Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/127/126. The contractual start date was in March 2014. The draft report began editorial review in August 2019 and was accepted for publication in July 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Perkins et al. This work was produced by Perkins et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Perkins et al
Chapter 1 Introduction
Description of condition
Out-of-hospital cardiac arrest (OHCA) is defined as the loss of functional cardiac mechanical activity, in association with an absence of systemic circulation, occurring outside a hospital setting. 1
The majority of OHCA events result from cardiac causes such as ischaemic heart disease, myocardial infarction and rhythm disturbances. Other causes of OHCA include trauma, submersion, drug overdose, asphyxia, exsanguination or other medical causes such as stroke or pulmonary embolism. 2,3
Cardiac arrest occurs through three different mechanisms: (1) lethal arrhythmias [ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT)] leading to loss of cardiac output, (2) insufficient myocardial contraction to produce cardiac output [pulseless electrical activity (PEA)] and (3) complete failure of the electrical conduction system of the heart (asystole).
Manifestation of OHCA is dramatic: blood supply to the brain and vital organs ceases within seconds, the patient loses consciousness and the process of cell death commences. The window of opportunity to achieve return of spontaneous circulation (ROSC) is very narrow: a matter of minutes. Delays in attempts to restart the heart have catastrophic consequences, increasing the likelihood of death or severe neurological injury. Prolonged duration of resuscitation attempts is associated with poor outcomes.
The Department of Health and Social Care’s Cardiovascular Disease Outcomes Strategy: Improving Outcomes for People With or at Risk of Cardiovascular Disease,4 published in 2013, estimated that an increase in the survival rate from OHCA in England of between 10% and 11% could save > 1000 lives each year. Resuscitation to Recovery: A National Framework to Improve Care of People with Out-of-Hospital Cardiac Arrest (OHCA) in England,5 was published in 2017, providing a consensus on the optimal pathway for OHCA in England. Research to improve understanding of resuscitation from OHCA was identified as a national priority. Similar initiatives have been published in the devolved nations. 6–8
Chain of survival
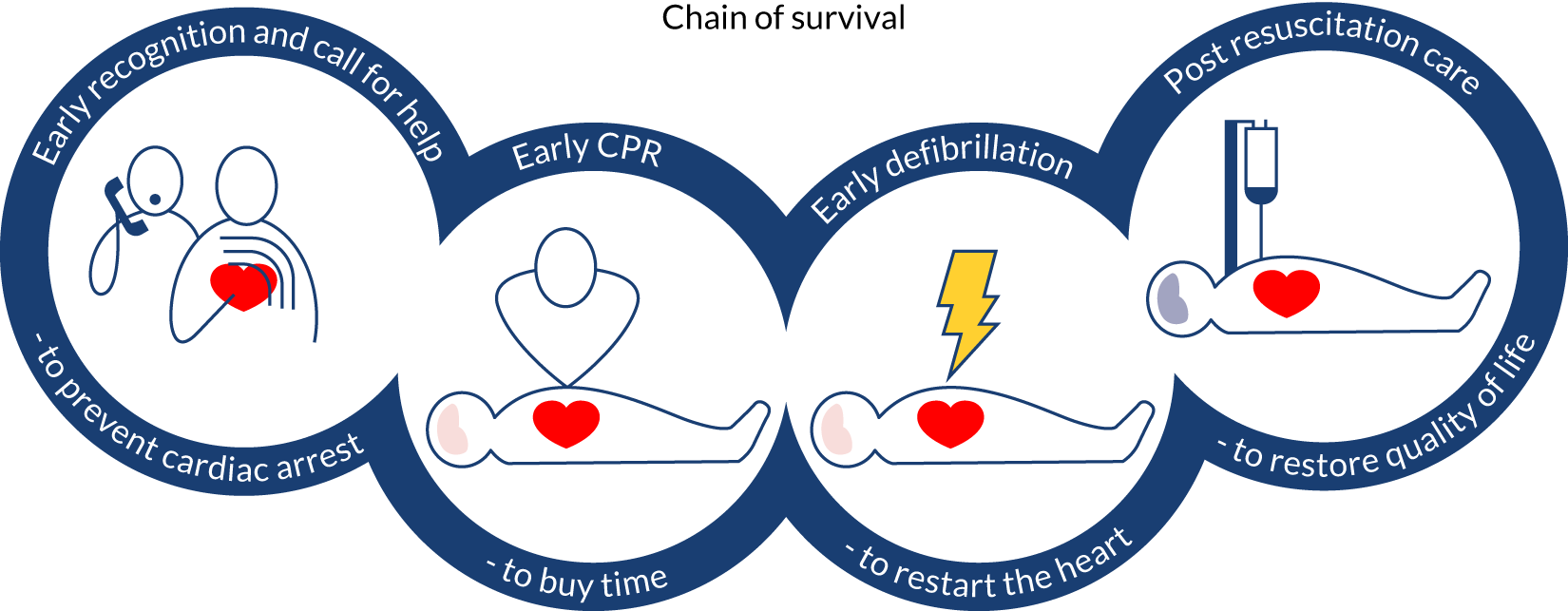
The chain of survival9 concept (Figure 1) is recognised internationally and summarises the key components of the response to OHCA to optimise the chances of survival. The links in the chain are discussed in more detail in the following sections.
FIGURE 1.
The chain of survival. 9 CPR, cardiopulmonary resuscitation. Reprinted from Resuscitation, Vol. 71, Nolan J, Soar J, Eikeland H, The chain of survival, pp. 270–1, Copyright 2006, with permission from Elsevier.
Early access
The first link in the chain highlights that it is important to identify a patient at risk of cardiac arrest (e.g. someone with an acute coronary syndrome) or a patient who has suffered a cardiac arrest (signs of which are loss of consciousness and absence of normal breathing). Rapid identification and calling for help early allow the ambulance service to send a trained advanced life support team to them as quickly as possible.
Raising public awareness of OHCA and the steps members of the public can take to increase chances of survival [calling 999 immediately and commencing cardiopulmonary resuscitation (CPR)] are the subject of major campaigns led by the British Heart Foundation and Resuscitation Council (UK), voluntary aid societies such as St John Ambulance and British Red Cross, and NHS ambulance services. World ‘Restart a Heart’ Day is an annual initiative that aims to train as many people as possible in CPR in 1 day. 10 NHS England has led work on improving ambulance call-taker’s recognition of life-threatening emergencies such as cardiac arrest, based on information provided by the person calling 999/111, as a component of the Ambulance Response Programme. 11 Ambulance telephone triage using NHS Pathways to identify OHCA accurately identifies 75% of adult OHCA {sensitivity 0.759 [95% confidence interval (CI) 0.473 to 0.773], specificity 0.986 (95% CI 0.9858 to 0.98647), positive predictive value 26.80% (95% CI 25.88 to 27.73)}. 12 This facilitates despatch of an ambulance response with the highest priority, and the provision of advice and support to the caller on how to perform CPR pending arrival of trained personnel. In other countries, such as Singapore, a comprehensive programme of ambulance dispatcher telephone support was associated with a doubling in bystander CPR rates. 13 When the ambulance service is aware of a nearby defibrillator, there is an opportunity to direct the caller and/or another responder to retrieve this, provided this does not delay or interrupt bystander CPR (see High-quality cardiopulmonary resuscitation).
High-quality cardiopulmonary resuscitation
Cardiopulmonary resuscitation is the combination of chest compressions and ventilations, and is optimally started by those initially at the scene of the collapse (bystander CPR). Bystander CPR increases the odds of survival by 1.23 (95% CI 0.71 to 2.11) in the studies with the highest baseline survival rates, to 5.01 (95% CI 2.57 to 9.78) in the studies with the lowest baseline rates. 14 When the emergency medical services (EMS) arrive on scene, they will take over CPR. Current resuscitation guidelines highlight the importance of high-quality CPR for ensuring optimal outcomes from cardiac arrest. 15 High-quality CPR is CPR that ensures an adequate chest compression depth is achieved (5–6 cm), that the compression rate is 100–120 per minute, that interruptions are minimised (for rhythm check/defibrillation and during extrication) and that the chest is allowed to recoil between chest compressions.
There are no randomised trials evaluating different compression parameters. Nevertheless, high-quality CPR appears to be important for outcomes. 16 Experimental studies show a linear increase in cardiac output and coronary perfusion pressure with increasing compression depths. 17,18 Observational studies in humans found improved defibrillator shock success19 and trends towards better ROSC rates and long-term survival with deeper chest compressions. 20 Faster chest compression rates (> 100 per minute) are associated with improved survival21,22 and ensuring that the chest is allowed to recoil between sequential chest compressions also appears to be important. 23
Interruptions in CPR are harmful. 24 A particularly critical time to minimise interruptions to CPR is around the time of attempted defibrillation. Prolonged pre-shock and peri-shock interruptions in CPR reduce the chances of shock success19 and survival. 25
Mechanical chest compression has not been shown in randomised trials to improve the outcome of OHCA. 26
Early defibrillation
Approximately one-quarter of OHCAs in the UK are due to an arrhythmia either VF or pulseless VT. These rhythms are referred to as shockable rhythms, as the arrhythmias may be terminated and cardiac function restored by the successful delivery of defibrillator shocks. The time from the onset of VF/VT to the delivery of a shock is critical to shock success and the chances of survival. For every 60–90 seconds that a shock is delayed, the chances of survival fall by approximately 10%. 27
If a defibrillator is immediately available at the scene of a cardiac arrest, defibrillation should be attempted without delay. When there is a delay in applying a defibrillator, there is a theoretical rationale that providing CPR before a shock improves coronary perfusion and, therefore, the chances of achieving sustained ROSC. 28
This concept was evaluated by the Resuscitation Outcomes Consortium (ROC) in a cluster randomised trial comparing early analysis (30–60 seconds of EMS-administered CPR before initial rhythm analysis) with later analysis (180 seconds of CPR before the initial electrocardiographic analysis). 29 The primary outcome was survival to hospital discharge with satisfactory functional status [a modified Rankin Scale (mRS) score of ≤ 3, on a scale of 0–6, with higher scores indicating greater disability]. The trial enrolled 9933 patients (5290 to early analysis and 4643 to late analysis) but found no difference in outcomes (cluster-adjusted difference of –0.2%, 95% CI –1.1% to 0.7%).
Public access defibrillation (PAD) has the potential to improve outcomes of OHCA. A national scheme led by the Department of Health and Social Care was launched in England in 1999 and was focused on busy public places such as railway stations. 30 ROSC was reported for 170 out of 437 (39%) patients, and hospital discharge was reported for 113 out of 437 (26%) patients. 31 A systematic review of observational studies reported an overall median survival of 40% (range 9–83.3%) in OHCA patients treated by PAD, with defibrillation by bystanders associated with median survival rates of 53% (range 26–72%). 32 In a further systematic review, bystander automated external defibrillator (AED) use was associated with survival to hospital discharge [odds ratio (OR) 1.66, 95% CI 1.54 to 1.79] and favourable neurological outcome (OR 2.37, 95% CI 1.58 to 3.57) in patients with shockable rhythms. However, the quality of the evidence quality was deemed to be low to very low. 33
Public access defibrillators are underutilised in OHCA. A retrospective review of OHCA in Hampshire reported that callers had access to an AED in 44 (4.2%) OHCA cases, and that AEDs were used before ambulance arrival in only 18 (1.7%) cases. 34 A systematic review of barriers to and facilitators of the use of PADs identified a range of themes, including knowledge and awareness, willingness to use, acquisition and maintenance, availability and accessibility, training issues, registration and regulation, medicolegal issues, EMS dispatch-assisted use of AEDs, AED locator systems, demographic factors, and other behavioural factors. The quality of the evidence was deemed to be very low. 35 Deakin et al. 36 recently mapped 4012 OHCAs to 2076 AEDs known to the South Central Ambulance Service, and reported that only 5.9% of the AEDs were within a 100 m radius of OHCA locations during daytime, falling to 1.59% during out of hours.
Adrenaline
Treatment with adrenaline has been an integral component of advanced life support from the birth of modern CPR in the early 1960s. In guidelines written originally in 1961, Safar37 recommended the use of adrenaline: 1 mg intravenously or 0.5 mg intracardiac. Adrenaline has been recommended in successive guidelines from around the world. An analysis of drug use across 264 EMS agencies in the USA and Canada reported that 81% (range 57–98%) of patients experiencing an OHCA received adrenaline. 38 The Out-of-Hospital Cardiac Arrest Outcomes (OHCAO) registry reported that 77.5% of patients in England and Wales received adrenaline as part of their treatment for OHCA. 39
Animal studies show that injection of adrenaline during cardiac arrest increases aortic tone, thereby augmenting coronary blood flow. 40,41 However, there are limited reliable data to assess the effects of adrenaline on long-term outcomes after cardiac arrest. The International Liaison Committee on Resuscitation (ILCOR) synthesised the available evidence for adrenaline in 2010 (also reassessed in October 2012) and noted that, although adrenaline may improve the rate of ROSC and short-term survival, there is insufficient evidence to suggest that adrenaline improves survival to discharge from hospital and neurological outcome. ILCOR stated that placebo-controlled trials to evaluate the use of any vasopressor in adult and paediatric cardiac arrest are needed. 42
Post-resuscitation care
The ROSC marks the start of the post-resuscitation care phase of treatment. 43 Unless the arrest has been relatively brief, most patients who achieve ROSC will have an obtunded consciousness level, necessitating admission to intensive care. The focus of the post-resuscitation care phase of treatment is stabilising cardiac function to prevent further cardiac arrest and minimising the consequences of the cardiac arrest on neurological outcome. This involves the use of targeted temperature management, the avoidance of hyperglycaemia and coronary angiography to guide coronary reperfusion, if required. Post-resuscitation care treatments are initiated by ambulance clinicians and continue after the patient’s arrival in the emergency department (ED) and in the intensive care unit (ICU). Patients with ROSC who have evidence of ST-segment elevation on a 12-lead electrocardiogram (ECG) are transferred directly to the cardiac catheter laboratory for urgent angiography revascularisation, if appropriate, in accordance with National Institute for Health and Care Excellence (NICE) guidance. 44 A recent randomised clinical trial suggested no benefit for urgent angiography in post-cardiac arrest patients without ST-segment elevation on a 12-lead ECG,45 but other trials evaluating the role of urgent coronary angiography in these patients are ongoing. 46–51
Incidence and burden of disease
The societal burden of OHCA has been described as equal to or greater than that of other leading causes of death. 52 Each year, an estimated 275,000 people in Europe experience an OHCA, with < 30,000 surviving to hospital discharge. 53 The UK OHCA outcome project, a prospective observational study involving all UK NHS ambulance services, reported that 28,729 patients were treated for OHCA by the 10 ambulance services in England in 2014 (53/100,000 population), with 7.9% surviving to hospital discharge. 2 Globally, estimates of OHCA outcomes vary across countries, with survival to hospital discharge rates of 7.6% in Europe, 6.8% in North America, 3% in Asia and 9.7% in Australia. 54
Out-of-hospital cardiac arrest exerts a major burden on NHS resources (ambulance response, emergency treatment, post-resuscitation care, rehabilitation) and years of life lost, but treatment currently has a low chance of success.
Existing evidence
A Cochrane review55 identified a single randomised, placebo-controlled trial of intravenous (i.v.) adrenaline in OHCA (the search was conducted in December 2012). The Pre-hospital Adrenaline for Cardiac Arrest (PACA) trial,56 conducted by our co-investigators Judith Finn and Ian G Jacobs, was undertaken in Western Australia. The study aimed to enrol 5000 patients, but, at the time the study closed, only 601 patients had been randomised. The relatively small numbers led to the results having large uncertainty. The rate of ROSC (short-term survival) was higher in those receiving adrenaline [64/272 (23.5%) vs. 22/262 (8.4%) patients; OR 3.4, 95% CI 2.0 to 5.6], but there was no clear evidence of a benefit in survival to hospital discharge (long-term survival) [adrenaline arm, 11 (4.0%) patients, vs. placebo arm, 5 (1.9%) patients; OR 2.2, 95% CI 0.7 to 6.3]. Two of the survivors in the adrenaline arm, but none in the placebo arm, had a poor neurological outcome. In addition to the trial’s imprecision, interpretation of the findings is limited by a large number of post-randomisation exclusions (n = 67, 11%).
A second randomised study, conducted in Oslo, Norway, compared i.v. cannulation and injection of drugs (including adrenaline) with no i.v. cannula or drugs among 851 patients experiencing an OHCA. 57 The patients in the i.v. arm had better short-term survival rates [ROSC: i.v. arm, 165/418 (40%), vs. no i.v. arm, 107/433 (25%); OR 1.99, 95% CI 1.48 to 2.67]; however, there was no clear difference between arms in long-term survival outcomes {survival to hospital discharge: i.v. arm, 44/418 (10.5%), vs. no i.v. arm, 40/433 (9.2%); OR 1.16, 95% CI 0.74 to 1.82; favourable neurological outcome [measured using the Cerebral Performance Category (CPC) 1 or 2]: i.v. arm, 9.8%, vs. no i.v. arm, 8.1%; OR 1.24, 95% CI 0.77 to 1.98}. The increase in the rate of ROSC was seen mainly in the patients with initial non-shockable rhythms (asystole and PEA): 29% in the i.v. arm versus 11% in the no i.v. arm. The rate of ROSC was 59% in the i.v. arm, compared with 53% in the no i.v. arm, in those patients with an initial rhythm of VF/VT.
In the post hoc analysis of the i.v. versus no-i.v. trial, outcomes were examined according to whether or not a patient had actually received adrenaline. 58 Treatment with adrenaline (n = 367) was associated with a greater chance of being admitted to hospital (OR 2.5, 95% CI 1.9 to 3.4). However, long-term survival outcomes were worse, with reduced survival to hospital discharge [adrenaline arm, 24/367 (7%), vs. no adrenaline arm, 60/481 (13%); OR 0.5, 95% CI 0.3 to 0.8] and reduced neurologically intact (CPC 1 or 2) survival [adrenaline arm, 19/367 (5%), vs. no adrenaline arm, 57/481 (11%); OR 0.4, 95% CI 0.2 to 0.7]. These effects persisted after adjustment for confounding factors (VF, response interval, witnessed arrest, sex, age and tracheal intubation).
At the time of developing the Pre-hospital Assessment of the Role of Adrenaline Measuring the Effectiveness of Drug administration In Cardiac arrest 2 (PARAMEDIC2) trial, three large observational studies59–61 suggested that adrenaline may cause worse long-term outcomes. The largest observational study of adrenaline use in cardiac arrest involves 417,188 OHCAs in Japan. 59 In propensity-matched patients, use of adrenaline was associated with an increased rate of ROSC [adjusted odds ratio (aOR) 2.51, 95% CI 2.24 to 2.80], but was also associated with a 1-month survival rate of approximately half that achieved among those not given adrenaline (aOR 0.54, 95% CI 0.43 to 0.68). In another observational study from the Osaka group in Japan,60 1013 (32.0%) of 3161 patients who were analysed received adrenaline. Those patients receiving adrenaline had a significantly lower rate of neurologically intact (CPC 1 or 2) 1-month survival than those not receiving adrenaline (4.1% vs. 6.1%, respectively; OR 0.69, 95% CI 0.48 to 0.98).
An analysis of registry data had shown reduced survival in those who received adrenaline; The North American ROC Epistry (n = 16,000) found an inverse association between adrenaline dose and survival to discharge (survival was > 20% for those not requiring adrenaline, and fell to < 5% for those requiring more than two doses). This finding persisted after adjustment for age, sex, EMS-witnessed arrest, bystander-witnessed arrest, bystander CPR, shockable initial rhythm, time from 911 call to EMS arrival, the duration of OHCA and study site. 38 This was similar to a previous analysis of the Swedish Registry (n = 10,000 patients; OR of long-term survival 0.43, 95% CI 0.27 to 0.66). 61
This creates the paradox of better short-term survival at the cost of worse long-term outcomes, in other words a ‘double-edged sword’. 62 However, observational studies are limited by the influence of confounding variables that may introduce bias, in particular the phenomenon known as resuscitation time bias, whereby an exposure is more likely to occur the longer the cardiac arrest continues. Because duration of resuscitation is strongly associated with worse outcome, this will bias the results towards a harmful effect of the exposure. 63 The importance of differences in analytical approaches and the way they control for resuscitation time bias is illustrated by the discordant findings from the analysis of the same data set, whereby one study shows benefit64 and the other demonstrates harm. 59
Mechanisms by which adrenaline may cause harm
There are a number of mechanisms by which adrenaline may cause harm. These can be considered under the following headings.
Reduced microvascular blood flow and exacerbation of cerebral injury
In animal models of cardiac arrest, adrenaline increases coronary perfusion pressure (which predicts restarting the heart), but impairs macrovascular and microvascular cerebral blood flow. Specifically, adrenaline was noted to reduce carotid blood flow65 and microvascular blood flow,29 causing worsening cerebral ischaemia. 66
Cardiovascular toxicity
In a further analysis of the Norwegian i.v. versus no i.v. trial,57 adrenaline increased the frequency of transitions from PEA to ROSC and extended the time window for ROSC, but at a cost of greater cardiovascular instability after ROSC, with a higher rate of re-arresting. These observations were consistent with other studies that linked adrenaline with ventricular arrhythmias and increased post-ROSC myocardial dysfunction. 67 In human studies with patients with sepsis68 or acute lung injury,69 beta-agonist stimulation was similarly linked to cardiovascular instability and reduced survival. 70 A systematic review of beta-blocker treatment in animal models of cardiac arrest found that fewer shocks were required for defibrillation; that myocardial oxygen demand was reduced; and that post-resuscitation myocardial stability improved, with less arrhythmia and improved survival. 71
Metabolic effects
Adrenaline causes lactic acidosis,72 which is associated with poor outcomes after cardiac arrest. 73,74 It also induces stress hyperglycaemia, which is also associated with poorer outcomes. 75
Immunomodulation and predisposition to infection
Infective complications, including bacteraemia and early-onset pneumonia, are common after OHCA, and are associated with worse outcomes. 76 The immune-modulatory effects of beta agonists have been well characterised and may reduce host defence against infection,77 which may contribute to an increased susceptibility to post-resuscitation sepsis.
Summary of effects
Use of adrenaline in cardiac arrest increases short-term survival (i.e. ROSC), but doubt remained about whether or not this translated into better long-term outcomes.
Rationale for intervention
Whether or not the practice of giving adrenaline is effective remained an important question that needed to be answered. Uncertainty about adrenaline has been raised by recent evidence59–61 suggesting that it may be harmful. Resolving this uncertainty was urgent, as adrenaline is used widely to treat cardiac arrests, and, if harmful, may be responsible for many avoidable deaths. There have been several precedents whereby treatments have been evaluated after years or decades of use and had been found to be ineffective or harmful, including pulmonary artery catheters in intensive care,78 beta agonists for acute respiratory distress syndrome69 and corticosteroids for head injury. 79 It was therefore possible that adrenaline for cardiac arrest might be a similar case.
The ILCOR appraised the evidence surrounding adrenaline use for OHCA in 201042 and again in October 2012. It concluded that there was an urgent need for randomised, placebo-controlled trials of adrenaline.
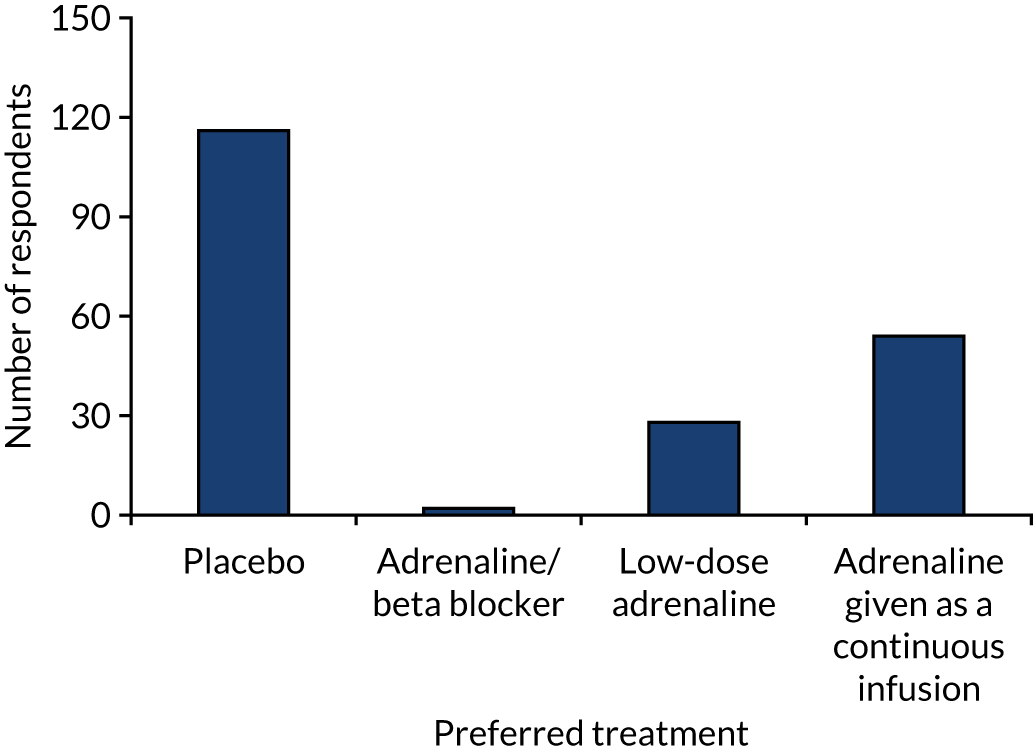
We conducted a written survey of 213 attendees (doctors, nurses, paramedics) of the Resuscitation Council (UK) Annual Scientific Symposium in September 2012 to assess the scientific and clinical communities’ current perspectives on the role of adrenaline in the treatment of cardiac arrest. Respondents expressed their agreement to a series of statements on a seven-point Likert scale (1 = strongly disagree, 7 = strongly agree). Respondents reported that adrenaline increased short-term survival [median score 6, interquartile range (IQR) 6–7], but disagreed that it improved long-term outcomes [median score 2 (IQR 2–3)]. The greatest uncertainty was around the balance of risks and the benefits of i.v. adrenaline (Figure 2). Respondents felt that the most pressing future research need for the NHS was a trial comparing adrenaline with placebo (Figure 3).
FIGURE 2.
Perspectives of the UK clinical community on the role of adrenaline for the treatment of cardiac arrest. 80 Reprinted from Resuscitation, Vol. 108, Perkins GD, Quinn T, Deakin CD, Nolan JP, Lall R, Slowther A, et al. , Pre-hospital Assessment of the Role of Adrenaline Measuring the Effectiveness of Drug administration In Cardiac arrest (PARAMEDIC-2): trial protocol, pp. 75–81, Copyright 2016, with permission from Elsevier.
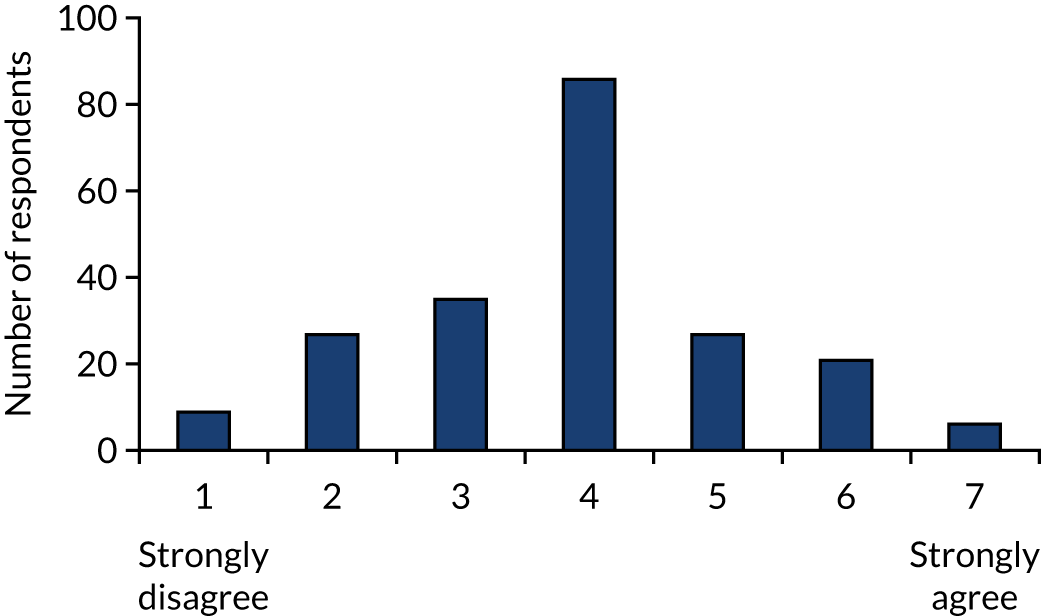
FIGURE 3.
Preferences of the UK clinical community. 80 Reprinted from Resuscitation, Vol. 108, Perkins GD, Quinn T, Deakin CD, Nolan JP, Lall R, Slowther A, et al. , Pre-hospital Assessment of the Role of Adrenaline Measuring the Effectiveness of Drug administration In Cardiac arrest (PARAMEDIC-2): trial protocol, pp. 75–81, Copyright 2016, with permission from Elsevier.
A trial addressing this question was timely, because of the recent publication of studies questioning the effectiveness of adrenaline, and calls for a large-scale randomised controlled trials to resolve this issue. There were no other completed, ongoing or planned trials in the ClinicalTrials.gov [https://clinicaltrials.gov/ (1 January 2012)] or controlled-trials.com (accessed 2012) databases. Moreover, research projects [e.g. the Prehospital Randomised Assessment of a Mechanical compression Device In Cardiac arrest (PaRAMeDIC) trial81 in 2010] had shown the feasibility of conducting large-scale OHCA trials in the UK. The learning from the PaRAMeDIC trial,81 undertaken by this group, helped to ensure efficient and successful recruitment.
The emerging data suggested that a number of experimental strategies could be considered, including comparing adrenaline with alpha 2 agonists, comparing adrenaline with beta blockade, lower-dose adrenaline or adrenaline as a continuous infusion. Preferences of the clinical community are summarised in Figure 3. The timing of adrenaline administration may also be important; however, this was primarily dependent on ambulance response times, which were difficult to control for in a randomised trial. We suggested that the most pressing need was for a definitive trial comparing standard-dose adrenaline (1 mg every 3–5 minutes) with placebo. Until there was clarity about the effect of adrenaline on long-term outcomes, the best comparator agent (placebo or standard-dose adrenaline) for trials of other agents remained unknown.
This randomised controlled trial of adrenaline had the support of key stakeholders, such as patient representatives, the College of Paramedics, the National Ambulance Services Medical Directors’ Group, the Joint Royal Colleges Ambulance Liaison Committee (JRCALC), the Resuscitation Council (UK) and the British Heart Foundation.
Objectives
Primary objective
The primary objective of this trial was to determine the clinical effectiveness of adrenaline in the treatment of OHCA, measured as a primary outcome of 30-day survival.
Secondary objective
The secondary objectives of the trial were to evaluate the effects of adrenaline on survival and on the cognitive and neurological outcomes of survivors, and to establish the cost-effectiveness of using adrenaline.
Chapter 2 Methods
Trial design
This was a pragmatic, randomised, allocation-concealed, placebo-controlled, parallel-group superiority trial and economic evaluation. Participants were randomised to either adrenaline (intervention) or placebo (control) in a 1 : 1 allocation ratio. Randomisation took place when a trial-trained paramedic opened an Investigational Medicinal Product (IMP) pack, which contained either 10 syringes of adrenaline (1 mg) or a matching placebo (0.9% saline). The primary outcome was survival to 30 days. Secondary outcomes focused on patient, clinical, resource and economic outcomes. Patients, clinical teams and those assessing patient outcomes were masked to the treatment allocation.
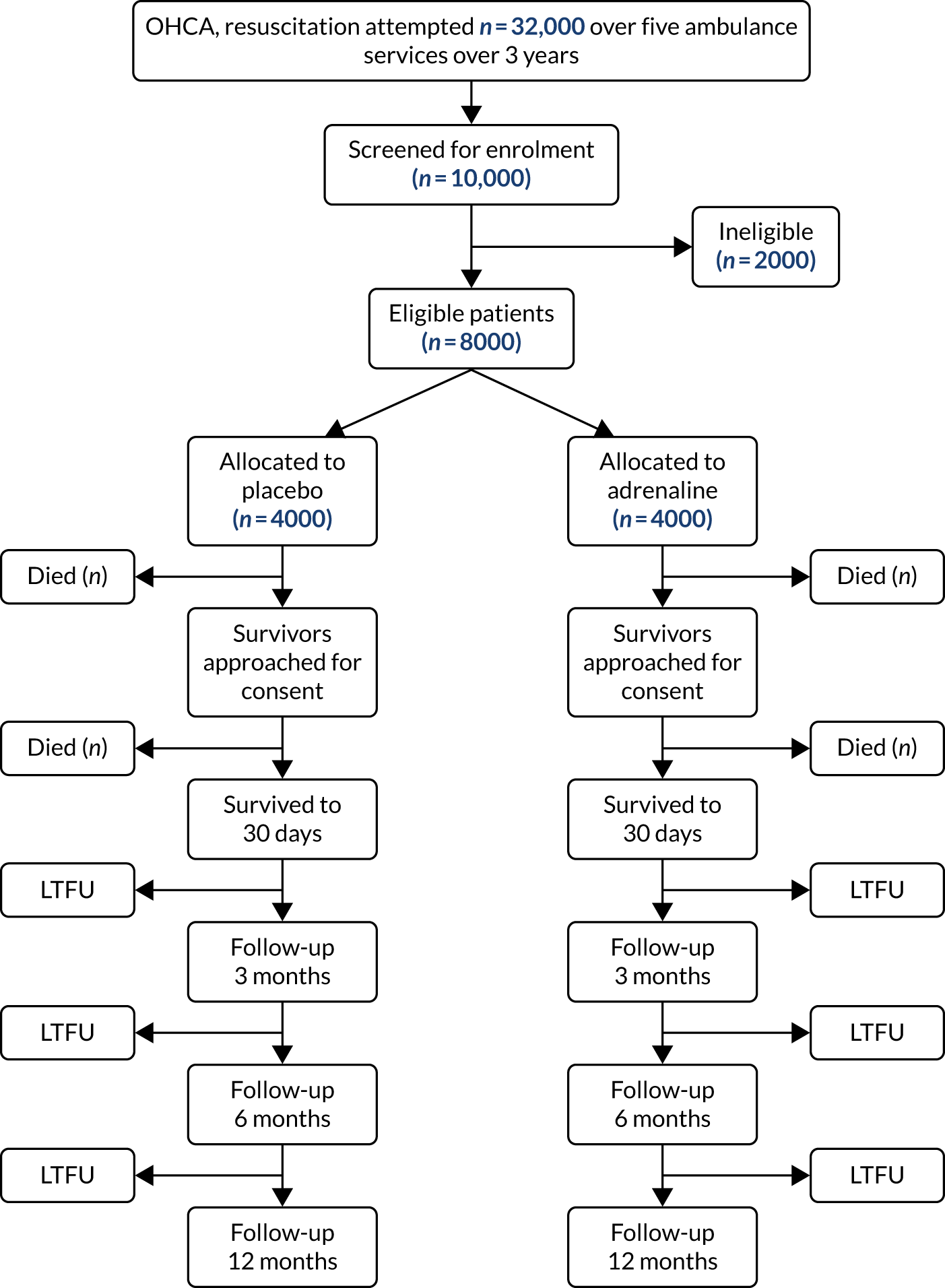
Figure 4 shows the planned flow of participants through the trial.
FIGURE 4.
Flow chart for PARAMEDIC-2 trial. 80 LTFU, lost to follow-up. Reprinted from Resuscitation, Vol. 108, Perkins GD, Quinn T, Deakin CD, Nolan JP, Lall R, Slowther A, et al. , Pre-hospital Assessment of the Role of Adrenaline Measuring the Effectiveness of Drug administration In Cardiac arrest (PARAMEDIC-2): trial protocol, pp. 75–81, Copyright 2016, with permission from Elsevier.
Pilot trial
An internal pilot was run to test that the components of this trial worked together. This pilot ran for 6 months. The data from this were included in the main trial. During the pilot, we measured recruitment rate and compliance with the allocated intervention, and checked that the approach to data collection and follow-up worked effectively. The pilot phase ran seamlessly into the main trial. The results of the pilot trial were reviewed with the Trial Steering Committee (TSC), the Data Monitoring Committee (DMC) and representatives from the Health Technology Assessment (HTA) programme, specifically considering the achievement of the following targets:
-
25% of ambulance staff trained [i.e. the majority (80%) of participating staff at 25% of stations]
-
181 patients recruited within 6 months of first randomisation
-
data available on primary outcome – > 98%
-
proportion of patients who are alive agreeing to follow–up – > 75%
-
reconcile IMP packs with participants enrolled in the trial
-
review of the approach to inform patients and relatives of trial participation
-
review of feasibility to collect secondary outcomes.
All pilot objectives were achieved, and the TSC approved continuation to the main phase of the trial on 7 May 2015.
Changes to trial design
There were no substantial changes to the trial design. Table 1 lists all amendments to the trial and details of the changes made. Reasons for changes to the trial design describes the reason for the change in exclusion criteria (amendment 6).
| Date of amendment | Number | Document(s) affected | Changes | New version number; date | Date approved by MHRA | Date approved by REC |
|---|---|---|---|---|---|---|
| 2 August 2018 | 21 | Unblinding response form | Unblinding response form updated | v2.0; 7 August 2018 | N/A | 14 August 2018 |
| Unblinding information sheet | Unblinding information sheet updated and separated into three versions (non-survivor, survivor with poor neurological outcome, and survivor with good neurological outcome) | v2.0; 2 August 2018 | ||||
| 25 July 2018 | 20 | Protocol |
|
v6.0; 25 July 2018 | N/A | 13 November 2018 |
| 26 March 2018 | 19 | Protocol |
|
v5.0; 28 March 2018 | N/A | 3 April 2018 |
| Unblinding information sheet | New document to be able to respond to requests for unblinding | v1.0; 28 March 2018 | ||||
| Unblinding response form | New document to be able to respond to requests for unblinding | v1.0; 28 March 2018 | ||||
| Unblinding cover letter for response form | New document to be able to respond to requests for unblinding | v1.0; 28 March 2018 | ||||
| Confirmation of treatment letter | New document to be able to respond to requests for unblinding | v1.0; 28 March 2018 | ||||
| 28 February 2018 | 18 | Protocol | Section 2.6.15.1 added to describe the process for responding to trial participation enquiries | v4.0; 28 February 2018 | N/A | 28 February 2018 |
| Cover letter (patient enrolled) | New document produced to be able to respond to trial participation enquiries | v1.0; 28 February 2018 | ||||
| Cover letter (patient not enrolled) | New document produced to be able to respond to trial participation enquiries | v1.0; 28 February 2018 | ||||
| Trial participation information sheet | New document produced to be able to respond to trial participation enquiries | v1.0; 28 February 2018 | ||||
| 13 March 2017 | 17 | N/A | Addition of hospital (Medway) | N/A | N/A | 17 March 2017 |
| 23 January 2017 | 16 | N/A | Addition of general practice surgeries as sites to allow mRS data collection under HRA approval | N/A | N/A | 23 January 2017 |
| 13 December 2016 | 15 | N/A | Addition of two hospital sites (Luton and Dunstable, and Bedford) | N/A | N/A | 20 December 2016 |
| 24 November 2016 | 14 | N/A | Translations and back translations of patient information sheet, consent forms and cover letters into Arabic, Gujarati, Hindi, Polish, Punjabi, Urdu and Welsh | N/A | N/A | 14 December 2016 |
| 2 November 2016 | 13 | GP letter (mRS) | New letters for GPs to collect mRS data (two versions – one for consent and one for passive follow-up) | v1.0; 1 November 2016 | N/A | 18 November 2016 |
| 21 October 2016 | 12 | Protocol | Addition of quality of CPR data collection | v3.0; 12 October 2016 | 27 October 2016 | 31 October 2016 |
| 1 June 2016 | 11 | Protocol |
|
v2.0; 4 May 2016 | N/A | 20 June 2016 |
| Patient information sheets |
|
v2.0; 8 April 2016 | ||||
| Patient information sheets cover letters | Separate versions created for patient and legal representative and approach in hospital or after hospital discharge | v2.0; 8 April 2016 | ||||
| Consent forms | Wording updated to include reference to Health and Social Care Information Centre (former name of NHS Digital) and Office for National Statistics | v2.0; 8 April 2016 (note that the legal representative consent form was amended to v2.1 because of a typographical error) | ||||
| 28 October 2015 | 10 | N/A | Addition of four hospitals (Burton Hospitals NHS Foundation Trust, Western Sussex Hospitals NHS Foundation Trust, University Hospitals Bristol and Weston NHS Foundation Trust, and Countess of Chester Hospital NHS Foundation Trust) | N/A | N/A | 6 November 2015 |
| 28 October 2015 | 9 | GP letter | GP letter about survival and mRS scores for non-responders | v1.0; October 2015 | N/A | 9 November 2015 |
| 8 September 2015 | 8 | IMP dossier | Revision of IMP dossier to extend IMP shelf life to 12 months | 4.0 | 15 October 2015 | 9 November 2015 |
| 10 July 2015 | 7 | N/A | Addition of Royal Surrey County Hospital | N/A | N/A | 23 July 2014 |
| 24 June 2015 | 6 | Protocol | Addition of ‘cardiac arrest secondary to life-threatening asthma’ as exclusion criterion | 1.1; 24 June 2015 | 13 July 2015 | 8 July 2015 |
| 11 May 2015 | 5 | Poster for public ‘10 Facts about the PARAMEDIC2 trial’ | New document to raise public awareness of trial | 1.0; 12 May 2015 | N/A | 22 May 2015 |
| Leaflet for general practice surgeries | New document to raise public awareness of trial | 1.0; 12 May 2015 | ||||
| Communication strategy | Revised document | 11 May 2015 | ||||
| 3 December 2014 | 4 | N/A | Clarification that prisoners would not be excluded from the trial | N/A | N/A | 4 December 2014 |
| 8 October 2014 | 3 | N/A | Addition of English hospitals | N/A | N/A | 8 October 2014 |
| 4 September 2014 | 2 | N/A | Addition of Welsh hospitals: Bronglais General Hospital, Glan Clwyd Hospital, Glangwili General Hospital, Morriston Hospital, Nevill Hall Hospital, Prince Charles Hospital, Princess of Wales Hospital, Prince Philip Hospital, Royal Glamorgan Hospital, Royal Gwent Hospital, University Hospital of Wales, Withybush General Hospital, Wrexham Maelor Hospital, Ysbyty Gwynedd, Singleton Hospital, West Wales General Hospital and Llandough Hospital | N/A | N/A | 9 September 2014 |
| 27 August 2014 | 1 | Patient information sheet | Updated following feedback from CAG |
|
N/A | 10 October 2014 |
| Consent forms (patient and legal representative) | Updated following feedback from CAG |
|
||||
| Cover letter (patient and legal representative) | Updated following feedback from CAG |
|
||||
| Poster for public ‘OK to Ask’ | New document to raise public awareness of trial |
|
||||
| REC form Part C | WAST principal investigator corrected to Nigel Rees | N/A |
Reasons for changes to the trial design
Amendment 4
This amendment updated the Research Ethics Committee (REC) application to confirm that participants who were prisoners or young offenders in the custody of Her Majesty’s Prison Service or who were offenders supervised by the probation service in England or Wales would be included in the trial. The rationale for not excluding prisoners was as follows:
-
There may be practical difficulties in identifying a prisoner in an emergency situation.
-
Attempting to establish if someone is a prisoner may lead to confusion and delay of treatment, which may cause harm.
-
Prisoners are not being recruited to the trial because of their position as prisoners, they are being recruited because of their interaction with the NHS in a cardiac arrest situation.
-
It would be unethical to deny prisoners the rights to the potential benefits of the trial.
Amendment 6
Cardiac arrest secondary to life-threatening asthma was added as a trial exclusion criterion following amendment 6 on 24 June 2015. Although the published literature points to there being equipoise about the use of i.v. adrenaline in asthma,82–85 there is evidence that adrenaline may be beneficial for patients with anaphylaxis. The rationale for the change was that, during the pilot trial, ambulance staff were concerned about potential overlap between the presentation of asthma and anaphylaxis (both may present with bronchospasm). Therefore, the Trial Management Group (TMG) felt that the safest option was to extend the exclusion for anaphylaxis to include cardiac arrests suspected to have been caused by asthma.
Eligibility criteria
Inclusion criteria
Patients were eligible if both the following criteria were met:
-
cardiac arrest in an out-of-hospital environment
-
advanced life support initiated and/or continued by an ambulance service clinician.
Exclusion criteria
The exclusion criteria at the time of arrest were as follows:
-
known or apparent pregnancy
-
known to be or apparently aged < 16 years
-
cardiac arrest caused by anaphylaxis or life-threatening asthma
-
adrenaline given prior to arrival of ambulance service clinician.
In London Ambulance Service, traumatic cardiac arrests were also excluded, in accordance with local protocols.
Trial setting
Recruitment took place in five NHS ambulance services in the UK (London Ambulance Service NHS Trust, North East Ambulance Service NHS Foundation Trust, South Central Ambulance Service NHS Foundation Trust, West Midlands Ambulance Service University NHS Foundation Trust and Welsh Ambulance Service NHS Trust). These ambulance services serve a mix of urban and rural locations in England and Wales, covering a population of 24 million people. They collectively attend ≈32,000 cases of cardiac arrest each year; resuscitation is attempted or continued by ambulance staff in approximately 45% of these cases.
The ambulance services are activated through a central emergency call number (999, 112 or 111), which directs the caller to the geographically relevant emergency operations and dispatch centre. Calls are received and processed by trained NHS ambulance dispatch staff. Dispatch staff use one of two dispatch support systems: NHS Pathways or Advanced Medical Priority Dispatch System. Cases identified as a cardiac arrest are assigned the highest priority response. At the time of the trial, ambulance services were expected to provide a defibrillation-capable response within 8 minutes for 75% of these cases. There was an expectation of having an ambulance on scene within 19 minutes in 95% of cases. A typical first response could be a community first responder, a rapid response vehicle (e.g. car, motorbike or bicycle) or an air/land ambulance. Clinically trained staff, such as paramedics or emergency medical technicians, typically attend cardiac arrests. They are often supported by emergency care assistants. Paramedics can deliver advanced life support (ALS) interventions (including advanced airway management and i.v. drugs) and, after trial-specific training, were able to recruit patients to the trial. Technicians, emergency care assistants and many community responders dispatched by the NHS ambulance service can deliver CPR and defibrillation, and some use supraglottic airways. When ambulance staff arrive at a cardiac arrest, one of the first steps is for them to assess the appropriateness of a full resuscitation attempt. If there is unequivocal evidence of death (major traumatic injuries, putrefaction, rigour mortis, post-mortem staining, etc.) NHS guidelines86 allow resuscitation to be withheld. Other situations in which resuscitation may be withheld are when a patient has a ‘do not attempt cardiopulmonary resuscitation’ instruction, if the patient is in asystole, if no bystander CPR has been performed or if > 15 minutes have elapsed since the time of collapse. 87 Ambulance services follow common national guidelines for resuscitation from the Resuscitation Council UK guidelines,86 which follow those provided by the European Resuscitation Council. 88 The overall UK rate of survival to discharge for all cases of OHCA for which resuscitation is attempted is 7.9%. 89
Trial intervention
Patients were enrolled in the trial by the attending ambulance service clinicians, who determined whether or not a resuscitation attempt was appropriate (according to the JRCALC guidelines87), and, if it was, whether or not the patient was eligible. Patients who met the eligibility criteria were randomised to the trial.
Patients received resuscitation according to the Resuscitation Council (UK) and JRCALC ALS guidelines. 86 All standard ALS interventions were provided, including chest compression, defibrillation and advanced airway management, as required, with the exception that standard adrenaline was substituted with trial IMP drawn from a single trial treatment pack. Vehicles also carried their standard supply of adrenaline, for use only with ineligible patients.
If the patient reached the point in the resuscitation protocol where pharmacological treatments were indicated, they were randomly assigned to receive either parenteral adrenaline or saline placebo by the opening of a trial drug pack. Each treatment pack contained 10 × 3-ml prefilled syringes, with each syringe containing either 1 mg of adrenaline (intervention) or 0.9% saline (control). All trial drug packs were labelled with a unique trial pack number and in accordance with EudraLex Volume 4 Annex 13 requirements. 90 The adrenaline and placebo packs and syringes were identical in appearance; therefore, clinicians, patients and trial personnel were unaware of whether any specific pack contained adrenaline or placebo.
Single doses of adrenaline or saline were administered every 3–5 minutes by an i.v. or intraosseous route. Clinicians were instructed to use only one treatment pack per patient (10 × 3-ml syringes). Treatments were continued until a sustained pulse was achieved, resuscitation was discontinued or care was handed over to the clinician at the receiving hospital.
Treatment after admission to hospital was not specified in the trial protocol, but was informed by national guidelines,91 which covered targeted temperature management, haemodynamic and ventilator criteria and prognostication.
Randomisation
As recruitment took place in an emergency situation, telephone or internet randomisation was impractical; therefore, the trial used a system of pre-randomised treatment packs. The trial IMP was packaged in numbered treatment packs. The pre-randomised sequence was prepared by the programmers at the Warwick Clinical Trials Unit (WCTU). The randomisation sequence was computer generated by the stratified randomisation method with concealed assignment, using ambulance service as a strata, with an allocation ratio of 1 : 1.
Treatment packs were supplied to each ambulance service in a central location, and were distributed from there to participating ambulance stations and vehicles. When ambulance service personnel identified an eligible patient, randomisation was achieved by opening one of the packs carried in the vehicle attending the arrest.
Post-randomisation withdrawals and exclusions
There were three main sources of post-randomisation withdrawal and exclusions. The first was when, between randomisation (opening the drug pack) and administering the trial intervention, a patient was identified as ineligible. Examples of this include when a patient achieved ROSC, when a patient was confirmed to have died or when a trial exclusion became known to the ambulance clinicians before drug administration. This group would not receive vasopressor drugs in clinical practice. No follow-up data were collected on this group of patients and they were identified in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram as post-randomisation exclusions.
The second situation is when, after drug administration, it subsequently became known that the patient was ineligible for the trial. An example of this would be discovering in hospital that the cause of the cardiac arrest was anaphylaxis. This group of patients would be exposed to the vasopressor drugs in clinical practice, as it is not until after drug administration that the exclusion is discovered. This group of patients were followed up in the normal manner and included in the intention-to-treat analysis.
The third scenario is when a patient or their legal representative withdrew their consent for ongoing data collection. This group of patients were eligible for and received the trial intervention, but incomplete data were obtained on their long-term outcomes. This group of patients were included in the intention-to-treat analysis, up until the point of withdrawal. The information sheet explained the trial and the data that were collected. The consent form separated out the different data that were collected, and gave the patient the option to decline. NHS records were continually used unless the patient explicitly refused permission for this, as were tracking of the patients via NHS Digital to determine survival to 12 months post cardiac arrest.
In the rare situation in which a patient had neither consented to nor refused follow-up, they were not included in the face-to-face follow-up, but data collection from NHS records and in-hospital data sets continued.
Blinding
Methods for ensuring blinding
The packaging and the labelling of the IMP packs did not reveal which IMP was being used; therefore, the patient, attending clinicians, hospital treating team, research paramedics and trial administration team were masked to treatment allocation. Only the statistician was able to link the IMP pack number to the allocation of adrenaline or placebo.
Methods for unblinding the trial
The chief investigator retained the right to break the code for serious adverse events (SAEs) that were unexpected and suspected to be causally related to an investigational product, and that potentially required expedited reporting to regulatory authorities. The chief investigator unblinded if requested to do so by a coroner as part of a death enquiry. In exceptional circumstances, the chief investigator also considered requests for unblinding from patients or, if they lacked capacity, family members/next of kin. In this scenario, the trial statisticians made the unblinding on the chief investigator’s request and the chief investigator was informed accordingly of the allocation. This occurred only if the request was made after the benefits and harms of disclosing this information to them had been discussed. Otherwise, treatment codes (IMP pack number) were broken only for the planned interim analyses of data by the statistician at the request of the DMC.
Methods for unblinding the trial: after trial completion
Requests for unblinding of treatment allocation were received (either from survivors or from the next of kin of trial participants who were deceased) after completion of the trial. When these requests were received, the PARAMEDIC2 trial office sent the enquirer (on behalf of the ambulance services) an information sheet about unblinding and a response form for completion if they wished to continue with unblinding after having read the information provided. Once a completed response form was received, requesting unblinding, the PARAMEDIC2 trial office responded, once checks had been performed to verify the case details, to confirm the treatment allocation on behalf of the ambulance services, with ambulance service contact details for further queries.
Outcome measures
Efficacy
Primary outcome
The primary outcome was survival to 30 days post cardiac arrest.
Secondary outcomes
-
Survived event (sustained ROSC, with spontaneous circulation until admission and transfer of care to medical staff at the receiving hospital).
-
Survival to hospital discharge (the point at which the patient is discharged from the hospital acute care unit, regardless of neurological status, outcome or destination) and to 3, 6 and 12 months.
-
Neurological outcome (mRS) at hospital discharge and at 3 and 6 months [assessed at discharge using the Rankin Focused Assessment (RFA), and completed at 3 and 6 months via the simplified modified Rankin Scale questionnaire (smRSq)].
-
Neurological outcomes [assessed using the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) and ‘Two Simple Questions’] at 3 and 6 months.
-
Health-related quality of life at 3 and 6 months [assessed using the Short Form questionnaire-12 items (SF-12) and the EuroQol-5 Dimensions, five-level version (EQ-5D-5L)].
-
Cognitive outcome at 3 months [assessed using the Mini Mental State Examination (MMSE)].
-
Anxiety and depression at 3 months [assessed using the Hospital Anxiety and Depression Scale (HADS)].
-
Post-traumatic stress at 3 months [Post-traumatic stress disorder Checklist – Civilian Version (PCL-C).
-
Hospital length of stay.
-
Intensive care unit length of stay.
The outcomes defined by the Utstein convention for reporting outcomes from cardiac arrest92 were reported.
Rationale for outcome measures
The mRS was administered at hospital discharge and at 3 and 6 months. The mRS was selected instead of the CPC as it is more sensitive to detecting mild cognitive impairment. It can be reliably extracted from medical records and is a predictor of long-term survival. There was emerging international consensus (Utstein 2012/1393) that the mRS should be the primary measure of neurological outcome in cardiac arrest trials. The mRS is a seven-point scale ranging from mRS 0 (no symptoms) to 6 (dead). The RFA was chosen as a framework for assessing the mRS score at discharge, as this could be completed using a variety of sources of information, such as a patient assessment, via relatives or hospital staff or using hospital notes, and has been shown to have high inter-rater reliability. 94 The smRSq95 was used to collect the mRS score at 3 and 6 months, as this could be easily self-completed by the patient or legal representative. The spectrum of impairment of health-related quality of life following cardiac arrest includes memory and cognitive dysfunction, affective disorders and post-traumatic stress disorder (PTSD). 96 The SF-12 is a standard quality-of-life measure that is short and easy to complete. In addition, the EuroQol-5 Dimensions (EQ-5D) questionnaire was used as a health utility measure for the health economic analysis. Cognitive function was assessed using the MMSE. 97 The IQCODE and the ‘Two Simple Questions’ tool98 formed supplementary assessments of cognitive function. The PCL-C99 is a 17-item self-administered questionnaire measuring the risk of developing PTSD and had been used in previous studies as a good surrogate for the clinical diagnosis of PTSD, which requires a face-to-face interview by a suitably trained professional. The HADS is a 14-item self-administered questionnaire that had been previously used successfully to measure affective disorders in cardiac arrest survivors. 100 Two of these measures (PCL-C and HADS) were used as part of a multicentre follow-up for people surviving a critical illness (Intensive Care Outcome Network study);101 the people from this study were used as a reference population.
Health economics
Primary economic outcome
The primary economic outcome was the incremental cost per quality-adjusted life-year (QALY) gained with use of adrenaline compared with the incremental cost per QALY gained with use of placebo from the perspective of the NHS and Personal Social Services (PSS) over a 6-month time horizon. QALYs were calculated using area-under-the-curve methods, assuming linear interpolation between baseline utility (set to zero) and utility values derived from a combination of mRS and EQ-5D-5L assessments at hospital discharge and at 3 and 6 months post randomisation.
Secondary economic outcomes
-
Incremental cost per QALY gained from the perspective of the NHS and PSS with 1-year and lifetime (via decision-analytic modelling) time horizons.
-
Incremental cost per unit increase in the proportion surviving to 6 months post cardiac arrest, estimated from an NHS/PSS perspective.
-
Incremental cost per unit increase in the proportion surviving with good neurological outcome at 6 months post cardiac arrest, estimated from an NHS/PSS perspective.
-
Cost of critical care stay, cost of hospital stay, use of NHS and PSS resources after discharge and broader resource use after discharge. Resource use and costs were estimated over the 6-month period from randomisation/cardiac arrest event onwards.
Sample size
Incidence of primary outcome
Most existing data refer to survival to hospital discharge rather than survival to 30 days, but as most mortality will occur in the first few days after cardiac arrest, we expect these two measures to be very similar. Estimates of long-term survival of patients who receive adrenaline during a resuscitation attempt vary between about 3.5% and 12%. From national data for England, overall survival to hospital discharge of patients for whom resuscitation is attempted is 7%. 89 However, this will include a small number of patients who achieve ROSC immediately and would not receive adrenaline, and hence would not be recruited to the trial. As these patients have much better outcomes, we expected that the survival among the trial population would be slightly lower. Estimates from the Norwegian trial of i.v. drugs and the Australian trial of adrenaline were 9%57 and 4%,56 respectively. We therefore expected a rate of survival to 30 days of ≈ 6% in the adrenaline group.
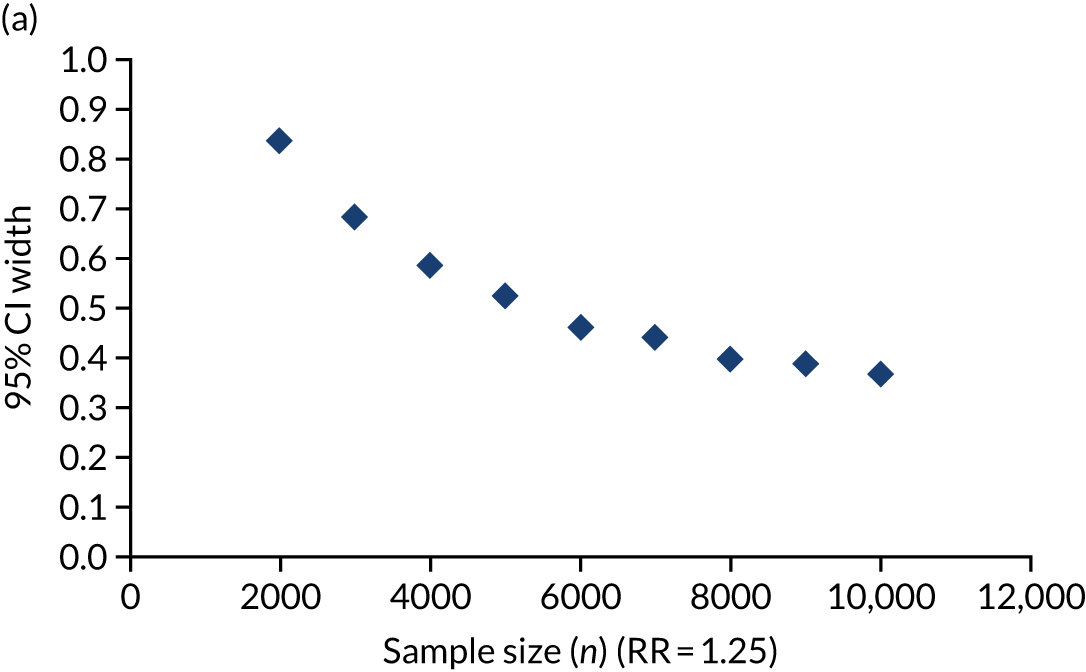
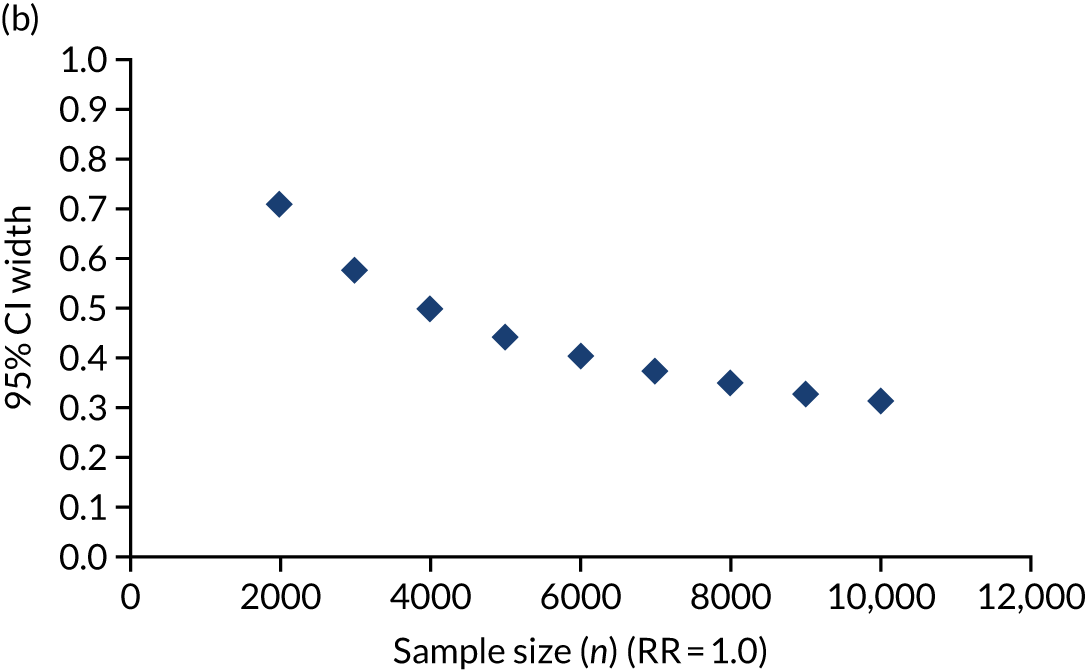
The trial’s primary aim was to estimate the treatment effect of adrenaline and the uncertainty around this; we therefore based the target sample size primarily on the precision of the estimate of the risk ratio (RR). 40 Figure 5 shows the precision that is achievable (width of the 95% CI for the RR) with different total sample sizes, for RRs (placebo vs. adrenaline) of 1.25 and 1.00. A RR of 1.25 corresponds to an increase in 30-day survival from 6% in the adrenaline group to 7.5% in the placebo group.
