Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/104/06. The contractual start date was in September 2012. The draft report began editorial review in November 2019 and was accepted for publication in July 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Richards et al. This work was produced by Richards et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 The authors
Chapter 1 Introduction
Scientific background
Anaemia is common in patients undergoing major surgery. Observational and database studies have suggested that both anaemia and blood transfusion are associated with increased patient risk and worse outcomes following surgery. 1
Intravenous iron can produce a rapid rise in haemoglobin (Hb). Small studies and case series have shown that, if intravenous iron is applied in the preoperative setting, patients could have their anaemia corrected by the time of surgery. This may reduce the need for blood transfusion and improve patient outcomes.
In 2016, National Institute for Health and Care Excellence (NICE) guidelines recommended that patients undergoing surgery with an expected blood loss of ≥ 500 ml should be screened for anaemia at least 2 weeks prior to surgery, and recommended treatment with oral or intravenous iron therapy. 2 These guidelines were endorsed and supported by the 2018 Frankfurt consensus on patient blood management,3 and recently the NHS England Commissioning for Quality and Innovation scheme4 for 2020–21 set targets for 60% of patients to be screened and treated for iron-deficiency anaemia (IDA) before major surgery. Nevertheless, these initiatives were based on very low quality evidence and no large randomised controlled trial (RCT) has shown superiority of intervention with preoperative iron therapy.
It is not known whether or not intravenous iron given to patients before major surgery can correct anaemia and reduce the associated risk to the patient at operation and in the postoperative period.
The problem of anaemia and surgery
The World Health Organization defines anaemia as insufficient red blood cell (RBC) mass circulating in the blood, a Hb level of < 130 g/l for men and < 120 g/l for women. Anaemia is associated with impaired physical function, reduced quality of life, infection, and patient morbidity and mortality. Preoperative anaemia is common, affecting 30–60% of all patients undergoing major elective surgery. 5,6 Preoperative anaemia is an independent risk factor for blood transfusion, inpatient complications, delayed hospital discharge and worse recovery. 7
The cause of anaemia in this patient group is often multifactorial: due to blood losses, nutritional deficiency, IDA, anaemia of chronic disease (ACD) (cancer and/or inflammatory disease) or a combination of these aetiologies. Two main types of anaemia mostly affect surgical patients, IDA and ACD; the latter is more common in chronically ill and hospitalised patients. 8 ACD can be difficult to diagnose, often being regarded as a diagnosis of exclusion; the key feature is a disruption of normal iron homeostasis initiated by a cytokine-mediated immune response, such as in chronic inflammatory disease, during infection or following surgery. 8,9
Although anaemia is diagnosed by low Hb level, it is blood indices including ferritin, mean corpuscular volume and mean corpuscular Hb that define the cause of anaemia. IDA is classically defined when there is insufficient iron for Hb manufacture, leading to small (microcytic) pale (hypochromic) red cells; this is also termed absolute iron deficiency (AID). ACD can also be regarded as a type of iron deficiency, whereby inflammation mediates a disruption in normal iron transport and iron is sequestered in ferritin as part of the ‘acute phase response’, an innate response to stress. 8,10,11 This has been recognised as a functional iron deficiency (FID), and others have suggested the term anaemia of inflammation. 12
In a pilot study of patients (n = 1511) undergoing major surgery, 245 had preoperative anaemia. Microcytosis was observed in only 13% of anaemic patients. Ferritin levels < 30 ng/ml were seen in 31% of anemic patients, and 64% had a ferritin level of < 100 ng/ml. If IDA was defined by using low ferritin and low mean corpuscular volume, this would mean that out of the 245 patients with anaemia only 13 would be defined as having ‘textbook’ IDA. Although low serum ferritin levels can reliably indicate reduced iron stores in the body, ferritin is an acute phase protein and may be elevated in the presence of inflammation. In surgical patients, the majority have underlying inflammation, even in the preoperative setting, with elevated mean C-reactive protein (CRP) levels of 23 mg/l in the pilot study. IDA may, therefore, be masked by ‘abnormally’ high or normal ferritin levels in this group of patients. Raised CRP levels correlated with anaemia, suggesting that FID was the most common cause of anaemia in this series of preoperative patients.
In a Cochrane Database review of iron therapy for the treatment of anaemia in non-chronic kidney disease populations,13 the definitions of anaemia and iron deficiency were extremely varied. 14 Data in the surgical population were notably lacking and there was no evidence on how to define IDA in surgical patients. 13,14 This was further highlighted in a prospective observational trial of patients with anaemia undergoing cardiac surgery, in which laboratory variables for iron deficiency including ferritin, transferrin saturation (TSAT) and hepcidin were compared with bone marrow analysis (from the sternum during open cardiac surgery). The results were confusing, with little correlation between variables. 15,16
In summary, the exact definition of anaemia in the surgical patient is confusing. Proposals exist for IDA, FID, AID and ACD. There is an inability to define ‘iron deficiency’ accurately in patients with anaemia before operation, which has meant that most patients are not currently managed with iron therapy and blood transfusion remains the standard of care in the perioperative period. 17
This problem of definitions among AID, FID and IDA was addressed in a clinical trial on patients with anaemia and heart failure. In the FAIR–HF (Ferinject® Assessment in patients with IRon deficiency and chronic Heart Failure) study, AID was diagnosed when the serum ferritin level was < 100 µg/l and FID was diagnosed when ferritin was between 100 and 299 µg/l and TSAT was < 20%. In this group of patients, the mean [standard deviation (SD)] CRP level was 7.46 mg/l (5.34 mg/l). 18 There was no difference in response between AID and FID to intravenous iron therapy. Those treated with intravenous iron had a significant improvement in patient quality of life, disease status and 6-minute walk test compared with those treated with placebo.
Two small RCTs in surgical patients assessed the use of intravenous iron preoperatively, with mixed results. In Australia, Froessler et al. 19 randomised 72 patients, 4–21 days preoperatively, to either intravenous iron or standard of care. The use of intravenous iron was associated with an increased preoperative Hb level and reduced blood transfusion at operation (12% vs. 31%), and a reduction in hospital length of stay (LOS), but no associated difference in patient morbidity, mortality or quality of life. 19 The Intravenous Iron in Colorectal Cancer Associated Anaemia (IVICA) trial20 from Nottingham, UK, looked at 116 patients undergoing colorectal cancer surgery; intravenous iron compared with oral iron, given at least 2 weeks before operation, did increase Hb levels, but there was no difference in blood transfusion use from recruitment to trial completion in terms of either volume of blood administered (p = 0.841) or number of patients transfused (p = 0.470). 20 Long-term follow-up suggested that those patients who received intravenous iron had improved quality of life at postoperative follow-up and this was associated with higher Hb levels. 21
Blood transfusion may be a poor treatment option
The current standard of care for anaemia in patients undergoing surgery is blood transfusion. The demand for blood products had steadily increased in the decade from 2000. In 2008/2009, 1.86 million units of blood were transfused in the UK; the cost per unit was £130 (in 2012), an overall cost of provision to the NHS of £247.4M. Although blood transfusion is a well-organised and well-provided service, there was £7.2M in waste and loss of productivity related to blood and related blood products. 22 Other concerns include the potential impact of universal prion screening for blood products. The NHS Blood and Transplant (NHSBT) national commission forecast that this would result in an increase of £15–25 per unit and an increase of nearly £41 per unit in filtration charges. 22 Although the cost to the NHS from NHSBT was £130 for one unit of blood, this was the provisional cost not including the cumulative total NHS cost of nursing time, patient transport and treatment costs, etc., which is likely to be considerably higher. 23
Transfusion is known to exert immunological and immunosuppressive effects, which include a decrease in T-cell and natural killer cell production. In addition, transfusion itself is associated with increased inflammatory response. Although anaemia increases the requirement for transfusion, blood transfusion has independently been associated with a worse patient outcome. 24,25 Prospective observational studies suggest that allogenic blood transfusion (ABT) increases the risk of fluid overload, postoperative infection and respiratory complications and reduces patients’ functionality, with an increased re-admission rate in intensive care units (ICUs) and longer hospital stay. 26
Transfusion has also been associated with increased relative risk of cancer recurrence. A systematic review and meta-analysis that investigated the effects of ABT and recurrence of colorectal cancer in 12,127 patients suggested a moderate association was to be found between rates of ABT and colorectal cancer recurrence [odds ratio 1.42, 95% confidence interval (CI) 1.20 to 1.67]. The conclusion was that ABT should be restricted to patients undergoing colorectal cancer resection with curative intent. 27
Treating anaemia preoperatively: why oral iron is not adequate
The 2015 NICE (NG24) guidelines recommended that anaemic patients are treated with oral therapy as a first-line option. 2 Oral iron is a common, cheap and effective method to replenish total body iron stores, and is effective in patients with IDA. However, oral iron is poorly absorbed, with absorption reduced by numerous factors including proton pump inhibitors, anti-inflammatory drugs, inflammation and gastrointestinal disease including Helicobacter pylori, all features common in patients undergoing surgery. Side effects of oral iron are common, reported by about half of patients, most commonly including abdominal pain, constipation or diarrhoea and heartburn. Compliance is a problem with oral iron therapy and only 20–40% of patients complete a full course of treatment. 28
Oral iron is absorbed in the duodenum at a rate of 2–16 mg per day. A formula based on body weight and Hb levels can be used to calculate the amount of iron needed to replenish iron stores: the Ganzoni formula. 29 Ganzoni calculated that most patients with anaemia resulting from iron deficiency will need between 100 and 1600 mg of iron to replenish body reserves. Therefore, oral iron is able to restore normal iron levels in 3–6 months; a period that is impractical and too long in the surgical setting. Hb levels may in fact increase before the replenishment of iron stores, but the loss of an equivalent of 1 blood unit during surgery represents > 400 mg of iron stores and may compromise this lengthy treatment.
Normal iron homeostasis is tightly regulated. The body is highly efficient at recycling iron from old red cells, with 50 ml of blood or 25 mg of iron being reused daily. The other main loss is from the skin (about 2 mg per day) or, in women, from menstrual losses. The hepatic peptide hormone, hepcidin, is responsible for regulating enteric iron absorption, plasma iron concentrations and tissue distribution. Hepcidin acts by inducing the degradation of the iron exporter ferroportin. 30 Ferroportin is responsible for the active transport of iron out of cells, whether from enterocytes or from iron stores in hepatocytes. In the blood, iron is moved to the site of need by transferrin. Hepcidin synthesis is transcriptionally regulated by extracellular and intracellular iron concentrations through a complex series of bone morphogenetic protein receptors, their iron-specific ligands, modulators and iron sensors. Hepcidin synthesis is also induced by inflammatory signals, including interleukin-6 and activin B. Hence, hepcidin synthesis is thought to play a role in host defence and inflammation, as well as being pathogenic in iron-restrictive anaemias associated with inflammation such as chronic diseases and some cancers or trauma. 10
The main problem in surgical patients is that the underlying disease process and concomitant inflammatory response evoke mediators that increase hepcidin levels with subsequent sequestration of iron, reduced iron absorption and a functional IDA. 31
Why is the study needed now?
The use of intravenous iron has been studied in a variety of clinical settings. Meta-analyses show proven efficacy in renal failure,32 cardiac failure,33 inflammatory bowel disease34 and women’s health. 35,36 Overall, end points from clinical trials have focused on change in Hb levels or correction of anaemia rather than the effect of intravenous iron on blood transfusion or patient outcomes. 37
Anaemia is common in all areas of surgical practice, with database and observational data linking preoperative anaemia to an associated increased patient risk. Risk has primarily focused on the need for blood transfusion, with secondary end points including associated risk of adverse events (AEs) in most organ systems [acute kidney injury (AKI), acute neurocognitive decline, cardiac risk, wound infection, etc.] as well as increased length of ICU or hospital stay, and increased overall mortality.
Major abdominal surgery includes upper gastrointestinal, hepatobiliary and pancreatic, colorectal, urological, vascular and gynaecological surgery. These are often lengthy and complex operations. The patient population is often older and patients frequently have comorbidities. Although laparoscopic surgery has increased in the last decade, with an associated reduction in blood transfusion use,38–43 large open operations remain a significant burden to patients and also to health-care utilisation. Interventions to reduce surgical risk would benefit patient outcomes and also reduce health-care costs.
The cause of anaemia in the preoperative setting is also not fully understood. It is frequently related to blood loss from the underlying condition, such as bowel cancer, or secondary to inflammation, leading to a state of FID. Therefore, it is highly likely that intravenous iron would be efficacious in this setting to increase Hb levels and to be an effective treatment for preoperative anaemia.
If intravenous iron can correct anaemia in a timely manner before major surgery, this may reasonably be predicted to improve patients’ energy levels and reduce fatigue, consequently helping patients to recover from surgery. In turn, this may be associated with a reduction in complications, faster recovery and reduced length of hospital stay.
Chapter 2 Methods
Study design
This was a randomised, double-blind, parallel-group, placebo-controlled, multicentre, Phase III study with 1 : 1 randomisation to either intravenous iron or placebo. All patients were treated according to protocol and received double-blind intravenous iron therapy or placebo. All patients were followed for 6 months from date of operation. As the active and placebo infusion fluids cannot be matched in appearance, unblinded study personnel not otherwise involved in the study or in patient management were responsible for investigational drug administration. PREVENTT is reported in accordance with Consolidated Standards of Reporting Trials (URL: www.consort-statement.org/).
Aim
This study aimed to assess whether or not giving a single dose of intravenous iron to patients with anaemia prior to major abdominal surgery reduced the need for transfusion or the likelihood of death in the perioperative period. In addition, the effect of the intervention on postoperative complications, hospital stay, re-admission to hospital and quality-of-life outcomes was assessed.
Important changes to methods
The initial working protocol included an additional hospital visit for patients for assessment before their operation. This additional visit was not acceptable to patients, with failure to recruit patients in the first 4 months of the trial. In a protocol amendment, the timing of the preoperative assessments was changed such that these took place at the patient’s admission for their operation, avoiding the need for any additional visits to the hospital for the purpose of the trial. The amended trial protocol was relaunched at the start of 2014.
After this, there were only four further protocol amendments during the course of the trial, including reducing the timeline to surgery from 14 days to 10 days, revising the description for major surgery, adjusting the diagnosis of anaemia in line with the World Health Organization’s definitions (120 g/l for women and 130 g/l for men), and removing the need for preoperative liver function testing and updates as a result of revised Medicines and Healthcare products Regulatory Agency guidelines (see Appendix 1).
Participants
Inclusion criteria
Patients who met the following criteria at the start of treatment were eligible for the study:
-
Aged at least 18 years and providing signed written informed consent.
-
Undergoing elective major open abdominal surgery
-
benign or malignant disease
-
undergoing major surgery, defined as an operation of anticipated duration > 1 hour.
-
-
screening Hb level of ≥ 90 g/l (9.0 g/dl) but ≤ 120 g/l (12.0 g/dl) in women or 130 g/l (13.0 g/dl) in men within 4 weeks of randomisation.
-
Randomisation and administration of study infusion a minimum of 10 days and maximum of 42 days before the planned operation.
-
Women of childbearing potential had a negative pregnancy test (within last 7 days) and agreed to use effective form of contraception until 6 weeks post treatment.
-
Laboratory data used for the determination of eligibility at the baseline visit were not older than 4 weeks.
Exclusion criteria
Patients who, at the start of treatment, met any of the following criteria were not eligible for the study:
-
Undergoing laparoscopic surgery.
-
Body weight < 50 kg.
-
Known history of acquired iron overload, or family history of haemochromatosis or thalassaemia or TSAT > 50%.
-
Known reason for anaemia (e.g. untreated vitamin B12 or folate deficiency or haemoglobinopathy).
-
Known hypersensitivity to ferric carboxymaltose (Ferinject®; Vifor Pharma UK Limited, Staines-upon-Thames, UK) or its excipients.
-
Temperature > 37.5 °C or patient on non-prophylactic antibiotics.
-
Known chronic liver disease.
-
If clinically indicated for the patient to have liver function tests (LFTs) as part of pre-assessment for surgery and at this screening, alanine transaminase or aspartate transaminase was above three times the upper limit of the normal range.
-
Received erythropoietin or intravenous iron therapy in the previous 12 weeks.
-
Immunosuppressive therapy (for organ transplantation) or renal dialysis (current or planned) within the next 12 months.
-
Severe asthma or severe allergy (requiring hospitalisation within the last 12 months).
-
Unfit for elective surgery.
-
Pregnant or lactating.
-
Unable to fully comprehend and/or perform study procedures in the investigator’s opinion.
-
Patient involvement in another investigational medicinal product (IMP) trial within the previous 4 weeks, prior to randomisation. Involvement in another IMP trial, following randomisation, that may impact on the results of the PREVENTT trial.
Setting
Forty-six hospitals in the UK participated: University College London Hospitals NHS Foundation Trust; Royal Free London NHS Foundation Trust; Royal Cornwall Hospitals NHS Trust; Royal Devon and Exeter NHS Foundation Trust; Royal Marsden NHS Foundation Trust; The Hillingdon Hospitals NHS Foundation Trust; Swansea Bay University Health Board – Morriston Hospital; York Teaching Hospital NHS Foundation Trust; Dorset County Hospital NHS Foundation Trust; Maidstone and Tunbridge Wells NHS Trust; Newcastle Hospitals – Freeman Hospital; Southmead Hospital Bristol; The Royal London Hospital – Barts Health NHS Trust; Sheffield Teaching Hospital – Northern General Hospital; University Hospital Southampton NHS Foundation Trust; University Hospital of North Staffordshire NHS Trust; University Hospitals Bristol NHS Foundation Trust; Royal Sussex County Hospital; St James’s University Hospital; Guy’s and St Thomas’ NHS Foundation Trust; Central Manchester University Hospitals NHS Foundation Trust; Blackpool Teaching Hospitals; West Suffolk NHS Foundation Trust; Royal Surrey County Hospital; Wythenshawe Hospital; James Cook University Hospital; Broomfield Hospital – Mid Essex Hospital Trust; Royal Liverpool and Broadgreen University Hospitals NHS Trust; Salford Royal NHS Foundation Trust; The County Hospital, Wye Valley NHS Trust; Northampton General Hospital; Imperial College Healthcare NHS Trust; John Radcliffe Hospital – Oxford University Hospitals; Queen’s Medical Centre, Nottingham University Hospitals NHS Trust; Aintree University Hospital NHS Foundation Trust; Queen Elizabeth Hospital, NHS Gateshead; Royal Infirmary of Edinburgh; The Pennine Acute Hospitals; Norfolk and Norwich University Hospital; Peterborough and Stamford Hospitals; Russells Hall Hospital, Dudley; King’s College Hospital; Liverpool Women’s NHS Foundation Trust; Basildon University Hospital; Countess of Chester Hospital; Hinchinbrook Hospital – North West Anglia Foundation Trust; and Cheltenham and Gloucester Hospital, Gloucestershire Hospitals NHS Foundation Trust.
Randomisation
Randomisation was by a secure web-based service through the Clinical Trials Unit (CTU) at the London School of Hygiene & Tropical Medicine (LSHTM), provided by Sealed Envelope (London, UK). Randomisation used minimisation, taking into account baseline Hb level (< 100 g/l or ≥ 100 g/l), age (< 70 or ≥ 70 years), centre and operation type (major/major+/complex). Patients were randomised to receive either active treatment (intravenous iron as ferric carboxymaltose 1000 mg) or placebo. The web-based database allocated the participant a unique trial identification number and their identification details were entered onto the trial patient identification log < 100 g/l or ≥ 100 g/l kept in the investigator site file. Once this number was assigned to a patient, it was not reused, even in the case of participant withdrawal from the study.
Randomisation was performed at the trial sites by the unblinded member of staff who was delegated this responsibility by the principal investigator (PI) only, as evidenced by documentation in the delegation log. Each unblinded member of staff was trained in the use of the web-based randomisation service at the site initiation visit, and was then provided with their own individual password and personal identification number (PIN) to access the service.
