Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/50/49. The contractual start date was in March 2013. The draft report began editorial review in November 2019 and was accepted for publication in June 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Adamson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
There are > 9000 new cases of oesophageal cancer and 8000 oesophageal cancer deaths in the UK each year. 1 Oesophageal cancer is the seventh most common cause of cancer deaths (the fourth most common in men) and accounts for 5% of the UK total. The majority of cases occur in those aged ≥ 60 years and overall prognosis is poor, with 5-year survival rates of only 15%. 1 Worldwide there are > 450,000 new cases of oesophageal cancer per year and > 400,000 deaths. Over 80% of new cases, and deaths, occur in low- and middle-income nations. 2
The majority of patients present with incurable disease and mean survival in advanced disease is 3–5 months. 3 The emphasis of treatment for most patients is therefore on effective palliation. Between 70% and 90% will require intervention for dysphagia. 4,5 This particular symptom has a profound impact on physical independence, social functioning and other aspects of health-related quality of life. Interventions to improve swallowing must, therefore, aim to produce prompt and lasting palliation of dysphagia while minimising the need for late reinterventions and hospitalisation. Interventions should produce these benefits without causing significant impairment of other aspects of quality of life and be accessible at scale to this patient population.
Evidence base for palliation of dysphagia in advanced oesophageal cancer
Although the optimal palliative intervention remains to be established, oesophageal stenting using self-expanding metal stents (SEMSs) is the most widely used treatment option for providing prompt relief of severe dysphagia. Shenfine et al. ’s6 2005 Health Technology Assessment (HTA) evidence review and prospective study confirmed the efficacy of SEMS placement and also highlighted that delayed complications are common and result in later reinterventions. The pragmatic randomised controlled trial (RCT) randomised 215 patients between SEMS and a variety of non-SEMS treatments. 7 It found that SEMS is an effective one-treatment strategy for palliation of dysphagia but highlighted higher initial pain scores and lower quality of life in the SEMS-treated arm.
Notably, 35% (38/108) of SEMS patients in the study required reintervention, with the risk of stent-related complications increasing over time. The analysis of survival data in Shenfine et al. ’s7 study suggested that there was longer survival in patients treated with non-stent therapies (median survival: non-stent, 172 days; 18-mm SEMS, 86 days; 24-mm SEMS, 94 days; p = 0.04).
The study also confirmed the need for better evidence relating to combination therapies, survival – particularly where treatment combinations are investigated – and more robust evidence on quality of life. A literature review conducted as part of the Shenfine et al. 7 report found that only 0.55% of papers published since 1966 on oesophageal cancer considered quality-of-life issues, despite the inherently palliative nature of treatment options for the majority of patients. It also highlighted that the methodological quality of studies reporting quality-of-life data was very poor.
The two most recent Cochrane systematic reviews8,9 confirm the efficacy of SEMS in providing safe, effective and quicker initial relief of dysphagia when compared with other intervention modalities, such as rigid plastic tube insertion, endoscopic chemical and thermal ablation or external beam radiotherapy/chemoradiotherapy. However, they too highlight a continuous decline in quality of life in SEMS-treated patients, possibly related to poorer pain scores, and that non-stent modalities may be associated with improved survival. Dai et al. ’s9 updated review in 2014 included 3684 patients in 53 RCTs. It reported significant variability in the quality of evidence, with only 25% of studies graded as high quality. 9 In general, studies recruited small numbers and used varied scales and outcomes – which precluded meta-analysis – and few studies provided data on combined interventions. It also reported a lack of well-designed studies containing robust assessment of health-related quality of life (HRQoL) or costs. The conclusion of the review was that no absolute superiority of any intervention was shown, but that combinations of different modalities might provide better treatment outcomes. Like Shenfine et al. 6 and Sreedharan et al. ,8 Dai et al. 9 called for high-quality RCTs of combination treatments, particularly of SEMS with either brachytherapy or external beam radiotherapy (EBRT).
Dai et al. 9 concluded that, of the non-stent interventions, high-dose intraluminal brachytherapy may be considered an appropriate alternative to SEMS, particularly in patients with longer survival. This conclusion was based on two studies comparing SEMS insertion with brachytherapy using validated patient-reported measures of dysphagia and HRQoL. 10,11 SEMS provided more rapid onset of relief. In Homs et al. ’s10 larger study of 209 patients, 18% of patients receiving brachytherapy alone had persistent problems with dysphagia 2–4 weeks after the procedure, compared with none in the SEMS arm. However, dysphagia-free survival was longer in the brachytherapy arm [median of 115 days, compared with 82 days with SEMS; mean difference 33 days, 95% confidence interval (CI) 1 to 64 days; p < 0.05]. There was also evidence in Homs et al. ’s10 study that late upper gastrointestinal (GI) haemorrhage occurred significantly less frequently in the brachytherapy arm than in the SEMS arm. In both studies, other aspects of HRQoL appeared to be more stable in the brachytherapy arm over time.
More recently, Rosenblatt et al. 12 reported a multicentre international RCT of EBRT at a dose of 30 Gy in 10 fractions combined with high-dose-rate intraluminal brachytherapy compared with high-dose-rate intraluminal brachytherapy alone in 219 patients. The study reported an absolute difference in dysphagia relief experience of 16% at 100 days and 18% at 200 days in favour of the combination arm. There was no difference in survival between the two arms. Description of the toxicities was limited, although this was reported as not significantly different between the arms. Importantly, only patients with limited locoregional disease were eligible, limiting the relevance of the finding to the broader population of patients who were receiving a stent.
There have been no large-scale trials combining stent plus brachytherapy. In a single-arm safety study of brachytherapy followed by biodegradable stent placement, Hirdes et al. 13 reported early termination of the study as the safety threshold was exceeded, with 47% of patients suffering major complications. By contrast, Amdal et al. 14 have reported a RCT of brachytherapy following SEMS placement versus brachytherapy alone. However, the trial was closed because of slow recruitment, with only 42 patients randomised and insufficient statistical power to draw robust conclusions.
One prospective multicentre Chinese RCT combining endoluminal radiotherapy and stent in a single modality has been reported by Zhu et al. 15 Compared with conventional stenting (n = 75), patients treated with 125I irradiation stents (n = 73) had longer survival (median 177 days vs. 147 days) and consistently lower dysphagia scores over time, although recurrent dysphagia due to occlusion was not different between arms (28% vs. 27%). No health economic analysis was included and the expertise required for irradiation stent placement and care is not widely available. Tian et al. ’s16 non-randomised study in the same health-care economy suggested that the cost associated with irradiation stents is almost four times that of conventional stents.
Implications for NHS practice
Therefore, the available evidence suggests that SEMS is an appropriate intervention for rapid dysphagia relief in incurable oesophageal cancer, and is widely implemented as first-line management of dysphagia in the NHS in this group. 17
Brachytherapy represents an appropriate alternative to SEMS, particularly in those with longer predicted survival, as, although the onset of dysphagia relief is slower, brachytherapy provides a longer duration of dysphagia relief, improves quality of life over time and requires fewer reinterventions.
However, throughout the UK, experience of and access to brachytherapy is very limited. Indeed, only 2.5% of all cancer patients requiring radiotherapy,18 including those with oesophageal cancer, are treated with brachytherapy. National Oesophago-Gastric Cancer Audits17,19 consistently report that only 15% of English Cancer Alliances treating these patients have access to brachytherapy, with no improvement over a 5-year period, and in the 2017 report17 < 30 brachytherapy episodes were recorded compared with > 2000 stent insertions for palliation. Brachytherapy, therefore, does not currently have a role in the routine palliation of oesophageal cancer dysphagia in NHS settings either alone or as an option for combination with stenting.
The efficacy of SEMS alone, however, is limited by early problems with pain, decline in general aspects of quality of life and later complications, such as haemorrhage and tumour overgrowth. Median time to recurrent dysphagia in stent comparator studies20,21 and in Homs et al. ’s10 SEMS versus brachytherapy study is 11–12 weeks. Reinterventions not only impose a significant burden on NHS resources but also decrease the quality of life and functioning of an unwell, predominantly elderly, population with a median survival of 12–20 weeks. This is consistent with estimations that health-care costs in general in the last year of life account for 20–30% of overall health-care budgets. 22 This is also consistent with data that demonstrate that, among cancer patients, patients with upper GI cancer have high rates of health and social care usage in the last 12 months of life. 23
Research rationale
In line with Cochrane Review research recommendations,8,9 the combination of SEMS with other treatments might reduce costs and patient burden by reducing adverse events and reinterventions at a time when patients are approaching the last weeks of life.
This study was developed in response to a National Institute for Health Research (NIHR) call for research proposals into aspects of palliative care to address uncertainties in the evidence base for interventions combined with SEMS.
In contrast with the lack of availability and higher cost of brachytherapy, EBRT is readily accessible by patients at regional cancer centres across the UK, although its use in the immediate post-stent period has not been rigorously studied.
Only one prospective RCT of EBRT in combination with SEMS versus SEMS alone has been reported. 24 Javed et al. ’s24 single-centre Indian study recruited 84 patients and reported more sustained dysphagia relief in the SEMS plus EBRT group (7 months vs. 3 months) and longer survival (180 vs. 120 days). However, there was no a priori statistical plan described, no power calculation and no reporting of missing data, which resulted in low-quality evidence.
Study aim and objectives
This study aimed to assess whether or not the addition of EBRT reduces the risk of recurrent dysphagia, improves quality of life and reduces health economic and personal burden in patients undergoing SEMS placement.
The Radiotherapy after Oesophageal Cancer Stenting (ROCS) study was funded by NIHR as part of its HTA programme. The study was composed of:
-
a RCT, with internal pilot, in which palliative radiotherapy at a dose of 20 Gy in five fractions or 30 Gy in 10 fractions delivered following SEMS placement was compared with SEMS placement alone
-
an embedded qualitative study to explore patient and carer experience of participating in the randomised study, of receiving the radiotherapy intervention, and of their lived experience of advanced oesophageal cancer and dysphagia
-
an evaluation of the cost-effectiveness of delivering the radiotherapy intervention, and of the resource use associated with advanced oesophageal cancer.
Intervention development
The radiotherapy intervention was intended to reflect current practice in the centres recruiting to this trial, rather than to be closely prescriptive. This would allow the findings to be readily and directly applicable to current UK practice. EBRT is routinely available at regional cancer centres across the UK. For palliation of oesophageal cancer, a radiotherapy course delivering a tumour-absorbed dose of 20 Gy in five fractions or 30 Gy in 10 fractions is generally recommended by the Royal College of Radiologists. 25 The study team suggested a dose of 20 Gy in five fractions, which was the most commonly used dose across the UK at the time of study design,19 with the 30 Gy in 10 fraction dose chosen at the discretion of the treating physician. Recent audit data suggest that both of these doses remain the most commonly used palliative regimens in advanced oesophageal cancer in England, accounting for 63% of palliative radiotherapy delivered. 17
Self-expanding metal stents are the most common intervention used in the UK and internationally for the palliation of malignant dysphagia. Audit data for 2014–16 confirm that they constituted 94.5% of all stents placed for oesophageal cancer dysphagia in England. 17 They can be placed at a single endoscopic or radiological session. There are several designs with a variety of delivery devices and, for this trial, SEMS insertion was undertaken in accordance with standard local protocols. Covered or partially covered metal stents were permitted and the length, type and mode of stent placement were selected by the treating clinician.
Chapter 2 Methods
Study design
The study was designed as a pragmatic RCT of EBRT in addition to stent insertions versus stent insertion alone. Participants were those clinically assessed as requiring stent insertion for relief of dysphagia caused by inoperable oesophageal cancer. An internal pilot was included to assess rates and methods of recruitment as it was expected that the trial would be challenging in studying a palliative population approaching the last months of life.
To ensure a multiperspective analysis of efficacy and address previously highlighted evidence gaps, the trial also included an embedded qualitative study and a health economics component to interrogate the cost-effectiveness of radiotherapy intervention, and to capture in detail health and social care resource use across both arms of the trial.
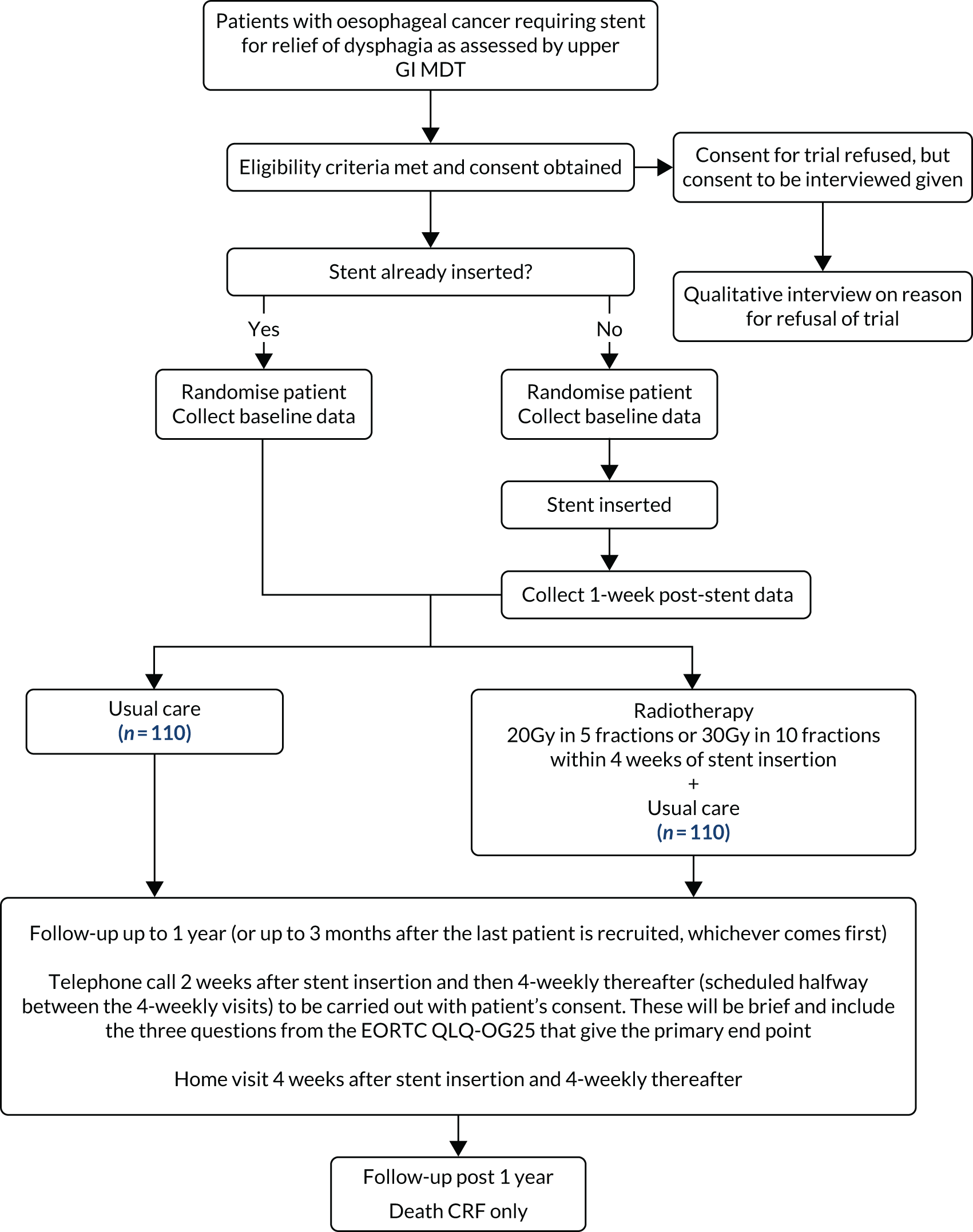
A description of the trial protocol has previously been published. 26 The trial schema is detailed in Figure 1. A full summary of protocol amendments is included in Table 1.
FIGURE 1.
Trial schema showing randomisation and follow-up. CRF, case report form; EORTC QLQ-OG25, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire oesophago-gastric module; MDT, multidisciplinary team.
| Change to protocol | Date |
|---|---|
| Exclusion criterion removed: planned endoscopic treatment of the tumour (e.g. laser) in the immediate peri-stenting period | April 2013 |
| Inclusion criterion added: patient has completed baseline QLQs added in the inclusion criteria | April 2013 |
| Companion PIS and consent form introduced in the qualitative study | September 2014 |
| Dedicated face-to-face follow-up specified as preferred to ensure optimum support for patients; telephone or postal follow-up for questionnaire completion also permitted depending on patient’s choice | September 2014 |
| Additional level of withdrawal added: option for participants to stop home visits and questionnaires but follow-up | September 2014 |
| Qualitative study added interviews with research nurses responsible for recruiting patients to the trial | September 2014 |
| Randomisation allowed within 2 weeks after stent insertion, but preferably within 1 week of stent insertion | April 2015 |
| Baseline assessments for those patients consented following stent insertion, ideally baseline assessments will occur within 1 week, but not > 2 weeks, following the procedure | April 2015 |
| Clarification of time zero owing to consent also possible after stent insertion | April 2015 |
| Secondary outcome added: determine the haemostatic effect of radiotherapy on tumour bleeding | March 2016 |
| Inclusion criterion of oesophageal carcinoma widened to include clinical and/or radiological evidence of invasive tumour (as agreed by MDT consensus) and at least high grade dysplasia of a non-small cell type on histology | March 2016 |
| Interim telephone calls introduced 2 weeks after stent insertion and 4-weekly thereafter to be scheduled halfway between the 4-weekly assessment visits | March 2016 |
| Dysphagia card was introduced with a list of questions asked during the telephone calls | March 2016 |
| Follow-up until death reduced to 1 year, follow-up post 1 year is death CRF only | March 2016 |
| Primary outcome amended: assess the impact that radiotherapy has in addition to stent placement on time to progression of patient-reported dysphagia or other dysphagia-related event in a patient population unable to undergo surgery | February 2017 |
| Final accrual reduced from original 496 to 220 | December 2017 |
| Primary outcome amended: to assess the impact that radiotherapy has in addition to stent placement on difference in event rate of patient-reported dysphagia or other dysphagia-related event at 12 weeks following stent insertion in a patient population unable to undergo surgery | December 2017 |
| Originally it was intended to perform a time-to-event analysis. However, in collaboration with the IDMC in response to recruitment difficulties, the primary outcome analysis has been amended and will now be based on proportion of events at week 12. An event is defined as a progression in self-reported dysphagia (see above) or other dysphagia-related event | |
| Follow-up for 1 year or up to 3 months after the last patient is recruited, whichever comes first | December 2017 |
| New secondary outcome – measure hospital admission rates | December 2017 |
Ethics approval and research governance
Ethics approval for the study was given by the Wales Research Ethics Committee 2 in October 2012 (reference number 12/WA/0230). Global Research and Development approval was given in January 2013. The trial was registered with the International Standard Randomised Controlled Trial Registry (ISRCTN) under the reference number ISRCTN12376468 and also with Clinicaltrials.gov under the reference number NCT01915693.
Participants
The study recruited patients referred for an oesophageal stent as primary palliation for advanced oesophageal cancer dysphagia. Patients were recruited from 23 cancer centres and acute hospitals across Scotland, England and Wales (see Appendix 1). Recruitment was limited to five sites in the first 18 months to allow for the embedded pilot phase and review.
Inclusion criteria
Patients were considered for inclusion if they:
-
had oesophageal carcinoma with either of the following –
-
histological confirmation (excluding small cell carcinoma)
-
clinical and/or radiological evidence of invasive tumour [as agreed by multidisciplinary team (MDT) consensus] and at least high-grade dysplasia of a non-small cell type on histology
-
-
were not suitable for radical treatment (oesophagectomy or radical chemoradiotherapy) because of either patient choice or medical reasons
-
had dysphagia clinically assessed as needing a stent insertion as primary treatment of the dysphagia
-
were aged ≥ 16 years
-
had a treatment decision made by discussion with an upper GI MDT for stent insertion
-
were deemed suitable for radiotherapy
-
had an expected survival of at least 12 weeks
-
could provide written informed consent
-
had completed baseline quality-of-life questionnaires (as a minimum patients had to have completed the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ) oesophago-gastric module (OG25).
Exclusion criteria
Patients were not considered for exclusion if they:
-
had small-cell carcinoma
-
had tumour length of > 12 cm
-
had tumour growth within 2 cm of the upper oesophageal sphincter
-
had endoscopic treatment of the tumour, other than dilatation, planned in the peri-stent period
-
had a tracheo-oesophageal fistula
-
had a pacemaker in proposed radiotherapy field
-
had previous radiotherapy to the area of the proposed radiotherapy field
-
were pregnant.
Recruitment procedure
Patients were identified in secondary care by their treating clinician or by members of the local upper GI MDT. Patients were then approached prior to stent insertion by the local research nurse, who introduced the ROCS study, provided them with a participant information sheet (PIS) and completed eligibility checks. The research nurses received specific training on information provision to include the radiotherapy intervention and the consent process.
Comprehensive screening log data were collected throughout the trial to track proportions of patients who were eligible, approached and randomised. This was used to feed back to participating centres, the Trial Management Group (TMG), the Independent Data Monitoring Committee (IDMC) and the funding body throughout the duration of the study.
During the trial pilot phase, screening logs demonstrated that the number of patients requiring stent insertion was as predicted by the trial team and that the 64% acceptance rate was above the 50% initially predicted. However, eligibility was 33% compared with the 70% predicted. The reasons for ineligibility included previous radiotherapy and capacity to consent issues. The screening data also highlighted that a significant number of patients were being stented before trial teams became aware (14%). Data from the qualitative study additionally identified individual patient distress at being approached prior to stenting. Together these data resulted in a protocol amendment to allow randomisation up to 2 weeks after stent insertion, and eventually 66% of the overall 220 patients were randomised post stent insertion. Following review of the comprehensive pilot data, the funder approved trial continuation and the number of centres was increased.
Informed consent
Once trial eligibility was confirmed, informed written consent was obtained after a full explanation had been given and further questions answered. The consent was taken by an appropriately trained research nurse or delegate. The original signed and dated consent forms were held securely as part of the trial site file, with a copy provided to the participant. Patients and informal caregivers were consented separately for the embedded qualitative study by the qualitative researcher.
Patients were also asked to consent to NHS Information Centre flagging so that the date and cause of death could be collected without longer-term follow-up. This was optional and additional to the standard informed consent.
Randomisation and concealment
Eligible and consenting participants were randomised centrally via the Wales Cancer Trials Unit [now the Centre for Trials Research (CTR), Cardiff University] randomisation telephone line using an online trial management database with a manual backup available. The outcome of the randomisation procedure was communicated to the participant by the research nurse together with details of the allocated treatment. As indicated, the initial protocol stipulated randomisation prior to stent insertion, but was amended to allow post-stent randomisation after the pilot phase.
Participants were randomised to a trial arm using the method of minimisation with a random element (80 : 20). Minimisation was stratified to ensure balanced allocation for a number of potential confounding factors: centre, stage at diagnosis (I–III vs. IV), histology (squamous or other) and MDT intent to give chemotherapy (yes or no). Randomisation was carried out using a 1 : 1 allocation ratio.
Study interventions
Self-expanding metal stents: both arms
Following the decision by the MDT to proceed with stenting as the primary treatment for oesophageal cancer-related dysphagia, stent insertion was performed as per the standard procedures of the treating centre. The length and type were determined and recorded by the treating clinician. It was advised that, where possible, the length of stent should be chosen to ensure that at least 2 cm of normal oesophagus was covered by the stent above and below the tumour stricture. The following were also recorded: whether the stent was inserted under sedation or general anaesthetic, whether dilatation was required before or after stent insertion and whether or not radiological imaging was used.
Patients who were offered any endoscopic treatment of the tumour, other than oesophageal dilatation used as part of the centre’s normal procedure for stent insertion, were excluded from the study, unless an emergency required such procedure. Use of such procedures was recorded on the case report form (CRF). Patients in whom brachytherapy or EBRT was planned routinely for after stent insertion were also excluded.
External beam radiotherapy trial arm: intervention
The study protocol mandated that the radiotherapy begin within 4 weeks of stent insertion and preferably within 2 weeks. Radiotherapy treatment was delivered to the primary tumour and significant treatable lymphadenopathy, as defined by the treating oncologist. Treatment dose was either 20 Gy in five fractions over 1 week or 30 Gy in 10 fractions over 2 weeks using daily fractionation and the centre’s normal radiotherapy treatment procedures. The 20 Gy in five fractions regimen was the preferred option but the dose and fractionation schedule were chosen by the treating clinical oncologist.
Patients were withdrawn from the trial if they missed > 7 consecutive calendar days during radiotherapy treatment and any further treatment given was at their treating clinician’s discretion. In the unlikely event of radiotherapy side effects severe enough to interfere with treatment delivery, the treating clinician had the option to temporarily stop treatment and allow a break of no more than 7 calendar days prior to recommencement.
Radiotherapy quality assurance
Radiotherapy quality assurance was carried out by the Cardiff National Cancer Research Institute (NCRI) Radiotherapy Trial Quality Assurance (RTTQA) Group. The ROCS radiotherapy quality assurance group consisted of a radiation oncologist and radiotherapy physicist from the Cardiff NCRI RTTQA Group, who gave information and guidance regarding implementation of the protocol, monitored compliance with the protocol and, where necessary, provided feedback on the radiotherapy quality assurance accreditation.
Pre-trial quality assurance
A process document containing information on set-up, verification and beam arrangement was required from all radiotherapy sites prior to being opened to recruitment. This was reviewed by the ROCS radiotherapy quality assurance group.
On-trial quality assurance
Following entry of the first patient into the trial at each radiotherapy treatment site, a set of computerised tomography (CT) images or simulator images, together with information concerning the treatment fields (Digital Imaging and Communications in Medicine-RadioTherapy file or hard copy) and treated volumes, were requested to ensure compliance.
Data collection and management
Pre-stent and 1 week post stent insertion
Patients randomised prior to stent insertion were seen before stenting for a pre-stent assessment at which the following data were collected: World Health Organization (WHO) performance status, questionnaires, EORTC QLQ-C30, EORTC QLQ-OG25, EuroQol-5 Dimensions, three-level version (EQ-5D-3L), Common Terminology Criteria for Adverse Events (CTCAE) v4.03. See Appendix 2 for the QLQs.
All patients, including those randomised post stent insertion, were seen 1 week post stent insertion, when the above data were collected, in addition to stent morbidity data, bleeding and transfusion episodes, and resource use. This formed the baseline against which future deterioration was measured.
Follow-up
Every 4 weeks after the 1-week post-stent insertion assessment, and until death, the following assessments were conducted and data were collected: WHO performance status, questionnaires (EORTC QLQ-C30, EORTC QLQ-OG25, EQ-5D-3L), toxicity assessment (CTCAE), stent morbidity data, bleeding and transfusion episodes, and resource use. Serious adverse events (SAEs) were monitored in real time.
The funded study included costs for additional research nurse time to allow all follow-up visits to occur in the home setting, or place of patient choice. The aim was to minimise the burden of study processes for patients and their families and to maximise data capture in participants who were at an advanced stage of illness. The challenges of capturing self-reported health data from patients with poor health and life expectancy are well documented,27 with recommendations for dedicated research staff collecting the quality-of-life (QoL) data via home visits where possible. 10 Face-to-face follow-up was preferred in our trial to ensure optimum support for patients in completing assessments and to minimise disruption for them; however, where patients specifically declined face-to-face follow-up visits but expressed a preference to have telephone or postal follow-up for questionnaire completion, follow-up assessments were undertaken in this way.
Information was captured in the CRFs on whether or not patients required support from research practitioners or family members/informal carers to complete patient-reported outcome questionnaires and reasons for missing data were also recorded.
As part of their regular data reviews, the IDMC highlighted that, despite excellent CRF returns, there were missing data owing to participants becoming too frail to complete questionnaires or dying without the ROCS primary outcome data being collected. Subsequently, the study protocol was amended in consultation with the research nurse teams to introduce interim telephone calls to participants scheduled halfway between the 4-weekly follow-up visits, aiming to ensure that dysphagia deterioration was assessed more frequently and that data were available in all patients. The telephone call assessments were brief and included the specific OG25 dysphagia questions only. For ease of call administration, participants were given a dysphagia card with details of the three questions that they were expected to answer.
Trial and data management
Paper-based CRFs were completed at sites within 4 weeks of the follow-up visit and a copy sent to the CTR for clinical database entry in MACRO Electronic Data Capture (InferMed, London, UK) version 4.9. A range of data validation checks were carried out to minimise erroneous and missing data throughout the trial. These consisted of checks programmed into MACRO Electronic Data Capture and more complex consistency checks (central monitoring) programmed in Stata® (StataCorp LP, College Station, TX, USA) version 16 (e.g. comparing toxicities reported on CRFs with toxicities reported through the SAE pharmacovigilance system). Data cleaning was an ongoing process and central monitoring was conducted prior to IDMC meetings and to commencing the final data analysis. Where central monitoring highlighted concerns at particular centres, site monitoring could be triggered and source data verification performed.
The TMG met once every 3 months to review screening and recruitment data and trials processes and receive Trial Steering Committee (TSC) and IDMC reports and advice. They oversaw all reporting and governance responsibilities and advised on all amendments to the protocol. The TSC and IDMC met once every 6 months to review trial progress and safety.
Investigator meetings were planned on a 6-monthly basis to facilitate peer support and motivate recruitment among principal investigators and particularly research nurses. The meetings allowed identification of both generic and site-specific trial process issues, sharing of good practice, and feedback from the qualitative researcher on patient experiences of being approached, informed about and consented into the trial. The knowledge and experience of the research nurses and the qualitative patient feedback resulted in real-time changes to, and improvements in, the protocol and data capture including:
-
changes to CRF design and content
-
changes to the wording of the PIS and description of the intervention by research nurses
-
changes to the timing of randomisation to include after stent placement
-
the inclusion of ‘between-visit’ telephone calls to capture primary outcome data
-
an additional level of withdrawal to allow ongoing case note data capture
-
the inclusion of research nurse interviews regarding non-consent.
Outcome measures
Primary outcome
The primary outcome in this trial was a revised binary measure of deterioration in dysphagia symptoms, or death, by 12 weeks.
Patient-reported dysphagia was measured at each time point using the EORTC QLQ-OG25,28 which amalgamates the widely used EORTC scales to assess HRQoL in patients with oesophageal and gastric cancer. 29,30 In the earlier EORTC scales, problems with the validity of the dysphagia scale were noted, with patients finding the response categories confusing. The EORTC QLQ-OG25 resolved this issue and combined both oesophageal and gastric modules to ensure that HRQoL issues relevant to both groups of patients and that patients with oesophagogastric (junctional) tumours were included. The questionnaire has six scales; the dysphagia scale is scored from 0 to 100 and a change of 10–15 points in mean score is considered to be clinically significant. 31
Relief of dysphagia is expected in the majority of participants following stent insertion so the dysphagia score taken 1 week after stent insertion (prior to EBRT in treatment arm) formed the time zero measurement for the main end point of the study. A worsening in score of 11 compared with time zero at any subsequent time point was taken as deterioration. However, it is possible that patients undergoing radiotherapy may have a temporary worsening of dysphagia secondary to radiation-induced oesophagitis and other temporary changes may occur. To ensure that EBRT did not bias the primary outcome, definitive deterioration in dysphagia was defined as an 11-point change on two consecutive occasions, with the first being taken as the event time point.
To ensure that all primary events were captured, all CRF data related to toxicities and SAEs were reviewed to identify any additional dysphagia-related primary events that may have occurred between assessments. These events were reviewed blindly by the two co-chief investigators and by a gastroenterologist independent of the study (see Primary analysis).
Secondary outcomes
Overall survival
Notification of death was collected and overall survival (OS) was calculated from the date of stent insertion to the date of death from any cause.
Dysphagia deterioration-free survival
Dysphagia deterioration-free survival (DDFS) was calculated from the date of stent insertion to the date of deterioration in dysphagia symptoms (as per the primary outcome definition).
Health-related quality of life
Quality of life was measured using the EORTC QLQ-C30, EORTC QLQ-OG25 and EQ-5D-3L at the specified assessment time points.
Health-related quality of life was assessed using generic instruments: the EORTC QLQ-C-30, which assesses global QoL, functional domains (physical, emotional, social, role and cognitive) and symptoms (fatigue, nausea and vomiting, pain, dyspnoea, insomnia, appetite loss, constipation, diarrhoea and financial difficulty) that commonly occur in patients with cancer; and the EORTC QLQ-OG25. Both tools (the EORTC QLQ-C30 and the EORTC QLQ-OG25) were employed, as validation of the EORTC QLQ-OG25 demonstrated that it measures separate HRQoL issues and it is likely that dysphagia accounts for only a proportion of the impact on QoL. 32 All of the scales and single-item measures range in score from 0 to 100; a higher score represents a higher (‘better’) level of functioning, or a higher (‘worse’) level of symptoms.
The prespecified main patient-reported outcome items that were identified a priori to be of relevance were the global health score from the EORTC QLQ-C30 and four scales from the EORTC QLQ OG25 questionnaire: odynophagia, pain/discomfort, eating restrictions and eating in front of others.
Toxicity
Toxicity data were scored using the National Cancer Institute CTCAE at baseline, during treatment and at the prespecified time points during follow-up.
Morbidity
Tumour bleeding
Upper GI bleed events were confirmed by the chief investigators, who were blinded to the study arm, and reviewed by an independent gastroenterologist. These included blood transfusion, haematemesis, upper GI haemorrhage or bleed, melaena and interventions related to bleeding (e.g. argon plasma coagulation or additional radiotherapy). If there was no clinical evidence that anaemia was due to a bleed, then it was not considered.
Dysphagia-related stent complications and reinterventions
Stent complications were defined as re-stenting, repeat endoscopy, overgrowth of stent, undergrowth of stent, stent blockage, stent fracture and stent slippage. Reinterventions were defined as additional stent insertion, stent removal, endoscopic intervention (including laser therapy and alcohol injection) and other palliative radiotherapy (including brachytherapy and additional EBRT for dysphagia).
Stent-related pain
A stent-related pain event was defined as grade 2+ stent-related pain reported on the toxicity CRF.
Cost-effectiveness
The economic valuation was in the form of a cost–utility analysis (CUA) assessing total costs against differences in health outcome expressed as quality-adjusted-life-years (QALYs), with utilities derived from the EQ-5D-3L questionnaire responses. 33–35 EQ-5D-3L has been used previously on patients with inoperable oesophageal cancer. 7 CUA is the health economic method preferred by the National Institute for Health and Care Excellence (NICE). 33 In line with NICE guidance, the analysis was undertaken from an NHS and Personal Social Services perspective. Details of the health economic evaluation are reported in Chapter 5.
Patient perspectives
The embedded qualitative study was designed to explore the feasibility of patients’ recruitment to the trial by examining their experience of consent and recruitment, to highlight the reasons for non-consent and to examine patients’ motivation to accept randomisation to an intervention that may include extra radiotherapy. It also provided the opportunity to understand patients’ experience of living with advanced oesophageal cancer and dysphagia and how they negotiated interventions – particularly in terms of trade-offs between perceived burdens and benefits. The qualitative methodology is described in detail in Chapter 4.
Sample size
Original sample size justification based on time to event
In a population with a median survival of approximately 4 months, an increase in median time to deterioration in self-reported dysphagia of 4 weeks was considered clinically meaningful. This was based on previous results10 and expert multidisciplinary clinical and service user opinion. Sample size was therefore originally calculated, based on a time-to-event analysis, to detect an increase in median time to deterioration in self-reported dysphagia of 4 weeks: from 12 to 16 weeks [equivalent to a hazard ratio (HR) of 0.75 and a difference in 12-week event rate of 50% vs. 60%]. For 80% power with alpha = 0.05 based on a two-sided log-rank test, 198 patients per arm would be required: 396 in total, which is a total of 384 events. Assuming 20% attrition, a total of 496 participants would be required.
Time to event would be calculated from the time of stent insertion to the time of deterioration or death. Patients who did not achieve an improvement from the pre-stent measure of at least 11 points31 on the OG25 dysphagia subscale at the first week assessment after stent insertion (time zero) would be included and followed up but assumed to have failed at time zero. Those who were deterioration free and alive would be censored at the time last seen.
Revised sample size justification based on proportions of events at 12 weeks post stent
In view of the challenging nature of the study in recruiting a palliative population towards the end of life, the IDMC undertook 6-monthly reviews of recruitment and data returns. Although the consent rate continued to approach the predicted rate of 50%, the proportion of eligible patients remained significantly lower than originally predicted (43.6% vs. the 70% predicted). Missing data also increased significantly beyond 12 weeks, despite high CRF returns, reflecting the increasing frailty of the patient population. In view of these combined issues the IDMC recommended a revised sample size calculation based on comparison of proportions with a dysphagia event at week 12. To reduce this proportion from 40% to 20% required 164 patients (80% power, 5% alpha two-sided). This difference in proportions is in line with the difference sought in other studies of stent or non-stent interventions for malignant dysphagia. 9,12,36 To allow for a 25% loss to follow-up required 220 patients to be randomised. This is equivalent to the largest sample size recruited into intervention RCTs for advanced oesophageal cancer dysphagia. The changes were approved by the independent TSC and ratified by the funder following independent review.
Statistical analysis
Analysis and reporting of this trial were undertaken in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines. 37 All statistical analyses were performed using Stata following a predefined statistical analysis plan agreed with the IDMC. The modified intention-to-treat (ITT) population was defined as all patients who had a stent inserted and returned a baseline EORTC QLQ-OG25 (OG25) questionnaire. The per-protocol population was defined as the subgroup of the modified ITT population that was alive and who had not withdrawn from trial treatment at 4 weeks post stent insertion, that was not found to be ineligible, had no protocol deviation or other reason for exclusion and (in the radiotherapy arm) received at least one fraction of radiotherapy.
Primary analysis
Analysis of the primary binary end points of deterioration in dysphagia symptoms by 12 weeks was primarily conducted in the modified ITT population with complete-case data. Complete cases were defined as having complete data for the dysphagia subscale of the EORTC QLQ-OG25 questionnaire at time 0, week 4, week 8 and week 12 or having died with complete data prior to week 12. Single missing time points were permitted under the rules described below.
Primary end point events were based on:
-
Two consecutive deteriorations in patient-reported dysphagia score.
-
One deterioration and no more data possible (i.e. patient withdrew completely or died before next visit).
-
One deterioration, missing next visit; withdrew or died within 4 weeks of missing visit.
-
Additional dysphagia-related primary events (additional stent insertion, dysphagia on clinical assessment or documented as reason for hospital admission, overgrowth or undergrowth of stent, grade 3+ dysphagia on toxicity form or SAE or additional radiotherapy to oesophagus/stent region). All additional primary events were assessed blindly and confirmed by the chief investigators and reviewed by an independent gastroenterologist, as a dysphagia-related event.
-
Death prior to week 12.
In the absence of a documented dysphagia-related event, missing dysphagia scores between two non-event dysphagia scores were assumed to be no event.
Multivariate logistic regression was used to adjust for randomisation stratification factors and the adjusted odds ratio (OR) was presented along with 95% CIs and p-values for the primary analysis and all sensitivity analyses. Where there were fewer than five patients per centre, these patients were combined into a new centre to ensure that all patients’ data were used in the adjusted model. Three sensitivity analyses were performed:
-
using the same complete-case population but treating death by 12 weeks without prior deterioration as no deterioration
-
imputing missing data using a best-case scenario that assumed no deterioration in a missing OG25 form immediately prior to an OG25 form that showed deterioration (or additional primary event confirmed by the chief investigator), or missing OG25 data immediately prior to death
-
imputing missing data using a worst-case scenario that assumed deterioration in a missing OG25 form immediately prior to an OG25 form that showed deterioration (or additional primary event confirmed by the chief investigator), or missing OG25 data immediately prior to death.
Additional sensitivity analyses of the main results and the three sensitivity analyses above were performed in the per-protocol population.
Secondary analyses
Overall survival, follow-up and dysphagia deterioration-free survival
Overall survival and DDFS were analysed by time-to-event methods (Kaplan–Meier curves and Cox regression), with those without events being censored at time last seen. The Cox regressions were adjusted for randomisation stratification factors with treating centre also included as a shared frailty. For those with uncensored data, the reverse Kaplan–Meier approach was used to present follow-up post-stent insertion in each arm. DDFS events were calculated as per the method used for the primary end point above. As a sensitivity analysis, the DDFS analysis was repeated, but data were censored at death with no prior dysphagia event.
Quality of life and World Health Organization performance status
All EORTC QLQ-C30 items were scored for each patient according to the EORTC QLQ-C30 scoring manual. 38 Data were imputed according to EORTC guidance if fewer than half of the items in a scale were missing. Where data were missing from more than half of the items in any scale, these scales were excluded from the analyses. When a complete questionnaire was missing, the reason for the missing questionnaire was ascertained and categorised. The same methods were used for analysing the QoL data and the WHO performance status scores.
The distribution of the baseline scores was tested for normality using kernel density plots, normal probability plots, normal quantile plots and the Shapiro–Wilk test to determine which graphical method to use to display scores over time [median and interquartile range (IQR) if not normal, mean and 95% CIs if normal]. Questionnaires that were returned within 2 weeks (plus or minus a specified time point) were selected for each time point measure.
Linear mixed models were used to compare differences between trial arms for each subscale or single item. Time was included as a categorical covariate using the week of observation from 0 (time zero) to 16, after which the proportion of missing data became too high. If an intermediate value was missing, the corresponding time was skipped. Covariates included trial arm, age, time zero score and randomisation stratification factors. For each subscale or single item, the interaction of treatment by time of assessment was tested.
To assess goodness of fit of the linear mixed models, the fixed effects for determining the selection of linear mixed model for each subscale or item were evaluated using Akaike information criterion (AIC) and Bayesian information criterion (BIC) and the model with the lowest AIC and BIC is presented.
Morbidity
The same analytical method was used for the following morbidity events:
-
upper GI bleed events
-
dysphagia-related stent complications and reinterventions
-
additional stent insertion
-
repeat endoscopy
-
overgrowth or undergrowth of stent
-
stent-related pain.
Time to first morbidity event was compared between trial arms using competing risks regression, with death as a competing risk, adjusted for randomisation stratification factors and cumulative incidence functions plotted by trial arm.
Missing data
The potential for missing data was significant in this trial given the study population. Plans to reduce the number and impact of missing data centred on:
-
maximising data capture
-
handling of missing data
-
analysis and reporting.
Significant efforts were made to reduce the number of missing data. As described, they included additional research nurse time to collect all follow-up data at home, multidisciplinary input to trial and CRF design to optimise the type and number of data captured and reasons for missing data, implementation of between-visit telephone calls, and an additional level of withdrawal to allow continued case note data capture.
The handling and investigation of the potential influence of missing data through sensitivity analyses are described in Primary analysis and in Chapter 3.
Patient and public involvement
Aim
The involvement of patients and members of the public as research partners was underpinned by a comprehensive strategy within the CTR and the Marie Curie Research Centre (MCRC) at Cardiff University, which was responsible for the conduct of the main trial and the embedded qualitative component. The ROCS study had research partner involvement from the initial concept and outline design phase, through study delivery as part of the TMG and as a core component of the dissemination phase. There were specific objectives in relation to:
-
research partner advertisement and recruitment onto the TMG by interview
-
research partner training and integration in to the trial team and TMG
-
ongoing support and assessment of impact of the contribution of the research partners
-
involvement in publications and dissemination plan.
Methods
One member of the public, Jim Fitzgibbon, had a lead role for development of patient and public involvement (PPI) strategy in the CTR alongside Annmarie Nelson as academic lead for PPI. Together they developed the strategy for PPI in the trial. They developed a role description and bespoke recruitment and training plans. An additional member, Stephen Thomas, was appointed following successful grant capture and set-up of the TMG.
Support
Research partners were introduced to, and integrated into, the trial team. Both research partners became familiar with the trial unit environment, and trial staff received training on the importance of the research partner role and specific aspects of support. Reimbursement of expenses was offered, as were honoraria for their time, in keeping with the local strategy for research partner support.
Impact
Both lay members of the TMG were fully involved as the trial progressed. Particular areas where they had an impact were:
-
Jim Fitzgibbon was involved in initial trial design and grant submission as co-applicant, as well as the protocol publication.
-
Jim Fitzgibbon and Stephen Thomas directly influenced protocol design and subsequent amendments, particularly in relation to patient-reported outcomes and recruitment strategy.
-
Jim Fitzgibbon and Stephen Thomas directly supported development of trial materials, particularly the PIS and consent form, and the formatting and content of CRFs.
-
They made sure that the trial was conducted in a participant-friendly and ethically acceptable way, which included changes in timing of consent, the introduction of a patient card containing the primary outcome dysphagia questions in support of telephone data capture, and introduction of additional 2-weekly telephone assessments between the scheduled 4-weekly face-to-face assessments.
-
They were involved in the interpretation and reporting of the trial results for this monograph.
It is anticipated that they will also be involved in:
-
disseminating results through publication, UK and international presentations, and knowledge transfer through national and regional clinical and academic organisations and patient groups.
Conclusions and reflections
The involvement of our lay research partners was core to the successful development and implementation of the ROCS study. Their inputs have had an impact throughout the trial and have supported wider integration of research partners in the trials unit environment. Although the involvement predates the NIHR standards, we followed forefront processes in recruitment, training and support. Although it has been possible to describe and list some of the impact that research partners have had, we recognise the need in future to utilise more considered and timely ways of recording it and to undertake a ‘study within the study’ of how best to capture and describe research partner impact. Work is proceeding in the CTR to develop a practical system and tool to enable this to happen.
Chapter 3 Results
Recruitment and randomisation
A total of 220 participants were recruited to the ROCS study by 23 centres (see Appendix 1) between 16 December 2013 and 24 August 2018. In total, 112 participants were allocated to the usual-care arm and 108 participants were allocated to the EBRT arm.
Flow of participants through the trial
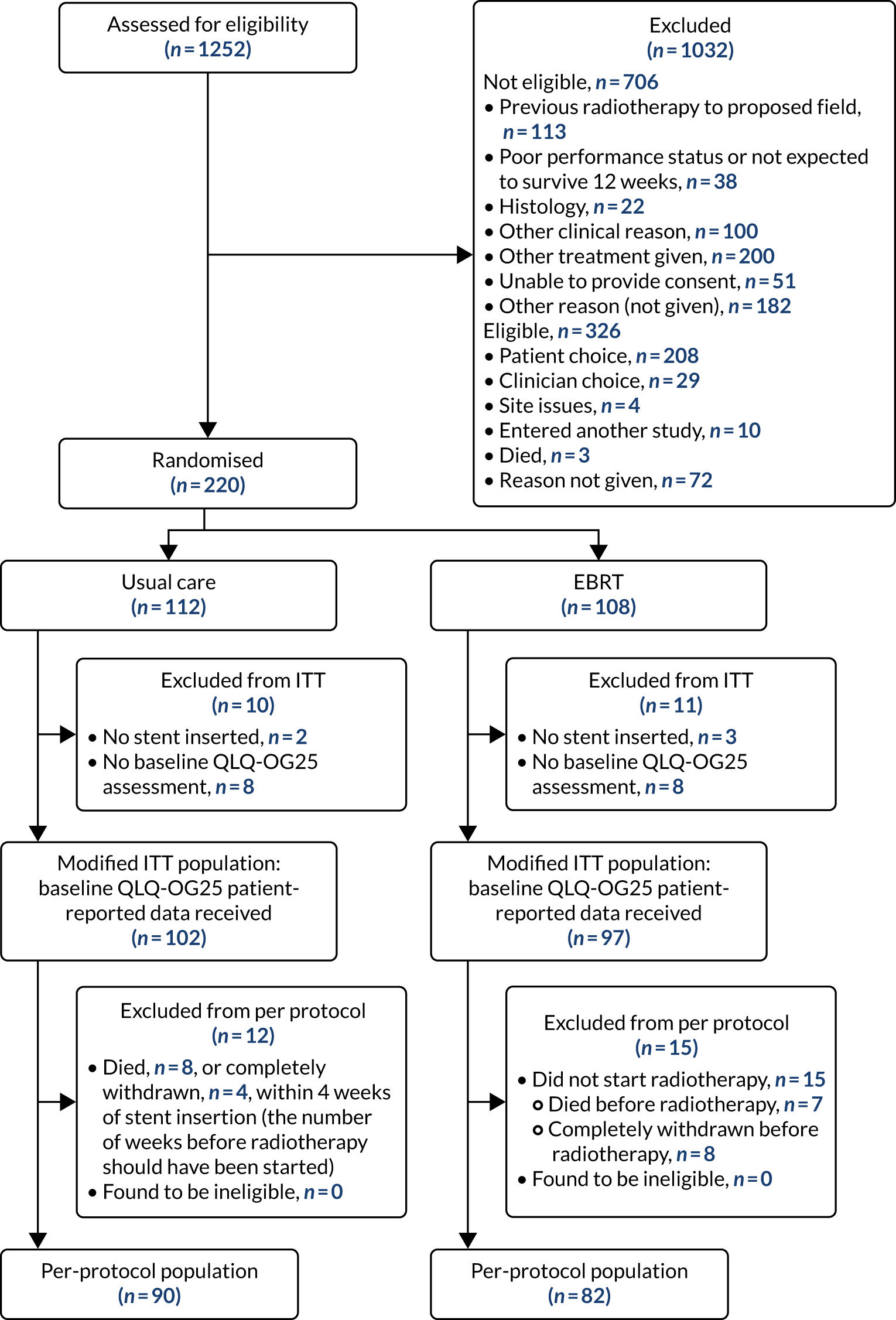
The CONSORT flow diagram is shown in Figure 2 and summarises patient flow from eligibility screening and randomisation to stent insertion (either before or after randomisation) and inclusion in the ITT and per-protocol populations. Unless otherwise stated, all analyses are for the modified ITT population. In total, 43.6% (546/1252) of screened patients were eligible and, of those, 40.3% (220/546) gave consent.
FIGURE 2.
The CONSORT flow diagram.