FIGURE 5.
Width of 95% CI for the RR against sample size. (a) RR 1.25; and (b) RR 1.0, with 6% survival in the adrenaline arm.
Sample size
The target sample size was 8000 participants, which was expected to give a width of the 95% CI for the RR of approximately 0.4 or slightly less; for a RR of 1.25, the 95% CI would be 1.07 to 1.46, and for a RR of 1.0 the 95% CI would be 0.84 to 1.19. There was a trade-off between precision and practicality in setting a target sample size at > 8000, there was only a small improvement in precision, but the difficulty and time needed to recruit this number increased significantly. We expected a very small number of missing data for survival outcomes. In the PaRAMeDIC trial,102 we ascertained survival status for > 99% of randomised patients; therefore, we have not adjusted the sample size estimates to account for missing data.
Using a conventional sample size calculation based on a significance test, a sample size of 8000 would have 93% power to achieve a statistically significant (p < 0.05) result if the true treatment difference is a RR of 1.33 (increase from 6% in the adrenaline group to 8% in the placebo group), or 75% power if the true treatment difference is a RR of 1.25 (increase from 6% in the adrenaline group to 7.5% in the placebo group).
Reconsideration of sample size after low survival rate
In July 2016, the DMC raised concerns regarding a lower than anticipated survival rate. We explored additional data sources and the current literature to assess if the original assumption was correct. Since the inception of the trial, we had access to two contemporary sources of information for the epidemiology and outcome of this patient group in the UK.
The first was a secondary analysis of the PaRAMeDIC trial. 102 The PaRAMeDIC trial102 enrolled adult patients experiencing OHCA in three English and the Welsh ambulance service regions. Patients were eligible for enrolment if they had an OHCA attended by a trial vehicle and resuscitation was continued by the EMS team. Patients who were pregnant or who sustained a traumatic cardiac arrest were excluded. We collected information on whether or not a patient received i.v. cardiac arrest medications per se, rather than specifically adrenaline. As resuscitation algorithms recommend that adrenaline and amiodarone (the only other recommended i.v. drug for cardiac arrest) are given together, we believed that this was a reasonable surrogate to the patient requiring i.v. adrenaline. For the PaRAMeDIC trial,102 this group comprised 3621 patients, with an overall 30-day survival rate of 2.8%.
The second source of data was the national OCHA registry (OHCAO) hosted by the University of Warwick. 103 This registry collects process and outcome information from all English ambulance services. Data were available covering the period from 2013 to 2015. These data were analysed for patients aged > 16 years who were given i.v. adrenaline (n = 28,939). In this group, 3.1% survived to discharge.
We extended the initial review of the literature to include studies that included standard-dose adrenaline as control, rather than limiting to intervention. Table 2 gives a brief detail of these studies, together with the percentage rates for survival to discharge for patients receiving standard-dose adrenaline (1 mg). This identified 10 studies in which high-dose adrenaline or vasopressin were given as comparator. In one trial, placebo was given as comparator. 56 The median rate of survival to discharge was 2.8%, and the weighted mean was 3.5%.
| Study | Number of survivors (n) | Participants receiving intervention (n) | Survival to discharge (%) | Comparator | Population | Setting | VF (%) |
|---|---|---|---|---|---|---|---|
| Brown et al.104 1992 | 26 | 632 | 4.1 | High-dose adrenaline | Adults | OHCA | 49 |
| Callaham et al.105 1992 | 3 | 270 | 1.1 | High-dose adrenaline | Adults | OHCA (non-trauma) | 21 |
| Gueugniaud et al.106 1998 | 46 | 1650 | 2.8 | High-dose adrenaline | Adults | OHCA (non-trauma) | 16 |
| Sherman et al.107 1997 | 0 | 62 | 0.0 | High-dose adrenaline | Adults | OHCA (non-trauma), on arrival ED | 16 |
| Steill et al.108 1992 | 2 | 165 | 1.2 | High-dose adrenaline | Adults | IHCA/OHCA | 43 |
| Jacobs et al.56 2011 | 11 | 272 | 4.0 | Placebo | Adults | OHCA | 43 |
| Ducros et al.109 2011 | 2 | 16 | 12.5 | Vaso/Epi | Adults | Witnessed OHCA | 0 |
| Gueugniaud et al.110 2008 | 33 | 1452 | 2.3 | Vasopressin/epinephrine | Adults | OHCA (non-trauma) | 9 |
| Lindner et al.111 1997 | 3 | 20 | 15.0 | Vasopressin/epinephrine | Adults | OHCA, refractory VF | 100 |
| Ong et al.112 2012 | 8 | 353 | 2.3 | Vasopressin/epinephrine | Adults | ED | 8 |
| Wenzel et al.113 2004 | 58 | 597 | 9.7 | Vasopressin/epinephrine | Adults | OHCA (non-trauma) | 41 |
Considering this information and the survival outcomes for PARAMEDIC2 at the time of our review, we concluded, as a trial team, that our initial estimates of survival to discharge/30-day survival, as detailed in the protocol, were overly optimistic. An adjusted sample size calculation based on the collected data indicated that, if we assumed a rate of survival to 30 days of 2% in the control arm, we would need > 24,000 patients for a RR of 1.33 at 90% power.
In addition, we estimated treatment effects detected by the sample size of 8000, as seen in Table 3.
| Power (%) | Proportion on control (%) | Proportion on intervention (%) | ARR (%) | RR |
|---|---|---|---|---|
| 80 | 2 | 2.98 | 0.98 | 1.49 |
| 90 | 2 | 3.15 | 1.15 | 1.57 |
| 93 | 2 | 3.23 | 1.23 | 1.62 |
We believed that revising the sample size to 24,000 was unachievable (and unaffordable) at that point in the trial.
We noted that the Jacobs et al. 56 trial showed an OR of 2.2 in the survival to discharge outcome, but with wide CIs (95% CI 0.7 to 6.3). Furthermore, a threshold of 1% in absolute risk reduction for outcomes has been used widely in resuscitation science as the threshold that defines the minimal clinically important difference. 114,115 We therefore believed that the trial would still yield valuable information about the safety and effectiveness of adrenaline if the observed survival rates continued to the end of the trial.
Consent
Obtaining consent
The ambulance service research teams received training on informed consent and assessing capacity, good clinical practice (GCP) guidelines, relevant legislation, and the trial-related procedures around consent.
Informing the patient about participation in the trial
For all patients who were transported to hospital for further treatment, ambulance service research teams conducted checks with local hospitals to determine whether or not a patient had survived. The first attempt to contact the patient and inform them of their enrolment in the trial was during their stay in hospital. The ambulance service research paramedics made contact with the patient as soon as practicable after the initial emergency had passed, taking the utmost care and sensitivity in doing so. Following our experience from an OHCA study of 4400 patients (PaRAMeDIC trial),81 and from discussions with fellow researchers from the REVIVE AIRWAYS cardiac arrest study116 and discussions with patient and public representatives, we believed that the earliest practicable time to approach patients and relatives was once the patient was discharged from the ICU and was on a hospital ward. This allowed sufficient time for the research team to be made aware of enrolment, to identify who the patient was, to check which hospital the patient was transferred to and whether or not they were still alive and to verify with the hospital team where the patient was in the hospital. Transfer to a ward indicated that the initial emergency had passed and the patient’s condition had stabilised. It was also considered more likely that the patient had regained consciousness and it would avoid any confusion or additional distress of making an approach while the patient remained critically ill in intensive care.
For patients or legal representatives who did not speak English, patient information sheets and consent forms were translated into some common languages. When printed translations were not available in the required language, Language Line Services (now known as LanguageLine Solutions, Monterey, CA, USA) was used to translate the patient information sheets and consent forms for the patient or their legal representative.
Procedure for taking consent
The research paramedic assessed if the patient had capacity to consent, with advice from the hospital team. If the patient had capacity, they were provided with the information sheet explaining the trial and the options for their involvement. The patient was allowed time to consider the information provided and had the opportunity to ask questions and discuss with others. The research paramedic or hospital team then asked when the patient would like someone to come back to discuss participation further and potentially take consent. The patient could decide that it was not an appropriate time to discuss the trial or they could decide that they did not want to be involved, in which case their feelings were respected and their decision about continuing in the trial was recorded.
The consent form listed the different sorts of information that were collected. Specific consent was not sought to use the data already collected. If the patient did not want the trial team to continue to collect data about survival, or to access the patient’s health records, then they indicated this on the consent form by not initialling the corresponding boxes or told the trial team verbally.
Research paramedics confirmed with the patient or legal representative their willingness to continue with the trial at each contact point.
In the event that a patient lacked capacity to consent, the research paramedic worked with the hospital team to identify a legal representative, defined as:
-
personal legal representative – a person independent of the trial, who, by virtue of their relationship with the potential study participant, is suitable to act as their legal representative for the purposes of that trial, and who is available and willing to so act for those purposes.
Or, if there is no such person:
-
professional legal representative – a person independent of the trial, who is the doctor primarily responsible for the medical treatment provided to that adult
-
a person nominated by the relevant health-care provider.
The legal representative was approached and provided with the information sheet explaining the trial and the options for their and the patient’s involvement, including the need for them to give consent on behalf of the patient and complete questionnaires on behalf of the patient. The legal representative was given time to consider the information provided. The research paramedic or hospital team then asked when the legal representative would like someone to come back to discuss participation further and potentially take consent.
The legal representative could decide that it was not an appropriate time to discuss the trial or they could decide that the patient would not want to take part, in which case their feelings were respected and their decision about taking part was recorded.
In exceptional circumstances, if consent was not obtained during the hospital stay, the patient or their legal representative was sent an invitation letter by post and written consent was taken at the 3-month follow-up visit, if the patient or legal representative agreed.
It is possible that the patient could have regained capacity by the time the 3-month visit was due. When contacting the legal representative to arrange the 3-month visit, the ambulance service research team asked if they could speak with the patient. If, on assessment of the patient, either on the telephone or at the visit, it was found that the patient still lacked capacity, the legal representative was asked to complete the questionnaires on behalf of the patient. If the patient had regained capacity, then information was provided about the trial and consent for further data collection was sought.
General information about the trial and contact details for further information was made freely available throughout the trial. Information about the trial was placed on ambulance trust and University of Warwick websites, ambulance service public newsletters, posters and information leaflets were shared with general practices, pharmacies, hospital accident and EDs and waiting areas, totalling 6500 mail-outs, which included 2356 general practices and 3181 pharmacies. Prior to the start of the trial, a press release was issued providing information about the trial. This was followed by periodic regional press releases as the trial progressed. The trial website was updated with information throughout the trial [www.warwick.ac.uk/paramedic2 (accessed 1 August 2019)], and was accessed > 178,000 times during the trial.
Although not required by the relevant regulations, the trial team developed a system to allow a patient to decline participation in the trial in the event that they sustained a cardiac arrest.
Requests not to participate were sent to and managed by the WCTU trial team. An online form could be completed on the website or the team could be contacted by telephone or e-mail. A stainless steel ‘No Study’ bracelet was issued to the person’s home address and, with the person’s permission, their home address was passed to the ambulance service to register the person’s wishes by placing an address flag on dispatcher systems. Those requesting a ‘No Study’ bracelet were also told to tell those close to them their wishes and told that those wishes would be respected by the treating paramedics. Paramedics were trained to look for the bracelet when attending a cardiac arrest.
Enquiries regarding trial participation
The following process was introduced at the end of the trial to deal with trial participation enquiries from members of the public.
If a member of the public enquired as to whether or not their next of kin was enrolled in the PARAMEDIC2 trial, they completed a trial participation enquiry form. On receipt of a completed trial participation enquiry form, the PARAMEDIC2 trial office worked with the appropriate site to check whether or not the next of kin of the enquirer was enrolled in the PARAMEDIC2 trial. Once it was confirmed, a letter was sent from the appropriate ambulance service to confirm whether or not the enquirer’s next of kin was enrolled in the trial, along with an information sheet about the trial, which included contact details for the PARAMEDIC2 trial office.
Serious adverse events
Events that were related to cardiac arrest and that were expected in patients undergoing attempted resuscitation were not reported. These included:
-
death
-
hospitalisation
-
persistent or significant disability or incapacity
-
organ failure.
All events categorised as serious [SAE/serious adverse reaction (SAR)/suspected unexpected serious adverse reaction (SUSAR)] were required to be reported to the WCTU within 24 hours of becoming aware of the event. All reports of SAEs/SARs/SUSARs were reviewed on receipt by the chief investigators or delegated clinical members of the TMG; the main REC, the Medicines and Healthcare products Regulatory Agency (MHRA) and the sponsor were notified within 7 or 15 days of receipt of those that were considered to satisfy the criteria for being related to the IMP and unexpected, in accordance with regulatory requirements. Reports of SAEs/SARs/SUSARs were also reviewed by the DMC at its regular meetings, or more frequently if requested by the DMC chairperson.
Procedures in case of pregnancy
Known pregnancy at the time of the cardiac arrest was an exclusion criterion for this trial. However, should the patient later be known to have been pregnant at the time of cardiac arrest and trial intervention, the protocol specified that the outcome of the pregnancy would be followed up and documented, even if the subject was discontinued from the trial. All reports of congenital abnormalities or birth defects were to be reported and followed up as a SAE.
Protocol non-compliances
Any deviations from or violations of the trial protocol or GCP were reported to the WCTU trial team promptly via a paper case report form (CRF) or an electronic case report form (eCRF). Any reports were assessed by the trial team on the day of receipt, or the following working day if received on a weekend, and escalated to the chief investigator (or their delegate), the quality assurance team and the WCTU manager if the non-compliance was a new or an exceptional event. All non-compliances, including cumulative numbers, trends and frequency over time, were reviewed at monthly trial management meetings. All violations and serious breaches were reported to the sponsor, and serious breaches were reported to the MHRA within 7 days (Figure 6). The ambulance service trial teams put in place corrective and preventative actions to mitigate the risk, and these actions were reviewed by the WCTU trial team to ensure that all had been completed.
FIGURE 6.
Process for receipt of a non-compliance report. CTU, Clinical Trials Unit.
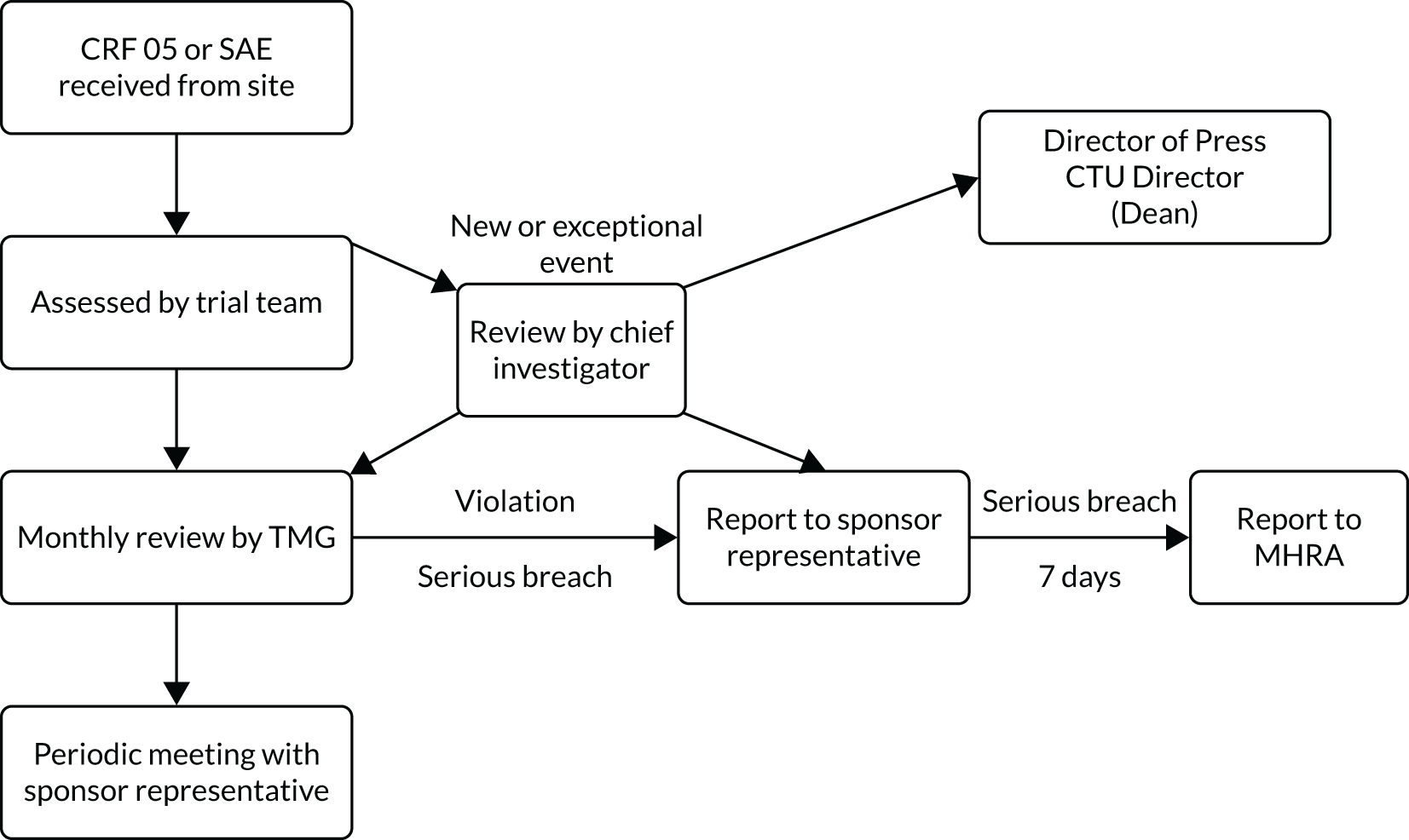
A protocol deviation was defined as a change or departure from the clinical trial protocol and/or GCP that did not result in harm to the trial participants or significantly affect the scientific value of the reported results of the trial.
A protocol violation was defined as a serious non-compliance with the approved protocol resulting from error, fraud or misconduct.
A serious breach was defined as a breach (deviation or violation) that was likely to affect, to a significant degree, either the safety or physical or mental integrity of the participants of the trial, or the scientific value of the trial.
Data collection
Screening data
Data were collected on a screening log of all cardiac arrests, which were attended by a trial-trained paramedic in the trial areas. These data consisted of the case identifier, the age and sex of the patient and the reason why the patient was not enrolled in the trial. This allowed assessment of the proportion of missed enrolments and the reasons, to ensure that the trial sample was representative of the trial population. The reasons for missed enrolments were established either from reviewing the patient’s clinical record, or from speaking to the attending clinician. Several strategies were used during the trial to reinforce the message that all eligible patients should be enrolled in the trial, and data on the numbers and reasons for missed enrolments were reviewed throughout the trial and fed back to sites.
Patient enrolment
All cardiac arrests for which a trial pack was opened were reported to the ambulance service trial teams by the recruiting clinician. The method for notification of enrolled patients was specific to each research site, but was usually done via telephone to the control centre or to a dedicated telephone number. Once ambulance service research teams were notified of an enrolled patient, the patient details were registered promptly on the trial database via an online web application hosted by the University of Warwick.
Baseline cardiac arrest data
Baseline cardiac arrest data were obtained retrospectively from ambulance service clinical records and the OHCAO registry (University of Warwick) and transcribed directly onto eCRFs via the PARAMEDIC2 web application. All ambulance service staff were trained on how to collect and enter trial data, and all data definitions followed Utstein recommendations. 92 Follow-up data were then obtained from hospitals, general practitioner (GP) surgeries, NHS Personal Demographics Service and patient follow-up questionnaires and were entered into the PARAMEDIC2 web application as they became available.
Hospital
Patients were taken to any hospital in the trial regions. Although hospital clinicians did not have a role in delivering the trial interventions, they were informed about the trial and were provided with information about the trial for any clinicians or patients who needed it.
Hospitals were contacted initially to ascertain survival of patients handed over from ambulance services to the ED. If the patient had survived, the research paramedics liaised with the hospital clinicians to visit the patient and seek consent for continuation in the trial (see Consent).
For any patient taken to hospital, data were collected on survival, length of stay in hospital and ICU, targeted temperature management, adrenaline use and mRS score at discharge, as well as discharge address and GP details. As some patients were found in a public place without any identifiers, or only part of their details were known to the ambulance service at the time of arrest, hospitals also, when necessary, provided missing information such as name, address and date of birth.
Survival checks
Survival checks were completed by the ambulance service research teams or the WCTU trial team using a variety of sources:
-
hospital data
-
Summary Care Record/Welsh Demographic Service
-
GP surgeries.
Follow-up
Survivors willing to take part were followed up approximately 3 months and 6 months after their cardiac arrest, as per Figure 4. Whenever possible, the 3-month assessments were a home visit, but, if the patient preferred postal questionnaires or to go through the questionnaires over the telephone, this was arranged, although the MMSE was not completed over the telephone. Questionnaires at the 6-month time point were sent by the WCTU and returned by post.
Following the approach to the patient, in the unlikely event that we had not obtained a response from the patient, the WCTU trial team approached the patient’s GP or hospital or ambulance service for information on their mRS score as close to the 3- and 6-month time points as possible. If the 3-month booklet had not been completed by the time the 6-month visit was due, the patient was sent the 3-month booklet to complete instead, so that outcomes that are only collected at 3 months were not missed.
Data linkage with other data sets
Data on each patient’s stay in hospital were collected retrospectively from the following electronic data sets to provide data on length of stay in hospital and hospital interventions:
-
Intensive Care National Audit and Research Centre (ICNARC).
-
Patient Episode Database for Wales (PEDW).
-
Hospital Episode Statistics (HES).
-
UK Transplant Registry (UKTR).
An application was made to receive data from the National Institute for Cardiovascular Outcomes Research data set. However, the application was not approved in time for data to be received in time for analysis.
Quality of cardiopulmonary resuscitation
Information on CPR quality is increasingly being viewed as an international reporting standard for cardiac arrest research.
The executive summary of the European Resuscitation Council Guidelines for Resuscitation 201088 identifies four critical components of high-quality CPR: minimise interruptions in chest compressions (as measured by chest compression fraction), provide compressions of adequate rate, provide compressions of adequate depth and allow full chest recoil between compressions.
Because a way to measure chest recoil was unavailable, the first three parameters were measured:
-
chest compression fraction, also known as chest compression ratio
-
chest compression rate
-
chest compression depth.
A preliminary review of existing literature showed that these three variables are typically studied using measurements covering two time periods:
-
initial 5 minutes of recorded CPR treatment
-
whole episode of recorded CPR treatment.
To measure these indicators of the quality of CPR for PARAMEDIC2 trial cases, data were downloaded from defibrillators, when possible. Three models of defibrillators were used across two ambulance services:
-
London Ambulance Service –
-
LIFEPAK® 15 monitor/defibrillator (Physio-Control, Inc., Redmond, WA, USA)
-
LIFEPAK® 1000 defibrillator (Physio-Control, Inc.).
-
-
North East Ambulance Service –
-
X Series® monitor/defibrillator (ZOLL Medical Corporation, Chelmsford, MA, USA).
-
When it was not possible to download quality of CPR data using existing means, a CPR card was used as a substitute. These Conformité Européenne-marked CPR cards, supplied by Laerdal Medical (Stavanger, Norway), measured compression fraction, compression rate and depth. CPR cards were operated by being switched on and placed centrally on a patient’s chest. Chest compressions were then performed as usual. Quality of CPR data were recorded during the resuscitation attempt, but the card did not provide real-time feedback to the paramedics.
In total, 1000 CPR cards were distributed to South Central Ambulance Service, the Welsh Ambulance Service and the West Midlands Ambulance Service. Trial-trained paramedics were taught how to use the CPR cards. Trial sites used site-specific processes to return used CPR cards from paramedics to site research teams. Site research teams downloaded data from the returned used CPR cards and provided the quality of CPR data securely to the WCTU. Data from both the defibrillators and CPR cards were entered into the trial database for analysis.
Table 4 compares the feasibility of acquiring the needed parameters and measurement periods from the devices used for the PARAMEDIC2 trial. The X Series defibrillator records all three parameters; an arithmetic mean (or average) value is given for the whole treatment episode and arithmetic mean values are also given for 1-minute intervals. The CPR cards record all three parameters, with an arithmetic mean value given for the whole treatment episode only; 1-minute intervals are not possible. The LIFEPAK 15 and LIFEPAK 1000 defibrillators record compression rate and compression fraction only (compression depth is not possible without an additional puck-sized sensor). Instead of the arithmetic mean, a ‘trimmed mean’ value (namely a mean that removes the extreme observations) is given by the LIFEPAK defibrillators for the whole treatment episode, and also for each 1-minute interval.
| Parameter | Period | Device | ||
|---|---|---|---|---|
| CPR cards (Laerdal Medical) (SCAS, WAST and WMAS) | LIFEPAK 15 and LIFEPAK 1000 defibrillators (Physio-Control, Inc.) (LAS) | X Series defibrillator (ZOLL Medical Corporation) (NEAS) | ||
| Compression depth | 1-minute intervals | Not feasible | Not feasible | Feasible |
| Whole episode | Feasible | Not feasible | Feasible | |
| Compression rate | 1-minute intervals | Not feasible | Feasible | Feasible |
| Whole episode | Feasible | Feasible | Feasible | |
| Compression fraction or ratio | 1-minute intervals | Not feasible | Feasible | Feasible |
| Whole episode | Feasible | Feasible | Feasible | |
Statistical analyses
Primary and secondary outcomes
The primary analysis was performed with and without adjustment in the modified intention-to-treat population, which included all the patients who had undergone randomisation and were confirmed to have received the assigned intervention.
Other approaches, for instance per protocol and complier-average causal effect, were not considered because of the negligible proportion of non-compliance. For data with a normal distribution, means and standard deviations (SDs) are presented. For data that were non-normally distributed, medians and IQRs are presented. Categorical data are summarised as frequency and percentage.
Fixed-effect regression models were used to examine survival outcomes with and without adjustment. Variables included in adjusted analyses were age, sex, the time between the 999 call and the ambulance arriving at the scene, the time between the ambulance arriving and trial drug administration, the suspected aetiology of the cardiac arrest, the initial heart rhythm, whether or not the event was witnessed and whether or not a bystander undertook CPR.
Length-of-stay outcomes were examined by the Hodges–Lehmann117 method, and were reported as estimated median differences with 95% CIs. When modelling the mRS, scores of 0–3 were classified as ‘good’ and scores of 4–6 were classified as ‘poor’; that is scores of 0–3 were regarded as a ‘good’ outcome and scores of 4–6 were regarded as a ‘poor’ outcome. If the proportional odds assumption was violated in modelling the mRS, partial proportional odds models118,119 were used. Other secondary outcomes (including quality of life and neurological and cognitive functions) were summarised by treatment arm.
Sensitivity
A sensitivity analysis was prespecified for mRS scores at discharge and at 3 and 6 months if > 10% of the data of survivors at each time point were missing. We used last observation carried forward and best- and worst-case scenarios for imputation. For the best-case scenario, missing data are considered as having no symptoms. For the worst-case scenario, missing data are considered as having severe disability in those confirmed to be alive at follow-up, and dead in those with confirmed death or missing survival status at follow-up. Post hoc sensitivity analyses were conducted for survival at 30 days, survival at hospital discharge, and survival with a good neurological outcome at discharge and at 3 and 6 months. Best-case and worst-case scenarios and multiple imputation were used.
Unadjusted and adjusted analyses were conducted for the primary and secondary outcomes, apart from the Two Simple Questions tool. Unadjusted and adjusted ORs with 95% CIs and mean differences with 95% CIs were reported for categorical and continuous outcomes, respectively. The number needed to treat and its 95% CI were calculated for survival at 30 days.
Quality of cardiopulmonary resuscitation
We used only the data on quality of CPR collected by London Ambulance Service because insufficient data were collected from the other sites. The Mann–Whitney U-test was used to compare treatment differences in hospital- and ICU-free survival because of their non-normal distributions.
Subgroups
Prespecified subgroup analyses included a patient’s age, cause of cardiac arrest, initial cardiac rhythm, whether or not the cardiac arrest was witnessed, whether or not CPR was performed by a bystander, interval between the emergency call and ambulance arrival at the scene, interval between ambulance arrival and the trial-agent administration, and the interval between the emergency call and trial-agent administration. A p-value for interaction was reported in each analysis.
Reporting of analyses followed CONSORT guidelines.
Additional analyses
Interim analyses
Prior to the start of the trial, we used the Lan–DeMets,120 O’Brien–Fleming and Pocock alpha spending methods to determine the upper and lower stopping boundaries for the primary outcome, with no adjustment in the final analysis. Interim analyses were carried out 10 times on a quarterly basis during the trial recruitment. Reports were delivered confidentially to the DMC. The DMC reviewed these results and made recommendations to the TSC about continuation of recruitment or any modification to the trial that may have been necessary.
The DMC monitored the accumulating outcome data; one of its roles was to recommend cessation of recruitment if a clear result had been reached (i.e. if either adrenaline or placebo was clearly superior). We suggested that different thresholds of evidence for early termination were adopted if adrenaline or placebo was being more effective, as it was probable that stronger evidence would be needed to change current practice (adrenaline use) if adrenaline was found to be inferior. We therefore proposed that interim analyses were conducted frequently in the early stages of the trial, so that, if adrenaline was superior, this was detected earlier. Thus, we minimised any risks to patients while producing robust evidence that will change practice if adrenaline is inferior.
The outcomes of primary interest for the interim analyses were 30-day survival and neurological status. We prepared reports for the DMC initially every 3 months. The exact schedule of interim analyses and the nature of any early-stopping rules were determined by the DMC, in discussion with the investigators, before the start of recruitment.
Bayesian analysis
To aid in interpretation, we included a Bayesian analysis for the primary outcome and for survival with a favourable neurological outcome. The Bayesian statistical analyses were performed using RStan. All other statistical analyses were performed with the use of SAS® software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
We performed unadjusted Bayesian analyses for two outcomes, 30-day survival and survival with favourable neurological outcome, to enable us to quantify the probability of treatment effects of different sizes. We modelled the adrenaline and placebo arms separately, with the outcome for each participant being a Bernoulli (0/1) variable, with treatment arm-specific probabilities of ‘success’. Non-informative beta(1, 1) priors (Figure 7) were used for both groups, initially. We also performed a sensitivity analysis assuming more realistic, informative priors. These were beta(5, 150) for the adrenaline group (Figure 8), reflecting previous knowledge that survival in the adrenaline group was very unlikely to exceed 10%, and was most likely to be around 3%, and beta(2, 20) for the placebo group (Figure 9), because a survival rate exceeding 20% in the placebo group was very unlikely.
FIGURE 7.
Beta(1, 1).
FIGURE 8.
Beta(5, 150).
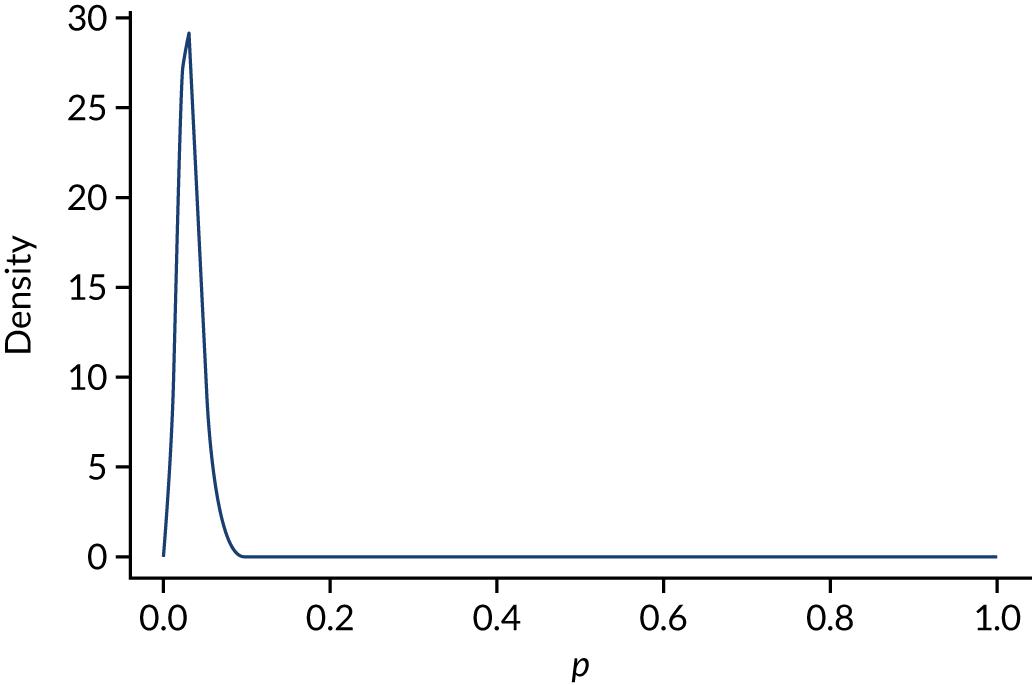
FIGURE 9.
Beta(2, 20).
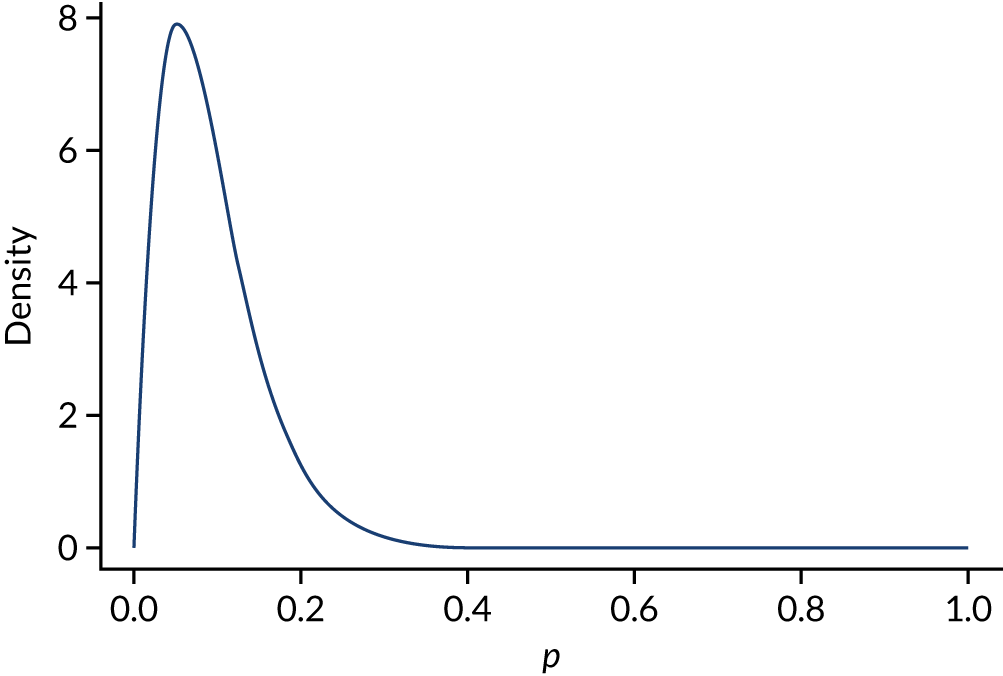
We calculated the 95% and 80% highest-density intervals, and, for risk differences, the probability that the treatment effect exceeded 0%, 1% and 2% (which have been suggested as representing clinically important differences).
We present the results for the analyses of risk differences for both outcomes with the non-informative prior. The informative priors made only very minor differences to the results.
Economic evaluation
A within-trial economic evaluation and decision-analytic model that extrapolated economic outcomes beyond the trial-follow-up period were based on the trial design. The economic evaluation was conducted from the recommended NHS and PSS perspective. 121 Further details of the economic evaluation methods are presented in Chapter 4.
Quality control of protocol non-compliances
Protocol violations were monitored using statistical process control charts. The monthly number of violations and the proportion as a percentage of recruitment were plotted. The moving range, defined as the absolute value of month-to-month change, was plotted against the recruitment month. Any out-of-control conditions, defined as outside the prespecified limits, were investigated for quality control.
Ethics and regulatory approvals
The trial was approved by the South Central Oxford C REC (reference number 14/SC/0157) and the MHRA (EudraCT number 2014-000792-11). The trial was sponsored by the University of Warwick and was conducted in accordance with the Directive 2001/20/EC122 of the European Parliament and of the Council of 4 April 2001 and The Medicines for Human Use (Clinical Trials) Regulations act,123 statutory instrument 2004 No. 1031 and amendment (No. 2) statutory instrument 2006 No. 2984.
The Confidentiality Advisory Group provided approval under regulation 5 of the Health Service (Control of Patient Information) Regulations 2002124 to process patient-identifiable information without consent (reference number 14/CAG/1009).
Several changes to the protocol and procedures were made during the trial (see Table 1). When these fulfilled the definition of substantial amendments according to the Medicines for Human Use (Clinical Trials) Regulations act,123 they were reviewed and approved by the REC, the MHRA (when applicable) and ambulance trusts. Approvals were not sought at hospital level, but those trusts were notified of all amendments via the Clinical Research Network.
The funders had no role in the trial design, in the collection or analysis of the data, or in the writing of the manuscript.
Ethics considerations
In designing and conducting this trial, a number of specific ethics issues were identified that required careful consideration.
Conducting a placebo-controlled trial
As adrenaline is currently part of standard clinical treatment in cardiac arrest, a placebo controlled trial necessitates some patients not receiving standard treatment. To justify the use of a placebo in place of standard treatment, it was necessary to demonstrate that current evidence on the use of adrenaline in cardiac arrest raised sufficient doubt about its efficacy and concern about its potential harm to patients, to justify a trial that involved withholding adrenaline from some participants. There is an ethical obligation for health-care professionals to provide treatment that imparts the most benefit and least harm for the patient, based on the best available evidence. Our review of the available evidence concluded that adrenaline, as currently used, improves the rate of ROSC), but increases the likelihood of neurological damage in those patients who survive and leave hospital. Thus, adrenaline can cause benefit and harm, and it was not clear from available evidence whether or not it caused more harm than benefit.
Given the uncertainty of the evidence, and the potential life-threatening nature of the condition being treated, it is ethically important to obtain the best evidence we can to justify treatment, while ensuring that the interests of the research participants remain paramount. Based on available evidence, the risks and benefits from participating in the trial were reasonably balanced (if a participant was randomised to placebo, they may have a reduced chance of immediate survival, but an increased chance of surviving to leave hospital neurologically intact). Our initial patient and public involvement (PPI) work suggested that members of the public value long-term survival more than short-term recovery of spontaneous circulation, so it would seem reasonable to assume that potential participants might agree to take part in a placebo-controlled trial, if they were able to do so.
Using a model of deferred consent
Enrolment in a clinical trial of a medicinal product normally requires informed consent from the participant or, if the participant lacks capacity, their legal representative. A decision about participating in a trial with potentially life or death consequences is particularly challenging, and, when treatment is not urgently required, the consent process may take considerable time. The unpredictability and immediately incapacitating nature of cardiac arrest (sudden loss of consciousness) means that it is not possible to obtain prospective informed consent from participants. Because of the need for immediate treatment, it is also not possible to obtain consent from the patient’s legal representative. This situation is ethically challenging as it is not possible to respect the participant’s autonomy by enabling them to make an informed choice regarding participation. The ethical justification for over-riding the usual requirement for consent must therefore be substantial with regard to the potential for the research to provide future benefit to patients or to protect them from future harm. All clinical trials of IMPs in the UK are governed by the EU Clinical Trials Directive (2001/20/EC)122 and the Statutory Instrument 2004/1031 [The Medicines for Human Use (Clinical Trials) Regulations 2004]. 123 In designing and conducting this trial, we also complied with the 2006 amendment to these regulations, relating to emergency care research that permits enrolment without consent in the emergency situation, subject to approval by an appropriate REC, and with a requirement that consent is obtained once it is no longer necessary to take action as a matter of urgency (deferred consent). Our approach was based on the template outlined at the Health Research Authority Workshop 2012 on conducting emergency research in patients who lack capacity. 125
The ethics implications of the deferred consent model [referred to as ‘exception from informed consent’ (EFIC) in the USA] in relation to the constraints on the principle of respect for autonomy, and approaches to mitigate these, have been discussed in the academic literature. 126–130 Providing information to, or undertaking consultation with, the relevant population prior to the research taking place can assist in the assessment of whether or not the research is sufficiently important in terms of the clinical benefit to patients to warrant the use of deferred consent. In the initial stages of trial design, we consulted PPI members from the first PARAMEDIC trial, and held a community engagement event where the rationale of the trial was presented to 280 lay people with an interest in first aid (see Patient and public involvement).
Providing information prior to and during the trial also allows the opportunity for potential participants to register their wish not to participate, and for researchers to respect their autonomous prior refusal. In the USA, some RECs (Institutional Review Boards) require researchers in EFIC studies to provide a mechanism for potential participants to opt out. 131 In the UK, it is rare for trials in emergency care to provide this option. For the PARAMEDIC2 trial, we developed a substantial public communications policy and included a mechanism for members of the public to register their wish not to participate, which included provision of ‘opt-out’ bracelets to be worn during the trial period (see Procedure for taking consent).
Approaching patients or their relatives (or legal representative) to inform them about enrolment in the trial and to seek consent for continuation in the trial also required careful thought in balancing the importance of providing information as soon as possible following the emergency and minimising distress caused by approaching relatives of patients critically ill in intensive care. Many patients lacked capacity to consent to participation in the early stages of recovery and some patients regained capacity over the period of the trial. Our consent processes were responsive to these complexities within the legal framework of the European Directive122 (see Procedure for taking consent).
Informing families of patients who do not survive cardiac arrest
The sad reality of an OHCA is that fewer than 1 in 10 people survive. Experiencing the sudden unexpected loss of a loved one because of cardiac arrest is a traumatic event that frequently leads to symptoms of anxiety, depression and post-traumatic distress. Careful consideration therefore needed to be given to how, when and if the relatives of non-survivors were to be informed about participation in the trial. By the time a patient’s death occurred, the trial intervention would have been implemented and no further follow-up would occur. Thus, there would be no requirement to seek consent to continue, and nor would it be useful to do so. The purpose of any communication with the family/next of kin of the deceased would be to inform them about the patient’s involvement in the trial, demonstrating that the process of trial recruitment is open and transparent. In addition, it mitigates any potential harm to family members from discovering at a later date that their relative or friend had been involved in a trial without their knowledge. However, knowledge of trial participation after the event may also place a significant burden on the next of kin at a time of heightened emotional distress due to the loss of their relative or friend. Any strategy to inform the family or next of kin following a patient’s death needs to balance the need for transparency with the need to minimise this distress.
The research team, including the trial ethicist, considered this issue with great care and discussed different approaches with patient representatives, clinicians caring for patients who had experienced a cardiac arrest and their families, the Resuscitation Council, and the REC. Approaches to informing the relatives of participants who do not survive can be broadly categorised as passive or active. Passive methods include placing information about the trial in publicly accessible places (e.g. websites, newsletters) and targeted sites likely to be attended by relatives of the deceased (e.g. hospitals, general practice surgeries, Registrar of Births and Deaths offices), with a contact telephone number and address for further information. This approach allows people to make a choice about whether or not and when they wish to seek further information. However, it is uncertain whether or not relatives of participants will see them. Discussion with investigators of previous UK trials in emergency of life-threatening conditions suggested that passive strategies, although not formally evaluated, had been used successfully.
Active strategies involve making direct contact with relatives through a face-to-face meeting, a telephone call or written communication. To our knowledge, this approach has not been used in previous UK OHCA trials, so we were unable to draw on relatives’ or researchers’ experiences of this process. There are practical barriers to providing information actively. The sudden and unpredicted nature of cardiac arrest mean that the relatives/next of kin are neither universally present nor identifiable at the time of the cardiac arrest. Information on the identity of the relatives/next of kin are also not held by ambulance services, so follow-up at a later date or time may not be possible. For people for whom resuscitation efforts are terminated in the home (≈ 40% of total cases), it is not possible for the attending paramedic to spend the necessary time to explain about the trial, answer questions and provide support to relatives who will be extremely distressed. Alternatively, providing unsolicited written information by post could exacerbate an already traumatic and stressful experience.
Having assessed the balance of benefits and burdens for relatives of the different strategies, and taking into account the importance of transparency, we concluded that the burden to families of adopting an active information strategy outweighed the potential benefits. We therefore developed a passive information strategy with a clear process for responding to enquiries from relatives of participants (and the general public) (see Procedure for taking consent and Enquiries regarding trial participation).
Patient and public involvement
During the planning and development phase, we worked with the PPI members of the PaRAMeDIC trial, who contributed to the trial design and proposed follow-up processes. Specific contributions related to the selection of outcome measures and a summary/presentation of the research in plain English. We held a community engagement event (supported by West Midlands South Comprehensive Local Research Network) in late November 2012, at which we presented the scientific rationale behind this trial to a group of 280 lay people who were interested in first aid. After preparing the talk in collaboration with one of our PPI representatives (John Long), to ensure that concepts were presented in plain, understandable English, we delivered the presentation and addressed any questions from the group. We explained the concept of short-term and longer-term outcomes and briefly sought community views about priorities for outcomes and their views on a trial of adrenaline for OHCA. We received responses from 243 participants. Ninety-five per cent of respondents indicated that long-term survival was important to them, whereas short-term survival was not. Participants broadly agreed that there was a need for further research about adrenaline as a treatment for cardiac arrest (86% agreed, 8% neither agree nor disagreed and 6% disagreed).
Prior to ethics approval for the trial being obtained, the views of the Resuscitation Council (UK) Patient Advisory Group were sought on the appropriateness of our approach to informing relatives of patients who do not survive. These views and comments supported the approach described in the protocol and ethics application.
John Long, our PPI co-applicant, with extensive experience of working with charities dedicated to reducing death from cardiac arrest, was a member of the TMG and lead PPI representative. John Long attended the REC meeting and provided important input from a patient perspective on the ethics of the trial. Since then, he has been heavily involved in TMG meetings, developing the trial procedures and processes, and reviewing public-facing information (e.g. website text and press releases) for readability and relevance. John Long has volunteered on several occasions to present the concept of the trial to several interested user groups/conferences. For example, on request by the Patient Voice Group, NHS North and West Reading Clinical Commissioning Group, John Long, one of our co-investigators and a local research fellow attended its meeting on 10 March 2015. John Long also presented the trial at several Lifesaving Foundation conferences during the trial. We also included two PPI representatives in the TSC.
In addition, the WCTU team formed a PPI group comprising eight members of the public, and chaired by John Long, to give advice on the content and distribution of patient- and public-facing documents. The group was put together through the University of Warwick’s Universities/User Teaching and Research Action Partnership (UNTRAP) group and included a cross section of age, sex, religion and experience. The group have met on six occasions throughout the trial, on at least an annual basis, and have provided input on the following:
-
the patient information sheet and consent forms
-
process for informing patients or their relatives of their involvement in the trial
-
the trial communication strategy (ways in which information about the trial can be shared in relevant communities)
-
wording and layout of the trial website, trial posters, information leaflets and press releases
-
issues regarding seeking/obtaining consent
-
co-enrolment of participants
-
end-of-trial communications, including how to communicate the results to trial participants.
Following the completion of the trial, the results were presented to the trial investigators group, and our PPI representatives were invited to attend this meeting. Here, an infographics leaflet summarising the results of the trial for patients and members of the public was presented and the PPI representatives provided feedback. Our PPI representative, John Long, also attended a Science Media Centre briefing in London to present the results of the trial to journalists and to be on hand to answer any questions from a patient or public perspective.
Chapter 3 Results
Participant flow
Recruitment took place between 23 December 2014 and 17 October 2017. The trial team assessed 10,623 patients in regions served by the London Ambulance Service NHS Trust, the West Midlands Ambulance Service University NHS Foundation Trust, the North East Ambulance Service NHS Foundation Trust, the South Central Ambulance Service NHS Foundation Trust and the Welsh Ambulance Service NHS Trust. A total of 8103 (76.3%) patients met the inclusion criteria and were randomised to the adrenaline or placebo arm by ambulance service paramedics. Within a short period of randomisation (confirming eligibility) and administration of the trial drug, 87 patients were found to be ineligible after the trial drug pack was opened, and were thus excluded post randomisation. Another two patients had an unknown allocation because of missing trial drug packs. Therefore, 4015 patients were allocated to the adrenaline arm and 3999 were allocated to the placebo arm. Details of the CONSORT flow diagram are presented in Figure 10. Patient flow in hospital is presented in Figure 11.
FIGURE 10.
The CONSORT flow diagram. a, Cumulative loss to follow-up; b, hospital discharge was not a fixed time point, compared with the other survival outcomes and hence it was listed separately. DNAR, do not attempt resuscitation; LTFU, lost to follow-up.
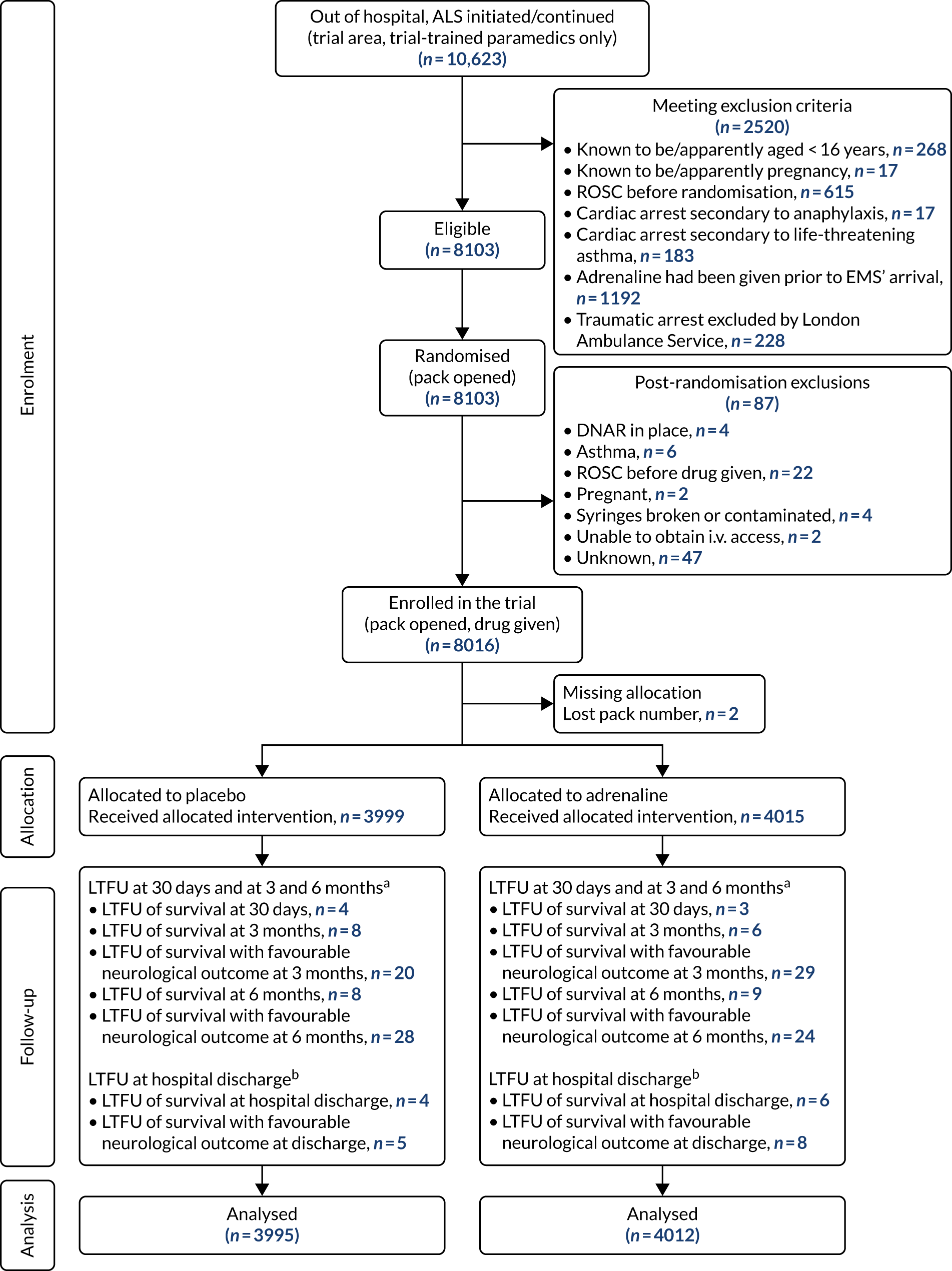
FIGURE 11.
Patient flow through the trial from recruitment to hospital discharge. Note that percentages of transportation to in-hospital status are based on enrolled patients in each arm. a, A total of 213 and 391 patients are included in placebo and adrenaline boxes, respectively, as a result of unknown ICU/ward admission information.
Losses and exclusions
Of all patients approached for the eligibility assessment, 2520 (23.7%) met the exclusion criteria. Having adrenaline before EMS’ arrival was the major reason for exclusion, accounting for 1192 (47.3%) exclusions.
In practice, administration of the trial drug occurred shortly after randomisation. Within this short period, 87 patients were found to be ineligible for various reasons after the trial drug pack had been opened (see Figure 10). Another two patients were given the drug, but were excluded from the analysis because the drug pack number could not be verified.
After survival to hospital ward admission, patients might become lost to follow-up (LTFU) because of consent decline/withdrawal or because they were lost track of in the trial. For the primary outcome, seven patients were LTFU. A further 10 patients became LTFU within 6 months follow-up, leaving 14 and 17 with missing survival status at 3 and 6 months, respectively.
After survival to hospital ward admission, patients might become LTFU if they declined consent, withdrew consent or could not be traced by the research paramedics. Ten patients were LTFU before hospital discharge. This number cannot be compared directly with other survival outcomes measured, as the discharge time is not a fixed time point and hospital stay varied from days to months. Nine patients survived for > 30 days in hospital and died before discharge, whereas seven were discharged from hospital and later died before reaching 30 days post randomisation. In addition, six patients were LTFU in the period from 30 days to hospital discharge, and three were LTFU in the period from discharge to 30 days.
Trial participation enquiries and unblinding requests
Warwick Clinical Trials Unit received five enquiries from members of the public asking whether they or their relative had been enrolled in the trial. Of the five enquiries, two patients or their relatives were confirmed to have been enrolled.
During the recruitment phase of the trial, we received six requests for unblinding (two from HM coroners, two from relatives of deceased patients as part of complaints investigations and two as part of non-compliance investigations). In all cases, requests occurred after collection of primary outcome data. Following closure of recruitment, we received nine requests from patients or their relatives.
Overview of recruitment
Recruitment began in a staggered fashion in five ambulance services. Figure 12 shows the cumulative recruitment at each ambulance service, as well as funder (i.e. HTA programme) targets for recruitment. The HTA programme recruitment targets were revised in July 2015 to allow a more gradual increase in recruitment, following delays in starting recruitment. Table 5 shows the number of patients at each ambulance service who were randomised and received the trial drug (not including post-randomisation exclusions).
FIGURE 12.
Cumulative recruitment from December 2014 to October 2017.