The blinded staff did not have access to the randomisation system, and, therefore, remained blinded to the treatment allocated.
Blinding and unblinding
Blinding
The iron carboxymaltose solution is dark brown in appearance; blinding was obtained by shielding the patients from seeing the preparation of the study drug, and having the unblinded study personnel who were not involved in any study assessments (efficacy or safety) as those responsible for preparing and administering the study treatment. This unblinded member of staff was present throughout administration of the trial drug. Patient shielding was achieved by preparing and administering the study drug behind a screen or curtain. The drug was shielded from vision (light protection bags) and administered through black tubing.
The unblinded member of staff disposed of the administration kit in a concealed way. All patients were monitored during the trial drug administration as per normal clinical practice; any AEs were documented.
Unblinding
The blinding of patients or other medical staff could be broken for valid medical or safety reasons (e.g. in the case of a severe AE, when it is necessary for a treating health-care professional to know which treatment the patient has received).
Incidents of unblinding were recorded in the randomisation system, and reports on unblinding were sent to the sponsor and the Data Safety and Monitoring Committee (DSMC).
Interventions
Patients who conformed to all eligibility criteria and provided written informed consent were randomised to receive either intravenous iron or placebo 10–42 days before the planned date of their surgery, as described in Randomisation.
Administration of the IMP was carried out in a hospital in line with local protocols. The study medication was administered to patients by the unblinded member of staff. An intravenous line was sited for drug administration Following this, it was advised that the skin along the donor vein be wiped using an iodine swab to help maintain the blinding. The patient was shielded from seeing preparation of the study drug, drug administration, disconnection and removal of the intravenous line, as described above. Patients were monitored for AEs or signs of hypersensitivity during and for at least 30 minutes following the administration of the treatment.
In the intravenous iron group, 1000 mg of ferric carboxymaltose (Ferinject) was administered as an intravenous infusion (100 ml n/saline) over a minimum of 15 minutes using a black infusion kit.
In the placebo group, normal saline was administered as an intravenous infusion (100 ml n/saline) over a minimum of 15 minutes using a black infusion kit.
Adverse events occurring in connection with the administration of study medication were recorded. In the event of a patient having an allergic reaction or signs of intolerance during study drug administration, the investigator managed this in accordance with local protocol and submitted a completed serious adverse event (SAE) form to LSHTM within 24 hours of the event.
Data management
Confidentiality
All data were handled in accordance with the UK Data Protection Act 1998 and 2018.
The case report forms (CRFs) did not bear the patient’s name or other personal identifiable data. The patient’s date of birth and trial identification number were used for identification.
Data collection tools and source document identification
Trial data were collected electronically at each participating centre and transferred electronically to a secure server at the CTU at LSHTM, via a secure web-based system. Data collection and entry was carried out by trained investigators or research nurses at each site. Designated investigator staff entered the information required by the protocol onto the electronic case report forms (eCRFs) from the source documents. The following were used as source documents:
-
Medical notes.
-
Drug charts.
-
Anaesthetic records.
-
Electronic hospital systems for laboratory results.
-
Patient diaries.
-
Validated questionnaires (patients completed paper copies and these were considered source documents. Delegated members of staff transcribed the data into the eCRFs).
Details of all study staff involved in data processing were contained in the site-specific delegation log for each centre. Copies of these are held in the PREVENTT trial master file, which is held by the CTU at LSHTM.
It was the responsibility of each local investigator to ensure the accuracy of all data entered in the CRFs. The delegation log identified all those personnel with responsibilities for data collection and handling, including those who had access to the trial database.
Data handling and analysis
The PREVENTT database application was built on the popular open source web platform commonly referred to as LAMP (Linux, Apache, MySQL and PHP). It was hosted on a centralised application server at LSHTM and was accessed by users through a normal web browser [e.g. Internet Explorer (Microsoft Corporation, Redmond, WA, USA) or Firefox (Mozilla Corporation, Mountain View, CA, USA)]. Online forms (eCRFs) with built-in validation checks were used by investigators or research nurses at participating centres to enter data. The system was blinded so that treatment groups were not revealed to users of the database application.
Data were extracted from the system by exporting the database tables as CSV (comma-separated values) text files or other suitable format. Analyses were conducted by the trial statistician in a statistical package (such as Stata® version 15.0; StataCorp LP, College Station, TX, USA) after importing the database tables. For unblinded analyses, these files were combined with the CSV file exported from the unblinded randomisation system.
Electronic case report form requirements
Data were entered at each local site using an electronic data capture system and managed by the data manager at the LSHTM CTU. The data analysis was performed by the trial statistician based at LSHTM CTU.
Electronic data were monitored by central statistical monitoring and by site visits as outlined in a monitoring standard operating procedure (SOP). Control checks were programmed into the system and the electronic data capture system automatically flagged erroneous data points using range checks and validators (e.g. when date of death occurs before date of birth), as well as entered data points that were likely to be inaccurate or the result of a typing error (e.g. blood pressure of 80/120 instead of 120/80). The eCRF was also set up so that data could not be missed out or left blank. Any changes made to the electronic data were tracked to maintain an audit trail.
Patients completed the health-related quality-of-life (HRQoL) questionnaires at their hospital assessment visits. If patients did not attend these appointments, then blinded research staff were responsible for contacting them and encouraging them to complete these questionnaires and the patient diaries.
The data were transmitted securely from each local site to the LSTHM CTU, via password- or PIN-protected online data entry over an encrypted internet connection (Secure Sockets Layer). This transfer was in accordance with the Data Protection Acts 1998 and 2018, the University College London Information Security Policy and the Trust Information Governance Policy.
Monitoring and site visits
The conduct of the study was supervised by specifically trained monitors from the LSHTM CTU. A trial-specific monitoring plan was established following a risk assessment and full details are available in the PREVENTT Monitoring SOP. The trial was monitored according to this agreed plan.
Baseline assessment
Baseline assessments were planned to coincide with the routine hospital schedule such as outpatient, endoscopy or pre-assessment clinic attendance. The ‘baseline assessments’, including laboratory tests for the purposes of the PREVENTT trial, were the same as those for routine clinical care in pre assessment before major surgery, so as to follow routine clinical practice and surgical/anaesthetic pathways where possible. For convenience, patients could undergo ‘baseline’ assessments, randomisation and trial drug administrations at the same attendance.
Assessments included the following:
-
checking conformance with inclusion/exclusion criteria, including laboratory tests taken within the prior 4 weeks [full blood count (FBC), urea and electrolytes (UE), LFT where clinically indicated as part of pre assessment, iron studies, estimated glomerular filtration rate (eGFR), CRP, thyroid function tests, vitamin B12 and folate].
-
documentation of past medical history
-
vital signs (blood pressure, pulse rate, body weight, height and temperature)
-
12-lead ECG (electrocardiography)
-
additional blood samples for central laboratory analysis (FBC, iron studies, TSAT and total iron binding capacity)
-
HRQoL questionnaires
-
documentation of health resources used (HRU); patient diary issued.
Follow-up
Patients were initially followed up during their stay in hospital, up to discharge.
Follow-up assessments
-
The Post-Operative Morbidity Survey (POMS) was administered on days 3, 5, 7 and 14 after surgery, if the patient remained in hospital.
-
Documentation of postoperative complications [using the Clavien–Dindo (CD) system].
-
Transfusion of blood and blood components.
-
FBC, UE, eGFR and CRP (if collected as part of routine care).
-
On discharge, hand-out of documentation of HRU diaries.
Patients were subsequently followed up at 8 weeks (± 2 weeks) and 6 months (± 1 month) after their operation. If the operation did not take place, the follow-up visit was calculated from the planned surgery date. The following assessments were carried out at each follow-up visit.
Follow-up visit 1 [8 weeks (± 2 weeks) after operation]
-
Documentation of hospital admissions.
-
Transfusion of blood and blood components.
-
Vital signs (blood pressure, pulse rate, body weight).
-
FBC, UE, eGFR and CRP.
-
HRQoL questionnaires.
-
Documentation of HRU; diary collected and reissued.
Follow-up visit 2 [6 months (± 1 month) after operation]
-
Documentation of hospital admissions.
-
Transfusion of blood and blood components.
-
Vital signs (blood pressure, pulse rate, body weight).
-
FBC, UE, eGFR and CRP.
-
HRQoL questionnaires.
-
Collection of documentation of HRU; patient diary collected.
Safety assessments
Definitions
An AE was defined as any untoward medical occurrence in a patient or clinical trial patient to whom a medicinal product was administered, but not necessarily having a causal relationship with this treatment.
An adverse reaction (AR) was defined as any untoward and unintended response in a patient to an IMP that is related to any dose administered to that patient. This includes medication errors and uses outside protocol (including misuse and abuse of product).
An unexpected AR was defined as an AR the nature and severity of which is not consistent with the information about the medicinal product set out:
-
in the case of a product with a marketing authorisation, in the summary of product characteristics (SmPC) for that product
-
in the case of any other IMP, in the investigator’s brochure relating to the trial in question.
An important medical event was defined as an event that may jeopardise the subject or may require an intervention to prevent one of the above characteristics or consequences. Such events should also be considered ‘serious’.
Recording and reporting adverse events
All AEs were recorded in the medical records following consent. Any AEs that occurred within 30 days of the trial treatment were noted in the CRF, recorded in an AE form and reported to the LSHTM CTU. All AEs were recorded with clinical symptoms and accompanied with a simple (brief) description of the event, including dates. All AEs were reported to the sponsor at least once per year.
Assessment of adverse events
Each AE was assessed for causality, expectedness and seriousness.
Causality was defined as follows:
-
Suspected – there was at least some evidence to suggest a causal relationship (e.g. the event occurred within a reasonable time after administration of the trial medication).
-
Not suspected – there was little or no evidence to suggest there was a causal relationship (e.g. the event did not occur within a reasonable time after administration of the trial medication). There is another reasonable explanation for the event (e.g. the patient’s clinical condition, other concomitant treatments).
Expectedness is defined as follows:
-
Expected – an AE that was classed as serious in nature and that is consistent with the information about ferric carboxymaltose listed in the SmPC.
-
Unexpected – an AE that was classed in nature as serious and that is not consistent with the information about ferric carboxymaltose listed in the SmPC.
The reference document to be used to assess expectedness against the IMP is the SmPC, which can be found at https://beta.medicines.org.uk/emc/ (accessed 1 October 2020), under Ferinject. The protocol will be used as the reference document to assess disease-related and/or procedural expected events.
Seriousness is defined as an AE or AR that:
-
results in death
-
is life-threatening
-
requires hospitalisation or prolongation of existing hospitalisation
-
results in persistent or significant disability or incapacity
-
consists of a congenital anomaly or birth defect.
Patient and public involvement
The Trial Steering Committee (TSC) comprised two lay members; one had ulcerative colitis and had undergone two major open laparotomies with a total colectomy and ileorectal anastomosis, and the other had several gynaecological procedures cumulating in a total abdominal hysterectomy. The lay members attended TSC meetings as full, voting members and contributed to the protocol; specifically, they prioritised the secondary end-point selection and also reviewed the patient information sheet, patient poster and diaries. The lay members were also very helpful in advising the Project Management Group (PMG) about how to approach patients for recruitment into the trial.
Definition of the end of the trial
The end of the study is defined as the date at which the last patient completed their last study visit.
Chapter 3 Trial outcomes and outcome measures
Co-primary end points
The trial had two co-primary end points:
-
risk of the composite end point of blood transfusion or death from randomisation until 30 days following the index operation
-
blood transfusion rate (including repeat transfusions) from randomisation until 30 days following the index operation.
A blood transfusion event was defined as transfusion of any volume of 1 unit of blood or blood product. When more than 1 unit of packed red cells or any other blood product was intended to be received contiguously, this was regarded as a single blood transfusion. The blood transfusion rate was defined as the number of blood transfusions divided by the total patient time at risk.
The PREVENTT TSC trial sites were selected after compliance with NHS Blood and Transplant guidelines. 3,44 Patient blood management and transfusion practice was assessed in two independent audits during recruitment.
Key secondary end points
-
Total number of units of blood or blood components transfused between randomisation and 30 days postoperatively (also at 6 months postoperatively as other secondary end point), excluding large blood transfusions. A large blood transfusion was defined as a single transfusion consisting of ≥ 4 units of blood or blood products.
-
Days alive and out of hospital (DAOH) from the date of the planned surgery until 30 days post index operation.
-
Postoperative complications (from index operation to date of discharge) using CD classification.
-
HRQoL outcome:
-
Multidimensional Fatigue Inventory (MFI) questionnaire total score at 8 weeks postoperatively (also at the 10-day assessment and 6 months postoperatively as other secondary end points).
-
EuroQol-5 Dimensions, five-level version (EQ-5D-5L) questionnaire total score at 8 weeks postoperatively (also at the 10-day assessment and 6 months postoperatively as other secondary end points).
-
Single question outcome measure (SQOM) at 8 weeks postoperatively (also at the 10-day assessment and 6 months postoperatively as other secondary end points).
-
Other secondary end points
-
Change in Hb levels from randomisation to (1) day of index operation (prior to surgery), (2) 8 weeks and (3) 6 months post index operation.
-
Correction of anaemia (Hb level of ≤ 120 g/l for women/Hb level of ≤ 130 g/l for men) at day of index operation.
-
Risk of blood transfusion or death, excluding large blood transfusions, from randomisation to (1) 30 days and (2) 6 months post index operation. A large blood transfusion is defined as a single transfusion consisting of ≥ 4 units of blood or blood products.
-
POMS outcomes at 3, 5, 7 and 14 days following the index operation. Outcomes consist of the presence of morbidity defined by the domains of POMS (e.g. gastrointestinal or cardiovascular).
-
ICU and total hospital LOS from date of index operation until discharge.
-
Re-admission to hospital at (1) 8 weeks and (2) 6 months post operation.
-
All-cause mortality from randomisation to (1) 8 weeks and (2) 6 months post index operation.
-
Health economics outcomes:
-
change in health-care resource utilisation from baseline to 6 months post operation
-
change in calculated NHS and societal costs from baseline to 6 months post operation
-
change in quality-adjusted life-years (QALYs) from baseline to 6 months post operation
-
cost-effectiveness, measured in terms of the incremental cost per percentage reduction in patients receiving blood transfusions and incremental cost per QALY gained, using data from baseline to 6 months post operation.
-
-
Safety and related efficacy outcomes:
-
Large blood transfusion from randomisation to 30 days post index operation
-
Any reaction or side effect from trial therapy
-
Any reaction or side effect from blood or blood product (transfusion reaction)
-
SAEs and suspected unexpected serious adverse events (SUSARs)
-
Development of perioperative AKI
-
Concomitant medications
-
Vital signs. Change from randomisation to (1) day of index operation (prior to surgery), (2) 8 weeks and (3) 6 months post index operation
-
Laboratory results. Change from randomisation to (1) day of index operation (prior to surgery), (2) 8 weeks and (3) 6 months post index operation. Of particular interest will be changes in eGFR, creatinine, serum phosphate, ferritin and TSAT levels.
-
Subgroup analysis
A number of subgroup analyses for both co-primary end points were predefined; these were age (< 70 and ≥ 70 years), baseline Hb level (< 100 g/l and ≥ 100 g/l), sex (male and female), body mass index (< 30 kg/m2 and ≥ 30 kg/m2), ferritin (< 100 and ≥ 100), TSATS (< 20% and ≥ 20%) and type of operation (major, major+ and complex). The subgroups were analysed by inclusion of an interaction term between treatment group and the subgroup in the relevant regression model.
Change in end points over the time of the trial
There were no changes to the co-primary end points of the trial following publication of the trial protocol. DAOH was added as a secondary outcome.
Sample size
The sample size requirement was calculated for the composite co-primary end point of blood transfusion or death by 30 days post operation. Assumptions for the sample size calculations were based on data from the pilot study, observational trials and audits carried out previously.
The anticipated risk of blood transfusion in the control group was estimated as approximately 40%. On the basis that the trial would have a type 1 error rate of 5% and an estimated 5% loss to follow-up, an estimated sample size of 500 patients (250 in each group) will have 90% power to detect an absolute reduction in risk of 14% [equivalent to a 35% relative risk reduction, risk ratio (RR) = 0.65] in the treatment group or approximately 80% power to detect an absolute reduction of 12% (30% relative reduction).
The above sample size should also provide similar or greater power for the second co-primary end point of blood transfusion rate, which includes repeat transfusions.
Laparoscopic surgery
PREVENTT was planned for major open abdominal surgery, which represented high-risk surgery for blood transfusion and also patient risk. 39–43 Cochrane reviews have shown that laparoscopic surgery is associated with a lower risk of blood loss, improved postoperative recovery, reduced patient complications and reduced length of hospital stay. In 2010, at initial grant submission, it was envisaged that colorectal surgery patients would be the leading population recruited into PREVENTT; however, there has been a steady rise in laparoscopic surgery over the last 10 years. By 2017, the majority of procedures (63%, SD 18%) in the 162 centres in the national bowel cancer audit45 were being performed laparoscopically. 45 Although this means that one-third of abdominal surgeries that were performed by open laparotomy, recruiting centres found that most operations were listed as an intended laparoscopic procedure.
The TSC reviewed trial recruitment in detail and recalculated the power analysis to include laparoscopic surgery; aside from changing the protocol halfway through the study, the anticipated actual recruitment would need to double, such that two patients undergoing laparoscopic surgery would be needed for every one patient planned to undergo open surgery. Therefore, the TSC decided not to include patients who were due to undergo laparoscopic surgery.
Statistical methods
Co-primary end points
The trial had two co-primary end points: (1) risk of blood transfusion or death from randomisation until 30 days following the index operation and (2) number of blood transfusions from randomisation until 30 days following the index operation. For the first co-primary end point, a RR (intravenous iron vs. placebo) and 95% CI were calculated using binomial regression (binary outcome with a log link). A p-value was calculated using a likelihood ratio test (LRT). An absolute risk difference (treatment vs. placebo) and 95% CI was also calculated using binomial regression (binary outcome with an identity link).
The second co-primary end point took into account recurrent transfusions and different patient tendencies (frailties) for repeat transfusions. The number and percentage of patients with 0, 1, 2, 3, 4, 5 and ≥ 6 transfusions were reported by treatment group. A rate ratio and 95% CI were calculated using a negative binomial regression model and a LRT p-value was reported. As some patients died before the end of the study’s duration, the length of each patient’s period of observation was included as an exposure in the model. In the case that a difference in mortality was noted between treatment arms, an additional sensitivity analysis was planned with joint modelling to allow estimation of the rate ratio and 95% CI of deaths; however, as there were only a few deaths and no difference between the treatment groups was observed, this was not required.
Analysis of the co-primary end points was carried out in the intention-to-treat population. To account for multiple testing, a Benjamini–Hochberg46 procedure with a 5% false discovery rate was used to determine statistical significance for the co-primary end points.
No imputation of missing outcomes was carried out for the primary analysis of either of the co-primary end points, and no adjustment for baseline covariates was made.
The analysis of the co-primary end points was repeated for the per protocol population, excluding patients who did not have the planned operation or whose operation took place outside the specified time window (10–42 days post treatment).
The primary analysis was also repeated adjusting for the baseline covariate included in the stratification for randomisation (age, baseline Hb level and operation type). The analysis for the two co-primary end points was repeated for baseline to 6 months post index operation as secondary end points.
Intention-to-treat analysis included all patients who gave consent for inclusion in the trial and who were available for follow-up in the trial to 30 days.
Per protocol analysis excluded those patients who did not have the trial treatment, did not undergo surgery, had their operation outside the prescribed timelines, had an operation not classified as major open abdominal surgery, or who withdrew consent between randomisation and surgery. Reasons for protocol deviation are not mutually exclusive.
Key secondary end point analyses
The total number of units of blood or blood components transfused between randomisation to 30 days post index operation, excluding large blood transfusions, was counted.