Characteristics of the study sample
Baseline characteristics
Baseline characteristics are shown in Tables 2 and 3. The randomisation stratification factors of tumour type, stage at diagnosis and intended chemotherapy after stent insertion were well balanced between trial arms, as were other baseline characteristics.
| Characteristic | Usual care (N = 102) | EBRT (N = 97) |
|---|---|---|
| Age (years), median (IQR), n | 73.5 (65.4–81.5), 102 | 72.0 (65.3–79.9), 97 |
| Randomisation time point, n (%) | ||
| Before stent | 39 (38.2) | 36 (37.1) |
| After stent | 63 (61.8) | 61 (62.9) |
| WHO performance status, n (%) | ||
| 0 | 10 (9.8) | 10 (10.3) |
| 1 | 61 (59.8) | 59 (60.8) |
| 2 | 27 (26.5) | 27 (27.8) |
| 3 | 4 (3.9) | 1 (1.0) |
| 4 | 0 (0.0) | 0 (0.0) |
| Tumour type, n (%) | ||
| Adenocarcinoma | 68 (66.7) | 61 (62.9) |
| Squamous | 33 (32.4) | 34 (35.1) |
| Undifferentiated/other | 1 (1.0) | 2 (2.1) |
| Overall length of primary tumour (endoscopic assessment), median (IQR), n | ||
| Measured length (cm) | 5.0 (4.0–7.0), 64 | 6.0 (4.0–8.0), 57 |
| Estimated length (cm) | 6.0 (4.5–8.0), 28 | 7.0 (5.0–8.0), 33 |
| Measured/estimated length (cm) | 5.9 (4.0–7.0), 92 | 6.0 (4.5–8.0), 90 |
| Missing | 10 (9.8) | 7 (7.2) |
| Alternative method for assessing length, n (%) | ||
| PET | 5 (4.9) | 7 (7.2) |
| CT | 23 (22.5) | 23 (23.7) |
| Barium | 0 (0.0) | 0 (0.0) |
| Other | 1 (1.0) | 0 (0.0) |
| None | 1 (1.0) | 0 (0.0) |
| Site of predominant tumour, n (%) | ||
| Upper | 3 (2.9) | 3 (3.1) |
| Middle | 24 (23.5) | 25 (25.8) |
| Lower | 75 (73.5) | 68 (70.1) |
| If lower, involvement of GOJ | 38 (37.3) | 38 (39.2) |
| Unknown | 0 (0.0) | 1 (1.0) |
| Extension across GOJ (if involvement of GOJ), n (%) | ||
| Siewert type 1 | 21 (20.6) | 20 (20.6) |
| Siewert type 2 | 15 (14.7) | 13 (13.4) |
| Missing | 2 (2.0) | 5 (5.2) |
| T stage | ||
| 0 | 1 (1.0) | 0 (0.0) |
| 1 | 1 (1.0) | 1 (1.0) |
| 2 | 4 (3.9) | 7 (7.2) |
| 3 | 61 (59.8) | 54 (55.7) |
| 4 | 29 (28.4) | 31 (32.0) |
| Unknown | 2 (2.0) | 2 (2.1) |
| Missing | 4 (3.9) | 2 (2.1) |
| N stage, n (%) | ||
| 0 | 17 (16.7) | 10 (10.3) |
| 1 | 46 (45.1) | 46 (47.4) |
| 2 | 20 (19.6) | 20 (20.6) |
| 3 | 15 (14.7) | 17 (17.5) |
| Unknown | 1 (1.0) | 2 (2.1) |
| Missing | 3 (2.9) | 2 (2.1) |
| M stage, n (%) | ||
| 0 | 46 (45.1) | 41 (42.3) |
| 1 | 49 (48.0) | 50 (51.5) |
| Unknown | 2 (2.0) | 1 (1.0) |
| Missing | 5 (4.9) | 5 (5.2) |
| Overall stage, n (%) | ||
| 1–3 | 51 (50.0) | 46 (47.4) |
| 4 | 51 (50.0) | 51 (52.6) |
| Chemotherapy | Usual care (N = 102) | EBRT (N = 97) |
|---|---|---|
| Previous chemotherapy given, n (%) | ||
| No | 87 (85.3) | 74 (76.3) |
| Yes | 15 (14.7) | 23 (23.7) |
| EOX | 6 (5.9) | 7 (6.9) |
| ECX | 4 (3.9) | 3 (2.9) |
| Cisplatin + capecitabine | 1 (1.0) | 3 (2.9) |
| CX; OxCap | 0 (0.0) | 2 (2.0) |
| OxCap | 2 (2.0) | 0 (0.0) |
| Carboplatin + capecitabine + epirubicin | 0 (0.0) | 1 (1.0) |
| Carboplatin + paclitaxel | 0 (0.0) | 1 (1.0) |
| Cisplatin; 5FU | 0 (0.0) | 1 (1.0) |
| Cisplatin + epirubicin | 0 (0.0) | 1 (1.0) |
| CX | 1 (1.0) | 0 (0.0) |
| CX + herceptin; docetaxel | 0 (0.0) | 1 (1.0) |
| Docetaxel; irinotecan | 0 (0.0) | 1 (1.0) |
| ECF | 0 (0.0) | 1 (1.0) |
| ECX neoadjuvant; EOX | 0 (0.0) | 1 (1.0) |
| EOX; docetaxel | 1 (1.0) | 0 (0.0) |
| If had prior chemotherapy, intended number of cycles, median (IQR), n | 6.0 (4.0–8.0), 15 | 4.0 (3.0–6.0), 21 |
| Intended number of cycles missing, n (%) | 0 (0.0) | 2 (8.7) |
| Number of prior chemotherapy cycles given, median (IQR), n | 3.0 (2.0–6.0), 15 | 4.0 (3.0–6.0), 23 |
| MDT intended chemotherapy after stent? n (%) | ||
| Yes | 36 (35.3) | 34 (35.1) |
| No | 66 (64.7) | 63 (64.9) |
Allocation of treatments
Treatment adherence
Table 4 shows a summary of stent insertion, including clinical characteristics and complications. There were no reports of oesophageal or other GI tract perforation. Radiotherapy administration is shown in Table 5 for those randomised to stent plus radiotherapy. Of the 97 patients randomised to receive radiotherapy, 15 died or withdrew before radiotherapy treatment could be given. One participant chose a reduction to the planned radiotherapy, from 30 Gy in 10 fractions to 15 Gy in five fractions. All remaining participants received the planned radiotherapy except one participant who received 8 Gy in one dose, as this was the local practice. This was classified by the TMG as a minor deviation as this is an appropriate palliative dose and this patient was kept in the per-protocol population.
| Stent characteristic | Usual care (N = 102) | EBRT (N = 97) |
|---|---|---|
| Type of stent, n (%) | ||
| Fully covered stent | 31 (30.4) | 24 (24.7) |
| Covered stent with anti-reflux valve | 5 (4.9) | 3 (3.1) |
| Partially covered stent | 55 (53.9) | 58 (59.8) |
| Partially covered stent with anti-reflux valve | 0 (0.0) | 1 (1.0) |
| Uncovered | 9 (8.8) | 9 (9.3) |
| Missing | 2 (2.0) | 2 (2.1) |
| Length of stent (cm), median (IQR), n | 10.2 (8.0–13.0), 100 | 10.3 (8.0–13.0), 92 |
| Dilatation required, n (%) | ||
| Before stent insertion | 4 (3.9) | 10 (10.3) |
| After stent insertion | 0 (0.0) | 2 (2.1) |
| Not required | 95 (93.1) | 80 (82.5) |
| Missing | 3 (2.9) | 5 (5.2) |
| Radiological imaging used, n (%) | ||
| Yes | 69 (67.6) | 46 (47.4) |
| No | 33 (32.4) | 48 (49.5) |
| Missing | 0 (0.0) | 3 (3.1) |
| Post-insertion oesophagogram performed, n (%) | ||
| Yes | 10 (9.8) | 13 (13.4) |
| No | 92 (90.2) | 84 (86.6) |
| If yes, any stent slippage, n (%) | ||
| Yes | 0 (0.0) | 3 (23.1) |
| No | 10 (100.0) | 10 (76.9) |
| Number of nights in hospital post stent, median (IQR), n | 1.0 (0.0–2.0), 102 | 1.0 (0.0–2.0), 95 |
| Acute airway compression, n (%) | ||
| Yes | 0 (0.0) | 0 (0.0) |
| No | 99 (97.1) | 96 (99.0) |
| Missing | 3 (2.9) | 1 (1.0) |
| Oesophageal or other GI tract perforation, n (%) | ||
| Yes | 0 (0.0) | 0 (0.0) |
| No | 101 (99.0) | 97 (100.0) |
| Missing | 1 (1.0) | 0 (0.0) |
| CT scan performed post stent insertion, n (%) | ||
| Yes | 3 (2.9) | 7 (7.2) |
| No | 99 (97.1) | 90 (92.8) |
| Chest X-ray performed post stent insertion, n (%) | ||
| Yes | 23 (22.5) | 21 (21.6) |
| No | 79 (77.5) | 76 (78.4) |
| Radiotherapy compliance | EBRT (N = 97) |
|---|---|
| Number of patients receiving radiotherapy, n (%) | 82 (84.5) |
| Reasons if radiotherapy not received, n (%) | |
| Withdrew before radiotherapy | 7 (7.2) |
| Died before radiotherapy | 8 (8.2) |
| Planned dose 20 Gy in five fractions | 64 (78.0) |
| Planned dose 30 Gy in 10 fractions | 17 (20.7) |
| Planned dose 8 Gy in one fraction | 1 (1.2) |
| Reduction to planned dose | 1 (1.2) |
| Radiotherapy delays | 10 (12.2) |
| Toxicity | 1 (10.0) |
| Patient choice | 1 (10.0) |
| Logistical/machine breakdown | 3 (30.0) |
| Other | 5 (50.0) |
| Been hospitalised hydration | 1 (10.0) |
| Felt ill | 1 (10.0) |
| Weekend break | 1 (10.0) |
| Bank holidays | 1 (10.0) |
| Not known | 1 (10.0) |
| Average number of days delayed, median (IQR; range), n | 2.0 (1.0–2.0; 1.0–5.0), 10 |
| Field size (cm), median (IQR), n | |
| X | 12.0 (10.0–14.0), 81 |
| Y | 11.4 (8.3–14.0), 81 |
| Field definition, n (%) | |
| CT simulator | 77 (93.9) |
| Conventional simulator | 5 (6.1) |
| Field arrangement, n (%) | |
| Parallel pair (anteroposterior, posteroanterior) | 76 (92.7) |
| Other | 5 (6.1) |
| 3D conformal radiotherapy | 2 (40.0) |
| 4 – field | 1 (20.0) |
| 5 – field | 1 (20.0) |
| Conformal ‘4’ field box | 1 (20.0) |
| Missing | 1 (1.2) |
| Number of beams, median (IQR), n | 2.0 (2.0–2.0), 82 |
| Beam 1, n (%) | |
| 6 | 56 (68.3) |
| 10 | 25 (30.5) |
| Missing | 1 (1.2) |
| Beam 2, n (%) | |
| 6 | 56 (68.3) |
| 10 | 25 (30.5) |
| Missing | 1 (1.2) |
| Beam 3, n (%) | |
| 6 | 5 (6.1) |
| 10 | 1 (1.2) |
| Missing | 76 (92.7) |
| Beam 4, n (%) | |
| 6 | 5 (6.1) |
| 10 | 1 (1.2) |
| Missing | 76 (92.7) |
| Contrast applied, n (%) | |
| Yes | 17 (20.7) |
| No | 65 (79.3) |
| Dose calculation method used, n (%) | |
| Tables | 43 (52.4) |
| TPS | 37 (45.1) |
| Other | 2 (2.4) |
| First calculation check done in RADCALC (Computer) | 1 (50.0) |
| OMP planning system | 1 (50.0) |
| Inhomogeneity correction used, n (%) | |
| Yes | 18 (22.0) |
| No | 64 (78.0) |
Missing data, compliance with follow-up and return of European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 and OG25 questionnaires
The return of EORTC QLQ-OG25 and C30 questionnaires over time, including missing reasons where known, is shown in Table 6 and Appendix 3, Table 28, respectively. Although there was very good questionnaire return in early visits, missing questionnaire data substantially increased over time. Sensitivity analyses were used to take account of missing primary end-point data, and these are detailed in the primary outcome analysis below. Around 50% of participants required help to complete questionnaires, and this stayed fairly constant over time. Fewer participants needed a carer to complete the questionnaire for them (0–8% prior to week 32). The main reasons for missing questionnaires in both arms were that the patient was too ill, refused or withdrew consent, or the patient took the questionnaire away and did not return it. However, for many of the missing questionnaires no reason is given.
| Time point | Usual care (N = 102), n (%) | EBRT (N = 97), n (%) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Not expecteda | Expected | Actually received | Help needed to complete questionnaire | Carer completed | Missing reasons | Not expecteda | Expected | Actually received | Help needed to complete questionnaire | Carer completed | Missing reasons | |||||||
| Patient too ill, refused or withdrew consent | Patient did not return | Admin error | Reason missing | Patient too ill, refused or withdrew consent | Patient did not return | Admin error | Reason missing | |||||||||||
| Baseline | 0 | 102 | 102 (100.0) | 46 (45.1) | 5 (4.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 97 | 97 (100.0) | 38 (39.2) | 7 (7.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 1 week post stent insertion | 0 | 39 | 39 (100.0) | 21 (53.8) | 6 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 36 | 36 (100.0) | 11 (30.6) | 2 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 weeks post stent insertion | 11 | 91 | 78 (85.7) | 36 (46.2) | 3 (3.8) | 0 (0.0) | 3 (23.1) | 0 (0.0) | 10 (76.9) | 6 | 91 | 75 (82.4) | 28 (37.3) | 3 (4.0) | 2 (12.5) | 1 (6.3) | 1 (6.3) | 12 (75.0) |
| 8 weeks post stent insertion | 23 | 79 | 61 (77.2) | 32 (52.5) | 2 (3.3) | 2 (11.1) | 2 (11.1) | 0 (0.0) | 14 (77.8) | 17 | 80 | 62 (77.5) | 27 (43.5) | 4 (6.5) | 3 (16.7) | 1 (5.6) | 0 (0.0) | 14 (77.8) |
| 12 weeks post stent insertion | 37 | 65 | 46 (70.8) | 18 (39.1) | 0 (0.0) | 3 (15.8) | 2 (10.5) | 0 (0.0) | 14 (73.7) | 34 | 63 | 49 (77.8) | 21 (42.9) | 3 (6.1) | 2 (14.3) | 1 (7.1) | 0 (0.0) | 11 (78.6) |
| 16 weeks post stent insertion | 49 | 53 | 41 (77.4) | 24 (58.5) | 1 (2.4) | 2 (16.7) | 2 (16.7) | 0 (0.0) | 8 (66.7) | 45 | 52 | 40 (76.9) | 20 (50.0) | 3 (7.5) | 1 (8.3) | 2 (16.7) | 0 (0.0) | 9 (75.0) |
| 20 weeks post stent insertion | 57 | 45 | 34 (75.6) | 19 (55.9) | 0 (0.0) | 3 (27.3) | 0 (0.0) | 0 (0.0) | 8 (72.7) | 55 | 42 | 35 (83.3) | 15 (42.9) | 2 (5.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (100.0) |
| 24 weeks post stent insertion | 58 | 44 | 27 (61.4) | 14 (51.9) | 1 (3.7) | 3 (17.6) | 0 (0.0) | 0 (0.0) | 14 (82.4) | 58 | 39 | 26 (66.7) | 11 (42.3) | 1 (3.8) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 12 (92.3) |
| 28 weeks post stent insertion | 65 | 37 | 21 (56.8) | 11 (52.4) | 1 (4.8) | 3 (18.8) | 0 (0.0) | 0 (0.0) | 13 (81.3) | 70 | 27 | 19 (70.4) | 10 (52.6) | 1 (5.3) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 7 (87.5) |
| 32 weeks post stent insertion | 72 | 30 | 14 (46.7) | 9 (64.3) | 0 (0.0) | 6 (37.5) | 0 (0.0) | 0 (0.0) | 10 (62.5) | 76 | 21 | 12 (57.1) | 6 (50.0) | 2 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (100.0) |
| 36 weeks post stent insertion | 76 | 26 | 13 (50.0) | 7 (53.8) | 0 (0.0) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 12 (92.3) | 81 | 16 | 10 (62.5) | 7 (70.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (100.0) |
| 40 weeks post stent insertion | 79 | 23 | 12 (52.2) | 5 (41.7) | 0 (0.0) | 5 (45.5) | 0 (0.0) | 0 (0.0) | 6 (54.5) | 82 | 15 | 8 (53.3) | 5 (62.5) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (100.0) |
| 44 weeks post stent insertion | 85 | 17 | 6 (35.3) | 3 (50.0) | 0 (0.0) | 2 (18.2) | 0 (0.0) | 0 (0.0) | 9 (81.8) | 83 | 14 | 7 (50.0) | 4 (57.1) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (100.0) |
| 48 weeks post stent insertion | 84 | 18 | 7 (38.9) | 4 (57.1) | 0 (0.0) | 2 (18.2) | 0 (0.0) | 0 (0.0) | 9 (81.8) | 84 | 13 | 5 (38.5) | 3 (60.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 7 (87.5) |
| 52 weeks post stent insertion | 86 | 16 | 4 (25.0) | 2 (50.0) | 0 (0.0) | 2 (16.7) | 0 (0.0) | 0 (0.0) | 10 (83.3) | 85 | 12 | 5 (41.7) | 2 (40.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (100.0) |
Baseline characteristics of participants missing versus not missing primary end-point data
Baseline characteristics of those participants missing and those not missing primary end-point data were reasonably well balanced, as shown in Table 7 and in Appendix 3, Table 29.
| Characteristic | Complete data up to week 12 (N = 149) | Missing data up to week 12 (N = 50) |
|---|---|---|
| Age (years), median (IQR), n | 72.2 (64.8–80.2), 149 | 75.0 (68.4–83.3), 50 |
| Randomisation time point, n (%) | ||
| Before stent | 59 (39.6) | 16 (32.0) |
| After stent | 90 (60.4) | 34 (68.0) |
| WHO performance status, n (%) | ||
| 0 | 17 (11.4) | 3 (6.0) |
| 1 | 93 (62.4) | 27 (54.0) |
| 2 | 37 (24.8) | 17 (34.0) |
| 3 | 2 (1.3) | 3 (6.0) |
| Tumour type, n (%) | ||
| Adenocarcinoma | 96 (64.4) | 33 (66.0) |
| Squamous | 51 (34.2) | 16 (32.0) |
| Undifferentiated/other | 2 (1.3) | 1 (2.0) |
| Overall length of primary tumour (endoscopic assessment) | ||
| Measured length (cm), median (IQR), n | 5.0 (4.0–7.0), 93 | 5.3 (4.3–7.0), 28 |
| Estimated length (cm), median (IQR), n | 6.0 (5.0–8.0), 42 | 7.0 (5.5–9.0), 19 |
| Measured/estimated length (cm), median (IQR), n | 6.0 (4.0–8.0), 135 | 6.0 (5.0–8.0), 47 |
| Missing, n (%) | 14 (9.4) | 3 (6.0) |
| Alternative method for assessing length, n (%) | ||
| PET | 9 (6.0) | 3 (6.0) |
| CT | 33 (22.1) | 13 (26.0) |
| Barium | 0 (0.0) | 0 (0.0) |
| Other | 1 (0.7) | 0 (0.0) |
| None | 1 (0.7) | 0 (0.0) |
| Site of predominant tumour, n (%) | ||
| Upper | 5 (3.4) | 1 (2.0) |
| Middle | 34 (22.8) | 15 (30.0) |
| Lower | 109 (73.2) | 34 (68.0) |
| If lower, involvement of GOJ | 60 (40.3) | 16 (32.0) |
| Unknown | 1 (0.7) | 0 (0.0) |
| Extension across GOJ (if involvement of GOJ), n (%) | ||
| Siewert type 1 | 32 (21.5) | 9 (18.0) |
| Siewert type 2 | 26 (17.4) | 2 (4.0) |
| Missing | 2 (1.3) | 5 (10.0) |
| T stage, n (%) | ||
| 0 | 1 (0.7) | 0 (0.0) |
| 1 | 1 (0.7) | 1 (2.0) |
| 2 | 7 (4.7) | 4 (8.0) |
| 3 | 87 (58.4) | 28 (56.0) |
| 4 | 45 (30.2) | 15 (30.0) |
| Unknown | 2 (1.3) | 2 (4.0) |
| X | 6 (4.0) | 0 (0.0) |
| N stage, n (%) | ||
| 0 | 20 (13.4) | 7 (14.0) |
| 1 | 68 (45.6) | 24 (48.0) |
| 2 | 29 (19.5) | 11 (22.0) |
| 3 | 27 (18.1) | 5 (10.0) |
| Unknown | 1 (0.7) | 2 (4.0) |
| X | 4 (2.7) | 1 (2.0) |
| M stage, n (%) | ||
| 0 | 63 (42.3) | 24 (48.0) |
| 1 | 77 (51.7) | 22 (44.0) |
| Unknown | 1 (0.7) | 2 (4.0) |
| X | 8 (5.4) | 2 (4.0) |
| Overall stage, n (%) | ||
| 1–3 | 70 (47.0) | 27 (54.0) |
| 4 | 79 (53.0) | 23 (46.0) |
Primary outcome
Modified intention-to-treat population
There were 102 versus 97 patients (usual care vs. EBRT) in the modified ITT population with 74 versus 75 patients (usual care vs. EBRT) having complete data at week 12 for the primary end point (Table 8). The complete-case analysis, which included deaths up to week 12 with complete data as an event, showed no evidence of a difference in the proportion of patients experiencing a primary event up to week 12 post stent by trial arm [36/74 (48.6%) vs. 34/75 (45.3%); adjusted odds ratio (OR) 0.82, 95% CI 0.40 to 1.68; p = 0.587]. The sensitivity analysis, treating death by week 12 without prior event as no event, also showed no evidence of a difference between trial arms [21/74 (28.4%) vs. 21/75 (28.0%); adjusted OR 1.05, 95% CI 0.49 to 2.25; p = 0.893].
| Characteristic | Usual care (N = 102), n (%) | EBRT (N = 97), n (%) | Adjusted OR (95% CI; p-value, n) |
|---|---|---|---|
| Incomplete-case data at week 12 | |||
| Completely withdrew with no event | 6 (5.9) | 5 (5.2) | |
| Died with incomplete data and no event | 8 (7.8) | 6 (6.2) | |
| Alive at week 12 with incomplete data and no event | 14 (13.7) | 11 (11.3) | |
| Reasons for complete withdrawal | |||
| Participant choice | 3 (2.9) | 4 (4.1) | |
| Other | 3 (2.9) | 1 (1.0) | |
| Informed by CNS on family’s behalf | 1 (1.0) | 0 (0.0) | |
| Lost to follow-up | 1 (1.0) | 1 (1.0) | |
| Transferred to another area due to relocation | 1 (1.0) | 0 (0.0) | |
| Complete-case data at week 12 | |||
| Total with complete data | 74 (72.5) | 75 (77.3) | |
| Died with complete data | 20 (19.6) | 22 (22.7) | |
| Alive at week 12 with complete data | 54 (52.9) | 53 (54.6) | |
| Complete-case analysis (death as an event) | |||
| Number of primary events or deaths | 36 (48.6) | 34 (45.3) | 0.82 (0.40 to 1.68; 0.587, 149) |
| Sensitivity analyses | |||
| Complete-case analysis (death as non-event) | |||
| Number of primary events | 21 (28.4) | 21 (28.0) | 1.05 (0.49 to 2.25; 0.893, 149) |
| Best case | |||
| Total with complete data | 90 (88.2) | 88 (90.7) | |
| Number of primary events or deaths | 40 (44.4) | 36 (40.9) | 0.85 (0.44 to 1.62; 0.612, 178) |
| Worst case | |||
| Total with complete data | 90 (88.2) | 88 (90.7) | |
| Number of primary events or deaths | 53 (58.9) | 46 (52.3) | 0.73 (0.38 to 1.40; 0.345, 178) |
Imputation of missing data under the best- and worst-case scenarios resulted in 90 versus 88 patients in the denominator for the associated sensitivity analyses. Those with remaining missing data were missing two or more questionnaires and no deterioration had been confirmed prior to this. There was no evidence of a difference in the primary end point between trial arms under either the best-case (p = 0.612) or the worst-case (p = 0.345) scenario.
Per-protocol population
There were 90 versus 82 patients in the per-protocol population with 66 versus 64 (usual care vs. EBRT) having complete data at week 12 for the primary end point (Table 9). The complete-case analysis, which included deaths up to week 12 with complete data as an event, showed no evidence of a difference in the proportion of patients experiencing a primary event up to week 12 post stent by trial arm [28/66 (42.4%) vs. 26/64 (40.6%); adjusted OR 0.99, 95% CI 0.45 to 2.14; p = 0.972]. The sensitivity analysis, treating death by week 12 without prior event as no event, also showed no evidence of a difference between trial arms [19/66 (28.8%) vs. 19/64 (29.7%); adjusted OR 1.20, 95% CI 0.53 to 2.71; p = 0.666]. The complete cases in the per-protocol population all completed full planned radiotherapy.
| Characteristic | Stent (N = 90), n (%) | Stent plus radiotherapy (N = 82), n (%) | Adjusted OR (95% CI; p-value, n) |
|---|---|---|---|
| Incomplete case data at week 12 | |||
| Completely withdrew with no event | 2 (2.2) | 3 (3.7) | |
| Died with incomplete data and no event | 8 (8.9) | 5 (6.1) | |
| Alive at week 12 with incomplete data and no event | 14 (15.6) | 10 (12.2) | |
| Reasons for complete withdrawal | |||
| Participant choice | 1 (1.1) | 2 (2.4) | |
| Other | 1 (1.1) | 1 (1.2) | |
| Informed by CNS on family’s behalf | 1 (1.1) | 0 (0.0) | |
| Loss to follow-up | 0 (0.0) | 1 (1.2) | |
| Complete-case data at week 12 | |||
| Total with complete data | 66 (73.3) | 64 (78.0) | |
| Died with complete data | 12 (13.3) | 14 (17.1) | |
| Alive at week 12 with complete data | 54 (60.0) | 50 (61.0) | |
| Complete-case analysis (death as an event) | |||
| Number of primary events or deaths | 28 (42.4) | 26 (40.6) | 0.99 (0.45 to 2.14; 0.972; 130) |
| Complete-case analysis (death as non-event) | |||
| Number of primary events | 19 (28.8) | 19 (29.7) | 1.20 (0.53 to 2.71; 0.666; 130) |
| Best case | |||
| Total with complete data | 82 (91.1) | 76 (92.7) | |
| Number of primary events or deaths | 32 (39.0) | 28 (36.8) | 0.96 (0.48 to 1.93; 0.910; 158) |
| Worst case | |||
| Total with complete data | 82 (91.1) | 76 (92.7) | |
| Number of primary events or deaths | 45 (54.9) | 37 (48.7) | 0.80 (0.41 to 1.58; 0.527; 158) |
Imputation of missing data under the best- and worst-case scenarios resulted in 82 versus 76 patients in the denominator for the associated sensitivity analyses. Those with remaining missing data were missing two or more questionnaires and no deterioration had been confirmed prior to this. There was no evidence of a difference in the primary end point between trial arms under either the best-case (p = 0.910) or the worst-case (p = 0.527) scenario.
Dysphagia deterioration-free survival
Figure 3 shows secondary analysis of the original primary end point of DDFS over all time points. There was no evidence of a difference between the arms (adjusted HR 0.92; 95% CI 0.68 to 1.26; p = 0.618; n = 199). Overall, the median time to dysphagia event or death was 14.6 weeks (95% CI 12.1 to 17.4 weeks); median time to dysphagia event or death was 13.1 weeks (95% CI 10 to 17.9 weeks) and 14.7 weeks (95% CI 12.1 to 17.4 weeks) in the usual-care arm and the EBRT arm, respectively.
FIGURE 3.
Dysphagia deterioration-free survival post stent by trial arm.
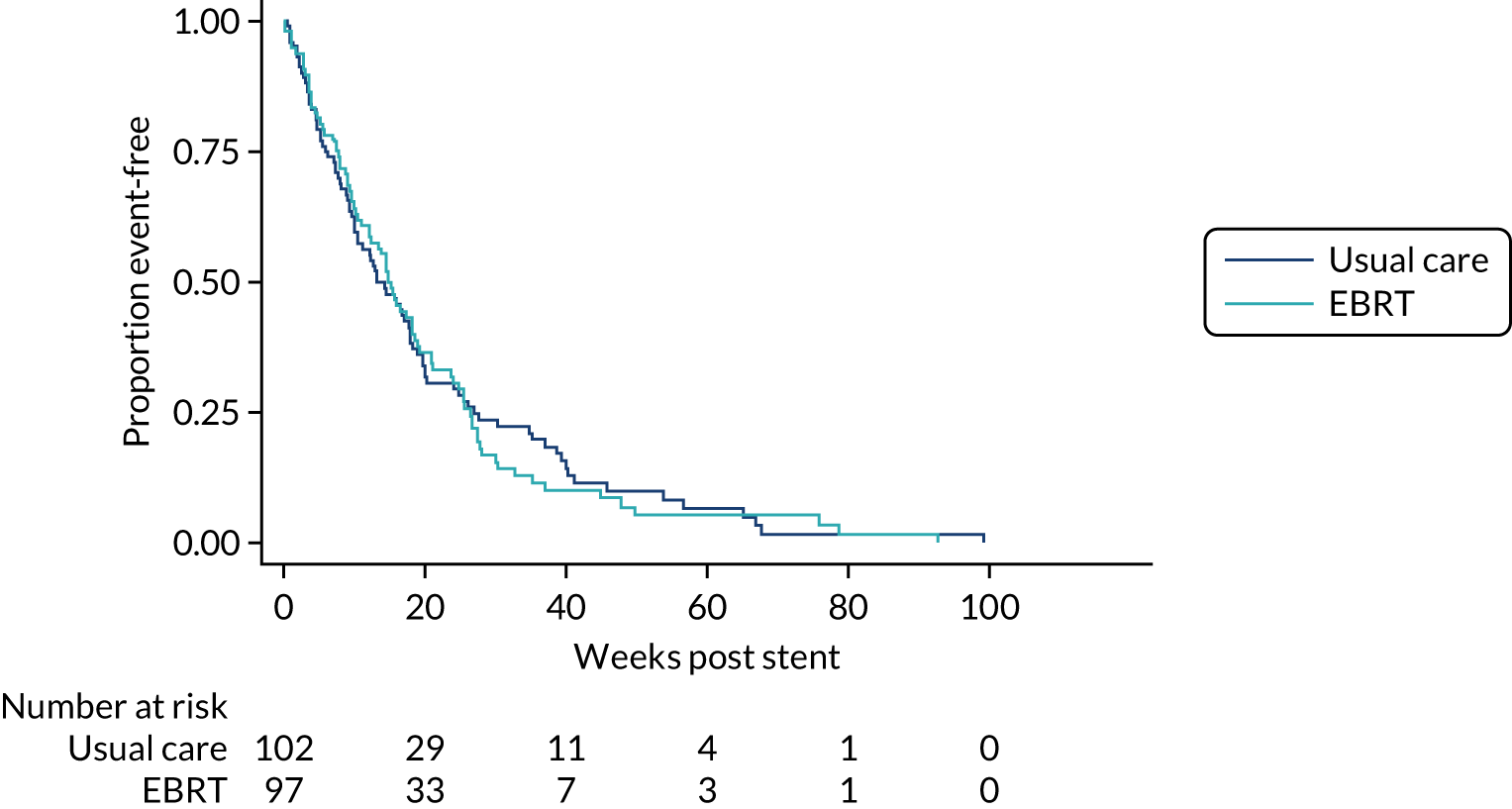
Censoring at death with no event resulted in an adjusted HR 0.97 (95% CI 0.59 to 1.60; p = 0.904; n = 199). Overall, median time to dysphagia event was 41.1 weeks (95% CI 28 weeks – not reached). Median time to dysphagia event was 41.1 weeks (95% CI 24.1 weeks – not reached) and 30.3 (95% CI 25.4 weeks – not reached) in the usual-care arm and the EBRT arm, respectively.
Secondary outcomes
Overall survival and follow-up
Overall survival and follow-up post stent insertion are shown in Table 10. Very few patients were still alive at the end of the study. Median follow-up in those still alive was 22.9 weeks (95% CI 4.0 to 41.9 weeks) (n = 16) versus 22.1 weeks (95% CI 8.0 to 34.7 weeks) (n = 15) (see Appendix 3, Figure 22). There was no evidence of difference in OS: median OS was 19.7 weeks (95% CI 14.4 to 27.7 weeks) versus 18.9 weeks (95% CI 14.7 to 25.6 weeks) in the usual-care arm versus the EBRT arm, respectively (adjusted HR 1.06; 95% CI 0.78 to 1.45; two-sided p-value 0.700) (Figure 4).
| Characteristic | Usual care (N = 102) | EBRT (N = 97) |
|---|---|---|
| Median follow-up post stent, weeks (95% CI), n | 22.9 (4.0 to 41.9), 16 | 22.1 (8.0 to 34.7), 15 |
| Median overall survival post stent, weeks (95% CI), n | 19.7 (14.4 to 27.7), 102 | 18.9 (14.7 to 25.6), 97 |
| Total deaths, n (%) | 86 (84.3) | 82 (84.5) |
| Causes of death, n (%) | ||
| Oesophageal cancer | 79 (91.9) | 72 (87.8) |
| Stent related | 2 (2.3) | 2 (2.4) |
| Radiotherapy related | 0 (0.0) | 1 (1.2) |
| Other, please specify | 5 (5.8) | 7 (8.5) |
| Acute kidney injury, bladder cancer | 0 (0.0) | 1 (1.2) |
| Cardiovascular | 1 (1.2) | 1 (1.2) |
| Chest sepsis | 2 (2.3) | 1 (1.2) |
| GI bleed | 0 (0.0) | 1 (1.2) |
| Neutropenic sepsis | 0 (0.0) | 1 (1.2) |
| Pulmonary embolism | 0 (0.0) | 2 (2.4) |
| Unknown | 2 (2.3) | 0 (0.0) |
FIGURE 4.
Overall survival post stent insertion by trial arm.
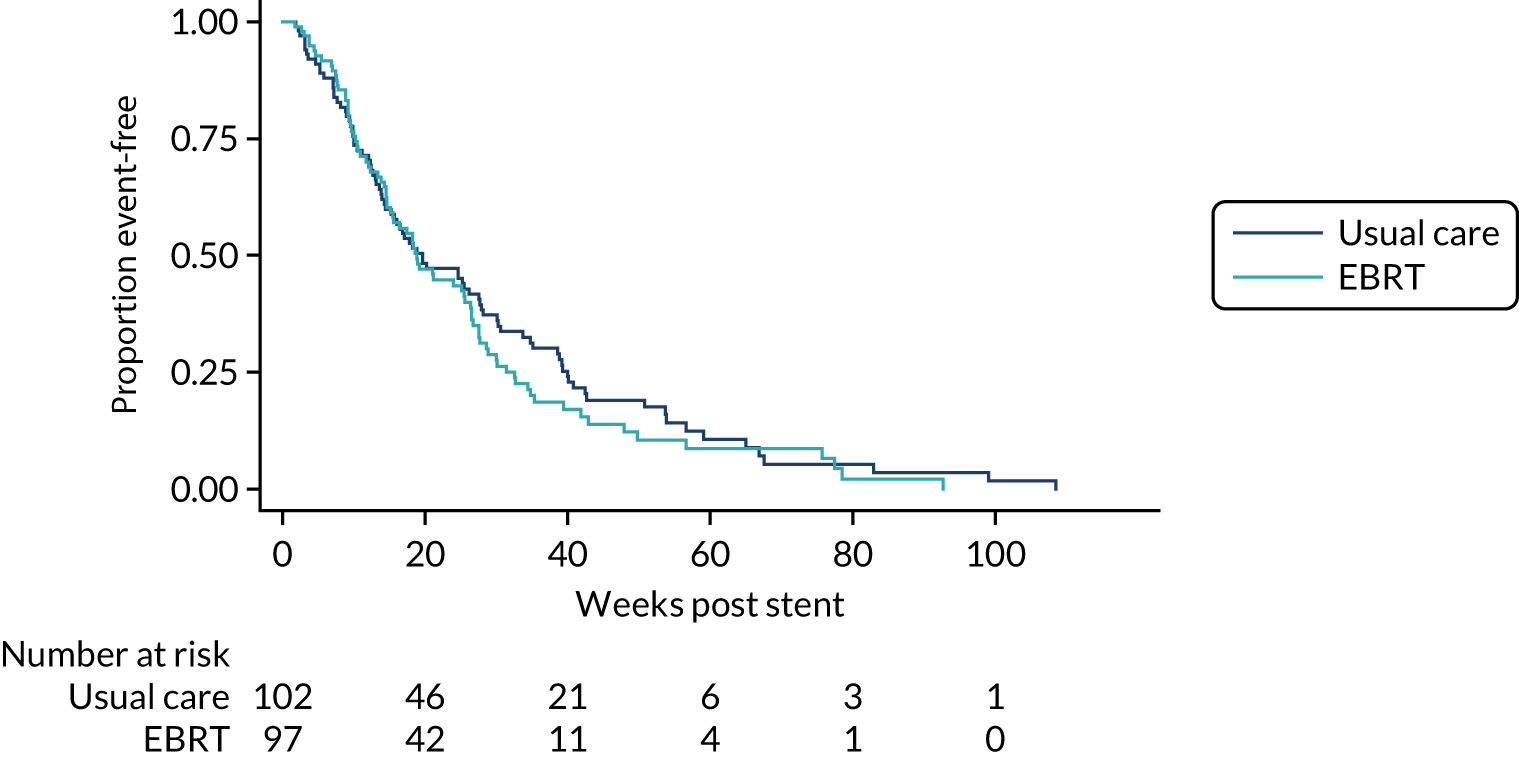
European Organisation for Research and Treatment of Cancer – Quality of Life Questionnaire C30 and OG25 questionnaire scales and World Health Organization performance status
The prespecified main subscales or items of interest were global health (Figure 5), odynophagia (Figure 6), pain/discomfort (OG25) (Figure 7), eating restrictions (Figure 8) and eating in front of others (Figure 9). There was no evidence of time versus treatment interactions for any of these (p-values all > 0.05).
FIGURE 5.
Mean global health scores and 95% CIs by time and treatment arm.
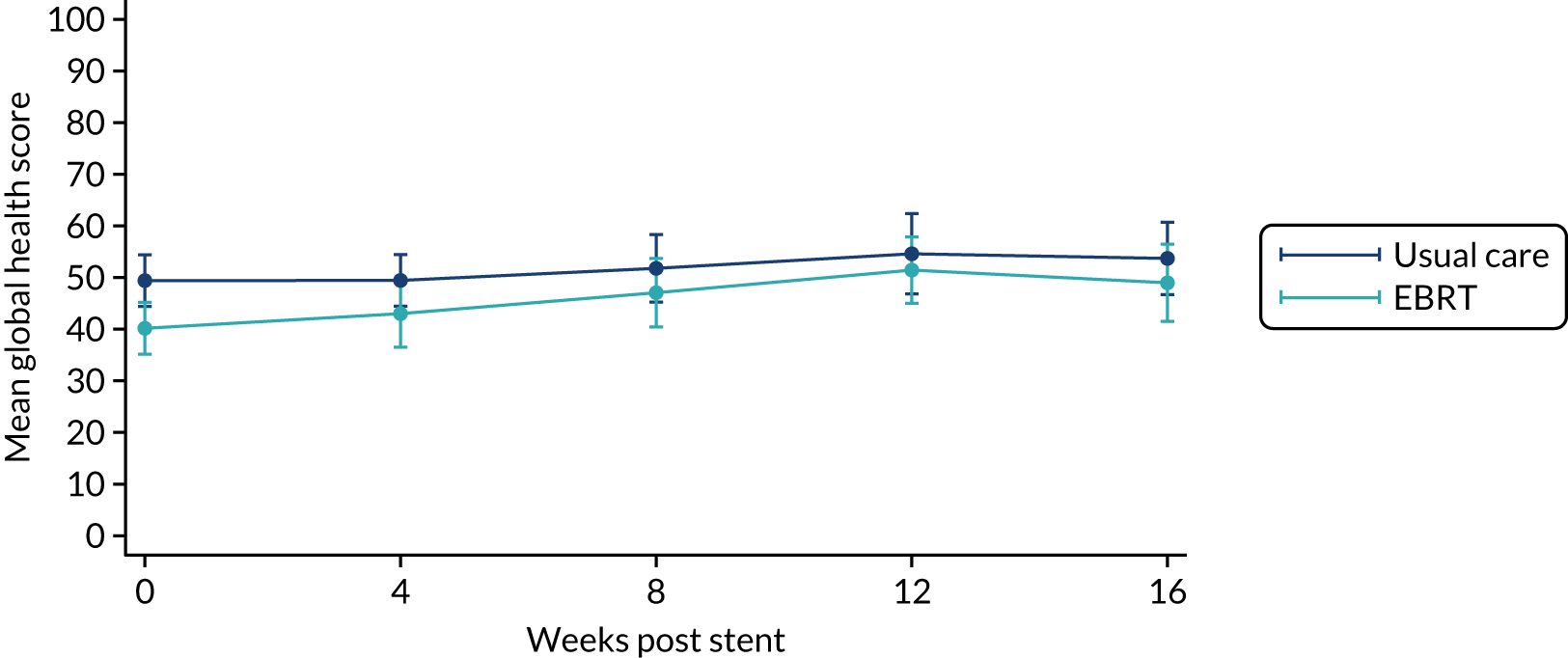
FIGURE 6.
Box plots of odynophagia scores by time and treatment arm.
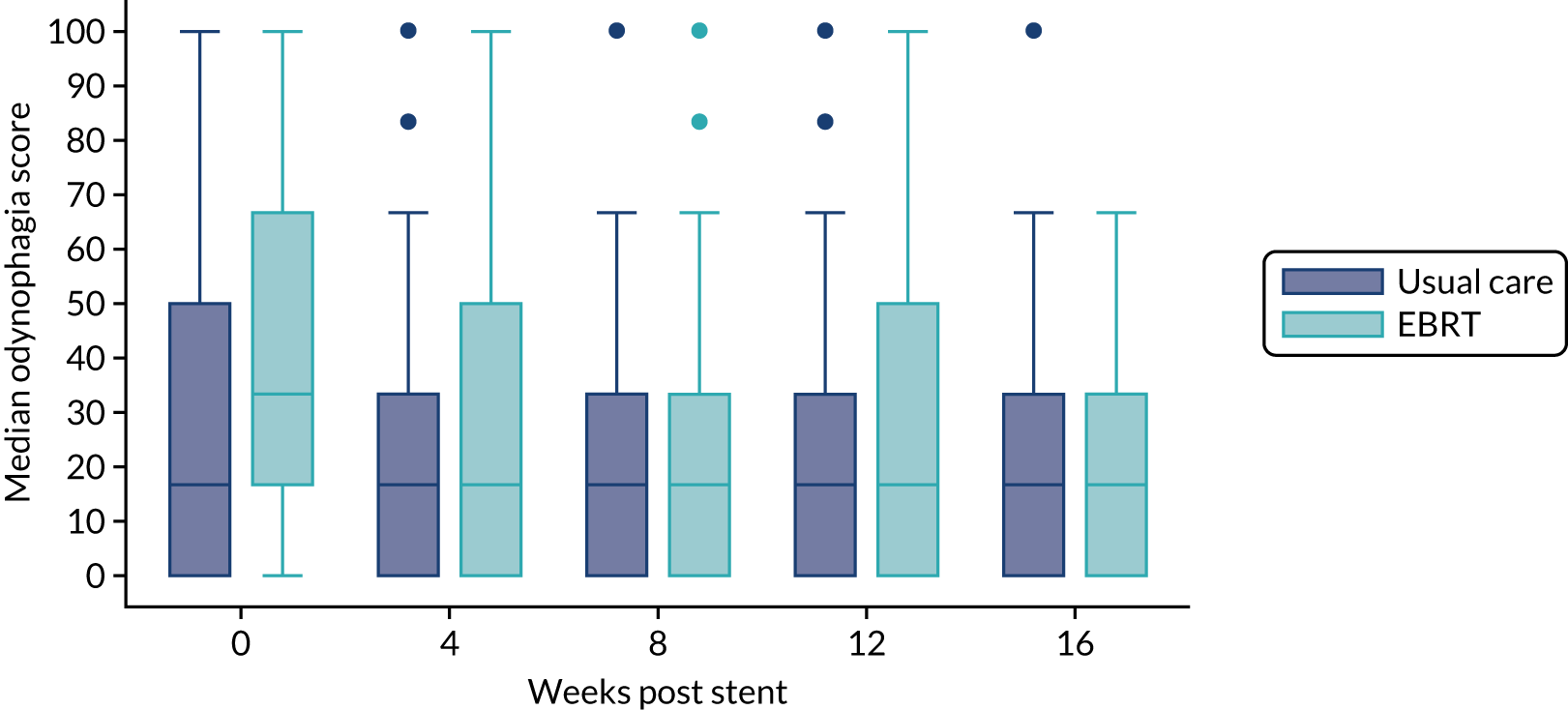
FIGURE 7.
Box plots of pain/discomfort scores (QLQ-OG25 questionnaire) by time and treatment arm.
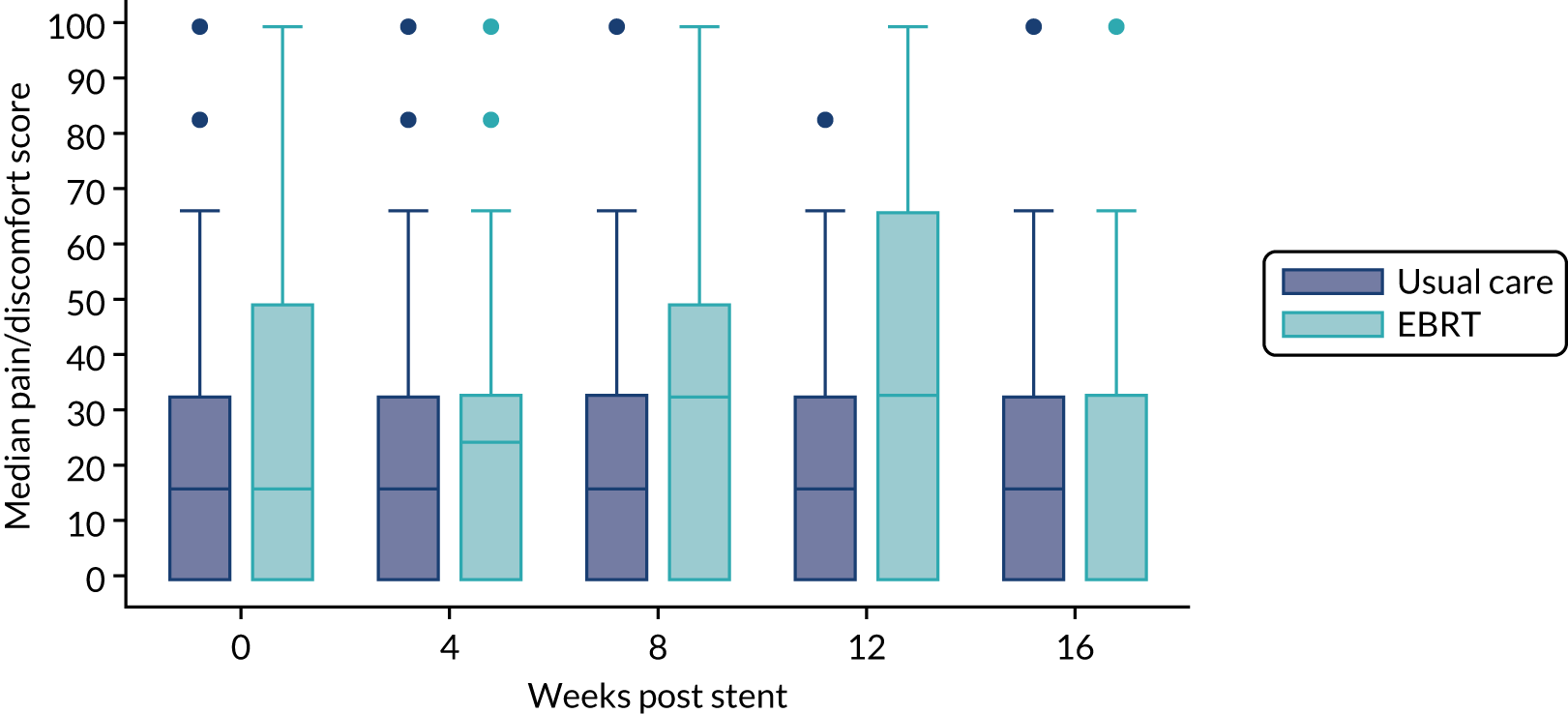
FIGURE 8.
Mean eating restrictions scores and 95% CIs by time and treatment arm.
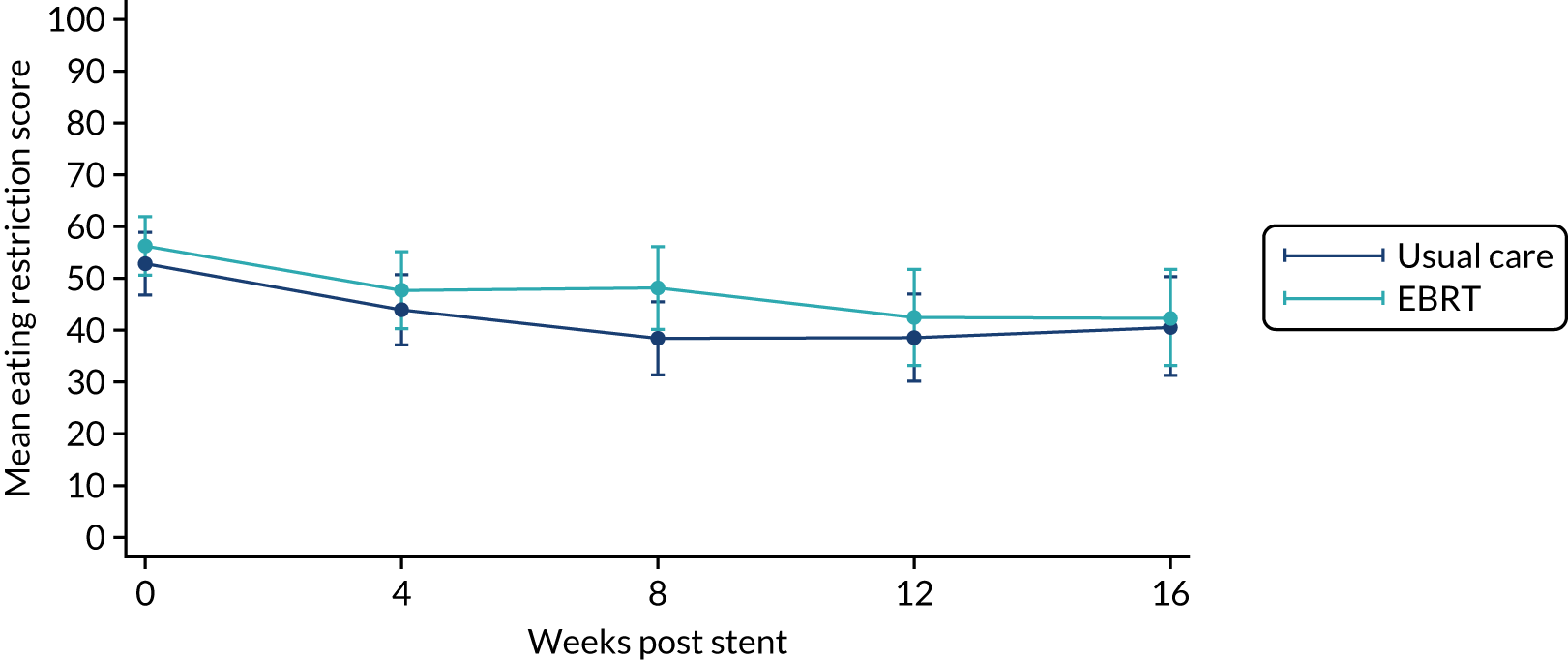
FIGURE 9.
Box plots of ‘eating in front of others’ scores by time and treatment arm.
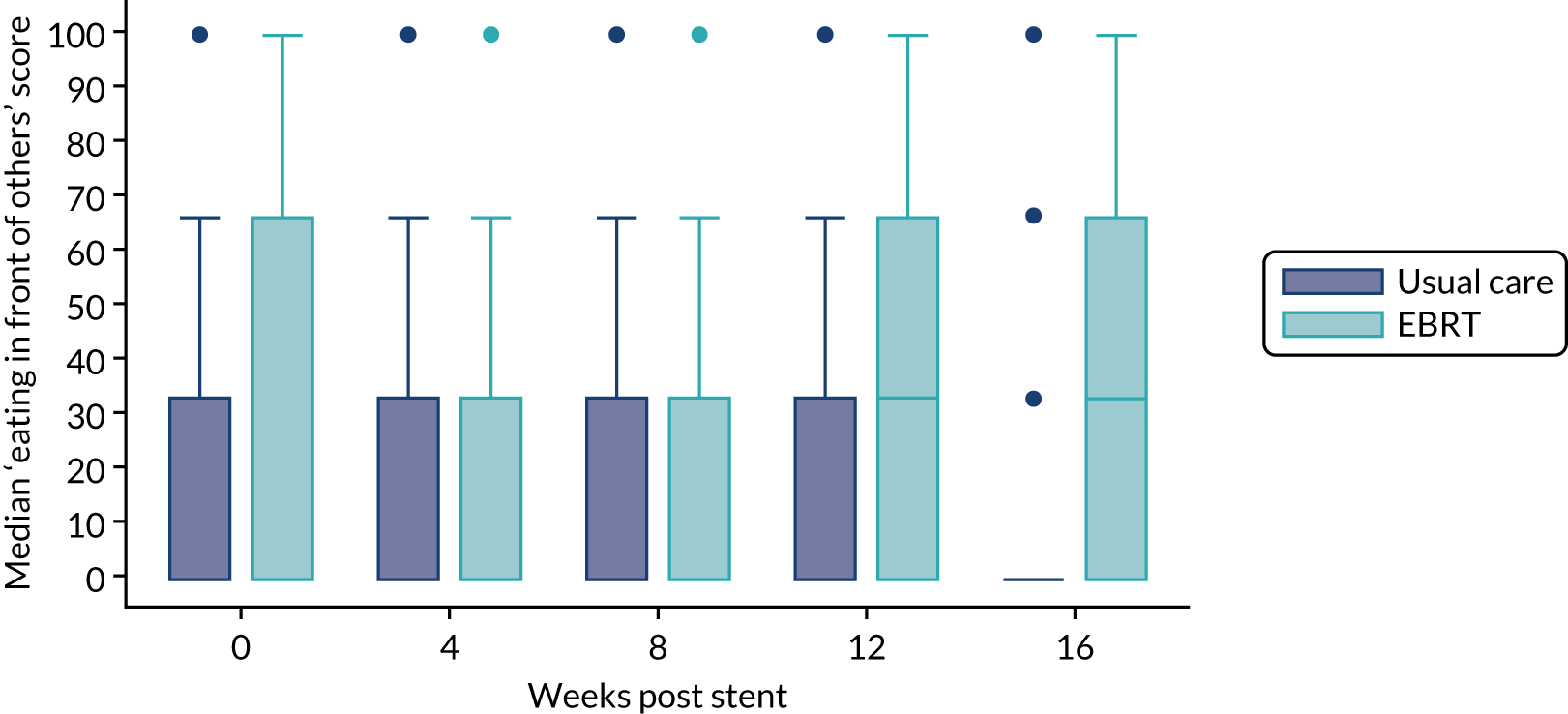
Appendix 3, Table 30, shows the results of the linear mixed models for the EORTC-QLQ-C30 and OG25 questionnaires scales and WHO performance status. As can be seen, the proportion of missing data at each time point was balanced across trial arms and increased from ≈ 0% at the first post-stent assessment to ≈ 25% at week 4, ≈ 40% at week 8, ≈ 55% at week 12 and > 60% at week 16.
There was evidence of time versus treatment interaction for dysphagia (p-value = 0.013), (Figure 10). It can be seen that at week 4 the median dysphagia was higher in the radiotherapy arm, but by week 8 scores were the same in both arms. This short-term deterioration was expected hence the requirement for two successive deteriorations needed in the definition of the primary end point.
FIGURE 10.
Box plots of dysphagia scores by time and treatment arm.