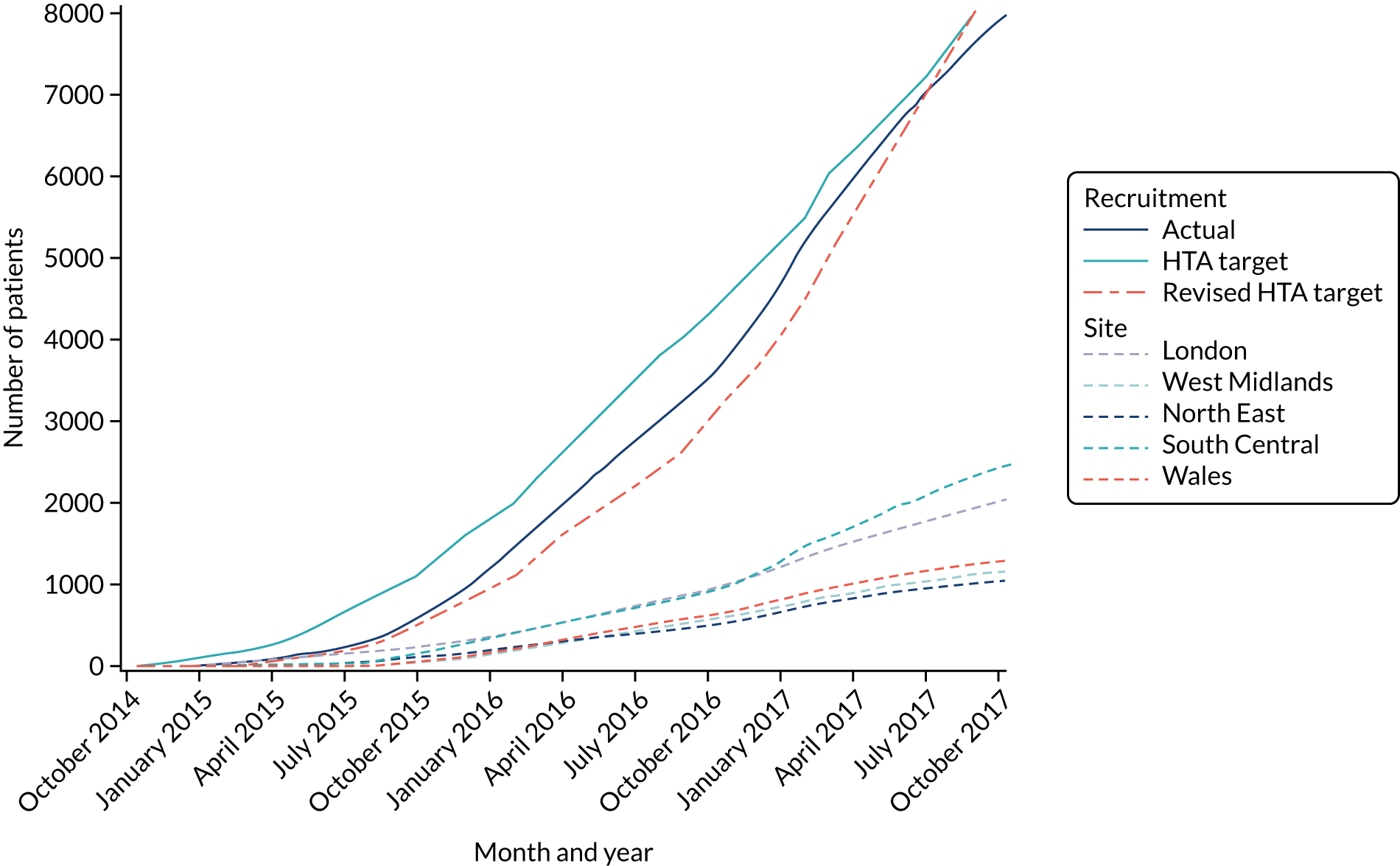
| Site | Start date | Number of recruited patients |
|---|---|---|
| London | 23 December 2014 | 2058 |
| South Central | 17 February 2015 | 2456 |
| North East | 10 April 2015 | 1049 |
| West Midlands | 16 June 2015 | 1163 |
| Wales | 14 July 2015 | 1290 |
Baseline data
Baseline data were collected for 8016 patients. Two patients had missing allocation and were not included in the analysis. Baseline patient and event characteristics (summarised in Tables 6 and 7) were well balanced between the trial arms.
| Characteristic | Adrenaline arm (N = 4015) | Placebo arm (N = 3999) |
|---|---|---|
| Age (years), mean (SD) | 69.7 (16.6) | 69.8 (16.4) |
| Sex, n (%) | ||
| Male | 2609 (65.0) | 2584 (64.6) |
| Female | 1406 (35.0) | 1415 (35.4) |
| Initial rhythm, n (%) | ||
| Shockable rhythm | 770 (19.2) | 748 (18.7) |
| VF | 716 (17.8) | 684 (17.1) |
| Pulseless VT | 25 (0.6) | 20 (0.5) |
| Not otherwise identified with AED | 29 (0.7) | 44 (1.1) |
| Non-shockable rhythm | 3149 (78.4) | 3181 (79.5) |
| Asystole | 2135 (53.2) | 2194 (54.9) |
| PEA/EMD | 955 (23.8) | 937 (23.4) |
| Bradycardia | 20 (0.5) | 16 (0.4) |
| Not otherwise identified with AED | 39 (1.0) | 34 (0.9) |
| Undetermined | 96 (2.4) | 70 (1.8) |
| Not identified | 4 (0.1) | 1 (< 0.1) |
| Missing | 92 (2.3) | 69 (1.7) |
| Initial aetiology, n (%) | ||
| Medical (presumed cardiac) | 3656 (91.1) | 3691 (92.3) |
| Traumatic cause | 66 (1.6) | 57 (1.4) |
| Drowning | 10 (0.2) | 10 (0.3) |
| Drug overdose | 74 (1.8) | 72 (1.8) |
| Electrocution | 0 (0.0) | 1 (< 0.1) |
| Asphyxia | 117 (2.9) | 81 (2.0) |
| Not identified | 1 (< 0.1) | 2 (0.1) |
| Missing | 91 (2.3) | 85 (2.1) |
| Witness, n (%) | ||
| None | 1498 (37.3) | 1505 (37.6) |
| Paramedic | 452 (11.3) | 470 (11.8) |
| Bystander | 2013 (50.1) | 1967 (49.2) |
| Not identified | 1 (< 0.1) | 1 (< 0.1) |
| Missing | 51 (1.3) | 56 (1.4) |
| Bystander commenced CPR, n (%) | ||
| Bystander CPR | 2382 (59.3) | 2349 (58.7) |
| No bystander CPR | 1111 (27.7) | 1094 (27.4) |
| By paramedic during witnessed event | 452 (11.3) | 470 (11.8) |
| Not identified | 1 (< 0.1) | 1 (< 0.1) |
| Missing | 69 (1.7) | 84 (2.1) |
| Adrenaline arm (N = 4015) | Placebo arm (N = 3999) | |
|---|---|---|
| Time from 999 call to EMS’ arrival at scene (minutes)a,b | ||
| Median (IQR) | 6.7 (4.3–9.7) | 6.6 (4.2–9.6) |
| Missing, n | 0 | 0 |
| Time from 999 call to administration of drug (minutes)a,b | ||
| Median (IQR) | 21.5 (16.0–27.3) | 21.1 (16.1–27.4) |
| Missing, n | 40 | 50 |
| Time from EMS’ arrival at scene to leaving scene (minutes) | ||
| Mean (SD) | 50.1 (21.8) | 44.5 (18.3) |
| Missing, n | 1976 | 2773 |
| Time from EMS leaving scene to arrival at hospital (minutes) | ||
| Mean (SD) | 12.9 (9.8) | 12.4 (8.9) |
| Missing, n | 1977 | 2774 |
| Time from starting ALS to cessation of resuscitation efforts (minutes)b | ||
| n | 1027 | 1457 |
| Median (IQR) | 47.5 (35.1–64.0) | 43.1 (33.5–56.1) |
| Missing, n | 947 | 1315 |
| ROSC, n (%) | ||
| Yes | 1457 (36.3) | 468 (11.7) |
| No | 2518 (62.7) | 3492 (87.3) |
| Missing | 40 (1.0) | 39 (1.0) |
| Patients transported to hospital, n (%) | ||
| Yes | 2041 (50.8) | 1227 (30.7) |
| No | 1974 (49.2) | 2772 (69.3) |
| Patient declared deceased by ED staff, n (%) | ||
| Yes | 988 (24.6) | 689 (17.2) |
| No | 614 (15.3) | 290 (7.3) |
| Not applicable (not transported) | 1974 (49.2) | 2772 (69.3) |
| Missing | 439 (10.9) | 248 (6.2) |
Pre-hospital treatment data
The summaries of pre-hospital treatments and the quality of EMS CPR are presented in Tables 8 and 9, respectively.
| Pre-hospital treatment | Adrenaline arm (N = 4015) | Placebo arm (N = 3999) |
|---|---|---|
| i.v. access, n (%) | 2739 (68.2) | 2763 (69.1) |
| Missing | 5 (0.1) | 3 (0.1) |
| Unknown | 76 (1.9) | 66 (1.7) |
| Intraosseous access, n (%) | 1340 (33.4) | 1,319 (33.0) |
| Missing | 5 (0.1) | 4 (0.1) |
| Unknown | 76 (1.9) | 64 (1.6) |
| Number of trial drug doses | ||
| Mean (SD) | 4.9 (2.5) | 5.1 (2.3) |
| Unknown | 7 | 9 |
| Administration of amiodarone, n (%) | 584 (14.5) | 368 (9.2) |
| Missing | 11 (0.3) | 14 (0.4) |
| Unknown | 47 (1.2) | 39 (1.0) |
| Supraglottic airway used, n (%) | 2844 (70.8) | 2847 (71.2) |
| Unobtainable | 5 (0.1) | 3 (0.1) |
| Unknown | 176 (4.4) | 164 (4.1) |
| Tracheal tube used, n (%) | 1206 (30.0) | 1125 (28.1) |
| Missing | 5 (0.1) | 4 (0.1) |
| Unknown | 167 (4.2) | 157 (3.9) |
| Number of shocks after randomisation (n) | 3980 | 3962 |
| Mean (SD) | 1.4 (3.0) | 0.9 (2.4) |
| Compression rate in the first 5 minutes (per minute)a (n) | 149 | 137 |
| Mean (SD) | 106.8 (14.4) | 106.5 (13.3) |
| Compression fraction in the first 5 minutes (%)b (n) | 149 | 137 |
| Mean (SD) | 76.2 (11.2) | 78.4 (13.0) |
| Quality of CPR | Trial arm, mean (SD) | |
|---|---|---|
| Adrenaline (n = 149) | Placebo (n = 137) | |
| Compression rate in the first 5 minutes (per minute)a | 106.8 (14.4) | 106.5 (13.3) |
| Compression fraction in the first 5 minutes (%)b | 76.2 (11.2) | 78.4 (13.0) |
Numbers of patients analysed
The number analysed for each outcome is listed in Table 10.
| Outcome | Number analysed |
|---|---|
| Survival at 30 days | 8007 |
| Survival until hospital admission | 7955 |
| Survival to hospital discharge | 8002 |
| Survival at 3 months | 8000 |
| Survival at 6 months | 7997 |
| Survival at 12 months | 7997 |
| mRS at hospital discharge | 8001 |
| mRS at 3 months | 7965 |
| mRS at 6 months | 7963 |
| IQCODE at 3 months | 132 |
| IQCODE at 6 months | 121 |
| Two Simple Questions at 3 months | 154 |
| Two Simple Questions at 6 months | 126 |
| EQ-5D-5L at 3 months | 154 |
| EQ-5D-5L at 6 months | 126 |
| SF-12 at 3 months | 137 |
| SF-12 at 6 months | 116 |
| MMSE at 3 months | 123 |
| HADS (anxiety) at 3 months | 144 |
| HADS (depression) at 3 months | 142 |
| Post-traumatic stress at 3 months | 137 |
| Hospital length of stay (discharged) | 218 |
| Hospital length of stay (deceased) | 7785 |
| Intensive care length of stay (discharged) | 238 |
| Intensive care length of stay (deceased) | 590 |
| Free of hospital stay | 8000 |
| Free of intensive care | 7999 |
Outcomes and estimations
Short- to medium-term outcomes are presented in Table 11. The survival rate at 30 days post randomisation was significantly higher in the adrenaline arm than in the placebo arm. The treatment difference of survival rate then decreased over time. Fewer patients who survived had a favourable neurological outcome, and differences between groups were smaller than for survival alone [0.3% (95% CI –0.3% to 0.9%) difference at discharge; 0.5% (95% CI –0.1% to 1.1%) difference at 3 and 6 months]. The number of patients with an unknown neurological outcome increased from discharge (n = 13) to 3 months (n = 49) and to 6 months (n = 51).
| Outcome | Adrenaline arm | Placebo arm | Difference (95% CI) | OR (95% CI) | |
|---|---|---|---|---|---|
| Unadjusted | Adjusted | ||||
| Survival | |||||
| Primary outcome, n/N (%) | |||||
| 30-day survival status | 130/4012 (3.2) | 94/3995 (2.4) | 0.9% (0.2% to 1.6%) | 1.39 (1.06 to 1.82) | 1.47 (1.09 to 1.97) |
| Secondary outcomes, n/N (%) | |||||
| Survived to hospital admissiona | 947/3973 (23.8) | 319/3982 (8.0) | 15.8% (14.3% to 17.4%) | 3.59 (3.14 to 4.12) | 3.83 (3.30 to 4.43) |
| Survived to hospital discharge | 128/4009 (3.2) | 91/3995 (2.3) | 0.9% (0.2% to 1.6%) | 1.41 (1.08 to 1.86) | 1.48 (1.10 to 2.00) |
| Survived at 3 months | 121/4009 (3.0) | 86/3991 (2.2) | 0.9% (0.2% to 1.6%) | 1.41 (1.07 to 1.87) | 1.47 (1.08 to 2.00) |
| Survived at 6 months | 117/4006 (2.9) | 85/3991 (2.1) | 0.8% (0.1% to 1.5%) | 1.38 (1.04 to 1.83) | 1.43 (1.05 to 1.96) |
| Survived at 12 months | 107/4006 (2.7) | 80/3991 (2.0) | 0.7% (0.0% to 1.3%) | 1.34 (1.00 to 1.80) | 1.38 (1.00 to 1.92) |
| Hospital stay, median (IQR) | |||||
| ICU length of stay of survivors | 7.5 (3.0–15.0) | 7.0 (3.5–12.5) | 0.0 (–1.0 to 2.0) | – | – |
| ICU length of stay of deceasedb | 2.0 (1.0–5.0) | 3.0 (1.0–5.0) | 0 (0 to 0) | ||
| Hospital length of stay of survivors | 21.0 (10.0–41.0) | 20.0 (9.0–38) | 1.0 (–3.0 to 6.0) | – | – |
| Hospital length of stay of deceasedb | 0 (0–0) | 0 (0–0) | 0 (0 to 0) | ||
| mRS | |||||
| Secondary outcomes | |||||
| Neurological outcome (mRS) at hospital discharge,c n (%) | |||||
| 0 – No symptoms | 12 (0.3) | 15 (0.4) | – | 0.80 (0.37 to 1.70) | 0.80 (0.36 to 1.77) |
| 1 – No significant disability | 17 (0.4) | 10 (0.3) | – | 1.16 (0.68 to 1.98) | 1.16 (0.66 to 2.03) |
| 2 – Slight disability | 23 (0.6) | 29 (0.7) | – | 0.96 (0.65 to 1.41) | 0.98 (0.65 to 1.48) |
| 3 – Moderate disability | 35 (0.9) | 20 (0.5) | – | 1.18 (0.86 to 1.61) | 1.23 (0.88 to 1.73) |
| 4 – Moderately severe disability | 12 (0.3) | 8 (0.2) | – | 1.21 (0.90 to 1.63) | 1.26 (0.91 to 1.75) |
| 5 – Severe disability | 27 (0.7) | 8 (0.2) | – | 1.41 (1.07 to 1.85) | 1.51 (1.12 to 2.04) |
| 6 – Dead | 3881 (96.7) | 3904 (97.6) | – | 1 | 1 |
| Unknown | 8 (0.2) | 5 (0.1) | – | ||
| Favourable neurological outcome at hospital discharge (0–3 vs. 4–6), n/N (%) | 87/4007 (2.2) | 74/3994 (1.9) | 0.3% (–0.3% to 0.9%) | 1.18 (0.86 to 1.61) | 1.19 (0.85 to 1.68) |
| Neurological outcome (mRS) at 3 months,c n (%) | |||||
| 0 – No symptoms | 20 (0.5) | 20 (0.5) | – | 1.33 (0.98 to 1.80) | 1.20 (0.62 to 2.33) |
| 1 – No significant disability | 30 (0.7) | 20 (0.5) | – | 1.39 (0.89 to 2.18) | |
| 2 – Slight disability | 10 (0.2) | 11 (0.3) | – | 1.27 (0.84 to 1.90) | |
| 3 – Moderate disability | 22 (0.5) | 12 (0.3) | – | 1.41 (0.99 to 2.02) | |
| 4 – Moderately severe disability | 6 (0.1) | 5 (0.1) | – | 1.41 (0.99 to 1.99) | |
| 5 – Severe disability | 10 (0.2) | 6 (0.2) | – | 1.45 (1.04 to 2.02) | |
| 6 – Dead | 3888 (96.8) | 3905 (97.6) | – | 1 | |
| Unknown | 29 (0.7) | 20 (0.5) | – | ||
| Favourable neurological outcome at 3 months (0–3 vs. 4–6), n/N (%) | 82/3986 (2.1) | 63/3979 (1.6) | 0.5% (–0.1% to 1.1%) | 1.39 (0.97 to 2.01) | |
| Neurological outcome (mRS) at 6 months,c n (%) | |||||
| 0 – No symptoms | 25 (0.6) | 19 (0.5) | – | 1.51 (1.11 to 2.06) | 1.38 (0.73 to 2.62) |
| 1 – No significant disability | 22 (0.5) | 13 (0.3) | – | 1.63 (1.01 to 2.65) | |
| 2 – Slight disability | 10 (0.2) | 9 (0.2) | – | 1.47 (0.95 to 2.26) | |
| 3 – Moderate disability | 21 (0.5) | 17 (0.4) | – | 1.40 (0.96 to 2.03) | |
| 4 – Moderately severe disability | 7 (0.2) | 2 (0.1) | – | 1.49 (1.04 to 2.15) | |
| 5 – Severe disability | 16 (0.4) | 7 (0.2) | – | 1.62 (1.15 to 2.28) | |
| 6 – Dead | 3889 (96.9) | 3906 (97.7) | – | 1 | |
| Unknown | 25 (0.6) | 26 (0.7) | – | ||
| Favourable neurological outcome at 6 months (0–3 vs. 4–6), n/N (%) | 78/3990 (2.0) | 58/3973 (1.5) | 0.5% (–0.1% to 1.1%) | 1.35 (0.96 to 1.90) | 1.35 (0.93 to 1.97) |
Intensive care unit- and hospital-free survival are summarised in Figures 13 and 14, respectively.
FIGURE 13.
Intensive care unit-free survival (days) in the 30 days post randomisation, by treatment arm. a, Mann–Whitney U-test was used.
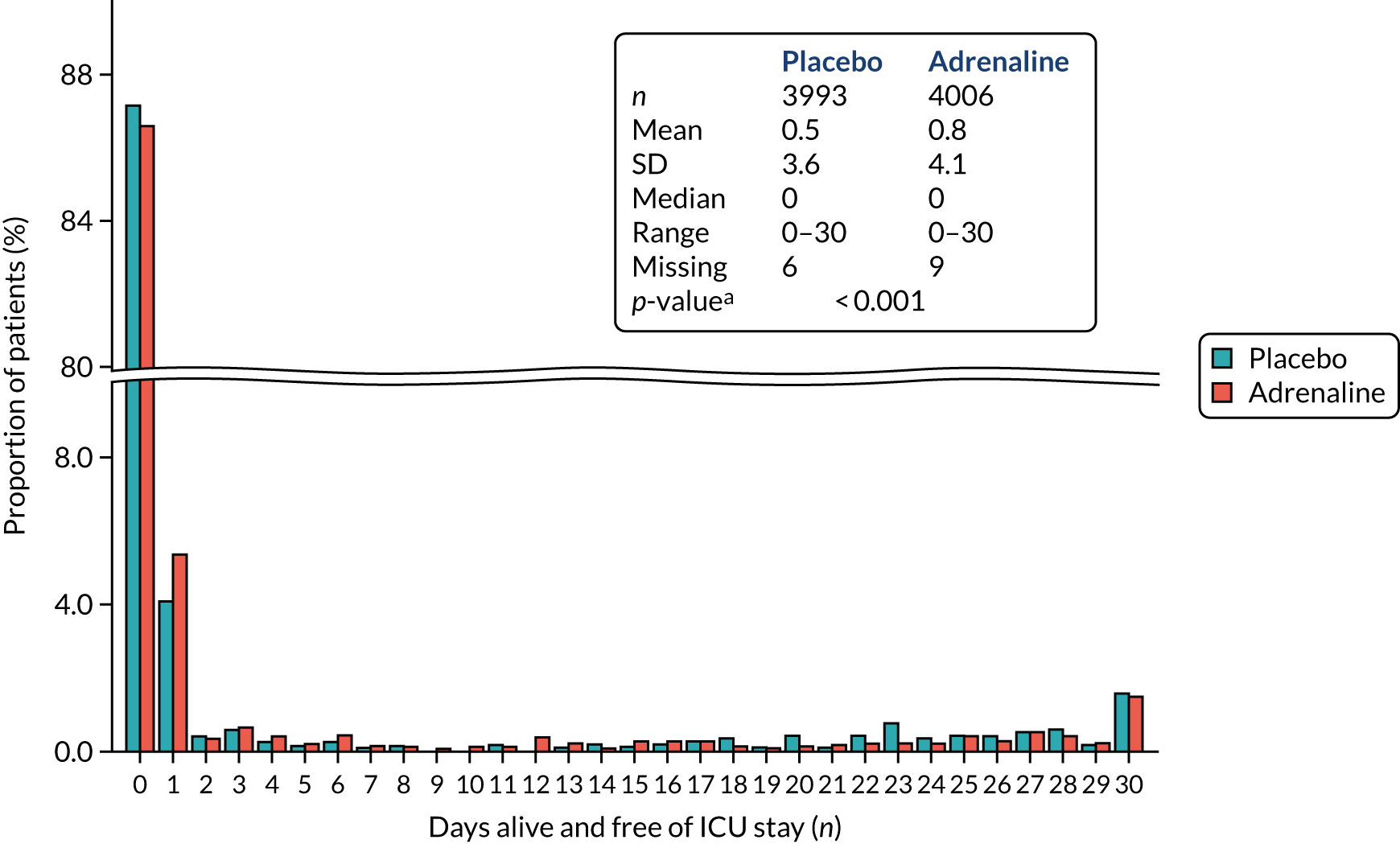
FIGURE 14.
Hospital-free survival (days) in the 30 days post randomisation, by trial arm. a, Mann–Whitney U-test was used.
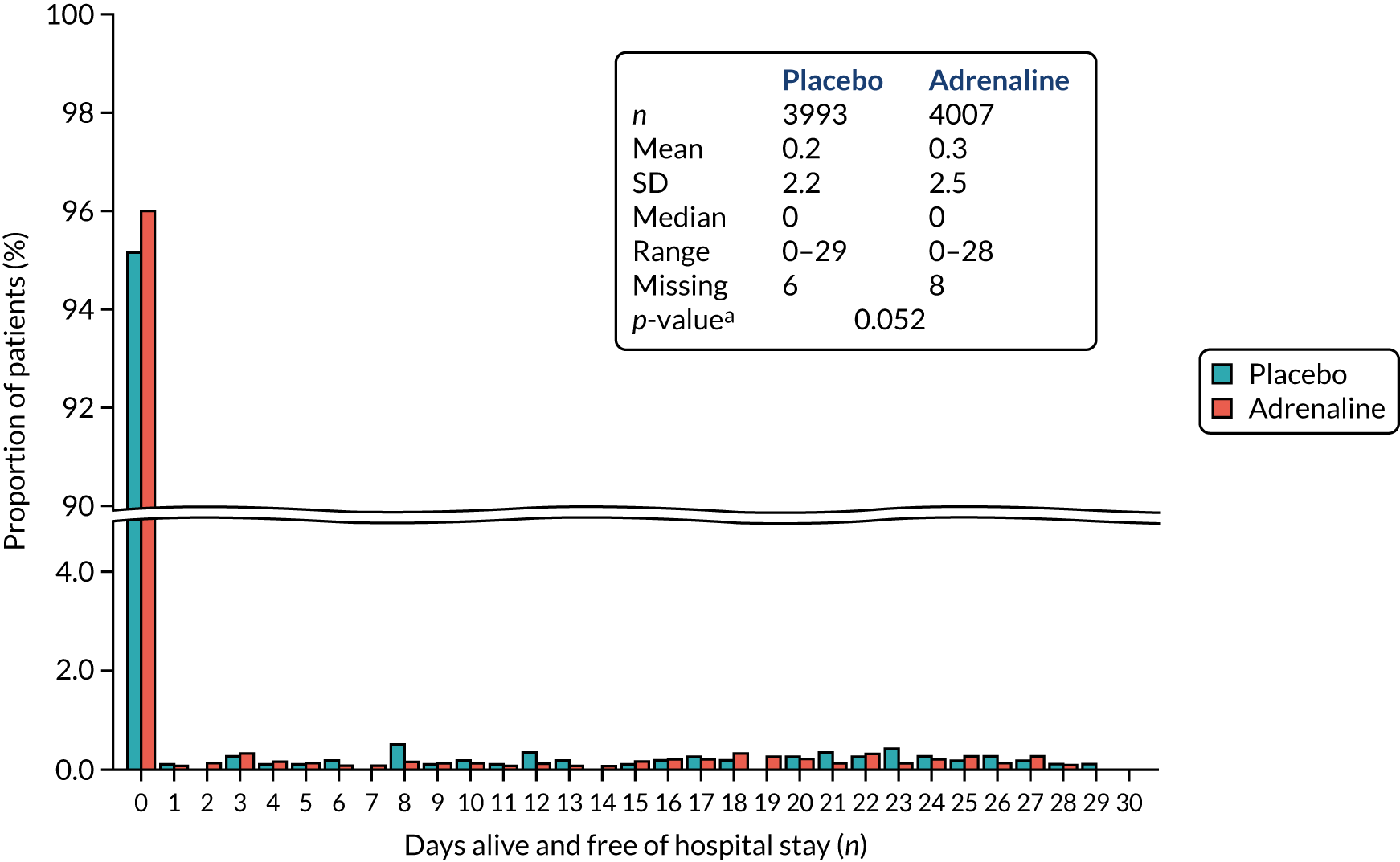
Longer-term neurocognitive and health-related quality-of-life outcomes among surviving patients are presented in Figures 15–17. Figure 15 summarises outcomes whereby a higher score is better. Figure 16 summarises outcomes whereby a lower score indicates a better health outcome. Figure 17 presents responses to Two Simple Questions relating to requirement for assistance and mental recovery after cardiac arrest.
FIGURE 15.
Summary of longer-term neurocognitive and health-related quality-of-life outcomes for the MMSE, the EQ-5D-5L and the SF-12 physical and mental health components. (a) Number of survivors; (b) mean (SD) MMSE score; (c) mean (SD) EQ-5D-5L score; (d) mean (SD) EQ-VAS score; (e) mean (SD) SF-12 physical health score; and (f) mean (SD) SF-12 mental health score. VAS, visual analogue scale. Note that error bars are ± 95% CIs of means. Frequency (rate) and mean (SD) are shown on top of the bars for categorical and continuous outcomes, respectively. Normal range or value of the outcomes: MMSE, 25–30 (122); HADS, 0–7 (123). The population mean is 50 (SD 10) for SF-12 and 0.86 (SD 0.23) for EQ-5D-5L index score. VAS, visual analogue scale.
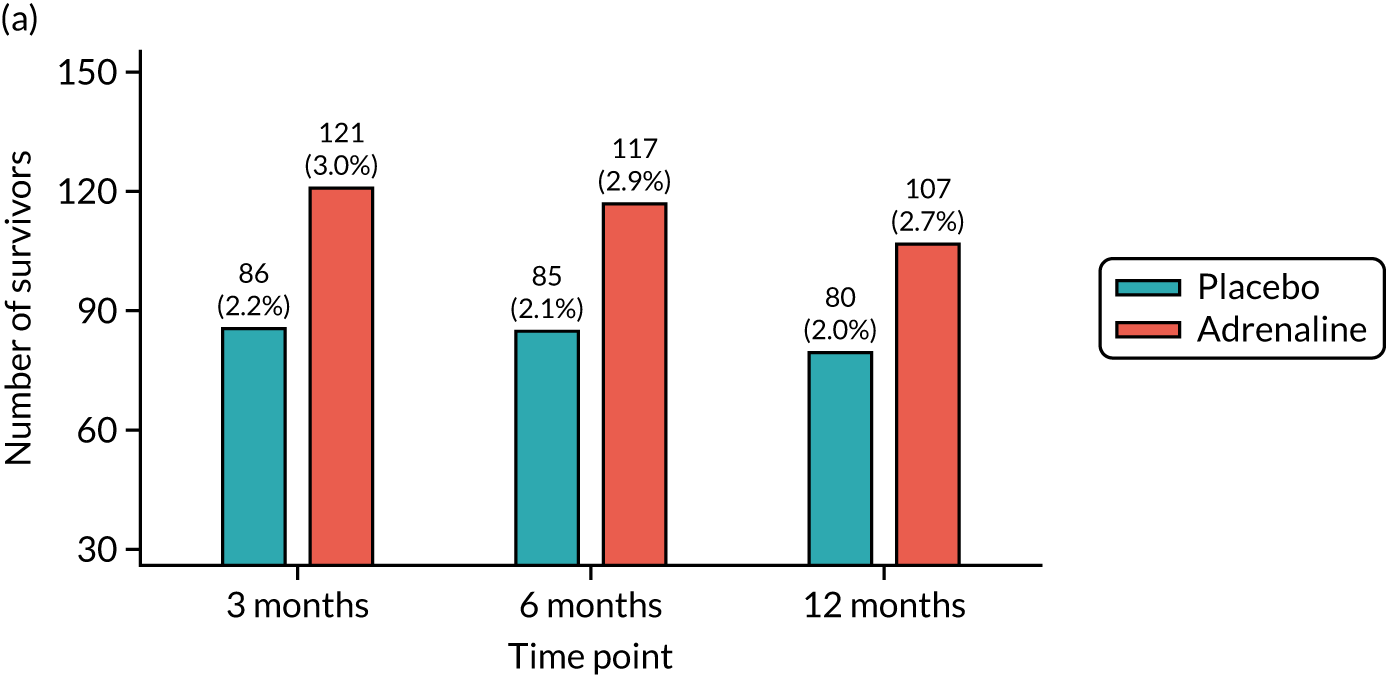
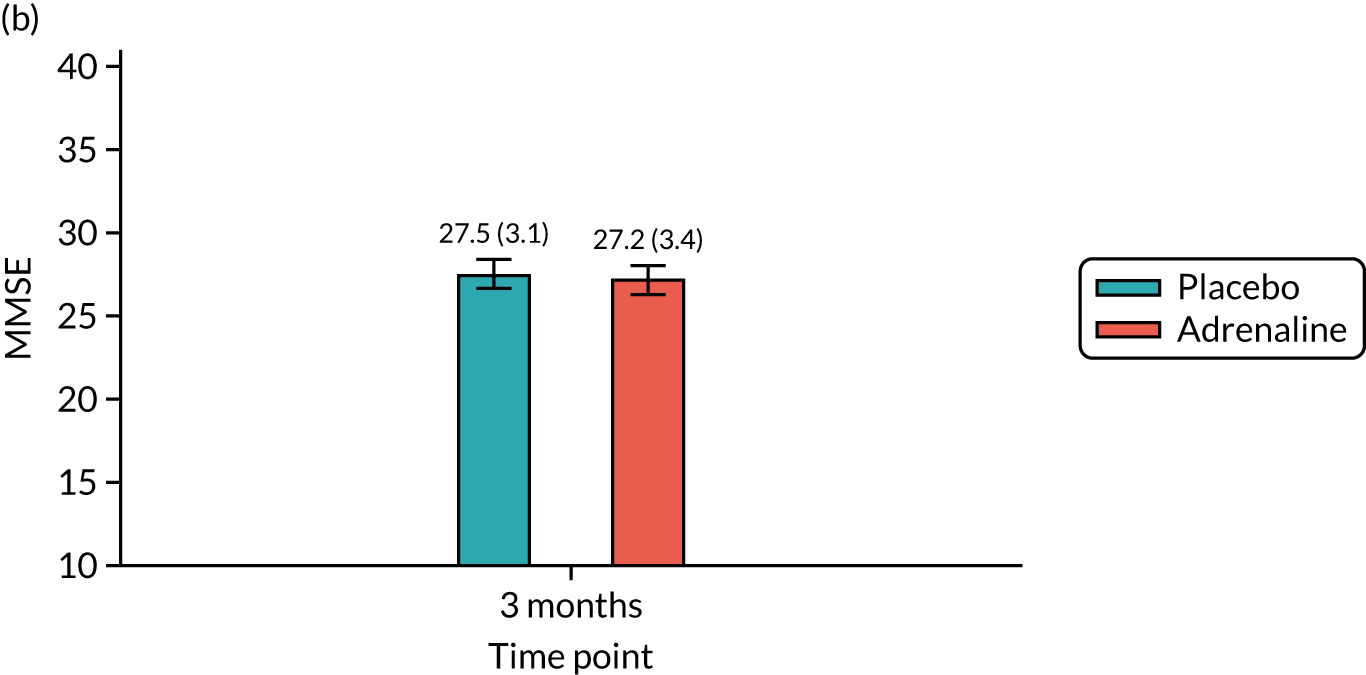
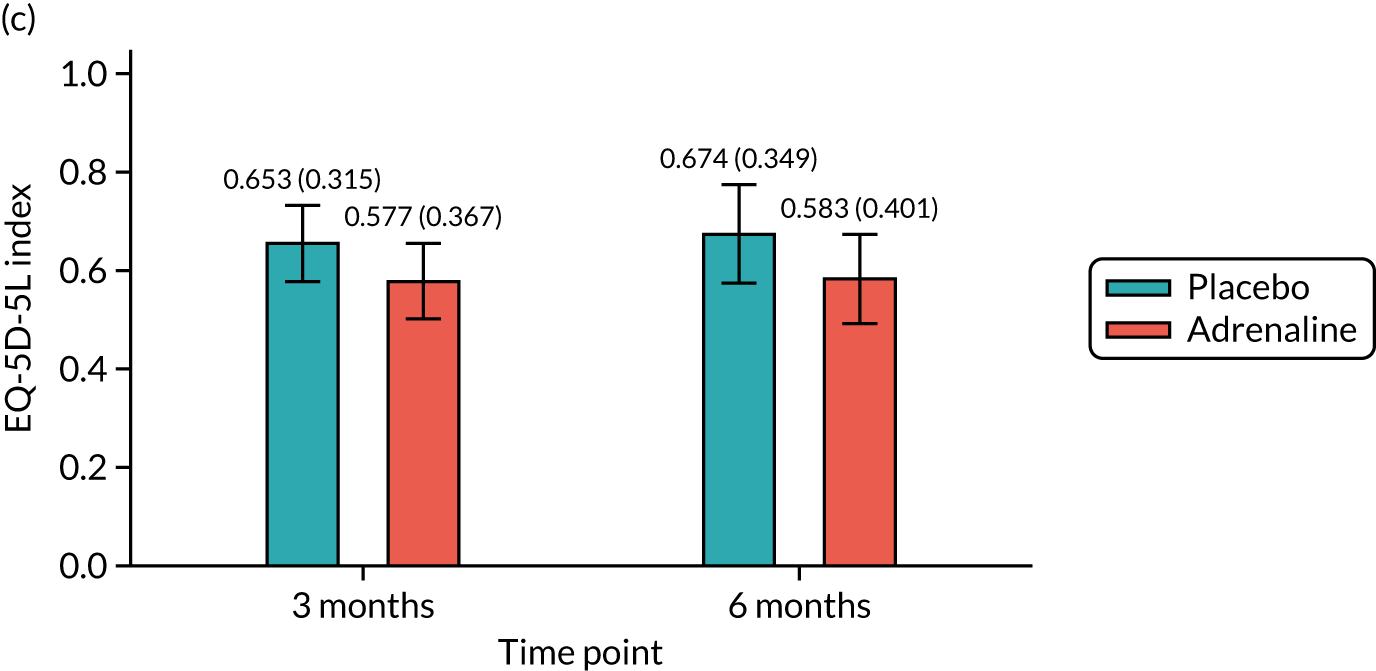
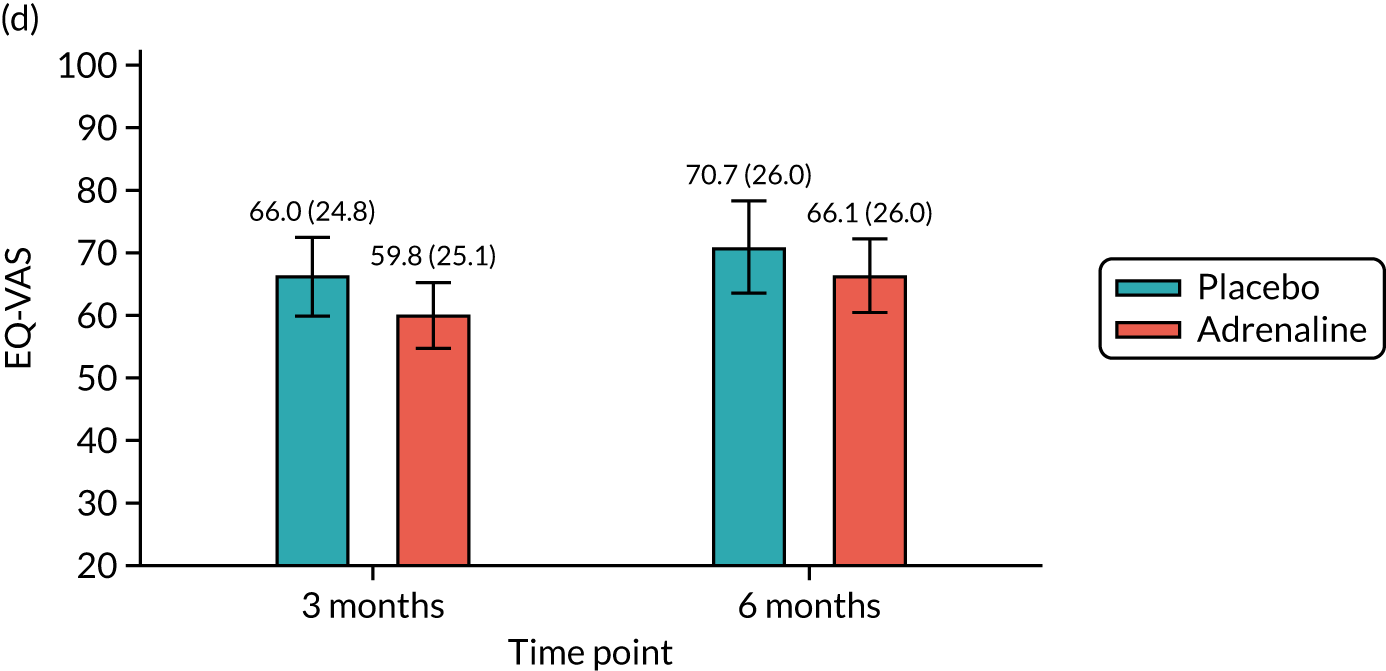
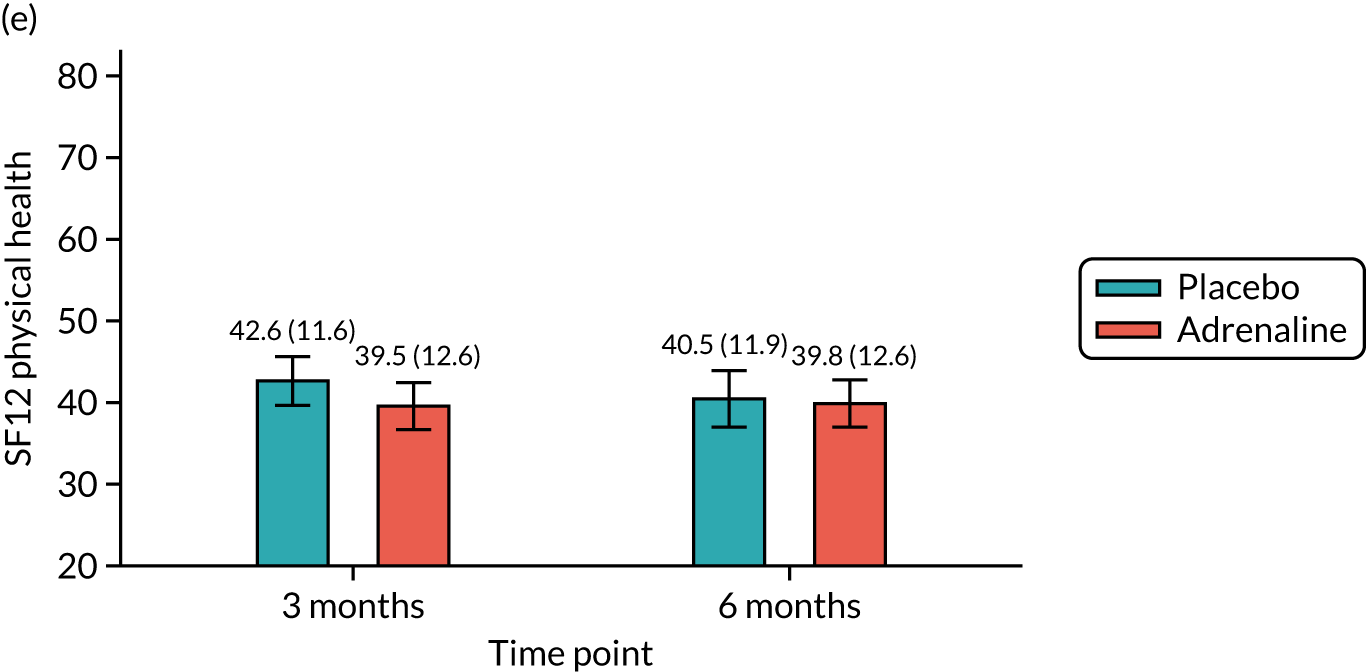
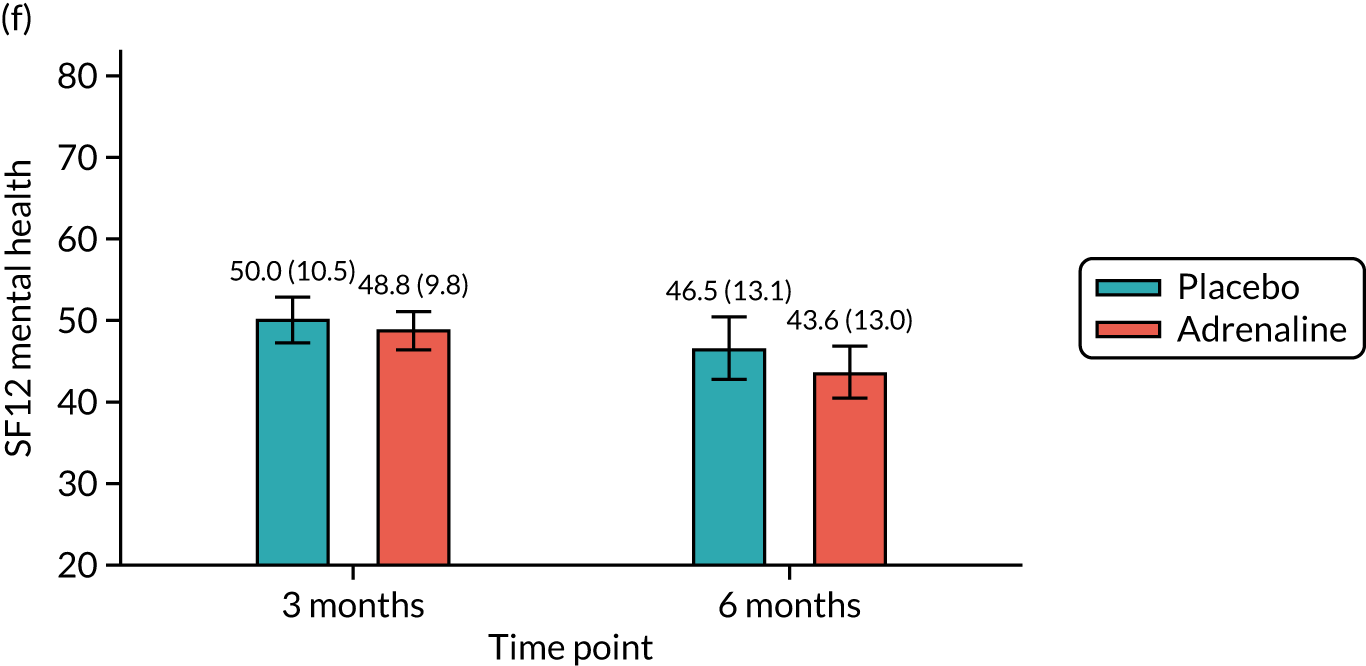
FIGURE 16.
Summary of longer-term neurocognitive and health-related quality of life for (a) IQCODE; (b) PCL-C; (c) HADS anxiety; and (d) HADS depression. Note that error bars are ± 95% CIs of means. Frequency (rate) and mean (SD) are shown on top of the bars for categorical and continuous outcomes, respectively. Normal range or value of the outcomes: PCL-C, no optimal range; IQCODE, < 3.04 (124).
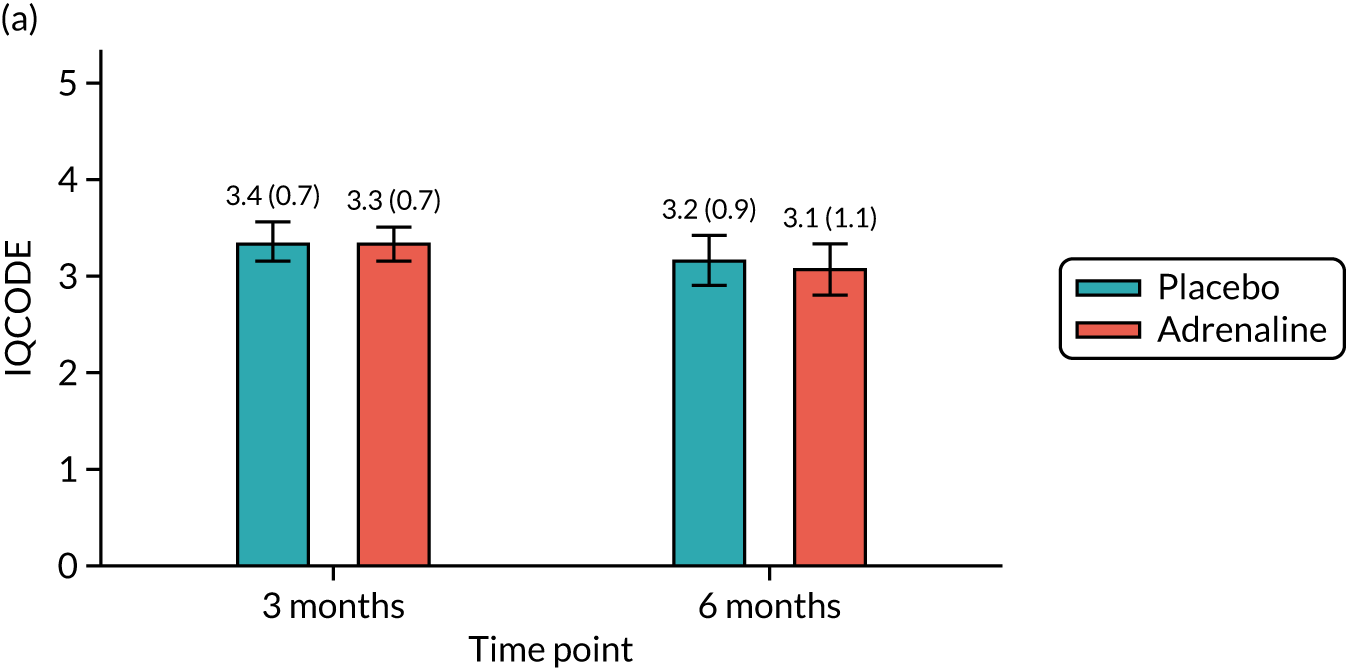
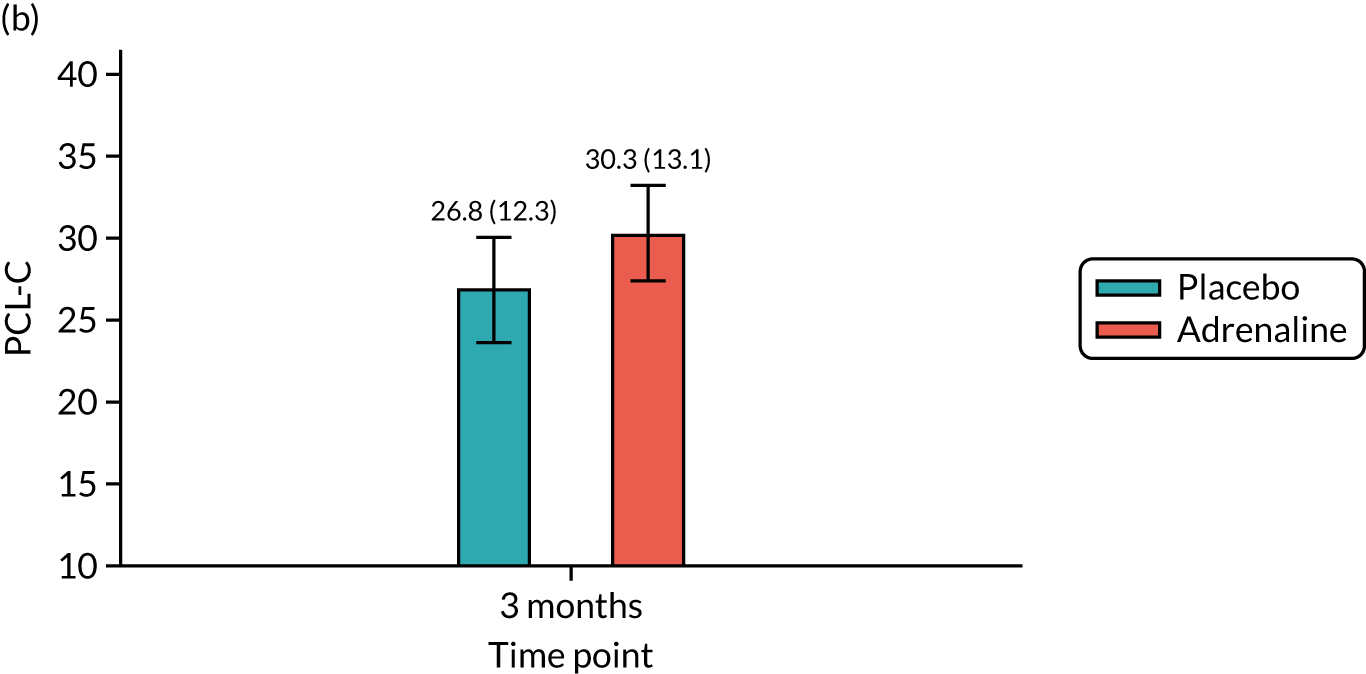
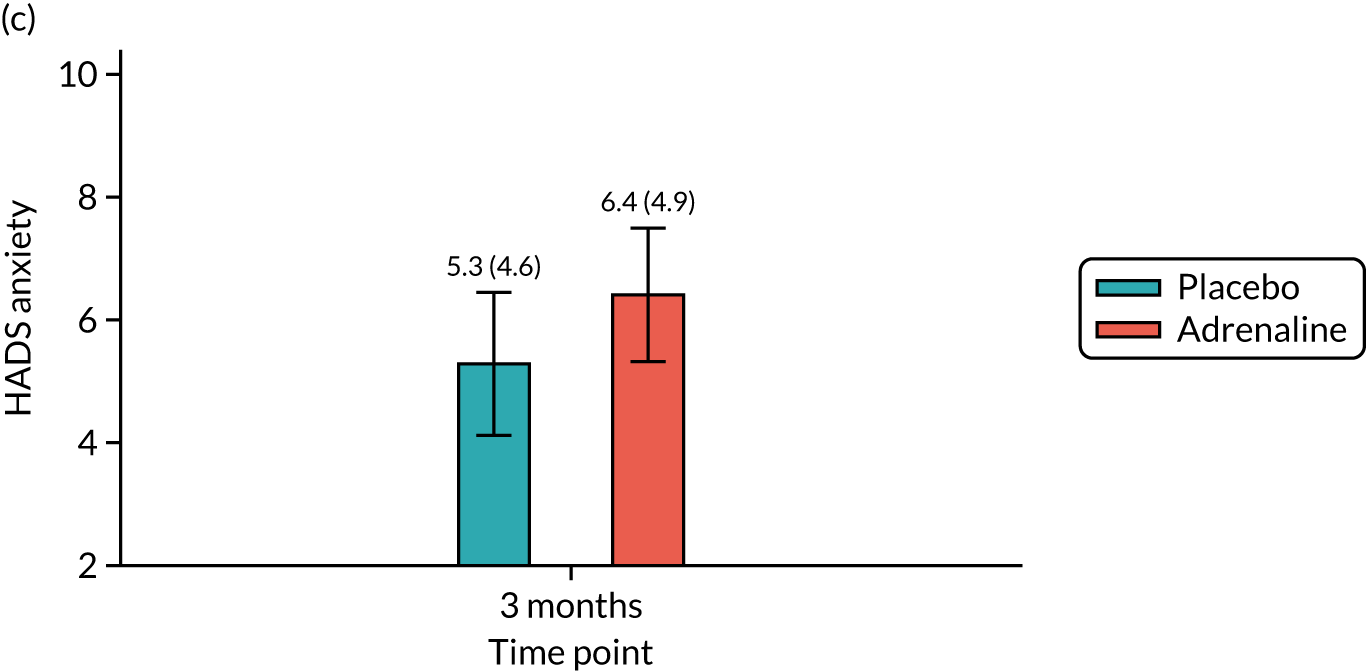
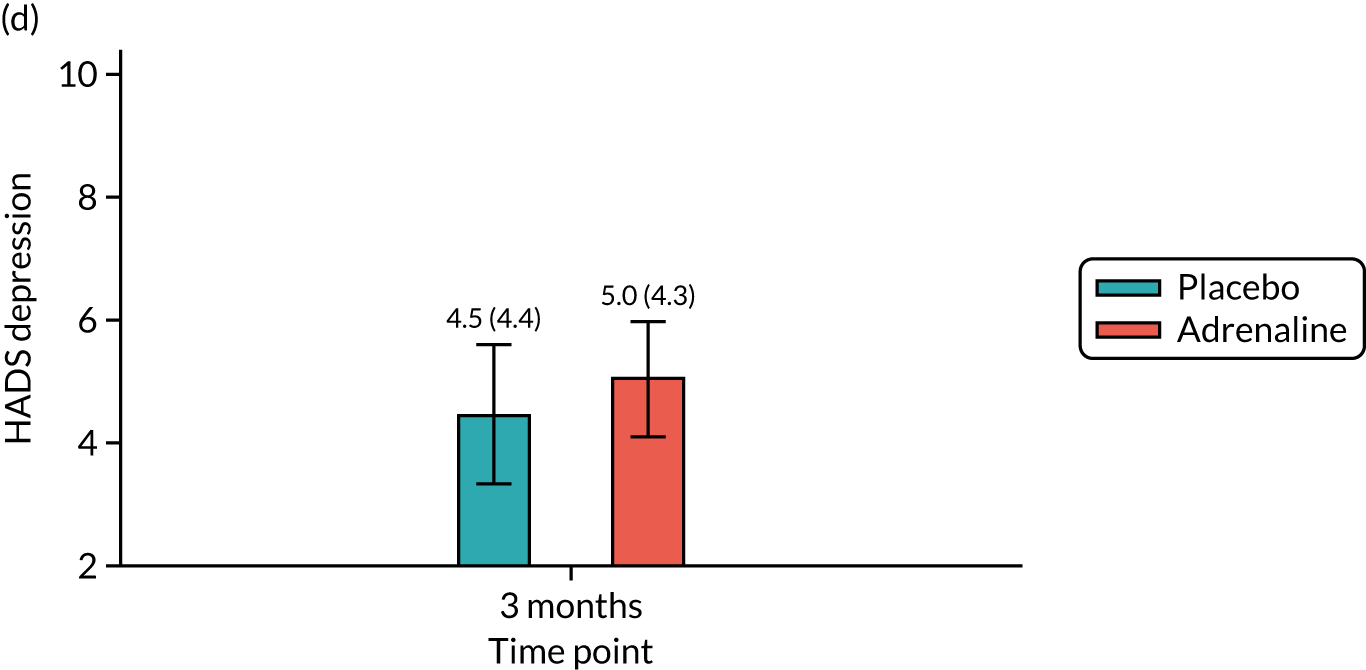
FIGURE 17.
Summary of Two Simple Questions. Q1: In the last 2 weeks, did you require help from another person for your everyday activities? Q1a: If yes, is this a new situation following your cardiac arrest? Q2: Do you feel that you have made a complete mental recovery from your cardiac arrest? The Q1 and Q2 bars show the proportion of yes answers among all followed-up patients at 3 and 6 months. The Q1a bars show the proportion of patients giving a yes answer of those who answered yes to Q1. Q, question.
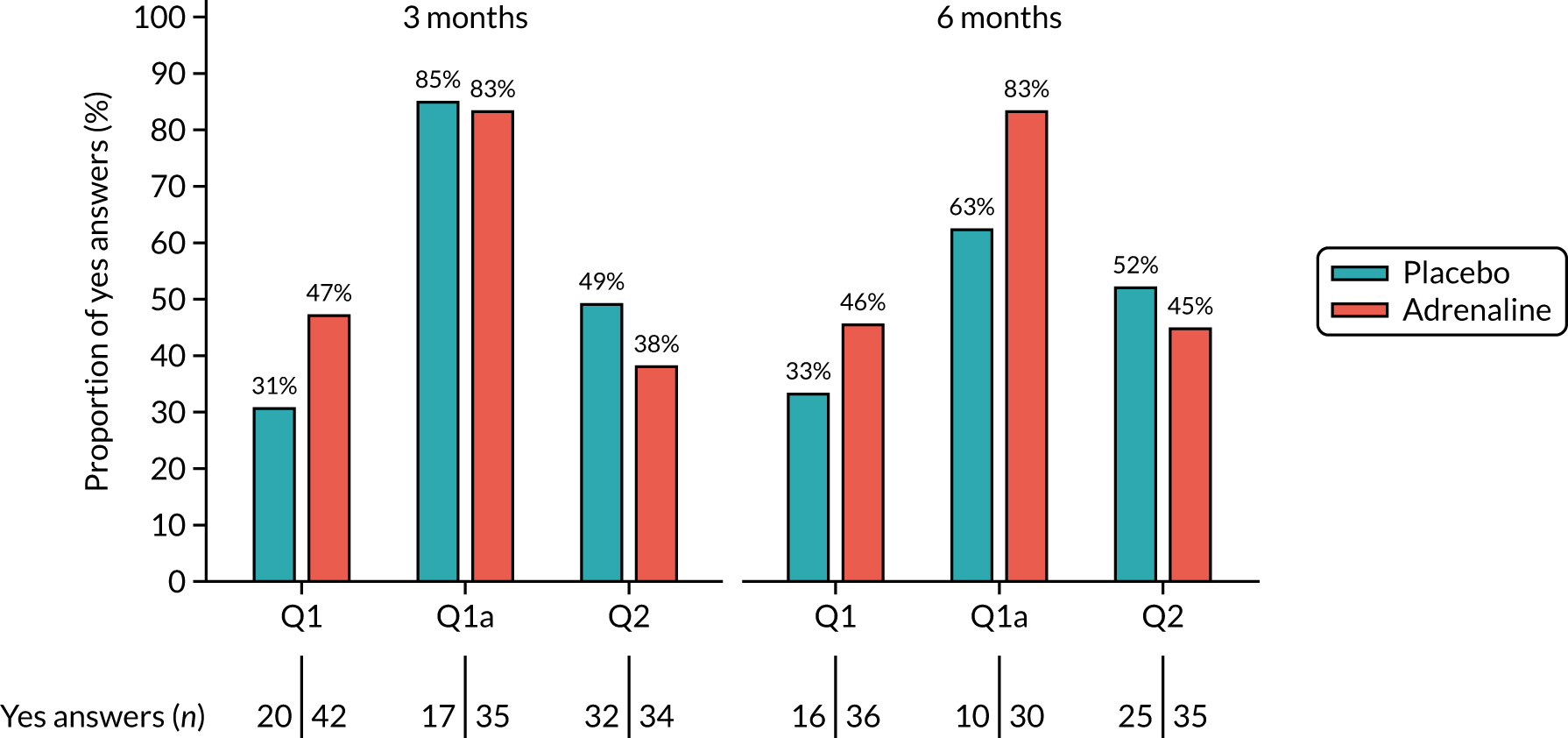
Ancillary analysis
Subgroup analysis
The treatment effect on survival to 30 days was further assessed in the prespecified subgroups. Survival rate was consistently higher in the adrenaline arm, but the results indicated no evidence of significant treatment difference across subgroups in both unadjusted (Figure 18) and adjusted analyses (Figures 19) (interaction p > 0.05).
FIGURE 18.
Unadjusted subgroup analysis.
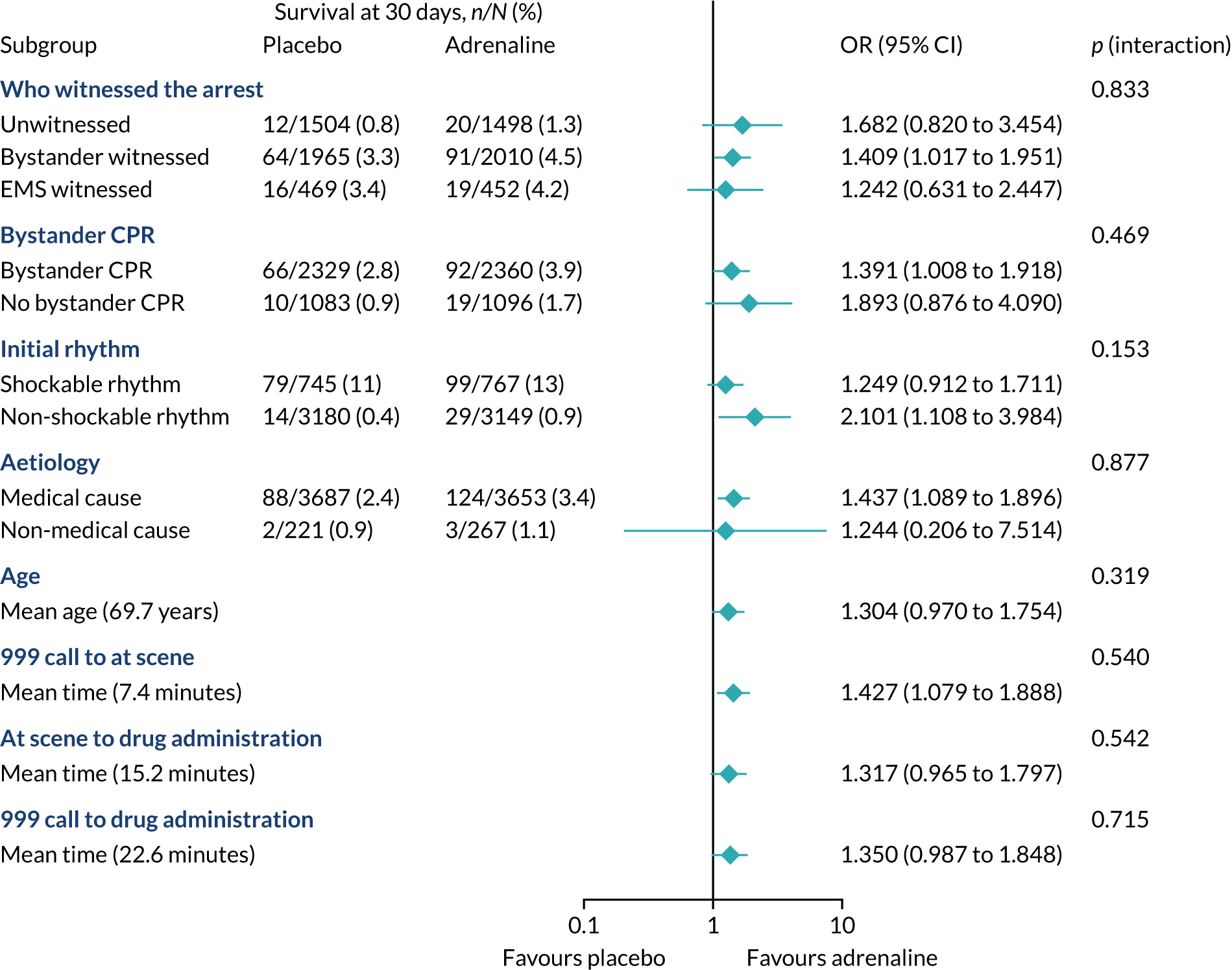
FIGURE 19.
Adjusted subgroup analysis.
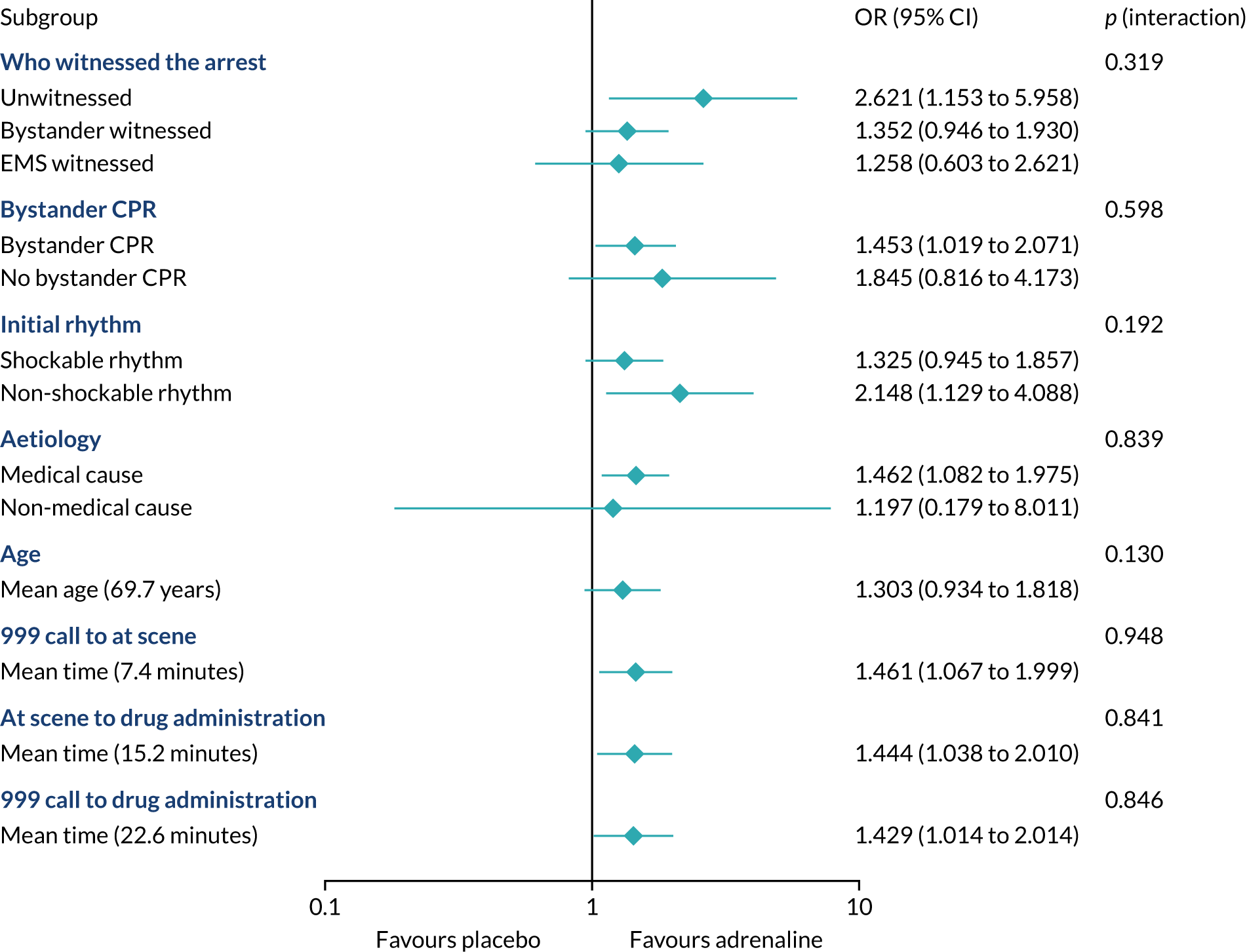
Data linkage
Patients who survived on the scene and were transported to hospital were included for data linkage (Figure 20). Data linkage to HES, the PEDW, the ICNARC data set and the UKTR was conducted for the health economic analysis. The overall match rate of the ICNARC data set and the UKTR are 22.9% and 5.5%, respectively.
FIGURE 20.
Data linkage to UKTR and ICNARC.