The mean (SD) and median [interquartile range (IQR)] number of units of blood were reported by treatment group. The total number of units transfused was also categorised (0, 1, 2, 3, 4, 5, ≥ 6) and the frequency and percentage of patients in each group were reported. Rate ratios (treatment vs. placebo) and 95% CIs were calculated using a negative binomial regression model. A p-value was calculated using a LRT. The same methods were also used for this outcome at 6 months as a secondary end point.
Days alive and out of hospital until 30 days post the index operation
The number of DAOH from the date of the index operation until 30 days post index operation was calculated for each patient using the method described by Ariti et al. 47 Discharge to a nursing home or other care facility was not considered as being out of hospital. The number and percentage of patients lost to follow-up before 30 days post index operation were reported for each treatment group; these patients were excluded from this analysis. The mean (SD) and median (IQR) DAOH were reported by treatment group. DAOH at 30 days post the index event was analysed with a linear regression model. A sensitivity analysis was also carried out comparing DAOH between the treatment groups using a Wilcoxon rank-sum test.
Postoperative complications from index operation to date of discharge
Postoperative complications during the inpatient period were classified using the CD system. For each patient, the most severe postoperative complication was identified. The number and proportion of patients with any moderate or severe postoperative complication (CD grade III or above) were reported by treatment group. A RR (treatment vs. placebo), 95% CI and p-value were calculated. In addition, the worst postoperative complication for each patient was categorised as none/mild (CD I or II), CD III, CD IV or CD V, and the number and percentage of patients in each category were reported by treatment group. Between-group comparisons were made using a test for trend.
Quality of life
The secondary end points included HRQoL as measured by the EQ-5D-5L, fatigue as measured by the MFI and the patient’s overall perception of outcome as measured by a SQOM. The EQ-5D-5L produces two scores: a ‘descriptive index’, which can range from –0.59 to 1.00, and a visual-analogue measure of overall health, which can range from 0–100. Higher scores on the EQ-5D-5L represent a better outcome. The MFI is a 20-item measure that produces domain-specific scores for general fatigue, physical fatigue, reduced activity, reduced motivation and mental fatigue, which can range from 4 to 20. A total MFI score, which can range from 20 to 100, can also be calculated. Higher MFI scores represent greater fatigue. The SQOM required the patient to record how their condition had changed since starting their participation in the study. Patients could choose from seven response options ranging from ‘has much improved’ to ‘is much worse’. Scores could range from –3 to +3, with higher scores representing greater improvement.
Continuous outcome measures at each follow-up time point for the two trial groups were compared using multivariate linear regression with adjustment for covariates, including baseline levels of the relevant outcome measure.
Other secondary end points
Haemoglobin
The change in Hb levels from randomisation to (1) day of operation (prior to surgery), (2) 8 weeks and (3) 6 months post index operation was measured.
Mean (SD) Hb level (g/l) at randomisation and at each visit was reported by treatment group. Mean (SD) change in Hb (g/l) was reported at each visit by treatment group. Differences between treatment groups in mean change in Hb levels from randomisation to day of index operation were analysed using analysis of covariance (ANCOVA) with baseline Hb level included in the model. It may be necessary to examine Hb for transformations to adhere to the assumptions of the ANCOVA model. The adjusted difference in mean change (treatment vs. placebo) in Hb was reported along with a 95% CI and p-value from an F-test.
Correction of anaemia at day of index operation
The number and percentage of patients with anaemia corrected (male Hb level of > 130 g/l and female Hb level of > 120 g/l) at the day of the index operation was reported by treatment group. A RR (treatment vs. placebo) and 95% CI for the correction of anaemia at the day of the index operation were estimated using binomial regression (binary outcome with a log link). A p-value was calculated using a LRT. Patients whose operations were cancelled were excluded from this analysis unless they had a Hb measurement on the planned day of surgery. The number and percentage of patients missing was reported by treatment group.
Risk of blood transfusion or death, excluding large blood transfusion, from randomisation to (1) 30 days and (2) 6 months post index operation.
The same statistical methods as described for the first co-primary end point were used for these analyses.
Intensive care unit and total hospital length of stay
Intensive care unit and total hospital LOS relating to the index operation were summarised using the median, IQR and range for each treatment group. Differences between groups were tested using the Mann–Whitney U-test.
Re-admission to hospital at (1) 8 weeks and (2) 6 months post the index operation
The number and percentage of patients re-admitted to hospital were reported by treatment group at each of the follow-up visits, and reasons for re-admission were reported. Two members of the writing committee who were blinded to the intervention, adjudicated causality for re-admission for all patients in the trial before data lock. Causality was divided into administrative or planned re-admission (such as for chemotherapy), or postoperative complication or unplanned re-admission (such as wound infection). The same statistical methods were used as described in Co-primary end points for the first co-primary end point. Kaplan–Meier curves were plotted to visually compare re-admission to hospital between treatment groups at 8 weeks and 6 months. For these analyses, the analysis time started from the date of discharge from the index operation. For any patients who did not have the index operation, the planned day of operation plus the median length of hospital stay in that treatment arm were used as the start of analysis time. A Cox proportional hazards model was used to obtain a hazard ratio (and 95% CI) comparing re-admissions in treatment groups at 8 weeks and 6 months; p-values were calculated using a log-rank test.
All-cause mortality at 8 weeks and 6 months post index operation
Risk ratios (treatment vs. placebo) and 95% CIs for all-cause mortality at 8 weeks and 6 months post the index operation were estimated using binomial regression (binary outcome with a log link). A p-value was calculated using a LRT.
Kaplan–Meier curves were plotted to visually compare mortality between treatment groups from randomisation to 8 weeks and 6 months post operation. A Cox proportional hazards model was used to obtain a hazard ratio (and 95% CI) comparing mortality in treatment groups at 8 weeks and 6 months.
Health-care resource utilisation from baseline to 6 months post operation were calculated
-
Calculated NHS and societal costs from baseline to 6 months post operation.
-
QALYs from baseline to 6 months post operation.
-
Cost-effectiveness, measured in terms of the incremental cost per percentage reduction in patients receiving blood transfusions and incremental cost per QALY gained, using data from baseline to 6 months post operation.
The cost and cost-effectiveness for the ‘within-trial’ period, up to 6 months post operation, was estimated. Costs were assessed from the perspective of the NHS and personal social services, and also from a societal perspective.
Multiple imputation by chained equations were used to deal with missing EQ-5D-5L and resource use values. Subsequent analyses of imputed data included variance correction factors to account for additional variability introduced into parameter values as a result of the imputation process.
Cost-effectiveness was calculated as the mean cost difference between intervention versus placebo divided by the mean difference in outcomes (% blood transfusion/QALYs) to give the incremental cost-effectiveness ratio (ICER). Non-parametric methods for calculating CIs around the ICER based on bootstrapped estimates of the mean cost and QALY differences were used. The bootstrap replications will also be used to construct a cost-effectiveness acceptability curve, which will show the probability that use of iron is cost-effective at 6 months post operation for different values of NHS willingness to pay for an additional QALY. The results were subjected to deterministic (one-way, two-way and multiway) sensitivity analysis.
Safety and other related efficacy outcomes
-
Large blood transfusion.
-
Any reaction or side effect from trial therapy.
-
Any reaction or side effect from blood or blood product (transfusion reaction).
-
SAEs and SUSARs.
-
Development of perioperative AKI.
-
Concomitant medications.
For each of the above safety outcomes, the number and percentage of patients were reported by treatment group for each outcome. Risk ratios and 95% CIs are reported, and p-values were calculated using chi-squared or Fisher’s exact tests, as appropriate.
Ethics considerations
Ethics approval for the study in the UK was given by the East of England – Cambridgeshire and Hertfordshire Research Ethics Committee on 5 November 2012 (reference number 12/EE/0445). The trial was registered with a National Clinical Trial number of NCT01692418.
The trial was overseen by three committees: the TSC, the DSMC and the PMG.
The TSC had overall responsibility for the scientific integrity and quality of the trial. This involved: ensuring the trial was conducted to the standards set out in the guidelines for Good Clinical Practice; ensuring adherence to protocol as far as possible; responsibility for overall patient safety; and considering new relevant information arising throughout the duration of the trial. The TSC also had responsibility to consider any recommendations made by the DSMC. The TSC met annually throughout PREVENTT to monitor the progress and quality of the trial, to review the recruitment rate and consider protocol amendments.
The DSMC had the responsibility to ensure the safety of patients in the trial. The DSMC was the only group to review interim analyses broken down by treatment group during the conduct of the trial. The DSMC performed interim safety analyses annually, but there were no interim efficacy analyses. The interim reports were semi-unblinded (i.e. summaries were presented by group A and group B) and contained details of patient recruitment, demographic and baseline characteristics, details of the intervention, primary safety end points, primary efficacy end point and other end points identified by the DSMC including AEs and SAEs. The chairperson of the DSMC reported directly to the chairperson of the TSC.
The PMG was responsible for the day-to-day running of the trial, meeting fortnightly during the setting up of PREVENTT and the early stages of recruitment, and then approximately monthly for the remainder of the trial.
Chapter 4 Trial recruitment
In total, 487 patients were recruited from 46 hospitals in the UK over 5 years from September 2013 to September 2018, with the first patient being enrolled in January 2014. One centre recruited 118 patients, 20 centres recruited more than five patients and five centres failed to recruit (see Appendix 2). Overall, throughout the 5 years of the trial, patient recruitment was a median number of eight patients, a minimum of two and a maximum of 18, per month. There were several factors that directly affected recruitment.
Timelines before surgery
In July 2015, screening data from 4979 patients were collected over a 3-month period from the active sites. Overall, 25% of those patients screened were eligible for PREVENTT, 47% were not anaemic and 28% were not undergoing major open elective surgery. Of those eligible, the timeline of 10–42 days before operation was not possible for 11% of patients, and 6% of patients did not have the relevant blood results for screening. These two factors, patients not having a Hb result or not being screened within the timelines for inclusion in the trial, were the biggest reasons for recruitment failure. There were several factors that contributed to this.
Preoperative pathways did not adequately allow for the recognition, diagnosis and treatment of anaemia before the planned operation date. Most patients did not have a recorded set of blood results (either in the notes or on the hospital system) at the time of surgical review. The surgical pathway was dictated by the 2-week rule for diagnosis to treatment in cancer surgery. The surgical decision for operation, and, therefore, the operation date, was predominantly made at the final multidisciplinary meeting, with the date for surgery often in the following week. Preoperative assessment clinics were frequently the first time that a FBC was performed to assess for anaemia, and this preoperative assessment clinic appointment was often within the 10 days before surgery.
Surgical practice and clinical care have changed significantly in the UK over recent years. In the last decade, hospitals and NHS trusts reorganised patient care with centralisation to high-volume centres, with the intent to improve outcomes. Consequently, cancer services such as upper gastrointestinal, hepatobiliary and urological surgery often involved patients being referred between hospitals. Sharing of clinical data between hospitals and NHS trusts was variable and presented a challenge to local research teams. Often, patients referred to tertiary hospitals were identified without full clinical data or laboratory results available.
Individual patient factors were not a major problem. Among the 384 patients eligible at that point, 159 were recruited, representing 41% of the potential population, a reasonable achievement for any surgical trial. Among those who were not recruited, the main reasons were that patients felt that there was too much going on, they did not want to make additional hospital visits, they had concerns over paperwork, they did not want additional tests and they did not want to receive the placebo.
National Institute for Health and Care Excellence guidance
In November 2015, NICE guidance on blood transfusion practice was released. 2 This was supported by the NICE Quality Standard 1382 in December 2016, which stated:
People should have their haemoglobin levels checked at least 2 weeks before surgery, if possible and necessary for the procedure they are having. If they have iron-deficiency anaemia, they should be offered iron supplementation. Oral iron should be offered initially, and started at least 2 weeks before surgery. If oral iron is not appropriate, intravenous iron should be considered.
National Institute for Health and Care Excellence guidelines2 also recommended that the blood transfusion threshold should be lowered to a Hb level of < 70 g/l (80 g/l in patients with ischaemic heart disease). This is also a significant change from the previous transfusion threshold of 90 g/l (which was the inclusion level for the trial) and may have contributed to the lower blood transfusion rate seen in this trial (29% compared with the predicted rate of 40%).
Through 2014 to 2016 there was steady recruitment to PREVENTT, with a median number of nine patients (range 2–18 patients) per month over 36 months. Following publication of the NICE guidance,2 there was considerable communication from sites with concerns about the need to follow this guidance and give iron therapy, and the lack of equipoise in the use of preoperative intravenous iron. There was also a steady drive in the UK and throughout Europe to increase preoperative intravenous iron clinics. 23,48,49
The overall impact of the 2015 NICE guidance was significant;2 several centres withdrew from PREVENTT and patient recruitment reduced to a median of 7 (range 4–15) patients per month over the subsequent 21 months through 2017 and 2018 (p = 0.01).
Owing to the difficulties with recruitment, the TSC decided to halt recruitment in September 2018 after 487 patients (compared with the 500 that were planned) had been enrolled and randomised to enable follow-up to be completed within the trial period. The effect of recruiting 13 fewer patients than planned was considered to be negligible, with sample size calculations indicating a small loss of power (88% vs. 90%) and loss to follow-up having been less than anticipated in the initial power calculations.
Chapter 5 Results
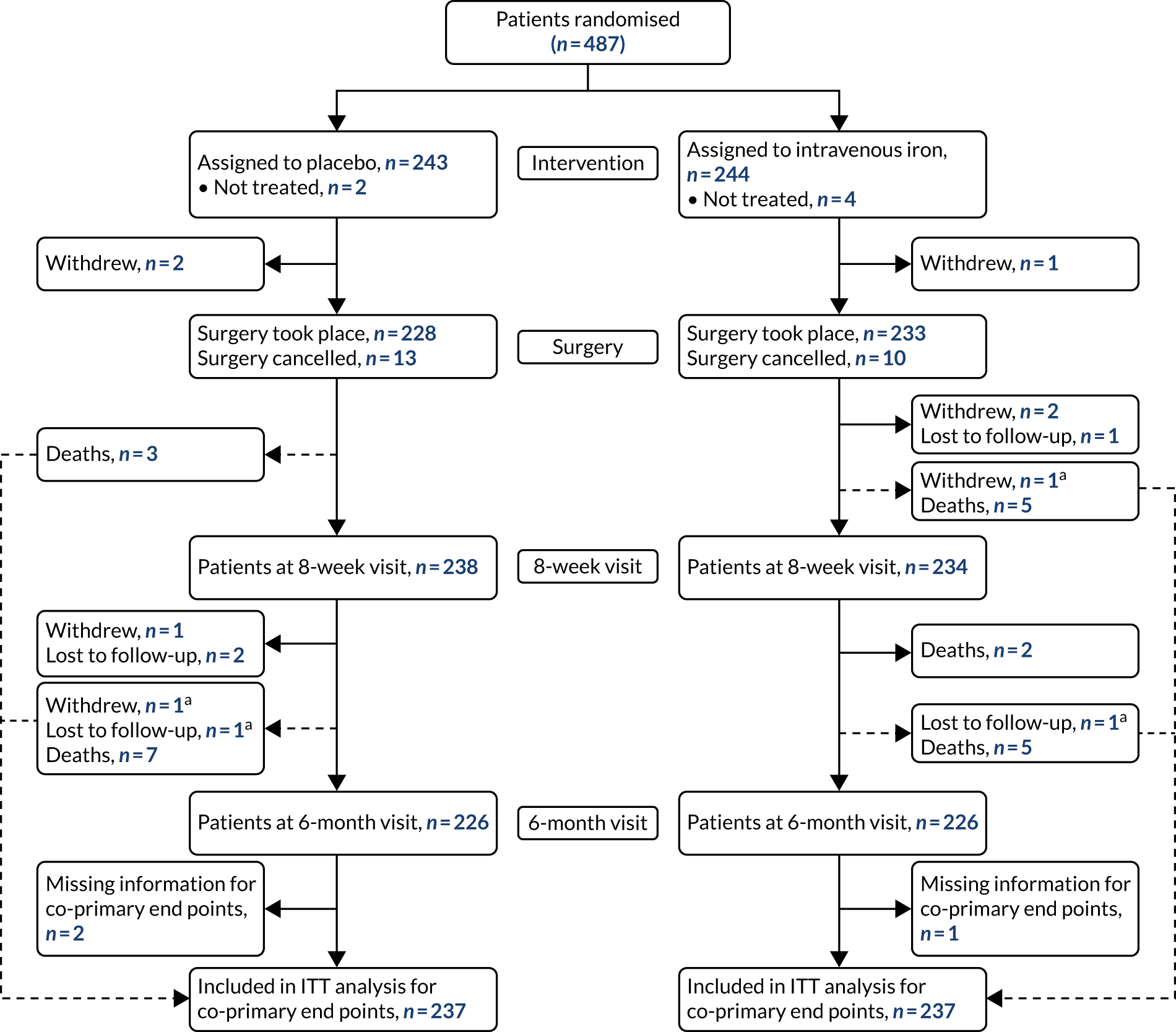
Patient flow
Between 6 January 2014 and 28 September 2018, 487 patients were randomised into PREVENTT (243 placebo, 244 intravenous iron) (Figure 1). Six patients (two placebo, four intravenous iron) did not receive their intended randomised treatment. In total, eight patients withdrew consent during follow-up. Three patients (two placebo, one intravenous iron) withdrew consent between randomisation and the planned operation, and five patients (two placebo, three intravenous iron) withdrew consent between surgery and the 6-month visit. Twenty-three patients did not undergo their planned operation (13 placebo, 10 intravenous iron). Of these, 10 patients’ operations were cancelled because of a clinical plan change, seven patients were deemed unfit for surgery and six patients had disease progression. A total of 228 patients in the placebo group and 233 patients in the intravenous iron group underwent their planned operation.
Eight patients (three placebo, five intravenous iron) died between surgery and the 8-week visit, and a further 14 patients (seven in each group) died between the 8-week and 6-month visits. Follow-up rates were very good, with only five patients (three placebo, two intravenous iron) who had not died or withdrawn consent missing the 6-month visit. Vital status at 6 months was known for 236 (97.1%) and 238 (97.5%) patients in the placebo and intravenous iron groups, respectively.
Protocol deviations
In addition to the patients described above who did not have the trial treatment or undergo their planned operation, 46 patients (26 placebo, 20 intravenous iron) had their surgery outside the prescribed timelines. Of these, 13 operations took place within 10 days of the trial treatment and 33 operations took place > 42 days after treatment. A further 20 patients underwent surgery that was not classified as major open abdominal surgery (12 placebo, eight intravenous iron). Of these, six patients underwent surgery that was open and close (i.e. abandoned), 13 patients had operations that were performed laparoscopically and one patient had a minor operation.
There were no cases reported of patients being unintentionally unblinded. At the time of surgery, patients were asked if they thought that they knew which treatment they had received. Overall, 47% of patients reported not knowing, 27% thought that they had received intravenous iron (of whom 58% actually did receive iron) and 25% thought that they had received placebo (of whom 60% actually did receive placebo).
FIGURE 1.
Enrolment, randomisation and follow-up. a, Patient has blood transfusion before withdrawal or loss to follow-up and is, therefore, included in the analysis of the co-primary end points. ITT, intention to treat.
Baseline characteristics
Overall, median (IQR) age was 66 (54–72) years and 267 (54.8%) patients were female. Most (87.9%) patients were white, 6.8% were African Caribbean and 4.9% were Asian. Most patients were American Society of Anesthesiologists grade II (61.0%) or III (25.6%). Comorbidities included that 182 patients had hypertension (37.4%), 75 (15.4%) were diabetic, 46 (9.4%) had had a previous heart attack, stroke or transient ischaemic attack, 76 (15.6%) reported renal problems and 64 (13.1%) reported respiratory problems. Half of patients had never smoked and 41 (8.5%) were current smokers. The two treatment groups were well balanced, with no major differences in any of the baseline characteristics (Table 1).