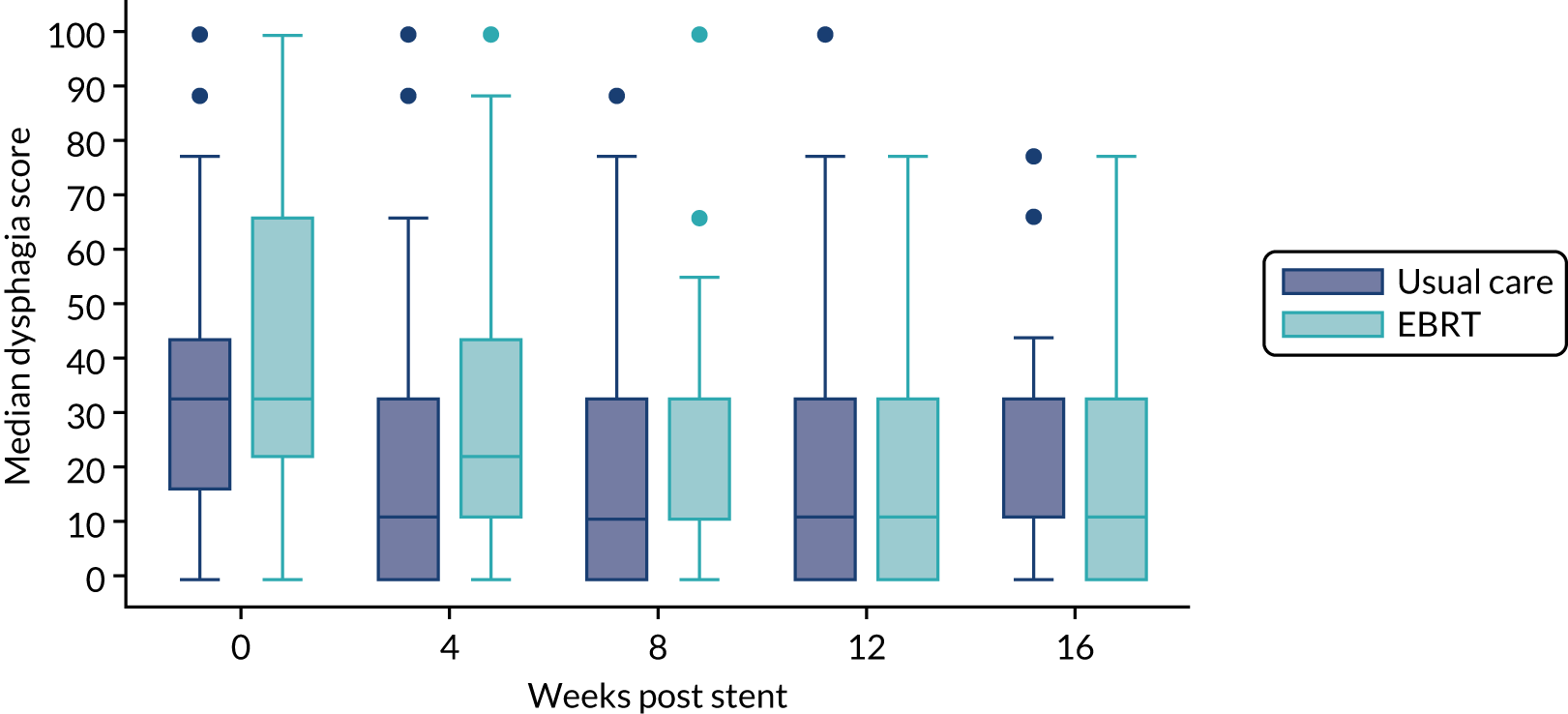
There was evidence of time versus treatment interactions for pain as measured by the EORTC QLQ-C30 (p = 0.005) (Figure 11). It can be seen that at weeks 8, 12 and 16 the median pain score was higher in the radiotherapy arm. There was also evidence of time versus treatment interactions for constipation (p = 0.009). The mean score at weeks 8, 12 and 16 was higher in the radiotherapy arm, mirroring the pain scales and possibly related to higher use of analgesia.
FIGURE 11.
Box plots of pain scores (QLQ-C30) by time and treatment arm.
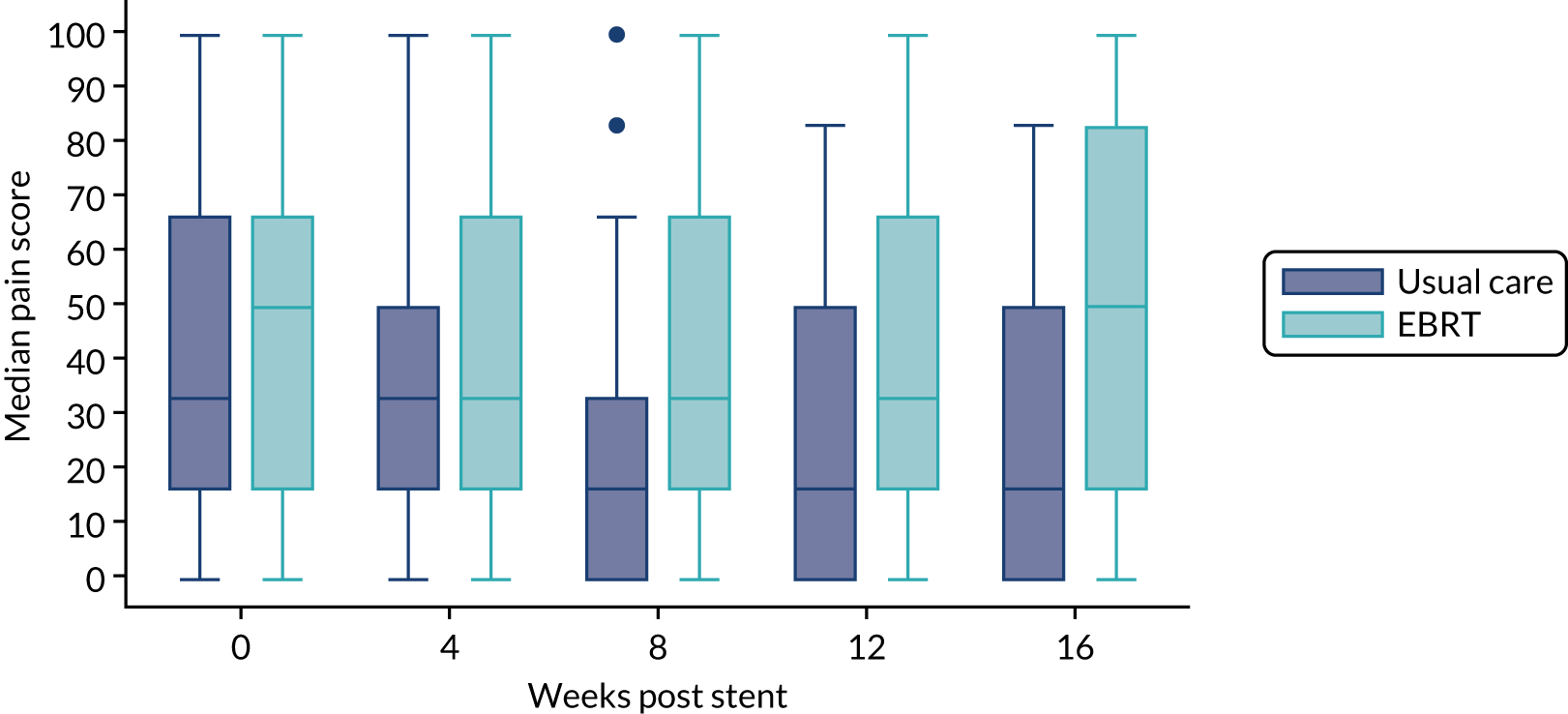
There was also evidence of time versus treatment interactions for cognitive functioning (p = 0.007, with higher median scores at weeks 12 and 16 in the radiotherapy arm), body image (p = 0.011, with higher median scores at weeks 8 and 16 in the radiotherapy arm), anxiety (p = 0.002, with higher mean scores at weeks 12 and 16 in the radiotherapy arm) and trouble swallowing saliva (p = 0.025, with higher median scores at week 16 in the radiotherapy arm). These findings are more difficult to understand and may be due to chance.
There was no evidence for time–treatment interactions for fatigue (p = 0.522; Figure 12), weight loss (p = 0.053), WHO performance status (p = 0.565; Appendix 3, Figure 23) or any of the other HRQoL scales.
FIGURE 12.
Box plots of fatigue scores by time and treatment arm.
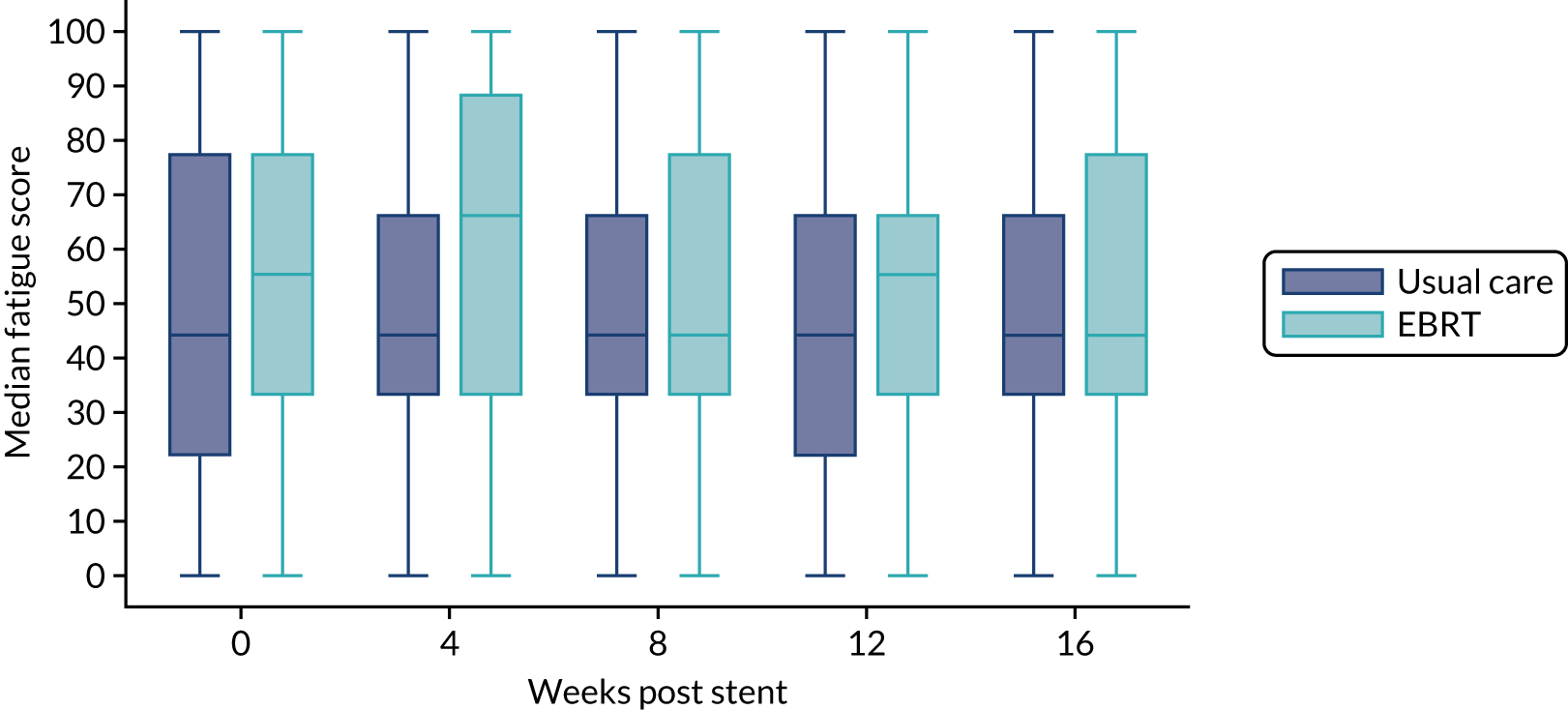
Upper gastrointestinal-related bleeding events
There was evidence of a difference in time to first upper GI-related bleed event or upper GI-related hospital admission between trial arms (Figure 13). Adjusted subhazard ratio was 0.52 (95% CI 0.28 to 0.97; p = 0.038, n = 199). Median time to first upper GI-related bleeding event or hospital admission for bleeding event was 49.0 weeks (95% CI 33.3 to not reached weeks) versus 65.9 weeks (95% CI 52.7 to not reached weeks) in the usual-care arm versus the EBRT arm, respectively. Three participants versus four participants (usual care vs. EBRT) had anticoagulant treatment within 4 weeks before the first upper GI-related bleeding event. Table 11 shows the analysis of upper GI-related bleeding events by trial arm, overall and up to week 16. Overall, up to week 52, 29 (28.4%) patients versus 16 (16.5%) patients had an upper GI-related bleed event (usual care vs. EBRT). The number needed to treat was therefore 8.4. Blood transfusion was the most common event (25.5% vs. 13.4%, usual care vs. EBRT), followed by haematemesis (4.9% vs. 6.2%) and upper GI haemorrhage or bleed (7.8% vs. 2.1%).
FIGURE 13.
Cumulative incidence function plot of time to first upper GI-related bleed event or hospital admission by trial arm, with death as a competing risk.
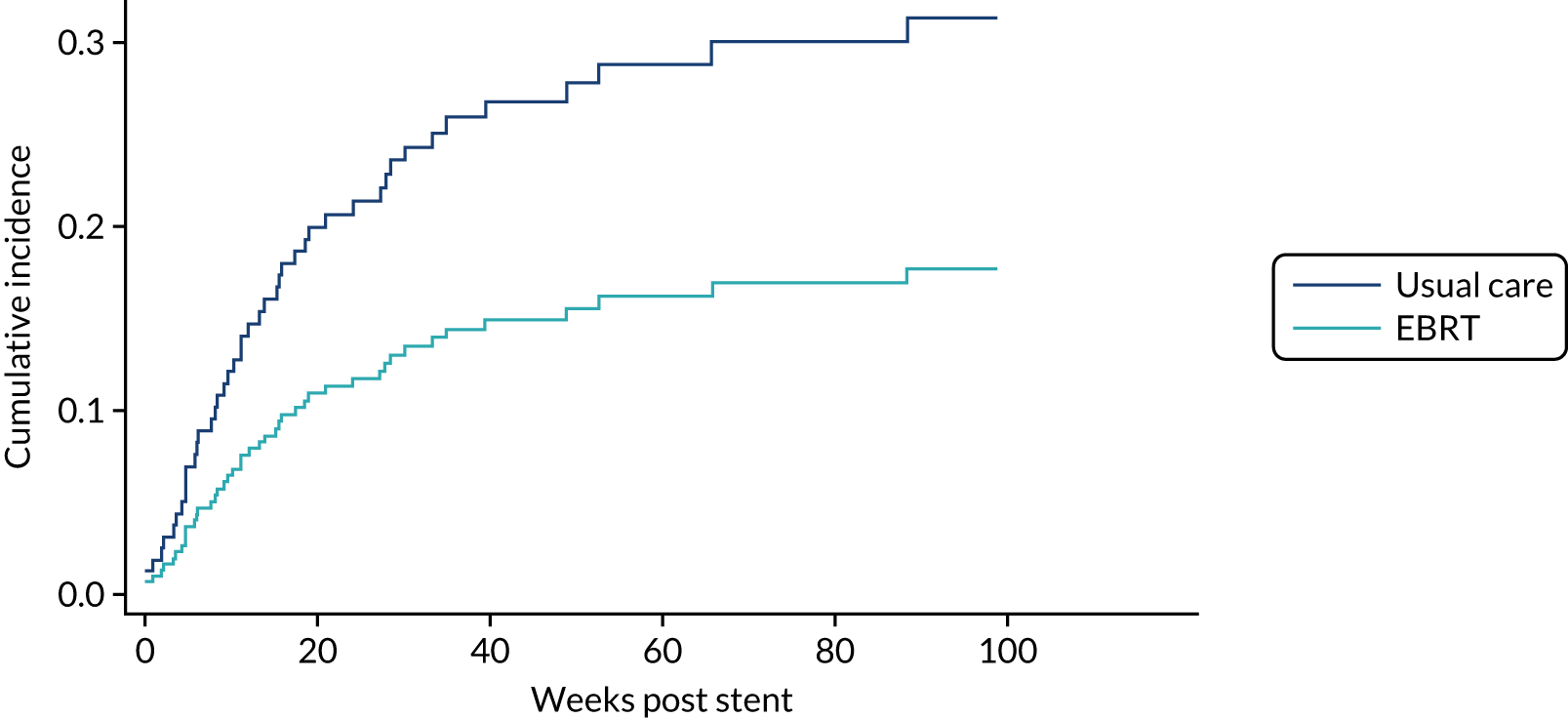
| Characteristic | Usual care (N = 102) | EBRT (N = 97) |
|---|---|---|
| Overall | ||
| Number of patients with at least one upper GI-related bleeding event, n (%) | 29 (28.4) | 16 (16.5) |
| Absolute risk reduction | 0.119 | |
| Number needed to treat | 8.4 | |
| Number of patients experiencing each type of upper GI-related bleeding event, n (%) | ||
| Blood transfusion | 26 (25.5) | 13 (13.4) |
| Haematemesis | 5 (4.9) | 6 (6.2) |
| Upper GI haemorrhage or bleed | 8 (7.8) | 2 (2.1) |
| Melaena | 4 (3.9) | 0 (0.0) |
| Argon plasma coagulation due to bleed | 0 (0.0) | 1 (1.0) |
| Additional radiotherapy due to bleed | 1 (1.0) | 0 (0.0) |
| Anaemia due to bleed | 4 (3.9) | 2 (2.1) |
| Number of patients with upper GI-related bleeding event who received antiplatelet drugsa | 0 (0.0) | 0 (0.0) |
| Number of patients with upper GI-related bleeding event who received anticoagulantsb | 7 (6.9) | 7 (7.2) |
| Number of patients with upper GI-related bleeding event who received NSAIDs other than aspirin | 4 (3.9) | 0 (0.0) |
| Up to week 16 | ||
| Number of patients with at least one upper GI-related bleeding event, n (%) | 19 (18.6) | 10 (10.3) |
| Absolute risk reduction | 0.083 | |
| Number needed to treat | 12.0 | |
| Number of patients experiencing each type of upper GI-related bleeding event, n (%) | ||
| Blood transfusion | 13 (12.7) | 9 (9.3) |
| Haematemesis | 4 (3.9) | 3 (3.1) |
| Upper GI haemorrhage or bleed | 6 (5.9) | 1 (1.0) |
| Melaena | 3 (2.9) | 0 (0.0) |
| Argon plasma coagulation due to bleed | 0 (0.0) | 0 (0.0) |
| Additional radiotherapy due to bleed | 0 (0.0) | 0 (0.0) |
| Anaemia due to bleed | 2 (2.0) | 2 (2.1) |
| Number of patients with upper GI-related bleeding event who received antiplatelet drugsa | 0 (0.0) | 0 (0.0) |
| Number of patients with upper GI-related bleeding event who received anticoagulantsb | 5 (4.9) | 4 (4.1) |
| Number of patients with upper GI-related bleeding event who received NSAIDs other than aspirin | 2 (2.0) | 0 (0.0) |
No patients in either arm who experienced an upper GI-related bleed event received antiplatelet drugs at any point over the 52 weeks, but 6.9% versus 7.2% received anticoagulants. A total of 4.9% in the usual-care arm received non-steroidal anti-inflammatory drugs (NSAIDs) other than aspirin, but none in the EBRT arm did so.
Up to week 16, 19 patients (18.6%) versus 10 patients (10.3%) had an upper GI-related bleed event (usual care vs. EBRT). The number needed to treat was therefore 12.0. Blood transfusion was the most common event (12.7% vs. 9.3%, usual care vs. EBRT), followed by haematemesis (3.9% vs. 3.1%) and upper GI haemorrhage or bleed (5.9% vs. 1%). No patients in either arm who experienced an upper GI-related bleed event received antiplatelet drugs at any point over the 16 weeks, but 4.9% versus 4.1% received anticoagulants. Two per cent in the usual-care arm received NSAIDs other than aspirin, but none in the EBRT arm did so.
A post hoc subgroup analysis was conducted to look for evidence of an interaction between treatment allocation and tumour length on time to first bleed but none was found (p-value for interaction 0.947; adjusted for randomisation stratification variables). The treatment effect was consistent in both subgroups: for baseline tumour length < 6 cm, adjusted subhazard ratio = 0.58 (95% CI 0.24 to 1.38; p-value 0.216; n = 88); for those patients with baseline tumour length ≥ 6 cm, adjusted subhazard ratio = 0.60 (95% CI 0.24 to 1.48; p-value 0.265; n = 94).
With or without an upper GI bleed event, antiplatelet and anticoagulant drug administration was reasonably balanced between trial arms (Table 12). Very few patients received antiplatelet treatment; however, 16.7% versus 18.6% of patients (usual care vs. EBRT) received anticoagulants. In total, six patients (5.9%) in the usual-care arm received tranexamic acid versus none in the EBRT arm. Overall, 38.2% versus 49.5% of patients (usual care vs. EBRT) received opioids.
| Treatment | Usual care (N = 102), n (%) | EBRT (N = 97), n (%) |
|---|---|---|
| Aspirin | 1 (1.0) | 2 (2.1) |
| Clopidogrel | 0 (0.0) | 2 (2.1) |
| Tranexamic acid | 6 (5.9) | 0 (0.0) |
| Anticoagulants | 21 (20.6) | 18 (18.6) |
| NSAIDs other than aspirin | 7 (6.9) | 1 (1.0) |
| Opioids | 39 (38.2) | 48 (49.5) |
Dysphagia-related stent complications and reinterventions
There was no evidence of a difference in time to first dysphagia-related stent complication or reintervention event between trial arms [see Appendix 3, Figure 24; adjusted subhazard ratio 0.79 (95% CI 0.37 to 1.66; p = 0.529; n = 199)]. Median time to first dysphagia-related stent complication or reintervention event was 45.7 weeks (95% CI 37 to not reached weeks) versus 58.9 weeks (95% CI 36.7 to not reached weeks) (usual care vs. EBRT).
Additional stent insertion
There was no evidence of a difference in time to first additional stent insertion between trial arms (adjusted subhazard ratio 0.58, 95% CI 0.23 to 1.46; p = 0.246; n = 199). Median time to first additional stent insertion was not reached versus 59.7 weeks (95% CI 43.3 to not reached weeks) stent versus stent plus radiotherapy.
Repeat endoscopy
There was no evidence of a difference in time to first repeat endoscopy between trial arms (see Appendix 3, Figure 25; adjusted subhazard ratio 1.72, 95% CI 0.86 to 3.47; p = 0.126; n = 199). Median time to first repeat endoscopy (in weeks) was not reached in either arm.
Overgrowth or undergrowth of stent
There was no evidence of a difference in time to first overgrowth or undergrowth of stent event between trial arms (see Appendix 3, Figure 26; adjusted subhazard ratio 0.83, 95% CI 0.21 to 3.24; p = 0.785). Median time to first overgrowth or undergrowth of stent event (in weeks) was not reached in either arm.
Stent-related pain
There was no evidence of a difference in time to first grade 2+ stent-related pain event between trial arms (Figure 14; adjusted subhazard ratio 1.27, 95% CI 0.80 to 2.03; p = 0.318; n = 199). Median time to first stent-related pain event was not reached versus 36.3 weeks (95% CI 20.0 to not reached weeks) stent versus stent plus radiotherapy.
FIGURE 14.
Cumulative incidence function plot of time to first grade 2+ stent-related pain event by trial arm, with death as a competing risk.
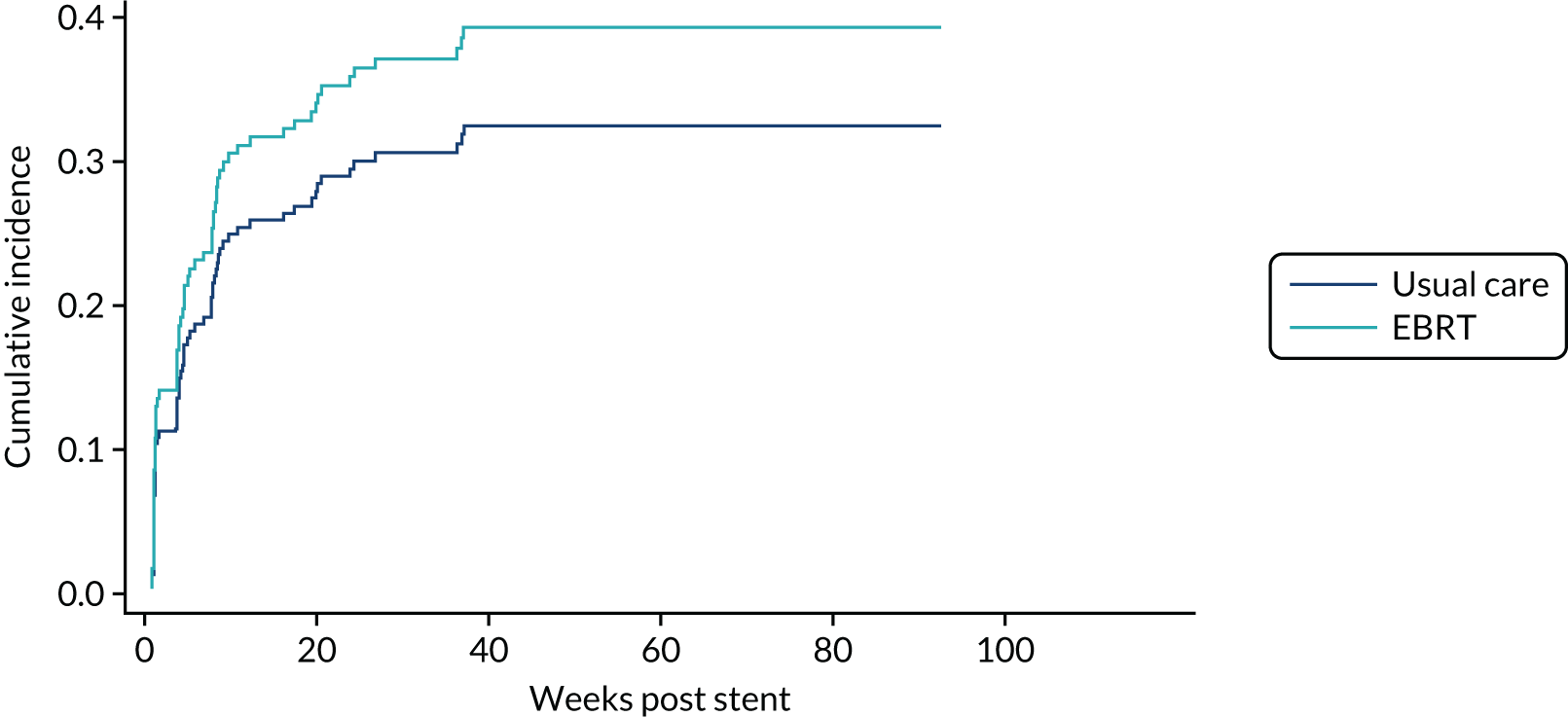
Toxicity
Figure 15 shows the percentage of patients experiencing each grade 3/4 toxicity by trial arm after week 1 up to week 16. There was more vomiting in the radiotherapy arm, primarily at week 4 (2.3% vs. 11.9%), and this was also seen at week 4 in the QLQ score (see Appendix 3, Table 30 and Figure 27). There was also more fatigue in the EBRT arm, particularly at week 12 (4.9% vs. 15.4%), consistent with the fatigue scores reported on the QLQ-C30 questionnaires at these time points (see Figure 12 and Appendix 3, Table 30). There was also more nausea, anorexia and stent-related pain in those receiving radiotherapy, but less anaemia and upper GI haemorrhage.
FIGURE 15.
Bar chart showing percentage of participants experiencing each grade 3/4 toxicity up to week 16, by trial arm.
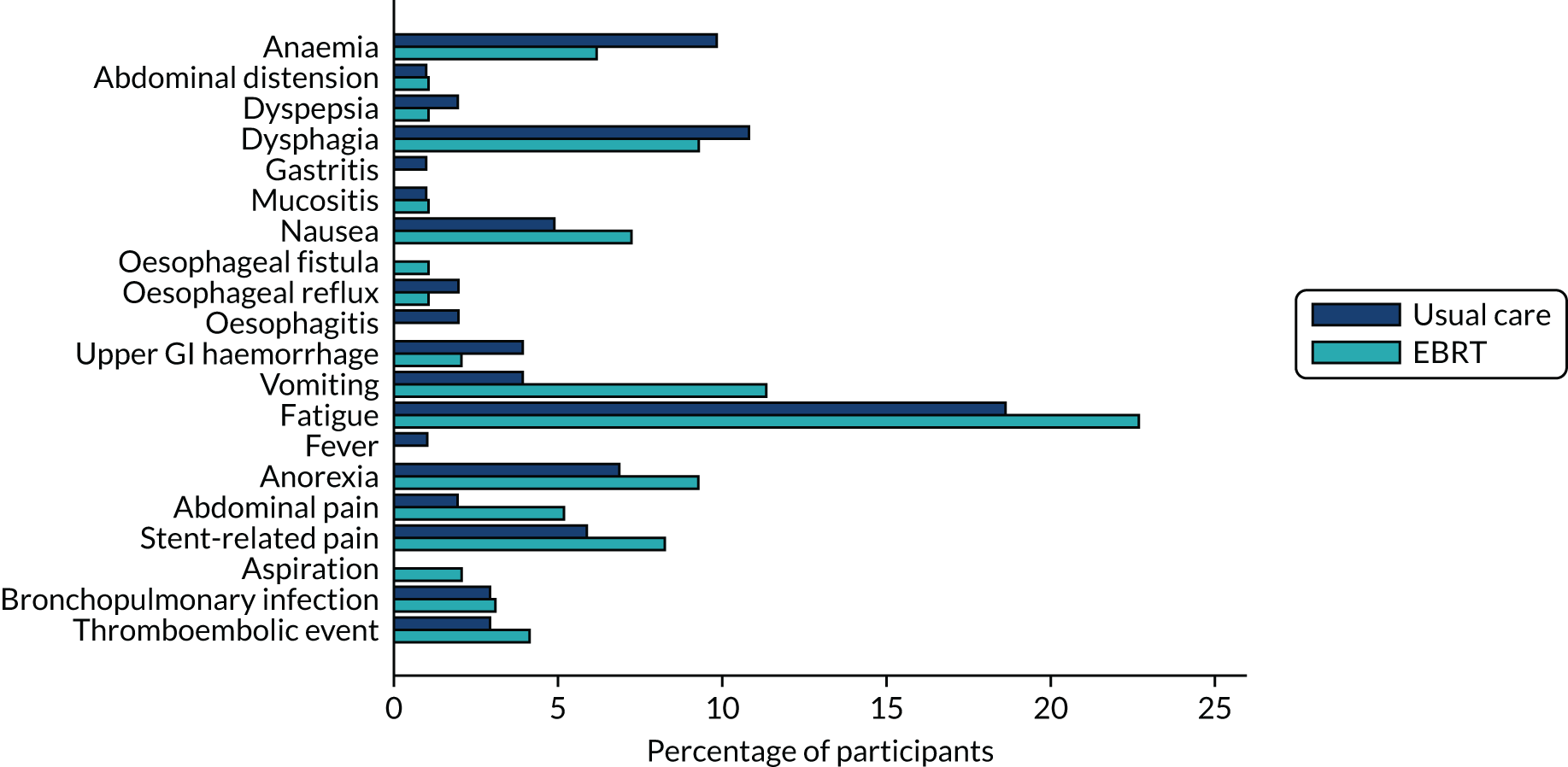
Three deaths (stent: one fall, one myocardial infarction; stent plus radiotherapy: one multifocal ischaemic stroke) were reported through the SAE system and none was thought to be related to treatment.
Additional palliative radiotherapy
In total, 19.6% versus 9.3% (usual care vs. EBRT) of patients received additional palliative radiotherapy (Table 13). In the usual-care arm, 80% of these received radiotherapy to the oesophagus, compared with 22.2% in the EBRT arm. The median dose and fractions were 20 Gy in five fractions in the usual-care arm and 8 Gy in one fraction in the EBRT arm.
| Characteristic | Usual care (N = 102) | EBRT (N = 97) |
|---|---|---|
| Additional palliative radiotherapy, n (%) | ||
| Yes | 20 (19.6) | 9 (9.3) |
| No | 82 (80.4) | 88 (90.7) |
| If yes, organ/region, n (%) | ||
| Oesophagus | 16 (80.0) | 2 (22.2) |
| Bilateral sub clavian fossa | 1 (5.0) | 0 (0.0) |
| Brain | 1 (5.0) | 1 (11.1) |
| Femur | 1 (5.0) | 0 (0.0) |
| Gastro-oesophageal junction | 1 (5.0) | 0 (0.0) |
| L1–L3 vertebrae and left pelvis | 0 (0.0) | 1 (11.1) |
| L4–S1 | 0 (0.0) | 1 (11.1) |
| Left lung | 1 (5.0) | 0 (0.0) |
| Left supraclavicular fossa | 0 (0.0) | 1 (11.1) |
| Lumbar spine | 0 (0.0) | 1 (11.1) |
| Posterior ribs | 0 (0.0) | 1 (11.1) |
| Right hip | 1 (5.0) | 0 (0.0) |
| Scapula | 0 (0.0) | 2 (22.2) |
| Total dose (Gy), median (IQR), na | 20.0 (8.0–30.0), 19 | 8.0 (8.0–20.0), 9 |
| Total fractions, median (IQR), na | 5.0 (3.0–10.0), 19 | 1.0 (1.0–5.0), 9 |
Post-stent insertion chemotherapy
Table 14 shows post-stent insertion chemotherapy given. The MDT intended to give chemotherapy to 36 out of 102 (35.3%) versus 34 out of 97 (35.1%) patients. Although 29 out of 36 (80.6%) of these in the usual-care arm were given chemotherapy, less than half of those [15/34 (44.1%)] in the EBRT arm were given chemotherapy. An additional four versus two patients, for whom the MDT had not originally intended to give chemotherapy, received chemotherapy post stent.
| Usual care (N = 102) | EBRT (N = 97) | |
|---|---|---|
| MDT intends to give chemotherapy, n (%) | 36 (35.3) | 34 (35.1) |
| If yes, did patient receive chemotherapy for oesophageal cancer post stent insertion? n (%) | ||
| Yes | 29 (80.6) | 15 (44.1) |
| Received chemotherapy for oesophageal cancer post stent insertion, n (%) | ||
| Yes | 33 (32.4) | 17 (16.7) |
| No | 68 (66.7) | 79 (77.5) |
| Missing | 1 (1.0) | 1 (1.0) |
| If yes, which?a n (%) | ||
| ECF | 2 (6.1) | 1 (5.9) |
| Capecitabine | 2 (6.1) | 0 (0.0) |
| EOX | 13 (39.4) | 8 (47.1) |
| 5FU | 1 (3.0) | 0 (0.0) |
| Other | 31 (93.9) | 13 (76.5) |
| (1) ECX × 3 cycles, (2) docetaxel × 3 cycles and (3) irinotecan | 0 (0.0) | 1 (5.9) |
| Trastuzumab, capecitabine + cisplatin | 1 (3.0) | 0 (0.0) |
| CX | 4 (12.1) | 1 (5.9) |
| CX + herceptin | 6 (18.2) | 2 (11.8) |
| Capecitabine | 2 (6.1) | 0 (0.0) |
| Carboplatin | 1 (3.0) | 0 (0.0) |
| Carboplatin + epirubicin | 1 (3.0) | 0 (0.0) |
| Carboplatin + herceptin | 0 (0.0) | 2 (11.8) |
| Carboplatin + paclitaxel | 1 (3.0) | 1 (5.9) |
| Cisplatin | 2 (6.1) | 1 (5.9) |
| Docetaxel | 4 (12.1) | 1 (5.9) |
| Durvalumab | 1 (3.0) | 0 (0.0) |
| ECX | 0 (0.0) | 1 (5.9) |
| Epirubicin + oxaliplatin | 1 (3.0) | 0 (0.0) |
| Folfiri | 1 (3.0) | 0 (0.0) |
| Herceptin | 0 (0.0) | 1 (5.9) |
| Irinotecan | 0 (0.0) | 1 (5.9) |
| OxCap | 2 (6.1) | 0 (0.0) |
| Oxaliplatin | 2 (6.1) | 0 (0.0) |
| Paclitaxel | 1 (3.0) | 0 (0.0) |
| Paclitaxel and ramucirumab | 1 (3.0) | 0 (0.0) |
| Raltitrexed and oxaliplatin | 0 (0.0) | 1 (5.9) |
| Intended number of cycles, median (IQR), n | 6.0 (4.0–6.0), 29 | 6.0 (4.0–6.0), 16 |
| Intended number of cycles missing, n (%) | 4 (12.1) | 1 (5.9) |
| Number of cycles given, median (IQR), n | 3.0 (2.0–4.0), 33 | 4.0 (3.0–4.0), 16 |
| Number of cycles given missing, n (%) | 0 (0.0) | 1 (5.9) |
Chapter 4 Qualitative study
Background
The prognosis for oesophageal cancer is often poor, and most patients present with incurable disease, with a mean survival of 3–5 months. Cancer Research UK has identified oesophageal cancer as a priority for clinical research,39 but there is limited literature about the experience of living with the disease. In qualitative research involving 13 patients,40 the information-seeking patterns and needs of patients with oesophageal cancer were elicited. Patients outlined the strategies used to manage uncertainty, such as trial and error and rationalisation, and emphasised the need to consider the whole family in delivering sufficient information. A recent systematic review41 described a lack of services designed to meet the needs of people with oesophageal cancer and recommended more bespoke support for complex cases. In addition, there are complex implications for the patient’s everyday life and idiosyncratic beliefs with challenges to role and identity. A study with five patients highlighted these issues through narrative interviews that were analysed using a phenomenological–hermeneutic approach. 42,43
Dysphagia is one of the most distressing manifestations of the disease and the main focus of treatment, while fatigue challenges activities of everyday life. 44 The psychosocial role of food adds a further dimension to the perceived challenges posed by oesophageal cancer to the mechanics of eating. Palliation of dysphagia remains the focus of treatment but may result in specialist palliative interventions being overlooked. Clinical outcomes among 131 patients who underwent palliative interventions highlighted the palliative limitations of treatments such as SEMSs. 45
Qualitative methods were used to explore the views and experiences of patients in this trial. The aim was to understand how patients understood and experienced the trial itself and what effect the insertion of a stent had on patients’ QoL. The study particularly explored experiences of patients who also received radiotherapy. The qualitative data form an essential part of the trial’s evaluation, provide in-depth patient-centred assessment and support further understanding of the quantitative trial’s results.
Aims
The qualitative component of the trial had two aims: (1) to explore the acceptability and feasibility of patients’ recruitment to the trial and (2) to explore participants’ experience of trial interventions. Patients’ experiences of consent and recruitment, including reasons for declining, were elicited, as were patients’ motivation to accept randomisation to an intervention that may have included extra radiotherapy. Recruitment to the qualitative component was optional and required separate consent. Patients who declined to participate in the trial, but who did consent to the qualitative component, were offered an interview about their reasons for declining as soon as possible after the approach to participate.
By drawing on the reported experiences of patients, the ROCS qualitative study focused on the following objectives to:
-
explore patients’ motivation for participation in the trial
-
explore patients’ perceptions of participation in the trial
-
explore reasons for non-consent to the trial
-
assess patient experience and perceptions of each trial arm
-
identify potential improvements to the recruitment process.
Methods
Data collection
Information about the optional qualitative study was given to trial participants (and those who declined) by research nurses at the research sites. Patients were provided with a tear-off slip and a stamped addressed envelope to return their contact details directly to the qualitative researcher. Patients were then contacted by the researcher to arrange a time and location for the interview to take place. The qualitative researcher thoroughly explained the study to the patient, giving them ample opportunity to ask questions and make an informed decision before signed consent was taken from each participant at the time of interview. All patients were made aware that they were able to withdraw from the study at any point, without explanation. Patient companions at the time of interview were also asked for their consent to record and use their interview data.
Subsequent to the consent process, the interviews were conducted at either the participant’s home or an alternative quiet location including hospital rooms. Interviews took between 19 and 116 minutes, with a median time of 68 minutes. For all of the interviews, semistructured topic guides were used to ensure consistency among participants. The interview guide was adapted following initial interviews to reflect participants’ concerns and perceptions. Participants were encouraged to discuss their wider experiences and perceptions in relation to the trial, treatment interventions, or oesophageal cancer generally. All interviews were audio-recorded and transcribed verbatim.
The interviewers were experienced researchers with backgrounds in occupational therapy and narrative analysis and sociology. Both interviewers have experience of palliative care research and conducting interviews concerning sensitive topics.
Data analysis was based on the analysis of 30 longitudinal interviews conducted with 15 patients over the first 8 weeks of their trial involvement (Table 15). Nine patients were randomised to the EBRT arm (received radiotherapy after stenting) and six to the usual-care arm (no radiotherapy). All patients were allocated pseudonyms to protect their identity. Each reference to a participant is followed by a letter, as follows: a, first interview; b, second interview; c, third interview. One patient declined to participate in the study and two research nurses also participated in interviews.
| Arm | Participant | Sex | Number of interviews per participant |
|---|---|---|---|
| EBRT | Elsie | F | 3 |
| May | F | 2 | |
| Emma | F | 1 | |
| Camilla | F | 1 | |
| Michael | M | 2 | |
| Penelope | F | 3 | |
| Stan | M | 2 | |
| Arthur | M | 1 | |
| Peter | M | 3 | |
| Total | 9 | 18 | |
| Usual care | Bernard | M | 3 |
| Janet | F | 3 | |
| Betty | F | 2 | |
| Calvin | M | 2 | |
| Joan | F | 1 | |
| Kenneth | M | 1 | |
| Total | 6 | 12 | |
Data analysis
Interviews were analysed using Braun and Clarke’s framework for thematic analysis. 46 Analysis moved from familiarisation with interviews to initial coding and identifying and defining themes. Coding comprised two approaches: first, data were coded to elicit specific interpretation around key areas of research study participation and comparison across the two study arms; and second, a coding framework was developed around coping and QoL to elicit a rich description of the experience of living with oesophageal cancer.
The coding process was managed using NVivo (QSR International, Warrington, UK) version 11 software and reflected the interview process, with the interviewer asking specific questions around trial experience but taking an inductive approach to describe accounts of the everyday experience of patients and carers. Ten per cent of coded data were checked for agreement by a member of the trial team (AN).
Themes were identified in 16 areas relating to trial experience, and overall coping and QoL when living with oesophageal cancer (see Table 16). These included symptoms and management, deciding to join the trial and experience of trial interventions. The next stage of analysis looked at higher-level themes, exploring what the nature of the strategies and barriers were and how patients constructed their experience of oesophageal cancer.
Once coding and the development of the themes were completed, two frameworks evolved: one further developing specific insights around trial experience and the other an interpretive account of living with oesophageal cancer. Supplementary materials are available in the appendices to illustrate the depth of data available for each theme.
Results
There was an overlap between the two frameworks, such as the experience of the stent, but the relationship between coding and themes was central (i.e. stent experience within the trial and stent within themes of living with oesophageal cancer, e.g. adaption, agency and uncertainty). Table 16 outlines the emergent themes and subthemes.
| Frameworks | Themes | Subthemes |
|---|---|---|
| Trial experience | Recruitment to trial | Decision-making and joining the trial |
| Patients’ perceptions of trial |
Information Trial understanding |
|
| Randomisation | Randomisation and equipoise | |
| Experiences of trial (burdens and benefits) |
Trial experience Questionnaires |
|
| Research practitioner perspective | Staff and NHS experience | |
| Living with oesophageal cancer | Experiences of each trial arm |
Chemotherapy Radiotherapy Information Symptoms and management |
| Life course (responses to social changes) |
Coping and QoL Information Diet and weight loss |
|
| Medical management (responses to physical changes) |
Diagnosis Coping and QoL Diet and weight loss Medication Staff and NHS experience Symptoms and management |
|
| Everyday life (responses to psychosocial changes) |
Coping and QoL Diet and weight loss Information Symptoms and management |
|
The following section highlights the experiences of patients and carers regarding their recruitment and participation in the trial. It outlines participants’ perceptions, including randomisation of each trial arm and perspectives from non-consenters. In addition, the experiences of participants, including the QLQs and burdens and benefits of the trial, and researchers’ perspectives are demonstrated.
Recruitment to the trial
Participants spoke at length about their experiences of recruitment to the trial, including their reasons for participating. Most patients expressed a desire to help others in the future through the research, implying a sense of altruism:
I can help somebody, someone else, whatever, kids, or something, yeah, that’s how I feel anyway, I said it’s not for me, for someone down the line.
Usual-care patient, Joan a
It seemed to be not be too much trouble and if it’s to help other people in the future that would be a bonus.
EBRT patient, May a
Patients reported that they wanted to give back to the health service: ‘A very tiny payback for an enormous amount of care I’m about to get’ (usual-care patient, Betty a). Other patients felt that they would potentially benefit from the trial itself, particularly if they received radiotherapy: ‘I was thinking radiography part of it might have helped me’ (usual-care patient, Bernard a). For some patients, participation offered ‘a bit a bit of hope’ (usual-care patient, Bernard b).
Perceptions of trial participation
In the initial stages of the qualitative study (the first 6 months of data collection), the time between the research nurse approaching the patient about the study and the stent procedure taking place was very short, and this had a significant impact on the participants’ recall of the recruitment process. Common across participants’ reflections were feelings of being too rushed:
She [research nurse] came to hospital just before I was having the stent because it was all rushed . . . I was ready to go to the procedure, so I was kind of [loud bang] mind going to other places.
EBRT patient, Arthur a
Participants also reported feeling very stressed and pressured at the time of receiving the information, recounting the volume of information at recruitment as being overwhelming and often repetitive:
It’s just a lot of information to take on board they just.
was in no state to read it.
EBRT patient, Elsie b
However, over the course of the research, which incorporated real-time reporting of participant interview data relating to trial processes at TMG meetings, the research team worked to improve patients’ experiences of recruitment. Participants reported in later interviews that recruitment was not overly rushed or confusing and information was given clearly and, typically, multiple times: ‘They gave me all the paperwork [quite easy] to read and I took it home I read it’ (EBRT patient, Stan a). Participants were aware that they could ask questions, whom they could ask and how they could contact them: ‘I’ve got all names local hospital telephone numbers and all local hospital’s telephone everybody’s telephone number’ (EBRT patient, May b).
All participants reported having some comprehension of the study. However, with probing it was evident that some patients were less clear about the details of the study than others. These points of confusion included when and for how long research visits would take place: ‘Does she [research nurse] carry on any length of time?’ (EBRT patient, Camilla a). Participants were at times uncertain about what researchers wanted to find out from them: ‘What are you learning from me like, you know’ (EBRT patient, Calvin b).
Others felt that better clarification and explanation was required of the questionnaires, as patients were unclear about the meaning of some of the questions and felt that they were repetitive:
There’s not much difference in their context as a question . . . and there’s not much difference in their context as an answer, if you know what I mean . . . I think there’s some that you should be able to trim down you know.
EBRT patient, Calvin b
This information was fed back to the research nurses at the regular investigator meetings and used to improve discussions with patients around trial design and processes.
Randomisation
Patients often expressed confusion regarding the randomisation process. Several participants struggled to understand what the different trial arms meant. Uncertainty was expressed regarding whether or not radiotherapy would be offered regardless of the randomisation outcomes, as patients did not understand the equipoise of the trial: ‘If I volunteer for it, I think I should go on it’ (EBRT patient, Stan a). Patients sometimes felt that disease status, general health or prognosis influenced the outcome:
[I] Wondered if I should be having radiotherapy but I’ve been told by other people friends who are doctors who say that it’s not suitable anyway.
Usual-care patient, Kenneth a
We presumed it was because of the tumour was better to react with the chemo, than radiotherapy and chemo.
EBRT carer, Elsie a
Having identified these points at an early stage of the qualitative study, research nurses were advised to increase patients’ comprehension of the potential outcomes of randomisation. Self-reported understanding subsequently improved, with patients reporting having a good comprehension of the process of randomisation.
Trial allocation: external beam radiotherapy arm
The EBRT arm was perceived favourably by some patients, who were satisfied with their allocation: ‘I feel pleased that I am getting radiotherapy’ (Emma b). Optimism regarding the possibility of shrinking or stopping the tumours growing was also reported:
We’re hoping it would have shrunk the tumour a bit and enable the stent to settle.
Usual-care patient, Kenneth a
. . . the radiotherapy what do you [think] that it would have [done]?
. . . stopped it may be growing hey . . . I think what you’re really looking for is a longer life.
EBRT carer, Elsie a
Trial allocation: usual-care arm
Interviews with research nurses highlighted that it was difficult at times to provide patients with the results of their randomisation allocation, most notably in relation to participants who were randomised to the usual-care arm of the study. Although particular care was taken to highlight the equipoise of the study, participants generally favoured the EBRT arm. A number of patients reported feeling ‘a little disappointed’ at their allocation (EBRT carer, Elsie a).
For some of these patients who received usual care, a general acceptance and rationalisation of the outcome tended to take place over time, in order for the participant to reposition any initial disappointment they may have felt: ‘We now feel that it’s positive that I didn’t get selected’ (usual-care patient, Bernard c).
Primary points that usual-care arm patients discussed were not having to deal with the side effects of radiotherapy, including a potential effect on chemotherapy:
I feel it’s a positive thing to be honest with you because I know if it had happened, there was other things that they couldn’t do . . . so you know and I mean I’m quite happy that my treatment is progressing the way it is.
. . . not been able to have all your chemo ’cos you wasn’t feeling well.
Usual-care arm, Bernard c
Not having to make additional trips to the hospital were also stated as a positive outcome of randomisation to the usual-care arm: ‘I’ve heard a few people having to travel so . . . having radiotherapy there was people going there [hospital] everyday’ (usual-care patient, Janet a).
Patients who declined the trial
This group was difficult to recruit to the qualitative study and only one patient was interviewed. This patient preferred to make the most of time that they had left; thus, they had a different set of priorities: ‘Mainly I got enough going on at the moment and I just want didn’t feel up to doing anything else taking part in that’ (patient declining trial).
Research nurses usefully gave anecdotal reports for the reasons expressed by patients, including concerns over extra travel, time and family commitments. In addition, nurses acknowledged the burden of treatment in relation to frailty: ‘2 weeks of daily radiotherapy is quite a commitment, especially for frail, elderly patients’ (research nurse 1). Concerns were reported about overburdening other family members who accompanied patients and worries over parking difficulties. One research nurse (research nurse 2) reflected that both patients who consented straight away were single and the fact that they did not need to consider others may have been a factor here. Recruitment was also more successful when researchers met people in person to discuss the study. Nurses did not recruit patients by telephone: ‘these people seem to be more unwell and haven’t been stented yet’ (research nurse 2).
Experiences of trial assessments
Discussions about the questionnaire completion raised valuable points. Patients enjoyed the research nurses’ visits, which were perceived as informal opportunities to ask questions and receive information. Visits sometimes provided therapeutic benefits, providing company and support for patients:
Talking to someone, yes it puts you at your ease . . . you can ask someone if you’re not certain . . . you’re there for someone to ask questions if they were worried and we got your phone number[s] . . . I’ll probably miss you both.
EBRT patient, Penelope c
There were instances where the burden or difficulties of the study were notable. Conducting the questionnaire over the telephone could be confusing: ‘It’s better to come here, then you can explain it . . . I’ve never been very good on the phone anyway’ (EBRT patient, Peter c). Others commented that the questionnaires were tedious, repetitive, irrelevant or too long.
Some patients felt that the questionnaires were confusing and required context. They also commented that the format caused difficulty in matching questions and answers to the same line and tick box:
At the time, there was a lot of irrelevant questions in there . . . It kind of doubles up on itself . . . You know when you are ticking the boxes and it becomes like one of them things and you start missing boxes, because actually it’s set out quite bad . . . if it was shaded you say answered that question and then you go across . . . and tick to box.
EBRT patient, Arthur a
Burdens of the study
Burdens associated with study participation were not directly identified by participants, but several issues were raised across the interviews. One of these was information overload at the time of consent:
I was going to have my stent [off] and spoke about the trial at the time it seemed a lot to take on board to make that decision on that very day . . . I wasn’t given much notification initially my thoughts were – I didn’t want to do that.
EBRT patient, Elsie a
The travel costs and practicalities in getting to hospital, regardless of study arm, were burdens:
It cost me five sixty [£5.60] . . . a real struggle to find a space in the hospital.
Usual-care patient, Bernard b
I just had a letter saying that there was an appointment at half past nine in [hospital], which is impossible . . . at that time of day.
Usual-care patient, Kenneth a
Time spent at different appointments was significant: ‘You just end up having so many different appointments don’t you can’t keep up’ (usual-care patient, Janet c). Patients described the physical impact of radiotherapy treatment including nausea, dizziness and tiredness: ‘I always felt a wee bit sickish in the morning’ (EBRT patient, Michael b).
Benefits of study participation
Participants reflected on the benefits of trial participation, including access to research nurses or practitioners:
[Research nurse] gives you a bit more insight . . . because when you see the consultant, he is very fact and figures . . . and when you see someone like yourselves, you obviously dealt with these people before who have been in the same position as myself and you get a bit more like . . . normality rather than clinical.