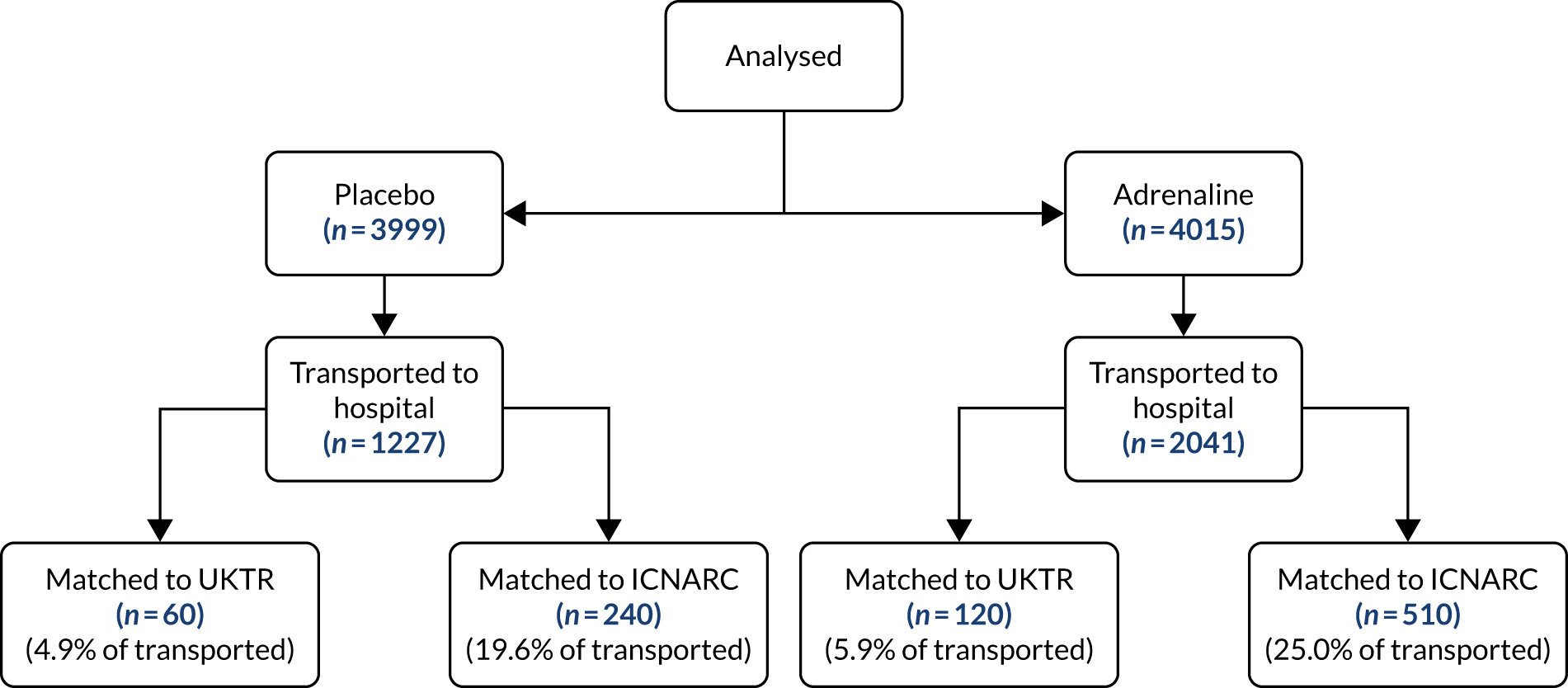
The linkage rates for the HES data sets are 96.4% for the PEWD (Welsh HES), 82.1% for NHS Digital and 92.2% across both data sets.
Sensitivity analysis
Four scenarios were evaluated in the analysis, including best-case scenario in both arms (A), worst-case scenario in both arms (B), best-case scenario in the placebo arm and worst-case scenario in the adrenaline arm (C), best-case scenario in the adrenaline arm and worst-case scenario in the placebo arm (D), and multiple imputation assuming missing at random (E). Best cases were defined as patients surviving and worst cases were defined as patients dying. Results were presented in Table 12. The longer-term outcomes are more affected by the individual imputation scenarios (C and D), but the majority of the results, including those of the multiple imputation approach, were in line with the main results.
| Scenario | Trial arm, n (%) | Difference (95% CI) (%) | Unadjusted OR (95% CI) | |
|---|---|---|---|---|
| Adrenaline (N = 4015) | Placebo (N = 3999) | |||
| 30-day survival status | ||||
| A | 133 (3.3) | 98 (2.5) | 0.9 (0.1 to 1.6) | 1.36 (1.05 to 1.78) |
| B | 130 (3.2) | 94 (2.4) | 0.9 (0.2 to 1.6) | 1.39 (1.06 to 1.82) |
| C | 130 (3.2) | 98 (2.5) | 0.8 (0.1 to 1.5) | 1.33 (1.02 to 1.74) |
| D | 133 (3.3) | 94 (2.4) | 1.0 (0.2 to 1.7) | 1.42 (1.09 to 1.86) |
| E | – | – | – | 1.38 (1.06 to 1.81) |
| Survived to hospital discharge | ||||
| A | 134 (3.3) | 95 (2.4) | 1.0 (0.2 to 1.7) | 1.42 (1.09 to 1.85) |
| B | 128 (3.2) | 91 (2.3) | 0.9 (0.2 to 1.6) | 1.41 (1.08 to 1.86) |
| C | 128 (3.2) | 95 (2.4) | 0.8 (0.1 to 1.5) | 1.35 (1.03 to 1.77) |
| D | 134 (3.3) | 91 (2.3) | 1.1 (0.3 to 1.8) | 1.48 (1.13 to 1.94) |
| E | – | – | – | 1.41 (1.07 to 1.85) |
| 90-day survival status | ||||
| A | 127 (3.2) | 94 (2.4) | 0.8 (0.1 to 1.5) | 1.36 (1.04 to 1.78) |
| B | 121 (3.0) | 86 (2.2) | 0.9 (0.2 to 1.6) | 1.41 (1.07 to 1.87) |
| C | 121 (3.0) | 94 (2.4) | 0.7 (–0.0 to 1.4) | 1.29 (0.98 to 1.70) |
| D | 127 (3.2) | 86 (2.2) | 1.0 (0.3 to 1.7) | 1.49 (1.13 to 1.96) |
| E | – | – | – | 1.41 (1.07 to 1.87) |
| 180-day survival status | ||||
| A | 126 (3.1) | 93 (2.3) | 0.8 (0.1 to 1.5) | 1.36 (1.04 to 1.79) |
| B | 117 (2.9) | 85 (2.1) | 0.8 (0.1 to 1.5) | 1.38 (1.04 to 1.83) |
| C | 117 (2.9) | 93 (2.3) | 0.6 (–0.1 to 1.3) | 1.26 (0.96 to 1.66) |
| D | 126 (3.1) | 85 (2.1) | 1.0 (0.3 to 1.7) | 1.49 (1.13 to 1.97) |
| E | – | – | – | 1.38 (1.04 to 1.83) |
| Survival with good neurological outcome at discharge (mRS score of ≤ 3) | ||||
| A | 95 (2.4) | 79 (2.0) | 0.4 (–0.2 to 1.0) | 1.20 (0.89 to 1.63) |
| B | 87 (2.2) | 74 (1.9) | 0.3 (–0.3 to 0.9) | 1.17 (0.86 to 1.61) |
| C | 87 (2.2) | 79 (2.0) | 0.2 (–0.4 to 0.8) | 1.10 (0.81 to 1.50) |
| D | 95 (2.4) | 74 (1.9) | 0.5 (–0.1 to 1.1) | 1.29 (0.95 to 1.75) |
| E | – | – | – | 1.18 (0.86 to 1.61) |
| Survival with good neurological outcome at 3 months (mRS score of ≤ 3) | ||||
| A | 111 (2.8) | 83 (2.1) | 0.7 (0.0 to 1.4) | 1.34 (1.01 to 1.79) |
| B | 82 (2.0) | 63 (1.6) | 0.5 (–0.1 to 1.1) | 1.30 (0.94 to 1.81) |
| C | 82 (2.0) | 83 (2.1) | –0.0 (–0.7 to 0.6) | 0.98 (0.72 to 1.34) |
| D | 111 (2.8) | 63 (1.6) | 1.2 (0.6 to 1.8) | 1.78 (1.30 to 2.43) |
| E | – | – | – | 1.30 (0.93 to 1.81) |
| Survival with good neurological outcome at 6 months (mRS score of ≤ 3) | ||||
| A | 103 (2.6) | 84 (2.1) | 0.5 (–0.2 to 1.1) | 1.23 (0.92 to 1.64) |
| B | 78 (2.0) | 58 (1.5) | 0.5 (–0.1 to 1.1) | 1.35 (0.96 to 1.90) |
| C | 78 (2.0) | 84 (2.1) | –0.1 (–0.8 to 0.5) | 0.92 (0.68 to 1.26) |
| D | 103 (2.6) | 58 (1.5) | 1.1 (0.5 to 1.7) | 1.79 (1.29 to 2.48) |
| E | – | – | – | 1.36 (0.97 to 1.92) |
Harms, serious adverse events
Serious adverse events
No events fulfilling the criteria for SAEs, SARs or SUSARs were reported during the trial.
Protocol deviations and violations
Table 13 shows the type and frequency of protocol deviations and violations throughout the trial, and Figure 21 shows the number of violations over time as a proportion of recruitment in each month. Overall, the proportions were small. The peak in October 2015 was mainly as a result of the small number of participants recruited by this early stage. The dotted line shows the decreasing trend of violations over time. Figure 22 is the statistical process control chart of the change of protocol violations over time, as a percentage of recruitment, from June 2015 to October 2017. The violation had large variation at the early stage, due to the peak in October 2015 (see Figure 22). The change then stabilised and was mostly limited to the upper one-sigma (SD) limit.
| Frequency (n) | |
|---|---|
| Type of deviation | |
| Expired trial drug pack used (used within 12-month shelf life) | 6 |
| Two trial packs opened for one patient (when all 10 doses from the first pack had been administered) | 4 |
| Open-label adrenaline given after 10 doses of the IMP | 19 |
| No active notification of patient enrolment to research team (no effect on follow-up of patient) | 359 |
| One pack used for two patients | 8 |
| Other | 32 |
| Total | 428 |
| Type of violation | |
| Expired trial drug pack used (used after end of 12-month shelf life) | 3 |
| Paramedic enrolled patient without completing trial training | 19 |
| Two trial packs opened for one patient (when < 10 doses had been administered from the first pack) | 10 |
| Ineligible patient enrolled (known to be ineligible at the point of randomisation) | 8 |
| Open-label adrenaline given after < 10 doses of the IMP | 40 |
| Other (IMP administered post ROSC; trained paramedic did not travel to A&E with untrained crew; patient’s relatives contacted after they had declined consent for follow-up) | 3 |
| Total | 83 |
FIGURE 21.
Frequency of protocol violations over time.
FIGURE 22.
Statistical process control chart of the frequency of protocol violations over time as a percentage of recruitment.
Serious breaches
Two serious breaches occurred during the trial, as outlined in the following sections.
Investigational Medicinal Product management
During scheduled reconciliation of the IMP (batches 6.2 and 6.3), the number of unaccounted packs in one ambulance service reached 4.4%. An investigation was instigated and the results were reviewed by the TMG. It was concluded that the risk to trial subjects, the public and to the scientific value of the trial was low. However, it was discussed with the MHRA and classified as a serious breach due to non-compliances associated with IMP management. A corrective and preventative actions plan was put in place, which included several communications to paramedic crews, thorough tracking of the drug pack journey for unaccounted packs and more regular drug audits. The rate of unaccounted-for packs reduced to 3.3% for future batch reconciliation and 2.7% overall. The serious breach was considered closed by the MHRA on 27 July 2017.
Enrolment error, trial number 7398
A patient was enrolled who met one of the trial exclusion criteria. The HM Coroner inquest found that the error did not contribute to the patient’s death. A root-cause analysis found that the paramedic did not adequately check the contents of the syringe before administering it; therefore, incorrect medicines were selected and given. This was reported to the MHRA as a potential serious breach, and was considered closed on 28 August 2018.
Corrective and preventative actions
The following preventative measures were in place prior to this incident occurring:
-
Face-to-face trial refresher training had been provided to clinicians on an ongoing basis.
-
Paramedics were provided with aide memoires detailing inclusion and exclusion criteria.
-
Eligibility criteria stickers had been added to all IMP packs to act as a reminder.
-
A question-and-answer exercise was undertaken at South Central Ambulance Service giving examples of non-compliances and checking that teams were aware of the correct course of action.
The following additional actions were put in place following the incident:
-
The enrolling paramedic was prevented from enrolling further patients in the trial until retraining had taken place.
-
A communication was sent on two separate occasions to all paramedics in the ambulance service to act as a reminder of eligibility criteria.
-
A message was sent to all ambulance service dashboards reminding clinicians to check patient eligibility before administering the PARAMEDIC2 trial IMP.
-
Refresher training slides were updated to emphasise eligibility criteria.
Assessment of impact of protocol non-compliances
It is the assessment of the TMG that the trial complied with GCP and that the deviations, violations and serious breaches did not have any material impact on the trial findings.
Chapter 4 Economic evaluation
Overview
A prospective within-trial economic evaluation was conducted to estimate the cost-effectiveness of adrenaline, compared with placebo, for OHCA. Costs are expressed in Great British pounds (2017 price year) and health outcomes are expressed in terms of QALYs, 6-month overall survival and 6-month survival with good neurological function (mRS score of ≤ 3). The base-case analysis used intention-to-treat data covering the 6-month period from randomisation and was conducted from the perspective of the UK NHS and PSS. 132
A decision-analytic model was used to extrapolate outcomes beyond the trial follow-up and to assess the cost-effectiveness of adrenaline over a lifetime time horizon. Costs and outcomes were discounted to present values at 3.5% per annum only when the time horizon of the analyses was > 1 year (i.e. the lifetime decision-analytic model). Sensitivity analyses explored the probable impact of alternative data inputs (e.g. as a result of adopting a broader societal perspective) and assumptions on cost-effectiveness outcomes. Subgroup analyses were conducted to estimate heterogeneity in cost-effectiveness outcomes. Findings are reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines. 133
Measurement and valuation of resource use
Economic costs were calculated using estimates of resource inputs associated with pre-hospital emergency response, including treatment costs, and using estimates of broader utilisation of hospital- and community-based health and social care services.
Initial emergency response
Resource inputs associated with the initial pre-hospital emergency response were extracted from the trial CRFs completed by research paramedics. These data included the number of emergency response staff/ambulance crew and vehicles in attendance, the duration of the emergency response and the cumulative adrenaline doses administered. Emergency response duration was defined as (1) the interval between the at-scene time (i.e. the time at which the emergency response team arrived at scene) and the left-scene time (or the time at which CPR was stopped if the left-scene time was not recorded) if the cardiac arrest occurred at home and the patient was pronounced dead at scene, or (2) the interval between at-scene time and arrival-at-hospital time if the patient survived and was taken to hospital.
It was assumed that one vehicle transported the patient to hospital if multiple vehicles were in attendance, with the remaining vehicles standing down and available for the next assignment. Consequently, a 1-hour stand-down time was added to the emergency response time for each patient to account for ambulance crew stand-down and restock time following discussions with the trial paramedics about duration of these activities under current UK clinical practice. Sensitivity analyses (see Table 14, sensitivity analyses 11 and 13) explored the impact of excluding the cost of emergency response crew stand-down and restock time on cost-effectiveness outcomes.
Initial emergency response data were generally collected for trial participants up to the point of death or hospital admission. This meant that trial data were not available to estimate some resource inputs for patients who died at scene. In particular, the trial CRFs did not record post-death ambulance crew activity, such as whether or not the deceased person was transported to a mortuary. Thus, it was assumed that patients pronounced dead at the scene of the cardiac arrest would be left at the scene if the location of the cardiac arrest was the patient’s home, and would be transported by emergency ambulance to the nearest hospital or public mortuary if the location of the cardiac arrest was a public place. Google Maps (Google Inc., Mountain View, CA, USA) [called from within R (The R Foundation for Statistical Computing, Vienna, Austria) via packages ‘gmapsdistance’ and ‘googleway’] was then used to estimate ambulance travel time to the nearest hospital mortuary for the subgroup of patients whose arrest occurred in a public place. Sensitivity analyses explored the impact of variations in this assumption on the base-case cost-effectiveness results (see Table 14, sensitivity analyses 12 and 13).
Hospital- and community-based health and social care service use
Data on the use of hospital- and community-based health and social care services covering the 6-month period from randomisation were collected for trial participants by three principal means:
-
Trial CRFs completed by research paramedics provided details of the initial hospitalisation episode, including ED assessment and treatment and inpatient length of stay in the ICU and cardiac and general adult wards.
-
Hospital Episode Statistics134 were requested for trial participants, covering the period from each participant’s cardiac arrest event to 31 March 2018. This provided a complete profile of resource use associated with all hospital episodes for all participants over the trial time horizon, including ED attendances, outpatient attendances, day-case admissions and critical care and other inpatient admissions covering procedures undertaken and lengths of stay. When HES data were not available, data collected using the trial CRFs and patient self-reports of hospital-based service use were used instead.
-
Economic questionnaires completed by trial participants or their proxies at the 3- and 6-month post-randomisation assessment points provided details of hospital re-admissions and use of hospital outpatient services, community health and social care services, prescribed medications, NHS supplies, child-care costs, time off work and out-of-pocket medical expenses.
Valuation of hospital resource use collected through trial case report forms
Hospital resource inputs were estimated for trial participants using the trial CRFs and valued by attaching unit costs drawn largely from secondary sources. Hospital-based services included inpatient admissions, outpatient visits and diagnostic tests and scans. Unit costs for these services were obtained primarily from the 2016/17 NHS reference costs main schedules,135 and acted as an alternative source of hospital cost values to the HES-generated data. Average daily unit costs were calculated for hospital-based services using a costing methodology previously described in a HTA report that informed an economic evaluation of a mechanical versus manual CPR for OHCA. 136 The costing method involves assigning identifying Healthcare Resource Group (HRG) codes for clinical care most likely to be received following OHCA and calculating a per diem cost weighted by the level of activity.
Valuation of Hospital Episode Statistics data
The HRG4+ 2016/17 Reference Costs Grouper137 was used to generate HRG codes for each record of admitted care and critical care episode, ED attendance, and outpatient visit extracted from HES for each trial participant. Records were matched to grouper costs in the 2016/17 NHS Reference Costs main schedules135 based on the HRG and combination variables describing the level of resource use associated with each episode of care. ED and outpatient attendances were matched to averaged reference costs based on the HRG code only. Admitted care episodes were matched at the full consultant episode level based on the HRG, patient classification (admitted vs. day case), type of admission (elective vs. non-elective) and length of stay (short stay vs. long stay). Inpatient length of stay was defined as short stay if the episode duration was 1 day and was described as long stay for episodes lasting ≥ 2 days, in line with national reference cost calculations (see pages 47 and 48 of the National Cost Collection guidance 2018138).
Unbundled HRGs associated with high-cost drugs, devices and investigations and excess bed-days were automatically generated by the Grouper software for each admitted care record. Reference costs were attached to unbundled HRGs and excess bed-day costs generated by multiplying the duration of excess bed-days by respective excess bed-day unit costs in the 2016/17 reference cost schedules. 135 Costs were summed across full consultant episodes within spells to generate total costs per admitted care spell covering the total time period between admission and hospital discharge. Critical care records were matched to reference costs based on the critical care HRG, and critical care unit function code describing type of critical care activity. Non-specific general adult critical care patients and the maximum number of organ systems supported at any one time during the critical care period predominate in the algorithms were used to cost critical care episodes. 139
Resource use and economic costs extracted from participant-completed questionnaires
Economic questionnaires completed by trial participants or their proxies at the 3- and 6-month post-randomisation assessment points provided resource use data that complemented the resource use data extracted from the trial CRFs and HES data sets. Details of hospital re-admissions, by type and duration of hospital admission and hospital outpatient service, by type and volume, were extracted from the economic questionnaires and valued using 2016/17 NHS reference costs main schedules. 135
Primary and community health and social care services included face-to-face or telephone contacts and/or home visits by a general practice doctor, practice nurse, community physiotherapist or other community health or social care professionals. Consultation costs were derived from the Personal Social Services Research Unit’s Unit Costs of Health and Social Care 2017. 140 The cost of prescribed medication was obtained primarily from the prescription cost analysis 2017 database141 and electronic searches of the British National Formulary (BNF) 2017 edition. 142 Typical dosages and durations of treatment reported in the BNF for each medication were used in calculating quantities of individual preparations if the daily dose and/or duration of a course of medication was not reported in the trial documentation.
The quantities of over-the-counter medicines were rounded to the nearest pack and unit costs were obtained from online sources. Costs of aids and supplies (e.g. walking aids, heart monitors) were either provided by the trial participants themselves (if self-purchased) or derived from the NHS supply chain catalogue for aids and supplies provided by health providers during the trial follow-up period. Unit costs for health-care resource inputs were inflated/deflated to 2016/17 prices when necessary using the NHS Hospital and Community Health Services Pay and Prices Index, and the Consumer Prices Index. 140
The economic questionnaires completed by trial participants or their proxies at the 3- and 6-month post-randomisation assessment points also provided details of broader societal (non-NHS/PSS) resource input and costs, such as out-of-pocket medical expenses (e.g. privately purchased medication, travel costs as a result of hospital and medical appointments), child-care costs, income lost, housework help and laundry services costs. Out-of-pocket medical expenditure (e.g. medication purchased by patients themselves and travel costs) were either provided in monetary terms or valued by attaching unit costs to resource inputs provided. Non-health-care costs such as child-care costs and lost income were generally provided by participants and/or their proxies in monetary terms; therefore, no requirement to value these outcomes was necessary.
Estimation of costs for the total 1-year period after randomisation
Hospital Episode Statistics134 were requested for trial participants covering the period from the cardiac arrest event to 31 March 2018. This meant that patients followed up after this date had incomplete HES records covering the 1-year period after the cardiac arrest event. The following methodology was used to calculate 1-year hospital costs estimates for all inpatients, including those with < 1 year of complete HES data:
-
Hospital Episode Statistics data were available for 1833 (45.7%) participants in the adrenaline group and 1094 (27.4%) participants in the placebo group. Of these, complete records of hospital resource use up to 1 year were available for 1802 (98.3%) of the 1833 in the adrenaline group and 1068 (97.6%) of the 1094 in the placebo group. These data were used to estimate costs of hospital care (initial hospitalisation episode, re-admissions and use of non-admitted hospital care services such as day-case admissions, ED attendances and outpatient attendances) covering the period between the cardiac arrest event to 1 year post event. The remaining 31 (1.7%) patients in the adrenaline group and 26 (2.4%) in the placebo group had HES-based records of hospital resource use for < 1 year. The 1-year costs of hospital resource use for these patients were treated as missing data.
-
Of the remaining 2182 (54.3%) trial participants in the adrenaline group and 2905 (72.6%) trial participants in the placebo group who did not have HES data, 1984 (90.9%) in the adrenaline group and 2778 (95.6%) in the placebo group died at the scene of the cardiac arrest or were not taken to hospital. We assigned zero hospitalisation costs to these patients. The remaining 198 (8.1%) and 127 (4.4%) participants in the adrenaline and placebo groups, respectively, who were taken to hospital but had no HES records were either treated as missing data (if they declined consent to continued participation) or assigned 1-year hospitalisation costs based on the CRFs and data collected through the economic questionnaires completed by trial participants or their proxies.
-
The cost of hospital resource use covering the 0–6 months post-randomisation period was estimated based on resource use extracted from the CRF and economic questionnaire data. The costs of hospital re-admissions and non-admitted care services in the 6- to 12-month window were estimated by extrapolating costs incurred in the 3- to 6-month period to 6–12 months. This essentially multiplied the 3- to 6-month costs by three for the calculation of 1-year hospital costs.
-
Finally, 1-year costs associated with the use of non-hospital-based health and social care services and societal costs were estimated by extrapolating costs incurred at 3–6 months post randomisation (derived from participant-completed questionnaires) to 6–12 months post randomisation for all trial participants.
Measurement of outcomes
Health-related quality of life and survival
Health-related quality of life was assessed using generic instruments (i.e. the EQ-5D-5L and SF-12 version 2) completed at 3 and 6 months post randomisation and the mRS instrument completed at hospital discharge and at 3 and 6 months post randomisation. Responses to the EQ-5D-5L descriptive system were converted into health utility (or preference-based health-related quality-of-life) values, based on the UK EuroQol-5 Dimensions, three-level version (EQ-5D-3L), tariff143 using the van Hout et al. 144 interim cross-walk algorithm, in line with current NICE recommendations. 145 The mRS scores were converted into health utility (or preference-based health-related quality-of-life) values, based on the UK EQ-5D-3L tariff,143 using a published mapping function developed by Rivero-Arias et al. 146 The SF-12 scores were converted into health utility values using the Short Form questionnaire-6 Dimensions (SF-6D) utility tariff for the UK published by Brazier et al. 147 Survival outcomes following cardiac arrest were also collected to 12 months post cardiac arrest for trial participants.
Quality-adjusted life-years
Quality-adjusted life-year profiles were generated for each trial participant using area-under-the-curve methods, assuming a baseline health utility value of zero and linear interpolation between utility measurements, taking into account survival over the 6-month time horizon of interest (base case). In the base-case analysis, we combined utility values from the mRS scores at hospital discharge and utility values derived from the EQ-5D-5L at 3 and 6 months to calculate QALY profiles over 6 months. We used mRS-derived health utilities in place of EQ-5D-5L values if the latter were missing at the 3- or 6-month post-randomisation assessment points. 146 Health utility values generated from the mRS were available at hospital discharge and at all assessment points over the 6-month base-case time horizon. Thus, the impact of combined EQ-5D-5L-/mRS-generated QALYs on the base-case cost-effectiveness results was assessed in a sensitivity analysis that used QALYs generated solely from the mRS scores. Patients who died were assigned a utility value of zero onwards from time of death for the calculation of QALYs.
We did not assess health-related quality of life at baseline, as the clinical context precluded collecting any patient-reported outcome data around the time of randomisation: patients were either unconscious or too critically ill to complete questionnaires. 148 Alternative assumptions around health-related quality of life at baseline explored the impact on the cost-effectiveness of adrenaline of setting the baseline utility value to zero. In the first of these, the baseline utility value in the adrenaline and placebo groups was set to –0.402, reflecting UK population preferences for an unconscious health state within the EQ-5D-3L A1 tariff set. 149
We also calculated QALY profiles over 12 months, under the assumption that patients’ health-related quality of life at 12 months was the same as their health-related quality of life at 6 months, if they had survived, and we used this information to inform a sensitivity analysis exploring longer-term cost-effectiveness.
Statistical analysis of economic data
Summary of resource use and costs
Patient-level costs were generated for each resource variable by multiplying the quantity reported by the respective unit cost, weighted by length of stay or duration of contact when appropriate. Summary statistics (means, standard errors and completion rates) were generated for the whole trial population and for those surviving to hospital discharge by treatment allocation and assessment point. Between-group differences in mean resource use and mean costs at each assessment point were compared using the two-sample t-test. Statistical significance was assessed at the 5% significance level. A non-parametric bootstrap routine was implemented, generating 1000 replications of the data. Estimates of standard errors surrounding mean resource use (or cost) estimates and 95% CIs surrounding between-group differences in mean resource use (or costs) were obtained from the bootstrapped samples.
Summary of health-related quality-of-life data
Responses to each health dimension of the EQ-5D-5L and SF-12 are presented by level of function. Comparisons of responses were conducted on the basis of optimal level of function (e.g. ‘no problem’ on the EQ-5D-5L) versus suboptimal level of function (indicating any functional impairment). Between-group differences in optimal versus suboptimal level of function for each health dimension were compared for each outcome measure using chi-squared (χ2) tests at each assessment point for each health-related quality-of-life instrument. Summary statistics (means, standard errors and completeness rates) for health utilities were generated for the whole trial population and for survivors to hospital discharge by treatment allocation, assessment point and health-related quality-of-life instrument. Estimates of between-group difference in mean health utility values and 95% bootstrapped CIs surrounding mean group differences were generated based on 1000 bootstrapped resamples of the data.
Missing data
In addition to trial-specific protocols and procedures to minimise missing data, a strategy was employed prior to commencement of recruitment to identify and collect resource use and health outcomes data from multiple sources, with the specific aim of reducing the level of missingness for the economic outcomes of interest. Participants were assumed to incur no health-care costs beyond the initial emergency response costs if cardiac arrest occurred at home and the patient was declared deceased at the scene of arrest. Costs associated with use of hospital-based services were generated primarily from HES data; information obtained through trial-specific procedures (trial CRFs and participant questionnaires) was used when HES data were not available.
Health-related quality of life was assessed using multiple instruments, including the mRS completed at hospital discharge and the mRS, EQ-5D-5L and SF-12 completed by trial participants at 3 and 6 months post randomisation. Survival information was collected through trial-specific procedures and Office for National Statistics data. This process of using multiple data sources to systematically fill in missing data provided a more complete profile of the main economic outcomes of interest, compared with relying on a single data source.
Nevertheless, multiple imputation by chained equations (MICE), implemented through the R package MICE,150 was used to predict values for any remaining missing items, assuming data were missing at random. Missing costs and health utility values were imputed at the level of resource category and health-related quality-of-life assessment, stratified by survival status at hospital discharge and treatment allocation in accordance with good-practice recommendations outlined in Faria et al. 151 Imputation was achieved using predictive mean matching, which has the advantage of preserving non-linear relationships and correlations between variables within the data. Twenty imputed data sets were generated and used to inform the base-case and subsequent sensitivity and subgroup analyses. Parameter estimates were pooled across the 20 imputed data sets using Rubin’s rules to account for between- and within-imputation components of variance terms associated with parameter estimates.
Base-case cost-effectiveness
The base-case cost-effectiveness analysis used the intention-to-treat data to estimate the cost–utility of adrenaline, compared with placebo, from the perspective of the UK NHS and PSS. 132 Economic costs and QALYs were calculated for each patient over a 6-month post-randomisation time horizon. Total costs were calculated by summing costs associated with the initial emergency response (including the cost of the intervention) and costs of broader hospital- and community-based health and social care services. QALYs were generated based on health utility data generated from the mRS score at hospital discharge and the EQ-5D-5L score at the 3- and 6-month assessments.
Two seemingly unrelated normal error regressions were fitted to imputed data using the systems fit implementation in R,152 which accounts for the correlation between patient-level costs and QALYs. The regressions controlled for treatment allocation, age, sex, time to first dose administration, cause of cardiac arrest, whether or not the cardiac arrest was witnessed, bystander CPR and rhythm. The base-case analyses were replicated using the Stata® version 15 (StataCorp LP, College Station, TX, USA) MICE and seemingly unrelated regressions SUR REG functions.
The incremental cost-effectiveness ratio (ICER) was calculated for adrenaline, compared with placebo, by dividing the between-group difference in adjusted mean total costs by the between-group difference in adjusted mean QALYs. Cost-effectiveness was assessed by comparing the ICER to cost-effectiveness thresholds of between £15,000 and £30,000 per QALY gained, in line with NICE guidance132 and the recent empirical threshold of £13,000 per QALY estimate suggested by Claxton et al. 153 Estimates were also generated assuming £50,000 and £100,000 values for the cost-effectiveness threshold for a QALY, reflecting potentially higher willingness-to-pay thresholds for a life-saving intervention. The incremental net (monetary) benefit of adrenaline compared with placebo was calculated for cost-effectiveness thresholds ranging from £15,000 to £200,000 per QALY gained. Net monetary benefit values reflect the opportunity cost of (or the benefits forgone from) adopting a new treatment when resources could be put to use elsewhere. A positive net monetary benefit suggests that, on average, adrenaline provides a net gain, compared with placebo, for the health service and can be considered cost-effective at the given cost-effectiveness threshold.
Uncertainty around the mean cost-effectiveness estimates was characterised through a Monte Carlo method. 154 This involved simulating 1000 replicates of the ICER from a joint distribution of the incremental costs and QALYs and plotting the simulated ICERs on the cost-effectiveness plane. A sensitivity analysis explored an alternative characterisation of uncertainty based on generating 1000 bootstrap replications of the ICER instead of the Monte Carlo method (see Table 14, sensitivity analysis 4). Cost-effectiveness acceptability curves (CEACs) were also plotted to give graphical display of the probability that adrenaline is cost-effective across a wide range of cost-effectiveness thresholds. A sensitivity analysis explored the impact of embedding the imputation model within the non-parametric bootstrap to simulate 1000 replicates of the ICER to calculate the probability that adrenaline is cost-effective at the cost-effectiveness thresholds specified previously.
Analysis of secondary health economic outcomes
Secondary health economic outcomes were analysed using logistic regression models that were embedded in the bootstrap and imputation models. The regression models estimated adjusted overall survival and neurologically intact survival probabilities for adrenaline and placebo at 6 months post randomisation. This preserved the correlation between patient-level costs and effects and allowed us to express the cost-effectiveness of adrenaline as incremental costs per unit increase in the proportion of patients surviving to 6 months post cardiac arrest.
Sensitivity analyses
Table 14 summarises methods and assumptions underlying the base-case and the sensitivity analyses conducted to explore the impact of alternative modelling assumptions and methods on the cost-effectiveness of adrenaline.
| Sensitivity analysis | Base-case methods/assumptions | Changes implemented in sensitivity analyses |
|---|---|---|
| Prespecified | ||
| 1 | Adjusted multiple imputation | Unadjusted multiple imputation |
| 2 | Adjusted multiple imputation | Adjusted complete-case analysis |
| 3 | Adjusted multiple imputation | Unadjusted complete-case analysis |
| 4 | Parameter estimates via seemingly unrelated linear regression | Parameter estimates via linear regression implemented within a boostrap |
| 5 | QALYs based on EQ-5D-5L/mRS data | QALYs based on mRS data only |
| 6 | QALYs derived assuming baseline utility of 0 (equivalent to dead) | QALYs derived assuming baseline utility of –0.042 (equivalent to unconscious health state) |
| 7 | QALYs based on EQ-5D-5L and mRS data, NHS/PSS costs | QALYs based on EQ-5D-5L and mRS data, 6 months’ societal costs |
| 8 | 6-month time horizon (QALYs based on EQ-5D-5L and mRS data, NHS/PSS costs) | 12-month time horizon (QALYs based on EQ-5D-5L and mRS data, NHS/PSS costs) |
| 9 | QALYs based on EQ-5D-5L and mRS data, 6-month time horizon | 12-month time horizon (QALYs based on mRS data, NHS/PSS costs) |
| 10 | QALYs based on EQ-5D-5L and mRS data, 6-month time horizon | 12-month time horizon (QALYs based on EQ-5D-5L and mRS data, societal costs) |
| Post hoc analyses | ||
| 11 | 1 hour added to emergency response cost calculations to account for stand-down and restock time | Excluded stand-down and restock time in emergency response cost calculations |
| 12 | Accounts for cost of transporting deceased patients to nearest hospital or local authority mortuary if patient died at scene of cardiac arrest and in a public place | Excluded estimated cost of transporting deceased patients to nearest hospital mortuary if patient died at scene of cardiac arrest and in a public place |
| 13 | Accounts for stand-down/restock time and transportation costs to nearest mortuary if patients died in a public place in emergency response cost calculations | Excluded stand-down/restock time and cost of transporting patients to nearest mortuary in emergency response cost calculations |
Subgroup analysis
Prespecified subgroup analyses were also conducted based on specifications in the PaRAMeDIC trial81 statistical analysis plan to explore whether or not there are subgroups of patients for whom adrenaline is likely to be cost-effective. In addition, one post hoc subgroup explored the treatment dose–response relationship and estimated the dose at which adrenaline is likely to be most cost-effective. Our subgroup analyses were as follows:
-
Cardiac arrest witnessed by paramedic versus cardiac arrest witnessed by bystander versus cardiac arrest not witnessed.
-
Bystander CPR versus no bystander CPR for bystander-witnessed and not-witnessed OHCAs.
-
Type of initial rhythm: shockable (VT/VF) versus non-shockable (PEA/asystole).
-
Aetiology of cardiac arrest (medical vs. non-medical).
-
Age (≤ 60 years vs. > 60 years).
-
Time interval from 999 call to EMS arrival (≤ 10 minutes vs. > 10 minutes) among those with a witnessed arrest.
-
Time interval from EMS arrival to administration of trial drug (≤ 10 minutes vs. > 10 minutes) among those with a witnessed arrest.
-
Time interval from 999 call to administration of trial drug (≤ 10 minutes vs. > 10 minutes) among those with a witnessed arrest.
-
The time to emergency treatment variables were categorised based on previous studies reporting that administration of adrenaline within 10 minutes following cardiac arrest is associated with neurologically improved survival outcomes. 155
Extrapolating beyond trial follow-up
A simple Markov state-transition model was built in R using the package heemod156 to extrapolate the within-trial results over the lifetimes of cardiac arrest survivors. The model as shown in Figure 23 comprises four health states (OHCA state, post-OHCA survival with good neurological function, post-OHCA survival with poor/impaired neurological function and death). The cycle length is 1 year and all individuals start in the OHCA state at the incidence of cardiac arrest event. Possible transitions from this state represent 1-year survival with good or poor neurological function and death. This implies that the OHCA state captures all the economic costs and benefits in the first year after randomisation and so corresponds to the within-trial cost-effectiveness analysis with a 1-year time horizon. In the PARAMEDIC2 trial, neurological function was assessed at hospital discharge and at 3 and 6 months post randomisation. This information was used to categorise post-OHCA survival as good if the score of the mRS assessment closest to the 1-year time point after the cardiac arrest was ≤ 3 and to categorise it as poor for scores of > 3. Probabilities governing these transitions were estimated separately for the adrenaline and placebo groups, based on these categorisations, and are summarised in Table 15.
FIGURE 23.
Model to extrapolate cost-effectiveness beyond the PARAMEDIC2 trial follow-up. C, complement (probability of remaining in good or poor neurological functional states); good, good neurological function; poor, poor neurological function; pGood2Dead, annual probability of death among survivors with good neurological function; pPoor2Dead, annual probability of death among survivors with poor neurological function; pOHCA2Dead, probability of death within first year post cardiac arrest/from randomisation; pOHCA2Good, probability of surviving to 1 year with good neurological function; pOHCA2Poor, probability of surviving to 1 year with poor neurological function.
| Parameter | Mean (SE) | Distribution | Source |
|---|---|---|---|
| Cohort characteristics | |||
| Mean age (years), 1-year post-cardiac arrest survivors | 60 | PARAMEDIC2 data | |
| Percentage of males among 1-year post-cardiac arrest survivors | 78 | Beta | PARAMEDIC2 data |
| Probabilities | |||
| 1-year survival with good function (mRS score of ≤ 3), adrenaline | 0.023 (0.0024) | Multinomial | PARAMEDIC2 data |
| 1-year survival with good function (mRS score of ≤ 3), placebo | 0.019 (0.0021) | Multinomial | PARAMEDIC2 data |
| 1-year survival with poor function (mRS score of > 3), adrenaline | 0.005 (0.0011) | Multinomial | PARAMEDIC2 data |
| 1-year survival with poor function (mRS score of > 3), placebo | 0.003 (0.0019) | Multinomial | PARAMEDIC2 data |
| 3-month mortality given alive with good function at 9 months post arrest | 0.029 (0.013) | Binomial | PARAMEDIC2 data |
| 3-month mortality given alive with poor function at 9 months post arrest | 0.061 (0.042) | Binomial | PARAMEDIC2 data |
| Annual mortality rate given survival to 1 year with good function | 0.111 (0.0457) | Derived | 0.111/UK mortality 2015157 |
| Annual mortality rate given survival to 1 year with poor function | 0.215 (0.1213) | Derived | 0.215/UK mortality 2015157 |
| Costs (£, 2017 prices) | |||
| OHCA, adjusted mean costs, 1 year post randomisation, adrenaline | 3741 (536) | Gamma | PARAMEDIC2 data |
| OHCA, adjusted mean costs, 1 year post randomisation, placebo | 2330 (541) | Gamma | PARAMEDIC2 data |
| Good functional state | 7907 (718) | Gamma | PARAMEDIC2 data |
| Poor functional state | 24,457 (2275) | Gamma | PARAMEDIC2 data |
| Utilities (EQ-5D-5L) | |||
| OHCA, adjusted mean utility, first year post randomisation, adrenaline | 0.0038 (0.0051) | Beta | PARAMEDIC2 data |
| OHCA, adjusted mean utility, first year post randomisation, placebo | 0.0061 (0.0051) | Beta | PARAMEDIC2 data |
| Good functional state | 0.707 (0.019) | Beta | PARAMEDIC2 data |
| Poor functional state | 0.0982 (0.0511) | Truncated normal | PARAMEDIC2 data |
Possible transitions beyond the first year after the cardiac arrest event were restricted to movement from the good and poor functional states to death. The probabilities governing these movements were assumed to be independent of treatment allocation and were estimated from two sources. First, we estimated the conditional probability of surviving to 12 months, given that an individual had survived the first 9 months with good or poor function, and converted these estimates to 1-year probabilities using standard formulae. 158 Second, we obtained age- and sex-adjusted UK mortality statistics,157 assuming a starting age of 60 years and 78% probability of being male, reflecting the average cohort characteristics of survivors at 1 year post randomisation. We used this information to extrapolate survival beyond the first year post cardiac arrest to a lifetime horizon by assigning individuals a probability of death equivalent to the larger of the two estimates.
We assumed that patients surviving beyond the first year after the cardiac arrest event either died or remained alive in the same functional state at each modelled cycle (functional status cannot improve or deteriorate after first year post cardiac arrest). The assumption that neurological function cannot improve or deteriorate after the first year post cardiac arrest was made because of a lack of robust data to estimate the between-state transitions, and is consistent with recent economic models evaluating the cost-effectiveness of intervention in cardiac arrest. 159
Patients in the OHCA state were assigned cost and utility values obtained from the within-trial sensitivity analysis in which the time horizon was extended to 1 year. This covered the full spectrum of costs and QALYs incurred in the first year after the cardiac arrest event, adjusted for demographic and clinical characteristics. Costs associated with the good and poor functional states were estimated from resource use estimates covering the 3- to 6-month post-randomisation period collected for trial participants. Utility values associated with good and poor functional states were collected through the EQ-5D-5L reported by trial participants and stratified by functional status at the 6-month assessment point (see Table 15). Costs generated from resource use covering the 3- to 6-month period after randomisation were converted to 1-year costs assuming that the rate of resource use in this 3-month window is maintained over a period of 1 year. Costs and utilities were discounted at 3.5% per annum over the lifetime of cardiac arrest survivors.
Chapter 5 Results
Trial population
A total of 8014 participants were randomised in the PARAMEDIC2 trial: 4015 to the adrenaline group and 3999 to the placebo group (see Figure 24). Of these, 219 participants survived to hospital discharge: 128 in the adrenaline group and 91 in the placebo group.
In our estimation of economic outcomes of interest, we assigned a nominal value of zero to resource use, costs and health utility for patients who died at the scene of the cardiac arrest or prior to the collection of health-related quality-of-life data (in which case zeros were assigned to QALYs, but not resource use and costs). Thus, for all participants based on HES and CRF sources, approximately 98.9% and 99.2% of all health resource use data (including the zero service utilisation and utility assigned to deaths) were available at 3 months for the adrenaline and placebo groups, respectively; this dropped to 98.7% and 98.8%, respectively, by 6 months (see Appendix 1, Tables 26 and 27). Similarly, a complete QALY profile over the 6-month time horizon was estimated for 7939 of the 8014 (99.1%) trial participants, based on utility weights derived from the EQ-5D-5L and mRS assessments at hospital discharge and at 3 and 6 months post randomisation (see Table 22).
When looking at the completeness rates for survivors (see Table 23), approximately 72.7% and 71.4% of all health resource use data reported on the CRFs were complete at 3 months for the adrenaline and placebo groups, respectively; this declined to 63.3% and 51.6%, respectively, by 6 months (see Appendix 1, Tables 30 and 31). A complete QALY profile based on the EQ-5D-5L and mRS utility scores was available for 132 out of 219 (60.3%) survivors at 6 months post randomisation.
Overall, approximately 1% of QALY data and between 1% and 2% of costs (at the component level) were missing (and subsequently imputed) for the primary analysis.
Resource use and costs extracted from Hospital Episode Statistics
Figure 24 displays the flow chart of data sources used to inform estimates of resource use and costs. HES data were requested for 2023 (50.4%) of the 4015 patients in the adrenaline arm and for 1214 (30.4%) of the 3999 patients in the placebo arm. This represented data for trial participants who survived to hospital admission and who did not withdraw consent to participate in the trial. Of those requested, HES records covering the 6-month (1 year for some patients) period from the cardiac arrest event were provided by NHS Digital and PEDW for 1833 patients in the adrenaline arm and 1094 in the placebo arm, generating linkage rates of 90.6% and 90.1% for the adrenaline and placebo arms, respectively. This represented 45.7% of the 4015 patients randomised to adrenaline and 27.4% of the 3999 patients randomised to placebo. For the remaining 2182 (54.3%) patients in the adrenaline arm who did not have HES data provided, 2171 (99.5%) died prior to hospital admission (and therefore incurred no costs beyond the initial emergency response costs) and 11 (0.5%) had no linkage records or survived to decline consent to continued participation in the trial. For the placebo arm, 2905 (72.6%) of the 3999 patients did not have HES data provided: 2893 (99.6%) died before they could be admitted to hospital and 12 (0.4%) either declined consent or had no linked records for analysis. Summaries of hospital resource use (length of stay in a hospital inpatient ward and ICU admissions, day-case admissions, and ED and outpatient attendances), by trial arm and follow-up period, extracted from the HES records are presented in Appendix 1, Table 26. Summary statistics were generated based on the available HES records at each level of hospital resource use, and therefore excluded trial participants who were not admitted to hospital (e.g. those who died at home), those who declined continued consent and those admitted to hospital but who had no record for a particular category or level of hospital resource use variable.
FIGURE 24.
Flow chart of data sources used to inform estimation of resource use and costs. Resource use data were collected through trial CRFs. S2hd, survive(d) to hospital discharge.
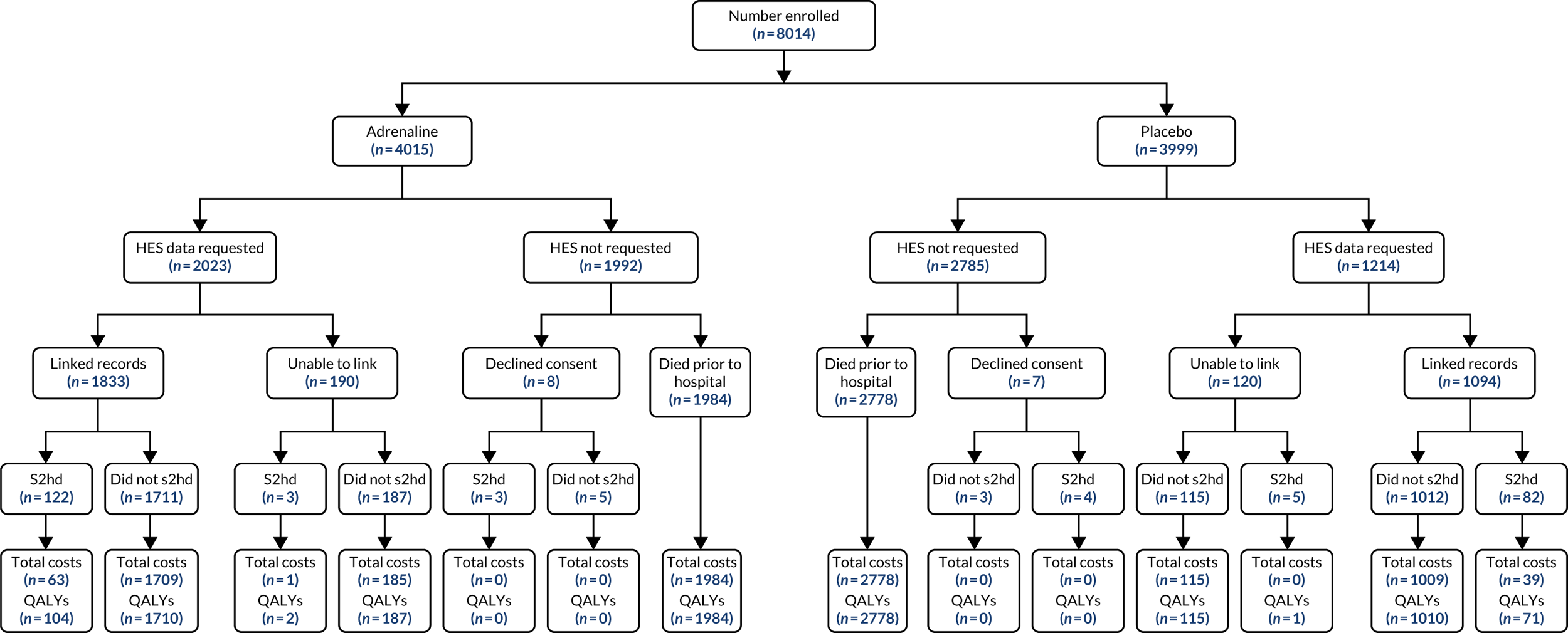
During the initial hospitalisation event among the 1833 (45.7%) and 1094 (27.4%) patients with HES data in the adrenaline and placebo arms, respectively (see Appendix 1, Table 26), there were no noticeable differences between the adrenaline and placebo arms in mean hospital length of stay (6.63 vs. 7.84 days, respectively; unadjusted mean difference –1.2 days, 95% bootstrapped CI –3.5 to 0.91 days) and ICU length of stay (5.75 vs. 6.29 days, respectively; mean difference –0.54 days, 95% CI –2.13 to 1.07 days). There were no noticeable differences between the adrenaline and placebo groups in length of stay in a general ward (18.55 vs. 16.69 days, respectively; mean difference 1.86 days, 95% CI –9.84 to 13.71 days) or an ICU (9.8 vs. 13.29 days, respectively; mean difference –3.49 days, 95% CI –12.96 to 6.31 days) for hospital re-admissions over the 6-month period after the cardiac arrest. Similarly, there were no differences between the adrenaline and placebo arms in ED attendances (1.05 vs. 1.04 visits, respectively; mean difference 0.01 visits, 95% CI –0.02 to 0.03 visits), day-case attendances (5.93 vs. 1.06 visits, respectively; mean difference 4.87 visits, 95% CI –0.12 to 15.8 visits) and outpatient attendances (5.12 vs. 6.07 visits, respectively; mean difference –0.95, 95% CI –2.58 to 0.68 visits).
Thirty-one (1.7%) of the 1833 trial participants with HES data in the adrenaline arm and 26 (2.4%) of the 1094 patients with HES data in the placebo arm who had their cardiac arrest event after 31 March 2017 were alive 1 year after arrest; therefore, these patients had partially incomplete 1-year records of hospital resource use based on the HES data. We assumed that hospital resource use data covering the 6- to 12-month period after the cardiac arrest event are missing for these patients because of incompleteness of follow-up. For the remaining 1802 (98.3%) of the 1833 patients in the adrenaline arm and 1068 (97.6%) of the 1094 in the placebo arm, complete records of hospital resource use were available for the 1-year period after the cardiac arrest event and are summarised in Appendix 1, Table 26. There were no noticeable differences between the adrenaline and placebo arms in hospital length of stay or ICU length of stay during hospital re-admissions in the 6- to 12-month period after the cardiac arrest event. Similarly, there were no differences between the two groups in ED attendance and day-case attendances, but the adrenaline arm had significantly more outpatient consultations, on average, than the placebo arm (7.45 vs. 4.3 visits, respectively; unadjusted mean difference 3.15 visits, 95% CI 0.31 to 6.74 visits).
Appendix 1, Table 27, presents hospital-related costs based on HES-only data for the 0- to 6-month and 0- to 12-month post-cardiac arrest periods by trial arm and resource category. As explained in Chapter 4, Valuation of hospital resource use collected through trial case report forms, these costs were derived by mapping HRG codes generated using the HRG4+ 2016/17 Reference Costs Grouper137 to national reference costs. 135 Across all categories of hospital services, the mean total cost per patient estimated from the HES data was £5224 for the adrenaline arm and £4777 for the placebo arm, generating a mean cost difference of £448 (95% CI –£718 to £1483; p = 0.412) during the 0- to 6-month period following the cardiac arrest event.
Costs of hospital-based services covering the period of 0–12 months after the cardiac arrest event are also summarised in Appendix 1, Table 27. As explained previously, only participants with complete 1-year HES records are included in the calculation of these costs. The mean total cost of hospital resource use over 12 months was £4913 for the adrenaline arm and £4350 for the placebo arm, generating a mean 1-year cost-difference of £563 (95% CI –£660 to £1644; p = 0.314).
Summaries of the NHS and PSS resource use values by trial allocation, resource category and trial period for complete cases collected through trial CRFs and participant-completed questionnaires are presented in Appendix 1, Table 28, for all patients and in Appendix 1, Table 30, for survivors. The equivalent summaries for non-NHS and PSS resource use information are also presented in Appendix 1, Table 29, for all patients and in Appendix 1, Table 31, for survivors. Resource use values are presented for subcategories of resource use, including emergency response, intervention doses administered, hospital inpatient and outpatient care, primary health care (residential care, community health and social care), prescribed medications, and equipment and aids. Broader societal resource inputs and costs included privately purchased medications, travel costs, child care, income lost, help with housework and laundry service costs (see Appendix 1, Tables 29 and 31).
In terms of specific resource use values from an NHS and PSS perspective for adrenaline versus placebo for all participants at 3 months (see Appendix 1, Table 28), notable differences were observed for number of syringes given (mean difference for adrenaline vs. placebo –0.152 syringes, 95% bootstrapped CI –0.266 to –0.052 syringes), ED stays (mean difference 0.201 days, 95% CI 0.179 to 0.224 days), general ward stays (mean difference 0.296 days, 95% CI 0.078 to 0.536 days) and ICU stays (mean difference 0.438 days, 95% CI 0.298 to 0.592 days). When looking at the equivalent information for survivors to hospital discharge at 3 months (see Appendix 1, Table 28), there were no noticeable differences between the trial arms.
Between 3 and 6 months post cardiac arrest, for all participants (see Appendix 1, Table 28), evidence of noticeable differences in resource use from an NHS and PSS perspective between adrenaline and placebo was observed for district nurse contacts (mean difference 0.055, 95% CI 0.003 to 0.141) and use of other aids and adaptations (mean difference 0.004, 95% CI 0.001 to 0.007). For survivors to hospital discharge (see Appendix 1, Table 30), there were noticeable differences in resource use for district nurse contacts (mean difference 2.494, 95% CI 0.013 to 6.315) only. For all other resource use items, no differences between the trial arms were apparent.
In terms of non-NHS/PSS resource use, encompassing expenditure incurred by patients, family members and lost income due to ill health as a result of the cardiac arrest, for all participants at 3 months (see Appendix 1, Table 27), no significant differences were observed during this time period. There were also no significant differences in non-NHS/PSS resource use for survivors to hospital discharge over this period (see Appendix 1, Table 31).
Between 3 and 6 months post randomisation, and for all participants (see Appendix 1, Table 29), differences in resource use from a broader perspective were observed for reliance on child care (mean difference 0.004 occurrences, 95% CI 0.001 to 0.007 occurrences), travel costs (mean difference 0.01, 95% CI 0.000 to 0.019) and other additional costs (mean difference 0.014, 95% CI 0.000 to 0.027). For survivors to hospital discharge (see Appendix 1, Table 31), there were no noticeable differences between the two trial arms for all other resource use items.
Unit costs
Unit costs, and data sources from which they were derived, of resource use variables are presented in Appendix 1, Table 32. The unit cost of emergency ambulance (including vehicle and crew) support was estimated at £8.80 per minute in 2017 prices. This was based on a 2008 estimate of £6.80 per minute of emergency ambulance response reported in the Unit Costs of Health and Social Care 2008160 compendium, published by the Personal Social Services Research Unit, and inflated to reflect the Hospital and Community Health Service pay and price inflation reported in the Unit Costs of Health and Social Care 2017. 140 The total cost of prehospital emergency response was calculated for each patient using the following formula:
Unit cost values and their sources are reported for all other resource inputs in Appendix 1, Table 32.
Economic cost estimates at level of resource use variable and category, trial data collection instruments
Tables 16 (all patients) and 17 (survivors) summarise the estimated economic costs for hospital- and community-based health and social care services (NHS/PSS costs), based on the CRF data by trial allocation, resource category and trial period. The equivalent, more disaggregated, information at the level of resource variables is presented in Appendix 1 (see Table 33 for NHS/PSS costs for all patients and Table 35 for NHS/PSS costs for survivors; see Table 34 for non-NHS/PSS costs for all patients and Table 36 for non-NHS/PSS costs for survivors). Economic costs from an NHS and PSS perspective for all participants are presented in Table 16 by trial arm, trial period and cost category. Between randomisation and 3 months post randomisation, the mean total emergency response costs were, on average, higher for the adrenaline arm than for the placebo arm: £1716 versus £1660, respectively (mean cost difference £56.41, 95% bootstrapped CI £18.54 to £94.42). Similarly, total inpatient costs were higher, on average, for the adrenaline arm than for the placebo arm: £2094 versus £1082 (mean cost difference £1011.78, 95% bootstrapped CI £729.39 to £1325.13). This was driven by higher costs for ED costs, general ward stays (£129 vs. £80 for the adrenaline vs. placebo arms, respectively; mean difference £49.19, 95% CI £13.40 to £87.45) and ICU stays (£1924 vs. £998 for the adrenaline vs. placebo arms, respectively; mean difference £926.88, 95% CI £629.78 to £1250.56) (see Appendix 1, Table 33). There were no significant differences in costs from an NHS and PSS perspective for survivors to hospital discharge at 3 months (see Table 17).
| Post-randomisation assessment period | Category | Adrenaline arm (N = 4015) | Placebo arm (N = 3999) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean cost (SE) (£) | Participants with complete data (n) | Mean cost (SE) (£) | Mean difference in costs (95% CI) (£) | p-value | ||
| 0–3 months | Emergency response costs | 4010 | 1716.03 (17.05) | 3992 | 1659.61 (10.41) | 56.41 (18.54 to 94.42) | < 0.001 |
| Intervention costs | 4008 | 33.82 (0.27) | 3990 | 0 (0) | 33.82 (33.27 to 34.32) | < 0.001 | |
| Inpatient costs | 3964 | 2093.96 (128.43) | 3967 | 1082.18 (88.63) | 1011.78 (729.39 to 1325.13) | < 0.001 | |
| Outpatient costs | 3967 | 18.62 (5.63) | 3961 | 10.63 (3.38) | 8 (–3.77 to 21.61) | 0.196 | |
| Community care costs | 3976 | 10.69 (4.76) | 3969 | 5.42 (1.67) | 5.27 (–2.75 to 17.05) | 0.308 | |
| Medication costs | 3975 | 6.8 (1.7) | 3970 | 4.98 (1.68) | 1.82 (–2.96 to 6.61) | 0.45 | |
| Aids and adaptations costs | 3967 | 1.78 (1.29) | 3964 | 1.75 (1.29) | 0.02 (–3.8 to 3.88) | 0.972 | |
| Total NHS and PSS costs | 3937 | 3764.73 (127.92) | 3936 | 2686.9 (90) | 1077.83 (790.49 to 1406.45) | < 0.001 | |
| 3–6 months | Inpatient costs | 3961 | 83.1 (50.84) | 3949 | 37.36 (19.5) | 45.73 (–37.8 to 172.54) | 0.388 |
| Outpatient costs | 3960 | 18.59 (6.93) | 3949 | 9.25 (4.02) | 9.34 (–4.73 to 25.67) | 0.218 | |
| Community care costs | 3964 | 6.54 (2.03) | 3953 | 1.55 (0.33) | 4.99 (1.36 to 9.26) | < 0.001 | |
| Medication | 3961 | 3.68 (1.2) | 3952 | 1.03 (0.26) | 2.65 (0.71 to 5.61) | 0.002 | |
| Aids and adaptations costs | 3959 | 4.78 (2.18) | 3954 | 1.24 (1.09) | 3.54 (–1 to 8.89) | 0.12 | |
| Total NHS and PSS costs | 3948 | 93.75 (50.8) | 3940 | 47.62 (20.86) | 46.13 (–38.68 to 169.18) | 0.382 | |
| 0–6 months | Total NHS and PSS costs | 3919 | 3641.84 (149.13) | 3914 | 2548.36 (84) | 1093.49 (800.43 to 1442) | < 0.001 |
| Assessment period | Category | Adrenaline arm (N = 128) | Placebo arm (N = 91) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean cost (SE) (£) | Participants with complete data (n) | Mean cost (SE) (£) | Mean difference in costs (bootstrap 95% CI) (£) | p-value | ||
| 0–3 months after randomisation | Emergency response costs | 127 | 1535.32 (45.62) | 91 | 1455.87 (53.8) | 79.45 (–51.87 to 222.5) | 0.242 |
| Intervention costs | 128 | 14.38 (0.99) | 91 | 0 (0) | 14.38 (12.44 to 16.33) | < 0.001 | |
| Inpatient costs | 88 | 29,425.72 (3032.74) | 65 | 24,356.65 (3055.72) | 5069.07 (–3985.61 to 13,777.15) | 0.244 | |
| Outpatient costs | 86 | 859.01 (241.89) | 57 | 738.40 (214.69) | 120.61 (–494.23 to 763.48) | 0.7 | |
| Community care costs | 95 | 447.36 (204.33) | 65 | 330.81 (89.27) | 116.55 (–250.21 to 580.86) | 0.684 | |
| Medications | 94 | 287.66 (64.88) | 66 | 299.49 (93.53) | –11.83 (–250.68 to 183.9) | 0.97 | |
| Aids and adaptations costs | 86 | 81.91 (63.42) | 60 | 115.82 (84.33) | –33.91 (–263.39 to 159.46) | 0.736 | |
| Total NHS and PSS costs | 72 | 32,669.22 (3589.54) | 50 | 27,956.52 (3795.11) | 4712.7 (–5527.48 to 14,967.31) | 0.382 | |
| 3–6 months after randomisation | Inpatient costs | 80 | 4114.25 (2466.27) | 45 | 1916.46 (957.58) | 2197.79 (–2031.75 to 8385.56) | 0.44 |
| Outpatient costs | 79 | 932.02 (328.67) | 45 | 812.04 (331.92) | 119.97 (–835.56 to 1002.03) | 0.736 | |
| Community care costs | 83 | 312.26 (86.32) | 49 | 125.27 (19.78) | 187 (29.94 to 378.04) | 0.02 | |
| Medications | 80 | 182.04 (54.94) | 48 | 84.86 (18.07) | 97.18 (2.60 to 225.3) | 0.044 | |
| Aids and adaptations costs | 78 | 242.69 (104.76) | 50 | 97.99 (87.6) | 144.7 (–117.87 to 409.13) | 0.296 | |
| Total NHS and PSS costs | 67 | 5524.18 (2914.88) | 36 | 3508.31 (1334.03) | 2015.87 (–3297.29 to 8742.91) | 0.598 | |
| 0–6 months | Total NHS and PSS costs | 54 | 33,385.85 (7293.82) | 29 | 29,144.43 (4609.34) | 4241.42 (–9982.79 to 22,538.49) | 0.698 |
Between 3 and 6 months, for all participants (see Table 16), the mean total primary health-care costs were significantly higher for the adrenaline arm (£6.54) than for the placebo arm (£1.55) (mean cost difference £4.99, 95% bootstrapped CI £1.36 to £9.26). Medications costs were, on average, significantly higher for the adrenaline arm (£3.68) than for the placebo arm (£1.03) (mean cost difference £2.65, 95% bootstrapped CI £0.71 to £5.61). For survivors to hospital discharge (see Table 17), the mean total primary health-care costs were significantly higher for the adrenaline arm (£312) than for the placebo arm (£125) (mean cost difference £187, 95% bootstrapped CI £29.94 to £378.04).
