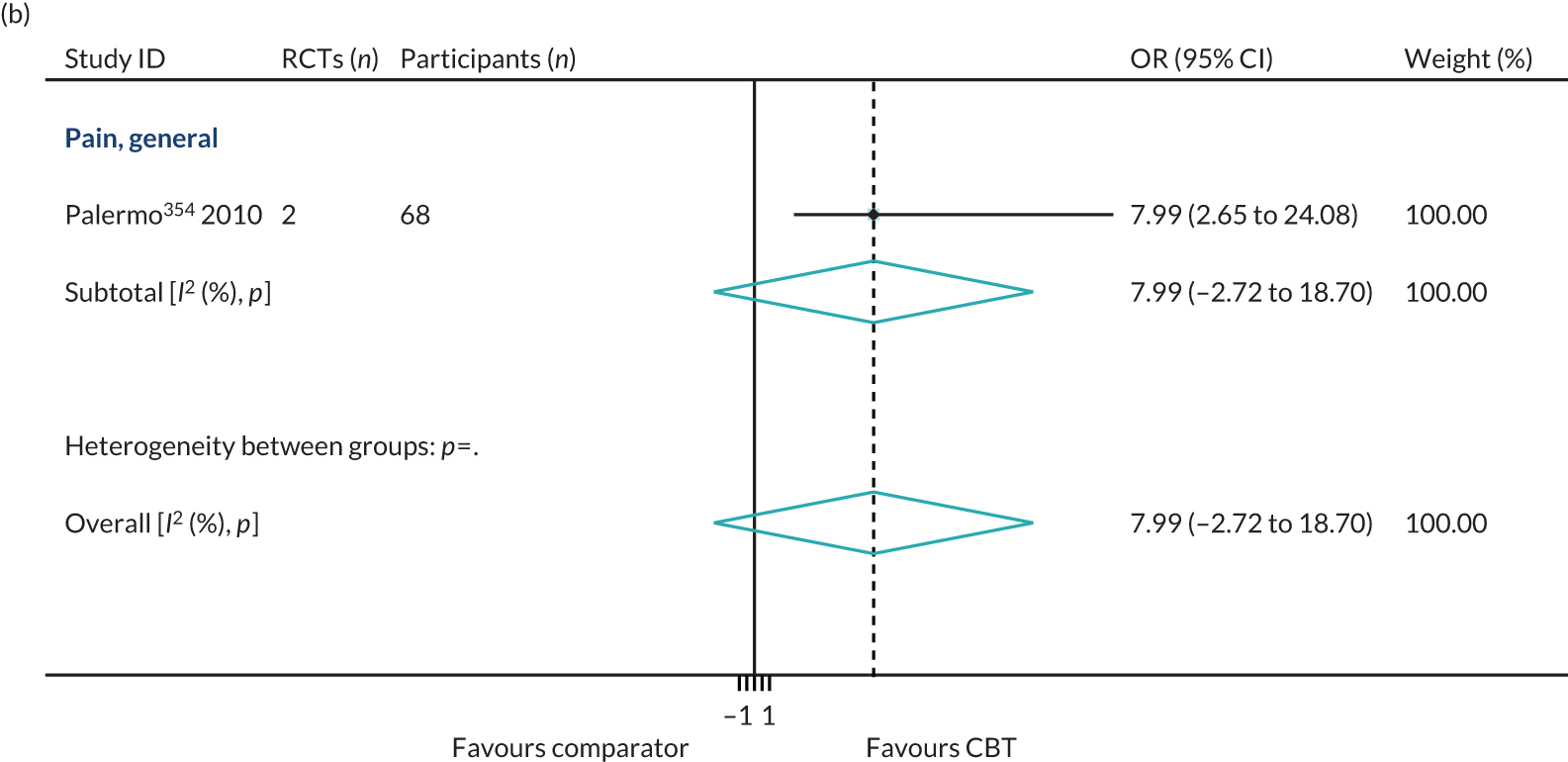
List of abbreviations
- ADHD
- attention deficit hyperactivity disorder
- AMSTAR
- A MeaSurement Tool to Assess systematic Reviews
- BAI
- Beck Anxiety Inventory
- CBT
- cognitive–behavioural therapy
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- COPD
- chronic obstructive pulmonary disease
- CRD
- Centre for Reviews and Dissemination
- DARE
- Database of Abstracts of Reviews of Effects
- ECG
- expert consultation group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICD-11
- International Classification of Diseases, Eleventh Revision
- NDORMS
- Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PMA
- panoramic meta-analysis
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
- randomised controlled trial
- RR
- risk ratio
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- SIGN
- Scottish Intercollegiate Guidelines Network
- SMD
- standardised mean difference
- TAU
- treatment as usual
- VAS
- visual analogue scale
- WHO
- World Health Organization
- WLC
- wait-list control