History of iron deficiency was reported by 139 (28.5%) patients. The groups were well matched with respect to potential aetiological factors that may have an impact on iron levels by reducing absorption (e.g. hiatus hernia or acid reflux), causing iron deficiency (bleeding tendency), or through inflammation (arthritis or inflammatory bowel disease). Similarly, the groups were similar with respect to the proportions that received preoperative chemotherapy or radiotherapy, which had been undertaken in 110 (22.6%) patients before their planned operation.
There was no difference in medications being taken preoperatively that may affect iron therapy or bleeding; overall, 95 (19.6%) patients were taking iron tablets, 42 (8.6%) patients were on anticoagulation therapy and 57 (11.7%) patients were on antiplatelet therapy (two on dual antiplatelets), and five patients were on both anticoagulation and antiplatelet therapy.
| Characteristic | Placebo (N = 243) | Intravenous iron (N = 244) |
|---|---|---|
| Demographics | ||
| Age (years), median (IQR) | 65 (50–72) | 66 (57–72) |
| Female, n (%) | 142 (58.4) | 125 (51.2) |
| Ethnicity, n (%) | ||
| White | 217 (89.3) | 211 (86.5) |
| African Caribbean | 19 (7.8) | 14 (5.7) |
| Asian | 6 (2.5) | 18 (7.4) |
| Other | 1 (0.4) | 1 (0.4) |
| Clinical measures, n (%) | ||
| American Society of Anesthesiologists grade | ||
| I | 31 (13.0) | 30 (12.8) |
| II | 141 (59.2) | 147 (62.8) |
| III | 65 (27.3) | 56 (23.9) |
| IV | 1 (0.4) | 1 (0.4) |
| Missing | 5 | 10 |
| Medical history, n (%) | ||
| Myocardial infarction | 20 (8.2) | 12 (4.9) |
| Angina/chest pain | 16 (6.6) | 15 (6.1) |
| Heart failure | 3 (1.2) | 9 (3.7) |
| Hypertension | 93 (38.3) | 89 (36.5) |
| Breathlessness | 28 (11.5) | 25 (10.2) |
| Liver disease | 8 (3.3) | 14 (5.7) |
| Kidney/urinary problems | 37 (15.2) | 39 (16.0) |
| Bleeding tendencies | 7 (2.9) | 11 (4.5) |
| Iron deficiency | 69 (28.4) | 70 (28.7) |
| COPD/bronchitis/asthma | 37 (15.2) | 27 (11.1) |
| Acid reflux/stomach ulcer | 54 (22.2) | 54 (22.1) |
| Hiatus hernia | 23 (9.5) | 17 (7.0) |
| Coeliac disease | 2 (0.8) | 0 (0.0) |
| Inflammatory bowel disease | 13 (5.3) | 13 (5.3) |
| CVA/TIA | 13 (5.3) | 4 (1.6) |
| Rheumatoid arthritis | 12 (4.9) | 10 (4.1) |
| Diabetes | 38 (15.6) | 37 (15.2) |
| Pre-operation chemotherapy | 59 (24.3) | 50 (20.5) |
| Radiotherapy | 6 (2.5) | 7 (2.9) |
| Smoking history, n (%) | ||
| Never | 116 (47.9) | 113 (46.5) |
| Ex-smoker | 107 (44.2) | 108 (44.4) |
| Current | 19 (7.9) | 22 (9.1) |
| Missing | 1 | 1 |
| Current medication that affects bleeding, n (%) | ||
| Warfarin | 4 (1.6) | 7 (2.9) |
| Aspirin | 28 (11.5) | 23 (9.4) |
| Clopidogrel | 5 (2.1) | 3 (1.2) |
| Other | 25 (10.3) | 22 (9.0) |
| Iron tablets | 49 (20.2) | 46 (18.9) |
| Missing | 0 | 1 |
Operation details
Of the 487 patients randomised, 461 (95%) underwent surgery (228 placebo, 233 intravenous iron). The median (IQR) time from randomisation to surgery was 15 (12–22) days and was similar in the two groups.
The planned date of operation was postponed for 18 patients (3.7%); for 15 patients, this was due to lack of bed availability.
The two groups were well balanced in terms of the complexity and type of surgery undertaken, with the most common surgeries being upper gastrointestinal (34%), gynaecological (30%) and colorectal (15%) (Table 2). Anaesthetic time and total procedure time were similar in the two groups. Overall median (IQR) total procedure time (anaesthesia, preparation and surgery) was 250 (175–355) minutes and median (IQR) total operation time (knife to skin to drapes removed) was 165 (110–277) minutes. Cell salvage was used in 21 (5%) patients (11 placebo, 10 intravenous iron) with the median (IQR) volume being reinfused 400 ml (200–658 ml).
Of the 461 patients who underwent their planned surgery, 286 (62%) went to ICU with median (IQR) ICU LOS of 2 (2–4) days. Overall, the median total hospital LOS was 9 (IQR 6–14) days. Three patients died following surgery without being discharged. Both ICU LOS and total hospital LOS were similar in the two groups.
Eighteen patients (3.9%) were returned to theatre, for the following documented reasons: bleeding (two patients), surgical airway (three patients), major wound revision (seven patients), surgical complicatons (three patients) and other (three patients).
| Surgery details | Placebo (N = 243) | Intravenous iron (N = 244) |
|---|---|---|
| Surgery took place, n (%) | 228 (93.8) | 233 (95.5) |
| Time from treatment to index operation | ||
| Median, days (IQR) | 15 (12–22) | 14 (12–20.5) |
| Range, days | 6–207 | 5–212 |
| n (%) < 10 days | 5 (2.2) | 8 (3.4) |
| n (%) > 42 days | 21 (9.2) | 12 (5.2) |
| Planned type of surgery, n (%) | ||
| CMO | 85 (35.0) | 89 (36.5) |
| Major | 89 (36.6) | 87 (35.7) |
| Major+ | 69 (28.4) | 68 (27.9) |
| Type of operation, n (%) | ||
| Abdominal aortic aneurysm | 4 (1.8) | 1 (0.4) |
| Colorectal | 33 (14.5) | 38 (16.3) |
| General | 17 (7.5) | 21 (9.0) |
| Gynaecological | 75 (32.9) | 63 (27.0) |
| Upper gastrointestinal | 77 (33.8) | 81 (34.8) |
| Urological | 22 (9.6) | 29 (12.4) |
| Surgery details | ||
| Anaesthetic time: minutes, median (IQR) | 240 (161–320) | 268 (180–376) |
| Surgery time: minutes, median (IQR) | 145 (98–230) | 179 (123–323) |
Efficacy of intravenous iron
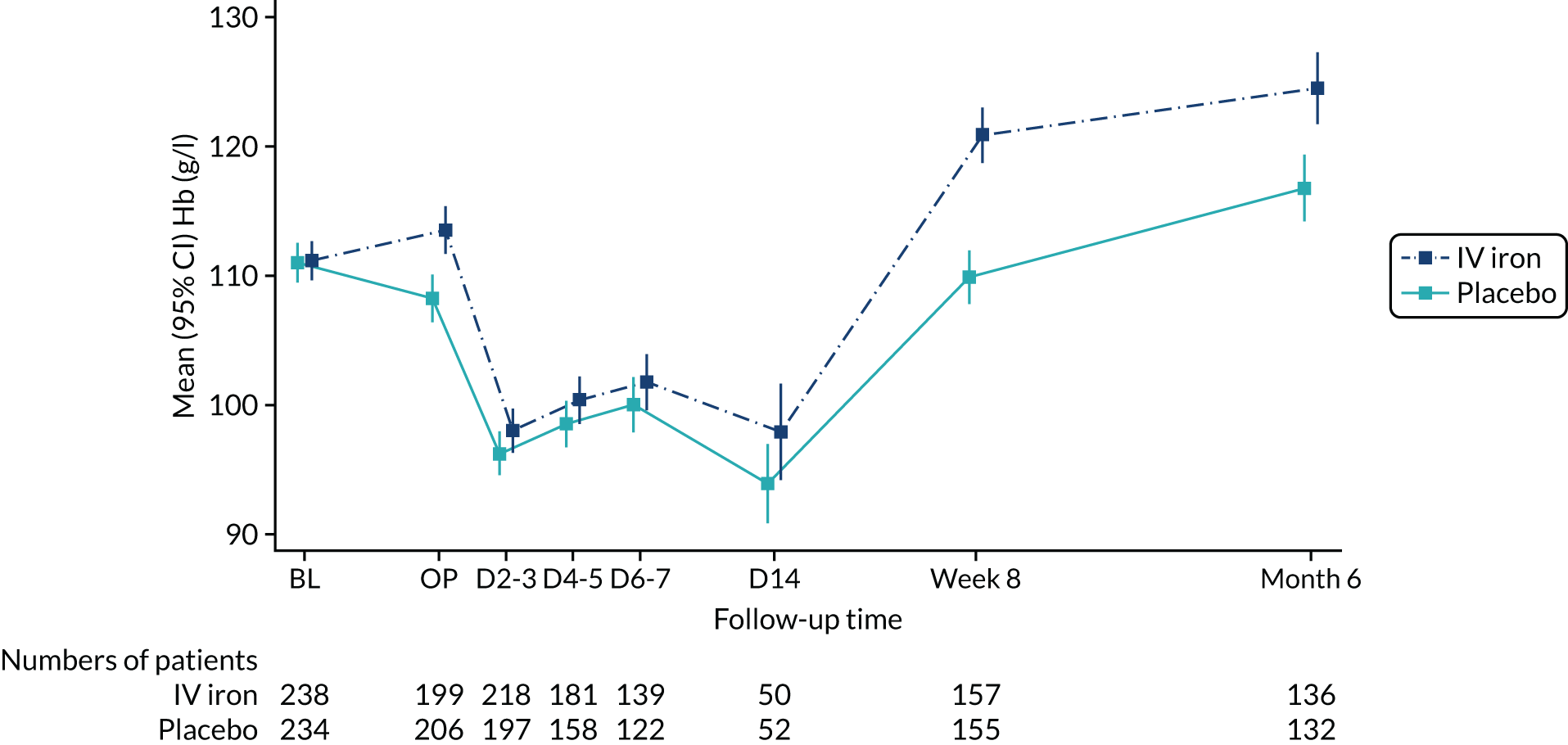
At randomisation, Hb levels were well balanced between the placebo and intravenous iron groups: mean (SD) 111.0 g/l (11.9 g/l) and 111.2 g/l (11.8 g/l), respectively.
At the time of surgery, mean Hb was significantly higher in the intravenous iron group (113.5 g/l vs. 108.2 g/l) with the difference being 4.7 g/l, 95% CI 2.7 to 6.8 g/l; p < 0.0001 (from ANCOVA model adjusting for baseline Hb). Anaemia was corrected in 42 (21%) patients in the intervention group compared with 21 (10%) patients in the placebo group (RR 2.06, 95% CI 1.27 to 3.35 g/l; p = 0.002).
Haemoglobin levels were not significantly different in the immediate postoperative days, but were significantly higher in the intravenous iron group at 8 weeks (mean difference 10.7 g/l, 95% CI 7.8 to 13.7 g/l; p < 0.0001) and at 6 months (mean difference 7.3 g/l, 95% CI 3.6 to 11.1 g/l; p < 0.001) (Figure 2).
FIGURE 2.
Haemoglobin levels of the trial participants by treatment group and visit. BL and OP were measured by the central laboratory; all other measurements are from local laboratories. D2–3, D4–5, D6–7 and D14 measurements are available only for patients still hospitalised at that time. BL, baseline prerandomised treatment; D, day post operation (e.g. D2–3 = day 2 or 3 post operation); IV, intravenous; OP, day of operation.
Co-primary end points
A total of 474 out of 487 (97.3%) patients (237 in each group) were included in the intention-to-treat analysis for the two co-primary end points. Overall, from randomisation to 30 days following their index operation, 136 patients (67 placebo, 69 intravenous iron) received at least one blood transfusion or died. There was no difference in the risk of transfusion or death at 30 days between the treatment groups (RR 1.03, 95% CI 0.78 to 1.37; p = 0.84; absolute risk difference +0.8%, 95% CI –7.3% to 9.0%). A total of 216 transfusion episodes (111 placebo, 105 intravenous iron) occurred between randomisation and 30 days following the index surgery. There was no difference between the groups in the rates of blood transfusion (rate ratio 0.98, 95% CI 0.68 to 1.43; p = 0.93; absolute rate difference 0.00, 95% CI –0.14 to 0.15) (Table 3). Excluding large blood transfusions did not alter these results.
Analyses of the co-primary end points adjusting for age, baseline Hb and type of surgery did not materially change the results (adjusted RR for blood transfusion or death to 30 days 1.06, 95% CI 0.81 to 1.38; p = 0.69 and adjusted rate ratio for transfusion episodes 0.99, 95% CI 0.69 to 1.42; p = 0.96). Similarly, per protocol analyses did not materially alter the results (RR for blood transfusion or death to 30 days 1.04, 95% CI 0.77 to 1.41; p = 0.79 and rate ratio for transfusion episodes 0.98, 95% CI 0.67 to 1.44; p = 0.93).
| Co-primary end point | Placebo (N = 243) | Intravenous iron (N = 244) | Treatment effect: intravenous iron vs. placebo (95% CI) | p-value |
|---|---|---|---|---|
| Blood transfusion or death n/N (%) | 67/237 (28.3) | 69/237 (29.1) | 1.03 (0.78 to 1.37)a | 0.84 |
| Transfusion, n | 67 | 68 | ||
| Death, n | 2 | 2 | ||
| Transfusion episodes, n (%) | 0.98 (0.68 to 1.43)b | 0.93 | ||
| 0 | 170 (71.7) | 169 (71.3) | ||
| 1 | 37 (15.6) | 49 (20.7) | ||
| 2 | 22 (9.3) | 9 (3.8) | ||
| 3 | 5 (2.1) | 5 (2.1) | ||
| 4 | 1 (0.4) | 3 (1.3) | ||
| 5 | 1 (0.4) | 1 (0.4) | ||
| 6 | 1 (0.4) | 1 (0.4) |
Key secondary end points
Total number of units of blood or blood products transfused
Overall, the blood transfusion rate was 29.3%, with 139/474 patients receiving at least one transfusion from randomisation until 6 months. Packed RBCs were transfused in 133 patients and six patients received a different blood product in isolation: three had platelets and three had fresh-frozen plasma (FFP) transfusions. Of those who had packed red cell transfusions, seven also received FFP, five received platelets and one received cryoprecipitate.
Excluding large blood transfusions (defined as a single transfusion consisting of ≥ 4 units of blood or blood products, of which there were five in the placebo group and seven in the intravenous iron group). A total of 130 patients (64 placebo, 66 intravenous iron) were transfused with a total of 300 units of blood or blood products between randomisation and 30 days post operation. The mean (SD) transfusion rate at 30 days was 0.65 (1.3) and 0.61 (1.3) in the placebo and intravenous iron groups, respectively (rate ratio 0.98, 95% CI 0.65 to 1.47; p = 0.92). The results at 6 months post operation were similar, with the mean (SD) rate being 0.94 (2.0) and 0.79 (1.6) in the placebo and intravenous iron groups, respectively (rate ratio 0.89, 95% CI 0.60 to 1.32; p = 0.56) (Table 4).
| Blood or blood products transfused | Placebo (N = 243) | Intravenous iron (N = 244) | Rate ratio: intravenous iron vs. placebo (95% CI) | p-value |
|---|---|---|---|---|
| Total units transfused at 30 days (excluding large transfusions), n (%) | 0.98 (0.65 to 1.47) | 0.92 | ||
| 0 | 173 (73.0) | 171 (72.2) | ||
| 1 | 14 (5.9) | 21 (8.9) | ||
| 2 | 28 (11.8) | 31 (13.1) | ||
| 3 | 12 (5.1) | 7 (3.0) | ||
| 4 | 6 (2.5) | 1 (0.4) | ||
| 5 | 2 (0.8) | 3 (1.3) | ||
| 6+ | 2 (0.8) | 3 (1.3) | ||
| Total units transfused at 6 months (excluding large transfusions), n (%) | 0.89 (0.60 to 1.32) | 0.56 | ||
| 0 | 151 (67.4) | 148 (67.3) | ||
| 1 | 13 (5.8) | 21 (9.5) | ||
| 2 | 31 (13.8) | 32 (14.5) | ||
| 3 | 15 (6.7) | 10 (4.5) | ||
| 4 | 7 (3.1) | 2 (0.9) | ||
| 5 | 2 (0.9) | 2 (0.9) | ||
| 6+ | 5 (2.2) | 5 (2.3) |
Postoperative complications
Postoperative complications were similar in the two groups, with 24 (11%) patients in the placebo group and 22 (9%) patients in the intravenous iron group experiencing significant (defined as CD classification grade III or greater) postoperative complications (RR 0.89, 95% CI 0.52 to 1.55; p = 0.69) (Table 5).
| CD grade | Placebo (N = 243), n (%) | Intravenous iron (N = 244), n (%) |
|---|---|---|
| None | 139 (61.2) | 138 (59.2) |
| Grade I | 24 (10.6) | 28 (12.0) |
| Grade II | 40 (17.6) | 45 (19.3) |
| Grade III | 17 (7.5) | 14 (6.1) |
| Grade IV | 5 (2.2) | 8 (3.4) |
| Grade V | 2 (0.9) | 0 (0.0) |
Days alive and out of hospital
Days alive and out of hospital to 30 days following surgery was similar in the two groups. Mean (SD) DAOH was 19.8 (7.5) days in the placebo group and 19.7 (7.0) days in the intravenous iron group (p = 0.84).
Quality of life
There were no significant between-group differences in HRQoL, fatigue or overall condition improvement at the 6-month assessment (Table 6). The mean (SD) EQ-5D-5L descriptive index was 0.82 (0.21) in the placebo group and 0.82 (0.22) in the intravenous iron group (adjusted difference, 0.02; p = 0.30). A comparison of the EuroQol visual analogue scores in both groups also revealed no differences. The mean total MFI score was 47.4 (SD 19.1) in the placebo group and 48.8 (SD 18.9) in the active treatment group (adjusted difference, –0.1; p = 0.94). There were no significant differences in any of the MFI domain-specific scores. The mean SQOM score was 1.26 (SD 1.83) in the placebo group and 1.35 (SD 1.70) in the active treatment group (adjusted difference, 0.08; p = 0.69). There were no significant differences in any of the quality-of-life measures at the 10-day or 8-week assessments.
| Outcome | Placebo (N = 243) | Intravenous iron (N = 244) | Mean difference: intravenous iron vs. placebo (95% CI) | p-value |
|---|---|---|---|---|
| EQ-5D-5L questionnaire, mean (SD) | ||||
| Utility score | 0.82 (0.21) | 0.82 (0.22) | 0.02 (–0.02 to 0.05) | 0.30 |
| Health score | 76.2 (19.2) | 75.0 (18.4) | 0.2 (–3.4 to 3.8) | 0.91 |
| Number of responses | 173 | 177 | ||
| MFI questionnaire, mean (SD) | ||||
| Total score | 47.4 (19.1) | 48.8 (18.9) | –0.1 (–3.5 to 3.2) | 0.94 |
| General fatigue | 10.9 (4.4) | 10.9 (4.3) | –0.2 (–1.1 to 0.6) | 0.56 |
| Physical fatigue | 10.5 (4.7) | 11.1 (4.5) | 0.2 (–0.6 to 1.1) | 0.58 |
| Reduced activity | 9.9 (4.9) | 10.1 (4.5) | –0.1 (–1.0 to 0.8) | 0.86 |
| Reduced motivation | 8.3 (3.9) | 8.6 (3.9) | 0.2 (–0.6 to 0.9) | 0.70 |
| Mental fatigue | 7.9 (4.3) | 8.1 (4.6) | 0.1 (–0.7 to 0.8) | 0.83 |
| Number of responses | 171 | 177 | ||
| SQOM questionnaire, mean (SD) | ||||
| Total score | 1.26 (1.83) | 1.35 (1.70) | 0.08 (–0.30 to 0.46) | 0.69 |
| Number of responses | 172 | 178 | ||
Other secondary end points
Length of intensive care unit and hospital stay
There was no difference in ICU LOS. Median (IQR) ICU LOS was 1 (0–3) day in the placebo group, and 2 (0–3) days in the intravenous iron group (p = 0.11). Overall, 75% of patients had their operation on the day of admission and there was no difference in total hospital LOS, with the median (IQR) total hospital LOS being 9 (7–14) days in the placebo group and 9 (5–14) days in the intravenous iron group (p = 0.14). The distribution of total hospital LOS by treatment group is shown in Figure 3. For three patients who died before discharge (two in the placebo group/one in the intravenous iron group), LOS was calculated as the number of days from date of admission for surgery until date of death.