EBRT patient, Arthur a
Patients appreciated the opportunity ‘to actually have some radiotherapy’ (EBRT patient, Elsie b). Some felt that it increased care including greater monitoring and contact:
Someone keeping tabs to make certain that everything’s alright which you might not necessarily get if you weren’t part of [it] . . . you’re all so friendly and helpful I can’t give you any criticism at all I think it’s beneficial full stop.
EBRT patient, Penelope c
Treatment at clinics was sometimes perceived by patients to be better than they would have received outside the study: ‘They couldn’t be nicer, can’t do enough for you, it makes such a difference’ (EBRT patient, Elsie c). Some patients felt that interviews provided time to talk and reflect. ‘It’s nice to talk to you actually’ (EBRT patient, Penelope c).
The research practitioner perspective
Research practitioners reflected on what patients had reported. They perceived the ROCS study to be challenging and struggled with this: ‘It’s quite a harrowing study’ (research nurse 1). Nurses were concerned when they perceived patients to be too unwell or too frail: ‘When they are more unwell it is an awful lot to ask of them . . . it is 15 pages of information sheets’ (research nurse 1). They felt that informed consent was sometimes conditional: ‘Some patients have said that they’ll consent and then if they don’t get the radiotherapy they’ll withdraw’ (research nurse 2). Likewise, families played a significant role in how and why patients participated in the study and were at times difficult to negotiate: ‘Most of them [patients] are also thinking about their families’ (research nurse 2).
Impact of treatments
This section reports the impact of stent insertion from the usual-care and EBRT arms and the impact of radiotherapy from the EBRT arm. It outlines the experiences patients reported in terms of chemotherapy and palliative care, overall.
Impact of the stent
Patients reported varying experiences of having a stent. Improvement in eating was the main positive outcome, along with improved ability to swallow and less reflux: ‘After the stent put in it was all easier . . . I take all the proper foods down . . . you know my body weight back up again’ (usual-care patient, Janet a).
Although pain was the most reported negative outcome, fear of blockage, damaging the stent and uncertainty about cause of discomfort were sources of anxiety for patients. In turn, this affected patients’ eating habits:
I’ve haven’t really tested it to any extent because I’m only eating [half] of [mashable] of food you know . . . but I’m scared to try and try and swallow you see.
Usual-care patient, Janet c
In some cases, it was difficult to differentiate between the impact that the stent had and the pain and discomfort created by the disease itself or the package of treatments including the stent and chemotherapy:
I got . . . second stent put in, ‘cos the first one slipped . . . I was in a lot of pain and being sick and other things so they took me in and . . . they tried pulling it out and things but couldn’t, so they just had to put another stent on the top. So, once they put that on the other and then the week after that I went for chemotherapy, which is just like you can imagine it’s like feeling pretty much like rubbish really . . .
But the stent is working.
Yeah and I think it’s important to not lose sight of that. I can swallow a lot more easily.
EBRT arm, Emma a
Pain and discomfort around the stent were significant across both trial arms, as were nausea and acid reflux, particularly immediately after the stent procedure:
I’ve got terrible indigestion . . . Night and day [coughs] . . . It’s like wind, like, wind just very cold around, up my throat and all around, but I am alright, it eases up sometimes . . . I think it’s worse actually . . . I think it’s worse than last week.
Usual-care patient, Joan a
Some patients explicitly compared their symptoms pre and post stent insertion, with most describing some benefits:
Before the stent went in, I had reflux . . . I know that reflux used to come up like soapy water. Bursting, you know come up in a bubble. Now since I have had that stent put in, I haven’t had that watery substance . . . but it clears in extra-long time . . . it really gives me pain, but it doesn’t last . . . as before.
Usual-care patient, Calvin a
Patients were uncertain about the long-term success of the stent and would have liked better follow-up information and contact with health-care professionals:
When I came out I didn’t know what I was supposed to be taking in the way of medication and I rang my GP [general practitioner] and I didn’t have a reply then he did reply and he said that he would send a district nurse . . . but nobody came. I didn’t have any contact with anybody for 5 days . . . and I got all this medication now and I don’t think some of it is really suitable for me but it’s not for me to decide.
Usual-care patient, Kenneth a
Greater support and advice for patients around diet, eating habits and postural adjustment were needed, as trial and error was the main strategy for managing the stent:
Then we got this stent in, it was very uncomfortable with the acid and then the hospital they had to give us stuff, they gave that Gaviscon [® GlaxoSmithKline plc, Brentford, UK] . . . but I didn’t like that I think it’s awful and then I used it . . . I just seemed to think it stuck there and it’s to me it was awfully gassy but may be if you persevere with it but when I first come out after getting a stent my brother had that bed, it’s in the hall there.
EBRT patient, Michael a
Impact of radiotherapy (external beam radiotherapy arm)
Patients who received radiotherapy reported a range of post-therapy negative side effects, which were more often described than any positive impact. Negative side effects included loss of appetite because of pain when swallowing. Physical symptoms occurred at varying times post therapy and for different time spans, and some patients reported feeling worse than before the radiotherapy:
My main concern is not eating . . . at the moment it’s even hard to swallow water because of the pain . . . Discomfort, that’s probably a better word . . . Last 2 days it really sort of hit me. I was running sick, tired, couldn’t swallow very well at all worse than what it was before. Even the first week of the radiotherapy I was walking up to the car park . . . I was doing it on the second week, I was thinking I’m not sure I can do that now but again I think that’s the radiotherapy doing that . . . so hopefully that will improve.
EBRT patient, Stan a
It was common for patients to generally feel more unwell, tired, dizzy: ‘Nausea [very] nauseous and nae appetite’ (EBRT patient, Michael b). Despite most patients describing negative side effects of having the radiotherapy, a few reported a corresponding improvement in longer-term symptoms or general well-being:
I didn’t feel that good, I was tired when I was having the therapy I didn’t want to eat. I lost a lot of weight but now it’s all over if you like and I’m beginning to get back I’m eating well.
EBRT patient, Stan b
However, some patients were relieved that the radiotherapy had not caused any further deterioration or side effects:
I thought it was going to make eating a bit more uncomfortable . . . so that kind of didn’t happen which I was really pleased about.
EBRT patient, Elsie b
Improvement in physical and mental well-being was described by one patient: ‘It has made an improvement to my symptoms and also my quality of life’ (EBRT patient, Elsie c).
A lack of knowledge regarding what would happen post therapy was apparent. Participants struggled with the uncertainty around when normality in their daily lives would be restored:
How long before this hard to swallow and can I possibly start eating properly again I don’t know? I struggle to eat anyway ‘cos I don’t I never feel hungry is the problem.
EBRT patient, Stan a
Some participants commented on logistical aspects, including the amount of extra travel and long journeys sometimes necessitated by radiotherapy appointments: ‘I have 8-mile-return journey from [place name] and 80-mile-return journey from here’ (EBRT patient, Camilla a).
Overall, however, patients felt that the treatment itself, especially compared with chemotherapy, ‘wasn’t long at all’ (EBRT patient, Peter b).
Impact of chemotherapy
The responses to chemotherapy among participants in both arms varied, with more patients in the EBRT arm undergoing treatment. Reported benefits of chemotherapy were improved ability to swallow and tumour shrinkage:
I went to the cancer clinic yesterday and they told me that the tumour’s shrunk, so something’s working.
Usual-care patient, Janet a
The wider positive physical and psychological effects of chemotherapy were described by participants. These constructive experiences were, however, often coupled with concerns around uncertainties about the future:
I don’t know what’s happened but I’m certainly feeling better than I have felt for a long time and people are commenting . . . I’m expecting after this next lot of chemo to be feeling better again . . . but I don’t know how much better . . . I mean a couple of other people have warned me that ‘oh well suddenly it’s going to hit you and you’re going to go down’ but that hasn’t happened at all so far . . . so I’m hoping it isn’t going to but maybe it will.
Usual-care patient, Bernard c
Other participants did not tolerate treatment because of unacceptable toxicities, feeling too ill to continue and feeling worse after chemotherapy than before:
I couldn’t deal with it. It was too much, too toxic. So, then they took me off . . . because chemotherapy can just be vicious can’t it?
EBRT patient, Stan b
Participants receiving chemotherapy discussed side effects; some compared the impact that different types of treatments have:
You know you get a reaction after chemo you get everything but I wasn’t expecting that [sickness]. I had radiotherapy before . . . in my mouth and my neck . . . but not the chemo that was the worst.
Usual-care patient, Janet c
At times patients placed hope around the outcomes of receiving chemotherapy:
I’m really hoping once we start the chemo things are going to change and change for the better . . . I’ve got to keep looking up because I can’t, I can’t look down.
Usual-care patient, Bernard a
Participants spoke about the need for further information regarding symptoms:
. . . my voice is annoying me, I can’t talk . . . you know for some reason, I don’t know why . . . I told them in the hospital.
And what have they said?
Er, nothing.
Usual-care patient, Bernard a
Practical implications of chemotherapy involved transport, parking, timing and the impact that it had on carer employment: ‘I would drop him off and pick him up, ‘cos there’s nowhere to park anyway’ (usual-care carer, Janet c).
Palliative care
Palliative care was framed around either personal awareness of a disease transition, or practical advice, usually initiated by the carer: ‘I got [Macmillan nurse] phone numbers, yeah I got contact numbers you know’ (usual-care carer, Janet c). Patients and carers referred to the specific organisations of Marie Curie (Marie Curie, London, UK), Macmillan (Macmillan Cancer Support, Somerset, UK) and Tenovus (Tenovus Cancer Care, Cardiff, UK), in addition to discussing hospice and palliative care and how it may relate to their future medical care. They also provided advice on finances, practical help and medication:
I saw one of the Macmillan benefits advisors as well and he’s given me other travel costs and um I’ve lost a lot of weight of course and had to buy a lot of clothes smaller size clothes so, I got clothing money and travel money from them so that was good.
EBRT patient, May a
Patients framed future disease trajectory around QoL and alleviation of distressing symptoms, particularly pain:
Because it’s not about the bloody length of your life it’s about what you do with it and you have to be out of pain and able to move around.
EBRT patient, Emma a
Patients narrated a transition time, where they were evaluating treatment responses and the potential impact on QoL. Patients still framed any participation in research around benefit to others but evaluated their own personal situation around maintaining QoL:
Nothing they can do, because I haven’t got the strength to take the treatment, neither the chemo nor the radiotherapy. So, all they’re doing is, trying to keep it calm and keep at bay . . . it didn’t seem as though it was going to be a long you know, it wasn’t go to increase life span to a great degree. So, we thought well why put myself through all that trauma and if I see nothing in the end you know? So uh, that’s what we are doing . . . I won’t say it’s a waste of time but it’s a waste of time as far as I’m concerned . . . but whatever I do, somebody in the future may get the benefits, so that’s how we have left it. We are not bothering to go any further.
Usual-care patient, Calvin b
Palliative medicine itself also at times had an impact on the QoL for patients:
You told me that the oral morphine has made you bit more sleepy, bit confused?
Yeah well . . .
Definitely sleepiness and then confusion . . . Ah but I suppose as a result of being sleepy . . . so his appetite probably dropped a little as well.
Usual-care arm, Calvin a
Where communication and symptom management were clear, patients were positive about being adequately supported, despite the paucity of treatment options. Palliative care pathways were considered useful by patients in dealing with the physical and psychological effects of the disease:
I got a feeling that’s going to come to an end because there’s no point, there’s nothing they can say. I got the hospice specialist, she’s probably come to take over from them . . . which is what’s happening now. So, any problems or anything I got now I talk to [hospice nurse], which is better anyway because saves me going there . . . said if you do get a break through pain then they’ll get it sorted.
EBRT patient, Stan b
Summary of the experiences of each trial arm
Overall, patients described varied experiences after receiving the stent, although most reported some level of pain or discomfort but improved ability to swallow. Radiotherapy mainly provided negative side effects including pain, nausea and tiredness, with minimal improvement to QoL. Chemotherapy did deliver benefits for some patients who were strong enough to receive it, although experiences differed significantly. These findings highlighted a need for practitioners to provide bespoke, timely and accurate advice and support relating to all arms of the study. Information such as potential and expected outcomes, pain management and palliative care were necessary for all patients. Importantly, patients required ongoing advice and health-care professional contact. The physical, psychological and financial impact of the extra radiotherapy and chemotherapy needs to be considered in relation to patients, carers and the health service more generally.
Patients’ experiences of living with oesophageal cancer
Patients were uncertain about the aims of treatment, with the majority of patients unsure about what would happen next. The long-term impact of stent intervention was unclear, as was how best to manage the stent in everyday life. Uncertainty affected self-management and access to support. Patients often responded by using coping strategies such as prioritisation around relationships and roles, experimentation around symptom management and physical manifestations of the disease, and rationalisation relating to navigating everyday life.
Responses to social changes
Patients and carers described how they dealt with social changes relating to oesophageal cancer. They highlighted how their plans for the future were disrupted, with retirement and work uncertainties, and their valued social roles compromised:
She [patient] was going to take early retirement as well . . .
. . . this year we were going to buy a get a new car and go round historic houses and whatever and do all this decorating, all so it’s all been put on hold.
EBRT arm, Elsie a
Patients described the diminishing of participation in previously valued activities. Daily living, including hobbies, was affected, with a consequent impact on social life. Marie Curie, Tenovus and Macmillan were the main external agencies mentioned by patients for providing support and advice on these matters. Financial and employment worries were expressed, with several participants expressing relief that they had retired. For some life course challenges, patients spoke of how they were prioritising relationships and valued activities, with a sense of actively considering what was most important to them.
The desire for relative normality and the importance of maintaining their identity, roles and interests were emphasised, as they expressed the wish to continue to be good neighbours, friends and family members themselves. Patients described how there was a need to negotiate and prioritise with their families when dealing with the uncertainties of the disease:
He [son] came and picked me up and I went down there for a few days then he brought me back and stayed with me for a night . . . I was feeling out of my environment a little bit, being there . . . I don’t know what it’s going to be like when he goes back . . . he hasn’t even mentioned it yet. I think it’s going to be when he can see that I have made some, if any, significant improvement . . . So I got a bit of strength, I can get about and do some things for myself.
Usual-care patient, Calvin b
Participants outlined family and social activities, especially regarding eating, that they valued and expressed sadness where they were no longer able to take part in them. They described the sources and also challenges of their support system:
[Wife’s name] has become, I know she’s the reason I want to beat this, but she’s also become like my gaoler if you like. She’ll keep on to me, don’t eat so fast, don’t do this do that, and consequently it seems sometimes like she’s holding me back, although I know that she isn’t . . .
Yeah but if I don’t do it you get blocked . . . So you know what else can I do? I don’t want to see you blocked and I don’t want to be rushing you into A&E [accident and emergency].
Usual-care patient, Bernard c
Patients’ feelings of self-consciousness over altered appearance sometimes led to avoidance of social situations and previously enjoyed activities, reinforcing the sense of a changed self and the impact that their changing physical self had on others:
I want to go out and I just feel so incredibly skinny and I don’t want people staring at me . . . yeah I do feel very self-conscious about how thin I am.
EBRT patient Elsie b
The stress placed on family and friends by oesophageal cancer was acknowledged by patients including feelings of guilt, reinforcing the uncertainty that they felt about their future:
I suppose it’s worrying for all them round about us . . . there’s a lot of people with the same as myself, that go quicker.
EBRT patient, Michael b
The social aspect of hospital clinics was discussed by patients, where at times they felt supported by other patients and the knowledge that there were other people undergoing similar experiences: ‘The majority of people I’ve met seem to be as positive as I am and that helps, doesn’t it?’ (usual-care patient, Bernard c). However, others felt that other patients lacked the capacity to socialise: ‘I don’t think they want talk to anybody anyway because they’re not well’ (usual-care patient, Janet a).
Patients were uncertain about treatment outcomes and their disease trajectory and prioritised the need for hope and for a positive manner, even when the prognosis was poor:
I feel that . . . I am coming out . . . on the good side of it now and that you know we’re going to hear . . . in the coming weeks that something positive has come out of all this and that we’ve actually you know we’re on the good side of it now and that things are going to get better . . . which is certainly what I’m expecting to hear and I’m not just saying that I honestly believe it.
Usual-care patient, Bernard c
Patients stressed the need for a positive and caring approach by clinicians, despite being fully aware of their terminal prognosis:
We went to see [general practitioner] . . . and he said ‘look’ he said ‘don’t think this is the end of your world’ he said ‘I get people in here all the time who been told they got cancer and they live for years and years’ he said ‘you’re not about to die imminently’ so that gave me hope.
Usual-care patient, Bernard a
Patient priorities were primarily concerning personal and professional relationships. Challenges for patients were in managing the expectations of others and in identifying supportive health-care professionals who could manage their prognosis realistically but positively.
Responses to physical changes
The overarching theme around clinical management was uncertainty. Patients were unclear about treatment aims and outcomes, disease trajectories, side effects and symptom management:
I just thought that [feeling sick] was part of the illness . . . I felt that [feeling full up] was part of the treatment because I don’t know, that’s the trouble and I’ve done a lot of research on it and I can’t find nothing, apart from saying you’ll feel nauseous, sick.
Usual-care patient, Janet b
Patients felt overwhelmed by the amount of information that they received, the number of appointments required and the number of different health-care professionals to whom they were introduced, making it challenging to retain information or know who to contact for specific issues: ‘I get confused with it all . . . there’s so many things . . . too many things going on’ (EBRT patient, Stan b). Carers and patients often experimented around practical ways of managing information, such as using diaries to record symptoms and to try to anticipate the likely impact of treatment or trial and error strategies:
When the next cycle came down we put it on the same page . . . so we were able to compare what happened . . . and when [patient] was feeling absolutely terrible I said to her hang on, last time we did this you felt like that. Well in 3 days you should be picking up . . . Hang on, there’s light at the end of the tunnel.
EBRT carer, Elsie c
The different communication styles of health-care professionals had an impact on relationships with patients and carers:
You feel a bit more positive when you see him [doctor]. When you see her [doctor] you don’t . . . and you know it’s bad but you know it’s not what you want to hear . . . you want to hear some of the positive sides . . . you got to focus on the positive.
EBRT patient, Elsie b
Patients and carers were uncertain about staff and their roles: ‘Somebody came from out of the blue one day for a blood test . . . that was about 4 weeks ago, I don’t know why he came’ (usual-care patient, Janet b). The follow-up process was also unclear for many patients:
I haven’t got an appointment . . . I thought I would have seen him [oncologist/stent consultant] again by this time but don’t know, I don’t know how he works his system.
Usual-care patient, Betty a
The role of the research nurse was perceived as multifaceted and research nurses acted as both a key worker and point of contact for some patients and carers, providing clarification on aspects of medication and symptoms:
I wouldn’t go the GP [general practitioner] because they haven’t got a clue, I would try [research nurse] first of all, even if she had to aim me to someone else, because she said phone her up anytime.
EBRT patient, Arthur a
Coping with side effects was complicated by uncertainty over whether disease or treatment was the cause. This had an impact on the ability of the patient to decide whether or not to seek help. Patients and carers adapted to changing situations mainly through trial and error. Areas of concern were primarily pain, nausea, managing poor appetite, nutrition and medication, and the impact that this had on activities of daily living when patients and carers experimented with retraining habits and making lifestyle changes.
Pain was a significant issue, and patients found it difficult to establish whether pain was related to specific activities such as eating or was a short-term consequence of treatment (e.g. the stent insertion) or a sign of advancing disease:
I’ve got almost like a continual heartburn . . . but I’m guessing it’s the stent probably settling in and my swallowing’s been fine. It’s just an irritation down my swallowing tube.
Usual-care patient, Bernard a
Patients were unsure about whether or not they should seek help for pain, drawing on previous experiences and trying to judge possible causes and sometimes opted to simply endure it:
Sometimes it’s been achy there but whether it was the stent or not I really don’t know because I had aches and pains beforehand . . . it could have been overeating or all sorts of things.
EBRT patient, Penelope b
At the end of the day well, you it’s just there’s not much else you can do but up and get on with it and give it another day and hope you get through it.
EBRT patient, Emma a
Patients had difficulty interpreting whether pain was directly due to the stent or other factors. At times, patients expected that the stent would take some time to settle down and described how lack of clarification had aided confusion:
I would have said the pain was based in my windpipe . . . but alas that turned out to be wrong. Pain was where the stent was pushing against the growth . . . it hasn’t done the job I think they were hoping . . . In fact [doctor] is the first one to actually use the word cancerous growth to me.
EBRT patient, Camilla a
Reasons for enduring symptoms such as pain sometimes related to fear of painkiller addiction, not being certain of how to assess pain severity and waiting for the stent to become more comfortable:
Still terrible, 6 days now, there’s not much change . . . it might calm down tomorrow . . . I want to give it a week ‘cos then I can ring up one of two nurses that I am dealing with like . . . I am expecting to see some good change within a week like, ‘cos you can’t rush. They don’t happen overnight like, or two, aahhhgg [patient in pain].
Usual-care patient, Joan a
Professional guidance helped in alleviating discomfort at times when patients were having difficulty judging pain and when it should be treated:
When I first told [research nurse] what the problems I was having, in other words quite severe nausea and almost inability to eat, she said ‘are you taking pain killers?’ I said ‘no’ . . . she says ‘start taking them now’ . . . so [laughs] I’m floating in paracetamol now and have been for a week and of course I feel much better [laughs].
Usual-care patient, Betty a
Patients and carers experimented with how to take medication and expressed difficulties with swallowing tablets that were too big and correct use of the syringe driver and were worried about side effects, in particular excessive sleepiness or confusion. The management of pain and the side effects of medication on everyday life was finely balanced:
I’m getting bad now. I can’t walk very far, not like I used to . . . it’s tiredness more than anything . . . I try not to, I don’t take that during the day that morphine, because it makes you tired if I go out driving . . . so I only take it at night.
EBRT patient, Peter b
Tiredness and sleep disturbance caused by pain had a major impact on the ability to carry out activities, with trial and error experiments around pain management:
It’s lovely when I sleep now. I lay dead flat . . . straight out and it’s lovely. That’s why I love to go on a bed at night because I can be sat there like this, I can be hurting and I can go to bed . . . hot water might ease it as well, having a nice hot shower at night . . . I think that eases it a bit.
EBRT patient, Peter b
Participants experimented with various strategies that prioritised maintaining or gaining weight for health purposes and recovering the enjoyment of eating. Challenges to accomplish these aims included lack of appetite, boredom with dietary restriction, confusing dietary advice and adapting diet, although consequences of eating such as regurgitation, pain and nausea also had an impact on motivation to eat:
Even a basic vegetable soup would bloat me . . . it was pretty bad. They couldn’t get the endoscope down, that’s how bad it was. So, we’re running out of options.
EBRT patient, Emma a
In contrast, good dietetic advice was highly valued and was used by both patients and carers to adapt meals:
Food is my medicine, this is what the dietitian told me, which is right.
She was very good, dietitian from [local hospital] was amazing . . . she gave us a lot more in-depth, what he could use and what he can eat whereas at the [names other local hospital] they kind of frightened me a little.
EBRT patient, Arthur a
However, patients expressed frustration if they were unable to see a dietitian or they were given conflicting or non-specific advice:
. . . one of the weaknesses up there [hospital] is that the dietitians only work on certain days . . . which means you never see the same dietitian and some of them are ok they know what’s going on, the others they just say ‘oh what happened last time?’
Yes, they are more generalised aren’t they?
Yes, and it’s like they got a script.
EBRT arm, Emma a
Strategies for managing diet and weight loss were characterised by trial and error. Participants described eating smaller meals, eating one big meal, adapting the hospital menu, keeping a food diary, avoiding gassy drinks, eating early, cutting out sugar, blending food, eating high-calorie food and boosting their diet with nutritional supplements:
I’ve been off the eating of course but I’m getting more down than I was certainly . . . it’s just whether you’re in the mood really for eating . . . I’ve been trying toast and egg and beans and stuff, just picking at stuff.
EBRT patient, May b
Preparation for eating was also important, with patients experimenting to alleviate consequences such as acid reflux:
I said when’s the right time to take it and he [doctor] said about all day release tablets and [names medication] and that you get to feel yourself when the right time is.
EBRT patient, Michael b
Patients were uncertain about treatment, disease trajectory and management of symptoms. Carers and patients experimented with strategies to manage pain, aid sleep and enable eating.
Responses to psychosocial changes
Patients described a variety of responses to their changed psychosocial situations. Central to patients’ experiences was uncertainty, with patients feeling that they could not plan for the future:
Everything seems to be altering all the time, you know what I mean . . . one day is different from the next, can’t sort of plan anything.
EBRT patient, Peter b
Patients responded by adapting routines, rationalising their situation and accepting their changed context. Many focused on achievable goals, placing cancer in the context of their overall life. They often prioritised a positive mindset in which cancer was framed mainly as an obstacle or challenge:
I’m happy that I can go out every day, quite happy with that. There’s still the illness, well you know you got cancer . . . you don’t really feel wonderful every day, but no one does. So that’s why I don’t often moan about it.
Usual-care patient, Janet b
In some cases, coming to terms with a loss of health and the rate at which change had occurred for patients was highly challenging, resulting in the need to re-evaluate their priorities:
People were saying to me then you’re losing weight, you’re not looking well and then I did decide to go to the doctor eventually and that was it . . . I can’t believe it’s happened to me so quickly because I was the one who took people everywhere and did everything, you know.
Usual-care patient, Kenneth a
The psychosocial aspect of food was a significant issue for patients and carers, who negotiated the physical and mechanical challenges of swallowing and stents while trying to retain the social and pleasurable aspect of eating. Patients described a sense of loss around appetite, mealtimes with others and food as a comfort:
It’s like a mind thing . . . you’ve got a stent down and you’re scared to eat in case. They warn you of it, if it chokes or that . . . but I’m getting there. I tried over the weekend a bit of bread and that was alright . . . and when you’re having the chemo and that you got a different taste and your appetite is different and your taste. You’re eating things you never used to eat and then you went off things that you liked . . . I was saying I’m getting back a bit of my appetite now . . . and I try not to eat later on at night because I think it maybe it lies when I’m sleeping and it gets uncomfortable.
EBRT patient, Michael a
Patients struggled with feelings of guilt over lack of appetite and the ongoing challenge of finding ways to make food physically and psychologically appetising, in an attempt to mitigate food as source of distress:
I’m sick and tired of the food that I’ve been eating at home and these bought things, I don’t know what it is about them. They’re tasteless . . . I tell myself well all you’ve got to do is to cook it yourself and can’t be bothered . . . I think the main thing is I can’t get out of the habit of . . . feeling guilty about wasting food.
EBRT patient, Penelope c
The practical support of family members was welcomed, and patients appreciated the alleviation of pressure around food preparation: ‘My partner is brilliant. She does the food for me . . . because it’s a pain just to go and do it yourself’ (EBRT patient, Stan b).
When families adapted to the patient, this reinstated the psychosocial aspect, as well as the nutritional function, of meals:
We all took our time eating. We must have been there over an hour and half wasn’t we? . . . and because we were all sort of eating at your pace and it was lovely wasn’t it? . . . It was really good.
Usual-care carer, Bernard b
Food could also be a source of contention. For example, some patients reported feeling pressurised by the strategies of others, such as being given unwanted gifts of food. Others felt pressure to conform to social expectations:
So, since the stent . . . I’ve got to try and improve because otherwise you go in that hole . . . we haven’t actually done a social thing, whereas before we would go for a lot of meals you know with friends and whatever but obviously since this, we haven’t been doing that you . . . I would go and have a small dish and they all know now.
The last time we went we had one between us didn’t we . . . we didn’t want to embarrass him you know . . .
Well unfortunately I’ve got two friends who always come down to see me and it’s great you know and the first thing they do is order food.
But they are always inviting us and they are always caring and uh, they are lovely but, he just feels a bit sort of self-conscious because he’s not eating.
EBRT arm, Arthur a
The psychosocial aspects of oesophageal cancer focused on patients and carers stoically dealing with the challenges of the disease including living with pain, dealing with the psychosocial aspects of eating and adapting their daily activities. Although patients and carers were successful in coping with some aspects, such as finding ways to reduce stent blockage, there was a significant overlay of uncertainty around when to seek help and who to approach for specific advice. This led to potentially unnecessary or prolonged discomfort around issues that could be helped by professional advice and guidance.
Patients described oesophageal cancer as obstructing their anticipated life course and accomplishment of everyday life, as described in Figure 16. A disease-orientated focus via medical management provided some relief of specific symptoms such as dysphagia, but potentially hindered a more holistic approach to mediating and managing a terminal prognosis. Patients described these issues in relation to strategies, uncertainties and challenges. In turn, patients explained responses to social, physical and psychological changes, which included maintaining or modifying everyday life, dealing with their own expectations and those of others, and coping with disease symptoms and treatment side effects.
FIGURE 16.
Patients’ experiences of living with oesophageal cancer.
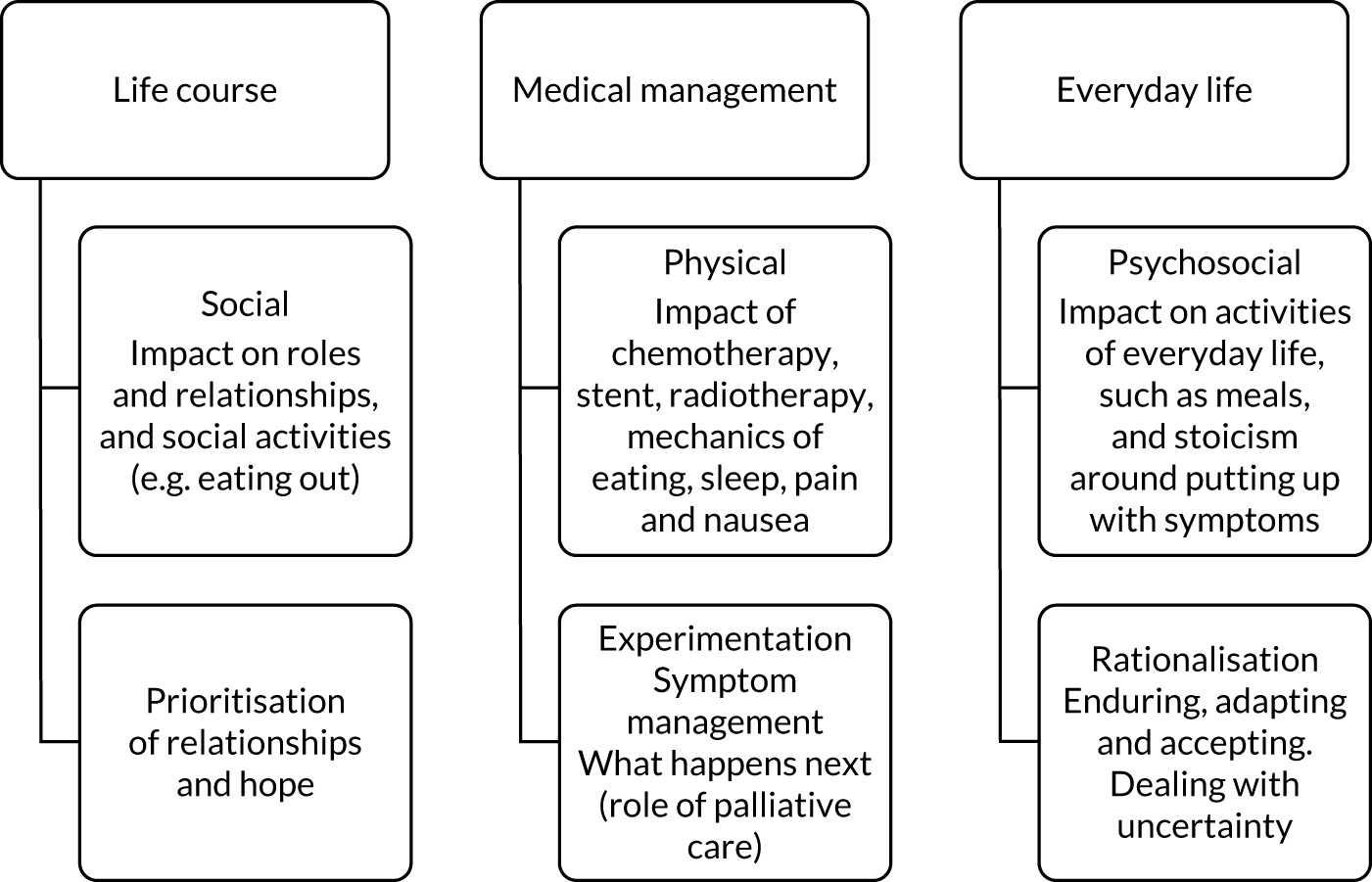
Potential improvements to the recruitment process
The results of the study show that, to improve the recruitment process, patients and carers need an appropriate amount of time and information to consider their involvement and to give informed consent. It is important that patients have the opportunity to discuss the study with friends and family and that patients are provided with contact details of an appropriate person should they wish to ask further questions. To minimise the burden of time, visits with the qualitative researcher and the research nurse may be timed to coincide with each other for future appointments.
Confusion over randomisation, particularly the equipoise of the treatments, illustrates that information should be given multiple times to ensure that the different trial arms are fully explained. Comprehension of the participant’s status in relation to the study needs to be regularly discussed and clarified.
It is important to discuss potential outcomes and burdens for patients, particularly relating to the value of radiotherapy when the participant’s health declines. In relation to the QLQs, it is evident that no single method of data collection will be suitable for all patients. Therefore, it is important that choices are made available to participants and that researchers are flexible in their approach to data collection.
Discussion
Participants’ accounts of recruitment to the trial itself, as well as their more general experiences of dealing with oesophageal cancer, highlighted key issues. Patients and carers sought more consistent, timely and clear guidance on the trial itself, including equipoise of randomisation, longer-term outcomes, the impact of chemotherapy and radiotherapy, and the levels of contact with practitioners.
Medical issues including stent management, especially blockage,47,48 symptom management, nausea, pain49 and tiredness were apparent for patients from both trial arms. These physical issues were often dealt with by patients and carers through processes of experimentation and were usually accompanied by overlapping social and practical everyday QoL challenges. These included uncertainties around their sense of purpose and identity, finances, accessing additional support from other agencies and task management. Personalised care plans with expected time scales and outcomes, could provide some benefit in these circumstances.
Medical management is limited in oesophageal cancer, and the provision of a stent to relieve swallowing difficulties is the most common intervention. However, as the study illustrated, stenting does not address the likely course of the disease and the many symptoms that the patient lives with. Information around diet,50 pain relief and general medical management needs to be provided throughout the course of the disease; it is not sufficient just to alleviate specific symptoms relating to stent provision. 51 Patients and carers emphasised the important social, psychosocial and physical aspects of nutrition and eating. 52 Where possible, patients should receive increased ongoing support with multifaceted eating and diet difficulties from professionals such as nutritionists and counsellors.
Stent insertion is the primary treatment intervention for patients on this clinical pathway and an assumption that it addresses all issues of food intake and nutrition risks inadvertently obstructing additional treatment options, including specialist palliative care, that would be timely earlier in the disease course. Patients have life-limiting disease and expressed the need to address their priorities in relation to their QoL more holistically. 53 Palliative care was viewed as helpful, where provided. A multidisciplinary approach is recommended, but the patient experience indicated that this was not consistently followed and referrals to palliative care services could have been made earlier. 54
Peer support interventions are recommended for oesophageal cancer patients treated surgically, but our patient population suggests that it is helpful only if the patient desires peer support, and there was some ambivalence around its value.
Patients and carers acknowledged the challenges faced by medical staff and services and showed willingness to rationalise and co-operate even when facing uncertainties. However, patient and carer insights also highlighted a need for co-ordinated, bespoke, timely advice and information provision55,56 by health services and third-sector services in relation to medical, life course and everyday life issues.
Chapter 5 Health economics evaluation
Aim
A model-based CUA was undertaken, which reflected the trial follow-up period. This analysis addressed the secondary trial objective of assessing costs and cost-effectiveness of EBRT in addition to stent placement to improve participant-reported dysphagia or other dysphagia-related events at 12 weeks and 12 months following stent insertion in a participant population unable to undergo surgery.
Methods
Prior to commencement of the analysis, a health economic analysis plan was produced, reviewed by the trial team in line with the statistical analysis plan. The health economics team followed this analysis plan during the conduct of the economic evaluation without deviation.
A UK NHS and Personal Social Services perspective was adopted in the analysis, in line with NICE methodological recommendations. 57 Health outcomes are expressed in terms of QALYs. No discounting was applied as the time horizon of the analysis did not exceed 1 year. The analysis undertaken was a CUA producing incremental cost-effectiveness ratios (ICERs) expressed as cost per QALY gained.
Cost data were analysed in SPSS (IBM Corporation, Armonk, NY, USA) version 26 and Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA). Outcomes were analysed using Stata decision-analytic modelling undertaken in Microsoft Excel [using Visual Basics for Applications (VBA)].
Costs included in the health economic analysis
The health economic analysis considered the following costs:
-
intervention implementation cost of EBRT
-
cost of subsequent health-care resource use (primary, secondary, hospice and social care including any cancer treatment as well as medications received).
All available cases (for the most complete overview) were used for descriptive purposes. Available cases were defined as all randomised patients with health-care resource use data for individual time points (i.e. for calculation of weekly costs for model). Available cases for total health-care resource use (i.e. for descriptives) were defined as patients with data for all four follow-up points available (to avoid underestimation of costs between baseline and 12 weeks due to missing data and deaths). All costs are expressed as 2017/18 Great British pounds, inflated and converted appropriately where required. 58
Missing cost data
The problems concerning missing data are particularly relevant to the health economic analysis as the main outcomes are cumulative measures collected over the trial period. Missing items relating to health-care service usage may underestimate the total costs while missing outcome data may be correlated to effects, as those individuals without information may be systematically different from those for whom all information is observed. 59 Therefore, using complete-case assessments and available cases analysis only could result in meaningful data being excluded. Descriptives and analysis of costs were based on available cases. For the CUA (based on the modified ITT population), patient-level mean imputation for all patients alive at each follow-up point was used to account for missing data. This imputation method was chosen owing to the availability of 14 follow-up observations. Considering that most patients had data for at least two follow-up periods, patient-level mean was considered more accurate than population-level mean. However, for patients without any follow-up data, population means were imputed. If a patient died, all further health-care costs were left blank for all follow-up periods after death.
Intervention implementation cost
Patients randomised to the EBRT arm of the ROCS study received EBRT in addition to stent insertion to improve their ability to swallow. Dose and number of fractions received were collected routinely as part of the trial. Information regarding the procedure and duration of radiotherapy planning was obtained from the trial team and clinical experts. Standard, weighted NHS reference costs were applied to all resource use items. 60
Intervention costs considered in the health economic analysis included:
-
costs of a standard consultant-led oncology outpatient appointment to discuss the radiotherapy and likely side effects and to take patient consent
-
costs related to planning of EBRT (parallel opposed beams), including CT scan and 2.5 hours of radiographer time on an outpatient basis to explain the treatment plan and mark the patient for treatment
-
cost of delivery of radiotherapy fractions.
No training costs were considered, as a straightforward, standard radiotherapy approach in keeping with local practices was used.
In accordance with the protocol,26 we explored different costing methods by microcosting individual cost items instead of using aggregated NHS reference costs for radiotherapy planning. This was considered in the deterministic sensitivity analysis.
Cost of subsequent health-care resource use
This included the costs of all health-care resource use accrued between baseline and 12 weeks and 12 months, respectively. Health-care resource use [including primary care consultations, accident and emergency (A&E) visits, outpatient appointments, inpatient stays, social care contacts, hospice stays and medications prescribed] was established using a client service receipt inventory (CSRI). 61 The CSRI was adapted for health-care resource use collection in oesophageal cancer patients and administered at baseline, at the end of weeks 1 and 4 and once every 4 weeks thereafter until the 52-week follow-up. As long as it was indicated on the CRF whether or not the patient had any health-care contacts by ticking either ‘Yes’ or ‘No’ to the question ‘Has the patient had contact with any other health-care provider for any reason in the previous 4 weeks?’ or as long as one or more items in any health-care consultations section of the CSRI were completed (values of ‘0’ or greater), the CSRI was assumed to have been fully completed and any missing items were imputed with zeros. If the CRF was marked as ‘not done’ or was otherwise incomplete, data were considered missing.
Each CSRI questionnaire asked for health and social care resource use in the past 4 weeks (except the week 1 and week 4 CSRIs, which asked for 7 days and 3 weeks, respectively). Therefore, the costs of the first four follow-up points were summated to calculate the 12-week costs and all 14 post-baseline time points were added to produce a total cost over the 12-month follow-up period post stent insertion. CSRI data were cross-checked and supplemented with data on stent complications, blood transfusion and hospital admission and discharge data as well as information on palliative radiotherapy and chemotherapy treatments from the main trial CRF. This was necessary to ensure that all health-care resource use was recorded while avoiding double-counting. Costs were assigned using published unit costs. 60,62,63 All research-related contacts were excluded.
The health-care costs in primary, secondary and social care for both the EBRT arm and the usual-care arm in the 12 weeks and 12 months post randomisation were then summated and mean cost difference per patient (including 95% CI and p-value) calculated using SPSS. Independent-samples t-tests were used for comparison with a 5% significance level. Bonferroni–Holm sequential corrections were used to adjust for type I error rate inflation in multiple comparisons. 64
Primary care costs
Primary care costs included general practitioner (GP) visits in surgery and home visits (including telephone consultations), as well as palliative nurse specialist appointments in surgery or the patient’s home, district nurse visits in surgery and other primary care contacts. Other contacts were costed as specified (e.g. phlebotomist, dietitian, physiotherapist). When no details on the nature of other contacts were available, it was assumed to be an interaction with a dietitian as this was the most common other primary care contact.
All unit costs used for costing primary care resource use can be found in Appendix 4, Table 31.
Secondary care costs
Secondary health-care contacts collected using the CSRI included A&E visits, outpatient consultations, day hospital visits, palliative nurse specialist appointments in hospital and inpatient stays. Furthermore, blood transfusions, radiotherapy fractions and chemotherapy cycles were recorded. Numbers of inpatient days were calculated from recorded hospital admission and discharge dates and compared with CSRI data. If CRF inpatient data did not match patient-reported CSRI data, the higher number was used. If hospital stays spanned two follow-up periods (e.g. patient admitted in week 16 and discharged in week 18), the entire duration of stay was assigned to the admission follow-up period only.
Inpatient stay unit costs were based on a weighted mean across all NHS reference cost entries for elective and emergency excess bed-days and multiplied by the number of bed-days recorded. Outpatient visit costs were calculated as the mean consultant-led outpatient appointment, weighted across all departments. 60
Blood transfusions were costed by unit based on published costs65 or as a day case60 where no information on number of units was available. Palliative radiotherapy was costed by multiplying the number of fractions recorded by the per fraction cost of £83.06 for same-day radiotherapy admission or attendance (excluding brachytherapy) taken from NHS reference costs. 60 If no information on fraction number was recorded, the population mean number of fractions in the relevant follow-up period was used. Chemotherapy treatment costs were established by adding all chemotherapy drug costs (according to band)60 as well as delivery of oral chemotherapy or simple (for one chemotherapy drug) and more complex (for two or more drugs) parenteral chemotherapy. 60 Where no information on the regimen used was available, the weighted mean cost of all chemotherapy day case options60 was used. Monoclonal antibody unit costs for immunochemotherapy treatments (e.g. durvalumab, ramucirumab) were taken from the British National Formulary. 63 Only one chemotherapy cycle was assumed to be administered per 4-week follow-up period.
All unit costs used for costing secondary care resource use can be found in Appendix 4, Table 31.
Social care and hospice costs
Social care costs included district nurse home visits and care assistant visits. District nurse and carer costs were obtained by multiplying the number of visits with the number of people attending (one or two) and the unit costs. All unit costs used for costing social care resource use can be found in Appendix 4, Table 31.
Hospice day cases and inpatient stays were costed using published information,66 inflated to 2018 prices. 58
Medication costs
Up to 20 medication prescriptions were recorded for every living patient in all 14 follow-up periods. Prescriptions were costed individually using standard unit costs23 for all licensed medications. Costs for unlicensed medications or other products (e.g. multivitamins, probiotics, udder cream) were obtained from alternative sources, such as online wholesalers. Where no dose information was available, the most common dose prescribed was used. Any chemotherapy drugs were excluded to avoid double-counting. Costs of all medications prescribed were summated to a total medication cost per follow-up period at an individual patient level.
All medication unit costs used for the economic evaluation are listed in Appendix 4, Table 32.
Total costs
By adding up the implementation costs of EBRT (in the EBRT arm only) and subsequent primary, secondary, social care, hospice and medication costs for all patients in both trial arms, total mean costs per patient (including 95% CIs) were calculated to derive the incremental costs of the intervention at the 12-week and 12-month follow-up compared with usual care.
Health outcomes
The QALYs required for the CUA were derived from EQ-5D-3L responses that were collected once every 4 weeks from trial participants (as described in Chapter 2). Individual-level utility scores were obtained at each assessment point using the EQ-5D-3L value set67,68 and summated for the EBRT and usual-care arms. During this procedure, missing EQ-5D-3L items were replaced with the trial population mean of each item if at least three items were complete. Where more than two items were missing, the questionnaire was considered ‘not completed’ and no utility value was calculated.
The CUA used the modified ITT population (n = 199) with missing utility values imputed as the patient-level mean of all available time points, in the base case. Sensitivity analyses used the complete cases of the modified ITT population and available cases (without imputation) to test the impact that missingness had on the analysis results. After the analysis populations were defined and patient-level mean imputation performed where required, QALYs for each individual patient were calculated according to an area-under-the-curve approach and linear interpolation, using all time points to estimate overall QALYs as a combined measure of patients’ QoL and survival over 12 weeks and 12 months.
Some patients were consented prior to stent insertion (n = 75); the remainder were consented after their stent was inserted (n = 124). This posed a difficulty when assigning any initial values to patient arms from the clinical data. For this reason, the first clinical assessment and first QoL measure were week 1 data in the case of the 124 patients who were consented after their stent insertion and baseline data in the case of the 75 patients who were consented prior to stent insertion.
Model overview
As a means to undertake the health economic analysis in a complex population with high mortality, a decision-analytic, mathematical model was constructed. Similar models have previously been used successfully to undertake evaluations in comparable patient populations. 68,69 Mathematical models are built to simulate reality as an efficient way to test the effect of new treatments on the patient population. A modelling-based approach enables exploration of the cost-effectiveness of a treatment by assessing the effectiveness and any costs incurred in the process and testing different options and scenarios without any patient risk. Operational research techniques, and in particular simulation models, have been employed with much success to optimise the arrangements of different health-care environments. 70
A de novo combined decision tree and Markov model was used to assess the cost-effectiveness of the use of EBRT compared with standard care for patients suffering with end-stage oesophageal cancer with a time horizon of 12 weeks in the base case as well as 12 months in sensitivity analysis.
The model, which follows patients through their journey in the cancer pathway, was constructed using Microsoft Excel with the modelling process coded in VBA. VBA is a computer programming language attached to Microsoft Excel enabling the user to automate specific procedures to be repeated thousands of times to attain a degree of variability around the results. This variability is important from a modelling perspective as not all patients necessarily fit the ‘average’ situation.
Patient population
The model simulates a cohort of 1000 patients based on fully anonymised, directly obtained, patient-level data collected for patients in the EBRT and comparator arms of the ROCS study.
Model description
A schematic representation of the model structure is depicted in Figure 17. Patients enter the model at time zero. For the first 2 weeks, patients are assumed to have a stent inserted and receive standard care, in accordance with the ROCS study, taking into account patients who died in this time period based on actual trial survival data. The stent insertion and any other treatment received was costed and patient QoL calculated. At the beginning of week 3, patients are split into the usual-care and EBRT arms, with the usual-care arm receiving usual care and the EBRT arm receiving usual care plus EBRT, following the ROCS study protocol. Weekly costs and utilities are calculated. At the end of week 4, all radiotherapy is assumed to be completed, and patients are assigned to one of three Markov states: dysphagia worsening, dysphagia stable or death. The patients then move through the model for 8 weeks with a cycle length of 1 week, based on transition probabilities defined by the dysphagia and survival data from the ROCS study, with costs and utilities calculated weekly. At the end of the 12-week time horizon (4 weeks in decision tree and 8 weeks in the Markov model), all costs and utilities are totalled for both the EBRT arm and the usual-care arm. An ICER was calculated to determine cost-effectiveness.
FIGURE 17.
Schematic of the decision-analytic model constructed to calculate the cost-effectiveness of EBRT. (a) Decision tree for weeks 0 to 4; and (b) Markov model for weeks 5 to 12.
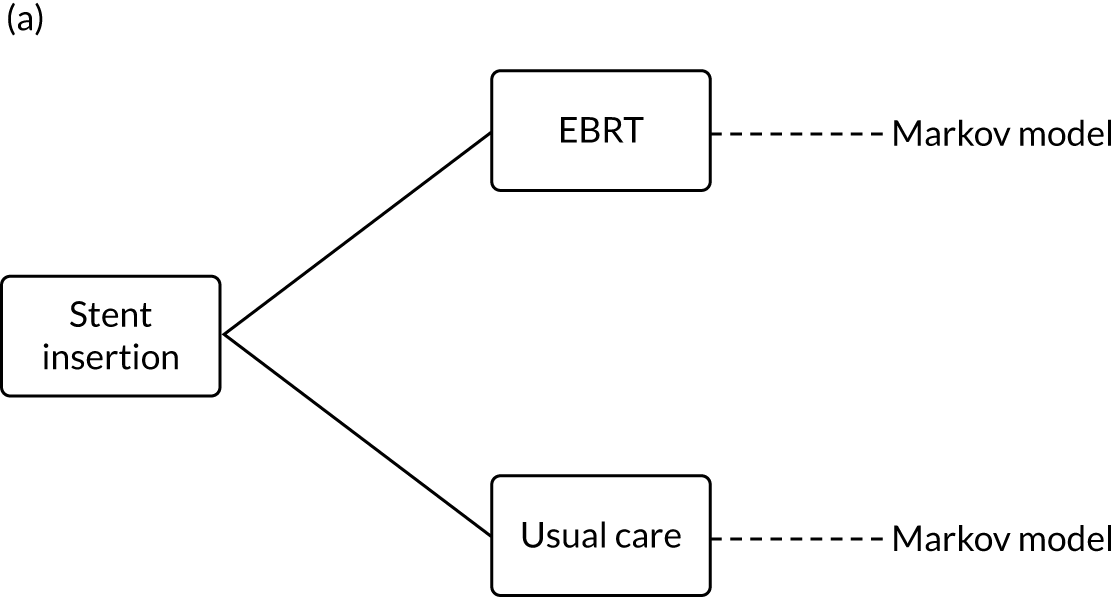
Key model assumptions
Owing to the complexity of cancer treatment and the patient population, the model had to rely on several simplifications and assumptions, all of which were discussed with and verified by the ROCS TMG prior to inclusion in the model.
These assumptions include:
-
Adverse events were not included as separate states within the model with an associated cost and utility decrement. This was done to reduce the potential for double-counting as, because of the fact that HRQoL and health-care resource use data were collected every 4 weeks throughout the trial, any utility decrements and costs associated with adverse events would be reflected in the actual trial data.
-
If a patient underwent stent reinsertion during the first 12 weeks, this would be accounted for by the individual costs and utilities calculated in the respective 4-week period.
-
Base-case analysis was based on the modified ITT population of 199 patients.
Model inputs
The model required weekly cost and utility data as well as transition probabilities derived from dysphagia scores and survival. All parameters used in the model were obtained from ROCS study data, which were analysed on an individual patient level.
Health-care costs
To populate the cost parameters required for the decision-analytic model, costs were converted into weekly costs. Weekly costs for weeks 1 and 2 were obtained by adding total week 1 costs and one-third of total week 4 costs and dividing by 2 to get the mean weekly cost. Week 3 and 4 costs were taken from the week 4 CSRI data divided by 3 (as this questionnaire covered a 3-week period). Total costs at all other time points up to 12 weeks in the base case were divided by 4 to acquire weekly costs for both trial arms. After 12 weeks, the model uses mean weekly costs of all further follow-up points (weeks 13–52) for sensitivity analysis.
Allocation to initial Markov states and transition probabilities
The health economic model required relevant clinical evidence such as dysphagia scores and survival to calculate transition probabilities between health states.
Dysphagia scores were collected every 4 weeks at the home visit and 2-weekly by telephone in accordance with the trial protocol. 26 Detailed analysis of the dysphagia data can be found in Chapter 2. Dysphagia is a scale variable ranging from zero (no issues with swallowing) to 100 (severe issues with swallowing). However, to enable transitions between the health states of stable and worsening dysphagia, a cut-off point had to be defined for the model. Therefore, if a patient’s dysphagia score increases by ≥ 11 points, the dysphagia for that patient is defined as ‘worsening’; otherwise a patient is defined as ‘remaining the same or improving’.
Missing dysphagia scale scores were replaced with the trial population mean of each score if at least two scores were complete. Where more than one score was missing, the questionnaire was considered ‘not completed’ and no dysphagia score was calculated. When a patient died, the dysphagia score for the remainder of the data collection period was set to missing.
As dysphagia scores were collected at 4-week intervals, the data required transformation to enable the calculation of weekly transition probabilities. It was assumed that a Poisson distribution would be most likely (i.e. with a constant rate and independent of the time since the last event). This followed Fleurence and Hollenbeak71 to enable conversion of the probability of an event in the 4-week period to an instantaneous rate that can then be converted into weekly transition probabilities without loss of data integrity.