Economic costs from a broader societal perspective are presented in Appendix 1 (see Table 34 for all participants and Table 36 for survivors) by trial arm, trial period and cost category. There were no significant differences in costs for the period between randomisation and 3 months (see Appendix 1, Table 34). There were also no noticeable differences in the costs when looking at the information for survivors to hospital discharge (see Appendix 1, Table 36). Between 3 and 6 months post randomisation, for all participants (see Appendix 1, Table 34), mean total additional health-care-related costs (out-of-pocket medical expenditure on items such as over-the-counter medications, travel to medical appointments and aids) and non-health-care costs (e.g. child care and lost income as a result of the cardiac arrest) were, on average, significantly higher for the adrenaline group (£18.52) than for the placebo group (£5.02) (mean cost difference £13.50, 95% bootstrapped CI £0.43 to £33.45).
Total economic cost estimates over trial follow-up, and trial data collection instruments
Total costs estimated based on the trial data collection instruments for all participants are presented in Table 18 by trial arm, trial period and aggregate cost category. Based on total costs, adrenaline was, on average, more costly. Between randomisation and 3 months, the total mean cost was higher, from an NHS and PSS perspective, for the adrenaline arm than for the placebo arm: £3765 versus £2687, respectively (mean cost difference £1078, bootstrapped 95% CI £790 to £1406). This was also the case when costs were considered from a broader societal perspective: £3774 versus £2698 for the adrenaline and placebo arms, respectively (mean cost difference £1076, bootstrapped 95% CI £782 to £1403). There were no significant differences between the trial arms in total mean costs for survivors to hospital discharge (Table 19). Between 3 and 6 months, for all participants, the total mean non-NHS/PSS costs were significantly higher for the adrenaline arm (£18.51) than for the placebo arm (£4.95) (mean cost difference £13.56, bootstrapped 95% CI £0.49 to £33.62). There were no significant differences between the trial arms in total mean costs for survivors to hospital discharge (see Table 19). Over the trial time horizon of the first 6 months post randomisation, for all participants, adrenaline was, on average, more costly (see Table 18). The total mean cost from an NHS and PSS perspective was higher for the adrenaline arm than for the placebo arm: £3642 versus £2548, respectively (mean cost difference £1093, bootstrapped 95% CI £800 to £1442). This was also the case when costs were considered from a broader societal perspective: £3672 versus £2535 for the adrenaline and placebo arms, respectively (mean cost difference £1136, bootstrapped 95% CI £840 to £1484). There were no differences between the groups for total mean costs for survivors to hospital discharge (see Table 19).
| Assessment period | Category | Adrenaline arm (N = 4015) | Placebo arm (N = 3999) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean cost (SE) (£) | Participants with complete data (n) | Mean cost (SE) (£) | Mean cost difference (bootstrap 95% CI) (£) | p-value | ||
| 0–3 months post randomisation | Total NHS and PSS costs | 3937 | 3764.73 (127.92) | 3936 | 2686.9 (90) | 1077.83 (790.49 to 1406.45) | < 0.001 |
| Total non-NHS and PSS costs | 3973 | 37.34 (10.32) | 3970 | 19.93 (6.45) | 17.4 (–6.71 to 40.77) | 0.156 | |
| Total societal costs | 3936 | 3774.22 (127.5) | 3936 | 2698.06 (91.12) | 1076.16 (782.48 to 1403.42) | < 0.001 | |
| 3–6 months post randomisation | Total NHS and PSS costs | 3948 | 93.75 (50.8) | 3940 | 47.62 (20.86) | 46.13 (–38.68 to 169.18) | 0.382 |
| Total non-NHS and PSS costs | 3962 | 18.51 (8.13) | 3953 | 4.95 (2.25) | 13.56 (0.49 to 33.62) | 0.038 | |
| Total societal costs | 3948 | 110.4 (52.28) | 3939 | 46.22 (20.39) | 64.18 (–21.67 to 188.32) | 0.192 | |
| 0–6 months post randomisation | Total NHS and PSS costs | 3919 | 3641.84 (149.13) | 3914 | 2548.36 (84) | 1093.49 (800.43 to 1442) | < 0.001 |
| Total non-NHS and PSS costs | 3956 | 50.84 (14.62) | 3952 | 15.07 (5.59) | 35.77 (8.1 to 68.61) | 0.01 | |
| Total societal costs | 3919 | 3671.5 (150.28) | 3913 | 2535.36 (82.89) | 1136.14 (840.32 to 1484.33) | < 0.001 | |
| Assessment period | Category | Adrenaline arm (N = 128) | Placebo arm (N = 91) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean cost (SE) (£) | Participants with complete data (n) | Mean cost (SE) (£) | Mean cost difference (bootstrap 95% CI) (£) | p-value | ||
| 0–3 months post randomisation | Total NHS and PSS costs | 72 | 32,669.22 (3589.54) | 50 | 27,956.52 (3795.11) | 4712.7 (–5527.48 to 14,967.31) | 0.382 |
| Total non-NHS and PSS costs | 92 | 1612.33 (420.67) | 66 | 1199 (372.51) | 413.33 (–686.8 to 1566.93) | 0.444 | |
| Total societal costs | 71 | 33,602.52 (3614.7) | 50 | 28,835.07 (3848.78) | 4767.45 (–5096.05 to 15,071.74) | 0.394 | |
| 3–6 months post randomisation | Total NHS and PSS costs | 67 | 5524.18 (2914.88) | 36 | 3508.31 (1334.03) | 2015.87 (–3297.29 to 8742.91) | 0.598 |
| Total non-NHS and PSS costs | 81 | 905.61 (372.83) | 49 | 399.36 (174.22) | 506.25 (–196.78 to 1464.21) | 0.204 | |
| Total societal costs | 67 | 6505.26 (3014.44) | 35 | 3450.12 (1332.71) | 3055.14 (–2433.59 to 9949.4) | 0.374 | |
| 0–6 months post randomisation | Total NHS and PSS costs | 54 | 33,385.85 (7293.82) | 29 | 29,144.43 (4609.34) | 4241.42 (–9982.79 to 22,538.49) | 0.698 |
| Total non-NHS and PSS costs | 75 | 2681.64 (703.79) | 49 | 1215.1 (432.91) | 1466.54 (–171.05 to 3200.25) | 0.082 | |
| Total societal costs | 54 | 35,538.15 (7286.24) | 28 | 28,277.49 (4592.94) | 7260.66 (–6800.38 to 24,616) | 0.422 | |
Economic cost estimates, combined Hospital Episode Statistics and trial instrument data in the 0–6 months post-randomisation period
Economic costs from an NHS and PSS perspective for all participants based on combining resource use data from HES and trial instrument sources are presented in Table 20 by trial arm, cost period and cost category. For the whole trial population, HES data were used to estimate only the cost of hospital-based services (inpatient admissions and ED and outpatient attendances) during the period 0–6 months after randomisation for 1833 (45.7%) of the 4015 participants in the adrenaline arm and 1094 (27.4%) of the 3999 participants in the placebo arm. Hospitalisation costs for the remaining participants were either set at zero (primarily as a result of patients dying at the scene of cardiac arrest: adrenaline, n = 1985; placebo, n = 2768) and trial CRFs completed by paramedics and trial participants (adrenaline, n = 188 and placebo, n = 126), with any remaining hospitalisation costs classed as missing (adrenaline, n = 9 and placebo, n = 11). Non-hospital-based costs of health and social care services were estimated from resource use data collected using economic questionnaires completed by trial participants.
| Assessment period | Category | Adrenaline arm (N = 4015) | Placebo arm (N = 3999) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean cost (SE) (£) | Participants with complete data (n) | Mean cost (SE) (£) | Mean cost difference (bootstrap 95% CI) (£) | p-value | ||
| 0–6 months | Hospitalisation costs (NHS and PSS) | 4006 | 2669 (179) | 3988 | 1460 (129) | 1209 (804 to 1660) | < 0.001 |
| Non hospitalisation costs (NHS and PSS) | 3934 | 1785 (21) | 3926 | 1678 (13) | 107 (61 to 155) | < 0.001 | |
| Non-NHS and PSS costs | 3956 | 51 (15) | 3952 | 15 (6) | 36 (8 to 69) | 0.01 | |
| Total NHS and PSS costs | 3934 | 3789 (121) | 3926 | 2698 (94) | 1091 (807 to 1398) | < 0.001 | |
| Total societal costs | 3933 | 3829 (124) | 3925 | 2687 (92) | 1143 (861 to 1451) | < 0.001 | |
| 0–12 monthsa | Hospitalisation costs (NHS and PSS) | 3971 | 2419 (173) | 3961 | 1322 (131) | 1096 (692 to 1537) | < 0.001 |
| Non hospitalisation costs (NHS and PSS) | 3922 | 1822 (32) | 3915 | 1677 (13) | 145 (87 to 215) | < 0.001 | |
| Non-NHS and PSS costs | 3941 | 63 (26) | 3939 | 16 (8) | 47 (2 to 111) | 0.042 | |
| Total NHS and PSS costs | 3921 | 3748 (126) | 3915 | 2651 (95) | 1096 (804 to 1415) | < 0.001 | |
| Total societal costs | 3920 | 3793 (132) | 3915 | 2662 (96) | 1131 (820 to 1473) | < 0.001 | |
Between randomisation and 6 months (see Table 20), the total mean cost estimates based on the combined HES and trial data collection instrument data were higher, from an NHS and PSS perspective, for the adrenaline arm (£3789) than for the placebo arm (£2698) (mean cost difference £1091, bootstrapped 95% CI £807 to £1398). This was also the case when costs were considered from a broader societal perspective: £3829 versus £2687 for the adrenaline and placebo arms, respectively (mean cost difference £1143, bootstrapped 95% CI £861 to £1451).
There were no significant differences in total mean costs for survivors to hospital discharge between trial arms (Table 21). Over the trial time horizon of 6 months, for all participants, the adrenaline intervention was, on average, more costly, but the difference in total costs was not statistically significant. The mean total cost was higher, from an NHS and PSS perspective, for the adrenaline arm (£33,554) than for the placebo arm (£33,348) (mean cost difference £205, bootstrapped 95% CI –£10,849 to £10,159). This was also the case when costs were considered from a broader societal perspective: £34,994 versus £31,557 for the adrenaline and placebo arms, respectively (mean cost difference £3438, bootstrapped 95% CI –£7222 to £13,343).
| Assessment period | Category | Adrenaline arm, (N = 128) | Placebo arm, (N = 91) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean cost (SE) (£) | Participants with complete data (n) | Mean cost (SE) (£) | Mean cost difference (bootstrap 95% CI) (£) | p-value | ||
| 0–6 months | Hospitalisation costs (NHS and PSS) | 123 | 37,153 (3780) | 83 | 33,258 (3162) | 3894 (–5364 to 14,798) | 0.408 |
| Non-hospitalisation costs (NHS and PSS) | 64 | 3467 (686) | 39 | 3011 (645) | 456 (–1357 to 2388) | 0.58 | |
| Non-NHS and PSS costs | 75 | 2682 (716) | 49 | 1215 (429) | 1467 (–91 to 3115) | 0.07 | |
| Total NHS and PSS costs | 64 | 33,554 (2956) | 39 | 33,348 (4263) | 205 (–10,849 to 10,159) | 0.9 | |
| Total societal costs | 63 | 36,522 (3211) | 38 | 32,988 (4153) | 3534 (–7106 to 13,535) | 0.49 | |
| 0–12 monthsa | Hospitalisation costs (NHS and PSS) | 89 | 39,577 (5273) | 57 | 38,447 (4801) | 1130 (–11,842 to 15,807) | 0.856 |
| Non-hospitalisation costs (NHS and PSS) | 52 | 6671 (1978) | 28 | 3432 (927) | 3240 (–656 to 7924) | 0.11 | |
| Non-NHS and PSS costs | 60 | 4150 (1641) | 36 | 1756 (799) | 2394 (–748 to 6580) | 0.152 | |
| Total NHS and PSS costs | 51 | 37,962 (4448) | 28 | 38,868 (6258) | –906 (–17,196 to 13,318) | 0.982 | |
| Total societal costs | 50 | 42,204 (5143) | 28 | 40,401 (6340) | 1804 (–15,835 to 16,999) | 0.766 | |
Economic costs, combined Hospital Episode Statistics and trial data collection form based resource use data, 12-month period from randomisation
Cost estimates covering the 1-year period from randomisation are also presented in Table 20 (whole trial population) and Table 21 (survivors to hospital discharge) by trial allocation and resource category. These costs were derived using the methodology described in Chapter 4, Estimation of costs for the total 1-year period after randomisation, taking into account the fact that not all patients had complete HES-derived hospital care data covering the 1-year period from randomisation.
Between randomisation and 12 months, and for the whole trial population, the estimated total mean costs based on complete cases from the combined HES and trial data collection instrument data were higher, from an NHS and PSS perspective, for the adrenaline arm (£3748) than for the placebo arm (£2651) (mean total cost difference £1096, bootstrapped 95% CI £804 to £1415). This was also the case when costs were considered from a broader societal perspective: £3793 versus £2662 for the adrenaline and placebo arms, respectively (mean total cost difference £1131, bootstrapped 95% CI £820 to £1473). There were no significant differences in total mean costs between the trial arms for survivors to hospital discharge (see Table 21) during the first 12 months after randomisation. During this period, adrenaline was, on average, associated with increased costs, but the difference in total costs was not statistically significant.
Health-related quality-of-life and quality-adjusted life-year outcomes
The distribution of the responses to the EQ-5D-5L and SF-12 health-related quality-of-life questionnaires by trial arm and trial period for survivors to hospital discharge are presented in Appendix 1, Tables 37 and 38, respectively. The comparisons of responses were conducted on the basis of optimal level of function (e.g. ‘no problem’ on the EQ-5D-5L) versus suboptimal level of function (indicating any functional impairment). At the 3-month assessment point, the only statistically significant differences in levels of function in health-related quality of life was for the self-care dimension of the EQ-5D-5L (p = 0.020). There were no statistically significant differences in levels of function in health-related quality of life for participant-reported dimensions of the EQ-5D-5L or SF-12 measures between the adrenaline and placebo groups at the 6-month assessment point (see Appendix 1, Tables 37 and 38).
The mean health utility scores from alternative sources [mRS mapped to the EQ-5D, EQ-5D-5L and SF-6D (derived from the SF-12)] by trial arm and trial period for all participants are presented in Tables 22 and 23, and displayed in Appendix 1, Figure 25 (utility generated via the mRS and EQ-5D-5L). There were no statistically significant differences in mean utility scores between the adrenaline arm and placebo arm for the health utility scores from different sources at the trial assessment points. However, the mean health utility scores, based on mRS mapping to the EQ-5D-3L for survivors to hospital discharge (see Table 23), were statistically significantly lower at hospital discharge for the adrenaline arm (0.48) than for the placebo arm (0.60) (unadjusted mean difference –0.118, 95% CI –0.196 to –0.032).
| Assessment period | Category | Adrenaline arm (N = 4015) | Placebo arm (N = 3999) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean (SE) utility (£) | Participants with complete data (n) | Mean (SE) utility (£) | Mean difference (bootstrapped 95% CI) utility (£) | p-value | ||
| Hospital discharge | mRS mapped to EQ-5D | 4009 | 0.015 (0.002) | 3994 | 0.013 (0.002) | 0.002 (–0.002 to 0.006) | 0.582 |
| 0–3 months after randomisation | EQ-5D-5L | 3976 | 0.013 (0.002) | 3970 | 0.011 (0.001) | 0.002 (–0.002 to 0.006) | 0.472 |
| mRS mapped to EQ-5D | 3991 | 0.016 (0.002) | 3981 | 0.012 (0.002) | 0.003 (–0.001 to 0.008) | 0.228 | |
| SF-12 (SF-6D) | 3968 | 0.013 (0.002) | 3968 | 0.011 (0.001) | 0.002 (–0.002 to 0.007) | 0.406 | |
| 3–6 months post randomisation assessment period | EQ-5D-5L | 3963 | 0.011 (0.002) | 3976 | 0.008 (0.001) | 0.002 (–0.002 to 0.006) | 0.420 |
| mRS mapped to EQ-5D | 3993 | 0.015 (0.002) | 3975 | 0.011 (0.001) | 0.004 (0 to 0.009) | 0.118 | |
| SF-12 (SF-6D) | 3963 | 0.012 (0.001) | 3955 | 0.008 (0.001) | 0.004 (0 to 0.007) | 0.092 | |
| Assessment period | Category | Adrenaline arm (N = 128) | Placebo arm (N = 91) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean (SE) costs (£) | Participants with complete data (n) | Mean (SE) costs (£) | Mean difference (bootstrapped 95% CI) costs (£) | p-value | ||
| Hospital discharge | mRS mapped to EQ-5D | 126 | 0.479 (0.031) | 89 | 0.597 (0.029) | –0.118 (–0.196 to -0.032) | 0.002 |
| 0–3 months after randomisation | EQ-5D-5L | 95 | 0.533 (0.039) | 66 | 0.643 (0.038) | –0.11 (–0.211 to 0.004) | 0.056 |
| mRS mapped to EQ-5D | 109 | 0.576 (0.032) | 76 | 0.651 (0.033) | –0.075 (–0.163 to 0.016) | 0.104 | |
| SF-12 (SF-6D) | 86 | 0.609 (0.022) | 64 | 0.669 (0.021) | –0.06 (–0.116 to 0.003) | 0.066 | |
| 3–6 months post randomisation | EQ-5D-5L | 81 | 0.517 (0.046) | 51 | 0.648 (0.051) | –0.131 (–0.257 to 0.01) | 0.064 |
| mRS mapped to EQ-5D | 111 | 0.538 (0.035) | 70 | 0.62 (0.037) | –0.082 (–0.181 to 0.021) | 0.116 | |
| SF-12 (SF-6D) | 80 | 0.584 (0.026) | 51 | 0.634 (0.027) | –0.05 (–0.116 to 0.026) | 0.202 | |
Cost-effectiveness results
Base-case analysis
The incremental cost-effectiveness of adrenaline is shown in Table 24 for the participants with costs and health outcomes data subject to multiple imputation. When an NHS and PSS perspective was adopted (i.e. that adopted for the baseline analysis), and health outcomes were measured in terms of QALYs based on the EQ-5D and mRS measures, the adjusted mean total cost was £3591 for the adrenaline arm, and £2285 for the placebo arm, generating a mean incremental cost of £1306 (95% CI £837 to £1774) over the first 6-month post-randomisation period. The adjusted mean QALYs was 0.0025 in the adrenaline arm and 0.0017 in the placebo arm, generating a mean difference in QALYs of 0.0008 (95% CI –0.0014 to 0.003). The ICER was £1,693,003. The probability that adrenaline was cost-effective at a cost-effectiveness threshold of £30,000 per QALY gained was zero. Extending the within-trial analysis to cover the 1-year period from randomisation by extrapolating costs and utilities measured at 6 months to 12 months reduced the base-case ICER from £1,693,003 per QALY gained to £644,308 per QALY gained for adrenaline, compared with placebo (mean incremental costs of £1411 and mean incremental QALYs of 0.0022). Extrapolating the within-trial analysis to cover the lifetime of survivors (see the model described in Chapter 4, Extrapolating beyond trial follow-up) reduced the ICER further to £81,070 per QALY gained (mean incremental costs of £1775 and mean incremental QALYs of 0.022).
| Analysis model | Adrenaline arm, mean (SE) | Placebo arm, mean (SE) | Mean difference (95% CI) | Cost-effectiveness | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£)a | QALYsa | ICER (£/QALY) | INMB at £30,000/QALYb (95% CI (£)) | Probability of being cost-effective at £30,000/QALYb | |
| Within-trial base case (adjustedc multiple imputation), 6 months post cardiac arrest event | 3591 (540) | 0.0025 (0.0025) | 2285 (544) | 0.0017 (0.0025) | 1306 (837 to 1774) | 0.0008 (–0.0014 to 0.003) | 1,693,003 | –1282 (–1733 to –831) | 0 |
| Extrapolation to 12 months post cardiac arrest event | 3741 (536) | 0.006 (0.0049) | 2330 (541) | 0.0038 (0.005) | 1411 (946 to 1876) | 0.0022 (–0.0021 to 0.0065) | 644,308 | –1346 (–1784 to –907) | 0 |
| Extrapolation to lifetime horizon (decision-analytic model) | 5308 (797) | 0.111 (0.037) | 3534 (736) | 0.089 (0.031) | 1775 (250 to 3394) | 0.022 (–0.011 to 0.063) | 81,070 | –1118 (–2776 to 487) | 0.098 |
| Outcome | Adrenaline arm, mean (SE) | Placebo arm, mean (SE) | Mean difference (bootstrapped 95% CI) | Cost-effectiveness | |||||
|---|---|---|---|---|---|---|---|---|---|
| Costs (£) | Probability | Costs (£) | Probability | Costs (£) | Probability | ICER (£) | INMB at £30,000/QALY (95% CI) (£) | Probability of being cost-effective at £30,000/QALY | |
| 6 months’ survival | 3654 (388) | 0.0051 (0.0022) | 2284 (420) | 0.0037 (0.0016) | 1370 (954 to 1840) | 0.0014 (–0.0003 to 0.003) | 982,880 | –1328 (–1794 to –935) | 0 |
| 6 months’ neurologically intact survival | 3654 (388) | 0.0026 (0.0022) | 2284 (420) | 0.0023 (0.0016) | 1370 (954 to 1840) | 0.0004 (–0.0003 to 0.003) | 3,772,322 | –1359 (–1794 to –935) | 0 |
Incremental net monetary benefits
The associated adjusted mean incremental net monetary benefits (INMBs) of adrenaline at cost-effectiveness thresholds of £30,000 per QALY were as follows: –£1282 for the within-trial base-case analysis (adjusted multiple imputation), 6 months post cardiac arrest event; –£1346 for extrapolation to 12 months post cardiac arrest event; and –£1118 for extrapolation to a lifetime time horizon (decision-analytic model) (see Table 24). The base-case mean INMB was < £0, suggesting that adopting the adrenaline protocol would result in a net economic loss of £1258, on average, from an NHS and PSS perspective [INMB –£1250 (95% CI –£1686 to –£815) at 6 months and –£1118 (95% CI –£2776 to £487) over the lifetime of survivors, assuming a cost-effectiveness threshold of £30,000 per QALY]. The CEAC shows that this results in a probability of cost-effectiveness of < 1% at cost-effectiveness thresholds of < £200,000 (see Appendix 1, Figure 25); that is, if decision-makers are willing to pay £30,000 for an additional QALY, the probability that adrenaline is cost-effective remains < 1% over the 6-month base-case within-trial time horizon and 11% over a lifetime time horizon (see Table 24).
Sensitivity analysis
Several sensitivity analyses were undertaken to assess the impact of uncertainty surrounding key parameters or methodological features on the cost-effectiveness results. The base-case analyses were replicated using Stata software and the results were consistent with the primary analyses. The adjusted and unadjusted complete-case analyses also exhibit the same pattern of results as the base-case analyses (see Appendix 1, Table 39). The probability that adrenaline is cost-effective remained relatively static (at about 0%) for the majority of the sensitivity analyses (i.e. societal costs, QALYs based on the mRS, 12-month time horizon, 12-month time horizon and societal perspective, and 12-month time horizon and QALYs based on the mRS). All sensitivity analyses showed that the average INMB value always remains < £0 (see Appendix 1, Table 39).
In sensitivity analyses that explored the impact of the missing not at random assumption on the within-trial base-case results, imputed utility values and costs were systematically decreased and increased while holding imputed values for other variables constant. The results are displayed in Appendix 1, Tables 40 and 41. They suggest that doubling the value of imputed utilities favoured adrenaline (the ICER decreased to £1,021,684 per QALY), whereas halving them favoured placebo (the ICER increased to £2,067,739 per QALY gained). Similarly, doubling imputed costs favoured adrenaline and decreased the ICER to £1,186,037 per QALY, whereas halving them had little effect on the within-trial base-case cost-effectiveness results (£1,688,008 per QALY). The probability that adrenaline is cost-effective was zero at cost-per-QALY thresholds of < £100,000 across alternative assessments, in which missing values for the main economic costs and outcomes were not assumed to be missing at random (see Appendix 1, Figures 28 and 29).
Subgroup analyses
To explore the heterogeneity in the cost-effectiveness results, 10 variables were considered: cause of cardiac arrest (medical, non-medical), age (≤ 60 years, > 60 years), sex (female, male), time from EMS arrival at scene to administration of first dose (≤ 10 minutes, > 10 minutes), time from 999 call received to EMS arrival at scene (≤ 10 minutes, > 10 minutes), shockable rhythm (yes, no), number of syringes given out of two (≤ 2, > 2), number of syringes given out of four (≤ 4, > 4), witnessed by (not witnessed, EMS, bystander), and bystander CPR (no, yes, unknown). All subgroup analyses were based on the patient-reported EQ-5D and mRS measures and used multiple imputation and covariate adjustments, as per the primary analyses (see Appendix 1, Table 42). Subgroups for which the probability of cost-effectiveness was > 10% at £30,000 per QALY gained over the 6 months within-trial time horizon were cardiac arrest of medical aetiology (probability of cost-effectiveness 15%), EMS witnessed (15%), aged > 60 years (20%), two or more doses of adrenaline administered (27%) and four or more doses of adrenaline administered (32%). All other subgroups generated probabilities of cost-effectiveness for adrenaline of < 10% at a cost-effectiveness threshold of £30,000 per QALY gained.
Secondary health economic outcomes
Results are presented in Table 25 for the cost-effectiveness of adrenaline expressed as incremental cost per unit increase in the proportion surviving to 6 months post cardiac arrest and the proportion surviving with good neurological function (i.e. neurologically intact survivor). The adjusted mean cost over 6 months was £3654 in the adrenaline arm and £2284 in the placebo arm, generating a cost-difference of £1370 (95% bootstrapped CI £954 to £1840). The 6-month adjusted survival probability was 0.0051 in the adrenaline arm and 0.0037 in the placebo arm, generating a difference in survival probability of 0.0014 (95% CI –0.0003 to 0.003) in favour of adrenaline. The 6-month adjusted probability of survival with good functional outcome was 0.0026 for adrenaline and 0.0023 for placebo, generating a difference in probability of 0.0014 (95% CI –0.0003 to 0.003) in favour of adrenaline. The ICER was £982,880 per percentage point increase in overall survival and £3,772,322 per percentage point increase in neurological survival.
Chapter 6 Discussion
Overall evidence
The use of adrenaline during resuscitation for OHCA resulted in a significantly higher rate of survival to hospital admission (23.6% vs. 8.0% for the adrenaline and placebo arms, respectively; aOR 3.83, 95% CI 3.30 to 4.43) than the use of placebo. Thirty-day survival was also higher (3.2% vs. 2.4% for the adrenaline and placebo arms, respectively; aOR 1.47, 95% CI 1.09 to 1.97), but the rate of favourable neurological outcome was not significantly different (2.2% vs. 1.9% for the adrenaline and placebo arms, respectively; aOR 1.19, 95% CI 0.85 to 1.68). The pattern of improved survival but no significant improvement in neurological outcomes continued through to 6 months. By 12 months, survival in the adrenaline arm was 1.5%, compared with 1% in the placebo arm (aOR 1.40, 95% CI 1.00 to 1.96). A Bayesian analysis found that the probability that the risk difference for survival was > 1%, favouring the adrenaline arm, was 37%, and the probability that favourable neurological outcome was improved by > 1% was 1.9%. An adjusted subgroup analysis did not identify a significant interaction for any of the subgroups studied.
More survivors in the adrenaline group than in the placebo group had severe neurological impairment at hospital discharge. The number with severe neurological impairment decreased through to 6 months, although evaluation was limited by greater loss to follow-up. Examining health-related quality of life up to 6 months after randomisation, and cognitive function, anxiety/depression or post-traumatic stress to 3 months, showed significant functional impairment in cardiac arrest survivors, compared with the normal population. One-third to half of patients reported needing help from someone for everyday activities. For the majority, this was a new situation after their cardiac arrest. Fewer than half reported having made a full mental recovery after their cardiac arrest. Although underpowered, the pattern of impairment suggested greater disability in the adrenaline group.
The ICER for adrenaline was estimated at £1,693,003 per QALY gained over the first 6 months after the cardiac arrest event and £81,070 per QALY gained over a lifetime time horizon. The associated adjusted mean INMBs of adrenaline at a cost-effectiveness threshold of £30,000 per QALY gained was –£1282 at 6 months and –£1118 over a lifetime of survivors, suggesting that adopting the adrenaline protocol would result in a net economic loss. The CEAC shows that these INMBs result in a probability of cost-effectiveness of < 1% for cost-effectiveness thresholds of < £200,000 for an additional QALY over a time horizon of up to 1 year after the cardiac arrest. Over the lifetime of survivors, the probability of cost-effectiveness approaches 50% at a £100,000 per QALY cost-effectiveness threshold.
Interpretation of results
Clinical outcomes
The use of adrenaline, in comparison with placebo, resulted in almost four times the number of patients being admitted to hospital after OHCA. This finding is broadly consistent with the findings of the PACA trial,56 which documented an OR of 3.4 (95% CI 2.0 to 5.6) for ROSC in favour of adrenaline over placebo for OHCA.
Many more patients in the adrenaline group than in the placebo group were admitted to hospital but did not survive to discharge: 2016 (50.2%) and 1209 (30.2%) patients in the adrenaline and placebo arms, respectively, were admitted, and 128 (3.2%) and 91 (2.3%) patients in the adrenaline and placebo arms, respectively, survived to discharge. Thus, 1888 patients in the adrenaline arm died in hospital, compared with 1118 in the placebo arm. We do not have data on the mode of death, but the majority of deaths occurred in the ED. These early deaths are more likely to be caused by circulatory failure or limitation of treatment due to the presence of severe comorbidities and functional impairment, as withdrawal of life-sustaining therapy resulting from perceived severe neurological injury should not normally occur until after 72 hours. However, although several studies have documented the mode of death among post-cardiac arrest patients admitted to an intensive trauma unit,161–163 this has not been well documented for those dying in the ED.
In the adrenaline group, 414 of the 566 (73.1%) patients admitted to the ICU died there, compared with 176 of the 270 (65.1%) placebo group patients admitted to the ICU. Thus, 238 additional patients in the adrenaline group were admitted to an ICU, but died there. Surviving patients spent, on average, 7 days in intensive care and 21 days in hospital. By the time of hospital discharge, 128 (3.2%) patients were alive in the adrenaline group, compared with 91 (2.3%) in the placebo group, of whom 87 (2.2%) and 74 (1.9%), respectively, survived with a favourable neurological outcome. Although, at the time of discharge, 39 survivors in the adrenaline group and 16 in the placebo group had severe neurological injury, by 6 months, those with severe neurological injury numbered 23 and 9, respectively. It is well established that the functional status of cardiac arrest survivors can improve over the first few months and that some with the most severe neurological injury will die. 164 Ultimately, long-term follow-up at 12 months after cardiac arrest is ideal, but is challenging because of the resources required and the increasing loss to follow-up over time, which results in attrition bias. Data on 30-day survival were available for 4012 and 3995 patients in the adrenaline and placebo groups, respectively; by 6 months, these numbers had decreased to 4006 and 3991 for the adrenaline and placebo groups, respectively, representing a loss to follow-up for survival of just six and four patients, respectively. However, neurological outcome is more difficult to collect after hospital discharge. Data on neurological outcome at discharge were available for 4007 and 3994 patients in the adrenaline and placebo groups, respectively, but, by 6 months, these numbers had decreased to 3991 and 3973, respectively, which is a loss to follow-up for neurological outcome of 16 and 21 patients, respectively. A series of sensitivity analyses were undertaken to explore the potential impact of these missing data; the results of most of these were consistent with the main results.
Patients, clinicians and researchers prioritised health-related quality of life as a core outcome for evaluation following cardiac arrest. 165 In PARAMEDIC2, health-related quality of life was impaired among survivors at 3 and 6 months, compared with the UK general population, suggesting large differences between the well-being of OHCA survivors and that of the general population. Moreover, a reduction in both mental and, to a lesser extent, physical well-being was observed at 6 months, highlighting the importance of detailed assessment over the longer term. Impairment spanned physical and mental health domains, leading to reduced health utility scores. Compared with other studies,166 the extent of functional impairment is greater, which is likely to be reflective of the PARAMEDIC2 cohort involving only patients refractory to initial attempts at resuscitation. Although there were no pronounced differences between the adrenaline and placebo groups, the overall level of impairment highlights an unmet need for this group of patients.
Meta-analyses of the PARAMEDIC2 and PACA56 trials were, as expected, consistent with adrenaline increasing survival to hospital admission (RR 2.51, 95% CI 1.67 to 3.76; two studies, 8489 participants; an increase in the rate of ROSC at hospital admission from 83 to 209 per 1000, 95% CI 139 to 313) and survival to hospital discharge (RR 1.44, 95% CI 1.11 to 1.86; two studies, 8538 participants; an increase in survival to hospital discharge from 23 to 32 per 1000, 95% CI 25 to 42). Survival rates with a favourable neurological outcome at discharge were similar (RR 1.21, 95% CI 0.9 to 1.62; two studies, 8535 participants; an increase from 19 to 22 per 1000, 95% CI 17 to 30). 55
A meta-analysis of the PARAMEDIC2 trial with the PACA56 trial according to initial cardiac arrest rhythm found very low survival-to-discharge rates with an initial non-shockable rhythm, but a significant effect for adrenaline (0.39% for placebo vs. 1.03% for adrenaline; OR 2.57, 95% CI 1.36 to 4.83). For shockable rhythms, although survival rates were higher (9.43% vs. 11.66% in placebo and adrenaline groups, respectively), the incremental effect of adrenaline was less pronounced (OR 1.26, 95% CI 0.93 to 1.71; between-group heterogeneity p = 0.05). A similar pattern was observed for survival to discharge with a favourable neurological outcome, but the CI for the OR crossed 1 for both shockable and non-shockable rhythms. 167
Two explanations for the much higher rate of ROSC and survival to hospital admission with adrenaline, and yet no significant improvement in neurological outcome, are as follows: (1) the heart is much more resilient than the brain when exposed to a period of hypoxia-ischaemia – thus, the heart can be ‘restarted’ but the brain has already been damaged irreversibly; and (2) even though adrenaline increases coronary and cerebral perfusion pressure,40 it may reduce microcirculatory flow in the brain. 29,66
Minimum clinically important difference
An international survey of key respondents from 46 countries identified that the minimal clinically important difference for survival with a favourable neurological outcome is 3% (when the baseline rate for the population is 2%). 168 Other trials have used minimal clinically important differences of 1.4%,169 2%56,170 or larger (6.3%). 171 The Universal Termination of Resuscitation Rule uses a false-positive threshold of 1%, whereas neuroprognostication algorithms172 in intensive care allow a false-positive rate for a favourable neurological outcome of up to 5%. 91,173 The 6-month difference for survival (0.8%, 95% CI 0.1% to 1.5%) and survival with a favourable neurological outcome (0.5%, 95% CI –0.1% to 1.1%) fall below the thresholds set for a minimal clinically important difference in previous studies.
Clinical effectiveness in the context of the chain of survival
The chain of survival describes the system of care required to optimise survival after OHCA. 9 It comprises four links: early access (identifying cardiac arrest early and activating the emergency services), early CPR (to maintain perfusion to the brain and vital organs), early defibrillation (to restart the heart for patients with an initially shockable rhythm) and early post-resuscitation care. The primary PARAMEDIC2 trial publication174 reported that 112 patients would need to be treated with adrenaline to prevent one death after cardiac arrest. This is substantially more than the number needed to be treated with other interventions delivered earlier in the chain of survival,175,176 for example early recognition of cardiac arrest, number needed to treat = 11;177 bystander CPR, number needed to treat = 15;178 and rapid defibrillation, number needed to treat = 5. 179
Cost-effectiveness
Economic evaluations in cardiac arrest research are relatively sparse. An economic model for public-access defibrillation reported an ICER of US$53,797 per QALY gained. 159 An economic evaluation of a mechanical chest compression device found that manual CPR dominated in health economic terms (mechanical CPR cost more and led to a reduction in QALYs, on average). 136 An extracorporeal CPR strategy in Sydney reported an ICER of €16,890 per QALY gained. A nurse-led post-cardiac arrest neurorehabilitation programme found a mean health benefit (0.04 additional QALYs) for minimal additional costs (€89), giving a 76% probability that the intervention would be cost-effective at a cost-effectiveness threshold of €80,000. 180 The NICE methods guideline132 manual notes that, in general, interventions with an ICER of < £20,000 per QALY gained are considered to be cost-effective. ICER values of > £30,000 per QALY gained require consideration of the certainty of the ICER and evidence that the assessment of the change in the health-related quality of life is inadequately captured and that the intervention adds demonstrable and distinct substantial benefits that may not have been adequately captured in the measurement of health gain.
In the present study, the ICER for the base-case analysis is 55-fold higher than the accepted cost-effectiveness threshold of £30,000. There was a low probability of cost-effectiveness (< 1%) up to a threshold of £200,000 per QALY gained. Subgroup analyses covering cause of cardiac arrest, age, sex, ambulance response time, time to drug administration, initial rhythm, witness status and bystander CPR did not identify any specific subgroups for which the ICER substantially improved. Sensitivity analyses that included societal costs, QALYs based on mRS values, a 12-month time horizon, a 12-month time horizon and societal perspective, and a 12-month time horizon and QALYs based on mRS showed that the average INMB value is always < £0.
Furthermore, our separate decision-analytic model demonstrated that the survival benefits associated with adrenaline generate additional QALY benefits, and therefore improved cost-effectiveness, over an extended time horizon. Nevertheless, even over a lifetime time horizon, the mean ICER associated with adrenaline exceeds cost-effectiveness thresholds widely accepted by decision-makers and health technology assessment agencies. Alternative extrapolations of cost-effectiveness, beyond the parameters of the PARAMEDIC2 trial, that focus solely on individuals experiencing OHCA are unlikely to alter decisions to recommend adrenaline on cost-effectiveness grounds.
It is possible that some benefits of admission to intensive care following cardiac arrest were incompletely captured through focusing on survival and health-related quality-of-life outcomes. The prevalence of post-traumatic stress among relatives of patients who die from cardiac arrest is high. 181,182 It is possible that admission to hospital allows the family time to say goodbye and to be present at the time of death. 183 Whether or not this has a beneficial effect, and any economic gains therein, remains to be determined. Approximately 10% of patients who die in ICU following OHCA become solid organ donors. 184 This may yield additional economic and health-related quality-of-life gains to the organ recipient and is worthy of further evaluation.
Ethics considerations
The design and conduct of this trial required particularly careful ethics consideration, given the life-threatening and emergency nature of OHCA. The lack of conclusive evidence of benefit, uncertainty about potential harm from adrenaline, and the presence of clinical equipoise provided the ethics justification of the need for a placebo-controlled trial. The implementation of the trial generated public debate on the ethics implications of conducting research in emergency situations without prior consent from participants or their legal representative. The public information strategy and process for enabling people to register their wish not to participate that we developed is, so far as we are aware, the first such initiative in the UK.
The implications of the trial findings for patients, clinicians, policy-makers and commissioners of health care also require careful ethics consideration. How an individual balances the chance of survival with the chance of having severe neurological impairment will be shaped by their personal values, but in the moment of a cardiac arrest, it is not possible to take into account these values when making a treatment decision. Thus, the decision about whether or not adrenaline is given will be directed by a policy or guideline applicable to all patients, informed by available evidence. A further consideration for policy-makers and commissioners of health care is the overall cost and cost-effectiveness of any treatment. The NHS is a publicly funded health service with limited financial resources and an obligation to distribute these resources fairly for everyone in response to health-care need. Our health economic analysis suggests that adrenaline treatment would not meet the current NICE criteria for cost-effectiveness. Developing a policy recommendation for the use of adrenaline for OHCA requires a complex balancing of current clinical practice norms, empirical evidence, and values. Understanding the range of population perspectives regarding this decision will be an important element of developing such a policy.
Interpretation by the International Liaison Committee on Resuscitation
The ILCOR is a collaboration of resuscitation councils from around the world that collaborate to ‘save more lives globally through resuscitation’,185 through the use of transparent evaluation of scientific data to promote, disseminate, and implement international consensus guidelines for resuscitation and first aid. The publication of the PARAMEDIC2 trial prompted the ILCOR to prioritise a review of vasopressors as treatment for cardiac arrest. 186 The ILCOR commissioned a systematic review and meta-analysis focusing on the use of standard-dose adrenaline (1 mg), compared with placebo, vasopressin, or adrenaline and vasopressin. 187 Similar to our Cochrane review,55 the review found moderate-quality evidence that adrenaline improves the rate of ROSC, survival to hospital discharge and 3-month survival among those experiencing OHCA. Subgroup analyses found that the improvement in short-term outcomes was more pronounced for non-shockable rhythms. The systematic review authors noted, in the pooled analysis, no improvement in mid-term (hospital discharge) neurological outcomes, but interpret it as showing a signal towards improved outcomes for 3-month survival, based on the findings from the PARAMEDIC2 trial [2.1% (82/3986) in the adrenaline group, compared with 1.6% (63/3979) in the placebo group; RR 1.30, 95% CI 0.94 to 1.80; absolute risk difference: 5 more per 1000 people, 95% CI 1 fewer to 13 more]. The authors acknowledge the loss to follow-up and very low event rates, leading to very low confidence in the effect estimate.
The ILCOR Advanced Life Support Task Force assessed the findings from the systematic review using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Evidence to Decision Framework. 188 The Task Force comprised 19 health-care professionals from around the world. No patient or public representatives participated in the Evidence to Decision Famework or in the development of treatment recommendations. The assessors considered the problem of OHCA as high priority. After considering the effect of adrenaline on ROSC, survival and neurological outcomes, the assessors judged the desirable effects of using adrenaline as moderate and judged the undesirable effects as small. The overall certainty of evidence was assessed to be moderate, noting that certainty varies according to outcome (high for ROSC, low for neurological outcomes). The ILCOR assessed that there was possibly important uncertainty or variability in how much people value the main outcomes, noting that the Core Outcome Set for Cardiac Arrest investigators reported that patients valued survival with favourable neurological outcome most highly. 165 The ILCOR’s overall judgement of the balance between desirable and undesirable effects probably favours using adrenaline. They reported that the intervention was probably acceptable to key stakeholders and considered it feasible to implement, noting that, although there was no research identified about patient acceptability, adrenaline use is currently the standard of care in many settings.
The writing group concluded by recommending that adrenaline should be administered as soon as possible during CPR (strong recommendation for non-shockable rhythms, weak recommendation for shockable rhythms). 186
Internal validity and methodological limitations
The internal validity of the trial is strengthened by the randomised, blinded, placebo-controlled design. Selection bias was minimised by the computer-generated randomisation sequence using the stratification method with concealed assignment, using ambulance service as a strata, with an allocation ratio of 1 : 1. This resulted in almost identical baseline characteristics of the key variables associated with outcome (age, sex, initial rhythm, cause of cardiac arrest, witnessed status, bystander CPR, ambulance response time and time to administration of the study intervention). Performance bias was reduced through the use of treatment packs that were identical in appearance. Laboratory testing of the trial IMP showed no changes in appearance throughout the duration of the trial. Trial participants were unconscious at the time of enrolment. No requests for unblinding were received during the treatment phase of the trial. Although not formally tested for, it is possible that some of the effects of adrenaline (e.g. rhythm transitions,189,190 higher rate of ROSC, greater cardiovascular instability after ROSC67,70,190) may have led to paramedics forming a view on the composition of the trial drug. As the acute effects of adrenaline are short lived, it is less likely to have influenced those treating the patient in hospital. Detection bias was minimised by the use of outcome assessors who were not involved in the initial treatment of the participant. Many outcomes (e.g. ROSC, survival status) are objective and would not be influenced by knowledge of treatment allocation. No unblinding of participants or legal representatives occurred before outcome assessments were complete. The trial had very high rates of follow-up for survival up to 12 months after enrolment in the trial. By contrast, beyond hospital discharge, loss to follow-up for neurological outcomes and health-related quality of life increased significantly. Consistent with previous studies,102,136,191 those with worse neurological outcomes were more likely to be LTFU. The trial was registered on the International Standard Randomised Controlled Trial Number (ISRCTN) registry and outcomes were defined prior to the enrolment of the first patient. The trial protocol was finalised and approved prior to commencement of randomisation. This report contains all the trial outcomes, thus eliminating selective reporting as a source of bias.
External validity and generalisability
External validity is a question of whether or not the results of a study can be applied to patients other than those enrolled in the study. 192 External validity is a matter of judgement and depends on the characteristics of the participants included in the trial, the trial setting, the treatment regimens tested and the outcomes assessed. 193
The PARAMEDIC2 trial was a pragmatic trial design, intended to show the real-world effectiveness of the intervention, and maximising the generalisability of findings. 194 Patient eligibility for inclusion was broad, with no exclusion based on age (other than < 16 years), sex, comorbidity (other than pregnancy), cause of arrest (other than anaphylaxis/asthma), location of arrest, witnessed status of arrest or initial cardiac arrest rhythm. The patients included in the trial were ‘representative’ of all OHCA patients attended by the respective ambulance services and where ALS is initiated. Similarly, all standard ALS interventions were provided to the trial participants, including chest compression, defibrillation and advanced airway management, as required, with the exception that standard adrenaline was substituted with trial drug drawn from a single trial treatment pack. The primary outcome of ‘survival to 30 days post cardiac arrest’ was an objective measurement, directly relevant to participants.
Ambulance system configuration is similar throughout the NHS and is based on a single-tier response system led by paramedics. Community first responders support emergency response systems. Physician involvement in initial phases of out-of-hospital resuscitation is relatively rare. NHS paramedics operate according to national clinical guidelines for cardiac arrest produced by the JRCALC. 87 These guidelines and those used in hospital for post-resuscitation care are based on the NICE-accredited Resuscitation Council (UK) guidelines,86 which are consistent with the European Resuscitation Council guidelines. 88 Although resuscitation guidelines around the world are broadly similar and based on recommendations from the ILCOR, emergency system response configurations vary. In many parts of the USA, the paramedic-based system is supplemented by basic life support response units, meaning that there are often more emergency care workers at the scene of a cardiac arrest than in the UK. In urbanised areas, emergency response times are quicker, although there is wide variation.
Analysis of the national OHCA registry103 and PARAMEDIC trial102,136 databases for patients treated for OCHA revealed similar patient characteristics and outcomes to those reported in PARAMEDIC2. 39 An evaluation of adrenaline use in the London Ambulance Service prior to the trial (2012–13) showed similar findings. 195 Outside the UK, compared with the Norwegian i.v. versus no i.v. trial,57 patients enrolled in PARAMEDIC2 were, on average, 4 years older (69 vs. 65 years) and were less likely to have a bystander-witnessed cardiac arrest (50% vs. 65%) or to have an initial shockable rhythm (19% vs. 33%). Compared with the PACA trial,56 patients enrolled in the PARAMEDIC2 trial were also, on average, 4 years older and were as likely to have a bystander-witnessed arrest, but were less likely to have an initially shockable rhythm (45% in the PACA trial56). Observational data from the US Resuscitation Outcome Consortium for 13,053 patients from 264 EMS agencies across 11 geographically distinct sites, who sustained an OHCA and were treated with adrenaline between 2005–7, similarly showed a younger cohort of patients (mean age 65 years); the rate of bystander-witnessed cardiac arrest was lower (39%) than that seen in the PARAMEDIC2 trial, but the proportion of patients with an initially shockable rhythm was higher (23%). 38 A similar pattern is seen in data from Seattle for cardiac arrests (from 2006 to 2012). 196
Some studies suggest that the timing of drug administration is critical to its effectiveness. 60,197–199 Adrenaline is recommended in European guidelines after the third attempt at defibrillation for shockable rhythms and after initiating ALS (CPR and airway management) for non-shockable rhythms. With OHCA, drug administration is usually delayed while paramedics travel to the scene of collapse, initiate CPR and defibrillation (when indicated) and obtain vascular access. In an evaluation of repeated doses of adrenaline conducted by the London Ambulance Service, the average time to the first dose of adrenaline was 21–26 minutes. 195 The time from collapse to drug treatment in the PARAMEDIC2 trial was, on average, 21 minutes. This is longer than the interval during in-hospital cardiac arrest (average 3 minutes)197,200 and in the majority of animal cardiac arrest models (average 9.5 minutes). 201 The timing is, however, similar to a systematic review of time to drug administration across 17 studies (19.4 minutes, 95% CI 12.8 to 25.9 minutes),202 and in more recent studies (range 13–24 minutes). 199,203–205 A subgroup analysis of time to drug administration and paramedic-witnessed cardiac arrest (in which case, adrenaline is likely to be given earlier) did not find evidence of an interaction. The practicalities of delivering early drug administration and its influence on outcomes remains to be determined.
Post-resuscitation care across acute NHS hospitals is informed by the NICE-accredited Resuscitation Council (UK) guidelines. 206 These guidelines follow the ILCOR and European Resuscitation Council/European Society of Intensive Care Medicine recommendations. 91 Key components of these guidelines are the use of targeted temperature management, maintenance of physiological targets (arterial blood oxygen saturations of 94–98%, normocarbia, normoglycaemia, systolic blood pressure of > 100 mmHg), targeted temperature management, early percutaneous coronary intervention and delaying multimodal neuroprognostication to at least 72 hours after cardiac arrest. Although not formally protocolised or evaluated in the PARAMEDIC2 trial, an observational study across 286 NHS ICUs (29,621 OHCAs) suggested up to 60% use of targeted temperature management and a median time to treatment withdrawal of 5 days. 184 Evaluation of the Myocardial Ischaemia National Audit Project database through to 2016 showed percutaneous coronary intervention use in ST elevation myocardial infarction to be in the region of 75%, and 15% for non-ST elevation myocardial infarction. 207 Future data with the Intensive Care National Audit Project and HES may provide more granular insights into the post-resuscitation care of patients enrolled in the PARAMEDIC2 trial.
Chapter 7 Conclusions
Implications for health care
Cardiac arrest remains a common and important condition. The PARAMEDIC2 trial found that adrenaline is effective at restarting the heart, leading to a 3.6-fold higher proportion of patients being admitted to hospital than if adrenaline was not given. The effect on survival to 30 days was more modest: 1.5-fold higher, corresponding to a 0.8% absolute increase in survival, giving a number needed to treat to save one life of 112. Survival with a favourable neurological outcome did not significantly differ between the adrenaline and placebo groups from hospital discharge. This pattern of better survival but no significant improvement in neurological outcomes continued through to the 6-month follow-up. Health-related quality of life was significantly impaired among survivors of OHCA and did not differ by treatment arm. The ICER for adrenaline was estimated at £1,675,976 per QALY gained. Based on current standards, this indicates that adrenaline is not a cost-effective intervention for the treatment of cardiac arrest. Consultation with the wider patient and public community will be important when considering the implications of the PARAMEDIC2 trial for clinical practice.
Recommendations for research
Unanswered questions about the use of vasopressors in OHCA remain. These include questions about timing, dosage, rate of administration, route of administration and concomitant therapies. Given the uncertainty about the effect of adrenaline on neurological outcomes, further research is required to better understand patients’ preferences in relation to survival and neurological outcome after OHCA. Further research should explore how patients and the public interpret the findings from the trial, the priorities of patients and the public, and the implications for practice of the findings.
The NHS guidelines support the use of the intraosseous route for drug administration if initial attempts at i.v. access fail. In the PARAMEDIC2 trial, one-third of patients received delayed drug administration through the intraosseous route. It is possible that an intraosseous-first strategy may have enabled drug treatments to be delivered earlier. However, recent observational studies63,208 raise doubt about the effectiveness of the intraosseous route for drug administration. Such studies are nevertheless limited by resuscitation time bias and other unmeasured confounders. The inclusion of a placebo arm in the PARAMEDIC2 trial should allow a less biased comparison of the i.v. and intraosseous route, which may guide the need for further research on the role of i.v. and intraosseous drug administration.
The evidence produced by the PARAMEDIC2 trial demonstrates that adrenaline is highly effective at restarting the heart and sustaining survival to hospital admission. Despite active treatments in intensive care, two-thirds of these patients will die prior to hospital discharge. The majority of these patients (65%) die from the devastating consequences of post-cardiac arrest brain injury. Of those who survive the initial cardiac arrest, the majority experience some neurocognitive functional impairment, ranging from mild cognitive problems to survival in a persistent vegetative state. There is very limited evidence about effective neuroprotective strategies beyond targeted temperature management. A key priority for future research is to identify effective treatment strategies to mitigate the effects of post-resuscitation brain injury. For those who survive but are left with significant functional impairment, further research in defining the optimal post-resuscitation rehabilitation care pathway would be beneficial.
Finally, the ethics and clinical implications of vasopressor use in OHCA and its effects on organ donation also need to be explored:
-
What value do patients and the public place on survival, survival with a favourable neurological outcome and survival with an unfavourable neurological outcome?
-
How do the communities served by NHS ambulance services interpret the findings from the PARAMEDIC2 trial and what are their views on the implications for practice?
-
In the context of cardiac arrest, where do patients and their relatives prefer to die (home or hospital)?
-
Does the effect of adrenaline differ according to the time of administration?
-
Does the effect of adrenaline differ according to the dose delivered or the strategy for delivery (bolus vs. infusion)?
-
Does the route of administration (i.v. or intraosseous) influence outcomes?
-
What post-resuscitation interventions can improve survival with a favourable neurological outcome?
-
What effect does the use of adrenaline have on the rate of organ donation?
Acknowledgements
Ambulance Services
London Ambulance Service NHS Trust
-
Alexander Boda (Research Paramedic).
-
Joshua Golding (Research Paramedic).
-
Johanna Lazarus (Research Paramedic).
-
Helen Werts (Research Paramedic).
North East Ambulance Service NHS Foundation Trust
-
Emma Bell (Research and Development Assistant).
-
Sonia Byers (Research and Development Manager).
-
Michelle Jackson (Research Administrator).
-
Matt Limmer (Research Paramedic).
-
Gary Shaw (Research Paramedic).
-
Craig Wynne (Research Paramedic).
South Central Ambulance Service NHS Foundation Trust
-
Nikki Brock (Research Paramedic).
-
Ed England (Pharmacy Advisor and Research Lead).
-
Claire Godfrey (Research Paramedic).
-
Claire King (Research Paramedic).
-
Isabel Rodriguez-Bachiller (Research Paramedic).
-
Sarah Taylor (Research Administrator).
-
Michelle Thomson (Research Paramedic).
-
Marie Stevens (Research Paramedic).
Welsh Ambulance Service NHS Trust
-
Jennifer Bradley (Research Paramedic).
-
Laura Galligan (Research Officer).
-
Robert Lovett (Research Support Officer).
-
Richard Whitfield (Research and Development Manager).
West Midlands Ambulance Service University NHS Foundation Trust
-
Rhiannon Boldy (Clinical Audit and Research Assistant).
-
Emma Harris (Clinical Audit and Research Assistant).
-
Jenny Lumley Holmes (Clinical Audit Manager).
-
Prudence Horwood (Research Nurse).
-
Mindy Jhamat (Research Paramedic).
-
Madison Larden (Research Nurse).
-
Garry Parcell (Research Paramedic).
-
Alice Pretty (Clinical Audit and Research Assistant).
-
Gill Price (Research and Development Manager).
-
Jenny Sears Brown (Research Paramedic).
Trial Steering Committee
-
Robert Andrews (Member).
-
Father Neil Baylis (Member).
-
Jonathan Benger (Member).
-
Bob Ewings (Member).
-
Matthew Gane (Member).
-
Stephen Jarvis (Member).
-
Claire Kenna (Member).
-
William Lee (Member).
-
Jon Nicholl (Chairperson).
-
David Pitcher (Member).
-
Helen Snooks (Member).
-
Matt Wise (Member).
Data Monitoring Committee
-
Marion Campbell (Chairperson).
-
Sue Mason (Member).
-
Kathy Rowan (Member).
-
Jasmeet Soar (Member).
Sponsor representative
Sponsor: Ms Jane Prewitt (Warwick Medical School, University of Warwick).
University of Warwick Clinical Trials Unit
-
James Buck (Recruitment Facilitator).
-
Nicola Cashin (Data Entry Clerk).
-
Louise Clarkson (Clinical Trial Monitor).
-
Matthew Cooke (Co-Investigator).
-
Claire Daffern (Quality Assurance Manager).
-
Sarah Duggan (Clinical Trials Unit Manager).
-
Hayley Johnson (Trial Administrator).
-
Programming team.
-
Malvenia Richmond (Data Entry Clerk).
-
Sarah Rumble (Clinical Trials Co-ordinator).
-
Natalie Strickland (Clinical Trials Unit Manager).
-
Martin Underwood (Clinical Trials Unit Director).
-
Eva Vernon (Trial Co-ordinator).
-
Jill Wood (Quality Assurance Manager).
MODEPHARMA Ltd (Beckenham, UK)
-
Oliver Gupta (Project Manager – IMP supplies).
-
Rima Gupta (Project Manager – IMP supplies).
We would like to thank the patients and legal representatives.
Copyright
Tables 19–21, 26 and 27 contain NHS Digital data. Copyright © 2019, the Health and Social Care Information Centre. Re-used with the permission of the Health and Social Care Information Centre [now NHS Digital]. All rights reserved.
Contributions of authors
Gavin D Perkins (https://orcid.org/0000-0003-3027-7548) (Professor of Critical Care Medicine) was the chief investigator of the PARAMEDIC2 trial. He led the team of co-applicants, researchers and trial support staff who conceived and designed the trial, and contributed to data collection, analysis and interpretation of the trial findings. He led drafting the report and revised it critically for important intellectual content.
Chen Ji (https://orcid.org/0000-0003-4919-3299) (Statistician) contributed to the design of the work, performed the statistical analyses, assisted with interpretation of data, and drafted the work and revised it critically for important intellectual content.
Felix Achana (https://orcid.org/0000-0002-8727-9125) (Health Economist) conducted the main body of the analyses of the health economic data.
John JM Black (https://orcid.org/0000-0002-1167-1550) (Emergency Physician and Medical Director of the South Central Ambulance Service) was involved in the design of the trial, secured the support of the National Ambulance Services Medical Directors’ Group for the trial, was the South Central Ambulance Service board sponsor for the trial, assisted in the interpretation of the trial findings, and helped revise the report critically for important intellectual content.
Karl Charlton (https://orcid.org/0000-0002-9601-1083) (Research Paramedic) was involved in the day-to-day management of the trial at the North East Ambulance Service; was involved in the acquisition, analysis and interpretation of data; and revised the work critically for important intellectual content.
James Crawford (https://orcid.org/0000-0003-1600-2652) (Trial Administrator) was involved in the design of the trial and acquisition of data, and revised the work critically for important intellectual content.
Adam de Paeztron (https://orcid.org/0000-0001-7001-8626) (Trial Manager) co-ordinated the trial and was involved in the design of the study, the acquisition of data, drafting the work and revising it critically for important intellectual content.
Charles Deakin (https://orcid.org/0000-0002-2565-9771) (Consultant in Cardiac Anaesthesia and Intensive Care) was principal investigator at the South Central Ambulance Service; was involved in the acquisition, analysis and interpretation of data; and revised the work critically for important intellectual content.
Mark Docherty (https://orcid.org/0000-0002-0147-4617) (Director of Nursing, Quality and Clinical Commissioning) was principal investigator at the West Midlands Ambulance Service; was involved in the acquisition, analysis and interpretation of data; and revised the work critically for important intellectual content.
Judith Finn (https://orcid.org/0000-0002-7307-7944) (Professor, Director of the Prehospital, Resuscitation and Emergency Care Research Unit) was involved in the conception and design of the work, contributed to the acquisition and interpretation of the data, and revised the work critically for important intellectual content.
Rachael T Fothergill (https://orcid.org/0000-0003-1341-6200) (Head of Clinical Audit and Research) was involved in the day-to-day management of the trial at the London Ambulance Service; was involved in the acquisition, analysis and interpretation of data; and revised the work critically for important intellectual content.
Simon Gates (https://orcid.org/0000-0003-3193-0975) (Professor of Biostatistics and Clinical Trials) was involved in the conception and design of the work, contributed to the acquisition and interpretation of the data, and revised the work critically for important intellectual content.
Imogen Gunson (https://orcid.org/0000-0001-8335-3335) (Research Paramedic) was involved in the day-to-day management of the trial at the West Midlands Ambulance Service; was involved in the acquisition, analysis and interpretation of data; and revised the work critically for important intellectual content.
Kyee Han (https://orcid.org/0000-0002-5206-5856) (Medical Director) was principal investigator at the North East Ambulance Service; was involved in the acquisition, analysis and interpretation of data; and revised the work critically for important intellectual content.
Susie Hennings (https://orcid.org/0000-0002-8160-6658) (Senior Project Manager) was involved in the design of the study and acquisition of data, and revised the work critically for important intellectual content.
Jessica Horton (https://orcid.org/0000-0003-0868-4761) (Trial Manager) co-ordinated the trial and was involved in the design of the trial, the acquisition of data, drafting the work and revising it critically for important intellectual content.
Kamran Khan (https://orcid.org/0000-0002-4322-9695) (Health Economist) contributed to the design of the economic research instruments and the collection of unit cost data.