FIGURE 3.
Distribution of total hospital LOS for patients undergoing surgery in (a) the placebo group; and (b) the intravenous iron group.
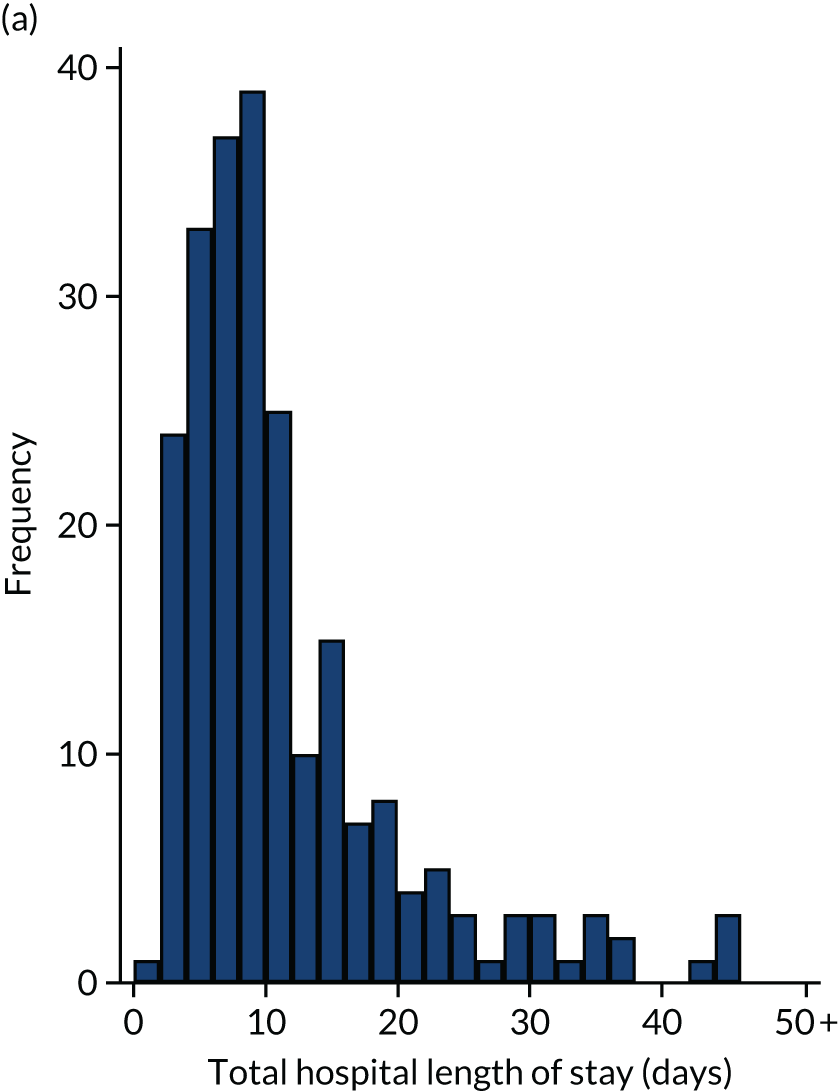
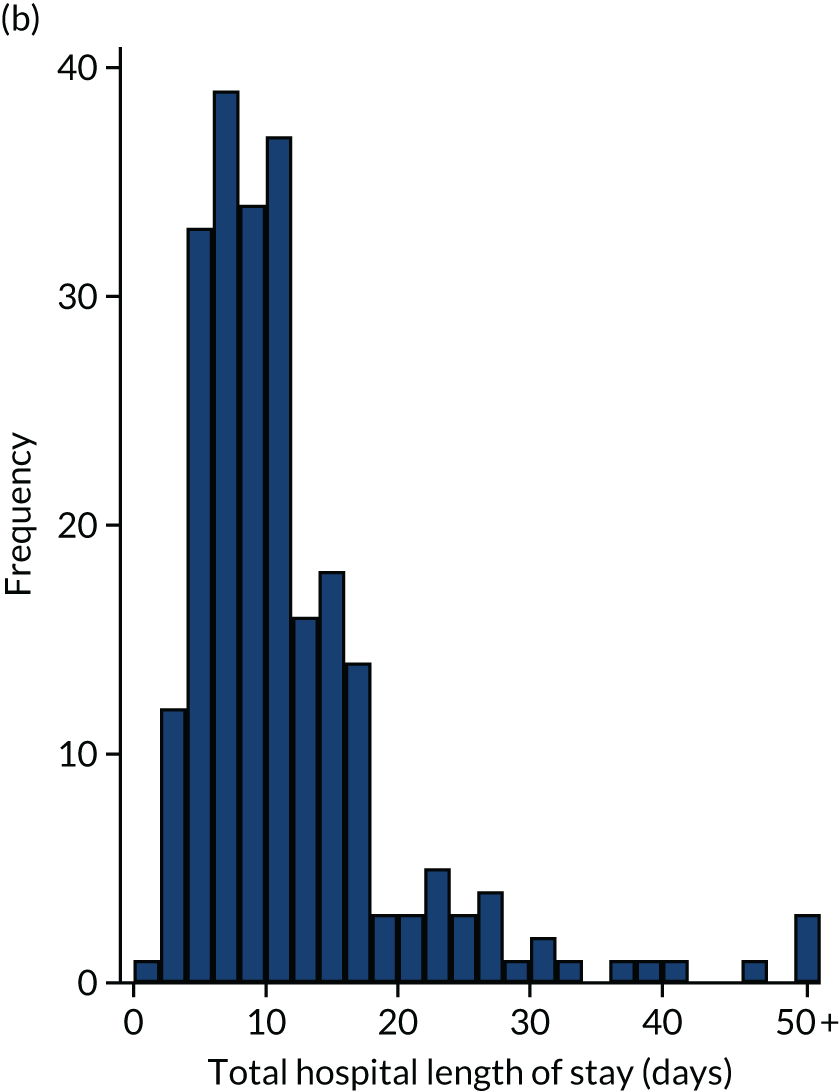
Re-admissions
Following discharge, a number of patients were re-admitted to hospital. Re-admissions were adjudicated blind to treatment allocation and classified as postoperative complications or planned re-admissions (e.g. adjuvant chemotherapy or radiotherapy and administrative).
The number of re-admissions for complications was significantly lower in the intravenous iron group in the first 8 weeks following index operation. The number (%) of patients re-admitted for postoperative complications was 51 (22%) in the placebo group and 31 (13%) in the intravenous iron group (risk ratio 0.61, 95% CI 0.40 to 0.91, p = 0.015). Including repeat re-admissions, there were a total of 71 re-admissions in the placebo group compared with 38 re-admissions in the intravenous iron group (rate ratio 0.54, 95% CI 0.34 to 0.85; p = 0.009) (Table 7). At 6 months, the picture was similar, with significantly fewer total re-admissions in the intravenous iron group, although the number of patients with any re-admission was not statistically significant.
| Re-admission | Placebo | Intravenous iron | RRa (95% CI) | p-value |
|---|---|---|---|---|
| Discharge to 8 weeks | ||||
| 1+ re-admission, n/N (%) | 51/234 (21.8) | 31/234 (13.2) | 0.61 (0.40 to 0.91) | 0.015 |
| Number of re-admissions, n (%) | ||||
| 1 | 37 (15.8) | 24 (10.3) | ||
| 2 | 11 (4.7) | 7 (3.0) | ||
| 3 | 1 (0.4) | 0 (0.0) | ||
| 4 | 1 (0.4) | 0 (0.0) | ||
| 5 | 1 (0.4) | 0 (0.0) | ||
| Total number of re-admissions | 71 | 38 | 0.54 (0.34 to 0.85) | 0.0086 |
| Discharge to 6 months | ||||
| 1+ re-admission, n/N (%) | 73/223 (32.7) | 58/227 (25.6) | 0.78 (0.58 to 1.04) | 0.093 |
| Number of re-admissions, n (%) | ||||
| 1 | 46 (20.6) | 38 (16.7) | ||
| 2 | 15 (6.7) | 16 (7.0) | ||
| 3 | 4 (1.8) | 2 (0.9) | ||
| 4 | 3 (1.3) | 2 (0.9) | ||
| 5 | 2 (0.9) | 0 (0.0) | ||
| 6 | 1 (0.4) | 0 (0.0) | ||
| 7 | 2 (0.9) | 0 (0.0) | ||
| Total number of re-admissions | 130 | 84 | 0.64 (0.44 to 0.92) | 0.018 |
Mortality and adverse events
There were no significant differences between the two groups in terms of mortality or any of the prespecified safety end points (Table 8). At 30 days post operation, four (< 1%) patients had died (two in each group), three of whom died in hospital and one after discharge; at 6 months, 22 (4.5%) patients had died (10 in the placebo group and 12 in the intravenous iron group).
A total of 16 patients (five placebo group and 11 intravenous iron group) had ARs that were reported as being directly related to the IMP administration (RR 2.19, 95% CI 0.77 to 6.21; p = 0.20). One patient had a blood transfusion stopped because of an AR.
| Safety end points | Placebo (N = 243) | Intravenous iron (N = 244) | RR (95% CI) | p-value |
|---|---|---|---|---|
| Mortality, n (%) | ||||
| 30 days post operation | 2 (0.8) | 2 (0.8) | 1.01 (0.14 to 7.10) | 0.99 |
| 6 months post operation | 10 (4.2) | 12 (5.0) | 1.19 (0.52 to 2.70) | 0.68 |
| Intention-to-treat population, n/N (%) | ||||
| Large blood transfusion within 6 months | 5/200 (2.5) | 7/202 (3.5) | 1.39 (0.45 to 4.29) | 0.77 |
| ARs to trial therapy | 5/243 (2.1) | 11/244 (4.5) | 2.19 (0.77 to 6.21) | 0.20 |
| SAEs and SUSARs | 24/243 (9.9) | 22/244 (9.0) | 0.91 (0.53 to 1.58) | 0.75 |
| Development of perioperative AKI | 13/122 (10.7) | 11/140 (7.9) | 0.74 (0.34 to 1.59) | 0.52 |
| Safety population, n/N (%) | ||||
| Large blood transfusion within 6 months | 5/199 (2.5) | 7/198 (3.5) | 1.41 (0.45 to 4.36) | 0.57 |
| ARs to trial therapy | 5/240 (2.1) | 11/240 (4.6) | 2.20 (0.78 to 6.24) | 0.20 |
| SAEs and SUSARs | 23/240 (9.6) | 22/240 (9.2) | 0.96 (0.55 to 1.67) | 0.88 |
| Development of perioperative AKI | 13/122 (10.7) | 11/137 (8.0) | 0.75 (0.35 to 1.62) | 0.52 |
All site-reported AEs (serious and non-serious) were reported using the MedDRA (Medical Dictionary for Regulatory Activities) system organ class. There were more metabolism and nutrition disorders reported as AEs in the intravenous iron group than in the placebo group (0 vs. 6; p = 0.03) (hypoalbuminaemia, n = 2; hypoglycaemia, n = 2; hypokalaemia, n = 1; hypophosphataemia, n = 1) and there were more renal and urinary disorders reported in the placebo group than the intravenous iron group (7 vs. 0; p = 0.007) (acute renal failure, n = 4; renal pain, n = 1; urethral discharge, n = 1; urethritis, n = 1) (Table 9). There were no differences between the groups for SAEs.
| System organ class | Placebo, n (%) [NE] | Intravenous iron, n (%) [NE] | p-value |
|---|---|---|---|
| Cardiac disorders | 6 (2.5) [6] | 5 (2.1) [5] | 0.771 |
| Gastrointestinal disorders | 18 (7.5) [28] | 20 (8.3) [27] | 0.866 |
| General disorders and administration site conditions | 6 (2.5) [7] | 8 (3.3) [9] | 0.787 |
| Hepatobiliary disorders | 1 (0.4) [1] | 0 (0.0) [0] | 0.497 |
| Immune system disorders | 0 (0.0) [0] | 1 (0.4) [1] | 1.000 |
| Infections and infestations | 19 (7.9) [21] | 17 (7.0) [17] | 0.732 |
| Injury, poisoning and procedural complications | 14 (5.9) [17] | 18 (7.4) [19] | 0.584 |
| Investigations | 9 (3.8) [11] | 11 (4.5) [16] | 0.820 |
| Metabolism and nutrition disorders | 0 (0.0) [0] | 6 (2.5) [6] | 0.030 |
| Musculoskeletal and connective tissue disorders | 3 (1.3) [5] | 4 (1.7) [4] | 1.000 |
| Neoplasms: benign, malignant and unspecified (including cysts and polyps) | 1 (0.4) [1] | 0 (0.0) [0] | 0.497 |
| Nervous system disorders | 8 (3.3) [9] | 11 (4.5) [14] | 0.641 |
| Psychiatric disorders | 3 (1.3) [3] | 6 (2.5) [6] | 0.504 |
| Renal and urinary disorders | 7 (2.9) [7] | 0 (0.0) [0] | 0.007 |
| Reproductive system and breast disorders | 2 (0.8) [2] | 1 (0.4) [1] | 0.622 |
| Respiratory, thoracic and mediastinal disorders | 5 (2.1) [5] | 7 (2.9) [7] | 0.772 |
| Skin and subcutaneous tissue disorders | 2 (0.8) [2] | 3 (1.2) [4] | 1.000 |
| Surgical and medical procedures | 2 (0.8) [2] | 2 (0.8) [2] | 1.000 |
| Vascular disorders | 3 (1.3) [3] | 3 (1.2) [3] | 1.000 |
Predefined subgroup analyses
Predefined subgroup analyses for the co-primary end points were performed for age (< 70 vs. ≥ 70 years), sex (male vs. female), body mass index (< 30 km/m2 vs. ≥ 30 kg/m2), operation type (complex major operation, major, major+), Hb (< 100 g/l vs. ≥ 100 g/l), ferritin (< 100 ng/ml vs. ≥ 100 ng/ml) and TSATs (< 20% vs. ≥ 20%). No interactions were observed (Table 10).
| End point | Placebo (N = 243) | Intravenous iron (N = 244) | Treatment effect (95% CI) | Interaction, p-value |
|---|---|---|---|---|
| Blood transfusion or death within 30 days, n/N (%) | ||||
| Age (years) | ||||
| < 70 | 44/156 (28.2) | 41/157 (26.1) | 0.93 (0.64 to 1.33) | |
| ≥ 70 | 23/81 (28.4) | 28/80 (35.0) | 1.23 (0.78 to 1.95) | 0.34 |
| Central lab Hb (g/l) | ||||
| < 100 | 23/42 (54.8) | 23/41 (56.1) | 1.02 (0.70 to 1.51) | |
| ≥ 100 | 44/187 (23.5) | 45/190 (23.7) | 1.01 (0.70 to 1.45) | 0.95 |
| Sex | ||||
| Female | 42/139 (30.2) | 39/122 (32.0) | 1.06 (0.74 to 1.52) | |
| Male | 25/98 (25.5) | 30/115 (26.1) | 1.02 (0.65 to 1.62) | 0.91 |
| Body mass index (kg/m2) | ||||
| < 30 | 52/178 (29.2) | 51/161 (31.7) | 1.08 (0.79 to 1.50) | |
| ≥ 30 | 15/57 (26.3) | 18/75 (24.0) | 0.91 (0.50 to 1.65) | 0.62 |
| Central lab ferritin (ng/ml) | ||||
| < 100 | 34/132 (25.8) | 34/128 (26.6) | 1.03 (0.69 to 1.55) | |
| ≥ 100 | 32/98 (32.7) | 31/94 (33.0) | 1.01 (0.67 to 1.51) | 0.94 |
| Central lab TSAT (%) | ||||
| < 20 | 55/174 (31.6) | 49/163 (30.1) | 0.95 (0.69 to 1.31) | |
| ≥ 20 | 8/50 (16.0) | 15/53 (28.3) | 1.77 (0.82 to 3.81) | 0.13 |
| Type of surgery | ||||
| CMO | 25/83 (30.1) | 20/87 (23.0) | 0.76 (0.46 to 1.26) | |
| Major | 17/86 (19.8) | 22/85 (25.9) | 1.31 (0.75 to 2.29) | |
| Major+ | 25/68 (36.8) | 27/65 (41.5) | 1.13 (0.74 to 1.73) | 0.32 |
| Blood transfusion episodes within 30 days, mean (SD) | ||||
| Age (years) | ||||
| < 70 | 0.5 (1.0) | 0.4 (0.8) | 0.79 (0.50 to 1.24) | |
| ≥ 70 | 0.4 (0.7) | 0.6 (1.1) | 1.48 (0.79 to 2.77) | 0.11 |
| Central lab Hb (g/l) | ||||
| < 100 | 0.8 (1.1) | 0.8 (1.2) | 1.07 (0.51 to 2.24) | |
| ≥ 100 | 0.4 (0.9) | 0.4 (0.8) | 0.93 (0.61 to 1.41) | 0.74 |
| Sex | ||||
| Female | 0.5 (0.9) | 0.4 (0.7) | 0.92 (0.55 to 1.51) | |
| Male | 0.4 (0.9) | 0.5 (1.1) | 1.07 (0.61 to 1.86) | 0.69 |
| Body mass index (kg/m2) | ||||
| < 30 | 0.5 (0.9) | 0.5 (1.0) | 1.12 (0.73 to 1.72) | |
| ≥ 30 | 0.5 (1.0) | 0.3 (0.6) | 0.68 (0.32 to 1.42) | 0.25 |
| Central lab ferritin (ng/ml) | ||||
| < 100 | 0.5 (1.0) | 0.4 (0.9) | 0.92 (0.55 to 1.52) | |
| ≥ 100 | 0.5 (0.8) | 0.5 (0.9) | 1.07 (0.61 to 1.88) | 0.70 |
| Central lab TSAT (%) | ||||
| < 20 | 0.5 (1.0) | 0.5 (1.0) | 0.92 (0.60 to 1.41) | |
| ≥ 20 | 0.3 (0.7) | 0.4 (0.7) | 1.55 (0.64 to 3.75) | 0.29 |
| Type of surgery | ||||
| CMO | 0.6 (1.1) | 0.4 (1.0) | 0.77 (0.43 to 1.40) | |
| Major | 0.3 (0.7) | 0.3 (0.6) | 1.24 (0.62 to 2.45) | |
| Major+ | 0.6 (0.9) | 0.6 (1.0) | 1.09 (0.57 to 2.08) | 0.56 |
Chapter 6 Discussion
The use of intravenous iron in patients with anaemia before major abdominal surgery did not reduce the need for a blood transfusion or the risk of death in the perioperative period. The use of intravenous iron did increase Hb levels before major abdominal surgery, but did not reduce the risk of surgical complications in hospital or affect length of hospital stay. Following discharge from hospital, there was a greater increase in Hb levels in the intravenous iron group and an associated reduction in patients’ re-admission rates to hospital for surgical complications.
The primary end point results from PREVENTT resolves existing equipoise from two previous small trials on the use of preoperative intravenous iron, and confirm that preoperative intravenous iron does not reduce the need for blood transfusion in patients undergoing open major abdominal surgery. The IVICA trial,50 from Nottingham, UK, looked at 116 patients undergoing colorectal cancer surgery and found that intravenous iron similarly increased Hb levels, but there was no difference in blood transfusion use from recruitment to trial completion in terms of either volume of blood administered (p = 0.841) or number of patients transfused (p = 0.470). However, in a smaller trial of 72 patients in Australia,19 intravenous iron did reduce blood transfusion at operation (12% vs. 31%), with an associated reduction in hospital LOS but no associated difference in patient morbidity, mortality or quality of life.