Survival data were analysed by totalling the number of deaths in each 4-week period for each of the dysphagia categories. This 4-weekly probability was transformed in the same way as the swallowing probabilities above to obtain weekly probabilities of a patient dying for each of the first 12 weeks in the base case and subsequently for the remainder of the year for sensitivity analysis.
All transition probabilities used in the model can be found in Appendix 4, Table 34.
Health-related quality-of-life outcomes
Patient-level utilities were calculated every 4 weeks for the different Markov states within the model.
To use utility scores in the Markov model, we calculated utilities for each Markov state at each particular time point. Although patient mean interpolation was used to populate any missing data while the patient was alive, when a patient died, a utility score of zero was assumed for that patient for the remainder of the time horizon. Utilities were calculated based on the individual 4-week follow-up periods between baseline and week 12 for each of the states in the Markov model. For all time points beyond 12 weeks, one mean weekly value was calculated for each arm and health state for the remaining time horizon. This was deemed the most appropriate course of action as no clear pattern in utility scores from 12 weeks onwards emerged and the sample size diminished rapidly owing to high mortality in this patient population as time progressed. Although this is a limitation of the model, the variability is accounted for in probabilistic sensitivity analysis. All utility values used in the model can be found in Appendix 4, Table 35.
Cost–utility analysis
The model was used to conduct the within-trial CUA to assess the incremental costs per QALY gained as a result of the use of EBRT. The base case investigated the cost-effectiveness of EBRT at 12 weeks, with total costs and QALYs based on the modified ITT population (n = 199). Results of the comparative analysis of incremental costs and effects were summarised in terms of ICERs. An ICER can be represented as:
where C1 and E1 are the costs and effects of the EBRT arm and C0 and E0 are the cost and effects of the usual-care arm with ΔC and ΔE being the incremental costs and effects of the intervention compared with the control.
The ICER of a CUA represents the incremental cost per QALY gained. A QALY is a measure that combines quantity of life (i.e. additional life expectancy) and QoL in one outcome. The cost per QALY gained is calculated between EBRT and usual-care arms by dividing the difference in costs by the difference in QALYs. Generally, NICE considers an intervention to be cost-effective if one of the following applies:57
-
The intervention is less costly and more clinically effective than all other relevant alternatives. In this case, no ICER is calculated as the strategy in question dominates the alternatives.
-
The intervention has an ICER of < £20,000 per QALY gained compared with the next best alternative. This means that an investment of up to £20,000 to achieve an additional QALY is considered cost-effective.
However, NICE considers an intervention not to be cost-effective if:
-
The intervention is more costly and less clinically effective than all other relevant alternatives. In this case, no ICER is calculated as the strategy in question is dominated by the alternatives.
-
The intervention has an ICER of > £20,000 per QALY gained compared with the next best alternative, with the maximum threshold increasing depending on the circumstances (e.g. for orphan drugs or end-of-life interventions).
The ICER resulting from the CUA was compared with the willingness-to-pay threshold of £20,000 per QALY gained as standardised by NICE. Results are reported as ICERs showing the extra cost of producing one extra QALY or the extra savings achieved by sacrificing one additional QALY.
Sensitivity analyses
Sensitivity analyses were undertaken to test the robustness of the CUA considering the uncertainty in input parameters such as costs and outcomes and in different scenarios. Deterministic, univariate sensitivity analyses changed intervention and health-care costs and outcomes individually within plausible ranges (using ± 10%, ± 20% and ± 30% of the mean value). Scenario analyses tested different assumptions and recalculated the ICER (e.g. based on different populations: complete cases, all available cases). In addition, the time horizon was extended to 12 months to explore longer-term effects of the intervention. For this, costs and QALYs beyond 12 weeks were based on all available cases, as sample size was limited after 28 weeks due to high mortality. Considering that, after week 28, sample size in both arms was small (owing to the high mortality in this patient arm), we also tested different costs for the post-12-week period in sensitivity analysis (e.g. using weeks 13–16 and weeks 13–28 costs as proxies for the rest of the year). A summary of all sensitivity analyses undertaken as a part of this analysis can be found in Appendix 4, Table 33.
Probabilistic sensitivity analysis used non-parametric bootstrapping to address joint parameter uncertainty and assess the impact on the ICER during 1000 simulations that were undertaken using random sampling of the distributions of costs and outcomes, with results presented on cost-effectiveness planes and as cost-effectiveness acceptability curves. The cost-effectiveness plane is a scatterplot of the point estimates obtained as a result of the 1000 simulations depicted in four quadrants representing the probability of the intervention being more/less costly and more/less effective than usual care. A cost-effectiveness acceptability curve is a curve that describes the probability of the intervention being cost-effective at different willingness-to-pay thresholds based on the probabilistic sensitivity analysis.
Results
Intervention implementation costs
Planning EBRT (oncology appointment, CT scan and radiographer time) was costed as £377.15 based on the weighted average cost of all external, simple and superficial radiotherapy planning procedures available in NHS reference costs. 60 Cost per fraction delivered was £155.58, calculated as the weighted mean of all external and superficial radiotherapy delivery costs. The mean number of fractions delivered per patient was 6.06 [standard deviation (SD) 2.16]. Nineteen patients received 10 fractions, 64 patients received five fractions and one patient each received four fractions and one fraction, respectively. No information on the intervention EBRT treatment was available for 23 patients. Based on all available cases, total mean EBRT intervention cost was £1304.42 (SD £364.99) per patient.
Cost of subsequent health-care resource use
The results reported in this chapter represent mean cost per patient based on all available cases. Total health-care costs between baseline and 12 weeks were calculated based on data from 51 patients in the EBRT arm and 61 patients in the usual-care arm who had data available for all four follow-up points in the 12 weeks post randomisation.
Primary care costs
A summary of the available primary care costs for the follow-up periods between baseline and 12 weeks can be found in Table 17.
| Health-care resource | EBRT arm, n (SD) | Usual-care arm, n (SD) | Difference; 95% CI | p-value |
|---|---|---|---|---|
| Total primary care costs between baseline and 12 weeks (all four follow-up points available) | ||||
| Sample size | 51 | 61 | n/a | n/a |
| GP visits at home | 103.74 (222.83) | 41.73 (41.73) | 62.01; –2.96 to 126.99 | 0.061 |
| GP visits at surgery | 49.87 (51.10) | 59.61 (76.14) | –9.75; –34.54 to 15.05 | 0.438 |
| Palliative nurse specialist visits at home | 129.19 (207.03) | 126.37 (207.57) | 2.82; –75.14 to 80.78 | 0.943 |
| Palliative nurse specialist visits at surgery | 0.73 (5.18) | 1.82 (8.07) | –1.09; –3.69 to 1.50 | 0.406 |
| District nurse visits at surgery | 1.69 (7.81) | 0.20 (1.58) | 1.49; –0.54 to 3.52 | 0.148 |
| Other contacts | 30.17 (57.28) | 17.71 (33.78) | 12.46; –4.82 to 29.75 | 0.156 |
| Total cost of primary care use per patient | 315.39 (397.12) | 247.44 (261.83) | 67.95; –61.18 to 197.08 | 0.298 |
| Primary care costs between baseline and 1 week | ||||
| Sample size | 100 | 100 | n/a | n/a |
| GP visits at home | 13.88 (42.20) | 11.28 (43.85) | 2.60; –9.40 to 14.60 | 0.669 |
| GP visits at surgery | 8.60 (19.06) | 9.35 (17.93) | –0.75; –5.91 to 4.41 | 0.775 |
| Palliative nurse specialist visits at home | 9.38 (29.96) | 13.54 (52.67) | –4.17; –16.12 to 7.78 | 0.492 |
| Palliative nurse specialist visits at surgery | 0.00 (0.00) | 0.74 (5.21) | –0.74; –1.77 to 0.29 | 0.157 |
| District nurse visits at surgery | 0.00 (0.00) | 0.12 (1.23) | –0.12; –0.37 to 0.12 | 0.319 |
| Other contacts | 10.02 (47.23) | 3.79 (18.47) | 6.23; –3.77 to 16.24 | 0.220 |
| Total cost of primary care use per patient | 41.88 (73.91) | 38.82 (84.68) | 3.06; –19.11 to 25.22 | 0.786 |
| Primary care costs in weeks 2 to 4 | ||||
| Sample size | 91 | 92 | n/a | n/a |
| GP visits at home | 25.73 (59.94) | 21.68 (58.40) | 4.05; –13.21 to 21.31 | 0.644 |
| GP visits at surgery | 11.92 (26.08) | 19.11 (39.01) | –7.19; –16.88 to 2.50 | 0.145 |
| Palliative nurse specialist visits at home | 33.20 (90.39) | 46.42 (93.13) | –13.23; –40.00 to 13.55 | 0.331 |
| Palliative nurse specialist visits at surgery | 0.00 (0.00) | 0.40 (3.86) | –0.40; –1.20 to 0.40 | 0.321 |
| District nurse visits at surgery | 0.00 (0.00) | 0.13 (1.29) | –0.13; –0.40 to 0.13 | 0.321 |
| Other contacts | 6.64 (23.55) | 6.81 (26.62) | –0.17; –7.50 to 7.16 | 0.963 |
| Total cost of primary care use per patient | 77.49 (129.61) | 94.56 (132.66) | –17.07; –55.33 to 21.19 | 0.380 |
| Primary care costs in weeks 5 to 8 | ||||
| Sample size | 79 | 83 | n/a | n/a |
| GP visits at home | 27.45 (80.44) | 19.85 (51.27) | 7.59; –13.23 to 28.42 | 0.473 |
| GP visits at surgery | 12.31 (24.49) | 14.42 (30.81) | –2.11; –10.77 to 6.55 | 0.631 |
| Palliative nurse specialist visits at home | 40.88 (100.66) | 70.28 (142.86) | –29.41; –67.93 to 9.11 | 0.134 |
| Palliative nurse specialist visits at surgery | 0.47 (4.16) | 0.45 (4.06) | 0.02; –1.25 to 1.30 | 0.972 |
| District nurse visits at surgery | 0.00 (0.00) | 0.15 (1.35) | –0.15; –0.45 to 0.15 | 0.331 |
| Other contacts | 10.68 (38.50) | 7.89 (22.97) | 2.79; –6.99 to 12.58 | 0.574 |
| Total cost of primary care use per patient | 91.78 (138.76) | 113.04 (175.31) | –21.26; –70.47 to 27.96 | 0.395 |
| Primary care costs in weeks 9 to 12 | ||||
| Sample size | 55 | 62 | n/a | n/a |
| GP visits at home | 39.42 (123.59) | 13.08 (34.61) | 26.35; –6.07 to 58.77 | 0.110 |
| GP visits at surgery | 14.28 (25.44) | 10.39 (27.78) | 3.89; –5.91 to 13.68 | 0.434 |
| Palliative nurse specialist visits at home | 49.72 (91.77) | 31.92 (74.47) | 17.79; –12.68 to 48.26 | 0.250 |
| Palliative nurse specialist visits at surgery | 0.00 (0.00) | 0.00 (0.00) | 0.00 | n/a |
| District nurse visits at surgery | 1.57 (7.53) | 0.00 (0.00) | 1.57; –0.32 to 3.46 | 0.103 |
| Other contacts | 6.08 (21.90) | 3.54 (14.71) | 2.54; –4.23 to 9.30 | 0.459 |
| Total cost of primary care use per patient (SD) | 111.07 (175.79) | 58.93 (89.02) | 52.14; –0.15 to 104.42 | 0.051 |
Primary care costs in the EBRT arm were lower in weeks 2–8 and higher in weeks 9–12 than in the usual-care arm. Notably, EBRT patients had more GP home visits throughout the 12-week follow-up period. However, none of the differences reached statistical significance.
The total primary care costs between baseline and week 12 for all patients who provided data for all four follow-up points amounted to £315.39 (SD £397.12) per patient in the EBRT arm and £247.44 (SD £261.83) per patient in the usual-care arm. The mean difference of £67.95 (95% CI –£61.18 to £197.08) in favour of the usual-care arm was not statistically significant (p = 0.298).
Secondary care costs
A summary of the available secondary care costs for the follow-up periods between baseline and 12 weeks can be found in Table 18. It should be noted that total costs do not match individual item costs as data were obtained from two different data sets with different numbers of available cases.
| Health-care resource | EBRT arm, n (SD) | Usual-care arm, n (SD) | Difference; 95% CI | p-value |
|---|---|---|---|---|
| Total secondary care costs between baseline and 12 weeks (all four follow-up points available) | ||||
| Sample size | 51 | 61 | n/a | n/a |
| A&E visits | 70.45 (111.24) | 91.04 (173.91) | –20.59; –76.52 to 35.34 | 0.467 |
| Outpatient visits | 568.36 (522.29) | 449.13 (437.02) | 119.23; –60.38 to 298.84 | 0.191 |
| Palliative nurse visits at hospital | 18.14 (44.01) | 19.41 (57.56) | –1.27; –20.77 to 18.22 | 0.897 |
| Day surgery visits | 378.03 (527.23) | 236.20 (448.04) | 141.83; –40.78 to 324.44 | 0.127 |
| Inpatient stays | 1832.33 (3654.49) | 2659.16 (4511.55) | –826.83; –2385.06 to 731.40 | 0.295 |
| Blood transfusions | 41.53 (167.80) | 107.57 (262.07) | –66.03; –150.33 to 18.27 | 0.123 |
| Palliative radiotherapy | n = 6 | n = 19 | 0.00 | n/a |
| 0.00 (0.00) | 0.00 (0.00) | |||
| Chemotherapy | n = 6 | n = 19 | 258.33; –1585.56 to 2102.21 | 0.775 |
| 3114.60 (3027.96) | 2856.27 (1443.07) | |||
| Total cost of secondary care use per patient | 3623.23 (4437.86) | 4917.96 (4803.16) | –1294.73; 3039.72 to 450.26 | 0.144 |
| Secondary care costs between baseline and 1 week | ||||
| Sample size | 100 | 100 | n/a | n/a |
| A&E visits | 38.30 (84.93) | 22.50 (60.49) | 15.79; –4.77 to 36.35 | 0.131 |
| Outpatient visits | 123.47 (188.09) | 133.40 (210.16) | –9.92; –65.54 to 45.69 | 0.725 |
| Palliative nurse visits at hospital | 6.66 (21.28) | 6.29 (24.13) | 0.37; –5.98 to 6.71 | 0.909 |
| Day surgery visits | 149.94 (319.75) | 111.80 (337.97) | 38.14; –53.61 to 129.89 | 0.413 |
| Inpatient stays | 573.38 (1106.45) | 452.67 (907.57) | 120.71; –161.49 to 402.92 | 0.400 |
| Blood transfusions | 9.04 (64.90) | 0.00 (0.00) | 9.04; –3.76 to 21.84 | 0.165 |
| Palliative radiotherapy | n = 101 | n = 99 | 0.00 | n/a |
| 0.00 (0.00) | 0.00 (0.00) | |||
| Chemotherapy | n = 101 | n = 99 | 51.03; –147.36 to 249.42 | 0.613 |
| 136.68 (948.40) | 85.65 (323.29) | |||
| Total cost of secondary care use per patient | n = 101 | n = 100 | 217.10; –148.93 to 583.13 | 0.244 |
| 1028.56 (1549.20) | 811.46 (1027.52) | |||
| Secondary care costs in weeks 2 to 4 | ||||
| Sample size | 91 | 92 | n/a | n/a |
| A&E visits | 16.93 (61.90) | 17.17 (68.00) | –0.23; –19.21 to 18.74 | 0.981 |
| Outpatient visits | 201.76 (366.00) | 65.48 (96.08) | 136.28; 58.41 to 214.15 | 0.001a |
| Palliative nurse visits at hospital | 10.16 (43.45) | 8.04 (23.80) | 2.12; –8.08 to 12.33 | 0.682 |
| Day surgery visits | 114.02 (453.41) | 36.46 (163.78) | 77.56; –21.70 to 176.79 | 0.125 |
| Inpatient stays | 744.05 (2421.60) | 549.65 (1683.39) | 194.40; –413.39 to 802.18 | 0.529 |
| Blood transfusions | 23.18 (101.98) | 22.93 (104.61) | 0.25; –29.89 to 30.39 | 0.987 |
| Palliative radiotherapy | n = 32 | n = 45 | 15.57; –6.46 to 37.61 | 0.163 |
| 15.57 (74.40) | 0.00 (0.00) | |||
| Chemotherapy | n = 32 | n = 45 | –91.18; –415.05 to 232.75 | 0.577 |
| 366.55 (833.80) | 457.73 (593.92) | |||
| Total cost of secondary care use per patient | 1244.48 (2472.70) | 923.62 (1816.24) | 320.86; –311.50 to 953.21 | 0.318 |
| Secondary care costs in weeks 5 to 8 | ||||
| Sample size | 79 | 83 | n/a | n/a |
| A&E visits | 19.99 (62.46) | 17.28 (62.14) | 2.71; –16.63 to 22.05 | 0.782 |
| Outpatient visits | 112.57 (234.99) | 93.32 (175.58) | 19.25; –44.91 to 83.41 | 0.554 |
| Palliative nurse visits at hospital | 5.62 (23.76) | 7.13 (39.81) | –1.51; –11.75 to 8.72 | 0.771 |
| Day surgery visits | 75.15 (254.72) | 26.82 (139.35) | 48.33; –14.97 to 111.63 | 0.134 |
| Inpatient stays | 1101.05 (2444.29) | 720.49 (1794.11) | 380.56; –282.48 to 1034.60 | 0.259 |
| Blood transfusions | 31.50 (153.36) | 21.16 (110.49) | 10.34; –30.99 to 51.66 | 0.622 |
| Palliative radiotherapy | n = 37 | n = 48 | –28.38; –63.73 to 6.95 | 0.114 |
| 4.49 (19.04) | 32.88 (106.64) | |||
| Chemotherapy | n = 37 | n = 48 | –275.04; –573.93 to 23.84 | 0.071 |
| 370.95 (772.90) | 645.99 (612.90) | |||
| Total cost of secondary care use per patient | 1521.72 (2587.36) | 1278.81 (2051.69) | 242.91; –479.83 to 965.65 | 0.508 |
| Secondary care costs in weeks 9 to 12 | ||||
| Sample size | 55 | 62 | n/a | n/a |
| A&E visits | 19.14 (57.05) | 21.65 (58.51) | –2.51; –23.73 to 18.71 | 0.815 |
| Outpatient visits | 91.28 (139.02) | 101.80 (196.76) | –10.52; –73.65 to 52.62 | 0.742 |
| Palliative nurse visits at hospital | 4.04 (18.39) | 3.58 (17.35) | 0.46; –6.09 to 7.00 | 0.891 |
| Day surgery visits | 28.88 (140.52) | 45.24 (163.80) | –16.36; –72.10 to 39.89 | 0.566 |
| Inpatient stays | 359.43 (1520.19) | 964.53 (2192.82) | –605.10; –1304.70 to 94.51 | 0.089 |
| Blood transfusions | 0.00 (0.00) | 66.11 (198.79) | –66.11; –119.23 to –12.99 | 0.015b |
| Palliative radiotherapy | n = 24 | n = 42 | –4.45; –49.51 to 40.61 | 0.844 |
| 17.30 (84.77) | 21.75 (89.98) | |||
| Chemotherapy | n = 24 | n = 42 | –105.26; –437.21 to 226.68 | 0.529 |
| 513.12 (684.89) | 618.38 (628.56) | |||
| Total cost of secondary care use per patient | 734.22 (1714.83) | 1636.55 (2175.52) | –902.32; –1.626 to –178.54 | 0.015b |
Secondary care costs in the EBRT arm were slightly higher between baseline and week 8 but lower between weeks 9 and 12 than for usual-care arm patients (p = 0.015, not statistically significant after Bonferroni–Holm correction for multiple comparison). This lower cost was mainly due to reduced inpatient stay, blood transfusion and chemotherapy costs compared with the usual-care arm.
Total secondary care costs between baseline and the 12-week follow-up point per patient with available data for all four follow-up periods amounted to £3623.23 (SD £4437.86) in the EBRT arm and £4917.96 (SD £4803.16) in the usual-care arm. The mean difference of –£1294.73 (95% CI –£3039.72 to £450.26) was not statistically significant (p = 0.144).
Hospice and social care costs
A summary of the available hospice and social care costs for the follow-up periods between baseline and 12 weeks is presented in Table 19.
| Health-care resource | EBRT arm, n (SD) | Usual-care arm, n (SD) | Difference; 95% CI | p-value |
|---|---|---|---|---|
| Total hospice and social care costs between baseline and 12 weeks (all four follow-up points available) | ||||
| Sample size | 51 | 61 | n/a | n/a |
| Hospice days | 25.89 (145.28) | 178.57 (835.74) | –152.68; –387.68 to 82.32 | 0.201 |
| District nurse home visits | 180.96 (524.86) | 94.56 (310.08) | 86.40; –72.09 to 244.90 | 0.282 |
| Carer visits | 220.24 (1281.88) | 2.66 (12.76) | 217.58; –107.41 to 542.57 | 0.187 |
| Total cost of social care use per patient | 401.20 (1360.64) | 97.22 (309.52) | 303.98; –51.51 to 659.47 | 0.093 |
| Hospice and social care costs between baseline and 1 week | ||||
| Sample size | 100 | 100 | n/a | n/a |
| Hospice days | 0.00 (0.00) | 26.41 (264.06) | –26.41; –78.48 to 25.67 | 0.319 |
| District nurse home visits | 23.46 (162.68) | 5.38 (35.01) | 18.07; –14.74 to 50.89 | 0.279 |
| Carer visits | 45.36 (336.72) | 8.18 (76.12) | 37.18; –31.23 to 105.59 | 0.285 |
| Total cost of social care use per patient | 68.82 (389.61) | 13.48 (82.91) | 55.33; –23.22 to 133.89 | 0.166 |
| Hospice and social care costs in weeks 2 to 4 | ||||
| Sample size | 91 | 92 | n/a | n/a |
| Hospice days | 16.32 (115.54) | 82.52 (467.76) | –66.20; –165.83 to 33.44 | 0.192 |
| District nurse home visits | 79.87 (398.65) | 33.44 (180.21) | 46.43; –43.65 to 136.51 | 0.311 |
| Carer visits | 34.42 (197.10) | 51.39 (331.35) | –16.97; –96.49 to 62.55 | 0.674 |
| Total cost of social care use per patient | 114.28 (506.77) | 85.38 (469.73) | 28.90; –113.61 to 171.41 | 0.690 |
| Hospice and social care costs in weeks 5 to 8 | ||||
| Sample size | 79 | 83 | n/a | n/a |
| Hospice days | 133.70 (529.26) | 99.42 (407.49) | 34.28; –111.87 to 180.43 | 0.644 |
| District nurse home visits | 76.91 (342.12) | 98.68 (389.45) | –21.78; –135.74 to 92.19 | 0.706 |
| Carer visits | 118.94 (702.98) | 10.41 (83.68) | 108.53; –44.97 to 262.02 | 0.165 |
| Total cost of social care use per patient | 195.85 (815.02) | 109.09 (403.83) | 86.75; –111.67 to 284.89 | 0.389 |
| Hospice and social care costs in weeks 9 to 12 | ||||
| Sample size | 55 | 62 | n/a | n/a |
| Hospice days | 15.00 (91.35) | 13.31 (86.09) | 1.69; –30.81 to 34.20 | 0.918 |
| District nurse home visits | 170.60 (665.80) | 19.23 (88.83) | 151.37; –17.70 to 320.44 | 0.079 |
| Carer visits | 74.62 (417.70) | 1.74 (10.79) | 72.88; –32.18 to 177.94 | 0.172 |
| Total cost of social care use per patient | 245.22 (772.10) | 20.97 (89.10) | 224.25; 28.67 to 419.83 | 0.025a |
Total hospice costs (including day cases and inpatient days) between baseline and the 12-week follow-up point per patient who contributed data to all four follow-up points amounted to £25.89 (SD £145.28) in the EBRT arm and £178.57 (SD £835.75) in the usual-care arm. The mean difference of –£152.68 (95% CI –£387.68 to £82.32) was not statistically significant (p = 0.201).
Social care costs were higher in the EBRT arm at all follow-up points with higher costs between 9 and 12 weeks (p = 0.025; difference not significant after Bonferroni–Holm correction for multiple comparison).
Total per patient social care costs (including district nurse home visits and carer visits) in the 12-week follow-up period amounted to £401.20 (SD = £1360.64) in the EBRT arm and £97.22 (SD = £309.52) in the usual-care arm. The mean difference of £303.98 (95% CI –£51.51 to £659.47) was not statistically significant (p = 0.093).
Medication costs
A summary of the available medication costs for the follow-up periods between baseline and 12 weeks is presented in Table 20.
| Health-care resource | EBRT arm, n (SD) | Usual-care arm, n (SD) | Difference; 95% CI | p-value |
|---|---|---|---|---|
| Medication costs between baseline and 12 weeks (all four follow-up points available) | ||||
| Sample size | 51 | 61 | n/a | n/a |
| Total cost of medication use per patient | 187.76 (166.35) | 257.83 (197.28) | –70.07; –139.21 to –0.93 | 0.047 |
| Medication costs between baseline and 1 week | ||||
| Sample size | 100 | 100 | n/a | n/a |
| Total cost of medication use per patient | 40.09 (63.68) | 48.24 (56.26) | –8.15; –24.91 to 8.60 | 0.339 |
| Medication costs in weeks 2 to 4 | ||||
| Sample size | 91 | 92 | n/a | n/a |
| Total cost of medication use per patient | 43.05 (56.22) | 51.15 (78.75) | –8.11; –28.08 to 11.87 | 0.424 |
| Medication costs in weeks 5 to 8 | ||||
| Sample size | 79 | 83 | n/a | n/a |
| Total cost of medication use per patient | 74.74 (110.22) | 74.56 (76.10) | 0.18; –29.09 to 29.45 | 0.990 |
| Medication costs in weeks 9 to 12 | ||||
| Sample size | 55 | 62 | n/a | n/a |
| Total cost of medication use per patient | 60.96 (70.32) | 79.14 (84.83) | –18.18; –46.93 to 10.57 | 0.213 |
Medication costs were slightly lower in the EBRT arm than in the usual-care arm, but no evidence of significant differences was found.
Total per patient medication costs in the 12-week follow-up period amounted to £187.76 (SD £166.35) in the EBRT arm and £257.83 (SD £197.28) in the usual-care arm. The mean difference of –£70.07 (95% CI –£139.21 to –£0.93) reached statistical significance at the 0.05 level (p = 0.047).
Total health-care costs
Total health-care costs (based on available cases) in the 12-week follow-up period included the cost of all primary, secondary and social care, and any medications prescribed (Table 21). Patients in the EBRT arm had higher health-care costs than those in the usual-care arm in the first 8 weeks after baseline. Between 8 and 12 weeks, total health-care costs were lower in the EBRT arm. None of the differences was statistically significant.
| Health-care resource | EBRT arm, n (SD) | Usual-care arm, n (SD) | Difference; 95% CI | p-value |
|---|---|---|---|---|
| Total health-care costs between baseline and 1 week | ||||
| Sample size | 101 | 100 | n/a | n/a |
| Total cost of health-care use per patient | 1177.85 (1660.00) | 938.41 (1094.15) | 239.44; –152.03 to 630.91 | 0.229 |
| Total health-care costs in weeks 2 to 4 | ||||
| Sample size | 91 | 92 | n/a | n/a |
| Total cost of health-care use per patient | 1495.62 (2625.95) | 1237.24 (1951.38) | 258.38; –415.95 to 932.72 | 0.451 |
| Total health-care costs in weeks 5 to 8 | ||||
| Sample size | 79 | 83 | n/a | n/a |
| Total cost of health-care use per patient | 2017.80 (2710.67) | 1674.93 (2066.76) | 342.86; –402.87 to 1088.60 | 0.365 |
| Total health-care costs in weeks 9 to 12 | ||||
| Sample size | 55 | 62 | n/a | n/a |
| Total cost of health-care use per patient | 1166.47 (1902.23) | 1808.90 (2206.20) | –642.43; –1401.57 to 116.72 | 0.094 |
If we considered only patients with data for all four follow-up periods in the first 12 weeks following randomisation, patients in the EBRT arm accrued mean per patient health-care costs of £4553.47 (SD £4746.01), compared with £5699.02 (SD £5024.49) in the usual-care arm. The incremental cost of EBRT was –£1145.55 (95% CI –£2988.00 to £696.90). The lower cost was mainly caused by lower inpatient and chemotherapy costs in the weeks 9–12 time period, but the difference was not statistically significant (p = 0.221).
After adding the intervention implementation costs for all patients who had data for all four follow-up periods in the 12 weeks from baseline, patients in the EBRT arm accrued a mean cost of £5854.10 (SD £4768.79), compared with £5699.02 (SD £5024.49) in the usual-care arm. The difference of £155.08 (95% CI –£1691.15 to £2001.31) was not statistically significant (p = 0.868).
Total costs of the modified intention-to-treat population
Consistent with the statistical analysis, the CUA was based on the modified ITT population (n = 199). This included 97 patients in the EBRT arm and 102 patients in the usual-care arm. Because not all cost data were recorded at all time points for every patient, the population mean for every parameter at individual follow-up points was used for imputation of missing data.
A breakdown of all costs in the modified ITT population can be found in Table 22. In the 12-week follow-up period, costs of secondary and social care were higher in the EBRT arm whereas primary care, hospice and medication costs were slightly lower (Figure 18). The higher costs mainly occurred in the first 8 weeks and could not be offset by lower costs, especially for secondary care, in weeks 9–12. Total mean health-care cost (including primary, secondary, hospice and social care, as well as medication costs) in the modified ITT population therefore amounted to £5202.16 (SD £5613.63) in the EBRT arm and £4667.73 (SD £4719.99) in the usual-care arm with a mean difference of £534.43 (95% CI –£912.86 to £1981.73; p = 0.467) in favour of the usual-care arm.
| Health-care resource | EBRT arm, n (SD) | Usual-care arm, n (SD) | Difference; 95% CI | p-value |
|---|---|---|---|---|
| Health-care costs between baseline and 1 week | ||||
| Sample size | 97 | 102 | n/a | n/a |
| Primary care | 52.82 (87.19) | 41.08 (84.08) | 11.74; –12.20 to 35.67 | 0.335 |
| Secondary care | 1070.00 (1621.15) | 838.74 (1057.42) | 231.26; –149.55 to 612.08 | 0.233 |
| Hospice care | 0.00 (0.00) | 27.23 (261.39) | –27.23; –79.57 to 25.12 | 0.306 |
| Social care | 77.29 (398.14) | 14.64 (82.14) | 62.65; –16.80 to 142.10 | 0.122 |
| Medication | 41.51 (66.40) | 48.85 (55.01) | –7.34; –24.35 to 9.67 | 0.396 |
| Total cost of health-care use per patient | 1241.62 (1741.88) | 970.54 (1120.28) | 271.09; –136.33 to 678.50 | 0.191 |
| Health-care costs in weeks 2–4 | ||||
| Sample size | 90 | 92 | n/a | n/a |
| Primary care | 99.41 (197.39) | 110.75 (165.58) | –11.34; –64.58 to 41.91 | 0.675 |
| Secondary care | 1080.91 (2119.35) | 915.50 (1816.67) | 165.41; –411.53 to 742.36 | 0.572 |
| Hospice care | 17.12 (116.23) | 83.67 (467.62) | –66.55; –166.72 to 33.61 | 0.192 |
| Social care | 134.92 (528.08) | 87.69 (469.56) | 47.23; –98.85 to 193.32 | 0.524 |
| Medication | 42.92 (55.54) | 52.72 (78.29) | –9.79; –29.69 to 10.10 | 0.333 |
| Total cost of health-care use per patient | 1375.28 (2343.80) | 1250.32 (1962.63) | 124.96; –506.80 to 756.73 | 0.697 |
| Health-care costs in weeks 5–8 | ||||
| Sample size | 83 | 86 | n/a | n/a |
| Primary care | 102.86 (165.67) | 148.84 (324.27) | –45.99; –124.62 to 32.65 | 0.250 |
| Secondary care | 1549.66 (2571.33) | 1241.40 (2028.24) | 308.26; –393.75 to 1010.27 | 0.387 |
| Hospice care | 127.26 (516.99) | 98.76 (400.39) | 28.50; –111.65 to 168.65 | 0.689 |
| Social care | 190.11 (795.85) | 103.51 (395.17) | 86.60; –103.22 to 276.43 | 0.369 |
| Medication | 72.54 (108.02) | 73.35 (74.73) | –0.81; –28.94 to 27.32 | 0.955 |
| Total cost of health-care use per patient | 2042.44 (2710.97) | 1665.87 (2078.36) | 376.57; –355.49 to 1180.63 | 0.311 |
| Health-care costs in weeks 9–12 | ||||
| Sample size | 67 | 74 | n/a | n/a |
| Primary care | 112.32 (170.89) | 64.69 (90.53) | 47.63; 2.68 to 92.57 | 0.038a |
| Secondary care | 897.68 (1740.03) | 1464.16 (2041.91) | –566.48; –1201.51 to 68.55 | 0.080 |
| Hospice care | 37.77 (165.10) | 11.54 (78.83) | 26.23; –16.21 to 68.68 | 0.224 |
| Social care | 246.15 (718.81) | 22.60 (84.03) | 223.54; 57.15 to 389.94 | 0.009b |
| Medication | 62.41 (69.61) | 72.97 (80.02) | –10.56; –35.65 to 14.53 | 0.407 |
| Total cost of health-care use per patient | 1356.33 (1968.01) | 1635.97 (2069.81) | –279.63; –953.86 to 394.60 | 0.414 |
| Total health-care costs between baseline and week 12 | ||||
| Sample size | 97 | 102 | n/a | n/a |
| Primary care | 310.65 (400.09) | 312.01 (410.02) | –1.36; –114.69 to 111.97 | 0.981 |
| Secondary care | 4018.96 (5000.51) | 3756.21 (4410.26) | 262.75; –1053.76 to 1579.26 | 0.694 |
| Hospice care | 150.86 (605.37) | 193.67 (746.57) | –42.80; –233.39 to 147.78 | 0.658 |
| Social care | 535.17 (1552.36) | 195.61 (621.18) | 339.57; 11.95 to 667.19 | 0.042a |
| Medication | 186.52 (186.82) | 210.24 (183.42) | –23.72; –75.49 to 28.04 | 0.367 |
| Total cost of health-care use per patient | 5202.16 (5613.63) | 4667.73 (4719.99) | 534.43; –912.86 to 1981.73 | 0.467 |
Mean EBRT intervention costs were £1297.34 (SD £296.38) in the modified ITT population. Therefore, total mean cost for this population (including all health-care costs and intervention costs) was £6499.50 (SD £5593.65) in the EBRT arm. The mean difference of £1831.77 (95% CI £387.43 to £3276.11) favouring usual-care treatment was statistically significant (p = 0.013).
FIGURE 18.
Mean cost differences between EBRT and usual-care patients in the modified ITT population in the 12-week follow-up period.
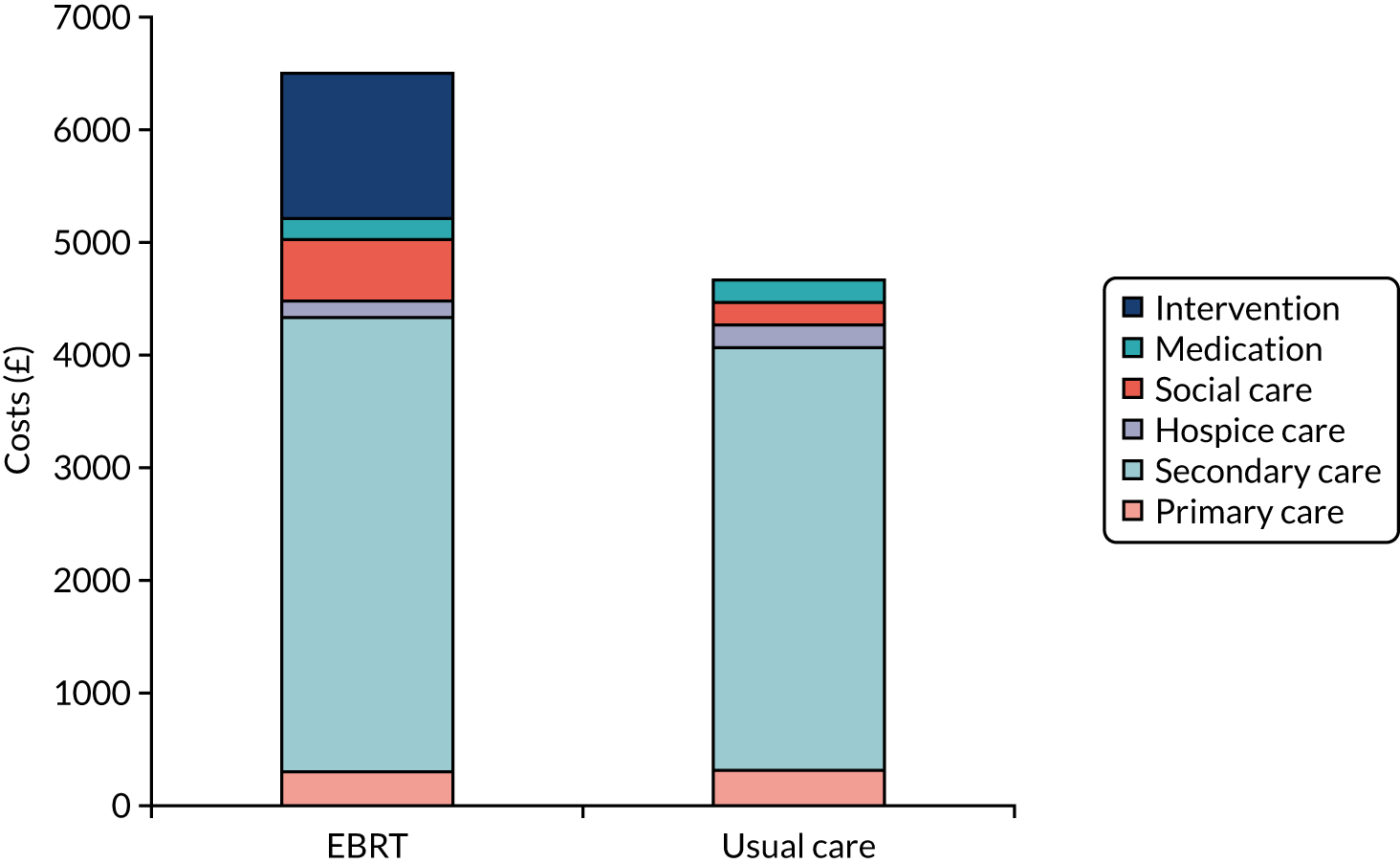
Total cost at 12 months
Total health-care costs at 52 weeks were based on all available cases who contributed at least one health-care resource use data set at one follow-up point within the 1-year trial period. This included 104 people in the EBRT arm and 102 in the usual-care arm. The total 12-month health-care costs in this population were £7440.43 (SD £7643.21) in the EBRT arm and £9087.14 (SD £8890.75) in the usual-care arm. The mean difference of –£1646.70 (95% CI –£3922.89 to £629.49) was not statistically significant (p = 0.155). Adding the implementation cost of EBRT on an individual patient level decreases the cost difference to –£568.05 (95% CI –£2858.80 to £1722.71; p = 0.625).
Figure 19 illustrates the changes in health-care costs over time. It is apparent from the graph that the difference in costs between the two trial arms is mainly attributed to the follow-up points from 28 weeks onwards, when health-care costs were consistently lower in the EBRT arm, especially in weeks 45–52. However, none of these cost differences reached statistical significance, and sample sizes had fallen below 25 patients per trial arm. When considering health-care costs (including intervention cost) between baseline and 28 weeks only (with more representative sample sizes), patients in the EBRT arm (n = 104) accrued £8092.74 (SD £7768.58), compared with £7443.79 (SD £7169.20) in the usual-care arm (n = 102), with a mean incremental cost of EBRT patients of £648.95 (95% CI –£1405.64 to £2703.54; p = 0.534). The cost savings in the EBRT arm, based on the 12-month follow-up period, should therefore be interpreted with caution. However, between weeks 9 and 16, patients in the EBRT arm (n = 57) had lower health-care costs (£2382.48, SD £3429.09) than the usual-care arm (n = 66) (£3325.41, SD £3234.21). The mean difference of –£942.93 (95% CI –£2133.51 to £247.64) may be attributed to a short-term effect of the intervention on the need for health care in this period but was not statistically significant (p = 0.119).
FIGURE 19.
Total health-care costs (excluding intervention costs) in EBRT and usual-care arms over 52 weeks’ follow-up (based on available cases).
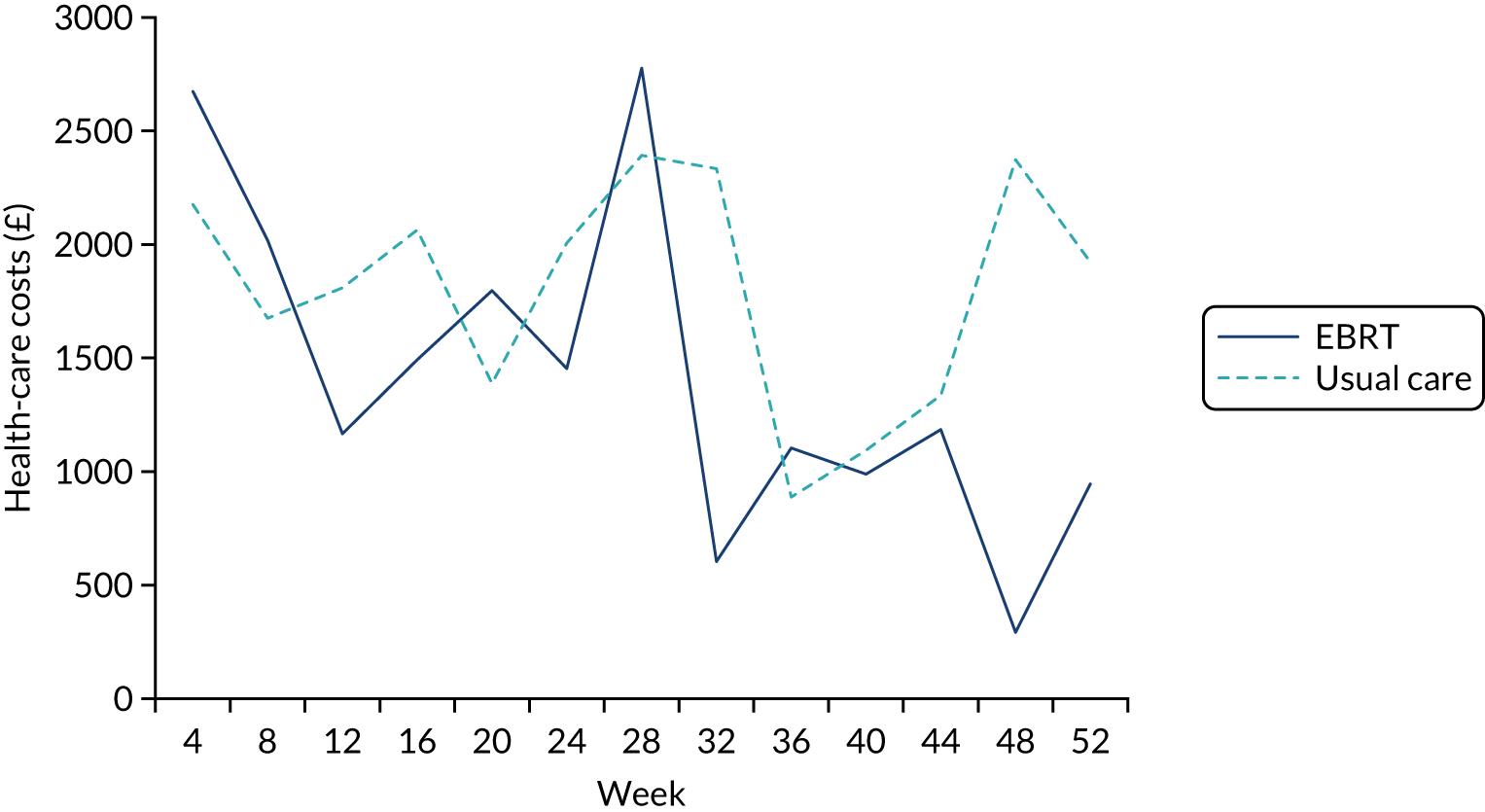
Although the annual costs reported above represent the mean costs over 1 year for a palliative population with poor prognosis, they underestimate costs for patients with longer survival. We therefore calculated mean annual costs for five EBRT patients and 11 usual-care patients who were still alive at the 52-week follow-up point and completed the CSRI questionnaire. Based on the small sample size of 16 patients in the ROCS study population at 52 weeks, the mean annual health-care cost (excluding trial intervention) for this palliative patient population is £16,129.59 (SD £10,721.58).
Weekly costs used in the model
Costs were converted into weekly costs based on the modified ITT population for the 12-week time horizon and based on available cases for costs between weeks 13 and 52 for the 1-year horizon used as part of sensitivity analysis. Weekly costs used in the model can be found in Table 23.
| Time period | EBRT arm | Usual-care arm |
|---|---|---|
| Weeks 1 and 2 | 770.02 | 770.02 |
| Weeks 3 and 4 (HRU) | 458.43 | 416.77 |
| Weeks 3 and 4 (EBRT) | 648.67 | 0.00 |
| Weeks 5 to 8 | 510.61 | 416.47 |
| Weeks 9 to 12 | 339.08 | 408.99 |
| Weeks 12 onwards | 163.99 | 240.77 |
Dysphagia and survival
At week 5, patients are split into dysphagia categories by treatment for the Markov model (see Appendix 4, Table 34). Initially, the proportion of patients whose ability to swallow improves or remains the same is higher in the EBRT arm than in the usual-care arm (0.77 and 0.76 respectively). The probability of death is higher in the usual-care arm (p = 0.11) than in the EBRT arm (p = 0.06). Considering patients beginning in the ‘ability to swallow improved’ state, the probability that patients in the EBRT arm remain in that state for the next cycle decreases each month, beginning at 0.92 for weeks 5–8, then increasing to 0.90 for weeks 9–12 and to 0.82 for weeks 13 onwards; the corresponding figures for the usual-care arm are 0.91, 0.91 and 0.88, respectively. Similarly, the pattern repeats for patients beginning in a worsening state of dysphagia, with probabilities for improving their dysphagia decreasing over time. The probability of death increased every month in both arms for most time points (see Appendix 4, Table 34). In the usual-care arm, the probability of dying decreased in weeks 9–12 among those whose ability to swallow improved. However, this anomaly could be due to a diminishing sample size and sample variation.
Health-related quality of life and quality-adjusted life-years
No significant differences in QALYs between the EBRT arm and the usual-care arm were found at baseline (p = 0.1345). This picture remains the same at 12 weeks and 12 months, with no significant differences in the instantaneous utility score between the EBRT arm and the usual-care arm (p = 0.460 and p = 0.294 for 12 weeks and 12 months, respectively). Table 24 summarises the QALY gains by arm over the 12-week and 12-month follow-up periods.
| Time horizon | Allocation | Mean | SD | Minimum | Median | Maximum | IQR |
|---|---|---|---|---|---|---|---|
| 12 weeks | EBRT | 0.1602 | 0.0831 | –0.0172 | 0.1794 | 0.3077 | 0.1516 |
| Usual care | 0.1485 | 0.0861 | –0.0382 | 0.1510 | 0.3077 | 0.1447 | |
| 12 months | EBRT | 0.2529 | 0.2092 | –0.0382 | 0.2023 | 0.9533 | – |
| Usual care | 0.3004 | 0.2471 | –0.0603 | 0.2189 | 0.8969 | 0.3767 |
The changes in transition probabilities over time according to which state of the Markov model the patients begin in are illustrated in Appendix 4, Figures 28 and 29.
Correlation analysis conducted as part of the model validation process found a negative correlation that exists between dysphagia and EQ-5D-3L score. At 12 weeks, by running Spearman’s rank tests, there is a highly significant moderate negative correlation for both the EBRT arm and the usual-care arm (ρ = –0.4410; p = 0.0031 and ρ = –0.4319; p = 0.0.0054, respectively). This conclusion follows intuition showing that poor ability to swallow negatively affects QoL for both the EBRT and usual-care patients.
Cost–utility analysis
Based on the incremental cost of stent insertion and EBRT compared with stent insertion alone, the base case ICER is –£549,200 per QALY gained at the 12-week follow-up. This means that EBRT is more costly and less effective than the usual-care treatment and thus dominated by usual care.
Table 25 summarises the incremental results for the deterministic base-case CUA. The difference in QALYs between the arms is minimal over the 12 weeks (1.3919). However, the costs for the EBRT arm are over £750,000 higher (for 500 simulated patients in the EBRT arm), resulting in a large negative ICER.
| Characteristic | Usual care | EBRT | Difference |
|---|---|---|---|
| Total cost | £2,313,998 | £3,078,427 | £764,429 |
| Total QALYs | 55.32 | 53.93 | –1.3919 |
| ICER (cost per QALY gained) | Dominated (–£549,200) |
Sensitivity analyses
No change to the base-case conclusion was found in the one-way sensitivity analyses conducted. EBRT was dominated, and thus found not to be a cost-effective treatment for end-stage oesophageal cancer, in all one-way sensitivity analyses (Table 26). Scenario analysis showed that extending the analysis to a 12-month time horizon does not have an impact on the conclusion as EBRT remains dominated in all cases.
| SA-ID | Parameter | Change | ICER | |
|---|---|---|---|---|
| 12 weeks | 12 months | |||
| Base case | n/a | n/a | Dominated | Dominated |
| SA1 | Costs | –10% | Dominated | Dominated |
| SA2 | Costs | –20% | Dominated | Dominated |
| SA3 | Costs | –30% | Dominated | Dominated |
| SA4 | Costs | +10% | Dominated | Dominated |
| SA5 | Costs | +20% | Dominated | Dominated |
| SA6 | Costs | +30% | Dominated | Dominated |
| SA7 | Costs | Intervention cost microcosted | Dominated | Dominated |
| SA8 | Utilities | –10% | Dominated | Dominated |
| SA9 | Utilities | –20% | Dominated | Dominated |
| SA10 | Utilities | –30% | Dominated | Dominated |
| SA11 | Utilities | +10% | Dominated | Dominated |
| SA12 | Utilities | +20% | Dominated | Dominated |
| SA13 | Utilities | +30% | Dominated | Dominated |
| SA14 | All parameters | Complete cases used | Dominated | Dominated |
| SA15 | All parameters | All available cases used | Dominated | Dominated |
| SA16 | All parameters post 12 weeks | Weeks 13–16 used | Dominated | Dominated |
| SA17 | All parameters post 12 weeks | Weeks 13–28 used | Dominated | Dominated |
Figure 20 displays the probabilistic sensitivity analysis results. In all 1000 iterations, EBRT was found to be more costly than usual care (with all points in the north-east and north-west quadrants of the cost-effectiveness plane). Overall, the probability that EBRT is cost-effective at the willingness-to-pay threshold of £20,000 per QALY gained is 1.4% (Figure 21). If we extend the time horizon to 12 months, this probability increases to 10.5%.
FIGURE 20.
Cost-effectiveness plane illustrating the distribution of incremental cost per QALY gained over 1000 analysis iterations following parameter resampling within predefined ranges and distributions based on a simulation cohort of 1000 patients.
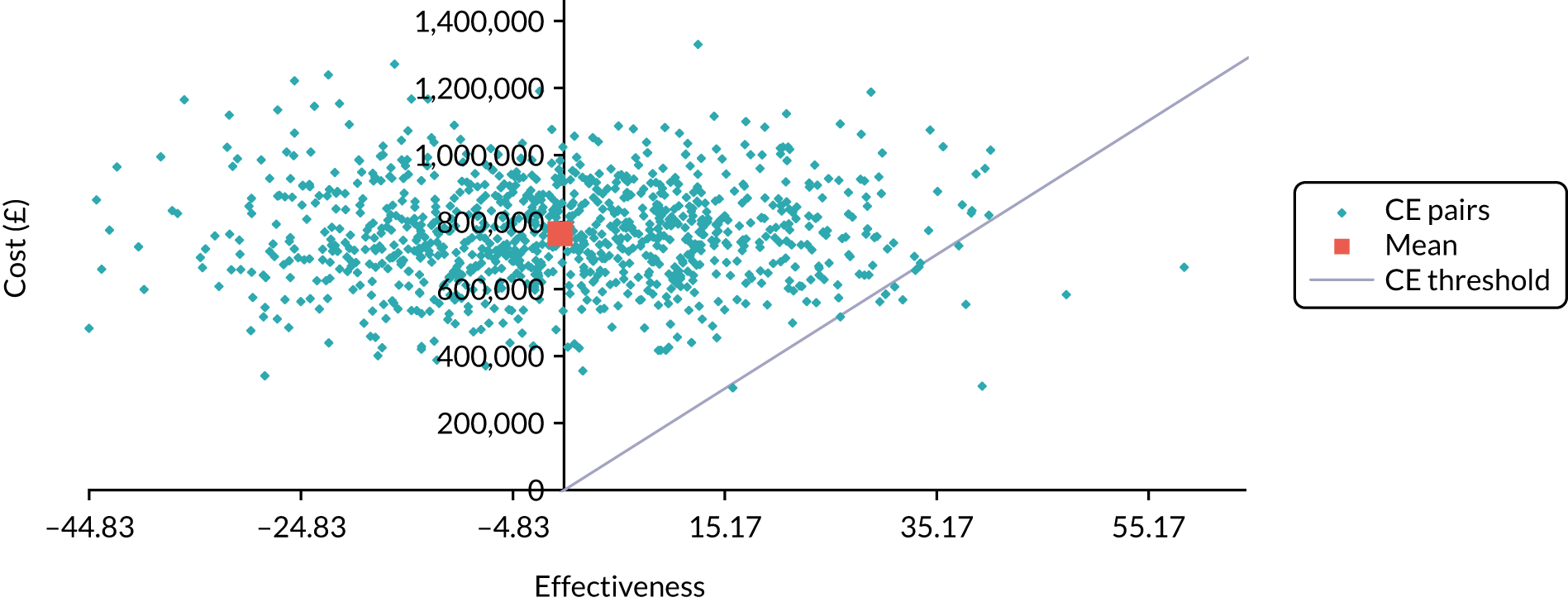
FIGURE 21.