Sarah Lamb (https://orcid.org/0000-0003-4349-7195) (Professor of Trauma Rehabilitation) was involved in the conception and design of the work, contributed to the acquisition and interpretation of the data, and revised the work critically for important intellectual content.
John Long (https://orcid.org/0000-0003-0674-2972) (PPI Representative) was involved in the design of the trial and acquisition of data, and revised the work critically for important intellectual content.
Joshua Miller (https://orcid.org/0000-0003-1990-4029) (Research Paramedic) was involved in the day-to-day management of the trial at the West Midlands Ambulance Service and in the acquisition, analysis and interpretation of data, and revised the work critically for important intellectual content.
Fionna Moore (https://orcid.org/0000-0002-9575-6884) (Medical Director) was principal investigator at the London Ambulance Service; was involved in the acquisition, analysis and interpretation of data; and revised the work critically for important intellectual content.
Jerry Nolan (https://orcid.org/0000-0003-3141-3812) (Consultant Anaesthetist) was involved in the conception and design of the work, contributed to the acquisition and interpretation of the data, and revised the work critically for important intellectual content.
Lyndsey O’Shea (https://orcid.org/0000-0003-2619-7596) (Research Support Officer) was involved in the day-to-day management of the trial at the Welsh Ambulance Service and in the acquisition, analysis and interpretation of data, and revised the work critically for important intellectual content.
Stavros Petrou (https://orcid.org/0000-0003-3121-6050) (Professor of Health Economics) provided oversight of all aspects of the economic evaluation, including its design, conduct, analysis and reporting.
Helen Pocock (https://orcid.org/0000-0001-7648-5313) (Research Paramedic) was involved in the day-to-day management of the trial at the South Central Ambulance Service and in the acquisition, analysis and interpretation of data, and revised the work critically for important intellectual content.
Tom Quinn (https://orcid.org/0000-0002-5116-0034) (Professor of Cardiovascular Nursing) was involved in the conception and design of the work, contributed to the acquisition and interpretation of the data, and revised the work critically for important intellectual content.
Nigel Rees (https://orcid.org/0000-0001-8799-5335) (Senior Research Lead) was principal investigator at the Welsh Ambulance Service; was involved in the acquisition, analysis and interpretation of data; and revised the work critically for important intellectual content.
Scott Regan (https://orcid.org/0000-0002-1235-0767) (Senior Project Manager) was involved in the design of the trial and acquisition of data, and revised the work critically for important intellectual content.
Andy Rosser (https://orcid.org/0000-0002-5477-4269) (Clinical Support Officer) was involved in the day-to-day management of the trial at the West Midlands Ambulance Service and in the acquisition, analysis and interpretation of data, and revised the work critically for important intellectual content.
Charlotte Scomparin (https://orcid.org/0000-0001-6693-8104) (Trial Manager) co-ordinated the trial and was involved in the design of the trial, the acquisition of data, drafting the work and revising it critically for important intellectual content.
Anne Slowther (https://orcid.org/0000-0002-3338-8457) (Professor of Clinical Ethics) was involved in the conception and design of the work, contributed to the acquisition and interpretation of the data, and revised the work critically for important intellectual content.
Ranjit Lall (https://orcid.org/0000-0003-1737-2866) (Lead Statistician) was involved in the conception and design of the work, supervised the statistical analyses reported, assisted with interpretation of data, drafted the work and revised it critically for important intellectual content.
Publications
Nolan JP. Does adrenaline improve long-term outcomes after out-of-hospital cardiac arrest? J Paramedic Pract 2015;7:16–18.
Perkins GD, Quinn T, Deakin CD, Nolan JP, Lall R, Slowther A, et al. Pre-hospital Assessment of the Role of Adrenaline: Measuring the Effectiveness of Drug administration In Cardiac arrest (PARAMEDIC-2): trial protocol. Resuscitation 2016;108:75–81.
Perkins GD, Ji C, Deakin CD, Quinn T, Nolan JP, Scomparin C, et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Eng J Med 2018;379:711–21.
Perkins GD, Kenna C, Ji C, Deakin CD, Nolan JP, Quinn T, et al. The effects of adrenaline in out of hospital cardiac arrest with shockable and non-shockable rhythms: findings from the PACA and PARAMEDIC-2 randomised controlled trials. Resuscitation 2019;140:55–63.
Perkins GD, Kenna C, Ji C, Deakin CD, Nolan JP, Quinn T, et al. The influence of time to adrenaline administration in the PARAMEDIC 2 randomised controlled trial. Intensive Care Med 2020;46:426–36.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Myat A, Song KJ, Rea T. Out-of-hospital cardiac arrest: current concepts. Lancet 2018;391:970-9. https://doi.org/10.1016/S0140-6736(18)30472-0.
- Hawkes C, Booth S, Ji C, Brace-McDonnell SJ, Whittington A, Mapstone J, et al. Epidemiology and outcomes from out-of-hospital cardiac arrests in England. Resuscitation 2017;110:133-40. https://doi.org/10.1016/j.resuscitation.2016.10.030.
- Olasveengen TM, de Caen AR, Mancini ME, Maconochie IK, Aickin R, Atkins DL, et al. 2017 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations summary. Resuscitation 2017;121:201-14. https://doi.org/10.1016/j.resuscitation.2017.10.021.
- Department of Health and Social Care Cardiovascular Disease Team . Cardiovascular Disease Outcomes Strategy: Improving Outcomes for People With or at Risk of Cardiovascular Disease 2013.
- OHCA Steering Group . Resuscitation to Recovery: A National Framework to Improve Care of People With Out-of-Hospital Cardiac Arrest (OHCA) in England 2017. www.resus.org.uk/library/publications/publication-resuscitation-recovery (accessed 3 December 2020).
- Department of Health and Social Care . Community Resuscitation Strategy for Northern Ireland 2014. www.health-ni.gov.uk/publications/community-resuscitation-strategy-and-reports (accessed 3 December 2020).
- Scottish Government . Out-of-Hospital Cardiac Arrest: A Strategy for Scotland. 2015.
- NHS Wales, Welsh Government . Out of Hospital Cardiac Arrest Plan: Improving the Care of People With an Out of Hospital Cardiac Arrest (OHCA) in Wales 2017. https://gov.wales/sites/default/files/publications/2019-03/out-of-hospital-cardiac-arrest-plan.pdf (accessed 3 December 2020).
- Nolan J, Soar J, Eikeland H. The chain of survival. Resuscitation 2006;71:270-1. https://doi.org/10.1016/j.resuscitation.2006.09.001.
- Bottiger BW, Lockey A, Aickin R, Castren M, de Caen A, Escalante R, et al. ‘All citizens of the world can save a life’ – The World Restart a Heart (WRAH) initiative starts in 2018. Resuscitation 2018;128:188-90. https://doi.org/10.1016/j.resuscitation.2018.04.015.
- Crocker R. Ambulance Response Programme (ARP) Impact Assessment. South of England: NHS South, Central and West Commissioning Support Unit; 2017.
- Deakin CD, England S, Diffey D. Ambulance telephone triage using ‘NHS Pathways’ to identify adult cardiac arrest. Heart 2017;103:738-44. https://doi.org/10.1136/heartjnl-2016-310651.
- Harjanto S, Na MX, Hao Y, Ng YY, Doctor N, Goh ES, et al. A before-after interventional trial of dispatcher-assisted cardio-pulmonary resuscitation for out-of-hospital cardiac arrests in Singapore. Resuscitation 2016;102:85-93. https://doi.org/10.1016/j.resuscitation.2016.02.014.
- Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2010;3:63-81. https://doi.org/10.1161/CIRCOUTCOMES.109.889576.
- Koster RW, Baubin MA, Bossaert LL, Caballero A, Cassan P, Castrén M, et al. European Resuscitation Council guidelines for resuscitation 2010 section 2. Adult basic life support and use of automated external defibrillators. Resuscitation 2010;81:1277-92. https://doi.org/10.1016/j.resuscitation.2010.08.009.
- Wik L, Steen PA, Bircher NG. Quality of bystander cardiopulmonary resuscitation influences outcome after prehospital cardiac arrest. Resuscitation 1994;28:195-203. https://doi.org/10.1016/0300-9572(94)90064-7.
- Babbs CF, Voorhees WD, Fitzgerald KR, Holmes HR, Geddes LA. Relationship of blood pressure and flow during CPR to chest compression amplitude: evidence for an effective compression threshold. Ann Emerg Med 1983;12:527-32. https://doi.org/10.1016/S0196-0644(83)80290-X.
- Ornato JP, Levine RL, Young DS, Racht EM, Garnett AR, Gonzalez ER. The effect of applied chest compression force on systemic arterial pressure and end-tidal carbon dioxide concentration during CPR in human beings. Ann Emerg Med 1989;18:732-7. https://doi.org/10.1016/S0196-0644(89)80005-8.
- Edelson DP, Abella BS, Kramer-Johansen J, Wik L, Myklebust H, Barry AM, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation 2006;71:137-45. https://doi.org/10.1016/j.resuscitation.2006.04.008.
- Stiell IG, Brown SP, Christenson J, Cheskes S, Nichol G, Powell J, et al. What is the role of chest compression depth during out-of-hospital cardiac arrest resuscitation?. Crit Care Med 2012;40:1192-8. https://doi.org/10.1097/CCM.0b013e31823bc8bb.
- Stiell IG, Brown SP, Nichol G, Cheskes S, Vaillancourt C, Callaway CW, et al. What is the optimal chest compression depth during out-of-hospital cardiac arrest resuscitation of adult patients?. Circulation 2014;130:1962-70. https://doi.org/10.1161/CIRCULATIONAHA.114.008671.
- Abella BS, Sandbo N, Vassilatos P, Alvarado JP, O’Hearn N, Wigder HN, et al. Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during in-hospital cardiac arrest. Circulation 2005;111:428-34. https://doi.org/10.1161/01.CIR.0000153811.84257.59.
- Idris AH, Guffey D, Aufderheide TP, Brown S, Morrison LJ, Nichols P, et al. Relationship between chest compression rates and outcomes from cardiac arrest. Circulation 2012;125:3004-12. https://doi.org/10.1161/CIRCULATIONAHA.111.059535.
- Idris AH, Guffey D, Pepe PE, Brown SP, Brooks SC, Callaway CW, et al. Chest compression rates and survival following out-of-hospital cardiac arrest. Crit Care Med 2015;43:840-8. https://doi.org/10.1097/CCM.0000000000000824.
- Zuercher M, Hilwig RW, Ranger-Moore J, Nysaether J, Nadkarni VM, Berg MD, et al. Leaning during chest compressions impairs cardiac output and left ventricular myocardial blood flow in piglet cardiac arrest. Crit Care Med 2010;38:1141-6. https://doi.org/10.1097/CCM.0b013e3181ce1fe2.
- Gates S, Quinn T, Deakin CD, Blair L, Couper K, Perkins GD. Mechanical chest compression for out of hospital cardiac arrest: systematic review and meta-analysis. Resuscitation 2015;94:91-7. https://doi.org/10.1016/j.resuscitation.2015.07.002.
- Vaillancourt C, Everson-Stewart S, Christenson J, Andrusiek D, Powell J, Nichol G, et al. The impact of increased chest compression fraction on return of spontaneous circulation for out-of-hospital cardiac arrest patients not in ventricular fibrillation. Resuscitation 2011;82:1501-7. https://doi.org/10.1016/j.resuscitation.2011.07.011.
- Cheskes S, Schmicker RH, Christenson J, Salcido DD, Rea T, Powell J, et al. Perishock pause: an independent predictor of survival from out-of-hospital shockable cardiac arrest. Circulation 2011;124:58-66. https://doi.org/10.1161/CIRCULATIONAHA.110.010736.
- Fries M, Weil MH, Chang YT, Castillo C, Tang W. Microcirculation during cardiac arrest and resuscitation. Crit Care Med 2006;34:454-7. https://doi.org/10.1097/01.CCM.0000247717.81480.B2.
- Davies CS, Colquhoun M, Graham S, Evans T, Chamberlain D. Defibrillator Advisory Committee . Defibrillators in public places: the introduction of a national scheme for public access defibrillation in England. Resuscitation 2002;52:13-21. https://doi.org/10.1016/S0300-9572(01)00439-7.
- Colquhoun MC, Chamberlain DA, Newcombe RG, Harris R, Harris S, Peel K, et al. A national scheme for public access defibrillation in England and Wales: early results. Resuscitation 2008;78:275-80. https://doi.org/10.1016/j.resuscitation.2008.03.226.
- Bækgaard JS, Viereck S, Møller TP, Ersbøll AK, Lippert F, Folke F. The effects of public access defibrillation on survival after out-of-hospital cardiac arrest: a systematic review of observational studies. Circulation 2017;136:954-65. https://doi.org/10.1161/CIRCULATIONAHA.117.029067.
- Holmberg MJ, Vognsen M, Andersen MS, Donnino MW, Andersen LW. Bystander automated external defibrillator use and clinical outcomes after out-of-hospital cardiac arrest: a systematic review and meta-analysis. Resuscitation 2017;120:77-8. https://doi.org/10.1016/j.resuscitation.2017.09.003.
- Deakin CD, Shewry E, Gray HH. Public access defibrillation remains out of reach for most victims of out-of-hospital sudden cardiac arrest. Heart 2014;100:619-23. https://doi.org/10.1136/heartjnl-2013-305030.
- Smith CM, Lim Choi Keung SN, Khan MO, Arvanitis TN, Fothergill R, Hartley-Sharpe C, et al. Barriers and facilitators to public access defibrillation in out-of-hospital cardiac arrest: a systematic review. Eur Heart J Qual Care Clin Outcomes 2017;3:264-73. https://doi.org/10.1093/ehjqcco/qcx023.
- Deakin CD, Anfield S, Hodgetts GA. Underutilisation of public access defibrillation is related to retrieval distance and time-dependent availability. Heart 2018;104:1339-43. https://doi.org/10.1136/heartjnl-2018-312998.
- Safar P. Community-wide cardiopulmonary resuscitation. J Iowa Med Soc 1964;54:629-35.
- Glover BM, Brown SP, Morrison L, Davis D, Kudenchuk PJ, Van Ottingham L, et al. Wide variability in drug use in out-of-hospital cardiac arrest: a report from the resuscitation outcomes consortium. Resuscitation 2012;83:1324-30. https://doi.org/10.1016/j.resuscitation.2012.07.008.
- Booth S, Ji C, Soar J, Siriwardena AN, Fothergill R, Spaight R, et al. Prehospital adrenaline administration for out-of-hospital cardiac arrest: the picture in England and Wales. Resuscitation 2018;130. https://doi.org/10.1016/j.resuscitation.2018.07.211.
- Michael JR, Guerci AD, Koehler RC, Shi AY, Tsitlik J, Chandra N, et al. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation 1984;69:822-35. https://doi.org/10.1161/01.cir.69.4.822.
- Brown CG, Werman HA, Davis EA, Hobson J, Hamlin RL. The effects of graded doses of epinephrine on regional myocardial blood flow during cardiopulmonary resuscitation in swine. Circulation 1987;75:491-7. https://doi.org/10.1161/01.cir.75.2.491.
- Deakin CD, Morrison LJ, Morley PT, Callaway CW, Kerber RE, Kronick SL, et al. Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2010;81:e93-e174. https://doi.org/10.1016/j.resuscitation.2010.08.027.
- Stiell IG, Nichol G, Leroux BG, Rea TD, Ornato JP, Powell J, et al. Early versus later rhythm analysis in patients with out-of-hospital cardiac arrest. N Engl J Med 2011;365:787-97. https://doi.org/10.1056/NEJMoa1010076.
- National Institute for Health and Care Excellence . Myocardial Infarction With ST-Segment Elevation: Acute Management 2013.
- Lemkes JS, Janssens GN, van der Hoeven NW, Jewbali LSD, Dubois EA, Meuwissen M, et al. Coronary angiography after cardiac arrest without ST-segment elevation. N Engl J Med 2019;380:1397-407. https://doi.org/10.1056/NEJMoa1816897.
- Patterson T, Perkins A, Perkins GD, Clayton T, Evans R, Nguyen H, et al. Rationale and design of: A Randomized tRial of Expedited transfer to a cardiac arrest center for non-ST elevation out-of-hospital cardiac arrest: the ARREST randomized controlled trial. Am Heart J 2018;204:92-101. https://doi.org/10.1016/j.ahj.2018.06.016.
- ClinicalTrials.gov . Direct or Subacute Coronary Angiography in Out-of-Hospital Cardiac Arrest (DISCO) n.d. https://clinicaltrials.gov/ct2/show/NCT02309151 (accessed 5 October 2020).
- ClinicalTrials.gov . Coronariography in OUt of HosPital Cardiac ArrEst (COUPE) n.d. https://clinicaltrials.gov/ct2/show/NCT02641626 (accessed 5 October 2020).
- ClinicalTrials.gov . Immediate Unselected Coronary Angiography Versus Delayed Triage in Survivors of Out-of-Hospital Cardiac Arrest Without ST-Segment Elevation (TOMAHAWK) n.d. https://clinicaltrials.gov/ct2/show/NCT02750462 (accessed 5 October 2020).
- ClinicalTrials.gov . Early Coronary Angiography Versus Delayed Coronary Angiography (PEARL) n.d. www.clinicaltrials.gov/ct2/show/NCT02387398 (accessed 5 October 2020).
- ClinicalTrials.gov . EMERGEncy Versus Delayed Coronary Angiogram in Survivors of Out-of-Hospital Cardiac Arrest (EMERGE) n.d. https://clinicaltrials.gov/ct2/show/NCT02876458 (accessed 5 October 2020).
- Stecker EC, Reinier K, Marijon E, Narayanan K, Teodorescu C, Uy-Evanado A, et al. Public health burden of sudden cardiac death in the United States. Circ Arrhythm Electrophysiol 2014;7:212-17. https://doi.org/10.1161/CIRCEP.113.001034.
- Atwood C, Eisenberg MS, Herlitz J, Rea TD. Incidence of EMS-treated out-of-hospital cardiac arrest in Europe. Resuscitation 2005;67:75-80. https://doi.org/10.1016/j.resuscitation.2005.03.021.
- Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation 2010;81:1479-87. https://doi.org/10.1016/j.resuscitation.2010.08.006.
- Finn J, Jacobs I, Williams TA, Gates S, Perkins GD. Adrenaline and vasopressin for cardiac arrest. Cochrane Database Syst Rev 2019;1. https://doi.org/10.1002/14651858.CD003179.pub2.
- Jacobs IG, Finn JC, Jelinek GA, Oxer HF, Thompson PL. Effect of adrenaline on survival in out-of-hospital cardiac arrest: a randomised double-blind placebo-controlled trial. Resuscitation 2011;82:1138-43. https://doi.org/10.1016/j.resuscitation.2011.06.029.
- Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA 2009;302:2222-9. https://doi.org/10.1001/jama.2009.1729.
- Olasveengen TM, Wik L, Sunde K, Steen PA. Outcome when adrenaline (epinephrine) was actually given vs. not given – post hoc analysis of a randomized clinical trial. Resuscitation 2012;83:327-32. https://doi.org/10.1016/j.resuscitation.2011.11.011.
- Hagihara A, Hasegawa M, Abe T, Nagata T, Wakata Y, Miyazaki S. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA 2012;307:1161-8. https://doi.org/10.1001/jama.2012.294.
- Hayashi Y, Iwami T, Kitamura T, Nishiuchi T, Kajino K, Sakai T, et al. Impact of early intravenous epinephrine administration on outcomes following out-of-hospital cardiac arrest. Circ J 2012;76:1639-45. https://doi.org/10.1253/circj.CJ-11-1433.
- Holmberg M, Holmberg S, Herlitz J. Low chance of survival among patients requiring adrenaline (epinephrine) or intubation after out-of-hospital cardiac arrest in Sweden. Resuscitation 2002;54:37-45. https://doi.org/10.1016/S0300-9572(02)00048-5.
- Arntz HR, Breckwoldt J. Cardiac resuscitation: epinephrine to treat cardiac arrest – a double-edged sword. Nat Rev Cardiol 2012;9:380-2. https://doi.org/10.1038/nrcardio.2012.71.
- Andersen LW, Grossestreuer AV, Donnino MW. ‘Resuscitation time bias’ – a unique challenge for observational cardiac arrest research. Resuscitation 2018;125:79-82. https://doi.org/10.1016/j.resuscitation.2018.02.006.
- Nakahara S, Tomio J, Takahashi H, Ichikawa M, Nishida M, Morimura N, et al. Evaluation of pre-hospital administration of adrenaline (epinephrine) by emergency medical services for patients with out of hospital cardiac arrest in Japan: controlled propensity matched retrospective cohort study. BMJ 2013;347. https://doi.org/10.1136/bmj.f6829.
- Burnett AM, Segal N, Salzman JG, McKnite MS, Frascone RJ. Potential negative effects of epinephrine on carotid blood flow and ETCO2 during active compression-decompression CPR utilizing an impedance threshold device. Resuscitation 2012;83:1021-4. https://doi.org/10.1016/j.resuscitation.2012.03.018.
- Ristagno G, Tang W, Huang L, Fymat A, Chang YT, Sun S, et al. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit Care Med 2009;37:1408-15. https://doi.org/10.1097/CCM.0b013e31819cedc9.
- Sun S, Tang W, Song F, Yu T, Ristagno G, Shan Y, et al. The effects of epinephrine on outcomes of normothermic and therapeutic hypothermic cardiopulmonary resuscitation. Crit Care Med 2010;38:2175-80. https://doi.org/10.1097/CCM.0b013e3181eedad6.
- Schmittinger CA, Torgersen C, Luckner G, Schröder DC, Lorenz I, Dünser MW. Adverse cardiac events during catecholamine vasopressor therapy: a prospective observational study. Intensive Care Med 2012;38:950-8. https://doi.org/10.1007/s00134-012-2531-2.
- Gao Smith F, Perkins GD, Gates S, Young D, McAuley DF, Tunnicliffe W, et al. Effect of intravenous β-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012;379:229-35. https://doi.org/10.1016/S0140-6736(11)61623-1.
- McQueen C, Gates S, Perkins GD. Adrenaline for the pharmacological treatment of cardiac arrest . . . going, going, gone?. Resuscitation 2012;83:921-2. https://doi.org/10.1016/j.resuscitation.2012.06.001.
- de Oliveira FC, Feitosa-Filho GS, Ritt LE. Use of beta-blockers for the treatment of cardiac arrest due to ventricular fibrillation/pulseless ventricular tachycardia: a systematic review. Resuscitation 2012;83:674-83. https://doi.org/10.1016/j.resuscitation.2012.01.025.
- Totaro RJ, Raper RF. Epinephrine-induced lactic acidosis following cardiopulmonary bypass. Crit Care Med 1997;25:1693-9. https://doi.org/10.1097/00003246-199710000-00019.
- Cocchi MN, Miller J, Hunziker S, Carney E, Salciccioli J, Farris S, et al. The association of lactate and vasopressor need for mortality prediction in survivors of cardiac arrest. Minerva Anestesiol 2011;77:1063-71.
- Shinozaki K, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Watanabe E, et al. Blood ammonia and lactate levels on hospital arrival as a predictive biomarker in patients with out-of-hospital cardiac arrest. Resuscitation 2011;82:404-9. https://doi.org/10.1016/j.resuscitation.2010.10.026.
- Beiser DG, Carr GE, Edelson DP, Peberdy MA, Hoek TL. Derangements in blood glucose following initial resuscitation from in-hospital cardiac arrest: a report from the national registry of cardiopulmonary resuscitation. Resuscitation 2009;80:624-30. https://doi.org/10.1016/j.resuscitation.2009.02.011.
- Coba V, Jaehne AK, Suarez A, Dagher GA, Brown SC, Yang JJ, et al. The incidence and significance of bacteremia in out of hospital cardiac arrest. Resuscitation 2014;85:196-202. https://doi.org/10.1016/j.resuscitation.2013.09.022.
- Bassford CR, Thickett DR, Perkins GD. The rise and fall of β-agonists in the treatment of ARDS. Crit Care 2012;16. https://doi.org/10.1186/cc11221.
- Rajaram SS, Desai NK, Kalra A, Gajera M, Cavanaugh SK, Brampton W, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev 2013;2. https://doi.org/10.1002/14651858.CD003408.pub3.
- Edwards P, Arango M, Balica L, Cottingham R, El-Sayed H, Farrell B, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet 2005;365:1957-9. https://doi.org/10.1016/S0140-6736(05)66552-X.
- Perkins GD, Quinn T, Deakin CD, Nolan JP, Lall R, Slowther AM, et al. Pre-hospital Assessment of the Role of Adrenaline: Measuring the Effectiveness of Drug administration In Cardiac arrest (PARAMEDIC-2): trial protocol. Resuscitation 2016;108:75-81. https://doi.org/10.1016/j.resuscitation.2016.08.029.
- Perkins GD, Woollard M, Cooke MW, Deakin C, Horton J, Lall R, et al. Prehospital randomised assessment of a mechanical compression device in cardiac arrest (PaRAMeDIC) trial protocol. Scand J Trauma Resusc Emerg Med 2010;18. https://doi.org/10.1186/1757-7241-18-58.
- Lazarus SC. Emergency treatment of asthma. New Engl J Med 2010;363:755-64. https://doi.org/10.1056/NEJMcp1003469.
- Green SM. Intravenous epinephrine in asthma? A word of caution. Ann Emerg Med 2003;41:712-13. https://doi.org/10.1067/mem.2003.145.
- Smith D, Riel J, Tilles I, Kino R, Lis J, Hoffman JR. Intravenous epinephrine in life-threatening asthma. Ann Emerg Med 2003;41:706-11. https://doi.org/10.1067/mem.2003.144.
- Putland M, Kerr D, Kelly AM. Adverse events associated with the use of intravenous epinephrine in emergency department patients presenting with severe asthma. Ann Emerg Med 2006;47:559-63. https://doi.org/10.1016/j.annemergmed.2006.01.022.
- Deakin C, Brown S, Jewkes F, Lockey D, Lyon R, Moore F, et al. Guidelines: Prehospital Resuscitation n.d. www.resus.org.uk/library/2015-resuscitation-guidelines/prehospital-resuscitation (accessed 5 October 2020).
- Brown SN, Kumar D, Millins M, Mark J. UK Ambulance Services Clinical Practice Guidelines 2016. Bridgwater: Class Professional Publishing; 2016.
- Nolan JP, Soar J, Zideman DA, Biarent D, Bossaert LL, Deakin C, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation 2010;81:1219-76. https://doi.org/10.1016/j.resuscitation.2010.08.021.
- Perkins GD, Cooke MW. Variability in cardiac arrest survival: the NHS Ambulance Service quality indicators. Emerg Med J 2012;29:3-5. https://doi.org/10.1136/emermed-2011-200758.
- European Commission . Guidelines: Detailed Commission Guidelines on Good Manufacturing Practice for Investigational Medicinal Products for Human Use, Pursuant to the Second Subparagraph of Article 63(1) of Regulation (EU) No 536 2014 n.d. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-10/guideline_adopted_1_en_act_part1_v3.pdf (accessed 5 October 2020).
- Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines for post-resuscitation care 2015: section 5 of the European Resuscitation Council guidelines for resuscitation 2015. Resuscitation 2015;95:202-22. https://doi.org/10.1016/j.resuscitation.2015.07.018.
- Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa). Resuscitation 2004;63:233-49. https://doi.org/10.1016/j.resuscitation.2004.09.008.
- Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation 2015;132:1286-300. https://doi.org/10.1161/CIR.0000000000000144.
- Saver JL, Filip B, Hamilton S, Yanes A, Craig S, Cho M, et al. Improving the reliability of stroke disability grading in clinical trials and clinical practice: the Rankin Focused Assessment (RFA). Stroke 2010;41:992-5. https://doi.org/10.1161/STROKEAHA.109.571364.
- Bruno A, Akinwuntan AE, Lin C, Close B, Davis K, Baute V, et al. Simplified modified rankin scale questionnaire: reproducibility over the telephone and validation with quality of life. Stroke 2011;42:2276-9. https://doi.org/10.1161/STROKEAHA.111.613273.
- Arawwawala D, Brett SJ. Clinical review: beyond immediate survival from resuscitation-long-term outcome considerations after cardiac arrest. Crit Care 2007;11. https://doi.org/10.1186/cc6139.
- Sunnerhagen KS, Johansson O, Herlitz J, Grimby G. Life after cardiac arrest; a retrospective study. Resuscitation 1996;31:135-40. https://doi.org/10.1016/0300-9572(95)00903-5.
- Longstreth WT, Nichol G, Van Ottingham L, Hallstrom AP. Two simple questions to assess neurologic outcomes at 3 months after out-of-hospital cardiac arrest: experience from the public access defibrillation trial. Resuscitation 2010;81:530-3. https://doi.org/10.1016/j.resuscitation.2010.01.011.
- Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): Reliability, Validity and Diagnostic Utility n.d.
- O’Reilly SM, Grubb N, O’Carroll RE. Long-term emotional consequences of in-hospital cardiac arrest and myocardial infarction. Br J Clin Psychol 2004;43:83-95. https://doi.org/10.1348/014466504772812986.
- Griffiths JA, Morgan K, Barber VS, Young JD. Study protocol: the Intensive Care Outcome Network (‘ICON’) study. BMC Health Serv Res 2008;8. https://doi.org/10.1186/1472-6963-8-132.
- Perkins GD, Lall R, Quinn T, Deakin CD, Cooke MW, Horton J, et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet 2015;385:947-55. https://doi.org/10.1016/S0140-6736(14)61886-9.
- Perkins GD, Brace-McDonnell SJ. OHCAO Project Group . The UK Out of Hospital Cardiac Arrest Outcome (OHCAO) project. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008736.
- Brown CG, Martin DR, Pepe PE, Stueven H, Cummins RO, Gonzalez E, et al. A comparison of standard-dose and high-dose epinephrine in cardiac arrest outside the hospital. The Multicenter High-Dose Epinephrine Study Group. N Engl J Med 1992;327:1051-5. https://doi.org/10.1056/NEJM199210083271503.
- Callaham M, Madsen CD, Barton CW, Saunders CE, Pointer J. A randomized clinical trial of high-dose epinephrine and norepinephrine vs standard-dose epinephrine in prehospital cardiac arrest. JAMA 1992;268:2667-72. https://doi.org/10.1001/jama.1992.03490190067033.
- Gueugniaud PY, Mols P, Goldstein P, Pham E, Dubien PY, Deweerdt C, et al. A comparison of repeated high doses and repeated standard doses of epinephrine for cardiac arrest outside the hospital. European Epinephrine Study Group. N Engl J Med 1998;339:1595-601. https://doi.org/10.1056/NEJM199811263392204.
- Sherman BW, Munger MA, Foulke GE, Rutherford WF, Panacek EA. High-dose versus standard-dose epinephrine treatment of cardiac arrest after failure of standard therapy. Pharmacotherapy 1997;17:242-7.
- Stiell IG, Hebert PC, Weitzman BN, Wells GA, Raman S, Stark RM, et al. High-dose epinephrine in adult cardiac arrest. N Engl J Med 1992;327:1045-50. https://doi.org/10.1056/NEJM199210083271502.
- Ducros L, Vicaut E, Soleil C, Le Guen M, Gueye P, Poussant T, et al. Effect of the addition of vasopressin or vasopressin plus nitroglycerin to epinephrine on arterial blood pressure during cardiopulmonary resuscitation in humans. J Emerg Med 2011;41:453-9. https://doi.org/10.1016/j.jemermed.2010.02.030.
- Gueugniaud PY, David JS, Chanzy E, Hubert H, Dubien PY, Mauriaucourt P, et al. Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation. N Engl J Med 2008;359:21-30. https://doi.org/10.1056/NEJMoa0706873.
- Lindner KH, Dirks B, Strohmenger HU, Prengel AW, Lindner IM, Lurie KG. Randomised comparison of epinephrine and vasopressin in patients with out-of-hospital ventricular fibrillation. Lancet 1997;349:535-7. https://doi.org/10.1016/S0140-6736(97)80087-6.
- Ong ME, Tiah L, Leong BS, Tan EC, Ong VY, Tan EA, et al. A randomised, double-blind, multi-centre trial comparing vasopressin and adrenaline in patients with cardiac arrest presenting to or in the emergency department. Resuscitation 2012;83:953-60. https://doi.org/10.1016/j.resuscitation.2012.02.005.
- Wenzel V, Krismer AC, Arntz HR, Sitter H, Stadlbauer KH, Lindner KH. European Resuscitation Council Vasopressor during Cardiopulmonary Resuscitation Study Group . A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N Engl J Med 2004;350:105-13. https://doi.org/10.1056/NEJMoa025431.
- Schneiderman LJ. Defining medical futility and improving medical care. J Bioeth Inq 2011;8:123-31. https://doi.org/10.1007/s11673-011-9293-3.
- Sasson C, Hegg AJ, Macy M, Park A, Kellermann A, McNally B. CARES Surveillance Group . Prehospital termination of resuscitation in cases of refractory out-of-hospital cardiac arrest. JAMA 2008;300:1432-8. https://doi.org/10.1001/jama.300.12.1432.
- Benger J, Coates D, Davies S, Greenwood R, Nolan J, Rhys M, et al. Randomised comparison of the effectiveness of the laryngeal mask airway supreme, i-gel and current practice in the initial airway management of out of hospital cardiac arrest: a feasibility study. Br J Anaesth 2016;116:262-8. https://doi.org/10.1093/bja/aev477.
- Hodges JL, Lehmann EL. Estimates of location based on rank tests. Ann Math Statist 1963;34:598-611. https://doi.org/10.1214/aoms/1177704172.
- Peterson B, Harrell FE. Partial proportional odds models for ordinal response variables. J Royal Stat Soc 1990;39:205-17. https://doi.org/10.2307/2347760.
- Williams R. Generalized ordered logit/partial proportional odds models for ordinal dependent variables. Stata J 2006;6:58-82. https://doi.org/10.1177/1536867X0600600104.
- DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med 1994;13:1341-52. https://doi.org/10.1002/sim.4780131308.
- National Institute for Health and Care Excellence . Guide to the Methods of Health Technology Appraisal 2008.
- European Commission . Clinical Trials – Directive 2001 20 EC n.d. https://ec.europa.eu/health/human-use/clinical-trials/directive_en (accessed 23 September 2020).
- Great Britain . The Medicines for Human Use (Clinical Trials) Regulations 2004 2004.
- Great Britain . The Health Service (Control of Patient Information) Regulations 2002 2002.
- Davies H, Shakur H, Padkin A, Roberts I, Slowther AM, Perkins GD. Guide to the design and review of emergency research when it is proposed that consent and consultation be waived. Emerg Med J 2014;31:794-5. https://doi.org/10.1136/emermed-2014-203675.
- Whitesides LW, Baren JM, Biros MH, Fleischman RJ, Govindarajan PR, Jones EB, et al. Impact of individual clinical outcomes on trial participants’ perspectives on enrollment in emergency research without consent. Clin Trials 2017;14:180-6. https://doi.org/10.1177/1740774516677276.
- Baker FX, Merz JF. What gives them the right? Legal privilege and waivers of consent for research. Clin Trials 2018;15:579-86. https://doi.org/10.1177/1740774518803122.
- Chin TL, Moore EE, Coors ME, Chandler JG, Ghasabyan A, Harr JN, et al. Exploring ethical conflicts in emergency trauma research: the COMBAT (Control of Major Bleeding after Trauma) study experience. Surgery 2015;157:10-9. https://doi.org/10.1016/j.surg.2014.05.021.
- Dickert NW, Mah VA, Baren JM, Biros MH, Govindarajan P, Pancioli A, et al. Enrollment in research under exception from informed consent: the Patients’ Experiences in Emergency Research (PEER) study. Resuscitation 2013;84:1416-21. https://doi.org/10.1016/j.resuscitation.2013.04.006.
- Kämäräinen A, Silfvast T, Saarinen S, Virta J, Virkkunen I. Conduct of emergency research in patients unable to give consent – experiences and perceptions of patients, their consent providing next of kin, and treating physicians following a prehospital resuscitation trial. Resuscitation 2012;83:81-5. https://doi.org/10.1016/j.resuscitation.2011.07.018.
- Nelson MJ, Deiorio NM, Schmidt TA, Zive DM, Griffiths D, Newgard CD. Why persons choose to opt out of an exception from informed consent cardiac arrest trial. Resuscitation 2013;84:825-30. https://doi.org/10.1016/j.resuscitation.2013.01.030.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. https://doi.org/10.1136/bmj.f1049.
- NHS Digital . Hospital Episode Statistics (HES) n.d. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (accessed 10 December 2019).
- NHS Improvement . National Schedule of Reference Costs, 2016–17: The Main Schedule n.d. https://improvement.nhs.uk/resources/reference-costs/#archive (accessed 10 December 2019).
- Gates S, Lall R, Quinn T, Deakin CD, Cooke MW, Horton J, et al. Prehospital randomised assessment of a mechanical compression device in out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised trial and economic evaluation. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21110.
- NHS Digital . HRG4+ 2016 17 Reference Costs Grouper 2017. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/grouper-and-tools-archive/costing-hrg4-2016-17-reference-costs-grouper (accessed 10 December 2019).
- NHS Improvement . National Cost Collection Guidance 2018 n.d. https://improvement.nhs.uk/documents/2359/National_cost_collection_guidance_2018_April.pdf (accessed 10 December 2019).
- NHS Digital . HES Data Dictionary: Adult Critical Care – Adult Critical Care (ACC) Hospital Episodes Statistics (HES) Data Dictionary 2017. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary (accessed 10 December 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- NHS Digital . Prescription Cost Analysis – England, 2017 [PAS] 2017. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/prescription-cost-analysis-england-2017 (accessed 10 December 2019).
- Joint Formulary Committee . British National Formulary 2017.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. https://doi.org/10.1136/bmj.316.7133.736.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Valuation Set for England (Updated November 2018) n.d. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 10 December 2019).
- Rivero-Arias O, Ouellet M, Gray A, Wolstenholme J, Rothwell PM, Luengo-Fernandez R. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Making 2010;30:341-54. https://doi.org/10.1177/0272989X09349961.
- Brazier J, Czoski-Murray C, Roberts J, Brown M, Symonds T, Kelleher C. Estimation of a preference-based index from a condition-specific measure: the King’s Health Questionnaire. Med Decis Making 2008;28:113-26. https://doi.org/10.1177/0272989X07301820.
- Dritsaki M, Achana F, Mason J, Petrou S. Methodological issues surrounding the use of baseline health-related quality of life data to inform trial-based economic evaluations of interventions within emergency and critical care settings: a systematic literature review. PharmacoEconomics 2017;35:501-15. https://doi.org/10.1007/s40273-016-0485-x.
- MVH Group . The Measurement and Valuation of Health: Final Report on the Modelling of Valuation Tariffs 1995.
- van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1-67. https://doi.org/10.18637/jss.v045.i03.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Henningsen A, Hamann JD. Systemfit: a package for estimating systems of simultaneous equations in R. J Stat Softw 2007;23:1-40. https://doi.org/10.18637/jss.v023.i04.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- Kudenchuk PJ. Early epinephrine in out-of-hospital cardiac arrest: is sooner better than none at all?. Resuscitation 2013;84:861-2. https://doi.org/10.1016/j.resuscitation.2013.05.003.
- Filipovic-Pierucci A, Zarca K, Durand-Zaleski I. Markov models for health economic evaluation modelling in R with the heemod package. Value Health 2016;19. https://doi.org/10.1016/j.jval.2016.09.133.
- Office for National Statistics . National Life Tables: UK, 1980–82 to 2015–17 n.d. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed 25 September 2019).
- Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling. PharmacoEconomics 2007;25:3-6. https://doi.org/10.2165/00019053-200725010-00002.
- Andersen LW, Holmberg MJ, Granfeldt A, James LP, Caulley L. Cost-effectiveness of public automated external defibrillators. Resuscitation 2019;138:250-8. https://doi.org/10.1016/j.resuscitation.2019.03.029.
- Curtis L. Unit Costs of Health and Social Care 2008. Canterbury: PSSRU, University of Kent; 2008.
- Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med 2004;30:2126-8. https://doi.org/10.1007/s00134-004-2425-z.
- Lemiale V, Dumas F, Mongardon N, Giovanetti O, Charpentier J, Chiche JD, et al. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med 2013;39:1972-80. https://doi.org/10.1007/s00134-013-3043-4.
- Dragancea I, Rundgren M, Englund E, Friberg H, Cronberg T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation 2013;84:337-42. https://doi.org/10.1016/j.resuscitation.2012.09.015.
- Arrich J, Zeiner A, Sterz F, Janata A, Uray T, Richling N, et al. Factors associated with a change in functional outcome between one month and six months after cardiac arrest: a retrospective cohort study. Resuscitation 2009;80:876-80. https://doi.org/10.1016/j.resuscitation.2009.04.045.
- Haywood K, Whitehead L, Nadkarni VM, Achana F, Beesems S, Böttiger BW, et al. COSCA (Core Outcome Set for Cardiac Arrest) in adults: an advisory statement from the International Liaison Committee on Resuscitation. Resuscitation 2018;127:147-63. https://doi.org/10.1016/j.resuscitation.2018.03.022.
- Smith K, Andrew E, Lijovic M, Nehme Z, Bernard S. Quality of life and functional outcomes 12 months after out-of-hospital cardiac arrest. Circulation 2015;131:174-81. https://doi.org/10.1161/CIRCULATIONAHA.114.011200.
- Perkins GD, Kenna C, Ji C, Deakin CD, Nolan JP, Quinn T, et al. The effects of adrenaline in out of hospital cardiac arrest with shockable and non-shockable rhythms: findings from the PACA and PARAMEDIC-2 randomised controlled trials. Resuscitation 2019;140:55-63. https://doi.org/10.1016/j.resuscitation.2019.05.007.
- Nichol G, Brown SP, Perkins GD, Kim F, Sterz F, Broeckel Elrod JA, et al. What change in outcomes after cardiac arrest is necessary to change practice? Results of an international survey. Resuscitation 2016;107:115-20. https://doi.org/10.1016/j.resuscitation.2016.08.004.
- Nichol G, Leroux B, Wang H, Callaway CW, Sopko G, Weisfeldt M, et al. Trial of continuous or interrupted chest compressions during CPR. N Engl J Med 2015;373:2203-14. https://doi.org/10.1056/NEJMoa1509139.
- Benger JR, Kirby K, Black S, Brett SJ, Clout M, Lazaroo MJ, et al. Effect of a strategy of a supraglottic airway device vs tracheal intubation during out-of-hospital cardiac arrest on functional outcome: the AIRWAYS-2 randomized clinical trial. JAMA 2018;320:779-91. https://doi.org/10.1001/jama.2018.11597.
- Kudenchuk PJ, Daya M, Dorian P. Resuscitation Outcomes Consortium Investigators . Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med 2016;375:802-3. https://doi.org/10.1056/NEJMc1608041.
- Morrison LJ, Visentin LM, Kiss A, Theriault R, Eby D, Vermeulen M, et al. Validation of a rule for termination of resuscitation in out-of-hospital cardiac arrest. N Engl J Med 2006;355:478-87. https://doi.org/10.1056/NEJMoa052620.
- Sandroni C, D’Arrigo S, Nolan JP. Prognostication after cardiac arrest. Crit Care 2018;22. https://doi.org/10.1186/s13054-018-2060-7.
- Perkins GD, Ji C, Deakin CD, Quinn T, Nolan JP, Scomparin C, et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med 2018;379:711-21. https://doi.org/10.1056/NEJMoa1806842.
- Ong MEH, Perkins GD, Cariou A. Out-of-hospital cardiac arrest: prehospital management. Lancet 2018;391:980-8. https://doi.org/10.1016/S0140-6736(18)30316-7.
- Deakin CD. The chain of survival: not all links are equal. Resuscitation 2018;126:80-2. https://doi.org/10.1016/j.resuscitation.2018.02.012.
- Berdowski J, Beekhuis F, Zwinderman AH, Tijssen JG, Koster RW. Importance of the first link: description and recognition of an out-of-hospital cardiac arrest in an emergency call. Circulation 2009;119:2096-102. https://doi.org/10.1161/CIRCULATIONAHA.108.768325.
- Hasselqvist-Ax I, Riva G, Herlitz J, Rosenqvist M, Hollenberg J, Nordberg P, et al. Early cardiopulmonary resuscitation in out-of-hospital cardiac arrest. N Engl J Med 2015;372:2307-15. https://doi.org/10.1056/NEJMoa1405796.
- Kitamura T, Kiyohara K, Sakai T, Matsuyama T, Hatakeyama T, Shimamoto T, et al. Public-access defibrillation and out-of-hospital cardiac arrest in Japan. N Engl J Med 2016;375:1649-59. https://doi.org/10.1056/NEJMsa1600011.
- Moulaert VR, Goossens M, Heijnders IL, Verbunt JA, Heugten CM. Early neurologically focused follow-up after cardiac arrest is cost-effective: a trial-based economic evaluation. Resuscitation 2016;106:30-6. https://doi.org/10.1016/j.resuscitation.2016.06.015.
- Zimmerli M, Tisljar K, Balestra GM, Langewitz W, Marsch S, Hunziker S. Prevalence and risk factors for post-traumatic stress disorder in relatives of out-of-hospital cardiac arrest patients. Resuscitation 2014;85:801-8. https://doi.org/10.1016/j.resuscitation.2014.02.022.
- Compton S, Grace H, Madgy A, Swor RA. Post-traumatic stress disorder symptomology associated with witnessing unsuccessful out-of-hospital cardiopulmonary resuscitation. Acad Emerg Med 2009;16:226-9. https://doi.org/10.1111/j.1553-2712.2008.00336.x.
- Kentish-Barnes N, Chaize M, Seegers V, Legriel S, Cariou A, Jaber S, et al. Complicated grief after death of a relative in the intensive care unit. Eur Respir J 2015;45:1341-52. https://doi.org/10.1183/09031936.00160014.
- Nolan JP, Ferrando P, Soar J, Benger J, Thomas M, Harrison DA, et al. Increasing survival after admission to UK critical care units following cardiopulmonary resuscitation. Crit Care 2016;20. https://doi.org/10.1186/s13054-016-1390-6.
- Perkins GD, Neumar R, Monsieurs KG, Lim SH, Castren M, Nolan JP, et al. The International Liaison Committee on Resuscitation – review of the last 25 years and vision for the future. Resuscitation 2017;121:104-16. https://doi.org/10.1016/j.resuscitation.2017.09.029.
- International Liaison Committee on Resuscitation . Vasopressors in Adult Cardiac Arrest (ALS): Systematic Review n.d. https://costr.ilcor.org/document/vasopressors-in-adult-cardiac-arrest (accessed 23 September 2020).
- Holmberg MJ, Issa MS, Moskowitz A, Morley P, Welsford M, Neumar RW, et al. Vasopressors during adult cardiac arrest: a systematic review and meta-analysis. Resuscitation 2019;139:106-21. https://doi.org/10.1016/j.resuscitation.2019.04.008.
- Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016;353. https://doi.org/10.1136/bmj.i2016.
- Nordseth T, Olasveengen TM, Kvaløy JT, Wik L, Steen PA, Skogvoll E. Dynamic effects of adrenaline (epinephrine) in out-of-hospital cardiac arrest with initial pulseless electrical activity (PEA). Resuscitation 2012;83:946-52. https://doi.org/10.1016/j.resuscitation.2012.02.031.
- Neset A, Nordseth T, Kramer-Johansen J, Wik L, Olasveengen TM. Effects of adrenaline on rhythm transitions in out-of-hospital cardiac arrest. Acta Anaesthesiol Scand 2013;57:1260-7. https://doi.org/10.1111/aas.12184.
- Nichol G, Guffey D, Stiell IG, Leroux B, Cheskes S, Idris A, et al. Post-discharge outcomes after resuscitation from out-of-hospital cardiac arrest: a ROC PRIMED substudy. Resuscitation 2015;93:74-81. https://doi.org/10.1016/j.resuscitation.2015.05.011.
- Murad MH, Katabi A, Benkhadra R, Montori VM. External validity, generalisability, applicability and directness: a brief primer. BMJ Evid Based Med 2018;23:17-9. https://doi.org/10.1136/ebmed-2017-110800.
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c869.
- Ford I, Norrie J. Pragmatic trials. N Engl J Med 2016;375:454-63. https://doi.org/10.1056/NEJMra1510059.
- Fothergill RT, Emmerson AC, Iyer R, Lazarus J, Whitbread M, Nolan JP, et al. Repeated adrenaline doses and survival from an out-of-hospital cardiac arrest. Resuscitation 2019;138:316-21. https://doi.org/10.1016/j.resuscitation.2019.01.022.
- Fisk CA, Olsufka M, Yin L, McCoy AM, Latimer AJ, Maynard C, et al. Lower-dose epinephrine administration and out-of-hospital cardiac arrest outcomes. Resuscitation 2018;124:43-8. https://doi.org/10.1016/j.resuscitation.2018.01.004.
- Donnino MW, Salciccioli JD, Howell MD, Cocchi MN, Giberson B, Berg K, et al. Time to administration of epinephrine and outcome after in-hospital cardiac arrest with non-shockable rhythms: retrospective analysis of large in-hospital data registry. BMJ 2014;348. https://doi.org/10.1136/bmj.g3028.
- Hansen M, Schmicker RH, Newgard CD, Grunau B, Scheuermeyer F, Cheskes S, et al. Time to epinephrine administration and survival from nonshockable out-of-hospital cardiac arrest among children and adults. Circulation 2018;137:2032-40. https://doi.org/10.1161/CIRCULATIONAHA.117.033067.
- Ewy GA, Bobrow BJ, Chikani V, Sanders AB, Otto CW, Spaite DW, et al. The time dependent association of adrenaline administration and survival from out-of-hospital cardiac arrest. Resuscitation 2015;96:180-5. https://doi.org/10.1016/j.resuscitation.2015.08.011.
- Andersen LW, Kurth T, Chase M, Berg KM, Cocchi MN, Callaway C, et al. Early administration of epinephrine (adrenaline) in patients with cardiac arrest with initial shockable rhythm in hospital: propensity score matched analysis. BMJ 2016;353. https://doi.org/10.1136/bmj.i1577.
- Reynolds JC, Rittenberger JC, Menegazzi JJ. Drug administration in animal studies of cardiac arrest does not reflect human clinical experience. Resuscitation 2007;74:13-26. https://doi.org/10.1016/j.resuscitation.2006.10.032.
- Rittenberger JC, Bost JE, Menegazzi JJ. Time to give the first medication during resuscitation in out-of-hospital cardiac arrest. Resuscitation 2006;70:201-6. https://doi.org/10.1016/j.resuscitation.2005.12.006.
- Kudenchuk PJ, Brown SP, Daya M, Rea T, Nichol G, Morrison LJ, et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med 2016;374:1711-22. https://doi.org/10.1056/NEJMoa1514204.
- Tanaka H, Takyu H, Sagisaka R, Ueta H, Shirakawa T, Kinoshi T, et al. Favorable neurological outcomes by early epinephrine administration within 19 minutes after EMS call for out-of-hospital cardiac arrest patients. Am J Emerg Med 2016;34:2284-90. https://doi.org/10.1016/j.ajem.2016.08.026.
- Hubble MW, Tyson C. Impact of early vasopressor administration on neurological outcomes after prolonged out-of-hospital cardiac arrest. Prehosp Disaster Med 2017;32:297-304. https://doi.org/10.1017/S1049023X17000115.
- Nolan J, Deakin, C, Lockey, A, Perkins, G, Soar J. Post-resuscitation Care Guidelines 2015. London: Resuscitation Council (UK); 2015.
- Couper K, Kimani PK, Gale CP, Quinn T, Squire IB, Marshall A, et al. Patient, health service factors and variation in mortality following resuscitated out-of-hospital cardiac arrest in acute coronary syndrome: analysis of the Myocardial Ischaemia National Audit Project. Resuscitation 2018;124:49-57. https://doi.org/10.1016/j.resuscitation.2018.01.011.