The PREVENTT results are also comparable with a large RCT by Spahn et al. 51 in patients with anaemia or iron deficiency before cardiac surgery. They used a combined package of interventions with intravenous iron including erythropoietin, vitamin B12 and folic acid with an average volume of packed red cells per patient but found no difference in the number of patients transfused between groups and found no impact on postoperative complications or length of hospital stay.
PREVENTT defined blood transfusion as all or part of any unit of blood or blood component; it was not specific to packed RBCs. 52,53 Our rationale was that the use of blood products outside packed RBCs is unusual. This was the case, as only six patients in the trial received either platelets or FFP. These interventions were equally distributed between the groups and unlikely to affect the overall results or message from this study. In addition, a blood transfusion does not contain clotting factors and packed RBCs are often combined with other blood products in routine clinical practice. Finally, a complication of any blood product transfusion, with impact on patient outcome, is transfusion-associated circulatory overload, so it was important to be inclusive of any AE related to transfusion.
Power calculation
The initial power calculation in the PREVENTT protocol1 was based on 19 observational studies on the use of intravenous iron in surgical patients, with a total of 3043 patients and an average increase in Hb of 9.6 g/l. The combined results showed a reduction in blood transfusion from 39.6% in the control groups (n = 1127) to 29.6% in those who received intravenous iron (n = 657).
In a prospective observational pilot study for PREVENTT, we reviewed all patients (n = 718) undergoing major surgery at University College Hospital (UCH) [now University College London Hospital (UCLH)] who spent 48 hours or more in hospital, with an average LOS of 6 days. Average Hb was 133 g/l, but 154 (21%) patients were anaemic. Blood transfusion occurred in 64 (41.5%) patients with anaemia, who received a total of 190 units, compared with 58 (10.2%) patients without anaemia, who received 121 units.
A crude projection of results from the above data in a population of 250 patients with anaemia before surgery, where intravenous iron successfully increased preoperative Hb by 10 g/l in 80% of patients, would result in only 114 of the 250 patients being anaemic at the time of operation [i.e. we expected that intravenous iron would correct anaemia in 136 (54.4%) patients]. Consequently, the projected results would have been that intravenous iron reduced the frequency of blood transfusion from 98 patients to 59 patients.
These projections were not realised in PREVENTT. The intervention of intravenous iron did not result in a 10 g/l rise in Hb levels in 80% of patients, and the average Hb rise was significantly lower; only 21.1% of patients had their anaemia corrected before their operation, less than half than the number that was expected (54.4%).
This may reflect those patients who did not have iron deficiency or where the diagnosis of FID was incorrect. The impact of inflammation on FID and on bone marrow suppression merits further mechanistic investigation.
Efficacy
The Hb rise was less than anticipated and may be the reason that there was no difference in the co-primary end points. For there to be a reduction in transfusion, the difference in operative Hb required between the two groups would need to have been much greater. The rise in Hb levels seen in PREVENTT was lower than expected, with a mean difference between intravenous iron and placebo of 4.7 g/l (95% CI 2.7 to 6.8 g/l) over a median 2-week period. Anaemia was corrected in only 42 (21.1%) patients before operation, with no difference seen between the intention-to-treat and per protocol analyses. In the IVICA trial,50 patients had colorectal cancer and were randomised an average of 21 (15–34) days before surgery, with a mean Hb level rise of 5.0 g/l with oral iron and 15.5 g/l with intravenous iron. This rise was lower in the Australian trial,19 in which preoperative intravenous iron patients were treated a median (IQR) of 8 (6–13) days before their operations and their Hb level rose from a baseline mean (SD) of 107 g/l (13 g/l) to 115 g/l (13 g/l). In a meta-analysis of 9004 patients in 65 studies of the role of iron to treat anaemia, intravenous iron resulted in higher Hb levels than control (mean difference 1.04, 95% CI 0.52 to 1.57 g/l; I2 = 93%; χ2 test for heterogeneity; p < 0.00001). 1 However, there was considerable heterogeneity (I2 = 93%), with a point estimate of the mean difference in Hb levels ranging from −7 g/l to 30 g/l.
The results from PREVENTT may have been affected by the use of oral iron in 19.5% of patients. In the Cochrane analysis,13 oral iron increased Hb levels (mean difference 9.1 g/l, 95% CI 4.8 to 13.5 g/l; I2 = 67%; χ2 test for heterogeneity; p = 0.0004) but with significant heterogeneity (I2 = 67%), with point estimates of the mean difference in Hb levels ranging from 2.0 to 22 g/l higher in the oral iron group than controls. When comparing intravenous iron to oral iron, Hb concentration was higher in the intravenous iron group (mean difference 0.53, 95% CI 0.31 to 0.75), which is comparable to that seen in PREVENTT.
Another reason could be that PREVENTT included only those with a Hb level of > 90 g/l. In the pilot studies, only 5% of those with anaemia had a Hb level of < 90 g/l, and these patients often had more comorbidities and represented a generally sicker population of patients, so this may have added confounders to the analysis. Overall, the average Hb at recruitment in PREVENTT was not dissimilar to previous RCTs, but in many preoperative clinics in the UK the greatest use of intravenous iron is often for those with a Hb level of < 100 g/l. 54 By excluding severely anaemic patients, we may have excluded those who could have benefited the most. However, in the predefined subgroup analysis of patients with a Hb level of < 100 g/l, there was no clinically significant difference in the preoperative Hb rise compared with those with a Hb level of > 100 g/l and there was no interaction for either of the co-primary end points.
The use of intravenous iron therapy is for the treatment of iron deficiency. Our assumption was that iron deficiency would be the predominant cause for anaemia,14 whether AID or FID, which would respond to intravenous iron therapy as treatment. This could be a factor in the lack of efficacy seen because iron deficiency was not specifically diagnosed as being causal for anaemia in the eligibility criteria. Nevertheless, we did predefine a subgroup analysis in those patients with a ferritin level of < 100 ng/ml and TSAT < 20% in line with current guidelines. 55 At inclusion and randomisation, the majority of patients (76%) had TSAT < 20%, 57% had a ferritin level of < 100 ng/ml and 32% a ferritin level of < 30 ng/ml. There was no evidence of any interaction between treatment in those patients who were iron deficient and anaemic and these predefined subgroups for the co-primary end points of the study. It may be that the causal mechanism behind anaemia in the preoperative setting requires treatment with concurrent erythropoietin, as reported by Spahn et al. 51 in cardiac surgery. Erythropoietin is not licensed for such patients in the UK, but erythropoietin and intravenous iron have been recommended for anaemia in patients before orthopaedic surgery. 3
The lack of efficacy in terms of Hb rise in the treatment group meant that, in fact, the difference in Hb between the groups at the time of surgery was small. The transfusion of RBCs during the perioperative period is triggered by low Hb concentration, the trigger level of which may vary from institution to institution, and from practitioner to practitioner. Drops in Hb during surgery are due to either haemorrhage or haemodilution. Major haemorrhage will mostly prompt transfusion, often without measuring Hb, and this was the same in both groups, resulting in the decision to exclude large blood transfusion episodes from analyses. More minor haemorrhage or haemodilution will reduce Hb proportionally, and lower Hb levels are more likely to result in transfusion. Therefore, the fact that there was only a very small difference in Hb between the two groups may account for the lack of difference in transfusion rates and number of units of red cells transfused. In addition, we have shown that the major rise in Hb occurred postoperatively. This may account for the differences seen in re-admissions for complications in this period, as lower Hb is known to be associated with infection and other complications, and re-admission. 56 Therefore, it may be surmised that the only possible benefit demonstrated by the preoperative administration of intravenous iron is not during or immediately after surgery, but some weeks to months after surgery, where Hb rises more quickly in the patients treated initially with intravenous iron. This may also reflect the mechanism of anaemia, induced by blood loss during surgery. Oral iron is known to be ineffective in the first 4–6 weeks after surgery when hepcidin levels are high as part of the inflammatory response to surgery;57 hence, iron is not absorbed from the intestine, and red cell production is reliant on iron stores already in the liver, which were much greater in the patients treated with intravenous iron.
This trial has a number of important clinical implications. It would seem that there is no benefit in treating patients with this dose of intravenous iron in the immediate preoperative period. Future trials should examine the effect of treating patients with intravenous iron much earlier before surgery. However, this may be very challenging in the current NHS system, where preoperative assessment is carried out only 1 or 2 weeks before surgery, particularly for patients who have cancer, for whom surgery is a priority in terms of time and urgency. Another very interesting hypothesis generated from this study is whether or not the use of intravenous iron given in the postoperative setting, before discharge from hospital, results in an earlier correction of postoperative anaemia. This would be cheaper than giving it preoperatively because the patient would already be in hospital, being nursed and monitored in a hospital bed and probably with a cannula in situ. Such a trial should focus on the reduction of postoperative complications, longer-term, patient-oriented end points, and assessing whether or not reducing re-admissions to hospital for postoperative complications may be cost-effective. Other future trials might examine particular diagnoses, such as surgery for cancer, and focus on cancer outcomes, such as tolerance on adjuvant treatment or patient-reported outcomes, as well as rate of recurrence.
Generalisability
PREVENTT has addressed the common problem of patients presenting with anaemia before surgery.
The population studied was those patients undergoing major surgery, which would include major cancer and non-cancer surgery. Many of the patients were elderly and had comorbidities; therefore, this population represents those at highest risk of needing a blood transfusion and also at higher risk of postoperative complications.
The intervention was a total dose of intravenous iron of 1000 mg; this should be adequate to replenish a patient’s iron stores even in the presence of anaemia. The timing of the intervention, at least 10 days before an operation, should have provided adequate time for haematopoiesis. In addition, this is, realistically, the earliest feasible time for administration in the current UK preoperative pathway.
The control was a placebo and essentially standard care in the UK.
Outcomes were primarily risk of blood transfusion, but also included the important patient-related end points of postoperative complications, length of hospital stay and patient quality of life.
PREVENTT is, therefore, generalisable to current practice in the UK because if intravenous iron is clinically ineffective in this setting, it is unlikely to be effective in lower-risk or laparoscopic surgery. Similarly, the effect would be lessened in timelines closer to surgery. Indeed, the PREVENTT population is very similar to the target population for the NHS England Commissioning for Quality and Innovation framework, and consideration should be given to revising this framework.
The issue of restoring iron levels to improve recovery after surgery needs to be addressed in future research.
Overall evidence
We will add these data at a later date. The PREVENTT fellow who conducted a full systematic review and update of previous Cochrane review cannot complete this work until February 2021; thereafter, we will present the PREVENTT results in context.
Chapter 7 Conclusions
PREVENTT found no benefit from the use of intravenous iron to treat anaemia when given preoperatively before major abdominal surgery.
Implications for health care
The primary results of the trial demonstrate no evidence of clinical benefit in giving intravenous iron preoperatively to patients undergoing major surgery (non-cardiac).
There was no evidence of impact in terms of blood transfusion rate or risk in the perioperative period, up to 6 months postoperatively, from giving intravenous iron to this group of patients preoperatively. Furthermore, there was no clear benefit demonstrated in terms of quality of life from this treatment, even at 6 months postoperatively. The current NHS England Commissioning for Quality and Innovation targets should be withdrawn.
There was, however, demonstrable improvement of the Hb level, in terms of correcting anaemia compared with placebo in both the preoperative and postoperative periods, and there were no negative effects seen or increases in AE reporting, particularly infection, with the use of intravenous iron. The post discharge increase in Hb levels and associated reduction in re-admissions for postoperative complications merit further research.
Recommendations for research
There will be further analyses carried out on the data obtained from the trial to assess the causality of anaemia in this patient population, particularly the study of levels of hepcidin and iron studies of serum samples, to assess the inflammatory states of patients and their relation to the study outcomes.
Further follow-up in the cancer patients will also be carried out to assess longer-term follow-up in relation to giving iron to this group of patients.
Postoperative treatment of anaemic patients with intravenous iron also needs to be studied further to assess improved benefits.
Acknowledgements
Trial Steering Committee
Andrew Bradbury (Chairperson), Toby Richards (Chief Investigator), Trevor Burley, Shelley Van Loen, Stefan Anker, Andrew Klein, Iain Macdougall, Gavin Murphy, Martin Besser and Isabel Unsworth.
Observers: Tim Clayton, Tim Collier, Kimberley Potter, Sandy Abeysiri, Richard Evans, Rosemary Knight, Rebecca Swinson, Laura Van Dyck and Jane Keidan.
Data Safety and Monitoring Committee
Lorna Williamson (Chairperson), Angela Crook and John Pepper.
Unblinded statisticians supporting the DSMC were Joanna Dobson, Simon Newsome, Tom Godec and Matthew Dodd.
Project Management Group
Toby Richards, Laura Van Dyck, Richard Evans, Sandy Abeysiri, Ben Clevenger, Anna Butcher, Rebecca Swinson, Tim Collier and Kimberley Potter.
Study design and support
Stefan Anker, John Kelly, Steven Morris, John Browne, Jane Keidan, Michael Grocott, Marisa Chau and Rosemary Knight.
Trial statistician
Timothy Collier.
Randomisation service
Sealed Envelope.
Blood sample analysis
The Doctors Laboratory, London, UK.
Final report preparation
Megan Knight.
Contributing sites
Royal Marsden NHS Foundation Trust (118 patients, opened 4 April 2014)
Ravishankar Rao Baikady (PI), Ethel Black, Helen Lawrence, Maria Kouthra, Katherine Horner, Sham Jhanji, Ed Todman, Zoe Keon-Cohen, Martin Rooms, Judith Tomlinson, Ian Bailes, Susanna Walker, Katrina Pirie, Michelle Gerstman, Ramanathan Kasivisvanathan, Sophie Uren, David Magee, Alex Eeles, Rob Anker, Jamie McCanny, Michelle O’Mahony, Toby Reynolds, Sian Batley, Aoife Hegarty, Simon Trundle, Francesca Mazzola, Kate Tatham, Alina Balint, Ben Morrison, Matthew Evans, Ching Ling Pang, Lorna Smith, Charlotte Wilson, Victoria Sjorin, Poonam Khatri, Marco Wilson and David Parkinson.
University College London Hospitals NHS Foundation Trust (38 patients, opened 1 September 2013)
James Crosbie (PI), Khaled Dawas (PI), Deborah Smyth, Georgia Bercades, Jung Ryu, Anna Reyes, Gladys Martir, Laura Gallego, Alison Macklin, Magda Rocha, Karen Tam and David Brealey.
Guy’s and St Thomas’ NHS Foundation Trust (25 patients, opened 7 May 2014)
Jugdeep Dhesi (PI), Catriona Morrison, Joanna Hardwick, Jude Partridge, Philip Braude, Andrew Rogerson, Nymah Jahangir, Clare Thomson, Lizzie Biswell, Jason Cross, Ffion Pritchard, Aminata Mohammed, Deirdre Wallace, Ma Gem Galat, Jane Okello, Rebecca Symes, Mollika, Chakravorty, Josette Leon, Angela Cape (pharmacy) and Charlotte Gibbs (pharmacy).
Sheffield Teaching Hospitals NHS Foundation Trust (24 patients, opened 11 November 2013)
Sumayer Sanghera (PI), Andy Dennis, Faith Kibutu, Joyce Fofie, Sarah Bird, Abiola Alli and Yvonne Jackson.
North Bristol NHS Trust (23 patients, opened 4 February 2014)
Salah Albuheissi (PI), Carol Brain and Connie Shiridzinomwa.
Royal Cornwall Hospitals NHS Trust (23 patients, opened 19 September 2013)
Catherine Ralph (PI), Belinda Wroath, Fiona Hammonds, Benita Adams, John Faulds and Sara Staddon.
King’s College Hospital NHS Foundation Trust (18 patients, opened 26 February 2016)
Timothy Hughes (PI), Sian Saha, Clare Finney, Clair Harris, Clare Mellis, Lucy Johnson, Paul Riozzi, Adam Yarnold, Fraser Buchanan, Philip Hopkins, Louise Greig and Harriet Noble.
University Hospital Southampton NHS Foundation Trust (18 patients, opened 7 January 2014)
Mark Edwards (PI), Mike Grocott, James Plumb, David Harvie, Ahilanandan Dushianthan, Mai Wakatsuki, Samantha Leggett, Karen Salmon, Clare Bolger, Rachel Burnish, James Otto, Gurinder Rayat, Kim Golder, Pauline Bartlett, Sitara Bali, Leanne Seaward, Beverley Wadams, Bryony Tyrell, Hannah Collins, Natasha Tantony, Rosie Geale, Amber Wilson and Darran Ball.
Oxford University Hospitals NHS Foundation Trust (15 patients, opened 22 October 2015)
Ian Lindsey (PI), Debbie Barker and Madeleine Thyseen.
South Tees Hospitals NHS Foundation Trust (11 patients, opened 20 November 2014)
Patrick Chiam (PI), Carol Hannaway and Kerry Colling.
The Hillingdon Hospitals NHS Foundation Trust (11 patients, opened 4 October 2013)
Cheryl Messer (PI), Neil Verma, Mariam Nasseri, Gail Poonawala, Abbie Sellars, James Harris (PI) and Pratyuja Mainali.
Mid Essex Hospital Services NHS Trust (10 patients, opened 11 March 2015)
Mr Toby Hammond (PI), Al Hughes, David O’Hara, Fiona McNeela and Lauren Shillito.
Leeds Teaching Hospitals NHS Trust (10 patients, opened 12 November 2014)
Alwyn Kotze (PI) and Catherine Moriarty.
York Teaching Hospital NHS Foundation Trust (10 patients, opened 17 December 2013)
Jonathan Wilson (PI), Simon Davies, David Yates, Joe Carter, Jon Redman, Sara Ma, Kate Howard, Heidi Redfearn and Danielle Wilcock.
Imperial College Healthcare NHS Trust (nine patients, opened 6 November 2015)
Justine Lowe (PI), Asela Dharmadasa (PI), Tamara Alexander, Jasmine Jose, Gillian Hornzee, Fatima Akbar, Severine Rey, Anoop Patel, Samantha Coulson, Rajan Saini, Joseph Santipillai, Thomas McCretton, Ian Bailes (PI), Jamie Mccanny, Kiran Chima, Karen Collins, Byiravey Pathmanathan, Anjalee Chattersingh, Laura McLeavy and Zayneb Al-Saadi.
Salford Royal NHS Foundation Trust (nine patients, opened 17 November 2014)
Manju Patel (PI), Sofia Skampardoni, Rajkumar Chinnadurai, Vicky Thomas, Anne Keen, Katherine Pagett, Clare Keatley, Jason Howard, Leigh Willoughby (PI) and Marie Greenhalgh.
The Dudley Group NHS Foundation Trust (eight patients, opened 8 March 2016)
Stephen Jenkins (PI), Ranjit Gidda and Angela Watts.
Royal Liverpool and Broadgreen University Hospitals NHS Trust (eight patients, opened 20 February 2015)
Chris Breaton (PI) and Jane Parker.
Royal Free London NHS Foundation Trust (eight patients, opened 19 December 2013)
Susan Mallett (PI) and Sarah James.
Wye Valley NHS Trust (seven patients, opened 20 May 2015)
Lisa Penny (PI), Kim Chan, Tamsin Reeves, Marisa Catterall, Sue Williams, Janine Birch, Kate Hammerton, Nicola Williamson, Anni Thomas, Melanie Evans, Lily Mercer, Gill Horsfield and Claire Hughes.
Blackpool Teaching Hospitals NHS Foundation Trust (seven patients, opened 1 September 2014)
Jason Cupitt (PI) and Emma Stoddard.
Liverpool Women’s NHS Foundation Trust (six patients, opened 4 May 2018)
Helen McNamara (PI) and Chloe Birt.