Cost-effectiveness acceptability curve depicting the probability of EBRT being cost-effective at different willingness-to-pay thresholds based on 1000 iterations for a simulated cohort of 1000 patients.
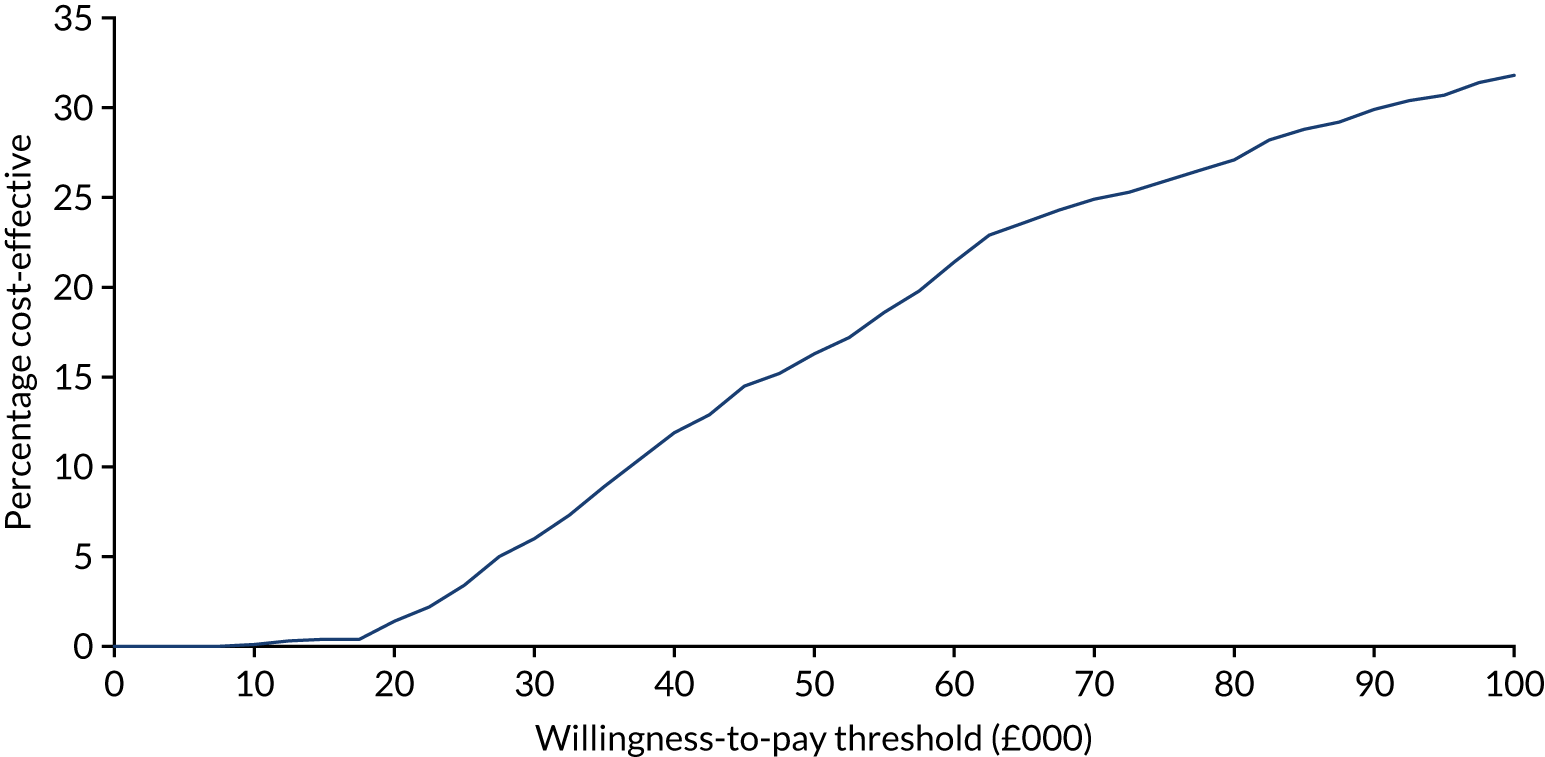
Discussion
This chapter describes the methods and results of the health economic evaluation undertaken as part of the ROCS study, providing a thorough and transparent account of the economic analysis, with potential for the economic model to be adapted in future trials and studies and to inform decision-making. To our knowledge, this represents the first reported economic analysis of the cost-effectiveness of the addition of EBRT in patients with advanced oesophageal cancer undergoing stent placement compared with stent placement alone, based on one of the largest reported intervention trials involving stents.
The ROCS study enabled the collection of detailed patient-level resource use and clinical and health utilities reflecting interventions that replicate current practice in the UK NHS sites. This provided a comprehensive assessment of costs and health utilities, capturing in-depth data on intervention and subsequent use of health and personal social care resources over the course of the follow-up period. The data collection of economic outcomes benefited from the attention that the ROCS study design paid to capturing precise and regular resource use information from patients and including reasons for missing data. These individual patient-level data were used to undertake a CUA, employing economic modelling as the vehicle for analysis. The construction of a complex model, populated entirely with trial data, allowed a thorough examination of the costs and consequences of EBRT in addition to stent placement in the context of routine clinical practice with careful attention paid to managing the challenges of undertaking an economic evaluation in a palliative population with limited prognosis.
External beam radiotherapy is an expected up-front intervention cost, which represented an additional £1304 per patient in addition to stent insertion. As a result, the combined intervention was more expensive than stent placement alone. Despite lower health-care costs of £943 in the EBRT arm between weeks 9 and 16, which may be attributed to a short-term effect of the intervention on the need for health care in this period, little difference in health resource use and associated costs was evidenced across both arms in the first 12 weeks. At 12 months, potential savings were noted in subsequent health and social care costs in the EBRT arm, which offset the EBRT costs and resulted in savings of £568 per patient still alive at 52 weeks. These savings were mainly attributed to the follow-up points from 28 weeks onwards, at which time health-care costs were consistently lower in the EBRT arm, especially in weeks 45–52. However, none of these cost differences reached statistical significance, and sample sizes had fallen below 25 patients per trial arm. Furthermore, between baseline and 28 weeks, with more representative sample sizes, patients in the EBRT arm accrued a mean incremental cost of £648. The cost savings in the EBRT arm based on the 12-month follow-up period should therefore be interpreted with caution.
When health outcomes were assessed, there were no statistically significant differences in health utilities and survival between arms, which reflects the findings from the primary and secondary clinical and QoL outcomes of the ROCS study. Although the clinical analysis observed significantly fewer bleeding events in the ERBT arm over the time horizon, this did not result in any significant differences in costs associated with admissions or interventions in the EBRT arm. However, given the potential for EBRT to reduce bleeding in selected patients and a significantly lower cost for blood transfusions in the EBRT arm between weeks 9 and 12, further examination of the association of bleeding risk and impact on resource use and costs is warranted. Based on the QALYs generated from the ROCS study, there was no health benefit for EBRT when added to stent placement at 12 weeks.
The base-case analysis (12-week time horizon) found that EBRT is not a cost-effective treatment at a willingness-to-pay threshold of £20,000 per QALY gained when used in addition to stent placement in an advanced oesophageal cancer population. The findings remain robust when tested in a range of sensitivity analyses and the conclusion did not change when a 12-month time horizon was considered.
Although there are no directly comparable economic evaluations to the ROCS study population, the findings from the ROCS study can be placed in the wider context of palliative interventions in advanced oesophageal cancer, although the economic evidence base is limited. Such studies have considered the potential for upfront intervention costs to be offset by reducing the need for further (re-)interventions. However, prior studies72,73 have been constrained by limitations such as capturing initial interventions costs only and lack of corresponding health utilities. Although limited in providing a ‘single trial’ estimation of data inputs, the ROCS study data have provided detailed assessment of costs and outcomes related to a range of different health states associated with the patient pathway following EBRT in addition to stenting and stenting alone. The 12-month time horizon is likely to have captured all relevant costs and health consequences, with longer-term extrapolation unlikely to affect the findings (i.e. given that the ROCS study population reflects the wider clinical population, this could be considered appropriate in capturing a lifetime horizon for this population).
A recent costing study72 discussed the direct medical costs to the Canadian health-care system. Although this compared costs across cancer stages and treatment, radiotherapy was a predictor of higher net costs. This was also observed in an Australian study of resource use and costs in oesophageal cancer. 73 Thien et al. 72 highlighted the importance of capturing phase-specific costs to estimate the cumulative costs over the patient pathway. The ROCS study data set provides detailed assessment of health and social care resource use during the ROCS study treatment pathways, which in turn could enable further assessment of the costs associated with the management of people with advanced oesophageal cancer.
There are several important caveats to the economic evaluation. The focus on an NHS/Personal Social Services perspective will not have captured the impact on patients, family and society. The CUA was based on the EQ-5D-3L. Although this reflects appropriate methods for national decision-making,57 the inherent insensitivity of the generic EQ-5D questionnaire may not have reflected the more precise estimates of HRQoL changes, captured by the other patient-reported measures used in the trial. However, the findings from the EQ-5D-3L derived utilities are comparable to the clinical measures of HRQoL in that no differences were observed in QoL outcomes. The trade-off between costs and benefits focused on the interventions tested in the ROCS study and may not fully reflect the other potential treatment options such as specialist palliative care. No regard has been made as to whether EBRT would be cost-effective taking into account any specific decision criteria (e.g. in relation to end-of-life care). Although it is ultimately the jurisdiction of decision-makers to appraise the evidence from the ROCS study, given that there is no survival advantage shown, it is unlikely that formal consideration of such criteria would alter the conclusion.
In conclusion, the addition of EBRT to stent placement is not a cost-effective option, at a willingness-to-pay threshold of £20,000 per QALY gained, in the management of patients with advanced oesophageal cancer.
Chapter 6 Discussion and conclusions
The most common intervention for relief of severe dysphagia in advanced oesophageal cancer is insertion of a SEMS. Although this provides rapid relief, it is associated with deteriorations in quality of life and increased reintervention rates over time, with average stent patency reported as 11–12 weeks. 10,20,21 Systematic reviews8,9 have highlighted the need for studies that test interventions in combination with SEMS to improve dysphagia outcomes and for such studies to include robust health economic and qualitative evaluation of participant experiences.
Any combined intervention should not have too great an impact on other aspects of quality of life and, to be useful, should be readily available reasonably close to home for a frail patient population. Such availability will be dependent on the expertise and infrastructure available locally. EBRT is a treatment modality that is widely available across the UK and accounts for over 95% of all radiotherapy given. 18 It is commonly used as a palliative oncological treatment for oesophageal cancer symptoms. This contrasts starkly with the lack of infrastructure and expertise for palliative oesophageal brachytherapy. 17 Therefore, EBRT represents a readily available modality for combination with SEMS to improve palliation in the setting of advanced oesophageal cancer in the UK. Given its wide availability internationally, it also represents a viable option in middle- and low-income countries, which account for > 80% of oesophageal cancer incidence and deaths. 2
The ROCS study is the first large-scale prospective, pragmatic RCT to test the impact of combining palliative EBRT with stent insertion versus stent insertion alone on dysphagia, quality of life and bleeding. Its mixed-methodological approach has allowed a robust multiperspective analysis of efficacy.
Summary of key findings
This study clearly shows that EBRT did not improve the proportion of patients with a stent who experienced recurrent dysphagia up to and including 12 weeks. The median overall survival of 19 weeks confirmed that the participant population accurately reflects the wider clinical population of these patients. 3 Only 31 patients were alive at the end of the study, with a median follow-up of those still alive of 22.9 weeks (n = 16) versus 22.1 (n = 15). Nonetheless, the majority of the recruited patients had a performance status (PS) of 2 or better, and thus were among the fitter patients with this disease, so our results may underestimate burdens to those with poorer PS.
The trial was powered on 82 patients per arm to reduce the proportion of recurrent dysphagia events from 40% to 20% at week 12 (80% power, 5% alpha two-sided). Our complete-case analysis revealed an average event rate across arms of 46%, similar to that predicted.
Our modified ITT population had 102 (usual care) and 97 (EBRT plus stent) patients per arm, with 74 versus 75 complete data sets up to week 12. Sensitivity analysis by imputing for missing data under best- and worst-case scenarios resulted in 90 versus 88 patients in the denominator. The robustness of the primary outcome result was confirmed across all of these data sets as well as a per-protocol data set. Whether or not death was treated as an event did not alter the primary outcome.
Secondary sensitivity analysis of DDFS again showed no difference.
For those undergoing EBRT, the qualitative data set importantly uncovered trade-offs in terms of fatigue, pain and burden associated with travel to the radiotherapy sessions. Similarly, QoL data showed a trend towards increased fatigue in the radiotherapy arm, and fatigue was noticeably more frequently described as a grade 3+ toxicity in the radiotherapy arm although this finding was not statistically significant.
It is noteworthy that, although there was no difference in other QoL outcomes between arms, global HRQoL scores were low across the study population. Eating restriction scores improved from baseline, reflecting SEMS insertion, but never returned to normal and eating remained a significant issue over time. These outcomes reflect the challenges that patients described in the qualitative study of their lived experiences of eating restrictions, concerns over nutrition and diet, and a trial and error approach to combating these, with important implications for practice.
The health economic evaluation of complete cases did not demonstrate a significant difference in combined health and social care costs between arms at 12 weeks, and cost–utility analysis of the full ITT arm confirmed that EBRT was a less cost-effective approach than stent insertion alone.
At 12 months, potential savings were noted in subsequent health and social care costs in the EBRT arm, which offset the intervention costs and resulted in savings of £568 per patient still alive at 52 weeks. These savings were mainly attributed to the period after 28 weeks, when health-care costs were consistently lower in the EBRT arm. However, none of the differences reached statistical significance, and sample sizes had fallen below 25 patients per arm. So few patients survive for 1 year in this situation that it is not appropriate to propose radiotherapy on this basis.
Radiotherapy is often offered to try to help palliate bleeding, although there are no prospective studies to show that it is effective for this. Although there was no evidence of a difference between arms for the primary outcome, those in the radiotherapy arm had significantly fewer bleeding events. Up to week 16, in the usual-care arm 19 (18.6%) patients had a bleeding-related event compared with 10 (10.3%) patients in the EBRT arm, giving a number needed to treat of 12. The effect persisted and increased over time: at 52 weeks, 29 (28.4%) patients in the usual-care arm, compared with 16 (16.5%) patients in the radiotherapy arm, had an event, giving a number needed to treat of eight. These are the first prospective randomised data on radiotherapy for palliation of upper GI cancer bleeding risk that we are aware of.
There were no differences between arms in antiplatelet or anticoagulant use. We did not capture data on visual descriptions of tumour at endoscopy, but there was no association between tumour length and bleeding risk.
These data suggest that, for those patients with good performance status deemed at higher risk of bleeding at initial endoscopy, prophylactic radiotherapy may reduce later bleeding risk and associated admissions/interventions. The health economic data, for example, showed lower health-care costs in the EBRT arm between weeks 9 and 12, associated in particular with lower blood transfusion costs.
Patients in the EBRT arm were also much less likely to undergo subsequent palliative radiotherapy to the oesophagus. In the usual-care arm, 20 patients had subsequent palliative radiotherapy (overall median dose was 20 Gy in five fractions), in 16 (80%) cases to the oesophagus; in the EBRT arm, nine (9.3%) patients had additional radiotherapy, with only two of these receiving further oesophageal radiotherapy. It would, however, be expected that clinicians are more reluctant to repeat radiotherapy treatment in the EBRT patients because of the risk of exceeding normal tissue tolerance.
Patients’ and carers’ descriptions of living with advanced oesophageal cancer and dysphagia revealed ongoing challenges with eating despite stent placement. The ROCS study clearly illustrated that the technical intervention of stenting does not address the multidimensional eating concerns and symptoms that the patient lives with. Information around diet, pain relief and general medical management through the course of the disease was often missing, with patients having to adopt a trial and error approach to their daily life, and gradually adopting a process of ‘reframing hope’ to address their quality of life rather than survival. Both patients and carers emphasised the important social, psychosocial and physical aspects of nutrition and eating, as well as the need for holistic support in dealing with uncertainty.
Strengths and limitations
The ROCS study successfully recruited to its revised target of 220 patients, making it one of the largest reported intervention studies involving stents for advanced oesophageal cancer. Recruitment in this population is extremely challenging,10,32 and specific design elements were important in helping recruitment and reducing the risk of missing data.
Additional research nurse time and training to allow for data capture to take place in the patient’s home ensured that CRF returns were high, and were able to describe reasons for missing data. CRF analysis confirmed that patients requested support from the research nurse on approximately 50% of occasions to complete questionnaires, highlighting the importance of the nurse’s presence in capturing robust data and the frailty of this patient population.
Regular face-to-face meetings of the research nurse teams allowed sharing of good practice as well as rapid feedback of emerging qualitative data to support approaches to information-giving, consent and follow-up. The meetings produced practical solutions that informed protocol amendments and changes to process such as timing of randomisation, changes to CRF design, and the timing and content of ‘between-visit’ telephone calls.
The ROCS study also specifically addressed the identified gaps in qualitative and health economic outcomes consistently called for. 7–9 The trial had a comprehensive assessment of HRQoL issues with both generic and disease-specific components and a patient-reported HRQoL primary outcome. The embedded qualitative study acted as a ‘study within a study’ to help improve trial processes and resulted in improvements such as timing of randomisation, options for follow-up and study description to help understanding of equipoise. It also captured the perceived trade-offs of the radiotherapy intervention in terms of additional burden. The qualitative data also described the experience across both trial arms of living with advanced oesophageal cancer, and it was possible to correlate findings in relation to symptoms such as fatigue across quantitative QoL and toxicity data.
The attention paid to capturing precise and regular resource use information from patients, including reasons for missing data, provided a rich data set for comprehensive assessment of costs and health utilities. The construction of a complex model, populated entirely with trial data, allowed a thorough examination of the costs and consequences of EBRT in addition to stent insertion in the context of routine clinical practice.
The study was originally powered to detect a difference in time to event of 4 weeks, from 12 to 16 weeks. During the course of the study, it became clear that it would not be possible to reach the recruitment target and that missing data increased significantly beyond 12 weeks, reflecting the frailty of the patient population. On the advice of the IDMC, and with independent TSC and funder support, we therefore revised the primary outcome to a binary outcome of differences in proportions of recurrent dysphagia at 12 weeks. Although it is possible that this affected the ability of the study to detect a true effect for EBRT, we believe that the consistency of the results across the sensitivity analyses is robust, including the secondary analysis of DDFS. In addition, the event rate in the study reflected that used in the power calculation. Careful attention to data capture meant that, of a required population of 164, our modified ITT population of 199 yielded 149 complete data sets to week 12, and 178 data sets when using a sensitivity analysis of best- and worst-case scenario appropriate to the data set.
Implications for health-care practice
-
Patients with advanced oesophageal cancer requiring SEMSs to improve dysphagia will not benefit further from the addition of concurrent palliative EBRT and are likely to find the trade-offs of fatigue and additional hospital visits too burdensome. For those with a longer prognosis and who are considered to have an increased risk of tumour bleeding, concurrent EBRT may reduce the risk of bleeding and associated interventions. However, when offering patients this intervention, information about the impact that it can have on QoL and trade-offs would be important in informing decision-making.
-
Insertion of a stent for dysphagia does not address the experience of patients in relation to eating concerns, symptoms and adapting to uncertainty. Patients and carers require timely and ongoing support from multidisciplinary professionals on the important psychosocial and physical aspects of nutrition and eating. Patients also require help in negotiating uncertainty and the reframing of hope towards quality of life rather than survival.
-
A significant proportion of the patients who took part in the ROCS study were planned to have systemic anticancer treatment despite very limited prognosis and with wide variation across the UK in regimen used. The study confirms the need for greater consistency in the assessment of suitability for systemic treatment, being mindful of the trade-offs between survival advantage and burdens of treatment. The wide variation in the type of regimen used reflects the current clinical uncertainty about the most effective palliative chemotherapy approach.
Implications for future research
-
Future studies are required to assess other interventions that may usefully be combined with a SEMS to improve the patient’s ability to swallow. Such studies may benefit from insights gained during the ROCS study on trial conduct in this setting. Specifically, we recommend investment in additional research practitioner time and training to capture follow-up data in the home setting, randomisation after stent insertion to allow more time for patient identification and trial consideration and regular meeting up of multisite research practitioners to consider barriers and share best practice as trial processes that can all improve trial conduct. Embedded qualitative methods can also ensure that intervention combinations are robustly assessed in terms of patient experience and perceptions of trade-offs between treatment benefits and burdens.
-
The ROCS study has highlighted significant unmet supportive and palliative care needs of patients with advanced oesophageal cancer, including multifaceted aspects of eating and nutrition. Further research is required to assess the benefits of early (at time of diagnosis) palliative interventions and to specify the effective domains of multidisciplinary supportive care. In particular, research is required on the types of nutritional support that will best address the practical challenges and psychosocial distress around eating and nutritional intake for both patients and carers.
-
The ROCS study has also identified how oesophageal cancer patients and their carers adapt their expectations over time and engage in their own process of ‘reframing hope’. Further research would be useful to define how this is best supported, and how the concept of reframing hope may influence treatment discussions and subsequent choices.
Acknowledgements
We would like to thank the independent members of the ROCS TSC for their regular expert advice and support throughout this project: Professor Barry Hancock OBE (Emeritus Professor, University of Sheffield) – chairperson, Professor Tim Maughan (University of Oxford), Dr Martin Forster (University College London), Dr Stephen Shepherd (Gloucestershire Hospital), Professor Robert Huddart (The Institute of Cancer Research, London), Professor Sarah Brown (University of Leeds), Ms Elaine Robinson (Research Partner) and Mrs Julie Hepburn (Research Partner).
We would also like to thank the IDMC for their guidance and support: Professor Susan Dutton (University of Oxford) – chairperson, Professor Marie Fallon (University of Edinburgh) and Professor John Dewar (Tayside Cancer Centre, Ninewells Hospital, Dundee).
We are very grateful to all of the participants for considering the study, for giving their time to participate and for sharing their experiences with us. Thanks also to all of the clinical teams who supported the study throughout its duration.
We would like to express our gratitude to Mrs Sarah Townsend, research and development manager, Velindre University NHS Trust, which was the study sponsor, for unwavering support throughout the project, and to the entire staff of the CTR at Cardiff University.
We are indebted to the principal investigators for their dedication in identifying and recruiting patients at each site: Dr Andrew Bateman, Dr Olivia Chan, Dr Mathilda Cominos, Dr Serena Hilman, Dr Eleanor James, Dr Daniella A Power, Professor Ashraf Rasheed, Dr Angus Robinson, Dr Martin Scott-Brown, Dr Elizabeth Selvaduri, Dr Paul Shaw, Dr David Tsang, Dr Ravi Vohra, Dr Nick Wadd, Dr Jonathan Wadsley and Dr Julie Walther. A full list of recruiting sites is included in Appendix 1, Table 27.
Our thanks also to all of the research nurses/practitioners who recruited patients to the study and attended study meetings to share their experiences and support colleagues with advice: Debbie Forbes, Mim Evans, Rebecca Cloudsdale, Anita Willicombe, Liane Davis, Alice Johnson, Kay Nicholson, Joanne Taylor, Stephanie Brown, Jeanette Gilbert, Lauren McCrisken, Gail Pottinger, Rebecca Houlihan, Joanna Nicklin, Amanda Portal, Harvey Dymond, Dianne Thomas, Kelly Andrews, Stella Wright, Emma Hall, Rachel Williams, Debbie Barnett, Emma Thompson, Keith Harland, Gillian Brown, Kathryn Yousaf, Alison Chedham, Fatima Akbar, Jasmine Jose, Gillian Brown, Rebecca Burt, Sarah Wood, Lesley Nichols, Kerry Goodsell, Rebecca Burt, Catherine Gibson, Rachel Roberts and Lisa Roche.
Contributions of authors
Douglas Adamson (https://orcid.org/0000-0002-4026-1593) (Consultant Clinical Oncologist), co-chief investigator, led on the conception and design of the study, the development of the study protocol and organisation of study conduct, interpretation of the data and the writing of the report.
Jane Blazeby (https://orcid.org/0000-0002-3354-3330) (Professor of Surgery) contributed to the design of the study; the development of the study protocol and study implementation, including the national research nurse meetings, the interpretation of the data and the writing of the report.
Catharine Porter (https://orcid.org/0000-0003-1851-0677) (Research Associate in Statistics) contributed to the development of the study protocol, statistical analysis plan, data analysis, interpretation of the data and the writing of the report.
Christopher Hurt (https://orcid.org/0000-0003-1206-8355) (Senior Research Fellow in Statistics) led on the statistical analysis and contributed to the development of the study protocol, statistical analysis plan, data analysis, interpretation of the data and the writing of the report.
Gareth Griffiths (https://orcid.org/0000-0002-9579-8021) (Professor of Clinical Trials within Medicine) contributed to the conception and design of the study, the development of the study protocol and organisation of study conduct, interpretation of the data and the writing of the report.
Annmarie Nelson (https://orcid.org/0000-0002-6075-8425) (Scientific Director) led on the development and conduct of the embedded qualitative study and contributed to the development of the study protocol, interpretation of the data and the writing of the report.
Bernadette Sewell (https://orcid.org/0000-0001-5471-922X) (Senior Lecturer) led on the development of the health economic evaluation and data analysis, and contributed to interpretation of the data and the writing of the report.
Mari Jones (https://orcid.org/0000-0001-9661-4899) (Research Officer) contributed to the development of the health economic model and data analysis, interpretation of the data and the writing of the report.
Martina Svobodova (https://orcid.org/0000-0001-7949-4039) (Trial Manager) managed the study and contributed to data collection, site monitoring, data cleaning and the drafting and writing of the report.
Deborah Fitzsimmons (https://orcid.org/0000-0002-7286-8410) (Professor, Public Health Policy and Social Sciences) contributed to the development of the health economic evaluation and data analysis, interpretation of the data and the writing of the report.
Lisette Nixon (https://orcid.org/0000-0002-1270-6970) (Senior Trial Manager) contributed to the study development, protocol design and study conduct, data collection, study monitoring and the writing of the report.
Jim Fitzgibbon (https://orcid.org/0000-0002-3899-8510) (Lay Research Partner) contributed to the study design, protocol development, study conduct and the writing of the report.
Stephen Thomas (https://orcid.org/0000-0002-1824-8448) (Lay Research Partner) contributed to the protocol development, study conduct and the writing of the report.
Anthony Millin (https://orcid.org/0000-0002-3138-8779) (Head of Radiotherapy Physics) led on radiotherapy quality assurance for the trial and contributed to the protocol development, study conduct and the writing of the report.
Tom Crosby (https://orcid.org/0000-0003-2232-1756) (Consultant Clinical Oncologist) contributed to the study design, protocol development, interpretation of results and the writing of the report.
John Staffurth (https://orcid.org/0000-0002-7834-3172) (Consultant Clinical Oncologist) led on radiotherapy quality assurance for the study and contributed to the study design, protocol development, study conduct and the writing of the report.
Anthony Byrne (https://orcid.org/0000-0002-1413-1496) (Clinical Director), co-chief investigator, led on the conception and design of the study, the development of the study protocol and organisation of study conduct, interpretation of the data and the writing of the report.
All authors approved the final version of the report.
Publications
Adamson D, Blazeby J, Nelson A, Hurt C, Nixon L, Fitzgibbon J, et al. Palliative radiotherapy in addition to self-expanding metal stent for improving dysphagia and survival in advanced oesophageal cancer (ROCS: Radiotherapy after Oesophageal Cancer Stenting): study protocol for a randomized controlled trial. Trials 2014;15:402.
Adamson D, Byrne A, Porter C, Blazeby J, Griffiths G, Nelson A, et al. Palliative radiotherapy after oesophageal cancer stenting (ROCS): a multicentre, open-label, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol 2021;6:292–303.
Data-sharing statement
The study team are committed to the sharing of relevant data to maximise its use in support of delivering patient benefit. Data will be shared in accordance with the data-sharing policies of the CTR and the study sponsor, governed by Research Ethics Committee guidance and the General Data Protection Regulation. Please contact the corresponding author to request data sharing.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Cancer Research UK . Oesophageal Cancer Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/oesophageal-cancer (accessed 12 October 2019).
- Wong MCS, Hamilton W, Whiteman DC, Jiang JY, Qiao Y, Fung FDH, et al. Global Incidence and mortality of oesophageal cancer and their correlation with socioeconomic indicators temporal patterns and trends in 41 countries. Sci Rep 2018;8. https://doi.org/10.1038/s41598-018-19819-8.
- O’Hanlon DM, Callanan K, Karat D, Crisp W, Griffin M. Outcome, survival, and costs in patients undergoing intubation for carcinoma of the oesophagus. Am J Surg 1997;174:316-19. https://doi.org/10.1016/S0002-9610(97)00104-9.
- Watkinson AF, Ellul J, Entwisle K, Mason RC, Adam A. Esophageal carcinoma: initial results of palliative treatment with covered self-expanding endoprostheses. Radiology 1995;195:821-7. https://doi.org/10.1148/radiology.195.3.7538682.
- Scottish Audit of Gastric and Oesophageal Cancer . Report 1997–2000: A Prospective Audit. 2002.
- Shenfine J, McNamee P, Steen N, Bond J, Griffin SM. A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9050.
- Shenfine J, McNamee P, Steen N, Bond J, Griffin SM. A randomized controlled clinical trial of palliative therapies for patients with inoperable esophageal cancer. Am J Gastroenterol 2009;104:1674-85. https://doi.org/10.1038/ajg.2009.155.
- Sreedharan A, Harris K, Crellin A, Forman D, Everett SM. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev 2009;4. https://doi.org/10.1002/14651858.CD005048.pub2.
- Dai Y, Li C, Xie Y, Liu X, Zhang J, Zhou J, et al. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev 2014;10. https://doi.org/10.1002/14651858.CD005048.pub4.
- Homs MY, Steyerberg EW, Eijkenboom WM, Tilanus HW, Stalpers LJ, Bartelsman JF, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364:1497-504. https://doi.org/10.1016/S0140-6736(04)17272-3.
- Bergquist H, Wenger U, Johnsson E, Nyman J, Ejnell H, Hammerlid E, et al. Stent insertion or endoluminal brachytherapy as palliation of patients with advanced cancer of the esophagus and gastroesophageal junction. Results of a randomized, controlled clinical trial. Dis Esophagus 2005;18:131-9. https://doi.org/10.1111/j.1442-2050.2005.00467.x.
- Rosenblatt E, Jones G, Sur RK, Donde B, Salvajoli JV, Ghosh-Laskar S, et al. Adding external beam radiotherapy to intraluminal brachytherapy improves palliation in obstructive squamous cell oesophageal cancer: a prospective multi-centre randomised trial of the International Atomic Energy Agency. Radiother Oncol 2010;97:488-94. https://doi.org/10.1016/j.radonc.2010.09.001.
- Hirdes MMC, van Hooft JE, Wijrdeman HK, Hulshof MCCM, Fockens P, Reerink O, et al. Combination of biodegradable stent placement and single-dose brachytherapy is associated with an unacceptably high complication rate in the treatment of dysphagia from esophogeal cancer. Gastrointest Endosc 2012;76:267-74. https://doi.org/10.1016/j.gie.2012.04.442.
- Amdal CD, Jacobsen AB, Sandstad B, Warloe T, Bjordal K. Palliative brachytherapy with or without primary stent placement in patients with oesophageal cancer, a randomised phase III trial. Radiother Oncol 2013;107:428-33. https://doi.org/10.1016/j.radonc.2013.04.008.
- Zhu H-D, Guo J-H, Mao A-W, Lv W-F, Ji J-S, Wang W-H, et al. Conventional stents versus stents loaded with 125iodine seeds for the treatment of unresectable oesophageal cancer: a multicentre, randomised phase 3 trial. Lancet Oncol 2014;15:612-19. https://doi.org/10.1016/S1470-2045(14)70131-7.
- Tian D, Wen H, Fu M. Comparative study of self-expanding metal stent and intraluminal radioactive stent for inoperable esophageal squamous cell carcinoma. World J Surg Oncol 2016;14. https://doi.org/10.1186/s12957-016-0768-x.
- Healthcare Quality Improvement Partnership Ltd (HQP) . An Audit of the Care Received by People With Oesophago-Gastric Cancer in England and Wales 2017 Annual Report 2017.
- Royal College of Radiologists . The Role and Development of Brachytherapy Services in the United Kingdom 2007.
- Healthcare Quality Improvement Partnership Ltd (HQP) . An Audit of the Care Received by People With Oesophago-Gastric Cancer in England and Wales 2013 Annual Report 2013.
- Conio M, Repici A, Battaglia G, De Pretis G, Ghezzo L, Bittinger M, et al. A randomised prospective comparison of self-expandable metal stents in the palliation of malignant esophageal dysphagia. Am J Gastroenterol 2007;102:2667-77. https://doi.org/10.1111/j.1572-0241.2007.01565.x.
- Verschuur EM, Repici A, Kuipers EJ, Steyerberg EW, Siersema PD. New design esophageal stents for the palliation of dysphagia from esophageal or gastric cardia cancer: a randomized trial. Am J Gastroenterol 2008;103:304-12. https://doi.org/10.1111/j.1572-0241.2007.01542.x.
- Hoover DR, Crystal S, Kumar R, Sambamoorthi U, Cantor JC. Medical expenditures during the last year of life: findings from the 1992-1996 Medicare Current Beneficiary Survey. Health Serv Res 2002;37:1625-42. https://doi.org/10.1111/1475-6773.01113.
- Chitris X, Steventon A, Glaser A, Bardsley M. Use of Health and Social Care by People with Cancer. London: Nuffield Trust; 2014.
- Javed A, Pal S, Dash NR, Ahuja V, Mohanti BK, Vishnubhatla S, et al. Palliative stenting with or without radiotherapy for inoperable esophageal carcinoma: a randomized trial. J Gastrointest Cancer 2012;43:63-9. https://doi.org/10.1007/s12029-010-9206-4.
- Royal College of Radiologists . Radiotherapy Dose Fractionation 2017.
- Adamson D, Blazeby J, Neslon A, Hurt C, Nixon L, Fitzgibbon J, et al. Palliative radiotherapy in addition to self-expanding metal stent for improving dysphagia and survival in advanced oesophageal cancer (ROCS: Radiotherapy after Oesophageal Cancer Stenting): protocol for a randomised controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-402.
- Blazeby JM, Nicklin J, Brookes ST, Winstone K, Alderson D. Feasibility of quality of life assessment in patients with upper gastrointestinal tract cancer. Br J Cancer 2003;89:497-501. https://doi.org/10.1038/sj.bjc.6601146.
- Lagergren P, Fayers P, Conroy T, Stein HJ, Sezer O, Hardwick R, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer 2007;43:2066-73. https://doi.org/10.1016/j.ejca.2007.07.005.
- Blazeby JM, Conroy T, Hammerlid E, Fayers P, Sezer O, Koller M, et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer 2003;39:1384-94. https://doi.org/10.1016/S0959-8049(03)00270-3.
- Blazeby JM, Conroy T, Bottomley A, Vickery C, Arraras J, Sezer O, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer 2004;40:2260-8. https://doi.org/10.1016/j.ejca.2004.05.023.
- Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol 2011;29:89-96. https://doi.org/10.1200/JCO.2010.28.0107.
- Blazeby JM, Williams MH, Brookes ST, Alderson D, Farndon JR. Quality of life measurement in patients with oesophageal cancer. Gut 1995;37:505-8. https://doi.org/10.1136/gut.37.4.505.
- National Institute for Health and Care Excellence (NICE) . Methods for Technology Appraisals 2009.
- EuroQol Group . EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- White RE, Chepkwony R, Mwachiro M, Burgert SL, Enders FT, Topazian M. Randomized trial of small-diameter versus large-diameter esophageal stents for palliation of malignant esophageal obstruction. J Clin Gastroenterol 2015;49:660-5. https://doi.org/10.1097/MCG.0000000000000333.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. Open Med 2010;4:e60-8. https://doi.org/10.1016/j.ijsu.2010.09.006.
- European Organisation for Research and Treatment of Cancer . The EORTC QLQ-C30 Scoring Manual n.d. www.academia.edu/18887698/The_EORTC_QLQ-C30_Scoring_Manual_Published_by_European_Organisation_for_Research_and_Treatment_of_Cancer (accessed 18 October 2019).
- Cancer Research UK . Beating Cancer Sooner. Our Research Strategy 2014.
- Andreassen S, Randers I, Näslund E, Stockeld D, Mattiasson AC. Patients’ experiences of living with oesophageal cancer. J Clin Nurs 2006;15:685-95. https://doi.org/10.1111/j.1365-2702.2006.01412.x.
- Cowley A, Evans C, Bath-Hextall F, Cooper J. Patient, nursing and medical staff experiences and perceptions of the care of people with palliative esophagogastric cancer: a systematic review of the qualitative evidence. JBI Database System Rev Implement Rep 2016;14:134-66. https://doi.org/10.11124/JBISRIR-2016-003168.
- Clarke C, McCorry NK, Dempster M. The role of identity in adjustment among survivors of oesophageal cancer. J Health Psychol 2011;16:99-108. https://doi.org/10.1177/1359105310368448.
- Missel M, Birkelund R. Living with incurable oesophageal cancer. A phenomenological hermeneutical interpretation of patient stories. Eur J Oncol Nurs 2011;15:296-301. https://doi.org/10.1016/j.ejon.2010.10.006.
- Watt E, Whyte F. The experience of dysphagia and its effect on the quality of life of patients with oesophageal cancer. Eur J Cancer Care 2003;12:183-93. https://doi.org/10.1046/j.1365-2354.2003.00376.x.
- Kakuta T, Kosugi SI, Ichikawa H, Hanyu T, Ishikawa T, Kanda T, et al. Palliative interventions for patients with incurable locally advanced or metastatic thoracic esophageal carcinoma. Esophagus 2019;16:278-84. https://doi.org/10.1007/s10388-019-00665-0.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- McGrath JP, Browne M, Riordan C. Expandable metal stents in the palliation of malignant dysphagia and oesophageal respiratory fistula. Ir Med J 2001;94:270-2.
- Rozanes I, Poyanli A, Acunaş B. Palliative treatment of inoperable malignant esophageal strictures with metal stents: one center’s experience with four different stents. Eur J Radiol 2002;43:196-203. https://doi.org/10.1016/S0720-048X(02)00154-7.
- Madhusudhan C, Saluja SS, Pal S, Ahuja V, Saran P, Dash NR, et al. Palliative stenting for relief of dysphagia in patients with inoperable esophageal cancer: impact on quality of life. Dis Esophagus 2009;22:331-6. https://doi.org/10.1111/j.1442-2050.2008.00906.x.
- Qiu Y, You J, Lv Q, Yuan L. Effect of whole-course nutrition management on patients with esophageal cancer undergoing concurrent chemoradiotherapy: a randomized control trial (P05-031-19). Curr Dev Nutr 2019;3. https://doi.org/10.1093/cdn/nzz030.P05-031-19.
- World Health Organization . WHO Definition of Palliative Care n.d. www.who.int/cancer/palliative/definition/en/ (accessed 12 October 2019).
- Laursen L, Schønau MN, Bergenholtz HM, Siemsen M, Christensen M, Missel M. Table in the Corner: a qualitative study of life situation and perspectives of the everyday lives of oesophageal cancer patients in palliative care. BMC Palliat Care 2019;18. https://doi.org/10.1186/s12904-019-0445-2.
- Sampson C, Finlay I, Byrne A, Snow V, Nelson A. The practice of palliative care from the perspective of patients and carers. BMJ Support Palliat Care 2014;4:291-8. https://doi.org/10.1136/bmjspcare-2013-000551.
- Dixon J, King D, Matosevic T, Clark M, Knapp M. Equity in the Provision of Palliative Care in the UK: Review of Evidence. London: Personal Social Services Research Unit, London School of Economics and Political Science; 2015.
- Harrop E, Byrne A, Nelson A. ‘It’s alright to ask for help’: findings from a qualitative study exploring the information and support needs for family carers at the end of life. BMC Palliat Care 2014;13. https://doi.org/10.1186/1472-684X-13-22.
- Bee PE, Barnes P, Luker KA. A systematic review of informal caregivers’ needs in providing home-based end-of-life care to people with cancer. J Clin Nurs 2009;18:1379-93. https://doi.org/10.1111/j.1365-2702.2008.02405.x.
- National Institute for Health and Care Excellence (NICE) . Developing NICE Guidelines: The Manual. Process and Methods 2017. www.nice.org.uk/process/pmg20/chapter/introduction-and-overview (accessed 19 July 2019).
- CCEMG-EPPI-Centre Cost Converter 2019. http://eppi.ioe.ac.uk/costconversion/default.aspx (accessed 30 September 2019).
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2017 to 2018 2018. https://improvement.nhs.uk/resources/reference-costs/ (accessed 20 October 2019).
- Beecham J, Knapp M. Client Service Receipt Inventory 1997. www.dirum.org/assets/downloads/634462388066137028-CSRI.pdf (accessed 19 October 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 20 October 2019).
- Eichstaedt KE, Kovatch K, Maroof DA. A less conservative method to adjust for familywise error rate in neuropsychological research: the Holm’s sequential Bonferroni procedure. NeuroRehabil 2013;32:693-6. https://doi.org/10.3233/NRE-130893.
- National Institute for Health and Care Excellence (NICE) . Costing Statement: Blood Transfusion 2015. www.nice.org.uk/guidance/ng24/resources/costing-statement-2177158141 (accessed 18 October 2019).
- McBride T, Morton A, Nichols A, van Stolk C. Comparing the costs of alternative models of end-of-life care. J Palliat Care 2011;27:126-33. https://doi.org/10.1177/082585971102700208.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Rao C, Haycock A, Zacharakis E, Krasopoulos G, Yakoub D, Protopapas A, et al. Economic analysis of esophageal stenting for management of malignant dysphagia. Dis Esophagus 2009;22:337-47. https://doi.org/10.1111/j.1442-2050.2008.00916.x.
- da Silveira EB, Artifon EL. Cost-effectiveness of palliation of unresectable esophageal cancer. Dig Dis Sci 2008;53:3103-11. https://doi.org/10.1007/s10620-008-0302-2.
- Katsaliaki K, Mustafee N. Applications of simulation within the healthcare context. J Oper Res 2011;62:1431-51. https://doi.org/10.1057/jors.2010.20.
- Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling. PharmacoEconomics 2007;25:3-6. https://doi.org/10.2165/00019053-200725010-00002.
- Thein HH, Jembere N, Thavorn K, Chan KKW, Coyte PC, de Oliveira C, et al. Estimates and predictors of health care costs of esophageal adenocarcinoma: a population-based cohort study. BMC Cancer 2018;18. https://doi.org/10.1186/s12885-018-4620-2.
- Gordon LG, Eckermann S, Hirst NG, Watson DI, Mayne GC, Fahey P, et al. Australian Cancer Study Clinical Follow-Up Study . Healthcare resource use and medical costs for the management of oesophageal cancer. Br J Surg 2011;98:1589-98. https://doi.org/10.1002/bjs.7599.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J Natl Cancer Inst 1993;85:365-76. https://doi.org/10.1093/jnci/85.5.365.
Appendix 1 Recruiting sites
| Site | Principal investigator | Patients randomised |
|---|---|---|
| Ninewells Hospital, NHS Tayside, Dundee | Dr Douglas Adamson | 61 |
| University Hospitals Bristol NHS Foundation Trust Royal Infirmary | Professor Jane Blazeby | 27 |
| Weston Park, Sheffield Teaching Hospitals NHS Foundation Trust | Dr Jonathan Wadsley | 18 |
| University Hospital of Wales, Cardiff and Vale University Health Board | Dr Anthony Byrne | 12 |
| University Hospital Coventry, Coventry and Warwickshire NHS Trust | Dr Martin Scott-Brown | 10 |
| University Hospital Llandough, Cardiff and Vale University Health Board | Dr Anthony Byrne | 9 |
| Musgrove Park Hospital, Taunton and Somerset NHS Foundation Trust | Dr Julie Walther | 9 |
| Basildon and Thurrock University Hospitals Foundation NHS Trust | Dr Olivia Chan | 8 |
| St Mary’s Hospital, Imperial College Healthcare NHS Trust | Dr Daniella A Power | 8 |
| Nottingham University Hospitals NHS Trust | Dr Ravi Vohra/Dr Eleanor James | 7 |
| Royal Glamorgan Hospital, Cwm Taff Morgannwg University Health Board | Dr Paul Shaw | 7 |
| Weston Super Mare Hospital, Weston Area Health Trust | Dr Serena Hilman | 7 |
| Worthing Hospital, Western Sussex Hospitals NHS Foundation Trust | Dr Angus Robinson/Dr Elizabeth Selvaduri | 6 |
| Conquest Hospital Hastings, East Sussex Healthcare NHS Trust | Dr Angus Robinson | 5 |
| Royal Sussex Hospital Brighton, East Sussex Healthcare NHS Trust | Dr Angus Robinson | 4 |
| University Hospital Southampton Foundation Trust | Dr Andrew Bateman | 3 |
| George Eliot Hospital NHS Trust | Dr Martin Scott-Brown | 3 |
| Kent and Canterbury Hospital, East Kent Hospitals NHS Foundation Trust | Dr Mathilda Cominos | 3 |
| King’s Mill Hospital, Sherwood Forest Hospitals NHS Foundation Trust | Dr Eleanor James | 3 |
| Doncaster and Bassetlaw Hospitals Hospitals NHS Foundation Trust | Dr Jonathan Wadsley | 3 |
| Southend University Hospital Hospitals NHS Foundation Trust | Dr Olivia Chan/Dr David Tsang | 3 |
| Royal Gwent Hospital, Aneurin Bevan University Health Board | Professor Ashraf Rasheed | 2 |
| James Cook Hospital, South Tees Hospitals NHS Foundation Trust | Dr Nick Wadd | 2 |
| Total | 220 | |
Appendix 2 Quality-of-life questionnaires
Reproduced from The EORTC Quality of Life Group. 74 Any person wishing to use EORTC measures must contact the EORTC Quality of Life Department. Details available at https://www.qol.eortc.org/questionnaires.