- Granfeldt A, Avis SR, Lind PC, Holmberg MJ, Kleinman M, Maconochie I, et al. Intravenous vs. intraosseous administration of drugs during cardiac arrest: a systematic review. Resuscitation 2020;149:150-7. https://doi.org/10.1016/j.resuscitation.2020.02.025.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
Appendix 1 Health economics tables and figures
| Assessment period | Category | Adrenaline arm (N = 1833)a | Placebo arm (N = 1094)a | Adrenaline vs. placebo | |||||
|---|---|---|---|---|---|---|---|---|---|
| n b | Participants with complete data (n) | Mean (SE) | n b | Participants with complete data (n) | Mean (SE) | Mean difference (95% CI) | p-value | ||
| 0–6 months after randomisation | Inpatient stay – initial admission | ||||||||
| Inpatient ward (days) | 670 | 670 | 6.63 (0.54) | 317 | 317 | 7.84 (0.9) | –1.2 (–3.5 to 0.91) | 0.264 | |
| ICU (days) | 506 | 505 | 5.75 (0.43) | 234 | 233 | 6.29 (0.69) | –0.54 (–2.13 to 1.07) | 0.518 | |
| Inpatient stay, re-admissions | |||||||||
| Inpatient ward (days) | 56 | 56 | 18.55 (3.74) | 37 | 36 | 16.69 (4.8) | 1.86 (–9.84 to 13.71) | 0.746 | |
| ICU (days) | 15 | 15 | 9.8 (3.48) | 7 | 7 | 13.29 (3.79) | –3.49 (–12.96 to 6.31) | 0.478 | |
| Other hospitalisation episodes | |||||||||
| Accident and emergency (visits) | 1700 | 1700 | 1.05 (0.01) | 1040 | 1040 | 1.04 (0.01) | 0.01 (–0.02 to 0.03) | 0.95 | |
| Day case (visits) | 15 | 15 | 5.93 (4.95) | 16 | 16 | 1.06 (0.06) | 4.87 (–0.12 to 15.8) | 0.402 | |
| Outpatient attendance (visits) | 281 | 281 | 5.12 (0.43) | 144 | 144 | 6.07 (0.66) | –0.95 (–2.58 to 0.68) | 0.224 | |
| 6–12 months after randomisationc | Inpatient stay – re-admissions | ||||||||
| Inpatient ward (days) | 26 | 23 | 5.87 (1.24) | 16 | 11 | 9.36 (4.84) | –3.49 (–14.42 to 4.32) | 0.494 | |
| ICU (days) | 6 | 5 | 5.8 (2.27) | 2 | 1 | 1 (0) | 4.8 (1 to 12) | 0.79 | |
| Other hospitalisation episodes | |||||||||
| Accident and emergency (visits) | 30 | 26 | 2.27 (0.28) | 19 | 13 | 1.54 (0.28) | 0.73 (–0.08 to 1.45) | 0.084 | |
| Day case (visits) | 10 | 10 | 9.1 (7.99) | 5 | 4 | 1 (0) | 8.1 (0.11 to 26.05) | 0.062 | |
| Outpatient attendance (visits) | 91 | 71 | 7.45 (1.48) | 62 | 40 | 4.3 (0.64) | 3.15 (0.31 to 6.74) | 0.028 | |
| Assessment period | Category | Adrenaline arm (N = 1833)a | Placebo arm (N = 1094)a | Adrenaline vs. placebo | |||||
|---|---|---|---|---|---|---|---|---|---|
| n b | Number of participants with data | Mean (SE) costs (£) | n b | Number of participants with data | Mean (SE) costs (£) | Mean difference (95% CI) (£) | p-value | ||
| 0–6 months after randomisation | Inpatient stay – initial admission | ||||||||
| Admitted patient care | 670 | 670 | 4655 (227) | 317 | 316 | 5536 (410) | –881 (–1854 to 9) | 0.06 | |
| Critical care | 506 | 505 | 10,162 (608) | 234 | 233 | 11,338 (1203) | –1175 (–3900 to 1494) | 0.406 | |
| Inpatient stay, re-admissions | |||||||||
| Admitted patient care | 56 | 56 | 9906 (1168) | 37 | 36 | 10,353 (1984) | –447 (–4856 to 3848) | 0.904 | |
| Critical care | 15 | 15 | 13,450 (4493) | 7 | 7 | 19,395 (4947) | –5945 (–17,765 to 6726) | 0.368 | |
| Other hospitalisation episodes | |||||||||
| ED | 1700 | 1700 | 233 (2) | 1040 | 1040 | 234 (3) | –1 (–8 to 6) | 0.684 | |
| Day case | 15 | 15 | 2405 (1841) | 16 | 16 | 925 (288) | 1480 (–814 to 5634) | 0.652 | |
| Outpatient care | 281 | 280 | 605 (45) | 144 | 144 | 741 (77) | –136 (–311 to 33) | 0.14 | |
| Total costs | 1833 | 1831 | 5224 (295) | 1094 | 1091 | 4777 (454) | 448 (–718 to 1483) | 0.412 | |
| 0–12 months after randomisationc | Inpatient stay, re-admissions | ||||||||
| Admitted patient care | 736 | 692 | 4840 (266) | 361 | 319 | 5855 (587) | –1015 (–2351 to 130) | 0.11 | |
| Critical care | 525 | 492 | 9802 (602) | 242 | 220 | 11,164 (1297) | –1362 (–4413 to 1248) | 0.35 | |
| Other hospitalisation episodes | |||||||||
| ED | 1706 | 1683 | 237 (3) | 1041 | 1020 | 236 (3) | 2 (–6 to 10) | 0.762 | |
| Day case | 22 | 19 | 4062 (2891) | 21 | 18 | 807 (258) | 3255 (–194 to 9952) | 0.124 | |
| Outpatient care | 284 | 253 | 790 (94) | 145 | 119 | 757 (83) | 34 (–198 to 291) | 0.76 | |
| Total costs | 1833 | 1801 | 4913 (303) | 1094 | 1066 | 4350 (468) | 563 (–660 to 1644) | 0.314 | |
| Assessment period | Category | Adrenaline arm (N = 4015) | Placebo arm (N = 3999) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean (SE) | Participants with complete data (n) | Mean (SE) | Mean difference (bootstrapped 95% CI) | p-value | ||
| 0–3 months after randomisation | Emergency response (minutes) | ||||||
| Time 1 (arrival at scene to departure) (minutes) | 4013 | 60.279 (0.779) | 3999 | 58.745 (0.548) | 1.534 (–0.2 to 3.392) | 0.1 | |
| Time 2a (departure from scene to arrival at hospital or mortuary) (minutes) | 4011 | 70.457 (0.234) | 3992 | 70.04 (0.298) | 0.418 (–0.332 to 1.151) | 0.244 | |
| Intervention dose | |||||||
| Number of syringes given | 4008 | 4.923 (0.04) | 3990 | 5.075 (0.037) | –0.152 (–0.266 to 0.052) | 0.004 | |
| Inpatient stay – initial admission (days) | |||||||
| ED | 4006 | 0.51 (0.009) | 3996 | 0.309 (0.008) | 0.201 (0.179 to 0.224) | < 0.001 | |
| General ward | 4013 | 0.792 (0.098) | 3998 | 0.496 (0.072) | 0.296 (0.078 to 0.536) | 0.008 | |
| ICU | 4013 | 0.91 (0.059) | 3999 | 0.472 (0.047) | 0.438 (0.298 to 0.592) | < 0.001 | |
| Inpatient stay – repeat admissions (days) | |||||||
| ED | 3974 | 0.005 (0.001) | 3970 | 0.003 (0.001) | 0.001 (–0.002 to 0.004) | 0.652 | |
| General ward | 3973 | 0.048 (0.017) | 3970 | 0.024 (0.011) | 0.024 (–0.013 to 0.066) | 0.214 | |
| ICU | |||||||
| Outpatient attendance (visits) | |||||||
| Cardiology | 3972 | 0.02 (0.004) | 3968 | 0.016 (0.003) | 0.005 (–0.005 to 0.014) | 0.392 | |
| Cardiac rehabilitation | 3974 | 0.036 (0.009) | 3969 | 0.033 (0.011) | 0.003 (–0.025 to 0.029) | 0.85 | |
| Nursing/residential home | 3974 | 0.062 (0.03) | 3970 | 0.035 (0.02) | 0.027 (–0.039 to 0.107) | 0.422 | |
| Other outpatient attendance | 3974 | 0.029 (0.009) | 3968 | 0.038 (0.018) | –0.009 (–0.051 to 0.027) | 0.738 | |
| Primary health-care contact (number of contacts) | |||||||
| District nurse | 3976 | 0.025 (0.008) | 3970 | 0.017 (0.006) | 0.007 (–0.012 to 0.026) | 0.466 | |
| GP, surgery visit | 3976 | 0.034 (0.005) | 3970 | 0.029 (0.005) | 0.005 (–0.011 to 0.019) | 0.558 | |
| GP, home visit | 3976 | 0.003 (0.001) | 3970 | 0.003 (0.001) | 0 (–0.004 to 0.003) | 0.834 | |
| GP, telephone consultation | 3976 | 0.002 (0.001) | 3969 | 0.002 (0.001) | 0 (–0.002 to 0.002) | 0.668 | |
| Practice nurse | 3976 | 0.001 (0.001) | 3969 | 0 (0) | 0.001 (0 to 0.002) | 0.916 | |
| Physiotherapy | 3976 | 0.037 (0.035) | 3969 | 0 (0) | 0.037 (0 to 0.111) | 0.144 | |
| Occupational therapy | 3976 | 0.001 (0.001) | 3969 | 0.001 (0.001) | 0 (–0.001 to 0.001) | 0.442 | |
| Social worker | 3976 | 0.003 (0.001) | 3970 | 0.002 (0.001) | 0.001 (–0.003 to 0.004) | 0.854 | |
| Speech therapy | 3976 | 0.009 (0.006) | 3970 | 0.012 (0.008) | –0.003 (–0.024 to 0.016) | 0.772 | |
| Psychiatrist | 3976 | 0.001 (0.001) | 3970 | 0.001 (0.001) | 0 (–0.002 to 0.002) | 0.646 | |
| Psychology | 3976 | 0.005 (0.004) | 3970 | 0.006 (0.004) | –0.001 (–0.012 to 0.011) | 0.786 | |
| Counsellor | 3976 | 0.001 (0.001) | 3970 | 0.001 (0.001) | 0.001 (–0.001 to 0.002) | 0.982 | |
| Home care worker | 3976 | 0.107 (0.06) | 3970 | 0.051 (0.038) | 0.056 (–0.07 to 0.22) | 0.4 | |
| Lunch or social club | 3976 | 0.003 (0.003) | 3970 | 0.007 (0.004) | –0.003 (–0.013 to 0.006) | 0.498 | |
| Self-help groups | 3976 | 0.046 (0.033) | 3970 | 0.005 (0.003) | 0.041 (–0.007 to 0.112) | 0.274 | |
| Meals and laundry | 3976 | 0.046 (0.036) | 3970 | 0.026 (0.016) | 0.02 (–0.045 to 0.11) | 0.716 | |
| Other community care | 3976 | 0 (0) | 3969 | 0.002 (0.002) | –0.002 (–0.006 to 0.001) | 0.184 | |
| Medications | |||||||
| Medication, number of items | 3975 | 2.119 (0.016) | 3970 | 2.088 (0.012) | 0.031 (–0.009 to 0.07) | 0.132 | |
| Aids and adaptations (per item/pair when appropriate) | |||||||
| Defibrillator | 3974 | 0 (0) | 3969 | 0 (0) | 0 (–0.001 to 0) | < 0.001 | |
| Heart monitor | 3974 | 0.001 (0.001) | 3969 | 0 (0) | 0 (–0.001 to 0.001) | 0.62 | |
| PEG pump | 3974 | 0 (0) | 3969 | 0 (0) | 0 (0 to 0.001) | 0.432 | |
| Hoist | 3974 | 0.001 (0.001) | 3969 | 0 (0) | 0.001 (0 to 0.001) | 0.954 | |
| Wheelchair | 3974 | 0.001 (0.001) | 3969 | 0 (0) | 0.001 (0 to 0.002) | 0.258 | |
| Walking aid | 3974 | 0.003 (0.001) | 3969 | 0.003 (0.001) | 0 (–0.003 to 0.002) | 0.558 | |
| Hand aid | 3974 | 0 (0) | 3969 | 0.001 (0.001) | –0.001 (–0.002 to 0) | 0.022 | |
| Stair rail | 3974 | 0 (0) | 3969 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Other aids and adaptations | 3974 | 0.005 (0.002) | 3969 | 0.003 (0.001) | 0.003 (0 to 0.006) | 0.158 | |
| 3–6 months after randomisation | Inpatient stay (days) | ||||||
| ED | 3965 | 0.005 (0.002) | 3953 | 0.004 (0.001) | 0.002 (–0.002 to 0.006) | 0.62 | |
| General ward | 4004 | 0.114 (0.059) | 3988 | 0.078 (0.043) | 0.036 (–0.094 to 0.195) | 0.638 | |
| ICU | 4006 | 0.008 (0.008) | 3989 | 0 (0) | 0.008 (0 to 0.025) | 0.276 | |
| Outpatient attendance (visits) | |||||||
| Cardiology | 3964 | 0.017 (0.003) | 3951 | 0.011 (0.003) | 0.006 (–0.002 to 0.013) | 0.172 | |
| Cardiac rehabilitation | 3964 | 0.036 (0.011) | 3953 | 0.034 (0.012) | 0.002 (–0.029 to 0.033) | 0.97 | |
| Nursing/residential home | 3964 | 0.102 (0.047) | 3952 | 0.028 (0.024) | 0.074 (–0.019 to 0.182) | 0.124 | |
| Other outpatient attendance | 3963 | 0.005 (0.002) | 3953 | 0.005 (0.002) | –0.001 (–0.006 to 0.005) | 0.784 | |
| Primary health-care contact (number of contacts) | |||||||
| District nurse | 3965 | 0.059 (0.038) | 3954 | 0.004 (0.001) | 0.055 (0.003 to 0.141) | 0.004 | |
| GP, surgery visit | 3965 | 0.035 (0.007) | 3954 | 0.022 (0.005) | 0.013 (–0.003 to 0.029) | 0.134 | |
| GP, home visit | 3965 | 0.005 (0.002) | 3954 | 0.002 (0.001) | 0.004 (–0.001 to 0.008) | 0.154 | |
| GP, telephone consultation | 3964 | 0.001 (0.001) | 3953 | 0.002 (0.001) | –0.001 (–0.003 to 0.002) | 0.384 | |
| Practice nurse | 3964 | 0 (0) | 3953 | 0 (0) | 0 (0 to 0.001) | 0.42 | |
| Physiotherapy | 3964 | 0.001 (0.001) | 3953 | 0 (0) | 0.001 (0 to 0.003) | 0.772 | |
| Occupational therapy | 3964 | 0 (0) | 3953 | 0.001 (0.001) | –0.001 (–0.002 to 0) | < 0.001 | |
| Social worker | 3965 | 0.005 (0.002) | 3954 | 0.002 (0.001) | 0.003 (–0.001 to 0.008) | 0.276 | |
| Speech therapy | 3965 | 0.003 (0.002) | 3954 | 0 (0) | 0.002 (0 to 0.006) | 0.238 | |
| Psychiatrist | 3965 | 0.001 (0.001) | 3954 | 0 (0) | 0 (–0.001 to 0.001) | 0.606 | |
| Psychology | 3965 | 0.002 (0.001) | 3954 | 0.002 (0.001) | 0 (–0.002 to 0.003) | 0.84 | |
| Counsellor | 3965 | 0.003 (0.003) | 3954 | 0.001 (0.001) | 0.002 (–0.002 to 0.008) | 0.786 | |
| Home care worker | 3965 | 0.069 (0.05) | 3954 | 0.002 (0.002) | 0.067 (–0.003 to 0.181) | 0.218 | |
| Lunch or social club | 3965 | 0.001 (0.001) | 3954 | 0 (0) | 0.001 (0 to 0.004) | 0.762 | |
| Self-help groups | 3965 | 0.027 (0.024) | 3954 | 0.004 (0.003) | 0.023 (–0.006 to 0.084) | 0.344 | |
| Meals and laundry | 3965 | 0.047 (0.033) | 3954 | 0.001 (0.001) | 0.046 (–0.001 to 0.116) | 0.174 | |
| Other community care | 3964 | 0.007 (0.006) | 3953 | 0 (0) | 0.007 (0 to 0.024) | 0.146 | |
| Medications | |||||||
| Medication, number of items | 3961 | 2.063 (0.012) | 3952 | 2.03 (0.007) | 0.033 (0.008 to 0.058) | 0.006 | |
| Aids and adaptations (per item/pair when appropriate) | |||||||
| Defibrillator | 3964 | 0 (0) | 3954 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Heart monitor | 3964 | 0 (0) | 3954 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| PEG pump | 3964 | 0 (0) | 3954 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Hoist | 3964 | 0.001 (0.001) | 3954 | 0 (0) | 0.001 (0 to 0.002) | 0.358 | |
| Wheelchair | 3964 | 0.002 (0.001) | 3954 | 0.001 (0.001) | 0.001 (–0.001 to 0.002) | 0.68 | |
| Walking aid | 3964 | 0.001 (0.001) | 3954 | 0.001 (0.001) | 0.001 (–0.001 to 0.002) | 0.954 | |
| Hand aid | 3964 | 0 (0) | 3954 | 0 (0) | 0 (–0.001 to 0) | < 0.001 | |
| Stair rail | 3964 | 0 (0) | 3954 | 0 (0) | 0 (–0.001 to 0) | < 0.001 | |
| Other aids and adaptations | 3964 | 0.005 (0.002) | 3954 | 0.001 (0.001) | 0.004 (0.001 to 0.007) | 0.03 | |
| Assessment period | Category | Adrenaline arm (N = 4015) | Placebo arm (N = 3999) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean (SE) | Participants with complete data (n) | Mean (SE) | Mean difference (bootstrapped 95% CI) | p-value | ||
| 0- to 3-month post-randomisation assessment period | Medications (item) | ||||||
| Medication, number of items | 3975 | 2.001 (0.001) | 3970 | 2 (0) | 0.001 (0 to 0.001) | 0.952 | |
| Additional costs (number of occurrences) | |||||||
| Child care | 3976 | 0.013 (0.002) | 3970 | 0.008 (0.001) | 0.005 (0.001 to 0.01) | 0.048 | |
| House work | 3976 | 0.014 (0.002) | 3970 | 0.01 (0.002) | 0.004 (–0.001 to 0.009) | 0.144 | |
| Travel costs | 3977 | 0.024 (0.004) | 3970 | 0.015 (0.003) | 0.01 (0 to 0.019) | 0.052 | |
| Other additional costs | 3977 | 0.037 (0.005) | 3970 | 0.024 (0.004) | 0.014 (0 to 0.027) | 0.062 | |
| 3–6 months after randomisation | Medications (item) | ||||||
| Medication, number of items | 3962 | 2 (0) | 3953 | 2 (0) | 0 (0 to 0.001) | 0.456 | |
| Additional costs (number of occurrences) | |||||||
| Child care | 3964 | 0.008 (0.001) | 3955 | 0.004 (0.001) | 0.004 (0.001 to 0.007) | 0.044 | |
| House work | 3964 | 0.009 (0.002) | 3955 | 0.005 (0.001) | 0.004 (0 to 0.008) | 0.086 | |
| Travel costs | 3964 | 0.015 (0.003) | 3955 | 0.008 (0.002) | 0.007 (0.001 to 0.014) | 0.036 | |
| Other additional costs | 3964 | 0.022 (0.004) | 3955 | 0.011 (0.003) | 0.011 (0.002 to 0.022) | 0.024 | |
| Assessment period | Category | Adrenaline arm (N = 128) | Placebo arm (N = 91) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean (SE) | Participants with complete data (n) | Mean (SE) | Mean difference (bootstrapped 95% CI) | p-value | ||
| 0–3 months after randomisation | Emergency response (minutes) | ||||||
| Time 1 (arrival at scene to departure) | 128 | 44.388 (1.529) | 91 | 40.24 (1.607) | 4.148 (–0.245 to 8.459) | 0.064 | |
| Time 2a (departure from scene to arrival at hospital or mortuary) | 128 | 74.017 (0.95) | 91 | 73.859 (1.387) | 0.158 (–3.18 to 3.237) | 0.84 | |
| Intervention dose | |||||||
| Number of syringes given | 128 | 2.094 (0.144) | 91 | 2.385 (0.224) | –0.291 (–0.825 to 0.223) | 0.264 | |
| Inpatient stay – initial admission (days) | |||||||
| ED | 124 | 0.992 (0.014) | 90 | 1.089 (0.058) | –0.097 (–0.227 to 0.003) | 0.042 | |
| General ward | 127 | 22.118 (2.297) | 90 | 19.233 (2.266) | 2.885 (–3.624 to 8.857) | 0.368 | |
| ICU | 127 | 10.283 (1.101) | 91 | 8.143 (1.123) | 2.141 (–0.911 to 5.027) | 0.182 | |
| Inpatient stay – repeat admissions (days) | |||||||
| ED | 93 | 0.194 (0.05) | 66 | 0.197 (0.058) | –0.003 (–0.16 to 0.15) | 0.99 | |
| General ward | 92 | 2.065 (0.692) | 66 | 1.455 (0.656) | 0.611 (–1.258 to 2.511) | 0.546 | |
| ICU | |||||||
| Outpatient attendance (visits) | |||||||
| Cardiology | 91 | 0.879 (0.132) | 64 | 0.969 (0.169) | –0.09 (–0.526 to 0.331) | 0.666 | |
| Cardiac rehabilitation | 93 | 1.527 (0.349) | 65 | 2.015 (0.627) | –0.489 (–2.08 to 0.731) | 0.502 | |
| Nursing/residential home | 93 | 2.634 (1.273) | 66 | 2.106 (1.213) | 0.528 (–2.754 to 3.844) | 0.73 | |
| Other outpatient attendance | 93 | 1.237 (0.374) | 64 | 2.359 (1.068) | –1.123 (–3.328 to 0.83) | 0.304 | |
| Primary health-care contact (number of contacts) | |||||||
| District nurse | 95 | 1.032 (0.299) | 66 | 1.045 (0.352) | –0.014 (–0.926 to 0.884) | 0.992 | |
| GP, surgery visit | 95 | 1.411 (0.182) | 66 | 1.727 (0.241) | –0.317 (–0.943 to 0.287) | 0.31 | |
| GP, home visit | 95 | 0.116 (0.036) | 66 | 0.167 (0.088) | –0.051 (–0.263 to 0.108) | 0.644 | |
| GP, telephone consultation | 95 | 0.084 (0.033) | 65 | 0.123 (0.051) | –0.039 (–0.165 to 0.077) | 0.544 | |
| Practice nurse | 95 | 0.021 (0.022) | 65 | 0 (0) | 0.021 (0 to 0.078) | 0.76 | |
| Physiotherapy | 95 | 1.568 (1.596) | 65 | 0 (0) | 1.568 (0.01 to 5.117) | 0.042 | |
| Occupational therapy | 95 | 0.021 (0.015) | 65 | 0.031 (0.031) | –0.01 (–0.085 to 0.049) | 0.852 | |
| Social worker | 95 | 0.126 (0.043) | 66 | 0.136 (0.078) | –0.01 (–0.192 to 0.149) | 0.986 | |
| Speech therapy | 95 | 0.389 (0.221) | 66 | 0.742 (0.463) | –0.353 (–1.485 to 0.518) | 0.548 | |
| Psychiatrist | 95 | 0.053 (0.028) | 66 | 0.076 (0.047) | –0.023 (–0.136 to 0.08) | 0.724 | |
| Psychology | 95 | 0.221 (0.159) | 66 | 0.364 (0.236) | –0.143 (–0.709 to 0.368) | 0.628 | |
| Counsellor | 95 | 0.042 (0.025) | 66 | 0.03 (0.032) | 0.012 (–0.073 to 0.087) | 0.738 | |
| Home care worker | 95 | 4.495 (2.346) | 66 | 3.076 (2.291) | 1.419 (–5.2 to 7.616) | 0.676 | |
| Lunch or social club | 95 | 0.137 (0.114) | 66 | 0.394 (0.271) | –0.257 (–0.884 to 0.261) | 0.408 | |
| Self-help groups | 95 | 1.916 (1.321) | 66 | 0.273 (0.201) | 1.643 (–0.426 to 4.5) | 0.27 | |
| Meals and laundry | 95 | 1.926 (1.609) | 66 | 1.576 (0.938) | 0.351 (–2.92 to 4.276) | 0.916 | |
| Other community care | 95 | 0.011 (0.011) | 65 | 0.123 (0.129) | –0.113 (–0.414 to 0.029) | 0.474 | |
| Medications | |||||||
| Medication, number of items | 94 | 7.043 (0.414) | 66 | 7.318 (0.401) | –0.276 (–1.36 to 0.881) | 0.596 | |
| Aids and adaptations (per item/pair when appropriate) | |||||||
| Defibrillator | 93 | 0 (0) | 65 | 0.015 (0.015) | –0.015 (–0.05 to 0) | < 0.001 | |
| Heart monitor | 93 | 0.022 (0.015) | 65 | 0.015 (0.015) | 0.006 (–0.037 to 0.047) | 0.794 | |
| PEG pump | 93 | 0.011 (0.01) | 65 | 0 (0) | 0.011 (0 to 0.034) | 0.758 | |
| Hoist | 93 | 0.022 (0.015) | 65 | 0 (0) | 0.022 (0 to 0.052) | 0.306 | |
| Wheelchair | 93 | 0.043 (0.022) | 65 | 0 (0) | 0.043 (0 to 0.09) | 0.052 | |
| Walking aid | 93 | 0.118 (0.033) | 65 | 0.185 (0.057) | –0.066 (–0.208 to 0.057) | 0.296 | |
| Hand aid | 93 | 0.011 (0.011) | 65 | 0.062 (0.03) | –0.051 (–0.118 to 0.005) | 0.066 | |
| Stair rail | 93 | 0 (0) | 65 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Other aids and adaptations | 93 | 0.226 (0.056) | 65 | 0.154 (0.045) | 0.072 (–0.067 to 0.211) | 0.292 | |
| 3–6 months after randomisation | Inpatient stay (days) | ||||||
| ED, visits | 84 | 0.25 (0.072) | 49 | 0.306 (0.097) | –0.056 (–0.298 to 0.154) | 0.616 | |
| General ward | 122 | 3.746 (1.826) | 83 | 3.741 (2.092) | 0.005 (–5.431 to 5.353) | 0.98 | |
| ICU | 124 | 0.274 (0.263) | 84 | 0 (0) | 0.274 (0 to 0.992) | 0.094 | |
| Outpatient attendance (visits) | |||||||
| Cardiology | 83 | 0.807 (0.106) | 47 | 0.936 (0.219) | –0.129 (–0.617 to 0.295) | 0.596 | |
| Cardiac rehabilitation | 83 | 1.711 (0.485) | 49 | 2.755 (0.925) | –1.044 (–3.262 to 0.755) | 0.298 | |
| Nursing/residential home | 83 | 4.867 (2.177) | 48 | 2.271 (1.905) | 2.597 (–3.022 to 8.345) | 0.344 | |
| Other outpatient attendance | 82 | 0.232 (0.085) | 49 | 0.429 (0.18) | –0.197 (–0.637 to 0.154) | 0.328 | |
| Primary health-care contact (number of contacts) | |||||||
| District nurse | 84 | 2.774 (1.745) | 50 | 0.28 (0.091) | 2.494 (0.013 to 6.315) | 0.046 | |
| GP, surgery visit | 84 | 1.631 (0.252) | 50 | 1.72 (0.293) | –0.089 (–0.934 to 0.643) | 0.826 | |
| GP, home visit | 84 | 0.25 (0.095) | 50 | 0.12 (0.052) | 0.13 (–0.072 to 0.342) | 0.254 | |
| GP, telephone consultation | 83 | 0.06 (0.03) | 49 | 0.143 (0.079) | –0.083 (–0.264 to 0.059) | 0.348 | |
| Practice nurse | 83 | 0.012 (0.012) | 49 | 0 (0) | 0.012 (0 to 0.039) | 0.714 | |
| Physiotherapy | 83 | 0.048 (0.049) | 49 | 0 (0) | 0.048 (0 to 0.16) | 0.71 | |
| Occupational therapy | 83 | 0 (0) | 49 | 0.061 (0.058) | –0.061 (–0.18 to 0) | < 0.001 | |
| Social worker | 84 | 0.214 (0.096) | 50 | 0.14 (0.064) | 0.074 (–0.143 to 0.298) | 0.512 | |
| Speech therapy | 84 | 0.119 (0.075) | 50 | 0.02 (0.02) | 0.099 (–0.022 to 0.272) | 0.136 | |
| Psychiatrist | 84 | 0.024 (0.017) | 50 | 0.02 (0.019) | 0.004 (–0.048 to 0.053) | 0.902 | |
| Psychology | 84 | 0.083 (0.049) | 50 | 0.12 (0.074) | –0.037 (–0.212 to 0.128) | 0.7 | |
| Counsellor | 84 | 0.119 (0.118) | 50 | 0.06 (0.046) | 0.059 (–0.13 to 0.345) | 0.794 | |
| Home care worker | 84 | 3.262 (2.398) | 50 | 0.16 (0.127) | 3.102 (–0.208 to 8.501) | 0.196 | |
| Lunch or social club | 84 | 0.048 (0.047) | 50 | 0 (0) | 0.048 (0 to 0.158) | 0.708 | |
| Self-help groups | 84 | 1.274 (1.111) | 50 | 0.32 (0.286) | 0.954 (–0.704 to 3.612) | 0.454 | |
| Meals and laundry | 84 | 2.214 (1.531) | 50 | 0.1 (0.064) | 2.114 (–0.1 to 5.762) | 0.168 | |
| Other community care | 83 | 0.337 (0.299) | 49 | 0 (0) | 0.337 (0 to 1.043) | 0.084 | |
| Medications | |||||||
| Medication, number of items | 80 | 5.138 (0.435) | 48 | 4.479 (0.4) | 0.658 (–0.549 to 1.807) | 0.272 | |
| Aids and adaptations (per item/pair when appropriate) | |||||||
| Defibrillator | 83 | 0 (0) | 50 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Heart monitor | 83 | 0 (0) | 50 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| PEG pump | 83 | 0 (0) | 50 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Hoist | 83 | 0.06 (0.025) | 50 | 0.02 (0.02) | 0.04 (–0.021 to 0.105) | 0.228 | |
| Wheelchair | 83 | 0.072 (0.029) | 50 | 0.06 (0.034) | 0.012 (–0.079 to 0.093) | 0.842 | |
| Walking aid | 83 | 0.06 (0.032) | 50 | 0.06 (0.034) | 0 (–0.097 to 0.092) | 0.974 | |
| Hand aid | 83 | 0 (0) | 50 | 0.02 (0.02) | –0.02 (–0.071 to 0) | < 0.001 | |
| Stair rail | 83 | 0 (0) | 50 | 0.02 (0.02) | –0.02 (–0.066 to 0) | < 0.001 | |
| Other aids and adaptations | 83 | 0.241 (0.072) | 50 | 0.1 (0.057) | 0.141 (–0.028 to 0.323) | 0.112 | |
| Assessment period | Category | Adrenaline arm (N = 128) | Placebo arm (N = 128) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean (SE) | Participants with complete data (n) | Mean (SE) | Mean difference (bootstrapped 95% CI) | p-value | ||
| 0- to 3-month post-randomisation assessment period | Medications | ||||||
| Medication, number of items | 94 | 2.021 (0.015) | 66 | 2 (0) | 0.021 (0 to 0.054) | 0.274 | |
| Additional costs (number of occurrences) | |||||||
| Child care | 95 | 0.558 (0.051) | 66 | 0.5 (0.062) | 0.058 (–0.102 to 0.208) | 0.512 | |
| House work | 95 | 0.579 (0.054) | 66 | 0.576 (0.082) | 0.003 (–0.195 to 0.178) | 0.994 | |
| Travel costs | 96 | 1.01 (0.099) | 66 | 0.894 (0.128) | 0.116 (–0.22 to 0.422) | 0.514 | |
| Other additional costs | 96 | 1.552 (0.15) | 66 | 1.424 (0.202) | 0.128 (–0.381 to 0.585) | 0.596 | |
| 3–6 months after randomisation | Medications | ||||||
| Medication, number of items | 81 | 2.012 (0.013) | 49 | 2 (0) | 0.012 (0 to 0.042) | 0.726 | |
| Additional costs (number of occurrences) | |||||||
| Child care | 83 | 0.361 (0.053) | 51 | 0.294 (0.069) | 0.067 (–0.117 to 0.242) | 0.472 | |
| House work | 83 | 0.41 (0.062) | 51 | 0.353 (0.082) | 0.057 (–0.157 to 0.272) | 0.64 | |
| Travel costs | 83 | 0.735 (0.103) | 51 | 0.627 (0.123) | 0.107 (–0.224 to 0.433) | 0.582 | |
| Other additional costs | 83 | 1.048 (0.154) | 51 | 0.824 (0.2) | 0.225 (–0.306 to 0.757) | 0.44 | |
| Type of resource | Unit cost (£) | Unit | Source | Notes |
|---|---|---|---|---|
| Emergency response | ||||
| Emergency ambulance | 8.00 | Minute | PSSRU 2008,160 p. 82 | £6.80 (2008) prices inflated to £8.00 (2017 prices) |
| Adrenaline injection | 6.87 | Syringe | BNF 2016/17142 | 1 mg/10 ml (1 in 10,000) dilute solution for injection pre-filled syringes (Martindale Pharmaceuticals Ltd, Wooburn Green, UK) |
| Primary source of unit cost of hospital services (raw resource use data derived from HES), mean (SD), range | ||||
| Inpatients (initial): £6022.47 (£20,869.58), £352–683,381 | Inpatient spell | National Schedule of Reference Costs, 2016–17 135 | ||
| Inpatients (re-admission): £3804.74 (£20,877.28), £211–41,151 | Inpatient spell | National Schedule of Reference Costs, 2016–17 135 | ||
| Critical care (initial): £11,289.47 (£19,539.52), £348–364,247 | Inpatient spell | National Schedule of Reference Costs, 2016–17 135 | ||
| Critical care (re-admissions): £13,817.98 (£18,635.79), £1202–64,739 | Inpatient spell | National Schedule of Reference Costs, 2016–17 135 | ||
| Accident and emergency: £233.79 (£93.93), £83–1045 | Visit | National Schedule of Reference Costs, 2016–17 135 | ||
| Outpatients (first appointment): £291.41 (£423.15), £32–1714 | Attendance | National Schedule of Reference Costs, 2016–17 135 | ||
| Outpatients (follow-up): £353.95 (£423.73), £32–5659 | Attendance | National Schedule of Reference Costs, 2016–17 135 | ||
| Secondary source of unit costs for hospital services (raw resource use data derived from trial CRFs) | ||||
| Inpatient care | ||||
| ED visit | 389.81 | Visit | National Schedule of Reference Costs, 2016–17 135 | Emergency medicine (service code T01A; currency code VB02ZZ) |
| ICU bed-day | 2114.13 | Bed-day | National Schedule of Reference Costs, 2016–17 135 | Average cost of 0–6 or more organs supported (CCU01 and XC01Z-XC07Z) weighted by activity |
| Cardiac ward bed-day | 305.85 | Bed-day | National Schedule of Reference Costs, 2016–17 135 | Non-elective inpatients; excess bed-days |
| Secondary (outpatient services) | ||||
| Cardiology | 137.07 | Attendance | National Schedule of Reference Costs, 2016–17 135 | Service code 320 |
| Cardiac rehabilitation | 79.70 | Attendance | National Schedule of Reference Costs, 2016–17 135 | Service code 327 |
| Surgery | 141.19 | Attendance | National Schedule of Reference Costs, 2016–17 135 | Service code 100 |
| Neurology | 171.98 | Attendance | National Schedule of Reference Costs, 2016–17 135 | Service code 400 |
| Ophthalmology | 95.15 | Attendance | National Schedule of Reference Costs, 2016–17 135 | Service code 130 |
| Urology | 111.61 | Attendance | National Schedule of Reference Costs, 2016–17 135 | Service code 101 |
| Angiography | 169.00 | Attendance | National Schedule of Reference Costs, 2016–17 135 | RD32Z contrast fluoroscopy procedures with duration of > 40 minutes |
| Echocardiography | 74.00 | Attendance | National Schedule of Reference Costs, 2016–17 135 | RD51A |
| Haematology/oncology | 176.24 | Attendance | National Schedule of Reference Costs, 2016–17 135 | 370 |
| Podiatry | 45.81 | Attendance | National Schedule of Reference Costs, 2016–17 135 | 653 |
| Nursing/residential home | 162.00 | Attendance | PSSRU 2017140 | Local authority own-provision residential care for older people (£162/day, p. 35140) |
| Home carers twice a day | 27.00 | Attendance | PSSRU 2017140 | p. 178.140 Face to face: £26 per hour on a weekday (£27 per hour on weekend day, £27 per hour on night-time weekday); assumed 1 hour of care provided each day at £27 per hour |
| Other outpatient services: mean £155.78 (SD £244.19), range £1–2114 | ||||
| Community health and social care services | ||||
| Counsellor | 53.00 | Contact | PSSRU 2017140 | Community-based professional (band 7) costing £53 per hour (pages 153–155); average length of surgery consultation (PSSRU 2013, page 54) |
| District nurse | 41.04 | Contact | PSSRU 2013209 | The mean average cost for a face-to-face contact in district nursing services for 2012/2013 was £39, inflated to 2017 prices |
| GP, home visit | 38.00 | Contact | PSSRU 2017140 | Per patient contact lasting 9.22 minutes including CO2 emissions (page 162) |
| GP, surgery visit | 37.00 | Contact | PSSRU 2017140 | Per surgery consultation lasting 9.22 minutes (page 162) |
| GP, telephone consultation | 28.49 | Contact | PSSRU 2017140 | Per telephone consultation lasting 7.1 minutes (PSSRU 2013, page 191) |
| Home care worker | 13.50 | Contact | PSSRU 2017140 | Page 178. Face to face: £26 per hour weekday (£27 per day-time weekend, £27 per night-time weekday, £27 per night-time weekend). Assumed 30 minutes contact at £27 per hour |
| Meals and laundry | 6.94 | One meal | PSSRU 2014210 | The average cost per meal on wheels was £6.60 for the local authority in 2012/13 (PSSRU Unit Costs 2014, page 127). Inflated to 2017 prices using the Hospital & Community Health Services (HCHS) Pay and Prices index |
| Occupational therapy | 64.99 | Contact | National Schedule of Reference Costs, 2016–17 135 | Occupational Therapy (service code 951) |
| Physiotherapy | 48.00 | Contact | National Schedule of Reference Costs, 2016–17 135 | Service code 650 |
| Practice nurse | 10.85 | Contact | PSSRU 2017140 | Practice nurse hourly costs including qualifications £42 (page 160); duration of contact 15.5 minutes (PSSRU 2013, page 188) |
| Psychiatrist | 84.57 | Contact | National Schedule of Reference Costs, 2016–17 135 | Liaison Psychiatry (service code 722) |
| Psychology | 168.65 | Contact | National Schedule of Reference Costs, 2016–17 135 | Clinical Psychology (service code 656) |
| Lunch or social club | 6.94 | One meal | PSSRU 2014210 | The average cost per meal on wheels was £6.60 for the local authority in 2012/13 (PSSRU 2014, page 127). Inflated to 2017 prices using the Hospital & Community Health Services (HCHS) Pay and Prices index |
| Social worker | 43.07 | Contact | PSSRU 2017140 | Social worker (adult services) with qualifications cost £59 per hour, page 174; assumed 73% of time is spent on client-related activities (PSSRU 2017, page 174) including direct contact (includes travel) |
| Speech therapy | 96.52 | Contact | National Schedule of Reference Costs, 2016–17 135 | Service code 652 |
| Other primary care services: mean £58.98 (SD £19.43), range £21–98 | ||||
| Additional costsa over 6 months for the adrenaline group, n; mean (SD), range | 61; £3634 (£6590.44), £0.93–31,400 | |||
| Additional costs over 6 months for the adrenaline group, n; mean (SD), range | 40; £2468 (£3805.61), £0.20–16,040 | |||
| Assessment period | Category | Adrenaline arm (N = 4015) | Placebo arm (N = 3999) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean cost (SE) (£) | Participants with complete data (n) | Mean cost (SE) (£) | Mean difference in costs (95% CI) (£) | p-value | ||
| 0–3 months after randomisation | Emergency response costs | ||||||
| Time 1 (arrival at scene to departure) | 4012 | 1152.27 (17.13) | 3999 | 1099.38 (10.71) | 52.89 (14.73 to 91.44) | 0.002 | |
| Time 2a (arrival at scene to departure) | 4011 | 563.66 (1.87) | 3992 | 560.32 (2.38) | 3.34 (–2.66 to 9.21) | 0.244 | |
| Total emergency response costs | 4010 | 1716.03 (17.05) | 3992 | 1659.61 (10.41) | 56.41 (18.54 to 94.42) | < 0.001 | |
| Intervention costs | |||||||
| Syringes given | 4008 | 33.82 (0.27) | 3990 | 0 (0) | 33.82 (33.27 to 34.32) | < 0.001 | |
| Inpatient costs | |||||||
| ED | 7980 | 99.26 (1.6) | 7966 | 60.43 (1.52) | 38.83 (34.66 to 43.08) | < 0.001 | |
| General ward | 7986 | 129.07 (15.65) | 7968 | 79.88 (11.44) | 49.19 (13.4 to 87.45) | 0.006 | |
| ICU | 4013 | 1924.47 (124.11) | 3999 | 997.59 (99.04) | 926.88 (629.78 to 1250.56) | < 0.001 | |
| Total inpatient costs | 3964 | 2093.96 (128.43) | 3967 | 1082.18 (88.63) | 1011.78 (729.39 to 1325.13) | < 0.001 | |
| Outpatient costs | |||||||
| Cardiology | 3972 | 2.76 (0.5) | 3968 | 2.14 (0.45) | 0.62 (–0.64 to 1.91) | 0.326 | |
| Cardiac rehabilitation | 3974 | 2.85 (0.68) | 3969 | 2.63 (0.88) | 0.22 (–2.03 to 2.31) | 0.83 | |
| Nursing/residential home | 3974 | 9.99 (4.91) | 3970 | 5.67 (3.24) | 4.32 (–6.28 to 17.34) | 0.412 | |
| Other outpatient costs | 3972 | 3.12 (1.14) | 3964 | 2.63 (1.16) | 0.49 (–2.59 to 3.6) | 0.674 | |
| Total outpatient costs | 3967 | 18.62 (5.63) | 3961 | 10.63 (3.38) | 8 (–3.77 to 21.61) | 0.196 | |
| Primary health-care costs | |||||||
| GP, surgery visit | 3976 | 1.25 (0.2) | 3970 | 1.06 (0.2) | 0.18 (–0.39 to 0.7) | 0.53 | |
| GP, home visit | 3976 | 0.11 (0.03) | 3970 | 0.11 (0.05) | 0 (–0.13 to 0.11) | 0.98 | |
| GP, telephone consultation | 3976 | 0.06 (0.02) | 3969 | 0.06 (0.02) | 0 (–0.07 to 0.06) | 0.936 | |
| District nurse | 3976 | 1.13 (0.33) | 3970 | 0.71 (0.25) | 0.41 (–0.39 to 1.24) | 0.326 | |
| Practice nurse | 3976 | 0.01 (0.01) | 3969 | 0 (0) | 0.01 (0 to 0.02) | 0.644 | |
| Physiotherapy | 3976 | 1.8 (1.7) | 3969 | 0 (0) | 1.8 (0.01 to 5.34) | 0.034 | |
| Occupational therapy | 3976 | 0.03 (0.02) | 3969 | 0.03 (0.03) | 0 (–0.08 to 0.07) | 0.898 | |
| Social worker | 3976 | 0.13 (0.05) | 3970 | 0.1 (0.06) | 0.03 (–0.13 to 0.17) | 0.69 | |
| Speech therapy | 3976 | 0.9 (0.56) | 3970 | 1.19 (0.76) | –0.29 (–2.28 to 1.55) | 0.8 | |
| Psychiatrist | 3976 | 0.11 (0.06) | 3970 | 0.11 (0.07) | 0 (–0.17 to 0.15) | 0.898 | |
| Psychology | 3976 | 0.89 (0.69) | 3970 | 1.02 (0.71) | –0.13 (–2.03 to 1.87) | 0.848 | |
| Home care worker | 3976 | 1.45 (0.82) | 3970 | 0.69 (0.51) | 0.76 (–0.95 to 2.97) | 0.4 | |
| Lunch or social club | 3976 | 0.02 (0.02) | 3970 | 0.05 (0.03) | –0.02 (–0.09 to 0.04) | 0.448 | |
| Self-help groups | 3976 | 0 (0) | 3970 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Meals and laundry | 3976 | 0.32 (0.25) | 3970 | 0.18 (0.11) | 0.14 (–0.31 to 0.76) | 0.72 | |
| Counsellor | 3976 | 0.05 (0.03) | 3970 | 0.03 (0.03) | 0.03 (–0.05 to 0.12) | 0.606 | |
| Other community care costs | 3976 | 2.45 (2.31) | 3969 | 0.09 (0.07) | 2.35 (–0.13 to 7.2) | 0.414 | |
| Total community care costs | 3976 | 10.69 (4.76) | 3969 | 5.42 (1.67) | 5.27 (–2.75 to 17.05) | 0.308 | |
| Medication costs | |||||||
| Medication | 3975 | 6.8 (1.7) | 3970 | 4.98 (1.68) | 1.82 (–2.96 to 6.61) | 0.45 | |
| Aids and adaptations costs | |||||||
| Defibrillator | 3974 | 0 (0) | 3968 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Heart monitor | 3974 | 0 (0) | 3969 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| PEG pump | 3973 | 0 (0) | 3969 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Hoist | 3974 | 2.11 (1.46) | 3969 | 0 (0) | 2.11 (0 to 5.27) | 0.262 | |
| Wheelchair | 3974 | 0.2 (0.1) | 3969 | 0 (0) | 0.2 (0.05 to 0.43) | 0.038 | |
| Walking aid | 3974 | 0.1 (0.04) | 3969 | 0.09 (0.04) | 0.01 (–0.09 to 0.12) | 0.882 | |
| Hand aid | 3974 | 0 (0) | 3969 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Stair rail | 3974 | 0 (0) | 3969 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Other aids and adaptations costs | 3968 | 0.49 (0.29) | 3965 | 1.68 (1.29) | –1.19 (–4.25 to 0.69) | 0.476 | |
| Total aids and adaptations costs | 3967 | 1.78 (1.29) | 3964 | 1.75 (1.29) | 0.02 (–3.8 to 3.88) | 0.972 | |
| 0–3 months: total NHS and PSS costs | 3937 | 3764.73 (127.92) | 3936 | 2686.9 (90) | 1077.83 (790.49 to 1406.45) | < 0.001 | |
| 3–6 months after randomisation | Inpatient costs | ||||||
| ED | 3965 | 2.06 (0.65) | 3953 | 1.48 (0.51) | 0.59 (–0.83 to 2.23) | 0.454 | |
| General ward | 4003 | 44.09 (15.06) | 3987 | 36.02 (14.8) | 8.07 (–36.25 to 50.39) | 0.662 | |
| ICU | 4005 | 65.98 (51.66) | 3989 | 15.37 (15.4) | 50.61 (–29.07 to 182.2) | 0.234 | |
| Total inpatient costs | 3961 | 83.1 (50.84) | 3949 | 37.36 (19.5) | 45.73 (–37.8 to 172.54) | 0.388 | |
| Outpatient costs | |||||||
| Cardiology | 3964 | 2.32 (0.41) | 3951 | 1.53 (0.4) | 0.79 (–0.32 to 1.82) | 0.136 | |
| Cardiac rehabilitation | 3964 | 2.86 (0.86) | 3953 | 2.72 (0.95) | 0.13 (–2.29 to 2.66) | 0.948 | |
| Nursing/residential home | 3964 | 16.51 (7.68) | 3952 | 4.47 (3.81) | 12.04 (–3.12 to 29.51) | 0.118 | |
| Other outpatient costs | 3963 | 0.6 (0.23) | 3953 | 0.65 (0.31) | –0.05 (–0.85 to 0.67) | 0.938 | |
| Total outpatient costs | 3960 | 18.59 (6.93) | 3949 | 9.25 (4.02) | 9.34 (–4.73 to 25.67) | 0.218 | |
| Primary health-care costs | |||||||
| GP, surgery visit | 3965 | 1.29 (0.25) | 3954 | 0.8 (0.18) | 0.48 (–0.1 to 1.08) | 0.116 | |
| GP, home visit | 3965 | 0.21 (0.08) | 3954 | 0.06 (0.03) | 0.15 (–0.01 to 0.34) | 0.082 | |
| GP, telephone consultation | 3964 | 0.04 (0.02) | 3953 | 0.05 (0.03) | –0.01 (–0.09 to 0.04) | 0.646 | |
| District nurse | 3965 | 2.41 (1.57) | 3954 | 0.15 (0.05) | 2.27 (0.13 to 5.8) | 0.002 | |
| Practice nurse | 3964 | 0 (0) | 3953 | 0 (0) | 0 (0 to 0.01) | 0.494 | |
| Physiotherapy | 3964 | 0.05 (0.05) | 3953 | 0 (0) | 0.05 (0 to 0.15) | 0.772 | |
| Occupational therapy | 3964 | 0 (0) | 3953 | 0.05 (0.05) | –0.05 (–0.15 to 0) | < 0.001 | |
| Social worker | 3965 | 0.2 (0.09) | 3954 | 0.08 (0.04) | 0.12 (–0.04 to 0.35) | 0.198 | |
| Speech therapy | 3965 | 0.24 (0.16) | 3954 | 0.02 (0.02) | 0.22 (0 to 0.58) | 0.09 | |
| Psychiatrist | 3965 | 0.13 (0.09) | 3954 | 0.02 (0.02) | 0.11 (–0.04 to 0.3) | 0.29 | |
| Psychology | 3965 | 0.3 (0.18) | 3954 | 0.26 (0.16) | 0.04 (–0.4 to 0.55) | 0.95 | |
| Home care worker | 3965 | 0.93 (0.68) | 3954 | 0.03 (0.02) | 0.91 (–0.03 to 2.45) | 0.206 | |
| Lunch or social club | 3965 | 0.01 (0.01) | 3954 | 0 (0) | 0.01 (0 to 0.03) | 0.762 | |
| Self-help groups | 3965 | 0 (0) | 3954 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Meals and laundry | 3965 | 0.33 (0.23) | 3954 | 0.01 (0.01) | 0.32 (–0.01 to 0.81) | 0.182 | |
| Counsellor | 3965 | 0.13 (0.14) | 3954 | 0.04 (0.03) | 0.09 (–0.09 to 0.41) | 0.762 | |
| Other community care costs | 3964 | 0.28 (0.2) | 3953 | 0 (0) | 0.28 (0.03 to 0.78) | 0.004 | |