North West Anglia NHS Foundation Trust (Peterborough) (six patients, opened 2 December 2015)
Alexander Hardy (PI), Robert Dennis, Deborah Butcher, Susie O’Sullivan and Alan Pope.
University Hospital of South Manchester NHS Foundation Trust (now Manchester University NHS Foundation Trust) (six patients, opened 29 September 2014)
Sumaya Elhanash (PI), Stephen Preston, Helen Officer, Andrea Stoker, Stuart Moss, Alison Walker, Anna Gipson, Julie Melville, Joanne Bradley-Potts, Richard McCormac, Vivienne Benson, Kirsty Melia, Julie Fielding and Wendy Guest.
Swansea Bay University Health Board (six patients, opened 8 July 2014)
Simon Ford (PI).
Gloucestershire Hospitals NHS Foundation Trust (five patients, opened 22 February 2018)
Henry Murdoch (PI), Susan Beames, Paula Townshend, Kayleigh Collins, Jon Glass and Bethan Cartwright.
Aintree University Hospital NHS Foundation Trust (now the Liverpool University Hospitals NHS Foundation Trust) (five patients, opened 11 March 2016)
Balsam Altemimi (PI) and Lucy Berresford.
Royal Surrey County Hospital, Royal Surrey NHS Foundation Trust (five patients, opened 19 September 2014)
Dr Chris Jones (PI), Dr Leigh Kelliher, Dr Sam de Silva and Dr Katie Blightman.
Central Manchester University Hospitals NHS Foundation Trust (five patients, opened 27 June 2014)
Kate Pendry (PI) and Lebina Pinto.
Barts Health NHS Trust (five patients, opened 9 May 2014)
Shubha Allard (PI) and Louise Taylor.
The Newcastle upon Tyne Hospitals NHS Foundation Trust (five patients, opened 7 March 2014)
Ahmed Chishti (PI) and Julia Scott.
Norfolk and Norwich University Hospitals NHS Foundation Trust (four patients, opened 6 November 2015)
Debbie O’Hare (PI) and Michael Lewis.
Pennine Acute Hospitals NHS Trust (four patients, opened 22 July 2015)
Zahid Hussain (PI), Karen Hallett, Susan Dermody, Carolyn Corbett and Louise Morby.
Dorset County Hospital NHS Foundation Trust (four patients, opened 6 December 2013)
Matthew Hough (PI), Sarah Williams, Patricia Williams, Sarah Horton, Pauline Ashcroft and Anthony Homer.
Royal Edinburgh Hospital – NHS Lothian (three patients, opened 4 March 2016)
Alastair Lang, Heidi Dawson and Ewen Harrison (PI).
Royal Devon and Exeter NHS Foundation Trust (three patients, opened 12 May 2014)
John Thompson (PI).
North West Anglia NHS Foundation Trust (Hinchingbrooke) (one patient, opened 1 February 2018)
Vimal Hariharan (PI) and Vanessa Goss.
Basildon and Thurrock University Hospitals NHS Foundation Trust (one patient, opened 4 October 2017)
Ramachandran Ravi (PI), Georgina Butt and Mark Vertue.
Nottingham University Hospitals NHS Trust (one patient, opened 27 May 2016)
Austin Acheson (PI), Oliver Ng, Debbie Bush, Edward Dickson and Amy Ward.
Brighton and Sussex University Hospitals NHS Trust (one patient, opened 7 March 2014)
Sophie Morris (PI) and research and development management.
Maidstone and Tunbridge Wells NHS Trust (one patient, opened 10 October 2013)
Andrew Taylor (PI) and Rebecca Casey.
Countess of Chester Hospital NHS Foundation Trust (no patients, opened 26 April 2017)
Lawrence Wilson (PI), Dale Vimalachandran, Maria Faulkner, Helen Jeffrey, Claire Gabrielle and Sharon Martin.
Gateshead Health NHS Foundation Trust (no patients, opened 23 November 2015)
Andrew Bracewell (PI), Jenny Ritzema and David Sproates.
Northampton General Hospital NHS Trust (no patients, opened 22 December 2014)
Farhad Alexander-Sefre (PI).
West Suffolk NHS Foundation Trust (no patients, opened 16 September 2014)
Christiane Kubitzek (PI), Sally Humphreys, James Curtis, Paul Oats, Sandra Swann, Abbie Holden and Claire Adam.
University Hospitals Bristol NHS Foundation Trust (no patients, opened 19 May 2014)
Louise Flintoff, Claudia Paoloni (PI) and Karen Bobruk.
Contributions of authors
Toby Richards (https://orcid.org/0000-0001-9872-4653) (Head of Department of Surgery) was the chief investigator and was involved in the trial concept and design, the conduct of the research, interpretation and reporting of results, and writing and reviewing the manuscript.
Ravishankar Rao Baikady (https://orcid.org/0000-0002-6827-9250) (Consultant Anaesthetist) was the Principal Investigator at the site with the highest recruitment levels, and was involved in the trial design, conduct of research, and reviewing the manuscript.
Ben Clevenger (https://orcid.org/0000-0001-5659-6323) (Consultant Anaesthetist) was a NIHR PREVENTT research fellow involved with the conduct of the research, the sites set-up and management, and reviewing the manuscript.
Anna Butcher (https://orcid.org/0000-0002-1454-9403) (Clinical Research Associate) was a NIHR PREVENTT research fellow involved with the conduct of the research, the sites set-up and management, and reviewing the manuscript.
Sandy Abeysiri (https://orcid.org/0000-0002-4258-2585) (Clinical Research Associate) was a NIHR PREVENTT research fellow involved with the conduct of the research, the sites set-up and management, and reviewing the manuscript.
Marisa Chau (https://orcid.org/0000-0003-2965-1870) (Clinical Project Manager) was a trial manager involved with the conduct of the research and produced the references for the manuscript.
Rebecca Swinson (https://orcid.org/0000-0002-4997-5998) (Trial Manager) was the trial manager and was involved in the trial set-up, site set-up, the conduct of the research and reviewing the manuscript.
Tim Collier (https://orcid.org/0000-0002-3095-277X) (Associate Professor, Medical Statistics) was the lead statistician and was involved in the trial design and conduct, analysing the data, and writing and reviewing the manuscript.
Matthew Dodd (https://orcid.org/0000-0002-6207-6604) (Research Fellow in Medical Statistics) was the unblinded trial statistician and a member of the Data Monitoring Committee, analysed the data and was involved in reviewing the manuscript.
Laura Van Dyck (https://orcid.org/0000-0002-5720-8633) (Data Manager) was the data manager and was involved in the conduct of the research and reviewing the manuscript.
Iain Macdougall (https://orcid.org/0000-0001-9098-8611) (Consultant Nephrologist) is an expert in clinical trials on intravenous iron, was a member of the TSC and was involved in writing and reviewing the manuscript.
Gavin Murphy (https://orcid.org/0000-0003-0976-1482) (Professor of Cardiac Surgery) is an expert in clinical trials on surgery and blood transfusion, was a member of the TSC and was involved in writing and reviewing the manuscript.
John Browne (https://orcid.org/0000-0003-3494-4336) (Director of the National Health Services Research Institute) is an expert in clinical trials and quality of life analysis, was a member of the TSC, was involved in the trial design, and in writing and reviewing the manuscript.
Andrew Bradbury (https://orcid.org/0000-0003-0530-9667) (Professor of Vascular Surgery) is an expert in clinical trials on surgery, was the chair of the TSC, and was involved in writing and reviewing the manuscript.
Andrew Klein (https://orcid.org/0000-0001-9913-6570) (Consultant Anaesthetist) is an expert in clinical trials on intravenous iron and blood transfusion, was a member of the TSC, and was involved in the trial concept and writing and reviewing the manuscript.
Publications
Richards T, Clevenger B, Keidan J, Collier T, Klein AA, Anker SD, Kelly JD. PREVENTT: preoperative intravenous iron to treat anaemia in major surgery: study protocol for a randomised controlled trial. Trials 2015;16:254.
Butcher A, Clevenger B, Swinson R, van Dyck L, Klein A, Richards T. PREVENTT trial: a snapshot of preoperative anaemia in the United Kingdom. J Clin Trials 2016;6:3.
Butcher A, Swinson R, Van Dyck L, Collier T, Richards T. The role of general practice in surgical trials. Br J Gen Pract 2017;67:157–8.
Richards T, Baikady RR, Clevenger B, Butcher A, Abeysiri S, Macdougall IC, et al. Preoperative intravenous iron to treat anaemia before major abdominal surgery (PREVENTT): a randomised, double-blind, controlled trial [published online ahead of print 4 September 2020]. Lancet 2020;396:1353–61.
Data-sharing statement
Research data will be made available to the scientific community with as few restrictions as possible so as to maximise the value of the data for research and for eventual patient and public benefit. All data requests should be submitted to the corresponding author for consideration.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Richards T, Clevenger B, Keidan J, Collier T, Klein AA, Anker SD, et al. PREVENTT: preoperative intravenous iron to treat anaemia in major surgery: study protocol for a randomised controlled trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-0774-2.
- National Institute for Health and Care Excellence (NICE) . Blood Transfusion. NICE Guideline (NG24) 2015. www.nice.org.uk/guidance/ng24 (accessed 1 April 2020).
- Mueller MM, Van Remoortel H, Meybohm P, Aranko K, Aubron C, Burger R, et al. Patient blood management: recommendations from the 2018 Frankfurt Consensus Conference. JAMA 2019;321:983-97. https://doi.org/10.1001/jama.2019.0554.
- NHS . NHS Commissioning for Quality and Innovation. 2020 21 CQUIN 2020. www.england.nhs.uk/nhs-standard-contract/cquin/cquin-20-21/ (accessed 1 April 2020).
- Fowler AJ, Ahmad T, Phull MK, Allard S, Gillies MA, Pearse RM. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg 2015;102:1314-24. https://doi.org/10.1002/bjs.9861.
- Musallam KM, Tamim HM, Richards T, Spahn DR, Rosendaal FR, Habbal A, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet 2011;378:1396-407. https://doi.org/10.1016/S0140-6736(11)61381-0.
- Muñoz M, García-Erce JA, Díez-Lobo AI, Campos A, Sebastianes C, Bisbe E. Anaemia Working Group España (AWGE) . Usefulness of the administration of intravenous iron sucrose for the correction of preoperative anemia in major surgery patients. Med Clin 2009;132:303-6. https://doi.org/10.1016/j.medcli.2008.04.011.
- Weiss G, Goodnough L. Anemia of chronic disease. N Engl J Med 2005;352:1011-23.
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 2004;113:1271-6. https://doi.org/10.1172/JCI20945.
- Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol 2009;122:78-86. https://doi.org/10.1159/000243791.
- Ganz T, Nemeth E. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol 2006;290:G199-203. https://doi.org/10.1152/ajpgi.00412.2005.
- Muñoz M, Romero A, Morales M, Campos A, García-Erce JA, Ramírez G. Iron metabolism, inflammation and anemia in critically ill patients. A cross-sectional study. Nutr Hosp 2005;20:115-20.
- Gurusamy KS, Nagendran M, Broadhurst JF, Anker SD, Richards T. Iron therapy in anaemic adults without chronic kidney disease. Cochrane Database Syst Rev 2014;12. https://doi.org/10.1002/14651858.CD010640.pub2.
- Clevenger B, Richards T. Pre-operative anaemia. Anaesthesia 2015;70:e6-8. https://doi.org/10.1111/anae.12918.
- Martin-Cabrera P, Hung M, Ortmann E, Richards T, Ghosh M, Bottrill F, et al. Clinical use of low haemoglobin density, transferrin saturation, bone marrow morphology, Perl’s stain and other plasma markers in the identification of treatable anaemia presenting for cardiac surgery in a prospective cohort study. J Clin Pathol 2015;68:923-30. https://doi.org/10.1136/jclinpath-2015-203024.
- Hung M, Ortmann E, Besser M, Martin-Cabrera P, Richards T, Ghosh M, et al. A prospective observational cohort study to identify the causes of anaemia and association with outcome in cardiac surgical patients. Heart 2015;101:107-12. https://doi.org/10.1136/heartjnl-2014-305856.
- Gombotz H, Rehak PH, Shander A, Hofmann A. Blood use in elective surgery: the Austrian benchmark study. Transfusion 2007;47:1468-80. https://doi.org/10.1111/j.1537-2995.2007.01286.x.
- Anker SD, Colet JC, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Rationale and design of Ferinject assessment in patients with IRon deficiency and chronic Heart Failure (FAIR-HF) study: a randomized, placebo-controlled study of intravenous iron supplementation in patients with and without anaemia. Eur J Heart Fail 2009;11:1084-91. https://doi.org/10.1093/eurjhf/hfp140.
- Froessler B, Palm P, Weber I, Hodyl NA, Singh R, Murphy EM. The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: a randomized controlled trial. Ann Surg 2016;264:41-6. https://doi.org/10.1097/SLA.0000000000001646.
- Keeler BD, Simpson JA, Ng O, Padmanabhan H, Brookes MJ, Acheson AG. IVICA Trial Group . Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br J Surg 2017;104:214-21. https://doi.org/10.1002/bjs.10328.
- Keeler BD, Dickson EA, Simpson JA, Ng O, Padmanabhan H, Brookes MJ, et al. IVICA Trial Group . The impact of pre-operative intravenous iron on quality of life after colorectal cancer surgery: outcomes from the intravenous iron in colorectal cancer-associated anaemia (IVICA) trial. Anaesthesia 2019;74:714-25. https://doi.org/10.1111/anae.14659.
- Bradburn R. National Health Service Blood and Transplant (NHSBT). NHS; 2009.
- Leahy MF, Hofmann A, Towler S, Trentino KM, Burrows SA, Swain SG, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion 2017;57:1347-58. https://doi.org/10.1111/trf.14006.
- Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res 2002;102:237-44. https://doi.org/10.1006/jsre.2001.6330.
- Leichtle SW, Mouawad NJ, Lampman R, Singal B, Cleary RK. Does preoperative anemia adversely affect colon and rectal surgery outcomes?. J Am Coll Surg 2011;212:187-94. https://doi.org/10.1016/j.jamcollsurg.2010.09.013.
- Narayan S, Bellamy M, Bolton-Maggs P. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group . Annual SHOT Report 2018 n.d.:16-35. www.shotuk.org/wp-content/uploads/myimages/SHOT-Report-2018_Web_Version-1.pdf (accessed 1 April 2020).
- Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg 2012;256:235-44. https://doi.org/10.1097/SLA.0b013e31825b35d5.
- Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0117383.
- Ganzoni AM. New aspects of iron deficiency. Schweiz Med Wochenschr 1970;100:691-7.
- Ganz T. Systemic iron homeostasis. Physiol Rev 2013;93:1721-41. https://doi.org/10.1152/physrev.00008.2013.
- Theusinger OM, Leyvraz PF, Schanz U, Seifert B, Spahn DR. Treatment of iron deficiency anemia in orthopedic surgery with intravenous iron: efficacy and limits: a prospective study. Anesthesiology 2007;107:923-7. https://doi.org/10.1097/01.anes.0000291441.10704.82.
- Shepshelovich D, Rozen-Zvi B, Avni T, Gafter U, Gafter-Gvili A. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: an updated systematic review and meta-analysis. Am J Kidney Dis 2016;68:677-90. https://doi.org/10.1053/j.ajkd.2016.04.018.
- Jankowska EA, Tkaczyszyn M, Suchocki T, Drozd M, von Haehling S, Doehner W, et al. Effects of intravenous iron therapy in iron-deficient patients with systolic heart failure: a meta-analysis of randomized controlled trials. Eur J Heart Fail 2016;18:786-95. https://doi.org/10.1002/ejhf.473.
- Bonovas S, Fiorino G, Allocca M, Lytras T, Tsantes A, Peyrin-Biroulet L, et al. Intravenous versus oral iron for the treatment of anemia in inflammatory bowel disease: a systematic review and meta-analysis of randomized controlled trials. Medicine 2016;95. https://doi.org/10.1097/MD.0000000000002308.
- Sultan P, Bampoe S, Shah R, Guo N, Estes J, Stave C, et al. Oral vs intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am J Obstet Gynecol 2019;221:19-29.e3. https://doi.org/10.1016/j.ajog.2018.12.016.
- Lewkowitz AK, Gupta A, Simon L, Sabol BA, Stoll C, Cooke E, et al. Intravenous compared with oral iron for the treatment of iron-deficiency anemia in pregnancy: a systematic review and meta-analysis. J Perinatol 2019;39:519-32. https://doi.org/10.1038/s41372-019-0320-2.
- Clevenger B, Gurusamy K, Klein AA, Murphy GJ, Anker SD, Richards T. Systematic review and meta-analysis of iron therapy in anaemic adults without chronic kidney disease: updated and abridged Cochrane review. Eur J Heart Fail 2016;18:774-85. https://doi.org/10.1002/ejhf.514.
- Owusu-Ansah R, Gatongi D, Chien PF. Health technology assessment of surgical therapies for benign gynaecological disease. Best Pract Res Clin Obstet Gynaecol 2006;20:841-79. https://doi.org/10.1016/j.bpobgyn.2006.11.006.
- Medeiros LR, Rosa DD, Bozzetti MC, Fachel JM, Furness S, Garry R, et al. Laparoscopy versus laparotomy for benign ovarian tumour. Cochrane Database Syst Rev 2009;2. https://doi.org/10.1002/14651858.CD004751.pub3.
- Aarts JW, Nieboer TE, Johnson N, Tavender E, Garry R, Mol BW, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev 2015;8. https://doi.org/10.1002/14651858.CD003677.pub5.
- Ahmed Ali U, Keus F, Heikens JT, Bemelman WA, Berdah SV, Gooszen HG, et al. Open versus laparoscopic (assisted) ileo pouch anal anastomosis for ulcerative colitis and familial adenomatous polyposis. Cochrane Database Syst Rev 2009;1. https://doi.org/10.1002/14651858.CD006267.pub2.
- Vennix S, Pelzers L, Bouvy N, Beets GL, Pierie JP, Wiggers T, et al. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev 2014;4. https://doi.org/10.1002/14651858.CD005200.pub3.
- Schwenk W, Haase O, Neudecker J, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev 2005;3. https://doi.org/10.1002/14651858.CD003145.pub2.
- NHS Blood and Transplant . National Comparative Audit of Blood Transfusion 2018. https://hospital.blood.co.uk/audits/national-comparative-audit/# (accessed 1 October 2020).
- Healthcare Quality Improvement Partnership . National Bowel Cancer Audit – Annual Report 2017 Version 2 n.d. www.hqip.org.uk/resource/national-bowel-cancer-audit-annual-report-2017/ (accessed 1 April 2020).
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B 1995;57:289-300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x.
- Ariti CA, Cleland JG, Pocock SJ, Pfeffer MA, Swedberg K, Granger CB, et al. Days alive and out of hospital and the patient journey in patients with heart failure: Insights from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Am Heart J 2011;162:900-6. https://doi.org/10.1016/j.ahj.2011.08.003.
- Sinclair RC, Duffield KE, de Pennington JH. Improving preoperative haemoglobin using a quality improvement approach to treat iron deficiency anaemia. BMJ Open Qual 2020;9. https://doi.org/10.1136/bmjoq-2019-000776.
- Jung-König, Füllenbach C, Murphy M, Manzini P, Laspina S, Pendry K, et al. Programmes for the management of preoperative anaemia: audit in ten European hospitals within the PaBloE (Patient Blood Management in Europe) Working Group. Vox Sang 2020;115:182-91. https://doi.org/10.1111/vox.12872.
- Keeler BD, Simpson JA, Ng O, Padmanabhan H, Brookes MJ. An open-label, randomised controlled trial comparing the efficacy of intravenous and oral iron in the preoperative management of colorectal cancer anaemia: IVICA trial. Gut 2015;1. https://doi.org/10.1136/gutjnl-2015-309861.736.
- Spahn DR, Schoenrath F, Spahn GH, Seifert B, Stein P, Theusinger OM, et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet 2019;393:2201-12. https://doi.org/10.1016/S0140-6736(18)32555-8.