Appendix 3 Main results additional tables and figures
| Time point | Usual care (N = 102) | EBRT (N = 97) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Not expected,a n | Expected, n | Actually received, n (%) | Help needed to complete questionnaire, n (%) | Carer completed, n (%) | Missing reasons | Not expected,a n | Expected, n | Actually received, n (%) | Help needed to complete questionnaire, n (%) | Carer completed, n (%) | Missing reasons | |||||||
| Patient too ill, refused or withdrew consent, n (%) | Patient did not return, n (%) | Admin error, n (%) | Reason missing, n (%) | Patient too ill, refused or withdrew consent, n (%) | Patient did not return, n (%) | Admin error, n (%) | Reason missing, n (%) | |||||||||||
| Baseline | 0 | 102 | 101 (99.0) | 46 (45.5) | 5 (5.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 97 | 96 (99.0) | 38 (39.6) | 7 (7.3) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 1 week post stent insertion | 0 | 39 | 39 (100.0) | 21 (53.8) | 6 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 36 | 34 (94.4) | 11 (32.4) | 2 (5.9) | 2 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 4 weeks post stent insertion | 11 | 91 | 77 (84.6) | 36 (46.8) | 3 (3.9) | 0 (0.0) | 3 (21.4) | 0 (0.0) | 11 (78.6) | 6 | 91 | 73 (80.2) | 28 (38.4) | 3 (4.1) | 4 (22.2) | 1 (5.6) | 1 (5.6) | 12 (66.7) |
| 8 weeks post stent insertion | 23 | 79 | 60 (75.9) | 32 (53.3) | 2 (3.3) | 4 (21.1) | 2 (10.5) | 0 (0.0) | 13 (68.4) | 17 | 80 | 57 (71.3) | 27 (47.4) | 4 (7.0) | 8 (34.8) | 1 (4.3) | 0 (0.0) | 14 (60.9) |
| 12 weeks post stent insertion | 37 | 65 | 42 (64.6) | 18 (42.9) | 0 (0.0) | 7 (30.4) | 2 (8.7) | 0 (0.0) | 14 (60.9) | 34 | 63 | 47 (74.6) | 21 (44.7) | 3 (6.4) | 4 (25.0) | 1 (6.3) | 0 (0.0) | 11 (68.8) |
| 16 weeks post stent insertion | 49 | 53 | 36 (67.9) | 24 (66.7) | 1 (2.8) | 7 (41.2) | 2 (11.8) | 0 (0.0) | 8 (47.1) | 45 | 52 | 37 (71.2) | 20 (54.1) | 3 (8.1) | 4 (26.7) | 2 (13.3) | 0 (0.0) | 9 (60.0) |
| 20 weeks post stent insertion | 57 | 45 | 28 (62.2) | 19 (67.9) | 0 (0.0) | 9 (52.9) | 0 (0.0) | 0 (0.0) | 8 (47.1) | 55 | 42 | 31 (73.8) | 15 (48.4) | 2 (6.5) | 4 (36.4) | 0 (0.0) | 0 (0.0) | 7 (63.6) |
| 24 weeks post stent insertion | 58 | 44 | 24 (54.5) | 14 (58.3) | 1 (4.2) | 6 (30.0) | 0 (0.0) | 0 (0.0) | 14 (70.0) | 58 | 39 | 25 (64.1) | 11 (44.0) | 1 (4.0) | 2 (14.3) | 0 (0.0) | 0 (0.0) | 12 (85.7) |
| 28 weeks post stent insertion | 65 | 37 | 20 (54.1) | 11 (55.0) | 1 (5.0) | 4 (23.5) | 0 (0.0) | 0 (0.0) | 13 (76.5) | 70 | 27 | 16 (59.3) | 10 (62.5) | 1 (6.3) | 4 (36.4) | 0 (0.0) | 0 (0.0) | 7 (63.6) |
| 32 weeks post stent insertion | 72 | 30 | 11 (36.7) | 9 (81.8) | 0 (0.0) | 9 (47.4) | 0 (0.0) | 0 (0.0) | 10 (52.6) | 76 | 21 | 10 (47.6) | 6 (60.0) | 2 (20.0) | 2 (18.2) | 0 (0.0) | 0 (0.0) | 9 (81.8) |
| 36 weeks post stent insertion | 76 | 26 | 11 (42.3) | 7 (63.6) | 0 (0.0) | 3 (20.0) | 0 (0.0) | 0 (0.0) | 12 (80.0) | 81 | 16 | 9 (56.3) | 7 (77.8) | 1 (11.1) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 6 (85.7) |
| 40 weeks post stent insertion | 79 | 23 | 12 (52.2) | 5 (41.7) | 0 (0.0) | 5 (45.5) | 0 (0.0) | 0 (0.0) | 6 (54.5) | 82 | 15 | 8 (53.3) | 5 (62.5) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (100.0) |
| 44 weeks post stent insertion | 85 | 17 | 6 (35.3) | 3 (50.0) | 0 (0.0) | 2 (18.2) | 0 (0.0) | 0 (0.0) | 9 (81.8) | 83 | 14 | 6 (42.9) | 4 (66.7) | 1 (16.7) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 7 (87.5) |
| 48 weeks post stent insertion | 84 | 18 | 6 (33.3) | 4 (66.7) | 0 (0.0) | 3 (25.0) | 0 (0.0) | 0 (0.0) | 9 (75.0) | 84 | 13 | 4 (30.8) | 3 (75.0) | 0 (0.0) | 2 (22.2) | 0 (0.0) | 0 (0.0) | 7 (77.8) |
| 52 weeks post stent insertion | 86 | 16 | 3 (18.8) | 2 (66.7) | 0 (0.0) | 3 (23.1) | 0 (0.0) | 0 (0.0) | 10 (76.9) | 85 | 12 | 5 (41.7) | 2 (40.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (100.0) |
| Chemotherapy | Complete data up to week 12a (N = 149) | Missing data up to week 12a (N = 50) |
|---|---|---|
| Previous chemotherapy given | ||
| None | 118 (79.2) | 43 (86.0) |
| EOX | 11 (7.4) | 2 (4.0) |
| ECX | 6 (4.0) | 1 (2.0) |
| Cisplatin + capecitabine | 4 (2.7) | 0 (0.0) |
| CX; OxCap | 2 (1.3) | 0 (0.0) |
| OxCap | 1 (0.7) | 1 (2.0) |
| Carboplatin + capecitabine + epirubicin | 0 (0.0) | 1 (2.0) |
| Carboplatin + paclitaxel | 1 (0.7) | 0 (0.0) |
| Cisplatin; 5FU | 1 (0.7) | 0 (0.0) |
| Cisplatin + epirubicin | 1 (0.7) | 0 (0.0) |
| CX | 1 (0.7) | 0 (2.0) |
| CX + herceptin; docetaxel | 1 (0.7) | 0 (0.0) |
| Docetaxel; irinotecan | 1 (0.7) | 0 (0.0) |
| ECF | 0 (0.0) | 1 (2.0) |
| ECX neoadjuvant; EOX | 0 (0.0) | 1 (2.0) |
| EOX; docetaxel | 1 (0.7) | 0 (0.0) |
| If had prior chemotherapy, intended number of cycles – median (IQR), n | 6.0 (3.0–6.0), 29 | 3.0 (3.0–8.0), 7 |
| Number of prior chemotherapy cycles given – median (IQR), n | 4.0 (3.0–6.0), 31 | 3.0 (1.0–3.0), 7 |
| MDT intended chemotherapy after stent? | ||
| Yes | 52 (34.9) | 18 (36.0) |
| No | 97 (65.1) | 32 (64.0) |
| Time point | Treatment arm | Effect | Treatment × time effect (p-value) | ||||
|---|---|---|---|---|---|---|---|
| Usual care | EBRT | Time | Treatment | ||||
| Mean (95% CI), n or median (IQR), n | Mean (95% CI), n or median (IQR), n | Adjusted mean difference (95% CI)a | p-value | Adjusted mean difference (95% CI)a | p-value | ||
| EORTC-QLQ-C30/QLQ OG25 facet | |||||||
| Global health (mean) | |||||||
| Baseline | 49.4 (44.2 to 54.4), 99 | 40.2 (35.1 to 45.2), 95 | 0.12 (–0.25 to 0.49) | 0.516 | –1.99 (–6.59 to 2.62) | 0.398 | 0.660 |
| Week 4 | 49.4 (44.5 to 54.4), 77 | 43.0 (36.5 to 49.5), 73 | |||||
| Week 8 | 51.8 (45.3 to 58.3), 57 | 47.1 (40.4 to 53.7), 56 | |||||
| Week 12 | 54.6 (46.8 to 62.4), 41 | 51.4 (45.0 to 57.9), 46 | |||||
| Week 16 | 53.7 (46.7 to 60.7), 34 | 49.0 (41.5 to 56.5), 37 | |||||
| Odynophagia (median) | |||||||
| Baseline | 16.7 (0–50), 101 | 33.3 (16.7–66.7), 95 | –0.65 (–1.06 to –0.23) | 0.002 | 2.69 (–2.52 to 7.91) | 0.311 | 0.694 |
| Week 4 | 16.7 (0–33.3), 77 | 16.7 (0–50), 74 | |||||
| Week 8 | 16.7 (0–33.3), 58 | 16.7 (0–33.3), 59 | |||||
| Week 12 | 16.7 (0–33.3), 43 | 16.7 (0–50), 46 | |||||
| Week 16 | 16.7 (0–33.3), 34 | 16.7 (0–33.3), 35 | |||||
| Dysphagia (median) | |||||||
| Baseline | 33.3 (16.6–44.4), 102 | 33.3 (22.2–66.7), 97 | –0.75(–1.12 to –0.38) | < 0.001 | 4.21 (–0.56 to 8.98) | 0.084 | 0.013 |
| Week 4 | 11.1 (0–33.3), 78 | 22.2 (11.1–44.4), 75 | |||||
| Week 8 | 11.1 (0–33.3), 60 | 11.1 (11.1–33.3), 62 | |||||
| Week 12 | 11.1 (0–33.3), 46 | 11.1 (0–33.3), 49 | |||||
| Week 16 | 11.1 (11.1–33.3), 37 | 11.1 (0–33.3), 37 | |||||
| Pain/discomfort OG25 (median) | |||||||
| Baseline | 16.7 (0–33.3), 102 | 16.7 (0–50), 94 | –0.15 (–0.58 to 0.29) | 0.514 | 2.08 (–3.50 to 7.66) | 0.466 | 0.579 |
| Week 4 | 16.7 (0–33.3), 77 | 25 (0–33.3), 74 | |||||
| Week 8 | 16.7 (0–33.3), 59 | 33.3 (0–50), 59 | |||||
| Week 12 | 16.7 (0–33.3), 44 | 33.3 (0–66.7), 46 | |||||
| Week 16 | 16.7 (0–33.3), 34 | 33.3 (0–33.3), 35 | |||||
| Eating restriction (mean) | |||||||
| Baseline | 52.9 (46.9 to 58.9), 102 | 56.3 (50.6 to 61.9), 95 | –0.45 (–0.87 to –0.02) | 0.040 | 1.86 (–3.73 to 7.47) | 0.513 | 0.555 |
| Week 4 | 44 (37.2 to 50.8), 75 | 47.8 (40.4 to 55.2), 69 | |||||
| Week 8 | 38.6 (31.6 to 45.6), 59 | 48.2 (40.3 to 56.1), 57 | |||||
| Week 12 | 38.8 (30.4 to 47.1), 40 | 42.6 (33.3 to 51.8), 46 | |||||
| Week 16 | 40.8 (31.4 to 50.2), 40 | 42.5 (33.3 to 51.6), 42 | |||||
| Eating in front of others (median) | |||||||
| Baseline | 0 (0–33.3), 98 | 0 (0–66.7), 95 | –0.37 (–0.86 to 0.12) | 0.138 | 1.51 (–4.47 to 7.49) | 0.622 | 0.117 |
| Week 4 | 0 (0–33.3), 74 | 0 (0–33.3), 73 | |||||
| Week 8 | 0 (0–33.3), 58 | 0 (0–33.3), 58 | |||||
| Week 12 | 0 (0–33.3), 42 | 33.3 (0–66.7), 45 | |||||
| Week 16 | 0 (0–0), 33 | 33.3 (0–66.7), 35 | |||||
| Physical functioning (median) | |||||||
| Baseline | 26.7 (8.3–53.3), 102 | 33.3 (13.3–53.3), 95 | 0.68 (0.34 to1.03) | < 0.001 | 1.22 (–3.40 to 5.84) | 0.604 | 0.643 |
| Week 4 | 26.7 (13.3–53.3), 77 | 40 (13.3–60), 73 | |||||
| Week 8 | 33.3 (20–46.7), 59 | 40 (13.3–60), 57 | |||||
| Week 12 | 33.3 (13.3–46.7), 41 | 33.3 (20–53.3), 46 | |||||
| Week 16 | 33.3 (13.3–53.3), 30 | 33.3 (20–58.3), 34 | |||||
| Role functioning (mean) | |||||||
| Baseline | 44.6 (37.4 to 51.8), 102 | 48.6 (41.5 to 55.7), 95 | 0.41 (–0.09 to 0.91) | 0.110 | 2.49 (–4.09 to 9.06) | 0.459 | 0.750 |
| Week 4 | 45.5 (37.5 to 53.6), 75 | 50 (42.0 to 58.0), 68 | |||||
| Week 8 | 43.7 (34.5 to 52.8), 58 | 51.9 (42.7 to 61.0), 54 | |||||
| Week 12 | 38.6 (27.6 to 49.6), 38 | 47.1 (37.1 to 57.1), 46 | |||||
| Week 16 | 44.9 (33.6 to 56.3), 36 | 47.1 (36.1 to 58.2), 41 | |||||
| Emotional functioning (median) | |||||||
| Baseline | 25 (8.3–41.7), 99 | 25 (0–50), 95 | –0.26 (–0.59 to 0.06) | 0.114 | 0.50 (–3.65 to 4.64) | 0.815 | 0.225 |
| Week 4 | 25 (8.3–41.7), 77 | 25 (8.3–58.3), 73 | |||||
| Week 8 | 25 (0–33.3), 58 | 20.8 (8.3–41.7), 56 | |||||
| Week 12 | 16.7 (8.3–33.3), 41 | 25 (8.3–33.3), 46 | |||||
| Week 16 | 16.7 (0–33.3), 30 | 20.8 (8.3–50), 34 | |||||
| Cognitive functioning (median) | |||||||
| Baseline | 16.7 (0–33.3), 99 | 16.7 (0–50), 95 | –0.14 (–0.47 to 0.18) | 0.392 | –0.35 (–4.64 to 3.94) | 0.874 | 0.007 |
| Week 4 | 16.7 (0–33.3), 77 | 16.7 (0–50), 73 | |||||
| Week 8 | 16.7 (0–33.3), 58 | 16.7 (0–41.7), 56 | |||||
| Week 12 | 0 (0–16.7), 41 | 16.7 (0–33.3), 46 | |||||
| Week 16 | 0 (0–16.7), 30 | 25 (0–50), 34 | |||||
| Social functioning (median) | |||||||
| Baseline | 33.3 (0–66.7), 99 | 33.3 (0.66.7), 95 | 0.62 (0.15 to 1.09) | 0.010 | 2.43 (–3.84 to 8.71) | 0.447 | 0.351 |
| Week 4 | 33.3 (0–66.7), 77 | 33.3 (0.66.7), 73 | |||||
| Week 8 | 33.3 (0.66.7), 57 | 33.3 (8.3–66.7), 56 | |||||
| Week 12 | 33.3 (0–50), 41 | 33.3 (16.7–66.7), 46 | |||||
| Week 16 | 33.3 (0–66.7), 30 | 33.3 (16.7–66.7), 34 | |||||
| Fatigue (median) | |||||||
| Baseline | 44.4 (22.2–77.8), 102 | 55.6 (33.3–77.8), 95 | 0.37 (–0.02 to 0.77) | 0.065 | 3.50 (–1.68 to 8.68) | 0.186 | 0.522 |
| Week 4 | 44.4 (33.3–66.7), 77 | 66.7 (33.3–88.9), 73 | |||||
| Week 8 | 44.4 (33.3–66.7), 59 | 44.4 (33.3–77.8), 57 | |||||
| Week 12 | 44.4 (22.2–66.7), 41 | 55.6 (33.3–66.7), 46 | |||||
| Week 16 | 44.4 (33.3–66.7), 30 | 44.4 (33.3–77.8), 34 | |||||
| Nausea/vomiting (median) | |||||||
| Baseline | 16.7 (0–50), 102 | 33.3 (16.7–66.7), 95 | –0.54 (–0.98 to –0.10) | 0.017 | 5.60 (0.11 to 11.08) | 0.046 | 0.195 |
| Week 4 | 16.7 (0–33.3), 77 | 33.3 (0–50), 73 | |||||
| Week 8 | 16.7 (0–33.3), 59 | 16.7 (0–33.3), 57 | |||||
| Week 12 | 16.7 (0–33.3), 41 | 16.7 (0–33.3), 46 | |||||
| Week 16 | 0 (0–33.3), 30 | 16.7 (0–33.3), 34 | |||||
| Pain C30 (median) | |||||||
| Baseline | 33.3 (16.7–66.7), 102 | 50 (16.7–66.7), 95 | –0.56 (–1.01 to –0.11) | 0.015 | 2.39 (–3.25 to 8.03) | 0.406 | 0.005 |
| Week 4 | 33.3 (16.7–50), 77 | 33.3 (16.7–66.7), 73 | |||||
| Week 8 | 16.7 (0–33.3), 58 | 33.3 (16.7–66.7), 57 | |||||
| Week 12 | 16.7 (0–50), 41 | 33.3 (16.7–66.7), 46 | |||||
| Week 16 | 16.7 (0–50), 30 | 50 (16.7–83.3), 34 | |||||
| Dyspnoea (median) | |||||||
| Baseline | 33.3 (0–33.3), 102 | 33.3 (0–66.7), 94 | 0.17 (–0.25 to 0.59) | 0.427 | 2.50 (–3.03 to 8.02) | 0.376 | 0.169 |
| Week 4 | 33.3 (0–33.3), 77 | 33.3 (0–66.7), 73 | |||||
| Week 8 | 33.3 (0–33.3), 59 | 33.3 (0–33.3), 57 | |||||
| Week 12 | 33.3 (0–33.3), 41 | 33.3 (0–66.7), 46 | |||||
| Week 16 | 33.3 (0–33.3), 30 | 33.3 (0–66.7), 34 | |||||
| Insomnia (mean) | |||||||
| Baseline | 37.6 (30.8 to 44.4), 102 | 44.2 (36.7 to 51.7), 95 | –0.70 (–1.21 to –0.18) | 0.009 | 6.76 (–0.10 to 13.42) | 0.047 | 0.688 |
| Week 4 | 32.9 (25.2 to 40.6), 74 | 42.3 (32.7 to 51.8), 67 | |||||
| Week 8 | 25.9 (17.6 to 34.1), 58 | 36.4 (26.4 to 46.5), 54 | |||||
| Week 12 | 21.6 (11.4 to 31.8), 37 | 31.9 (21.8 to 41.9), 45 | |||||
| Week 16 | 29.6 (18.9 to 40.3), 36 | 37.5 (25.6 to 49.4), 40 | |||||
| Appetite loss (mean) | |||||||
| Baseline | 50.8 (43.6 to 58.0), 101 | 62.8 (55.4 to 70.2), 94 | –0.30 (–0.90 to 0.30) | 0.560 | 6.42 (–1.18 to 14.02) | 0.098 | 0.612 |
| Week 4 | 52.9 (45.1 to 60.7), 75 | 55.9 (46.6 to 65.2), 68 | |||||
| Week 8 | 37.9 (29.0 to 46.8), 58 | 55.8 (46.4 to 65.1), 55 | |||||
| Week 12 | 45.9 (33.0 to 58.9), 37 | 47.8 (36.9 to 58.8), 46 | |||||
| Week 16 | 41.7 (31.1 to 52.2), 36 | 49.6 (38.8 to 60.4), 41 | |||||
| Constipation (mean) | |||||||
| Baseline | 42.4 (35.9 to 48.9), 99 | 41.1 (34.2 to 47.9), 95 | –0.95 (–1.49 to –0.41) | 0.003 | –4.33 (–10.78 to 2.12) | 0.240 | 0.009 |
| Week 4 | 48.0 (40.3 to 55.7), 75 | 44.1 (35.1 to 53.1), 68 | |||||
| Week 8 | 30.4 (22.5 to 38.3), 57 | 35.8 (27.2 to 44.4), 54 | |||||
| Week 12 | 22.8 (13.6 to 32.0), 38 | 37.7 (28.7 to 46.7), 46 | |||||
| Week 16 | 27.8 (18.6 to 36.9), 36 | 41.5 (31.0 to 51.9), 41 | |||||
| Diarrhoea (median) | |||||||
| Baseline | 0 (0–0), 98 | 0 (0–33.3), 95 | 0.15 (–0.28 to 0.59) | 0.493 | 2.81 (–2.27 to 7.89) | 0.279 | 0.147 |
| Week 4 | 0 (0–0), 77 | 0 (0–33.3), 72 | |||||
| Week 8 | 0 (0–33.3), 57 | 0 (0–0), 56 | |||||
| Week 12 | 0 (0–33.3), 41 | 0 (0–0), 45 | |||||
| Week 16 | 0 (0–0), 30 | 0 (0–33.3), 34 | |||||
| Financial difficulties (median) | |||||||
| Baseline | 0 (0–0), 99 | 0 (0–33.3), 94 | –0.15 (–0.47 to 0.17) | 0.400 | –0.27 (–4.46 to 3.91) | 0.899 | 0.445 |
| Week 4 | 0 (0–0), 76 | 0 (0–0), 73 | |||||
| Week 8 | 0 (0–0), 56 | 0 (0–0), 56 | |||||
| Week 12 | 0 (0–0), 41 | 0 (0–0), 46 | |||||
| Week 16 | 0 (0–0), 29 | 0 (0–33.3), 34 | |||||
| Body image (median) | |||||||
| Baseline | 0 (0–33.3), 101 | 0 (0–66.7), 94 | –0.15 (–0.66 to 0.36) | 0.920 | –2.23 (–8.66 to 4.20) | 0.496 | 0.011 |
| Week 4 | 0 (0–33.3), 75 | 0 (0–33.3), 74 | |||||
| Week 8 | 0 (0–33.3), 59 | 33.3 (0–66.7), 59 | |||||
| Week 12 | 0 (0–33.3), 44 | 0 (0–66.7), 45 | |||||
| Week 16 | 0 (0–0), 33 | 33.3 (0–66.7), 34 | |||||
| Reflux (median) | |||||||
| Baseline | 33.3 (0–50), 102 | 33.3 (0–66.7), 95 | –0.55 (–0.96 to –0.15) | 0.007 | 0.72 (–4.47 to 5.92) | 0.785 | 0.825 |
| Week 4 | 16.7 (0–33.3), 77 | 33.3 (0–50), 74 | |||||
| Week 8 | 16.7 (0–50), 59 | 16.7 (0–33.3), 59 | |||||
| Week 12 | 16.7 (0–50), 44 | 16.7 (0–50), 46 | |||||
| Week 16 | 8.33 (0–33.3), 34 | 33.3 (0–50), 35 | |||||
| Anxiety (mean) | |||||||
| Baseline | 55.4 (49.2 to 61.6), 102 | 51.2 (44.6 to 57.9), 95 | –0.91 (–1.32 to –0.50) | < 0.001 | –2.87 (–8.45 to 2.71) | 0.313 | 0.002 |
| Week 4 | 49.6 (42.4 to 56.7), 75 | 47.6 (39.3 to 55.9), 69 | |||||
| Week 8 | 45.5 (38.1 to 52.8), 59 | 44.2 (36.1 to 52.2), 57 | |||||
| Week 12 | 38.8 (29.4 to 48.1), 40 | 49.3 (40.1 to 58.4), 46 | |||||
| Week 16 | 40 (30.5 to 49.5), 40 | 46.8 (35.9 to 57.8), 42 | |||||
| Dry mouth (median) | |||||||
| Baseline | 33.3 (0–66.7), 100 | 33.3 (0–66.7), 95 | –0.58 (–1.08 to 0.09) | 0.022 | 0.64 (–5.61 to 6.89) | 0.840 | 0.816 |
| Week 4 | 33.3 (0–66.7), 77 | 33.3 (0–66.7), 74 | |||||
| Week 8 | 0 (0–33.3), 59 | 33.3 (0–66.7), 59 | |||||
| Week 12 | 16.7 (0–33.3), 44 | 33.3 (0–33.3), 43 | |||||
| Week 16 | 0 (0–33.3), 34 | 33.3 (0–66.7), 34 | |||||
| Trouble with taste (median) | |||||||
| Baseline | 0 (0–33.3), 101 | 33.3 (0–66.7), 95 | 0.44 (–0.05 to 0.94) | 0.079 | 1.08 (–5.39 to 7.55) | 0.744 | 0.547 |
| Week 4 | 0 (0–66.7), 76 | 0 (0–66.7), 73 | |||||
| Week 8 | 0 (0–33.3), 59 | 0 (0–66.7), 59 | |||||
| Week 12 | 16.7 (0–50), 44 | 33.3 (0–33.3), 46 | |||||
| Week 16 | 0 (0–33.3), 33 | 33.3 (0–66.7), 34 | |||||
| Trouble swallowing saliva (median) | |||||||
| Baseline | 0 (0–33.3), 102 | 0 (0–33.3), 95 | –0.79 (–1.22 to –0.36) | < 0.001 | –1.84 (–7.31 to 3.63) | 0.511 | 0.025 |
| Week 4 | 0 (0–33.3), 76 | 0 (0–33.3), 74 | |||||
| Week 8 | 0 (0–33.3), 59 | 0 (0–33.3), 59 | |||||
| Week 12 | 0 (0–33.3), 44 | 0 (0–33.3), 46 | |||||
| Week 16 | 0 (0–0), 34 | 0 (0–33.3), 35 | |||||
| Choked when swallowing (median) | |||||||
| Baseline | 0 (0–33.3), 102 | 0 (0–33.3), 95 | –0.49 (–0.86 to 0.11) | 0.011 | –0.74 (–5.29 to 3.80) | 0.749 | 0.321 |
| Week 4 | 0 (0–0), 77 | 0 (0–33.3), 74 | |||||
| Week 8 | 0 (0–0), 59 | 0 (0–33.3), 59 | |||||
| Week 12 | 0 (0–0), 44 | 0 (0–0), 46 | |||||
| Week 16 | 0 (0–0), 34 | 0 (0–0), 35 | |||||
| Trouble with coughing (median) | |||||||
| Baseline | 33.3 (0–33.3), 101 | 33.3 (0–33.3), 95 | –0.28 (–0.71 to 0.15) | 0.202 | –0.62 (–5.94 to 4.70) | 0.820 | 0.370 |
| Week 4 | 33.3 (0–33.3), 77 | 33.3 (0–33.3), 74 | |||||
| Week 8 | 33.3 (0–33.3), 59 | 33.3 (0–33.3), 57 | |||||
| Week 12 | 33.3 (0–33.3), 43 | 33.3 (0–33.3), 46 | |||||
| Week 16 | 33.3 (0–33.3), 34 | 33.3 (0–33.3), 34 | |||||
| Trouble talking (median) | |||||||
| Baseline | 0 (0–0), 99 | 0 (0–33.3), 94 | 0.00 (–0.33 to 0.33) | 0.990 | 0.56 (–3.55 to 4.67) | 0.790 | 0.487 |
| Week 4 | 0 (0–0), 75 | 0 (0–33.3), 74 | |||||
| Week 8 | 0 (0–0), 59 | 0 (0–0), 58 | |||||
| Week 12 | 0 (0–0), 44 | 0 (0–0), 45 | |||||
| Week 16 | 0 (0–0), 34 | 0 (0–33.3), 35 | |||||
| Weight loss (mean) | |||||||
| Baseline | 40.6 (33.2 to 48.0), 101 | 41.8 (34.0 to 49.5), 95 | –0.56 (–1.06 to 0.05) | 0.030 | –1.01 (–7.71 to 5.69) | 0.767 | 0.053 |
| Week 4 | 43.7 (35.3 to 52.1), 74 | 45.4 (36.6 to 54.2), 69 | |||||
| Week 8 | 32.8 (23.9 to 41.7), 59 | 36.3 (27.6 to 45.0), 56 | |||||
| Week 12 | 30.0 (18.7 to 41.3), 40 | 42.8 (33.3 to 52.2), 46 | |||||
| Week 16 | 30.8 (19.2 to 42.5), 40 | 42.1 (30.8 to 53.3), 42 | |||||
| Hair loss (median) | |||||||
| Baseline | 0 (0–0), 17 | 0 (0–33.3), 21 | 0.10 (–0.58 to 0.77) | 0.781 | 0.61 (–7.98 to 9.20) | 0.889 | 0.160 |
| Week 4 | 0 (0–0), 13 | 0 (0–16.7), 16 | |||||
| Week 8 | 0 (0–33.3), 15 | 0 (0–33.3), 11 | |||||
| Week 12 | 33.3 (0–33.3), 10 | 16.7 (0–33.3), 12 | |||||
| Week 16 | 0 (0–0), 11 | 0 (0–33.3), 8 | |||||
| WHO performance status (mean) | |||||||
| Baseline | 1.30 (1.16 to 1.45), 102 | 1.32 (1.19 to 1.45), 97 | 0.03 (0.02 to 0.04) | < 0.001 | 0.06 (–0.09 to 0.20) | 0.457 | 0.565 |
| Week 4 | 1.47 (1.30 to 1.64), 76 | 1.61 (1.44 to 1.79), 75 | |||||
| Week 8 | 1.57 (1.34 to 1.80), 63 | 1.73 (1.52 to 1.95), 60 | |||||
| Week 12 | 1.44 (1.21 to 1.67), 48 | 1.55 (1.35 to 1.75), 49 | |||||
| Week 16 | 1.52 (1.27 to 1.77), 42 | 1.77 (1.55 to 2.00), 44 | |||||
FIGURE 22.
Follow-up in participants still alive by trial arm.
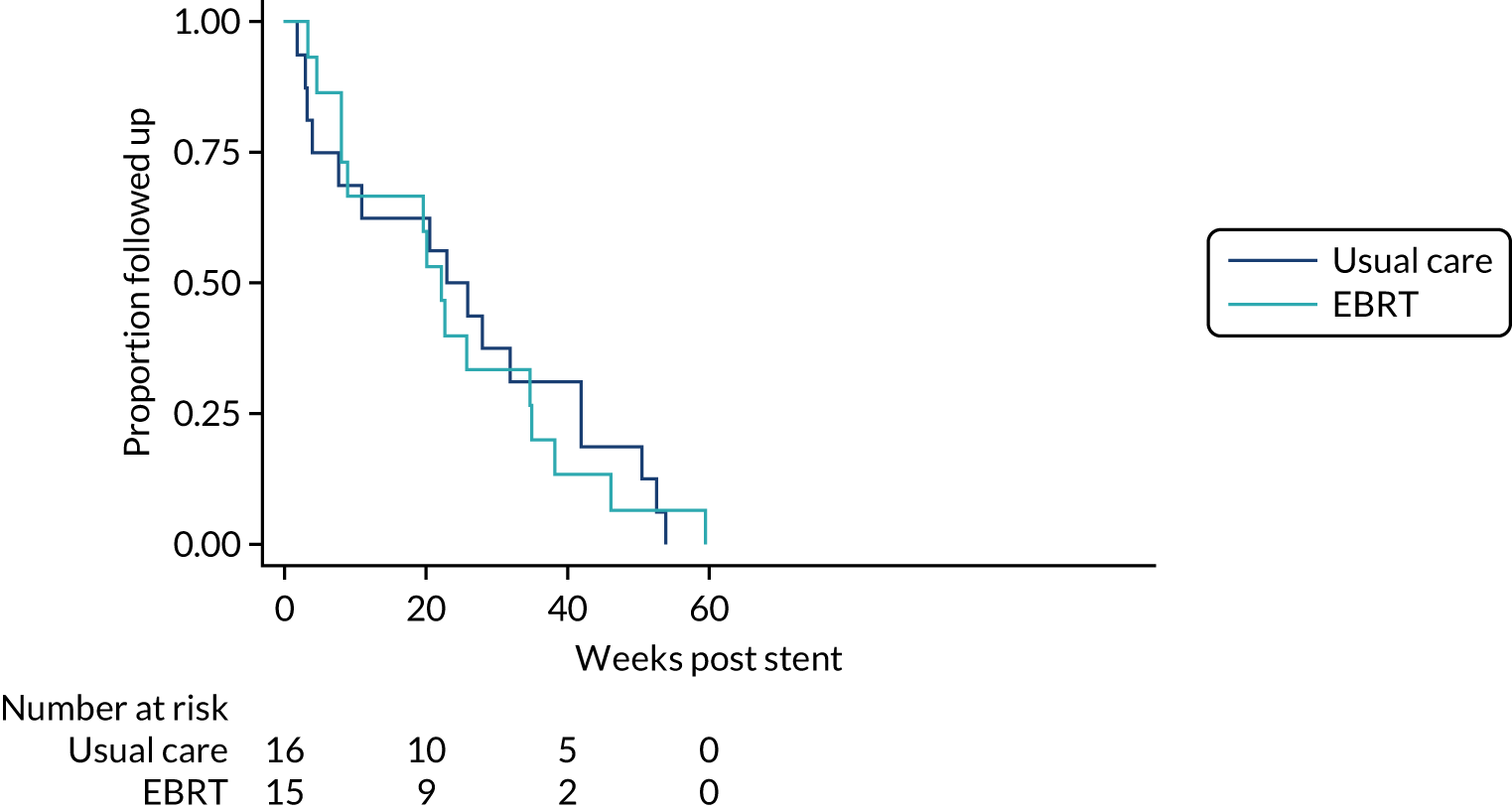
FIGURE 23.
Mean WHO performance status scores and 95% CIs by time and treatment arm.
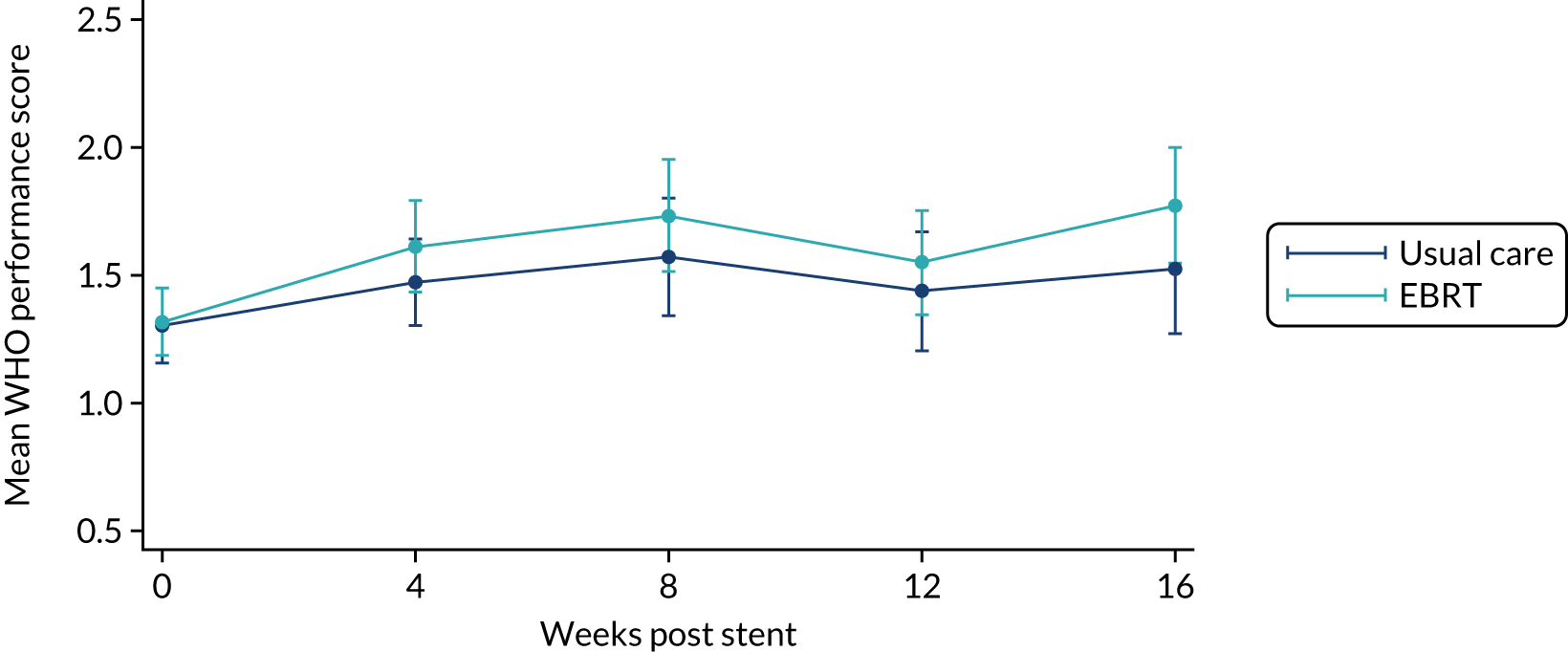
FIGURE 24.
Cumulative incidence function plot of time to first dysphagia-related stent complication or reintervention by trial arm, with death as a competing risk.
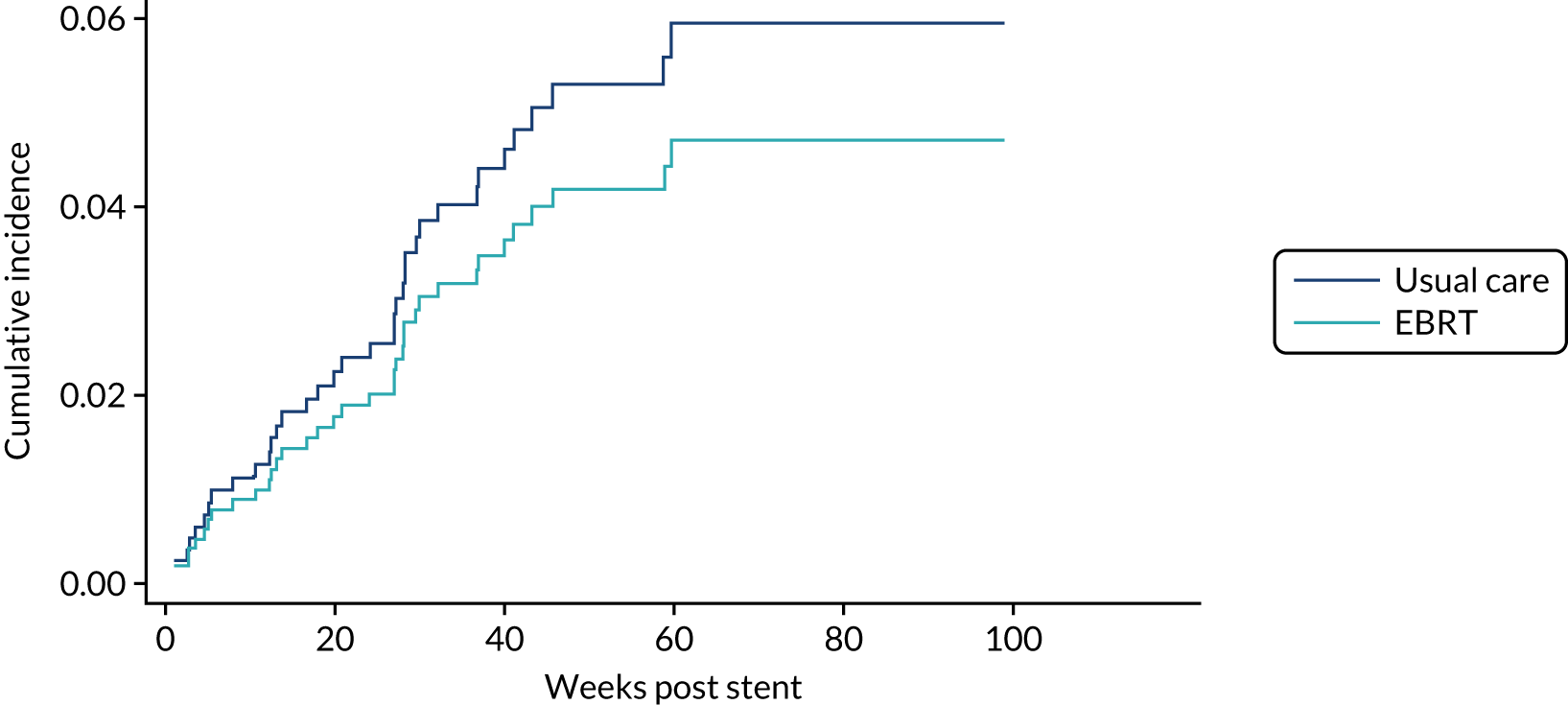
FIGURE 25.
Cumulative incidence function plot of time to first repeat endoscopy by trial arm, with death as a competing risk.
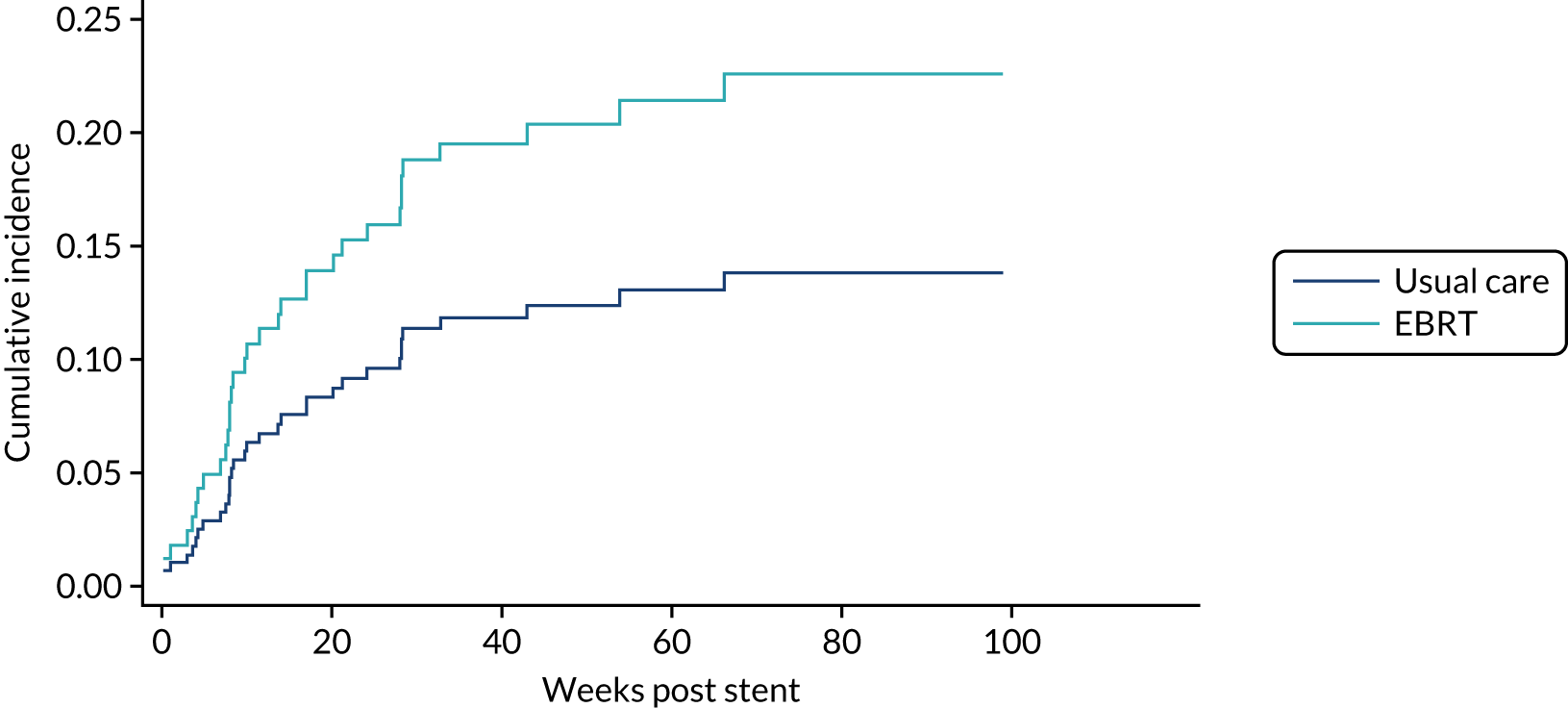
FIGURE 26.
Cumulative incidence function plot of time to overgrowth or undergrowth by trial arm, with death as a competing risk.
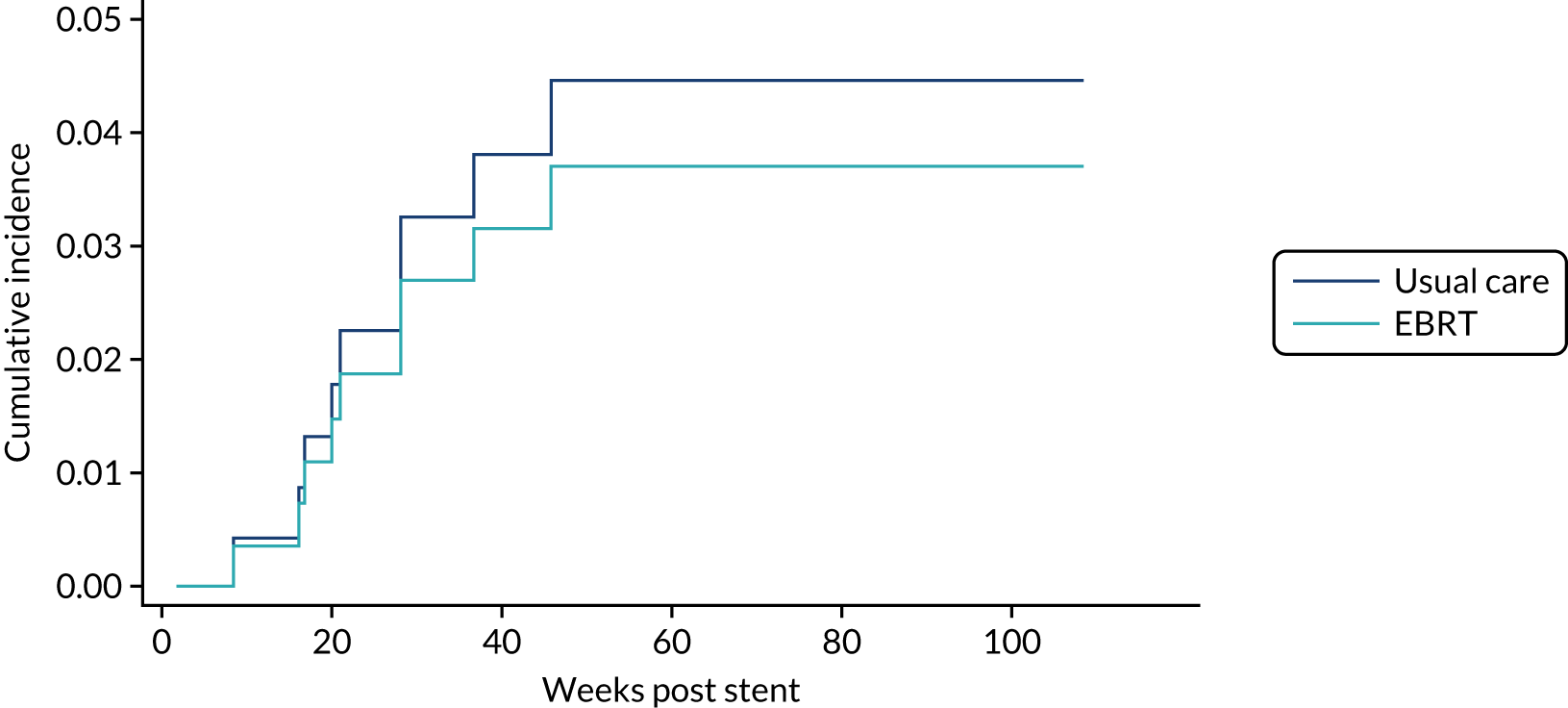
FIGURE 27.
Box plots of nausea/vomiting scores by time and treatment arm.