| Total community care costs | 3964 | 6.54 (2.03) | 3953 | 1.55 (0.33) | 4.99 (1.36 to 9.26) | < 0.001 | |
| Medications | |||||||
| Medication | 3961 | 3.68 (1.2) | 3952 | 1.03 (0.26) | 2.65 (0.71 to 5.61) | 0.002 | |
| Aids and adaptations costs | |||||||
| Defibrillator | 3964 | 0 (0) | 3954 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Heart monitor | 3964 | 0 (0) | 3954 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| PEG pump | 3964 | 0 (0) | 3954 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Hoist | 3964 | 5.28 (2.4) | 3954 | 1.06 (1.04) | 4.22 (–0.11 to 9.53) | 0.106 | |
| Wheelchair | 3964 | 0.3 (0.12) | 3954 | 0.15 (0.08) | 0.15 (–0.1 to 0.45) | 0.348 | |
| Walking aid | 3964 | 0.02 (0.02) | 3954 | 0.02 (0.02) | 0 (–0.05 to 0.05) | 0.752 | |
| Hand aid | 3964 | 0 (0) | 3954 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Stair rail | 3964 | 0 (0) | 3954 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Other aids and adaptations costs | 3959 | 0.24 (0.2) | 3954 | 0.01 (0.01) | 0.22 (0 to 0.66) | 0.15 | |
| Total aids and adaptations costs | 3959 | 4.78 (2.18) | 3954 | 1.24 (1.09) | 3.54 (–1 to 8.89) | 0.12 | |
| Total costs | |||||||
| 3–6 months: total NHS and PSS costs | 3948 | 93.75 (50.8) | 3940 | 47.62 (20.86) | 46.13 (–38.68 to 169.18) | 0.382 | |
| 0–6 months: total NHS and PSS costs | 3919 | 3641.84 (149.13) | 3914 | 2548.36 (84) | 1093.49 (800.43 to 1442) | < 0.001 | |
| Assessment period | Category | Adrenaline arm (N = 4015) | Placebo arm (N = 3999) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean cost (SE) (£) | Participants with complete data (n) | Mean cost (SE) (£) | Mean difference in costs (95% CI) (£) | p-value | ||
| 0–3 months after randomisation | Medications | ||||||
| Medication | 3975 | 0.04 (0.03) | 3970 | 0 (0) | 0.04 (0 to 0.09) | 0.26 | |
| Additional costs | |||||||
| Child care | 3976 | 0 (0) | 3970 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Housework | 3976 | 0.1 (0.09) | 3970 | 0.24 (0.16) | –0.15 (–0.55 to 0.18) | 0.364 | |
| Travel costs | 3977 | 1.66 (0.74) | 3970 | 1.38 (0.63) | 0.28 (–1.44 to 2.29) | 0.73 | |
| Other additional costs | 3977 | 37.61 (10.35) | 3970 | 18.3 (6.25) | 19.31 (–4.21 to 42.52) | 0.116 | |
| Total additional costs | 3976 | 39.19 (10.49) | 3970 | 19.93 (6.45) | 19.26 (–4.94 to 43.39) | 0.124 | |
| Total costs | |||||||
| 0–3 months: total non-NHS and PSS costs | 3973 | 37.34 (10.32) | 3970 | 19.93 (6.45) | 17.4 (–6.71 to 40.77) | 0.156 | |
| 0–3 months: total societal costs | 3936 | 3774.22 (127.5) | 3936 | 2698.06 (91.12) | 1076.16 (782.48 to 1403.42) | < 0.001 | |
| 3–6 months after randomisation | Medications | ||||||
| Medication | 3962 | 0 (0) | 3953 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Additional costs | |||||||
| Child care | 3964 | 0 (0) | 3955 | 0.08 (0.08) | –0.08 (–0.23 to 0) | < 0.001 | |
| Housework | 3964 | 0.47 (0.32) | 3955 | 0.07 (0.04) | 0.39 (–0.06 to 1.1) | 0.19 | |
| Travel costs | 3964 | 1.99 (1.02) | 3955 | 0.4 (0.15) | 1.59 (0 to 3.98) | 0.052 | |
| Other additional costs | 3964 | 16.06 (7.56) | 3955 | 4.47 (2.19) | 11.59 (–0.73 to 30.2) | 0.076 | |
| Total additional costs | 3964 | 18.52 (8.12) | 3955 | 5.02 (2.25) | 13.5 (0.43 to 33.45) | 0.042 | |
| Total costs | |||||||
| 3–6 months: total on-NHS and PSS costs | 3962 | 18.51 (8.13) | 3953 | 4.95 (2.25) | 13.56 (0.49 to 33.62) | 0.038 | |
| 3–6 months: total societal costs | 3948 | 110.4 (52.28) | 3939 | 46.22 (20.39) | 64.18 (–21.67 to 188.32) | 0.192 | |
| 0–6 months: total non-NHS and PSS costs | 3956 | 50.84 (14.62) | 3952 | 15.07 (5.59) | 35.77 (8.1 to 68.61) | 0.01 | |
| 0–6 months: total societal costs | 3919 | 3671.5 (150.28) | 3913 | 2535.36 (82.89) | 1136.14 (840.32 to 1484.33) | < 0.001 | |
| Assessment period | Category | Adrenaline arm (n = 128) | Placebo arm (n = 91) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean cost (SE) (£) | Participants with complete data (n) | Mean cost (SE) (£) | Mean difference in costs (bootstrapped 95% CI) (£) | p-value | ||
| 0–3 months after randomisation | Emergency response costs | ||||||
| Time 1 (arrival at scene to departure) | 127 | 942.98 (43.08) | 91 | 865 (50.55) | 77.98 (–49.91 to 214.72) | 0.23 | |
| Time 2a (arrival at scene to departure) | 128 | 592.13 (7.6) | 91 | 590.87 (11.09) | 1.26 (–25.44 to 25.9) | 0.84 | |
| Total emergency response costs | 127 | 1535.32 (45.62) | 91 | 1455.87 (53.8) | 79.45 (–51.87 to 222.5) | 0.242 | |
| Intervention costs | |||||||
| Intervention costs | 128 | 14.38 (0.99) | 91 | 0 (0) | 14.38 (12.44 to 16.33) | < 0.001 | |
| Inpatient costs | |||||||
| ED | 217 | 251.49 (9.81) | 156 | 254.88 (11.24) | –3.39 (–35.3 to 24.12) | 0.8 | |
| General ward | 219 | 4188.33 (433.9) | 156 | 3581.97 (426.11) | 606.36 (–597.29 to 1729.24) | 0.322 | |
| ICU | 127 | 21,740.58 (2328.42) | 91 | 17,215.06 (2373.78) | 4525.52 (–1925.88 to 10,628.52) | 0.182 | |
| Total inpatient costs | 88 | 29,425.72 (3032.74) | 65 | 24,356.65 (3055.72) | 5069.07 (–3985.61 to 13,777.15) | 0.244 | |
| Outpatient costs | |||||||
| Cardiology | 91 | 120.5 (18.14) | 64 | 132.79 (23.19) | –12.29 (–72.07 to 45.37) | 0.67 | |
| Cardiac rehabilitation | 93 | 121.69 (27.84) | 65 | 160.63 (49.99) | –38.93 (–165.76 to 58.3) | 0.506 | |
| Nursing/residential home | 93 | 426.77 (206.19) | 66 | 341.18 (196.55) | 85.59 (–446.07 to 622.75) | 0.73 | |
| Other outpatient costs | 91 | 136.08 (47.11) | 60 | 173.61 (72.69) | –37.53 (–218.24 to 115.95) | 0.676 | |
| Total outpatient costs | 86 | 859.01 (241.89) | 57 | 738.4 (214.69) | 120.61 (–494.23 to 763.48) | 0.7 | |
| Primary health-care costs | |||||||
| GP, surgery visit | 95 | 52.19 (6.72) | 66 | 63.91 (8.93) | –11.72 (–34.89 to 10.61) | 0.312 | |
| GP, home visit | 95 | 4.4 (1.38) | 66 | 6.33 (3.34) | –1.93 (–9.99 to 4.11) | 0.644 | |
| GP, telephone consultation | 95 | 2.4 (0.93) | 65 | 3.51 (1.44) | –1.11 (–4.69 to 2.19) | 0.548 | |
| District nurse | 95 | 47.09 (12.98) | 66 | 42.91 (14.46) | 4.18 (–34.82 to 41.28) | 0.826 | |
| Practice nurse | 95 | 0.23 (0.24) | 65 | 0 (0) | 0.23 (0 to 0.85) | 0.76 | |
| Physiotherapy | 95 | 75.28 (76.62) | 65 | 0 (0) | 75.28 (0.47 to 245.62) | 0.042 | |
| Occupational therapy | 95 | 1.37 (0.98) | 65 | 2 (2) | –0.63 (–5.52 to 3.17) | 0.858 | |
| Social worker | 95 | 5.44 (1.84) | 66 | 5.87 (3.38) | –0.43 (–8.28 to 6.41) | 0.98 | |
| Speech therapy | 95 | 37.59 (21.36) | 66 | 71.66 (44.72) | –34.07 (–143.35 to 50.01) | 0.548 | |
| Psychiatrist | 95 | 4.45 (2.39) | 66 | 6.41 (3.96) | –1.96 (–11.47 to 6.74) | 0.728 | |
| Psychology | 95 | 37.28 (26.86) | 66 | 61.33 (39.74) | –24.05 (–119.63 to 62.08) | 0.632 | |
| Home care worker | 95 | 60.68 (31.67) | 66 | 41.52 (30.93) | 19.16 (–70.19 to 102.83) | 0.676 | |
| Lunch or social club | 95 | 0.95 (0.79) | 66 | 2.84 (1.88) | –1.89 (–6.25 to 1.71) | 0.376 | |
| Self-help groupsb | 95 | – | 66 | – | – | < 0.001 | |
| Meals and laundry | 95 | 13.37 (11.17) | 66 | 10.94 (6.51) | 2.43 (–20.26 to 29.67) | 0.918 | |
| Counsellor | 95 | 2.23 (1.35) | 66 | 1.61 (1.68) | 0.63 (–3.86 to 4.63) | 0.73 | |
| Other community care costs | 95 | 102.41 (103.78) | 65 | 5.71 (4.21) | 96.71 (–9.68 to 329.22) | 0.542 | |
| Total community care costs | 95 | 447.36 (204.33) | 65 | 330.81 (89.27) | 116.55 (–250.21 to 580.86) | 0.684 | |
| Medications | |||||||
| Medication | 94 | 287.66 (64.88) | 66 | 299.49 (93.53) | –11.83 (–250.68 to 183.9) | 0.97 | |
| Aids and adaptations costs | |||||||
| Hoist | 93 | 90.01 (61.56) | 65 | 0 (0) | 90.01 (0 to 215.81) | 0.306 | |
| Wheelchair | 93 | 8.39 (4.31) | 65 | 0 (0) | 8.39 (0 to 17.53) | 0.052 | |
| Walking aid | 93 | 4.36 (1.59) | 65 | 5.41 (2.22) | –1.05 (–6.56 to 4.07) | 0.726 | |
| Hand aid | 93 | 0.02 (0.02) | 65 | 0.13 (0.07) | –0.11 (–0.25 to 0.01) | 0.052 | |
| Other aids and adaptations costs | 87 | 22.24 (13.52) | 61 | 109.1 (82.98) | –86.87 (–271.51 to 24.64) | 0.336 | |
| Total aids and adaptations costs | 86 | 81.91 (63.42) | 60 | 115.82 (84.33) | –33.91 (–263.39 to 159.46) | 0.736 | |
| Total costs | |||||||
| 0–3 months: total NHS and PSS costs | 72 | 32,669.22 (3589.54) | 50 | 27,956.52 (3795.11) | 4712.7 (–5527.48 to 14,967.31) | 0.382 | |
| 3–6 months after randomisation | Inpatient costs | ||||||
| ED | 84 | 97.45 (27.96) | 49 | 119.33 (37.73) | –21.88 (–116.02 to 60.14) | 0.616 | |
| General ward | 121 | 1458.47 (450.89) | 82 | 1,687.77 (697.84) | –229.3 (–1938.34 to 1295.45) | 0.76 | |
| ICU | 123 | 2148.51 (1649.51) | 84 | 0 (0) | 2148.51 (17.47 to 5940.07) | 0.032 | |
| Total inpatient costs | 80 | 4114.25 (2466.27) | 45 | 1916.46 (957.58) | 2197.79 (–2031.75 to 8385.56) | 0.44 | |
| Outpatient costs | |||||||
| Cardiology | 83 | 110.65 (14.53) | 47 | 128.32 (30.04) | –17.67 (–84.6 to 40.45) | 0.596 | |
| Cardiac rehabilitation | 83 | 136.35 (38.67) | 49 | 219.58 (73.76) | –83.23 (–260.01 to 60.19) | 0.298 | |
| Nursing/residential home | 83 | 788.53 (352.6) | 48 | 367.88 (308.67) | 420.66 (–489.54 to 1352) | 0.344 | |
| Other outpatient costs | 82 | 29.24 (10.35) | 49 | 52.8 (25.37) | –23.56 (–86.71 to 19.04) | 0.384 | |
| Total outpatient costs | 79 | 932.02 (328.67) | 45 | 812.04 (331.92) | 119.97 (–835.56 to 1002.03) | 0.736 | |
| Primary health-care costs | |||||||
| GP, surgery visit | 84 | 60.79 (9.33) | 50 | 63.64 (10.85) | –2.85 (–34.14 to 24.32) | 0.864 | |
| GP, home visit | 84 | 9.95 (3.62) | 50 | 4.56 (1.99) | 5.39 (–2.17 to 13.33) | 0.19 | |
| GP, telephone consultation | 83 | 1.72 (0.86) | 49 | 4.07 (2.25) | –2.35 (–7.51 to 1.67) | 0.348 | |
| District nurse | 84 | 113.84 (71.63) | 50 | 11.49 (3.73) | 102.35 (0.54 to 259.15) | 0.046 | |
| Practice nurse | 83 | 0.13 (0.13) | 49 | 0 (0) | 0.13 (0 to 0.42) | 0.714 | |
| Physiotherapy | 83 | 2.31 (2.34) | 49 | 0 (0) | 2.31 (0 to 7.68) | 0.71 | |
| Occupational therapy | 83 | 0 (0) | 49 | 3.98 (3.77) | –3.98 (–11.7 to 0) | < 0.001 | |
| Social worker | 84 | 9.23 (4.12) | 50 | 6.03 (2.77) | 3.2 (–6.16 to 12.84) | 0.51 | |
| Speech therapy | 84 | 11.49 (7.22) | 50 | 1.93 (1.93) | 9.56 (–2.09 to 26.22) | 0.134 | |
| Psychiatrist | 84 | 6.04 (4.24) | 50 | 1.69 (1.63) | 4.35 (–2.54 to 14.61) | 0.316 | |
| Psychology | 84 | 14.05 (8.31) | 50 | 20.24 (12.44) | –6.18 (–35.83 to 21.53) | 0.702 | |
| Home care worker | 84 | 44.04 (32.37) | 50 | 2.16 (1.71) | 41.88 (–2.81 to 114.76) | 0.196 | |
| Lunch or social club | 84 | 0.33 (0.33) | 50 | 0 (0) | 0.33 (0 to 1.1) | 0.708 | |
| Self-help groupsb | 84 | – | 50 | – | – | – | |
| Meals and laundry | 84 | 15.37 (10.63) | 50 | 0.69 (0.45) | 14.67 (–0.69 to 39.99) | 0.168 | |
| Counsellor | 84 | 6.31 (6.26) | 50 | 3.18 (2.42) | 3.13 (–6.87 to 18.28) | 0.794 | |
| Other community care costs | 83 | 13.16 (9.54) | 49 | 0 (0) | 13.16 (1.47 to 36.46) | 0.004 | |
| Total community care costs | 83 | 312.26 (86.32) | 49 | 125.27 (19.78) | 187 (29.94 to 378.04) | 0.02 | |
| Medications | |||||||
| Medication | 80 | 182.04 (54.94) | 48 | 84.86 (18.07) | 97.18 (2.6 to 225.3) | 0.044 | |
| Aids and adaptations costs | |||||||
| Hoist | 83 | 252.14 (105.74) | 50 | 83.71 (83.57) | 168.43 (–89.17 to 438.34) | 0.222 | |
| Wheelchair | 83 | 14.1 (5.57) | 50 | 11.7 (6.71) | 2.4 (–15.49 to 18.19) | 0.836 | |
| Walking aid | 83 | 0.97 (0.82) | 50 | 1.45 (1.3) | –0.48 (–3.77 to 2.37) | 0.768 | |
| Hand aid | 83 | 0 (0) | 50 | 0.03 (0.03) | –0.03 (–0.12 to 0) | < 0.001 | |
| Other aids and adaptations costs | 78 | 12.07 (10.15) | 50 | 1.07 (0.62) | 11 (–0.77 to 34.02) | 0.192 | |
| Total aids and adaptations costs | 78 | 242.69 (104.76) | 50 | 97.99 (87.6) | 144.7 (–117.87 to 409.13) | 0.296 | |
| Total costs | |||||||
| 3–6 months: total NHS and PSS costs | 67 | 5524.18 (2914.88) | 36 | 3508.31 (1334.03) | 2015.87 (–3297.29 to 8742.91) | 0.598 | |
| 0–6 months: total NHS and PSS costs | 54 | 33,385.85 (7293.82) | 29 | 29,144.43 (4609.34) | 4241.42 (–9982.79 to 22,538.49) | 0.698 | |
| Assessment period | Category | Adrenaline arm (n = 128) | Placebo arm (n = 91) | Adrenaline vs. placebo | |||
|---|---|---|---|---|---|---|---|
| Participants with complete data (n) | Mean cost (SE) (£) | Participants with complete data (n) | Mean cost (SE) (£) | Mean difference in costs (bootstrapped 95% CI) (£) | p-value | ||
| 0–3 months after randomisation | Medications | ||||||
| Medications (privately purchased) | 94 | 1.64 (1.13) | 66 | 0.14 (0.07) | 1.5 (–0.14 to 4.01) | 0.216 | |
| Additional costs | |||||||
| Child care | 95 | 0 (0) | 66 | 0 (0) | 0 (0 to 0) | < 0.001 | |
| Housework | 95 | 4 (3.55) | 66 | 14.7 (9.18) | –10.7 (–33.51 to 4.85) | 0.24 | |
| Travel costs | 96 | 68.8 (28.7) | 66 | 83.18 (35.48) | –14.38 (–106.34 to 73.34) | 0.762 | |
| Other additional costs | 96 | 1558.05 (405.25) | 66 | 1100.98 (366.5) | 457.06 (–640.29 to 1562.61) | 0.404 | |
| Total additional costs | 95 | 1640.32 (411.74) | 66 | 1198.86 (372.51) | 441.46 (–667.47 to 1553.23) | 0.416 | |
| 0–3 months: total non-NHS and PSS costs | 92 | 1612.33 (420.67) | 66 | 1199 (372.51) | 413.33 (–686.8 to 1566.93) | 0.444 | |
| 3–6 months post randomisation assessment period | Medications | ||||||
| Medications (privately purchased) | 81 | 0.07 (0.03) | 49 | 0.12 (0.11) | –0.04 (–0.32 to 0.12) | 0.818 | |
| Additional costs | |||||||
| Child care | 83 | 0 (0) | 51 | 5.88 (5.69) | –5.88 (–18.37 to 0) | < 0.001 | |
| Housework | 83 | 22.29 (14.06) | 51 | 5.67 (3.15) | 16.62 (–5.81 to 47.85) | 0.298 | |
| Travel costs | 83 | 95.07 (45.79) | 51 | 31.11 (11.3) | 63.96 (–13.94 to 165.39) | 0.128 | |
| Other additional costs | 83 | 766.96 (343.22) | 51 | 346.71 (163.69) | 420.26 (–219.52 to 1305.15) | 0.27 | |
| Total additional costs | 83 | 884.33 (363.5) | 51 | 389.36 (167.28) | 494.96 (–182.1 to 1441.7) | 0.192 | |
| 3–6 months: total non-NHS and PSS costs | 81 | 905.61 (372.83) | 49 | 399.36 (174.22) | 506.25 (–196.78 to 1464.21) | 0.204 | |
| EQ-5D domain | 3-month assessment | 6-month assessment | ||||
|---|---|---|---|---|---|---|
| Adrenaline arm (N = 128) | Placebo arm (N = 91) | p-valuea | Adrenaline arm (N = 128) | Placebo arm (N = 91) | p-valuea | |
| Mobility, n (%) | 0.449 | 0.554 | ||||
| Level 1: no problems | 36 (28.1) | 31 (34.1) | 37 (28.9) | 21 (23.1) | ||
| Level 2: slight problems | 24 (18.8) | 13 (14.3) | 11 (8.6) | 10 (11) | ||
| Level 3: moderate problems | 13 (10.2) | 13 (14.3) | 11 (8.6) | 15 (16.5) | ||
| Level 4: severe problems | 7 (5.5) | 5 (5.5) | 5 (3.9) | 1 (1.1) | ||
| Level 5: unable to walk | 8 (6.2) | 2 (2.2) | 10 (7.8) | 2 (2.2) | ||
| Missing | 40 (31.2) | 27 (29.7) | 54 (42.2) | 42 (46.2) | ||
| Self-care, n (%) | 0.020 | 0.420 | ||||
| Level 1: no problems | 55 (43) | 52 (57.1) | 48 (37.5) | 36 (39.6) | ||
| Level 2: slight problems | 13 (10.2) | 2 (2.2) | 9 (7) | 7 (7.7) | ||
| Level 3: moderate problems | 10 (7.8) | 5 (5.5) | 5 (3.9) | 3 (3.3) | ||
| Level 4: severe problems | 1 (0.8) | 1 (1.1) | 2 (1.6) | 0 (0) | ||
| Level 5: unable to wash or dress | 9 (7) | 4 (4.4) | 10 (7.8) | 3 (3.3) | ||
| Missing | 40 (31.2) | 27 (29.7) | 54 (42.2) | 42 (46.2) | ||
| Usual activities, n (%) | 0.161 | 0.547 | ||||
| Level 1: no problems | 25 (19.5) | 26 (28.6) | 25 (19.5) | 20 (22) | ||
| Level 2: slight problems | 25 (19.5) | 16 (17.6) | 17 (13.3) | 11 (12.1) | ||
| Level 3: moderate problems | 15 (11.7) | 13 (14.3) | 16 (12.5) | 11 (12.1) | ||
| Level 4: severe problems | 6 (4.7) | 1 (1.1) | 2 (1.6) | 2 (2.2) | ||
| Level 5: unable to perform usual activities | 17 (13.3) | 8 (8.8) | 14 (10.9) | 5 (5.5) | ||
| Missing | 40 (31.2) | 27 (29.7) | 54 (42.2) | 42 (46.2) | ||
| Pain/discomfort, n (%) | 0.814 | 0.299 | ||||
| Level 1: no pain or discomfort | 37 (28.9) | 29 (31.9) | 28 (21.9) | 24 (26.4) | ||
| Level 2: slight pain or discomfort | 25 (19.5) | 19 (20.9) | 25 (19.5) | 13 (14.3) | ||
| Level 3: moderate pain or discomfort | 18 (14.1) | 8 (8.8) | 14 (10.9) | 8 (8.8) | ||
| Level 4: severe pain or discomfort | 4 (3.1) | 5 (5.5) | 5 (3.9) | 3 (3.3) | ||
| Level 5: extreme pain or discomfort | 4 (3.1) | 3 (3.3) | 2 (1.6) | 1 (1.1) | ||
| Missing | 40 (31.2) | 27 (29.7) | 54 (42.2) | 42 (46.2) | ||
| Anxiety/depression, n (%) | 1 | 0.757 | ||||
| Level 1: not anxious or depressed | 40 (31.2) | 29 (31.9) | 28 (21.9) | 21 (23.1) | ||
| Level 2: slightly anxious or depressed | 22 (17.2) | 17 (18.7) | 25 (19.5) | 16 (17.6) | ||
| Level 3: moderately anxious or depressed | 15 (11.7) | 13 (14.3) | 12 (9.4) | 8 (8.8) | ||
| Level 4: severely anxious or depressed | 8 (6.2) | 4 (4.4) | 4 (3.1) | 0 (0) | ||
| Level 5: extremely anxious or depressed | 3 (2.3) | 1 (1.1) | 4 (3.1) | 4 (4.4) | ||
| Missing | 40 (31.2) | 27 (29.7) | 55 (43) | 42 (46.2) | ||
| SF-12 domain | 3-month assessment | 6-month assessment | ||||
|---|---|---|---|---|---|---|
| Adrenaline arm (N = 128) | Placebo arm (N = 91) | p-valuea | Adrenaline arm (N = 128) | Placebo arm (N = 91) | p-valuea | |
| General health, n (%) | 0.590 | 0.693 | ||||
| Excellent | 5 (3.9) | 6 (6.6) | 4 (3.1) | 1 (1.1) | ||
| Very good | 10 (7.8) | 16 (17.6) | 8 (6.2) | 13 (14.3) | ||
| Good | 32 (25) | 24 (26.4) | 29 (22.7) | 15 (16.5) | ||
| Fair | 22 (17.2) | 10 (11) | 22 (17.2) | 12 (13.2) | ||
| Poor | 17 (13.3) | 7 (7.7) | 14 (10.9) | 7 (7.7) | ||
| Missing | 42 (32.8) | 28 (30.8) | 51 (39.8) | 43 (47.3) | ||
| Moderate activities, n (%) | 0.321 | 1 | ||||
| Yes, limited a lot | 31 (24.2) | 17 (18.7) | 21 (16.4) | 14 (15.4) | ||
| Yes, limited a little | 26 (20.3) | 18 (19.8) | 25 (19.5) | 18 (19.8) | ||
| No, not limited at all | 29 (22.7) | 28 (30.8) | 30 (23.4) | 17 (18.7) | ||
| Missing | 42 (32.8) | 28 (30.8) | 52 (40.6) | 42 (46.2) | ||
| Climbing stairs, n (%) | 0.171 | 0.970 | ||||
| Yes, limited a lot | 38 (29.7) | 20 (22) | 26 (20.3) | 16 (17.6) | ||
| Yes, limited a little | 19 (14.8) | 16 (17.6) | 26 (20.3) | 19 (20.9) | ||
| No, not limited at all | 29 (22.7) | 27 (29.7) | 23 (18) | 14 (15.4) | ||
| Missing | 42 (32.8) | 28 (30.8) | 53 (41.4) | 42 (46.2) | ||
| Accomplished less physically, n (%) | 0.519 | 1 | ||||
| All of the time | 19 (14.8) | 11 (12.1) | 17 (13.3) | 11 (12.1) | ||
| Most of the time | 11 (8.6) | 10 (11) | 8 (6.2) | 6 (6.6) | ||
| Some of the time | 18 (14.1) | 14 (15.4) | 20 (15.6) | 16 (17.6) | ||
| A little of the time | 16 (12.5) | 8 (8.8) | 12 (9.4) | 5 (5.5) | ||
| None of the time | 19 (14.8) | 21 (23.1) | 19 (14.8) | 11 (12.1) | ||
| Missing | 45 (35.2) | 27 (29.7) | 52 (40.6) | 42 (46.2) | ||
| Limited physically, n (%) | 0.193 | 0.786 | ||||
| All of the time | 23 (18) | 11 (12.1) | 18 (14.1) | 14 (15.4) | ||
| Most of the time | 9 (7) | 7 (7.7) | 8 (6.2) | 7 (7.7) | ||
| Some of the time | 16 (12.5) | 17 (18.7) | 18 (14.1) | 13 (14.3) | ||
| A little of the time | 19 (14.8) | 8 (8.8) | 13 (10.2) | 4 (4.4) | ||
| None of the time | 16 (12.5) | 21 (23.1) | 16 (12.5) | 11 (12.1) | ||
| Missing | 45 (35.2) | 27 (29.7) | 55 (43) | 42 (46.2) | ||
| Did less work,b n (%) | 0.691 | 1 | ||||
| All of the time | 5 (3.9) | 6 (6.6) | 13 (10.2) | 9 (9.9) | ||
| Most of the time | 8 (6.2) | 3 (3.3) | 7 (5.5) | 2 (2.2) | ||
| Some of the time | 12 (9.4) | 12 (13.2) | 15 (11.7) | 12 (13.2) | ||
| A little of the time | 20 (15.6) | 9 (9.9) | 13 (10.2) | 5 (5.5) | ||
| None of the time | 33 (25.8) | 32 (35.2) | 29 (22.7) | 21 (23.1) | ||
| Missing | 50 (39.1) | 29 (31.9) | 51 (39.8) | 42 (46.2) | ||
| Accomplished less emotionally, n (%) | 0.495 | 0.885 | ||||
| All of the time | 3 (2.3) | 5 (5.5) | 14 (10.9) | 8 (8.8) | ||
| Most of the time | 4 (3.1) | 2 (2.2) | 7 (5.5) | 2 (2.2) | ||
| Some of the time | 8 (6.2) | 10 (11) | 10 (7.8) | 10 (11) | ||
| A little of the time | 16 (12.5) | 7 (7.7) | 11 (8.6) | 6 (6.6) | ||
| None of the time | 46 (35.9) | 38 (41.8) | 30 (23.4) | 22 (24.2) | ||
| Missing | 51 (39.8) | 29 (31.9) | 56 (43.8) | 43 (47.3) | ||
| Pain, n (%) | 0.603 | 1 | ||||
| Not at all | 39 (30.5) | 26 (28.6) | 32 (25) | 21 (23.1) | ||
| A little bit | 20 (15.6) | 17 (18.7) | 17 (13.3) | 10 (11) | ||
| Moderately | 10 (7.8) | 12 (13.2) | 8 (6.2) | 8 (8.8) | ||
| Quite a bit | 8 (6.2) | 7 (7.7) | 11 (8.6) | 7 (7.7) | ||
| Extremely | 6 (4.7) | 1 (1.1) | 9 (7) | 3 (3.3) | ||
| Missing | 45 (35.2) | 28 (30.8) | 51 (39.8) | 42 (46.2) | ||
| Calm, n (%) | 0.385 | 1 | ||||
| All of the time | 11 (8.6) | 13 (14.3) | 9 (7) | 5 (5.5) | ||
| Most of the time | 40 (31.2) | 34 (37.4) | 30 (23.4) | 20 (22) | ||
| Some of the time | 16 (12.5) | 11 (12.1) | 22 (17.2) | 14 (15.4) | ||
| A little of the time | 11 (8.6) | 3 (3.3) | 11 (8.6) | 7 (7.7) | ||
| None of the time | 2 (1.6) | 2 (2.2) | 5 (3.9) | 3 (3.3) | ||
| Missing | 48 (37.5) | 28 (30.8) | 51 (39.8) | 42 (46.2) | ||
| Energy, n (%) | 0.178 | 0.886 | ||||
| All of the time | 5 (3.9) | 9 (9.9) | 3 (2.3) | 3 (3.3) | ||
| Most of the time | 20 (15.6) | 20 (22) | 19 (14.8) | 13 (14.3) | ||
| Some of the time | 21 (16.4) | 18 (19.8) | 19 (14.8) | 17 (18.7) | ||
| A little of the time | 20 (15.6) | 5 (5.5) | 19 (14.8) | 6 (6.6) | ||
| None of the time | 15 (11.7) | 11 (12.1) | 17 (13.3) | 10 (11) | ||
| Missing | 47 (36.7) | 28 (30.8) | 51 (39.8) | 42 (46.2) | ||
| Feeling downhearted, n (%) | 1 | 0.859 | ||||
| All of the time | 5 (3.9) | 3 (3.3) | 5 (3.9) | 2 (2.2) | ||
| Most of the time | 6 (4.7) | 3 (3.3) | 12 (9.4) | 8 (8.8) | ||
| Some of the time | 24 (18.8) | 17 (18.7) | 19 (14.8) | 12 (13.2) | ||
| A little of the time | 22 (17.2) | 19 (20.9) | 24 (18.8) | 11 (12.1) | ||
| None of the time | 24 (18.8) | 21 (23.1) | 17 (13.3) | 16 (17.6) | ||
| Missing | 47 (36.7) | 28 (30.8) | 51 (39.8) | 42 (46.2) | ||
| Social activities, n (%) | 1 | 0.953 | ||||
| All of the time | 10 (7.8) | 8 (8.8) | 11 (8.6) | 6 (6.6) | ||
| Most of the time | 10 (7.8) | 4 (4.4) | 6 (4.7) | 6 (6.6) | ||
| Some of the time | 19 (14.8) | 13 (14.3) | 18 (14.1) | 10 (11) | ||
| A little of the time | 7 (5.5) | 10 (11) | 9 (7) | 7 (7.7) | ||
| None of the time | 35 (27.3) | 28 (30.8) | 33 (25.8) | 20 (22) | ||
| Missing | 47 (36.7) | 28 (30.8) | 51 (39.8) | 42 (46.2) | ||
| Analysis model | Adrenaline arm | Placebo arm | Mean difference (95% CI) | Cost-effectiveness | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean costs (SE) (£) | Mean QALYs (SE) | Mean costs (SE) (£) | Mean QALYs (SE) | Costs (£) | QALYs | ICER (£/QALY) | INMB at £30,000/QALY (95% CI) | Probability of being cost-effective at £30,000/QALY | |
| Unadjusted multiple imputation | 4566 (166) | 0.0076 (0.0008) | 3229 (166) | 0.0067 (0.0008) | 1337 (877 to 1797) | 0.001 (–0.0012 to 0.0031) | 1,401,872 | –1308 (–1748 to –868) | 0 |
| Adjusted complete case | 3082 (369) | –0.0007 (0.002) | 2067 (371) | –0.0014 (0.002) | 1015 (725 to 1306) | 0.0007 (–0.0008 to 0.0023) | 1,377,745 | –993 (–1270 to –716) | 0 |
| Unadjusted complete case | 3943 (106) | 0.0064 (0.0006) | 2905 (106) | 0.0057 (0.0006) | 1038 (745 to 1332) | 0.0007 (–0.0009 to 0.0022) | 1,521,586 | –1018 (–1297 to –739) | 0 |
| Parameter estimates via linear regression implemented within a bootstrap | 3592 (522) | 0.0024 (0.0025) | 2292 (527) | 0.0017 (0.0025) | 1300 (846 to 1755) | 0.0007 (–0.0014 to 0.0029) | 1,784,993 | –1279 (–1737 to –820) | 0 |
| QALYs based on mRS data 6 months | 3591 (541) | 0.0031 (0.0026) | 2285 (545) | 0.0018 (0.0026) | 1306 (836 to 1776) | 0.0012 (–0.001 to 0.0035) | 1,069,119 | –1269 (–1719 to –819) | 0 |
| QALYs derived assuming baseline utility of –0.042 (unconscious state) | 3592 (530) | 0.0018 (0.0024) | 2292 (535) | 0.0015 (0.0024) | 1300 (839 to 1761) | 0.0003 (–0.0018 to 0.0024) | 4,218,224 | –1291 (–1738 to –844) | 0 |
| Societal costs, 6 months | 3638 (541) | 0.0025 (0.0025) | 2228 (545) | 0.0017 (0.0026) | 1410 (940 to 1880) | 0.0008 (–0.0014 to 0.003) | 1,828,271 | –1387 (–1837 to –937) | |
| QALYs based on EQ-5D/mRS, 12 months NHS/PSS costs | 3741 (536) | 0.006 (0.0049) | 2330 (541) | 0.0038 (0.005) | 1411 (946 to 1876) | 0.0022 (–0.0021 to 0.0065) | 644,308 | –1346 (–1784 to –907) | |
| QALYs based on mRS data, 12-month NHS/PSS costs | 3742 (538) | 0.0068 (0.005) | 2327 (542) | 0.0041 (0.0051) | 1415 (948 to 1881) | 0.0027 (–0.0017 to 0.007) | 528,477 | –1334 (–1771 to –898) | 0 |
| QALYs based on EQ-5D/mRS data, 12-month societal costs | 3768 (571) | 0.0059 (0.005) | 2259 (576) | 0.0037 (0.005) | 1509 (1014 to 2004) | 0.0022 (–0.0021 to 0.0066) | 679,317 | –1442 (–1908 to –977) | 0 |
| Excluded stand-down and restock time in emergency response cost calculations | 2989 (526) | 0.002 (0.0025) | 1684 (530) | 0.0012 (0.0025) | 1305 (844 to 1766) | 0.0007 (–0.0015 to 0.0029) | 1,835,618 | –1284 (–1727 to –841) | 0 |
| Excluded estimated cost of transporting deceased patients to nearest hospital mortuary if patient died at scene of cardiac arrest and in a public place | 3445 (525) | 0.0019 (0.0025) | 2146 (529) | 0.0012 (0.0025) | 1299 (838 to 1760) | 0.0007 (–0.0014 to 0.0029) | 1,794,795 | –1277 (–1720 to –835) | 0 |
| Excluded stand-down/restock time and cost of transporting patients to nearest mortuary in emergency response cost calculations | 2989 (526) | 0.002 (0.0025) | 1684 (530) | 0.0012 (0.0025) | 1305 (844 to 1766) | 0.0007 (–0.0015 to 0.0029) | 1,835,618 | –1284 (–1727 to –841) | 0 |
| Missing-not-at-random assumption | Adrenaline arm | Placebo arm | Mean difference (95% CI) | Cost-effectiveness | ||||
|---|---|---|---|---|---|---|---|---|
| Mean costs (SE) (£) | Mean QALYs (SE) | Mean costs (SE) (£) | Mean QALYs (SE) | Costs (£) | QALYs | ICER (£/QALY) | INMB at £30,000/QALY (95% CI) (£) | |
| Increased imputed utility by | ||||||||
| 100% | 3601 (529) | 0.0022 (0.0036) | 2320 (534) | 0.0009 (0.0036) | 1281 (821 to 1741) | 0.0013 (–0.0018 to 0.0043) | 1,021,684 | –1243 (–1681 to –805) |
| 75% | 3594 (534) | 0.0021 (0.0033) | 2307 (538) | 0.0009 (0.0034) | 1287 (823 to 1750) | 0.0012 (–0.0017 to 0.0041) | 1,072,646 | –1251 (–1693 to –808) |
| 50% | 3606 (530) | 0.0022 (0.0031) | 2322 (534) | 0.001 (0.0031) | 1284 (823 to 1744) | 0.0011 (–0.0015 to 0.0038) | 1,120,679 | –1249 (–1689 to –810) |
| 25% | 3595 (540) | 0.0024 (0.0029) | 2302 (545) | 0.0012 (0.0029) | 1294 (824 to 1763) | 0.0011 (–0.0014 to 0.0036) | 1,158,930 | –1260 (–1709 to –811) |
| 0.0% | 3582 (532) | 0.0024 (0.0025) | 2292 (537) | 0.0016 (0.0025) | 1291 (828 to 1754) | 0.0008 (–0.0014 to 0.003) | 1,670,014 | –1268 (–1712 to –823) |
| Decreased imputed utility by | ||||||||
| 25% | 3594 (522) | 0.0027 (0.0023) | 2298 (527) | 0.002 (0.0023) | 1296 (842 to 1750) | 0.0006 (–0.0013 to 0.0026) | 2,036,654 | –1277 (–1717 to –837) |
| 50% | 3580 (538) | 0.0026 (0.0023) | 2262 (543) | 0.002 (0.0023) | 1318 (850 to 1786) | 0.0006 (–0.0014 to 0.0026) | 2,114,049 | –1299 (–1753 to –845) |
| 75% | 3574 (539) | 0.0027 (0.0023) | 2269 (543) | 0.002 (0.0023) | 1305 (837 to 1773) | 0.0006 (–0.0014 to 0.0026) | 2,067,739 | –1286 (–1740 to –832) |
| 100% | 3574 (539) | 0.0027 (0.0023) | 2269 (543) | 0.002 (0.0023) | 1305 (837 to 1773) | 0.0006 (–0.0014 to 0.0026) | 2,067,739 | –1286 (–1740 to –832) |
| Missing-not-at-random assumption | Adrenaline arm | Placebo arm | Mean difference (95% CI) | Cost-effectiveness | ||||
|---|---|---|---|---|---|---|---|---|
| Mean costs (SE) (£) | Mean QALYs (SE) | Mean costs (SE) (£) | Mean QALYs (SE) | Costs (£) | QALYs | ICER (£/QALY) | INMB at £30,000/QALY (£) | |
| Increased imputed costs by | ||||||||
| 100% | 3175 (874) | 0.0024 (0.0028) | 2285 (881) | 0.0017 (0.0028) | 890 (131 to 1650) | 0.0008 (–0.0017 to 0.0032) | 1,186,037 | 0.008 |
| 75% | 3196 (802) | 0.0024 (0.0027) | 2238 (808) | 0.0017 (0.0027) | 958 (261 to 1655) | 0.0007 (–0.0016 to 0.0031) | 1,301,145 | 0.003 |
| 50% | 3300 (703) | 0.0025 (0.0026) | 2331 (709) | 0.0017 (0.0026) | 969 (358 to 1580) | 0.0008 (–0.0015 to 0.0031) | 1,246,202 | 0 |
| 25% | 3384 (621) | 0.0024 (0.0026) | 2335 (626) | 0.0017 (0.0026) | 1049 (509 to 1589) | 0.0008 (–0.0015 to 0.003) | 1,363,580 | 0 |
| 0.0% | 3582 (532) | 0.0024 (0.0025) | 2292 (537) | 0.0016 (0.0025) | 1291 (828 to 1754) | 0.0008 (–0.0014 to 0.003) | 1,670,014 | 0 |
| Decreased imputed costs by | ||||||||
| 25% | 3634 (516) | 0.0024 (0.0025) | 2299 (520) | 0.0017 (0.0025) | 1335 (887 to 1783) | 0.0008 (–0.0014 to 0.0029) | 1,746,277 | 0 |
| 50% | 3650 (507) | 0.0024 (0.0025) | 2334 (512) | 0.0017 (0.0025) | 1315 (874 to 1756) | 0.0008 (–0.0014 to 0.0029) | 1,687,983 | 0 |
| 75% | 3663 (511) | 0.0024 (0.0025) | 2359 (515) | 0.0016 (0.0025) | 1304 (860 to 1747) | 0.0008 (–0.0014 to 0.0029) | 1,688,008 | 0 |
| 100% | 3663 (511) | 0.0024 (0.0025) | 2359 (515) | 0.0016 (0.0025) | 1304 (860 to 1747) | 0.0008 (–0.0014 to 0.0029) | 1,688,008 | 0 |
| Subgroup | Adrenaline arm | Placebo arm | Mean difference (95% CI) | Cost-effectiveness | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean costs (SE) (£) | Mean QALYs (SE) | Mean costs (SE) (£) | Mean QALYs (SE) | Costs (£) | QALYs | ICER (£/QALY) | INMB at £30,000/QALY (95% CI) (£) | Probability of being cost-effective at £30,000/QALY | |
| Cause of cardiac arrest | |||||||||
| Non-medical | 3491 (660) | 0.0014 (0.0031) | 2403 (702) | 0.0029 (0.0033) | 1088 (–563 to 2740) | –0.0015 (–0.0092 to 0.0062) | Dominated | –1134 (–2722 to 455) | 0.079 |
| Medical | 3763 (383) | 0.005 (0.0018) | 2438 (702) | 0.004 (0.0033) | 1325 (–1062 to 3713) | 0.001 (–0.0102 to 0.0122) | 1,356,042 | –1296 (–3702 to 1110) | 0.147 |
| Age (years) | |||||||||
| ≤ 60 | 5550 (552) | 0.0087 (0.0026) | 3234 (561) | 0.0059 (0.0026) | 2316 (1614 to 3018) | 0.0028 (–0.0005 to 0.0061) | 823,606 | –2232 (–2906 to –1557) | 0 |
| > 60 | 2729 (570) | 0.0001 (0.0027) | 2272 (561) | 0.0011 (0.0026) | 457 (–722 to 1636) | –0.001 (–0.0065 to 0.0046) | Dominated | –486 (–1660 to 688) | 0.195 |
| Sex | |||||||||
| Female | 3434 (563) | 0.0022 (0.0026) | 2432 (565) | 0.002 (0.0026) | 1002 (213 to 1790) | 0.0002 (–0.0034 to 0.0039) | 4,145,700 | –995 (–1753 to –236) | 0.004 |
| Male | 3690 (521) | 0.0035 (0.0024) | 2220 (565) | 0.0024 (0.0026) | 1470 (213 to 2728) | 0.0011 (–0.0048 to 0.0069) | 1,389,015 | –1439 (–2696 to –182) | 0.016 |
| EMS time from arrival at scene to administration of first dose (minutes) | |||||||||
| ≤ 10 | 3399 (600) | 0.0031 (0.0028) | 2478 (605) | 0.001 (0.0028) | 922 (–72 to 1916) | 0.0021 (–0.0026 to 0.0067) | 439,679 | –859 (–1815 to 97) | 0.04 |
| > 10 | 3220 (531) | –0.0037 (0.0025) | 1795 (605) | –0.0041 (0.0028) | 1425 (–86 to 2937) | 0.0004 (–0.0067 to 0.0074) | 3,989,128 | –1415 (–2931 to 102) | 0.027 |
| Time from 999 call received to EMS arrival at scene (minutes) | |||||||||
| ≤ 10 | 3676 (544) | 0.0024 (0.0025) | 2203 (548) | 0.0018 (0.0026) | 1473 (925 to 2021) | 0.0006 (–0.002 to 0.0032) | 2,427,288 | –1455 (–1982 to –927) | 0 |
| > 10 | 2188 (599) | –0.001 (0.0028) | 1335 (548) | –0.0022 (0.0026) | 853 (–336 to 2042) | 0.0012 (–0.0043 to 0.0068) | 701,635 | –817 (–1985 to 352) | 0.086 |
| Shockable rhythm | |||||||||
| No | 3435 (548) | 0.0025 (0.0026) | 2425 (552) | 0.0017 (0.0026) | 1010 (483 to 1536) | 0.0008 (–0.0016 to 0.0033) | 1,201,680 | –984 (–1491 to –478) | 0 |
| Yes | 9166 (684) | 0.0306 (0.0032) | 6620 (552) | 0.0302 (0.0026) | 2546 (1236 to 3857) | 0.0005 (–0.0057 to 0.0066) | 5,282,677 | –2532 (–3813 to –1251) | 0.001 |
| Number of syringes given (out of two) | |||||||||
| ≤ 2 | 9767 (751) | 0.0238 (0.0035) | 7641 (827) | 0.0319 (0.0039) | 2127 (690 to 3564) | –0.008 (–0.0148 to -0.0013) | Dominated | –2367 (–3758 to –977) | 0.001 |
| > 2 | 2031 (637) | –0.0029 (0.003) | 1347 (827) | –0.0032 (0.0039) | 685 (–1432 to 2801) | 0.0002 (–0.0097 to 0.0102) | 2,995,310 | –678 (–2809 to 1453) | 0.269 |
| Number of syringes given (out of four) | |||||||||
| ≤ 4 | 5315 (556) | 0.0081 (0.0026) | 3036 (572) | 0.0068 (0.0027) | 2279 (1582 to 2976) | 0.0013 (–0.002 to 0.0046) | 1,782,438 | –2241 (–2912 to –1569) | 0 |
| > 4 | 936 (573) | –0.0068 (0.0027) | 686 (572) | –0.0061 (0.0027) | 251 (–920 to 1422) | –0.0007 (–0.0062 to 0.0048) | Dominated | –272 (–1439 to 896) | 0.319 |
| Witnessed by | |||||||||
| Not witnessed | 3470 (560) | 0.002 (0.0026) | 2409 (568) | 0.0022 (0.0027) | 1061 (299 to 1823) | –0.0002 (–0.0038 to 0.0034) | Dominated | –1067 (–1800 to –334) | 0.002 |
| EMS | 4738 (787) | 0.0117 (0.0037) | 3804 (568) | 0.0125 (0.0027) | 934 (–829 to 2696) | –0.0009 (–0.0091 to 0.0074) | Dominated | –959 (–2687 to 768) | 0.153 |
| Bystander | 4762 (572) | 0.0068 (0.0027) | 3189 (568) | 0.0049 (0.0027) | 1573 (308 to 2837) | 0.0019 (–0.0041 to 0.0078) | 843,463 | –1517 (–2777 to –256) | 0.007 |
| Bystander CPR | |||||||||
| No | 3325 (582) | 0.0032 (0.0027) | 2411 (545) | 0.0029 (0.0026) | 915 (–219 to 2048) | 0.0003 (–0.005 to 0.0056) | 2,859,086 | –905 (–2026 to 216) | 0.048 |
| Yes | 4022 (538) | 0.0033 (0.0025) | 2456 (545) | 0.0022 (0.0026) | 1566 (964 to 2169) | 0.0011 (–0.0018 to 0.0039) | 1,475,919 | –1535 (–2114 to –955) | 0 |
FIGURE 25.
Health-related quality of life at baseline, at hospital discharge and at 3 and 6 months after randomisation. (a) EQ-5D/mRS (whole trial); (b) mRS (whole trial); (c) EQ-5D/mRS (survivors); and (d) mRS (survivors).
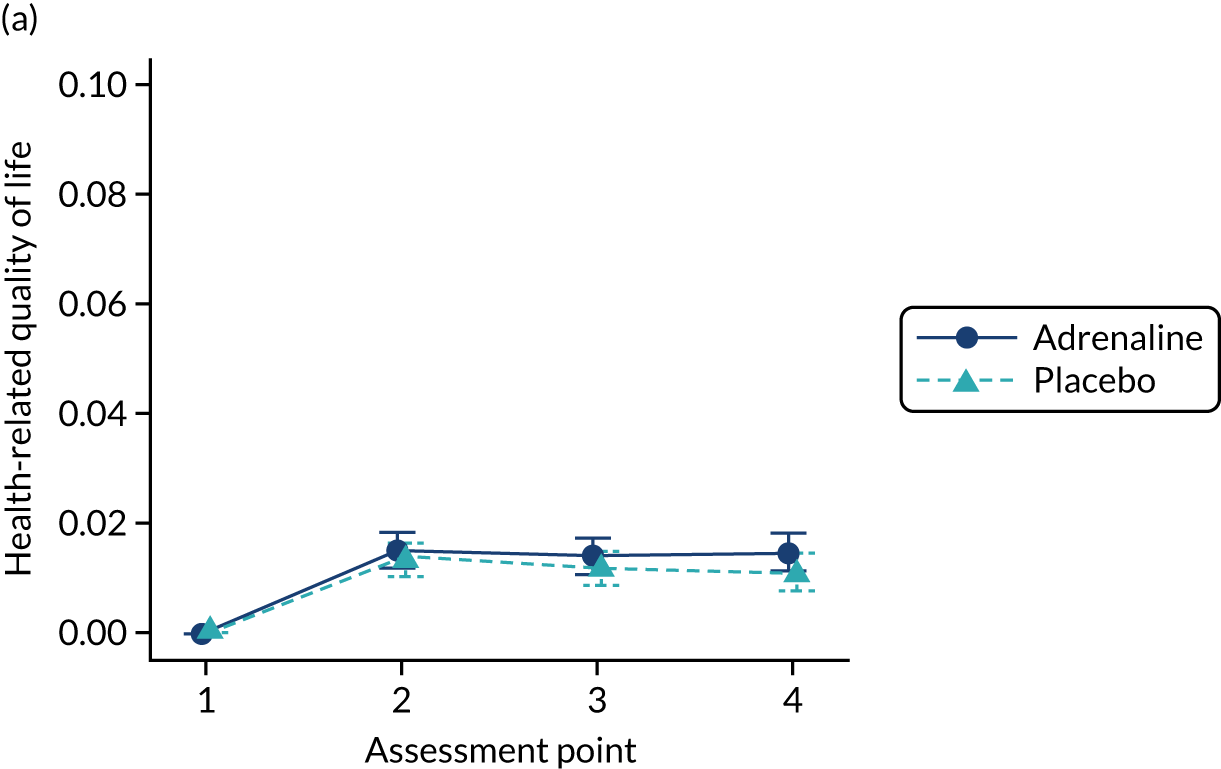
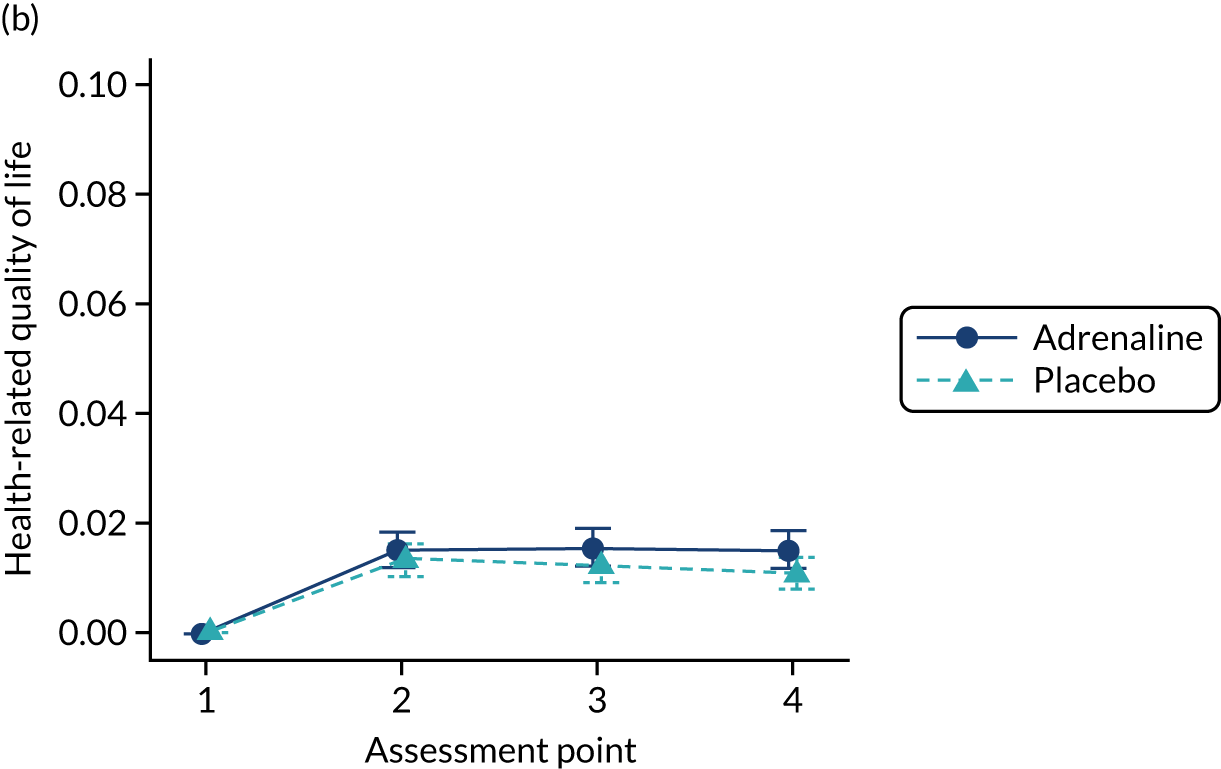
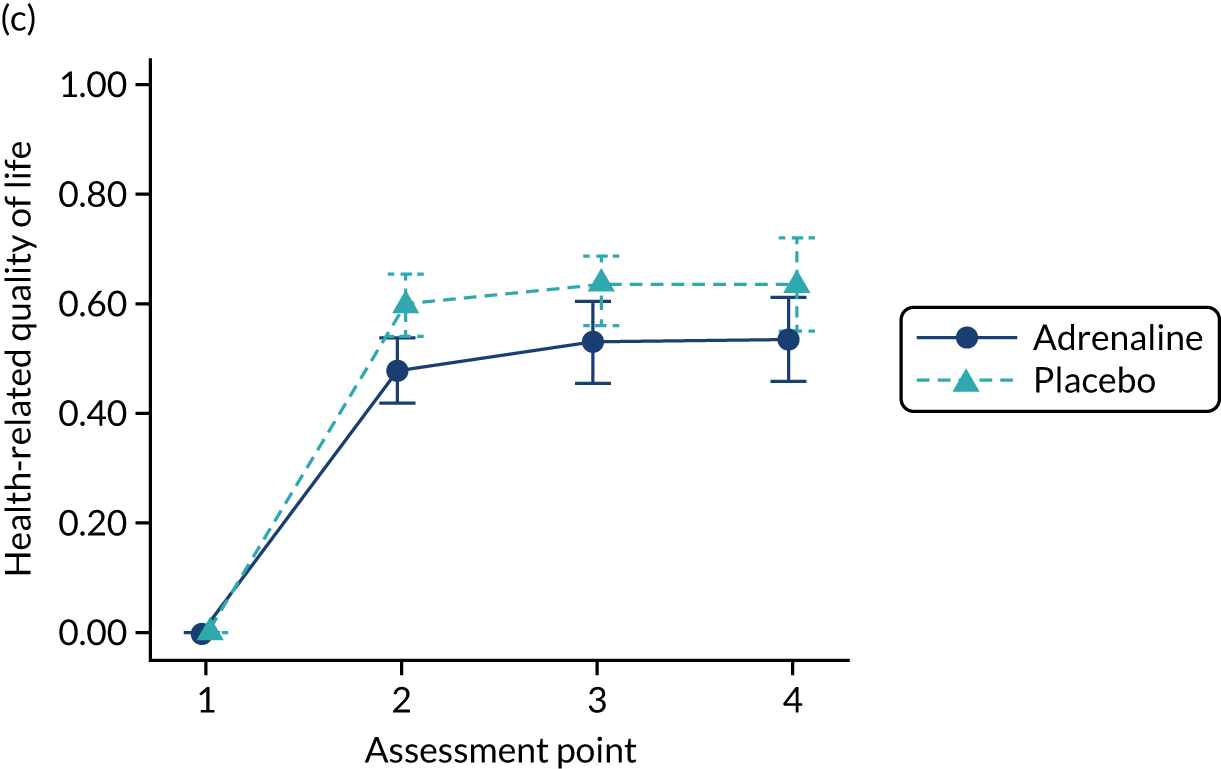
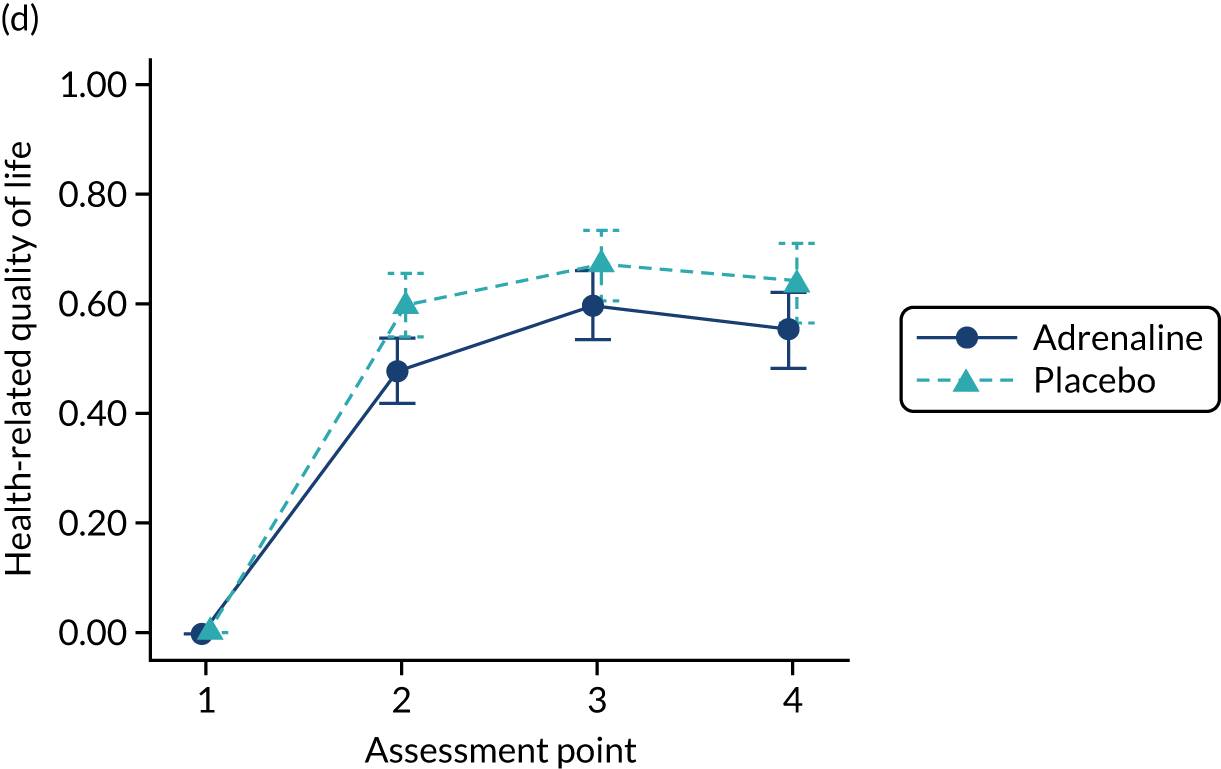
FIGURE 26.
Adjusted multiple imputation (base case). (a) Cost-effectiveness plane; and (b) CEAC.
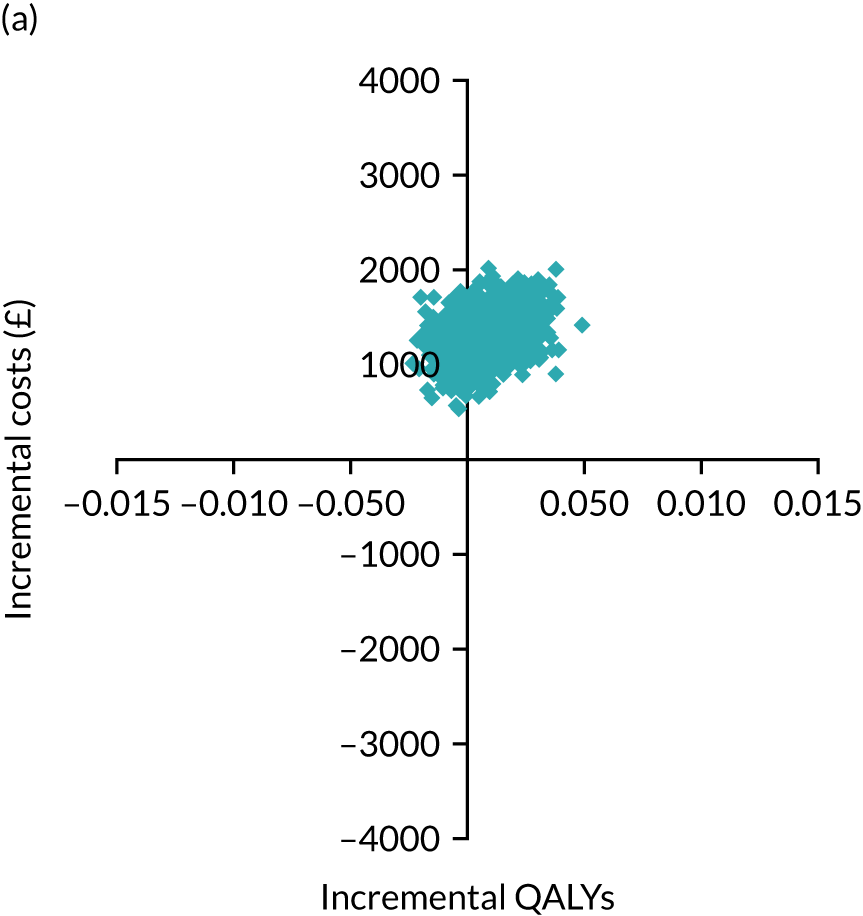
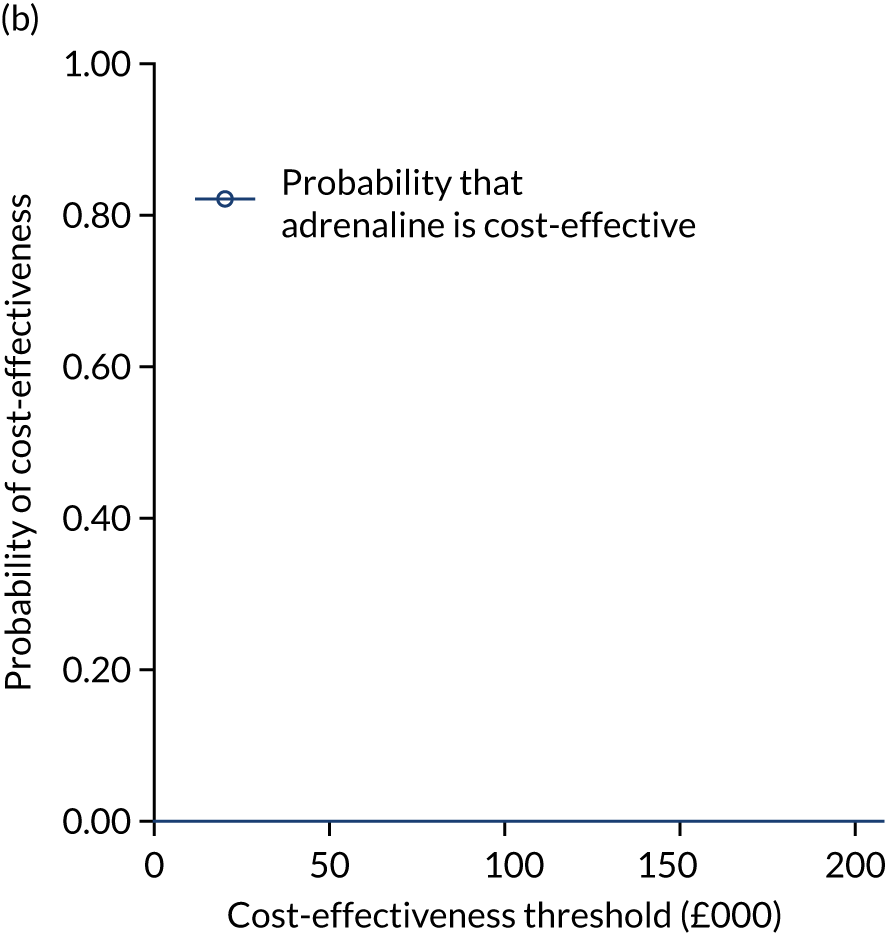
FIGURE 27.
The 12-month time horizon (QALYs based on EQ-5D-5L and mRS data, NHS/PSS costs). (a) Cost-effectiveness plane; and (b) CEAC.
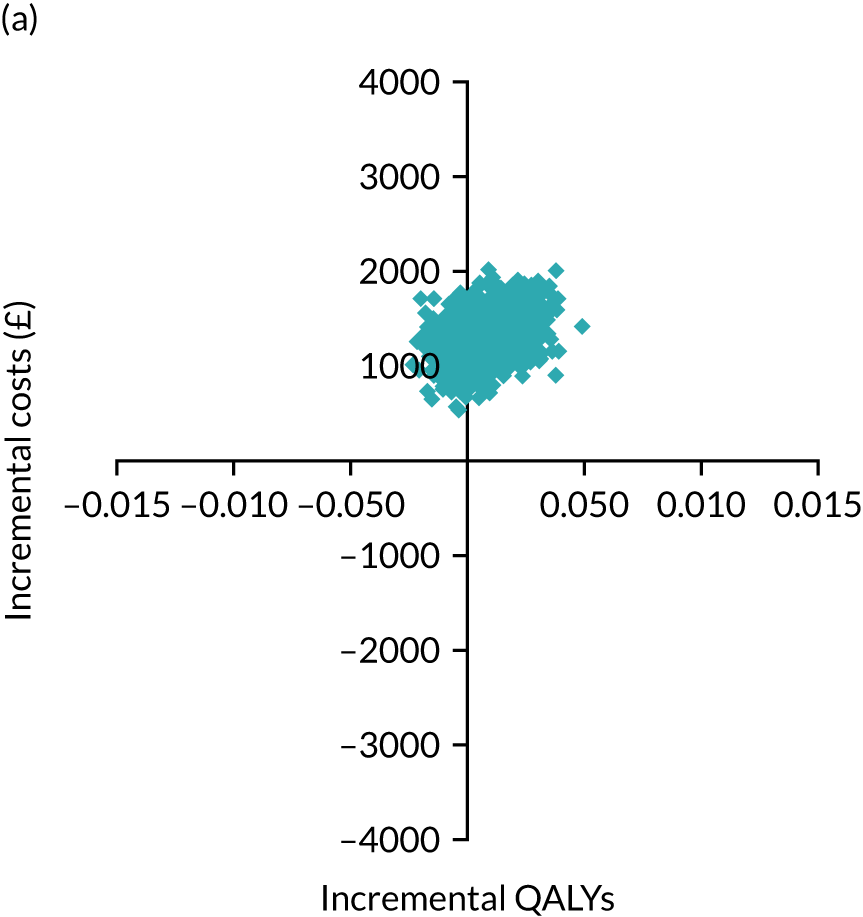
FIGURE 28.
The lifetime time horizon (QALYs based on mRS data, NHS/PSS costs). (a) Cost-effectiveness plane; and (b) CEAC.
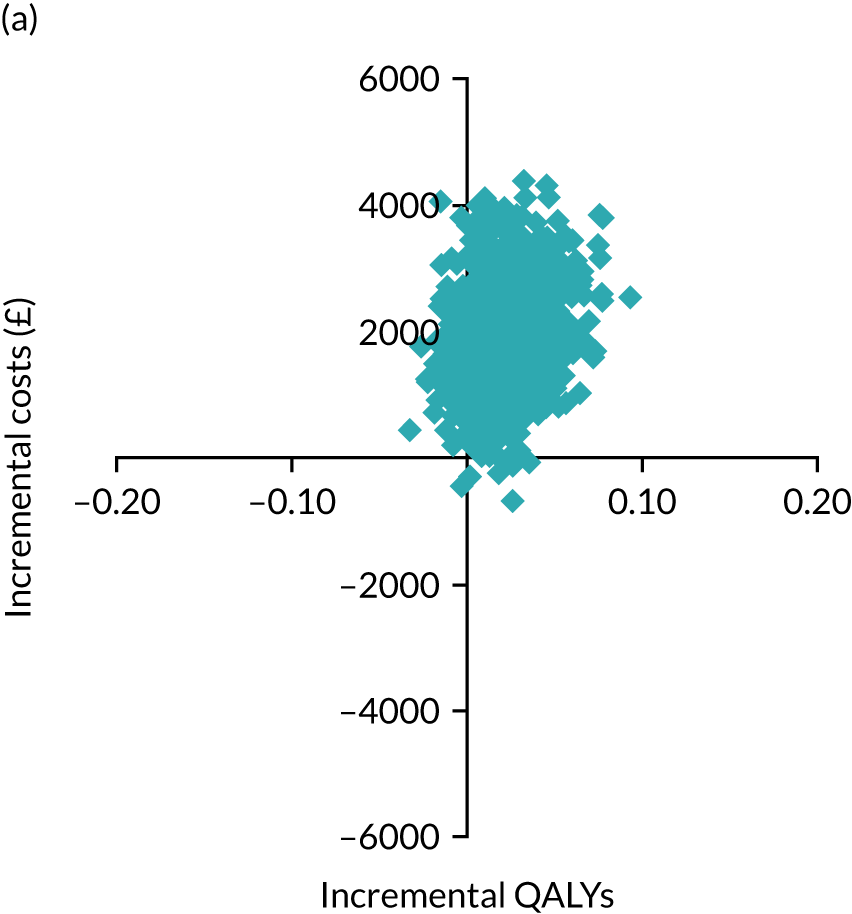
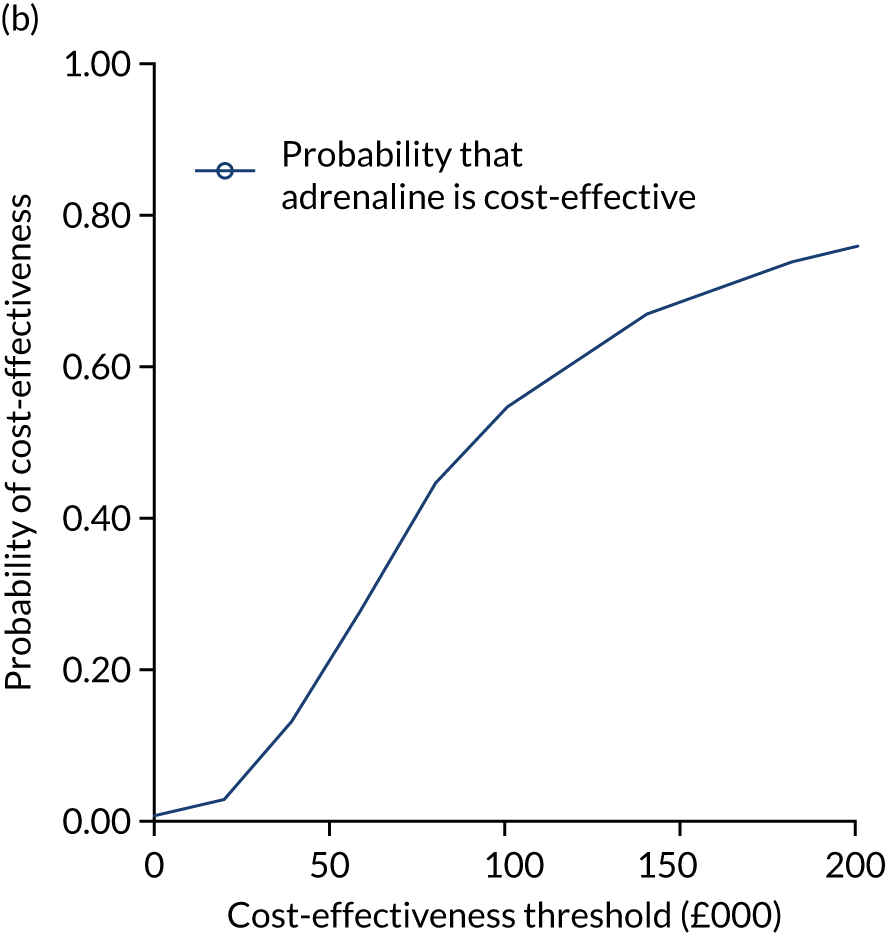
FIGURE 29.
The CEAC from sensitivity analyses assessing the impact of missing-not-at-random assumptions on imputed utilities (QALYs based on mRS data, NHS/PSS costs over 6 months).
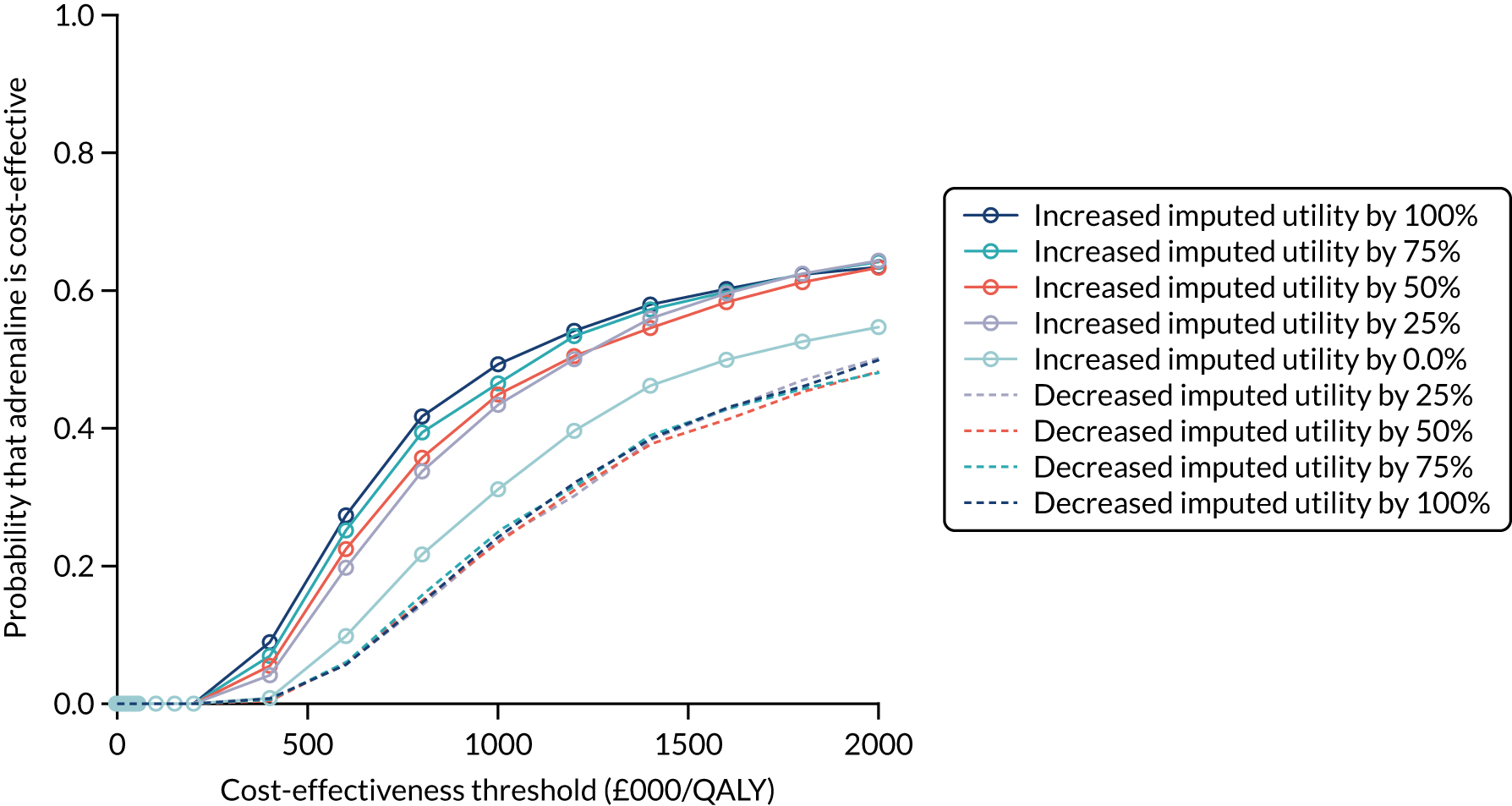
FIGURE 30.
The CEAC from sensitivity analyses assessing the impact of missing-not-at-random assumptions on imputed costs (QALYs based on mRS data, NHS/PSS costs over 6 months).
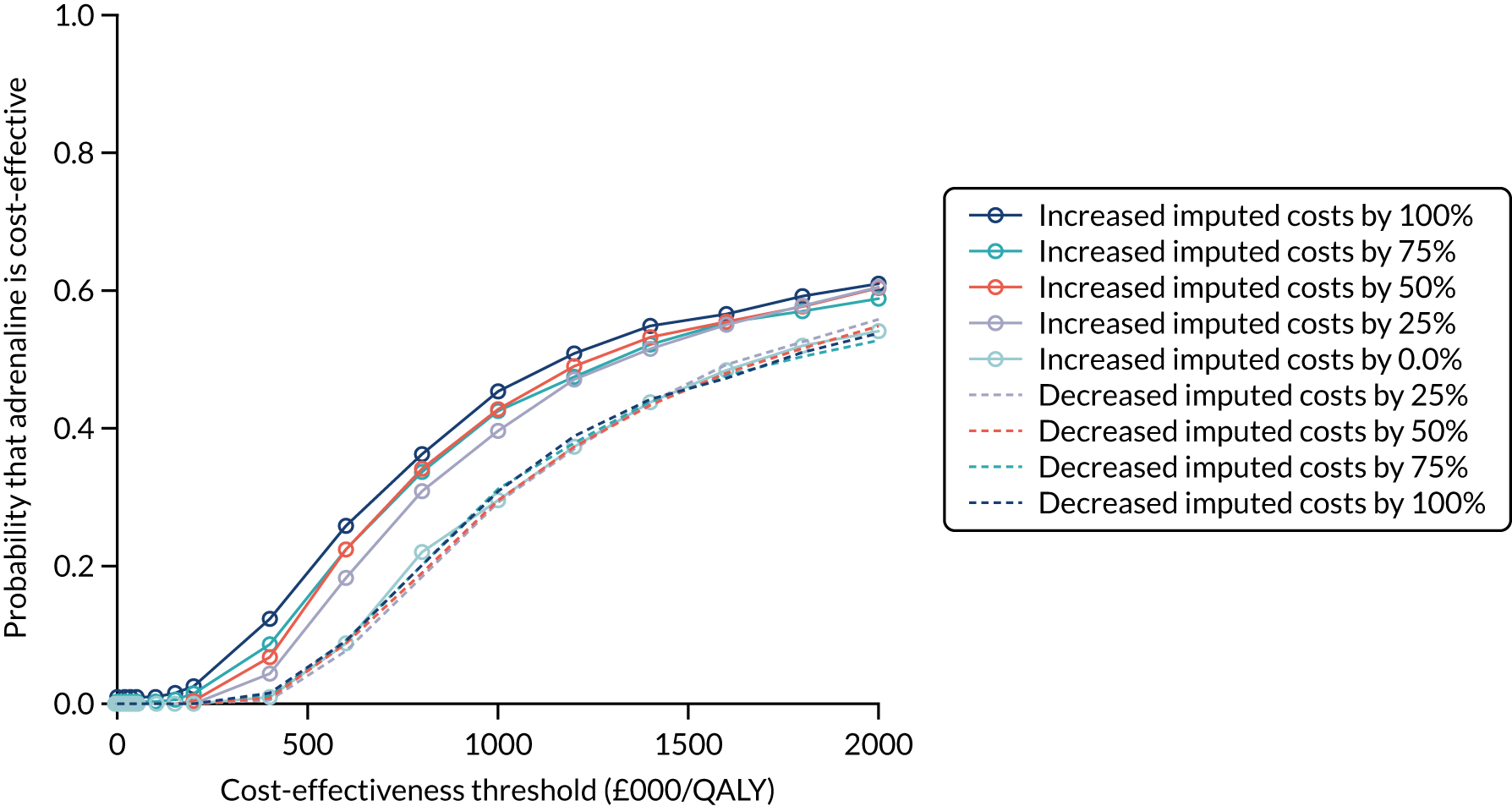
List of abbreviations
- AED
- automated external defibrillator
- ALS
- advanced life support
- aOR
- adjusted odds ratio
- BNF
- British National Formulary
- CEAC
- cost-effectiveness acceptability curve
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CPC
- Cerebral Performance Category
- CPR
- cardiopulmonary resuscitation
- CRF
- case report form
- DMC
- Data Monitoring Committee
- ECG
- electrocardiogram
- eCRF
- electronic case report form
- ED
- emergency department
- EFIC
- exception from informed consent
- EMS
- emergency medical services
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GCP
- good clinical practice
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HES
- Hospital Episode Statistics
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICNARC
- Intensive Care National Audit and Research Centre
- ICU
- intensive care unit
- ILCOR
- International Liaison Committee on Resuscitation
- IMP
- Investigational Medicinal Product
- INMB
- incremental net monetary benefit
- IQCODE
- Informant Questionnaire on Cognitive Decline in the Elderly
- IQR
- interquartile range
- i.v.
- intravenous
- JRCALC
- Joint Royal Colleges Ambulance Liaison Committee
- LTFU
- lost to follow-up
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MICE
- multiple imputation by chained equations
- MMSE
- Mini Mental State Examination
- mRS
- modified Rankin Scale
- NICE
- National Institute for Health and Care Excellence
- OHCA
- out-of-hospital cardiac arrest
- OHCAO
- Out-of-Hospital Cardiac Arrest Outcomes
- OR
- odds ratio
- PACA
- Pre-hospital Adrenaline for Cardiac Arrest
- PAD
- public access defibrillation
- PaRAMeDIC
- Prehospital Randomised Assessment of a Mechanical compression Device In Cardiac arrest
- PARAMEDIC2
- Pre-hospital Assessment of the Role of Adrenaline Measuring the Effectiveness of Drug administration In Cardiac arrest 2
- PCL-C
- Post-traumatic stress disorder Checklist – Civilian Version
- PEA
- pulseless electrical activity
- PEDW
- Patient Episode Database for Wales
- PPI
- patient and public involvement
- PSS
- Personal Social Services
- PTSD
- post-traumatic stress disorder
- QALY
- quality-adjusted life-year
- REC
- Research Ethics Committee
- RFA
- Rankin Focused Assessment
- ROC
- Resuscitation Outcomes Consortium
- ROSC
- return of spontaneous circulation
- RR
- risk ratio
- SAE
- serious adverse event
- SAR
- serious adverse reaction
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-12
- Short Form questionnaire-12 items
- smRSq
- simplified modified Rankin Scale questionnaire
- SUSAR
- suspected unexpected serious adverse reaction
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UKTR
- UK Transplant Registry
- VF
- ventricular fibrillation
- VT
- ventricular tachycardia
- WCTU
- Warwick Clinical Trials Unit