- Mazer CD, Whitlock RP, Fergusson DA, Hall J, Belley-Cote E, Connolly K, et al. Restrictive or liberal red-cell transfusion for cardiac surgery. N Engl J Med 2017;377:2133-44. https://doi.org/10.1056/NEJMoa1711818.
- Afifi A, Simry W. Transfusion Indication Threshold Reduction (TITRe2) trial: when to transfuse and what to give?. Glob Cardiol Sci Pract 2015;2015. https://doi.org/10.5339/gcsp.2015.61.
- Faulds J, Whately-Smith C, Clarke K. Transfusion requirement and length of stay of anaemic surgical patients associated with a patient blood management service: a single-centre retrospective study. Transfus Med 2019;29:311-18. https://doi.org/10.1111/tme.12617.
- Muñoz M, Acheson AG, Bisbe E, Butcher A, Gómez-Ramírez S, Khalafallah AA, et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018;73:1418-31. https://doi.org/10.1111/anae.14358.
- Roubinian NH, Murphy EL, Mark DG, Triulzi DJ, Carson JL, Lee C, et al. Long-term outcomes among patients discharged from the hospital with moderate anemia: a retrospective cohort study. Ann Intern Med 2019;170:81-9. https://doi.org/10.7326/M17-3253.
- Litton E, Baker S, Erber W, Farmer S, Ferrier J, French C, et al. Hepcidin predicts response to IV iron therapy in patients admitted to the intensive care unit: a nested cohort study. J Intensive Care 2018;6. https://doi.org/10.1186/s40560-018-0328-2.
Appendix 1 Summary of changes to the PREVENTT protocol
Version 1 of the protocol completed on 3 September 2012.
The following changes were made to protocol version 2 (5 October 2012), which was effective from 5 November 2012:
-
The IMP administration was clarified, to ensure that it was carried out in an inpatient setting with appropriate resuscitation facilities and staff available.
-
The protocol was amended to clarify that the study treatment must stop immediately in the event of intolerance or allergic reaction during administration.
-
The flow chart was improved and minor errors corrected.
-
A change to the safety reporting procedure was made, from this point SAEs should be reported to LSHTM CTU within 24 hours of the local principal investigator’s knowledge.
-
The unblinding procedure was clarified. The treating clinical physician makes the final decision to unblind, but, before unblinding, the reason and rationale will be discussed with the PREVENTT office at University College London.
The following changes were made to protocol version 3 (17 April 2013), which was effective from 23 May 2013:
-
Physical examination was removed as these data will not be collected or needed, and ECG monitoring removed from postbaseline assessments as ECG monitoring was not required by the safety profile of IMP and some sites do not routinely perform this.
-
Clarification was made to co-primary end point relating to risk of blood transfusion or death, and added that deaths will be adjusted for in the analysis.
-
POMS – these data also need to be collected at day 5 because patients are discharged much sooner.
-
Health economics – these sections were revised following review and update from the trial Health Economist.
-
TSC and DSMC – membership of both committees were added, now they have been approved by the HTA.
-
AE section was revised to reflect the LSHTM CTU AE processes (because the sponsor has delegated this responsibility to the LSHTM CTU).
-
Restarting treatment – following on from review by the TSC, it was agreed that treatment could be restarted under certain conditions.
The following changes were made to protocol version 4 (16 December 2013), which was effective from 14 January 2014:
-
Changes were made in the light of updated advice from the Medicines and Healthcare products Regulatory Agency on intravenous iron. Two new exclusion criteria were added (patients with severe asthma or severe allergy, and patients unfit for elective surgery) and patients should now be monitored for at least 30 minutes following treatment.
-
The side effects were updated to reflect changes in the latest version of the SmPC.
-
Changes were made to the preoperative visit, as sites were having difficulty fitting in this extra hospital visit. The visit was removed and the preoperative quality-of-life forms could be completed at home. The central blood samples were taken on admission to hospital.
-
Changes to the timelines from treatment to surgery – the lower limit of this timeline was shortened from 14 to 10 days, as sites found this timeline restrictive when trying to recruit, and it also meant cancer patients were excluded (it was agreed by the TSC that 10 days was sufficient time for the intravenous iron to take effect).
-
Further clarified documentation for drug returns and drug destruction to ensure that blinding was not compromised by blinded staff at site, and the wording relating to destruction of the remaining drug was removed.
-
Included the system that will be used to document the postoperative complications (Clavien–Dindo).
-
Included UE tests as a requirement, rather than if available in follow-up visits, as this is needed to calculate eGFR.
-
Further clarification to Hb measurements, immunosuppressive therapy in exclusion criteria, timing of screening pregnancy test, vital signs assessment, development safety update report and Annual Progress Report (APR) anniversary dates and non-exclusion of those on oral iron supplements.
-
Clarified IMP administration masking with iodine so that it allows flexibility of other methods in the event any patients being allergic to iodine.
-
Added additional details so that sites can call patients prior to consenting (as requested by site research and development departments).
The following changes were made to protocol version 5 (5 March 2015), which was effective from 10 April 2015:
-
Changed inclusion criteria to increase the upper limit of Hb for men to match the World Health Organization’s definition of anaemia and to help aid recruitment.
-
Amended the definition of major surgery so that sites can include patients having surgery which does not include the removal of an organ.
-
Changed the exclusion criteria so that is it clear that only untreated vitamin B12/folate deficiency would make a patient ineligible.
-
Added definition of severe asthma/allergy to the exclusion criteria.
-
Increased the number of sites from 20 to 35, to help aid recruitment.
-
Removed brand names of masking intravenous bags and giving sets, to allow flexibility across sites.
-
Included 200-ml vials of Ferinject to allow flexibility in use of hospital stocks across sites.
-
SAE form can be submitted via online AE database.
-
Data monitoring changed to reflect new monitoring SOP requirements.
-
Administrative changes to update details of TSC members and observers.
The following changes were made to protocol version 6 (27 July 2016), which was effective from 5 September 2016:
-
Changed exclusion criteria for LFTs to reflect local hospital practice and pathways.
-
Changed exclusion criteria so that patients who have had a previous blood transfusion within the previous 12 weeks can still be included in the trial.
-
Changed assessment of LFTs at baseline to clarify this is only done if clinically indicated, according to local hospital practice and pathways.
-
Updated the risk/benefits section of the protocol to reflect the change to the exclusion criteria for patients who have not had their LFTs checked.
-
Increased the number of sites from 35 to 40 to help with recruitment.
-
Updated to reflect the approval by the funder of an additional 2 years of recruitment.
-
Updated SmPC.
Appendix 2 Recruitment by site and by month
Recruitment by site
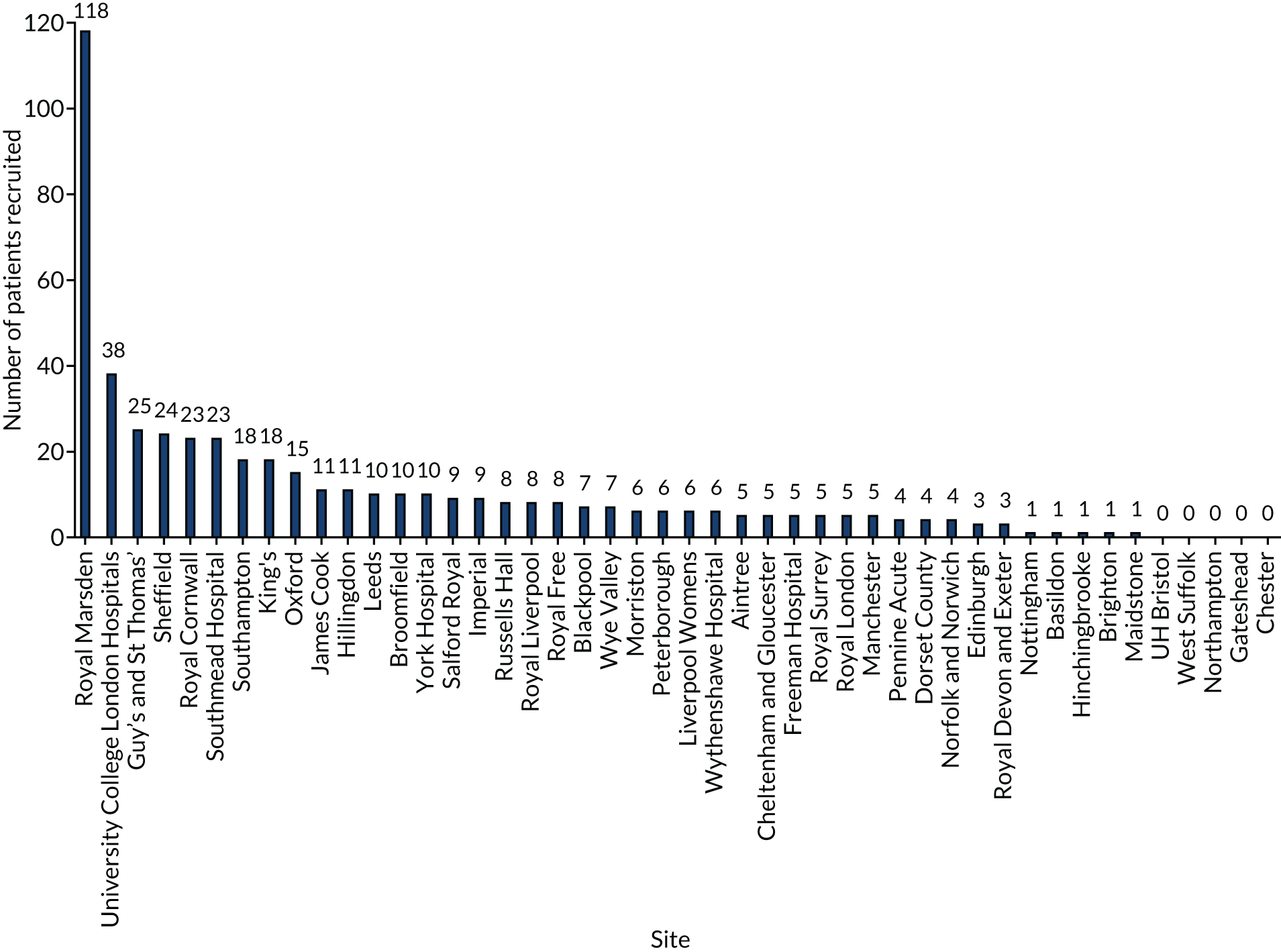
Recruitment by month
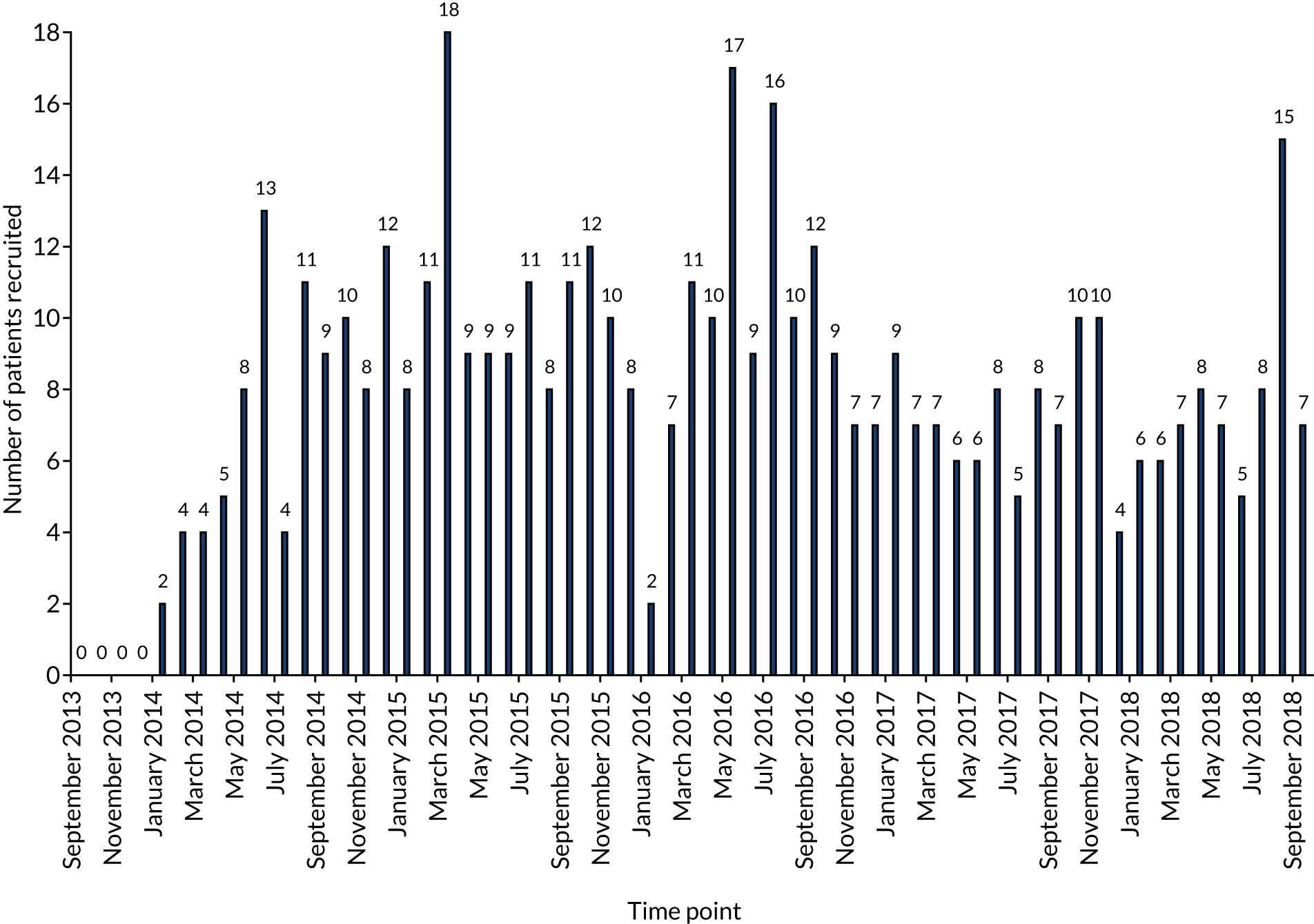
Recruitment curve
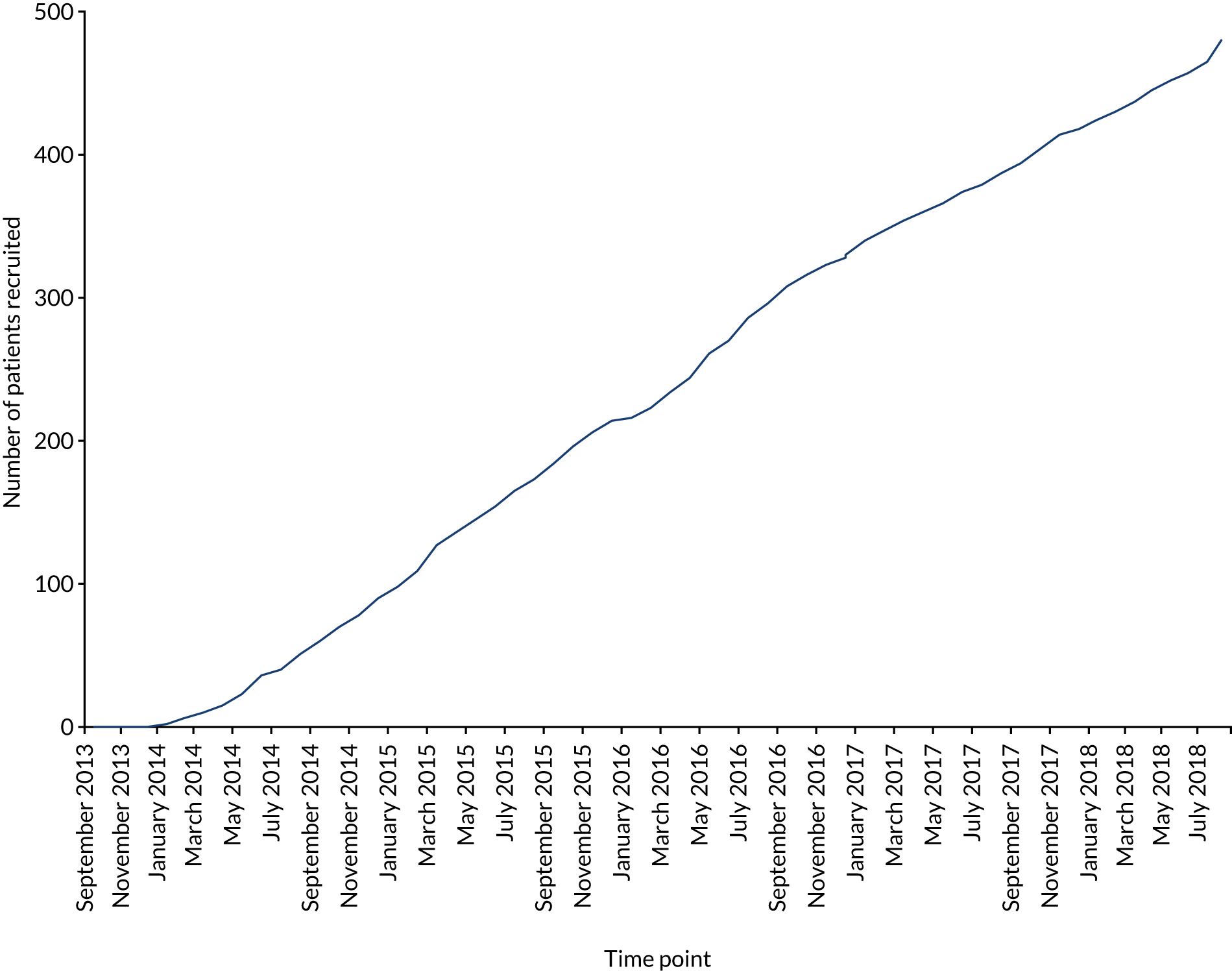
List of abbreviations
- ABT
- allogenic blood transfusion
- ACD
- anaemia of chronic disease
- AE
- adverse event
- AID
- absolute iron deficiency
- AKI
- acute kidney injury
- ANCOVA
- analysis of covariance
- AR
- adverse reaction
- CD
- Clavien–Dindo
- CI
- confidence interval
- CRF
- case report form
- CRP
- C-reactive protein
- CSV
- comma-separated values
- CTU
- Clinical Trials Unit
- DAOH
- days alive and out of hospital
- DSMC
- Data Safety and Monitoring Committee
- ECG
- electrocardiogram
- eCRF
- electronic case report form
- eGFR
- estimated glomerular filtration rate
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FBC
- full blood count
- FFP
- fresh-frozen plasma
- FID
- functional iron deficiency
- Hb
- haemoglobin
- HRQoL
- health-related quality of life
- HRU
- health resources used
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- IDA
- iron-deficiency anaemia
- IMP
- investigational medicinal product
- IQR
- interquartile range
- IVICA
- Intravenous Iron in Colorectal Cancer Associated Anaemia
- LFT
- liver function test
- LOS
- length of stay
- LRT
- likelihood ratio test
- LSHTM
- London School of Hygiene & Tropical Medicine
- MFI
- Multidimensional Fatigue Inventory
- NICE
- National Institute for Health and Care Excellence
- PI
- principal investigator
- PIN
- personal identification number
- PMG
- Project Management Group
- POMS
- Post-Operative Morbidity Survey
- PREVENTT
- preoperative intravenous iron to treat anaemia in major surgery
- QALY
- quality-adjusted life-year
- RBC
- red blood cell
- RCT
- randomised control trial
- RR
- risk ratio
- SAE
- serious adverse event
- SD
- standard deviation
- SmPC
- summary of product characteristics
- SOP
- standard operating procedure
- SQOM
- single question outcome measure
- SUSAR
- suspected unexpected serious adverse reaction
- TSAT
- transferrin saturation
- TSC
- Trial Steering Committee
- UE
- urea and electrolytes