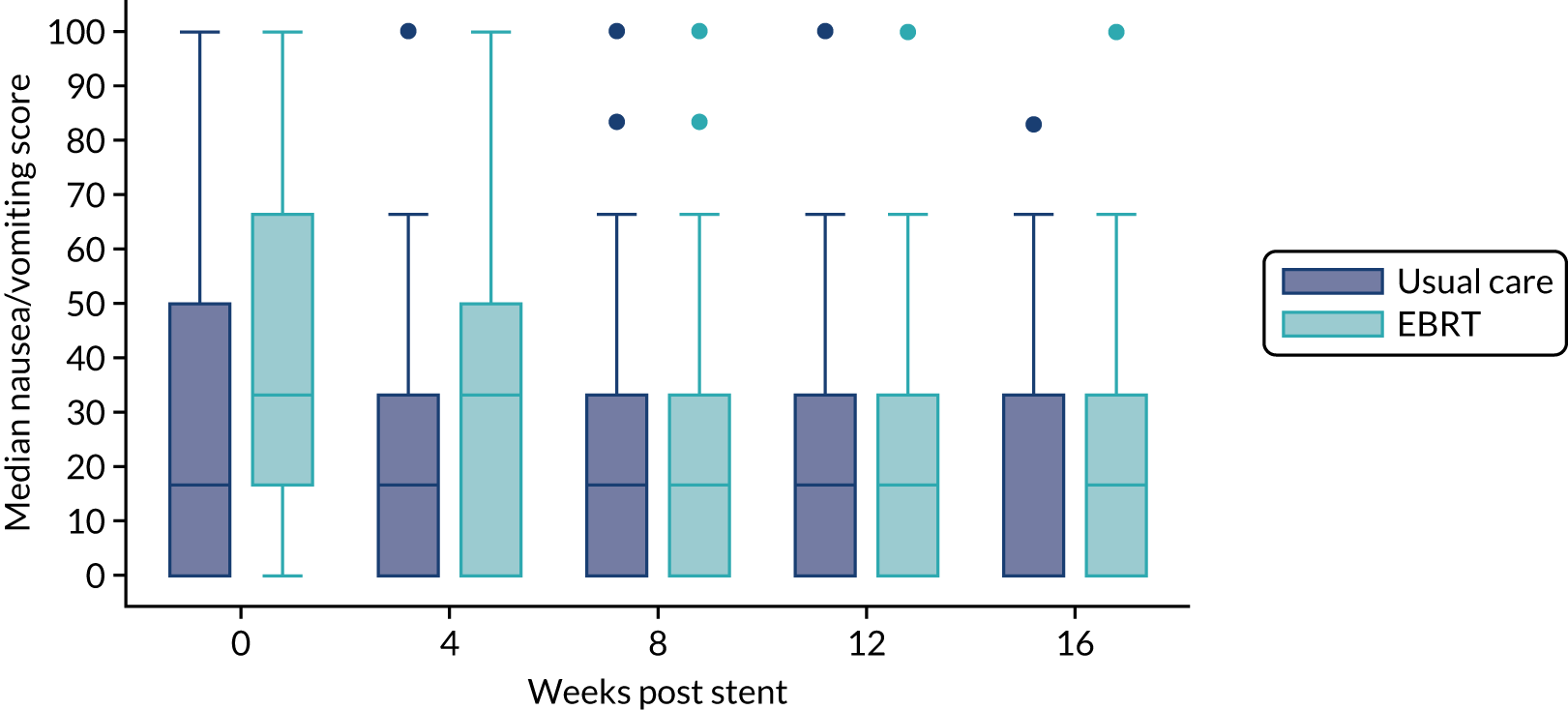
Appendix 4 Health economic evaluation additional tables and figures
| Resource | Currency code/HRG | Unit cost (£) | Notes |
|---|---|---|---|
| Primary care | |||
| GP consultation at surgery | n/a | 37.40a | 9.22 minutes’ duration, including direct care staff and qualifications |
| GP consultation at home | n/a | 86.73a | GP home visit (9.22 minutes) + 20-minute travel time (indirect, £148 per hour) |
| GP consultation by telephone | n/a | 15.10a | 4 minutes’ duration |
| Palliative nurse specialist at surgery | n/a | 37.00a | Band 6; 30-minute visit assumed (£74 per hour) |
| Palliative nurse specialist at home | N21AF | 104.17b | Specialist nursing, palliative/respite care, adult, face to face |
| District nurse consultation at surgery | n/a | 12.33a | Band 6; 10-minute visit (£74 per hour) |
| Dietitian | A03 | 85.76b | Community Health Services |
| Podiatrist | A09A-A09F | 43.39b | Weighted across all options |
| Practice nurse consultation at surgery | n/a | 7.00a | £42.00 per hour (including qualifications), 10-minute appointment assumed |
| Physiotherapist | A08A1 | 57.26b | Physiotherapist, adult, one to one |
| Occupational therapist | A06A1 | 81.31b | Occupational therapist, adult, one to one |
| Social worker in surgery | n/a | 28.00a | £60/hour; £84 per hour of client-related work; 20-minute visit assumed |
| Phlebotomist | n/a | 5.09a | Including £1.93b (weighted average blood test cost) |
| Health visitor | N03F | 52.97b | Health visitor, other clinical intervention |
| Pharmacist | n/a | 7.33a | £44 per hour; 10 minutes assumed |
| Secondary care | |||
| A&E attendance (admitted) | n/a | 236.59b | Weighted across all admitted A&E entries (patient dead on arrival excluded) |
| A&E attendance (discharged) | n/a | 144.90b | Weighted across all discharged A&E entries (patient dead on arrival excluded) |
| Inpatient day (all) | n/a | 494.22b | Weighted across all elective and emergency excess bed-days (paediatrics excluded) |
| Inpatient day (elective) | n/a | 431.11b | Weighted across all elective excess bed-days (paediatrics excluded) |
| Inpatient day (emergency) | n/a | 557.33b | Weighted across all emergency excess bed-days (paediatrics excluded) |
| Outpatient appointment (unspecified) | n/a | 143.44b | Weighted across all consultant-led outpatient visits |
| Outpatient appointment (oncologist) | n/a | 182.45b | Weighted for first and follow-up attendance |
| Outpatient appointment (general surgeon) | n/a | 149.24b | Weighted for first and follow-up attendance |
| Day hospital | n/a | 742.09b | Weighted across all day case entries |
| Specialist nurse in hospital | n/a | 37.00a | Band 6; £111 per hour patient contact; 20-minute appointment assumed |
| Procedures and imaging | |||
| Stenting | n/a | 877.09b | Based on endoscopic insertion of luminal stent (weighted for all options) |
| Blood transfusion (day case) | SA44A | 499.00b | Single plasma exchange or other intravenous blood transfusion, aged ≥ 19 years |
| Blood transfusion (per unit) | n/a | 176.52c | Weighted for first and subsequent units; inflated from 2015 costs |
| Endoscopy | FE21Z, FE22Z, FE50A | 329.74b | Weighted across all relevant options |
| CT scan | RD20A-RD28Z | 103.95b | Weighted for all options in a number of areas |
| Oesophageal biopsy | FE21Z | 476.19b | Diagnostic endoscopic upper gastrointestinal tract procedures with biopsy, aged ≥ 19 years |
| Radiotherapy | SC96Z | 83.06b | Same day radiotherapy admission or attendance (excluding brachytherapy) |
| Chemotherapy (per cycle) | |||
| Chemotherapy as day case (unspecified) | n/a | 1099.77b | Includes drug procurement and delivery (weighted across all day case options) |
| 5-FU chemotherapy | n/a | 1445.49b | Includes procurement of two drugs (bands 5 and 6) and delivery |
| Carboplatin chemotherapy | n/a | 654.41b | Includes procurement of one drug (band 6) and delivery |
| Carboplatin/capecitabine chemotherapy | n/a | 1021.73b | Includes procurement of two drugs (bands 1 and 6) and delivery |
| Capecitabine chemotherapy | n/a | 468.79b | Includes procurement of one drug (band 1) and oral delivery |
| CAP chemotherapy | n/a | 1550.48b | Includes procurement of three drugs (bands 2, 5 and 6) and delivery |
| Cisplatin chemotherapy | n/a | 815.88b | Includes procurement of one drug (band 5) and delivery |
| Cisplatin/capecitabine chemotherapy | n/a | 1121.71b | Includes procurement of two drugs (bands 1 and 5) and delivery |
| Docetaxel chemotherapy | n/a | 654.41b | Includes procurement of one drug (band 6) and delivery |
| Durvalumab chemotherapy | n/a | 5427.48b,d | Includes procurement and delivery every 2 weeks |
| ECF chemotherapy | n/a | 1711.94b | Includes procurement of three drugs (bands 2, 5 and 5) and delivery |
| ECX chemotherapy | n/a | 1449.64b | Includes procurement of three drugs (bands 1, 2 and 5) and delivery |
| EOX chemotherapy | n/a | 1159.65b | Includes procurement of three drugs (bands 1, 2 and 3) and delivery |
| Epirubicin/oxaliplatin chemotherapy | n/a | 853.82b | Includes procurement of two drugs (bands 2 and 3) and delivery |
| HCX chemotherapy | n/a | 1449.64b | Includes procurement of three drugs (bands 1, 2 and 5) and delivery |
| Irinotecan chemotherapy | n/a | 654.41b | Includes procurement of one drug (band 6) and delivery |
| Oxaliplatin chemotherapy | n/a | 525.89b | Includes procurement of one drug (band 3) and delivery |
| Oxaliplatin/capecitabine chemotherapy | n/a | 893.20b | Includes procurement of two drugs (bands 1 and 3) and delivery |
| Paclitaxel/carboplatin chemotherapy | n/a | 982.34b | Includes procurement of two drugs (bands 2 and 6) and delivery |
| Paclitaxel/ramucirumab | n/a | 5823.41b,d | Includes procurement of two drugs (band 2 and ramucirumab) and delivery at day 1 and ramucirumab and single delivery on day 15 |
| Raltitrexed/oxaliplatin chemotherapy | n/a | 1155.50b | Includes procurement of two drugs (bands 3 and 5) and delivery |
| Trastuzumab (Herceptin) chemotherapy | n/a | 514.19b | Includes procurement of one drug (band 2) and delivery |
| Social care | |||
| District nurse consultation at home | N02AF | 38.45b | District nurse, adult, face to face |
| Care assistant | n/a | 27.00a | £27 per hour; 1 hour assumed (based on home care worker, face to face) |
| Social worker | n/a | 48.00a | £60 per hour; £84 per hour of client-related work; 20-minute visit; 20-minute travel assumed |
| Hospice day | n/a | 165.04e | Per day, inflated to 2018 prices |
| Medication type | Dose | Pack size | Unit cost |
|---|---|---|---|
| Accrete D3 | 1.5 g/400 units | 60 | £2.95 |
| Acidophilus extra | 10 billion | 60 | £19.95a |
| Aclidinium bromide/formoterol (Duaklir Genuair) | 340/12 µg | 60 puffs | £32.50 |
| Actrapid Insulin | 100 units/ml | 10 ml | £15.68 |
| Adcal-D3 Dissolve | 750 mg/200 unit | 112 | £2.95 |
| Adcal-D3 Dissolve | 1500 mg/400 unit | 56 | £5.99 |
| Akynzeo | 300 mg/0.5 mg | 1 | £69.00 |
| Alendronic acid | 70 mg | 4 | £0.86 |
| Alfentanil infection | 5 mg/ml | 10 | £21.95 |
| Allopurinol | 100 mg | 28 | £1.72 |
| Allopurinol | 300 mg | 28 | £6.35 |
| Alprazolam (Xanax) | 0.5 mg | 60 | £6.09 |
| Amiodarone | 100 mg | 28 | £1.63 |
| Amiodarone | 200 mg | 28 | £2.91 |
| Amitriptyline | 10 mg | 28 | £1.01 |
| Amitriptyline | 25 mg | 28 | £0.72 |
| Amlodipine | 5 mg | 28 | £0.71 |
| Amlodipine | 10 mg | 28 | £0.71 |
| Amoxicillin | 250 mg | 21 | £1.05 |
| Amoxicillin | 500 mg | 21 | £1.08 |
| Amoxicillin oral suspension | 50 mg/ml | 100 ml | £1.26 |
| Amoxicillin IV | 1 g | 10 | £10.96 |
| Anastrozole | 1 mg | 28 | £1.84 |
| Antacid and oxetacaine suspension | 10 mg/5 ml | 150 ml | £19.00 |
| Apixaban | 5 mg | 56 | £53.20 |
| Aprepitant | 80 mg | 2 | £31.61 |
| Aprepitant | 125 mg | 5 | £79.03 |
| Aspirin | 75 mg | 28 | £0.71 |
| Aspirin dispersable | 75 mg | 28 | £0.77 |
| Atenolol | 25 mg | 28 | £0.57 |
| Atenolol | 50 mg | 28 | £0.63 |
| Atorvastatin | 10 mg | 28 | £0.69 |
| Atorvastatin | 20 mg | 28 | £0.81 |
| Atorvastatin | 40 mg | 28 | £0.98 |
| Atorvastatin | 80 mg | 28 | £1.65 |
| Atropine sulfate injection | 300 µg | 1 | £7.29 |
| Atropine sulfate injection | 500 µg | 1 | £13.00 |
| Azithromycin | 500 mg | 3 | £1.09 |
| Baclofen | 10 mg | 84 | £1.54 |
| Beconase nasal spray | £4.00 | ||
| Beclomethasone nasal spray | 50 µg/puff | 200 puffs | £3.02 |
| Beclomethasone/formoterol (Fostair) inhaler | 100 µg/6 µg | 120 puffs | £29.32 |
| Beclomethasone/formoterol (Fostair) inhaler | 200 µg/6 µg | 120 puffs | £29.32 |
| Betahistine dihydrochloride | 8 mg | 84 | £1.16 |
| Betamethasone (Betnovate) cream | 0.10% | 30 g | £1.61 |
| Bendroflumethiazide | 2.5 mg | 28 | £0.54 |
| Benzydamine hydrochloride spray | 0.15% | 30 ml | £4.02 |
| Benzydamine hydrochloride mouthwash | 0.15% | 300 ml | £6.95 |
| Benzylpenicillin sodium | 1.2 g | 25 | £109.49 |
| Bimatoprost eye drops | 100 µg/ml | 3 ml | £11.71 |
| Biotene Oralbalance gel | 50 g | £4.46 | |
| Bisacodyl | 5 mg | 60 | £5.39 |
| Bisacodyl suppository | 10 mg | 12 | £3.53 |
| Bisoprolol fumarate | 1.25 mg | 28 | £0.76 |
| Bisoprolol fumarate | 2.5 mg | 28 | £0.69 |
| Bisoprolol fumarate | 3.75 mg | 28 | £0.93 |
| Bisoprolol fumarate | 5 mg | 28 | £0.68 |
| Bisoprolol fumarate | 10 mg | 28 | £0.75 |
| Bonjela choline salicylate | 87 mg/1 g | 15 g | £2.91 |
| Brinzolamide eye drops | 10 mg/ml | 5 ml | £2.00 |
| Budesonide | 3 mg | 100 | £75.05 |
| Buprenorphine transdermal patch | 5 µg/h | 4 | £17.60 |
| Buprenorphine transdermal patch | 10 µg/h | 4 | £31.55 |
| Buprenorphine transdermal patch | 15 µg/h | 4 | £49.15 |
| Buprenorphine transdermal patch (Butec) | 20 µg/h | 4 | £57.46 |
| Buprenorphine transdermal patch | 35 µg/h | 4 | £15.80 |
| Buscopan (hyoscine butylbromide) | 10 mg | 56 | £3.00 |
| Buscopan IV | 20 mg | 10 | £2.92 |
| Calceos chewable (colecaliferol) | 500 mg/400 units | 60 | £4.24 |
| Calcichew D3 Forte | 60 | £4.24 | |
| Calcipotriol topical | 50 µg/1 g | 30 g | £6.64 |
| Calmurid cream | 100 g | £5.75 | |
| Calogen extra | 200 ml | £4.98 | |
| Camellose sodium (optive) eye drops | 1% | 30 | £3.00 |
| Candesartan cilexetil | 4 mg | 28 | £0.54 |
| Candesartan cilexetil | 8 mg | 28 | £4.72 |
| Candesartan cilexetil | 16 mg | 28 | £1.78 |
| Carbamazepine | 100 mg | 84 | £2.07 |
| Carbocisteine | 375 mg | 120 | £4.60 |
| Carbocisteine oral solution | 250 mg/5 ml | 300 ml | £9.49 |
| Carbomer 980 0.2% eye gel | 2 mg/g | 10 g | £2.80 |
| Carvedilol | 6.25 mg | 28 | £0.97 |
| Carvedilol | 25 mg | 28 | £1.17 |
| Cefalexin | 500 mg | 21 | £2.15 |
| Cetirizine | 10 mg | 30 | £0.86 |
| Cetraben ointment | 125 g | £3.49 | |
| Chloramphenicol 0.5% eye drops | 5 mg/ml | 10 ml | £2.96 |
| Chlorhexidine gluconate mouthwash | 0.20% | 300 ml | £4.18 |
| Chlorphenamine maleate | 4 mg | 28 | £0.76 |
| Chlorphenamine maleate (Piriton) | 4 mg | 30 | £2.06 |
| Chlorphenamine maleate injection | 10 mg/1 ml | 5 | £22.49 |
| Cinnarizine | 15 mg | 84 | £5.05 |
| Ciprofloxacin | 500 mg | 10 | £0.91 |
| Citalopram | 10 mg | 28 | £0.89 |
| Citalopram | 20 mg | 28 | £0.88 |
| Clarithromycin | 250 mg | 14 | £1.29 |
| Clarithromycin | 500 mg | 14 | £2.10 |
| Clarithromycin oral suspension | 250 mg/5 ml | 70 ml | £6.07 |
| Clarithromycin IV | 500 mg | 1 | £9.45 |
| Clenil Modutile (beclomethasone inhaler) | 100 µg/puff | 200 | £7.42 |
| Clexane (enoxaparin sodium) IV | 40 mg/0.4 ml | 10 | £30.27 |
| Clexane (enoxaparin sodium) IV | 100 mg/ml | 10 | £72.30 |
| Clexane (enoxaparin sodium) IV | 120 mg/0.8 ml | 10 | £87.93 |
| Clexane (enoxaparin sodium) IV | 150 mg/1 ml | 10 | £99.91 |
| Clopidogrel | 75 mg | 28 | £1.40 |
| Clotrimazole 1% cream | 10 mg/g | 30 g | £2.89 |
| Co-Amilofruse | 5/40 mg | 28 | £5.29 |
| Co-amociclav | 250/125 mg | 21 | £1.79 |
| Co-amociclav | 500/125 mg | 21 | £2.31 |
| Co-amociclav (Augmentin) injection | 1.2 g powder | 10 | £10.60 |
| Co-codamol | 8/500 mg | 30 | £0.81 |
| Co-codamol | 15/500 mg | 100 | £4.12 |
| Co-codamol | 30/500 mg | 100 | £3.63 |
| Co-codamol effervescent tablets | 30/500 mg | 100 | £7.19 |
| Co-danthramer | 25 mg/200 mg/5 ml | 300 ml | £180.00 |
| Co-danthrusate | 12 mg/1 ml | 200 ml | £202.50 |
| Codeine linctus | 3 mg/1 ml | 200 ml | £1.90 |
| Codeine phosphate | 15 mg | 28 | £0.83 |
| Codeine phosphate | 30 mg | 28 | £0.93 |
| Codeine phosphate | 60 mg | 28 | £1.82 |
| Co-dydramol (dihydrocodeine/paracetamol) | 500/7.46 mg | 32 | £4.52 |
| Colchicine | 500 µg | 100 | £8.70 |
| Colestyramine | 4 g | 50 | £10.76 |
| Complan shakes | 57 g | 4 sachets | £2.80 |
| Coracten XL (nifedipine) | 60 mg | 28 | £7.34 |
| Co-trimoxazole | 80/400 mg | 28 | £2.01 |
| Co-trimoxazole IV infusion | 960 mg | 10 | £35.00 |
| Covonia cough mixture (pholcodine) | 5 mg/5 ml | 150 ml | £3.30 |
| Cyclizine | 50 mg | 100 | £6.81 |
| Cyclizine injection | 50 mg/ml | 5 | £17.02 |
| Dalteparin | 2500 units/0.2 ml | 10 | £18.58 |
| Dalteparin (Fragmin) | 5000 units/0.2 ml | 10 | £28.23 |
| Dalteparin (Fragmin) | 7500 units/0.3 ml | 10 | £42.34 |
| Dalteparin | 12,500 units/0.5 ml | 5 | £35.29 |
| Dalteparin (Fragmin) | 15,000 units/0.6 ml | 5 | £42.34 |
| Desunin 800 (colecalciferol) | 800 units | 30 | £3.60 |
| Dexamethasone | 500 µg | 28 | £10.27 |
| Dexamethasone | 2 mg | 50 | £30.01 |
| Dexamethasone | 4 mg | 50 | £60.01 |
| Dexamethasone | 8 mg | 30 | £72.00 |
| Dexamethasone oral solution | 2 mg/5 ml | 150 ml | £42.30 |
| Dexamethasone for injection | 3.3 mg/1 ml | 10 | £23.21 |
| Dexamethasone for injection | 6.6 mg/2 ml | 10 | £22.00 |
| Dexamethasone for injection | 3.8 mg/1 ml | 10 | £20.00 |
| Diamorphine hydrochloride | 5 mg | 5 | £13.76 |
| Diamorphine hydrochloride | 10 mg | 5 | £16.76 |
| Diamorphine hydrochloride | 30 mg | 5 | £16.14 |
| Diazepam | 2 mg | 28 | £0.71 |
| Diazepam | 5 mg | 28 | £0.61 |
| Diclofenac sodium (Voltarol) gel | 1.16% | 100 g | £4.63 |
| Difflam (benzydamine hydrochloride) | 1.5 mg/ml | 300 ml | £6.50 |
| Diffundox XL (tamsulosin hydrochloride) | 400 µg | 30 | £3.87 |
| Digoxin | 62.5 µg | 28 | £1.39 |
| Digoxin | 125 µg | 28 | £1.40 |
| Digoxin | 250 µg | 28 | £1.44 |
| Dihydrocodeine tartrate | 30 mg | 28 | £0.94 |
| Dihydrocodeine tartrate | 60 mg | 56 | £5.20 |
| Diltiazem hydrochloride | 60 mg | 90 | £7.96 |
| Diltiazem hydrochloride | 120 mg | 56 | £7.15 |
| Docusate sodium oral solution | 50 mg/5 ml | 300 ml | £9.19 |
| Docusate sodium | 100 mg | 30 | £2.09 |
| Domperidone | 10 mg | 30 | £0.94 |
| Domperidone oral suspension | 1 mg/ml | 200 ml | £23.60 |
| Dorzolamide eye drops | 20 mg/ml | 5 ml | £2.38 |
| Dorzolamide/timolol eye drops | 20 and 5 mg/ml | 5 ml | £2.04 |
| Doxazosin | 2 mg | 28 | £0.86 |
| Doxazosin | 4 mg | 28 | £1.00 |
| Doxazosin | 8 mg | 28 | £1.92 |
| Doxycycline | 50 mg | 28 | £1.21 |
| Doxycycline | 100 mg | 8 | £0.91 |
| E45 cream | 100 g | £4.28 | |
| Elantan LA25 (isosorbide mononitrate) | 25 mg | 28 | £3.40 |
| Emollient cream (Dermol) | 1 mg | 100 g | £2.86 |
| Enalapril | 10 mg | 28 | £1.81 |
| Enoxaparin | 20 mg/0.2 ml | 10 | £20.86 |
| Enoxaparin | 40 mg/0.4 ml | 10 | £30.37 |
| Enoxaparin | 80 mg/0.8 ml | 10 | £55.13 |
| Enoxaparin | 100 mg/ml | 10 | £72.30 |
| Ensure Supplement | 250 ml | £2.39 | |
| Epimax cream | 500 g | £2.49 | |
| Epirubicin hydrochloride infusion | 100 mg/50 ml | 1 | £201.76 |
| Ertapenem injection | 1 g | 10 | £31.65 |
| Erythromycin | 250 mg | 28 | £1.40 |
| Esomeprazole | 20 mg | 28 | £2.28 |
| Esomeprazole | 40 mg | 28 | £2.27 |
| Exanatide | 10 µg/0.04 ml | 1 | £81.89 |
| Ezetimibe | 10 mg | 28 | £2.17 |
| Felodipine | 5 mg | 28 | £4.21 |
| Felodipine | 10 mg | 28 | £5.66 |
| Fentanyl patch | 12 µg | 5 | £12.59 |
| Fentanyl patch | 25 µg | 5 | £17.99 |
| Fentanyl patch | 37.5 µg | 5 | £15.46 |
| Fentanyl patch | 50 µg | 5 | £33.66 |
| Fentanyl patch | 75 µg | 5 | £46.99 |
| Fentanyl patch | 100 µg | 5 | £57.86 |
| Ferric carboxymaltose (Ferinject) | 500 mg | 5 | £405.88 |
| Ferric carboxymaltose (Ferinject) | 1000 mg/20 ml | 1 | £154.23 |
| Ferrous fumarate | 210 mg | 84 | £3.50 |
| Ferrous fumarate | 140 mg/5 ml | 200 ml | £0.72 |
| Ferrous gluconate | 300 mg | 28 | £1.00 |
| Ferrous sulphate | 200 mg | 28 | £1.06 |
| Fexofenadine hydrochloride | 120 mg | 30 | £2.05 |
| Filgrastim | 30 million units/ml | 5 | £263.52 |
| Filgrastim | £304.59 | ||
| Finasteride | 5 mg | 28 | £1.18 |
| Fluconazole | 50 mg | 7 | £0.72 |
| Fluconazole | 200 mg | 7 | £3.92 |
| Fluconazole oral suspension | 50 mg/5 ml | 35 ml | £25.50 |
| Fluconazole IV | 200 mg/100 ml | 5 | £19.45 |
| Fludrocortisone acetate | 100 µg | 30 | £13.60 |
| Fluoxetine | 20 mg | 30 | £0.93 |
| Fluoxetine | 40 mg | 30 | £1.80 |
| Fluticasone | 50 µg | 120 | £6.53 |
| Fluticasone (Flixotide Accuhaler) | 250 µg | 60 puffs | £25.51 |
| Fluticasone fuorate nasal spray (Avamys) | 27.5 µg | 120 puffs | £6.44 |
| Fluticasone/vilanterol (Relvar Ellipta) inhaler | 92 µg/22 µg | 30 doses | £22.00 |
| Folic acid | 5 mg | 28 | £0.73 |
| Forceval vitamins | 30 | £9.92 | |
| Formoterol Easyhaler | 12 µg | 120 | £23.75 |
| Fortijuce | 200 ml | £2.02 | |
| Fortisip Compact | 500 ml | £5.32 | |
| Fortisip Standard | 200 ml | £1.12 | |
| Fostair NEXTHaler | 100/6 µg | 120 puffs | £29.32 |
| Fresubin Energy | 200 ml | £1.40 | |
| Fultium D3 oral solution | 3000 units/ml | 100 ml | £144.00 |
| Fultium D3 capsules | 800 units | 30 | £3.60 |
| Furosemide IV | 20 mg/2 ml | 10 | £13.42 |
| Furosemide tablets | 20 mg | 28 | £1.48 |
| Furosemide tablets | 40 mg | 28 | £2.09 |
| Furosemide | 20 mg/5 ml | 150 ml | £14.81 |
| Gabapentin | 300 mg | 100 | £3.15 |
| Gaviscon Advance | 5 ml | 500 ml | £5.12 |
| Gentamicin IV | 360 mg/120 ml | 20 | £174.07 |
| Gliclazide | 40 mg | 28 | £1.53 |
| Gliclazide | 80 mg | 28 | £0.82 |
| Glycerol suppository | 4 g | 12 | £1.16 |
| Glyceryl trinitrate transdermal patch | 5 mg/24 hours | 28 | £12.77 |
| Glyceryl trinitrate spray | 400 µg/dose | 200 | £3.83 |
| Haloperidol | 500 µg | 28 | £29.88 |
| Haloperidol | 1.5 mg | 28 | £14.81 |
| Haloperidol injection | 5 mg/1 ml | 10 | £35.00 |
| Hexetidine (Gelclair) | 200 ml | £2.92 | |
| Humalog (insulin lispro) | 100 units/ml | 10 ml | £16.61 |
| Humulin M3 insulin | 100 units/ml | 1 | £15.68 |
| Hydrocortisone injection | 100 mg/ml | 5 | £10.60 |
| Hydroxocobalamin injection | 1 mg/1 ml | 5 | £10.60 |
| Hydroxychloroquine | 200 mg | 60 | £3.70 |
| Hylo Tear | 0.10% | 10 ml | £8.50 |
| Hyoscine butylbromide tablet | 10 mg | 56 | £3.00 |
| Hyoscine butylbromide injection | 20 mg/ml | 10 | £2.92 |
| Hyoscine hydrobromide patch | 1 mg/72 hours | 2 | £12.87 |
| Hypovase (prazosin) | 500 µg | 60 | £2.69 |
| Ibuprofen | 200 mg | 16 | £1.18 |
| Ibuprofen | 400 mg | 24 | £0.91 |
| Ibuprofen 10% gel (Fenbid Forte) | 100 g | £4.00 | |
| Ibuprofen oral suspension | 100 mg/5 ml | 100 | £1.49 |
| Indoramin | 25 mg | 84 | £60.26 |
| Influenza vaccine | Prefilled | 1 | £8.00 |
| Inositol nicotinate | 1 g | 50 | £13.12b |
| Ipratropium bromide (Atrovent inhaler) | 20 µg/puff | 200 | £5.56 |
| Irbesartan | 300 mg | 28 | £2.77 |
| Iron dextran infusion | 500 mg/10 ml | 2 | £79.70 |
| Isosorbide mononitrate | 10 mg | 56 | £1.07 |
| Isosorbide mononitrate | 40 mg | 56 | £1.30 |
| Isotard 25XL (iIsosorbide mononitrate) | 25 mg | 28 | £6.75 |
| Isotard 50XL (isosorbide mononitrate) | 50 mg | 28 | £6.75 |
| Isotard 60XL (isosorbide mononitrate) | 60 mg | 28 | £10.50 |
| Ispaghula Husk Granules (Fybogel) | 3.5 g | 30 | £2.83 |
| Ivabradine | 2.5 mg | 56 | £91.75 |
| Januvia (sitagliptin) | 100 mg | 28 | £33.26 |
| Ketorolac trometamol 0.5% eye drops | 5 mg/ml | 5 ml | £3.57 |
| Lacidipine | 2 mg | 28 | £2.64 |
| Lacidipine | 4 mg | 28 | £2.57 |
| Lacri-lube eye ointment | 3.5 g | £3.01 | |
| Lactulose oral solution | 3.1–3.7 g/5 ml | 500 ml | £2.40 |
| Lactulose oral solution (sachets) | 10 g/15 ml | 10 | £2.51 |
| Lansoprazole | 15 mg | 28 | £0.76 |
| Lansoprazole | 30 mg | 28 | £1.01 |
| Lansoprazole orodispersible | 15 mg | 28 | £3.12 |
| Latanoprost eye drops | 50 µg/ml | 2.5 ml | £7.92 |
| Laxido oral powder | 30 | £3.84 | |
| Lercanidipine hydrochloride | 20 mg | 28 | £3.83 |
| Letrozole | 2.5 mg | 14 | £0.95 |
| Levemir insulin detemir | 100 units/ml | 5 | £42.00 |
| Levomepromazine | 25 mg | 84 | £20.26 |
| Levomepromazine IV | 25 mg/ml | 10 | £20.13 |
| Levonorgestrel/ethinylestradiol | 150 µg/30 µg | 63 | £2.82 |
| Levothyroxine | 12.5 µg | 28 | £1.78 |
| Levothyroxine | 25 µg | 28 | £1.63 |
| Levothyroxine | 50 µg | 28 | £1.03 |
| Levothyroxine | 75 µg | 28 | £2.73 |
| Levothyroxine | 100 µg | 28 | £1.03 |
| Levothyroxine oral solution | 25 µg/5 ml | 100 ml | £94.99 |
| Levothyroxine oral solution | 125 µg/5 ml | 100 ml | £185.00 |
| Lisinopril | 2.5 mg | 28 | £0.80 |
| Lisinopril | 5 mg | 28 | £0.80 |
| Lisinopril | 10 mg | 28 | £0.80 |
| Lisinopril | 20 mg | 28 | £0.88 |
| Loperamide hydrochloride | 2 mg | 30 | £1.83 |
| Loratadine | 10 mg | 30 | £0.88 |
| Lorazepam | 500 mg | 28 | £12.00 |
| Lorazepam | 1 mg | 28 | £3.38 |
| Losartan | 50 mg | 28 | £2.07 |
| Losartan | 100 mg | 28 | £1.95 |
| Lumigan 0.1% eye drops (bimatoprost) | 100 µg/ml | 3 ml | £11.71 |
| Macrogol 3350 powder | 85 g/l | 28 | £29.85 |
| Magnesium aspartate powder | 243 mg | 10 | £8.95 |
| Magnesium Hydroxide | 79 mg/ml | 500 ml | £5.31 |
| Mebeverine | 200 mg | 60 | £9.22 |
| Meropenem IV | 500 mg | 10 | £88.90 |
| Meropenem IV | 1 g | 10 | £206.28 |
| Metformin (Sukkarto SR) | 500 mg | 56 | £2.38 |
| Metformin (Glucient SR, Glucophage SR) | 500 mg | 56 | £4.00 |
| Metformin (Glucient SR) | 1 g | 56 | £6.40 |
| Metformin (oral solution) | 500 mg/5 ml | 150 ml | £6.80 |
| Metoclopramide hydrochloride | 10 mg | 28 | £0.61 |
| Metoclopramide hydrochloride oral solution | 5 mg/5 ml | 150 ml | £19.77 |
| Metoclopramide hydrochloride IV | 10 mg/2 ml | 10 | £2.65 |
| Methotrexate | 2.5 mg | 28 | £1.77 |
| Metronidazole | 400 mg | 21 | £5.47 |
| Metronidazole IV | 500 mg/100 ml | 20 | £63.86 |
| Microlax enema | 5 ml | 12 | £4.87 |
| Midazolam injection | 2 mg/2 ml | 10 | £6.00 |
| Midazolam | 5 mg/5 ml | 10 | £9.63 |
| Midazolam injection | 10 mg/2 ml | 10 | £6.43 |
| Mirabegron | 50 mg | 30 | £29.00 |
| Mirtazapine | 15 mg | 28 | £1.23 |
| Mirtazapine | 30 mg | 28 | £7.28 |
| Misoprostol (Cytotec) | 200 mg | 60 | £10.03 |
| Montelukast | 10 mg | 28 | £1.17 |
| Morphine (Sevredol) | 10 mg | 56 | £5.31 |
| Morphine (Sevredol) | 20 mg | 56 | £10.61 |
| Morphine sulphate oral solution | 10 mg/5 ml | 300 ml | £5.32 |
| Morphine sulphate injection | 1 mg/1 ml | 10 | £31.90 |
| Morphine sulphate Injection | 5 mg/5 ml | 10 | £44.00 |
| Morphine sulphate Injection | 10 mg/ml | 10 | £11.13 |
| Morphine sulphate Injection | 15 mg/ml | 10 | £10.74 |
| Morphine sulphate injection | 20 mg/ml | 10 | £68.95 |
| Morphine sulphate infusion | 50 mg/50 ml | 1 | £5.78 |
| Movicol Ready to Take | 13.7 g | 30 sachets | £7.72 |
| MST Continus | 5 mg | 60 | £3.29 |
| MST Continus | 10 mg | 60 | £5.20 |
| MST Continus | 15 mg | 60 | £9.10 |
| MST Continus | 30 mg | 60 | £12.47 |
| MST Continus | 60 mg | 60 | £24.32 |
| MST Continuous | 100 mg | 60 | £38.50 |
| MST Continus modified-release granules | 20 mg | 30 sachets | £24.58 |
| Mucodyne (carbocisteine) | 250 mg/5 ml | 300 ml | £8.55 |
| Multivitamin (Centrum assumed) | £8.33a | ||
| Mupirocin cream 2% | 20 mg/1g | 15 g | £5.26 |
| MXL capsules | 90 mg | 28 | £22.04 |
| Nebivolol | 5 mg | 28 | £4.37 |
| Nefopam hydrochloride | 30 mg | 90 | £7.77 |
| Nicorandil | 5 mg | 60 | £4.29 |
| Nicotine Inhalator | 15 mg | 4 | £4.87 |
| Nicotine patch | 7 mg | 7 | £9.12 |
| Nifedipine | 10 mg | 56 | £7.34 |
| Nitrofurantoin | 50 mg | 28 | £8.21 |
| Nitrofurantoin (Macrobid) | 100 mg | 14 | £9.50 |
| NovoMix 30 (biphasic insulin aspart) | 100 units/ml | 5 | £29.89 |
| Nutrison concentrated | 500 ml | £5.87 | |
| Nystatin | 100,000 units/ml | 30 ml | £2.03 |
| Olmesartan | 20 mg | 28 | £1.52 |
| Omeprazole | 10 mg | 28 | £0.82 |
| Omeprazole | 20 mg | 28 | £0.83 |
| Omeprazole | 40 mg | 28 | £2.24 |
| Omeprazole infusion | 40 mg | 5 | £26.00 |
| Ondansetrone | 4 mg | 10 | £9.94 |
| Ondansetrone | 8 mg | 10 | £10.31 |
| Ondansetrone oral solution | 4 mg/5 ml | 50 ml | £38.10 |
| Ondansetrone injection | 4 mg/2 ml | 5 | £29.97 |
| Ondansetrone injection | 8 mg/4 ml | 5 | £15.00 |
| Oramorph | 10 mg/5 ml | 300 ml | £5.32 |
| Oramorph | 20 mg/ml | 120 ml | £19.50 |
| Oxazepam | 10 mg | 28 | £7.68 |
| Oxybutynin hydrochloride | 2.5 mg | 56 | £2.17 |
| Oxycodone hydrochloride tablets | 10 mg | 56 | £25.04 |
| Oxycodon hydrochloride (OxyNorm) capsules | 5 mg | 56 | £11.43 |
| Oxycodon hydrochloride (OxyNorm) capsules | 10 mg | 56 | £22.86 |
| Oxycodone hydrochloride (OxyNorm) capsules | 20 mg | 56 | £45.71 |
| Oxycodone hydrochloride (OxyNorm) oral solution | 5 mg/5 ml | 250 | £9.71 |
| Oxycodone hydrochloride prolonged release | 15 mg | 56 | £19.06 |
| Oxycodone hydrochloride modified release | 5 mg | 28 | £12.52 |
| Oxycodone hydrochloride modified release | 10 mg | 56 | £25.04 |
| Oxycodone hydrochloride modified release | 15 mg | 56 | £38.12 |
| Oxycodone hydrochloride modified release | 20 mg | 56 | £50.08 |
| Oxycodone hydrochloride modified release | 30 mg | 56 | £76.23 |
| Oxycodone hydrochloride modified release | 40 mg | 56 | £100.19 |
| Oxycodone hydrochloride injection | 20 mg/2 ml | 5 | £16.00 |
| Pabrinex | 5 ml and 2 ml | 20 | £22.53 |
| Palonosetron | 500 µg | 1 | £55.89 |
| Pancreatine (Creon) | 25,000 units | 100 | £28.25 |
| Pantoprazole | 40 mg | 28 | £1.06 |
| Paracetamol | 250 mg/5 ml | 100 ml | £1.75 |
| Paracetamol | 500 mg | 100 | £1.81 |
| Paracetamol | 1 g | 100 | £2.49 |
| Paracetamol orodispersible | 250 mg | 24 | £4.12 |
| Paracetamol soluble | 500 mg | 100 | £6.80 |
| Paracetamol soluble | 1 g | 50 | £6.59 |
| Paracetamol oral solution | 250 mg/5 ml | 200 ml | £4.40 |
| Paracetamol oral solution | 500 mg/5 ml | 200 ml | £18.00 |
| Paracetamol infusion | 1 g/100 ml | 10 | £12.00 |
| Parenteral nutrition supplement | Infusion | 1 | £3.55 |
| Paroxetine | 30 mg | 30 | £1.79 |
| Peppermint oil capsules | 0.2 ml | 84 | £7.04 |
| Peptac Liquid | 500 ml | £1.95 | |
| Perindopril erbumine | 2 mg | 30 | £2.04 |
| Perindopril erbumine | 8 mg | 30 | £2.53 |
| Phenytoin | 300 mg | 28 | £9.11 |
| Phorpain 5% ibuprofen gel | 50 mg/g | 100 g | £2.26 |
| Phosphate Sandoz | 1.93 6g | 100 | £19.39 |
| Pizotifen | 500 µg | 28 | £1.02 |
| Potassium chloride | 600 mg | 100 | £13.12b |
| Pramipexole | 0.088 mg | 30 | £5.18 |
| Prednisolone | 1 mg | 28 | £0.63 |
| Prednisolone | 5 mg | 28 | £0.74 |
| Prednisolone | 10 mg | 28 | £1.77 |
| Pregabalin | 25 mg | 56 | £2.79 |
| Pregabalin | 50 mg | 84 | £3.25 |
| Pregabalin | 75 mg | 56 | £3.25 |
| Pregabalin | 100 mg | 84 | £4.27 |
| Pregabalin | 150 mg | 56 | £3.67 |
| Pregabalin | 300 mg | 56 | £5.55 |
| Probiotic tabletsb | 60 | £9.49† | |
| Pro-Cal Shot | 720 ml | 1 | £15.26 |
| Prochlorperazine | 5 mg | 28 | £0.73 |
| Prochlorperazine oral solution | 5 mg/5 ml | 100 ml | £3.34 |
| Propranolol hydrochloride | 40 mg | 28 | £2.05 |
| Propranolol hydrochloride | 80 mg | 56 | £2.98 |
| Propranolol hydrochloride oral solution | 10 mg/5 ml | 150 ml | £28.45 |
| ProSource Plus | 30 ml | 100 | £149.08 |
| Prostap SR DCS (leuprorelin acetate) | 3.75 mg | 1 | £75.24 |
| Prostap 3 DCS (leuprorelin acetate) | 11.25 mg | 1 | £225.72 |
| Quinine bisulphate | 300 mg | 28 | £2.78 |
| Qvar 50 Inhaler (beclometasone) | 50 µg/puff | 200 puffs | £3.70 |
| Ramipril | 1.25 mg | 28 | £1.93 |
| Ramipril | 2.5 mg | 28 | £4.24 |
| Ramipril | 5 mg | 28 | £4.63 |
| Ramipril | 10 mg | 28 | £5.12 |
| Ramipril oral solution | 2.5 mg/5 ml | 150 ml | £96.00 |
| Ranitidine | 75 mg | 12 | £2.80 |
| Ranitidine | 150 mg | 60 | £1.07 |
| Ranitidine | 300 mg | 30 | £1.08 |
| Ranitidine | 75 mg/5 ml | 300 ml | £6.39 |
| Ranitidine injection | 50 mg/2 ml | 5 | £4.40 |
| Rivaroxaban | 10 mg | 30 | £54.00 |
| Rivaroxaban | 15 mg | 28 | £50.40 |
| Rivaroxaban | 20 mg | 28 | £50.40 |
| Rolapitant | 90 mg | 2 | £47.42 |
| Rosuvastatin | 10 mg | 28 | £1.41 |
| Rosuvastatin | 20 mg | 28 | £2.01 |
| Salbutamol | 5 mg/2.5 ml | 20 | £3.90 |
| Salbutamol Easyhaler | 100 µg | 200 puffs | £3.31 |
| Salbutamol Easyhaler | 200 µg | 200 puffs | £6.63 |
| Salbutamol Airomir Autohaler | 100 µg | 200 puffs | £6.02 |
| Sando K potassium | 600/400 mg | 100 | £9.95 |
| Sativex oromucosal spray (cannabis extract) | 270 | £375.00 | |
| Scandishake | 85 g | 6 | £15.54 |
| Secura cream | 3.25 oz | £6.60a | |
| Seebri Breezhaler (glycopyrronium) | 44 µg | 30 | £27.50 |
| Senna | 7.5 mg | 60 | £2.01 |
| Senna (Senokot MaxStrength tablets) | 15 mg | 48 | £5.69 |
| Senna (Senokot) | 7.5 mg/5 ml | 500 ml | £4.76 |
| Seretide 250 Evohaler | 25/250 µg | 120 | £29.32 |
| Seretide 250 Accuhaler | 50/250 µg | 60 | £35.00 |
| Sertraline | 50 mg | 28 | £0.82 |
| Sertraline | 100 mg | 28 | £1.08 |
| Simeticone (Maalox) | 250 ml | £2.91 | |
| Simvastatin | 10 mg | 28 | £0.90 |
| Simvastatin | 20 mg | 28 | £0.75 |
| Simvastatin | 40 mg | 28 | £0.87 |
| Sodium acid phosphate/sodium phosphate | enema | 1 | £3.98 |
| Sodium bicarbonate | 500 mg | 56 | £1.47 |
| Sodium chloride | 0.009 | 1 l | £3.59 |
| Sodium chloride with glucose | 5%/0.45% | 500 ml | £2.34 |
| Sodium chloride (nebuliser solution) | 4 ml | 60 | £24.30 |
| Sodium chloride nebuliser liquid | 2.5 ml | 20 | £4.36 |
| Sodium picosulfate | 5 mg/5 ml | 300 ml | £7.10 |
| Solifenacin succinate | 5 mg | 30 | £27.62 |
| Solpadeine (co-codamol) | 500 mg/12.8 mg | 20 | £3.61 |
| Solpadol (co-codamol) | 30/500 mg | 30 | £1.09 |
| Spatone | 560 ml | 28 | £12.00a |
| Spironolactone | 25 mg | 28 | £1.05 |
| Sucralfate (unlicensed) | 1 g | 100 | £31.71c |
| Sulfasalazine | 500 mg | 112 | £8.12 |
| Sulfasalazine | 250 mg/5 ml | 500 ml | £44.51 |
| Symbicort 100/6 Turbohaler | 100/6 µg | 120 puffs | £28.00 |
| Symbicort 200/6 Turbohaler | 200/6 µg | 120 puffs | £28.00 |
| Tamsulosin hydrochloride | 400 µg | 30 | £10.47 |
| Tazocin IV | 4 g/0.5 g | 1 | £15.17 |
| Teicoplanin | 200 mg | 1 | £3.93 |
| Teicoplanin | 400 mg | 1 | £7.32 |
| Temazepam | 10 mg | 28 | £3.00 |
| Testosterone (Testogel) | 50 mg/5 g | 30 | £31.11 |
| Terbinafine | 250 mg | 14 | £1.20 |
| Terbutaline | 500 µg | 120 puffs | £8.30 |
| Theophylline (Uniphylline Continus) | 400 mg | 56 | £5.65 |
| Thiamine | 50 mg | 100 | £3.99 |
| Thiamine | 100 mg | 100 | £5.74 |
| Ticagrelor | 90 mg | 56 | £54.60 |
| Timolol maleate (Timoptol 0.5%) | 5 mg/ml | 5 ml | £3.12 |
| Tinzaparin sodium | 3500 units/0.35 ml | 10 | £27.71 |
| Tinzaparin sodium | 10,000 units/0.5 ml | 10 | £59.50 |
| Tinzaparin sodium | 12,000 units/0.6 ml | 10 | £71.40 |
| Tiotropium (Braltus) inhaler | 10 µg/dose | 30 | £25.80 |
| Tiotropium (Spritiva Respimat) inhaler | 2.5 µg/dose | 60 | £23.00 |
| Tiotropium bromide (Spiriva) | 18 µg | 30 | £33.50 |
| Tramadol hydrochloride (Zydol) | 50 mg | 60 | £4.60 |
| Tramadol hydrochloride Injection | 100 mg/2 ml | 5 | £4.00 |
| Tranexamic acid | 500 mg | 60 | £5.42 |
| Tranexamic acid injection | 500 mg/5 ml | 10 | £15.47 |
| Travadan (travoprost) eye drops | 40 µg/ml | 2.5 ml | £3.24 |
| Trazodone hydrochloride | 100 mg/5 ml | 120 | £185.45 |
| Trimethoprim | 200 mg | 14 | £0.89 |
| Udder cream | 340 g | £8.89a | |
| Ultibro Breezhaler (glycopyrronium/indacaterol) | 85 mg/43 mg | 30 | £32.50 |
| Uniphyllin Continus | 200 mg | 56 | £2.96 |
| Uniphyllin Continus | 400 mg | 56 | £5.65 |
| Urea moisturising cream | 50 mg/1 g | 500 | £14.99 |
| Ursodeoxycholic acid | 300 mg | 60 | £55.08 |
| Venlafaxine | 75 mg | 56 | £3.40 |
| Ventolin Evohaler (salbumatol) | 100 µg/puff | 200 puffs | £1.50 |
| Ventolin Accuhaler (salbumatol) | 200 µg/puff | 60 puffs | £3.60 |
| Viscopaste bandage | 7.5 cm × 6 m | 1 | £3.78 |
| Vitamin B compound | 15/1/1 mg | 28 | £26.63 |
| Vitamin K (menadiol phosphate) | 5 mg | 100 | £204.49 |
| Warfarin sodium | 500 µg | 28 | £1.34 |
| Warfarin sodium | 1 mg | 28 | £0.67 |
| Warfarin sodium | 3 mg | 28 | £0.74 |
| Warfarin sodium | 5 mg | 28 | £0.78 |
| Xerotin (mucilage) | 100 ml | £6.86 | |
| Zimovane (zopiclone) | 7.5 mg | 28 | £0.87 |
| Zolpidem tartrate | 10 mg | 28 | £0.92 |
| Zomorph | 10 mg | 60 | £3.47 |
| Zomorph | 30 mg | 60 | £8.30 |
| Zomorph | 60 mg | 60 | £16.20 |
| Zopiclone | 3.75 mg | 28 | £0.88 |
| Zopiclone | 7.5 mg | 28 | £0.87 |
| Zoton FasTab (Lansoprazole) | 15 mg | 28 | £2.90 |
| Zoton FasTab (Lansoprazole) | 30 mg | 28 | £4.26 |
| SA-ID | Parameter | Change |
|---|---|---|
| SA1 | Costs | –10% |
| SA2 | Costs | –20% |
| SA3 | Costs | –30% |
| SA4 | Costs | +10% |
| SA5 | Costs | +20% |
| SA6 | Costs | +30% |
| SA7 | Costs | Intervention cost microcosted |
| SA8 | Utilities | –10% |
| SA9 | Utilities | –20% |
| SA10 | Utilities | –30% |
| SA11 | Utilities | +10% |
| SA12 | Utilities | +20% |
| SA13 | Utilities | +30% |
| SA14 | All parameters | Complete cases used |
| SA15 | All parameters | All available cases used |
| SA16 | All parameters post 12 weeks | Weeks 13 to 16 used |
| SA17 | All parameters post 12 weeks | Weeks 13 to 28 used |
| Model stage | Arm | Parameter | Used |
|---|---|---|---|
| Weeks 1 and 2 | Both | Probability of death | 0.01 |
| Total = 199 | |||
| Weeks 3 and 4 | EBRT | Probability of death | 0.03 |
| Usual care | Probability of death | 0.05 | |
| Total = 193 | |||
| Entering Markov cycles | EBRT | Probability of starting in ‘ability to swallow improved’ stage | 0.77 |
| Probability of starting in ‘progression/not improved’ stage | 0.16 | ||
| Probability of death | 0.06 | ||
| Total = 91 | |||
| Usual care | Probability of starting in ‘ability to swallow improved’ stage | 0.76 | |
| Probability of starting in ‘progression/not improved’ stage | 0.13 | ||
| Probability of death | 0.11 | ||
| Total = 91 | |||
| Markov: ability to swallow improved – weeks 4 to 8 | EBRT | Probability of remaining in ability to swallow improved | 0.92 |
| Probability of progression of dysphagia (move to progressive state) | 0.06 | ||
| Probability of death (move to absorbing state) | 0.02 | ||
| Total = 65 | |||
| Usual care | Probability of remaining in ability to swallow improved | 0.91 | |
| Probability of progression of dysphagia (move to progressive state) | 0.06 | ||
| Probability of death (move to absorbing state) | 0.03 | ||
| Total = 61 | |||
| Markov: dysphagia progression – weeks 4 to 8 | EBRT | Probability of improvement (move to improved ability to swallow state) | 0.34 |
| Probability of remaining in progressive state | 0.63 | ||
| Probability of death (move to absorbing state) | 0.03 | ||
| Total = 17 | |||
| Usual care | Probability of improvement (move to improved ability to swallow state) | 0.21 | |
| Probability of remaining in progressive state | 0.75 | ||
| Probability of death (move to absorbing state) | 0.04 | ||
| Total = 19 | |||
| Markov: ability to swallow improved – weeks 8 to 12 | EBRT | Probability of remaining in ability to swallow improved | 0.90 |
| Probability of progression of dysphagia (move to progressive state) | 0.05 | ||
| Probability of death (move to absorbing state) | 0.05 | ||
| Total = 50 | |||
| Usual care | Probability of remaining in ability to swallow improved | 0.91 | |
| Probability of progression of dysphagia (move to progressive state) | 0.06 | ||
| Probability of death (move to absorbing state) | 0.03 | ||
| Total = 50 | |||
| Markov: dysphagia progression – weeks 8 to 12 | EBRT | Probability of improvement (move to improved ability to swallow state) | 0.20 |
| Probability of remaining in progressive state | 0.70 | ||
| Probability of death (move to absorbing state) | 0.10 | ||
| Total = 14 | |||
| Usual care | Probability of improvement (move to improved ability to swallow state) | 0.15 | |
| Probability of remaining in progressive state | 0.78 | ||
| Probability of death (move to absorbing state) | 0.07 | ||
| Total = 18 | |||
| Markov: ability to swallow improved – weeks 12 onward | EBRT | Probability of remaining in ability to swallow improved | 0.82 |
| Probability of progression of dysphagia (move to progressive state) | 0.08 | ||
| Probability of death (move to absorbing state) | 0.09 | ||
| Usual care | Probability of remaining in ability to swallow improved | 0.88 | |
| Probability of progression of dysphagia (move to progressive state) | 0.06 | ||
| Probability of death (move to absorbing state) | 0.06 | ||
| Markov: dysphagia progression – weeks 12 onward | EBRT | Probability of improvement (move to improved ability to swallow state) | 0.11 |
| Probability of remaining in progressive state | 0.79 | ||
| Probability of death (move to absorbing state) | 0.09 | ||
| Usual care | Probability of improvement (move to improved ability to swallow state) | 0.15 | |
| Probability of remaining in progressive state | 0.73 | ||
| Probability of death (move to absorbing state) | 0.12 | ||
| Model stage | Arm | Mean | SD | n |
|---|---|---|---|---|
| Weeks 1 and 2 | Both | 0.6141 | 0.2831 | 199 |
| Weeks 3 and 4 | EBRT | 0.5610 | 0.3214 | 91 |
| Usual care | 0.5860 | 0.3020 | 91 | |
| Markov: ability to swallow improved – weeks 5 to 8 | EBRT | 0.6090 | 0.2957 | 75 |
| Usual care | 0.6006 | 0.2896 | 78 | |
| Markov: dysphagia progression – weeks 5 to 8 | EBRT | 0.3359 | 0.3505 | 16 |
| Usual care | 0.4980 | 0.3688 | 13 | |
| Markov: ability to swallow improved – weeks 9 to 12 | EBRT | 0.5439 | 0.3149 | 65 |
| Usual care | 0.6602 | 0.2437 | 61 | |
| Markov: dysphagia progression – weeks 9 to 12 | EBRT | 0.4624 | 0.3090 | 17 |
| Usual care | 0.5230 | 0.3160 | 19 | |
| Markov: ability to swallow improved – weeks 13 onwards | EBRT | 0.6264 | 0.2634 | 148 |
| Usual care | 0.6659 | 0.1718 | 220 | |
| Markov: dysphagia progression – weeks 13 onwards | EBRT | 0.5674 | 0.3226 | 72 |
| Usual care | 0.6239 | 0.1924 | 65 |
FIGURE 28.
Comparing the transition probabilities for patients who begin in a worsening or improving state of dysphagia and the different probabilities for the next state: radiotherapy.
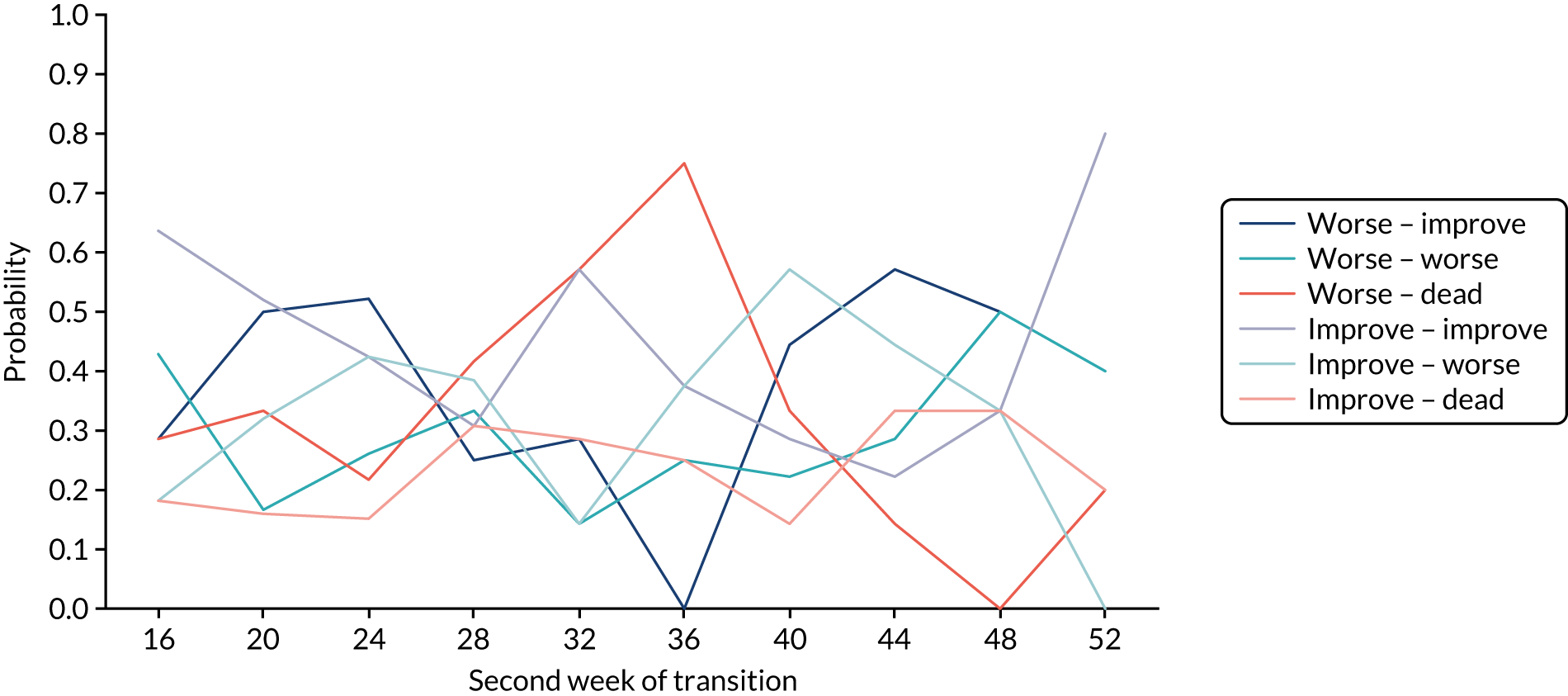
FIGURE 29.
Comparing the transition probabilities for patients who begin in a worsening or improving state of dysphagia and the different probabilities for the next state: usual care.
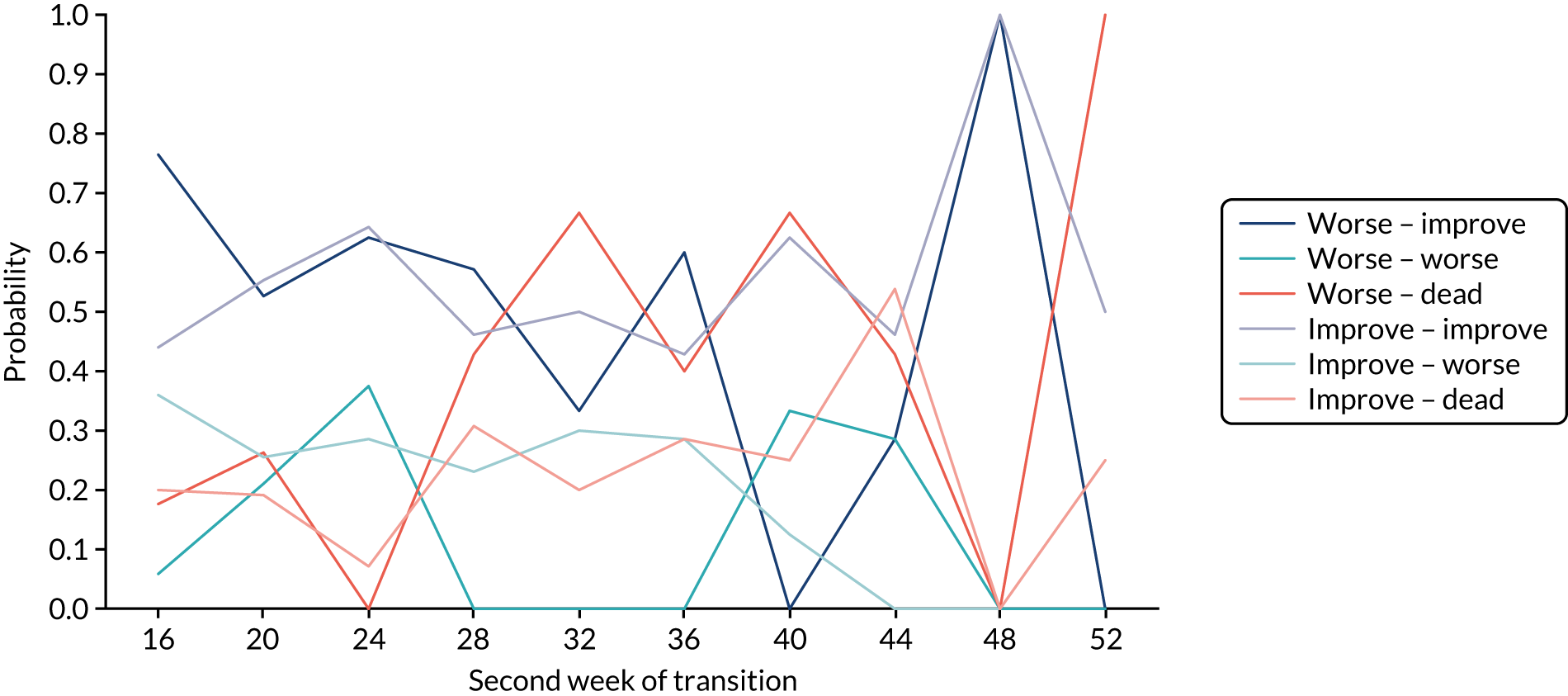
List of abbreviations
- A&E
- accident and emergency
- AIC
- Akaike information criterion
- BIC
- Bayesian information criterion
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CSRI
- client service receipt inventory
- CT
- computerised tomography
- CTCAE
- Common Terminology Criteria for Adverse Events
- CTR
- Centre for Trials Research
- CUA
- cost–utility analysis
- DDFS
- dysphagia deterioration-free survival
- EBRT
- external beam radiotherapy
- EORTC
- European Organisation for Research and Treatment of Cancer
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GI
- gastrointestinal
- GP
- general practitioner
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IDMC
- Independent Data Monitoring Committee
- IQR
- interquartile range
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ITT
- intention to treat
- MDT
- multidisciplinary team
- NCRI
- National Cancer Research Institute
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NSAID
- non-steroidal anti-inflammatory drug
- OG25
- oesophago-gastric module
- OR
- odds ratio
- OS
- overall survival
- PIS
- participant information sheet
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QLQ
- Quality of Life Questionnaire
- QoL
- quality of life
- RCT
- randomised controlled trial
- ROCS
- Radiotherapy after Oesophageal Cancer Stenting
- RTTQA
- Radiotherapy Trials Quality Assurance
- SAE
- serious adverse event
- SD
- standard deviation
- SEMS
- self-expanding metal stent
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VBA
- Visual Basics for Applications
- WHO
- World Health Organization
